User login
Tularemia: A Rare But Nationally Notifiable Disease
The pediatrician’s first patient of the day was an 8-year-old boy, accompanied by both of his parents. It was the boy’s third visit in just over a week for fever and left-sided neck swelling, and the family was understandably anxious for answers.
“The antibiotics don’t seem to be working,” the mother explained. “He still has fever every day, as high as 104, and his neck looks just as swollen.”
A quick review of the chart revealed the boy’s initial diagnosis had been bacterial lymphadenitis, for which amoxicillin-clavulanate had been prescribed. Three days later, given lack of clinical improvement, therapy was transitioned to clindamycin. On examination, the boy was febrile and ill-appearing with a 3-cm by 5-cm tender, non-fluctuant swelling over the left sternocleidomastoid muscle.
The pediatrician ran through a quick mental checklist of diagnostic possibilities for his patient’s continued symptoms. Staphylococcal lymphadenitis still seemed possible. Could the boy be infected with methicillin-resistant Staphylococcus aureus that was also clindamycin resistant? Alternately, perhaps the problem was “source control” and the boy had developed an occult neck abscess that needed to be drained. An ultrasound could help sort that out. Finally, the pediatrician considered less common bacterial causes of lymph node swelling and fever. He placed Bartonella henselae, the cause of cat scratch disease, near the top of his list. “I’ve never seen it,” he told the parents, “But we could also consider tularemia.”
On average, 200 cases of tularemia are reported in the United States each year, and the incidence of disease is increasing, according to a surveillance report released by the Centers for Disease Control and Prevention in December 2023.1
Between 2011 and 2022, 2462 tularemia cases were reported in the United States. That translated to an average annual incidence of 0.064 per 100,000 population, an increase of 56% compared with 2001-2010. Forty-seven states reported at least one case of tularemia, although half of all reported cases came from four states — Arkansas (18%), Kansas (11%), Missouri (11%), and Oklahoma (10%). The incidence of tularemia was highest in children ages 5-9 years old, older men, and American Indian or Alaska Natives individuals. Although cases occurred year-round, 78% had symptom onset May through September.
In the United States, most human cases of tularemia have been arthropod borne, transmitted by the bite of an infected tick or deer fly. Infection also can be spread through contact with infected animals or animal tissue, particularly rabbits, hares, muskrats, prairie dogs, and other rodents, including hamsters. Outbreaks of tularemia have occurred among pet store hamsters, and at least one child in the United States developed tularemia after being bitten by a pet hamster.
Tularemia is almost always associated with fever but other clinical manifestations vary by the type of exposure. Ulceroglandular disease occurs after a tick or deer fly bite or after handling an infected animal. An ulcer develops at the site where the bacteria entered the body, along with enlargement of regional lymph nodes. Less commonly, lymph node swelling can occur without the development of an ulcer. If the bacteria enter through the eye, symptoms include conjunctivitis and swelling of pre-auricular lymph nodes. Eating or drinking contaminated food or water is associated with sore throat, mouth ulcers, tonsillitis, and swelling of lymph glands in the neck. Pneumonic tularemia, the most serious form of the disease, typically happens after inhaling bacteria-containing dust or aerosols and is associated with cough, chest pain, and difficulty breathing. Pneumonic tularemia can develop if other forms of tularemia are untreated, and the bacteria spread to the lung.
Back in the exam room, the pediatrician carefully re-examined the boy’s scalp. A 1-cm poorly healing ulcer on the left occiput added support for the diagnosis of ulceroglandular tularemia, the most common form of the disease in children. Serologic testing ultimately confirmed the diagnosis and the boy’s symptoms resolved with treatment.
Gentamicin administered intravenously or intramuscularly is the drug of choice for the treatment of tularemia in children. Ciprofloxacin is considered an alternative but is not approved by the U.S. Food and Drug Administration for this indication.
The pediatrician reported the case of tularemia to his local health department. Tularemia is a nationally notifiable disease in the United States; state health departments report to the CDC through the National Notifiable Diseases Surveillance System. In turn, public health authorities shared information to prevent tularemia. Steps to prevent tick and deer fly bites include the use of an Environmental Protection Agency–registered insect repellent. Individuals who hunt, trap, or skin animals are encouraged to wear gloves when handling animals —especially rabbits, muskrats, and prairie dogs — and cook game meat thoroughly. Tularemia can be inadvertently aerosolized if an infected animal or carcass is run over with a tractor or lawnmower. Checking for carcasses before mowing may reduce the risk.
Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
Reference
1. Rich SN et al. Tularemia—United States, 2011-2022. MMWR Morb Mortal Wkly Rep 2025;73:1152–1156. doi:
The pediatrician’s first patient of the day was an 8-year-old boy, accompanied by both of his parents. It was the boy’s third visit in just over a week for fever and left-sided neck swelling, and the family was understandably anxious for answers.
“The antibiotics don’t seem to be working,” the mother explained. “He still has fever every day, as high as 104, and his neck looks just as swollen.”
A quick review of the chart revealed the boy’s initial diagnosis had been bacterial lymphadenitis, for which amoxicillin-clavulanate had been prescribed. Three days later, given lack of clinical improvement, therapy was transitioned to clindamycin. On examination, the boy was febrile and ill-appearing with a 3-cm by 5-cm tender, non-fluctuant swelling over the left sternocleidomastoid muscle.
The pediatrician ran through a quick mental checklist of diagnostic possibilities for his patient’s continued symptoms. Staphylococcal lymphadenitis still seemed possible. Could the boy be infected with methicillin-resistant Staphylococcus aureus that was also clindamycin resistant? Alternately, perhaps the problem was “source control” and the boy had developed an occult neck abscess that needed to be drained. An ultrasound could help sort that out. Finally, the pediatrician considered less common bacterial causes of lymph node swelling and fever. He placed Bartonella henselae, the cause of cat scratch disease, near the top of his list. “I’ve never seen it,” he told the parents, “But we could also consider tularemia.”
On average, 200 cases of tularemia are reported in the United States each year, and the incidence of disease is increasing, according to a surveillance report released by the Centers for Disease Control and Prevention in December 2023.1
Between 2011 and 2022, 2462 tularemia cases were reported in the United States. That translated to an average annual incidence of 0.064 per 100,000 population, an increase of 56% compared with 2001-2010. Forty-seven states reported at least one case of tularemia, although half of all reported cases came from four states — Arkansas (18%), Kansas (11%), Missouri (11%), and Oklahoma (10%). The incidence of tularemia was highest in children ages 5-9 years old, older men, and American Indian or Alaska Natives individuals. Although cases occurred year-round, 78% had symptom onset May through September.
In the United States, most human cases of tularemia have been arthropod borne, transmitted by the bite of an infected tick or deer fly. Infection also can be spread through contact with infected animals or animal tissue, particularly rabbits, hares, muskrats, prairie dogs, and other rodents, including hamsters. Outbreaks of tularemia have occurred among pet store hamsters, and at least one child in the United States developed tularemia after being bitten by a pet hamster.
Tularemia is almost always associated with fever but other clinical manifestations vary by the type of exposure. Ulceroglandular disease occurs after a tick or deer fly bite or after handling an infected animal. An ulcer develops at the site where the bacteria entered the body, along with enlargement of regional lymph nodes. Less commonly, lymph node swelling can occur without the development of an ulcer. If the bacteria enter through the eye, symptoms include conjunctivitis and swelling of pre-auricular lymph nodes. Eating or drinking contaminated food or water is associated with sore throat, mouth ulcers, tonsillitis, and swelling of lymph glands in the neck. Pneumonic tularemia, the most serious form of the disease, typically happens after inhaling bacteria-containing dust or aerosols and is associated with cough, chest pain, and difficulty breathing. Pneumonic tularemia can develop if other forms of tularemia are untreated, and the bacteria spread to the lung.
Back in the exam room, the pediatrician carefully re-examined the boy’s scalp. A 1-cm poorly healing ulcer on the left occiput added support for the diagnosis of ulceroglandular tularemia, the most common form of the disease in children. Serologic testing ultimately confirmed the diagnosis and the boy’s symptoms resolved with treatment.
Gentamicin administered intravenously or intramuscularly is the drug of choice for the treatment of tularemia in children. Ciprofloxacin is considered an alternative but is not approved by the U.S. Food and Drug Administration for this indication.
The pediatrician reported the case of tularemia to his local health department. Tularemia is a nationally notifiable disease in the United States; state health departments report to the CDC through the National Notifiable Diseases Surveillance System. In turn, public health authorities shared information to prevent tularemia. Steps to prevent tick and deer fly bites include the use of an Environmental Protection Agency–registered insect repellent. Individuals who hunt, trap, or skin animals are encouraged to wear gloves when handling animals —especially rabbits, muskrats, and prairie dogs — and cook game meat thoroughly. Tularemia can be inadvertently aerosolized if an infected animal or carcass is run over with a tractor or lawnmower. Checking for carcasses before mowing may reduce the risk.
Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
Reference
1. Rich SN et al. Tularemia—United States, 2011-2022. MMWR Morb Mortal Wkly Rep 2025;73:1152–1156. doi:
The pediatrician’s first patient of the day was an 8-year-old boy, accompanied by both of his parents. It was the boy’s third visit in just over a week for fever and left-sided neck swelling, and the family was understandably anxious for answers.
“The antibiotics don’t seem to be working,” the mother explained. “He still has fever every day, as high as 104, and his neck looks just as swollen.”
A quick review of the chart revealed the boy’s initial diagnosis had been bacterial lymphadenitis, for which amoxicillin-clavulanate had been prescribed. Three days later, given lack of clinical improvement, therapy was transitioned to clindamycin. On examination, the boy was febrile and ill-appearing with a 3-cm by 5-cm tender, non-fluctuant swelling over the left sternocleidomastoid muscle.
The pediatrician ran through a quick mental checklist of diagnostic possibilities for his patient’s continued symptoms. Staphylococcal lymphadenitis still seemed possible. Could the boy be infected with methicillin-resistant Staphylococcus aureus that was also clindamycin resistant? Alternately, perhaps the problem was “source control” and the boy had developed an occult neck abscess that needed to be drained. An ultrasound could help sort that out. Finally, the pediatrician considered less common bacterial causes of lymph node swelling and fever. He placed Bartonella henselae, the cause of cat scratch disease, near the top of his list. “I’ve never seen it,” he told the parents, “But we could also consider tularemia.”
On average, 200 cases of tularemia are reported in the United States each year, and the incidence of disease is increasing, according to a surveillance report released by the Centers for Disease Control and Prevention in December 2023.1
Between 2011 and 2022, 2462 tularemia cases were reported in the United States. That translated to an average annual incidence of 0.064 per 100,000 population, an increase of 56% compared with 2001-2010. Forty-seven states reported at least one case of tularemia, although half of all reported cases came from four states — Arkansas (18%), Kansas (11%), Missouri (11%), and Oklahoma (10%). The incidence of tularemia was highest in children ages 5-9 years old, older men, and American Indian or Alaska Natives individuals. Although cases occurred year-round, 78% had symptom onset May through September.
In the United States, most human cases of tularemia have been arthropod borne, transmitted by the bite of an infected tick or deer fly. Infection also can be spread through contact with infected animals or animal tissue, particularly rabbits, hares, muskrats, prairie dogs, and other rodents, including hamsters. Outbreaks of tularemia have occurred among pet store hamsters, and at least one child in the United States developed tularemia after being bitten by a pet hamster.
Tularemia is almost always associated with fever but other clinical manifestations vary by the type of exposure. Ulceroglandular disease occurs after a tick or deer fly bite or after handling an infected animal. An ulcer develops at the site where the bacteria entered the body, along with enlargement of regional lymph nodes. Less commonly, lymph node swelling can occur without the development of an ulcer. If the bacteria enter through the eye, symptoms include conjunctivitis and swelling of pre-auricular lymph nodes. Eating or drinking contaminated food or water is associated with sore throat, mouth ulcers, tonsillitis, and swelling of lymph glands in the neck. Pneumonic tularemia, the most serious form of the disease, typically happens after inhaling bacteria-containing dust or aerosols and is associated with cough, chest pain, and difficulty breathing. Pneumonic tularemia can develop if other forms of tularemia are untreated, and the bacteria spread to the lung.
Back in the exam room, the pediatrician carefully re-examined the boy’s scalp. A 1-cm poorly healing ulcer on the left occiput added support for the diagnosis of ulceroglandular tularemia, the most common form of the disease in children. Serologic testing ultimately confirmed the diagnosis and the boy’s symptoms resolved with treatment.
Gentamicin administered intravenously or intramuscularly is the drug of choice for the treatment of tularemia in children. Ciprofloxacin is considered an alternative but is not approved by the U.S. Food and Drug Administration for this indication.
The pediatrician reported the case of tularemia to his local health department. Tularemia is a nationally notifiable disease in the United States; state health departments report to the CDC through the National Notifiable Diseases Surveillance System. In turn, public health authorities shared information to prevent tularemia. Steps to prevent tick and deer fly bites include the use of an Environmental Protection Agency–registered insect repellent. Individuals who hunt, trap, or skin animals are encouraged to wear gloves when handling animals —especially rabbits, muskrats, and prairie dogs — and cook game meat thoroughly. Tularemia can be inadvertently aerosolized if an infected animal or carcass is run over with a tractor or lawnmower. Checking for carcasses before mowing may reduce the risk.
Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
Reference
1. Rich SN et al. Tularemia—United States, 2011-2022. MMWR Morb Mortal Wkly Rep 2025;73:1152–1156. doi:
Group A Streptococcal Pharyngitis Diagnosis
It’s wintertime, peak season for GAS pharyngitis, and you’d think that this far into the 21st century we would have a foolproof process for diagnosing which among the many patients with pharyngitis have true GAS pharyngitis. Thinking back to the 1980s, we have come a long way from simple throat cultures for detecting GAS, e.g., numerous point of care (POC) Clinical Laboratory Improvement Amendments (CLIA), waved rapid antigen detection tests (RADT), and numerous highly sensitive molecular assays, e.g. nucleic acid amplification tests (NAAT). But if you think the issues surrounding management of GAS pharyngitis have been solved by these newer tests, think again.
Several good reviews1-3 are excellent resources for those wishing a refresher on GAS diagnosis/management issues. They present nitty gritty details on comparative advantages/disadvantages of the many testing options while reminding us of the nuts and bolts of GAS pharyngitis. The following are a few nuggets from these articles.
Properly collected throat specimen. A quality throat specimen involves swabbing both tonsillar pillars plus posterior pharynx without touching tongue or inner cheeks. Two swab collections increase sensitivity by almost 10% compared with a single swab. Transport media is preferred if samples will not be cultured within 24 hours. Caveat: RADT testing of a transport media-diluted sample lowers sensitivity compared with direct swab use.
Reliable GAS detection. Commercially available tests in 2025 are well studied. Culture is considered a gold standard for detecting clinically relevant GAS by CDC.4 Culture has good sensitivity (estimated 80%-90% varying among studies and by quality of specimens) and 99% specificity but requires 16-24 hours for results. RADT solves the time-delay issues and has near 100% specificity but sensitivity used to be as low as 65%, hence the 2012 Infectious Diseases Society of America guideline recommendation for backup throat culture for negative tests.5 However, current RADT have sensitivities in the 85%-90% range.3,4 So a positive RADT reliably and quickly indicates GAS antigens are present. NAAT have the highest combined sensitivity and specificity, near 100% for each, and a positive reliably indicates GAS nucleic acids are present.
So why not simply always use NAAT? First, it’s a “be careful what you wish for” scenario. NAAT can, and do, detect dead remnants and colonizing GAS way more than culture.2,3 So NAAT are overly sensitive, adding an extra layer of interpretation difficulty, ie, as many as 20% of positive NAAT detections may be carriers or dead GAS. Second, NAAT often requires special instrumentation and kits are more expensive. That said, reimbursement is often higher for NAAT.
Choice based on accuracy in detecting GAS. If time delays were not a problem, culture would still seem the answer. If more rapid detection is needed, either RADT with culture back up or NAAT could be the answer. That said, consider that in the real world, throat cultures are less sensitive and RADT are less specific than indicated by some published data.6 So, the ideal answer, it seems, would be NAAT GAS detection coupled with a confirmatory biomarker of GAS infection. Such innate immune biomarkers may be on the horizon.3
But first, pretest screening. In 2025 what do we do with a positive result? Do we prescribe antibiotics? Do we think the detected GAS bacteria/antigens/nucleic acids represent the cause of the pharyngitis? Or did we just detect dead GAS or even a carrier, while a virus is the true cause? Challenges for this decision include most pharyngitis (up to 70%) being due to viruses, not GAS, plus up to 20% of GAS detections even by less sensitive culture or RADT can be carriers, plus an added 10%-20% of RADT and NAAT detections are dead GAS. Thus, with indiscriminate testing of all pharyngitis patients, the number of truly positive GAS detections that are actually “false positives” (GAS in some form is present but not causing pharyngitis) may be almost as high as for those representing true GAS pharyngitis.
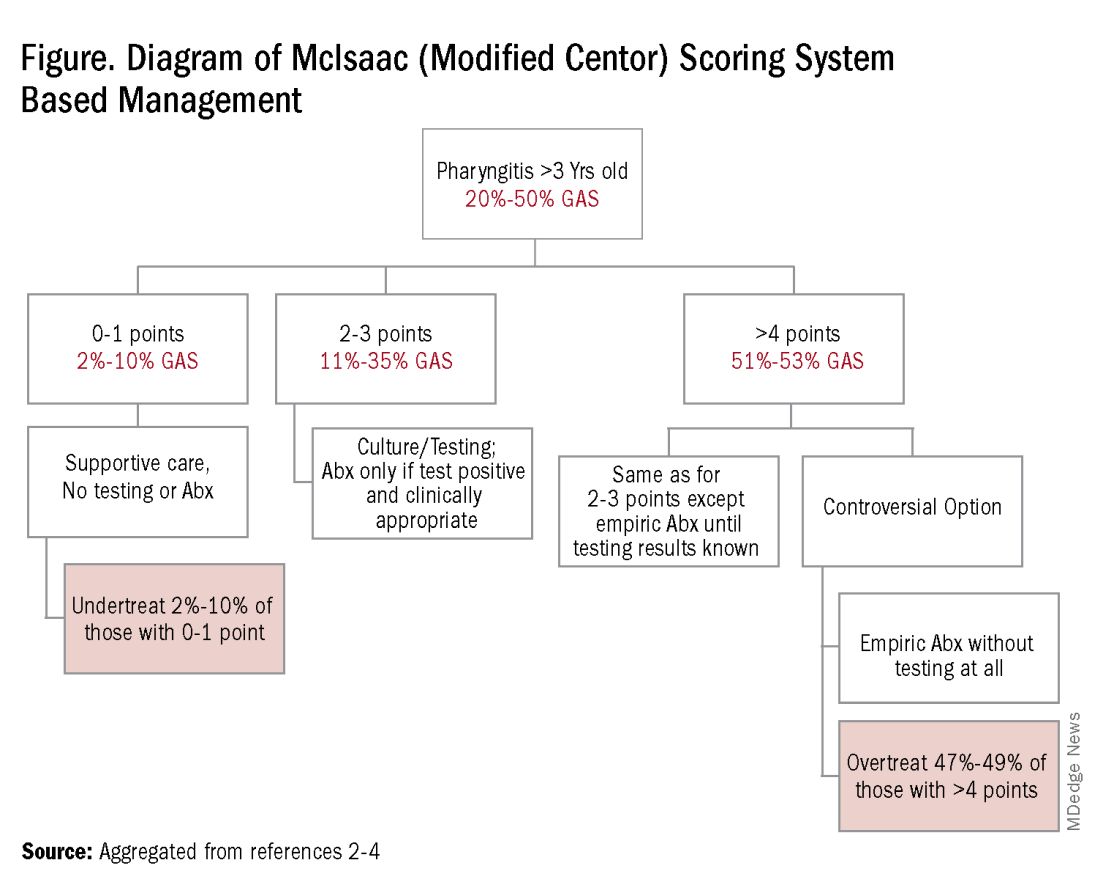
Some tool is needed to minimize testing patients who are likely to have viral pharyngitis to reduce test-positive/GAS-pharyngitis-negative scenarios. Pretest patient screening therefore is critical to increase the positive predictive value of positive GAS testing results. The history and physical can be helpful. In the simplest form of pretest screening, eliminate those younger than 3 years old* or those with viral type sign/symptoms, eg conjunctivitis, cough, coryza.7 This could cut “false” positives by as much as a half. More complete validated scoring systems are also available but remain imperfect. The most published is the McIsaac score (modified Centor score).3-5,8 (See Table and Figure.)
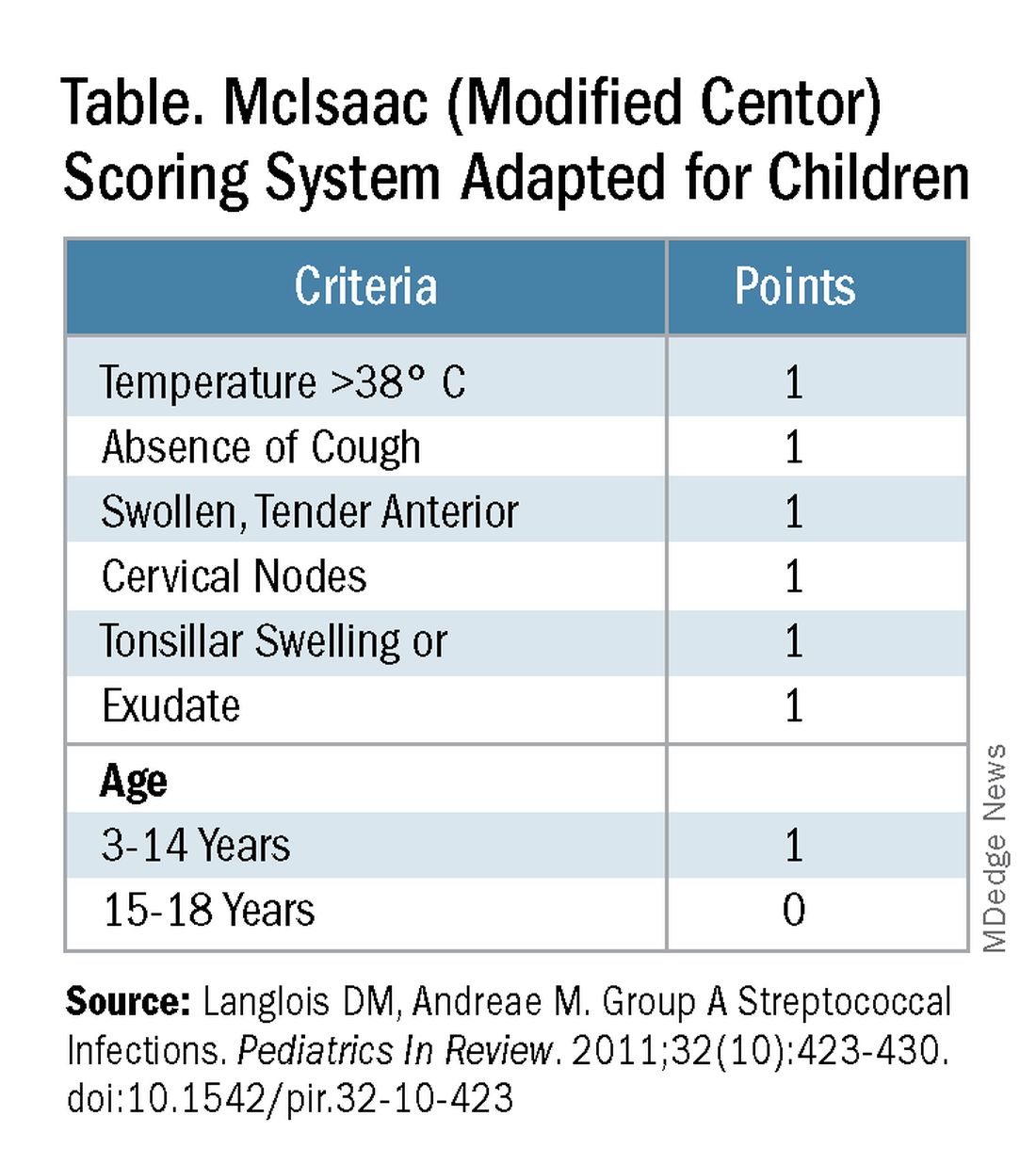
However, even with this validated scoring system, misdiagnoses and some antibiotic misuse will likely occur, particularly if the controversial option to treat a patient with a score above 4 without testing is used. For example, a 2004 study in patients older than 3 years old revealed that 45% with a score above 4 points did not have GAS pharyngitis. (McIsaac et al.) A 2012 study showed similar potential overdiagnosis from using the score without testing (45% with > 4 points did not have GAS pharyngitis). Of note, clinical scores of below 2 comprised up to 10% and would be neither tested nor treated. (Figure.)
Best clinical judgment. Regardless of the chosen test, we still need to interpret positive results, ie, use best clinical judgment. We know that even with pretest screening some positives tests will represent carriers or nonviable GAS. Yet true GAS pharyngitis needs antibiotic treatment to minimize nonpyogenic and pyogenic complications, plus reduce contagion/transmission risk and days of illness. Thus, we are forced to use best clinical judgment when considering if what could be GAS pharyngitis, particularly exudative pharyngitis, could actually be due to EBV, adenovirus, or gonococcus, each of which can mimic GAS findings. Differentiating these requires discussion beyond the scope of this article, but clues are often found in the history, the patient’s age, associated symptoms and distribution of tonsillopharyngeal exudate. Likewise Group C and G streptococcal pharyngitis can mimic GAS. Note: A comprehensive throat culture can identify these streptococci but requires a special order and likely a call to the laboratory.
Summary: The age-old problem persists, ie, differentiating the minority (~30%) of pharyngitis cases needing antibiotics from the majority that do not. We all wish to promptly treat true GAS pharyngitis; however our current tools remain imperfect. That said, we should strive to correctly diagnose/manage as many patients with pharyngitis as possible. I, for one, can’t wait until we get a validated biomarker that confirms GAS as the culprit in pharyngitis episodes. In the meantime, most providers likely have clinic or hospital approved pathways for managing GAS pharyngitis, many of which are at least in part based on data from sources for this discussion. If not, a firm foundation for creating one can be found in sources among the reference list below. Finally, if you think such pathways somehow interfere with patient flow, consider that a busy multi-provider private practice successfully integrated pretest screening and a pathway while maintaining patient flow and improving antibiotic stewardship.7
*Focal pharyngotonsillar GAS infection is rare in children younger than 3 years old, when GAS nasal passage infection may manifest as streptococcosis.9
Dr Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Bannerjee D, Selvarangan RS. The Evolution of Group A Streptococcus Pharyngitis Testing. Association for Diagnostics and Laboratory Medicine, 2018, Sep 1.
2. Cohen JF et al. Group A Streptococcus Pharyngitis in Children: New Perspectives on Rapid Diagnostic Testing and Antimicrobial Stewardship. J Pediatric Infect Dis Soc. 2024 Apr 24;13(4):250-256. doi: 10.1093/jpids/piae0223.
3. Boyanton Jr BL et al. Current Laboratory and Point-of-Care Pharyngitis Diagnostic Testing and Knowledge Gaps. J Infect Dis. 2024 Oct 23;230(Suppl 3):S182–S189. doi: 10.1093/infdis/jiae415.
4. Group A Strep Infection. Centers for Disease Control and Prevention, 2024, Mar 1.
5. Shulman ST et al. Clinical Practice Guideline for the Diagnosis and Management of Group A Streptococcal Pharyngitis: 2012 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2012 Nov 15;55(10):e86-102. doi: 10.1093/cid/cis629.
6. Rao A et al. Diagnosis and Antibiotic Treatment of Group A Streptococcal Pharyngitis in Children in a Primary Care Setting: Impact of Point-of-Care Polymerase Chain Reaction. BMC Pediatr. 2019 Jan 16;19(1):24. doi: 10.1186/s12887-019-1393-y.
7. Norton LE et al. Improving Guideline-Based Streptococcal Pharyngitis Testing: A Quality Improvement Initiative. Pediatrics. 2018 Jul;142(1):e20172033. doi: 10.1542/peds.2017-2033.
8. MD+ Calc website. Centor Score (Modified/McIsaac) for Strep Pharyngitis.
9. Langlois DM, Andreae M. Group A Streptococcal Infections. Pediatr Rev. 2011 Oct;32(10):423-9; quiz 430. doi: 10.1542/pir.32-10-423.
It’s wintertime, peak season for GAS pharyngitis, and you’d think that this far into the 21st century we would have a foolproof process for diagnosing which among the many patients with pharyngitis have true GAS pharyngitis. Thinking back to the 1980s, we have come a long way from simple throat cultures for detecting GAS, e.g., numerous point of care (POC) Clinical Laboratory Improvement Amendments (CLIA), waved rapid antigen detection tests (RADT), and numerous highly sensitive molecular assays, e.g. nucleic acid amplification tests (NAAT). But if you think the issues surrounding management of GAS pharyngitis have been solved by these newer tests, think again.
Several good reviews1-3 are excellent resources for those wishing a refresher on GAS diagnosis/management issues. They present nitty gritty details on comparative advantages/disadvantages of the many testing options while reminding us of the nuts and bolts of GAS pharyngitis. The following are a few nuggets from these articles.
Properly collected throat specimen. A quality throat specimen involves swabbing both tonsillar pillars plus posterior pharynx without touching tongue or inner cheeks. Two swab collections increase sensitivity by almost 10% compared with a single swab. Transport media is preferred if samples will not be cultured within 24 hours. Caveat: RADT testing of a transport media-diluted sample lowers sensitivity compared with direct swab use.
Reliable GAS detection. Commercially available tests in 2025 are well studied. Culture is considered a gold standard for detecting clinically relevant GAS by CDC.4 Culture has good sensitivity (estimated 80%-90% varying among studies and by quality of specimens) and 99% specificity but requires 16-24 hours for results. RADT solves the time-delay issues and has near 100% specificity but sensitivity used to be as low as 65%, hence the 2012 Infectious Diseases Society of America guideline recommendation for backup throat culture for negative tests.5 However, current RADT have sensitivities in the 85%-90% range.3,4 So a positive RADT reliably and quickly indicates GAS antigens are present. NAAT have the highest combined sensitivity and specificity, near 100% for each, and a positive reliably indicates GAS nucleic acids are present.
So why not simply always use NAAT? First, it’s a “be careful what you wish for” scenario. NAAT can, and do, detect dead remnants and colonizing GAS way more than culture.2,3 So NAAT are overly sensitive, adding an extra layer of interpretation difficulty, ie, as many as 20% of positive NAAT detections may be carriers or dead GAS. Second, NAAT often requires special instrumentation and kits are more expensive. That said, reimbursement is often higher for NAAT.
Choice based on accuracy in detecting GAS. If time delays were not a problem, culture would still seem the answer. If more rapid detection is needed, either RADT with culture back up or NAAT could be the answer. That said, consider that in the real world, throat cultures are less sensitive and RADT are less specific than indicated by some published data.6 So, the ideal answer, it seems, would be NAAT GAS detection coupled with a confirmatory biomarker of GAS infection. Such innate immune biomarkers may be on the horizon.3
But first, pretest screening. In 2025 what do we do with a positive result? Do we prescribe antibiotics? Do we think the detected GAS bacteria/antigens/nucleic acids represent the cause of the pharyngitis? Or did we just detect dead GAS or even a carrier, while a virus is the true cause? Challenges for this decision include most pharyngitis (up to 70%) being due to viruses, not GAS, plus up to 20% of GAS detections even by less sensitive culture or RADT can be carriers, plus an added 10%-20% of RADT and NAAT detections are dead GAS. Thus, with indiscriminate testing of all pharyngitis patients, the number of truly positive GAS detections that are actually “false positives” (GAS in some form is present but not causing pharyngitis) may be almost as high as for those representing true GAS pharyngitis.
Some tool is needed to minimize testing patients who are likely to have viral pharyngitis to reduce test-positive/GAS-pharyngitis-negative scenarios. Pretest patient screening therefore is critical to increase the positive predictive value of positive GAS testing results. The history and physical can be helpful. In the simplest form of pretest screening, eliminate those younger than 3 years old* or those with viral type sign/symptoms, eg conjunctivitis, cough, coryza.7 This could cut “false” positives by as much as a half. More complete validated scoring systems are also available but remain imperfect. The most published is the McIsaac score (modified Centor score).3-5,8 (See Table and Figure.)

However, even with this validated scoring system, misdiagnoses and some antibiotic misuse will likely occur, particularly if the controversial option to treat a patient with a score above 4 without testing is used. For example, a 2004 study in patients older than 3 years old revealed that 45% with a score above 4 points did not have GAS pharyngitis. (McIsaac et al.) A 2012 study showed similar potential overdiagnosis from using the score without testing (45% with > 4 points did not have GAS pharyngitis). Of note, clinical scores of below 2 comprised up to 10% and would be neither tested nor treated. (Figure.)

Best clinical judgment. Regardless of the chosen test, we still need to interpret positive results, ie, use best clinical judgment. We know that even with pretest screening some positives tests will represent carriers or nonviable GAS. Yet true GAS pharyngitis needs antibiotic treatment to minimize nonpyogenic and pyogenic complications, plus reduce contagion/transmission risk and days of illness. Thus, we are forced to use best clinical judgment when considering if what could be GAS pharyngitis, particularly exudative pharyngitis, could actually be due to EBV, adenovirus, or gonococcus, each of which can mimic GAS findings. Differentiating these requires discussion beyond the scope of this article, but clues are often found in the history, the patient’s age, associated symptoms and distribution of tonsillopharyngeal exudate. Likewise Group C and G streptococcal pharyngitis can mimic GAS. Note: A comprehensive throat culture can identify these streptococci but requires a special order and likely a call to the laboratory.
Summary: The age-old problem persists, ie, differentiating the minority (~30%) of pharyngitis cases needing antibiotics from the majority that do not. We all wish to promptly treat true GAS pharyngitis; however our current tools remain imperfect. That said, we should strive to correctly diagnose/manage as many patients with pharyngitis as possible. I, for one, can’t wait until we get a validated biomarker that confirms GAS as the culprit in pharyngitis episodes. In the meantime, most providers likely have clinic or hospital approved pathways for managing GAS pharyngitis, many of which are at least in part based on data from sources for this discussion. If not, a firm foundation for creating one can be found in sources among the reference list below. Finally, if you think such pathways somehow interfere with patient flow, consider that a busy multi-provider private practice successfully integrated pretest screening and a pathway while maintaining patient flow and improving antibiotic stewardship.7
*Focal pharyngotonsillar GAS infection is rare in children younger than 3 years old, when GAS nasal passage infection may manifest as streptococcosis.9
Dr Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Bannerjee D, Selvarangan RS. The Evolution of Group A Streptococcus Pharyngitis Testing. Association for Diagnostics and Laboratory Medicine, 2018, Sep 1.
2. Cohen JF et al. Group A Streptococcus Pharyngitis in Children: New Perspectives on Rapid Diagnostic Testing and Antimicrobial Stewardship. J Pediatric Infect Dis Soc. 2024 Apr 24;13(4):250-256. doi: 10.1093/jpids/piae0223.
3. Boyanton Jr BL et al. Current Laboratory and Point-of-Care Pharyngitis Diagnostic Testing and Knowledge Gaps. J Infect Dis. 2024 Oct 23;230(Suppl 3):S182–S189. doi: 10.1093/infdis/jiae415.
4. Group A Strep Infection. Centers for Disease Control and Prevention, 2024, Mar 1.
5. Shulman ST et al. Clinical Practice Guideline for the Diagnosis and Management of Group A Streptococcal Pharyngitis: 2012 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2012 Nov 15;55(10):e86-102. doi: 10.1093/cid/cis629.
6. Rao A et al. Diagnosis and Antibiotic Treatment of Group A Streptococcal Pharyngitis in Children in a Primary Care Setting: Impact of Point-of-Care Polymerase Chain Reaction. BMC Pediatr. 2019 Jan 16;19(1):24. doi: 10.1186/s12887-019-1393-y.
7. Norton LE et al. Improving Guideline-Based Streptococcal Pharyngitis Testing: A Quality Improvement Initiative. Pediatrics. 2018 Jul;142(1):e20172033. doi: 10.1542/peds.2017-2033.
8. MD+ Calc website. Centor Score (Modified/McIsaac) for Strep Pharyngitis.
9. Langlois DM, Andreae M. Group A Streptococcal Infections. Pediatr Rev. 2011 Oct;32(10):423-9; quiz 430. doi: 10.1542/pir.32-10-423.
It’s wintertime, peak season for GAS pharyngitis, and you’d think that this far into the 21st century we would have a foolproof process for diagnosing which among the many patients with pharyngitis have true GAS pharyngitis. Thinking back to the 1980s, we have come a long way from simple throat cultures for detecting GAS, e.g., numerous point of care (POC) Clinical Laboratory Improvement Amendments (CLIA), waved rapid antigen detection tests (RADT), and numerous highly sensitive molecular assays, e.g. nucleic acid amplification tests (NAAT). But if you think the issues surrounding management of GAS pharyngitis have been solved by these newer tests, think again.
Several good reviews1-3 are excellent resources for those wishing a refresher on GAS diagnosis/management issues. They present nitty gritty details on comparative advantages/disadvantages of the many testing options while reminding us of the nuts and bolts of GAS pharyngitis. The following are a few nuggets from these articles.
Properly collected throat specimen. A quality throat specimen involves swabbing both tonsillar pillars plus posterior pharynx without touching tongue or inner cheeks. Two swab collections increase sensitivity by almost 10% compared with a single swab. Transport media is preferred if samples will not be cultured within 24 hours. Caveat: RADT testing of a transport media-diluted sample lowers sensitivity compared with direct swab use.
Reliable GAS detection. Commercially available tests in 2025 are well studied. Culture is considered a gold standard for detecting clinically relevant GAS by CDC.4 Culture has good sensitivity (estimated 80%-90% varying among studies and by quality of specimens) and 99% specificity but requires 16-24 hours for results. RADT solves the time-delay issues and has near 100% specificity but sensitivity used to be as low as 65%, hence the 2012 Infectious Diseases Society of America guideline recommendation for backup throat culture for negative tests.5 However, current RADT have sensitivities in the 85%-90% range.3,4 So a positive RADT reliably and quickly indicates GAS antigens are present. NAAT have the highest combined sensitivity and specificity, near 100% for each, and a positive reliably indicates GAS nucleic acids are present.
So why not simply always use NAAT? First, it’s a “be careful what you wish for” scenario. NAAT can, and do, detect dead remnants and colonizing GAS way more than culture.2,3 So NAAT are overly sensitive, adding an extra layer of interpretation difficulty, ie, as many as 20% of positive NAAT detections may be carriers or dead GAS. Second, NAAT often requires special instrumentation and kits are more expensive. That said, reimbursement is often higher for NAAT.
Choice based on accuracy in detecting GAS. If time delays were not a problem, culture would still seem the answer. If more rapid detection is needed, either RADT with culture back up or NAAT could be the answer. That said, consider that in the real world, throat cultures are less sensitive and RADT are less specific than indicated by some published data.6 So, the ideal answer, it seems, would be NAAT GAS detection coupled with a confirmatory biomarker of GAS infection. Such innate immune biomarkers may be on the horizon.3
But first, pretest screening. In 2025 what do we do with a positive result? Do we prescribe antibiotics? Do we think the detected GAS bacteria/antigens/nucleic acids represent the cause of the pharyngitis? Or did we just detect dead GAS or even a carrier, while a virus is the true cause? Challenges for this decision include most pharyngitis (up to 70%) being due to viruses, not GAS, plus up to 20% of GAS detections even by less sensitive culture or RADT can be carriers, plus an added 10%-20% of RADT and NAAT detections are dead GAS. Thus, with indiscriminate testing of all pharyngitis patients, the number of truly positive GAS detections that are actually “false positives” (GAS in some form is present but not causing pharyngitis) may be almost as high as for those representing true GAS pharyngitis.
Some tool is needed to minimize testing patients who are likely to have viral pharyngitis to reduce test-positive/GAS-pharyngitis-negative scenarios. Pretest patient screening therefore is critical to increase the positive predictive value of positive GAS testing results. The history and physical can be helpful. In the simplest form of pretest screening, eliminate those younger than 3 years old* or those with viral type sign/symptoms, eg conjunctivitis, cough, coryza.7 This could cut “false” positives by as much as a half. More complete validated scoring systems are also available but remain imperfect. The most published is the McIsaac score (modified Centor score).3-5,8 (See Table and Figure.)

However, even with this validated scoring system, misdiagnoses and some antibiotic misuse will likely occur, particularly if the controversial option to treat a patient with a score above 4 without testing is used. For example, a 2004 study in patients older than 3 years old revealed that 45% with a score above 4 points did not have GAS pharyngitis. (McIsaac et al.) A 2012 study showed similar potential overdiagnosis from using the score without testing (45% with > 4 points did not have GAS pharyngitis). Of note, clinical scores of below 2 comprised up to 10% and would be neither tested nor treated. (Figure.)

Best clinical judgment. Regardless of the chosen test, we still need to interpret positive results, ie, use best clinical judgment. We know that even with pretest screening some positives tests will represent carriers or nonviable GAS. Yet true GAS pharyngitis needs antibiotic treatment to minimize nonpyogenic and pyogenic complications, plus reduce contagion/transmission risk and days of illness. Thus, we are forced to use best clinical judgment when considering if what could be GAS pharyngitis, particularly exudative pharyngitis, could actually be due to EBV, adenovirus, or gonococcus, each of which can mimic GAS findings. Differentiating these requires discussion beyond the scope of this article, but clues are often found in the history, the patient’s age, associated symptoms and distribution of tonsillopharyngeal exudate. Likewise Group C and G streptococcal pharyngitis can mimic GAS. Note: A comprehensive throat culture can identify these streptococci but requires a special order and likely a call to the laboratory.
Summary: The age-old problem persists, ie, differentiating the minority (~30%) of pharyngitis cases needing antibiotics from the majority that do not. We all wish to promptly treat true GAS pharyngitis; however our current tools remain imperfect. That said, we should strive to correctly diagnose/manage as many patients with pharyngitis as possible. I, for one, can’t wait until we get a validated biomarker that confirms GAS as the culprit in pharyngitis episodes. In the meantime, most providers likely have clinic or hospital approved pathways for managing GAS pharyngitis, many of which are at least in part based on data from sources for this discussion. If not, a firm foundation for creating one can be found in sources among the reference list below. Finally, if you think such pathways somehow interfere with patient flow, consider that a busy multi-provider private practice successfully integrated pretest screening and a pathway while maintaining patient flow and improving antibiotic stewardship.7
*Focal pharyngotonsillar GAS infection is rare in children younger than 3 years old, when GAS nasal passage infection may manifest as streptococcosis.9
Dr Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Bannerjee D, Selvarangan RS. The Evolution of Group A Streptococcus Pharyngitis Testing. Association for Diagnostics and Laboratory Medicine, 2018, Sep 1.
2. Cohen JF et al. Group A Streptococcus Pharyngitis in Children: New Perspectives on Rapid Diagnostic Testing and Antimicrobial Stewardship. J Pediatric Infect Dis Soc. 2024 Apr 24;13(4):250-256. doi: 10.1093/jpids/piae0223.
3. Boyanton Jr BL et al. Current Laboratory and Point-of-Care Pharyngitis Diagnostic Testing and Knowledge Gaps. J Infect Dis. 2024 Oct 23;230(Suppl 3):S182–S189. doi: 10.1093/infdis/jiae415.
4. Group A Strep Infection. Centers for Disease Control and Prevention, 2024, Mar 1.
5. Shulman ST et al. Clinical Practice Guideline for the Diagnosis and Management of Group A Streptococcal Pharyngitis: 2012 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2012 Nov 15;55(10):e86-102. doi: 10.1093/cid/cis629.
6. Rao A et al. Diagnosis and Antibiotic Treatment of Group A Streptococcal Pharyngitis in Children in a Primary Care Setting: Impact of Point-of-Care Polymerase Chain Reaction. BMC Pediatr. 2019 Jan 16;19(1):24. doi: 10.1186/s12887-019-1393-y.
7. Norton LE et al. Improving Guideline-Based Streptococcal Pharyngitis Testing: A Quality Improvement Initiative. Pediatrics. 2018 Jul;142(1):e20172033. doi: 10.1542/peds.2017-2033.
8. MD+ Calc website. Centor Score (Modified/McIsaac) for Strep Pharyngitis.
9. Langlois DM, Andreae M. Group A Streptococcal Infections. Pediatr Rev. 2011 Oct;32(10):423-9; quiz 430. doi: 10.1542/pir.32-10-423.
Varicella Outbreaks: 2022-2024
Practitioners providing care to children are familiar with the childhood immunization schedule and routinely administer varicella vaccine at the 12-month and 4- to 5-year visits. However, when is the last time most of us or any of the current trainees have seen a case?
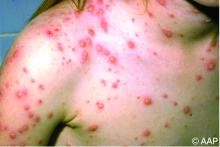
Briefly, varicella is a highly contagious disease caused by varicella-zoster virus (VZV). It is characterized by a generalized pruritic erythematous rash in various stages of development beginning as macules, progressing to papules, and ultimately becoming vesicular lesions on an erythematous base (“dewdrop on a rose petal”) and resolves with crusting of the lesion (Figure 1). It has an incubation period of 10-21 days with symptoms usually developing within 14-16 days after exposure. The vesicular rash must be differentiated from enterovirus, Staphylococcus aureus, contact dermatitis, or insect bites, which initially may be difficult. Approximately 50% of children can have symptoms including fever, malaise, anorexia, headache, and occasionally, mild abdominal pain in the 24-48 hours prior to the appearance of rash. Lesions usually first appear on the scalp, face, or trunk in successive crops over several days. A person with varicella has lesions in various stages.
In a normal host, new vesicle formation usually stops within 4 days, and most lesions have fully crusted by day 6. VZV establishes latency in sensory ganglia and may reactivate years or decades later to cause herpes zoster (HZ). Most healthy children with varicella recover without sequelae so the disease is generally regarded as benign. However, varicella can lead to serious complications and deaths in healthy as well as immunocompromised persons.
Complications of Varicella: bacterial superinfection of skin lesions most often with Streptococcus pyogenes or S aureus manifested as cellulitis, myositis, or necrotizing fasciitis; neurologic complications include cerebellar ataxia and encephalitis with the latter seen most often in adults. Pneumonia occurs most often in adults, especially those infected during pregnancy. Another concern, infection during the first 20 weeks of pregnancy can lead to fetal death or severe birth defects, including limb hypoplasia, cutaneous scarring, ocular abnormalities, and central nervous system damage (congenital varicella syndrome).
The risk for development of severe disseminated disease was first noted in the 1960s as treatments for leukemia in children improved. They were surviving their cancer only to develop severe and often fatal varicella. Today it is recognized that development of disseminated disease is a risk for all infected persons with impaired T cell function, malignancies, HIV, or receiving immunosuppressive therapy.
Reye’s syndrome is rarely seen today since taking salicylates while infected with VZV was identified as a predisposing factor for development.
VZV is only found in humans and transmission is person to person or airborne. The secondary household attack rate is approximately 90%. In contrast, the secondary attack rates in classrooms may be as low as 12%-33%. Transmission rates in the tropics for unexplained reasons are also lower.
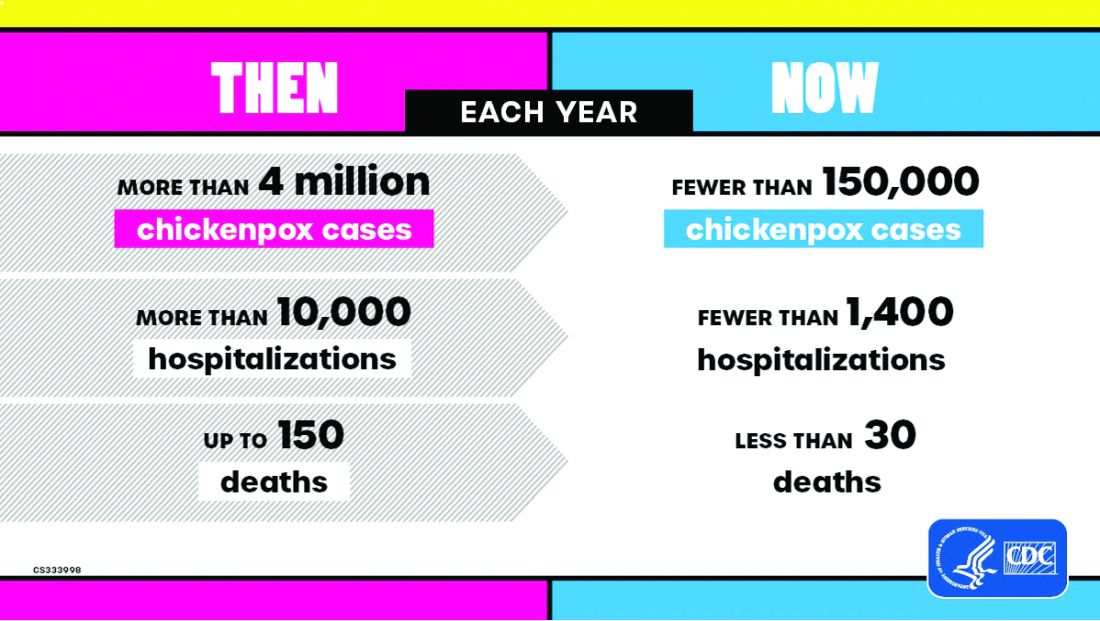
Vaccine History: Why do we rarely see this disease anymore? Varicella, a live attenuated vaccine, was developed in 1974 by Dr. Michiaki Takahashi. It remains the only vaccine directed against a herpes group virus. In 1979, the Collaborative Varicella Vaccine Study Group was established at the National Institutes of Health (NIH) and additional safety and efficacy trials were conducted in the United States initially in leukemic patients in remission and later in healthy children, which supported Takahashi’s data. Licensure of varicella vaccine was granted in 1995. That same year, due to continuing disease and societal burden, the United States was the first country to incorporate varicella into the routine childhood immunization schedule, which resulted in significant reductions in cases. To further improve control of varicella, in 2007 vaccine recommendations were revised and a routine two-dose schedule was implemented. The impact of varicella disease pre- and post-vaccine licensure is illustrated in Figure 2. Not listed, is that in the pre-vaccine era, there were approximately 44 cases of congenital varicella syndrome annually.
As of 2023 only 23% (45/195) of nations routinely administer this vaccine and 4% (8/195) have restricted recommendations. The remaining 73% of countries do not offer the vaccine, including all countries on the African continent, and Cuba, Guatemala, Haiti, Honduras, India, Jordan, Lebanon, Philippines, Portugal, and Venezuela to list a few.
Varicella Outbreak: In October 2022, New York City (NYC) identified a varicella outbreak primarily involving persons who recently migrated from Central and South America and lived in a shelter in NYC or residential facility (n = 105); the outbreak is ongoing. As of March 8, 2024, 873 cases (53%) were among children aged 4-18 years and 91.9% had no documentation of varicella vaccine at time of symptom onset. There were 28 hospitalizations, and no deaths reported. The most common sources of transmission were the residential facilities (41.3%) and importation or possible importation (39.4%). School transmission accounted for only 1.2% of cases.
Most migrants arrived from countries where varicella vaccination is not part of the routine childhood immunization schedule. Although most cases occurred in children, almost 30% occurred in adults. Many of the migrants arrived from tropical countries where susceptibility rates are also higher in adults. This outbreak is a reminder of the importance of limiting disease transmission by maintaining high vaccination rates. To curtail this outbreak, approximately 27,000 doses of varicella vaccine were administered to the arriving migrants. In addition, MMR, COVID-19, influenza, and all routine pediatric vaccines required for school entry were administered. Temporary closure of the residential facilities were required. Education was provided to residents regarding immunizations as well as assistance to help them establish a primary care home. Multiple agencies were mobilized to successfully coordinate these efforts.
Take Home Message
1. Each country has its own routine immunization schedule. It may not include all vaccines recommended in the US schedule. When questioned I’m frequently told that immunizations are up to date, only to review records and find they are not, especially when it is related to MMR. It is often administered at 9 months and/or MR or MM is administered depending on the country. As reported here, varicella is a routine vaccine in only 45 countries.
2.
3. Once an outbreak has been identified, the infrastructure to manage and contain it must already be established. In most instances there will be a need for a rapid and often large-scale effort involving multiple agencies including local health care providers.
4. Not all diseases are reportable. Only deaths by varicella are nationally notifiable. Otherwise, cases are reported voluntarily. As of November 2, 2024, there have been 5,157 cases of varicella reported, excluding any cases from NYC.
Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
CDC. Nationally Notifiable Infectious Diseases and Conditions, United States: Weekly Tables. https://wonder.cdc.gov/nndss/nndss_weekly_tables_menu.asp.
Graham KA et al. Varicella Outbreak Among Recent Arrivals to New York City, 2022-2024. MMWR Morb Mortal Wkly Rep. 2024 May 30;73(21):478-483. doi: 10.15585/mmwr.mm7321a1.
Marin M et al. Health and Economic Impact of the United States Varicella Vaccination Program, 1996-2020. J Infect Dis. 2022 Oct 21;226(Suppl 4):S463-S469. doi: 10.1093/infdis/jiac271.
Varicella-Zoster Virus Infections in Kimberkin DW et al, eds. Red Book: 2024 Report of the Committee on Infectious Diseases, 33rd Edition. American Academy of Pediatrics, 2024:938-951. https://www.aap.org/Red-Book-2024-Report-of-the-Committee-on-Infectious-Diseases-33rd-Edition-Paperback?srsltid=AfmBOoqyF60rR9ZwQ5jA8AouNhtRRTyPLnc_r7HWw7JVYV8v33Hr2vQS.
Practitioners providing care to children are familiar with the childhood immunization schedule and routinely administer varicella vaccine at the 12-month and 4- to 5-year visits. However, when is the last time most of us or any of the current trainees have seen a case?
Briefly, varicella is a highly contagious disease caused by varicella-zoster virus (VZV). It is characterized by a generalized pruritic erythematous rash in various stages of development beginning as macules, progressing to papules, and ultimately becoming vesicular lesions on an erythematous base (“dewdrop on a rose petal”) and resolves with crusting of the lesion (Figure 1). It has an incubation period of 10-21 days with symptoms usually developing within 14-16 days after exposure. The vesicular rash must be differentiated from enterovirus, Staphylococcus aureus, contact dermatitis, or insect bites, which initially may be difficult. Approximately 50% of children can have symptoms including fever, malaise, anorexia, headache, and occasionally, mild abdominal pain in the 24-48 hours prior to the appearance of rash. Lesions usually first appear on the scalp, face, or trunk in successive crops over several days. A person with varicella has lesions in various stages.
In a normal host, new vesicle formation usually stops within 4 days, and most lesions have fully crusted by day 6. VZV establishes latency in sensory ganglia and may reactivate years or decades later to cause herpes zoster (HZ). Most healthy children with varicella recover without sequelae so the disease is generally regarded as benign. However, varicella can lead to serious complications and deaths in healthy as well as immunocompromised persons.
Complications of Varicella: bacterial superinfection of skin lesions most often with Streptococcus pyogenes or S aureus manifested as cellulitis, myositis, or necrotizing fasciitis; neurologic complications include cerebellar ataxia and encephalitis with the latter seen most often in adults. Pneumonia occurs most often in adults, especially those infected during pregnancy. Another concern, infection during the first 20 weeks of pregnancy can lead to fetal death or severe birth defects, including limb hypoplasia, cutaneous scarring, ocular abnormalities, and central nervous system damage (congenital varicella syndrome).
The risk for development of severe disseminated disease was first noted in the 1960s as treatments for leukemia in children improved. They were surviving their cancer only to develop severe and often fatal varicella. Today it is recognized that development of disseminated disease is a risk for all infected persons with impaired T cell function, malignancies, HIV, or receiving immunosuppressive therapy.
Reye’s syndrome is rarely seen today since taking salicylates while infected with VZV was identified as a predisposing factor for development.
VZV is only found in humans and transmission is person to person or airborne. The secondary household attack rate is approximately 90%. In contrast, the secondary attack rates in classrooms may be as low as 12%-33%. Transmission rates in the tropics for unexplained reasons are also lower.
Vaccine History: Why do we rarely see this disease anymore? Varicella, a live attenuated vaccine, was developed in 1974 by Dr. Michiaki Takahashi. It remains the only vaccine directed against a herpes group virus. In 1979, the Collaborative Varicella Vaccine Study Group was established at the National Institutes of Health (NIH) and additional safety and efficacy trials were conducted in the United States initially in leukemic patients in remission and later in healthy children, which supported Takahashi’s data. Licensure of varicella vaccine was granted in 1995. That same year, due to continuing disease and societal burden, the United States was the first country to incorporate varicella into the routine childhood immunization schedule, which resulted in significant reductions in cases. To further improve control of varicella, in 2007 vaccine recommendations were revised and a routine two-dose schedule was implemented. The impact of varicella disease pre- and post-vaccine licensure is illustrated in Figure 2. Not listed, is that in the pre-vaccine era, there were approximately 44 cases of congenital varicella syndrome annually.

As of 2023 only 23% (45/195) of nations routinely administer this vaccine and 4% (8/195) have restricted recommendations. The remaining 73% of countries do not offer the vaccine, including all countries on the African continent, and Cuba, Guatemala, Haiti, Honduras, India, Jordan, Lebanon, Philippines, Portugal, and Venezuela to list a few.
Varicella Outbreak: In October 2022, New York City (NYC) identified a varicella outbreak primarily involving persons who recently migrated from Central and South America and lived in a shelter in NYC or residential facility (n = 105); the outbreak is ongoing. As of March 8, 2024, 873 cases (53%) were among children aged 4-18 years and 91.9% had no documentation of varicella vaccine at time of symptom onset. There were 28 hospitalizations, and no deaths reported. The most common sources of transmission were the residential facilities (41.3%) and importation or possible importation (39.4%). School transmission accounted for only 1.2% of cases.
Most migrants arrived from countries where varicella vaccination is not part of the routine childhood immunization schedule. Although most cases occurred in children, almost 30% occurred in adults. Many of the migrants arrived from tropical countries where susceptibility rates are also higher in adults. This outbreak is a reminder of the importance of limiting disease transmission by maintaining high vaccination rates. To curtail this outbreak, approximately 27,000 doses of varicella vaccine were administered to the arriving migrants. In addition, MMR, COVID-19, influenza, and all routine pediatric vaccines required for school entry were administered. Temporary closure of the residential facilities were required. Education was provided to residents regarding immunizations as well as assistance to help them establish a primary care home. Multiple agencies were mobilized to successfully coordinate these efforts.
Take Home Message
1. Each country has its own routine immunization schedule. It may not include all vaccines recommended in the US schedule. When questioned I’m frequently told that immunizations are up to date, only to review records and find they are not, especially when it is related to MMR. It is often administered at 9 months and/or MR or MM is administered depending on the country. As reported here, varicella is a routine vaccine in only 45 countries.
2.
3. Once an outbreak has been identified, the infrastructure to manage and contain it must already be established. In most instances there will be a need for a rapid and often large-scale effort involving multiple agencies including local health care providers.
4. Not all diseases are reportable. Only deaths by varicella are nationally notifiable. Otherwise, cases are reported voluntarily. As of November 2, 2024, there have been 5,157 cases of varicella reported, excluding any cases from NYC.
Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
CDC. Nationally Notifiable Infectious Diseases and Conditions, United States: Weekly Tables. https://wonder.cdc.gov/nndss/nndss_weekly_tables_menu.asp.
Graham KA et al. Varicella Outbreak Among Recent Arrivals to New York City, 2022-2024. MMWR Morb Mortal Wkly Rep. 2024 May 30;73(21):478-483. doi: 10.15585/mmwr.mm7321a1.
Marin M et al. Health and Economic Impact of the United States Varicella Vaccination Program, 1996-2020. J Infect Dis. 2022 Oct 21;226(Suppl 4):S463-S469. doi: 10.1093/infdis/jiac271.
Varicella-Zoster Virus Infections in Kimberkin DW et al, eds. Red Book: 2024 Report of the Committee on Infectious Diseases, 33rd Edition. American Academy of Pediatrics, 2024:938-951. https://www.aap.org/Red-Book-2024-Report-of-the-Committee-on-Infectious-Diseases-33rd-Edition-Paperback?srsltid=AfmBOoqyF60rR9ZwQ5jA8AouNhtRRTyPLnc_r7HWw7JVYV8v33Hr2vQS.
Practitioners providing care to children are familiar with the childhood immunization schedule and routinely administer varicella vaccine at the 12-month and 4- to 5-year visits. However, when is the last time most of us or any of the current trainees have seen a case?
Briefly, varicella is a highly contagious disease caused by varicella-zoster virus (VZV). It is characterized by a generalized pruritic erythematous rash in various stages of development beginning as macules, progressing to papules, and ultimately becoming vesicular lesions on an erythematous base (“dewdrop on a rose petal”) and resolves with crusting of the lesion (Figure 1). It has an incubation period of 10-21 days with symptoms usually developing within 14-16 days after exposure. The vesicular rash must be differentiated from enterovirus, Staphylococcus aureus, contact dermatitis, or insect bites, which initially may be difficult. Approximately 50% of children can have symptoms including fever, malaise, anorexia, headache, and occasionally, mild abdominal pain in the 24-48 hours prior to the appearance of rash. Lesions usually first appear on the scalp, face, or trunk in successive crops over several days. A person with varicella has lesions in various stages.
In a normal host, new vesicle formation usually stops within 4 days, and most lesions have fully crusted by day 6. VZV establishes latency in sensory ganglia and may reactivate years or decades later to cause herpes zoster (HZ). Most healthy children with varicella recover without sequelae so the disease is generally regarded as benign. However, varicella can lead to serious complications and deaths in healthy as well as immunocompromised persons.
Complications of Varicella: bacterial superinfection of skin lesions most often with Streptococcus pyogenes or S aureus manifested as cellulitis, myositis, or necrotizing fasciitis; neurologic complications include cerebellar ataxia and encephalitis with the latter seen most often in adults. Pneumonia occurs most often in adults, especially those infected during pregnancy. Another concern, infection during the first 20 weeks of pregnancy can lead to fetal death or severe birth defects, including limb hypoplasia, cutaneous scarring, ocular abnormalities, and central nervous system damage (congenital varicella syndrome).
The risk for development of severe disseminated disease was first noted in the 1960s as treatments for leukemia in children improved. They were surviving their cancer only to develop severe and often fatal varicella. Today it is recognized that development of disseminated disease is a risk for all infected persons with impaired T cell function, malignancies, HIV, or receiving immunosuppressive therapy.
Reye’s syndrome is rarely seen today since taking salicylates while infected with VZV was identified as a predisposing factor for development.
VZV is only found in humans and transmission is person to person or airborne. The secondary household attack rate is approximately 90%. In contrast, the secondary attack rates in classrooms may be as low as 12%-33%. Transmission rates in the tropics for unexplained reasons are also lower.
Vaccine History: Why do we rarely see this disease anymore? Varicella, a live attenuated vaccine, was developed in 1974 by Dr. Michiaki Takahashi. It remains the only vaccine directed against a herpes group virus. In 1979, the Collaborative Varicella Vaccine Study Group was established at the National Institutes of Health (NIH) and additional safety and efficacy trials were conducted in the United States initially in leukemic patients in remission and later in healthy children, which supported Takahashi’s data. Licensure of varicella vaccine was granted in 1995. That same year, due to continuing disease and societal burden, the United States was the first country to incorporate varicella into the routine childhood immunization schedule, which resulted in significant reductions in cases. To further improve control of varicella, in 2007 vaccine recommendations were revised and a routine two-dose schedule was implemented. The impact of varicella disease pre- and post-vaccine licensure is illustrated in Figure 2. Not listed, is that in the pre-vaccine era, there were approximately 44 cases of congenital varicella syndrome annually.

As of 2023 only 23% (45/195) of nations routinely administer this vaccine and 4% (8/195) have restricted recommendations. The remaining 73% of countries do not offer the vaccine, including all countries on the African continent, and Cuba, Guatemala, Haiti, Honduras, India, Jordan, Lebanon, Philippines, Portugal, and Venezuela to list a few.
Varicella Outbreak: In October 2022, New York City (NYC) identified a varicella outbreak primarily involving persons who recently migrated from Central and South America and lived in a shelter in NYC or residential facility (n = 105); the outbreak is ongoing. As of March 8, 2024, 873 cases (53%) were among children aged 4-18 years and 91.9% had no documentation of varicella vaccine at time of symptom onset. There were 28 hospitalizations, and no deaths reported. The most common sources of transmission were the residential facilities (41.3%) and importation or possible importation (39.4%). School transmission accounted for only 1.2% of cases.
Most migrants arrived from countries where varicella vaccination is not part of the routine childhood immunization schedule. Although most cases occurred in children, almost 30% occurred in adults. Many of the migrants arrived from tropical countries where susceptibility rates are also higher in adults. This outbreak is a reminder of the importance of limiting disease transmission by maintaining high vaccination rates. To curtail this outbreak, approximately 27,000 doses of varicella vaccine were administered to the arriving migrants. In addition, MMR, COVID-19, influenza, and all routine pediatric vaccines required for school entry were administered. Temporary closure of the residential facilities were required. Education was provided to residents regarding immunizations as well as assistance to help them establish a primary care home. Multiple agencies were mobilized to successfully coordinate these efforts.
Take Home Message
1. Each country has its own routine immunization schedule. It may not include all vaccines recommended in the US schedule. When questioned I’m frequently told that immunizations are up to date, only to review records and find they are not, especially when it is related to MMR. It is often administered at 9 months and/or MR or MM is administered depending on the country. As reported here, varicella is a routine vaccine in only 45 countries.
2.
3. Once an outbreak has been identified, the infrastructure to manage and contain it must already be established. In most instances there will be a need for a rapid and often large-scale effort involving multiple agencies including local health care providers.
4. Not all diseases are reportable. Only deaths by varicella are nationally notifiable. Otherwise, cases are reported voluntarily. As of November 2, 2024, there have been 5,157 cases of varicella reported, excluding any cases from NYC.
Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
CDC. Nationally Notifiable Infectious Diseases and Conditions, United States: Weekly Tables. https://wonder.cdc.gov/nndss/nndss_weekly_tables_menu.asp.
Graham KA et al. Varicella Outbreak Among Recent Arrivals to New York City, 2022-2024. MMWR Morb Mortal Wkly Rep. 2024 May 30;73(21):478-483. doi: 10.15585/mmwr.mm7321a1.
Marin M et al. Health and Economic Impact of the United States Varicella Vaccination Program, 1996-2020. J Infect Dis. 2022 Oct 21;226(Suppl 4):S463-S469. doi: 10.1093/infdis/jiac271.
Varicella-Zoster Virus Infections in Kimberkin DW et al, eds. Red Book: 2024 Report of the Committee on Infectious Diseases, 33rd Edition. American Academy of Pediatrics, 2024:938-951. https://www.aap.org/Red-Book-2024-Report-of-the-Committee-on-Infectious-Diseases-33rd-Edition-Paperback?srsltid=AfmBOoqyF60rR9ZwQ5jA8AouNhtRRTyPLnc_r7HWw7JVYV8v33Hr2vQS.
Anticipated Effects of Pneumococcal Vaccines on Otitis
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).
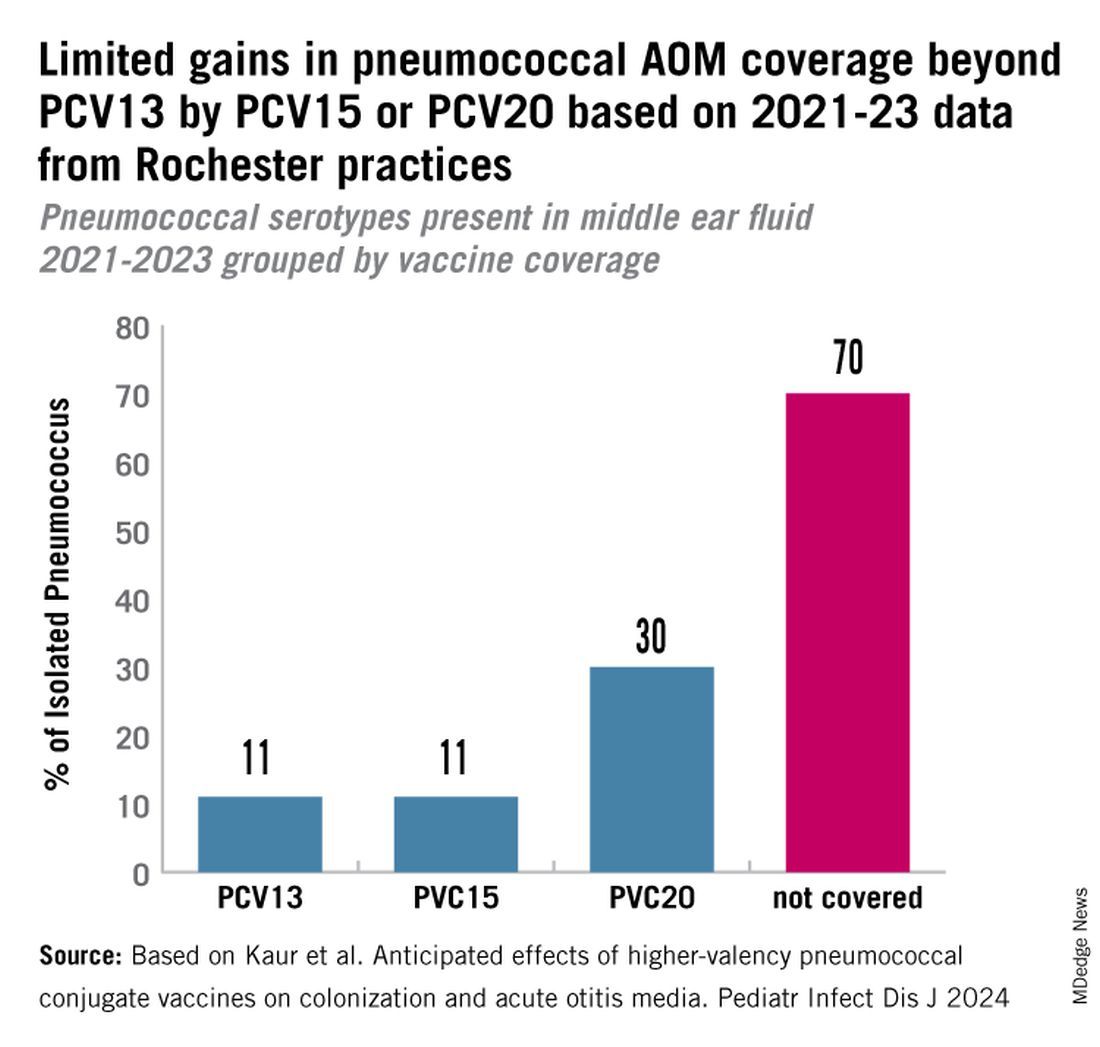
Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).

Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).

Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
Oropouche Virus
The pediatrician’s first patient of the day was a 15-year-old boy complaining of fever, chills, and profound arthralgias. His exam, including a careful assessment of his joints, yielded no clues, and the pediatrician was ready to diagnose this as a routine viral illness. An additional bit of history provided by the patient’s mother prompted the pediatrician to pause and reconsider.
“A week ago, we returned from a visit to Cuba,” the mother reported. “Could this be Oropouche virus infection?”
Oropouche virus disease is an arboviral disease caused by the Oropouche virus (OROV). It is transmitted to humans through midge or mosquito bites. Although largely unknown to most United States clinicians until recently, this vector-borne virus is not new. The first human Oropouche virus infection was identified in Trinidad and Tobago in 1955 and since then, there have been intermittent outbreaks in the Amazon region. In recent months, though, the epidemiology of Oropouche virus infections has changed. Infections are being identified in new geographic areas, including Cuba. According to the Pan American Health Organization, 506 cases of Oropouche virus infection have been identified in Cuba since May 27, 2024.
Two deaths from Oropouche virus infection have been reported in previously healthy people. Evolving data suggests adverse outcomes associated with vertical transmission during pregnancy. One fetal death and child with congenital anomalies have been reported in Brazil. Additional fetal deaths, miscarriages, and congenital anomalies are under investigation.
Travel-associated cases have been reported in the United States. As of September 10, 2024, 52 Oropouche virus disease cases had been reported from five states in the United States. The Centers for Disease Control and Prevention confirmed that the first 31 of these cases were travelers returning from Cuba. The CDC issued a health advisory on August 16, 2024: Increased Oropouche Virus Activity and Associated Risk to Travelers.
The pediatrician quickly reviewed the signs and symptoms of Oropouche virus infection. Disease typically presents as an abrupt onset of fever, severe headache, chills, myalgia, and arthralgia 3 to 10 days after the bite of infected mosquito. Some patients develop a maculopapular rash that starts on the trunk and spreads to the extremities. Meningitis and encephalitis develop in less than 1 in 20 people. The symptoms of Oropouche virus infection overlap with those of other arboviruses such as dengue, chikungunya, and Zika viruses. The disease can also mimic malaria or rickettsial infection. Approximately 60% of people with Oropouche virus infection experience a recurrence of symptoms within days to weeks of the initial resolution of symptoms.
Testing for Oropouche virus infection is available through the CDC’s Arbovirus Diagnostic Laboratory. In people who are acutely ill, reverse transcription-polymerase chain reaction testing can be used to identify the virus in serum and cerebrospinal fluid. Serologic testing is also available for people who have been symptomatic for at least 6 days.
The pediatrician contacted his local health department to discuss the possibility of Oropouche virus infection. After reviewing the case definition, public health authorities recommended laboratory testing for Oropouche virus, dengue, and Zika virus.
Back in the exam room, the pediatrician provided anticipatory guidance to the patient and his mother. There are no antiviral medications to treat Oropouche virus infection, so the pediatrician recommended supportive care, including acetaminophen for fever and pain. He also advised avoiding aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) until dengue could be ruled out to reduce the risk of bleeding. After confirming that no one else in the home was sick with similar symptoms, he counseled about prevention strategies.
To date, transmission of Oropouche virus in the United States has not been documented, but vectors potentially capable of transmitting the virus are present in some areas of the United States. When people who are infected with Oropouche are bitten, they can spread the virus through their blood to biting midges or mosquitoes. The insects can then spread the virus to other people. To reduce to potential for local transmission, people who are sick with suspected Oropouche virus infection are advised to avoid biting-midge and mosquito bites for the first week of their illness. Any person who has recently traveled to an area where Oropouche virus transmission is occurring should also avoid insect bites for 3 weeks after returning home to account for the potential incubation period of the virus. This includes wearing an EPA-registered insect repellent.
A suspect case is a patient who has been in an area with documented or suspected OROV circulation* within 2 weeks of initial symptom onset (as patients may experience recurrent symptoms) and the following:
- Abrupt onset of reported fever, headache, and one or more of the following: myalgia, arthralgia, photophobia, retro-orbital/eye pain, or signs and symptoms of neuroinvasive disease (eg, stiff neck, altered mental status, seizures, limb weakness, or cerebrospinal fluid pleocytosis).
- Tested negative for other possible diseases, in particular dengue.†
- Absence of a more likely clinical explanation.
*If concern exists for local transmission in a nonendemic area, consider if the patient shared an exposure location with a person with confirmed OROV infection, lives in an area where travel-related cases have been identified, or has known vector exposure (eg, mosquitoes or biting midges).
†If strong suspicion of OROV disease exists based on the patient’s clinical features and history of travel to an area with virus circulation, do not wait on negative testing before sending specimens to CDC.
Adapted from: Centers for Disease Control and Prevention. Response to Oropouche Virus Disease Cases in U.S. States and Territories in the Americas. Available at: https.//www.cdc.gov/oropouche/media/pdfs/2024/09/response-to-oropouche-virus-disease.pdf
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu)
The pediatrician’s first patient of the day was a 15-year-old boy complaining of fever, chills, and profound arthralgias. His exam, including a careful assessment of his joints, yielded no clues, and the pediatrician was ready to diagnose this as a routine viral illness. An additional bit of history provided by the patient’s mother prompted the pediatrician to pause and reconsider.
“A week ago, we returned from a visit to Cuba,” the mother reported. “Could this be Oropouche virus infection?”
Oropouche virus disease is an arboviral disease caused by the Oropouche virus (OROV). It is transmitted to humans through midge or mosquito bites. Although largely unknown to most United States clinicians until recently, this vector-borne virus is not new. The first human Oropouche virus infection was identified in Trinidad and Tobago in 1955 and since then, there have been intermittent outbreaks in the Amazon region. In recent months, though, the epidemiology of Oropouche virus infections has changed. Infections are being identified in new geographic areas, including Cuba. According to the Pan American Health Organization, 506 cases of Oropouche virus infection have been identified in Cuba since May 27, 2024.
Two deaths from Oropouche virus infection have been reported in previously healthy people. Evolving data suggests adverse outcomes associated with vertical transmission during pregnancy. One fetal death and child with congenital anomalies have been reported in Brazil. Additional fetal deaths, miscarriages, and congenital anomalies are under investigation.
Travel-associated cases have been reported in the United States. As of September 10, 2024, 52 Oropouche virus disease cases had been reported from five states in the United States. The Centers for Disease Control and Prevention confirmed that the first 31 of these cases were travelers returning from Cuba. The CDC issued a health advisory on August 16, 2024: Increased Oropouche Virus Activity and Associated Risk to Travelers.
The pediatrician quickly reviewed the signs and symptoms of Oropouche virus infection. Disease typically presents as an abrupt onset of fever, severe headache, chills, myalgia, and arthralgia 3 to 10 days after the bite of infected mosquito. Some patients develop a maculopapular rash that starts on the trunk and spreads to the extremities. Meningitis and encephalitis develop in less than 1 in 20 people. The symptoms of Oropouche virus infection overlap with those of other arboviruses such as dengue, chikungunya, and Zika viruses. The disease can also mimic malaria or rickettsial infection. Approximately 60% of people with Oropouche virus infection experience a recurrence of symptoms within days to weeks of the initial resolution of symptoms.
Testing for Oropouche virus infection is available through the CDC’s Arbovirus Diagnostic Laboratory. In people who are acutely ill, reverse transcription-polymerase chain reaction testing can be used to identify the virus in serum and cerebrospinal fluid. Serologic testing is also available for people who have been symptomatic for at least 6 days.
The pediatrician contacted his local health department to discuss the possibility of Oropouche virus infection. After reviewing the case definition, public health authorities recommended laboratory testing for Oropouche virus, dengue, and Zika virus.
Back in the exam room, the pediatrician provided anticipatory guidance to the patient and his mother. There are no antiviral medications to treat Oropouche virus infection, so the pediatrician recommended supportive care, including acetaminophen for fever and pain. He also advised avoiding aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) until dengue could be ruled out to reduce the risk of bleeding. After confirming that no one else in the home was sick with similar symptoms, he counseled about prevention strategies.
To date, transmission of Oropouche virus in the United States has not been documented, but vectors potentially capable of transmitting the virus are present in some areas of the United States. When people who are infected with Oropouche are bitten, they can spread the virus through their blood to biting midges or mosquitoes. The insects can then spread the virus to other people. To reduce to potential for local transmission, people who are sick with suspected Oropouche virus infection are advised to avoid biting-midge and mosquito bites for the first week of their illness. Any person who has recently traveled to an area where Oropouche virus transmission is occurring should also avoid insect bites for 3 weeks after returning home to account for the potential incubation period of the virus. This includes wearing an EPA-registered insect repellent.
A suspect case is a patient who has been in an area with documented or suspected OROV circulation* within 2 weeks of initial symptom onset (as patients may experience recurrent symptoms) and the following:
- Abrupt onset of reported fever, headache, and one or more of the following: myalgia, arthralgia, photophobia, retro-orbital/eye pain, or signs and symptoms of neuroinvasive disease (eg, stiff neck, altered mental status, seizures, limb weakness, or cerebrospinal fluid pleocytosis).
- Tested negative for other possible diseases, in particular dengue.†
- Absence of a more likely clinical explanation.
*If concern exists for local transmission in a nonendemic area, consider if the patient shared an exposure location with a person with confirmed OROV infection, lives in an area where travel-related cases have been identified, or has known vector exposure (eg, mosquitoes or biting midges).
†If strong suspicion of OROV disease exists based on the patient’s clinical features and history of travel to an area with virus circulation, do not wait on negative testing before sending specimens to CDC.
Adapted from: Centers for Disease Control and Prevention. Response to Oropouche Virus Disease Cases in U.S. States and Territories in the Americas. Available at: https.//www.cdc.gov/oropouche/media/pdfs/2024/09/response-to-oropouche-virus-disease.pdf
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu)
The pediatrician’s first patient of the day was a 15-year-old boy complaining of fever, chills, and profound arthralgias. His exam, including a careful assessment of his joints, yielded no clues, and the pediatrician was ready to diagnose this as a routine viral illness. An additional bit of history provided by the patient’s mother prompted the pediatrician to pause and reconsider.
“A week ago, we returned from a visit to Cuba,” the mother reported. “Could this be Oropouche virus infection?”
Oropouche virus disease is an arboviral disease caused by the Oropouche virus (OROV). It is transmitted to humans through midge or mosquito bites. Although largely unknown to most United States clinicians until recently, this vector-borne virus is not new. The first human Oropouche virus infection was identified in Trinidad and Tobago in 1955 and since then, there have been intermittent outbreaks in the Amazon region. In recent months, though, the epidemiology of Oropouche virus infections has changed. Infections are being identified in new geographic areas, including Cuba. According to the Pan American Health Organization, 506 cases of Oropouche virus infection have been identified in Cuba since May 27, 2024.
Two deaths from Oropouche virus infection have been reported in previously healthy people. Evolving data suggests adverse outcomes associated with vertical transmission during pregnancy. One fetal death and child with congenital anomalies have been reported in Brazil. Additional fetal deaths, miscarriages, and congenital anomalies are under investigation.
Travel-associated cases have been reported in the United States. As of September 10, 2024, 52 Oropouche virus disease cases had been reported from five states in the United States. The Centers for Disease Control and Prevention confirmed that the first 31 of these cases were travelers returning from Cuba. The CDC issued a health advisory on August 16, 2024: Increased Oropouche Virus Activity and Associated Risk to Travelers.
The pediatrician quickly reviewed the signs and symptoms of Oropouche virus infection. Disease typically presents as an abrupt onset of fever, severe headache, chills, myalgia, and arthralgia 3 to 10 days after the bite of infected mosquito. Some patients develop a maculopapular rash that starts on the trunk and spreads to the extremities. Meningitis and encephalitis develop in less than 1 in 20 people. The symptoms of Oropouche virus infection overlap with those of other arboviruses such as dengue, chikungunya, and Zika viruses. The disease can also mimic malaria or rickettsial infection. Approximately 60% of people with Oropouche virus infection experience a recurrence of symptoms within days to weeks of the initial resolution of symptoms.
Testing for Oropouche virus infection is available through the CDC’s Arbovirus Diagnostic Laboratory. In people who are acutely ill, reverse transcription-polymerase chain reaction testing can be used to identify the virus in serum and cerebrospinal fluid. Serologic testing is also available for people who have been symptomatic for at least 6 days.
The pediatrician contacted his local health department to discuss the possibility of Oropouche virus infection. After reviewing the case definition, public health authorities recommended laboratory testing for Oropouche virus, dengue, and Zika virus.
Back in the exam room, the pediatrician provided anticipatory guidance to the patient and his mother. There are no antiviral medications to treat Oropouche virus infection, so the pediatrician recommended supportive care, including acetaminophen for fever and pain. He also advised avoiding aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) until dengue could be ruled out to reduce the risk of bleeding. After confirming that no one else in the home was sick with similar symptoms, he counseled about prevention strategies.
To date, transmission of Oropouche virus in the United States has not been documented, but vectors potentially capable of transmitting the virus are present in some areas of the United States. When people who are infected with Oropouche are bitten, they can spread the virus through their blood to biting midges or mosquitoes. The insects can then spread the virus to other people. To reduce to potential for local transmission, people who are sick with suspected Oropouche virus infection are advised to avoid biting-midge and mosquito bites for the first week of their illness. Any person who has recently traveled to an area where Oropouche virus transmission is occurring should also avoid insect bites for 3 weeks after returning home to account for the potential incubation period of the virus. This includes wearing an EPA-registered insect repellent.
A suspect case is a patient who has been in an area with documented or suspected OROV circulation* within 2 weeks of initial symptom onset (as patients may experience recurrent symptoms) and the following:
- Abrupt onset of reported fever, headache, and one or more of the following: myalgia, arthralgia, photophobia, retro-orbital/eye pain, or signs and symptoms of neuroinvasive disease (eg, stiff neck, altered mental status, seizures, limb weakness, or cerebrospinal fluid pleocytosis).
- Tested negative for other possible diseases, in particular dengue.†
- Absence of a more likely clinical explanation.
*If concern exists for local transmission in a nonendemic area, consider if the patient shared an exposure location with a person with confirmed OROV infection, lives in an area where travel-related cases have been identified, or has known vector exposure (eg, mosquitoes or biting midges).
†If strong suspicion of OROV disease exists based on the patient’s clinical features and history of travel to an area with virus circulation, do not wait on negative testing before sending specimens to CDC.
Adapted from: Centers for Disease Control and Prevention. Response to Oropouche Virus Disease Cases in U.S. States and Territories in the Americas. Available at: https.//www.cdc.gov/oropouche/media/pdfs/2024/09/response-to-oropouche-virus-disease.pdf
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu)
Predicting RSV’s Role in the Upcoming Winter Respiratory Season
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV, influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6 
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV, influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6 
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV, influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6 
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
Summer Is Not Over: Let's Talk About Recreational Water–Associated Illnesses
Recently I was in Wyoming. As I rode down the Snake River, the guide pointed out tree trunks that had been chewed on by beavers. Days later I joined a local friend for a hike to Taggart Lake. Upon reaching the end of the trail as I began to cast my eyes on the magnificent scenery, I could not help but notice several children, including toddlers, playing in the fresh warm water. The next thing out of my friend’s mouth was “You know there is Giardia in there.” Little did she know, she and the guide had just helped me select a topic for ID Consult.
Giardia, aka ”beaver fever,” was discussed in detail in this column as part of the differential of a diarrheal illness by Christopher J. Harrison, MD. However, it is the perfect time of year to revisit other recreational water–associated illnesses.
Infections acquired during recreational water activity can lead to illnesses involving the gastrointestinal tract, central nervous system, respiratory tract, skin, eyes, and ears. Pathogens, chemicals, and toxins are transmitted by ingestion, contact with contaminated water or a sick individual or animal, and inhalation of aerosols. The National Waterborne Disease and Outbreak Surveillance System (WBDOSS) collects data on waterborne disease and outbreaks associated with recreational water, drinking water, and environmental and undetermined exposures to water. All reporting to the Centers for Disease Control and Prevention (CDC) is voluntary. However, mandatory pathogen reporting requirements can vary by state. Ideally, once an agency has completed the outbreak investigation, the definitive cause and source will be determined, and interventions to prevent future outbreaks implemented.
Treated Versus Untreated Water
One useful way to help narrow the etiology of a patient’s symptoms is to consider those illnesses associated with treated water venues (e.g., pools, hot tubs, water parks) versus untreated water venues (e.g., rivers, lakes, oceans). Parents may forget to offer that information since they may not perceive a connection between water exposure and the illness, especially if they traveled within the US.
In 2021, the CDC reported results of data submitted between 2015 and 2019 from treated recreational water facilities. Of the 208 outbreaks, most (96%) were associated with public pools, hot tubs, or water playgrounds. These outbreaks resulted in at least 3,646 cases of illness, 286 hospitalizations, and 13 deaths. Overall infectious etiologies were the primary cause of illness. Of the 155 outbreaks with a confirmed etiology, Cryptosporidium was the causative pathogen in 49% of the outbreaks and accounted for 84% (2,492) of cases, while Legionella caused 42% of outbreaks, accounted for 13% (354) of cases, and was responsible for all 13 deaths. Slightly more than half (107 of 208) of the outbreaks started between June-August with Cryptosporidium accounting for 63 of the outbreaks during that period. A little more than one-third were associated with a hotel or resort. The majority of hotel recreational water–associated illnesses was associated with hot tubs. Of the 53 outbreaks without a confirmed etiology, 20 were suspected to have a chemical related etiology (excess chlorine, altered pool chemistry).
In contrast, there were 140 untreated recreational water outbreaks reported between 2000 and 2014 from 35 states and Guam involving 4,958 cases and 2 deaths. The etiology was confirmed for 103 (74%) outbreaks including 5 that had multiple etiologies and 8 due to toxins or chemicals; 7 of 8 toxins were from harmful algal blooms. Enteric pathogens were the etiology in 84% of outbreaks including: Norovirus (n = 1459), Shigella (n = 362) Avian schistosomes (n = 345), Cryptosporidium (n = 314) and Escherichia coli (n = 155).There were 24 cases of Giardia. The two deaths were due to Naegleria fowleri. The top 2 settings for these outbreaks were public parks (36%) and beaches (32%) with most outbreaks (n = 117) being associated with a lake /pond venue. Most outbreaks began between June and August.
The major differences between the two types of recreational water–associated illnesses are their most common settings and etiologies. With that in mind, let us briefly review the most common etiology from each venue.
Treated Water Venue: Cryptosporidiosis
Cryptosporidium is an oocyst-forming protozoa that causes a self-limited watery, nonbloody diarrhea which usually resolves within 10-14 days. Most patients have associated abdominal cramps, fever, and vomiting although infected persons can be asymptomatic. Infection in the immunocompromised potentially can lead to profuse and prolonged diarrhea. Oocysts are excreted in the feces of infected hosts and as little as 10 can cause infection. They can survive extreme environmental conditions in water and soil for several months and even survive up to 7 days in a properly chlorinated pool. Transmission occurs between humans via contaminated food and water or from infected animals. Oocysts have been isolated in raw or unpasteurized milk and apple cider. Incidence is highest in children 1 through 4 years of age.
Diagnosis today is usually via molecular methods (nucleic acid amplification tests, aka NAATs), due to their high sensitivity and specificity and is the preferred method. These tests can identify multiple gastrointestinal tract pathogens with a single assay. Diagnosis by microscopy or fecal immunoassay antigens are still available. Treatment is supportive in most cases. If needed, a 3-day course of nitazoxanide can be prescribed. Immunocompromised patients should be managed in consultation with an infectious disease specialist.
Untreated Water Venue: Norovirus
Norovirus is a viral illness characterized by the abrupt onset of vomiting and/or watery diarrhea, usually associated with nausea and abdominal cramps. Symptoms persist 24-72 hours, however they may be prolonged in the immunocompromised and persons at the extremes of the age spectrum. Norovirus has replaced rotavirus as the major cause of medically attended gastroenteritis. While a major cause of recreational water–associated illnesses, high attack rates also occur in semi closed communities including cruise ships, childcare centers, and schools. Transmission is fecal-oral, vomitus oral, person to person, by ingestion of contaminated food and water or touching contaminated surfaces with subsequent touching of the mouth. Asymptomatic viral shedding may occur, especially in children. Prolonged shedding (> 6 mos.) has been reported in immunocompromised hosts.
Molecular diagnosis with stool is utilized most often. Treatment is supportive.
Take Home Message
When evaluating your patients for an acute gastrointestinal illness, consider water-related activities and their potential for being the source. Encourage patients not to ignore posted advisories on beaches, to not swim if they have diarrhea, not to swallow the water they swim in and to minimize water entering their nose while swimming in warm freshwater. If you start seeing several patients with similar symptoms and/or etiology, consider contacting your local or state health department. It could be the beginning of an outbreak.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She has no relevant financial disclosures.
Suggested Readings
Graciaa DS et al. Outbreaks Associated with Untreated Recreational Water — United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018 Jun 29;67(25):701-706. doi: 10.15585/mmwr.mm6725a1.
Hlavsa MC et al. Outbreaks Associated with Treated Recreational Water — United States, 2015–2019. MMWR Morb Mortal Wkly Rep. 2021;70:733–738. doi: 10.15585/mmwr.mm7020a1.
Kimberlin DW et al., eds. Red Book Report of the Committee on Infectious Diseases. 33rd ed. American Academy of Pediatrics. 2024. Cryptosporidiosis, p 338-40 and Norovirus, p 622-624.Waterborne Outbreaks Summary Reports. CDC. 2024 April 18.
Recently I was in Wyoming. As I rode down the Snake River, the guide pointed out tree trunks that had been chewed on by beavers. Days later I joined a local friend for a hike to Taggart Lake. Upon reaching the end of the trail as I began to cast my eyes on the magnificent scenery, I could not help but notice several children, including toddlers, playing in the fresh warm water. The next thing out of my friend’s mouth was “You know there is Giardia in there.” Little did she know, she and the guide had just helped me select a topic for ID Consult.
Giardia, aka ”beaver fever,” was discussed in detail in this column as part of the differential of a diarrheal illness by Christopher J. Harrison, MD. However, it is the perfect time of year to revisit other recreational water–associated illnesses.
Infections acquired during recreational water activity can lead to illnesses involving the gastrointestinal tract, central nervous system, respiratory tract, skin, eyes, and ears. Pathogens, chemicals, and toxins are transmitted by ingestion, contact with contaminated water or a sick individual or animal, and inhalation of aerosols. The National Waterborne Disease and Outbreak Surveillance System (WBDOSS) collects data on waterborne disease and outbreaks associated with recreational water, drinking water, and environmental and undetermined exposures to water. All reporting to the Centers for Disease Control and Prevention (CDC) is voluntary. However, mandatory pathogen reporting requirements can vary by state. Ideally, once an agency has completed the outbreak investigation, the definitive cause and source will be determined, and interventions to prevent future outbreaks implemented.
Treated Versus Untreated Water
One useful way to help narrow the etiology of a patient’s symptoms is to consider those illnesses associated with treated water venues (e.g., pools, hot tubs, water parks) versus untreated water venues (e.g., rivers, lakes, oceans). Parents may forget to offer that information since they may not perceive a connection between water exposure and the illness, especially if they traveled within the US.
In 2021, the CDC reported results of data submitted between 2015 and 2019 from treated recreational water facilities. Of the 208 outbreaks, most (96%) were associated with public pools, hot tubs, or water playgrounds. These outbreaks resulted in at least 3,646 cases of illness, 286 hospitalizations, and 13 deaths. Overall infectious etiologies were the primary cause of illness. Of the 155 outbreaks with a confirmed etiology, Cryptosporidium was the causative pathogen in 49% of the outbreaks and accounted for 84% (2,492) of cases, while Legionella caused 42% of outbreaks, accounted for 13% (354) of cases, and was responsible for all 13 deaths. Slightly more than half (107 of 208) of the outbreaks started between June-August with Cryptosporidium accounting for 63 of the outbreaks during that period. A little more than one-third were associated with a hotel or resort. The majority of hotel recreational water–associated illnesses was associated with hot tubs. Of the 53 outbreaks without a confirmed etiology, 20 were suspected to have a chemical related etiology (excess chlorine, altered pool chemistry).
In contrast, there were 140 untreated recreational water outbreaks reported between 2000 and 2014 from 35 states and Guam involving 4,958 cases and 2 deaths. The etiology was confirmed for 103 (74%) outbreaks including 5 that had multiple etiologies and 8 due to toxins or chemicals; 7 of 8 toxins were from harmful algal blooms. Enteric pathogens were the etiology in 84% of outbreaks including: Norovirus (n = 1459), Shigella (n = 362) Avian schistosomes (n = 345), Cryptosporidium (n = 314) and Escherichia coli (n = 155).There were 24 cases of Giardia. The two deaths were due to Naegleria fowleri. The top 2 settings for these outbreaks were public parks (36%) and beaches (32%) with most outbreaks (n = 117) being associated with a lake /pond venue. Most outbreaks began between June and August.
The major differences between the two types of recreational water–associated illnesses are their most common settings and etiologies. With that in mind, let us briefly review the most common etiology from each venue.
Treated Water Venue: Cryptosporidiosis
Cryptosporidium is an oocyst-forming protozoa that causes a self-limited watery, nonbloody diarrhea which usually resolves within 10-14 days. Most patients have associated abdominal cramps, fever, and vomiting although infected persons can be asymptomatic. Infection in the immunocompromised potentially can lead to profuse and prolonged diarrhea. Oocysts are excreted in the feces of infected hosts and as little as 10 can cause infection. They can survive extreme environmental conditions in water and soil for several months and even survive up to 7 days in a properly chlorinated pool. Transmission occurs between humans via contaminated food and water or from infected animals. Oocysts have been isolated in raw or unpasteurized milk and apple cider. Incidence is highest in children 1 through 4 years of age.
Diagnosis today is usually via molecular methods (nucleic acid amplification tests, aka NAATs), due to their high sensitivity and specificity and is the preferred method. These tests can identify multiple gastrointestinal tract pathogens with a single assay. Diagnosis by microscopy or fecal immunoassay antigens are still available. Treatment is supportive in most cases. If needed, a 3-day course of nitazoxanide can be prescribed. Immunocompromised patients should be managed in consultation with an infectious disease specialist.
Untreated Water Venue: Norovirus
Norovirus is a viral illness characterized by the abrupt onset of vomiting and/or watery diarrhea, usually associated with nausea and abdominal cramps. Symptoms persist 24-72 hours, however they may be prolonged in the immunocompromised and persons at the extremes of the age spectrum. Norovirus has replaced rotavirus as the major cause of medically attended gastroenteritis. While a major cause of recreational water–associated illnesses, high attack rates also occur in semi closed communities including cruise ships, childcare centers, and schools. Transmission is fecal-oral, vomitus oral, person to person, by ingestion of contaminated food and water or touching contaminated surfaces with subsequent touching of the mouth. Asymptomatic viral shedding may occur, especially in children. Prolonged shedding (> 6 mos.) has been reported in immunocompromised hosts.
Molecular diagnosis with stool is utilized most often. Treatment is supportive.
Take Home Message
When evaluating your patients for an acute gastrointestinal illness, consider water-related activities and their potential for being the source. Encourage patients not to ignore posted advisories on beaches, to not swim if they have diarrhea, not to swallow the water they swim in and to minimize water entering their nose while swimming in warm freshwater. If you start seeing several patients with similar symptoms and/or etiology, consider contacting your local or state health department. It could be the beginning of an outbreak.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She has no relevant financial disclosures.
Suggested Readings
Graciaa DS et al. Outbreaks Associated with Untreated Recreational Water — United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018 Jun 29;67(25):701-706. doi: 10.15585/mmwr.mm6725a1.
Hlavsa MC et al. Outbreaks Associated with Treated Recreational Water — United States, 2015–2019. MMWR Morb Mortal Wkly Rep. 2021;70:733–738. doi: 10.15585/mmwr.mm7020a1.
Kimberlin DW et al., eds. Red Book Report of the Committee on Infectious Diseases. 33rd ed. American Academy of Pediatrics. 2024. Cryptosporidiosis, p 338-40 and Norovirus, p 622-624.Waterborne Outbreaks Summary Reports. CDC. 2024 April 18.
Recently I was in Wyoming. As I rode down the Snake River, the guide pointed out tree trunks that had been chewed on by beavers. Days later I joined a local friend for a hike to Taggart Lake. Upon reaching the end of the trail as I began to cast my eyes on the magnificent scenery, I could not help but notice several children, including toddlers, playing in the fresh warm water. The next thing out of my friend’s mouth was “You know there is Giardia in there.” Little did she know, she and the guide had just helped me select a topic for ID Consult.
Giardia, aka ”beaver fever,” was discussed in detail in this column as part of the differential of a diarrheal illness by Christopher J. Harrison, MD. However, it is the perfect time of year to revisit other recreational water–associated illnesses.
Infections acquired during recreational water activity can lead to illnesses involving the gastrointestinal tract, central nervous system, respiratory tract, skin, eyes, and ears. Pathogens, chemicals, and toxins are transmitted by ingestion, contact with contaminated water or a sick individual or animal, and inhalation of aerosols. The National Waterborne Disease and Outbreak Surveillance System (WBDOSS) collects data on waterborne disease and outbreaks associated with recreational water, drinking water, and environmental and undetermined exposures to water. All reporting to the Centers for Disease Control and Prevention (CDC) is voluntary. However, mandatory pathogen reporting requirements can vary by state. Ideally, once an agency has completed the outbreak investigation, the definitive cause and source will be determined, and interventions to prevent future outbreaks implemented.
Treated Versus Untreated Water
One useful way to help narrow the etiology of a patient’s symptoms is to consider those illnesses associated with treated water venues (e.g., pools, hot tubs, water parks) versus untreated water venues (e.g., rivers, lakes, oceans). Parents may forget to offer that information since they may not perceive a connection between water exposure and the illness, especially if they traveled within the US.
In 2021, the CDC reported results of data submitted between 2015 and 2019 from treated recreational water facilities. Of the 208 outbreaks, most (96%) were associated with public pools, hot tubs, or water playgrounds. These outbreaks resulted in at least 3,646 cases of illness, 286 hospitalizations, and 13 deaths. Overall infectious etiologies were the primary cause of illness. Of the 155 outbreaks with a confirmed etiology, Cryptosporidium was the causative pathogen in 49% of the outbreaks and accounted for 84% (2,492) of cases, while Legionella caused 42% of outbreaks, accounted for 13% (354) of cases, and was responsible for all 13 deaths. Slightly more than half (107 of 208) of the outbreaks started between June-August with Cryptosporidium accounting for 63 of the outbreaks during that period. A little more than one-third were associated with a hotel or resort. The majority of hotel recreational water–associated illnesses was associated with hot tubs. Of the 53 outbreaks without a confirmed etiology, 20 were suspected to have a chemical related etiology (excess chlorine, altered pool chemistry).
In contrast, there were 140 untreated recreational water outbreaks reported between 2000 and 2014 from 35 states and Guam involving 4,958 cases and 2 deaths. The etiology was confirmed for 103 (74%) outbreaks including 5 that had multiple etiologies and 8 due to toxins or chemicals; 7 of 8 toxins were from harmful algal blooms. Enteric pathogens were the etiology in 84% of outbreaks including: Norovirus (n = 1459), Shigella (n = 362) Avian schistosomes (n = 345), Cryptosporidium (n = 314) and Escherichia coli (n = 155).There were 24 cases of Giardia. The two deaths were due to Naegleria fowleri. The top 2 settings for these outbreaks were public parks (36%) and beaches (32%) with most outbreaks (n = 117) being associated with a lake /pond venue. Most outbreaks began between June and August.
The major differences between the two types of recreational water–associated illnesses are their most common settings and etiologies. With that in mind, let us briefly review the most common etiology from each venue.
Treated Water Venue: Cryptosporidiosis
Cryptosporidium is an oocyst-forming protozoa that causes a self-limited watery, nonbloody diarrhea which usually resolves within 10-14 days. Most patients have associated abdominal cramps, fever, and vomiting although infected persons can be asymptomatic. Infection in the immunocompromised potentially can lead to profuse and prolonged diarrhea. Oocysts are excreted in the feces of infected hosts and as little as 10 can cause infection. They can survive extreme environmental conditions in water and soil for several months and even survive up to 7 days in a properly chlorinated pool. Transmission occurs between humans via contaminated food and water or from infected animals. Oocysts have been isolated in raw or unpasteurized milk and apple cider. Incidence is highest in children 1 through 4 years of age.
Diagnosis today is usually via molecular methods (nucleic acid amplification tests, aka NAATs), due to their high sensitivity and specificity and is the preferred method. These tests can identify multiple gastrointestinal tract pathogens with a single assay. Diagnosis by microscopy or fecal immunoassay antigens are still available. Treatment is supportive in most cases. If needed, a 3-day course of nitazoxanide can be prescribed. Immunocompromised patients should be managed in consultation with an infectious disease specialist.
Untreated Water Venue: Norovirus
Norovirus is a viral illness characterized by the abrupt onset of vomiting and/or watery diarrhea, usually associated with nausea and abdominal cramps. Symptoms persist 24-72 hours, however they may be prolonged in the immunocompromised and persons at the extremes of the age spectrum. Norovirus has replaced rotavirus as the major cause of medically attended gastroenteritis. While a major cause of recreational water–associated illnesses, high attack rates also occur in semi closed communities including cruise ships, childcare centers, and schools. Transmission is fecal-oral, vomitus oral, person to person, by ingestion of contaminated food and water or touching contaminated surfaces with subsequent touching of the mouth. Asymptomatic viral shedding may occur, especially in children. Prolonged shedding (> 6 mos.) has been reported in immunocompromised hosts.
Molecular diagnosis with stool is utilized most often. Treatment is supportive.
Take Home Message
When evaluating your patients for an acute gastrointestinal illness, consider water-related activities and their potential for being the source. Encourage patients not to ignore posted advisories on beaches, to not swim if they have diarrhea, not to swallow the water they swim in and to minimize water entering their nose while swimming in warm freshwater. If you start seeing several patients with similar symptoms and/or etiology, consider contacting your local or state health department. It could be the beginning of an outbreak.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She has no relevant financial disclosures.
Suggested Readings
Graciaa DS et al. Outbreaks Associated with Untreated Recreational Water — United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018 Jun 29;67(25):701-706. doi: 10.15585/mmwr.mm6725a1.
Hlavsa MC et al. Outbreaks Associated with Treated Recreational Water — United States, 2015–2019. MMWR Morb Mortal Wkly Rep. 2021;70:733–738. doi: 10.15585/mmwr.mm7020a1.
Kimberlin DW et al., eds. Red Book Report of the Committee on Infectious Diseases. 33rd ed. American Academy of Pediatrics. 2024. Cryptosporidiosis, p 338-40 and Norovirus, p 622-624.Waterborne Outbreaks Summary Reports. CDC. 2024 April 18.
Predicting and Understanding Vaccine Response Determinants
In this column, I recently discussed the impact of the microbiome on childhood vaccine responses. My group has been expanding our research on the topic of childhood vaccine response and its relationship to infection proneness. Therefore, I want to share new research findings.
Immune responsiveness to vaccines varies among children, leaving some susceptible to infections. We also have evidence that the immune deficiencies that contribute to poor vaccine responsiveness also manifest in children as respiratory infection proneness.
Predicting Vaccine Response in the Neonatal Period
The first 100 days of life is an amazing transition time in early life. During that time, the immune system is highly influenced by environmental factors that generate epigenetic changes affecting vaccine responsiveness. Some publications have used the term “window of opportunity,” because it is thought that interventions to change a negative trajectory to a positive one for vaccine responsiveness have a better potential to be effective. Predicting which children will be poorly responsive to vaccines would be desirable, so those children could be specifically identified for intervention. Doing so in the neonatal age time frame using easy-to-obtain clinical samples would be a bonus.
In our most recent study, we sought to identify cytokine biosignatures in the neonatal period, measured in convenient nasopharyngeal secretions, that predict vaccine responses, measured as antibody levels to various vaccines at 1 year of life. Secondly, we assessed the effect of antibiotic exposures on vaccine responses in the study cohort. Third, we tested for induction of CD4+ T-cell vaccine-specific immune memory at infant age 1 year. Fourth, we studied antigen presenting cells (APCs) at rest and in response to an adjuvant called R848, known to stimulate toll-like receptor (TLR) 7/8 agonist, to assess its effects on the immune cells of low vaccine responder children, compared with other children.1
The study population consisted of 101 infants recruited from two primary care pediatric practices in/near Rochester, New York. Children lived in suburban and rural environments. Enrollment and sampling occurred during 2017-2020. All participants received regularly scheduled childhood vaccinations according to the recommendations by US Centers for Disease Control. Nasopharyngeal swabs were used to collect nasal secretions. Antibody titers against six antigens were measured at approximately 1 year of age from all 72 available blood samples. The protective threshold of the corresponding vaccine antigen divided each vaccine-induced antibody level and the ratio considered a normalized titer. The normalized antibody titers were used to define vaccine responsiveness groups as Low Vaccine Responder (bottom 25th percentile of vaccine responders, n = 18 children), as Normal Vaccine Responder (25-75th percentile of vaccine responders, n = 36 children) and as High Vaccine Responder (top 25th percentile of vaccine responders, n = 18 children).
We found that specific nasal cytokine levels measured at newborn age 1 week old, 2 weeks old, and 3 weeks old were predictive of the vaccine response groupings measured at child age 1 year old, following their primary series of vaccinations. The P values varied between less than .05 to .001.
Five newborns had antibiotic exposure at/near the time of birth; 4 [80%] of the 5 were Low Vaccine Responders vs 1 [2%] of 60 Normal+High Vaccine Responder children, P = .006. Also, the cumulative days of antibiotic exposure up to 1 year was highly associated with low vaccine responders, compared with Normal+High Vaccine Responder children (P = 2 x 10-16).
We found that Low Vaccine Responder infants had reduced vaccine-specific T-helper memory cells producing INFg and IL-2 (Th1 cytokines) and IL-4 (Th2 cytokines), compared with Normal+High Vaccine Responder children. In the absence of sufficient numbers of antigen-specific memory CD4+ T-cells, a child would become unprotected from the target infection that the vaccines were intended to prevent after the antibody levels wane.
We found that Low Vaccine Responder antigen-presenting cells are different from those in normal vaccine responders and they can be distinguished when at rest and when stimulated by a specific adjuvant — R848. Our previous findings suggested that Low Vaccine Responder children have a prolonged neonatal-like immune profile (PNIP).2 Therefore, stimulating the immune system of a Low Vaccine Responder could shift their cellular immune responses to behave like cells of Normal+High Vaccine Responder children.
In summary, we identified cytokine biosignatures measured in nasopharyngeal secretions in the neonatal period that predicted vaccine response groups measured as antibody levels at 1 year of life. We showed that reduced vaccine responsiveness was associated with antibiotic exposure at/near birth and with cumulative exposure during the first year of life. We found that Low Vaccine Responder children at 1 year old have fewer vaccine-specific memory CD4+ Th1 and Th2-cells and that antigen-presenting cells at rest and in response to R848 antigen stimulation differ, compared with Normal+High Vaccine Responder children.
Future work by our group will focus on exploring early-life risk factors that influence differences in vaccine responsiveness and interventions that might shift a child’s responsiveness from low to normal or high.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (New York) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. Variability of Vaccine Responsiveness in Young Children. J Infect Dis. 2023 Nov 22:jiad524. doi: 10.1093/infdis/jiad524.
2. Pichichero ME et al. Functional Immune Cell Differences Associated with Low Vaccine Responses in Infants. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
In this column, I recently discussed the impact of the microbiome on childhood vaccine responses. My group has been expanding our research on the topic of childhood vaccine response and its relationship to infection proneness. Therefore, I want to share new research findings.
Immune responsiveness to vaccines varies among children, leaving some susceptible to infections. We also have evidence that the immune deficiencies that contribute to poor vaccine responsiveness also manifest in children as respiratory infection proneness.
Predicting Vaccine Response in the Neonatal Period
The first 100 days of life is an amazing transition time in early life. During that time, the immune system is highly influenced by environmental factors that generate epigenetic changes affecting vaccine responsiveness. Some publications have used the term “window of opportunity,” because it is thought that interventions to change a negative trajectory to a positive one for vaccine responsiveness have a better potential to be effective. Predicting which children will be poorly responsive to vaccines would be desirable, so those children could be specifically identified for intervention. Doing so in the neonatal age time frame using easy-to-obtain clinical samples would be a bonus.
In our most recent study, we sought to identify cytokine biosignatures in the neonatal period, measured in convenient nasopharyngeal secretions, that predict vaccine responses, measured as antibody levels to various vaccines at 1 year of life. Secondly, we assessed the effect of antibiotic exposures on vaccine responses in the study cohort. Third, we tested for induction of CD4+ T-cell vaccine-specific immune memory at infant age 1 year. Fourth, we studied antigen presenting cells (APCs) at rest and in response to an adjuvant called R848, known to stimulate toll-like receptor (TLR) 7/8 agonist, to assess its effects on the immune cells of low vaccine responder children, compared with other children.1
The study population consisted of 101 infants recruited from two primary care pediatric practices in/near Rochester, New York. Children lived in suburban and rural environments. Enrollment and sampling occurred during 2017-2020. All participants received regularly scheduled childhood vaccinations according to the recommendations by US Centers for Disease Control. Nasopharyngeal swabs were used to collect nasal secretions. Antibody titers against six antigens were measured at approximately 1 year of age from all 72 available blood samples. The protective threshold of the corresponding vaccine antigen divided each vaccine-induced antibody level and the ratio considered a normalized titer. The normalized antibody titers were used to define vaccine responsiveness groups as Low Vaccine Responder (bottom 25th percentile of vaccine responders, n = 18 children), as Normal Vaccine Responder (25-75th percentile of vaccine responders, n = 36 children) and as High Vaccine Responder (top 25th percentile of vaccine responders, n = 18 children).
We found that specific nasal cytokine levels measured at newborn age 1 week old, 2 weeks old, and 3 weeks old were predictive of the vaccine response groupings measured at child age 1 year old, following their primary series of vaccinations. The P values varied between less than .05 to .001.
Five newborns had antibiotic exposure at/near the time of birth; 4 [80%] of the 5 were Low Vaccine Responders vs 1 [2%] of 60 Normal+High Vaccine Responder children, P = .006. Also, the cumulative days of antibiotic exposure up to 1 year was highly associated with low vaccine responders, compared with Normal+High Vaccine Responder children (P = 2 x 10-16).
We found that Low Vaccine Responder infants had reduced vaccine-specific T-helper memory cells producing INFg and IL-2 (Th1 cytokines) and IL-4 (Th2 cytokines), compared with Normal+High Vaccine Responder children. In the absence of sufficient numbers of antigen-specific memory CD4+ T-cells, a child would become unprotected from the target infection that the vaccines were intended to prevent after the antibody levels wane.
We found that Low Vaccine Responder antigen-presenting cells are different from those in normal vaccine responders and they can be distinguished when at rest and when stimulated by a specific adjuvant — R848. Our previous findings suggested that Low Vaccine Responder children have a prolonged neonatal-like immune profile (PNIP).2 Therefore, stimulating the immune system of a Low Vaccine Responder could shift their cellular immune responses to behave like cells of Normal+High Vaccine Responder children.
In summary, we identified cytokine biosignatures measured in nasopharyngeal secretions in the neonatal period that predicted vaccine response groups measured as antibody levels at 1 year of life. We showed that reduced vaccine responsiveness was associated with antibiotic exposure at/near birth and with cumulative exposure during the first year of life. We found that Low Vaccine Responder children at 1 year old have fewer vaccine-specific memory CD4+ Th1 and Th2-cells and that antigen-presenting cells at rest and in response to R848 antigen stimulation differ, compared with Normal+High Vaccine Responder children.
Future work by our group will focus on exploring early-life risk factors that influence differences in vaccine responsiveness and interventions that might shift a child’s responsiveness from low to normal or high.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (New York) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. Variability of Vaccine Responsiveness in Young Children. J Infect Dis. 2023 Nov 22:jiad524. doi: 10.1093/infdis/jiad524.
2. Pichichero ME et al. Functional Immune Cell Differences Associated with Low Vaccine Responses in Infants. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
In this column, I recently discussed the impact of the microbiome on childhood vaccine responses. My group has been expanding our research on the topic of childhood vaccine response and its relationship to infection proneness. Therefore, I want to share new research findings.
Immune responsiveness to vaccines varies among children, leaving some susceptible to infections. We also have evidence that the immune deficiencies that contribute to poor vaccine responsiveness also manifest in children as respiratory infection proneness.
Predicting Vaccine Response in the Neonatal Period
The first 100 days of life is an amazing transition time in early life. During that time, the immune system is highly influenced by environmental factors that generate epigenetic changes affecting vaccine responsiveness. Some publications have used the term “window of opportunity,” because it is thought that interventions to change a negative trajectory to a positive one for vaccine responsiveness have a better potential to be effective. Predicting which children will be poorly responsive to vaccines would be desirable, so those children could be specifically identified for intervention. Doing so in the neonatal age time frame using easy-to-obtain clinical samples would be a bonus.
In our most recent study, we sought to identify cytokine biosignatures in the neonatal period, measured in convenient nasopharyngeal secretions, that predict vaccine responses, measured as antibody levels to various vaccines at 1 year of life. Secondly, we assessed the effect of antibiotic exposures on vaccine responses in the study cohort. Third, we tested for induction of CD4+ T-cell vaccine-specific immune memory at infant age 1 year. Fourth, we studied antigen presenting cells (APCs) at rest and in response to an adjuvant called R848, known to stimulate toll-like receptor (TLR) 7/8 agonist, to assess its effects on the immune cells of low vaccine responder children, compared with other children.1
The study population consisted of 101 infants recruited from two primary care pediatric practices in/near Rochester, New York. Children lived in suburban and rural environments. Enrollment and sampling occurred during 2017-2020. All participants received regularly scheduled childhood vaccinations according to the recommendations by US Centers for Disease Control. Nasopharyngeal swabs were used to collect nasal secretions. Antibody titers against six antigens were measured at approximately 1 year of age from all 72 available blood samples. The protective threshold of the corresponding vaccine antigen divided each vaccine-induced antibody level and the ratio considered a normalized titer. The normalized antibody titers were used to define vaccine responsiveness groups as Low Vaccine Responder (bottom 25th percentile of vaccine responders, n = 18 children), as Normal Vaccine Responder (25-75th percentile of vaccine responders, n = 36 children) and as High Vaccine Responder (top 25th percentile of vaccine responders, n = 18 children).
We found that specific nasal cytokine levels measured at newborn age 1 week old, 2 weeks old, and 3 weeks old were predictive of the vaccine response groupings measured at child age 1 year old, following their primary series of vaccinations. The P values varied between less than .05 to .001.
Five newborns had antibiotic exposure at/near the time of birth; 4 [80%] of the 5 were Low Vaccine Responders vs 1 [2%] of 60 Normal+High Vaccine Responder children, P = .006. Also, the cumulative days of antibiotic exposure up to 1 year was highly associated with low vaccine responders, compared with Normal+High Vaccine Responder children (P = 2 x 10-16).
We found that Low Vaccine Responder infants had reduced vaccine-specific T-helper memory cells producing INFg and IL-2 (Th1 cytokines) and IL-4 (Th2 cytokines), compared with Normal+High Vaccine Responder children. In the absence of sufficient numbers of antigen-specific memory CD4+ T-cells, a child would become unprotected from the target infection that the vaccines were intended to prevent after the antibody levels wane.
We found that Low Vaccine Responder antigen-presenting cells are different from those in normal vaccine responders and they can be distinguished when at rest and when stimulated by a specific adjuvant — R848. Our previous findings suggested that Low Vaccine Responder children have a prolonged neonatal-like immune profile (PNIP).2 Therefore, stimulating the immune system of a Low Vaccine Responder could shift their cellular immune responses to behave like cells of Normal+High Vaccine Responder children.
In summary, we identified cytokine biosignatures measured in nasopharyngeal secretions in the neonatal period that predicted vaccine response groups measured as antibody levels at 1 year of life. We showed that reduced vaccine responsiveness was associated with antibiotic exposure at/near birth and with cumulative exposure during the first year of life. We found that Low Vaccine Responder children at 1 year old have fewer vaccine-specific memory CD4+ Th1 and Th2-cells and that antigen-presenting cells at rest and in response to R848 antigen stimulation differ, compared with Normal+High Vaccine Responder children.
Future work by our group will focus on exploring early-life risk factors that influence differences in vaccine responsiveness and interventions that might shift a child’s responsiveness from low to normal or high.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (New York) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. Variability of Vaccine Responsiveness in Young Children. J Infect Dis. 2023 Nov 22:jiad524. doi: 10.1093/infdis/jiad524.
2. Pichichero ME et al. Functional Immune Cell Differences Associated with Low Vaccine Responses in Infants. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
Highly Pathogenic Avian Influenza (HPAI)
Imagine this: A 15-year-old male presents to an urgent care center with a one-day history of fever, cough, and shortness of breath. He is mildly tachypneic with bilateral scattered crackles on lung exam. A rapid test for COVID-19 and influenza is positive for influenza A — a surprising result in June.
An oxygen saturation of 90% prompts transfer to the emergency department at the local children’s hospital. The emergency medicine fellow is skeptical of the presumptive diagnosis. Influenza in the summer in a boy who had not traveled outside his small hometown in the southeastern United States? A respiratory viral panel also detected influenza A, but the specimen did not type as influenza A H1 or H3. This result prompted the laboratory technician to place a call to the ordering physician. “Does this patient have risk factors for avian flu?” the tech asked.
Highly pathogenic avian influenza (HPAI) A(H5N1) is not a new virus. It was discovered in waterfowl in China in 1996 and has since evolved into multiple clades and subclades, spreading to every continent on the globe except Oceania. It is called highly pathogenic because it kills a large number of the birds that it infects. In 2021, Clade 2.3.4.4b HPAI A(H5N1) viruses emerged in North America, causing large outbreaks in wild birds and farmed poultry populations, including backyard flocks. Sporadic infections have been identified in a diverse group of mammals, including foxes, raccoons, baby goats, bears, and harbor seals. In March of this year, HPAI A(H5N1) was detected for the first time in United States dairy cattle. As we go to press, the United States Department of Agriculture has detected HPAI A(H5N1) in dairy cattle on 36 farms in 9 states.
Human infections are rare, but often severe. Following a 1997 outbreak of HPAI A(H5N1) in Hong Kong, 18 people were infected and 6 died. Since then, more than 900 cases have been reported in humans and approximately half of these have been fatal. The spectrum of disease includes asymptomatic infection and mild disease, as occurred recently in Texas. A dairy farm worker who was exposed to dairy cattle presumed to be infected with HPAI A(H5N1) developed conjunctivitis and no other symptoms. An individual infected in Colorado in 2022 had no symptoms other than fatigue and recovered.
Human-to-human transmission was not identified with either of these cases, although very limited, non-sustained transmission has been observed in the past, usually in family members of infected people after prolonged close exposure.
Right now, most people in the United States are not at risk for HPAI A(H5N1) infection.
Careful history taking with our illustrative and hypothetical case revealed exposure to farm animals but in a state without known cases of HPAI A(H5N1) in dairy cattle. State health department officials nevertheless agreed with further testing of the patient. Some influenza diagnostic tests cleared by the US Food and Drug Administration (FDA) can detect some novel influenza A viruses such as HPAI A(H5N1) but cannot distinguish between infection with seasonal influenza A or novel influenza A viruses. Molecular assays may give an “influenza A untypeable” result, as in our case. The CDC urges further testing on these untypeable specimens at local or state public health laboratories. When HPAI A(H5N1) is suspected, a negative result on a commercially available test is not considered sufficient to exclude the possibility of infection.
Our patient was admitted to the hospital and droplet, contact, and airborne precautions were instituted along with antiviral treatment with oseltamivir. Preliminary analysis of HPAI A(H5N1) viruses predicts susceptibility to currently available antivirals. The admitting physician confirmed that the boy had received influenza vaccine in the preceding season but, unfortunately, seasonal vaccines do not protect against HPAI A(H5N1) infection.
Advice for Clinicians
Given the recent media attention and public health focus on HPAI A(H5N1), frontline clinicians may start receiving questions from patients and families and perhaps requests for testing. At this point, testing is generally recommended only for individuals with risk factors or known exposures. Healthcare providers with questions about testing are encouraged to reach out to their local or state health departments.
Public health authorities have provided recommendations for protection from HPAI. These include avoiding unprotected exposures to sick or dead wild birds, poultry, other domesticated birds, and wild or domesticated animals (including cattle). People should avoid unprotected contact with animals with suspected or confirmed HPAI A(H5N1)-virus infection or products from these animals, including raw or unpasteurized milk and raw milk products.
We can, however, reassure families that the commercial milk supply is safe. In late April, the FDA reported that HPAI viral fragments were found in one of five retail milk samples by polymerase chain reaction testing. Additional testing did not detect any live, infectious virus, indicating the effectiveness of pasteurization at inactivating the virus. Of importance to pediatricians and others pediatric clinicians, limited sampling of retail powdered infant formula and powdered milk products marketed as toddler formula revealed no viral fragments or viable virus.
The million-dollar question is whether HPAI A(H5N1) could start a new pandemic. To date, the virus has not acquired the mutations that would make it easily transmissible from person to person. If that changes and the virus does start spreading more widely, candidate vaccines that could protect against HPAI A(H5N1) have been developed and are part of the national stockpile. Let’s hope we don’t need them.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the American Academy of Pediatrics’ Committee on Infectious Diseases and the physician lead for Red Book Online. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
Imagine this: A 15-year-old male presents to an urgent care center with a one-day history of fever, cough, and shortness of breath. He is mildly tachypneic with bilateral scattered crackles on lung exam. A rapid test for COVID-19 and influenza is positive for influenza A — a surprising result in June.
An oxygen saturation of 90% prompts transfer to the emergency department at the local children’s hospital. The emergency medicine fellow is skeptical of the presumptive diagnosis. Influenza in the summer in a boy who had not traveled outside his small hometown in the southeastern United States? A respiratory viral panel also detected influenza A, but the specimen did not type as influenza A H1 or H3. This result prompted the laboratory technician to place a call to the ordering physician. “Does this patient have risk factors for avian flu?” the tech asked.
Highly pathogenic avian influenza (HPAI) A(H5N1) is not a new virus. It was discovered in waterfowl in China in 1996 and has since evolved into multiple clades and subclades, spreading to every continent on the globe except Oceania. It is called highly pathogenic because it kills a large number of the birds that it infects. In 2021, Clade 2.3.4.4b HPAI A(H5N1) viruses emerged in North America, causing large outbreaks in wild birds and farmed poultry populations, including backyard flocks. Sporadic infections have been identified in a diverse group of mammals, including foxes, raccoons, baby goats, bears, and harbor seals. In March of this year, HPAI A(H5N1) was detected for the first time in United States dairy cattle. As we go to press, the United States Department of Agriculture has detected HPAI A(H5N1) in dairy cattle on 36 farms in 9 states.
Human infections are rare, but often severe. Following a 1997 outbreak of HPAI A(H5N1) in Hong Kong, 18 people were infected and 6 died. Since then, more than 900 cases have been reported in humans and approximately half of these have been fatal. The spectrum of disease includes asymptomatic infection and mild disease, as occurred recently in Texas. A dairy farm worker who was exposed to dairy cattle presumed to be infected with HPAI A(H5N1) developed conjunctivitis and no other symptoms. An individual infected in Colorado in 2022 had no symptoms other than fatigue and recovered.
Human-to-human transmission was not identified with either of these cases, although very limited, non-sustained transmission has been observed in the past, usually in family members of infected people after prolonged close exposure.
Right now, most people in the United States are not at risk for HPAI A(H5N1) infection.
Careful history taking with our illustrative and hypothetical case revealed exposure to farm animals but in a state without known cases of HPAI A(H5N1) in dairy cattle. State health department officials nevertheless agreed with further testing of the patient. Some influenza diagnostic tests cleared by the US Food and Drug Administration (FDA) can detect some novel influenza A viruses such as HPAI A(H5N1) but cannot distinguish between infection with seasonal influenza A or novel influenza A viruses. Molecular assays may give an “influenza A untypeable” result, as in our case. The CDC urges further testing on these untypeable specimens at local or state public health laboratories. When HPAI A(H5N1) is suspected, a negative result on a commercially available test is not considered sufficient to exclude the possibility of infection.
Our patient was admitted to the hospital and droplet, contact, and airborne precautions were instituted along with antiviral treatment with oseltamivir. Preliminary analysis of HPAI A(H5N1) viruses predicts susceptibility to currently available antivirals. The admitting physician confirmed that the boy had received influenza vaccine in the preceding season but, unfortunately, seasonal vaccines do not protect against HPAI A(H5N1) infection.
Advice for Clinicians
Given the recent media attention and public health focus on HPAI A(H5N1), frontline clinicians may start receiving questions from patients and families and perhaps requests for testing. At this point, testing is generally recommended only for individuals with risk factors or known exposures. Healthcare providers with questions about testing are encouraged to reach out to their local or state health departments.
Public health authorities have provided recommendations for protection from HPAI. These include avoiding unprotected exposures to sick or dead wild birds, poultry, other domesticated birds, and wild or domesticated animals (including cattle). People should avoid unprotected contact with animals with suspected or confirmed HPAI A(H5N1)-virus infection or products from these animals, including raw or unpasteurized milk and raw milk products.
We can, however, reassure families that the commercial milk supply is safe. In late April, the FDA reported that HPAI viral fragments were found in one of five retail milk samples by polymerase chain reaction testing. Additional testing did not detect any live, infectious virus, indicating the effectiveness of pasteurization at inactivating the virus. Of importance to pediatricians and others pediatric clinicians, limited sampling of retail powdered infant formula and powdered milk products marketed as toddler formula revealed no viral fragments or viable virus.
The million-dollar question is whether HPAI A(H5N1) could start a new pandemic. To date, the virus has not acquired the mutations that would make it easily transmissible from person to person. If that changes and the virus does start spreading more widely, candidate vaccines that could protect against HPAI A(H5N1) have been developed and are part of the national stockpile. Let’s hope we don’t need them.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the American Academy of Pediatrics’ Committee on Infectious Diseases and the physician lead for Red Book Online. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
Imagine this: A 15-year-old male presents to an urgent care center with a one-day history of fever, cough, and shortness of breath. He is mildly tachypneic with bilateral scattered crackles on lung exam. A rapid test for COVID-19 and influenza is positive for influenza A — a surprising result in June.
An oxygen saturation of 90% prompts transfer to the emergency department at the local children’s hospital. The emergency medicine fellow is skeptical of the presumptive diagnosis. Influenza in the summer in a boy who had not traveled outside his small hometown in the southeastern United States? A respiratory viral panel also detected influenza A, but the specimen did not type as influenza A H1 or H3. This result prompted the laboratory technician to place a call to the ordering physician. “Does this patient have risk factors for avian flu?” the tech asked.
Highly pathogenic avian influenza (HPAI) A(H5N1) is not a new virus. It was discovered in waterfowl in China in 1996 and has since evolved into multiple clades and subclades, spreading to every continent on the globe except Oceania. It is called highly pathogenic because it kills a large number of the birds that it infects. In 2021, Clade 2.3.4.4b HPAI A(H5N1) viruses emerged in North America, causing large outbreaks in wild birds and farmed poultry populations, including backyard flocks. Sporadic infections have been identified in a diverse group of mammals, including foxes, raccoons, baby goats, bears, and harbor seals. In March of this year, HPAI A(H5N1) was detected for the first time in United States dairy cattle. As we go to press, the United States Department of Agriculture has detected HPAI A(H5N1) in dairy cattle on 36 farms in 9 states.
Human infections are rare, but often severe. Following a 1997 outbreak of HPAI A(H5N1) in Hong Kong, 18 people were infected and 6 died. Since then, more than 900 cases have been reported in humans and approximately half of these have been fatal. The spectrum of disease includes asymptomatic infection and mild disease, as occurred recently in Texas. A dairy farm worker who was exposed to dairy cattle presumed to be infected with HPAI A(H5N1) developed conjunctivitis and no other symptoms. An individual infected in Colorado in 2022 had no symptoms other than fatigue and recovered.
Human-to-human transmission was not identified with either of these cases, although very limited, non-sustained transmission has been observed in the past, usually in family members of infected people after prolonged close exposure.
Right now, most people in the United States are not at risk for HPAI A(H5N1) infection.
Careful history taking with our illustrative and hypothetical case revealed exposure to farm animals but in a state without known cases of HPAI A(H5N1) in dairy cattle. State health department officials nevertheless agreed with further testing of the patient. Some influenza diagnostic tests cleared by the US Food and Drug Administration (FDA) can detect some novel influenza A viruses such as HPAI A(H5N1) but cannot distinguish between infection with seasonal influenza A or novel influenza A viruses. Molecular assays may give an “influenza A untypeable” result, as in our case. The CDC urges further testing on these untypeable specimens at local or state public health laboratories. When HPAI A(H5N1) is suspected, a negative result on a commercially available test is not considered sufficient to exclude the possibility of infection.
Our patient was admitted to the hospital and droplet, contact, and airborne precautions were instituted along with antiviral treatment with oseltamivir. Preliminary analysis of HPAI A(H5N1) viruses predicts susceptibility to currently available antivirals. The admitting physician confirmed that the boy had received influenza vaccine in the preceding season but, unfortunately, seasonal vaccines do not protect against HPAI A(H5N1) infection.
Advice for Clinicians
Given the recent media attention and public health focus on HPAI A(H5N1), frontline clinicians may start receiving questions from patients and families and perhaps requests for testing. At this point, testing is generally recommended only for individuals with risk factors or known exposures. Healthcare providers with questions about testing are encouraged to reach out to their local or state health departments.
Public health authorities have provided recommendations for protection from HPAI. These include avoiding unprotected exposures to sick or dead wild birds, poultry, other domesticated birds, and wild or domesticated animals (including cattle). People should avoid unprotected contact with animals with suspected or confirmed HPAI A(H5N1)-virus infection or products from these animals, including raw or unpasteurized milk and raw milk products.
We can, however, reassure families that the commercial milk supply is safe. In late April, the FDA reported that HPAI viral fragments were found in one of five retail milk samples by polymerase chain reaction testing. Additional testing did not detect any live, infectious virus, indicating the effectiveness of pasteurization at inactivating the virus. Of importance to pediatricians and others pediatric clinicians, limited sampling of retail powdered infant formula and powdered milk products marketed as toddler formula revealed no viral fragments or viable virus.
The million-dollar question is whether HPAI A(H5N1) could start a new pandemic. To date, the virus has not acquired the mutations that would make it easily transmissible from person to person. If that changes and the virus does start spreading more widely, candidate vaccines that could protect against HPAI A(H5N1) have been developed and are part of the national stockpile. Let’s hope we don’t need them.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the American Academy of Pediatrics’ Committee on Infectious Diseases and the physician lead for Red Book Online. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
Worldwide Uptick in Invasive Group A Streptococcus Disease Post Pandemic — What Should We Know?
Invasive group A streptococcus (iGAS) infections are rare (4-9 cases/100,000 US population annually) but potentially devastating (approximately 2,300 deaths annually in US), and affect all ages. Cases increase in winter-spring, paralleling the “season” of increased noninvasive GAS, e.g., pharyngitis and scarlet fever. iGAS case rates are lower in children than adults. That said, one well-known pediatric iGAS scenario has been deep cellulitis and necrotizing fasciitis during the healing phase of varicella. Other forms of iGAS include bacteremia, pneumonia (particularly when empyema is present), lymphangitis, erysipelas, and toxic shock syndrome. iGAS can occur with/after influenza but has also occurred concurrently with other viral respiratory infections.
Persons with underlying conditions (cancer or immune compromised status; chronic diseases of the heart, kidney or lung; diabetes mellitus) are at higher risk. Other subpopulations at risk for iGAS are illicit drug users, the elderly, homeless persons, nursing home residents, American Indian persons, and Alaska Native persons. Most experts feel that highly toxigenic strains of GAS are responsible for most iGAS. Indeed, most iGAS isolates produce (sometimes hyper-produce) superantigens that cause exaggerated innate immune responses, higher levels of inflammation, and often times tissue destruction, e.g., “flesh eating bacteria.” And who can forget that Jim Henson, creator of the Muppets, died of iGAS?
But why discuss iGAS in 2024? The pattern for iGAS has fluctuated more than usual in the last decade. So much so that the recent upsurge has caught the collective eye of the lay press. So, patients and friends may have questions about why and how iGAS is increasing lately. The bottom line is that no one knows for sure. However, the most recent 2 years of uptick may reflect GAS circulating at relatively high levels even when taking into account that GAS season occurs in winter-spring most years. Yet it seems likely that additional factors may have played a role in the fluctuations noted this past decade, e.g., temporary changes in societal behavior, a new GAS strain with over two dozen mutations, and possibly rapid waning of protection against GAS exotoxins.
Social Behavior Factor
The SARS-CoV-2 pandemic brought extremes of disease and death to the world and dramatic changes in social behavior. A byproduct was dramatic decreases in nearly all infectious diseases, with numerous reports of near absence of many respiratory and gastrointestinal viruses in the 2020-2021 seasons. Interestingly, we did not see a drop in human rhinovirus infections, justifying its nickname as the cockroach of viruses. Reports also emerged about drops in bacterial diseases during 2020-2021 (although not so much for STIs), including noninvasive and invasive GAS disease, and also GAS-associated deaths (lowest since 2016).1 The drop in iGAS during social restrictions makes sense because GAS is spread by direct contact with infected persons or their secretions, and social contact had dramatically decreased particularly in the first 6 months of the pandemic.
However, since 2022 and the return to “normal” social behaviors, both viral diseases (e.g., RSV, influenza, and Norovirus), and some bacterial diseases have rebounded. That said, something else must be contributing, because iGAS rates had increased 4-5 years pre pandemic. In fact, the fluctuating pattern included “normal” annual rates in the early 2000s rising in ~2015 followed by the explainable pandemic drop (by nearly 25%), and not-too-unexpected 2-year postpandemic rise. But interestingly enough, the rebound is higher than might be expected for iGAS and children were overrepresented in first year’s rise (2022 rate for pediatric iGAS was the highest since 1997) while those older than 65 were overrepresented in second year (2023).1
Emergence of M1UK
One potential factor for the prepandemic rise in iGAS infections worldwide is the emergence and worldwide spread of a new GAS emm type variant designated M1UK.2 GAS isolates can be typed into categories designated as emm types based on DNA sequence. There are more than 240 emm types, with 6 being most common — M1, M3, and M28 (each up to 20% of GAS isolates) and M12, M82, and M89 (each up to 10%). M1, M3 and M28 have also been particularly associated with invasive disease. While emm types vary year to year and region by region, the overall emm type distribution among iGAS isolates in the United States had not been unusual since the turn of the century and the US M1 strain was the same as that which had been predominant worldwide (designated M1GLOBAL). This new M1UK sublineage had emerged around 2010 and had been increasing pre pandemic. The M1UK sequence contained a specific set of 27 SNPs (single nucleoside polymorphisms, i.e., single base mutations) and was associated with an uptick in scarlet fever in the United Kingdom starting around 2010. Its prevalence increased up to around 2015 while spreading internationally. It also had enhanced expression of SpeA, a phage-encoded superantigen. Some of the M1UK mutations also appear to alter GAS metabolic processes to allow better survival (better “fitness”) compared with other GAS. So, a more virulent hardier GAS had arisen and seems a reasonable candidate for contributing to the increased iGAS rates.
Waning Antibody to GAS As Potential Factor in Rebound
No consensus exists on correlates of protection from iGAS. However, adults seem to have less noninvasive GAS than children. One potential reason is that frequent GAS re-exposure, regardless of whether disease results, likely boosts anti-GAS antibodies. Pandemic social restrictions temporarily prevented such boosts. In children with developing antibody repertoires, anti-GAS antibodies may have waned below protective levels faster during a year without frequent boosting. Thus, children were iGAS susceptible soon after pandemic restrictions were dropped (2022). Increased iGAS rates in the elderly in 2023 may have occurred because of diminished GAS exposures accelerating immune senescence with anti-GAS antibodies dropping, but less quickly than in children. These speculations are simply hypotheses until future studies can test them.
All that said, how do we use information on increased iGAS in our daily practices? In addition to standard preventive strategies for viral coinfections (e.g., varicella and influenza vaccine), reminding families about rigorous attention to wound care is the one high-risk scenario we have not yet discussed. During 2024, a time of expected increased prevalence of iGAS, early wound care needs to be fastidious. Further, share warning signs with families (e.g., rapidly expanding painful erythema), “streaks” ascending from extremity wounds, fever and a highly painful wound, darkening almost purple color within cellulitis or soft tissue infection, or loss of sensation in the middle of an otherwise painful soft tissue infection. These presentations require immediate medical attention.
If such a patient presents, the Centers for Disease Control and Prevention (CDC) recommends admission along with blood and, where possible, wound cultures. If in the context of pneumonia with pleural effusion, culturing pleural fluid is also important. Remember, leading edge cultures are not often positive for GAS, seemingly because GAS exotoxins are found at erythema’s leading edge, not the bacteria. The bacteria are somewhere more central in the inflammatory process. Despite not being prominent among recent iGAS cases, another scenario that could sneak up on you is the infected surgical wound as nascent iGAS.
Finally, remember that nationally increasing numbers of iGAS isolates are resistant to erythromycin and clindamycin, the latter usually recommended to reduce tissue damage in iGAS.3 So, it is important to be aware of susceptibility patterns in your locale and consider an ID consultation. My hope is that you do not see an iGAS case this year, but we all need to remain alert. With a high index of suspicion and rapid diagnosis, you can minimize long-term sequelae and potential fatalities.
While it is too early to tell how the rest of 2024 will turn out, preliminary indications are that GAS is circulating at higher than usual levels (30%-35% GAS positive throat swabs in early April 2024 in Kansas City area) and iGAS rates will likely also be relatively high, particularly if Ontario, Canada, data are any indication.4 
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Current Group A Strep Activity, Centers for Disease Control and Prevention. April 2024. CDC webpage on current invasive GAS disease. April 2024.
2. Li Y et al. Expansion of Invasive Group A Streptococcus M1UK Lineage in Active Bacterial Core Surveillance, United States, 2019-2021 Emerg Infect Dis. 2023;29(10):2116-2120. doi: 10.3201/eid2910.230675.
3. Andreoni F et al. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J Infect Dis. 2017 Jan 15;215(2):269-277. doi: 10.1093/infdis/jiw229.
4. Group A Streptococcal Disease, Invasive (iGAS), Public Health Ontario.
Invasive group A streptococcus (iGAS) infections are rare (4-9 cases/100,000 US population annually) but potentially devastating (approximately 2,300 deaths annually in US), and affect all ages. Cases increase in winter-spring, paralleling the “season” of increased noninvasive GAS, e.g., pharyngitis and scarlet fever. iGAS case rates are lower in children than adults. That said, one well-known pediatric iGAS scenario has been deep cellulitis and necrotizing fasciitis during the healing phase of varicella. Other forms of iGAS include bacteremia, pneumonia (particularly when empyema is present), lymphangitis, erysipelas, and toxic shock syndrome. iGAS can occur with/after influenza but has also occurred concurrently with other viral respiratory infections.
Persons with underlying conditions (cancer or immune compromised status; chronic diseases of the heart, kidney or lung; diabetes mellitus) are at higher risk. Other subpopulations at risk for iGAS are illicit drug users, the elderly, homeless persons, nursing home residents, American Indian persons, and Alaska Native persons. Most experts feel that highly toxigenic strains of GAS are responsible for most iGAS. Indeed, most iGAS isolates produce (sometimes hyper-produce) superantigens that cause exaggerated innate immune responses, higher levels of inflammation, and often times tissue destruction, e.g., “flesh eating bacteria.” And who can forget that Jim Henson, creator of the Muppets, died of iGAS?
But why discuss iGAS in 2024? The pattern for iGAS has fluctuated more than usual in the last decade. So much so that the recent upsurge has caught the collective eye of the lay press. So, patients and friends may have questions about why and how iGAS is increasing lately. The bottom line is that no one knows for sure. However, the most recent 2 years of uptick may reflect GAS circulating at relatively high levels even when taking into account that GAS season occurs in winter-spring most years. Yet it seems likely that additional factors may have played a role in the fluctuations noted this past decade, e.g., temporary changes in societal behavior, a new GAS strain with over two dozen mutations, and possibly rapid waning of protection against GAS exotoxins.
Social Behavior Factor
The SARS-CoV-2 pandemic brought extremes of disease and death to the world and dramatic changes in social behavior. A byproduct was dramatic decreases in nearly all infectious diseases, with numerous reports of near absence of many respiratory and gastrointestinal viruses in the 2020-2021 seasons. Interestingly, we did not see a drop in human rhinovirus infections, justifying its nickname as the cockroach of viruses. Reports also emerged about drops in bacterial diseases during 2020-2021 (although not so much for STIs), including noninvasive and invasive GAS disease, and also GAS-associated deaths (lowest since 2016).1 The drop in iGAS during social restrictions makes sense because GAS is spread by direct contact with infected persons or their secretions, and social contact had dramatically decreased particularly in the first 6 months of the pandemic.
However, since 2022 and the return to “normal” social behaviors, both viral diseases (e.g., RSV, influenza, and Norovirus), and some bacterial diseases have rebounded. That said, something else must be contributing, because iGAS rates had increased 4-5 years pre pandemic. In fact, the fluctuating pattern included “normal” annual rates in the early 2000s rising in ~2015 followed by the explainable pandemic drop (by nearly 25%), and not-too-unexpected 2-year postpandemic rise. But interestingly enough, the rebound is higher than might be expected for iGAS and children were overrepresented in first year’s rise (2022 rate for pediatric iGAS was the highest since 1997) while those older than 65 were overrepresented in second year (2023).1
Emergence of M1UK
One potential factor for the prepandemic rise in iGAS infections worldwide is the emergence and worldwide spread of a new GAS emm type variant designated M1UK.2 GAS isolates can be typed into categories designated as emm types based on DNA sequence. There are more than 240 emm types, with 6 being most common — M1, M3, and M28 (each up to 20% of GAS isolates) and M12, M82, and M89 (each up to 10%). M1, M3 and M28 have also been particularly associated with invasive disease. While emm types vary year to year and region by region, the overall emm type distribution among iGAS isolates in the United States had not been unusual since the turn of the century and the US M1 strain was the same as that which had been predominant worldwide (designated M1GLOBAL). This new M1UK sublineage had emerged around 2010 and had been increasing pre pandemic. The M1UK sequence contained a specific set of 27 SNPs (single nucleoside polymorphisms, i.e., single base mutations) and was associated with an uptick in scarlet fever in the United Kingdom starting around 2010. Its prevalence increased up to around 2015 while spreading internationally. It also had enhanced expression of SpeA, a phage-encoded superantigen. Some of the M1UK mutations also appear to alter GAS metabolic processes to allow better survival (better “fitness”) compared with other GAS. So, a more virulent hardier GAS had arisen and seems a reasonable candidate for contributing to the increased iGAS rates.
Waning Antibody to GAS As Potential Factor in Rebound
No consensus exists on correlates of protection from iGAS. However, adults seem to have less noninvasive GAS than children. One potential reason is that frequent GAS re-exposure, regardless of whether disease results, likely boosts anti-GAS antibodies. Pandemic social restrictions temporarily prevented such boosts. In children with developing antibody repertoires, anti-GAS antibodies may have waned below protective levels faster during a year without frequent boosting. Thus, children were iGAS susceptible soon after pandemic restrictions were dropped (2022). Increased iGAS rates in the elderly in 2023 may have occurred because of diminished GAS exposures accelerating immune senescence with anti-GAS antibodies dropping, but less quickly than in children. These speculations are simply hypotheses until future studies can test them.
All that said, how do we use information on increased iGAS in our daily practices? In addition to standard preventive strategies for viral coinfections (e.g., varicella and influenza vaccine), reminding families about rigorous attention to wound care is the one high-risk scenario we have not yet discussed. During 2024, a time of expected increased prevalence of iGAS, early wound care needs to be fastidious. Further, share warning signs with families (e.g., rapidly expanding painful erythema), “streaks” ascending from extremity wounds, fever and a highly painful wound, darkening almost purple color within cellulitis or soft tissue infection, or loss of sensation in the middle of an otherwise painful soft tissue infection. These presentations require immediate medical attention.
If such a patient presents, the Centers for Disease Control and Prevention (CDC) recommends admission along with blood and, where possible, wound cultures. If in the context of pneumonia with pleural effusion, culturing pleural fluid is also important. Remember, leading edge cultures are not often positive for GAS, seemingly because GAS exotoxins are found at erythema’s leading edge, not the bacteria. The bacteria are somewhere more central in the inflammatory process. Despite not being prominent among recent iGAS cases, another scenario that could sneak up on you is the infected surgical wound as nascent iGAS.
Finally, remember that nationally increasing numbers of iGAS isolates are resistant to erythromycin and clindamycin, the latter usually recommended to reduce tissue damage in iGAS.3 So, it is important to be aware of susceptibility patterns in your locale and consider an ID consultation. My hope is that you do not see an iGAS case this year, but we all need to remain alert. With a high index of suspicion and rapid diagnosis, you can minimize long-term sequelae and potential fatalities.
While it is too early to tell how the rest of 2024 will turn out, preliminary indications are that GAS is circulating at higher than usual levels (30%-35% GAS positive throat swabs in early April 2024 in Kansas City area) and iGAS rates will likely also be relatively high, particularly if Ontario, Canada, data are any indication.4 
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Current Group A Strep Activity, Centers for Disease Control and Prevention. April 2024. CDC webpage on current invasive GAS disease. April 2024.
2. Li Y et al. Expansion of Invasive Group A Streptococcus M1UK Lineage in Active Bacterial Core Surveillance, United States, 2019-2021 Emerg Infect Dis. 2023;29(10):2116-2120. doi: 10.3201/eid2910.230675.
3. Andreoni F et al. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J Infect Dis. 2017 Jan 15;215(2):269-277. doi: 10.1093/infdis/jiw229.
4. Group A Streptococcal Disease, Invasive (iGAS), Public Health Ontario.
Invasive group A streptococcus (iGAS) infections are rare (4-9 cases/100,000 US population annually) but potentially devastating (approximately 2,300 deaths annually in US), and affect all ages. Cases increase in winter-spring, paralleling the “season” of increased noninvasive GAS, e.g., pharyngitis and scarlet fever. iGAS case rates are lower in children than adults. That said, one well-known pediatric iGAS scenario has been deep cellulitis and necrotizing fasciitis during the healing phase of varicella. Other forms of iGAS include bacteremia, pneumonia (particularly when empyema is present), lymphangitis, erysipelas, and toxic shock syndrome. iGAS can occur with/after influenza but has also occurred concurrently with other viral respiratory infections.
Persons with underlying conditions (cancer or immune compromised status; chronic diseases of the heart, kidney or lung; diabetes mellitus) are at higher risk. Other subpopulations at risk for iGAS are illicit drug users, the elderly, homeless persons, nursing home residents, American Indian persons, and Alaska Native persons. Most experts feel that highly toxigenic strains of GAS are responsible for most iGAS. Indeed, most iGAS isolates produce (sometimes hyper-produce) superantigens that cause exaggerated innate immune responses, higher levels of inflammation, and often times tissue destruction, e.g., “flesh eating bacteria.” And who can forget that Jim Henson, creator of the Muppets, died of iGAS?
But why discuss iGAS in 2024? The pattern for iGAS has fluctuated more than usual in the last decade. So much so that the recent upsurge has caught the collective eye of the lay press. So, patients and friends may have questions about why and how iGAS is increasing lately. The bottom line is that no one knows for sure. However, the most recent 2 years of uptick may reflect GAS circulating at relatively high levels even when taking into account that GAS season occurs in winter-spring most years. Yet it seems likely that additional factors may have played a role in the fluctuations noted this past decade, e.g., temporary changes in societal behavior, a new GAS strain with over two dozen mutations, and possibly rapid waning of protection against GAS exotoxins.
Social Behavior Factor
The SARS-CoV-2 pandemic brought extremes of disease and death to the world and dramatic changes in social behavior. A byproduct was dramatic decreases in nearly all infectious diseases, with numerous reports of near absence of many respiratory and gastrointestinal viruses in the 2020-2021 seasons. Interestingly, we did not see a drop in human rhinovirus infections, justifying its nickname as the cockroach of viruses. Reports also emerged about drops in bacterial diseases during 2020-2021 (although not so much for STIs), including noninvasive and invasive GAS disease, and also GAS-associated deaths (lowest since 2016).1 The drop in iGAS during social restrictions makes sense because GAS is spread by direct contact with infected persons or their secretions, and social contact had dramatically decreased particularly in the first 6 months of the pandemic.
However, since 2022 and the return to “normal” social behaviors, both viral diseases (e.g., RSV, influenza, and Norovirus), and some bacterial diseases have rebounded. That said, something else must be contributing, because iGAS rates had increased 4-5 years pre pandemic. In fact, the fluctuating pattern included “normal” annual rates in the early 2000s rising in ~2015 followed by the explainable pandemic drop (by nearly 25%), and not-too-unexpected 2-year postpandemic rise. But interestingly enough, the rebound is higher than might be expected for iGAS and children were overrepresented in first year’s rise (2022 rate for pediatric iGAS was the highest since 1997) while those older than 65 were overrepresented in second year (2023).1
Emergence of M1UK
One potential factor for the prepandemic rise in iGAS infections worldwide is the emergence and worldwide spread of a new GAS emm type variant designated M1UK.2 GAS isolates can be typed into categories designated as emm types based on DNA sequence. There are more than 240 emm types, with 6 being most common — M1, M3, and M28 (each up to 20% of GAS isolates) and M12, M82, and M89 (each up to 10%). M1, M3 and M28 have also been particularly associated with invasive disease. While emm types vary year to year and region by region, the overall emm type distribution among iGAS isolates in the United States had not been unusual since the turn of the century and the US M1 strain was the same as that which had been predominant worldwide (designated M1GLOBAL). This new M1UK sublineage had emerged around 2010 and had been increasing pre pandemic. The M1UK sequence contained a specific set of 27 SNPs (single nucleoside polymorphisms, i.e., single base mutations) and was associated with an uptick in scarlet fever in the United Kingdom starting around 2010. Its prevalence increased up to around 2015 while spreading internationally. It also had enhanced expression of SpeA, a phage-encoded superantigen. Some of the M1UK mutations also appear to alter GAS metabolic processes to allow better survival (better “fitness”) compared with other GAS. So, a more virulent hardier GAS had arisen and seems a reasonable candidate for contributing to the increased iGAS rates.
Waning Antibody to GAS As Potential Factor in Rebound
No consensus exists on correlates of protection from iGAS. However, adults seem to have less noninvasive GAS than children. One potential reason is that frequent GAS re-exposure, regardless of whether disease results, likely boosts anti-GAS antibodies. Pandemic social restrictions temporarily prevented such boosts. In children with developing antibody repertoires, anti-GAS antibodies may have waned below protective levels faster during a year without frequent boosting. Thus, children were iGAS susceptible soon after pandemic restrictions were dropped (2022). Increased iGAS rates in the elderly in 2023 may have occurred because of diminished GAS exposures accelerating immune senescence with anti-GAS antibodies dropping, but less quickly than in children. These speculations are simply hypotheses until future studies can test them.
All that said, how do we use information on increased iGAS in our daily practices? In addition to standard preventive strategies for viral coinfections (e.g., varicella and influenza vaccine), reminding families about rigorous attention to wound care is the one high-risk scenario we have not yet discussed. During 2024, a time of expected increased prevalence of iGAS, early wound care needs to be fastidious. Further, share warning signs with families (e.g., rapidly expanding painful erythema), “streaks” ascending from extremity wounds, fever and a highly painful wound, darkening almost purple color within cellulitis or soft tissue infection, or loss of sensation in the middle of an otherwise painful soft tissue infection. These presentations require immediate medical attention.
If such a patient presents, the Centers for Disease Control and Prevention (CDC) recommends admission along with blood and, where possible, wound cultures. If in the context of pneumonia with pleural effusion, culturing pleural fluid is also important. Remember, leading edge cultures are not often positive for GAS, seemingly because GAS exotoxins are found at erythema’s leading edge, not the bacteria. The bacteria are somewhere more central in the inflammatory process. Despite not being prominent among recent iGAS cases, another scenario that could sneak up on you is the infected surgical wound as nascent iGAS.
Finally, remember that nationally increasing numbers of iGAS isolates are resistant to erythromycin and clindamycin, the latter usually recommended to reduce tissue damage in iGAS.3 So, it is important to be aware of susceptibility patterns in your locale and consider an ID consultation. My hope is that you do not see an iGAS case this year, but we all need to remain alert. With a high index of suspicion and rapid diagnosis, you can minimize long-term sequelae and potential fatalities.
While it is too early to tell how the rest of 2024 will turn out, preliminary indications are that GAS is circulating at higher than usual levels (30%-35% GAS positive throat swabs in early April 2024 in Kansas City area) and iGAS rates will likely also be relatively high, particularly if Ontario, Canada, data are any indication.4 
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Current Group A Strep Activity, Centers for Disease Control and Prevention. April 2024. CDC webpage on current invasive GAS disease. April 2024.
2. Li Y et al. Expansion of Invasive Group A Streptococcus M1UK Lineage in Active Bacterial Core Surveillance, United States, 2019-2021 Emerg Infect Dis. 2023;29(10):2116-2120. doi: 10.3201/eid2910.230675.
3. Andreoni F et al. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J Infect Dis. 2017 Jan 15;215(2):269-277. doi: 10.1093/infdis/jiw229.
4. Group A Streptococcal Disease, Invasive (iGAS), Public Health Ontario.