User login
COVID-19 vaccinations lag in youngest children
Case: A 3-year-old girl presented to the emergency department after a brief seizure at home. She looked well on physical exam except for a fever of 103° F and thick rhinorrhea.
The intern on duty methodically worked through the standard list of questions. “Immunizations up to date?” she asked.
“Absolutely,” the child’s mom responded. “She’s had everything that’s recommended.”
“Including COVID-19 vaccine?” the intern prompted.
“No.” The mom responded with a shake of her head. “We don’t do that vaccine.”
That mom is not alone.
COVID-19 vaccines for children as young as 6 months were given emergency-use authorization by the Food and Drug Administration in June 2022 and in February 2023, the Advisory Committee on Immunization Practices included COVID-19 vaccine on the routine childhood immunization schedule.
COVID-19 vaccines are safe in young children, and they prevent the most severe outcomes associated with infection, including hospitalization. Newly released data confirm that the COVID-19 vaccines produced by Moderna and Pfizer also provide protection against symptomatic infection for at least 4 months after completion of the monovalent primary series.
In a Morbidity and Mortality Weekly Report released on Feb. 17, 2023, the Centers for Disease Control and Prevention reported the results of a test-negative design case-control study that enrolled symptomatic children tested for SARS-CoV-2 infection through Feb. 5, 2023, as part of the Increasing Community Access to Testing (ICATT) program.1 ICATT provides SARS-CoV-2 testing to persons aged at least 3 years at pharmacy and community-based testing sites nationwide.
Two doses of monovalent Moderna vaccine (complete primary series) was 60% effective against symptomatic infection (95% confidence interval, 49%-68%) 2 weeks to 2 months after receipt of the second dose. Vaccine effectiveness dropped to 36% (95% CI, 15%-52%) 3-4 months after the second dose. Three doses of monovalent Pfizer-BioNTech vaccine (complete primary series) was 31% effective (95% CI, 7%-49%) at preventing symptomatic infection 2 weeks to 4 months after receipt of the third dose. A bivalent vaccine dose for eligible children is expected to provide more protection against currently circulating SARS-CoV-2 variants.
Despite evidence of vaccine efficacy, very few parents are opting to protect their young children with the COVID-19 vaccine. The CDC reports that, as of March 1, 2023, only 8% of children under 2 years and 10.5% of children aged 2-4 years have initiated a COVID vaccine series. The American Academy of Pediatrics has emphasized that 15.0 million children between the ages of 6 months and 4 years have not yet received their first COVID-19 vaccine dose.
While the reasons underlying low COVID-19 vaccination rates in young children are complex, themes emerge. Socioeconomic disparities contributing to low vaccination rates in young children were highlighted in another recent MMWR article.2 Through Dec. 1, 2022, vaccination coverage was lower in rural counties (3.4%) than in urban counties (10.5%). Rates were lower in Black and Hispanic children than in White and Asian children.
According to the CDC, high rates of poverty in Black and Hispanic communities may affect vaccination coverage by affecting caregivers’ access to vaccination sites or ability to leave work to take their child to be vaccinated. Pediatric care providers have repeatedly been identified by parents as a source of trusted vaccine information and a strong provider recommendation is associated with vaccination, but not all families are receiving vaccine advice. In a 2022 Kaiser Family Foundation survey, parents of young children with annual household incomes above $90,000 were more likely to talk to their pediatrician about a COVID-19 vaccine than families with lower incomes.3Vaccine hesitancy, fueled by general confusion and skepticism, is another factor contributing to low vaccination rates. Admittedly, the recommendations are complex and on March 14, 2023, the FDA again revised the emergency-use authorization for young children. Some caregivers continue to express concerns about vaccine side effects as well as the belief that the vaccine won’t prevent their child from getting sick.
Kendall Purcell, MD, a pediatrician with Norton Children’s Medical Group in Louisville, Ky., recommends COVID-19 vaccination for her patients because it reduces the risk of severe disease. That factored into her own decision to vaccinate her 4-year-old son and 1-year-old daughter, but she hasn’t been able to convince the parents of all her patients. “Some feel that COVID-19 is not as severe for children, so the risks don’t outweigh the benefits when it comes to vaccinating their children.” Back to our case: In the ED the intern reviewed the laboratory testing she had ordered. She then sat down with the mother of the 3-year-old girl to discuss the diagnosis: febrile seizure associated with COVID-19 infection. Febrile seizures are a well-recognized but uncommon complication of COVID-19 in children. In a retrospective cohort study using electronic health record data, febrile seizures occurred in 0.5% of 8,854 children aged 0-5 years with COVID-19 infection.4 About 9% of these children required critical care services. In another cohort of hospitalized children, neurologic complications occurred in 7% of children hospitalized with COVID-19.5 Febrile and nonfebrile seizures were most commonly observed.
“I really thought COVID-19 was no big deal in young kids,” the mom said. “Parents need the facts.”
The facts are these: Through Dec. 2, 2022, more than 3 million cases of COVID-19 have been reported in children aged younger than 5 years. While COVID is generally less severe in young children than older adults, it is difficult to predict which children will become seriously ill. When children are hospitalized, one in four requires intensive care. COVID-19 is now a vaccine-preventable disease, but too many children remain unprotected.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at pdnews@mdedge.com. Ms. Ezell is a recent graduate from Indiana University Southeast with a Bachelor of Arts in English. They have no conflicts of interest.
References
1. Fleming-Dutra KE et al. Morb Mortal Wkly Rep. 2023;72:177-182.
2. Murthy BP et al. Morb Mortal Wkly Rep. 2023;72:183-9.
3. Lopes L et al. KFF COVID-19 vaccine monitor: July 2022. San Francisco: Kaiser Family Foundation, 2022.
4. Cadet K et al. J Child Neurol. 2022 Apr;37(5):410-5.
5. Antoon JW et al. Pediatrics. 2022 Nov 1;150(5):e2022058167.
Case: A 3-year-old girl presented to the emergency department after a brief seizure at home. She looked well on physical exam except for a fever of 103° F and thick rhinorrhea.
The intern on duty methodically worked through the standard list of questions. “Immunizations up to date?” she asked.
“Absolutely,” the child’s mom responded. “She’s had everything that’s recommended.”
“Including COVID-19 vaccine?” the intern prompted.
“No.” The mom responded with a shake of her head. “We don’t do that vaccine.”
That mom is not alone.
COVID-19 vaccines for children as young as 6 months were given emergency-use authorization by the Food and Drug Administration in June 2022 and in February 2023, the Advisory Committee on Immunization Practices included COVID-19 vaccine on the routine childhood immunization schedule.
COVID-19 vaccines are safe in young children, and they prevent the most severe outcomes associated with infection, including hospitalization. Newly released data confirm that the COVID-19 vaccines produced by Moderna and Pfizer also provide protection against symptomatic infection for at least 4 months after completion of the monovalent primary series.
In a Morbidity and Mortality Weekly Report released on Feb. 17, 2023, the Centers for Disease Control and Prevention reported the results of a test-negative design case-control study that enrolled symptomatic children tested for SARS-CoV-2 infection through Feb. 5, 2023, as part of the Increasing Community Access to Testing (ICATT) program.1 ICATT provides SARS-CoV-2 testing to persons aged at least 3 years at pharmacy and community-based testing sites nationwide.
Two doses of monovalent Moderna vaccine (complete primary series) was 60% effective against symptomatic infection (95% confidence interval, 49%-68%) 2 weeks to 2 months after receipt of the second dose. Vaccine effectiveness dropped to 36% (95% CI, 15%-52%) 3-4 months after the second dose. Three doses of monovalent Pfizer-BioNTech vaccine (complete primary series) was 31% effective (95% CI, 7%-49%) at preventing symptomatic infection 2 weeks to 4 months after receipt of the third dose. A bivalent vaccine dose for eligible children is expected to provide more protection against currently circulating SARS-CoV-2 variants.
Despite evidence of vaccine efficacy, very few parents are opting to protect their young children with the COVID-19 vaccine. The CDC reports that, as of March 1, 2023, only 8% of children under 2 years and 10.5% of children aged 2-4 years have initiated a COVID vaccine series. The American Academy of Pediatrics has emphasized that 15.0 million children between the ages of 6 months and 4 years have not yet received their first COVID-19 vaccine dose.
While the reasons underlying low COVID-19 vaccination rates in young children are complex, themes emerge. Socioeconomic disparities contributing to low vaccination rates in young children were highlighted in another recent MMWR article.2 Through Dec. 1, 2022, vaccination coverage was lower in rural counties (3.4%) than in urban counties (10.5%). Rates were lower in Black and Hispanic children than in White and Asian children.
According to the CDC, high rates of poverty in Black and Hispanic communities may affect vaccination coverage by affecting caregivers’ access to vaccination sites or ability to leave work to take their child to be vaccinated. Pediatric care providers have repeatedly been identified by parents as a source of trusted vaccine information and a strong provider recommendation is associated with vaccination, but not all families are receiving vaccine advice. In a 2022 Kaiser Family Foundation survey, parents of young children with annual household incomes above $90,000 were more likely to talk to their pediatrician about a COVID-19 vaccine than families with lower incomes.3Vaccine hesitancy, fueled by general confusion and skepticism, is another factor contributing to low vaccination rates. Admittedly, the recommendations are complex and on March 14, 2023, the FDA again revised the emergency-use authorization for young children. Some caregivers continue to express concerns about vaccine side effects as well as the belief that the vaccine won’t prevent their child from getting sick.
Kendall Purcell, MD, a pediatrician with Norton Children’s Medical Group in Louisville, Ky., recommends COVID-19 vaccination for her patients because it reduces the risk of severe disease. That factored into her own decision to vaccinate her 4-year-old son and 1-year-old daughter, but she hasn’t been able to convince the parents of all her patients. “Some feel that COVID-19 is not as severe for children, so the risks don’t outweigh the benefits when it comes to vaccinating their children.” Back to our case: In the ED the intern reviewed the laboratory testing she had ordered. She then sat down with the mother of the 3-year-old girl to discuss the diagnosis: febrile seizure associated with COVID-19 infection. Febrile seizures are a well-recognized but uncommon complication of COVID-19 in children. In a retrospective cohort study using electronic health record data, febrile seizures occurred in 0.5% of 8,854 children aged 0-5 years with COVID-19 infection.4 About 9% of these children required critical care services. In another cohort of hospitalized children, neurologic complications occurred in 7% of children hospitalized with COVID-19.5 Febrile and nonfebrile seizures were most commonly observed.
“I really thought COVID-19 was no big deal in young kids,” the mom said. “Parents need the facts.”
The facts are these: Through Dec. 2, 2022, more than 3 million cases of COVID-19 have been reported in children aged younger than 5 years. While COVID is generally less severe in young children than older adults, it is difficult to predict which children will become seriously ill. When children are hospitalized, one in four requires intensive care. COVID-19 is now a vaccine-preventable disease, but too many children remain unprotected.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at pdnews@mdedge.com. Ms. Ezell is a recent graduate from Indiana University Southeast with a Bachelor of Arts in English. They have no conflicts of interest.
References
1. Fleming-Dutra KE et al. Morb Mortal Wkly Rep. 2023;72:177-182.
2. Murthy BP et al. Morb Mortal Wkly Rep. 2023;72:183-9.
3. Lopes L et al. KFF COVID-19 vaccine monitor: July 2022. San Francisco: Kaiser Family Foundation, 2022.
4. Cadet K et al. J Child Neurol. 2022 Apr;37(5):410-5.
5. Antoon JW et al. Pediatrics. 2022 Nov 1;150(5):e2022058167.
Case: A 3-year-old girl presented to the emergency department after a brief seizure at home. She looked well on physical exam except for a fever of 103° F and thick rhinorrhea.
The intern on duty methodically worked through the standard list of questions. “Immunizations up to date?” she asked.
“Absolutely,” the child’s mom responded. “She’s had everything that’s recommended.”
“Including COVID-19 vaccine?” the intern prompted.
“No.” The mom responded with a shake of her head. “We don’t do that vaccine.”
That mom is not alone.
COVID-19 vaccines for children as young as 6 months were given emergency-use authorization by the Food and Drug Administration in June 2022 and in February 2023, the Advisory Committee on Immunization Practices included COVID-19 vaccine on the routine childhood immunization schedule.
COVID-19 vaccines are safe in young children, and they prevent the most severe outcomes associated with infection, including hospitalization. Newly released data confirm that the COVID-19 vaccines produced by Moderna and Pfizer also provide protection against symptomatic infection for at least 4 months after completion of the monovalent primary series.
In a Morbidity and Mortality Weekly Report released on Feb. 17, 2023, the Centers for Disease Control and Prevention reported the results of a test-negative design case-control study that enrolled symptomatic children tested for SARS-CoV-2 infection through Feb. 5, 2023, as part of the Increasing Community Access to Testing (ICATT) program.1 ICATT provides SARS-CoV-2 testing to persons aged at least 3 years at pharmacy and community-based testing sites nationwide.
Two doses of monovalent Moderna vaccine (complete primary series) was 60% effective against symptomatic infection (95% confidence interval, 49%-68%) 2 weeks to 2 months after receipt of the second dose. Vaccine effectiveness dropped to 36% (95% CI, 15%-52%) 3-4 months after the second dose. Three doses of monovalent Pfizer-BioNTech vaccine (complete primary series) was 31% effective (95% CI, 7%-49%) at preventing symptomatic infection 2 weeks to 4 months after receipt of the third dose. A bivalent vaccine dose for eligible children is expected to provide more protection against currently circulating SARS-CoV-2 variants.
Despite evidence of vaccine efficacy, very few parents are opting to protect their young children with the COVID-19 vaccine. The CDC reports that, as of March 1, 2023, only 8% of children under 2 years and 10.5% of children aged 2-4 years have initiated a COVID vaccine series. The American Academy of Pediatrics has emphasized that 15.0 million children between the ages of 6 months and 4 years have not yet received their first COVID-19 vaccine dose.
While the reasons underlying low COVID-19 vaccination rates in young children are complex, themes emerge. Socioeconomic disparities contributing to low vaccination rates in young children were highlighted in another recent MMWR article.2 Through Dec. 1, 2022, vaccination coverage was lower in rural counties (3.4%) than in urban counties (10.5%). Rates were lower in Black and Hispanic children than in White and Asian children.
According to the CDC, high rates of poverty in Black and Hispanic communities may affect vaccination coverage by affecting caregivers’ access to vaccination sites or ability to leave work to take their child to be vaccinated. Pediatric care providers have repeatedly been identified by parents as a source of trusted vaccine information and a strong provider recommendation is associated with vaccination, but not all families are receiving vaccine advice. In a 2022 Kaiser Family Foundation survey, parents of young children with annual household incomes above $90,000 were more likely to talk to their pediatrician about a COVID-19 vaccine than families with lower incomes.3Vaccine hesitancy, fueled by general confusion and skepticism, is another factor contributing to low vaccination rates. Admittedly, the recommendations are complex and on March 14, 2023, the FDA again revised the emergency-use authorization for young children. Some caregivers continue to express concerns about vaccine side effects as well as the belief that the vaccine won’t prevent their child from getting sick.
Kendall Purcell, MD, a pediatrician with Norton Children’s Medical Group in Louisville, Ky., recommends COVID-19 vaccination for her patients because it reduces the risk of severe disease. That factored into her own decision to vaccinate her 4-year-old son and 1-year-old daughter, but she hasn’t been able to convince the parents of all her patients. “Some feel that COVID-19 is not as severe for children, so the risks don’t outweigh the benefits when it comes to vaccinating their children.” Back to our case: In the ED the intern reviewed the laboratory testing she had ordered. She then sat down with the mother of the 3-year-old girl to discuss the diagnosis: febrile seizure associated with COVID-19 infection. Febrile seizures are a well-recognized but uncommon complication of COVID-19 in children. In a retrospective cohort study using electronic health record data, febrile seizures occurred in 0.5% of 8,854 children aged 0-5 years with COVID-19 infection.4 About 9% of these children required critical care services. In another cohort of hospitalized children, neurologic complications occurred in 7% of children hospitalized with COVID-19.5 Febrile and nonfebrile seizures were most commonly observed.
“I really thought COVID-19 was no big deal in young kids,” the mom said. “Parents need the facts.”
The facts are these: Through Dec. 2, 2022, more than 3 million cases of COVID-19 have been reported in children aged younger than 5 years. While COVID is generally less severe in young children than older adults, it is difficult to predict which children will become seriously ill. When children are hospitalized, one in four requires intensive care. COVID-19 is now a vaccine-preventable disease, but too many children remain unprotected.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at pdnews@mdedge.com. Ms. Ezell is a recent graduate from Indiana University Southeast with a Bachelor of Arts in English. They have no conflicts of interest.
References
1. Fleming-Dutra KE et al. Morb Mortal Wkly Rep. 2023;72:177-182.
2. Murthy BP et al. Morb Mortal Wkly Rep. 2023;72:183-9.
3. Lopes L et al. KFF COVID-19 vaccine monitor: July 2022. San Francisco: Kaiser Family Foundation, 2022.
4. Cadet K et al. J Child Neurol. 2022 Apr;37(5):410-5.
5. Antoon JW et al. Pediatrics. 2022 Nov 1;150(5):e2022058167.
Young children quickly outgrow the need for ear tubes
About half a million children between the ages of 1 and 3 years old have ear tube surgery in the United States every year at an annual cost exceeding $2 billion. It is the most common childhood surgery performed with anesthesia. It is a surgery commonly performed on children in most other high- and middle-income countries.
My group recently published a paper on the timing and necessity of tympanostomy tubes for recurrent otitis media in young children. The primary objective was to quantitatively examine recurrent acute otitis media (AOM) incidence with respect to age of occurrence, the influence of daycare attendance, and other risk factors in individual children. We introduced the concept of a “window of susceptibility” to AOM as new terminology referring to a child who has two or more closely spaced AOM occurrences during a window of time. We sought to know what to expect and how to advise the parent when a child presents with closely spaced AOMs.
A secondary objective was to develop models to predict the risk and timing of AOM recurrences based on the natural history of disease in young children who do not get tympanostomy tubes. Prediction models were developed to assist clinicians in understanding and explaining to parents the benefit of tympanostomy tubes based on the child’s age and number of AOMs.
The children were all from a primary care pediatric practice in Rochester, N.Y., which comprised a typical mixed demographic of largely middle-class, health care–insured families that was broadly representative of the racial/ethnic diversity in the community. The sample included both wealthy families and those living below the poverty line. The diagnosis of AOM was made based on the American Academy of Pediatrics guidance in which a presumed middle ear effusion and a full or bulging tympanic membrane were required. Almost all episodes (> 85%) of clinically diagnosed AOM cases were confirmed by culture of middle ear fluid collected by tympanocentesis to ensure diagnostic accuracy.
286 children who had ear infections were studied. We found that 80% of ear infections occurred during a very narrow window of susceptibility – age 6-21 months. About 72% of children had a window of susceptibility to ear infections that lasted 5 months or less; 97% of children had a window of susceptibility that lasted 10 months or less.
From this result, we observed that about 90% of children have a window of time lasting about 10 months when they get repeated ear infections. By the time a child gets three ear infections in 6 months (a period of time recommended by the AAP and American Academy of Otolaryngology–Head and Neck Surgery when ear tubes might be considered) and then a referral for ear tubes is made and the child gets an appointment with the ear, nose, and throat doctor, and surgery is scheduled, the ear infections were going to stop anyway.
In other words, millions of children worldwide have been getting ear tubes and physicians and parents saw that the ear infections stopped. So they concluded the ear tubes stopped the infections. We found the infections were going to stop anyway even if the child did not receive ear tubes because their susceptibility to ear infections is over by the time the surgery is performed. The child outgrew ear infections.
An exception was children in daycare at an early age. Our study found that children in daycare who are around 6 months old and start getting ear infections at that age are likely destined to have three or more ear infections in the first year of life. If children are going to be in daycare, perhaps those who need them should receive ear tubes early. Analysis of other demographic and risk factor covariates – sex, race/ethnicity, breastfeeding, siblings in the home, smoking in the home, atopy, and family history of otitis media – were not significantly associated with the number of AOMs in the child population we studied.
We developed a prediction model for doctors, so they could input a child’s age, number of ear infections, and daycare attendance and receive back an estimate of the number of likely future ear infections for that child. With that knowledge, physicians and parents can make more informed decisions.
Our message to clinicians and parents is to reconsider the necessity and timing of ear tube surgery for children with recurrent ear infections because the future is not predicted by the past. Children having several ear infections in a short time does not predict that they will have a similar number of ear infections in the future.
The study was supported by the National Institutes of Health awarded to Rochester Regional Health. Dr. Pichichero was principal investigator for the award.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
About half a million children between the ages of 1 and 3 years old have ear tube surgery in the United States every year at an annual cost exceeding $2 billion. It is the most common childhood surgery performed with anesthesia. It is a surgery commonly performed on children in most other high- and middle-income countries.
My group recently published a paper on the timing and necessity of tympanostomy tubes for recurrent otitis media in young children. The primary objective was to quantitatively examine recurrent acute otitis media (AOM) incidence with respect to age of occurrence, the influence of daycare attendance, and other risk factors in individual children. We introduced the concept of a “window of susceptibility” to AOM as new terminology referring to a child who has two or more closely spaced AOM occurrences during a window of time. We sought to know what to expect and how to advise the parent when a child presents with closely spaced AOMs.
A secondary objective was to develop models to predict the risk and timing of AOM recurrences based on the natural history of disease in young children who do not get tympanostomy tubes. Prediction models were developed to assist clinicians in understanding and explaining to parents the benefit of tympanostomy tubes based on the child’s age and number of AOMs.
The children were all from a primary care pediatric practice in Rochester, N.Y., which comprised a typical mixed demographic of largely middle-class, health care–insured families that was broadly representative of the racial/ethnic diversity in the community. The sample included both wealthy families and those living below the poverty line. The diagnosis of AOM was made based on the American Academy of Pediatrics guidance in which a presumed middle ear effusion and a full or bulging tympanic membrane were required. Almost all episodes (> 85%) of clinically diagnosed AOM cases were confirmed by culture of middle ear fluid collected by tympanocentesis to ensure diagnostic accuracy.
286 children who had ear infections were studied. We found that 80% of ear infections occurred during a very narrow window of susceptibility – age 6-21 months. About 72% of children had a window of susceptibility to ear infections that lasted 5 months or less; 97% of children had a window of susceptibility that lasted 10 months or less.
From this result, we observed that about 90% of children have a window of time lasting about 10 months when they get repeated ear infections. By the time a child gets three ear infections in 6 months (a period of time recommended by the AAP and American Academy of Otolaryngology–Head and Neck Surgery when ear tubes might be considered) and then a referral for ear tubes is made and the child gets an appointment with the ear, nose, and throat doctor, and surgery is scheduled, the ear infections were going to stop anyway.
In other words, millions of children worldwide have been getting ear tubes and physicians and parents saw that the ear infections stopped. So they concluded the ear tubes stopped the infections. We found the infections were going to stop anyway even if the child did not receive ear tubes because their susceptibility to ear infections is over by the time the surgery is performed. The child outgrew ear infections.
An exception was children in daycare at an early age. Our study found that children in daycare who are around 6 months old and start getting ear infections at that age are likely destined to have three or more ear infections in the first year of life. If children are going to be in daycare, perhaps those who need them should receive ear tubes early. Analysis of other demographic and risk factor covariates – sex, race/ethnicity, breastfeeding, siblings in the home, smoking in the home, atopy, and family history of otitis media – were not significantly associated with the number of AOMs in the child population we studied.
We developed a prediction model for doctors, so they could input a child’s age, number of ear infections, and daycare attendance and receive back an estimate of the number of likely future ear infections for that child. With that knowledge, physicians and parents can make more informed decisions.
Our message to clinicians and parents is to reconsider the necessity and timing of ear tube surgery for children with recurrent ear infections because the future is not predicted by the past. Children having several ear infections in a short time does not predict that they will have a similar number of ear infections in the future.
The study was supported by the National Institutes of Health awarded to Rochester Regional Health. Dr. Pichichero was principal investigator for the award.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
About half a million children between the ages of 1 and 3 years old have ear tube surgery in the United States every year at an annual cost exceeding $2 billion. It is the most common childhood surgery performed with anesthesia. It is a surgery commonly performed on children in most other high- and middle-income countries.
My group recently published a paper on the timing and necessity of tympanostomy tubes for recurrent otitis media in young children. The primary objective was to quantitatively examine recurrent acute otitis media (AOM) incidence with respect to age of occurrence, the influence of daycare attendance, and other risk factors in individual children. We introduced the concept of a “window of susceptibility” to AOM as new terminology referring to a child who has two or more closely spaced AOM occurrences during a window of time. We sought to know what to expect and how to advise the parent when a child presents with closely spaced AOMs.
A secondary objective was to develop models to predict the risk and timing of AOM recurrences based on the natural history of disease in young children who do not get tympanostomy tubes. Prediction models were developed to assist clinicians in understanding and explaining to parents the benefit of tympanostomy tubes based on the child’s age and number of AOMs.
The children were all from a primary care pediatric practice in Rochester, N.Y., which comprised a typical mixed demographic of largely middle-class, health care–insured families that was broadly representative of the racial/ethnic diversity in the community. The sample included both wealthy families and those living below the poverty line. The diagnosis of AOM was made based on the American Academy of Pediatrics guidance in which a presumed middle ear effusion and a full or bulging tympanic membrane were required. Almost all episodes (> 85%) of clinically diagnosed AOM cases were confirmed by culture of middle ear fluid collected by tympanocentesis to ensure diagnostic accuracy.
286 children who had ear infections were studied. We found that 80% of ear infections occurred during a very narrow window of susceptibility – age 6-21 months. About 72% of children had a window of susceptibility to ear infections that lasted 5 months or less; 97% of children had a window of susceptibility that lasted 10 months or less.
From this result, we observed that about 90% of children have a window of time lasting about 10 months when they get repeated ear infections. By the time a child gets three ear infections in 6 months (a period of time recommended by the AAP and American Academy of Otolaryngology–Head and Neck Surgery when ear tubes might be considered) and then a referral for ear tubes is made and the child gets an appointment with the ear, nose, and throat doctor, and surgery is scheduled, the ear infections were going to stop anyway.
In other words, millions of children worldwide have been getting ear tubes and physicians and parents saw that the ear infections stopped. So they concluded the ear tubes stopped the infections. We found the infections were going to stop anyway even if the child did not receive ear tubes because their susceptibility to ear infections is over by the time the surgery is performed. The child outgrew ear infections.
An exception was children in daycare at an early age. Our study found that children in daycare who are around 6 months old and start getting ear infections at that age are likely destined to have three or more ear infections in the first year of life. If children are going to be in daycare, perhaps those who need them should receive ear tubes early. Analysis of other demographic and risk factor covariates – sex, race/ethnicity, breastfeeding, siblings in the home, smoking in the home, atopy, and family history of otitis media – were not significantly associated with the number of AOMs in the child population we studied.
We developed a prediction model for doctors, so they could input a child’s age, number of ear infections, and daycare attendance and receive back an estimate of the number of likely future ear infections for that child. With that knowledge, physicians and parents can make more informed decisions.
Our message to clinicians and parents is to reconsider the necessity and timing of ear tube surgery for children with recurrent ear infections because the future is not predicted by the past. Children having several ear infections in a short time does not predict that they will have a similar number of ear infections in the future.
The study was supported by the National Institutes of Health awarded to Rochester Regional Health. Dr. Pichichero was principal investigator for the award.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
Measles
I received a call late one night from a colleague in the emergency department of the children’s hospital. “This 2-year-old has a fever, cough, red eyes, and an impressive rash. I’ve personally never seen a case of measles, but I’m worried given that this child has never received the MMR vaccine.”
By the end of the call, I was worried too. Measles is a febrile respiratory illness classically accompanied by cough, coryza, conjunctivitis, and a characteristic maculopapular rash that begins on the face and spreads to the trunk and limbs. It is also highly contagious: 90% percent of susceptible, exposed individuals become infected.
Admittedly, measles is rare. Just 118 cases were reported in the United States in 2022, but 83 of those were in Columbus just 3 hours from where my colleague and I live and work. According to City of Columbus officials, the outbreak occurred almost exclusively in unimmunized children, the majority of whom were 5 years and younger. An unexpectedly high number of children were hospitalized. Typically, one in five people with measles will require hospitalization. In this outbreak, 33 children have been hospitalized as of Jan. 10.
Public health experts warn that 2023 could be much worse unless we increase measles immunization rates in the United States and globally. Immunization of around 95% of eligible people with two doses of measles-containing vaccine is associated with herd immunity. Globally, we’re falling short. Only 81% of the world’s children have received their first measle vaccine dose and only 71% have received the second dose. These are the lowest coverage rates for measles vaccine since 2008.
A 2022 joint press release from the Centers for Disease Control and Prevention and the World Health Organization noted that “measles anywhere is a threat everywhere, as the virus can quickly spread to multiple communities and across international borders.” Some prior measles outbreaks in the United States have started with a case in an international traveler or a U.S. resident who contracted measles during travel abroad.
In the United States, the number of children immunized with multiple routine vaccines has fallen in the last couple of years, in part because of pandemic-related disruptions in health care delivery. Increasing vaccine hesitancy, fueled by debates over the COVID-19 vaccine, may be slowing catch-up immunization in kids who fell behind.
Investigators from Emory University, Atlanta, and Marshfield Clinic Research Institute recently estimated that 9,145,026 U.S. children are susceptible to measles. If pandemic-level immunization rates continue without effective catch-up immunization, that number could rise to more than 15 million.
School vaccination requirements support efforts to ensure that kids are protected against vaccine-preventable diseases, but some data suggest that opposition to requiring MMR vaccine to attend public school is growing. According to a 2022 Kaiser Family Foundation Vaccine Monitor survey, 28% of U.S. adults – and 35% of parents of children under 18 – now say that parents should be able to decide to not vaccinate their children for measles, mumps, and rubella. That’s up from 16% of adults and 23% of parents in a 2019 Pew Research Center poll.
Public confidence in the benefits of MMR has also dropped modestly. About 85% of adults surveyed said that the benefits of MMR vaccine outweigh the risk, down from 88% in 2019. Among adults not vaccinated against COVID-19, only 70% said that benefits of these vaccines outweigh the risks.
While the WHO ramps up efforts to improve measles vaccination globally, pediatric clinicians can take steps now to mitigate the risk of measles outbreaks in their own communities. Query health records to understand how many eligible children in your practice have not yet received MMR vaccine. Notify families that vaccination is strongly recommended and make scheduling an appointment to receive vaccine easy. Some practices may have the bandwidth to offer evening and weekend hours for vaccine catch-up visits.
Curious about immunization rates in your state? The American Academy of Pediatrics has an interactive map that reports immunization coverage levels by state and provides comparisons to national rates and goals.
Prompt recognition and isolation of individuals with measles, along with prophylaxis of susceptible contacts, can limit community transmission. Measles can resemble other illnesses associated with fever and rash. Washington state has developed a screening tool to assist with recognition of measles. The CDC also has a measles outbreak toolkit that includes resources that outline clinical features and diagnoses, as well as strategies for talking to parents about vaccines.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant disclosed that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at pdnews@mdedge.com.
I received a call late one night from a colleague in the emergency department of the children’s hospital. “This 2-year-old has a fever, cough, red eyes, and an impressive rash. I’ve personally never seen a case of measles, but I’m worried given that this child has never received the MMR vaccine.”
By the end of the call, I was worried too. Measles is a febrile respiratory illness classically accompanied by cough, coryza, conjunctivitis, and a characteristic maculopapular rash that begins on the face and spreads to the trunk and limbs. It is also highly contagious: 90% percent of susceptible, exposed individuals become infected.
Admittedly, measles is rare. Just 118 cases were reported in the United States in 2022, but 83 of those were in Columbus just 3 hours from where my colleague and I live and work. According to City of Columbus officials, the outbreak occurred almost exclusively in unimmunized children, the majority of whom were 5 years and younger. An unexpectedly high number of children were hospitalized. Typically, one in five people with measles will require hospitalization. In this outbreak, 33 children have been hospitalized as of Jan. 10.
Public health experts warn that 2023 could be much worse unless we increase measles immunization rates in the United States and globally. Immunization of around 95% of eligible people with two doses of measles-containing vaccine is associated with herd immunity. Globally, we’re falling short. Only 81% of the world’s children have received their first measle vaccine dose and only 71% have received the second dose. These are the lowest coverage rates for measles vaccine since 2008.
A 2022 joint press release from the Centers for Disease Control and Prevention and the World Health Organization noted that “measles anywhere is a threat everywhere, as the virus can quickly spread to multiple communities and across international borders.” Some prior measles outbreaks in the United States have started with a case in an international traveler or a U.S. resident who contracted measles during travel abroad.
In the United States, the number of children immunized with multiple routine vaccines has fallen in the last couple of years, in part because of pandemic-related disruptions in health care delivery. Increasing vaccine hesitancy, fueled by debates over the COVID-19 vaccine, may be slowing catch-up immunization in kids who fell behind.
Investigators from Emory University, Atlanta, and Marshfield Clinic Research Institute recently estimated that 9,145,026 U.S. children are susceptible to measles. If pandemic-level immunization rates continue without effective catch-up immunization, that number could rise to more than 15 million.
School vaccination requirements support efforts to ensure that kids are protected against vaccine-preventable diseases, but some data suggest that opposition to requiring MMR vaccine to attend public school is growing. According to a 2022 Kaiser Family Foundation Vaccine Monitor survey, 28% of U.S. adults – and 35% of parents of children under 18 – now say that parents should be able to decide to not vaccinate their children for measles, mumps, and rubella. That’s up from 16% of adults and 23% of parents in a 2019 Pew Research Center poll.
Public confidence in the benefits of MMR has also dropped modestly. About 85% of adults surveyed said that the benefits of MMR vaccine outweigh the risk, down from 88% in 2019. Among adults not vaccinated against COVID-19, only 70% said that benefits of these vaccines outweigh the risks.
While the WHO ramps up efforts to improve measles vaccination globally, pediatric clinicians can take steps now to mitigate the risk of measles outbreaks in their own communities. Query health records to understand how many eligible children in your practice have not yet received MMR vaccine. Notify families that vaccination is strongly recommended and make scheduling an appointment to receive vaccine easy. Some practices may have the bandwidth to offer evening and weekend hours for vaccine catch-up visits.
Curious about immunization rates in your state? The American Academy of Pediatrics has an interactive map that reports immunization coverage levels by state and provides comparisons to national rates and goals.
Prompt recognition and isolation of individuals with measles, along with prophylaxis of susceptible contacts, can limit community transmission. Measles can resemble other illnesses associated with fever and rash. Washington state has developed a screening tool to assist with recognition of measles. The CDC also has a measles outbreak toolkit that includes resources that outline clinical features and diagnoses, as well as strategies for talking to parents about vaccines.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant disclosed that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at pdnews@mdedge.com.
I received a call late one night from a colleague in the emergency department of the children’s hospital. “This 2-year-old has a fever, cough, red eyes, and an impressive rash. I’ve personally never seen a case of measles, but I’m worried given that this child has never received the MMR vaccine.”
By the end of the call, I was worried too. Measles is a febrile respiratory illness classically accompanied by cough, coryza, conjunctivitis, and a characteristic maculopapular rash that begins on the face and spreads to the trunk and limbs. It is also highly contagious: 90% percent of susceptible, exposed individuals become infected.
Admittedly, measles is rare. Just 118 cases were reported in the United States in 2022, but 83 of those were in Columbus just 3 hours from where my colleague and I live and work. According to City of Columbus officials, the outbreak occurred almost exclusively in unimmunized children, the majority of whom were 5 years and younger. An unexpectedly high number of children were hospitalized. Typically, one in five people with measles will require hospitalization. In this outbreak, 33 children have been hospitalized as of Jan. 10.
Public health experts warn that 2023 could be much worse unless we increase measles immunization rates in the United States and globally. Immunization of around 95% of eligible people with two doses of measles-containing vaccine is associated with herd immunity. Globally, we’re falling short. Only 81% of the world’s children have received their first measle vaccine dose and only 71% have received the second dose. These are the lowest coverage rates for measles vaccine since 2008.
A 2022 joint press release from the Centers for Disease Control and Prevention and the World Health Organization noted that “measles anywhere is a threat everywhere, as the virus can quickly spread to multiple communities and across international borders.” Some prior measles outbreaks in the United States have started with a case in an international traveler or a U.S. resident who contracted measles during travel abroad.
In the United States, the number of children immunized with multiple routine vaccines has fallen in the last couple of years, in part because of pandemic-related disruptions in health care delivery. Increasing vaccine hesitancy, fueled by debates over the COVID-19 vaccine, may be slowing catch-up immunization in kids who fell behind.
Investigators from Emory University, Atlanta, and Marshfield Clinic Research Institute recently estimated that 9,145,026 U.S. children are susceptible to measles. If pandemic-level immunization rates continue without effective catch-up immunization, that number could rise to more than 15 million.
School vaccination requirements support efforts to ensure that kids are protected against vaccine-preventable diseases, but some data suggest that opposition to requiring MMR vaccine to attend public school is growing. According to a 2022 Kaiser Family Foundation Vaccine Monitor survey, 28% of U.S. adults – and 35% of parents of children under 18 – now say that parents should be able to decide to not vaccinate their children for measles, mumps, and rubella. That’s up from 16% of adults and 23% of parents in a 2019 Pew Research Center poll.
Public confidence in the benefits of MMR has also dropped modestly. About 85% of adults surveyed said that the benefits of MMR vaccine outweigh the risk, down from 88% in 2019. Among adults not vaccinated against COVID-19, only 70% said that benefits of these vaccines outweigh the risks.
While the WHO ramps up efforts to improve measles vaccination globally, pediatric clinicians can take steps now to mitigate the risk of measles outbreaks in their own communities. Query health records to understand how many eligible children in your practice have not yet received MMR vaccine. Notify families that vaccination is strongly recommended and make scheduling an appointment to receive vaccine easy. Some practices may have the bandwidth to offer evening and weekend hours for vaccine catch-up visits.
Curious about immunization rates in your state? The American Academy of Pediatrics has an interactive map that reports immunization coverage levels by state and provides comparisons to national rates and goals.
Prompt recognition and isolation of individuals with measles, along with prophylaxis of susceptible contacts, can limit community transmission. Measles can resemble other illnesses associated with fever and rash. Washington state has developed a screening tool to assist with recognition of measles. The CDC also has a measles outbreak toolkit that includes resources that outline clinical features and diagnoses, as well as strategies for talking to parents about vaccines.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant disclosed that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at pdnews@mdedge.com.
Immunity debt and the tripledemic
Respiratory syncytial virus (RSV) and influenza cases are surging to record numbers this winter in the wake of the COVID-19 pandemic when children were sheltering in the home, receiving virtual education, masking, and hand sanitizing, and when other precautionary health measures were in place.
RSV and flu illness in children now have hospital emergency rooms and pediatric ICUs and wards over capacity. As these respiratory infections increase and variants of SARS-CoV-2 come to dominate, we may expect the full impact of a tripledemic (RSV + flu + SARS-CoV-2).
It has been estimated that RSV causes 33 million lower respiratory infections and 3.6 million hospitalizations annually worldwide in children younger than 5 years old (Lancet. 2022 May 19. doi: 10.1016/S0140-6736(22)00478-0). RSV is typically a seasonal respiratory infection occurring in late fall through early winter, when it gives way to dominance by flu. Thus, we have experienced an out-of-season surge in RSV since it began in early fall 2022, and it persists. A likely explanation for the early and persisting surge in RSV is immunity debt (Infect Dis Now. 2021 Aug. doi: 10.1016/j.idnow.2021.05.004).
Immunity debt is an unintended consequence of prevention of infections that occurred because of public health measures to prevent spread of SARS-CoV-2 infections. The COVID-19 lockdown undoubtedly saved many lives. However, while we were sheltering from SARS-CoV-2 infections, we also were avoiding other infections, especially other respiratory infections such as RSV and flu.
Our group studied this in community-based pediatric practices in Rochester, N.Y. Physician-diagnosed, medically attended infectious disease illness visits were assessed in two child cohorts, age 6-36 months from March 15 to Dec. 31, 2020 (the pandemic period), compared with the same months in 2019 (prepandemic). One hundred forty-four children were included in the pandemic cohort and 215 in the prepandemic cohort. Visits for bronchiolitis were 7.4-fold lower (P = .04), acute otitis media 3.7-fold lower (P < .0001), viral upper respiratory infections (URI) 3.8-fold lower (P < .0001), and croup 27.5-fold lower (P < .0001) in the pandemic than the prepandemic cohort (Front Pediatr. 2021 Sep 13. doi: 10.3389/fped.2021.72248).
The significant reduction in respiratory illness during the COVID-19 epidemic we and others observed resulted in a large pool of children who did not experience RSV or flu infections for an entire year or more. Herd immunity dropped. The susceptible child population increased, including children older than typically seen. We had an immunity debt that had to be repaid, and the repayment is occurring now.
As a consequence of the surge in RSV, interest in prevention has gained more attention. In 1966, tragically, two infant deaths and hospitalization of 80% of the participating infants occurred during a clinical trial of an experimental candidate RSV vaccine, which contained an inactivated version of the entire virus. The severe side effect was later found to be caused by both an antibody and a T-cell problem. The antibody produced in response to the inactivated whole virus didn’t have very good functional activity at blocking or neutralizing the virus. That led to deposition of immune complexes and activation of complement that damaged the airways. The vaccine also triggered a T-cell response with inflammatory cytokine release that added to airway obstruction and lack of clearance of the virus. RSV vaccine development was halted and the bar for further studies was raised very high to ensure safety of any future RSV vaccines. Now, 55 years later, two RSV vaccines and a new preventive monoclonal antibody are nearing licensure.
GlaxoSmithKline (GSK) and Pfizer are in phase 3 clinical trials of a safer RSV vaccine that contains only the RSV surface protein known as protein F. Protein F changes its structure when the virus infects and fuses with human respiratory epithelial cells. The GSK and Pfizer vaccines use a molecular strategy developed at the National Institutes of Health to lock protein F into its original, prefusion configuration. A similar strategy was used by Pfizer/BioNTech and Moderna in their design of mRNA vaccines to the SARS-CoV-2 spike surface protein.
A vaccine with the F protein in its prefusion form takes care of the antibody problem that caused the severe side-effects from the 1966 version of inactivated whole virus vaccine because it induces very high-efficiency, high-potency antibodies that neutralize the RSV. The T-cell response is not as well understood and that is why studies are being done in adults first and then moving to young infants.
The new RSV vaccines are being developed for use in adults over age 60, adults with comorbidities, maternal immunization, and infants. Encouraging results were recently reported by GSK and Pfizer from adult trials. In an interim analysis, Pfizer also recently reported that maternal immunization in the late second or third trimester with their vaccine had an efficacy of 82% within a newborn’s first 90 days of life against severe lower respiratory tract illness. At age 6 months, the efficacy was sustained at 69%. So far, both the GSK and Pfizer RSV vaccines have shown a favorable safety profile.
Another strategy in the RSV prevention field has been a monoclonal antibody. Palivizumab (Synagis, AstraZeneca) is used to prevent severe RSV infections in prematurely born and other infants who are at higher risk of mortality and severe morbidity. Soon there will likely be another monoclonal antibody, called nirsevimab (Beyfortus, AstraZeneca and Sanofi). It is approved in Europe but not yet approved in the United States as I prepare this column. Nirsevimab may be even better than palivizumab – based on phase 3 trial data – and a single injection lasts through an entire normal RSV season while palivizumab requires monthly injections.
Similar to the situation with RSV, the flu season started earlier than usual in fall 2022 and has been picking up steam, likely also because of immunity debt. The WHO estimates that annual epidemics of influenza cause 1 billion infections, 3 million to 5 million severe cases, and 300,000-500,000 deaths. Seasonal flu vaccines provide modest protection. Current flu vaccine formulations consist of the hemagglutinin (H) and neuraminidase (N) proteins but those proteins change sufficiently (called antigenic drift) such that production of the vaccines based on a best guess each year often is not correct for the influenza A or influenza B strains that circulate in a given year (antigenic mismatch).
Public health authorities have long worried about a major change in the composition of the H and N proteins of the influenza virus (called antigenic shift). Preparedness and response to the COVID-19 pandemic was based on preparedness and response to an anticipated influenza pandemic similar to the 1918 flu pandemic. For flu, new “universal” vaccines are in development. Among the candidate vaccines are mRNA vaccines, building on the success of the SARS-CoV-2 mRNA vaccines (Science. 2022 Nov 24. doi: 10.1126/science.abm0271).
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
Respiratory syncytial virus (RSV) and influenza cases are surging to record numbers this winter in the wake of the COVID-19 pandemic when children were sheltering in the home, receiving virtual education, masking, and hand sanitizing, and when other precautionary health measures were in place.
RSV and flu illness in children now have hospital emergency rooms and pediatric ICUs and wards over capacity. As these respiratory infections increase and variants of SARS-CoV-2 come to dominate, we may expect the full impact of a tripledemic (RSV + flu + SARS-CoV-2).
It has been estimated that RSV causes 33 million lower respiratory infections and 3.6 million hospitalizations annually worldwide in children younger than 5 years old (Lancet. 2022 May 19. doi: 10.1016/S0140-6736(22)00478-0). RSV is typically a seasonal respiratory infection occurring in late fall through early winter, when it gives way to dominance by flu. Thus, we have experienced an out-of-season surge in RSV since it began in early fall 2022, and it persists. A likely explanation for the early and persisting surge in RSV is immunity debt (Infect Dis Now. 2021 Aug. doi: 10.1016/j.idnow.2021.05.004).
Immunity debt is an unintended consequence of prevention of infections that occurred because of public health measures to prevent spread of SARS-CoV-2 infections. The COVID-19 lockdown undoubtedly saved many lives. However, while we were sheltering from SARS-CoV-2 infections, we also were avoiding other infections, especially other respiratory infections such as RSV and flu.
Our group studied this in community-based pediatric practices in Rochester, N.Y. Physician-diagnosed, medically attended infectious disease illness visits were assessed in two child cohorts, age 6-36 months from March 15 to Dec. 31, 2020 (the pandemic period), compared with the same months in 2019 (prepandemic). One hundred forty-four children were included in the pandemic cohort and 215 in the prepandemic cohort. Visits for bronchiolitis were 7.4-fold lower (P = .04), acute otitis media 3.7-fold lower (P < .0001), viral upper respiratory infections (URI) 3.8-fold lower (P < .0001), and croup 27.5-fold lower (P < .0001) in the pandemic than the prepandemic cohort (Front Pediatr. 2021 Sep 13. doi: 10.3389/fped.2021.72248).
The significant reduction in respiratory illness during the COVID-19 epidemic we and others observed resulted in a large pool of children who did not experience RSV or flu infections for an entire year or more. Herd immunity dropped. The susceptible child population increased, including children older than typically seen. We had an immunity debt that had to be repaid, and the repayment is occurring now.
As a consequence of the surge in RSV, interest in prevention has gained more attention. In 1966, tragically, two infant deaths and hospitalization of 80% of the participating infants occurred during a clinical trial of an experimental candidate RSV vaccine, which contained an inactivated version of the entire virus. The severe side effect was later found to be caused by both an antibody and a T-cell problem. The antibody produced in response to the inactivated whole virus didn’t have very good functional activity at blocking or neutralizing the virus. That led to deposition of immune complexes and activation of complement that damaged the airways. The vaccine also triggered a T-cell response with inflammatory cytokine release that added to airway obstruction and lack of clearance of the virus. RSV vaccine development was halted and the bar for further studies was raised very high to ensure safety of any future RSV vaccines. Now, 55 years later, two RSV vaccines and a new preventive monoclonal antibody are nearing licensure.
GlaxoSmithKline (GSK) and Pfizer are in phase 3 clinical trials of a safer RSV vaccine that contains only the RSV surface protein known as protein F. Protein F changes its structure when the virus infects and fuses with human respiratory epithelial cells. The GSK and Pfizer vaccines use a molecular strategy developed at the National Institutes of Health to lock protein F into its original, prefusion configuration. A similar strategy was used by Pfizer/BioNTech and Moderna in their design of mRNA vaccines to the SARS-CoV-2 spike surface protein.
A vaccine with the F protein in its prefusion form takes care of the antibody problem that caused the severe side-effects from the 1966 version of inactivated whole virus vaccine because it induces very high-efficiency, high-potency antibodies that neutralize the RSV. The T-cell response is not as well understood and that is why studies are being done in adults first and then moving to young infants.
The new RSV vaccines are being developed for use in adults over age 60, adults with comorbidities, maternal immunization, and infants. Encouraging results were recently reported by GSK and Pfizer from adult trials. In an interim analysis, Pfizer also recently reported that maternal immunization in the late second or third trimester with their vaccine had an efficacy of 82% within a newborn’s first 90 days of life against severe lower respiratory tract illness. At age 6 months, the efficacy was sustained at 69%. So far, both the GSK and Pfizer RSV vaccines have shown a favorable safety profile.
Another strategy in the RSV prevention field has been a monoclonal antibody. Palivizumab (Synagis, AstraZeneca) is used to prevent severe RSV infections in prematurely born and other infants who are at higher risk of mortality and severe morbidity. Soon there will likely be another monoclonal antibody, called nirsevimab (Beyfortus, AstraZeneca and Sanofi). It is approved in Europe but not yet approved in the United States as I prepare this column. Nirsevimab may be even better than palivizumab – based on phase 3 trial data – and a single injection lasts through an entire normal RSV season while palivizumab requires monthly injections.
Similar to the situation with RSV, the flu season started earlier than usual in fall 2022 and has been picking up steam, likely also because of immunity debt. The WHO estimates that annual epidemics of influenza cause 1 billion infections, 3 million to 5 million severe cases, and 300,000-500,000 deaths. Seasonal flu vaccines provide modest protection. Current flu vaccine formulations consist of the hemagglutinin (H) and neuraminidase (N) proteins but those proteins change sufficiently (called antigenic drift) such that production of the vaccines based on a best guess each year often is not correct for the influenza A or influenza B strains that circulate in a given year (antigenic mismatch).
Public health authorities have long worried about a major change in the composition of the H and N proteins of the influenza virus (called antigenic shift). Preparedness and response to the COVID-19 pandemic was based on preparedness and response to an anticipated influenza pandemic similar to the 1918 flu pandemic. For flu, new “universal” vaccines are in development. Among the candidate vaccines are mRNA vaccines, building on the success of the SARS-CoV-2 mRNA vaccines (Science. 2022 Nov 24. doi: 10.1126/science.abm0271).
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
Respiratory syncytial virus (RSV) and influenza cases are surging to record numbers this winter in the wake of the COVID-19 pandemic when children were sheltering in the home, receiving virtual education, masking, and hand sanitizing, and when other precautionary health measures were in place.
RSV and flu illness in children now have hospital emergency rooms and pediatric ICUs and wards over capacity. As these respiratory infections increase and variants of SARS-CoV-2 come to dominate, we may expect the full impact of a tripledemic (RSV + flu + SARS-CoV-2).
It has been estimated that RSV causes 33 million lower respiratory infections and 3.6 million hospitalizations annually worldwide in children younger than 5 years old (Lancet. 2022 May 19. doi: 10.1016/S0140-6736(22)00478-0). RSV is typically a seasonal respiratory infection occurring in late fall through early winter, when it gives way to dominance by flu. Thus, we have experienced an out-of-season surge in RSV since it began in early fall 2022, and it persists. A likely explanation for the early and persisting surge in RSV is immunity debt (Infect Dis Now. 2021 Aug. doi: 10.1016/j.idnow.2021.05.004).
Immunity debt is an unintended consequence of prevention of infections that occurred because of public health measures to prevent spread of SARS-CoV-2 infections. The COVID-19 lockdown undoubtedly saved many lives. However, while we were sheltering from SARS-CoV-2 infections, we also were avoiding other infections, especially other respiratory infections such as RSV and flu.
Our group studied this in community-based pediatric practices in Rochester, N.Y. Physician-diagnosed, medically attended infectious disease illness visits were assessed in two child cohorts, age 6-36 months from March 15 to Dec. 31, 2020 (the pandemic period), compared with the same months in 2019 (prepandemic). One hundred forty-four children were included in the pandemic cohort and 215 in the prepandemic cohort. Visits for bronchiolitis were 7.4-fold lower (P = .04), acute otitis media 3.7-fold lower (P < .0001), viral upper respiratory infections (URI) 3.8-fold lower (P < .0001), and croup 27.5-fold lower (P < .0001) in the pandemic than the prepandemic cohort (Front Pediatr. 2021 Sep 13. doi: 10.3389/fped.2021.72248).
The significant reduction in respiratory illness during the COVID-19 epidemic we and others observed resulted in a large pool of children who did not experience RSV or flu infections for an entire year or more. Herd immunity dropped. The susceptible child population increased, including children older than typically seen. We had an immunity debt that had to be repaid, and the repayment is occurring now.
As a consequence of the surge in RSV, interest in prevention has gained more attention. In 1966, tragically, two infant deaths and hospitalization of 80% of the participating infants occurred during a clinical trial of an experimental candidate RSV vaccine, which contained an inactivated version of the entire virus. The severe side effect was later found to be caused by both an antibody and a T-cell problem. The antibody produced in response to the inactivated whole virus didn’t have very good functional activity at blocking or neutralizing the virus. That led to deposition of immune complexes and activation of complement that damaged the airways. The vaccine also triggered a T-cell response with inflammatory cytokine release that added to airway obstruction and lack of clearance of the virus. RSV vaccine development was halted and the bar for further studies was raised very high to ensure safety of any future RSV vaccines. Now, 55 years later, two RSV vaccines and a new preventive monoclonal antibody are nearing licensure.
GlaxoSmithKline (GSK) and Pfizer are in phase 3 clinical trials of a safer RSV vaccine that contains only the RSV surface protein known as protein F. Protein F changes its structure when the virus infects and fuses with human respiratory epithelial cells. The GSK and Pfizer vaccines use a molecular strategy developed at the National Institutes of Health to lock protein F into its original, prefusion configuration. A similar strategy was used by Pfizer/BioNTech and Moderna in their design of mRNA vaccines to the SARS-CoV-2 spike surface protein.
A vaccine with the F protein in its prefusion form takes care of the antibody problem that caused the severe side-effects from the 1966 version of inactivated whole virus vaccine because it induces very high-efficiency, high-potency antibodies that neutralize the RSV. The T-cell response is not as well understood and that is why studies are being done in adults first and then moving to young infants.
The new RSV vaccines are being developed for use in adults over age 60, adults with comorbidities, maternal immunization, and infants. Encouraging results were recently reported by GSK and Pfizer from adult trials. In an interim analysis, Pfizer also recently reported that maternal immunization in the late second or third trimester with their vaccine had an efficacy of 82% within a newborn’s first 90 days of life against severe lower respiratory tract illness. At age 6 months, the efficacy was sustained at 69%. So far, both the GSK and Pfizer RSV vaccines have shown a favorable safety profile.
Another strategy in the RSV prevention field has been a monoclonal antibody. Palivizumab (Synagis, AstraZeneca) is used to prevent severe RSV infections in prematurely born and other infants who are at higher risk of mortality and severe morbidity. Soon there will likely be another monoclonal antibody, called nirsevimab (Beyfortus, AstraZeneca and Sanofi). It is approved in Europe but not yet approved in the United States as I prepare this column. Nirsevimab may be even better than palivizumab – based on phase 3 trial data – and a single injection lasts through an entire normal RSV season while palivizumab requires monthly injections.
Similar to the situation with RSV, the flu season started earlier than usual in fall 2022 and has been picking up steam, likely also because of immunity debt. The WHO estimates that annual epidemics of influenza cause 1 billion infections, 3 million to 5 million severe cases, and 300,000-500,000 deaths. Seasonal flu vaccines provide modest protection. Current flu vaccine formulations consist of the hemagglutinin (H) and neuraminidase (N) proteins but those proteins change sufficiently (called antigenic drift) such that production of the vaccines based on a best guess each year often is not correct for the influenza A or influenza B strains that circulate in a given year (antigenic mismatch).
Public health authorities have long worried about a major change in the composition of the H and N proteins of the influenza virus (called antigenic shift). Preparedness and response to the COVID-19 pandemic was based on preparedness and response to an anticipated influenza pandemic similar to the 1918 flu pandemic. For flu, new “universal” vaccines are in development. Among the candidate vaccines are mRNA vaccines, building on the success of the SARS-CoV-2 mRNA vaccines (Science. 2022 Nov 24. doi: 10.1126/science.abm0271).
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
More children should be getting flu vaccines
Cold and flu season came early in 2022.
On Nov. 4, 2022, the Centers for Disease Control and Prevention issued a Health Alert Network Health Advisory about early, elevated respiratory disease incidence caused by multiple viruses other than SARS-CoV-2.
Interseasonal spread of respiratory syncytial virus has continued in 2022, with RSV-associated hospitalizations increasing in the late spring and continuing throughout the summer and into the fall. In October, some regions of the country were seeing RSV activity near the peak seasonal levels typically observed in December and January.
Cases of severe respiratory infection in children who tested positive for rhinovirus or enterovirus spiked in August; further testing confirmed the presence of EV-D68 in some children. Rhinovirus and enterovirus continue to circulate and are isolated in hospitalized children with respiratory illness.
In some parts of the country, influenza cases have rapidly increased ahead of what we normally anticipate. According to preliminary estimates from the CDC, between Oct. 1 and Oct. 22, 880,000 people were sickened with flu, 420,000 people visited a health care provider for flu illness, and 6,900 people were hospitalized for flu. The cumulative hospitalization rate is higher than observed at this time of year in every previous flu season since 2010-2011. Hospitalization rates are highest in children aged 0-4 years and adults 65 years and older.
Of course, this report came as no surprise to pediatric health care providers. Many children’s hospitals had been operating at or over capacity for weeks. While a systematic assessment of the surge on children’s hospitals has not been published, anecdotally, hospitals from around the country have described record emergency department visits and inpatient census numbers. Some have set up tents or other temporary facilities to see ambulatory patients and have canceled elective surgeries because of a lack of beds.
There is no quick or easy solution to stem the tide of RSV-related or enterovirus/rhinovirus admissions, but many flu-related hospitalizations are vaccine preventable. Unfortunately, too few children are receiving influenza vaccine. As of the week ending Oct. 15, only about 22.1% of eligible children had been immunized. The American Academy of Pediatrics and the CDC recommend that all children are vaccinated, preferably by the end of October so they have time to develop immunity before influenza starts circulating. As it stands now, the majority of the nation’s children are facing a flu season without the benefits of vaccine.
There is still time to take steps to prevent this flu season from becoming one of the worst in recent memory. A strong provider recommendation for influenza vaccine is consistently associated with higher rates of vaccine acceptance. We need to recommend influenza vaccine to all eligible patients at every visit and in every setting. It will help if we can say it like we mean it. Some of us are tired of debating the merits of COVID-19 vaccine with families and may be leery of additional debates about flu. Some of us may just be tired, as many practices have already expanded office hours to care for the influx of kids with respiratory illness. On the heels of two atypical flu seasons, a few of us may be quietly complacent about the importance of flu vaccines for children.
Anyone in need of a little motivation should check out a paper recently published in Clinical Infectious Diseases that reinforces the value of flu vaccine, even in a year when there is a poor match between the vaccine and circulating viruses.
The 2019-2020 flu season was a bad flu season for children. Two antigenically drifted influenza viruses predominated and cases of influenza soared, resulting in the largest influenza epidemic in children in the United States since 1992. Pediatric Intensive Care Influenza Study investigators used a test-negative design to estimate the effectiveness of influenza vaccine in preventing critical and life-threatening influenza in children during that season. The good news: vaccination reduced the risk of critical influenza by 78% against H1N1pdm09 viruses that were well-matched to vaccine and by 47% against mismatched viruses. Vaccination was estimated to be 75% protective against antigenically drifted B-Victoria viruses. Overall vaccine effectiveness against critical illness from any influenza virus was 63% (95% confidence interval, 38%-78%).
While it might be tempting to attribute suboptimal immunization rates to vaccine hesitancy, ready availability remains an issue for some families. We need to eliminate barriers to access. While the AAP continues to emphasize immunization in the medical home, especially for the youngest infants, the 2022 policy statement suggests that vaccinating children in schools, pharmacies, and other nontraditional settings could improve immunization rates. To the extent feasible, we need to work with partners to support community-based initiatives and promote these to families who struggle to make it into the office.
Improving access is just one potential way to reduce health disparities related to influenza and influenza vaccination. Over 10 influenza seasons, higher rates of influenza-associated hospitalizations and intensive care unit admissions were observed in Black, Hispanic, and American Indian/Alaska Native people. These disparities were highest in children aged younger than 4 years and influenza-associated in-hospital deaths were three- to fourfold higher in Black, Hispanic, and Asian/Pacific Islander children, compared with White children. The reason for the disparities isn’t completely clear but increasing immunization rates may be part of the solution. During the 2020-2021 influenza season, flu immunization rates in Black children (51.6%) were lower than those seen in White (57.4%) and Hispanic children (58.9%).
The AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023, highlight a variety of evidence-based strategies to increase influenza immunization rates. These may provide a little inspiration for clinicians looking to try a new approach. If you wish to share your experience with increasing influenza immunization rates in your practice setting, please email me at Kristina.bryant@louisville.edu.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead.
Cold and flu season came early in 2022.
On Nov. 4, 2022, the Centers for Disease Control and Prevention issued a Health Alert Network Health Advisory about early, elevated respiratory disease incidence caused by multiple viruses other than SARS-CoV-2.
Interseasonal spread of respiratory syncytial virus has continued in 2022, with RSV-associated hospitalizations increasing in the late spring and continuing throughout the summer and into the fall. In October, some regions of the country were seeing RSV activity near the peak seasonal levels typically observed in December and January.
Cases of severe respiratory infection in children who tested positive for rhinovirus or enterovirus spiked in August; further testing confirmed the presence of EV-D68 in some children. Rhinovirus and enterovirus continue to circulate and are isolated in hospitalized children with respiratory illness.
In some parts of the country, influenza cases have rapidly increased ahead of what we normally anticipate. According to preliminary estimates from the CDC, between Oct. 1 and Oct. 22, 880,000 people were sickened with flu, 420,000 people visited a health care provider for flu illness, and 6,900 people were hospitalized for flu. The cumulative hospitalization rate is higher than observed at this time of year in every previous flu season since 2010-2011. Hospitalization rates are highest in children aged 0-4 years and adults 65 years and older.
Of course, this report came as no surprise to pediatric health care providers. Many children’s hospitals had been operating at or over capacity for weeks. While a systematic assessment of the surge on children’s hospitals has not been published, anecdotally, hospitals from around the country have described record emergency department visits and inpatient census numbers. Some have set up tents or other temporary facilities to see ambulatory patients and have canceled elective surgeries because of a lack of beds.
There is no quick or easy solution to stem the tide of RSV-related or enterovirus/rhinovirus admissions, but many flu-related hospitalizations are vaccine preventable. Unfortunately, too few children are receiving influenza vaccine. As of the week ending Oct. 15, only about 22.1% of eligible children had been immunized. The American Academy of Pediatrics and the CDC recommend that all children are vaccinated, preferably by the end of October so they have time to develop immunity before influenza starts circulating. As it stands now, the majority of the nation’s children are facing a flu season without the benefits of vaccine.
There is still time to take steps to prevent this flu season from becoming one of the worst in recent memory. A strong provider recommendation for influenza vaccine is consistently associated with higher rates of vaccine acceptance. We need to recommend influenza vaccine to all eligible patients at every visit and in every setting. It will help if we can say it like we mean it. Some of us are tired of debating the merits of COVID-19 vaccine with families and may be leery of additional debates about flu. Some of us may just be tired, as many practices have already expanded office hours to care for the influx of kids with respiratory illness. On the heels of two atypical flu seasons, a few of us may be quietly complacent about the importance of flu vaccines for children.
Anyone in need of a little motivation should check out a paper recently published in Clinical Infectious Diseases that reinforces the value of flu vaccine, even in a year when there is a poor match between the vaccine and circulating viruses.
The 2019-2020 flu season was a bad flu season for children. Two antigenically drifted influenza viruses predominated and cases of influenza soared, resulting in the largest influenza epidemic in children in the United States since 1992. Pediatric Intensive Care Influenza Study investigators used a test-negative design to estimate the effectiveness of influenza vaccine in preventing critical and life-threatening influenza in children during that season. The good news: vaccination reduced the risk of critical influenza by 78% against H1N1pdm09 viruses that were well-matched to vaccine and by 47% against mismatched viruses. Vaccination was estimated to be 75% protective against antigenically drifted B-Victoria viruses. Overall vaccine effectiveness against critical illness from any influenza virus was 63% (95% confidence interval, 38%-78%).
While it might be tempting to attribute suboptimal immunization rates to vaccine hesitancy, ready availability remains an issue for some families. We need to eliminate barriers to access. While the AAP continues to emphasize immunization in the medical home, especially for the youngest infants, the 2022 policy statement suggests that vaccinating children in schools, pharmacies, and other nontraditional settings could improve immunization rates. To the extent feasible, we need to work with partners to support community-based initiatives and promote these to families who struggle to make it into the office.
Improving access is just one potential way to reduce health disparities related to influenza and influenza vaccination. Over 10 influenza seasons, higher rates of influenza-associated hospitalizations and intensive care unit admissions were observed in Black, Hispanic, and American Indian/Alaska Native people. These disparities were highest in children aged younger than 4 years and influenza-associated in-hospital deaths were three- to fourfold higher in Black, Hispanic, and Asian/Pacific Islander children, compared with White children. The reason for the disparities isn’t completely clear but increasing immunization rates may be part of the solution. During the 2020-2021 influenza season, flu immunization rates in Black children (51.6%) were lower than those seen in White (57.4%) and Hispanic children (58.9%).
The AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023, highlight a variety of evidence-based strategies to increase influenza immunization rates. These may provide a little inspiration for clinicians looking to try a new approach. If you wish to share your experience with increasing influenza immunization rates in your practice setting, please email me at Kristina.bryant@louisville.edu.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead.
Cold and flu season came early in 2022.
On Nov. 4, 2022, the Centers for Disease Control and Prevention issued a Health Alert Network Health Advisory about early, elevated respiratory disease incidence caused by multiple viruses other than SARS-CoV-2.
Interseasonal spread of respiratory syncytial virus has continued in 2022, with RSV-associated hospitalizations increasing in the late spring and continuing throughout the summer and into the fall. In October, some regions of the country were seeing RSV activity near the peak seasonal levels typically observed in December and January.
Cases of severe respiratory infection in children who tested positive for rhinovirus or enterovirus spiked in August; further testing confirmed the presence of EV-D68 in some children. Rhinovirus and enterovirus continue to circulate and are isolated in hospitalized children with respiratory illness.
In some parts of the country, influenza cases have rapidly increased ahead of what we normally anticipate. According to preliminary estimates from the CDC, between Oct. 1 and Oct. 22, 880,000 people were sickened with flu, 420,000 people visited a health care provider for flu illness, and 6,900 people were hospitalized for flu. The cumulative hospitalization rate is higher than observed at this time of year in every previous flu season since 2010-2011. Hospitalization rates are highest in children aged 0-4 years and adults 65 years and older.
Of course, this report came as no surprise to pediatric health care providers. Many children’s hospitals had been operating at or over capacity for weeks. While a systematic assessment of the surge on children’s hospitals has not been published, anecdotally, hospitals from around the country have described record emergency department visits and inpatient census numbers. Some have set up tents or other temporary facilities to see ambulatory patients and have canceled elective surgeries because of a lack of beds.
There is no quick or easy solution to stem the tide of RSV-related or enterovirus/rhinovirus admissions, but many flu-related hospitalizations are vaccine preventable. Unfortunately, too few children are receiving influenza vaccine. As of the week ending Oct. 15, only about 22.1% of eligible children had been immunized. The American Academy of Pediatrics and the CDC recommend that all children are vaccinated, preferably by the end of October so they have time to develop immunity before influenza starts circulating. As it stands now, the majority of the nation’s children are facing a flu season without the benefits of vaccine.
There is still time to take steps to prevent this flu season from becoming one of the worst in recent memory. A strong provider recommendation for influenza vaccine is consistently associated with higher rates of vaccine acceptance. We need to recommend influenza vaccine to all eligible patients at every visit and in every setting. It will help if we can say it like we mean it. Some of us are tired of debating the merits of COVID-19 vaccine with families and may be leery of additional debates about flu. Some of us may just be tired, as many practices have already expanded office hours to care for the influx of kids with respiratory illness. On the heels of two atypical flu seasons, a few of us may be quietly complacent about the importance of flu vaccines for children.
Anyone in need of a little motivation should check out a paper recently published in Clinical Infectious Diseases that reinforces the value of flu vaccine, even in a year when there is a poor match between the vaccine and circulating viruses.
The 2019-2020 flu season was a bad flu season for children. Two antigenically drifted influenza viruses predominated and cases of influenza soared, resulting in the largest influenza epidemic in children in the United States since 1992. Pediatric Intensive Care Influenza Study investigators used a test-negative design to estimate the effectiveness of influenza vaccine in preventing critical and life-threatening influenza in children during that season. The good news: vaccination reduced the risk of critical influenza by 78% against H1N1pdm09 viruses that were well-matched to vaccine and by 47% against mismatched viruses. Vaccination was estimated to be 75% protective against antigenically drifted B-Victoria viruses. Overall vaccine effectiveness against critical illness from any influenza virus was 63% (95% confidence interval, 38%-78%).
While it might be tempting to attribute suboptimal immunization rates to vaccine hesitancy, ready availability remains an issue for some families. We need to eliminate barriers to access. While the AAP continues to emphasize immunization in the medical home, especially for the youngest infants, the 2022 policy statement suggests that vaccinating children in schools, pharmacies, and other nontraditional settings could improve immunization rates. To the extent feasible, we need to work with partners to support community-based initiatives and promote these to families who struggle to make it into the office.
Improving access is just one potential way to reduce health disparities related to influenza and influenza vaccination. Over 10 influenza seasons, higher rates of influenza-associated hospitalizations and intensive care unit admissions were observed in Black, Hispanic, and American Indian/Alaska Native people. These disparities were highest in children aged younger than 4 years and influenza-associated in-hospital deaths were three- to fourfold higher in Black, Hispanic, and Asian/Pacific Islander children, compared with White children. The reason for the disparities isn’t completely clear but increasing immunization rates may be part of the solution. During the 2020-2021 influenza season, flu immunization rates in Black children (51.6%) were lower than those seen in White (57.4%) and Hispanic children (58.9%).
The AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023, highlight a variety of evidence-based strategies to increase influenza immunization rates. These may provide a little inspiration for clinicians looking to try a new approach. If you wish to share your experience with increasing influenza immunization rates in your practice setting, please email me at Kristina.bryant@louisville.edu.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead.
Congenital syphilis: It’s still a significant public health problem
You’re rounding in the nursery and informed of the following about one of your new patients: He’s a 38-week-old infant delivered to a mother diagnosed with syphilis at 12 weeks’ gestation at her initial prenatal visit. Her rapid plasma reagin (RPR) was 1:64 and the fluorescent treponemal antibody–absorption (FTA-ABS) test was positive. By report she was appropriately treated. Maternal RPRs obtained at 18 and 28 weeks’ gestation were 1:16 and 1:4, respectively. Maternal RPR at delivery and the infant’s RPR obtained shortly after birth were both 1:4. The mother wants to know if her baby is infected.
One result of syphilis during pregnancy is intrauterine infection and resultant congenital disease in the infant. Before you answer this mother, let’s discuss syphilis.
Congenital syphilis is a significant public health problem. In 2021, there were a total of 2,677 cases reported for a rate of 74.1 per 100,000 live births. Between 2020 and 2021, the number of cases of congenital syphilis increased 24.1% (2,158-2,677 cases), concurrent with a 45.8% increase (10.7-15.6 per 100,000) in the rate of primary and secondary syphilis in women aged 15-44 years. Between 2012 and 2021, the number of cases of congenital syphilis increased 701.5% (334-2,677 cases) and the increase in rates of primary and secondary syphilis in women aged 15-44 was 642.9% over the same period.
Why are the rates of congenital syphilis increasing? Most cases result from a lack of prenatal care and thus no testing for syphilis. The next most common cause is inadequate maternal treatment.
Congenital syphilis usually is acquired through transplacental transmission of spirochetes in the maternal bloodstream. Occasionally, it occurs at delivery via direct contact with maternal lesions. It is not transmitted in breast milk. Transmission of syphilis:
- Can occur any time during pregnancy.
- Is more likely to occur in women with untreated primary or secondary disease (60%-100%).
- Is approximately 40% in those with early latent syphilis and less than 8% in mothers with late latent syphilis.
- Is higher in women coinfected with HIV since they more frequently receive no prenatal care and their disease is inadequately treated.
Coinfection with syphilis may also increase the rate of mother-to-child transmission of HIV.
Untreated early syphilis during pregnancy results in spontaneous abortion, stillbirth, or perinatal death in up to 40% of cases. Infected newborns with early congenital syphilis can be asymptomatic or have evidence of hepatosplenomegaly, generalized lymphadenopathy, nasal discharge that is occasionally bloody, rash, and skeletal abnormalities (osteochondritis and periostitis). Other manifestations include edema, hemolytic anemia, jaundice, pneumonia, pseudoparalysis, and thrombocytopenia. Asymptomatic infants may have abnormal cerebrospinal fluid findings including elevated CSF white cell count, elevated protein, and a reactive venereal disease research laboratory test.
Late congenital syphilis, defined as the onset of symptoms after 2 years of age is secondary to scarring or persistent inflammation and gumma formation in a variety of tissues. It occurs in up to 40% of cases of untreated maternal disease. Most cases can be prevented by maternal treatment and treatment of the infant within the first 3 months of life. Common clinical manifestations include interstitial keratitis, sensorineural hearing loss, frontal bossing, saddle nose, Hutchinson teeth, mulberry molars, perforation of the hard palate, anterior bowing of the tibia (saber shins), and other skeletal abnormalities.
Diagnostic tests. Maternal diagnosis is dependent upon knowing the results of both a nontreponemal (RPR, VDRL) and a confirmatory treponemal test (TP-PA, TP-EIA, TP-CIA, FTA-ABS,) before or at delivery. TP-PA is the preferred test. When maternal disease is confirmed, the newborn should have the same quantitative nontreponemal test as the mother. A confirmatory treponemal test is not required
Evaluation and treatment. It’s imperative that children born to mothers with a reactive test, regardless of their treatment status, have a thorough exam performed before hospital discharge. The provider must determine what additional interventions should be performed.
The American Academy of Pediatrics and the Centers for Disease Control and Prevention (www.cdc.gov/std/treatment-guidelines/congenital-syphilis.htm) have developed standard algorithms for the diagnostic approach and treatment of infants born to mothers with reactive serologic tests for syphilis. It is available in the Red Book for AAP members (https://publications.aap.org/redbook). Recommendations based on various scenarios for neonates up to 1 month of age include proven or highly probable congenital syphilis, possible congenital syphilis, congenital syphilis less likely, and congenital syphilis unlikely. It is beyond the scope of this article to list the criteria and evaluation for each scenario. The reader is referred to the algorithm.
If syphilis is suspected in infants or children older than 1 month, the challenge is to determine if it is untreated congenital syphilis or acquired syphilis. Maternal syphilis status should be determined. Evaluation for congenital syphilis in this age group includes CSF analysis for VDRL, cell count and protein, CBC with differential and platelets, hepatic panel, abdominal ultrasound, long-bone radiographs, chest radiograph, neuroimaging, auditory brain stem response, and HIV testing.
Let’s go back to your patient. The mother was diagnosed with syphilis during pregnancy. You confirm that she was treated with benzathine penicillin G, and the course was completed at least 4 weeks before delivery. Treatment with any other drug during pregnancy is not appropriate. The RPR has declined, and the infant’s titer is equal to or less than four times the maternal titer. The exam is significant for generalized adenopathy and slightly bloody nasal discharge. This infant has two findings consistent with congenital syphilis regardless of RPR titer or treatment status. This places him in the proven or highly probable congenital syphilis group. Management includes CSF analysis (VDRL, cell count, and protein), CBC with differential and platelet count, and treatment with penicillin G for 10 days. Additional tests as clinically indicated include: long-bone radiograph, chest radiography, aspartate aminotranferase and alanine aminotransferase levels, neuroimaging, ophthalmologic exam, and auditory brain stem response. Despite maternal treatment, this newborn has congenital syphilis. The same nontreponemal test should be obtained every 2-3 months until it is nonreactive. It should be nonreactive by 6 months. If the infection persists to 6-12 months post treatment, reevaluation including CSF analysis and retreatment may be indicated.
Congenital syphilis can be prevented by maternal screening, diagnosis, and treatment. When that fails it is up to us to diagnosis and adequately treat our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
You’re rounding in the nursery and informed of the following about one of your new patients: He’s a 38-week-old infant delivered to a mother diagnosed with syphilis at 12 weeks’ gestation at her initial prenatal visit. Her rapid plasma reagin (RPR) was 1:64 and the fluorescent treponemal antibody–absorption (FTA-ABS) test was positive. By report she was appropriately treated. Maternal RPRs obtained at 18 and 28 weeks’ gestation were 1:16 and 1:4, respectively. Maternal RPR at delivery and the infant’s RPR obtained shortly after birth were both 1:4. The mother wants to know if her baby is infected.
One result of syphilis during pregnancy is intrauterine infection and resultant congenital disease in the infant. Before you answer this mother, let’s discuss syphilis.
Congenital syphilis is a significant public health problem. In 2021, there were a total of 2,677 cases reported for a rate of 74.1 per 100,000 live births. Between 2020 and 2021, the number of cases of congenital syphilis increased 24.1% (2,158-2,677 cases), concurrent with a 45.8% increase (10.7-15.6 per 100,000) in the rate of primary and secondary syphilis in women aged 15-44 years. Between 2012 and 2021, the number of cases of congenital syphilis increased 701.5% (334-2,677 cases) and the increase in rates of primary and secondary syphilis in women aged 15-44 was 642.9% over the same period.
Why are the rates of congenital syphilis increasing? Most cases result from a lack of prenatal care and thus no testing for syphilis. The next most common cause is inadequate maternal treatment.
Congenital syphilis usually is acquired through transplacental transmission of spirochetes in the maternal bloodstream. Occasionally, it occurs at delivery via direct contact with maternal lesions. It is not transmitted in breast milk. Transmission of syphilis:
- Can occur any time during pregnancy.
- Is more likely to occur in women with untreated primary or secondary disease (60%-100%).
- Is approximately 40% in those with early latent syphilis and less than 8% in mothers with late latent syphilis.
- Is higher in women coinfected with HIV since they more frequently receive no prenatal care and their disease is inadequately treated.
Coinfection with syphilis may also increase the rate of mother-to-child transmission of HIV.
Untreated early syphilis during pregnancy results in spontaneous abortion, stillbirth, or perinatal death in up to 40% of cases. Infected newborns with early congenital syphilis can be asymptomatic or have evidence of hepatosplenomegaly, generalized lymphadenopathy, nasal discharge that is occasionally bloody, rash, and skeletal abnormalities (osteochondritis and periostitis). Other manifestations include edema, hemolytic anemia, jaundice, pneumonia, pseudoparalysis, and thrombocytopenia. Asymptomatic infants may have abnormal cerebrospinal fluid findings including elevated CSF white cell count, elevated protein, and a reactive venereal disease research laboratory test.
Late congenital syphilis, defined as the onset of symptoms after 2 years of age is secondary to scarring or persistent inflammation and gumma formation in a variety of tissues. It occurs in up to 40% of cases of untreated maternal disease. Most cases can be prevented by maternal treatment and treatment of the infant within the first 3 months of life. Common clinical manifestations include interstitial keratitis, sensorineural hearing loss, frontal bossing, saddle nose, Hutchinson teeth, mulberry molars, perforation of the hard palate, anterior bowing of the tibia (saber shins), and other skeletal abnormalities.
Diagnostic tests. Maternal diagnosis is dependent upon knowing the results of both a nontreponemal (RPR, VDRL) and a confirmatory treponemal test (TP-PA, TP-EIA, TP-CIA, FTA-ABS,) before or at delivery. TP-PA is the preferred test. When maternal disease is confirmed, the newborn should have the same quantitative nontreponemal test as the mother. A confirmatory treponemal test is not required
Evaluation and treatment. It’s imperative that children born to mothers with a reactive test, regardless of their treatment status, have a thorough exam performed before hospital discharge. The provider must determine what additional interventions should be performed.
The American Academy of Pediatrics and the Centers for Disease Control and Prevention (www.cdc.gov/std/treatment-guidelines/congenital-syphilis.htm) have developed standard algorithms for the diagnostic approach and treatment of infants born to mothers with reactive serologic tests for syphilis. It is available in the Red Book for AAP members (https://publications.aap.org/redbook). Recommendations based on various scenarios for neonates up to 1 month of age include proven or highly probable congenital syphilis, possible congenital syphilis, congenital syphilis less likely, and congenital syphilis unlikely. It is beyond the scope of this article to list the criteria and evaluation for each scenario. The reader is referred to the algorithm.
If syphilis is suspected in infants or children older than 1 month, the challenge is to determine if it is untreated congenital syphilis or acquired syphilis. Maternal syphilis status should be determined. Evaluation for congenital syphilis in this age group includes CSF analysis for VDRL, cell count and protein, CBC with differential and platelets, hepatic panel, abdominal ultrasound, long-bone radiographs, chest radiograph, neuroimaging, auditory brain stem response, and HIV testing.
Let’s go back to your patient. The mother was diagnosed with syphilis during pregnancy. You confirm that she was treated with benzathine penicillin G, and the course was completed at least 4 weeks before delivery. Treatment with any other drug during pregnancy is not appropriate. The RPR has declined, and the infant’s titer is equal to or less than four times the maternal titer. The exam is significant for generalized adenopathy and slightly bloody nasal discharge. This infant has two findings consistent with congenital syphilis regardless of RPR titer or treatment status. This places him in the proven or highly probable congenital syphilis group. Management includes CSF analysis (VDRL, cell count, and protein), CBC with differential and platelet count, and treatment with penicillin G for 10 days. Additional tests as clinically indicated include: long-bone radiograph, chest radiography, aspartate aminotranferase and alanine aminotransferase levels, neuroimaging, ophthalmologic exam, and auditory brain stem response. Despite maternal treatment, this newborn has congenital syphilis. The same nontreponemal test should be obtained every 2-3 months until it is nonreactive. It should be nonreactive by 6 months. If the infection persists to 6-12 months post treatment, reevaluation including CSF analysis and retreatment may be indicated.
Congenital syphilis can be prevented by maternal screening, diagnosis, and treatment. When that fails it is up to us to diagnosis and adequately treat our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
You’re rounding in the nursery and informed of the following about one of your new patients: He’s a 38-week-old infant delivered to a mother diagnosed with syphilis at 12 weeks’ gestation at her initial prenatal visit. Her rapid plasma reagin (RPR) was 1:64 and the fluorescent treponemal antibody–absorption (FTA-ABS) test was positive. By report she was appropriately treated. Maternal RPRs obtained at 18 and 28 weeks’ gestation were 1:16 and 1:4, respectively. Maternal RPR at delivery and the infant’s RPR obtained shortly after birth were both 1:4. The mother wants to know if her baby is infected.
One result of syphilis during pregnancy is intrauterine infection and resultant congenital disease in the infant. Before you answer this mother, let’s discuss syphilis.
Congenital syphilis is a significant public health problem. In 2021, there were a total of 2,677 cases reported for a rate of 74.1 per 100,000 live births. Between 2020 and 2021, the number of cases of congenital syphilis increased 24.1% (2,158-2,677 cases), concurrent with a 45.8% increase (10.7-15.6 per 100,000) in the rate of primary and secondary syphilis in women aged 15-44 years. Between 2012 and 2021, the number of cases of congenital syphilis increased 701.5% (334-2,677 cases) and the increase in rates of primary and secondary syphilis in women aged 15-44 was 642.9% over the same period.
Why are the rates of congenital syphilis increasing? Most cases result from a lack of prenatal care and thus no testing for syphilis. The next most common cause is inadequate maternal treatment.
Congenital syphilis usually is acquired through transplacental transmission of spirochetes in the maternal bloodstream. Occasionally, it occurs at delivery via direct contact with maternal lesions. It is not transmitted in breast milk. Transmission of syphilis:
- Can occur any time during pregnancy.
- Is more likely to occur in women with untreated primary or secondary disease (60%-100%).
- Is approximately 40% in those with early latent syphilis and less than 8% in mothers with late latent syphilis.
- Is higher in women coinfected with HIV since they more frequently receive no prenatal care and their disease is inadequately treated.
Coinfection with syphilis may also increase the rate of mother-to-child transmission of HIV.
Untreated early syphilis during pregnancy results in spontaneous abortion, stillbirth, or perinatal death in up to 40% of cases. Infected newborns with early congenital syphilis can be asymptomatic or have evidence of hepatosplenomegaly, generalized lymphadenopathy, nasal discharge that is occasionally bloody, rash, and skeletal abnormalities (osteochondritis and periostitis). Other manifestations include edema, hemolytic anemia, jaundice, pneumonia, pseudoparalysis, and thrombocytopenia. Asymptomatic infants may have abnormal cerebrospinal fluid findings including elevated CSF white cell count, elevated protein, and a reactive venereal disease research laboratory test.
Late congenital syphilis, defined as the onset of symptoms after 2 years of age is secondary to scarring or persistent inflammation and gumma formation in a variety of tissues. It occurs in up to 40% of cases of untreated maternal disease. Most cases can be prevented by maternal treatment and treatment of the infant within the first 3 months of life. Common clinical manifestations include interstitial keratitis, sensorineural hearing loss, frontal bossing, saddle nose, Hutchinson teeth, mulberry molars, perforation of the hard palate, anterior bowing of the tibia (saber shins), and other skeletal abnormalities.
Diagnostic tests. Maternal diagnosis is dependent upon knowing the results of both a nontreponemal (RPR, VDRL) and a confirmatory treponemal test (TP-PA, TP-EIA, TP-CIA, FTA-ABS,) before or at delivery. TP-PA is the preferred test. When maternal disease is confirmed, the newborn should have the same quantitative nontreponemal test as the mother. A confirmatory treponemal test is not required
Evaluation and treatment. It’s imperative that children born to mothers with a reactive test, regardless of their treatment status, have a thorough exam performed before hospital discharge. The provider must determine what additional interventions should be performed.
The American Academy of Pediatrics and the Centers for Disease Control and Prevention (www.cdc.gov/std/treatment-guidelines/congenital-syphilis.htm) have developed standard algorithms for the diagnostic approach and treatment of infants born to mothers with reactive serologic tests for syphilis. It is available in the Red Book for AAP members (https://publications.aap.org/redbook). Recommendations based on various scenarios for neonates up to 1 month of age include proven or highly probable congenital syphilis, possible congenital syphilis, congenital syphilis less likely, and congenital syphilis unlikely. It is beyond the scope of this article to list the criteria and evaluation for each scenario. The reader is referred to the algorithm.
If syphilis is suspected in infants or children older than 1 month, the challenge is to determine if it is untreated congenital syphilis or acquired syphilis. Maternal syphilis status should be determined. Evaluation for congenital syphilis in this age group includes CSF analysis for VDRL, cell count and protein, CBC with differential and platelets, hepatic panel, abdominal ultrasound, long-bone radiographs, chest radiograph, neuroimaging, auditory brain stem response, and HIV testing.
Let’s go back to your patient. The mother was diagnosed with syphilis during pregnancy. You confirm that she was treated with benzathine penicillin G, and the course was completed at least 4 weeks before delivery. Treatment with any other drug during pregnancy is not appropriate. The RPR has declined, and the infant’s titer is equal to or less than four times the maternal titer. The exam is significant for generalized adenopathy and slightly bloody nasal discharge. This infant has two findings consistent with congenital syphilis regardless of RPR titer or treatment status. This places him in the proven or highly probable congenital syphilis group. Management includes CSF analysis (VDRL, cell count, and protein), CBC with differential and platelet count, and treatment with penicillin G for 10 days. Additional tests as clinically indicated include: long-bone radiograph, chest radiography, aspartate aminotranferase and alanine aminotransferase levels, neuroimaging, ophthalmologic exam, and auditory brain stem response. Despite maternal treatment, this newborn has congenital syphilis. The same nontreponemal test should be obtained every 2-3 months until it is nonreactive. It should be nonreactive by 6 months. If the infection persists to 6-12 months post treatment, reevaluation including CSF analysis and retreatment may be indicated.
Congenital syphilis can be prevented by maternal screening, diagnosis, and treatment. When that fails it is up to us to diagnosis and adequately treat our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Polio in 2022: Some concerns but vaccine still works
Who would have thought we would need to refresh our knowledge on polio virus in 2022? Fate seems cruel to add this concern on the heels of SARS-CoV-2, monkeypox, abnormal seasons for RSV, acute flaccid myelitis (AFM) linked to enteroviruses, and a summer of parechovirus causing infant meningitis. But confirmation that indeed an adult had polio with paralytic disease raises concerns among public health groups and ordinary citizens alike, particularly those who remember polio in its heyday.
History: In the summer of 1952, polio was among the most feared diseases on the planet. Families were advised to not allow children to congregate in groups or use public swimming pools; little league baseball games were being canceled and there was talk of not opening schools for the fall. Every parent’s nightmare seemed to be the nonspecific febrile summer illness that led to paralytic sequelae. TV news included videos of the iron lung wards in hospitals across the country. Medical providers felt powerless, only able to give nonspecific preventive advice. There was no specific antiviral (there still isn’t) and vaccines seemed a long way off.
Then came the news that Dr. Jonas Salk’s group had gotten an inactivated polio vaccine (IPV) approved for general use in 1955. Families were excited to have their children vaccinated. Paralytic polio cases dropped like a rock from approximately 22,000/year in 1952 to approximately 2,200 in 1956. A surge to near 6,000 cases in 1959 led to Dr. Albert Sabin’s oral polio vaccine (OPV), which supplanted IPV in 1961. OPV had the advantages of: 1) Inducing mucosal as well as serum antibodies, 2) more durable responses, and 3) immunity in unvaccinated persons exposed to vaccine virus that had been shed in stools into wastewater and rivers.
By 1964, polio had nearly disappeared. The last wild-type indigenous U.S. case was in 1979. By 1994, all the Americas were declared polio free. Because the only U.S. paralytic polio cases thereafter were foreign imports or were associated with oral vaccine strains (so-called vaccine-associated paralytic polio [VAPP]), OPV was replaced by an enhanced IPV in 2000 to prevent further VAPP.
Polio facts: Polio is asymptomatic in about 70% of infections. Among the 30% with symptoms, paralysis occurs infrequently, with the overall rate of paralytic infections being 0.5% (rate varies by virus type with type 3 having the highest rate).1 Why then was the world so afraid of polio? If every person in a U.S. birth cohort (about 3.7 million) was unvaccinated and became infected with poliovirus, more than 18,000 would get paralytic polio and almost 1,300 would die. Of note, adults have a higher chance of paralytic polio after infection than children.
Concerns in 2022: Persons vaccinated with at least three doses of either IPV or OPV have historically been protected from paralytic polio (99% protection). But are we sure that the United States remains protected against polio after 2 decades of IPV being the only vaccine? Polio could be reintroduced at any time to the United States from countries with reported cases that likely arose because of low vaccination rates related to war, famine, or political upheavals (Malawi, Mozambique, Nigeria, Pakistan, and Afghanistan).2 The proof? The recent confirmed New York case.
International efforts resulted in global eradication of two polio wild-types viruses (type 2 in 2015 and type 3 in 2019). Nevertheless, vaccine-derived, virulent polio virus (VDPV) type 2 and VDPV-3 still circulate in some areas, particularly Africa (VDPV-2) and Israel (VDPV-3). The above-mentioned U.S. case is an unvaccinated adult traveler who went to an area where VDPV-2 circulates and developed disease after returning home.3 So, it was not an indigenous reappearance in the United States and it was not a breakthrough case in a vaccinated person. But it is sobering to realize that all who are unvaccinated remain at risk for paralytic polio in 2022, particularly because vaccination rates declined nearly everywhere during the initial COVID-19 pandemic. We are still catching up, with vaccination rates under 50% in some ZIP codes.4
Are VDPVs circulating in some parts of the United States? Interestingly, wastewater surveillance programs may be the most economical and practical way to perform polio surveillance. Such a program detected polio virus in London wastewater in June 2022.5 New York has recently detected polio in wastewater during testing begun because of the recent case.6
Good news: For paralytic polio, seropositivity at any titer indicates protection, so U.S. serosurveillance data would also be informative. How durable is polio protection in the IPV era? Available data suggest that even though we have used only IPV these past 20 years, seropositivity rates among vaccinees with at least three doses of either IPV or OPV should persist for decades and likely for life. Even before polio became a concern this year, the Centers for Disease Control and Prevention, being proactive, wanted to ensure that the enhanced IPV was producing durable immunity and that persons of all ages remained seropositive to the three polio virus types over 10 years after discontinuing OPV use in 2012.
The CDC collaborated with investigators in Kansas City, Mo., to evaluate titers and seropositivity to all three types in a 2- to 85-year-old otherwise healthy cohort with demographics that mirrored the 2010 census for the Kansas City region, which in turn mirrored the national 2021 census data.7 There were approximately 100 persons in each age cohort, with 200 below age 11 years (the cohort that had received only IPV). Serology was performed at the CDC.
Overall seropositivity rates were high, but lower for type 3 (83.3%) and type 2 (90.7%) than type 1 (94.4%). Of note, most of those seronegative for one or more types were among 2- to 3-year-olds who had not completed their full IPV series, with most seronegative results being against polio types 1 and 3. Further, five, who were confirmed as having received no polio vaccine, were seronegative for all three types. Two with no available vaccine records (over 18 years old) were also seronegative for all three types.
So, regardless of the era in which one got polio vaccine, vaccine protection appears to persist indefinitely after three doses. Even 80-year-olds were still seropositive if they had three doses. We can confidently reassure our patients that the vaccine still works; the persons who need to fear polio in 2022 are those who are not vaccinated or have had fewer than three doses, particularly if they travel to areas of persistent polio. Wild type 1 virus persists in a few countries as does VDPV type 2 and VDPV type 3. Importantly, wild type 2 and wild type 3 (with the lowest seropositivity in 2012 study) have been eliminated globally so the only circulating type 2 and type 3 polio virus is VDPV in a few countries. Travel to these countries warrants review of polio vaccine records and CDC or WHO current recommendations for travelers to those countries.
Dr. Harrison is a professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at pdnews@mdedge.com.
References
1. Poliomyelitis. World Health Organization fact sheet, 2022 Jul 4..
2. Franco-Paredes C et al. Lancet Infect Dis. 2022 Aug 16. doi: 10.1016/S1473-3099(22)00548-5.
3. Link-Gelles R et al. MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33):1065-8.
4. “Polio vaccination rate for 2-year-olds is as low as 37% in parts of N.Y. county where paralysis case was found,” NBC News, Erika Edwards, 2022 Aug 16. 5. Vaccine-derived poliovirus type 2 (VDPV2) detected in environmental samples in London. Polioeradication.org. 2022 Jun 22.
6. “NYSDOH and NYCDOHMH wastewater monitoring identifies polio in New York City and urges unvaccinated New Yorkers to get vaccinated now,” nyc.gov. 2022 Aug 12.
7. Wallace GS et al. Hum Vaccin Immunother. 2017;13(4):776-83.
Who would have thought we would need to refresh our knowledge on polio virus in 2022? Fate seems cruel to add this concern on the heels of SARS-CoV-2, monkeypox, abnormal seasons for RSV, acute flaccid myelitis (AFM) linked to enteroviruses, and a summer of parechovirus causing infant meningitis. But confirmation that indeed an adult had polio with paralytic disease raises concerns among public health groups and ordinary citizens alike, particularly those who remember polio in its heyday.
History: In the summer of 1952, polio was among the most feared diseases on the planet. Families were advised to not allow children to congregate in groups or use public swimming pools; little league baseball games were being canceled and there was talk of not opening schools for the fall. Every parent’s nightmare seemed to be the nonspecific febrile summer illness that led to paralytic sequelae. TV news included videos of the iron lung wards in hospitals across the country. Medical providers felt powerless, only able to give nonspecific preventive advice. There was no specific antiviral (there still isn’t) and vaccines seemed a long way off.
Then came the news that Dr. Jonas Salk’s group had gotten an inactivated polio vaccine (IPV) approved for general use in 1955. Families were excited to have their children vaccinated. Paralytic polio cases dropped like a rock from approximately 22,000/year in 1952 to approximately 2,200 in 1956. A surge to near 6,000 cases in 1959 led to Dr. Albert Sabin’s oral polio vaccine (OPV), which supplanted IPV in 1961. OPV had the advantages of: 1) Inducing mucosal as well as serum antibodies, 2) more durable responses, and 3) immunity in unvaccinated persons exposed to vaccine virus that had been shed in stools into wastewater and rivers.
By 1964, polio had nearly disappeared. The last wild-type indigenous U.S. case was in 1979. By 1994, all the Americas were declared polio free. Because the only U.S. paralytic polio cases thereafter were foreign imports or were associated with oral vaccine strains (so-called vaccine-associated paralytic polio [VAPP]), OPV was replaced by an enhanced IPV in 2000 to prevent further VAPP.
Polio facts: Polio is asymptomatic in about 70% of infections. Among the 30% with symptoms, paralysis occurs infrequently, with the overall rate of paralytic infections being 0.5% (rate varies by virus type with type 3 having the highest rate).1 Why then was the world so afraid of polio? If every person in a U.S. birth cohort (about 3.7 million) was unvaccinated and became infected with poliovirus, more than 18,000 would get paralytic polio and almost 1,300 would die. Of note, adults have a higher chance of paralytic polio after infection than children.
Concerns in 2022: Persons vaccinated with at least three doses of either IPV or OPV have historically been protected from paralytic polio (99% protection). But are we sure that the United States remains protected against polio after 2 decades of IPV being the only vaccine? Polio could be reintroduced at any time to the United States from countries with reported cases that likely arose because of low vaccination rates related to war, famine, or political upheavals (Malawi, Mozambique, Nigeria, Pakistan, and Afghanistan).2 The proof? The recent confirmed New York case.
International efforts resulted in global eradication of two polio wild-types viruses (type 2 in 2015 and type 3 in 2019). Nevertheless, vaccine-derived, virulent polio virus (VDPV) type 2 and VDPV-3 still circulate in some areas, particularly Africa (VDPV-2) and Israel (VDPV-3). The above-mentioned U.S. case is an unvaccinated adult traveler who went to an area where VDPV-2 circulates and developed disease after returning home.3 So, it was not an indigenous reappearance in the United States and it was not a breakthrough case in a vaccinated person. But it is sobering to realize that all who are unvaccinated remain at risk for paralytic polio in 2022, particularly because vaccination rates declined nearly everywhere during the initial COVID-19 pandemic. We are still catching up, with vaccination rates under 50% in some ZIP codes.4
Are VDPVs circulating in some parts of the United States? Interestingly, wastewater surveillance programs may be the most economical and practical way to perform polio surveillance. Such a program detected polio virus in London wastewater in June 2022.5 New York has recently detected polio in wastewater during testing begun because of the recent case.6
Good news: For paralytic polio, seropositivity at any titer indicates protection, so U.S. serosurveillance data would also be informative. How durable is polio protection in the IPV era? Available data suggest that even though we have used only IPV these past 20 years, seropositivity rates among vaccinees with at least three doses of either IPV or OPV should persist for decades and likely for life. Even before polio became a concern this year, the Centers for Disease Control and Prevention, being proactive, wanted to ensure that the enhanced IPV was producing durable immunity and that persons of all ages remained seropositive to the three polio virus types over 10 years after discontinuing OPV use in 2012.
The CDC collaborated with investigators in Kansas City, Mo., to evaluate titers and seropositivity to all three types in a 2- to 85-year-old otherwise healthy cohort with demographics that mirrored the 2010 census for the Kansas City region, which in turn mirrored the national 2021 census data.7 There were approximately 100 persons in each age cohort, with 200 below age 11 years (the cohort that had received only IPV). Serology was performed at the CDC.
Overall seropositivity rates were high, but lower for type 3 (83.3%) and type 2 (90.7%) than type 1 (94.4%). Of note, most of those seronegative for one or more types were among 2- to 3-year-olds who had not completed their full IPV series, with most seronegative results being against polio types 1 and 3. Further, five, who were confirmed as having received no polio vaccine, were seronegative for all three types. Two with no available vaccine records (over 18 years old) were also seronegative for all three types.
So, regardless of the era in which one got polio vaccine, vaccine protection appears to persist indefinitely after three doses. Even 80-year-olds were still seropositive if they had three doses. We can confidently reassure our patients that the vaccine still works; the persons who need to fear polio in 2022 are those who are not vaccinated or have had fewer than three doses, particularly if they travel to areas of persistent polio. Wild type 1 virus persists in a few countries as does VDPV type 2 and VDPV type 3. Importantly, wild type 2 and wild type 3 (with the lowest seropositivity in 2012 study) have been eliminated globally so the only circulating type 2 and type 3 polio virus is VDPV in a few countries. Travel to these countries warrants review of polio vaccine records and CDC or WHO current recommendations for travelers to those countries.
Dr. Harrison is a professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at pdnews@mdedge.com.
References
1. Poliomyelitis. World Health Organization fact sheet, 2022 Jul 4..
2. Franco-Paredes C et al. Lancet Infect Dis. 2022 Aug 16. doi: 10.1016/S1473-3099(22)00548-5.
3. Link-Gelles R et al. MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33):1065-8.
4. “Polio vaccination rate for 2-year-olds is as low as 37% in parts of N.Y. county where paralysis case was found,” NBC News, Erika Edwards, 2022 Aug 16. 5. Vaccine-derived poliovirus type 2 (VDPV2) detected in environmental samples in London. Polioeradication.org. 2022 Jun 22.
6. “NYSDOH and NYCDOHMH wastewater monitoring identifies polio in New York City and urges unvaccinated New Yorkers to get vaccinated now,” nyc.gov. 2022 Aug 12.
7. Wallace GS et al. Hum Vaccin Immunother. 2017;13(4):776-83.
Who would have thought we would need to refresh our knowledge on polio virus in 2022? Fate seems cruel to add this concern on the heels of SARS-CoV-2, monkeypox, abnormal seasons for RSV, acute flaccid myelitis (AFM) linked to enteroviruses, and a summer of parechovirus causing infant meningitis. But confirmation that indeed an adult had polio with paralytic disease raises concerns among public health groups and ordinary citizens alike, particularly those who remember polio in its heyday.
History: In the summer of 1952, polio was among the most feared diseases on the planet. Families were advised to not allow children to congregate in groups or use public swimming pools; little league baseball games were being canceled and there was talk of not opening schools for the fall. Every parent’s nightmare seemed to be the nonspecific febrile summer illness that led to paralytic sequelae. TV news included videos of the iron lung wards in hospitals across the country. Medical providers felt powerless, only able to give nonspecific preventive advice. There was no specific antiviral (there still isn’t) and vaccines seemed a long way off.
Then came the news that Dr. Jonas Salk’s group had gotten an inactivated polio vaccine (IPV) approved for general use in 1955. Families were excited to have their children vaccinated. Paralytic polio cases dropped like a rock from approximately 22,000/year in 1952 to approximately 2,200 in 1956. A surge to near 6,000 cases in 1959 led to Dr. Albert Sabin’s oral polio vaccine (OPV), which supplanted IPV in 1961. OPV had the advantages of: 1) Inducing mucosal as well as serum antibodies, 2) more durable responses, and 3) immunity in unvaccinated persons exposed to vaccine virus that had been shed in stools into wastewater and rivers.
By 1964, polio had nearly disappeared. The last wild-type indigenous U.S. case was in 1979. By 1994, all the Americas were declared polio free. Because the only U.S. paralytic polio cases thereafter were foreign imports or were associated with oral vaccine strains (so-called vaccine-associated paralytic polio [VAPP]), OPV was replaced by an enhanced IPV in 2000 to prevent further VAPP.
Polio facts: Polio is asymptomatic in about 70% of infections. Among the 30% with symptoms, paralysis occurs infrequently, with the overall rate of paralytic infections being 0.5% (rate varies by virus type with type 3 having the highest rate).1 Why then was the world so afraid of polio? If every person in a U.S. birth cohort (about 3.7 million) was unvaccinated and became infected with poliovirus, more than 18,000 would get paralytic polio and almost 1,300 would die. Of note, adults have a higher chance of paralytic polio after infection than children.
Concerns in 2022: Persons vaccinated with at least three doses of either IPV or OPV have historically been protected from paralytic polio (99% protection). But are we sure that the United States remains protected against polio after 2 decades of IPV being the only vaccine? Polio could be reintroduced at any time to the United States from countries with reported cases that likely arose because of low vaccination rates related to war, famine, or political upheavals (Malawi, Mozambique, Nigeria, Pakistan, and Afghanistan).2 The proof? The recent confirmed New York case.
International efforts resulted in global eradication of two polio wild-types viruses (type 2 in 2015 and type 3 in 2019). Nevertheless, vaccine-derived, virulent polio virus (VDPV) type 2 and VDPV-3 still circulate in some areas, particularly Africa (VDPV-2) and Israel (VDPV-3). The above-mentioned U.S. case is an unvaccinated adult traveler who went to an area where VDPV-2 circulates and developed disease after returning home.3 So, it was not an indigenous reappearance in the United States and it was not a breakthrough case in a vaccinated person. But it is sobering to realize that all who are unvaccinated remain at risk for paralytic polio in 2022, particularly because vaccination rates declined nearly everywhere during the initial COVID-19 pandemic. We are still catching up, with vaccination rates under 50% in some ZIP codes.4
Are VDPVs circulating in some parts of the United States? Interestingly, wastewater surveillance programs may be the most economical and practical way to perform polio surveillance. Such a program detected polio virus in London wastewater in June 2022.5 New York has recently detected polio in wastewater during testing begun because of the recent case.6
Good news: For paralytic polio, seropositivity at any titer indicates protection, so U.S. serosurveillance data would also be informative. How durable is polio protection in the IPV era? Available data suggest that even though we have used only IPV these past 20 years, seropositivity rates among vaccinees with at least three doses of either IPV or OPV should persist for decades and likely for life. Even before polio became a concern this year, the Centers for Disease Control and Prevention, being proactive, wanted to ensure that the enhanced IPV was producing durable immunity and that persons of all ages remained seropositive to the three polio virus types over 10 years after discontinuing OPV use in 2012.
The CDC collaborated with investigators in Kansas City, Mo., to evaluate titers and seropositivity to all three types in a 2- to 85-year-old otherwise healthy cohort with demographics that mirrored the 2010 census for the Kansas City region, which in turn mirrored the national 2021 census data.7 There were approximately 100 persons in each age cohort, with 200 below age 11 years (the cohort that had received only IPV). Serology was performed at the CDC.
Overall seropositivity rates were high, but lower for type 3 (83.3%) and type 2 (90.7%) than type 1 (94.4%). Of note, most of those seronegative for one or more types were among 2- to 3-year-olds who had not completed their full IPV series, with most seronegative results being against polio types 1 and 3. Further, five, who were confirmed as having received no polio vaccine, were seronegative for all three types. Two with no available vaccine records (over 18 years old) were also seronegative for all three types.
So, regardless of the era in which one got polio vaccine, vaccine protection appears to persist indefinitely after three doses. Even 80-year-olds were still seropositive if they had three doses. We can confidently reassure our patients that the vaccine still works; the persons who need to fear polio in 2022 are those who are not vaccinated or have had fewer than three doses, particularly if they travel to areas of persistent polio. Wild type 1 virus persists in a few countries as does VDPV type 2 and VDPV type 3. Importantly, wild type 2 and wild type 3 (with the lowest seropositivity in 2012 study) have been eliminated globally so the only circulating type 2 and type 3 polio virus is VDPV in a few countries. Travel to these countries warrants review of polio vaccine records and CDC or WHO current recommendations for travelers to those countries.
Dr. Harrison is a professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at pdnews@mdedge.com.
References
1. Poliomyelitis. World Health Organization fact sheet, 2022 Jul 4..
2. Franco-Paredes C et al. Lancet Infect Dis. 2022 Aug 16. doi: 10.1016/S1473-3099(22)00548-5.
3. Link-Gelles R et al. MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33):1065-8.
4. “Polio vaccination rate for 2-year-olds is as low as 37% in parts of N.Y. county where paralysis case was found,” NBC News, Erika Edwards, 2022 Aug 16. 5. Vaccine-derived poliovirus type 2 (VDPV2) detected in environmental samples in London. Polioeradication.org. 2022 Jun 22.
6. “NYSDOH and NYCDOHMH wastewater monitoring identifies polio in New York City and urges unvaccinated New Yorkers to get vaccinated now,” nyc.gov. 2022 Aug 12.
7. Wallace GS et al. Hum Vaccin Immunother. 2017;13(4):776-83.
Acute otitis media pneumococcal disease burden in children due to serotypes not included in vaccines
My group in Rochester, N.Y., examined the current pneumococcal serotypes causing AOM in children. From our data, we can determine the PCV13 vaccine types that escape prevention and cause AOM and understand what effect to expect from the new pneumococcal conjugate vaccines (PCVs) that will be coming soon. There are limited data from middle ear fluid (MEF) cultures on which to base such analyses. Tympanocentesis is the preferred method for securing MEF for culture and our group is unique in providing such data to the Centers for Disease Control and publishing our results on a periodic basis to inform clinicians.
Pneumococci are the second most common cause of acute otitis media (AOM) since the introduction of pneumococcal conjugate vaccines (PCVs) more than 2 decades ago.1,2 Pneumococcal AOM causes more severe acute disease and more often causes suppurative complications than Haemophilus influenzae, which is the most common cause of AOM. Prevention of pneumococcal AOM will be a highly relevant contributor to cost-effectiveness analyses for the anticipated introduction of PCV15 (Merck) and PCV20 (Pfizer). Both PCV15 and PCV20 have been licensed for adult use; PCV15 licensure for infants and children occurred in June 2022 for invasive pneumococcal disease and is anticipated in the near future for PCV20. They are improvements over PCV13 because they add serotypes that cause invasive pneumococcal diseases, although less so for prevention of AOM, on the basis of our data.
Nasopharyngeal colonization is a necessary pathogenic step in progression to pneumococcal disease. However, not all strains of pneumococci expressing different capsular serotypes are equally virulent and likely to cause disease. In PCV-vaccinated populations, vaccine pressure and antibiotic resistance drive PCV serotype replacement with nonvaccine serotypes (NVTs), gradually reducing the net effectiveness of the vaccines. Therefore, knowledge of prevalent NVTs colonizing the nasopharynx identifies future pneumococcal serotypes most likely to emerge as pathogenic.
We published an effectiveness study of PCV13.3 A relative reduction of 86% in AOM caused by strains expressing PCV13 serotypes was observed in the first few years after PCV13 introduction. The greatest reduction in MEF samples was in serotype 19A, with a relative reduction of 91%. However, over time the vaccine type efficacy of PCV13 against MEF-positive pneumococcal AOM has eroded. There was no clear efficacy against serotype 3, and we still observed cases of serotype 19A and 19F. PCV13 vaccine failures have been even more frequent in Europe (nearly 30% of pneumococcal AOM in Europe is caused by vaccine serotypes) than our data indicate, where about 10% of AOM is caused by PCV13 serotypes.
In our most recent publication covering 2015-2019, we described results from 589 children, aged 6-36 months, from whom we collected 2,042 nasopharyngeal samples.2,4 During AOM, 495 MEF samples from 319 AOM-infected children were collected (during bilateral infections, tympanocentesis was performed in both ears). Whether bacteria were isolated was based per AOM case, not per tap. The average age of children with AOM was 15 months (range 6-31 months). The three most prevalent nasopharyngeal pneumococcal serotypes were 35B, 23B, and 15B/C. Serotype 35B was the most common at AOM visits in both the nasopharynx and MEF samples followed by serotype 15B/C. Nonsusceptibility among pneumococci to penicillin, azithromycin, and multiple other antibiotics was high. Increasing resistance to ceftriaxone was also observed.
Based on our results, if PCV15 (PCV13 + 22F and 33F) effectiveness is identical to PCV13 for the included serotypes and 100% efficacy for the added serotypes is presumed, PCV15 will reduce pneumococcal AOMs by 8%, pneumococcal nasopharyngeal colonization events at onset of AOM by 6%, and pneumococcal nasopharyngeal colonization events during health by 3%. As for the projected reductions brought about by PCV20 (PCV15 + 8, 10A, 11A, 12F, and 15B), presuming serotype 15B is efficacious against serotype 15C and 100% efficacy for the added serotypes, PCV20 will reduce pneumococcal AOMs by 22%, pneumococcal nasopharyngeal colonization events at onset of AOM by 20%, and pneumococcal nasopharyngeal colonization events during health by 3% (Figure).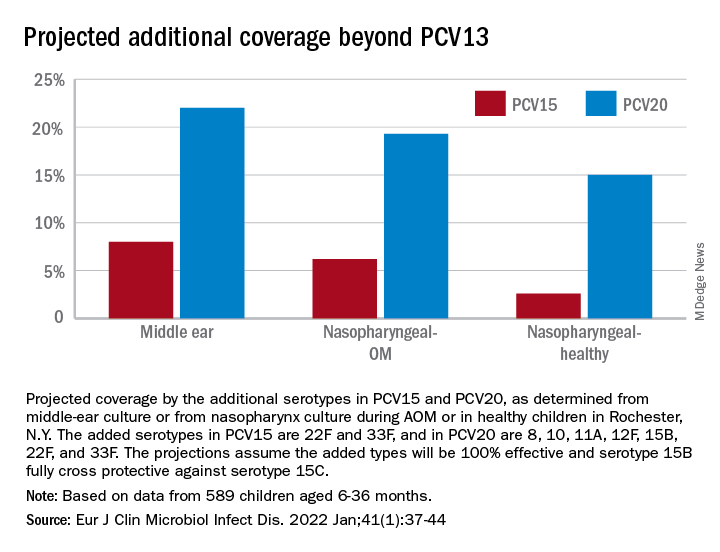
The CDC estimated that, in 2004, pneumococcal disease in the United States caused 4 million illness episodes, 22,000 deaths, 445,000 hospitalizations, 774,000 emergency department visits, 5 million outpatient visits, and 4.1 million outpatient antibiotic prescriptions. Direct medical costs totaled $3.5 billion. Pneumonia (866,000 cases) accounted for 22% of all cases and 72% of pneumococcal costs. AOM and sinusitis (1.5 million cases each) composed 75% of cases and 16% of direct medical costs.5 However, if indirect costs are taken into account, such as work loss by parents of young children, the cost of pneumococcal disease caused by AOM alone may exceed $6 billion annually6 and become dominant in the cost-effectiveness analysis in high-income countries.
Despite widespread use of PCV13, Pneumococcus has shown its resilience under vaccine pressure such that the organism remains a very common AOM pathogen. All-cause AOM has declined modestly and pneumococcal AOM caused by the specific serotypes in PCVs has declined dramatically since the introduction of PCVs. However, the burden of pneumococcal AOM disease is still considerable.
The notion that strains expressing serotypes that were not included in PCV7 were less virulent was proven wrong within a few years after introduction of PCV7, with the emergence of strains expressing serotype 19A, and others. The same cycle occurred after introduction of PCV13. It appears to take about 4 years after introduction of a PCV before peak effectiveness is achieved – which then begins to erode with emergence of NVTs. First, the NVTs are observed to colonize the nasopharynx as commensals and then from among those strains new disease-causing strains emerge.
At the most recent meeting of the International Society of Pneumococci and Pneumococcal Diseases in Toronto in June, many presentations focused on the fact that PCVs elicit highly effective protective serotype-specific antibodies to the capsular polysaccharides of included types. However, 100 serotypes are known. The limitations of PCVs are becoming increasingly apparent. They are costly and consume a large portion of the Vaccines for Children budget. Children in the developing world remain largely unvaccinated because of the high cost. NVTs that have emerged to cause disease vary by country, vary by adult vs. pediatric populations, and are dynamically changing year to year. Forthcoming PCVs of 15 and 20 serotypes will be even more costly than PCV13, will not include many newly emerged serotypes, and will probably likewise encounter “serotype replacement” because of high immune evasion by pneumococci.
When Merck and Pfizer made their decisions on serotype composition for PCV15 and PCV20, respectively, they were based on available data at the time regarding predominant serotypes causing invasive pneumococcal disease in countries that had the best data and would be the market for their products. However, from the time of the decision to licensure of vaccine is many years, and during that time the pneumococcal serotypes have changed, more so for AOM, and I predict more change will occur in the future.
In the past 3 years, Dr. Pichichero has received honoraria from Merck to attend 1-day consulting meetings and his institution has received investigator-initiated research grants to study aspects of PCV15. In the past 3 years, he was reimbursed for expenses to attend the ISPPD meeting in Toronto to present a poster on potential efficacy of PCV20 to prevent complicated AOM.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital.
References
1. Kaur R et al. Pediatrics. 2017;140(3).
2. Kaur R et al. Eur J Clin Microbiol Infect Dis. 2021;41:37-44..
3. Pichichero M et al. Lancet Child Adolesc Health. 2018;2(8):561-8.
4. Zhou F et al. Pediatrics. 2008;121(2):253-60.
5. Huang SS et al. Vaccine. 2011;29(18):3398-412.
6. Casey JR and Pichichero ME. Clin Pediatr (Phila). 2014;53(9):865-73. .
My group in Rochester, N.Y., examined the current pneumococcal serotypes causing AOM in children. From our data, we can determine the PCV13 vaccine types that escape prevention and cause AOM and understand what effect to expect from the new pneumococcal conjugate vaccines (PCVs) that will be coming soon. There are limited data from middle ear fluid (MEF) cultures on which to base such analyses. Tympanocentesis is the preferred method for securing MEF for culture and our group is unique in providing such data to the Centers for Disease Control and publishing our results on a periodic basis to inform clinicians.
Pneumococci are the second most common cause of acute otitis media (AOM) since the introduction of pneumococcal conjugate vaccines (PCVs) more than 2 decades ago.1,2 Pneumococcal AOM causes more severe acute disease and more often causes suppurative complications than Haemophilus influenzae, which is the most common cause of AOM. Prevention of pneumococcal AOM will be a highly relevant contributor to cost-effectiveness analyses for the anticipated introduction of PCV15 (Merck) and PCV20 (Pfizer). Both PCV15 and PCV20 have been licensed for adult use; PCV15 licensure for infants and children occurred in June 2022 for invasive pneumococcal disease and is anticipated in the near future for PCV20. They are improvements over PCV13 because they add serotypes that cause invasive pneumococcal diseases, although less so for prevention of AOM, on the basis of our data.
Nasopharyngeal colonization is a necessary pathogenic step in progression to pneumococcal disease. However, not all strains of pneumococci expressing different capsular serotypes are equally virulent and likely to cause disease. In PCV-vaccinated populations, vaccine pressure and antibiotic resistance drive PCV serotype replacement with nonvaccine serotypes (NVTs), gradually reducing the net effectiveness of the vaccines. Therefore, knowledge of prevalent NVTs colonizing the nasopharynx identifies future pneumococcal serotypes most likely to emerge as pathogenic.
We published an effectiveness study of PCV13.3 A relative reduction of 86% in AOM caused by strains expressing PCV13 serotypes was observed in the first few years after PCV13 introduction. The greatest reduction in MEF samples was in serotype 19A, with a relative reduction of 91%. However, over time the vaccine type efficacy of PCV13 against MEF-positive pneumococcal AOM has eroded. There was no clear efficacy against serotype 3, and we still observed cases of serotype 19A and 19F. PCV13 vaccine failures have been even more frequent in Europe (nearly 30% of pneumococcal AOM in Europe is caused by vaccine serotypes) than our data indicate, where about 10% of AOM is caused by PCV13 serotypes.
In our most recent publication covering 2015-2019, we described results from 589 children, aged 6-36 months, from whom we collected 2,042 nasopharyngeal samples.2,4 During AOM, 495 MEF samples from 319 AOM-infected children were collected (during bilateral infections, tympanocentesis was performed in both ears). Whether bacteria were isolated was based per AOM case, not per tap. The average age of children with AOM was 15 months (range 6-31 months). The three most prevalent nasopharyngeal pneumococcal serotypes were 35B, 23B, and 15B/C. Serotype 35B was the most common at AOM visits in both the nasopharynx and MEF samples followed by serotype 15B/C. Nonsusceptibility among pneumococci to penicillin, azithromycin, and multiple other antibiotics was high. Increasing resistance to ceftriaxone was also observed.
Based on our results, if PCV15 (PCV13 + 22F and 33F) effectiveness is identical to PCV13 for the included serotypes and 100% efficacy for the added serotypes is presumed, PCV15 will reduce pneumococcal AOMs by 8%, pneumococcal nasopharyngeal colonization events at onset of AOM by 6%, and pneumococcal nasopharyngeal colonization events during health by 3%. As for the projected reductions brought about by PCV20 (PCV15 + 8, 10A, 11A, 12F, and 15B), presuming serotype 15B is efficacious against serotype 15C and 100% efficacy for the added serotypes, PCV20 will reduce pneumococcal AOMs by 22%, pneumococcal nasopharyngeal colonization events at onset of AOM by 20%, and pneumococcal nasopharyngeal colonization events during health by 3% (Figure).
The CDC estimated that, in 2004, pneumococcal disease in the United States caused 4 million illness episodes, 22,000 deaths, 445,000 hospitalizations, 774,000 emergency department visits, 5 million outpatient visits, and 4.1 million outpatient antibiotic prescriptions. Direct medical costs totaled $3.5 billion. Pneumonia (866,000 cases) accounted for 22% of all cases and 72% of pneumococcal costs. AOM and sinusitis (1.5 million cases each) composed 75% of cases and 16% of direct medical costs.5 However, if indirect costs are taken into account, such as work loss by parents of young children, the cost of pneumococcal disease caused by AOM alone may exceed $6 billion annually6 and become dominant in the cost-effectiveness analysis in high-income countries.
Despite widespread use of PCV13, Pneumococcus has shown its resilience under vaccine pressure such that the organism remains a very common AOM pathogen. All-cause AOM has declined modestly and pneumococcal AOM caused by the specific serotypes in PCVs has declined dramatically since the introduction of PCVs. However, the burden of pneumococcal AOM disease is still considerable.
The notion that strains expressing serotypes that were not included in PCV7 were less virulent was proven wrong within a few years after introduction of PCV7, with the emergence of strains expressing serotype 19A, and others. The same cycle occurred after introduction of PCV13. It appears to take about 4 years after introduction of a PCV before peak effectiveness is achieved – which then begins to erode with emergence of NVTs. First, the NVTs are observed to colonize the nasopharynx as commensals and then from among those strains new disease-causing strains emerge.
At the most recent meeting of the International Society of Pneumococci and Pneumococcal Diseases in Toronto in June, many presentations focused on the fact that PCVs elicit highly effective protective serotype-specific antibodies to the capsular polysaccharides of included types. However, 100 serotypes are known. The limitations of PCVs are becoming increasingly apparent. They are costly and consume a large portion of the Vaccines for Children budget. Children in the developing world remain largely unvaccinated because of the high cost. NVTs that have emerged to cause disease vary by country, vary by adult vs. pediatric populations, and are dynamically changing year to year. Forthcoming PCVs of 15 and 20 serotypes will be even more costly than PCV13, will not include many newly emerged serotypes, and will probably likewise encounter “serotype replacement” because of high immune evasion by pneumococci.
When Merck and Pfizer made their decisions on serotype composition for PCV15 and PCV20, respectively, they were based on available data at the time regarding predominant serotypes causing invasive pneumococcal disease in countries that had the best data and would be the market for their products. However, from the time of the decision to licensure of vaccine is many years, and during that time the pneumococcal serotypes have changed, more so for AOM, and I predict more change will occur in the future.
In the past 3 years, Dr. Pichichero has received honoraria from Merck to attend 1-day consulting meetings and his institution has received investigator-initiated research grants to study aspects of PCV15. In the past 3 years, he was reimbursed for expenses to attend the ISPPD meeting in Toronto to present a poster on potential efficacy of PCV20 to prevent complicated AOM.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital.
References
1. Kaur R et al. Pediatrics. 2017;140(3).
2. Kaur R et al. Eur J Clin Microbiol Infect Dis. 2021;41:37-44..
3. Pichichero M et al. Lancet Child Adolesc Health. 2018;2(8):561-8.
4. Zhou F et al. Pediatrics. 2008;121(2):253-60.
5. Huang SS et al. Vaccine. 2011;29(18):3398-412.
6. Casey JR and Pichichero ME. Clin Pediatr (Phila). 2014;53(9):865-73. .
My group in Rochester, N.Y., examined the current pneumococcal serotypes causing AOM in children. From our data, we can determine the PCV13 vaccine types that escape prevention and cause AOM and understand what effect to expect from the new pneumococcal conjugate vaccines (PCVs) that will be coming soon. There are limited data from middle ear fluid (MEF) cultures on which to base such analyses. Tympanocentesis is the preferred method for securing MEF for culture and our group is unique in providing such data to the Centers for Disease Control and publishing our results on a periodic basis to inform clinicians.
Pneumococci are the second most common cause of acute otitis media (AOM) since the introduction of pneumococcal conjugate vaccines (PCVs) more than 2 decades ago.1,2 Pneumococcal AOM causes more severe acute disease and more often causes suppurative complications than Haemophilus influenzae, which is the most common cause of AOM. Prevention of pneumococcal AOM will be a highly relevant contributor to cost-effectiveness analyses for the anticipated introduction of PCV15 (Merck) and PCV20 (Pfizer). Both PCV15 and PCV20 have been licensed for adult use; PCV15 licensure for infants and children occurred in June 2022 for invasive pneumococcal disease and is anticipated in the near future for PCV20. They are improvements over PCV13 because they add serotypes that cause invasive pneumococcal diseases, although less so for prevention of AOM, on the basis of our data.
Nasopharyngeal colonization is a necessary pathogenic step in progression to pneumococcal disease. However, not all strains of pneumococci expressing different capsular serotypes are equally virulent and likely to cause disease. In PCV-vaccinated populations, vaccine pressure and antibiotic resistance drive PCV serotype replacement with nonvaccine serotypes (NVTs), gradually reducing the net effectiveness of the vaccines. Therefore, knowledge of prevalent NVTs colonizing the nasopharynx identifies future pneumococcal serotypes most likely to emerge as pathogenic.
We published an effectiveness study of PCV13.3 A relative reduction of 86% in AOM caused by strains expressing PCV13 serotypes was observed in the first few years after PCV13 introduction. The greatest reduction in MEF samples was in serotype 19A, with a relative reduction of 91%. However, over time the vaccine type efficacy of PCV13 against MEF-positive pneumococcal AOM has eroded. There was no clear efficacy against serotype 3, and we still observed cases of serotype 19A and 19F. PCV13 vaccine failures have been even more frequent in Europe (nearly 30% of pneumococcal AOM in Europe is caused by vaccine serotypes) than our data indicate, where about 10% of AOM is caused by PCV13 serotypes.
In our most recent publication covering 2015-2019, we described results from 589 children, aged 6-36 months, from whom we collected 2,042 nasopharyngeal samples.2,4 During AOM, 495 MEF samples from 319 AOM-infected children were collected (during bilateral infections, tympanocentesis was performed in both ears). Whether bacteria were isolated was based per AOM case, not per tap. The average age of children with AOM was 15 months (range 6-31 months). The three most prevalent nasopharyngeal pneumococcal serotypes were 35B, 23B, and 15B/C. Serotype 35B was the most common at AOM visits in both the nasopharynx and MEF samples followed by serotype 15B/C. Nonsusceptibility among pneumococci to penicillin, azithromycin, and multiple other antibiotics was high. Increasing resistance to ceftriaxone was also observed.
Based on our results, if PCV15 (PCV13 + 22F and 33F) effectiveness is identical to PCV13 for the included serotypes and 100% efficacy for the added serotypes is presumed, PCV15 will reduce pneumococcal AOMs by 8%, pneumococcal nasopharyngeal colonization events at onset of AOM by 6%, and pneumococcal nasopharyngeal colonization events during health by 3%. As for the projected reductions brought about by PCV20 (PCV15 + 8, 10A, 11A, 12F, and 15B), presuming serotype 15B is efficacious against serotype 15C and 100% efficacy for the added serotypes, PCV20 will reduce pneumococcal AOMs by 22%, pneumococcal nasopharyngeal colonization events at onset of AOM by 20%, and pneumococcal nasopharyngeal colonization events during health by 3% (Figure).
The CDC estimated that, in 2004, pneumococcal disease in the United States caused 4 million illness episodes, 22,000 deaths, 445,000 hospitalizations, 774,000 emergency department visits, 5 million outpatient visits, and 4.1 million outpatient antibiotic prescriptions. Direct medical costs totaled $3.5 billion. Pneumonia (866,000 cases) accounted for 22% of all cases and 72% of pneumococcal costs. AOM and sinusitis (1.5 million cases each) composed 75% of cases and 16% of direct medical costs.5 However, if indirect costs are taken into account, such as work loss by parents of young children, the cost of pneumococcal disease caused by AOM alone may exceed $6 billion annually6 and become dominant in the cost-effectiveness analysis in high-income countries.
Despite widespread use of PCV13, Pneumococcus has shown its resilience under vaccine pressure such that the organism remains a very common AOM pathogen. All-cause AOM has declined modestly and pneumococcal AOM caused by the specific serotypes in PCVs has declined dramatically since the introduction of PCVs. However, the burden of pneumococcal AOM disease is still considerable.
The notion that strains expressing serotypes that were not included in PCV7 were less virulent was proven wrong within a few years after introduction of PCV7, with the emergence of strains expressing serotype 19A, and others. The same cycle occurred after introduction of PCV13. It appears to take about 4 years after introduction of a PCV before peak effectiveness is achieved – which then begins to erode with emergence of NVTs. First, the NVTs are observed to colonize the nasopharynx as commensals and then from among those strains new disease-causing strains emerge.
At the most recent meeting of the International Society of Pneumococci and Pneumococcal Diseases in Toronto in June, many presentations focused on the fact that PCVs elicit highly effective protective serotype-specific antibodies to the capsular polysaccharides of included types. However, 100 serotypes are known. The limitations of PCVs are becoming increasingly apparent. They are costly and consume a large portion of the Vaccines for Children budget. Children in the developing world remain largely unvaccinated because of the high cost. NVTs that have emerged to cause disease vary by country, vary by adult vs. pediatric populations, and are dynamically changing year to year. Forthcoming PCVs of 15 and 20 serotypes will be even more costly than PCV13, will not include many newly emerged serotypes, and will probably likewise encounter “serotype replacement” because of high immune evasion by pneumococci.
When Merck and Pfizer made their decisions on serotype composition for PCV15 and PCV20, respectively, they were based on available data at the time regarding predominant serotypes causing invasive pneumococcal disease in countries that had the best data and would be the market for their products. However, from the time of the decision to licensure of vaccine is many years, and during that time the pneumococcal serotypes have changed, more so for AOM, and I predict more change will occur in the future.
In the past 3 years, Dr. Pichichero has received honoraria from Merck to attend 1-day consulting meetings and his institution has received investigator-initiated research grants to study aspects of PCV15. In the past 3 years, he was reimbursed for expenses to attend the ISPPD meeting in Toronto to present a poster on potential efficacy of PCV20 to prevent complicated AOM.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital.
References
1. Kaur R et al. Pediatrics. 2017;140(3).
2. Kaur R et al. Eur J Clin Microbiol Infect Dis. 2021;41:37-44..
3. Pichichero M et al. Lancet Child Adolesc Health. 2018;2(8):561-8.
4. Zhou F et al. Pediatrics. 2008;121(2):253-60.
5. Huang SS et al. Vaccine. 2011;29(18):3398-412.
6. Casey JR and Pichichero ME. Clin Pediatr (Phila). 2014;53(9):865-73. .
To vaccinate 6-month- to 5-year-olds against SARS-CoV-2 or not to vaccinate
A family’s decision to vaccinate their child is best made jointly with a trusted medical provider who knows the child and family. The American Academy of Pediatrics created a toolkit with resources for answering questions about the recently authorized SARS-CoV-2 mRNA vaccines (Pfizer and Moderna) for 6-month- to 5-year-olds with science-backed vaccine facts, including links to other useful AAP information websites, talking points, graphics, and videos.1
SARS-CoV-2 seasonality
SARS-CoV-2 is now endemic, not a once-a-year seasonal virus. Seasons (aka surges) will occur whenever a new variant arises (twice yearly since 2020, Omicron BA.4/BA.5 currently), or when enough vaccine holdouts, newborns, and/or those with waning of prior immunity (vaccine or infection induced) accrue.
Emergency use authorization submission data for mRNA vaccine responses in young children2,3
Moderna in 6-month- through 5-year-olds. Two 25-mcg doses given 4-8 weeks apart produced 37.8% (95% confidence interval, 20.9%-51.1%) protection against symptomatic Omicron SARS-CoV-2 infections through 3 months of follow-up. Immunobridging analysis of antibody responses compared to 18- to 25-year-olds (100-mcg doses) showed the children’s responses were noninferior. Thus, the committee inferred that vaccine effectiveness in children should be similar to that in 18- to 25-year-olds. Fever, irritability, or local reaction/pain occurred in two-thirds after the second dose. Grade 3 reactions were noted in less than 5%.
Pfizer in 6-month- through 4-year-olds. Three 3-mcg doses, two doses 3-8 weeks apart and the third dose at least 8 weeks later (median 16 weeks), produced 80.3% (95% CI, 13.9%-96.7%) protection against symptomatic COVID-19 during the 6 weeks after the third dose. Local and systemic reactions occurred in 63.8%; less than 5% had grade 3 reactions (fever in about 3%, irritability in 1.3%, fatigue in 0.8%) mostly after second dose.
Neither duration of follow-up is very long. The Moderna data tell me that a third primary dose would have been better but restarting the trial to evaluate third doses would have delayed Moderna’s EUA another 4-6 months. The three-dose Pfizer data look better but may not have been as good with another 6 weeks of follow-up.
Additional post-EUA data will be collected. Boosters will be needed when immunity from both vaccines wanes (one estimate is about 6 months after the primary series). The Advisory Committee on Immunization Practices noted in their deliberations that vaccine-induced antibody responses are higher and cross-neutralize variants (even Omicron) better than infection-induced immunity.4
Are there downsides to the vaccines? Naysayers question vaccinating children less than 5 years old with reasons containing enough “truth” that they catch people’s attention, for example, “young children don’t get very sick with COVID-19,” “most have been infected already,” “RNA for the spike protein stays in the body for months,” or “myocarditis.” Naysayers can quote references in reputable journals but seem to spin selected data out of context or quote unconfirmed data from the Vaccine Adverse Event Reporting System.
Reasons to vaccinate
- While children have milder disease than adults, mid-June 2022 surveillance indicated 50 hospitalizations and 1 pediatric death each day from SARS-CoV-2.5
- Vaccinating young children endows a foundation of vaccine-induced SARS-CoV-2 immunity that is superior to infection-induced immunity.4
- Long-term effects of large numbers of SARS-CoV-2 particles that enter every organ of a developing child have not been determined.
- Viral loads are lowered by prior vaccine; fewer viral replications lessen chances for newer variants to arise.
- Transmission is less in breakthrough infections than infections in the unvaccinated.
- Thirty percent of 5- to 11-year-olds hospitalized for SARS-CoV-2 had no underlying conditions;6 hospitalization rates in newborn to 4-year-olds have been the highest in the Omicron surge.7
- No myocarditis or pericarditis episodes have been detected in 6-month- to 11-year-old trials.
- The AAP and ACIP recommend the mRNA vaccines.
My thoughts are that SARS-CoV-2 vaccine is just another “routine” childhood vaccine that prepares children for healthier futures, pandemic or not, and the vaccines are as safe as other routine vaccines.
And like other pediatric vaccines, it should be no surprise that boosters will be needed, even if no newer variants than Omicron BA.4/BA.5 arise. But we know newer variants will arise and, similar to influenza vaccine, new formulations, perhaps with multiple SARS-CoV-2 strain antigens, will be needed every year or so. Everyone will get SARS-CoV-2 multiple times in their lives no matter how careful they are. So isn’t it good medical practice to establish early the best available foundation for maintaining lifelong SARS-CoV-2 immunity?
To me it is like pertussis. Most pertussis-infected children are sick enough to be hospitalized; very few die. They are miserable with illnesses that take weeks to months to subside. The worst disease usually occurs in unvaccinated young children or those with underlying conditions. Reactogenicity was reduced with acellular vaccine but resulted in less immunogenicity, so we give boosters at intervals that best match waning immunity. Circulating strains can be different than the vaccine strain, so protection against infection is 80%. Finally, even the safest vaccine may very rarely have sequelae. That is why The National Vaccine Injury Compensation Program was created. Yet the benefit-to-harm ratio for children and society favors universal pertussis vaccine use. And we vaccinate even those who have had pertussis because even infection-based immunity is incomplete and protection wanes. If arguments similar to those by SARS-CoV-2 vaccine naysayers were applied to acellular pertussis vaccine, it seems they would argue against pertussis vaccine for young children.
Another major issue has been “safety concerns” about the vaccines’ small amount of mRNA for the spike protein encased in microscopic lipid bubbles injected in the arm or leg. This mRNA is picked up by human cells, and in the cytoplasm (not the nucleus where our DNA resides) produces a limited supply of spike protein that is then picked up by antigen-presenting cells for short-lived distribution (days to 2 weeks at most) to regional lymph nodes where immune-memory processes are jump-started. Contrast that to even asymptomatic SARS-CoV-2 infection where multibillions of virus particles are produced for up to 14 days with access to every bodily organ that contains ACE-2 receptors (they all do). Each virus particle hijacks a human cell producing thousands of mRNA for spike protein (and multiple other SARS-CoV-2 proteins), eventually releasing multibillions of lipid fragments from the ruptured cell. Comparing the amount of these components in the mRNA vaccines to those from infection is like comparing a campfire to the many-thousand-acre wildfire. So, if one is worried about the effects of spike protein and lipid fragments, the limited localized amounts in mRNA vaccines should make one much less concerned than the enormous amounts circulating throughout the body as a result of a SARS-CoV-2 infection.
My take is that children 6-months to 5-years-old deserve SARS-CoV-2–induced vaccine protection and we can and should strongly recommend it as medical providers and child advocates.
*Dr. Harrison is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at pdnews@mdedge.com.
References
1. AAP. 2022 Jun 21. As COVID-19 vaccines become available for children ages 6 months to 4 years, AAP urges families to reach out to pediatricians to ask questions and access vaccine. www.aap.org.
2. CDC. Grading of recommendations, assessment, development, and evaluation (GRADE): Moderna COVID-19 vaccine for children aged 6 months–5 years. www.cdc.gov.
3. CDC. ACIP evidence to recommendations for use of Moderna COVID-19 vaccine in children ages 6 months–5 years and Pfizer-BioNTech COVID-19 vaccine in children ages 6 months–4 years under an emergency use authorization. www.cdc.gov.
4. Tang J et al. Nat Commun. 2022;13:2979.
5. Children and COVID-19: State Data Report. 2022 Jun 30. www.aap.org.
6. Shi DS et al. MMWR Morb Mortal Wkly Rep. 2022;71:574-81.
7. Marks KJ et al. MMWR Morb Mortal Wkly Rep. 2022;71:429-36.
Other good resources for families are https://getvaccineanswers.org/ or www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-in-babies-and-children/art-20484405.
*This story was updated on July 19, 2022.
A family’s decision to vaccinate their child is best made jointly with a trusted medical provider who knows the child and family. The American Academy of Pediatrics created a toolkit with resources for answering questions about the recently authorized SARS-CoV-2 mRNA vaccines (Pfizer and Moderna) for 6-month- to 5-year-olds with science-backed vaccine facts, including links to other useful AAP information websites, talking points, graphics, and videos.1
SARS-CoV-2 seasonality
SARS-CoV-2 is now endemic, not a once-a-year seasonal virus. Seasons (aka surges) will occur whenever a new variant arises (twice yearly since 2020, Omicron BA.4/BA.5 currently), or when enough vaccine holdouts, newborns, and/or those with waning of prior immunity (vaccine or infection induced) accrue.
Emergency use authorization submission data for mRNA vaccine responses in young children2,3
Moderna in 6-month- through 5-year-olds. Two 25-mcg doses given 4-8 weeks apart produced 37.8% (95% confidence interval, 20.9%-51.1%) protection against symptomatic Omicron SARS-CoV-2 infections through 3 months of follow-up. Immunobridging analysis of antibody responses compared to 18- to 25-year-olds (100-mcg doses) showed the children’s responses were noninferior. Thus, the committee inferred that vaccine effectiveness in children should be similar to that in 18- to 25-year-olds. Fever, irritability, or local reaction/pain occurred in two-thirds after the second dose. Grade 3 reactions were noted in less than 5%.
Pfizer in 6-month- through 4-year-olds. Three 3-mcg doses, two doses 3-8 weeks apart and the third dose at least 8 weeks later (median 16 weeks), produced 80.3% (95% CI, 13.9%-96.7%) protection against symptomatic COVID-19 during the 6 weeks after the third dose. Local and systemic reactions occurred in 63.8%; less than 5% had grade 3 reactions (fever in about 3%, irritability in 1.3%, fatigue in 0.8%) mostly after second dose.
Neither duration of follow-up is very long. The Moderna data tell me that a third primary dose would have been better but restarting the trial to evaluate third doses would have delayed Moderna’s EUA another 4-6 months. The three-dose Pfizer data look better but may not have been as good with another 6 weeks of follow-up.
Additional post-EUA data will be collected. Boosters will be needed when immunity from both vaccines wanes (one estimate is about 6 months after the primary series). The Advisory Committee on Immunization Practices noted in their deliberations that vaccine-induced antibody responses are higher and cross-neutralize variants (even Omicron) better than infection-induced immunity.4
Are there downsides to the vaccines? Naysayers question vaccinating children less than 5 years old with reasons containing enough “truth” that they catch people’s attention, for example, “young children don’t get very sick with COVID-19,” “most have been infected already,” “RNA for the spike protein stays in the body for months,” or “myocarditis.” Naysayers can quote references in reputable journals but seem to spin selected data out of context or quote unconfirmed data from the Vaccine Adverse Event Reporting System.
Reasons to vaccinate
- While children have milder disease than adults, mid-June 2022 surveillance indicated 50 hospitalizations and 1 pediatric death each day from SARS-CoV-2.5
- Vaccinating young children endows a foundation of vaccine-induced SARS-CoV-2 immunity that is superior to infection-induced immunity.4
- Long-term effects of large numbers of SARS-CoV-2 particles that enter every organ of a developing child have not been determined.
- Viral loads are lowered by prior vaccine; fewer viral replications lessen chances for newer variants to arise.
- Transmission is less in breakthrough infections than infections in the unvaccinated.
- Thirty percent of 5- to 11-year-olds hospitalized for SARS-CoV-2 had no underlying conditions;6 hospitalization rates in newborn to 4-year-olds have been the highest in the Omicron surge.7
- No myocarditis or pericarditis episodes have been detected in 6-month- to 11-year-old trials.
- The AAP and ACIP recommend the mRNA vaccines.
My thoughts are that SARS-CoV-2 vaccine is just another “routine” childhood vaccine that prepares children for healthier futures, pandemic or not, and the vaccines are as safe as other routine vaccines.
And like other pediatric vaccines, it should be no surprise that boosters will be needed, even if no newer variants than Omicron BA.4/BA.5 arise. But we know newer variants will arise and, similar to influenza vaccine, new formulations, perhaps with multiple SARS-CoV-2 strain antigens, will be needed every year or so. Everyone will get SARS-CoV-2 multiple times in their lives no matter how careful they are. So isn’t it good medical practice to establish early the best available foundation for maintaining lifelong SARS-CoV-2 immunity?
To me it is like pertussis. Most pertussis-infected children are sick enough to be hospitalized; very few die. They are miserable with illnesses that take weeks to months to subside. The worst disease usually occurs in unvaccinated young children or those with underlying conditions. Reactogenicity was reduced with acellular vaccine but resulted in less immunogenicity, so we give boosters at intervals that best match waning immunity. Circulating strains can be different than the vaccine strain, so protection against infection is 80%. Finally, even the safest vaccine may very rarely have sequelae. That is why The National Vaccine Injury Compensation Program was created. Yet the benefit-to-harm ratio for children and society favors universal pertussis vaccine use. And we vaccinate even those who have had pertussis because even infection-based immunity is incomplete and protection wanes. If arguments similar to those by SARS-CoV-2 vaccine naysayers were applied to acellular pertussis vaccine, it seems they would argue against pertussis vaccine for young children.
Another major issue has been “safety concerns” about the vaccines’ small amount of mRNA for the spike protein encased in microscopic lipid bubbles injected in the arm or leg. This mRNA is picked up by human cells, and in the cytoplasm (not the nucleus where our DNA resides) produces a limited supply of spike protein that is then picked up by antigen-presenting cells for short-lived distribution (days to 2 weeks at most) to regional lymph nodes where immune-memory processes are jump-started. Contrast that to even asymptomatic SARS-CoV-2 infection where multibillions of virus particles are produced for up to 14 days with access to every bodily organ that contains ACE-2 receptors (they all do). Each virus particle hijacks a human cell producing thousands of mRNA for spike protein (and multiple other SARS-CoV-2 proteins), eventually releasing multibillions of lipid fragments from the ruptured cell. Comparing the amount of these components in the mRNA vaccines to those from infection is like comparing a campfire to the many-thousand-acre wildfire. So, if one is worried about the effects of spike protein and lipid fragments, the limited localized amounts in mRNA vaccines should make one much less concerned than the enormous amounts circulating throughout the body as a result of a SARS-CoV-2 infection.
My take is that children 6-months to 5-years-old deserve SARS-CoV-2–induced vaccine protection and we can and should strongly recommend it as medical providers and child advocates.
*Dr. Harrison is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at pdnews@mdedge.com.
References
1. AAP. 2022 Jun 21. As COVID-19 vaccines become available for children ages 6 months to 4 years, AAP urges families to reach out to pediatricians to ask questions and access vaccine. www.aap.org.
2. CDC. Grading of recommendations, assessment, development, and evaluation (GRADE): Moderna COVID-19 vaccine for children aged 6 months–5 years. www.cdc.gov.
3. CDC. ACIP evidence to recommendations for use of Moderna COVID-19 vaccine in children ages 6 months–5 years and Pfizer-BioNTech COVID-19 vaccine in children ages 6 months–4 years under an emergency use authorization. www.cdc.gov.
4. Tang J et al. Nat Commun. 2022;13:2979.
5. Children and COVID-19: State Data Report. 2022 Jun 30. www.aap.org.
6. Shi DS et al. MMWR Morb Mortal Wkly Rep. 2022;71:574-81.
7. Marks KJ et al. MMWR Morb Mortal Wkly Rep. 2022;71:429-36.
Other good resources for families are https://getvaccineanswers.org/ or www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-in-babies-and-children/art-20484405.
*This story was updated on July 19, 2022.
A family’s decision to vaccinate their child is best made jointly with a trusted medical provider who knows the child and family. The American Academy of Pediatrics created a toolkit with resources for answering questions about the recently authorized SARS-CoV-2 mRNA vaccines (Pfizer and Moderna) for 6-month- to 5-year-olds with science-backed vaccine facts, including links to other useful AAP information websites, talking points, graphics, and videos.1
SARS-CoV-2 seasonality
SARS-CoV-2 is now endemic, not a once-a-year seasonal virus. Seasons (aka surges) will occur whenever a new variant arises (twice yearly since 2020, Omicron BA.4/BA.5 currently), or when enough vaccine holdouts, newborns, and/or those with waning of prior immunity (vaccine or infection induced) accrue.
Emergency use authorization submission data for mRNA vaccine responses in young children2,3
Moderna in 6-month- through 5-year-olds. Two 25-mcg doses given 4-8 weeks apart produced 37.8% (95% confidence interval, 20.9%-51.1%) protection against symptomatic Omicron SARS-CoV-2 infections through 3 months of follow-up. Immunobridging analysis of antibody responses compared to 18- to 25-year-olds (100-mcg doses) showed the children’s responses were noninferior. Thus, the committee inferred that vaccine effectiveness in children should be similar to that in 18- to 25-year-olds. Fever, irritability, or local reaction/pain occurred in two-thirds after the second dose. Grade 3 reactions were noted in less than 5%.
Pfizer in 6-month- through 4-year-olds. Three 3-mcg doses, two doses 3-8 weeks apart and the third dose at least 8 weeks later (median 16 weeks), produced 80.3% (95% CI, 13.9%-96.7%) protection against symptomatic COVID-19 during the 6 weeks after the third dose. Local and systemic reactions occurred in 63.8%; less than 5% had grade 3 reactions (fever in about 3%, irritability in 1.3%, fatigue in 0.8%) mostly after second dose.
Neither duration of follow-up is very long. The Moderna data tell me that a third primary dose would have been better but restarting the trial to evaluate third doses would have delayed Moderna’s EUA another 4-6 months. The three-dose Pfizer data look better but may not have been as good with another 6 weeks of follow-up.
Additional post-EUA data will be collected. Boosters will be needed when immunity from both vaccines wanes (one estimate is about 6 months after the primary series). The Advisory Committee on Immunization Practices noted in their deliberations that vaccine-induced antibody responses are higher and cross-neutralize variants (even Omicron) better than infection-induced immunity.4
Are there downsides to the vaccines? Naysayers question vaccinating children less than 5 years old with reasons containing enough “truth” that they catch people’s attention, for example, “young children don’t get very sick with COVID-19,” “most have been infected already,” “RNA for the spike protein stays in the body for months,” or “myocarditis.” Naysayers can quote references in reputable journals but seem to spin selected data out of context or quote unconfirmed data from the Vaccine Adverse Event Reporting System.
Reasons to vaccinate
- While children have milder disease than adults, mid-June 2022 surveillance indicated 50 hospitalizations and 1 pediatric death each day from SARS-CoV-2.5
- Vaccinating young children endows a foundation of vaccine-induced SARS-CoV-2 immunity that is superior to infection-induced immunity.4
- Long-term effects of large numbers of SARS-CoV-2 particles that enter every organ of a developing child have not been determined.
- Viral loads are lowered by prior vaccine; fewer viral replications lessen chances for newer variants to arise.
- Transmission is less in breakthrough infections than infections in the unvaccinated.
- Thirty percent of 5- to 11-year-olds hospitalized for SARS-CoV-2 had no underlying conditions;6 hospitalization rates in newborn to 4-year-olds have been the highest in the Omicron surge.7
- No myocarditis or pericarditis episodes have been detected in 6-month- to 11-year-old trials.
- The AAP and ACIP recommend the mRNA vaccines.
My thoughts are that SARS-CoV-2 vaccine is just another “routine” childhood vaccine that prepares children for healthier futures, pandemic or not, and the vaccines are as safe as other routine vaccines.
And like other pediatric vaccines, it should be no surprise that boosters will be needed, even if no newer variants than Omicron BA.4/BA.5 arise. But we know newer variants will arise and, similar to influenza vaccine, new formulations, perhaps with multiple SARS-CoV-2 strain antigens, will be needed every year or so. Everyone will get SARS-CoV-2 multiple times in their lives no matter how careful they are. So isn’t it good medical practice to establish early the best available foundation for maintaining lifelong SARS-CoV-2 immunity?
To me it is like pertussis. Most pertussis-infected children are sick enough to be hospitalized; very few die. They are miserable with illnesses that take weeks to months to subside. The worst disease usually occurs in unvaccinated young children or those with underlying conditions. Reactogenicity was reduced with acellular vaccine but resulted in less immunogenicity, so we give boosters at intervals that best match waning immunity. Circulating strains can be different than the vaccine strain, so protection against infection is 80%. Finally, even the safest vaccine may very rarely have sequelae. That is why The National Vaccine Injury Compensation Program was created. Yet the benefit-to-harm ratio for children and society favors universal pertussis vaccine use. And we vaccinate even those who have had pertussis because even infection-based immunity is incomplete and protection wanes. If arguments similar to those by SARS-CoV-2 vaccine naysayers were applied to acellular pertussis vaccine, it seems they would argue against pertussis vaccine for young children.
Another major issue has been “safety concerns” about the vaccines’ small amount of mRNA for the spike protein encased in microscopic lipid bubbles injected in the arm or leg. This mRNA is picked up by human cells, and in the cytoplasm (not the nucleus where our DNA resides) produces a limited supply of spike protein that is then picked up by antigen-presenting cells for short-lived distribution (days to 2 weeks at most) to regional lymph nodes where immune-memory processes are jump-started. Contrast that to even asymptomatic SARS-CoV-2 infection where multibillions of virus particles are produced for up to 14 days with access to every bodily organ that contains ACE-2 receptors (they all do). Each virus particle hijacks a human cell producing thousands of mRNA for spike protein (and multiple other SARS-CoV-2 proteins), eventually releasing multibillions of lipid fragments from the ruptured cell. Comparing the amount of these components in the mRNA vaccines to those from infection is like comparing a campfire to the many-thousand-acre wildfire. So, if one is worried about the effects of spike protein and lipid fragments, the limited localized amounts in mRNA vaccines should make one much less concerned than the enormous amounts circulating throughout the body as a result of a SARS-CoV-2 infection.
My take is that children 6-months to 5-years-old deserve SARS-CoV-2–induced vaccine protection and we can and should strongly recommend it as medical providers and child advocates.
*Dr. Harrison is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at pdnews@mdedge.com.
References
1. AAP. 2022 Jun 21. As COVID-19 vaccines become available for children ages 6 months to 4 years, AAP urges families to reach out to pediatricians to ask questions and access vaccine. www.aap.org.
2. CDC. Grading of recommendations, assessment, development, and evaluation (GRADE): Moderna COVID-19 vaccine for children aged 6 months–5 years. www.cdc.gov.
3. CDC. ACIP evidence to recommendations for use of Moderna COVID-19 vaccine in children ages 6 months–5 years and Pfizer-BioNTech COVID-19 vaccine in children ages 6 months–4 years under an emergency use authorization. www.cdc.gov.
4. Tang J et al. Nat Commun. 2022;13:2979.
5. Children and COVID-19: State Data Report. 2022 Jun 30. www.aap.org.
6. Shi DS et al. MMWR Morb Mortal Wkly Rep. 2022;71:574-81.
7. Marks KJ et al. MMWR Morb Mortal Wkly Rep. 2022;71:429-36.
Other good resources for families are https://getvaccineanswers.org/ or www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-in-babies-and-children/art-20484405.
*This story was updated on July 19, 2022.
Monkeypox: What’s a pediatrician to do?
Not long ago, a pediatrician working in a local urgent care clinic called me about a teenage girl with a pruritic rash. She described vesicles and pustules located primarily on the face and arms with no surrounding cellulitis or other exam findings.
“She probably has impetigo,” my colleague said. “But I took a travel and exposure history and learned that her grandma had recently returned home from visiting family in the Congo. Do you think I need to worry about monkeypox?”
While most pediatricians in the United States have never seen a case of monkeypox, the virus is not new. An orthopox, it belongs to the same genus that includes smallpox and cowpox viruses. It was discovered in 1958 when two colonies of monkeys kept for research developed pox-like rashes. The earliest human case was reported in 1970 in the Democratic Republic of Congo and now the virus is endemic in some counties in Central and West Africa.
Monkeypox virus is a zoonotic disease – it can spread from animals to people. Rodents and other small mammals – not monkeys – are thought to be the most likely reservoir. The virus typically spreads from person to person through close contact with skin or respiratory secretions or contact with contaminated fomites. Typical infection begins with fever, lymphadenopathy, and flulike symptoms that include headache and malaise. One to four days after the onset of fever, the characteristic rash begins as macular lesions that evolve into papules, then vesicles, and finally pustules. Pustular lesions are deep-seated, well circumscribed, and are usually the same size and in the same stage of development on a given body site. The rash often starts on the face or the mouth, and then moves to the extremities, including the palms and soles. Over time, the lesions umbilicate and ultimately crust over.
On May 20, the Centers for Disease Control and Prevention issued a Health Advisory describing a case of monkeypox in a patient in Massachusetts. A single case normally wouldn’t cause too much alarm. In fact, there were two cases reported in the United States in 2021, both in travelers returning to the United States from Nigeria, a country in which the virus is endemic. No transmissions from these individuals to close contacts were identified.
The Massachusetts case was remarkable for two reasons. It occurred in an individual who had recently returned from a trip to Canada, which is not a country in which the virus is endemic. Additionally, it occurred in the context of a global outbreak of monkey pox that has, to date, disproportionately affected individuals who identify as men who have sex with men. Patients have often lacked the characteristic prodrome and many have had rash localized to the perianal and genital area, with or without symptoms of proctitis (anorectal pain, tenesmus, and bleeding). Clinically, some lesions mimicked sexually transmitted infections that the occur in the anogenital area, including herpes, syphilis, and lymphogranuloma venereum.
As of May 31, 2022, 17 persons in nine states had been diagnosed with presumed monkeypox virus infection. They ranged in age from 28 to 61 years and 16/17 identified as MSM. Fourteen reported international travel in the 3 weeks before developing symptoms. As of June 12, that number had grown to 53, while worldwide the number of confirmed and suspected cases reached 1,584. Up-to-date case counts are available at https://ourworldindata.org/monkeypox.
Back on the phone, my colleague laughed a little nervously. “I guess I’m not really worried about monkeypox in my patient.” She paused and then asked, “This isn’t going to be the next pandemic, is it?”
Public health experts at the Centers for Disease Control and Prevention and the World Health Organization have been reassuring in that regard. Two vaccines are available for the prevention of monkeypox. JYNNEOS is a nonreplicating live viral vaccine licensed as a two-dose series to prevent both monkeypox and smallpox. ACAM 2000 is a live Vaccinia virus preparation licensed to prevent smallpox. These vaccines are effective when given before exposure but are thought to also beneficial when given as postexposure prophylaxis. According to the CDC, vaccination within 4 days of exposure can prevent the development of disease. Vaccination within 14 days of exposure may not prevent the development of disease but may lessen symptoms. Treatment is generally supportive but antiviral therapy could be considered for individuals with severe disease. Tecovirmat is Food and Drug Administration approved for the treatment of smallpox but is available under nonresearch Expanded Access Investigational New Drug (EA-IND) protocol for the treatment of children and adults with severe orthopox infections, including monkeypox.
So, what’s a pediatrician to do? Take a good travel history, as my colleague did, because that is good medicine. At this point in an outbreak though, a lack of travel does not exclude the diagnosis. Perform a thorough exam of skin and mucosal areas. When there are rashes in the genital or perianal area, consider the possibility of monkeypox in addition to typical sexually transmitted infections. Ask about exposure to other persons with similar rashes, as well as close or intimate contact with a persons in a social network experiencing monkeypox infections. This includes MSM who meet partners through an online website, app, or at social events. Monkeypox can also be spread through contact with an animal (dead or alive) that is an African endemic species or use of a product derived from such animals. Public health experts encourage clinicians to be alert for rash illnesses consistent with monkeypox, regardless of a patient’s gender or sexual orientation, history of international travel, or specific risk factors.
Pediatricians see many kids with rashes, and while cases of monkeypox climb daily, the disease is still very rare. Given the media coverage of the outbreak, pediatricians should be prepared for questions from patients and their parents. Clinicians who suspect a case of monkeypox should contact their local or state health department for guidance and the need for testing. Tips for recognizing monkeypox and distinguishing it from more common viral illnesses such as chicken pox are available at www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Not long ago, a pediatrician working in a local urgent care clinic called me about a teenage girl with a pruritic rash. She described vesicles and pustules located primarily on the face and arms with no surrounding cellulitis or other exam findings.
“She probably has impetigo,” my colleague said. “But I took a travel and exposure history and learned that her grandma had recently returned home from visiting family in the Congo. Do you think I need to worry about monkeypox?”
While most pediatricians in the United States have never seen a case of monkeypox, the virus is not new. An orthopox, it belongs to the same genus that includes smallpox and cowpox viruses. It was discovered in 1958 when two colonies of monkeys kept for research developed pox-like rashes. The earliest human case was reported in 1970 in the Democratic Republic of Congo and now the virus is endemic in some counties in Central and West Africa.
Monkeypox virus is a zoonotic disease – it can spread from animals to people. Rodents and other small mammals – not monkeys – are thought to be the most likely reservoir. The virus typically spreads from person to person through close contact with skin or respiratory secretions or contact with contaminated fomites. Typical infection begins with fever, lymphadenopathy, and flulike symptoms that include headache and malaise. One to four days after the onset of fever, the characteristic rash begins as macular lesions that evolve into papules, then vesicles, and finally pustules. Pustular lesions are deep-seated, well circumscribed, and are usually the same size and in the same stage of development on a given body site. The rash often starts on the face or the mouth, and then moves to the extremities, including the palms and soles. Over time, the lesions umbilicate and ultimately crust over.
On May 20, the Centers for Disease Control and Prevention issued a Health Advisory describing a case of monkeypox in a patient in Massachusetts. A single case normally wouldn’t cause too much alarm. In fact, there were two cases reported in the United States in 2021, both in travelers returning to the United States from Nigeria, a country in which the virus is endemic. No transmissions from these individuals to close contacts were identified.
The Massachusetts case was remarkable for two reasons. It occurred in an individual who had recently returned from a trip to Canada, which is not a country in which the virus is endemic. Additionally, it occurred in the context of a global outbreak of monkey pox that has, to date, disproportionately affected individuals who identify as men who have sex with men. Patients have often lacked the characteristic prodrome and many have had rash localized to the perianal and genital area, with or without symptoms of proctitis (anorectal pain, tenesmus, and bleeding). Clinically, some lesions mimicked sexually transmitted infections that the occur in the anogenital area, including herpes, syphilis, and lymphogranuloma venereum.
As of May 31, 2022, 17 persons in nine states had been diagnosed with presumed monkeypox virus infection. They ranged in age from 28 to 61 years and 16/17 identified as MSM. Fourteen reported international travel in the 3 weeks before developing symptoms. As of June 12, that number had grown to 53, while worldwide the number of confirmed and suspected cases reached 1,584. Up-to-date case counts are available at https://ourworldindata.org/monkeypox.
Back on the phone, my colleague laughed a little nervously. “I guess I’m not really worried about monkeypox in my patient.” She paused and then asked, “This isn’t going to be the next pandemic, is it?”
Public health experts at the Centers for Disease Control and Prevention and the World Health Organization have been reassuring in that regard. Two vaccines are available for the prevention of monkeypox. JYNNEOS is a nonreplicating live viral vaccine licensed as a two-dose series to prevent both monkeypox and smallpox. ACAM 2000 is a live Vaccinia virus preparation licensed to prevent smallpox. These vaccines are effective when given before exposure but are thought to also beneficial when given as postexposure prophylaxis. According to the CDC, vaccination within 4 days of exposure can prevent the development of disease. Vaccination within 14 days of exposure may not prevent the development of disease but may lessen symptoms. Treatment is generally supportive but antiviral therapy could be considered for individuals with severe disease. Tecovirmat is Food and Drug Administration approved for the treatment of smallpox but is available under nonresearch Expanded Access Investigational New Drug (EA-IND) protocol for the treatment of children and adults with severe orthopox infections, including monkeypox.
So, what’s a pediatrician to do? Take a good travel history, as my colleague did, because that is good medicine. At this point in an outbreak though, a lack of travel does not exclude the diagnosis. Perform a thorough exam of skin and mucosal areas. When there are rashes in the genital or perianal area, consider the possibility of monkeypox in addition to typical sexually transmitted infections. Ask about exposure to other persons with similar rashes, as well as close or intimate contact with a persons in a social network experiencing monkeypox infections. This includes MSM who meet partners through an online website, app, or at social events. Monkeypox can also be spread through contact with an animal (dead or alive) that is an African endemic species or use of a product derived from such animals. Public health experts encourage clinicians to be alert for rash illnesses consistent with monkeypox, regardless of a patient’s gender or sexual orientation, history of international travel, or specific risk factors.
Pediatricians see many kids with rashes, and while cases of monkeypox climb daily, the disease is still very rare. Given the media coverage of the outbreak, pediatricians should be prepared for questions from patients and their parents. Clinicians who suspect a case of monkeypox should contact their local or state health department for guidance and the need for testing. Tips for recognizing monkeypox and distinguishing it from more common viral illnesses such as chicken pox are available at www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Not long ago, a pediatrician working in a local urgent care clinic called me about a teenage girl with a pruritic rash. She described vesicles and pustules located primarily on the face and arms with no surrounding cellulitis or other exam findings.
“She probably has impetigo,” my colleague said. “But I took a travel and exposure history and learned that her grandma had recently returned home from visiting family in the Congo. Do you think I need to worry about monkeypox?”
While most pediatricians in the United States have never seen a case of monkeypox, the virus is not new. An orthopox, it belongs to the same genus that includes smallpox and cowpox viruses. It was discovered in 1958 when two colonies of monkeys kept for research developed pox-like rashes. The earliest human case was reported in 1970 in the Democratic Republic of Congo and now the virus is endemic in some counties in Central and West Africa.
Monkeypox virus is a zoonotic disease – it can spread from animals to people. Rodents and other small mammals – not monkeys – are thought to be the most likely reservoir. The virus typically spreads from person to person through close contact with skin or respiratory secretions or contact with contaminated fomites. Typical infection begins with fever, lymphadenopathy, and flulike symptoms that include headache and malaise. One to four days after the onset of fever, the characteristic rash begins as macular lesions that evolve into papules, then vesicles, and finally pustules. Pustular lesions are deep-seated, well circumscribed, and are usually the same size and in the same stage of development on a given body site. The rash often starts on the face or the mouth, and then moves to the extremities, including the palms and soles. Over time, the lesions umbilicate and ultimately crust over.
On May 20, the Centers for Disease Control and Prevention issued a Health Advisory describing a case of monkeypox in a patient in Massachusetts. A single case normally wouldn’t cause too much alarm. In fact, there were two cases reported in the United States in 2021, both in travelers returning to the United States from Nigeria, a country in which the virus is endemic. No transmissions from these individuals to close contacts were identified.
The Massachusetts case was remarkable for two reasons. It occurred in an individual who had recently returned from a trip to Canada, which is not a country in which the virus is endemic. Additionally, it occurred in the context of a global outbreak of monkey pox that has, to date, disproportionately affected individuals who identify as men who have sex with men. Patients have often lacked the characteristic prodrome and many have had rash localized to the perianal and genital area, with or without symptoms of proctitis (anorectal pain, tenesmus, and bleeding). Clinically, some lesions mimicked sexually transmitted infections that the occur in the anogenital area, including herpes, syphilis, and lymphogranuloma venereum.
As of May 31, 2022, 17 persons in nine states had been diagnosed with presumed monkeypox virus infection. They ranged in age from 28 to 61 years and 16/17 identified as MSM. Fourteen reported international travel in the 3 weeks before developing symptoms. As of June 12, that number had grown to 53, while worldwide the number of confirmed and suspected cases reached 1,584. Up-to-date case counts are available at https://ourworldindata.org/monkeypox.
Back on the phone, my colleague laughed a little nervously. “I guess I’m not really worried about monkeypox in my patient.” She paused and then asked, “This isn’t going to be the next pandemic, is it?”
Public health experts at the Centers for Disease Control and Prevention and the World Health Organization have been reassuring in that regard. Two vaccines are available for the prevention of monkeypox. JYNNEOS is a nonreplicating live viral vaccine licensed as a two-dose series to prevent both monkeypox and smallpox. ACAM 2000 is a live Vaccinia virus preparation licensed to prevent smallpox. These vaccines are effective when given before exposure but are thought to also beneficial when given as postexposure prophylaxis. According to the CDC, vaccination within 4 days of exposure can prevent the development of disease. Vaccination within 14 days of exposure may not prevent the development of disease but may lessen symptoms. Treatment is generally supportive but antiviral therapy could be considered for individuals with severe disease. Tecovirmat is Food and Drug Administration approved for the treatment of smallpox but is available under nonresearch Expanded Access Investigational New Drug (EA-IND) protocol for the treatment of children and adults with severe orthopox infections, including monkeypox.
So, what’s a pediatrician to do? Take a good travel history, as my colleague did, because that is good medicine. At this point in an outbreak though, a lack of travel does not exclude the diagnosis. Perform a thorough exam of skin and mucosal areas. When there are rashes in the genital or perianal area, consider the possibility of monkeypox in addition to typical sexually transmitted infections. Ask about exposure to other persons with similar rashes, as well as close or intimate contact with a persons in a social network experiencing monkeypox infections. This includes MSM who meet partners through an online website, app, or at social events. Monkeypox can also be spread through contact with an animal (dead or alive) that is an African endemic species or use of a product derived from such animals. Public health experts encourage clinicians to be alert for rash illnesses consistent with monkeypox, regardless of a patient’s gender or sexual orientation, history of international travel, or specific risk factors.
Pediatricians see many kids with rashes, and while cases of monkeypox climb daily, the disease is still very rare. Given the media coverage of the outbreak, pediatricians should be prepared for questions from patients and their parents. Clinicians who suspect a case of monkeypox should contact their local or state health department for guidance and the need for testing. Tips for recognizing monkeypox and distinguishing it from more common viral illnesses such as chicken pox are available at www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.








