User login
Modified Atkins diet beneficial in drug-resistant epilepsy
, new research shows.
In a randomized prospective study, the number of seizures per month dropped by more than half in one-quarter of patients following the high-fat, low-carb diet; and 5% of the group were free from all seizure activity after 6 months.
Both adults and adolescents reported benefits from the diet, which is a less strict version of a traditional ketogenic diet that many patients find difficult to follow. The modified Atkins diet includes foods such as leafy green vegetables and eggs, chicken, fish, bacon, and other animal proteins.
“The use of an exchange list and recipe booklet with local recipes and spices helped in the initiation of modified Atkins diet with the flexibility of meal choices and ease of administration,” said coinvestigator Manjari Tripathi, MD, DM, department of neurology, All India Institute of Medical Science, New Delhi.
“As items were everyday household ingredients in proportion to the requirement of the modified Atkins diet, this diet is possible in low-income countries also,” Dr. Tripathi added.
The findings were published online in the journal Neurology.
Low carbs, high benefit
The modified Atkins diet includes around 65% fat, 25% protein, and 10% carbohydrates. Unlike a traditional ketogenic diet, the modified Atkins diet includes no restrictions on protein, calories, or fluids.
Researchers have long known that ketogenic and Atkins diets are associated with reduced seizure activity in adolescents with epilepsy. But previous studies were small, and many were retrospective analyses.
The current investigators enrolled 160 patients (80 adults, 80 adolescents) aged 10-55 years whose epilepsy was not controlled despite using at least three antiseizure medications at maximum tolerated doses.
The intervention group received training in the modified Atkins diet and were given a food exchange list, sample menu, and recipe booklet. Carbohydrate intake was restricted to 20 grams per day.
Participants took supplemental multivitamins and minerals, kept a food diary, logged seizure activity, and measured urine ketone levels three times a day. They also received weekly check-up phone calls to ensure diet adherence.
The control group received a normal diet with no carbohydrate restrictions. All participants continued their prescribed antiseizure therapy throughout the trial.
Primary outcome met
The primary study outcome was a reduction in seizures of more than 50%. At 6 months, 26.2% of the intervention group had reached that goal, compared with just 2.5% of the control group (P < .001).
When the median number of seizures in the modified Atkins diet group was analyzed, the frequency dropped in the intervention group from 37.5 per month at baseline to 27.5 per month after 3 months of the modified Atkins diet and to 21.5 per month after 6 months.
Adding the modified Atkins diet had a larger effect on seizure activity in adults than in adolescents. At the end of 6 months, 36% of adolescents on the modified Atkins diet had more than a 50% reduction in seizures, while 57.1% of adults on the diet reached that level.
Quality-of-life scores were also higher in the intervention group.
By the end of the trial, 5% of patients on the modified Atkins diet had no seizure activity at all versus none of the control group. In fact, the median number of seizures increased in the control group during the study.
The mean morning and evening levels of urine ketosis in the intervention group were 58.3 ± 8.0 mg/dL and 62.2 ± 22.6 mg/dL, respectively, suggesting satisfactory diet adherence. There was no significant difference between groups in weight loss.
Dr. Tripathi noted that 33% of participants did not complete the study because of poor tolerance of the diet, lack of benefit, or the inability to follow up – in part due to COVID-19. However, she said tolerance of the modified Atkins diet was better than what has been reported with the ketogenic diet.
“Though the exact mechanism by which such a diet protects against seizures is unknown, there is evidence that it causes effects on intermediary metabolism that influences the dynamics of the major inhibitory and excitatory neurotransmitter systems in the brain,” Dr. Tripathi said.
Benefits outweigh cost
Commenting on the research findings, Mackenzie Cervenka, MD, professor of neurology and director of the Adult Epilepsy Diet Center at Johns Hopkins University, Baltimore, noted that the study is the first randomized controlled trial of this size to demonstrate a benefit from adding the modified Atkins diet to standard antiseizure therapy in treatment-resistant epilepsy.
“Importantly, the study also showed improvement in quality of life and behavior over standard-of-care therapies without significant adverse effects,” said Dr. Cervenka, who was not part of the research.
The investigators noted that the flexibility of the modified Atkins diet allows more variation in menu options and a greater intake of protein, making it easier to follow than a traditional ketogenic diet.
One area of debate, however, is whether these diets are manageable for individuals with low income. Poultry, meat, and fish, all of which are staples of a modified Atkins diet, can be more expensive than other high-carb options such as pasta and rice.
“While some of the foods such as protein sources that patients purchase when they are on a ketogenic diet therapy can be more expensive, if you take into account the cost of antiseizure medications and other antiseizure treatments, hospital visits, and missed work related to seizures, et cetera, the overall financial benefits of seizure reduction with incorporating a ketogenic diet therapy may outweigh these costs,” Dr. Cervenka said.
“There are also low-cost foods that can be used since there is a great deal of flexibility with a modified Atkins diet,” she added.
The study was funded by the Centre of Excellence for Epilepsy, which is funded by the Department of Biotechnology, Government of India. Dr. Tripathi and Dr. Cervenka report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
In a randomized prospective study, the number of seizures per month dropped by more than half in one-quarter of patients following the high-fat, low-carb diet; and 5% of the group were free from all seizure activity after 6 months.
Both adults and adolescents reported benefits from the diet, which is a less strict version of a traditional ketogenic diet that many patients find difficult to follow. The modified Atkins diet includes foods such as leafy green vegetables and eggs, chicken, fish, bacon, and other animal proteins.
“The use of an exchange list and recipe booklet with local recipes and spices helped in the initiation of modified Atkins diet with the flexibility of meal choices and ease of administration,” said coinvestigator Manjari Tripathi, MD, DM, department of neurology, All India Institute of Medical Science, New Delhi.
“As items were everyday household ingredients in proportion to the requirement of the modified Atkins diet, this diet is possible in low-income countries also,” Dr. Tripathi added.
The findings were published online in the journal Neurology.
Low carbs, high benefit
The modified Atkins diet includes around 65% fat, 25% protein, and 10% carbohydrates. Unlike a traditional ketogenic diet, the modified Atkins diet includes no restrictions on protein, calories, or fluids.
Researchers have long known that ketogenic and Atkins diets are associated with reduced seizure activity in adolescents with epilepsy. But previous studies were small, and many were retrospective analyses.
The current investigators enrolled 160 patients (80 adults, 80 adolescents) aged 10-55 years whose epilepsy was not controlled despite using at least three antiseizure medications at maximum tolerated doses.
The intervention group received training in the modified Atkins diet and were given a food exchange list, sample menu, and recipe booklet. Carbohydrate intake was restricted to 20 grams per day.
Participants took supplemental multivitamins and minerals, kept a food diary, logged seizure activity, and measured urine ketone levels three times a day. They also received weekly check-up phone calls to ensure diet adherence.
The control group received a normal diet with no carbohydrate restrictions. All participants continued their prescribed antiseizure therapy throughout the trial.
Primary outcome met
The primary study outcome was a reduction in seizures of more than 50%. At 6 months, 26.2% of the intervention group had reached that goal, compared with just 2.5% of the control group (P < .001).
When the median number of seizures in the modified Atkins diet group was analyzed, the frequency dropped in the intervention group from 37.5 per month at baseline to 27.5 per month after 3 months of the modified Atkins diet and to 21.5 per month after 6 months.
Adding the modified Atkins diet had a larger effect on seizure activity in adults than in adolescents. At the end of 6 months, 36% of adolescents on the modified Atkins diet had more than a 50% reduction in seizures, while 57.1% of adults on the diet reached that level.
Quality-of-life scores were also higher in the intervention group.
By the end of the trial, 5% of patients on the modified Atkins diet had no seizure activity at all versus none of the control group. In fact, the median number of seizures increased in the control group during the study.
The mean morning and evening levels of urine ketosis in the intervention group were 58.3 ± 8.0 mg/dL and 62.2 ± 22.6 mg/dL, respectively, suggesting satisfactory diet adherence. There was no significant difference between groups in weight loss.
Dr. Tripathi noted that 33% of participants did not complete the study because of poor tolerance of the diet, lack of benefit, or the inability to follow up – in part due to COVID-19. However, she said tolerance of the modified Atkins diet was better than what has been reported with the ketogenic diet.
“Though the exact mechanism by which such a diet protects against seizures is unknown, there is evidence that it causes effects on intermediary metabolism that influences the dynamics of the major inhibitory and excitatory neurotransmitter systems in the brain,” Dr. Tripathi said.
Benefits outweigh cost
Commenting on the research findings, Mackenzie Cervenka, MD, professor of neurology and director of the Adult Epilepsy Diet Center at Johns Hopkins University, Baltimore, noted that the study is the first randomized controlled trial of this size to demonstrate a benefit from adding the modified Atkins diet to standard antiseizure therapy in treatment-resistant epilepsy.
“Importantly, the study also showed improvement in quality of life and behavior over standard-of-care therapies without significant adverse effects,” said Dr. Cervenka, who was not part of the research.
The investigators noted that the flexibility of the modified Atkins diet allows more variation in menu options and a greater intake of protein, making it easier to follow than a traditional ketogenic diet.
One area of debate, however, is whether these diets are manageable for individuals with low income. Poultry, meat, and fish, all of which are staples of a modified Atkins diet, can be more expensive than other high-carb options such as pasta and rice.
“While some of the foods such as protein sources that patients purchase when they are on a ketogenic diet therapy can be more expensive, if you take into account the cost of antiseizure medications and other antiseizure treatments, hospital visits, and missed work related to seizures, et cetera, the overall financial benefits of seizure reduction with incorporating a ketogenic diet therapy may outweigh these costs,” Dr. Cervenka said.
“There are also low-cost foods that can be used since there is a great deal of flexibility with a modified Atkins diet,” she added.
The study was funded by the Centre of Excellence for Epilepsy, which is funded by the Department of Biotechnology, Government of India. Dr. Tripathi and Dr. Cervenka report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
In a randomized prospective study, the number of seizures per month dropped by more than half in one-quarter of patients following the high-fat, low-carb diet; and 5% of the group were free from all seizure activity after 6 months.
Both adults and adolescents reported benefits from the diet, which is a less strict version of a traditional ketogenic diet that many patients find difficult to follow. The modified Atkins diet includes foods such as leafy green vegetables and eggs, chicken, fish, bacon, and other animal proteins.
“The use of an exchange list and recipe booklet with local recipes and spices helped in the initiation of modified Atkins diet with the flexibility of meal choices and ease of administration,” said coinvestigator Manjari Tripathi, MD, DM, department of neurology, All India Institute of Medical Science, New Delhi.
“As items were everyday household ingredients in proportion to the requirement of the modified Atkins diet, this diet is possible in low-income countries also,” Dr. Tripathi added.
The findings were published online in the journal Neurology.
Low carbs, high benefit
The modified Atkins diet includes around 65% fat, 25% protein, and 10% carbohydrates. Unlike a traditional ketogenic diet, the modified Atkins diet includes no restrictions on protein, calories, or fluids.
Researchers have long known that ketogenic and Atkins diets are associated with reduced seizure activity in adolescents with epilepsy. But previous studies were small, and many were retrospective analyses.
The current investigators enrolled 160 patients (80 adults, 80 adolescents) aged 10-55 years whose epilepsy was not controlled despite using at least three antiseizure medications at maximum tolerated doses.
The intervention group received training in the modified Atkins diet and were given a food exchange list, sample menu, and recipe booklet. Carbohydrate intake was restricted to 20 grams per day.
Participants took supplemental multivitamins and minerals, kept a food diary, logged seizure activity, and measured urine ketone levels three times a day. They also received weekly check-up phone calls to ensure diet adherence.
The control group received a normal diet with no carbohydrate restrictions. All participants continued their prescribed antiseizure therapy throughout the trial.
Primary outcome met
The primary study outcome was a reduction in seizures of more than 50%. At 6 months, 26.2% of the intervention group had reached that goal, compared with just 2.5% of the control group (P < .001).
When the median number of seizures in the modified Atkins diet group was analyzed, the frequency dropped in the intervention group from 37.5 per month at baseline to 27.5 per month after 3 months of the modified Atkins diet and to 21.5 per month after 6 months.
Adding the modified Atkins diet had a larger effect on seizure activity in adults than in adolescents. At the end of 6 months, 36% of adolescents on the modified Atkins diet had more than a 50% reduction in seizures, while 57.1% of adults on the diet reached that level.
Quality-of-life scores were also higher in the intervention group.
By the end of the trial, 5% of patients on the modified Atkins diet had no seizure activity at all versus none of the control group. In fact, the median number of seizures increased in the control group during the study.
The mean morning and evening levels of urine ketosis in the intervention group were 58.3 ± 8.0 mg/dL and 62.2 ± 22.6 mg/dL, respectively, suggesting satisfactory diet adherence. There was no significant difference between groups in weight loss.
Dr. Tripathi noted that 33% of participants did not complete the study because of poor tolerance of the diet, lack of benefit, or the inability to follow up – in part due to COVID-19. However, she said tolerance of the modified Atkins diet was better than what has been reported with the ketogenic diet.
“Though the exact mechanism by which such a diet protects against seizures is unknown, there is evidence that it causes effects on intermediary metabolism that influences the dynamics of the major inhibitory and excitatory neurotransmitter systems in the brain,” Dr. Tripathi said.
Benefits outweigh cost
Commenting on the research findings, Mackenzie Cervenka, MD, professor of neurology and director of the Adult Epilepsy Diet Center at Johns Hopkins University, Baltimore, noted that the study is the first randomized controlled trial of this size to demonstrate a benefit from adding the modified Atkins diet to standard antiseizure therapy in treatment-resistant epilepsy.
“Importantly, the study also showed improvement in quality of life and behavior over standard-of-care therapies without significant adverse effects,” said Dr. Cervenka, who was not part of the research.
The investigators noted that the flexibility of the modified Atkins diet allows more variation in menu options and a greater intake of protein, making it easier to follow than a traditional ketogenic diet.
One area of debate, however, is whether these diets are manageable for individuals with low income. Poultry, meat, and fish, all of which are staples of a modified Atkins diet, can be more expensive than other high-carb options such as pasta and rice.
“While some of the foods such as protein sources that patients purchase when they are on a ketogenic diet therapy can be more expensive, if you take into account the cost of antiseizure medications and other antiseizure treatments, hospital visits, and missed work related to seizures, et cetera, the overall financial benefits of seizure reduction with incorporating a ketogenic diet therapy may outweigh these costs,” Dr. Cervenka said.
“There are also low-cost foods that can be used since there is a great deal of flexibility with a modified Atkins diet,” she added.
The study was funded by the Centre of Excellence for Epilepsy, which is funded by the Department of Biotechnology, Government of India. Dr. Tripathi and Dr. Cervenka report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Screen all patients for cannabis use before surgery: Guideline
All patients who undergo procedures that require regional or general anesthesia should be asked if, how often, and in what forms they use the drug, according to recommendations from the American Society of Regional Anesthesia and Pain Medicine.
One reason: Patients who regularly use cannabis may experience worse pain and nausea after surgery and may require more opioid analgesia, the group said.
The society’s recommendations – published in Regional Anesthesia and Pain Medicine – are the first guidelines in the United States to cover cannabis use as it relates to surgery, the group said.
Possible interactions
Use of cannabis has increased in recent years, and researchers have been concerned that the drug may interact with anesthesia and complicate pain management. Few studies have evaluated interactions between cannabis and anesthetic agents, however, according to the authors of the new guidelines.
“With the rising prevalence of both medical and recreational cannabis use in the general population, anesthesiologists, surgeons, and perioperative physicians must have an understanding of the effects of cannabis on physiology in order to provide safe perioperative care,” the guideline said.
“Before surgery, anesthesiologists should ask patients if they use cannabis – whether medicinally or recreationally – and be prepared to possibly change the anesthesia plan or delay the procedure in certain situations,” Samer Narouze, MD, PhD, ASRA president and senior author of the guidelines, said in a news release about the recommendations.
Although some patients may use cannabis to relieve pain, research shows that “regular users may have more pain and nausea after surgery, not less, and may need more medications, including opioids, to manage the discomfort,” said Dr. Narouze, chairman of the Center for Pain Medicine at Western Reserve Hospital in Cuyahoga Falls, Ohio.
Risks for vomiting, heart attack
The new recommendations were created by a committee of 13 experts, including anesthesiologists, chronic pain physicians, and a patient advocate. Shalini Shah, MD, vice chair of anesthesiology at the University of California, Irvine, was lead author of the document.
Four of 21 recommendations were classified as grade A, meaning that following them would be expected to provide substantial benefits. Those recommendations are to screen all patients before surgery; postpone elective surgery for patients who have altered mental status or impaired decision-making capacity at the time of surgery; counsel frequent, heavy users about the potential for cannabis use to impair postoperative pain control; and counsel pregnant patients about the risks of cannabis use to unborn children.
The authors cited studies to support their recommendations, including one showing that long-term cannabis use was associated with a 20% increase in the incidence of postoperative nausea and vomiting, a leading complaint of surgery patients. Other research has shown that cannabis use is linked to more pain and use of opioids after surgery.
Other recommendations include delaying elective surgery for at least 2 hours after a patient has smoked cannabis, owing to an increased risk for heart attack, and considering adjustment of ventilation settings during surgery for regular smokers of cannabis. Research has shown that smoking cannabis may be a rare trigger for myocardial infarction and is associated with airway inflammation and self-reported respiratory symptoms.
Nevertheless, doctors should not conduct universal toxicology screening, given a lack of evidence supporting this practice, the guideline stated.
The authors did not have enough information to make recommendations about reducing cannabis use before surgery or adjusting opioid prescriptions after surgery for patients who use cannabis, they said.
Kenneth Finn, MD, president of the American Board of Pain Medicine, welcomed the publication of the new guidelines. Dr. Finn, who practices at Springs Rehabilitation in Colorado Springs, has edited a textbook about cannabis in medicine and founded the International Academy on the Science and Impact of Cannabis.
“The vast majority of medical providers really have no idea about cannabis and what its impacts are on the human body,” Dr. Finn said.
For one, it can interact with numerous other drugs, including warfarin.
Guideline coauthor Eugene R. Viscusi, MD, professor of anesthesiology at the Sidney Kimmel Medical College, Philadelphia, emphasized that, while cannabis may be perceived as “natural,” it should not be considered differently from manufactured drugs.
Cannabis and cannabinoids represent “a class of very potent and pharmacologically active compounds,” Dr. Viscusi said in an interview. While researchers continue to assess possible medically beneficial effects of cannabis compounds, clinicians also need to be aware of the risks.
“The literature continues to emerge, and while we are always hopeful for good news, as physicians, we need to be very well versed on potential risks, especially in a high-risk situation like surgery,” he said.
Dr. Shah has consulted for companies that develop medical devices and drugs. Dr. Finn is the editor of the textbook, “Cannabis in Medicine: An Evidence-Based Approach” (Springer: New York, 2020), for which he receives royalties.
A version of this article first appeared on Medscape.com.
All patients who undergo procedures that require regional or general anesthesia should be asked if, how often, and in what forms they use the drug, according to recommendations from the American Society of Regional Anesthesia and Pain Medicine.
One reason: Patients who regularly use cannabis may experience worse pain and nausea after surgery and may require more opioid analgesia, the group said.
The society’s recommendations – published in Regional Anesthesia and Pain Medicine – are the first guidelines in the United States to cover cannabis use as it relates to surgery, the group said.
Possible interactions
Use of cannabis has increased in recent years, and researchers have been concerned that the drug may interact with anesthesia and complicate pain management. Few studies have evaluated interactions between cannabis and anesthetic agents, however, according to the authors of the new guidelines.
“With the rising prevalence of both medical and recreational cannabis use in the general population, anesthesiologists, surgeons, and perioperative physicians must have an understanding of the effects of cannabis on physiology in order to provide safe perioperative care,” the guideline said.
“Before surgery, anesthesiologists should ask patients if they use cannabis – whether medicinally or recreationally – and be prepared to possibly change the anesthesia plan or delay the procedure in certain situations,” Samer Narouze, MD, PhD, ASRA president and senior author of the guidelines, said in a news release about the recommendations.
Although some patients may use cannabis to relieve pain, research shows that “regular users may have more pain and nausea after surgery, not less, and may need more medications, including opioids, to manage the discomfort,” said Dr. Narouze, chairman of the Center for Pain Medicine at Western Reserve Hospital in Cuyahoga Falls, Ohio.
Risks for vomiting, heart attack
The new recommendations were created by a committee of 13 experts, including anesthesiologists, chronic pain physicians, and a patient advocate. Shalini Shah, MD, vice chair of anesthesiology at the University of California, Irvine, was lead author of the document.
Four of 21 recommendations were classified as grade A, meaning that following them would be expected to provide substantial benefits. Those recommendations are to screen all patients before surgery; postpone elective surgery for patients who have altered mental status or impaired decision-making capacity at the time of surgery; counsel frequent, heavy users about the potential for cannabis use to impair postoperative pain control; and counsel pregnant patients about the risks of cannabis use to unborn children.
The authors cited studies to support their recommendations, including one showing that long-term cannabis use was associated with a 20% increase in the incidence of postoperative nausea and vomiting, a leading complaint of surgery patients. Other research has shown that cannabis use is linked to more pain and use of opioids after surgery.
Other recommendations include delaying elective surgery for at least 2 hours after a patient has smoked cannabis, owing to an increased risk for heart attack, and considering adjustment of ventilation settings during surgery for regular smokers of cannabis. Research has shown that smoking cannabis may be a rare trigger for myocardial infarction and is associated with airway inflammation and self-reported respiratory symptoms.
Nevertheless, doctors should not conduct universal toxicology screening, given a lack of evidence supporting this practice, the guideline stated.
The authors did not have enough information to make recommendations about reducing cannabis use before surgery or adjusting opioid prescriptions after surgery for patients who use cannabis, they said.
Kenneth Finn, MD, president of the American Board of Pain Medicine, welcomed the publication of the new guidelines. Dr. Finn, who practices at Springs Rehabilitation in Colorado Springs, has edited a textbook about cannabis in medicine and founded the International Academy on the Science and Impact of Cannabis.
“The vast majority of medical providers really have no idea about cannabis and what its impacts are on the human body,” Dr. Finn said.
For one, it can interact with numerous other drugs, including warfarin.
Guideline coauthor Eugene R. Viscusi, MD, professor of anesthesiology at the Sidney Kimmel Medical College, Philadelphia, emphasized that, while cannabis may be perceived as “natural,” it should not be considered differently from manufactured drugs.
Cannabis and cannabinoids represent “a class of very potent and pharmacologically active compounds,” Dr. Viscusi said in an interview. While researchers continue to assess possible medically beneficial effects of cannabis compounds, clinicians also need to be aware of the risks.
“The literature continues to emerge, and while we are always hopeful for good news, as physicians, we need to be very well versed on potential risks, especially in a high-risk situation like surgery,” he said.
Dr. Shah has consulted for companies that develop medical devices and drugs. Dr. Finn is the editor of the textbook, “Cannabis in Medicine: An Evidence-Based Approach” (Springer: New York, 2020), for which he receives royalties.
A version of this article first appeared on Medscape.com.
All patients who undergo procedures that require regional or general anesthesia should be asked if, how often, and in what forms they use the drug, according to recommendations from the American Society of Regional Anesthesia and Pain Medicine.
One reason: Patients who regularly use cannabis may experience worse pain and nausea after surgery and may require more opioid analgesia, the group said.
The society’s recommendations – published in Regional Anesthesia and Pain Medicine – are the first guidelines in the United States to cover cannabis use as it relates to surgery, the group said.
Possible interactions
Use of cannabis has increased in recent years, and researchers have been concerned that the drug may interact with anesthesia and complicate pain management. Few studies have evaluated interactions between cannabis and anesthetic agents, however, according to the authors of the new guidelines.
“With the rising prevalence of both medical and recreational cannabis use in the general population, anesthesiologists, surgeons, and perioperative physicians must have an understanding of the effects of cannabis on physiology in order to provide safe perioperative care,” the guideline said.
“Before surgery, anesthesiologists should ask patients if they use cannabis – whether medicinally or recreationally – and be prepared to possibly change the anesthesia plan or delay the procedure in certain situations,” Samer Narouze, MD, PhD, ASRA president and senior author of the guidelines, said in a news release about the recommendations.
Although some patients may use cannabis to relieve pain, research shows that “regular users may have more pain and nausea after surgery, not less, and may need more medications, including opioids, to manage the discomfort,” said Dr. Narouze, chairman of the Center for Pain Medicine at Western Reserve Hospital in Cuyahoga Falls, Ohio.
Risks for vomiting, heart attack
The new recommendations were created by a committee of 13 experts, including anesthesiologists, chronic pain physicians, and a patient advocate. Shalini Shah, MD, vice chair of anesthesiology at the University of California, Irvine, was lead author of the document.
Four of 21 recommendations were classified as grade A, meaning that following them would be expected to provide substantial benefits. Those recommendations are to screen all patients before surgery; postpone elective surgery for patients who have altered mental status or impaired decision-making capacity at the time of surgery; counsel frequent, heavy users about the potential for cannabis use to impair postoperative pain control; and counsel pregnant patients about the risks of cannabis use to unborn children.
The authors cited studies to support their recommendations, including one showing that long-term cannabis use was associated with a 20% increase in the incidence of postoperative nausea and vomiting, a leading complaint of surgery patients. Other research has shown that cannabis use is linked to more pain and use of opioids after surgery.
Other recommendations include delaying elective surgery for at least 2 hours after a patient has smoked cannabis, owing to an increased risk for heart attack, and considering adjustment of ventilation settings during surgery for regular smokers of cannabis. Research has shown that smoking cannabis may be a rare trigger for myocardial infarction and is associated with airway inflammation and self-reported respiratory symptoms.
Nevertheless, doctors should not conduct universal toxicology screening, given a lack of evidence supporting this practice, the guideline stated.
The authors did not have enough information to make recommendations about reducing cannabis use before surgery or adjusting opioid prescriptions after surgery for patients who use cannabis, they said.
Kenneth Finn, MD, president of the American Board of Pain Medicine, welcomed the publication of the new guidelines. Dr. Finn, who practices at Springs Rehabilitation in Colorado Springs, has edited a textbook about cannabis in medicine and founded the International Academy on the Science and Impact of Cannabis.
“The vast majority of medical providers really have no idea about cannabis and what its impacts are on the human body,” Dr. Finn said.
For one, it can interact with numerous other drugs, including warfarin.
Guideline coauthor Eugene R. Viscusi, MD, professor of anesthesiology at the Sidney Kimmel Medical College, Philadelphia, emphasized that, while cannabis may be perceived as “natural,” it should not be considered differently from manufactured drugs.
Cannabis and cannabinoids represent “a class of very potent and pharmacologically active compounds,” Dr. Viscusi said in an interview. While researchers continue to assess possible medically beneficial effects of cannabis compounds, clinicians also need to be aware of the risks.
“The literature continues to emerge, and while we are always hopeful for good news, as physicians, we need to be very well versed on potential risks, especially in a high-risk situation like surgery,” he said.
Dr. Shah has consulted for companies that develop medical devices and drugs. Dr. Finn is the editor of the textbook, “Cannabis in Medicine: An Evidence-Based Approach” (Springer: New York, 2020), for which he receives royalties.
A version of this article first appeared on Medscape.com.
FROM REGIONAL ANETHESIA AND MEDICINE
Is thrombolysis safe for stroke patients on DOACs?
, a new study has found.
The study, the largest ever regarding the safety of thrombolysis in patients on DOACs, actually found a lower rate of sICH among patients taking DOACs than among those not taking anticoagulants.
“Thrombolysis is a backbone therapy in stroke, but the large population of patients who take DOACs are currently excluded from this treatment because DOAC use is a contraindication to treatment with thrombolysis. This is based on the presumption of an increased risk of sICH, but data to support or refute this presumption are lacking,” said senior author David J. Seiffge, MD, Bern University Hospital, Switzerland.
“Our results suggest that current guidelines need to be revised to remove the absolute contraindication of thrombolysis in patients on DOACs. The guidelines need to be more liberal on the use of thrombolysis in these patients,” he added.
“This study provides the basis for extending vital thrombolysis treatment to this substantial population of patients who take DOACs,” Dr. Seiffge said.
He estimates that 1 of every 6 stroke patients are taking a DOAC and that 1% to 2% of patients taking DOACs have a stroke each year. “As millions of patients are on DOACs, this is a large number of people who are not getting potentially life-saving thrombolysis therapy.”
Dr. Seiffge comments: “In our hospital we see at least one stroke patient on DOACs every day. It is a very frequent scenario. With this new data, we believe many of these patients could now benefit from thrombolysis without an increased bleeding risk.”
The study was published online in JAMA Neurology.
An international investigation
While thrombolysis is currently contraindicated for patients taking DOACs, some clinicians still administer thrombolysis to these patients. Different selection strategies are used, including the use of DOAC reversal agents prior to thrombolysis or the selection of patients with low anticoagulant activity, the authors noted.
The current study involved an international collaboration. The investigators compared the risk of sICH among patients who had recently taken DOACs and who underwent thrombolysis as treatment for acute ischemic stroke with the risk among control stroke patients who underwent thrombolysis but who had not been taking DOACs.
Potential contributing centers were identified by a systematic search of the literature based on published studies on the use of thrombolysis for patients who had recently taken DOACs or prospective stroke registries that may include patients who had recently taken DOACs.
The study included 832 patients from 64 centers worldwide who were confirmed to have taken a DOAC within 48 hours of receiving thrombolysis for acute ischemic stroke. The comparison group was made up of 32,375 patients who had experienced ischemic stroke that was treated with thrombolysis but who had received no prior anticoagulation therapy.
Compared with control patients, patients who had recently taken DOACs were older; the incidence of hypertension among them was higher; they had a higher degree of prestroke disability; they were less likely to be smokers; the time from symptom onset to treatment was longer; they had experienced more severe stroke; and they were more likely to have a large-vessel occlusion.
Of the patients taking DOACs, 30.3% received DOAC reversal prior to thrombolysis. For 27.0%, DOAC plasma levels were measured. The remainder were treated with thrombolysis without either of these selection methods.
Results showed that the unadjusted rate of sICH was 2.5% among patients taking DOACs, compared with 4.1% among control patients who were not taking anticoagulants.
After adjustment for stroke severity and other baseline sICH predictors, patients who had recently taken DOACs and who received thrombolysis had lower odds of developing sICH (adjusted odds ratio, 0.57; 95% confidence interval, 0.36-0.92; P = .02).
There was no difference between the selection strategies, and results were consistent in different sensitivity analyses.
The secondary outcome of any ICH occurred in 18.0% in patients taking DOACs, compared with 17.4% among control patients who used no anticoagulants. After adjustment, there was no difference in the odds for any ICH between the groups (aOR, 1.18; 95% CI, 0.95-1.45; P = .14).
The unadjusted rate of functional independence was 45% among patients taking DOACs, compared with 57% among control patients. After adjustment, patients who had recently taken DOACs and who underwent thrombolysis had numerically higher odds of being functionally independent than control patients, although this difference did not reach statistical significance (aOR, 1.13; 95% CI, 0.94-1.36; P = .20).
The association of DOAC therapy with lower odds of sICH remained when mechanical thrombectomy, large-vessel occlusion, or concomitant antiplatelet therapy was added to the model.
“This is by far the largest study to look at this issue of thrombolytic use in patients on DOACs, and we did not find any group on DOACs that had an excess ICH rate with thrombolysis,” Dr. Seiffge said,
He explained that receiving warfarin was at one time an absolute contraindication for thrombolysis, but after a 2014 study suggested that the risk was not increased for patients with an international normalized ratio below 117, this was downgraded to a relative contraindication.
“We think our study is comparable and should lead to a guideline change,” Dr. Seiffge commented.
“A relative contraindication allows clinicians the space to make a considered decision on an individual basis,” he added.
Dr. Seiffge said that at his hospital, local guidelines regarding this issue have already been changed on the basis of these data, and use of DOACs is now considered a relative contraindication.
“International guidelines can take years to update, so in the meantime, I think other centers will also go ahead with a more liberal approach. There are always some centers that are ahead of the guidelines,” he added.
Although the lower risk of sICH seen in patients who have recently used DOACs seems counterintuitive at first glance, there could be a pathophysiologic explanation for this finding, the authors suggest.
They point out that thrombin inhibition, either directly or via the coagulation cascade, might be protective against the occurrence of sICH.
“Anticoagulants may allow the clot to respond better to thrombolysis – the clot is not as solid and is easier to recanalize. This leads to smaller strokes and a lower bleeding risk. Thrombin generation is also a major driver for blood brain barrier breakdown. DOACs reduce thrombin generation, so reduce blood brain barrier breakdown and reduce bleeding,” Dr. Seiffge explained. “But these are hypotheses,” he added.
Study ‘meaningfully advances the field’
In an accompanying editorial, Eva A. Mistry, MBBS, University of Cincinnati, said the current study “meaningfully advances the field” and provides an estimation of safety of intravenous thrombolysis among patients who have taken DOACs within 48 hours of hospital admission.
She lists strengths of the study as inclusion of a large number of patients across several geographically diverse institutions with heterogeneous standard practices for thrombolysis with recent DOAC use and narrow confidence intervals regarding observed rates of sICH.
“Further, the upper bound of this confidence interval for the DOAC group is below 4%, which is a welcome result and provides supportive data for clinicians who already practice thrombolysis for patients with recent DOAC ingestion,” Dr. Mistry adds.
However, she points out several study limitations, which she says limit immediate, widespread clinical applicability.
These include use of a nonconcurrent control population, which included patients from centers that did not contribute to the DOAC group and the inclusion of Asian patients who likely received a lower thrombolytic dose.
Dr. Seiffge noted that the researchers did adjust for Asian patients but not for the thrombolytic dosage. “I personally do not think this affects the results, as Asian patients have a lower dosage because they have a higher bleeding risk. The lower bleeding risk with DOACs was seen in all continents.”
Dr. Mistry also suggests that the DOAC group itself is prone to selection bias from preferential thrombolysis of patients receiving DOAC who are at lower risk of sICH.
But Dr. Seiffge argued: “I think, actually, the opposite is true. The DOAC patients were older, had more severe comorbidities, and an increased bleeding risk.”
Dr. Mistry concluded, “Despite the limitations of the study design and enrolled population, these data may be used by clinicians to make individualized decisions regarding thrombolysis among patients with recent DOAC use. Importantly, this study lays the foundation for prospective, well-powered studies that definitively determine the safety of thrombolysis in this population.”
The study was supported by a grant from the Bangerter-Rhyner Foundation. Dr. Seiffge received grants from Bangerter Rhyner Foundation during the conduct of the study and personal fees from Bayer, Alexion, and VarmX outside the submitted work. Dr. Mistry receives grant funding from the National Institute of Neurological Disorders and Stroke and serves as a consultant for RAPID AI.
A version of this article first appeared on Medscape.com.
, a new study has found.
The study, the largest ever regarding the safety of thrombolysis in patients on DOACs, actually found a lower rate of sICH among patients taking DOACs than among those not taking anticoagulants.
“Thrombolysis is a backbone therapy in stroke, but the large population of patients who take DOACs are currently excluded from this treatment because DOAC use is a contraindication to treatment with thrombolysis. This is based on the presumption of an increased risk of sICH, but data to support or refute this presumption are lacking,” said senior author David J. Seiffge, MD, Bern University Hospital, Switzerland.
“Our results suggest that current guidelines need to be revised to remove the absolute contraindication of thrombolysis in patients on DOACs. The guidelines need to be more liberal on the use of thrombolysis in these patients,” he added.
“This study provides the basis for extending vital thrombolysis treatment to this substantial population of patients who take DOACs,” Dr. Seiffge said.
He estimates that 1 of every 6 stroke patients are taking a DOAC and that 1% to 2% of patients taking DOACs have a stroke each year. “As millions of patients are on DOACs, this is a large number of people who are not getting potentially life-saving thrombolysis therapy.”
Dr. Seiffge comments: “In our hospital we see at least one stroke patient on DOACs every day. It is a very frequent scenario. With this new data, we believe many of these patients could now benefit from thrombolysis without an increased bleeding risk.”
The study was published online in JAMA Neurology.
An international investigation
While thrombolysis is currently contraindicated for patients taking DOACs, some clinicians still administer thrombolysis to these patients. Different selection strategies are used, including the use of DOAC reversal agents prior to thrombolysis or the selection of patients with low anticoagulant activity, the authors noted.
The current study involved an international collaboration. The investigators compared the risk of sICH among patients who had recently taken DOACs and who underwent thrombolysis as treatment for acute ischemic stroke with the risk among control stroke patients who underwent thrombolysis but who had not been taking DOACs.
Potential contributing centers were identified by a systematic search of the literature based on published studies on the use of thrombolysis for patients who had recently taken DOACs or prospective stroke registries that may include patients who had recently taken DOACs.
The study included 832 patients from 64 centers worldwide who were confirmed to have taken a DOAC within 48 hours of receiving thrombolysis for acute ischemic stroke. The comparison group was made up of 32,375 patients who had experienced ischemic stroke that was treated with thrombolysis but who had received no prior anticoagulation therapy.
Compared with control patients, patients who had recently taken DOACs were older; the incidence of hypertension among them was higher; they had a higher degree of prestroke disability; they were less likely to be smokers; the time from symptom onset to treatment was longer; they had experienced more severe stroke; and they were more likely to have a large-vessel occlusion.
Of the patients taking DOACs, 30.3% received DOAC reversal prior to thrombolysis. For 27.0%, DOAC plasma levels were measured. The remainder were treated with thrombolysis without either of these selection methods.
Results showed that the unadjusted rate of sICH was 2.5% among patients taking DOACs, compared with 4.1% among control patients who were not taking anticoagulants.
After adjustment for stroke severity and other baseline sICH predictors, patients who had recently taken DOACs and who received thrombolysis had lower odds of developing sICH (adjusted odds ratio, 0.57; 95% confidence interval, 0.36-0.92; P = .02).
There was no difference between the selection strategies, and results were consistent in different sensitivity analyses.
The secondary outcome of any ICH occurred in 18.0% in patients taking DOACs, compared with 17.4% among control patients who used no anticoagulants. After adjustment, there was no difference in the odds for any ICH between the groups (aOR, 1.18; 95% CI, 0.95-1.45; P = .14).
The unadjusted rate of functional independence was 45% among patients taking DOACs, compared with 57% among control patients. After adjustment, patients who had recently taken DOACs and who underwent thrombolysis had numerically higher odds of being functionally independent than control patients, although this difference did not reach statistical significance (aOR, 1.13; 95% CI, 0.94-1.36; P = .20).
The association of DOAC therapy with lower odds of sICH remained when mechanical thrombectomy, large-vessel occlusion, or concomitant antiplatelet therapy was added to the model.
“This is by far the largest study to look at this issue of thrombolytic use in patients on DOACs, and we did not find any group on DOACs that had an excess ICH rate with thrombolysis,” Dr. Seiffge said,
He explained that receiving warfarin was at one time an absolute contraindication for thrombolysis, but after a 2014 study suggested that the risk was not increased for patients with an international normalized ratio below 117, this was downgraded to a relative contraindication.
“We think our study is comparable and should lead to a guideline change,” Dr. Seiffge commented.
“A relative contraindication allows clinicians the space to make a considered decision on an individual basis,” he added.
Dr. Seiffge said that at his hospital, local guidelines regarding this issue have already been changed on the basis of these data, and use of DOACs is now considered a relative contraindication.
“International guidelines can take years to update, so in the meantime, I think other centers will also go ahead with a more liberal approach. There are always some centers that are ahead of the guidelines,” he added.
Although the lower risk of sICH seen in patients who have recently used DOACs seems counterintuitive at first glance, there could be a pathophysiologic explanation for this finding, the authors suggest.
They point out that thrombin inhibition, either directly or via the coagulation cascade, might be protective against the occurrence of sICH.
“Anticoagulants may allow the clot to respond better to thrombolysis – the clot is not as solid and is easier to recanalize. This leads to smaller strokes and a lower bleeding risk. Thrombin generation is also a major driver for blood brain barrier breakdown. DOACs reduce thrombin generation, so reduce blood brain barrier breakdown and reduce bleeding,” Dr. Seiffge explained. “But these are hypotheses,” he added.
Study ‘meaningfully advances the field’
In an accompanying editorial, Eva A. Mistry, MBBS, University of Cincinnati, said the current study “meaningfully advances the field” and provides an estimation of safety of intravenous thrombolysis among patients who have taken DOACs within 48 hours of hospital admission.
She lists strengths of the study as inclusion of a large number of patients across several geographically diverse institutions with heterogeneous standard practices for thrombolysis with recent DOAC use and narrow confidence intervals regarding observed rates of sICH.
“Further, the upper bound of this confidence interval for the DOAC group is below 4%, which is a welcome result and provides supportive data for clinicians who already practice thrombolysis for patients with recent DOAC ingestion,” Dr. Mistry adds.
However, she points out several study limitations, which she says limit immediate, widespread clinical applicability.
These include use of a nonconcurrent control population, which included patients from centers that did not contribute to the DOAC group and the inclusion of Asian patients who likely received a lower thrombolytic dose.
Dr. Seiffge noted that the researchers did adjust for Asian patients but not for the thrombolytic dosage. “I personally do not think this affects the results, as Asian patients have a lower dosage because they have a higher bleeding risk. The lower bleeding risk with DOACs was seen in all continents.”
Dr. Mistry also suggests that the DOAC group itself is prone to selection bias from preferential thrombolysis of patients receiving DOAC who are at lower risk of sICH.
But Dr. Seiffge argued: “I think, actually, the opposite is true. The DOAC patients were older, had more severe comorbidities, and an increased bleeding risk.”
Dr. Mistry concluded, “Despite the limitations of the study design and enrolled population, these data may be used by clinicians to make individualized decisions regarding thrombolysis among patients with recent DOAC use. Importantly, this study lays the foundation for prospective, well-powered studies that definitively determine the safety of thrombolysis in this population.”
The study was supported by a grant from the Bangerter-Rhyner Foundation. Dr. Seiffge received grants from Bangerter Rhyner Foundation during the conduct of the study and personal fees from Bayer, Alexion, and VarmX outside the submitted work. Dr. Mistry receives grant funding from the National Institute of Neurological Disorders and Stroke and serves as a consultant for RAPID AI.
A version of this article first appeared on Medscape.com.
, a new study has found.
The study, the largest ever regarding the safety of thrombolysis in patients on DOACs, actually found a lower rate of sICH among patients taking DOACs than among those not taking anticoagulants.
“Thrombolysis is a backbone therapy in stroke, but the large population of patients who take DOACs are currently excluded from this treatment because DOAC use is a contraindication to treatment with thrombolysis. This is based on the presumption of an increased risk of sICH, but data to support or refute this presumption are lacking,” said senior author David J. Seiffge, MD, Bern University Hospital, Switzerland.
“Our results suggest that current guidelines need to be revised to remove the absolute contraindication of thrombolysis in patients on DOACs. The guidelines need to be more liberal on the use of thrombolysis in these patients,” he added.
“This study provides the basis for extending vital thrombolysis treatment to this substantial population of patients who take DOACs,” Dr. Seiffge said.
He estimates that 1 of every 6 stroke patients are taking a DOAC and that 1% to 2% of patients taking DOACs have a stroke each year. “As millions of patients are on DOACs, this is a large number of people who are not getting potentially life-saving thrombolysis therapy.”
Dr. Seiffge comments: “In our hospital we see at least one stroke patient on DOACs every day. It is a very frequent scenario. With this new data, we believe many of these patients could now benefit from thrombolysis without an increased bleeding risk.”
The study was published online in JAMA Neurology.
An international investigation
While thrombolysis is currently contraindicated for patients taking DOACs, some clinicians still administer thrombolysis to these patients. Different selection strategies are used, including the use of DOAC reversal agents prior to thrombolysis or the selection of patients with low anticoagulant activity, the authors noted.
The current study involved an international collaboration. The investigators compared the risk of sICH among patients who had recently taken DOACs and who underwent thrombolysis as treatment for acute ischemic stroke with the risk among control stroke patients who underwent thrombolysis but who had not been taking DOACs.
Potential contributing centers were identified by a systematic search of the literature based on published studies on the use of thrombolysis for patients who had recently taken DOACs or prospective stroke registries that may include patients who had recently taken DOACs.
The study included 832 patients from 64 centers worldwide who were confirmed to have taken a DOAC within 48 hours of receiving thrombolysis for acute ischemic stroke. The comparison group was made up of 32,375 patients who had experienced ischemic stroke that was treated with thrombolysis but who had received no prior anticoagulation therapy.
Compared with control patients, patients who had recently taken DOACs were older; the incidence of hypertension among them was higher; they had a higher degree of prestroke disability; they were less likely to be smokers; the time from symptom onset to treatment was longer; they had experienced more severe stroke; and they were more likely to have a large-vessel occlusion.
Of the patients taking DOACs, 30.3% received DOAC reversal prior to thrombolysis. For 27.0%, DOAC plasma levels were measured. The remainder were treated with thrombolysis without either of these selection methods.
Results showed that the unadjusted rate of sICH was 2.5% among patients taking DOACs, compared with 4.1% among control patients who were not taking anticoagulants.
After adjustment for stroke severity and other baseline sICH predictors, patients who had recently taken DOACs and who received thrombolysis had lower odds of developing sICH (adjusted odds ratio, 0.57; 95% confidence interval, 0.36-0.92; P = .02).
There was no difference between the selection strategies, and results were consistent in different sensitivity analyses.
The secondary outcome of any ICH occurred in 18.0% in patients taking DOACs, compared with 17.4% among control patients who used no anticoagulants. After adjustment, there was no difference in the odds for any ICH between the groups (aOR, 1.18; 95% CI, 0.95-1.45; P = .14).
The unadjusted rate of functional independence was 45% among patients taking DOACs, compared with 57% among control patients. After adjustment, patients who had recently taken DOACs and who underwent thrombolysis had numerically higher odds of being functionally independent than control patients, although this difference did not reach statistical significance (aOR, 1.13; 95% CI, 0.94-1.36; P = .20).
The association of DOAC therapy with lower odds of sICH remained when mechanical thrombectomy, large-vessel occlusion, or concomitant antiplatelet therapy was added to the model.
“This is by far the largest study to look at this issue of thrombolytic use in patients on DOACs, and we did not find any group on DOACs that had an excess ICH rate with thrombolysis,” Dr. Seiffge said,
He explained that receiving warfarin was at one time an absolute contraindication for thrombolysis, but after a 2014 study suggested that the risk was not increased for patients with an international normalized ratio below 117, this was downgraded to a relative contraindication.
“We think our study is comparable and should lead to a guideline change,” Dr. Seiffge commented.
“A relative contraindication allows clinicians the space to make a considered decision on an individual basis,” he added.
Dr. Seiffge said that at his hospital, local guidelines regarding this issue have already been changed on the basis of these data, and use of DOACs is now considered a relative contraindication.
“International guidelines can take years to update, so in the meantime, I think other centers will also go ahead with a more liberal approach. There are always some centers that are ahead of the guidelines,” he added.
Although the lower risk of sICH seen in patients who have recently used DOACs seems counterintuitive at first glance, there could be a pathophysiologic explanation for this finding, the authors suggest.
They point out that thrombin inhibition, either directly or via the coagulation cascade, might be protective against the occurrence of sICH.
“Anticoagulants may allow the clot to respond better to thrombolysis – the clot is not as solid and is easier to recanalize. This leads to smaller strokes and a lower bleeding risk. Thrombin generation is also a major driver for blood brain barrier breakdown. DOACs reduce thrombin generation, so reduce blood brain barrier breakdown and reduce bleeding,” Dr. Seiffge explained. “But these are hypotheses,” he added.
Study ‘meaningfully advances the field’
In an accompanying editorial, Eva A. Mistry, MBBS, University of Cincinnati, said the current study “meaningfully advances the field” and provides an estimation of safety of intravenous thrombolysis among patients who have taken DOACs within 48 hours of hospital admission.
She lists strengths of the study as inclusion of a large number of patients across several geographically diverse institutions with heterogeneous standard practices for thrombolysis with recent DOAC use and narrow confidence intervals regarding observed rates of sICH.
“Further, the upper bound of this confidence interval for the DOAC group is below 4%, which is a welcome result and provides supportive data for clinicians who already practice thrombolysis for patients with recent DOAC ingestion,” Dr. Mistry adds.
However, she points out several study limitations, which she says limit immediate, widespread clinical applicability.
These include use of a nonconcurrent control population, which included patients from centers that did not contribute to the DOAC group and the inclusion of Asian patients who likely received a lower thrombolytic dose.
Dr. Seiffge noted that the researchers did adjust for Asian patients but not for the thrombolytic dosage. “I personally do not think this affects the results, as Asian patients have a lower dosage because they have a higher bleeding risk. The lower bleeding risk with DOACs was seen in all continents.”
Dr. Mistry also suggests that the DOAC group itself is prone to selection bias from preferential thrombolysis of patients receiving DOAC who are at lower risk of sICH.
But Dr. Seiffge argued: “I think, actually, the opposite is true. The DOAC patients were older, had more severe comorbidities, and an increased bleeding risk.”
Dr. Mistry concluded, “Despite the limitations of the study design and enrolled population, these data may be used by clinicians to make individualized decisions regarding thrombolysis among patients with recent DOAC use. Importantly, this study lays the foundation for prospective, well-powered studies that definitively determine the safety of thrombolysis in this population.”
The study was supported by a grant from the Bangerter-Rhyner Foundation. Dr. Seiffge received grants from Bangerter Rhyner Foundation during the conduct of the study and personal fees from Bayer, Alexion, and VarmX outside the submitted work. Dr. Mistry receives grant funding from the National Institute of Neurological Disorders and Stroke and serves as a consultant for RAPID AI.
A version of this article first appeared on Medscape.com.
From JAMA Neurology
Antiepileptic drugs tied to increased Parkinson’s disease risk
, new research suggests.
Drawing on data from the UK Biobank, investigators compared more than 1,400 individuals diagnosed with Parkinson’s disease with matched control persons and found a considerably higher risk of developing Parkinson’s disease among those who had taken AEDs in comparison with those who had not. There was a trend linking a greater number of AED prescriptions and multiple AEDs associated with a greater risk for Parkinson’s disease.
“We observed an association between the most commonly prescribed antiepileptic drugs in the U.K. and Parkinson’s disease using data from UK Biobank,” said senior author Alastair Noyce, PhD, professor of neurology and neuroepidemiology and honorary consultant neurologist, Queen Mary University of London.
“This is the first time that a comprehensive study of the link between AEDs and Parkinson’s disease has been undertaken,” said Dr. Noyce.
He added that the findings have no immediate clinical implications, “but further research is definitely needed, [as] this is an interesting observation made in a research setting.”
The study was published online in JAMA Neurology.
Plausible, but unclear link
Recent observational studies have found a “temporal association” between epilepsy and incident Parkinson’s disease, but the mechanism underlying this association is “unclear,” the authors wrote.
It is “plausible” that AEDs “may account for some or all of the apparent association between epilepsy and Parkinson’s disease” and that movement disorders are potential side effects of AEDs, but the association between AEDs and Parkinson’s disease has “not been well studied,” so it remains “unclear” whether AEDs play a role in the association.
“We have previously reported an association between epilepsy and Parkinson’s disease in several different datasets. Here, we wanted to see if it could be explained by an association with the drugs used to treat epilepsy rather than epilepsy per se,” Dr. Noyce explained.
Are AEDs the culprit?
The researchers used data from the UK Biobank, a longitudinal cohort study with more than 500,000 participants, as well as linked primary care medication data to conduct a nested case-control study to investigate this potential association. Participants ranged in age from 40 to 69 years and were recruited between 2006 and 2010.
The researchers compared 1,433 individuals diagnosed with Parkinson’s disease with 8,598 control persons who were matched in a 6:1 ratio for age, sex, race, ethnicity, and socioeconomic status (median [interquartile range] age, 71 [65-75] years; 60.9% men; 97.5% White).
Of those with Parkinson’s disease, 4.3% had been prescribed an AED prior to the date of their being diagnosed with Parkinson’s disease, compared with 2.5% in the control group; 4.4% had been diagnosed with epilepsy, compared with 1% of the control persons.
The strongest evidence was for the association between lamotrigine, levetiracetam, and sodium valproate and Parkinson’s disease. There was “weaker evidence” for carbamazepine, although all the AEDs were associated with a higher risk of Parkinson’s disease.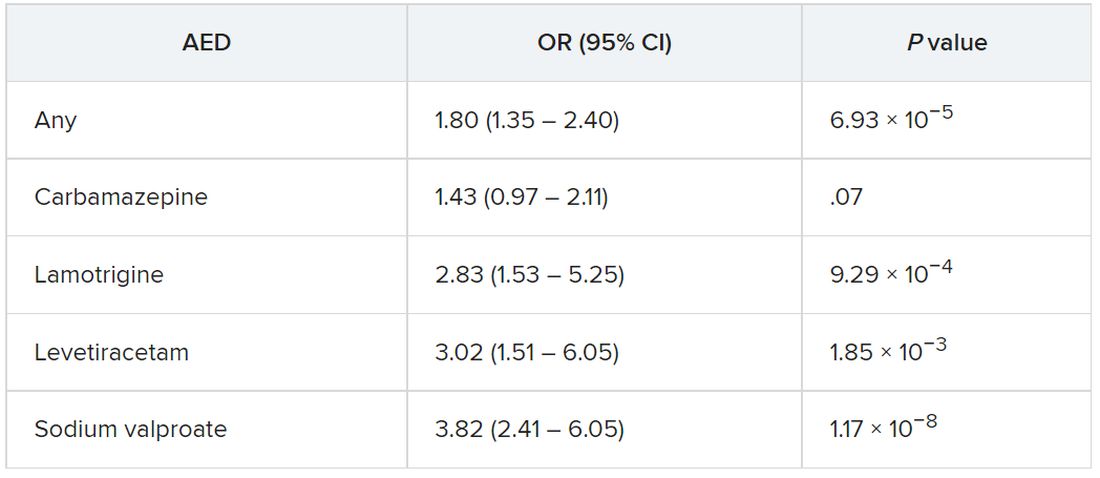
The odds of incident Parkinson’s disease were higher among those who were prescribed one or more AEDs and among individuals who were issued a higher number of prescriptions, the authors reported.
It is possible that it is the epilepsy itself that is associated with the risk of Parkinson’s disease, rather than the drugs, and that “likely explains part of the association we are seeing,” said Dr. Noyce.
“The bottom line is that more research into the links between epilepsy – and drugs used to treat epilepsy – and Parkinson’s disease is needed,” he said.
Moreover, “only with time will we work out whether the findings hold any real clinical relevance,” he added.
Alternative explanations
Commenting on the research, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, said, “It has been established in prior research that there is an association between epilepsy and Parkinson’s disease.” The current study “shows that having had a prescription written for one of four antiepileptic medications was associated with subsequently receiving a diagnosis of Parkinson’s disease.”
Although one possible conclusion is that the AEDs themselves increase the risk of developing Parkinson’s disease, “there seem to be other alternative explanations as to why a person who had been prescribed AEDs has an increased risk of receiving a diagnosis of Parkinson’s disease,” said Dr. Gilbert, an associate professor of neurology at Bellevue Hospital Center, New York, who was not involved with the current study.
For example, pre-motor changes in the brain of persons with Parkinson’s disease “may increase the risk of requiring an AED by potentially increasing the risk of having a seizure,” and “changes in the brain caused by the seizures for which AEDs are prescribed may increase the risk of Parkinson’s disease.”
Moreover, psychiatric changes related to Parkinson’s disease may have led to the prescription for AEDs, because at least two of the AEDs are also prescribed for mood stabilization, Dr. Gilbert suggested.
“An unanswered question that the paper acknowledges is, what about people who receive AEDs for reasons other than seizures? Do they also have an increased risk of Parkinson’s disease? This would be an interesting population to focus on because it would remove the link between AEDs and seizure and focus on the association between AEDs and Parkinson’s disease,” Dr. Gilbert said.
She emphasized that people who take AEDs for seizures “should not jump to the conclusion that they must come off these medications so as not to increase their risk of developing Parkinson’s disease.” She noted that having seizures “can be dangerous – injuries can occur during a seizure, and if a seizure can’t be stopped or a number occur in rapid succession, brain injury may result.”
For these reasons, people with “a tendency to have seizures need to protect themselves with AEDs” and “should certainly reach out to their neurologists with any questions,” Dr. Gilbert said.
The Preventive Neurology Unit is funded by Barts Charity. The Apocrita High Performance Cluster facility, supported by Queen Mary University London Research–IT Services, was used for this research. Dr. Noyce has received grants from Barts Charity, Parkinson’s UK, Cure Parkinson’s, the Michael J. Fox Foundation, Innovate UK, Solvemed, and Alchemab and personal fees from AstraZeneca, AbbVie, Zambon, BIAL, uMedeor, Alchemab, Britannia, and Charco Neurotech outside the submitted work. The other authors’ disclosures are listed on the original article. Dr. Gilbert reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Drawing on data from the UK Biobank, investigators compared more than 1,400 individuals diagnosed with Parkinson’s disease with matched control persons and found a considerably higher risk of developing Parkinson’s disease among those who had taken AEDs in comparison with those who had not. There was a trend linking a greater number of AED prescriptions and multiple AEDs associated with a greater risk for Parkinson’s disease.
“We observed an association between the most commonly prescribed antiepileptic drugs in the U.K. and Parkinson’s disease using data from UK Biobank,” said senior author Alastair Noyce, PhD, professor of neurology and neuroepidemiology and honorary consultant neurologist, Queen Mary University of London.
“This is the first time that a comprehensive study of the link between AEDs and Parkinson’s disease has been undertaken,” said Dr. Noyce.
He added that the findings have no immediate clinical implications, “but further research is definitely needed, [as] this is an interesting observation made in a research setting.”
The study was published online in JAMA Neurology.
Plausible, but unclear link
Recent observational studies have found a “temporal association” between epilepsy and incident Parkinson’s disease, but the mechanism underlying this association is “unclear,” the authors wrote.
It is “plausible” that AEDs “may account for some or all of the apparent association between epilepsy and Parkinson’s disease” and that movement disorders are potential side effects of AEDs, but the association between AEDs and Parkinson’s disease has “not been well studied,” so it remains “unclear” whether AEDs play a role in the association.
“We have previously reported an association between epilepsy and Parkinson’s disease in several different datasets. Here, we wanted to see if it could be explained by an association with the drugs used to treat epilepsy rather than epilepsy per se,” Dr. Noyce explained.
Are AEDs the culprit?
The researchers used data from the UK Biobank, a longitudinal cohort study with more than 500,000 participants, as well as linked primary care medication data to conduct a nested case-control study to investigate this potential association. Participants ranged in age from 40 to 69 years and were recruited between 2006 and 2010.
The researchers compared 1,433 individuals diagnosed with Parkinson’s disease with 8,598 control persons who were matched in a 6:1 ratio for age, sex, race, ethnicity, and socioeconomic status (median [interquartile range] age, 71 [65-75] years; 60.9% men; 97.5% White).
Of those with Parkinson’s disease, 4.3% had been prescribed an AED prior to the date of their being diagnosed with Parkinson’s disease, compared with 2.5% in the control group; 4.4% had been diagnosed with epilepsy, compared with 1% of the control persons.
The strongest evidence was for the association between lamotrigine, levetiracetam, and sodium valproate and Parkinson’s disease. There was “weaker evidence” for carbamazepine, although all the AEDs were associated with a higher risk of Parkinson’s disease.
The odds of incident Parkinson’s disease were higher among those who were prescribed one or more AEDs and among individuals who were issued a higher number of prescriptions, the authors reported.
It is possible that it is the epilepsy itself that is associated with the risk of Parkinson’s disease, rather than the drugs, and that “likely explains part of the association we are seeing,” said Dr. Noyce.
“The bottom line is that more research into the links between epilepsy – and drugs used to treat epilepsy – and Parkinson’s disease is needed,” he said.
Moreover, “only with time will we work out whether the findings hold any real clinical relevance,” he added.
Alternative explanations
Commenting on the research, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, said, “It has been established in prior research that there is an association between epilepsy and Parkinson’s disease.” The current study “shows that having had a prescription written for one of four antiepileptic medications was associated with subsequently receiving a diagnosis of Parkinson’s disease.”
Although one possible conclusion is that the AEDs themselves increase the risk of developing Parkinson’s disease, “there seem to be other alternative explanations as to why a person who had been prescribed AEDs has an increased risk of receiving a diagnosis of Parkinson’s disease,” said Dr. Gilbert, an associate professor of neurology at Bellevue Hospital Center, New York, who was not involved with the current study.
For example, pre-motor changes in the brain of persons with Parkinson’s disease “may increase the risk of requiring an AED by potentially increasing the risk of having a seizure,” and “changes in the brain caused by the seizures for which AEDs are prescribed may increase the risk of Parkinson’s disease.”
Moreover, psychiatric changes related to Parkinson’s disease may have led to the prescription for AEDs, because at least two of the AEDs are also prescribed for mood stabilization, Dr. Gilbert suggested.
“An unanswered question that the paper acknowledges is, what about people who receive AEDs for reasons other than seizures? Do they also have an increased risk of Parkinson’s disease? This would be an interesting population to focus on because it would remove the link between AEDs and seizure and focus on the association between AEDs and Parkinson’s disease,” Dr. Gilbert said.
She emphasized that people who take AEDs for seizures “should not jump to the conclusion that they must come off these medications so as not to increase their risk of developing Parkinson’s disease.” She noted that having seizures “can be dangerous – injuries can occur during a seizure, and if a seizure can’t be stopped or a number occur in rapid succession, brain injury may result.”
For these reasons, people with “a tendency to have seizures need to protect themselves with AEDs” and “should certainly reach out to their neurologists with any questions,” Dr. Gilbert said.
The Preventive Neurology Unit is funded by Barts Charity. The Apocrita High Performance Cluster facility, supported by Queen Mary University London Research–IT Services, was used for this research. Dr. Noyce has received grants from Barts Charity, Parkinson’s UK, Cure Parkinson’s, the Michael J. Fox Foundation, Innovate UK, Solvemed, and Alchemab and personal fees from AstraZeneca, AbbVie, Zambon, BIAL, uMedeor, Alchemab, Britannia, and Charco Neurotech outside the submitted work. The other authors’ disclosures are listed on the original article. Dr. Gilbert reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Drawing on data from the UK Biobank, investigators compared more than 1,400 individuals diagnosed with Parkinson’s disease with matched control persons and found a considerably higher risk of developing Parkinson’s disease among those who had taken AEDs in comparison with those who had not. There was a trend linking a greater number of AED prescriptions and multiple AEDs associated with a greater risk for Parkinson’s disease.
“We observed an association between the most commonly prescribed antiepileptic drugs in the U.K. and Parkinson’s disease using data from UK Biobank,” said senior author Alastair Noyce, PhD, professor of neurology and neuroepidemiology and honorary consultant neurologist, Queen Mary University of London.
“This is the first time that a comprehensive study of the link between AEDs and Parkinson’s disease has been undertaken,” said Dr. Noyce.
He added that the findings have no immediate clinical implications, “but further research is definitely needed, [as] this is an interesting observation made in a research setting.”
The study was published online in JAMA Neurology.
Plausible, but unclear link
Recent observational studies have found a “temporal association” between epilepsy and incident Parkinson’s disease, but the mechanism underlying this association is “unclear,” the authors wrote.
It is “plausible” that AEDs “may account for some or all of the apparent association between epilepsy and Parkinson’s disease” and that movement disorders are potential side effects of AEDs, but the association between AEDs and Parkinson’s disease has “not been well studied,” so it remains “unclear” whether AEDs play a role in the association.
“We have previously reported an association between epilepsy and Parkinson’s disease in several different datasets. Here, we wanted to see if it could be explained by an association with the drugs used to treat epilepsy rather than epilepsy per se,” Dr. Noyce explained.
Are AEDs the culprit?
The researchers used data from the UK Biobank, a longitudinal cohort study with more than 500,000 participants, as well as linked primary care medication data to conduct a nested case-control study to investigate this potential association. Participants ranged in age from 40 to 69 years and were recruited between 2006 and 2010.
The researchers compared 1,433 individuals diagnosed with Parkinson’s disease with 8,598 control persons who were matched in a 6:1 ratio for age, sex, race, ethnicity, and socioeconomic status (median [interquartile range] age, 71 [65-75] years; 60.9% men; 97.5% White).
Of those with Parkinson’s disease, 4.3% had been prescribed an AED prior to the date of their being diagnosed with Parkinson’s disease, compared with 2.5% in the control group; 4.4% had been diagnosed with epilepsy, compared with 1% of the control persons.
The strongest evidence was for the association between lamotrigine, levetiracetam, and sodium valproate and Parkinson’s disease. There was “weaker evidence” for carbamazepine, although all the AEDs were associated with a higher risk of Parkinson’s disease.
The odds of incident Parkinson’s disease were higher among those who were prescribed one or more AEDs and among individuals who were issued a higher number of prescriptions, the authors reported.
It is possible that it is the epilepsy itself that is associated with the risk of Parkinson’s disease, rather than the drugs, and that “likely explains part of the association we are seeing,” said Dr. Noyce.
“The bottom line is that more research into the links between epilepsy – and drugs used to treat epilepsy – and Parkinson’s disease is needed,” he said.
Moreover, “only with time will we work out whether the findings hold any real clinical relevance,” he added.
Alternative explanations
Commenting on the research, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, said, “It has been established in prior research that there is an association between epilepsy and Parkinson’s disease.” The current study “shows that having had a prescription written for one of four antiepileptic medications was associated with subsequently receiving a diagnosis of Parkinson’s disease.”
Although one possible conclusion is that the AEDs themselves increase the risk of developing Parkinson’s disease, “there seem to be other alternative explanations as to why a person who had been prescribed AEDs has an increased risk of receiving a diagnosis of Parkinson’s disease,” said Dr. Gilbert, an associate professor of neurology at Bellevue Hospital Center, New York, who was not involved with the current study.
For example, pre-motor changes in the brain of persons with Parkinson’s disease “may increase the risk of requiring an AED by potentially increasing the risk of having a seizure,” and “changes in the brain caused by the seizures for which AEDs are prescribed may increase the risk of Parkinson’s disease.”
Moreover, psychiatric changes related to Parkinson’s disease may have led to the prescription for AEDs, because at least two of the AEDs are also prescribed for mood stabilization, Dr. Gilbert suggested.
“An unanswered question that the paper acknowledges is, what about people who receive AEDs for reasons other than seizures? Do they also have an increased risk of Parkinson’s disease? This would be an interesting population to focus on because it would remove the link between AEDs and seizure and focus on the association between AEDs and Parkinson’s disease,” Dr. Gilbert said.
She emphasized that people who take AEDs for seizures “should not jump to the conclusion that they must come off these medications so as not to increase their risk of developing Parkinson’s disease.” She noted that having seizures “can be dangerous – injuries can occur during a seizure, and if a seizure can’t be stopped or a number occur in rapid succession, brain injury may result.”
For these reasons, people with “a tendency to have seizures need to protect themselves with AEDs” and “should certainly reach out to their neurologists with any questions,” Dr. Gilbert said.
The Preventive Neurology Unit is funded by Barts Charity. The Apocrita High Performance Cluster facility, supported by Queen Mary University London Research–IT Services, was used for this research. Dr. Noyce has received grants from Barts Charity, Parkinson’s UK, Cure Parkinson’s, the Michael J. Fox Foundation, Innovate UK, Solvemed, and Alchemab and personal fees from AstraZeneca, AbbVie, Zambon, BIAL, uMedeor, Alchemab, Britannia, and Charco Neurotech outside the submitted work. The other authors’ disclosures are listed on the original article. Dr. Gilbert reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Stem cell transplant superior to DMTs for secondary progressive MS
new research suggests.
Results from a retrospective study show that more than 60% of patients with SPMS who received AHSCT were free from disability progression at 5 years. Also for these patients, improvement was more likely to be maintained for years after treatment.
The investigators noted that patients with secondary progressive disease often show little benefit from other DMTs, so interest in other treatments is high. While AHSCT is known to offer good results for patients with relapsing remitting MS, studies of its efficacy for SPMS have yielded conflicting results.
The new findings suggest it may be time to take another look at this therapy for patients with active, more severe disease, the researchers wrote.
“AHSCT may become a treatment option in secondary progressive MS patients with inflammatory activity who have failed available treatments,” said coinvestigator Matilde Inglese, MD, PhD, professor of neurology at the University of Genoa (Italy).
“Patients selection is very important to ensure the best treatment response and minimize safety issues, including transplant-related mortality,” Dr. Inglese added.
The findings were published online in Neurology.
Class III evidence
In the retrospective, propensity-matching study, researchers used two Italian registries to identify 79 patients who were treated off label with AHSCT and 1,975 patients who received another therapy.
Other DMTs included in the control-group analysis were beta-interferons, azathioprine, glatiramer acetate, mitoxantrone, fingolimod, natalizumab, methotrexate, teriflunomide, cyclophosphamide, dimethyl fumarate, or alemtuzumab.
Results showed that time to first disability progression was significantly longer for patients who had received transplants (hazard ratio, 0.5; P = .005); 61.7% of the AHSCT group were free of disability progression at 5 years versus 46.3% of the control group.
Among patients who received AHSCT, relapse rates were lower in comparison with those who received other DMTs (P < .001), and disability scores were lower over 10 years (P < .001).
The transplant group was also significantly more likely than the other-DMTs group to achieve sustained improvement in disability 3 years after treatment (34.7% vs. 4.6%; P < .001).
“This study provides Class III evidence that autologous hematopoietic stem cell transplants prolonged the time to confirmed disability progression compared to other disease-modifying therapies,” the investigators wrote.
Extends the treatment population
Commenting on the study, Jeff Cohen, MD, director of experimental therapeutics at the Mellen Center for Multiple Sclerosis Treatment and Research at the Cleveland Clinic, said the research “extends the population for which hematopoietic stem cell transplant should be considered.”
Although previous studies did not show a benefit for patients with severe progressive MS, participants in the current study had secondary progressive MS and superimposed relapse activity, said Dr. Cohen, who was not involved with the research.
“We think that indicates a greater likelihood of benefit” from AHSCT, he noted. “The fact that someone has overt progression or somewhat more severe disability doesn’t preclude the use of stem cell transplant.”
Dr. Cohen pointed out, however, that the study is not without limitations. The exclusion of patients taking B-cell therapies from the SPMS control group raises the question of whether similar results would come from a comparison with AHSCT.
In addition, Dr. Cohen noted there are safety concerns about the therapy, which has yielded higher transplant-related mortality among patients with SPMS – although only one patient in the current study died following the transplant.
Still, the findings are promising, Dr. Cohen added.
“I think as more data accumulate that supports its benefit and reasonable safety in a variety of populations, we’ll see it used more,” he said.
The study was funded by the Italian Multiple Sclerosis Foundation. Dr. Inglese has received fees for consultation from Roche, Genzyme, Merck, Biogen, and Novartis. Dr. Cohen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Results from a retrospective study show that more than 60% of patients with SPMS who received AHSCT were free from disability progression at 5 years. Also for these patients, improvement was more likely to be maintained for years after treatment.
The investigators noted that patients with secondary progressive disease often show little benefit from other DMTs, so interest in other treatments is high. While AHSCT is known to offer good results for patients with relapsing remitting MS, studies of its efficacy for SPMS have yielded conflicting results.
The new findings suggest it may be time to take another look at this therapy for patients with active, more severe disease, the researchers wrote.
“AHSCT may become a treatment option in secondary progressive MS patients with inflammatory activity who have failed available treatments,” said coinvestigator Matilde Inglese, MD, PhD, professor of neurology at the University of Genoa (Italy).
“Patients selection is very important to ensure the best treatment response and minimize safety issues, including transplant-related mortality,” Dr. Inglese added.
The findings were published online in Neurology.
Class III evidence
In the retrospective, propensity-matching study, researchers used two Italian registries to identify 79 patients who were treated off label with AHSCT and 1,975 patients who received another therapy.
Other DMTs included in the control-group analysis were beta-interferons, azathioprine, glatiramer acetate, mitoxantrone, fingolimod, natalizumab, methotrexate, teriflunomide, cyclophosphamide, dimethyl fumarate, or alemtuzumab.
Results showed that time to first disability progression was significantly longer for patients who had received transplants (hazard ratio, 0.5; P = .005); 61.7% of the AHSCT group were free of disability progression at 5 years versus 46.3% of the control group.
Among patients who received AHSCT, relapse rates were lower in comparison with those who received other DMTs (P < .001), and disability scores were lower over 10 years (P < .001).
The transplant group was also significantly more likely than the other-DMTs group to achieve sustained improvement in disability 3 years after treatment (34.7% vs. 4.6%; P < .001).
“This study provides Class III evidence that autologous hematopoietic stem cell transplants prolonged the time to confirmed disability progression compared to other disease-modifying therapies,” the investigators wrote.
Extends the treatment population
Commenting on the study, Jeff Cohen, MD, director of experimental therapeutics at the Mellen Center for Multiple Sclerosis Treatment and Research at the Cleveland Clinic, said the research “extends the population for which hematopoietic stem cell transplant should be considered.”
Although previous studies did not show a benefit for patients with severe progressive MS, participants in the current study had secondary progressive MS and superimposed relapse activity, said Dr. Cohen, who was not involved with the research.
“We think that indicates a greater likelihood of benefit” from AHSCT, he noted. “The fact that someone has overt progression or somewhat more severe disability doesn’t preclude the use of stem cell transplant.”
Dr. Cohen pointed out, however, that the study is not without limitations. The exclusion of patients taking B-cell therapies from the SPMS control group raises the question of whether similar results would come from a comparison with AHSCT.
In addition, Dr. Cohen noted there are safety concerns about the therapy, which has yielded higher transplant-related mortality among patients with SPMS – although only one patient in the current study died following the transplant.
Still, the findings are promising, Dr. Cohen added.
“I think as more data accumulate that supports its benefit and reasonable safety in a variety of populations, we’ll see it used more,” he said.
The study was funded by the Italian Multiple Sclerosis Foundation. Dr. Inglese has received fees for consultation from Roche, Genzyme, Merck, Biogen, and Novartis. Dr. Cohen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Results from a retrospective study show that more than 60% of patients with SPMS who received AHSCT were free from disability progression at 5 years. Also for these patients, improvement was more likely to be maintained for years after treatment.
The investigators noted that patients with secondary progressive disease often show little benefit from other DMTs, so interest in other treatments is high. While AHSCT is known to offer good results for patients with relapsing remitting MS, studies of its efficacy for SPMS have yielded conflicting results.
The new findings suggest it may be time to take another look at this therapy for patients with active, more severe disease, the researchers wrote.
“AHSCT may become a treatment option in secondary progressive MS patients with inflammatory activity who have failed available treatments,” said coinvestigator Matilde Inglese, MD, PhD, professor of neurology at the University of Genoa (Italy).
“Patients selection is very important to ensure the best treatment response and minimize safety issues, including transplant-related mortality,” Dr. Inglese added.
The findings were published online in Neurology.
Class III evidence
In the retrospective, propensity-matching study, researchers used two Italian registries to identify 79 patients who were treated off label with AHSCT and 1,975 patients who received another therapy.
Other DMTs included in the control-group analysis were beta-interferons, azathioprine, glatiramer acetate, mitoxantrone, fingolimod, natalizumab, methotrexate, teriflunomide, cyclophosphamide, dimethyl fumarate, or alemtuzumab.
Results showed that time to first disability progression was significantly longer for patients who had received transplants (hazard ratio, 0.5; P = .005); 61.7% of the AHSCT group were free of disability progression at 5 years versus 46.3% of the control group.
Among patients who received AHSCT, relapse rates were lower in comparison with those who received other DMTs (P < .001), and disability scores were lower over 10 years (P < .001).
The transplant group was also significantly more likely than the other-DMTs group to achieve sustained improvement in disability 3 years after treatment (34.7% vs. 4.6%; P < .001).
“This study provides Class III evidence that autologous hematopoietic stem cell transplants prolonged the time to confirmed disability progression compared to other disease-modifying therapies,” the investigators wrote.
Extends the treatment population
Commenting on the study, Jeff Cohen, MD, director of experimental therapeutics at the Mellen Center for Multiple Sclerosis Treatment and Research at the Cleveland Clinic, said the research “extends the population for which hematopoietic stem cell transplant should be considered.”
Although previous studies did not show a benefit for patients with severe progressive MS, participants in the current study had secondary progressive MS and superimposed relapse activity, said Dr. Cohen, who was not involved with the research.
“We think that indicates a greater likelihood of benefit” from AHSCT, he noted. “The fact that someone has overt progression or somewhat more severe disability doesn’t preclude the use of stem cell transplant.”
Dr. Cohen pointed out, however, that the study is not without limitations. The exclusion of patients taking B-cell therapies from the SPMS control group raises the question of whether similar results would come from a comparison with AHSCT.
In addition, Dr. Cohen noted there are safety concerns about the therapy, which has yielded higher transplant-related mortality among patients with SPMS – although only one patient in the current study died following the transplant.
Still, the findings are promising, Dr. Cohen added.
“I think as more data accumulate that supports its benefit and reasonable safety in a variety of populations, we’ll see it used more,” he said.
The study was funded by the Italian Multiple Sclerosis Foundation. Dr. Inglese has received fees for consultation from Roche, Genzyme, Merck, Biogen, and Novartis. Dr. Cohen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Prodromal Parkinson’s disease tied to significant functional impairment
new research shows.
The new findings come from a large case-control study that analyzed Medicare claims data to evaluate functional limitations in prodromal Parkinson’s disease, leading the investigators to suggest prodromal Parkinson’s disease should be recognized as a distinct disease stage.
“It’s increasingly recognized as a stage of Parkinson’s and there is an argument here for that,” said lead investigator Cameron Miller-Patterson, MD, assistant professor of neurology at Virginia Commonwealth University, Richmond. “Because we’re finding that people with prodromal Parkinson’s disease may have functional limitations, identifying them sooner and getting them the appropriate symptomatic therapy could be helpful.”
The findings were published online in JAMA Neurology.
Improving quality of life
Individuals with prodromal Parkinson’s disease have symptoms of Parkinson’s disease, but not enough to meet diagnostic criteria. However, all patients with prodromal Parkinson’s disease eventually meet that threshold.
To evaluate whether functional limitations are present in individuals with Parkinson’s disease prior to diagnosis versus the general population, researchers analyzed Medicare-linked data on 6,674 individuals aged 65 years and older who participated in the National Health and Aging Trends Study, a longitudinal survey in the United States. Survey questions evaluated dexterity, eating, mobility, mood, pain, sleep, speech, strength, and vision.
Patients with incident Parkinson’s disease were defined as having two or more Medicare diagnoses. Controls were defined as those with Medicare eligibility at baseline and 2 or more years prior, with no diagnosis.
Compared with individuals who never had Parkinson’s disease, those who eventually received a diagnosis were less likely to report being able to walk 6 blocks (odds ratio, 0.34; 95% confidence interval, 0.15-0.82), stand independently from kneeling (OR, 0.30; 95% CI, 0.11-0.85) or lift a heavy object overhead (OR, 0.36; 95% CI, 0.15-0.87). They were also more likely to report imbalance (OR, 2.77; 95% CI, 1.24-6.20) 3 years prior to diagnosis.
“Generally, we don’t start treating people until we see them in the clinic and give them a diagnosis of Parkinson’s disease,” Dr. Miller-Patterson said. “If we identify them earlier, even before diagnosis, we may be able to improve their quality of life by treating them sooner.”
Serving patients better
Better recognition of prodromal Parkinson’s disease could also help identify participants for clinical trials of therapeutics that could slow disease progression, something that is beyond the ability of currently approved medications.
This, and growing support for distinguishing prodromal Parkinson’s disease as an official stage of Parkinson’s disease, makes findings such as these both timely and important, the authors of an accompanying commentary wrote .
“The recognition of a prodromal period has been viewed as potentially critical to the success of disease-modifying interventions, on the argument that it may be too late to enact meaningful clinical change once symptoms clinically manifest given the degree of neurodegeneration already present,” Ian O. Bledsoe, MD, Weill Institute for Neurosciences, University of California, San Francisco, and coauthors wrote.
One limitation, however, is that the study design didn’t allow researchers to determine if individuals with eventual Parkinson’s disease who reported parkinsonian symptoms had prodromal Parkinson’s disease or undiagnosed disease. The answer would clarify whether prodromal Parkinson’s disease is more common than previously thought or if Parkinson’s disease diagnosis is often delayed for years – or both.
“Despite the limitations of this study, its broader point and importance remain: People appear to have some markers of functional decline before they are diagnosed with Parkinson’s disease,” the editorialists wrote. “Additionally, motor dysfunction may arise at an earlier time point in the disease than we typically think. There is a potential opportunity to serve this population better.”
The study was funded by the National Institutes of Health. Dr. Miller-Patterson reported receiving other NIH grants during the course of the study. Dr. Bledsoe reported personal fees from Boston Scientific, Amneal Pharmaceuticals, IDEO, Accorda, Humancraft.com, and Putnam Associates, as well as grants from the National Institutes of Health, the Michael J. Fox Foundation, and Dystonia Medical.
A version of this article first appeared on Medscape.com.
new research shows.
The new findings come from a large case-control study that analyzed Medicare claims data to evaluate functional limitations in prodromal Parkinson’s disease, leading the investigators to suggest prodromal Parkinson’s disease should be recognized as a distinct disease stage.
“It’s increasingly recognized as a stage of Parkinson’s and there is an argument here for that,” said lead investigator Cameron Miller-Patterson, MD, assistant professor of neurology at Virginia Commonwealth University, Richmond. “Because we’re finding that people with prodromal Parkinson’s disease may have functional limitations, identifying them sooner and getting them the appropriate symptomatic therapy could be helpful.”
The findings were published online in JAMA Neurology.
Improving quality of life
Individuals with prodromal Parkinson’s disease have symptoms of Parkinson’s disease, but not enough to meet diagnostic criteria. However, all patients with prodromal Parkinson’s disease eventually meet that threshold.
To evaluate whether functional limitations are present in individuals with Parkinson’s disease prior to diagnosis versus the general population, researchers analyzed Medicare-linked data on 6,674 individuals aged 65 years and older who participated in the National Health and Aging Trends Study, a longitudinal survey in the United States. Survey questions evaluated dexterity, eating, mobility, mood, pain, sleep, speech, strength, and vision.
Patients with incident Parkinson’s disease were defined as having two or more Medicare diagnoses. Controls were defined as those with Medicare eligibility at baseline and 2 or more years prior, with no diagnosis.
Compared with individuals who never had Parkinson’s disease, those who eventually received a diagnosis were less likely to report being able to walk 6 blocks (odds ratio, 0.34; 95% confidence interval, 0.15-0.82), stand independently from kneeling (OR, 0.30; 95% CI, 0.11-0.85) or lift a heavy object overhead (OR, 0.36; 95% CI, 0.15-0.87). They were also more likely to report imbalance (OR, 2.77; 95% CI, 1.24-6.20) 3 years prior to diagnosis.
“Generally, we don’t start treating people until we see them in the clinic and give them a diagnosis of Parkinson’s disease,” Dr. Miller-Patterson said. “If we identify them earlier, even before diagnosis, we may be able to improve their quality of life by treating them sooner.”
Serving patients better
Better recognition of prodromal Parkinson’s disease could also help identify participants for clinical trials of therapeutics that could slow disease progression, something that is beyond the ability of currently approved medications.
This, and growing support for distinguishing prodromal Parkinson’s disease as an official stage of Parkinson’s disease, makes findings such as these both timely and important, the authors of an accompanying commentary wrote .
“The recognition of a prodromal period has been viewed as potentially critical to the success of disease-modifying interventions, on the argument that it may be too late to enact meaningful clinical change once symptoms clinically manifest given the degree of neurodegeneration already present,” Ian O. Bledsoe, MD, Weill Institute for Neurosciences, University of California, San Francisco, and coauthors wrote.
One limitation, however, is that the study design didn’t allow researchers to determine if individuals with eventual Parkinson’s disease who reported parkinsonian symptoms had prodromal Parkinson’s disease or undiagnosed disease. The answer would clarify whether prodromal Parkinson’s disease is more common than previously thought or if Parkinson’s disease diagnosis is often delayed for years – or both.
“Despite the limitations of this study, its broader point and importance remain: People appear to have some markers of functional decline before they are diagnosed with Parkinson’s disease,” the editorialists wrote. “Additionally, motor dysfunction may arise at an earlier time point in the disease than we typically think. There is a potential opportunity to serve this population better.”
The study was funded by the National Institutes of Health. Dr. Miller-Patterson reported receiving other NIH grants during the course of the study. Dr. Bledsoe reported personal fees from Boston Scientific, Amneal Pharmaceuticals, IDEO, Accorda, Humancraft.com, and Putnam Associates, as well as grants from the National Institutes of Health, the Michael J. Fox Foundation, and Dystonia Medical.
A version of this article first appeared on Medscape.com.
new research shows.
The new findings come from a large case-control study that analyzed Medicare claims data to evaluate functional limitations in prodromal Parkinson’s disease, leading the investigators to suggest prodromal Parkinson’s disease should be recognized as a distinct disease stage.
“It’s increasingly recognized as a stage of Parkinson’s and there is an argument here for that,” said lead investigator Cameron Miller-Patterson, MD, assistant professor of neurology at Virginia Commonwealth University, Richmond. “Because we’re finding that people with prodromal Parkinson’s disease may have functional limitations, identifying them sooner and getting them the appropriate symptomatic therapy could be helpful.”
The findings were published online in JAMA Neurology.
Improving quality of life
Individuals with prodromal Parkinson’s disease have symptoms of Parkinson’s disease, but not enough to meet diagnostic criteria. However, all patients with prodromal Parkinson’s disease eventually meet that threshold.
To evaluate whether functional limitations are present in individuals with Parkinson’s disease prior to diagnosis versus the general population, researchers analyzed Medicare-linked data on 6,674 individuals aged 65 years and older who participated in the National Health and Aging Trends Study, a longitudinal survey in the United States. Survey questions evaluated dexterity, eating, mobility, mood, pain, sleep, speech, strength, and vision.
Patients with incident Parkinson’s disease were defined as having two or more Medicare diagnoses. Controls were defined as those with Medicare eligibility at baseline and 2 or more years prior, with no diagnosis.
Compared with individuals who never had Parkinson’s disease, those who eventually received a diagnosis were less likely to report being able to walk 6 blocks (odds ratio, 0.34; 95% confidence interval, 0.15-0.82), stand independently from kneeling (OR, 0.30; 95% CI, 0.11-0.85) or lift a heavy object overhead (OR, 0.36; 95% CI, 0.15-0.87). They were also more likely to report imbalance (OR, 2.77; 95% CI, 1.24-6.20) 3 years prior to diagnosis.
“Generally, we don’t start treating people until we see them in the clinic and give them a diagnosis of Parkinson’s disease,” Dr. Miller-Patterson said. “If we identify them earlier, even before diagnosis, we may be able to improve their quality of life by treating them sooner.”
Serving patients better
Better recognition of prodromal Parkinson’s disease could also help identify participants for clinical trials of therapeutics that could slow disease progression, something that is beyond the ability of currently approved medications.
This, and growing support for distinguishing prodromal Parkinson’s disease as an official stage of Parkinson’s disease, makes findings such as these both timely and important, the authors of an accompanying commentary wrote .
“The recognition of a prodromal period has been viewed as potentially critical to the success of disease-modifying interventions, on the argument that it may be too late to enact meaningful clinical change once symptoms clinically manifest given the degree of neurodegeneration already present,” Ian O. Bledsoe, MD, Weill Institute for Neurosciences, University of California, San Francisco, and coauthors wrote.
One limitation, however, is that the study design didn’t allow researchers to determine if individuals with eventual Parkinson’s disease who reported parkinsonian symptoms had prodromal Parkinson’s disease or undiagnosed disease. The answer would clarify whether prodromal Parkinson’s disease is more common than previously thought or if Parkinson’s disease diagnosis is often delayed for years – or both.
“Despite the limitations of this study, its broader point and importance remain: People appear to have some markers of functional decline before they are diagnosed with Parkinson’s disease,” the editorialists wrote. “Additionally, motor dysfunction may arise at an earlier time point in the disease than we typically think. There is a potential opportunity to serve this population better.”
The study was funded by the National Institutes of Health. Dr. Miller-Patterson reported receiving other NIH grants during the course of the study. Dr. Bledsoe reported personal fees from Boston Scientific, Amneal Pharmaceuticals, IDEO, Accorda, Humancraft.com, and Putnam Associates, as well as grants from the National Institutes of Health, the Michael J. Fox Foundation, and Dystonia Medical.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Cluster headache tied to high risk of mental and neurologic disorders
, leading to significant disability and absenteeism, new research shows.
Results from a Swedish register-based study also showed that patients with cluster headache had a sixfold increased risk for central nervous system disorders and a twofold increased risk for musculoskeletal disorders.
Although cluster headaches are often more prevalent in men, researchers found that multimorbidity rates were significantly higher in women. In addition, rates of external injuries were significantly higher among individuals with cluster headache than among persons without cluster headache.
“The findings very clearly indicate that cluster headache patients suffer from other health issues as well and that they are at risk of having longer periods of times when they cannot work,” said lead investigator Caroline Ran, PhD, a research specialist in the department of neuroscience at the Karolinska Institutet, Stockholm.
“It’s really important for clinicians to look at cluster headache from a broader perspective and make sure that patients are followed up so that they don’t risk ending up in a situation where they have several comorbidities,” Dr. Ran added.
The findings were published online in Neurology.
‘Striking’ finding
Cluster headache is one of the most severe and debilitating types of headache. It causes intense pain behind the eyes, which has been described as being worse than pain associated with childbirth or kidney stones.
Attacks can occur multiple times in a single day and can last up to 3 hours. Cluster headache is rare, occurring in about 1 in 1,000 individuals, and is more common in men. Underdiagnosis is common – especially in women.
The study drew on two Swedish population-based registries and included 3,240 patients with cluster headache aged 16-64 years and 16,200 matched control persons. The analysis covered medical visits from 2001 to 2010.
Results showed that 91.9% of participants with cluster headache had some type of multimorbidity. By comparison, 77.6% of the control group had some type of multimorbidity (odds ratio, 3.26; P < .0001).
Prior studies have shown a higher incidence of mental health and behavioral disorders among patients with cluster headache. However, when the researchers removed those conditions along with external injuries from the dataset, patients with headache were still significantly more likely to have multiple co-occurring illnesses (86.7% vs. 68.8%; OR, 2.95; P < .0001).
The most common comorbid conditions in the overall cluster headache group were diseases of the nervous system (OR, 5.9; 95% CI, 5.46 -6.42); 51.8% of the cluster headache group reported these disorders, compared with just 15.4% of the control group.
Diseases of the eye, the respiratory, gastrointestinal, and musculoskeletal systems, and connective tissue were also significantly more common among patients with cluster headache.
“For each diagnosis that we investigated, we found a higher incidence in the cluster headache group, and we thought this was a very striking finding and worth discussing in the clinical setting that these patients are at risk of general ill health,” Dr. Ran said.
Risky behavior?
Another novel finding was the higher rate of external injuries among the cluster headache group, compared with the control group. The finding seems to back up the theory that patients with cluster headache are more likely to engage in risky behaviors, the researchers noted.
In the cluster headache group, external injuries were reported by 47.1% of men and 41% of women, versus 34.9% and 26.0%, respectively, in the control group.
“Now we can also show that cluster headache patients have more injuries and that is totally unrelated to the biological health of the individuals, so that could also indicate higher risk taking,” Dr. Ran said.
Overall multimorbidity rates and diagnoses in each medical category except external injury were higher among women with cluster headache than men with headaches. In addition, the mean number of days on sick leave and disability pension was higher among women with cluster headache than among men with cluster headache (83.71 days vs. 52.56 days).
Overall, the mean number of sickness absence and disability pension net days in 2010 was nearly twice as high in the cluster headache group as in the control group (63.15 days vs. 34.08 days).
Removing mental and behavioral health disorders from the mix did not lower those numbers.
“Our numbers indicate that the mental health issues that are related to cluster headache might not impact their work situation as much as the other comorbidities,” Dr. Ran said.
Struggle is real
Commenting on the findings, Heidi Schwarz, MD, professor of clinical neurology at the University of Rochester (N.Y.) Medical Center, called the study a “valuable contribution” to the field and to the treatment of cluster headache.
“It’s a good study that addresses factors that really need to be considered as you take care of these patients,” said Dr. Schwarz, who was not involved with the research.
“The most salient features of this is that cluster headache is quite disabling, and if you add a comorbidity to it, it’s even more disabling,” she said.
Dr. Schwarz noted that cluster headache is often misdiagnosed as migraine or is overlooked altogether, especially in women. These data underscore that, although cluster headache is more common in men, it affects women too and could lead to even greater disability.
“This has a direct impact on patient quality of life, and in the end, that really should be what we’re looking to enhance,” Dr. Schwarz said. “When a patient with cluster comes in and they tell you they’re really struggling, believe them because it’s quite real.”
The findings also fill a gap in the literature and offer the kind of data that could not be collected in the United States, she noted. Sweden provides paid sick time for all workers aged 16 and older and offers a disability pension to all workers whose ability to work is temporarily or permanently inhibited because of illness or injury.
“You will never get this kind of data in the United States because this kind of data comes from two datasets that are extremely inclusive and detailed in a society, Sweden, where they have a social support system,” Dr. Schwarz said.
The study was funded by the Swedish Research Council, the Swedish Brain Foundation, and Mellby Gård, Region Stockholm, Märta Lundkvist stiftelse and Karolinska Institutet research funds. Dr. Ran and Dr. Schwarz report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, leading to significant disability and absenteeism, new research shows.
Results from a Swedish register-based study also showed that patients with cluster headache had a sixfold increased risk for central nervous system disorders and a twofold increased risk for musculoskeletal disorders.
Although cluster headaches are often more prevalent in men, researchers found that multimorbidity rates were significantly higher in women. In addition, rates of external injuries were significantly higher among individuals with cluster headache than among persons without cluster headache.
“The findings very clearly indicate that cluster headache patients suffer from other health issues as well and that they are at risk of having longer periods of times when they cannot work,” said lead investigator Caroline Ran, PhD, a research specialist in the department of neuroscience at the Karolinska Institutet, Stockholm.
“It’s really important for clinicians to look at cluster headache from a broader perspective and make sure that patients are followed up so that they don’t risk ending up in a situation where they have several comorbidities,” Dr. Ran added.
The findings were published online in Neurology.
‘Striking’ finding
Cluster headache is one of the most severe and debilitating types of headache. It causes intense pain behind the eyes, which has been described as being worse than pain associated with childbirth or kidney stones.
Attacks can occur multiple times in a single day and can last up to 3 hours. Cluster headache is rare, occurring in about 1 in 1,000 individuals, and is more common in men. Underdiagnosis is common – especially in women.
The study drew on two Swedish population-based registries and included 3,240 patients with cluster headache aged 16-64 years and 16,200 matched control persons. The analysis covered medical visits from 2001 to 2010.
Results showed that 91.9% of participants with cluster headache had some type of multimorbidity. By comparison, 77.6% of the control group had some type of multimorbidity (odds ratio, 3.26; P < .0001).
Prior studies have shown a higher incidence of mental health and behavioral disorders among patients with cluster headache. However, when the researchers removed those conditions along with external injuries from the dataset, patients with headache were still significantly more likely to have multiple co-occurring illnesses (86.7% vs. 68.8%; OR, 2.95; P < .0001).
The most common comorbid conditions in the overall cluster headache group were diseases of the nervous system (OR, 5.9; 95% CI, 5.46 -6.42); 51.8% of the cluster headache group reported these disorders, compared with just 15.4% of the control group.
Diseases of the eye, the respiratory, gastrointestinal, and musculoskeletal systems, and connective tissue were also significantly more common among patients with cluster headache.
“For each diagnosis that we investigated, we found a higher incidence in the cluster headache group, and we thought this was a very striking finding and worth discussing in the clinical setting that these patients are at risk of general ill health,” Dr. Ran said.
Risky behavior?
Another novel finding was the higher rate of external injuries among the cluster headache group, compared with the control group. The finding seems to back up the theory that patients with cluster headache are more likely to engage in risky behaviors, the researchers noted.
In the cluster headache group, external injuries were reported by 47.1% of men and 41% of women, versus 34.9% and 26.0%, respectively, in the control group.
“Now we can also show that cluster headache patients have more injuries and that is totally unrelated to the biological health of the individuals, so that could also indicate higher risk taking,” Dr. Ran said.
Overall multimorbidity rates and diagnoses in each medical category except external injury were higher among women with cluster headache than men with headaches. In addition, the mean number of days on sick leave and disability pension was higher among women with cluster headache than among men with cluster headache (83.71 days vs. 52.56 days).
Overall, the mean number of sickness absence and disability pension net days in 2010 was nearly twice as high in the cluster headache group as in the control group (63.15 days vs. 34.08 days).
Removing mental and behavioral health disorders from the mix did not lower those numbers.
“Our numbers indicate that the mental health issues that are related to cluster headache might not impact their work situation as much as the other comorbidities,” Dr. Ran said.
Struggle is real
Commenting on the findings, Heidi Schwarz, MD, professor of clinical neurology at the University of Rochester (N.Y.) Medical Center, called the study a “valuable contribution” to the field and to the treatment of cluster headache.
“It’s a good study that addresses factors that really need to be considered as you take care of these patients,” said Dr. Schwarz, who was not involved with the research.
“The most salient features of this is that cluster headache is quite disabling, and if you add a comorbidity to it, it’s even more disabling,” she said.
Dr. Schwarz noted that cluster headache is often misdiagnosed as migraine or is overlooked altogether, especially in women. These data underscore that, although cluster headache is more common in men, it affects women too and could lead to even greater disability.
“This has a direct impact on patient quality of life, and in the end, that really should be what we’re looking to enhance,” Dr. Schwarz said. “When a patient with cluster comes in and they tell you they’re really struggling, believe them because it’s quite real.”
The findings also fill a gap in the literature and offer the kind of data that could not be collected in the United States, she noted. Sweden provides paid sick time for all workers aged 16 and older and offers a disability pension to all workers whose ability to work is temporarily or permanently inhibited because of illness or injury.
“You will never get this kind of data in the United States because this kind of data comes from two datasets that are extremely inclusive and detailed in a society, Sweden, where they have a social support system,” Dr. Schwarz said.
The study was funded by the Swedish Research Council, the Swedish Brain Foundation, and Mellby Gård, Region Stockholm, Märta Lundkvist stiftelse and Karolinska Institutet research funds. Dr. Ran and Dr. Schwarz report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, leading to significant disability and absenteeism, new research shows.
Results from a Swedish register-based study also showed that patients with cluster headache had a sixfold increased risk for central nervous system disorders and a twofold increased risk for musculoskeletal disorders.
Although cluster headaches are often more prevalent in men, researchers found that multimorbidity rates were significantly higher in women. In addition, rates of external injuries were significantly higher among individuals with cluster headache than among persons without cluster headache.
“The findings very clearly indicate that cluster headache patients suffer from other health issues as well and that they are at risk of having longer periods of times when they cannot work,” said lead investigator Caroline Ran, PhD, a research specialist in the department of neuroscience at the Karolinska Institutet, Stockholm.
“It’s really important for clinicians to look at cluster headache from a broader perspective and make sure that patients are followed up so that they don’t risk ending up in a situation where they have several comorbidities,” Dr. Ran added.
The findings were published online in Neurology.
‘Striking’ finding
Cluster headache is one of the most severe and debilitating types of headache. It causes intense pain behind the eyes, which has been described as being worse than pain associated with childbirth or kidney stones.
Attacks can occur multiple times in a single day and can last up to 3 hours. Cluster headache is rare, occurring in about 1 in 1,000 individuals, and is more common in men. Underdiagnosis is common – especially in women.
The study drew on two Swedish population-based registries and included 3,240 patients with cluster headache aged 16-64 years and 16,200 matched control persons. The analysis covered medical visits from 2001 to 2010.
Results showed that 91.9% of participants with cluster headache had some type of multimorbidity. By comparison, 77.6% of the control group had some type of multimorbidity (odds ratio, 3.26; P < .0001).
Prior studies have shown a higher incidence of mental health and behavioral disorders among patients with cluster headache. However, when the researchers removed those conditions along with external injuries from the dataset, patients with headache were still significantly more likely to have multiple co-occurring illnesses (86.7% vs. 68.8%; OR, 2.95; P < .0001).
The most common comorbid conditions in the overall cluster headache group were diseases of the nervous system (OR, 5.9; 95% CI, 5.46 -6.42); 51.8% of the cluster headache group reported these disorders, compared with just 15.4% of the control group.
Diseases of the eye, the respiratory, gastrointestinal, and musculoskeletal systems, and connective tissue were also significantly more common among patients with cluster headache.
“For each diagnosis that we investigated, we found a higher incidence in the cluster headache group, and we thought this was a very striking finding and worth discussing in the clinical setting that these patients are at risk of general ill health,” Dr. Ran said.
Risky behavior?
Another novel finding was the higher rate of external injuries among the cluster headache group, compared with the control group. The finding seems to back up the theory that patients with cluster headache are more likely to engage in risky behaviors, the researchers noted.
In the cluster headache group, external injuries were reported by 47.1% of men and 41% of women, versus 34.9% and 26.0%, respectively, in the control group.
“Now we can also show that cluster headache patients have more injuries and that is totally unrelated to the biological health of the individuals, so that could also indicate higher risk taking,” Dr. Ran said.
Overall multimorbidity rates and diagnoses in each medical category except external injury were higher among women with cluster headache than men with headaches. In addition, the mean number of days on sick leave and disability pension was higher among women with cluster headache than among men with cluster headache (83.71 days vs. 52.56 days).
Overall, the mean number of sickness absence and disability pension net days in 2010 was nearly twice as high in the cluster headache group as in the control group (63.15 days vs. 34.08 days).
Removing mental and behavioral health disorders from the mix did not lower those numbers.
“Our numbers indicate that the mental health issues that are related to cluster headache might not impact their work situation as much as the other comorbidities,” Dr. Ran said.
Struggle is real
Commenting on the findings, Heidi Schwarz, MD, professor of clinical neurology at the University of Rochester (N.Y.) Medical Center, called the study a “valuable contribution” to the field and to the treatment of cluster headache.
“It’s a good study that addresses factors that really need to be considered as you take care of these patients,” said Dr. Schwarz, who was not involved with the research.
“The most salient features of this is that cluster headache is quite disabling, and if you add a comorbidity to it, it’s even more disabling,” she said.
Dr. Schwarz noted that cluster headache is often misdiagnosed as migraine or is overlooked altogether, especially in women. These data underscore that, although cluster headache is more common in men, it affects women too and could lead to even greater disability.
“This has a direct impact on patient quality of life, and in the end, that really should be what we’re looking to enhance,” Dr. Schwarz said. “When a patient with cluster comes in and they tell you they’re really struggling, believe them because it’s quite real.”
The findings also fill a gap in the literature and offer the kind of data that could not be collected in the United States, she noted. Sweden provides paid sick time for all workers aged 16 and older and offers a disability pension to all workers whose ability to work is temporarily or permanently inhibited because of illness or injury.
“You will never get this kind of data in the United States because this kind of data comes from two datasets that are extremely inclusive and detailed in a society, Sweden, where they have a social support system,” Dr. Schwarz said.
The study was funded by the Swedish Research Council, the Swedish Brain Foundation, and Mellby Gård, Region Stockholm, Märta Lundkvist stiftelse and Karolinska Institutet research funds. Dr. Ran and Dr. Schwarz report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Annual U.S. Parkinson’s disease incidence 50% higher than earlier estimates
according to new research that investigators say highlights the growing strain on clinical services and the need for more research funding.
In an analysis of five databases and more than 15 million people, about 60,000-90,000 individuals older than 45 years are estimated to be diagnosed with Parkinson’s disease each year – which is far more than the previous estimate of around 40,000-60,000 new cases annually.
This is the latest study to update decades-old epidemiologic data on Parkinson’s disease incidence and prevalence. Previous incidence rates came from small, single-population studies that are now more than 25 years old.
“In the advocacy community, we’ve been earnest about the impact of people living with Parkinson’s disease, and what we really lacked was sufficient data to be able to demonstrate the urgency of our need,” said study coinvestigator James Beck, PhD, chief scientific officer at the Parkinson’s Foundation, New York.
“We wanted to revise these numbers, highlight that they are larger than people anticipated, and use it as a call to action to change the approach we have toward Parkinson’s,” Dr. Beck said.
The findings were published online in NPJ Parkinson’s Disease.
Updating an outdated model
The study builds on the Parkinson’s Prevalence Project, a 2018 initiative that used a new model to calculate Parkinson’s disease prevalence. Before then, federal prevalence data was based on a 40-year-old study of just 26 Parkinson’s disease cases in one small county in rural Mississippi.
Dr. Beck and others used a more sophisticated model, using data from five separate cohort studies. They estimated the total number of patients living with Parkinson’s disease in the United States to be 930,000, which is far higher than the 650,000 the old model predicted.
Researchers then moved on to the current project, developing a new method to estimate Parkinson’s disease incidence.
The project included 2012 data on more than 15 million individuals in the United States and Canada. The investigators drew from three large insurance databases (Kaiser Permanente Northern California, Ontario Health Care, and Medicare) and two long-term epidemiologic studies (the Honolulu-Asia Aging Study and the Rochester Epidemiology Project).
On the basis of their analysis, the investigators proposed a working Parkinson’s disease incident rate estimate of 47-77 cases per 100,000 people aged 45 years or older. Limiting the analysis to those aged 65 or older raised the incidence to 108-212 per 100,000 people.
That translates to 60,000-95,000 new cases each year among adults aged 45 years or older. Using the Medicare administrative database alone for this same time period suggests an annual incidence of nearly 90,000 for individuals aged 65 or older.
“The numbers we’re proposing are conservative,” Dr. Beck said. “The true numbers are probably north of 90,000.”
Incidence rates increased with age and were higher in men. The researchers also identified clusters of counties with higher incidence rates in parts of the country called the “Parkinson’s belt.”
That geographic area mirrors the Rust Belt and includes parts of the Northeastern and Midwestern United States with a long history of industrial manufacturing that used heavy metals and industrial solvents, which are environmental factors linked to risk for Parkinson’s disease.
Cases were also higher in southern California, southeastern Texas, and Florida – agricultural regions with high pesticide use, which is also a risk factor for Parkinson’s disease. Central Pennsylvania also had higher incidence rates.
Why the increase?
The increase in cases could be the result of the more comprehensive estimation model used, the researchers noted. Or it could be improved detection, the aging population, a rise in sedentary lifestyles, increased exposure to environmental risk factors, or even the sharp decline in smoking in the United States, as some studies have shown that smokers have a lower Parkinson’s disease risk.
“The short answer is, we don’t know; and the long answer is, it’s all the above,” Dr. Beck said.
Although about 15% of Parkinson’s disease cases have a genetic basis, the cause is unknown in the majority of cases. In addition, diagnosis is difficult because there is no blood test or scan that detects the disease.
“Diagnosis requires a skilled clinician with real familiarity with Parkinson’s. And we have a real shortage of neurologists in this country to not only be able to diagnose but also to treat the condition,” Dr. Beck said.
That was one motivation for doing the study: to highlight what experts say is a pending clinical crisis for patients with Parkinson’s disease, he added.
The investigators also wanted to raise awareness about the scope of the disorder – not just about prevalence and incidence but also what those data mean for the health care industry, research aims, drug development and health care coverage, and policies.
In a 2020 study, the same researchers calculated a cost of $52 billion per year for medical and nonmedical costs related to Parkinson’s disease, which works out to about $26,000 per year per patient. That figure is expected to surpass $79 billion by 2030.
“This is an urgent condition for many people who live with the disease. And to the extent we can get our country to recognize that and really make the investment now, this is an area where a stitch in time saves nine,” Dr. Beck said.
“If we can invest some money now, we have a chance to really make a difference in the future,” he added.
‘Groundbreaking’ findings
Commenting on the findings, Jori Fleisher, MD, MSCE, associate professor of neurological sciences at Rush University Medical Center, Chicago, called the results “groundbreaking” and said that they validate what clinicians have been seeing in real-world practice.
“The findings reflect what a lot of us in practice have been appreciating anecdotally, which is that it seems that Parkinson’s is being diagnosed more frequently and that the incidence has been rising,” said Dr. Fleisher, who was not involved with the study.
She noted that the use of multiple datasets is one element of the methodology that makes the data so significant.
“There has been great work out of individual centers; but no matter how good your study methods are within that one population, you’re drawing conclusions based on that one population,” Dr. Fleisher said.
This research, together with the previous work by the group on prevalence data, could go a long way toward raising awareness about the scope of Parkinson’s disease in the United States – which could lead to earlier diagnosis, more research funding, and increased attention on the need for more clinicians who specialize in movement disorders, she added.
“This should increase research funding across the spectrum, including everything from the basic science to translational research, clinical research and implementation, and health services research,” Dr. Fleisher said.
The study was supported by the Parkinson’s Foundation, The Michael J. Fox Foundation for Parkinson’s Research, and the Institute for Clinical Evaluative Sciences. Dr. Beck and Dr. Fleisher reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to new research that investigators say highlights the growing strain on clinical services and the need for more research funding.
In an analysis of five databases and more than 15 million people, about 60,000-90,000 individuals older than 45 years are estimated to be diagnosed with Parkinson’s disease each year – which is far more than the previous estimate of around 40,000-60,000 new cases annually.
This is the latest study to update decades-old epidemiologic data on Parkinson’s disease incidence and prevalence. Previous incidence rates came from small, single-population studies that are now more than 25 years old.
“In the advocacy community, we’ve been earnest about the impact of people living with Parkinson’s disease, and what we really lacked was sufficient data to be able to demonstrate the urgency of our need,” said study coinvestigator James Beck, PhD, chief scientific officer at the Parkinson’s Foundation, New York.
“We wanted to revise these numbers, highlight that they are larger than people anticipated, and use it as a call to action to change the approach we have toward Parkinson’s,” Dr. Beck said.
The findings were published online in NPJ Parkinson’s Disease.
Updating an outdated model
The study builds on the Parkinson’s Prevalence Project, a 2018 initiative that used a new model to calculate Parkinson’s disease prevalence. Before then, federal prevalence data was based on a 40-year-old study of just 26 Parkinson’s disease cases in one small county in rural Mississippi.
Dr. Beck and others used a more sophisticated model, using data from five separate cohort studies. They estimated the total number of patients living with Parkinson’s disease in the United States to be 930,000, which is far higher than the 650,000 the old model predicted.
Researchers then moved on to the current project, developing a new method to estimate Parkinson’s disease incidence.
The project included 2012 data on more than 15 million individuals in the United States and Canada. The investigators drew from three large insurance databases (Kaiser Permanente Northern California, Ontario Health Care, and Medicare) and two long-term epidemiologic studies (the Honolulu-Asia Aging Study and the Rochester Epidemiology Project).
On the basis of their analysis, the investigators proposed a working Parkinson’s disease incident rate estimate of 47-77 cases per 100,000 people aged 45 years or older. Limiting the analysis to those aged 65 or older raised the incidence to 108-212 per 100,000 people.
That translates to 60,000-95,000 new cases each year among adults aged 45 years or older. Using the Medicare administrative database alone for this same time period suggests an annual incidence of nearly 90,000 for individuals aged 65 or older.
“The numbers we’re proposing are conservative,” Dr. Beck said. “The true numbers are probably north of 90,000.”
Incidence rates increased with age and were higher in men. The researchers also identified clusters of counties with higher incidence rates in parts of the country called the “Parkinson’s belt.”
That geographic area mirrors the Rust Belt and includes parts of the Northeastern and Midwestern United States with a long history of industrial manufacturing that used heavy metals and industrial solvents, which are environmental factors linked to risk for Parkinson’s disease.
Cases were also higher in southern California, southeastern Texas, and Florida – agricultural regions with high pesticide use, which is also a risk factor for Parkinson’s disease. Central Pennsylvania also had higher incidence rates.
Why the increase?
The increase in cases could be the result of the more comprehensive estimation model used, the researchers noted. Or it could be improved detection, the aging population, a rise in sedentary lifestyles, increased exposure to environmental risk factors, or even the sharp decline in smoking in the United States, as some studies have shown that smokers have a lower Parkinson’s disease risk.
“The short answer is, we don’t know; and the long answer is, it’s all the above,” Dr. Beck said.
Although about 15% of Parkinson’s disease cases have a genetic basis, the cause is unknown in the majority of cases. In addition, diagnosis is difficult because there is no blood test or scan that detects the disease.
“Diagnosis requires a skilled clinician with real familiarity with Parkinson’s. And we have a real shortage of neurologists in this country to not only be able to diagnose but also to treat the condition,” Dr. Beck said.
That was one motivation for doing the study: to highlight what experts say is a pending clinical crisis for patients with Parkinson’s disease, he added.
The investigators also wanted to raise awareness about the scope of the disorder – not just about prevalence and incidence but also what those data mean for the health care industry, research aims, drug development and health care coverage, and policies.
In a 2020 study, the same researchers calculated a cost of $52 billion per year for medical and nonmedical costs related to Parkinson’s disease, which works out to about $26,000 per year per patient. That figure is expected to surpass $79 billion by 2030.
“This is an urgent condition for many people who live with the disease. And to the extent we can get our country to recognize that and really make the investment now, this is an area where a stitch in time saves nine,” Dr. Beck said.
“If we can invest some money now, we have a chance to really make a difference in the future,” he added.
‘Groundbreaking’ findings
Commenting on the findings, Jori Fleisher, MD, MSCE, associate professor of neurological sciences at Rush University Medical Center, Chicago, called the results “groundbreaking” and said that they validate what clinicians have been seeing in real-world practice.
“The findings reflect what a lot of us in practice have been appreciating anecdotally, which is that it seems that Parkinson’s is being diagnosed more frequently and that the incidence has been rising,” said Dr. Fleisher, who was not involved with the study.
She noted that the use of multiple datasets is one element of the methodology that makes the data so significant.
“There has been great work out of individual centers; but no matter how good your study methods are within that one population, you’re drawing conclusions based on that one population,” Dr. Fleisher said.
This research, together with the previous work by the group on prevalence data, could go a long way toward raising awareness about the scope of Parkinson’s disease in the United States – which could lead to earlier diagnosis, more research funding, and increased attention on the need for more clinicians who specialize in movement disorders, she added.
“This should increase research funding across the spectrum, including everything from the basic science to translational research, clinical research and implementation, and health services research,” Dr. Fleisher said.
The study was supported by the Parkinson’s Foundation, The Michael J. Fox Foundation for Parkinson’s Research, and the Institute for Clinical Evaluative Sciences. Dr. Beck and Dr. Fleisher reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to new research that investigators say highlights the growing strain on clinical services and the need for more research funding.
In an analysis of five databases and more than 15 million people, about 60,000-90,000 individuals older than 45 years are estimated to be diagnosed with Parkinson’s disease each year – which is far more than the previous estimate of around 40,000-60,000 new cases annually.
This is the latest study to update decades-old epidemiologic data on Parkinson’s disease incidence and prevalence. Previous incidence rates came from small, single-population studies that are now more than 25 years old.
“In the advocacy community, we’ve been earnest about the impact of people living with Parkinson’s disease, and what we really lacked was sufficient data to be able to demonstrate the urgency of our need,” said study coinvestigator James Beck, PhD, chief scientific officer at the Parkinson’s Foundation, New York.
“We wanted to revise these numbers, highlight that they are larger than people anticipated, and use it as a call to action to change the approach we have toward Parkinson’s,” Dr. Beck said.
The findings were published online in NPJ Parkinson’s Disease.
Updating an outdated model
The study builds on the Parkinson’s Prevalence Project, a 2018 initiative that used a new model to calculate Parkinson’s disease prevalence. Before then, federal prevalence data was based on a 40-year-old study of just 26 Parkinson’s disease cases in one small county in rural Mississippi.
Dr. Beck and others used a more sophisticated model, using data from five separate cohort studies. They estimated the total number of patients living with Parkinson’s disease in the United States to be 930,000, which is far higher than the 650,000 the old model predicted.
Researchers then moved on to the current project, developing a new method to estimate Parkinson’s disease incidence.
The project included 2012 data on more than 15 million individuals in the United States and Canada. The investigators drew from three large insurance databases (Kaiser Permanente Northern California, Ontario Health Care, and Medicare) and two long-term epidemiologic studies (the Honolulu-Asia Aging Study and the Rochester Epidemiology Project).
On the basis of their analysis, the investigators proposed a working Parkinson’s disease incident rate estimate of 47-77 cases per 100,000 people aged 45 years or older. Limiting the analysis to those aged 65 or older raised the incidence to 108-212 per 100,000 people.
That translates to 60,000-95,000 new cases each year among adults aged 45 years or older. Using the Medicare administrative database alone for this same time period suggests an annual incidence of nearly 90,000 for individuals aged 65 or older.
“The numbers we’re proposing are conservative,” Dr. Beck said. “The true numbers are probably north of 90,000.”
Incidence rates increased with age and were higher in men. The researchers also identified clusters of counties with higher incidence rates in parts of the country called the “Parkinson’s belt.”
That geographic area mirrors the Rust Belt and includes parts of the Northeastern and Midwestern United States with a long history of industrial manufacturing that used heavy metals and industrial solvents, which are environmental factors linked to risk for Parkinson’s disease.
Cases were also higher in southern California, southeastern Texas, and Florida – agricultural regions with high pesticide use, which is also a risk factor for Parkinson’s disease. Central Pennsylvania also had higher incidence rates.
Why the increase?
The increase in cases could be the result of the more comprehensive estimation model used, the researchers noted. Or it could be improved detection, the aging population, a rise in sedentary lifestyles, increased exposure to environmental risk factors, or even the sharp decline in smoking in the United States, as some studies have shown that smokers have a lower Parkinson’s disease risk.
“The short answer is, we don’t know; and the long answer is, it’s all the above,” Dr. Beck said.
Although about 15% of Parkinson’s disease cases have a genetic basis, the cause is unknown in the majority of cases. In addition, diagnosis is difficult because there is no blood test or scan that detects the disease.
“Diagnosis requires a skilled clinician with real familiarity with Parkinson’s. And we have a real shortage of neurologists in this country to not only be able to diagnose but also to treat the condition,” Dr. Beck said.
That was one motivation for doing the study: to highlight what experts say is a pending clinical crisis for patients with Parkinson’s disease, he added.
The investigators also wanted to raise awareness about the scope of the disorder – not just about prevalence and incidence but also what those data mean for the health care industry, research aims, drug development and health care coverage, and policies.
In a 2020 study, the same researchers calculated a cost of $52 billion per year for medical and nonmedical costs related to Parkinson’s disease, which works out to about $26,000 per year per patient. That figure is expected to surpass $79 billion by 2030.
“This is an urgent condition for many people who live with the disease. And to the extent we can get our country to recognize that and really make the investment now, this is an area where a stitch in time saves nine,” Dr. Beck said.
“If we can invest some money now, we have a chance to really make a difference in the future,” he added.
‘Groundbreaking’ findings
Commenting on the findings, Jori Fleisher, MD, MSCE, associate professor of neurological sciences at Rush University Medical Center, Chicago, called the results “groundbreaking” and said that they validate what clinicians have been seeing in real-world practice.
“The findings reflect what a lot of us in practice have been appreciating anecdotally, which is that it seems that Parkinson’s is being diagnosed more frequently and that the incidence has been rising,” said Dr. Fleisher, who was not involved with the study.
She noted that the use of multiple datasets is one element of the methodology that makes the data so significant.
“There has been great work out of individual centers; but no matter how good your study methods are within that one population, you’re drawing conclusions based on that one population,” Dr. Fleisher said.
This research, together with the previous work by the group on prevalence data, could go a long way toward raising awareness about the scope of Parkinson’s disease in the United States – which could lead to earlier diagnosis, more research funding, and increased attention on the need for more clinicians who specialize in movement disorders, she added.
“This should increase research funding across the spectrum, including everything from the basic science to translational research, clinical research and implementation, and health services research,” Dr. Fleisher said.
The study was supported by the Parkinson’s Foundation, The Michael J. Fox Foundation for Parkinson’s Research, and the Institute for Clinical Evaluative Sciences. Dr. Beck and Dr. Fleisher reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NPJ PARKINSON’S DISEASE
Four-gene signature linked to increased PML risk
a team of European and U.S. investigators reported.
The four-gene signature could be used to screen patients who are currently taking or are candidates for drugs know to increase risk for PML, a rare but frequently lethal demyelinating disorder of the central nervous system, according to Eli Hatchwell, MD, PhD, from Population BIO UK in Oxfordshire, England, and colleagues.
“Due to the seriousness of a PML diagnosis – particularly because it often leads to life-threatening outcomes and the lack of treatment options once it develops – it would seem unethical not to test individuals considering immunosuppressive therapies with PML risk for our top four variants, and advising those with a positive result to consider an alternative therapy or treatment strategy,” they wrote in a study published in Frontiers in Neurology.
Benign virus, bad disease
PML is caused by reactivation of the otherwise benign JC virus (JCV), also known as human polyomavirus 2. (The “J” and “C” in the virus’ common name stand for John Cunningham, a man with Hodgkin lymphoma from whose brain the virus was first isolated, in 1971.)
The estimated prevalence of JCV infection ranges from 40% to 70% of the population worldwide, although PML itself is rare, with an incidence of approximately 1 in 200,000.
PML is a complication of treatment with targeted monoclonal antibodies, such as natalizumab (Tysabri), rituximab (Rituxan), alemtuzumab (Campath; Lemtrada), and other agents with immunosuppressive properties, such as dimethyl fumarate and mycophenolate mofetil.
In addition, PML can occur among patients with diseases that disrupt or inhibit natural immunity, such as HIV/AIDS, hematologic cancers, and autoimmune diseases.
Predisposing variants suspected
Dr. Hatchwell and colleagues hypothesized that some patients may have rare genetic variants in immune-system genes that predispose them to increased risk for PML. The researchers had previously shown an association between PML and 19 genetic risk variants among 184 patients with PML.
In the current study, they looked at variants in an additional 152 patients with PML who served as a validation sample. Of the 19 risk variants they had previously identified, the investigators narrowed the field down to 4 variants in both population controls and in a matched control set consisting of patients with multiple sclerosis (MS) who were positive for JCV and who were on therapy with a PML-linked drug for at least 2 years.
The four variants they identified, all linked to immune viral defense, were C8B, 1-57409459-C-A, rs139498867; LY9 (a checkpoint regulator also known as SLAMF3), 1-160769595-AG-A, rs763811636; FCN2, 9-137779251-G-A, rs76267164; and STXBP2, 19-7712287-G-C, rs35490401.
In all, 10.9% of patients with PML carried at least one of the variants.
The investigators reported that carriers of any one of the variants has a nearly ninefold risk for developing PML after exposure to a PML-linked drug compared with non-carriers with similar drug exposures (odds ratio, 8.7; P < .001).
“Measures of clinical validity and utility compare favorably to other genetic risk tests, such as BRCA1 and BRCA2 screening for breast cancer risk and HLA-B_15:02 pharmacogenetic screening for pharmacovigilance of carbamazepine to prevent Stevens-Johnson syndrome and toxic epidermal necrolysis,” the authors noted.
Screening? Maybe
In a press release, Lawrence Steinman, MD, from Stanford (Calif.) University, who was not involved in the study, stated that “preventative screening for these variants should become part of the standard of care. I wish we had more powerful tools like this for other therapies.”
But another neurologist who was not involved in the study commented that the finding, while “exciting” as a confirmation study, is not as yet practice changing.
“It does give us very good confidence that these four genes are indeed risk factors that increase the risk of this brain infection by quite a bit, so that makes it very exciting,” said Robert Fox, MD, from the Neurological Institute at the Cleveland Clinic.
“Indeed, we are trying to risk-stratify patients to try to reduce the risk of PML in the patients treated with our MS drugs. So for natalizumab we risk stratify by testing them for JC virus serology. Half of people don’t have it and we say ‘OK, you’re good to go.’ With other drugs like Tecfidera – dimethyl fumarate – we follow their lymphocyte counts, so when their lymphocyte counts drop too low we say ‘OK, you need to come off the drug because of the risk of PML,’ ” he said in an interview.
The four-gene signature, however, only identifies about 11% of patients with PML, which is not a sufficiently large enough effect to be clinically useful. For example, the risk for PML in patients treated with natalizumab is about 1%, and if the test can only detect enhanced risk in about 11% of those patients, the risk would drop from 1% to 0.9%, which “doesn’t really the move needle much,” he pointed out.
Dr. Fox also noted that neurologists now have a large formulary of drugs to offer their patients, including agents (such as interferon-beta and corticosteroids that are not associated with increased risk for PML).
The study was funded by Emerald Lake Safety and Population Bio. Dr. Hatchwell and several coauthors are employees of the respective companies, and several are inventors of genetic screening methods for PML. Dr. Steiman has disclosed consulting for TG Therapeutics. Dr. Fox reported consulting for manufacturers of MS therapies.
a team of European and U.S. investigators reported.
The four-gene signature could be used to screen patients who are currently taking or are candidates for drugs know to increase risk for PML, a rare but frequently lethal demyelinating disorder of the central nervous system, according to Eli Hatchwell, MD, PhD, from Population BIO UK in Oxfordshire, England, and colleagues.
“Due to the seriousness of a PML diagnosis – particularly because it often leads to life-threatening outcomes and the lack of treatment options once it develops – it would seem unethical not to test individuals considering immunosuppressive therapies with PML risk for our top four variants, and advising those with a positive result to consider an alternative therapy or treatment strategy,” they wrote in a study published in Frontiers in Neurology.
Benign virus, bad disease
PML is caused by reactivation of the otherwise benign JC virus (JCV), also known as human polyomavirus 2. (The “J” and “C” in the virus’ common name stand for John Cunningham, a man with Hodgkin lymphoma from whose brain the virus was first isolated, in 1971.)
The estimated prevalence of JCV infection ranges from 40% to 70% of the population worldwide, although PML itself is rare, with an incidence of approximately 1 in 200,000.
PML is a complication of treatment with targeted monoclonal antibodies, such as natalizumab (Tysabri), rituximab (Rituxan), alemtuzumab (Campath; Lemtrada), and other agents with immunosuppressive properties, such as dimethyl fumarate and mycophenolate mofetil.
In addition, PML can occur among patients with diseases that disrupt or inhibit natural immunity, such as HIV/AIDS, hematologic cancers, and autoimmune diseases.
Predisposing variants suspected
Dr. Hatchwell and colleagues hypothesized that some patients may have rare genetic variants in immune-system genes that predispose them to increased risk for PML. The researchers had previously shown an association between PML and 19 genetic risk variants among 184 patients with PML.
In the current study, they looked at variants in an additional 152 patients with PML who served as a validation sample. Of the 19 risk variants they had previously identified, the investigators narrowed the field down to 4 variants in both population controls and in a matched control set consisting of patients with multiple sclerosis (MS) who were positive for JCV and who were on therapy with a PML-linked drug for at least 2 years.
The four variants they identified, all linked to immune viral defense, were C8B, 1-57409459-C-A, rs139498867; LY9 (a checkpoint regulator also known as SLAMF3), 1-160769595-AG-A, rs763811636; FCN2, 9-137779251-G-A, rs76267164; and STXBP2, 19-7712287-G-C, rs35490401.
In all, 10.9% of patients with PML carried at least one of the variants.
The investigators reported that carriers of any one of the variants has a nearly ninefold risk for developing PML after exposure to a PML-linked drug compared with non-carriers with similar drug exposures (odds ratio, 8.7; P < .001).
“Measures of clinical validity and utility compare favorably to other genetic risk tests, such as BRCA1 and BRCA2 screening for breast cancer risk and HLA-B_15:02 pharmacogenetic screening for pharmacovigilance of carbamazepine to prevent Stevens-Johnson syndrome and toxic epidermal necrolysis,” the authors noted.
Screening? Maybe
In a press release, Lawrence Steinman, MD, from Stanford (Calif.) University, who was not involved in the study, stated that “preventative screening for these variants should become part of the standard of care. I wish we had more powerful tools like this for other therapies.”
But another neurologist who was not involved in the study commented that the finding, while “exciting” as a confirmation study, is not as yet practice changing.
“It does give us very good confidence that these four genes are indeed risk factors that increase the risk of this brain infection by quite a bit, so that makes it very exciting,” said Robert Fox, MD, from the Neurological Institute at the Cleveland Clinic.
“Indeed, we are trying to risk-stratify patients to try to reduce the risk of PML in the patients treated with our MS drugs. So for natalizumab we risk stratify by testing them for JC virus serology. Half of people don’t have it and we say ‘OK, you’re good to go.’ With other drugs like Tecfidera – dimethyl fumarate – we follow their lymphocyte counts, so when their lymphocyte counts drop too low we say ‘OK, you need to come off the drug because of the risk of PML,’ ” he said in an interview.
The four-gene signature, however, only identifies about 11% of patients with PML, which is not a sufficiently large enough effect to be clinically useful. For example, the risk for PML in patients treated with natalizumab is about 1%, and if the test can only detect enhanced risk in about 11% of those patients, the risk would drop from 1% to 0.9%, which “doesn’t really the move needle much,” he pointed out.
Dr. Fox also noted that neurologists now have a large formulary of drugs to offer their patients, including agents (such as interferon-beta and corticosteroids that are not associated with increased risk for PML).
The study was funded by Emerald Lake Safety and Population Bio. Dr. Hatchwell and several coauthors are employees of the respective companies, and several are inventors of genetic screening methods for PML. Dr. Steiman has disclosed consulting for TG Therapeutics. Dr. Fox reported consulting for manufacturers of MS therapies.
a team of European and U.S. investigators reported.
The four-gene signature could be used to screen patients who are currently taking or are candidates for drugs know to increase risk for PML, a rare but frequently lethal demyelinating disorder of the central nervous system, according to Eli Hatchwell, MD, PhD, from Population BIO UK in Oxfordshire, England, and colleagues.
“Due to the seriousness of a PML diagnosis – particularly because it often leads to life-threatening outcomes and the lack of treatment options once it develops – it would seem unethical not to test individuals considering immunosuppressive therapies with PML risk for our top four variants, and advising those with a positive result to consider an alternative therapy or treatment strategy,” they wrote in a study published in Frontiers in Neurology.
Benign virus, bad disease
PML is caused by reactivation of the otherwise benign JC virus (JCV), also known as human polyomavirus 2. (The “J” and “C” in the virus’ common name stand for John Cunningham, a man with Hodgkin lymphoma from whose brain the virus was first isolated, in 1971.)
The estimated prevalence of JCV infection ranges from 40% to 70% of the population worldwide, although PML itself is rare, with an incidence of approximately 1 in 200,000.
PML is a complication of treatment with targeted monoclonal antibodies, such as natalizumab (Tysabri), rituximab (Rituxan), alemtuzumab (Campath; Lemtrada), and other agents with immunosuppressive properties, such as dimethyl fumarate and mycophenolate mofetil.
In addition, PML can occur among patients with diseases that disrupt or inhibit natural immunity, such as HIV/AIDS, hematologic cancers, and autoimmune diseases.
Predisposing variants suspected
Dr. Hatchwell and colleagues hypothesized that some patients may have rare genetic variants in immune-system genes that predispose them to increased risk for PML. The researchers had previously shown an association between PML and 19 genetic risk variants among 184 patients with PML.
In the current study, they looked at variants in an additional 152 patients with PML who served as a validation sample. Of the 19 risk variants they had previously identified, the investigators narrowed the field down to 4 variants in both population controls and in a matched control set consisting of patients with multiple sclerosis (MS) who were positive for JCV and who were on therapy with a PML-linked drug for at least 2 years.
The four variants they identified, all linked to immune viral defense, were C8B, 1-57409459-C-A, rs139498867; LY9 (a checkpoint regulator also known as SLAMF3), 1-160769595-AG-A, rs763811636; FCN2, 9-137779251-G-A, rs76267164; and STXBP2, 19-7712287-G-C, rs35490401.
In all, 10.9% of patients with PML carried at least one of the variants.
The investigators reported that carriers of any one of the variants has a nearly ninefold risk for developing PML after exposure to a PML-linked drug compared with non-carriers with similar drug exposures (odds ratio, 8.7; P < .001).
“Measures of clinical validity and utility compare favorably to other genetic risk tests, such as BRCA1 and BRCA2 screening for breast cancer risk and HLA-B_15:02 pharmacogenetic screening for pharmacovigilance of carbamazepine to prevent Stevens-Johnson syndrome and toxic epidermal necrolysis,” the authors noted.
Screening? Maybe
In a press release, Lawrence Steinman, MD, from Stanford (Calif.) University, who was not involved in the study, stated that “preventative screening for these variants should become part of the standard of care. I wish we had more powerful tools like this for other therapies.”
But another neurologist who was not involved in the study commented that the finding, while “exciting” as a confirmation study, is not as yet practice changing.
“It does give us very good confidence that these four genes are indeed risk factors that increase the risk of this brain infection by quite a bit, so that makes it very exciting,” said Robert Fox, MD, from the Neurological Institute at the Cleveland Clinic.
“Indeed, we are trying to risk-stratify patients to try to reduce the risk of PML in the patients treated with our MS drugs. So for natalizumab we risk stratify by testing them for JC virus serology. Half of people don’t have it and we say ‘OK, you’re good to go.’ With other drugs like Tecfidera – dimethyl fumarate – we follow their lymphocyte counts, so when their lymphocyte counts drop too low we say ‘OK, you need to come off the drug because of the risk of PML,’ ” he said in an interview.
The four-gene signature, however, only identifies about 11% of patients with PML, which is not a sufficiently large enough effect to be clinically useful. For example, the risk for PML in patients treated with natalizumab is about 1%, and if the test can only detect enhanced risk in about 11% of those patients, the risk would drop from 1% to 0.9%, which “doesn’t really the move needle much,” he pointed out.
Dr. Fox also noted that neurologists now have a large formulary of drugs to offer their patients, including agents (such as interferon-beta and corticosteroids that are not associated with increased risk for PML).
The study was funded by Emerald Lake Safety and Population Bio. Dr. Hatchwell and several coauthors are employees of the respective companies, and several are inventors of genetic screening methods for PML. Dr. Steiman has disclosed consulting for TG Therapeutics. Dr. Fox reported consulting for manufacturers of MS therapies.
FROM FRONTIERS IN NEUROLOGY
Strong two-way link between epilepsy and depression
, with implications for diagnosis and patient care. The findings “strongly support previous observations of a bidirectional association between these two brain disorders,” said Eva Bølling-Ladegaard, MD, a PhD student, department of clinical medicine (Neurology), Aarhus (Denmark) University.
“We add to the existing evidence in temporal range, showing that the increased risks of depression following epilepsy, and vice versa, are sustained over a much more extended time period than previously shown; that is, 20 years on both sides of receiving a diagnosis of the index disorder,” Ms. Bølling-Ladegaard said.
The study was published online in Neurology.
Epilepsy then depression
The researchers examined the magnitude and long-term temporal association between epilepsy and depression. They compared the risk of the two brain disorders following another chronic disorder (asthma) in a nationwide, register-based, matched cohort study.
In a population of more than 8.7 million people, they identified 139,014 persons with epilepsy (54% males; median age at diagnosis, 43 years), 219,990 with depression (37% males; median age at diagnosis, 43 years), and 358,821 with asthma (49% males; median age at diagnosis, 29 years).
The rate of developing depression was increased nearly twofold among people with epilepsy compared with the matched population who did not have epilepsy (adjusted hazard ratio, 1.88; 95% confidence interval, 1.82-1.95).
The rate of depression was highest during the first months and years after epilepsy diagnosis. It declined over time, yet remained significantly elevated throughout the 20+ years of observation.
The cumulative incidence of depression at 5 and 35 years’ follow-up in the epilepsy cohort was 1.37% and 6.05%, respectively, compared with 0.59% and 3.92% in the reference population.
The highest rate of depression after epilepsy was among individuals aged 40-59 years, and the lowest was among those aged 0-19 years at first epilepsy diagnosis.
Depression then epilepsy
The rate of developing epilepsy was increased more than twofold among patients with incident depression compared with the matched population who were without depression (aHR, 2.35; 95% CI, 2.25-2.44).
As in the opposite analysis, the rate of epilepsy was highest during the first months and years after depression diagnosis and declined over time.
The cumulative incidence of epilepsy at 5 and 35 years after depression diagnosis was 1.10% and 4.19%, respectively, compared with 0.32% and 2.06% in the reference population.
The rate of epilepsy was highest among those aged 0-19 years at time of first depression diagnosis and was lowest among those aged 80+ at first depression diagnosis.
For comparison, after asthma diagnosis, rates of depression and epilepsy were increased 1.63-fold (95% CI, 1.59-1.67) and 1.48-fold (95% CI, 1.44-1.53), respectively, compared with matched individuals without asthma.
Using admission with seizures as a proxy for treatment failure, the researchers observed an increased risk of treatment failure among people with epilepsy who were diagnosed with depression.
“Our results support previous findings indicating worse seizure outcomes in people with epilepsy and coexisting depression,” said Ms. Bølling-Ladegaard.
“Increased clinical awareness of the association between epilepsy and depression is therefore needed in order to increase the proportion of patients that receive appropriate treatment and improve outcomes for these patient groups,” she said.
Clinical implications
Reached for comment, Zulfi Haneef, MBBS, MD, associate professor of neurology, Baylor College of Medicine, Houston, noted that the link between epilepsy and depression is “well-known.”
“However, typically one thinks of epilepsy as leading to depression, not vice versa. Here they show the risk of epilepsy following depression to be high (highest of the risks given), which is thought provoking. However, association does not imply causation,” Dr. Haneef said.
“Prima facie, there is no biological rationale for depression to lead to epilepsy,” he said. He noted that some antidepressants can reduce the seizure threshold.
The findings do have implications for care, he said.
“For neurologists, this is another study that exhorts them to screen for depression and treat adequately in all patients with epilepsy,” Dr. Haneef said.
“For psychiatrists, this study may give guidance to watch more carefully for seizures in patients with depression, especially when using antidepressant medications that induce seizures.
“For the general public with either epilepsy or depression, it would help them be aware about this bidirectional association,” Dr. Haneef said.
The study was funded by the Lundbeck Foundation, the Danish Epilepsy Association, and the Novo Nordisk Foundation. Ms. Bølling-Ladegaard and Dr. Haneef have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, with implications for diagnosis and patient care. The findings “strongly support previous observations of a bidirectional association between these two brain disorders,” said Eva Bølling-Ladegaard, MD, a PhD student, department of clinical medicine (Neurology), Aarhus (Denmark) University.
“We add to the existing evidence in temporal range, showing that the increased risks of depression following epilepsy, and vice versa, are sustained over a much more extended time period than previously shown; that is, 20 years on both sides of receiving a diagnosis of the index disorder,” Ms. Bølling-Ladegaard said.
The study was published online in Neurology.
Epilepsy then depression
The researchers examined the magnitude and long-term temporal association between epilepsy and depression. They compared the risk of the two brain disorders following another chronic disorder (asthma) in a nationwide, register-based, matched cohort study.
In a population of more than 8.7 million people, they identified 139,014 persons with epilepsy (54% males; median age at diagnosis, 43 years), 219,990 with depression (37% males; median age at diagnosis, 43 years), and 358,821 with asthma (49% males; median age at diagnosis, 29 years).
The rate of developing depression was increased nearly twofold among people with epilepsy compared with the matched population who did not have epilepsy (adjusted hazard ratio, 1.88; 95% confidence interval, 1.82-1.95).
The rate of depression was highest during the first months and years after epilepsy diagnosis. It declined over time, yet remained significantly elevated throughout the 20+ years of observation.
The cumulative incidence of depression at 5 and 35 years’ follow-up in the epilepsy cohort was 1.37% and 6.05%, respectively, compared with 0.59% and 3.92% in the reference population.
The highest rate of depression after epilepsy was among individuals aged 40-59 years, and the lowest was among those aged 0-19 years at first epilepsy diagnosis.
Depression then epilepsy
The rate of developing epilepsy was increased more than twofold among patients with incident depression compared with the matched population who were without depression (aHR, 2.35; 95% CI, 2.25-2.44).
As in the opposite analysis, the rate of epilepsy was highest during the first months and years after depression diagnosis and declined over time.
The cumulative incidence of epilepsy at 5 and 35 years after depression diagnosis was 1.10% and 4.19%, respectively, compared with 0.32% and 2.06% in the reference population.
The rate of epilepsy was highest among those aged 0-19 years at time of first depression diagnosis and was lowest among those aged 80+ at first depression diagnosis.
For comparison, after asthma diagnosis, rates of depression and epilepsy were increased 1.63-fold (95% CI, 1.59-1.67) and 1.48-fold (95% CI, 1.44-1.53), respectively, compared with matched individuals without asthma.
Using admission with seizures as a proxy for treatment failure, the researchers observed an increased risk of treatment failure among people with epilepsy who were diagnosed with depression.
“Our results support previous findings indicating worse seizure outcomes in people with epilepsy and coexisting depression,” said Ms. Bølling-Ladegaard.
“Increased clinical awareness of the association between epilepsy and depression is therefore needed in order to increase the proportion of patients that receive appropriate treatment and improve outcomes for these patient groups,” she said.
Clinical implications
Reached for comment, Zulfi Haneef, MBBS, MD, associate professor of neurology, Baylor College of Medicine, Houston, noted that the link between epilepsy and depression is “well-known.”
“However, typically one thinks of epilepsy as leading to depression, not vice versa. Here they show the risk of epilepsy following depression to be high (highest of the risks given), which is thought provoking. However, association does not imply causation,” Dr. Haneef said.
“Prima facie, there is no biological rationale for depression to lead to epilepsy,” he said. He noted that some antidepressants can reduce the seizure threshold.
The findings do have implications for care, he said.
“For neurologists, this is another study that exhorts them to screen for depression and treat adequately in all patients with epilepsy,” Dr. Haneef said.
“For psychiatrists, this study may give guidance to watch more carefully for seizures in patients with depression, especially when using antidepressant medications that induce seizures.
“For the general public with either epilepsy or depression, it would help them be aware about this bidirectional association,” Dr. Haneef said.
The study was funded by the Lundbeck Foundation, the Danish Epilepsy Association, and the Novo Nordisk Foundation. Ms. Bølling-Ladegaard and Dr. Haneef have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, with implications for diagnosis and patient care. The findings “strongly support previous observations of a bidirectional association between these two brain disorders,” said Eva Bølling-Ladegaard, MD, a PhD student, department of clinical medicine (Neurology), Aarhus (Denmark) University.
“We add to the existing evidence in temporal range, showing that the increased risks of depression following epilepsy, and vice versa, are sustained over a much more extended time period than previously shown; that is, 20 years on both sides of receiving a diagnosis of the index disorder,” Ms. Bølling-Ladegaard said.
The study was published online in Neurology.
Epilepsy then depression
The researchers examined the magnitude and long-term temporal association between epilepsy and depression. They compared the risk of the two brain disorders following another chronic disorder (asthma) in a nationwide, register-based, matched cohort study.
In a population of more than 8.7 million people, they identified 139,014 persons with epilepsy (54% males; median age at diagnosis, 43 years), 219,990 with depression (37% males; median age at diagnosis, 43 years), and 358,821 with asthma (49% males; median age at diagnosis, 29 years).
The rate of developing depression was increased nearly twofold among people with epilepsy compared with the matched population who did not have epilepsy (adjusted hazard ratio, 1.88; 95% confidence interval, 1.82-1.95).
The rate of depression was highest during the first months and years after epilepsy diagnosis. It declined over time, yet remained significantly elevated throughout the 20+ years of observation.
The cumulative incidence of depression at 5 and 35 years’ follow-up in the epilepsy cohort was 1.37% and 6.05%, respectively, compared with 0.59% and 3.92% in the reference population.
The highest rate of depression after epilepsy was among individuals aged 40-59 years, and the lowest was among those aged 0-19 years at first epilepsy diagnosis.
Depression then epilepsy
The rate of developing epilepsy was increased more than twofold among patients with incident depression compared with the matched population who were without depression (aHR, 2.35; 95% CI, 2.25-2.44).
As in the opposite analysis, the rate of epilepsy was highest during the first months and years after depression diagnosis and declined over time.
The cumulative incidence of epilepsy at 5 and 35 years after depression diagnosis was 1.10% and 4.19%, respectively, compared with 0.32% and 2.06% in the reference population.
The rate of epilepsy was highest among those aged 0-19 years at time of first depression diagnosis and was lowest among those aged 80+ at first depression diagnosis.
For comparison, after asthma diagnosis, rates of depression and epilepsy were increased 1.63-fold (95% CI, 1.59-1.67) and 1.48-fold (95% CI, 1.44-1.53), respectively, compared with matched individuals without asthma.
Using admission with seizures as a proxy for treatment failure, the researchers observed an increased risk of treatment failure among people with epilepsy who were diagnosed with depression.
“Our results support previous findings indicating worse seizure outcomes in people with epilepsy and coexisting depression,” said Ms. Bølling-Ladegaard.
“Increased clinical awareness of the association between epilepsy and depression is therefore needed in order to increase the proportion of patients that receive appropriate treatment and improve outcomes for these patient groups,” she said.
Clinical implications
Reached for comment, Zulfi Haneef, MBBS, MD, associate professor of neurology, Baylor College of Medicine, Houston, noted that the link between epilepsy and depression is “well-known.”
“However, typically one thinks of epilepsy as leading to depression, not vice versa. Here they show the risk of epilepsy following depression to be high (highest of the risks given), which is thought provoking. However, association does not imply causation,” Dr. Haneef said.
“Prima facie, there is no biological rationale for depression to lead to epilepsy,” he said. He noted that some antidepressants can reduce the seizure threshold.
The findings do have implications for care, he said.
“For neurologists, this is another study that exhorts them to screen for depression and treat adequately in all patients with epilepsy,” Dr. Haneef said.
“For psychiatrists, this study may give guidance to watch more carefully for seizures in patients with depression, especially when using antidepressant medications that induce seizures.
“For the general public with either epilepsy or depression, it would help them be aware about this bidirectional association,” Dr. Haneef said.
The study was funded by the Lundbeck Foundation, the Danish Epilepsy Association, and the Novo Nordisk Foundation. Ms. Bølling-Ladegaard and Dr. Haneef have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY