User login
Cardiovascular disease is implicated in link between air pollution and dementia
Virtually all of the association between air pollution and dementia seemed to occur through the presence or the development of cardiovascular disease, which suggests a need to optimize treatment of concurrent cardiovascular disease and risk-factor control in older adults at higher risk for dementia and living in polluted urban areas, said lead author Giulia Grande, MD, a researcher at the Aging Research Center, Karolinska Institutet and Stockholm University, in Solna, Sweden.
In the longitudinal, population-based cohort study, investigators studied 2,927 randomly selected residents in a district of Stockholm who were aged 60 years or older (mean, 74.1 years), lived at home or in institutions, and were free of dementia at baseline (March 2001 through August 2004).
The investigators assessed the participants’ exposure to two major air pollutants – particulate matter ≤2.5 mcm and nitrogen oxide – yearly starting in 1990, from outdoor levels at their residential addresses. Both pollutants are generated by road traffic, among other sources.
Results reported in JAMA Neurology showed that, with a mean follow-up of 6.01 years, 12.4% of the older adults received a dementia diagnosis.
Dementia risk increased with the level of air pollutants at their residential address in the past, with strongest associations seen for exposure in the preceding 5 years: The hazard ratio (HR) for dementia was 1.54 for an interquartile range difference of 0.88 mcg/m3 in particulate matter ≤2.5 mcm and 1.14 for an interquartile range difference of 8.35 mcg/m3 in nitrogen oxide during that time period.
Of note, the study cohort lived in an area having “comparatively good ambient air quality” in which restrictions on air pollution have increased in recent decades, Dr. Grande and coinvestigators noted. “Interestingly, the higher limit reported herein is not only below the current European limit for fine particulate matter but also below the US standard. In other words, we were able to establish harmful effects at levels below current standards,” they wrote.
In analyses of effect modification, the elevation of risk related to particulate matter ≤2.5 mcm exposure and nitrogen oxide exposure was significantly greater among older adults who had heart failure (HRs, 1.93 and 1.43, respectively). Risk was marginally greater among those with ischemic heart disease (HRs, 1.67 and 1.36, respectively).
Analyses of potential mediators showed that preceding stroke accounted for the largest share of all dementia cases related to particulate matter ≤2.5 mcm exposure, at 49.4%.
The stronger association for exposure in the past 5 years is noteworthy for the big picture, they added. “From a policy point of view, this result is encouraging because it might imply that reducing air pollutant levels today could yield better outcomes already in the shorter term, reinforcing the need for appropriately set air quality standards,” they said.
Dr. Grande disclosed no relevant conflicts of interest. The study was funded by the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K); the Swedish Ministry of Health and Social Affairs; the participating County Councils and Municipalities; the Swedish Research Council; funding for doctoral education from the Karolinska Institutet; and the Swedish Research Council for Health, Working Life and Welfare.
SOURCE: Grande G et al. JAMA Neurol. 2020. doi:10.1001/jamaneurol.2019.4914.
Virtually all of the association between air pollution and dementia seemed to occur through the presence or the development of cardiovascular disease, which suggests a need to optimize treatment of concurrent cardiovascular disease and risk-factor control in older adults at higher risk for dementia and living in polluted urban areas, said lead author Giulia Grande, MD, a researcher at the Aging Research Center, Karolinska Institutet and Stockholm University, in Solna, Sweden.
In the longitudinal, population-based cohort study, investigators studied 2,927 randomly selected residents in a district of Stockholm who were aged 60 years or older (mean, 74.1 years), lived at home or in institutions, and were free of dementia at baseline (March 2001 through August 2004).
The investigators assessed the participants’ exposure to two major air pollutants – particulate matter ≤2.5 mcm and nitrogen oxide – yearly starting in 1990, from outdoor levels at their residential addresses. Both pollutants are generated by road traffic, among other sources.
Results reported in JAMA Neurology showed that, with a mean follow-up of 6.01 years, 12.4% of the older adults received a dementia diagnosis.
Dementia risk increased with the level of air pollutants at their residential address in the past, with strongest associations seen for exposure in the preceding 5 years: The hazard ratio (HR) for dementia was 1.54 for an interquartile range difference of 0.88 mcg/m3 in particulate matter ≤2.5 mcm and 1.14 for an interquartile range difference of 8.35 mcg/m3 in nitrogen oxide during that time period.
Of note, the study cohort lived in an area having “comparatively good ambient air quality” in which restrictions on air pollution have increased in recent decades, Dr. Grande and coinvestigators noted. “Interestingly, the higher limit reported herein is not only below the current European limit for fine particulate matter but also below the US standard. In other words, we were able to establish harmful effects at levels below current standards,” they wrote.
In analyses of effect modification, the elevation of risk related to particulate matter ≤2.5 mcm exposure and nitrogen oxide exposure was significantly greater among older adults who had heart failure (HRs, 1.93 and 1.43, respectively). Risk was marginally greater among those with ischemic heart disease (HRs, 1.67 and 1.36, respectively).
Analyses of potential mediators showed that preceding stroke accounted for the largest share of all dementia cases related to particulate matter ≤2.5 mcm exposure, at 49.4%.
The stronger association for exposure in the past 5 years is noteworthy for the big picture, they added. “From a policy point of view, this result is encouraging because it might imply that reducing air pollutant levels today could yield better outcomes already in the shorter term, reinforcing the need for appropriately set air quality standards,” they said.
Dr. Grande disclosed no relevant conflicts of interest. The study was funded by the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K); the Swedish Ministry of Health and Social Affairs; the participating County Councils and Municipalities; the Swedish Research Council; funding for doctoral education from the Karolinska Institutet; and the Swedish Research Council for Health, Working Life and Welfare.
SOURCE: Grande G et al. JAMA Neurol. 2020. doi:10.1001/jamaneurol.2019.4914.
Virtually all of the association between air pollution and dementia seemed to occur through the presence or the development of cardiovascular disease, which suggests a need to optimize treatment of concurrent cardiovascular disease and risk-factor control in older adults at higher risk for dementia and living in polluted urban areas, said lead author Giulia Grande, MD, a researcher at the Aging Research Center, Karolinska Institutet and Stockholm University, in Solna, Sweden.
In the longitudinal, population-based cohort study, investigators studied 2,927 randomly selected residents in a district of Stockholm who were aged 60 years or older (mean, 74.1 years), lived at home or in institutions, and were free of dementia at baseline (March 2001 through August 2004).
The investigators assessed the participants’ exposure to two major air pollutants – particulate matter ≤2.5 mcm and nitrogen oxide – yearly starting in 1990, from outdoor levels at their residential addresses. Both pollutants are generated by road traffic, among other sources.
Results reported in JAMA Neurology showed that, with a mean follow-up of 6.01 years, 12.4% of the older adults received a dementia diagnosis.
Dementia risk increased with the level of air pollutants at their residential address in the past, with strongest associations seen for exposure in the preceding 5 years: The hazard ratio (HR) for dementia was 1.54 for an interquartile range difference of 0.88 mcg/m3 in particulate matter ≤2.5 mcm and 1.14 for an interquartile range difference of 8.35 mcg/m3 in nitrogen oxide during that time period.
Of note, the study cohort lived in an area having “comparatively good ambient air quality” in which restrictions on air pollution have increased in recent decades, Dr. Grande and coinvestigators noted. “Interestingly, the higher limit reported herein is not only below the current European limit for fine particulate matter but also below the US standard. In other words, we were able to establish harmful effects at levels below current standards,” they wrote.
In analyses of effect modification, the elevation of risk related to particulate matter ≤2.5 mcm exposure and nitrogen oxide exposure was significantly greater among older adults who had heart failure (HRs, 1.93 and 1.43, respectively). Risk was marginally greater among those with ischemic heart disease (HRs, 1.67 and 1.36, respectively).
Analyses of potential mediators showed that preceding stroke accounted for the largest share of all dementia cases related to particulate matter ≤2.5 mcm exposure, at 49.4%.
The stronger association for exposure in the past 5 years is noteworthy for the big picture, they added. “From a policy point of view, this result is encouraging because it might imply that reducing air pollutant levels today could yield better outcomes already in the shorter term, reinforcing the need for appropriately set air quality standards,” they said.
Dr. Grande disclosed no relevant conflicts of interest. The study was funded by the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K); the Swedish Ministry of Health and Social Affairs; the participating County Councils and Municipalities; the Swedish Research Council; funding for doctoral education from the Karolinska Institutet; and the Swedish Research Council for Health, Working Life and Welfare.
SOURCE: Grande G et al. JAMA Neurol. 2020. doi:10.1001/jamaneurol.2019.4914.
FROM JAMA NEUROLOGY
Sleep-disordered breathing linked with Alzheimer’s disease biomarkers in cognitively normal older adults
investigators have found.
Among 127 adults enrolled in a randomized clinical trial of interventions to promote mental well-being in older adults, those with sleep-disordered breathing had significantly greater amyloid burden and gray-matter volume, as well as increased perfusion and metabolism in parietal-occipital regions, reported Claire André, PhD, from the French Institute of Health and Medical Research (INSERM) unit in Caen, and colleagues.
“Our findings highlight the need to treat sleep disorders in the older population, even in the absence of cognitive or behavioral manifestations,” they wrote in a study published in JAMA Neurology.
Previous studies of the possible association between sleep-disordered breathing and dementia risk have shown conflicting or inconsistent results, the authors noted.
“These discrepancies may be explained by the characteristics of patients with sleep-disordered breathing (e.g., recruited from sleep clinics versus from the community, differences in age and disease duration), the scoring criteria of respiratory events, sample sizes, or the lack of controls for possibly biasing covariates,” they wrote.
To see whether they could clear up the confusion, the investigators conducted a retrospective analysis of 127 patients who were enrolled in the Age-Well randomized, controlled trial of the Medit-Ageing European project. The participants were community-dwelling adults (mean age, 69.1 years; 63% women), who were enrolled in the trial and underwent evaluation from 2016 to 2018 at the Cyceron Cancer Center in Caen.
The participants, all of whom were cognitively unimpaired at baseline, underwent neuropsychological assessment, polysomnography, MRI, plus florbetapir- and fluorodeoxyglucose-labeled PET.
The investigators defined sleep-disordered breathing as 15 apnea-hypopnea index events per hour or higher, and compared results between those with sleep-disordered breathing and those without for each imaging modality.
Participants with sleep-disordered breathing has significantly greater amyloid burden (P = .04), gray-matter volume (P = .04), perfusion (P = .04), and metabolism (P = .001), primarily overlapping the posterior cingulate cortex and precuneus, areas known to be significantly involved in Alzheimer’s disease.
When the investigators looked for behavioral and cognitive correlates of sleep-disordered breathing severity with associated brain changes, however, they found no associations with either cognitive performance, self-reported cognitive or sleep difficulties, or symptoms of daytime sleepiness.
“Importantly, to the best of our knowledge, our results show in vivo for the first time that greater amyloid burden colocalizes with greater gray-matter volume, perfusion, and metabolism in older participants with sleep-disordered breathing who are cognitively unimpaired. We believe that these overlapping patterns reinforce the likelihood of common underlying mechanisms,” they wrote.
The Age-Well randomized clinical trial is part of the Medit-Ageing project and is funded through the European Union’s Horizon 2020 Research and Innovation Program, INSERM, and Fondation d’ Entreprise MMA des Entrepreneurs du Futur. Dr. André reported no conflicts of interest to disclose.
SOURCE: André C et al. JAMA Neurol. 2020 Mar 23. doi: 10.1001/jamaneurol.2020.0311.
investigators have found.
Among 127 adults enrolled in a randomized clinical trial of interventions to promote mental well-being in older adults, those with sleep-disordered breathing had significantly greater amyloid burden and gray-matter volume, as well as increased perfusion and metabolism in parietal-occipital regions, reported Claire André, PhD, from the French Institute of Health and Medical Research (INSERM) unit in Caen, and colleagues.
“Our findings highlight the need to treat sleep disorders in the older population, even in the absence of cognitive or behavioral manifestations,” they wrote in a study published in JAMA Neurology.
Previous studies of the possible association between sleep-disordered breathing and dementia risk have shown conflicting or inconsistent results, the authors noted.
“These discrepancies may be explained by the characteristics of patients with sleep-disordered breathing (e.g., recruited from sleep clinics versus from the community, differences in age and disease duration), the scoring criteria of respiratory events, sample sizes, or the lack of controls for possibly biasing covariates,” they wrote.
To see whether they could clear up the confusion, the investigators conducted a retrospective analysis of 127 patients who were enrolled in the Age-Well randomized, controlled trial of the Medit-Ageing European project. The participants were community-dwelling adults (mean age, 69.1 years; 63% women), who were enrolled in the trial and underwent evaluation from 2016 to 2018 at the Cyceron Cancer Center in Caen.
The participants, all of whom were cognitively unimpaired at baseline, underwent neuropsychological assessment, polysomnography, MRI, plus florbetapir- and fluorodeoxyglucose-labeled PET.
The investigators defined sleep-disordered breathing as 15 apnea-hypopnea index events per hour or higher, and compared results between those with sleep-disordered breathing and those without for each imaging modality.
Participants with sleep-disordered breathing has significantly greater amyloid burden (P = .04), gray-matter volume (P = .04), perfusion (P = .04), and metabolism (P = .001), primarily overlapping the posterior cingulate cortex and precuneus, areas known to be significantly involved in Alzheimer’s disease.
When the investigators looked for behavioral and cognitive correlates of sleep-disordered breathing severity with associated brain changes, however, they found no associations with either cognitive performance, self-reported cognitive or sleep difficulties, or symptoms of daytime sleepiness.
“Importantly, to the best of our knowledge, our results show in vivo for the first time that greater amyloid burden colocalizes with greater gray-matter volume, perfusion, and metabolism in older participants with sleep-disordered breathing who are cognitively unimpaired. We believe that these overlapping patterns reinforce the likelihood of common underlying mechanisms,” they wrote.
The Age-Well randomized clinical trial is part of the Medit-Ageing project and is funded through the European Union’s Horizon 2020 Research and Innovation Program, INSERM, and Fondation d’ Entreprise MMA des Entrepreneurs du Futur. Dr. André reported no conflicts of interest to disclose.
SOURCE: André C et al. JAMA Neurol. 2020 Mar 23. doi: 10.1001/jamaneurol.2020.0311.
investigators have found.
Among 127 adults enrolled in a randomized clinical trial of interventions to promote mental well-being in older adults, those with sleep-disordered breathing had significantly greater amyloid burden and gray-matter volume, as well as increased perfusion and metabolism in parietal-occipital regions, reported Claire André, PhD, from the French Institute of Health and Medical Research (INSERM) unit in Caen, and colleagues.
“Our findings highlight the need to treat sleep disorders in the older population, even in the absence of cognitive or behavioral manifestations,” they wrote in a study published in JAMA Neurology.
Previous studies of the possible association between sleep-disordered breathing and dementia risk have shown conflicting or inconsistent results, the authors noted.
“These discrepancies may be explained by the characteristics of patients with sleep-disordered breathing (e.g., recruited from sleep clinics versus from the community, differences in age and disease duration), the scoring criteria of respiratory events, sample sizes, or the lack of controls for possibly biasing covariates,” they wrote.
To see whether they could clear up the confusion, the investigators conducted a retrospective analysis of 127 patients who were enrolled in the Age-Well randomized, controlled trial of the Medit-Ageing European project. The participants were community-dwelling adults (mean age, 69.1 years; 63% women), who were enrolled in the trial and underwent evaluation from 2016 to 2018 at the Cyceron Cancer Center in Caen.
The participants, all of whom were cognitively unimpaired at baseline, underwent neuropsychological assessment, polysomnography, MRI, plus florbetapir- and fluorodeoxyglucose-labeled PET.
The investigators defined sleep-disordered breathing as 15 apnea-hypopnea index events per hour or higher, and compared results between those with sleep-disordered breathing and those without for each imaging modality.
Participants with sleep-disordered breathing has significantly greater amyloid burden (P = .04), gray-matter volume (P = .04), perfusion (P = .04), and metabolism (P = .001), primarily overlapping the posterior cingulate cortex and precuneus, areas known to be significantly involved in Alzheimer’s disease.
When the investigators looked for behavioral and cognitive correlates of sleep-disordered breathing severity with associated brain changes, however, they found no associations with either cognitive performance, self-reported cognitive or sleep difficulties, or symptoms of daytime sleepiness.
“Importantly, to the best of our knowledge, our results show in vivo for the first time that greater amyloid burden colocalizes with greater gray-matter volume, perfusion, and metabolism in older participants with sleep-disordered breathing who are cognitively unimpaired. We believe that these overlapping patterns reinforce the likelihood of common underlying mechanisms,” they wrote.
The Age-Well randomized clinical trial is part of the Medit-Ageing project and is funded through the European Union’s Horizon 2020 Research and Innovation Program, INSERM, and Fondation d’ Entreprise MMA des Entrepreneurs du Futur. Dr. André reported no conflicts of interest to disclose.
SOURCE: André C et al. JAMA Neurol. 2020 Mar 23. doi: 10.1001/jamaneurol.2020.0311.
FROM JAMA NEUROLOGY
Do urgent care centers use optimal medications for acute migraine?
according to a study published in the March issue of Headache. Pain and nausea or vomiting associated with migraine may go undertreated, and treatment may not be consistent with American Headache Society (AHS) guidelines for EDs, said Mia T. Minen, MD, of the department of neurology and population health at NYU Langone Health in New York and colleagues.
“Our study findings raise the question as to whether the patients with migraine in the urgent care setting should be managed similarly to the ED, and whether the AHS guidelines for the ED should be revisited and applied to urgent care,” the researchers noted.
Relative to the ED, urgent care centers may provide cost savings and emerge “as a preferred place for treatment for people with migraine, perhaps as they are potentially more quiet medical settings where people with migraine might expeditiously receive care,” the authors said.
Dr. Minen and colleagues conducted a retrospective chart review to assess migraine management at two urgent care centers in New York. They examined the number of urgent care visits for migraine, treatments used, and how closely clinicians followed the AHS recommendations for administration of antiemetic medication and triptans, among other outcomes.
The study population included adults diagnosed with migraine at the NYU Langone Medhattan Urgent Care center between Dec. 1, 2015, and Dec. 1, 2018, or at the NYU Langone Ambulatory Care Urgent Care West Side center between May 1, 2017, and Dec. 1, 2018. Of more than 32,000 urgent care visits during the study period, 78 patients received a migraine diagnosis. Patients with migraine had an average age of 32.5 years, and 79.5% were female. More than half had a documented history of migraine. Two of the patients (2.6%) had been to an emergency department for headache or migraine.
Less than half of the patients who presented with pain (46.6%) were given medication, most commonly ketorolac injection. Most patients (78.2%) received prescriptions, and 25.6% received a triptan prescription. About 60% of patients were told to follow up with a neurologist. In addition, 11.5% revisited urgent care with a migraine or headache or to request a prescription refill.
“Patients in this study appeared to be using the urgent care centers specifically for acute care,” the researchers said. “The patients generally had infrequent headaches and the majority would not have qualified for migraine preventive treatment.”
Although AHS guidelines include three “should offer” medications for acute management of migraine in the ED – intravenous metoclopramide, intravenous prochlorperazine, and subcutaneous sumatriptan – two of the medications, subcutaneous sumatriptan and intravenous prochlorperazine, were not available in the urgent care pharmacy. “Of the level B migraine medications, only metoclopramide IV was in the pharmacy, and only 12.3% was given this at their urgent care visit,” the researchers said. “There was also likely undertreatment of nausea/vomiting; despite 39 patients with recorded nausea or vomiting with their migraine, less than half (46.2%) received an antiemetic at the visit,” including metoclopramide or ondansetron through oral or intravenous administration.
Future studies should look at headache and migraine visits at urgent care centers across the United States, the investigators suggested.
One of the authors of the study (Leslie Miller, MD) is the head of the NYU Langone Health Urgent Care Centers. Dr. Minen has received grant support, honoraria, or travel funds from the National Institutes of Health, the American Academy of Neurology, the American Brain Foundation, the National Multiple Sclerosis Society, the National Headache Foundation, the American Headache Society, Barnard College, and NYU. Dr. Minen is associate editor of Headache.
SOURCE: Minen MT et al. Headache. 2020;60(3):542-52.
according to a study published in the March issue of Headache. Pain and nausea or vomiting associated with migraine may go undertreated, and treatment may not be consistent with American Headache Society (AHS) guidelines for EDs, said Mia T. Minen, MD, of the department of neurology and population health at NYU Langone Health in New York and colleagues.
“Our study findings raise the question as to whether the patients with migraine in the urgent care setting should be managed similarly to the ED, and whether the AHS guidelines for the ED should be revisited and applied to urgent care,” the researchers noted.
Relative to the ED, urgent care centers may provide cost savings and emerge “as a preferred place for treatment for people with migraine, perhaps as they are potentially more quiet medical settings where people with migraine might expeditiously receive care,” the authors said.
Dr. Minen and colleagues conducted a retrospective chart review to assess migraine management at two urgent care centers in New York. They examined the number of urgent care visits for migraine, treatments used, and how closely clinicians followed the AHS recommendations for administration of antiemetic medication and triptans, among other outcomes.
The study population included adults diagnosed with migraine at the NYU Langone Medhattan Urgent Care center between Dec. 1, 2015, and Dec. 1, 2018, or at the NYU Langone Ambulatory Care Urgent Care West Side center between May 1, 2017, and Dec. 1, 2018. Of more than 32,000 urgent care visits during the study period, 78 patients received a migraine diagnosis. Patients with migraine had an average age of 32.5 years, and 79.5% were female. More than half had a documented history of migraine. Two of the patients (2.6%) had been to an emergency department for headache or migraine.
Less than half of the patients who presented with pain (46.6%) were given medication, most commonly ketorolac injection. Most patients (78.2%) received prescriptions, and 25.6% received a triptan prescription. About 60% of patients were told to follow up with a neurologist. In addition, 11.5% revisited urgent care with a migraine or headache or to request a prescription refill.
“Patients in this study appeared to be using the urgent care centers specifically for acute care,” the researchers said. “The patients generally had infrequent headaches and the majority would not have qualified for migraine preventive treatment.”
Although AHS guidelines include three “should offer” medications for acute management of migraine in the ED – intravenous metoclopramide, intravenous prochlorperazine, and subcutaneous sumatriptan – two of the medications, subcutaneous sumatriptan and intravenous prochlorperazine, were not available in the urgent care pharmacy. “Of the level B migraine medications, only metoclopramide IV was in the pharmacy, and only 12.3% was given this at their urgent care visit,” the researchers said. “There was also likely undertreatment of nausea/vomiting; despite 39 patients with recorded nausea or vomiting with their migraine, less than half (46.2%) received an antiemetic at the visit,” including metoclopramide or ondansetron through oral or intravenous administration.
Future studies should look at headache and migraine visits at urgent care centers across the United States, the investigators suggested.
One of the authors of the study (Leslie Miller, MD) is the head of the NYU Langone Health Urgent Care Centers. Dr. Minen has received grant support, honoraria, or travel funds from the National Institutes of Health, the American Academy of Neurology, the American Brain Foundation, the National Multiple Sclerosis Society, the National Headache Foundation, the American Headache Society, Barnard College, and NYU. Dr. Minen is associate editor of Headache.
SOURCE: Minen MT et al. Headache. 2020;60(3):542-52.
according to a study published in the March issue of Headache. Pain and nausea or vomiting associated with migraine may go undertreated, and treatment may not be consistent with American Headache Society (AHS) guidelines for EDs, said Mia T. Minen, MD, of the department of neurology and population health at NYU Langone Health in New York and colleagues.
“Our study findings raise the question as to whether the patients with migraine in the urgent care setting should be managed similarly to the ED, and whether the AHS guidelines for the ED should be revisited and applied to urgent care,” the researchers noted.
Relative to the ED, urgent care centers may provide cost savings and emerge “as a preferred place for treatment for people with migraine, perhaps as they are potentially more quiet medical settings where people with migraine might expeditiously receive care,” the authors said.
Dr. Minen and colleagues conducted a retrospective chart review to assess migraine management at two urgent care centers in New York. They examined the number of urgent care visits for migraine, treatments used, and how closely clinicians followed the AHS recommendations for administration of antiemetic medication and triptans, among other outcomes.
The study population included adults diagnosed with migraine at the NYU Langone Medhattan Urgent Care center between Dec. 1, 2015, and Dec. 1, 2018, or at the NYU Langone Ambulatory Care Urgent Care West Side center between May 1, 2017, and Dec. 1, 2018. Of more than 32,000 urgent care visits during the study period, 78 patients received a migraine diagnosis. Patients with migraine had an average age of 32.5 years, and 79.5% were female. More than half had a documented history of migraine. Two of the patients (2.6%) had been to an emergency department for headache or migraine.
Less than half of the patients who presented with pain (46.6%) were given medication, most commonly ketorolac injection. Most patients (78.2%) received prescriptions, and 25.6% received a triptan prescription. About 60% of patients were told to follow up with a neurologist. In addition, 11.5% revisited urgent care with a migraine or headache or to request a prescription refill.
“Patients in this study appeared to be using the urgent care centers specifically for acute care,” the researchers said. “The patients generally had infrequent headaches and the majority would not have qualified for migraine preventive treatment.”
Although AHS guidelines include three “should offer” medications for acute management of migraine in the ED – intravenous metoclopramide, intravenous prochlorperazine, and subcutaneous sumatriptan – two of the medications, subcutaneous sumatriptan and intravenous prochlorperazine, were not available in the urgent care pharmacy. “Of the level B migraine medications, only metoclopramide IV was in the pharmacy, and only 12.3% was given this at their urgent care visit,” the researchers said. “There was also likely undertreatment of nausea/vomiting; despite 39 patients with recorded nausea or vomiting with their migraine, less than half (46.2%) received an antiemetic at the visit,” including metoclopramide or ondansetron through oral or intravenous administration.
Future studies should look at headache and migraine visits at urgent care centers across the United States, the investigators suggested.
One of the authors of the study (Leslie Miller, MD) is the head of the NYU Langone Health Urgent Care Centers. Dr. Minen has received grant support, honoraria, or travel funds from the National Institutes of Health, the American Academy of Neurology, the American Brain Foundation, the National Multiple Sclerosis Society, the National Headache Foundation, the American Headache Society, Barnard College, and NYU. Dr. Minen is associate editor of Headache.
SOURCE: Minen MT et al. Headache. 2020;60(3):542-52.
FROM HEADACHE
Vision symptoms are common and often life-altering for patients with Parkinson’s disease
according to the results of a multicenter, cross-sectional cohort study.
“Ophthalmologic symptoms are underreported by patients with Parkinson’s disease and often overlooked by their treating physicians,” noted the investigators, who were led by Carlijn D.J.M. Borm, MD, Parkinson Centre Nijmegen (the Netherlands), Department of Neurology, Donders Institute for Brain, Cognition and Behaviour at Radboud University Medical Centre. “Importantly, intact vision is especially vital for patients with Parkinson’s disease to compensate (through visual guidance) for their common loss of motor automaticity that is caused by basal ganglia dysfunction.”
The investigators studied 848 patients with Parkinson’s disease in the Netherlands and Austria who were recruited by e-mail or in outpatient clinics and 250 age-matched healthy controls drawn from partners and acquaintances, comparing the groups on symptoms assessed with the Visual Impairment in Parkinson’s Disease Questionnaire (VIPD-Q).
Results reported in Neurology showed that 82% of patients with Parkinson’s disease reported at least one ophthalmologic symptom, compared with 48% of age-matched healthy controls (P < .001). Symptoms related to the ocular surface – blurry near vision, a burning or gritty sensation, mucus or particles, and watering of the eyes – were the most common.
Moreover, 68% of patients reported having ophthalmologic symptoms that interfered with daily activities, compared with 35% of healthy controls (P < .001).
The study’s findings suggest “that either Parkinson’s disease itself or its treatment has an effect on ophthalmologic functions beyond the normal aging process,” Dr. Borm and coinvestigators wrote. “The high prevalence of ophthalmologic symptoms and their effect on daily life is striking, and emphasizes the need to address this subject in both research and clinical practice.”
“Patients who report ophthalmologic symptoms need a referral for further evaluation. For those patients who do not volunteer problems themselves, a screening questionnaire such as the VIPD-Q may help with identifying ophthalmologic symptoms in patients with Parkinson’s disease that might otherwise be missed, thereby enabling timely referral and treatment,” they noted.
Study details
The study participants were 70 years old, on average, and the patients with Parkinson’s disease had had the disease for a median duration of 7 years. Compared with the healthy control group, the patient group more often reported that they used visual aids (95% vs. 88%; P = .001) and had visited an ophthalmologist (35% vs. 19%; P < .001). The median score on the VIPD-Q, out of a possible 51 points, was 10 among the patients with Parkinson’s disease, compared with 2 among the healthy controls (P < .001).
Patients most commonly reported symptoms related to the ocular surface (63% vs. 24% among controls; P < .001). But they also often reported symptoms in the intraocular domain (54% vs. 25%; P < .001), the oculomotor domain (44% vs. 10%; P < .001), and the optic nerve domain (44% vs. 19%; P < .001). Fully 22% of the patients reported visual hallucinations, compared with just 2% of the healthy controls (P < .001).
As VIPD-Q score increased, so did the likelihood of falls (odds ratio, 1.043; P < .001). In addition, patients with Parkinson’s disease more often reported that ophthalmologic symptoms had a moderate or severe impact on their quality of life (53% vs. 16%; P < .001).
Dr. Borm disclosed no relevant conflicts of interest. The study was funded by the Stichting Parkinson Fonds.
Awareness is key to spotting these treatable symptoms
“This study confirms a lot of what we already knew about Parkinson’s disease, but it gives more numbers to it and also the patient’s perspective rather than the doctor’s perspective,” Andrew G. Lee, MD, commented in an interview. “We know that patients with Parkinson’s disease have a lot of ophthalmologic symptoms – probably more than we recognize or ask about in the clinic – and their symptoms predominantly are out of proportion to what we see on exam,” said Dr. Lee, who is chairman of the Department of Ophthalmology at Blanton Eye Institute, Houston Methodist Hospital.
In fact, patients may have normal acuity, normal visual fields, and a normal structural eye exam, yet still report vision problems because of the central neurodegeneration occurring, he noted. “Ophthalmologists cannot rely on just the eye exam when examining patients with Parkinson’s disease. They have to take symptoms into consideration. It’s really important to be aware of how brain disease can affect the eyes symptom-wise, even though the eye exam is normal.”
Administering the questionnaire used in the study is not very difficult but is somewhat time consuming, so most ophthalmologists and neurologists are unlikely to use it, according to Dr. Lee. But knowing common symptoms and asking about them can ensure they are promptly recognized, the first step in addressing them.
“None of the visual complaints in patients with Parkinson’s disease are curable because Parkinson’s disease is not curable and the disease is the underlying major etiology for the problems. However, all of the symptoms have treatments,” he said.
For example, dry eye, caused by decreased blinking, can be treated with drops. Convergence insufficiency, which generates double vision when focusing on nearby objects, can be managed with prisms, exercises, and if needed, eye muscle surgery. Impaired eye movement is best treated with different sets of glasses specific to tasks, such as one pair for reading and one pair for distance. And visual hallucinations can be addressed with changes to existing medications or new medication, or simple reassurance that the hallucinations aren’t harmful.
“It’s important that this kind of study increases awareness in the medical community,” concluded Dr. Lee, who disclosed no relevant conflicts of interest. “The take-home messages are that eye doctors know that these are real complaints, that the eye exam is not going to give the answer, and that all of the symptoms have treatments even though there is no cure.”
SOURCE: Borm CDJM et al. Neurology. 2020 Mar 11. doi: 10.1212/WNL.0000000000009214.
according to the results of a multicenter, cross-sectional cohort study.
“Ophthalmologic symptoms are underreported by patients with Parkinson’s disease and often overlooked by their treating physicians,” noted the investigators, who were led by Carlijn D.J.M. Borm, MD, Parkinson Centre Nijmegen (the Netherlands), Department of Neurology, Donders Institute for Brain, Cognition and Behaviour at Radboud University Medical Centre. “Importantly, intact vision is especially vital for patients with Parkinson’s disease to compensate (through visual guidance) for their common loss of motor automaticity that is caused by basal ganglia dysfunction.”
The investigators studied 848 patients with Parkinson’s disease in the Netherlands and Austria who were recruited by e-mail or in outpatient clinics and 250 age-matched healthy controls drawn from partners and acquaintances, comparing the groups on symptoms assessed with the Visual Impairment in Parkinson’s Disease Questionnaire (VIPD-Q).
Results reported in Neurology showed that 82% of patients with Parkinson’s disease reported at least one ophthalmologic symptom, compared with 48% of age-matched healthy controls (P < .001). Symptoms related to the ocular surface – blurry near vision, a burning or gritty sensation, mucus or particles, and watering of the eyes – were the most common.
Moreover, 68% of patients reported having ophthalmologic symptoms that interfered with daily activities, compared with 35% of healthy controls (P < .001).
The study’s findings suggest “that either Parkinson’s disease itself or its treatment has an effect on ophthalmologic functions beyond the normal aging process,” Dr. Borm and coinvestigators wrote. “The high prevalence of ophthalmologic symptoms and their effect on daily life is striking, and emphasizes the need to address this subject in both research and clinical practice.”
“Patients who report ophthalmologic symptoms need a referral for further evaluation. For those patients who do not volunteer problems themselves, a screening questionnaire such as the VIPD-Q may help with identifying ophthalmologic symptoms in patients with Parkinson’s disease that might otherwise be missed, thereby enabling timely referral and treatment,” they noted.
Study details
The study participants were 70 years old, on average, and the patients with Parkinson’s disease had had the disease for a median duration of 7 years. Compared with the healthy control group, the patient group more often reported that they used visual aids (95% vs. 88%; P = .001) and had visited an ophthalmologist (35% vs. 19%; P < .001). The median score on the VIPD-Q, out of a possible 51 points, was 10 among the patients with Parkinson’s disease, compared with 2 among the healthy controls (P < .001).
Patients most commonly reported symptoms related to the ocular surface (63% vs. 24% among controls; P < .001). But they also often reported symptoms in the intraocular domain (54% vs. 25%; P < .001), the oculomotor domain (44% vs. 10%; P < .001), and the optic nerve domain (44% vs. 19%; P < .001). Fully 22% of the patients reported visual hallucinations, compared with just 2% of the healthy controls (P < .001).
As VIPD-Q score increased, so did the likelihood of falls (odds ratio, 1.043; P < .001). In addition, patients with Parkinson’s disease more often reported that ophthalmologic symptoms had a moderate or severe impact on their quality of life (53% vs. 16%; P < .001).
Dr. Borm disclosed no relevant conflicts of interest. The study was funded by the Stichting Parkinson Fonds.
Awareness is key to spotting these treatable symptoms
“This study confirms a lot of what we already knew about Parkinson’s disease, but it gives more numbers to it and also the patient’s perspective rather than the doctor’s perspective,” Andrew G. Lee, MD, commented in an interview. “We know that patients with Parkinson’s disease have a lot of ophthalmologic symptoms – probably more than we recognize or ask about in the clinic – and their symptoms predominantly are out of proportion to what we see on exam,” said Dr. Lee, who is chairman of the Department of Ophthalmology at Blanton Eye Institute, Houston Methodist Hospital.
In fact, patients may have normal acuity, normal visual fields, and a normal structural eye exam, yet still report vision problems because of the central neurodegeneration occurring, he noted. “Ophthalmologists cannot rely on just the eye exam when examining patients with Parkinson’s disease. They have to take symptoms into consideration. It’s really important to be aware of how brain disease can affect the eyes symptom-wise, even though the eye exam is normal.”
Administering the questionnaire used in the study is not very difficult but is somewhat time consuming, so most ophthalmologists and neurologists are unlikely to use it, according to Dr. Lee. But knowing common symptoms and asking about them can ensure they are promptly recognized, the first step in addressing them.
“None of the visual complaints in patients with Parkinson’s disease are curable because Parkinson’s disease is not curable and the disease is the underlying major etiology for the problems. However, all of the symptoms have treatments,” he said.
For example, dry eye, caused by decreased blinking, can be treated with drops. Convergence insufficiency, which generates double vision when focusing on nearby objects, can be managed with prisms, exercises, and if needed, eye muscle surgery. Impaired eye movement is best treated with different sets of glasses specific to tasks, such as one pair for reading and one pair for distance. And visual hallucinations can be addressed with changes to existing medications or new medication, or simple reassurance that the hallucinations aren’t harmful.
“It’s important that this kind of study increases awareness in the medical community,” concluded Dr. Lee, who disclosed no relevant conflicts of interest. “The take-home messages are that eye doctors know that these are real complaints, that the eye exam is not going to give the answer, and that all of the symptoms have treatments even though there is no cure.”
SOURCE: Borm CDJM et al. Neurology. 2020 Mar 11. doi: 10.1212/WNL.0000000000009214.
according to the results of a multicenter, cross-sectional cohort study.
“Ophthalmologic symptoms are underreported by patients with Parkinson’s disease and often overlooked by their treating physicians,” noted the investigators, who were led by Carlijn D.J.M. Borm, MD, Parkinson Centre Nijmegen (the Netherlands), Department of Neurology, Donders Institute for Brain, Cognition and Behaviour at Radboud University Medical Centre. “Importantly, intact vision is especially vital for patients with Parkinson’s disease to compensate (through visual guidance) for their common loss of motor automaticity that is caused by basal ganglia dysfunction.”
The investigators studied 848 patients with Parkinson’s disease in the Netherlands and Austria who were recruited by e-mail or in outpatient clinics and 250 age-matched healthy controls drawn from partners and acquaintances, comparing the groups on symptoms assessed with the Visual Impairment in Parkinson’s Disease Questionnaire (VIPD-Q).
Results reported in Neurology showed that 82% of patients with Parkinson’s disease reported at least one ophthalmologic symptom, compared with 48% of age-matched healthy controls (P < .001). Symptoms related to the ocular surface – blurry near vision, a burning or gritty sensation, mucus or particles, and watering of the eyes – were the most common.
Moreover, 68% of patients reported having ophthalmologic symptoms that interfered with daily activities, compared with 35% of healthy controls (P < .001).
The study’s findings suggest “that either Parkinson’s disease itself or its treatment has an effect on ophthalmologic functions beyond the normal aging process,” Dr. Borm and coinvestigators wrote. “The high prevalence of ophthalmologic symptoms and their effect on daily life is striking, and emphasizes the need to address this subject in both research and clinical practice.”
“Patients who report ophthalmologic symptoms need a referral for further evaluation. For those patients who do not volunteer problems themselves, a screening questionnaire such as the VIPD-Q may help with identifying ophthalmologic symptoms in patients with Parkinson’s disease that might otherwise be missed, thereby enabling timely referral and treatment,” they noted.
Study details
The study participants were 70 years old, on average, and the patients with Parkinson’s disease had had the disease for a median duration of 7 years. Compared with the healthy control group, the patient group more often reported that they used visual aids (95% vs. 88%; P = .001) and had visited an ophthalmologist (35% vs. 19%; P < .001). The median score on the VIPD-Q, out of a possible 51 points, was 10 among the patients with Parkinson’s disease, compared with 2 among the healthy controls (P < .001).
Patients most commonly reported symptoms related to the ocular surface (63% vs. 24% among controls; P < .001). But they also often reported symptoms in the intraocular domain (54% vs. 25%; P < .001), the oculomotor domain (44% vs. 10%; P < .001), and the optic nerve domain (44% vs. 19%; P < .001). Fully 22% of the patients reported visual hallucinations, compared with just 2% of the healthy controls (P < .001).
As VIPD-Q score increased, so did the likelihood of falls (odds ratio, 1.043; P < .001). In addition, patients with Parkinson’s disease more often reported that ophthalmologic symptoms had a moderate or severe impact on their quality of life (53% vs. 16%; P < .001).
Dr. Borm disclosed no relevant conflicts of interest. The study was funded by the Stichting Parkinson Fonds.
Awareness is key to spotting these treatable symptoms
“This study confirms a lot of what we already knew about Parkinson’s disease, but it gives more numbers to it and also the patient’s perspective rather than the doctor’s perspective,” Andrew G. Lee, MD, commented in an interview. “We know that patients with Parkinson’s disease have a lot of ophthalmologic symptoms – probably more than we recognize or ask about in the clinic – and their symptoms predominantly are out of proportion to what we see on exam,” said Dr. Lee, who is chairman of the Department of Ophthalmology at Blanton Eye Institute, Houston Methodist Hospital.
In fact, patients may have normal acuity, normal visual fields, and a normal structural eye exam, yet still report vision problems because of the central neurodegeneration occurring, he noted. “Ophthalmologists cannot rely on just the eye exam when examining patients with Parkinson’s disease. They have to take symptoms into consideration. It’s really important to be aware of how brain disease can affect the eyes symptom-wise, even though the eye exam is normal.”
Administering the questionnaire used in the study is not very difficult but is somewhat time consuming, so most ophthalmologists and neurologists are unlikely to use it, according to Dr. Lee. But knowing common symptoms and asking about them can ensure they are promptly recognized, the first step in addressing them.
“None of the visual complaints in patients with Parkinson’s disease are curable because Parkinson’s disease is not curable and the disease is the underlying major etiology for the problems. However, all of the symptoms have treatments,” he said.
For example, dry eye, caused by decreased blinking, can be treated with drops. Convergence insufficiency, which generates double vision when focusing on nearby objects, can be managed with prisms, exercises, and if needed, eye muscle surgery. Impaired eye movement is best treated with different sets of glasses specific to tasks, such as one pair for reading and one pair for distance. And visual hallucinations can be addressed with changes to existing medications or new medication, or simple reassurance that the hallucinations aren’t harmful.
“It’s important that this kind of study increases awareness in the medical community,” concluded Dr. Lee, who disclosed no relevant conflicts of interest. “The take-home messages are that eye doctors know that these are real complaints, that the eye exam is not going to give the answer, and that all of the symptoms have treatments even though there is no cure.”
SOURCE: Borm CDJM et al. Neurology. 2020 Mar 11. doi: 10.1212/WNL.0000000000009214.
FROM NEUROLOGY
Young women with insomnia at higher risk for car accidents
and reported daytime sleepiness represent a subpopulation at specific risk, according to an analysis of a 5-year population sample. The new research was published online in Sleep and led by Charles Morin, PhD, of Laval University, Quebec City.
The risks of daytime sleepiness and MVA are generally thought of in the context of obstructive sleep apnea (OSA) or men, but the results of the new work suggest that insomnia should not be overlooked, according to Krishna Sundar, MD, clinical professor of pulmonary, critical care, and sleep medicine, and medical director of the Sleep-Wake Center, at the University of Utah, Salt Lake City.
“The notion has been that it may keep them more hypervigilant and less prone to motor vehicle accidents because they are less able to fall asleep even if they want to during the daytime, as compared to other conditions like sleep apnea where there is a higher tendency to doze off,” Dr. Sundar said in an interview.
It should also be remembered that patients aren’t always completely reliable when it comes to self-assessment, according to Brandon M. Seay, MD, a pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta. “Most people with insomnia won’t say they are sleepy in the daytime, but when you objectively look, you do see an element of daytime sleepiness even if it’s not perceived that well by insomnia patients,” said Dr. Seay.
The heightened risks in young women with insomnia is notable, according to Dr. Sundar. Insomnia is more common in women, and they may also be more susceptible to unintended consequences of sleep medications because they metabolize them more slowly. “Especially for younger women, if they are insomniac and on prescription medicines, and if they have excess daytime sleepiness, this [risk of MVA] needs to be factored in,” said Dr. Sundar.
Insomnia is a condition that waxes and wanes over time, and can vary in its presentation across age groups, which is why the authors chose to conduct a prospective longitudinal study in a Canadian sample. They recruited 3,413 adults with insomnia (median age, 49.0 years; range, 18-96; 61.5% female). After 5 years, the retention rate was 68.7%.
After filling out baseline information, participants were asked every 6 months about MVAs and what role they believed daytime consequences of insomnia played if an accident occurred. Prescription and over-the-counter medication use were also self-reported.
In the first 2 years of the study, 8.2% of women aged 18-29 reported MVAs, which was the highest of any demographic (range, 2.3%-4.3%). By the third year, the frequency in this group overlapped that of men in the same age group, and both remained higher than older age groups.
Participants judged that insomnia consequences played a role in 39.4% of reported MVA. In 17.2% of accidents, participants said insomnia consequences contributed at least 50% of the cause.
MVA risk was associated individually with presence of insomnia symptoms (hazard ratio [HR], 1.20; 95% confidence interval, 1.00-1.45) and daytime fatigue (HR, 1.21; 95% CI, 1.01-1.47), but there were only trends toward associations with sleeping fewer than 6 hours (P = .16) and excessive daytime sleepiness (P = .06). MVAs were associated with reported past-year use of prescribed sleep medications (HR, 1.50; 95% CI, 1.17-1.91) and reported use of OTC medications (HR, 1.42; 95% CI, 1.02-1.98).
In women aged 18-29, MVAs were associated with insomnia symptoms (HR, 1.83; 95% CI, 1.13-2.98) and excessive daytime sleepiness (HR, 2.42; 95% CI, 1.11-5.24).
The study was limited by its reliance on self-reporting and lack of data on specific medications used.
The study was funded by the Canadian Institutes of Health.
SOURCE: Morin C et al. Sleep. 2020 Feb 29. DOI: 10.1093/sleep/zsaa032.
and reported daytime sleepiness represent a subpopulation at specific risk, according to an analysis of a 5-year population sample. The new research was published online in Sleep and led by Charles Morin, PhD, of Laval University, Quebec City.
The risks of daytime sleepiness and MVA are generally thought of in the context of obstructive sleep apnea (OSA) or men, but the results of the new work suggest that insomnia should not be overlooked, according to Krishna Sundar, MD, clinical professor of pulmonary, critical care, and sleep medicine, and medical director of the Sleep-Wake Center, at the University of Utah, Salt Lake City.
“The notion has been that it may keep them more hypervigilant and less prone to motor vehicle accidents because they are less able to fall asleep even if they want to during the daytime, as compared to other conditions like sleep apnea where there is a higher tendency to doze off,” Dr. Sundar said in an interview.
It should also be remembered that patients aren’t always completely reliable when it comes to self-assessment, according to Brandon M. Seay, MD, a pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta. “Most people with insomnia won’t say they are sleepy in the daytime, but when you objectively look, you do see an element of daytime sleepiness even if it’s not perceived that well by insomnia patients,” said Dr. Seay.
The heightened risks in young women with insomnia is notable, according to Dr. Sundar. Insomnia is more common in women, and they may also be more susceptible to unintended consequences of sleep medications because they metabolize them more slowly. “Especially for younger women, if they are insomniac and on prescription medicines, and if they have excess daytime sleepiness, this [risk of MVA] needs to be factored in,” said Dr. Sundar.
Insomnia is a condition that waxes and wanes over time, and can vary in its presentation across age groups, which is why the authors chose to conduct a prospective longitudinal study in a Canadian sample. They recruited 3,413 adults with insomnia (median age, 49.0 years; range, 18-96; 61.5% female). After 5 years, the retention rate was 68.7%.
After filling out baseline information, participants were asked every 6 months about MVAs and what role they believed daytime consequences of insomnia played if an accident occurred. Prescription and over-the-counter medication use were also self-reported.
In the first 2 years of the study, 8.2% of women aged 18-29 reported MVAs, which was the highest of any demographic (range, 2.3%-4.3%). By the third year, the frequency in this group overlapped that of men in the same age group, and both remained higher than older age groups.
Participants judged that insomnia consequences played a role in 39.4% of reported MVA. In 17.2% of accidents, participants said insomnia consequences contributed at least 50% of the cause.
MVA risk was associated individually with presence of insomnia symptoms (hazard ratio [HR], 1.20; 95% confidence interval, 1.00-1.45) and daytime fatigue (HR, 1.21; 95% CI, 1.01-1.47), but there were only trends toward associations with sleeping fewer than 6 hours (P = .16) and excessive daytime sleepiness (P = .06). MVAs were associated with reported past-year use of prescribed sleep medications (HR, 1.50; 95% CI, 1.17-1.91) and reported use of OTC medications (HR, 1.42; 95% CI, 1.02-1.98).
In women aged 18-29, MVAs were associated with insomnia symptoms (HR, 1.83; 95% CI, 1.13-2.98) and excessive daytime sleepiness (HR, 2.42; 95% CI, 1.11-5.24).
The study was limited by its reliance on self-reporting and lack of data on specific medications used.
The study was funded by the Canadian Institutes of Health.
SOURCE: Morin C et al. Sleep. 2020 Feb 29. DOI: 10.1093/sleep/zsaa032.
and reported daytime sleepiness represent a subpopulation at specific risk, according to an analysis of a 5-year population sample. The new research was published online in Sleep and led by Charles Morin, PhD, of Laval University, Quebec City.
The risks of daytime sleepiness and MVA are generally thought of in the context of obstructive sleep apnea (OSA) or men, but the results of the new work suggest that insomnia should not be overlooked, according to Krishna Sundar, MD, clinical professor of pulmonary, critical care, and sleep medicine, and medical director of the Sleep-Wake Center, at the University of Utah, Salt Lake City.
“The notion has been that it may keep them more hypervigilant and less prone to motor vehicle accidents because they are less able to fall asleep even if they want to during the daytime, as compared to other conditions like sleep apnea where there is a higher tendency to doze off,” Dr. Sundar said in an interview.
It should also be remembered that patients aren’t always completely reliable when it comes to self-assessment, according to Brandon M. Seay, MD, a pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta. “Most people with insomnia won’t say they are sleepy in the daytime, but when you objectively look, you do see an element of daytime sleepiness even if it’s not perceived that well by insomnia patients,” said Dr. Seay.
The heightened risks in young women with insomnia is notable, according to Dr. Sundar. Insomnia is more common in women, and they may also be more susceptible to unintended consequences of sleep medications because they metabolize them more slowly. “Especially for younger women, if they are insomniac and on prescription medicines, and if they have excess daytime sleepiness, this [risk of MVA] needs to be factored in,” said Dr. Sundar.
Insomnia is a condition that waxes and wanes over time, and can vary in its presentation across age groups, which is why the authors chose to conduct a prospective longitudinal study in a Canadian sample. They recruited 3,413 adults with insomnia (median age, 49.0 years; range, 18-96; 61.5% female). After 5 years, the retention rate was 68.7%.
After filling out baseline information, participants were asked every 6 months about MVAs and what role they believed daytime consequences of insomnia played if an accident occurred. Prescription and over-the-counter medication use were also self-reported.
In the first 2 years of the study, 8.2% of women aged 18-29 reported MVAs, which was the highest of any demographic (range, 2.3%-4.3%). By the third year, the frequency in this group overlapped that of men in the same age group, and both remained higher than older age groups.
Participants judged that insomnia consequences played a role in 39.4% of reported MVA. In 17.2% of accidents, participants said insomnia consequences contributed at least 50% of the cause.
MVA risk was associated individually with presence of insomnia symptoms (hazard ratio [HR], 1.20; 95% confidence interval, 1.00-1.45) and daytime fatigue (HR, 1.21; 95% CI, 1.01-1.47), but there were only trends toward associations with sleeping fewer than 6 hours (P = .16) and excessive daytime sleepiness (P = .06). MVAs were associated with reported past-year use of prescribed sleep medications (HR, 1.50; 95% CI, 1.17-1.91) and reported use of OTC medications (HR, 1.42; 95% CI, 1.02-1.98).
In women aged 18-29, MVAs were associated with insomnia symptoms (HR, 1.83; 95% CI, 1.13-2.98) and excessive daytime sleepiness (HR, 2.42; 95% CI, 1.11-5.24).
The study was limited by its reliance on self-reporting and lack of data on specific medications used.
The study was funded by the Canadian Institutes of Health.
SOURCE: Morin C et al. Sleep. 2020 Feb 29. DOI: 10.1093/sleep/zsaa032.
FROM SLEEP
American Headache Society updates guideline on neuroimaging for migraine
Migraine with atypical features may require neuroimaging, according to the guideline. These include an unusual aura; change in clinical features; a first or worst migraine; a migraine that presents with brainstem aura, confusion, or motor manifestation; migraine accompaniments in later life; headaches that are side-locked or posttraumatic; and aura that presents without headache.
Assessing the evidence
The recommendation to avoid MRI or CT in otherwise neurologically normal patients with migraine carried a grade A recommendation from the American Headache Society, while the specific considerations for neuroimaging was based on consensus and carried a grade C recommendation, according to lead author Randolph W. Evans, MD, of the department of neurology at Baylor College of Medicine in Houston, and colleagues.
The recommendations, published in the journal Headache (2020 Feb;60(2):318-36), came from a systematic review of 23 studies of adults at least 18 years old who underwent MRI or CT during outpatient treatment for migraine between 1973 and 2018. Ten studies looked at CT neuroimaging in patients with migraine, nine studies examined MRI neuroimaging alone in patients with migraine, and four studies contained adults with headache or migraine who underwent either MRI or CT. The majority of studies analyzed were retrospective or cross-sectional in nature, while four studies were prospective observational studies.
Dr. Evans and colleagues noted that neuroimaging for patients with suspected migraine is ordered for a variety of reasons, such as excluding conditions that aren’t migraine, diagnostic certainty, cognitive bias, practice workflow, medicolegal concerns, addressing patient and family anxiety, and addressing clinician anxiety. Neuroimaging also can be costly, they said, adding up to an estimated $1 billion annually according to one study, and can lead to additional testing from findings that may not be clinically significant.
Good advice, with caveats
In an interview, Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews, said that while he generally does not like broad guideline recommendations, the recommendation made by the American Headache Society to avoid neuroimaging in patients with a normal neurological examination without any atypical features and red flags “takes most of the important factors into consideration and will work almost all the time.” The recommendation made by consensus for specific considerations of neuroimaging was issued by top headache specialists in the United States who reviewed the data, and it is unlikely a patient with a migraine as diagnosed by the International Classification of Headache Disorders with a normal neurological examination would have a significant abnormality that would appear with imaging, Dr. Rapoport said.
“If everyone caring for migraine patients knew these recommendations, and used them unless the patients fit the exclusions mentioned, we would have more efficient clinical practice and save lots of money on unnecessary scanning,” he said.
However, Dr. Rapoport, clinical professor of neurology at the University of California, Los Angeles, founder of the New England Center for Headache, and past president of The International Headache Society, said that not all clinicians will be convinced by the American Headache Society’s recommendations.
“Various third parties often jump on society recommendations or guidelines and prevent smart clinicians from doing what they need to do when they want to disregard the recommendation or guideline,” he explained. “More importantly, if a physician feels the need to think out of the box and image a patient without a clear reason, and the patient cannot pay for the scan when a medical insurance company refuses to authorize it, there can be a bad result if the patient does not get the study.”
Dr. Rapoport noted that the guideline does not address situations where neuroimaging may not pick up conditions that lead to migraine, such as a subarachnoid or subdural hemorrhage, reversible cerebral vasoconstriction syndrome, or early aspects of low cerebrospinal fluid pressure syndrome. Anxiety on the part of the patient or the clinician is another area that can be addressed by future research, he said.
“If the clinician does a good job of explaining the odds of anything significant being found with a typical migraine history and normal examination, and the patient says [they] need an MRI with contrast to be sure, it will be difficult to dissuade them,” said Dr. Rapoport. “If you don’t order one, they will find a way to get one. If it is abnormal, you could be in trouble. Also, if the clinician has no good reason to do a scan but has anxiety about what is being missed, it will probably get done.”
There was no funding source for the guidelines. The authors reported personal and institutional relationships in the form of advisory board memberships, investigator appointments, speakers bureau positions, research support, and consultancies for a variety of pharmaceutical companies, agencies, institutions, publishers, and other organizations.
Migraine with atypical features may require neuroimaging, according to the guideline. These include an unusual aura; change in clinical features; a first or worst migraine; a migraine that presents with brainstem aura, confusion, or motor manifestation; migraine accompaniments in later life; headaches that are side-locked or posttraumatic; and aura that presents without headache.
Assessing the evidence
The recommendation to avoid MRI or CT in otherwise neurologically normal patients with migraine carried a grade A recommendation from the American Headache Society, while the specific considerations for neuroimaging was based on consensus and carried a grade C recommendation, according to lead author Randolph W. Evans, MD, of the department of neurology at Baylor College of Medicine in Houston, and colleagues.
The recommendations, published in the journal Headache (2020 Feb;60(2):318-36), came from a systematic review of 23 studies of adults at least 18 years old who underwent MRI or CT during outpatient treatment for migraine between 1973 and 2018. Ten studies looked at CT neuroimaging in patients with migraine, nine studies examined MRI neuroimaging alone in patients with migraine, and four studies contained adults with headache or migraine who underwent either MRI or CT. The majority of studies analyzed were retrospective or cross-sectional in nature, while four studies were prospective observational studies.
Dr. Evans and colleagues noted that neuroimaging for patients with suspected migraine is ordered for a variety of reasons, such as excluding conditions that aren’t migraine, diagnostic certainty, cognitive bias, practice workflow, medicolegal concerns, addressing patient and family anxiety, and addressing clinician anxiety. Neuroimaging also can be costly, they said, adding up to an estimated $1 billion annually according to one study, and can lead to additional testing from findings that may not be clinically significant.
Good advice, with caveats
In an interview, Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews, said that while he generally does not like broad guideline recommendations, the recommendation made by the American Headache Society to avoid neuroimaging in patients with a normal neurological examination without any atypical features and red flags “takes most of the important factors into consideration and will work almost all the time.” The recommendation made by consensus for specific considerations of neuroimaging was issued by top headache specialists in the United States who reviewed the data, and it is unlikely a patient with a migraine as diagnosed by the International Classification of Headache Disorders with a normal neurological examination would have a significant abnormality that would appear with imaging, Dr. Rapoport said.
“If everyone caring for migraine patients knew these recommendations, and used them unless the patients fit the exclusions mentioned, we would have more efficient clinical practice and save lots of money on unnecessary scanning,” he said.
However, Dr. Rapoport, clinical professor of neurology at the University of California, Los Angeles, founder of the New England Center for Headache, and past president of The International Headache Society, said that not all clinicians will be convinced by the American Headache Society’s recommendations.
“Various third parties often jump on society recommendations or guidelines and prevent smart clinicians from doing what they need to do when they want to disregard the recommendation or guideline,” he explained. “More importantly, if a physician feels the need to think out of the box and image a patient without a clear reason, and the patient cannot pay for the scan when a medical insurance company refuses to authorize it, there can be a bad result if the patient does not get the study.”
Dr. Rapoport noted that the guideline does not address situations where neuroimaging may not pick up conditions that lead to migraine, such as a subarachnoid or subdural hemorrhage, reversible cerebral vasoconstriction syndrome, or early aspects of low cerebrospinal fluid pressure syndrome. Anxiety on the part of the patient or the clinician is another area that can be addressed by future research, he said.
“If the clinician does a good job of explaining the odds of anything significant being found with a typical migraine history and normal examination, and the patient says [they] need an MRI with contrast to be sure, it will be difficult to dissuade them,” said Dr. Rapoport. “If you don’t order one, they will find a way to get one. If it is abnormal, you could be in trouble. Also, if the clinician has no good reason to do a scan but has anxiety about what is being missed, it will probably get done.”
There was no funding source for the guidelines. The authors reported personal and institutional relationships in the form of advisory board memberships, investigator appointments, speakers bureau positions, research support, and consultancies for a variety of pharmaceutical companies, agencies, institutions, publishers, and other organizations.
Migraine with atypical features may require neuroimaging, according to the guideline. These include an unusual aura; change in clinical features; a first or worst migraine; a migraine that presents with brainstem aura, confusion, or motor manifestation; migraine accompaniments in later life; headaches that are side-locked or posttraumatic; and aura that presents without headache.
Assessing the evidence
The recommendation to avoid MRI or CT in otherwise neurologically normal patients with migraine carried a grade A recommendation from the American Headache Society, while the specific considerations for neuroimaging was based on consensus and carried a grade C recommendation, according to lead author Randolph W. Evans, MD, of the department of neurology at Baylor College of Medicine in Houston, and colleagues.
The recommendations, published in the journal Headache (2020 Feb;60(2):318-36), came from a systematic review of 23 studies of adults at least 18 years old who underwent MRI or CT during outpatient treatment for migraine between 1973 and 2018. Ten studies looked at CT neuroimaging in patients with migraine, nine studies examined MRI neuroimaging alone in patients with migraine, and four studies contained adults with headache or migraine who underwent either MRI or CT. The majority of studies analyzed were retrospective or cross-sectional in nature, while four studies were prospective observational studies.
Dr. Evans and colleagues noted that neuroimaging for patients with suspected migraine is ordered for a variety of reasons, such as excluding conditions that aren’t migraine, diagnostic certainty, cognitive bias, practice workflow, medicolegal concerns, addressing patient and family anxiety, and addressing clinician anxiety. Neuroimaging also can be costly, they said, adding up to an estimated $1 billion annually according to one study, and can lead to additional testing from findings that may not be clinically significant.
Good advice, with caveats
In an interview, Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews, said that while he generally does not like broad guideline recommendations, the recommendation made by the American Headache Society to avoid neuroimaging in patients with a normal neurological examination without any atypical features and red flags “takes most of the important factors into consideration and will work almost all the time.” The recommendation made by consensus for specific considerations of neuroimaging was issued by top headache specialists in the United States who reviewed the data, and it is unlikely a patient with a migraine as diagnosed by the International Classification of Headache Disorders with a normal neurological examination would have a significant abnormality that would appear with imaging, Dr. Rapoport said.
“If everyone caring for migraine patients knew these recommendations, and used them unless the patients fit the exclusions mentioned, we would have more efficient clinical practice and save lots of money on unnecessary scanning,” he said.
However, Dr. Rapoport, clinical professor of neurology at the University of California, Los Angeles, founder of the New England Center for Headache, and past president of The International Headache Society, said that not all clinicians will be convinced by the American Headache Society’s recommendations.
“Various third parties often jump on society recommendations or guidelines and prevent smart clinicians from doing what they need to do when they want to disregard the recommendation or guideline,” he explained. “More importantly, if a physician feels the need to think out of the box and image a patient without a clear reason, and the patient cannot pay for the scan when a medical insurance company refuses to authorize it, there can be a bad result if the patient does not get the study.”
Dr. Rapoport noted that the guideline does not address situations where neuroimaging may not pick up conditions that lead to migraine, such as a subarachnoid or subdural hemorrhage, reversible cerebral vasoconstriction syndrome, or early aspects of low cerebrospinal fluid pressure syndrome. Anxiety on the part of the patient or the clinician is another area that can be addressed by future research, he said.
“If the clinician does a good job of explaining the odds of anything significant being found with a typical migraine history and normal examination, and the patient says [they] need an MRI with contrast to be sure, it will be difficult to dissuade them,” said Dr. Rapoport. “If you don’t order one, they will find a way to get one. If it is abnormal, you could be in trouble. Also, if the clinician has no good reason to do a scan but has anxiety about what is being missed, it will probably get done.”
There was no funding source for the guidelines. The authors reported personal and institutional relationships in the form of advisory board memberships, investigator appointments, speakers bureau positions, research support, and consultancies for a variety of pharmaceutical companies, agencies, institutions, publishers, and other organizations.
FROM HEADACHE
TBI deaths from falls on the rise
A 17% surge in mortality from fall-related traumatic brain injuries from 2008 to 2017 was driven largely by increases among those aged 75 years and older, according to investigators from the Centers for Disease Control and Prevention.
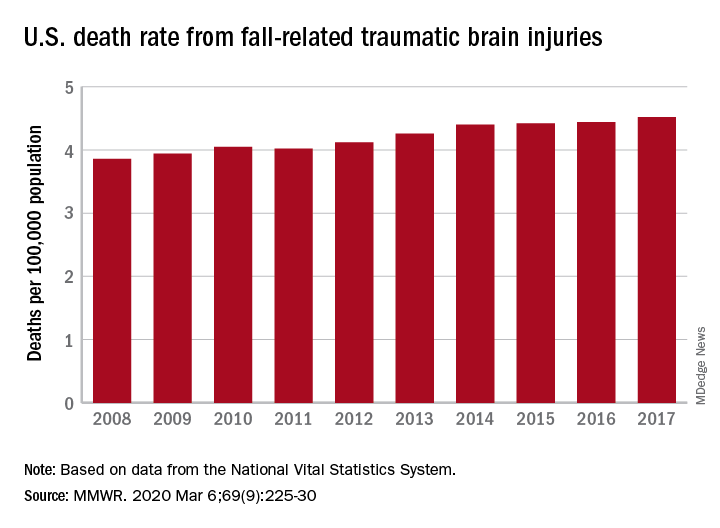
Nationally, the rate of deaths from traumatic brain injuries (TBIs) caused by unintentional falls rose from 3.86 per 100,000 population in 2008 to 4.52 per 100,000 in 2017, as the number of deaths went from 12,311 to 17,408, said Alexis B. Peterson, PhD, and Scott R. Kegler, PhD, of the CDC’s National Center for Injury Prevention and Control in Atlanta.
“This increase might be explained by longer survival following the onset of common diseases such as stroke, cancer, and heart disease or be attributable to the increasing population of older adults in the United States,” they suggested in the Mortality and Morbidity Weekly Report.
The rate of fall-related TBI among Americans aged 75 years and older increased by an average of 2.6% per year from 2008 to 2017, compared with 1.8% in those aged 55-74. Over that same time, death rates dropped for those aged 35-44 (–0.3%), 18-34 (–1.1%), and 0-17 (–4.3%), they said, based on data from the National Vital Statistics System’s multiple cause-of-death database.
The death rate increased fastest in residents of rural areas (2.9% per year), but deaths from fall-related TBI were up at all levels of urbanization. The largest central cities and fringe metro areas were up by 1.4% a year, with larger annual increases seen in medium-size cities (2.1%), small cities (2.2%), and small towns (2.1%), Dr. Peterson and Dr. Kegler said.
Rates of TBI-related mortality in general are higher in rural areas, they noted, and “heterogeneity in the availability and accessibility of resources (e.g., access to high-level trauma centers and rehabilitative services) can result in disparities in postinjury outcomes.”
State-specific rates increased in 45 states, although Alaska was excluded from the analysis because of its small number of cases (less than 20). Increases were significant in 29 states, but none of the changes were significant in the 4 states with lower rates at the end of the study period, the investigators reported.
“In older adults, evidence-based fall prevention strategies can prevent falls and avert costly medical expenditures,” Dr. Peterson and Dr. Kegler said, suggesting that health care providers “consider prescribing exercises that incorporate balance, strength and gait activities, such as tai chi, and reviewing and managing medications linked to falls.”
SOURCE: Peterson AB, Kegler SR. MMWR. 2019 Mar 6;69(9):225-30.
A 17% surge in mortality from fall-related traumatic brain injuries from 2008 to 2017 was driven largely by increases among those aged 75 years and older, according to investigators from the Centers for Disease Control and Prevention.

Nationally, the rate of deaths from traumatic brain injuries (TBIs) caused by unintentional falls rose from 3.86 per 100,000 population in 2008 to 4.52 per 100,000 in 2017, as the number of deaths went from 12,311 to 17,408, said Alexis B. Peterson, PhD, and Scott R. Kegler, PhD, of the CDC’s National Center for Injury Prevention and Control in Atlanta.
“This increase might be explained by longer survival following the onset of common diseases such as stroke, cancer, and heart disease or be attributable to the increasing population of older adults in the United States,” they suggested in the Mortality and Morbidity Weekly Report.
The rate of fall-related TBI among Americans aged 75 years and older increased by an average of 2.6% per year from 2008 to 2017, compared with 1.8% in those aged 55-74. Over that same time, death rates dropped for those aged 35-44 (–0.3%), 18-34 (–1.1%), and 0-17 (–4.3%), they said, based on data from the National Vital Statistics System’s multiple cause-of-death database.
The death rate increased fastest in residents of rural areas (2.9% per year), but deaths from fall-related TBI were up at all levels of urbanization. The largest central cities and fringe metro areas were up by 1.4% a year, with larger annual increases seen in medium-size cities (2.1%), small cities (2.2%), and small towns (2.1%), Dr. Peterson and Dr. Kegler said.
Rates of TBI-related mortality in general are higher in rural areas, they noted, and “heterogeneity in the availability and accessibility of resources (e.g., access to high-level trauma centers and rehabilitative services) can result in disparities in postinjury outcomes.”
State-specific rates increased in 45 states, although Alaska was excluded from the analysis because of its small number of cases (less than 20). Increases were significant in 29 states, but none of the changes were significant in the 4 states with lower rates at the end of the study period, the investigators reported.
“In older adults, evidence-based fall prevention strategies can prevent falls and avert costly medical expenditures,” Dr. Peterson and Dr. Kegler said, suggesting that health care providers “consider prescribing exercises that incorporate balance, strength and gait activities, such as tai chi, and reviewing and managing medications linked to falls.”
SOURCE: Peterson AB, Kegler SR. MMWR. 2019 Mar 6;69(9):225-30.
A 17% surge in mortality from fall-related traumatic brain injuries from 2008 to 2017 was driven largely by increases among those aged 75 years and older, according to investigators from the Centers for Disease Control and Prevention.

Nationally, the rate of deaths from traumatic brain injuries (TBIs) caused by unintentional falls rose from 3.86 per 100,000 population in 2008 to 4.52 per 100,000 in 2017, as the number of deaths went from 12,311 to 17,408, said Alexis B. Peterson, PhD, and Scott R. Kegler, PhD, of the CDC’s National Center for Injury Prevention and Control in Atlanta.
“This increase might be explained by longer survival following the onset of common diseases such as stroke, cancer, and heart disease or be attributable to the increasing population of older adults in the United States,” they suggested in the Mortality and Morbidity Weekly Report.
The rate of fall-related TBI among Americans aged 75 years and older increased by an average of 2.6% per year from 2008 to 2017, compared with 1.8% in those aged 55-74. Over that same time, death rates dropped for those aged 35-44 (–0.3%), 18-34 (–1.1%), and 0-17 (–4.3%), they said, based on data from the National Vital Statistics System’s multiple cause-of-death database.
The death rate increased fastest in residents of rural areas (2.9% per year), but deaths from fall-related TBI were up at all levels of urbanization. The largest central cities and fringe metro areas were up by 1.4% a year, with larger annual increases seen in medium-size cities (2.1%), small cities (2.2%), and small towns (2.1%), Dr. Peterson and Dr. Kegler said.
Rates of TBI-related mortality in general are higher in rural areas, they noted, and “heterogeneity in the availability and accessibility of resources (e.g., access to high-level trauma centers and rehabilitative services) can result in disparities in postinjury outcomes.”
State-specific rates increased in 45 states, although Alaska was excluded from the analysis because of its small number of cases (less than 20). Increases were significant in 29 states, but none of the changes were significant in the 4 states with lower rates at the end of the study period, the investigators reported.
“In older adults, evidence-based fall prevention strategies can prevent falls and avert costly medical expenditures,” Dr. Peterson and Dr. Kegler said, suggesting that health care providers “consider prescribing exercises that incorporate balance, strength and gait activities, such as tai chi, and reviewing and managing medications linked to falls.”
SOURCE: Peterson AB, Kegler SR. MMWR. 2019 Mar 6;69(9):225-30.
FROM MMWR
Stress-related disorders linked to later neurodegenerative diseases
Individuals with posttraumatic stress disorder (PTSD), acute stress reaction, adjustment disorder, or other stress reactions had an 80% increased risk of vascular neurodegenerative diseases, according to results of the study, which was based on Swedish population registry data.
Risk of primary neurodegenerative diseases was increased as well in people with those conditions, but only by 31%, according to lead author Huan Song, MD, PhD, of Sichuan University in Chengdu, China.
“The stronger association observed for neurodegenerative diseases with a vascular component, compared with primary neurodegenerative diseases, suggested a considerable role of a possible cerebrovascular pathway,” Dr. Song and coauthors said in a report on the study appearing in JAMA Neurology.
While some previous studies have linked stress-related disorders to neurodegenerative diseases – particularly PTSD and dementia – this is believed to be the first, according to the investigators, to comprehensively evaluate all stress-related disorders in relation to the most common neurodegenerative conditions.
When considering neurodegenerative conditions separately, they found a statistically significant association between stress-related disorders and Alzheimer’s disease, while linkages with Parkinson’s disease and amyotrophic lateral sclerosis (ALS) were “comparable” but associations did not reach statistical significance, according to investigators.
Based on these findings, stress reduction should be recommended in addition to daily physical activity, mental activity, and a heart-healthy diet to potentially reduce risk of onset or worsening of cognitive decline, according to Chun Lim, MD, PhD, medical director of the cognitive neurology unit at Beth Israel Deaconess Medical Center in Boston.
“We don’t really have great evidence that anything slows down the progression of Alzheimer’s disease, but there are some suggestions that for people who lead heart-healthy lifestyles or adhere to a Mediterranean diet, fewer develop cognitive issues over 5-10 years,” Dr. Lim said in an interview. “Because of this paper, stress reduction may be one additional way to hopefully help these patients these patients that have or are concerned about cognitive issues.”
The population-matched cohort of the study included 61,748 individuals with stress-related disorders and 595,335 matched individuals without those disorders, while the sibling-matched cohort included 44,839 individuals with those disorders and 78,482 without. The median age at the start of follow-up was 47 years and 39.4% of those with stress-related disorders were male.
During follow-up, the incidence of neurodegenerative diseases per 1,000 person-years was 1.50 for individuals with stress-related disorders, versus 0.82 for those without stress-related disorders, according to the report. Risk of primary neurodegenerative diseases was increased among those with stress-related disorders, compared with those without, with a hazard ratio of 1.31 (95% confidence interval, 1.15-1.48). However, the risk of vascular neurodegenerative diseases was significantly higher, with an HR of 1.80 (95% CI, 1.40-2.31; P = .03 for the difference between hazard ratios).
Results of the matched sibling cohort supported results of the population-matched cohort, though the elevated risk of vascular neurodegenerative diseases among those with stress-related disorders was “slightly lower” than in the population-based cohort, Dr. Song and coauthors wrote in their report.
Beyond causing a host of hormonal and medical issues, stress can lead to sleep issues that may have long-term consequences, Dr. Lim noted in the interview.
“There’s some thought that quality sleep is important for memory formation, and if people are under a fair amount of stress and they have really poor sleep, that can also lead to cognitive issues including memory impairment,” he said.
“There are these multiple avenues that may be contributing to the accelerated development of these kinds of issues,” he added, “so I think this paper suggests more ways to counsel the patients about using lifestyle modifications to slow down the development of these cognitive impairments.”
Funding for the study came from the Swedish Research Council, Icelandic Research Fund; ,European Research Council the Karolinska Institutet, Swedish Research Council, and West China Hospital. Authors of the study provided disclosures related to those organizations as well as Shire/Takeda and Evolan.
SOURCE: Song H et al. JAMA Neurol. 2020 Mar 9. doi: 10.1001/jamaneurol.2020.0117.
Individuals with posttraumatic stress disorder (PTSD), acute stress reaction, adjustment disorder, or other stress reactions had an 80% increased risk of vascular neurodegenerative diseases, according to results of the study, which was based on Swedish population registry data.
Risk of primary neurodegenerative diseases was increased as well in people with those conditions, but only by 31%, according to lead author Huan Song, MD, PhD, of Sichuan University in Chengdu, China.
“The stronger association observed for neurodegenerative diseases with a vascular component, compared with primary neurodegenerative diseases, suggested a considerable role of a possible cerebrovascular pathway,” Dr. Song and coauthors said in a report on the study appearing in JAMA Neurology.
While some previous studies have linked stress-related disorders to neurodegenerative diseases – particularly PTSD and dementia – this is believed to be the first, according to the investigators, to comprehensively evaluate all stress-related disorders in relation to the most common neurodegenerative conditions.
When considering neurodegenerative conditions separately, they found a statistically significant association between stress-related disorders and Alzheimer’s disease, while linkages with Parkinson’s disease and amyotrophic lateral sclerosis (ALS) were “comparable” but associations did not reach statistical significance, according to investigators.
Based on these findings, stress reduction should be recommended in addition to daily physical activity, mental activity, and a heart-healthy diet to potentially reduce risk of onset or worsening of cognitive decline, according to Chun Lim, MD, PhD, medical director of the cognitive neurology unit at Beth Israel Deaconess Medical Center in Boston.
“We don’t really have great evidence that anything slows down the progression of Alzheimer’s disease, but there are some suggestions that for people who lead heart-healthy lifestyles or adhere to a Mediterranean diet, fewer develop cognitive issues over 5-10 years,” Dr. Lim said in an interview. “Because of this paper, stress reduction may be one additional way to hopefully help these patients these patients that have or are concerned about cognitive issues.”
The population-matched cohort of the study included 61,748 individuals with stress-related disorders and 595,335 matched individuals without those disorders, while the sibling-matched cohort included 44,839 individuals with those disorders and 78,482 without. The median age at the start of follow-up was 47 years and 39.4% of those with stress-related disorders were male.
During follow-up, the incidence of neurodegenerative diseases per 1,000 person-years was 1.50 for individuals with stress-related disorders, versus 0.82 for those without stress-related disorders, according to the report. Risk of primary neurodegenerative diseases was increased among those with stress-related disorders, compared with those without, with a hazard ratio of 1.31 (95% confidence interval, 1.15-1.48). However, the risk of vascular neurodegenerative diseases was significantly higher, with an HR of 1.80 (95% CI, 1.40-2.31; P = .03 for the difference between hazard ratios).
Results of the matched sibling cohort supported results of the population-matched cohort, though the elevated risk of vascular neurodegenerative diseases among those with stress-related disorders was “slightly lower” than in the population-based cohort, Dr. Song and coauthors wrote in their report.
Beyond causing a host of hormonal and medical issues, stress can lead to sleep issues that may have long-term consequences, Dr. Lim noted in the interview.
“There’s some thought that quality sleep is important for memory formation, and if people are under a fair amount of stress and they have really poor sleep, that can also lead to cognitive issues including memory impairment,” he said.
“There are these multiple avenues that may be contributing to the accelerated development of these kinds of issues,” he added, “so I think this paper suggests more ways to counsel the patients about using lifestyle modifications to slow down the development of these cognitive impairments.”
Funding for the study came from the Swedish Research Council, Icelandic Research Fund; ,European Research Council the Karolinska Institutet, Swedish Research Council, and West China Hospital. Authors of the study provided disclosures related to those organizations as well as Shire/Takeda and Evolan.
SOURCE: Song H et al. JAMA Neurol. 2020 Mar 9. doi: 10.1001/jamaneurol.2020.0117.
Individuals with posttraumatic stress disorder (PTSD), acute stress reaction, adjustment disorder, or other stress reactions had an 80% increased risk of vascular neurodegenerative diseases, according to results of the study, which was based on Swedish population registry data.
Risk of primary neurodegenerative diseases was increased as well in people with those conditions, but only by 31%, according to lead author Huan Song, MD, PhD, of Sichuan University in Chengdu, China.
“The stronger association observed for neurodegenerative diseases with a vascular component, compared with primary neurodegenerative diseases, suggested a considerable role of a possible cerebrovascular pathway,” Dr. Song and coauthors said in a report on the study appearing in JAMA Neurology.
While some previous studies have linked stress-related disorders to neurodegenerative diseases – particularly PTSD and dementia – this is believed to be the first, according to the investigators, to comprehensively evaluate all stress-related disorders in relation to the most common neurodegenerative conditions.
When considering neurodegenerative conditions separately, they found a statistically significant association between stress-related disorders and Alzheimer’s disease, while linkages with Parkinson’s disease and amyotrophic lateral sclerosis (ALS) were “comparable” but associations did not reach statistical significance, according to investigators.
Based on these findings, stress reduction should be recommended in addition to daily physical activity, mental activity, and a heart-healthy diet to potentially reduce risk of onset or worsening of cognitive decline, according to Chun Lim, MD, PhD, medical director of the cognitive neurology unit at Beth Israel Deaconess Medical Center in Boston.
“We don’t really have great evidence that anything slows down the progression of Alzheimer’s disease, but there are some suggestions that for people who lead heart-healthy lifestyles or adhere to a Mediterranean diet, fewer develop cognitive issues over 5-10 years,” Dr. Lim said in an interview. “Because of this paper, stress reduction may be one additional way to hopefully help these patients these patients that have or are concerned about cognitive issues.”
The population-matched cohort of the study included 61,748 individuals with stress-related disorders and 595,335 matched individuals without those disorders, while the sibling-matched cohort included 44,839 individuals with those disorders and 78,482 without. The median age at the start of follow-up was 47 years and 39.4% of those with stress-related disorders were male.
During follow-up, the incidence of neurodegenerative diseases per 1,000 person-years was 1.50 for individuals with stress-related disorders, versus 0.82 for those without stress-related disorders, according to the report. Risk of primary neurodegenerative diseases was increased among those with stress-related disorders, compared with those without, with a hazard ratio of 1.31 (95% confidence interval, 1.15-1.48). However, the risk of vascular neurodegenerative diseases was significantly higher, with an HR of 1.80 (95% CI, 1.40-2.31; P = .03 for the difference between hazard ratios).
Results of the matched sibling cohort supported results of the population-matched cohort, though the elevated risk of vascular neurodegenerative diseases among those with stress-related disorders was “slightly lower” than in the population-based cohort, Dr. Song and coauthors wrote in their report.
Beyond causing a host of hormonal and medical issues, stress can lead to sleep issues that may have long-term consequences, Dr. Lim noted in the interview.
“There’s some thought that quality sleep is important for memory formation, and if people are under a fair amount of stress and they have really poor sleep, that can also lead to cognitive issues including memory impairment,” he said.
“There are these multiple avenues that may be contributing to the accelerated development of these kinds of issues,” he added, “so I think this paper suggests more ways to counsel the patients about using lifestyle modifications to slow down the development of these cognitive impairments.”
Funding for the study came from the Swedish Research Council, Icelandic Research Fund; ,European Research Council the Karolinska Institutet, Swedish Research Council, and West China Hospital. Authors of the study provided disclosures related to those organizations as well as Shire/Takeda and Evolan.
SOURCE: Song H et al. JAMA Neurol. 2020 Mar 9. doi: 10.1001/jamaneurol.2020.0117.
FROM JAMA NEUROLOGY
USPSTF again deems evidence insufficient to recommend cognitive impairment screening in older adults
The U.S. Preventive Services Task Force has deemed the current evidence “insufficient” to make a recommendation in regard to screening for cognitive impairment in adults aged 65 years or older.
“More research is needed on the effect of screening and early detection of cognitive impairment on important patient, caregiver, and societal outcomes, including decision making, advance planning, and caregiver outcomes,” wrote lead author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the task force. The statement was published in JAMA.
To update a 2014 recommendation from the USPSTF, which also found insufficient evidence to properly assess cognitive screening’s benefits and harms, the task force commissioned a systematic review of studies applicable to community-dwelling older adults who are not exhibiting signs or symptoms of cognitive impairment. For their statement, “cognitive impairment” is defined as mild cognitive impairment and mild to moderate dementia.
Ultimately, they determined several factors that limited the overall evidence, including the short duration of most trials and the heterogenous nature of interventions and inconsistencies in outcomes reported. Any evidence that suggested improvements was mostly applicable to patients with moderate dementia, meaning “its applicability to a screen-detected population is uncertain.”
Updating 2014 recommendations
Their statement was based on an evidence report, also published in JAMA, in which a team of researchers reviewed 287 studies that included more than 285,000 older adults; 92 of the studies were newly identified, while the other 195 were carried forward from the 2014 recommendation’s review. The researchers sought the answers to five key questions, carrying over the framework from the previous review.
“Despite the accumulation of new data, the conclusions for these key questions are essentially unchanged from the prior review,” wrote lead author Carrie D. Patnode, PhD, of the Kaiser Permanente Center for Health Research in Portland, Ore., and coauthors.
Of the questions – which concerned the accuracy of screening instruments; the harms of screening; the harms of interventions; and if screening or interventions improved decision making or outcomes for the patient, family/caregiver, or society – moderate evidence was found to support the accuracy of the instruments, treatment with acetylcholinesterase inhibitors and memantine for patients with moderate dementia, and psychoeducation interventions for caregivers of patients with moderate dementia. At the same time, there was moderate evidence of adverse effects from acetylcholinesterase inhibitors and memantine in patients with moderate dementia.
“I think, eventually, there will be sufficient evidence to justify screening, once we have what I call a tiered approach,” Marwan Sabbagh, MD, of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, said in an interview. “The very near future will include blood tests for Alzheimer’s, or PET scans, or genetics, or something else. Right now, the cognitive screens lack the specificity and sensitivity, and the secondary screening infrastructure that would improve the accuracy doesn’t exist yet.
“I think this is a ‘not now,’ ” he added, “but I wouldn’t say ‘not ever.’ ”
Dr. Patnode and coauthors noted specific limitations in the evidence, including a lack of studies on how screening for and treating cognitive impairment affects decision making. In addition, details like quality of life and institutionalization were inconsistently reported, and “consistent and standardized reporting of results according to meaningful thresholds of clinical significance” would have been valuable across all measures.
Clinical implications
The implications of this report’s conclusions are substantial, especially as the rising prevalence of mild cognitive impairment and dementia becomes a worldwide concern, wrote Ronald C. Petersen, PhD, MD, of the Mayo Clinic in Rochester, Minn., and Kristine Yaffe, MD, of the University of California, San Francisco, in an accompanying editorial.
Though the data does not explicitly support screening, Dr. Petersen and Dr. Yaffe noted that it still may have benefits. An estimated 10% of cognitive impairment is caused by at least somewhat reversible causes, and screening could also be used to improve care in medical problems that are worsened by cognitive impairment. To find the true value of these efforts, they wrote, researchers need to design and execute additional clinical trials that “answer many of the important questions surrounding screening and treatment of cognitive impairment.”
“The absence of evidence for benefit may lead to inaction,” they added, noting that clinicians screening should still consider the value of screening on a case-by-case basis in order to keep up with the impact of new disease-modifying therapies for certain neurodegenerative diseases.
All members of the USPSTF received travel reimbursement and an honorarium for participating in meetings. One member reported receiving grants and personal fees from Healthwise. The study was funded by the Department of Health & Human Services. One of the authors reported receiving grants from the National Institutes of Health and the Food and Drug Administration. Dr. Petersen and Dr. Yaffe reported consulting for, and receiving funding from, various pharmaceutical companies, foundations, and government organizations.
SOURCES: Owens DK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0435; Patnode CD et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2019.22258.
The U.S. Preventive Services Task Force has deemed the current evidence “insufficient” to make a recommendation in regard to screening for cognitive impairment in adults aged 65 years or older.
“More research is needed on the effect of screening and early detection of cognitive impairment on important patient, caregiver, and societal outcomes, including decision making, advance planning, and caregiver outcomes,” wrote lead author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the task force. The statement was published in JAMA.
To update a 2014 recommendation from the USPSTF, which also found insufficient evidence to properly assess cognitive screening’s benefits and harms, the task force commissioned a systematic review of studies applicable to community-dwelling older adults who are not exhibiting signs or symptoms of cognitive impairment. For their statement, “cognitive impairment” is defined as mild cognitive impairment and mild to moderate dementia.
Ultimately, they determined several factors that limited the overall evidence, including the short duration of most trials and the heterogenous nature of interventions and inconsistencies in outcomes reported. Any evidence that suggested improvements was mostly applicable to patients with moderate dementia, meaning “its applicability to a screen-detected population is uncertain.”
Updating 2014 recommendations
Their statement was based on an evidence report, also published in JAMA, in which a team of researchers reviewed 287 studies that included more than 285,000 older adults; 92 of the studies were newly identified, while the other 195 were carried forward from the 2014 recommendation’s review. The researchers sought the answers to five key questions, carrying over the framework from the previous review.
“Despite the accumulation of new data, the conclusions for these key questions are essentially unchanged from the prior review,” wrote lead author Carrie D. Patnode, PhD, of the Kaiser Permanente Center for Health Research in Portland, Ore., and coauthors.
Of the questions – which concerned the accuracy of screening instruments; the harms of screening; the harms of interventions; and if screening or interventions improved decision making or outcomes for the patient, family/caregiver, or society – moderate evidence was found to support the accuracy of the instruments, treatment with acetylcholinesterase inhibitors and memantine for patients with moderate dementia, and psychoeducation interventions for caregivers of patients with moderate dementia. At the same time, there was moderate evidence of adverse effects from acetylcholinesterase inhibitors and memantine in patients with moderate dementia.
“I think, eventually, there will be sufficient evidence to justify screening, once we have what I call a tiered approach,” Marwan Sabbagh, MD, of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, said in an interview. “The very near future will include blood tests for Alzheimer’s, or PET scans, or genetics, or something else. Right now, the cognitive screens lack the specificity and sensitivity, and the secondary screening infrastructure that would improve the accuracy doesn’t exist yet.
“I think this is a ‘not now,’ ” he added, “but I wouldn’t say ‘not ever.’ ”
Dr. Patnode and coauthors noted specific limitations in the evidence, including a lack of studies on how screening for and treating cognitive impairment affects decision making. In addition, details like quality of life and institutionalization were inconsistently reported, and “consistent and standardized reporting of results according to meaningful thresholds of clinical significance” would have been valuable across all measures.
Clinical implications
The implications of this report’s conclusions are substantial, especially as the rising prevalence of mild cognitive impairment and dementia becomes a worldwide concern, wrote Ronald C. Petersen, PhD, MD, of the Mayo Clinic in Rochester, Minn., and Kristine Yaffe, MD, of the University of California, San Francisco, in an accompanying editorial.
Though the data does not explicitly support screening, Dr. Petersen and Dr. Yaffe noted that it still may have benefits. An estimated 10% of cognitive impairment is caused by at least somewhat reversible causes, and screening could also be used to improve care in medical problems that are worsened by cognitive impairment. To find the true value of these efforts, they wrote, researchers need to design and execute additional clinical trials that “answer many of the important questions surrounding screening and treatment of cognitive impairment.”
“The absence of evidence for benefit may lead to inaction,” they added, noting that clinicians screening should still consider the value of screening on a case-by-case basis in order to keep up with the impact of new disease-modifying therapies for certain neurodegenerative diseases.
All members of the USPSTF received travel reimbursement and an honorarium for participating in meetings. One member reported receiving grants and personal fees from Healthwise. The study was funded by the Department of Health & Human Services. One of the authors reported receiving grants from the National Institutes of Health and the Food and Drug Administration. Dr. Petersen and Dr. Yaffe reported consulting for, and receiving funding from, various pharmaceutical companies, foundations, and government organizations.
SOURCES: Owens DK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0435; Patnode CD et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2019.22258.
The U.S. Preventive Services Task Force has deemed the current evidence “insufficient” to make a recommendation in regard to screening for cognitive impairment in adults aged 65 years or older.
“More research is needed on the effect of screening and early detection of cognitive impairment on important patient, caregiver, and societal outcomes, including decision making, advance planning, and caregiver outcomes,” wrote lead author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the task force. The statement was published in JAMA.
To update a 2014 recommendation from the USPSTF, which also found insufficient evidence to properly assess cognitive screening’s benefits and harms, the task force commissioned a systematic review of studies applicable to community-dwelling older adults who are not exhibiting signs or symptoms of cognitive impairment. For their statement, “cognitive impairment” is defined as mild cognitive impairment and mild to moderate dementia.
Ultimately, they determined several factors that limited the overall evidence, including the short duration of most trials and the heterogenous nature of interventions and inconsistencies in outcomes reported. Any evidence that suggested improvements was mostly applicable to patients with moderate dementia, meaning “its applicability to a screen-detected population is uncertain.”
Updating 2014 recommendations
Their statement was based on an evidence report, also published in JAMA, in which a team of researchers reviewed 287 studies that included more than 285,000 older adults; 92 of the studies were newly identified, while the other 195 were carried forward from the 2014 recommendation’s review. The researchers sought the answers to five key questions, carrying over the framework from the previous review.
“Despite the accumulation of new data, the conclusions for these key questions are essentially unchanged from the prior review,” wrote lead author Carrie D. Patnode, PhD, of the Kaiser Permanente Center for Health Research in Portland, Ore., and coauthors.
Of the questions – which concerned the accuracy of screening instruments; the harms of screening; the harms of interventions; and if screening or interventions improved decision making or outcomes for the patient, family/caregiver, or society – moderate evidence was found to support the accuracy of the instruments, treatment with acetylcholinesterase inhibitors and memantine for patients with moderate dementia, and psychoeducation interventions for caregivers of patients with moderate dementia. At the same time, there was moderate evidence of adverse effects from acetylcholinesterase inhibitors and memantine in patients with moderate dementia.
“I think, eventually, there will be sufficient evidence to justify screening, once we have what I call a tiered approach,” Marwan Sabbagh, MD, of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, said in an interview. “The very near future will include blood tests for Alzheimer’s, or PET scans, or genetics, or something else. Right now, the cognitive screens lack the specificity and sensitivity, and the secondary screening infrastructure that would improve the accuracy doesn’t exist yet.
“I think this is a ‘not now,’ ” he added, “but I wouldn’t say ‘not ever.’ ”
Dr. Patnode and coauthors noted specific limitations in the evidence, including a lack of studies on how screening for and treating cognitive impairment affects decision making. In addition, details like quality of life and institutionalization were inconsistently reported, and “consistent and standardized reporting of results according to meaningful thresholds of clinical significance” would have been valuable across all measures.
Clinical implications
The implications of this report’s conclusions are substantial, especially as the rising prevalence of mild cognitive impairment and dementia becomes a worldwide concern, wrote Ronald C. Petersen, PhD, MD, of the Mayo Clinic in Rochester, Minn., and Kristine Yaffe, MD, of the University of California, San Francisco, in an accompanying editorial.
Though the data does not explicitly support screening, Dr. Petersen and Dr. Yaffe noted that it still may have benefits. An estimated 10% of cognitive impairment is caused by at least somewhat reversible causes, and screening could also be used to improve care in medical problems that are worsened by cognitive impairment. To find the true value of these efforts, they wrote, researchers need to design and execute additional clinical trials that “answer many of the important questions surrounding screening and treatment of cognitive impairment.”
“The absence of evidence for benefit may lead to inaction,” they added, noting that clinicians screening should still consider the value of screening on a case-by-case basis in order to keep up with the impact of new disease-modifying therapies for certain neurodegenerative diseases.
All members of the USPSTF received travel reimbursement and an honorarium for participating in meetings. One member reported receiving grants and personal fees from Healthwise. The study was funded by the Department of Health & Human Services. One of the authors reported receiving grants from the National Institutes of Health and the Food and Drug Administration. Dr. Petersen and Dr. Yaffe reported consulting for, and receiving funding from, various pharmaceutical companies, foundations, and government organizations.
SOURCES: Owens DK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0435; Patnode CD et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2019.22258.
FROM JAMA
As costs for neurologic drugs rise, adherence to therapy drops
For their study, published online Feb. 19 in Neurology, Brian C. Callaghan, MD, of the University of Michigan, Ann Arbor, and colleagues looked at claims records from a large national private insurer to identify new cases of dementia, Parkinson’s disease, and neuropathy between 2001 and 2016, along with pharmacy records following diagnoses.
The researchers identified more than 52,000 patients with neuropathy on gabapentinoids and another 5,000 treated with serotonin-norepinephrine reuptake inhibitors for the same. They also identified some 20,000 patients with dementia taking cholinesterase inhibitors, and 3,000 with Parkinson’s disease taking dopamine agonists. Dr. Callaghan and colleagues compared patient adherence over 6 months for pairs of drugs in the same class with similar or equal efficacy, but with different costs to the patient.
Such cost differences can be stark: The researchers noted that the average 2016 out-of-pocket cost for 30 days of pregabalin, a drug used in the treatment of peripheral neuropathy, was $65.70, compared with $8.40 for gabapentin. With two common dementia drugs the difference was even more pronounced: $79.30 for rivastigmine compared with $3.10 for donepezil, both cholinesterase inhibitors with similar efficacy and tolerability.
Dr. Callaghan and colleagues found that such cost differences bore significantly on patient adherence. An increase of $50 in patient costs was seen decreasing adherence by 9% for neuropathy patients on gabapentinoids (adjusted incidence rate ratio [IRR] 0.91, 0.89-0.93) and by 12% for dementia patients on cholinesterase inhibitors (adjusted IRR 0.88, 0.86-0.91, P less than .05 for both). Similar price-linked decreases were seen for neuropathy patients on SNRIs and Parkinson’s patients on dopamine agonists, but the differences did not reach statistical significance.
Black, Asian, and Hispanic patients saw greater drops in adherence than did white patients associated with the same out-of-pocket cost differences, leading the researchers to note that special care should be taken in prescribing decisions for these populations.
“When choosing among medications with differential [out-of-pocket] costs, prescribing the medication with lower [out-of-pocket] expense will likely improve medication adherence while reducing overall costs,” Dr. Callaghan and colleagues wrote in their analysis. “For example, prescribing gabapentin or venlafaxine to patients with newly diagnosed neuropathy is likely to lead to higher adherence compared with pregabalin or duloxetine, and therefore, there is a higher likelihood of relief from neuropathic pain.” The researchers noted that while combination pills and extended-release formulations may be marketed as a way to increase adherence, the higher out-of-pocket costs of such medicines could offset any adherence benefit.
Dr. Callaghan and his colleagues described as strengths of their study its large sample and statistical approach that “allowed us to best estimate the causal relationship between [out-of-pocket] costs and medication adherence by limiting selection bias, residual confounding, and the confounding inherent to medication choice.” Nonadherence – patients who never filled a prescription after diagnosis – was not captured in the study.
The American Academy of Neurology funded the study. Two of its authors reported financial conflicts of interest in the form of compensation from pharmaceutical or device companies. Its lead author, Dr. Callaghan, reported funding for a device maker and performing medical legal consultations.
SOURCE: Reynolds EL et al. Neurology. 2020 Feb 19. doi/10.1212/WNL.0000000000009039.
For their study, published online Feb. 19 in Neurology, Brian C. Callaghan, MD, of the University of Michigan, Ann Arbor, and colleagues looked at claims records from a large national private insurer to identify new cases of dementia, Parkinson’s disease, and neuropathy between 2001 and 2016, along with pharmacy records following diagnoses.
The researchers identified more than 52,000 patients with neuropathy on gabapentinoids and another 5,000 treated with serotonin-norepinephrine reuptake inhibitors for the same. They also identified some 20,000 patients with dementia taking cholinesterase inhibitors, and 3,000 with Parkinson’s disease taking dopamine agonists. Dr. Callaghan and colleagues compared patient adherence over 6 months for pairs of drugs in the same class with similar or equal efficacy, but with different costs to the patient.
Such cost differences can be stark: The researchers noted that the average 2016 out-of-pocket cost for 30 days of pregabalin, a drug used in the treatment of peripheral neuropathy, was $65.70, compared with $8.40 for gabapentin. With two common dementia drugs the difference was even more pronounced: $79.30 for rivastigmine compared with $3.10 for donepezil, both cholinesterase inhibitors with similar efficacy and tolerability.
Dr. Callaghan and colleagues found that such cost differences bore significantly on patient adherence. An increase of $50 in patient costs was seen decreasing adherence by 9% for neuropathy patients on gabapentinoids (adjusted incidence rate ratio [IRR] 0.91, 0.89-0.93) and by 12% for dementia patients on cholinesterase inhibitors (adjusted IRR 0.88, 0.86-0.91, P less than .05 for both). Similar price-linked decreases were seen for neuropathy patients on SNRIs and Parkinson’s patients on dopamine agonists, but the differences did not reach statistical significance.
Black, Asian, and Hispanic patients saw greater drops in adherence than did white patients associated with the same out-of-pocket cost differences, leading the researchers to note that special care should be taken in prescribing decisions for these populations.
“When choosing among medications with differential [out-of-pocket] costs, prescribing the medication with lower [out-of-pocket] expense will likely improve medication adherence while reducing overall costs,” Dr. Callaghan and colleagues wrote in their analysis. “For example, prescribing gabapentin or venlafaxine to patients with newly diagnosed neuropathy is likely to lead to higher adherence compared with pregabalin or duloxetine, and therefore, there is a higher likelihood of relief from neuropathic pain.” The researchers noted that while combination pills and extended-release formulations may be marketed as a way to increase adherence, the higher out-of-pocket costs of such medicines could offset any adherence benefit.
Dr. Callaghan and his colleagues described as strengths of their study its large sample and statistical approach that “allowed us to best estimate the causal relationship between [out-of-pocket] costs and medication adherence by limiting selection bias, residual confounding, and the confounding inherent to medication choice.” Nonadherence – patients who never filled a prescription after diagnosis – was not captured in the study.
The American Academy of Neurology funded the study. Two of its authors reported financial conflicts of interest in the form of compensation from pharmaceutical or device companies. Its lead author, Dr. Callaghan, reported funding for a device maker and performing medical legal consultations.
SOURCE: Reynolds EL et al. Neurology. 2020 Feb 19. doi/10.1212/WNL.0000000000009039.
For their study, published online Feb. 19 in Neurology, Brian C. Callaghan, MD, of the University of Michigan, Ann Arbor, and colleagues looked at claims records from a large national private insurer to identify new cases of dementia, Parkinson’s disease, and neuropathy between 2001 and 2016, along with pharmacy records following diagnoses.
The researchers identified more than 52,000 patients with neuropathy on gabapentinoids and another 5,000 treated with serotonin-norepinephrine reuptake inhibitors for the same. They also identified some 20,000 patients with dementia taking cholinesterase inhibitors, and 3,000 with Parkinson’s disease taking dopamine agonists. Dr. Callaghan and colleagues compared patient adherence over 6 months for pairs of drugs in the same class with similar or equal efficacy, but with different costs to the patient.
Such cost differences can be stark: The researchers noted that the average 2016 out-of-pocket cost for 30 days of pregabalin, a drug used in the treatment of peripheral neuropathy, was $65.70, compared with $8.40 for gabapentin. With two common dementia drugs the difference was even more pronounced: $79.30 for rivastigmine compared with $3.10 for donepezil, both cholinesterase inhibitors with similar efficacy and tolerability.
Dr. Callaghan and colleagues found that such cost differences bore significantly on patient adherence. An increase of $50 in patient costs was seen decreasing adherence by 9% for neuropathy patients on gabapentinoids (adjusted incidence rate ratio [IRR] 0.91, 0.89-0.93) and by 12% for dementia patients on cholinesterase inhibitors (adjusted IRR 0.88, 0.86-0.91, P less than .05 for both). Similar price-linked decreases were seen for neuropathy patients on SNRIs and Parkinson’s patients on dopamine agonists, but the differences did not reach statistical significance.
Black, Asian, and Hispanic patients saw greater drops in adherence than did white patients associated with the same out-of-pocket cost differences, leading the researchers to note that special care should be taken in prescribing decisions for these populations.
“When choosing among medications with differential [out-of-pocket] costs, prescribing the medication with lower [out-of-pocket] expense will likely improve medication adherence while reducing overall costs,” Dr. Callaghan and colleagues wrote in their analysis. “For example, prescribing gabapentin or venlafaxine to patients with newly diagnosed neuropathy is likely to lead to higher adherence compared with pregabalin or duloxetine, and therefore, there is a higher likelihood of relief from neuropathic pain.” The researchers noted that while combination pills and extended-release formulations may be marketed as a way to increase adherence, the higher out-of-pocket costs of such medicines could offset any adherence benefit.
Dr. Callaghan and his colleagues described as strengths of their study its large sample and statistical approach that “allowed us to best estimate the causal relationship between [out-of-pocket] costs and medication adherence by limiting selection bias, residual confounding, and the confounding inherent to medication choice.” Nonadherence – patients who never filled a prescription after diagnosis – was not captured in the study.
The American Academy of Neurology funded the study. Two of its authors reported financial conflicts of interest in the form of compensation from pharmaceutical or device companies. Its lead author, Dr. Callaghan, reported funding for a device maker and performing medical legal consultations.
SOURCE: Reynolds EL et al. Neurology. 2020 Feb 19. doi/10.1212/WNL.0000000000009039.
FROM NEUROLOGY




