User login
Many EMS protocols for status epilepticus do not follow evidence-based guidelines
“Many protocols did not follow evidence-based guidelines and did not accurately define generalized convulsive status epilepticus,” said John P. Betjemann, MD, associate professor of neurology at the University of California, San Francisco, and his colleagues. They reported their findings in the March 26 issue of JAMA.
Generalized convulsive status epilepticus is a neurologic emergency, and trials published in 2001 and 2012 found that benzodiazepines are effective prehospital treatments for patients with generalized convulsive status epilepticus. These trials informed a 2016 evidence-based guideline that cites level A evidence for intramuscular midazolam, IV lorazepam, and IV diazepam as initial treatment options for adults.
To determine whether EMS system protocols follow these recommendations, the investigators reviewed treatment protocols from 33 EMS systems that cover the 58 counties in California. The researchers reviewed EMS system protocols between May and June 2018 to determine when they were last updated and whether they defined generalized convulsive status epilepticus according to the guideline (namely, 5 or more minutes of continuous seizure or two or more discrete seizures between which a patient has incomplete recovery of consciousness). They also determined whether the protocols included any of the three benzodiazepines in the guideline and, if so, at what dose and using which route of administration.
Protocols’ most recent revision dates ranged between 2007 and 2018. Twenty-seven protocols (81.8%) were revised after the second clinical trial was published in 2012, and 17 (51.5%) were revised after the 2016 guideline. Seven EMS system protocols (21.2%) defined generalized convulsive status epilepticus according to the guideline. Thirty-two protocols (97.0%) included intramuscular midazolam, 2 (6.1%) included IV lorazepam, and 5 (15.2%) included IV diazepam.
Although the protocols “appropriately emphasized” intramuscular midazolam, the protocol doses often were lower than those used in the trials or recommended in the guideline. In addition, most protocols listed IV and intraosseous midazolam as options, although these treatments were not studied in the trials nor recommended in the guideline. In all, six of the protocols (18.2%) recommended at least one medication by the route and dose suggested in the trials or in the guideline.
“Why EMS system protocols deviate from the evidence and how this affects patient outcomes deserves further study,” the authors said.
The researchers noted that they examined EMS protocols in only one state and that “protocols may not necessarily reflect what emergency medical technicians actually do in practice.” In addition, the researchers accessed the most recent protocols by consulting EMS system websites rather than by contacting each EMS system for its most up-to-date protocol.
The authors reported personal compensation from JAMA Neurology and from Continuum Audio unrelated to the present study, as well as grants from the National Institutes of Health.
SOURCE: Betjemann JP et al. JAMA. 2019 Mar 26.
“Many protocols did not follow evidence-based guidelines and did not accurately define generalized convulsive status epilepticus,” said John P. Betjemann, MD, associate professor of neurology at the University of California, San Francisco, and his colleagues. They reported their findings in the March 26 issue of JAMA.
Generalized convulsive status epilepticus is a neurologic emergency, and trials published in 2001 and 2012 found that benzodiazepines are effective prehospital treatments for patients with generalized convulsive status epilepticus. These trials informed a 2016 evidence-based guideline that cites level A evidence for intramuscular midazolam, IV lorazepam, and IV diazepam as initial treatment options for adults.
To determine whether EMS system protocols follow these recommendations, the investigators reviewed treatment protocols from 33 EMS systems that cover the 58 counties in California. The researchers reviewed EMS system protocols between May and June 2018 to determine when they were last updated and whether they defined generalized convulsive status epilepticus according to the guideline (namely, 5 or more minutes of continuous seizure or two or more discrete seizures between which a patient has incomplete recovery of consciousness). They also determined whether the protocols included any of the three benzodiazepines in the guideline and, if so, at what dose and using which route of administration.
Protocols’ most recent revision dates ranged between 2007 and 2018. Twenty-seven protocols (81.8%) were revised after the second clinical trial was published in 2012, and 17 (51.5%) were revised after the 2016 guideline. Seven EMS system protocols (21.2%) defined generalized convulsive status epilepticus according to the guideline. Thirty-two protocols (97.0%) included intramuscular midazolam, 2 (6.1%) included IV lorazepam, and 5 (15.2%) included IV diazepam.
Although the protocols “appropriately emphasized” intramuscular midazolam, the protocol doses often were lower than those used in the trials or recommended in the guideline. In addition, most protocols listed IV and intraosseous midazolam as options, although these treatments were not studied in the trials nor recommended in the guideline. In all, six of the protocols (18.2%) recommended at least one medication by the route and dose suggested in the trials or in the guideline.
“Why EMS system protocols deviate from the evidence and how this affects patient outcomes deserves further study,” the authors said.
The researchers noted that they examined EMS protocols in only one state and that “protocols may not necessarily reflect what emergency medical technicians actually do in practice.” In addition, the researchers accessed the most recent protocols by consulting EMS system websites rather than by contacting each EMS system for its most up-to-date protocol.
The authors reported personal compensation from JAMA Neurology and from Continuum Audio unrelated to the present study, as well as grants from the National Institutes of Health.
SOURCE: Betjemann JP et al. JAMA. 2019 Mar 26.
“Many protocols did not follow evidence-based guidelines and did not accurately define generalized convulsive status epilepticus,” said John P. Betjemann, MD, associate professor of neurology at the University of California, San Francisco, and his colleagues. They reported their findings in the March 26 issue of JAMA.
Generalized convulsive status epilepticus is a neurologic emergency, and trials published in 2001 and 2012 found that benzodiazepines are effective prehospital treatments for patients with generalized convulsive status epilepticus. These trials informed a 2016 evidence-based guideline that cites level A evidence for intramuscular midazolam, IV lorazepam, and IV diazepam as initial treatment options for adults.
To determine whether EMS system protocols follow these recommendations, the investigators reviewed treatment protocols from 33 EMS systems that cover the 58 counties in California. The researchers reviewed EMS system protocols between May and June 2018 to determine when they were last updated and whether they defined generalized convulsive status epilepticus according to the guideline (namely, 5 or more minutes of continuous seizure or two or more discrete seizures between which a patient has incomplete recovery of consciousness). They also determined whether the protocols included any of the three benzodiazepines in the guideline and, if so, at what dose and using which route of administration.
Protocols’ most recent revision dates ranged between 2007 and 2018. Twenty-seven protocols (81.8%) were revised after the second clinical trial was published in 2012, and 17 (51.5%) were revised after the 2016 guideline. Seven EMS system protocols (21.2%) defined generalized convulsive status epilepticus according to the guideline. Thirty-two protocols (97.0%) included intramuscular midazolam, 2 (6.1%) included IV lorazepam, and 5 (15.2%) included IV diazepam.
Although the protocols “appropriately emphasized” intramuscular midazolam, the protocol doses often were lower than those used in the trials or recommended in the guideline. In addition, most protocols listed IV and intraosseous midazolam as options, although these treatments were not studied in the trials nor recommended in the guideline. In all, six of the protocols (18.2%) recommended at least one medication by the route and dose suggested in the trials or in the guideline.
“Why EMS system protocols deviate from the evidence and how this affects patient outcomes deserves further study,” the authors said.
The researchers noted that they examined EMS protocols in only one state and that “protocols may not necessarily reflect what emergency medical technicians actually do in practice.” In addition, the researchers accessed the most recent protocols by consulting EMS system websites rather than by contacting each EMS system for its most up-to-date protocol.
The authors reported personal compensation from JAMA Neurology and from Continuum Audio unrelated to the present study, as well as grants from the National Institutes of Health.
SOURCE: Betjemann JP et al. JAMA. 2019 Mar 26.
FROM JAMA
Key clinical point: Many emergency medical services (EMS) system protocols may not follow evidence-based guidelines or accurately define generalized convulsive status epilepticus.
Major finding: In all, 18.2% of the protocols recommended at least one medication by the route and at the dose suggested in clinical trials or in an evidence-based guideline.
Study details: A review of treatment protocols from 33 EMS systems that cover the 58 counties in California.
Disclosures: The authors reported personal compensation from JAMA Neurology and Continuum Audio unrelated to the present study and grants from the National Institutes of Health.
Source: Betjemann JP et al. JAMA. 2019 March 26.
Can intraputamenal infusions of GDNF treat Parkinson’s disease?
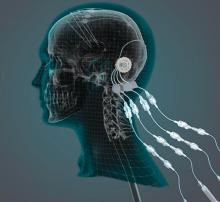
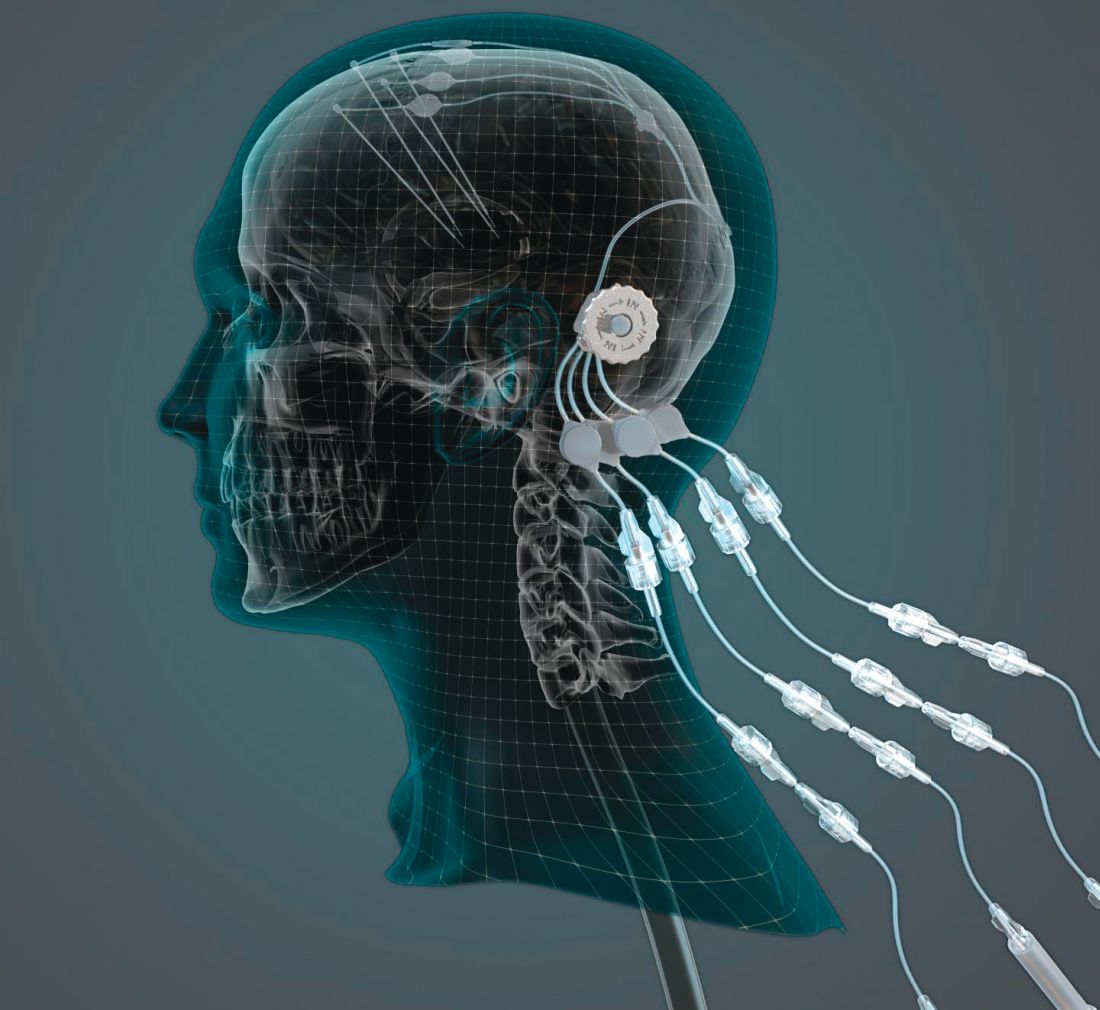
researchers reported. The investigational therapy, delivered through a skull-mounted port, was well tolerated in a 40-week, randomized, controlled trial and a 40-week, open-label extension.
Neither study met its primary endpoint, but post hoc analyses suggest possible clinical benefits. In addition, PET imaging after the 40-week, randomized trial found significantly increased 18F-DOPA uptake in patients who received GDNF. The randomized trial was published in the March 2019 issue of Brain; data from the open-label extension were published online ahead of print Feb. 26, 2019, in the Journal of Parkinson’s Disease.
“The spatial and relative magnitude of the improvement in the brain scans is beyond anything seen previously in trials of surgically delivered growth-factor treatments for Parkinson’s [disease],” said principal investigator Alan L. Whone, MBChB, PhD, of the University of Bristol (England) and North Bristol National Health Service Trust. “This represents some of the most compelling evidence yet that we may have a means to possibly reawaken and restore the dopamine brain cells that are gradually destroyed in Parkinson’s [disease].”
Nevertheless, the trial did not confirm clinical benefits. The hypothesis that growth factors can benefit patients with Parkinson’s disease may be incorrect, the researchers acknowledged. It also is possible that the hypothesis is valid and that a trial with a higher GDNF dose, longer treatment duration, patients with an earlier disease stage, or different outcome measures would yield positive results. GDNF warrants further study, they wrote.
The findings could have implications for other neurologic disorders as well.
“This trial has shown that we can safely and repeatedly infuse drugs directly into patients’ brains over months or years. This is a significant breakthrough in our ability to treat neurologic conditions ... because most drugs that might work cannot cross from the bloodstream into the brain,” said Steven Gill, MB, MS. Mr. Gill, of the North Bristol NHS Trust and the U.K.-based engineering firm Renishaw, designed the convection-enhanced delivery system used in the studies.
A neurotrophic protein
GDNF has neurorestorative and neuroprotective effects in animal models of Parkinson’s disease. In open-label studies, continuous, low-rate intraputamenal administration of GDNF has shown signs of potential efficacy, but a placebo-controlled trial did not replicate clinical benefits. In the present studies, the researchers assessed intermittent GDNF administration using convection-enhanced delivery, which can achieve wider and more even distribution of GDNF, compared with the previous approach.
The researchers conducted a single-center, randomized, double-blind, placebo-controlled trial to study this novel administration approach. Patients were aged 35-75 years, had motor symptoms for at least 5 years, and had moderate disease severity in the off state (that is, Hoehn and Yahr stage 2-3 and Unified Parkinson’s Disease Rating Scale motor score–part III [UPDRS-III] of 25-45).
In a pilot stage of the trial, six patients were randomized 2:1 to receive GDNF (120 mcg per putamen) or placebo. In the primary stage, another 35 patients were randomized 1:1 to GDNF or placebo. The primary outcome was the percentage change from baseline to week 40 in the off-state UPDRS-III among patients from the primary stage of the trial. Further analyses included all 41 patients from the pilot and primary stages.
Patients in the primary analysis had a mean age of 56.4 years and mean disease duration of 10.9 years. About half were female.
Results on primary and secondary clinical endpoints did not significantly differ between the groups. Average off state UPDRS motor score decreased by 17.3 in the active treatment group, compared with 11.8 in the placebo group.
A post hoc analysis, however, found that nine patients (43%) in the active-treatment group had a large, clinically important motor improvement of 10 or more points in the off state, whereas no placebo patients did. These “10-point responders in the GDNF group are a potential focus of interest; however, as this is a post hoc finding we would not wish to overinterpret its meaning,” Dr. Whone and his colleagues wrote. Among patients who received GDNF, PET imaging demonstrated significantly increased 18F-DOPA uptake throughout the putamen, ranging from a 25% increase in the left anterior putamen to a 100% increase in both posterior putamena, whereas patients who received placebo did not have significantly increased uptake.
No drug-related serious adverse events were reported. “The majority of device-related adverse events were port site associated, most commonly local hypertrophic scarring or infections, amenable to antibiotics,” the investigators wrote. “The frequency of these declined during the trial as surgical and device handling experience improved.”
Open-label extension
By week 80, when all participants had received GDNF, both groups showed moderate to large improvement in symptoms, compared with baseline. From baseline to week 80, percentage change in UPDRS motor score in the off state did not significantly differ between patients who received GDNF for 80 weeks and patients who received placebo followed by GDNF (26.7% vs. 27.6%). Secondary endpoints also did not differ between the groups. Treatment compliance was 97.8%; no patients discontinued the study.
The trials were funded by Parkinson’s UK with support from the Cure Parkinson’s Trust and in association with the North Bristol NHS Trust. GDNF and additional resources and funding were provided by MedGenesis Therapeutix, which owns the license for GDNF and received funding from the Michael J. Fox Foundation for Parkinson’s Research. Renishaw manufactured the convection-enhanced delivery device on behalf of North Bristol NHS Trust. The Gatsby Foundation provided a 3T MRI scanner. Some study authors are employed by and have shares or share options with MedGenesis Therapeutix. Other authors are employees of Renishaw. Dr. Gill is Renishaw’s medical director and may have a future royalty share from the drug delivery system that he invented.
SOURCES: Whone AL et al. Brain. 2019 Feb 26. doi: 10.1093/brain/awz023; Whone AL et al. J Parkinsons Dis. 2019 Feb 26. doi: 10.3233/JPD-191576.
researchers reported. The investigational therapy, delivered through a skull-mounted port, was well tolerated in a 40-week, randomized, controlled trial and a 40-week, open-label extension.
Neither study met its primary endpoint, but post hoc analyses suggest possible clinical benefits. In addition, PET imaging after the 40-week, randomized trial found significantly increased 18F-DOPA uptake in patients who received GDNF. The randomized trial was published in the March 2019 issue of Brain; data from the open-label extension were published online ahead of print Feb. 26, 2019, in the Journal of Parkinson’s Disease.
“The spatial and relative magnitude of the improvement in the brain scans is beyond anything seen previously in trials of surgically delivered growth-factor treatments for Parkinson’s [disease],” said principal investigator Alan L. Whone, MBChB, PhD, of the University of Bristol (England) and North Bristol National Health Service Trust. “This represents some of the most compelling evidence yet that we may have a means to possibly reawaken and restore the dopamine brain cells that are gradually destroyed in Parkinson’s [disease].”
Nevertheless, the trial did not confirm clinical benefits. The hypothesis that growth factors can benefit patients with Parkinson’s disease may be incorrect, the researchers acknowledged. It also is possible that the hypothesis is valid and that a trial with a higher GDNF dose, longer treatment duration, patients with an earlier disease stage, or different outcome measures would yield positive results. GDNF warrants further study, they wrote.
The findings could have implications for other neurologic disorders as well.
“This trial has shown that we can safely and repeatedly infuse drugs directly into patients’ brains over months or years. This is a significant breakthrough in our ability to treat neurologic conditions ... because most drugs that might work cannot cross from the bloodstream into the brain,” said Steven Gill, MB, MS. Mr. Gill, of the North Bristol NHS Trust and the U.K.-based engineering firm Renishaw, designed the convection-enhanced delivery system used in the studies.
A neurotrophic protein
GDNF has neurorestorative and neuroprotective effects in animal models of Parkinson’s disease. In open-label studies, continuous, low-rate intraputamenal administration of GDNF has shown signs of potential efficacy, but a placebo-controlled trial did not replicate clinical benefits. In the present studies, the researchers assessed intermittent GDNF administration using convection-enhanced delivery, which can achieve wider and more even distribution of GDNF, compared with the previous approach.
The researchers conducted a single-center, randomized, double-blind, placebo-controlled trial to study this novel administration approach. Patients were aged 35-75 years, had motor symptoms for at least 5 years, and had moderate disease severity in the off state (that is, Hoehn and Yahr stage 2-3 and Unified Parkinson’s Disease Rating Scale motor score–part III [UPDRS-III] of 25-45).
In a pilot stage of the trial, six patients were randomized 2:1 to receive GDNF (120 mcg per putamen) or placebo. In the primary stage, another 35 patients were randomized 1:1 to GDNF or placebo. The primary outcome was the percentage change from baseline to week 40 in the off-state UPDRS-III among patients from the primary stage of the trial. Further analyses included all 41 patients from the pilot and primary stages.
Patients in the primary analysis had a mean age of 56.4 years and mean disease duration of 10.9 years. About half were female.
Results on primary and secondary clinical endpoints did not significantly differ between the groups. Average off state UPDRS motor score decreased by 17.3 in the active treatment group, compared with 11.8 in the placebo group.
A post hoc analysis, however, found that nine patients (43%) in the active-treatment group had a large, clinically important motor improvement of 10 or more points in the off state, whereas no placebo patients did. These “10-point responders in the GDNF group are a potential focus of interest; however, as this is a post hoc finding we would not wish to overinterpret its meaning,” Dr. Whone and his colleagues wrote. Among patients who received GDNF, PET imaging demonstrated significantly increased 18F-DOPA uptake throughout the putamen, ranging from a 25% increase in the left anterior putamen to a 100% increase in both posterior putamena, whereas patients who received placebo did not have significantly increased uptake.
No drug-related serious adverse events were reported. “The majority of device-related adverse events were port site associated, most commonly local hypertrophic scarring or infections, amenable to antibiotics,” the investigators wrote. “The frequency of these declined during the trial as surgical and device handling experience improved.”
Open-label extension
By week 80, when all participants had received GDNF, both groups showed moderate to large improvement in symptoms, compared with baseline. From baseline to week 80, percentage change in UPDRS motor score in the off state did not significantly differ between patients who received GDNF for 80 weeks and patients who received placebo followed by GDNF (26.7% vs. 27.6%). Secondary endpoints also did not differ between the groups. Treatment compliance was 97.8%; no patients discontinued the study.
The trials were funded by Parkinson’s UK with support from the Cure Parkinson’s Trust and in association with the North Bristol NHS Trust. GDNF and additional resources and funding were provided by MedGenesis Therapeutix, which owns the license for GDNF and received funding from the Michael J. Fox Foundation for Parkinson’s Research. Renishaw manufactured the convection-enhanced delivery device on behalf of North Bristol NHS Trust. The Gatsby Foundation provided a 3T MRI scanner. Some study authors are employed by and have shares or share options with MedGenesis Therapeutix. Other authors are employees of Renishaw. Dr. Gill is Renishaw’s medical director and may have a future royalty share from the drug delivery system that he invented.
SOURCES: Whone AL et al. Brain. 2019 Feb 26. doi: 10.1093/brain/awz023; Whone AL et al. J Parkinsons Dis. 2019 Feb 26. doi: 10.3233/JPD-191576.
researchers reported. The investigational therapy, delivered through a skull-mounted port, was well tolerated in a 40-week, randomized, controlled trial and a 40-week, open-label extension.
Neither study met its primary endpoint, but post hoc analyses suggest possible clinical benefits. In addition, PET imaging after the 40-week, randomized trial found significantly increased 18F-DOPA uptake in patients who received GDNF. The randomized trial was published in the March 2019 issue of Brain; data from the open-label extension were published online ahead of print Feb. 26, 2019, in the Journal of Parkinson’s Disease.
“The spatial and relative magnitude of the improvement in the brain scans is beyond anything seen previously in trials of surgically delivered growth-factor treatments for Parkinson’s [disease],” said principal investigator Alan L. Whone, MBChB, PhD, of the University of Bristol (England) and North Bristol National Health Service Trust. “This represents some of the most compelling evidence yet that we may have a means to possibly reawaken and restore the dopamine brain cells that are gradually destroyed in Parkinson’s [disease].”
Nevertheless, the trial did not confirm clinical benefits. The hypothesis that growth factors can benefit patients with Parkinson’s disease may be incorrect, the researchers acknowledged. It also is possible that the hypothesis is valid and that a trial with a higher GDNF dose, longer treatment duration, patients with an earlier disease stage, or different outcome measures would yield positive results. GDNF warrants further study, they wrote.
The findings could have implications for other neurologic disorders as well.
“This trial has shown that we can safely and repeatedly infuse drugs directly into patients’ brains over months or years. This is a significant breakthrough in our ability to treat neurologic conditions ... because most drugs that might work cannot cross from the bloodstream into the brain,” said Steven Gill, MB, MS. Mr. Gill, of the North Bristol NHS Trust and the U.K.-based engineering firm Renishaw, designed the convection-enhanced delivery system used in the studies.
A neurotrophic protein
GDNF has neurorestorative and neuroprotective effects in animal models of Parkinson’s disease. In open-label studies, continuous, low-rate intraputamenal administration of GDNF has shown signs of potential efficacy, but a placebo-controlled trial did not replicate clinical benefits. In the present studies, the researchers assessed intermittent GDNF administration using convection-enhanced delivery, which can achieve wider and more even distribution of GDNF, compared with the previous approach.
The researchers conducted a single-center, randomized, double-blind, placebo-controlled trial to study this novel administration approach. Patients were aged 35-75 years, had motor symptoms for at least 5 years, and had moderate disease severity in the off state (that is, Hoehn and Yahr stage 2-3 and Unified Parkinson’s Disease Rating Scale motor score–part III [UPDRS-III] of 25-45).
In a pilot stage of the trial, six patients were randomized 2:1 to receive GDNF (120 mcg per putamen) or placebo. In the primary stage, another 35 patients were randomized 1:1 to GDNF or placebo. The primary outcome was the percentage change from baseline to week 40 in the off-state UPDRS-III among patients from the primary stage of the trial. Further analyses included all 41 patients from the pilot and primary stages.
Patients in the primary analysis had a mean age of 56.4 years and mean disease duration of 10.9 years. About half were female.
Results on primary and secondary clinical endpoints did not significantly differ between the groups. Average off state UPDRS motor score decreased by 17.3 in the active treatment group, compared with 11.8 in the placebo group.
A post hoc analysis, however, found that nine patients (43%) in the active-treatment group had a large, clinically important motor improvement of 10 or more points in the off state, whereas no placebo patients did. These “10-point responders in the GDNF group are a potential focus of interest; however, as this is a post hoc finding we would not wish to overinterpret its meaning,” Dr. Whone and his colleagues wrote. Among patients who received GDNF, PET imaging demonstrated significantly increased 18F-DOPA uptake throughout the putamen, ranging from a 25% increase in the left anterior putamen to a 100% increase in both posterior putamena, whereas patients who received placebo did not have significantly increased uptake.
No drug-related serious adverse events were reported. “The majority of device-related adverse events were port site associated, most commonly local hypertrophic scarring or infections, amenable to antibiotics,” the investigators wrote. “The frequency of these declined during the trial as surgical and device handling experience improved.”
Open-label extension
By week 80, when all participants had received GDNF, both groups showed moderate to large improvement in symptoms, compared with baseline. From baseline to week 80, percentage change in UPDRS motor score in the off state did not significantly differ between patients who received GDNF for 80 weeks and patients who received placebo followed by GDNF (26.7% vs. 27.6%). Secondary endpoints also did not differ between the groups. Treatment compliance was 97.8%; no patients discontinued the study.
The trials were funded by Parkinson’s UK with support from the Cure Parkinson’s Trust and in association with the North Bristol NHS Trust. GDNF and additional resources and funding were provided by MedGenesis Therapeutix, which owns the license for GDNF and received funding from the Michael J. Fox Foundation for Parkinson’s Research. Renishaw manufactured the convection-enhanced delivery device on behalf of North Bristol NHS Trust. The Gatsby Foundation provided a 3T MRI scanner. Some study authors are employed by and have shares or share options with MedGenesis Therapeutix. Other authors are employees of Renishaw. Dr. Gill is Renishaw’s medical director and may have a future royalty share from the drug delivery system that he invented.
SOURCES: Whone AL et al. Brain. 2019 Feb 26. doi: 10.1093/brain/awz023; Whone AL et al. J Parkinsons Dis. 2019 Feb 26. doi: 10.3233/JPD-191576.
Does adherence to a Mediterranean diet reduce the risk of Parkinson’s disease?
Among older adults, adherence to a Mediterranean diet is associated with lower probability of prodromal Parkinson’s disease, according to research published in Movement Disorders.
“Recommending the Mediterranean diet pattern, either to reduce the risk or lessen the effects ... of prodromal Parkinson’s disease, needs to be considered and further explored,” said lead author Maria I. Maraki, PhD, of the department of nutrition and dietetics at Harokopio University in Athens, Greece, and her research colleagues.
Evidence regarding the effect of a Mediterranean diet on Parkinson’s disease risk remains limited, however, and physicians should be cautious in interpreting the data, researchers noted in accompanying editorials.
“There is a puzzling constellation of information and data that cannot be reconciled with a simple model accounting for the role of diet, vascular risk factors, and the neurodegenerative process and mechanisms underlying Parkinson’s disease,” Connie Marras, MD, PhD, and Jose A. Obeso, MD, PhD, said in an editorial. Given Maraki et al.’s findings, “most of us would be glad to accept that such a causal inverse association exists and can therefore be strongly recommended to our patients,” but “further work is needed before definitive conclusions can be reached,” Dr. Marras and Dr. Obeso wrote. Dr. Marras is affiliated with the University Health Network and the University of Toronto. Dr. Obeso is affiliated with University Hospital HM Puerta del Sur, CEU San Pablo University, Móstoles, Spain.
The role of diet
Prior research has suggested that adherence to the Mediterranean diet – characterized by consumption of nonrefined cereals, fruits, vegetables, legumes, potatoes, fish, and olive oil – may be associated with reduced risk of Parkinson’s disease. In addition, studies have found that adherence to the Mediterranean diet may be protective in other diseases, including dementia and cardiovascular disease. Dr. Maraki and her colleagues sought to assess whether adherence to the Mediterranean diet is associated with the likelihood of prodromal Parkinson’s disease or its manifestations. To calculate the probability of prodromal Parkinson’s disease, the investigators used a tool created by the International Parkinson and Movement Disorder Society (MDS) that takes into account baseline risk factors as well as prodromal markers such as constipation and motor slowing.
They analyzed data from 1,731 participants in the population-based Hellenic Longitudinal Investigation of Aging and Diet (HELIAD) cohort in Greece. Participants, 41% of whom were male, were aged 65 years or older and did not have Parkinson’s disease. They completed a detailed food frequency questionnaire, and the researchers calculated how closely each participant’s diet adhered to the Mediterranean diet. Diet adherence scores ranged from 0 to 55, with higher scores indicating greater adherence.
The median probability of prodromal Parkinson’s disease was 1.9% (range, 0.2%-96.7%), and the probability was lower among those with greater adherence to the Mediterranean diet. This difference was “driven mostly by nonmotor markers of prodromal Parkinson’s disease,” including depression, constipation, urinary dysfunction, and daytime somnolence, the researchers said. “Each unit increase in Mediterranean diet score was associated with a 2% decreased probability for prodromal Parkinson’s disease.” Compared with participants in the lowest quartile of Mediterranean diet adherence, those in the highest quartile had an approximately 21% lower probability for prodromal Parkinson’s disease.
Potential confounding
“This study pushes the prodromal criteria into performing a job they were never designed to do,” which presents potential pitfalls, Ronald B. Postuma, MD, of the department of neurology at Montreal General Hospital in Quebec, said in an accompanying editorial.
While the MDS criteria were designed to assess the likelihood that any person over age 50 years is in a state of prodromal Parkinson’s disease, the present study aimed to evaluate whether a single putative risk factor for Parkinson’s disease is associated with the likelihood of its prodromal state.
In addition, the analysis did not include some of the prodromal markers that are part of the MDS criteria, including olfaction, polysomnographic-proven REM sleep behavior disorder, and dopaminergic functional neuroimaging.
“As pointed out by the researchers, many of the risk factors in the prodromal criteria are potentially confounded by factors other than Parkinson’s disease; for example, one could imagine that older people, men, or farmers (with their higher pesticide exposure) are less likely to follow the Mediterranean diet simply because of different cultural lifestyle patterns,” Dr. Postuma said.
It is also possible that the Mediterranean diet affects prodromal markers such as constipation, sleep, or depression without affecting underlying neurodegenerative disease. In any case, the effect sizes observed in the study were small, and there was no evidence that participants who adhered most closely to a Mediterranean diet had less parkinsonism, Dr. Postuma said.
These limitations do not preclude physicians from recommending the diet for other reasons. “Numerous studies, reviews, meta-analyses, and randomized controlled trials consistently rank the Mediterranean diet as among the healthiest diets available,” Dr. Postuma said. “So, one can clearly recommend diets such as these, even if not necessarily for Parkinson’s disease prevention.”
Adding insights
The researchers used a Mediterranean diet score that was developed in a population of adults from metropolitan Athens, “an area not unlike the one in which the score is being applied in the HELIAD study,” Christy C. Tangney, PhD, professor of clinical nutrition and preventive medicine and associate dean for research at Rush University Medical Center, Chicago, said in a separate editorial. As expected, the average Mediterranean diet adherence score in this study was higher than that in the Chicago Health and Aging Project (33.2 vs. 28.2).
“If we can identify differences in diet or lifestyle patterns and risk of this latent phase of Parkinson’s disease neurodegeneration, we may be one step closer to identifying preventive measures,” she said. Follow-up reports from HELIAD and other cohorts may allow researchers to assess how changes in dietary patterns relate to changes in Parkinson’s disease markers, the probability of prodromal Parkinson’s disease, and incident Parkinson’s disease, Dr. Tangney said.
The study authors had no conflicts of interest or financial disclosures. The study was supported by a grant from the Alzheimer’s Association, an ESPA‐EU grant cofunded by the European Social Fund and Greek National resources, and a grant from the Ministry for Health and Social Solidarity (Greece). Dr. Maraki and a coauthor have received financial support from the Greek State Scholarships Foundation. Dr. Tangney and Dr. Postuma had no conflicts of interest.
SOURCE: Maraki MI et al. Mov Disord. 2018 Oct 10. doi: 10.1002/mds.27489.
Among older adults, adherence to a Mediterranean diet is associated with lower probability of prodromal Parkinson’s disease, according to research published in Movement Disorders.
“Recommending the Mediterranean diet pattern, either to reduce the risk or lessen the effects ... of prodromal Parkinson’s disease, needs to be considered and further explored,” said lead author Maria I. Maraki, PhD, of the department of nutrition and dietetics at Harokopio University in Athens, Greece, and her research colleagues.
Evidence regarding the effect of a Mediterranean diet on Parkinson’s disease risk remains limited, however, and physicians should be cautious in interpreting the data, researchers noted in accompanying editorials.
“There is a puzzling constellation of information and data that cannot be reconciled with a simple model accounting for the role of diet, vascular risk factors, and the neurodegenerative process and mechanisms underlying Parkinson’s disease,” Connie Marras, MD, PhD, and Jose A. Obeso, MD, PhD, said in an editorial. Given Maraki et al.’s findings, “most of us would be glad to accept that such a causal inverse association exists and can therefore be strongly recommended to our patients,” but “further work is needed before definitive conclusions can be reached,” Dr. Marras and Dr. Obeso wrote. Dr. Marras is affiliated with the University Health Network and the University of Toronto. Dr. Obeso is affiliated with University Hospital HM Puerta del Sur, CEU San Pablo University, Móstoles, Spain.
The role of diet
Prior research has suggested that adherence to the Mediterranean diet – characterized by consumption of nonrefined cereals, fruits, vegetables, legumes, potatoes, fish, and olive oil – may be associated with reduced risk of Parkinson’s disease. In addition, studies have found that adherence to the Mediterranean diet may be protective in other diseases, including dementia and cardiovascular disease. Dr. Maraki and her colleagues sought to assess whether adherence to the Mediterranean diet is associated with the likelihood of prodromal Parkinson’s disease or its manifestations. To calculate the probability of prodromal Parkinson’s disease, the investigators used a tool created by the International Parkinson and Movement Disorder Society (MDS) that takes into account baseline risk factors as well as prodromal markers such as constipation and motor slowing.
They analyzed data from 1,731 participants in the population-based Hellenic Longitudinal Investigation of Aging and Diet (HELIAD) cohort in Greece. Participants, 41% of whom were male, were aged 65 years or older and did not have Parkinson’s disease. They completed a detailed food frequency questionnaire, and the researchers calculated how closely each participant’s diet adhered to the Mediterranean diet. Diet adherence scores ranged from 0 to 55, with higher scores indicating greater adherence.
The median probability of prodromal Parkinson’s disease was 1.9% (range, 0.2%-96.7%), and the probability was lower among those with greater adherence to the Mediterranean diet. This difference was “driven mostly by nonmotor markers of prodromal Parkinson’s disease,” including depression, constipation, urinary dysfunction, and daytime somnolence, the researchers said. “Each unit increase in Mediterranean diet score was associated with a 2% decreased probability for prodromal Parkinson’s disease.” Compared with participants in the lowest quartile of Mediterranean diet adherence, those in the highest quartile had an approximately 21% lower probability for prodromal Parkinson’s disease.
Potential confounding
“This study pushes the prodromal criteria into performing a job they were never designed to do,” which presents potential pitfalls, Ronald B. Postuma, MD, of the department of neurology at Montreal General Hospital in Quebec, said in an accompanying editorial.
While the MDS criteria were designed to assess the likelihood that any person over age 50 years is in a state of prodromal Parkinson’s disease, the present study aimed to evaluate whether a single putative risk factor for Parkinson’s disease is associated with the likelihood of its prodromal state.
In addition, the analysis did not include some of the prodromal markers that are part of the MDS criteria, including olfaction, polysomnographic-proven REM sleep behavior disorder, and dopaminergic functional neuroimaging.
“As pointed out by the researchers, many of the risk factors in the prodromal criteria are potentially confounded by factors other than Parkinson’s disease; for example, one could imagine that older people, men, or farmers (with their higher pesticide exposure) are less likely to follow the Mediterranean diet simply because of different cultural lifestyle patterns,” Dr. Postuma said.
It is also possible that the Mediterranean diet affects prodromal markers such as constipation, sleep, or depression without affecting underlying neurodegenerative disease. In any case, the effect sizes observed in the study were small, and there was no evidence that participants who adhered most closely to a Mediterranean diet had less parkinsonism, Dr. Postuma said.
These limitations do not preclude physicians from recommending the diet for other reasons. “Numerous studies, reviews, meta-analyses, and randomized controlled trials consistently rank the Mediterranean diet as among the healthiest diets available,” Dr. Postuma said. “So, one can clearly recommend diets such as these, even if not necessarily for Parkinson’s disease prevention.”
Adding insights
The researchers used a Mediterranean diet score that was developed in a population of adults from metropolitan Athens, “an area not unlike the one in which the score is being applied in the HELIAD study,” Christy C. Tangney, PhD, professor of clinical nutrition and preventive medicine and associate dean for research at Rush University Medical Center, Chicago, said in a separate editorial. As expected, the average Mediterranean diet adherence score in this study was higher than that in the Chicago Health and Aging Project (33.2 vs. 28.2).
“If we can identify differences in diet or lifestyle patterns and risk of this latent phase of Parkinson’s disease neurodegeneration, we may be one step closer to identifying preventive measures,” she said. Follow-up reports from HELIAD and other cohorts may allow researchers to assess how changes in dietary patterns relate to changes in Parkinson’s disease markers, the probability of prodromal Parkinson’s disease, and incident Parkinson’s disease, Dr. Tangney said.
The study authors had no conflicts of interest or financial disclosures. The study was supported by a grant from the Alzheimer’s Association, an ESPA‐EU grant cofunded by the European Social Fund and Greek National resources, and a grant from the Ministry for Health and Social Solidarity (Greece). Dr. Maraki and a coauthor have received financial support from the Greek State Scholarships Foundation. Dr. Tangney and Dr. Postuma had no conflicts of interest.
SOURCE: Maraki MI et al. Mov Disord. 2018 Oct 10. doi: 10.1002/mds.27489.
Among older adults, adherence to a Mediterranean diet is associated with lower probability of prodromal Parkinson’s disease, according to research published in Movement Disorders.
“Recommending the Mediterranean diet pattern, either to reduce the risk or lessen the effects ... of prodromal Parkinson’s disease, needs to be considered and further explored,” said lead author Maria I. Maraki, PhD, of the department of nutrition and dietetics at Harokopio University in Athens, Greece, and her research colleagues.
Evidence regarding the effect of a Mediterranean diet on Parkinson’s disease risk remains limited, however, and physicians should be cautious in interpreting the data, researchers noted in accompanying editorials.
“There is a puzzling constellation of information and data that cannot be reconciled with a simple model accounting for the role of diet, vascular risk factors, and the neurodegenerative process and mechanisms underlying Parkinson’s disease,” Connie Marras, MD, PhD, and Jose A. Obeso, MD, PhD, said in an editorial. Given Maraki et al.’s findings, “most of us would be glad to accept that such a causal inverse association exists and can therefore be strongly recommended to our patients,” but “further work is needed before definitive conclusions can be reached,” Dr. Marras and Dr. Obeso wrote. Dr. Marras is affiliated with the University Health Network and the University of Toronto. Dr. Obeso is affiliated with University Hospital HM Puerta del Sur, CEU San Pablo University, Móstoles, Spain.
The role of diet
Prior research has suggested that adherence to the Mediterranean diet – characterized by consumption of nonrefined cereals, fruits, vegetables, legumes, potatoes, fish, and olive oil – may be associated with reduced risk of Parkinson’s disease. In addition, studies have found that adherence to the Mediterranean diet may be protective in other diseases, including dementia and cardiovascular disease. Dr. Maraki and her colleagues sought to assess whether adherence to the Mediterranean diet is associated with the likelihood of prodromal Parkinson’s disease or its manifestations. To calculate the probability of prodromal Parkinson’s disease, the investigators used a tool created by the International Parkinson and Movement Disorder Society (MDS) that takes into account baseline risk factors as well as prodromal markers such as constipation and motor slowing.
They analyzed data from 1,731 participants in the population-based Hellenic Longitudinal Investigation of Aging and Diet (HELIAD) cohort in Greece. Participants, 41% of whom were male, were aged 65 years or older and did not have Parkinson’s disease. They completed a detailed food frequency questionnaire, and the researchers calculated how closely each participant’s diet adhered to the Mediterranean diet. Diet adherence scores ranged from 0 to 55, with higher scores indicating greater adherence.
The median probability of prodromal Parkinson’s disease was 1.9% (range, 0.2%-96.7%), and the probability was lower among those with greater adherence to the Mediterranean diet. This difference was “driven mostly by nonmotor markers of prodromal Parkinson’s disease,” including depression, constipation, urinary dysfunction, and daytime somnolence, the researchers said. “Each unit increase in Mediterranean diet score was associated with a 2% decreased probability for prodromal Parkinson’s disease.” Compared with participants in the lowest quartile of Mediterranean diet adherence, those in the highest quartile had an approximately 21% lower probability for prodromal Parkinson’s disease.
Potential confounding
“This study pushes the prodromal criteria into performing a job they were never designed to do,” which presents potential pitfalls, Ronald B. Postuma, MD, of the department of neurology at Montreal General Hospital in Quebec, said in an accompanying editorial.
While the MDS criteria were designed to assess the likelihood that any person over age 50 years is in a state of prodromal Parkinson’s disease, the present study aimed to evaluate whether a single putative risk factor for Parkinson’s disease is associated with the likelihood of its prodromal state.
In addition, the analysis did not include some of the prodromal markers that are part of the MDS criteria, including olfaction, polysomnographic-proven REM sleep behavior disorder, and dopaminergic functional neuroimaging.
“As pointed out by the researchers, many of the risk factors in the prodromal criteria are potentially confounded by factors other than Parkinson’s disease; for example, one could imagine that older people, men, or farmers (with their higher pesticide exposure) are less likely to follow the Mediterranean diet simply because of different cultural lifestyle patterns,” Dr. Postuma said.
It is also possible that the Mediterranean diet affects prodromal markers such as constipation, sleep, or depression without affecting underlying neurodegenerative disease. In any case, the effect sizes observed in the study were small, and there was no evidence that participants who adhered most closely to a Mediterranean diet had less parkinsonism, Dr. Postuma said.
These limitations do not preclude physicians from recommending the diet for other reasons. “Numerous studies, reviews, meta-analyses, and randomized controlled trials consistently rank the Mediterranean diet as among the healthiest diets available,” Dr. Postuma said. “So, one can clearly recommend diets such as these, even if not necessarily for Parkinson’s disease prevention.”
Adding insights
The researchers used a Mediterranean diet score that was developed in a population of adults from metropolitan Athens, “an area not unlike the one in which the score is being applied in the HELIAD study,” Christy C. Tangney, PhD, professor of clinical nutrition and preventive medicine and associate dean for research at Rush University Medical Center, Chicago, said in a separate editorial. As expected, the average Mediterranean diet adherence score in this study was higher than that in the Chicago Health and Aging Project (33.2 vs. 28.2).
“If we can identify differences in diet or lifestyle patterns and risk of this latent phase of Parkinson’s disease neurodegeneration, we may be one step closer to identifying preventive measures,” she said. Follow-up reports from HELIAD and other cohorts may allow researchers to assess how changes in dietary patterns relate to changes in Parkinson’s disease markers, the probability of prodromal Parkinson’s disease, and incident Parkinson’s disease, Dr. Tangney said.
The study authors had no conflicts of interest or financial disclosures. The study was supported by a grant from the Alzheimer’s Association, an ESPA‐EU grant cofunded by the European Social Fund and Greek National resources, and a grant from the Ministry for Health and Social Solidarity (Greece). Dr. Maraki and a coauthor have received financial support from the Greek State Scholarships Foundation. Dr. Tangney and Dr. Postuma had no conflicts of interest.
SOURCE: Maraki MI et al. Mov Disord. 2018 Oct 10. doi: 10.1002/mds.27489.
FROM MOVEMENT DISORDERS
Key clinical point: Adherence to a Mediterranean diet is associated with lower probability of prodromal Parkinson’s disease.
Major finding: Each 1-unit increase in Mediterranean diet score was associated with a 2% decreased probability for prodromal Parkinson’s disease.
Study details: A study of 1,731 older adults in the population-based Hellenic Longitudinal Investigation of Aging and Diet (HELIAD) cohort in Greece.
Disclosures: The study authors had no conflicts of interest or financial disclosures. The study was supported by a grant from the Alzheimer’s Association, an ESPA‐EU grant cofunded by the European Social Fund and Greek National resources, and a grant from the Ministry for Health and Social Solidarity (Greece). Dr. Maraki and a coauthor have received financial support from the Greek State Scholarships Foundation.
Source: Maraki MI et al. Mov Disord. 2018 Oct 10. doi:10.1002/mds.27489.
International survey probes oxygen’s efficacy for cluster headache
According to the results, triptans also are highly effective, with some side effects. Newer medications deserve further study, the researchers said.
To assess the effectiveness and adverse effects of acute cluster headache medications in a large international sample, Stuart M. Pearson, a researcher in the department of psychology at the University of West Georgia in Carrollton, and his coauthors analyzed data from the Cluster Headache Questionnaire. Respondents from more than 50 countries completed the online survey; most were from the United States, the United Kingdom, and Canada. The survey included questions about cluster headache diagnostic criteria and medication effectiveness, complications, and access to medications.
In all, 3,251 subjects participated in the questionnaire, and 2,193 respondents met criteria for the study; 1,604 had cluster headache, and 589 had probable cluster headache. Among the respondents with cluster headache, 68.8% were male, 78.0% had episodic cluster headache, and the average age was 46 years. More than half of respondents reported complete or very effective treatment for triptans (54%) and oxygen (also 54%). The proportion of respondents who reported that ergot derivatives, caffeine or energy drinks, and intranasal ketamine were completely or very effective ranged from 14% to 25%. Patients were less likely to report high levels of efficacy for opioids (6%), intranasal capsaicin (5%), and intranasal lidocaine (2%).
Participants experienced few complications from oxygen, with 99% reporting no or minimal physical and medical complications, and 97% reporting no or minimal psychological and emotional complications. Patients also reported few complications from intranasal lidocaine, intranasal ketamine, intranasal capsaicin, and caffeine and energy drinks. For triptans, 74% of respondents reported no or minimal physical and medical complications, and 85% reported no or minimal psychological and emotional complications.
Among the 139 participants with cluster headache who were aged 65 years or older, responses were similar to those for the entire population. In addition, the 589 respondents with probable cluster headache reported similar efficacy data, compared with respondents with a full diagnosis of cluster headache.
“Oxygen in particular had a high rate of complete effectiveness, a low rate of ineffectiveness, and a low rate of physical, medical, emotional, and psychological side effects,” the investigators said. “However, respondents reported that it was difficult to obtain.”
Limited insurance coverage of oxygen may affect access, even though the treatment has a Level A recommendation for the acute treatment of cluster headache in the American Headache Society guidelines, the authors said. Physicians also may pose a barrier. A prior study found that 12% of providers did not prescribe oxygen for cluster headache because they doubted its efficacy or did not know about it. In addition, there may be concerns that the treatment could be a fire hazard in a patient population that has high rates of smoking, the researchers said.
Limitations of the study include the survey’s use of nonvalidated questions, the lack of a formal clinical diagnosis of cluster headache, and the grouping of all triptans, rather than assessing individual triptan medications, such as sumatriptan subcutaneous, alone.
The study received funding from Autonomic Technologies and Clusterbusters. One of the authors has served as a paid consultant to Eli Lilly as a member of the data monitoring committee for clinical trials of galcanezumab for cluster headache and migraine.
This article was updated 3/7/2019.
SOURCE: Pearson SM et al. Headache. 2019 Jan 11. doi: 10.1111/head.13473.
According to the results, triptans also are highly effective, with some side effects. Newer medications deserve further study, the researchers said.
To assess the effectiveness and adverse effects of acute cluster headache medications in a large international sample, Stuart M. Pearson, a researcher in the department of psychology at the University of West Georgia in Carrollton, and his coauthors analyzed data from the Cluster Headache Questionnaire. Respondents from more than 50 countries completed the online survey; most were from the United States, the United Kingdom, and Canada. The survey included questions about cluster headache diagnostic criteria and medication effectiveness, complications, and access to medications.
In all, 3,251 subjects participated in the questionnaire, and 2,193 respondents met criteria for the study; 1,604 had cluster headache, and 589 had probable cluster headache. Among the respondents with cluster headache, 68.8% were male, 78.0% had episodic cluster headache, and the average age was 46 years. More than half of respondents reported complete or very effective treatment for triptans (54%) and oxygen (also 54%). The proportion of respondents who reported that ergot derivatives, caffeine or energy drinks, and intranasal ketamine were completely or very effective ranged from 14% to 25%. Patients were less likely to report high levels of efficacy for opioids (6%), intranasal capsaicin (5%), and intranasal lidocaine (2%).
Participants experienced few complications from oxygen, with 99% reporting no or minimal physical and medical complications, and 97% reporting no or minimal psychological and emotional complications. Patients also reported few complications from intranasal lidocaine, intranasal ketamine, intranasal capsaicin, and caffeine and energy drinks. For triptans, 74% of respondents reported no or minimal physical and medical complications, and 85% reported no or minimal psychological and emotional complications.
Among the 139 participants with cluster headache who were aged 65 years or older, responses were similar to those for the entire population. In addition, the 589 respondents with probable cluster headache reported similar efficacy data, compared with respondents with a full diagnosis of cluster headache.
“Oxygen in particular had a high rate of complete effectiveness, a low rate of ineffectiveness, and a low rate of physical, medical, emotional, and psychological side effects,” the investigators said. “However, respondents reported that it was difficult to obtain.”
Limited insurance coverage of oxygen may affect access, even though the treatment has a Level A recommendation for the acute treatment of cluster headache in the American Headache Society guidelines, the authors said. Physicians also may pose a barrier. A prior study found that 12% of providers did not prescribe oxygen for cluster headache because they doubted its efficacy or did not know about it. In addition, there may be concerns that the treatment could be a fire hazard in a patient population that has high rates of smoking, the researchers said.
Limitations of the study include the survey’s use of nonvalidated questions, the lack of a formal clinical diagnosis of cluster headache, and the grouping of all triptans, rather than assessing individual triptan medications, such as sumatriptan subcutaneous, alone.
The study received funding from Autonomic Technologies and Clusterbusters. One of the authors has served as a paid consultant to Eli Lilly as a member of the data monitoring committee for clinical trials of galcanezumab for cluster headache and migraine.
This article was updated 3/7/2019.
SOURCE: Pearson SM et al. Headache. 2019 Jan 11. doi: 10.1111/head.13473.
According to the results, triptans also are highly effective, with some side effects. Newer medications deserve further study, the researchers said.
To assess the effectiveness and adverse effects of acute cluster headache medications in a large international sample, Stuart M. Pearson, a researcher in the department of psychology at the University of West Georgia in Carrollton, and his coauthors analyzed data from the Cluster Headache Questionnaire. Respondents from more than 50 countries completed the online survey; most were from the United States, the United Kingdom, and Canada. The survey included questions about cluster headache diagnostic criteria and medication effectiveness, complications, and access to medications.
In all, 3,251 subjects participated in the questionnaire, and 2,193 respondents met criteria for the study; 1,604 had cluster headache, and 589 had probable cluster headache. Among the respondents with cluster headache, 68.8% were male, 78.0% had episodic cluster headache, and the average age was 46 years. More than half of respondents reported complete or very effective treatment for triptans (54%) and oxygen (also 54%). The proportion of respondents who reported that ergot derivatives, caffeine or energy drinks, and intranasal ketamine were completely or very effective ranged from 14% to 25%. Patients were less likely to report high levels of efficacy for opioids (6%), intranasal capsaicin (5%), and intranasal lidocaine (2%).
Participants experienced few complications from oxygen, with 99% reporting no or minimal physical and medical complications, and 97% reporting no or minimal psychological and emotional complications. Patients also reported few complications from intranasal lidocaine, intranasal ketamine, intranasal capsaicin, and caffeine and energy drinks. For triptans, 74% of respondents reported no or minimal physical and medical complications, and 85% reported no or minimal psychological and emotional complications.
Among the 139 participants with cluster headache who were aged 65 years or older, responses were similar to those for the entire population. In addition, the 589 respondents with probable cluster headache reported similar efficacy data, compared with respondents with a full diagnosis of cluster headache.
“Oxygen in particular had a high rate of complete effectiveness, a low rate of ineffectiveness, and a low rate of physical, medical, emotional, and psychological side effects,” the investigators said. “However, respondents reported that it was difficult to obtain.”
Limited insurance coverage of oxygen may affect access, even though the treatment has a Level A recommendation for the acute treatment of cluster headache in the American Headache Society guidelines, the authors said. Physicians also may pose a barrier. A prior study found that 12% of providers did not prescribe oxygen for cluster headache because they doubted its efficacy or did not know about it. In addition, there may be concerns that the treatment could be a fire hazard in a patient population that has high rates of smoking, the researchers said.
Limitations of the study include the survey’s use of nonvalidated questions, the lack of a formal clinical diagnosis of cluster headache, and the grouping of all triptans, rather than assessing individual triptan medications, such as sumatriptan subcutaneous, alone.
The study received funding from Autonomic Technologies and Clusterbusters. One of the authors has served as a paid consultant to Eli Lilly as a member of the data monitoring committee for clinical trials of galcanezumab for cluster headache and migraine.
This article was updated 3/7/2019.
SOURCE: Pearson SM et al. Headache. 2019 Jan 11. doi: 10.1111/head.13473.
FROM HEADACHE
Key clinical point: Oxygen is a highly effective treatment for cluster headache with few complications.
Major finding: More than half of respondents (54%) reported that triptans and oxygen were completely or very effective.
Study details: Analysis of data from 1,604 people with cluster headache who completed the online Cluster Headache Questionnaire.
Disclosures: The study received funding from Autonomic Technologies and Clusterbusters. One of the authors has served as a paid consultant to Eli Lilly as a member of the data monitoring committee for clinical trials of galcanezumab for cluster headache and migraine.
Source: Pearson SM et al. Headache. 2019 Jan 11. doi: 10.1111/head.13473.
Cloud of inconsistency hangs over cannabis data
More people are using medical cannabis as it becomes legal in more states, but the lack of standardization in states’ data collection hindered investigators’ efforts to track that use.
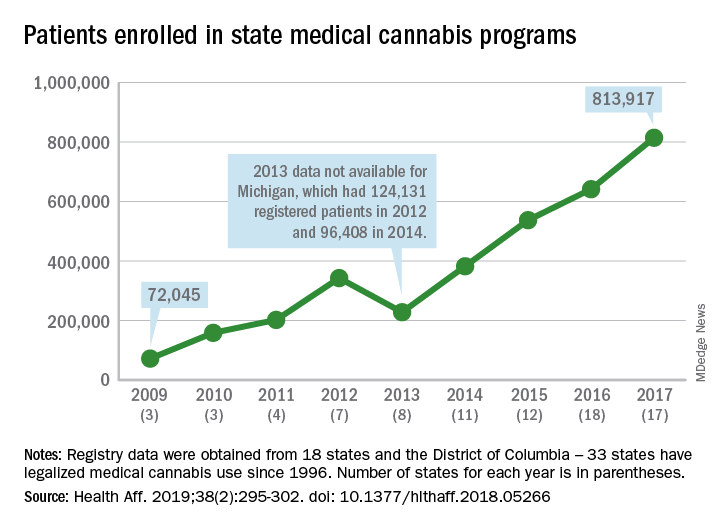
Legalized medical cannabis is now available in 33 states and the District of Columbia, and the number of users has risen from just over 72,000 in 2009 to almost 814,000 in 2017. That 814,000, however, covers only 16 states and D.C., since 1 state (Connecticut) does not publish reports on medical cannabis use, 12 did not have statistics available, 2 (New York and Vermont) didn’t report data for 2017, and 2 (California and Maine) have voluntary registries that are unlikely to be accurate, according to Kevin F. Boehnke, PhD, of the University of Michigan, Ann Arbor, and his associates.
Michigan had the largest reported number of patients enrolled in its medical cannabis program in 2017, almost 270,000. California – the state with the oldest medical cannabis legislation (passed in 1996) and the largest overall population but a voluntary cannabis registry – reported its highest number of enrollees, 12,659, in 2009-2010, the investigators said. Colorado had more than 116,000 patients in its medical cannabis program in 2010 (Health Aff. 2019;38[2]:295-302).
The “many inconsistencies in data quality across states [suggest] the need for further standardization of data collection. Such standardization would add transparency to understanding how medical cannabis programs are used, which would help guide both research and policy needs,” Dr. Boehnke and his associates wrote.
More consistency was seen in the reasons for using medical cannabis. Chronic pain made up 62.2% of all qualifying conditions reported by patients during 1999-2016, with the annual average varying between 33.3% and 73%. Multiple sclerosis spasticity symptoms had the second-highest number of reports over the study period, followed by chemotherapy-induced nausea and vomiting, posttraumatic stress disorder, and cancer, they reported.
The investigators also looked at the appropriateness of cannabis and determined that its use in 85.5% of patient-reported conditions was “supported by conclusive or substantial evidence of therapeutic effectiveness, according to the 2017 National Academies report” on the health effects of cannabis.
“We believe not only that it is inappropriate for cannabis to remain a Schedule I substance, but also that state and federal policy makers should begin evaluating evidence-based ways for safely integrating cannabis research and products into the health care system,” they concluded.
SOURCE: Boehnke KF et al. Health Aff. 2019;38(2):295-302.
More people are using medical cannabis as it becomes legal in more states, but the lack of standardization in states’ data collection hindered investigators’ efforts to track that use.
Legalized medical cannabis is now available in 33 states and the District of Columbia, and the number of users has risen from just over 72,000 in 2009 to almost 814,000 in 2017. That 814,000, however, covers only 16 states and D.C., since 1 state (Connecticut) does not publish reports on medical cannabis use, 12 did not have statistics available, 2 (New York and Vermont) didn’t report data for 2017, and 2 (California and Maine) have voluntary registries that are unlikely to be accurate, according to Kevin F. Boehnke, PhD, of the University of Michigan, Ann Arbor, and his associates.
Michigan had the largest reported number of patients enrolled in its medical cannabis program in 2017, almost 270,000. California – the state with the oldest medical cannabis legislation (passed in 1996) and the largest overall population but a voluntary cannabis registry – reported its highest number of enrollees, 12,659, in 2009-2010, the investigators said. Colorado had more than 116,000 patients in its medical cannabis program in 2010 (Health Aff. 2019;38[2]:295-302).
The “many inconsistencies in data quality across states [suggest] the need for further standardization of data collection. Such standardization would add transparency to understanding how medical cannabis programs are used, which would help guide both research and policy needs,” Dr. Boehnke and his associates wrote.
More consistency was seen in the reasons for using medical cannabis. Chronic pain made up 62.2% of all qualifying conditions reported by patients during 1999-2016, with the annual average varying between 33.3% and 73%. Multiple sclerosis spasticity symptoms had the second-highest number of reports over the study period, followed by chemotherapy-induced nausea and vomiting, posttraumatic stress disorder, and cancer, they reported.
The investigators also looked at the appropriateness of cannabis and determined that its use in 85.5% of patient-reported conditions was “supported by conclusive or substantial evidence of therapeutic effectiveness, according to the 2017 National Academies report” on the health effects of cannabis.
“We believe not only that it is inappropriate for cannabis to remain a Schedule I substance, but also that state and federal policy makers should begin evaluating evidence-based ways for safely integrating cannabis research and products into the health care system,” they concluded.
SOURCE: Boehnke KF et al. Health Aff. 2019;38(2):295-302.
More people are using medical cannabis as it becomes legal in more states, but the lack of standardization in states’ data collection hindered investigators’ efforts to track that use.
Legalized medical cannabis is now available in 33 states and the District of Columbia, and the number of users has risen from just over 72,000 in 2009 to almost 814,000 in 2017. That 814,000, however, covers only 16 states and D.C., since 1 state (Connecticut) does not publish reports on medical cannabis use, 12 did not have statistics available, 2 (New York and Vermont) didn’t report data for 2017, and 2 (California and Maine) have voluntary registries that are unlikely to be accurate, according to Kevin F. Boehnke, PhD, of the University of Michigan, Ann Arbor, and his associates.
Michigan had the largest reported number of patients enrolled in its medical cannabis program in 2017, almost 270,000. California – the state with the oldest medical cannabis legislation (passed in 1996) and the largest overall population but a voluntary cannabis registry – reported its highest number of enrollees, 12,659, in 2009-2010, the investigators said. Colorado had more than 116,000 patients in its medical cannabis program in 2010 (Health Aff. 2019;38[2]:295-302).
The “many inconsistencies in data quality across states [suggest] the need for further standardization of data collection. Such standardization would add transparency to understanding how medical cannabis programs are used, which would help guide both research and policy needs,” Dr. Boehnke and his associates wrote.
More consistency was seen in the reasons for using medical cannabis. Chronic pain made up 62.2% of all qualifying conditions reported by patients during 1999-2016, with the annual average varying between 33.3% and 73%. Multiple sclerosis spasticity symptoms had the second-highest number of reports over the study period, followed by chemotherapy-induced nausea and vomiting, posttraumatic stress disorder, and cancer, they reported.
The investigators also looked at the appropriateness of cannabis and determined that its use in 85.5% of patient-reported conditions was “supported by conclusive or substantial evidence of therapeutic effectiveness, according to the 2017 National Academies report” on the health effects of cannabis.
“We believe not only that it is inappropriate for cannabis to remain a Schedule I substance, but also that state and federal policy makers should begin evaluating evidence-based ways for safely integrating cannabis research and products into the health care system,” they concluded.
SOURCE: Boehnke KF et al. Health Aff. 2019;38(2):295-302.
FROM HEALTH AFFAIRS
Clinical benefits persist 5 years after thymectomy for myasthenia gravis
Thymectomy may continue to benefit patients with myasthenia gravis 5 years after the procedure, according to an extension study published in Lancet Neurology.
The study evaluated the clinical status, medication requirements, and adverse events of patients with myasthenia gravis who completed a randomized controlled trial of thymectomy plus prednisone versus prednisone alone and agreed to participate in a rater-blinded 2-year extension.
“Thymectomy within the first few years of the disease course in addition to prednisone therapy confers benefits that persist for 5 years ... in patients with generalized nonthymomatous myasthenia gravis,” said lead study author Gil I. Wolfe, MD, chair of the department of neurology at the University at Buffalo in New York, and his research colleagues. “Results from the extension study provide further support for the use of thymectomy in management of myasthenia gravis and should encourage serious consideration of this treatment option in discussions between clinicians and their patients,” they wrote. “Our results should lead to revision of clinical guidelines in favor of thymectomy and could potentially reverse downward trends in the use of thymectomy in overall management of myasthenia gravis.”
The main 3-year results of the Thymectomy Trial in Nonthymomatous Myasthenia Gravis Patients Receiving Prednisone (MGTX) were reported in 2016; the international trial found that thymectomy plus prednisone was superior to prednisone alone at 3 years (N Engl J Med. 2016 Aug 11;375[6]:511-22). The extension study aimed to assess the durability of the treatment response.
MGTX enrolled patients aged 18-65 years who had generalized nonthymomatous myasthenia gravis of less than 5 years’ duration and Myasthenia Gravis Foundation of America Clinical Classification Class II-IV disease. Of 111 patients who completed MGTX, 68 entered the extension study, and 50 completed the 60-month assessment (24 patients in the prednisone alone group and 26 patients in the prednisone plus thymectomy group).
At 5 years, patients in the thymectomy plus prednisone group had significantly lower time-weighted average Quantitative Myasthenia Gravis (QMG) scores (5.47 vs. 9.34) and mean alternate-day prednisone doses (24 mg vs. 48 mg), compared with patients who received prednisone alone. Twelve of 35 patients in the thymectomy group and 14 of 33 patients in the prednisone group had at least one adverse event by month 60. No treatment-related deaths occurred in the extension phase.
At 5 years, significantly more patients who underwent thymectomy had minimal manifestation status (i.e., no functional limitations from the disease other than some muscle weakness) – 88% versus 58%. The corresponding figures at 3 years were 67% and 47%.
In addition, 3-year and 5-year data indicate that the need for hospitalization is reduced after surgery, compared with medical therapy alone, Dr. Wolfe said.
Two patients in each treatment arm had an increase of 2 points or more in the QMG score, indicating clinical worsening.
“Our current findings reinforce the benefit of thymectomy seen in [MGTX], dispelling doubts about the procedure’s benefits and how long those benefits last,” said Dr. Wolfe. “We do hope that the new findings help reverse the apparent reluctance to do thymectomy and that the proportion of patients with myasthenia gravis who undergo thymectomy will increase.”
The authors noted that the small sample size of the extension study may limit its generalizability.
The study received funding from the National Institutes of Health. Dr. Wolfe reported grants from the NIH, the Muscular Dystrophy Association, the Myasthenia Gravis Foundation of America, CSL-Behring, and ArgenX, as well as personal fees from Grifols, Shire, and Alexion Pharmaceuticals. Coauthors reported working with and receiving funds from agencies, foundations, and pharmaceutical companies.
SOURCE: Wolfe GI et al. Lancet Neurol. 2019 Jan 25. doi: 10.1016/S1474-4422(18)30392-2.
Thymectomy may continue to benefit patients with myasthenia gravis 5 years after the procedure, according to an extension study published in Lancet Neurology.
The study evaluated the clinical status, medication requirements, and adverse events of patients with myasthenia gravis who completed a randomized controlled trial of thymectomy plus prednisone versus prednisone alone and agreed to participate in a rater-blinded 2-year extension.
“Thymectomy within the first few years of the disease course in addition to prednisone therapy confers benefits that persist for 5 years ... in patients with generalized nonthymomatous myasthenia gravis,” said lead study author Gil I. Wolfe, MD, chair of the department of neurology at the University at Buffalo in New York, and his research colleagues. “Results from the extension study provide further support for the use of thymectomy in management of myasthenia gravis and should encourage serious consideration of this treatment option in discussions between clinicians and their patients,” they wrote. “Our results should lead to revision of clinical guidelines in favor of thymectomy and could potentially reverse downward trends in the use of thymectomy in overall management of myasthenia gravis.”
The main 3-year results of the Thymectomy Trial in Nonthymomatous Myasthenia Gravis Patients Receiving Prednisone (MGTX) were reported in 2016; the international trial found that thymectomy plus prednisone was superior to prednisone alone at 3 years (N Engl J Med. 2016 Aug 11;375[6]:511-22). The extension study aimed to assess the durability of the treatment response.
MGTX enrolled patients aged 18-65 years who had generalized nonthymomatous myasthenia gravis of less than 5 years’ duration and Myasthenia Gravis Foundation of America Clinical Classification Class II-IV disease. Of 111 patients who completed MGTX, 68 entered the extension study, and 50 completed the 60-month assessment (24 patients in the prednisone alone group and 26 patients in the prednisone plus thymectomy group).
At 5 years, patients in the thymectomy plus prednisone group had significantly lower time-weighted average Quantitative Myasthenia Gravis (QMG) scores (5.47 vs. 9.34) and mean alternate-day prednisone doses (24 mg vs. 48 mg), compared with patients who received prednisone alone. Twelve of 35 patients in the thymectomy group and 14 of 33 patients in the prednisone group had at least one adverse event by month 60. No treatment-related deaths occurred in the extension phase.
At 5 years, significantly more patients who underwent thymectomy had minimal manifestation status (i.e., no functional limitations from the disease other than some muscle weakness) – 88% versus 58%. The corresponding figures at 3 years were 67% and 47%.
In addition, 3-year and 5-year data indicate that the need for hospitalization is reduced after surgery, compared with medical therapy alone, Dr. Wolfe said.
Two patients in each treatment arm had an increase of 2 points or more in the QMG score, indicating clinical worsening.
“Our current findings reinforce the benefit of thymectomy seen in [MGTX], dispelling doubts about the procedure’s benefits and how long those benefits last,” said Dr. Wolfe. “We do hope that the new findings help reverse the apparent reluctance to do thymectomy and that the proportion of patients with myasthenia gravis who undergo thymectomy will increase.”
The authors noted that the small sample size of the extension study may limit its generalizability.
The study received funding from the National Institutes of Health. Dr. Wolfe reported grants from the NIH, the Muscular Dystrophy Association, the Myasthenia Gravis Foundation of America, CSL-Behring, and ArgenX, as well as personal fees from Grifols, Shire, and Alexion Pharmaceuticals. Coauthors reported working with and receiving funds from agencies, foundations, and pharmaceutical companies.
SOURCE: Wolfe GI et al. Lancet Neurol. 2019 Jan 25. doi: 10.1016/S1474-4422(18)30392-2.
Thymectomy may continue to benefit patients with myasthenia gravis 5 years after the procedure, according to an extension study published in Lancet Neurology.
The study evaluated the clinical status, medication requirements, and adverse events of patients with myasthenia gravis who completed a randomized controlled trial of thymectomy plus prednisone versus prednisone alone and agreed to participate in a rater-blinded 2-year extension.
“Thymectomy within the first few years of the disease course in addition to prednisone therapy confers benefits that persist for 5 years ... in patients with generalized nonthymomatous myasthenia gravis,” said lead study author Gil I. Wolfe, MD, chair of the department of neurology at the University at Buffalo in New York, and his research colleagues. “Results from the extension study provide further support for the use of thymectomy in management of myasthenia gravis and should encourage serious consideration of this treatment option in discussions between clinicians and their patients,” they wrote. “Our results should lead to revision of clinical guidelines in favor of thymectomy and could potentially reverse downward trends in the use of thymectomy in overall management of myasthenia gravis.”
The main 3-year results of the Thymectomy Trial in Nonthymomatous Myasthenia Gravis Patients Receiving Prednisone (MGTX) were reported in 2016; the international trial found that thymectomy plus prednisone was superior to prednisone alone at 3 years (N Engl J Med. 2016 Aug 11;375[6]:511-22). The extension study aimed to assess the durability of the treatment response.
MGTX enrolled patients aged 18-65 years who had generalized nonthymomatous myasthenia gravis of less than 5 years’ duration and Myasthenia Gravis Foundation of America Clinical Classification Class II-IV disease. Of 111 patients who completed MGTX, 68 entered the extension study, and 50 completed the 60-month assessment (24 patients in the prednisone alone group and 26 patients in the prednisone plus thymectomy group).
At 5 years, patients in the thymectomy plus prednisone group had significantly lower time-weighted average Quantitative Myasthenia Gravis (QMG) scores (5.47 vs. 9.34) and mean alternate-day prednisone doses (24 mg vs. 48 mg), compared with patients who received prednisone alone. Twelve of 35 patients in the thymectomy group and 14 of 33 patients in the prednisone group had at least one adverse event by month 60. No treatment-related deaths occurred in the extension phase.
At 5 years, significantly more patients who underwent thymectomy had minimal manifestation status (i.e., no functional limitations from the disease other than some muscle weakness) – 88% versus 58%. The corresponding figures at 3 years were 67% and 47%.
In addition, 3-year and 5-year data indicate that the need for hospitalization is reduced after surgery, compared with medical therapy alone, Dr. Wolfe said.
Two patients in each treatment arm had an increase of 2 points or more in the QMG score, indicating clinical worsening.
“Our current findings reinforce the benefit of thymectomy seen in [MGTX], dispelling doubts about the procedure’s benefits and how long those benefits last,” said Dr. Wolfe. “We do hope that the new findings help reverse the apparent reluctance to do thymectomy and that the proportion of patients with myasthenia gravis who undergo thymectomy will increase.”
The authors noted that the small sample size of the extension study may limit its generalizability.
The study received funding from the National Institutes of Health. Dr. Wolfe reported grants from the NIH, the Muscular Dystrophy Association, the Myasthenia Gravis Foundation of America, CSL-Behring, and ArgenX, as well as personal fees from Grifols, Shire, and Alexion Pharmaceuticals. Coauthors reported working with and receiving funds from agencies, foundations, and pharmaceutical companies.
SOURCE: Wolfe GI et al. Lancet Neurol. 2019 Jan 25. doi: 10.1016/S1474-4422(18)30392-2.
FROM LANCET NEUROLOGY
Key clinical point: The benefits of thymectomy for myasthenia gravis persist 5 years after the procedure.
Major finding: Patients who undergo thymectomy and receive prednisone have lower time-weighted average Quantitative Myasthenia Gravis scores (5.47 vs. 9.34) and mean alternate-day prednisone doses (24 mg vs. 48 mg), compared with patients who receive prednisone alone.
Study details: A rater-blinded 2-year extension study that enrolled 68 patients who had completed a 3-year randomized controlled trial.
Disclosures: The study received funding from the National Institutes of Health. Dr. Wolfe reported grants from the NIH, the Muscular Dystrophy Association, the Myasthenia Gravis Foundation of America, CSL-Behring, and ArgenX, as well as personal fees from Grifols, Shire, and Alexion Pharmaceuticals. Other authors reported working with and receiving funds from various agencies, foundations, and pharmaceutical companies.
Source: Wolfe GI et al. Lancet Neurol. 2019 Jan 25. doi: 10.1016/S1474-4422(18)30392-2.
No evidence for disease-modifying effect of levodopa in Parkinson’s disease
investigators reported. The disease course was not significantly different for patients who had a full 80 weeks of levodopa/carbodopa therapy, compared with that seen with those who started treatment after a 40-week delay, according to the investigators.
“These findings imply that levodopa had no disease-modifying effect on Parkinson’s disease over the period of the trial,” wrote investigator Rob M. A. de Bie, MD, PhD, professor of movement disorders at the University of Amsterdam, and his colleagues in the New England Journal of Medicine.
By contrast, results of an earlier randomized, placebo-controlled trial suggested that levodopa had disease-modifying effects, though the findings of that study were inconclusive, according to authors of an editorial (see Views on the News).
In the current multicenter trial, known as LEAP (Levodopa in Early Parkinson’s Disease) a total of 445 patients with early Parkinson’s disease were randomized to either 80 weeks of levodopa and carbodopa or to 40 weeks of placebo followed by 40 weeks of levodopa/carbodopa.
Levodopa was dosed at 100 mg three times per day, and carbodopa at 25 mg three times per day, according to the report.
There was no significant difference between the early and delayed treatment groups for primary outcome of the trial, which was change in the Unified Parkinson’s Disease Rating Scale (UPDRS) from baseline to week 80.
The mean change in UPDRS was –1.0 in the group of patients who had the full 80 weeks of levodopa/carbodopa and –2.0 for those who had delayed therapy, for a difference of 1 point (P = .44). Higher scores on the UPDRS signify worse disease.
At week 40, there was a change in UPDRS favoring the early-initiation strategy, which reflected the effects of levodopa on disease symptoms, investigators added.
Nausea was more common in the early-start group during the first 40 weeks of the trial. However, there were no differences between groups in other adverse events of particular interest, including dyskinesias and motor fluctuations related to levodopa, Dr. de Bie and his colleagues reported.
Taken together, these results suggest no beneficial or detrimental disease-modifying effect for an early treatment strategy, although further trials are warranted to evaluate other strategies, such as higher levodopa doses, longer administration, or starting the drug at later stages of disease, they wrote.
Dr. de Bie reported grants from ZonMw, Parkinson Vereniging, and Stichting Parkinsonfonds during the conduct of the study, as well as grants from GE Health and Medtronic outside the submitted work. Study authors provided disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
SOURCE: Verschuur CVM et al. N Engl J Med. 2019;380:315-24.
This trial supports current clinical practice in two ways, according to Susan Bressman, MD, and Rachel Saunders‑Pullman, MD, MPH. On one hand, the study provides no evidence to suggest that levodopa slows Parkinson’s disease progression, Dr. Bressman and Dr. Saunders-Pullman wrote in an editorial accompanying the study. On the other hand, they added, it provides no evidence that clinicians should delay therapy when it is clinically indicated.
The LEAP trial (Levodopa in Early Parkinson’s Disease) was designed to resolve uncertainty over the potential effects of levodopa on disease progression, they noted. This was necessary because of the results of the placebo-controlled ELLDOPA trial, which was published about 14 years ago and suggested that patients randomized to 40 weeks of levodopa did not deteriorate clinically to the degree that was observed in patients randomized to placebo.
The primary end point of that trial was Unified Parkinson’s Disease Rating Scale (UPDRS) scores after a 2-week washout period.
While one interpretation of the UPDRS results from ELLDOPA was that levodopa slowed disease progression, another was that the 2-week washout period was too short, allowing for residual effects of levodopa on symptoms, suggested Dr. Bressman and Dr. Saunders-Pullman.
The randomized LEAP study now shows not only that there were no differences in UPDRS scores when using a delayed start trial design – which implies that there was no disease-modifying effect – but also that starting levodopa early did not have negative effects, the editorial authors wrote.
In particular, the researchers showed no differences in rates of dyskinesia or levodopa-related fluctuations in those started early versus those started later.
“The results of the current trial, taken together with those of other trials, support treatment that is guided by clinical need and that uses the lowest dose that provides a satisfactory clinical effect,” wrote the editorial’s authors.
Dr. Bressman and Dr. Saunders‑Pullman are with the Icahn School of Medicine at Mt. Sinai, New York. Their editorial appears in the New England Journal of Medicine (2019;380:389-90). Dr. Bressman reported disclosures related to Denali Therapeutics, the Michael J. Fox Foundation, and Prevail Therapeutics, while Dr. Saunders-Pullman reported disclosures with Denali Therapeutics, the National Institutes of Health, Genzyme Sanofi, and the Bigglesworth Family Foundation.
This trial supports current clinical practice in two ways, according to Susan Bressman, MD, and Rachel Saunders‑Pullman, MD, MPH. On one hand, the study provides no evidence to suggest that levodopa slows Parkinson’s disease progression, Dr. Bressman and Dr. Saunders-Pullman wrote in an editorial accompanying the study. On the other hand, they added, it provides no evidence that clinicians should delay therapy when it is clinically indicated.
The LEAP trial (Levodopa in Early Parkinson’s Disease) was designed to resolve uncertainty over the potential effects of levodopa on disease progression, they noted. This was necessary because of the results of the placebo-controlled ELLDOPA trial, which was published about 14 years ago and suggested that patients randomized to 40 weeks of levodopa did not deteriorate clinically to the degree that was observed in patients randomized to placebo.
The primary end point of that trial was Unified Parkinson’s Disease Rating Scale (UPDRS) scores after a 2-week washout period.
While one interpretation of the UPDRS results from ELLDOPA was that levodopa slowed disease progression, another was that the 2-week washout period was too short, allowing for residual effects of levodopa on symptoms, suggested Dr. Bressman and Dr. Saunders-Pullman.
The randomized LEAP study now shows not only that there were no differences in UPDRS scores when using a delayed start trial design – which implies that there was no disease-modifying effect – but also that starting levodopa early did not have negative effects, the editorial authors wrote.
In particular, the researchers showed no differences in rates of dyskinesia or levodopa-related fluctuations in those started early versus those started later.
“The results of the current trial, taken together with those of other trials, support treatment that is guided by clinical need and that uses the lowest dose that provides a satisfactory clinical effect,” wrote the editorial’s authors.
Dr. Bressman and Dr. Saunders‑Pullman are with the Icahn School of Medicine at Mt. Sinai, New York. Their editorial appears in the New England Journal of Medicine (2019;380:389-90). Dr. Bressman reported disclosures related to Denali Therapeutics, the Michael J. Fox Foundation, and Prevail Therapeutics, while Dr. Saunders-Pullman reported disclosures with Denali Therapeutics, the National Institutes of Health, Genzyme Sanofi, and the Bigglesworth Family Foundation.
This trial supports current clinical practice in two ways, according to Susan Bressman, MD, and Rachel Saunders‑Pullman, MD, MPH. On one hand, the study provides no evidence to suggest that levodopa slows Parkinson’s disease progression, Dr. Bressman and Dr. Saunders-Pullman wrote in an editorial accompanying the study. On the other hand, they added, it provides no evidence that clinicians should delay therapy when it is clinically indicated.
The LEAP trial (Levodopa in Early Parkinson’s Disease) was designed to resolve uncertainty over the potential effects of levodopa on disease progression, they noted. This was necessary because of the results of the placebo-controlled ELLDOPA trial, which was published about 14 years ago and suggested that patients randomized to 40 weeks of levodopa did not deteriorate clinically to the degree that was observed in patients randomized to placebo.
The primary end point of that trial was Unified Parkinson’s Disease Rating Scale (UPDRS) scores after a 2-week washout period.
While one interpretation of the UPDRS results from ELLDOPA was that levodopa slowed disease progression, another was that the 2-week washout period was too short, allowing for residual effects of levodopa on symptoms, suggested Dr. Bressman and Dr. Saunders-Pullman.
The randomized LEAP study now shows not only that there were no differences in UPDRS scores when using a delayed start trial design – which implies that there was no disease-modifying effect – but also that starting levodopa early did not have negative effects, the editorial authors wrote.
In particular, the researchers showed no differences in rates of dyskinesia or levodopa-related fluctuations in those started early versus those started later.
“The results of the current trial, taken together with those of other trials, support treatment that is guided by clinical need and that uses the lowest dose that provides a satisfactory clinical effect,” wrote the editorial’s authors.
Dr. Bressman and Dr. Saunders‑Pullman are with the Icahn School of Medicine at Mt. Sinai, New York. Their editorial appears in the New England Journal of Medicine (2019;380:389-90). Dr. Bressman reported disclosures related to Denali Therapeutics, the Michael J. Fox Foundation, and Prevail Therapeutics, while Dr. Saunders-Pullman reported disclosures with Denali Therapeutics, the National Institutes of Health, Genzyme Sanofi, and the Bigglesworth Family Foundation.
investigators reported. The disease course was not significantly different for patients who had a full 80 weeks of levodopa/carbodopa therapy, compared with that seen with those who started treatment after a 40-week delay, according to the investigators.
“These findings imply that levodopa had no disease-modifying effect on Parkinson’s disease over the period of the trial,” wrote investigator Rob M. A. de Bie, MD, PhD, professor of movement disorders at the University of Amsterdam, and his colleagues in the New England Journal of Medicine.
By contrast, results of an earlier randomized, placebo-controlled trial suggested that levodopa had disease-modifying effects, though the findings of that study were inconclusive, according to authors of an editorial (see Views on the News).
In the current multicenter trial, known as LEAP (Levodopa in Early Parkinson’s Disease) a total of 445 patients with early Parkinson’s disease were randomized to either 80 weeks of levodopa and carbodopa or to 40 weeks of placebo followed by 40 weeks of levodopa/carbodopa.
Levodopa was dosed at 100 mg three times per day, and carbodopa at 25 mg three times per day, according to the report.
There was no significant difference between the early and delayed treatment groups for primary outcome of the trial, which was change in the Unified Parkinson’s Disease Rating Scale (UPDRS) from baseline to week 80.
The mean change in UPDRS was –1.0 in the group of patients who had the full 80 weeks of levodopa/carbodopa and –2.0 for those who had delayed therapy, for a difference of 1 point (P = .44). Higher scores on the UPDRS signify worse disease.
At week 40, there was a change in UPDRS favoring the early-initiation strategy, which reflected the effects of levodopa on disease symptoms, investigators added.
Nausea was more common in the early-start group during the first 40 weeks of the trial. However, there were no differences between groups in other adverse events of particular interest, including dyskinesias and motor fluctuations related to levodopa, Dr. de Bie and his colleagues reported.
Taken together, these results suggest no beneficial or detrimental disease-modifying effect for an early treatment strategy, although further trials are warranted to evaluate other strategies, such as higher levodopa doses, longer administration, or starting the drug at later stages of disease, they wrote.
Dr. de Bie reported grants from ZonMw, Parkinson Vereniging, and Stichting Parkinsonfonds during the conduct of the study, as well as grants from GE Health and Medtronic outside the submitted work. Study authors provided disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
SOURCE: Verschuur CVM et al. N Engl J Med. 2019;380:315-24.
investigators reported. The disease course was not significantly different for patients who had a full 80 weeks of levodopa/carbodopa therapy, compared with that seen with those who started treatment after a 40-week delay, according to the investigators.
“These findings imply that levodopa had no disease-modifying effect on Parkinson’s disease over the period of the trial,” wrote investigator Rob M. A. de Bie, MD, PhD, professor of movement disorders at the University of Amsterdam, and his colleagues in the New England Journal of Medicine.
By contrast, results of an earlier randomized, placebo-controlled trial suggested that levodopa had disease-modifying effects, though the findings of that study were inconclusive, according to authors of an editorial (see Views on the News).
In the current multicenter trial, known as LEAP (Levodopa in Early Parkinson’s Disease) a total of 445 patients with early Parkinson’s disease were randomized to either 80 weeks of levodopa and carbodopa or to 40 weeks of placebo followed by 40 weeks of levodopa/carbodopa.
Levodopa was dosed at 100 mg three times per day, and carbodopa at 25 mg three times per day, according to the report.
There was no significant difference between the early and delayed treatment groups for primary outcome of the trial, which was change in the Unified Parkinson’s Disease Rating Scale (UPDRS) from baseline to week 80.
The mean change in UPDRS was –1.0 in the group of patients who had the full 80 weeks of levodopa/carbodopa and –2.0 for those who had delayed therapy, for a difference of 1 point (P = .44). Higher scores on the UPDRS signify worse disease.
At week 40, there was a change in UPDRS favoring the early-initiation strategy, which reflected the effects of levodopa on disease symptoms, investigators added.
Nausea was more common in the early-start group during the first 40 weeks of the trial. However, there were no differences between groups in other adverse events of particular interest, including dyskinesias and motor fluctuations related to levodopa, Dr. de Bie and his colleagues reported.
Taken together, these results suggest no beneficial or detrimental disease-modifying effect for an early treatment strategy, although further trials are warranted to evaluate other strategies, such as higher levodopa doses, longer administration, or starting the drug at later stages of disease, they wrote.
Dr. de Bie reported grants from ZonMw, Parkinson Vereniging, and Stichting Parkinsonfonds during the conduct of the study, as well as grants from GE Health and Medtronic outside the submitted work. Study authors provided disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
SOURCE: Verschuur CVM et al. N Engl J Med. 2019;380:315-24.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Levodopa did not have any significant disease-modifying effects in patients with early Parkinson’s disease.
Major finding: Change in the Unified Parkinson’s Disease Rating Scale (UPDRS) was –1.0 after 80 weeks of levodopa/carbodopa versus –2.0 for 40 weeks of placebo followed by 40 weeks of treatment (P = .44).
Study details: A delayed-start trial including 445 patients with early Parkinson’s disease randomized to 80 weeks of treatment or 40 weeks of placebo plus 40 weeks of treatment.
Disclosures: Study authors reported disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
Source: Verschuur CVM et al. N Engl J Med. 2019;380:315-324.
Study hints at lacosamide’s efficacy for small fiber neuropathy
As a treatment for small fiber neuropathy (SFN), lacosamide decreased pain and had a positive effect on sleep quality with minimal adverse events in patients with mutations in the gene SCN9A that encodes the voltage-gated sodium channel Nav1.7, according to a randomized, placebo-controlled, double-blind, crossover-design study published in Brain.
“This is the first study that investigated the efficacy of lacosamide [Vimpat] in patients with SFN,” wrote lead author Bianca T.A. de Greef, MD, of Maastricht University Medical Center, the Netherlands, and her coauthors. “Compared with placebo, lacosamide appeared to be safe to use and well tolerated in this cohort of patients.”
Lacosamide, which is approved in the United States to treat partial-onset seizures in people aged 4 years and older, has been shown to bind to and inhibit Nav1.7.
The investigators randomized 25 Dutch patients with Nav1.7-related SFN into the Lacosamide-Efficacy-’N’-Safety in SFN (LENSS) study to receive lacosamide followed by placebo, or vice versa. The patients were recruited between November 2014 and July 2016; 1 patient dropped out before treatment and another after the first treatment period, leaving 24 patients who received lacosamide and 23 patients who received placebo. They went through a 3-week titration period, an 8-week treatment period, a 2-week tapering period, and a washout period of at least 2 weeks, after which they switched to the other treatment arm and repeated the same schedule.
Through the daily pain intensity numerical rating scale and the daily sleep interference scale (DSIS), among other questionnaires, the investigators sought to determine if lacosamide reduced pain and thereby improved sleep quality. Lacosamide treatment led to a decrease in mean average pain by at least 1 point in 50.0% of patients, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213). In addition, 25.0% of the lacosamide group reported at least a 2-point decrease in mean average pain versus 8.7% in the placebo group. There was also a notable difference in pain’s impact on sleep quality between the two, with the lacosamide period seeing a DSIS median value of 5.3, compared with 5.7 for the placebo period.
According to the patients’ global impression of change questionnaire, 33.3% felt better while using lacosamide versus 4.3% who felt better while using placebo (P = .0156). Six serious adverse events occurred during the study, though only two occurred during the lacosamide period. The most common adverse events for patients taking lacosamide included dizziness, headache, and nausea, all of which were comparable with adverse events in patients taking placebo.
Dr. de Greef and her colleagues noted the study’s potential limitations, including a carryover effect that could have confounded direct treatment effects (which they attempted to mitigate via a lengthier washout period) and a small cohort that was limited to very specific patients. However, the authors chose this particular cohort because “our aim was to demonstrate proof of-concept, which can be used for future studies involving larger groups of patients diagnosed with SFN.” They observed that their response rates were slightly lower than expected, but they noted that “lacosamide appears to be as effective as currently available neuropathic pain treatment.”
The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
SOURCE: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
As a treatment for small fiber neuropathy (SFN), lacosamide decreased pain and had a positive effect on sleep quality with minimal adverse events in patients with mutations in the gene SCN9A that encodes the voltage-gated sodium channel Nav1.7, according to a randomized, placebo-controlled, double-blind, crossover-design study published in Brain.
“This is the first study that investigated the efficacy of lacosamide [Vimpat] in patients with SFN,” wrote lead author Bianca T.A. de Greef, MD, of Maastricht University Medical Center, the Netherlands, and her coauthors. “Compared with placebo, lacosamide appeared to be safe to use and well tolerated in this cohort of patients.”
Lacosamide, which is approved in the United States to treat partial-onset seizures in people aged 4 years and older, has been shown to bind to and inhibit Nav1.7.
The investigators randomized 25 Dutch patients with Nav1.7-related SFN into the Lacosamide-Efficacy-’N’-Safety in SFN (LENSS) study to receive lacosamide followed by placebo, or vice versa. The patients were recruited between November 2014 and July 2016; 1 patient dropped out before treatment and another after the first treatment period, leaving 24 patients who received lacosamide and 23 patients who received placebo. They went through a 3-week titration period, an 8-week treatment period, a 2-week tapering period, and a washout period of at least 2 weeks, after which they switched to the other treatment arm and repeated the same schedule.
Through the daily pain intensity numerical rating scale and the daily sleep interference scale (DSIS), among other questionnaires, the investigators sought to determine if lacosamide reduced pain and thereby improved sleep quality. Lacosamide treatment led to a decrease in mean average pain by at least 1 point in 50.0% of patients, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213). In addition, 25.0% of the lacosamide group reported at least a 2-point decrease in mean average pain versus 8.7% in the placebo group. There was also a notable difference in pain’s impact on sleep quality between the two, with the lacosamide period seeing a DSIS median value of 5.3, compared with 5.7 for the placebo period.
According to the patients’ global impression of change questionnaire, 33.3% felt better while using lacosamide versus 4.3% who felt better while using placebo (P = .0156). Six serious adverse events occurred during the study, though only two occurred during the lacosamide period. The most common adverse events for patients taking lacosamide included dizziness, headache, and nausea, all of which were comparable with adverse events in patients taking placebo.
Dr. de Greef and her colleagues noted the study’s potential limitations, including a carryover effect that could have confounded direct treatment effects (which they attempted to mitigate via a lengthier washout period) and a small cohort that was limited to very specific patients. However, the authors chose this particular cohort because “our aim was to demonstrate proof of-concept, which can be used for future studies involving larger groups of patients diagnosed with SFN.” They observed that their response rates were slightly lower than expected, but they noted that “lacosamide appears to be as effective as currently available neuropathic pain treatment.”
The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
SOURCE: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
As a treatment for small fiber neuropathy (SFN), lacosamide decreased pain and had a positive effect on sleep quality with minimal adverse events in patients with mutations in the gene SCN9A that encodes the voltage-gated sodium channel Nav1.7, according to a randomized, placebo-controlled, double-blind, crossover-design study published in Brain.
“This is the first study that investigated the efficacy of lacosamide [Vimpat] in patients with SFN,” wrote lead author Bianca T.A. de Greef, MD, of Maastricht University Medical Center, the Netherlands, and her coauthors. “Compared with placebo, lacosamide appeared to be safe to use and well tolerated in this cohort of patients.”
Lacosamide, which is approved in the United States to treat partial-onset seizures in people aged 4 years and older, has been shown to bind to and inhibit Nav1.7.
The investigators randomized 25 Dutch patients with Nav1.7-related SFN into the Lacosamide-Efficacy-’N’-Safety in SFN (LENSS) study to receive lacosamide followed by placebo, or vice versa. The patients were recruited between November 2014 and July 2016; 1 patient dropped out before treatment and another after the first treatment period, leaving 24 patients who received lacosamide and 23 patients who received placebo. They went through a 3-week titration period, an 8-week treatment period, a 2-week tapering period, and a washout period of at least 2 weeks, after which they switched to the other treatment arm and repeated the same schedule.
Through the daily pain intensity numerical rating scale and the daily sleep interference scale (DSIS), among other questionnaires, the investigators sought to determine if lacosamide reduced pain and thereby improved sleep quality. Lacosamide treatment led to a decrease in mean average pain by at least 1 point in 50.0% of patients, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213). In addition, 25.0% of the lacosamide group reported at least a 2-point decrease in mean average pain versus 8.7% in the placebo group. There was also a notable difference in pain’s impact on sleep quality between the two, with the lacosamide period seeing a DSIS median value of 5.3, compared with 5.7 for the placebo period.
According to the patients’ global impression of change questionnaire, 33.3% felt better while using lacosamide versus 4.3% who felt better while using placebo (P = .0156). Six serious adverse events occurred during the study, though only two occurred during the lacosamide period. The most common adverse events for patients taking lacosamide included dizziness, headache, and nausea, all of which were comparable with adverse events in patients taking placebo.
Dr. de Greef and her colleagues noted the study’s potential limitations, including a carryover effect that could have confounded direct treatment effects (which they attempted to mitigate via a lengthier washout period) and a small cohort that was limited to very specific patients. However, the authors chose this particular cohort because “our aim was to demonstrate proof of-concept, which can be used for future studies involving larger groups of patients diagnosed with SFN.” They observed that their response rates were slightly lower than expected, but they noted that “lacosamide appears to be as effective as currently available neuropathic pain treatment.”
The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
SOURCE: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
FROM BRAIN
Key clinical point:
Major finding: In the lacosamide group, 50.0% of patients reported mean average pain decreasing by at least 1 point, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213).
Study details: A randomized, placebo-controlled, double-blind, crossover-design study of 25 patients with Nav1.7-related small fiber neuropathy who received lacosamide followed by placebo, or vice versa.
Disclosures: The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
Source: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
Frailty may affect the expression of dementia
according to research published online ahead of print Jan. 17 in Lancet Neurology. Data suggest that frailty reduces the threshold for Alzheimer’s disease pathology to cause cognitive decline. Frailty also may contribute to other mechanisms that cause dementia, such as inflammation and immunosenescence, said the investigators.
“While more research is needed, given that frailty is potentially reversible, it is possible that helping people to maintain function and independence in later life could reduce both dementia risk and the severity of debilitating symptoms common in this disease,” said Professor Kenneth Rockwood, MD, of the Nova Scotia Health Authority and Dalhousie University in Halifax, N.S., in a press release.
More susceptible to dementia?
The presence of amyloid plaques and neurofibrillary tangles is not a sufficient condition for the clinical expression of dementia. Some patients with a high degree of Alzheimer’s disease pathology have no apparent cognitive decline. Other factors therefore may modify the relationship between pathology and dementia.
Most people who develop Alzheimer’s disease dementia are older than 65 years, and many of these patients are frail. Frailty is understood as a decreased physiologic reserve and an increased risk for adverse health outcomes. Dr. Rockwood and his colleagues hypothesized that frailty moderates the clinical expression of dementia in relation to Alzheimer’s disease pathology.
To test their hypothesis, the investigators performed a cross-sectional analysis of data from the Rush Memory and Aging Project, which collects clinical and pathologic data from adults older than 59 years without dementia at baseline who live in Illinois. Since 1997, participants have undergone annual clinical and neuropsychological evaluations, and the cohort has been followed for 21 years. For their analysis, Dr. Rockwood and his colleagues included participants without dementia or with Alzheimer’s dementia at their last clinical assessment. Eligible participants had died, and complete autopsy data were available for them.
The researchers measured Alzheimer’s disease pathology using a summary measure of neurofibrillary tangles and neuritic and diffuse plaques. Clinical diagnoses of Alzheimer’s dementia were based on clinician consensus. Dr. Rockwood and his colleagues retrospectively created a 41-item frailty index from variables (e.g., symptoms, signs, comorbidities, and function) that were obtained at each clinical evaluation.
Logistic regression and moderation modeling allowed the investigators to evaluate relationships between Alzheimer’s disease pathology, frailty, and Alzheimer’s dementia. Dr. Rockwood and hus colleagues adjusted all analyses for age, sex, and education.
In all, 456 participants were included in the analysis. The sample’s mean age at death was 89.7 years, and 69% of participants were women. At participants’ last clinical assessment, 242 (53%) had possible or probable Alzheimer’s dementia.
The sample’s mean frailty index was 0.42. The median frailty index was 0.41, a value similar to the threshold commonly used to distinguish between moderate and severe frailty. People with high frailty index scores (i.e., 0.41 or greater) were older, had lower Mini-Mental State Examination scores, were more likely to have a diagnosis of dementia, and had a higher Braak stage than those with moderate or low frailty index scores.
Significant interaction between frailty and Alzheimer’s disease
After the investigators adjusted for age, sex, and education, frailty (odds ratio, 1.76) and Alzheimer’s disease pathology (OR, 4.81) were independently associated with Alzheimer’s dementia. When the investigators added frailty to the model for the relationship between Alzheimer’s disease pathology and Alzheimer’s dementia, the model fit improved. They found a significant interaction between frailty and Alzheimer’s disease pathology (OR, 0.73). People with a low amount of frailty were better able to tolerate Alzheimer’s disease pathology, and people with higher amounts of frailty were more likely to have more Alzheimer’s disease pathology and clinical dementia.
One of the study’s limitations is that it is a secondary analysis, according to Dr. Rockwood and his colleagues. In addition, frailty was measured close to participants’ time of death, and the measurements may thus reflect terminal decline. Participant deaths resulting from causes other than those related to dementia might have confounded the results. Finally, the sample came entirely from people living in retirement homes in Illinois, which might have introduced bias. Future research should use a population-based sample, said the authors.
Frailty could be a basis for risk stratification and could inform the management and treatment of older adults, said Dr. Rockwood and his colleagues. The study results have “the potential to improve our understanding of disease expression, explain failures in pharmacologic treatment, and aid in the development of more appropriate therapeutic targets, approaches, and measurements of success,” they concluded.
The study had no source of funding. The authors reported receiving fees and grants from DGI Clinical, GlaxoSmithKline, Pfizer, and Sanofi. Authors also received support from governmental bodies such as the National Institutes of Health and the Canadian Institutes of Health Research.
SOURCE: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
The results of the study by Rockwood and colleagues confirm the strong links between frailty and Alzheimer’s disease and other dementias, said Francesco Panza, MD, PhD, of the University of Bari (Italy) Aldo Moro, and his colleagues in an accompanying editorial.
Frailty is primary or preclinical when it is not directly associated with a specific disease or when the patient has no substantial disability. Frailty is considered secondary or clinical when it is associated with known comorbidities (e.g., cardiovascular disease or depression). “This distinction is central in identifying frailty phenotypes with the potential to predict and prevent dementia, using novel models of risk that introduce modifiable factors,” wrote Dr. Panza and his colleagues.
“In light of current knowledge on the cognitive frailty phenotype, secondary preventive strategies for cognitive impairment and physical frailty can be suggested,” they added. “For instance, individualized multidomain interventions can target physical, nutritional, cognitive, and psychological domains that might delay the progression to overt dementia and secondary occurrence of adverse health-related outcomes, such as disability, hospitalization, and mortality.”
Dr. Panza, Madia Lozupone, MD, PhD , and Giancarlo Logroscino, MD, PhD , are affiliated with the neurodegenerative disease unit in the department of basic medicine, neuroscience, and sense organs at the University of Bari (Italy) Aldo Moro. The above remarks come from an editorial that these authors wrote to accompany the study by Rockwood et al. The authors declared no competing interests.
The results of the study by Rockwood and colleagues confirm the strong links between frailty and Alzheimer’s disease and other dementias, said Francesco Panza, MD, PhD, of the University of Bari (Italy) Aldo Moro, and his colleagues in an accompanying editorial.
Frailty is primary or preclinical when it is not directly associated with a specific disease or when the patient has no substantial disability. Frailty is considered secondary or clinical when it is associated with known comorbidities (e.g., cardiovascular disease or depression). “This distinction is central in identifying frailty phenotypes with the potential to predict and prevent dementia, using novel models of risk that introduce modifiable factors,” wrote Dr. Panza and his colleagues.
“In light of current knowledge on the cognitive frailty phenotype, secondary preventive strategies for cognitive impairment and physical frailty can be suggested,” they added. “For instance, individualized multidomain interventions can target physical, nutritional, cognitive, and psychological domains that might delay the progression to overt dementia and secondary occurrence of adverse health-related outcomes, such as disability, hospitalization, and mortality.”
Dr. Panza, Madia Lozupone, MD, PhD , and Giancarlo Logroscino, MD, PhD , are affiliated with the neurodegenerative disease unit in the department of basic medicine, neuroscience, and sense organs at the University of Bari (Italy) Aldo Moro. The above remarks come from an editorial that these authors wrote to accompany the study by Rockwood et al. The authors declared no competing interests.
The results of the study by Rockwood and colleagues confirm the strong links between frailty and Alzheimer’s disease and other dementias, said Francesco Panza, MD, PhD, of the University of Bari (Italy) Aldo Moro, and his colleagues in an accompanying editorial.
Frailty is primary or preclinical when it is not directly associated with a specific disease or when the patient has no substantial disability. Frailty is considered secondary or clinical when it is associated with known comorbidities (e.g., cardiovascular disease or depression). “This distinction is central in identifying frailty phenotypes with the potential to predict and prevent dementia, using novel models of risk that introduce modifiable factors,” wrote Dr. Panza and his colleagues.
“In light of current knowledge on the cognitive frailty phenotype, secondary preventive strategies for cognitive impairment and physical frailty can be suggested,” they added. “For instance, individualized multidomain interventions can target physical, nutritional, cognitive, and psychological domains that might delay the progression to overt dementia and secondary occurrence of adverse health-related outcomes, such as disability, hospitalization, and mortality.”
Dr. Panza, Madia Lozupone, MD, PhD , and Giancarlo Logroscino, MD, PhD , are affiliated with the neurodegenerative disease unit in the department of basic medicine, neuroscience, and sense organs at the University of Bari (Italy) Aldo Moro. The above remarks come from an editorial that these authors wrote to accompany the study by Rockwood et al. The authors declared no competing interests.
according to research published online ahead of print Jan. 17 in Lancet Neurology. Data suggest that frailty reduces the threshold for Alzheimer’s disease pathology to cause cognitive decline. Frailty also may contribute to other mechanisms that cause dementia, such as inflammation and immunosenescence, said the investigators.
“While more research is needed, given that frailty is potentially reversible, it is possible that helping people to maintain function and independence in later life could reduce both dementia risk and the severity of debilitating symptoms common in this disease,” said Professor Kenneth Rockwood, MD, of the Nova Scotia Health Authority and Dalhousie University in Halifax, N.S., in a press release.
More susceptible to dementia?
The presence of amyloid plaques and neurofibrillary tangles is not a sufficient condition for the clinical expression of dementia. Some patients with a high degree of Alzheimer’s disease pathology have no apparent cognitive decline. Other factors therefore may modify the relationship between pathology and dementia.
Most people who develop Alzheimer’s disease dementia are older than 65 years, and many of these patients are frail. Frailty is understood as a decreased physiologic reserve and an increased risk for adverse health outcomes. Dr. Rockwood and his colleagues hypothesized that frailty moderates the clinical expression of dementia in relation to Alzheimer’s disease pathology.
To test their hypothesis, the investigators performed a cross-sectional analysis of data from the Rush Memory and Aging Project, which collects clinical and pathologic data from adults older than 59 years without dementia at baseline who live in Illinois. Since 1997, participants have undergone annual clinical and neuropsychological evaluations, and the cohort has been followed for 21 years. For their analysis, Dr. Rockwood and his colleagues included participants without dementia or with Alzheimer’s dementia at their last clinical assessment. Eligible participants had died, and complete autopsy data were available for them.
The researchers measured Alzheimer’s disease pathology using a summary measure of neurofibrillary tangles and neuritic and diffuse plaques. Clinical diagnoses of Alzheimer’s dementia were based on clinician consensus. Dr. Rockwood and his colleagues retrospectively created a 41-item frailty index from variables (e.g., symptoms, signs, comorbidities, and function) that were obtained at each clinical evaluation.
Logistic regression and moderation modeling allowed the investigators to evaluate relationships between Alzheimer’s disease pathology, frailty, and Alzheimer’s dementia. Dr. Rockwood and hus colleagues adjusted all analyses for age, sex, and education.
In all, 456 participants were included in the analysis. The sample’s mean age at death was 89.7 years, and 69% of participants were women. At participants’ last clinical assessment, 242 (53%) had possible or probable Alzheimer’s dementia.
The sample’s mean frailty index was 0.42. The median frailty index was 0.41, a value similar to the threshold commonly used to distinguish between moderate and severe frailty. People with high frailty index scores (i.e., 0.41 or greater) were older, had lower Mini-Mental State Examination scores, were more likely to have a diagnosis of dementia, and had a higher Braak stage than those with moderate or low frailty index scores.
Significant interaction between frailty and Alzheimer’s disease
After the investigators adjusted for age, sex, and education, frailty (odds ratio, 1.76) and Alzheimer’s disease pathology (OR, 4.81) were independently associated with Alzheimer’s dementia. When the investigators added frailty to the model for the relationship between Alzheimer’s disease pathology and Alzheimer’s dementia, the model fit improved. They found a significant interaction between frailty and Alzheimer’s disease pathology (OR, 0.73). People with a low amount of frailty were better able to tolerate Alzheimer’s disease pathology, and people with higher amounts of frailty were more likely to have more Alzheimer’s disease pathology and clinical dementia.
One of the study’s limitations is that it is a secondary analysis, according to Dr. Rockwood and his colleagues. In addition, frailty was measured close to participants’ time of death, and the measurements may thus reflect terminal decline. Participant deaths resulting from causes other than those related to dementia might have confounded the results. Finally, the sample came entirely from people living in retirement homes in Illinois, which might have introduced bias. Future research should use a population-based sample, said the authors.
Frailty could be a basis for risk stratification and could inform the management and treatment of older adults, said Dr. Rockwood and his colleagues. The study results have “the potential to improve our understanding of disease expression, explain failures in pharmacologic treatment, and aid in the development of more appropriate therapeutic targets, approaches, and measurements of success,” they concluded.
The study had no source of funding. The authors reported receiving fees and grants from DGI Clinical, GlaxoSmithKline, Pfizer, and Sanofi. Authors also received support from governmental bodies such as the National Institutes of Health and the Canadian Institutes of Health Research.
SOURCE: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
according to research published online ahead of print Jan. 17 in Lancet Neurology. Data suggest that frailty reduces the threshold for Alzheimer’s disease pathology to cause cognitive decline. Frailty also may contribute to other mechanisms that cause dementia, such as inflammation and immunosenescence, said the investigators.
“While more research is needed, given that frailty is potentially reversible, it is possible that helping people to maintain function and independence in later life could reduce both dementia risk and the severity of debilitating symptoms common in this disease,” said Professor Kenneth Rockwood, MD, of the Nova Scotia Health Authority and Dalhousie University in Halifax, N.S., in a press release.
More susceptible to dementia?
The presence of amyloid plaques and neurofibrillary tangles is not a sufficient condition for the clinical expression of dementia. Some patients with a high degree of Alzheimer’s disease pathology have no apparent cognitive decline. Other factors therefore may modify the relationship between pathology and dementia.
Most people who develop Alzheimer’s disease dementia are older than 65 years, and many of these patients are frail. Frailty is understood as a decreased physiologic reserve and an increased risk for adverse health outcomes. Dr. Rockwood and his colleagues hypothesized that frailty moderates the clinical expression of dementia in relation to Alzheimer’s disease pathology.
To test their hypothesis, the investigators performed a cross-sectional analysis of data from the Rush Memory and Aging Project, which collects clinical and pathologic data from adults older than 59 years without dementia at baseline who live in Illinois. Since 1997, participants have undergone annual clinical and neuropsychological evaluations, and the cohort has been followed for 21 years. For their analysis, Dr. Rockwood and his colleagues included participants without dementia or with Alzheimer’s dementia at their last clinical assessment. Eligible participants had died, and complete autopsy data were available for them.
The researchers measured Alzheimer’s disease pathology using a summary measure of neurofibrillary tangles and neuritic and diffuse plaques. Clinical diagnoses of Alzheimer’s dementia were based on clinician consensus. Dr. Rockwood and his colleagues retrospectively created a 41-item frailty index from variables (e.g., symptoms, signs, comorbidities, and function) that were obtained at each clinical evaluation.
Logistic regression and moderation modeling allowed the investigators to evaluate relationships between Alzheimer’s disease pathology, frailty, and Alzheimer’s dementia. Dr. Rockwood and hus colleagues adjusted all analyses for age, sex, and education.
In all, 456 participants were included in the analysis. The sample’s mean age at death was 89.7 years, and 69% of participants were women. At participants’ last clinical assessment, 242 (53%) had possible or probable Alzheimer’s dementia.
The sample’s mean frailty index was 0.42. The median frailty index was 0.41, a value similar to the threshold commonly used to distinguish between moderate and severe frailty. People with high frailty index scores (i.e., 0.41 or greater) were older, had lower Mini-Mental State Examination scores, were more likely to have a diagnosis of dementia, and had a higher Braak stage than those with moderate or low frailty index scores.
Significant interaction between frailty and Alzheimer’s disease
After the investigators adjusted for age, sex, and education, frailty (odds ratio, 1.76) and Alzheimer’s disease pathology (OR, 4.81) were independently associated with Alzheimer’s dementia. When the investigators added frailty to the model for the relationship between Alzheimer’s disease pathology and Alzheimer’s dementia, the model fit improved. They found a significant interaction between frailty and Alzheimer’s disease pathology (OR, 0.73). People with a low amount of frailty were better able to tolerate Alzheimer’s disease pathology, and people with higher amounts of frailty were more likely to have more Alzheimer’s disease pathology and clinical dementia.
One of the study’s limitations is that it is a secondary analysis, according to Dr. Rockwood and his colleagues. In addition, frailty was measured close to participants’ time of death, and the measurements may thus reflect terminal decline. Participant deaths resulting from causes other than those related to dementia might have confounded the results. Finally, the sample came entirely from people living in retirement homes in Illinois, which might have introduced bias. Future research should use a population-based sample, said the authors.
Frailty could be a basis for risk stratification and could inform the management and treatment of older adults, said Dr. Rockwood and his colleagues. The study results have “the potential to improve our understanding of disease expression, explain failures in pharmacologic treatment, and aid in the development of more appropriate therapeutic targets, approaches, and measurements of success,” they concluded.
The study had no source of funding. The authors reported receiving fees and grants from DGI Clinical, GlaxoSmithKline, Pfizer, and Sanofi. Authors also received support from governmental bodies such as the National Institutes of Health and the Canadian Institutes of Health Research.
SOURCE: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
FROM LANCET NEUROLOGY
Key clinical point: Frailty modifies the association between Alzheimer’s disease pathology and Alzheimer dementia.
Major finding: Frailty index score (odds ratio, 1.76) is independently associated with dementia status.
Study details: A cross-sectional analysis of 456 deceased participants in the Rush Memory and Aging Project.
Disclosures: The study had no outside funding.
Source: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
DMTs, stem cell transplants both reduce disease progression in MS
Disease-modifying therapies give patients with relapsing-remitting multiple sclerosis a lower risk of developing secondary progressive disease that may only be topped in specific patients with highly active disease by the use of nonmyeloablative hematopoietic stem cell transplantation, according to findings from two studies published online Jan. 15 in JAMA.
The first study found that interferon-beta, glatiramer acetate (Copaxone), fingolimod (Gilenya), natalizumab (Tysabri), and alemtuzumab (Lemtrada) are associated with a lower risk of conversion to secondary progressive MS, compared with no treatment. Initial treatment with the newer therapies provided a greater risk reduction, compared with initial treatment with interferon-beta or glatiramer acetate.
The second study, described as “the first randomized trial of HSCT [nonmyeloablative hematopoietic stem cell transplantation] in patients with relapsing-remitting MS,” suggests that HSCT prolongs the time to disease progression, compared with disease-modifying therapies (DMTs). It also suggests that HSCT can lead to clinical improvement.
DMTs reduced risk of conversion to secondary progressive MS
Few previous studies have examined the association between DMTs and the risk of conversion from relapsing-remitting MS to secondary progressive MS. Those that have analyzed this association have not used a validated definition of secondary progressive MS. J. William L. Brown, MD, of the University of Cambridge, England, and his colleagues used a validated definition of secondary progressive MS that was published in 2016 to investigate how DMTs affect the rate of conversion, compared with no treatment. The researchers also compared the risk reduction provided by fingolimod, alemtuzumab, or natalizumab with that provided by interferon-beta or glatiramer acetate.
Dr. Brown and his colleagues analyzed prospectively collected clinical data from an international observational cohort study called MSBase. Eligible participants had relapsing-remitting MS, the complete MSBase minimum data set, at least one Expanded Disability Status Scale (EDSS) score recorded within 6 months before baseline, and at least two EDSS scores recorded after baseline. Participants initiated a DMT or began clinical monitoring during 1988-2012. The population had a minimum follow-up duration of 4 years. Patients who stopped their initial therapy within 6 months and those participating in clinical trials were excluded.
The primary outcome was conversion to secondary progressive MS. Dr. Brown and his colleagues defined this outcome as an EDSS increase of 1 point for participants with a baseline EDSS score of 5.5 or less and as an increase of 0.5 points for participants with a baseline EDSS score higher than 5.5. This increase had to occur in the absence of relapses and be confirmed at a subsequent visit 3 or fewer months later. In addition, the increased EDSS score had to be 4 or more.
After excluding ineligible participants, the investigators matched 1,555 patients from 68 centers in 21 countries. Each therapy analyzed was associated with reduced risk of converting to secondary progressive MS, compared with no treatment. The hazard ratios for conversion were 0.71 for interferon-beta or glatiramer acetate, 0.37 for fingolimod, 0.61 for natalizumab, and 0.52 for alemtuzumab, compared with no treatment.
Treatment with interferon-beta or glatiramer acetate within 5 years of disease onset was associated with a reduced risk of conversion (HR, 0.77), compared with treatment later than 5 years after disease onset. Similarly, patients who escalated treatment from interferon-beta or glatiramer acetate to any of the other three DMTs within 5 years of disease onset had a significantly lower risk of conversion (HR, 0.76) than did those who escalated later. Furthermore, initial treatment with fingolimod, alemtuzumab, or natalizumab was associated with a significantly reduced risk of conversion (HR, 0.66), compared with initial treatment with interferon-beta or glatiramer acetate.
One of the study’s limitations is its observational design, which precludes the determination of causality, Dr. Brown and his colleagues said. In addition, functional score subcomponents of the EDSS were unavailable, which prevented the researchers from using the definition of secondary progressive MS with the best combination of sensitivity, specificity, and accuracy. Some analyses were limited by small numbers of patients, and the study did not evaluate the risks associated with DMTs. Nevertheless, “these findings, considered along with these therapies’ risks, may help inform decisions about DMT selection,” the authors concluded.
Financial support for this study was provided by the National Health and Medical Research Council of Australia and the University of Melbourne. Dr. Brown received a Next Generation Fellowship funded by the Grand Charity of the Freemasons and an MSBase 2017 Fellowship. Alemtuzumab studies conducted in Cambridge were supported by the National Institute for Health Research Cambridge Biomedical Research Centre and the MS Society UK.
HSCT delayed disease progression
In a previous case series, Richard K. Burt, MD, of Northwestern University in Chicago, and his colleagues found that patients with relapsing-remitting MS who underwent nonmyeloablative HSCT had neurologic improvement and a 70% likelihood of having a 4-year period of disease remission. Dr. Burt and his colleagues undertook the MS international stem cell transplant trial to compare the effects of nonmyeloablative HSCT with those of continued DMT treatment on disease progression in participants with highly active relapsing-remitting MS.
The researchers enrolled 110 participants at four international centers into their open-label trial. Eligible participants had two or more clinical relapses or one relapse and at least one gadolinium-enhancing lesion at a separate time within the previous 12 months, despite DMT treatment. The investigators also required participants to have an EDSS score between 2.0 and 6.0. Patients with primary or secondary progressive MS were excluded.
Dr. Burt and his colleagues randomized participants to receive HSCT or an approved DMT that was more effective or in a different class than the one they were receiving at baseline. Ocrelizumab (Ocrevus) was not administered during the study because it had not yet been approved. The investigators excluded alemtuzumab because of its association with persistent lymphopenia and autoimmune disorders. After 1 year of treatment, patients receiving a DMT who had disability progression could cross over to the HSCT arm. Patients randomized to HSCT stopped taking their usual DMT.
Time to disease progression was the study’s primary endpoint. The investigators defined disease progression as an increase in EDSS score of at least 1 point on two evaluations 6 months apart after at least 1 year of treatment. The increase was required to result from MS. The neurologist who recorded participants’ EDSS evaluations was blinded to treatment group assignment.
The researchers randomized 55 patients to each study arm. Approximately 66% of participants were women, and the sample’s mean age was 36 years. There were no significant baseline differences between groups on demographic, clinical, or imaging characteristics. Three patients in the HSCT group were withdrawn from the study, and four in the DMT group were lost to follow-up after seeking HSCT at outside facilities.
Three patients in the HSCT group and 34 patients in the DMT group had disease progression. Mean follow-up duration was 2.8 years. The investigators could not calculate the median time to progression in the HSCT group because too few events occurred. Median time to progression was 24 months in the DMT group (HR, 0.07). During the first year, mean EDSS scores decreased (indicating improvement) from 3.38 to 2.36 in the HSCT group. Mean EDSS scores increased from 3.31 to 3.98 in the DMT group. No participants died, and no patients who received HSCT-developed nonhematopoietic grade 4 toxicities.
“To our knowledge, this is the first randomized trial of HSCT in patients with relapsing-remitting MS,” Dr. Burt and his colleagues said. Although observational studies have found similar EDSS improvements following HSCT, “this degree of improvement has not been demonstrated in pharmaceutical trials even with more intensive DMT such as alemtuzumab,” they concluded.
The Danhakl Family Foundation, the Cumming Foundation, the McNamara Purcell Foundation, Morgan Stanley, and the National Institute for Health Research Sheffield Clinical Research Facility provided financial support for this study. No pharmaceutical companies supported the study.
SOURCEs: Brown JWL et al. JAMA. 2019;321(2):175-87. doi: 10.1001/jama.2018.20588.; and Burt RK et al. JAMA. 2019;321(2):165-74. doi: 10.1001/jama.2018.18743.
The study by Brown et al. provides evidence that DMTs slow the appearance of persistent disabilities in patients with multiple sclerosis (MS), Harold Atkins, MD, wrote in an accompanying editorial (JAMA. 2019 Jan 15;321[2]:153-4). Although disease-modifying therapies (DMTs) may suppress clinical signs of disease activity for long periods in some patients, these therapies slow MS rather than halt it. DMTs require long-term administration and may cause intolerable side effects that impair patients’ quality of life. These therapies also may result in complications such as severe depression or progressive multifocal leukoencephalopathy.
“The study by Burt et al. ... provides a rigorous indication that HSCT [hematopoietic stem cell transplantation] can be an effective treatment for selected patients with MS,” Dr. Atkins said. Treating physicians, however, have concerns about this procedure, which is resource-intensive and “requires specialized medical and nursing expertise and dedicated hospital infrastructure to minimize its risks.” Many patients in the study had moderate to severe acute toxicity following treatment, and patient selection thus requires caution.
An important limitation of the study is that participants did not have access to alemtuzumab or ocrelizumab, which arguably are the most effective DMTs, Dr. Atkins said. The study began in 2005, when fewer DMTs were available. “The inclusion of patients who were less than optimally treated in the DMT group needs to be considered when interpreting the results of this study,” Dr. Atkins said.
Furthermore, Burt and colleagues studied patients with highly active MS, but “only a small proportion of the MS patient population exhibits this degree of activity,” he added. The results therefore may not be generalizable. Nevertheless, “even with the limitations of the trial, the results support a role for HSCT delivered at centers that are experienced in the clinical care of patients with highly active MS,” Dr. Atkins concluded.
Dr. Atkins is affiliated with the Ottawa Hospital Blood and Marrow Transplant Program at the University of Ottawa in Ontario. He reported no conflicts of interest.
The study by Brown et al. provides evidence that DMTs slow the appearance of persistent disabilities in patients with multiple sclerosis (MS), Harold Atkins, MD, wrote in an accompanying editorial (JAMA. 2019 Jan 15;321[2]:153-4). Although disease-modifying therapies (DMTs) may suppress clinical signs of disease activity for long periods in some patients, these therapies slow MS rather than halt it. DMTs require long-term administration and may cause intolerable side effects that impair patients’ quality of life. These therapies also may result in complications such as severe depression or progressive multifocal leukoencephalopathy.
“The study by Burt et al. ... provides a rigorous indication that HSCT [hematopoietic stem cell transplantation] can be an effective treatment for selected patients with MS,” Dr. Atkins said. Treating physicians, however, have concerns about this procedure, which is resource-intensive and “requires specialized medical and nursing expertise and dedicated hospital infrastructure to minimize its risks.” Many patients in the study had moderate to severe acute toxicity following treatment, and patient selection thus requires caution.
An important limitation of the study is that participants did not have access to alemtuzumab or ocrelizumab, which arguably are the most effective DMTs, Dr. Atkins said. The study began in 2005, when fewer DMTs were available. “The inclusion of patients who were less than optimally treated in the DMT group needs to be considered when interpreting the results of this study,” Dr. Atkins said.
Furthermore, Burt and colleagues studied patients with highly active MS, but “only a small proportion of the MS patient population exhibits this degree of activity,” he added. The results therefore may not be generalizable. Nevertheless, “even with the limitations of the trial, the results support a role for HSCT delivered at centers that are experienced in the clinical care of patients with highly active MS,” Dr. Atkins concluded.
Dr. Atkins is affiliated with the Ottawa Hospital Blood and Marrow Transplant Program at the University of Ottawa in Ontario. He reported no conflicts of interest.
The study by Brown et al. provides evidence that DMTs slow the appearance of persistent disabilities in patients with multiple sclerosis (MS), Harold Atkins, MD, wrote in an accompanying editorial (JAMA. 2019 Jan 15;321[2]:153-4). Although disease-modifying therapies (DMTs) may suppress clinical signs of disease activity for long periods in some patients, these therapies slow MS rather than halt it. DMTs require long-term administration and may cause intolerable side effects that impair patients’ quality of life. These therapies also may result in complications such as severe depression or progressive multifocal leukoencephalopathy.
“The study by Burt et al. ... provides a rigorous indication that HSCT [hematopoietic stem cell transplantation] can be an effective treatment for selected patients with MS,” Dr. Atkins said. Treating physicians, however, have concerns about this procedure, which is resource-intensive and “requires specialized medical and nursing expertise and dedicated hospital infrastructure to minimize its risks.” Many patients in the study had moderate to severe acute toxicity following treatment, and patient selection thus requires caution.
An important limitation of the study is that participants did not have access to alemtuzumab or ocrelizumab, which arguably are the most effective DMTs, Dr. Atkins said. The study began in 2005, when fewer DMTs were available. “The inclusion of patients who were less than optimally treated in the DMT group needs to be considered when interpreting the results of this study,” Dr. Atkins said.
Furthermore, Burt and colleagues studied patients with highly active MS, but “only a small proportion of the MS patient population exhibits this degree of activity,” he added. The results therefore may not be generalizable. Nevertheless, “even with the limitations of the trial, the results support a role for HSCT delivered at centers that are experienced in the clinical care of patients with highly active MS,” Dr. Atkins concluded.
Dr. Atkins is affiliated with the Ottawa Hospital Blood and Marrow Transplant Program at the University of Ottawa in Ontario. He reported no conflicts of interest.
Disease-modifying therapies give patients with relapsing-remitting multiple sclerosis a lower risk of developing secondary progressive disease that may only be topped in specific patients with highly active disease by the use of nonmyeloablative hematopoietic stem cell transplantation, according to findings from two studies published online Jan. 15 in JAMA.
The first study found that interferon-beta, glatiramer acetate (Copaxone), fingolimod (Gilenya), natalizumab (Tysabri), and alemtuzumab (Lemtrada) are associated with a lower risk of conversion to secondary progressive MS, compared with no treatment. Initial treatment with the newer therapies provided a greater risk reduction, compared with initial treatment with interferon-beta or glatiramer acetate.
The second study, described as “the first randomized trial of HSCT [nonmyeloablative hematopoietic stem cell transplantation] in patients with relapsing-remitting MS,” suggests that HSCT prolongs the time to disease progression, compared with disease-modifying therapies (DMTs). It also suggests that HSCT can lead to clinical improvement.
DMTs reduced risk of conversion to secondary progressive MS
Few previous studies have examined the association between DMTs and the risk of conversion from relapsing-remitting MS to secondary progressive MS. Those that have analyzed this association have not used a validated definition of secondary progressive MS. J. William L. Brown, MD, of the University of Cambridge, England, and his colleagues used a validated definition of secondary progressive MS that was published in 2016 to investigate how DMTs affect the rate of conversion, compared with no treatment. The researchers also compared the risk reduction provided by fingolimod, alemtuzumab, or natalizumab with that provided by interferon-beta or glatiramer acetate.
Dr. Brown and his colleagues analyzed prospectively collected clinical data from an international observational cohort study called MSBase. Eligible participants had relapsing-remitting MS, the complete MSBase minimum data set, at least one Expanded Disability Status Scale (EDSS) score recorded within 6 months before baseline, and at least two EDSS scores recorded after baseline. Participants initiated a DMT or began clinical monitoring during 1988-2012. The population had a minimum follow-up duration of 4 years. Patients who stopped their initial therapy within 6 months and those participating in clinical trials were excluded.
The primary outcome was conversion to secondary progressive MS. Dr. Brown and his colleagues defined this outcome as an EDSS increase of 1 point for participants with a baseline EDSS score of 5.5 or less and as an increase of 0.5 points for participants with a baseline EDSS score higher than 5.5. This increase had to occur in the absence of relapses and be confirmed at a subsequent visit 3 or fewer months later. In addition, the increased EDSS score had to be 4 or more.
After excluding ineligible participants, the investigators matched 1,555 patients from 68 centers in 21 countries. Each therapy analyzed was associated with reduced risk of converting to secondary progressive MS, compared with no treatment. The hazard ratios for conversion were 0.71 for interferon-beta or glatiramer acetate, 0.37 for fingolimod, 0.61 for natalizumab, and 0.52 for alemtuzumab, compared with no treatment.
Treatment with interferon-beta or glatiramer acetate within 5 years of disease onset was associated with a reduced risk of conversion (HR, 0.77), compared with treatment later than 5 years after disease onset. Similarly, patients who escalated treatment from interferon-beta or glatiramer acetate to any of the other three DMTs within 5 years of disease onset had a significantly lower risk of conversion (HR, 0.76) than did those who escalated later. Furthermore, initial treatment with fingolimod, alemtuzumab, or natalizumab was associated with a significantly reduced risk of conversion (HR, 0.66), compared with initial treatment with interferon-beta or glatiramer acetate.
One of the study’s limitations is its observational design, which precludes the determination of causality, Dr. Brown and his colleagues said. In addition, functional score subcomponents of the EDSS were unavailable, which prevented the researchers from using the definition of secondary progressive MS with the best combination of sensitivity, specificity, and accuracy. Some analyses were limited by small numbers of patients, and the study did not evaluate the risks associated with DMTs. Nevertheless, “these findings, considered along with these therapies’ risks, may help inform decisions about DMT selection,” the authors concluded.
Financial support for this study was provided by the National Health and Medical Research Council of Australia and the University of Melbourne. Dr. Brown received a Next Generation Fellowship funded by the Grand Charity of the Freemasons and an MSBase 2017 Fellowship. Alemtuzumab studies conducted in Cambridge were supported by the National Institute for Health Research Cambridge Biomedical Research Centre and the MS Society UK.
HSCT delayed disease progression
In a previous case series, Richard K. Burt, MD, of Northwestern University in Chicago, and his colleagues found that patients with relapsing-remitting MS who underwent nonmyeloablative HSCT had neurologic improvement and a 70% likelihood of having a 4-year period of disease remission. Dr. Burt and his colleagues undertook the MS international stem cell transplant trial to compare the effects of nonmyeloablative HSCT with those of continued DMT treatment on disease progression in participants with highly active relapsing-remitting MS.
The researchers enrolled 110 participants at four international centers into their open-label trial. Eligible participants had two or more clinical relapses or one relapse and at least one gadolinium-enhancing lesion at a separate time within the previous 12 months, despite DMT treatment. The investigators also required participants to have an EDSS score between 2.0 and 6.0. Patients with primary or secondary progressive MS were excluded.
Dr. Burt and his colleagues randomized participants to receive HSCT or an approved DMT that was more effective or in a different class than the one they were receiving at baseline. Ocrelizumab (Ocrevus) was not administered during the study because it had not yet been approved. The investigators excluded alemtuzumab because of its association with persistent lymphopenia and autoimmune disorders. After 1 year of treatment, patients receiving a DMT who had disability progression could cross over to the HSCT arm. Patients randomized to HSCT stopped taking their usual DMT.
Time to disease progression was the study’s primary endpoint. The investigators defined disease progression as an increase in EDSS score of at least 1 point on two evaluations 6 months apart after at least 1 year of treatment. The increase was required to result from MS. The neurologist who recorded participants’ EDSS evaluations was blinded to treatment group assignment.
The researchers randomized 55 patients to each study arm. Approximately 66% of participants were women, and the sample’s mean age was 36 years. There were no significant baseline differences between groups on demographic, clinical, or imaging characteristics. Three patients in the HSCT group were withdrawn from the study, and four in the DMT group were lost to follow-up after seeking HSCT at outside facilities.
Three patients in the HSCT group and 34 patients in the DMT group had disease progression. Mean follow-up duration was 2.8 years. The investigators could not calculate the median time to progression in the HSCT group because too few events occurred. Median time to progression was 24 months in the DMT group (HR, 0.07). During the first year, mean EDSS scores decreased (indicating improvement) from 3.38 to 2.36 in the HSCT group. Mean EDSS scores increased from 3.31 to 3.98 in the DMT group. No participants died, and no patients who received HSCT-developed nonhematopoietic grade 4 toxicities.
“To our knowledge, this is the first randomized trial of HSCT in patients with relapsing-remitting MS,” Dr. Burt and his colleagues said. Although observational studies have found similar EDSS improvements following HSCT, “this degree of improvement has not been demonstrated in pharmaceutical trials even with more intensive DMT such as alemtuzumab,” they concluded.
The Danhakl Family Foundation, the Cumming Foundation, the McNamara Purcell Foundation, Morgan Stanley, and the National Institute for Health Research Sheffield Clinical Research Facility provided financial support for this study. No pharmaceutical companies supported the study.
SOURCEs: Brown JWL et al. JAMA. 2019;321(2):175-87. doi: 10.1001/jama.2018.20588.; and Burt RK et al. JAMA. 2019;321(2):165-74. doi: 10.1001/jama.2018.18743.
Disease-modifying therapies give patients with relapsing-remitting multiple sclerosis a lower risk of developing secondary progressive disease that may only be topped in specific patients with highly active disease by the use of nonmyeloablative hematopoietic stem cell transplantation, according to findings from two studies published online Jan. 15 in JAMA.
The first study found that interferon-beta, glatiramer acetate (Copaxone), fingolimod (Gilenya), natalizumab (Tysabri), and alemtuzumab (Lemtrada) are associated with a lower risk of conversion to secondary progressive MS, compared with no treatment. Initial treatment with the newer therapies provided a greater risk reduction, compared with initial treatment with interferon-beta or glatiramer acetate.
The second study, described as “the first randomized trial of HSCT [nonmyeloablative hematopoietic stem cell transplantation] in patients with relapsing-remitting MS,” suggests that HSCT prolongs the time to disease progression, compared with disease-modifying therapies (DMTs). It also suggests that HSCT can lead to clinical improvement.
DMTs reduced risk of conversion to secondary progressive MS
Few previous studies have examined the association between DMTs and the risk of conversion from relapsing-remitting MS to secondary progressive MS. Those that have analyzed this association have not used a validated definition of secondary progressive MS. J. William L. Brown, MD, of the University of Cambridge, England, and his colleagues used a validated definition of secondary progressive MS that was published in 2016 to investigate how DMTs affect the rate of conversion, compared with no treatment. The researchers also compared the risk reduction provided by fingolimod, alemtuzumab, or natalizumab with that provided by interferon-beta or glatiramer acetate.
Dr. Brown and his colleagues analyzed prospectively collected clinical data from an international observational cohort study called MSBase. Eligible participants had relapsing-remitting MS, the complete MSBase minimum data set, at least one Expanded Disability Status Scale (EDSS) score recorded within 6 months before baseline, and at least two EDSS scores recorded after baseline. Participants initiated a DMT or began clinical monitoring during 1988-2012. The population had a minimum follow-up duration of 4 years. Patients who stopped their initial therapy within 6 months and those participating in clinical trials were excluded.
The primary outcome was conversion to secondary progressive MS. Dr. Brown and his colleagues defined this outcome as an EDSS increase of 1 point for participants with a baseline EDSS score of 5.5 or less and as an increase of 0.5 points for participants with a baseline EDSS score higher than 5.5. This increase had to occur in the absence of relapses and be confirmed at a subsequent visit 3 or fewer months later. In addition, the increased EDSS score had to be 4 or more.
After excluding ineligible participants, the investigators matched 1,555 patients from 68 centers in 21 countries. Each therapy analyzed was associated with reduced risk of converting to secondary progressive MS, compared with no treatment. The hazard ratios for conversion were 0.71 for interferon-beta or glatiramer acetate, 0.37 for fingolimod, 0.61 for natalizumab, and 0.52 for alemtuzumab, compared with no treatment.
Treatment with interferon-beta or glatiramer acetate within 5 years of disease onset was associated with a reduced risk of conversion (HR, 0.77), compared with treatment later than 5 years after disease onset. Similarly, patients who escalated treatment from interferon-beta or glatiramer acetate to any of the other three DMTs within 5 years of disease onset had a significantly lower risk of conversion (HR, 0.76) than did those who escalated later. Furthermore, initial treatment with fingolimod, alemtuzumab, or natalizumab was associated with a significantly reduced risk of conversion (HR, 0.66), compared with initial treatment with interferon-beta or glatiramer acetate.
One of the study’s limitations is its observational design, which precludes the determination of causality, Dr. Brown and his colleagues said. In addition, functional score subcomponents of the EDSS were unavailable, which prevented the researchers from using the definition of secondary progressive MS with the best combination of sensitivity, specificity, and accuracy. Some analyses were limited by small numbers of patients, and the study did not evaluate the risks associated with DMTs. Nevertheless, “these findings, considered along with these therapies’ risks, may help inform decisions about DMT selection,” the authors concluded.
Financial support for this study was provided by the National Health and Medical Research Council of Australia and the University of Melbourne. Dr. Brown received a Next Generation Fellowship funded by the Grand Charity of the Freemasons and an MSBase 2017 Fellowship. Alemtuzumab studies conducted in Cambridge were supported by the National Institute for Health Research Cambridge Biomedical Research Centre and the MS Society UK.
HSCT delayed disease progression
In a previous case series, Richard K. Burt, MD, of Northwestern University in Chicago, and his colleagues found that patients with relapsing-remitting MS who underwent nonmyeloablative HSCT had neurologic improvement and a 70% likelihood of having a 4-year period of disease remission. Dr. Burt and his colleagues undertook the MS international stem cell transplant trial to compare the effects of nonmyeloablative HSCT with those of continued DMT treatment on disease progression in participants with highly active relapsing-remitting MS.
The researchers enrolled 110 participants at four international centers into their open-label trial. Eligible participants had two or more clinical relapses or one relapse and at least one gadolinium-enhancing lesion at a separate time within the previous 12 months, despite DMT treatment. The investigators also required participants to have an EDSS score between 2.0 and 6.0. Patients with primary or secondary progressive MS were excluded.
Dr. Burt and his colleagues randomized participants to receive HSCT or an approved DMT that was more effective or in a different class than the one they were receiving at baseline. Ocrelizumab (Ocrevus) was not administered during the study because it had not yet been approved. The investigators excluded alemtuzumab because of its association with persistent lymphopenia and autoimmune disorders. After 1 year of treatment, patients receiving a DMT who had disability progression could cross over to the HSCT arm. Patients randomized to HSCT stopped taking their usual DMT.
Time to disease progression was the study’s primary endpoint. The investigators defined disease progression as an increase in EDSS score of at least 1 point on two evaluations 6 months apart after at least 1 year of treatment. The increase was required to result from MS. The neurologist who recorded participants’ EDSS evaluations was blinded to treatment group assignment.
The researchers randomized 55 patients to each study arm. Approximately 66% of participants were women, and the sample’s mean age was 36 years. There were no significant baseline differences between groups on demographic, clinical, or imaging characteristics. Three patients in the HSCT group were withdrawn from the study, and four in the DMT group were lost to follow-up after seeking HSCT at outside facilities.
Three patients in the HSCT group and 34 patients in the DMT group had disease progression. Mean follow-up duration was 2.8 years. The investigators could not calculate the median time to progression in the HSCT group because too few events occurred. Median time to progression was 24 months in the DMT group (HR, 0.07). During the first year, mean EDSS scores decreased (indicating improvement) from 3.38 to 2.36 in the HSCT group. Mean EDSS scores increased from 3.31 to 3.98 in the DMT group. No participants died, and no patients who received HSCT-developed nonhematopoietic grade 4 toxicities.
“To our knowledge, this is the first randomized trial of HSCT in patients with relapsing-remitting MS,” Dr. Burt and his colleagues said. Although observational studies have found similar EDSS improvements following HSCT, “this degree of improvement has not been demonstrated in pharmaceutical trials even with more intensive DMT such as alemtuzumab,” they concluded.
The Danhakl Family Foundation, the Cumming Foundation, the McNamara Purcell Foundation, Morgan Stanley, and the National Institute for Health Research Sheffield Clinical Research Facility provided financial support for this study. No pharmaceutical companies supported the study.
SOURCEs: Brown JWL et al. JAMA. 2019;321(2):175-87. doi: 10.1001/jama.2018.20588.; and Burt RK et al. JAMA. 2019;321(2):165-74. doi: 10.1001/jama.2018.18743.
FROM JAMA