User login
Cluster headache is associated with increased suicidality
Short- and long-term cluster headache disease burden, as well as depressive symptoms, contributes to suicidality, according to research published online Cephalalgia. Development of treatments that reduce the headache-related burden and prevent future bouts could reduce suicidality, said the researchers.
Although cluster headache has been called the “suicide headache,” few studies have examined suicidality in patients with cluster headache. Research by Rozen et al. found that the rate of suicidal attempt among patients was similar to that among the general population. The results have not been replicated, however, and the investigators did not examine whether suicidality varied according to the phases of the disorder.
A prospective, multicenter study
Mi Ji Lee, MD, PhD, clinical assistant professor of neurology at Samsung Medical Center in Seoul, South Korea, and colleagues conducted a prospective study to investigate the suicidality associated with cluster headache and the factors associated with increased suicidality in that disorder. The researchers enrolled 193 consecutive patients with cluster headache between September 2016 and August 2018 at 15 hospitals. They examined the patients and used the Patient Health Questionnaire–9 (PHQ-9) and the General Anxiety Disorder–7 item scale (GAD-7) screening tools. During the ictal and interictal phases, the researchers asked the patients whether they had had passive suicidal ideation, active suicidal ideation, suicidal planning, or suicidal attempt. Dr. Ji Lee and colleagues performed univariable and multivariable logistic regression analyses to evaluate the factors associated with high ictal suicidality, which was defined as two or more positive responses during the ictal phase. Participants were followed up during the between-bout phase.
The researchers excluded 18 patients from analysis because they were between bouts at enrollment. The mean age of the remaining 175 patients was 38.4 years. Mean age at onset was 29.9 years. About 85% of the patients were male. The diagnosis was definite cluster headache for 87.4% of the sample and probable cluster headache for 12.6%. In addition, 88% of the population had episodic cluster headache.
Suicidal ideation increased during the ictal phase
During the ictal phase, 64.2% of participants reported passive suicidal ideation, and 35.8% reported active suicidal ideation. Furthermore, 5.8% of patients had a suicidal plan, and 2.3% attempted suicide. In the interictal phase, 4.0% of patients reported passive suicidal ideation, and 3.5% reported active suicidal ideation. Interictal suicidal planning was reported by 2.9% of participants, and 1.2% of participants attempted suicide interictally. The results were similar between patients with definite and probable cluster headache.
The ictal phase increased the odds of passive suicidal ideation (odds ratio [OR], 42.46), active suicidal ideation (OR, 15.55), suicidal planning (OR, 2.06), and suicidal attempt (OR, 2.02), compared with the interictal phase. The differences in suicidal planning and suicidal attempt between the ictal and interictal phases, however, were not statistically significant.
Longer disease duration, higher attack intensity, higher Headache Impact Test–6 (HIT-6) score, GAD-7 score, and PHQ-9 score were associated with high ictal suicidality. Disease duration, HIT-6, and PHQ-9 remained significantly associated with high ictal suicidality in the multivariate analysis. Younger age at onset, longer disease duration, total number of lifetime bouts, and higher GAD-7 and PHQ-9 scores were significantly associated with interictal suicidality in the univariable analysis. The total number of lifetime bouts and the PHQ-9 scores remained significant in the multivariable analysis.
In all, 54 patients were followed up between bouts. None reported passive suicidal ideation, 1.9% reported active suicidal ideation, 1.9% reported suicidal planning, and none reported suicidal attempt. Compared with the between-bouts period, the ictal phase was associated with significantly higher odds of active suicidal ideation (OR, 37.32) and nonsignificantly increased suicidal planning (OR, 3.20).
Patients need a disease-modifying treatment
Taken together, the study results underscore the importance of proper management of cluster headache to reduce its burden, said the authors. “Given that greater headache-related impact was independently associated with ictal suicidality, an intensive treatment to reduce the headache-related impact might be beneficial to prevent suicide in cluster headache patients,” they said. In addition to reducing headache-related impact and headache intensity, “a disease-modifying treatment to prevent further bouts is warranted to decrease suicidality in cluster headache patients.”
Although patients with cluster headache had increased suicidality in the ictal and interictal phases, they had lower suicidality between bouts, compared with the general population. This result suggests that patients remain mentally healthy when the bouts are over, and that “a strategy to shorten the length of bout is warranted,” said Dr. Ji Lee and colleagues. Furthermore, the fact that suicidality did not differ significantly between patients with definite cluster headache and those with probable cluster headache “prompts clinicians for an increased identification and intensive treatment strategy for probable cluster headache.”
The current study is the first prospective investigation of suicidality in the various phases of cluster headache, according to the investigators. It nevertheless has several limitations. The prevalence of chronic cluster headache was low in the study population, and not all patients presented for follow-up during the period between bouts. In addition, the data were obtained from recall, and consequently may be less accurate than those gained from prospective recording. Finally, Dr. Ji Lee and colleagues did not gather information on personality disorders, insomnia, substance abuse, or addiction, even though these factors can influence suicidality in patients with chronic pain.
The investigators reported no conflicts of interest related to their research. The study was supported by a grant from the Korean Neurological Association.
SOURCE: Ji Lee M et al. Cephalalgia. 2019 Apr 24. doi: 10.1177/0333102419845660.
Short- and long-term cluster headache disease burden, as well as depressive symptoms, contributes to suicidality, according to research published online Cephalalgia. Development of treatments that reduce the headache-related burden and prevent future bouts could reduce suicidality, said the researchers.
Although cluster headache has been called the “suicide headache,” few studies have examined suicidality in patients with cluster headache. Research by Rozen et al. found that the rate of suicidal attempt among patients was similar to that among the general population. The results have not been replicated, however, and the investigators did not examine whether suicidality varied according to the phases of the disorder.
A prospective, multicenter study
Mi Ji Lee, MD, PhD, clinical assistant professor of neurology at Samsung Medical Center in Seoul, South Korea, and colleagues conducted a prospective study to investigate the suicidality associated with cluster headache and the factors associated with increased suicidality in that disorder. The researchers enrolled 193 consecutive patients with cluster headache between September 2016 and August 2018 at 15 hospitals. They examined the patients and used the Patient Health Questionnaire–9 (PHQ-9) and the General Anxiety Disorder–7 item scale (GAD-7) screening tools. During the ictal and interictal phases, the researchers asked the patients whether they had had passive suicidal ideation, active suicidal ideation, suicidal planning, or suicidal attempt. Dr. Ji Lee and colleagues performed univariable and multivariable logistic regression analyses to evaluate the factors associated with high ictal suicidality, which was defined as two or more positive responses during the ictal phase. Participants were followed up during the between-bout phase.
The researchers excluded 18 patients from analysis because they were between bouts at enrollment. The mean age of the remaining 175 patients was 38.4 years. Mean age at onset was 29.9 years. About 85% of the patients were male. The diagnosis was definite cluster headache for 87.4% of the sample and probable cluster headache for 12.6%. In addition, 88% of the population had episodic cluster headache.
Suicidal ideation increased during the ictal phase
During the ictal phase, 64.2% of participants reported passive suicidal ideation, and 35.8% reported active suicidal ideation. Furthermore, 5.8% of patients had a suicidal plan, and 2.3% attempted suicide. In the interictal phase, 4.0% of patients reported passive suicidal ideation, and 3.5% reported active suicidal ideation. Interictal suicidal planning was reported by 2.9% of participants, and 1.2% of participants attempted suicide interictally. The results were similar between patients with definite and probable cluster headache.
The ictal phase increased the odds of passive suicidal ideation (odds ratio [OR], 42.46), active suicidal ideation (OR, 15.55), suicidal planning (OR, 2.06), and suicidal attempt (OR, 2.02), compared with the interictal phase. The differences in suicidal planning and suicidal attempt between the ictal and interictal phases, however, were not statistically significant.
Longer disease duration, higher attack intensity, higher Headache Impact Test–6 (HIT-6) score, GAD-7 score, and PHQ-9 score were associated with high ictal suicidality. Disease duration, HIT-6, and PHQ-9 remained significantly associated with high ictal suicidality in the multivariate analysis. Younger age at onset, longer disease duration, total number of lifetime bouts, and higher GAD-7 and PHQ-9 scores were significantly associated with interictal suicidality in the univariable analysis. The total number of lifetime bouts and the PHQ-9 scores remained significant in the multivariable analysis.
In all, 54 patients were followed up between bouts. None reported passive suicidal ideation, 1.9% reported active suicidal ideation, 1.9% reported suicidal planning, and none reported suicidal attempt. Compared with the between-bouts period, the ictal phase was associated with significantly higher odds of active suicidal ideation (OR, 37.32) and nonsignificantly increased suicidal planning (OR, 3.20).
Patients need a disease-modifying treatment
Taken together, the study results underscore the importance of proper management of cluster headache to reduce its burden, said the authors. “Given that greater headache-related impact was independently associated with ictal suicidality, an intensive treatment to reduce the headache-related impact might be beneficial to prevent suicide in cluster headache patients,” they said. In addition to reducing headache-related impact and headache intensity, “a disease-modifying treatment to prevent further bouts is warranted to decrease suicidality in cluster headache patients.”
Although patients with cluster headache had increased suicidality in the ictal and interictal phases, they had lower suicidality between bouts, compared with the general population. This result suggests that patients remain mentally healthy when the bouts are over, and that “a strategy to shorten the length of bout is warranted,” said Dr. Ji Lee and colleagues. Furthermore, the fact that suicidality did not differ significantly between patients with definite cluster headache and those with probable cluster headache “prompts clinicians for an increased identification and intensive treatment strategy for probable cluster headache.”
The current study is the first prospective investigation of suicidality in the various phases of cluster headache, according to the investigators. It nevertheless has several limitations. The prevalence of chronic cluster headache was low in the study population, and not all patients presented for follow-up during the period between bouts. In addition, the data were obtained from recall, and consequently may be less accurate than those gained from prospective recording. Finally, Dr. Ji Lee and colleagues did not gather information on personality disorders, insomnia, substance abuse, or addiction, even though these factors can influence suicidality in patients with chronic pain.
The investigators reported no conflicts of interest related to their research. The study was supported by a grant from the Korean Neurological Association.
SOURCE: Ji Lee M et al. Cephalalgia. 2019 Apr 24. doi: 10.1177/0333102419845660.
Short- and long-term cluster headache disease burden, as well as depressive symptoms, contributes to suicidality, according to research published online Cephalalgia. Development of treatments that reduce the headache-related burden and prevent future bouts could reduce suicidality, said the researchers.
Although cluster headache has been called the “suicide headache,” few studies have examined suicidality in patients with cluster headache. Research by Rozen et al. found that the rate of suicidal attempt among patients was similar to that among the general population. The results have not been replicated, however, and the investigators did not examine whether suicidality varied according to the phases of the disorder.
A prospective, multicenter study
Mi Ji Lee, MD, PhD, clinical assistant professor of neurology at Samsung Medical Center in Seoul, South Korea, and colleagues conducted a prospective study to investigate the suicidality associated with cluster headache and the factors associated with increased suicidality in that disorder. The researchers enrolled 193 consecutive patients with cluster headache between September 2016 and August 2018 at 15 hospitals. They examined the patients and used the Patient Health Questionnaire–9 (PHQ-9) and the General Anxiety Disorder–7 item scale (GAD-7) screening tools. During the ictal and interictal phases, the researchers asked the patients whether they had had passive suicidal ideation, active suicidal ideation, suicidal planning, or suicidal attempt. Dr. Ji Lee and colleagues performed univariable and multivariable logistic regression analyses to evaluate the factors associated with high ictal suicidality, which was defined as two or more positive responses during the ictal phase. Participants were followed up during the between-bout phase.
The researchers excluded 18 patients from analysis because they were between bouts at enrollment. The mean age of the remaining 175 patients was 38.4 years. Mean age at onset was 29.9 years. About 85% of the patients were male. The diagnosis was definite cluster headache for 87.4% of the sample and probable cluster headache for 12.6%. In addition, 88% of the population had episodic cluster headache.
Suicidal ideation increased during the ictal phase
During the ictal phase, 64.2% of participants reported passive suicidal ideation, and 35.8% reported active suicidal ideation. Furthermore, 5.8% of patients had a suicidal plan, and 2.3% attempted suicide. In the interictal phase, 4.0% of patients reported passive suicidal ideation, and 3.5% reported active suicidal ideation. Interictal suicidal planning was reported by 2.9% of participants, and 1.2% of participants attempted suicide interictally. The results were similar between patients with definite and probable cluster headache.
The ictal phase increased the odds of passive suicidal ideation (odds ratio [OR], 42.46), active suicidal ideation (OR, 15.55), suicidal planning (OR, 2.06), and suicidal attempt (OR, 2.02), compared with the interictal phase. The differences in suicidal planning and suicidal attempt between the ictal and interictal phases, however, were not statistically significant.
Longer disease duration, higher attack intensity, higher Headache Impact Test–6 (HIT-6) score, GAD-7 score, and PHQ-9 score were associated with high ictal suicidality. Disease duration, HIT-6, and PHQ-9 remained significantly associated with high ictal suicidality in the multivariate analysis. Younger age at onset, longer disease duration, total number of lifetime bouts, and higher GAD-7 and PHQ-9 scores were significantly associated with interictal suicidality in the univariable analysis. The total number of lifetime bouts and the PHQ-9 scores remained significant in the multivariable analysis.
In all, 54 patients were followed up between bouts. None reported passive suicidal ideation, 1.9% reported active suicidal ideation, 1.9% reported suicidal planning, and none reported suicidal attempt. Compared with the between-bouts period, the ictal phase was associated with significantly higher odds of active suicidal ideation (OR, 37.32) and nonsignificantly increased suicidal planning (OR, 3.20).
Patients need a disease-modifying treatment
Taken together, the study results underscore the importance of proper management of cluster headache to reduce its burden, said the authors. “Given that greater headache-related impact was independently associated with ictal suicidality, an intensive treatment to reduce the headache-related impact might be beneficial to prevent suicide in cluster headache patients,” they said. In addition to reducing headache-related impact and headache intensity, “a disease-modifying treatment to prevent further bouts is warranted to decrease suicidality in cluster headache patients.”
Although patients with cluster headache had increased suicidality in the ictal and interictal phases, they had lower suicidality between bouts, compared with the general population. This result suggests that patients remain mentally healthy when the bouts are over, and that “a strategy to shorten the length of bout is warranted,” said Dr. Ji Lee and colleagues. Furthermore, the fact that suicidality did not differ significantly between patients with definite cluster headache and those with probable cluster headache “prompts clinicians for an increased identification and intensive treatment strategy for probable cluster headache.”
The current study is the first prospective investigation of suicidality in the various phases of cluster headache, according to the investigators. It nevertheless has several limitations. The prevalence of chronic cluster headache was low in the study population, and not all patients presented for follow-up during the period between bouts. In addition, the data were obtained from recall, and consequently may be less accurate than those gained from prospective recording. Finally, Dr. Ji Lee and colleagues did not gather information on personality disorders, insomnia, substance abuse, or addiction, even though these factors can influence suicidality in patients with chronic pain.
The investigators reported no conflicts of interest related to their research. The study was supported by a grant from the Korean Neurological Association.
SOURCE: Ji Lee M et al. Cephalalgia. 2019 Apr 24. doi: 10.1177/0333102419845660.
FROM CEPHALAGIA
Key clinical point: Cluster headache is associated with increased suicidality during attacks and within the active period.
Major finding: Cluster headache attacks increased the risk of active suicidal ideation (odds ratio, 15.55).
Study details: A prospective, multicenter study of 175 patients with cluster headache.
Disclosures: The study was supported by a grant from the Korean Neurological Association.
Source: Ji Lee M et al. Cephalalgia. 2019 Apr 24. doi: 10.1177/0333102419845660.
Out-of-pocket costs for neurologic medications rise sharply
The out-of-pocket cost of multiple sclerosis (MS) treatments increased the most, with a 20-fold increase during that time. The average out-of-pocket cost for MS therapy was $15/month in 2004, compared with $309/month in 2016. Patients also had to pay more for brand name medications for peripheral neuropathy, dementia, and Parkinson’s disease, researchers said.
“Out-of-pocket costs vary widely both across and within conditions,” said study author Brian C. Callaghan, MD, an assistant professor of neurology at the University of Michigan in Ann Arbor, and research colleagues. “To minimize patient financial burden, neurologists require access to precise cost information when making treatment decisions.”
Prior studies have found that high drug costs “can create burdens such as medical debt, skipping food or other essentials, or even not taking drugs as often as necessary,” Dr. Callaghan said in a news release.
To assess how out-of-pocket costs affect patients with neurologic conditions, the investigators analyzed data from a large, privately insured health care claims database. They determined medication costs for patients with MS, peripheral neuropathy, epilepsy, dementia, and Parkinson’s disease who were seen by outpatient neurologists. They also compared costs for high-deductible and traditional plans and explored cumulative out-of-pocket costs during the first 2 years after diagnosis.
The analysis examined the five most commonly prescribed drugs by neurologists for each condition based on Medicare data. In addition, the researchers included in their analysis all approved MS medications, lacosamide as a brand name epilepsy drug, and venlafaxine, a peripheral neuropathy medication that transitioned from brand to generic.
In all, the study population included 105,355 patients with MS, 314,530 with peripheral neuropathy, 281,073 with epilepsy, 120,720 with dementia, and 90,801 with Parkinson’s disease.
In 2016, patients in high-deductible health plans had an average monthly out-of-pocket expense that was approximately twice that of patients not in those plans – $661 versus $246 among patients with MS, and $40 versus $18 among patients with epilepsy.
In the 2 years after diagnosis in 2012 or 2013, cumulative out-of-pocket costs for patients with MS were a mean of $2,238, but costs varied widely. Cumulative costs were no more than $90 for patients in the bottom 5% of expenses, whereas they exceeded $9,800 for patients in the top 5% of expenses. Among patients with epilepsy, cumulative out-of-pocket costs were $230 in the 2 years after diagnosis.
“In 2004, out-of-pocket costs were of such low magnitude that physicians could typically ignore these costs for most patients and not adversely affect the financial status of patients or their adherence to medications. However, by 2016, out-of-pockets costs have risen to the point where neurologists should consider out-of-pocket costs for most medications and for most patients,” Dr. Callaghan and colleagues wrote.
Ralph L. Sacco, MD, president of the American Academy of Neurology (AAN), said in a news release that the AAN has created a Neurology Drug Pricing Task Force and is advocating for better drug-pricing policies. “This study provides important information to help us better understand how these problems can directly affect our patients,” Dr. Sacco said.
“Everyone deserves affordable access to the medications that will be most beneficial, but if the drugs are too expensive, people may simply not take them, possibly leading to medical complications and higher costs later,” Dr. Sacco said.
The study was supported by the AAN. Several authors are supported by National Institutes of Health grants. Dr. Callaghan receives research support from Impeto Medical and performs consulting work.
SOURCE: Callaghan BC et al. Neurology. 2019 May 1. doi: 10.1212/WNL.0000000000007564.
The out-of-pocket cost of multiple sclerosis (MS) treatments increased the most, with a 20-fold increase during that time. The average out-of-pocket cost for MS therapy was $15/month in 2004, compared with $309/month in 2016. Patients also had to pay more for brand name medications for peripheral neuropathy, dementia, and Parkinson’s disease, researchers said.
“Out-of-pocket costs vary widely both across and within conditions,” said study author Brian C. Callaghan, MD, an assistant professor of neurology at the University of Michigan in Ann Arbor, and research colleagues. “To minimize patient financial burden, neurologists require access to precise cost information when making treatment decisions.”
Prior studies have found that high drug costs “can create burdens such as medical debt, skipping food or other essentials, or even not taking drugs as often as necessary,” Dr. Callaghan said in a news release.
To assess how out-of-pocket costs affect patients with neurologic conditions, the investigators analyzed data from a large, privately insured health care claims database. They determined medication costs for patients with MS, peripheral neuropathy, epilepsy, dementia, and Parkinson’s disease who were seen by outpatient neurologists. They also compared costs for high-deductible and traditional plans and explored cumulative out-of-pocket costs during the first 2 years after diagnosis.
The analysis examined the five most commonly prescribed drugs by neurologists for each condition based on Medicare data. In addition, the researchers included in their analysis all approved MS medications, lacosamide as a brand name epilepsy drug, and venlafaxine, a peripheral neuropathy medication that transitioned from brand to generic.
In all, the study population included 105,355 patients with MS, 314,530 with peripheral neuropathy, 281,073 with epilepsy, 120,720 with dementia, and 90,801 with Parkinson’s disease.
In 2016, patients in high-deductible health plans had an average monthly out-of-pocket expense that was approximately twice that of patients not in those plans – $661 versus $246 among patients with MS, and $40 versus $18 among patients with epilepsy.
In the 2 years after diagnosis in 2012 or 2013, cumulative out-of-pocket costs for patients with MS were a mean of $2,238, but costs varied widely. Cumulative costs were no more than $90 for patients in the bottom 5% of expenses, whereas they exceeded $9,800 for patients in the top 5% of expenses. Among patients with epilepsy, cumulative out-of-pocket costs were $230 in the 2 years after diagnosis.
“In 2004, out-of-pocket costs were of such low magnitude that physicians could typically ignore these costs for most patients and not adversely affect the financial status of patients or their adherence to medications. However, by 2016, out-of-pockets costs have risen to the point where neurologists should consider out-of-pocket costs for most medications and for most patients,” Dr. Callaghan and colleagues wrote.
Ralph L. Sacco, MD, president of the American Academy of Neurology (AAN), said in a news release that the AAN has created a Neurology Drug Pricing Task Force and is advocating for better drug-pricing policies. “This study provides important information to help us better understand how these problems can directly affect our patients,” Dr. Sacco said.
“Everyone deserves affordable access to the medications that will be most beneficial, but if the drugs are too expensive, people may simply not take them, possibly leading to medical complications and higher costs later,” Dr. Sacco said.
The study was supported by the AAN. Several authors are supported by National Institutes of Health grants. Dr. Callaghan receives research support from Impeto Medical and performs consulting work.
SOURCE: Callaghan BC et al. Neurology. 2019 May 1. doi: 10.1212/WNL.0000000000007564.
The out-of-pocket cost of multiple sclerosis (MS) treatments increased the most, with a 20-fold increase during that time. The average out-of-pocket cost for MS therapy was $15/month in 2004, compared with $309/month in 2016. Patients also had to pay more for brand name medications for peripheral neuropathy, dementia, and Parkinson’s disease, researchers said.
“Out-of-pocket costs vary widely both across and within conditions,” said study author Brian C. Callaghan, MD, an assistant professor of neurology at the University of Michigan in Ann Arbor, and research colleagues. “To minimize patient financial burden, neurologists require access to precise cost information when making treatment decisions.”
Prior studies have found that high drug costs “can create burdens such as medical debt, skipping food or other essentials, or even not taking drugs as often as necessary,” Dr. Callaghan said in a news release.
To assess how out-of-pocket costs affect patients with neurologic conditions, the investigators analyzed data from a large, privately insured health care claims database. They determined medication costs for patients with MS, peripheral neuropathy, epilepsy, dementia, and Parkinson’s disease who were seen by outpatient neurologists. They also compared costs for high-deductible and traditional plans and explored cumulative out-of-pocket costs during the first 2 years after diagnosis.
The analysis examined the five most commonly prescribed drugs by neurologists for each condition based on Medicare data. In addition, the researchers included in their analysis all approved MS medications, lacosamide as a brand name epilepsy drug, and venlafaxine, a peripheral neuropathy medication that transitioned from brand to generic.
In all, the study population included 105,355 patients with MS, 314,530 with peripheral neuropathy, 281,073 with epilepsy, 120,720 with dementia, and 90,801 with Parkinson’s disease.
In 2016, patients in high-deductible health plans had an average monthly out-of-pocket expense that was approximately twice that of patients not in those plans – $661 versus $246 among patients with MS, and $40 versus $18 among patients with epilepsy.
In the 2 years after diagnosis in 2012 or 2013, cumulative out-of-pocket costs for patients with MS were a mean of $2,238, but costs varied widely. Cumulative costs were no more than $90 for patients in the bottom 5% of expenses, whereas they exceeded $9,800 for patients in the top 5% of expenses. Among patients with epilepsy, cumulative out-of-pocket costs were $230 in the 2 years after diagnosis.
“In 2004, out-of-pocket costs were of such low magnitude that physicians could typically ignore these costs for most patients and not adversely affect the financial status of patients or their adherence to medications. However, by 2016, out-of-pockets costs have risen to the point where neurologists should consider out-of-pocket costs for most medications and for most patients,” Dr. Callaghan and colleagues wrote.
Ralph L. Sacco, MD, president of the American Academy of Neurology (AAN), said in a news release that the AAN has created a Neurology Drug Pricing Task Force and is advocating for better drug-pricing policies. “This study provides important information to help us better understand how these problems can directly affect our patients,” Dr. Sacco said.
“Everyone deserves affordable access to the medications that will be most beneficial, but if the drugs are too expensive, people may simply not take them, possibly leading to medical complications and higher costs later,” Dr. Sacco said.
The study was supported by the AAN. Several authors are supported by National Institutes of Health grants. Dr. Callaghan receives research support from Impeto Medical and performs consulting work.
SOURCE: Callaghan BC et al. Neurology. 2019 May 1. doi: 10.1212/WNL.0000000000007564.
FROM NEUROLOGY
Long-term antibiotic use may heighten stroke, CHD risk
, according to a study in the European Heart Journal.
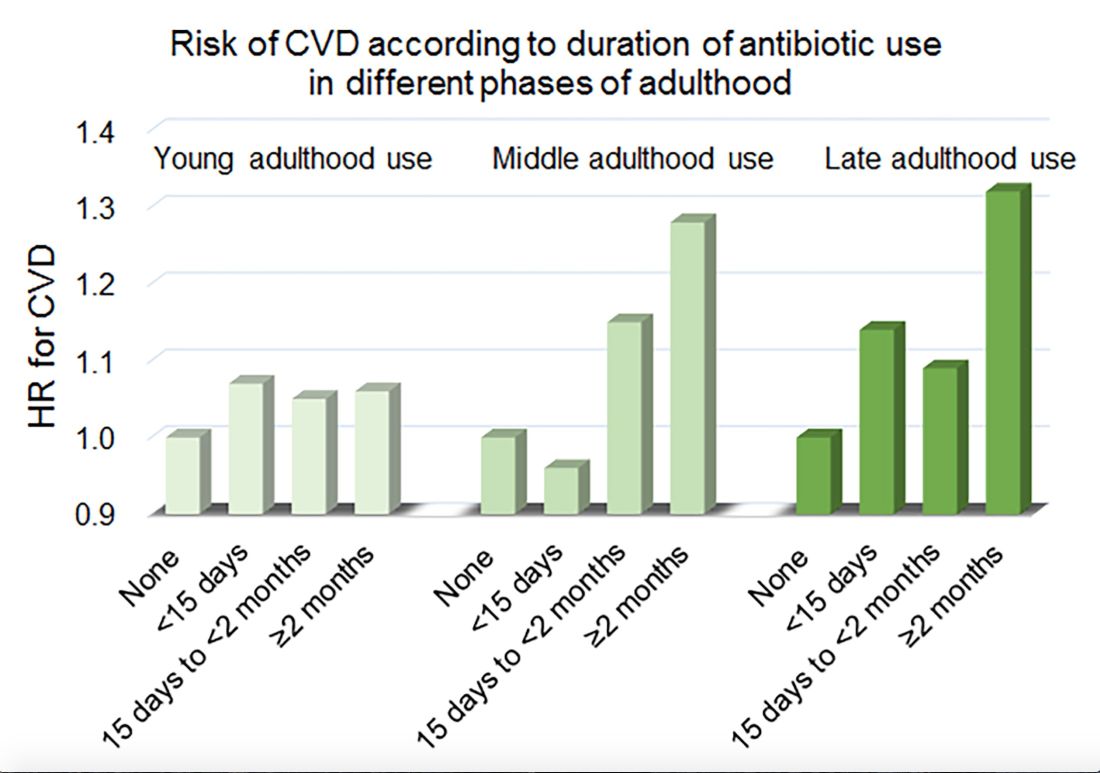
Women in the Nurses’ Health Study who used antibiotics for 2 or more months between ages 40 and 59 years or at age 60 years and older had a significantly increased risk of cardiovascular disease, compared with those who did not use antibiotics. Antibiotic use between 20 and 39 years old was not significantly related to cardiovascular disease.
Prior research has found that antibiotics may have long-lasting effects on gut microbiota and relate to cardiovascular disease risk.
“Antibiotic use is the most critical factor in altering the balance of microorganisms in the gut,” said lead investigator Lu Qi, MD, PhD, in a news release. “Previous studies have shown a link between alterations in the microbiotic environment of the gut and inflammation and narrowing of the blood vessels, stroke, and heart disease,” said Dr. Qi, who is the director of the Tulane University Obesity Research Center in New Orleans and an adjunct professor of nutrition at Harvard T.C. Chan School of Public Health in Boston.
To evaluate associations between life stage, antibiotic exposure, and subsequent cardiovascular disease, researchers analyzed data from 36,429 participants in the Nurses’ Health Study. The women were at least 60 years old and had no history of cardiovascular disease or cancer when they completed a 2004 questionnaire about antibiotic usage during young, middle, and late adulthood. The questionnaire asked participants to indicate the total time using antibiotics with eight categories ranging from none to 5 or more years.
The researchers defined incident cardiovascular disease as a composite endpoint of coronary heart disease (nonfatal myocardial infarction or fatal coronary heart disease) and stroke (nonfatal or fatal). They calculated person-years of follow-up from the questionnaire return date until date of cardiovascular disease diagnosis, death, or end of follow-up in 2012.
Women with longer duration of antibiotic use were more likely to use other medications and have unfavorable cardiovascular risk profiles, including family history of myocardial infarction and higher body mass index. Antibiotics most often were used to treat respiratory infections. During an average follow-up of 7.6 years, 1,056 participants developed cardiovascular disease.
In a multivariable model that adjusted for demographics, diet, lifestyle, reason for antibiotic use, medications, overweight status, and other factors, long-term antibiotic use – 2 months or more – in late adulthood was associated with significantly increased risk of cardiovascular disease (hazard ratio, 1.32), as was long-term antibiotic use in middle adulthood (HR, 1.28).
Although antibiotic use was self-reported, which could lead to misclassification, the participants were health professionals, which may mitigate this limitation, the authors noted. Whether these findings apply to men and other populations requires further study, they said.
Because of the study’s observational design, the results “cannot show that antibiotics cause heart disease and stroke, only that there is a link between them,” Dr. Qi said. “It’s possible that women who reported more antibiotic use might be sicker in other ways that we were unable to measure, or there may be other factors that could affect the results that we have not been able take account of.”
“Our study suggests that antibiotics should be used only when they are absolutely needed,” he concluded. “Considering the potentially cumulative adverse effects, the shorter time of antibiotic use the better.”
The study was supported by National Institutes of Health grants, the Boston Obesity Nutrition Research Center, and the United States–Israel Binational Science Foundation. One author received support from the Japan Society for the Promotion of Science. The authors had no conflicts of interest.
SOURCE: Heianza Y et al. Eur Heart J. 2019 Apr 24. doi: 10.1093/eurheartj/ehz231.
, according to a study in the European Heart Journal.

Women in the Nurses’ Health Study who used antibiotics for 2 or more months between ages 40 and 59 years or at age 60 years and older had a significantly increased risk of cardiovascular disease, compared with those who did not use antibiotics. Antibiotic use between 20 and 39 years old was not significantly related to cardiovascular disease.
Prior research has found that antibiotics may have long-lasting effects on gut microbiota and relate to cardiovascular disease risk.
“Antibiotic use is the most critical factor in altering the balance of microorganisms in the gut,” said lead investigator Lu Qi, MD, PhD, in a news release. “Previous studies have shown a link between alterations in the microbiotic environment of the gut and inflammation and narrowing of the blood vessels, stroke, and heart disease,” said Dr. Qi, who is the director of the Tulane University Obesity Research Center in New Orleans and an adjunct professor of nutrition at Harvard T.C. Chan School of Public Health in Boston.
To evaluate associations between life stage, antibiotic exposure, and subsequent cardiovascular disease, researchers analyzed data from 36,429 participants in the Nurses’ Health Study. The women were at least 60 years old and had no history of cardiovascular disease or cancer when they completed a 2004 questionnaire about antibiotic usage during young, middle, and late adulthood. The questionnaire asked participants to indicate the total time using antibiotics with eight categories ranging from none to 5 or more years.
The researchers defined incident cardiovascular disease as a composite endpoint of coronary heart disease (nonfatal myocardial infarction or fatal coronary heart disease) and stroke (nonfatal or fatal). They calculated person-years of follow-up from the questionnaire return date until date of cardiovascular disease diagnosis, death, or end of follow-up in 2012.
Women with longer duration of antibiotic use were more likely to use other medications and have unfavorable cardiovascular risk profiles, including family history of myocardial infarction and higher body mass index. Antibiotics most often were used to treat respiratory infections. During an average follow-up of 7.6 years, 1,056 participants developed cardiovascular disease.
In a multivariable model that adjusted for demographics, diet, lifestyle, reason for antibiotic use, medications, overweight status, and other factors, long-term antibiotic use – 2 months or more – in late adulthood was associated with significantly increased risk of cardiovascular disease (hazard ratio, 1.32), as was long-term antibiotic use in middle adulthood (HR, 1.28).
Although antibiotic use was self-reported, which could lead to misclassification, the participants were health professionals, which may mitigate this limitation, the authors noted. Whether these findings apply to men and other populations requires further study, they said.
Because of the study’s observational design, the results “cannot show that antibiotics cause heart disease and stroke, only that there is a link between them,” Dr. Qi said. “It’s possible that women who reported more antibiotic use might be sicker in other ways that we were unable to measure, or there may be other factors that could affect the results that we have not been able take account of.”
“Our study suggests that antibiotics should be used only when they are absolutely needed,” he concluded. “Considering the potentially cumulative adverse effects, the shorter time of antibiotic use the better.”
The study was supported by National Institutes of Health grants, the Boston Obesity Nutrition Research Center, and the United States–Israel Binational Science Foundation. One author received support from the Japan Society for the Promotion of Science. The authors had no conflicts of interest.
SOURCE: Heianza Y et al. Eur Heart J. 2019 Apr 24. doi: 10.1093/eurheartj/ehz231.
, according to a study in the European Heart Journal.

Women in the Nurses’ Health Study who used antibiotics for 2 or more months between ages 40 and 59 years or at age 60 years and older had a significantly increased risk of cardiovascular disease, compared with those who did not use antibiotics. Antibiotic use between 20 and 39 years old was not significantly related to cardiovascular disease.
Prior research has found that antibiotics may have long-lasting effects on gut microbiota and relate to cardiovascular disease risk.
“Antibiotic use is the most critical factor in altering the balance of microorganisms in the gut,” said lead investigator Lu Qi, MD, PhD, in a news release. “Previous studies have shown a link between alterations in the microbiotic environment of the gut and inflammation and narrowing of the blood vessels, stroke, and heart disease,” said Dr. Qi, who is the director of the Tulane University Obesity Research Center in New Orleans and an adjunct professor of nutrition at Harvard T.C. Chan School of Public Health in Boston.
To evaluate associations between life stage, antibiotic exposure, and subsequent cardiovascular disease, researchers analyzed data from 36,429 participants in the Nurses’ Health Study. The women were at least 60 years old and had no history of cardiovascular disease or cancer when they completed a 2004 questionnaire about antibiotic usage during young, middle, and late adulthood. The questionnaire asked participants to indicate the total time using antibiotics with eight categories ranging from none to 5 or more years.
The researchers defined incident cardiovascular disease as a composite endpoint of coronary heart disease (nonfatal myocardial infarction or fatal coronary heart disease) and stroke (nonfatal or fatal). They calculated person-years of follow-up from the questionnaire return date until date of cardiovascular disease diagnosis, death, or end of follow-up in 2012.
Women with longer duration of antibiotic use were more likely to use other medications and have unfavorable cardiovascular risk profiles, including family history of myocardial infarction and higher body mass index. Antibiotics most often were used to treat respiratory infections. During an average follow-up of 7.6 years, 1,056 participants developed cardiovascular disease.
In a multivariable model that adjusted for demographics, diet, lifestyle, reason for antibiotic use, medications, overweight status, and other factors, long-term antibiotic use – 2 months or more – in late adulthood was associated with significantly increased risk of cardiovascular disease (hazard ratio, 1.32), as was long-term antibiotic use in middle adulthood (HR, 1.28).
Although antibiotic use was self-reported, which could lead to misclassification, the participants were health professionals, which may mitigate this limitation, the authors noted. Whether these findings apply to men and other populations requires further study, they said.
Because of the study’s observational design, the results “cannot show that antibiotics cause heart disease and stroke, only that there is a link between them,” Dr. Qi said. “It’s possible that women who reported more antibiotic use might be sicker in other ways that we were unable to measure, or there may be other factors that could affect the results that we have not been able take account of.”
“Our study suggests that antibiotics should be used only when they are absolutely needed,” he concluded. “Considering the potentially cumulative adverse effects, the shorter time of antibiotic use the better.”
The study was supported by National Institutes of Health grants, the Boston Obesity Nutrition Research Center, and the United States–Israel Binational Science Foundation. One author received support from the Japan Society for the Promotion of Science. The authors had no conflicts of interest.
SOURCE: Heianza Y et al. Eur Heart J. 2019 Apr 24. doi: 10.1093/eurheartj/ehz231.
FROM THE EUROPEAN HEART JOURNAL
Key clinical point: Among middle-aged and older women, 2 or more months’ exposure to antibiotics is associated with an increased risk of coronary heart disease or stroke.
Major finding: Long-term antibiotic use in late adulthood was associated with significantly increased risk of cardiovascular disease (hazard ratio, 1.32), as was long-term antibiotic use in middle adulthood (HR, 1.28).
Study details: An analysis of data from nearly 36,500 women in the Nurses’ Health Study.
Disclosures: The study was supported by National Institutes of Health grants, the Boston Obesity Nutrition Research Center, and the United States–Israel Binational Science Foundation. One author received support from the Japan Society for the Promotion of Science. The authors had no conflicts of interest.
Source: Heianza Y et al. Eur Heart J. 2019 Apr 24. doi: 10.1093/eurheartj/ehz231.
TMS is associated with improved recollection in older adults
according to a small pilot study published online ahead of print April 17 in Neurology. Stimulation also increased functional MRI signals associated with recollection throughout the hippocampal-cortical network.
“Disruption and abnormal functioning of the hippocampal-cortical network, the region of the brain involved in memory formation, has been linked to age-related memory decline, so it’s exciting to see that, by targeting this region, magnetic stimulation may help improve memory in older adults,” said Joel L. Voss, PhD, of Northwestern University in Chicago. “These results may help us better understand how this network supports memory.”
Recollection is the type of memory most impaired during normal aging. Other types of memory, such as recognition, are relatively spared. Research indicates that multiple sessions of TMS improve recollection and hippocampal-cortical network function in young adults.
Dr. Voss and colleagues performed a pilot study to evaluate whether TMS could improve recollection in older adults. They enrolled 15 cognitively normal older adults (mean age, 72.46 years) into a sham-controlled, single-blind, counterbalanced experiment. Eleven subjects were women. Participants underwent fMRI while learning objects paired with scenes and locations. Investigators administered TMS to lateral parietal locations that were based on each participant’s fMRI connectivity with the hippocampus. Using a within-subjects crossover design, Dr. Voss’s group assessed participants’ recollection and recognition memory at baseline and 24 hours and also 1 week after five consecutive daily sessions of full-intensity stimulation, compared with low-intensity sham stimulation.
At baseline, participants had impaired recollection, but not impaired recognition, compared with a historical sample of younger adults. At 24 hours, TMS provided robust recollection improvement and weak recognition improvement, compared with sham. TMS improved recollection by 31.1% from baseline, and sham yielded a nonsignificant change of −3.1%. Recollection improvements after TMS were consistent across participants. Recognition changed by a nonsignificant 2.8% following TMS and by a nonsignificant −2.9% following sham.
The investigators also found a significant and consistent increase in fMRI recollection activity for the targeted hippocampal-cortical network, but not for the control frontal-parietal network. They observed no increased fMRI activity for recognition.
“These findings demonstrate a causal link between recollection and the hippocampal-cortical network in older adults,” said Dr. Voss. “While our small study examined age-related memory loss, it did not examine this stimulation in people with memory loss from more serious conditions such as mild cognitive impairment or Alzheimer’s disease.” Furthermore, the study was designed to test for neural and behavioral target engagement, but not clinical efficacy, he added.
The study’s limitations included its small sample size, its single-site design, and its lack of active control stimulation. Nevertheless, it identified specific and consistent effects of stimulation across participants that were consistent with previous findings in younger adults. “These findings motivate future studies to optimize the effectiveness of noninvasive stimulation for treatment of age-related memory impairment and to improve mechanistic understanding of the hippocampal-cortical networks that support episodic memory across the lifespan,” said Dr. Voss.
The National Institute on Aging, as well as the Northwestern University Cognitive Neurology and Alzheimer’s Disease Center, supported the study.
SOURCE: Nilakantan AS et al. Neurology. 2019 Apr 17 doi: 10.1212/WNL.0000000000007502.
according to a small pilot study published online ahead of print April 17 in Neurology. Stimulation also increased functional MRI signals associated with recollection throughout the hippocampal-cortical network.
“Disruption and abnormal functioning of the hippocampal-cortical network, the region of the brain involved in memory formation, has been linked to age-related memory decline, so it’s exciting to see that, by targeting this region, magnetic stimulation may help improve memory in older adults,” said Joel L. Voss, PhD, of Northwestern University in Chicago. “These results may help us better understand how this network supports memory.”
Recollection is the type of memory most impaired during normal aging. Other types of memory, such as recognition, are relatively spared. Research indicates that multiple sessions of TMS improve recollection and hippocampal-cortical network function in young adults.
Dr. Voss and colleagues performed a pilot study to evaluate whether TMS could improve recollection in older adults. They enrolled 15 cognitively normal older adults (mean age, 72.46 years) into a sham-controlled, single-blind, counterbalanced experiment. Eleven subjects were women. Participants underwent fMRI while learning objects paired with scenes and locations. Investigators administered TMS to lateral parietal locations that were based on each participant’s fMRI connectivity with the hippocampus. Using a within-subjects crossover design, Dr. Voss’s group assessed participants’ recollection and recognition memory at baseline and 24 hours and also 1 week after five consecutive daily sessions of full-intensity stimulation, compared with low-intensity sham stimulation.
At baseline, participants had impaired recollection, but not impaired recognition, compared with a historical sample of younger adults. At 24 hours, TMS provided robust recollection improvement and weak recognition improvement, compared with sham. TMS improved recollection by 31.1% from baseline, and sham yielded a nonsignificant change of −3.1%. Recollection improvements after TMS were consistent across participants. Recognition changed by a nonsignificant 2.8% following TMS and by a nonsignificant −2.9% following sham.
The investigators also found a significant and consistent increase in fMRI recollection activity for the targeted hippocampal-cortical network, but not for the control frontal-parietal network. They observed no increased fMRI activity for recognition.
“These findings demonstrate a causal link between recollection and the hippocampal-cortical network in older adults,” said Dr. Voss. “While our small study examined age-related memory loss, it did not examine this stimulation in people with memory loss from more serious conditions such as mild cognitive impairment or Alzheimer’s disease.” Furthermore, the study was designed to test for neural and behavioral target engagement, but not clinical efficacy, he added.
The study’s limitations included its small sample size, its single-site design, and its lack of active control stimulation. Nevertheless, it identified specific and consistent effects of stimulation across participants that were consistent with previous findings in younger adults. “These findings motivate future studies to optimize the effectiveness of noninvasive stimulation for treatment of age-related memory impairment and to improve mechanistic understanding of the hippocampal-cortical networks that support episodic memory across the lifespan,” said Dr. Voss.
The National Institute on Aging, as well as the Northwestern University Cognitive Neurology and Alzheimer’s Disease Center, supported the study.
SOURCE: Nilakantan AS et al. Neurology. 2019 Apr 17 doi: 10.1212/WNL.0000000000007502.
according to a small pilot study published online ahead of print April 17 in Neurology. Stimulation also increased functional MRI signals associated with recollection throughout the hippocampal-cortical network.
“Disruption and abnormal functioning of the hippocampal-cortical network, the region of the brain involved in memory formation, has been linked to age-related memory decline, so it’s exciting to see that, by targeting this region, magnetic stimulation may help improve memory in older adults,” said Joel L. Voss, PhD, of Northwestern University in Chicago. “These results may help us better understand how this network supports memory.”
Recollection is the type of memory most impaired during normal aging. Other types of memory, such as recognition, are relatively spared. Research indicates that multiple sessions of TMS improve recollection and hippocampal-cortical network function in young adults.
Dr. Voss and colleagues performed a pilot study to evaluate whether TMS could improve recollection in older adults. They enrolled 15 cognitively normal older adults (mean age, 72.46 years) into a sham-controlled, single-blind, counterbalanced experiment. Eleven subjects were women. Participants underwent fMRI while learning objects paired with scenes and locations. Investigators administered TMS to lateral parietal locations that were based on each participant’s fMRI connectivity with the hippocampus. Using a within-subjects crossover design, Dr. Voss’s group assessed participants’ recollection and recognition memory at baseline and 24 hours and also 1 week after five consecutive daily sessions of full-intensity stimulation, compared with low-intensity sham stimulation.
At baseline, participants had impaired recollection, but not impaired recognition, compared with a historical sample of younger adults. At 24 hours, TMS provided robust recollection improvement and weak recognition improvement, compared with sham. TMS improved recollection by 31.1% from baseline, and sham yielded a nonsignificant change of −3.1%. Recollection improvements after TMS were consistent across participants. Recognition changed by a nonsignificant 2.8% following TMS and by a nonsignificant −2.9% following sham.
The investigators also found a significant and consistent increase in fMRI recollection activity for the targeted hippocampal-cortical network, but not for the control frontal-parietal network. They observed no increased fMRI activity for recognition.
“These findings demonstrate a causal link between recollection and the hippocampal-cortical network in older adults,” said Dr. Voss. “While our small study examined age-related memory loss, it did not examine this stimulation in people with memory loss from more serious conditions such as mild cognitive impairment or Alzheimer’s disease.” Furthermore, the study was designed to test for neural and behavioral target engagement, but not clinical efficacy, he added.
The study’s limitations included its small sample size, its single-site design, and its lack of active control stimulation. Nevertheless, it identified specific and consistent effects of stimulation across participants that were consistent with previous findings in younger adults. “These findings motivate future studies to optimize the effectiveness of noninvasive stimulation for treatment of age-related memory impairment and to improve mechanistic understanding of the hippocampal-cortical networks that support episodic memory across the lifespan,” said Dr. Voss.
The National Institute on Aging, as well as the Northwestern University Cognitive Neurology and Alzheimer’s Disease Center, supported the study.
SOURCE: Nilakantan AS et al. Neurology. 2019 Apr 17 doi: 10.1212/WNL.0000000000007502.
FROM NEUROLOGY
Plasma levels of neurofilament light track neurodegeneration in MCI and Alzheimer’s disease
Plasma levels of the axonal protein also correlated with its presence in cerebrospinal fluid, and mirrored the changes in amyloid beta (Abeta) 42, Niklas Mattsson, MD, and colleagues wrote in JAMA Neurology.
“Taken together, these findings suggest that the neurofilament light level is a dynamic biomarker that changes throughout the course of Alzheimer’s disease and is sensitive to progressive neurodegeneration,” wrote Dr. Mattsson of Lund (Sweden) University and his coauthors. “This has important implications, given the unmet need for noninvasive blood-based methods to objectively track longitudinal neurodegeneration in Alzheimer’s disease.”
A blood-based biomarker of Alzheimer’s disease progression could open a new door for drug trials, the authors noted. Previously, NfL levels have only been available by lumbar puncture – a invasive and expensive test that many patients resist. If NfL plasma levels do reliably track dementia progression, the test could become a standard part of clinical trials, providing regular drug response data as the study progresses.
Neurofilaments are polypeptides that give structure to the neuronal cytoskeleton and regulate microtubule function. Injured cells release the protein very quickly. Neurofilaments are elevated in traumatic brain injury, multiple sclerosis, and some psychiatric illnesses. Neurofilament levels have even been used to predict neurologic recovery after cardiac arrest.
Dr. Mattsson and his team used data obtained over 11 years from 1,583 subjects enrolled in the Alzheimer’s Disease Neuroimaging Initiative study. The sample comprised three groups: cognitively unimpaired controls (401), patients with MCI (855), and patients with Alzheimer’s dementia (327). The investigators analyzed 4,326 samples.
In addition to the NfL measurements, they tracked Abeta and tau in cerebrospinal fluid (CSF), structural brain changes by 18fluorodeoxyglucose (FDG)–PET and MRI, and cognitive and functional performance. The primary outcome was NfL’s association with these changes. The team set the lower limit of NfL as 6.7 ng/L and the upper, 1,620 ng/L.
At baseline, only advancing age correlated with NfL levels. But it was significantly higher in patients with MCI (37.9 ng/L) and Alzheimer’s dementia (45.9 ng/L) than it was in the control subjects (32.1 ng/L). Over the years of follow-up, levels increased in all groups, but NfL increased more rapidly among patients with MCI and Alzheimer’s dementia than controls (2.7 vs. 2.4 ng/L per year). The difference was most pronounced when comparing levels in patients with Alzheimer’s dementia and MCI with controls. However, control subjects who were Abeta positive by CSF had greater NfL changes than Abeta-negative controls.
Baseline measures of CSF Abeta and tau, as well as hippocampal and ventricular volume and cortical thickness, also correlated with NfL levels, as did cognition and functional scores.
During follow-up, the diagnostic groups showed different NfL trajectories, which correlated strongly with the other measures.
In the control group, NfL increases correlated with lower FDG-PET measures, lower CSF Abeta, reduced hippocampal volume, and higher ventricular volume. Among patients with MCI, NfL increases correlated most strongly with hippocampal volume, temporal region, and cognition. Among patients with Alzheimer’s dementia, the NfL increase most strongly tracked cognitive decline,
When the investigators applied these findings to the A/T/N (amyloid, tau, neurodegeneration) classification system, NfL most often correlated with neurodegeneration, but not always. This might suggest that the neuronal damage occurred separately from Abeta changes. In all three groups, rapid NfL increases mirrored the rate of change in most other measures.
“In controls and patients with MCI and Alzheimer’s dementia, greater rates of NfL were associated with accelerated reduction in FDG-PET measures … expansion of ventricular volume,” and a reduction in cognitive and functional performance, Dr. Mattsson and his colleagues wrote.“ In addition, greater increases in NfL levels were associated with accelerated loss of hippocampal volume and entorhinal cortical thickness in controls and patients with MCI and with accelerated increases in total tau level, phosphorylated tau level, and white matter lesions in patients with MCI. In general, the concentrations of blood-based NfL appears to reflect the intensity of the neuronal injury.”
Dr Mattsson reported being a consultant for the Alzheimer’s Disease Neuroimaging Initiative.
SOURCE: Mattsson N et al. JAMA Neurol. 2019 Apr 22. doi: 10.1001/jamaneurol.2019.0765.
This is an impressive study that convincingly demonstrates the sensitivity of plasma neurofilament light (NfL) to disease progression in patients with mild cognitive impairment and dementia in the Alzheimer’s Disease Neuroimaging Initiative cohort.
It is known that NfL is sensitive to neuronal damage that can result from a variety of pathologies, not limited to Alzheimer’s disease, so it is not diagnostically specific. In the present study there was even overlap of values between the cognitively unimpaired and mild cognitive impairment groups at baseline as well, although the levels separated over time.
In the right setting, NfL might still be a clinically useful diagnostic marker, especially for mild-stage disease when other clinical measures such as mental status scores and structural brain scans are inconclusive, and further study seems warranted for such a possibility.
Its greatest utility, however, will likely be in clinical trials. It would have been of the greatest interest to know how NfL levels changed in the setting of demonstrated cerebral amyloid clearance by agents such as aducanumab that failed to halt dementia progression, or in the setting of BACE1 inhibitor-related worsening of cognition. Did NfL levels remain static in the former and rise in the latter? Looking ahead, it seems likely that this easily accessed biomarker will become an integral part of clinical trial design. Assuming cost is not overly burdensome, it may even find its way eventually into clinical practice.
Dr. Caselli is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
This is an impressive study that convincingly demonstrates the sensitivity of plasma neurofilament light (NfL) to disease progression in patients with mild cognitive impairment and dementia in the Alzheimer’s Disease Neuroimaging Initiative cohort.
It is known that NfL is sensitive to neuronal damage that can result from a variety of pathologies, not limited to Alzheimer’s disease, so it is not diagnostically specific. In the present study there was even overlap of values between the cognitively unimpaired and mild cognitive impairment groups at baseline as well, although the levels separated over time.
In the right setting, NfL might still be a clinically useful diagnostic marker, especially for mild-stage disease when other clinical measures such as mental status scores and structural brain scans are inconclusive, and further study seems warranted for such a possibility.
Its greatest utility, however, will likely be in clinical trials. It would have been of the greatest interest to know how NfL levels changed in the setting of demonstrated cerebral amyloid clearance by agents such as aducanumab that failed to halt dementia progression, or in the setting of BACE1 inhibitor-related worsening of cognition. Did NfL levels remain static in the former and rise in the latter? Looking ahead, it seems likely that this easily accessed biomarker will become an integral part of clinical trial design. Assuming cost is not overly burdensome, it may even find its way eventually into clinical practice.
Dr. Caselli is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
This is an impressive study that convincingly demonstrates the sensitivity of plasma neurofilament light (NfL) to disease progression in patients with mild cognitive impairment and dementia in the Alzheimer’s Disease Neuroimaging Initiative cohort.
It is known that NfL is sensitive to neuronal damage that can result from a variety of pathologies, not limited to Alzheimer’s disease, so it is not diagnostically specific. In the present study there was even overlap of values between the cognitively unimpaired and mild cognitive impairment groups at baseline as well, although the levels separated over time.
In the right setting, NfL might still be a clinically useful diagnostic marker, especially for mild-stage disease when other clinical measures such as mental status scores and structural brain scans are inconclusive, and further study seems warranted for such a possibility.
Its greatest utility, however, will likely be in clinical trials. It would have been of the greatest interest to know how NfL levels changed in the setting of demonstrated cerebral amyloid clearance by agents such as aducanumab that failed to halt dementia progression, or in the setting of BACE1 inhibitor-related worsening of cognition. Did NfL levels remain static in the former and rise in the latter? Looking ahead, it seems likely that this easily accessed biomarker will become an integral part of clinical trial design. Assuming cost is not overly burdensome, it may even find its way eventually into clinical practice.
Dr. Caselli is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
Plasma levels of the axonal protein also correlated with its presence in cerebrospinal fluid, and mirrored the changes in amyloid beta (Abeta) 42, Niklas Mattsson, MD, and colleagues wrote in JAMA Neurology.
“Taken together, these findings suggest that the neurofilament light level is a dynamic biomarker that changes throughout the course of Alzheimer’s disease and is sensitive to progressive neurodegeneration,” wrote Dr. Mattsson of Lund (Sweden) University and his coauthors. “This has important implications, given the unmet need for noninvasive blood-based methods to objectively track longitudinal neurodegeneration in Alzheimer’s disease.”
A blood-based biomarker of Alzheimer’s disease progression could open a new door for drug trials, the authors noted. Previously, NfL levels have only been available by lumbar puncture – a invasive and expensive test that many patients resist. If NfL plasma levels do reliably track dementia progression, the test could become a standard part of clinical trials, providing regular drug response data as the study progresses.
Neurofilaments are polypeptides that give structure to the neuronal cytoskeleton and regulate microtubule function. Injured cells release the protein very quickly. Neurofilaments are elevated in traumatic brain injury, multiple sclerosis, and some psychiatric illnesses. Neurofilament levels have even been used to predict neurologic recovery after cardiac arrest.
Dr. Mattsson and his team used data obtained over 11 years from 1,583 subjects enrolled in the Alzheimer’s Disease Neuroimaging Initiative study. The sample comprised three groups: cognitively unimpaired controls (401), patients with MCI (855), and patients with Alzheimer’s dementia (327). The investigators analyzed 4,326 samples.
In addition to the NfL measurements, they tracked Abeta and tau in cerebrospinal fluid (CSF), structural brain changes by 18fluorodeoxyglucose (FDG)–PET and MRI, and cognitive and functional performance. The primary outcome was NfL’s association with these changes. The team set the lower limit of NfL as 6.7 ng/L and the upper, 1,620 ng/L.
At baseline, only advancing age correlated with NfL levels. But it was significantly higher in patients with MCI (37.9 ng/L) and Alzheimer’s dementia (45.9 ng/L) than it was in the control subjects (32.1 ng/L). Over the years of follow-up, levels increased in all groups, but NfL increased more rapidly among patients with MCI and Alzheimer’s dementia than controls (2.7 vs. 2.4 ng/L per year). The difference was most pronounced when comparing levels in patients with Alzheimer’s dementia and MCI with controls. However, control subjects who were Abeta positive by CSF had greater NfL changes than Abeta-negative controls.
Baseline measures of CSF Abeta and tau, as well as hippocampal and ventricular volume and cortical thickness, also correlated with NfL levels, as did cognition and functional scores.
During follow-up, the diagnostic groups showed different NfL trajectories, which correlated strongly with the other measures.
In the control group, NfL increases correlated with lower FDG-PET measures, lower CSF Abeta, reduced hippocampal volume, and higher ventricular volume. Among patients with MCI, NfL increases correlated most strongly with hippocampal volume, temporal region, and cognition. Among patients with Alzheimer’s dementia, the NfL increase most strongly tracked cognitive decline,
When the investigators applied these findings to the A/T/N (amyloid, tau, neurodegeneration) classification system, NfL most often correlated with neurodegeneration, but not always. This might suggest that the neuronal damage occurred separately from Abeta changes. In all three groups, rapid NfL increases mirrored the rate of change in most other measures.
“In controls and patients with MCI and Alzheimer’s dementia, greater rates of NfL were associated with accelerated reduction in FDG-PET measures … expansion of ventricular volume,” and a reduction in cognitive and functional performance, Dr. Mattsson and his colleagues wrote.“ In addition, greater increases in NfL levels were associated with accelerated loss of hippocampal volume and entorhinal cortical thickness in controls and patients with MCI and with accelerated increases in total tau level, phosphorylated tau level, and white matter lesions in patients with MCI. In general, the concentrations of blood-based NfL appears to reflect the intensity of the neuronal injury.”
Dr Mattsson reported being a consultant for the Alzheimer’s Disease Neuroimaging Initiative.
SOURCE: Mattsson N et al. JAMA Neurol. 2019 Apr 22. doi: 10.1001/jamaneurol.2019.0765.
Plasma levels of the axonal protein also correlated with its presence in cerebrospinal fluid, and mirrored the changes in amyloid beta (Abeta) 42, Niklas Mattsson, MD, and colleagues wrote in JAMA Neurology.
“Taken together, these findings suggest that the neurofilament light level is a dynamic biomarker that changes throughout the course of Alzheimer’s disease and is sensitive to progressive neurodegeneration,” wrote Dr. Mattsson of Lund (Sweden) University and his coauthors. “This has important implications, given the unmet need for noninvasive blood-based methods to objectively track longitudinal neurodegeneration in Alzheimer’s disease.”
A blood-based biomarker of Alzheimer’s disease progression could open a new door for drug trials, the authors noted. Previously, NfL levels have only been available by lumbar puncture – a invasive and expensive test that many patients resist. If NfL plasma levels do reliably track dementia progression, the test could become a standard part of clinical trials, providing regular drug response data as the study progresses.
Neurofilaments are polypeptides that give structure to the neuronal cytoskeleton and regulate microtubule function. Injured cells release the protein very quickly. Neurofilaments are elevated in traumatic brain injury, multiple sclerosis, and some psychiatric illnesses. Neurofilament levels have even been used to predict neurologic recovery after cardiac arrest.
Dr. Mattsson and his team used data obtained over 11 years from 1,583 subjects enrolled in the Alzheimer’s Disease Neuroimaging Initiative study. The sample comprised three groups: cognitively unimpaired controls (401), patients with MCI (855), and patients with Alzheimer’s dementia (327). The investigators analyzed 4,326 samples.
In addition to the NfL measurements, they tracked Abeta and tau in cerebrospinal fluid (CSF), structural brain changes by 18fluorodeoxyglucose (FDG)–PET and MRI, and cognitive and functional performance. The primary outcome was NfL’s association with these changes. The team set the lower limit of NfL as 6.7 ng/L and the upper, 1,620 ng/L.
At baseline, only advancing age correlated with NfL levels. But it was significantly higher in patients with MCI (37.9 ng/L) and Alzheimer’s dementia (45.9 ng/L) than it was in the control subjects (32.1 ng/L). Over the years of follow-up, levels increased in all groups, but NfL increased more rapidly among patients with MCI and Alzheimer’s dementia than controls (2.7 vs. 2.4 ng/L per year). The difference was most pronounced when comparing levels in patients with Alzheimer’s dementia and MCI with controls. However, control subjects who were Abeta positive by CSF had greater NfL changes than Abeta-negative controls.
Baseline measures of CSF Abeta and tau, as well as hippocampal and ventricular volume and cortical thickness, also correlated with NfL levels, as did cognition and functional scores.
During follow-up, the diagnostic groups showed different NfL trajectories, which correlated strongly with the other measures.
In the control group, NfL increases correlated with lower FDG-PET measures, lower CSF Abeta, reduced hippocampal volume, and higher ventricular volume. Among patients with MCI, NfL increases correlated most strongly with hippocampal volume, temporal region, and cognition. Among patients with Alzheimer’s dementia, the NfL increase most strongly tracked cognitive decline,
When the investigators applied these findings to the A/T/N (amyloid, tau, neurodegeneration) classification system, NfL most often correlated with neurodegeneration, but not always. This might suggest that the neuronal damage occurred separately from Abeta changes. In all three groups, rapid NfL increases mirrored the rate of change in most other measures.
“In controls and patients with MCI and Alzheimer’s dementia, greater rates of NfL were associated with accelerated reduction in FDG-PET measures … expansion of ventricular volume,” and a reduction in cognitive and functional performance, Dr. Mattsson and his colleagues wrote.“ In addition, greater increases in NfL levels were associated with accelerated loss of hippocampal volume and entorhinal cortical thickness in controls and patients with MCI and with accelerated increases in total tau level, phosphorylated tau level, and white matter lesions in patients with MCI. In general, the concentrations of blood-based NfL appears to reflect the intensity of the neuronal injury.”
Dr Mattsson reported being a consultant for the Alzheimer’s Disease Neuroimaging Initiative.
SOURCE: Mattsson N et al. JAMA Neurol. 2019 Apr 22. doi: 10.1001/jamaneurol.2019.0765.
FROM JAMA NEUROLOGY
Can immune checkpoint inhibitors treat PML?
investigators reported in the New England Journal of Medicine.
Three research teams described 10 cases in which patients with PML received pembrolizumab or nivolumab.
In one study, researchers administered pembrolizumab to eight adults with PML. Five patients had clinical improvement or stabilization, whereas 3 patients did not. Among the patients with clinical improvement, treatment led to reduced JC viral load in cerebrospinal fluid (CSF) and increased CD4+ and CD8+ anti–JC virus activity in vitro. Among patients without clinical improvement, treatment did not meaningfully change viral load or antiviral cellular immune response.
In a separate letter, researchers in Germany described an additional patient with PML who had clinical stabilization and no disease progression on MRI after treatment with pembrolizumab.
In another letter, researchers in France described a patient with PML whose condition improved after treatment with nivolumab.
“Do pembrolizumab and nivolumab fit the bill for treatment of PML? The current reports are encouraging but suggest that the presence of JC virus–specific T cells in the blood is a prerequisite for their use,” said Igor J. Koralnik, MD, of the department of neurological sciences at Rush University Medical Center in Chicago, in an accompanying editorial. “A controlled trial may be needed to determine whether immune checkpoint inhibitors are indeed able to keep JC virus in check in patients with PML.”
Reinvigorating T cells
Both monoclonal antibodies target programmed cell death protein 1 (PD-1), which inhibits T-cell proliferation and cytokine production when it binds its associated ligand, Dr. Koralnik said. Pembrolizumab and nivolumab block this inhibition and have been used to spur T-cell activity against tumors in patients with cancer.
PML, an often fatal brain infection caused by the JC virus in patients with immunosuppression, has no specific treatment. Management hinges on “recovery of the immune system, either by treating the underlying cause of immunosuppression or by discontinuing the use of immunosuppressive medications,” said Dr. Koralnik.
Pembrolizumab
Prior studies have found that PD-1 expression is elevated on T lymphocytes of patients with PML. To determine whether PD-1 blockade with pembrolizumab reinvigorates anti–JC virus immune activity in patients with PML, Irene Cortese, MD, of the National Institutes of Health’s Neuroimmunology Clinic and her research colleagues administered pembrolizumab at a dose of 2 mg/kg of body weight every 4-6 weeks to eight adults with PML. The patients received 1-3 doses, and each patient had a different underlying condition.
In all patients, treatment induced down-regulation of PD-1 expression on lymphocytes in CSF and peripheral blood, and five of the eight patients had clinical stabilization or improvement. Of the other three patients who did not improve, one had stabilized prior to treatment and remained stable. The other two patients died from PML.
Additional reports
Separately, Sebastian Rauer, MD, of Albert Ludwigs University in Freiburg, Germany, and his colleagues reported that a patient with PML whose symptoms culminated in mutism in February 2018 began speaking again after receiving five infusions of pembrolizumab over 10 weeks. “In addition, the size and number of lesions on MRI decreased, and JCV was no longer detectable in CSF,” Dr. Rauer and his colleagues wrote. “The patient has remained stable as of the end of March 2019, with persistent but abating psychomotor slowing, aphasia, and disorientation.”
Finally, Ondine Walter, of Toulouse (France) University Hospital and colleagues described the case of a 60-year-old woman with PML who received nivolumab on a compassionate-use basis. Two weeks after treatment, JC viral load in CSF and blood had decreased. “Starting 8 weeks after the initiation of nivolumab therapy, the patient’s neurologic symptoms and signs stabilized, and subsequently she showed improved alertness, and the ptosis and hemiplegia abated.”
Reason for caution
Prior studies, however, give reasons for caution when considering the potential use of immune checkpoint inhibitors to treat PML, Dr. Koralnik noted. In one case, a patient developed an inflammatory form of PML known as immune reconstitution inflammatory syndrome after receiving nivolumab (J Neurovirol. 2019 March 12. doi: 10.1007/s13365-019-00738-x). In addition, researchers have reported a case of PML that occurred after 1 year of nivolumab treatment, and four cases of PML related to nivolumab have been reported in pharmacovigilance databases (Emerg Infect Dis. 2018;24:1594-6). The cost and safety profiles of the medications also may be considerations, Dr. Koralnik said.
The study by Dr. Cortese and colleagues was funded by the National Institutes of Health, and the authors had no relevant disclosures. Some of the research letter authors disclosed grants and personal fees from pharmaceutical companies.
SOURCES: Cortese I et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMoa1815039; Rauer S et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1817193; Walter O et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1816198; Koralnik IJ. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMe1904140.
investigators reported in the New England Journal of Medicine.
Three research teams described 10 cases in which patients with PML received pembrolizumab or nivolumab.
In one study, researchers administered pembrolizumab to eight adults with PML. Five patients had clinical improvement or stabilization, whereas 3 patients did not. Among the patients with clinical improvement, treatment led to reduced JC viral load in cerebrospinal fluid (CSF) and increased CD4+ and CD8+ anti–JC virus activity in vitro. Among patients without clinical improvement, treatment did not meaningfully change viral load or antiviral cellular immune response.
In a separate letter, researchers in Germany described an additional patient with PML who had clinical stabilization and no disease progression on MRI after treatment with pembrolizumab.
In another letter, researchers in France described a patient with PML whose condition improved after treatment with nivolumab.
“Do pembrolizumab and nivolumab fit the bill for treatment of PML? The current reports are encouraging but suggest that the presence of JC virus–specific T cells in the blood is a prerequisite for their use,” said Igor J. Koralnik, MD, of the department of neurological sciences at Rush University Medical Center in Chicago, in an accompanying editorial. “A controlled trial may be needed to determine whether immune checkpoint inhibitors are indeed able to keep JC virus in check in patients with PML.”
Reinvigorating T cells
Both monoclonal antibodies target programmed cell death protein 1 (PD-1), which inhibits T-cell proliferation and cytokine production when it binds its associated ligand, Dr. Koralnik said. Pembrolizumab and nivolumab block this inhibition and have been used to spur T-cell activity against tumors in patients with cancer.
PML, an often fatal brain infection caused by the JC virus in patients with immunosuppression, has no specific treatment. Management hinges on “recovery of the immune system, either by treating the underlying cause of immunosuppression or by discontinuing the use of immunosuppressive medications,” said Dr. Koralnik.
Pembrolizumab
Prior studies have found that PD-1 expression is elevated on T lymphocytes of patients with PML. To determine whether PD-1 blockade with pembrolizumab reinvigorates anti–JC virus immune activity in patients with PML, Irene Cortese, MD, of the National Institutes of Health’s Neuroimmunology Clinic and her research colleagues administered pembrolizumab at a dose of 2 mg/kg of body weight every 4-6 weeks to eight adults with PML. The patients received 1-3 doses, and each patient had a different underlying condition.
In all patients, treatment induced down-regulation of PD-1 expression on lymphocytes in CSF and peripheral blood, and five of the eight patients had clinical stabilization or improvement. Of the other three patients who did not improve, one had stabilized prior to treatment and remained stable. The other two patients died from PML.
Additional reports
Separately, Sebastian Rauer, MD, of Albert Ludwigs University in Freiburg, Germany, and his colleagues reported that a patient with PML whose symptoms culminated in mutism in February 2018 began speaking again after receiving five infusions of pembrolizumab over 10 weeks. “In addition, the size and number of lesions on MRI decreased, and JCV was no longer detectable in CSF,” Dr. Rauer and his colleagues wrote. “The patient has remained stable as of the end of March 2019, with persistent but abating psychomotor slowing, aphasia, and disorientation.”
Finally, Ondine Walter, of Toulouse (France) University Hospital and colleagues described the case of a 60-year-old woman with PML who received nivolumab on a compassionate-use basis. Two weeks after treatment, JC viral load in CSF and blood had decreased. “Starting 8 weeks after the initiation of nivolumab therapy, the patient’s neurologic symptoms and signs stabilized, and subsequently she showed improved alertness, and the ptosis and hemiplegia abated.”
Reason for caution
Prior studies, however, give reasons for caution when considering the potential use of immune checkpoint inhibitors to treat PML, Dr. Koralnik noted. In one case, a patient developed an inflammatory form of PML known as immune reconstitution inflammatory syndrome after receiving nivolumab (J Neurovirol. 2019 March 12. doi: 10.1007/s13365-019-00738-x). In addition, researchers have reported a case of PML that occurred after 1 year of nivolumab treatment, and four cases of PML related to nivolumab have been reported in pharmacovigilance databases (Emerg Infect Dis. 2018;24:1594-6). The cost and safety profiles of the medications also may be considerations, Dr. Koralnik said.
The study by Dr. Cortese and colleagues was funded by the National Institutes of Health, and the authors had no relevant disclosures. Some of the research letter authors disclosed grants and personal fees from pharmaceutical companies.
SOURCES: Cortese I et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMoa1815039; Rauer S et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1817193; Walter O et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1816198; Koralnik IJ. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMe1904140.
investigators reported in the New England Journal of Medicine.
Three research teams described 10 cases in which patients with PML received pembrolizumab or nivolumab.
In one study, researchers administered pembrolizumab to eight adults with PML. Five patients had clinical improvement or stabilization, whereas 3 patients did not. Among the patients with clinical improvement, treatment led to reduced JC viral load in cerebrospinal fluid (CSF) and increased CD4+ and CD8+ anti–JC virus activity in vitro. Among patients without clinical improvement, treatment did not meaningfully change viral load or antiviral cellular immune response.
In a separate letter, researchers in Germany described an additional patient with PML who had clinical stabilization and no disease progression on MRI after treatment with pembrolizumab.
In another letter, researchers in France described a patient with PML whose condition improved after treatment with nivolumab.
“Do pembrolizumab and nivolumab fit the bill for treatment of PML? The current reports are encouraging but suggest that the presence of JC virus–specific T cells in the blood is a prerequisite for their use,” said Igor J. Koralnik, MD, of the department of neurological sciences at Rush University Medical Center in Chicago, in an accompanying editorial. “A controlled trial may be needed to determine whether immune checkpoint inhibitors are indeed able to keep JC virus in check in patients with PML.”
Reinvigorating T cells
Both monoclonal antibodies target programmed cell death protein 1 (PD-1), which inhibits T-cell proliferation and cytokine production when it binds its associated ligand, Dr. Koralnik said. Pembrolizumab and nivolumab block this inhibition and have been used to spur T-cell activity against tumors in patients with cancer.
PML, an often fatal brain infection caused by the JC virus in patients with immunosuppression, has no specific treatment. Management hinges on “recovery of the immune system, either by treating the underlying cause of immunosuppression or by discontinuing the use of immunosuppressive medications,” said Dr. Koralnik.
Pembrolizumab
Prior studies have found that PD-1 expression is elevated on T lymphocytes of patients with PML. To determine whether PD-1 blockade with pembrolizumab reinvigorates anti–JC virus immune activity in patients with PML, Irene Cortese, MD, of the National Institutes of Health’s Neuroimmunology Clinic and her research colleagues administered pembrolizumab at a dose of 2 mg/kg of body weight every 4-6 weeks to eight adults with PML. The patients received 1-3 doses, and each patient had a different underlying condition.
In all patients, treatment induced down-regulation of PD-1 expression on lymphocytes in CSF and peripheral blood, and five of the eight patients had clinical stabilization or improvement. Of the other three patients who did not improve, one had stabilized prior to treatment and remained stable. The other two patients died from PML.
Additional reports
Separately, Sebastian Rauer, MD, of Albert Ludwigs University in Freiburg, Germany, and his colleagues reported that a patient with PML whose symptoms culminated in mutism in February 2018 began speaking again after receiving five infusions of pembrolizumab over 10 weeks. “In addition, the size and number of lesions on MRI decreased, and JCV was no longer detectable in CSF,” Dr. Rauer and his colleagues wrote. “The patient has remained stable as of the end of March 2019, with persistent but abating psychomotor slowing, aphasia, and disorientation.”
Finally, Ondine Walter, of Toulouse (France) University Hospital and colleagues described the case of a 60-year-old woman with PML who received nivolumab on a compassionate-use basis. Two weeks after treatment, JC viral load in CSF and blood had decreased. “Starting 8 weeks after the initiation of nivolumab therapy, the patient’s neurologic symptoms and signs stabilized, and subsequently she showed improved alertness, and the ptosis and hemiplegia abated.”
Reason for caution
Prior studies, however, give reasons for caution when considering the potential use of immune checkpoint inhibitors to treat PML, Dr. Koralnik noted. In one case, a patient developed an inflammatory form of PML known as immune reconstitution inflammatory syndrome after receiving nivolumab (J Neurovirol. 2019 March 12. doi: 10.1007/s13365-019-00738-x). In addition, researchers have reported a case of PML that occurred after 1 year of nivolumab treatment, and four cases of PML related to nivolumab have been reported in pharmacovigilance databases (Emerg Infect Dis. 2018;24:1594-6). The cost and safety profiles of the medications also may be considerations, Dr. Koralnik said.
The study by Dr. Cortese and colleagues was funded by the National Institutes of Health, and the authors had no relevant disclosures. Some of the research letter authors disclosed grants and personal fees from pharmaceutical companies.
SOURCES: Cortese I et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMoa1815039; Rauer S et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1817193; Walter O et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1816198; Koralnik IJ. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMe1904140.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Low LDL cholesterol may increase women’s risk of hemorrhagic stroke
, according to research published in Neurology.
“Women with very low LDL cholesterol or low triglycerides should be monitored by their doctors for other stroke risk factors that can be modified, like high blood pressure and smoking, in order to reduce their risk of hemorrhagic stroke,” said Pamela M. Rist, ScD, instructor in epidemiology at Harvard Medical School, Boston. “Additional research is needed to determine how to lower the risk of hemorrhagic stroke in women with very low LDL and low triglycerides.”
Several meta-analyses have indicated that LDL cholesterol levels are inversely associated with the risk of hemorrhagic stroke. Because lipid-lowering treatments are used to prevent cardiovascular disease, this potential association has implications for clinical practice. Most of the studies included in these meta-analyses had low numbers of events among women, which prevented researchers from stratifying their results by sex. Because women are at greater risk of stroke than men, Dr. Rist and her colleagues sought to evaluate the association between lipid levels and risk of hemorrhagic stroke.
An analysis of the Women’s Health Study
The investigators examined data from the Women’s Health Study, a randomized, double-blind, placebo-controlled trial of low-dose aspirin and vitamin E for the primary prevention of cardiovascular disease and cancer among female American health professionals aged 45 years or older. The study ended in March 2004, but follow-up is ongoing. At regular intervals, the women complete a questionnaire about disease outcomes, including stroke. Some participants agreed to provide a fasting venous blood sample before randomization. With the subjects’ permission, a committee of physicians examined medical records for women who reported a stroke on a follow-up questionnaire.
Dr. Rist and her colleagues analyzed 27,937 samples for levels of LDL cholesterol, HDL cholesterol, total cholesterol, and triglycerides. They assigned each sample to one of five cholesterol level categories that were based on Adult Treatment Panel III guidelines. Cox proportional hazards models enabled the researchers to calculate the hazard ratio of incident hemorrhagic stroke events. They adjusted their results for covariates such as age, smoking status, menopausal status, body mass index, and alcohol consumption.
A U-shaped association
Women in the lowest category of LDL cholesterol level (less than 70 mg/dL) were younger, less likely to have a history of hypertension, and less likely to use cholesterol-lowering drugs than women in the reference group (100.0-129.9 mg/dL). Women with the lowest LDL cholesterol level were more likely to consume alcohol, have a normal weight, engage in physical activity, and be premenopausal than women in the reference group. The investigators confirmed 137 incident hemorrhagic stroke events during a mean 19.3 years of follow-up.
After data adjustment, the researchers found that women with the lowest level of LDL cholesterol had 2.17 times the risk of hemorrhagic stroke, compared with participants in the reference group. They found a trend toward increased risk among women with an LDL cholesterol level of 160 mg/dL or higher, but the result was not statistically significant. The highest risk for intracerebral hemorrhage (ICH) was among women with an LDL cholesterol level of less than 70 mg/dL (relative risk, 2.32), followed by women with a level of 160 mg/dL or higher (RR, 1.71).
In addition, after multivariable adjustment, women in the lowest quartile of triglycerides (less than or equal to 74 mg/dL for fasting and less than or equal to 85 mg/dL for nonfasting) had a significantly increased risk of hemorrhagic stroke, compared with women in the highest quartile (RR, 2.00). Low triglyceride levels were associated with an increased risk of subarachnoid hemorrhage, but not with an increased risk of ICH. Neither HDL cholesterol nor total cholesterol was associated with risk of hemorrhagic stroke, the researchers wrote.
Mechanism of increased risk unclear
The researchers do not yet know how low triglyceride and LDL cholesterol levels increase the risk of hemorrhagic stroke. One hypothesis is that low cholesterol promotes necrosis of the arterial medial layer’s smooth muscle cells. This impaired endothelium might be more susceptible to microaneurysms, which are common in patients with ICH, said the researchers.
The prospective design and the large sample size were two of the study’s strengths, but the study had important weaknesses as well, the researchers wrote. For example, few women were premenopausal at baseline, so the investigators could not evaluate whether menopausal status modifies the association between lipid levels and risk of hemorrhagic stroke. In addition, lipid levels were measured only at baseline, which prevented an analysis of whether change in lipid levels over time modifies the risk of hemorrhagic stroke.
Dr. Rist reported receiving a grant from the National Institutes of Health.
SOURCE: Rist PM et al. Neurology. 2019 April 10. doi: 10.1212/WNL.0000000000007454.
, according to research published in Neurology.
“Women with very low LDL cholesterol or low triglycerides should be monitored by their doctors for other stroke risk factors that can be modified, like high blood pressure and smoking, in order to reduce their risk of hemorrhagic stroke,” said Pamela M. Rist, ScD, instructor in epidemiology at Harvard Medical School, Boston. “Additional research is needed to determine how to lower the risk of hemorrhagic stroke in women with very low LDL and low triglycerides.”
Several meta-analyses have indicated that LDL cholesterol levels are inversely associated with the risk of hemorrhagic stroke. Because lipid-lowering treatments are used to prevent cardiovascular disease, this potential association has implications for clinical practice. Most of the studies included in these meta-analyses had low numbers of events among women, which prevented researchers from stratifying their results by sex. Because women are at greater risk of stroke than men, Dr. Rist and her colleagues sought to evaluate the association between lipid levels and risk of hemorrhagic stroke.
An analysis of the Women’s Health Study
The investigators examined data from the Women’s Health Study, a randomized, double-blind, placebo-controlled trial of low-dose aspirin and vitamin E for the primary prevention of cardiovascular disease and cancer among female American health professionals aged 45 years or older. The study ended in March 2004, but follow-up is ongoing. At regular intervals, the women complete a questionnaire about disease outcomes, including stroke. Some participants agreed to provide a fasting venous blood sample before randomization. With the subjects’ permission, a committee of physicians examined medical records for women who reported a stroke on a follow-up questionnaire.
Dr. Rist and her colleagues analyzed 27,937 samples for levels of LDL cholesterol, HDL cholesterol, total cholesterol, and triglycerides. They assigned each sample to one of five cholesterol level categories that were based on Adult Treatment Panel III guidelines. Cox proportional hazards models enabled the researchers to calculate the hazard ratio of incident hemorrhagic stroke events. They adjusted their results for covariates such as age, smoking status, menopausal status, body mass index, and alcohol consumption.
A U-shaped association
Women in the lowest category of LDL cholesterol level (less than 70 mg/dL) were younger, less likely to have a history of hypertension, and less likely to use cholesterol-lowering drugs than women in the reference group (100.0-129.9 mg/dL). Women with the lowest LDL cholesterol level were more likely to consume alcohol, have a normal weight, engage in physical activity, and be premenopausal than women in the reference group. The investigators confirmed 137 incident hemorrhagic stroke events during a mean 19.3 years of follow-up.
After data adjustment, the researchers found that women with the lowest level of LDL cholesterol had 2.17 times the risk of hemorrhagic stroke, compared with participants in the reference group. They found a trend toward increased risk among women with an LDL cholesterol level of 160 mg/dL or higher, but the result was not statistically significant. The highest risk for intracerebral hemorrhage (ICH) was among women with an LDL cholesterol level of less than 70 mg/dL (relative risk, 2.32), followed by women with a level of 160 mg/dL or higher (RR, 1.71).
In addition, after multivariable adjustment, women in the lowest quartile of triglycerides (less than or equal to 74 mg/dL for fasting and less than or equal to 85 mg/dL for nonfasting) had a significantly increased risk of hemorrhagic stroke, compared with women in the highest quartile (RR, 2.00). Low triglyceride levels were associated with an increased risk of subarachnoid hemorrhage, but not with an increased risk of ICH. Neither HDL cholesterol nor total cholesterol was associated with risk of hemorrhagic stroke, the researchers wrote.
Mechanism of increased risk unclear
The researchers do not yet know how low triglyceride and LDL cholesterol levels increase the risk of hemorrhagic stroke. One hypothesis is that low cholesterol promotes necrosis of the arterial medial layer’s smooth muscle cells. This impaired endothelium might be more susceptible to microaneurysms, which are common in patients with ICH, said the researchers.
The prospective design and the large sample size were two of the study’s strengths, but the study had important weaknesses as well, the researchers wrote. For example, few women were premenopausal at baseline, so the investigators could not evaluate whether menopausal status modifies the association between lipid levels and risk of hemorrhagic stroke. In addition, lipid levels were measured only at baseline, which prevented an analysis of whether change in lipid levels over time modifies the risk of hemorrhagic stroke.
Dr. Rist reported receiving a grant from the National Institutes of Health.
SOURCE: Rist PM et al. Neurology. 2019 April 10. doi: 10.1212/WNL.0000000000007454.
, according to research published in Neurology.
“Women with very low LDL cholesterol or low triglycerides should be monitored by their doctors for other stroke risk factors that can be modified, like high blood pressure and smoking, in order to reduce their risk of hemorrhagic stroke,” said Pamela M. Rist, ScD, instructor in epidemiology at Harvard Medical School, Boston. “Additional research is needed to determine how to lower the risk of hemorrhagic stroke in women with very low LDL and low triglycerides.”
Several meta-analyses have indicated that LDL cholesterol levels are inversely associated with the risk of hemorrhagic stroke. Because lipid-lowering treatments are used to prevent cardiovascular disease, this potential association has implications for clinical practice. Most of the studies included in these meta-analyses had low numbers of events among women, which prevented researchers from stratifying their results by sex. Because women are at greater risk of stroke than men, Dr. Rist and her colleagues sought to evaluate the association between lipid levels and risk of hemorrhagic stroke.
An analysis of the Women’s Health Study
The investigators examined data from the Women’s Health Study, a randomized, double-blind, placebo-controlled trial of low-dose aspirin and vitamin E for the primary prevention of cardiovascular disease and cancer among female American health professionals aged 45 years or older. The study ended in March 2004, but follow-up is ongoing. At regular intervals, the women complete a questionnaire about disease outcomes, including stroke. Some participants agreed to provide a fasting venous blood sample before randomization. With the subjects’ permission, a committee of physicians examined medical records for women who reported a stroke on a follow-up questionnaire.
Dr. Rist and her colleagues analyzed 27,937 samples for levels of LDL cholesterol, HDL cholesterol, total cholesterol, and triglycerides. They assigned each sample to one of five cholesterol level categories that were based on Adult Treatment Panel III guidelines. Cox proportional hazards models enabled the researchers to calculate the hazard ratio of incident hemorrhagic stroke events. They adjusted their results for covariates such as age, smoking status, menopausal status, body mass index, and alcohol consumption.
A U-shaped association
Women in the lowest category of LDL cholesterol level (less than 70 mg/dL) were younger, less likely to have a history of hypertension, and less likely to use cholesterol-lowering drugs than women in the reference group (100.0-129.9 mg/dL). Women with the lowest LDL cholesterol level were more likely to consume alcohol, have a normal weight, engage in physical activity, and be premenopausal than women in the reference group. The investigators confirmed 137 incident hemorrhagic stroke events during a mean 19.3 years of follow-up.
After data adjustment, the researchers found that women with the lowest level of LDL cholesterol had 2.17 times the risk of hemorrhagic stroke, compared with participants in the reference group. They found a trend toward increased risk among women with an LDL cholesterol level of 160 mg/dL or higher, but the result was not statistically significant. The highest risk for intracerebral hemorrhage (ICH) was among women with an LDL cholesterol level of less than 70 mg/dL (relative risk, 2.32), followed by women with a level of 160 mg/dL or higher (RR, 1.71).
In addition, after multivariable adjustment, women in the lowest quartile of triglycerides (less than or equal to 74 mg/dL for fasting and less than or equal to 85 mg/dL for nonfasting) had a significantly increased risk of hemorrhagic stroke, compared with women in the highest quartile (RR, 2.00). Low triglyceride levels were associated with an increased risk of subarachnoid hemorrhage, but not with an increased risk of ICH. Neither HDL cholesterol nor total cholesterol was associated with risk of hemorrhagic stroke, the researchers wrote.
Mechanism of increased risk unclear
The researchers do not yet know how low triglyceride and LDL cholesterol levels increase the risk of hemorrhagic stroke. One hypothesis is that low cholesterol promotes necrosis of the arterial medial layer’s smooth muscle cells. This impaired endothelium might be more susceptible to microaneurysms, which are common in patients with ICH, said the researchers.
The prospective design and the large sample size were two of the study’s strengths, but the study had important weaknesses as well, the researchers wrote. For example, few women were premenopausal at baseline, so the investigators could not evaluate whether menopausal status modifies the association between lipid levels and risk of hemorrhagic stroke. In addition, lipid levels were measured only at baseline, which prevented an analysis of whether change in lipid levels over time modifies the risk of hemorrhagic stroke.
Dr. Rist reported receiving a grant from the National Institutes of Health.
SOURCE: Rist PM et al. Neurology. 2019 April 10. doi: 10.1212/WNL.0000000000007454.
FROM NEUROLOGY
BACE-1 inhibition worsens cognition in patients with prodromal Alzheimer’s disease
More bad news for Alzheimer’s research. Two more BACE inhibitors fall far short of the finish line.
Declining cognitive scores, falls, suicidal ideation, and liver enzyme abnormalities were all seen in clinical trials.
The news doesn’t bode well for the therapeutic target of BACE (beta-site APP cleaving enzyme) inhibition. BACE is one of the enzymes that trims the amyloid precursor protein (APP). Inhibiting it does reduce the amount of toxic amyloid-beta in cerebrospinal fluid, and amyloid plaque in the brain. But none of these molecules has shown clinical benefit in dementia patients, whether their disease is mild, or moderate or – now – prodromal. And it is now apparent that inhibiting BACE also produces serious off-target problems.
“BACE-1 inhibition certainly seemed to have a sound rationale assuming the basis for amyloid’s role in Alzheimer’s disease pathogenesis is a gain of toxicity,” Richard J. Caselli, MD, of the Mayo Clinic, Rochester, Minn., said in an interview. “That APP is important for Alzheimer’s pathogenesis still seems clear but whether amyloid-beta toxicity is the driving force is no longer clear. Further, interruption of the APP system disrupts more than amyloid-beta peptide, possibly explaining the adverse cognitive effects of BACE-1 inhibition shown exhibited now by three different BACE-1 inhibitors.”
Verubecestat
Researchers got their first dose of bad news regarding verubecestat at the 2017 Clinical Trials in Alzheimer’s Disease meeting. There, Michael F. Egan, MD, Merck’s associate vice president of clinical neuroscience, discussed the molecule’s failure to slow cognitive decline in patients with mild to moderate Alzheimer’s disease. There was plenty of biomarker evidence that the drug did block amyloid-beta production, but there also was a plethora of concerning adverse events, Dr. Egan said in an interview.
However, verubecestat still was being pursued in patients with prodromal Alzheimer’s disease. In February, Merck stopped the trial after a futility analysis and announced that the company was terminating studies of verubecestat in that population as well. In the April 11 issue of the New England Journal of Medicine, Dr. Egan and his colleagues report the full extent of verubecestat’s failure in prodromal patients, and the accompanying adverse events.
At the time of termination, 1,454 patients had been enrolled. Of these, 485 received 12 mg/day, 484 received 40 mg/day, and 485 received placebo. About half of each group completed 104 weeks of treatment in the study, which was designed to extend up to 5 years.
The primary outcome was change in the Clinical Dementia Rating Scale–Sum of Boxes score (CDR-SB). Seven secondary outcomes examined other cognitive and functional end points, along with changes in hippocampal volume on MRI and amyloid burden as determined in PET imaging.
Not only did verubecestat fail to slow cognitive decline, it appeared to exacerbate it. The mean change on the CDR-SB was 1.65 in the 12-mg group, 2.02 in the 40-mg group, and 1.58 in the placebo group, favoring placebo.
“In an exploratory analysis according to time point, scores on the CDR-SB were also higher [signifying more impairment of cognition and daily functioning] in the 40-mg group than in the placebo group at 13, 26, and 52 weeks ... suggesting but not confirming the possibility of worse performance at these earlier time points in the high-dose verubecestat group,” the investigators said.
Verubecestat also was associated with more conversions to Alzheimer’s disease. Per 100 patient-years, the Alzheimer’s disease event rates were 24.5 in the 12-mg group, 25.5 in the 40-mg group, and 19.3 in the placebo group. Compared with placebo, those taking 12-mg doses were 30% more likely to develop Alzheimer’s disease and those taking 40-mg doses were 38% more likely. The findings suggest that “verubecestat may have accelerated the progression to diagnosis of dementia due to Alzheimer’s disease,” the investigators said.
The negative impact of verubecestat was apparent quite early in the study. “In exploratory analyses, both dose levels of verubecestat were associated with poorer outcomes on the [Composite Cognition Score-3 Domain] and the ADAS-Cog [Alzheimer’s Disease Assessment Scale–Cognitive Subscale] measures of cognition that, relative to placebo, appeared worse at week 13 and did not appear to progress thereafter.”
Results of the secondary end points, including the ADAS-Cog and the Mini-Mental State Exam, also indicated that verubecestat may have worsened cognitive performance.
Imaging outcomes were positive, however. Hippocampal volume was 6,448 mL in the 12-mg group, 6,469 mL in the 40-mg group, and 6,435 mL in the placebo group. Brain amyloid increased in the placebo group, as expected, and decreased in the active groups. The small group of patients who underwent cerebrospinal fluid sampling showed reductions of more than 60% in amyloid-beta and soluble APP-beta associated with verubecestat. These results show that the molecule was indeed hitting its intended target, but that doing so was not clinically beneficial.
Adverse events were more common in the verubecestat groups. These included rash, dermatitis, urticaria, sleep disturbance, weight loss, and cough. Hair coloring changed in 2.5% of patients in the 12-mg group and 5% of the 40-mg group, but in none of the subjects taking placebo.
Patients taking verubecestat were more likely to sustain falls and injuries and to express suicidal ideation.
The results of this trial differ from the study of verubecestat in mild to moderate Alzheimer’s disease, the investigators noted. Those patients did not decline cognitively as did those with prodromal disease.
“Patients at an earlier stage of the disease may be more sensitive to the effects of substantial BACE-1 inhibition, perhaps because of a role of BACE-1 in normal synaptic function. It is also possible that the effects of verubecestat are due to inhibition of BACE-2,” they said.
Atabecestat
In a research letter in the same issue of the New England Journal of Medicine (2019 Apr 11;380:1483-5), David Henley, MD, senior director of Janssen’s Alzheimer’s clinical development core, released similarly negative results from an interim analysis of EARLY (Efficacy and Safety Study of Atabecestat in Participants Who Are Asymptomatic at Risk for Developing Alzheimer’s Dementia) trial, a randomized study of the BACE-1 inhibitor candidate, atabecestat.
The phase 2 trial enrolled 557 patients with prodromal Alzheimer’s disease. The primary cognitive end point was change from baseline in the Preclinical Alzheimer’s Cognitive Composite (PACC) score.
This trial was discontinued in May 2018 because of liver-related adverse events, although safety follow-up continues. The research letter did not disclose details of the hepatic events, but a company press release from May 2018 referred to them in a general sense.
“Elevations of liver enzymes, which were serious in nature, have been observed in some study participants who received the Janssen BACE inhibitor, atabecestat. After a thorough evaluation of all available liver safety data from our studies, Janssen has concluded that the benefit-risk ratio is no longer favorable to continue development of atabecestat for people who have late-onset preclinical stage Alzheimer’s disease.”
Patients in EARLY were randomized to 5 mg, 25 mg, or placebo. As in the verubecestat trial, those randomized to placebo did better. The mean changes from baseline in the PACC score were −1.44 in the 25-mg group, −0.58 in the 5-mg group, and −0.32 in the placebo group.
“At month 6, the difference between the 25-mg group and the placebo group was −1.12 and the difference between the 5-mg group and the placebo group was −0.26, favoring placebo over the higher dose,” the authors said.
This theme reemerged in a secondary end point, the Repeatable Battery for the Assessment of Neuropsychological Status. The 25-mg group declined 3.58 points more than placebo, and the 5-mg group, 1.43 points more.
Adverse events were more common in the active groups and included depression, effects on sleep and dreams, and anxiety.
“The differences in cognitive performance between the groups are of uncertain clinical significance; however, given similar findings favoring placebo over BACE-1 inhibitors in other trial, we are communicating this potential signal of worsening cognitive function in the treated groups,” Dr. Henley said.
SOURCE: Egan MF et al. N Engl J Med. 2019 Apr 11;380:1408-20.
“Some trials fail because the experimental treatment proves to be no different than a control or standard intervention,” David Knopman, MD, wrote in an accompanying editorial (N Engl J Med. 2019 Apr 11;380:1476-8). “Others fail because of unacceptable side effects. In this issue of the Journal, an article by Egan et al. and a letter to the editor by Henley et al. (N Engl J Med. 2019 Apr 11;380:1483-5) describe a third reason for failure – a treatment worsens the target symptoms.
Certainly, beta-site amyloid precursor protein-cleaving enzyme 1 (BACE-1) inhibition makes sense when viewed in the light of the current understanding of Alzheimer’s disease neuropathology. The amyloid cascade hypothesis holds that toxic amyloid-beta fragments accumulate in the brain, form dense neuritic plaques, and lead to neuronal death and cognitive decline.
“The model is rooted in the inseparability of Alzheimer’s disease from abundant amyloid-beta pathologic features,” Dr. Knopman wrote. But, “Over the past 2 decades, the amyloid-beta–lowering strategy has been put to the test in trials of antiamyloid antibodies, none of which have been successful.”
Therefore, hitting amyloid at the source – the transmembrane cleavage domain – seemed important and, potentially, effective. But three BACE inhibitors (verubecestat, atabecestat, and lanabecestat) have shown similarly negative cognitive effects. “Together, these results suggest that preserved BACE-1 activity may be critical to normal synaptic functions. These observations place a limitation on how amyloid-beta lowering can be accomplished.”
It is possible that decreasing the level of BACE inhibition might ameliorate off-target effects and neuronal compromise but still be enough to reduce the generation of toxic amyloid-beta fragments, Dr. Knopman said. But, “Adjustments in the dose to a narrow window of BACE-1 inhibition would be difficult to accomplish in a clinical trial until there are peripheral biomarkers that reflect the activity of the agent in the brain.”
Thus far, most of the studied antiamyloid drugs have indeed reduced amyloid-beta levels, but none of those reductions affected cognition. A rethinking of amyloid-beta’s place in dementia progression may be in order.
“The dissociation between amyloid-beta lowering and cognitive benefits with both BACE-1 inhibition and antiamyloid antibody therapy is troubling. To be blunt, amyloid-beta lowering seems to be an ineffective approach, and it is time to focus on other targets to move therapeutics for Alzheimer’s disease forward.”
Dr. Knopman is a clinical neurologist and Alzheimer’s researcher at the Mayo Clinic, Rochester, Minn.
“Some trials fail because the experimental treatment proves to be no different than a control or standard intervention,” David Knopman, MD, wrote in an accompanying editorial (N Engl J Med. 2019 Apr 11;380:1476-8). “Others fail because of unacceptable side effects. In this issue of the Journal, an article by Egan et al. and a letter to the editor by Henley et al. (N Engl J Med. 2019 Apr 11;380:1483-5) describe a third reason for failure – a treatment worsens the target symptoms.
Certainly, beta-site amyloid precursor protein-cleaving enzyme 1 (BACE-1) inhibition makes sense when viewed in the light of the current understanding of Alzheimer’s disease neuropathology. The amyloid cascade hypothesis holds that toxic amyloid-beta fragments accumulate in the brain, form dense neuritic plaques, and lead to neuronal death and cognitive decline.
“The model is rooted in the inseparability of Alzheimer’s disease from abundant amyloid-beta pathologic features,” Dr. Knopman wrote. But, “Over the past 2 decades, the amyloid-beta–lowering strategy has been put to the test in trials of antiamyloid antibodies, none of which have been successful.”
Therefore, hitting amyloid at the source – the transmembrane cleavage domain – seemed important and, potentially, effective. But three BACE inhibitors (verubecestat, atabecestat, and lanabecestat) have shown similarly negative cognitive effects. “Together, these results suggest that preserved BACE-1 activity may be critical to normal synaptic functions. These observations place a limitation on how amyloid-beta lowering can be accomplished.”
It is possible that decreasing the level of BACE inhibition might ameliorate off-target effects and neuronal compromise but still be enough to reduce the generation of toxic amyloid-beta fragments, Dr. Knopman said. But, “Adjustments in the dose to a narrow window of BACE-1 inhibition would be difficult to accomplish in a clinical trial until there are peripheral biomarkers that reflect the activity of the agent in the brain.”
Thus far, most of the studied antiamyloid drugs have indeed reduced amyloid-beta levels, but none of those reductions affected cognition. A rethinking of amyloid-beta’s place in dementia progression may be in order.
“The dissociation between amyloid-beta lowering and cognitive benefits with both BACE-1 inhibition and antiamyloid antibody therapy is troubling. To be blunt, amyloid-beta lowering seems to be an ineffective approach, and it is time to focus on other targets to move therapeutics for Alzheimer’s disease forward.”
Dr. Knopman is a clinical neurologist and Alzheimer’s researcher at the Mayo Clinic, Rochester, Minn.
“Some trials fail because the experimental treatment proves to be no different than a control or standard intervention,” David Knopman, MD, wrote in an accompanying editorial (N Engl J Med. 2019 Apr 11;380:1476-8). “Others fail because of unacceptable side effects. In this issue of the Journal, an article by Egan et al. and a letter to the editor by Henley et al. (N Engl J Med. 2019 Apr 11;380:1483-5) describe a third reason for failure – a treatment worsens the target symptoms.
Certainly, beta-site amyloid precursor protein-cleaving enzyme 1 (BACE-1) inhibition makes sense when viewed in the light of the current understanding of Alzheimer’s disease neuropathology. The amyloid cascade hypothesis holds that toxic amyloid-beta fragments accumulate in the brain, form dense neuritic plaques, and lead to neuronal death and cognitive decline.
“The model is rooted in the inseparability of Alzheimer’s disease from abundant amyloid-beta pathologic features,” Dr. Knopman wrote. But, “Over the past 2 decades, the amyloid-beta–lowering strategy has been put to the test in trials of antiamyloid antibodies, none of which have been successful.”
Therefore, hitting amyloid at the source – the transmembrane cleavage domain – seemed important and, potentially, effective. But three BACE inhibitors (verubecestat, atabecestat, and lanabecestat) have shown similarly negative cognitive effects. “Together, these results suggest that preserved BACE-1 activity may be critical to normal synaptic functions. These observations place a limitation on how amyloid-beta lowering can be accomplished.”
It is possible that decreasing the level of BACE inhibition might ameliorate off-target effects and neuronal compromise but still be enough to reduce the generation of toxic amyloid-beta fragments, Dr. Knopman said. But, “Adjustments in the dose to a narrow window of BACE-1 inhibition would be difficult to accomplish in a clinical trial until there are peripheral biomarkers that reflect the activity of the agent in the brain.”
Thus far, most of the studied antiamyloid drugs have indeed reduced amyloid-beta levels, but none of those reductions affected cognition. A rethinking of amyloid-beta’s place in dementia progression may be in order.
“The dissociation between amyloid-beta lowering and cognitive benefits with both BACE-1 inhibition and antiamyloid antibody therapy is troubling. To be blunt, amyloid-beta lowering seems to be an ineffective approach, and it is time to focus on other targets to move therapeutics for Alzheimer’s disease forward.”
Dr. Knopman is a clinical neurologist and Alzheimer’s researcher at the Mayo Clinic, Rochester, Minn.
More bad news for Alzheimer’s research. Two more BACE inhibitors fall far short of the finish line.
Declining cognitive scores, falls, suicidal ideation, and liver enzyme abnormalities were all seen in clinical trials.
The news doesn’t bode well for the therapeutic target of BACE (beta-site APP cleaving enzyme) inhibition. BACE is one of the enzymes that trims the amyloid precursor protein (APP). Inhibiting it does reduce the amount of toxic amyloid-beta in cerebrospinal fluid, and amyloid plaque in the brain. But none of these molecules has shown clinical benefit in dementia patients, whether their disease is mild, or moderate or – now – prodromal. And it is now apparent that inhibiting BACE also produces serious off-target problems.
“BACE-1 inhibition certainly seemed to have a sound rationale assuming the basis for amyloid’s role in Alzheimer’s disease pathogenesis is a gain of toxicity,” Richard J. Caselli, MD, of the Mayo Clinic, Rochester, Minn., said in an interview. “That APP is important for Alzheimer’s pathogenesis still seems clear but whether amyloid-beta toxicity is the driving force is no longer clear. Further, interruption of the APP system disrupts more than amyloid-beta peptide, possibly explaining the adverse cognitive effects of BACE-1 inhibition shown exhibited now by three different BACE-1 inhibitors.”
Verubecestat
Researchers got their first dose of bad news regarding verubecestat at the 2017 Clinical Trials in Alzheimer’s Disease meeting. There, Michael F. Egan, MD, Merck’s associate vice president of clinical neuroscience, discussed the molecule’s failure to slow cognitive decline in patients with mild to moderate Alzheimer’s disease. There was plenty of biomarker evidence that the drug did block amyloid-beta production, but there also was a plethora of concerning adverse events, Dr. Egan said in an interview.
However, verubecestat still was being pursued in patients with prodromal Alzheimer’s disease. In February, Merck stopped the trial after a futility analysis and announced that the company was terminating studies of verubecestat in that population as well. In the April 11 issue of the New England Journal of Medicine, Dr. Egan and his colleagues report the full extent of verubecestat’s failure in prodromal patients, and the accompanying adverse events.
At the time of termination, 1,454 patients had been enrolled. Of these, 485 received 12 mg/day, 484 received 40 mg/day, and 485 received placebo. About half of each group completed 104 weeks of treatment in the study, which was designed to extend up to 5 years.
The primary outcome was change in the Clinical Dementia Rating Scale–Sum of Boxes score (CDR-SB). Seven secondary outcomes examined other cognitive and functional end points, along with changes in hippocampal volume on MRI and amyloid burden as determined in PET imaging.
Not only did verubecestat fail to slow cognitive decline, it appeared to exacerbate it. The mean change on the CDR-SB was 1.65 in the 12-mg group, 2.02 in the 40-mg group, and 1.58 in the placebo group, favoring placebo.
“In an exploratory analysis according to time point, scores on the CDR-SB were also higher [signifying more impairment of cognition and daily functioning] in the 40-mg group than in the placebo group at 13, 26, and 52 weeks ... suggesting but not confirming the possibility of worse performance at these earlier time points in the high-dose verubecestat group,” the investigators said.
Verubecestat also was associated with more conversions to Alzheimer’s disease. Per 100 patient-years, the Alzheimer’s disease event rates were 24.5 in the 12-mg group, 25.5 in the 40-mg group, and 19.3 in the placebo group. Compared with placebo, those taking 12-mg doses were 30% more likely to develop Alzheimer’s disease and those taking 40-mg doses were 38% more likely. The findings suggest that “verubecestat may have accelerated the progression to diagnosis of dementia due to Alzheimer’s disease,” the investigators said.
The negative impact of verubecestat was apparent quite early in the study. “In exploratory analyses, both dose levels of verubecestat were associated with poorer outcomes on the [Composite Cognition Score-3 Domain] and the ADAS-Cog [Alzheimer’s Disease Assessment Scale–Cognitive Subscale] measures of cognition that, relative to placebo, appeared worse at week 13 and did not appear to progress thereafter.”
Results of the secondary end points, including the ADAS-Cog and the Mini-Mental State Exam, also indicated that verubecestat may have worsened cognitive performance.
Imaging outcomes were positive, however. Hippocampal volume was 6,448 mL in the 12-mg group, 6,469 mL in the 40-mg group, and 6,435 mL in the placebo group. Brain amyloid increased in the placebo group, as expected, and decreased in the active groups. The small group of patients who underwent cerebrospinal fluid sampling showed reductions of more than 60% in amyloid-beta and soluble APP-beta associated with verubecestat. These results show that the molecule was indeed hitting its intended target, but that doing so was not clinically beneficial.
Adverse events were more common in the verubecestat groups. These included rash, dermatitis, urticaria, sleep disturbance, weight loss, and cough. Hair coloring changed in 2.5% of patients in the 12-mg group and 5% of the 40-mg group, but in none of the subjects taking placebo.
Patients taking verubecestat were more likely to sustain falls and injuries and to express suicidal ideation.
The results of this trial differ from the study of verubecestat in mild to moderate Alzheimer’s disease, the investigators noted. Those patients did not decline cognitively as did those with prodromal disease.
“Patients at an earlier stage of the disease may be more sensitive to the effects of substantial BACE-1 inhibition, perhaps because of a role of BACE-1 in normal synaptic function. It is also possible that the effects of verubecestat are due to inhibition of BACE-2,” they said.
Atabecestat
In a research letter in the same issue of the New England Journal of Medicine (2019 Apr 11;380:1483-5), David Henley, MD, senior director of Janssen’s Alzheimer’s clinical development core, released similarly negative results from an interim analysis of EARLY (Efficacy and Safety Study of Atabecestat in Participants Who Are Asymptomatic at Risk for Developing Alzheimer’s Dementia) trial, a randomized study of the BACE-1 inhibitor candidate, atabecestat.
The phase 2 trial enrolled 557 patients with prodromal Alzheimer’s disease. The primary cognitive end point was change from baseline in the Preclinical Alzheimer’s Cognitive Composite (PACC) score.
This trial was discontinued in May 2018 because of liver-related adverse events, although safety follow-up continues. The research letter did not disclose details of the hepatic events, but a company press release from May 2018 referred to them in a general sense.
“Elevations of liver enzymes, which were serious in nature, have been observed in some study participants who received the Janssen BACE inhibitor, atabecestat. After a thorough evaluation of all available liver safety data from our studies, Janssen has concluded that the benefit-risk ratio is no longer favorable to continue development of atabecestat for people who have late-onset preclinical stage Alzheimer’s disease.”
Patients in EARLY were randomized to 5 mg, 25 mg, or placebo. As in the verubecestat trial, those randomized to placebo did better. The mean changes from baseline in the PACC score were −1.44 in the 25-mg group, −0.58 in the 5-mg group, and −0.32 in the placebo group.
“At month 6, the difference between the 25-mg group and the placebo group was −1.12 and the difference between the 5-mg group and the placebo group was −0.26, favoring placebo over the higher dose,” the authors said.
This theme reemerged in a secondary end point, the Repeatable Battery for the Assessment of Neuropsychological Status. The 25-mg group declined 3.58 points more than placebo, and the 5-mg group, 1.43 points more.
Adverse events were more common in the active groups and included depression, effects on sleep and dreams, and anxiety.
“The differences in cognitive performance between the groups are of uncertain clinical significance; however, given similar findings favoring placebo over BACE-1 inhibitors in other trial, we are communicating this potential signal of worsening cognitive function in the treated groups,” Dr. Henley said.
SOURCE: Egan MF et al. N Engl J Med. 2019 Apr 11;380:1408-20.
More bad news for Alzheimer’s research. Two more BACE inhibitors fall far short of the finish line.
Declining cognitive scores, falls, suicidal ideation, and liver enzyme abnormalities were all seen in clinical trials.
The news doesn’t bode well for the therapeutic target of BACE (beta-site APP cleaving enzyme) inhibition. BACE is one of the enzymes that trims the amyloid precursor protein (APP). Inhibiting it does reduce the amount of toxic amyloid-beta in cerebrospinal fluid, and amyloid plaque in the brain. But none of these molecules has shown clinical benefit in dementia patients, whether their disease is mild, or moderate or – now – prodromal. And it is now apparent that inhibiting BACE also produces serious off-target problems.
“BACE-1 inhibition certainly seemed to have a sound rationale assuming the basis for amyloid’s role in Alzheimer’s disease pathogenesis is a gain of toxicity,” Richard J. Caselli, MD, of the Mayo Clinic, Rochester, Minn., said in an interview. “That APP is important for Alzheimer’s pathogenesis still seems clear but whether amyloid-beta toxicity is the driving force is no longer clear. Further, interruption of the APP system disrupts more than amyloid-beta peptide, possibly explaining the adverse cognitive effects of BACE-1 inhibition shown exhibited now by three different BACE-1 inhibitors.”
Verubecestat
Researchers got their first dose of bad news regarding verubecestat at the 2017 Clinical Trials in Alzheimer’s Disease meeting. There, Michael F. Egan, MD, Merck’s associate vice president of clinical neuroscience, discussed the molecule’s failure to slow cognitive decline in patients with mild to moderate Alzheimer’s disease. There was plenty of biomarker evidence that the drug did block amyloid-beta production, but there also was a plethora of concerning adverse events, Dr. Egan said in an interview.
However, verubecestat still was being pursued in patients with prodromal Alzheimer’s disease. In February, Merck stopped the trial after a futility analysis and announced that the company was terminating studies of verubecestat in that population as well. In the April 11 issue of the New England Journal of Medicine, Dr. Egan and his colleagues report the full extent of verubecestat’s failure in prodromal patients, and the accompanying adverse events.
At the time of termination, 1,454 patients had been enrolled. Of these, 485 received 12 mg/day, 484 received 40 mg/day, and 485 received placebo. About half of each group completed 104 weeks of treatment in the study, which was designed to extend up to 5 years.
The primary outcome was change in the Clinical Dementia Rating Scale–Sum of Boxes score (CDR-SB). Seven secondary outcomes examined other cognitive and functional end points, along with changes in hippocampal volume on MRI and amyloid burden as determined in PET imaging.
Not only did verubecestat fail to slow cognitive decline, it appeared to exacerbate it. The mean change on the CDR-SB was 1.65 in the 12-mg group, 2.02 in the 40-mg group, and 1.58 in the placebo group, favoring placebo.
“In an exploratory analysis according to time point, scores on the CDR-SB were also higher [signifying more impairment of cognition and daily functioning] in the 40-mg group than in the placebo group at 13, 26, and 52 weeks ... suggesting but not confirming the possibility of worse performance at these earlier time points in the high-dose verubecestat group,” the investigators said.
Verubecestat also was associated with more conversions to Alzheimer’s disease. Per 100 patient-years, the Alzheimer’s disease event rates were 24.5 in the 12-mg group, 25.5 in the 40-mg group, and 19.3 in the placebo group. Compared with placebo, those taking 12-mg doses were 30% more likely to develop Alzheimer’s disease and those taking 40-mg doses were 38% more likely. The findings suggest that “verubecestat may have accelerated the progression to diagnosis of dementia due to Alzheimer’s disease,” the investigators said.
The negative impact of verubecestat was apparent quite early in the study. “In exploratory analyses, both dose levels of verubecestat were associated with poorer outcomes on the [Composite Cognition Score-3 Domain] and the ADAS-Cog [Alzheimer’s Disease Assessment Scale–Cognitive Subscale] measures of cognition that, relative to placebo, appeared worse at week 13 and did not appear to progress thereafter.”
Results of the secondary end points, including the ADAS-Cog and the Mini-Mental State Exam, also indicated that verubecestat may have worsened cognitive performance.
Imaging outcomes were positive, however. Hippocampal volume was 6,448 mL in the 12-mg group, 6,469 mL in the 40-mg group, and 6,435 mL in the placebo group. Brain amyloid increased in the placebo group, as expected, and decreased in the active groups. The small group of patients who underwent cerebrospinal fluid sampling showed reductions of more than 60% in amyloid-beta and soluble APP-beta associated with verubecestat. These results show that the molecule was indeed hitting its intended target, but that doing so was not clinically beneficial.
Adverse events were more common in the verubecestat groups. These included rash, dermatitis, urticaria, sleep disturbance, weight loss, and cough. Hair coloring changed in 2.5% of patients in the 12-mg group and 5% of the 40-mg group, but in none of the subjects taking placebo.
Patients taking verubecestat were more likely to sustain falls and injuries and to express suicidal ideation.
The results of this trial differ from the study of verubecestat in mild to moderate Alzheimer’s disease, the investigators noted. Those patients did not decline cognitively as did those with prodromal disease.
“Patients at an earlier stage of the disease may be more sensitive to the effects of substantial BACE-1 inhibition, perhaps because of a role of BACE-1 in normal synaptic function. It is also possible that the effects of verubecestat are due to inhibition of BACE-2,” they said.
Atabecestat
In a research letter in the same issue of the New England Journal of Medicine (2019 Apr 11;380:1483-5), David Henley, MD, senior director of Janssen’s Alzheimer’s clinical development core, released similarly negative results from an interim analysis of EARLY (Efficacy and Safety Study of Atabecestat in Participants Who Are Asymptomatic at Risk for Developing Alzheimer’s Dementia) trial, a randomized study of the BACE-1 inhibitor candidate, atabecestat.
The phase 2 trial enrolled 557 patients with prodromal Alzheimer’s disease. The primary cognitive end point was change from baseline in the Preclinical Alzheimer’s Cognitive Composite (PACC) score.
This trial was discontinued in May 2018 because of liver-related adverse events, although safety follow-up continues. The research letter did not disclose details of the hepatic events, but a company press release from May 2018 referred to them in a general sense.
“Elevations of liver enzymes, which were serious in nature, have been observed in some study participants who received the Janssen BACE inhibitor, atabecestat. After a thorough evaluation of all available liver safety data from our studies, Janssen has concluded that the benefit-risk ratio is no longer favorable to continue development of atabecestat for people who have late-onset preclinical stage Alzheimer’s disease.”
Patients in EARLY were randomized to 5 mg, 25 mg, or placebo. As in the verubecestat trial, those randomized to placebo did better. The mean changes from baseline in the PACC score were −1.44 in the 25-mg group, −0.58 in the 5-mg group, and −0.32 in the placebo group.
“At month 6, the difference between the 25-mg group and the placebo group was −1.12 and the difference between the 5-mg group and the placebo group was −0.26, favoring placebo over the higher dose,” the authors said.
This theme reemerged in a secondary end point, the Repeatable Battery for the Assessment of Neuropsychological Status. The 25-mg group declined 3.58 points more than placebo, and the 5-mg group, 1.43 points more.
Adverse events were more common in the active groups and included depression, effects on sleep and dreams, and anxiety.
“The differences in cognitive performance between the groups are of uncertain clinical significance; however, given similar findings favoring placebo over BACE-1 inhibitors in other trial, we are communicating this potential signal of worsening cognitive function in the treated groups,” Dr. Henley said.
SOURCE: Egan MF et al. N Engl J Med. 2019 Apr 11;380:1408-20.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Polyglutamine diseases are rare, but not the mutations that cause them
Polyglutamine diseases are a group of hereditary neurodegenerative disorders caused by mutations in which a trinucleotide repeat expands pathologically on a disease-associated gene. The diseases are rare, with the most common among them – Huntington disease – affecting between 10 and 14 per 100,000 people in Western countries, where prevalence is highest.
In polyglutamine diseases, which include the spinocerebellar ataxias, dentatorubral-pallidoluysian atrophy, and spinal bulbar muscular atrophy, higher CAG (cytosine-adenine-guanine) repeat numbers are associated with greater disease severity, faster progression, or earlier age at onset.
In research published April 1 in JAMA Neurology, investigators report that more than one-tenth of the European population carries CAG expansions that fall short of the repeats needed to cause any of 9 polyglutamine diseases – but are enough to put them at risk of having children who develop one. A smaller number of people – about 1% – carry enough CAG repeats to cause one of the diseases late in life.
For their research, Sarah L. Gardiner, MD, of Leiden (the Netherlands) University, and her colleagues looked at polyglutamine expansion variants for nine diseases in samples from 14,196 adults (56% of whom were women) from the Netherlands, Scotland, and Ireland. The samples were taken from five population-based cohort studies conducted between 1997 and 2012, and all subjects were without a history of polyglutamine disease or major depression.
Of these, 10.7% had a CAG repeat number on a disease-associated gene that was in the intermediate range, defined as a number of repeats that cannot cause disease but for which “expansion into the fully pathological range has been observed on intergenerational transmission,” Dr. Gardiner and her colleagues wrote. And some 1.3% of subjects were found to have CAG repeats within the disease-causing range, “mostly in the lower range associated with elderly onset.”
The investigators found no differences in sex, age, or body mass index between individuals with CAG repeat numbers within the pathological range and individuals with CAG repeat numbers within the normal or intermediate range.
Whether carriers of immediate or lower-range pathological CAG repeats went on to develop disease could not be measured, as follow-up data were not available. Another limitation of the study, the investigators acknowledged, was that the genotyping method used “did not allow us to determine the presence of trinucleotide interruptions,” which can affect disease penetrance.
“A late age at onset, a reduced penetrance, or the presence of interruptions could all explain the asymptomatic status of our carriers of intermediate and pathological polyglutamine disease–associated alleles at the time of assessment,” Dr. Gardiner and her colleagues wrote.
This study was funded by the European Union and Dutch government agencies; one of the population-based cohort studies from which the study sample was taken received some support from Bristol-Myers-Squibb. One of Dr. Gardiner’s coauthors, Raymund A. C. Roos, MD, PhD, disclosed being an adviser for UniQure, a gene-therapy firm, and no other conflicts of interest were reported.
SOURCE: Gardiner et al. JAMA Neurol. 2019 Apr 1. doi: 10.001/jamaneurol.2019.042.
Gardiner et al. describe the results of an appraisal of polyglutamine expansion variants in more than 14,000 individuals from the Netherlands, Scotland, and Ireland. Given the relative rarity of polyglutamine repeat disease, the first question that comes to mind is why were so many individuals identified with repeats in the pathogenic range? Based on our understanding of disease prevalence, it is unlikely that each of these individuals will become affected; therefore, this work suggests a reduced penetrance of these mutations. The findings are illustrative of a growing theme in human disease genetics: There are a very large number of apparently healthy individuals in the general population who carry mutations associated with various diseases. The phenomenon of reduced penetrance, where mutations cause disease in some but not all carriers, overlaps and arguably may be the same as that of variable expressivity, where the same mutation can lead to very different disease outcomes in different individuals. It is extremely likely that second-generation sequencing and population-scale screening will continue to reveal similar themes. We continue to appreciate the increasing complexity of the human genome and its relationship to disease, even those diseases we thought of previously as simple “single-gene” disorders.
Monia B. Hammer, PhD, and Andrew B. Singleton, PhD, are with the National Institute on Aging, National Institutes of Health, Bethesda, Md. Dr. Hammer and Dr. Singleton report no financial conflicts of interest related to their editorial.
Gardiner et al. describe the results of an appraisal of polyglutamine expansion variants in more than 14,000 individuals from the Netherlands, Scotland, and Ireland. Given the relative rarity of polyglutamine repeat disease, the first question that comes to mind is why were so many individuals identified with repeats in the pathogenic range? Based on our understanding of disease prevalence, it is unlikely that each of these individuals will become affected; therefore, this work suggests a reduced penetrance of these mutations. The findings are illustrative of a growing theme in human disease genetics: There are a very large number of apparently healthy individuals in the general population who carry mutations associated with various diseases. The phenomenon of reduced penetrance, where mutations cause disease in some but not all carriers, overlaps and arguably may be the same as that of variable expressivity, where the same mutation can lead to very different disease outcomes in different individuals. It is extremely likely that second-generation sequencing and population-scale screening will continue to reveal similar themes. We continue to appreciate the increasing complexity of the human genome and its relationship to disease, even those diseases we thought of previously as simple “single-gene” disorders.
Monia B. Hammer, PhD, and Andrew B. Singleton, PhD, are with the National Institute on Aging, National Institutes of Health, Bethesda, Md. Dr. Hammer and Dr. Singleton report no financial conflicts of interest related to their editorial.
Gardiner et al. describe the results of an appraisal of polyglutamine expansion variants in more than 14,000 individuals from the Netherlands, Scotland, and Ireland. Given the relative rarity of polyglutamine repeat disease, the first question that comes to mind is why were so many individuals identified with repeats in the pathogenic range? Based on our understanding of disease prevalence, it is unlikely that each of these individuals will become affected; therefore, this work suggests a reduced penetrance of these mutations. The findings are illustrative of a growing theme in human disease genetics: There are a very large number of apparently healthy individuals in the general population who carry mutations associated with various diseases. The phenomenon of reduced penetrance, where mutations cause disease in some but not all carriers, overlaps and arguably may be the same as that of variable expressivity, where the same mutation can lead to very different disease outcomes in different individuals. It is extremely likely that second-generation sequencing and population-scale screening will continue to reveal similar themes. We continue to appreciate the increasing complexity of the human genome and its relationship to disease, even those diseases we thought of previously as simple “single-gene” disorders.
Monia B. Hammer, PhD, and Andrew B. Singleton, PhD, are with the National Institute on Aging, National Institutes of Health, Bethesda, Md. Dr. Hammer and Dr. Singleton report no financial conflicts of interest related to their editorial.
Polyglutamine diseases are a group of hereditary neurodegenerative disorders caused by mutations in which a trinucleotide repeat expands pathologically on a disease-associated gene. The diseases are rare, with the most common among them – Huntington disease – affecting between 10 and 14 per 100,000 people in Western countries, where prevalence is highest.
In polyglutamine diseases, which include the spinocerebellar ataxias, dentatorubral-pallidoluysian atrophy, and spinal bulbar muscular atrophy, higher CAG (cytosine-adenine-guanine) repeat numbers are associated with greater disease severity, faster progression, or earlier age at onset.
In research published April 1 in JAMA Neurology, investigators report that more than one-tenth of the European population carries CAG expansions that fall short of the repeats needed to cause any of 9 polyglutamine diseases – but are enough to put them at risk of having children who develop one. A smaller number of people – about 1% – carry enough CAG repeats to cause one of the diseases late in life.
For their research, Sarah L. Gardiner, MD, of Leiden (the Netherlands) University, and her colleagues looked at polyglutamine expansion variants for nine diseases in samples from 14,196 adults (56% of whom were women) from the Netherlands, Scotland, and Ireland. The samples were taken from five population-based cohort studies conducted between 1997 and 2012, and all subjects were without a history of polyglutamine disease or major depression.
Of these, 10.7% had a CAG repeat number on a disease-associated gene that was in the intermediate range, defined as a number of repeats that cannot cause disease but for which “expansion into the fully pathological range has been observed on intergenerational transmission,” Dr. Gardiner and her colleagues wrote. And some 1.3% of subjects were found to have CAG repeats within the disease-causing range, “mostly in the lower range associated with elderly onset.”
The investigators found no differences in sex, age, or body mass index between individuals with CAG repeat numbers within the pathological range and individuals with CAG repeat numbers within the normal or intermediate range.
Whether carriers of immediate or lower-range pathological CAG repeats went on to develop disease could not be measured, as follow-up data were not available. Another limitation of the study, the investigators acknowledged, was that the genotyping method used “did not allow us to determine the presence of trinucleotide interruptions,” which can affect disease penetrance.
“A late age at onset, a reduced penetrance, or the presence of interruptions could all explain the asymptomatic status of our carriers of intermediate and pathological polyglutamine disease–associated alleles at the time of assessment,” Dr. Gardiner and her colleagues wrote.
This study was funded by the European Union and Dutch government agencies; one of the population-based cohort studies from which the study sample was taken received some support from Bristol-Myers-Squibb. One of Dr. Gardiner’s coauthors, Raymund A. C. Roos, MD, PhD, disclosed being an adviser for UniQure, a gene-therapy firm, and no other conflicts of interest were reported.
SOURCE: Gardiner et al. JAMA Neurol. 2019 Apr 1. doi: 10.001/jamaneurol.2019.042.
Polyglutamine diseases are a group of hereditary neurodegenerative disorders caused by mutations in which a trinucleotide repeat expands pathologically on a disease-associated gene. The diseases are rare, with the most common among them – Huntington disease – affecting between 10 and 14 per 100,000 people in Western countries, where prevalence is highest.
In polyglutamine diseases, which include the spinocerebellar ataxias, dentatorubral-pallidoluysian atrophy, and spinal bulbar muscular atrophy, higher CAG (cytosine-adenine-guanine) repeat numbers are associated with greater disease severity, faster progression, or earlier age at onset.
In research published April 1 in JAMA Neurology, investigators report that more than one-tenth of the European population carries CAG expansions that fall short of the repeats needed to cause any of 9 polyglutamine diseases – but are enough to put them at risk of having children who develop one. A smaller number of people – about 1% – carry enough CAG repeats to cause one of the diseases late in life.
For their research, Sarah L. Gardiner, MD, of Leiden (the Netherlands) University, and her colleagues looked at polyglutamine expansion variants for nine diseases in samples from 14,196 adults (56% of whom were women) from the Netherlands, Scotland, and Ireland. The samples were taken from five population-based cohort studies conducted between 1997 and 2012, and all subjects were without a history of polyglutamine disease or major depression.
Of these, 10.7% had a CAG repeat number on a disease-associated gene that was in the intermediate range, defined as a number of repeats that cannot cause disease but for which “expansion into the fully pathological range has been observed on intergenerational transmission,” Dr. Gardiner and her colleagues wrote. And some 1.3% of subjects were found to have CAG repeats within the disease-causing range, “mostly in the lower range associated with elderly onset.”
The investigators found no differences in sex, age, or body mass index between individuals with CAG repeat numbers within the pathological range and individuals with CAG repeat numbers within the normal or intermediate range.
Whether carriers of immediate or lower-range pathological CAG repeats went on to develop disease could not be measured, as follow-up data were not available. Another limitation of the study, the investigators acknowledged, was that the genotyping method used “did not allow us to determine the presence of trinucleotide interruptions,” which can affect disease penetrance.
“A late age at onset, a reduced penetrance, or the presence of interruptions could all explain the asymptomatic status of our carriers of intermediate and pathological polyglutamine disease–associated alleles at the time of assessment,” Dr. Gardiner and her colleagues wrote.
This study was funded by the European Union and Dutch government agencies; one of the population-based cohort studies from which the study sample was taken received some support from Bristol-Myers-Squibb. One of Dr. Gardiner’s coauthors, Raymund A. C. Roos, MD, PhD, disclosed being an adviser for UniQure, a gene-therapy firm, and no other conflicts of interest were reported.
SOURCE: Gardiner et al. JAMA Neurol. 2019 Apr 1. doi: 10.001/jamaneurol.2019.042.
FROM JAMA NEUROLOGY
Amyloid brain imaging changed clinical management in 60% of MCI and dementia patients
.
Diagnoses changed from Alzheimer’s disease to non–Alzheimer’s disease in 25% of 11,409 patients and from non–Alzheimer’s disease to Alzheimer’s disease in 10.5%, reported Gil Rabinovici, MD, and his colleagues. The use of Alzheimer’s disease drugs doubled in amyloid-positive MCI patients, and increased by a third in amyloid-positive dementia patients. Physicians involved in the study said the scans provided key clinical information in 82% of cases with post-scan management changes.
Scans also benefited amyloid-negative patients. Before the scan, 71% of these carried an Alzheimer’s disease diagnosis; afterward, just 10% did, opening the way for an accurate diagnosis and more effective treatment.
The study was powered to detect a 30% or greater change in the MCI and dementia groups. The 60% change emphasize how useful amyloid PET scans could be in clinical practice, Dr. Rabinovici, the study’s lead author and principal investigator, said in a press statement.
“We are impressed by the magnitude of these results, which make it clear that amyloid PET imaging can have a major impact on how we diagnose and care for patients with Alzheimer’s disease and other forms of cognitive decline,” said Dr. Rabinovici of the University of California, San Francisco.
Alzheimer’s Association leaders were similarly pleased.
“These results present highly credible, large-scale evidence that amyloid PET imaging can be a powerful tool to improve the accuracy of Alzheimer’s diagnosis and lead to better medical management, especially in difficult-to-diagnose cases,” said Maria C. Carrillo, PhD, chief science officer of the Alzheimer’s Association and a coauthor of the study. “It is important that amyloid PET imaging be more broadly accessible to those who need it.”
Next steps
Ultimately, investigators hope the nationwide-wide, open-label study will prove the clinical value of amyloid PET scanning and convince the Centers for Medicare & Medicaid Services to make the test a fully covered service for those who meet the appropriate use criteria set forth by the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging.
IDEAS’ second goal – showing that the scans improve health outcomes – is scheduled for 2020. These data are a key component of the CMS decision, but they might be a tough sell, Clifford R. Jack Jr., MD, and Ronald C. Petersen, MD, PhD, wrote in an accompanying editorial. Dr. Jack and Dr. Petersen are affiliated with the Mayo Clinic in Rochester, Minn.
“For CMS to cover the cost of amyloid PET, it must be demonstrated that the result of a scan has an effect on patient outcomes, not just patient care processes – and, without a disease-modifying therapy available, that might be a challenge,” they wrote.
IDEAS is a funding collaboration of the CMS, the Alzheimer’s Association, Avid Radiopharmaceuticals/Eli Lilly, General Electric Healthcare, Piramal Imaging, and the American College of Radiology. Dr. Rabinovici had no financial disclosures.
SOURCE: Rabinovici GD et al. JAMA. 2019 Apr 2. doi: 10.1001/jama.2019.2000.
Current clinical practice does not routinely include biomarkers, and if given a choice, most patients would prefer brain imaging to spinal fluid-based testing, so IDEAS may be making imaging-based biomarker characterization a real possibility in the future.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center. He made these comments in an interview.
Current clinical practice does not routinely include biomarkers, and if given a choice, most patients would prefer brain imaging to spinal fluid-based testing, so IDEAS may be making imaging-based biomarker characterization a real possibility in the future.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center. He made these comments in an interview.
Current clinical practice does not routinely include biomarkers, and if given a choice, most patients would prefer brain imaging to spinal fluid-based testing, so IDEAS may be making imaging-based biomarker characterization a real possibility in the future.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center. He made these comments in an interview.
.
Diagnoses changed from Alzheimer’s disease to non–Alzheimer’s disease in 25% of 11,409 patients and from non–Alzheimer’s disease to Alzheimer’s disease in 10.5%, reported Gil Rabinovici, MD, and his colleagues. The use of Alzheimer’s disease drugs doubled in amyloid-positive MCI patients, and increased by a third in amyloid-positive dementia patients. Physicians involved in the study said the scans provided key clinical information in 82% of cases with post-scan management changes.
Scans also benefited amyloid-negative patients. Before the scan, 71% of these carried an Alzheimer’s disease diagnosis; afterward, just 10% did, opening the way for an accurate diagnosis and more effective treatment.
The study was powered to detect a 30% or greater change in the MCI and dementia groups. The 60% change emphasize how useful amyloid PET scans could be in clinical practice, Dr. Rabinovici, the study’s lead author and principal investigator, said in a press statement.
“We are impressed by the magnitude of these results, which make it clear that amyloid PET imaging can have a major impact on how we diagnose and care for patients with Alzheimer’s disease and other forms of cognitive decline,” said Dr. Rabinovici of the University of California, San Francisco.
Alzheimer’s Association leaders were similarly pleased.
“These results present highly credible, large-scale evidence that amyloid PET imaging can be a powerful tool to improve the accuracy of Alzheimer’s diagnosis and lead to better medical management, especially in difficult-to-diagnose cases,” said Maria C. Carrillo, PhD, chief science officer of the Alzheimer’s Association and a coauthor of the study. “It is important that amyloid PET imaging be more broadly accessible to those who need it.”
Next steps
Ultimately, investigators hope the nationwide-wide, open-label study will prove the clinical value of amyloid PET scanning and convince the Centers for Medicare & Medicaid Services to make the test a fully covered service for those who meet the appropriate use criteria set forth by the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging.
IDEAS’ second goal – showing that the scans improve health outcomes – is scheduled for 2020. These data are a key component of the CMS decision, but they might be a tough sell, Clifford R. Jack Jr., MD, and Ronald C. Petersen, MD, PhD, wrote in an accompanying editorial. Dr. Jack and Dr. Petersen are affiliated with the Mayo Clinic in Rochester, Minn.
“For CMS to cover the cost of amyloid PET, it must be demonstrated that the result of a scan has an effect on patient outcomes, not just patient care processes – and, without a disease-modifying therapy available, that might be a challenge,” they wrote.
IDEAS is a funding collaboration of the CMS, the Alzheimer’s Association, Avid Radiopharmaceuticals/Eli Lilly, General Electric Healthcare, Piramal Imaging, and the American College of Radiology. Dr. Rabinovici had no financial disclosures.
SOURCE: Rabinovici GD et al. JAMA. 2019 Apr 2. doi: 10.1001/jama.2019.2000.
.
Diagnoses changed from Alzheimer’s disease to non–Alzheimer’s disease in 25% of 11,409 patients and from non–Alzheimer’s disease to Alzheimer’s disease in 10.5%, reported Gil Rabinovici, MD, and his colleagues. The use of Alzheimer’s disease drugs doubled in amyloid-positive MCI patients, and increased by a third in amyloid-positive dementia patients. Physicians involved in the study said the scans provided key clinical information in 82% of cases with post-scan management changes.
Scans also benefited amyloid-negative patients. Before the scan, 71% of these carried an Alzheimer’s disease diagnosis; afterward, just 10% did, opening the way for an accurate diagnosis and more effective treatment.
The study was powered to detect a 30% or greater change in the MCI and dementia groups. The 60% change emphasize how useful amyloid PET scans could be in clinical practice, Dr. Rabinovici, the study’s lead author and principal investigator, said in a press statement.
“We are impressed by the magnitude of these results, which make it clear that amyloid PET imaging can have a major impact on how we diagnose and care for patients with Alzheimer’s disease and other forms of cognitive decline,” said Dr. Rabinovici of the University of California, San Francisco.
Alzheimer’s Association leaders were similarly pleased.
“These results present highly credible, large-scale evidence that amyloid PET imaging can be a powerful tool to improve the accuracy of Alzheimer’s diagnosis and lead to better medical management, especially in difficult-to-diagnose cases,” said Maria C. Carrillo, PhD, chief science officer of the Alzheimer’s Association and a coauthor of the study. “It is important that amyloid PET imaging be more broadly accessible to those who need it.”
Next steps
Ultimately, investigators hope the nationwide-wide, open-label study will prove the clinical value of amyloid PET scanning and convince the Centers for Medicare & Medicaid Services to make the test a fully covered service for those who meet the appropriate use criteria set forth by the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging.
IDEAS’ second goal – showing that the scans improve health outcomes – is scheduled for 2020. These data are a key component of the CMS decision, but they might be a tough sell, Clifford R. Jack Jr., MD, and Ronald C. Petersen, MD, PhD, wrote in an accompanying editorial. Dr. Jack and Dr. Petersen are affiliated with the Mayo Clinic in Rochester, Minn.
“For CMS to cover the cost of amyloid PET, it must be demonstrated that the result of a scan has an effect on patient outcomes, not just patient care processes – and, without a disease-modifying therapy available, that might be a challenge,” they wrote.
IDEAS is a funding collaboration of the CMS, the Alzheimer’s Association, Avid Radiopharmaceuticals/Eli Lilly, General Electric Healthcare, Piramal Imaging, and the American College of Radiology. Dr. Rabinovici had no financial disclosures.
SOURCE: Rabinovici GD et al. JAMA. 2019 Apr 2. doi: 10.1001/jama.2019.2000.
FROM THE JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION



