User login
Skin Cancer in Military Pilots: A Special Population With Special Risk Factors
Military dermatologists are charged with caring for a diverse population of active-duty members, civilian dependents, and military retirees. Although certain risk factors for cutaneous malignancies are common in all of these groups, the active-duty population experiences unique exposures to be considered when determining their risk for skin cancer. One subset that may be at a higher risk is military pilots who fly at high altitudes on irregular schedules in austere environments. Through the unparalleled comradeship inherent in many military units, pilots “hear” from their fellow pilots that they are at increased risk for skin cancer. Do their occupational exposures translate into increased risk for cutaneous malignancy? This article will survey the literature pertaining to pilots and skin cancer so that all dermatologists may better care for this unique population.
Epidemiology
Anecdotally, we have observed basal cell carcinoma in pilots in their 20s and early 30s, earlier than would be expected in an otherwise healthy prescreened military population.1 Woolley and Hughes2 published a case report of skin cancer in a young military aviator. The patient was a 32-year-old male helicopter pilot with Fitzpatrick skin type II and no personal or family history of skin cancer who was diagnosed with a periocular nodular basal cell carcinoma. He deployed to locations with high UV radiation (UVR) indices, and his vacation time also was spent in such areas.2 UV radiation exposure and Fitzpatrick skin type are known risk factors across occupations, but are there special exposures that come with military aviation service?
To better understand the risk for malignancy in this special population, the US Air Force examined the rates of all cancer types among a cohort of flying versus nonflying officers.3 Aviation personnel showed increased incidence of testicular, bladder, and all-site cancers combined. Noticeably absent was a statistically significant increased risk for malignant melanoma (MM) and nonmelanoma skin cancer (NMSC). Other epidemiological studies examined the incidence rates of MM in the US Armed Forces compared with age- and race-matched civilian populations and showed mixed results: 2 studies showed increased risk,4,5 while a third showed decreased risk.6 Despite finding opposite results of MM rates in military members versus the civilian population, 2 of these studies showed US Air Force members to have higher rates of MM than those in the US Army or Navy.4,6 Interestingly, the air force has the highest number of pilots among all the services, with 4000 more pilots than the army and navy.7 Further studies are needed to determine if the higher air force MM rates occur in pilots.
Although there are mixed and limited data pertaining to military flight crews, there is more robust literature concerning civilian flight personnel. One meta-analysis pooled studies related to cancer risk in cabin crews and civil and military pilots.8 In military pilots, they found a standardized incidence ratio (SIR) of 1.43 (95% confidence interval [CI], 1.09-1.87) for MM and 1.80 (95% CI, 1.25-2.80) for NMSC. The SIRs were higher for male cabin attendants (3.42 and 7.46, respectively) and civil pilots (2.18 and 1.88, respectively). They also found the most common cause of mortality in civilian cabin crews was AIDS, possibly explaining the higher SIRs for all types of malignancy in that population.8 In the United States, many civilian pilots previously were military pilots9 who likely served in the military for at least 10 years.10 A 2015 meta-analysis of 19 studies of more than 266,000 civil pilots and aircrew members found an SIR for MM of 2.22 (95% CI, 1.67-2.93) for civil pilots and 2.09 (95% CI, 1.67-2.62) for aircrews, stating the risk for MM is at least twice that of the general population.11
Risk Factors
UV Radiation
These studies suggest flight duties increase the risk for cutaneous malignancy. UV radiation is a known risk factor for skin cancer.12 The main body of the aircraft may protect the cabin’s crew and passengers from UVR, but pilots are exposed to more UVR, especially in aircraft with larger windshields. A government study in 2007 examined the transmittance of UVR through windscreens of 8 aircraft: 3 commercial jets, 2 commercial propeller planes, 1 private jet, and 2 small propeller planes.13 UVB was attenuated by all the windscreens (<1% transmittance), but 43% to 54% of UVA was transmitted, with plastic windshields attenuating more than glass. Sanlorenzo et al14 measured UVA irradiance at the pilot’s seat of a turboprop aircraft at 30,000-ft altitude. They compared this exposure to a UVA tanning bed and estimated that 57 minutes of flight at 30,000-ft altitude was equivalent to 20 minutes inside a UVA tanning booth, a startling finding.14
Cosmic Radiation
Cosmic radiation consists of neutrons and gamma rays that originate outside Earth’s atmosphere. Pilots are exposed to higher doses of cosmic radiation than nonpilots, but the health effects are difficult to study. Boice et al15 described how factors such as altitude, latitude, and flight time determine pilots’ cumulative exposure. With longer flight times at higher altitudes, a pilot’s exposure to cosmic radiation is increasing over the years.15 A 2012 review found that aircrews have low-level cosmic radiation exposure. Despite increases in MM and NMSC in pilots and increased rates of breast cancer in female aircrew, overall cancer-related mortality was lower in flying versus nonflying controls.16 Thus, cosmic radiation may not be as onerous of an occupational hazard for pilots as has been postulated.
Altered Circadian Rhythms
Aviation duties, especially in the military, require irregular work schedules that repeatedly interfere with normal sleep-wake cycles, disrupt circadian rhythms, and lead to reduced melatonin levels.8 Evidence suggests that low levels of melatonin could increase the risk for breast and prostate cancer—both cancers that occur more frequently in female aircrew and male pilots, respectively—by reducing melatonin’s natural protective role in such malignancies.17,18 A World Health Organization working group categorized shift work as “probably carcinogenic” and cited alterations of melatonin levels, changes in other circadian rhythm–related gene pathways, and relative immunosuppression as likely causative factors.19 In a 2011 study, exposing mice to UVR during times when nucleotide excision repair mechanisms were at their lowest activity caused an increased rate of skin cancers.20 A 2014 review discussed how epidemiological studies of shift workers such as nurses, firefighters, pilots, and flight crews found contradictory data, but molecular studies show that circadian rhythm–linked repair and tumorigenesis mechanisms are altered by aberrations in the normal sleep-wake cycle.21
Cockpit Instrumentation
Electromagnetic energy from the flight instruments in the cockpit also could influence malignancy risk. Nicholas et al22 found magnetic field measurements within the cockpit to be 2 to 10 times that experienced within the home or office. However, no studies examining the health effects of cockpit flight instruments and magnetic fields were found.
Final Thoughts
It is important to counsel pilots on the generally recognized, nonaviation-specific risk factors of family history, skin type, and UVR exposure in the development of skin cancer. Additionally, it is important to explain the possible role of exposure to UVR at higher altitudes, cosmic radiation, and electromagnetic energy from cockpit instruments, as well as altered sleep-wake cycles. A pilot’s risk for MM may be twice that of matched controls, and the risk for NMSC could be higher.8,11 Although the literature lacks specific recommendations for pilots, it is reasonable to screen pilots once per year to better assess their individual risk and encourage diligent use of sunscreen and sun-protective measures when flying. It also may be important to advocate for the development of engineering controls that decrease UVR transmittance through windscreens, particularly for aircraft flying at higher altitudes for longer flights. More research is needed to determine if changes in circadian rhythm and decreases in melatonin increase skin cancer risk, which could impact how pilots’ schedules are managed. Together, we can ensure adequate surveillance, diagnosis, and treatment in this at-risk population.
- Roewert‐Huber J, Lange-Asschenfeldt B, Stockfleth E, et al. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2007;157(suppl 2):47-51.
- Woolley SD, Hughes C. A young military pilot presents with a periocular basal cell carcinoma: a case report. Travel Med Infect Dis. 2013;11:435-437.
- Grayson JK, Lyons TJ. Cancer incidence in United States Air Force aircrew, 1975-89. Aviat Space Environ Med. 1996;67:101-104.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Garland FC, White MR, Garland CF, et al. Occupational sunlight exposure and melanoma in the US Navy. Arc Environ Health. 1990;45:261-267.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the US military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Active Duty Master Personnel File: Active Duty Tactical Operations Officers. Seaside, CA: Defense Manpower Data Center; August 31, 2017. Accessed September 22, 2017.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Jansen HS, Oster CV, eds. Taking Flight: Education and Training for Aviation Careers. Washington, DC: National Academy Press; 1997.
- About AFROTC Service Commitment. US Air Force ROTC website. https://www.afrotc.com/about/service. Accessed September 20, 2017.
- Sanlorenzo M, Wehner MR, Linos E, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol. 2015;151:51-58.
- Ananthaswamy HN, Pierceall WE. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem Photobiol. 1990;52:1119-1136.
- Nakagawara VB, Montgomery RW, Marshall WJ. Optical Radiation Transmittance of Aircraft Windscreens and Pilot Vision. Oklahoma City, OK: Federal Aviation Administration; 2007.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Boice JD, Blettner M, Auvinen A. Epidemiologic studies of pilots and aircrew. Health Phys. 2000;79:576-584.
- Zeeb H, Hammer GP, Blettner M. Epidemiological investigations of aircrew: an occupational group with low-level cosmic radiation exposure [published online March 6, 2012]. J Radiol Prot. 2012;32:N15-N19.
- Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005;16:254-258.
- Siu SW, Lau KW, Tam PC, et al. Melatonin and prostate cancer cell proliferation: interplay with castration, epidermal growth factor, and androgen sensitivity. Prostate. 2002;52:106-122.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. Lyon, France: World Health Organization International Agency for Research on Cancer; 2010.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci. 2011;108:18790-18795.
- Markova-Car EP, Jurišic´ D, Ilic´ N, et al. Running for time: circadian rhythms and melanoma. Tumour Biol. 2014;35:8359-8368.
- Nicholas JS, Lackland DT, Butler GC, et al. Cosmic radiation and magnetic field exposure to airline flight crews. Am J Ind Med. 1998;34:574-580.
Military dermatologists are charged with caring for a diverse population of active-duty members, civilian dependents, and military retirees. Although certain risk factors for cutaneous malignancies are common in all of these groups, the active-duty population experiences unique exposures to be considered when determining their risk for skin cancer. One subset that may be at a higher risk is military pilots who fly at high altitudes on irregular schedules in austere environments. Through the unparalleled comradeship inherent in many military units, pilots “hear” from their fellow pilots that they are at increased risk for skin cancer. Do their occupational exposures translate into increased risk for cutaneous malignancy? This article will survey the literature pertaining to pilots and skin cancer so that all dermatologists may better care for this unique population.
Epidemiology
Anecdotally, we have observed basal cell carcinoma in pilots in their 20s and early 30s, earlier than would be expected in an otherwise healthy prescreened military population.1 Woolley and Hughes2 published a case report of skin cancer in a young military aviator. The patient was a 32-year-old male helicopter pilot with Fitzpatrick skin type II and no personal or family history of skin cancer who was diagnosed with a periocular nodular basal cell carcinoma. He deployed to locations with high UV radiation (UVR) indices, and his vacation time also was spent in such areas.2 UV radiation exposure and Fitzpatrick skin type are known risk factors across occupations, but are there special exposures that come with military aviation service?
To better understand the risk for malignancy in this special population, the US Air Force examined the rates of all cancer types among a cohort of flying versus nonflying officers.3 Aviation personnel showed increased incidence of testicular, bladder, and all-site cancers combined. Noticeably absent was a statistically significant increased risk for malignant melanoma (MM) and nonmelanoma skin cancer (NMSC). Other epidemiological studies examined the incidence rates of MM in the US Armed Forces compared with age- and race-matched civilian populations and showed mixed results: 2 studies showed increased risk,4,5 while a third showed decreased risk.6 Despite finding opposite results of MM rates in military members versus the civilian population, 2 of these studies showed US Air Force members to have higher rates of MM than those in the US Army or Navy.4,6 Interestingly, the air force has the highest number of pilots among all the services, with 4000 more pilots than the army and navy.7 Further studies are needed to determine if the higher air force MM rates occur in pilots.
Although there are mixed and limited data pertaining to military flight crews, there is more robust literature concerning civilian flight personnel. One meta-analysis pooled studies related to cancer risk in cabin crews and civil and military pilots.8 In military pilots, they found a standardized incidence ratio (SIR) of 1.43 (95% confidence interval [CI], 1.09-1.87) for MM and 1.80 (95% CI, 1.25-2.80) for NMSC. The SIRs were higher for male cabin attendants (3.42 and 7.46, respectively) and civil pilots (2.18 and 1.88, respectively). They also found the most common cause of mortality in civilian cabin crews was AIDS, possibly explaining the higher SIRs for all types of malignancy in that population.8 In the United States, many civilian pilots previously were military pilots9 who likely served in the military for at least 10 years.10 A 2015 meta-analysis of 19 studies of more than 266,000 civil pilots and aircrew members found an SIR for MM of 2.22 (95% CI, 1.67-2.93) for civil pilots and 2.09 (95% CI, 1.67-2.62) for aircrews, stating the risk for MM is at least twice that of the general population.11
Risk Factors
UV Radiation
These studies suggest flight duties increase the risk for cutaneous malignancy. UV radiation is a known risk factor for skin cancer.12 The main body of the aircraft may protect the cabin’s crew and passengers from UVR, but pilots are exposed to more UVR, especially in aircraft with larger windshields. A government study in 2007 examined the transmittance of UVR through windscreens of 8 aircraft: 3 commercial jets, 2 commercial propeller planes, 1 private jet, and 2 small propeller planes.13 UVB was attenuated by all the windscreens (<1% transmittance), but 43% to 54% of UVA was transmitted, with plastic windshields attenuating more than glass. Sanlorenzo et al14 measured UVA irradiance at the pilot’s seat of a turboprop aircraft at 30,000-ft altitude. They compared this exposure to a UVA tanning bed and estimated that 57 minutes of flight at 30,000-ft altitude was equivalent to 20 minutes inside a UVA tanning booth, a startling finding.14
Cosmic Radiation
Cosmic radiation consists of neutrons and gamma rays that originate outside Earth’s atmosphere. Pilots are exposed to higher doses of cosmic radiation than nonpilots, but the health effects are difficult to study. Boice et al15 described how factors such as altitude, latitude, and flight time determine pilots’ cumulative exposure. With longer flight times at higher altitudes, a pilot’s exposure to cosmic radiation is increasing over the years.15 A 2012 review found that aircrews have low-level cosmic radiation exposure. Despite increases in MM and NMSC in pilots and increased rates of breast cancer in female aircrew, overall cancer-related mortality was lower in flying versus nonflying controls.16 Thus, cosmic radiation may not be as onerous of an occupational hazard for pilots as has been postulated.
Altered Circadian Rhythms
Aviation duties, especially in the military, require irregular work schedules that repeatedly interfere with normal sleep-wake cycles, disrupt circadian rhythms, and lead to reduced melatonin levels.8 Evidence suggests that low levels of melatonin could increase the risk for breast and prostate cancer—both cancers that occur more frequently in female aircrew and male pilots, respectively—by reducing melatonin’s natural protective role in such malignancies.17,18 A World Health Organization working group categorized shift work as “probably carcinogenic” and cited alterations of melatonin levels, changes in other circadian rhythm–related gene pathways, and relative immunosuppression as likely causative factors.19 In a 2011 study, exposing mice to UVR during times when nucleotide excision repair mechanisms were at their lowest activity caused an increased rate of skin cancers.20 A 2014 review discussed how epidemiological studies of shift workers such as nurses, firefighters, pilots, and flight crews found contradictory data, but molecular studies show that circadian rhythm–linked repair and tumorigenesis mechanisms are altered by aberrations in the normal sleep-wake cycle.21
Cockpit Instrumentation
Electromagnetic energy from the flight instruments in the cockpit also could influence malignancy risk. Nicholas et al22 found magnetic field measurements within the cockpit to be 2 to 10 times that experienced within the home or office. However, no studies examining the health effects of cockpit flight instruments and magnetic fields were found.
Final Thoughts
It is important to counsel pilots on the generally recognized, nonaviation-specific risk factors of family history, skin type, and UVR exposure in the development of skin cancer. Additionally, it is important to explain the possible role of exposure to UVR at higher altitudes, cosmic radiation, and electromagnetic energy from cockpit instruments, as well as altered sleep-wake cycles. A pilot’s risk for MM may be twice that of matched controls, and the risk for NMSC could be higher.8,11 Although the literature lacks specific recommendations for pilots, it is reasonable to screen pilots once per year to better assess their individual risk and encourage diligent use of sunscreen and sun-protective measures when flying. It also may be important to advocate for the development of engineering controls that decrease UVR transmittance through windscreens, particularly for aircraft flying at higher altitudes for longer flights. More research is needed to determine if changes in circadian rhythm and decreases in melatonin increase skin cancer risk, which could impact how pilots’ schedules are managed. Together, we can ensure adequate surveillance, diagnosis, and treatment in this at-risk population.
Military dermatologists are charged with caring for a diverse population of active-duty members, civilian dependents, and military retirees. Although certain risk factors for cutaneous malignancies are common in all of these groups, the active-duty population experiences unique exposures to be considered when determining their risk for skin cancer. One subset that may be at a higher risk is military pilots who fly at high altitudes on irregular schedules in austere environments. Through the unparalleled comradeship inherent in many military units, pilots “hear” from their fellow pilots that they are at increased risk for skin cancer. Do their occupational exposures translate into increased risk for cutaneous malignancy? This article will survey the literature pertaining to pilots and skin cancer so that all dermatologists may better care for this unique population.
Epidemiology
Anecdotally, we have observed basal cell carcinoma in pilots in their 20s and early 30s, earlier than would be expected in an otherwise healthy prescreened military population.1 Woolley and Hughes2 published a case report of skin cancer in a young military aviator. The patient was a 32-year-old male helicopter pilot with Fitzpatrick skin type II and no personal or family history of skin cancer who was diagnosed with a periocular nodular basal cell carcinoma. He deployed to locations with high UV radiation (UVR) indices, and his vacation time also was spent in such areas.2 UV radiation exposure and Fitzpatrick skin type are known risk factors across occupations, but are there special exposures that come with military aviation service?
To better understand the risk for malignancy in this special population, the US Air Force examined the rates of all cancer types among a cohort of flying versus nonflying officers.3 Aviation personnel showed increased incidence of testicular, bladder, and all-site cancers combined. Noticeably absent was a statistically significant increased risk for malignant melanoma (MM) and nonmelanoma skin cancer (NMSC). Other epidemiological studies examined the incidence rates of MM in the US Armed Forces compared with age- and race-matched civilian populations and showed mixed results: 2 studies showed increased risk,4,5 while a third showed decreased risk.6 Despite finding opposite results of MM rates in military members versus the civilian population, 2 of these studies showed US Air Force members to have higher rates of MM than those in the US Army or Navy.4,6 Interestingly, the air force has the highest number of pilots among all the services, with 4000 more pilots than the army and navy.7 Further studies are needed to determine if the higher air force MM rates occur in pilots.
Although there are mixed and limited data pertaining to military flight crews, there is more robust literature concerning civilian flight personnel. One meta-analysis pooled studies related to cancer risk in cabin crews and civil and military pilots.8 In military pilots, they found a standardized incidence ratio (SIR) of 1.43 (95% confidence interval [CI], 1.09-1.87) for MM and 1.80 (95% CI, 1.25-2.80) for NMSC. The SIRs were higher for male cabin attendants (3.42 and 7.46, respectively) and civil pilots (2.18 and 1.88, respectively). They also found the most common cause of mortality in civilian cabin crews was AIDS, possibly explaining the higher SIRs for all types of malignancy in that population.8 In the United States, many civilian pilots previously were military pilots9 who likely served in the military for at least 10 years.10 A 2015 meta-analysis of 19 studies of more than 266,000 civil pilots and aircrew members found an SIR for MM of 2.22 (95% CI, 1.67-2.93) for civil pilots and 2.09 (95% CI, 1.67-2.62) for aircrews, stating the risk for MM is at least twice that of the general population.11
Risk Factors
UV Radiation
These studies suggest flight duties increase the risk for cutaneous malignancy. UV radiation is a known risk factor for skin cancer.12 The main body of the aircraft may protect the cabin’s crew and passengers from UVR, but pilots are exposed to more UVR, especially in aircraft with larger windshields. A government study in 2007 examined the transmittance of UVR through windscreens of 8 aircraft: 3 commercial jets, 2 commercial propeller planes, 1 private jet, and 2 small propeller planes.13 UVB was attenuated by all the windscreens (<1% transmittance), but 43% to 54% of UVA was transmitted, with plastic windshields attenuating more than glass. Sanlorenzo et al14 measured UVA irradiance at the pilot’s seat of a turboprop aircraft at 30,000-ft altitude. They compared this exposure to a UVA tanning bed and estimated that 57 minutes of flight at 30,000-ft altitude was equivalent to 20 minutes inside a UVA tanning booth, a startling finding.14
Cosmic Radiation
Cosmic radiation consists of neutrons and gamma rays that originate outside Earth’s atmosphere. Pilots are exposed to higher doses of cosmic radiation than nonpilots, but the health effects are difficult to study. Boice et al15 described how factors such as altitude, latitude, and flight time determine pilots’ cumulative exposure. With longer flight times at higher altitudes, a pilot’s exposure to cosmic radiation is increasing over the years.15 A 2012 review found that aircrews have low-level cosmic radiation exposure. Despite increases in MM and NMSC in pilots and increased rates of breast cancer in female aircrew, overall cancer-related mortality was lower in flying versus nonflying controls.16 Thus, cosmic radiation may not be as onerous of an occupational hazard for pilots as has been postulated.
Altered Circadian Rhythms
Aviation duties, especially in the military, require irregular work schedules that repeatedly interfere with normal sleep-wake cycles, disrupt circadian rhythms, and lead to reduced melatonin levels.8 Evidence suggests that low levels of melatonin could increase the risk for breast and prostate cancer—both cancers that occur more frequently in female aircrew and male pilots, respectively—by reducing melatonin’s natural protective role in such malignancies.17,18 A World Health Organization working group categorized shift work as “probably carcinogenic” and cited alterations of melatonin levels, changes in other circadian rhythm–related gene pathways, and relative immunosuppression as likely causative factors.19 In a 2011 study, exposing mice to UVR during times when nucleotide excision repair mechanisms were at their lowest activity caused an increased rate of skin cancers.20 A 2014 review discussed how epidemiological studies of shift workers such as nurses, firefighters, pilots, and flight crews found contradictory data, but molecular studies show that circadian rhythm–linked repair and tumorigenesis mechanisms are altered by aberrations in the normal sleep-wake cycle.21
Cockpit Instrumentation
Electromagnetic energy from the flight instruments in the cockpit also could influence malignancy risk. Nicholas et al22 found magnetic field measurements within the cockpit to be 2 to 10 times that experienced within the home or office. However, no studies examining the health effects of cockpit flight instruments and magnetic fields were found.
Final Thoughts
It is important to counsel pilots on the generally recognized, nonaviation-specific risk factors of family history, skin type, and UVR exposure in the development of skin cancer. Additionally, it is important to explain the possible role of exposure to UVR at higher altitudes, cosmic radiation, and electromagnetic energy from cockpit instruments, as well as altered sleep-wake cycles. A pilot’s risk for MM may be twice that of matched controls, and the risk for NMSC could be higher.8,11 Although the literature lacks specific recommendations for pilots, it is reasonable to screen pilots once per year to better assess their individual risk and encourage diligent use of sunscreen and sun-protective measures when flying. It also may be important to advocate for the development of engineering controls that decrease UVR transmittance through windscreens, particularly for aircraft flying at higher altitudes for longer flights. More research is needed to determine if changes in circadian rhythm and decreases in melatonin increase skin cancer risk, which could impact how pilots’ schedules are managed. Together, we can ensure adequate surveillance, diagnosis, and treatment in this at-risk population.
- Roewert‐Huber J, Lange-Asschenfeldt B, Stockfleth E, et al. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2007;157(suppl 2):47-51.
- Woolley SD, Hughes C. A young military pilot presents with a periocular basal cell carcinoma: a case report. Travel Med Infect Dis. 2013;11:435-437.
- Grayson JK, Lyons TJ. Cancer incidence in United States Air Force aircrew, 1975-89. Aviat Space Environ Med. 1996;67:101-104.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Garland FC, White MR, Garland CF, et al. Occupational sunlight exposure and melanoma in the US Navy. Arc Environ Health. 1990;45:261-267.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the US military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Active Duty Master Personnel File: Active Duty Tactical Operations Officers. Seaside, CA: Defense Manpower Data Center; August 31, 2017. Accessed September 22, 2017.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Jansen HS, Oster CV, eds. Taking Flight: Education and Training for Aviation Careers. Washington, DC: National Academy Press; 1997.
- About AFROTC Service Commitment. US Air Force ROTC website. https://www.afrotc.com/about/service. Accessed September 20, 2017.
- Sanlorenzo M, Wehner MR, Linos E, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol. 2015;151:51-58.
- Ananthaswamy HN, Pierceall WE. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem Photobiol. 1990;52:1119-1136.
- Nakagawara VB, Montgomery RW, Marshall WJ. Optical Radiation Transmittance of Aircraft Windscreens and Pilot Vision. Oklahoma City, OK: Federal Aviation Administration; 2007.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Boice JD, Blettner M, Auvinen A. Epidemiologic studies of pilots and aircrew. Health Phys. 2000;79:576-584.
- Zeeb H, Hammer GP, Blettner M. Epidemiological investigations of aircrew: an occupational group with low-level cosmic radiation exposure [published online March 6, 2012]. J Radiol Prot. 2012;32:N15-N19.
- Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005;16:254-258.
- Siu SW, Lau KW, Tam PC, et al. Melatonin and prostate cancer cell proliferation: interplay with castration, epidermal growth factor, and androgen sensitivity. Prostate. 2002;52:106-122.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. Lyon, France: World Health Organization International Agency for Research on Cancer; 2010.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci. 2011;108:18790-18795.
- Markova-Car EP, Jurišic´ D, Ilic´ N, et al. Running for time: circadian rhythms and melanoma. Tumour Biol. 2014;35:8359-8368.
- Nicholas JS, Lackland DT, Butler GC, et al. Cosmic radiation and magnetic field exposure to airline flight crews. Am J Ind Med. 1998;34:574-580.
- Roewert‐Huber J, Lange-Asschenfeldt B, Stockfleth E, et al. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2007;157(suppl 2):47-51.
- Woolley SD, Hughes C. A young military pilot presents with a periocular basal cell carcinoma: a case report. Travel Med Infect Dis. 2013;11:435-437.
- Grayson JK, Lyons TJ. Cancer incidence in United States Air Force aircrew, 1975-89. Aviat Space Environ Med. 1996;67:101-104.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Garland FC, White MR, Garland CF, et al. Occupational sunlight exposure and melanoma in the US Navy. Arc Environ Health. 1990;45:261-267.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the US military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Active Duty Master Personnel File: Active Duty Tactical Operations Officers. Seaside, CA: Defense Manpower Data Center; August 31, 2017. Accessed September 22, 2017.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Jansen HS, Oster CV, eds. Taking Flight: Education and Training for Aviation Careers. Washington, DC: National Academy Press; 1997.
- About AFROTC Service Commitment. US Air Force ROTC website. https://www.afrotc.com/about/service. Accessed September 20, 2017.
- Sanlorenzo M, Wehner MR, Linos E, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol. 2015;151:51-58.
- Ananthaswamy HN, Pierceall WE. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem Photobiol. 1990;52:1119-1136.
- Nakagawara VB, Montgomery RW, Marshall WJ. Optical Radiation Transmittance of Aircraft Windscreens and Pilot Vision. Oklahoma City, OK: Federal Aviation Administration; 2007.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Boice JD, Blettner M, Auvinen A. Epidemiologic studies of pilots and aircrew. Health Phys. 2000;79:576-584.
- Zeeb H, Hammer GP, Blettner M. Epidemiological investigations of aircrew: an occupational group with low-level cosmic radiation exposure [published online March 6, 2012]. J Radiol Prot. 2012;32:N15-N19.
- Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005;16:254-258.
- Siu SW, Lau KW, Tam PC, et al. Melatonin and prostate cancer cell proliferation: interplay with castration, epidermal growth factor, and androgen sensitivity. Prostate. 2002;52:106-122.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. Lyon, France: World Health Organization International Agency for Research on Cancer; 2010.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci. 2011;108:18790-18795.
- Markova-Car EP, Jurišic´ D, Ilic´ N, et al. Running for time: circadian rhythms and melanoma. Tumour Biol. 2014;35:8359-8368.
- Nicholas JS, Lackland DT, Butler GC, et al. Cosmic radiation and magnetic field exposure to airline flight crews. Am J Ind Med. 1998;34:574-580.
Practice Points
- Military and civilian pilots have an increased risk for melanoma and nonmelanoma skin cancer, likely due to unique occupational exposures.
- We recommend annual skin cancer screening for all pilots to help assess their individual risk.
- Pilots should be educated on their increased risk for skin cancer and encouraged to use sun-protective measures during their flying duties and leisure activities.
Management of Trauma and Burn Scars: The Dermatologist’s Role in Expanding Patient Access to Care
Hypertrophic scarring secondary to trauma, burns, and surgical interventions is a major source of morbidity worldwide and often is mechanically, aesthetically, and symptomatically debilitating. Modern advances in acute trauma care protocols have resulted in survival rates greater than 90% in both civilian and military populations.1,2 Patients with wounds that have historically proven fatal are now surviving and are confronted with the long-term sequelae of their injuries. With more than 52,000 service members injured in military engagements from 2001 to 2015 and 8.5 million civilians presenting annually with injury patterns at risk for hypertrophic scarring, it is paramount that we ensure access to safe and effective long-term scar care.2,3
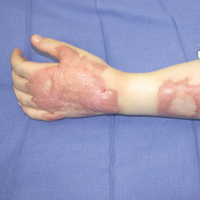
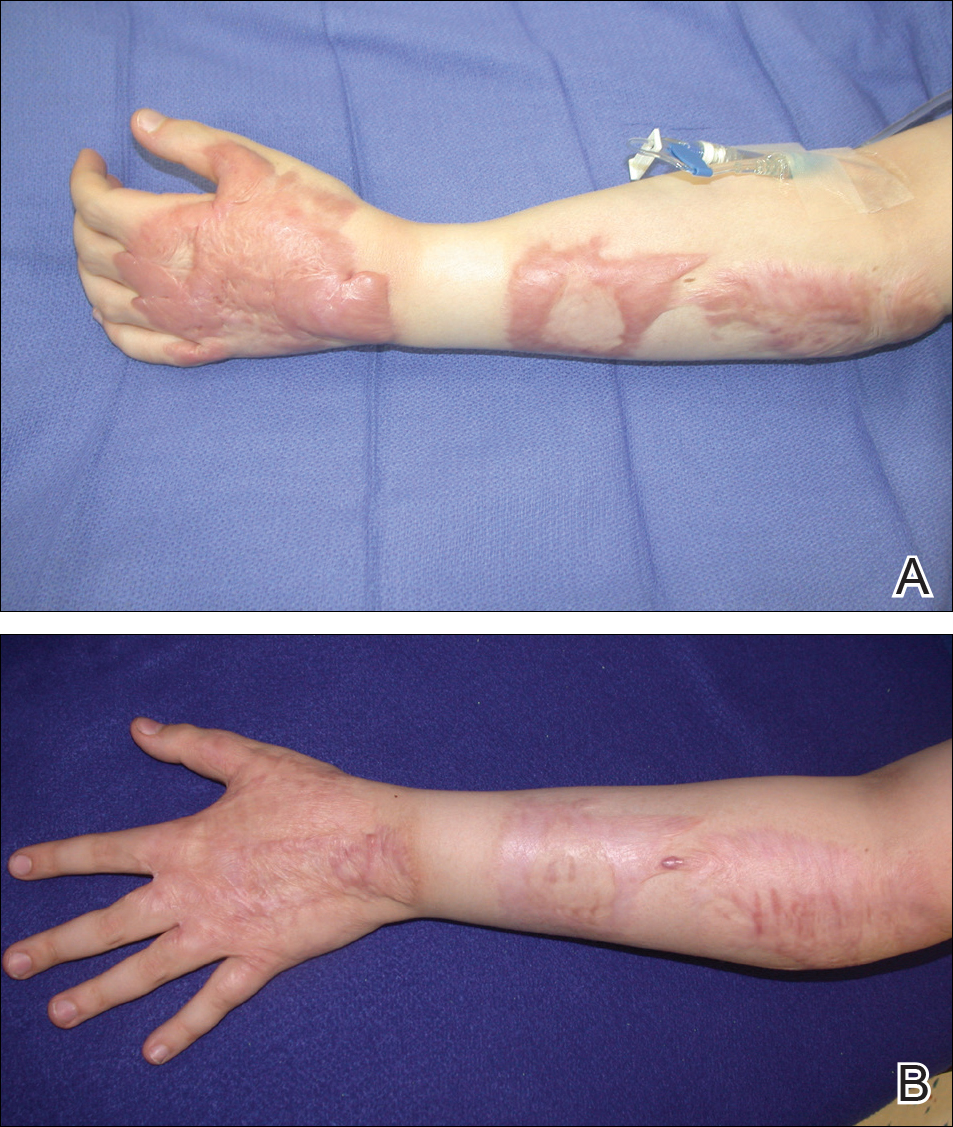
At its simplest level, hypertrophic scarring is believed to result from a disequilibrium between collagen production and degradation. This failure to properly transition through the stages of wound healing results in bothersome symptoms, a disfigured appearance, and mechanical dysfunction of the skin (Figure, A). Decreased elasticity and extensibility, increased dermal thickness, and scar contractures impair patient range of motion and functional mobility. Those affected commonly experience varying degrees of pruritus and dysesthesia along the scar. Combined with aesthetic variations in pigmentation, erythema, texture, and thickness, hypertrophic scarring often leads to long-term psychosocial impairment and decreased health-related quality of life.4
Treatment Approach
Treatment of hypertrophic scars requires a multimodal approach due to the spectrum of associated concerns and the natural recalcitrance of the scar to therapy. Protocols should be tailored to the individual but generally begin with tissue-conserving surgical interventions followed by selective photothermolysis of the scar vasculature. Subsequently, deep and superficial ablative fractional laser (AFL) treatment and local pharmacotherapy also are employed. Treatment can be accomplished in the outpatient setting under local anesthesia in a serial fashion. In the authors’ experience, these therapies behave in a synergistic fashion, achieving outcomes that far exceed the sum of their parts, often obviating the need for scar excision in the majority of cases (Figure, B).
Tissue-Conserving Surgical Intervention
Z-plasty is an indispensable surgical tool due to its ability to lengthen scars and reduce wound tension. Treatment is easily customizable to the patient and can be performed using the individual or multiple Z-plasty techniques. Undermining and step-off correction while suturing allow the physician to lower raised scars, elevate depressed scars, and obscure scar presence by minimizing the straight lines that draw the eye to the scar. Z-plasties rely on the creation and transposition of 2 triangular flaps and permit a 75% increase in length along the desired tension vector. As such, Z-plasties decrease wound tension and facilitate scar maturation.
Selective Photothermolysis of the Vasculature
Although there are several devices available to treat vascular and immature hypertrophic scars, the majority of studies have been conducted with the 595-nm pulsed dye laser. By preferentially heating oxyhemoglobin within the dermal microvasculature, the pulsed dye laser irreparably injures the vascular endothelium. The subsequent tissue hypoxia and collagen fiber heating results in collagen fiber realignment, normalization of collagen subtypes, and neocollagenesis.5 Pulsed dye laser therapy most effectively reduces erythema and pruritus; however, improvements in scar volume, pliability, and elasticity also have been reported.5 When targeting the fine vasculature of the scar, thermal confinement is critical to prevent injury to the surrounding dermis. As such, pulse widths of 0.45 to 1.5 milliseconds are routinely utilized with a fluence just sufficient to elicit transient purpura lasting 3 to 5 seconds. Employing a spot size of 7 to 10 mm, typical fluences range from 4.5 to 6.5 J/cm2. Engagement of the dynamic cooling device reduces the risk for complications, allowing the patient to proceed to the next step in their therapy regimen: the AFL.
Ablative Fractional Laser
The AFL creates a pixilated pattern of injury throughout the epidermis and dermis of the treatment area. Ablative fractional laser platforms include the 10,600-nm CO2 and 2940-nm erbium-doped YAG lasers, both targeting intracellular water. The AFL vaporizes columns of tissue, leaving minute vertical channels with narrow rims of protein coagulation referred to as microscopic treatment zones (MTZs).6 Scar collagen analysis after AFL treatment has shown a profile resembling unaffected skin.7 Consistently, patients report improvements in stiffness, range of motion, pain, pruritus, pigmentation, and erythema.Physician observers also have reported similar improvements in these end points.8,9 Recently, interim data from a prospective controlled trial were presented showing objective improvements in dermal thickness, elasticity, and extensibility after 3 treatments with the CO2 AFL.6 The UltraPulse CO2 laser (Lumenis) is the most well-studied and widely available AFL for scar therapy and as such we will outline common treatment parameters with this device. Of note, treatment end points may be generalized to any AFL.
The DeepFX UltraPulse configuration is utilized to achieve deep AFL therapy and has a fixed pulse width of 0.8 milliseconds, slightly less than the thermal relaxation time of the skin. The diameter of the MTZs is 120 µm, and MTZ density for scar treatment ranges from 1% to 10% with a goal depth of at least 80% of scar thickness. Maximal penetration of the AFL is 4 mm, which is directly proportional to fluence. The goal of deep AFL is the removal of scar tissue to facilitate remodeling and neocollagenesis. Superficial fractional ablation can then be achieved utilizing the ActiveFX UltraPulse configuration generating a 1.3-mm MTZ spot size. We commonly use a treatment level of 3 (82% density). Typical treatment energy ranges from 80 to 125 mJ, which correlates with depths of approximately 50 to 115 µm. With both configurations, the size and shape of the treatment area can be customized to the scar. In addition, frequency may be adjusted to control the speed of treatment while balancing the risk of bulk heating. The goal of superficial AFL is to minimize scar surface irregularities and ensure blending of deep AFL treatment. Once AFL treatment is complete, local pharmacotherapy can then be employed.
Pharmacotherapy
Intralesional corticosteroids have long represented the standard of care for hypertrophic scars, with concentrations between 2.5 and 40 mg/mL that are titrated to scar thickness and location to avoid unwanted atrophy. Visual blanching of the scar represents the clinical end point for treatment. Corticosteroids act by inhibiting fibroblast proliferation and enhancing collagen degradation.10 5-Fluorouracil (5-FU) also is used in scar management. In addition to inhibiting fibroblast proliferation and inducing fibroblast apoptosis, 5-FU inhibits myofibroblast proliferation, which is helpful in the prevention and treatment of scar contracture.11 As monotherapy, weekly injections with 1 to 3 mL of 50 mg/mL 5-FU has been safe and effective. Combination intralesional corticosteroid and 5-FU therapy has been reported and is associated with improved scar regression, reduced reoccurrence, and fewer side effects.11 In our experience, a 1:1 suspension is effective with appropriate titration of the corticosteroid component. Although less well defined, topical application of pharmacotherapy and massage to the newly created MTZs appears beneficial and offers another option for delivery of corticosteroids and 5-FU, in addition to a number of promising medications such as bimatoprost, poly-L-lactic acid, timolol, and rapamycin.12
Conclusion
Advances in laser surgery and our understanding of wound healing have created a paradigm shift in the treatment approach to trauma and burn scars. In lieu of extensive scar excisions, the summarized multimodal regimen emphasizing tissue conservation and autologous remodeling is gaining favor in the military, academic medical centers, and scar centers of excellence, but patients are finding local access to care difficult. Dermatologists are uniquely positioned to cost-effectively deliver this care in the outpatient setting utilizing devices and techniques they already possess. With the end goal of optimization of functional, symptomatic, and aesthetic state of the patient, it is critical that dermatologists seize this opportunity to truly make a difference for the military and civilian patients that need it most.
- American Burn Association, National Burn Repository. 2015 National burn repository report of data from 2005-2014. http://www.ameriburn.org/2015NBRAnnualReport.pdf. Accessed May 10, 2017.
- Centers for Disease Control and Prevention. 2013 National hospital ambulatory medical care survey emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf. Accessed May 10, 2017.
- Fischer H. A guide to U.S. Military casualty statistics: Operation Freedom’s Sentinel, Operation Inherent Resolve, Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. Congressional Research Service website. https://fas.org/sgp/crs/natsec/RS22452.pdf. Published August 7, 2015. Accessed May 10, 2017.
- Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol. 2003;4:245-272.
- Vrijman C, van Drooge AM, Limpens J, et al. Laser and intense pulsed light therapy for the treatment of hypertrophic scars: a systematic review. Br J Dermatol. 2011;165:934-942.
- Miletta N, Lee K, Siwy K, et al. Objective improvement in burn scars after treatment with fractionated CO2 laser. Paper presented at: American Society for Laser Medicine and Surgery 36th Annual Conference; April 1-3, 2016; Boston, MA.
- Ozog DM, Liu A, Chaffins ML, et al. Evaluation of clinical results, histological architecture, and collagen expression following treatment of mature burn scars with a fraction carbon dioxide laser. JAMA Dermatol. 2013;149:50-57.
- Levi B, Ibrahim A, Mathews K, et al. The use of CO2 fractional photothermolysis for the treatment of burn scars. J Burn Care Res. 2016;37:106-114.
- van Drooge AM, Vrijman C, van der Veen W, et al. A randomized controlled pilot study on ablative fractional CO2 laser for consecutive patients presenting with various scar types. Dermatol Surg. 2015;41:371-377.
- Wang XQ, Lui YK, Wang ZY, et al. Antimitotic drug injections and radiotherapy: a review of the effectiveness of treatment for hypertrophic scars and keloids. Int J Low Extrem Wounds. 2008;7:151-159.
- Gupta S, Kalra A. Efficacy and safety of intralesional 5-fluorouracil in the treatment of keloids. Dermatology. 2002;204:130-132.
- Haedersdal M, Erlendsson AM, Paasch U, et al. Translational medicine in the field of AFL (AFXL)-assisted drug delivery: a critical review from basics to current clinical status. J Am Acad Dermatol. 2016;74:981-1004.
Hypertrophic scarring secondary to trauma, burns, and surgical interventions is a major source of morbidity worldwide and often is mechanically, aesthetically, and symptomatically debilitating. Modern advances in acute trauma care protocols have resulted in survival rates greater than 90% in both civilian and military populations.1,2 Patients with wounds that have historically proven fatal are now surviving and are confronted with the long-term sequelae of their injuries. With more than 52,000 service members injured in military engagements from 2001 to 2015 and 8.5 million civilians presenting annually with injury patterns at risk for hypertrophic scarring, it is paramount that we ensure access to safe and effective long-term scar care.2,3
At its simplest level, hypertrophic scarring is believed to result from a disequilibrium between collagen production and degradation. This failure to properly transition through the stages of wound healing results in bothersome symptoms, a disfigured appearance, and mechanical dysfunction of the skin (Figure, A). Decreased elasticity and extensibility, increased dermal thickness, and scar contractures impair patient range of motion and functional mobility. Those affected commonly experience varying degrees of pruritus and dysesthesia along the scar. Combined with aesthetic variations in pigmentation, erythema, texture, and thickness, hypertrophic scarring often leads to long-term psychosocial impairment and decreased health-related quality of life.4

Treatment Approach
Treatment of hypertrophic scars requires a multimodal approach due to the spectrum of associated concerns and the natural recalcitrance of the scar to therapy. Protocols should be tailored to the individual but generally begin with tissue-conserving surgical interventions followed by selective photothermolysis of the scar vasculature. Subsequently, deep and superficial ablative fractional laser (AFL) treatment and local pharmacotherapy also are employed. Treatment can be accomplished in the outpatient setting under local anesthesia in a serial fashion. In the authors’ experience, these therapies behave in a synergistic fashion, achieving outcomes that far exceed the sum of their parts, often obviating the need for scar excision in the majority of cases (Figure, B).
Tissue-Conserving Surgical Intervention
Z-plasty is an indispensable surgical tool due to its ability to lengthen scars and reduce wound tension. Treatment is easily customizable to the patient and can be performed using the individual or multiple Z-plasty techniques. Undermining and step-off correction while suturing allow the physician to lower raised scars, elevate depressed scars, and obscure scar presence by minimizing the straight lines that draw the eye to the scar. Z-plasties rely on the creation and transposition of 2 triangular flaps and permit a 75% increase in length along the desired tension vector. As such, Z-plasties decrease wound tension and facilitate scar maturation.
Selective Photothermolysis of the Vasculature
Although there are several devices available to treat vascular and immature hypertrophic scars, the majority of studies have been conducted with the 595-nm pulsed dye laser. By preferentially heating oxyhemoglobin within the dermal microvasculature, the pulsed dye laser irreparably injures the vascular endothelium. The subsequent tissue hypoxia and collagen fiber heating results in collagen fiber realignment, normalization of collagen subtypes, and neocollagenesis.5 Pulsed dye laser therapy most effectively reduces erythema and pruritus; however, improvements in scar volume, pliability, and elasticity also have been reported.5 When targeting the fine vasculature of the scar, thermal confinement is critical to prevent injury to the surrounding dermis. As such, pulse widths of 0.45 to 1.5 milliseconds are routinely utilized with a fluence just sufficient to elicit transient purpura lasting 3 to 5 seconds. Employing a spot size of 7 to 10 mm, typical fluences range from 4.5 to 6.5 J/cm2. Engagement of the dynamic cooling device reduces the risk for complications, allowing the patient to proceed to the next step in their therapy regimen: the AFL.
Ablative Fractional Laser
The AFL creates a pixilated pattern of injury throughout the epidermis and dermis of the treatment area. Ablative fractional laser platforms include the 10,600-nm CO2 and 2940-nm erbium-doped YAG lasers, both targeting intracellular water. The AFL vaporizes columns of tissue, leaving minute vertical channels with narrow rims of protein coagulation referred to as microscopic treatment zones (MTZs).6 Scar collagen analysis after AFL treatment has shown a profile resembling unaffected skin.7 Consistently, patients report improvements in stiffness, range of motion, pain, pruritus, pigmentation, and erythema.Physician observers also have reported similar improvements in these end points.8,9 Recently, interim data from a prospective controlled trial were presented showing objective improvements in dermal thickness, elasticity, and extensibility after 3 treatments with the CO2 AFL.6 The UltraPulse CO2 laser (Lumenis) is the most well-studied and widely available AFL for scar therapy and as such we will outline common treatment parameters with this device. Of note, treatment end points may be generalized to any AFL.
The DeepFX UltraPulse configuration is utilized to achieve deep AFL therapy and has a fixed pulse width of 0.8 milliseconds, slightly less than the thermal relaxation time of the skin. The diameter of the MTZs is 120 µm, and MTZ density for scar treatment ranges from 1% to 10% with a goal depth of at least 80% of scar thickness. Maximal penetration of the AFL is 4 mm, which is directly proportional to fluence. The goal of deep AFL is the removal of scar tissue to facilitate remodeling and neocollagenesis. Superficial fractional ablation can then be achieved utilizing the ActiveFX UltraPulse configuration generating a 1.3-mm MTZ spot size. We commonly use a treatment level of 3 (82% density). Typical treatment energy ranges from 80 to 125 mJ, which correlates with depths of approximately 50 to 115 µm. With both configurations, the size and shape of the treatment area can be customized to the scar. In addition, frequency may be adjusted to control the speed of treatment while balancing the risk of bulk heating. The goal of superficial AFL is to minimize scar surface irregularities and ensure blending of deep AFL treatment. Once AFL treatment is complete, local pharmacotherapy can then be employed.
Pharmacotherapy
Intralesional corticosteroids have long represented the standard of care for hypertrophic scars, with concentrations between 2.5 and 40 mg/mL that are titrated to scar thickness and location to avoid unwanted atrophy. Visual blanching of the scar represents the clinical end point for treatment. Corticosteroids act by inhibiting fibroblast proliferation and enhancing collagen degradation.10 5-Fluorouracil (5-FU) also is used in scar management. In addition to inhibiting fibroblast proliferation and inducing fibroblast apoptosis, 5-FU inhibits myofibroblast proliferation, which is helpful in the prevention and treatment of scar contracture.11 As monotherapy, weekly injections with 1 to 3 mL of 50 mg/mL 5-FU has been safe and effective. Combination intralesional corticosteroid and 5-FU therapy has been reported and is associated with improved scar regression, reduced reoccurrence, and fewer side effects.11 In our experience, a 1:1 suspension is effective with appropriate titration of the corticosteroid component. Although less well defined, topical application of pharmacotherapy and massage to the newly created MTZs appears beneficial and offers another option for delivery of corticosteroids and 5-FU, in addition to a number of promising medications such as bimatoprost, poly-L-lactic acid, timolol, and rapamycin.12
Conclusion
Advances in laser surgery and our understanding of wound healing have created a paradigm shift in the treatment approach to trauma and burn scars. In lieu of extensive scar excisions, the summarized multimodal regimen emphasizing tissue conservation and autologous remodeling is gaining favor in the military, academic medical centers, and scar centers of excellence, but patients are finding local access to care difficult. Dermatologists are uniquely positioned to cost-effectively deliver this care in the outpatient setting utilizing devices and techniques they already possess. With the end goal of optimization of functional, symptomatic, and aesthetic state of the patient, it is critical that dermatologists seize this opportunity to truly make a difference for the military and civilian patients that need it most.
Hypertrophic scarring secondary to trauma, burns, and surgical interventions is a major source of morbidity worldwide and often is mechanically, aesthetically, and symptomatically debilitating. Modern advances in acute trauma care protocols have resulted in survival rates greater than 90% in both civilian and military populations.1,2 Patients with wounds that have historically proven fatal are now surviving and are confronted with the long-term sequelae of their injuries. With more than 52,000 service members injured in military engagements from 2001 to 2015 and 8.5 million civilians presenting annually with injury patterns at risk for hypertrophic scarring, it is paramount that we ensure access to safe and effective long-term scar care.2,3
At its simplest level, hypertrophic scarring is believed to result from a disequilibrium between collagen production and degradation. This failure to properly transition through the stages of wound healing results in bothersome symptoms, a disfigured appearance, and mechanical dysfunction of the skin (Figure, A). Decreased elasticity and extensibility, increased dermal thickness, and scar contractures impair patient range of motion and functional mobility. Those affected commonly experience varying degrees of pruritus and dysesthesia along the scar. Combined with aesthetic variations in pigmentation, erythema, texture, and thickness, hypertrophic scarring often leads to long-term psychosocial impairment and decreased health-related quality of life.4

Treatment Approach
Treatment of hypertrophic scars requires a multimodal approach due to the spectrum of associated concerns and the natural recalcitrance of the scar to therapy. Protocols should be tailored to the individual but generally begin with tissue-conserving surgical interventions followed by selective photothermolysis of the scar vasculature. Subsequently, deep and superficial ablative fractional laser (AFL) treatment and local pharmacotherapy also are employed. Treatment can be accomplished in the outpatient setting under local anesthesia in a serial fashion. In the authors’ experience, these therapies behave in a synergistic fashion, achieving outcomes that far exceed the sum of their parts, often obviating the need for scar excision in the majority of cases (Figure, B).
Tissue-Conserving Surgical Intervention
Z-plasty is an indispensable surgical tool due to its ability to lengthen scars and reduce wound tension. Treatment is easily customizable to the patient and can be performed using the individual or multiple Z-plasty techniques. Undermining and step-off correction while suturing allow the physician to lower raised scars, elevate depressed scars, and obscure scar presence by minimizing the straight lines that draw the eye to the scar. Z-plasties rely on the creation and transposition of 2 triangular flaps and permit a 75% increase in length along the desired tension vector. As such, Z-plasties decrease wound tension and facilitate scar maturation.
Selective Photothermolysis of the Vasculature
Although there are several devices available to treat vascular and immature hypertrophic scars, the majority of studies have been conducted with the 595-nm pulsed dye laser. By preferentially heating oxyhemoglobin within the dermal microvasculature, the pulsed dye laser irreparably injures the vascular endothelium. The subsequent tissue hypoxia and collagen fiber heating results in collagen fiber realignment, normalization of collagen subtypes, and neocollagenesis.5 Pulsed dye laser therapy most effectively reduces erythema and pruritus; however, improvements in scar volume, pliability, and elasticity also have been reported.5 When targeting the fine vasculature of the scar, thermal confinement is critical to prevent injury to the surrounding dermis. As such, pulse widths of 0.45 to 1.5 milliseconds are routinely utilized with a fluence just sufficient to elicit transient purpura lasting 3 to 5 seconds. Employing a spot size of 7 to 10 mm, typical fluences range from 4.5 to 6.5 J/cm2. Engagement of the dynamic cooling device reduces the risk for complications, allowing the patient to proceed to the next step in their therapy regimen: the AFL.
Ablative Fractional Laser
The AFL creates a pixilated pattern of injury throughout the epidermis and dermis of the treatment area. Ablative fractional laser platforms include the 10,600-nm CO2 and 2940-nm erbium-doped YAG lasers, both targeting intracellular water. The AFL vaporizes columns of tissue, leaving minute vertical channels with narrow rims of protein coagulation referred to as microscopic treatment zones (MTZs).6 Scar collagen analysis after AFL treatment has shown a profile resembling unaffected skin.7 Consistently, patients report improvements in stiffness, range of motion, pain, pruritus, pigmentation, and erythema.Physician observers also have reported similar improvements in these end points.8,9 Recently, interim data from a prospective controlled trial were presented showing objective improvements in dermal thickness, elasticity, and extensibility after 3 treatments with the CO2 AFL.6 The UltraPulse CO2 laser (Lumenis) is the most well-studied and widely available AFL for scar therapy and as such we will outline common treatment parameters with this device. Of note, treatment end points may be generalized to any AFL.
The DeepFX UltraPulse configuration is utilized to achieve deep AFL therapy and has a fixed pulse width of 0.8 milliseconds, slightly less than the thermal relaxation time of the skin. The diameter of the MTZs is 120 µm, and MTZ density for scar treatment ranges from 1% to 10% with a goal depth of at least 80% of scar thickness. Maximal penetration of the AFL is 4 mm, which is directly proportional to fluence. The goal of deep AFL is the removal of scar tissue to facilitate remodeling and neocollagenesis. Superficial fractional ablation can then be achieved utilizing the ActiveFX UltraPulse configuration generating a 1.3-mm MTZ spot size. We commonly use a treatment level of 3 (82% density). Typical treatment energy ranges from 80 to 125 mJ, which correlates with depths of approximately 50 to 115 µm. With both configurations, the size and shape of the treatment area can be customized to the scar. In addition, frequency may be adjusted to control the speed of treatment while balancing the risk of bulk heating. The goal of superficial AFL is to minimize scar surface irregularities and ensure blending of deep AFL treatment. Once AFL treatment is complete, local pharmacotherapy can then be employed.
Pharmacotherapy
Intralesional corticosteroids have long represented the standard of care for hypertrophic scars, with concentrations between 2.5 and 40 mg/mL that are titrated to scar thickness and location to avoid unwanted atrophy. Visual blanching of the scar represents the clinical end point for treatment. Corticosteroids act by inhibiting fibroblast proliferation and enhancing collagen degradation.10 5-Fluorouracil (5-FU) also is used in scar management. In addition to inhibiting fibroblast proliferation and inducing fibroblast apoptosis, 5-FU inhibits myofibroblast proliferation, which is helpful in the prevention and treatment of scar contracture.11 As monotherapy, weekly injections with 1 to 3 mL of 50 mg/mL 5-FU has been safe and effective. Combination intralesional corticosteroid and 5-FU therapy has been reported and is associated with improved scar regression, reduced reoccurrence, and fewer side effects.11 In our experience, a 1:1 suspension is effective with appropriate titration of the corticosteroid component. Although less well defined, topical application of pharmacotherapy and massage to the newly created MTZs appears beneficial and offers another option for delivery of corticosteroids and 5-FU, in addition to a number of promising medications such as bimatoprost, poly-L-lactic acid, timolol, and rapamycin.12
Conclusion
Advances in laser surgery and our understanding of wound healing have created a paradigm shift in the treatment approach to trauma and burn scars. In lieu of extensive scar excisions, the summarized multimodal regimen emphasizing tissue conservation and autologous remodeling is gaining favor in the military, academic medical centers, and scar centers of excellence, but patients are finding local access to care difficult. Dermatologists are uniquely positioned to cost-effectively deliver this care in the outpatient setting utilizing devices and techniques they already possess. With the end goal of optimization of functional, symptomatic, and aesthetic state of the patient, it is critical that dermatologists seize this opportunity to truly make a difference for the military and civilian patients that need it most.
- American Burn Association, National Burn Repository. 2015 National burn repository report of data from 2005-2014. http://www.ameriburn.org/2015NBRAnnualReport.pdf. Accessed May 10, 2017.
- Centers for Disease Control and Prevention. 2013 National hospital ambulatory medical care survey emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf. Accessed May 10, 2017.
- Fischer H. A guide to U.S. Military casualty statistics: Operation Freedom’s Sentinel, Operation Inherent Resolve, Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. Congressional Research Service website. https://fas.org/sgp/crs/natsec/RS22452.pdf. Published August 7, 2015. Accessed May 10, 2017.
- Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol. 2003;4:245-272.
- Vrijman C, van Drooge AM, Limpens J, et al. Laser and intense pulsed light therapy for the treatment of hypertrophic scars: a systematic review. Br J Dermatol. 2011;165:934-942.
- Miletta N, Lee K, Siwy K, et al. Objective improvement in burn scars after treatment with fractionated CO2 laser. Paper presented at: American Society for Laser Medicine and Surgery 36th Annual Conference; April 1-3, 2016; Boston, MA.
- Ozog DM, Liu A, Chaffins ML, et al. Evaluation of clinical results, histological architecture, and collagen expression following treatment of mature burn scars with a fraction carbon dioxide laser. JAMA Dermatol. 2013;149:50-57.
- Levi B, Ibrahim A, Mathews K, et al. The use of CO2 fractional photothermolysis for the treatment of burn scars. J Burn Care Res. 2016;37:106-114.
- van Drooge AM, Vrijman C, van der Veen W, et al. A randomized controlled pilot study on ablative fractional CO2 laser for consecutive patients presenting with various scar types. Dermatol Surg. 2015;41:371-377.
- Wang XQ, Lui YK, Wang ZY, et al. Antimitotic drug injections and radiotherapy: a review of the effectiveness of treatment for hypertrophic scars and keloids. Int J Low Extrem Wounds. 2008;7:151-159.
- Gupta S, Kalra A. Efficacy and safety of intralesional 5-fluorouracil in the treatment of keloids. Dermatology. 2002;204:130-132.
- Haedersdal M, Erlendsson AM, Paasch U, et al. Translational medicine in the field of AFL (AFXL)-assisted drug delivery: a critical review from basics to current clinical status. J Am Acad Dermatol. 2016;74:981-1004.
- American Burn Association, National Burn Repository. 2015 National burn repository report of data from 2005-2014. http://www.ameriburn.org/2015NBRAnnualReport.pdf. Accessed May 10, 2017.
- Centers for Disease Control and Prevention. 2013 National hospital ambulatory medical care survey emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf. Accessed May 10, 2017.
- Fischer H. A guide to U.S. Military casualty statistics: Operation Freedom’s Sentinel, Operation Inherent Resolve, Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. Congressional Research Service website. https://fas.org/sgp/crs/natsec/RS22452.pdf. Published August 7, 2015. Accessed May 10, 2017.
- Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol. 2003;4:245-272.
- Vrijman C, van Drooge AM, Limpens J, et al. Laser and intense pulsed light therapy for the treatment of hypertrophic scars: a systematic review. Br J Dermatol. 2011;165:934-942.
- Miletta N, Lee K, Siwy K, et al. Objective improvement in burn scars after treatment with fractionated CO2 laser. Paper presented at: American Society for Laser Medicine and Surgery 36th Annual Conference; April 1-3, 2016; Boston, MA.
- Ozog DM, Liu A, Chaffins ML, et al. Evaluation of clinical results, histological architecture, and collagen expression following treatment of mature burn scars with a fraction carbon dioxide laser. JAMA Dermatol. 2013;149:50-57.
- Levi B, Ibrahim A, Mathews K, et al. The use of CO2 fractional photothermolysis for the treatment of burn scars. J Burn Care Res. 2016;37:106-114.
- van Drooge AM, Vrijman C, van der Veen W, et al. A randomized controlled pilot study on ablative fractional CO2 laser for consecutive patients presenting with various scar types. Dermatol Surg. 2015;41:371-377.
- Wang XQ, Lui YK, Wang ZY, et al. Antimitotic drug injections and radiotherapy: a review of the effectiveness of treatment for hypertrophic scars and keloids. Int J Low Extrem Wounds. 2008;7:151-159.
- Gupta S, Kalra A. Efficacy and safety of intralesional 5-fluorouracil in the treatment of keloids. Dermatology. 2002;204:130-132.
- Haedersdal M, Erlendsson AM, Paasch U, et al. Translational medicine in the field of AFL (AFXL)-assisted drug delivery: a critical review from basics to current clinical status. J Am Acad Dermatol. 2016;74:981-1004.
Practice Points
- Burn and trauma scarring represents a major source of morbidity in both the civilian and military populations worldwide and often is mechanically, aesthetically, and symptomatically debilitating.
- Advances in laser surgery and our understanding of wound healing have resulted in a scar therapy paradigm shift from large scar excisions and repair to a multimodal, tissue-conserving approach that relies on remodeling of the existing tissue.
- Dermatologists are uniquely positioned to increase patient access to cost-effective, outpatient-based burn and trauma scar care utilizing devices and techniques that they currently possess.
Total-Body Photography in Skin Cancer Screening: The Clinical Utility of Standardized Imaging
Skin cancer is an important public health issue in the United States, as 1 in 5 Americans are projected to develop a cutaneous malignancy during their lifetime. Currently, 75% of all skin cancer–related deaths are due to malignant melanomas (MMs), though melanomas account for less than 5% of all skin cancers.1 Early detection of MM is essential, as prognosis depends on tumor stage, particularly the depth of the melanoma.2-4 In general, patients with thin, early-stage melanomas have a more than 96% survival rate, which drops to 14% in late-stage disease.5,6 Five percent to 30% of all melanomas are identified incidentally on total-body skin examinations (TBSEs) performed by a trained provider and thus would not have been caught with only a focused skin examination or patient self-examination.7,8 Nonetheless, the clinical utility of skin cancer screening with TBSEs remains controversial, largely due to the poor quality of data available to establish a notable mortality benefit from skin cancer screening. As a result, obtaining endorsement from the larger medical community, federal government, and health insurance industry to include routine TBSEs as part of a preventive care health care strategy has not occurred. The absence of definitive clinical care guidelines mandating routine TBSEs is one of the greatest barriers preventing access to appropriate dermatologic screening along with the paucity of trained providers; however, standardized total-body photography (TBP) promises to provide a way forward by lowering the costs of dermatologic screening while simultaneously leveraging technology to increase availability.
Impact on Biopsy Efficiency
Current US Preventive Services Task Force (USPSTF) guidelines state that evidence is insufficient to assess the balance of benefits and harms of visual skin examination by a clinician to screen for skin cancer in adults. The USPSTF noted that “[d]irect evidence on the effectiveness of screening in reducing melanoma morbidity and mortality is limited to a single fair-quality ecologic study with important methodological limitations” (ie, the Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany [SCREEN] study), and although information on harm is similarly sparse, “[t]he potential for harm clearly exists, including a high rate of unnecessary biopsies, possibly resulting in cosmetic or, more rarely, functional adverse effects, and the risk of overdiagnosis and overtreatment.”9 The majority of suspicious skin lesions excised during screenings are not cancerous. For example, the SCREEN study found that 20 to 55 excisions were performed to detect 1 case of melanoma.10 At that rate, the USPSTF also noted that approximately 4000 excisions would be required to prevent a single death from melanoma.9 Following the lead of the USPSTF, the Patient Protection and Affordable Care Act did not mandate that skin examinations be included as essential preventive coverage in its requirements for insurance coverage of primary care prevention. As such, dermatologists face financial pressure to avoid performing time-consuming TBSEs, regardless of their perceived utility.11
As the USPSTF points out, the value of TBSEs relies on the examiner’s ability to correctly identify malignant lesions and minimize biopsies of benign lesions, a concept known as biopsy efficiency.9 Secondarily, a TBSE is time consuming, and the time required for a dermatologist to complete a TBSE given the high rate of benign findings may not be financially viable. We argue that the routine use of total-body skin imaging may offer a way forward in addressing these issues. Total-body photography involves a photographic system that can allow dermatologists to acquire standardized images that can be used for primary diagnosis and to track individual lesions over time. Nonmedical personnel and medical assistants can be easily trained to use standardized photography devices to quickly obtain high-quality clinical images, thereby greatly reducing the time and cost of obtaining these images. Studies have found that the use of photographic monitoring may improve biopsy efficiency.12-15 A recent study by Truong et al16 found that TBP used to monitor all existing melanocytic lesions on patients substantially reduced the number of biopsies that patients required. These results reflect that most nevi, including clinically atypical nevi, are usually stable and unlikely to exhibit suspicious changes over time.17,18 For this reason, the use of TBP could minimize unnecessary biopsies because clinically suspicious but stable nevi can be objectively documented and followed over time.
Standardized TBP also offers the ability for dermatologists to work synergistically with modern computer technology involving algorithms capable of analyzing high-quality images to autodiagnose or flag concerning lesions that may require biopsy. Esteva et al19 described their development of a deep learning algorithm that relies on a convolutional neural network (CNN). This CNN was trained to identify melanomas using a large data set of clinical dermatologic images and subsequently was able to distinguish MMs from benign nevi at a rate on par with a board-certified dermatologist.19 Widespread use of total-body imaging would create an enormous database of high-resolution images that would be ideally suited to the development of such computerized algorithms, which could then be applied to future images by way of artificial intelligence. Convolutional neural networks that use a single patient’s imaging over time could be developed to assess the change in number or size of benign nevi and identify lesions that are concerning for MM while simultaneously comparing them to a population-based data set.
On a large scale, such a capability would minimize the inefficiency and subjectivity of TBSEs as a tool for identifying malignancy. Currently, dermatologists are only able to track and document a few concerning lesions on a patient’s body, rendering the choice of which lesions require biopsy more subjective. Total-body photography, particularly if used with an algorithm capable of quickly analyzing all the nevi on a person’s body, largely eliminates such subjectivity by creating a standardized set of images that can be tracked over time and flagging concerning lesions prior to the dermatologist examining the patient. In this way, the specialty of dermatology could achieve the same model of objective evaluation of standardized clinical images as those employed in radiology, cardiology, and other clinical disciplines. The additional benefit of such a system would be lower costs, as the images could be acquired by nonmedical personnel and then undergo initial assessment by an algorithm, which would flag concerning lesions, similar to a modern electrocardiogram machine, allowing the dermatologist to use his/her time more efficiently by only focusing on concerning lesions with the confidence that the patient’s entire body has already been rigorously screened.
By using TBP to improve biopsy efficiency and the objectivity of the TBSE as a tool to detect skin cancer, we propose that the benefit-to-harm ratio of the TBSE would remarkably improve. Ultimately, this type of screening would meet the strict requirements to be included in preventive health care strategies and thereby improve access to dermatologic care.
The Use of TBP in the Military
Total-body photography has several specific applications in the military. Standardized imaging has the potential to improve dermatologic care for active-duty soldiers across space and time. First, a large percentage of deployment medical care is devoted to dermatologic issues. From 2008 to 2015, 5% of all medical encounters in the combat theaters of Iraq and Afghanistan involved dermatologic concerns.20 Access to appropriate dermatologic care in a combat theater is important, as poorly controlled dermatologic conditions (eg, psoriasis, eczema) often require evacuation when left untreated. Although current TBP systems may not be portable or durable enough to survive in an austere deployment environment, we propose it would be feasible to have skin imaging booths at larger forward operating bases. The images could then be transported to a remote dermatologist to assess and recommend treatment. The expense of transporting and maintaining the imaging system in country would be offset by the expenses spared by not requiring a dermatologist in country and the reductions in costly medical evacuations from theater.
Although the US military population is younger and generally healthier than the general adult population due to extensive medical screening on admission, age limitations for active-duty service, a mandated active lifestyle, and access to good health care, there are still a substantial number of service members diagnosed with skin cancer each year.21 From 2005 through 2014, MM was the most common non–gender-specific cancer (n=1571); in men, only testicular cancer was more prevalent (1591 vs 1298 cases), and in women, only breast cancer was more prevalent (773 vs 273 cases). Furthermore, from 2004 to 2013, the incidence rates of melanoma have increased by 1.4%, while with other cancer rates have declined during the same time period.21 Thus, TBP as a screening modality across the military population is a promising method for improving detection of skin cancer and reducing morbidity and mortality.
Military medicine often is on the forefront of medical advances in technology, disease understanding, and clinical care due to the unique resources available in the military health care system, which allow investigators the ability to obtain vast amounts of epidemiologic data.22 The military health care system also is unique in its ability to mandate that its members obtain preventive health services. Thus, it would be possible for the military to mandate TBP at accession and retirement, for instance, or more frequently for annual screening. The implementation of such a program would improve the health of the military population and be a public health service by pioneering the use of a standardized TBP system across a large health care system to improve skin cancer detection.
Current Studies in the Military
The Dermatology Service at the Walter Reed National Military Medical Center (WRNMMC)(Bethesda, Maryland) is evaluating the use of a total-body digital skin imaging system under a grant from the Telemedicine and Advanced Technology Research Center of the US Army. The system in use was found to be particularly well suited for military dermatology because it offers standardized TBP processing, produces a report that can be uploaded to the US Department of Defense (DoD) electronic medical record system, and requires relatively brief training for ancillary personnel to operate. Regardless of the platform the DoD ultimately finds most suitable, it is critical that a standard exist for TBP to ensure that uniform data sets are generated to allow military and other DoD dermatologists as well as civilian health care providers to share clinical information. The goal of the current study at WRNMMC is to vet TBP platforms at WRNMMC so the military can then develop standards to procure additional platforms for placement throughout the Military Health System, Military Entrance Processing Stations, operational environments, and collaborating health care systems (eg, the Veterans Health Administration).
Once deployed broadly across the Military Health System, these TBP platforms would be part of a network of telehealth care. For acute dermatologic issues, diagnoses provided via teledermatology platforms can then be managed by health care providers utilizing appropriate clinical practice guidelines or by non–health care providers utilizing general medical knowledge databases. Such a system with TBP information collected at multiple access points across a service member’s career would build a repository of data that would be immensely useful to patients and to clinical research. Of particular interest to military researchers is that TBP data could be used to study which patients require in-person examinations or more careful monitoring; the proper intervals for skin cancer screening; and the assessment of the benefits of TBP in improving morbidity, mortality, and biopsy efficiency in the detection of MM as well as nonmelanoma skin cancers.
Limitations to Progress
Currently, there are multiple limitations to the implementation of TBP as a part of TBSE screening. First, the potential improvement in biopsy efficiency using TBP is predicated on its ability to prove nevi stability over time, but in younger populations, benign nevi are more likely to change or increase in number, which may reduce the biopsy efficiency of screening in a younger population, thereby negating some of the benefit of imaging and CNN assessment. For instance, Truong et al16 found that younger age (<30 years) did not show the same improvement in biopsy efficiency with the use of TBP, which the authors theorized may reflect “the dynamic nature of nevi in younger patients” that has been documented in other studies.23,24 Approximately 65% of the active-duty military population is aged 18 to 30 years, and 98% of accessions to active duty occur in individuals aged 17 to 30 years.25 As such, TBP may not improve biopsy efficiency in the active-duty military population as dramatically as it would across the general population.
A second limitation of the use of TBP in the active-duty military population is the ethics of implementing DoD-wide mandatory TBP. Although the TBP platform will be compliant with the Health Insurance Portability and Accountability Act, mandating that soldiers contribute their TBP to a repository of data that will then be used for research without explicitly requesting their consent is ethically problematic; however, since the 1950s, the DoD has collected serum samples from its service members for force protection and operations reasons as well as for the purpose of research.22,26 Currently, the DoD Serum Repository collects serum samples as part of a mandatory human immunodeficiency virus screening program that evaluates service members every 2 years; this repository of human serum samples is accessible for research purposes without the consent of the individuals being studied.27 These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Thus, although there is precedent in the DoD for such mass data collection, it is an ongoing ethical consideration.28
RELATED ARTICLE: Gigapixel Photography for Skin Cancer Surveillance
Finally, although the potential use of TBP and computer algorithms to improve the efficiency and affordability of TBSEs is exciting, there are no existing computer algorithms that we are aware of that can be used with existing TBP platforms in the manner we proposed. However, we feel that computer algorithms, such as the one created by Esteva et al,19 are just the beginning and that the use of artificial intelligence is not far off. Even after the creation of a TBP-compatible algorithm adept at analyzing malignant lesions, however, this technology would need to be further evaluated in the clinical setting to determine its capability and practicality. Current TBP platforms also are limited by their large size, cost, and complexity. As TBP platforms improve, it is likely that more streamlined and less expensive versions of current models will greatly enhance the field of teledermatology, particularly in the military setting.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131-149; quiz 182-184.
- Eisemann N, Jansen L, Holleczek B, et al. Up-to-date results on survival of patients with melanoma in Germany [published online July 19, 2012]. Br J Dermatol. 2012;167:606-612.
- MacKie RM, Bray C, Vestey J, et al. Melanoma incidence and mortality in Scotland 1979-2003 [published online May 29, 2007]. Br J Cancer. 2007;96:1772-1777.
- Dickson PV, Gershenwald JE. Staging and prognosis of cutaneous melanoma. Surg Oncol Clin N Am. 2011;20:1-17.
- Balch CM, Gershenwald JE, Soong SL, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
- Kingsley-Loso JL, Grey KR, Hanson JL, et al. Incidental lesions found in veterans referred to dermatology: the value of a dermatologic examination [published online January 23, 2015]. J Am Acad Dermatol. 2015;72:651.e1-655.e1.
- Grant-Kels JM, Stoff B. Total body skin exams (TBSEs): saving lives or wasting time? J Am Acad Dermatol. 2017;76:183-185.
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Robinson JK, Halpern AC. Cost-effective melanoma screening. JAMA Dermatol. 2016;152:19-21.
- Feit NE, Dusza SW, Marghoob AA. Melanomas detected with the aid of total cutaneous photography. Br J Dermatol. 2004;150:706-714.
- Haenssle HA, Krueger U, Vente C, et al. Results from an observational trial: digital epiluminescence microscopy follow-up of atypical nevi increases the sensitivity and the chance of success of conventional dermoscopy in detecting melanoma. J Invest Dermatol. 2006;126:980-985.
- Salerni G, Carrera C, Lovatto L, et al. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. J Am Acad Dermatol. 2012;67:E17-E27.
- Rice ZP, Weiss FJ, DeLong LK, et al. Utilization and rationale for the implementation of total body (digital) photography as an adjunct screening measure for melanoma. Melanoma Res. 2010;20:417-421.
- Truong A, Strazzulla L, March J, et al. Reduction in nevus biopsies in patients monitored by total body photography [published online March 3, 2016]. J Am Acad Dermatol. 2016;75:135.e5-143.e5.
- Lucas CR, Sanders LL, Murray JC, et al. Early melanoma detection: nonuniform dermoscopic features and growth. J Am Acad Dermatol. 2003;48:663-671.
- Fuller SR, Bowen GM, Tanner B, et al. Digital dermoscopic monitoring of atypical nevi in patients at risk for melanoma. Dermatol Surg. 2007;33:1198-1206; discussion 1205-1206.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks [published online January 25, 2017]. Nature. 2017;542:115-118.
- Defense Medical Epidemiology Database. Military Health System website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Epidemiology-Database. Accessed April 10, 2017.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Helmandollar KJ, Meyerle JH. Exploration of modern military research resources. Cutis. 2016;98:231-234.
- Goodson AG, Grossman D. Strategies for early melanoma detection: approaches to the patient with nevi. J Am Acad Dermatol. 2009;60:719-735; quiz 736-738.
- Bajaj S, Dusza SW, Marchetti MA, et al. Growth-curve modeling of nevi with a peripheral globular pattern. JAMA Dermatol. 2015;151:1338-1345.
- Niebuhr DW, Gubata ME, Cowan DN, et al. Accession Medical Standards Analysis & Research Activity (AMSARA) 2011 Annual Report. Silver Spring, MD: Division of Preventive Medicine, Walter Reed Army Institute of Research; 2012.
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD Serum Repository. Mil Med. 2015;180:10-12.
- Pavlin JA, Welch RA. Ethics, human use, and the Department of Defense Serum Repository. Mil Med. 2015;180:49-56.
Skin cancer is an important public health issue in the United States, as 1 in 5 Americans are projected to develop a cutaneous malignancy during their lifetime. Currently, 75% of all skin cancer–related deaths are due to malignant melanomas (MMs), though melanomas account for less than 5% of all skin cancers.1 Early detection of MM is essential, as prognosis depends on tumor stage, particularly the depth of the melanoma.2-4 In general, patients with thin, early-stage melanomas have a more than 96% survival rate, which drops to 14% in late-stage disease.5,6 Five percent to 30% of all melanomas are identified incidentally on total-body skin examinations (TBSEs) performed by a trained provider and thus would not have been caught with only a focused skin examination or patient self-examination.7,8 Nonetheless, the clinical utility of skin cancer screening with TBSEs remains controversial, largely due to the poor quality of data available to establish a notable mortality benefit from skin cancer screening. As a result, obtaining endorsement from the larger medical community, federal government, and health insurance industry to include routine TBSEs as part of a preventive care health care strategy has not occurred. The absence of definitive clinical care guidelines mandating routine TBSEs is one of the greatest barriers preventing access to appropriate dermatologic screening along with the paucity of trained providers; however, standardized total-body photography (TBP) promises to provide a way forward by lowering the costs of dermatologic screening while simultaneously leveraging technology to increase availability.
Impact on Biopsy Efficiency
Current US Preventive Services Task Force (USPSTF) guidelines state that evidence is insufficient to assess the balance of benefits and harms of visual skin examination by a clinician to screen for skin cancer in adults. The USPSTF noted that “[d]irect evidence on the effectiveness of screening in reducing melanoma morbidity and mortality is limited to a single fair-quality ecologic study with important methodological limitations” (ie, the Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany [SCREEN] study), and although information on harm is similarly sparse, “[t]he potential for harm clearly exists, including a high rate of unnecessary biopsies, possibly resulting in cosmetic or, more rarely, functional adverse effects, and the risk of overdiagnosis and overtreatment.”9 The majority of suspicious skin lesions excised during screenings are not cancerous. For example, the SCREEN study found that 20 to 55 excisions were performed to detect 1 case of melanoma.10 At that rate, the USPSTF also noted that approximately 4000 excisions would be required to prevent a single death from melanoma.9 Following the lead of the USPSTF, the Patient Protection and Affordable Care Act did not mandate that skin examinations be included as essential preventive coverage in its requirements for insurance coverage of primary care prevention. As such, dermatologists face financial pressure to avoid performing time-consuming TBSEs, regardless of their perceived utility.11
As the USPSTF points out, the value of TBSEs relies on the examiner’s ability to correctly identify malignant lesions and minimize biopsies of benign lesions, a concept known as biopsy efficiency.9 Secondarily, a TBSE is time consuming, and the time required for a dermatologist to complete a TBSE given the high rate of benign findings may not be financially viable. We argue that the routine use of total-body skin imaging may offer a way forward in addressing these issues. Total-body photography involves a photographic system that can allow dermatologists to acquire standardized images that can be used for primary diagnosis and to track individual lesions over time. Nonmedical personnel and medical assistants can be easily trained to use standardized photography devices to quickly obtain high-quality clinical images, thereby greatly reducing the time and cost of obtaining these images. Studies have found that the use of photographic monitoring may improve biopsy efficiency.12-15 A recent study by Truong et al16 found that TBP used to monitor all existing melanocytic lesions on patients substantially reduced the number of biopsies that patients required. These results reflect that most nevi, including clinically atypical nevi, are usually stable and unlikely to exhibit suspicious changes over time.17,18 For this reason, the use of TBP could minimize unnecessary biopsies because clinically suspicious but stable nevi can be objectively documented and followed over time.
Standardized TBP also offers the ability for dermatologists to work synergistically with modern computer technology involving algorithms capable of analyzing high-quality images to autodiagnose or flag concerning lesions that may require biopsy. Esteva et al19 described their development of a deep learning algorithm that relies on a convolutional neural network (CNN). This CNN was trained to identify melanomas using a large data set of clinical dermatologic images and subsequently was able to distinguish MMs from benign nevi at a rate on par with a board-certified dermatologist.19 Widespread use of total-body imaging would create an enormous database of high-resolution images that would be ideally suited to the development of such computerized algorithms, which could then be applied to future images by way of artificial intelligence. Convolutional neural networks that use a single patient’s imaging over time could be developed to assess the change in number or size of benign nevi and identify lesions that are concerning for MM while simultaneously comparing them to a population-based data set.
On a large scale, such a capability would minimize the inefficiency and subjectivity of TBSEs as a tool for identifying malignancy. Currently, dermatologists are only able to track and document a few concerning lesions on a patient’s body, rendering the choice of which lesions require biopsy more subjective. Total-body photography, particularly if used with an algorithm capable of quickly analyzing all the nevi on a person’s body, largely eliminates such subjectivity by creating a standardized set of images that can be tracked over time and flagging concerning lesions prior to the dermatologist examining the patient. In this way, the specialty of dermatology could achieve the same model of objective evaluation of standardized clinical images as those employed in radiology, cardiology, and other clinical disciplines. The additional benefit of such a system would be lower costs, as the images could be acquired by nonmedical personnel and then undergo initial assessment by an algorithm, which would flag concerning lesions, similar to a modern electrocardiogram machine, allowing the dermatologist to use his/her time more efficiently by only focusing on concerning lesions with the confidence that the patient’s entire body has already been rigorously screened.
By using TBP to improve biopsy efficiency and the objectivity of the TBSE as a tool to detect skin cancer, we propose that the benefit-to-harm ratio of the TBSE would remarkably improve. Ultimately, this type of screening would meet the strict requirements to be included in preventive health care strategies and thereby improve access to dermatologic care.
The Use of TBP in the Military
Total-body photography has several specific applications in the military. Standardized imaging has the potential to improve dermatologic care for active-duty soldiers across space and time. First, a large percentage of deployment medical care is devoted to dermatologic issues. From 2008 to 2015, 5% of all medical encounters in the combat theaters of Iraq and Afghanistan involved dermatologic concerns.20 Access to appropriate dermatologic care in a combat theater is important, as poorly controlled dermatologic conditions (eg, psoriasis, eczema) often require evacuation when left untreated. Although current TBP systems may not be portable or durable enough to survive in an austere deployment environment, we propose it would be feasible to have skin imaging booths at larger forward operating bases. The images could then be transported to a remote dermatologist to assess and recommend treatment. The expense of transporting and maintaining the imaging system in country would be offset by the expenses spared by not requiring a dermatologist in country and the reductions in costly medical evacuations from theater.
Although the US military population is younger and generally healthier than the general adult population due to extensive medical screening on admission, age limitations for active-duty service, a mandated active lifestyle, and access to good health care, there are still a substantial number of service members diagnosed with skin cancer each year.21 From 2005 through 2014, MM was the most common non–gender-specific cancer (n=1571); in men, only testicular cancer was more prevalent (1591 vs 1298 cases), and in women, only breast cancer was more prevalent (773 vs 273 cases). Furthermore, from 2004 to 2013, the incidence rates of melanoma have increased by 1.4%, while with other cancer rates have declined during the same time period.21 Thus, TBP as a screening modality across the military population is a promising method for improving detection of skin cancer and reducing morbidity and mortality.
Military medicine often is on the forefront of medical advances in technology, disease understanding, and clinical care due to the unique resources available in the military health care system, which allow investigators the ability to obtain vast amounts of epidemiologic data.22 The military health care system also is unique in its ability to mandate that its members obtain preventive health services. Thus, it would be possible for the military to mandate TBP at accession and retirement, for instance, or more frequently for annual screening. The implementation of such a program would improve the health of the military population and be a public health service by pioneering the use of a standardized TBP system across a large health care system to improve skin cancer detection.
Current Studies in the Military
The Dermatology Service at the Walter Reed National Military Medical Center (WRNMMC)(Bethesda, Maryland) is evaluating the use of a total-body digital skin imaging system under a grant from the Telemedicine and Advanced Technology Research Center of the US Army. The system in use was found to be particularly well suited for military dermatology because it offers standardized TBP processing, produces a report that can be uploaded to the US Department of Defense (DoD) electronic medical record system, and requires relatively brief training for ancillary personnel to operate. Regardless of the platform the DoD ultimately finds most suitable, it is critical that a standard exist for TBP to ensure that uniform data sets are generated to allow military and other DoD dermatologists as well as civilian health care providers to share clinical information. The goal of the current study at WRNMMC is to vet TBP platforms at WRNMMC so the military can then develop standards to procure additional platforms for placement throughout the Military Health System, Military Entrance Processing Stations, operational environments, and collaborating health care systems (eg, the Veterans Health Administration).
Once deployed broadly across the Military Health System, these TBP platforms would be part of a network of telehealth care. For acute dermatologic issues, diagnoses provided via teledermatology platforms can then be managed by health care providers utilizing appropriate clinical practice guidelines or by non–health care providers utilizing general medical knowledge databases. Such a system with TBP information collected at multiple access points across a service member’s career would build a repository of data that would be immensely useful to patients and to clinical research. Of particular interest to military researchers is that TBP data could be used to study which patients require in-person examinations or more careful monitoring; the proper intervals for skin cancer screening; and the assessment of the benefits of TBP in improving morbidity, mortality, and biopsy efficiency in the detection of MM as well as nonmelanoma skin cancers.
Limitations to Progress
Currently, there are multiple limitations to the implementation of TBP as a part of TBSE screening. First, the potential improvement in biopsy efficiency using TBP is predicated on its ability to prove nevi stability over time, but in younger populations, benign nevi are more likely to change or increase in number, which may reduce the biopsy efficiency of screening in a younger population, thereby negating some of the benefit of imaging and CNN assessment. For instance, Truong et al16 found that younger age (<30 years) did not show the same improvement in biopsy efficiency with the use of TBP, which the authors theorized may reflect “the dynamic nature of nevi in younger patients” that has been documented in other studies.23,24 Approximately 65% of the active-duty military population is aged 18 to 30 years, and 98% of accessions to active duty occur in individuals aged 17 to 30 years.25 As such, TBP may not improve biopsy efficiency in the active-duty military population as dramatically as it would across the general population.
A second limitation of the use of TBP in the active-duty military population is the ethics of implementing DoD-wide mandatory TBP. Although the TBP platform will be compliant with the Health Insurance Portability and Accountability Act, mandating that soldiers contribute their TBP to a repository of data that will then be used for research without explicitly requesting their consent is ethically problematic; however, since the 1950s, the DoD has collected serum samples from its service members for force protection and operations reasons as well as for the purpose of research.22,26 Currently, the DoD Serum Repository collects serum samples as part of a mandatory human immunodeficiency virus screening program that evaluates service members every 2 years; this repository of human serum samples is accessible for research purposes without the consent of the individuals being studied.27 These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Thus, although there is precedent in the DoD for such mass data collection, it is an ongoing ethical consideration.28
RELATED ARTICLE: Gigapixel Photography for Skin Cancer Surveillance
Finally, although the potential use of TBP and computer algorithms to improve the efficiency and affordability of TBSEs is exciting, there are no existing computer algorithms that we are aware of that can be used with existing TBP platforms in the manner we proposed. However, we feel that computer algorithms, such as the one created by Esteva et al,19 are just the beginning and that the use of artificial intelligence is not far off. Even after the creation of a TBP-compatible algorithm adept at analyzing malignant lesions, however, this technology would need to be further evaluated in the clinical setting to determine its capability and practicality. Current TBP platforms also are limited by their large size, cost, and complexity. As TBP platforms improve, it is likely that more streamlined and less expensive versions of current models will greatly enhance the field of teledermatology, particularly in the military setting.
Skin cancer is an important public health issue in the United States, as 1 in 5 Americans are projected to develop a cutaneous malignancy during their lifetime. Currently, 75% of all skin cancer–related deaths are due to malignant melanomas (MMs), though melanomas account for less than 5% of all skin cancers.1 Early detection of MM is essential, as prognosis depends on tumor stage, particularly the depth of the melanoma.2-4 In general, patients with thin, early-stage melanomas have a more than 96% survival rate, which drops to 14% in late-stage disease.5,6 Five percent to 30% of all melanomas are identified incidentally on total-body skin examinations (TBSEs) performed by a trained provider and thus would not have been caught with only a focused skin examination or patient self-examination.7,8 Nonetheless, the clinical utility of skin cancer screening with TBSEs remains controversial, largely due to the poor quality of data available to establish a notable mortality benefit from skin cancer screening. As a result, obtaining endorsement from the larger medical community, federal government, and health insurance industry to include routine TBSEs as part of a preventive care health care strategy has not occurred. The absence of definitive clinical care guidelines mandating routine TBSEs is one of the greatest barriers preventing access to appropriate dermatologic screening along with the paucity of trained providers; however, standardized total-body photography (TBP) promises to provide a way forward by lowering the costs of dermatologic screening while simultaneously leveraging technology to increase availability.
Impact on Biopsy Efficiency
Current US Preventive Services Task Force (USPSTF) guidelines state that evidence is insufficient to assess the balance of benefits and harms of visual skin examination by a clinician to screen for skin cancer in adults. The USPSTF noted that “[d]irect evidence on the effectiveness of screening in reducing melanoma morbidity and mortality is limited to a single fair-quality ecologic study with important methodological limitations” (ie, the Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany [SCREEN] study), and although information on harm is similarly sparse, “[t]he potential for harm clearly exists, including a high rate of unnecessary biopsies, possibly resulting in cosmetic or, more rarely, functional adverse effects, and the risk of overdiagnosis and overtreatment.”9 The majority of suspicious skin lesions excised during screenings are not cancerous. For example, the SCREEN study found that 20 to 55 excisions were performed to detect 1 case of melanoma.10 At that rate, the USPSTF also noted that approximately 4000 excisions would be required to prevent a single death from melanoma.9 Following the lead of the USPSTF, the Patient Protection and Affordable Care Act did not mandate that skin examinations be included as essential preventive coverage in its requirements for insurance coverage of primary care prevention. As such, dermatologists face financial pressure to avoid performing time-consuming TBSEs, regardless of their perceived utility.11
As the USPSTF points out, the value of TBSEs relies on the examiner’s ability to correctly identify malignant lesions and minimize biopsies of benign lesions, a concept known as biopsy efficiency.9 Secondarily, a TBSE is time consuming, and the time required for a dermatologist to complete a TBSE given the high rate of benign findings may not be financially viable. We argue that the routine use of total-body skin imaging may offer a way forward in addressing these issues. Total-body photography involves a photographic system that can allow dermatologists to acquire standardized images that can be used for primary diagnosis and to track individual lesions over time. Nonmedical personnel and medical assistants can be easily trained to use standardized photography devices to quickly obtain high-quality clinical images, thereby greatly reducing the time and cost of obtaining these images. Studies have found that the use of photographic monitoring may improve biopsy efficiency.12-15 A recent study by Truong et al16 found that TBP used to monitor all existing melanocytic lesions on patients substantially reduced the number of biopsies that patients required. These results reflect that most nevi, including clinically atypical nevi, are usually stable and unlikely to exhibit suspicious changes over time.17,18 For this reason, the use of TBP could minimize unnecessary biopsies because clinically suspicious but stable nevi can be objectively documented and followed over time.
Standardized TBP also offers the ability for dermatologists to work synergistically with modern computer technology involving algorithms capable of analyzing high-quality images to autodiagnose or flag concerning lesions that may require biopsy. Esteva et al19 described their development of a deep learning algorithm that relies on a convolutional neural network (CNN). This CNN was trained to identify melanomas using a large data set of clinical dermatologic images and subsequently was able to distinguish MMs from benign nevi at a rate on par with a board-certified dermatologist.19 Widespread use of total-body imaging would create an enormous database of high-resolution images that would be ideally suited to the development of such computerized algorithms, which could then be applied to future images by way of artificial intelligence. Convolutional neural networks that use a single patient’s imaging over time could be developed to assess the change in number or size of benign nevi and identify lesions that are concerning for MM while simultaneously comparing them to a population-based data set.
On a large scale, such a capability would minimize the inefficiency and subjectivity of TBSEs as a tool for identifying malignancy. Currently, dermatologists are only able to track and document a few concerning lesions on a patient’s body, rendering the choice of which lesions require biopsy more subjective. Total-body photography, particularly if used with an algorithm capable of quickly analyzing all the nevi on a person’s body, largely eliminates such subjectivity by creating a standardized set of images that can be tracked over time and flagging concerning lesions prior to the dermatologist examining the patient. In this way, the specialty of dermatology could achieve the same model of objective evaluation of standardized clinical images as those employed in radiology, cardiology, and other clinical disciplines. The additional benefit of such a system would be lower costs, as the images could be acquired by nonmedical personnel and then undergo initial assessment by an algorithm, which would flag concerning lesions, similar to a modern electrocardiogram machine, allowing the dermatologist to use his/her time more efficiently by only focusing on concerning lesions with the confidence that the patient’s entire body has already been rigorously screened.
By using TBP to improve biopsy efficiency and the objectivity of the TBSE as a tool to detect skin cancer, we propose that the benefit-to-harm ratio of the TBSE would remarkably improve. Ultimately, this type of screening would meet the strict requirements to be included in preventive health care strategies and thereby improve access to dermatologic care.
The Use of TBP in the Military
Total-body photography has several specific applications in the military. Standardized imaging has the potential to improve dermatologic care for active-duty soldiers across space and time. First, a large percentage of deployment medical care is devoted to dermatologic issues. From 2008 to 2015, 5% of all medical encounters in the combat theaters of Iraq and Afghanistan involved dermatologic concerns.20 Access to appropriate dermatologic care in a combat theater is important, as poorly controlled dermatologic conditions (eg, psoriasis, eczema) often require evacuation when left untreated. Although current TBP systems may not be portable or durable enough to survive in an austere deployment environment, we propose it would be feasible to have skin imaging booths at larger forward operating bases. The images could then be transported to a remote dermatologist to assess and recommend treatment. The expense of transporting and maintaining the imaging system in country would be offset by the expenses spared by not requiring a dermatologist in country and the reductions in costly medical evacuations from theater.
Although the US military population is younger and generally healthier than the general adult population due to extensive medical screening on admission, age limitations for active-duty service, a mandated active lifestyle, and access to good health care, there are still a substantial number of service members diagnosed with skin cancer each year.21 From 2005 through 2014, MM was the most common non–gender-specific cancer (n=1571); in men, only testicular cancer was more prevalent (1591 vs 1298 cases), and in women, only breast cancer was more prevalent (773 vs 273 cases). Furthermore, from 2004 to 2013, the incidence rates of melanoma have increased by 1.4%, while with other cancer rates have declined during the same time period.21 Thus, TBP as a screening modality across the military population is a promising method for improving detection of skin cancer and reducing morbidity and mortality.
Military medicine often is on the forefront of medical advances in technology, disease understanding, and clinical care due to the unique resources available in the military health care system, which allow investigators the ability to obtain vast amounts of epidemiologic data.22 The military health care system also is unique in its ability to mandate that its members obtain preventive health services. Thus, it would be possible for the military to mandate TBP at accession and retirement, for instance, or more frequently for annual screening. The implementation of such a program would improve the health of the military population and be a public health service by pioneering the use of a standardized TBP system across a large health care system to improve skin cancer detection.
Current Studies in the Military
The Dermatology Service at the Walter Reed National Military Medical Center (WRNMMC)(Bethesda, Maryland) is evaluating the use of a total-body digital skin imaging system under a grant from the Telemedicine and Advanced Technology Research Center of the US Army. The system in use was found to be particularly well suited for military dermatology because it offers standardized TBP processing, produces a report that can be uploaded to the US Department of Defense (DoD) electronic medical record system, and requires relatively brief training for ancillary personnel to operate. Regardless of the platform the DoD ultimately finds most suitable, it is critical that a standard exist for TBP to ensure that uniform data sets are generated to allow military and other DoD dermatologists as well as civilian health care providers to share clinical information. The goal of the current study at WRNMMC is to vet TBP platforms at WRNMMC so the military can then develop standards to procure additional platforms for placement throughout the Military Health System, Military Entrance Processing Stations, operational environments, and collaborating health care systems (eg, the Veterans Health Administration).
Once deployed broadly across the Military Health System, these TBP platforms would be part of a network of telehealth care. For acute dermatologic issues, diagnoses provided via teledermatology platforms can then be managed by health care providers utilizing appropriate clinical practice guidelines or by non–health care providers utilizing general medical knowledge databases. Such a system with TBP information collected at multiple access points across a service member’s career would build a repository of data that would be immensely useful to patients and to clinical research. Of particular interest to military researchers is that TBP data could be used to study which patients require in-person examinations or more careful monitoring; the proper intervals for skin cancer screening; and the assessment of the benefits of TBP in improving morbidity, mortality, and biopsy efficiency in the detection of MM as well as nonmelanoma skin cancers.
Limitations to Progress
Currently, there are multiple limitations to the implementation of TBP as a part of TBSE screening. First, the potential improvement in biopsy efficiency using TBP is predicated on its ability to prove nevi stability over time, but in younger populations, benign nevi are more likely to change or increase in number, which may reduce the biopsy efficiency of screening in a younger population, thereby negating some of the benefit of imaging and CNN assessment. For instance, Truong et al16 found that younger age (<30 years) did not show the same improvement in biopsy efficiency with the use of TBP, which the authors theorized may reflect “the dynamic nature of nevi in younger patients” that has been documented in other studies.23,24 Approximately 65% of the active-duty military population is aged 18 to 30 years, and 98% of accessions to active duty occur in individuals aged 17 to 30 years.25 As such, TBP may not improve biopsy efficiency in the active-duty military population as dramatically as it would across the general population.
A second limitation of the use of TBP in the active-duty military population is the ethics of implementing DoD-wide mandatory TBP. Although the TBP platform will be compliant with the Health Insurance Portability and Accountability Act, mandating that soldiers contribute their TBP to a repository of data that will then be used for research without explicitly requesting their consent is ethically problematic; however, since the 1950s, the DoD has collected serum samples from its service members for force protection and operations reasons as well as for the purpose of research.22,26 Currently, the DoD Serum Repository collects serum samples as part of a mandatory human immunodeficiency virus screening program that evaluates service members every 2 years; this repository of human serum samples is accessible for research purposes without the consent of the individuals being studied.27 These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Thus, although there is precedent in the DoD for such mass data collection, it is an ongoing ethical consideration.28
RELATED ARTICLE: Gigapixel Photography for Skin Cancer Surveillance
Finally, although the potential use of TBP and computer algorithms to improve the efficiency and affordability of TBSEs is exciting, there are no existing computer algorithms that we are aware of that can be used with existing TBP platforms in the manner we proposed. However, we feel that computer algorithms, such as the one created by Esteva et al,19 are just the beginning and that the use of artificial intelligence is not far off. Even after the creation of a TBP-compatible algorithm adept at analyzing malignant lesions, however, this technology would need to be further evaluated in the clinical setting to determine its capability and practicality. Current TBP platforms also are limited by their large size, cost, and complexity. As TBP platforms improve, it is likely that more streamlined and less expensive versions of current models will greatly enhance the field of teledermatology, particularly in the military setting.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131-149; quiz 182-184.
- Eisemann N, Jansen L, Holleczek B, et al. Up-to-date results on survival of patients with melanoma in Germany [published online July 19, 2012]. Br J Dermatol. 2012;167:606-612.
- MacKie RM, Bray C, Vestey J, et al. Melanoma incidence and mortality in Scotland 1979-2003 [published online May 29, 2007]. Br J Cancer. 2007;96:1772-1777.
- Dickson PV, Gershenwald JE. Staging and prognosis of cutaneous melanoma. Surg Oncol Clin N Am. 2011;20:1-17.
- Balch CM, Gershenwald JE, Soong SL, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
- Kingsley-Loso JL, Grey KR, Hanson JL, et al. Incidental lesions found in veterans referred to dermatology: the value of a dermatologic examination [published online January 23, 2015]. J Am Acad Dermatol. 2015;72:651.e1-655.e1.
- Grant-Kels JM, Stoff B. Total body skin exams (TBSEs): saving lives or wasting time? J Am Acad Dermatol. 2017;76:183-185.
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Robinson JK, Halpern AC. Cost-effective melanoma screening. JAMA Dermatol. 2016;152:19-21.
- Feit NE, Dusza SW, Marghoob AA. Melanomas detected with the aid of total cutaneous photography. Br J Dermatol. 2004;150:706-714.
- Haenssle HA, Krueger U, Vente C, et al. Results from an observational trial: digital epiluminescence microscopy follow-up of atypical nevi increases the sensitivity and the chance of success of conventional dermoscopy in detecting melanoma. J Invest Dermatol. 2006;126:980-985.
- Salerni G, Carrera C, Lovatto L, et al. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. J Am Acad Dermatol. 2012;67:E17-E27.
- Rice ZP, Weiss FJ, DeLong LK, et al. Utilization and rationale for the implementation of total body (digital) photography as an adjunct screening measure for melanoma. Melanoma Res. 2010;20:417-421.
- Truong A, Strazzulla L, March J, et al. Reduction in nevus biopsies in patients monitored by total body photography [published online March 3, 2016]. J Am Acad Dermatol. 2016;75:135.e5-143.e5.
- Lucas CR, Sanders LL, Murray JC, et al. Early melanoma detection: nonuniform dermoscopic features and growth. J Am Acad Dermatol. 2003;48:663-671.
- Fuller SR, Bowen GM, Tanner B, et al. Digital dermoscopic monitoring of atypical nevi in patients at risk for melanoma. Dermatol Surg. 2007;33:1198-1206; discussion 1205-1206.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks [published online January 25, 2017]. Nature. 2017;542:115-118.
- Defense Medical Epidemiology Database. Military Health System website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Epidemiology-Database. Accessed April 10, 2017.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Helmandollar KJ, Meyerle JH. Exploration of modern military research resources. Cutis. 2016;98:231-234.
- Goodson AG, Grossman D. Strategies for early melanoma detection: approaches to the patient with nevi. J Am Acad Dermatol. 2009;60:719-735; quiz 736-738.
- Bajaj S, Dusza SW, Marchetti MA, et al. Growth-curve modeling of nevi with a peripheral globular pattern. JAMA Dermatol. 2015;151:1338-1345.
- Niebuhr DW, Gubata ME, Cowan DN, et al. Accession Medical Standards Analysis & Research Activity (AMSARA) 2011 Annual Report. Silver Spring, MD: Division of Preventive Medicine, Walter Reed Army Institute of Research; 2012.
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD Serum Repository. Mil Med. 2015;180:10-12.
- Pavlin JA, Welch RA. Ethics, human use, and the Department of Defense Serum Repository. Mil Med. 2015;180:49-56.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131-149; quiz 182-184.
- Eisemann N, Jansen L, Holleczek B, et al. Up-to-date results on survival of patients with melanoma in Germany [published online July 19, 2012]. Br J Dermatol. 2012;167:606-612.
- MacKie RM, Bray C, Vestey J, et al. Melanoma incidence and mortality in Scotland 1979-2003 [published online May 29, 2007]. Br J Cancer. 2007;96:1772-1777.
- Dickson PV, Gershenwald JE. Staging and prognosis of cutaneous melanoma. Surg Oncol Clin N Am. 2011;20:1-17.
- Balch CM, Gershenwald JE, Soong SL, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
- Kingsley-Loso JL, Grey KR, Hanson JL, et al. Incidental lesions found in veterans referred to dermatology: the value of a dermatologic examination [published online January 23, 2015]. J Am Acad Dermatol. 2015;72:651.e1-655.e1.
- Grant-Kels JM, Stoff B. Total body skin exams (TBSEs): saving lives or wasting time? J Am Acad Dermatol. 2017;76:183-185.
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Robinson JK, Halpern AC. Cost-effective melanoma screening. JAMA Dermatol. 2016;152:19-21.
- Feit NE, Dusza SW, Marghoob AA. Melanomas detected with the aid of total cutaneous photography. Br J Dermatol. 2004;150:706-714.
- Haenssle HA, Krueger U, Vente C, et al. Results from an observational trial: digital epiluminescence microscopy follow-up of atypical nevi increases the sensitivity and the chance of success of conventional dermoscopy in detecting melanoma. J Invest Dermatol. 2006;126:980-985.
- Salerni G, Carrera C, Lovatto L, et al. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. J Am Acad Dermatol. 2012;67:E17-E27.
- Rice ZP, Weiss FJ, DeLong LK, et al. Utilization and rationale for the implementation of total body (digital) photography as an adjunct screening measure for melanoma. Melanoma Res. 2010;20:417-421.
- Truong A, Strazzulla L, March J, et al. Reduction in nevus biopsies in patients monitored by total body photography [published online March 3, 2016]. J Am Acad Dermatol. 2016;75:135.e5-143.e5.
- Lucas CR, Sanders LL, Murray JC, et al. Early melanoma detection: nonuniform dermoscopic features and growth. J Am Acad Dermatol. 2003;48:663-671.
- Fuller SR, Bowen GM, Tanner B, et al. Digital dermoscopic monitoring of atypical nevi in patients at risk for melanoma. Dermatol Surg. 2007;33:1198-1206; discussion 1205-1206.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks [published online January 25, 2017]. Nature. 2017;542:115-118.
- Defense Medical Epidemiology Database. Military Health System website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Epidemiology-Database. Accessed April 10, 2017.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Helmandollar KJ, Meyerle JH. Exploration of modern military research resources. Cutis. 2016;98:231-234.
- Goodson AG, Grossman D. Strategies for early melanoma detection: approaches to the patient with nevi. J Am Acad Dermatol. 2009;60:719-735; quiz 736-738.
- Bajaj S, Dusza SW, Marchetti MA, et al. Growth-curve modeling of nevi with a peripheral globular pattern. JAMA Dermatol. 2015;151:1338-1345.
- Niebuhr DW, Gubata ME, Cowan DN, et al. Accession Medical Standards Analysis & Research Activity (AMSARA) 2011 Annual Report. Silver Spring, MD: Division of Preventive Medicine, Walter Reed Army Institute of Research; 2012.
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD Serum Repository. Mil Med. 2015;180:10-12.
- Pavlin JA, Welch RA. Ethics, human use, and the Department of Defense Serum Repository. Mil Med. 2015;180:49-56.
Practice Points
- Advances in technology have the potential to provide affordable standardized total-body photography platforms.
- Total-body photography augments the clinical examination and plays a role in clinical decision-making.
- Total-body photography has the potential to become a part of the total-body skin examination and increase access to dermatologic care.
Photoprotection Prevents Skin Cancer: Let’s Make It Fashionable to Wear Sun-Protective Clothing
Photoprotection is the foundation of all skin cancer prevention, as UV radiation (UVR) exposure is the only known modifiable risk factor for skin cancer. With the majority of UVR exposure–induced skin cancers found on the scalp, ears, face, and neck, public health initiatives call for wise choices in personal fashion that emphasize the importance of covering these areas.1-3 From a science of fashion perspective, research has shown that wide-brimmed hats provide better means of ensuring the largest area of coverage compared to standard baseball-style hats.4 Thus, for maximum protection, wide-brimmed hats should be favored. However, in academic and military settings, individual style is not optional and is instead influenced or directed by policy, which may not be aligned with the goal of providing photoprotection and raises additional concern for individuals working in environments with longer periods of peak daylight UVR exposure.
In all military branches, service members don uniforms that include head coverage when operating outdoors; however, the choice of headgear is not always aimed at reducing UVR exposure. Similarly, in our counterpart civilian populations, wearing hats that provide the best photoprotection may be influenced by school policies, which frequently mandate clothing choices for children, or by the press or fashion industry in the general public, which might portray sun-protective garments as unfashionable or in some cases threatening if perceived as demonstrating gang affiliation.5 This article serves to encourage health care providers to not only discuss the use of sunscreen when educating patients on sun protection but also to emphasize the benefits of wearing photoprotective garments, particularly wide-brimmed hats given their simplicity, reusability, and affordability. Hat use is particularly important for men with comorbid androgenetic alopecia.6
Skin Cancer Risk
Unfortunately, the incidence of most common types of skin cancer, specifically nonmelanoma skin cancers such basal cell carcinomas and squamous cell carcinomas (ie, keratinocyte carcinomas [KCs]), is difficult to estimate properly, as these cases are not required to be reported to worldwide cancer registries. However, more than 5.4 million cases of skin cancers were diagnosed among 3.3 million Americans in 2016, with an estimated 13,650 deaths associated with skin cancers (not including KCs).3 Tracking and data analyses of cases diagnosed in the active and reserve component populations of the US Armed Forces reflect parallel findings.7 Keratinocyte carcinomas could be considered largely preventable, as most are the result of UVR exposure.1 Additionally, it has been suggested that the vast majority of mutations in melanoma skin cancers (up to 86%) are caused by UVR exposure.8
Prevention
United States–based national public health services such as the American Cancer Society, the Centers for Disease Control and Prevention, and the American Academy of Dermatology embrace photoprotection as the central practice in reducing risk factors for skin cancers. Guidelines put forth by these and other national preventive medical institutions specifically recommend the use of wide-brimmed hats as the best option for protection of the face, head, ears, and neck, in addition to more common recommendations such as seeking shade, avoiding sunlight during peak hours of the day, and using sunscreen.1-3 At state and local levels, policies should be adapted from these recommendations to support protective practices and skin cancer education that begins early for school-aged children. Unfortunately, in some school districts, wearing hats of any kind may be perceived as disruptive or in some cases baseball hats may be a sign of gang affiliation and are therefore banned in the schoolyard.5 The opposite is true in certain parts of the world where sun protection is embraced by the population as a whole, such as Australia where the widely accepted “slip, slop and slap, seek and slide” campaign has extended to some school policymakers who have considered adopting a “no hat, no play” policy.9,10
Sunscreen use as a primary component of photoprotection has its disadvantages in comparison to wearing protective clothing, as sunscreen cannot be reused and proper usage requires reapplication after swimming, when sweating, and following 2 hours of application.1-3 The need for reapplication of sunscreen can lead to considerable expense as well as time spent in application and reapplication. Additionally, for individuals who are physically active (eg, operationally engaged service members, outdoor athletes), sunscreen applied to the face may become a hindrance to function, as it may drip or enter the eyes with excessive sweating, possibly impairing vision. Some individuals may be averse to applying lotions or creams to the skin in general, as they do not prefer the textural changes or appearance of the skin after application. The application of sunscreen also could impair use of lifesaving military gear (eg, gas masks, helmets) from fitting or securing appropriately.
Patient Education
From a military perspective, a review of a recent targeted pilot study in which skin cancer patients at a US Veterans Administration hospital were surveyed on personal knowledge of UVR protection showed that respondents who had a history of skin cancer diagnosis did not feel that they had ever been at an increased risk for skin cancers and did not receive skin cancer prevention education during their tours of service. The overwhelming majority of all participants in this study agreed that the military should issue sun-protective clothing and sunscreen to active-duty personnel.11 Another 2015 survey of 356 current US Air Force flight line personnel noted that active-duty service members tend not to use sunscreen when at work or while at home, and 43% of participants reported using no sun-protective methods while working outdoors.12 Although these studies focused on military personal, the data mirror findings within the general public, as it was shown in a survey by the Centers for Disease Control and Prevention that Americans do not fully take advantage of the benefits of UVR protection, specifically with regard to sunscreen use. Little to no usage was correlated with low socioeconomic status, suggesting that a reusable form of protection could be preferred.13
Public health initiatives typically promote education on the use of sunscreen in populations that spend a considerable amount of time working outdoors (eg, construction workers, farmers, military personnel); however, we feel emphasis should be placed on the benefits of wearing hats, as the UVR exposure protection they provide does not wear off, is cost effective, does not require reapplication, and has the advantage of being a recyclable and affordable form of photoprotection.
History of the Military-Grade Wide-Brimmed Hat
One military-specific example of a sun-protective hat is the boonie hat, known at the time of its inception as the tropical or hot-weather hat, which first became popular during the Vietnam War. This hat option was initially proposed on April 7, 1966, when it was realized that a full-brimmed field hat was needed to protect soldiers’ faces and necks from rain and sun in harsh tropical climates.14 Unfortunately, despite the protective advantages of this style of head covering and favorable support from service members themselves, the boonie hat was not widely accepted, as commanders disliked its “unmilitary appearance.” Fervent protests by units throughout Vietnam eventually led to a compromise in policy that allowed unit-level commanders to authorize the use of boonie hats for units in combat or combat support field operations.14 Today, the boonie hat continues to garnish mixed emotions from unit commanders, as wearing this garment often is interpreted as not being in line with an appropriate military appearance, which is similar to the public fashion zeitgeist that also does not openly endorse the use of sun-protective garments. A change in fashion culture and policy (both military and civilian) that promotes sun-protective measures is needed.
Wide-Brimmed Hats Are Superior to Baseball Hats
The distribution of skin cancers across anatomic sites is consistent and proportional with the level and frequency of chronic UVR exposure, with the occurrence of most skin cancers being greatest on the nose, forehead/temples, cheeks/perioral areas, and ears.15 Additionally, higher incidences of skin cancers have been noted in chronically sun-exposed areas of the head and neck in men versus women. It is thought that hair distribution in these areas may be the causal factor.6
Baseball-style hats are worn by all branches of the US military as part of standard training and work duty uniform requirements, primarily for the sake of tradition by maintaining a standard appearance and uniform dress code but also to provide photoprotection to these vulnerable areas of the body. Standard, nonmilitary, baseball-style hats have been shown to provide UV protection factor (UPF) equivalents ranging from 2 to 10 on sites known for the highest levels of exposure.16 Military “patrol caps,” fashioned similar to the baseball-style hat but constructed from military-grade textiles, provide greater levels of photoprotection with UPF ratings from 35 to 50 and higher depending on the fabric color.17 Although patrol caps have a favorable UPF rating and are advantageous compared to former military headgear styles (eg, berets), wide-brimmed hats would provide greater overall coverage.4,6 Studies in school environments also revealed that wide-brimmed hats come out ahead in side-by-side testing against baseball hats and are shown to provide greater photoprotection for the cheeks, chin, ears, and neck.16
Final Thoughts
The battle to educate the public about adequate photoprotection to prevent skin cancers caused by UVR exposure applies to all providers, both military and civilian. Our ongoing initiatives should not only sustain current practices but should further stress the importance of wearing wide-brimmed hats as a vital part of coverage of the skin and protection from UVR. We must combat the public perception that wearing wide-brimmed hats is a detractor of personal fashion and that instead it is desirable to reduce the risk for skin cancer. The wide-brimmed hat is a simple, reusable, and easily executed recommendation that should be made to all patients, both military and civilian, young and old. In conclusion, by improving patients’ perceptions and acknowledgment of the importance of photoprotection as well as making a concerted effort to integrate our knowledge in the fashion industry, in policies at schools, in the military, and in popular culture, we will undoubtedly come to agree that it is not unfashionable to wear a wide-brimmed hat, but it is unfashionable to risk developing skin cancer.
- Prevent skin cancer. American Academy of Dermatology website. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent. Accessed January 4, 2017.
- What can I do to reduce my risk of skin cancer? Centers for Disease Control and Prevention website. http://www.cdc.gov/cancer/skin/basic_info/prevention.htm. Accessed January 4, 2017.
- Cancer facts & figures 2016. American Cancer Society website. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed January 4, 2017.
- Diffey BL, Cheeseman J. Sun protection with hats. Br J Dermatol. 1992;127:10-12.
- Bray FN. Florida school boards restrict access to outdoor sun protection: an observational study. J Am Acad Dermatol. 2016;75:642-644.
- Yeung H, Luk KM, Chen SC. Focal photodamage on the occipital scalp. JAMA Dermatol. 2016;152:1060-1062.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Parkin DM, Mesher D, Sasieni P. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer. 2011;105(suppl 2):S66-S69.
- Casper K. Elementary schools consider “no hat no play policy.” Coolibar website. http://blog.coolibar.com/elementary-schools-consider-no-hat-no-play-policy/. Published March 27, 2012. Accessed January 4, 2017.
- Slip, slop, slap, seek & slide: Sid Seagull. SunSmart Victoria website. http://www.sunsmart.com.au/tools/videos/current-tv-campaigns/slip-slop-slap-seek-slide-sid-seagull.html. Accessed January 4, 2017.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns - a pilot study. Am J Prev Med. 2016;50:E62-E63.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Mil Med. 2015;180:26-31.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults [published online May 19, 2015]. J Am Acad Dermatol. 2015;73:83-92.e1.
- Stanton SL. Headgear. In: Stanton SL. U.S. Army Uniforms of the Vietnam War. Harrisburg, PA: Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population [published online July 31, 2008]. J Invest Dermatol. 2009;129:323-328.
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Winterhalter C, DiLuna K, Bide M. Characterization of the Ultraviolet Protection of Combat Uniform Fabrics. Natick, MA: US Army Solider and Biological Chemical Command; 2002. Technical report 02/006.
Photoprotection is the foundation of all skin cancer prevention, as UV radiation (UVR) exposure is the only known modifiable risk factor for skin cancer. With the majority of UVR exposure–induced skin cancers found on the scalp, ears, face, and neck, public health initiatives call for wise choices in personal fashion that emphasize the importance of covering these areas.1-3 From a science of fashion perspective, research has shown that wide-brimmed hats provide better means of ensuring the largest area of coverage compared to standard baseball-style hats.4 Thus, for maximum protection, wide-brimmed hats should be favored. However, in academic and military settings, individual style is not optional and is instead influenced or directed by policy, which may not be aligned with the goal of providing photoprotection and raises additional concern for individuals working in environments with longer periods of peak daylight UVR exposure.
In all military branches, service members don uniforms that include head coverage when operating outdoors; however, the choice of headgear is not always aimed at reducing UVR exposure. Similarly, in our counterpart civilian populations, wearing hats that provide the best photoprotection may be influenced by school policies, which frequently mandate clothing choices for children, or by the press or fashion industry in the general public, which might portray sun-protective garments as unfashionable or in some cases threatening if perceived as demonstrating gang affiliation.5 This article serves to encourage health care providers to not only discuss the use of sunscreen when educating patients on sun protection but also to emphasize the benefits of wearing photoprotective garments, particularly wide-brimmed hats given their simplicity, reusability, and affordability. Hat use is particularly important for men with comorbid androgenetic alopecia.6
Skin Cancer Risk
Unfortunately, the incidence of most common types of skin cancer, specifically nonmelanoma skin cancers such basal cell carcinomas and squamous cell carcinomas (ie, keratinocyte carcinomas [KCs]), is difficult to estimate properly, as these cases are not required to be reported to worldwide cancer registries. However, more than 5.4 million cases of skin cancers were diagnosed among 3.3 million Americans in 2016, with an estimated 13,650 deaths associated with skin cancers (not including KCs).3 Tracking and data analyses of cases diagnosed in the active and reserve component populations of the US Armed Forces reflect parallel findings.7 Keratinocyte carcinomas could be considered largely preventable, as most are the result of UVR exposure.1 Additionally, it has been suggested that the vast majority of mutations in melanoma skin cancers (up to 86%) are caused by UVR exposure.8
Prevention
United States–based national public health services such as the American Cancer Society, the Centers for Disease Control and Prevention, and the American Academy of Dermatology embrace photoprotection as the central practice in reducing risk factors for skin cancers. Guidelines put forth by these and other national preventive medical institutions specifically recommend the use of wide-brimmed hats as the best option for protection of the face, head, ears, and neck, in addition to more common recommendations such as seeking shade, avoiding sunlight during peak hours of the day, and using sunscreen.1-3 At state and local levels, policies should be adapted from these recommendations to support protective practices and skin cancer education that begins early for school-aged children. Unfortunately, in some school districts, wearing hats of any kind may be perceived as disruptive or in some cases baseball hats may be a sign of gang affiliation and are therefore banned in the schoolyard.5 The opposite is true in certain parts of the world where sun protection is embraced by the population as a whole, such as Australia where the widely accepted “slip, slop and slap, seek and slide” campaign has extended to some school policymakers who have considered adopting a “no hat, no play” policy.9,10
Sunscreen use as a primary component of photoprotection has its disadvantages in comparison to wearing protective clothing, as sunscreen cannot be reused and proper usage requires reapplication after swimming, when sweating, and following 2 hours of application.1-3 The need for reapplication of sunscreen can lead to considerable expense as well as time spent in application and reapplication. Additionally, for individuals who are physically active (eg, operationally engaged service members, outdoor athletes), sunscreen applied to the face may become a hindrance to function, as it may drip or enter the eyes with excessive sweating, possibly impairing vision. Some individuals may be averse to applying lotions or creams to the skin in general, as they do not prefer the textural changes or appearance of the skin after application. The application of sunscreen also could impair use of lifesaving military gear (eg, gas masks, helmets) from fitting or securing appropriately.
Patient Education
From a military perspective, a review of a recent targeted pilot study in which skin cancer patients at a US Veterans Administration hospital were surveyed on personal knowledge of UVR protection showed that respondents who had a history of skin cancer diagnosis did not feel that they had ever been at an increased risk for skin cancers and did not receive skin cancer prevention education during their tours of service. The overwhelming majority of all participants in this study agreed that the military should issue sun-protective clothing and sunscreen to active-duty personnel.11 Another 2015 survey of 356 current US Air Force flight line personnel noted that active-duty service members tend not to use sunscreen when at work or while at home, and 43% of participants reported using no sun-protective methods while working outdoors.12 Although these studies focused on military personal, the data mirror findings within the general public, as it was shown in a survey by the Centers for Disease Control and Prevention that Americans do not fully take advantage of the benefits of UVR protection, specifically with regard to sunscreen use. Little to no usage was correlated with low socioeconomic status, suggesting that a reusable form of protection could be preferred.13
Public health initiatives typically promote education on the use of sunscreen in populations that spend a considerable amount of time working outdoors (eg, construction workers, farmers, military personnel); however, we feel emphasis should be placed on the benefits of wearing hats, as the UVR exposure protection they provide does not wear off, is cost effective, does not require reapplication, and has the advantage of being a recyclable and affordable form of photoprotection.
History of the Military-Grade Wide-Brimmed Hat
One military-specific example of a sun-protective hat is the boonie hat, known at the time of its inception as the tropical or hot-weather hat, which first became popular during the Vietnam War. This hat option was initially proposed on April 7, 1966, when it was realized that a full-brimmed field hat was needed to protect soldiers’ faces and necks from rain and sun in harsh tropical climates.14 Unfortunately, despite the protective advantages of this style of head covering and favorable support from service members themselves, the boonie hat was not widely accepted, as commanders disliked its “unmilitary appearance.” Fervent protests by units throughout Vietnam eventually led to a compromise in policy that allowed unit-level commanders to authorize the use of boonie hats for units in combat or combat support field operations.14 Today, the boonie hat continues to garnish mixed emotions from unit commanders, as wearing this garment often is interpreted as not being in line with an appropriate military appearance, which is similar to the public fashion zeitgeist that also does not openly endorse the use of sun-protective garments. A change in fashion culture and policy (both military and civilian) that promotes sun-protective measures is needed.
Wide-Brimmed Hats Are Superior to Baseball Hats
The distribution of skin cancers across anatomic sites is consistent and proportional with the level and frequency of chronic UVR exposure, with the occurrence of most skin cancers being greatest on the nose, forehead/temples, cheeks/perioral areas, and ears.15 Additionally, higher incidences of skin cancers have been noted in chronically sun-exposed areas of the head and neck in men versus women. It is thought that hair distribution in these areas may be the causal factor.6
Baseball-style hats are worn by all branches of the US military as part of standard training and work duty uniform requirements, primarily for the sake of tradition by maintaining a standard appearance and uniform dress code but also to provide photoprotection to these vulnerable areas of the body. Standard, nonmilitary, baseball-style hats have been shown to provide UV protection factor (UPF) equivalents ranging from 2 to 10 on sites known for the highest levels of exposure.16 Military “patrol caps,” fashioned similar to the baseball-style hat but constructed from military-grade textiles, provide greater levels of photoprotection with UPF ratings from 35 to 50 and higher depending on the fabric color.17 Although patrol caps have a favorable UPF rating and are advantageous compared to former military headgear styles (eg, berets), wide-brimmed hats would provide greater overall coverage.4,6 Studies in school environments also revealed that wide-brimmed hats come out ahead in side-by-side testing against baseball hats and are shown to provide greater photoprotection for the cheeks, chin, ears, and neck.16
Final Thoughts
The battle to educate the public about adequate photoprotection to prevent skin cancers caused by UVR exposure applies to all providers, both military and civilian. Our ongoing initiatives should not only sustain current practices but should further stress the importance of wearing wide-brimmed hats as a vital part of coverage of the skin and protection from UVR. We must combat the public perception that wearing wide-brimmed hats is a detractor of personal fashion and that instead it is desirable to reduce the risk for skin cancer. The wide-brimmed hat is a simple, reusable, and easily executed recommendation that should be made to all patients, both military and civilian, young and old. In conclusion, by improving patients’ perceptions and acknowledgment of the importance of photoprotection as well as making a concerted effort to integrate our knowledge in the fashion industry, in policies at schools, in the military, and in popular culture, we will undoubtedly come to agree that it is not unfashionable to wear a wide-brimmed hat, but it is unfashionable to risk developing skin cancer.
Photoprotection is the foundation of all skin cancer prevention, as UV radiation (UVR) exposure is the only known modifiable risk factor for skin cancer. With the majority of UVR exposure–induced skin cancers found on the scalp, ears, face, and neck, public health initiatives call for wise choices in personal fashion that emphasize the importance of covering these areas.1-3 From a science of fashion perspective, research has shown that wide-brimmed hats provide better means of ensuring the largest area of coverage compared to standard baseball-style hats.4 Thus, for maximum protection, wide-brimmed hats should be favored. However, in academic and military settings, individual style is not optional and is instead influenced or directed by policy, which may not be aligned with the goal of providing photoprotection and raises additional concern for individuals working in environments with longer periods of peak daylight UVR exposure.
In all military branches, service members don uniforms that include head coverage when operating outdoors; however, the choice of headgear is not always aimed at reducing UVR exposure. Similarly, in our counterpart civilian populations, wearing hats that provide the best photoprotection may be influenced by school policies, which frequently mandate clothing choices for children, or by the press or fashion industry in the general public, which might portray sun-protective garments as unfashionable or in some cases threatening if perceived as demonstrating gang affiliation.5 This article serves to encourage health care providers to not only discuss the use of sunscreen when educating patients on sun protection but also to emphasize the benefits of wearing photoprotective garments, particularly wide-brimmed hats given their simplicity, reusability, and affordability. Hat use is particularly important for men with comorbid androgenetic alopecia.6
Skin Cancer Risk
Unfortunately, the incidence of most common types of skin cancer, specifically nonmelanoma skin cancers such basal cell carcinomas and squamous cell carcinomas (ie, keratinocyte carcinomas [KCs]), is difficult to estimate properly, as these cases are not required to be reported to worldwide cancer registries. However, more than 5.4 million cases of skin cancers were diagnosed among 3.3 million Americans in 2016, with an estimated 13,650 deaths associated with skin cancers (not including KCs).3 Tracking and data analyses of cases diagnosed in the active and reserve component populations of the US Armed Forces reflect parallel findings.7 Keratinocyte carcinomas could be considered largely preventable, as most are the result of UVR exposure.1 Additionally, it has been suggested that the vast majority of mutations in melanoma skin cancers (up to 86%) are caused by UVR exposure.8
Prevention
United States–based national public health services such as the American Cancer Society, the Centers for Disease Control and Prevention, and the American Academy of Dermatology embrace photoprotection as the central practice in reducing risk factors for skin cancers. Guidelines put forth by these and other national preventive medical institutions specifically recommend the use of wide-brimmed hats as the best option for protection of the face, head, ears, and neck, in addition to more common recommendations such as seeking shade, avoiding sunlight during peak hours of the day, and using sunscreen.1-3 At state and local levels, policies should be adapted from these recommendations to support protective practices and skin cancer education that begins early for school-aged children. Unfortunately, in some school districts, wearing hats of any kind may be perceived as disruptive or in some cases baseball hats may be a sign of gang affiliation and are therefore banned in the schoolyard.5 The opposite is true in certain parts of the world where sun protection is embraced by the population as a whole, such as Australia where the widely accepted “slip, slop and slap, seek and slide” campaign has extended to some school policymakers who have considered adopting a “no hat, no play” policy.9,10
Sunscreen use as a primary component of photoprotection has its disadvantages in comparison to wearing protective clothing, as sunscreen cannot be reused and proper usage requires reapplication after swimming, when sweating, and following 2 hours of application.1-3 The need for reapplication of sunscreen can lead to considerable expense as well as time spent in application and reapplication. Additionally, for individuals who are physically active (eg, operationally engaged service members, outdoor athletes), sunscreen applied to the face may become a hindrance to function, as it may drip or enter the eyes with excessive sweating, possibly impairing vision. Some individuals may be averse to applying lotions or creams to the skin in general, as they do not prefer the textural changes or appearance of the skin after application. The application of sunscreen also could impair use of lifesaving military gear (eg, gas masks, helmets) from fitting or securing appropriately.
Patient Education
From a military perspective, a review of a recent targeted pilot study in which skin cancer patients at a US Veterans Administration hospital were surveyed on personal knowledge of UVR protection showed that respondents who had a history of skin cancer diagnosis did not feel that they had ever been at an increased risk for skin cancers and did not receive skin cancer prevention education during their tours of service. The overwhelming majority of all participants in this study agreed that the military should issue sun-protective clothing and sunscreen to active-duty personnel.11 Another 2015 survey of 356 current US Air Force flight line personnel noted that active-duty service members tend not to use sunscreen when at work or while at home, and 43% of participants reported using no sun-protective methods while working outdoors.12 Although these studies focused on military personal, the data mirror findings within the general public, as it was shown in a survey by the Centers for Disease Control and Prevention that Americans do not fully take advantage of the benefits of UVR protection, specifically with regard to sunscreen use. Little to no usage was correlated with low socioeconomic status, suggesting that a reusable form of protection could be preferred.13
Public health initiatives typically promote education on the use of sunscreen in populations that spend a considerable amount of time working outdoors (eg, construction workers, farmers, military personnel); however, we feel emphasis should be placed on the benefits of wearing hats, as the UVR exposure protection they provide does not wear off, is cost effective, does not require reapplication, and has the advantage of being a recyclable and affordable form of photoprotection.
History of the Military-Grade Wide-Brimmed Hat
One military-specific example of a sun-protective hat is the boonie hat, known at the time of its inception as the tropical or hot-weather hat, which first became popular during the Vietnam War. This hat option was initially proposed on April 7, 1966, when it was realized that a full-brimmed field hat was needed to protect soldiers’ faces and necks from rain and sun in harsh tropical climates.14 Unfortunately, despite the protective advantages of this style of head covering and favorable support from service members themselves, the boonie hat was not widely accepted, as commanders disliked its “unmilitary appearance.” Fervent protests by units throughout Vietnam eventually led to a compromise in policy that allowed unit-level commanders to authorize the use of boonie hats for units in combat or combat support field operations.14 Today, the boonie hat continues to garnish mixed emotions from unit commanders, as wearing this garment often is interpreted as not being in line with an appropriate military appearance, which is similar to the public fashion zeitgeist that also does not openly endorse the use of sun-protective garments. A change in fashion culture and policy (both military and civilian) that promotes sun-protective measures is needed.
Wide-Brimmed Hats Are Superior to Baseball Hats
The distribution of skin cancers across anatomic sites is consistent and proportional with the level and frequency of chronic UVR exposure, with the occurrence of most skin cancers being greatest on the nose, forehead/temples, cheeks/perioral areas, and ears.15 Additionally, higher incidences of skin cancers have been noted in chronically sun-exposed areas of the head and neck in men versus women. It is thought that hair distribution in these areas may be the causal factor.6
Baseball-style hats are worn by all branches of the US military as part of standard training and work duty uniform requirements, primarily for the sake of tradition by maintaining a standard appearance and uniform dress code but also to provide photoprotection to these vulnerable areas of the body. Standard, nonmilitary, baseball-style hats have been shown to provide UV protection factor (UPF) equivalents ranging from 2 to 10 on sites known for the highest levels of exposure.16 Military “patrol caps,” fashioned similar to the baseball-style hat but constructed from military-grade textiles, provide greater levels of photoprotection with UPF ratings from 35 to 50 and higher depending on the fabric color.17 Although patrol caps have a favorable UPF rating and are advantageous compared to former military headgear styles (eg, berets), wide-brimmed hats would provide greater overall coverage.4,6 Studies in school environments also revealed that wide-brimmed hats come out ahead in side-by-side testing against baseball hats and are shown to provide greater photoprotection for the cheeks, chin, ears, and neck.16
Final Thoughts
The battle to educate the public about adequate photoprotection to prevent skin cancers caused by UVR exposure applies to all providers, both military and civilian. Our ongoing initiatives should not only sustain current practices but should further stress the importance of wearing wide-brimmed hats as a vital part of coverage of the skin and protection from UVR. We must combat the public perception that wearing wide-brimmed hats is a detractor of personal fashion and that instead it is desirable to reduce the risk for skin cancer. The wide-brimmed hat is a simple, reusable, and easily executed recommendation that should be made to all patients, both military and civilian, young and old. In conclusion, by improving patients’ perceptions and acknowledgment of the importance of photoprotection as well as making a concerted effort to integrate our knowledge in the fashion industry, in policies at schools, in the military, and in popular culture, we will undoubtedly come to agree that it is not unfashionable to wear a wide-brimmed hat, but it is unfashionable to risk developing skin cancer.
- Prevent skin cancer. American Academy of Dermatology website. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent. Accessed January 4, 2017.
- What can I do to reduce my risk of skin cancer? Centers for Disease Control and Prevention website. http://www.cdc.gov/cancer/skin/basic_info/prevention.htm. Accessed January 4, 2017.
- Cancer facts & figures 2016. American Cancer Society website. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed January 4, 2017.
- Diffey BL, Cheeseman J. Sun protection with hats. Br J Dermatol. 1992;127:10-12.
- Bray FN. Florida school boards restrict access to outdoor sun protection: an observational study. J Am Acad Dermatol. 2016;75:642-644.
- Yeung H, Luk KM, Chen SC. Focal photodamage on the occipital scalp. JAMA Dermatol. 2016;152:1060-1062.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Parkin DM, Mesher D, Sasieni P. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer. 2011;105(suppl 2):S66-S69.
- Casper K. Elementary schools consider “no hat no play policy.” Coolibar website. http://blog.coolibar.com/elementary-schools-consider-no-hat-no-play-policy/. Published March 27, 2012. Accessed January 4, 2017.
- Slip, slop, slap, seek & slide: Sid Seagull. SunSmart Victoria website. http://www.sunsmart.com.au/tools/videos/current-tv-campaigns/slip-slop-slap-seek-slide-sid-seagull.html. Accessed January 4, 2017.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns - a pilot study. Am J Prev Med. 2016;50:E62-E63.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Mil Med. 2015;180:26-31.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults [published online May 19, 2015]. J Am Acad Dermatol. 2015;73:83-92.e1.
- Stanton SL. Headgear. In: Stanton SL. U.S. Army Uniforms of the Vietnam War. Harrisburg, PA: Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population [published online July 31, 2008]. J Invest Dermatol. 2009;129:323-328.
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Winterhalter C, DiLuna K, Bide M. Characterization of the Ultraviolet Protection of Combat Uniform Fabrics. Natick, MA: US Army Solider and Biological Chemical Command; 2002. Technical report 02/006.
- Prevent skin cancer. American Academy of Dermatology website. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent. Accessed January 4, 2017.
- What can I do to reduce my risk of skin cancer? Centers for Disease Control and Prevention website. http://www.cdc.gov/cancer/skin/basic_info/prevention.htm. Accessed January 4, 2017.
- Cancer facts & figures 2016. American Cancer Society website. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed January 4, 2017.
- Diffey BL, Cheeseman J. Sun protection with hats. Br J Dermatol. 1992;127:10-12.
- Bray FN. Florida school boards restrict access to outdoor sun protection: an observational study. J Am Acad Dermatol. 2016;75:642-644.
- Yeung H, Luk KM, Chen SC. Focal photodamage on the occipital scalp. JAMA Dermatol. 2016;152:1060-1062.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Parkin DM, Mesher D, Sasieni P. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer. 2011;105(suppl 2):S66-S69.
- Casper K. Elementary schools consider “no hat no play policy.” Coolibar website. http://blog.coolibar.com/elementary-schools-consider-no-hat-no-play-policy/. Published March 27, 2012. Accessed January 4, 2017.
- Slip, slop, slap, seek & slide: Sid Seagull. SunSmart Victoria website. http://www.sunsmart.com.au/tools/videos/current-tv-campaigns/slip-slop-slap-seek-slide-sid-seagull.html. Accessed January 4, 2017.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns - a pilot study. Am J Prev Med. 2016;50:E62-E63.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Mil Med. 2015;180:26-31.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults [published online May 19, 2015]. J Am Acad Dermatol. 2015;73:83-92.e1.
- Stanton SL. Headgear. In: Stanton SL. U.S. Army Uniforms of the Vietnam War. Harrisburg, PA: Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population [published online July 31, 2008]. J Invest Dermatol. 2009;129:323-328.
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Winterhalter C, DiLuna K, Bide M. Characterization of the Ultraviolet Protection of Combat Uniform Fabrics. Natick, MA: US Army Solider and Biological Chemical Command; 2002. Technical report 02/006.
Practice Points
- Routine wear of wide-brimmed hats is the simplest, most inexpensive, and only reusable form of photoprotection for the head and neck and should be an everyday practice for reducing the risk for preventable skin cancers.
- The regular wear of clothing and head cover with adequate UV protection factor is equally as important to utilize in the prevention of UV-induced skin cancers as the application of topical sunscreens and sunblocks.
- The medical community should make a concerted effort to dispel any public policy or fashion trend that does not promote personal protection from sun-induced skin cancers. Policies that restrict wearing photoprotective garments, such as in schools and in the military, need to be changed.
Psoriasis Treatment Considerations in Military Patients: Unique Patients, Unique Drugs
Psoriasis is a common dermatologic problem with nearly 5% prevalence in the United States. There is a bimodal distribution with peak onset between 20 and 30 years of age and 50 and 60 years, which means that this condition can arise before, during, or after military service.1 Unfortunately, for many prospective recruits psoriasis is a medically disqualifying condition that can prevent entry into active duty unless a medical waiver is granted. For active-duty military, new-onset psoriasis and its treatment can impair affected service members’ ability to perform mission-critical work and can prevent them from deploying to remote or austere locations. In this way, psoriasis presents a unique challenge for active-duty service members.
Many therapies are available that can effectively treat psoriasis, but these treatments often carry a side-effect profile that limits their use during travel or in austere settings. Herein, we discuss the unique challenges of treating psoriasis patients who are in the military at a time when global mobility is critical to mission success. Although in some ways these challenges truly are unique to the military population, we strongly believe that similar but perhaps underappreciated challenges exist in the civilian sector. Close examination of these challenges may reveal that alternative treatment choices are sometimes indicated for reasons beyond just efficacy, side-effect profile, and cost.
Treatment Considerations
The medical treatment of psoriasis has undergone substantial change in recent decades. Before the turn of the century, the mainstays of medical treatment were steroids, methotrexate, and phototherapy. Today, a wide array of biologics and other systemic drugs are altering the impact of psoriasis in our society. With so many treatment options currently available, the question becomes, “Which one is best for my patient?” Immediate considerations are efficacy versus side effects as well as cost; however, in military dermatology, the ability to store, transport, and administer the treatment can be just as important.
Although these problems may at first seem unique to active-duty military members, they also affect a substantial segment of the civilian sector. Take for instance the government contractor who deploys in support of military contingency actions, or the foreign aid workers, international businessmen, and diplomats around the world. In fact, any person who travels extensively might have difficulty carrying and storing their medications (Table) or encounter barriers that prevent routine access to care. Travel also may increase the risk of exposure to virulent pathogens such as Mycobacterium tuberculosis, which may further limit treatment options. This group of world travelers together comprises a minority of psoriasis patients who may be better treated with novel agents rather than with what might be considered the standard of care in a domestic setting.
Options for Care
Methotrexate
In many ways, methotrexate is the gold standard of psoriasis treatment. It is a first-line medication for many patients because it is typically well tolerated, has well-established efficacy, is easy to administer, and is relatively inexpensive.12 Although it is easy to store, transport, and administer, it requires regular laboratory monitoring at 3-month intervals or more frequently with dosage changes. It also is contraindicated in women of childbearing age who plan to become pregnant, which can be a considerable hindrance in the young active-duty population.
Cyclosporine
Cyclosporine is another inexpensive medication that can produce excellent results in the treatment of psoriasis.1,12 Although long-term use of cyclosporine in transplant patients has been well studied, its use for the treatment of dermatologic conditions is usually limited to 1 year. The need for monthly blood pressure checks and at least quarterly laboratory monitoring means it is not an optimal choice for a deployed service member.
Acitretin
Acitretin is another systemic medication with an established track record in psoriasis treatment. Although close follow-up and laboratory monitoring is required for both males and females, use of this medication can have a greater effect on women of childbearing age, as it is absolutely contraindicated in any female trying to conceive.13 In addition, acitretin is stored in fat cells, and traces of the drug can be found in the blood for up to 3 years. During this period, patients are advised to strictly avoid pregnancy and are even restricted from donating blood.13 Given these concerns, acitretin is not always a reasonable treatment option for the military service member.
Biologics
Biologics are the newest agents in the treatment of psoriasis. They require less laboratory monitoring and can provide excellent results. Adalimumab is a reasonable first-line biologic treatment for some patients. We find the laboratory monitoring is minimally obtrusive, side effects usually are limited, and the efficacy is great enough that most patients elect to continue treatment. Unfortunately, adalimumab has some major drawbacks in our specific use scenario in that it requires nearly continuous refrigeration and is never to exceed 25°C (77°F), it has a relatively close-interval dosing schedule, and it can cause immunosuppression. However, for short trips to nonaustere locations with an acceptable risk for pathogenic exposure, adalimumab may remain a viable option for many travelers, as it can be stored at room temperature for up to 14 days.2 Ustekinumab also is a reasonable choice for many travelers because dosing is every 12 weeks and it carries a lower risk of immunosuppression.2,3 Ustekinumab, however, has the major drawback of high cost.12 Newer IL-17A inhibitors such as secukinumab or ixekizumab also can offer excellent results, but long-term infection rates have not been reported. Overall, the infection rates are comparable to ustekinumab.14,15 After the loading phase, secukinumab is dosed monthly and logistically could still pose a problem due to the need for continued refrigeration.14
Apremilast
Although it is not the best first-line treatment for every patient, apremilast carries 3 distinct advantages in treating the military patient population: (1) laboratory monitoring is required only once per year, (2) it is easy to store, and (3) it is easy to administer. However, the major downside is that apremilast is less effective than other systemic agents in the treatment of psoriasis.16 As with other systemic drugs, adjunctive topical treatment can provide additional therapeutic effects, and for many patients, this combined approach is sufficient to reach their therapeutic goals.
For these reasons, in the special case of deployable, active-duty military members we often consider starting treatment with apremilast versus other systemic agents. As with all systemic psoriasis treatments, we generally advise patients to return 16 weeks after initiating treatment to assess efficacy and evaluate their deployment status. Although apremilast may take longer to reach full efficacy than many other systemic agents, one clinical trial suggested this time frame is sufficient to evaluate response to treatment.16 After this initial assessment, we revert to yearly monitoring, and the patient is usually cleared to deploy with minimal restrictions.
Final Considerations
The manifestation of psoriasis is different in every patient, and military service poses additional treatment challenges. For all of our military patients, we recommend an initial period of close follow-up after starting any new systemic agent, which is necessary to ensure the treatment is effective and well tolerated and also that we are good stewards of our resources. Once efficacy is established and side effects remain tolerable, we generally endorse continued treatment without specific travel or work restrictions.
We are cognizant of the unique nature of military service, and all too often we find ourselves trying to practice good medicine in bad places. As military physicians, we serve a population that is eager to do their job and willing to make incredible sacrifices to do so. After considering the wide range of circumstances unique to the military, our responsibility as providers is to do our best to improve service members’ quality of life as they carry out their missions.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Stelara [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
- Humira [package insert]. North Chicago, IL: AbbVie Inc; 2007.
- Cosentyx [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016.
- Otezla [package insert]. Summit, NJ: Celgene Corporation; 2014.
- Enbrel [package insert]. Thousand Oaks, CA: Amgen; 2015.
- Taltz [package insert]. Indianapolis, IN: Eli Lilly and Company; 2016.
- Methotrexate [package insert]. Morgantown, WV: Mylan Pharmaceuticals Inc; 2016.
- Gengraf [package insert]. North Chicago, IL: Abbvie Inc; 2015.
- Acitretin [package insert]. Mason, OH: Prasco Laboratories; 2015.
- Beyer V, Wolverton SE. Recent trends in systemic psoriasis treatment costs. Arch Dermatol. 2010;146:46-54.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis [published online June 8, 2016]. N Engl J Med. 2016;375:345-356.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, anoral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
Psoriasis is a common dermatologic problem with nearly 5% prevalence in the United States. There is a bimodal distribution with peak onset between 20 and 30 years of age and 50 and 60 years, which means that this condition can arise before, during, or after military service.1 Unfortunately, for many prospective recruits psoriasis is a medically disqualifying condition that can prevent entry into active duty unless a medical waiver is granted. For active-duty military, new-onset psoriasis and its treatment can impair affected service members’ ability to perform mission-critical work and can prevent them from deploying to remote or austere locations. In this way, psoriasis presents a unique challenge for active-duty service members.
Many therapies are available that can effectively treat psoriasis, but these treatments often carry a side-effect profile that limits their use during travel or in austere settings. Herein, we discuss the unique challenges of treating psoriasis patients who are in the military at a time when global mobility is critical to mission success. Although in some ways these challenges truly are unique to the military population, we strongly believe that similar but perhaps underappreciated challenges exist in the civilian sector. Close examination of these challenges may reveal that alternative treatment choices are sometimes indicated for reasons beyond just efficacy, side-effect profile, and cost.
Treatment Considerations
The medical treatment of psoriasis has undergone substantial change in recent decades. Before the turn of the century, the mainstays of medical treatment were steroids, methotrexate, and phototherapy. Today, a wide array of biologics and other systemic drugs are altering the impact of psoriasis in our society. With so many treatment options currently available, the question becomes, “Which one is best for my patient?” Immediate considerations are efficacy versus side effects as well as cost; however, in military dermatology, the ability to store, transport, and administer the treatment can be just as important.
Although these problems may at first seem unique to active-duty military members, they also affect a substantial segment of the civilian sector. Take for instance the government contractor who deploys in support of military contingency actions, or the foreign aid workers, international businessmen, and diplomats around the world. In fact, any person who travels extensively might have difficulty carrying and storing their medications (Table) or encounter barriers that prevent routine access to care. Travel also may increase the risk of exposure to virulent pathogens such as Mycobacterium tuberculosis, which may further limit treatment options. This group of world travelers together comprises a minority of psoriasis patients who may be better treated with novel agents rather than with what might be considered the standard of care in a domestic setting.

Options for Care
Methotrexate
In many ways, methotrexate is the gold standard of psoriasis treatment. It is a first-line medication for many patients because it is typically well tolerated, has well-established efficacy, is easy to administer, and is relatively inexpensive.12 Although it is easy to store, transport, and administer, it requires regular laboratory monitoring at 3-month intervals or more frequently with dosage changes. It also is contraindicated in women of childbearing age who plan to become pregnant, which can be a considerable hindrance in the young active-duty population.
Cyclosporine
Cyclosporine is another inexpensive medication that can produce excellent results in the treatment of psoriasis.1,12 Although long-term use of cyclosporine in transplant patients has been well studied, its use for the treatment of dermatologic conditions is usually limited to 1 year. The need for monthly blood pressure checks and at least quarterly laboratory monitoring means it is not an optimal choice for a deployed service member.
Acitretin
Acitretin is another systemic medication with an established track record in psoriasis treatment. Although close follow-up and laboratory monitoring is required for both males and females, use of this medication can have a greater effect on women of childbearing age, as it is absolutely contraindicated in any female trying to conceive.13 In addition, acitretin is stored in fat cells, and traces of the drug can be found in the blood for up to 3 years. During this period, patients are advised to strictly avoid pregnancy and are even restricted from donating blood.13 Given these concerns, acitretin is not always a reasonable treatment option for the military service member.
Biologics
Biologics are the newest agents in the treatment of psoriasis. They require less laboratory monitoring and can provide excellent results. Adalimumab is a reasonable first-line biologic treatment for some patients. We find the laboratory monitoring is minimally obtrusive, side effects usually are limited, and the efficacy is great enough that most patients elect to continue treatment. Unfortunately, adalimumab has some major drawbacks in our specific use scenario in that it requires nearly continuous refrigeration and is never to exceed 25°C (77°F), it has a relatively close-interval dosing schedule, and it can cause immunosuppression. However, for short trips to nonaustere locations with an acceptable risk for pathogenic exposure, adalimumab may remain a viable option for many travelers, as it can be stored at room temperature for up to 14 days.2 Ustekinumab also is a reasonable choice for many travelers because dosing is every 12 weeks and it carries a lower risk of immunosuppression.2,3 Ustekinumab, however, has the major drawback of high cost.12 Newer IL-17A inhibitors such as secukinumab or ixekizumab also can offer excellent results, but long-term infection rates have not been reported. Overall, the infection rates are comparable to ustekinumab.14,15 After the loading phase, secukinumab is dosed monthly and logistically could still pose a problem due to the need for continued refrigeration.14
Apremilast
Although it is not the best first-line treatment for every patient, apremilast carries 3 distinct advantages in treating the military patient population: (1) laboratory monitoring is required only once per year, (2) it is easy to store, and (3) it is easy to administer. However, the major downside is that apremilast is less effective than other systemic agents in the treatment of psoriasis.16 As with other systemic drugs, adjunctive topical treatment can provide additional therapeutic effects, and for many patients, this combined approach is sufficient to reach their therapeutic goals.
For these reasons, in the special case of deployable, active-duty military members we often consider starting treatment with apremilast versus other systemic agents. As with all systemic psoriasis treatments, we generally advise patients to return 16 weeks after initiating treatment to assess efficacy and evaluate their deployment status. Although apremilast may take longer to reach full efficacy than many other systemic agents, one clinical trial suggested this time frame is sufficient to evaluate response to treatment.16 After this initial assessment, we revert to yearly monitoring, and the patient is usually cleared to deploy with minimal restrictions.
Final Considerations
The manifestation of psoriasis is different in every patient, and military service poses additional treatment challenges. For all of our military patients, we recommend an initial period of close follow-up after starting any new systemic agent, which is necessary to ensure the treatment is effective and well tolerated and also that we are good stewards of our resources. Once efficacy is established and side effects remain tolerable, we generally endorse continued treatment without specific travel or work restrictions.
We are cognizant of the unique nature of military service, and all too often we find ourselves trying to practice good medicine in bad places. As military physicians, we serve a population that is eager to do their job and willing to make incredible sacrifices to do so. After considering the wide range of circumstances unique to the military, our responsibility as providers is to do our best to improve service members’ quality of life as they carry out their missions.
Psoriasis is a common dermatologic problem with nearly 5% prevalence in the United States. There is a bimodal distribution with peak onset between 20 and 30 years of age and 50 and 60 years, which means that this condition can arise before, during, or after military service.1 Unfortunately, for many prospective recruits psoriasis is a medically disqualifying condition that can prevent entry into active duty unless a medical waiver is granted. For active-duty military, new-onset psoriasis and its treatment can impair affected service members’ ability to perform mission-critical work and can prevent them from deploying to remote or austere locations. In this way, psoriasis presents a unique challenge for active-duty service members.
Many therapies are available that can effectively treat psoriasis, but these treatments often carry a side-effect profile that limits their use during travel or in austere settings. Herein, we discuss the unique challenges of treating psoriasis patients who are in the military at a time when global mobility is critical to mission success. Although in some ways these challenges truly are unique to the military population, we strongly believe that similar but perhaps underappreciated challenges exist in the civilian sector. Close examination of these challenges may reveal that alternative treatment choices are sometimes indicated for reasons beyond just efficacy, side-effect profile, and cost.
Treatment Considerations
The medical treatment of psoriasis has undergone substantial change in recent decades. Before the turn of the century, the mainstays of medical treatment were steroids, methotrexate, and phototherapy. Today, a wide array of biologics and other systemic drugs are altering the impact of psoriasis in our society. With so many treatment options currently available, the question becomes, “Which one is best for my patient?” Immediate considerations are efficacy versus side effects as well as cost; however, in military dermatology, the ability to store, transport, and administer the treatment can be just as important.
Although these problems may at first seem unique to active-duty military members, they also affect a substantial segment of the civilian sector. Take for instance the government contractor who deploys in support of military contingency actions, or the foreign aid workers, international businessmen, and diplomats around the world. In fact, any person who travels extensively might have difficulty carrying and storing their medications (Table) or encounter barriers that prevent routine access to care. Travel also may increase the risk of exposure to virulent pathogens such as Mycobacterium tuberculosis, which may further limit treatment options. This group of world travelers together comprises a minority of psoriasis patients who may be better treated with novel agents rather than with what might be considered the standard of care in a domestic setting.

Options for Care
Methotrexate
In many ways, methotrexate is the gold standard of psoriasis treatment. It is a first-line medication for many patients because it is typically well tolerated, has well-established efficacy, is easy to administer, and is relatively inexpensive.12 Although it is easy to store, transport, and administer, it requires regular laboratory monitoring at 3-month intervals or more frequently with dosage changes. It also is contraindicated in women of childbearing age who plan to become pregnant, which can be a considerable hindrance in the young active-duty population.
Cyclosporine
Cyclosporine is another inexpensive medication that can produce excellent results in the treatment of psoriasis.1,12 Although long-term use of cyclosporine in transplant patients has been well studied, its use for the treatment of dermatologic conditions is usually limited to 1 year. The need for monthly blood pressure checks and at least quarterly laboratory monitoring means it is not an optimal choice for a deployed service member.
Acitretin
Acitretin is another systemic medication with an established track record in psoriasis treatment. Although close follow-up and laboratory monitoring is required for both males and females, use of this medication can have a greater effect on women of childbearing age, as it is absolutely contraindicated in any female trying to conceive.13 In addition, acitretin is stored in fat cells, and traces of the drug can be found in the blood for up to 3 years. During this period, patients are advised to strictly avoid pregnancy and are even restricted from donating blood.13 Given these concerns, acitretin is not always a reasonable treatment option for the military service member.
Biologics
Biologics are the newest agents in the treatment of psoriasis. They require less laboratory monitoring and can provide excellent results. Adalimumab is a reasonable first-line biologic treatment for some patients. We find the laboratory monitoring is minimally obtrusive, side effects usually are limited, and the efficacy is great enough that most patients elect to continue treatment. Unfortunately, adalimumab has some major drawbacks in our specific use scenario in that it requires nearly continuous refrigeration and is never to exceed 25°C (77°F), it has a relatively close-interval dosing schedule, and it can cause immunosuppression. However, for short trips to nonaustere locations with an acceptable risk for pathogenic exposure, adalimumab may remain a viable option for many travelers, as it can be stored at room temperature for up to 14 days.2 Ustekinumab also is a reasonable choice for many travelers because dosing is every 12 weeks and it carries a lower risk of immunosuppression.2,3 Ustekinumab, however, has the major drawback of high cost.12 Newer IL-17A inhibitors such as secukinumab or ixekizumab also can offer excellent results, but long-term infection rates have not been reported. Overall, the infection rates are comparable to ustekinumab.14,15 After the loading phase, secukinumab is dosed monthly and logistically could still pose a problem due to the need for continued refrigeration.14
Apremilast
Although it is not the best first-line treatment for every patient, apremilast carries 3 distinct advantages in treating the military patient population: (1) laboratory monitoring is required only once per year, (2) it is easy to store, and (3) it is easy to administer. However, the major downside is that apremilast is less effective than other systemic agents in the treatment of psoriasis.16 As with other systemic drugs, adjunctive topical treatment can provide additional therapeutic effects, and for many patients, this combined approach is sufficient to reach their therapeutic goals.
For these reasons, in the special case of deployable, active-duty military members we often consider starting treatment with apremilast versus other systemic agents. As with all systemic psoriasis treatments, we generally advise patients to return 16 weeks after initiating treatment to assess efficacy and evaluate their deployment status. Although apremilast may take longer to reach full efficacy than many other systemic agents, one clinical trial suggested this time frame is sufficient to evaluate response to treatment.16 After this initial assessment, we revert to yearly monitoring, and the patient is usually cleared to deploy with minimal restrictions.
Final Considerations
The manifestation of psoriasis is different in every patient, and military service poses additional treatment challenges. For all of our military patients, we recommend an initial period of close follow-up after starting any new systemic agent, which is necessary to ensure the treatment is effective and well tolerated and also that we are good stewards of our resources. Once efficacy is established and side effects remain tolerable, we generally endorse continued treatment without specific travel or work restrictions.
We are cognizant of the unique nature of military service, and all too often we find ourselves trying to practice good medicine in bad places. As military physicians, we serve a population that is eager to do their job and willing to make incredible sacrifices to do so. After considering the wide range of circumstances unique to the military, our responsibility as providers is to do our best to improve service members’ quality of life as they carry out their missions.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Stelara [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
- Humira [package insert]. North Chicago, IL: AbbVie Inc; 2007.
- Cosentyx [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016.
- Otezla [package insert]. Summit, NJ: Celgene Corporation; 2014.
- Enbrel [package insert]. Thousand Oaks, CA: Amgen; 2015.
- Taltz [package insert]. Indianapolis, IN: Eli Lilly and Company; 2016.
- Methotrexate [package insert]. Morgantown, WV: Mylan Pharmaceuticals Inc; 2016.
- Gengraf [package insert]. North Chicago, IL: Abbvie Inc; 2015.
- Acitretin [package insert]. Mason, OH: Prasco Laboratories; 2015.
- Beyer V, Wolverton SE. Recent trends in systemic psoriasis treatment costs. Arch Dermatol. 2010;146:46-54.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis [published online June 8, 2016]. N Engl J Med. 2016;375:345-356.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, anoral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Stelara [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
- Humira [package insert]. North Chicago, IL: AbbVie Inc; 2007.
- Cosentyx [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016.
- Otezla [package insert]. Summit, NJ: Celgene Corporation; 2014.
- Enbrel [package insert]. Thousand Oaks, CA: Amgen; 2015.
- Taltz [package insert]. Indianapolis, IN: Eli Lilly and Company; 2016.
- Methotrexate [package insert]. Morgantown, WV: Mylan Pharmaceuticals Inc; 2016.
- Gengraf [package insert]. North Chicago, IL: Abbvie Inc; 2015.
- Acitretin [package insert]. Mason, OH: Prasco Laboratories; 2015.
- Beyer V, Wolverton SE. Recent trends in systemic psoriasis treatment costs. Arch Dermatol. 2010;146:46-54.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis [published online June 8, 2016]. N Engl J Med. 2016;375:345-356.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, anoral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
Practice Points
- Establishing goals of treatment with each patient is a critical step in treating the patient rather than the diagnosis.
- A good social history can reveal job-related impact of disease and potential logistical roadblocks to treatment.
- Efficacy must be weighed against the burden of logistical constraints for each patient; potential issues include difficulty complying with follow-up visits, access to laboratory monitoring, exposure to pathogens, and adequacy of medication transport and storage.
Exploration of Modern Military Research Resources
Advances in medical biotechnologies, data-gathering techniques, and -omics technologies have resulted in the broader understanding of disease pathology and treatment and have facilitated the individualization of health care plans to meet the unique needs of each patient. Military medicine often has been on the forefront of medical technology, disease understanding, and clinical care both on and off the battlefield, in large part due to the unique resources available in the military health care system. These resources allow investigators the ability to integrate vast amounts of epidemiologic data with an extensive biological sample database of its service members, which in the modern age has translated into advances in the understanding of melanoma and the treatment of scars.
History of Research in the Military
Starting in the 1950s, the US Department of Defense (DoD) started to collect serum samples of its service members for the purpose of research.1 It was not until 1985 that the DoD implemented a long-term frozen storage system for serum samples obtained through mandatory screening for human immunodeficiency virus (HIV) in service members.2 Subsequently, the Department of Defense Serum Repository (DoDSR) was officially established in 1989 as a central archive for the long-term storage of serum obtained from active-duty and reserve service members in the US Navy, Army, and Marines.2,3 In the mid-1990s, the DoDSR expanded its capabilities to include the storage of serum samples from all military members, including the US Air Force, obtained predeployment and postdeployment.3,4 At that time, a records-keeping system was established, now known as the Defense Medical Surveillance System (DMSS). The creation of the DMSS provided an extensive epidemiologic database that provided valuable information such as demographic data, service records, deployment data, reportable medical events, exposure history, and vaccination records, which could be linked to the serum samples of each service member.2-4 Since 2008, the responsibilities of maintaining the DoDSR and the DMSS were transferred to the Armed Forces Health Surveillance Center (AFHSC).5
There have been several other databases created over the years that provide additional support and resources to military investigators. The Automated Central Tumor Registry and Department of Pathology and Area Laboratory Services both help investigators to track the incidence of specific cancers in the military population and provide them with pathologic specimens. Additionally, electronic medical records including the composite health care system and the Armed Forces Health Longitudinal Technology Application supplemented with insurance claims data accessible from the Military Health System Management and Reporting Tool (M2) database have made it possible to track patient data.
Utilization of Military Research Resources
Today, the DoDSR is a secure facility that maintains more than 56 million serum specimens from more than 11 million individuals in –30°C freezers, making it one of the largest repositories in the world.3,6 Each serum sample is linked with an individual’s DMSS record, providing a way for investigators to study how external factors such as deployment history, occupation, and exposure history relate to an individual’s unique genetic and physiological makeup. Furthermore, these data can be used for seroepidemiologic investigations that contribute to all facets of clinical care. The AFHSC routinely publishes findings related to notifiable diseases, disease outbreaks, and disease trends in a monthly report.7
There are strict guidelines in place that limit access to the DoDSR and service members’ data. Use of the repository for information directly related to a patient’s health care is one reason for access, such as analyzing serum for antibodies and seroconversion to assist in the diagnosis of a disease such as HIV. Another reason would be to obtain information needed for criminal investigations and prosecution. Typically, these types of requests require a judge-issued court order and approval by the Assistant Secretary of Defense for Health Affairs.4 The DoDSR also is used to study force health protection issues, such as infectious disease incidence and disease prevalence in the military population.
Obtaining access to the DoDSR and service members’ data for research purposes requires that the principal investigator be a DoD employee. Each research proposal is reviewed by members of the AFHSC to determine if the DoDSR is able to meet the demands of the project, including having the appropriate number of serum samples and supporting epidemiologic data available. The AFHSC provides a letter of support if it deems the project to be in line with its current resources and capabilities. Each research proposal is then sent to an institutional review board (IRB) to determine if the study is exempt or needs to go through a full IRB review process. A study might be exempt if the investigators are not obtaining data through interaction with living individuals or not having access to any identifiable protected health information associated with the samples.6 Regardless of whether the study is exempt or not exempt, the AFHSC will de-identify each sample before releasing the samples to the investigators by using a coding system to shield the patient’s identity from the investigator.
Resources within the military medical research system provide investigators with access to an extensive biorepository of serum and linked epidemiological data. Samples from the DoDSR have been used in no less than 75 peer-reviewed publications since 1985.8,9 Several of these studies have been influential in expanding knowledge about conditions seen more commonly in the military population such as stress fractures, traumatic brain injuries, posttraumatic stress disorder, and suicide.8 Additionally, DoDSR samples have been used to form military vaccination policies and track both infectious and noninfectious conditions in the military; for example, during the H1N1 influenza virus outbreak of 2009, AFHSC was essential in helping to limit the spread of the virus within the military community by using its data and collaborating with groups such as the Centers for Disease Control and Prevention to develop a plan for disease surveillance and control.5
Several military research resources are currently being used for a melanoma study that aims to assess if specific phenotypic features, melanoma risk alleles, and environmental factors (eg, duty station location, occupation, amount of UV exposure) can be used to develop better screening models to identify individuals who are at risk for developing melanoma. Secondarily, the study aims to determine if recently developed multimarker diagnostic and prognostic assays for melanoma will prove useful in the diagnostic and prognostic assessment of melanocytic neoplasms in the military population. For this study, one of the authors (J.H.M) is utilizing DoDSR serum from 1700 retrospective cases of invasive melanoma and 1700 matched controls. Additionally, the Automated Central Tumor Registry and Department of Pathology and Area Laboratory Services databases are being used to obtain tissue from more than 300 melanoma cases and nevi controls.
Limitations of the Current System
Despite the impressive capabilities of the current system, there are some issues that limit its potential. One such limitation is associated with the way that the serum samples at the DoDSR are utilized. Through 2012, the DoDSR had 54,542,658 serum specimens available, of which only 228,610 (0.42%) had ever been accessed for study.8 With such a wealth of information and relative availability, why are the serum samples not being accessed more frequently for studies? The inherent nature of the DoDSR being a restricted facility and only accessible to DoD-affiliated investigators may contribute, which allows the DoDSR to fulfill its primary purpose of contributing to military-relevant investigations but at the same time limits the number and type of investigations that can be performed. One idea that has been proposed is allowing civilian investigator access to the DoDSR if it can be proven that the research is targeted toward military-relevant issues.8 However, the current AFHSC access guidelines would need revision and would require additional safeguards to ensure that military-protected health information is not compromised. Nonetheless, such a change may result in more extensive use of DoDSR resources in the future.
An ethical issue that needs to be addressed pertains to how the DoDSR permits use of human serum samples for research purposes without getting consent from the individuals being studied. The serum samples are collected as part of mandatory predeployment and postdeployment examinations for HIV screening of all military members. These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Although it is true that military members must comply with specific requirements pertaining to military readiness (eg, receiving appropriate vaccinations, drug testing, regular medical screening), it is debated whether they still retain the right as patients to refuse participating in research and clinical trials.10 The AFHSC does have several regulatory steps in place to ensure that military members’ samples are used in an appropriate manner, including requiring a DoD primary investigator, IRB review of every research proposal, and de-identification of samples. At a minimum, giving military members the ability to provide informed consent would ensure that the military system is adhering to evolving human research standards.
The current lack of biological specimens other than serum in the DoDSR is another limitation of the current system. Recent advances in molecular analyses are impacted by expanding -omics techniques, such as epigenomics, transcriptomics, and proteomics. The field of epigenomics is the study of reversible changes to DNA (eg, methylation) associated with specific disease states or following specific environmental exposures.9,11 Transcriptomics, which analyzes messenger RNA transcript levels of expressed genes, and proteomics, which uses expression of proteins, are 2 techniques being used to develop biomarkers associated with specific diseases and environmental exposures.9,11 Serum alone does not provide the high-quality nucleic acids needed for many of these studies to take place. Adding whole-blood specimens or blood spot samples of military service members to the DoDSR would allow researchers to use these techniques to investigate many new biomarkers associated with military-relevant diseases and exposures. These techniques also can be used in the expanding field of personalized medicine so that health care providers are able to tailor all phases of care, including diagnosis and treatment, to an individual’s genetic profile.
Conclusion
The history of research in military medicine has been built on achieving the primary goal of serving those men and women who put their lives in danger to protect this country. In an evolving environment of new technologies that have led to changes in service members’ injuries, exposures, and diseases, military medicine also must adapt. Resources such as the DoDSR and DMSS, which provide investigators with the unique ability to link epidemiological data with serum samples, have been invaluable contributors to this overall mission. As with any large system, there are always improvements that can be made. Improving access to the DoDSR serum samples, educating and obtaining consent from military service members to use their samples in research, and adding specimens to the DoDSR that can be used for -omics techniques are 3 changes that should be considered to maximize
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Rubertone MV, Brundage JF. The defense medical surveillance system and the department of defense serum repository: glimpses of the future of public health surveillance. Am J Public Health. 2002;92:1900-1904.
- Department of Defense Serum Repository. Military Health System and the Defense Health Agency website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Department-of-Defense-Serum-Repository. Accessed August 2, 2016.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD serum repository. Mil Med. 2015;180(10 suppl):10-12.
- DeFraites RF. The Armed Forces Health Surveillance Center: enhancing the Military Health System’s public health capabilities. BMC Public Health. 2011;11(suppl 2):S1.
- Pavlin JA, Welch RA. Ethics, human use, and the department of defense serum repository. Mil Med. 2015;180:49-56.
- Defense Medical Surveillance System. Military Health System and the Defense Health Agency website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Surveillance-System. Accessed August 2, 2016.
- Perdue CL, Eick-Cost AA, Rubertone MV, et al. Description and utilization of the United States Department of Defense Serum Repository: a review of published studies, 1985-2012. Plos One. 2015;10:1-16.
- Mancuso JD, Mallon TM, Gaydos JC. Maximizing the capabilities of the DoD serum repository to meet current and future needs: report of the needs panel. Mil Med. 2015;180:14-24.
- Department of Defense. Department of Defense Instruction. http://www.dtic.mil/whs/directives/corres/pdf/600014p.pdf. Posted September 26, 2001. Updated October 3, 2013. Accessed August 2, 2016.
- Lindler LE. Building a DoD biorepository for the future: potential benefits and way forward. Mil Med. 2015;180:90-94.
Advances in medical biotechnologies, data-gathering techniques, and -omics technologies have resulted in the broader understanding of disease pathology and treatment and have facilitated the individualization of health care plans to meet the unique needs of each patient. Military medicine often has been on the forefront of medical technology, disease understanding, and clinical care both on and off the battlefield, in large part due to the unique resources available in the military health care system. These resources allow investigators the ability to integrate vast amounts of epidemiologic data with an extensive biological sample database of its service members, which in the modern age has translated into advances in the understanding of melanoma and the treatment of scars.
History of Research in the Military
Starting in the 1950s, the US Department of Defense (DoD) started to collect serum samples of its service members for the purpose of research.1 It was not until 1985 that the DoD implemented a long-term frozen storage system for serum samples obtained through mandatory screening for human immunodeficiency virus (HIV) in service members.2 Subsequently, the Department of Defense Serum Repository (DoDSR) was officially established in 1989 as a central archive for the long-term storage of serum obtained from active-duty and reserve service members in the US Navy, Army, and Marines.2,3 In the mid-1990s, the DoDSR expanded its capabilities to include the storage of serum samples from all military members, including the US Air Force, obtained predeployment and postdeployment.3,4 At that time, a records-keeping system was established, now known as the Defense Medical Surveillance System (DMSS). The creation of the DMSS provided an extensive epidemiologic database that provided valuable information such as demographic data, service records, deployment data, reportable medical events, exposure history, and vaccination records, which could be linked to the serum samples of each service member.2-4 Since 2008, the responsibilities of maintaining the DoDSR and the DMSS were transferred to the Armed Forces Health Surveillance Center (AFHSC).5
There have been several other databases created over the years that provide additional support and resources to military investigators. The Automated Central Tumor Registry and Department of Pathology and Area Laboratory Services both help investigators to track the incidence of specific cancers in the military population and provide them with pathologic specimens. Additionally, electronic medical records including the composite health care system and the Armed Forces Health Longitudinal Technology Application supplemented with insurance claims data accessible from the Military Health System Management and Reporting Tool (M2) database have made it possible to track patient data.
Utilization of Military Research Resources
Today, the DoDSR is a secure facility that maintains more than 56 million serum specimens from more than 11 million individuals in –30°C freezers, making it one of the largest repositories in the world.3,6 Each serum sample is linked with an individual’s DMSS record, providing a way for investigators to study how external factors such as deployment history, occupation, and exposure history relate to an individual’s unique genetic and physiological makeup. Furthermore, these data can be used for seroepidemiologic investigations that contribute to all facets of clinical care. The AFHSC routinely publishes findings related to notifiable diseases, disease outbreaks, and disease trends in a monthly report.7
There are strict guidelines in place that limit access to the DoDSR and service members’ data. Use of the repository for information directly related to a patient’s health care is one reason for access, such as analyzing serum for antibodies and seroconversion to assist in the diagnosis of a disease such as HIV. Another reason would be to obtain information needed for criminal investigations and prosecution. Typically, these types of requests require a judge-issued court order and approval by the Assistant Secretary of Defense for Health Affairs.4 The DoDSR also is used to study force health protection issues, such as infectious disease incidence and disease prevalence in the military population.
Obtaining access to the DoDSR and service members’ data for research purposes requires that the principal investigator be a DoD employee. Each research proposal is reviewed by members of the AFHSC to determine if the DoDSR is able to meet the demands of the project, including having the appropriate number of serum samples and supporting epidemiologic data available. The AFHSC provides a letter of support if it deems the project to be in line with its current resources and capabilities. Each research proposal is then sent to an institutional review board (IRB) to determine if the study is exempt or needs to go through a full IRB review process. A study might be exempt if the investigators are not obtaining data through interaction with living individuals or not having access to any identifiable protected health information associated with the samples.6 Regardless of whether the study is exempt or not exempt, the AFHSC will de-identify each sample before releasing the samples to the investigators by using a coding system to shield the patient’s identity from the investigator.
Resources within the military medical research system provide investigators with access to an extensive biorepository of serum and linked epidemiological data. Samples from the DoDSR have been used in no less than 75 peer-reviewed publications since 1985.8,9 Several of these studies have been influential in expanding knowledge about conditions seen more commonly in the military population such as stress fractures, traumatic brain injuries, posttraumatic stress disorder, and suicide.8 Additionally, DoDSR samples have been used to form military vaccination policies and track both infectious and noninfectious conditions in the military; for example, during the H1N1 influenza virus outbreak of 2009, AFHSC was essential in helping to limit the spread of the virus within the military community by using its data and collaborating with groups such as the Centers for Disease Control and Prevention to develop a plan for disease surveillance and control.5
Several military research resources are currently being used for a melanoma study that aims to assess if specific phenotypic features, melanoma risk alleles, and environmental factors (eg, duty station location, occupation, amount of UV exposure) can be used to develop better screening models to identify individuals who are at risk for developing melanoma. Secondarily, the study aims to determine if recently developed multimarker diagnostic and prognostic assays for melanoma will prove useful in the diagnostic and prognostic assessment of melanocytic neoplasms in the military population. For this study, one of the authors (J.H.M) is utilizing DoDSR serum from 1700 retrospective cases of invasive melanoma and 1700 matched controls. Additionally, the Automated Central Tumor Registry and Department of Pathology and Area Laboratory Services databases are being used to obtain tissue from more than 300 melanoma cases and nevi controls.
Limitations of the Current System
Despite the impressive capabilities of the current system, there are some issues that limit its potential. One such limitation is associated with the way that the serum samples at the DoDSR are utilized. Through 2012, the DoDSR had 54,542,658 serum specimens available, of which only 228,610 (0.42%) had ever been accessed for study.8 With such a wealth of information and relative availability, why are the serum samples not being accessed more frequently for studies? The inherent nature of the DoDSR being a restricted facility and only accessible to DoD-affiliated investigators may contribute, which allows the DoDSR to fulfill its primary purpose of contributing to military-relevant investigations but at the same time limits the number and type of investigations that can be performed. One idea that has been proposed is allowing civilian investigator access to the DoDSR if it can be proven that the research is targeted toward military-relevant issues.8 However, the current AFHSC access guidelines would need revision and would require additional safeguards to ensure that military-protected health information is not compromised. Nonetheless, such a change may result in more extensive use of DoDSR resources in the future.
An ethical issue that needs to be addressed pertains to how the DoDSR permits use of human serum samples for research purposes without getting consent from the individuals being studied. The serum samples are collected as part of mandatory predeployment and postdeployment examinations for HIV screening of all military members. These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Although it is true that military members must comply with specific requirements pertaining to military readiness (eg, receiving appropriate vaccinations, drug testing, regular medical screening), it is debated whether they still retain the right as patients to refuse participating in research and clinical trials.10 The AFHSC does have several regulatory steps in place to ensure that military members’ samples are used in an appropriate manner, including requiring a DoD primary investigator, IRB review of every research proposal, and de-identification of samples. At a minimum, giving military members the ability to provide informed consent would ensure that the military system is adhering to evolving human research standards.
The current lack of biological specimens other than serum in the DoDSR is another limitation of the current system. Recent advances in molecular analyses are impacted by expanding -omics techniques, such as epigenomics, transcriptomics, and proteomics. The field of epigenomics is the study of reversible changes to DNA (eg, methylation) associated with specific disease states or following specific environmental exposures.9,11 Transcriptomics, which analyzes messenger RNA transcript levels of expressed genes, and proteomics, which uses expression of proteins, are 2 techniques being used to develop biomarkers associated with specific diseases and environmental exposures.9,11 Serum alone does not provide the high-quality nucleic acids needed for many of these studies to take place. Adding whole-blood specimens or blood spot samples of military service members to the DoDSR would allow researchers to use these techniques to investigate many new biomarkers associated with military-relevant diseases and exposures. These techniques also can be used in the expanding field of personalized medicine so that health care providers are able to tailor all phases of care, including diagnosis and treatment, to an individual’s genetic profile.
Conclusion
The history of research in military medicine has been built on achieving the primary goal of serving those men and women who put their lives in danger to protect this country. In an evolving environment of new technologies that have led to changes in service members’ injuries, exposures, and diseases, military medicine also must adapt. Resources such as the DoDSR and DMSS, which provide investigators with the unique ability to link epidemiological data with serum samples, have been invaluable contributors to this overall mission. As with any large system, there are always improvements that can be made. Improving access to the DoDSR serum samples, educating and obtaining consent from military service members to use their samples in research, and adding specimens to the DoDSR that can be used for -omics techniques are 3 changes that should be considered to maximize
Advances in medical biotechnologies, data-gathering techniques, and -omics technologies have resulted in the broader understanding of disease pathology and treatment and have facilitated the individualization of health care plans to meet the unique needs of each patient. Military medicine often has been on the forefront of medical technology, disease understanding, and clinical care both on and off the battlefield, in large part due to the unique resources available in the military health care system. These resources allow investigators the ability to integrate vast amounts of epidemiologic data with an extensive biological sample database of its service members, which in the modern age has translated into advances in the understanding of melanoma and the treatment of scars.
History of Research in the Military
Starting in the 1950s, the US Department of Defense (DoD) started to collect serum samples of its service members for the purpose of research.1 It was not until 1985 that the DoD implemented a long-term frozen storage system for serum samples obtained through mandatory screening for human immunodeficiency virus (HIV) in service members.2 Subsequently, the Department of Defense Serum Repository (DoDSR) was officially established in 1989 as a central archive for the long-term storage of serum obtained from active-duty and reserve service members in the US Navy, Army, and Marines.2,3 In the mid-1990s, the DoDSR expanded its capabilities to include the storage of serum samples from all military members, including the US Air Force, obtained predeployment and postdeployment.3,4 At that time, a records-keeping system was established, now known as the Defense Medical Surveillance System (DMSS). The creation of the DMSS provided an extensive epidemiologic database that provided valuable information such as demographic data, service records, deployment data, reportable medical events, exposure history, and vaccination records, which could be linked to the serum samples of each service member.2-4 Since 2008, the responsibilities of maintaining the DoDSR and the DMSS were transferred to the Armed Forces Health Surveillance Center (AFHSC).5
There have been several other databases created over the years that provide additional support and resources to military investigators. The Automated Central Tumor Registry and Department of Pathology and Area Laboratory Services both help investigators to track the incidence of specific cancers in the military population and provide them with pathologic specimens. Additionally, electronic medical records including the composite health care system and the Armed Forces Health Longitudinal Technology Application supplemented with insurance claims data accessible from the Military Health System Management and Reporting Tool (M2) database have made it possible to track patient data.
Utilization of Military Research Resources
Today, the DoDSR is a secure facility that maintains more than 56 million serum specimens from more than 11 million individuals in –30°C freezers, making it one of the largest repositories in the world.3,6 Each serum sample is linked with an individual’s DMSS record, providing a way for investigators to study how external factors such as deployment history, occupation, and exposure history relate to an individual’s unique genetic and physiological makeup. Furthermore, these data can be used for seroepidemiologic investigations that contribute to all facets of clinical care. The AFHSC routinely publishes findings related to notifiable diseases, disease outbreaks, and disease trends in a monthly report.7
There are strict guidelines in place that limit access to the DoDSR and service members’ data. Use of the repository for information directly related to a patient’s health care is one reason for access, such as analyzing serum for antibodies and seroconversion to assist in the diagnosis of a disease such as HIV. Another reason would be to obtain information needed for criminal investigations and prosecution. Typically, these types of requests require a judge-issued court order and approval by the Assistant Secretary of Defense for Health Affairs.4 The DoDSR also is used to study force health protection issues, such as infectious disease incidence and disease prevalence in the military population.
Obtaining access to the DoDSR and service members’ data for research purposes requires that the principal investigator be a DoD employee. Each research proposal is reviewed by members of the AFHSC to determine if the DoDSR is able to meet the demands of the project, including having the appropriate number of serum samples and supporting epidemiologic data available. The AFHSC provides a letter of support if it deems the project to be in line with its current resources and capabilities. Each research proposal is then sent to an institutional review board (IRB) to determine if the study is exempt or needs to go through a full IRB review process. A study might be exempt if the investigators are not obtaining data through interaction with living individuals or not having access to any identifiable protected health information associated with the samples.6 Regardless of whether the study is exempt or not exempt, the AFHSC will de-identify each sample before releasing the samples to the investigators by using a coding system to shield the patient’s identity from the investigator.
Resources within the military medical research system provide investigators with access to an extensive biorepository of serum and linked epidemiological data. Samples from the DoDSR have been used in no less than 75 peer-reviewed publications since 1985.8,9 Several of these studies have been influential in expanding knowledge about conditions seen more commonly in the military population such as stress fractures, traumatic brain injuries, posttraumatic stress disorder, and suicide.8 Additionally, DoDSR samples have been used to form military vaccination policies and track both infectious and noninfectious conditions in the military; for example, during the H1N1 influenza virus outbreak of 2009, AFHSC was essential in helping to limit the spread of the virus within the military community by using its data and collaborating with groups such as the Centers for Disease Control and Prevention to develop a plan for disease surveillance and control.5
Several military research resources are currently being used for a melanoma study that aims to assess if specific phenotypic features, melanoma risk alleles, and environmental factors (eg, duty station location, occupation, amount of UV exposure) can be used to develop better screening models to identify individuals who are at risk for developing melanoma. Secondarily, the study aims to determine if recently developed multimarker diagnostic and prognostic assays for melanoma will prove useful in the diagnostic and prognostic assessment of melanocytic neoplasms in the military population. For this study, one of the authors (J.H.M) is utilizing DoDSR serum from 1700 retrospective cases of invasive melanoma and 1700 matched controls. Additionally, the Automated Central Tumor Registry and Department of Pathology and Area Laboratory Services databases are being used to obtain tissue from more than 300 melanoma cases and nevi controls.
Limitations of the Current System
Despite the impressive capabilities of the current system, there are some issues that limit its potential. One such limitation is associated with the way that the serum samples at the DoDSR are utilized. Through 2012, the DoDSR had 54,542,658 serum specimens available, of which only 228,610 (0.42%) had ever been accessed for study.8 With such a wealth of information and relative availability, why are the serum samples not being accessed more frequently for studies? The inherent nature of the DoDSR being a restricted facility and only accessible to DoD-affiliated investigators may contribute, which allows the DoDSR to fulfill its primary purpose of contributing to military-relevant investigations but at the same time limits the number and type of investigations that can be performed. One idea that has been proposed is allowing civilian investigator access to the DoDSR if it can be proven that the research is targeted toward military-relevant issues.8 However, the current AFHSC access guidelines would need revision and would require additional safeguards to ensure that military-protected health information is not compromised. Nonetheless, such a change may result in more extensive use of DoDSR resources in the future.
An ethical issue that needs to be addressed pertains to how the DoDSR permits use of human serum samples for research purposes without getting consent from the individuals being studied. The serum samples are collected as part of mandatory predeployment and postdeployment examinations for HIV screening of all military members. These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Although it is true that military members must comply with specific requirements pertaining to military readiness (eg, receiving appropriate vaccinations, drug testing, regular medical screening), it is debated whether they still retain the right as patients to refuse participating in research and clinical trials.10 The AFHSC does have several regulatory steps in place to ensure that military members’ samples are used in an appropriate manner, including requiring a DoD primary investigator, IRB review of every research proposal, and de-identification of samples. At a minimum, giving military members the ability to provide informed consent would ensure that the military system is adhering to evolving human research standards.
The current lack of biological specimens other than serum in the DoDSR is another limitation of the current system. Recent advances in molecular analyses are impacted by expanding -omics techniques, such as epigenomics, transcriptomics, and proteomics. The field of epigenomics is the study of reversible changes to DNA (eg, methylation) associated with specific disease states or following specific environmental exposures.9,11 Transcriptomics, which analyzes messenger RNA transcript levels of expressed genes, and proteomics, which uses expression of proteins, are 2 techniques being used to develop biomarkers associated with specific diseases and environmental exposures.9,11 Serum alone does not provide the high-quality nucleic acids needed for many of these studies to take place. Adding whole-blood specimens or blood spot samples of military service members to the DoDSR would allow researchers to use these techniques to investigate many new biomarkers associated with military-relevant diseases and exposures. These techniques also can be used in the expanding field of personalized medicine so that health care providers are able to tailor all phases of care, including diagnosis and treatment, to an individual’s genetic profile.
Conclusion
The history of research in military medicine has been built on achieving the primary goal of serving those men and women who put their lives in danger to protect this country. In an evolving environment of new technologies that have led to changes in service members’ injuries, exposures, and diseases, military medicine also must adapt. Resources such as the DoDSR and DMSS, which provide investigators with the unique ability to link epidemiological data with serum samples, have been invaluable contributors to this overall mission. As with any large system, there are always improvements that can be made. Improving access to the DoDSR serum samples, educating and obtaining consent from military service members to use their samples in research, and adding specimens to the DoDSR that can be used for -omics techniques are 3 changes that should be considered to maximize
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Rubertone MV, Brundage JF. The defense medical surveillance system and the department of defense serum repository: glimpses of the future of public health surveillance. Am J Public Health. 2002;92:1900-1904.
- Department of Defense Serum Repository. Military Health System and the Defense Health Agency website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Department-of-Defense-Serum-Repository. Accessed August 2, 2016.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD serum repository. Mil Med. 2015;180(10 suppl):10-12.
- DeFraites RF. The Armed Forces Health Surveillance Center: enhancing the Military Health System’s public health capabilities. BMC Public Health. 2011;11(suppl 2):S1.
- Pavlin JA, Welch RA. Ethics, human use, and the department of defense serum repository. Mil Med. 2015;180:49-56.
- Defense Medical Surveillance System. Military Health System and the Defense Health Agency website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Surveillance-System. Accessed August 2, 2016.
- Perdue CL, Eick-Cost AA, Rubertone MV, et al. Description and utilization of the United States Department of Defense Serum Repository: a review of published studies, 1985-2012. Plos One. 2015;10:1-16.
- Mancuso JD, Mallon TM, Gaydos JC. Maximizing the capabilities of the DoD serum repository to meet current and future needs: report of the needs panel. Mil Med. 2015;180:14-24.
- Department of Defense. Department of Defense Instruction. http://www.dtic.mil/whs/directives/corres/pdf/600014p.pdf. Posted September 26, 2001. Updated October 3, 2013. Accessed August 2, 2016.
- Lindler LE. Building a DoD biorepository for the future: potential benefits and way forward. Mil Med. 2015;180:90-94.
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Rubertone MV, Brundage JF. The defense medical surveillance system and the department of defense serum repository: glimpses of the future of public health surveillance. Am J Public Health. 2002;92:1900-1904.
- Department of Defense Serum Repository. Military Health System and the Defense Health Agency website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Department-of-Defense-Serum-Repository. Accessed August 2, 2016.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD serum repository. Mil Med. 2015;180(10 suppl):10-12.
- DeFraites RF. The Armed Forces Health Surveillance Center: enhancing the Military Health System’s public health capabilities. BMC Public Health. 2011;11(suppl 2):S1.
- Pavlin JA, Welch RA. Ethics, human use, and the department of defense serum repository. Mil Med. 2015;180:49-56.
- Defense Medical Surveillance System. Military Health System and the Defense Health Agency website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Surveillance-System. Accessed August 2, 2016.
- Perdue CL, Eick-Cost AA, Rubertone MV, et al. Description and utilization of the United States Department of Defense Serum Repository: a review of published studies, 1985-2012. Plos One. 2015;10:1-16.
- Mancuso JD, Mallon TM, Gaydos JC. Maximizing the capabilities of the DoD serum repository to meet current and future needs: report of the needs panel. Mil Med. 2015;180:14-24.
- Department of Defense. Department of Defense Instruction. http://www.dtic.mil/whs/directives/corres/pdf/600014p.pdf. Posted September 26, 2001. Updated October 3, 2013. Accessed August 2, 2016.
- Lindler LE. Building a DoD biorepository for the future: potential benefits and way forward. Mil Med. 2015;180:90-94.
Practice Points
- Large patient databases and tissue repositories are increasingly being used to improve patient care through the use of clinical data, genomics, proteinomics, and metabolomics.
- The US Military has an established electronic medical record as well as tissue and serum repositories that can be leveraged to study melanoma and other dermatologic diseases.
Managing Residual Limb Hyperhidrosis in Wounded Warriors
We live in a time when young, otherwise healthy, active-duty individuals are undergoing traumatic amputations at an exceedingly high rate due to ongoing military engagements. According to US military casualty statistics through September 1, 2014, Operation Iraqi Freedom, Operation Enduring Freedom, and Operation New Dawn veterans have undergone a total of 1573 amputations.1 Walter Reed National Military Medical Center (WRNMMC) is one of several military facilities that has managed the care of these unique patients returning from the many ongoing conflicts around the globe. Multidisciplinary teams composed of surgeons, anesthesiologists, physical therapists, prosthetists, and others have joined forces to provide extraordinary emergency and recovery care for these patients. Even in the best hands, however, these traumatic amputee patients often experience long-term and lifelong sequelae of their injuries. As dermatologists at this facility (S.P. was at WRNMMC for 3 years before transferring to Madigan Army Medical Center), we are often asked to assist in the management of a subset of these sequelae: residual limb dermatoses. Residual limb dermatoses such as recurrent bacterial and fungal infections, cysts, abrasions, blistering, irritant and allergic dermatitis, pressure ulcers, acroangiodermatitis, stump edema, and many others have a high prevalence in our wounded warrior population and impact both amputee quality of life and utilization of medical resources. As many as 73% of amputees will experience a variety of residual limb dermatoses at some point in their life, with the highest prevalence in younger, more active patients.2,3 We have observed that many, if not most, of these cutaneous problems can be attributed to or are exacerbated by hyperhidrosis of the residual limb. Hyperhidrosis in this population of patients can be related to excessive sweat production, but more commonly, it is attributed to the lack of evaporation of normal perspiration.
Excess Sweat and the Prosthesis
To understand hyperhidrosis in amputee patients, it is important to understand the anatomy of the prosthesis. There are a variety of materials that are used to create prosthetic limbs. The most commonly used materials are a combination of plastic and carbon graphite/carbon fiber. The modern prosthetic limb uses a suspension system that attaches the prosthetic limb to the residual limb by creating a vacuum. There are several mechanisms to create this vacuum; however, they all depend on a liner that fits snugly over the residual limb. This liner-limb interface is responsible for protection, mitigation of sheering forces, and comfort, and it is the anchor for a good fit in the prosthesis. Unfortunately, this liner is the primary factor contributing to residual limb dermatologic problems. The liner usually is made of silicone or polyurethane and is designed to be water and sweat resistant; any excess water that finds its way into the liner-prosthetic interface will affect the seal of the device and cause slippage of the prosthesis.4 This water-resistant barrier is what induces the hyperhidrotic environment over the residual limb that is covered. These patients sweat with exertion, and because of the water-resistant liner, there is no mechanism for sweat evaporation. This leads to a localized environment of hyperhidrosis, increasing patients’ susceptibility to chronic skin conditions. In addition to the dermatologic pathology of the residual limb, there are notable functional concerns caused by excessive sweating. Increased moisture due to sweating not only leads to pathologic dermatoses but also to impaired fit and loss of suction by leaking into the prosthetic-limb interface, which in turn can lead to decreased stamina in the prosthesis, falls, and in severe cases even prosthetic abandonment.5
Treating Hyperhidrosis
While working with wounded warriors in the dermatology, prosthetics, and wound care clinics at WRNMMC, it repeatedly became clear that our current treatment options for hyperhidrosis in this population were not routinely tolerated or efficacious. Although hyperhidrosis of the axillary or palmoplantar region is a commonly encountered problem with clear treatment algorithms and management strategies, hyperhidrosis in the setting of a residual limb following amputation is somewhat unique and without definitive permanent cure. In approaching this problem, our institution has implemented a variety of therapies to the residual limb that have been well described and effective in the treatment in the axillary region.
Topical antiperspirants (ie, aluminum chloride) are well-documented treatments of hyperhidrosis and work by the formation of a metal iron precipitate when binding with mucopolysaccharides. These complexes cause damage to epithelial cells lining the ostia of eccrine glands, forming a plug in the lumen of the eccrine duct.6 Unfortunately, irritant contact dermatitis has affected the majority of our residual limb patients who have used topical antiperspirants and has led to poor compliance. Glycopyrrolate, an antimuscarinic agent, often is used with varying degrees of success. It works as a competitive antagonist blocking the acetylcholine muscarinic receptors that are responsible for the innervation of eccrine sweat glands. Several of our residual limb patients have a history of global hyperhidrosis and have responded favorably to 1- to 2-mg doses of glycopyrrolate administered twice daily. The side-effect profile headlined by xerostomia, urinary retention, and constipation has, as it often does, limited the dosing. We have observed that with the use of glycopyrrolate, these patients admit to less overall sweating but experience only a mild decrease in the cutaneous problems they experience over the residual limbs, which is likely attributed to the prosthetic liner that induces hyperhidrosis by preventing sweat evaporation from the residual limb. Patients may not be sweating as much, but they are still sweating and that sweat is unable to evaporate from under the liner.
Botulinum toxin is a common treatment of axillary hyperhidrosis and its effects on residual limbs are the same.5,7 Botulinum toxin types A, B, and E specifically cleave soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes, which prevents neurosecretory vesicles from fusing with the interior surface of the plasma membrane of the nerve synapse, thereby blocking release of acetylcholine.8 In inhibiting acetylcholine release, the signal for eccrine secretion is blocked. This therapy has been effective in our residual limb patients who tolerate the treatment. It typically involves the injection of 300 to 500 U of botulinum toxin type A diluted with 0.9% saline at 2 to 5 U per 0.1 mL into the residual limb.5 As with the other treatments, there are side effects that complicate compliance. Most residual limb treatments require 150 injections, which can be uncomfortable for this patient cohort. The majority of these wounded warriors have abnormal anatomy because of the traumatic nature of their injuries (ie, improvised explosive device attacks, artillery injury), and they often experience hyperesthesia, phantom limb pain, and notable scarring. These injections can be extremely painful, which often limits their utility. In addition, the therapy only provides 3 to 6 months of symptom relief. Our compliance rate for returning patients has not been good and we suspect that it is likely due to the discomfort associated with the injections.
Other therapies such as laser hair removal and iontophoresis have been attempted but have not yielded great results or compliance. In addition to the limitations of these treatment methods, the residual limbs have presented their own unique set of challenges; complications have included varied anatomy of the residual limbs, scarring, sensitivities, and heterotopic ossification. Temporary remedies such as botulinum toxin injections also present logistical complications because they require repetitive procedural appointments that can be quite burdensome to attend when these patients move back home, often far away from our large military treatment facilities.
A New Therapy With Exciting Potential
With the recent advent of microwave thermal ablation technology, the potential for a different, possibly permanent treatment was discovered. Microwave thermal ablation of the eccrine coils has been proven safe and effective in the prolonged reduction of hyperhidrosis of the axillae and has presented as a potential therapy for our residual limb hyperhidrosis patient population. This technology produces heat that is targeted to a specific depth in the treated tissue while cooling the epidermis. There are various treatment levels that can be used to deliver graded intensities of heat. When the deep dermis is targeted, adnexal structures are denatured and destroyed, causing diminished or eliminated function. Eccrine sweat glands, apocrine glands, and even hair follicles are affected by the therapy. The manufacturer of the only microwave thermal ablation device on the market that is approved by the US Food and Drug Administration to treat axillary hyperhidrosis has suggested that these effects are long-term and possibly permanent.9 After several iterations with this technology, we have been able to successfully apply microwave thermal ablation of eccrine coils to 5 residual limbs and are excited about the promise that this technique possesses. A report of our index case will be published soon,10 and we are looking forward to launching our protocol treating traumatic lower extremity amputee patients that have hyperhidrosis with microwave therapy ablation technology here at WRNMMC.
Final Thoughts
Amputation residual limb dermatoses have a high prevalence and impact on amputee quality of life, particularly among young military members who strive to maintain a highly active lifestyle. Many of these dermatoses are directly related to hyperhidrosis of the residual limb that is covered by the prosthetic device and the liner that interfaces with the skin. Although many treatments for residual limb hyperhidrosis have been used with varying efficacy, none have offered a cost-effective or sustained response. Many of our wounded warriors in this amputee population have or will be transitioning out of the military in the coming years. It is imperative to our government, our institution, and most importantly our patients that efforts are made to develop a more permanent and efficacious treatment application to provide relief to these wounded heroes. This amputee population is unique in that they are younger, healthier, and highly motivated to live as “normal” of a life as possible. The ability to ambulate in a prosthetic device can have a huge social and psychological impact, and providing a therapy that minimizes complications associated with prosthetic use is invaluable. We are excited about the results we have seen with the microwave thermal ablation device and feel that there is potential benefit for other amputee populations if the procedure is perfected.
It is an exciting age in medicine where technology and biology have remarkably honed our diagnostic and treatment capabilities. We hope that everyone in the dermatology community shares our enthusiasm and will continue to explore and test these new technologies to improve and better the lives of the patients we treat.
- Fischer H. A guide to U.S. military casualty statistics: Operation Inherent Resolve, Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. http://ibiblio.org/hyperwar/NHC/CasualtyStats2014Nov/CasualtyStats2014Nov.htm. Congressional Research Service 7-5700; RS22452. Published November 20, 2014. Accessed May 13, 2016.
- Yang NB, Garza LA, Foote CE, et al. High prevalence of stump dermatoses 38 years or more after amputation. Arch Dermatol. 2012;148:1283-1286.
- Koc E, Tunca M, Akar A, et al. Skin problems in amputees: a descriptive study. Int J Dermatol. 2008;47:463-466.
- Ghoseiri K, Safari MR. Prevalence of heat and perspiration discomfort inside prostheses: literature review. J Rehabil Res Dev. 2014;51:855-868.
- Gratrix M, Hivnor C. Botulinum A toxin treatment for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Hölzle E. Topical pharmacological treatment. Curr Probl Dermatol. 2002;30:30-43.
- Lee KYC, Levell NJ. Turning the tide: a history and review of hyperhidrosis treatment. JRSM Open. 2014;5. doi:10.1177/2042533313505511.
- Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3-9.
- Lupin M, Hong HC, O’Shaughnessy KF. Long-term efficacy and quality of life assessment for treatment of axillary hyperhidrosis with a microwave device. Dermatol Surg. 2014;40:805-807.
- Mula K, Winston J, Pace S, et al. Use of microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. In press.
We live in a time when young, otherwise healthy, active-duty individuals are undergoing traumatic amputations at an exceedingly high rate due to ongoing military engagements. According to US military casualty statistics through September 1, 2014, Operation Iraqi Freedom, Operation Enduring Freedom, and Operation New Dawn veterans have undergone a total of 1573 amputations.1 Walter Reed National Military Medical Center (WRNMMC) is one of several military facilities that has managed the care of these unique patients returning from the many ongoing conflicts around the globe. Multidisciplinary teams composed of surgeons, anesthesiologists, physical therapists, prosthetists, and others have joined forces to provide extraordinary emergency and recovery care for these patients. Even in the best hands, however, these traumatic amputee patients often experience long-term and lifelong sequelae of their injuries. As dermatologists at this facility (S.P. was at WRNMMC for 3 years before transferring to Madigan Army Medical Center), we are often asked to assist in the management of a subset of these sequelae: residual limb dermatoses. Residual limb dermatoses such as recurrent bacterial and fungal infections, cysts, abrasions, blistering, irritant and allergic dermatitis, pressure ulcers, acroangiodermatitis, stump edema, and many others have a high prevalence in our wounded warrior population and impact both amputee quality of life and utilization of medical resources. As many as 73% of amputees will experience a variety of residual limb dermatoses at some point in their life, with the highest prevalence in younger, more active patients.2,3 We have observed that many, if not most, of these cutaneous problems can be attributed to or are exacerbated by hyperhidrosis of the residual limb. Hyperhidrosis in this population of patients can be related to excessive sweat production, but more commonly, it is attributed to the lack of evaporation of normal perspiration.
Excess Sweat and the Prosthesis
To understand hyperhidrosis in amputee patients, it is important to understand the anatomy of the prosthesis. There are a variety of materials that are used to create prosthetic limbs. The most commonly used materials are a combination of plastic and carbon graphite/carbon fiber. The modern prosthetic limb uses a suspension system that attaches the prosthetic limb to the residual limb by creating a vacuum. There are several mechanisms to create this vacuum; however, they all depend on a liner that fits snugly over the residual limb. This liner-limb interface is responsible for protection, mitigation of sheering forces, and comfort, and it is the anchor for a good fit in the prosthesis. Unfortunately, this liner is the primary factor contributing to residual limb dermatologic problems. The liner usually is made of silicone or polyurethane and is designed to be water and sweat resistant; any excess water that finds its way into the liner-prosthetic interface will affect the seal of the device and cause slippage of the prosthesis.4 This water-resistant barrier is what induces the hyperhidrotic environment over the residual limb that is covered. These patients sweat with exertion, and because of the water-resistant liner, there is no mechanism for sweat evaporation. This leads to a localized environment of hyperhidrosis, increasing patients’ susceptibility to chronic skin conditions. In addition to the dermatologic pathology of the residual limb, there are notable functional concerns caused by excessive sweating. Increased moisture due to sweating not only leads to pathologic dermatoses but also to impaired fit and loss of suction by leaking into the prosthetic-limb interface, which in turn can lead to decreased stamina in the prosthesis, falls, and in severe cases even prosthetic abandonment.5
Treating Hyperhidrosis
While working with wounded warriors in the dermatology, prosthetics, and wound care clinics at WRNMMC, it repeatedly became clear that our current treatment options for hyperhidrosis in this population were not routinely tolerated or efficacious. Although hyperhidrosis of the axillary or palmoplantar region is a commonly encountered problem with clear treatment algorithms and management strategies, hyperhidrosis in the setting of a residual limb following amputation is somewhat unique and without definitive permanent cure. In approaching this problem, our institution has implemented a variety of therapies to the residual limb that have been well described and effective in the treatment in the axillary region.
Topical antiperspirants (ie, aluminum chloride) are well-documented treatments of hyperhidrosis and work by the formation of a metal iron precipitate when binding with mucopolysaccharides. These complexes cause damage to epithelial cells lining the ostia of eccrine glands, forming a plug in the lumen of the eccrine duct.6 Unfortunately, irritant contact dermatitis has affected the majority of our residual limb patients who have used topical antiperspirants and has led to poor compliance. Glycopyrrolate, an antimuscarinic agent, often is used with varying degrees of success. It works as a competitive antagonist blocking the acetylcholine muscarinic receptors that are responsible for the innervation of eccrine sweat glands. Several of our residual limb patients have a history of global hyperhidrosis and have responded favorably to 1- to 2-mg doses of glycopyrrolate administered twice daily. The side-effect profile headlined by xerostomia, urinary retention, and constipation has, as it often does, limited the dosing. We have observed that with the use of glycopyrrolate, these patients admit to less overall sweating but experience only a mild decrease in the cutaneous problems they experience over the residual limbs, which is likely attributed to the prosthetic liner that induces hyperhidrosis by preventing sweat evaporation from the residual limb. Patients may not be sweating as much, but they are still sweating and that sweat is unable to evaporate from under the liner.
Botulinum toxin is a common treatment of axillary hyperhidrosis and its effects on residual limbs are the same.5,7 Botulinum toxin types A, B, and E specifically cleave soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes, which prevents neurosecretory vesicles from fusing with the interior surface of the plasma membrane of the nerve synapse, thereby blocking release of acetylcholine.8 In inhibiting acetylcholine release, the signal for eccrine secretion is blocked. This therapy has been effective in our residual limb patients who tolerate the treatment. It typically involves the injection of 300 to 500 U of botulinum toxin type A diluted with 0.9% saline at 2 to 5 U per 0.1 mL into the residual limb.5 As with the other treatments, there are side effects that complicate compliance. Most residual limb treatments require 150 injections, which can be uncomfortable for this patient cohort. The majority of these wounded warriors have abnormal anatomy because of the traumatic nature of their injuries (ie, improvised explosive device attacks, artillery injury), and they often experience hyperesthesia, phantom limb pain, and notable scarring. These injections can be extremely painful, which often limits their utility. In addition, the therapy only provides 3 to 6 months of symptom relief. Our compliance rate for returning patients has not been good and we suspect that it is likely due to the discomfort associated with the injections.
Other therapies such as laser hair removal and iontophoresis have been attempted but have not yielded great results or compliance. In addition to the limitations of these treatment methods, the residual limbs have presented their own unique set of challenges; complications have included varied anatomy of the residual limbs, scarring, sensitivities, and heterotopic ossification. Temporary remedies such as botulinum toxin injections also present logistical complications because they require repetitive procedural appointments that can be quite burdensome to attend when these patients move back home, often far away from our large military treatment facilities.
A New Therapy With Exciting Potential
With the recent advent of microwave thermal ablation technology, the potential for a different, possibly permanent treatment was discovered. Microwave thermal ablation of the eccrine coils has been proven safe and effective in the prolonged reduction of hyperhidrosis of the axillae and has presented as a potential therapy for our residual limb hyperhidrosis patient population. This technology produces heat that is targeted to a specific depth in the treated tissue while cooling the epidermis. There are various treatment levels that can be used to deliver graded intensities of heat. When the deep dermis is targeted, adnexal structures are denatured and destroyed, causing diminished or eliminated function. Eccrine sweat glands, apocrine glands, and even hair follicles are affected by the therapy. The manufacturer of the only microwave thermal ablation device on the market that is approved by the US Food and Drug Administration to treat axillary hyperhidrosis has suggested that these effects are long-term and possibly permanent.9 After several iterations with this technology, we have been able to successfully apply microwave thermal ablation of eccrine coils to 5 residual limbs and are excited about the promise that this technique possesses. A report of our index case will be published soon,10 and we are looking forward to launching our protocol treating traumatic lower extremity amputee patients that have hyperhidrosis with microwave therapy ablation technology here at WRNMMC.
Final Thoughts
Amputation residual limb dermatoses have a high prevalence and impact on amputee quality of life, particularly among young military members who strive to maintain a highly active lifestyle. Many of these dermatoses are directly related to hyperhidrosis of the residual limb that is covered by the prosthetic device and the liner that interfaces with the skin. Although many treatments for residual limb hyperhidrosis have been used with varying efficacy, none have offered a cost-effective or sustained response. Many of our wounded warriors in this amputee population have or will be transitioning out of the military in the coming years. It is imperative to our government, our institution, and most importantly our patients that efforts are made to develop a more permanent and efficacious treatment application to provide relief to these wounded heroes. This amputee population is unique in that they are younger, healthier, and highly motivated to live as “normal” of a life as possible. The ability to ambulate in a prosthetic device can have a huge social and psychological impact, and providing a therapy that minimizes complications associated with prosthetic use is invaluable. We are excited about the results we have seen with the microwave thermal ablation device and feel that there is potential benefit for other amputee populations if the procedure is perfected.
It is an exciting age in medicine where technology and biology have remarkably honed our diagnostic and treatment capabilities. We hope that everyone in the dermatology community shares our enthusiasm and will continue to explore and test these new technologies to improve and better the lives of the patients we treat.
We live in a time when young, otherwise healthy, active-duty individuals are undergoing traumatic amputations at an exceedingly high rate due to ongoing military engagements. According to US military casualty statistics through September 1, 2014, Operation Iraqi Freedom, Operation Enduring Freedom, and Operation New Dawn veterans have undergone a total of 1573 amputations.1 Walter Reed National Military Medical Center (WRNMMC) is one of several military facilities that has managed the care of these unique patients returning from the many ongoing conflicts around the globe. Multidisciplinary teams composed of surgeons, anesthesiologists, physical therapists, prosthetists, and others have joined forces to provide extraordinary emergency and recovery care for these patients. Even in the best hands, however, these traumatic amputee patients often experience long-term and lifelong sequelae of their injuries. As dermatologists at this facility (S.P. was at WRNMMC for 3 years before transferring to Madigan Army Medical Center), we are often asked to assist in the management of a subset of these sequelae: residual limb dermatoses. Residual limb dermatoses such as recurrent bacterial and fungal infections, cysts, abrasions, blistering, irritant and allergic dermatitis, pressure ulcers, acroangiodermatitis, stump edema, and many others have a high prevalence in our wounded warrior population and impact both amputee quality of life and utilization of medical resources. As many as 73% of amputees will experience a variety of residual limb dermatoses at some point in their life, with the highest prevalence in younger, more active patients.2,3 We have observed that many, if not most, of these cutaneous problems can be attributed to or are exacerbated by hyperhidrosis of the residual limb. Hyperhidrosis in this population of patients can be related to excessive sweat production, but more commonly, it is attributed to the lack of evaporation of normal perspiration.
Excess Sweat and the Prosthesis
To understand hyperhidrosis in amputee patients, it is important to understand the anatomy of the prosthesis. There are a variety of materials that are used to create prosthetic limbs. The most commonly used materials are a combination of plastic and carbon graphite/carbon fiber. The modern prosthetic limb uses a suspension system that attaches the prosthetic limb to the residual limb by creating a vacuum. There are several mechanisms to create this vacuum; however, they all depend on a liner that fits snugly over the residual limb. This liner-limb interface is responsible for protection, mitigation of sheering forces, and comfort, and it is the anchor for a good fit in the prosthesis. Unfortunately, this liner is the primary factor contributing to residual limb dermatologic problems. The liner usually is made of silicone or polyurethane and is designed to be water and sweat resistant; any excess water that finds its way into the liner-prosthetic interface will affect the seal of the device and cause slippage of the prosthesis.4 This water-resistant barrier is what induces the hyperhidrotic environment over the residual limb that is covered. These patients sweat with exertion, and because of the water-resistant liner, there is no mechanism for sweat evaporation. This leads to a localized environment of hyperhidrosis, increasing patients’ susceptibility to chronic skin conditions. In addition to the dermatologic pathology of the residual limb, there are notable functional concerns caused by excessive sweating. Increased moisture due to sweating not only leads to pathologic dermatoses but also to impaired fit and loss of suction by leaking into the prosthetic-limb interface, which in turn can lead to decreased stamina in the prosthesis, falls, and in severe cases even prosthetic abandonment.5
Treating Hyperhidrosis
While working with wounded warriors in the dermatology, prosthetics, and wound care clinics at WRNMMC, it repeatedly became clear that our current treatment options for hyperhidrosis in this population were not routinely tolerated or efficacious. Although hyperhidrosis of the axillary or palmoplantar region is a commonly encountered problem with clear treatment algorithms and management strategies, hyperhidrosis in the setting of a residual limb following amputation is somewhat unique and without definitive permanent cure. In approaching this problem, our institution has implemented a variety of therapies to the residual limb that have been well described and effective in the treatment in the axillary region.
Topical antiperspirants (ie, aluminum chloride) are well-documented treatments of hyperhidrosis and work by the formation of a metal iron precipitate when binding with mucopolysaccharides. These complexes cause damage to epithelial cells lining the ostia of eccrine glands, forming a plug in the lumen of the eccrine duct.6 Unfortunately, irritant contact dermatitis has affected the majority of our residual limb patients who have used topical antiperspirants and has led to poor compliance. Glycopyrrolate, an antimuscarinic agent, often is used with varying degrees of success. It works as a competitive antagonist blocking the acetylcholine muscarinic receptors that are responsible for the innervation of eccrine sweat glands. Several of our residual limb patients have a history of global hyperhidrosis and have responded favorably to 1- to 2-mg doses of glycopyrrolate administered twice daily. The side-effect profile headlined by xerostomia, urinary retention, and constipation has, as it often does, limited the dosing. We have observed that with the use of glycopyrrolate, these patients admit to less overall sweating but experience only a mild decrease in the cutaneous problems they experience over the residual limbs, which is likely attributed to the prosthetic liner that induces hyperhidrosis by preventing sweat evaporation from the residual limb. Patients may not be sweating as much, but they are still sweating and that sweat is unable to evaporate from under the liner.
Botulinum toxin is a common treatment of axillary hyperhidrosis and its effects on residual limbs are the same.5,7 Botulinum toxin types A, B, and E specifically cleave soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes, which prevents neurosecretory vesicles from fusing with the interior surface of the plasma membrane of the nerve synapse, thereby blocking release of acetylcholine.8 In inhibiting acetylcholine release, the signal for eccrine secretion is blocked. This therapy has been effective in our residual limb patients who tolerate the treatment. It typically involves the injection of 300 to 500 U of botulinum toxin type A diluted with 0.9% saline at 2 to 5 U per 0.1 mL into the residual limb.5 As with the other treatments, there are side effects that complicate compliance. Most residual limb treatments require 150 injections, which can be uncomfortable for this patient cohort. The majority of these wounded warriors have abnormal anatomy because of the traumatic nature of their injuries (ie, improvised explosive device attacks, artillery injury), and they often experience hyperesthesia, phantom limb pain, and notable scarring. These injections can be extremely painful, which often limits their utility. In addition, the therapy only provides 3 to 6 months of symptom relief. Our compliance rate for returning patients has not been good and we suspect that it is likely due to the discomfort associated with the injections.
Other therapies such as laser hair removal and iontophoresis have been attempted but have not yielded great results or compliance. In addition to the limitations of these treatment methods, the residual limbs have presented their own unique set of challenges; complications have included varied anatomy of the residual limbs, scarring, sensitivities, and heterotopic ossification. Temporary remedies such as botulinum toxin injections also present logistical complications because they require repetitive procedural appointments that can be quite burdensome to attend when these patients move back home, often far away from our large military treatment facilities.
A New Therapy With Exciting Potential
With the recent advent of microwave thermal ablation technology, the potential for a different, possibly permanent treatment was discovered. Microwave thermal ablation of the eccrine coils has been proven safe and effective in the prolonged reduction of hyperhidrosis of the axillae and has presented as a potential therapy for our residual limb hyperhidrosis patient population. This technology produces heat that is targeted to a specific depth in the treated tissue while cooling the epidermis. There are various treatment levels that can be used to deliver graded intensities of heat. When the deep dermis is targeted, adnexal structures are denatured and destroyed, causing diminished or eliminated function. Eccrine sweat glands, apocrine glands, and even hair follicles are affected by the therapy. The manufacturer of the only microwave thermal ablation device on the market that is approved by the US Food and Drug Administration to treat axillary hyperhidrosis has suggested that these effects are long-term and possibly permanent.9 After several iterations with this technology, we have been able to successfully apply microwave thermal ablation of eccrine coils to 5 residual limbs and are excited about the promise that this technique possesses. A report of our index case will be published soon,10 and we are looking forward to launching our protocol treating traumatic lower extremity amputee patients that have hyperhidrosis with microwave therapy ablation technology here at WRNMMC.
Final Thoughts
Amputation residual limb dermatoses have a high prevalence and impact on amputee quality of life, particularly among young military members who strive to maintain a highly active lifestyle. Many of these dermatoses are directly related to hyperhidrosis of the residual limb that is covered by the prosthetic device and the liner that interfaces with the skin. Although many treatments for residual limb hyperhidrosis have been used with varying efficacy, none have offered a cost-effective or sustained response. Many of our wounded warriors in this amputee population have or will be transitioning out of the military in the coming years. It is imperative to our government, our institution, and most importantly our patients that efforts are made to develop a more permanent and efficacious treatment application to provide relief to these wounded heroes. This amputee population is unique in that they are younger, healthier, and highly motivated to live as “normal” of a life as possible. The ability to ambulate in a prosthetic device can have a huge social and psychological impact, and providing a therapy that minimizes complications associated with prosthetic use is invaluable. We are excited about the results we have seen with the microwave thermal ablation device and feel that there is potential benefit for other amputee populations if the procedure is perfected.
It is an exciting age in medicine where technology and biology have remarkably honed our diagnostic and treatment capabilities. We hope that everyone in the dermatology community shares our enthusiasm and will continue to explore and test these new technologies to improve and better the lives of the patients we treat.
- Fischer H. A guide to U.S. military casualty statistics: Operation Inherent Resolve, Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. http://ibiblio.org/hyperwar/NHC/CasualtyStats2014Nov/CasualtyStats2014Nov.htm. Congressional Research Service 7-5700; RS22452. Published November 20, 2014. Accessed May 13, 2016.
- Yang NB, Garza LA, Foote CE, et al. High prevalence of stump dermatoses 38 years or more after amputation. Arch Dermatol. 2012;148:1283-1286.
- Koc E, Tunca M, Akar A, et al. Skin problems in amputees: a descriptive study. Int J Dermatol. 2008;47:463-466.
- Ghoseiri K, Safari MR. Prevalence of heat and perspiration discomfort inside prostheses: literature review. J Rehabil Res Dev. 2014;51:855-868.
- Gratrix M, Hivnor C. Botulinum A toxin treatment for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Hölzle E. Topical pharmacological treatment. Curr Probl Dermatol. 2002;30:30-43.
- Lee KYC, Levell NJ. Turning the tide: a history and review of hyperhidrosis treatment. JRSM Open. 2014;5. doi:10.1177/2042533313505511.
- Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3-9.
- Lupin M, Hong HC, O’Shaughnessy KF. Long-term efficacy and quality of life assessment for treatment of axillary hyperhidrosis with a microwave device. Dermatol Surg. 2014;40:805-807.
- Mula K, Winston J, Pace S, et al. Use of microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. In press.
- Fischer H. A guide to U.S. military casualty statistics: Operation Inherent Resolve, Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. http://ibiblio.org/hyperwar/NHC/CasualtyStats2014Nov/CasualtyStats2014Nov.htm. Congressional Research Service 7-5700; RS22452. Published November 20, 2014. Accessed May 13, 2016.
- Yang NB, Garza LA, Foote CE, et al. High prevalence of stump dermatoses 38 years or more after amputation. Arch Dermatol. 2012;148:1283-1286.
- Koc E, Tunca M, Akar A, et al. Skin problems in amputees: a descriptive study. Int J Dermatol. 2008;47:463-466.
- Ghoseiri K, Safari MR. Prevalence of heat and perspiration discomfort inside prostheses: literature review. J Rehabil Res Dev. 2014;51:855-868.
- Gratrix M, Hivnor C. Botulinum A toxin treatment for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Hölzle E. Topical pharmacological treatment. Curr Probl Dermatol. 2002;30:30-43.
- Lee KYC, Levell NJ. Turning the tide: a history and review of hyperhidrosis treatment. JRSM Open. 2014;5. doi:10.1177/2042533313505511.
- Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3-9.
- Lupin M, Hong HC, O’Shaughnessy KF. Long-term efficacy and quality of life assessment for treatment of axillary hyperhidrosis with a microwave device. Dermatol Surg. 2014;40:805-807.
- Mula K, Winston J, Pace S, et al. Use of microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. In press.
Practice Points
- Hyperhidrosis of residual limbs often is induced by the occlusive effects of the water-resistant prosthetic liner that fits snugly over the limb.
- Commonly accepted treatments of hyperhidrosis often are less effective or poorly tolerated by these patients. The microwave thermal ablation device is a promising tool that may provide long-term relief of symptoms.