User login
Acne Keloidalis Nuchae in the Armed Forces
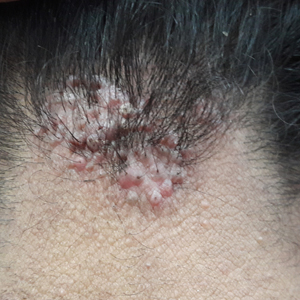
Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder most commonly involving the occipital scalp and posterior neck characterized by the development of keloidlike papules, pustules, and plaques. If left untreated, this condition may progress to scarring alopecia. It primarily affects males of African descent, but it also may occur in females and in other ethnic groups. Although the exact underlying pathogenesis is unclear, close haircuts and chronic mechanical irritation to the posterior neck and scalp are known inciting factors. For this reason, AKN disproportionately affects active-duty military servicemembers who are held to strict grooming standards. The US Military maintains these grooming standards to ensure uniformity, self-discipline, and serviceability in operational settings.1 Regulations dictate short tapered hair, particularly on the back of the neck, which can require weekly to biweekly haircuts to maintain.1-5
First-line treatment of AKN is prevention by avoiding short haircuts and other forms of mechanical irritation.1,6,7 However, there are considerable barriers to this strategy within the military due to uniform regulations as well as personal appearance and grooming standards. Early identification and treatment are of utmost importance in managing AKN in the military population to ensure reduction of morbidity, prevention of late-stage disease, and continued fitness for duty. This article reviews the clinical features, epidemiology, and treatments available for management of AKN, with a special focus on the active-duty military population.
Clinical Features and Epidemiology
Acne keloidalis nuchae is a chronic inflammatory disorder characterized by the development of keloidlike papules, pustules, and plaques on the posterior neck and occipital scalp.6 Also known as folliculitis keloidalis nuchae, AKN is seen primarily in men of African descent, though cases also have been reported in females and in a few other ethnic groups.6,7 In black males, the AKN prevalence worldwide ranges from 0.5% to 13.6%. The male to female ratio is 20 to 1.7 Although the exact cause is unknown, AKN appears to develop from chronic irritation and inflammation following localized skin injury and/or trauma. Chronic irritation from close-shaved haircuts, tight-fitting shirt collars, caps, and helmets have all been implicated as considerable risk factors.6-8
Symptoms generally develop hours to days following a close haircut and begin with the early formation of inflamed irritated papules and notable erythema.6,7 These papules may become secondarily infected and develop into pustules and/or abscesses, especially in cases in which the affected individual continues to have the hair shaved. Continued use of shared razors increases the risk for secondary infection and also raises the concern for transmission of blood-borne pathogens, as AKN lesions are quick to bleed with minor trauma.7
Over time, chronic inflammation and continued trauma of the AKN papules leads to widespread fibrosis and scar formation, as the papules coalesce into larger plaques and nodules. If left untreated, these later stages of disease can progress to chronic scarring alopecia.6
Prevention
In the general population, first-line therapy of AKN is preventative. The goal is to break the cycle of chronic inflammation, thereby preventing the development of additional lesions and subsequent scarring.7 Patients should be encouraged to avoid frequent haircuts, close shaves, hats, helmets, and tight shirt collars.6-8
A 2017 cross-sectional study by Adotama et al9 investigated recognition and management of AKN in predominantly black barbershops in an urban setting. Fifty barbers from barbershops in Oklahoma City, Oklahoma, were enrolled and interviewed for the study. Of these barbers, only 44% (22/50) were able to properly identify AKN from a photograph. Although the vast majority (94% [47/50]) were aware that razor use would aggravate the condition, only 46% (23/50) reported avoidance of cutting hair for clients with active AKN.9 This study, while limited by its small sample size, showed that many barbers may be unaware of AKN and therefore unknowingly contribute to the disease process by performing haircuts on actively inflamed scalps. For this reason, it is important to educate patients about their condition and strongly recommend lifestyle and hairstyle modifications in the management of their disease.
Acne keloidalis nuchae that is severe enough to interfere with the proper use and wear of military equipment (eg, Kevlar helmets) or maintenance of regulation grooming standards does not meet military admission standards.10,11 However, mild undiagnosed cases may be overlooked during entrance physical examinations, while many servicemembers develop AKN after entering the military.10 For these individuals, long-term avoidance of haircuts is not a realistic or obtainable therapeutic option.
Treatment
Topical Therapy
Early mild to moderate cases of AKN—papules less than 3 mm, no nodules present—may be treated with potent topical steroids. Studies have shown 2-week alternating cycles of high-potency topical steroids (2 weeks of twice-daily application followed by 2 weeks without application) for 8 to 12 weeks to be effective in reducing AKN lesions.8,12 Topical clindamycin also may be added and has demonstrated efficacy particularly when pustules are present.7,8
Intralesional Steroids
For moderate cases of AKN—papules more than 3 mm, plaques, and nodules—intralesional steroid injections may be considered. Triamcinolone may be used at a dose of 5 to 40 mg/mL administered at 4-week intervals.7 More concentrated doses will produce faster responses but also carry the known risk of side effects such as hypopigmentation in darker-skinned individuals and skin atrophy.
Systemic Therapy
Systemic therapy with oral antibiotics may be warranted as an adjunct to mild to moderate cases of AKN or in cases with clear evidence of secondary infection. Long-term tetracycline antibiotics, such as minocycline and doxycycline, may be used concurrently with topical and/or intralesional steroids.6,7 Their antibacterial and anti-inflammatory effects are useful in controlling secondary infections and reducing overall chronic inflammation.
When selecting an appropriate antibiotic for long-term use in active-duty military patients, it is important to consider their effects on duty status. Doxycycline is preferred for active-duty servicemembers because it is not duty limiting or medically disqualifying.10,13-15 However, minocycline, is restricted for use in aviators and aircrew members due to the risk for central nervous system side effects, which may include light-headedness, dizziness, and vertigo.
UV Light Therapy
UV radiation has known anti-inflammatory, immunosuppressive, and antifibrotic effects and commonly is used in the treatment of many dermatologic conditions.16 Within the last decade, targeted UVB (tUVB) radiation has shown promise as an effective alternative therapy for AKN. In 2014, Okoye et al16 conducted a prospective, randomized, split-scalp study in 11 patients with AKN. Each patient underwent treatment with a tUVB device (with peaks at 303 and 313 nm) to a randomly selected side of the scalp 3 times weekly for 16 weeks. Significant reductions in lesion count were seen on the treated side after 8 (P=.03) and 16 weeks (P=.04), with no change noted on the control side. Aside from objective lesion counts, patients completed questionnaires (n=6) regarding their treatment outcomes. Notably, 83.3% (5/6) reported marked improvement in their condition. Aside from mild transient burning and erythema of the treated area, no serious side effects were reported.16
Targeted UVB phototherapy has limited utility in an operational setting due to accessibility and operational tempo. Phototherapy units typically are available only at commands in close proximity to large medical treatment facilities. Further, the vast majority of servicemembers have duty hours that are not amenable to multiple treatment sessions per week for several months. For servicemembers in administrative roles or serving in garrison or shore billets, tUVB or narrowband UV phototherapy may be viable treatment options.
Laser Therapy
Various lasers have been used to treat AKN, including the CO2 laser, pulsed dye laser, 810-nm diode laser, and 1064-nm Nd:YAG laser.6 Kantor et al17 utilized a CO2 laser with a focused beam for surgical excision of a late-stage AKN case as early as 1986. In these patients, it was demonstrated that focused CO2 laser could be used to remove fibrotic lesions in an outpatient setting with only local anesthesia. Although only 8 patients were treated in this report, no relapses occurred.17
CO2 laser evaporation using the unfocused beam setting with 130 to 150 J/cm2 has been less successful, with relapses reported in multiple cases.6 Dragoni et al18 attempted treatment with a 595-nm pulsed dye laser with 6.5-J/cm2 fluence and 0.5-millisecond pulse but faced similar results, with lesions returning within 1 month.
There have been numerous reports of clinical improvement of AKN with the use of the 1064-nm Nd:YAG laser.6,19 Esmat et al19 treated 16 patients with a fluence of 35 to 45 J/cm2 and pulse duration of 10 to 30 milliseconds adjusted to skin type and hair thickness. An overall 82% reduction in lesion count was observed after 5 treatment sessions. Biopsies following the treatment course demonstrated a significant reduction in papule and plaque count (P=.001 and P=.011, respectively), and no clinical recurrences were noted at 12 months posttreatment.19 Similarly, Woo et al20 conducted a single-blinded, randomized, controlled trial to assess the efficacy of the Nd:YAG laser in combination with topical corticosteroid therapy vs topical corticosteroid monotherapy. Of the 20 patients treated, there was a statistically significant improvement in patients with papule-only AKN who received the laser and topical combination treatment (P=.031).20
Laser therapy may be an available treatment option for military servicemembers stationed within close proximity to military treatment facilities, with the Nd:YAG laser typically having the widest availability. Although laser therapy may be effective in early stages of disease, servicemembers would have to be amenable to limitation of future hair growth in the treated areas.
Surgical Excision
Surgical excision may be considered for large, extensive, disfiguring, and/or refractory lesions. Excision is a safe and effective method to remove tender, inflamed, keloidlike masses. Techniques for excision include electrosurgical excision with secondary intention healing, excision of a horizontal ellipse involving the posterior hairline with either primary closure or secondary intention healing, and use of a semilunar tissue expander prior to excision and closure.6 Regardless of the technique, it is important to ensure that affected tissue is excised at a depth that includes the base of the hair follicles to prevent recurrence.21
Final Thoughts
Acne keloidalis nuchae is a chronic inflammatory disease that causes considerable morbidity and can lead to chronic infection, alopecia, and disfigurement of the occipital scalp and posterior neck. Although easily preventable through the avoidance of mechanical trauma, irritation, and frequent short haircuts, the active-duty military population is restricted in their preventive measures due to current grooming and uniform standards. In this population, early identification and treatment are necessary to manage the disease to reduce patient morbidity and ensure continued operational and medical readiness. Topical and intralesional steroids may be used in mild to moderate cases. Topical and/or systemic antibiotics may be added to the treatment regimen in cases of secondary bacterial infection. For more severe refractory cases, laser therapy or complete surgical excision may be warranted.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328, 331-333.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed April 14, 2020.
- U.S. Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. Quantico, VA: United States Marine Corps, 2018. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137. Accessed April 14, 2020.
- Grooming standards. In: US Department of the Navy. United States Navy Uniform Regulations: NAVPERS 15665I. https://www.public.navy.mil/bupers-npc/support/uniforms/uniformregulations/chapter2/Pages/2201PersonalAppearance.aspx. Updated May 2019. Accessed April 14, 2020.
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Washington, DC: Department of the Air Force, 2019. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf. Accessed April 14, 2020.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systemic review of the literature. Dermatol Ther (Heidelb). 2016;6:362-378.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Adotama P, Tinker D, Mitchell K, et al. Barber knowledge and recommendations regarding pseudofolliculitis barbae and acne keloidalis nuchae in an urban setting. JAMA Dermatol. 2017;12:1325.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with sin disease. Cutis. 2019;6:329-332.
- Medical standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf. Accessed April 27, 2020.
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- US Department of the Army. Standards of medical fitness. https://www.qmo.amedd.army.mil/diabetes/AR40_5012011.pdf. Published December 14, 2007. Accessed April 27, 2020.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed April 27, 2020.
- US Navy Aeromedical Reference and Waiver Guide. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed April 14, 2020.
- Okoye GA, Rainer BM, Leung SG, et al. Improving acne keloidalis nuchae with targeted ultraviolet B treatment: a prospective, randomized split-scalp study. Br J Dermatol. 2014;17:1156-1163.
- Kantor GR, Ratz JL, Wheeland RG. Treatment of acne keloidalis nuchae with carbon dioxide laser. J Am Acad Dermatol. 1986;14(2, pt 1):263-267.
- 18. Dragoni F, Bassi A, Cannarozzo G, et al. Successful treatment of acne keloidalis nuchae resistant to conventional therapy with 1064-nm Nd:YAG laser. G Ital Dermatol Venereol. 2013;148:231-232.
- Esmat SM, Hay RMA, Zeid OMA, et al. The efficacy of laser assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650.
- Woo DK, Treyger G, Henderson M, et al. Prospective controlled trial for the treatment of acne keloidalis nuchae with a long-pulsed neodymium-doped yttrium-aluminum-garnet laser. J Cutan Med Surg. 2018;22:236-238.
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder most commonly involving the occipital scalp and posterior neck characterized by the development of keloidlike papules, pustules, and plaques. If left untreated, this condition may progress to scarring alopecia. It primarily affects males of African descent, but it also may occur in females and in other ethnic groups. Although the exact underlying pathogenesis is unclear, close haircuts and chronic mechanical irritation to the posterior neck and scalp are known inciting factors. For this reason, AKN disproportionately affects active-duty military servicemembers who are held to strict grooming standards. The US Military maintains these grooming standards to ensure uniformity, self-discipline, and serviceability in operational settings.1 Regulations dictate short tapered hair, particularly on the back of the neck, which can require weekly to biweekly haircuts to maintain.1-5
First-line treatment of AKN is prevention by avoiding short haircuts and other forms of mechanical irritation.1,6,7 However, there are considerable barriers to this strategy within the military due to uniform regulations as well as personal appearance and grooming standards. Early identification and treatment are of utmost importance in managing AKN in the military population to ensure reduction of morbidity, prevention of late-stage disease, and continued fitness for duty. This article reviews the clinical features, epidemiology, and treatments available for management of AKN, with a special focus on the active-duty military population.
Clinical Features and Epidemiology
Acne keloidalis nuchae is a chronic inflammatory disorder characterized by the development of keloidlike papules, pustules, and plaques on the posterior neck and occipital scalp.6 Also known as folliculitis keloidalis nuchae, AKN is seen primarily in men of African descent, though cases also have been reported in females and in a few other ethnic groups.6,7 In black males, the AKN prevalence worldwide ranges from 0.5% to 13.6%. The male to female ratio is 20 to 1.7 Although the exact cause is unknown, AKN appears to develop from chronic irritation and inflammation following localized skin injury and/or trauma. Chronic irritation from close-shaved haircuts, tight-fitting shirt collars, caps, and helmets have all been implicated as considerable risk factors.6-8
Symptoms generally develop hours to days following a close haircut and begin with the early formation of inflamed irritated papules and notable erythema.6,7 These papules may become secondarily infected and develop into pustules and/or abscesses, especially in cases in which the affected individual continues to have the hair shaved. Continued use of shared razors increases the risk for secondary infection and also raises the concern for transmission of blood-borne pathogens, as AKN lesions are quick to bleed with minor trauma.7
Over time, chronic inflammation and continued trauma of the AKN papules leads to widespread fibrosis and scar formation, as the papules coalesce into larger plaques and nodules. If left untreated, these later stages of disease can progress to chronic scarring alopecia.6
Prevention
In the general population, first-line therapy of AKN is preventative. The goal is to break the cycle of chronic inflammation, thereby preventing the development of additional lesions and subsequent scarring.7 Patients should be encouraged to avoid frequent haircuts, close shaves, hats, helmets, and tight shirt collars.6-8
A 2017 cross-sectional study by Adotama et al9 investigated recognition and management of AKN in predominantly black barbershops in an urban setting. Fifty barbers from barbershops in Oklahoma City, Oklahoma, were enrolled and interviewed for the study. Of these barbers, only 44% (22/50) were able to properly identify AKN from a photograph. Although the vast majority (94% [47/50]) were aware that razor use would aggravate the condition, only 46% (23/50) reported avoidance of cutting hair for clients with active AKN.9 This study, while limited by its small sample size, showed that many barbers may be unaware of AKN and therefore unknowingly contribute to the disease process by performing haircuts on actively inflamed scalps. For this reason, it is important to educate patients about their condition and strongly recommend lifestyle and hairstyle modifications in the management of their disease.
Acne keloidalis nuchae that is severe enough to interfere with the proper use and wear of military equipment (eg, Kevlar helmets) or maintenance of regulation grooming standards does not meet military admission standards.10,11 However, mild undiagnosed cases may be overlooked during entrance physical examinations, while many servicemembers develop AKN after entering the military.10 For these individuals, long-term avoidance of haircuts is not a realistic or obtainable therapeutic option.
Treatment
Topical Therapy
Early mild to moderate cases of AKN—papules less than 3 mm, no nodules present—may be treated with potent topical steroids. Studies have shown 2-week alternating cycles of high-potency topical steroids (2 weeks of twice-daily application followed by 2 weeks without application) for 8 to 12 weeks to be effective in reducing AKN lesions.8,12 Topical clindamycin also may be added and has demonstrated efficacy particularly when pustules are present.7,8
Intralesional Steroids
For moderate cases of AKN—papules more than 3 mm, plaques, and nodules—intralesional steroid injections may be considered. Triamcinolone may be used at a dose of 5 to 40 mg/mL administered at 4-week intervals.7 More concentrated doses will produce faster responses but also carry the known risk of side effects such as hypopigmentation in darker-skinned individuals and skin atrophy.
Systemic Therapy
Systemic therapy with oral antibiotics may be warranted as an adjunct to mild to moderate cases of AKN or in cases with clear evidence of secondary infection. Long-term tetracycline antibiotics, such as minocycline and doxycycline, may be used concurrently with topical and/or intralesional steroids.6,7 Their antibacterial and anti-inflammatory effects are useful in controlling secondary infections and reducing overall chronic inflammation.
When selecting an appropriate antibiotic for long-term use in active-duty military patients, it is important to consider their effects on duty status. Doxycycline is preferred for active-duty servicemembers because it is not duty limiting or medically disqualifying.10,13-15 However, minocycline, is restricted for use in aviators and aircrew members due to the risk for central nervous system side effects, which may include light-headedness, dizziness, and vertigo.
UV Light Therapy
UV radiation has known anti-inflammatory, immunosuppressive, and antifibrotic effects and commonly is used in the treatment of many dermatologic conditions.16 Within the last decade, targeted UVB (tUVB) radiation has shown promise as an effective alternative therapy for AKN. In 2014, Okoye et al16 conducted a prospective, randomized, split-scalp study in 11 patients with AKN. Each patient underwent treatment with a tUVB device (with peaks at 303 and 313 nm) to a randomly selected side of the scalp 3 times weekly for 16 weeks. Significant reductions in lesion count were seen on the treated side after 8 (P=.03) and 16 weeks (P=.04), with no change noted on the control side. Aside from objective lesion counts, patients completed questionnaires (n=6) regarding their treatment outcomes. Notably, 83.3% (5/6) reported marked improvement in their condition. Aside from mild transient burning and erythema of the treated area, no serious side effects were reported.16
Targeted UVB phototherapy has limited utility in an operational setting due to accessibility and operational tempo. Phototherapy units typically are available only at commands in close proximity to large medical treatment facilities. Further, the vast majority of servicemembers have duty hours that are not amenable to multiple treatment sessions per week for several months. For servicemembers in administrative roles or serving in garrison or shore billets, tUVB or narrowband UV phototherapy may be viable treatment options.
Laser Therapy
Various lasers have been used to treat AKN, including the CO2 laser, pulsed dye laser, 810-nm diode laser, and 1064-nm Nd:YAG laser.6 Kantor et al17 utilized a CO2 laser with a focused beam for surgical excision of a late-stage AKN case as early as 1986. In these patients, it was demonstrated that focused CO2 laser could be used to remove fibrotic lesions in an outpatient setting with only local anesthesia. Although only 8 patients were treated in this report, no relapses occurred.17
CO2 laser evaporation using the unfocused beam setting with 130 to 150 J/cm2 has been less successful, with relapses reported in multiple cases.6 Dragoni et al18 attempted treatment with a 595-nm pulsed dye laser with 6.5-J/cm2 fluence and 0.5-millisecond pulse but faced similar results, with lesions returning within 1 month.
There have been numerous reports of clinical improvement of AKN with the use of the 1064-nm Nd:YAG laser.6,19 Esmat et al19 treated 16 patients with a fluence of 35 to 45 J/cm2 and pulse duration of 10 to 30 milliseconds adjusted to skin type and hair thickness. An overall 82% reduction in lesion count was observed after 5 treatment sessions. Biopsies following the treatment course demonstrated a significant reduction in papule and plaque count (P=.001 and P=.011, respectively), and no clinical recurrences were noted at 12 months posttreatment.19 Similarly, Woo et al20 conducted a single-blinded, randomized, controlled trial to assess the efficacy of the Nd:YAG laser in combination with topical corticosteroid therapy vs topical corticosteroid monotherapy. Of the 20 patients treated, there was a statistically significant improvement in patients with papule-only AKN who received the laser and topical combination treatment (P=.031).20
Laser therapy may be an available treatment option for military servicemembers stationed within close proximity to military treatment facilities, with the Nd:YAG laser typically having the widest availability. Although laser therapy may be effective in early stages of disease, servicemembers would have to be amenable to limitation of future hair growth in the treated areas.
Surgical Excision
Surgical excision may be considered for large, extensive, disfiguring, and/or refractory lesions. Excision is a safe and effective method to remove tender, inflamed, keloidlike masses. Techniques for excision include electrosurgical excision with secondary intention healing, excision of a horizontal ellipse involving the posterior hairline with either primary closure or secondary intention healing, and use of a semilunar tissue expander prior to excision and closure.6 Regardless of the technique, it is important to ensure that affected tissue is excised at a depth that includes the base of the hair follicles to prevent recurrence.21
Final Thoughts
Acne keloidalis nuchae is a chronic inflammatory disease that causes considerable morbidity and can lead to chronic infection, alopecia, and disfigurement of the occipital scalp and posterior neck. Although easily preventable through the avoidance of mechanical trauma, irritation, and frequent short haircuts, the active-duty military population is restricted in their preventive measures due to current grooming and uniform standards. In this population, early identification and treatment are necessary to manage the disease to reduce patient morbidity and ensure continued operational and medical readiness. Topical and intralesional steroids may be used in mild to moderate cases. Topical and/or systemic antibiotics may be added to the treatment regimen in cases of secondary bacterial infection. For more severe refractory cases, laser therapy or complete surgical excision may be warranted.
Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder most commonly involving the occipital scalp and posterior neck characterized by the development of keloidlike papules, pustules, and plaques. If left untreated, this condition may progress to scarring alopecia. It primarily affects males of African descent, but it also may occur in females and in other ethnic groups. Although the exact underlying pathogenesis is unclear, close haircuts and chronic mechanical irritation to the posterior neck and scalp are known inciting factors. For this reason, AKN disproportionately affects active-duty military servicemembers who are held to strict grooming standards. The US Military maintains these grooming standards to ensure uniformity, self-discipline, and serviceability in operational settings.1 Regulations dictate short tapered hair, particularly on the back of the neck, which can require weekly to biweekly haircuts to maintain.1-5
First-line treatment of AKN is prevention by avoiding short haircuts and other forms of mechanical irritation.1,6,7 However, there are considerable barriers to this strategy within the military due to uniform regulations as well as personal appearance and grooming standards. Early identification and treatment are of utmost importance in managing AKN in the military population to ensure reduction of morbidity, prevention of late-stage disease, and continued fitness for duty. This article reviews the clinical features, epidemiology, and treatments available for management of AKN, with a special focus on the active-duty military population.
Clinical Features and Epidemiology
Acne keloidalis nuchae is a chronic inflammatory disorder characterized by the development of keloidlike papules, pustules, and plaques on the posterior neck and occipital scalp.6 Also known as folliculitis keloidalis nuchae, AKN is seen primarily in men of African descent, though cases also have been reported in females and in a few other ethnic groups.6,7 In black males, the AKN prevalence worldwide ranges from 0.5% to 13.6%. The male to female ratio is 20 to 1.7 Although the exact cause is unknown, AKN appears to develop from chronic irritation and inflammation following localized skin injury and/or trauma. Chronic irritation from close-shaved haircuts, tight-fitting shirt collars, caps, and helmets have all been implicated as considerable risk factors.6-8
Symptoms generally develop hours to days following a close haircut and begin with the early formation of inflamed irritated papules and notable erythema.6,7 These papules may become secondarily infected and develop into pustules and/or abscesses, especially in cases in which the affected individual continues to have the hair shaved. Continued use of shared razors increases the risk for secondary infection and also raises the concern for transmission of blood-borne pathogens, as AKN lesions are quick to bleed with minor trauma.7
Over time, chronic inflammation and continued trauma of the AKN papules leads to widespread fibrosis and scar formation, as the papules coalesce into larger plaques and nodules. If left untreated, these later stages of disease can progress to chronic scarring alopecia.6
Prevention
In the general population, first-line therapy of AKN is preventative. The goal is to break the cycle of chronic inflammation, thereby preventing the development of additional lesions and subsequent scarring.7 Patients should be encouraged to avoid frequent haircuts, close shaves, hats, helmets, and tight shirt collars.6-8
A 2017 cross-sectional study by Adotama et al9 investigated recognition and management of AKN in predominantly black barbershops in an urban setting. Fifty barbers from barbershops in Oklahoma City, Oklahoma, were enrolled and interviewed for the study. Of these barbers, only 44% (22/50) were able to properly identify AKN from a photograph. Although the vast majority (94% [47/50]) were aware that razor use would aggravate the condition, only 46% (23/50) reported avoidance of cutting hair for clients with active AKN.9 This study, while limited by its small sample size, showed that many barbers may be unaware of AKN and therefore unknowingly contribute to the disease process by performing haircuts on actively inflamed scalps. For this reason, it is important to educate patients about their condition and strongly recommend lifestyle and hairstyle modifications in the management of their disease.
Acne keloidalis nuchae that is severe enough to interfere with the proper use and wear of military equipment (eg, Kevlar helmets) or maintenance of regulation grooming standards does not meet military admission standards.10,11 However, mild undiagnosed cases may be overlooked during entrance physical examinations, while many servicemembers develop AKN after entering the military.10 For these individuals, long-term avoidance of haircuts is not a realistic or obtainable therapeutic option.
Treatment
Topical Therapy
Early mild to moderate cases of AKN—papules less than 3 mm, no nodules present—may be treated with potent topical steroids. Studies have shown 2-week alternating cycles of high-potency topical steroids (2 weeks of twice-daily application followed by 2 weeks without application) for 8 to 12 weeks to be effective in reducing AKN lesions.8,12 Topical clindamycin also may be added and has demonstrated efficacy particularly when pustules are present.7,8
Intralesional Steroids
For moderate cases of AKN—papules more than 3 mm, plaques, and nodules—intralesional steroid injections may be considered. Triamcinolone may be used at a dose of 5 to 40 mg/mL administered at 4-week intervals.7 More concentrated doses will produce faster responses but also carry the known risk of side effects such as hypopigmentation in darker-skinned individuals and skin atrophy.
Systemic Therapy
Systemic therapy with oral antibiotics may be warranted as an adjunct to mild to moderate cases of AKN or in cases with clear evidence of secondary infection. Long-term tetracycline antibiotics, such as minocycline and doxycycline, may be used concurrently with topical and/or intralesional steroids.6,7 Their antibacterial and anti-inflammatory effects are useful in controlling secondary infections and reducing overall chronic inflammation.
When selecting an appropriate antibiotic for long-term use in active-duty military patients, it is important to consider their effects on duty status. Doxycycline is preferred for active-duty servicemembers because it is not duty limiting or medically disqualifying.10,13-15 However, minocycline, is restricted for use in aviators and aircrew members due to the risk for central nervous system side effects, which may include light-headedness, dizziness, and vertigo.
UV Light Therapy
UV radiation has known anti-inflammatory, immunosuppressive, and antifibrotic effects and commonly is used in the treatment of many dermatologic conditions.16 Within the last decade, targeted UVB (tUVB) radiation has shown promise as an effective alternative therapy for AKN. In 2014, Okoye et al16 conducted a prospective, randomized, split-scalp study in 11 patients with AKN. Each patient underwent treatment with a tUVB device (with peaks at 303 and 313 nm) to a randomly selected side of the scalp 3 times weekly for 16 weeks. Significant reductions in lesion count were seen on the treated side after 8 (P=.03) and 16 weeks (P=.04), with no change noted on the control side. Aside from objective lesion counts, patients completed questionnaires (n=6) regarding their treatment outcomes. Notably, 83.3% (5/6) reported marked improvement in their condition. Aside from mild transient burning and erythema of the treated area, no serious side effects were reported.16
Targeted UVB phototherapy has limited utility in an operational setting due to accessibility and operational tempo. Phototherapy units typically are available only at commands in close proximity to large medical treatment facilities. Further, the vast majority of servicemembers have duty hours that are not amenable to multiple treatment sessions per week for several months. For servicemembers in administrative roles or serving in garrison or shore billets, tUVB or narrowband UV phototherapy may be viable treatment options.
Laser Therapy
Various lasers have been used to treat AKN, including the CO2 laser, pulsed dye laser, 810-nm diode laser, and 1064-nm Nd:YAG laser.6 Kantor et al17 utilized a CO2 laser with a focused beam for surgical excision of a late-stage AKN case as early as 1986. In these patients, it was demonstrated that focused CO2 laser could be used to remove fibrotic lesions in an outpatient setting with only local anesthesia. Although only 8 patients were treated in this report, no relapses occurred.17
CO2 laser evaporation using the unfocused beam setting with 130 to 150 J/cm2 has been less successful, with relapses reported in multiple cases.6 Dragoni et al18 attempted treatment with a 595-nm pulsed dye laser with 6.5-J/cm2 fluence and 0.5-millisecond pulse but faced similar results, with lesions returning within 1 month.
There have been numerous reports of clinical improvement of AKN with the use of the 1064-nm Nd:YAG laser.6,19 Esmat et al19 treated 16 patients with a fluence of 35 to 45 J/cm2 and pulse duration of 10 to 30 milliseconds adjusted to skin type and hair thickness. An overall 82% reduction in lesion count was observed after 5 treatment sessions. Biopsies following the treatment course demonstrated a significant reduction in papule and plaque count (P=.001 and P=.011, respectively), and no clinical recurrences were noted at 12 months posttreatment.19 Similarly, Woo et al20 conducted a single-blinded, randomized, controlled trial to assess the efficacy of the Nd:YAG laser in combination with topical corticosteroid therapy vs topical corticosteroid monotherapy. Of the 20 patients treated, there was a statistically significant improvement in patients with papule-only AKN who received the laser and topical combination treatment (P=.031).20
Laser therapy may be an available treatment option for military servicemembers stationed within close proximity to military treatment facilities, with the Nd:YAG laser typically having the widest availability. Although laser therapy may be effective in early stages of disease, servicemembers would have to be amenable to limitation of future hair growth in the treated areas.
Surgical Excision
Surgical excision may be considered for large, extensive, disfiguring, and/or refractory lesions. Excision is a safe and effective method to remove tender, inflamed, keloidlike masses. Techniques for excision include electrosurgical excision with secondary intention healing, excision of a horizontal ellipse involving the posterior hairline with either primary closure or secondary intention healing, and use of a semilunar tissue expander prior to excision and closure.6 Regardless of the technique, it is important to ensure that affected tissue is excised at a depth that includes the base of the hair follicles to prevent recurrence.21
Final Thoughts
Acne keloidalis nuchae is a chronic inflammatory disease that causes considerable morbidity and can lead to chronic infection, alopecia, and disfigurement of the occipital scalp and posterior neck. Although easily preventable through the avoidance of mechanical trauma, irritation, and frequent short haircuts, the active-duty military population is restricted in their preventive measures due to current grooming and uniform standards. In this population, early identification and treatment are necessary to manage the disease to reduce patient morbidity and ensure continued operational and medical readiness. Topical and intralesional steroids may be used in mild to moderate cases. Topical and/or systemic antibiotics may be added to the treatment regimen in cases of secondary bacterial infection. For more severe refractory cases, laser therapy or complete surgical excision may be warranted.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328, 331-333.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed April 14, 2020.
- U.S. Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. Quantico, VA: United States Marine Corps, 2018. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137. Accessed April 14, 2020.
- Grooming standards. In: US Department of the Navy. United States Navy Uniform Regulations: NAVPERS 15665I. https://www.public.navy.mil/bupers-npc/support/uniforms/uniformregulations/chapter2/Pages/2201PersonalAppearance.aspx. Updated May 2019. Accessed April 14, 2020.
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Washington, DC: Department of the Air Force, 2019. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf. Accessed April 14, 2020.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systemic review of the literature. Dermatol Ther (Heidelb). 2016;6:362-378.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Adotama P, Tinker D, Mitchell K, et al. Barber knowledge and recommendations regarding pseudofolliculitis barbae and acne keloidalis nuchae in an urban setting. JAMA Dermatol. 2017;12:1325.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with sin disease. Cutis. 2019;6:329-332.
- Medical standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf. Accessed April 27, 2020.
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- US Department of the Army. Standards of medical fitness. https://www.qmo.amedd.army.mil/diabetes/AR40_5012011.pdf. Published December 14, 2007. Accessed April 27, 2020.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed April 27, 2020.
- US Navy Aeromedical Reference and Waiver Guide. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed April 14, 2020.
- Okoye GA, Rainer BM, Leung SG, et al. Improving acne keloidalis nuchae with targeted ultraviolet B treatment: a prospective, randomized split-scalp study. Br J Dermatol. 2014;17:1156-1163.
- Kantor GR, Ratz JL, Wheeland RG. Treatment of acne keloidalis nuchae with carbon dioxide laser. J Am Acad Dermatol. 1986;14(2, pt 1):263-267.
- 18. Dragoni F, Bassi A, Cannarozzo G, et al. Successful treatment of acne keloidalis nuchae resistant to conventional therapy with 1064-nm Nd:YAG laser. G Ital Dermatol Venereol. 2013;148:231-232.
- Esmat SM, Hay RMA, Zeid OMA, et al. The efficacy of laser assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650.
- Woo DK, Treyger G, Henderson M, et al. Prospective controlled trial for the treatment of acne keloidalis nuchae with a long-pulsed neodymium-doped yttrium-aluminum-garnet laser. J Cutan Med Surg. 2018;22:236-238.
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328, 331-333.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed April 14, 2020.
- U.S. Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. Quantico, VA: United States Marine Corps, 2018. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137. Accessed April 14, 2020.
- Grooming standards. In: US Department of the Navy. United States Navy Uniform Regulations: NAVPERS 15665I. https://www.public.navy.mil/bupers-npc/support/uniforms/uniformregulations/chapter2/Pages/2201PersonalAppearance.aspx. Updated May 2019. Accessed April 14, 2020.
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Washington, DC: Department of the Air Force, 2019. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf. Accessed April 14, 2020.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systemic review of the literature. Dermatol Ther (Heidelb). 2016;6:362-378.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Adotama P, Tinker D, Mitchell K, et al. Barber knowledge and recommendations regarding pseudofolliculitis barbae and acne keloidalis nuchae in an urban setting. JAMA Dermatol. 2017;12:1325.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with sin disease. Cutis. 2019;6:329-332.
- Medical standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf. Accessed April 27, 2020.
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- US Department of the Army. Standards of medical fitness. https://www.qmo.amedd.army.mil/diabetes/AR40_5012011.pdf. Published December 14, 2007. Accessed April 27, 2020.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed April 27, 2020.
- US Navy Aeromedical Reference and Waiver Guide. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed April 14, 2020.
- Okoye GA, Rainer BM, Leung SG, et al. Improving acne keloidalis nuchae with targeted ultraviolet B treatment: a prospective, randomized split-scalp study. Br J Dermatol. 2014;17:1156-1163.
- Kantor GR, Ratz JL, Wheeland RG. Treatment of acne keloidalis nuchae with carbon dioxide laser. J Am Acad Dermatol. 1986;14(2, pt 1):263-267.
- 18. Dragoni F, Bassi A, Cannarozzo G, et al. Successful treatment of acne keloidalis nuchae resistant to conventional therapy with 1064-nm Nd:YAG laser. G Ital Dermatol Venereol. 2013;148:231-232.
- Esmat SM, Hay RMA, Zeid OMA, et al. The efficacy of laser assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650.
- Woo DK, Treyger G, Henderson M, et al. Prospective controlled trial for the treatment of acne keloidalis nuchae with a long-pulsed neodymium-doped yttrium-aluminum-garnet laser. J Cutan Med Surg. 2018;22:236-238.
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
Practice Points
- Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder of the occipital scalp and posterior neck characterized by keloidlike papules, pustules, and plaques that develop following mechanical irritation.
- Military members are required to maintain short haircuts and may be disproportionately affected by AKN.
- In the military population, early identification and treatment, which includes topical steroids, oral antibiotics, UV light therapy, lasers, and surgical excision, can prevent further scarring, permanent hair loss, and disfigurement from AKN.
Hyperbaric Oxygen Therapy in Dermatology
Hyperbaric oxygen therapy (HOT) is a treatment modality dating to 1861 in the United States.1 Today, there are 14 indications2 for HOT (Table), issued by the Undersea & Hyperbaric Medical Society, which also administers an accreditation program for facilities providing HOT.3 The 14 indications also are relevant because it is unlikely that HOT will be covered by insurance for unapproved indications.4

Although HOT is not commonly seen as a first-line intervention in dermatology, there are scenarios in which it can be used to good effect: compromised grafts and flaps; poorly healing ulceration related to vasculitis and autoimmune disorders; and possibly for vascular compromise, including cutaneous ischemia caused by fillers. We review its indications, dermatologic applications, and potential complications.
Overview of HOT
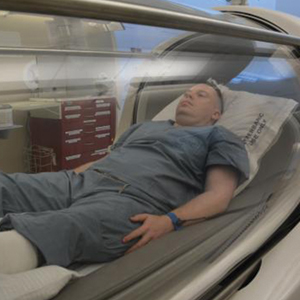
Hyperbaric oxygen therapy involves sitting or lying in a special chamber that allows for controlled levels of oxygen (O2) at increased atmospheric pressure, which specifically involves breathing near 100% O2 while inside a monoplace or multiplace chamber5 that is pressurized to greater than sea level pressure (≥1.4 atmosphere absolute).2
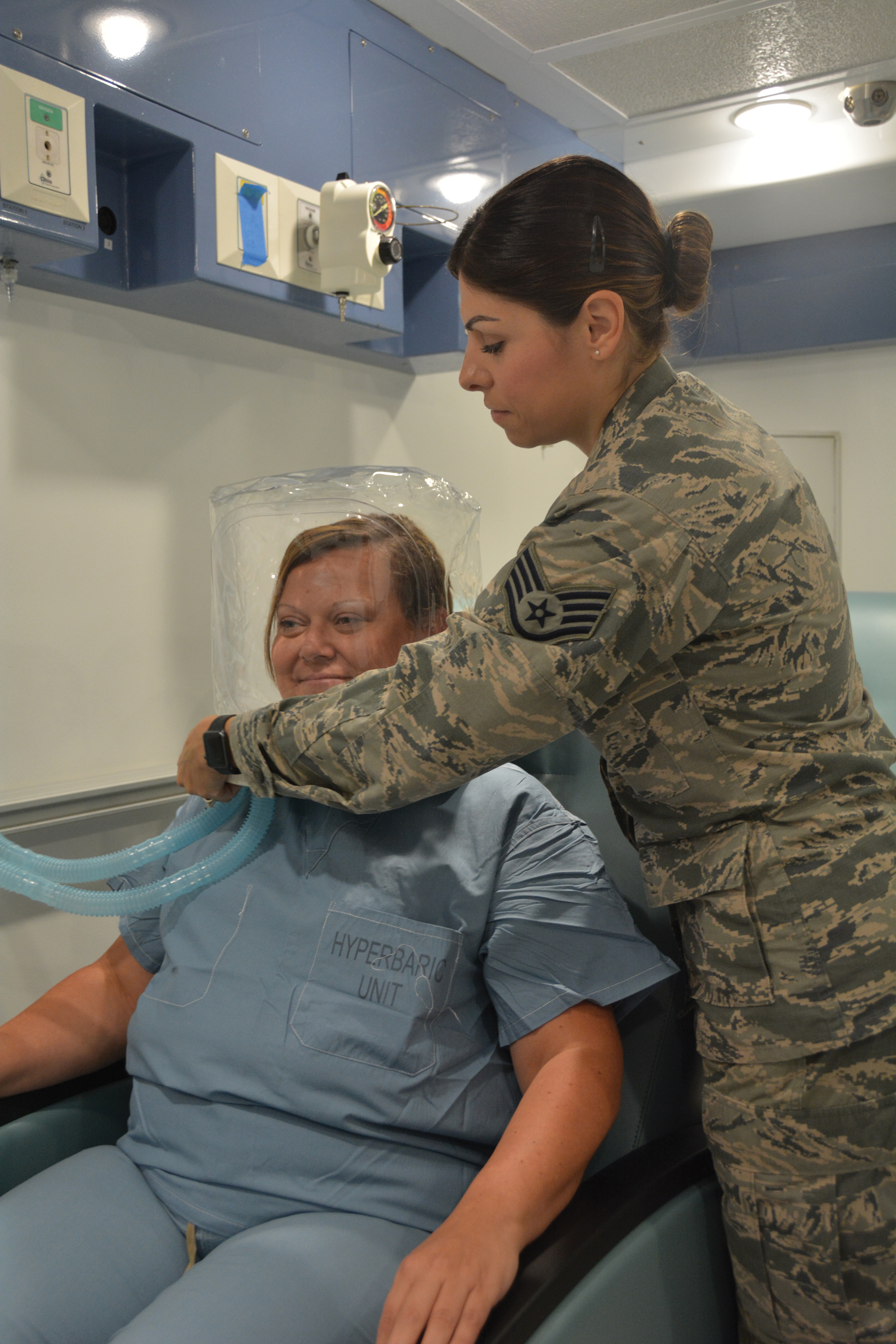
A monoplace chamber is designed to treat a single person (Figure 1); a multiplace chamber (Figure 2) accommodates as many as 5 to 25 patients.5,6 The chambers also accommodate hospital beds and medical attendants, if needed. Hyperbaric O2 is inhaled through a mask, a tight-fitting hood, or an endotracheal tube, depending on the patient’s status.7 Treatment ranges from only 1 or 2 iterations for acute conditions to 30 sessions or more for chronic conditions. Individual sessions last 45 minutes to 5 hours; 120 minutes is considered a safe maximum duration.7 A television often is provided to help the patient pass the time.8

Long-standing Use in Decompression Sickness
Hyperbaric oxygen therapy is best known for its effectiveness in treating decompression sickness (DCS) and carbon monoxide poisoning. Decompression sickness involves liberation of free gas from tissue, in the form of bubbles, when a person experiences a relative decrease in atmospheric pressure, which results in an imbalance in the sum of gas tensions in tissue compared to ambient pressure.
Decompression sickness has special military significance because it can affect divers and pilots, particularly those flying at high altitude. Over the course of 12 years, approximately 50 pilot trainees at an Air Force training site in Colorado required HOT when ground-level O2 failed to resolve their DCS symptoms.10
Symptoms of DCS range from musculoskeletal pain to severe neurologic and pulmonary complications. First-line therapy for DCS is 100% O2 at ground level. When symptoms are severe or persistent, HOT is the treatment of choice. It works by decreasing the volume of air bubbles (as predicted by Boyle’s Law), providing oxygenation to hypoxic tissue and mitigating inflammatory responses implicated in tissue injury9; HOT can be considered salvage treatment for rare, severe, or unresponsive complications of DCS during common activities such as diving and flying.
The emergent nature of DCS often necessitates an on-call, on-site HOT facility or contracted community services. Although DCS is a rare complication, it can be devastating, as was the case for a military pilot flying an ultrahigh altitude reconnaissance aircraft.11 He developed a near fatal case of neurologic DCS during a military mission and required treatment with emergent HOT. Although his symptoms were reduced with therapy, he has persistent cognitive deficits.11
Other Indications
Dermatologic Flaps and Grafts
Although less commonly discussed in dermatologic literature, the use of HOT in compromised grafts and flaps has been addressed in the plastic surgery literature. In a large multicenter study, researchers evaluated 20,821 Mohs micrographic surgery procedures and reported 149 adverse events, of which 20.1% were dehiscence and partial or full necrosis.12 These complications, though rare, are potentially devastating, particularly in cosmetically sensitive locations such as the face. Traditional care for compromised grafts and flaps includes local wound care, surgical debridement, and additional reconstructive procedures. These interventions can be expensive and uncomfortable for patients and carry risk for further morbidity.13
Grafts become compromised when their metabolic demand outpaces the ability of the recipient bed due to characteristics of the graft or the recipient bed or both. Flaps carry their own blood supply, which can be compromised if the flap is too long or too large for the pedicle, there is notable tension on the wound, or blood flow is mechanically obstructed by kinking or twisting. Under these conditions, HOT can be beneficial, as O2 dissolves in plasma, thus improving the O2 tissue cellular diffusion gradient.7 An increased level of systemic O2 promotes wound healing and graft or flap survival by improving fibroblast function, blood flow, and vascularity, and by mitigating ischemia-reperfusion injury.13
Radiation-Induced Ulceration
Radionecrosis, a complication of radiotherapy, is caused by progressive obliterating endarteritis with resultant vascular stenosis and fibroatrophy, which eventually cause stromal fibrosis.15 In a study that looked at 1267 nonmelanoma skin cancers that had been treated with radiotherapy, the ulceration rate was 6.3%. Most of the ulcerated lesions were treatable conservatively, but some were more treatment resistant.16 Hampson et al17 reported on 58 patients with cutaneous wounds due to soft-tissue radionecrosis who were treated with HOT as part of a larger observational case series in which investigators looked at multiple types of radionecrosis. They found that 76% of these patients improved: 26% showed complete resolution and the remaining 50% had 50% to 90% improvement.17
Vasculitis or Autoimmune Ulceration
Vasculitis and vasculopathy can occur independent of, or in association with, connective tissue disease and can result in chronic ulceration. At our institution, a patient with antimelanoma differentiation-associated protein 5 dermatomyositis who had refractory digital ulcerations despite intensive systemic therapy had an excellent response to HOT; ulcerations resolved after 37 treatments.18
Efrati et al19 reported on 35 patients who had chronic nonhealing vasculitic ulcerations despite immunosuppression medication who were treated with HOT. Twenty-eight patients completely healed, 4 had partial healing, and 3 had no improvement.
Mirasoglu et al20 reported on a case series of 6 systemic sclerosis patients who had ulcerations that persisted despite other treatments. After initiation of HOT, 4 patients experienced complete response and 2 experienced partial response, which is notable because such ulcerations are often extremely difficult to treat and have usually failed multiple therapies before being addressed with HOT.
Cutaneous Vascular Compromise
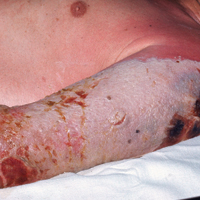
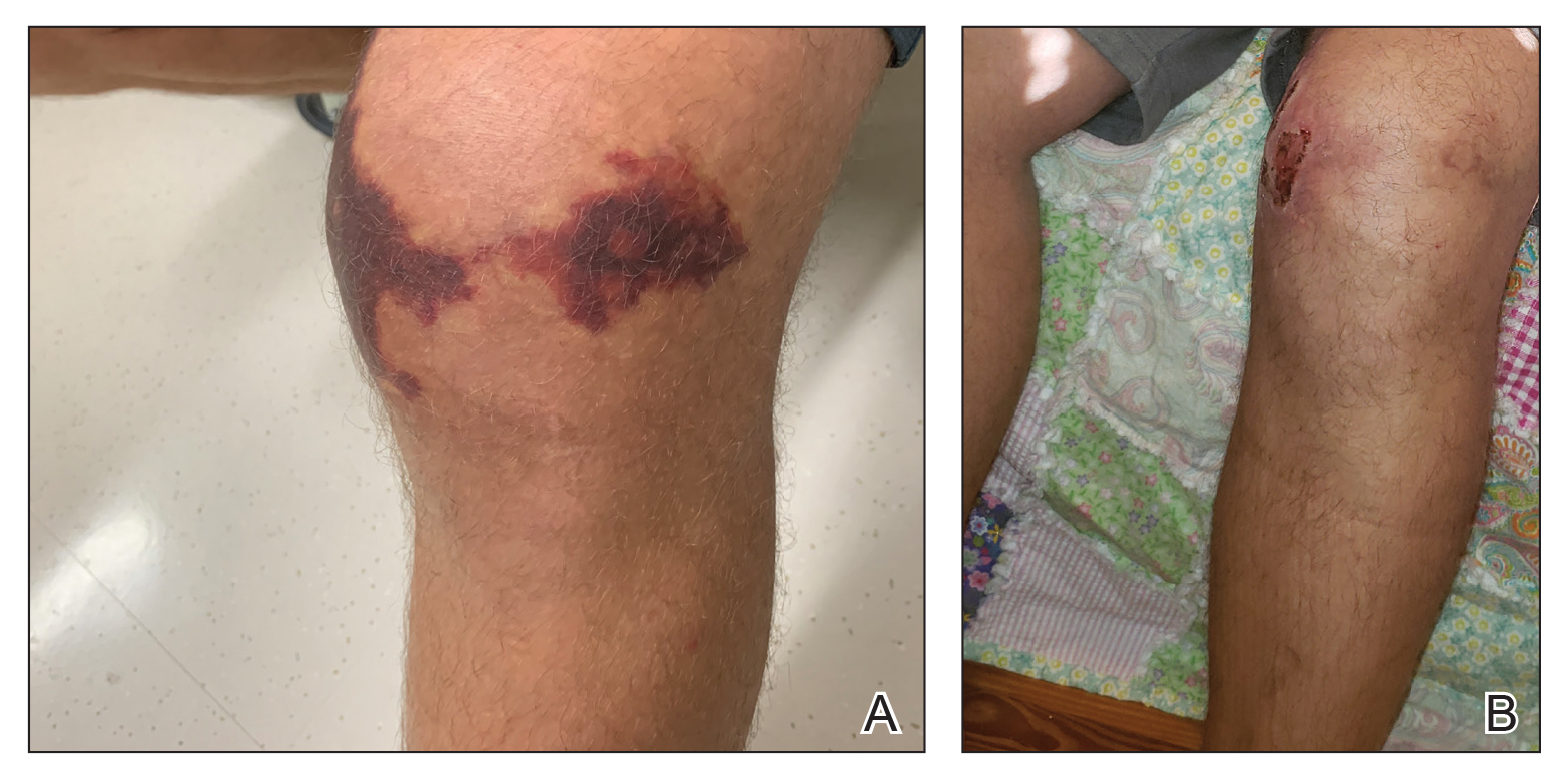
At our institution, a 36-year-old man was referred to the dermatology clinic 2 days after undergoing embolization of a symptomatic arteriovenous malformation in the right knee (Figure 3A). The procedure was complicated by cutaneous purpura concerning for necrosis, a known complication of this procedure. We referred the patient for evaluation to consider HOT. Although he was outside the ideal window for starting treatment, HOT was initiated. With a late start in treatment, areas of skin had already progressed to full necrosis, which did not respond to treatment; however, contiguous areas that initially looked very similar clinically did respond to treatment (Figure 3B). This case suggests a penumbralike effect in which vulnerable tissue that would most likely have been lost was salvaged by HOT.
Ischemia
Hyperbaric oxygen therapy has been used to treat ischemia caused by injection of cosmetic filler. Henderson et al21 described a 37-year-old woman who experienced occlusion of the left superficial temporal artery while self-injecting a hyaluronic acid filler around the temples. The problem was complicated by left-sided hearing loss, cutaneous blanching of the left face, and pain. She was treated with enoxaparin, aspirin, dexamethasone, antibiotics, and intradermal lidocaine. Additionally, she was urgently referred to a HOT facility and was treated with 6 HOT treatments in 3 days, with the first treatment provided 15 hours after the initial insult. The patient showed a decrease in ischemic discoloration over the course of the treatment. Eventually, her hearing returned to baseline and she achieved an acceptable cosmetic outcome.21
Uittenbogaard et al22 reported the treatment of a patient who experienced dermal ischemia after receiving calcium hydroxylapatite at an aesthetic clinic. She did not improve with standard treatment but subsequently experienced resolution of symptoms after treatment with HOT. She had an excellent cosmetic outcome at 6-month follow-up.22
Complications and a Contraindication
Hyperbaric oxygen therapy generally is safe, but there is potential for complications.
Fire
This rare risk has a catastrophic outcome.23 Standards for fire prevention in hyperbaric facilities are issued by the National Fire Protection Association, covering construction and building materials, lighting, electrical wiring, exposure to flammable materials, and other possible ignition sources.24
Middle Ear Barotrauma
The incidence of the most common adverse effect of HOT is reported at 2% to 30%.7,25 Middle ear barotrauma occurs most commonly during the compression phase of treatment. It is more common in patients treated in a monoplace chamber because they are kept supine and are less able to regulate middle ear pressure.26 Symptoms of middle ear barotrauma can be relieved by teaching patients autoinflation technique, such as the Valsalva maneuver, or by placing tympanoplasty tubes.27
Reversible Myopia
Caused by direct O2 toxicity to the lens, this complication can last for weeks, though it eventually resolves spontaneously. Reversible myopia has been reported to be at least as common as middle ear barotrauma.27
Other Complications
Central nervous system complications, such as seizures, and pulmonary O2 toxicity are rare, more serious complications.27
Untreated Pneumothorax
The only absolute contraindication to HOT, pneumothorax can decompensate during HOT if left untreated. However, HOT can proceed once pneumothorax is addressed.7
Conclusion
Hyperbaric O2 therapy can make a positive contribution to the dermatologic therapeutic armamentarium, in specific patients, for impending graft or flap failure, chronic wounds and ulcerations, and cutaneous vascular compromise. Although HOT is not a commonly needed treatment in dermatology, it is important to be aware of its potential because delay in treatment can decrease its effectiveness. It is recommended that dermatologists locate the nearest HOT facility and become familiar with its capabilities.
- Carney AY. Hyperbaric oxygen therapy: an introduction. Crit Care Nurs Q. 2013;36:274-279.
- Weaver LK, ed. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report. 13th ed. Undersea and Hyperbaric Medical Society. 2014.https://www.uhms.
org/images/indications/UHMS_HBO2_Indications
_13th_Ed._Front_Matter__References.pdf. Accessed December 18, 2019. - Undersea & Hyperbaric Medical Society. UHMS Hyperbaric Facility Accreditation Program. https://www.uhms.org/about/accreditation/accreditation-for-hyperbaric-medicine.html. Accessed December 18, 2019.
- Hyperbaric oxygen (HBO) therapy. US Centers for Medicare & Medicaid Services. https://www.medicare.gov/coverage/hyperbaric-oxygen-hbo-therapy. Accessed December 18, 2019.
- Gracia L, Perez-Vidal C, de Paco JM, et al. Identification and control of a multiplace hyperbaric chamber. PLoS One. 2018;13:e0200407.
- Monoplace vs multiplace hyperbaric chamber. CutisCare. https://cutiscareusa.com/hyperbaric-oxygen-therapy/monoplace-vs-multiplace-hyperbaric-chamber/. Published August 31, 2018. Accessed December 18, 2019.
- Leach RM, Rees PJ, Wilmshurst PP. Hyperbaric oxygen therapy. BMJ. 1998;317:1140-1143.
- Health Quality Ontario. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1-142.
- Vann RD, Butler FK, Mitchell SJ, et al. Decompression illness. Lancet. 2011;377:153-164.
- Rhodes WC, Hertner G, Price R, et al. Treating decompression sickness: military flight simulation site-community hospital partnership. Mil Med. 2017;182:e1718-e1721.
- Jersey SL, Baril RT, McCarty RD, et al. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med. 2010;81:64-68.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23-32.
- Bowersox JC, Strauss MB, Hart GB. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperb Med. 1986;1:141-149.
- Fernández Canedo I, Padilla España L, Francisco Millán Cayetano J, et al. Hyperbaric oxygen therapy: an alternative treatment for radiation-induced cutaneous ulcers. Australas J Dermatol. 2018;59:e203-e207.
- Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53:993-1001.
- Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
- Jeter J, Wolf EG, Richards M, et al. Successful treatment of anti-MDA5 dermatomyositis associated cutaneous digital pulp ulcerations with hyperbaric oxygen therapy [published online August 21, 2019]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001114.
- Efrati S, Bergan J, Fishlev G, et al. Hyperbaric oxygen therapy for nonhealing vasculitic ulcers. Clin Exp Dermatol. 2007;32:12-17.
- Mirasoglu B, Bagli BS, Aktas S. Hyperbaric oxygen therapy for chronic ulcers in systemic sclerosis—case series. Int J Dermatol. 2017;56:636-640.
- Henderson R, Reilly DA, Cooper JS. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: case report and issues. Plast Reconstr Surg Glob Open. 2018;6:e1618.
- Uittenbogaard D, Lansdorp CA, Bauland CG, et al. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med. 2019;46:207-210.
- Schorow S. The air in there. NFPA Journal. January 3, 2017. https://www.nfpa.org/News-and-Research/Publications-and-media/NFPA-Journal/2017/January-February-2017/Features/Hyperbaric-chambers. Accessed December 18, 2019.
- National Fire Protection Association. NFPA 99: Health Care Facilities Code 2018. https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=99. Accessed December 18, 2019.
- Blanshard J, Toma A, Bryson P, et al. Middle ear barotrauma in patients undergoing hyperbaric oxygen therapy. Clin Otolaryngol. 1996;21:400-403.
- Lima MA, Farage L, Cury MC, et al. Update on middle ear barotrauma after hyperbaric oxygen therapy—insights on pathophysiology. Int Arch Otorhinolaryngol. 2014;18:204-209.
- Heyboer M, Sharma D, Santiago W, et al. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6:210-224.
Hyperbaric oxygen therapy (HOT) is a treatment modality dating to 1861 in the United States.1 Today, there are 14 indications2 for HOT (Table), issued by the Undersea & Hyperbaric Medical Society, which also administers an accreditation program for facilities providing HOT.3 The 14 indications also are relevant because it is unlikely that HOT will be covered by insurance for unapproved indications.4

Although HOT is not commonly seen as a first-line intervention in dermatology, there are scenarios in which it can be used to good effect: compromised grafts and flaps; poorly healing ulceration related to vasculitis and autoimmune disorders; and possibly for vascular compromise, including cutaneous ischemia caused by fillers. We review its indications, dermatologic applications, and potential complications.
Overview of HOT
Hyperbaric oxygen therapy involves sitting or lying in a special chamber that allows for controlled levels of oxygen (O2) at increased atmospheric pressure, which specifically involves breathing near 100% O2 while inside a monoplace or multiplace chamber5 that is pressurized to greater than sea level pressure (≥1.4 atmosphere absolute).2
A monoplace chamber is designed to treat a single person (Figure 1); a multiplace chamber (Figure 2) accommodates as many as 5 to 25 patients.5,6 The chambers also accommodate hospital beds and medical attendants, if needed. Hyperbaric O2 is inhaled through a mask, a tight-fitting hood, or an endotracheal tube, depending on the patient’s status.7 Treatment ranges from only 1 or 2 iterations for acute conditions to 30 sessions or more for chronic conditions. Individual sessions last 45 minutes to 5 hours; 120 minutes is considered a safe maximum duration.7 A television often is provided to help the patient pass the time.8


Long-standing Use in Decompression Sickness
Hyperbaric oxygen therapy is best known for its effectiveness in treating decompression sickness (DCS) and carbon monoxide poisoning. Decompression sickness involves liberation of free gas from tissue, in the form of bubbles, when a person experiences a relative decrease in atmospheric pressure, which results in an imbalance in the sum of gas tensions in tissue compared to ambient pressure.
Decompression sickness has special military significance because it can affect divers and pilots, particularly those flying at high altitude. Over the course of 12 years, approximately 50 pilot trainees at an Air Force training site in Colorado required HOT when ground-level O2 failed to resolve their DCS symptoms.10
Symptoms of DCS range from musculoskeletal pain to severe neurologic and pulmonary complications. First-line therapy for DCS is 100% O2 at ground level. When symptoms are severe or persistent, HOT is the treatment of choice. It works by decreasing the volume of air bubbles (as predicted by Boyle’s Law), providing oxygenation to hypoxic tissue and mitigating inflammatory responses implicated in tissue injury9; HOT can be considered salvage treatment for rare, severe, or unresponsive complications of DCS during common activities such as diving and flying.
The emergent nature of DCS often necessitates an on-call, on-site HOT facility or contracted community services. Although DCS is a rare complication, it can be devastating, as was the case for a military pilot flying an ultrahigh altitude reconnaissance aircraft.11 He developed a near fatal case of neurologic DCS during a military mission and required treatment with emergent HOT. Although his symptoms were reduced with therapy, he has persistent cognitive deficits.11
Other Indications
Dermatologic Flaps and Grafts
Although less commonly discussed in dermatologic literature, the use of HOT in compromised grafts and flaps has been addressed in the plastic surgery literature. In a large multicenter study, researchers evaluated 20,821 Mohs micrographic surgery procedures and reported 149 adverse events, of which 20.1% were dehiscence and partial or full necrosis.12 These complications, though rare, are potentially devastating, particularly in cosmetically sensitive locations such as the face. Traditional care for compromised grafts and flaps includes local wound care, surgical debridement, and additional reconstructive procedures. These interventions can be expensive and uncomfortable for patients and carry risk for further morbidity.13
Grafts become compromised when their metabolic demand outpaces the ability of the recipient bed due to characteristics of the graft or the recipient bed or both. Flaps carry their own blood supply, which can be compromised if the flap is too long or too large for the pedicle, there is notable tension on the wound, or blood flow is mechanically obstructed by kinking or twisting. Under these conditions, HOT can be beneficial, as O2 dissolves in plasma, thus improving the O2 tissue cellular diffusion gradient.7 An increased level of systemic O2 promotes wound healing and graft or flap survival by improving fibroblast function, blood flow, and vascularity, and by mitigating ischemia-reperfusion injury.13
Radiation-Induced Ulceration
Radionecrosis, a complication of radiotherapy, is caused by progressive obliterating endarteritis with resultant vascular stenosis and fibroatrophy, which eventually cause stromal fibrosis.15 In a study that looked at 1267 nonmelanoma skin cancers that had been treated with radiotherapy, the ulceration rate was 6.3%. Most of the ulcerated lesions were treatable conservatively, but some were more treatment resistant.16 Hampson et al17 reported on 58 patients with cutaneous wounds due to soft-tissue radionecrosis who were treated with HOT as part of a larger observational case series in which investigators looked at multiple types of radionecrosis. They found that 76% of these patients improved: 26% showed complete resolution and the remaining 50% had 50% to 90% improvement.17
Vasculitis or Autoimmune Ulceration
Vasculitis and vasculopathy can occur independent of, or in association with, connective tissue disease and can result in chronic ulceration. At our institution, a patient with antimelanoma differentiation-associated protein 5 dermatomyositis who had refractory digital ulcerations despite intensive systemic therapy had an excellent response to HOT; ulcerations resolved after 37 treatments.18
Efrati et al19 reported on 35 patients who had chronic nonhealing vasculitic ulcerations despite immunosuppression medication who were treated with HOT. Twenty-eight patients completely healed, 4 had partial healing, and 3 had no improvement.
Mirasoglu et al20 reported on a case series of 6 systemic sclerosis patients who had ulcerations that persisted despite other treatments. After initiation of HOT, 4 patients experienced complete response and 2 experienced partial response, which is notable because such ulcerations are often extremely difficult to treat and have usually failed multiple therapies before being addressed with HOT.
Cutaneous Vascular Compromise
At our institution, a 36-year-old man was referred to the dermatology clinic 2 days after undergoing embolization of a symptomatic arteriovenous malformation in the right knee (Figure 3A). The procedure was complicated by cutaneous purpura concerning for necrosis, a known complication of this procedure. We referred the patient for evaluation to consider HOT. Although he was outside the ideal window for starting treatment, HOT was initiated. With a late start in treatment, areas of skin had already progressed to full necrosis, which did not respond to treatment; however, contiguous areas that initially looked very similar clinically did respond to treatment (Figure 3B). This case suggests a penumbralike effect in which vulnerable tissue that would most likely have been lost was salvaged by HOT.

Ischemia
Hyperbaric oxygen therapy has been used to treat ischemia caused by injection of cosmetic filler. Henderson et al21 described a 37-year-old woman who experienced occlusion of the left superficial temporal artery while self-injecting a hyaluronic acid filler around the temples. The problem was complicated by left-sided hearing loss, cutaneous blanching of the left face, and pain. She was treated with enoxaparin, aspirin, dexamethasone, antibiotics, and intradermal lidocaine. Additionally, she was urgently referred to a HOT facility and was treated with 6 HOT treatments in 3 days, with the first treatment provided 15 hours after the initial insult. The patient showed a decrease in ischemic discoloration over the course of the treatment. Eventually, her hearing returned to baseline and she achieved an acceptable cosmetic outcome.21
Uittenbogaard et al22 reported the treatment of a patient who experienced dermal ischemia after receiving calcium hydroxylapatite at an aesthetic clinic. She did not improve with standard treatment but subsequently experienced resolution of symptoms after treatment with HOT. She had an excellent cosmetic outcome at 6-month follow-up.22
Complications and a Contraindication
Hyperbaric oxygen therapy generally is safe, but there is potential for complications.
Fire
This rare risk has a catastrophic outcome.23 Standards for fire prevention in hyperbaric facilities are issued by the National Fire Protection Association, covering construction and building materials, lighting, electrical wiring, exposure to flammable materials, and other possible ignition sources.24
Middle Ear Barotrauma
The incidence of the most common adverse effect of HOT is reported at 2% to 30%.7,25 Middle ear barotrauma occurs most commonly during the compression phase of treatment. It is more common in patients treated in a monoplace chamber because they are kept supine and are less able to regulate middle ear pressure.26 Symptoms of middle ear barotrauma can be relieved by teaching patients autoinflation technique, such as the Valsalva maneuver, or by placing tympanoplasty tubes.27
Reversible Myopia
Caused by direct O2 toxicity to the lens, this complication can last for weeks, though it eventually resolves spontaneously. Reversible myopia has been reported to be at least as common as middle ear barotrauma.27
Other Complications
Central nervous system complications, such as seizures, and pulmonary O2 toxicity are rare, more serious complications.27
Untreated Pneumothorax
The only absolute contraindication to HOT, pneumothorax can decompensate during HOT if left untreated. However, HOT can proceed once pneumothorax is addressed.7
Conclusion
Hyperbaric O2 therapy can make a positive contribution to the dermatologic therapeutic armamentarium, in specific patients, for impending graft or flap failure, chronic wounds and ulcerations, and cutaneous vascular compromise. Although HOT is not a commonly needed treatment in dermatology, it is important to be aware of its potential because delay in treatment can decrease its effectiveness. It is recommended that dermatologists locate the nearest HOT facility and become familiar with its capabilities.
Hyperbaric oxygen therapy (HOT) is a treatment modality dating to 1861 in the United States.1 Today, there are 14 indications2 for HOT (Table), issued by the Undersea & Hyperbaric Medical Society, which also administers an accreditation program for facilities providing HOT.3 The 14 indications also are relevant because it is unlikely that HOT will be covered by insurance for unapproved indications.4

Although HOT is not commonly seen as a first-line intervention in dermatology, there are scenarios in which it can be used to good effect: compromised grafts and flaps; poorly healing ulceration related to vasculitis and autoimmune disorders; and possibly for vascular compromise, including cutaneous ischemia caused by fillers. We review its indications, dermatologic applications, and potential complications.
Overview of HOT
Hyperbaric oxygen therapy involves sitting or lying in a special chamber that allows for controlled levels of oxygen (O2) at increased atmospheric pressure, which specifically involves breathing near 100% O2 while inside a monoplace or multiplace chamber5 that is pressurized to greater than sea level pressure (≥1.4 atmosphere absolute).2
A monoplace chamber is designed to treat a single person (Figure 1); a multiplace chamber (Figure 2) accommodates as many as 5 to 25 patients.5,6 The chambers also accommodate hospital beds and medical attendants, if needed. Hyperbaric O2 is inhaled through a mask, a tight-fitting hood, or an endotracheal tube, depending on the patient’s status.7 Treatment ranges from only 1 or 2 iterations for acute conditions to 30 sessions or more for chronic conditions. Individual sessions last 45 minutes to 5 hours; 120 minutes is considered a safe maximum duration.7 A television often is provided to help the patient pass the time.8


Long-standing Use in Decompression Sickness
Hyperbaric oxygen therapy is best known for its effectiveness in treating decompression sickness (DCS) and carbon monoxide poisoning. Decompression sickness involves liberation of free gas from tissue, in the form of bubbles, when a person experiences a relative decrease in atmospheric pressure, which results in an imbalance in the sum of gas tensions in tissue compared to ambient pressure.
Decompression sickness has special military significance because it can affect divers and pilots, particularly those flying at high altitude. Over the course of 12 years, approximately 50 pilot trainees at an Air Force training site in Colorado required HOT when ground-level O2 failed to resolve their DCS symptoms.10
Symptoms of DCS range from musculoskeletal pain to severe neurologic and pulmonary complications. First-line therapy for DCS is 100% O2 at ground level. When symptoms are severe or persistent, HOT is the treatment of choice. It works by decreasing the volume of air bubbles (as predicted by Boyle’s Law), providing oxygenation to hypoxic tissue and mitigating inflammatory responses implicated in tissue injury9; HOT can be considered salvage treatment for rare, severe, or unresponsive complications of DCS during common activities such as diving and flying.
The emergent nature of DCS often necessitates an on-call, on-site HOT facility or contracted community services. Although DCS is a rare complication, it can be devastating, as was the case for a military pilot flying an ultrahigh altitude reconnaissance aircraft.11 He developed a near fatal case of neurologic DCS during a military mission and required treatment with emergent HOT. Although his symptoms were reduced with therapy, he has persistent cognitive deficits.11
Other Indications
Dermatologic Flaps and Grafts
Although less commonly discussed in dermatologic literature, the use of HOT in compromised grafts and flaps has been addressed in the plastic surgery literature. In a large multicenter study, researchers evaluated 20,821 Mohs micrographic surgery procedures and reported 149 adverse events, of which 20.1% were dehiscence and partial or full necrosis.12 These complications, though rare, are potentially devastating, particularly in cosmetically sensitive locations such as the face. Traditional care for compromised grafts and flaps includes local wound care, surgical debridement, and additional reconstructive procedures. These interventions can be expensive and uncomfortable for patients and carry risk for further morbidity.13
Grafts become compromised when their metabolic demand outpaces the ability of the recipient bed due to characteristics of the graft or the recipient bed or both. Flaps carry their own blood supply, which can be compromised if the flap is too long or too large for the pedicle, there is notable tension on the wound, or blood flow is mechanically obstructed by kinking or twisting. Under these conditions, HOT can be beneficial, as O2 dissolves in plasma, thus improving the O2 tissue cellular diffusion gradient.7 An increased level of systemic O2 promotes wound healing and graft or flap survival by improving fibroblast function, blood flow, and vascularity, and by mitigating ischemia-reperfusion injury.13
Radiation-Induced Ulceration
Radionecrosis, a complication of radiotherapy, is caused by progressive obliterating endarteritis with resultant vascular stenosis and fibroatrophy, which eventually cause stromal fibrosis.15 In a study that looked at 1267 nonmelanoma skin cancers that had been treated with radiotherapy, the ulceration rate was 6.3%. Most of the ulcerated lesions were treatable conservatively, but some were more treatment resistant.16 Hampson et al17 reported on 58 patients with cutaneous wounds due to soft-tissue radionecrosis who were treated with HOT as part of a larger observational case series in which investigators looked at multiple types of radionecrosis. They found that 76% of these patients improved: 26% showed complete resolution and the remaining 50% had 50% to 90% improvement.17
Vasculitis or Autoimmune Ulceration
Vasculitis and vasculopathy can occur independent of, or in association with, connective tissue disease and can result in chronic ulceration. At our institution, a patient with antimelanoma differentiation-associated protein 5 dermatomyositis who had refractory digital ulcerations despite intensive systemic therapy had an excellent response to HOT; ulcerations resolved after 37 treatments.18
Efrati et al19 reported on 35 patients who had chronic nonhealing vasculitic ulcerations despite immunosuppression medication who were treated with HOT. Twenty-eight patients completely healed, 4 had partial healing, and 3 had no improvement.
Mirasoglu et al20 reported on a case series of 6 systemic sclerosis patients who had ulcerations that persisted despite other treatments. After initiation of HOT, 4 patients experienced complete response and 2 experienced partial response, which is notable because such ulcerations are often extremely difficult to treat and have usually failed multiple therapies before being addressed with HOT.
Cutaneous Vascular Compromise
At our institution, a 36-year-old man was referred to the dermatology clinic 2 days after undergoing embolization of a symptomatic arteriovenous malformation in the right knee (Figure 3A). The procedure was complicated by cutaneous purpura concerning for necrosis, a known complication of this procedure. We referred the patient for evaluation to consider HOT. Although he was outside the ideal window for starting treatment, HOT was initiated. With a late start in treatment, areas of skin had already progressed to full necrosis, which did not respond to treatment; however, contiguous areas that initially looked very similar clinically did respond to treatment (Figure 3B). This case suggests a penumbralike effect in which vulnerable tissue that would most likely have been lost was salvaged by HOT.

Ischemia
Hyperbaric oxygen therapy has been used to treat ischemia caused by injection of cosmetic filler. Henderson et al21 described a 37-year-old woman who experienced occlusion of the left superficial temporal artery while self-injecting a hyaluronic acid filler around the temples. The problem was complicated by left-sided hearing loss, cutaneous blanching of the left face, and pain. She was treated with enoxaparin, aspirin, dexamethasone, antibiotics, and intradermal lidocaine. Additionally, she was urgently referred to a HOT facility and was treated with 6 HOT treatments in 3 days, with the first treatment provided 15 hours after the initial insult. The patient showed a decrease in ischemic discoloration over the course of the treatment. Eventually, her hearing returned to baseline and she achieved an acceptable cosmetic outcome.21
Uittenbogaard et al22 reported the treatment of a patient who experienced dermal ischemia after receiving calcium hydroxylapatite at an aesthetic clinic. She did not improve with standard treatment but subsequently experienced resolution of symptoms after treatment with HOT. She had an excellent cosmetic outcome at 6-month follow-up.22
Complications and a Contraindication
Hyperbaric oxygen therapy generally is safe, but there is potential for complications.
Fire
This rare risk has a catastrophic outcome.23 Standards for fire prevention in hyperbaric facilities are issued by the National Fire Protection Association, covering construction and building materials, lighting, electrical wiring, exposure to flammable materials, and other possible ignition sources.24
Middle Ear Barotrauma
The incidence of the most common adverse effect of HOT is reported at 2% to 30%.7,25 Middle ear barotrauma occurs most commonly during the compression phase of treatment. It is more common in patients treated in a monoplace chamber because they are kept supine and are less able to regulate middle ear pressure.26 Symptoms of middle ear barotrauma can be relieved by teaching patients autoinflation technique, such as the Valsalva maneuver, or by placing tympanoplasty tubes.27
Reversible Myopia
Caused by direct O2 toxicity to the lens, this complication can last for weeks, though it eventually resolves spontaneously. Reversible myopia has been reported to be at least as common as middle ear barotrauma.27
Other Complications
Central nervous system complications, such as seizures, and pulmonary O2 toxicity are rare, more serious complications.27
Untreated Pneumothorax
The only absolute contraindication to HOT, pneumothorax can decompensate during HOT if left untreated. However, HOT can proceed once pneumothorax is addressed.7
Conclusion
Hyperbaric O2 therapy can make a positive contribution to the dermatologic therapeutic armamentarium, in specific patients, for impending graft or flap failure, chronic wounds and ulcerations, and cutaneous vascular compromise. Although HOT is not a commonly needed treatment in dermatology, it is important to be aware of its potential because delay in treatment can decrease its effectiveness. It is recommended that dermatologists locate the nearest HOT facility and become familiar with its capabilities.
- Carney AY. Hyperbaric oxygen therapy: an introduction. Crit Care Nurs Q. 2013;36:274-279.
- Weaver LK, ed. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report. 13th ed. Undersea and Hyperbaric Medical Society. 2014.https://www.uhms.
org/images/indications/UHMS_HBO2_Indications
_13th_Ed._Front_Matter__References.pdf. Accessed December 18, 2019. - Undersea & Hyperbaric Medical Society. UHMS Hyperbaric Facility Accreditation Program. https://www.uhms.org/about/accreditation/accreditation-for-hyperbaric-medicine.html. Accessed December 18, 2019.
- Hyperbaric oxygen (HBO) therapy. US Centers for Medicare & Medicaid Services. https://www.medicare.gov/coverage/hyperbaric-oxygen-hbo-therapy. Accessed December 18, 2019.
- Gracia L, Perez-Vidal C, de Paco JM, et al. Identification and control of a multiplace hyperbaric chamber. PLoS One. 2018;13:e0200407.
- Monoplace vs multiplace hyperbaric chamber. CutisCare. https://cutiscareusa.com/hyperbaric-oxygen-therapy/monoplace-vs-multiplace-hyperbaric-chamber/. Published August 31, 2018. Accessed December 18, 2019.
- Leach RM, Rees PJ, Wilmshurst PP. Hyperbaric oxygen therapy. BMJ. 1998;317:1140-1143.
- Health Quality Ontario. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1-142.
- Vann RD, Butler FK, Mitchell SJ, et al. Decompression illness. Lancet. 2011;377:153-164.
- Rhodes WC, Hertner G, Price R, et al. Treating decompression sickness: military flight simulation site-community hospital partnership. Mil Med. 2017;182:e1718-e1721.
- Jersey SL, Baril RT, McCarty RD, et al. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med. 2010;81:64-68.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23-32.
- Bowersox JC, Strauss MB, Hart GB. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperb Med. 1986;1:141-149.
- Fernández Canedo I, Padilla España L, Francisco Millán Cayetano J, et al. Hyperbaric oxygen therapy: an alternative treatment for radiation-induced cutaneous ulcers. Australas J Dermatol. 2018;59:e203-e207.
- Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53:993-1001.
- Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
- Jeter J, Wolf EG, Richards M, et al. Successful treatment of anti-MDA5 dermatomyositis associated cutaneous digital pulp ulcerations with hyperbaric oxygen therapy [published online August 21, 2019]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001114.
- Efrati S, Bergan J, Fishlev G, et al. Hyperbaric oxygen therapy for nonhealing vasculitic ulcers. Clin Exp Dermatol. 2007;32:12-17.
- Mirasoglu B, Bagli BS, Aktas S. Hyperbaric oxygen therapy for chronic ulcers in systemic sclerosis—case series. Int J Dermatol. 2017;56:636-640.
- Henderson R, Reilly DA, Cooper JS. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: case report and issues. Plast Reconstr Surg Glob Open. 2018;6:e1618.
- Uittenbogaard D, Lansdorp CA, Bauland CG, et al. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med. 2019;46:207-210.
- Schorow S. The air in there. NFPA Journal. January 3, 2017. https://www.nfpa.org/News-and-Research/Publications-and-media/NFPA-Journal/2017/January-February-2017/Features/Hyperbaric-chambers. Accessed December 18, 2019.
- National Fire Protection Association. NFPA 99: Health Care Facilities Code 2018. https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=99. Accessed December 18, 2019.
- Blanshard J, Toma A, Bryson P, et al. Middle ear barotrauma in patients undergoing hyperbaric oxygen therapy. Clin Otolaryngol. 1996;21:400-403.
- Lima MA, Farage L, Cury MC, et al. Update on middle ear barotrauma after hyperbaric oxygen therapy—insights on pathophysiology. Int Arch Otorhinolaryngol. 2014;18:204-209.
- Heyboer M, Sharma D, Santiago W, et al. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6:210-224.
- Carney AY. Hyperbaric oxygen therapy: an introduction. Crit Care Nurs Q. 2013;36:274-279.
- Weaver LK, ed. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report. 13th ed. Undersea and Hyperbaric Medical Society. 2014.https://www.uhms.
org/images/indications/UHMS_HBO2_Indications
_13th_Ed._Front_Matter__References.pdf. Accessed December 18, 2019. - Undersea & Hyperbaric Medical Society. UHMS Hyperbaric Facility Accreditation Program. https://www.uhms.org/about/accreditation/accreditation-for-hyperbaric-medicine.html. Accessed December 18, 2019.
- Hyperbaric oxygen (HBO) therapy. US Centers for Medicare & Medicaid Services. https://www.medicare.gov/coverage/hyperbaric-oxygen-hbo-therapy. Accessed December 18, 2019.
- Gracia L, Perez-Vidal C, de Paco JM, et al. Identification and control of a multiplace hyperbaric chamber. PLoS One. 2018;13:e0200407.
- Monoplace vs multiplace hyperbaric chamber. CutisCare. https://cutiscareusa.com/hyperbaric-oxygen-therapy/monoplace-vs-multiplace-hyperbaric-chamber/. Published August 31, 2018. Accessed December 18, 2019.
- Leach RM, Rees PJ, Wilmshurst PP. Hyperbaric oxygen therapy. BMJ. 1998;317:1140-1143.
- Health Quality Ontario. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1-142.
- Vann RD, Butler FK, Mitchell SJ, et al. Decompression illness. Lancet. 2011;377:153-164.
- Rhodes WC, Hertner G, Price R, et al. Treating decompression sickness: military flight simulation site-community hospital partnership. Mil Med. 2017;182:e1718-e1721.
- Jersey SL, Baril RT, McCarty RD, et al. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med. 2010;81:64-68.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23-32.
- Bowersox JC, Strauss MB, Hart GB. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperb Med. 1986;1:141-149.
- Fernández Canedo I, Padilla España L, Francisco Millán Cayetano J, et al. Hyperbaric oxygen therapy: an alternative treatment for radiation-induced cutaneous ulcers. Australas J Dermatol. 2018;59:e203-e207.
- Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53:993-1001.
- Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
- Jeter J, Wolf EG, Richards M, et al. Successful treatment of anti-MDA5 dermatomyositis associated cutaneous digital pulp ulcerations with hyperbaric oxygen therapy [published online August 21, 2019]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001114.
- Efrati S, Bergan J, Fishlev G, et al. Hyperbaric oxygen therapy for nonhealing vasculitic ulcers. Clin Exp Dermatol. 2007;32:12-17.
- Mirasoglu B, Bagli BS, Aktas S. Hyperbaric oxygen therapy for chronic ulcers in systemic sclerosis—case series. Int J Dermatol. 2017;56:636-640.
- Henderson R, Reilly DA, Cooper JS. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: case report and issues. Plast Reconstr Surg Glob Open. 2018;6:e1618.
- Uittenbogaard D, Lansdorp CA, Bauland CG, et al. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med. 2019;46:207-210.
- Schorow S. The air in there. NFPA Journal. January 3, 2017. https://www.nfpa.org/News-and-Research/Publications-and-media/NFPA-Journal/2017/January-February-2017/Features/Hyperbaric-chambers. Accessed December 18, 2019.
- National Fire Protection Association. NFPA 99: Health Care Facilities Code 2018. https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=99. Accessed December 18, 2019.
- Blanshard J, Toma A, Bryson P, et al. Middle ear barotrauma in patients undergoing hyperbaric oxygen therapy. Clin Otolaryngol. 1996;21:400-403.
- Lima MA, Farage L, Cury MC, et al. Update on middle ear barotrauma after hyperbaric oxygen therapy—insights on pathophysiology. Int Arch Otorhinolaryngol. 2014;18:204-209.
- Heyboer M, Sharma D, Santiago W, et al. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6:210-224.
Practice Points
- Hyperbaric oxygen therapy can be considered for the treatment of failing cutaneous grafts and flaps, chronic ulcerations caused by vasculitis or autoimmune disorders, and vascular compromise, including cutaneous ischemia caused by fillers.
- Hyperbaric oxygen therapy involves 1- to 2-hour treatments, 5 days a week, for as long as 1 month.
- Hyperbaric oxygen therapy is safe and well-tolerated, with few contraindications. The sooner therapy is started, the greater the potential for benefit.
Atopic Dermatitis in the US Military
Dermatologic conditions historically have affected military members’ ability to serve during times of peace and conflict. These conditions range from chronic dermatologic diseases to environment- or occupation-related dermatologic diseases. Mild to moderate atopic dermatitis (AD) typically is a manageable skin condition. However, in a deployed setting, a flare of AD can result in the inability of a member to perform their military duty, which directly compromises mission safety and effectiveness. The military developed and updates medical standards for entry and retention of service members. These standards are designed to ensure the greatest potential for a military member to successfully serve at home station and during combat operations.
Impact of Injuries in Military
Historically, disease and nonbattle injuries have resulted in notably more hospitalizations and time lost than injuries sustained on the battlefield.1 A review of major conflicts dating from World War II shows approximately 10% of all dermatologic concerns were related to eczematous dermatitis, with 2% specifically related to AD. These numbers varied remarkably depending on the location and environment of the conflict, with eczema accounting for 25% of dermatologic concerns during the Gulf War.2 During the initial phases of Operation Iraqi Freedom, approximately 75% of hospitalizations were from disease and nonbattle injuries, of which dermatologic disease accounted for 3%.1 From 2003 to 2006 in Iraq, 35 service members were evacuated from combat zones specifically for uncontrolled AD.3 In a deployed environment, each member is critical to the unit’s success in completing their mission. A single member of a unit often is the only person qualified to perform a function for that team. There are rarely extra people with similar skills to replace a member unable to complete his/her duties. The loss of a single member compromises the effectiveness and safety of the team and can lead to mission failure. Therefore, AD can have a profound impact on military operations in a deployed environment.
Military Medical Standards for Accession and Retention
There are 2 main goals of the military medical standards. First, the individual health of the applicant or military member is of utmost importance. Applicants with medical conditions that will be exacerbated by military service or that limit the ability for successful military operations are not accepted for military service. Once an active-duty member is diagnosed with a medical condition, the military determines if limitations are needed for military assignments and deployments based on available medical care in those locations. Second, mission accomplishment in combat operations requires that healthy military members are able to complete their jobs in extreme environments and under notable stress. If an applicant has a medical condition unsuitable for military service, it is in the best interest of the applicant and the military to deny entry.
The Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03) lists conditions that are disqualifying for military service.4 Section 5.21 lists the following as disqualifying for military service in relation to eczematous dermatitis:
d. History of AD or eczema after the 12th birthday. History of residual or recurrent lesions in characteristic areas (face, neck, antecubital or popliteal fossae, occasionally wrists and hands).
e. History of recurrent or chronic nonspecific dermatitis within the past 2 years to include contact (irritant or allergic) or dyshidrotic dermatitis requiring more than treatment with topical corticosteroid.4
Although cases of incorrect diagnosis or very mild AD can be considered for a waiver, the process can be laborious and consideration or approval is not guaranteed. For current military members with new chronic eczematous dermatitis, each service has a process for evaluation and treatment. Some special operational jobs, such as aircrew, missile operators, and divers, have more restrictive medical requirements that are monitored by physicians with special training in these populations.
Atopic dermatitis affects 25% of children and 2% to 3% of adults.5 Approximately 60% of patients with AD will develop their first eruption by 1 year of age, and 90% by 5 years of age. Although the majority of patients will have resolution of their disease during childhood, 10% to 30% will have persistent disease into adulthood.5 Because the majority of AD resolves in childhood, it is understandable that asymptomatic individuals with a history of AD before 12 years of age meet military entrance medical standards.
Provoking Factors
The US Military maintains stringent medical standards because of the nature of the dynamic, rapidly changing military environment and its demands. Whether training for readiness in an austere location, deploying to extreme climates, or being stationed overseas, service members must be prepared to encounter a myriad of environmental extremes, physical stress, and psychological stressors. Environmental factors commonly experienced in the military can provoke or exacerbate symptoms of AD (Figures 1 and 2). Ideally, an individual with AD lives in a stable climate, has access to moisturizers and topical steroids, bathes regularly to remove dust and debris, wears 100% cotton garments to avoid irritation, and avoids using gear that would cause exacerbations. Service members rarely have such accommodations in deployed settings. A recent article in Military Medicine explained quite well, “If someone wanted to design an experience with the explicit goal to flare a person with otherwise well controlled atopic dermatitis it would probably look like a military deployment.”3


The United States has a military presence in countries with extreme temperature and humidity variations all over the world. Uniforms are standardized, and members are required to wear prescribed clothing with no alternatives. Uniforms are made of durable sturdy material. If uniforms can be laundered, they often are grouped together, and sensitive detergent cannot be specified. Bathing is challenging in deployed locations, with troops often going weeks using baby wipes for self-hygiene. These conditions increase risk for development of contact allergens, and little access to proper hygiene practices also increases risk for secondary infections in members with AD.
In addition to environmental challenges, the military gear and equipment used can flare AD. Service members must wear protective gear such as body armor. These heavy hard pieces of material are bulky; difficult to wash; and cause friction, sweating, and irritation. The military prepares for operations in chemical, biological, radiological, or nuclear environments, which requires wearing a rubber mask, multiple layers of boots and gloves, and thick charcoal impregnated over garments for many hours. Such conditions may flare AD or make it intolerable.
Although stress is a part of any deployment experience, excessive or prolonged stress can lead to combat operational stress reactions that inhibit a service member’s ability to function.6 Stressors during deployment can accumulate and may be caused by the operational environment, loss of fellow service members to injury or death, illness, leadership demands, personal choices, issues on the home front, interpersonal conflicts, and sleep loss.7 Atopic dermatitis can be exacerbated by such stress, leading to increased pruritus and scratching.7-9 Symptomatic AD also can play a role in worsening combat stress. Although severe pruritus may affect attentiveness to job duties during the day, these symptoms, if uncontrolled, also can negatively affect sleep. As many as 60% of patients with AD at baseline and 83% of patients with exacerbations experience sleep disturbance due to their disease.5 These stressors experienced by deployed military personnel can contribute to combat stress reactions, which may vary from simple inattentiveness to more serious behaviors such as suicidal ideation.6 Combat stress reactions inhibit a military member’s ability to function properly in the deployed environment and can lead to notable safety concerns and potential mission failure.
Vaccinations
Military members deploying overseas are required to receive specific vaccinations, including the smallpox vaccine. Although the virus was eradicated in 1980, the concern for smallpox to be used as a biological weapon in certain areas of the world necessitates continued vaccination of military populations. According to the Centers for Disease Control and Prevention, the only known reservoir for the virus is humans, and the disease has a mortality rate of 30%.10 A history of or present AD is a contraindication for primary smallpox vaccination and revaccination for nonemergency use because of the risk for eczema vaccinatum.11 The risk also applies to close contacts of vaccinated members. For 30 days after vaccination, service members must avoid skin-to-skin contact with anyone who has active AD.12 Eczema vaccinatum in vaccinated individuals is typically self-limited; however, eczema vaccinatum in nonvaccinated contacts can be severe. One case report described a 28-month-old child with refractory AD who developed severe eczema vaccinatum after contact with her recently vaccinated military parent. The child required a 48-day admission to the intensive care unit and multiple skin grafts; fortunately, the child did not develop any apparent long-term sequelae.13 This case highlights the importance of understanding the risks associated with smallpox vaccination in military members with AD and the responsibility of health care providers to properly screen and counsel individuals prior to administering smallpox vaccines.
Treatment
Treatment of mild to moderate AD is relatively straightforward in developed countries with good access to medical care. The most recent American Academy of Dermatology clinical guidelines for AD focus on minimizing irritants and triggers, regularly using moisturizers soon after bathing, and using topical steroids as needed.5 Military members face specific challenges regarding treatment of AD, particularly when deployed to remote locations without access to treatment facilities or medications. Military members are required to carry all necessary personal medications with them for at least 6 months and preferably the duration of the deployment, sometimes up to 1 year. Military members carry a large amount of gear for deployments, and it is not feasible to pack an additional 10 to 20 lb worth of emollients and topical steroids to last the entire deployment. Routine laboratory monitoring is limited or completely unavailable. Refrigeration typically is not available, making use of systemic medications nearly impossible during deployments. In the event of complications such as eczema herpeticum or secondary bacterial infection, service members could require evacuation from the deployed location to a larger field hospital or to the United States, which is costly and also removes a valuable team member from the deployed unit. These limitations in access to care, medications, and treatment options make AD a difficult condition to treat in the deployed setting.
Nonmilitary Medical Providers
Civilian providers play an important role in diagnosing and treating AD. It is vital to completely and accurately document treatment of all skin diseases; however, it is especially important for those who desire to or currently serve in the military. Military primary care providers or military dermatologists must review the information from civilian providers to aid in determining suitability for entry or retention in the military. Clearly documenting the morphology, extent of disease involvement (eg, body surface area), treatment plan, response to treatment, and exacerbating factors will aid in ensuring the patient’s medical record accurately reflects their skin disease. Ultimately, this record often is the only information available to make health determinations regarding military service.
Conclusion
A career in the military is challenging and rewarding for those who volunteer to serve. Because of the demanding and unpredictable lifestyle inherent with military service, the Department of Defense maintains strict medical standards for entrance and retention. These standards ensure members are capable of safely completing training and deploying anywhere in the world. Although AD is a relatively common and treatable skin disease in locations with well-established medical care, it can pose a notable problem for service members while deployed to austere locations with variable environments around the world. Environmental factors and gear requirements, coupled with limited access to treatment facilities and medications, render AD a potentially serious issue. Atopic dermatitis in military members can affect individual medical readiness and unit success. It is important that all providers understand the myriad effects that AD can have on an individual who wishes to join or continue service in the military.
- Zouris JM, Wade AL, Magno CP. Injury and illness casualty distributions among U.S. Army and Marine Corps personnel during Operation Iraqi Freedom. Mil Med. 2008;173:247-252.
- Gelman, AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:177-182.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 8, 2019.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Force Health Protection (Army Techniques Publication No. 4-02.8). Washington, DC: Department of the Army; March 2016. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/atp4_02x8.pdf. Accessed August 19, 2019.
- Judkins JL, Bradley DL. A review of the effectiveness of a combat and operational stress control restoration center in Afghanistan. Mil Med. 2017;182:1755-1762.
- Suarez AL, Feramisco JD, Koo J, et al. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Dermatol Venereol. 2012;92:7-15.
- Mochizuki H, Lavery MJ, Nattkemper LA, et al. Impact of acute stress on itch sensation and scratching behaviour in patients with atopic dermatitis and healthy controls. Br J Dermatol. 2019;180:821-827.
- Centers for Disease Control and Prevention. Smallpox: contraindications to vaccination. https://www.cdc.gov/smallpox/clinicians/vaccination-contraindications1.html. Updated December 5, 2016. Accessed August 19, 2019.
- Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;5:84-90.
- Reed JL, Scott DE, Bray M. Eczema vaccinatum. Clin Infect Dis. 2012;54:832-840.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccine. Clin Infect Dis. 2008;46:1555-1561.
Dermatologic conditions historically have affected military members’ ability to serve during times of peace and conflict. These conditions range from chronic dermatologic diseases to environment- or occupation-related dermatologic diseases. Mild to moderate atopic dermatitis (AD) typically is a manageable skin condition. However, in a deployed setting, a flare of AD can result in the inability of a member to perform their military duty, which directly compromises mission safety and effectiveness. The military developed and updates medical standards for entry and retention of service members. These standards are designed to ensure the greatest potential for a military member to successfully serve at home station and during combat operations.
Impact of Injuries in Military
Historically, disease and nonbattle injuries have resulted in notably more hospitalizations and time lost than injuries sustained on the battlefield.1 A review of major conflicts dating from World War II shows approximately 10% of all dermatologic concerns were related to eczematous dermatitis, with 2% specifically related to AD. These numbers varied remarkably depending on the location and environment of the conflict, with eczema accounting for 25% of dermatologic concerns during the Gulf War.2 During the initial phases of Operation Iraqi Freedom, approximately 75% of hospitalizations were from disease and nonbattle injuries, of which dermatologic disease accounted for 3%.1 From 2003 to 2006 in Iraq, 35 service members were evacuated from combat zones specifically for uncontrolled AD.3 In a deployed environment, each member is critical to the unit’s success in completing their mission. A single member of a unit often is the only person qualified to perform a function for that team. There are rarely extra people with similar skills to replace a member unable to complete his/her duties. The loss of a single member compromises the effectiveness and safety of the team and can lead to mission failure. Therefore, AD can have a profound impact on military operations in a deployed environment.
Military Medical Standards for Accession and Retention
There are 2 main goals of the military medical standards. First, the individual health of the applicant or military member is of utmost importance. Applicants with medical conditions that will be exacerbated by military service or that limit the ability for successful military operations are not accepted for military service. Once an active-duty member is diagnosed with a medical condition, the military determines if limitations are needed for military assignments and deployments based on available medical care in those locations. Second, mission accomplishment in combat operations requires that healthy military members are able to complete their jobs in extreme environments and under notable stress. If an applicant has a medical condition unsuitable for military service, it is in the best interest of the applicant and the military to deny entry.
The Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03) lists conditions that are disqualifying for military service.4 Section 5.21 lists the following as disqualifying for military service in relation to eczematous dermatitis:
d. History of AD or eczema after the 12th birthday. History of residual or recurrent lesions in characteristic areas (face, neck, antecubital or popliteal fossae, occasionally wrists and hands).
e. History of recurrent or chronic nonspecific dermatitis within the past 2 years to include contact (irritant or allergic) or dyshidrotic dermatitis requiring more than treatment with topical corticosteroid.4
Although cases of incorrect diagnosis or very mild AD can be considered for a waiver, the process can be laborious and consideration or approval is not guaranteed. For current military members with new chronic eczematous dermatitis, each service has a process for evaluation and treatment. Some special operational jobs, such as aircrew, missile operators, and divers, have more restrictive medical requirements that are monitored by physicians with special training in these populations.
Atopic dermatitis affects 25% of children and 2% to 3% of adults.5 Approximately 60% of patients with AD will develop their first eruption by 1 year of age, and 90% by 5 years of age. Although the majority of patients will have resolution of their disease during childhood, 10% to 30% will have persistent disease into adulthood.5 Because the majority of AD resolves in childhood, it is understandable that asymptomatic individuals with a history of AD before 12 years of age meet military entrance medical standards.
Provoking Factors
The US Military maintains stringent medical standards because of the nature of the dynamic, rapidly changing military environment and its demands. Whether training for readiness in an austere location, deploying to extreme climates, or being stationed overseas, service members must be prepared to encounter a myriad of environmental extremes, physical stress, and psychological stressors. Environmental factors commonly experienced in the military can provoke or exacerbate symptoms of AD (Figures 1 and 2). Ideally, an individual with AD lives in a stable climate, has access to moisturizers and topical steroids, bathes regularly to remove dust and debris, wears 100% cotton garments to avoid irritation, and avoids using gear that would cause exacerbations. Service members rarely have such accommodations in deployed settings. A recent article in Military Medicine explained quite well, “If someone wanted to design an experience with the explicit goal to flare a person with otherwise well controlled atopic dermatitis it would probably look like a military deployment.”3


The United States has a military presence in countries with extreme temperature and humidity variations all over the world. Uniforms are standardized, and members are required to wear prescribed clothing with no alternatives. Uniforms are made of durable sturdy material. If uniforms can be laundered, they often are grouped together, and sensitive detergent cannot be specified. Bathing is challenging in deployed locations, with troops often going weeks using baby wipes for self-hygiene. These conditions increase risk for development of contact allergens, and little access to proper hygiene practices also increases risk for secondary infections in members with AD.
In addition to environmental challenges, the military gear and equipment used can flare AD. Service members must wear protective gear such as body armor. These heavy hard pieces of material are bulky; difficult to wash; and cause friction, sweating, and irritation. The military prepares for operations in chemical, biological, radiological, or nuclear environments, which requires wearing a rubber mask, multiple layers of boots and gloves, and thick charcoal impregnated over garments for many hours. Such conditions may flare AD or make it intolerable.
Although stress is a part of any deployment experience, excessive or prolonged stress can lead to combat operational stress reactions that inhibit a service member’s ability to function.6 Stressors during deployment can accumulate and may be caused by the operational environment, loss of fellow service members to injury or death, illness, leadership demands, personal choices, issues on the home front, interpersonal conflicts, and sleep loss.7 Atopic dermatitis can be exacerbated by such stress, leading to increased pruritus and scratching.7-9 Symptomatic AD also can play a role in worsening combat stress. Although severe pruritus may affect attentiveness to job duties during the day, these symptoms, if uncontrolled, also can negatively affect sleep. As many as 60% of patients with AD at baseline and 83% of patients with exacerbations experience sleep disturbance due to their disease.5 These stressors experienced by deployed military personnel can contribute to combat stress reactions, which may vary from simple inattentiveness to more serious behaviors such as suicidal ideation.6 Combat stress reactions inhibit a military member’s ability to function properly in the deployed environment and can lead to notable safety concerns and potential mission failure.
Vaccinations
Military members deploying overseas are required to receive specific vaccinations, including the smallpox vaccine. Although the virus was eradicated in 1980, the concern for smallpox to be used as a biological weapon in certain areas of the world necessitates continued vaccination of military populations. According to the Centers for Disease Control and Prevention, the only known reservoir for the virus is humans, and the disease has a mortality rate of 30%.10 A history of or present AD is a contraindication for primary smallpox vaccination and revaccination for nonemergency use because of the risk for eczema vaccinatum.11 The risk also applies to close contacts of vaccinated members. For 30 days after vaccination, service members must avoid skin-to-skin contact with anyone who has active AD.12 Eczema vaccinatum in vaccinated individuals is typically self-limited; however, eczema vaccinatum in nonvaccinated contacts can be severe. One case report described a 28-month-old child with refractory AD who developed severe eczema vaccinatum after contact with her recently vaccinated military parent. The child required a 48-day admission to the intensive care unit and multiple skin grafts; fortunately, the child did not develop any apparent long-term sequelae.13 This case highlights the importance of understanding the risks associated with smallpox vaccination in military members with AD and the responsibility of health care providers to properly screen and counsel individuals prior to administering smallpox vaccines.
Treatment
Treatment of mild to moderate AD is relatively straightforward in developed countries with good access to medical care. The most recent American Academy of Dermatology clinical guidelines for AD focus on minimizing irritants and triggers, regularly using moisturizers soon after bathing, and using topical steroids as needed.5 Military members face specific challenges regarding treatment of AD, particularly when deployed to remote locations without access to treatment facilities or medications. Military members are required to carry all necessary personal medications with them for at least 6 months and preferably the duration of the deployment, sometimes up to 1 year. Military members carry a large amount of gear for deployments, and it is not feasible to pack an additional 10 to 20 lb worth of emollients and topical steroids to last the entire deployment. Routine laboratory monitoring is limited or completely unavailable. Refrigeration typically is not available, making use of systemic medications nearly impossible during deployments. In the event of complications such as eczema herpeticum or secondary bacterial infection, service members could require evacuation from the deployed location to a larger field hospital or to the United States, which is costly and also removes a valuable team member from the deployed unit. These limitations in access to care, medications, and treatment options make AD a difficult condition to treat in the deployed setting.
Nonmilitary Medical Providers
Civilian providers play an important role in diagnosing and treating AD. It is vital to completely and accurately document treatment of all skin diseases; however, it is especially important for those who desire to or currently serve in the military. Military primary care providers or military dermatologists must review the information from civilian providers to aid in determining suitability for entry or retention in the military. Clearly documenting the morphology, extent of disease involvement (eg, body surface area), treatment plan, response to treatment, and exacerbating factors will aid in ensuring the patient’s medical record accurately reflects their skin disease. Ultimately, this record often is the only information available to make health determinations regarding military service.
Conclusion
A career in the military is challenging and rewarding for those who volunteer to serve. Because of the demanding and unpredictable lifestyle inherent with military service, the Department of Defense maintains strict medical standards for entrance and retention. These standards ensure members are capable of safely completing training and deploying anywhere in the world. Although AD is a relatively common and treatable skin disease in locations with well-established medical care, it can pose a notable problem for service members while deployed to austere locations with variable environments around the world. Environmental factors and gear requirements, coupled with limited access to treatment facilities and medications, render AD a potentially serious issue. Atopic dermatitis in military members can affect individual medical readiness and unit success. It is important that all providers understand the myriad effects that AD can have on an individual who wishes to join or continue service in the military.
Dermatologic conditions historically have affected military members’ ability to serve during times of peace and conflict. These conditions range from chronic dermatologic diseases to environment- or occupation-related dermatologic diseases. Mild to moderate atopic dermatitis (AD) typically is a manageable skin condition. However, in a deployed setting, a flare of AD can result in the inability of a member to perform their military duty, which directly compromises mission safety and effectiveness. The military developed and updates medical standards for entry and retention of service members. These standards are designed to ensure the greatest potential for a military member to successfully serve at home station and during combat operations.
Impact of Injuries in Military
Historically, disease and nonbattle injuries have resulted in notably more hospitalizations and time lost than injuries sustained on the battlefield.1 A review of major conflicts dating from World War II shows approximately 10% of all dermatologic concerns were related to eczematous dermatitis, with 2% specifically related to AD. These numbers varied remarkably depending on the location and environment of the conflict, with eczema accounting for 25% of dermatologic concerns during the Gulf War.2 During the initial phases of Operation Iraqi Freedom, approximately 75% of hospitalizations were from disease and nonbattle injuries, of which dermatologic disease accounted for 3%.1 From 2003 to 2006 in Iraq, 35 service members were evacuated from combat zones specifically for uncontrolled AD.3 In a deployed environment, each member is critical to the unit’s success in completing their mission. A single member of a unit often is the only person qualified to perform a function for that team. There are rarely extra people with similar skills to replace a member unable to complete his/her duties. The loss of a single member compromises the effectiveness and safety of the team and can lead to mission failure. Therefore, AD can have a profound impact on military operations in a deployed environment.
Military Medical Standards for Accession and Retention
There are 2 main goals of the military medical standards. First, the individual health of the applicant or military member is of utmost importance. Applicants with medical conditions that will be exacerbated by military service or that limit the ability for successful military operations are not accepted for military service. Once an active-duty member is diagnosed with a medical condition, the military determines if limitations are needed for military assignments and deployments based on available medical care in those locations. Second, mission accomplishment in combat operations requires that healthy military members are able to complete their jobs in extreme environments and under notable stress. If an applicant has a medical condition unsuitable for military service, it is in the best interest of the applicant and the military to deny entry.
The Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03) lists conditions that are disqualifying for military service.4 Section 5.21 lists the following as disqualifying for military service in relation to eczematous dermatitis:
d. History of AD or eczema after the 12th birthday. History of residual or recurrent lesions in characteristic areas (face, neck, antecubital or popliteal fossae, occasionally wrists and hands).
e. History of recurrent or chronic nonspecific dermatitis within the past 2 years to include contact (irritant or allergic) or dyshidrotic dermatitis requiring more than treatment with topical corticosteroid.4
Although cases of incorrect diagnosis or very mild AD can be considered for a waiver, the process can be laborious and consideration or approval is not guaranteed. For current military members with new chronic eczematous dermatitis, each service has a process for evaluation and treatment. Some special operational jobs, such as aircrew, missile operators, and divers, have more restrictive medical requirements that are monitored by physicians with special training in these populations.
Atopic dermatitis affects 25% of children and 2% to 3% of adults.5 Approximately 60% of patients with AD will develop their first eruption by 1 year of age, and 90% by 5 years of age. Although the majority of patients will have resolution of their disease during childhood, 10% to 30% will have persistent disease into adulthood.5 Because the majority of AD resolves in childhood, it is understandable that asymptomatic individuals with a history of AD before 12 years of age meet military entrance medical standards.
Provoking Factors
The US Military maintains stringent medical standards because of the nature of the dynamic, rapidly changing military environment and its demands. Whether training for readiness in an austere location, deploying to extreme climates, or being stationed overseas, service members must be prepared to encounter a myriad of environmental extremes, physical stress, and psychological stressors. Environmental factors commonly experienced in the military can provoke or exacerbate symptoms of AD (Figures 1 and 2). Ideally, an individual with AD lives in a stable climate, has access to moisturizers and topical steroids, bathes regularly to remove dust and debris, wears 100% cotton garments to avoid irritation, and avoids using gear that would cause exacerbations. Service members rarely have such accommodations in deployed settings. A recent article in Military Medicine explained quite well, “If someone wanted to design an experience with the explicit goal to flare a person with otherwise well controlled atopic dermatitis it would probably look like a military deployment.”3


The United States has a military presence in countries with extreme temperature and humidity variations all over the world. Uniforms are standardized, and members are required to wear prescribed clothing with no alternatives. Uniforms are made of durable sturdy material. If uniforms can be laundered, they often are grouped together, and sensitive detergent cannot be specified. Bathing is challenging in deployed locations, with troops often going weeks using baby wipes for self-hygiene. These conditions increase risk for development of contact allergens, and little access to proper hygiene practices also increases risk for secondary infections in members with AD.
In addition to environmental challenges, the military gear and equipment used can flare AD. Service members must wear protective gear such as body armor. These heavy hard pieces of material are bulky; difficult to wash; and cause friction, sweating, and irritation. The military prepares for operations in chemical, biological, radiological, or nuclear environments, which requires wearing a rubber mask, multiple layers of boots and gloves, and thick charcoal impregnated over garments for many hours. Such conditions may flare AD or make it intolerable.
Although stress is a part of any deployment experience, excessive or prolonged stress can lead to combat operational stress reactions that inhibit a service member’s ability to function.6 Stressors during deployment can accumulate and may be caused by the operational environment, loss of fellow service members to injury or death, illness, leadership demands, personal choices, issues on the home front, interpersonal conflicts, and sleep loss.7 Atopic dermatitis can be exacerbated by such stress, leading to increased pruritus and scratching.7-9 Symptomatic AD also can play a role in worsening combat stress. Although severe pruritus may affect attentiveness to job duties during the day, these symptoms, if uncontrolled, also can negatively affect sleep. As many as 60% of patients with AD at baseline and 83% of patients with exacerbations experience sleep disturbance due to their disease.5 These stressors experienced by deployed military personnel can contribute to combat stress reactions, which may vary from simple inattentiveness to more serious behaviors such as suicidal ideation.6 Combat stress reactions inhibit a military member’s ability to function properly in the deployed environment and can lead to notable safety concerns and potential mission failure.
Vaccinations
Military members deploying overseas are required to receive specific vaccinations, including the smallpox vaccine. Although the virus was eradicated in 1980, the concern for smallpox to be used as a biological weapon in certain areas of the world necessitates continued vaccination of military populations. According to the Centers for Disease Control and Prevention, the only known reservoir for the virus is humans, and the disease has a mortality rate of 30%.10 A history of or present AD is a contraindication for primary smallpox vaccination and revaccination for nonemergency use because of the risk for eczema vaccinatum.11 The risk also applies to close contacts of vaccinated members. For 30 days after vaccination, service members must avoid skin-to-skin contact with anyone who has active AD.12 Eczema vaccinatum in vaccinated individuals is typically self-limited; however, eczema vaccinatum in nonvaccinated contacts can be severe. One case report described a 28-month-old child with refractory AD who developed severe eczema vaccinatum after contact with her recently vaccinated military parent. The child required a 48-day admission to the intensive care unit and multiple skin grafts; fortunately, the child did not develop any apparent long-term sequelae.13 This case highlights the importance of understanding the risks associated with smallpox vaccination in military members with AD and the responsibility of health care providers to properly screen and counsel individuals prior to administering smallpox vaccines.
Treatment
Treatment of mild to moderate AD is relatively straightforward in developed countries with good access to medical care. The most recent American Academy of Dermatology clinical guidelines for AD focus on minimizing irritants and triggers, regularly using moisturizers soon after bathing, and using topical steroids as needed.5 Military members face specific challenges regarding treatment of AD, particularly when deployed to remote locations without access to treatment facilities or medications. Military members are required to carry all necessary personal medications with them for at least 6 months and preferably the duration of the deployment, sometimes up to 1 year. Military members carry a large amount of gear for deployments, and it is not feasible to pack an additional 10 to 20 lb worth of emollients and topical steroids to last the entire deployment. Routine laboratory monitoring is limited or completely unavailable. Refrigeration typically is not available, making use of systemic medications nearly impossible during deployments. In the event of complications such as eczema herpeticum or secondary bacterial infection, service members could require evacuation from the deployed location to a larger field hospital or to the United States, which is costly and also removes a valuable team member from the deployed unit. These limitations in access to care, medications, and treatment options make AD a difficult condition to treat in the deployed setting.
Nonmilitary Medical Providers
Civilian providers play an important role in diagnosing and treating AD. It is vital to completely and accurately document treatment of all skin diseases; however, it is especially important for those who desire to or currently serve in the military. Military primary care providers or military dermatologists must review the information from civilian providers to aid in determining suitability for entry or retention in the military. Clearly documenting the morphology, extent of disease involvement (eg, body surface area), treatment plan, response to treatment, and exacerbating factors will aid in ensuring the patient’s medical record accurately reflects their skin disease. Ultimately, this record often is the only information available to make health determinations regarding military service.
Conclusion
A career in the military is challenging and rewarding for those who volunteer to serve. Because of the demanding and unpredictable lifestyle inherent with military service, the Department of Defense maintains strict medical standards for entrance and retention. These standards ensure members are capable of safely completing training and deploying anywhere in the world. Although AD is a relatively common and treatable skin disease in locations with well-established medical care, it can pose a notable problem for service members while deployed to austere locations with variable environments around the world. Environmental factors and gear requirements, coupled with limited access to treatment facilities and medications, render AD a potentially serious issue. Atopic dermatitis in military members can affect individual medical readiness and unit success. It is important that all providers understand the myriad effects that AD can have on an individual who wishes to join or continue service in the military.
- Zouris JM, Wade AL, Magno CP. Injury and illness casualty distributions among U.S. Army and Marine Corps personnel during Operation Iraqi Freedom. Mil Med. 2008;173:247-252.
- Gelman, AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:177-182.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 8, 2019.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Force Health Protection (Army Techniques Publication No. 4-02.8). Washington, DC: Department of the Army; March 2016. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/atp4_02x8.pdf. Accessed August 19, 2019.
- Judkins JL, Bradley DL. A review of the effectiveness of a combat and operational stress control restoration center in Afghanistan. Mil Med. 2017;182:1755-1762.
- Suarez AL, Feramisco JD, Koo J, et al. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Dermatol Venereol. 2012;92:7-15.
- Mochizuki H, Lavery MJ, Nattkemper LA, et al. Impact of acute stress on itch sensation and scratching behaviour in patients with atopic dermatitis and healthy controls. Br J Dermatol. 2019;180:821-827.
- Centers for Disease Control and Prevention. Smallpox: contraindications to vaccination. https://www.cdc.gov/smallpox/clinicians/vaccination-contraindications1.html. Updated December 5, 2016. Accessed August 19, 2019.
- Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;5:84-90.
- Reed JL, Scott DE, Bray M. Eczema vaccinatum. Clin Infect Dis. 2012;54:832-840.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccine. Clin Infect Dis. 2008;46:1555-1561.
- Zouris JM, Wade AL, Magno CP. Injury and illness casualty distributions among U.S. Army and Marine Corps personnel during Operation Iraqi Freedom. Mil Med. 2008;173:247-252.
- Gelman, AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:177-182.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 8, 2019.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Force Health Protection (Army Techniques Publication No. 4-02.8). Washington, DC: Department of the Army; March 2016. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/atp4_02x8.pdf. Accessed August 19, 2019.
- Judkins JL, Bradley DL. A review of the effectiveness of a combat and operational stress control restoration center in Afghanistan. Mil Med. 2017;182:1755-1762.
- Suarez AL, Feramisco JD, Koo J, et al. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Dermatol Venereol. 2012;92:7-15.
- Mochizuki H, Lavery MJ, Nattkemper LA, et al. Impact of acute stress on itch sensation and scratching behaviour in patients with atopic dermatitis and healthy controls. Br J Dermatol. 2019;180:821-827.
- Centers for Disease Control and Prevention. Smallpox: contraindications to vaccination. https://www.cdc.gov/smallpox/clinicians/vaccination-contraindications1.html. Updated December 5, 2016. Accessed August 19, 2019.
- Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;5:84-90.
- Reed JL, Scott DE, Bray M. Eczema vaccinatum. Clin Infect Dis. 2012;54:832-840.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccine. Clin Infect Dis. 2008;46:1555-1561.
Practice Points
- The US Military follows strict medical eligibility requirements for enlistment and retention. Atopic dermatitis (AD) and chronic eczematous conditions after 12 years of age is disqualifying for military service, but waivers may be possible for mild cases.
- Unpredictable and rigorous environmental and occupational stressors associated with military service as well as limited access to medical care make AD a challenging condition to manage for service members, particularly during military deployment.
- Accurate diagnosis and documentation of AD in childhood and adolescence by nonmilitary providers are essential, as they will aid in appropriately determining an applicant’s potential to successfully serve in the military.
- For current service members, nonmilitary providers play a vital role in diagnosis and management where military dermatologists are not readily available.
Treatment Consideration for US Military Members With Skin Disease
The National Defense Authorization Act for Fiscal Year 20171 has changed military medicine, including substantial reduction in military medical personnel as positions are converted to combat functions. As a result, there will be fewer military dermatologists, which means many US soldiers, sailors, airmen, and marines will seek medical care outside of military treatment facilities. This article highlights some unique treatment considerations in this patient population for our civilian dermatology colleagues.
Medical Readiness
In 2015, General Joseph F. Dunford Jr, 19th Chairman of the Joint Chiefs of Staff, made readiness his top priority for the US Armed Forces.2 Readiness refers to service members’ ability to deploy to locations across the globe and perform their military duties with little advanced notice, which requires personnel to be medically prepared at all times to leave home and perform their duties in locations with limited medical support.
Medical readiness is maintaining a unit that is medically able to perform its military function both at home and in a deployed environment. Military members’ medical readiness status is carefully tracked and determined via annual physical, dental, hearing, and vision examinations, as well as human immunodeficiency virus status and immunizations. The readiness status of the unit (ie, the number of troops ready to deploy at any given time) is available to commanders at all levels at any time. Each military branch has tracking systems that allow commanders to know when a member is past due for an examination or if a member’s medical status has changed, making them nondeployable. When a member is nondeployable, it affects the unit’s ability to perform its mission and degrades its readiness. If readiness is suboptimal, the military cannot deploy and complete its missions, which is why readiness is a top priority. The primary function of military medicine is to support the medical readiness of the force.
Deployment Eligibility
A unique aspect of military medicine that can be foreign to civilian physicians is the unit commanders’ authority to request and receive information on military members’ medical conditions as they relate to readiness. Under most circumstances, an individual’s medical information is his/her private information; however, that is not always the case in the military. If a member’s medical status changes and he/she becomes nondeployable, by regulation the commander can be privy to pertinent aspects of that member’s medical condition as it affects unit readiness, including the diagnosis, treatment plan, and prognosis. Commanders need this information to aid in the member’s recovery, ensure training does not impact his/her care, and identify possible need of replacement.
Published accession guidelines are used to determine medical eligibility for service.3 These instructions are organized by major organ systems and broad disease categories. They provide guidance on medically disqualifying conditions. The Table outlines those conditions that apply to the skin.3 Individual military branches may have additional regulations with guidance on medically disqualifying conditions that are job specific. Additional regulations also are available based on an area of military operation that can be more restrictive and specific to those locations.4
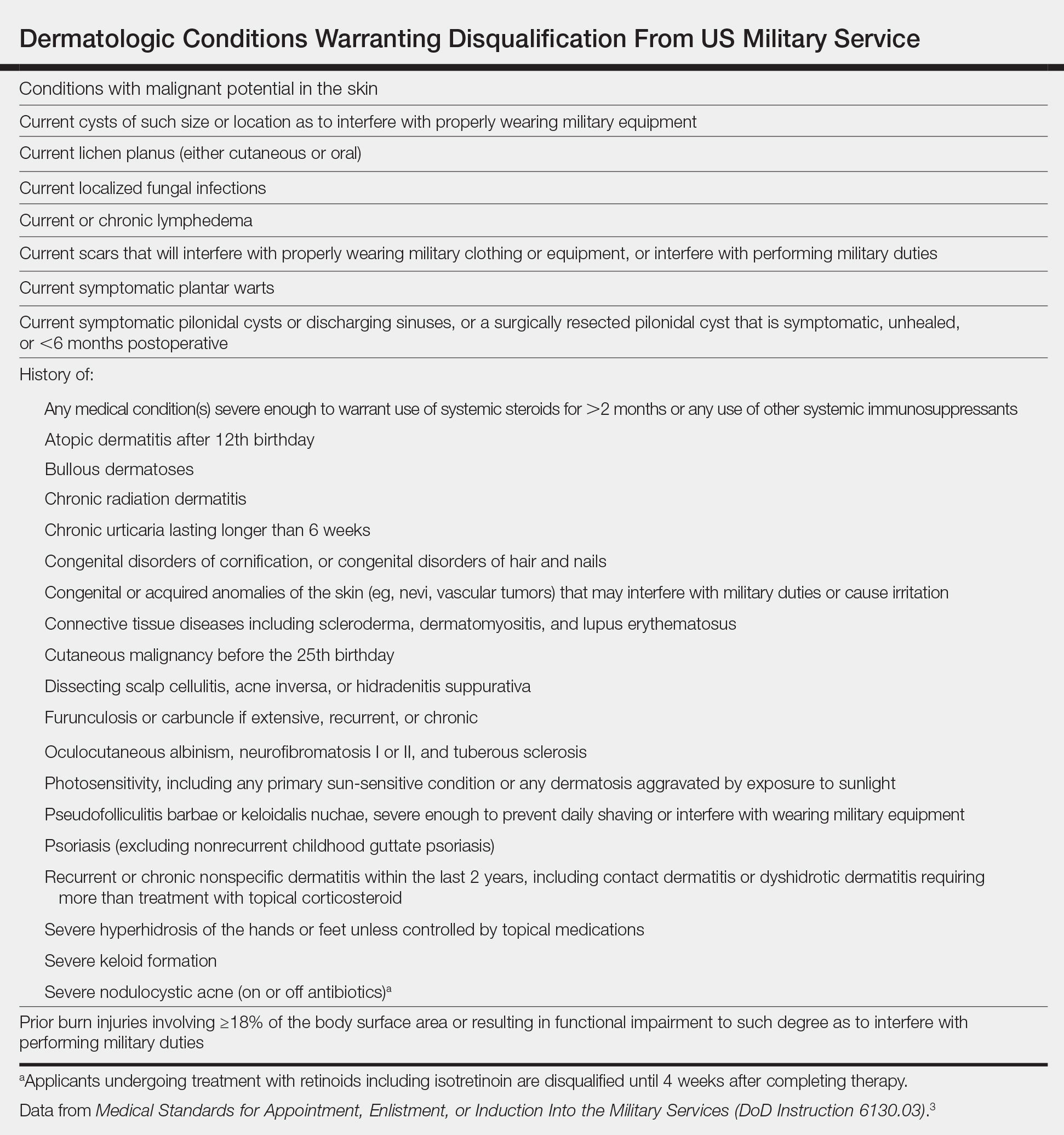
Similarly, each military branch has its own retention standards.5,6 Previously healthy individuals can develop new medical conditions, and commanders are notified if a service member becomes medically nondeployable. If a medical condition limits a service member’s ability to deploy, he/she will be evaluated for retention by a medical evaluation board (MEB). Three outcomes are possible: return in current function, retain the service member but retrain in another military occupation, or separate from military service.7 Rarely, waivers are provided so that the service member can return to duty.
Readiness and Patient Care
Importantly, readiness should not be seen as a roadblock to appropriate patient care. Patients should receive treatment that is appropriate for their medical condition. Much of the difficulty within military medicine is understanding and communicating how the natural disease history, prognosis, and treatment of their respective medical conditions will impact members’ service.
In some cases, the condition and/or treatment is incompatible with military service. Consider the following scenario: A 23-year-old active-duty soldier with a history of psoriasis developed widespread disease of 1 year’s duration and was referred to a civilian dermatologist due to nonavailability of a military dermatologist. After topical and light-based therapies failed, he was started on ustekinumab, which cleared the psoriasis. He wanted to continue on ustekinumab due to its good efficacy, but his unit was set to deploy in the coming year, and the drug made him medically nondeployable due to its immunosuppressive nature.
This real-life example was a difficult case to disposition. The service member was unsure if he could perform his military duties and deploy without continuing treatment with ustekinumab. His prior dermatology notes were requested to better assess the severity of his baseline disease, followed by a candid discussion between the military dermatologist and the patient about treatment options and their respective ramifications to his military career. One option included continuing ustekinumab, which would initiate an MEB evaluation and likely result in separation. Another option was UV therapy, which would not prompt an MEB evaluation but would not be available in deployed environments. Apremilast was offered as a third treatment option and could be used in place of UV therapy during deployment along with topical medications. This patient opted to continue treatment with ustekinumab, resulting in MEB review and separation from military service.
Dermatology Treatment Considerations
Civilian dermatologists should be aware of specific considerations when treating active US service members with common cutaneous diagnoses such as acne, atopic dermatitis (AD), psoriasis, dissecting cellulitis of the scalp (DCS), and lupus erythematosus (LE). This discussion is not meant to be all-inclusive but provides information and examples related to common treatment challenges in this patient population.
Acne
Acne is common in the active-duty military population. Typically, acne should be treated per recommended guidelines based on type and severity.8 Medical evaluation board review is warranted in cases of severe acne that is unresponsive to treatment and interferes with a service member’s performance.5,6 Unique situations in the active-duty military population include the following:
• Use of minocycline. Aircrew members have unique restrictions on many medications,6 including minocycline, which is restricted in this population due to vestibular side effects. Doxycycline is an acceptable alternative for aircrew members; however, even this medication may require a ground trial to ensure there are no idiosyncratic effects.
• Use of isotretinoin, which is not permitted in aircrew members, submariners, or divers. If they take this medication, they will be temporarily removed from duty for the duration of treatment and for a period of time after completion (1–3 months, depending on service). Isotretinoin also is not used during deployment due to potential side effects, the need for laboratory monitoring, and iPLEDGE system requirements.
Atopic Dermatitis
A history of AD after the 12th birthday is considered a disqualifying condition with regard to military service,3 though mild and well-controlled disease can easily be overlooked during entrance physical examinations. Members frequently present with eczema flares following field training exercises where they are outdoors for many hours and have been exposed to grass or other environmental triggers while wearing military gear that is heavy and occlusive, which is further exacerbated by being unable to bathe or care for their skin as they would at home.
Separation from the military is considered when AD is moderate to severe, is unresponsive to treatment, and/or interferes with performance of duty. Severity often can be evaluated based on the impact of AD on performance of duties in addition to clinical appearance. A pilot who is distracted by itching presents a potentially dangerous situation. A soldier whose AD flares every time he/she goes to the field, requiring him/her to return home early to control symptoms, can be considered moderate to severe due to lack of ability to do his/her job away from home base.
Response to treatment is more often where trouble lies for military members with AD, as patients are only permitted to take emollients, preferred cleansers, and topical medications to field training exercises and deployments. UV therapy is used to control disease in the military population but is not an option in deployed environments. Classic immunosuppressants (eg, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine) may result in a good response to treatment; however, due to their side-effect profiles, need for laboratory monitoring, and immunosuppressive nature, long-term use of those medications will result in a nondeployable status. Dupilumab does not appear to have the immunosuppressive effects of other biologics; however, the medication requires refrigeration,9 which currently precludes its use in the deployed environment, as it would be difficult to ensure supply and storage in remote areas.
Service members with a history of AD are exempt from the smallpox vaccine due to concerns about eczema vaccinatum.10
Psoriasis
Psoriasis is another dermatologic condition that does not meet military admission standards,3 and mild undiagnosed cases may be overlooked during the entrance physical examination. Because psoriasis commonly affects young adults, it may manifest in service members after entering service. If psoriasis is extensive or refractory to treatment, an MEB evaluation may be required.5,6 Widespread psoriasis can result in considerable discomfort when wearing body armor and other military gear. Severe localized disease can have duty implications; service members with treatment-resistant scalp psoriasis or pustular psoriasis of the feet may have difficulty wearing helmets or military boots, respectively.
Most service members with limited psoriasis vulgaris can be managed with topical steroids and steroid-sparing agents such as calcipotriene. Some service members opt not to aggressively treat their psoriasis if it is limited in nature and not symptomatic.
When discussing systemic treatments beyond light therapy in those with refractory disease, apremilast can be a good first-line treatment option.11 It is an oral medication, has minimal monitoring requirements, and lacks immunosuppressive side effects; therefore, it does not adversely impact deployability. If patients do not improve in 4 months with apremilast, biologics should then be considered; however, biologics have service implications, the most important being inability to deploy while taking the medication. In rare circumstances, military dermatologists may discuss utilizing biologic therapy only in the nondeployed setting. In these cases, service members are counseled that biologic therapy will be discontinued if they deploy in the future and treatment will be sustained with topicals and/or apremilast through the deployment. The treatment plan also should be communicated to the patient’s primary care provider to ensure that he/she is in agreement.
Dissecting Cellulitis of the Scalp
Dissecting cellulitis of the scalp may result in separation if the condition is unresponsive to treatment and/or interferes with satisfactory performance of duty.5 In addition to causing considerable pain, this condition can prevent service members from wearing combat helmets, which limits their ability to train and deploy. One of the authors (S.C.) has had more service members undergo an MEB evaluation for DCS than any of the other conditions mentioned.
Topical tretinoin and topical antibiotics can be used in conjunction with either doxycycline or minocycline to treat DCS, with the addition of intralesional corticosteroids for painful nodules. Fluctuant lesions are treated with incision and drainage. If there is inadequate response to treatment after 2 to 3 months, oral clindamycin and rifampin can be tried for 3 months. As an alternative measure or if the condition is refractory to oral clindamycin and rifampin, isotretinoin can then be used. One of the authors (S.C.) typically recommends a temporary no-helmet profile to the patient’s primary care provider until his/her next dermatology appointment. If the patient still has substantial disease despite these treatment options, it is recommended that the patient be issued a permanent profile for no helmet wear, which will prompt an MEB evaluation. Although tumor necrosis factor α inhibitors can work well in patients with DCS, the use of biologics is not conducive to continued service.
Lupus Erythematosus
A history of LE is disqualifying from military service. Patients who develop LE while on active duty will be referred for MEB evaluation if their disease is unresponsive to treatment and/or interferes with the satisfactory performance of duty.5,6 In general, connective tissue diseases have an array of physical implications that can affect military service, including photosensitivity, joint inflammation, and internal organ involvement. Similar to the other dermatologic conditions described, treatment of connective tissue diseases also can present challenges to continued military service. Considerations in the case of LE that are unique to military service members include the following:
• Sun exposure. Most military service members are required to work outside in all manners of conditions, which include hot, sunny, humid, and/or dry climates. Often physicians might counsel sun-sensitive patients with LE to avoid being outside during daylight hours, limit window exposure at work, and avoid daytime driving when possible; however, these recommendations are not possible for many, if not most, service members.
• Immunosuppressive therapies are incompatible with military deployment; therefore, prescribing methotrexate, cyclosporine, mycophenolate mofetil, rituximab, or belimumab for treatment of LE would prompt an MEB evaluation if the treatment is necessary to control the disease.
Final Thoughts
The recent changes to military medicine are needed to meet our country’s defense requirements and will ultimately result in civilian specialists playing a larger role in the care of our military population. This article highlights unique factors civilian dermatologists must consider when treating active-duty military patients to ensure they remain deployable during treatment.
- National Defense Authorization Act for Fiscal Year 2017, S 2943, 114th Congress, 2nd Sess (2016).
- Garamone J. Dunford sends message to joint force, stresses readiness, warfighting, education [news release]. Washington, DC: US Department of Defense; October 2, 2015. https://dod.defense.gov/News/Article/Article/621725/dunford-sends-message-to-joint-force-stresses-readiness-warfighting-education/. Accessed May 17, 2019.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; March 30, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 17, 2019.
- Force health protection guidance for deployment in USSOUTHCOM as of 7 December 2017. US Southern Command website. https://www.southcom.mil/Portals/7/Documents/Operational%20Contract%20Support/USSOUTHCOM_Force_Health_Protection_Guidance_AS_OF_7_DEC_2017.pdf?ver=2018-01-29-100603-957. Published December 7, 2017. Accessed May 28, 2019.
- US Department of the Army. Standards of medical fitness. http://www.au.af.mil/au/awc/awcgate/army/r40_501.pdf. Published August 26, 2003. Accessed May 17, 2019.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed May 17, 2019.
- Medical and physical evaluation boards (MEB/PEB). US Army Warrior Care and Transition website. https://wct.army.mil/modules/soldier/s6-medicalBoards.html. Accessed May 28, 2019.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Dupixent [package insert]. Tarrytown, NY: Regeneron, Inc; 2017.
- Departments of the Army, the Navy, the Air Force, and the Coast Guard. Immunizations and chemoprophylaxis for the prevention of infectious diseases. https://health.mil/Reference-Center/Policies/2013/10/07/Immunizations-and-Chemoprophylaxis-for-the-Prevention-of-Infectious-Diseases. Published October 7, 2013. Accessed May 28, 2019.
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631.
The National Defense Authorization Act for Fiscal Year 20171 has changed military medicine, including substantial reduction in military medical personnel as positions are converted to combat functions. As a result, there will be fewer military dermatologists, which means many US soldiers, sailors, airmen, and marines will seek medical care outside of military treatment facilities. This article highlights some unique treatment considerations in this patient population for our civilian dermatology colleagues.
Medical Readiness
In 2015, General Joseph F. Dunford Jr, 19th Chairman of the Joint Chiefs of Staff, made readiness his top priority for the US Armed Forces.2 Readiness refers to service members’ ability to deploy to locations across the globe and perform their military duties with little advanced notice, which requires personnel to be medically prepared at all times to leave home and perform their duties in locations with limited medical support.
Medical readiness is maintaining a unit that is medically able to perform its military function both at home and in a deployed environment. Military members’ medical readiness status is carefully tracked and determined via annual physical, dental, hearing, and vision examinations, as well as human immunodeficiency virus status and immunizations. The readiness status of the unit (ie, the number of troops ready to deploy at any given time) is available to commanders at all levels at any time. Each military branch has tracking systems that allow commanders to know when a member is past due for an examination or if a member’s medical status has changed, making them nondeployable. When a member is nondeployable, it affects the unit’s ability to perform its mission and degrades its readiness. If readiness is suboptimal, the military cannot deploy and complete its missions, which is why readiness is a top priority. The primary function of military medicine is to support the medical readiness of the force.
Deployment Eligibility
A unique aspect of military medicine that can be foreign to civilian physicians is the unit commanders’ authority to request and receive information on military members’ medical conditions as they relate to readiness. Under most circumstances, an individual’s medical information is his/her private information; however, that is not always the case in the military. If a member’s medical status changes and he/she becomes nondeployable, by regulation the commander can be privy to pertinent aspects of that member’s medical condition as it affects unit readiness, including the diagnosis, treatment plan, and prognosis. Commanders need this information to aid in the member’s recovery, ensure training does not impact his/her care, and identify possible need of replacement.
Published accession guidelines are used to determine medical eligibility for service.3 These instructions are organized by major organ systems and broad disease categories. They provide guidance on medically disqualifying conditions. The Table outlines those conditions that apply to the skin.3 Individual military branches may have additional regulations with guidance on medically disqualifying conditions that are job specific. Additional regulations also are available based on an area of military operation that can be more restrictive and specific to those locations.4

Similarly, each military branch has its own retention standards.5,6 Previously healthy individuals can develop new medical conditions, and commanders are notified if a service member becomes medically nondeployable. If a medical condition limits a service member’s ability to deploy, he/she will be evaluated for retention by a medical evaluation board (MEB). Three outcomes are possible: return in current function, retain the service member but retrain in another military occupation, or separate from military service.7 Rarely, waivers are provided so that the service member can return to duty.
Readiness and Patient Care
Importantly, readiness should not be seen as a roadblock to appropriate patient care. Patients should receive treatment that is appropriate for their medical condition. Much of the difficulty within military medicine is understanding and communicating how the natural disease history, prognosis, and treatment of their respective medical conditions will impact members’ service.
In some cases, the condition and/or treatment is incompatible with military service. Consider the following scenario: A 23-year-old active-duty soldier with a history of psoriasis developed widespread disease of 1 year’s duration and was referred to a civilian dermatologist due to nonavailability of a military dermatologist. After topical and light-based therapies failed, he was started on ustekinumab, which cleared the psoriasis. He wanted to continue on ustekinumab due to its good efficacy, but his unit was set to deploy in the coming year, and the drug made him medically nondeployable due to its immunosuppressive nature.
This real-life example was a difficult case to disposition. The service member was unsure if he could perform his military duties and deploy without continuing treatment with ustekinumab. His prior dermatology notes were requested to better assess the severity of his baseline disease, followed by a candid discussion between the military dermatologist and the patient about treatment options and their respective ramifications to his military career. One option included continuing ustekinumab, which would initiate an MEB evaluation and likely result in separation. Another option was UV therapy, which would not prompt an MEB evaluation but would not be available in deployed environments. Apremilast was offered as a third treatment option and could be used in place of UV therapy during deployment along with topical medications. This patient opted to continue treatment with ustekinumab, resulting in MEB review and separation from military service.
Dermatology Treatment Considerations
Civilian dermatologists should be aware of specific considerations when treating active US service members with common cutaneous diagnoses such as acne, atopic dermatitis (AD), psoriasis, dissecting cellulitis of the scalp (DCS), and lupus erythematosus (LE). This discussion is not meant to be all-inclusive but provides information and examples related to common treatment challenges in this patient population.
Acne
Acne is common in the active-duty military population. Typically, acne should be treated per recommended guidelines based on type and severity.8 Medical evaluation board review is warranted in cases of severe acne that is unresponsive to treatment and interferes with a service member’s performance.5,6 Unique situations in the active-duty military population include the following:
• Use of minocycline. Aircrew members have unique restrictions on many medications,6 including minocycline, which is restricted in this population due to vestibular side effects. Doxycycline is an acceptable alternative for aircrew members; however, even this medication may require a ground trial to ensure there are no idiosyncratic effects.
• Use of isotretinoin, which is not permitted in aircrew members, submariners, or divers. If they take this medication, they will be temporarily removed from duty for the duration of treatment and for a period of time after completion (1–3 months, depending on service). Isotretinoin also is not used during deployment due to potential side effects, the need for laboratory monitoring, and iPLEDGE system requirements.
Atopic Dermatitis
A history of AD after the 12th birthday is considered a disqualifying condition with regard to military service,3 though mild and well-controlled disease can easily be overlooked during entrance physical examinations. Members frequently present with eczema flares following field training exercises where they are outdoors for many hours and have been exposed to grass or other environmental triggers while wearing military gear that is heavy and occlusive, which is further exacerbated by being unable to bathe or care for their skin as they would at home.
Separation from the military is considered when AD is moderate to severe, is unresponsive to treatment, and/or interferes with performance of duty. Severity often can be evaluated based on the impact of AD on performance of duties in addition to clinical appearance. A pilot who is distracted by itching presents a potentially dangerous situation. A soldier whose AD flares every time he/she goes to the field, requiring him/her to return home early to control symptoms, can be considered moderate to severe due to lack of ability to do his/her job away from home base.
Response to treatment is more often where trouble lies for military members with AD, as patients are only permitted to take emollients, preferred cleansers, and topical medications to field training exercises and deployments. UV therapy is used to control disease in the military population but is not an option in deployed environments. Classic immunosuppressants (eg, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine) may result in a good response to treatment; however, due to their side-effect profiles, need for laboratory monitoring, and immunosuppressive nature, long-term use of those medications will result in a nondeployable status. Dupilumab does not appear to have the immunosuppressive effects of other biologics; however, the medication requires refrigeration,9 which currently precludes its use in the deployed environment, as it would be difficult to ensure supply and storage in remote areas.
Service members with a history of AD are exempt from the smallpox vaccine due to concerns about eczema vaccinatum.10
Psoriasis
Psoriasis is another dermatologic condition that does not meet military admission standards,3 and mild undiagnosed cases may be overlooked during the entrance physical examination. Because psoriasis commonly affects young adults, it may manifest in service members after entering service. If psoriasis is extensive or refractory to treatment, an MEB evaluation may be required.5,6 Widespread psoriasis can result in considerable discomfort when wearing body armor and other military gear. Severe localized disease can have duty implications; service members with treatment-resistant scalp psoriasis or pustular psoriasis of the feet may have difficulty wearing helmets or military boots, respectively.
Most service members with limited psoriasis vulgaris can be managed with topical steroids and steroid-sparing agents such as calcipotriene. Some service members opt not to aggressively treat their psoriasis if it is limited in nature and not symptomatic.
When discussing systemic treatments beyond light therapy in those with refractory disease, apremilast can be a good first-line treatment option.11 It is an oral medication, has minimal monitoring requirements, and lacks immunosuppressive side effects; therefore, it does not adversely impact deployability. If patients do not improve in 4 months with apremilast, biologics should then be considered; however, biologics have service implications, the most important being inability to deploy while taking the medication. In rare circumstances, military dermatologists may discuss utilizing biologic therapy only in the nondeployed setting. In these cases, service members are counseled that biologic therapy will be discontinued if they deploy in the future and treatment will be sustained with topicals and/or apremilast through the deployment. The treatment plan also should be communicated to the patient’s primary care provider to ensure that he/she is in agreement.
Dissecting Cellulitis of the Scalp
Dissecting cellulitis of the scalp may result in separation if the condition is unresponsive to treatment and/or interferes with satisfactory performance of duty.5 In addition to causing considerable pain, this condition can prevent service members from wearing combat helmets, which limits their ability to train and deploy. One of the authors (S.C.) has had more service members undergo an MEB evaluation for DCS than any of the other conditions mentioned.
Topical tretinoin and topical antibiotics can be used in conjunction with either doxycycline or minocycline to treat DCS, with the addition of intralesional corticosteroids for painful nodules. Fluctuant lesions are treated with incision and drainage. If there is inadequate response to treatment after 2 to 3 months, oral clindamycin and rifampin can be tried for 3 months. As an alternative measure or if the condition is refractory to oral clindamycin and rifampin, isotretinoin can then be used. One of the authors (S.C.) typically recommends a temporary no-helmet profile to the patient’s primary care provider until his/her next dermatology appointment. If the patient still has substantial disease despite these treatment options, it is recommended that the patient be issued a permanent profile for no helmet wear, which will prompt an MEB evaluation. Although tumor necrosis factor α inhibitors can work well in patients with DCS, the use of biologics is not conducive to continued service.
Lupus Erythematosus
A history of LE is disqualifying from military service. Patients who develop LE while on active duty will be referred for MEB evaluation if their disease is unresponsive to treatment and/or interferes with the satisfactory performance of duty.5,6 In general, connective tissue diseases have an array of physical implications that can affect military service, including photosensitivity, joint inflammation, and internal organ involvement. Similar to the other dermatologic conditions described, treatment of connective tissue diseases also can present challenges to continued military service. Considerations in the case of LE that are unique to military service members include the following:
• Sun exposure. Most military service members are required to work outside in all manners of conditions, which include hot, sunny, humid, and/or dry climates. Often physicians might counsel sun-sensitive patients with LE to avoid being outside during daylight hours, limit window exposure at work, and avoid daytime driving when possible; however, these recommendations are not possible for many, if not most, service members.
• Immunosuppressive therapies are incompatible with military deployment; therefore, prescribing methotrexate, cyclosporine, mycophenolate mofetil, rituximab, or belimumab for treatment of LE would prompt an MEB evaluation if the treatment is necessary to control the disease.
Final Thoughts
The recent changes to military medicine are needed to meet our country’s defense requirements and will ultimately result in civilian specialists playing a larger role in the care of our military population. This article highlights unique factors civilian dermatologists must consider when treating active-duty military patients to ensure they remain deployable during treatment.
The National Defense Authorization Act for Fiscal Year 20171 has changed military medicine, including substantial reduction in military medical personnel as positions are converted to combat functions. As a result, there will be fewer military dermatologists, which means many US soldiers, sailors, airmen, and marines will seek medical care outside of military treatment facilities. This article highlights some unique treatment considerations in this patient population for our civilian dermatology colleagues.
Medical Readiness
In 2015, General Joseph F. Dunford Jr, 19th Chairman of the Joint Chiefs of Staff, made readiness his top priority for the US Armed Forces.2 Readiness refers to service members’ ability to deploy to locations across the globe and perform their military duties with little advanced notice, which requires personnel to be medically prepared at all times to leave home and perform their duties in locations with limited medical support.
Medical readiness is maintaining a unit that is medically able to perform its military function both at home and in a deployed environment. Military members’ medical readiness status is carefully tracked and determined via annual physical, dental, hearing, and vision examinations, as well as human immunodeficiency virus status and immunizations. The readiness status of the unit (ie, the number of troops ready to deploy at any given time) is available to commanders at all levels at any time. Each military branch has tracking systems that allow commanders to know when a member is past due for an examination or if a member’s medical status has changed, making them nondeployable. When a member is nondeployable, it affects the unit’s ability to perform its mission and degrades its readiness. If readiness is suboptimal, the military cannot deploy and complete its missions, which is why readiness is a top priority. The primary function of military medicine is to support the medical readiness of the force.
Deployment Eligibility
A unique aspect of military medicine that can be foreign to civilian physicians is the unit commanders’ authority to request and receive information on military members’ medical conditions as they relate to readiness. Under most circumstances, an individual’s medical information is his/her private information; however, that is not always the case in the military. If a member’s medical status changes and he/she becomes nondeployable, by regulation the commander can be privy to pertinent aspects of that member’s medical condition as it affects unit readiness, including the diagnosis, treatment plan, and prognosis. Commanders need this information to aid in the member’s recovery, ensure training does not impact his/her care, and identify possible need of replacement.
Published accession guidelines are used to determine medical eligibility for service.3 These instructions are organized by major organ systems and broad disease categories. They provide guidance on medically disqualifying conditions. The Table outlines those conditions that apply to the skin.3 Individual military branches may have additional regulations with guidance on medically disqualifying conditions that are job specific. Additional regulations also are available based on an area of military operation that can be more restrictive and specific to those locations.4

Similarly, each military branch has its own retention standards.5,6 Previously healthy individuals can develop new medical conditions, and commanders are notified if a service member becomes medically nondeployable. If a medical condition limits a service member’s ability to deploy, he/she will be evaluated for retention by a medical evaluation board (MEB). Three outcomes are possible: return in current function, retain the service member but retrain in another military occupation, or separate from military service.7 Rarely, waivers are provided so that the service member can return to duty.
Readiness and Patient Care
Importantly, readiness should not be seen as a roadblock to appropriate patient care. Patients should receive treatment that is appropriate for their medical condition. Much of the difficulty within military medicine is understanding and communicating how the natural disease history, prognosis, and treatment of their respective medical conditions will impact members’ service.
In some cases, the condition and/or treatment is incompatible with military service. Consider the following scenario: A 23-year-old active-duty soldier with a history of psoriasis developed widespread disease of 1 year’s duration and was referred to a civilian dermatologist due to nonavailability of a military dermatologist. After topical and light-based therapies failed, he was started on ustekinumab, which cleared the psoriasis. He wanted to continue on ustekinumab due to its good efficacy, but his unit was set to deploy in the coming year, and the drug made him medically nondeployable due to its immunosuppressive nature.
This real-life example was a difficult case to disposition. The service member was unsure if he could perform his military duties and deploy without continuing treatment with ustekinumab. His prior dermatology notes were requested to better assess the severity of his baseline disease, followed by a candid discussion between the military dermatologist and the patient about treatment options and their respective ramifications to his military career. One option included continuing ustekinumab, which would initiate an MEB evaluation and likely result in separation. Another option was UV therapy, which would not prompt an MEB evaluation but would not be available in deployed environments. Apremilast was offered as a third treatment option and could be used in place of UV therapy during deployment along with topical medications. This patient opted to continue treatment with ustekinumab, resulting in MEB review and separation from military service.
Dermatology Treatment Considerations
Civilian dermatologists should be aware of specific considerations when treating active US service members with common cutaneous diagnoses such as acne, atopic dermatitis (AD), psoriasis, dissecting cellulitis of the scalp (DCS), and lupus erythematosus (LE). This discussion is not meant to be all-inclusive but provides information and examples related to common treatment challenges in this patient population.
Acne
Acne is common in the active-duty military population. Typically, acne should be treated per recommended guidelines based on type and severity.8 Medical evaluation board review is warranted in cases of severe acne that is unresponsive to treatment and interferes with a service member’s performance.5,6 Unique situations in the active-duty military population include the following:
• Use of minocycline. Aircrew members have unique restrictions on many medications,6 including minocycline, which is restricted in this population due to vestibular side effects. Doxycycline is an acceptable alternative for aircrew members; however, even this medication may require a ground trial to ensure there are no idiosyncratic effects.
• Use of isotretinoin, which is not permitted in aircrew members, submariners, or divers. If they take this medication, they will be temporarily removed from duty for the duration of treatment and for a period of time after completion (1–3 months, depending on service). Isotretinoin also is not used during deployment due to potential side effects, the need for laboratory monitoring, and iPLEDGE system requirements.
Atopic Dermatitis
A history of AD after the 12th birthday is considered a disqualifying condition with regard to military service,3 though mild and well-controlled disease can easily be overlooked during entrance physical examinations. Members frequently present with eczema flares following field training exercises where they are outdoors for many hours and have been exposed to grass or other environmental triggers while wearing military gear that is heavy and occlusive, which is further exacerbated by being unable to bathe or care for their skin as they would at home.
Separation from the military is considered when AD is moderate to severe, is unresponsive to treatment, and/or interferes with performance of duty. Severity often can be evaluated based on the impact of AD on performance of duties in addition to clinical appearance. A pilot who is distracted by itching presents a potentially dangerous situation. A soldier whose AD flares every time he/she goes to the field, requiring him/her to return home early to control symptoms, can be considered moderate to severe due to lack of ability to do his/her job away from home base.
Response to treatment is more often where trouble lies for military members with AD, as patients are only permitted to take emollients, preferred cleansers, and topical medications to field training exercises and deployments. UV therapy is used to control disease in the military population but is not an option in deployed environments. Classic immunosuppressants (eg, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine) may result in a good response to treatment; however, due to their side-effect profiles, need for laboratory monitoring, and immunosuppressive nature, long-term use of those medications will result in a nondeployable status. Dupilumab does not appear to have the immunosuppressive effects of other biologics; however, the medication requires refrigeration,9 which currently precludes its use in the deployed environment, as it would be difficult to ensure supply and storage in remote areas.
Service members with a history of AD are exempt from the smallpox vaccine due to concerns about eczema vaccinatum.10
Psoriasis
Psoriasis is another dermatologic condition that does not meet military admission standards,3 and mild undiagnosed cases may be overlooked during the entrance physical examination. Because psoriasis commonly affects young adults, it may manifest in service members after entering service. If psoriasis is extensive or refractory to treatment, an MEB evaluation may be required.5,6 Widespread psoriasis can result in considerable discomfort when wearing body armor and other military gear. Severe localized disease can have duty implications; service members with treatment-resistant scalp psoriasis or pustular psoriasis of the feet may have difficulty wearing helmets or military boots, respectively.
Most service members with limited psoriasis vulgaris can be managed with topical steroids and steroid-sparing agents such as calcipotriene. Some service members opt not to aggressively treat their psoriasis if it is limited in nature and not symptomatic.
When discussing systemic treatments beyond light therapy in those with refractory disease, apremilast can be a good first-line treatment option.11 It is an oral medication, has minimal monitoring requirements, and lacks immunosuppressive side effects; therefore, it does not adversely impact deployability. If patients do not improve in 4 months with apremilast, biologics should then be considered; however, biologics have service implications, the most important being inability to deploy while taking the medication. In rare circumstances, military dermatologists may discuss utilizing biologic therapy only in the nondeployed setting. In these cases, service members are counseled that biologic therapy will be discontinued if they deploy in the future and treatment will be sustained with topicals and/or apremilast through the deployment. The treatment plan also should be communicated to the patient’s primary care provider to ensure that he/she is in agreement.
Dissecting Cellulitis of the Scalp
Dissecting cellulitis of the scalp may result in separation if the condition is unresponsive to treatment and/or interferes with satisfactory performance of duty.5 In addition to causing considerable pain, this condition can prevent service members from wearing combat helmets, which limits their ability to train and deploy. One of the authors (S.C.) has had more service members undergo an MEB evaluation for DCS than any of the other conditions mentioned.
Topical tretinoin and topical antibiotics can be used in conjunction with either doxycycline or minocycline to treat DCS, with the addition of intralesional corticosteroids for painful nodules. Fluctuant lesions are treated with incision and drainage. If there is inadequate response to treatment after 2 to 3 months, oral clindamycin and rifampin can be tried for 3 months. As an alternative measure or if the condition is refractory to oral clindamycin and rifampin, isotretinoin can then be used. One of the authors (S.C.) typically recommends a temporary no-helmet profile to the patient’s primary care provider until his/her next dermatology appointment. If the patient still has substantial disease despite these treatment options, it is recommended that the patient be issued a permanent profile for no helmet wear, which will prompt an MEB evaluation. Although tumor necrosis factor α inhibitors can work well in patients with DCS, the use of biologics is not conducive to continued service.
Lupus Erythematosus
A history of LE is disqualifying from military service. Patients who develop LE while on active duty will be referred for MEB evaluation if their disease is unresponsive to treatment and/or interferes with the satisfactory performance of duty.5,6 In general, connective tissue diseases have an array of physical implications that can affect military service, including photosensitivity, joint inflammation, and internal organ involvement. Similar to the other dermatologic conditions described, treatment of connective tissue diseases also can present challenges to continued military service. Considerations in the case of LE that are unique to military service members include the following:
• Sun exposure. Most military service members are required to work outside in all manners of conditions, which include hot, sunny, humid, and/or dry climates. Often physicians might counsel sun-sensitive patients with LE to avoid being outside during daylight hours, limit window exposure at work, and avoid daytime driving when possible; however, these recommendations are not possible for many, if not most, service members.
• Immunosuppressive therapies are incompatible with military deployment; therefore, prescribing methotrexate, cyclosporine, mycophenolate mofetil, rituximab, or belimumab for treatment of LE would prompt an MEB evaluation if the treatment is necessary to control the disease.
Final Thoughts
The recent changes to military medicine are needed to meet our country’s defense requirements and will ultimately result in civilian specialists playing a larger role in the care of our military population. This article highlights unique factors civilian dermatologists must consider when treating active-duty military patients to ensure they remain deployable during treatment.
- National Defense Authorization Act for Fiscal Year 2017, S 2943, 114th Congress, 2nd Sess (2016).
- Garamone J. Dunford sends message to joint force, stresses readiness, warfighting, education [news release]. Washington, DC: US Department of Defense; October 2, 2015. https://dod.defense.gov/News/Article/Article/621725/dunford-sends-message-to-joint-force-stresses-readiness-warfighting-education/. Accessed May 17, 2019.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; March 30, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 17, 2019.
- Force health protection guidance for deployment in USSOUTHCOM as of 7 December 2017. US Southern Command website. https://www.southcom.mil/Portals/7/Documents/Operational%20Contract%20Support/USSOUTHCOM_Force_Health_Protection_Guidance_AS_OF_7_DEC_2017.pdf?ver=2018-01-29-100603-957. Published December 7, 2017. Accessed May 28, 2019.
- US Department of the Army. Standards of medical fitness. http://www.au.af.mil/au/awc/awcgate/army/r40_501.pdf. Published August 26, 2003. Accessed May 17, 2019.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed May 17, 2019.
- Medical and physical evaluation boards (MEB/PEB). US Army Warrior Care and Transition website. https://wct.army.mil/modules/soldier/s6-medicalBoards.html. Accessed May 28, 2019.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Dupixent [package insert]. Tarrytown, NY: Regeneron, Inc; 2017.
- Departments of the Army, the Navy, the Air Force, and the Coast Guard. Immunizations and chemoprophylaxis for the prevention of infectious diseases. https://health.mil/Reference-Center/Policies/2013/10/07/Immunizations-and-Chemoprophylaxis-for-the-Prevention-of-Infectious-Diseases. Published October 7, 2013. Accessed May 28, 2019.
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631.
- National Defense Authorization Act for Fiscal Year 2017, S 2943, 114th Congress, 2nd Sess (2016).
- Garamone J. Dunford sends message to joint force, stresses readiness, warfighting, education [news release]. Washington, DC: US Department of Defense; October 2, 2015. https://dod.defense.gov/News/Article/Article/621725/dunford-sends-message-to-joint-force-stresses-readiness-warfighting-education/. Accessed May 17, 2019.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; March 30, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 17, 2019.
- Force health protection guidance for deployment in USSOUTHCOM as of 7 December 2017. US Southern Command website. https://www.southcom.mil/Portals/7/Documents/Operational%20Contract%20Support/USSOUTHCOM_Force_Health_Protection_Guidance_AS_OF_7_DEC_2017.pdf?ver=2018-01-29-100603-957. Published December 7, 2017. Accessed May 28, 2019.
- US Department of the Army. Standards of medical fitness. http://www.au.af.mil/au/awc/awcgate/army/r40_501.pdf. Published August 26, 2003. Accessed May 17, 2019.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed May 17, 2019.
- Medical and physical evaluation boards (MEB/PEB). US Army Warrior Care and Transition website. https://wct.army.mil/modules/soldier/s6-medicalBoards.html. Accessed May 28, 2019.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Dupixent [package insert]. Tarrytown, NY: Regeneron, Inc; 2017.
- Departments of the Army, the Navy, the Air Force, and the Coast Guard. Immunizations and chemoprophylaxis for the prevention of infectious diseases. https://health.mil/Reference-Center/Policies/2013/10/07/Immunizations-and-Chemoprophylaxis-for-the-Prevention-of-Infectious-Diseases. Published October 7, 2013. Accessed May 28, 2019.
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631.
Practice Points
- Certain conditions and treatments are incompatible with military service and may result in separation.
- Dermatologists must consider a patient’s profession when choosing a treatment modality.
The Dermatologist’s Role in Amputee Skin Care
Limb amputation is a major life-changing event that markedly affects a patient’s quality of life as well as his/her ability to participate in activities of daily living. The most prevalent causes for amputation include vascular diseases, diabetes mellitus, trauma, and cancer, respectively.1,2 For amputees, maintaining prosthetic use is a major physical and psychological undertaking that benefits from a multidisciplinary team approach. Although individuals with lower limb amputations are disproportionately impacted by skin disease due to the increased mechanical forces exerted over the lower limbs, patients with upper limb amputations also develop dermatologic conditions secondary to wearing prostheses.
Approximately 185,000 amputations occur each year in the United States.3 Although amputations resulting from peripheral vascular disease or diabetes mellitus tend to occur in older individuals, amputations in younger patients usually occur from trauma.2 The US military has experienced increasing numbers of amputations from trauma due to the ongoing combat operations in the Middle East. Although improvements in body armor and tactical combat casualty care have reduced the number of preventable deaths, the number of casualties surviving with extremity injuries requiring amputation has increased.4,5 As of October 2017, 1705 US servicemembers underwent major limb amputations, with 1914 lower limb amputations and 302 upper limb amputations. These amputations mainly impacted men aged 21 to 29 years, but female servicemembers also were affected, and a small group of servicemembers had multiple amputations.6
One of the most common medical problems that amputees face during long-term care is skin disease, with approximately 75% of amputees using a lower limb prosthesis experiencing skin problems. In general, amputees experience nearly 65% more dermatologic concerns than the general population.7 In one study of 97 individuals with transfemoral amputations, some of the most common issues associated with socket prosthetics included heat and sweating in the prosthetic socket (72%) as well as sores and skin irritation from the socket (62%).8 Given the high incidence of skin disease on residual limbs, dermatologists are uniquely positioned to keep the amputee in his/her prosthesis and prevent prosthetic abandonment.
Complications Following Amputation
Although US military servicemembers who undergo amputations receive the very best prosthetic devices and rehabilitation resources, they still experience prosthesis abandonment.9 Despite the fact that prosthetic limbs and prosthesis technology have substantially improved over the last 2 decades, one study indicated that the high frequency of problems affecting tissue viability at residual limbs is due to the age-old problem of prosthetic fit.10 In patients with the most advanced prostheses, poor fit still results in mechanical damage to the skin, as the residual limb is exposed to unequal and shearing forces across the amputation site as well as high pressures that cause a vaso-occlusive effect.11,12 Issues with poor fit are especially important for more active patients, as they normally want to immediately return to their vigorous preinjury lifestyles. In these patients, even a properly fitting prosthetic may not be able to overcome the fact that the residual limb skin is not well suited for the mechanical forces generated by the prosthesis and the humid environment of the socket.1,13 Another complicating factor is the dynamic nature of the residual limb. Muscle atrophy, changes in gait, and weight gain or loss can lead to an ill-fitting prosthetic and subsequent skin breakdown.
There are many case reports and review articles describing the skin problems in amputees.1,14-17 The Table summarizes these conditions and outlines treatment options for each.15,18-20
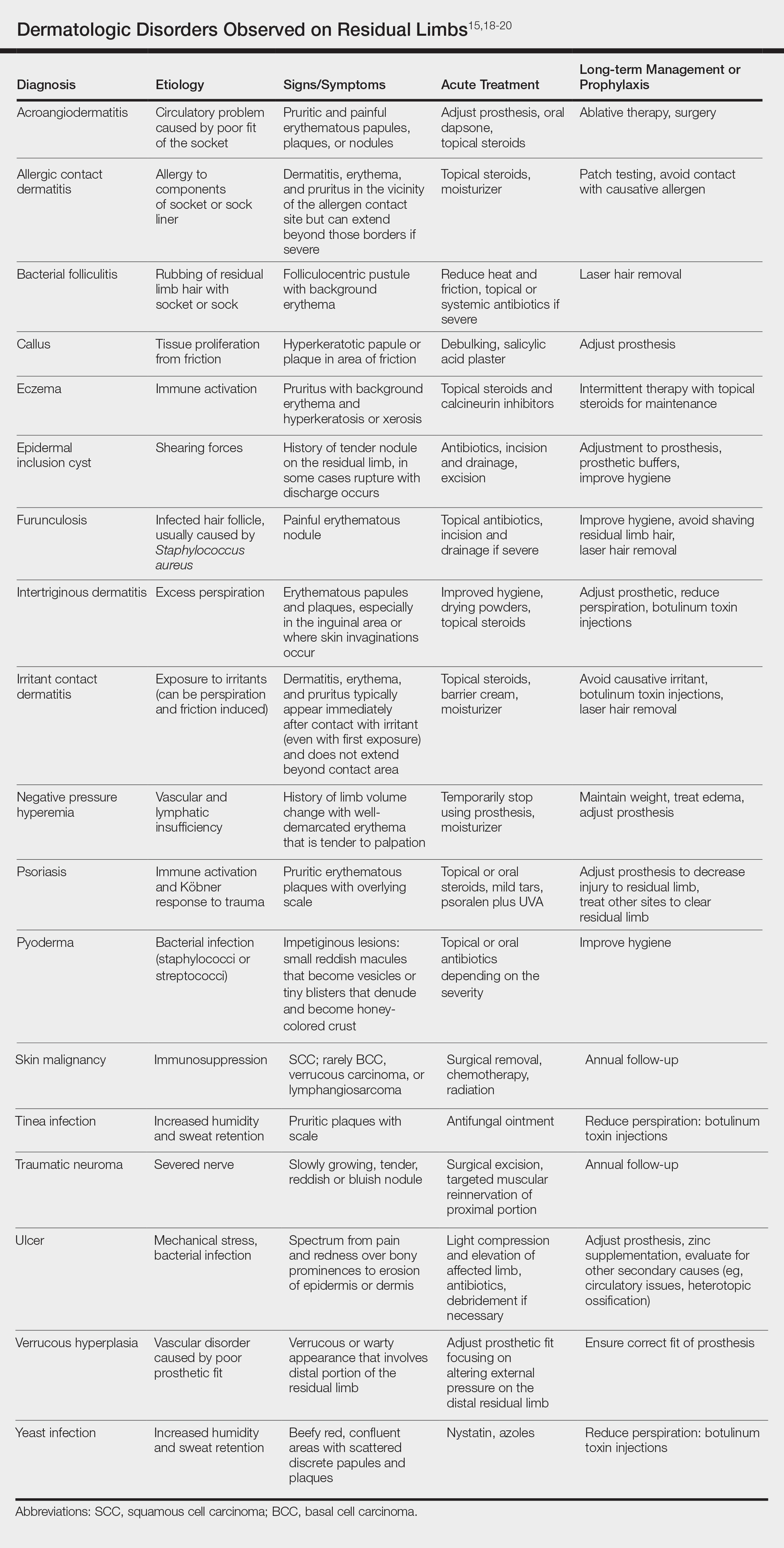
Most skin diseases on residual limbs are the result of mechanical skin breakdown, inflammation, infection, or combinations of these processes. Overall, amputees with diabetes mellitus and peripheral vascular disease tend to have skin disease related to poor perfusion, whereas amputees who are active and healthy tend to have conditions related to mechanical stress.7,13,14,17,21,22 Bui et al17 reported ulcers, abscesses, and blisters as the most common skin conditions that occur at the site of residual limbs; however, other less common dermatologic disorders such as skin malignancies, verrucous hyperplasia and carcinoma, granulomatous cutaneous lesions, acroangiodermatitis, and bullous pemphigoid also are seen.23-26 Buikema and Meyerle15 hypothesize that these conditions, as well as the more common skin diseases, are partly from the amputation disrupting blood and lymphatic flow in the residual limb, which causes the site to act as an immunocompromised district that induces dysregulation of neuroimmune regulators.
It is important to note that skin disease on residual limbs is not just an acute problem. Long-term follow-up of 247 traumatic amputees from the Vietnam War showed that almost half of prosthesis users (48.2%) reported a skin problem in the preceding year, more than 38 years after the amputation. Additionally, one-quarter of these individuals experienced skin problems approximately 50% of the time, which unfortunately led to limited use or total abandonment of the prosthesis for the preceding year in 56% of the veterans surveyed.21
Other complications following amputation indirectly lead to skin problems. Heterotopic ossification, or the formation of bone at extraskeletal sites, has been observed in up to 65% of military amputees from recent operations in Iraq and Afghanistan.27,28 If symptomatic, heterotopic ossification can lead to poor prosthetic fit and subsequent skin breakdown. As a result, it has been reported that up to 40% of combat-related lower extremity amputations may require excision of heterotopic ossificiation.29
Amputation also can result in psychologic concerns that indirectly affect skin health. A systematic review by Mckechnie and John30 suggested that despite heterogeneity between studies, even using the lowest figures demonstrated the significance anxiety and depression play in the lives of traumatic amputees. If left untreated, these mental health issues can lead to poor residual limb hygiene and prosthetic maintenance due to reductions in the patient’s energy and motivation. Studies have shown that proper hygiene of residual limbs and silicone liners reduces associated skin problems.19,31
Role of the Dermatologist
Routine care and conservative management of amputee skin problems often are accomplished by prosthetists, primary care physicians, nurses, and physical therapists. In one study, more than 80% of the most common skin problems affecting amputees could be attributed to the prosthesis itself, which highlights the importance of the continued involvement of the prosthetist beyond the initial fitting period.13 However, when a skin problem becomes refractory to conservative management, referral to a dermatologist is prudent; therefore, the dermatologist is an integral member of the multidisciplinary team that provides care for amputees.
The dermatologist often is best positioned to diagnose skin diseases that result from wearing prostheses and is well versed in treatments for short-term and long-term management of skin disease on residual limbs. The dermatologist also can offer prophylactic treatments to decrease sweating and hair growth to prevent potential infections and subsequent skin breakdown. Additionally, proper education on self-care has been shown to decrease the amount of skin problems and increase functional status and quality of life for amputees.32,33 Dermatologists can assist with the patient education process as well as refer amputees to a useful resource from the Amputee Coalition website (www.amputee-coalition.org) to provide specific patient education on how to maintain skin on the residual limb to prevent skin disease.
Current Treatments and Future Directions
Skin disorders affecting residual limbs usually are conditions that dermatologists commonly encounter and are comfortable managing in general practice. Additionally, dermatologists routinely treat hyperhidrosis and conduct laser hair removal, both of which are effective prophylactic adjuncts for amputee skin health. There are a few treatments for reducing residual limb hyperhidrosis that are particularly useful. Although first-line treatment of residual limb hyperhidrosis often is topical aluminum chloride, it requires frequent application and often causes considerable skin irritation when applied to residual limbs. Alternatively, intradermal botulinum toxin has been shown to successfully reduce sweat production in individuals with residual limb hyperhidrosis and is well tolerated.34 A 2017 case report discussed the use of microwave thermal ablation of eccrine coils using a noninvasive 3-step hyperhidrosis treatment system on a bilateral below-the-knee amputee. The authors reported the patient tolerated the procedure well with decreased dermatitis and folliculitis, leading to his ability to wear a prosthetic for longer periods of time.35
Ablative fractional resurfacing with a CO2 laser is another key treatment modality central to amputees, more specifically to traumatic amputees. A CO2 laser can decrease skin tension and increase skin mobility associated with traumatic scars as well as decrease skin vulnerability to biofilms present in chronic wounds on residual limbs. It is believed that the pattern of injury caused by ablative fractional lasers disrupts biofilms and stimulates growth factor secretion and collagen remodeling through the concept of photomicrodebridement.36 The ablative fractional resurfacing approach to scar therapy and chronic wound debridement can result in less skin injury, allowing the amputee to continue rehabilitation and return more quickly to prosthetic use.37
One interesting area of research in amputee care involves the study of novel ways to increase the skin’s ability to adapt to mechanical stress and load bearing and accelerate wound healing on the residual limb. Multiple studies have identified collagen fibril enlargement as an important component of skin adaptation, and biomolecules such as decorin may enhance this process.38-40 The concept of increasing these biomolecules at the correct time during wound healing to strengthen the residual limb tissue currently is being studied.39
Another encouraging area of research is the involvement of fibroblasts in cutaneous wound healing and their role in determining the phenotype of residual limb skin in amputees. The clinical application of autologous fibroblasts is approved by the US Food and Drug Administration for cosmetic use as a filler material and currently is under research for other applications, such as skin regeneration after surgery or manipulating skin characteristics to enhance the durability of residual limbs.41
Future preventative care of amputee skin may rely on tracking residual limb health before severe tissue injury occurs. For instance, Rink et al42 described an approach to monitor residual limb health using noninvasive imaging (eg, hyperspectral imaging, laser speckle imaging) and noninvasive probes that measure oxygenation, perfusion, skin barrier function, and skin hydration to the residual limb. Although these limb surveillance sensors would be employed by prosthetists, the dermatologist, as part of the multispecialty team, also could leverage the data for diagnosis and treatment considerations.
Final Thoughts
The dermatologist is an important member of the multidisciplinary team involved in the care of amputees. Skin disease is prevalent in amputees throughout their lives and often leads to abandonment of prostheses. Although current therapies and preventative treatments are for the most part successful, future research involving advanced technology to monitor skin health, increasing residual limb skin durability at the molecular level, and targeted laser therapies are promising. Through engagement and effective collaboration with the entire multidisciplinary team, dermatologists will have a considerable impact on amputee skin health.
- Dudek NL, Marks MB, Marshall SC, et al. Dermatologic conditions associated with use of a lower-extremity prosthesis. Arch Phys Med Rehabil. 2005;86:659-663.
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422-429.
- Kozak LJ. Ambulatory and Inpatient Procedures in the United States, 1995. Hyattsville, MD: US Department of Health and Human Services; 1998.
- Epstein RA, Heinemann AW, McFarland LV. Quality of life for veterans and servicemembers with major traumatic limb loss from Vietnam and OIF/OEF conflicts. J Rehabil Res Dev. 2010;47:373-385.
- Dougherty AL, Mohrle CR, Galarneau MR, et al. Battlefield extremity injuries in Operation Iraqi Freedom. Injury. 2009;40:772-777.
- Farrokhi S, Perez K, Eskridge S, et al. Major deployment-related amputations of lower and upper limbs, active and reserve components, U.S. Armed Forces, 2001-2017. MSMR. 2018;25:10-16.
- Highsmith MJ, Highsmith JT. Identifying and managing skin issues with lower-limb prosthetic use. Amputee Coalition website. https://www.amputee-coalition.org/wp-content/uploads/2015/.../skin_issues_lower.pdf. Accessed January 4, 2019.
- Hagberg K, Brånemark R. Consequences of non-vascular trans-femoral amputation: a survey of quality of life, prosthetic use and problems. Prosthet Orthot Int. 2001;25:186-194.
- Gajewski D, Granville R. The United States Armed Forces Amputee Patient Care Program. J Am Acad Orthop Surg. 2006;14(10 spec no):S183-S187.
- Butler K, Bowen C, Hughes AM, et al. A systematic review of the key factors affecting tissue viability and rehabilitation outcomes of the residual limb in lower extremity traumatic amputees. J Tissue Viability. 2014;23:81-93.
- Mak AF, Zhang M, Boone DA. State-of-the-art research in lower-limb prosthetic biomechanics-socket interface: a review. J Rehabil Res Dev. 2001;38:161-174.
- Silver-Thorn MB, Steege JW. A review of prosthetic interface stress investigations. J Rehabil Res Dev. 1996;33:253-266.
- Dudek NL, Marks MB, Marshall SC. Skin problems in an amputee clinic. Am J Phys Med Rehabil. 2006;85:424-429.
- Meulenbelt HE, Geertzen JH, Dijkstra PU, et al. Skin problems in lower limb amputees: an overview by case reports. J Eur Acad Dermatol Venereol. 2007;21:147-155.
- Buikema KE, Meyerle JH. Amputation stump: privileged harbor for infections, tumors, and immune disorders. Clin Dermatol. 2014;32:670-677.
- Highsmith JT, Highsmith MJ. Common skin pathology in LE prosthesis users. JAAPA. 2007;20:33-36, 47.
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090.
- Levy SW. Skin Problems of the Amputee. St. Louis, MO: Warren H. Green Inc; 1983.
- Levy SW, Allende MF, Barnes GH. Skin problems of the leg amputee. Arch Dermatol. 1962;85:65-81.
- Dumanian GA, Potter BK, Mioton LM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial [published October 26, 2018]. Ann Surg. 2018. doi:10.1097/SLA.0000000000003088.
- Yang NB, Garza LA, Foote CE, et al. High prevalence of stump dermatoses 38 years or more after amputation. Arch Dermatol. 2012;148:1283-1286.
- Meulenbelt HE, Geertzen JH, Jonkman MF, et al. Determinants of skin problems of the stump in lower-limb amputees. Arch Phys Med Rehabil. 2009;90:74-81.
- Lin CH, Ma H, Chung MT, et al. Granulomatous cutaneous lesions associated with risperidone-induced hyperprolactinemia in an amputated upper limb: risperidone-induced cutaneous granulomas. Int J Dermatol. 2012;51:75-78.
- Schwartz RA, Bagley MP, Janniger CK, et al. Verrucous carcinoma of a leg amputation stump. Dermatology. 1991;182:193-195.
- Reilly GD, Boulton AJ, Harrington CI. Stump pemphigoid: a new complication of the amputee. Br Med J. 1983;287:875-876.
- Turan H, Bas¸kan EB, Adim SB, et al. Acroangiodermatitis in a below-knee amputation stump: correspondence. Clin Exp Dermatol. 2011;36:560-561.
- Edwards DS, Kuhn KM, Potter BK, et al. Heterotopic ossification: a review of current understanding, treatment, and future. J Orthop Trauma. 2016;30(suppl 3):S27-S30.
- Potter BK, Burns TC, Lacap AP, et al. Heterotopic ossification following traumatic and combat-related amputations: prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476-486.
- Tintle SM, Shawen SB, Forsberg JA, et al. Reoperation after combat-related major lower extremity amputations. J Orthop Trauma. 2014;28:232-237.
- Mckechnie PS, John A. Anxiety and depression following traumatic limb amputation: a systematic review. Injury. 2014;45:1859-1866.
- Hachisuka K, Nakamura T, Ohmine S, et al. Hygiene problems of residual limb and silicone liners in transtibial amputees wearing the total surface bearing socket. Arch Phys Med Rehabil. 2001;82:1286-1290.
- Pantera E, Pourtier-Piotte C, Bensoussan L, et al. Patient education after amputation: systematic review and experts’ opinions. Ann Phys Rehabil Med. 2014;57:143-158.
- Blum C, Ehrler S, Isner ME. Assessment of therapeutic education in 135 lower limb amputees. Ann Phys Rehabil Med. 2016;59:E161.
- Pasquina PF, Perry BN, Alphonso AL, et al. Residual limb hyperhidrosis and rimabotulinumtoxinB: a randomized, placebo-controlled study. Arch Phys Med Rehabil. 2015;97:659-664.e2.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- Shumaker PR, Kwan JM, Badiavas EV, et al. Rapid healing of scar-associated chronic wounds after ablative fractional resurfacing. Arch Dermatol. 2012;148:1289-1293.
- Anderson RR, Donelan MB, Hivnor C, et al. Laser treatment of traumatic scars with an emphasis on ablative fractional laser resurfacing: consensus report. JAMA Dermatol. 2014;150:187-193.
- Sanders JE, Mitchell SB, Wang YN, et al. An explant model for the investigation of skin adaptation to mechanical stress. IEEE Trans Biomed Eng. 2002;49(12 pt 2):1626-1631.
- Wang YN, Sanders JE. How does skin adapt to repetitive mechanical stress to become load tolerant? Med Hypotheses. 2003;61:29-35.
- Sanders JE, Goldstein BS. Collagen fibril diameters increase and fibril densities decrease in skin subjected to repetitive compressive and shear stresses. J Biomech. 2001;34:1581-1587.
- Thangapazham R, Darling T, Meyerle J. Alteration of skin properties with autologous dermal fibroblasts. Int J Mol Sci. 2014;15:8407-8427.
- Rink CL, Wernke MM, Powell HM, et al. Standardized approach to quantitatively measure residual limb skin health in individuals with lower limb amputation. Adv Wound Care. 2017;6:225-232.
Limb amputation is a major life-changing event that markedly affects a patient’s quality of life as well as his/her ability to participate in activities of daily living. The most prevalent causes for amputation include vascular diseases, diabetes mellitus, trauma, and cancer, respectively.1,2 For amputees, maintaining prosthetic use is a major physical and psychological undertaking that benefits from a multidisciplinary team approach. Although individuals with lower limb amputations are disproportionately impacted by skin disease due to the increased mechanical forces exerted over the lower limbs, patients with upper limb amputations also develop dermatologic conditions secondary to wearing prostheses.
Approximately 185,000 amputations occur each year in the United States.3 Although amputations resulting from peripheral vascular disease or diabetes mellitus tend to occur in older individuals, amputations in younger patients usually occur from trauma.2 The US military has experienced increasing numbers of amputations from trauma due to the ongoing combat operations in the Middle East. Although improvements in body armor and tactical combat casualty care have reduced the number of preventable deaths, the number of casualties surviving with extremity injuries requiring amputation has increased.4,5 As of October 2017, 1705 US servicemembers underwent major limb amputations, with 1914 lower limb amputations and 302 upper limb amputations. These amputations mainly impacted men aged 21 to 29 years, but female servicemembers also were affected, and a small group of servicemembers had multiple amputations.6
One of the most common medical problems that amputees face during long-term care is skin disease, with approximately 75% of amputees using a lower limb prosthesis experiencing skin problems. In general, amputees experience nearly 65% more dermatologic concerns than the general population.7 In one study of 97 individuals with transfemoral amputations, some of the most common issues associated with socket prosthetics included heat and sweating in the prosthetic socket (72%) as well as sores and skin irritation from the socket (62%).8 Given the high incidence of skin disease on residual limbs, dermatologists are uniquely positioned to keep the amputee in his/her prosthesis and prevent prosthetic abandonment.
Complications Following Amputation
Although US military servicemembers who undergo amputations receive the very best prosthetic devices and rehabilitation resources, they still experience prosthesis abandonment.9 Despite the fact that prosthetic limbs and prosthesis technology have substantially improved over the last 2 decades, one study indicated that the high frequency of problems affecting tissue viability at residual limbs is due to the age-old problem of prosthetic fit.10 In patients with the most advanced prostheses, poor fit still results in mechanical damage to the skin, as the residual limb is exposed to unequal and shearing forces across the amputation site as well as high pressures that cause a vaso-occlusive effect.11,12 Issues with poor fit are especially important for more active patients, as they normally want to immediately return to their vigorous preinjury lifestyles. In these patients, even a properly fitting prosthetic may not be able to overcome the fact that the residual limb skin is not well suited for the mechanical forces generated by the prosthesis and the humid environment of the socket.1,13 Another complicating factor is the dynamic nature of the residual limb. Muscle atrophy, changes in gait, and weight gain or loss can lead to an ill-fitting prosthetic and subsequent skin breakdown.
There are many case reports and review articles describing the skin problems in amputees.1,14-17 The Table summarizes these conditions and outlines treatment options for each.15,18-20

Most skin diseases on residual limbs are the result of mechanical skin breakdown, inflammation, infection, or combinations of these processes. Overall, amputees with diabetes mellitus and peripheral vascular disease tend to have skin disease related to poor perfusion, whereas amputees who are active and healthy tend to have conditions related to mechanical stress.7,13,14,17,21,22 Bui et al17 reported ulcers, abscesses, and blisters as the most common skin conditions that occur at the site of residual limbs; however, other less common dermatologic disorders such as skin malignancies, verrucous hyperplasia and carcinoma, granulomatous cutaneous lesions, acroangiodermatitis, and bullous pemphigoid also are seen.23-26 Buikema and Meyerle15 hypothesize that these conditions, as well as the more common skin diseases, are partly from the amputation disrupting blood and lymphatic flow in the residual limb, which causes the site to act as an immunocompromised district that induces dysregulation of neuroimmune regulators.
It is important to note that skin disease on residual limbs is not just an acute problem. Long-term follow-up of 247 traumatic amputees from the Vietnam War showed that almost half of prosthesis users (48.2%) reported a skin problem in the preceding year, more than 38 years after the amputation. Additionally, one-quarter of these individuals experienced skin problems approximately 50% of the time, which unfortunately led to limited use or total abandonment of the prosthesis for the preceding year in 56% of the veterans surveyed.21
Other complications following amputation indirectly lead to skin problems. Heterotopic ossification, or the formation of bone at extraskeletal sites, has been observed in up to 65% of military amputees from recent operations in Iraq and Afghanistan.27,28 If symptomatic, heterotopic ossification can lead to poor prosthetic fit and subsequent skin breakdown. As a result, it has been reported that up to 40% of combat-related lower extremity amputations may require excision of heterotopic ossificiation.29
Amputation also can result in psychologic concerns that indirectly affect skin health. A systematic review by Mckechnie and John30 suggested that despite heterogeneity between studies, even using the lowest figures demonstrated the significance anxiety and depression play in the lives of traumatic amputees. If left untreated, these mental health issues can lead to poor residual limb hygiene and prosthetic maintenance due to reductions in the patient’s energy and motivation. Studies have shown that proper hygiene of residual limbs and silicone liners reduces associated skin problems.19,31
Role of the Dermatologist
Routine care and conservative management of amputee skin problems often are accomplished by prosthetists, primary care physicians, nurses, and physical therapists. In one study, more than 80% of the most common skin problems affecting amputees could be attributed to the prosthesis itself, which highlights the importance of the continued involvement of the prosthetist beyond the initial fitting period.13 However, when a skin problem becomes refractory to conservative management, referral to a dermatologist is prudent; therefore, the dermatologist is an integral member of the multidisciplinary team that provides care for amputees.
The dermatologist often is best positioned to diagnose skin diseases that result from wearing prostheses and is well versed in treatments for short-term and long-term management of skin disease on residual limbs. The dermatologist also can offer prophylactic treatments to decrease sweating and hair growth to prevent potential infections and subsequent skin breakdown. Additionally, proper education on self-care has been shown to decrease the amount of skin problems and increase functional status and quality of life for amputees.32,33 Dermatologists can assist with the patient education process as well as refer amputees to a useful resource from the Amputee Coalition website (www.amputee-coalition.org) to provide specific patient education on how to maintain skin on the residual limb to prevent skin disease.
Current Treatments and Future Directions
Skin disorders affecting residual limbs usually are conditions that dermatologists commonly encounter and are comfortable managing in general practice. Additionally, dermatologists routinely treat hyperhidrosis and conduct laser hair removal, both of which are effective prophylactic adjuncts for amputee skin health. There are a few treatments for reducing residual limb hyperhidrosis that are particularly useful. Although first-line treatment of residual limb hyperhidrosis often is topical aluminum chloride, it requires frequent application and often causes considerable skin irritation when applied to residual limbs. Alternatively, intradermal botulinum toxin has been shown to successfully reduce sweat production in individuals with residual limb hyperhidrosis and is well tolerated.34 A 2017 case report discussed the use of microwave thermal ablation of eccrine coils using a noninvasive 3-step hyperhidrosis treatment system on a bilateral below-the-knee amputee. The authors reported the patient tolerated the procedure well with decreased dermatitis and folliculitis, leading to his ability to wear a prosthetic for longer periods of time.35
Ablative fractional resurfacing with a CO2 laser is another key treatment modality central to amputees, more specifically to traumatic amputees. A CO2 laser can decrease skin tension and increase skin mobility associated with traumatic scars as well as decrease skin vulnerability to biofilms present in chronic wounds on residual limbs. It is believed that the pattern of injury caused by ablative fractional lasers disrupts biofilms and stimulates growth factor secretion and collagen remodeling through the concept of photomicrodebridement.36 The ablative fractional resurfacing approach to scar therapy and chronic wound debridement can result in less skin injury, allowing the amputee to continue rehabilitation and return more quickly to prosthetic use.37
One interesting area of research in amputee care involves the study of novel ways to increase the skin’s ability to adapt to mechanical stress and load bearing and accelerate wound healing on the residual limb. Multiple studies have identified collagen fibril enlargement as an important component of skin adaptation, and biomolecules such as decorin may enhance this process.38-40 The concept of increasing these biomolecules at the correct time during wound healing to strengthen the residual limb tissue currently is being studied.39
Another encouraging area of research is the involvement of fibroblasts in cutaneous wound healing and their role in determining the phenotype of residual limb skin in amputees. The clinical application of autologous fibroblasts is approved by the US Food and Drug Administration for cosmetic use as a filler material and currently is under research for other applications, such as skin regeneration after surgery or manipulating skin characteristics to enhance the durability of residual limbs.41
Future preventative care of amputee skin may rely on tracking residual limb health before severe tissue injury occurs. For instance, Rink et al42 described an approach to monitor residual limb health using noninvasive imaging (eg, hyperspectral imaging, laser speckle imaging) and noninvasive probes that measure oxygenation, perfusion, skin barrier function, and skin hydration to the residual limb. Although these limb surveillance sensors would be employed by prosthetists, the dermatologist, as part of the multispecialty team, also could leverage the data for diagnosis and treatment considerations.
Final Thoughts
The dermatologist is an important member of the multidisciplinary team involved in the care of amputees. Skin disease is prevalent in amputees throughout their lives and often leads to abandonment of prostheses. Although current therapies and preventative treatments are for the most part successful, future research involving advanced technology to monitor skin health, increasing residual limb skin durability at the molecular level, and targeted laser therapies are promising. Through engagement and effective collaboration with the entire multidisciplinary team, dermatologists will have a considerable impact on amputee skin health.
Limb amputation is a major life-changing event that markedly affects a patient’s quality of life as well as his/her ability to participate in activities of daily living. The most prevalent causes for amputation include vascular diseases, diabetes mellitus, trauma, and cancer, respectively.1,2 For amputees, maintaining prosthetic use is a major physical and psychological undertaking that benefits from a multidisciplinary team approach. Although individuals with lower limb amputations are disproportionately impacted by skin disease due to the increased mechanical forces exerted over the lower limbs, patients with upper limb amputations also develop dermatologic conditions secondary to wearing prostheses.
Approximately 185,000 amputations occur each year in the United States.3 Although amputations resulting from peripheral vascular disease or diabetes mellitus tend to occur in older individuals, amputations in younger patients usually occur from trauma.2 The US military has experienced increasing numbers of amputations from trauma due to the ongoing combat operations in the Middle East. Although improvements in body armor and tactical combat casualty care have reduced the number of preventable deaths, the number of casualties surviving with extremity injuries requiring amputation has increased.4,5 As of October 2017, 1705 US servicemembers underwent major limb amputations, with 1914 lower limb amputations and 302 upper limb amputations. These amputations mainly impacted men aged 21 to 29 years, but female servicemembers also were affected, and a small group of servicemembers had multiple amputations.6
One of the most common medical problems that amputees face during long-term care is skin disease, with approximately 75% of amputees using a lower limb prosthesis experiencing skin problems. In general, amputees experience nearly 65% more dermatologic concerns than the general population.7 In one study of 97 individuals with transfemoral amputations, some of the most common issues associated with socket prosthetics included heat and sweating in the prosthetic socket (72%) as well as sores and skin irritation from the socket (62%).8 Given the high incidence of skin disease on residual limbs, dermatologists are uniquely positioned to keep the amputee in his/her prosthesis and prevent prosthetic abandonment.
Complications Following Amputation
Although US military servicemembers who undergo amputations receive the very best prosthetic devices and rehabilitation resources, they still experience prosthesis abandonment.9 Despite the fact that prosthetic limbs and prosthesis technology have substantially improved over the last 2 decades, one study indicated that the high frequency of problems affecting tissue viability at residual limbs is due to the age-old problem of prosthetic fit.10 In patients with the most advanced prostheses, poor fit still results in mechanical damage to the skin, as the residual limb is exposed to unequal and shearing forces across the amputation site as well as high pressures that cause a vaso-occlusive effect.11,12 Issues with poor fit are especially important for more active patients, as they normally want to immediately return to their vigorous preinjury lifestyles. In these patients, even a properly fitting prosthetic may not be able to overcome the fact that the residual limb skin is not well suited for the mechanical forces generated by the prosthesis and the humid environment of the socket.1,13 Another complicating factor is the dynamic nature of the residual limb. Muscle atrophy, changes in gait, and weight gain or loss can lead to an ill-fitting prosthetic and subsequent skin breakdown.
There are many case reports and review articles describing the skin problems in amputees.1,14-17 The Table summarizes these conditions and outlines treatment options for each.15,18-20

Most skin diseases on residual limbs are the result of mechanical skin breakdown, inflammation, infection, or combinations of these processes. Overall, amputees with diabetes mellitus and peripheral vascular disease tend to have skin disease related to poor perfusion, whereas amputees who are active and healthy tend to have conditions related to mechanical stress.7,13,14,17,21,22 Bui et al17 reported ulcers, abscesses, and blisters as the most common skin conditions that occur at the site of residual limbs; however, other less common dermatologic disorders such as skin malignancies, verrucous hyperplasia and carcinoma, granulomatous cutaneous lesions, acroangiodermatitis, and bullous pemphigoid also are seen.23-26 Buikema and Meyerle15 hypothesize that these conditions, as well as the more common skin diseases, are partly from the amputation disrupting blood and lymphatic flow in the residual limb, which causes the site to act as an immunocompromised district that induces dysregulation of neuroimmune regulators.
It is important to note that skin disease on residual limbs is not just an acute problem. Long-term follow-up of 247 traumatic amputees from the Vietnam War showed that almost half of prosthesis users (48.2%) reported a skin problem in the preceding year, more than 38 years after the amputation. Additionally, one-quarter of these individuals experienced skin problems approximately 50% of the time, which unfortunately led to limited use or total abandonment of the prosthesis for the preceding year in 56% of the veterans surveyed.21
Other complications following amputation indirectly lead to skin problems. Heterotopic ossification, or the formation of bone at extraskeletal sites, has been observed in up to 65% of military amputees from recent operations in Iraq and Afghanistan.27,28 If symptomatic, heterotopic ossification can lead to poor prosthetic fit and subsequent skin breakdown. As a result, it has been reported that up to 40% of combat-related lower extremity amputations may require excision of heterotopic ossificiation.29
Amputation also can result in psychologic concerns that indirectly affect skin health. A systematic review by Mckechnie and John30 suggested that despite heterogeneity between studies, even using the lowest figures demonstrated the significance anxiety and depression play in the lives of traumatic amputees. If left untreated, these mental health issues can lead to poor residual limb hygiene and prosthetic maintenance due to reductions in the patient’s energy and motivation. Studies have shown that proper hygiene of residual limbs and silicone liners reduces associated skin problems.19,31
Role of the Dermatologist
Routine care and conservative management of amputee skin problems often are accomplished by prosthetists, primary care physicians, nurses, and physical therapists. In one study, more than 80% of the most common skin problems affecting amputees could be attributed to the prosthesis itself, which highlights the importance of the continued involvement of the prosthetist beyond the initial fitting period.13 However, when a skin problem becomes refractory to conservative management, referral to a dermatologist is prudent; therefore, the dermatologist is an integral member of the multidisciplinary team that provides care for amputees.
The dermatologist often is best positioned to diagnose skin diseases that result from wearing prostheses and is well versed in treatments for short-term and long-term management of skin disease on residual limbs. The dermatologist also can offer prophylactic treatments to decrease sweating and hair growth to prevent potential infections and subsequent skin breakdown. Additionally, proper education on self-care has been shown to decrease the amount of skin problems and increase functional status and quality of life for amputees.32,33 Dermatologists can assist with the patient education process as well as refer amputees to a useful resource from the Amputee Coalition website (www.amputee-coalition.org) to provide specific patient education on how to maintain skin on the residual limb to prevent skin disease.
Current Treatments and Future Directions
Skin disorders affecting residual limbs usually are conditions that dermatologists commonly encounter and are comfortable managing in general practice. Additionally, dermatologists routinely treat hyperhidrosis and conduct laser hair removal, both of which are effective prophylactic adjuncts for amputee skin health. There are a few treatments for reducing residual limb hyperhidrosis that are particularly useful. Although first-line treatment of residual limb hyperhidrosis often is topical aluminum chloride, it requires frequent application and often causes considerable skin irritation when applied to residual limbs. Alternatively, intradermal botulinum toxin has been shown to successfully reduce sweat production in individuals with residual limb hyperhidrosis and is well tolerated.34 A 2017 case report discussed the use of microwave thermal ablation of eccrine coils using a noninvasive 3-step hyperhidrosis treatment system on a bilateral below-the-knee amputee. The authors reported the patient tolerated the procedure well with decreased dermatitis and folliculitis, leading to his ability to wear a prosthetic for longer periods of time.35
Ablative fractional resurfacing with a CO2 laser is another key treatment modality central to amputees, more specifically to traumatic amputees. A CO2 laser can decrease skin tension and increase skin mobility associated with traumatic scars as well as decrease skin vulnerability to biofilms present in chronic wounds on residual limbs. It is believed that the pattern of injury caused by ablative fractional lasers disrupts biofilms and stimulates growth factor secretion and collagen remodeling through the concept of photomicrodebridement.36 The ablative fractional resurfacing approach to scar therapy and chronic wound debridement can result in less skin injury, allowing the amputee to continue rehabilitation and return more quickly to prosthetic use.37
One interesting area of research in amputee care involves the study of novel ways to increase the skin’s ability to adapt to mechanical stress and load bearing and accelerate wound healing on the residual limb. Multiple studies have identified collagen fibril enlargement as an important component of skin adaptation, and biomolecules such as decorin may enhance this process.38-40 The concept of increasing these biomolecules at the correct time during wound healing to strengthen the residual limb tissue currently is being studied.39
Another encouraging area of research is the involvement of fibroblasts in cutaneous wound healing and their role in determining the phenotype of residual limb skin in amputees. The clinical application of autologous fibroblasts is approved by the US Food and Drug Administration for cosmetic use as a filler material and currently is under research for other applications, such as skin regeneration after surgery or manipulating skin characteristics to enhance the durability of residual limbs.41
Future preventative care of amputee skin may rely on tracking residual limb health before severe tissue injury occurs. For instance, Rink et al42 described an approach to monitor residual limb health using noninvasive imaging (eg, hyperspectral imaging, laser speckle imaging) and noninvasive probes that measure oxygenation, perfusion, skin barrier function, and skin hydration to the residual limb. Although these limb surveillance sensors would be employed by prosthetists, the dermatologist, as part of the multispecialty team, also could leverage the data for diagnosis and treatment considerations.
Final Thoughts
The dermatologist is an important member of the multidisciplinary team involved in the care of amputees. Skin disease is prevalent in amputees throughout their lives and often leads to abandonment of prostheses. Although current therapies and preventative treatments are for the most part successful, future research involving advanced technology to monitor skin health, increasing residual limb skin durability at the molecular level, and targeted laser therapies are promising. Through engagement and effective collaboration with the entire multidisciplinary team, dermatologists will have a considerable impact on amputee skin health.
- Dudek NL, Marks MB, Marshall SC, et al. Dermatologic conditions associated with use of a lower-extremity prosthesis. Arch Phys Med Rehabil. 2005;86:659-663.
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422-429.
- Kozak LJ. Ambulatory and Inpatient Procedures in the United States, 1995. Hyattsville, MD: US Department of Health and Human Services; 1998.
- Epstein RA, Heinemann AW, McFarland LV. Quality of life for veterans and servicemembers with major traumatic limb loss from Vietnam and OIF/OEF conflicts. J Rehabil Res Dev. 2010;47:373-385.
- Dougherty AL, Mohrle CR, Galarneau MR, et al. Battlefield extremity injuries in Operation Iraqi Freedom. Injury. 2009;40:772-777.
- Farrokhi S, Perez K, Eskridge S, et al. Major deployment-related amputations of lower and upper limbs, active and reserve components, U.S. Armed Forces, 2001-2017. MSMR. 2018;25:10-16.
- Highsmith MJ, Highsmith JT. Identifying and managing skin issues with lower-limb prosthetic use. Amputee Coalition website. https://www.amputee-coalition.org/wp-content/uploads/2015/.../skin_issues_lower.pdf. Accessed January 4, 2019.
- Hagberg K, Brånemark R. Consequences of non-vascular trans-femoral amputation: a survey of quality of life, prosthetic use and problems. Prosthet Orthot Int. 2001;25:186-194.
- Gajewski D, Granville R. The United States Armed Forces Amputee Patient Care Program. J Am Acad Orthop Surg. 2006;14(10 spec no):S183-S187.
- Butler K, Bowen C, Hughes AM, et al. A systematic review of the key factors affecting tissue viability and rehabilitation outcomes of the residual limb in lower extremity traumatic amputees. J Tissue Viability. 2014;23:81-93.
- Mak AF, Zhang M, Boone DA. State-of-the-art research in lower-limb prosthetic biomechanics-socket interface: a review. J Rehabil Res Dev. 2001;38:161-174.
- Silver-Thorn MB, Steege JW. A review of prosthetic interface stress investigations. J Rehabil Res Dev. 1996;33:253-266.
- Dudek NL, Marks MB, Marshall SC. Skin problems in an amputee clinic. Am J Phys Med Rehabil. 2006;85:424-429.
- Meulenbelt HE, Geertzen JH, Dijkstra PU, et al. Skin problems in lower limb amputees: an overview by case reports. J Eur Acad Dermatol Venereol. 2007;21:147-155.
- Buikema KE, Meyerle JH. Amputation stump: privileged harbor for infections, tumors, and immune disorders. Clin Dermatol. 2014;32:670-677.
- Highsmith JT, Highsmith MJ. Common skin pathology in LE prosthesis users. JAAPA. 2007;20:33-36, 47.
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090.
- Levy SW. Skin Problems of the Amputee. St. Louis, MO: Warren H. Green Inc; 1983.
- Levy SW, Allende MF, Barnes GH. Skin problems of the leg amputee. Arch Dermatol. 1962;85:65-81.
- Dumanian GA, Potter BK, Mioton LM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial [published October 26, 2018]. Ann Surg. 2018. doi:10.1097/SLA.0000000000003088.
- Yang NB, Garza LA, Foote CE, et al. High prevalence of stump dermatoses 38 years or more after amputation. Arch Dermatol. 2012;148:1283-1286.
- Meulenbelt HE, Geertzen JH, Jonkman MF, et al. Determinants of skin problems of the stump in lower-limb amputees. Arch Phys Med Rehabil. 2009;90:74-81.
- Lin CH, Ma H, Chung MT, et al. Granulomatous cutaneous lesions associated with risperidone-induced hyperprolactinemia in an amputated upper limb: risperidone-induced cutaneous granulomas. Int J Dermatol. 2012;51:75-78.
- Schwartz RA, Bagley MP, Janniger CK, et al. Verrucous carcinoma of a leg amputation stump. Dermatology. 1991;182:193-195.
- Reilly GD, Boulton AJ, Harrington CI. Stump pemphigoid: a new complication of the amputee. Br Med J. 1983;287:875-876.
- Turan H, Bas¸kan EB, Adim SB, et al. Acroangiodermatitis in a below-knee amputation stump: correspondence. Clin Exp Dermatol. 2011;36:560-561.
- Edwards DS, Kuhn KM, Potter BK, et al. Heterotopic ossification: a review of current understanding, treatment, and future. J Orthop Trauma. 2016;30(suppl 3):S27-S30.
- Potter BK, Burns TC, Lacap AP, et al. Heterotopic ossification following traumatic and combat-related amputations: prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476-486.
- Tintle SM, Shawen SB, Forsberg JA, et al. Reoperation after combat-related major lower extremity amputations. J Orthop Trauma. 2014;28:232-237.
- Mckechnie PS, John A. Anxiety and depression following traumatic limb amputation: a systematic review. Injury. 2014;45:1859-1866.
- Hachisuka K, Nakamura T, Ohmine S, et al. Hygiene problems of residual limb and silicone liners in transtibial amputees wearing the total surface bearing socket. Arch Phys Med Rehabil. 2001;82:1286-1290.
- Pantera E, Pourtier-Piotte C, Bensoussan L, et al. Patient education after amputation: systematic review and experts’ opinions. Ann Phys Rehabil Med. 2014;57:143-158.
- Blum C, Ehrler S, Isner ME. Assessment of therapeutic education in 135 lower limb amputees. Ann Phys Rehabil Med. 2016;59:E161.
- Pasquina PF, Perry BN, Alphonso AL, et al. Residual limb hyperhidrosis and rimabotulinumtoxinB: a randomized, placebo-controlled study. Arch Phys Med Rehabil. 2015;97:659-664.e2.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- Shumaker PR, Kwan JM, Badiavas EV, et al. Rapid healing of scar-associated chronic wounds after ablative fractional resurfacing. Arch Dermatol. 2012;148:1289-1293.
- Anderson RR, Donelan MB, Hivnor C, et al. Laser treatment of traumatic scars with an emphasis on ablative fractional laser resurfacing: consensus report. JAMA Dermatol. 2014;150:187-193.
- Sanders JE, Mitchell SB, Wang YN, et al. An explant model for the investigation of skin adaptation to mechanical stress. IEEE Trans Biomed Eng. 2002;49(12 pt 2):1626-1631.
- Wang YN, Sanders JE. How does skin adapt to repetitive mechanical stress to become load tolerant? Med Hypotheses. 2003;61:29-35.
- Sanders JE, Goldstein BS. Collagen fibril diameters increase and fibril densities decrease in skin subjected to repetitive compressive and shear stresses. J Biomech. 2001;34:1581-1587.
- Thangapazham R, Darling T, Meyerle J. Alteration of skin properties with autologous dermal fibroblasts. Int J Mol Sci. 2014;15:8407-8427.
- Rink CL, Wernke MM, Powell HM, et al. Standardized approach to quantitatively measure residual limb skin health in individuals with lower limb amputation. Adv Wound Care. 2017;6:225-232.
- Dudek NL, Marks MB, Marshall SC, et al. Dermatologic conditions associated with use of a lower-extremity prosthesis. Arch Phys Med Rehabil. 2005;86:659-663.
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422-429.
- Kozak LJ. Ambulatory and Inpatient Procedures in the United States, 1995. Hyattsville, MD: US Department of Health and Human Services; 1998.
- Epstein RA, Heinemann AW, McFarland LV. Quality of life for veterans and servicemembers with major traumatic limb loss from Vietnam and OIF/OEF conflicts. J Rehabil Res Dev. 2010;47:373-385.
- Dougherty AL, Mohrle CR, Galarneau MR, et al. Battlefield extremity injuries in Operation Iraqi Freedom. Injury. 2009;40:772-777.
- Farrokhi S, Perez K, Eskridge S, et al. Major deployment-related amputations of lower and upper limbs, active and reserve components, U.S. Armed Forces, 2001-2017. MSMR. 2018;25:10-16.
- Highsmith MJ, Highsmith JT. Identifying and managing skin issues with lower-limb prosthetic use. Amputee Coalition website. https://www.amputee-coalition.org/wp-content/uploads/2015/.../skin_issues_lower.pdf. Accessed January 4, 2019.
- Hagberg K, Brånemark R. Consequences of non-vascular trans-femoral amputation: a survey of quality of life, prosthetic use and problems. Prosthet Orthot Int. 2001;25:186-194.
- Gajewski D, Granville R. The United States Armed Forces Amputee Patient Care Program. J Am Acad Orthop Surg. 2006;14(10 spec no):S183-S187.
- Butler K, Bowen C, Hughes AM, et al. A systematic review of the key factors affecting tissue viability and rehabilitation outcomes of the residual limb in lower extremity traumatic amputees. J Tissue Viability. 2014;23:81-93.
- Mak AF, Zhang M, Boone DA. State-of-the-art research in lower-limb prosthetic biomechanics-socket interface: a review. J Rehabil Res Dev. 2001;38:161-174.
- Silver-Thorn MB, Steege JW. A review of prosthetic interface stress investigations. J Rehabil Res Dev. 1996;33:253-266.
- Dudek NL, Marks MB, Marshall SC. Skin problems in an amputee clinic. Am J Phys Med Rehabil. 2006;85:424-429.
- Meulenbelt HE, Geertzen JH, Dijkstra PU, et al. Skin problems in lower limb amputees: an overview by case reports. J Eur Acad Dermatol Venereol. 2007;21:147-155.
- Buikema KE, Meyerle JH. Amputation stump: privileged harbor for infections, tumors, and immune disorders. Clin Dermatol. 2014;32:670-677.
- Highsmith JT, Highsmith MJ. Common skin pathology in LE prosthesis users. JAAPA. 2007;20:33-36, 47.
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090.
- Levy SW. Skin Problems of the Amputee. St. Louis, MO: Warren H. Green Inc; 1983.
- Levy SW, Allende MF, Barnes GH. Skin problems of the leg amputee. Arch Dermatol. 1962;85:65-81.
- Dumanian GA, Potter BK, Mioton LM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial [published October 26, 2018]. Ann Surg. 2018. doi:10.1097/SLA.0000000000003088.
- Yang NB, Garza LA, Foote CE, et al. High prevalence of stump dermatoses 38 years or more after amputation. Arch Dermatol. 2012;148:1283-1286.
- Meulenbelt HE, Geertzen JH, Jonkman MF, et al. Determinants of skin problems of the stump in lower-limb amputees. Arch Phys Med Rehabil. 2009;90:74-81.
- Lin CH, Ma H, Chung MT, et al. Granulomatous cutaneous lesions associated with risperidone-induced hyperprolactinemia in an amputated upper limb: risperidone-induced cutaneous granulomas. Int J Dermatol. 2012;51:75-78.
- Schwartz RA, Bagley MP, Janniger CK, et al. Verrucous carcinoma of a leg amputation stump. Dermatology. 1991;182:193-195.
- Reilly GD, Boulton AJ, Harrington CI. Stump pemphigoid: a new complication of the amputee. Br Med J. 1983;287:875-876.
- Turan H, Bas¸kan EB, Adim SB, et al. Acroangiodermatitis in a below-knee amputation stump: correspondence. Clin Exp Dermatol. 2011;36:560-561.
- Edwards DS, Kuhn KM, Potter BK, et al. Heterotopic ossification: a review of current understanding, treatment, and future. J Orthop Trauma. 2016;30(suppl 3):S27-S30.
- Potter BK, Burns TC, Lacap AP, et al. Heterotopic ossification following traumatic and combat-related amputations: prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476-486.
- Tintle SM, Shawen SB, Forsberg JA, et al. Reoperation after combat-related major lower extremity amputations. J Orthop Trauma. 2014;28:232-237.
- Mckechnie PS, John A. Anxiety and depression following traumatic limb amputation: a systematic review. Injury. 2014;45:1859-1866.
- Hachisuka K, Nakamura T, Ohmine S, et al. Hygiene problems of residual limb and silicone liners in transtibial amputees wearing the total surface bearing socket. Arch Phys Med Rehabil. 2001;82:1286-1290.
- Pantera E, Pourtier-Piotte C, Bensoussan L, et al. Patient education after amputation: systematic review and experts’ opinions. Ann Phys Rehabil Med. 2014;57:143-158.
- Blum C, Ehrler S, Isner ME. Assessment of therapeutic education in 135 lower limb amputees. Ann Phys Rehabil Med. 2016;59:E161.
- Pasquina PF, Perry BN, Alphonso AL, et al. Residual limb hyperhidrosis and rimabotulinumtoxinB: a randomized, placebo-controlled study. Arch Phys Med Rehabil. 2015;97:659-664.e2.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- Shumaker PR, Kwan JM, Badiavas EV, et al. Rapid healing of scar-associated chronic wounds after ablative fractional resurfacing. Arch Dermatol. 2012;148:1289-1293.
- Anderson RR, Donelan MB, Hivnor C, et al. Laser treatment of traumatic scars with an emphasis on ablative fractional laser resurfacing: consensus report. JAMA Dermatol. 2014;150:187-193.
- Sanders JE, Mitchell SB, Wang YN, et al. An explant model for the investigation of skin adaptation to mechanical stress. IEEE Trans Biomed Eng. 2002;49(12 pt 2):1626-1631.
- Wang YN, Sanders JE. How does skin adapt to repetitive mechanical stress to become load tolerant? Med Hypotheses. 2003;61:29-35.
- Sanders JE, Goldstein BS. Collagen fibril diameters increase and fibril densities decrease in skin subjected to repetitive compressive and shear stresses. J Biomech. 2001;34:1581-1587.
- Thangapazham R, Darling T, Meyerle J. Alteration of skin properties with autologous dermal fibroblasts. Int J Mol Sci. 2014;15:8407-8427.
- Rink CL, Wernke MM, Powell HM, et al. Standardized approach to quantitatively measure residual limb skin health in individuals with lower limb amputation. Adv Wound Care. 2017;6:225-232.
Practice Points
- Amputees have an increased risk for skin disease occurring on residual limbs.
- It is important to educate patients about proper hygiene techniques for residual limbs and prostheses as well as common signs and symptoms of skin disease at the amputation site.
- Amputees should see a dermatologist within the first year after amputation and often benefit from annual follow-up examinations.
- Early referral to a dermatologist for skin disease affecting residual limbs is warranted.
Combat Dermatology: The Role of the Deployed Army Dermatologist
Military dermatologists complete their residency training at 1 of 3 large military medical centers across the country: Walter Reed National Military Medical Center (Bethesda, Maryland), San Antonio Military Health System (San Antonio, Texas), or Naval Medical Center San Diego (San Diego, California). While in training, army dermatology residents in particular fall under the US Army Medical Command, or MEDCOM, which provides command and control of the army’s medical, dental, and veterinary treatment facilities. Upon graduating from residency, army dermatologists often are stationed with MEDCOM units but become eligible for deployment with US Army Forces Command (FORSCOM) units to both combat and noncombat zones depending on each individual FORSCOM unit’s mission.
The process by which dermatologists and other army physicians are tasked to a deploying FORSCOM unit is referred to as the Professional Filler System, or PROFIS, which was designed to help alleviate the financial cost and specialty skill degradation of having a physician assigned to a FORSCOM unit while not deployed.1 In general, the greater the amount of time that an army medical officer has not been deployed, the more likely they are to be selected for deployment with a FORSCOM unit. For the army dermatologist, deployment often comes shortly after completing residency or fellowship.
In this article, we review the various functions of the deployed dermatologist and also highlight the importance of maintaining basic emergency medical skills that could be generalized to the civilian population in case of local or national emergencies.
THE FIELD SURGEON
With rare exceptions, the US Army does not deploy dermatologists for their expertise in diagnosing and managing cutaneous diseases. Typically, a dermatologist will be assigned to a FORSCOM unit in the role of field surgeon. Other medical specialties including emergency medicine, family practice, internal medicine, pediatrics, and obstetrics and gynecology also are eligible for deployment as field surgeons.2 Field surgeons typically are assigned to a battalion-sized element of 300 to 1000 soldiers and are responsible for all medical care rendered under their supervision. Duties include combat resuscitation, primary care services, preventive medicine, medical training of battalion medical personnel, and serving as the medical adviser to the battalion commander.1 In some instances, a field surgeon will be stationed at a higher level of care co-located with a trauma surgeon; in those cases, the field surgeon also may be expected to assist in trauma surgery cases.
ARMY DEPLOYMENT MEDICAL SYSTEM
To better understand the responsibilities of a field surgeon, it is important to discuss the structure of the army’s deployment medical system. The US Military, including the army, has adopted a system of “roles” that have specific requirements regarding their associated medical capabilities.3 There are 4 roles designated within the army. Role 1 facilities are known as battalion aid stations (BASs).

Role of the Field Surgeon
Within the broader structure of the army, approximately 5 battalions (each composed of 300 to 1000 soldiers) comprise a single brigade combat team. Role 1 medical facilities typically have a single battalion surgeon assigned to them. Field surgeons most commonly serve in this battalion surgeon position. Additionally, Role 2 facilities may have slots for up to 2 battalion surgeons; however, field surgeons are less commonly tasked with this assignment.1 Occasionally, in one author’s (N.R.M.) personal experience, these roles are more fluid than one might expect. A field surgeon tasked initially with a Role 1 position may be shifted to a Role 2 assignment on an as-needed basis. This ability for rapid change in roles and responsibilities underscores the need for a fluid mind-set and thorough predeployment training for the field surgeon.
PREDEPLOYMENT TRAINING
As one might expect, dermatologists who have just graduated residency or fellowship are unlikely to have honed their trauma support skills to the degree needed to support a deployed battalion actively engaging in combat. Fortunately, there are many opportunities for military dermatologists to practice these skills prior to joining their FORSCOM colleagues. The initial exposure to trauma support comes during medical internship at the mandatory Combat Casualty Care Course (C4), an 8-day program designed to enhance the operational medical readiness and predeployment trauma training skills of medical officers.4 The C4 program includes 3 days of classroom training and 5 days of intensive field training. During C4, medical officers become certified in Advanced Trauma Life Support, a 3-day course organized by the American College of Surgeons.5 This course teaches medical officers how to quickly and judiciously triage, treat, and transport patients who have sustained potentially life-threatening traumas.
The next components of predeployment training, Tactical Combat Casualty Care and Tactical Combat Medical Care, occur in the months to weeks immediately preceding deployment.1,6 Tactical Combat Casualty Care prepares participants in the initial stabilization of trauma to occur at the point of injury.6 Tactical Combat Casualty Care principles generally are employed by medics (enlisted personnel trained in point-of-care medical support) rather than physicians; however, these principles are still critical for medical officers to be aware of when encountering severe traumas.6 In addition, the physician is responsible for ensuring his/her medics are fully trained in Tactical Combat Casualty Care. Tactical Combat Medical Care is geared more toward the direct preparation of medical officers. During the 5-day course, medical officers learn the gold standard for trauma care in both the classroom and in hands-on scenarios.1 This training not only allows medical officers to be self-sufficient in providing trauma support, but it also enables them to better maintain quality control of the performance of their medics continuously throughout the deployment.1
DEPLOYMENT RESPONSIBILITIES
Dermatologists who have completed the above training typically are subsequently deployed as field surgeons to a Role 1 facility. Field surgeons are designated as the officer in charge of the BAS and assume the position of medical platoon leader. A field surgeon usually will have both a physician assistant and a field medical assistant/medical plans officer (MEDO) to assist in running the BAS. The overarching goal of the field surgeon is to maintain the health and readiness of the battalion. In addition to addressing the day-to-day health care needs of individual soldiers, a field surgeon is expected to attend all staff meetings, advise the commander on preventative health and epidemiological trends, identify the scope of practice of the medics, ensure the BAS is prepared for mass casualties, and take responsibility for all controlled substances.
To illustrate the value that the properly trained dermatologist can provide in the deployed setting, we will outline field surgeon responsibilities and provide case examples of the first-hand experiences of one of the authors (N.R.M.) as a Role 2 officer in charge and field surgeon. The information presented in the case examples may have been altered to ensure continued operational security and out of respect to US servicemembers and coalition forces while still conveying important learning points.
Sick Call
In the deployed environment, military sick call functions as an urgent care center that is open continuously and serves the active-duty population, US government civilians and contractors, and coalition forces. In general, the physician assistant should treat approximately two-thirds of sick call patients under the supervision of the field surgeon, allowing the field surgeon to focus on his/her ancillary duties and ensure overall medical supervision of the unit. As a safeguard, patients with more than 2 visits for the same concern must be evaluated by the field surgeon. Sick call concerns range from minor traumas and illnesses to much more serious disease processes and injuries (as outlined in Medical Emergencies). As a field surgeon, it is critical to track disease nonbattle illnesses to ensure medical readiness of the unit. In the deployed environment, close quarters and austere environments commonly lend themselves to gastrointestinal illnesses, respiratory diseases, heat injuries, vector-borne diseases, and sexually transmitted infections.
Case Examples
During an 8-month deployment in Afghanistan, one of the authors (N.R.M.) provided or assisted in the care of more than 2300 routine sick call appointments, or approximately 10 patients per day. Epidemiology of disease was tracked, and the condition of the unit was presented daily to the battalion commander for consideration in upcoming operations. The top 5 most common categories of diagnoses included musculoskeletal injuries, gastrointestinal diseases, dermatologic concerns (eg, dermatitis, bacterial infections [cellulitis/abscess], fungal infections, arthropod assault, abrasions, lacerations, verruca vulgaris), respiratory illnesses, and mental health care, respectively. Maintaining a familiarity with general medicine is critical for the military dermatologist, and an adequate medical library or access to online medical review sources is critical for day-to-day sick call.
Medical Emergencies
In the event of a more serious injury or illness, a Role 1 BAS has very little capability in performing anything beyond the most basic interventions. Part of the art of being an effective field surgeon lies in stabilization, triage, and transport of these sometimes very ill patients. Both the decision to transport to a higher level of care (eg, Role 2 or 3 facility) as well as selection of the means of transportation falls on the field surgeon. The MEDO plays an essential role in assisting in the coordination of the transfer; however, the responsibility ultimately falls on the field surgeon.1,6 The field surgeon at the Role 2 BAS may be expected to perform more advanced medical and surgical interventions. More advanced pharmacotherapies include thrombolytics, antivenin, and vasopressors. Some procedural interventions include intubations, central lines, and laceration repairs. The Role 2 BAS has the capability to hold patients for up to 72 hours.
Case Examples
Specific conditions one of the authors (N.R.M.) treated include heat injury, myocardial infarction, disseminated tuberculosis, appendicitis, testicular torsion, malaria, suicidal ideation, burns, and status epilepticus. Over 8 months, the Role 2 BAS received 91 medical emergencies, with 53 necessitating evacuation to a higher level of care. Often, the more serious or rare conditions presented in the foreign contractor and coalition force populations working alongside US troops.
In one particular case, a 35-year-old man with an electrocardiogram-confirmed acute ST-segment elevation myocardial infarction was administered standard therapy consisting of intravenous morphine, oxygen, sublingual nitroglycerin, an angiotensin-converting enzyme inhibitor, and a beta-blocker. Given the lack of a cardiac catheterization laboratory at the next highest level of care as well as a low suspicion for aortic dissection (based on the patient’s history, physical examination, and chest radiograph), fibrinolysis with tenecteplase was performed in the deployed environment. After a very short observation for potential hemorrhage, the patient was then evacuated to the Role 3 hospital, where he made a near-complete recovery. Preparation with advanced cardiac life support courses and a thorough algorithmic review of the 10 most common causes of presentation to the emergency department helped adequately prepare the dermatologist to succeed.
Trauma Emergencies
The same principles of triage and transport apply to trauma emergencies. Mass casualties are an inevitable reality in combat, so appropriate training translating into efficient action is essential to ensure the lowest possible mortality. This training and the actions that stem from it are an additional responsibility that the field surgeon must maintain. During deployment, continued training organized by the field surgeon could quite literally mean the difference between life and death. In addition to the organizational responsibilities, field surgeons should be prepared to perform initial stabilization in trauma patients, including application of tourniquets, establishment of central lines, reading abdominal ultrasounds for free fluid, placement of chest tubes, intubation, and ventilator management. The Joint Trauma System Clinical Practice Guidelines also offer extensive and invaluable guidance on the most up-to-date approach to common trauma conditions arising in the deployed environment.7 At the Role 2 level, the field surgeon also must be prepared to coordinate ancillary services, manage the Role 2/forward surgical team intensive care unit, and serve as first assist in the operating room, as needed (Figure 2).

Case Examples
One of the authors (N.R.M.) assisted or provided care in approximately 225 trauma cases while deployed. A mass casualty event occurred, in which the Role 2 BAS received 34 casualties; of these casualties, 11 were immediate, 10 were delayed, 11 were minimal, and 2 were expectant. Injury patterns included mounted and dismounted improvised explosive device injuries (eg, blast, shrapnel, and traumatic brain injuries) as well as gunshot wounds. Direct care was provided for 13 casualties, including 10 abdominal ultrasound examinations for free fluid, placement of 2 chest tubes, 1 intubation, establishment of 3 central lines, and first-assisting 1 exploratory laparotomy. Of the casualties, 22 were evacuated to the Role 3 hospital, 8 were dispositioned to a coalition hospital, 2 were returned to active duty, and 2 died due to their injuries. The military trauma preparation as outlined in the predeployment training can help adequately prepare the military dermatologist to assist in these cases.
Ancillary Services
An important part of the efficacy of initial evaluation and stabilization of both medical and traumatic emergencies involves expedited laboratory tests, imaging, and the delivery of life-saving blood products to affected patients. The field surgeon is responsible for the readiness of these services and may play a critical role in streamlining these tasks for situations where a delay in care by minutes can be lethal. The MEDO assists the field surgeon to ensure the readiness of the medical equipment, and the field surgeon must ensure the readiness of the medics and technicians utilizing the equipment. In a deployed environment, only a finite amount of blood products may be stored. As a result, the design and implementation of an efficient and precise walking blood bank is critical. To help mitigate this issue, servicemembers are prescreened for their blood types and bloodborne illnesses. If a situation arises in which whole blood is needed, the prescreened individuals are screened again, and their blood is collected and transfused to the patient under the supervision of the physician. This task is critical in saving lives, and this process is the primary responsibility of the field surgeon.
Case Example
A 37-year-old man presented to the BAS with abdominal and pelvic gunshot wounds, as well as tachycardia, rapidly decreasing blood pressure, and altered consciousness. An exploratory laparotomy was performed to look for the sources of bleeding. The patient’s blood type was confirmed with a portable testing kit. Due to the injury pattern and clinical presentation, a call was immediately placed to begin screening and preparing servicemembers to donate blood for the walking blood bank. As expected, the Role 2 supply of blood products was exhausted during the exploratory laparotomy. With servicemembers in place and screened, an additional 12 units of whole blood were collected and administered in a timely fashion. The patient was stabilized and transported to the next highest level of care. Due to the process optimization performed by the laboratory team, whole-blood transfusions were ready within an average of 22 minutes, well ahead of the 45-minute standard of care.
Operating Room First Assist
If a field surgeon is stationed at a Role 2 BAS with a forward surgical team, he/she may be required to adopt the role of operating room first assist for the trauma surgeon or orthopedic surgeon on the team, which is especially true for isolated major traumas when triage and initial stabilization measures for multiple patients are of less concern. Dermatologists receive surgical training as part of the Accreditation Council for Graduate Medical Education requirements to graduate residency, making them more than capable of surgical assisting when needed.8 In particular, dermatologists’ ability to utilize instruments appropriately and think procedurally as well as their skills in suturing are helpful.
Case Example
A 22-year-old man with several shrapnel wounds to the abdomen demonstrated free fluid in the left lower quadrant. The field surgeon (N.R.M.) assisted the trauma surgeon in opening the abdomen and running the bowel for sources of bleeding. The trauma surgeon identified the bleed and performed a ligation. The patient was then packed, closed, and prepared for transfer to a higher level of care.
Preventive Medicine
As a result of the field surgeon being on the front line of medical care in an austere environment, implementation of preventive medicine practices and disease pattern recognition are his/her responsibility. Responsibilities may include stray animal euthanasia due to prevalence of rabies, enforcement of malaria prophylaxis, medical training and maintenance of snake antivenin, and assistance with other local endemic disease. The unique skill set of dermatologists in organism identification can further bolster the speed with which vector-borne diseases are recognized and prevention and treatment measures are implemented.
Case Example
As coalition forces executed a mission in Afghanistan, US servicemembers began experiencing abdominal distress, chills, fevers (temperature >40°C), debilitating headaches, myalgia, arthralgia, and tachycardia. Initially, these patients were evacuated to the Role 2 BAS, hindering the mission. Upon inspection, patients had numerous bug bites; one astute soldier collected the arthropod guilty of the assault and brought it to the aid station. Upon inspection, the offender was identified as the Phlebotomus genus of sandflies, organisms that are well known to dermatologists as a cause of leishmaniasis. Clinical correlation resulted in the presumed diagnosis of Pappataci fever, and vector-borne disease prevention measures were then able to be further emphasized and implemented in at-risk areas, allowing the mission to continue.9 Subsequent infectious disease laboratory testing confirmed the Phlebovirus transmitted by the sandfly as the underlying cause of the illness.
CONCLUSION
The diverse role of the field surgeon in the deployed setting makes any one specialist underprepared to completely take on the role from the outset; however, with appropriate and rigorous trauma training prior to deployment, dermatologists will continue to perform as invaluable assets to the US military in conflicts now and in the future.
1. Moawad FJ, Wilson R, Kunar MT, et al. Role of the battalion surgeon in the Iraq and Afghanistan War. Mil Med. 2012;177:412-416.
2. AR 601-142: Army Medical Department Professional Filler System. Washington, DC: US Department of the Army; 2015. http://cdm16635.contentdm.oclc.org/cdm/ref/collection/p16635coll11/id/4592. Accessed December 19, 2018.
3. Roles of medical care (United States). Emergency War Surgery. 4th ed. Fort Sam Houston, Texas: Office of the Surgeon General; 2013:17-28.
4. Combat Casualty Care Course (C4). Military Health System website. https://health.mil/Training-Center/Defense-Medical-Readiness-Training-Institute/Combat-Casualty-Care-Course. Accessed December 7, 2018.
5. Advanced Trauma Life Support. American College of Surgeons website. https://www.facs.org/quality-programs/trauma/atls. Accessed December 7, 2018.
6. Tactical Combat Casualty Care Course. Military Health System website. https://health.mil/Training-Center/Defense-Medical-Readiness-Training-Institute/Tactical-Combat-Casualty-Care-Course. Accessed December 18, 2018.
7. Joint Trauma System: The Department of Defense Center of Excellence for Trauma. Clinical Practice Guidelines.
8. ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Revised July 1, 2017. Accessed December 7, 2018.
9. Downs JW, Flood DT, Orr NH, et al. Sandfly fever in Afghanistan-a sometimes overlooked disease of military importance: a case series and review of the literature. US Army Med Dep J. 2017:60-66.
Military dermatologists complete their residency training at 1 of 3 large military medical centers across the country: Walter Reed National Military Medical Center (Bethesda, Maryland), San Antonio Military Health System (San Antonio, Texas), or Naval Medical Center San Diego (San Diego, California). While in training, army dermatology residents in particular fall under the US Army Medical Command, or MEDCOM, which provides command and control of the army’s medical, dental, and veterinary treatment facilities. Upon graduating from residency, army dermatologists often are stationed with MEDCOM units but become eligible for deployment with US Army Forces Command (FORSCOM) units to both combat and noncombat zones depending on each individual FORSCOM unit’s mission.
The process by which dermatologists and other army physicians are tasked to a deploying FORSCOM unit is referred to as the Professional Filler System, or PROFIS, which was designed to help alleviate the financial cost and specialty skill degradation of having a physician assigned to a FORSCOM unit while not deployed.1 In general, the greater the amount of time that an army medical officer has not been deployed, the more likely they are to be selected for deployment with a FORSCOM unit. For the army dermatologist, deployment often comes shortly after completing residency or fellowship.
In this article, we review the various functions of the deployed dermatologist and also highlight the importance of maintaining basic emergency medical skills that could be generalized to the civilian population in case of local or national emergencies.
THE FIELD SURGEON
With rare exceptions, the US Army does not deploy dermatologists for their expertise in diagnosing and managing cutaneous diseases. Typically, a dermatologist will be assigned to a FORSCOM unit in the role of field surgeon. Other medical specialties including emergency medicine, family practice, internal medicine, pediatrics, and obstetrics and gynecology also are eligible for deployment as field surgeons.2 Field surgeons typically are assigned to a battalion-sized element of 300 to 1000 soldiers and are responsible for all medical care rendered under their supervision. Duties include combat resuscitation, primary care services, preventive medicine, medical training of battalion medical personnel, and serving as the medical adviser to the battalion commander.1 In some instances, a field surgeon will be stationed at a higher level of care co-located with a trauma surgeon; in those cases, the field surgeon also may be expected to assist in trauma surgery cases.
ARMY DEPLOYMENT MEDICAL SYSTEM
To better understand the responsibilities of a field surgeon, it is important to discuss the structure of the army’s deployment medical system. The US Military, including the army, has adopted a system of “roles” that have specific requirements regarding their associated medical capabilities.3 There are 4 roles designated within the army. Role 1 facilities are known as battalion aid stations (BASs).

Role of the Field Surgeon
Within the broader structure of the army, approximately 5 battalions (each composed of 300 to 1000 soldiers) comprise a single brigade combat team. Role 1 medical facilities typically have a single battalion surgeon assigned to them. Field surgeons most commonly serve in this battalion surgeon position. Additionally, Role 2 facilities may have slots for up to 2 battalion surgeons; however, field surgeons are less commonly tasked with this assignment.1 Occasionally, in one author’s (N.R.M.) personal experience, these roles are more fluid than one might expect. A field surgeon tasked initially with a Role 1 position may be shifted to a Role 2 assignment on an as-needed basis. This ability for rapid change in roles and responsibilities underscores the need for a fluid mind-set and thorough predeployment training for the field surgeon.
PREDEPLOYMENT TRAINING
As one might expect, dermatologists who have just graduated residency or fellowship are unlikely to have honed their trauma support skills to the degree needed to support a deployed battalion actively engaging in combat. Fortunately, there are many opportunities for military dermatologists to practice these skills prior to joining their FORSCOM colleagues. The initial exposure to trauma support comes during medical internship at the mandatory Combat Casualty Care Course (C4), an 8-day program designed to enhance the operational medical readiness and predeployment trauma training skills of medical officers.4 The C4 program includes 3 days of classroom training and 5 days of intensive field training. During C4, medical officers become certified in Advanced Trauma Life Support, a 3-day course organized by the American College of Surgeons.5 This course teaches medical officers how to quickly and judiciously triage, treat, and transport patients who have sustained potentially life-threatening traumas.
The next components of predeployment training, Tactical Combat Casualty Care and Tactical Combat Medical Care, occur in the months to weeks immediately preceding deployment.1,6 Tactical Combat Casualty Care prepares participants in the initial stabilization of trauma to occur at the point of injury.6 Tactical Combat Casualty Care principles generally are employed by medics (enlisted personnel trained in point-of-care medical support) rather than physicians; however, these principles are still critical for medical officers to be aware of when encountering severe traumas.6 In addition, the physician is responsible for ensuring his/her medics are fully trained in Tactical Combat Casualty Care. Tactical Combat Medical Care is geared more toward the direct preparation of medical officers. During the 5-day course, medical officers learn the gold standard for trauma care in both the classroom and in hands-on scenarios.1 This training not only allows medical officers to be self-sufficient in providing trauma support, but it also enables them to better maintain quality control of the performance of their medics continuously throughout the deployment.1
DEPLOYMENT RESPONSIBILITIES
Dermatologists who have completed the above training typically are subsequently deployed as field surgeons to a Role 1 facility. Field surgeons are designated as the officer in charge of the BAS and assume the position of medical platoon leader. A field surgeon usually will have both a physician assistant and a field medical assistant/medical plans officer (MEDO) to assist in running the BAS. The overarching goal of the field surgeon is to maintain the health and readiness of the battalion. In addition to addressing the day-to-day health care needs of individual soldiers, a field surgeon is expected to attend all staff meetings, advise the commander on preventative health and epidemiological trends, identify the scope of practice of the medics, ensure the BAS is prepared for mass casualties, and take responsibility for all controlled substances.
To illustrate the value that the properly trained dermatologist can provide in the deployed setting, we will outline field surgeon responsibilities and provide case examples of the first-hand experiences of one of the authors (N.R.M.) as a Role 2 officer in charge and field surgeon. The information presented in the case examples may have been altered to ensure continued operational security and out of respect to US servicemembers and coalition forces while still conveying important learning points.
Sick Call
In the deployed environment, military sick call functions as an urgent care center that is open continuously and serves the active-duty population, US government civilians and contractors, and coalition forces. In general, the physician assistant should treat approximately two-thirds of sick call patients under the supervision of the field surgeon, allowing the field surgeon to focus on his/her ancillary duties and ensure overall medical supervision of the unit. As a safeguard, patients with more than 2 visits for the same concern must be evaluated by the field surgeon. Sick call concerns range from minor traumas and illnesses to much more serious disease processes and injuries (as outlined in Medical Emergencies). As a field surgeon, it is critical to track disease nonbattle illnesses to ensure medical readiness of the unit. In the deployed environment, close quarters and austere environments commonly lend themselves to gastrointestinal illnesses, respiratory diseases, heat injuries, vector-borne diseases, and sexually transmitted infections.
Case Examples
During an 8-month deployment in Afghanistan, one of the authors (N.R.M.) provided or assisted in the care of more than 2300 routine sick call appointments, or approximately 10 patients per day. Epidemiology of disease was tracked, and the condition of the unit was presented daily to the battalion commander for consideration in upcoming operations. The top 5 most common categories of diagnoses included musculoskeletal injuries, gastrointestinal diseases, dermatologic concerns (eg, dermatitis, bacterial infections [cellulitis/abscess], fungal infections, arthropod assault, abrasions, lacerations, verruca vulgaris), respiratory illnesses, and mental health care, respectively. Maintaining a familiarity with general medicine is critical for the military dermatologist, and an adequate medical library or access to online medical review sources is critical for day-to-day sick call.
Medical Emergencies
In the event of a more serious injury or illness, a Role 1 BAS has very little capability in performing anything beyond the most basic interventions. Part of the art of being an effective field surgeon lies in stabilization, triage, and transport of these sometimes very ill patients. Both the decision to transport to a higher level of care (eg, Role 2 or 3 facility) as well as selection of the means of transportation falls on the field surgeon. The MEDO plays an essential role in assisting in the coordination of the transfer; however, the responsibility ultimately falls on the field surgeon.1,6 The field surgeon at the Role 2 BAS may be expected to perform more advanced medical and surgical interventions. More advanced pharmacotherapies include thrombolytics, antivenin, and vasopressors. Some procedural interventions include intubations, central lines, and laceration repairs. The Role 2 BAS has the capability to hold patients for up to 72 hours.
Case Examples
Specific conditions one of the authors (N.R.M.) treated include heat injury, myocardial infarction, disseminated tuberculosis, appendicitis, testicular torsion, malaria, suicidal ideation, burns, and status epilepticus. Over 8 months, the Role 2 BAS received 91 medical emergencies, with 53 necessitating evacuation to a higher level of care. Often, the more serious or rare conditions presented in the foreign contractor and coalition force populations working alongside US troops.
In one particular case, a 35-year-old man with an electrocardiogram-confirmed acute ST-segment elevation myocardial infarction was administered standard therapy consisting of intravenous morphine, oxygen, sublingual nitroglycerin, an angiotensin-converting enzyme inhibitor, and a beta-blocker. Given the lack of a cardiac catheterization laboratory at the next highest level of care as well as a low suspicion for aortic dissection (based on the patient’s history, physical examination, and chest radiograph), fibrinolysis with tenecteplase was performed in the deployed environment. After a very short observation for potential hemorrhage, the patient was then evacuated to the Role 3 hospital, where he made a near-complete recovery. Preparation with advanced cardiac life support courses and a thorough algorithmic review of the 10 most common causes of presentation to the emergency department helped adequately prepare the dermatologist to succeed.
Trauma Emergencies
The same principles of triage and transport apply to trauma emergencies. Mass casualties are an inevitable reality in combat, so appropriate training translating into efficient action is essential to ensure the lowest possible mortality. This training and the actions that stem from it are an additional responsibility that the field surgeon must maintain. During deployment, continued training organized by the field surgeon could quite literally mean the difference between life and death. In addition to the organizational responsibilities, field surgeons should be prepared to perform initial stabilization in trauma patients, including application of tourniquets, establishment of central lines, reading abdominal ultrasounds for free fluid, placement of chest tubes, intubation, and ventilator management. The Joint Trauma System Clinical Practice Guidelines also offer extensive and invaluable guidance on the most up-to-date approach to common trauma conditions arising in the deployed environment.7 At the Role 2 level, the field surgeon also must be prepared to coordinate ancillary services, manage the Role 2/forward surgical team intensive care unit, and serve as first assist in the operating room, as needed (Figure 2).

Case Examples
One of the authors (N.R.M.) assisted or provided care in approximately 225 trauma cases while deployed. A mass casualty event occurred, in which the Role 2 BAS received 34 casualties; of these casualties, 11 were immediate, 10 were delayed, 11 were minimal, and 2 were expectant. Injury patterns included mounted and dismounted improvised explosive device injuries (eg, blast, shrapnel, and traumatic brain injuries) as well as gunshot wounds. Direct care was provided for 13 casualties, including 10 abdominal ultrasound examinations for free fluid, placement of 2 chest tubes, 1 intubation, establishment of 3 central lines, and first-assisting 1 exploratory laparotomy. Of the casualties, 22 were evacuated to the Role 3 hospital, 8 were dispositioned to a coalition hospital, 2 were returned to active duty, and 2 died due to their injuries. The military trauma preparation as outlined in the predeployment training can help adequately prepare the military dermatologist to assist in these cases.
Ancillary Services
An important part of the efficacy of initial evaluation and stabilization of both medical and traumatic emergencies involves expedited laboratory tests, imaging, and the delivery of life-saving blood products to affected patients. The field surgeon is responsible for the readiness of these services and may play a critical role in streamlining these tasks for situations where a delay in care by minutes can be lethal. The MEDO assists the field surgeon to ensure the readiness of the medical equipment, and the field surgeon must ensure the readiness of the medics and technicians utilizing the equipment. In a deployed environment, only a finite amount of blood products may be stored. As a result, the design and implementation of an efficient and precise walking blood bank is critical. To help mitigate this issue, servicemembers are prescreened for their blood types and bloodborne illnesses. If a situation arises in which whole blood is needed, the prescreened individuals are screened again, and their blood is collected and transfused to the patient under the supervision of the physician. This task is critical in saving lives, and this process is the primary responsibility of the field surgeon.
Case Example
A 37-year-old man presented to the BAS with abdominal and pelvic gunshot wounds, as well as tachycardia, rapidly decreasing blood pressure, and altered consciousness. An exploratory laparotomy was performed to look for the sources of bleeding. The patient’s blood type was confirmed with a portable testing kit. Due to the injury pattern and clinical presentation, a call was immediately placed to begin screening and preparing servicemembers to donate blood for the walking blood bank. As expected, the Role 2 supply of blood products was exhausted during the exploratory laparotomy. With servicemembers in place and screened, an additional 12 units of whole blood were collected and administered in a timely fashion. The patient was stabilized and transported to the next highest level of care. Due to the process optimization performed by the laboratory team, whole-blood transfusions were ready within an average of 22 minutes, well ahead of the 45-minute standard of care.
Operating Room First Assist
If a field surgeon is stationed at a Role 2 BAS with a forward surgical team, he/she may be required to adopt the role of operating room first assist for the trauma surgeon or orthopedic surgeon on the team, which is especially true for isolated major traumas when triage and initial stabilization measures for multiple patients are of less concern. Dermatologists receive surgical training as part of the Accreditation Council for Graduate Medical Education requirements to graduate residency, making them more than capable of surgical assisting when needed.8 In particular, dermatologists’ ability to utilize instruments appropriately and think procedurally as well as their skills in suturing are helpful.
Case Example
A 22-year-old man with several shrapnel wounds to the abdomen demonstrated free fluid in the left lower quadrant. The field surgeon (N.R.M.) assisted the trauma surgeon in opening the abdomen and running the bowel for sources of bleeding. The trauma surgeon identified the bleed and performed a ligation. The patient was then packed, closed, and prepared for transfer to a higher level of care.
Preventive Medicine
As a result of the field surgeon being on the front line of medical care in an austere environment, implementation of preventive medicine practices and disease pattern recognition are his/her responsibility. Responsibilities may include stray animal euthanasia due to prevalence of rabies, enforcement of malaria prophylaxis, medical training and maintenance of snake antivenin, and assistance with other local endemic disease. The unique skill set of dermatologists in organism identification can further bolster the speed with which vector-borne diseases are recognized and prevention and treatment measures are implemented.
Case Example
As coalition forces executed a mission in Afghanistan, US servicemembers began experiencing abdominal distress, chills, fevers (temperature >40°C), debilitating headaches, myalgia, arthralgia, and tachycardia. Initially, these patients were evacuated to the Role 2 BAS, hindering the mission. Upon inspection, patients had numerous bug bites; one astute soldier collected the arthropod guilty of the assault and brought it to the aid station. Upon inspection, the offender was identified as the Phlebotomus genus of sandflies, organisms that are well known to dermatologists as a cause of leishmaniasis. Clinical correlation resulted in the presumed diagnosis of Pappataci fever, and vector-borne disease prevention measures were then able to be further emphasized and implemented in at-risk areas, allowing the mission to continue.9 Subsequent infectious disease laboratory testing confirmed the Phlebovirus transmitted by the sandfly as the underlying cause of the illness.
CONCLUSION
The diverse role of the field surgeon in the deployed setting makes any one specialist underprepared to completely take on the role from the outset; however, with appropriate and rigorous trauma training prior to deployment, dermatologists will continue to perform as invaluable assets to the US military in conflicts now and in the future.
Military dermatologists complete their residency training at 1 of 3 large military medical centers across the country: Walter Reed National Military Medical Center (Bethesda, Maryland), San Antonio Military Health System (San Antonio, Texas), or Naval Medical Center San Diego (San Diego, California). While in training, army dermatology residents in particular fall under the US Army Medical Command, or MEDCOM, which provides command and control of the army’s medical, dental, and veterinary treatment facilities. Upon graduating from residency, army dermatologists often are stationed with MEDCOM units but become eligible for deployment with US Army Forces Command (FORSCOM) units to both combat and noncombat zones depending on each individual FORSCOM unit’s mission.
The process by which dermatologists and other army physicians are tasked to a deploying FORSCOM unit is referred to as the Professional Filler System, or PROFIS, which was designed to help alleviate the financial cost and specialty skill degradation of having a physician assigned to a FORSCOM unit while not deployed.1 In general, the greater the amount of time that an army medical officer has not been deployed, the more likely they are to be selected for deployment with a FORSCOM unit. For the army dermatologist, deployment often comes shortly after completing residency or fellowship.
In this article, we review the various functions of the deployed dermatologist and also highlight the importance of maintaining basic emergency medical skills that could be generalized to the civilian population in case of local or national emergencies.
THE FIELD SURGEON
With rare exceptions, the US Army does not deploy dermatologists for their expertise in diagnosing and managing cutaneous diseases. Typically, a dermatologist will be assigned to a FORSCOM unit in the role of field surgeon. Other medical specialties including emergency medicine, family practice, internal medicine, pediatrics, and obstetrics and gynecology also are eligible for deployment as field surgeons.2 Field surgeons typically are assigned to a battalion-sized element of 300 to 1000 soldiers and are responsible for all medical care rendered under their supervision. Duties include combat resuscitation, primary care services, preventive medicine, medical training of battalion medical personnel, and serving as the medical adviser to the battalion commander.1 In some instances, a field surgeon will be stationed at a higher level of care co-located with a trauma surgeon; in those cases, the field surgeon also may be expected to assist in trauma surgery cases.
ARMY DEPLOYMENT MEDICAL SYSTEM
To better understand the responsibilities of a field surgeon, it is important to discuss the structure of the army’s deployment medical system. The US Military, including the army, has adopted a system of “roles” that have specific requirements regarding their associated medical capabilities.3 There are 4 roles designated within the army. Role 1 facilities are known as battalion aid stations (BASs).

Role of the Field Surgeon
Within the broader structure of the army, approximately 5 battalions (each composed of 300 to 1000 soldiers) comprise a single brigade combat team. Role 1 medical facilities typically have a single battalion surgeon assigned to them. Field surgeons most commonly serve in this battalion surgeon position. Additionally, Role 2 facilities may have slots for up to 2 battalion surgeons; however, field surgeons are less commonly tasked with this assignment.1 Occasionally, in one author’s (N.R.M.) personal experience, these roles are more fluid than one might expect. A field surgeon tasked initially with a Role 1 position may be shifted to a Role 2 assignment on an as-needed basis. This ability for rapid change in roles and responsibilities underscores the need for a fluid mind-set and thorough predeployment training for the field surgeon.
PREDEPLOYMENT TRAINING
As one might expect, dermatologists who have just graduated residency or fellowship are unlikely to have honed their trauma support skills to the degree needed to support a deployed battalion actively engaging in combat. Fortunately, there are many opportunities for military dermatologists to practice these skills prior to joining their FORSCOM colleagues. The initial exposure to trauma support comes during medical internship at the mandatory Combat Casualty Care Course (C4), an 8-day program designed to enhance the operational medical readiness and predeployment trauma training skills of medical officers.4 The C4 program includes 3 days of classroom training and 5 days of intensive field training. During C4, medical officers become certified in Advanced Trauma Life Support, a 3-day course organized by the American College of Surgeons.5 This course teaches medical officers how to quickly and judiciously triage, treat, and transport patients who have sustained potentially life-threatening traumas.
The next components of predeployment training, Tactical Combat Casualty Care and Tactical Combat Medical Care, occur in the months to weeks immediately preceding deployment.1,6 Tactical Combat Casualty Care prepares participants in the initial stabilization of trauma to occur at the point of injury.6 Tactical Combat Casualty Care principles generally are employed by medics (enlisted personnel trained in point-of-care medical support) rather than physicians; however, these principles are still critical for medical officers to be aware of when encountering severe traumas.6 In addition, the physician is responsible for ensuring his/her medics are fully trained in Tactical Combat Casualty Care. Tactical Combat Medical Care is geared more toward the direct preparation of medical officers. During the 5-day course, medical officers learn the gold standard for trauma care in both the classroom and in hands-on scenarios.1 This training not only allows medical officers to be self-sufficient in providing trauma support, but it also enables them to better maintain quality control of the performance of their medics continuously throughout the deployment.1
DEPLOYMENT RESPONSIBILITIES
Dermatologists who have completed the above training typically are subsequently deployed as field surgeons to a Role 1 facility. Field surgeons are designated as the officer in charge of the BAS and assume the position of medical platoon leader. A field surgeon usually will have both a physician assistant and a field medical assistant/medical plans officer (MEDO) to assist in running the BAS. The overarching goal of the field surgeon is to maintain the health and readiness of the battalion. In addition to addressing the day-to-day health care needs of individual soldiers, a field surgeon is expected to attend all staff meetings, advise the commander on preventative health and epidemiological trends, identify the scope of practice of the medics, ensure the BAS is prepared for mass casualties, and take responsibility for all controlled substances.
To illustrate the value that the properly trained dermatologist can provide in the deployed setting, we will outline field surgeon responsibilities and provide case examples of the first-hand experiences of one of the authors (N.R.M.) as a Role 2 officer in charge and field surgeon. The information presented in the case examples may have been altered to ensure continued operational security and out of respect to US servicemembers and coalition forces while still conveying important learning points.
Sick Call
In the deployed environment, military sick call functions as an urgent care center that is open continuously and serves the active-duty population, US government civilians and contractors, and coalition forces. In general, the physician assistant should treat approximately two-thirds of sick call patients under the supervision of the field surgeon, allowing the field surgeon to focus on his/her ancillary duties and ensure overall medical supervision of the unit. As a safeguard, patients with more than 2 visits for the same concern must be evaluated by the field surgeon. Sick call concerns range from minor traumas and illnesses to much more serious disease processes and injuries (as outlined in Medical Emergencies). As a field surgeon, it is critical to track disease nonbattle illnesses to ensure medical readiness of the unit. In the deployed environment, close quarters and austere environments commonly lend themselves to gastrointestinal illnesses, respiratory diseases, heat injuries, vector-borne diseases, and sexually transmitted infections.
Case Examples
During an 8-month deployment in Afghanistan, one of the authors (N.R.M.) provided or assisted in the care of more than 2300 routine sick call appointments, or approximately 10 patients per day. Epidemiology of disease was tracked, and the condition of the unit was presented daily to the battalion commander for consideration in upcoming operations. The top 5 most common categories of diagnoses included musculoskeletal injuries, gastrointestinal diseases, dermatologic concerns (eg, dermatitis, bacterial infections [cellulitis/abscess], fungal infections, arthropod assault, abrasions, lacerations, verruca vulgaris), respiratory illnesses, and mental health care, respectively. Maintaining a familiarity with general medicine is critical for the military dermatologist, and an adequate medical library or access to online medical review sources is critical for day-to-day sick call.
Medical Emergencies
In the event of a more serious injury or illness, a Role 1 BAS has very little capability in performing anything beyond the most basic interventions. Part of the art of being an effective field surgeon lies in stabilization, triage, and transport of these sometimes very ill patients. Both the decision to transport to a higher level of care (eg, Role 2 or 3 facility) as well as selection of the means of transportation falls on the field surgeon. The MEDO plays an essential role in assisting in the coordination of the transfer; however, the responsibility ultimately falls on the field surgeon.1,6 The field surgeon at the Role 2 BAS may be expected to perform more advanced medical and surgical interventions. More advanced pharmacotherapies include thrombolytics, antivenin, and vasopressors. Some procedural interventions include intubations, central lines, and laceration repairs. The Role 2 BAS has the capability to hold patients for up to 72 hours.
Case Examples
Specific conditions one of the authors (N.R.M.) treated include heat injury, myocardial infarction, disseminated tuberculosis, appendicitis, testicular torsion, malaria, suicidal ideation, burns, and status epilepticus. Over 8 months, the Role 2 BAS received 91 medical emergencies, with 53 necessitating evacuation to a higher level of care. Often, the more serious or rare conditions presented in the foreign contractor and coalition force populations working alongside US troops.
In one particular case, a 35-year-old man with an electrocardiogram-confirmed acute ST-segment elevation myocardial infarction was administered standard therapy consisting of intravenous morphine, oxygen, sublingual nitroglycerin, an angiotensin-converting enzyme inhibitor, and a beta-blocker. Given the lack of a cardiac catheterization laboratory at the next highest level of care as well as a low suspicion for aortic dissection (based on the patient’s history, physical examination, and chest radiograph), fibrinolysis with tenecteplase was performed in the deployed environment. After a very short observation for potential hemorrhage, the patient was then evacuated to the Role 3 hospital, where he made a near-complete recovery. Preparation with advanced cardiac life support courses and a thorough algorithmic review of the 10 most common causes of presentation to the emergency department helped adequately prepare the dermatologist to succeed.
Trauma Emergencies
The same principles of triage and transport apply to trauma emergencies. Mass casualties are an inevitable reality in combat, so appropriate training translating into efficient action is essential to ensure the lowest possible mortality. This training and the actions that stem from it are an additional responsibility that the field surgeon must maintain. During deployment, continued training organized by the field surgeon could quite literally mean the difference between life and death. In addition to the organizational responsibilities, field surgeons should be prepared to perform initial stabilization in trauma patients, including application of tourniquets, establishment of central lines, reading abdominal ultrasounds for free fluid, placement of chest tubes, intubation, and ventilator management. The Joint Trauma System Clinical Practice Guidelines also offer extensive and invaluable guidance on the most up-to-date approach to common trauma conditions arising in the deployed environment.7 At the Role 2 level, the field surgeon also must be prepared to coordinate ancillary services, manage the Role 2/forward surgical team intensive care unit, and serve as first assist in the operating room, as needed (Figure 2).

Case Examples
One of the authors (N.R.M.) assisted or provided care in approximately 225 trauma cases while deployed. A mass casualty event occurred, in which the Role 2 BAS received 34 casualties; of these casualties, 11 were immediate, 10 were delayed, 11 were minimal, and 2 were expectant. Injury patterns included mounted and dismounted improvised explosive device injuries (eg, blast, shrapnel, and traumatic brain injuries) as well as gunshot wounds. Direct care was provided for 13 casualties, including 10 abdominal ultrasound examinations for free fluid, placement of 2 chest tubes, 1 intubation, establishment of 3 central lines, and first-assisting 1 exploratory laparotomy. Of the casualties, 22 were evacuated to the Role 3 hospital, 8 were dispositioned to a coalition hospital, 2 were returned to active duty, and 2 died due to their injuries. The military trauma preparation as outlined in the predeployment training can help adequately prepare the military dermatologist to assist in these cases.
Ancillary Services
An important part of the efficacy of initial evaluation and stabilization of both medical and traumatic emergencies involves expedited laboratory tests, imaging, and the delivery of life-saving blood products to affected patients. The field surgeon is responsible for the readiness of these services and may play a critical role in streamlining these tasks for situations where a delay in care by minutes can be lethal. The MEDO assists the field surgeon to ensure the readiness of the medical equipment, and the field surgeon must ensure the readiness of the medics and technicians utilizing the equipment. In a deployed environment, only a finite amount of blood products may be stored. As a result, the design and implementation of an efficient and precise walking blood bank is critical. To help mitigate this issue, servicemembers are prescreened for their blood types and bloodborne illnesses. If a situation arises in which whole blood is needed, the prescreened individuals are screened again, and their blood is collected and transfused to the patient under the supervision of the physician. This task is critical in saving lives, and this process is the primary responsibility of the field surgeon.
Case Example
A 37-year-old man presented to the BAS with abdominal and pelvic gunshot wounds, as well as tachycardia, rapidly decreasing blood pressure, and altered consciousness. An exploratory laparotomy was performed to look for the sources of bleeding. The patient’s blood type was confirmed with a portable testing kit. Due to the injury pattern and clinical presentation, a call was immediately placed to begin screening and preparing servicemembers to donate blood for the walking blood bank. As expected, the Role 2 supply of blood products was exhausted during the exploratory laparotomy. With servicemembers in place and screened, an additional 12 units of whole blood were collected and administered in a timely fashion. The patient was stabilized and transported to the next highest level of care. Due to the process optimization performed by the laboratory team, whole-blood transfusions were ready within an average of 22 minutes, well ahead of the 45-minute standard of care.
Operating Room First Assist
If a field surgeon is stationed at a Role 2 BAS with a forward surgical team, he/she may be required to adopt the role of operating room first assist for the trauma surgeon or orthopedic surgeon on the team, which is especially true for isolated major traumas when triage and initial stabilization measures for multiple patients are of less concern. Dermatologists receive surgical training as part of the Accreditation Council for Graduate Medical Education requirements to graduate residency, making them more than capable of surgical assisting when needed.8 In particular, dermatologists’ ability to utilize instruments appropriately and think procedurally as well as their skills in suturing are helpful.
Case Example
A 22-year-old man with several shrapnel wounds to the abdomen demonstrated free fluid in the left lower quadrant. The field surgeon (N.R.M.) assisted the trauma surgeon in opening the abdomen and running the bowel for sources of bleeding. The trauma surgeon identified the bleed and performed a ligation. The patient was then packed, closed, and prepared for transfer to a higher level of care.
Preventive Medicine
As a result of the field surgeon being on the front line of medical care in an austere environment, implementation of preventive medicine practices and disease pattern recognition are his/her responsibility. Responsibilities may include stray animal euthanasia due to prevalence of rabies, enforcement of malaria prophylaxis, medical training and maintenance of snake antivenin, and assistance with other local endemic disease. The unique skill set of dermatologists in organism identification can further bolster the speed with which vector-borne diseases are recognized and prevention and treatment measures are implemented.
Case Example
As coalition forces executed a mission in Afghanistan, US servicemembers began experiencing abdominal distress, chills, fevers (temperature >40°C), debilitating headaches, myalgia, arthralgia, and tachycardia. Initially, these patients were evacuated to the Role 2 BAS, hindering the mission. Upon inspection, patients had numerous bug bites; one astute soldier collected the arthropod guilty of the assault and brought it to the aid station. Upon inspection, the offender was identified as the Phlebotomus genus of sandflies, organisms that are well known to dermatologists as a cause of leishmaniasis. Clinical correlation resulted in the presumed diagnosis of Pappataci fever, and vector-borne disease prevention measures were then able to be further emphasized and implemented in at-risk areas, allowing the mission to continue.9 Subsequent infectious disease laboratory testing confirmed the Phlebovirus transmitted by the sandfly as the underlying cause of the illness.
CONCLUSION
The diverse role of the field surgeon in the deployed setting makes any one specialist underprepared to completely take on the role from the outset; however, with appropriate and rigorous trauma training prior to deployment, dermatologists will continue to perform as invaluable assets to the US military in conflicts now and in the future.
1. Moawad FJ, Wilson R, Kunar MT, et al. Role of the battalion surgeon in the Iraq and Afghanistan War. Mil Med. 2012;177:412-416.
2. AR 601-142: Army Medical Department Professional Filler System. Washington, DC: US Department of the Army; 2015. http://cdm16635.contentdm.oclc.org/cdm/ref/collection/p16635coll11/id/4592. Accessed December 19, 2018.
3. Roles of medical care (United States). Emergency War Surgery. 4th ed. Fort Sam Houston, Texas: Office of the Surgeon General; 2013:17-28.
4. Combat Casualty Care Course (C4). Military Health System website. https://health.mil/Training-Center/Defense-Medical-Readiness-Training-Institute/Combat-Casualty-Care-Course. Accessed December 7, 2018.
5. Advanced Trauma Life Support. American College of Surgeons website. https://www.facs.org/quality-programs/trauma/atls. Accessed December 7, 2018.
6. Tactical Combat Casualty Care Course. Military Health System website. https://health.mil/Training-Center/Defense-Medical-Readiness-Training-Institute/Tactical-Combat-Casualty-Care-Course. Accessed December 18, 2018.
7. Joint Trauma System: The Department of Defense Center of Excellence for Trauma. Clinical Practice Guidelines.
8. ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Revised July 1, 2017. Accessed December 7, 2018.
9. Downs JW, Flood DT, Orr NH, et al. Sandfly fever in Afghanistan-a sometimes overlooked disease of military importance: a case series and review of the literature. US Army Med Dep J. 2017:60-66.
1. Moawad FJ, Wilson R, Kunar MT, et al. Role of the battalion surgeon in the Iraq and Afghanistan War. Mil Med. 2012;177:412-416.
2. AR 601-142: Army Medical Department Professional Filler System. Washington, DC: US Department of the Army; 2015. http://cdm16635.contentdm.oclc.org/cdm/ref/collection/p16635coll11/id/4592. Accessed December 19, 2018.
3. Roles of medical care (United States). Emergency War Surgery. 4th ed. Fort Sam Houston, Texas: Office of the Surgeon General; 2013:17-28.
4. Combat Casualty Care Course (C4). Military Health System website. https://health.mil/Training-Center/Defense-Medical-Readiness-Training-Institute/Combat-Casualty-Care-Course. Accessed December 7, 2018.
5. Advanced Trauma Life Support. American College of Surgeons website. https://www.facs.org/quality-programs/trauma/atls. Accessed December 7, 2018.
6. Tactical Combat Casualty Care Course. Military Health System website. https://health.mil/Training-Center/Defense-Medical-Readiness-Training-Institute/Tactical-Combat-Casualty-Care-Course. Accessed December 18, 2018.
7. Joint Trauma System: The Department of Defense Center of Excellence for Trauma. Clinical Practice Guidelines.
8. ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Revised July 1, 2017. Accessed December 7, 2018.
9. Downs JW, Flood DT, Orr NH, et al. Sandfly fever in Afghanistan-a sometimes overlooked disease of military importance: a case series and review of the literature. US Army Med Dep J. 2017:60-66.
Practice Points
- Army dermatologists routinely deploy to combat zones as field surgeons. In this role, they provide routine, emergency, and trauma care for active-duty soldiers and coalition forces.
- With 5 years of general medical training, army dermatologists often are the most prepared to provide advanced care when compared to co-located physician assistants and combat medics.
- Maintaining basic medical skills would serve any dermatologist in case of local or national emergencies.
Military Grooming Standards and Their Impact on Skin Diseases of the Head and Neck
The US military enforces grooming standards to ensure the professional appearance and serviceability of soldiers in all operational settings. Although most individuals are able to uphold these regulations without incident, there is a growing cohort of servicemembers with skin diseases that were exacerbated or even initiated by haircuts, hairstyling, and shaving required to conform to these grooming standards. These skin diseases, which can affect both sexes and may not be appreciated until years into a soldier's service commitment, can have consequences related to individual morbidity and medical readiness for deployment, making it an important issue for medical practitioners to recognize and manage in servicemembers.
This review highlights several disorders of the pilosebaceous unit of the head and neck that can be caused or exacerbated by military grooming standards, including inflammatory hair disorders, traction alopecia, and pseudofolliculitis barbae. Discussion of each entity will include a review of susceptibility and causality as well as initial treatment options to consider (Table).
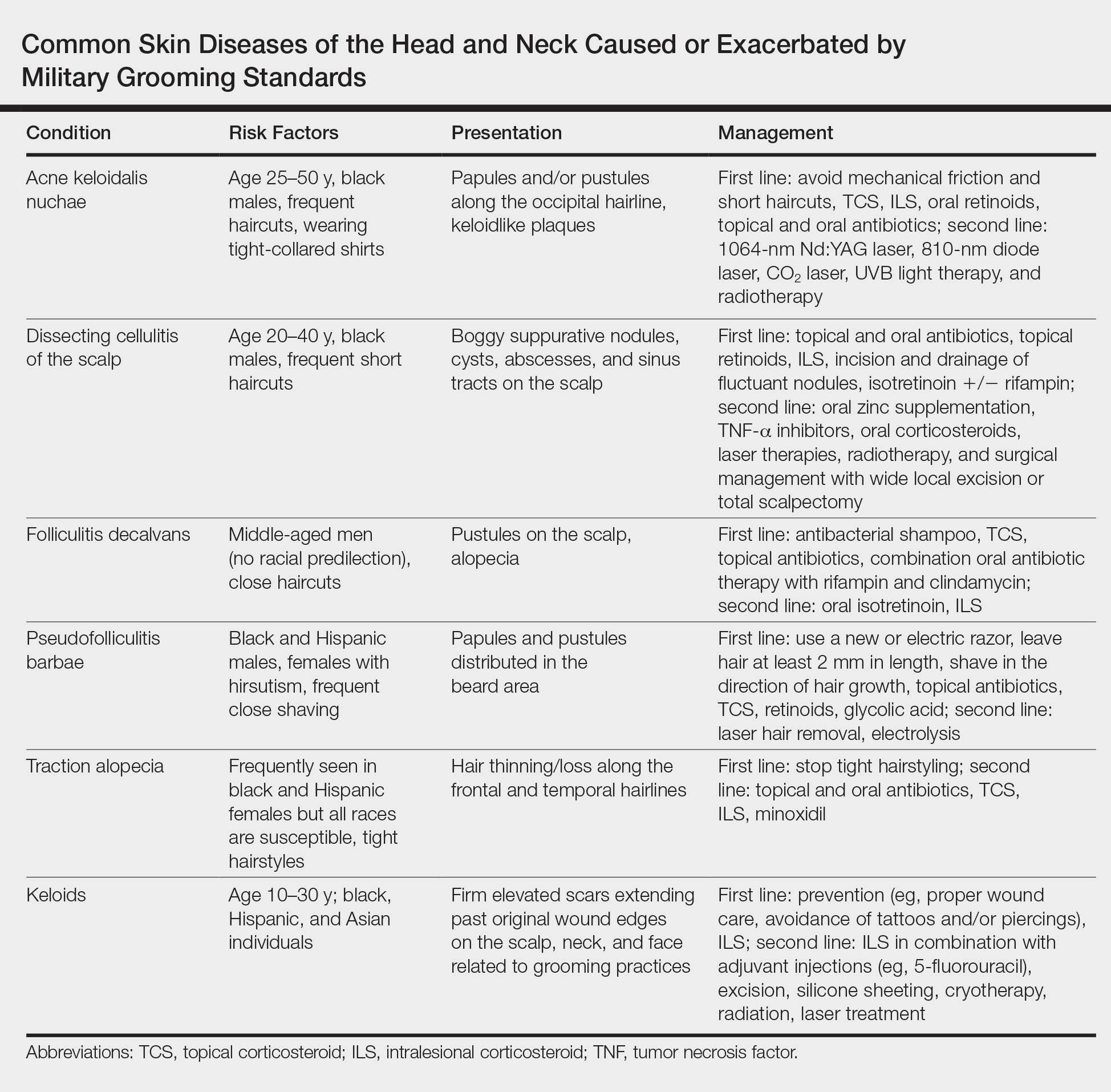
Inflammatory Hair Disorders
The proper appearance of servicemembers in uniform represents self-discipline and conformity to the high standards of the military. This transition occurs as a rite of passage for many new male recruits who receive shaved haircuts during their first days of basic training. Thereafter, male servicemembers are required to maintain a tapered appearance of the hair per military regulations.1 Clipping hair closely to the scalp or shaving the head entirely are authorized and often encouraged; therefore, high and tight haircuts and buzz cuts are popular among male soldiers due to the general ease of care and ability to maintain the haircut themselves. Conversely, these styles require servicemembers to get weekly or biweekly haircuts that in turn can lead to chronic trauma and irritation. In more susceptible populations, inflammatory hair disorders such as acne keloidalis nuchae (AKN), dissecting cellulitis of the scalp, and folliculitis decalvans may be incited.
Acne Keloidalis Nuchae
Acne keloidalis nuchae, also called folliculitis keloidalis, is a chronic scarring folliculitis presenting with papules and plaques on the occiput and nape of the neck that may merge to form hypertrophic scars or keloids. This disorder most commonly develops in young black men but also can be seen in black females and white patients of both sexes.2 Acne keloidalis nuchae shares many histologic features with central centrifugal cicatricial alopecia, which may suggest a similar pathogenesis. Apart from frequent haircuts, tight-collared shirts, such as those on military service uniforms, also have been associated with AKN. Because of these suspected etiologies, first-line treatment focuses on preventing further trauma by avoiding mechanical irritation and short haircuts, which may be difficult in the military setting. For earlier disease stages, topical and intralesional corticosteroids, oral retinoids, and topical and oral antibiotics are used for their anti-inflammatory properties.3 In refractory cases, surgical excision with healing by secondary intention may be attempted.4 Additional treatment options include the 1064-nm Nd:YAG and 810-nm diode lasers,3 UVB light therapy, CO2 laser, and radiotherapy.
Dissecting Cellulitis of the Scalp
Similar to AKN, dissecting cellulitis of the scalp is another inflammatory hair disorder that is worsened by frequent short haircuts.5 Dissecting cellulitis of the scalp is a primary cicatricial alopecia proposed to be secondary to follicular occlusion. It often is seen in black males aged 20 to 40 years and is characterized by boggy suppurative nodules and cysts with draining sinus tracts, abscesses, and resultant scarring alopecia. Dissecting cellulitis of the scalp is part of the follicular occlusion tetrad, which also includes hidradenitis suppurativa, acne conglobata, and pilonidal cysts. First-line therapies include topical and oral antibiotics, topical retinoids, intralesional corticosteroids, incision and drainage of fluctuant nodules, and oral isotretinoin with or without rifampin. Alternative treatments include oral zinc supplementation, oral corticosteroids, tumor necrosis factor α inhibitors, laser therapies, radiotherapy, and surgical management with wide local excision or total scalpectomy.6,7
Folliculitis Decalvans
Folliculitis decalvans is a primary cicatricial alopecia of the scalp that most commonly presents in middle-aged men without racial predilection.8 Folliculitis decalvans presents with multiple pustules, crusts, tufted hairs, and perifollicular hyperkeratosis, leading to scarring of the scalp, which often is most severe on the posterior vertex. Staphylococcus aureus is a presumed player in the pathogenesis of folliculitis decalvans with superantigens causing release of cytokines stimulating follicular destruction. Close haircuts in conformation with military grooming standards can contribute to this condition due to mechanical trauma and subsequent inflammation. It typically is diagnosed clinically, but if histologic confirmation is desired, a sample from the periphery of early lesions is preferred.9 Initial treatment consists of antibacterial shampoos, topical corticosteroids, topical antibiotics, and combination oral antibiotic therapy with rifampin and clindamycin. Studies using oral isotretinoin have shown variable results,10,11 and the most effective treatment of recalcitrant lesions appears to be intralesional corticosteroids.12
Follicular and Scarring Disorders
In addition to inflammatory hair disorders, military grooming standards have been linked to the pathogenesis of diseases such as pseudofolliculitis barbae, traction alopecia, and keloids, specifically through irritation of the face, neck, and scalp, as well as damage to the follicular unit.5 These conditions develop because grooming regulations necessitate certain hair practices such as close shaving of facial and neck hair and keeping long hair secured relatively tightly to the scalp.
Pseudofolliculitis Barbae
Males in the military are obligated to keep their faces clean-shaven.1 They may acquire a medical waiver for a specified beard length if deemed appropriate by the treating physician,1 which often leads to the need for continual waiver renewal and also may warrant possible negative perception from peers, subordinates, and leadership. One of the most prevalent conditions that is closely associated with shaving is pseudofolliculitis barbae. The combination of close shaving and tightly coiled hairs causes the hairs to grow toward and penetrate the skin, particularly on the neck.13 In some cases, the hairs never actually exit the skin and simply curl within the superficial epidermis. A foreign body reaction often arises, leading to inflamed follicular papules and pustules. Affected individuals may experience pain, pruritus, and secondary infections. Postinflammatory hyperpigmentation, hypertrophic scarring, and keloid formation are common sequelae in cases of untreated disease. Pseudofolliculitis barbae also is exacerbated by pulling the skin taut and shaving against the grain, making behavioral interventions a key component in management of this condition. Preliminary recommendations include using a new or electric razor, leaving hair at least 2 mm in length, and shaving in the direction of hair growth. Other treatment options with varying effectiveness include daily alternation of a mild topical corticosteroid and one of the following: a topical retinoid, topical antibiotics, or glycolic acid. The only treatments that approach definitive cure are laser hair removal and electrolysis for which patient skin type plays an important role in laser selection.5
Traction Alopecia
Similar to their male counterparts, female military members must also present a conservative professional appearance, including hair that is neatly groomed.1 If the length of the hair extends beyond the uniform collar, it must be inconspicuously fastened or pinned above the collar. As a result, loosely tied hair is unauthorized, and females with long hair must secure their hair tightly on a daily basis. Traction alopecia results from tight hairstyling over a prolonged period and commonly affects female soldiers. The etiology is presumed to be mechanical loosening of hair within the follicles, leading to inflammation. Although traditionally seen in black women along the frontal and temporal hairlines, traction alopecia has been identified in individuals of all races and can occur anywhere on the scalp.5 Perifollicular erythema may be the first sign, and papules and pustules may be visible. Although the hair loss in traction alopecia usually is reversible if the traction is ceased, end-stage disease may be permanent.6 Halting traction-inducing practices is paramount, and other treatment options that may slow progression include topical or oral antibiotics and topical or intralesional corticosteroids. Recovery of hair loss also may be aided by topical minoxidil.5
Keloids
Keloid formation is an important pathology to address, as it may result from several of the aforementioned conditions. Keloids are most commonly seen in black individuals but also can occur in Hispanic and Asian patients. The cause has not been fully elucidated but is thought to be a combination of dysfunctional fibroblasts with a genetic component based on racial predilection and twin concordance studies.5 The chest, shoulders, upper back, neck, and earlobes are particularly susceptible to keloid formation, which can appear from 1 to 24 years following dermal trauma.5 Unlike hypertrophic scars, keloids generally do not regress and frequently cause discomfort, pruritus, and emotional distress. They also can hinder wearing a military uniform. Sustained remission is problematic, making prevention a first-line approach, including proper care of wounds when they occur and avoiding elective procedures such as piercings and tattoos. Intralesional corticosteroids, adjuvant injections (eg, 5-fluorouracil), silicone sheeting, cryotherapy, radiation, laser therapy, and excision are some of the treatment options when keloids have formed.5
Final Comment
It is important to recognize military grooming standards as a cause or contributor to several diseases of the head and neck in military servicemembers. Specifically, frequent haircuts in male soldiers are associated with several inflammatory hair disorders, including AKN, dissecting cellulitis of the scalp, and folliculitis decalvans, while daily shaving predisposes individuals to pseudofolliculitis barbae with possible keloid formation. Females may develop traction alopecia from chronically tight, pulled back hairstyles. All of these conditions have health implications for the affected individuals and can compromise the military mission. Awareness, prevention, and recognition are key along with the knowledge base to provide anticipatory avoidance and initiate appropriate treatments, thereby mitigating these potential consequences.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed October 11, 2018.
- East-Innis AD, Stylianou K, Paolino A, et al. Acne keloidalis nuchae: risk factors and associated disorders--a retrospective study. Int J Dermatol. 2017;56:828-832.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systematic review of the literature. Dermatol Ther (Heidelb). 2016;6:363-378.
- Glenn MJ, Bennett RG, Kelly AP. Acne keloidalis nuchae: treatment with excision and second-intention healing. J Am Acad Dermatol. 1995;33:243-246.
- Madu P, Kundu RV. Follicular and scarring disorders in skin of color: presentation and management. Am J Clin Dermatol. 2014;15:307-321.
- Rodney IJ, Onwudiwe OC. Hair and scalp disorders in ethnic populations. J Drugs Dermatol. 2013;12:420-427.
- Lindsey SF, Tosti A. Ethnic hair disorders. Curr Probl Dermatol. 2015;47:139-148.
- Whiting DA. Cicatricial alopecia: clinico-pathological findings and treatment. Clin Dermatol. 2001;19:211-225.
- Sperling LC, Cowper SE, Knopp EA. An Atlas of Hair Pathology with Clinical Correlations. 2nd ed. Boca Raton, FL: CRC Press; 2012.
- Gemmeke A, Wollina U. Folliculitis decalvans of the scalp: response to triple therapy with isotretinoin, clindamycin, and prednisolone. Acta Dermatovenerol Alp Pannonica Adriat. 2006;15:184-186.
- Hallai N, Thompson I, Williams P, et al. Folliculitis spinulosa decalvans: failure to respond to oral isotretinoin. J Eur Acad Dermatol Venereol. 2006;20:223-224.
- Bolduc C, Sperling LC, Shapiro J. Primary cicatricial alopecia. J Am Acad Dermatol. 2016;75:101-117.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl):S113-S119.
The US military enforces grooming standards to ensure the professional appearance and serviceability of soldiers in all operational settings. Although most individuals are able to uphold these regulations without incident, there is a growing cohort of servicemembers with skin diseases that were exacerbated or even initiated by haircuts, hairstyling, and shaving required to conform to these grooming standards. These skin diseases, which can affect both sexes and may not be appreciated until years into a soldier's service commitment, can have consequences related to individual morbidity and medical readiness for deployment, making it an important issue for medical practitioners to recognize and manage in servicemembers.
This review highlights several disorders of the pilosebaceous unit of the head and neck that can be caused or exacerbated by military grooming standards, including inflammatory hair disorders, traction alopecia, and pseudofolliculitis barbae. Discussion of each entity will include a review of susceptibility and causality as well as initial treatment options to consider (Table).

Inflammatory Hair Disorders
The proper appearance of servicemembers in uniform represents self-discipline and conformity to the high standards of the military. This transition occurs as a rite of passage for many new male recruits who receive shaved haircuts during their first days of basic training. Thereafter, male servicemembers are required to maintain a tapered appearance of the hair per military regulations.1 Clipping hair closely to the scalp or shaving the head entirely are authorized and often encouraged; therefore, high and tight haircuts and buzz cuts are popular among male soldiers due to the general ease of care and ability to maintain the haircut themselves. Conversely, these styles require servicemembers to get weekly or biweekly haircuts that in turn can lead to chronic trauma and irritation. In more susceptible populations, inflammatory hair disorders such as acne keloidalis nuchae (AKN), dissecting cellulitis of the scalp, and folliculitis decalvans may be incited.
Acne Keloidalis Nuchae
Acne keloidalis nuchae, also called folliculitis keloidalis, is a chronic scarring folliculitis presenting with papules and plaques on the occiput and nape of the neck that may merge to form hypertrophic scars or keloids. This disorder most commonly develops in young black men but also can be seen in black females and white patients of both sexes.2 Acne keloidalis nuchae shares many histologic features with central centrifugal cicatricial alopecia, which may suggest a similar pathogenesis. Apart from frequent haircuts, tight-collared shirts, such as those on military service uniforms, also have been associated with AKN. Because of these suspected etiologies, first-line treatment focuses on preventing further trauma by avoiding mechanical irritation and short haircuts, which may be difficult in the military setting. For earlier disease stages, topical and intralesional corticosteroids, oral retinoids, and topical and oral antibiotics are used for their anti-inflammatory properties.3 In refractory cases, surgical excision with healing by secondary intention may be attempted.4 Additional treatment options include the 1064-nm Nd:YAG and 810-nm diode lasers,3 UVB light therapy, CO2 laser, and radiotherapy.
Dissecting Cellulitis of the Scalp
Similar to AKN, dissecting cellulitis of the scalp is another inflammatory hair disorder that is worsened by frequent short haircuts.5 Dissecting cellulitis of the scalp is a primary cicatricial alopecia proposed to be secondary to follicular occlusion. It often is seen in black males aged 20 to 40 years and is characterized by boggy suppurative nodules and cysts with draining sinus tracts, abscesses, and resultant scarring alopecia. Dissecting cellulitis of the scalp is part of the follicular occlusion tetrad, which also includes hidradenitis suppurativa, acne conglobata, and pilonidal cysts. First-line therapies include topical and oral antibiotics, topical retinoids, intralesional corticosteroids, incision and drainage of fluctuant nodules, and oral isotretinoin with or without rifampin. Alternative treatments include oral zinc supplementation, oral corticosteroids, tumor necrosis factor α inhibitors, laser therapies, radiotherapy, and surgical management with wide local excision or total scalpectomy.6,7
Folliculitis Decalvans
Folliculitis decalvans is a primary cicatricial alopecia of the scalp that most commonly presents in middle-aged men without racial predilection.8 Folliculitis decalvans presents with multiple pustules, crusts, tufted hairs, and perifollicular hyperkeratosis, leading to scarring of the scalp, which often is most severe on the posterior vertex. Staphylococcus aureus is a presumed player in the pathogenesis of folliculitis decalvans with superantigens causing release of cytokines stimulating follicular destruction. Close haircuts in conformation with military grooming standards can contribute to this condition due to mechanical trauma and subsequent inflammation. It typically is diagnosed clinically, but if histologic confirmation is desired, a sample from the periphery of early lesions is preferred.9 Initial treatment consists of antibacterial shampoos, topical corticosteroids, topical antibiotics, and combination oral antibiotic therapy with rifampin and clindamycin. Studies using oral isotretinoin have shown variable results,10,11 and the most effective treatment of recalcitrant lesions appears to be intralesional corticosteroids.12
Follicular and Scarring Disorders
In addition to inflammatory hair disorders, military grooming standards have been linked to the pathogenesis of diseases such as pseudofolliculitis barbae, traction alopecia, and keloids, specifically through irritation of the face, neck, and scalp, as well as damage to the follicular unit.5 These conditions develop because grooming regulations necessitate certain hair practices such as close shaving of facial and neck hair and keeping long hair secured relatively tightly to the scalp.
Pseudofolliculitis Barbae
Males in the military are obligated to keep their faces clean-shaven.1 They may acquire a medical waiver for a specified beard length if deemed appropriate by the treating physician,1 which often leads to the need for continual waiver renewal and also may warrant possible negative perception from peers, subordinates, and leadership. One of the most prevalent conditions that is closely associated with shaving is pseudofolliculitis barbae. The combination of close shaving and tightly coiled hairs causes the hairs to grow toward and penetrate the skin, particularly on the neck.13 In some cases, the hairs never actually exit the skin and simply curl within the superficial epidermis. A foreign body reaction often arises, leading to inflamed follicular papules and pustules. Affected individuals may experience pain, pruritus, and secondary infections. Postinflammatory hyperpigmentation, hypertrophic scarring, and keloid formation are common sequelae in cases of untreated disease. Pseudofolliculitis barbae also is exacerbated by pulling the skin taut and shaving against the grain, making behavioral interventions a key component in management of this condition. Preliminary recommendations include using a new or electric razor, leaving hair at least 2 mm in length, and shaving in the direction of hair growth. Other treatment options with varying effectiveness include daily alternation of a mild topical corticosteroid and one of the following: a topical retinoid, topical antibiotics, or glycolic acid. The only treatments that approach definitive cure are laser hair removal and electrolysis for which patient skin type plays an important role in laser selection.5
Traction Alopecia
Similar to their male counterparts, female military members must also present a conservative professional appearance, including hair that is neatly groomed.1 If the length of the hair extends beyond the uniform collar, it must be inconspicuously fastened or pinned above the collar. As a result, loosely tied hair is unauthorized, and females with long hair must secure their hair tightly on a daily basis. Traction alopecia results from tight hairstyling over a prolonged period and commonly affects female soldiers. The etiology is presumed to be mechanical loosening of hair within the follicles, leading to inflammation. Although traditionally seen in black women along the frontal and temporal hairlines, traction alopecia has been identified in individuals of all races and can occur anywhere on the scalp.5 Perifollicular erythema may be the first sign, and papules and pustules may be visible. Although the hair loss in traction alopecia usually is reversible if the traction is ceased, end-stage disease may be permanent.6 Halting traction-inducing practices is paramount, and other treatment options that may slow progression include topical or oral antibiotics and topical or intralesional corticosteroids. Recovery of hair loss also may be aided by topical minoxidil.5
Keloids
Keloid formation is an important pathology to address, as it may result from several of the aforementioned conditions. Keloids are most commonly seen in black individuals but also can occur in Hispanic and Asian patients. The cause has not been fully elucidated but is thought to be a combination of dysfunctional fibroblasts with a genetic component based on racial predilection and twin concordance studies.5 The chest, shoulders, upper back, neck, and earlobes are particularly susceptible to keloid formation, which can appear from 1 to 24 years following dermal trauma.5 Unlike hypertrophic scars, keloids generally do not regress and frequently cause discomfort, pruritus, and emotional distress. They also can hinder wearing a military uniform. Sustained remission is problematic, making prevention a first-line approach, including proper care of wounds when they occur and avoiding elective procedures such as piercings and tattoos. Intralesional corticosteroids, adjuvant injections (eg, 5-fluorouracil), silicone sheeting, cryotherapy, radiation, laser therapy, and excision are some of the treatment options when keloids have formed.5
Final Comment
It is important to recognize military grooming standards as a cause or contributor to several diseases of the head and neck in military servicemembers. Specifically, frequent haircuts in male soldiers are associated with several inflammatory hair disorders, including AKN, dissecting cellulitis of the scalp, and folliculitis decalvans, while daily shaving predisposes individuals to pseudofolliculitis barbae with possible keloid formation. Females may develop traction alopecia from chronically tight, pulled back hairstyles. All of these conditions have health implications for the affected individuals and can compromise the military mission. Awareness, prevention, and recognition are key along with the knowledge base to provide anticipatory avoidance and initiate appropriate treatments, thereby mitigating these potential consequences.
The US military enforces grooming standards to ensure the professional appearance and serviceability of soldiers in all operational settings. Although most individuals are able to uphold these regulations without incident, there is a growing cohort of servicemembers with skin diseases that were exacerbated or even initiated by haircuts, hairstyling, and shaving required to conform to these grooming standards. These skin diseases, which can affect both sexes and may not be appreciated until years into a soldier's service commitment, can have consequences related to individual morbidity and medical readiness for deployment, making it an important issue for medical practitioners to recognize and manage in servicemembers.
This review highlights several disorders of the pilosebaceous unit of the head and neck that can be caused or exacerbated by military grooming standards, including inflammatory hair disorders, traction alopecia, and pseudofolliculitis barbae. Discussion of each entity will include a review of susceptibility and causality as well as initial treatment options to consider (Table).

Inflammatory Hair Disorders
The proper appearance of servicemembers in uniform represents self-discipline and conformity to the high standards of the military. This transition occurs as a rite of passage for many new male recruits who receive shaved haircuts during their first days of basic training. Thereafter, male servicemembers are required to maintain a tapered appearance of the hair per military regulations.1 Clipping hair closely to the scalp or shaving the head entirely are authorized and often encouraged; therefore, high and tight haircuts and buzz cuts are popular among male soldiers due to the general ease of care and ability to maintain the haircut themselves. Conversely, these styles require servicemembers to get weekly or biweekly haircuts that in turn can lead to chronic trauma and irritation. In more susceptible populations, inflammatory hair disorders such as acne keloidalis nuchae (AKN), dissecting cellulitis of the scalp, and folliculitis decalvans may be incited.
Acne Keloidalis Nuchae
Acne keloidalis nuchae, also called folliculitis keloidalis, is a chronic scarring folliculitis presenting with papules and plaques on the occiput and nape of the neck that may merge to form hypertrophic scars or keloids. This disorder most commonly develops in young black men but also can be seen in black females and white patients of both sexes.2 Acne keloidalis nuchae shares many histologic features with central centrifugal cicatricial alopecia, which may suggest a similar pathogenesis. Apart from frequent haircuts, tight-collared shirts, such as those on military service uniforms, also have been associated with AKN. Because of these suspected etiologies, first-line treatment focuses on preventing further trauma by avoiding mechanical irritation and short haircuts, which may be difficult in the military setting. For earlier disease stages, topical and intralesional corticosteroids, oral retinoids, and topical and oral antibiotics are used for their anti-inflammatory properties.3 In refractory cases, surgical excision with healing by secondary intention may be attempted.4 Additional treatment options include the 1064-nm Nd:YAG and 810-nm diode lasers,3 UVB light therapy, CO2 laser, and radiotherapy.
Dissecting Cellulitis of the Scalp
Similar to AKN, dissecting cellulitis of the scalp is another inflammatory hair disorder that is worsened by frequent short haircuts.5 Dissecting cellulitis of the scalp is a primary cicatricial alopecia proposed to be secondary to follicular occlusion. It often is seen in black males aged 20 to 40 years and is characterized by boggy suppurative nodules and cysts with draining sinus tracts, abscesses, and resultant scarring alopecia. Dissecting cellulitis of the scalp is part of the follicular occlusion tetrad, which also includes hidradenitis suppurativa, acne conglobata, and pilonidal cysts. First-line therapies include topical and oral antibiotics, topical retinoids, intralesional corticosteroids, incision and drainage of fluctuant nodules, and oral isotretinoin with or without rifampin. Alternative treatments include oral zinc supplementation, oral corticosteroids, tumor necrosis factor α inhibitors, laser therapies, radiotherapy, and surgical management with wide local excision or total scalpectomy.6,7
Folliculitis Decalvans
Folliculitis decalvans is a primary cicatricial alopecia of the scalp that most commonly presents in middle-aged men without racial predilection.8 Folliculitis decalvans presents with multiple pustules, crusts, tufted hairs, and perifollicular hyperkeratosis, leading to scarring of the scalp, which often is most severe on the posterior vertex. Staphylococcus aureus is a presumed player in the pathogenesis of folliculitis decalvans with superantigens causing release of cytokines stimulating follicular destruction. Close haircuts in conformation with military grooming standards can contribute to this condition due to mechanical trauma and subsequent inflammation. It typically is diagnosed clinically, but if histologic confirmation is desired, a sample from the periphery of early lesions is preferred.9 Initial treatment consists of antibacterial shampoos, topical corticosteroids, topical antibiotics, and combination oral antibiotic therapy with rifampin and clindamycin. Studies using oral isotretinoin have shown variable results,10,11 and the most effective treatment of recalcitrant lesions appears to be intralesional corticosteroids.12
Follicular and Scarring Disorders
In addition to inflammatory hair disorders, military grooming standards have been linked to the pathogenesis of diseases such as pseudofolliculitis barbae, traction alopecia, and keloids, specifically through irritation of the face, neck, and scalp, as well as damage to the follicular unit.5 These conditions develop because grooming regulations necessitate certain hair practices such as close shaving of facial and neck hair and keeping long hair secured relatively tightly to the scalp.
Pseudofolliculitis Barbae
Males in the military are obligated to keep their faces clean-shaven.1 They may acquire a medical waiver for a specified beard length if deemed appropriate by the treating physician,1 which often leads to the need for continual waiver renewal and also may warrant possible negative perception from peers, subordinates, and leadership. One of the most prevalent conditions that is closely associated with shaving is pseudofolliculitis barbae. The combination of close shaving and tightly coiled hairs causes the hairs to grow toward and penetrate the skin, particularly on the neck.13 In some cases, the hairs never actually exit the skin and simply curl within the superficial epidermis. A foreign body reaction often arises, leading to inflamed follicular papules and pustules. Affected individuals may experience pain, pruritus, and secondary infections. Postinflammatory hyperpigmentation, hypertrophic scarring, and keloid formation are common sequelae in cases of untreated disease. Pseudofolliculitis barbae also is exacerbated by pulling the skin taut and shaving against the grain, making behavioral interventions a key component in management of this condition. Preliminary recommendations include using a new or electric razor, leaving hair at least 2 mm in length, and shaving in the direction of hair growth. Other treatment options with varying effectiveness include daily alternation of a mild topical corticosteroid and one of the following: a topical retinoid, topical antibiotics, or glycolic acid. The only treatments that approach definitive cure are laser hair removal and electrolysis for which patient skin type plays an important role in laser selection.5
Traction Alopecia
Similar to their male counterparts, female military members must also present a conservative professional appearance, including hair that is neatly groomed.1 If the length of the hair extends beyond the uniform collar, it must be inconspicuously fastened or pinned above the collar. As a result, loosely tied hair is unauthorized, and females with long hair must secure their hair tightly on a daily basis. Traction alopecia results from tight hairstyling over a prolonged period and commonly affects female soldiers. The etiology is presumed to be mechanical loosening of hair within the follicles, leading to inflammation. Although traditionally seen in black women along the frontal and temporal hairlines, traction alopecia has been identified in individuals of all races and can occur anywhere on the scalp.5 Perifollicular erythema may be the first sign, and papules and pustules may be visible. Although the hair loss in traction alopecia usually is reversible if the traction is ceased, end-stage disease may be permanent.6 Halting traction-inducing practices is paramount, and other treatment options that may slow progression include topical or oral antibiotics and topical or intralesional corticosteroids. Recovery of hair loss also may be aided by topical minoxidil.5
Keloids
Keloid formation is an important pathology to address, as it may result from several of the aforementioned conditions. Keloids are most commonly seen in black individuals but also can occur in Hispanic and Asian patients. The cause has not been fully elucidated but is thought to be a combination of dysfunctional fibroblasts with a genetic component based on racial predilection and twin concordance studies.5 The chest, shoulders, upper back, neck, and earlobes are particularly susceptible to keloid formation, which can appear from 1 to 24 years following dermal trauma.5 Unlike hypertrophic scars, keloids generally do not regress and frequently cause discomfort, pruritus, and emotional distress. They also can hinder wearing a military uniform. Sustained remission is problematic, making prevention a first-line approach, including proper care of wounds when they occur and avoiding elective procedures such as piercings and tattoos. Intralesional corticosteroids, adjuvant injections (eg, 5-fluorouracil), silicone sheeting, cryotherapy, radiation, laser therapy, and excision are some of the treatment options when keloids have formed.5
Final Comment
It is important to recognize military grooming standards as a cause or contributor to several diseases of the head and neck in military servicemembers. Specifically, frequent haircuts in male soldiers are associated with several inflammatory hair disorders, including AKN, dissecting cellulitis of the scalp, and folliculitis decalvans, while daily shaving predisposes individuals to pseudofolliculitis barbae with possible keloid formation. Females may develop traction alopecia from chronically tight, pulled back hairstyles. All of these conditions have health implications for the affected individuals and can compromise the military mission. Awareness, prevention, and recognition are key along with the knowledge base to provide anticipatory avoidance and initiate appropriate treatments, thereby mitigating these potential consequences.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed October 11, 2018.
- East-Innis AD, Stylianou K, Paolino A, et al. Acne keloidalis nuchae: risk factors and associated disorders--a retrospective study. Int J Dermatol. 2017;56:828-832.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systematic review of the literature. Dermatol Ther (Heidelb). 2016;6:363-378.
- Glenn MJ, Bennett RG, Kelly AP. Acne keloidalis nuchae: treatment with excision and second-intention healing. J Am Acad Dermatol. 1995;33:243-246.
- Madu P, Kundu RV. Follicular and scarring disorders in skin of color: presentation and management. Am J Clin Dermatol. 2014;15:307-321.
- Rodney IJ, Onwudiwe OC. Hair and scalp disorders in ethnic populations. J Drugs Dermatol. 2013;12:420-427.
- Lindsey SF, Tosti A. Ethnic hair disorders. Curr Probl Dermatol. 2015;47:139-148.
- Whiting DA. Cicatricial alopecia: clinico-pathological findings and treatment. Clin Dermatol. 2001;19:211-225.
- Sperling LC, Cowper SE, Knopp EA. An Atlas of Hair Pathology with Clinical Correlations. 2nd ed. Boca Raton, FL: CRC Press; 2012.
- Gemmeke A, Wollina U. Folliculitis decalvans of the scalp: response to triple therapy with isotretinoin, clindamycin, and prednisolone. Acta Dermatovenerol Alp Pannonica Adriat. 2006;15:184-186.
- Hallai N, Thompson I, Williams P, et al. Folliculitis spinulosa decalvans: failure to respond to oral isotretinoin. J Eur Acad Dermatol Venereol. 2006;20:223-224.
- Bolduc C, Sperling LC, Shapiro J. Primary cicatricial alopecia. J Am Acad Dermatol. 2016;75:101-117.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl):S113-S119.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed October 11, 2018.
- East-Innis AD, Stylianou K, Paolino A, et al. Acne keloidalis nuchae: risk factors and associated disorders--a retrospective study. Int J Dermatol. 2017;56:828-832.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systematic review of the literature. Dermatol Ther (Heidelb). 2016;6:363-378.
- Glenn MJ, Bennett RG, Kelly AP. Acne keloidalis nuchae: treatment with excision and second-intention healing. J Am Acad Dermatol. 1995;33:243-246.
- Madu P, Kundu RV. Follicular and scarring disorders in skin of color: presentation and management. Am J Clin Dermatol. 2014;15:307-321.
- Rodney IJ, Onwudiwe OC. Hair and scalp disorders in ethnic populations. J Drugs Dermatol. 2013;12:420-427.
- Lindsey SF, Tosti A. Ethnic hair disorders. Curr Probl Dermatol. 2015;47:139-148.
- Whiting DA. Cicatricial alopecia: clinico-pathological findings and treatment. Clin Dermatol. 2001;19:211-225.
- Sperling LC, Cowper SE, Knopp EA. An Atlas of Hair Pathology with Clinical Correlations. 2nd ed. Boca Raton, FL: CRC Press; 2012.
- Gemmeke A, Wollina U. Folliculitis decalvans of the scalp: response to triple therapy with isotretinoin, clindamycin, and prednisolone. Acta Dermatovenerol Alp Pannonica Adriat. 2006;15:184-186.
- Hallai N, Thompson I, Williams P, et al. Folliculitis spinulosa decalvans: failure to respond to oral isotretinoin. J Eur Acad Dermatol Venereol. 2006;20:223-224.
- Bolduc C, Sperling LC, Shapiro J. Primary cicatricial alopecia. J Am Acad Dermatol. 2016;75:101-117.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl):S113-S119.
Practice Points
- The short frequent haircuts required to maintain a tapered appearance of the hair per US military regulations may lead to inflammatory hair disorders such as acne keloidalis nuchae, dissecting cellulitis of the scalp, and folliculitis decalvans.
- The mainstay of prevention for these conditions is avoidance of inciting factors such as short haircuts, tight-collared shirts, frequent shaving, or tight hairstyles.
- Early identification and treatment of inflammatory follicular and scarring disorders can prevent further scarring, pigmentation changes, and/or disfigurement.
Laser Scar Management: Focused and High-Intensity Medical Exchange in Vietnam
Over the last decade the treatment of traumatic scars with lasers has emerged as a core component of multidisciplinary management. Military dermatologists have played a fundamental role in this shift by helping to develop new applications for existing technology and promulgate the techniques to reach additional providers and patients. Beyond scar management, the repurposing of adjunctive procedural techniques, such as sweat and hair reduction in amputees, also promises to enhance rehabilitation for many patients.
International engagement is a prominent and highly attractive feature of military practice, and military dermatologists routinely participate in disaster response missions, such as the 2010 Haiti earthquake,1 and ongoing planned operations, such as Pacific Partnership in the Indo-Asia-Pacific region led by the US Navy.2 In this article, I present a military perspective on the emerging niche of trauma dermatology and outline my more than 5 years of experience leveraging these skills to lead a multidisciplinary exchange in restorative medicine and burn scar management in Vietnam.
Trauma Dermatology
Over the course of the last decade, traumatic scar management has emerged as a staple of dermatologic surgery practice in some centers. Dermatologists hold the key to increasing patient access to effective outpatient care for symptomatic traumatic scars and other related issues using devices and techniques initially conceived for cosmetic applications.3 A major impetus for the considerable remodeling in our collective thoughts about traumatic scar management was the emergence of fractional laser technology in the mid-2000s. The remarkable, safe, reproducible, and durable benefits of fractional laser treatment of various scar types have created substantial momentum in recent years. The Naval Medical Center San Diego in California houses 1 of 3 centers of excellence in rehabilitation in the US military. Mastery of minimally invasive procedures to manage scars and other issues associated with trauma for the first time has established dermatologists as important partners in the overall rehabilitative effort.
My perspective on laser scar management has been previously described.4,5 Ablative fractional laser resurfacing is the backbone of rehabilitative scar management.6 Although the literature in this field is still relatively immature, higher-quality studies are accumulating rapidly as the burn and surgical communities adopt the procedure more widely.7-10 A considerable step forward in the dissemination of the procedure occurred recently with the development of category III Current Procedural Terminology (CPT) codes for ablative laser treatment of traumatic scars.11 Category III CPT codes are temporary codes used for emerging procedures that have not yet been deemed medically necessary. Although individual insurance carriers can determine whether to cover these procedures and the corresponding level of reimbursement, regular use is important for ultimate elevation to category I codes by the American Medical Association over a 5-year observation period. The CPT codes 0479T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; first 100 cm2 or part thereof, or 1% of body surface area of infants and children) and 0480T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; each additional 100 cm2, or each additional 1% of body surface area of infants and children, or part thereof [list separately in addition to code for primary procedure]) are examples of these category III codes.11
Nonablative fractional lasers; vascular-specific devices for erythematous scars; and long- and short-pulsed pigment-specific devices for hair and traumatic tattoo treatment, respectively, round out the commonly used laser platforms. For example, laser hair reduction can help improve the fit and comfort of prosthetic devices and has been shown to improve the overall quality of life for amputees.12 Botulinum toxin can be an important component of treatment of excessive sweating induced by occlusive liners in prosthetics, and microwave eccrine ablation is an emerging potential option for longer-lasting sweat reduction in this population.13-15 In addition to providing direct dermatology care and education, having members of the specialty in uniform has been a key to adopting new practical solutions to unsolved problems.
Pacific Partnership
Pacific Partnership is the largest annual multinational humanitarian assistance and disaster preparedness mission in the Indo-Asia-Pacific region.16 It was started in 2006 following the tsunami that devastated parts of South and Southeast Asia in 2004. The recently concluded Pacific Partnership 2018 marked the 13th iteration of the annual mission led by the US Navy in collaboration with other partner nations, which in 2018 included Japan, Australia, Canada, the United Kingdom, France, Singapore, Korea, and Peru, as well as nongovernmental organizations and international governmental agencies. Host nation mission locations vary somewhat from year to year, but 2018 included visits of the hospital ship USNS Mercy and more than 800 personnel to Indonesia, Malaysia, Sri Lanka, and Vietnam. Medical/dental, engineering, and veterinary teams join with their counterparts in each host nation to conduct civic action projects, community health exchanges, medical care, and disaster response training activities.16
Rehabilitation As a Vehicle for Medical Exchange
Since approximately 2012 there has been an evolving paradigm in Pacific Partnership from an emphasis on maximizing direct patient care in changing locations to one focused on building lasting partnerships through subject matter expert exchange. Multidisciplinary scar management, including surgical and laser scar revision and physical and occupational therapy, is a very promising model for engagement. Potential advantages of this type of exchange include the following: developing nations have relatively high rates of burns and other forms of trauma as well as uneven access to acute and ongoing rehabilitative care; patients often are otherwise healthy and young; results are frequently profound and readily demonstrable; and it is a skill set that has become highly developed in the military system. Just as dermatologists are illustrating their utility in trauma rehabilitation at home, these procedural skills provide fertile ground for exchange overseas.
The Overseas Humanitarian Assistance Shared Information System is an online platform that allows users to apply for grants under the Asia-Pacific Regional Initiative. In 2013, I started the Burn Scar Treatment/Restorative Medicine exchange with a grant under this program. A multidisciplinary team representing the specialties of dermatology, hand surgery, plastic surgery, physical medicine and rehabilitation, and pulmonary critical care participated in the 2013 Asia Pacific Burn Congress hosted by the National Institute of Burns (NIB) in Hanoi, Vietnam, and then followed up with didactics and patient care alongside Vietnamese physicians in the management of disfiguring and debilitating scars from burns and other trauma. This pilot project consisted of three 2- to 3-week phases: 2 at the NIB in Hanoi and 1 with a delegation from the NIB visiting the Naval Medical Center San Diego. When initial project funds expired in 2014, the exchange was absorbed into Pacific Partnership 2014, which began a string of 4 consecutive annual Pacific Partnership engagements at Da Nang General Hospital in Vietnam. The 2 most recent exchanges, including the exchange associated with Pacific Partnership 2018, have taken place at Khanh Hoa General Hospital in Nha Trang, Vietnam. During this time the team has grown to include physical and occupational therapists as well as a wound care nurse.
The Burn Scar Treatment/Restorative Medicine exchange consists of side-by-side laser and surgical scar revision performed with our Vietnamese hosts in their own hospital. Our Vietnamese partners perform a large volume of reconstructive surgeries in their usual practice, so it truly has been a bilateral exchange incorporating some advanced technology and techniques with an emphasis on longitudinal multidisciplinary care. Importantly, the procedures are supplemented with preoperative and postoperative care as well as instruction provided by physical and occupational therapy and wound care professionals working alongside host nation support staff. Because the areas of involvement often are extensive and a patient may only be seen once in this setting, laser and surgical procedures often are performed concurrently in the host nation operating room. Anesthesia support is provided by the host nation. Basic consumable surgical supplies (eg, sutures, gloves, marking pens, staplers) are supplemented with mission funds. Special adjuncts for the most severe contractures have included negative pressure wound therapy and a collagen-based bilayer matrix wound dressing. Laser treatments have been performed on the vast majority of patients with an ablative fractional CO2 laser and laser-assisted delivery of corticosteroid in hypertrophic areas. Of note, use of the laser has been provided to our hosts by the manufacturer for each of the 7 iterations of the exchange, and the wound dressing manufacturer also has donated some of their product to the exchange through the nongovernmental organization Project Hope for 2 missions. To date, more than 300 patients have safely received life-changing treatment during the exchange, with some receiving multiple treatments (Figure). Although multiple treatments over time are ideal, even a single treatment session can result in considerable and lasting improvements in function and symptoms.17 The hospital ship USNS Mercy has the same laser technology and has brought advanced scar treatment techniques to the far corners of the Pacific.
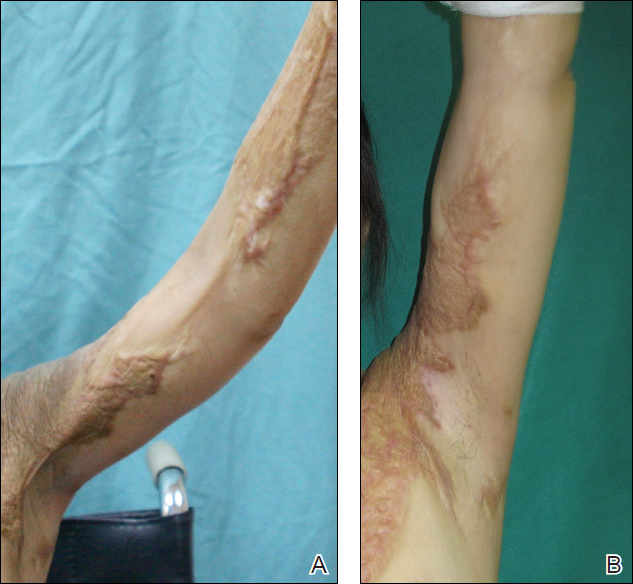
Measuring overall success—treatment and international relations—in this setting can be challenging. On an individual patient level, the benefits of restoring the ability to walk and work as well as reducing pain and itching are manifest and transformative for both the patient and family; however, aggregating this information into high-quality outcome data is difficult given the heterogeneous nature of traumatic injuries, which is compounded in the setting of international engagement where the intersection between patient and visiting provider may be singular or difficult to predict, funding is limited, language frequently is a barrier, and documentation, privacy, and medical research guidelines may be unfamiliar or contradictory. The cumulative impact of these types of exchanges on the relationship between nations also is critical but difficult to measure. It is common sense that deepening personal and professional relationships in the medical setting over time can increase trust and mutual understanding, perhaps setting the stage for broader engagement in other more sensitive areas. Trust and understanding are rather nebulous concepts, but earlier this year marked the first visit of an American aircraft carrier to Da Nang since 1975, following 4 consecutive annual Pacific Partnership missions in the same city, which does carry the patina of successful engagement on a systemic level.
Final Thoughts
Based on my personal experience, I provide the following tips for building a successful, focused, long-term medical exchange.
- Leverage your strengths and respect the strengths and style of practice of your hosts. A mind-set of exchange and not simply humanitarian care will be more successful. Your hosts are experts in a style of practice adapted to their surroundings and introducing new techniques that are grounded in the local practice patterns are more likely to be perpetuated.
- Collaboration with nongovernmental organizations and industry can be extremely helpful. Military and governmental organizations often are limited in funding, in the ways they can spend available funding, and in the receipt of donations. Appropriate coordination with civilian entities can elevate the exchange considerably by adding expertise and available assets as well as broadening the overall impact.
- Engage the support staff as well as the physicians. You will leverage contact with families and enhance care over the long-term.
- The benefits of multiple interactions over time are manifest, for both the patients and the participants. Personal and professional relationships are intertwined and naturally mature over time. Go for singles and doubles first before swinging for the fences.
- Multidisciplinary work overseas informs and enhances collaboration at home.
- Adding regional experts in international research and assessment to these specialized medical teams may better capture the impact of future exchanges of any flavor.
- The model of creating a focused exchange with independent funding followed by incorporation of successful concepts into larger missions seems to be a worthy and reproducible approach for future projects of any variety.
- Galeckas K. Dermatology aboard the USNS Comfort: disaster relief operations in Haiti after the 2010 earthquake. Dermatol Clin. 2011;29:15-19.
- Satter EK. The role of the dermatologist on military humanitarian missions. Cutis. 2010;85:85-89.
- Miletta NR, Donelan MB, Hivnor CM. Management of trauma and burn scars; the dermatologist's role in expanding patient access to care. Cutis. 2017;100:18-20.
- Shumaker PR. Laser treatment of traumatic scars: a military perspective. Semin Cutan Med Surg. 2015;34:17-23.
- Shumaker PR, Beachkofsky T, Basnett A, et al. A military perspective. In: Krakowski AC, Shumaker PR, eds. The Scar Book: Formation, Mitigation, Rehabilitation and Prevention. Philadelphia, PA: Wolters Kluwer; 2017:327-338.
- Anderson RR, Donelan MB, Greeson E, et al. Consensus report: laser treatment of traumatic scars with an emphasis on ablative fractional resurfacing. JAMA Dermatol. 2014;150:187-193.
- Hultman CS, Friedstat JS, Edkins RE, et al. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before and after cohort study, with long-term follow-up. Ann Surg. 2014;260:519-532.
- Blome-Eberwein S, Gogal C, Weiss MJ, et al. Prospective evaluation of fractional CO2 laser treatment of mature burn scars. J Burn Care Res. 2016;37:379-387.
- Issler-Fisher AC, Fisher OM, Smialkowski AO, et al. Ablative fractional CO2 laser for burn scar reconstruction: an extensive subjective and objective short-term outcome analysis of a prospective treatment cohort. Burns. 2017;43:573-582.
- Zuccaro J, Zlolkowski N, Fish J. A systematic review of the effectiveness of laser therapy for hypertrophic burn scars. Clin Plast Surg. 2017;44:767-779.
- Miller A. CPT 2018: What's new, part 2. American Academy of Dermatology website. https://www.aad.org/dw/monthly/2018/january/cpt-2018-whats-new-part-2. Accessed July 24, 2018.
- Miletta NR, Kim S, Lezanski-Gujda A, et al. Improving health-related quality of life in wounded warriors: the promising benefits of laser hair removal to the residual limb-prosthetic interface. Dermatol Surg. 2016;42:1182-1187.
- Gratrix M, Hivnor C. Botulinum toxin for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- USNS Mercy deploys in support of Pacific Partnership 2018 [news release]. Washington, DC: US Department of Defense; February 26, 2018. https://www.defense.gov/News/Article/Article/1450292/usns-mercy-deploys-in-support-of-pacific-partnership-2018/. Accessed July 11, 2018.
- Burns C, Basnett A, Valentine J, et al. Ablative fractional resurfacing: a powerful tool to help restore form and function during international medical exchange. Lasers Surg Med. 2017;49:471-474.
Over the last decade the treatment of traumatic scars with lasers has emerged as a core component of multidisciplinary management. Military dermatologists have played a fundamental role in this shift by helping to develop new applications for existing technology and promulgate the techniques to reach additional providers and patients. Beyond scar management, the repurposing of adjunctive procedural techniques, such as sweat and hair reduction in amputees, also promises to enhance rehabilitation for many patients.
International engagement is a prominent and highly attractive feature of military practice, and military dermatologists routinely participate in disaster response missions, such as the 2010 Haiti earthquake,1 and ongoing planned operations, such as Pacific Partnership in the Indo-Asia-Pacific region led by the US Navy.2 In this article, I present a military perspective on the emerging niche of trauma dermatology and outline my more than 5 years of experience leveraging these skills to lead a multidisciplinary exchange in restorative medicine and burn scar management in Vietnam.
Trauma Dermatology
Over the course of the last decade, traumatic scar management has emerged as a staple of dermatologic surgery practice in some centers. Dermatologists hold the key to increasing patient access to effective outpatient care for symptomatic traumatic scars and other related issues using devices and techniques initially conceived for cosmetic applications.3 A major impetus for the considerable remodeling in our collective thoughts about traumatic scar management was the emergence of fractional laser technology in the mid-2000s. The remarkable, safe, reproducible, and durable benefits of fractional laser treatment of various scar types have created substantial momentum in recent years. The Naval Medical Center San Diego in California houses 1 of 3 centers of excellence in rehabilitation in the US military. Mastery of minimally invasive procedures to manage scars and other issues associated with trauma for the first time has established dermatologists as important partners in the overall rehabilitative effort.
My perspective on laser scar management has been previously described.4,5 Ablative fractional laser resurfacing is the backbone of rehabilitative scar management.6 Although the literature in this field is still relatively immature, higher-quality studies are accumulating rapidly as the burn and surgical communities adopt the procedure more widely.7-10 A considerable step forward in the dissemination of the procedure occurred recently with the development of category III Current Procedural Terminology (CPT) codes for ablative laser treatment of traumatic scars.11 Category III CPT codes are temporary codes used for emerging procedures that have not yet been deemed medically necessary. Although individual insurance carriers can determine whether to cover these procedures and the corresponding level of reimbursement, regular use is important for ultimate elevation to category I codes by the American Medical Association over a 5-year observation period. The CPT codes 0479T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; first 100 cm2 or part thereof, or 1% of body surface area of infants and children) and 0480T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; each additional 100 cm2, or each additional 1% of body surface area of infants and children, or part thereof [list separately in addition to code for primary procedure]) are examples of these category III codes.11
Nonablative fractional lasers; vascular-specific devices for erythematous scars; and long- and short-pulsed pigment-specific devices for hair and traumatic tattoo treatment, respectively, round out the commonly used laser platforms. For example, laser hair reduction can help improve the fit and comfort of prosthetic devices and has been shown to improve the overall quality of life for amputees.12 Botulinum toxin can be an important component of treatment of excessive sweating induced by occlusive liners in prosthetics, and microwave eccrine ablation is an emerging potential option for longer-lasting sweat reduction in this population.13-15 In addition to providing direct dermatology care and education, having members of the specialty in uniform has been a key to adopting new practical solutions to unsolved problems.
Pacific Partnership
Pacific Partnership is the largest annual multinational humanitarian assistance and disaster preparedness mission in the Indo-Asia-Pacific region.16 It was started in 2006 following the tsunami that devastated parts of South and Southeast Asia in 2004. The recently concluded Pacific Partnership 2018 marked the 13th iteration of the annual mission led by the US Navy in collaboration with other partner nations, which in 2018 included Japan, Australia, Canada, the United Kingdom, France, Singapore, Korea, and Peru, as well as nongovernmental organizations and international governmental agencies. Host nation mission locations vary somewhat from year to year, but 2018 included visits of the hospital ship USNS Mercy and more than 800 personnel to Indonesia, Malaysia, Sri Lanka, and Vietnam. Medical/dental, engineering, and veterinary teams join with their counterparts in each host nation to conduct civic action projects, community health exchanges, medical care, and disaster response training activities.16
Rehabilitation As a Vehicle for Medical Exchange
Since approximately 2012 there has been an evolving paradigm in Pacific Partnership from an emphasis on maximizing direct patient care in changing locations to one focused on building lasting partnerships through subject matter expert exchange. Multidisciplinary scar management, including surgical and laser scar revision and physical and occupational therapy, is a very promising model for engagement. Potential advantages of this type of exchange include the following: developing nations have relatively high rates of burns and other forms of trauma as well as uneven access to acute and ongoing rehabilitative care; patients often are otherwise healthy and young; results are frequently profound and readily demonstrable; and it is a skill set that has become highly developed in the military system. Just as dermatologists are illustrating their utility in trauma rehabilitation at home, these procedural skills provide fertile ground for exchange overseas.
The Overseas Humanitarian Assistance Shared Information System is an online platform that allows users to apply for grants under the Asia-Pacific Regional Initiative. In 2013, I started the Burn Scar Treatment/Restorative Medicine exchange with a grant under this program. A multidisciplinary team representing the specialties of dermatology, hand surgery, plastic surgery, physical medicine and rehabilitation, and pulmonary critical care participated in the 2013 Asia Pacific Burn Congress hosted by the National Institute of Burns (NIB) in Hanoi, Vietnam, and then followed up with didactics and patient care alongside Vietnamese physicians in the management of disfiguring and debilitating scars from burns and other trauma. This pilot project consisted of three 2- to 3-week phases: 2 at the NIB in Hanoi and 1 with a delegation from the NIB visiting the Naval Medical Center San Diego. When initial project funds expired in 2014, the exchange was absorbed into Pacific Partnership 2014, which began a string of 4 consecutive annual Pacific Partnership engagements at Da Nang General Hospital in Vietnam. The 2 most recent exchanges, including the exchange associated with Pacific Partnership 2018, have taken place at Khanh Hoa General Hospital in Nha Trang, Vietnam. During this time the team has grown to include physical and occupational therapists as well as a wound care nurse.
The Burn Scar Treatment/Restorative Medicine exchange consists of side-by-side laser and surgical scar revision performed with our Vietnamese hosts in their own hospital. Our Vietnamese partners perform a large volume of reconstructive surgeries in their usual practice, so it truly has been a bilateral exchange incorporating some advanced technology and techniques with an emphasis on longitudinal multidisciplinary care. Importantly, the procedures are supplemented with preoperative and postoperative care as well as instruction provided by physical and occupational therapy and wound care professionals working alongside host nation support staff. Because the areas of involvement often are extensive and a patient may only be seen once in this setting, laser and surgical procedures often are performed concurrently in the host nation operating room. Anesthesia support is provided by the host nation. Basic consumable surgical supplies (eg, sutures, gloves, marking pens, staplers) are supplemented with mission funds. Special adjuncts for the most severe contractures have included negative pressure wound therapy and a collagen-based bilayer matrix wound dressing. Laser treatments have been performed on the vast majority of patients with an ablative fractional CO2 laser and laser-assisted delivery of corticosteroid in hypertrophic areas. Of note, use of the laser has been provided to our hosts by the manufacturer for each of the 7 iterations of the exchange, and the wound dressing manufacturer also has donated some of their product to the exchange through the nongovernmental organization Project Hope for 2 missions. To date, more than 300 patients have safely received life-changing treatment during the exchange, with some receiving multiple treatments (Figure). Although multiple treatments over time are ideal, even a single treatment session can result in considerable and lasting improvements in function and symptoms.17 The hospital ship USNS Mercy has the same laser technology and has brought advanced scar treatment techniques to the far corners of the Pacific.

Measuring overall success—treatment and international relations—in this setting can be challenging. On an individual patient level, the benefits of restoring the ability to walk and work as well as reducing pain and itching are manifest and transformative for both the patient and family; however, aggregating this information into high-quality outcome data is difficult given the heterogeneous nature of traumatic injuries, which is compounded in the setting of international engagement where the intersection between patient and visiting provider may be singular or difficult to predict, funding is limited, language frequently is a barrier, and documentation, privacy, and medical research guidelines may be unfamiliar or contradictory. The cumulative impact of these types of exchanges on the relationship between nations also is critical but difficult to measure. It is common sense that deepening personal and professional relationships in the medical setting over time can increase trust and mutual understanding, perhaps setting the stage for broader engagement in other more sensitive areas. Trust and understanding are rather nebulous concepts, but earlier this year marked the first visit of an American aircraft carrier to Da Nang since 1975, following 4 consecutive annual Pacific Partnership missions in the same city, which does carry the patina of successful engagement on a systemic level.
Final Thoughts
Based on my personal experience, I provide the following tips for building a successful, focused, long-term medical exchange.
- Leverage your strengths and respect the strengths and style of practice of your hosts. A mind-set of exchange and not simply humanitarian care will be more successful. Your hosts are experts in a style of practice adapted to their surroundings and introducing new techniques that are grounded in the local practice patterns are more likely to be perpetuated.
- Collaboration with nongovernmental organizations and industry can be extremely helpful. Military and governmental organizations often are limited in funding, in the ways they can spend available funding, and in the receipt of donations. Appropriate coordination with civilian entities can elevate the exchange considerably by adding expertise and available assets as well as broadening the overall impact.
- Engage the support staff as well as the physicians. You will leverage contact with families and enhance care over the long-term.
- The benefits of multiple interactions over time are manifest, for both the patients and the participants. Personal and professional relationships are intertwined and naturally mature over time. Go for singles and doubles first before swinging for the fences.
- Multidisciplinary work overseas informs and enhances collaboration at home.
- Adding regional experts in international research and assessment to these specialized medical teams may better capture the impact of future exchanges of any flavor.
- The model of creating a focused exchange with independent funding followed by incorporation of successful concepts into larger missions seems to be a worthy and reproducible approach for future projects of any variety.
Over the last decade the treatment of traumatic scars with lasers has emerged as a core component of multidisciplinary management. Military dermatologists have played a fundamental role in this shift by helping to develop new applications for existing technology and promulgate the techniques to reach additional providers and patients. Beyond scar management, the repurposing of adjunctive procedural techniques, such as sweat and hair reduction in amputees, also promises to enhance rehabilitation for many patients.
International engagement is a prominent and highly attractive feature of military practice, and military dermatologists routinely participate in disaster response missions, such as the 2010 Haiti earthquake,1 and ongoing planned operations, such as Pacific Partnership in the Indo-Asia-Pacific region led by the US Navy.2 In this article, I present a military perspective on the emerging niche of trauma dermatology and outline my more than 5 years of experience leveraging these skills to lead a multidisciplinary exchange in restorative medicine and burn scar management in Vietnam.
Trauma Dermatology
Over the course of the last decade, traumatic scar management has emerged as a staple of dermatologic surgery practice in some centers. Dermatologists hold the key to increasing patient access to effective outpatient care for symptomatic traumatic scars and other related issues using devices and techniques initially conceived for cosmetic applications.3 A major impetus for the considerable remodeling in our collective thoughts about traumatic scar management was the emergence of fractional laser technology in the mid-2000s. The remarkable, safe, reproducible, and durable benefits of fractional laser treatment of various scar types have created substantial momentum in recent years. The Naval Medical Center San Diego in California houses 1 of 3 centers of excellence in rehabilitation in the US military. Mastery of minimally invasive procedures to manage scars and other issues associated with trauma for the first time has established dermatologists as important partners in the overall rehabilitative effort.
My perspective on laser scar management has been previously described.4,5 Ablative fractional laser resurfacing is the backbone of rehabilitative scar management.6 Although the literature in this field is still relatively immature, higher-quality studies are accumulating rapidly as the burn and surgical communities adopt the procedure more widely.7-10 A considerable step forward in the dissemination of the procedure occurred recently with the development of category III Current Procedural Terminology (CPT) codes for ablative laser treatment of traumatic scars.11 Category III CPT codes are temporary codes used for emerging procedures that have not yet been deemed medically necessary. Although individual insurance carriers can determine whether to cover these procedures and the corresponding level of reimbursement, regular use is important for ultimate elevation to category I codes by the American Medical Association over a 5-year observation period. The CPT codes 0479T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; first 100 cm2 or part thereof, or 1% of body surface area of infants and children) and 0480T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; each additional 100 cm2, or each additional 1% of body surface area of infants and children, or part thereof [list separately in addition to code for primary procedure]) are examples of these category III codes.11
Nonablative fractional lasers; vascular-specific devices for erythematous scars; and long- and short-pulsed pigment-specific devices for hair and traumatic tattoo treatment, respectively, round out the commonly used laser platforms. For example, laser hair reduction can help improve the fit and comfort of prosthetic devices and has been shown to improve the overall quality of life for amputees.12 Botulinum toxin can be an important component of treatment of excessive sweating induced by occlusive liners in prosthetics, and microwave eccrine ablation is an emerging potential option for longer-lasting sweat reduction in this population.13-15 In addition to providing direct dermatology care and education, having members of the specialty in uniform has been a key to adopting new practical solutions to unsolved problems.
Pacific Partnership
Pacific Partnership is the largest annual multinational humanitarian assistance and disaster preparedness mission in the Indo-Asia-Pacific region.16 It was started in 2006 following the tsunami that devastated parts of South and Southeast Asia in 2004. The recently concluded Pacific Partnership 2018 marked the 13th iteration of the annual mission led by the US Navy in collaboration with other partner nations, which in 2018 included Japan, Australia, Canada, the United Kingdom, France, Singapore, Korea, and Peru, as well as nongovernmental organizations and international governmental agencies. Host nation mission locations vary somewhat from year to year, but 2018 included visits of the hospital ship USNS Mercy and more than 800 personnel to Indonesia, Malaysia, Sri Lanka, and Vietnam. Medical/dental, engineering, and veterinary teams join with their counterparts in each host nation to conduct civic action projects, community health exchanges, medical care, and disaster response training activities.16
Rehabilitation As a Vehicle for Medical Exchange
Since approximately 2012 there has been an evolving paradigm in Pacific Partnership from an emphasis on maximizing direct patient care in changing locations to one focused on building lasting partnerships through subject matter expert exchange. Multidisciplinary scar management, including surgical and laser scar revision and physical and occupational therapy, is a very promising model for engagement. Potential advantages of this type of exchange include the following: developing nations have relatively high rates of burns and other forms of trauma as well as uneven access to acute and ongoing rehabilitative care; patients often are otherwise healthy and young; results are frequently profound and readily demonstrable; and it is a skill set that has become highly developed in the military system. Just as dermatologists are illustrating their utility in trauma rehabilitation at home, these procedural skills provide fertile ground for exchange overseas.
The Overseas Humanitarian Assistance Shared Information System is an online platform that allows users to apply for grants under the Asia-Pacific Regional Initiative. In 2013, I started the Burn Scar Treatment/Restorative Medicine exchange with a grant under this program. A multidisciplinary team representing the specialties of dermatology, hand surgery, plastic surgery, physical medicine and rehabilitation, and pulmonary critical care participated in the 2013 Asia Pacific Burn Congress hosted by the National Institute of Burns (NIB) in Hanoi, Vietnam, and then followed up with didactics and patient care alongside Vietnamese physicians in the management of disfiguring and debilitating scars from burns and other trauma. This pilot project consisted of three 2- to 3-week phases: 2 at the NIB in Hanoi and 1 with a delegation from the NIB visiting the Naval Medical Center San Diego. When initial project funds expired in 2014, the exchange was absorbed into Pacific Partnership 2014, which began a string of 4 consecutive annual Pacific Partnership engagements at Da Nang General Hospital in Vietnam. The 2 most recent exchanges, including the exchange associated with Pacific Partnership 2018, have taken place at Khanh Hoa General Hospital in Nha Trang, Vietnam. During this time the team has grown to include physical and occupational therapists as well as a wound care nurse.
The Burn Scar Treatment/Restorative Medicine exchange consists of side-by-side laser and surgical scar revision performed with our Vietnamese hosts in their own hospital. Our Vietnamese partners perform a large volume of reconstructive surgeries in their usual practice, so it truly has been a bilateral exchange incorporating some advanced technology and techniques with an emphasis on longitudinal multidisciplinary care. Importantly, the procedures are supplemented with preoperative and postoperative care as well as instruction provided by physical and occupational therapy and wound care professionals working alongside host nation support staff. Because the areas of involvement often are extensive and a patient may only be seen once in this setting, laser and surgical procedures often are performed concurrently in the host nation operating room. Anesthesia support is provided by the host nation. Basic consumable surgical supplies (eg, sutures, gloves, marking pens, staplers) are supplemented with mission funds. Special adjuncts for the most severe contractures have included negative pressure wound therapy and a collagen-based bilayer matrix wound dressing. Laser treatments have been performed on the vast majority of patients with an ablative fractional CO2 laser and laser-assisted delivery of corticosteroid in hypertrophic areas. Of note, use of the laser has been provided to our hosts by the manufacturer for each of the 7 iterations of the exchange, and the wound dressing manufacturer also has donated some of their product to the exchange through the nongovernmental organization Project Hope for 2 missions. To date, more than 300 patients have safely received life-changing treatment during the exchange, with some receiving multiple treatments (Figure). Although multiple treatments over time are ideal, even a single treatment session can result in considerable and lasting improvements in function and symptoms.17 The hospital ship USNS Mercy has the same laser technology and has brought advanced scar treatment techniques to the far corners of the Pacific.

Measuring overall success—treatment and international relations—in this setting can be challenging. On an individual patient level, the benefits of restoring the ability to walk and work as well as reducing pain and itching are manifest and transformative for both the patient and family; however, aggregating this information into high-quality outcome data is difficult given the heterogeneous nature of traumatic injuries, which is compounded in the setting of international engagement where the intersection between patient and visiting provider may be singular or difficult to predict, funding is limited, language frequently is a barrier, and documentation, privacy, and medical research guidelines may be unfamiliar or contradictory. The cumulative impact of these types of exchanges on the relationship between nations also is critical but difficult to measure. It is common sense that deepening personal and professional relationships in the medical setting over time can increase trust and mutual understanding, perhaps setting the stage for broader engagement in other more sensitive areas. Trust and understanding are rather nebulous concepts, but earlier this year marked the first visit of an American aircraft carrier to Da Nang since 1975, following 4 consecutive annual Pacific Partnership missions in the same city, which does carry the patina of successful engagement on a systemic level.
Final Thoughts
Based on my personal experience, I provide the following tips for building a successful, focused, long-term medical exchange.
- Leverage your strengths and respect the strengths and style of practice of your hosts. A mind-set of exchange and not simply humanitarian care will be more successful. Your hosts are experts in a style of practice adapted to their surroundings and introducing new techniques that are grounded in the local practice patterns are more likely to be perpetuated.
- Collaboration with nongovernmental organizations and industry can be extremely helpful. Military and governmental organizations often are limited in funding, in the ways they can spend available funding, and in the receipt of donations. Appropriate coordination with civilian entities can elevate the exchange considerably by adding expertise and available assets as well as broadening the overall impact.
- Engage the support staff as well as the physicians. You will leverage contact with families and enhance care over the long-term.
- The benefits of multiple interactions over time are manifest, for both the patients and the participants. Personal and professional relationships are intertwined and naturally mature over time. Go for singles and doubles first before swinging for the fences.
- Multidisciplinary work overseas informs and enhances collaboration at home.
- Adding regional experts in international research and assessment to these specialized medical teams may better capture the impact of future exchanges of any flavor.
- The model of creating a focused exchange with independent funding followed by incorporation of successful concepts into larger missions seems to be a worthy and reproducible approach for future projects of any variety.
- Galeckas K. Dermatology aboard the USNS Comfort: disaster relief operations in Haiti after the 2010 earthquake. Dermatol Clin. 2011;29:15-19.
- Satter EK. The role of the dermatologist on military humanitarian missions. Cutis. 2010;85:85-89.
- Miletta NR, Donelan MB, Hivnor CM. Management of trauma and burn scars; the dermatologist's role in expanding patient access to care. Cutis. 2017;100:18-20.
- Shumaker PR. Laser treatment of traumatic scars: a military perspective. Semin Cutan Med Surg. 2015;34:17-23.
- Shumaker PR, Beachkofsky T, Basnett A, et al. A military perspective. In: Krakowski AC, Shumaker PR, eds. The Scar Book: Formation, Mitigation, Rehabilitation and Prevention. Philadelphia, PA: Wolters Kluwer; 2017:327-338.
- Anderson RR, Donelan MB, Greeson E, et al. Consensus report: laser treatment of traumatic scars with an emphasis on ablative fractional resurfacing. JAMA Dermatol. 2014;150:187-193.
- Hultman CS, Friedstat JS, Edkins RE, et al. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before and after cohort study, with long-term follow-up. Ann Surg. 2014;260:519-532.
- Blome-Eberwein S, Gogal C, Weiss MJ, et al. Prospective evaluation of fractional CO2 laser treatment of mature burn scars. J Burn Care Res. 2016;37:379-387.
- Issler-Fisher AC, Fisher OM, Smialkowski AO, et al. Ablative fractional CO2 laser for burn scar reconstruction: an extensive subjective and objective short-term outcome analysis of a prospective treatment cohort. Burns. 2017;43:573-582.
- Zuccaro J, Zlolkowski N, Fish J. A systematic review of the effectiveness of laser therapy for hypertrophic burn scars. Clin Plast Surg. 2017;44:767-779.
- Miller A. CPT 2018: What's new, part 2. American Academy of Dermatology website. https://www.aad.org/dw/monthly/2018/january/cpt-2018-whats-new-part-2. Accessed July 24, 2018.
- Miletta NR, Kim S, Lezanski-Gujda A, et al. Improving health-related quality of life in wounded warriors: the promising benefits of laser hair removal to the residual limb-prosthetic interface. Dermatol Surg. 2016;42:1182-1187.
- Gratrix M, Hivnor C. Botulinum toxin for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- USNS Mercy deploys in support of Pacific Partnership 2018 [news release]. Washington, DC: US Department of Defense; February 26, 2018. https://www.defense.gov/News/Article/Article/1450292/usns-mercy-deploys-in-support-of-pacific-partnership-2018/. Accessed July 11, 2018.
- Burns C, Basnett A, Valentine J, et al. Ablative fractional resurfacing: a powerful tool to help restore form and function during international medical exchange. Lasers Surg Med. 2017;49:471-474.
- Galeckas K. Dermatology aboard the USNS Comfort: disaster relief operations in Haiti after the 2010 earthquake. Dermatol Clin. 2011;29:15-19.
- Satter EK. The role of the dermatologist on military humanitarian missions. Cutis. 2010;85:85-89.
- Miletta NR, Donelan MB, Hivnor CM. Management of trauma and burn scars; the dermatologist's role in expanding patient access to care. Cutis. 2017;100:18-20.
- Shumaker PR. Laser treatment of traumatic scars: a military perspective. Semin Cutan Med Surg. 2015;34:17-23.
- Shumaker PR, Beachkofsky T, Basnett A, et al. A military perspective. In: Krakowski AC, Shumaker PR, eds. The Scar Book: Formation, Mitigation, Rehabilitation and Prevention. Philadelphia, PA: Wolters Kluwer; 2017:327-338.
- Anderson RR, Donelan MB, Greeson E, et al. Consensus report: laser treatment of traumatic scars with an emphasis on ablative fractional resurfacing. JAMA Dermatol. 2014;150:187-193.
- Hultman CS, Friedstat JS, Edkins RE, et al. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before and after cohort study, with long-term follow-up. Ann Surg. 2014;260:519-532.
- Blome-Eberwein S, Gogal C, Weiss MJ, et al. Prospective evaluation of fractional CO2 laser treatment of mature burn scars. J Burn Care Res. 2016;37:379-387.
- Issler-Fisher AC, Fisher OM, Smialkowski AO, et al. Ablative fractional CO2 laser for burn scar reconstruction: an extensive subjective and objective short-term outcome analysis of a prospective treatment cohort. Burns. 2017;43:573-582.
- Zuccaro J, Zlolkowski N, Fish J. A systematic review of the effectiveness of laser therapy for hypertrophic burn scars. Clin Plast Surg. 2017;44:767-779.
- Miller A. CPT 2018: What's new, part 2. American Academy of Dermatology website. https://www.aad.org/dw/monthly/2018/january/cpt-2018-whats-new-part-2. Accessed July 24, 2018.
- Miletta NR, Kim S, Lezanski-Gujda A, et al. Improving health-related quality of life in wounded warriors: the promising benefits of laser hair removal to the residual limb-prosthetic interface. Dermatol Surg. 2016;42:1182-1187.
- Gratrix M, Hivnor C. Botulinum toxin for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- USNS Mercy deploys in support of Pacific Partnership 2018 [news release]. Washington, DC: US Department of Defense; February 26, 2018. https://www.defense.gov/News/Article/Article/1450292/usns-mercy-deploys-in-support-of-pacific-partnership-2018/. Accessed July 11, 2018.
- Burns C, Basnett A, Valentine J, et al. Ablative fractional resurfacing: a powerful tool to help restore form and function during international medical exchange. Lasers Surg Med. 2017;49:471-474.
Teledermatology in the US Military: A Historic Foundation for Current and Future Applications
Telemedicine arose from the need to provide critical and timely advice directly to health care providers and patients in remote or resource-scarce settings. Whether by radio, telephone, or other means of telecommunication technology, the US military has long utilized telemedicine. What started as a way to expedite the delivery of emergency consultations and medical expertise to remote populations in need has since evolved into a billion-dollar innovation industry that is poised to improve health care efficiency and access to specialist care as well as to lower health care costs for all patients.
Teledermatology in the Military
A primary mission of military medicine is to keep service members anywhere in the world in good health on the job during training, combat, and humanitarian operations.1 Telemedicine greatly supports this mission by bringing the expertise of medical specialists to service members in the field without the cost or risks of travel for physicians. Telemedicine also is effective in promoting timely triage of patients and administration of the most appropriate levels of care. With the advent and globalization of high-speed wireless networks, advancements in telemedicine continue to develop and are becoming increasingly useful in military medicine.
As a specialty, dermatology is heavily reliant on visual information and therefore is particularly amenable to telemedicine applications. The rising popularity of such services has led to the development of the term teledermatology. While early teledermatology services were provided using radio, telephone, fax, and videoconferencing,2 three distinct visual methods typically are used today, including (1) store-and-forward (S&F), (2) live-interactive, and (3) a hybrid of the two.3 Military dermatology predominantly utilizes an S&F system, as still photographs of lesions generally are preferred over video for more focused visualization.
In 2004, the US Army Medical Department established a centralized telemedicine program using Army Knowledge Online,1 an S&F system that allows providers in remote locations to store and forward information about a patient’s clinical history along with digital photographs of the patient’s condition to a military dermatologist to review and make a diagnosis or suggest a treatment from a different location at a later time. Using this platform to provide asynchronous teledermatology services avoids the logistics required to schedule appointments and promotes convenience and more efficient use of physicians’ time and resources.
Given the ease of use of S&F systems among military practitioners, dermatology became one of the most heavily utilized teleconsultation specialties within the Army Knowledge Online system, accounting for 40% of the 10,817 consultations initiated from April 2004 to December 2012.5 It also is important to note that skin conditions historically account for 15% to 75% of outpatient visits during wartime; therefore, there is a need for dermatologic consultations, as primary care providers typically are responsible for providing dermatologic care to these patients.6 Because of the high demand for and low volume of US military dermatologists, the use of teledermatology (ie, Amy Knowledge Online) in the US military became a helpful educational tool and specialist extender for many primary care providers in the military.
Teledermatology in the military has evolved to not only provide timely and efficient care but also to reduce health care costs.
Advances in Teledermatology
While the military continues to use S&F teleconsultations—a model in which a deployed referring clinician sends information to a military dermatologist for diagnosis and/or management recommendations—a number of teledermatology programs have been developed for civilians that provide additional advantages over standard face-to-face dermatology care. The advantages of S&F teledermatology applications are many, including faster communication with dermatology providers, diagnostic concordance comparable to face-to-face appointments, cost-effective care for patients, the ability to educate providers remotely,8 and similar outcomes to in-person care.9 However, as to be expected, in-person care remains the gold standard, especially when diagnostic accuracy depends on biopsy findings.
The development of the smartphone along with advances in digital photography and consumer-friendly mobile applications has allowed for the emergence of direct-to-consumer (DTC) teledermatology applications. Regardless of the user’s ability, the quality of photographs taken with smartphones has improved, as standard features such as high-resolution cameras with image stabilization, automatic focus, and lighting have become commonplace. The popularity of smartphone technology also has increased, with nearly 75% of all adults and more than 90% of adults younger than 35 years of age owning a smartphone according to a 2016 survey.11
In 2015, there were at least 29 DTC teledermatology applications available on various mobile platforms,12 accounting for an estimated 1.25 million teleconsultations with providers.13 Teledermatology platforms such as DermatologistOnCall and Spruce Health have made accessing dermatologic care convenient, timely, and affordable for patients via patient-friendly mobile applications.
Regular access to dermatologic care is especially important for patients who have chronic skin conditions. Several unique practice models have emerged as innovative solutions to providing more convenient and timely care. For example, Curology (https://curology.com) is an online teledermatology practice specializing in acne treatment.
Although DTC teledermatology practices are convenient for many patients and providers, some have been criticized for providing poor quality of care12 or facilitating fragmented care by not integrating with established electronic health record (EHR) systems.15 As a result, recommended practice guidelines for DTC teledermatology have been developed by the American Academy of Dermatology and some state medical boards.16 Moreover, several EHR systems, such as Epic (www.epic.com) and Modernizing Medicine’s EMA (www.modmed.com), have developed fully integrated S&F teledermatology platforms to be incorporated with established brick-and-mortar care.17
The Future of Teledermatology in the Military
The Army Knowledge Online telemedicine platform used by the US military has continued to be useful, particularly when treating patients in remote locations, and shows promise for improving routine domestic dermatology care. It has reduced the number of medical evacuations and improved care for those who do not have access to a dermatologist.4 Furthermore, one study noted that most consultations submitted via teledermatology applications from a combat zone received a diagnosis and treatment recommendation from a military dermatologist faster than they would have stateside, where the wait often is 4 to 8 weeks. On average, a teledermatology consultation from Afghanistan was answered in less than 6 hours.4 Although this response time might not be realistic for all dermatology practices, there clearly is potential in certain situations and utilizing certain models of care to diagnose and treat more patients more efficiently utilizing teledermatology applications than in an in-person office visit. A review of 658 teledermatology consultations in the US military from January 2011 to December 2012 revealed that the leading diagnoses were eczematous dermatitis (14%), contact dermatitis (9%), nonmelanoma skin cancer (5%), psoriasis (4%), and urticaria (4%).4 Increased use of teledermatology evaluation of these conditions in routine US-based military practice could help expedite care, decrease patient travel time, and utilize in-clinic dermatologist time more efficiently. Teledermatology visits for postoperative concerns also have demonstrated utility and convenience for triage and management of patients in the civilian setting and may be an additional novel use of teledermatology in the military setting.18 With the use of an integrated S&F teledermatology platform within an existing EHR system that is paired with a secure patient mobile application that allows easy upload of photos, medical history, and messaging, it can be argued that quality of life could greatly be enhanced for both military patients and providers.
Limitations of Teledermatology
Certainly, there are and will always be limitations to teledermatology. Even as digital photography improves, the quality and context of clinical images are user dependent, and key associated skin findings in other locations of the body can be missed. The ability to palpate the skin also is lacking in virtual encounters. Therefore, teledermatology might be considered most appropriate for specific diseases and conditions (eg, acne, psoriasis, eczema). Embracing teledermatology does not mean replacing in-person care; rather, it should be seen as an adjunct used to manage the high demand for dermatology expertise in military and civilian practice. For the US military, the promise and potential to embrace innovation in providing dermatologic care is there, as long as there are leaders to continue to champion it. In the current state of health care, many of the perceived barriers of teledermatology applications have already been overcome, including lack of training, lack of reimbursement, and perceived medicolegal risks.19
The US Federal Government is a large entity, and it will undoubtedly take time and effort to implement new and innovative programs such as the ones described here in the military. The first step in implementation is awareness that the possibilities exist; then, with the cooperation of dermatologists and support from the chain of command, it will be possible to incorporate advances in teledermatology and cultivate new ones.
Final Thoughts
The S&F teledermatology method used in the military setting has become commonplace in both military and civilian settings alike. Newer innovations in telemedicine, particularly in teledermatology, will continue to shape the future of military and civilian medicine for years to come.
- Vidmar DA. The history of teledermatology in the Department of Defense. Dermatol Clin. 1999;17:113-124.
- McManus J, Salinas J, Morton M, et al. Teleconsultation program for deployed soldiers and healthcare professionals in remote and austere environments. Prehosp Disaster Med. 2008;23:210-216.
- Tensen E, Van Der Heijden JP, Jaspers MW, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the U.S. military in a deployed setting. Mil Med. 2014;179:1347-1353.
- McGraw TA, Norton SA. Military aeromedical evacuations from central and southwest Asia for ill-defined dermatologic diseases. Arch Dermatol. 2009;145:165-170.
- Shissel DJ, Wilde J. Operational dermatology. Mil Med. 2004;169:444-447.
- Henning JS, Wohltmann W, Hivnor C. Teledermatology from a combat zone. Arch Dermatol. 2010;146:676-677.
- Whited JD, Hall RP, Simel DL, et al. Reliability and accuracy of dermatologists’ clinic-based and digital image consultations. J Am Acad Dermatol. 1999;41:693-702.
- Pak H, Triplett CA, Lindquist JH, et al. Store-and-forward teledermatology results in similar clinical outcomes to conventional clinic-based care. J Telemed Telecare. 2007;13:26-30.
- Finnane A, Dallest K, Janda M, et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327.
- Poushter J. Smartphone ownership and internet usage continues to climb in emerging economies. Washington, DC: Pew Research Center. www.pewglobal.org/2016/02/22/smartphone-ownership-and-internet-usage-continues-to-climbin-emerging-economies/. Published February 22, 2016. Accessed February 2, 2018.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Huff C. Medical diagnosis by webcam? Washington, DC: American Association of Retired Persons. www.aarp.org/health/conditions-treatments/info-2015/telemedicine-health-symptoms-diagnosis.html. Published December 2015. Accessed February 2, 2018.
- Mehrotra A. The convenience revolution for the treatment of low-acuity conditions. JAMA. 2013;310:35-36.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Teledermatology toolkit. American Academy of Dermatology website. https://www.aad.org/practicecenter/managing-a-practice/teledermatology. Accessed April 24, 2018.
- Carter ZA, Goldman S, Anderson K, et al. Creation of an internalteledermatology store-and-forward system in an existing electronic health record: a pilot study in a safety-net public health and hospital system. JAMA Dermatol. 2017;153:644-650.
- Jeyamohan SR, Moye MS, Srivastava D, et al. Patient-acquired photographs for the management of postoperative concerns. JAMA Dermatol. 2017;153:226-227.
- Edison KE, Dyer JA, Whited JD, et al. Practice gaps. the barriers and the promise of teledermatology. Arch Dermatol. 2012;148:650-651.
Telemedicine arose from the need to provide critical and timely advice directly to health care providers and patients in remote or resource-scarce settings. Whether by radio, telephone, or other means of telecommunication technology, the US military has long utilized telemedicine. What started as a way to expedite the delivery of emergency consultations and medical expertise to remote populations in need has since evolved into a billion-dollar innovation industry that is poised to improve health care efficiency and access to specialist care as well as to lower health care costs for all patients.
Teledermatology in the Military
A primary mission of military medicine is to keep service members anywhere in the world in good health on the job during training, combat, and humanitarian operations.1 Telemedicine greatly supports this mission by bringing the expertise of medical specialists to service members in the field without the cost or risks of travel for physicians. Telemedicine also is effective in promoting timely triage of patients and administration of the most appropriate levels of care. With the advent and globalization of high-speed wireless networks, advancements in telemedicine continue to develop and are becoming increasingly useful in military medicine.
As a specialty, dermatology is heavily reliant on visual information and therefore is particularly amenable to telemedicine applications. The rising popularity of such services has led to the development of the term teledermatology. While early teledermatology services were provided using radio, telephone, fax, and videoconferencing,2 three distinct visual methods typically are used today, including (1) store-and-forward (S&F), (2) live-interactive, and (3) a hybrid of the two.3 Military dermatology predominantly utilizes an S&F system, as still photographs of lesions generally are preferred over video for more focused visualization.
In 2004, the US Army Medical Department established a centralized telemedicine program using Army Knowledge Online,1 an S&F system that allows providers in remote locations to store and forward information about a patient’s clinical history along with digital photographs of the patient’s condition to a military dermatologist to review and make a diagnosis or suggest a treatment from a different location at a later time. Using this platform to provide asynchronous teledermatology services avoids the logistics required to schedule appointments and promotes convenience and more efficient use of physicians’ time and resources.
Given the ease of use of S&F systems among military practitioners, dermatology became one of the most heavily utilized teleconsultation specialties within the Army Knowledge Online system, accounting for 40% of the 10,817 consultations initiated from April 2004 to December 2012.5 It also is important to note that skin conditions historically account for 15% to 75% of outpatient visits during wartime; therefore, there is a need for dermatologic consultations, as primary care providers typically are responsible for providing dermatologic care to these patients.6 Because of the high demand for and low volume of US military dermatologists, the use of teledermatology (ie, Amy Knowledge Online) in the US military became a helpful educational tool and specialist extender for many primary care providers in the military.
Teledermatology in the military has evolved to not only provide timely and efficient care but also to reduce health care costs.
Advances in Teledermatology
While the military continues to use S&F teleconsultations—a model in which a deployed referring clinician sends information to a military dermatologist for diagnosis and/or management recommendations—a number of teledermatology programs have been developed for civilians that provide additional advantages over standard face-to-face dermatology care. The advantages of S&F teledermatology applications are many, including faster communication with dermatology providers, diagnostic concordance comparable to face-to-face appointments, cost-effective care for patients, the ability to educate providers remotely,8 and similar outcomes to in-person care.9 However, as to be expected, in-person care remains the gold standard, especially when diagnostic accuracy depends on biopsy findings.
The development of the smartphone along with advances in digital photography and consumer-friendly mobile applications has allowed for the emergence of direct-to-consumer (DTC) teledermatology applications. Regardless of the user’s ability, the quality of photographs taken with smartphones has improved, as standard features such as high-resolution cameras with image stabilization, automatic focus, and lighting have become commonplace. The popularity of smartphone technology also has increased, with nearly 75% of all adults and more than 90% of adults younger than 35 years of age owning a smartphone according to a 2016 survey.11
In 2015, there were at least 29 DTC teledermatology applications available on various mobile platforms,12 accounting for an estimated 1.25 million teleconsultations with providers.13 Teledermatology platforms such as DermatologistOnCall and Spruce Health have made accessing dermatologic care convenient, timely, and affordable for patients via patient-friendly mobile applications.
Regular access to dermatologic care is especially important for patients who have chronic skin conditions. Several unique practice models have emerged as innovative solutions to providing more convenient and timely care. For example, Curology (https://curology.com) is an online teledermatology practice specializing in acne treatment.
Although DTC teledermatology practices are convenient for many patients and providers, some have been criticized for providing poor quality of care12 or facilitating fragmented care by not integrating with established electronic health record (EHR) systems.15 As a result, recommended practice guidelines for DTC teledermatology have been developed by the American Academy of Dermatology and some state medical boards.16 Moreover, several EHR systems, such as Epic (www.epic.com) and Modernizing Medicine’s EMA (www.modmed.com), have developed fully integrated S&F teledermatology platforms to be incorporated with established brick-and-mortar care.17
The Future of Teledermatology in the Military
The Army Knowledge Online telemedicine platform used by the US military has continued to be useful, particularly when treating patients in remote locations, and shows promise for improving routine domestic dermatology care. It has reduced the number of medical evacuations and improved care for those who do not have access to a dermatologist.4 Furthermore, one study noted that most consultations submitted via teledermatology applications from a combat zone received a diagnosis and treatment recommendation from a military dermatologist faster than they would have stateside, where the wait often is 4 to 8 weeks. On average, a teledermatology consultation from Afghanistan was answered in less than 6 hours.4 Although this response time might not be realistic for all dermatology practices, there clearly is potential in certain situations and utilizing certain models of care to diagnose and treat more patients more efficiently utilizing teledermatology applications than in an in-person office visit. A review of 658 teledermatology consultations in the US military from January 2011 to December 2012 revealed that the leading diagnoses were eczematous dermatitis (14%), contact dermatitis (9%), nonmelanoma skin cancer (5%), psoriasis (4%), and urticaria (4%).4 Increased use of teledermatology evaluation of these conditions in routine US-based military practice could help expedite care, decrease patient travel time, and utilize in-clinic dermatologist time more efficiently. Teledermatology visits for postoperative concerns also have demonstrated utility and convenience for triage and management of patients in the civilian setting and may be an additional novel use of teledermatology in the military setting.18 With the use of an integrated S&F teledermatology platform within an existing EHR system that is paired with a secure patient mobile application that allows easy upload of photos, medical history, and messaging, it can be argued that quality of life could greatly be enhanced for both military patients and providers.
Limitations of Teledermatology
Certainly, there are and will always be limitations to teledermatology. Even as digital photography improves, the quality and context of clinical images are user dependent, and key associated skin findings in other locations of the body can be missed. The ability to palpate the skin also is lacking in virtual encounters. Therefore, teledermatology might be considered most appropriate for specific diseases and conditions (eg, acne, psoriasis, eczema). Embracing teledermatology does not mean replacing in-person care; rather, it should be seen as an adjunct used to manage the high demand for dermatology expertise in military and civilian practice. For the US military, the promise and potential to embrace innovation in providing dermatologic care is there, as long as there are leaders to continue to champion it. In the current state of health care, many of the perceived barriers of teledermatology applications have already been overcome, including lack of training, lack of reimbursement, and perceived medicolegal risks.19
The US Federal Government is a large entity, and it will undoubtedly take time and effort to implement new and innovative programs such as the ones described here in the military. The first step in implementation is awareness that the possibilities exist; then, with the cooperation of dermatologists and support from the chain of command, it will be possible to incorporate advances in teledermatology and cultivate new ones.
Final Thoughts
The S&F teledermatology method used in the military setting has become commonplace in both military and civilian settings alike. Newer innovations in telemedicine, particularly in teledermatology, will continue to shape the future of military and civilian medicine for years to come.
Telemedicine arose from the need to provide critical and timely advice directly to health care providers and patients in remote or resource-scarce settings. Whether by radio, telephone, or other means of telecommunication technology, the US military has long utilized telemedicine. What started as a way to expedite the delivery of emergency consultations and medical expertise to remote populations in need has since evolved into a billion-dollar innovation industry that is poised to improve health care efficiency and access to specialist care as well as to lower health care costs for all patients.
Teledermatology in the Military
A primary mission of military medicine is to keep service members anywhere in the world in good health on the job during training, combat, and humanitarian operations.1 Telemedicine greatly supports this mission by bringing the expertise of medical specialists to service members in the field without the cost or risks of travel for physicians. Telemedicine also is effective in promoting timely triage of patients and administration of the most appropriate levels of care. With the advent and globalization of high-speed wireless networks, advancements in telemedicine continue to develop and are becoming increasingly useful in military medicine.
As a specialty, dermatology is heavily reliant on visual information and therefore is particularly amenable to telemedicine applications. The rising popularity of such services has led to the development of the term teledermatology. While early teledermatology services were provided using radio, telephone, fax, and videoconferencing,2 three distinct visual methods typically are used today, including (1) store-and-forward (S&F), (2) live-interactive, and (3) a hybrid of the two.3 Military dermatology predominantly utilizes an S&F system, as still photographs of lesions generally are preferred over video for more focused visualization.
In 2004, the US Army Medical Department established a centralized telemedicine program using Army Knowledge Online,1 an S&F system that allows providers in remote locations to store and forward information about a patient’s clinical history along with digital photographs of the patient’s condition to a military dermatologist to review and make a diagnosis or suggest a treatment from a different location at a later time. Using this platform to provide asynchronous teledermatology services avoids the logistics required to schedule appointments and promotes convenience and more efficient use of physicians’ time and resources.
Given the ease of use of S&F systems among military practitioners, dermatology became one of the most heavily utilized teleconsultation specialties within the Army Knowledge Online system, accounting for 40% of the 10,817 consultations initiated from April 2004 to December 2012.5 It also is important to note that skin conditions historically account for 15% to 75% of outpatient visits during wartime; therefore, there is a need for dermatologic consultations, as primary care providers typically are responsible for providing dermatologic care to these patients.6 Because of the high demand for and low volume of US military dermatologists, the use of teledermatology (ie, Amy Knowledge Online) in the US military became a helpful educational tool and specialist extender for many primary care providers in the military.
Teledermatology in the military has evolved to not only provide timely and efficient care but also to reduce health care costs.
Advances in Teledermatology
While the military continues to use S&F teleconsultations—a model in which a deployed referring clinician sends information to a military dermatologist for diagnosis and/or management recommendations—a number of teledermatology programs have been developed for civilians that provide additional advantages over standard face-to-face dermatology care. The advantages of S&F teledermatology applications are many, including faster communication with dermatology providers, diagnostic concordance comparable to face-to-face appointments, cost-effective care for patients, the ability to educate providers remotely,8 and similar outcomes to in-person care.9 However, as to be expected, in-person care remains the gold standard, especially when diagnostic accuracy depends on biopsy findings.
The development of the smartphone along with advances in digital photography and consumer-friendly mobile applications has allowed for the emergence of direct-to-consumer (DTC) teledermatology applications. Regardless of the user’s ability, the quality of photographs taken with smartphones has improved, as standard features such as high-resolution cameras with image stabilization, automatic focus, and lighting have become commonplace. The popularity of smartphone technology also has increased, with nearly 75% of all adults and more than 90% of adults younger than 35 years of age owning a smartphone according to a 2016 survey.11
In 2015, there were at least 29 DTC teledermatology applications available on various mobile platforms,12 accounting for an estimated 1.25 million teleconsultations with providers.13 Teledermatology platforms such as DermatologistOnCall and Spruce Health have made accessing dermatologic care convenient, timely, and affordable for patients via patient-friendly mobile applications.
Regular access to dermatologic care is especially important for patients who have chronic skin conditions. Several unique practice models have emerged as innovative solutions to providing more convenient and timely care. For example, Curology (https://curology.com) is an online teledermatology practice specializing in acne treatment.
Although DTC teledermatology practices are convenient for many patients and providers, some have been criticized for providing poor quality of care12 or facilitating fragmented care by not integrating with established electronic health record (EHR) systems.15 As a result, recommended practice guidelines for DTC teledermatology have been developed by the American Academy of Dermatology and some state medical boards.16 Moreover, several EHR systems, such as Epic (www.epic.com) and Modernizing Medicine’s EMA (www.modmed.com), have developed fully integrated S&F teledermatology platforms to be incorporated with established brick-and-mortar care.17
The Future of Teledermatology in the Military
The Army Knowledge Online telemedicine platform used by the US military has continued to be useful, particularly when treating patients in remote locations, and shows promise for improving routine domestic dermatology care. It has reduced the number of medical evacuations and improved care for those who do not have access to a dermatologist.4 Furthermore, one study noted that most consultations submitted via teledermatology applications from a combat zone received a diagnosis and treatment recommendation from a military dermatologist faster than they would have stateside, where the wait often is 4 to 8 weeks. On average, a teledermatology consultation from Afghanistan was answered in less than 6 hours.4 Although this response time might not be realistic for all dermatology practices, there clearly is potential in certain situations and utilizing certain models of care to diagnose and treat more patients more efficiently utilizing teledermatology applications than in an in-person office visit. A review of 658 teledermatology consultations in the US military from January 2011 to December 2012 revealed that the leading diagnoses were eczematous dermatitis (14%), contact dermatitis (9%), nonmelanoma skin cancer (5%), psoriasis (4%), and urticaria (4%).4 Increased use of teledermatology evaluation of these conditions in routine US-based military practice could help expedite care, decrease patient travel time, and utilize in-clinic dermatologist time more efficiently. Teledermatology visits for postoperative concerns also have demonstrated utility and convenience for triage and management of patients in the civilian setting and may be an additional novel use of teledermatology in the military setting.18 With the use of an integrated S&F teledermatology platform within an existing EHR system that is paired with a secure patient mobile application that allows easy upload of photos, medical history, and messaging, it can be argued that quality of life could greatly be enhanced for both military patients and providers.
Limitations of Teledermatology
Certainly, there are and will always be limitations to teledermatology. Even as digital photography improves, the quality and context of clinical images are user dependent, and key associated skin findings in other locations of the body can be missed. The ability to palpate the skin also is lacking in virtual encounters. Therefore, teledermatology might be considered most appropriate for specific diseases and conditions (eg, acne, psoriasis, eczema). Embracing teledermatology does not mean replacing in-person care; rather, it should be seen as an adjunct used to manage the high demand for dermatology expertise in military and civilian practice. For the US military, the promise and potential to embrace innovation in providing dermatologic care is there, as long as there are leaders to continue to champion it. In the current state of health care, many of the perceived barriers of teledermatology applications have already been overcome, including lack of training, lack of reimbursement, and perceived medicolegal risks.19
The US Federal Government is a large entity, and it will undoubtedly take time and effort to implement new and innovative programs such as the ones described here in the military. The first step in implementation is awareness that the possibilities exist; then, with the cooperation of dermatologists and support from the chain of command, it will be possible to incorporate advances in teledermatology and cultivate new ones.
Final Thoughts
The S&F teledermatology method used in the military setting has become commonplace in both military and civilian settings alike. Newer innovations in telemedicine, particularly in teledermatology, will continue to shape the future of military and civilian medicine for years to come.
- Vidmar DA. The history of teledermatology in the Department of Defense. Dermatol Clin. 1999;17:113-124.
- McManus J, Salinas J, Morton M, et al. Teleconsultation program for deployed soldiers and healthcare professionals in remote and austere environments. Prehosp Disaster Med. 2008;23:210-216.
- Tensen E, Van Der Heijden JP, Jaspers MW, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the U.S. military in a deployed setting. Mil Med. 2014;179:1347-1353.
- McGraw TA, Norton SA. Military aeromedical evacuations from central and southwest Asia for ill-defined dermatologic diseases. Arch Dermatol. 2009;145:165-170.
- Shissel DJ, Wilde J. Operational dermatology. Mil Med. 2004;169:444-447.
- Henning JS, Wohltmann W, Hivnor C. Teledermatology from a combat zone. Arch Dermatol. 2010;146:676-677.
- Whited JD, Hall RP, Simel DL, et al. Reliability and accuracy of dermatologists’ clinic-based and digital image consultations. J Am Acad Dermatol. 1999;41:693-702.
- Pak H, Triplett CA, Lindquist JH, et al. Store-and-forward teledermatology results in similar clinical outcomes to conventional clinic-based care. J Telemed Telecare. 2007;13:26-30.
- Finnane A, Dallest K, Janda M, et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327.
- Poushter J. Smartphone ownership and internet usage continues to climb in emerging economies. Washington, DC: Pew Research Center. www.pewglobal.org/2016/02/22/smartphone-ownership-and-internet-usage-continues-to-climbin-emerging-economies/. Published February 22, 2016. Accessed February 2, 2018.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Huff C. Medical diagnosis by webcam? Washington, DC: American Association of Retired Persons. www.aarp.org/health/conditions-treatments/info-2015/telemedicine-health-symptoms-diagnosis.html. Published December 2015. Accessed February 2, 2018.
- Mehrotra A. The convenience revolution for the treatment of low-acuity conditions. JAMA. 2013;310:35-36.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Teledermatology toolkit. American Academy of Dermatology website. https://www.aad.org/practicecenter/managing-a-practice/teledermatology. Accessed April 24, 2018.
- Carter ZA, Goldman S, Anderson K, et al. Creation of an internalteledermatology store-and-forward system in an existing electronic health record: a pilot study in a safety-net public health and hospital system. JAMA Dermatol. 2017;153:644-650.
- Jeyamohan SR, Moye MS, Srivastava D, et al. Patient-acquired photographs for the management of postoperative concerns. JAMA Dermatol. 2017;153:226-227.
- Edison KE, Dyer JA, Whited JD, et al. Practice gaps. the barriers and the promise of teledermatology. Arch Dermatol. 2012;148:650-651.
- Vidmar DA. The history of teledermatology in the Department of Defense. Dermatol Clin. 1999;17:113-124.
- McManus J, Salinas J, Morton M, et al. Teleconsultation program for deployed soldiers and healthcare professionals in remote and austere environments. Prehosp Disaster Med. 2008;23:210-216.
- Tensen E, Van Der Heijden JP, Jaspers MW, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the U.S. military in a deployed setting. Mil Med. 2014;179:1347-1353.
- McGraw TA, Norton SA. Military aeromedical evacuations from central and southwest Asia for ill-defined dermatologic diseases. Arch Dermatol. 2009;145:165-170.
- Shissel DJ, Wilde J. Operational dermatology. Mil Med. 2004;169:444-447.
- Henning JS, Wohltmann W, Hivnor C. Teledermatology from a combat zone. Arch Dermatol. 2010;146:676-677.
- Whited JD, Hall RP, Simel DL, et al. Reliability and accuracy of dermatologists’ clinic-based and digital image consultations. J Am Acad Dermatol. 1999;41:693-702.
- Pak H, Triplett CA, Lindquist JH, et al. Store-and-forward teledermatology results in similar clinical outcomes to conventional clinic-based care. J Telemed Telecare. 2007;13:26-30.
- Finnane A, Dallest K, Janda M, et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327.
- Poushter J. Smartphone ownership and internet usage continues to climb in emerging economies. Washington, DC: Pew Research Center. www.pewglobal.org/2016/02/22/smartphone-ownership-and-internet-usage-continues-to-climbin-emerging-economies/. Published February 22, 2016. Accessed February 2, 2018.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Huff C. Medical diagnosis by webcam? Washington, DC: American Association of Retired Persons. www.aarp.org/health/conditions-treatments/info-2015/telemedicine-health-symptoms-diagnosis.html. Published December 2015. Accessed February 2, 2018.
- Mehrotra A. The convenience revolution for the treatment of low-acuity conditions. JAMA. 2013;310:35-36.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Teledermatology toolkit. American Academy of Dermatology website. https://www.aad.org/practicecenter/managing-a-practice/teledermatology. Accessed April 24, 2018.
- Carter ZA, Goldman S, Anderson K, et al. Creation of an internalteledermatology store-and-forward system in an existing electronic health record: a pilot study in a safety-net public health and hospital system. JAMA Dermatol. 2017;153:644-650.
- Jeyamohan SR, Moye MS, Srivastava D, et al. Patient-acquired photographs for the management of postoperative concerns. JAMA Dermatol. 2017;153:226-227.
- Edison KE, Dyer JA, Whited JD, et al. Practice gaps. the barriers and the promise of teledermatology. Arch Dermatol. 2012;148:650-651.
Practice Points
- Teledermatology is increasing in its use and applications in both military and civilian medicine.
- The increased availability of high-quality digital photography as a result of smartphone technology lends itself well to store-and-forward (S&F) teledermatology applications.
- In the civilian community, new methods and platforms for teledermatology have been created based largely on those used by the military to maximize access to and efficiency of health care, including secure direct-to-consumer (DTC) mobile applications, live interactive methods, and integrated S&F platforms within electronic health record (EHR) systems.
Smallpox Vaccine Complications: The Dermatologist’s Role in Diagnosis and Management
The practice of variolation, or inoculation of the smallpox virus from a pustule into a healthy person, was described as early as 1500
Immunization
Vaccinia is an orthopoxvirus, distinct from the smallpox virus variola, with cross-protective immunity after infection. The smallpox vaccine that is available today is a second-generation vaccinia virus derived from plaque purification cloning from the first-generation version originally licensed in 1932, which was central to eradication.5 Today’s vaccine is administered using a bifurcated needle to puncture the epidermis 15 times. Ideally, a papule forms at the inoculation site 3 to 5 days later, progresses to a vesicle and then a pustule, and finally crusts and reaches maximum size by day 10. The crust separates from the skin at 14 to 21 days, at which time the virus can no longer be isolated from the wound. United States Department of Defense surveillance of the first 450,000 vaccinated personnel noted 1% of recipients developed cutaneous eruptions beyond the vaccination site, 5% developed a localized rash, and 1% experienced a generalized eruption.2 Adverse reactions included generalized vaccinia, erythema multiforme (EM), autoinoculation (including ocular vaccinia), and contact vaccinia. There were no cases of eczema vaccinatum (EV) or progressive vaccinia (PV) reported, and no deaths were attributed to these initial vaccines.2
Immunologic Response
Vaccinia replicates in keratinocytes, spreading from cell to cell, resulting in necrosis and vesicle formation. Components of both cellular and humoral immune responses are in place by 10 days after immunization. Deficiencies in these responses result in vaccine complications secondary to vaccine escape and replication beyond the inoculation site.6 A helper T cell TH2-predominant cytokine response in atopic individuals is the likely pathogenesis required for the rapid viral spread for EV.7 Similarly, patients with cell-mediated immunity deficiencies cannot sufficiently produce enough cytotoxic T cells to eliminate an established infection, which can result in PV. Despite the effectiveness of intravenous vaccinia immunoglobulins (VIGIVs) when administered to patients with certain vaccine complications, observations that children with severe X-linked agammaglobulinemia (Bruton disease) have normal responses to vaccination suggest that antibody production is least important in viral control.8 Simian models also suggest that B-cell depletion has no impact on lesion dissemination, as lesion size is inversely correlated with T-cell count.9
Eczema Vaccinatum
A national survey estimated the prevalence of eczema in the United States at 31.6 million individuals,10 with 2- to 3-fold increases in incidence since the 1970s.11 Due to the risk for developing EV, the Advisory Committee on Immunization Practices considers personal history of eczema or contact with a family member who has eczema (either currently or in the past) contraindications to nonemergency administration of the vaccine.12,13 However, atopic conditions in general are underrecognized, with only approximately one-third of patients carrying an official diagnosis from a physician.10 Despite a large atopic and vaccinated population, EV remains relatively uncommon at 10 to 39 cases per million vaccines.6
The EV rash classically involves the midface, neck, and antecubital and popliteal fossae but can present in any location. The lesions start as papules that quickly progress to vesicles and pustules with crusting on an erythematous base. Given the extent of denudation of the epidermis, impetiginization can occur. Death rates as high as 30% have been reported14 but have only occurred in instances of secondary contact transmission with no deaths occurring in the primary vaccinees.15 In a case published in 2008, a 2-year-old boy developed the first documented EV case under the new program after exposure to his father’s predeployment vaccine.16 A similar rash is shown in Figure 1 with notable vesicles and pustules. The child required burn patient–type management, VIGIV, and treatment with cidofovir and an investigational antiorthopox agent. He was discharged from the hospital after 48 days without sequelae or considerable scarring.16 If a family member has a contraindication barring secondary contact with the vaccine, the US Department of Defense’s policy defers vaccination in active-duty members until they reach their deployment destination, at which point the inoculation is administered.
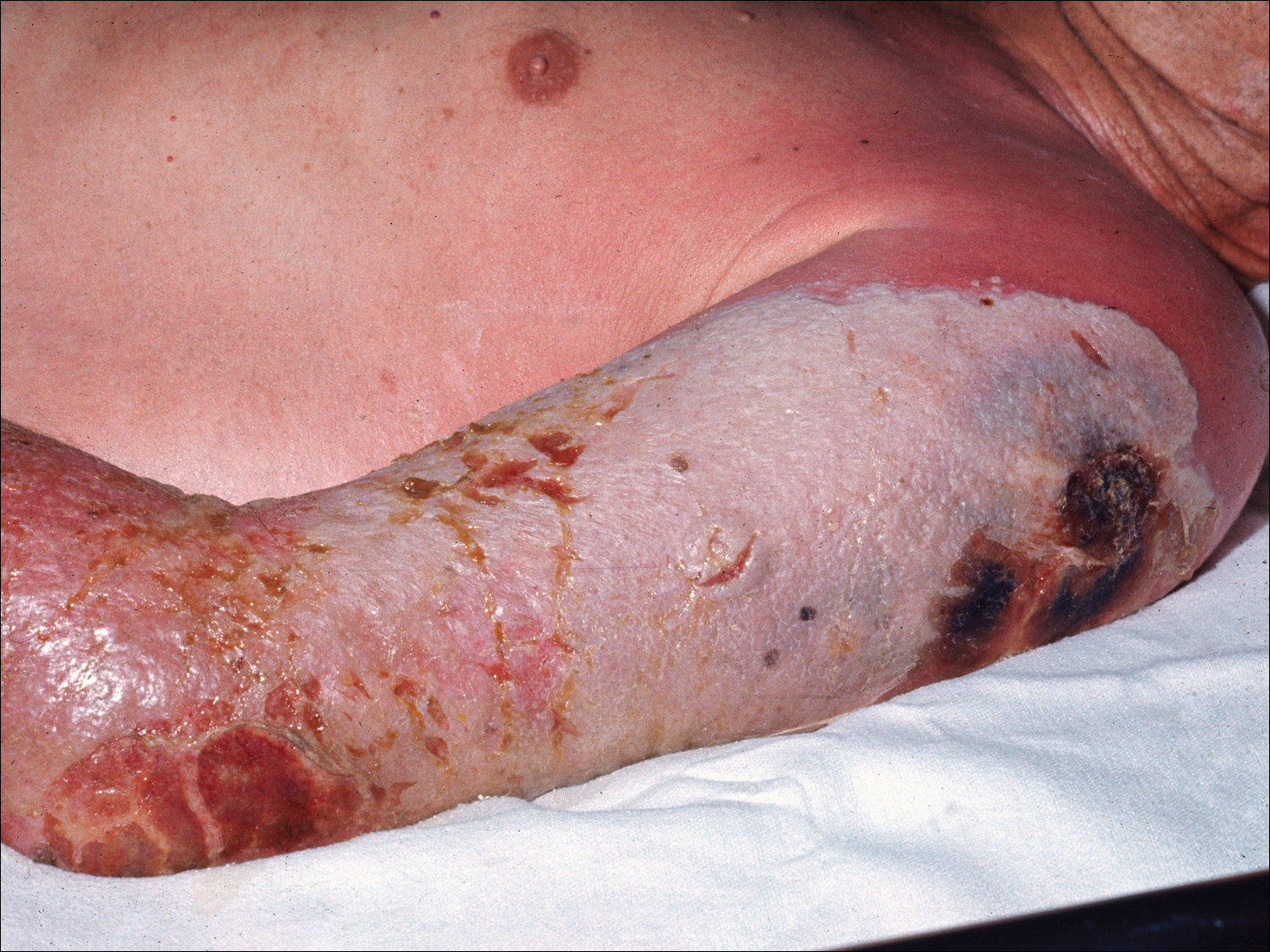
Progressive Vaccinia
Progressive vaccinia is also known as vaccinia necrosum or vaccinia gangrenosum. It is a dreaded but uncommon complication, occurring once in every 1 million vaccinations. It carries an overall case fatality rate of 15%,17 but it nearly always is fatal in patients with severe T-cell defects.18 Progressive vaccinia occurs exclusively in patients with cell-mediated immunodeficiency, with the severity of the acute illness correlating with the severity of immunodeficiency. In patients with cell-mediated immunodeficiency but intact humoral immunity, progression can be limited to expansion of the lesion, as it is thought that antibody production restricts viremia.18 Progressive vaccinia should be suspected in a patient if the vaccine site shows no signs of improvement by 14 days.19 The PV lesions do not heal and may progress or recur in patients with signs of prior healing. The leading edge has confluent vesicles, and the center of the lesion develops necrosis with thick black eschar formation. Most specifically, there is no surrounding inflammation; however, inflammation can develop later as a response to treatment or secondary infection. Figure 2 shows a PV lesion with black eschar and a transition to intact dermis without inflammation.
The first known case of PV since the 1960s vaccination campaign occurred in an active-duty Marine vaccinated with vaccinia before a diagnosis of acute myelogenous leukemia was recognized 2 weeks later.19 The vaccine site was stable in size and crusted when he received neutropenia-inducing chemotherapy 6.5 weeks after vaccination. The site then progressed in a manner typical for PV with central necrosis and a lack of inflammation at the expanding painless wound edge.19 This classic appearance with progression of satellite lesions prompted the treatment team to obtain wound and serum samples, which yielded the orthopox virus from polymerase chain reaction and viral culture. He required 2 months of care in an intensive care unit and received treatment with topical imiquimod, VIGIV, a topical and intravenous antiorthopox agent, and a second investigational antiorthopox agent; the patient ultimately survived.17,20
Generalized Vaccinia
Generalized vaccinia (GV) typically is a benign vaccine complication resulting from viremic spread from the initial inoculation site and is most commonly seen in healthy patients. Generalized vaccinia is only life threatening in immunocompromised patients. The incidence of GV is 23.4 to 241.5 patients per million vaccines.6 The majority of GV cases occur 5 to 12 days after vaccination when small distant pustules or vesicles appear on any part of the body, including the palms and soles. The lesions usually are smaller than the primary vaccination site and resolve more quickly. Generalized vaccinia can have a few to several hundred pocks, though the rash is rarely as diffuse as EV presentations.3 Given that EV can present diffusely on skin unaffected by atopic dermatitis, GV can be difficult to distinguish from EV. Features more common to EV include more systemically ill patients, increased numbers of lesions, and lesions that become confluent in an atopic distribution. It has been suggested that GV can be differentiated from vesicular or vesiculopapular EM because GV does not develop flaccid bullae and EM typically has targetoid lesions.18 Mild GV disease requires no treatment, but VIGIV can be used in more extensive cases.
Localized Reactions Due to Viral Replication
Accidental autoinoculation can occur when patients touch the vaccination site and then themselves, transferring virus particles to areas of compromised skin integrity, most commonly on the face, eyes, hands, genitalia, anus, or any other broken skin. Autoinoculation happens with some frequency and is of limited clinical concern unless there is ocular involvement. Keratitis develops in 6% of ocular vaccinia cases, and VIGIV is contraindicated, as rabbit models suggest that antigen-antibody precipitates in the cornea can cause scarring.21 Instead, trifluorothymidine is an effective topical treatment available for ocular vaccinia.
A robust response or “take” is defined as a reaction having redness, swelling, and warmth more than 3 inches in diameter at the inoculation site, peaking 6 to 12 days after inoculation with spontaneous regression occurring 1 to 3 days after.22,23 A robust take frequently is of concern to the clinician, as it can be difficult to discern from secondary infection. Secondary infections are uncommon, and a robust take is secondary to viral, not bacterial, cellulitis. Unfortunately, there are no diagnostics that have utility in distinguishing between the two, and the decision to administer empiric antibiotics might be unavoidable in light of the consequences of an untreated, rapidly progressive bacterial cellulitis. Milder cases in the setting of no constitutional symptoms could be safely monitored if close follow-up is assured.
Generalized Skin Reactions Without Viral Replication
Development of erythematous, pruritic, urticarial, and diffuse targetlike lesions of EM is common in first-time vaccinees. Often misdiagnosed as GV, EM is an immunologically mediated, not virally mediated, process. The most common infectious cause prompting EM is herpes simplex virus type 1. In the setting of a live-virus vaccine, it is difficult to determine if the vaccine prompted herpes simplex virus type 1 viral shedding and associated EM or if the vaccinia vaccine is more directly the cause of EM.24 Symptoms typically are mild, but more severe reactions may require treatment with corticosteroids. Stevens-Johnson syndrome with a severe bullous eruption has been linked to vaccinia24 but fortunately is rare. Morbilliform eruptions, urticaria, and angioedema also can occur.
Final Thoughts
Given current world events and ongoing bioterrorism threats, the smallpox vaccine program continues indefinitely. With a brisk military deployment tempo, a larger population of new vaccinees naturally will yield more cutaneous reactions. Military members, civilian health care workers, and members of the National Guard and National Reserves will develop complications and present to dermatologists for care. The historical pool of providers accustomed to seeing these complications from the 1960s eradication campaign is scant. Military and civilian dermatologists alike are uniquely poised to be the experts on protean manifestations of vaccinia reactions.
- Voigt EA, Kennedy RB, Poland GA. Defending against smallpox: a focus on vaccines. Expert Rev Vaccines. 2016;15:1197-1211.
- Grabenstein J, Wikenwerder W Jr. US military smallpox vaccination program experience. JAMA. 2003;289:3278-3282.
- Kelly CD, Egan C, Davis SW, et al. Laboratory confirmation of generalized vaccinia following smallpox vaccination. J Clin Microbiol. 2004;42:1373-1375.
- Slike BM, Creegan M, Marovich M, et al. Humoral immunity to primary smallpox vaccination: impact of childhood versus adult immunization on vaccinia vector vaccine development in military populations. PLoS One. 2017;12:E0169247.
- Notice to readers: newly licensed vaccine to replace old smallpox vaccine. MMWR. 2008;57:207-208.
- Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;58:101-114.
- Engler R, Kenner J, Leung D. Smallpox vaccination: risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357-365.
- Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36:766-774.
- Gordon S, Cecchinato V, Andresen V, et al. Smallpox vaccine safety is dependent on T cells and not B cells. J Infect Dis. 2011;203:1043-1053.
- Hanifin J, Reed M. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;82:82-91.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52:1-16.
- Petersen BW, Harms TJ, Reynolds MG, et al. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupation exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunizations Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016;65:257-262.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Eczema Vaccinatum. Eczema vaccinatum as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection analysis, and presentation of immunization safety data. Vaccine. 2007:25;5725-5734.
- Aragón TJ, Ulrich S, Fernyak S, et al. Risks of serious complications and death from smallpox vaccination: a systematic review of the United States experience, 1963-1968. BMC Public Health. 2003;3:26.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555-1561.
- Centers for Disease Control and Prevention (CDC). Progressive vaccinia in a military smallpox vaccinee—United States 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Fulginiti VA, Papier A, Lane M, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Progressive Vaccinia. Progressive vaccinia as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5735-5744.
- Lederman ER, Davidson W, Groff HL, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:E1372-E1385.
- Lane ML, Goldstein J. Adverse events occurring after smallpox vaccination. Semin Ped Infect Dis. 2003;14:189-195.
- Vaccine adverse events. CDC website. http://www.cdc.gov/smallpox/clinicians/vaccine-adverse-events5.html. Accessed January 3, 2018.
- Cono J, Casey CG, Bell DM. Smallpox vaccination and adversereactions, guidance for clinicians. CDC website. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Accessed January 3, 2018.
- Rosenblatt AE, Stein SL. Cutaneous reactions to vaccinations. Clin Dermatol. 2015;33:327-332.
The practice of variolation, or inoculation of the smallpox virus from a pustule into a healthy person, was described as early as 1500
Immunization
Vaccinia is an orthopoxvirus, distinct from the smallpox virus variola, with cross-protective immunity after infection. The smallpox vaccine that is available today is a second-generation vaccinia virus derived from plaque purification cloning from the first-generation version originally licensed in 1932, which was central to eradication.5 Today’s vaccine is administered using a bifurcated needle to puncture the epidermis 15 times. Ideally, a papule forms at the inoculation site 3 to 5 days later, progresses to a vesicle and then a pustule, and finally crusts and reaches maximum size by day 10. The crust separates from the skin at 14 to 21 days, at which time the virus can no longer be isolated from the wound. United States Department of Defense surveillance of the first 450,000 vaccinated personnel noted 1% of recipients developed cutaneous eruptions beyond the vaccination site, 5% developed a localized rash, and 1% experienced a generalized eruption.2 Adverse reactions included generalized vaccinia, erythema multiforme (EM), autoinoculation (including ocular vaccinia), and contact vaccinia. There were no cases of eczema vaccinatum (EV) or progressive vaccinia (PV) reported, and no deaths were attributed to these initial vaccines.2
Immunologic Response
Vaccinia replicates in keratinocytes, spreading from cell to cell, resulting in necrosis and vesicle formation. Components of both cellular and humoral immune responses are in place by 10 days after immunization. Deficiencies in these responses result in vaccine complications secondary to vaccine escape and replication beyond the inoculation site.6 A helper T cell TH2-predominant cytokine response in atopic individuals is the likely pathogenesis required for the rapid viral spread for EV.7 Similarly, patients with cell-mediated immunity deficiencies cannot sufficiently produce enough cytotoxic T cells to eliminate an established infection, which can result in PV. Despite the effectiveness of intravenous vaccinia immunoglobulins (VIGIVs) when administered to patients with certain vaccine complications, observations that children with severe X-linked agammaglobulinemia (Bruton disease) have normal responses to vaccination suggest that antibody production is least important in viral control.8 Simian models also suggest that B-cell depletion has no impact on lesion dissemination, as lesion size is inversely correlated with T-cell count.9
Eczema Vaccinatum
A national survey estimated the prevalence of eczema in the United States at 31.6 million individuals,10 with 2- to 3-fold increases in incidence since the 1970s.11 Due to the risk for developing EV, the Advisory Committee on Immunization Practices considers personal history of eczema or contact with a family member who has eczema (either currently or in the past) contraindications to nonemergency administration of the vaccine.12,13 However, atopic conditions in general are underrecognized, with only approximately one-third of patients carrying an official diagnosis from a physician.10 Despite a large atopic and vaccinated population, EV remains relatively uncommon at 10 to 39 cases per million vaccines.6
The EV rash classically involves the midface, neck, and antecubital and popliteal fossae but can present in any location. The lesions start as papules that quickly progress to vesicles and pustules with crusting on an erythematous base. Given the extent of denudation of the epidermis, impetiginization can occur. Death rates as high as 30% have been reported14 but have only occurred in instances of secondary contact transmission with no deaths occurring in the primary vaccinees.15 In a case published in 2008, a 2-year-old boy developed the first documented EV case under the new program after exposure to his father’s predeployment vaccine.16 A similar rash is shown in Figure 1 with notable vesicles and pustules. The child required burn patient–type management, VIGIV, and treatment with cidofovir and an investigational antiorthopox agent. He was discharged from the hospital after 48 days without sequelae or considerable scarring.16 If a family member has a contraindication barring secondary contact with the vaccine, the US Department of Defense’s policy defers vaccination in active-duty members until they reach their deployment destination, at which point the inoculation is administered.

Progressive Vaccinia
Progressive vaccinia is also known as vaccinia necrosum or vaccinia gangrenosum. It is a dreaded but uncommon complication, occurring once in every 1 million vaccinations. It carries an overall case fatality rate of 15%,17 but it nearly always is fatal in patients with severe T-cell defects.18 Progressive vaccinia occurs exclusively in patients with cell-mediated immunodeficiency, with the severity of the acute illness correlating with the severity of immunodeficiency. In patients with cell-mediated immunodeficiency but intact humoral immunity, progression can be limited to expansion of the lesion, as it is thought that antibody production restricts viremia.18 Progressive vaccinia should be suspected in a patient if the vaccine site shows no signs of improvement by 14 days.19 The PV lesions do not heal and may progress or recur in patients with signs of prior healing. The leading edge has confluent vesicles, and the center of the lesion develops necrosis with thick black eschar formation. Most specifically, there is no surrounding inflammation; however, inflammation can develop later as a response to treatment or secondary infection. Figure 2 shows a PV lesion with black eschar and a transition to intact dermis without inflammation.

The first known case of PV since the 1960s vaccination campaign occurred in an active-duty Marine vaccinated with vaccinia before a diagnosis of acute myelogenous leukemia was recognized 2 weeks later.19 The vaccine site was stable in size and crusted when he received neutropenia-inducing chemotherapy 6.5 weeks after vaccination. The site then progressed in a manner typical for PV with central necrosis and a lack of inflammation at the expanding painless wound edge.19 This classic appearance with progression of satellite lesions prompted the treatment team to obtain wound and serum samples, which yielded the orthopox virus from polymerase chain reaction and viral culture. He required 2 months of care in an intensive care unit and received treatment with topical imiquimod, VIGIV, a topical and intravenous antiorthopox agent, and a second investigational antiorthopox agent; the patient ultimately survived.17,20
Generalized Vaccinia
Generalized vaccinia (GV) typically is a benign vaccine complication resulting from viremic spread from the initial inoculation site and is most commonly seen in healthy patients. Generalized vaccinia is only life threatening in immunocompromised patients. The incidence of GV is 23.4 to 241.5 patients per million vaccines.6 The majority of GV cases occur 5 to 12 days after vaccination when small distant pustules or vesicles appear on any part of the body, including the palms and soles. The lesions usually are smaller than the primary vaccination site and resolve more quickly. Generalized vaccinia can have a few to several hundred pocks, though the rash is rarely as diffuse as EV presentations.3 Given that EV can present diffusely on skin unaffected by atopic dermatitis, GV can be difficult to distinguish from EV. Features more common to EV include more systemically ill patients, increased numbers of lesions, and lesions that become confluent in an atopic distribution. It has been suggested that GV can be differentiated from vesicular or vesiculopapular EM because GV does not develop flaccid bullae and EM typically has targetoid lesions.18 Mild GV disease requires no treatment, but VIGIV can be used in more extensive cases.
Localized Reactions Due to Viral Replication
Accidental autoinoculation can occur when patients touch the vaccination site and then themselves, transferring virus particles to areas of compromised skin integrity, most commonly on the face, eyes, hands, genitalia, anus, or any other broken skin. Autoinoculation happens with some frequency and is of limited clinical concern unless there is ocular involvement. Keratitis develops in 6% of ocular vaccinia cases, and VIGIV is contraindicated, as rabbit models suggest that antigen-antibody precipitates in the cornea can cause scarring.21 Instead, trifluorothymidine is an effective topical treatment available for ocular vaccinia.
A robust response or “take” is defined as a reaction having redness, swelling, and warmth more than 3 inches in diameter at the inoculation site, peaking 6 to 12 days after inoculation with spontaneous regression occurring 1 to 3 days after.22,23 A robust take frequently is of concern to the clinician, as it can be difficult to discern from secondary infection. Secondary infections are uncommon, and a robust take is secondary to viral, not bacterial, cellulitis. Unfortunately, there are no diagnostics that have utility in distinguishing between the two, and the decision to administer empiric antibiotics might be unavoidable in light of the consequences of an untreated, rapidly progressive bacterial cellulitis. Milder cases in the setting of no constitutional symptoms could be safely monitored if close follow-up is assured.
Generalized Skin Reactions Without Viral Replication
Development of erythematous, pruritic, urticarial, and diffuse targetlike lesions of EM is common in first-time vaccinees. Often misdiagnosed as GV, EM is an immunologically mediated, not virally mediated, process. The most common infectious cause prompting EM is herpes simplex virus type 1. In the setting of a live-virus vaccine, it is difficult to determine if the vaccine prompted herpes simplex virus type 1 viral shedding and associated EM or if the vaccinia vaccine is more directly the cause of EM.24 Symptoms typically are mild, but more severe reactions may require treatment with corticosteroids. Stevens-Johnson syndrome with a severe bullous eruption has been linked to vaccinia24 but fortunately is rare. Morbilliform eruptions, urticaria, and angioedema also can occur.
Final Thoughts
Given current world events and ongoing bioterrorism threats, the smallpox vaccine program continues indefinitely. With a brisk military deployment tempo, a larger population of new vaccinees naturally will yield more cutaneous reactions. Military members, civilian health care workers, and members of the National Guard and National Reserves will develop complications and present to dermatologists for care. The historical pool of providers accustomed to seeing these complications from the 1960s eradication campaign is scant. Military and civilian dermatologists alike are uniquely poised to be the experts on protean manifestations of vaccinia reactions.
The practice of variolation, or inoculation of the smallpox virus from a pustule into a healthy person, was described as early as 1500
Immunization
Vaccinia is an orthopoxvirus, distinct from the smallpox virus variola, with cross-protective immunity after infection. The smallpox vaccine that is available today is a second-generation vaccinia virus derived from plaque purification cloning from the first-generation version originally licensed in 1932, which was central to eradication.5 Today’s vaccine is administered using a bifurcated needle to puncture the epidermis 15 times. Ideally, a papule forms at the inoculation site 3 to 5 days later, progresses to a vesicle and then a pustule, and finally crusts and reaches maximum size by day 10. The crust separates from the skin at 14 to 21 days, at which time the virus can no longer be isolated from the wound. United States Department of Defense surveillance of the first 450,000 vaccinated personnel noted 1% of recipients developed cutaneous eruptions beyond the vaccination site, 5% developed a localized rash, and 1% experienced a generalized eruption.2 Adverse reactions included generalized vaccinia, erythema multiforme (EM), autoinoculation (including ocular vaccinia), and contact vaccinia. There were no cases of eczema vaccinatum (EV) or progressive vaccinia (PV) reported, and no deaths were attributed to these initial vaccines.2
Immunologic Response
Vaccinia replicates in keratinocytes, spreading from cell to cell, resulting in necrosis and vesicle formation. Components of both cellular and humoral immune responses are in place by 10 days after immunization. Deficiencies in these responses result in vaccine complications secondary to vaccine escape and replication beyond the inoculation site.6 A helper T cell TH2-predominant cytokine response in atopic individuals is the likely pathogenesis required for the rapid viral spread for EV.7 Similarly, patients with cell-mediated immunity deficiencies cannot sufficiently produce enough cytotoxic T cells to eliminate an established infection, which can result in PV. Despite the effectiveness of intravenous vaccinia immunoglobulins (VIGIVs) when administered to patients with certain vaccine complications, observations that children with severe X-linked agammaglobulinemia (Bruton disease) have normal responses to vaccination suggest that antibody production is least important in viral control.8 Simian models also suggest that B-cell depletion has no impact on lesion dissemination, as lesion size is inversely correlated with T-cell count.9
Eczema Vaccinatum
A national survey estimated the prevalence of eczema in the United States at 31.6 million individuals,10 with 2- to 3-fold increases in incidence since the 1970s.11 Due to the risk for developing EV, the Advisory Committee on Immunization Practices considers personal history of eczema or contact with a family member who has eczema (either currently or in the past) contraindications to nonemergency administration of the vaccine.12,13 However, atopic conditions in general are underrecognized, with only approximately one-third of patients carrying an official diagnosis from a physician.10 Despite a large atopic and vaccinated population, EV remains relatively uncommon at 10 to 39 cases per million vaccines.6
The EV rash classically involves the midface, neck, and antecubital and popliteal fossae but can present in any location. The lesions start as papules that quickly progress to vesicles and pustules with crusting on an erythematous base. Given the extent of denudation of the epidermis, impetiginization can occur. Death rates as high as 30% have been reported14 but have only occurred in instances of secondary contact transmission with no deaths occurring in the primary vaccinees.15 In a case published in 2008, a 2-year-old boy developed the first documented EV case under the new program after exposure to his father’s predeployment vaccine.16 A similar rash is shown in Figure 1 with notable vesicles and pustules. The child required burn patient–type management, VIGIV, and treatment with cidofovir and an investigational antiorthopox agent. He was discharged from the hospital after 48 days without sequelae or considerable scarring.16 If a family member has a contraindication barring secondary contact with the vaccine, the US Department of Defense’s policy defers vaccination in active-duty members until they reach their deployment destination, at which point the inoculation is administered.

Progressive Vaccinia
Progressive vaccinia is also known as vaccinia necrosum or vaccinia gangrenosum. It is a dreaded but uncommon complication, occurring once in every 1 million vaccinations. It carries an overall case fatality rate of 15%,17 but it nearly always is fatal in patients with severe T-cell defects.18 Progressive vaccinia occurs exclusively in patients with cell-mediated immunodeficiency, with the severity of the acute illness correlating with the severity of immunodeficiency. In patients with cell-mediated immunodeficiency but intact humoral immunity, progression can be limited to expansion of the lesion, as it is thought that antibody production restricts viremia.18 Progressive vaccinia should be suspected in a patient if the vaccine site shows no signs of improvement by 14 days.19 The PV lesions do not heal and may progress or recur in patients with signs of prior healing. The leading edge has confluent vesicles, and the center of the lesion develops necrosis with thick black eschar formation. Most specifically, there is no surrounding inflammation; however, inflammation can develop later as a response to treatment or secondary infection. Figure 2 shows a PV lesion with black eschar and a transition to intact dermis without inflammation.

The first known case of PV since the 1960s vaccination campaign occurred in an active-duty Marine vaccinated with vaccinia before a diagnosis of acute myelogenous leukemia was recognized 2 weeks later.19 The vaccine site was stable in size and crusted when he received neutropenia-inducing chemotherapy 6.5 weeks after vaccination. The site then progressed in a manner typical for PV with central necrosis and a lack of inflammation at the expanding painless wound edge.19 This classic appearance with progression of satellite lesions prompted the treatment team to obtain wound and serum samples, which yielded the orthopox virus from polymerase chain reaction and viral culture. He required 2 months of care in an intensive care unit and received treatment with topical imiquimod, VIGIV, a topical and intravenous antiorthopox agent, and a second investigational antiorthopox agent; the patient ultimately survived.17,20
Generalized Vaccinia
Generalized vaccinia (GV) typically is a benign vaccine complication resulting from viremic spread from the initial inoculation site and is most commonly seen in healthy patients. Generalized vaccinia is only life threatening in immunocompromised patients. The incidence of GV is 23.4 to 241.5 patients per million vaccines.6 The majority of GV cases occur 5 to 12 days after vaccination when small distant pustules or vesicles appear on any part of the body, including the palms and soles. The lesions usually are smaller than the primary vaccination site and resolve more quickly. Generalized vaccinia can have a few to several hundred pocks, though the rash is rarely as diffuse as EV presentations.3 Given that EV can present diffusely on skin unaffected by atopic dermatitis, GV can be difficult to distinguish from EV. Features more common to EV include more systemically ill patients, increased numbers of lesions, and lesions that become confluent in an atopic distribution. It has been suggested that GV can be differentiated from vesicular or vesiculopapular EM because GV does not develop flaccid bullae and EM typically has targetoid lesions.18 Mild GV disease requires no treatment, but VIGIV can be used in more extensive cases.
Localized Reactions Due to Viral Replication
Accidental autoinoculation can occur when patients touch the vaccination site and then themselves, transferring virus particles to areas of compromised skin integrity, most commonly on the face, eyes, hands, genitalia, anus, or any other broken skin. Autoinoculation happens with some frequency and is of limited clinical concern unless there is ocular involvement. Keratitis develops in 6% of ocular vaccinia cases, and VIGIV is contraindicated, as rabbit models suggest that antigen-antibody precipitates in the cornea can cause scarring.21 Instead, trifluorothymidine is an effective topical treatment available for ocular vaccinia.
A robust response or “take” is defined as a reaction having redness, swelling, and warmth more than 3 inches in diameter at the inoculation site, peaking 6 to 12 days after inoculation with spontaneous regression occurring 1 to 3 days after.22,23 A robust take frequently is of concern to the clinician, as it can be difficult to discern from secondary infection. Secondary infections are uncommon, and a robust take is secondary to viral, not bacterial, cellulitis. Unfortunately, there are no diagnostics that have utility in distinguishing between the two, and the decision to administer empiric antibiotics might be unavoidable in light of the consequences of an untreated, rapidly progressive bacterial cellulitis. Milder cases in the setting of no constitutional symptoms could be safely monitored if close follow-up is assured.
Generalized Skin Reactions Without Viral Replication
Development of erythematous, pruritic, urticarial, and diffuse targetlike lesions of EM is common in first-time vaccinees. Often misdiagnosed as GV, EM is an immunologically mediated, not virally mediated, process. The most common infectious cause prompting EM is herpes simplex virus type 1. In the setting of a live-virus vaccine, it is difficult to determine if the vaccine prompted herpes simplex virus type 1 viral shedding and associated EM or if the vaccinia vaccine is more directly the cause of EM.24 Symptoms typically are mild, but more severe reactions may require treatment with corticosteroids. Stevens-Johnson syndrome with a severe bullous eruption has been linked to vaccinia24 but fortunately is rare. Morbilliform eruptions, urticaria, and angioedema also can occur.
Final Thoughts
Given current world events and ongoing bioterrorism threats, the smallpox vaccine program continues indefinitely. With a brisk military deployment tempo, a larger population of new vaccinees naturally will yield more cutaneous reactions. Military members, civilian health care workers, and members of the National Guard and National Reserves will develop complications and present to dermatologists for care. The historical pool of providers accustomed to seeing these complications from the 1960s eradication campaign is scant. Military and civilian dermatologists alike are uniquely poised to be the experts on protean manifestations of vaccinia reactions.
- Voigt EA, Kennedy RB, Poland GA. Defending against smallpox: a focus on vaccines. Expert Rev Vaccines. 2016;15:1197-1211.
- Grabenstein J, Wikenwerder W Jr. US military smallpox vaccination program experience. JAMA. 2003;289:3278-3282.
- Kelly CD, Egan C, Davis SW, et al. Laboratory confirmation of generalized vaccinia following smallpox vaccination. J Clin Microbiol. 2004;42:1373-1375.
- Slike BM, Creegan M, Marovich M, et al. Humoral immunity to primary smallpox vaccination: impact of childhood versus adult immunization on vaccinia vector vaccine development in military populations. PLoS One. 2017;12:E0169247.
- Notice to readers: newly licensed vaccine to replace old smallpox vaccine. MMWR. 2008;57:207-208.
- Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;58:101-114.
- Engler R, Kenner J, Leung D. Smallpox vaccination: risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357-365.
- Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36:766-774.
- Gordon S, Cecchinato V, Andresen V, et al. Smallpox vaccine safety is dependent on T cells and not B cells. J Infect Dis. 2011;203:1043-1053.
- Hanifin J, Reed M. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;82:82-91.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52:1-16.
- Petersen BW, Harms TJ, Reynolds MG, et al. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupation exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunizations Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016;65:257-262.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Eczema Vaccinatum. Eczema vaccinatum as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection analysis, and presentation of immunization safety data. Vaccine. 2007:25;5725-5734.
- Aragón TJ, Ulrich S, Fernyak S, et al. Risks of serious complications and death from smallpox vaccination: a systematic review of the United States experience, 1963-1968. BMC Public Health. 2003;3:26.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555-1561.
- Centers for Disease Control and Prevention (CDC). Progressive vaccinia in a military smallpox vaccinee—United States 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Fulginiti VA, Papier A, Lane M, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Progressive Vaccinia. Progressive vaccinia as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5735-5744.
- Lederman ER, Davidson W, Groff HL, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:E1372-E1385.
- Lane ML, Goldstein J. Adverse events occurring after smallpox vaccination. Semin Ped Infect Dis. 2003;14:189-195.
- Vaccine adverse events. CDC website. http://www.cdc.gov/smallpox/clinicians/vaccine-adverse-events5.html. Accessed January 3, 2018.
- Cono J, Casey CG, Bell DM. Smallpox vaccination and adversereactions, guidance for clinicians. CDC website. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Accessed January 3, 2018.
- Rosenblatt AE, Stein SL. Cutaneous reactions to vaccinations. Clin Dermatol. 2015;33:327-332.
- Voigt EA, Kennedy RB, Poland GA. Defending against smallpox: a focus on vaccines. Expert Rev Vaccines. 2016;15:1197-1211.
- Grabenstein J, Wikenwerder W Jr. US military smallpox vaccination program experience. JAMA. 2003;289:3278-3282.
- Kelly CD, Egan C, Davis SW, et al. Laboratory confirmation of generalized vaccinia following smallpox vaccination. J Clin Microbiol. 2004;42:1373-1375.
- Slike BM, Creegan M, Marovich M, et al. Humoral immunity to primary smallpox vaccination: impact of childhood versus adult immunization on vaccinia vector vaccine development in military populations. PLoS One. 2017;12:E0169247.
- Notice to readers: newly licensed vaccine to replace old smallpox vaccine. MMWR. 2008;57:207-208.
- Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;58:101-114.
- Engler R, Kenner J, Leung D. Smallpox vaccination: risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357-365.
- Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36:766-774.
- Gordon S, Cecchinato V, Andresen V, et al. Smallpox vaccine safety is dependent on T cells and not B cells. J Infect Dis. 2011;203:1043-1053.
- Hanifin J, Reed M. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;82:82-91.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52:1-16.
- Petersen BW, Harms TJ, Reynolds MG, et al. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupation exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunizations Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016;65:257-262.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Eczema Vaccinatum. Eczema vaccinatum as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection analysis, and presentation of immunization safety data. Vaccine. 2007:25;5725-5734.
- Aragón TJ, Ulrich S, Fernyak S, et al. Risks of serious complications and death from smallpox vaccination: a systematic review of the United States experience, 1963-1968. BMC Public Health. 2003;3:26.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555-1561.
- Centers for Disease Control and Prevention (CDC). Progressive vaccinia in a military smallpox vaccinee—United States 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Fulginiti VA, Papier A, Lane M, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Progressive Vaccinia. Progressive vaccinia as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5735-5744.
- Lederman ER, Davidson W, Groff HL, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:E1372-E1385.
- Lane ML, Goldstein J. Adverse events occurring after smallpox vaccination. Semin Ped Infect Dis. 2003;14:189-195.
- Vaccine adverse events. CDC website. http://www.cdc.gov/smallpox/clinicians/vaccine-adverse-events5.html. Accessed January 3, 2018.
- Cono J, Casey CG, Bell DM. Smallpox vaccination and adversereactions, guidance for clinicians. CDC website. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Accessed January 3, 2018.
- Rosenblatt AE, Stein SL. Cutaneous reactions to vaccinations. Clin Dermatol. 2015;33:327-332.
Practice Points
- Dermatologists should be aware that smallpox vaccinations are being administered to patients and may present with a myriad of cutaneous complications.
- Progressive vaccinia should be suspected if a smallpox inoculation has not healed after 14 days and, most specifically, if there is no inflammation surrounding the site.
- Generalized vaccinia generally is a benign condition seen in otherwise healthy patients and usually requires no treatment.
Atopic patients should be educated to avoid receiving routine smallpox vaccinations if they would be considered at risk for requiring the inoculation.