User login
The Essential Guide to Estate Planning for Physicians: Securing Your Legacy and Protecting Your Wealth
As a physician, you’ve spent years building a career that not only provides financial security for your family but also allows you to make a meaningful impact in your community. However, without a comprehensive estate plan in place, much of what you’ve worked so hard to build may not be preserved according to your wishes.
Many physicians delay estate planning, assuming it’s something to consider later in life. However, the most successful estate plans are those that are established early and evolve over time. Proper planning ensures that your assets are protected, your loved ones are provided for, and your legacy is preserved in the most tax-efficient and legally-sound manner possible.1
This article explores why estate planning is particularly crucial for physicians, the key elements of a strong estate plan, and how beginning early can create long-term financial advantages.
Why Estate Planning Matters for Physicians
Physicians are in a unique financial position compared to many other professionals. With high earning potential, specialized assets, and significant liability exposure, their estate planning needs differ from those of the average individual. A well-structured estate plan not only facilitates the smooth transfer of wealth but also protects assets from excessive taxation, legal complications, and potential risks such as malpractice claims.
1. High Net-Worth Considerations
Physicians often accumulate substantial wealth over time. Without a clear estate plan, your estate could face excessive taxation, with a large portion of your assets potentially going to the government rather than your heirs. Estate taxes, probate costs, and legal fees can significantly erode your legacy if not properly planned for.
2. Asset Protection from Liability Risks
Unlike most professionals, physicians are at a higher risk of litigation. A comprehensive estate plan can incorporate asset protection strategies, such as irrevocable trusts, family limited partnerships, or liability insurance, to shield your wealth from lawsuits or creditor claims.
3. Family and Generational Wealth Planning
Many physicians prioritize ensuring their family’s financial stability. Whether you want to provide for your spouse, children, or even charitable causes, estate planning allows you to dictate how your wealth is distributed. Establishing trusts for your children or grandchildren can help manage how and when they receive their inheritance, preventing mismanagement and ensuring financial responsibility.
4. Business and Practice Continuity
If you own a medical practice, succession planning should be part of your estate plan. Without clear directives, the future of your practice may be uncertain in the event of your passing or incapacitation. A well-drafted estate plan provides a roadmap for ownership transition, ensuring continuity for patients, employees, and business partners.
Key Elements of an Effective Estate Plan
Every estate plan should be customized based on your financial situation, goals, and family dynamics. However, certain fundamental components apply to nearly all high-net-worth individuals, including physicians.
1. Revocable Living Trusts
A revocable living trust allows you to manage your assets during your lifetime while providing a clear path for distribution after your passing. Unlike a will, a trust helps your estate avoid probate, ensuring a smoother and more private transition of wealth. You maintain control over your assets while also establishing clear rules for distribution, particularly useful if you have minor children or complex family structures.2
2. Irrevocable Trusts for Asset Protection
For physicians concerned about lawsuits or estate tax exposure, irrevocable trusts can offer robust asset protection. Since assets placed in these trusts are no longer legally owned by you, they are shielded from creditors and legal claims while also reducing your taxable estate.2
3. Powers of Attorney and Healthcare Directives
Estate planning isn’t just about what happens after your passing—it’s also about protecting you and your family if you become incapacitated. A durable power of attorney allows a trusted individual to manage your financial affairs, while a healthcare directive ensures your medical decisions align with your wishes.3
4. Life Insurance Planning
Life insurance is an essential estate planning tool for physicians, providing liquidity to cover estate taxes, debts, or income replacement for your family. A properly structured life insurance trust can help ensure that policy proceeds remain outside of your taxable estate while being efficiently distributed according to your wishes.4
5. Business Succession Planning
If you own a medical practice, a well-designed succession plan can ensure that your business continues to operate smoothly in your absence. This may involve buy-sell agreements, key-person insurance, or identifying a successor to take over your role.5
The Long-Term Benefits of Early Estate Planning
Estate planning is not a one-time event—it’s a process that should evolve with your career, financial growth, and family dynamics. The earlier you begin, the more control you have over your financial future. Here’s why starting early is a strategic advantage:
1. Maximizing Tax Efficiency
Many estate planning strategies, such as gifting assets or establishing irrevocable trusts, are most effective when implemented over time. By spreading out wealth transfers and taking advantage of annual gift exclusions, you can significantly reduce estate tax liability while maintaining financial security.
2. Adjusting for Life Changes
Your financial situation and family needs will change over the years. Marriages, births, career advancements, and new investments all impact your estate planning needs. By starting early, you can make gradual adjustments rather than facing an overwhelming restructuring later in life.1
3. Ensuring Asset Protection Strategies Are in Place
Many asset protection strategies require time to be effective. For instance, certain types of trusts must be in place for a number of years before they fully shield assets from legal claims. Delaying planning could leave your wealth unnecessarily exposed.
4. Creating a Legacy Beyond Wealth
Estate planning is not just about finances—it’s about legacy. Whether you want to support a charitable cause, endow a scholarship, or establish a foundation, early planning gives you the ability to shape your long-term impact.
5. Adapt to Ever Changing Legislation
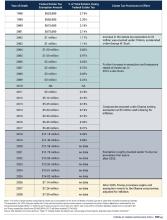
Estate planning needs to be adaptable. The federal government can change the estate tax exemption at any time; this was even a topic of the last election cycle. Early planning allows you to implement necessary changes throughout your life to minimize estate taxes. At present, unless new policy is enacted, the exemption per individual will reduce by half in 2026 (see Figure 1).
Final Thoughts: Taking Action Today
The complexity of physician finances—ranging from high income and significant assets to legal risks—makes individualized estate planning an absolute necessity.
By taking proactive steps today, you can maximize tax efficiency, safeguard your assets, and ensure your wishes are carried out without unnecessary delays or legal battles. Working with a financial advisor and estate planning attorney who understands the unique needs of physicians can help you craft a plan that aligns with your goals and evolves as your career progresses.
Mr. Gardner is a financial advisor at Lifetime Financial Growth, LLC, in Columbus, Ohio, one of the largest privately held wealth management firms in the country. John has had a passion for finance since his early years in college when his tennis coach introduced him. He also has a passion for helping physicians, as his wife is a gastroenterologist at Ohio State University. He reports no relevant disclosures relevant to this article. If you have additional questions, please contact John at 740-403-4891 or john_s_gardner@glic.com.
References
1. The Law Offices of Diron Rutty, LLC. https://www.dironruttyllc.com/reasons-to-start-estate-planning-early/.
2. Physician Side Gigs. https://www.physiciansidegigs.com/estateplanning.
3. Afshar, A & MacBeth, S. https://www.schwabe.com/publication/estate-planning-for-physicians-why-its-important-and-how-to-get-started/. December 2024.
4. Skeeles, JC. https://ohioline.osu.edu/factsheet/ep-1. July 2012.
5. Rosenfeld, J. Physician estate planning guide. Medical Economics. 2022 Nov. https://www.medicaleconomics.com/view/physician-estate-planning-guide.
As a physician, you’ve spent years building a career that not only provides financial security for your family but also allows you to make a meaningful impact in your community. However, without a comprehensive estate plan in place, much of what you’ve worked so hard to build may not be preserved according to your wishes.
Many physicians delay estate planning, assuming it’s something to consider later in life. However, the most successful estate plans are those that are established early and evolve over time. Proper planning ensures that your assets are protected, your loved ones are provided for, and your legacy is preserved in the most tax-efficient and legally-sound manner possible.1
This article explores why estate planning is particularly crucial for physicians, the key elements of a strong estate plan, and how beginning early can create long-term financial advantages.
Why Estate Planning Matters for Physicians
Physicians are in a unique financial position compared to many other professionals. With high earning potential, specialized assets, and significant liability exposure, their estate planning needs differ from those of the average individual. A well-structured estate plan not only facilitates the smooth transfer of wealth but also protects assets from excessive taxation, legal complications, and potential risks such as malpractice claims.
1. High Net-Worth Considerations
Physicians often accumulate substantial wealth over time. Without a clear estate plan, your estate could face excessive taxation, with a large portion of your assets potentially going to the government rather than your heirs. Estate taxes, probate costs, and legal fees can significantly erode your legacy if not properly planned for.
2. Asset Protection from Liability Risks
Unlike most professionals, physicians are at a higher risk of litigation. A comprehensive estate plan can incorporate asset protection strategies, such as irrevocable trusts, family limited partnerships, or liability insurance, to shield your wealth from lawsuits or creditor claims.
3. Family and Generational Wealth Planning
Many physicians prioritize ensuring their family’s financial stability. Whether you want to provide for your spouse, children, or even charitable causes, estate planning allows you to dictate how your wealth is distributed. Establishing trusts for your children or grandchildren can help manage how and when they receive their inheritance, preventing mismanagement and ensuring financial responsibility.
4. Business and Practice Continuity
If you own a medical practice, succession planning should be part of your estate plan. Without clear directives, the future of your practice may be uncertain in the event of your passing or incapacitation. A well-drafted estate plan provides a roadmap for ownership transition, ensuring continuity for patients, employees, and business partners.
Key Elements of an Effective Estate Plan
Every estate plan should be customized based on your financial situation, goals, and family dynamics. However, certain fundamental components apply to nearly all high-net-worth individuals, including physicians.
1. Revocable Living Trusts
A revocable living trust allows you to manage your assets during your lifetime while providing a clear path for distribution after your passing. Unlike a will, a trust helps your estate avoid probate, ensuring a smoother and more private transition of wealth. You maintain control over your assets while also establishing clear rules for distribution, particularly useful if you have minor children or complex family structures.2
2. Irrevocable Trusts for Asset Protection
For physicians concerned about lawsuits or estate tax exposure, irrevocable trusts can offer robust asset protection. Since assets placed in these trusts are no longer legally owned by you, they are shielded from creditors and legal claims while also reducing your taxable estate.2
3. Powers of Attorney and Healthcare Directives
Estate planning isn’t just about what happens after your passing—it’s also about protecting you and your family if you become incapacitated. A durable power of attorney allows a trusted individual to manage your financial affairs, while a healthcare directive ensures your medical decisions align with your wishes.3
4. Life Insurance Planning
Life insurance is an essential estate planning tool for physicians, providing liquidity to cover estate taxes, debts, or income replacement for your family. A properly structured life insurance trust can help ensure that policy proceeds remain outside of your taxable estate while being efficiently distributed according to your wishes.4
5. Business Succession Planning
If you own a medical practice, a well-designed succession plan can ensure that your business continues to operate smoothly in your absence. This may involve buy-sell agreements, key-person insurance, or identifying a successor to take over your role.5
The Long-Term Benefits of Early Estate Planning
Estate planning is not a one-time event—it’s a process that should evolve with your career, financial growth, and family dynamics. The earlier you begin, the more control you have over your financial future. Here’s why starting early is a strategic advantage:
1. Maximizing Tax Efficiency
Many estate planning strategies, such as gifting assets or establishing irrevocable trusts, are most effective when implemented over time. By spreading out wealth transfers and taking advantage of annual gift exclusions, you can significantly reduce estate tax liability while maintaining financial security.
2. Adjusting for Life Changes
Your financial situation and family needs will change over the years. Marriages, births, career advancements, and new investments all impact your estate planning needs. By starting early, you can make gradual adjustments rather than facing an overwhelming restructuring later in life.1
3. Ensuring Asset Protection Strategies Are in Place
Many asset protection strategies require time to be effective. For instance, certain types of trusts must be in place for a number of years before they fully shield assets from legal claims. Delaying planning could leave your wealth unnecessarily exposed.
4. Creating a Legacy Beyond Wealth
Estate planning is not just about finances—it’s about legacy. Whether you want to support a charitable cause, endow a scholarship, or establish a foundation, early planning gives you the ability to shape your long-term impact.
5. Adapt to Ever Changing Legislation
Estate planning needs to be adaptable. The federal government can change the estate tax exemption at any time; this was even a topic of the last election cycle. Early planning allows you to implement necessary changes throughout your life to minimize estate taxes. At present, unless new policy is enacted, the exemption per individual will reduce by half in 2026 (see Figure 1).
Final Thoughts: Taking Action Today
The complexity of physician finances—ranging from high income and significant assets to legal risks—makes individualized estate planning an absolute necessity.
By taking proactive steps today, you can maximize tax efficiency, safeguard your assets, and ensure your wishes are carried out without unnecessary delays or legal battles. Working with a financial advisor and estate planning attorney who understands the unique needs of physicians can help you craft a plan that aligns with your goals and evolves as your career progresses.
Mr. Gardner is a financial advisor at Lifetime Financial Growth, LLC, in Columbus, Ohio, one of the largest privately held wealth management firms in the country. John has had a passion for finance since his early years in college when his tennis coach introduced him. He also has a passion for helping physicians, as his wife is a gastroenterologist at Ohio State University. He reports no relevant disclosures relevant to this article. If you have additional questions, please contact John at 740-403-4891 or john_s_gardner@glic.com.
References
1. The Law Offices of Diron Rutty, LLC. https://www.dironruttyllc.com/reasons-to-start-estate-planning-early/.
2. Physician Side Gigs. https://www.physiciansidegigs.com/estateplanning.
3. Afshar, A & MacBeth, S. https://www.schwabe.com/publication/estate-planning-for-physicians-why-its-important-and-how-to-get-started/. December 2024.
4. Skeeles, JC. https://ohioline.osu.edu/factsheet/ep-1. July 2012.
5. Rosenfeld, J. Physician estate planning guide. Medical Economics. 2022 Nov. https://www.medicaleconomics.com/view/physician-estate-planning-guide.
As a physician, you’ve spent years building a career that not only provides financial security for your family but also allows you to make a meaningful impact in your community. However, without a comprehensive estate plan in place, much of what you’ve worked so hard to build may not be preserved according to your wishes.
Many physicians delay estate planning, assuming it’s something to consider later in life. However, the most successful estate plans are those that are established early and evolve over time. Proper planning ensures that your assets are protected, your loved ones are provided for, and your legacy is preserved in the most tax-efficient and legally-sound manner possible.1
This article explores why estate planning is particularly crucial for physicians, the key elements of a strong estate plan, and how beginning early can create long-term financial advantages.
Why Estate Planning Matters for Physicians
Physicians are in a unique financial position compared to many other professionals. With high earning potential, specialized assets, and significant liability exposure, their estate planning needs differ from those of the average individual. A well-structured estate plan not only facilitates the smooth transfer of wealth but also protects assets from excessive taxation, legal complications, and potential risks such as malpractice claims.
1. High Net-Worth Considerations
Physicians often accumulate substantial wealth over time. Without a clear estate plan, your estate could face excessive taxation, with a large portion of your assets potentially going to the government rather than your heirs. Estate taxes, probate costs, and legal fees can significantly erode your legacy if not properly planned for.
2. Asset Protection from Liability Risks
Unlike most professionals, physicians are at a higher risk of litigation. A comprehensive estate plan can incorporate asset protection strategies, such as irrevocable trusts, family limited partnerships, or liability insurance, to shield your wealth from lawsuits or creditor claims.
3. Family and Generational Wealth Planning
Many physicians prioritize ensuring their family’s financial stability. Whether you want to provide for your spouse, children, or even charitable causes, estate planning allows you to dictate how your wealth is distributed. Establishing trusts for your children or grandchildren can help manage how and when they receive their inheritance, preventing mismanagement and ensuring financial responsibility.
4. Business and Practice Continuity
If you own a medical practice, succession planning should be part of your estate plan. Without clear directives, the future of your practice may be uncertain in the event of your passing or incapacitation. A well-drafted estate plan provides a roadmap for ownership transition, ensuring continuity for patients, employees, and business partners.
Key Elements of an Effective Estate Plan
Every estate plan should be customized based on your financial situation, goals, and family dynamics. However, certain fundamental components apply to nearly all high-net-worth individuals, including physicians.
1. Revocable Living Trusts
A revocable living trust allows you to manage your assets during your lifetime while providing a clear path for distribution after your passing. Unlike a will, a trust helps your estate avoid probate, ensuring a smoother and more private transition of wealth. You maintain control over your assets while also establishing clear rules for distribution, particularly useful if you have minor children or complex family structures.2
2. Irrevocable Trusts for Asset Protection
For physicians concerned about lawsuits or estate tax exposure, irrevocable trusts can offer robust asset protection. Since assets placed in these trusts are no longer legally owned by you, they are shielded from creditors and legal claims while also reducing your taxable estate.2
3. Powers of Attorney and Healthcare Directives
Estate planning isn’t just about what happens after your passing—it’s also about protecting you and your family if you become incapacitated. A durable power of attorney allows a trusted individual to manage your financial affairs, while a healthcare directive ensures your medical decisions align with your wishes.3
4. Life Insurance Planning
Life insurance is an essential estate planning tool for physicians, providing liquidity to cover estate taxes, debts, or income replacement for your family. A properly structured life insurance trust can help ensure that policy proceeds remain outside of your taxable estate while being efficiently distributed according to your wishes.4
5. Business Succession Planning
If you own a medical practice, a well-designed succession plan can ensure that your business continues to operate smoothly in your absence. This may involve buy-sell agreements, key-person insurance, or identifying a successor to take over your role.5
The Long-Term Benefits of Early Estate Planning
Estate planning is not a one-time event—it’s a process that should evolve with your career, financial growth, and family dynamics. The earlier you begin, the more control you have over your financial future. Here’s why starting early is a strategic advantage:
1. Maximizing Tax Efficiency
Many estate planning strategies, such as gifting assets or establishing irrevocable trusts, are most effective when implemented over time. By spreading out wealth transfers and taking advantage of annual gift exclusions, you can significantly reduce estate tax liability while maintaining financial security.
2. Adjusting for Life Changes
Your financial situation and family needs will change over the years. Marriages, births, career advancements, and new investments all impact your estate planning needs. By starting early, you can make gradual adjustments rather than facing an overwhelming restructuring later in life.1
3. Ensuring Asset Protection Strategies Are in Place
Many asset protection strategies require time to be effective. For instance, certain types of trusts must be in place for a number of years before they fully shield assets from legal claims. Delaying planning could leave your wealth unnecessarily exposed.
4. Creating a Legacy Beyond Wealth
Estate planning is not just about finances—it’s about legacy. Whether you want to support a charitable cause, endow a scholarship, or establish a foundation, early planning gives you the ability to shape your long-term impact.
5. Adapt to Ever Changing Legislation
Estate planning needs to be adaptable. The federal government can change the estate tax exemption at any time; this was even a topic of the last election cycle. Early planning allows you to implement necessary changes throughout your life to minimize estate taxes. At present, unless new policy is enacted, the exemption per individual will reduce by half in 2026 (see Figure 1).
Final Thoughts: Taking Action Today
The complexity of physician finances—ranging from high income and significant assets to legal risks—makes individualized estate planning an absolute necessity.
By taking proactive steps today, you can maximize tax efficiency, safeguard your assets, and ensure your wishes are carried out without unnecessary delays or legal battles. Working with a financial advisor and estate planning attorney who understands the unique needs of physicians can help you craft a plan that aligns with your goals and evolves as your career progresses.
Mr. Gardner is a financial advisor at Lifetime Financial Growth, LLC, in Columbus, Ohio, one of the largest privately held wealth management firms in the country. John has had a passion for finance since his early years in college when his tennis coach introduced him. He also has a passion for helping physicians, as his wife is a gastroenterologist at Ohio State University. He reports no relevant disclosures relevant to this article. If you have additional questions, please contact John at 740-403-4891 or john_s_gardner@glic.com.
References
1. The Law Offices of Diron Rutty, LLC. https://www.dironruttyllc.com/reasons-to-start-estate-planning-early/.
2. Physician Side Gigs. https://www.physiciansidegigs.com/estateplanning.
3. Afshar, A & MacBeth, S. https://www.schwabe.com/publication/estate-planning-for-physicians-why-its-important-and-how-to-get-started/. December 2024.
4. Skeeles, JC. https://ohioline.osu.edu/factsheet/ep-1. July 2012.
5. Rosenfeld, J. Physician estate planning guide. Medical Economics. 2022 Nov. https://www.medicaleconomics.com/view/physician-estate-planning-guide.
Improving Care for Patients from Historically Minoritized and Marginalized Communities with Disorders of Gut-Brain Interaction
Introduction: Cases
Patient 1: A 57-year-old man with post-prandial distress variant functional dyspepsia (FD) was recommended to start nortriptyline. He previously established primary care with a physician he met at a barbershop health fair in Harlem, who referred him for specialty evaluation. Today, he presents for follow-up and reports he did not take this medication because he heard it is an antidepressant. How would you counsel him?
Patient 2: A 61-year-old woman was previously diagnosed with mixed variant irritable bowel syndrome (IBS-M). Her symptoms have not significantly changed. Her prior workup has been reassuring and consistent with IBS-M. Despite this, the patient pushes to repeat a colonoscopy, fearful that something is being missed or that she is not being offered care because of her undocumented status. How do you respond?
Patient 3: A 36-year-old man is followed for the management of generalized anxiety disorder and functional heartburn. He was started on low-dose amitriptyline with some benefit, but follow-up has been sporadic. On further discussion, he reports financial stressors, time barriers, and difficulty scheduling a meeting with his union representative for work accommodations as he lives in a more rural community. How do you reply?
Patient 4: A 74-year-old man with Parkinson’s disease who uses a wheelchair has functional constipation that is well controlled on his current regimen. He has never undergone colon cancer screening. He occasionally notices blood in his stool, so a colonoscopy was recommended to confirm that his hematochezia reflects functional constipation complicated by hemorrhoids. He is concerned about the bowel preparation required for a colonoscopy given his limited mobility, as his insurance does not cover assistance at home. He does not have family members to help him. How can you assist him?
Social determinants of health, health disparities, and DGBIs
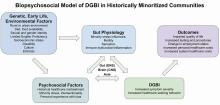
Social determinants of health affect all aspects of patient care, with an increasing body of published work looking at potential disparities in organ-based and structural diseases.1,2,3,4 However, little has been done to explore their influence on disorders of gut-brain interaction or DGBIs.
. As DGBIs cannot be diagnosed with a single laboratory or endoscopic test, the patient history is of the utmost importance and physician-patient rapport is paramount in their treatment. Such rapport may be more difficult to establish in patients coming from historically marginalized and minoritized communities who may be distrustful of healthcare as an institution of (discriminatory) power.
Potential DGBI management pitfalls in historically marginalized or minoritized communities
For racial and ethnic minorities in the United States, disparities in healthcare take on many forms. People from racial and ethnic minority communities are less likely to receive a gastroenterology consultation and those with IBS are more likely to undergo procedures as compared to White patients with IBS.6 Implicit bias may lead to fewer specialist referrals, and specialty care may be limited or unavailable in some areas. Patients may prefer seeing providers in their own community, with whom they share racial or ethnic identities, which could lead to fewer referrals to specialists outside of the community.
Historical discrimination contributes to a lack of trust in healthcare professionals, which may lead patients to favor more objective diagnostics such as endoscopy or view being counseled against invasive procedures as having necessary care denied. Due to a broader cultural stigma surrounding mental illness, patients may be more hesitant to utilize neuromodulators, which have historically been used for psychiatric diagnoses, as it may lead them to conflate their GI illness with mental illness.7,8
Since DGBIs cannot be diagnosed with a single test or managed with a single treatment modality, providing excellent care for patients with DGBIs requires clear communication. For patients with limited English proficiency (LEP), access to high-quality language assistance is the foundation of comprehensive care. Interpreter use (or lack thereof) may limit the ability to obtain a complete and accurate clinical history, which can lead to fewer referrals to specialists and increased reliance on endoscopic evaluations that may not be clinically indicated.
These language barriers affect patients on many levels – in their ability to understand instructions for medication administration, preparation for procedures, and return precautions – which may ultimately lead to poorer responses to therapy or delays in care. LEP alone is broadly associated with fewer referrals for outpatient follow-up, adverse health outcomes and complications, and longer hospital stays.9 These disparities can be mitigated by investing in high-quality interpreter services, providing instructions and forms in multiple languages, and engaging the patient’s family and social supports according to their preferences.
People experiencing poverty (urban and rural) face challenges across multiple domains including access to healthcare, health insurance, stable housing and employment, and more. Many patients seek care at federally qualified health centers, which may face greater difficulties coordinating care with external gastroenterologists.10
Insurance barriers limit access to essential medications, tests, and procedures, and create delays in establishing care with specialists. Significant psychological stress and higher rates of comorbid anxiety and depression contribute to increased IBS severity.11 Financial limitations may limit dietary choices, which can further exacerbate DGBI symptoms. Long work hours with limited flexibility may prohibit them from presenting for regular follow-ups and establishing advanced DGBI care such as with a dietitian or psychologist.
Patients with disabilities face many of the health inequities previously discussed, as well as additional challenges with physical accessibility, transportation, exclusion from education and employment, discrimination, and stigma. Higher prevalence of comorbid mental illness and higher rates of intimate partner violence and interpersonal violence all contribute to DGBI severity and challenges with access to care.12,13 Patients with disabilities may struggle to arrive at appointments, maneuver through the building or exam room, and ultimately follow recommended care plans.
How to approach DGBIs in historically marginalized and minoritized communities
Returning to the patients from the introduction, how would you counsel each of them?
Patient 1: We can discuss with the patient how nortriptyline and other typical antidepressants can and often are used for indications other than depression. These medications modify centrally-mediated pain signaling and many patients with functional dyspepsia experience a significant benefit. It is critical to build on the rapport that was established at the community health outreach event and to explore the patient’s concerns thoroughly.
Patient 2: We would begin by inquiring about her underlying fears associated with her symptoms and seek to understand her goals for repeat intervention. We can review the risks of endoscopy and shift the focus to improving her symptoms. If we can improve her bowel habits or her pain, her desire for further interventions may lessen.
Patient 3: It will be important to work within the realistic time and monetary constraints in this patient’s life. We can validate him and the challenges he is facing, provide positive reinforcement for the progress he has made so far, and avoid disparaging him for the aspects of the treatment plan he has been unable to follow through with. As he reported a benefit from amitriptyline, we can consider increasing his dose as a feasible next step.
Patient 4: We can encourage the patient to discuss with his primary care physician how they may be able to coordinate an inpatient admission for colonoscopy preparation. Given his co-morbidities, this avenue will provide him dedicated support to help him adequately prep to ensure a higher quality examination and limit the need for repeat procedures.
DGBI care in historically marginalized and minoritized communities: A call to action
Understanding cultural differences and existing disparities in care is essential to improving care for patients from historically minoritized communities with DGBIs. Motivational interviewing and shared decision-making, with acknowledgment of social and cultural differences, allow us to work together with patients and their support systems to set and achieve feasible goals.14
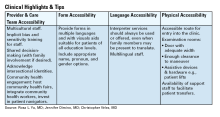
To address known health disparities, offices can take steps to ensure the accessibility of language, forms, physical space, providers, and care teams. Providing culturally sensitive care and lowering barriers to care are the first steps to effecting meaningful change for patients with DGBIs from historically minoritized communities.
Dr. Yu is based at Division of Gastroenterology and Hepatology, Boston Medical Center and Boston University, both in Boston, Massachusetts. Dr. Dimino and Dr. Vélez are based at the Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston, Massachusetts. Dr. Yu, Dr. Dimino, and Dr. Vélez do not have any conflicts of interest for this article.
Additional Online Resources
Form Accessibility
- Intake Form Guidance for Providers
- Making Your Clinic Welcoming to LGBTQ Patients
- Transgender data collection in the electronic health record: Current concepts and issues
Language Accessibility
Physical Accessibility
- Access to Medical Care for Individuals with Mobility Disabilities
- Making your medical office accessible
References
1. Zavala VA, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021 Jan. doi: 10.1038/s41416-020-01038-6.
2. Kardashian A, et al. Health disparities in chronic liver disease. Hepatology. 2023 Apr. doi: 10.1002/hep.32743.
3. Nephew LD, Serper M. Racial, Gender, and Socioeconomic Disparities in Liver Transplantation. Liver Transpl. 2021 Jun. doi: 10.1002/lt.25996.
4. Anyane-Yeboa A, et al. The Impact of the Social Determinants of Health on Disparities in Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2022 Nov. doi: 10.1016/j.cgh.2022.03.011.
5. Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. 2016 Feb. doi: 10.1053/j.gastro.2016.02.032.
6. Silvernale C, et al. Racial disparity in healthcare utilization among patients with Irritable Bowel Syndrome: results from a multicenter cohort. Neurogastroenterol Motil. 2021 May. doi: 10.1111/nmo.14039.
7. Hearn M, et al. Stigma and irritable bowel syndrome: a taboo subject? Lancet Gastroenterol Hepatol. 2020 Jun. doi: 10.1016/S2468-1253(19)30348-6.
8. Yan XJ, et al. The impact of stigma on medication adherence in patients with functional dyspepsia. Neurogastroenterol Motil. 2021 Feb. doi: 10.1111/nmo.13956.
9. Twersky SE, et al. The Impact of Limited English Proficiency on Healthcare Access and Outcomes in the U.S.: A Scoping Review. Healthcare (Basel). 2024 Jan. doi: 10.3390/healthcare12030364.
10. Bayly JE, et al. Limited English proficiency and reported receipt of colorectal cancer screening among adults 45-75 in 2019 and 2021. Prev Med Rep. 2024 Feb. doi: 10.1016/j.pmedr.2024.102638.
11. Cheng K, et al. Epidemiology of Irritable Bowel Syndrome in a Large Academic Safety-Net Hospital. J Clin Med. 2024 Feb. doi: 10.3390/jcm13051314.
12. Breiding MJ, Armour BS. The association between disability and intimate partner violence in the United States. Ann Epidemiol. 2015 Jun. doi: 10.1016/j.annepidem.2015.03.017.
13. Mitra M, et al. Prevalence and characteristics of sexual violence against men with disabilities. Am J Prev Med. 2016 Mar. doi: 10.1016/j.amepre.2015.07.030.
14. Bahafzallah L, et al. Motivational Interviewing in Ethnic Populations. J Immigr Minor Health. 2020 Aug. doi: 10.1007/s10903-019-00940-3.
Introduction: Cases
Patient 1: A 57-year-old man with post-prandial distress variant functional dyspepsia (FD) was recommended to start nortriptyline. He previously established primary care with a physician he met at a barbershop health fair in Harlem, who referred him for specialty evaluation. Today, he presents for follow-up and reports he did not take this medication because he heard it is an antidepressant. How would you counsel him?
Patient 2: A 61-year-old woman was previously diagnosed with mixed variant irritable bowel syndrome (IBS-M). Her symptoms have not significantly changed. Her prior workup has been reassuring and consistent with IBS-M. Despite this, the patient pushes to repeat a colonoscopy, fearful that something is being missed or that she is not being offered care because of her undocumented status. How do you respond?
Patient 3: A 36-year-old man is followed for the management of generalized anxiety disorder and functional heartburn. He was started on low-dose amitriptyline with some benefit, but follow-up has been sporadic. On further discussion, he reports financial stressors, time barriers, and difficulty scheduling a meeting with his union representative for work accommodations as he lives in a more rural community. How do you reply?
Patient 4: A 74-year-old man with Parkinson’s disease who uses a wheelchair has functional constipation that is well controlled on his current regimen. He has never undergone colon cancer screening. He occasionally notices blood in his stool, so a colonoscopy was recommended to confirm that his hematochezia reflects functional constipation complicated by hemorrhoids. He is concerned about the bowel preparation required for a colonoscopy given his limited mobility, as his insurance does not cover assistance at home. He does not have family members to help him. How can you assist him?
Social determinants of health, health disparities, and DGBIs
Social determinants of health affect all aspects of patient care, with an increasing body of published work looking at potential disparities in organ-based and structural diseases.1,2,3,4 However, little has been done to explore their influence on disorders of gut-brain interaction or DGBIs.
. As DGBIs cannot be diagnosed with a single laboratory or endoscopic test, the patient history is of the utmost importance and physician-patient rapport is paramount in their treatment. Such rapport may be more difficult to establish in patients coming from historically marginalized and minoritized communities who may be distrustful of healthcare as an institution of (discriminatory) power.
Potential DGBI management pitfalls in historically marginalized or minoritized communities
For racial and ethnic minorities in the United States, disparities in healthcare take on many forms. People from racial and ethnic minority communities are less likely to receive a gastroenterology consultation and those with IBS are more likely to undergo procedures as compared to White patients with IBS.6 Implicit bias may lead to fewer specialist referrals, and specialty care may be limited or unavailable in some areas. Patients may prefer seeing providers in their own community, with whom they share racial or ethnic identities, which could lead to fewer referrals to specialists outside of the community.
Historical discrimination contributes to a lack of trust in healthcare professionals, which may lead patients to favor more objective diagnostics such as endoscopy or view being counseled against invasive procedures as having necessary care denied. Due to a broader cultural stigma surrounding mental illness, patients may be more hesitant to utilize neuromodulators, which have historically been used for psychiatric diagnoses, as it may lead them to conflate their GI illness with mental illness.7,8
Since DGBIs cannot be diagnosed with a single test or managed with a single treatment modality, providing excellent care for patients with DGBIs requires clear communication. For patients with limited English proficiency (LEP), access to high-quality language assistance is the foundation of comprehensive care. Interpreter use (or lack thereof) may limit the ability to obtain a complete and accurate clinical history, which can lead to fewer referrals to specialists and increased reliance on endoscopic evaluations that may not be clinically indicated.
These language barriers affect patients on many levels – in their ability to understand instructions for medication administration, preparation for procedures, and return precautions – which may ultimately lead to poorer responses to therapy or delays in care. LEP alone is broadly associated with fewer referrals for outpatient follow-up, adverse health outcomes and complications, and longer hospital stays.9 These disparities can be mitigated by investing in high-quality interpreter services, providing instructions and forms in multiple languages, and engaging the patient’s family and social supports according to their preferences.
People experiencing poverty (urban and rural) face challenges across multiple domains including access to healthcare, health insurance, stable housing and employment, and more. Many patients seek care at federally qualified health centers, which may face greater difficulties coordinating care with external gastroenterologists.10
Insurance barriers limit access to essential medications, tests, and procedures, and create delays in establishing care with specialists. Significant psychological stress and higher rates of comorbid anxiety and depression contribute to increased IBS severity.11 Financial limitations may limit dietary choices, which can further exacerbate DGBI symptoms. Long work hours with limited flexibility may prohibit them from presenting for regular follow-ups and establishing advanced DGBI care such as with a dietitian or psychologist.
Patients with disabilities face many of the health inequities previously discussed, as well as additional challenges with physical accessibility, transportation, exclusion from education and employment, discrimination, and stigma. Higher prevalence of comorbid mental illness and higher rates of intimate partner violence and interpersonal violence all contribute to DGBI severity and challenges with access to care.12,13 Patients with disabilities may struggle to arrive at appointments, maneuver through the building or exam room, and ultimately follow recommended care plans.
How to approach DGBIs in historically marginalized and minoritized communities
Returning to the patients from the introduction, how would you counsel each of them?
Patient 1: We can discuss with the patient how nortriptyline and other typical antidepressants can and often are used for indications other than depression. These medications modify centrally-mediated pain signaling and many patients with functional dyspepsia experience a significant benefit. It is critical to build on the rapport that was established at the community health outreach event and to explore the patient’s concerns thoroughly.
Patient 2: We would begin by inquiring about her underlying fears associated with her symptoms and seek to understand her goals for repeat intervention. We can review the risks of endoscopy and shift the focus to improving her symptoms. If we can improve her bowel habits or her pain, her desire for further interventions may lessen.
Patient 3: It will be important to work within the realistic time and monetary constraints in this patient’s life. We can validate him and the challenges he is facing, provide positive reinforcement for the progress he has made so far, and avoid disparaging him for the aspects of the treatment plan he has been unable to follow through with. As he reported a benefit from amitriptyline, we can consider increasing his dose as a feasible next step.
Patient 4: We can encourage the patient to discuss with his primary care physician how they may be able to coordinate an inpatient admission for colonoscopy preparation. Given his co-morbidities, this avenue will provide him dedicated support to help him adequately prep to ensure a higher quality examination and limit the need for repeat procedures.
DGBI care in historically marginalized and minoritized communities: A call to action
Understanding cultural differences and existing disparities in care is essential to improving care for patients from historically minoritized communities with DGBIs. Motivational interviewing and shared decision-making, with acknowledgment of social and cultural differences, allow us to work together with patients and their support systems to set and achieve feasible goals.14
To address known health disparities, offices can take steps to ensure the accessibility of language, forms, physical space, providers, and care teams. Providing culturally sensitive care and lowering barriers to care are the first steps to effecting meaningful change for patients with DGBIs from historically minoritized communities.
Dr. Yu is based at Division of Gastroenterology and Hepatology, Boston Medical Center and Boston University, both in Boston, Massachusetts. Dr. Dimino and Dr. Vélez are based at the Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston, Massachusetts. Dr. Yu, Dr. Dimino, and Dr. Vélez do not have any conflicts of interest for this article.
Additional Online Resources
Form Accessibility
- Intake Form Guidance for Providers
- Making Your Clinic Welcoming to LGBTQ Patients
- Transgender data collection in the electronic health record: Current concepts and issues
Language Accessibility
Physical Accessibility
- Access to Medical Care for Individuals with Mobility Disabilities
- Making your medical office accessible
References
1. Zavala VA, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021 Jan. doi: 10.1038/s41416-020-01038-6.
2. Kardashian A, et al. Health disparities in chronic liver disease. Hepatology. 2023 Apr. doi: 10.1002/hep.32743.
3. Nephew LD, Serper M. Racial, Gender, and Socioeconomic Disparities in Liver Transplantation. Liver Transpl. 2021 Jun. doi: 10.1002/lt.25996.
4. Anyane-Yeboa A, et al. The Impact of the Social Determinants of Health on Disparities in Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2022 Nov. doi: 10.1016/j.cgh.2022.03.011.
5. Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. 2016 Feb. doi: 10.1053/j.gastro.2016.02.032.
6. Silvernale C, et al. Racial disparity in healthcare utilization among patients with Irritable Bowel Syndrome: results from a multicenter cohort. Neurogastroenterol Motil. 2021 May. doi: 10.1111/nmo.14039.
7. Hearn M, et al. Stigma and irritable bowel syndrome: a taboo subject? Lancet Gastroenterol Hepatol. 2020 Jun. doi: 10.1016/S2468-1253(19)30348-6.
8. Yan XJ, et al. The impact of stigma on medication adherence in patients with functional dyspepsia. Neurogastroenterol Motil. 2021 Feb. doi: 10.1111/nmo.13956.
9. Twersky SE, et al. The Impact of Limited English Proficiency on Healthcare Access and Outcomes in the U.S.: A Scoping Review. Healthcare (Basel). 2024 Jan. doi: 10.3390/healthcare12030364.
10. Bayly JE, et al. Limited English proficiency and reported receipt of colorectal cancer screening among adults 45-75 in 2019 and 2021. Prev Med Rep. 2024 Feb. doi: 10.1016/j.pmedr.2024.102638.
11. Cheng K, et al. Epidemiology of Irritable Bowel Syndrome in a Large Academic Safety-Net Hospital. J Clin Med. 2024 Feb. doi: 10.3390/jcm13051314.
12. Breiding MJ, Armour BS. The association between disability and intimate partner violence in the United States. Ann Epidemiol. 2015 Jun. doi: 10.1016/j.annepidem.2015.03.017.
13. Mitra M, et al. Prevalence and characteristics of sexual violence against men with disabilities. Am J Prev Med. 2016 Mar. doi: 10.1016/j.amepre.2015.07.030.
14. Bahafzallah L, et al. Motivational Interviewing in Ethnic Populations. J Immigr Minor Health. 2020 Aug. doi: 10.1007/s10903-019-00940-3.
Introduction: Cases
Patient 1: A 57-year-old man with post-prandial distress variant functional dyspepsia (FD) was recommended to start nortriptyline. He previously established primary care with a physician he met at a barbershop health fair in Harlem, who referred him for specialty evaluation. Today, he presents for follow-up and reports he did not take this medication because he heard it is an antidepressant. How would you counsel him?
Patient 2: A 61-year-old woman was previously diagnosed with mixed variant irritable bowel syndrome (IBS-M). Her symptoms have not significantly changed. Her prior workup has been reassuring and consistent with IBS-M. Despite this, the patient pushes to repeat a colonoscopy, fearful that something is being missed or that she is not being offered care because of her undocumented status. How do you respond?
Patient 3: A 36-year-old man is followed for the management of generalized anxiety disorder and functional heartburn. He was started on low-dose amitriptyline with some benefit, but follow-up has been sporadic. On further discussion, he reports financial stressors, time barriers, and difficulty scheduling a meeting with his union representative for work accommodations as he lives in a more rural community. How do you reply?
Patient 4: A 74-year-old man with Parkinson’s disease who uses a wheelchair has functional constipation that is well controlled on his current regimen. He has never undergone colon cancer screening. He occasionally notices blood in his stool, so a colonoscopy was recommended to confirm that his hematochezia reflects functional constipation complicated by hemorrhoids. He is concerned about the bowel preparation required for a colonoscopy given his limited mobility, as his insurance does not cover assistance at home. He does not have family members to help him. How can you assist him?
Social determinants of health, health disparities, and DGBIs
Social determinants of health affect all aspects of patient care, with an increasing body of published work looking at potential disparities in organ-based and structural diseases.1,2,3,4 However, little has been done to explore their influence on disorders of gut-brain interaction or DGBIs.
. As DGBIs cannot be diagnosed with a single laboratory or endoscopic test, the patient history is of the utmost importance and physician-patient rapport is paramount in their treatment. Such rapport may be more difficult to establish in patients coming from historically marginalized and minoritized communities who may be distrustful of healthcare as an institution of (discriminatory) power.
Potential DGBI management pitfalls in historically marginalized or minoritized communities
For racial and ethnic minorities in the United States, disparities in healthcare take on many forms. People from racial and ethnic minority communities are less likely to receive a gastroenterology consultation and those with IBS are more likely to undergo procedures as compared to White patients with IBS.6 Implicit bias may lead to fewer specialist referrals, and specialty care may be limited or unavailable in some areas. Patients may prefer seeing providers in their own community, with whom they share racial or ethnic identities, which could lead to fewer referrals to specialists outside of the community.
Historical discrimination contributes to a lack of trust in healthcare professionals, which may lead patients to favor more objective diagnostics such as endoscopy or view being counseled against invasive procedures as having necessary care denied. Due to a broader cultural stigma surrounding mental illness, patients may be more hesitant to utilize neuromodulators, which have historically been used for psychiatric diagnoses, as it may lead them to conflate their GI illness with mental illness.7,8
Since DGBIs cannot be diagnosed with a single test or managed with a single treatment modality, providing excellent care for patients with DGBIs requires clear communication. For patients with limited English proficiency (LEP), access to high-quality language assistance is the foundation of comprehensive care. Interpreter use (or lack thereof) may limit the ability to obtain a complete and accurate clinical history, which can lead to fewer referrals to specialists and increased reliance on endoscopic evaluations that may not be clinically indicated.
These language barriers affect patients on many levels – in their ability to understand instructions for medication administration, preparation for procedures, and return precautions – which may ultimately lead to poorer responses to therapy or delays in care. LEP alone is broadly associated with fewer referrals for outpatient follow-up, adverse health outcomes and complications, and longer hospital stays.9 These disparities can be mitigated by investing in high-quality interpreter services, providing instructions and forms in multiple languages, and engaging the patient’s family and social supports according to their preferences.
People experiencing poverty (urban and rural) face challenges across multiple domains including access to healthcare, health insurance, stable housing and employment, and more. Many patients seek care at federally qualified health centers, which may face greater difficulties coordinating care with external gastroenterologists.10
Insurance barriers limit access to essential medications, tests, and procedures, and create delays in establishing care with specialists. Significant psychological stress and higher rates of comorbid anxiety and depression contribute to increased IBS severity.11 Financial limitations may limit dietary choices, which can further exacerbate DGBI symptoms. Long work hours with limited flexibility may prohibit them from presenting for regular follow-ups and establishing advanced DGBI care such as with a dietitian or psychologist.
Patients with disabilities face many of the health inequities previously discussed, as well as additional challenges with physical accessibility, transportation, exclusion from education and employment, discrimination, and stigma. Higher prevalence of comorbid mental illness and higher rates of intimate partner violence and interpersonal violence all contribute to DGBI severity and challenges with access to care.12,13 Patients with disabilities may struggle to arrive at appointments, maneuver through the building or exam room, and ultimately follow recommended care plans.
How to approach DGBIs in historically marginalized and minoritized communities
Returning to the patients from the introduction, how would you counsel each of them?
Patient 1: We can discuss with the patient how nortriptyline and other typical antidepressants can and often are used for indications other than depression. These medications modify centrally-mediated pain signaling and many patients with functional dyspepsia experience a significant benefit. It is critical to build on the rapport that was established at the community health outreach event and to explore the patient’s concerns thoroughly.
Patient 2: We would begin by inquiring about her underlying fears associated with her symptoms and seek to understand her goals for repeat intervention. We can review the risks of endoscopy and shift the focus to improving her symptoms. If we can improve her bowel habits or her pain, her desire for further interventions may lessen.
Patient 3: It will be important to work within the realistic time and monetary constraints in this patient’s life. We can validate him and the challenges he is facing, provide positive reinforcement for the progress he has made so far, and avoid disparaging him for the aspects of the treatment plan he has been unable to follow through with. As he reported a benefit from amitriptyline, we can consider increasing his dose as a feasible next step.
Patient 4: We can encourage the patient to discuss with his primary care physician how they may be able to coordinate an inpatient admission for colonoscopy preparation. Given his co-morbidities, this avenue will provide him dedicated support to help him adequately prep to ensure a higher quality examination and limit the need for repeat procedures.
DGBI care in historically marginalized and minoritized communities: A call to action
Understanding cultural differences and existing disparities in care is essential to improving care for patients from historically minoritized communities with DGBIs. Motivational interviewing and shared decision-making, with acknowledgment of social and cultural differences, allow us to work together with patients and their support systems to set and achieve feasible goals.14
To address known health disparities, offices can take steps to ensure the accessibility of language, forms, physical space, providers, and care teams. Providing culturally sensitive care and lowering barriers to care are the first steps to effecting meaningful change for patients with DGBIs from historically minoritized communities.
Dr. Yu is based at Division of Gastroenterology and Hepatology, Boston Medical Center and Boston University, both in Boston, Massachusetts. Dr. Dimino and Dr. Vélez are based at the Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston, Massachusetts. Dr. Yu, Dr. Dimino, and Dr. Vélez do not have any conflicts of interest for this article.
Additional Online Resources
Form Accessibility
- Intake Form Guidance for Providers
- Making Your Clinic Welcoming to LGBTQ Patients
- Transgender data collection in the electronic health record: Current concepts and issues
Language Accessibility
Physical Accessibility
- Access to Medical Care for Individuals with Mobility Disabilities
- Making your medical office accessible
References
1. Zavala VA, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021 Jan. doi: 10.1038/s41416-020-01038-6.
2. Kardashian A, et al. Health disparities in chronic liver disease. Hepatology. 2023 Apr. doi: 10.1002/hep.32743.
3. Nephew LD, Serper M. Racial, Gender, and Socioeconomic Disparities in Liver Transplantation. Liver Transpl. 2021 Jun. doi: 10.1002/lt.25996.
4. Anyane-Yeboa A, et al. The Impact of the Social Determinants of Health on Disparities in Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2022 Nov. doi: 10.1016/j.cgh.2022.03.011.
5. Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. 2016 Feb. doi: 10.1053/j.gastro.2016.02.032.
6. Silvernale C, et al. Racial disparity in healthcare utilization among patients with Irritable Bowel Syndrome: results from a multicenter cohort. Neurogastroenterol Motil. 2021 May. doi: 10.1111/nmo.14039.
7. Hearn M, et al. Stigma and irritable bowel syndrome: a taboo subject? Lancet Gastroenterol Hepatol. 2020 Jun. doi: 10.1016/S2468-1253(19)30348-6.
8. Yan XJ, et al. The impact of stigma on medication adherence in patients with functional dyspepsia. Neurogastroenterol Motil. 2021 Feb. doi: 10.1111/nmo.13956.
9. Twersky SE, et al. The Impact of Limited English Proficiency on Healthcare Access and Outcomes in the U.S.: A Scoping Review. Healthcare (Basel). 2024 Jan. doi: 10.3390/healthcare12030364.
10. Bayly JE, et al. Limited English proficiency and reported receipt of colorectal cancer screening among adults 45-75 in 2019 and 2021. Prev Med Rep. 2024 Feb. doi: 10.1016/j.pmedr.2024.102638.
11. Cheng K, et al. Epidemiology of Irritable Bowel Syndrome in a Large Academic Safety-Net Hospital. J Clin Med. 2024 Feb. doi: 10.3390/jcm13051314.
12. Breiding MJ, Armour BS. The association between disability and intimate partner violence in the United States. Ann Epidemiol. 2015 Jun. doi: 10.1016/j.annepidem.2015.03.017.
13. Mitra M, et al. Prevalence and characteristics of sexual violence against men with disabilities. Am J Prev Med. 2016 Mar. doi: 10.1016/j.amepre.2015.07.030.
14. Bahafzallah L, et al. Motivational Interviewing in Ethnic Populations. J Immigr Minor Health. 2020 Aug. doi: 10.1007/s10903-019-00940-3.
A Practical Approach to Diagnosis and Management of Eosinophilic Esophagitis
Eosinophilic esophagitis (EoE) can be considered a “young” disease, with initial case series reported only about 30 years ago. Since that time, it has become a commonly encountered condition in both emergency and clinic settings. The most recent prevalence study estimates that 1 in 700 people in the U.S. have EoE,1 the volume of EoE-associated ED visits tripped between 2009 and 2019 and is projected to double again by 2030,2 and “new” gastroenterologists undoubtedly have learned about and seen this condition. As a chronic disease, EoE necessitates longitudinal follow-up and optimization of care to prevent complications. With increasing diagnostic delay, EoE progresses in most, but not all, patients from an inflammatory- to fibrostenotic-predominant condition.3
Diagnosis of EoE
The most likely area that you will encounter EoE is during an emergent middle-of-the-night endoscopy for food impaction. If called in for this, EoE will be the cause in more than 50% of patients.4 However, the diagnosis can only be made if esophageal biopsies are obtained at the time of the procedure. This is a critical time to decrease diagnostic delay, as half of patients are lost to follow-up after a food impaction.5 Unfortunately, although taking biopsies during index food impaction is guideline-recommended, a quality metric, and safe to obtain after the food bolus is cleared, this is infrequently done in practice.6, 7
The next most likely area for EoE detection is in the endoscopy suite where 15-23% of patients with dysphagia and 5-7% of patients undergoing upper endoscopy for any indication will have EoE.4 Sometimes EoE will be detected “incidentally” during an open-access case (for example, in a patient with diarrhea undergoing evaluation for celiac). In these cases, it is important to perform a careful history (as noted below) as subtle EoE symptoms can frequently be identified. Finally, when patients are seen in clinic for solid food dysphagia, EoE is clearly on the differential. A few percent of patients with refractory heartburn or chest pain will have EoE causing the symptoms rather than reflux,4 and all patients under consideration for antireflux surgery should have an endoscopy to assess for EoE.
When talking to patients with known or suspected EoE, the history must go beyond general questions about dysphagia or trouble swallowing. Many patients with EoE have overtly or subconsciously modified their eating behaviors over many years to minimize symptoms, may have adapted to chronic dysphagia, and will answer “no” when asked if they have trouble swallowing. Instead, use the acronym “IMPACT” to delve deeper into possible symptoms.8 Do they “Imbibe” fluids or liquids between each bite to help get food down? Do they “Modify” the way they eat (cut food into small bites; puree foods)? Do they “Prolong” mealtimes? Do they “Avoid” certain foods that stick? Do they “Chew’ until their food is a mush to get it down? And do they “Turn away” tablets or pills? Pill dysphagia is often a subtle symptom, and sometimes the only symptom elicited.
Additionally, it may be important to ask a partner or family member (if present) about their observations. They may provide insight (e.g. “yes – he chokes with every bite but never says it bothers him”) that the patient might not otherwise provide. The suspicion for EoE should also be increased in patients with concomitant atopic diseases and in those with a family history of dysphagia or who have family members needing esophageal dilation. It is important to remember that EoE can be seen across all ages, sexes, and races/ethnicities.
Diagnosis of EoE is based on the AGREE consensus,9 which is also echoed in the recently updated American College of Gastroenterology (ACG) guidelines.10 Diagnosis requires three steps. First, symptoms of esophageal dysfunction must be present. This will most typically be dysphagia in adolescents and adults, but symptoms are non-specific in children (e.g. poor growth and feeding, abdominal pain, vomiting, regurgitation, heartburn).
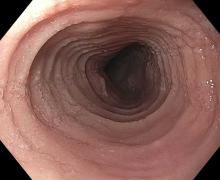
Second, at least 15 eosinophils per high-power field (eos/hpf) are required on esophageal biopsy, which implies that an endoscopy be performed. A high-quality endoscopic exam in EoE is of the utmost importance. The approach has been described elsewhere,11 but enough time on insertion should be taken to fully insufflate and examine the esophagus, including the areas of the gastroesophageal junction and upper esophageal sphincter where strictures can be missed, to gently wash debris, and to assess the endoscopic findings of EoE. Endoscopic findings should be reported using the validated EoE Endoscopy Reference Score (EREFS),12 which grades five key features. EREFS is reproducible, is responsive to treatment, and is guideline-recommended (see Figure 1).6, 10 The features are edema (present=1), rings (mild=1; moderate=2; severe=3), exudates (mild=1; severe=2), furrows (mild=1; severe=2), and stricture (present=1; also estimate diameter in mm) and are incorporated into many endoscopic reporting programs. Additionally, diffuse luminal narrowing and mucosal fragility (“crepe-paper” mucosa) should be assessed.
After this, biopsies should be obtained with at least 6 biopsy fragments from different locations in the esophagus. Any visible endoscopic abnormalities should be targeted (the highest yield is in exudates and furrows). The rationale is that EoE is patchy and at least 6 biopsies will maximize diagnostic yield.10 Ideally the initial endoscopy for EoE should be done off of treatments (like PPI or diet restriction) as these could mask the diagnosis. If a patient with suspected EoE has an endoscopy while on PPI, and the endoscopy is normal, a diagnosis of EoE cannot be made. In this case, consideration should be given as to stopping the PPI, allowing a wash out period (at least 1-2 months), and then repeating the endoscopy to confirm the diagnosis. This is important as EoE is a chronic condition necessitating life-long treatment and monitoring, so a definitive diagnosis is critical.
The third and final step in diagnosis is assessing for other conditions that could cause esophageal eosinophilia.9 The most common differential diagnosis is gastroesophageal reflux disease (GERD). In some cases, EoE and GERD overlap or can have a complex relationship.13 Unfortunately the location of the eosinophilia (i.e. distal only) and the level of the eosinophil counts are not useful in making this distinction, so all clinical features (symptoms, presence of erosive esophagitis, or a hiatal hernia endoscopically), and ancillary reflex testing when indicated may be required prior to a formal EoE diagnosis. After the diagnosis is established, there should be direct communication with the patient to review the diagnosis and select treatments. While it is possible to convey results electronically in a messaging portal or with a letter, a more formal interaction, such as a clinic visit, is recommended because this is a new diagnosis of a chronic condition. Similarly, a new diagnosis of inflammatory bowel disease would never be made in a pathology follow-up letter alone.
Treatment of EoE
When it comes to treatment, the new guidelines emphasize several points.10 First, there is the concept that anti-inflammatory treatment should be paired with assessment of fibrostenosis and esophageal dilation; to do either in isolation is incomplete treatment. It is safe to perform dilation both prior to anti-inflammatory treatment (for example, with a critical stricture in a patient with dysphagia) and after anti-inflammatory treatment has been prescribed (for example, during an endoscopy to assess treatment response).
Second, proton pump inhibitors (PPIs), swallowed topical corticosteroids (tCS), or dietary elimination are all acceptable first-line treatment options for EoE. A shared decision-making framework should be used for this discussion. If dietary elimination is selected,14 based on new clinical trial data, guidelines recommend using empiric elimination and starting with a less restrictive diet (either a one-food elimination diet with dairy alone or a two-food elimination with dairy and wheat elimination). If PPIs are selected, the dose should be double the standard reflux dose. Data are mixed as to whether to use twice daily dosing (i.e., omeprazole 20 mg twice daily) or once a day dosing (i.e., omeprazole 40 mg daily), but total dose and adherence may be more important than frequency.10
For tCS use, either budesonide or fluticasone can be selected, but budesonide oral suspension is the only FDA-approved tCS for EoE.15 Initial treatment length is usually 6-8 weeks for diet elimination and, 12 weeks for PPI and tCS. In general, it is best to pick a single treatment to start, and reserve combining therapies for patients who do not have a complete response to a single modality as there are few data to support combination therapy.
After initial treatment, it is critical to assess for treatment response.16 Goals of EoE treatment include improvement in symptoms, but also improvement in endoscopic and histologic features to prevent complications. Symptoms in EoE do not always correlate with underlying biologic disease activity: patients can minimize symptoms with careful eating; they may perceive no difference in symptoms despite histologic improvement if a stricture persists; and they may have minimal symptoms after esophageal dilation despite ongoing inflammation. Because of this, performing a follow-up endoscopy after initial treatment is guideline-recommended.10, 17 This allows assessing for endoscopic improvement, re-assessing for fibrostenosis and performing dilation if indicated, and obtaining esophageal biopsies. If there is non-response, options include switching between other first line treatments or considering “stepping-up” to dupilumab which is also an FDA-approved option for EoE that is recommended in the guidelines.10, 18 In some cases where patients have multiple severe atopic conditions such as asthma or eczema that would warrant dupilumab use, or if patients are intolerant to PPIs or tCS, dupilumab could be considered as an earlier treatment for EoE.
Long-Term Maintenance
If a patient has a good response (for example, improved symptoms, improved endoscopic features, and <15 eos/hpf on biopsy), treatment can be maintained long-term. In almost all cases, if treatment is stopped, EoE disease activity recurs.19 Patients could be seen back in clinic in 6-12 months, and then a discussion can be conducted about a follow-up endoscopy, with timing to be determined based on their individual disease features and severity.17
Patients with more severe strictures, however, may have to be seen in endoscopy for serial dilations. Continued follow-up is essential for optimal care. Just as patients can progress in their disease course with diagnostic delay, there are data that show they can also progress after diagnosis when there are gaps in care without regular follow-up.20 Unlike other chronic esophageal disorders such as GERD and Barrett’s esophagus and other chronic GI inflammatory conditions like inflammatory bowel disease, however, EoE is not associated with an increased risk of esophageal cancer.21, 22
Given its increasing frequency, EoE will be commonly encountered by gastroenterologists both new and established. Having a systematic approach for diagnosis, understanding how to elicit subtle symptoms, implementing a shared decision-making framework for treatment with a structured algorithm for assessing response, performing follow-up, maintaining treatment, and monitoring patients long-term will allow the large majority of EoE patients to be successfully managed.
Dr. Dellon is based at the Center for Esophageal Diseases and Swallowing, Center for Gastrointestinal Biology and Disease, Division of Gastroenterology and Hepatology, University of North Carolina School of Medicine, Chapel Hill. He disclosed research funding, consultant fees, and educational grants from multiple companies.
References
1. Thel HL, et al. Prevalence and Costs of Eosinophilic Esophagitis in the United States. Clin Gastroenterol Hepatol. 2025 Feb. doi: 10.1016/j.cgh.2024.09.031.
2. Lam AY, et al. Epidemiologic Burden and Projections for Eosinophilic Esophagitis-Associated Emergency Department Visits in the United States: 2009-2030. Clin Gastroenterol Hepatol. 2023 Nov. doi: 10.1016/j.cgh.2023.04.028.
3. Schoepfer AM, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013 Dec. doi: 10.1053/j.gastro.2013.08.015.
4. Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.06.067.
5. Chang JW, et al. Loss to follow-up after food impaction among patients with and without eosinophilic esophagitis. Dis Esophagus. 2019 Dec. doi: 10.1093/dote/doz056.
6. Aceves SS, et al. Endoscopic approach to eosinophilic esophagitis: American Society for Gastrointestinal Endoscopy Consensus Conference. Gastrointest Endosc. 2022 Aug. doi: 10.1016/j.gie.2022.05.013.
7. Leiman DA, et al. Quality Indicators for the Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2023 Jun. doi: 10.14309/ajg.0000000000002138.
8. Hirano I, Furuta GT. Approaches and Challenges to Management of Pediatric and Adult Patients With Eosinophilic Esophagitis. Gastroenterology. 2020 Mar. doi: 10.1053/j.gastro.2019.09.052.
9. Dellon ES, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: Proceedings of the AGREE conference. Gastroenterology. 2018 Oct. doi: 10.1053/j.gastro.2018.07.009.
10. Dellon ES, et al. ACG Clinical Guideline: Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2025 Jan. doi: 10.14309/ajg.0000000000003194.
11. Dellon ES. Optimizing the Endoscopic Examination in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2021 Dec. doi: 10.1016/j.cgh.2021.07.011.
12. Hirano I, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2012 May. doi: 10.1136/gutjnl-2011-301817.
13. Spechler SJ, et al. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007 Jun. doi: 10.1111/j.1572-0241.2007.01179.x.
14. Chang JW, et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul. doi: 10.1016/j.cgh.2023.03.006.
15. Hirano I, et al. Budesonide Oral Suspension Improves Outcomes in Patients With Eosinophilic Esophagitis: Results from a Phase 3 Trial. Clin Gastroenterol Hepatol. 2022 Mar. doi: 10.1016/j.cgh.2021.04.022.
16. Dellon ES, Gupta SK. A conceptual approach to understanding treatment response in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2019 Oct. doi: 10.1016/j.cgh.2019.01.030.
17. von Arnim U, et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep. doi: 10.1016/j.cgh.2022.12.018.
18. Dellon ES, et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N Engl J Med. 2022 Dec. doi: 10.1056/NEJMoa220598.
19. Dellon ES, et al. Rapid Recurrence of Eosinophilic Esophagitis Activity After Successful Treatment in the Observation Phase of a Randomized, Double-Blind, Double-Dummy Trial. Clin Gastroenterol Hepatol. 2020 Jun. doi: 10.1016/j.cgh.2019.08.050.
20. Chang NC, et al. A Gap in Care Leads to Progression of Fibrosis in Eosinophilic Esophagitis Patients. Clin Gastroenterol Hepatol. 2022 Aug. doi: 10.1016/j.cgh.2021.10.028.
21. Syed A, et al. The relationship between eosinophilic esophagitis and esophageal cancer. Dis Esophagus. 2017 Jul. doi: 10.1093/dote/dox050.
22. Albaneze N, et al. No Association Between Eosinophilic Oesophagitis and Oesophageal Cancer in US Adults: A Case-Control Study. Aliment Pharmacol Ther. 2025 Jan. doi: 10.1111/apt.18431.
Eosinophilic esophagitis (EoE) can be considered a “young” disease, with initial case series reported only about 30 years ago. Since that time, it has become a commonly encountered condition in both emergency and clinic settings. The most recent prevalence study estimates that 1 in 700 people in the U.S. have EoE,1 the volume of EoE-associated ED visits tripped between 2009 and 2019 and is projected to double again by 2030,2 and “new” gastroenterologists undoubtedly have learned about and seen this condition. As a chronic disease, EoE necessitates longitudinal follow-up and optimization of care to prevent complications. With increasing diagnostic delay, EoE progresses in most, but not all, patients from an inflammatory- to fibrostenotic-predominant condition.3
Diagnosis of EoE
The most likely area that you will encounter EoE is during an emergent middle-of-the-night endoscopy for food impaction. If called in for this, EoE will be the cause in more than 50% of patients.4 However, the diagnosis can only be made if esophageal biopsies are obtained at the time of the procedure. This is a critical time to decrease diagnostic delay, as half of patients are lost to follow-up after a food impaction.5 Unfortunately, although taking biopsies during index food impaction is guideline-recommended, a quality metric, and safe to obtain after the food bolus is cleared, this is infrequently done in practice.6, 7
The next most likely area for EoE detection is in the endoscopy suite where 15-23% of patients with dysphagia and 5-7% of patients undergoing upper endoscopy for any indication will have EoE.4 Sometimes EoE will be detected “incidentally” during an open-access case (for example, in a patient with diarrhea undergoing evaluation for celiac). In these cases, it is important to perform a careful history (as noted below) as subtle EoE symptoms can frequently be identified. Finally, when patients are seen in clinic for solid food dysphagia, EoE is clearly on the differential. A few percent of patients with refractory heartburn or chest pain will have EoE causing the symptoms rather than reflux,4 and all patients under consideration for antireflux surgery should have an endoscopy to assess for EoE.
When talking to patients with known or suspected EoE, the history must go beyond general questions about dysphagia or trouble swallowing. Many patients with EoE have overtly or subconsciously modified their eating behaviors over many years to minimize symptoms, may have adapted to chronic dysphagia, and will answer “no” when asked if they have trouble swallowing. Instead, use the acronym “IMPACT” to delve deeper into possible symptoms.8 Do they “Imbibe” fluids or liquids between each bite to help get food down? Do they “Modify” the way they eat (cut food into small bites; puree foods)? Do they “Prolong” mealtimes? Do they “Avoid” certain foods that stick? Do they “Chew’ until their food is a mush to get it down? And do they “Turn away” tablets or pills? Pill dysphagia is often a subtle symptom, and sometimes the only symptom elicited.
Additionally, it may be important to ask a partner or family member (if present) about their observations. They may provide insight (e.g. “yes – he chokes with every bite but never says it bothers him”) that the patient might not otherwise provide. The suspicion for EoE should also be increased in patients with concomitant atopic diseases and in those with a family history of dysphagia or who have family members needing esophageal dilation. It is important to remember that EoE can be seen across all ages, sexes, and races/ethnicities.
Diagnosis of EoE is based on the AGREE consensus,9 which is also echoed in the recently updated American College of Gastroenterology (ACG) guidelines.10 Diagnosis requires three steps. First, symptoms of esophageal dysfunction must be present. This will most typically be dysphagia in adolescents and adults, but symptoms are non-specific in children (e.g. poor growth and feeding, abdominal pain, vomiting, regurgitation, heartburn).
Second, at least 15 eosinophils per high-power field (eos/hpf) are required on esophageal biopsy, which implies that an endoscopy be performed. A high-quality endoscopic exam in EoE is of the utmost importance. The approach has been described elsewhere,11 but enough time on insertion should be taken to fully insufflate and examine the esophagus, including the areas of the gastroesophageal junction and upper esophageal sphincter where strictures can be missed, to gently wash debris, and to assess the endoscopic findings of EoE. Endoscopic findings should be reported using the validated EoE Endoscopy Reference Score (EREFS),12 which grades five key features. EREFS is reproducible, is responsive to treatment, and is guideline-recommended (see Figure 1).6, 10 The features are edema (present=1), rings (mild=1; moderate=2; severe=3), exudates (mild=1; severe=2), furrows (mild=1; severe=2), and stricture (present=1; also estimate diameter in mm) and are incorporated into many endoscopic reporting programs. Additionally, diffuse luminal narrowing and mucosal fragility (“crepe-paper” mucosa) should be assessed.
After this, biopsies should be obtained with at least 6 biopsy fragments from different locations in the esophagus. Any visible endoscopic abnormalities should be targeted (the highest yield is in exudates and furrows). The rationale is that EoE is patchy and at least 6 biopsies will maximize diagnostic yield.10 Ideally the initial endoscopy for EoE should be done off of treatments (like PPI or diet restriction) as these could mask the diagnosis. If a patient with suspected EoE has an endoscopy while on PPI, and the endoscopy is normal, a diagnosis of EoE cannot be made. In this case, consideration should be given as to stopping the PPI, allowing a wash out period (at least 1-2 months), and then repeating the endoscopy to confirm the diagnosis. This is important as EoE is a chronic condition necessitating life-long treatment and monitoring, so a definitive diagnosis is critical.
The third and final step in diagnosis is assessing for other conditions that could cause esophageal eosinophilia.9 The most common differential diagnosis is gastroesophageal reflux disease (GERD). In some cases, EoE and GERD overlap or can have a complex relationship.13 Unfortunately the location of the eosinophilia (i.e. distal only) and the level of the eosinophil counts are not useful in making this distinction, so all clinical features (symptoms, presence of erosive esophagitis, or a hiatal hernia endoscopically), and ancillary reflex testing when indicated may be required prior to a formal EoE diagnosis. After the diagnosis is established, there should be direct communication with the patient to review the diagnosis and select treatments. While it is possible to convey results electronically in a messaging portal or with a letter, a more formal interaction, such as a clinic visit, is recommended because this is a new diagnosis of a chronic condition. Similarly, a new diagnosis of inflammatory bowel disease would never be made in a pathology follow-up letter alone.
Treatment of EoE
When it comes to treatment, the new guidelines emphasize several points.10 First, there is the concept that anti-inflammatory treatment should be paired with assessment of fibrostenosis and esophageal dilation; to do either in isolation is incomplete treatment. It is safe to perform dilation both prior to anti-inflammatory treatment (for example, with a critical stricture in a patient with dysphagia) and after anti-inflammatory treatment has been prescribed (for example, during an endoscopy to assess treatment response).
Second, proton pump inhibitors (PPIs), swallowed topical corticosteroids (tCS), or dietary elimination are all acceptable first-line treatment options for EoE. A shared decision-making framework should be used for this discussion. If dietary elimination is selected,14 based on new clinical trial data, guidelines recommend using empiric elimination and starting with a less restrictive diet (either a one-food elimination diet with dairy alone or a two-food elimination with dairy and wheat elimination). If PPIs are selected, the dose should be double the standard reflux dose. Data are mixed as to whether to use twice daily dosing (i.e., omeprazole 20 mg twice daily) or once a day dosing (i.e., omeprazole 40 mg daily), but total dose and adherence may be more important than frequency.10
For tCS use, either budesonide or fluticasone can be selected, but budesonide oral suspension is the only FDA-approved tCS for EoE.15 Initial treatment length is usually 6-8 weeks for diet elimination and, 12 weeks for PPI and tCS. In general, it is best to pick a single treatment to start, and reserve combining therapies for patients who do not have a complete response to a single modality as there are few data to support combination therapy.
After initial treatment, it is critical to assess for treatment response.16 Goals of EoE treatment include improvement in symptoms, but also improvement in endoscopic and histologic features to prevent complications. Symptoms in EoE do not always correlate with underlying biologic disease activity: patients can minimize symptoms with careful eating; they may perceive no difference in symptoms despite histologic improvement if a stricture persists; and they may have minimal symptoms after esophageal dilation despite ongoing inflammation. Because of this, performing a follow-up endoscopy after initial treatment is guideline-recommended.10, 17 This allows assessing for endoscopic improvement, re-assessing for fibrostenosis and performing dilation if indicated, and obtaining esophageal biopsies. If there is non-response, options include switching between other first line treatments or considering “stepping-up” to dupilumab which is also an FDA-approved option for EoE that is recommended in the guidelines.10, 18 In some cases where patients have multiple severe atopic conditions such as asthma or eczema that would warrant dupilumab use, or if patients are intolerant to PPIs or tCS, dupilumab could be considered as an earlier treatment for EoE.
Long-Term Maintenance
If a patient has a good response (for example, improved symptoms, improved endoscopic features, and <15 eos/hpf on biopsy), treatment can be maintained long-term. In almost all cases, if treatment is stopped, EoE disease activity recurs.19 Patients could be seen back in clinic in 6-12 months, and then a discussion can be conducted about a follow-up endoscopy, with timing to be determined based on their individual disease features and severity.17
Patients with more severe strictures, however, may have to be seen in endoscopy for serial dilations. Continued follow-up is essential for optimal care. Just as patients can progress in their disease course with diagnostic delay, there are data that show they can also progress after diagnosis when there are gaps in care without regular follow-up.20 Unlike other chronic esophageal disorders such as GERD and Barrett’s esophagus and other chronic GI inflammatory conditions like inflammatory bowel disease, however, EoE is not associated with an increased risk of esophageal cancer.21, 22
Given its increasing frequency, EoE will be commonly encountered by gastroenterologists both new and established. Having a systematic approach for diagnosis, understanding how to elicit subtle symptoms, implementing a shared decision-making framework for treatment with a structured algorithm for assessing response, performing follow-up, maintaining treatment, and monitoring patients long-term will allow the large majority of EoE patients to be successfully managed.
Dr. Dellon is based at the Center for Esophageal Diseases and Swallowing, Center for Gastrointestinal Biology and Disease, Division of Gastroenterology and Hepatology, University of North Carolina School of Medicine, Chapel Hill. He disclosed research funding, consultant fees, and educational grants from multiple companies.
References
1. Thel HL, et al. Prevalence and Costs of Eosinophilic Esophagitis in the United States. Clin Gastroenterol Hepatol. 2025 Feb. doi: 10.1016/j.cgh.2024.09.031.
2. Lam AY, et al. Epidemiologic Burden and Projections for Eosinophilic Esophagitis-Associated Emergency Department Visits in the United States: 2009-2030. Clin Gastroenterol Hepatol. 2023 Nov. doi: 10.1016/j.cgh.2023.04.028.
3. Schoepfer AM, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013 Dec. doi: 10.1053/j.gastro.2013.08.015.
4. Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.06.067.
5. Chang JW, et al. Loss to follow-up after food impaction among patients with and without eosinophilic esophagitis. Dis Esophagus. 2019 Dec. doi: 10.1093/dote/doz056.
6. Aceves SS, et al. Endoscopic approach to eosinophilic esophagitis: American Society for Gastrointestinal Endoscopy Consensus Conference. Gastrointest Endosc. 2022 Aug. doi: 10.1016/j.gie.2022.05.013.
7. Leiman DA, et al. Quality Indicators for the Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2023 Jun. doi: 10.14309/ajg.0000000000002138.
8. Hirano I, Furuta GT. Approaches and Challenges to Management of Pediatric and Adult Patients With Eosinophilic Esophagitis. Gastroenterology. 2020 Mar. doi: 10.1053/j.gastro.2019.09.052.
9. Dellon ES, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: Proceedings of the AGREE conference. Gastroenterology. 2018 Oct. doi: 10.1053/j.gastro.2018.07.009.
10. Dellon ES, et al. ACG Clinical Guideline: Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2025 Jan. doi: 10.14309/ajg.0000000000003194.
11. Dellon ES. Optimizing the Endoscopic Examination in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2021 Dec. doi: 10.1016/j.cgh.2021.07.011.
12. Hirano I, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2012 May. doi: 10.1136/gutjnl-2011-301817.
13. Spechler SJ, et al. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007 Jun. doi: 10.1111/j.1572-0241.2007.01179.x.
14. Chang JW, et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul. doi: 10.1016/j.cgh.2023.03.006.
15. Hirano I, et al. Budesonide Oral Suspension Improves Outcomes in Patients With Eosinophilic Esophagitis: Results from a Phase 3 Trial. Clin Gastroenterol Hepatol. 2022 Mar. doi: 10.1016/j.cgh.2021.04.022.
16. Dellon ES, Gupta SK. A conceptual approach to understanding treatment response in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2019 Oct. doi: 10.1016/j.cgh.2019.01.030.
17. von Arnim U, et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep. doi: 10.1016/j.cgh.2022.12.018.
18. Dellon ES, et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N Engl J Med. 2022 Dec. doi: 10.1056/NEJMoa220598.
19. Dellon ES, et al. Rapid Recurrence of Eosinophilic Esophagitis Activity After Successful Treatment in the Observation Phase of a Randomized, Double-Blind, Double-Dummy Trial. Clin Gastroenterol Hepatol. 2020 Jun. doi: 10.1016/j.cgh.2019.08.050.
20. Chang NC, et al. A Gap in Care Leads to Progression of Fibrosis in Eosinophilic Esophagitis Patients. Clin Gastroenterol Hepatol. 2022 Aug. doi: 10.1016/j.cgh.2021.10.028.
21. Syed A, et al. The relationship between eosinophilic esophagitis and esophageal cancer. Dis Esophagus. 2017 Jul. doi: 10.1093/dote/dox050.
22. Albaneze N, et al. No Association Between Eosinophilic Oesophagitis and Oesophageal Cancer in US Adults: A Case-Control Study. Aliment Pharmacol Ther. 2025 Jan. doi: 10.1111/apt.18431.
Eosinophilic esophagitis (EoE) can be considered a “young” disease, with initial case series reported only about 30 years ago. Since that time, it has become a commonly encountered condition in both emergency and clinic settings. The most recent prevalence study estimates that 1 in 700 people in the U.S. have EoE,1 the volume of EoE-associated ED visits tripped between 2009 and 2019 and is projected to double again by 2030,2 and “new” gastroenterologists undoubtedly have learned about and seen this condition. As a chronic disease, EoE necessitates longitudinal follow-up and optimization of care to prevent complications. With increasing diagnostic delay, EoE progresses in most, but not all, patients from an inflammatory- to fibrostenotic-predominant condition.3
Diagnosis of EoE
The most likely area that you will encounter EoE is during an emergent middle-of-the-night endoscopy for food impaction. If called in for this, EoE will be the cause in more than 50% of patients.4 However, the diagnosis can only be made if esophageal biopsies are obtained at the time of the procedure. This is a critical time to decrease diagnostic delay, as half of patients are lost to follow-up after a food impaction.5 Unfortunately, although taking biopsies during index food impaction is guideline-recommended, a quality metric, and safe to obtain after the food bolus is cleared, this is infrequently done in practice.6, 7
The next most likely area for EoE detection is in the endoscopy suite where 15-23% of patients with dysphagia and 5-7% of patients undergoing upper endoscopy for any indication will have EoE.4 Sometimes EoE will be detected “incidentally” during an open-access case (for example, in a patient with diarrhea undergoing evaluation for celiac). In these cases, it is important to perform a careful history (as noted below) as subtle EoE symptoms can frequently be identified. Finally, when patients are seen in clinic for solid food dysphagia, EoE is clearly on the differential. A few percent of patients with refractory heartburn or chest pain will have EoE causing the symptoms rather than reflux,4 and all patients under consideration for antireflux surgery should have an endoscopy to assess for EoE.
When talking to patients with known or suspected EoE, the history must go beyond general questions about dysphagia or trouble swallowing. Many patients with EoE have overtly or subconsciously modified their eating behaviors over many years to minimize symptoms, may have adapted to chronic dysphagia, and will answer “no” when asked if they have trouble swallowing. Instead, use the acronym “IMPACT” to delve deeper into possible symptoms.8 Do they “Imbibe” fluids or liquids between each bite to help get food down? Do they “Modify” the way they eat (cut food into small bites; puree foods)? Do they “Prolong” mealtimes? Do they “Avoid” certain foods that stick? Do they “Chew’ until their food is a mush to get it down? And do they “Turn away” tablets or pills? Pill dysphagia is often a subtle symptom, and sometimes the only symptom elicited.
Additionally, it may be important to ask a partner or family member (if present) about their observations. They may provide insight (e.g. “yes – he chokes with every bite but never says it bothers him”) that the patient might not otherwise provide. The suspicion for EoE should also be increased in patients with concomitant atopic diseases and in those with a family history of dysphagia or who have family members needing esophageal dilation. It is important to remember that EoE can be seen across all ages, sexes, and races/ethnicities.
Diagnosis of EoE is based on the AGREE consensus,9 which is also echoed in the recently updated American College of Gastroenterology (ACG) guidelines.10 Diagnosis requires three steps. First, symptoms of esophageal dysfunction must be present. This will most typically be dysphagia in adolescents and adults, but symptoms are non-specific in children (e.g. poor growth and feeding, abdominal pain, vomiting, regurgitation, heartburn).
Second, at least 15 eosinophils per high-power field (eos/hpf) are required on esophageal biopsy, which implies that an endoscopy be performed. A high-quality endoscopic exam in EoE is of the utmost importance. The approach has been described elsewhere,11 but enough time on insertion should be taken to fully insufflate and examine the esophagus, including the areas of the gastroesophageal junction and upper esophageal sphincter where strictures can be missed, to gently wash debris, and to assess the endoscopic findings of EoE. Endoscopic findings should be reported using the validated EoE Endoscopy Reference Score (EREFS),12 which grades five key features. EREFS is reproducible, is responsive to treatment, and is guideline-recommended (see Figure 1).6, 10 The features are edema (present=1), rings (mild=1; moderate=2; severe=3), exudates (mild=1; severe=2), furrows (mild=1; severe=2), and stricture (present=1; also estimate diameter in mm) and are incorporated into many endoscopic reporting programs. Additionally, diffuse luminal narrowing and mucosal fragility (“crepe-paper” mucosa) should be assessed.
After this, biopsies should be obtained with at least 6 biopsy fragments from different locations in the esophagus. Any visible endoscopic abnormalities should be targeted (the highest yield is in exudates and furrows). The rationale is that EoE is patchy and at least 6 biopsies will maximize diagnostic yield.10 Ideally the initial endoscopy for EoE should be done off of treatments (like PPI or diet restriction) as these could mask the diagnosis. If a patient with suspected EoE has an endoscopy while on PPI, and the endoscopy is normal, a diagnosis of EoE cannot be made. In this case, consideration should be given as to stopping the PPI, allowing a wash out period (at least 1-2 months), and then repeating the endoscopy to confirm the diagnosis. This is important as EoE is a chronic condition necessitating life-long treatment and monitoring, so a definitive diagnosis is critical.
The third and final step in diagnosis is assessing for other conditions that could cause esophageal eosinophilia.9 The most common differential diagnosis is gastroesophageal reflux disease (GERD). In some cases, EoE and GERD overlap or can have a complex relationship.13 Unfortunately the location of the eosinophilia (i.e. distal only) and the level of the eosinophil counts are not useful in making this distinction, so all clinical features (symptoms, presence of erosive esophagitis, or a hiatal hernia endoscopically), and ancillary reflex testing when indicated may be required prior to a formal EoE diagnosis. After the diagnosis is established, there should be direct communication with the patient to review the diagnosis and select treatments. While it is possible to convey results electronically in a messaging portal or with a letter, a more formal interaction, such as a clinic visit, is recommended because this is a new diagnosis of a chronic condition. Similarly, a new diagnosis of inflammatory bowel disease would never be made in a pathology follow-up letter alone.
Treatment of EoE
When it comes to treatment, the new guidelines emphasize several points.10 First, there is the concept that anti-inflammatory treatment should be paired with assessment of fibrostenosis and esophageal dilation; to do either in isolation is incomplete treatment. It is safe to perform dilation both prior to anti-inflammatory treatment (for example, with a critical stricture in a patient with dysphagia) and after anti-inflammatory treatment has been prescribed (for example, during an endoscopy to assess treatment response).
Second, proton pump inhibitors (PPIs), swallowed topical corticosteroids (tCS), or dietary elimination are all acceptable first-line treatment options for EoE. A shared decision-making framework should be used for this discussion. If dietary elimination is selected,14 based on new clinical trial data, guidelines recommend using empiric elimination and starting with a less restrictive diet (either a one-food elimination diet with dairy alone or a two-food elimination with dairy and wheat elimination). If PPIs are selected, the dose should be double the standard reflux dose. Data are mixed as to whether to use twice daily dosing (i.e., omeprazole 20 mg twice daily) or once a day dosing (i.e., omeprazole 40 mg daily), but total dose and adherence may be more important than frequency.10
For tCS use, either budesonide or fluticasone can be selected, but budesonide oral suspension is the only FDA-approved tCS for EoE.15 Initial treatment length is usually 6-8 weeks for diet elimination and, 12 weeks for PPI and tCS. In general, it is best to pick a single treatment to start, and reserve combining therapies for patients who do not have a complete response to a single modality as there are few data to support combination therapy.
After initial treatment, it is critical to assess for treatment response.16 Goals of EoE treatment include improvement in symptoms, but also improvement in endoscopic and histologic features to prevent complications. Symptoms in EoE do not always correlate with underlying biologic disease activity: patients can minimize symptoms with careful eating; they may perceive no difference in symptoms despite histologic improvement if a stricture persists; and they may have minimal symptoms after esophageal dilation despite ongoing inflammation. Because of this, performing a follow-up endoscopy after initial treatment is guideline-recommended.10, 17 This allows assessing for endoscopic improvement, re-assessing for fibrostenosis and performing dilation if indicated, and obtaining esophageal biopsies. If there is non-response, options include switching between other first line treatments or considering “stepping-up” to dupilumab which is also an FDA-approved option for EoE that is recommended in the guidelines.10, 18 In some cases where patients have multiple severe atopic conditions such as asthma or eczema that would warrant dupilumab use, or if patients are intolerant to PPIs or tCS, dupilumab could be considered as an earlier treatment for EoE.
Long-Term Maintenance
If a patient has a good response (for example, improved symptoms, improved endoscopic features, and <15 eos/hpf on biopsy), treatment can be maintained long-term. In almost all cases, if treatment is stopped, EoE disease activity recurs.19 Patients could be seen back in clinic in 6-12 months, and then a discussion can be conducted about a follow-up endoscopy, with timing to be determined based on their individual disease features and severity.17
Patients with more severe strictures, however, may have to be seen in endoscopy for serial dilations. Continued follow-up is essential for optimal care. Just as patients can progress in their disease course with diagnostic delay, there are data that show they can also progress after diagnosis when there are gaps in care without regular follow-up.20 Unlike other chronic esophageal disorders such as GERD and Barrett’s esophagus and other chronic GI inflammatory conditions like inflammatory bowel disease, however, EoE is not associated with an increased risk of esophageal cancer.21, 22
Given its increasing frequency, EoE will be commonly encountered by gastroenterologists both new and established. Having a systematic approach for diagnosis, understanding how to elicit subtle symptoms, implementing a shared decision-making framework for treatment with a structured algorithm for assessing response, performing follow-up, maintaining treatment, and monitoring patients long-term will allow the large majority of EoE patients to be successfully managed.
Dr. Dellon is based at the Center for Esophageal Diseases and Swallowing, Center for Gastrointestinal Biology and Disease, Division of Gastroenterology and Hepatology, University of North Carolina School of Medicine, Chapel Hill. He disclosed research funding, consultant fees, and educational grants from multiple companies.
References
1. Thel HL, et al. Prevalence and Costs of Eosinophilic Esophagitis in the United States. Clin Gastroenterol Hepatol. 2025 Feb. doi: 10.1016/j.cgh.2024.09.031.
2. Lam AY, et al. Epidemiologic Burden and Projections for Eosinophilic Esophagitis-Associated Emergency Department Visits in the United States: 2009-2030. Clin Gastroenterol Hepatol. 2023 Nov. doi: 10.1016/j.cgh.2023.04.028.
3. Schoepfer AM, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013 Dec. doi: 10.1053/j.gastro.2013.08.015.
4. Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.06.067.
5. Chang JW, et al. Loss to follow-up after food impaction among patients with and without eosinophilic esophagitis. Dis Esophagus. 2019 Dec. doi: 10.1093/dote/doz056.
6. Aceves SS, et al. Endoscopic approach to eosinophilic esophagitis: American Society for Gastrointestinal Endoscopy Consensus Conference. Gastrointest Endosc. 2022 Aug. doi: 10.1016/j.gie.2022.05.013.
7. Leiman DA, et al. Quality Indicators for the Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2023 Jun. doi: 10.14309/ajg.0000000000002138.
8. Hirano I, Furuta GT. Approaches and Challenges to Management of Pediatric and Adult Patients With Eosinophilic Esophagitis. Gastroenterology. 2020 Mar. doi: 10.1053/j.gastro.2019.09.052.
9. Dellon ES, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: Proceedings of the AGREE conference. Gastroenterology. 2018 Oct. doi: 10.1053/j.gastro.2018.07.009.
10. Dellon ES, et al. ACG Clinical Guideline: Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2025 Jan. doi: 10.14309/ajg.0000000000003194.
11. Dellon ES. Optimizing the Endoscopic Examination in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2021 Dec. doi: 10.1016/j.cgh.2021.07.011.
12. Hirano I, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2012 May. doi: 10.1136/gutjnl-2011-301817.
13. Spechler SJ, et al. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007 Jun. doi: 10.1111/j.1572-0241.2007.01179.x.
14. Chang JW, et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul. doi: 10.1016/j.cgh.2023.03.006.
15. Hirano I, et al. Budesonide Oral Suspension Improves Outcomes in Patients With Eosinophilic Esophagitis: Results from a Phase 3 Trial. Clin Gastroenterol Hepatol. 2022 Mar. doi: 10.1016/j.cgh.2021.04.022.
16. Dellon ES, Gupta SK. A conceptual approach to understanding treatment response in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2019 Oct. doi: 10.1016/j.cgh.2019.01.030.
17. von Arnim U, et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep. doi: 10.1016/j.cgh.2022.12.018.
18. Dellon ES, et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N Engl J Med. 2022 Dec. doi: 10.1056/NEJMoa220598.
19. Dellon ES, et al. Rapid Recurrence of Eosinophilic Esophagitis Activity After Successful Treatment in the Observation Phase of a Randomized, Double-Blind, Double-Dummy Trial. Clin Gastroenterol Hepatol. 2020 Jun. doi: 10.1016/j.cgh.2019.08.050.
20. Chang NC, et al. A Gap in Care Leads to Progression of Fibrosis in Eosinophilic Esophagitis Patients. Clin Gastroenterol Hepatol. 2022 Aug. doi: 10.1016/j.cgh.2021.10.028.
21. Syed A, et al. The relationship between eosinophilic esophagitis and esophageal cancer. Dis Esophagus. 2017 Jul. doi: 10.1093/dote/dox050.
22. Albaneze N, et al. No Association Between Eosinophilic Oesophagitis and Oesophageal Cancer in US Adults: A Case-Control Study. Aliment Pharmacol Ther. 2025 Jan. doi: 10.1111/apt.18431.
Building Your Referral Base

In this video, Lisa Mathew, MD, of South Denver Gastroenterology in Denver, Colorado, and Raja Taunk, MD, of Anne Arundel Gastroenterology Associates, in Annapolis, Maryland, share insights on private practice gastroenterology and offer tips on building your practice – specifically improving your referral base.

In this video, Lisa Mathew, MD, of South Denver Gastroenterology in Denver, Colorado, and Raja Taunk, MD, of Anne Arundel Gastroenterology Associates, in Annapolis, Maryland, share insights on private practice gastroenterology and offer tips on building your practice – specifically improving your referral base.

In this video, Lisa Mathew, MD, of South Denver Gastroenterology in Denver, Colorado, and Raja Taunk, MD, of Anne Arundel Gastroenterology Associates, in Annapolis, Maryland, share insights on private practice gastroenterology and offer tips on building your practice – specifically improving your referral base.
Finding and Following Your Passion
Dear Friends,
Over the last year, I have been reading more about professional identity and professional branding, all of which have evolved in the setting of social media. However, the root of it remains constant — finding the intersection(s) of what you love. A common problem, especially as a trainee and early-career gastroenterologist, is that you may have many interests: various disease processes, innovation, medical education, leadership development, and much more. Since becoming faculty, I continue to define and refine my professional niche, trying to distinguish my “interests” from “passions.” It is a journey that my mentors advise me not to rush through and I am enjoying every moment of it!
In this issue’s “In Focus,” Dr. Hamza Salim, Dr. Anni Chowdhury, and Dr. Lavanya Viswanathan provide a practical guide for the clinical evaluation of chronic constipation and a systematic approach to treatment.
In the first of a two-part series in the “Short Clinical Review” section, Dr. Christopher Velez and Dr. Kara J. Jencks discuss the health inequities among sexual and gender minority (SGM) patients, particularly with disorders of brain-gut interaction (DBGI). They review common SGM terminology, sample verbiage for trauma-informed care, and case presentations to help guide our approach to providing care for SGM patients with DGBI.
The transition from trainee to early faculty may be difficult for those who are interested in research but struggle with the change from being a part of a research team to running one. In the “Early Career” section, Dr. Lauren Feld and colleagues describes her experience establishing a research lab as an early-career academic, from creating a niche to time management and mentorship.
The Federal Trade Commission’s noncompete ban made big news in April 2024 but there is still a lot of gray area for physicians. Dr. Timothy Craig Allen explains the ruling, what it means to physicians, the status of it today, and what the future may hold. Lastly, for “Private Practice Perspectives” in collaboration with Digestive Health Physicians Alliance, I interview Dr. Vasu Appalaneni on her use of artificial intelligence in private practice.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Polyethylene glycol was first used in the 1940s and 1950s to understand the physiology of the intestines, and first published as a compound for colonoscopy bowel preparation in 1981.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
Over the last year, I have been reading more about professional identity and professional branding, all of which have evolved in the setting of social media. However, the root of it remains constant — finding the intersection(s) of what you love. A common problem, especially as a trainee and early-career gastroenterologist, is that you may have many interests: various disease processes, innovation, medical education, leadership development, and much more. Since becoming faculty, I continue to define and refine my professional niche, trying to distinguish my “interests” from “passions.” It is a journey that my mentors advise me not to rush through and I am enjoying every moment of it!
In this issue’s “In Focus,” Dr. Hamza Salim, Dr. Anni Chowdhury, and Dr. Lavanya Viswanathan provide a practical guide for the clinical evaluation of chronic constipation and a systematic approach to treatment.
In the first of a two-part series in the “Short Clinical Review” section, Dr. Christopher Velez and Dr. Kara J. Jencks discuss the health inequities among sexual and gender minority (SGM) patients, particularly with disorders of brain-gut interaction (DBGI). They review common SGM terminology, sample verbiage for trauma-informed care, and case presentations to help guide our approach to providing care for SGM patients with DGBI.
The transition from trainee to early faculty may be difficult for those who are interested in research but struggle with the change from being a part of a research team to running one. In the “Early Career” section, Dr. Lauren Feld and colleagues describes her experience establishing a research lab as an early-career academic, from creating a niche to time management and mentorship.
The Federal Trade Commission’s noncompete ban made big news in April 2024 but there is still a lot of gray area for physicians. Dr. Timothy Craig Allen explains the ruling, what it means to physicians, the status of it today, and what the future may hold. Lastly, for “Private Practice Perspectives” in collaboration with Digestive Health Physicians Alliance, I interview Dr. Vasu Appalaneni on her use of artificial intelligence in private practice.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Polyethylene glycol was first used in the 1940s and 1950s to understand the physiology of the intestines, and first published as a compound for colonoscopy bowel preparation in 1981.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
Over the last year, I have been reading more about professional identity and professional branding, all of which have evolved in the setting of social media. However, the root of it remains constant — finding the intersection(s) of what you love. A common problem, especially as a trainee and early-career gastroenterologist, is that you may have many interests: various disease processes, innovation, medical education, leadership development, and much more. Since becoming faculty, I continue to define and refine my professional niche, trying to distinguish my “interests” from “passions.” It is a journey that my mentors advise me not to rush through and I am enjoying every moment of it!
In this issue’s “In Focus,” Dr. Hamza Salim, Dr. Anni Chowdhury, and Dr. Lavanya Viswanathan provide a practical guide for the clinical evaluation of chronic constipation and a systematic approach to treatment.
In the first of a two-part series in the “Short Clinical Review” section, Dr. Christopher Velez and Dr. Kara J. Jencks discuss the health inequities among sexual and gender minority (SGM) patients, particularly with disorders of brain-gut interaction (DBGI). They review common SGM terminology, sample verbiage for trauma-informed care, and case presentations to help guide our approach to providing care for SGM patients with DGBI.
The transition from trainee to early faculty may be difficult for those who are interested in research but struggle with the change from being a part of a research team to running one. In the “Early Career” section, Dr. Lauren Feld and colleagues describes her experience establishing a research lab as an early-career academic, from creating a niche to time management and mentorship.
The Federal Trade Commission’s noncompete ban made big news in April 2024 but there is still a lot of gray area for physicians. Dr. Timothy Craig Allen explains the ruling, what it means to physicians, the status of it today, and what the future may hold. Lastly, for “Private Practice Perspectives” in collaboration with Digestive Health Physicians Alliance, I interview Dr. Vasu Appalaneni on her use of artificial intelligence in private practice.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Polyethylene glycol was first used in the 1940s and 1950s to understand the physiology of the intestines, and first published as a compound for colonoscopy bowel preparation in 1981.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Journal Highlights: October-December 2024
Esophagus
Reed CC et al. Daily or Twice Daily Treatment with Topical Steroids Results in Similar Responses in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2024 Nov. doi: 10.1016/j.cgh.2024.10.016.
Patel RV et al. Functional Lumen Imaging Probe Provides an Accurate Assessment of Esophageal Diameter in Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.032.
Stomach
Shah SC et al. AGA Clinical Practice Update on Screening and Surveillance in Individuals at Increased Risk for Gastric Cancer in the United States: Expert Review. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.11.001.
IBD
Griffiths BJ et al. Hypercoagulation after Hospital Discharge in Acute Severe Ulcerative Colitis: A Prospective Study. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.031.
Liver
Lassailly G et al. Resolution of MASH with no worsening of fibrosis after bariatric surgery improves 15-year survival: a prospective cohort study. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.025.
Norman JS et al. Model for Urgency for Liver Transplantation in Hepatocellular Carcinoma: A Practical Model to Prioritize Patients With Hepatocellular Carcinoma on the Liver Transplant Waiting List. Gastroenterology. 2024 Nov. doi: 10.1053/j.gastro.2024.11.015.
Davis JPE et al. AGA Clinical Practice Update on Management of Portal Vein Thrombosis in Patients With Cirrhosis: Expert Review. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.10.038.
Pancreas
Drewes AM et al. Pain in Chronic Pancreatitis: Navigating the Maze of Blocked Tubes and Tangled Wires. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.11.026.
Endoscopy
Kindel TL et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity; Society of American Gastrointestinal and Endoscopic Surgeons. Multisociety Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.10.003.
Schmidt KA et al. Understanding Patients’ Current Acceptability of Artificial Intelligence During Colonoscopy for Polyp Detection: A Single-Center Study. Techniques and Innovations in Gastrointestinal Endoscopy. 2024 Dec. doi: 10.1016/j.tige.2024.250905.
Chandramouli S et al. Endoscopic Surveillance Patterns and Management of Helicobacter pylori in Newly Diagnosed Gastric Intestinal Metaplasia. Techniques and Innovations in Gastrointestinal Endoscopy. 2024 Dec. doi: 10.1016/j.tige.2024.250904.
Practice Management
Tsai C et al. Trauma-Informed Care in Gastroenterology: A Survey of Provider Attitudes, Knowledge, and Skills. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.09.015.
Mintz KM et al. Incorporating a GI Dietitian into Your GI Practice. Gastroenterology. 2024 Nov. doi: 10.1053/j.gastro.2024.10.022.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus
Reed CC et al. Daily or Twice Daily Treatment with Topical Steroids Results in Similar Responses in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2024 Nov. doi: 10.1016/j.cgh.2024.10.016.
Patel RV et al. Functional Lumen Imaging Probe Provides an Accurate Assessment of Esophageal Diameter in Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.032.
Stomach
Shah SC et al. AGA Clinical Practice Update on Screening and Surveillance in Individuals at Increased Risk for Gastric Cancer in the United States: Expert Review. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.11.001.
IBD
Griffiths BJ et al. Hypercoagulation after Hospital Discharge in Acute Severe Ulcerative Colitis: A Prospective Study. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.031.
Liver
Lassailly G et al. Resolution of MASH with no worsening of fibrosis after bariatric surgery improves 15-year survival: a prospective cohort study. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.025.
Norman JS et al. Model for Urgency for Liver Transplantation in Hepatocellular Carcinoma: A Practical Model to Prioritize Patients With Hepatocellular Carcinoma on the Liver Transplant Waiting List. Gastroenterology. 2024 Nov. doi: 10.1053/j.gastro.2024.11.015.
Davis JPE et al. AGA Clinical Practice Update on Management of Portal Vein Thrombosis in Patients With Cirrhosis: Expert Review. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.10.038.
Pancreas
Drewes AM et al. Pain in Chronic Pancreatitis: Navigating the Maze of Blocked Tubes and Tangled Wires. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.11.026.
Endoscopy
Kindel TL et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity; Society of American Gastrointestinal and Endoscopic Surgeons. Multisociety Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.10.003.
Schmidt KA et al. Understanding Patients’ Current Acceptability of Artificial Intelligence During Colonoscopy for Polyp Detection: A Single-Center Study. Techniques and Innovations in Gastrointestinal Endoscopy. 2024 Dec. doi: 10.1016/j.tige.2024.250905.
Chandramouli S et al. Endoscopic Surveillance Patterns and Management of Helicobacter pylori in Newly Diagnosed Gastric Intestinal Metaplasia. Techniques and Innovations in Gastrointestinal Endoscopy. 2024 Dec. doi: 10.1016/j.tige.2024.250904.
Practice Management
Tsai C et al. Trauma-Informed Care in Gastroenterology: A Survey of Provider Attitudes, Knowledge, and Skills. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.09.015.
Mintz KM et al. Incorporating a GI Dietitian into Your GI Practice. Gastroenterology. 2024 Nov. doi: 10.1053/j.gastro.2024.10.022.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus
Reed CC et al. Daily or Twice Daily Treatment with Topical Steroids Results in Similar Responses in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2024 Nov. doi: 10.1016/j.cgh.2024.10.016.
Patel RV et al. Functional Lumen Imaging Probe Provides an Accurate Assessment of Esophageal Diameter in Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.032.
Stomach
Shah SC et al. AGA Clinical Practice Update on Screening and Surveillance in Individuals at Increased Risk for Gastric Cancer in the United States: Expert Review. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.11.001.
IBD
Griffiths BJ et al. Hypercoagulation after Hospital Discharge in Acute Severe Ulcerative Colitis: A Prospective Study. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.031.
Liver
Lassailly G et al. Resolution of MASH with no worsening of fibrosis after bariatric surgery improves 15-year survival: a prospective cohort study. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.025.
Norman JS et al. Model for Urgency for Liver Transplantation in Hepatocellular Carcinoma: A Practical Model to Prioritize Patients With Hepatocellular Carcinoma on the Liver Transplant Waiting List. Gastroenterology. 2024 Nov. doi: 10.1053/j.gastro.2024.11.015.
Davis JPE et al. AGA Clinical Practice Update on Management of Portal Vein Thrombosis in Patients With Cirrhosis: Expert Review. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.10.038.
Pancreas
Drewes AM et al. Pain in Chronic Pancreatitis: Navigating the Maze of Blocked Tubes and Tangled Wires. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.11.026.
Endoscopy
Kindel TL et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity; Society of American Gastrointestinal and Endoscopic Surgeons. Multisociety Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.10.003.
Schmidt KA et al. Understanding Patients’ Current Acceptability of Artificial Intelligence During Colonoscopy for Polyp Detection: A Single-Center Study. Techniques and Innovations in Gastrointestinal Endoscopy. 2024 Dec. doi: 10.1016/j.tige.2024.250905.
Chandramouli S et al. Endoscopic Surveillance Patterns and Management of Helicobacter pylori in Newly Diagnosed Gastric Intestinal Metaplasia. Techniques and Innovations in Gastrointestinal Endoscopy. 2024 Dec. doi: 10.1016/j.tige.2024.250904.
Practice Management
Tsai C et al. Trauma-Informed Care in Gastroenterology: A Survey of Provider Attitudes, Knowledge, and Skills. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.09.015.
Mintz KM et al. Incorporating a GI Dietitian into Your GI Practice. Gastroenterology. 2024 Nov. doi: 10.1053/j.gastro.2024.10.022.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Improving Care for Sexual and Gender Minority Patients with Disorders of Gut-Brain Interaction
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.
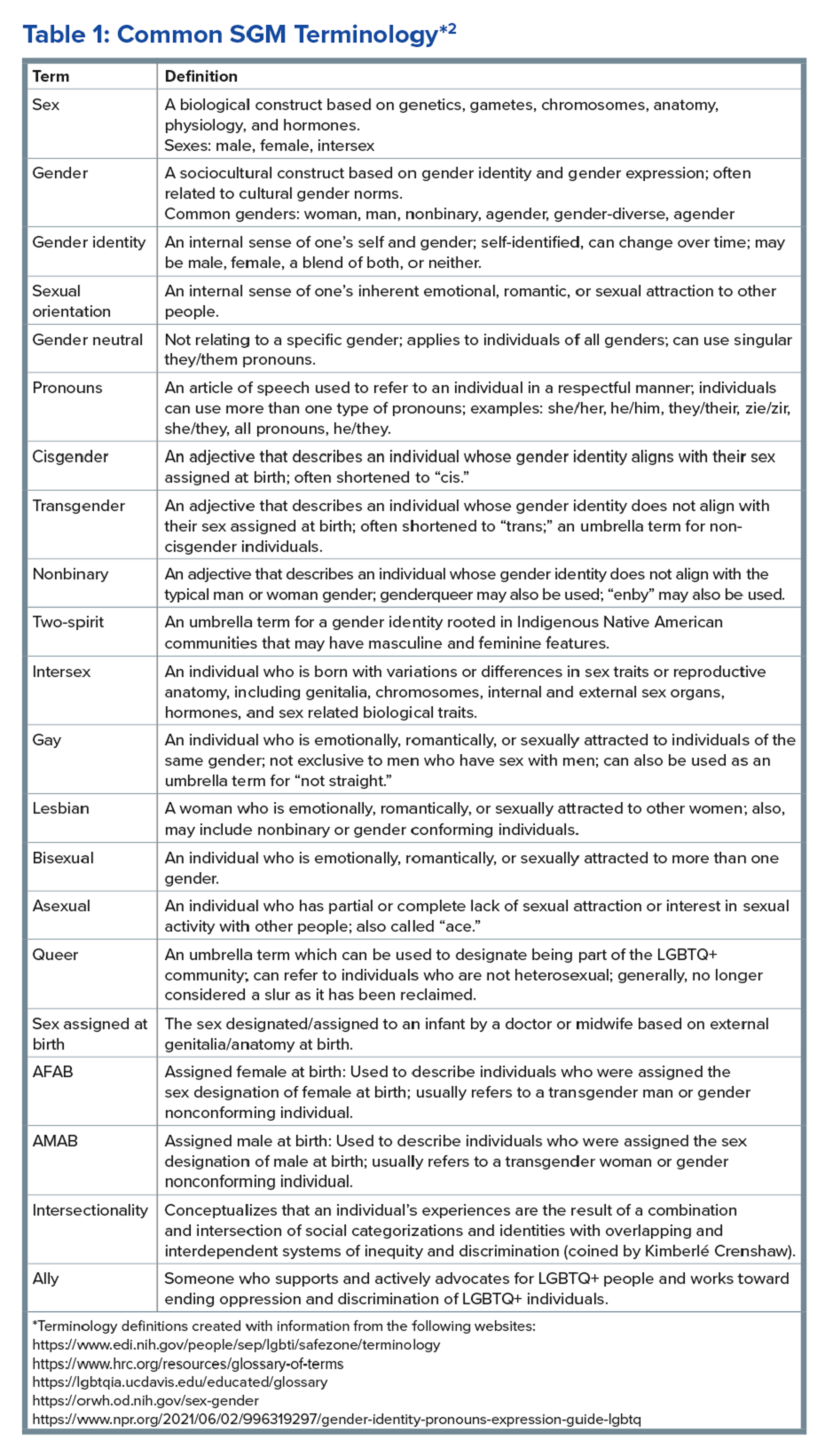
Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.
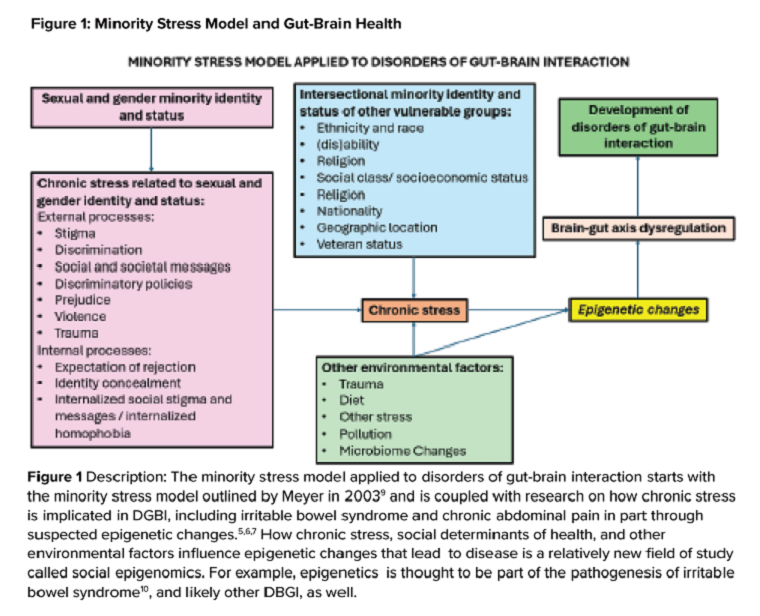
Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.

Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.

Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.

Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.

Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
Clinical Research in Early Career Academic Medicine
Conducting clinical research as an early career gastroenterologist can take on many forms and has varying definitions of success. This article focuses on key factors to consider and should be supplemented with mentorship tailored to personal interests, goals, and institutional criteria for success. In this article, we will discuss selected high-yield topics that assist in early-career research. We will briefly discuss 1. Defining your niche, 2. Collaboration, 3. Visibility, 4. Time management, 5. Funding, 6. Receiving mentorship, and 7. Providing mentorship. We will conclude with discussing several authors’ experience in the research lab of the first author (FELD Lab – Fostering Equity in Liver and Digestive disease).
Defining Your Niche
Defining your niche is an essential component of an early career, as when academicians must transition from a trainee, who is supporting the research of an established mentor, to defining their own subspeciality area of investigation. Early-career academics should build on their prior work, but should also explore their own passions and skill set to define what will be unique about their research program and contributions to the field. Of course, positioning oneself at the intersection of two or more seemingly unrelated fields opens much opportunity for large impact but comes at a cost of identifying mentorship and justifying the niche to funders.
Collaboration
Fostering a collaborative environment is essential for early-career physician-researchers. One effective approach is to establish collaboration circles with other early career academics. Expanding research endeavors beyond a single institution to a multi-center framework enriches both scope and impact. This collaborative approach not only amplifies the depth of research but also facilitates peer mentorship and sponsorship. Participation in such networks can significantly enhance scholarly output and broaden professional reach during this critical phase of academic progression. Furthermore, prioritizing the promotion of colleagues within these networks is crucial. Proactive sponsorship opportunities, such as inviting peers to present at institutional seminars, strengthen both individual and collective academic visibility.
Collaboration is also essential to foster between trainees involved in early-career investigators’ work. An interconnected lab environment ensures that trainees remain informed about concurrent projects, thereby fostering a culture of shared knowledge and optimized productivity. Encouraging trainees to spearhead research aligned with their interests, under mentor guidance, nurtures independent inquiry and leadership. By establishing explicit roles, responsibilities, and authorship agreements at the outset of collaborative projects, early career mentors can avoid future conflicts and preserve a collaborative culture within the lab. This structured approach cultivates a supportive ecosystem, advancing both individual and collective research achievements.
Visibility
Establishing visibility and developing name recognition are crucial components of career advancement for early-career academic physicians. By clearly defining their areas of expertise, faculty can position themselves as leaders within their discipline. Active participation in professional societies, both at the local and national level, engagement with interest groups, and frequent contributions to educational events can be effective strategies for gaining recognition. Leveraging social media platforms can be helpful in enhancing visibility by facilitating connections and promoting research to a broader audience.
Moreover, research visibility plays a vital role in academic promotion. A strong publication record, reflected by an increasing h-index, demonstrates the impact and relevance of one’s research. Self-citation, when appropriate, can reinforce the continuity and progression of scholarly contributions. While publishing in high-impact journals is desirable, adaptability in resubmitting to other journals following rejections ensures that research remains visible and accessible. It also clearly establishes by whom the work was first done, before someone else investigates the line of inquiry. Through a combination of strategic engagement and publication efforts, early-career physicians can effectively build their professional reputation and advance their academic careers.
Time Management
Time management is essential for any research, and particularly in early career when efficiency in clinical care duties is still being gained. Securing protected time for research is essential to develop a niche, build connections (both institutionally and beyond their institutions), and demonstrate productivity that can be utilized to support future grant efforts.
Similarly, using protected time efficiently is required. Without organization and planning, research time can be spent with scattered meetings and responding to various tasks that do not directly support your research. It is helpful to be introspective about the time of the day you are most productive in your research efforts and blocking off that time to focus on research tasks and minimizing distractions. Blocking monthly time for larger scale thinking and planning is also important. Weekly lab and individual one-on-one meetings also support time management for trainees and lab members, to ensure efficiency and progress. Additionally, robust clinical support is essential to ensure that research time remains protected and patient care moves forward. When negotiating for positions, and in regular meetings thereafter, it is important to advocate for sufficient clinical staffing such that non-physician tasks can be appropriately delegated to another member of the care team.
Funding
Securing adequate funding poses a significant challenge for all early-career physician-scientists, particularly because of the discrepancy between National Institutes of Health salary caps and the higher average salaries in academic gastroenterology. This financial gap can deter physicians from pursuing research-intensive careers altogether and can derail early investigators who do not obtain funding rapidly. To overcome this, early-career investigators may need to adopt flexible strategies, such as accepting a lower salary that aligns with grant funding limits or funneling incentive or bonus pay to research accounts. Alternatively, they can advocate for institutional support to bridge the salary gap, ensuring their research efforts remain financially viable.
Institutions committed to fostering research excellence may offer supplemental funding or bridge programs to retain talented physician-scientists, thereby mitigating the financial strain and encouraging long-term engagement in research. Regular meetings to review salary and support sources, including philanthropy, foundation grants, and other streams, should be undertaken with leadership to align the researcher’s timeline and available funding. If career development funding appears untenable, consideration of multi–principal investigator R01s or equivalent with senior established investigators can be a promising path.
Receiving Mentorship
Effective mentorship for early-career physician-scientists should be approached through a team-based model that leverages both internal and external mentors. Internal mentors, familiar with the specific culture, expectations, and advancement pathways of the institution, can provide invaluable guidance on navigating institutional metrics for success, such as promotion criteria, grant acquisition, and clinical-research balance. External mentors, on the other hand, bring a broader perspective by offering innovative career development strategies and solutions derived from experiences at their home institutions. This multimodal mentorship model ensures a well-rounded approach to professional growth.
All national gastroenterology societies, including the American Gastroenterological Association, the American College of Gastroenterology, and the American Society for Gastrointestinal Endoscopy, and American Association for the Study of Liver Disease, offer structured early-career mentorship programs designed to connect emerging researchers with experienced leaders in the field (see below). These programs typically require a formal application process and are highly regarded for their exceptional quality and impact. Participation in such initiatives can significantly enhance career development by expanding networks, fostering interdisciplinary collaboration, and providing tailored guidance that complements institutional support. Integrating both internal and external mentorship opportunities ensures a robust and dynamic foundation for long-term success in academic medicine.
- AGA Career Compass (https://gastro.org/fellows-and-early-career/mentoring/)
- ACG Early Career Leadership Program (https://gi.org/acg-institute/early-career-leadership-program/)
- AGA-AASLD Academic Skills Workshop (https://gastro.org/news/applications-now-being-accepted-for-the-2024-aga-aasld-academic-skills-workshop/)
- AASLD Women’s Initiative Committee Leadership Program (https://www.aasld.org/promoting-leadership-potential-women-hepatology)
- Scrubs n Heels Mentorship Matrix (https://scrubsandheels.com/matrix/)
Providing Mentorship
The trainee authors on this manuscript describe in this section what has been helpful for them as mentees in the FELD research lab.
Student doctor Nguyen describes her experience as a lab member and things she finds most helpful as a medical student in the lab:
- Upon joining the team, a one-to-one meeting to discuss trainee’s personal and professional goals, and availability, was crucial to building the mentor-mentee relationship. Establishing this meaningful mentorship early on clarified expectations on both sides, built trust, and increased motivation. As a trainee, it is essential for me to see how my work aligns with a long-term goal and to receive ample guidance throughout the process.
- One of the most impactful experiences has been joining informal lunch sessions where trainees discussed data collection protocols and exchanged insights. In doing so, Dr. Feld has cultivated a lab culture that encourages curiosity, constructive feedback, and collaborative learning.
- To increase productivity, our team of trainees created a useful group message thread where we coordinated more sessions to collaborate. This coordination formed stronger relationships between team members and fostered a sense of shared purpose.
Dr. Cooper, a third year internal medicine resident, describes her experience as both a research mentee and a mentor to the junior trainees: “As a resident pursuing a career in academic gastroenterology and hepatology, I have found three key elements to be most helpful: intentional mentorship, structured meetings, and leadership development.”
- Intentional mentorship: Prior to joining the lab, I met with Dr. Feld to discuss my research experience and my goals. She took the time to understand these within the context of my training timeline and tailored project opportunities that aligned with my interests and were both feasible and impactful for my next steps. This intentional approach not only fostered a productive research experience but also established a mentor-mentee relationship built on genuine care for my growth and development.
- Regular meetings: Frequent lab meetings promote accountability, teamwork, and shared problem-solving skills. The open exchange of ideas fosters collaboration and joint problem solving to elevate the quality of our research. They are also an opportunity to observe key decision-making points during the research process and have been a great way to learn more about solid methodology.
- Supervised leadership: I have had ample time to lead discussions and coordinate projects among the junior trainees. These monitored leadership experiences promote project management skills, mentorship, and team dynamic awareness while maintaining the safety net of senior guidance. This model helped me transition from a trainee supporting others’ research to a more independent role, contributing to multi-disciplinary projects while mentoring junior members.
Conclusion
In conclusion, many exciting opportunities and notable barriers exist to establishing a clinical research laboratory in the early career. While excellence in each of the areas outlined may evolve, some aspects will come easier than others and with time, persistence, and a bit of luck, the research world will be a better place because of your contributions!
Dr. Feld is assistant professor of gastroenterology and hepatology and physician executive of Diversity, Equity, Inclusion and Belonging for the department of medicine at the University of Massachusetts (UMass) Chan Medical School, Worcester. Ms. Nguyen is a medical student at UMass Chan Medical School. Dr. Cooper is a resident physician at UMass Chan Medical School. Dr. Rabinowitz is an attending physician in the Inflammatory Bowel Disease Center at Beth Israel Deaconess Medical Center, Boston, Mass. Dr. Uchida is codirector of the Multidisciplinary Eosinophilic Gastrointestinal Disease Clinic at the University of Utah School of Medicine, Salt Lake City.
Conducting clinical research as an early career gastroenterologist can take on many forms and has varying definitions of success. This article focuses on key factors to consider and should be supplemented with mentorship tailored to personal interests, goals, and institutional criteria for success. In this article, we will discuss selected high-yield topics that assist in early-career research. We will briefly discuss 1. Defining your niche, 2. Collaboration, 3. Visibility, 4. Time management, 5. Funding, 6. Receiving mentorship, and 7. Providing mentorship. We will conclude with discussing several authors’ experience in the research lab of the first author (FELD Lab – Fostering Equity in Liver and Digestive disease).
Defining Your Niche
Defining your niche is an essential component of an early career, as when academicians must transition from a trainee, who is supporting the research of an established mentor, to defining their own subspeciality area of investigation. Early-career academics should build on their prior work, but should also explore their own passions and skill set to define what will be unique about their research program and contributions to the field. Of course, positioning oneself at the intersection of two or more seemingly unrelated fields opens much opportunity for large impact but comes at a cost of identifying mentorship and justifying the niche to funders.
Collaboration
Fostering a collaborative environment is essential for early-career physician-researchers. One effective approach is to establish collaboration circles with other early career academics. Expanding research endeavors beyond a single institution to a multi-center framework enriches both scope and impact. This collaborative approach not only amplifies the depth of research but also facilitates peer mentorship and sponsorship. Participation in such networks can significantly enhance scholarly output and broaden professional reach during this critical phase of academic progression. Furthermore, prioritizing the promotion of colleagues within these networks is crucial. Proactive sponsorship opportunities, such as inviting peers to present at institutional seminars, strengthen both individual and collective academic visibility.
Collaboration is also essential to foster between trainees involved in early-career investigators’ work. An interconnected lab environment ensures that trainees remain informed about concurrent projects, thereby fostering a culture of shared knowledge and optimized productivity. Encouraging trainees to spearhead research aligned with their interests, under mentor guidance, nurtures independent inquiry and leadership. By establishing explicit roles, responsibilities, and authorship agreements at the outset of collaborative projects, early career mentors can avoid future conflicts and preserve a collaborative culture within the lab. This structured approach cultivates a supportive ecosystem, advancing both individual and collective research achievements.
Visibility
Establishing visibility and developing name recognition are crucial components of career advancement for early-career academic physicians. By clearly defining their areas of expertise, faculty can position themselves as leaders within their discipline. Active participation in professional societies, both at the local and national level, engagement with interest groups, and frequent contributions to educational events can be effective strategies for gaining recognition. Leveraging social media platforms can be helpful in enhancing visibility by facilitating connections and promoting research to a broader audience.
Moreover, research visibility plays a vital role in academic promotion. A strong publication record, reflected by an increasing h-index, demonstrates the impact and relevance of one’s research. Self-citation, when appropriate, can reinforce the continuity and progression of scholarly contributions. While publishing in high-impact journals is desirable, adaptability in resubmitting to other journals following rejections ensures that research remains visible and accessible. It also clearly establishes by whom the work was first done, before someone else investigates the line of inquiry. Through a combination of strategic engagement and publication efforts, early-career physicians can effectively build their professional reputation and advance their academic careers.
Time Management
Time management is essential for any research, and particularly in early career when efficiency in clinical care duties is still being gained. Securing protected time for research is essential to develop a niche, build connections (both institutionally and beyond their institutions), and demonstrate productivity that can be utilized to support future grant efforts.
Similarly, using protected time efficiently is required. Without organization and planning, research time can be spent with scattered meetings and responding to various tasks that do not directly support your research. It is helpful to be introspective about the time of the day you are most productive in your research efforts and blocking off that time to focus on research tasks and minimizing distractions. Blocking monthly time for larger scale thinking and planning is also important. Weekly lab and individual one-on-one meetings also support time management for trainees and lab members, to ensure efficiency and progress. Additionally, robust clinical support is essential to ensure that research time remains protected and patient care moves forward. When negotiating for positions, and in regular meetings thereafter, it is important to advocate for sufficient clinical staffing such that non-physician tasks can be appropriately delegated to another member of the care team.
Funding
Securing adequate funding poses a significant challenge for all early-career physician-scientists, particularly because of the discrepancy between National Institutes of Health salary caps and the higher average salaries in academic gastroenterology. This financial gap can deter physicians from pursuing research-intensive careers altogether and can derail early investigators who do not obtain funding rapidly. To overcome this, early-career investigators may need to adopt flexible strategies, such as accepting a lower salary that aligns with grant funding limits or funneling incentive or bonus pay to research accounts. Alternatively, they can advocate for institutional support to bridge the salary gap, ensuring their research efforts remain financially viable.
Institutions committed to fostering research excellence may offer supplemental funding or bridge programs to retain talented physician-scientists, thereby mitigating the financial strain and encouraging long-term engagement in research. Regular meetings to review salary and support sources, including philanthropy, foundation grants, and other streams, should be undertaken with leadership to align the researcher’s timeline and available funding. If career development funding appears untenable, consideration of multi–principal investigator R01s or equivalent with senior established investigators can be a promising path.
Receiving Mentorship
Effective mentorship for early-career physician-scientists should be approached through a team-based model that leverages both internal and external mentors. Internal mentors, familiar with the specific culture, expectations, and advancement pathways of the institution, can provide invaluable guidance on navigating institutional metrics for success, such as promotion criteria, grant acquisition, and clinical-research balance. External mentors, on the other hand, bring a broader perspective by offering innovative career development strategies and solutions derived from experiences at their home institutions. This multimodal mentorship model ensures a well-rounded approach to professional growth.
All national gastroenterology societies, including the American Gastroenterological Association, the American College of Gastroenterology, and the American Society for Gastrointestinal Endoscopy, and American Association for the Study of Liver Disease, offer structured early-career mentorship programs designed to connect emerging researchers with experienced leaders in the field (see below). These programs typically require a formal application process and are highly regarded for their exceptional quality and impact. Participation in such initiatives can significantly enhance career development by expanding networks, fostering interdisciplinary collaboration, and providing tailored guidance that complements institutional support. Integrating both internal and external mentorship opportunities ensures a robust and dynamic foundation for long-term success in academic medicine.
- AGA Career Compass (https://gastro.org/fellows-and-early-career/mentoring/)
- ACG Early Career Leadership Program (https://gi.org/acg-institute/early-career-leadership-program/)
- AGA-AASLD Academic Skills Workshop (https://gastro.org/news/applications-now-being-accepted-for-the-2024-aga-aasld-academic-skills-workshop/)
- AASLD Women’s Initiative Committee Leadership Program (https://www.aasld.org/promoting-leadership-potential-women-hepatology)
- Scrubs n Heels Mentorship Matrix (https://scrubsandheels.com/matrix/)
Providing Mentorship
The trainee authors on this manuscript describe in this section what has been helpful for them as mentees in the FELD research lab.
Student doctor Nguyen describes her experience as a lab member and things she finds most helpful as a medical student in the lab:
- Upon joining the team, a one-to-one meeting to discuss trainee’s personal and professional goals, and availability, was crucial to building the mentor-mentee relationship. Establishing this meaningful mentorship early on clarified expectations on both sides, built trust, and increased motivation. As a trainee, it is essential for me to see how my work aligns with a long-term goal and to receive ample guidance throughout the process.
- One of the most impactful experiences has been joining informal lunch sessions where trainees discussed data collection protocols and exchanged insights. In doing so, Dr. Feld has cultivated a lab culture that encourages curiosity, constructive feedback, and collaborative learning.
- To increase productivity, our team of trainees created a useful group message thread where we coordinated more sessions to collaborate. This coordination formed stronger relationships between team members and fostered a sense of shared purpose.
Dr. Cooper, a third year internal medicine resident, describes her experience as both a research mentee and a mentor to the junior trainees: “As a resident pursuing a career in academic gastroenterology and hepatology, I have found three key elements to be most helpful: intentional mentorship, structured meetings, and leadership development.”
- Intentional mentorship: Prior to joining the lab, I met with Dr. Feld to discuss my research experience and my goals. She took the time to understand these within the context of my training timeline and tailored project opportunities that aligned with my interests and were both feasible and impactful for my next steps. This intentional approach not only fostered a productive research experience but also established a mentor-mentee relationship built on genuine care for my growth and development.
- Regular meetings: Frequent lab meetings promote accountability, teamwork, and shared problem-solving skills. The open exchange of ideas fosters collaboration and joint problem solving to elevate the quality of our research. They are also an opportunity to observe key decision-making points during the research process and have been a great way to learn more about solid methodology.
- Supervised leadership: I have had ample time to lead discussions and coordinate projects among the junior trainees. These monitored leadership experiences promote project management skills, mentorship, and team dynamic awareness while maintaining the safety net of senior guidance. This model helped me transition from a trainee supporting others’ research to a more independent role, contributing to multi-disciplinary projects while mentoring junior members.
Conclusion
In conclusion, many exciting opportunities and notable barriers exist to establishing a clinical research laboratory in the early career. While excellence in each of the areas outlined may evolve, some aspects will come easier than others and with time, persistence, and a bit of luck, the research world will be a better place because of your contributions!
Dr. Feld is assistant professor of gastroenterology and hepatology and physician executive of Diversity, Equity, Inclusion and Belonging for the department of medicine at the University of Massachusetts (UMass) Chan Medical School, Worcester. Ms. Nguyen is a medical student at UMass Chan Medical School. Dr. Cooper is a resident physician at UMass Chan Medical School. Dr. Rabinowitz is an attending physician in the Inflammatory Bowel Disease Center at Beth Israel Deaconess Medical Center, Boston, Mass. Dr. Uchida is codirector of the Multidisciplinary Eosinophilic Gastrointestinal Disease Clinic at the University of Utah School of Medicine, Salt Lake City.
Conducting clinical research as an early career gastroenterologist can take on many forms and has varying definitions of success. This article focuses on key factors to consider and should be supplemented with mentorship tailored to personal interests, goals, and institutional criteria for success. In this article, we will discuss selected high-yield topics that assist in early-career research. We will briefly discuss 1. Defining your niche, 2. Collaboration, 3. Visibility, 4. Time management, 5. Funding, 6. Receiving mentorship, and 7. Providing mentorship. We will conclude with discussing several authors’ experience in the research lab of the first author (FELD Lab – Fostering Equity in Liver and Digestive disease).
Defining Your Niche
Defining your niche is an essential component of an early career, as when academicians must transition from a trainee, who is supporting the research of an established mentor, to defining their own subspeciality area of investigation. Early-career academics should build on their prior work, but should also explore their own passions and skill set to define what will be unique about their research program and contributions to the field. Of course, positioning oneself at the intersection of two or more seemingly unrelated fields opens much opportunity for large impact but comes at a cost of identifying mentorship and justifying the niche to funders.
Collaboration
Fostering a collaborative environment is essential for early-career physician-researchers. One effective approach is to establish collaboration circles with other early career academics. Expanding research endeavors beyond a single institution to a multi-center framework enriches both scope and impact. This collaborative approach not only amplifies the depth of research but also facilitates peer mentorship and sponsorship. Participation in such networks can significantly enhance scholarly output and broaden professional reach during this critical phase of academic progression. Furthermore, prioritizing the promotion of colleagues within these networks is crucial. Proactive sponsorship opportunities, such as inviting peers to present at institutional seminars, strengthen both individual and collective academic visibility.
Collaboration is also essential to foster between trainees involved in early-career investigators’ work. An interconnected lab environment ensures that trainees remain informed about concurrent projects, thereby fostering a culture of shared knowledge and optimized productivity. Encouraging trainees to spearhead research aligned with their interests, under mentor guidance, nurtures independent inquiry and leadership. By establishing explicit roles, responsibilities, and authorship agreements at the outset of collaborative projects, early career mentors can avoid future conflicts and preserve a collaborative culture within the lab. This structured approach cultivates a supportive ecosystem, advancing both individual and collective research achievements.
Visibility
Establishing visibility and developing name recognition are crucial components of career advancement for early-career academic physicians. By clearly defining their areas of expertise, faculty can position themselves as leaders within their discipline. Active participation in professional societies, both at the local and national level, engagement with interest groups, and frequent contributions to educational events can be effective strategies for gaining recognition. Leveraging social media platforms can be helpful in enhancing visibility by facilitating connections and promoting research to a broader audience.
Moreover, research visibility plays a vital role in academic promotion. A strong publication record, reflected by an increasing h-index, demonstrates the impact and relevance of one’s research. Self-citation, when appropriate, can reinforce the continuity and progression of scholarly contributions. While publishing in high-impact journals is desirable, adaptability in resubmitting to other journals following rejections ensures that research remains visible and accessible. It also clearly establishes by whom the work was first done, before someone else investigates the line of inquiry. Through a combination of strategic engagement and publication efforts, early-career physicians can effectively build their professional reputation and advance their academic careers.
Time Management
Time management is essential for any research, and particularly in early career when efficiency in clinical care duties is still being gained. Securing protected time for research is essential to develop a niche, build connections (both institutionally and beyond their institutions), and demonstrate productivity that can be utilized to support future grant efforts.
Similarly, using protected time efficiently is required. Without organization and planning, research time can be spent with scattered meetings and responding to various tasks that do not directly support your research. It is helpful to be introspective about the time of the day you are most productive in your research efforts and blocking off that time to focus on research tasks and minimizing distractions. Blocking monthly time for larger scale thinking and planning is also important. Weekly lab and individual one-on-one meetings also support time management for trainees and lab members, to ensure efficiency and progress. Additionally, robust clinical support is essential to ensure that research time remains protected and patient care moves forward. When negotiating for positions, and in regular meetings thereafter, it is important to advocate for sufficient clinical staffing such that non-physician tasks can be appropriately delegated to another member of the care team.
Funding
Securing adequate funding poses a significant challenge for all early-career physician-scientists, particularly because of the discrepancy between National Institutes of Health salary caps and the higher average salaries in academic gastroenterology. This financial gap can deter physicians from pursuing research-intensive careers altogether and can derail early investigators who do not obtain funding rapidly. To overcome this, early-career investigators may need to adopt flexible strategies, such as accepting a lower salary that aligns with grant funding limits or funneling incentive or bonus pay to research accounts. Alternatively, they can advocate for institutional support to bridge the salary gap, ensuring their research efforts remain financially viable.
Institutions committed to fostering research excellence may offer supplemental funding or bridge programs to retain talented physician-scientists, thereby mitigating the financial strain and encouraging long-term engagement in research. Regular meetings to review salary and support sources, including philanthropy, foundation grants, and other streams, should be undertaken with leadership to align the researcher’s timeline and available funding. If career development funding appears untenable, consideration of multi–principal investigator R01s or equivalent with senior established investigators can be a promising path.
Receiving Mentorship
Effective mentorship for early-career physician-scientists should be approached through a team-based model that leverages both internal and external mentors. Internal mentors, familiar with the specific culture, expectations, and advancement pathways of the institution, can provide invaluable guidance on navigating institutional metrics for success, such as promotion criteria, grant acquisition, and clinical-research balance. External mentors, on the other hand, bring a broader perspective by offering innovative career development strategies and solutions derived from experiences at their home institutions. This multimodal mentorship model ensures a well-rounded approach to professional growth.
All national gastroenterology societies, including the American Gastroenterological Association, the American College of Gastroenterology, and the American Society for Gastrointestinal Endoscopy, and American Association for the Study of Liver Disease, offer structured early-career mentorship programs designed to connect emerging researchers with experienced leaders in the field (see below). These programs typically require a formal application process and are highly regarded for their exceptional quality and impact. Participation in such initiatives can significantly enhance career development by expanding networks, fostering interdisciplinary collaboration, and providing tailored guidance that complements institutional support. Integrating both internal and external mentorship opportunities ensures a robust and dynamic foundation for long-term success in academic medicine.
- AGA Career Compass (https://gastro.org/fellows-and-early-career/mentoring/)
- ACG Early Career Leadership Program (https://gi.org/acg-institute/early-career-leadership-program/)
- AGA-AASLD Academic Skills Workshop (https://gastro.org/news/applications-now-being-accepted-for-the-2024-aga-aasld-academic-skills-workshop/)
- AASLD Women’s Initiative Committee Leadership Program (https://www.aasld.org/promoting-leadership-potential-women-hepatology)
- Scrubs n Heels Mentorship Matrix (https://scrubsandheels.com/matrix/)
Providing Mentorship
The trainee authors on this manuscript describe in this section what has been helpful for them as mentees in the FELD research lab.
Student doctor Nguyen describes her experience as a lab member and things she finds most helpful as a medical student in the lab:
- Upon joining the team, a one-to-one meeting to discuss trainee’s personal and professional goals, and availability, was crucial to building the mentor-mentee relationship. Establishing this meaningful mentorship early on clarified expectations on both sides, built trust, and increased motivation. As a trainee, it is essential for me to see how my work aligns with a long-term goal and to receive ample guidance throughout the process.
- One of the most impactful experiences has been joining informal lunch sessions where trainees discussed data collection protocols and exchanged insights. In doing so, Dr. Feld has cultivated a lab culture that encourages curiosity, constructive feedback, and collaborative learning.
- To increase productivity, our team of trainees created a useful group message thread where we coordinated more sessions to collaborate. This coordination formed stronger relationships between team members and fostered a sense of shared purpose.
Dr. Cooper, a third year internal medicine resident, describes her experience as both a research mentee and a mentor to the junior trainees: “As a resident pursuing a career in academic gastroenterology and hepatology, I have found three key elements to be most helpful: intentional mentorship, structured meetings, and leadership development.”
- Intentional mentorship: Prior to joining the lab, I met with Dr. Feld to discuss my research experience and my goals. She took the time to understand these within the context of my training timeline and tailored project opportunities that aligned with my interests and were both feasible and impactful for my next steps. This intentional approach not only fostered a productive research experience but also established a mentor-mentee relationship built on genuine care for my growth and development.
- Regular meetings: Frequent lab meetings promote accountability, teamwork, and shared problem-solving skills. The open exchange of ideas fosters collaboration and joint problem solving to elevate the quality of our research. They are also an opportunity to observe key decision-making points during the research process and have been a great way to learn more about solid methodology.
- Supervised leadership: I have had ample time to lead discussions and coordinate projects among the junior trainees. These monitored leadership experiences promote project management skills, mentorship, and team dynamic awareness while maintaining the safety net of senior guidance. This model helped me transition from a trainee supporting others’ research to a more independent role, contributing to multi-disciplinary projects while mentoring junior members.
Conclusion
In conclusion, many exciting opportunities and notable barriers exist to establishing a clinical research laboratory in the early career. While excellence in each of the areas outlined may evolve, some aspects will come easier than others and with time, persistence, and a bit of luck, the research world will be a better place because of your contributions!
Dr. Feld is assistant professor of gastroenterology and hepatology and physician executive of Diversity, Equity, Inclusion and Belonging for the department of medicine at the University of Massachusetts (UMass) Chan Medical School, Worcester. Ms. Nguyen is a medical student at UMass Chan Medical School. Dr. Cooper is a resident physician at UMass Chan Medical School. Dr. Rabinowitz is an attending physician in the Inflammatory Bowel Disease Center at Beth Israel Deaconess Medical Center, Boston, Mass. Dr. Uchida is codirector of the Multidisciplinary Eosinophilic Gastrointestinal Disease Clinic at the University of Utah School of Medicine, Salt Lake City.
The Federal Trade Commission’s Non-Compete Ban
Non-compete agreements (NCAs) in physician contracts, also termed “restrictive covenants” or “covenants not to compete,” have become a hot topic recently because of the Federal Trade Commission’s (FTC’s) April 2024 ruling invalidating almost all NCAs. But in fact, NCAs have long been controversial, and no more so than in the realm of physician NCAs, which involve substantial policy concerns.
how it relates to a physician employment contract currently, and its possible evolution.
What is It?
Generally speaking, an NCA, usually in the form of an employment contract clause, is an agreement between the employer and the employee that the employee will not enter into post-contract competition with that employer within the limitations of a specific duration, scope of practice, and/or geography. NCAs have traditionally been regulated under state statutory law and common law and have been permitted based on policy considerations that attempt to balance competing employee and employer interests. Physicians should understand their states’ statutory treatment of an NCA.
NCAs protect important employer business interests, including the protection of proprietary information, safeguarding trade secrets, reducing employee turnover, and protecting patient lists. Employees, though, have limited mobility in changing professional positions, have less bargaining power with the employer, and may find themselves with limited options for comparable professional positions.
The NCA ostensibly appears to greatly benefit the employer’s interests over the employee’s; however, NCA protection of employer interests may also substantially benefit employees by encouraging substantial employer investment in employees whom the employer recognizes as a stable and likely long-term human resource, ultimately fostering increased employee satisfaction and innovation. Indeed, one concern with the FTC’s non-compete ban is the potential for significant underinvestment in information sharing and employee training, because employers would, without a NCA, be less likely to recoup those employee investments and would have limited ability to keep competitors from free-riding on investments in employees who leave and join competitors. Ultimately, this would lead to decreased market efficiency.
What is Its Status Today?
Regulation of NCAs, including physician NCAs, has traditionally been based on state statutory law and by common law. Perhaps because of the increasing use of the NCA in professional settings, the NCA has been increasingly scrutinized by courts and state legislatures in the last few decades, with an overall increasing focus on NCA reasonableness and appropriate fit in individual employment settings, and with an emphasis on employer demonstration of legitimate and significant business interests for using a NCA.
States have evolved differently in their treatment of NCAs; some states ban NCAs altogether while others allow them with varying interpretation and enforceability, frequently focused upon the NCA’s duration, scope, and geography. Similarly, in common law, courts will frequently invalidate NCAs that are found to be unreasonably overbroad, either geographically, temporally, and/or in regard to scope.
On April 23, 2024, however, the FTC altered this existing state of affairs by issuing a rule banning new NCAs in all employment situations after September 3, 2024. The rule also holds that existing NCAs are not enforceable, with a small carve-out for some senior executives. It applies to for-profit businesses, and some, but not all, non-profit organizations. The FTC’s stated intent is to reduce healthcare spending by increasing employee compensation and mobility. The FTC’s ban is likely meant to reduce transaction costs by increasing physician mobility.
There have been several lawsuits regarding the FTC ruling, challenging it on different grounds. The US District Court for the Northern District of Texas in Ryan LLC v. FTC issued first a preliminary injunction, then a final decision overturning the FTC’s rule. The Court held that the FTC had exceeded its statutory authority, and further, that the rule was arbitrary and capricious. It noted that the rule’s “categorical ban” has no equivalent in state law, is “unreasonably overbroad without a reasonable explanation,” “provides no evidence or reasoned basis,” does not “consider the positive benefits of non-compete agreements,” and does not “address alternatives to the Rule.” The Ryan Court reasoned that as an administrative agency, the FTC can only act as Congress authorizes by statute. On Oct. 18, 2024, the FTC appealed the Court’s decision to the Fifth Circuit Court of Appeals, seeking to reverse the holding setting aside its NCA ban.
The United States District Court for the Eastern District of Pennsylvania in ATS Tree Services LLC v. FTC denied the plaintiff’s motion to stay enforcement of the rule, refusing to issue a preliminary injunction preventing its implementation. As in Ryan, the ATS Tree Services LLC v. FTC plaintiffs argued that the FTC had exceeded its statutory authority in issuing the rule. However, the Plaintiff did not appeal the holding.
The US District Court for the Middle District of Florida in Properties of the Villages, Inc. v. FTCheld, like Ryan, that the rule exceeds the FTC’s statutory authority, noting the FTC’s prior lack of any NCA enforcement actions; however, its reasoning differed from Ryan. The Florida Court held that the FTC in fact has statutory authority to issue such rules; however, the Court held that the FTC could not enforce its rule because it violates the “major questions doctrine.” The “major questions doctrine” requires an agency such as the FTC to “point to clear congressional authorization” for any rule it issues that has “extraordinary ... economic and political significance,” as the NCA ban rule certainly does.
What is Its Future?
The FTC’s NCA ban remains unsettled. State legislatures, in response to the recent court holdings, are reassessing their statutory law regarding NCAs. The Ryan Court’s holding prevented the FTC’s rule from going into effect on September 4, 2024. The Texas and Florida court decisions are awaiting 5th and 11th Circuit Court of Appeals review, respectively. Assuming affirmation of either of the cases on appeal, a circuit split regarding the NCA ban may occur. The US Supreme Court may be called upon to determine the validity of the FTC rule banning NCAs. The Circuit Court decisions are likely to occur in 2025, and any Supreme Court decision would not likely occur until 2026. Meanwhile, state statutory law and common law still apply to NCAs, and the FTC may challenge the validity of NCAs on a case-by-case basis.
US antitrust law remains a potential remedy to scrutinize and restrain inappropriate business practices, including NCA-related abuses. The Sherman Act allows federal and state actors and private citizens, to sue for redress. Antitrust cases are typically considered using the “rule of reason” formulated by the Supreme Court in 1911, which requires plaintiffs show that defendant businesses possessing market power did in fact undertake anticompetitive conduct that had or likely had anticompetitive effects. In other words, the court in an antitrust case will require that the plaintiff show that the business actually had a significant controlling market presence in the geographic area; and further, that the plaintiff show that the business’ actions in fact had an anticompetitive effect, or likely had one. The latter can be found by showing an anticompetitive effect such as abusive pricing
The FTC’s ruling is legally and academically controversial and in fact may not withstand court scrutiny. The rule was put forth by the FTC as an ambitious rule to reduce healthcare spending. But businesses survive only if their revenue surpasses their costs, including personnel costs. Further, maximization of capitalization is attained when businesses require NCAs. Businesses invest heavily in recruiting, hiring, and training personnel, and increased personnel turnover increases these expenditures. NCAs arguably provide a collective benefit by ensuring force continuity, mitigating the risk of the loss of highly trained personnel with proprietary knowledge. NCAs also help a business maintain a skilled workforce, helping maximize business valuation. If FTC’s NCA ban rule were ultimately upheld, businesses would likely respond by instituting longer-term employee contracts, extended termination notice periods, and disincentives for employees who do not fully serve their contract length, including substantial financial disincentives. Business valuation might decrease, reducing investment incentives.
NCAs have long been a method of balancing the interests of employees and employers. They protect businesses’ confidential information, trade secrets, and patient lists, at some cost to employees pursuing new opportunities. The employee, though, is also provided with some benefit from the NCA, albeit indirect. State statutory law and courts have traditionally worked to ensure an appropriate delicate balance between interests, with courts generally finding unbalanced NCAs unenforceable.
For now, physicians should understand the policy considerations of and recognize the uncertainty surrounding NCAs, become familiar with their state’s statutory NCA law, review employment contracts carefully for NCA reasonableness, and seek legal advice if necessary.
Perhaps the FTC’s approach is the correct one for our future. Or perhaps the appropriate future of NCA interpretation and enforcement should continue to rest on state statutory law and common law, where antitrust enforcement is on a case-by-case basis, rather than FTC rulemaking. The results of high court decisions, state statutory law changes in response to the FTC rule, and perhaps US congressional action will provide the final answer.
Dr. Allen is based at the University of Oklahoma Health Sciences Center in Oklahoma City. He has declared no conflicts of interest in relation to this article.
Non-compete agreements (NCAs) in physician contracts, also termed “restrictive covenants” or “covenants not to compete,” have become a hot topic recently because of the Federal Trade Commission’s (FTC’s) April 2024 ruling invalidating almost all NCAs. But in fact, NCAs have long been controversial, and no more so than in the realm of physician NCAs, which involve substantial policy concerns.
how it relates to a physician employment contract currently, and its possible evolution.
What is It?
Generally speaking, an NCA, usually in the form of an employment contract clause, is an agreement between the employer and the employee that the employee will not enter into post-contract competition with that employer within the limitations of a specific duration, scope of practice, and/or geography. NCAs have traditionally been regulated under state statutory law and common law and have been permitted based on policy considerations that attempt to balance competing employee and employer interests. Physicians should understand their states’ statutory treatment of an NCA.
NCAs protect important employer business interests, including the protection of proprietary information, safeguarding trade secrets, reducing employee turnover, and protecting patient lists. Employees, though, have limited mobility in changing professional positions, have less bargaining power with the employer, and may find themselves with limited options for comparable professional positions.
The NCA ostensibly appears to greatly benefit the employer’s interests over the employee’s; however, NCA protection of employer interests may also substantially benefit employees by encouraging substantial employer investment in employees whom the employer recognizes as a stable and likely long-term human resource, ultimately fostering increased employee satisfaction and innovation. Indeed, one concern with the FTC’s non-compete ban is the potential for significant underinvestment in information sharing and employee training, because employers would, without a NCA, be less likely to recoup those employee investments and would have limited ability to keep competitors from free-riding on investments in employees who leave and join competitors. Ultimately, this would lead to decreased market efficiency.
What is Its Status Today?
Regulation of NCAs, including physician NCAs, has traditionally been based on state statutory law and by common law. Perhaps because of the increasing use of the NCA in professional settings, the NCA has been increasingly scrutinized by courts and state legislatures in the last few decades, with an overall increasing focus on NCA reasonableness and appropriate fit in individual employment settings, and with an emphasis on employer demonstration of legitimate and significant business interests for using a NCA.
States have evolved differently in their treatment of NCAs; some states ban NCAs altogether while others allow them with varying interpretation and enforceability, frequently focused upon the NCA’s duration, scope, and geography. Similarly, in common law, courts will frequently invalidate NCAs that are found to be unreasonably overbroad, either geographically, temporally, and/or in regard to scope.
On April 23, 2024, however, the FTC altered this existing state of affairs by issuing a rule banning new NCAs in all employment situations after September 3, 2024. The rule also holds that existing NCAs are not enforceable, with a small carve-out for some senior executives. It applies to for-profit businesses, and some, but not all, non-profit organizations. The FTC’s stated intent is to reduce healthcare spending by increasing employee compensation and mobility. The FTC’s ban is likely meant to reduce transaction costs by increasing physician mobility.
There have been several lawsuits regarding the FTC ruling, challenging it on different grounds. The US District Court for the Northern District of Texas in Ryan LLC v. FTC issued first a preliminary injunction, then a final decision overturning the FTC’s rule. The Court held that the FTC had exceeded its statutory authority, and further, that the rule was arbitrary and capricious. It noted that the rule’s “categorical ban” has no equivalent in state law, is “unreasonably overbroad without a reasonable explanation,” “provides no evidence or reasoned basis,” does not “consider the positive benefits of non-compete agreements,” and does not “address alternatives to the Rule.” The Ryan Court reasoned that as an administrative agency, the FTC can only act as Congress authorizes by statute. On Oct. 18, 2024, the FTC appealed the Court’s decision to the Fifth Circuit Court of Appeals, seeking to reverse the holding setting aside its NCA ban.
The United States District Court for the Eastern District of Pennsylvania in ATS Tree Services LLC v. FTC denied the plaintiff’s motion to stay enforcement of the rule, refusing to issue a preliminary injunction preventing its implementation. As in Ryan, the ATS Tree Services LLC v. FTC plaintiffs argued that the FTC had exceeded its statutory authority in issuing the rule. However, the Plaintiff did not appeal the holding.
The US District Court for the Middle District of Florida in Properties of the Villages, Inc. v. FTCheld, like Ryan, that the rule exceeds the FTC’s statutory authority, noting the FTC’s prior lack of any NCA enforcement actions; however, its reasoning differed from Ryan. The Florida Court held that the FTC in fact has statutory authority to issue such rules; however, the Court held that the FTC could not enforce its rule because it violates the “major questions doctrine.” The “major questions doctrine” requires an agency such as the FTC to “point to clear congressional authorization” for any rule it issues that has “extraordinary ... economic and political significance,” as the NCA ban rule certainly does.
What is Its Future?
The FTC’s NCA ban remains unsettled. State legislatures, in response to the recent court holdings, are reassessing their statutory law regarding NCAs. The Ryan Court’s holding prevented the FTC’s rule from going into effect on September 4, 2024. The Texas and Florida court decisions are awaiting 5th and 11th Circuit Court of Appeals review, respectively. Assuming affirmation of either of the cases on appeal, a circuit split regarding the NCA ban may occur. The US Supreme Court may be called upon to determine the validity of the FTC rule banning NCAs. The Circuit Court decisions are likely to occur in 2025, and any Supreme Court decision would not likely occur until 2026. Meanwhile, state statutory law and common law still apply to NCAs, and the FTC may challenge the validity of NCAs on a case-by-case basis.
US antitrust law remains a potential remedy to scrutinize and restrain inappropriate business practices, including NCA-related abuses. The Sherman Act allows federal and state actors and private citizens, to sue for redress. Antitrust cases are typically considered using the “rule of reason” formulated by the Supreme Court in 1911, which requires plaintiffs show that defendant businesses possessing market power did in fact undertake anticompetitive conduct that had or likely had anticompetitive effects. In other words, the court in an antitrust case will require that the plaintiff show that the business actually had a significant controlling market presence in the geographic area; and further, that the plaintiff show that the business’ actions in fact had an anticompetitive effect, or likely had one. The latter can be found by showing an anticompetitive effect such as abusive pricing
The FTC’s ruling is legally and academically controversial and in fact may not withstand court scrutiny. The rule was put forth by the FTC as an ambitious rule to reduce healthcare spending. But businesses survive only if their revenue surpasses their costs, including personnel costs. Further, maximization of capitalization is attained when businesses require NCAs. Businesses invest heavily in recruiting, hiring, and training personnel, and increased personnel turnover increases these expenditures. NCAs arguably provide a collective benefit by ensuring force continuity, mitigating the risk of the loss of highly trained personnel with proprietary knowledge. NCAs also help a business maintain a skilled workforce, helping maximize business valuation. If FTC’s NCA ban rule were ultimately upheld, businesses would likely respond by instituting longer-term employee contracts, extended termination notice periods, and disincentives for employees who do not fully serve their contract length, including substantial financial disincentives. Business valuation might decrease, reducing investment incentives.
NCAs have long been a method of balancing the interests of employees and employers. They protect businesses’ confidential information, trade secrets, and patient lists, at some cost to employees pursuing new opportunities. The employee, though, is also provided with some benefit from the NCA, albeit indirect. State statutory law and courts have traditionally worked to ensure an appropriate delicate balance between interests, with courts generally finding unbalanced NCAs unenforceable.
For now, physicians should understand the policy considerations of and recognize the uncertainty surrounding NCAs, become familiar with their state’s statutory NCA law, review employment contracts carefully for NCA reasonableness, and seek legal advice if necessary.
Perhaps the FTC’s approach is the correct one for our future. Or perhaps the appropriate future of NCA interpretation and enforcement should continue to rest on state statutory law and common law, where antitrust enforcement is on a case-by-case basis, rather than FTC rulemaking. The results of high court decisions, state statutory law changes in response to the FTC rule, and perhaps US congressional action will provide the final answer.
Dr. Allen is based at the University of Oklahoma Health Sciences Center in Oklahoma City. He has declared no conflicts of interest in relation to this article.
Non-compete agreements (NCAs) in physician contracts, also termed “restrictive covenants” or “covenants not to compete,” have become a hot topic recently because of the Federal Trade Commission’s (FTC’s) April 2024 ruling invalidating almost all NCAs. But in fact, NCAs have long been controversial, and no more so than in the realm of physician NCAs, which involve substantial policy concerns.
how it relates to a physician employment contract currently, and its possible evolution.
What is It?
Generally speaking, an NCA, usually in the form of an employment contract clause, is an agreement between the employer and the employee that the employee will not enter into post-contract competition with that employer within the limitations of a specific duration, scope of practice, and/or geography. NCAs have traditionally been regulated under state statutory law and common law and have been permitted based on policy considerations that attempt to balance competing employee and employer interests. Physicians should understand their states’ statutory treatment of an NCA.
NCAs protect important employer business interests, including the protection of proprietary information, safeguarding trade secrets, reducing employee turnover, and protecting patient lists. Employees, though, have limited mobility in changing professional positions, have less bargaining power with the employer, and may find themselves with limited options for comparable professional positions.
The NCA ostensibly appears to greatly benefit the employer’s interests over the employee’s; however, NCA protection of employer interests may also substantially benefit employees by encouraging substantial employer investment in employees whom the employer recognizes as a stable and likely long-term human resource, ultimately fostering increased employee satisfaction and innovation. Indeed, one concern with the FTC’s non-compete ban is the potential for significant underinvestment in information sharing and employee training, because employers would, without a NCA, be less likely to recoup those employee investments and would have limited ability to keep competitors from free-riding on investments in employees who leave and join competitors. Ultimately, this would lead to decreased market efficiency.
What is Its Status Today?
Regulation of NCAs, including physician NCAs, has traditionally been based on state statutory law and by common law. Perhaps because of the increasing use of the NCA in professional settings, the NCA has been increasingly scrutinized by courts and state legislatures in the last few decades, with an overall increasing focus on NCA reasonableness and appropriate fit in individual employment settings, and with an emphasis on employer demonstration of legitimate and significant business interests for using a NCA.
States have evolved differently in their treatment of NCAs; some states ban NCAs altogether while others allow them with varying interpretation and enforceability, frequently focused upon the NCA’s duration, scope, and geography. Similarly, in common law, courts will frequently invalidate NCAs that are found to be unreasonably overbroad, either geographically, temporally, and/or in regard to scope.
On April 23, 2024, however, the FTC altered this existing state of affairs by issuing a rule banning new NCAs in all employment situations after September 3, 2024. The rule also holds that existing NCAs are not enforceable, with a small carve-out for some senior executives. It applies to for-profit businesses, and some, but not all, non-profit organizations. The FTC’s stated intent is to reduce healthcare spending by increasing employee compensation and mobility. The FTC’s ban is likely meant to reduce transaction costs by increasing physician mobility.
There have been several lawsuits regarding the FTC ruling, challenging it on different grounds. The US District Court for the Northern District of Texas in Ryan LLC v. FTC issued first a preliminary injunction, then a final decision overturning the FTC’s rule. The Court held that the FTC had exceeded its statutory authority, and further, that the rule was arbitrary and capricious. It noted that the rule’s “categorical ban” has no equivalent in state law, is “unreasonably overbroad without a reasonable explanation,” “provides no evidence or reasoned basis,” does not “consider the positive benefits of non-compete agreements,” and does not “address alternatives to the Rule.” The Ryan Court reasoned that as an administrative agency, the FTC can only act as Congress authorizes by statute. On Oct. 18, 2024, the FTC appealed the Court’s decision to the Fifth Circuit Court of Appeals, seeking to reverse the holding setting aside its NCA ban.
The United States District Court for the Eastern District of Pennsylvania in ATS Tree Services LLC v. FTC denied the plaintiff’s motion to stay enforcement of the rule, refusing to issue a preliminary injunction preventing its implementation. As in Ryan, the ATS Tree Services LLC v. FTC plaintiffs argued that the FTC had exceeded its statutory authority in issuing the rule. However, the Plaintiff did not appeal the holding.
The US District Court for the Middle District of Florida in Properties of the Villages, Inc. v. FTCheld, like Ryan, that the rule exceeds the FTC’s statutory authority, noting the FTC’s prior lack of any NCA enforcement actions; however, its reasoning differed from Ryan. The Florida Court held that the FTC in fact has statutory authority to issue such rules; however, the Court held that the FTC could not enforce its rule because it violates the “major questions doctrine.” The “major questions doctrine” requires an agency such as the FTC to “point to clear congressional authorization” for any rule it issues that has “extraordinary ... economic and political significance,” as the NCA ban rule certainly does.
What is Its Future?
The FTC’s NCA ban remains unsettled. State legislatures, in response to the recent court holdings, are reassessing their statutory law regarding NCAs. The Ryan Court’s holding prevented the FTC’s rule from going into effect on September 4, 2024. The Texas and Florida court decisions are awaiting 5th and 11th Circuit Court of Appeals review, respectively. Assuming affirmation of either of the cases on appeal, a circuit split regarding the NCA ban may occur. The US Supreme Court may be called upon to determine the validity of the FTC rule banning NCAs. The Circuit Court decisions are likely to occur in 2025, and any Supreme Court decision would not likely occur until 2026. Meanwhile, state statutory law and common law still apply to NCAs, and the FTC may challenge the validity of NCAs on a case-by-case basis.
US antitrust law remains a potential remedy to scrutinize and restrain inappropriate business practices, including NCA-related abuses. The Sherman Act allows federal and state actors and private citizens, to sue for redress. Antitrust cases are typically considered using the “rule of reason” formulated by the Supreme Court in 1911, which requires plaintiffs show that defendant businesses possessing market power did in fact undertake anticompetitive conduct that had or likely had anticompetitive effects. In other words, the court in an antitrust case will require that the plaintiff show that the business actually had a significant controlling market presence in the geographic area; and further, that the plaintiff show that the business’ actions in fact had an anticompetitive effect, or likely had one. The latter can be found by showing an anticompetitive effect such as abusive pricing
The FTC’s ruling is legally and academically controversial and in fact may not withstand court scrutiny. The rule was put forth by the FTC as an ambitious rule to reduce healthcare spending. But businesses survive only if their revenue surpasses their costs, including personnel costs. Further, maximization of capitalization is attained when businesses require NCAs. Businesses invest heavily in recruiting, hiring, and training personnel, and increased personnel turnover increases these expenditures. NCAs arguably provide a collective benefit by ensuring force continuity, mitigating the risk of the loss of highly trained personnel with proprietary knowledge. NCAs also help a business maintain a skilled workforce, helping maximize business valuation. If FTC’s NCA ban rule were ultimately upheld, businesses would likely respond by instituting longer-term employee contracts, extended termination notice periods, and disincentives for employees who do not fully serve their contract length, including substantial financial disincentives. Business valuation might decrease, reducing investment incentives.
NCAs have long been a method of balancing the interests of employees and employers. They protect businesses’ confidential information, trade secrets, and patient lists, at some cost to employees pursuing new opportunities. The employee, though, is also provided with some benefit from the NCA, albeit indirect. State statutory law and courts have traditionally worked to ensure an appropriate delicate balance between interests, with courts generally finding unbalanced NCAs unenforceable.
For now, physicians should understand the policy considerations of and recognize the uncertainty surrounding NCAs, become familiar with their state’s statutory NCA law, review employment contracts carefully for NCA reasonableness, and seek legal advice if necessary.
Perhaps the FTC’s approach is the correct one for our future. Or perhaps the appropriate future of NCA interpretation and enforcement should continue to rest on state statutory law and common law, where antitrust enforcement is on a case-by-case basis, rather than FTC rulemaking. The results of high court decisions, state statutory law changes in response to the FTC rule, and perhaps US congressional action will provide the final answer.
Dr. Allen is based at the University of Oklahoma Health Sciences Center in Oklahoma City. He has declared no conflicts of interest in relation to this article.
Avoid Getting Stuck: A Practical Guide to Managing Chronic Constipation
Introduction
Constipation affects one in six people worldwide and accounts for one third of outpatient visits.1 Chronic constipation is defined by difficult, infrequent, and/or incomplete defecation, quantified by less than three spontaneous bowel movements per week, persisting for at least 3 months. Patients may complain of straining during defecation, incomplete evacuation, hard stools (Bristol stool scale [BSS] type 1-2), and fullness or bloating. Chronic constipation can be subclassified as either a primary or secondary disorder.1,2
Primary Constipation Disorders
Primary constipation includes disorders of the colon or anorectum. This includes irritable bowel syndrome with constipation (IBS-C), chronic idiopathic constipation (CIC), slow transit constipation (STC), dyssynergic defecation, and pelvic floor disorders (see Figure 1).
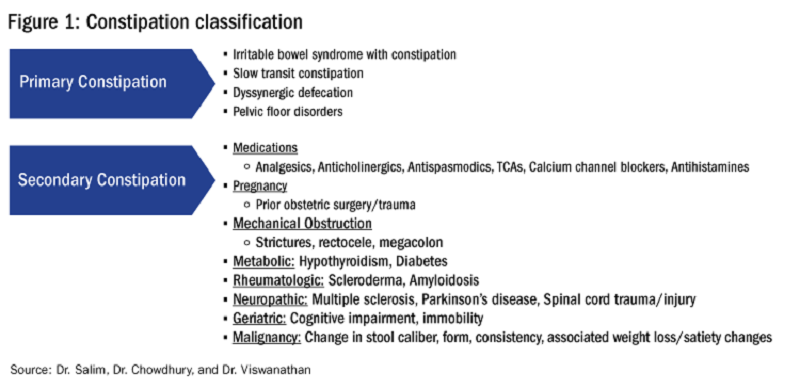
IBS-C
IBS-C is a chronic disorder of the gut-brain axis with a worldwide prevalence of 1.3% and a prevalence of 6%-16% in the United States, United Kingdom, and Canada, with females more likely to seek care than males.2 The economic impact of IBS-C is estimated to be $1.5 billion–$10 billion per year in the United States alone.3 The distinguishing characteristic is abdominal pain, however IBS-C can present with a constellation of symptoms. The diagnostic paradigm has shifted from IBS being a diagnosis of exclusion to now using a positive diagnostic strategy.2 Using this Rome IV criteria, one can make the diagnosis with > 95% accuracy.2,4
CIC
CIC, previously defined as functional constipation, is a disorder defined by incomplete defecation and difficult or infrequent stool. CIC is diagnosed in patients without an underlying anatomic or structural abnormality. Rome IV Criteria helps further classify the defining characteristics of chronic idiopathic constipation.2
Slow Transit Constipation
STC is characterized by impaired colonic transit time in the absence of pelvic floor dysfunction. It presents with infrequent bowel movements, diminished urgency, and/or straining with defecation.
Defecatory Disorders: Dyssynergic Defecation and Pelvic Floor Dysfunction
Defecatory disorders (DDs) result from alterations in the colonic-neural pathway with an unclear pathogenesis. A firm understanding of colonic physiology is necessary to identify DDs. The right colon helps to store and mix stool contents, the left colon helps add water to the stool, and the anal canal and rectum enable defecation and maintain continence. Any alteration along this physiologic pathway results in DDs.5
DDs primarily develop via maladaptive pelvic floor contraction during defecation or from muscle or nerve injury and include functional outlet obstruction, anorectal dyssynergia, and pelvic floor dysfunction. Increased resistance to defecation results from anismus, paradoxical anal sphincter contraction, or incomplete relaxation of the pelvic floor and external anal sphincter. This muscle incoordination is described as dyssynergia. DDs can involve either muscle or nerve dysfunction or a combination of the two. Reduced rectal sensation caused by reduced sensory triggers can cause stasis of stool, thus propagating the cycle of constipation. Over time, excessive straining can weaken the pelvic floor, increasing the risk of excessive perineal descent, rectal intussusception, solitary rectal ulcer syndrome, and pudendal neuropathy.5 Thus, identification of DDs is crucial in patients with chronic constipation.
Secondary Constipation Disorders
Secondary constipation disorders are a result of an alternate process and warrant a thorough review of outpatient medications and past medical history. Figure 1 outlines the most common causes of secondary constipation, which span a wide differential.
Clinical Evaluation
The evaluation of constipation begins with a thorough history. Description of bowel habits should include frequency, duration, straining, stool consistency using a Bristol stool chart, complete vs incomplete evacuation, pain, bloating, and use of digital maneuvers (vaginal splinting or digital stool removal). One should inquire about back trauma/surgeries and obstetric history to include vaginal forceps injury or episiotomy.
With increased smartphone use, toilet time on average has increased and can contribute to maladaptive bowel habits.6 Patients may not realize they are constipated, so patient education is critical. A patient with daily bowel movements ranging between BSS type 1-6 with incomplete evacuation might complain of diarrhea but may in fact have constipation with overflow diarrhea, for example. Past medical history is also clinically relevant, as systemic conditions can cause secondary constipation. A constipated patient should also be asked what therapies he/she has tried prior to gastroenterology referral as primary care referrals for constipation account for 8 million visits to gastroenterology per year.7
While a sensitive topic, inquire about abuse history, especially in those with childhood constipation symptoms. There is a positive correlation between childhood constipation and physical, emotional, and sexual abuse and, for any number of reasons, your patient may be reluctant to share this or undergo a digital rectal exam (DRE).8 In such cases, be sensitive in asking for this history in private rather than with other family members around and always perform this exam with a chaperone present.
A detailed physical exam is an indispensable tool all gastroenterologists must master when evaluating a constipated patient. Some key exam findings include abdominal distention, high-pitched bowel sounds, and presence of a succussion splash indicating obstructive pathology. Dry skin and brittle hair indicate hypothyroidism while hypermobile joints and skin laxity suggest connective tissue disease. Finally, a physical examination is incomplete without a DRE.
DRE
DRE is an often-overlooked physical exam component which provides helpful insight that can guide management. An informed DRE can help identify structural disorders such as fissure, hemorrhoids, anorectal mass, fecal impaction, rectal prolapse, and excessive perineal descent syndrome.9 Unless contraindicated, DRE should be a standard part of the workup of a patient with chronic constipation.
Workup
Colonoscopy
The role of colonoscopy in chronic constipation is low yield and only indicated if alarm signs are present.2 When no organic causes can be identified, the patient is deemed to have a functional bowel or motility disorder leading to constipation.
Colonic Transit Time
Colonic transit time (CTT) can be evaluated by assessing the presence of radio-opaque sitz markers in the colon with an abdominal x-ray 5 days after ingestion. The presence of five or more sitz markers may indicate STC. However, this can also signal an obstructive defecatory disorder. Colon scintigraphy can determine whether there is diffuse colonic dysmotility or dysfunction in a specific segment of the colon.10
Anorectal Function Testing (AFT)
AFT can evaluate DDs, such as fecal incontinence, dyssynergic defecation, rectal sensory disorders, anorectal pain, and rectal prolapse. AFT comprises three tests: anorectal manometry (ARM), balloon expulsion test (BET), and rectal sensory testing. These assess the defecation, continence, and sensory mechanisms of the rectum, respectively.
ARM testing employs a thin, flexible probe with an attached sensor that is inserted into the rectum to measure internal and external sphincter pressures while at rest, squeezing, and bearing down to give a functional assessment of sphincter tone.11 Cough or party balloon test assesses continence and sphincter strength. Rectal sensation is assessed by inflating a balloon incrementally and asking the patient to indicate first sensation, urgency to defecate, and discomfort. If both ARM and BET are abnormal, the patient meets diagnostic criteria for dyssynergic defecation.12
Pelvic floor disorders can be further assessed by MR defecography or barium defecography. Barium defecography is the more widely available of the two. MR defecography is a dynamic study that directly assesses pelvic floor muscles and endopelvic fascia during various stages of defecation and considered superior. This testing modality can distinguish between functional causes such as dyssynergia or pelvic floor dysfunction and structural causes of obstruction such as rectocele, rectal prolapse, or rectal intussusception. MR defecography is helpful when dyssynergia is suggested by ARM with a normal BET or if there is an absent recto-anal inhibitory reflex on ARM, which may suggest rectal intussusception.
Management
CIC
Incorporating 20-30 g of total soluble fiber, such as psyllium in individuals with low dietary fiber intake is the first-line recommendation for CIC.13 If response to a trial of fiber supplementation is inadequate, over-the-counter (OTC) osmotic laxatives such as polyethylene glycol and magnesium oxide can be incorporated. In the event of failure of OTC osmotic laxatives, lactulose can be considered. Stimulant laxatives such as senna, bisacodyl, or sodium picosulfate can be added as an adjunctive measure for short periods of time, defined as daily for 4 weeks or less.
If these measures are inadequate, pharmacological therapy with secretagogues and 5HT agonists can be considered. Prucalopride, a selective agonist of serotonin 5-HT4 receptors, is approved for CIC, prescribed 2 mg daily.14 It can also be used in patients with global motility delays, such as gastroparetics with constipation. The mechanism of action of secretagogues and specific dosing of these medications are discussed in Figure 2.15 Vibrant is a non-pharmacologic, orally ingested, vibrating, and programmable capsule device that has recently received Food and Drug Administration approval for treatment of chronic constipation by stimulating the intestinal wall, thereby promoting colonic contractile activity to achieve more spontaneous bowel movements. Further studies are required to assess its efficacy.16 Additionally, if there is inadequate response to all the above, it would be prudent to evaluate for the presence of pelvic floor dysfunction as well.
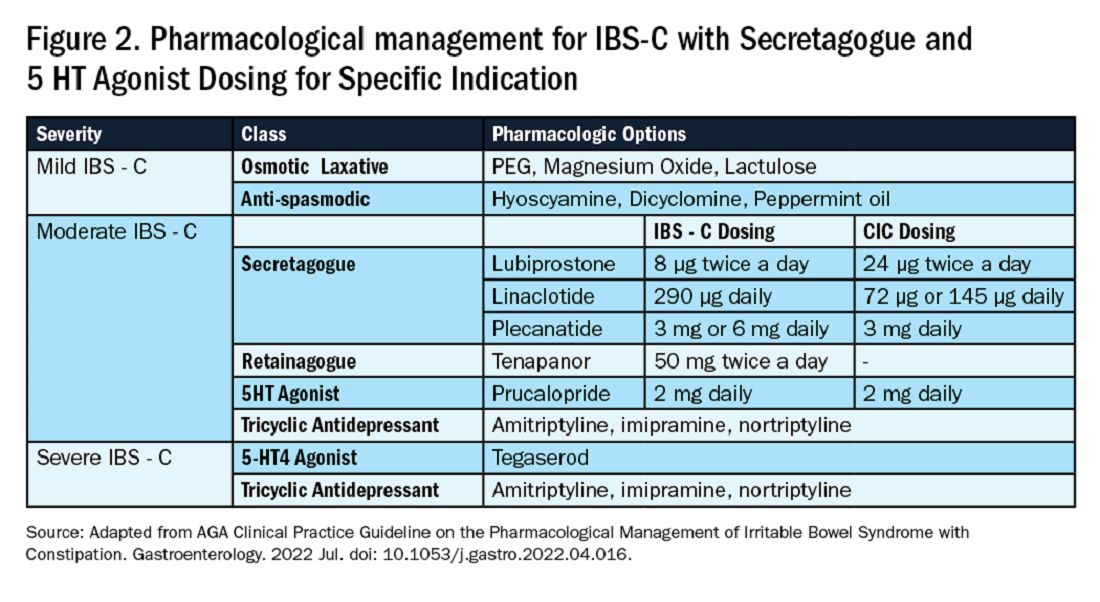
IBS-C
Similar to CIC, treatment for mild IBS-C starts with osmotic laxatives with the additional component of pain control. Antispasmodics can be used to manage the abdominal pain, cramping, and spasms associated with IBS-C. Antispasmodics available in the United States include anticholinergic agents that cause smooth muscle relaxation, such as dicyclomine or hyoscyamine or direct smooth muscle relaxants such as peppermint oil.17 IBS-C patients with moderate symptoms may need escalation of therapy to secretagogues or 5HT agonists (see Figure 2). Secretagogues increase fluid retention in the colonic lumen to promote bowel movements and improve visceral hypersensitivity. Lubiprostone is an intestinal chloride channel activator, indicated only for adult women with IBS-C. Linaclotide and plecanatide are guanylate cyclase-C activators which increase intestinal chloride and bicarbonate secretion, and both are indicated in IBS-C and CIC. Tenapanor inhibits the sodium/hydrogen exchanger in the GI tract, leading to increased water secretion, and is recommended for IBS-C in adults who have failed secretagogues.
All four of these drugs can be considered for moderate to severe IBS-C symptoms. In the case of severe IBS-C symptoms, Tegaserod, a 5-HT4 receptor partial agonist has been approved in women under 65 without significant cardiovascular or cerebrovascular disease.18 Regardless of IBS-C symptom severity, persistent visceral hypersensitivity can be treated with low-dose neuromodulators.19 Figure 2 provides treatment recommendations for IBS-C based on symptom severity.
Opioid-Induced Constipation (OIC)
In patients with OIC, peripherally acting mu-opioid receptor antagonists such as methylnaltrexone and naloxegol can be beneficial where stimulant laxatives are insufficient. Additionally, lubiprostone is indicated in OIC in non-cancer patients. At present, there are no head-to-head trials comparing efficacy of these medications.
Defecatory Disorders
Biofeedback therapy is the cornerstone of treatment for dyssynergic defecation, focusing on neuromuscular training to restore a normal pattern of defecation by teaching patients to tense the abdomen and relax the pelvic floor muscles and anal sphincter. It retrains the body to coordinate abdominal, rectal, and anal muscles to achieve synchronous contraction to achieve complete evacuation. It also increases awareness and response to rectal fullness or the need to defecate.
Biofeedback makes patients aware of counterproductive subconscious actions such as contracting of their anal sphincter during defecation followed by simulated defecation training with focus on how to tighten abdominal muscles and relax pelvic floor muscles to initiate and complete defecation.20 This is performed in the office with a physiotherapist or trained nurse for at least six sessions or at home where patients are encouraged to perform the exercises for 20 minutes, twice a day. These sessions utilize tools such as manometry probes, electromyography probes, simulated balloon, or home biofeedback training devices to provide visual feedback while practicing abdominophrenic breathing. Biofeedback is particularly helpful in patients suffering from constipation. Patients with defecatory disorders can also benefit from pelvic floor physical therapy which focuses on strengthening the pelvic and puborectal muscles, external anal sphincter, and pelvic muscles. This is more useful in patients with fecal incontinence. Despite all these treatments, a subset of patients may still not respond and may qualify for surgical evaluation.
Conclusion
While constipation is seldom life-threatening, it has a negative impact on patient quality of life and poses a significant financial burden on our overall healthcare system. The complexity of this condition should be appreciated and understood in order for a complete and thorough evaluation. We trust that our practical guide should serve as a useful tool in the evaluation of a chronically constipated patient.
Dr. Salim (@hamsalim07 on X) is based in the Department of Internal Medicine, University of Texas Medical Branch, Galveston. Dr. Chowdhury (annicho.med on Instagram) is a fellow in the Department of Gastroenterology, Hepatology, and Nutrition, University of Texas MD Anderson Cancer Center, Houston. Dr. Viswanathan (@LavanyaMD on X) is Associate Professor, University of Texas MD Anderson Cancer Center. The authors declare no conflict of interest.
References
1. Mugie S et al. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2010.12.010.
2. Almario CV et al. Gastroenterology. 2023 Dec. doi: 10.1053/j.gastro.2023.08.010.
3. Canavan C et al. Clin Epidemiol. 2014 Feb. doi: 10.2147/CLEP.S40245.
4. Rao SSC. Gastroenterol Clin North Am. 2007 Sep. doi: 10.1016/j.gtc.2007.07.013.
5. Bharucha AE et al. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
6. Cinquetti M et al. Clin Exp Pediatr. 2021 Sep. doi: 10.3345/cep.2020.01326.
7. Shah ND et al. Am J Gastroenterol. 2008 Jul. doi: 10.1111/j.1572-0241.2008.01910.x.
8. Rajindrajith S et al. J Pediatr Gastroenterol Nutr. 2014 Apr. doi: 10.1097/MPG.0000000000000249.
9. Talley NJ. Am J Gastroenterol. 2008 Apr. doi: 10.1111/j.1572-0241.2008.01832.x.
10. Maurer AH. J Nucl Med. 2015 Sep. doi: 10.2967/jnumed.113.134551.
11. Frye J et al. Am J Gastroenterol. 2024 Aug. doi: 10.14309/ajg.0000000000002670.
12. Rao SSC et al. J Neurogastroenterol Motil. 2016 Jun. doi: 10.5056/jnm16060.
13. Chang L et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.03.214.
14. Brenner DM et al. Am J Gastroenterol. 2021 Aug. doi: 10.14309/ajg.0000000000001266.
15. Chang L et al. Gastroenterology. 2022 Jul. doi: 10.1053/j.gastro.2022.04.016.
16. Rao SSC et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.02.013.
17. Lacy BE et al. Am J Gastroenterol. 2021 Jan. doi: 10.14309/ajg.0000000000001036.
18. Anderson JL et al. J Cardiovasc Pharmacol Ther. 2009 Sep. doi: 10.1177/1074248409340158.
19. Rahimi R et al. World J Gastroenterol. 2009 Apr. doi: 10.3748/wjg.15.1548.
20. Rao SSC. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2011.01.004.
Introduction
Constipation affects one in six people worldwide and accounts for one third of outpatient visits.1 Chronic constipation is defined by difficult, infrequent, and/or incomplete defecation, quantified by less than three spontaneous bowel movements per week, persisting for at least 3 months. Patients may complain of straining during defecation, incomplete evacuation, hard stools (Bristol stool scale [BSS] type 1-2), and fullness or bloating. Chronic constipation can be subclassified as either a primary or secondary disorder.1,2
Primary Constipation Disorders
Primary constipation includes disorders of the colon or anorectum. This includes irritable bowel syndrome with constipation (IBS-C), chronic idiopathic constipation (CIC), slow transit constipation (STC), dyssynergic defecation, and pelvic floor disorders (see Figure 1).

IBS-C
IBS-C is a chronic disorder of the gut-brain axis with a worldwide prevalence of 1.3% and a prevalence of 6%-16% in the United States, United Kingdom, and Canada, with females more likely to seek care than males.2 The economic impact of IBS-C is estimated to be $1.5 billion–$10 billion per year in the United States alone.3 The distinguishing characteristic is abdominal pain, however IBS-C can present with a constellation of symptoms. The diagnostic paradigm has shifted from IBS being a diagnosis of exclusion to now using a positive diagnostic strategy.2 Using this Rome IV criteria, one can make the diagnosis with > 95% accuracy.2,4
CIC
CIC, previously defined as functional constipation, is a disorder defined by incomplete defecation and difficult or infrequent stool. CIC is diagnosed in patients without an underlying anatomic or structural abnormality. Rome IV Criteria helps further classify the defining characteristics of chronic idiopathic constipation.2
Slow Transit Constipation
STC is characterized by impaired colonic transit time in the absence of pelvic floor dysfunction. It presents with infrequent bowel movements, diminished urgency, and/or straining with defecation.
Defecatory Disorders: Dyssynergic Defecation and Pelvic Floor Dysfunction
Defecatory disorders (DDs) result from alterations in the colonic-neural pathway with an unclear pathogenesis. A firm understanding of colonic physiology is necessary to identify DDs. The right colon helps to store and mix stool contents, the left colon helps add water to the stool, and the anal canal and rectum enable defecation and maintain continence. Any alteration along this physiologic pathway results in DDs.5
DDs primarily develop via maladaptive pelvic floor contraction during defecation or from muscle or nerve injury and include functional outlet obstruction, anorectal dyssynergia, and pelvic floor dysfunction. Increased resistance to defecation results from anismus, paradoxical anal sphincter contraction, or incomplete relaxation of the pelvic floor and external anal sphincter. This muscle incoordination is described as dyssynergia. DDs can involve either muscle or nerve dysfunction or a combination of the two. Reduced rectal sensation caused by reduced sensory triggers can cause stasis of stool, thus propagating the cycle of constipation. Over time, excessive straining can weaken the pelvic floor, increasing the risk of excessive perineal descent, rectal intussusception, solitary rectal ulcer syndrome, and pudendal neuropathy.5 Thus, identification of DDs is crucial in patients with chronic constipation.
Secondary Constipation Disorders
Secondary constipation disorders are a result of an alternate process and warrant a thorough review of outpatient medications and past medical history. Figure 1 outlines the most common causes of secondary constipation, which span a wide differential.
Clinical Evaluation
The evaluation of constipation begins with a thorough history. Description of bowel habits should include frequency, duration, straining, stool consistency using a Bristol stool chart, complete vs incomplete evacuation, pain, bloating, and use of digital maneuvers (vaginal splinting or digital stool removal). One should inquire about back trauma/surgeries and obstetric history to include vaginal forceps injury or episiotomy.
With increased smartphone use, toilet time on average has increased and can contribute to maladaptive bowel habits.6 Patients may not realize they are constipated, so patient education is critical. A patient with daily bowel movements ranging between BSS type 1-6 with incomplete evacuation might complain of diarrhea but may in fact have constipation with overflow diarrhea, for example. Past medical history is also clinically relevant, as systemic conditions can cause secondary constipation. A constipated patient should also be asked what therapies he/she has tried prior to gastroenterology referral as primary care referrals for constipation account for 8 million visits to gastroenterology per year.7
While a sensitive topic, inquire about abuse history, especially in those with childhood constipation symptoms. There is a positive correlation between childhood constipation and physical, emotional, and sexual abuse and, for any number of reasons, your patient may be reluctant to share this or undergo a digital rectal exam (DRE).8 In such cases, be sensitive in asking for this history in private rather than with other family members around and always perform this exam with a chaperone present.
A detailed physical exam is an indispensable tool all gastroenterologists must master when evaluating a constipated patient. Some key exam findings include abdominal distention, high-pitched bowel sounds, and presence of a succussion splash indicating obstructive pathology. Dry skin and brittle hair indicate hypothyroidism while hypermobile joints and skin laxity suggest connective tissue disease. Finally, a physical examination is incomplete without a DRE.
DRE
DRE is an often-overlooked physical exam component which provides helpful insight that can guide management. An informed DRE can help identify structural disorders such as fissure, hemorrhoids, anorectal mass, fecal impaction, rectal prolapse, and excessive perineal descent syndrome.9 Unless contraindicated, DRE should be a standard part of the workup of a patient with chronic constipation.
Workup
Colonoscopy
The role of colonoscopy in chronic constipation is low yield and only indicated if alarm signs are present.2 When no organic causes can be identified, the patient is deemed to have a functional bowel or motility disorder leading to constipation.
Colonic Transit Time
Colonic transit time (CTT) can be evaluated by assessing the presence of radio-opaque sitz markers in the colon with an abdominal x-ray 5 days after ingestion. The presence of five or more sitz markers may indicate STC. However, this can also signal an obstructive defecatory disorder. Colon scintigraphy can determine whether there is diffuse colonic dysmotility or dysfunction in a specific segment of the colon.10
Anorectal Function Testing (AFT)
AFT can evaluate DDs, such as fecal incontinence, dyssynergic defecation, rectal sensory disorders, anorectal pain, and rectal prolapse. AFT comprises three tests: anorectal manometry (ARM), balloon expulsion test (BET), and rectal sensory testing. These assess the defecation, continence, and sensory mechanisms of the rectum, respectively.
ARM testing employs a thin, flexible probe with an attached sensor that is inserted into the rectum to measure internal and external sphincter pressures while at rest, squeezing, and bearing down to give a functional assessment of sphincter tone.11 Cough or party balloon test assesses continence and sphincter strength. Rectal sensation is assessed by inflating a balloon incrementally and asking the patient to indicate first sensation, urgency to defecate, and discomfort. If both ARM and BET are abnormal, the patient meets diagnostic criteria for dyssynergic defecation.12
Pelvic floor disorders can be further assessed by MR defecography or barium defecography. Barium defecography is the more widely available of the two. MR defecography is a dynamic study that directly assesses pelvic floor muscles and endopelvic fascia during various stages of defecation and considered superior. This testing modality can distinguish between functional causes such as dyssynergia or pelvic floor dysfunction and structural causes of obstruction such as rectocele, rectal prolapse, or rectal intussusception. MR defecography is helpful when dyssynergia is suggested by ARM with a normal BET or if there is an absent recto-anal inhibitory reflex on ARM, which may suggest rectal intussusception.
Management
CIC
Incorporating 20-30 g of total soluble fiber, such as psyllium in individuals with low dietary fiber intake is the first-line recommendation for CIC.13 If response to a trial of fiber supplementation is inadequate, over-the-counter (OTC) osmotic laxatives such as polyethylene glycol and magnesium oxide can be incorporated. In the event of failure of OTC osmotic laxatives, lactulose can be considered. Stimulant laxatives such as senna, bisacodyl, or sodium picosulfate can be added as an adjunctive measure for short periods of time, defined as daily for 4 weeks or less.
If these measures are inadequate, pharmacological therapy with secretagogues and 5HT agonists can be considered. Prucalopride, a selective agonist of serotonin 5-HT4 receptors, is approved for CIC, prescribed 2 mg daily.14 It can also be used in patients with global motility delays, such as gastroparetics with constipation. The mechanism of action of secretagogues and specific dosing of these medications are discussed in Figure 2.15 Vibrant is a non-pharmacologic, orally ingested, vibrating, and programmable capsule device that has recently received Food and Drug Administration approval for treatment of chronic constipation by stimulating the intestinal wall, thereby promoting colonic contractile activity to achieve more spontaneous bowel movements. Further studies are required to assess its efficacy.16 Additionally, if there is inadequate response to all the above, it would be prudent to evaluate for the presence of pelvic floor dysfunction as well.

IBS-C
Similar to CIC, treatment for mild IBS-C starts with osmotic laxatives with the additional component of pain control. Antispasmodics can be used to manage the abdominal pain, cramping, and spasms associated with IBS-C. Antispasmodics available in the United States include anticholinergic agents that cause smooth muscle relaxation, such as dicyclomine or hyoscyamine or direct smooth muscle relaxants such as peppermint oil.17 IBS-C patients with moderate symptoms may need escalation of therapy to secretagogues or 5HT agonists (see Figure 2). Secretagogues increase fluid retention in the colonic lumen to promote bowel movements and improve visceral hypersensitivity. Lubiprostone is an intestinal chloride channel activator, indicated only for adult women with IBS-C. Linaclotide and plecanatide are guanylate cyclase-C activators which increase intestinal chloride and bicarbonate secretion, and both are indicated in IBS-C and CIC. Tenapanor inhibits the sodium/hydrogen exchanger in the GI tract, leading to increased water secretion, and is recommended for IBS-C in adults who have failed secretagogues.
All four of these drugs can be considered for moderate to severe IBS-C symptoms. In the case of severe IBS-C symptoms, Tegaserod, a 5-HT4 receptor partial agonist has been approved in women under 65 without significant cardiovascular or cerebrovascular disease.18 Regardless of IBS-C symptom severity, persistent visceral hypersensitivity can be treated with low-dose neuromodulators.19 Figure 2 provides treatment recommendations for IBS-C based on symptom severity.
Opioid-Induced Constipation (OIC)
In patients with OIC, peripherally acting mu-opioid receptor antagonists such as methylnaltrexone and naloxegol can be beneficial where stimulant laxatives are insufficient. Additionally, lubiprostone is indicated in OIC in non-cancer patients. At present, there are no head-to-head trials comparing efficacy of these medications.
Defecatory Disorders
Biofeedback therapy is the cornerstone of treatment for dyssynergic defecation, focusing on neuromuscular training to restore a normal pattern of defecation by teaching patients to tense the abdomen and relax the pelvic floor muscles and anal sphincter. It retrains the body to coordinate abdominal, rectal, and anal muscles to achieve synchronous contraction to achieve complete evacuation. It also increases awareness and response to rectal fullness or the need to defecate.
Biofeedback makes patients aware of counterproductive subconscious actions such as contracting of their anal sphincter during defecation followed by simulated defecation training with focus on how to tighten abdominal muscles and relax pelvic floor muscles to initiate and complete defecation.20 This is performed in the office with a physiotherapist or trained nurse for at least six sessions or at home where patients are encouraged to perform the exercises for 20 minutes, twice a day. These sessions utilize tools such as manometry probes, electromyography probes, simulated balloon, or home biofeedback training devices to provide visual feedback while practicing abdominophrenic breathing. Biofeedback is particularly helpful in patients suffering from constipation. Patients with defecatory disorders can also benefit from pelvic floor physical therapy which focuses on strengthening the pelvic and puborectal muscles, external anal sphincter, and pelvic muscles. This is more useful in patients with fecal incontinence. Despite all these treatments, a subset of patients may still not respond and may qualify for surgical evaluation.
Conclusion
While constipation is seldom life-threatening, it has a negative impact on patient quality of life and poses a significant financial burden on our overall healthcare system. The complexity of this condition should be appreciated and understood in order for a complete and thorough evaluation. We trust that our practical guide should serve as a useful tool in the evaluation of a chronically constipated patient.
Dr. Salim (@hamsalim07 on X) is based in the Department of Internal Medicine, University of Texas Medical Branch, Galveston. Dr. Chowdhury (annicho.med on Instagram) is a fellow in the Department of Gastroenterology, Hepatology, and Nutrition, University of Texas MD Anderson Cancer Center, Houston. Dr. Viswanathan (@LavanyaMD on X) is Associate Professor, University of Texas MD Anderson Cancer Center. The authors declare no conflict of interest.
References
1. Mugie S et al. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2010.12.010.
2. Almario CV et al. Gastroenterology. 2023 Dec. doi: 10.1053/j.gastro.2023.08.010.
3. Canavan C et al. Clin Epidemiol. 2014 Feb. doi: 10.2147/CLEP.S40245.
4. Rao SSC. Gastroenterol Clin North Am. 2007 Sep. doi: 10.1016/j.gtc.2007.07.013.
5. Bharucha AE et al. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
6. Cinquetti M et al. Clin Exp Pediatr. 2021 Sep. doi: 10.3345/cep.2020.01326.
7. Shah ND et al. Am J Gastroenterol. 2008 Jul. doi: 10.1111/j.1572-0241.2008.01910.x.
8. Rajindrajith S et al. J Pediatr Gastroenterol Nutr. 2014 Apr. doi: 10.1097/MPG.0000000000000249.
9. Talley NJ. Am J Gastroenterol. 2008 Apr. doi: 10.1111/j.1572-0241.2008.01832.x.
10. Maurer AH. J Nucl Med. 2015 Sep. doi: 10.2967/jnumed.113.134551.
11. Frye J et al. Am J Gastroenterol. 2024 Aug. doi: 10.14309/ajg.0000000000002670.
12. Rao SSC et al. J Neurogastroenterol Motil. 2016 Jun. doi: 10.5056/jnm16060.
13. Chang L et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.03.214.
14. Brenner DM et al. Am J Gastroenterol. 2021 Aug. doi: 10.14309/ajg.0000000000001266.
15. Chang L et al. Gastroenterology. 2022 Jul. doi: 10.1053/j.gastro.2022.04.016.
16. Rao SSC et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.02.013.
17. Lacy BE et al. Am J Gastroenterol. 2021 Jan. doi: 10.14309/ajg.0000000000001036.
18. Anderson JL et al. J Cardiovasc Pharmacol Ther. 2009 Sep. doi: 10.1177/1074248409340158.
19. Rahimi R et al. World J Gastroenterol. 2009 Apr. doi: 10.3748/wjg.15.1548.
20. Rao SSC. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2011.01.004.
Introduction
Constipation affects one in six people worldwide and accounts for one third of outpatient visits.1 Chronic constipation is defined by difficult, infrequent, and/or incomplete defecation, quantified by less than three spontaneous bowel movements per week, persisting for at least 3 months. Patients may complain of straining during defecation, incomplete evacuation, hard stools (Bristol stool scale [BSS] type 1-2), and fullness or bloating. Chronic constipation can be subclassified as either a primary or secondary disorder.1,2
Primary Constipation Disorders
Primary constipation includes disorders of the colon or anorectum. This includes irritable bowel syndrome with constipation (IBS-C), chronic idiopathic constipation (CIC), slow transit constipation (STC), dyssynergic defecation, and pelvic floor disorders (see Figure 1).

IBS-C
IBS-C is a chronic disorder of the gut-brain axis with a worldwide prevalence of 1.3% and a prevalence of 6%-16% in the United States, United Kingdom, and Canada, with females more likely to seek care than males.2 The economic impact of IBS-C is estimated to be $1.5 billion–$10 billion per year in the United States alone.3 The distinguishing characteristic is abdominal pain, however IBS-C can present with a constellation of symptoms. The diagnostic paradigm has shifted from IBS being a diagnosis of exclusion to now using a positive diagnostic strategy.2 Using this Rome IV criteria, one can make the diagnosis with > 95% accuracy.2,4
CIC
CIC, previously defined as functional constipation, is a disorder defined by incomplete defecation and difficult or infrequent stool. CIC is diagnosed in patients without an underlying anatomic or structural abnormality. Rome IV Criteria helps further classify the defining characteristics of chronic idiopathic constipation.2
Slow Transit Constipation
STC is characterized by impaired colonic transit time in the absence of pelvic floor dysfunction. It presents with infrequent bowel movements, diminished urgency, and/or straining with defecation.
Defecatory Disorders: Dyssynergic Defecation and Pelvic Floor Dysfunction
Defecatory disorders (DDs) result from alterations in the colonic-neural pathway with an unclear pathogenesis. A firm understanding of colonic physiology is necessary to identify DDs. The right colon helps to store and mix stool contents, the left colon helps add water to the stool, and the anal canal and rectum enable defecation and maintain continence. Any alteration along this physiologic pathway results in DDs.5
DDs primarily develop via maladaptive pelvic floor contraction during defecation or from muscle or nerve injury and include functional outlet obstruction, anorectal dyssynergia, and pelvic floor dysfunction. Increased resistance to defecation results from anismus, paradoxical anal sphincter contraction, or incomplete relaxation of the pelvic floor and external anal sphincter. This muscle incoordination is described as dyssynergia. DDs can involve either muscle or nerve dysfunction or a combination of the two. Reduced rectal sensation caused by reduced sensory triggers can cause stasis of stool, thus propagating the cycle of constipation. Over time, excessive straining can weaken the pelvic floor, increasing the risk of excessive perineal descent, rectal intussusception, solitary rectal ulcer syndrome, and pudendal neuropathy.5 Thus, identification of DDs is crucial in patients with chronic constipation.
Secondary Constipation Disorders
Secondary constipation disorders are a result of an alternate process and warrant a thorough review of outpatient medications and past medical history. Figure 1 outlines the most common causes of secondary constipation, which span a wide differential.
Clinical Evaluation
The evaluation of constipation begins with a thorough history. Description of bowel habits should include frequency, duration, straining, stool consistency using a Bristol stool chart, complete vs incomplete evacuation, pain, bloating, and use of digital maneuvers (vaginal splinting or digital stool removal). One should inquire about back trauma/surgeries and obstetric history to include vaginal forceps injury or episiotomy.
With increased smartphone use, toilet time on average has increased and can contribute to maladaptive bowel habits.6 Patients may not realize they are constipated, so patient education is critical. A patient with daily bowel movements ranging between BSS type 1-6 with incomplete evacuation might complain of diarrhea but may in fact have constipation with overflow diarrhea, for example. Past medical history is also clinically relevant, as systemic conditions can cause secondary constipation. A constipated patient should also be asked what therapies he/she has tried prior to gastroenterology referral as primary care referrals for constipation account for 8 million visits to gastroenterology per year.7
While a sensitive topic, inquire about abuse history, especially in those with childhood constipation symptoms. There is a positive correlation between childhood constipation and physical, emotional, and sexual abuse and, for any number of reasons, your patient may be reluctant to share this or undergo a digital rectal exam (DRE).8 In such cases, be sensitive in asking for this history in private rather than with other family members around and always perform this exam with a chaperone present.
A detailed physical exam is an indispensable tool all gastroenterologists must master when evaluating a constipated patient. Some key exam findings include abdominal distention, high-pitched bowel sounds, and presence of a succussion splash indicating obstructive pathology. Dry skin and brittle hair indicate hypothyroidism while hypermobile joints and skin laxity suggest connective tissue disease. Finally, a physical examination is incomplete without a DRE.
DRE
DRE is an often-overlooked physical exam component which provides helpful insight that can guide management. An informed DRE can help identify structural disorders such as fissure, hemorrhoids, anorectal mass, fecal impaction, rectal prolapse, and excessive perineal descent syndrome.9 Unless contraindicated, DRE should be a standard part of the workup of a patient with chronic constipation.
Workup
Colonoscopy
The role of colonoscopy in chronic constipation is low yield and only indicated if alarm signs are present.2 When no organic causes can be identified, the patient is deemed to have a functional bowel or motility disorder leading to constipation.
Colonic Transit Time
Colonic transit time (CTT) can be evaluated by assessing the presence of radio-opaque sitz markers in the colon with an abdominal x-ray 5 days after ingestion. The presence of five or more sitz markers may indicate STC. However, this can also signal an obstructive defecatory disorder. Colon scintigraphy can determine whether there is diffuse colonic dysmotility or dysfunction in a specific segment of the colon.10
Anorectal Function Testing (AFT)
AFT can evaluate DDs, such as fecal incontinence, dyssynergic defecation, rectal sensory disorders, anorectal pain, and rectal prolapse. AFT comprises three tests: anorectal manometry (ARM), balloon expulsion test (BET), and rectal sensory testing. These assess the defecation, continence, and sensory mechanisms of the rectum, respectively.
ARM testing employs a thin, flexible probe with an attached sensor that is inserted into the rectum to measure internal and external sphincter pressures while at rest, squeezing, and bearing down to give a functional assessment of sphincter tone.11 Cough or party balloon test assesses continence and sphincter strength. Rectal sensation is assessed by inflating a balloon incrementally and asking the patient to indicate first sensation, urgency to defecate, and discomfort. If both ARM and BET are abnormal, the patient meets diagnostic criteria for dyssynergic defecation.12
Pelvic floor disorders can be further assessed by MR defecography or barium defecography. Barium defecography is the more widely available of the two. MR defecography is a dynamic study that directly assesses pelvic floor muscles and endopelvic fascia during various stages of defecation and considered superior. This testing modality can distinguish between functional causes such as dyssynergia or pelvic floor dysfunction and structural causes of obstruction such as rectocele, rectal prolapse, or rectal intussusception. MR defecography is helpful when dyssynergia is suggested by ARM with a normal BET or if there is an absent recto-anal inhibitory reflex on ARM, which may suggest rectal intussusception.
Management
CIC
Incorporating 20-30 g of total soluble fiber, such as psyllium in individuals with low dietary fiber intake is the first-line recommendation for CIC.13 If response to a trial of fiber supplementation is inadequate, over-the-counter (OTC) osmotic laxatives such as polyethylene glycol and magnesium oxide can be incorporated. In the event of failure of OTC osmotic laxatives, lactulose can be considered. Stimulant laxatives such as senna, bisacodyl, or sodium picosulfate can be added as an adjunctive measure for short periods of time, defined as daily for 4 weeks or less.
If these measures are inadequate, pharmacological therapy with secretagogues and 5HT agonists can be considered. Prucalopride, a selective agonist of serotonin 5-HT4 receptors, is approved for CIC, prescribed 2 mg daily.14 It can also be used in patients with global motility delays, such as gastroparetics with constipation. The mechanism of action of secretagogues and specific dosing of these medications are discussed in Figure 2.15 Vibrant is a non-pharmacologic, orally ingested, vibrating, and programmable capsule device that has recently received Food and Drug Administration approval for treatment of chronic constipation by stimulating the intestinal wall, thereby promoting colonic contractile activity to achieve more spontaneous bowel movements. Further studies are required to assess its efficacy.16 Additionally, if there is inadequate response to all the above, it would be prudent to evaluate for the presence of pelvic floor dysfunction as well.

IBS-C
Similar to CIC, treatment for mild IBS-C starts with osmotic laxatives with the additional component of pain control. Antispasmodics can be used to manage the abdominal pain, cramping, and spasms associated with IBS-C. Antispasmodics available in the United States include anticholinergic agents that cause smooth muscle relaxation, such as dicyclomine or hyoscyamine or direct smooth muscle relaxants such as peppermint oil.17 IBS-C patients with moderate symptoms may need escalation of therapy to secretagogues or 5HT agonists (see Figure 2). Secretagogues increase fluid retention in the colonic lumen to promote bowel movements and improve visceral hypersensitivity. Lubiprostone is an intestinal chloride channel activator, indicated only for adult women with IBS-C. Linaclotide and plecanatide are guanylate cyclase-C activators which increase intestinal chloride and bicarbonate secretion, and both are indicated in IBS-C and CIC. Tenapanor inhibits the sodium/hydrogen exchanger in the GI tract, leading to increased water secretion, and is recommended for IBS-C in adults who have failed secretagogues.
All four of these drugs can be considered for moderate to severe IBS-C symptoms. In the case of severe IBS-C symptoms, Tegaserod, a 5-HT4 receptor partial agonist has been approved in women under 65 without significant cardiovascular or cerebrovascular disease.18 Regardless of IBS-C symptom severity, persistent visceral hypersensitivity can be treated with low-dose neuromodulators.19 Figure 2 provides treatment recommendations for IBS-C based on symptom severity.
Opioid-Induced Constipation (OIC)
In patients with OIC, peripherally acting mu-opioid receptor antagonists such as methylnaltrexone and naloxegol can be beneficial where stimulant laxatives are insufficient. Additionally, lubiprostone is indicated in OIC in non-cancer patients. At present, there are no head-to-head trials comparing efficacy of these medications.
Defecatory Disorders
Biofeedback therapy is the cornerstone of treatment for dyssynergic defecation, focusing on neuromuscular training to restore a normal pattern of defecation by teaching patients to tense the abdomen and relax the pelvic floor muscles and anal sphincter. It retrains the body to coordinate abdominal, rectal, and anal muscles to achieve synchronous contraction to achieve complete evacuation. It also increases awareness and response to rectal fullness or the need to defecate.
Biofeedback makes patients aware of counterproductive subconscious actions such as contracting of their anal sphincter during defecation followed by simulated defecation training with focus on how to tighten abdominal muscles and relax pelvic floor muscles to initiate and complete defecation.20 This is performed in the office with a physiotherapist or trained nurse for at least six sessions or at home where patients are encouraged to perform the exercises for 20 minutes, twice a day. These sessions utilize tools such as manometry probes, electromyography probes, simulated balloon, or home biofeedback training devices to provide visual feedback while practicing abdominophrenic breathing. Biofeedback is particularly helpful in patients suffering from constipation. Patients with defecatory disorders can also benefit from pelvic floor physical therapy which focuses on strengthening the pelvic and puborectal muscles, external anal sphincter, and pelvic muscles. This is more useful in patients with fecal incontinence. Despite all these treatments, a subset of patients may still not respond and may qualify for surgical evaluation.
Conclusion
While constipation is seldom life-threatening, it has a negative impact on patient quality of life and poses a significant financial burden on our overall healthcare system. The complexity of this condition should be appreciated and understood in order for a complete and thorough evaluation. We trust that our practical guide should serve as a useful tool in the evaluation of a chronically constipated patient.
Dr. Salim (@hamsalim07 on X) is based in the Department of Internal Medicine, University of Texas Medical Branch, Galveston. Dr. Chowdhury (annicho.med on Instagram) is a fellow in the Department of Gastroenterology, Hepatology, and Nutrition, University of Texas MD Anderson Cancer Center, Houston. Dr. Viswanathan (@LavanyaMD on X) is Associate Professor, University of Texas MD Anderson Cancer Center. The authors declare no conflict of interest.
References
1. Mugie S et al. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2010.12.010.
2. Almario CV et al. Gastroenterology. 2023 Dec. doi: 10.1053/j.gastro.2023.08.010.
3. Canavan C et al. Clin Epidemiol. 2014 Feb. doi: 10.2147/CLEP.S40245.
4. Rao SSC. Gastroenterol Clin North Am. 2007 Sep. doi: 10.1016/j.gtc.2007.07.013.
5. Bharucha AE et al. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
6. Cinquetti M et al. Clin Exp Pediatr. 2021 Sep. doi: 10.3345/cep.2020.01326.
7. Shah ND et al. Am J Gastroenterol. 2008 Jul. doi: 10.1111/j.1572-0241.2008.01910.x.
8. Rajindrajith S et al. J Pediatr Gastroenterol Nutr. 2014 Apr. doi: 10.1097/MPG.0000000000000249.
9. Talley NJ. Am J Gastroenterol. 2008 Apr. doi: 10.1111/j.1572-0241.2008.01832.x.
10. Maurer AH. J Nucl Med. 2015 Sep. doi: 10.2967/jnumed.113.134551.
11. Frye J et al. Am J Gastroenterol. 2024 Aug. doi: 10.14309/ajg.0000000000002670.
12. Rao SSC et al. J Neurogastroenterol Motil. 2016 Jun. doi: 10.5056/jnm16060.
13. Chang L et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.03.214.
14. Brenner DM et al. Am J Gastroenterol. 2021 Aug. doi: 10.14309/ajg.0000000000001266.
15. Chang L et al. Gastroenterology. 2022 Jul. doi: 10.1053/j.gastro.2022.04.016.
16. Rao SSC et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.02.013.
17. Lacy BE et al. Am J Gastroenterol. 2021 Jan. doi: 10.14309/ajg.0000000000001036.
18. Anderson JL et al. J Cardiovasc Pharmacol Ther. 2009 Sep. doi: 10.1177/1074248409340158.
19. Rahimi R et al. World J Gastroenterol. 2009 Apr. doi: 10.3748/wjg.15.1548.
20. Rao SSC. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2011.01.004.