User login
Cultural Competency and Treatment of Veteran and Military Patients With Mental Health Disorders
About 2.5 million U.S. service members have served in conflicts since September 11, 2001. Estimates of the numbers of service members who have deployed to Iraq and Afghanistan and have posttraumatic stress disorder (PTSD) range from 15% to 25%.1-3
This special issue contains several excellent articles about PTSD and comorbidities, including insomnia and depression. Although there are service members who have pure PTSD, in the experience of most clinicians, that is the exception rather than the rule.2 For example, insomnia may lead to patients’ excessive drinking to try to sleep. Numbing and avoidance from the excessive drinking leads to relationship problems and often divorce. Relationship problems are subsequently a key driver of suicide.4,5
Also included in this issue is a series of articles examining the case study of William, who has multiple sclerosis (MS), a disease usually in the domain of neurologists, rather than psychiatrists. However, given the physical, cognitive, and social stresses of MS, it is not surprising that comorbid depression is extremely common, appearing in about half of patients with MS over their lifetime.6 The multidisciplinary approach to care described in this series is critical for successful treatment.
There are well-established guidelines for the treatment of PTSD, developed by the American Psychiatric Association, DoD, and VA, often referred to as evidence-based treatments. However, there are many patients who are either unwilling or unable to adhere or who do not respond to the evidence-based treatments. Although these patients are often called treatment-resistant or refractory, it is also likely that the treatments are not engineered toward service members. That may be due to (1) unacceptable adverse effects from medication; (2) difficulties attending frequent appointments, especially for cognitivebehavioral treatments; (3) the reluctance of many service members to relive their trauma and/or talk about it; or
(4) the stigma of seeking treatment.2,7
The physical stresses of military service, including wounds and injuries, involve corresponding pain and disability. Alcohol, depression, PTSD, and traumatic brain injury have long been associated with one another, but sometimes musculoskeletal injuries are left out of the discussion. The musculoskeletal issues have led to service members being treated with opiates, which can cause dependence and addiction.4,5 In both military and civilian populations, many patients switch from legal opiates to illegal heroin. Many service members, especially after discharge from the military, thus start a slide into substance dependence, unemployment, and homelessness. Unfortunately, death by heroin overdose is increasingly common.8
Suicide rates among U.S. Army personnel have been increasing since 2004, surpassing comparable civilian suicide rates in 2008. The other service branches have not seen such a dramatic rise, but suicide is still a troubling problem. Suicide rates peaked in army active-duty troops over the past few years but are still rising in reservists. Suicides are most prevalent among young white males but have been increasing in older ages and females
as well.4,5
Risk factors for suicide among active-duty members are well known, because data are systemically collected. These include relationship difficulties, financial and occupational problems, pain and physical disability, and access to weapons.4,5
Cultural Compentency
The concept of moral injury is related to but different from PTSD, which is a medical diagnosis. In general, most authors conceptualize moral injury as an insult caused either by shame of killing or the guilt induced when fellow service members die while one has survived. Although not well studied by the medical community, most agree that it is a corrosive condition, which contributes to relationship difficulties and suicide.
A theme throughout military medicine is one of cultural competency: If you are not in the military, how can you understand the military culture? As a start, one of the easy ways is for a provider to ask patients about their military occupational specialty, basic and advanced training, and where they have been stationed. Ask when and where they have been deployed. Learn what their military rank is/was, and ask how they want to be addressed. Some will prefer to be addressed by rank, others by their first name. An important piece of advice for providers: Combat veterans do not want to be seen as victims. Treat them as battle-hardened or maybe battle-scarred, and respect their service.
At present, 15% of active-duty military, 17% of National Guard/Reserves, and 20% of new recruits are women. The recent wars in Iraq and Afghanistan have engendered a growing population of female veterans seeking health care through VA. Thus, women are among the fastest growing segments of new users of VA health care: As many as 40% of women returning from Iraq and Afghanistan may elect to use the VA, for a variety of medical and mental health reasons. In the civilian world, women experience PTSD at twice the rate than do men. In the military, available statistics suggest that the rate is about the same.
There are certain occupations that may lead to an increased rate of PTSD. Medical staff are exposed to horrifically wounded service members and local populations. They and others may have been involved with detainee medical issues. In addition, many service members, including individual augmentees and other reservists, were assigned to detainee missions, such as at Guantanamo Bay and Abu Ghraib. In general, reservists may not have the support of a cohesive unit.
Administrative Issues
Service members need to be physically and mentally fit for duty, according to various regulations.9 If service members have a severe mental illness, they usually will receive a medical evaluation to assess whether or not they are fit for duty. Service members may be medically discharged if found not fit for duty. They may also be medically retired, depending on the severity of their condition, which carries significant disability benefits. The Medical and Physical Evaluation Boards, now called the Integrated Disability Evaluation System, is a complex process.10
The diagnosis of PTSD does not necessarily lead to a medical discharge. If service members respond to treatment, they may be found fit for duty. Alternatively, with actual practice varying according to the service branch, unfortunately they may be administratively discharged without benefits.
Service members may or may not want to be assessed by a Medical Evaluation Board, which offers both benefits and potential shame. Those who want to stay in the military, in general, do not want to see a mental health care provider, because they fear for their jobs. However, those who are nearing the end of their enlistment or planning to retire have many pressures to endorse PTSD symptoms. These include the financial benefits of medical retirement (often at 50% of their base pay), including free medical care and other benefits.
Military, VA, and other providers need to know how to diagnose and treat these psychologic and neurologic brain injuries and disorders. They also need to know when and how to refer elsewhere for further evaluation and treatment. Finally, because PTSD is very much in the public discourse, providers should be prepared to engage in a dialogue with the public.
1. Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: Rand Corporation; 2008.
2. Treatment of posttraumatic stress disorder in military and veteran populations. Institute of Medicine Website. http://www.iom.edu/Reports/2014/Treatment-for-Posttraumatic-Stress-Disorder-in-Military-and-Veteran-Populations-Final-Assessment.aspx. Published June 20, 2014. Accessed March 9, 2015.
3. Joint mental health advisory team VII (J-MHAT 7) report. U.S. Army Website. http://armylive.dodlive.mil/index.php/2011/05/joint-mental-health-advisory-team-vii-j-mhat-7-report. Published May 24, 2011. Accessed March 9, 2015.
4. Ritchie EC. Suicides and the United States army: Perspectives from the former psychiatry consultant to the army surgeon general. Cerebrum. 2012(2012):1.
5. Black SA, Gallaway MS, Bell MR, Ritchie EC. Prevalence and risk factors associated with suicides of Army soldiers. Milit Psychol. 2011;23(4):433-451.
6. Wallin MT, Wilken JA, Turner AP, Williams RM, Kane R. Depression and multiple sclerosis: Review of a lethal combination. J Rehabil Res Dev. 2006;43(1):45-62.
7. Hoge C. DSM-5 PTSD screening may miss previously diagnosed soldiers. Healio Website. http://www.healio.com/psychiatry/ptsd/news/online/%7B4e137bbf-4bc0-4c31-b6b2-77e83e9b09d9%7D/dsm-5-ptsd-screening-may-miss-previously-diagnosed-soldiers. Published August 25, 2014. Accessed March 10, 2015.
8. Rudd RA, Paulozzi LJ, Burleson RW, et al; Centers for Disease Control (CDC). Increases in heroin overdose deaths—28 states, 2010 to 2012. MMWR Morb Mortal Wkly Rep. 2014;63(39):849-854.
9. U.S. Army. Standards of Medical Fitness, 2011. Army Regulation 40-501. U.S. Army Website. http://www.apd.army.mil/pdffiles/r40_501.pdf. Published August 4, 2011. Accessed March 10, 2015.
10. Army Physical Disability Evaluation System. The army integrated disability evaluation system. U.S. Army Website. http://usarmy.vo.llnwd.net/e2/rv5_downloads/features/readyandresilient/ARMY_IDES.pdf. Accessed March 10, 2015.
About 2.5 million U.S. service members have served in conflicts since September 11, 2001. Estimates of the numbers of service members who have deployed to Iraq and Afghanistan and have posttraumatic stress disorder (PTSD) range from 15% to 25%.1-3
This special issue contains several excellent articles about PTSD and comorbidities, including insomnia and depression. Although there are service members who have pure PTSD, in the experience of most clinicians, that is the exception rather than the rule.2 For example, insomnia may lead to patients’ excessive drinking to try to sleep. Numbing and avoidance from the excessive drinking leads to relationship problems and often divorce. Relationship problems are subsequently a key driver of suicide.4,5
Also included in this issue is a series of articles examining the case study of William, who has multiple sclerosis (MS), a disease usually in the domain of neurologists, rather than psychiatrists. However, given the physical, cognitive, and social stresses of MS, it is not surprising that comorbid depression is extremely common, appearing in about half of patients with MS over their lifetime.6 The multidisciplinary approach to care described in this series is critical for successful treatment.
There are well-established guidelines for the treatment of PTSD, developed by the American Psychiatric Association, DoD, and VA, often referred to as evidence-based treatments. However, there are many patients who are either unwilling or unable to adhere or who do not respond to the evidence-based treatments. Although these patients are often called treatment-resistant or refractory, it is also likely that the treatments are not engineered toward service members. That may be due to (1) unacceptable adverse effects from medication; (2) difficulties attending frequent appointments, especially for cognitivebehavioral treatments; (3) the reluctance of many service members to relive their trauma and/or talk about it; or
(4) the stigma of seeking treatment.2,7
The physical stresses of military service, including wounds and injuries, involve corresponding pain and disability. Alcohol, depression, PTSD, and traumatic brain injury have long been associated with one another, but sometimes musculoskeletal injuries are left out of the discussion. The musculoskeletal issues have led to service members being treated with opiates, which can cause dependence and addiction.4,5 In both military and civilian populations, many patients switch from legal opiates to illegal heroin. Many service members, especially after discharge from the military, thus start a slide into substance dependence, unemployment, and homelessness. Unfortunately, death by heroin overdose is increasingly common.8
Suicide rates among U.S. Army personnel have been increasing since 2004, surpassing comparable civilian suicide rates in 2008. The other service branches have not seen such a dramatic rise, but suicide is still a troubling problem. Suicide rates peaked in army active-duty troops over the past few years but are still rising in reservists. Suicides are most prevalent among young white males but have been increasing in older ages and females
as well.4,5
Risk factors for suicide among active-duty members are well known, because data are systemically collected. These include relationship difficulties, financial and occupational problems, pain and physical disability, and access to weapons.4,5
Cultural Compentency
The concept of moral injury is related to but different from PTSD, which is a medical diagnosis. In general, most authors conceptualize moral injury as an insult caused either by shame of killing or the guilt induced when fellow service members die while one has survived. Although not well studied by the medical community, most agree that it is a corrosive condition, which contributes to relationship difficulties and suicide.
A theme throughout military medicine is one of cultural competency: If you are not in the military, how can you understand the military culture? As a start, one of the easy ways is for a provider to ask patients about their military occupational specialty, basic and advanced training, and where they have been stationed. Ask when and where they have been deployed. Learn what their military rank is/was, and ask how they want to be addressed. Some will prefer to be addressed by rank, others by their first name. An important piece of advice for providers: Combat veterans do not want to be seen as victims. Treat them as battle-hardened or maybe battle-scarred, and respect their service.
At present, 15% of active-duty military, 17% of National Guard/Reserves, and 20% of new recruits are women. The recent wars in Iraq and Afghanistan have engendered a growing population of female veterans seeking health care through VA. Thus, women are among the fastest growing segments of new users of VA health care: As many as 40% of women returning from Iraq and Afghanistan may elect to use the VA, for a variety of medical and mental health reasons. In the civilian world, women experience PTSD at twice the rate than do men. In the military, available statistics suggest that the rate is about the same.
There are certain occupations that may lead to an increased rate of PTSD. Medical staff are exposed to horrifically wounded service members and local populations. They and others may have been involved with detainee medical issues. In addition, many service members, including individual augmentees and other reservists, were assigned to detainee missions, such as at Guantanamo Bay and Abu Ghraib. In general, reservists may not have the support of a cohesive unit.
Administrative Issues
Service members need to be physically and mentally fit for duty, according to various regulations.9 If service members have a severe mental illness, they usually will receive a medical evaluation to assess whether or not they are fit for duty. Service members may be medically discharged if found not fit for duty. They may also be medically retired, depending on the severity of their condition, which carries significant disability benefits. The Medical and Physical Evaluation Boards, now called the Integrated Disability Evaluation System, is a complex process.10
The diagnosis of PTSD does not necessarily lead to a medical discharge. If service members respond to treatment, they may be found fit for duty. Alternatively, with actual practice varying according to the service branch, unfortunately they may be administratively discharged without benefits.
Service members may or may not want to be assessed by a Medical Evaluation Board, which offers both benefits and potential shame. Those who want to stay in the military, in general, do not want to see a mental health care provider, because they fear for their jobs. However, those who are nearing the end of their enlistment or planning to retire have many pressures to endorse PTSD symptoms. These include the financial benefits of medical retirement (often at 50% of their base pay), including free medical care and other benefits.
Military, VA, and other providers need to know how to diagnose and treat these psychologic and neurologic brain injuries and disorders. They also need to know when and how to refer elsewhere for further evaluation and treatment. Finally, because PTSD is very much in the public discourse, providers should be prepared to engage in a dialogue with the public.
About 2.5 million U.S. service members have served in conflicts since September 11, 2001. Estimates of the numbers of service members who have deployed to Iraq and Afghanistan and have posttraumatic stress disorder (PTSD) range from 15% to 25%.1-3
This special issue contains several excellent articles about PTSD and comorbidities, including insomnia and depression. Although there are service members who have pure PTSD, in the experience of most clinicians, that is the exception rather than the rule.2 For example, insomnia may lead to patients’ excessive drinking to try to sleep. Numbing and avoidance from the excessive drinking leads to relationship problems and often divorce. Relationship problems are subsequently a key driver of suicide.4,5
Also included in this issue is a series of articles examining the case study of William, who has multiple sclerosis (MS), a disease usually in the domain of neurologists, rather than psychiatrists. However, given the physical, cognitive, and social stresses of MS, it is not surprising that comorbid depression is extremely common, appearing in about half of patients with MS over their lifetime.6 The multidisciplinary approach to care described in this series is critical for successful treatment.
There are well-established guidelines for the treatment of PTSD, developed by the American Psychiatric Association, DoD, and VA, often referred to as evidence-based treatments. However, there are many patients who are either unwilling or unable to adhere or who do not respond to the evidence-based treatments. Although these patients are often called treatment-resistant or refractory, it is also likely that the treatments are not engineered toward service members. That may be due to (1) unacceptable adverse effects from medication; (2) difficulties attending frequent appointments, especially for cognitivebehavioral treatments; (3) the reluctance of many service members to relive their trauma and/or talk about it; or
(4) the stigma of seeking treatment.2,7
The physical stresses of military service, including wounds and injuries, involve corresponding pain and disability. Alcohol, depression, PTSD, and traumatic brain injury have long been associated with one another, but sometimes musculoskeletal injuries are left out of the discussion. The musculoskeletal issues have led to service members being treated with opiates, which can cause dependence and addiction.4,5 In both military and civilian populations, many patients switch from legal opiates to illegal heroin. Many service members, especially after discharge from the military, thus start a slide into substance dependence, unemployment, and homelessness. Unfortunately, death by heroin overdose is increasingly common.8
Suicide rates among U.S. Army personnel have been increasing since 2004, surpassing comparable civilian suicide rates in 2008. The other service branches have not seen such a dramatic rise, but suicide is still a troubling problem. Suicide rates peaked in army active-duty troops over the past few years but are still rising in reservists. Suicides are most prevalent among young white males but have been increasing in older ages and females
as well.4,5
Risk factors for suicide among active-duty members are well known, because data are systemically collected. These include relationship difficulties, financial and occupational problems, pain and physical disability, and access to weapons.4,5
Cultural Compentency
The concept of moral injury is related to but different from PTSD, which is a medical diagnosis. In general, most authors conceptualize moral injury as an insult caused either by shame of killing or the guilt induced when fellow service members die while one has survived. Although not well studied by the medical community, most agree that it is a corrosive condition, which contributes to relationship difficulties and suicide.
A theme throughout military medicine is one of cultural competency: If you are not in the military, how can you understand the military culture? As a start, one of the easy ways is for a provider to ask patients about their military occupational specialty, basic and advanced training, and where they have been stationed. Ask when and where they have been deployed. Learn what their military rank is/was, and ask how they want to be addressed. Some will prefer to be addressed by rank, others by their first name. An important piece of advice for providers: Combat veterans do not want to be seen as victims. Treat them as battle-hardened or maybe battle-scarred, and respect their service.
At present, 15% of active-duty military, 17% of National Guard/Reserves, and 20% of new recruits are women. The recent wars in Iraq and Afghanistan have engendered a growing population of female veterans seeking health care through VA. Thus, women are among the fastest growing segments of new users of VA health care: As many as 40% of women returning from Iraq and Afghanistan may elect to use the VA, for a variety of medical and mental health reasons. In the civilian world, women experience PTSD at twice the rate than do men. In the military, available statistics suggest that the rate is about the same.
There are certain occupations that may lead to an increased rate of PTSD. Medical staff are exposed to horrifically wounded service members and local populations. They and others may have been involved with detainee medical issues. In addition, many service members, including individual augmentees and other reservists, were assigned to detainee missions, such as at Guantanamo Bay and Abu Ghraib. In general, reservists may not have the support of a cohesive unit.
Administrative Issues
Service members need to be physically and mentally fit for duty, according to various regulations.9 If service members have a severe mental illness, they usually will receive a medical evaluation to assess whether or not they are fit for duty. Service members may be medically discharged if found not fit for duty. They may also be medically retired, depending on the severity of their condition, which carries significant disability benefits. The Medical and Physical Evaluation Boards, now called the Integrated Disability Evaluation System, is a complex process.10
The diagnosis of PTSD does not necessarily lead to a medical discharge. If service members respond to treatment, they may be found fit for duty. Alternatively, with actual practice varying according to the service branch, unfortunately they may be administratively discharged without benefits.
Service members may or may not want to be assessed by a Medical Evaluation Board, which offers both benefits and potential shame. Those who want to stay in the military, in general, do not want to see a mental health care provider, because they fear for their jobs. However, those who are nearing the end of their enlistment or planning to retire have many pressures to endorse PTSD symptoms. These include the financial benefits of medical retirement (often at 50% of their base pay), including free medical care and other benefits.
Military, VA, and other providers need to know how to diagnose and treat these psychologic and neurologic brain injuries and disorders. They also need to know when and how to refer elsewhere for further evaluation and treatment. Finally, because PTSD is very much in the public discourse, providers should be prepared to engage in a dialogue with the public.
1. Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: Rand Corporation; 2008.
2. Treatment of posttraumatic stress disorder in military and veteran populations. Institute of Medicine Website. http://www.iom.edu/Reports/2014/Treatment-for-Posttraumatic-Stress-Disorder-in-Military-and-Veteran-Populations-Final-Assessment.aspx. Published June 20, 2014. Accessed March 9, 2015.
3. Joint mental health advisory team VII (J-MHAT 7) report. U.S. Army Website. http://armylive.dodlive.mil/index.php/2011/05/joint-mental-health-advisory-team-vii-j-mhat-7-report. Published May 24, 2011. Accessed March 9, 2015.
4. Ritchie EC. Suicides and the United States army: Perspectives from the former psychiatry consultant to the army surgeon general. Cerebrum. 2012(2012):1.
5. Black SA, Gallaway MS, Bell MR, Ritchie EC. Prevalence and risk factors associated with suicides of Army soldiers. Milit Psychol. 2011;23(4):433-451.
6. Wallin MT, Wilken JA, Turner AP, Williams RM, Kane R. Depression and multiple sclerosis: Review of a lethal combination. J Rehabil Res Dev. 2006;43(1):45-62.
7. Hoge C. DSM-5 PTSD screening may miss previously diagnosed soldiers. Healio Website. http://www.healio.com/psychiatry/ptsd/news/online/%7B4e137bbf-4bc0-4c31-b6b2-77e83e9b09d9%7D/dsm-5-ptsd-screening-may-miss-previously-diagnosed-soldiers. Published August 25, 2014. Accessed March 10, 2015.
8. Rudd RA, Paulozzi LJ, Burleson RW, et al; Centers for Disease Control (CDC). Increases in heroin overdose deaths—28 states, 2010 to 2012. MMWR Morb Mortal Wkly Rep. 2014;63(39):849-854.
9. U.S. Army. Standards of Medical Fitness, 2011. Army Regulation 40-501. U.S. Army Website. http://www.apd.army.mil/pdffiles/r40_501.pdf. Published August 4, 2011. Accessed March 10, 2015.
10. Army Physical Disability Evaluation System. The army integrated disability evaluation system. U.S. Army Website. http://usarmy.vo.llnwd.net/e2/rv5_downloads/features/readyandresilient/ARMY_IDES.pdf. Accessed March 10, 2015.
1. Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: Rand Corporation; 2008.
2. Treatment of posttraumatic stress disorder in military and veteran populations. Institute of Medicine Website. http://www.iom.edu/Reports/2014/Treatment-for-Posttraumatic-Stress-Disorder-in-Military-and-Veteran-Populations-Final-Assessment.aspx. Published June 20, 2014. Accessed March 9, 2015.
3. Joint mental health advisory team VII (J-MHAT 7) report. U.S. Army Website. http://armylive.dodlive.mil/index.php/2011/05/joint-mental-health-advisory-team-vii-j-mhat-7-report. Published May 24, 2011. Accessed March 9, 2015.
4. Ritchie EC. Suicides and the United States army: Perspectives from the former psychiatry consultant to the army surgeon general. Cerebrum. 2012(2012):1.
5. Black SA, Gallaway MS, Bell MR, Ritchie EC. Prevalence and risk factors associated with suicides of Army soldiers. Milit Psychol. 2011;23(4):433-451.
6. Wallin MT, Wilken JA, Turner AP, Williams RM, Kane R. Depression and multiple sclerosis: Review of a lethal combination. J Rehabil Res Dev. 2006;43(1):45-62.
7. Hoge C. DSM-5 PTSD screening may miss previously diagnosed soldiers. Healio Website. http://www.healio.com/psychiatry/ptsd/news/online/%7B4e137bbf-4bc0-4c31-b6b2-77e83e9b09d9%7D/dsm-5-ptsd-screening-may-miss-previously-diagnosed-soldiers. Published August 25, 2014. Accessed March 10, 2015.
8. Rudd RA, Paulozzi LJ, Burleson RW, et al; Centers for Disease Control (CDC). Increases in heroin overdose deaths—28 states, 2010 to 2012. MMWR Morb Mortal Wkly Rep. 2014;63(39):849-854.
9. U.S. Army. Standards of Medical Fitness, 2011. Army Regulation 40-501. U.S. Army Website. http://www.apd.army.mil/pdffiles/r40_501.pdf. Published August 4, 2011. Accessed March 10, 2015.
10. Army Physical Disability Evaluation System. The army integrated disability evaluation system. U.S. Army Website. http://usarmy.vo.llnwd.net/e2/rv5_downloads/features/readyandresilient/ARMY_IDES.pdf. Accessed March 10, 2015.
New Treatments for Hepatitis C
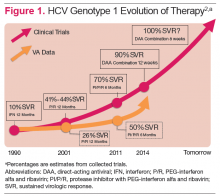
Hepatitis C virus (HCV) infection remains a significant problem in the VA system, with over 174,000 current actively infected patients.1 Despite the availability of antiviral treatment since the early 1990’s, only approximately 26% of patients have ever been treated. These treatments required the use of pegylated interferon alfa (PEG) and ribavirin (RBV), which are associated with significant adverse events (AEs) that prevented many from receiving the treatment. Of those treated, only a minority achieved a sustained virologic response (SVR), due to the limited efficacy of the treatments (Figure 1).2 With the advent of new direct-acting antiviral (DAA) treatments in 2011, treatment efficacy improved.
The first DAAs in use were the viral nonstructural protein 3/4A (NS3/4A) serine protease inhibitors (PIs) boceprevir and telaprevir, which were used with PEG and RBV for patients with HCV genotype 1 infection. This combination therapy improved SVR rates from about 26% to 50% in patients with HCV genotype 1 in the VA.3,4 However, due to the significant AEs with these combinations, relatively few patients were treated.
In late 2013, the FDA approved other DAAs, which allowed patients to be treated effectively without PEG. These included the nucleotide nonstructural protein 5B (NS5B) polymerase inhibitor sofosbuvir and a secondgeneration NS3/4A PI simeprevir.5-7 The first nucleotide analog NS5B polymerase inhibitor, sofosbuvir and the nonstructural protein 5A (NS5A) replication complex inhibitor, ledipasvir, was approved in October 2014.8-10 The recent developments in noninterferon treatments have been accompanied by revised treatment guidelines or recommendations by major professional societies. Current treatment recommendations will be reviewed here, but the recommendations will continue to evolve as new DAAs come to market.
DAA Sites of Action
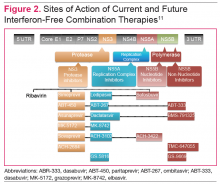
The HCV genome is a positive-stranded RNA molecule of about 9,500 nucleotides, which encodes a polyprotein of approximately 3,000 amino acids that form 10 individual viral proteins. These are composed of both structural and nonstructural (NS) proteins that are responsible for replication of the genome and formation of new viral particles. Understanding of the HCV-encoded proteins and their functions has permitted the development of different DAA therapies. In general, targeting a single protein is not effective, and combination therapy targeting 2 proteins is required for viral eradication (Figure 2).11 The 3 drug targets that are currently available include NS3/4A serine PIs (eg, simeprevir, boceprevir, telaprevir), NS5A replication complex inhibitors (eg, ledipasvir, daclatasvir), and NS5B RNA-dependent RNA polymerase inhibitors (eg, sofosbuvir).
Other DAA’s are in development that have targets in host rather than viral cells. These include cycolphilin A inhibitors and the micro-RNA (miR-122) antagonist miravirsen.12,13
The RNA-dependent RNA polymerase, encoded by the HCV NS5B is targeted by 2 classes of inhibitors: nucleoside or nucleotide analog inhibitors (NIs), and non-nucleoside inhibitors (NNIs).11 The only NI of the NS5B protein approved by the FDA is sofosbuvir. The resistance profiles of NIs and NNIs differ, because they bind to distinct sites on the NS5B protein. NIs are analogs of natural substrates and bind to the active site of the RNA polymerase, whereas NNIs are allosteric site inhibitors. NIs have activity in vitro against all HCV genotypes and have high barrier to resistance as the active site of NS5B polymerase is less tolerant of different amino acid substitutions.
In vitro studies have demonstrated that NIs are less likely to select for mutations compared with NNIs and PIs. The NNIs have limited genotypic coverage and have a lower barrier to resistance. Strategies for targeting HCV proteins include using a NI NS5B protein inhibitor as the backbone with a high barrier to resistance in combination with 1 or 2 other DAAs with lower barriers to resistance, or the combination of 3 DAAs with lower barriers to resistance.11 Ribavirin has broad-spectrum antiviral activity, one of which is anti-HCV activity. The mode of action of RBV against HCV is not well understood, but several mechanisms have been proposed, one of which is via inhibition of viral-dependent RNA polymerase.
Current HCV Treatment Recommendations
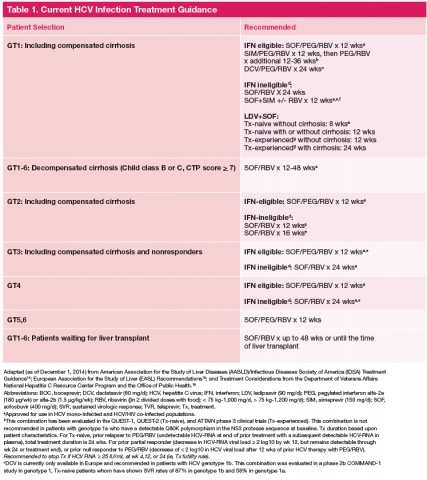
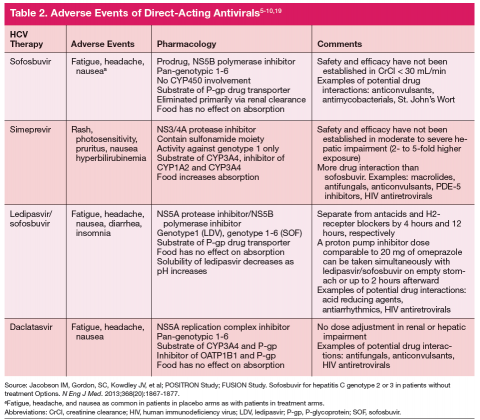
Current treatment recommendations are available from the American Association for the Study of the Liver Disease (AASLD) and the Infectious Diseases Society of America (IDSA) (http://www.hcvguidelines.org); the VA National Hepatitis C Resource Center Program and Office of Public Health (http://www.hepatitis.va.gov/pdf/2014hcv.pdf); and the European Association for the Study of the Liver (EASL) (http://www.easl.eu/_newsroom/latest-news/easl-recommendations-on-treatment-of-hepatitis-c-2014). These recommendations are updated frequently as new drugs enter the marketplace (Table 1).14-16
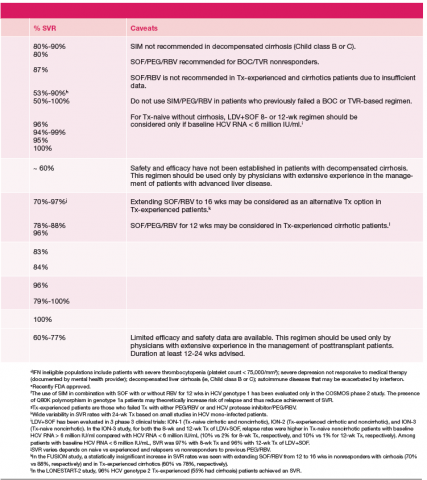
Since most patients are unable or unwilling to tolerate interferon, the majority of patients in treatment are currently receiving interferon-free combinations. Treatment of genotype 1 is currently dominated by the offlabel use of sofosbuvir in combination with simeprevir. Data have been published from a single phase II trial in patients with and without cirrhosis.7 Of note are emerging data from observational studies confirming the efficacy of over 80% SVR in patients with cirrhosis and genotype 1a with or without prior treatment.17 Treatment of patients with genotype 2 infection is dominated by the use of sofosbuvir and RBV combination. However, patients with cirrhosis do not respond as well as patients without cirrhosis, and it remains to be seen whether extending therapy is of any benefit.
One exception for the use of PEG is the recommendation for patients with HCV genotype 3 and cirrhosis to consider the combination of PEG, RBV, and sofosbuvir for 12 weeks.18 This has the advantage of being more effective, less costly, and shorter treatment duration compared with the sofosbuvir and RBV association but is only appropriate for patients who can tolerate interferon. Common AEs and potential contraindications to treatment are listed in Table 2.5-10,19
New Treatment Options for HCV in 2015
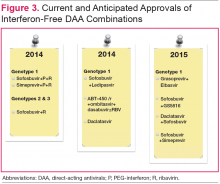
Figure 3 details DAA combinations currently in phase III trials that are expected to receive approval in 2015. This will expand the repertoire of drug combinations available and enable fine-tuning of regimens according to patient and viral characteristics and cost requirements.
Cost and Effectiveness
Cost-effectiveness issues are of immediate importance for health care systems and payors. The first generation PIs, boceprevir and telaprevir, were used in combination with PEG and RBV and entailed pharmacy costs on par with current noninterferon regimens.20,21 Studies generally demonstrated that these treatments are cost-effective, especially in patients with advanced fibrosis. Ollendorf and colleagues recently published an analysis of the costs of using interferon-free regimens for treatment of 540,000 patients with chronic HCV in California.22 Assuming that 50% of patients would present for treatment, the cost of the new DAAs would be immense and result in an increase in costs from $12 billion to $34 billion in the first year and net costs of $6 billion by the 20th year. If treatment were limited to patients with advanced cirrhosis, the first year costs would be increased by $7 billion, but at 20 years there would be approximately $1 billion in net cost savings.
For current treatments, a 12-week course of simeprevir/sofosbuvir has been shown to be more costeffective than 24 weeks of sofosbuvir/RBV for treatment
of genotype 1.23 Similarly, patients with genotype 1, no cirrhosis, and low viral load can be treated with 8 weeks of sofosbuvir/ledipasvir rather than other 12-week regimens, thereby reducing drug costs. The resources needed for upfront treatment of patients is of obvious concern, and various systems are struggling to determine how to provide access to these pharmaceuticals. Prioritizing patients according to risk for advanced fibrosis using noninvasive scoring systems should be used if there is limited access or resources.
Conclusion
Since the VA has a large population of patients with HCV infection, the advancement in HCV treatment is of paramount importance. The advent of new DAAs in 2011 improved treatment efficacy for patients with HCV, but few patients could be treated due to AEs related to PEG. In 2013, new DAAs were introduced that did not require conjunctive therapy with PEG, providing a treatment option for patients who could not tolerate PEG. New DAA combinations are currently in trials and, upon approval, will provide more options for patients with HCV infection.
Author disclosures
Dr. Ho has received research and grant support from Genentech, Inc. and Gilead; he is on the speakers’ bureau for Prime Education, Inc. The other authors report no actual or potential conflicts of interest with regard to this article.
Grant Support
Funding provided by VA HSR&D grant IIR-13-052-2, VA HIV/HCV QUERI program, and the Research Service of the Department of Veterans Affairs.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Backus LI, Belperio PS, Loomis TP, Yip GH, Mole LA. Hepatitis C virus screening and prevalence among US veterans in Department of Veterans Affairs care. JAMA Intern Med. 2013;173(16):1549-1552.
2. Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology. 2007;46(1):37-47.
3. Backus LI, Belperio PS, Shahoumian TA, Cheung R, Mole LA. Comparative effectiveness of the hepatitis C virus protease inhibitors boceprevir and telaprevir in a large U.S. cohort. Aliment Pharmacol Ther. 2014;39(1):93-103.
4. Ioannou GN, Beste LA, Green PK. Similar effectiveness of boceprevir and telaprevir treatment regimens for hepatitis C virus infection on the basis of a nationwide study of veterans. Clin Gastroenterol Hepatol. 2014;12(8):1371-1380.
5. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878-1887.
6. Jacobson IM, Gordon SC, Kowdley KV, et al; POSITRON Study; FUSION Study. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867-1877.
7. Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384(9956):1756-1765.
8. Afdhal N, Reddy KR, Nelson DR, et al; ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483-1493.
9. Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889-1898
10. Kowdley KV, Gordon SC, Reddy KR, et al; ION-3 Investigators. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879-1888.
11. Pawlotsky JM. New hepatitis C virus (HCV) drugs and the hope for a cure: Concepts in anti-HCV drug development. Semin Liver Dis. 2014;34(1):22-29.
12. Membreno FE, Espinales JC, Lawitz EJ. Cyclophilin inhibitors for hepatitis C therapy. Clin Liver Dis. 2013;17(1):129-139.
13. Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368(18):1685-1694.
14. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed November 25, 2014.
15. Department of Veterans Affairs. Chronic Hepatitis C Virus (HCV) Infection: Treatment considerations from the Department of Veterans Affairs National Hepatitis C Resource Center Program at the Office of Public Health. http://www.hepatitis.va.gov/pdf/2014hcv.pdf. Revised May 13, 2014. Accessed November 25, 2014.
16. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C in 2014. http://www.easl.eu/_newsroom/latest-news/easl-recommendations-on-treatment-of-hepatitis-c-2014. Accessed November 25, 2014.
17. Dieterich D, Bacon BR, Flamm SL, et al. Evaluation of sofosbuvir and simeprevir-based regimens in the TRIO network: academic and community treatment of a real-world, heterogeneous population. Hepatology. 2014;60(suppl S1):220A. Abstract 46.
18. Lawitz EJ, Poordad F, Brainard D, et al. Sofosbuvir with peginterferon-ribavirin for 12 weeks in previously treated patients with hepatitis C genotype 2 or 3 and cirrhosis [published online ahead of print October 16, 2014]. Hepatology. 2014; doi:10.1002/hep.27567.
19. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al; AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211-221.
20. Liu S, Cipriano LE, Holodniy M, Owens DK, Goldhaber-Fiebert JD. New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med. 2012;156(4):279-290.
21. Chan K, Lai MN, Groessl EJ, et al. Cost effectiveness of direct-acting antiviral
therapy for treatment-naive patients with chronic HCV genotype 1 infection in the veterans health administration. Clin Gastroenterol Hepatol. 2013;11(11):1503-1510.
22. Ollendorf DA, Tice JA, Pearson SD. The comparative clinical effectiveness and value of simeprevir and sofosbuvir for chronic hepatitis C virus infection. JAMA Intern Med. 2014;174(7):1170-1171.
23. Hagan LM, Sulkowski MS, Schinazi RF. Cost analysis of sofosbuvir/ribavirin versus sofosbuvir/simeprevir for genotype 1 hepatitis C virus in interferon-ineligible/intolerant individuals. Hepatology. 2014;60(1):37-45.
Hepatitis C virus (HCV) infection remains a significant problem in the VA system, with over 174,000 current actively infected patients.1 Despite the availability of antiviral treatment since the early 1990’s, only approximately 26% of patients have ever been treated. These treatments required the use of pegylated interferon alfa (PEG) and ribavirin (RBV), which are associated with significant adverse events (AEs) that prevented many from receiving the treatment. Of those treated, only a minority achieved a sustained virologic response (SVR), due to the limited efficacy of the treatments (Figure 1).2 With the advent of new direct-acting antiviral (DAA) treatments in 2011, treatment efficacy improved.
The first DAAs in use were the viral nonstructural protein 3/4A (NS3/4A) serine protease inhibitors (PIs) boceprevir and telaprevir, which were used with PEG and RBV for patients with HCV genotype 1 infection. This combination therapy improved SVR rates from about 26% to 50% in patients with HCV genotype 1 in the VA.3,4 However, due to the significant AEs with these combinations, relatively few patients were treated.
In late 2013, the FDA approved other DAAs, which allowed patients to be treated effectively without PEG. These included the nucleotide nonstructural protein 5B (NS5B) polymerase inhibitor sofosbuvir and a secondgeneration NS3/4A PI simeprevir.5-7 The first nucleotide analog NS5B polymerase inhibitor, sofosbuvir and the nonstructural protein 5A (NS5A) replication complex inhibitor, ledipasvir, was approved in October 2014.8-10 The recent developments in noninterferon treatments have been accompanied by revised treatment guidelines or recommendations by major professional societies. Current treatment recommendations will be reviewed here, but the recommendations will continue to evolve as new DAAs come to market.
DAA Sites of Action
The HCV genome is a positive-stranded RNA molecule of about 9,500 nucleotides, which encodes a polyprotein of approximately 3,000 amino acids that form 10 individual viral proteins. These are composed of both structural and nonstructural (NS) proteins that are responsible for replication of the genome and formation of new viral particles. Understanding of the HCV-encoded proteins and their functions has permitted the development of different DAA therapies. In general, targeting a single protein is not effective, and combination therapy targeting 2 proteins is required for viral eradication (Figure 2).11 The 3 drug targets that are currently available include NS3/4A serine PIs (eg, simeprevir, boceprevir, telaprevir), NS5A replication complex inhibitors (eg, ledipasvir, daclatasvir), and NS5B RNA-dependent RNA polymerase inhibitors (eg, sofosbuvir).
Other DAA’s are in development that have targets in host rather than viral cells. These include cycolphilin A inhibitors and the micro-RNA (miR-122) antagonist miravirsen.12,13
The RNA-dependent RNA polymerase, encoded by the HCV NS5B is targeted by 2 classes of inhibitors: nucleoside or nucleotide analog inhibitors (NIs), and non-nucleoside inhibitors (NNIs).11 The only NI of the NS5B protein approved by the FDA is sofosbuvir. The resistance profiles of NIs and NNIs differ, because they bind to distinct sites on the NS5B protein. NIs are analogs of natural substrates and bind to the active site of the RNA polymerase, whereas NNIs are allosteric site inhibitors. NIs have activity in vitro against all HCV genotypes and have high barrier to resistance as the active site of NS5B polymerase is less tolerant of different amino acid substitutions.
In vitro studies have demonstrated that NIs are less likely to select for mutations compared with NNIs and PIs. The NNIs have limited genotypic coverage and have a lower barrier to resistance. Strategies for targeting HCV proteins include using a NI NS5B protein inhibitor as the backbone with a high barrier to resistance in combination with 1 or 2 other DAAs with lower barriers to resistance, or the combination of 3 DAAs with lower barriers to resistance.11 Ribavirin has broad-spectrum antiviral activity, one of which is anti-HCV activity. The mode of action of RBV against HCV is not well understood, but several mechanisms have been proposed, one of which is via inhibition of viral-dependent RNA polymerase.
Current HCV Treatment Recommendations
Current treatment recommendations are available from the American Association for the Study of the Liver Disease (AASLD) and the Infectious Diseases Society of America (IDSA) (http://www.hcvguidelines.org); the VA National Hepatitis C Resource Center Program and Office of Public Health (http://www.hepatitis.va.gov/pdf/2014hcv.pdf); and the European Association for the Study of the Liver (EASL) (http://www.easl.eu/_newsroom/latest-news/easl-recommendations-on-treatment-of-hepatitis-c-2014). These recommendations are updated frequently as new drugs enter the marketplace (Table 1).14-16
Since most patients are unable or unwilling to tolerate interferon, the majority of patients in treatment are currently receiving interferon-free combinations. Treatment of genotype 1 is currently dominated by the offlabel use of sofosbuvir in combination with simeprevir. Data have been published from a single phase II trial in patients with and without cirrhosis.7 Of note are emerging data from observational studies confirming the efficacy of over 80% SVR in patients with cirrhosis and genotype 1a with or without prior treatment.17 Treatment of patients with genotype 2 infection is dominated by the use of sofosbuvir and RBV combination. However, patients with cirrhosis do not respond as well as patients without cirrhosis, and it remains to be seen whether extending therapy is of any benefit.
One exception for the use of PEG is the recommendation for patients with HCV genotype 3 and cirrhosis to consider the combination of PEG, RBV, and sofosbuvir for 12 weeks.18 This has the advantage of being more effective, less costly, and shorter treatment duration compared with the sofosbuvir and RBV association but is only appropriate for patients who can tolerate interferon. Common AEs and potential contraindications to treatment are listed in Table 2.5-10,19
New Treatment Options for HCV in 2015
Figure 3 details DAA combinations currently in phase III trials that are expected to receive approval in 2015. This will expand the repertoire of drug combinations available and enable fine-tuning of regimens according to patient and viral characteristics and cost requirements.
Cost and Effectiveness
Cost-effectiveness issues are of immediate importance for health care systems and payors. The first generation PIs, boceprevir and telaprevir, were used in combination with PEG and RBV and entailed pharmacy costs on par with current noninterferon regimens.20,21 Studies generally demonstrated that these treatments are cost-effective, especially in patients with advanced fibrosis. Ollendorf and colleagues recently published an analysis of the costs of using interferon-free regimens for treatment of 540,000 patients with chronic HCV in California.22 Assuming that 50% of patients would present for treatment, the cost of the new DAAs would be immense and result in an increase in costs from $12 billion to $34 billion in the first year and net costs of $6 billion by the 20th year. If treatment were limited to patients with advanced cirrhosis, the first year costs would be increased by $7 billion, but at 20 years there would be approximately $1 billion in net cost savings.
For current treatments, a 12-week course of simeprevir/sofosbuvir has been shown to be more costeffective than 24 weeks of sofosbuvir/RBV for treatment
of genotype 1.23 Similarly, patients with genotype 1, no cirrhosis, and low viral load can be treated with 8 weeks of sofosbuvir/ledipasvir rather than other 12-week regimens, thereby reducing drug costs. The resources needed for upfront treatment of patients is of obvious concern, and various systems are struggling to determine how to provide access to these pharmaceuticals. Prioritizing patients according to risk for advanced fibrosis using noninvasive scoring systems should be used if there is limited access or resources.
Conclusion
Since the VA has a large population of patients with HCV infection, the advancement in HCV treatment is of paramount importance. The advent of new DAAs in 2011 improved treatment efficacy for patients with HCV, but few patients could be treated due to AEs related to PEG. In 2013, new DAAs were introduced that did not require conjunctive therapy with PEG, providing a treatment option for patients who could not tolerate PEG. New DAA combinations are currently in trials and, upon approval, will provide more options for patients with HCV infection.
Author disclosures
Dr. Ho has received research and grant support from Genentech, Inc. and Gilead; he is on the speakers’ bureau for Prime Education, Inc. The other authors report no actual or potential conflicts of interest with regard to this article.
Grant Support
Funding provided by VA HSR&D grant IIR-13-052-2, VA HIV/HCV QUERI program, and the Research Service of the Department of Veterans Affairs.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Hepatitis C virus (HCV) infection remains a significant problem in the VA system, with over 174,000 current actively infected patients.1 Despite the availability of antiviral treatment since the early 1990’s, only approximately 26% of patients have ever been treated. These treatments required the use of pegylated interferon alfa (PEG) and ribavirin (RBV), which are associated with significant adverse events (AEs) that prevented many from receiving the treatment. Of those treated, only a minority achieved a sustained virologic response (SVR), due to the limited efficacy of the treatments (Figure 1).2 With the advent of new direct-acting antiviral (DAA) treatments in 2011, treatment efficacy improved.
The first DAAs in use were the viral nonstructural protein 3/4A (NS3/4A) serine protease inhibitors (PIs) boceprevir and telaprevir, which were used with PEG and RBV for patients with HCV genotype 1 infection. This combination therapy improved SVR rates from about 26% to 50% in patients with HCV genotype 1 in the VA.3,4 However, due to the significant AEs with these combinations, relatively few patients were treated.
In late 2013, the FDA approved other DAAs, which allowed patients to be treated effectively without PEG. These included the nucleotide nonstructural protein 5B (NS5B) polymerase inhibitor sofosbuvir and a secondgeneration NS3/4A PI simeprevir.5-7 The first nucleotide analog NS5B polymerase inhibitor, sofosbuvir and the nonstructural protein 5A (NS5A) replication complex inhibitor, ledipasvir, was approved in October 2014.8-10 The recent developments in noninterferon treatments have been accompanied by revised treatment guidelines or recommendations by major professional societies. Current treatment recommendations will be reviewed here, but the recommendations will continue to evolve as new DAAs come to market.
DAA Sites of Action
The HCV genome is a positive-stranded RNA molecule of about 9,500 nucleotides, which encodes a polyprotein of approximately 3,000 amino acids that form 10 individual viral proteins. These are composed of both structural and nonstructural (NS) proteins that are responsible for replication of the genome and formation of new viral particles. Understanding of the HCV-encoded proteins and their functions has permitted the development of different DAA therapies. In general, targeting a single protein is not effective, and combination therapy targeting 2 proteins is required for viral eradication (Figure 2).11 The 3 drug targets that are currently available include NS3/4A serine PIs (eg, simeprevir, boceprevir, telaprevir), NS5A replication complex inhibitors (eg, ledipasvir, daclatasvir), and NS5B RNA-dependent RNA polymerase inhibitors (eg, sofosbuvir).
Other DAA’s are in development that have targets in host rather than viral cells. These include cycolphilin A inhibitors and the micro-RNA (miR-122) antagonist miravirsen.12,13
The RNA-dependent RNA polymerase, encoded by the HCV NS5B is targeted by 2 classes of inhibitors: nucleoside or nucleotide analog inhibitors (NIs), and non-nucleoside inhibitors (NNIs).11 The only NI of the NS5B protein approved by the FDA is sofosbuvir. The resistance profiles of NIs and NNIs differ, because they bind to distinct sites on the NS5B protein. NIs are analogs of natural substrates and bind to the active site of the RNA polymerase, whereas NNIs are allosteric site inhibitors. NIs have activity in vitro against all HCV genotypes and have high barrier to resistance as the active site of NS5B polymerase is less tolerant of different amino acid substitutions.
In vitro studies have demonstrated that NIs are less likely to select for mutations compared with NNIs and PIs. The NNIs have limited genotypic coverage and have a lower barrier to resistance. Strategies for targeting HCV proteins include using a NI NS5B protein inhibitor as the backbone with a high barrier to resistance in combination with 1 or 2 other DAAs with lower barriers to resistance, or the combination of 3 DAAs with lower barriers to resistance.11 Ribavirin has broad-spectrum antiviral activity, one of which is anti-HCV activity. The mode of action of RBV against HCV is not well understood, but several mechanisms have been proposed, one of which is via inhibition of viral-dependent RNA polymerase.
Current HCV Treatment Recommendations
Current treatment recommendations are available from the American Association for the Study of the Liver Disease (AASLD) and the Infectious Diseases Society of America (IDSA) (http://www.hcvguidelines.org); the VA National Hepatitis C Resource Center Program and Office of Public Health (http://www.hepatitis.va.gov/pdf/2014hcv.pdf); and the European Association for the Study of the Liver (EASL) (http://www.easl.eu/_newsroom/latest-news/easl-recommendations-on-treatment-of-hepatitis-c-2014). These recommendations are updated frequently as new drugs enter the marketplace (Table 1).14-16
Since most patients are unable or unwilling to tolerate interferon, the majority of patients in treatment are currently receiving interferon-free combinations. Treatment of genotype 1 is currently dominated by the offlabel use of sofosbuvir in combination with simeprevir. Data have been published from a single phase II trial in patients with and without cirrhosis.7 Of note are emerging data from observational studies confirming the efficacy of over 80% SVR in patients with cirrhosis and genotype 1a with or without prior treatment.17 Treatment of patients with genotype 2 infection is dominated by the use of sofosbuvir and RBV combination. However, patients with cirrhosis do not respond as well as patients without cirrhosis, and it remains to be seen whether extending therapy is of any benefit.
One exception for the use of PEG is the recommendation for patients with HCV genotype 3 and cirrhosis to consider the combination of PEG, RBV, and sofosbuvir for 12 weeks.18 This has the advantage of being more effective, less costly, and shorter treatment duration compared with the sofosbuvir and RBV association but is only appropriate for patients who can tolerate interferon. Common AEs and potential contraindications to treatment are listed in Table 2.5-10,19
New Treatment Options for HCV in 2015
Figure 3 details DAA combinations currently in phase III trials that are expected to receive approval in 2015. This will expand the repertoire of drug combinations available and enable fine-tuning of regimens according to patient and viral characteristics and cost requirements.
Cost and Effectiveness
Cost-effectiveness issues are of immediate importance for health care systems and payors. The first generation PIs, boceprevir and telaprevir, were used in combination with PEG and RBV and entailed pharmacy costs on par with current noninterferon regimens.20,21 Studies generally demonstrated that these treatments are cost-effective, especially in patients with advanced fibrosis. Ollendorf and colleagues recently published an analysis of the costs of using interferon-free regimens for treatment of 540,000 patients with chronic HCV in California.22 Assuming that 50% of patients would present for treatment, the cost of the new DAAs would be immense and result in an increase in costs from $12 billion to $34 billion in the first year and net costs of $6 billion by the 20th year. If treatment were limited to patients with advanced cirrhosis, the first year costs would be increased by $7 billion, but at 20 years there would be approximately $1 billion in net cost savings.
For current treatments, a 12-week course of simeprevir/sofosbuvir has been shown to be more costeffective than 24 weeks of sofosbuvir/RBV for treatment
of genotype 1.23 Similarly, patients with genotype 1, no cirrhosis, and low viral load can be treated with 8 weeks of sofosbuvir/ledipasvir rather than other 12-week regimens, thereby reducing drug costs. The resources needed for upfront treatment of patients is of obvious concern, and various systems are struggling to determine how to provide access to these pharmaceuticals. Prioritizing patients according to risk for advanced fibrosis using noninvasive scoring systems should be used if there is limited access or resources.
Conclusion
Since the VA has a large population of patients with HCV infection, the advancement in HCV treatment is of paramount importance. The advent of new DAAs in 2011 improved treatment efficacy for patients with HCV, but few patients could be treated due to AEs related to PEG. In 2013, new DAAs were introduced that did not require conjunctive therapy with PEG, providing a treatment option for patients who could not tolerate PEG. New DAA combinations are currently in trials and, upon approval, will provide more options for patients with HCV infection.
Author disclosures
Dr. Ho has received research and grant support from Genentech, Inc. and Gilead; he is on the speakers’ bureau for Prime Education, Inc. The other authors report no actual or potential conflicts of interest with regard to this article.
Grant Support
Funding provided by VA HSR&D grant IIR-13-052-2, VA HIV/HCV QUERI program, and the Research Service of the Department of Veterans Affairs.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Backus LI, Belperio PS, Loomis TP, Yip GH, Mole LA. Hepatitis C virus screening and prevalence among US veterans in Department of Veterans Affairs care. JAMA Intern Med. 2013;173(16):1549-1552.
2. Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology. 2007;46(1):37-47.
3. Backus LI, Belperio PS, Shahoumian TA, Cheung R, Mole LA. Comparative effectiveness of the hepatitis C virus protease inhibitors boceprevir and telaprevir in a large U.S. cohort. Aliment Pharmacol Ther. 2014;39(1):93-103.
4. Ioannou GN, Beste LA, Green PK. Similar effectiveness of boceprevir and telaprevir treatment regimens for hepatitis C virus infection on the basis of a nationwide study of veterans. Clin Gastroenterol Hepatol. 2014;12(8):1371-1380.
5. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878-1887.
6. Jacobson IM, Gordon SC, Kowdley KV, et al; POSITRON Study; FUSION Study. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867-1877.
7. Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384(9956):1756-1765.
8. Afdhal N, Reddy KR, Nelson DR, et al; ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483-1493.
9. Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889-1898
10. Kowdley KV, Gordon SC, Reddy KR, et al; ION-3 Investigators. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879-1888.
11. Pawlotsky JM. New hepatitis C virus (HCV) drugs and the hope for a cure: Concepts in anti-HCV drug development. Semin Liver Dis. 2014;34(1):22-29.
12. Membreno FE, Espinales JC, Lawitz EJ. Cyclophilin inhibitors for hepatitis C therapy. Clin Liver Dis. 2013;17(1):129-139.
13. Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368(18):1685-1694.
14. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed November 25, 2014.
15. Department of Veterans Affairs. Chronic Hepatitis C Virus (HCV) Infection: Treatment considerations from the Department of Veterans Affairs National Hepatitis C Resource Center Program at the Office of Public Health. http://www.hepatitis.va.gov/pdf/2014hcv.pdf. Revised May 13, 2014. Accessed November 25, 2014.
16. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C in 2014. http://www.easl.eu/_newsroom/latest-news/easl-recommendations-on-treatment-of-hepatitis-c-2014. Accessed November 25, 2014.
17. Dieterich D, Bacon BR, Flamm SL, et al. Evaluation of sofosbuvir and simeprevir-based regimens in the TRIO network: academic and community treatment of a real-world, heterogeneous population. Hepatology. 2014;60(suppl S1):220A. Abstract 46.
18. Lawitz EJ, Poordad F, Brainard D, et al. Sofosbuvir with peginterferon-ribavirin for 12 weeks in previously treated patients with hepatitis C genotype 2 or 3 and cirrhosis [published online ahead of print October 16, 2014]. Hepatology. 2014; doi:10.1002/hep.27567.
19. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al; AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211-221.
20. Liu S, Cipriano LE, Holodniy M, Owens DK, Goldhaber-Fiebert JD. New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med. 2012;156(4):279-290.
21. Chan K, Lai MN, Groessl EJ, et al. Cost effectiveness of direct-acting antiviral
therapy for treatment-naive patients with chronic HCV genotype 1 infection in the veterans health administration. Clin Gastroenterol Hepatol. 2013;11(11):1503-1510.
22. Ollendorf DA, Tice JA, Pearson SD. The comparative clinical effectiveness and value of simeprevir and sofosbuvir for chronic hepatitis C virus infection. JAMA Intern Med. 2014;174(7):1170-1171.
23. Hagan LM, Sulkowski MS, Schinazi RF. Cost analysis of sofosbuvir/ribavirin versus sofosbuvir/simeprevir for genotype 1 hepatitis C virus in interferon-ineligible/intolerant individuals. Hepatology. 2014;60(1):37-45.
1. Backus LI, Belperio PS, Loomis TP, Yip GH, Mole LA. Hepatitis C virus screening and prevalence among US veterans in Department of Veterans Affairs care. JAMA Intern Med. 2013;173(16):1549-1552.
2. Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology. 2007;46(1):37-47.
3. Backus LI, Belperio PS, Shahoumian TA, Cheung R, Mole LA. Comparative effectiveness of the hepatitis C virus protease inhibitors boceprevir and telaprevir in a large U.S. cohort. Aliment Pharmacol Ther. 2014;39(1):93-103.
4. Ioannou GN, Beste LA, Green PK. Similar effectiveness of boceprevir and telaprevir treatment regimens for hepatitis C virus infection on the basis of a nationwide study of veterans. Clin Gastroenterol Hepatol. 2014;12(8):1371-1380.
5. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878-1887.
6. Jacobson IM, Gordon SC, Kowdley KV, et al; POSITRON Study; FUSION Study. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867-1877.
7. Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384(9956):1756-1765.
8. Afdhal N, Reddy KR, Nelson DR, et al; ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483-1493.
9. Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889-1898
10. Kowdley KV, Gordon SC, Reddy KR, et al; ION-3 Investigators. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879-1888.
11. Pawlotsky JM. New hepatitis C virus (HCV) drugs and the hope for a cure: Concepts in anti-HCV drug development. Semin Liver Dis. 2014;34(1):22-29.
12. Membreno FE, Espinales JC, Lawitz EJ. Cyclophilin inhibitors for hepatitis C therapy. Clin Liver Dis. 2013;17(1):129-139.
13. Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368(18):1685-1694.
14. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed November 25, 2014.
15. Department of Veterans Affairs. Chronic Hepatitis C Virus (HCV) Infection: Treatment considerations from the Department of Veterans Affairs National Hepatitis C Resource Center Program at the Office of Public Health. http://www.hepatitis.va.gov/pdf/2014hcv.pdf. Revised May 13, 2014. Accessed November 25, 2014.
16. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C in 2014. http://www.easl.eu/_newsroom/latest-news/easl-recommendations-on-treatment-of-hepatitis-c-2014. Accessed November 25, 2014.
17. Dieterich D, Bacon BR, Flamm SL, et al. Evaluation of sofosbuvir and simeprevir-based regimens in the TRIO network: academic and community treatment of a real-world, heterogeneous population. Hepatology. 2014;60(suppl S1):220A. Abstract 46.
18. Lawitz EJ, Poordad F, Brainard D, et al. Sofosbuvir with peginterferon-ribavirin for 12 weeks in previously treated patients with hepatitis C genotype 2 or 3 and cirrhosis [published online ahead of print October 16, 2014]. Hepatology. 2014; doi:10.1002/hep.27567.
19. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al; AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211-221.
20. Liu S, Cipriano LE, Holodniy M, Owens DK, Goldhaber-Fiebert JD. New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med. 2012;156(4):279-290.
21. Chan K, Lai MN, Groessl EJ, et al. Cost effectiveness of direct-acting antiviral
therapy for treatment-naive patients with chronic HCV genotype 1 infection in the veterans health administration. Clin Gastroenterol Hepatol. 2013;11(11):1503-1510.
22. Ollendorf DA, Tice JA, Pearson SD. The comparative clinical effectiveness and value of simeprevir and sofosbuvir for chronic hepatitis C virus infection. JAMA Intern Med. 2014;174(7):1170-1171.
23. Hagan LM, Sulkowski MS, Schinazi RF. Cost analysis of sofosbuvir/ribavirin versus sofosbuvir/simeprevir for genotype 1 hepatitis C virus in interferon-ineligible/intolerant individuals. Hepatology. 2014;60(1):37-45.
The State of Hepatitis C Care in the VA
As the largest single provider of hepatitis C virus infection (HCV) care in the U.S., the VA provided care to > 174,000 veterans with chronic HCV in 2013. Identifying veterans most likely to be infected with HCV, particularly those in the highrisk birth cohort born between 1945 and 1965, is a priority given recent CDC and U.S. Preventive Services Task Force (USPSTF) recommendations.1,2 The availability of new, all-oral HCV antiviral regimens with shorter treatment durations and improved tolerability are expected to greatly increase the number of veterans with HCV who could be treated successfully. In order to effectively reach those who are undiagnosed and to ensure that those diagnosed with HCV are evaluated and offered treatment, expanded reliance on primary care providers (PCPs) is essential. This article provides a population view of the current state of VA care for this large HCV-infected population and the important role PCPs share in disease identification and management.
Data Source
Data regarding the state of HCV care in the VA comes from the VA National Clinical Case Registry (CCR) for HCV.3 The VA HCV CCR is an extract of the VA electronic medical record that contains laboratory results, pharmacy information, provider information, and ICD-9 diagnosis codes from inpatient hospitalizations, outpatient visits, and problem lists of veterans with HCV seen at all VAMCs.
Screening and Prevalence of HCV
It is estimated that 2.3 to 2.7 million Americans are living with HCV, with 45% to 85% of those unaware of their infection.4,5 Nearly 75% of those infected are expected to have been born between 1945 and 1965; thus, the CDC and USPSTF now recommend onetime HCV screening for this birth cohort.1,2 Among nearly 5.6 million veterans with a VA outpatient visit in 2013, 56% have been screened for HCV. The HCV screening rate was 42% for those born prior to 1945, 65% for those born during 1945-1965, and 59% for those born after 1965. HCV infection prevalence overall in the VA was 5.8% but differed markedly among the birth cohorts: 1.6% for those born prior to 1945, 9.5% for those born during 1945-1965, and 1.2% for those born after 1965. The prevalence rate of veterans born in the 1945-1965 birth cohort (9.5%) is almost 3 times higher than that of the general U.S. population in this birth cohort (2.4%). The high prevalence serves as a reminder of the greater HCV disease burden in veterans and largely represents Vietman era veterans. Although HCV screening rates in VA have increased over 25% since 2002, the high prevalence among veterans in this birth cohort underscores the importance of continued screening efforts.
Veterans with Chronic HCV Infection
The VA Office of Public Health/Population Health generates national HCV reports annually from the HCV CCR describing the population of veterans with chronic HCV infection receiving VA care. These reports are intended to inform about patient care activities, clinician and patient education, prevention activities, and research directed at continuous improvement of veteran care. The first step in providing responsive care is understanding the affected population, and summarized herein is a description of the veterans with chronic HCV who received VA care in 2013.
In 2013, 174,302 veterans had laboratory evidence of HCV viremia and could be characterized as having chronic HCV. HCV treatment regimens and response depend on the viral genotype. Among veterans with genotype testing, 107,144 (80%) have genotype 1; 15,486 (12%) genotype 2; 9,745 (7%) genotype 3; 1333 (1%) genotype 4; and 63 (< 1%) genotype 5 or 6.
In terms of demographics, most veterans with chronic HCV in VA care in 2013 were men (97%); however, > 5,000 women veterans with chronic HCV received care from the VA. Over half (54%) of veterans with chronic HCV are white, and about one-third (34%) are black. The proportion of blacks within the HCV-infected veteran population is substantially greater than the proportion of blacks in the overall veteran population in VA care (15%) and highlights the disproportionately large burden of HCV that black veterans bear. A smaller proportion of the VA HCV population is Hispanic (6%), and the remaining veterans are other races, multiple races, or unknown.
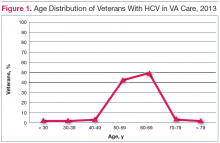
The HCV-infected veteran population is aging. The mean age of veterans with chronic HCV in 2013 was 59.7 years and for the first time, more veterans with HCV were aged 60 years (Figure 1).
Among the comorbidities that may have historically prevented veterans from receiving HCV antiviral therapy, 2 of the most pervasive are mental health conditions and alcohol use. The rates of mental illness among veterans overall is high, but mental illness is particularly high in veterans with HCV. Depression has affected 60%; of this population anxiety, 37%; posttraumatic stress disorder, 28%; and schizophrenia, 10%. Alcohol use disorders are also common among veterans with HCV in care. Active mental health conditions and substance use may affect medication adherence or follow-up visit adherence thereby limiting treatment candidacy. Integrating care of these individuals with mental health providers and substance-use treatment specialists is an important aspect of HCV care and is a priority in VA.
Three-quarters (76%) of the HCV-infected veteran population has been screened for HIV and HIV-HCV co-infection is present in 5733 (3%) of veterans with HCV. HIV-HCV co-infection is associated with an increased progression of liver disease and may have implications for the selection of HCV antiviral agents because of drug interactions. Hepatitis B virus (HBV)-HCV co-infection rates are higher at 7%. HBV vaccination or documentation of HBV immunity in those without HBV infection is 78%.
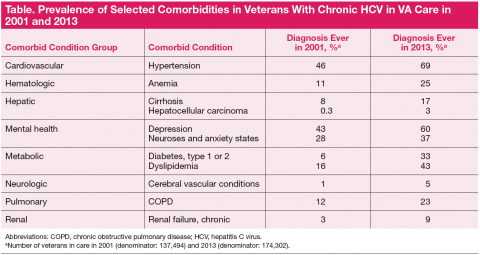
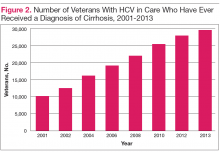
With regard to specific liver complications, 5% to 20% of those infected with chronic HCV will develop cirrhosis over a period of 20 to 30 years, and 1% to 5% will die of hepatocellular carcinoma (HCC) or cirrhosis.6 Given the natural history of chronic HCV and the aging HCV veteran cohort, increasing numbers of conditions related to progression of liver disease are expected over time. This is most evident in the number of veterans with a diagnosis of cirrhosis, which has increased from approximately 10,000 veterans (8%) in care in 2001 to nearly 30,000 veterans (17%) in care in 2013 (Figure 2).
Antiviral Therapy for Chronic HCV
Prior to mid-2011, the standard of care for HCV treatment was the combination of pegylated interferon and ribavirin. From 2011 through 2013, direct-acting antiviral (DAA) regimens containing boceprevir and telaprevir in combination with pegylated interferon and ribavirin became standard of care for genotype 1 while
the standard of care remained pegylated interferon and ribavirin for genotypes other than genotype 1. Recent advances in HCV antiviral therapy offer higher cure rates and fewer adverse events (AEs) compared with peginterferon-containing treatment. The expected ease and tolerability of these all-oral combination regimens is anticipated to greatly increase the number of veterans with HCV who could be treated successfully.
Because of the poor tolerability, prolonged treatment durations, serious AEs, and relative or absolute contraindications to peginterferon-based therapy, many veterans were not previously candidates for treatment. Of the 174,302 veterans with chronic HCV in care in 2013, 39,388 (23%) had received at least 1 course of HCV antiviral treatment. This largely reflects the time when peginterferon-based therapy was the standard of care. Since the approval of boceprevir and telaprevir 5,732 veterans (5.8%) in care in 2013 had ever received boceprevir or telaprevir-based regimens.
While recognizing that all veterans should be considered for HCV treatment, the urgency for treatment may be greater in those with advanced liver disease, because these patients are at the highest risk of developing decompensated cirrhosis or dying of liver-related disease. In 2013, there were 28,945 veterans in care that had advanced liver disease who might be considered potential HCV treatment candidates with an urgency to treat.
Duration of treatment and anticipated rates of treatment success with the all-oral regimens depend in part on a patient’s prior treatment status in addition to whether the patient has a diagnosis of advanced liver disease/cirrhosis. Regardless of HCV genotype, among all veterans approximately 85% are treatment-naïve and 15% are treatment-experienced. Advanced liver disease is present in 24% of treatment-naïve and 31% of treatment-experienced veterans with HCV genotype 1; 23% and 24% of veterans with HCV genotype 2, respectively; and 34% and 43% of veterans with HCV genotype 3, respectively.
Further understanding the population of veterans with HCV, including prior treatment status and stage of liver disease, is useful in identifying the target population for treatment. The VA uses these data to project treatment costs and assess capacity across the system in preparation for expected uptake of new regimens.
Sustained Virologic Response After HCV Antiviral Treatment
The goal of HCV antiviral therapy is to eradicate HCV and reduce the progression of liver disease and death from HCV infection. Successful antiviral treatment of HCV is determined by achieving a sustained virologic response (SVR) defined as an undetectable HCV viral load 12 weeks after the end of treatment. Of the 39,388 veterans in VA care in 2013 who have ever received antiviral therapy, SVR could be assessed in 32,815 veterans, and the overall SVR rate in this population was 42%. This SVR rate is similar to that observed in phase III trials of pegylated interferon-based regimens, where 42% to 46% of those infected with HCV genotype 1 achieved SVR.7,8 Although most veterans with genotype 1 infection received boceprevir or telaprevir-based regimens in 2013 and achieved higher SVRs of 50% to 52%, the overwhelming majority of veterans in care in 2013 received prior treatment with only peginterferon and ribavirin.9 Although SVR rates are expected to increase with newer all-oral HCV regimens, differences between clinical efficacy and real-world effectiveness will continue to be apparent,
and patient and provider expectations should be tempered accordingly.
The Role of Primary Care in HCV
Primary care providers have held the responsibility for multiple roles in HCV care since the discovery of the virus—particularly for HCV risk factor assessment, screening, and diagnosis. HCV antiviral treatment, however, was largely placed in the hands of specialists, given the complexities of patient selection, frequent reliance on a liver biopsy for determining need for treatment, and the toxicities of peginterferon and ribavirin therapy.
There are discussions both inside and outside the VA about potentially expanding the role of PCPs in HCV care. First, primary care is the major setting where the CDC and USPSTF recommendations for birth cohort screening are being implemented, and thus PCPs will be identifying veterans previously undiagnosed with HCV.1,2 Second, the ease and tolerability of the new all-oral combination regimens is causing a shift in the paradigm for HCV treatment, from a highly individualized approach, toward a more uniform approach.
Expanding the role of primary care would have multiple benefits to patients and the health care system as a whole. Only approximately 9% of HCV-infected veterans in VA care have been successfully treated at this time, largely due to low eligibility rates and the poor response rates, but other barriers have also contributed to the low success rate, one of which has been limited access to specialists. Furthermore, veterans who are referred to specialists are often noncompliant with the referral.10 If seeing an HCV specialist is required for treatment, the time to treat the HCV population will be much greater, more costly, and less efficient. Therefore, if the prospect of delivering HCV treatment to the majority of HCV patients is to be accomplished, it is necessary to consider providing treatment in the primary care setting as well as the specialist setting.
Treatment provided by nonspecialists has been evaluated in patients receiving peginterferon and ribavirin regimens and has shown that with adequate education and support, SVR rates were equivalent in the specialist and nonspecialist setting.11 To develop programs to provide primary care with such support, the VA has implemented the Specialty Care Access Network-Extension of Community Healthcare Outcomes program initiative, with casebased learning along with real-time consultation.
Currently, the majority of HCV-infected patients have never seen an HCV specialist, thus PCPs are already providing the majority of HCV care beyond HCV antiviral
treatment.12 Primary care providers are, therefore, key to addressing multiple important aspects of HCV care, including (1) counseling patients on transmission, prevention, lifestyle, and the role of substance use; (2) providing hepatitis A and B vaccination as well as appropriate general vaccinations for any patient with chronic liver disease; (3) modifying comorbidities that could accelerate fibrosis progression, such as diabetes mellitus, obesity and hyperlipidemia; (4) reducing risk from ongoing alcohol, drug, and tobacco use; (5) monitoring patients for fibrosis progression and identifying the presence of cirrhosis; and (6) providing general care for patients with cirrhosis, including HCC screening. These are critical aspects of HCV care, and many PCPs may still need additional education for these roles. The VA provides education and support for PCPs in their current role and is enhancing efforts to expand delivery of HCV treatment to the primary care setting as well.
Conclusions
In 2013, the typical veteran with chronic HCV was white, aged 60 years, and male, with a history of comorbidities, including hypertension, depression, and current or prior alcohol abuse. The proportion of veterans with advanced liver disease including cirrhosis (17%) and HCC (3%), has grown significantly over the past 10 years. By the end of 2013, almost 40,000 veterans had received antiviral therapy for HCV, more than 5,700 of whom had received DAAs. Overall SVR rates have been about 42% among those who were treated. Of veterans who are still potential treatment candidates, 85% are treatment-naive and about one-quarter have advanced liver disease.
Although HCV screening rates in veterans are higher than reported in other health care settings, particularly among those in the critical 1945-1965 birth cohort (65% screening rate), substantial numbers of veterans still require testing. The burden of disease, the lack of specialists, the ease and tolerability of new HCV antiviral medications, and the interplay of HCV with other traditional primary care efforts underly an increased role for PCPs in the care of veterans with HCV. Together, this information helps to construct a view of historical, current, and future HCV care in veterans.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patient.
1. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965 [published correction appears in MMWR Recomm Rep. 2012;61(43):886]. MMWR Recomm Rep. 2012;61(RR-4):1-32.
2. Moyer VA; U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
3. Backus LI, Gavrilov S, Loomis TP, et al. Clinical Case Registries: Simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc. 2009;16(6):775-783.
4. Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C virus infection in the United States: Model-based predictions. Ann Intern Med. 2014;161(3):170-180
5. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300
6. Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: A population-based study. Gastroenterology. 2004;127(5):1372-1380.
7. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358(9286):958-965.
8. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
9. Backus LI, Belperio PS, Shahoumian TA, Cheung R, Mole LA. Comparative effectiveness of the hepatitis C virus protease inhibitors boceprevir and telaprevir in a large U.S. cohort. Aliment Pharmacol Ther. 2014;39(1):93-103.
10. Brady CW, Coffman CJ, Provenzale D. Compliance with referral for hepatitis C evaluation among veterans. J Clin Gastroenterol. 2007;41(10):927-931.
11. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C infection by primary care providers. N Engl J Med. 2011;364(23):2199-2207.
12. Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368(20):1859-1861.
As the largest single provider of hepatitis C virus infection (HCV) care in the U.S., the VA provided care to > 174,000 veterans with chronic HCV in 2013. Identifying veterans most likely to be infected with HCV, particularly those in the highrisk birth cohort born between 1945 and 1965, is a priority given recent CDC and U.S. Preventive Services Task Force (USPSTF) recommendations.1,2 The availability of new, all-oral HCV antiviral regimens with shorter treatment durations and improved tolerability are expected to greatly increase the number of veterans with HCV who could be treated successfully. In order to effectively reach those who are undiagnosed and to ensure that those diagnosed with HCV are evaluated and offered treatment, expanded reliance on primary care providers (PCPs) is essential. This article provides a population view of the current state of VA care for this large HCV-infected population and the important role PCPs share in disease identification and management.
Data Source
Data regarding the state of HCV care in the VA comes from the VA National Clinical Case Registry (CCR) for HCV.3 The VA HCV CCR is an extract of the VA electronic medical record that contains laboratory results, pharmacy information, provider information, and ICD-9 diagnosis codes from inpatient hospitalizations, outpatient visits, and problem lists of veterans with HCV seen at all VAMCs.
Screening and Prevalence of HCV
It is estimated that 2.3 to 2.7 million Americans are living with HCV, with 45% to 85% of those unaware of their infection.4,5 Nearly 75% of those infected are expected to have been born between 1945 and 1965; thus, the CDC and USPSTF now recommend onetime HCV screening for this birth cohort.1,2 Among nearly 5.6 million veterans with a VA outpatient visit in 2013, 56% have been screened for HCV. The HCV screening rate was 42% for those born prior to 1945, 65% for those born during 1945-1965, and 59% for those born after 1965. HCV infection prevalence overall in the VA was 5.8% but differed markedly among the birth cohorts: 1.6% for those born prior to 1945, 9.5% for those born during 1945-1965, and 1.2% for those born after 1965. The prevalence rate of veterans born in the 1945-1965 birth cohort (9.5%) is almost 3 times higher than that of the general U.S. population in this birth cohort (2.4%). The high prevalence serves as a reminder of the greater HCV disease burden in veterans and largely represents Vietman era veterans. Although HCV screening rates in VA have increased over 25% since 2002, the high prevalence among veterans in this birth cohort underscores the importance of continued screening efforts.
Veterans with Chronic HCV Infection
The VA Office of Public Health/Population Health generates national HCV reports annually from the HCV CCR describing the population of veterans with chronic HCV infection receiving VA care. These reports are intended to inform about patient care activities, clinician and patient education, prevention activities, and research directed at continuous improvement of veteran care. The first step in providing responsive care is understanding the affected population, and summarized herein is a description of the veterans with chronic HCV who received VA care in 2013.
In 2013, 174,302 veterans had laboratory evidence of HCV viremia and could be characterized as having chronic HCV. HCV treatment regimens and response depend on the viral genotype. Among veterans with genotype testing, 107,144 (80%) have genotype 1; 15,486 (12%) genotype 2; 9,745 (7%) genotype 3; 1333 (1%) genotype 4; and 63 (< 1%) genotype 5 or 6.
In terms of demographics, most veterans with chronic HCV in VA care in 2013 were men (97%); however, > 5,000 women veterans with chronic HCV received care from the VA. Over half (54%) of veterans with chronic HCV are white, and about one-third (34%) are black. The proportion of blacks within the HCV-infected veteran population is substantially greater than the proportion of blacks in the overall veteran population in VA care (15%) and highlights the disproportionately large burden of HCV that black veterans bear. A smaller proportion of the VA HCV population is Hispanic (6%), and the remaining veterans are other races, multiple races, or unknown.
The HCV-infected veteran population is aging. The mean age of veterans with chronic HCV in 2013 was 59.7 years and for the first time, more veterans with HCV were aged 60 years (Figure 1).
Among the comorbidities that may have historically prevented veterans from receiving HCV antiviral therapy, 2 of the most pervasive are mental health conditions and alcohol use. The rates of mental illness among veterans overall is high, but mental illness is particularly high in veterans with HCV. Depression has affected 60%; of this population anxiety, 37%; posttraumatic stress disorder, 28%; and schizophrenia, 10%. Alcohol use disorders are also common among veterans with HCV in care. Active mental health conditions and substance use may affect medication adherence or follow-up visit adherence thereby limiting treatment candidacy. Integrating care of these individuals with mental health providers and substance-use treatment specialists is an important aspect of HCV care and is a priority in VA.
Three-quarters (76%) of the HCV-infected veteran population has been screened for HIV and HIV-HCV co-infection is present in 5733 (3%) of veterans with HCV. HIV-HCV co-infection is associated with an increased progression of liver disease and may have implications for the selection of HCV antiviral agents because of drug interactions. Hepatitis B virus (HBV)-HCV co-infection rates are higher at 7%. HBV vaccination or documentation of HBV immunity in those without HBV infection is 78%.
With regard to specific liver complications, 5% to 20% of those infected with chronic HCV will develop cirrhosis over a period of 20 to 30 years, and 1% to 5% will die of hepatocellular carcinoma (HCC) or cirrhosis.6 Given the natural history of chronic HCV and the aging HCV veteran cohort, increasing numbers of conditions related to progression of liver disease are expected over time. This is most evident in the number of veterans with a diagnosis of cirrhosis, which has increased from approximately 10,000 veterans (8%) in care in 2001 to nearly 30,000 veterans (17%) in care in 2013 (Figure 2).
Antiviral Therapy for Chronic HCV
Prior to mid-2011, the standard of care for HCV treatment was the combination of pegylated interferon and ribavirin. From 2011 through 2013, direct-acting antiviral (DAA) regimens containing boceprevir and telaprevir in combination with pegylated interferon and ribavirin became standard of care for genotype 1 while
the standard of care remained pegylated interferon and ribavirin for genotypes other than genotype 1. Recent advances in HCV antiviral therapy offer higher cure rates and fewer adverse events (AEs) compared with peginterferon-containing treatment. The expected ease and tolerability of these all-oral combination regimens is anticipated to greatly increase the number of veterans with HCV who could be treated successfully.
Because of the poor tolerability, prolonged treatment durations, serious AEs, and relative or absolute contraindications to peginterferon-based therapy, many veterans were not previously candidates for treatment. Of the 174,302 veterans with chronic HCV in care in 2013, 39,388 (23%) had received at least 1 course of HCV antiviral treatment. This largely reflects the time when peginterferon-based therapy was the standard of care. Since the approval of boceprevir and telaprevir 5,732 veterans (5.8%) in care in 2013 had ever received boceprevir or telaprevir-based regimens.
While recognizing that all veterans should be considered for HCV treatment, the urgency for treatment may be greater in those with advanced liver disease, because these patients are at the highest risk of developing decompensated cirrhosis or dying of liver-related disease. In 2013, there were 28,945 veterans in care that had advanced liver disease who might be considered potential HCV treatment candidates with an urgency to treat.
Duration of treatment and anticipated rates of treatment success with the all-oral regimens depend in part on a patient’s prior treatment status in addition to whether the patient has a diagnosis of advanced liver disease/cirrhosis. Regardless of HCV genotype, among all veterans approximately 85% are treatment-naïve and 15% are treatment-experienced. Advanced liver disease is present in 24% of treatment-naïve and 31% of treatment-experienced veterans with HCV genotype 1; 23% and 24% of veterans with HCV genotype 2, respectively; and 34% and 43% of veterans with HCV genotype 3, respectively.
Further understanding the population of veterans with HCV, including prior treatment status and stage of liver disease, is useful in identifying the target population for treatment. The VA uses these data to project treatment costs and assess capacity across the system in preparation for expected uptake of new regimens.
Sustained Virologic Response After HCV Antiviral Treatment
The goal of HCV antiviral therapy is to eradicate HCV and reduce the progression of liver disease and death from HCV infection. Successful antiviral treatment of HCV is determined by achieving a sustained virologic response (SVR) defined as an undetectable HCV viral load 12 weeks after the end of treatment. Of the 39,388 veterans in VA care in 2013 who have ever received antiviral therapy, SVR could be assessed in 32,815 veterans, and the overall SVR rate in this population was 42%. This SVR rate is similar to that observed in phase III trials of pegylated interferon-based regimens, where 42% to 46% of those infected with HCV genotype 1 achieved SVR.7,8 Although most veterans with genotype 1 infection received boceprevir or telaprevir-based regimens in 2013 and achieved higher SVRs of 50% to 52%, the overwhelming majority of veterans in care in 2013 received prior treatment with only peginterferon and ribavirin.9 Although SVR rates are expected to increase with newer all-oral HCV regimens, differences between clinical efficacy and real-world effectiveness will continue to be apparent,
and patient and provider expectations should be tempered accordingly.
The Role of Primary Care in HCV
Primary care providers have held the responsibility for multiple roles in HCV care since the discovery of the virus—particularly for HCV risk factor assessment, screening, and diagnosis. HCV antiviral treatment, however, was largely placed in the hands of specialists, given the complexities of patient selection, frequent reliance on a liver biopsy for determining need for treatment, and the toxicities of peginterferon and ribavirin therapy.
There are discussions both inside and outside the VA about potentially expanding the role of PCPs in HCV care. First, primary care is the major setting where the CDC and USPSTF recommendations for birth cohort screening are being implemented, and thus PCPs will be identifying veterans previously undiagnosed with HCV.1,2 Second, the ease and tolerability of the new all-oral combination regimens is causing a shift in the paradigm for HCV treatment, from a highly individualized approach, toward a more uniform approach.
Expanding the role of primary care would have multiple benefits to patients and the health care system as a whole. Only approximately 9% of HCV-infected veterans in VA care have been successfully treated at this time, largely due to low eligibility rates and the poor response rates, but other barriers have also contributed to the low success rate, one of which has been limited access to specialists. Furthermore, veterans who are referred to specialists are often noncompliant with the referral.10 If seeing an HCV specialist is required for treatment, the time to treat the HCV population will be much greater, more costly, and less efficient. Therefore, if the prospect of delivering HCV treatment to the majority of HCV patients is to be accomplished, it is necessary to consider providing treatment in the primary care setting as well as the specialist setting.
Treatment provided by nonspecialists has been evaluated in patients receiving peginterferon and ribavirin regimens and has shown that with adequate education and support, SVR rates were equivalent in the specialist and nonspecialist setting.11 To develop programs to provide primary care with such support, the VA has implemented the Specialty Care Access Network-Extension of Community Healthcare Outcomes program initiative, with casebased learning along with real-time consultation.
Currently, the majority of HCV-infected patients have never seen an HCV specialist, thus PCPs are already providing the majority of HCV care beyond HCV antiviral
treatment.12 Primary care providers are, therefore, key to addressing multiple important aspects of HCV care, including (1) counseling patients on transmission, prevention, lifestyle, and the role of substance use; (2) providing hepatitis A and B vaccination as well as appropriate general vaccinations for any patient with chronic liver disease; (3) modifying comorbidities that could accelerate fibrosis progression, such as diabetes mellitus, obesity and hyperlipidemia; (4) reducing risk from ongoing alcohol, drug, and tobacco use; (5) monitoring patients for fibrosis progression and identifying the presence of cirrhosis; and (6) providing general care for patients with cirrhosis, including HCC screening. These are critical aspects of HCV care, and many PCPs may still need additional education for these roles. The VA provides education and support for PCPs in their current role and is enhancing efforts to expand delivery of HCV treatment to the primary care setting as well.
Conclusions
In 2013, the typical veteran with chronic HCV was white, aged 60 years, and male, with a history of comorbidities, including hypertension, depression, and current or prior alcohol abuse. The proportion of veterans with advanced liver disease including cirrhosis (17%) and HCC (3%), has grown significantly over the past 10 years. By the end of 2013, almost 40,000 veterans had received antiviral therapy for HCV, more than 5,700 of whom had received DAAs. Overall SVR rates have been about 42% among those who were treated. Of veterans who are still potential treatment candidates, 85% are treatment-naive and about one-quarter have advanced liver disease.
Although HCV screening rates in veterans are higher than reported in other health care settings, particularly among those in the critical 1945-1965 birth cohort (65% screening rate), substantial numbers of veterans still require testing. The burden of disease, the lack of specialists, the ease and tolerability of new HCV antiviral medications, and the interplay of HCV with other traditional primary care efforts underly an increased role for PCPs in the care of veterans with HCV. Together, this information helps to construct a view of historical, current, and future HCV care in veterans.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patient.
As the largest single provider of hepatitis C virus infection (HCV) care in the U.S., the VA provided care to > 174,000 veterans with chronic HCV in 2013. Identifying veterans most likely to be infected with HCV, particularly those in the highrisk birth cohort born between 1945 and 1965, is a priority given recent CDC and U.S. Preventive Services Task Force (USPSTF) recommendations.1,2 The availability of new, all-oral HCV antiviral regimens with shorter treatment durations and improved tolerability are expected to greatly increase the number of veterans with HCV who could be treated successfully. In order to effectively reach those who are undiagnosed and to ensure that those diagnosed with HCV are evaluated and offered treatment, expanded reliance on primary care providers (PCPs) is essential. This article provides a population view of the current state of VA care for this large HCV-infected population and the important role PCPs share in disease identification and management.
Data Source
Data regarding the state of HCV care in the VA comes from the VA National Clinical Case Registry (CCR) for HCV.3 The VA HCV CCR is an extract of the VA electronic medical record that contains laboratory results, pharmacy information, provider information, and ICD-9 diagnosis codes from inpatient hospitalizations, outpatient visits, and problem lists of veterans with HCV seen at all VAMCs.
Screening and Prevalence of HCV
It is estimated that 2.3 to 2.7 million Americans are living with HCV, with 45% to 85% of those unaware of their infection.4,5 Nearly 75% of those infected are expected to have been born between 1945 and 1965; thus, the CDC and USPSTF now recommend onetime HCV screening for this birth cohort.1,2 Among nearly 5.6 million veterans with a VA outpatient visit in 2013, 56% have been screened for HCV. The HCV screening rate was 42% for those born prior to 1945, 65% for those born during 1945-1965, and 59% for those born after 1965. HCV infection prevalence overall in the VA was 5.8% but differed markedly among the birth cohorts: 1.6% for those born prior to 1945, 9.5% for those born during 1945-1965, and 1.2% for those born after 1965. The prevalence rate of veterans born in the 1945-1965 birth cohort (9.5%) is almost 3 times higher than that of the general U.S. population in this birth cohort (2.4%). The high prevalence serves as a reminder of the greater HCV disease burden in veterans and largely represents Vietman era veterans. Although HCV screening rates in VA have increased over 25% since 2002, the high prevalence among veterans in this birth cohort underscores the importance of continued screening efforts.
Veterans with Chronic HCV Infection
The VA Office of Public Health/Population Health generates national HCV reports annually from the HCV CCR describing the population of veterans with chronic HCV infection receiving VA care. These reports are intended to inform about patient care activities, clinician and patient education, prevention activities, and research directed at continuous improvement of veteran care. The first step in providing responsive care is understanding the affected population, and summarized herein is a description of the veterans with chronic HCV who received VA care in 2013.
In 2013, 174,302 veterans had laboratory evidence of HCV viremia and could be characterized as having chronic HCV. HCV treatment regimens and response depend on the viral genotype. Among veterans with genotype testing, 107,144 (80%) have genotype 1; 15,486 (12%) genotype 2; 9,745 (7%) genotype 3; 1333 (1%) genotype 4; and 63 (< 1%) genotype 5 or 6.
In terms of demographics, most veterans with chronic HCV in VA care in 2013 were men (97%); however, > 5,000 women veterans with chronic HCV received care from the VA. Over half (54%) of veterans with chronic HCV are white, and about one-third (34%) are black. The proportion of blacks within the HCV-infected veteran population is substantially greater than the proportion of blacks in the overall veteran population in VA care (15%) and highlights the disproportionately large burden of HCV that black veterans bear. A smaller proportion of the VA HCV population is Hispanic (6%), and the remaining veterans are other races, multiple races, or unknown.
The HCV-infected veteran population is aging. The mean age of veterans with chronic HCV in 2013 was 59.7 years and for the first time, more veterans with HCV were aged 60 years (Figure 1).
Among the comorbidities that may have historically prevented veterans from receiving HCV antiviral therapy, 2 of the most pervasive are mental health conditions and alcohol use. The rates of mental illness among veterans overall is high, but mental illness is particularly high in veterans with HCV. Depression has affected 60%; of this population anxiety, 37%; posttraumatic stress disorder, 28%; and schizophrenia, 10%. Alcohol use disorders are also common among veterans with HCV in care. Active mental health conditions and substance use may affect medication adherence or follow-up visit adherence thereby limiting treatment candidacy. Integrating care of these individuals with mental health providers and substance-use treatment specialists is an important aspect of HCV care and is a priority in VA.
Three-quarters (76%) of the HCV-infected veteran population has been screened for HIV and HIV-HCV co-infection is present in 5733 (3%) of veterans with HCV. HIV-HCV co-infection is associated with an increased progression of liver disease and may have implications for the selection of HCV antiviral agents because of drug interactions. Hepatitis B virus (HBV)-HCV co-infection rates are higher at 7%. HBV vaccination or documentation of HBV immunity in those without HBV infection is 78%.
With regard to specific liver complications, 5% to 20% of those infected with chronic HCV will develop cirrhosis over a period of 20 to 30 years, and 1% to 5% will die of hepatocellular carcinoma (HCC) or cirrhosis.6 Given the natural history of chronic HCV and the aging HCV veteran cohort, increasing numbers of conditions related to progression of liver disease are expected over time. This is most evident in the number of veterans with a diagnosis of cirrhosis, which has increased from approximately 10,000 veterans (8%) in care in 2001 to nearly 30,000 veterans (17%) in care in 2013 (Figure 2).
Antiviral Therapy for Chronic HCV
Prior to mid-2011, the standard of care for HCV treatment was the combination of pegylated interferon and ribavirin. From 2011 through 2013, direct-acting antiviral (DAA) regimens containing boceprevir and telaprevir in combination with pegylated interferon and ribavirin became standard of care for genotype 1 while
the standard of care remained pegylated interferon and ribavirin for genotypes other than genotype 1. Recent advances in HCV antiviral therapy offer higher cure rates and fewer adverse events (AEs) compared with peginterferon-containing treatment. The expected ease and tolerability of these all-oral combination regimens is anticipated to greatly increase the number of veterans with HCV who could be treated successfully.
Because of the poor tolerability, prolonged treatment durations, serious AEs, and relative or absolute contraindications to peginterferon-based therapy, many veterans were not previously candidates for treatment. Of the 174,302 veterans with chronic HCV in care in 2013, 39,388 (23%) had received at least 1 course of HCV antiviral treatment. This largely reflects the time when peginterferon-based therapy was the standard of care. Since the approval of boceprevir and telaprevir 5,732 veterans (5.8%) in care in 2013 had ever received boceprevir or telaprevir-based regimens.
While recognizing that all veterans should be considered for HCV treatment, the urgency for treatment may be greater in those with advanced liver disease, because these patients are at the highest risk of developing decompensated cirrhosis or dying of liver-related disease. In 2013, there were 28,945 veterans in care that had advanced liver disease who might be considered potential HCV treatment candidates with an urgency to treat.
Duration of treatment and anticipated rates of treatment success with the all-oral regimens depend in part on a patient’s prior treatment status in addition to whether the patient has a diagnosis of advanced liver disease/cirrhosis. Regardless of HCV genotype, among all veterans approximately 85% are treatment-naïve and 15% are treatment-experienced. Advanced liver disease is present in 24% of treatment-naïve and 31% of treatment-experienced veterans with HCV genotype 1; 23% and 24% of veterans with HCV genotype 2, respectively; and 34% and 43% of veterans with HCV genotype 3, respectively.
Further understanding the population of veterans with HCV, including prior treatment status and stage of liver disease, is useful in identifying the target population for treatment. The VA uses these data to project treatment costs and assess capacity across the system in preparation for expected uptake of new regimens.
Sustained Virologic Response After HCV Antiviral Treatment
The goal of HCV antiviral therapy is to eradicate HCV and reduce the progression of liver disease and death from HCV infection. Successful antiviral treatment of HCV is determined by achieving a sustained virologic response (SVR) defined as an undetectable HCV viral load 12 weeks after the end of treatment. Of the 39,388 veterans in VA care in 2013 who have ever received antiviral therapy, SVR could be assessed in 32,815 veterans, and the overall SVR rate in this population was 42%. This SVR rate is similar to that observed in phase III trials of pegylated interferon-based regimens, where 42% to 46% of those infected with HCV genotype 1 achieved SVR.7,8 Although most veterans with genotype 1 infection received boceprevir or telaprevir-based regimens in 2013 and achieved higher SVRs of 50% to 52%, the overwhelming majority of veterans in care in 2013 received prior treatment with only peginterferon and ribavirin.9 Although SVR rates are expected to increase with newer all-oral HCV regimens, differences between clinical efficacy and real-world effectiveness will continue to be apparent,
and patient and provider expectations should be tempered accordingly.
The Role of Primary Care in HCV
Primary care providers have held the responsibility for multiple roles in HCV care since the discovery of the virus—particularly for HCV risk factor assessment, screening, and diagnosis. HCV antiviral treatment, however, was largely placed in the hands of specialists, given the complexities of patient selection, frequent reliance on a liver biopsy for determining need for treatment, and the toxicities of peginterferon and ribavirin therapy.
There are discussions both inside and outside the VA about potentially expanding the role of PCPs in HCV care. First, primary care is the major setting where the CDC and USPSTF recommendations for birth cohort screening are being implemented, and thus PCPs will be identifying veterans previously undiagnosed with HCV.1,2 Second, the ease and tolerability of the new all-oral combination regimens is causing a shift in the paradigm for HCV treatment, from a highly individualized approach, toward a more uniform approach.
Expanding the role of primary care would have multiple benefits to patients and the health care system as a whole. Only approximately 9% of HCV-infected veterans in VA care have been successfully treated at this time, largely due to low eligibility rates and the poor response rates, but other barriers have also contributed to the low success rate, one of which has been limited access to specialists. Furthermore, veterans who are referred to specialists are often noncompliant with the referral.10 If seeing an HCV specialist is required for treatment, the time to treat the HCV population will be much greater, more costly, and less efficient. Therefore, if the prospect of delivering HCV treatment to the majority of HCV patients is to be accomplished, it is necessary to consider providing treatment in the primary care setting as well as the specialist setting.
Treatment provided by nonspecialists has been evaluated in patients receiving peginterferon and ribavirin regimens and has shown that with adequate education and support, SVR rates were equivalent in the specialist and nonspecialist setting.11 To develop programs to provide primary care with such support, the VA has implemented the Specialty Care Access Network-Extension of Community Healthcare Outcomes program initiative, with casebased learning along with real-time consultation.
Currently, the majority of HCV-infected patients have never seen an HCV specialist, thus PCPs are already providing the majority of HCV care beyond HCV antiviral
treatment.12 Primary care providers are, therefore, key to addressing multiple important aspects of HCV care, including (1) counseling patients on transmission, prevention, lifestyle, and the role of substance use; (2) providing hepatitis A and B vaccination as well as appropriate general vaccinations for any patient with chronic liver disease; (3) modifying comorbidities that could accelerate fibrosis progression, such as diabetes mellitus, obesity and hyperlipidemia; (4) reducing risk from ongoing alcohol, drug, and tobacco use; (5) monitoring patients for fibrosis progression and identifying the presence of cirrhosis; and (6) providing general care for patients with cirrhosis, including HCC screening. These are critical aspects of HCV care, and many PCPs may still need additional education for these roles. The VA provides education and support for PCPs in their current role and is enhancing efforts to expand delivery of HCV treatment to the primary care setting as well.
Conclusions
In 2013, the typical veteran with chronic HCV was white, aged 60 years, and male, with a history of comorbidities, including hypertension, depression, and current or prior alcohol abuse. The proportion of veterans with advanced liver disease including cirrhosis (17%) and HCC (3%), has grown significantly over the past 10 years. By the end of 2013, almost 40,000 veterans had received antiviral therapy for HCV, more than 5,700 of whom had received DAAs. Overall SVR rates have been about 42% among those who were treated. Of veterans who are still potential treatment candidates, 85% are treatment-naive and about one-quarter have advanced liver disease.
Although HCV screening rates in veterans are higher than reported in other health care settings, particularly among those in the critical 1945-1965 birth cohort (65% screening rate), substantial numbers of veterans still require testing. The burden of disease, the lack of specialists, the ease and tolerability of new HCV antiviral medications, and the interplay of HCV with other traditional primary care efforts underly an increased role for PCPs in the care of veterans with HCV. Together, this information helps to construct a view of historical, current, and future HCV care in veterans.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patient.
1. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965 [published correction appears in MMWR Recomm Rep. 2012;61(43):886]. MMWR Recomm Rep. 2012;61(RR-4):1-32.
2. Moyer VA; U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
3. Backus LI, Gavrilov S, Loomis TP, et al. Clinical Case Registries: Simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc. 2009;16(6):775-783.
4. Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C virus infection in the United States: Model-based predictions. Ann Intern Med. 2014;161(3):170-180
5. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300
6. Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: A population-based study. Gastroenterology. 2004;127(5):1372-1380.
7. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358(9286):958-965.
8. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
9. Backus LI, Belperio PS, Shahoumian TA, Cheung R, Mole LA. Comparative effectiveness of the hepatitis C virus protease inhibitors boceprevir and telaprevir in a large U.S. cohort. Aliment Pharmacol Ther. 2014;39(1):93-103.
10. Brady CW, Coffman CJ, Provenzale D. Compliance with referral for hepatitis C evaluation among veterans. J Clin Gastroenterol. 2007;41(10):927-931.
11. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C infection by primary care providers. N Engl J Med. 2011;364(23):2199-2207.
12. Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368(20):1859-1861.
1. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965 [published correction appears in MMWR Recomm Rep. 2012;61(43):886]. MMWR Recomm Rep. 2012;61(RR-4):1-32.
2. Moyer VA; U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
3. Backus LI, Gavrilov S, Loomis TP, et al. Clinical Case Registries: Simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc. 2009;16(6):775-783.
4. Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C virus infection in the United States: Model-based predictions. Ann Intern Med. 2014;161(3):170-180
5. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300
6. Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: A population-based study. Gastroenterology. 2004;127(5):1372-1380.
7. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358(9286):958-965.
8. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
9. Backus LI, Belperio PS, Shahoumian TA, Cheung R, Mole LA. Comparative effectiveness of the hepatitis C virus protease inhibitors boceprevir and telaprevir in a large U.S. cohort. Aliment Pharmacol Ther. 2014;39(1):93-103.
10. Brady CW, Coffman CJ, Provenzale D. Compliance with referral for hepatitis C evaluation among veterans. J Clin Gastroenterol. 2007;41(10):927-931.
11. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C infection by primary care providers. N Engl J Med. 2011;364(23):2199-2207.
12. Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368(20):1859-1861.
One Hundred Case Series of Vocal Cord Dysfunction in a Military Treatment Facility
Vocal cord dysfunction (VCD), also known as paradoxical vocal cord movement, is described as paroxysms of glottis obstruction due to true vocal cord adduction.1 Since VCD presents as a constellation of symptoms associated with dyspnea, it often is misdiagnosed as asthma.2 Vocal cord dysfunction often manifests as episodic dyspnea and wheezing, may occur with exercise, and may be minimally responsive to initial therapies. Flattened inspiratory curves may be noted on pulmonary function tests (PFTs), but direct laryngoscopy is the gold standard for diagnosis.3 A cohort of proven patients with VCD with a plateau in the inspiratory curve of PFTs also had a plateau on expiratory phase in 81% of cases.4
The differential diagnosis of patients presenting with upper airway symptoms is broad. It must include VCD, asthma, angioedema, laryngomalacia, vocal cord polyps, vocal cord tumors, and neurologic conditions such as brain stem compression or movement disorders. Essentially, all movement disorders of vocal cords must be considered, and organic causes of this movement disorder can be evaluated by visualization of the vocal cords. Triggers for VCD include exercise, airborne irritants, gastroesophageal reflux disease (GERD), allergic rhinitis, medications, and psychological conditions.5 Additionally, VCD can coexist with asthma, further complicating accurate diagnoses.6
Therapies are reported in case studies, but no large randomized controlled trials exist to evaluate current therapy options. Primary treatments of asthma therapy were largely ineffective, and ideal therapy includes a multidisciplinary approach, including speech therapy to optimize laryngeal control and treatment of all identified laryngeal irritants.6
The prevalence of VCD is unknown, with no prospective cohort studies completed to date and conflicting diagnostic criteria used in many case studies.7 A prevalence of 2.8% was noted in one particular cohort of 1,028 patients admitted to a rehabilitation center in a calendar year with the primary pulmonary diagnosis on admission.6 Females seemed to be affected at a higher ratio than were males, 2 to 3 females per 1 male diagnosis.7
In the military population, certain risk factors were noted in returning deployed members, including anxiety/high stress, exercise, and acute respiratory illnesses.8 In that particular cohort, 72% positive predictive value was noted for VCD if flattened inspiratory flow loops with negative methacholine challenge were present.
Diagnostic criteria are challenging, as symptoms such as dyspnea may be present acutely, last < 2 minutes, be self-limiting, and completely resolve outside of acute events. Stridor may be noted, primarily above the vocal cords, and less audible on chest auscultation.6 A goal of therapy, in addition to dedicated speech pathologist input, is optimizing comedical conditions, including GERD, allergic rhinitis, concomitant asthma, and any psychological diagnoses.9
Athletes are a particular subset of patients with VCD who are crucial to appropriately diagnose, including a detailed history and physical, PFTs, and proceeding to direct laryngoscopy to confirm diagnoses.10 Behavioral management includes rescue breathing techniques, and speech therapy programs focus on relaxation of the larynx and diaphragmatic breathing techniques, with the goal of establishing sense of control during acute events.10 Military service members are expected to operate at a high-intensity level similar to that of athletes, and treatments considered for athletes are applicable to military service members as well. Military strength and cardiovascular standards are measured by a combination of push-ups, sit-ups, and a run test, in addition to waist measurements. Some of the cohort were identified during physical fitness standard failures, usually in the run test, and ultimately received a pulmonology referral for wheezing or dyspnea with exertion. The objective of this retrospective cohort study was to evaluate 100 consecutively diagnosed cases of VCD in a military treatment facility.
Methods
The authors conducted a retrospective chart review of DoD military medical records of outpatient diagnoses in 100 consecutive diagnoses of VCD from January 2011 to February 2014. Institutional review board approval was obtained under Project RSM20130001E by the Exempt Determination Official at Eglin Air Force Base (AFB), Florida.
All cases were identified at time of VCD visualization and were diagnosed with video stroboscopy by speech therapy or by visual laryngoscopy by the otolaryngology or pulmonology departments via direct visualization.
Cases were collected chronologically, and all diagnosed cases at Eglin AFB hospital were included. Follow-up was scheduled with all patients diagnosed in Speech Therapy, and most patients were concurrently treated by Pulmonology or Allergy/Immunology. Pulmonary function tests were obtained in 98 of the 100 diagnosed cases. Patients eligible for care at Eglin AFB included active-duty and Reserve military members plus dependents and retirees.
The majority of patients diagnosed in this cohort were seen and diagnosed by Speech Therapy. Video stroboscopy is based on the principle that a movement of an object higher than a certain flicker rate appears to stand still to direct visualization, but with a rate of light exposure and imaging above the flicker rate by video, the true movement of the object can be identified.¹¹ Video stroboscopy is considered highly sensitive for organic disorders of vocal cords, but it is not specific for either organic or dysfunctional disorders.¹¹ It is still the gold standard above direct visualization, as it can detect abnormal movement of vocal cords above the critical rate that the human eye would perceive as not moving due to the frequency of movement (Figures 1 & 2).¹¹
In an older study, laryngoscopy was able to diagnose 100% of patients with symptomatic paradoxical vocal cord movement and additional 60% asymptomatic patients with a constellation of symptoms consistent with paradoxical vocal cord movement.¹²
Speech Therapy; Ear, Nose, and Throat (ENT); and Pulmonology may not perform direct visualization in these patients at initial presentation due to other suspected diagnoses. A more common test is the PFT, especially if asthma or other airway tract diseases are suspected (Figure 3).
Patient Descriptions
Study patients were referred for a variety of reasons, often from primary care clinics for concerns for asthma, episodic dyspnea, wheezing, or decreased exercise tolerance thought to be related to pulmonary or allergy causes. Pulmonology worked closely with Speech Therapy and referred VCD cases for speech evaluation, including video stroboscopy. Notably, of the patients in this cohort, although some were suspected to have asthma, those patients were ruled out during part of the pulmonology evaluation, both with PFT testing and methacholine challenges. An asthma diagnosis is important in a military treatment facility, as asthma is often grounds for discharge.
Patients ranged in age from 13 to 68 years, with a median age at 31 years diagnosis. Thirty-nine females and 61 males comprised the total case series. Speech Therapy diagnosed 97 patients, 96 were diagnosed at Eglin AFB hospital via stroboscopy. One patient was diagnosed off-base by Speech Therapy via direct visualization, 1 patient was diagnosed by Pulmonology on-base via direct visualization, and 2 patients were diagnosed by ENT on-base via direct visualization. These patients had direct laryngoscopy completed, often to rule out other organic causes for upper airway disease processes, and were found to have visual paradoxical vocal cord movement. Ninety-eight patients completed PFTs. Several patients were lost to follow-up, as can be common in a military population with frequent moves or members leaving service.
On record review, patient symptoms were present in the range of 2 months to 20 years, with a median duration of symptomatic reports lasting 2 years prior to diagnosis. Common diagnoses prior to visual VCD diagnosis included asthma, exercise-induced asthma, anxiety, and episodic wheezing. Risk factors that were evaluated in this case series included age, sex, body mass index (BMI), GERD, allergic rhinitis, postnasal drip, active smoker, previous smoker, and mental health diagnoses (Figure 4).
Pulmonary function test results were analyzed on 98 patients, including forced expiratory volume in 1 second (FEV1); forced vital capacity (FVC), FEV1/FVC ratio; peak inspiratory flow (PIF) and peak expiratory flow (PEF)—available in 97 studies; forced expiratory flow (FEF) at 25% to 75% of FVC (FEF 25%-75%)—available in 96 studies; and maximum voluntary ventilation (MVV) and MVV/FEV1 ratio—available in 60 of 98 PFTs.
Interventions
All patients diagnosed by Speech Therapy on-base were provided with laryngeal relaxation techniques, diaphragmatic breathing techniques, and controlled inhale/exhale techniques at time of diagnosis, with frequent follow-up scheduled with Speech Therapy and Pulmonology. All diagnoses potentially contributing to laryngeal irritation were treated, including GERD, allergic rhinitis, smoking cessation, weight loss, and exercise recommendations as needed.
Patients reported improvement on follow-up appointments with Speech Therapy in overall control of symptoms, subjectively categorized as poor improvement, partial improvement, and complete improvement. This was a subjective measurement of improvement and fully dependent on follow-up care and patient reporting for improvement. No predefined number of follow-ups was determined; patients were followed monthly until they declined further care, fully improved, moved out of the military treatment system, or were lost to follow-up.
Treatment included structured Speech Therapy sessions. Response to treatment was subjectively qualified by patient report. Fifteen patients reported complete resolution of symptoms, 57 reported partial improvement, 24 reported poor improvement, and 4 patients were lost to follow-up.
Results
Risk factors for the diagnosis of VCD included possible associations with GERD, allergic rhinitis, smoking, prior smoking, BMI, and mental health diagnoses. Body mass index ranged from 17 to 36 in the case series, with median BMI of 27. Mental health diagnoses were present in 35 patients and included diagnoses of anxiety, depression, and adjustment disorders. Gastroesophageal reflux disease diagnosis was present in 59 of the case series patients, 80 had the diagnosis of allergic rhinitis, 63 were diagnosed with postnasal drip. Sixteen case series patients were current smokers. An additional 26 were previous smokers (at least 100 cigarettes in lifetime) for a total of 42 patients that were current or prior smokers.
The chart review was completed to evaluate for the presence of these diagnoses, which included previous treatments; for example, proton pump inhibitors for GERD, antidepressants for depression, or intranasal steroids for allergic rhinitis. The diagnosis was counted as present if the patient was currently being treated for the particular diagnosis in question.
PFT Data
Data from PFTs were available for 98 of 100 cases diagnosed. Review of data across all 98 patients is noted for median FEV1 of 3.6, a median FVC of 4.5, with ratio of 0.80.
Since PFT values vary according to age, sex, and ethnicity, PFTs were analyzed for percent predicted values based on age, gender, and race. Notably, median values for FEV1, FVC, and PEF were all close to 100% of the predicted value. The MVV percent predicted was available in 60 cases and was 93% of predicted values. The most significant difference from expected values was FEF 25% to 75%, at 84% of expected results.
Flow-volume loop evaluations on the 97 PFTs available were completed, and 58 of the 97 were noted for variable extrathoracic airway obstruction consistent with inspiratory inhibition in the patient population. This is 60% of the available PFTs in this cohort study.
Discussion
This retrospective chart review of 100 consecutive VCD diagnoses in a military treatment facility reinforces many of the findings currently available in the literature. As illustrated in a Chest review article, the diagnosis of VCD on history, physical examination, or PFTs remains ellusive.1 The PFT evaluation contains some subjectivity regarding the flattening of inspiratory flow-volume loops and is not routinely reported in PFT results. In patients diagnosed with VCD, a clear consensus of treatment modalities remains lacking. Modification of risk factors (allergic rhinitis, GERD, smoking cessation, weight loss) assisted in self-reported patient improvement, as did focused speech therapy.
The median age of 31 years, likely reflected the younger military population served at Eglin AFB. Seventy-five of these patients were currently on active duty, 6 were retired from active duty (veterans), and 19 were dependents. The median time of symptoms to diagnosis was 2 years. Prior misdiagnosis with other diseases such as asthma was common. Also, referral to Pulmonology and Speech Therapy was usually completed after failed outpatient primary care management for the alternative diagnoses.
Improvement with therapy was mixed, and during the time of documented follow-up, 72 patients reported complete or partial improvement. Most active-duty patients in the partial improvement category based this subjective reporting on their ability to meet military physical fitness standards.
Previous data suggested a female predominance, but this study population was 61% male. Military populations are about 80% to 85% male, so an increase in male diagnosis is expected.
Many patients in the patient cohort arrived as a result of Pulmonology referrals with a presumptive diagnoses of asthma but were determined not to have asthma through PFT results inconsistent with asthma, no improvement with β-agonist therapies, and negative methacholine challenges (if performed). These results prompted evaluations for other conditions and eventually a VCD diagnosis. As noted, exclusion of asthma is of particular importance in a military population, as medical discharges often are pursued in service members with asthma whether controlled or uncontrolled. Lag time to referral also is possible in failures of military physical, which prompted medical evaluation once several failures had occurred over a 1- to 2-year time frame.
The PFT data evaluation was inconclusive for statistically significant changes when compared with age-matched normal PFT values. This also was noted in previous studies of VCD cases. Most notable was percent predicted values of FEF 25% to 75%, with 84% of expected values. The FEV1, FVC, and PEF all fell within predicted values of normal, despite wide ranges in age, sex, and ethnicity among the subjects. Inspiratory flattening consistent with extrathoracic obstruction was present in 58 of the 97 PFTs available for review at Eglin AFB.
Limitations
Limitations to this retrospective case series are illustrated here. Cases were found only when VCD was diagnosed and coded; and it is the authors’ suspicion that many have been misdiagnosed or improperly treated for asthma or other pulmonary/oropharynx conditions. If providers are not familiar with VCD or if PFT readings do not comment on inspiratory findings, diagnosis is less likely. Some of the authors’ colleagues already have determined that postdeployment prevalence of VCD seems to be elevated.8
This cohort was completed on all patients in a military treatment facility, with 75 active-duty personnel, 6 veterans, and 19 dependents of varying ages. This case series is retrospective and tabulates suspected risk factors; stronger and more informative studies could certainly be completed in prospective studies (although likely difficult with low prevalence) or in treatment comparison studies at the time of diagnosis.
Since the cohort had varied and lengthy time to diagnosis from onset of related symptoms, the treatment patients received prior to diagnosis differed extensively. Diagnosis was completed by numerous primary care managers or other subspecialties prior to arrival to Pulmonology and Speech Therapy at Eglin AFB. Once diagnosed in Speech Therapy, consistent treatment options were provided to patients in accordance with standard of care.
It is the authors’ suspicion that VCD may have a higher prevalence than previously reported in the literature. Military service members are tested annually or biannually on physical fitness standards and are evaluated for medical reasons for recurrent fitness standard failures. This selection of patients is more likely to have a VCD evaluation as part of a comprehensive evaluation than is a healthy adult in a civilian population. A prospective study in military service members would be more fruitful and possibly yield a higher prevalence postdeployment.
Conclusion
Vocal cord dysfunction remains a difficult diagnosis to treat, because multiple comorbidities likely contribute to the diagnosis. This retrospective case series attempted to compile common themes and noted that most of the patients had 2 or more risk factors of smoking, allergic rhinitis, GERD, or mental health diagnoses. A prospective trial would be ideal to evaluate VCD further. A focused trial in the particular communities of athletes or of military service members may be of increased benefit to better define VCD. It is notable that 100 cases were found in a relatively short period for a community hospital, and prevalence may be higher than previously reported.
1. Morris MJ, Christopher KL. Diagnostic criteria for the classification of vocal cord dysfunction. Chest. 2010;138(5):1213-1223.
2. National Heart, Lung, and Blood Institute. Expert panel report 3: guidelines for the diagnoses and management of asthma. Full report 2007. https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln .pdf. Published 2007.Accessed February 1, 2017.
3. Newman KB, Mason UG III, Schmaling KB. Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med. 1995;152(4, pt 1):1382-1386.
4. Sanz Santiago V, López Neyra A, Almería Gil E, Villa Asensi JR. Spirometry patterns in vocal cord dysfunction [in Spanish]. An Pediatr (Barc). 2013;78(3):173-177.
5. Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. 2010;81(2):156-159.
6. Benninger C, Parsons JP, Mastronarde JG. Vocal cord dysfunction and asthma. Curr Opin Pulm Med. 2011;17(1):45-49.
7. Campainha S, Ribeiro C, Guimar M, Lima R. Vocal cord dysfunction: a frequently forgotten entity. Case Rep Pulmonol. 2012;2012:525493.
8. Morris MJ, Oleszewski RT, Sterner JB, Allan PF. Vocal cord dysfunction related to combat deployment. Mil Med. 2013;178(11):1208-1212.
9. Al-Alwan A, Kaminsky D. Vocal cord dysfunction in athletes: clinical presentation and review of the literature. Phys Sportsmed. 2012;40(2):22-27.
10. Kenn K, Schmitz M. Prevalence of vocal cord dysfunction in patients with dyspnea. First prospective clinical study. Am J Respir Crit Care Med. 1997;155:A965.
11. Wendler, J, Nawka, T, Verges, D. Instructional course: videolaryngo-stroboscopy and phonetography—basic tools for diagnostics and documentation in the voice clinic. Poster presented at: 15th European Congress of Oto-Rhino-Laryngology, Head and Neck Surgery; September 11-16, 2004; Rodos-Kos, Greece.
12. Ibrahim WH, Gheriani HA, Almohamed AA, Raza T. Paradoxical vocal cord motion disorder: past, present and future. Postgrad Med J. 2007;83(977):164-172.
Vocal cord dysfunction (VCD), also known as paradoxical vocal cord movement, is described as paroxysms of glottis obstruction due to true vocal cord adduction.1 Since VCD presents as a constellation of symptoms associated with dyspnea, it often is misdiagnosed as asthma.2 Vocal cord dysfunction often manifests as episodic dyspnea and wheezing, may occur with exercise, and may be minimally responsive to initial therapies. Flattened inspiratory curves may be noted on pulmonary function tests (PFTs), but direct laryngoscopy is the gold standard for diagnosis.3 A cohort of proven patients with VCD with a plateau in the inspiratory curve of PFTs also had a plateau on expiratory phase in 81% of cases.4
The differential diagnosis of patients presenting with upper airway symptoms is broad. It must include VCD, asthma, angioedema, laryngomalacia, vocal cord polyps, vocal cord tumors, and neurologic conditions such as brain stem compression or movement disorders. Essentially, all movement disorders of vocal cords must be considered, and organic causes of this movement disorder can be evaluated by visualization of the vocal cords. Triggers for VCD include exercise, airborne irritants, gastroesophageal reflux disease (GERD), allergic rhinitis, medications, and psychological conditions.5 Additionally, VCD can coexist with asthma, further complicating accurate diagnoses.6
Therapies are reported in case studies, but no large randomized controlled trials exist to evaluate current therapy options. Primary treatments of asthma therapy were largely ineffective, and ideal therapy includes a multidisciplinary approach, including speech therapy to optimize laryngeal control and treatment of all identified laryngeal irritants.6
The prevalence of VCD is unknown, with no prospective cohort studies completed to date and conflicting diagnostic criteria used in many case studies.7 A prevalence of 2.8% was noted in one particular cohort of 1,028 patients admitted to a rehabilitation center in a calendar year with the primary pulmonary diagnosis on admission.6 Females seemed to be affected at a higher ratio than were males, 2 to 3 females per 1 male diagnosis.7
In the military population, certain risk factors were noted in returning deployed members, including anxiety/high stress, exercise, and acute respiratory illnesses.8 In that particular cohort, 72% positive predictive value was noted for VCD if flattened inspiratory flow loops with negative methacholine challenge were present.
Diagnostic criteria are challenging, as symptoms such as dyspnea may be present acutely, last < 2 minutes, be self-limiting, and completely resolve outside of acute events. Stridor may be noted, primarily above the vocal cords, and less audible on chest auscultation.6 A goal of therapy, in addition to dedicated speech pathologist input, is optimizing comedical conditions, including GERD, allergic rhinitis, concomitant asthma, and any psychological diagnoses.9
Athletes are a particular subset of patients with VCD who are crucial to appropriately diagnose, including a detailed history and physical, PFTs, and proceeding to direct laryngoscopy to confirm diagnoses.10 Behavioral management includes rescue breathing techniques, and speech therapy programs focus on relaxation of the larynx and diaphragmatic breathing techniques, with the goal of establishing sense of control during acute events.10 Military service members are expected to operate at a high-intensity level similar to that of athletes, and treatments considered for athletes are applicable to military service members as well. Military strength and cardiovascular standards are measured by a combination of push-ups, sit-ups, and a run test, in addition to waist measurements. Some of the cohort were identified during physical fitness standard failures, usually in the run test, and ultimately received a pulmonology referral for wheezing or dyspnea with exertion. The objective of this retrospective cohort study was to evaluate 100 consecutively diagnosed cases of VCD in a military treatment facility.
Methods
The authors conducted a retrospective chart review of DoD military medical records of outpatient diagnoses in 100 consecutive diagnoses of VCD from January 2011 to February 2014. Institutional review board approval was obtained under Project RSM20130001E by the Exempt Determination Official at Eglin Air Force Base (AFB), Florida.
All cases were identified at time of VCD visualization and were diagnosed with video stroboscopy by speech therapy or by visual laryngoscopy by the otolaryngology or pulmonology departments via direct visualization.
Cases were collected chronologically, and all diagnosed cases at Eglin AFB hospital were included. Follow-up was scheduled with all patients diagnosed in Speech Therapy, and most patients were concurrently treated by Pulmonology or Allergy/Immunology. Pulmonary function tests were obtained in 98 of the 100 diagnosed cases. Patients eligible for care at Eglin AFB included active-duty and Reserve military members plus dependents and retirees.
The majority of patients diagnosed in this cohort were seen and diagnosed by Speech Therapy. Video stroboscopy is based on the principle that a movement of an object higher than a certain flicker rate appears to stand still to direct visualization, but with a rate of light exposure and imaging above the flicker rate by video, the true movement of the object can be identified.¹¹ Video stroboscopy is considered highly sensitive for organic disorders of vocal cords, but it is not specific for either organic or dysfunctional disorders.¹¹ It is still the gold standard above direct visualization, as it can detect abnormal movement of vocal cords above the critical rate that the human eye would perceive as not moving due to the frequency of movement (Figures 1 & 2).¹¹
In an older study, laryngoscopy was able to diagnose 100% of patients with symptomatic paradoxical vocal cord movement and additional 60% asymptomatic patients with a constellation of symptoms consistent with paradoxical vocal cord movement.¹²
Speech Therapy; Ear, Nose, and Throat (ENT); and Pulmonology may not perform direct visualization in these patients at initial presentation due to other suspected diagnoses. A more common test is the PFT, especially if asthma or other airway tract diseases are suspected (Figure 3).
Patient Descriptions
Study patients were referred for a variety of reasons, often from primary care clinics for concerns for asthma, episodic dyspnea, wheezing, or decreased exercise tolerance thought to be related to pulmonary or allergy causes. Pulmonology worked closely with Speech Therapy and referred VCD cases for speech evaluation, including video stroboscopy. Notably, of the patients in this cohort, although some were suspected to have asthma, those patients were ruled out during part of the pulmonology evaluation, both with PFT testing and methacholine challenges. An asthma diagnosis is important in a military treatment facility, as asthma is often grounds for discharge.
Patients ranged in age from 13 to 68 years, with a median age at 31 years diagnosis. Thirty-nine females and 61 males comprised the total case series. Speech Therapy diagnosed 97 patients, 96 were diagnosed at Eglin AFB hospital via stroboscopy. One patient was diagnosed off-base by Speech Therapy via direct visualization, 1 patient was diagnosed by Pulmonology on-base via direct visualization, and 2 patients were diagnosed by ENT on-base via direct visualization. These patients had direct laryngoscopy completed, often to rule out other organic causes for upper airway disease processes, and were found to have visual paradoxical vocal cord movement. Ninety-eight patients completed PFTs. Several patients were lost to follow-up, as can be common in a military population with frequent moves or members leaving service.
On record review, patient symptoms were present in the range of 2 months to 20 years, with a median duration of symptomatic reports lasting 2 years prior to diagnosis. Common diagnoses prior to visual VCD diagnosis included asthma, exercise-induced asthma, anxiety, and episodic wheezing. Risk factors that were evaluated in this case series included age, sex, body mass index (BMI), GERD, allergic rhinitis, postnasal drip, active smoker, previous smoker, and mental health diagnoses (Figure 4).
Pulmonary function test results were analyzed on 98 patients, including forced expiratory volume in 1 second (FEV1); forced vital capacity (FVC), FEV1/FVC ratio; peak inspiratory flow (PIF) and peak expiratory flow (PEF)—available in 97 studies; forced expiratory flow (FEF) at 25% to 75% of FVC (FEF 25%-75%)—available in 96 studies; and maximum voluntary ventilation (MVV) and MVV/FEV1 ratio—available in 60 of 98 PFTs.
Interventions
All patients diagnosed by Speech Therapy on-base were provided with laryngeal relaxation techniques, diaphragmatic breathing techniques, and controlled inhale/exhale techniques at time of diagnosis, with frequent follow-up scheduled with Speech Therapy and Pulmonology. All diagnoses potentially contributing to laryngeal irritation were treated, including GERD, allergic rhinitis, smoking cessation, weight loss, and exercise recommendations as needed.
Patients reported improvement on follow-up appointments with Speech Therapy in overall control of symptoms, subjectively categorized as poor improvement, partial improvement, and complete improvement. This was a subjective measurement of improvement and fully dependent on follow-up care and patient reporting for improvement. No predefined number of follow-ups was determined; patients were followed monthly until they declined further care, fully improved, moved out of the military treatment system, or were lost to follow-up.
Treatment included structured Speech Therapy sessions. Response to treatment was subjectively qualified by patient report. Fifteen patients reported complete resolution of symptoms, 57 reported partial improvement, 24 reported poor improvement, and 4 patients were lost to follow-up.
Results
Risk factors for the diagnosis of VCD included possible associations with GERD, allergic rhinitis, smoking, prior smoking, BMI, and mental health diagnoses. Body mass index ranged from 17 to 36 in the case series, with median BMI of 27. Mental health diagnoses were present in 35 patients and included diagnoses of anxiety, depression, and adjustment disorders. Gastroesophageal reflux disease diagnosis was present in 59 of the case series patients, 80 had the diagnosis of allergic rhinitis, 63 were diagnosed with postnasal drip. Sixteen case series patients were current smokers. An additional 26 were previous smokers (at least 100 cigarettes in lifetime) for a total of 42 patients that were current or prior smokers.
The chart review was completed to evaluate for the presence of these diagnoses, which included previous treatments; for example, proton pump inhibitors for GERD, antidepressants for depression, or intranasal steroids for allergic rhinitis. The diagnosis was counted as present if the patient was currently being treated for the particular diagnosis in question.
PFT Data
Data from PFTs were available for 98 of 100 cases diagnosed. Review of data across all 98 patients is noted for median FEV1 of 3.6, a median FVC of 4.5, with ratio of 0.80.
Since PFT values vary according to age, sex, and ethnicity, PFTs were analyzed for percent predicted values based on age, gender, and race. Notably, median values for FEV1, FVC, and PEF were all close to 100% of the predicted value. The MVV percent predicted was available in 60 cases and was 93% of predicted values. The most significant difference from expected values was FEF 25% to 75%, at 84% of expected results.
Flow-volume loop evaluations on the 97 PFTs available were completed, and 58 of the 97 were noted for variable extrathoracic airway obstruction consistent with inspiratory inhibition in the patient population. This is 60% of the available PFTs in this cohort study.
Discussion
This retrospective chart review of 100 consecutive VCD diagnoses in a military treatment facility reinforces many of the findings currently available in the literature. As illustrated in a Chest review article, the diagnosis of VCD on history, physical examination, or PFTs remains ellusive.1 The PFT evaluation contains some subjectivity regarding the flattening of inspiratory flow-volume loops and is not routinely reported in PFT results. In patients diagnosed with VCD, a clear consensus of treatment modalities remains lacking. Modification of risk factors (allergic rhinitis, GERD, smoking cessation, weight loss) assisted in self-reported patient improvement, as did focused speech therapy.
The median age of 31 years, likely reflected the younger military population served at Eglin AFB. Seventy-five of these patients were currently on active duty, 6 were retired from active duty (veterans), and 19 were dependents. The median time of symptoms to diagnosis was 2 years. Prior misdiagnosis with other diseases such as asthma was common. Also, referral to Pulmonology and Speech Therapy was usually completed after failed outpatient primary care management for the alternative diagnoses.
Improvement with therapy was mixed, and during the time of documented follow-up, 72 patients reported complete or partial improvement. Most active-duty patients in the partial improvement category based this subjective reporting on their ability to meet military physical fitness standards.
Previous data suggested a female predominance, but this study population was 61% male. Military populations are about 80% to 85% male, so an increase in male diagnosis is expected.
Many patients in the patient cohort arrived as a result of Pulmonology referrals with a presumptive diagnoses of asthma but were determined not to have asthma through PFT results inconsistent with asthma, no improvement with β-agonist therapies, and negative methacholine challenges (if performed). These results prompted evaluations for other conditions and eventually a VCD diagnosis. As noted, exclusion of asthma is of particular importance in a military population, as medical discharges often are pursued in service members with asthma whether controlled or uncontrolled. Lag time to referral also is possible in failures of military physical, which prompted medical evaluation once several failures had occurred over a 1- to 2-year time frame.
The PFT data evaluation was inconclusive for statistically significant changes when compared with age-matched normal PFT values. This also was noted in previous studies of VCD cases. Most notable was percent predicted values of FEF 25% to 75%, with 84% of expected values. The FEV1, FVC, and PEF all fell within predicted values of normal, despite wide ranges in age, sex, and ethnicity among the subjects. Inspiratory flattening consistent with extrathoracic obstruction was present in 58 of the 97 PFTs available for review at Eglin AFB.
Limitations
Limitations to this retrospective case series are illustrated here. Cases were found only when VCD was diagnosed and coded; and it is the authors’ suspicion that many have been misdiagnosed or improperly treated for asthma or other pulmonary/oropharynx conditions. If providers are not familiar with VCD or if PFT readings do not comment on inspiratory findings, diagnosis is less likely. Some of the authors’ colleagues already have determined that postdeployment prevalence of VCD seems to be elevated.8
This cohort was completed on all patients in a military treatment facility, with 75 active-duty personnel, 6 veterans, and 19 dependents of varying ages. This case series is retrospective and tabulates suspected risk factors; stronger and more informative studies could certainly be completed in prospective studies (although likely difficult with low prevalence) or in treatment comparison studies at the time of diagnosis.
Since the cohort had varied and lengthy time to diagnosis from onset of related symptoms, the treatment patients received prior to diagnosis differed extensively. Diagnosis was completed by numerous primary care managers or other subspecialties prior to arrival to Pulmonology and Speech Therapy at Eglin AFB. Once diagnosed in Speech Therapy, consistent treatment options were provided to patients in accordance with standard of care.
It is the authors’ suspicion that VCD may have a higher prevalence than previously reported in the literature. Military service members are tested annually or biannually on physical fitness standards and are evaluated for medical reasons for recurrent fitness standard failures. This selection of patients is more likely to have a VCD evaluation as part of a comprehensive evaluation than is a healthy adult in a civilian population. A prospective study in military service members would be more fruitful and possibly yield a higher prevalence postdeployment.
Conclusion
Vocal cord dysfunction remains a difficult diagnosis to treat, because multiple comorbidities likely contribute to the diagnosis. This retrospective case series attempted to compile common themes and noted that most of the patients had 2 or more risk factors of smoking, allergic rhinitis, GERD, or mental health diagnoses. A prospective trial would be ideal to evaluate VCD further. A focused trial in the particular communities of athletes or of military service members may be of increased benefit to better define VCD. It is notable that 100 cases were found in a relatively short period for a community hospital, and prevalence may be higher than previously reported.
Vocal cord dysfunction (VCD), also known as paradoxical vocal cord movement, is described as paroxysms of glottis obstruction due to true vocal cord adduction.1 Since VCD presents as a constellation of symptoms associated with dyspnea, it often is misdiagnosed as asthma.2 Vocal cord dysfunction often manifests as episodic dyspnea and wheezing, may occur with exercise, and may be minimally responsive to initial therapies. Flattened inspiratory curves may be noted on pulmonary function tests (PFTs), but direct laryngoscopy is the gold standard for diagnosis.3 A cohort of proven patients with VCD with a plateau in the inspiratory curve of PFTs also had a plateau on expiratory phase in 81% of cases.4
The differential diagnosis of patients presenting with upper airway symptoms is broad. It must include VCD, asthma, angioedema, laryngomalacia, vocal cord polyps, vocal cord tumors, and neurologic conditions such as brain stem compression or movement disorders. Essentially, all movement disorders of vocal cords must be considered, and organic causes of this movement disorder can be evaluated by visualization of the vocal cords. Triggers for VCD include exercise, airborne irritants, gastroesophageal reflux disease (GERD), allergic rhinitis, medications, and psychological conditions.5 Additionally, VCD can coexist with asthma, further complicating accurate diagnoses.6
Therapies are reported in case studies, but no large randomized controlled trials exist to evaluate current therapy options. Primary treatments of asthma therapy were largely ineffective, and ideal therapy includes a multidisciplinary approach, including speech therapy to optimize laryngeal control and treatment of all identified laryngeal irritants.6
The prevalence of VCD is unknown, with no prospective cohort studies completed to date and conflicting diagnostic criteria used in many case studies.7 A prevalence of 2.8% was noted in one particular cohort of 1,028 patients admitted to a rehabilitation center in a calendar year with the primary pulmonary diagnosis on admission.6 Females seemed to be affected at a higher ratio than were males, 2 to 3 females per 1 male diagnosis.7
In the military population, certain risk factors were noted in returning deployed members, including anxiety/high stress, exercise, and acute respiratory illnesses.8 In that particular cohort, 72% positive predictive value was noted for VCD if flattened inspiratory flow loops with negative methacholine challenge were present.
Diagnostic criteria are challenging, as symptoms such as dyspnea may be present acutely, last < 2 minutes, be self-limiting, and completely resolve outside of acute events. Stridor may be noted, primarily above the vocal cords, and less audible on chest auscultation.6 A goal of therapy, in addition to dedicated speech pathologist input, is optimizing comedical conditions, including GERD, allergic rhinitis, concomitant asthma, and any psychological diagnoses.9
Athletes are a particular subset of patients with VCD who are crucial to appropriately diagnose, including a detailed history and physical, PFTs, and proceeding to direct laryngoscopy to confirm diagnoses.10 Behavioral management includes rescue breathing techniques, and speech therapy programs focus on relaxation of the larynx and diaphragmatic breathing techniques, with the goal of establishing sense of control during acute events.10 Military service members are expected to operate at a high-intensity level similar to that of athletes, and treatments considered for athletes are applicable to military service members as well. Military strength and cardiovascular standards are measured by a combination of push-ups, sit-ups, and a run test, in addition to waist measurements. Some of the cohort were identified during physical fitness standard failures, usually in the run test, and ultimately received a pulmonology referral for wheezing or dyspnea with exertion. The objective of this retrospective cohort study was to evaluate 100 consecutively diagnosed cases of VCD in a military treatment facility.
Methods
The authors conducted a retrospective chart review of DoD military medical records of outpatient diagnoses in 100 consecutive diagnoses of VCD from January 2011 to February 2014. Institutional review board approval was obtained under Project RSM20130001E by the Exempt Determination Official at Eglin Air Force Base (AFB), Florida.
All cases were identified at time of VCD visualization and were diagnosed with video stroboscopy by speech therapy or by visual laryngoscopy by the otolaryngology or pulmonology departments via direct visualization.
Cases were collected chronologically, and all diagnosed cases at Eglin AFB hospital were included. Follow-up was scheduled with all patients diagnosed in Speech Therapy, and most patients were concurrently treated by Pulmonology or Allergy/Immunology. Pulmonary function tests were obtained in 98 of the 100 diagnosed cases. Patients eligible for care at Eglin AFB included active-duty and Reserve military members plus dependents and retirees.
The majority of patients diagnosed in this cohort were seen and diagnosed by Speech Therapy. Video stroboscopy is based on the principle that a movement of an object higher than a certain flicker rate appears to stand still to direct visualization, but with a rate of light exposure and imaging above the flicker rate by video, the true movement of the object can be identified.¹¹ Video stroboscopy is considered highly sensitive for organic disorders of vocal cords, but it is not specific for either organic or dysfunctional disorders.¹¹ It is still the gold standard above direct visualization, as it can detect abnormal movement of vocal cords above the critical rate that the human eye would perceive as not moving due to the frequency of movement (Figures 1 & 2).¹¹
In an older study, laryngoscopy was able to diagnose 100% of patients with symptomatic paradoxical vocal cord movement and additional 60% asymptomatic patients with a constellation of symptoms consistent with paradoxical vocal cord movement.¹²
Speech Therapy; Ear, Nose, and Throat (ENT); and Pulmonology may not perform direct visualization in these patients at initial presentation due to other suspected diagnoses. A more common test is the PFT, especially if asthma or other airway tract diseases are suspected (Figure 3).
Patient Descriptions
Study patients were referred for a variety of reasons, often from primary care clinics for concerns for asthma, episodic dyspnea, wheezing, or decreased exercise tolerance thought to be related to pulmonary or allergy causes. Pulmonology worked closely with Speech Therapy and referred VCD cases for speech evaluation, including video stroboscopy. Notably, of the patients in this cohort, although some were suspected to have asthma, those patients were ruled out during part of the pulmonology evaluation, both with PFT testing and methacholine challenges. An asthma diagnosis is important in a military treatment facility, as asthma is often grounds for discharge.
Patients ranged in age from 13 to 68 years, with a median age at 31 years diagnosis. Thirty-nine females and 61 males comprised the total case series. Speech Therapy diagnosed 97 patients, 96 were diagnosed at Eglin AFB hospital via stroboscopy. One patient was diagnosed off-base by Speech Therapy via direct visualization, 1 patient was diagnosed by Pulmonology on-base via direct visualization, and 2 patients were diagnosed by ENT on-base via direct visualization. These patients had direct laryngoscopy completed, often to rule out other organic causes for upper airway disease processes, and were found to have visual paradoxical vocal cord movement. Ninety-eight patients completed PFTs. Several patients were lost to follow-up, as can be common in a military population with frequent moves or members leaving service.
On record review, patient symptoms were present in the range of 2 months to 20 years, with a median duration of symptomatic reports lasting 2 years prior to diagnosis. Common diagnoses prior to visual VCD diagnosis included asthma, exercise-induced asthma, anxiety, and episodic wheezing. Risk factors that were evaluated in this case series included age, sex, body mass index (BMI), GERD, allergic rhinitis, postnasal drip, active smoker, previous smoker, and mental health diagnoses (Figure 4).
Pulmonary function test results were analyzed on 98 patients, including forced expiratory volume in 1 second (FEV1); forced vital capacity (FVC), FEV1/FVC ratio; peak inspiratory flow (PIF) and peak expiratory flow (PEF)—available in 97 studies; forced expiratory flow (FEF) at 25% to 75% of FVC (FEF 25%-75%)—available in 96 studies; and maximum voluntary ventilation (MVV) and MVV/FEV1 ratio—available in 60 of 98 PFTs.
Interventions
All patients diagnosed by Speech Therapy on-base were provided with laryngeal relaxation techniques, diaphragmatic breathing techniques, and controlled inhale/exhale techniques at time of diagnosis, with frequent follow-up scheduled with Speech Therapy and Pulmonology. All diagnoses potentially contributing to laryngeal irritation were treated, including GERD, allergic rhinitis, smoking cessation, weight loss, and exercise recommendations as needed.
Patients reported improvement on follow-up appointments with Speech Therapy in overall control of symptoms, subjectively categorized as poor improvement, partial improvement, and complete improvement. This was a subjective measurement of improvement and fully dependent on follow-up care and patient reporting for improvement. No predefined number of follow-ups was determined; patients were followed monthly until they declined further care, fully improved, moved out of the military treatment system, or were lost to follow-up.
Treatment included structured Speech Therapy sessions. Response to treatment was subjectively qualified by patient report. Fifteen patients reported complete resolution of symptoms, 57 reported partial improvement, 24 reported poor improvement, and 4 patients were lost to follow-up.
Results
Risk factors for the diagnosis of VCD included possible associations with GERD, allergic rhinitis, smoking, prior smoking, BMI, and mental health diagnoses. Body mass index ranged from 17 to 36 in the case series, with median BMI of 27. Mental health diagnoses were present in 35 patients and included diagnoses of anxiety, depression, and adjustment disorders. Gastroesophageal reflux disease diagnosis was present in 59 of the case series patients, 80 had the diagnosis of allergic rhinitis, 63 were diagnosed with postnasal drip. Sixteen case series patients were current smokers. An additional 26 were previous smokers (at least 100 cigarettes in lifetime) for a total of 42 patients that were current or prior smokers.
The chart review was completed to evaluate for the presence of these diagnoses, which included previous treatments; for example, proton pump inhibitors for GERD, antidepressants for depression, or intranasal steroids for allergic rhinitis. The diagnosis was counted as present if the patient was currently being treated for the particular diagnosis in question.
PFT Data
Data from PFTs were available for 98 of 100 cases diagnosed. Review of data across all 98 patients is noted for median FEV1 of 3.6, a median FVC of 4.5, with ratio of 0.80.
Since PFT values vary according to age, sex, and ethnicity, PFTs were analyzed for percent predicted values based on age, gender, and race. Notably, median values for FEV1, FVC, and PEF were all close to 100% of the predicted value. The MVV percent predicted was available in 60 cases and was 93% of predicted values. The most significant difference from expected values was FEF 25% to 75%, at 84% of expected results.
Flow-volume loop evaluations on the 97 PFTs available were completed, and 58 of the 97 were noted for variable extrathoracic airway obstruction consistent with inspiratory inhibition in the patient population. This is 60% of the available PFTs in this cohort study.
Discussion
This retrospective chart review of 100 consecutive VCD diagnoses in a military treatment facility reinforces many of the findings currently available in the literature. As illustrated in a Chest review article, the diagnosis of VCD on history, physical examination, or PFTs remains ellusive.1 The PFT evaluation contains some subjectivity regarding the flattening of inspiratory flow-volume loops and is not routinely reported in PFT results. In patients diagnosed with VCD, a clear consensus of treatment modalities remains lacking. Modification of risk factors (allergic rhinitis, GERD, smoking cessation, weight loss) assisted in self-reported patient improvement, as did focused speech therapy.
The median age of 31 years, likely reflected the younger military population served at Eglin AFB. Seventy-five of these patients were currently on active duty, 6 were retired from active duty (veterans), and 19 were dependents. The median time of symptoms to diagnosis was 2 years. Prior misdiagnosis with other diseases such as asthma was common. Also, referral to Pulmonology and Speech Therapy was usually completed after failed outpatient primary care management for the alternative diagnoses.
Improvement with therapy was mixed, and during the time of documented follow-up, 72 patients reported complete or partial improvement. Most active-duty patients in the partial improvement category based this subjective reporting on their ability to meet military physical fitness standards.
Previous data suggested a female predominance, but this study population was 61% male. Military populations are about 80% to 85% male, so an increase in male diagnosis is expected.
Many patients in the patient cohort arrived as a result of Pulmonology referrals with a presumptive diagnoses of asthma but were determined not to have asthma through PFT results inconsistent with asthma, no improvement with β-agonist therapies, and negative methacholine challenges (if performed). These results prompted evaluations for other conditions and eventually a VCD diagnosis. As noted, exclusion of asthma is of particular importance in a military population, as medical discharges often are pursued in service members with asthma whether controlled or uncontrolled. Lag time to referral also is possible in failures of military physical, which prompted medical evaluation once several failures had occurred over a 1- to 2-year time frame.
The PFT data evaluation was inconclusive for statistically significant changes when compared with age-matched normal PFT values. This also was noted in previous studies of VCD cases. Most notable was percent predicted values of FEF 25% to 75%, with 84% of expected values. The FEV1, FVC, and PEF all fell within predicted values of normal, despite wide ranges in age, sex, and ethnicity among the subjects. Inspiratory flattening consistent with extrathoracic obstruction was present in 58 of the 97 PFTs available for review at Eglin AFB.
Limitations
Limitations to this retrospective case series are illustrated here. Cases were found only when VCD was diagnosed and coded; and it is the authors’ suspicion that many have been misdiagnosed or improperly treated for asthma or other pulmonary/oropharynx conditions. If providers are not familiar with VCD or if PFT readings do not comment on inspiratory findings, diagnosis is less likely. Some of the authors’ colleagues already have determined that postdeployment prevalence of VCD seems to be elevated.8
This cohort was completed on all patients in a military treatment facility, with 75 active-duty personnel, 6 veterans, and 19 dependents of varying ages. This case series is retrospective and tabulates suspected risk factors; stronger and more informative studies could certainly be completed in prospective studies (although likely difficult with low prevalence) or in treatment comparison studies at the time of diagnosis.
Since the cohort had varied and lengthy time to diagnosis from onset of related symptoms, the treatment patients received prior to diagnosis differed extensively. Diagnosis was completed by numerous primary care managers or other subspecialties prior to arrival to Pulmonology and Speech Therapy at Eglin AFB. Once diagnosed in Speech Therapy, consistent treatment options were provided to patients in accordance with standard of care.
It is the authors’ suspicion that VCD may have a higher prevalence than previously reported in the literature. Military service members are tested annually or biannually on physical fitness standards and are evaluated for medical reasons for recurrent fitness standard failures. This selection of patients is more likely to have a VCD evaluation as part of a comprehensive evaluation than is a healthy adult in a civilian population. A prospective study in military service members would be more fruitful and possibly yield a higher prevalence postdeployment.
Conclusion
Vocal cord dysfunction remains a difficult diagnosis to treat, because multiple comorbidities likely contribute to the diagnosis. This retrospective case series attempted to compile common themes and noted that most of the patients had 2 or more risk factors of smoking, allergic rhinitis, GERD, or mental health diagnoses. A prospective trial would be ideal to evaluate VCD further. A focused trial in the particular communities of athletes or of military service members may be of increased benefit to better define VCD. It is notable that 100 cases were found in a relatively short period for a community hospital, and prevalence may be higher than previously reported.
1. Morris MJ, Christopher KL. Diagnostic criteria for the classification of vocal cord dysfunction. Chest. 2010;138(5):1213-1223.
2. National Heart, Lung, and Blood Institute. Expert panel report 3: guidelines for the diagnoses and management of asthma. Full report 2007. https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln .pdf. Published 2007.Accessed February 1, 2017.
3. Newman KB, Mason UG III, Schmaling KB. Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med. 1995;152(4, pt 1):1382-1386.
4. Sanz Santiago V, López Neyra A, Almería Gil E, Villa Asensi JR. Spirometry patterns in vocal cord dysfunction [in Spanish]. An Pediatr (Barc). 2013;78(3):173-177.
5. Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. 2010;81(2):156-159.
6. Benninger C, Parsons JP, Mastronarde JG. Vocal cord dysfunction and asthma. Curr Opin Pulm Med. 2011;17(1):45-49.
7. Campainha S, Ribeiro C, Guimar M, Lima R. Vocal cord dysfunction: a frequently forgotten entity. Case Rep Pulmonol. 2012;2012:525493.
8. Morris MJ, Oleszewski RT, Sterner JB, Allan PF. Vocal cord dysfunction related to combat deployment. Mil Med. 2013;178(11):1208-1212.
9. Al-Alwan A, Kaminsky D. Vocal cord dysfunction in athletes: clinical presentation and review of the literature. Phys Sportsmed. 2012;40(2):22-27.
10. Kenn K, Schmitz M. Prevalence of vocal cord dysfunction in patients with dyspnea. First prospective clinical study. Am J Respir Crit Care Med. 1997;155:A965.
11. Wendler, J, Nawka, T, Verges, D. Instructional course: videolaryngo-stroboscopy and phonetography—basic tools for diagnostics and documentation in the voice clinic. Poster presented at: 15th European Congress of Oto-Rhino-Laryngology, Head and Neck Surgery; September 11-16, 2004; Rodos-Kos, Greece.
12. Ibrahim WH, Gheriani HA, Almohamed AA, Raza T. Paradoxical vocal cord motion disorder: past, present and future. Postgrad Med J. 2007;83(977):164-172.
1. Morris MJ, Christopher KL. Diagnostic criteria for the classification of vocal cord dysfunction. Chest. 2010;138(5):1213-1223.
2. National Heart, Lung, and Blood Institute. Expert panel report 3: guidelines for the diagnoses and management of asthma. Full report 2007. https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln .pdf. Published 2007.Accessed February 1, 2017.
3. Newman KB, Mason UG III, Schmaling KB. Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med. 1995;152(4, pt 1):1382-1386.
4. Sanz Santiago V, López Neyra A, Almería Gil E, Villa Asensi JR. Spirometry patterns in vocal cord dysfunction [in Spanish]. An Pediatr (Barc). 2013;78(3):173-177.
5. Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. 2010;81(2):156-159.
6. Benninger C, Parsons JP, Mastronarde JG. Vocal cord dysfunction and asthma. Curr Opin Pulm Med. 2011;17(1):45-49.
7. Campainha S, Ribeiro C, Guimar M, Lima R. Vocal cord dysfunction: a frequently forgotten entity. Case Rep Pulmonol. 2012;2012:525493.
8. Morris MJ, Oleszewski RT, Sterner JB, Allan PF. Vocal cord dysfunction related to combat deployment. Mil Med. 2013;178(11):1208-1212.
9. Al-Alwan A, Kaminsky D. Vocal cord dysfunction in athletes: clinical presentation and review of the literature. Phys Sportsmed. 2012;40(2):22-27.
10. Kenn K, Schmitz M. Prevalence of vocal cord dysfunction in patients with dyspnea. First prospective clinical study. Am J Respir Crit Care Med. 1997;155:A965.
11. Wendler, J, Nawka, T, Verges, D. Instructional course: videolaryngo-stroboscopy and phonetography—basic tools for diagnostics and documentation in the voice clinic. Poster presented at: 15th European Congress of Oto-Rhino-Laryngology, Head and Neck Surgery; September 11-16, 2004; Rodos-Kos, Greece.
12. Ibrahim WH, Gheriani HA, Almohamed AA, Raza T. Paradoxical vocal cord motion disorder: past, present and future. Postgrad Med J. 2007;83(977):164-172.
Combined Anterior-Posterior Decompression and Fusion for Cervical Spondylotic Myelopathy
Take-Home Points
- Surgical intervention for cervical spondylosis and radiculopathy classically involves either an anterior or posterior approach for adequate decompression of the spinal cord and associated nerve roots.
- Combined anterior-posterior surgery for cervical spondylotic myelopathy is a relatively new technique that has previously been used for disorders of the thoracolumbar spine.
- Combined anterior-posterior cervical decompression and fusion for the treatment of cervical spondylotic myelopathy is associated with minor complications and excellent neurologic outcomes.
- Combined surgery can either be performed in a single day or in a staged manner, with current literature showing that same-day surgery is superior with respect to estimated blood loss and length of stay.
Cervical spondylotic myelopathy (CSM) is a degenerative disease characterized by progressive compression of the spinal cord. CSM has been found to be the most common cause of spinal impairment as well as the most frequently acquired cause of spinal dysfunction in people over 55 years of age.1,2 If left untreated, this condition can reduce manual dexterity and cause gait disturbances, dysesthesias, and weakness in the extremities. When conservative treatments fail, surgical intervention often becomes the preferred course of action for CSM and/or myeloradiculopathy.
The surgical approach for CSM and other advanced cervical spine (CS) deformities varies and is often a source of debate. Being a relatively safe and effective procedure, anterior decompression with fusion is optimal in treating discogenic lesions causing myelopathy but is less effective in multilevel disease.3,4 When pseudarthrosis, adjacent segment degeneration (ASD), and hardware failure are of concern, posterior decompressive laminectomy with instrumentation is a promising option.5 However, this method is less effective in restoring lordosis and can increase the risk for later clinical deterioration.6 There is a select subset of patients for whom a combined anterior-posterior approach is ideal.7-9In cases in which a combined anterior-posterior approach is identified as the best treatment option, whether to perform the operation in a sequential or staged manner must be decided, and this question is another source of debate. Single-day surgery is sometimes anecdotally criticized as posing a greater risk to the patient. On the other hand, some comparative studies have shown no statistically significant difference in major complication rates between the 2 options.10,11 More descriptive studies of combined anterior-posterior decompression and fusion (CAPDF) are needed to explore the efficacy of the procedure. In this article, we describe a study we conducted to characterize the operative data, perioperative complications, and short-term outcomes associated with CAPDF for the treatment of CSM in a select group of patients.
Methods
After receiving Institutional Review Board approval for this study (formal consent was not required), we retrospectively reviewed the charts of 21 patients who underwent CAPDF for CSM at our institution. All patients underwent surgery between February 2010 and March 2015. Criteria for inclusion in the study included same-day CAPDF for CSM. Staged procedures were excluded, as were combined procedures for the treatment of other diseases (eg, malignancies). All patients were operated on by the same primary surgeon (Dr. Davis) and co-surgeon (Dr. Labiak). The 1 patient who was lost to follow-up was excluded from the postoperative outcome analysis.
We reviewed the patients’ medical records for surgical consultations, operative reports, intraoperative reports, progress notes, and postoperative office visit reports. Demographic information included age, sex, body mass index, and preoperative risk factors, such as diabetes and tobacco use. All patients had been diagnosed with myelopathy. Clinical data included previous history of CS surgery, levels fused (and number of levels fused) anteriorly and posteriorly, operative time, estimated blood loss (EBL), length of stay (LOS), and perioperative complications. Short-term (3-month follow-up) neurologic improvement was determined both objectively, with the Nurick grading system,12 and subjectively, with determination of patient quality of life before and after surgery and with neurologic examination.
Operative Technique: Anterior Approach
All operations were performed with continuous somatosensory evoked potential monitoring of both upper and lower extremities. Each patient, positioned supine with the head in a neutral position, underwent standard endotracheal intubation. Intubation was followed by a transverse incision and dissection down to the deep cervical fascia with maintenance of the carotid sheath laterally and tracheoesophageal complex medially. Interspaces were identified and later were confirmed with lateral radiographs. Discectomy, osteophytectomy, and removal of hypertrophied or calcified ligament were then performed until decompression was satisfactory. Corpectomies were not performed. Polyetheretherketone interbody spacers (Stryker) were used with autograft harvested from vertebral body resection. Low-profile screw-plate systems were placed. After completion of the anterior procedure, the patient was placed prone, with the head fixed in a Mayfield clamping device in neutral position and with all pressure points carefully padded.
Operative Technique: Posterior Approach
A midline incision was made through the skin and subcutaneous tissue to the level of the deep cervical fascia. Then, dissection was performed to the tips of the lateral masses. Instrumentation and fusion preceded spinal decompression. This order, chosen to preserve bony landmarks for guidance during instrumentation, did not interfere with subsequent decompression. Segmental spinal instrumentation was placed using lateral mass screw-rod fixation. After the laminae and ligamenta flava were bilaterally mobilized, the entire bony ligamentous complex spanning the area of fusion was removed en masse (most commonly C3–C7) in order to decrease the number of instrument passes near the spinal cord. Next, a modest foraminotomy was performed to extend the opening laterally and ensure adequate decompression of the nerve roots. Autograft harvested from the spinous processes and laminae was used. The posterior portion of the operation contributed significantly to blood loss and postoperative pain during the perioperative period. We recommend performing a very meticulous dissection to minimize these consequences. No patient in this study required a halo orthosis.
Results
Twenty-one patients with CSM were treated with CAPDF between February 2010 and March 2015 (Table 1).
Table 2 summarizes the operative data. Mean number of levels fused was 2 (range, 1-3) anteriorly and 3 (range, 1-4) posteriorly.
Of the 21 patients, 9 (42.3%) had at least 1 complication during the perioperative period. Table 3 summarizes all encountered complications. Neither neurologic instability nor mortality was observed after surgery.
Patient 7 was lost to follow-up. For the other 20 patients, mean time to “3-month follow-up” was 96 days (range, 51-149 days). The most commonly noted improvements in quality of life included resolution of numbness, improvement in gait, and return to previous activities, such as walking and even exercising.
Representative Case
Patient 15, a 53-year-old man, presented with complaints of dysesthesias of the hands. Focused neurologic evaluation at the time revealed limited CS range of motion on extension. The patient (Figures 2A-2D) was diffusely hyperreflexic and had pathologic spread in the upper extremities.
Discussion
Cervical myelopathy is a common yet frequently underdiagnosed disease, owing to the fact that many patients remain asymptomatic even after experiencing degenerative changes in the spinal column.14-16 The additive effects of spondylosis, osteophyte formation, ligamentous hypertrophy, and listhesis lead to progressive canal and intervertebral foraminal compromise, ultimately producing the clinical syndromes of myelopathy and radiculopathy.17 The characteristic symptoms of CSM are known to have an insidious onset. In the early stages, patients note a subtle gait disturbance and later experience manual dexterity reductions and upper extremity dysesthesias.18 As the condition progresses and conservative management fails, surgical intervention is sought.
Nevertheless, the pursuit of surgical treatment for CSM remains somewhat controversial. Some authors have found no statistically significant difference between conservative and surgical management of mild to moderate CSM,19 whereas others have found that surgically treated patients had much better outcomes than their medically treated counterparts.20 In 2010, Scardino and colleagues21 reported that CSM patients who were bedridden and/or wheelchair-bound with seemingly irreversible myelopathy were capable of neurologic improvement after surgical intervention. At the very least, what remains clear is that untreated CSM is known to follow an unpredictable course, with the condition deteriorating faster for some patients than others.22Traditional anterior or posterior approaches, which can be used in the majority of cases of cervical spondylosis and/or radiculopathy, have been compared extensively.23,24 The inverse relationship concerning the integrity of an anterior construct and the number of levels fused is a well-established clinical finding.3,4,8,25-28 Laminectomy with fusion is not without its disadvantages: Cervical instability secondary to mechanical loss of posterior cervical support, and subsequent post-laminectomy kyphosis, is a common complication.23 In cases in which more stability is required, the combined anterior-posterior approach is more promising than either approach alone. This technique has its roots in the treatment of several thoracolumbar spine disorders, including infections, scoliosis, trauma, and tumors.29-31 More recently, the technique has been applied to CS disorders.
In 2008, Gok and colleagues32 retrospectively compared the results of anterior-only fusion and CAPDF for CSM. Forty-six patients underwent anterior surgery only, and 21 underwent CAPDF. The groups’ complication rates were similar: 28.6% (anterior only) and 24% (CAPDF); the incidence of ASD was lower in the combined group. Song and colleagues33 conducted a similar study in 2010. They compared anterior fusion alone and CAPDF in treating degenerative cervical kyphosis. Results were strongly in favor of the combined technique, as it led to “greater correction of sagittal alignment, a better maintenance of correction angle, a higher rate of fusion, a lower rate of subsidence and lower complications.” Both studies established that, in a select group of patients, the benefits of CAPDF outweighed the risks. These findings, combined with our study’s findings of no major complications and the transience of minor complications, suggest CAPDF should not be considered too invasive or risky.
The results of our study also mirror those of 3 other studies on the use of CAPDF for CS disorders. In 1995, McAfee and colleagues34 reported on a group of 100 patients with follow-up of 2 years or more. In most cases, the surgical indication was trauma, but neoplasm, infection, rheumatoid arthritis, and CSM were found as well. Outcomes were very favorable: improvement in a previous neurologic deficit (57/75 patients), ability to walk again (21/35 patients), no new neurologic deficits, and no hardware failures. In 2000, Schultz and colleagues35 retrospectively reviewed the cases of 72 patients who underwent CAPDF for a variety of complex CS disorders. Two of the 72 experienced transient neurologic deficits, and, though the immediate complication rate was relatively high (32%), the long-term complication rate was down to 5%. In 2009, Konya and colleagues36 retrospectively reviewed the cases of 40 patients who underwent CAPDF, primarily for CSM. Within 1 week after surgery, neurologic deficits were reduced in 36 patients; by 1 year after surgery, neurologic deficits were reduced in all 40 patients, and fusion was achieved in 39. These 3 studies34-36 helped establish CAPDF of the CS as a viable and effective procedure that can be performed within a single day.
Although many physicians have achieved favorable results with single-day surgery, the decision to operate in a sequential or staged manner remains controversial. Some anecdotally claim CAPDF poses a greater operative risk to the patient. In 1991, the continuous procedure was found to involve less blood loss and shorter LOS while providing for better correction of severe spinal deformity in patients with scoliosis and rigid kyphosis.37 Three more recent comparative studies examining the same issue in the treatment of CS diseases found staging did not reduce the complication rate and may in fact have been associated with higher complication rates, more blood loss, and longer total operative time and LOS.10,11,38 Our study’s lower blood loss, shorter LOS, and lower major complication rate relative to the combined groups in all 3 of those studies are most likely attributable to our operating on a lower mean number of spinal levels and our restricting the surgical indication to CSM. The positive short-term outcomes and low rate of long-term complications in our study, combined with the data from these 3 comparative studies, suggest that same-day surgery is superior to staged surgery. A staged operation should be considered only if the patient cannot tolerate long periods under general anesthesia.
Many have advocated extending fusion down to T1 to prevent ASD at the C7–T1 disk space.35,39,40 We decided against this approach for 2 reasons. First, at C7, lateral mass screws were always chosen over pedicle screws. When possible, shorter lateral mass screws were used at this level, making C7 much less rigid. Second, the C7–T1 facet capsule was maintained to preserve joint integrity. We suggest extending fusion down to T1 only if there is prior evidence of spinal disease and/or listhesis at C7–T1. Although long-term (many-year) follow-up is often desired, we specifically assessed short-term (3-month) outcomes. We have anecdotally found that degree of improvement often follows a predictable course after 3-month follow-up. If myelopathy resolves even to a small extent during the first 3 postoperative months, later improvement will likely follow an upward course. Conversely, if myelopathy does not improve during the first 3 months, further improvement is much less likely.
This trend in neurologic improvement likely is directly related to degree of myelopathy before surgery. Patients with CSM generally experience symptoms over an extended period and try conservative management before any surgical consultation. Although spinal ischemia is often resolved by decompression, permanent ischemic damage to the cord is not uncommon. In this setting, postoperative neurologic improvement is minimal or even nonexistent, and decompression is preventive rather than curative. In our study, 1 patient had no subjective improvement after surgery. At 3-month follow-up, magnetic resonance imaging showed notable myelomalacia without residual spinal cord compression. We attribute the failure of the ischemic changes to resolve to long-standing preoperative damage to the cord. Nevertheless, surgery stabilized the myelopathy and prevented further ischemic damage and clinical deterioration.
As is the case with any operation, patients must be carefully selected for CAPDF. Indications for CAPDF, as described by Kim and Alexander,7 include acute spinal trauma, post-laminectomy kyphosis, kyphotic deformity with intact posterior tension band, multilevel spondylosis and OPLL, and preexisting risk factors for pseudarthrosis. Clearly, the severity of each varies, and the pathologies are not mutually exclusive. We emphasize that these indications provide only a guideline for performing CAPDF, and patients must be selected on a case-by-case basis. All the patients in our study were symptomatic and exhibited significant compression of the spinal cord anteriorly and posteriorly at multiple levels. Several presented with concomitant pathologies, such as cervical kyphotic deformity, congenital spinal stenosis, and OPLL. In each case, the indication for surgical intervention was undoubted. We sought both to improve the patient’s baseline symptoms and to prevent further damage to the spinal cord.
This study had its limitations. First, its retrospective design predisposed it to a higher degree of bias. Second, because CAPDF is not commonly performed, the sample size was relatively small. Third, although it provided a descriptive analysis of CAPDF for CSM, the study did not use a direct comparison group to establish whether treatment within a single day or staged treatment was more beneficial for our cohort in particular. On the basis of prior experience and observation, we think performing the operation within a single day is much more beneficial for the patient. Our discussion of studies that have compared same-day and staged surgery supports this observation. Therefore, staged treatment was not recommended to our patients.
Conclusion
Few descriptive studies have explored CAPDF for CSM. Our study’s results showed the procedure was associated with minor complications and provided symptomatic relief for a majority of patients as early as 3 months after surgery. In addition, CAPDF can be successfully performed sequentially within a single day. As such, it represents an excellent option for treating multilevel symptomatic CSM cases that require more extensive spinal decompression and more stability.
Am J Orthop. 2017;46(2):E97-E104. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 suppl):190S-197S.
2. Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19(4):409-421.
3. Sasso RC, Ruggiero RA Jr, Reilly TM, Hall PV. Early reconstruction failures after multilevel cervical corpectomy. Spine. 2003;28(2):140-142.
4. Zdeblick TA, Hughes SS, Riew KD, Bohlman HH. Failed anterior cervical discectomy and arthrodesis. Analysis and treatment of thirty-five patients. J Bone Joint Surg Am. 1997;79(4):523-532.
5. Zhu B, Xu Y, Liu X, Liu Z, Dang G. Anterior approach versus posterior approach for the treatment of multilevel cervical spondylotic myelopathy: a systemic review and meta-analysis. Eur Spine J. 2013;22(7):1583-1593.
6. Cabraja M, Abbushi A, Koeppen D, Kroppenstedt S, Woiciechowsky C. Comparison between anterior and posterior decompression with instrumentation for cervical spondylotic myelopathy: sagittal alignment and clinical outcome. Neurosurg Focus. 2010;28(3):E15.
7. Kim PK, Alexander JT. Indications for circumferential surgery for cervical spondylotic myelopathy. Spine J. 2006;6(6 suppl):299S-307S.
8. König SA, Ranguis S, Spetzger U. Management of complex cervical instability. J Neurol Surg A Cent Eur Neurosurg. 2015;76(2):119-125.
9. König SA, Spetzger U. Surgical management of cervical spondylotic myelopathy—indications for anterior, posterior or combined procedures for decompression and stabilisation. Acta Neurochir. 2014;156(2):253-258.
10. Harel R, Hwang R, Fakhar M, et al. Circumferential cervical surgery: to stage or not to stage? J Spinal Disord Tech. 2013;26(4):183-188.
11. Siemionow K, Tyrakowski M, Patel K, Neckrysh S. Comparison of perioperative complications following staged versus one-day anterior and posterior cervical decompression and fusion crossing the cervico-thoracic junction. Neurol Neurochir Pol. 2014;48(6):403-409.
12. Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95(1):87-100.
13. Chen CJ, Saulle D, Fu KM, Smith JS, Shaffrey CI. Dysphagia following combined anterior-posterior cervical spine surgeries. J Neurosurg Spine. 2013;19(3):279-287.
14. Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(8):1178-1184.
15. Gore DR, Sepic SB, Gardner GM. Roentgenographic findings of the cervical spine in asymptomatic people. Spine. 1986;11(6):521-524.
16. Law MD Jr, Bernhardt M, White AA 3rd. Cervical spondylotic myelopathy: a review of surgical indications and decision making. Yale J Biol Med. 1993;66(3):165-177.
17. Kelly JC, Groarke PJ, Butler JS, Poynton AR, O’Byrne JM. The natural history and clinical syndromes of degenerative cervical spondylosis. Adv Orthop. 2012;(2012):393642.
18. Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007;60(1 suppl 1):S35-S41.
19. Kadanka Z, Mares M, Bednarik J, et al. Approaches to spondylotic cervical myelopathy: conservative versus surgical results in a 3-year follow-up study. Spine. 2002;27(20):2205-2210.
20. Sampath P, Bendebba M, Davis JD, Ducker TB. Outcome of patients treated for cervical myelopathy. A prospective, multicenter study with independent clinical review. Spine. 2000;25(6):670-676.
21. Scardino FB, Rocha LP, Barcelos AC, Rotta JM, Botelho RV. Is there a benefit to operating on patients (bedridden or in wheelchairs) with advanced stage cervical spondylotic myelopathy? Eur Spine J. 2010;19(5):699-705.
22. Edwards CC 2nd, Riew KD, Anderson PA, Hilibrand AS, Vaccaro AF. Cervical myelopathy. Current diagnostic and treatment strategies. Spine J. 2003;3(1):68-81.
23. Herkowitz HN. A comparison of anterior cervical fusion, cervical laminectomy, and cervical laminoplasty for the surgical management of multiple level spondylotic radiculopathy. Spine. 1988;13(7):774-780.
24. Hukuda S, Mochizuki T, Ogata M, Shichikawa K, Shimomura Y. Operations for cervical spondylotic myelopathy. A comparison of the results of anterior and posterior procedures. J Bone Joint Surg Br. 1985;67(4):609-615.
25. Fernyhough JC, White JI, LaRocca H. Fusion rates in multilevel cervical spondylosis comparing allograft fibula with autograft fibula in 126 patients. Spine. 1991;16(10 suppl):S561-S564.
26. Macdonald RL, Fehlings MG, Tator CH, et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86(6):990-997.
27. Mayr MT, Subach BR, Comey CH, Rodts GE, Haid RW Jr. Cervical spinal stenosis: outcome after anterior corpectomy, allograft reconstruction, and instrumentation. J Neurosurg. 2002;96(1 suppl):10-16.
28. Swank ML, Lowery GL, Bhat AL, McDonough RF. Anterior cervical allograft arthrodesis and instrumentation: multilevel interbody grafting or strut graft reconstruction. Eur Spine J. 1997;6(2):138-143.
29. Böhm H, Harms J, Donk R, Zielke K. Correction and stabilization of angular kyphosis. Clin Orthop Relat Res. 1990;(258):56-61.
30. Spencer DL, DeWald RL. Simultaneous anterior and posterior surgical approach to the thoracic and lumbar spine. Spine. 1979;4(1):29-36.
31. Whitesides TE Jr, Shah SGA. On the management of unstable fractures of the thoracolumbar spine: rationale for use of anterior decompression and fusion and posterior stabilization. Spine. 1976;1(2):99-107.
32. Gok B, Sciubba DM, McLoughlin GS, et al. Surgical treatment of cervical spondylotic myelopathy with anterior compression: a review of 67 cases. J Neurosurg Spine. 2008;9(2):152-157.
33. Song KJ, Johnson JS, Choi BR, Wang JC, Lee KB. Anterior fusion alone compared with combined anterior and posterior fusion for the treatment of degenerative cervical kyphosis. J Bone Joint Surg Br. 2010;92(11):1548-1552.
34. McAfee PC, Bohlman HH, Ducker TB, Zeidman SM, Goldstein JA. One-stage anterior cervical decompression and posterior stabilization. A study of one hundred patients with a minimum of two years of follow-up. J Bone Joint Surg Am. 1995;77(12):1791-1800.
35. Schultz KD Jr, McLaughlin MR, Haid RW Jr, Comey CH, Rodts GE Jr, Alexander J. Single-stage anterior-posterior decompression and stabilization for complex cervical spine disorders. J Neurosurg. 2000;93(2 suppl):214-221.
36. Konya D, Ozgen S, Gercek A, Pamir MN. Outcomes for combined anterior and posterior surgical approaches for patients with multisegmental cervical spondylotic myelopathy. J Clin Neurosci. 2009;16(3):404-409.
37. Shufflebarger HL, Grimm JO, Bui V, Thomson JD. Anterior and posterior spinal fusion. Staged versus same-day surgery. Spine. 1991;16(8):930-933.
38. Ozturk C, Aydinli U, Vural R, Sehirlioglu A, Mutlu M. Simultaneous versus sequential one-stage combined anterior and posterior spinal surgery for spinal infections (outcomes and complications). Int Orthop. 2007;31(3):363-366.
39. Aryan HE, Sanchez-Mejia RO, Ben-Haim S, Ames CP. Successful treatment of cervical myelopathy with minimal morbidity by circumferential decompression and fusion. Eur Spine J. 2007;16(9):1401-1409.
40. Steinmetz MP, Miller J, Warbel A, Krishnaney AA, Bingaman W, Benzel EC. Regional instability following cervicothoracic junction surgery. J Neurosurg Spine. 2006;4(4):278-284.
Take-Home Points
- Surgical intervention for cervical spondylosis and radiculopathy classically involves either an anterior or posterior approach for adequate decompression of the spinal cord and associated nerve roots.
- Combined anterior-posterior surgery for cervical spondylotic myelopathy is a relatively new technique that has previously been used for disorders of the thoracolumbar spine.
- Combined anterior-posterior cervical decompression and fusion for the treatment of cervical spondylotic myelopathy is associated with minor complications and excellent neurologic outcomes.
- Combined surgery can either be performed in a single day or in a staged manner, with current literature showing that same-day surgery is superior with respect to estimated blood loss and length of stay.
Cervical spondylotic myelopathy (CSM) is a degenerative disease characterized by progressive compression of the spinal cord. CSM has been found to be the most common cause of spinal impairment as well as the most frequently acquired cause of spinal dysfunction in people over 55 years of age.1,2 If left untreated, this condition can reduce manual dexterity and cause gait disturbances, dysesthesias, and weakness in the extremities. When conservative treatments fail, surgical intervention often becomes the preferred course of action for CSM and/or myeloradiculopathy.
The surgical approach for CSM and other advanced cervical spine (CS) deformities varies and is often a source of debate. Being a relatively safe and effective procedure, anterior decompression with fusion is optimal in treating discogenic lesions causing myelopathy but is less effective in multilevel disease.3,4 When pseudarthrosis, adjacent segment degeneration (ASD), and hardware failure are of concern, posterior decompressive laminectomy with instrumentation is a promising option.5 However, this method is less effective in restoring lordosis and can increase the risk for later clinical deterioration.6 There is a select subset of patients for whom a combined anterior-posterior approach is ideal.7-9In cases in which a combined anterior-posterior approach is identified as the best treatment option, whether to perform the operation in a sequential or staged manner must be decided, and this question is another source of debate. Single-day surgery is sometimes anecdotally criticized as posing a greater risk to the patient. On the other hand, some comparative studies have shown no statistically significant difference in major complication rates between the 2 options.10,11 More descriptive studies of combined anterior-posterior decompression and fusion (CAPDF) are needed to explore the efficacy of the procedure. In this article, we describe a study we conducted to characterize the operative data, perioperative complications, and short-term outcomes associated with CAPDF for the treatment of CSM in a select group of patients.
Methods
After receiving Institutional Review Board approval for this study (formal consent was not required), we retrospectively reviewed the charts of 21 patients who underwent CAPDF for CSM at our institution. All patients underwent surgery between February 2010 and March 2015. Criteria for inclusion in the study included same-day CAPDF for CSM. Staged procedures were excluded, as were combined procedures for the treatment of other diseases (eg, malignancies). All patients were operated on by the same primary surgeon (Dr. Davis) and co-surgeon (Dr. Labiak). The 1 patient who was lost to follow-up was excluded from the postoperative outcome analysis.
We reviewed the patients’ medical records for surgical consultations, operative reports, intraoperative reports, progress notes, and postoperative office visit reports. Demographic information included age, sex, body mass index, and preoperative risk factors, such as diabetes and tobacco use. All patients had been diagnosed with myelopathy. Clinical data included previous history of CS surgery, levels fused (and number of levels fused) anteriorly and posteriorly, operative time, estimated blood loss (EBL), length of stay (LOS), and perioperative complications. Short-term (3-month follow-up) neurologic improvement was determined both objectively, with the Nurick grading system,12 and subjectively, with determination of patient quality of life before and after surgery and with neurologic examination.
Operative Technique: Anterior Approach
All operations were performed with continuous somatosensory evoked potential monitoring of both upper and lower extremities. Each patient, positioned supine with the head in a neutral position, underwent standard endotracheal intubation. Intubation was followed by a transverse incision and dissection down to the deep cervical fascia with maintenance of the carotid sheath laterally and tracheoesophageal complex medially. Interspaces were identified and later were confirmed with lateral radiographs. Discectomy, osteophytectomy, and removal of hypertrophied or calcified ligament were then performed until decompression was satisfactory. Corpectomies were not performed. Polyetheretherketone interbody spacers (Stryker) were used with autograft harvested from vertebral body resection. Low-profile screw-plate systems were placed. After completion of the anterior procedure, the patient was placed prone, with the head fixed in a Mayfield clamping device in neutral position and with all pressure points carefully padded.
Operative Technique: Posterior Approach
A midline incision was made through the skin and subcutaneous tissue to the level of the deep cervical fascia. Then, dissection was performed to the tips of the lateral masses. Instrumentation and fusion preceded spinal decompression. This order, chosen to preserve bony landmarks for guidance during instrumentation, did not interfere with subsequent decompression. Segmental spinal instrumentation was placed using lateral mass screw-rod fixation. After the laminae and ligamenta flava were bilaterally mobilized, the entire bony ligamentous complex spanning the area of fusion was removed en masse (most commonly C3–C7) in order to decrease the number of instrument passes near the spinal cord. Next, a modest foraminotomy was performed to extend the opening laterally and ensure adequate decompression of the nerve roots. Autograft harvested from the spinous processes and laminae was used. The posterior portion of the operation contributed significantly to blood loss and postoperative pain during the perioperative period. We recommend performing a very meticulous dissection to minimize these consequences. No patient in this study required a halo orthosis.
Results
Twenty-one patients with CSM were treated with CAPDF between February 2010 and March 2015 (Table 1).
Table 2 summarizes the operative data. Mean number of levels fused was 2 (range, 1-3) anteriorly and 3 (range, 1-4) posteriorly.
Of the 21 patients, 9 (42.3%) had at least 1 complication during the perioperative period. Table 3 summarizes all encountered complications. Neither neurologic instability nor mortality was observed after surgery.
Patient 7 was lost to follow-up. For the other 20 patients, mean time to “3-month follow-up” was 96 days (range, 51-149 days). The most commonly noted improvements in quality of life included resolution of numbness, improvement in gait, and return to previous activities, such as walking and even exercising.
Representative Case
Patient 15, a 53-year-old man, presented with complaints of dysesthesias of the hands. Focused neurologic evaluation at the time revealed limited CS range of motion on extension. The patient (Figures 2A-2D) was diffusely hyperreflexic and had pathologic spread in the upper extremities.
Discussion
Cervical myelopathy is a common yet frequently underdiagnosed disease, owing to the fact that many patients remain asymptomatic even after experiencing degenerative changes in the spinal column.14-16 The additive effects of spondylosis, osteophyte formation, ligamentous hypertrophy, and listhesis lead to progressive canal and intervertebral foraminal compromise, ultimately producing the clinical syndromes of myelopathy and radiculopathy.17 The characteristic symptoms of CSM are known to have an insidious onset. In the early stages, patients note a subtle gait disturbance and later experience manual dexterity reductions and upper extremity dysesthesias.18 As the condition progresses and conservative management fails, surgical intervention is sought.
Nevertheless, the pursuit of surgical treatment for CSM remains somewhat controversial. Some authors have found no statistically significant difference between conservative and surgical management of mild to moderate CSM,19 whereas others have found that surgically treated patients had much better outcomes than their medically treated counterparts.20 In 2010, Scardino and colleagues21 reported that CSM patients who were bedridden and/or wheelchair-bound with seemingly irreversible myelopathy were capable of neurologic improvement after surgical intervention. At the very least, what remains clear is that untreated CSM is known to follow an unpredictable course, with the condition deteriorating faster for some patients than others.22Traditional anterior or posterior approaches, which can be used in the majority of cases of cervical spondylosis and/or radiculopathy, have been compared extensively.23,24 The inverse relationship concerning the integrity of an anterior construct and the number of levels fused is a well-established clinical finding.3,4,8,25-28 Laminectomy with fusion is not without its disadvantages: Cervical instability secondary to mechanical loss of posterior cervical support, and subsequent post-laminectomy kyphosis, is a common complication.23 In cases in which more stability is required, the combined anterior-posterior approach is more promising than either approach alone. This technique has its roots in the treatment of several thoracolumbar spine disorders, including infections, scoliosis, trauma, and tumors.29-31 More recently, the technique has been applied to CS disorders.
In 2008, Gok and colleagues32 retrospectively compared the results of anterior-only fusion and CAPDF for CSM. Forty-six patients underwent anterior surgery only, and 21 underwent CAPDF. The groups’ complication rates were similar: 28.6% (anterior only) and 24% (CAPDF); the incidence of ASD was lower in the combined group. Song and colleagues33 conducted a similar study in 2010. They compared anterior fusion alone and CAPDF in treating degenerative cervical kyphosis. Results were strongly in favor of the combined technique, as it led to “greater correction of sagittal alignment, a better maintenance of correction angle, a higher rate of fusion, a lower rate of subsidence and lower complications.” Both studies established that, in a select group of patients, the benefits of CAPDF outweighed the risks. These findings, combined with our study’s findings of no major complications and the transience of minor complications, suggest CAPDF should not be considered too invasive or risky.
The results of our study also mirror those of 3 other studies on the use of CAPDF for CS disorders. In 1995, McAfee and colleagues34 reported on a group of 100 patients with follow-up of 2 years or more. In most cases, the surgical indication was trauma, but neoplasm, infection, rheumatoid arthritis, and CSM were found as well. Outcomes were very favorable: improvement in a previous neurologic deficit (57/75 patients), ability to walk again (21/35 patients), no new neurologic deficits, and no hardware failures. In 2000, Schultz and colleagues35 retrospectively reviewed the cases of 72 patients who underwent CAPDF for a variety of complex CS disorders. Two of the 72 experienced transient neurologic deficits, and, though the immediate complication rate was relatively high (32%), the long-term complication rate was down to 5%. In 2009, Konya and colleagues36 retrospectively reviewed the cases of 40 patients who underwent CAPDF, primarily for CSM. Within 1 week after surgery, neurologic deficits were reduced in 36 patients; by 1 year after surgery, neurologic deficits were reduced in all 40 patients, and fusion was achieved in 39. These 3 studies34-36 helped establish CAPDF of the CS as a viable and effective procedure that can be performed within a single day.
Although many physicians have achieved favorable results with single-day surgery, the decision to operate in a sequential or staged manner remains controversial. Some anecdotally claim CAPDF poses a greater operative risk to the patient. In 1991, the continuous procedure was found to involve less blood loss and shorter LOS while providing for better correction of severe spinal deformity in patients with scoliosis and rigid kyphosis.37 Three more recent comparative studies examining the same issue in the treatment of CS diseases found staging did not reduce the complication rate and may in fact have been associated with higher complication rates, more blood loss, and longer total operative time and LOS.10,11,38 Our study’s lower blood loss, shorter LOS, and lower major complication rate relative to the combined groups in all 3 of those studies are most likely attributable to our operating on a lower mean number of spinal levels and our restricting the surgical indication to CSM. The positive short-term outcomes and low rate of long-term complications in our study, combined with the data from these 3 comparative studies, suggest that same-day surgery is superior to staged surgery. A staged operation should be considered only if the patient cannot tolerate long periods under general anesthesia.
Many have advocated extending fusion down to T1 to prevent ASD at the C7–T1 disk space.35,39,40 We decided against this approach for 2 reasons. First, at C7, lateral mass screws were always chosen over pedicle screws. When possible, shorter lateral mass screws were used at this level, making C7 much less rigid. Second, the C7–T1 facet capsule was maintained to preserve joint integrity. We suggest extending fusion down to T1 only if there is prior evidence of spinal disease and/or listhesis at C7–T1. Although long-term (many-year) follow-up is often desired, we specifically assessed short-term (3-month) outcomes. We have anecdotally found that degree of improvement often follows a predictable course after 3-month follow-up. If myelopathy resolves even to a small extent during the first 3 postoperative months, later improvement will likely follow an upward course. Conversely, if myelopathy does not improve during the first 3 months, further improvement is much less likely.
This trend in neurologic improvement likely is directly related to degree of myelopathy before surgery. Patients with CSM generally experience symptoms over an extended period and try conservative management before any surgical consultation. Although spinal ischemia is often resolved by decompression, permanent ischemic damage to the cord is not uncommon. In this setting, postoperative neurologic improvement is minimal or even nonexistent, and decompression is preventive rather than curative. In our study, 1 patient had no subjective improvement after surgery. At 3-month follow-up, magnetic resonance imaging showed notable myelomalacia without residual spinal cord compression. We attribute the failure of the ischemic changes to resolve to long-standing preoperative damage to the cord. Nevertheless, surgery stabilized the myelopathy and prevented further ischemic damage and clinical deterioration.
As is the case with any operation, patients must be carefully selected for CAPDF. Indications for CAPDF, as described by Kim and Alexander,7 include acute spinal trauma, post-laminectomy kyphosis, kyphotic deformity with intact posterior tension band, multilevel spondylosis and OPLL, and preexisting risk factors for pseudarthrosis. Clearly, the severity of each varies, and the pathologies are not mutually exclusive. We emphasize that these indications provide only a guideline for performing CAPDF, and patients must be selected on a case-by-case basis. All the patients in our study were symptomatic and exhibited significant compression of the spinal cord anteriorly and posteriorly at multiple levels. Several presented with concomitant pathologies, such as cervical kyphotic deformity, congenital spinal stenosis, and OPLL. In each case, the indication for surgical intervention was undoubted. We sought both to improve the patient’s baseline symptoms and to prevent further damage to the spinal cord.
This study had its limitations. First, its retrospective design predisposed it to a higher degree of bias. Second, because CAPDF is not commonly performed, the sample size was relatively small. Third, although it provided a descriptive analysis of CAPDF for CSM, the study did not use a direct comparison group to establish whether treatment within a single day or staged treatment was more beneficial for our cohort in particular. On the basis of prior experience and observation, we think performing the operation within a single day is much more beneficial for the patient. Our discussion of studies that have compared same-day and staged surgery supports this observation. Therefore, staged treatment was not recommended to our patients.
Conclusion
Few descriptive studies have explored CAPDF for CSM. Our study’s results showed the procedure was associated with minor complications and provided symptomatic relief for a majority of patients as early as 3 months after surgery. In addition, CAPDF can be successfully performed sequentially within a single day. As such, it represents an excellent option for treating multilevel symptomatic CSM cases that require more extensive spinal decompression and more stability.
Am J Orthop. 2017;46(2):E97-E104. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Surgical intervention for cervical spondylosis and radiculopathy classically involves either an anterior or posterior approach for adequate decompression of the spinal cord and associated nerve roots.
- Combined anterior-posterior surgery for cervical spondylotic myelopathy is a relatively new technique that has previously been used for disorders of the thoracolumbar spine.
- Combined anterior-posterior cervical decompression and fusion for the treatment of cervical spondylotic myelopathy is associated with minor complications and excellent neurologic outcomes.
- Combined surgery can either be performed in a single day or in a staged manner, with current literature showing that same-day surgery is superior with respect to estimated blood loss and length of stay.
Cervical spondylotic myelopathy (CSM) is a degenerative disease characterized by progressive compression of the spinal cord. CSM has been found to be the most common cause of spinal impairment as well as the most frequently acquired cause of spinal dysfunction in people over 55 years of age.1,2 If left untreated, this condition can reduce manual dexterity and cause gait disturbances, dysesthesias, and weakness in the extremities. When conservative treatments fail, surgical intervention often becomes the preferred course of action for CSM and/or myeloradiculopathy.
The surgical approach for CSM and other advanced cervical spine (CS) deformities varies and is often a source of debate. Being a relatively safe and effective procedure, anterior decompression with fusion is optimal in treating discogenic lesions causing myelopathy but is less effective in multilevel disease.3,4 When pseudarthrosis, adjacent segment degeneration (ASD), and hardware failure are of concern, posterior decompressive laminectomy with instrumentation is a promising option.5 However, this method is less effective in restoring lordosis and can increase the risk for later clinical deterioration.6 There is a select subset of patients for whom a combined anterior-posterior approach is ideal.7-9In cases in which a combined anterior-posterior approach is identified as the best treatment option, whether to perform the operation in a sequential or staged manner must be decided, and this question is another source of debate. Single-day surgery is sometimes anecdotally criticized as posing a greater risk to the patient. On the other hand, some comparative studies have shown no statistically significant difference in major complication rates between the 2 options.10,11 More descriptive studies of combined anterior-posterior decompression and fusion (CAPDF) are needed to explore the efficacy of the procedure. In this article, we describe a study we conducted to characterize the operative data, perioperative complications, and short-term outcomes associated with CAPDF for the treatment of CSM in a select group of patients.
Methods
After receiving Institutional Review Board approval for this study (formal consent was not required), we retrospectively reviewed the charts of 21 patients who underwent CAPDF for CSM at our institution. All patients underwent surgery between February 2010 and March 2015. Criteria for inclusion in the study included same-day CAPDF for CSM. Staged procedures were excluded, as were combined procedures for the treatment of other diseases (eg, malignancies). All patients were operated on by the same primary surgeon (Dr. Davis) and co-surgeon (Dr. Labiak). The 1 patient who was lost to follow-up was excluded from the postoperative outcome analysis.
We reviewed the patients’ medical records for surgical consultations, operative reports, intraoperative reports, progress notes, and postoperative office visit reports. Demographic information included age, sex, body mass index, and preoperative risk factors, such as diabetes and tobacco use. All patients had been diagnosed with myelopathy. Clinical data included previous history of CS surgery, levels fused (and number of levels fused) anteriorly and posteriorly, operative time, estimated blood loss (EBL), length of stay (LOS), and perioperative complications. Short-term (3-month follow-up) neurologic improvement was determined both objectively, with the Nurick grading system,12 and subjectively, with determination of patient quality of life before and after surgery and with neurologic examination.
Operative Technique: Anterior Approach
All operations were performed with continuous somatosensory evoked potential monitoring of both upper and lower extremities. Each patient, positioned supine with the head in a neutral position, underwent standard endotracheal intubation. Intubation was followed by a transverse incision and dissection down to the deep cervical fascia with maintenance of the carotid sheath laterally and tracheoesophageal complex medially. Interspaces were identified and later were confirmed with lateral radiographs. Discectomy, osteophytectomy, and removal of hypertrophied or calcified ligament were then performed until decompression was satisfactory. Corpectomies were not performed. Polyetheretherketone interbody spacers (Stryker) were used with autograft harvested from vertebral body resection. Low-profile screw-plate systems were placed. After completion of the anterior procedure, the patient was placed prone, with the head fixed in a Mayfield clamping device in neutral position and with all pressure points carefully padded.
Operative Technique: Posterior Approach
A midline incision was made through the skin and subcutaneous tissue to the level of the deep cervical fascia. Then, dissection was performed to the tips of the lateral masses. Instrumentation and fusion preceded spinal decompression. This order, chosen to preserve bony landmarks for guidance during instrumentation, did not interfere with subsequent decompression. Segmental spinal instrumentation was placed using lateral mass screw-rod fixation. After the laminae and ligamenta flava were bilaterally mobilized, the entire bony ligamentous complex spanning the area of fusion was removed en masse (most commonly C3–C7) in order to decrease the number of instrument passes near the spinal cord. Next, a modest foraminotomy was performed to extend the opening laterally and ensure adequate decompression of the nerve roots. Autograft harvested from the spinous processes and laminae was used. The posterior portion of the operation contributed significantly to blood loss and postoperative pain during the perioperative period. We recommend performing a very meticulous dissection to minimize these consequences. No patient in this study required a halo orthosis.
Results
Twenty-one patients with CSM were treated with CAPDF between February 2010 and March 2015 (Table 1).
Table 2 summarizes the operative data. Mean number of levels fused was 2 (range, 1-3) anteriorly and 3 (range, 1-4) posteriorly.
Of the 21 patients, 9 (42.3%) had at least 1 complication during the perioperative period. Table 3 summarizes all encountered complications. Neither neurologic instability nor mortality was observed after surgery.
Patient 7 was lost to follow-up. For the other 20 patients, mean time to “3-month follow-up” was 96 days (range, 51-149 days). The most commonly noted improvements in quality of life included resolution of numbness, improvement in gait, and return to previous activities, such as walking and even exercising.
Representative Case
Patient 15, a 53-year-old man, presented with complaints of dysesthesias of the hands. Focused neurologic evaluation at the time revealed limited CS range of motion on extension. The patient (Figures 2A-2D) was diffusely hyperreflexic and had pathologic spread in the upper extremities.
Discussion
Cervical myelopathy is a common yet frequently underdiagnosed disease, owing to the fact that many patients remain asymptomatic even after experiencing degenerative changes in the spinal column.14-16 The additive effects of spondylosis, osteophyte formation, ligamentous hypertrophy, and listhesis lead to progressive canal and intervertebral foraminal compromise, ultimately producing the clinical syndromes of myelopathy and radiculopathy.17 The characteristic symptoms of CSM are known to have an insidious onset. In the early stages, patients note a subtle gait disturbance and later experience manual dexterity reductions and upper extremity dysesthesias.18 As the condition progresses and conservative management fails, surgical intervention is sought.
Nevertheless, the pursuit of surgical treatment for CSM remains somewhat controversial. Some authors have found no statistically significant difference between conservative and surgical management of mild to moderate CSM,19 whereas others have found that surgically treated patients had much better outcomes than their medically treated counterparts.20 In 2010, Scardino and colleagues21 reported that CSM patients who were bedridden and/or wheelchair-bound with seemingly irreversible myelopathy were capable of neurologic improvement after surgical intervention. At the very least, what remains clear is that untreated CSM is known to follow an unpredictable course, with the condition deteriorating faster for some patients than others.22Traditional anterior or posterior approaches, which can be used in the majority of cases of cervical spondylosis and/or radiculopathy, have been compared extensively.23,24 The inverse relationship concerning the integrity of an anterior construct and the number of levels fused is a well-established clinical finding.3,4,8,25-28 Laminectomy with fusion is not without its disadvantages: Cervical instability secondary to mechanical loss of posterior cervical support, and subsequent post-laminectomy kyphosis, is a common complication.23 In cases in which more stability is required, the combined anterior-posterior approach is more promising than either approach alone. This technique has its roots in the treatment of several thoracolumbar spine disorders, including infections, scoliosis, trauma, and tumors.29-31 More recently, the technique has been applied to CS disorders.
In 2008, Gok and colleagues32 retrospectively compared the results of anterior-only fusion and CAPDF for CSM. Forty-six patients underwent anterior surgery only, and 21 underwent CAPDF. The groups’ complication rates were similar: 28.6% (anterior only) and 24% (CAPDF); the incidence of ASD was lower in the combined group. Song and colleagues33 conducted a similar study in 2010. They compared anterior fusion alone and CAPDF in treating degenerative cervical kyphosis. Results were strongly in favor of the combined technique, as it led to “greater correction of sagittal alignment, a better maintenance of correction angle, a higher rate of fusion, a lower rate of subsidence and lower complications.” Both studies established that, in a select group of patients, the benefits of CAPDF outweighed the risks. These findings, combined with our study’s findings of no major complications and the transience of minor complications, suggest CAPDF should not be considered too invasive or risky.
The results of our study also mirror those of 3 other studies on the use of CAPDF for CS disorders. In 1995, McAfee and colleagues34 reported on a group of 100 patients with follow-up of 2 years or more. In most cases, the surgical indication was trauma, but neoplasm, infection, rheumatoid arthritis, and CSM were found as well. Outcomes were very favorable: improvement in a previous neurologic deficit (57/75 patients), ability to walk again (21/35 patients), no new neurologic deficits, and no hardware failures. In 2000, Schultz and colleagues35 retrospectively reviewed the cases of 72 patients who underwent CAPDF for a variety of complex CS disorders. Two of the 72 experienced transient neurologic deficits, and, though the immediate complication rate was relatively high (32%), the long-term complication rate was down to 5%. In 2009, Konya and colleagues36 retrospectively reviewed the cases of 40 patients who underwent CAPDF, primarily for CSM. Within 1 week after surgery, neurologic deficits were reduced in 36 patients; by 1 year after surgery, neurologic deficits were reduced in all 40 patients, and fusion was achieved in 39. These 3 studies34-36 helped establish CAPDF of the CS as a viable and effective procedure that can be performed within a single day.
Although many physicians have achieved favorable results with single-day surgery, the decision to operate in a sequential or staged manner remains controversial. Some anecdotally claim CAPDF poses a greater operative risk to the patient. In 1991, the continuous procedure was found to involve less blood loss and shorter LOS while providing for better correction of severe spinal deformity in patients with scoliosis and rigid kyphosis.37 Three more recent comparative studies examining the same issue in the treatment of CS diseases found staging did not reduce the complication rate and may in fact have been associated with higher complication rates, more blood loss, and longer total operative time and LOS.10,11,38 Our study’s lower blood loss, shorter LOS, and lower major complication rate relative to the combined groups in all 3 of those studies are most likely attributable to our operating on a lower mean number of spinal levels and our restricting the surgical indication to CSM. The positive short-term outcomes and low rate of long-term complications in our study, combined with the data from these 3 comparative studies, suggest that same-day surgery is superior to staged surgery. A staged operation should be considered only if the patient cannot tolerate long periods under general anesthesia.
Many have advocated extending fusion down to T1 to prevent ASD at the C7–T1 disk space.35,39,40 We decided against this approach for 2 reasons. First, at C7, lateral mass screws were always chosen over pedicle screws. When possible, shorter lateral mass screws were used at this level, making C7 much less rigid. Second, the C7–T1 facet capsule was maintained to preserve joint integrity. We suggest extending fusion down to T1 only if there is prior evidence of spinal disease and/or listhesis at C7–T1. Although long-term (many-year) follow-up is often desired, we specifically assessed short-term (3-month) outcomes. We have anecdotally found that degree of improvement often follows a predictable course after 3-month follow-up. If myelopathy resolves even to a small extent during the first 3 postoperative months, later improvement will likely follow an upward course. Conversely, if myelopathy does not improve during the first 3 months, further improvement is much less likely.
This trend in neurologic improvement likely is directly related to degree of myelopathy before surgery. Patients with CSM generally experience symptoms over an extended period and try conservative management before any surgical consultation. Although spinal ischemia is often resolved by decompression, permanent ischemic damage to the cord is not uncommon. In this setting, postoperative neurologic improvement is minimal or even nonexistent, and decompression is preventive rather than curative. In our study, 1 patient had no subjective improvement after surgery. At 3-month follow-up, magnetic resonance imaging showed notable myelomalacia without residual spinal cord compression. We attribute the failure of the ischemic changes to resolve to long-standing preoperative damage to the cord. Nevertheless, surgery stabilized the myelopathy and prevented further ischemic damage and clinical deterioration.
As is the case with any operation, patients must be carefully selected for CAPDF. Indications for CAPDF, as described by Kim and Alexander,7 include acute spinal trauma, post-laminectomy kyphosis, kyphotic deformity with intact posterior tension band, multilevel spondylosis and OPLL, and preexisting risk factors for pseudarthrosis. Clearly, the severity of each varies, and the pathologies are not mutually exclusive. We emphasize that these indications provide only a guideline for performing CAPDF, and patients must be selected on a case-by-case basis. All the patients in our study were symptomatic and exhibited significant compression of the spinal cord anteriorly and posteriorly at multiple levels. Several presented with concomitant pathologies, such as cervical kyphotic deformity, congenital spinal stenosis, and OPLL. In each case, the indication for surgical intervention was undoubted. We sought both to improve the patient’s baseline symptoms and to prevent further damage to the spinal cord.
This study had its limitations. First, its retrospective design predisposed it to a higher degree of bias. Second, because CAPDF is not commonly performed, the sample size was relatively small. Third, although it provided a descriptive analysis of CAPDF for CSM, the study did not use a direct comparison group to establish whether treatment within a single day or staged treatment was more beneficial for our cohort in particular. On the basis of prior experience and observation, we think performing the operation within a single day is much more beneficial for the patient. Our discussion of studies that have compared same-day and staged surgery supports this observation. Therefore, staged treatment was not recommended to our patients.
Conclusion
Few descriptive studies have explored CAPDF for CSM. Our study’s results showed the procedure was associated with minor complications and provided symptomatic relief for a majority of patients as early as 3 months after surgery. In addition, CAPDF can be successfully performed sequentially within a single day. As such, it represents an excellent option for treating multilevel symptomatic CSM cases that require more extensive spinal decompression and more stability.
Am J Orthop. 2017;46(2):E97-E104. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 suppl):190S-197S.
2. Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19(4):409-421.
3. Sasso RC, Ruggiero RA Jr, Reilly TM, Hall PV. Early reconstruction failures after multilevel cervical corpectomy. Spine. 2003;28(2):140-142.
4. Zdeblick TA, Hughes SS, Riew KD, Bohlman HH. Failed anterior cervical discectomy and arthrodesis. Analysis and treatment of thirty-five patients. J Bone Joint Surg Am. 1997;79(4):523-532.
5. Zhu B, Xu Y, Liu X, Liu Z, Dang G. Anterior approach versus posterior approach for the treatment of multilevel cervical spondylotic myelopathy: a systemic review and meta-analysis. Eur Spine J. 2013;22(7):1583-1593.
6. Cabraja M, Abbushi A, Koeppen D, Kroppenstedt S, Woiciechowsky C. Comparison between anterior and posterior decompression with instrumentation for cervical spondylotic myelopathy: sagittal alignment and clinical outcome. Neurosurg Focus. 2010;28(3):E15.
7. Kim PK, Alexander JT. Indications for circumferential surgery for cervical spondylotic myelopathy. Spine J. 2006;6(6 suppl):299S-307S.
8. König SA, Ranguis S, Spetzger U. Management of complex cervical instability. J Neurol Surg A Cent Eur Neurosurg. 2015;76(2):119-125.
9. König SA, Spetzger U. Surgical management of cervical spondylotic myelopathy—indications for anterior, posterior or combined procedures for decompression and stabilisation. Acta Neurochir. 2014;156(2):253-258.
10. Harel R, Hwang R, Fakhar M, et al. Circumferential cervical surgery: to stage or not to stage? J Spinal Disord Tech. 2013;26(4):183-188.
11. Siemionow K, Tyrakowski M, Patel K, Neckrysh S. Comparison of perioperative complications following staged versus one-day anterior and posterior cervical decompression and fusion crossing the cervico-thoracic junction. Neurol Neurochir Pol. 2014;48(6):403-409.
12. Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95(1):87-100.
13. Chen CJ, Saulle D, Fu KM, Smith JS, Shaffrey CI. Dysphagia following combined anterior-posterior cervical spine surgeries. J Neurosurg Spine. 2013;19(3):279-287.
14. Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(8):1178-1184.
15. Gore DR, Sepic SB, Gardner GM. Roentgenographic findings of the cervical spine in asymptomatic people. Spine. 1986;11(6):521-524.
16. Law MD Jr, Bernhardt M, White AA 3rd. Cervical spondylotic myelopathy: a review of surgical indications and decision making. Yale J Biol Med. 1993;66(3):165-177.
17. Kelly JC, Groarke PJ, Butler JS, Poynton AR, O’Byrne JM. The natural history and clinical syndromes of degenerative cervical spondylosis. Adv Orthop. 2012;(2012):393642.
18. Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007;60(1 suppl 1):S35-S41.
19. Kadanka Z, Mares M, Bednarik J, et al. Approaches to spondylotic cervical myelopathy: conservative versus surgical results in a 3-year follow-up study. Spine. 2002;27(20):2205-2210.
20. Sampath P, Bendebba M, Davis JD, Ducker TB. Outcome of patients treated for cervical myelopathy. A prospective, multicenter study with independent clinical review. Spine. 2000;25(6):670-676.
21. Scardino FB, Rocha LP, Barcelos AC, Rotta JM, Botelho RV. Is there a benefit to operating on patients (bedridden or in wheelchairs) with advanced stage cervical spondylotic myelopathy? Eur Spine J. 2010;19(5):699-705.
22. Edwards CC 2nd, Riew KD, Anderson PA, Hilibrand AS, Vaccaro AF. Cervical myelopathy. Current diagnostic and treatment strategies. Spine J. 2003;3(1):68-81.
23. Herkowitz HN. A comparison of anterior cervical fusion, cervical laminectomy, and cervical laminoplasty for the surgical management of multiple level spondylotic radiculopathy. Spine. 1988;13(7):774-780.
24. Hukuda S, Mochizuki T, Ogata M, Shichikawa K, Shimomura Y. Operations for cervical spondylotic myelopathy. A comparison of the results of anterior and posterior procedures. J Bone Joint Surg Br. 1985;67(4):609-615.
25. Fernyhough JC, White JI, LaRocca H. Fusion rates in multilevel cervical spondylosis comparing allograft fibula with autograft fibula in 126 patients. Spine. 1991;16(10 suppl):S561-S564.
26. Macdonald RL, Fehlings MG, Tator CH, et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86(6):990-997.
27. Mayr MT, Subach BR, Comey CH, Rodts GE, Haid RW Jr. Cervical spinal stenosis: outcome after anterior corpectomy, allograft reconstruction, and instrumentation. J Neurosurg. 2002;96(1 suppl):10-16.
28. Swank ML, Lowery GL, Bhat AL, McDonough RF. Anterior cervical allograft arthrodesis and instrumentation: multilevel interbody grafting or strut graft reconstruction. Eur Spine J. 1997;6(2):138-143.
29. Böhm H, Harms J, Donk R, Zielke K. Correction and stabilization of angular kyphosis. Clin Orthop Relat Res. 1990;(258):56-61.
30. Spencer DL, DeWald RL. Simultaneous anterior and posterior surgical approach to the thoracic and lumbar spine. Spine. 1979;4(1):29-36.
31. Whitesides TE Jr, Shah SGA. On the management of unstable fractures of the thoracolumbar spine: rationale for use of anterior decompression and fusion and posterior stabilization. Spine. 1976;1(2):99-107.
32. Gok B, Sciubba DM, McLoughlin GS, et al. Surgical treatment of cervical spondylotic myelopathy with anterior compression: a review of 67 cases. J Neurosurg Spine. 2008;9(2):152-157.
33. Song KJ, Johnson JS, Choi BR, Wang JC, Lee KB. Anterior fusion alone compared with combined anterior and posterior fusion for the treatment of degenerative cervical kyphosis. J Bone Joint Surg Br. 2010;92(11):1548-1552.
34. McAfee PC, Bohlman HH, Ducker TB, Zeidman SM, Goldstein JA. One-stage anterior cervical decompression and posterior stabilization. A study of one hundred patients with a minimum of two years of follow-up. J Bone Joint Surg Am. 1995;77(12):1791-1800.
35. Schultz KD Jr, McLaughlin MR, Haid RW Jr, Comey CH, Rodts GE Jr, Alexander J. Single-stage anterior-posterior decompression and stabilization for complex cervical spine disorders. J Neurosurg. 2000;93(2 suppl):214-221.
36. Konya D, Ozgen S, Gercek A, Pamir MN. Outcomes for combined anterior and posterior surgical approaches for patients with multisegmental cervical spondylotic myelopathy. J Clin Neurosci. 2009;16(3):404-409.
37. Shufflebarger HL, Grimm JO, Bui V, Thomson JD. Anterior and posterior spinal fusion. Staged versus same-day surgery. Spine. 1991;16(8):930-933.
38. Ozturk C, Aydinli U, Vural R, Sehirlioglu A, Mutlu M. Simultaneous versus sequential one-stage combined anterior and posterior spinal surgery for spinal infections (outcomes and complications). Int Orthop. 2007;31(3):363-366.
39. Aryan HE, Sanchez-Mejia RO, Ben-Haim S, Ames CP. Successful treatment of cervical myelopathy with minimal morbidity by circumferential decompression and fusion. Eur Spine J. 2007;16(9):1401-1409.
40. Steinmetz MP, Miller J, Warbel A, Krishnaney AA, Bingaman W, Benzel EC. Regional instability following cervicothoracic junction surgery. J Neurosurg Spine. 2006;4(4):278-284.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 suppl):190S-197S.
2. Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19(4):409-421.
3. Sasso RC, Ruggiero RA Jr, Reilly TM, Hall PV. Early reconstruction failures after multilevel cervical corpectomy. Spine. 2003;28(2):140-142.
4. Zdeblick TA, Hughes SS, Riew KD, Bohlman HH. Failed anterior cervical discectomy and arthrodesis. Analysis and treatment of thirty-five patients. J Bone Joint Surg Am. 1997;79(4):523-532.
5. Zhu B, Xu Y, Liu X, Liu Z, Dang G. Anterior approach versus posterior approach for the treatment of multilevel cervical spondylotic myelopathy: a systemic review and meta-analysis. Eur Spine J. 2013;22(7):1583-1593.
6. Cabraja M, Abbushi A, Koeppen D, Kroppenstedt S, Woiciechowsky C. Comparison between anterior and posterior decompression with instrumentation for cervical spondylotic myelopathy: sagittal alignment and clinical outcome. Neurosurg Focus. 2010;28(3):E15.
7. Kim PK, Alexander JT. Indications for circumferential surgery for cervical spondylotic myelopathy. Spine J. 2006;6(6 suppl):299S-307S.
8. König SA, Ranguis S, Spetzger U. Management of complex cervical instability. J Neurol Surg A Cent Eur Neurosurg. 2015;76(2):119-125.
9. König SA, Spetzger U. Surgical management of cervical spondylotic myelopathy—indications for anterior, posterior or combined procedures for decompression and stabilisation. Acta Neurochir. 2014;156(2):253-258.
10. Harel R, Hwang R, Fakhar M, et al. Circumferential cervical surgery: to stage or not to stage? J Spinal Disord Tech. 2013;26(4):183-188.
11. Siemionow K, Tyrakowski M, Patel K, Neckrysh S. Comparison of perioperative complications following staged versus one-day anterior and posterior cervical decompression and fusion crossing the cervico-thoracic junction. Neurol Neurochir Pol. 2014;48(6):403-409.
12. Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95(1):87-100.
13. Chen CJ, Saulle D, Fu KM, Smith JS, Shaffrey CI. Dysphagia following combined anterior-posterior cervical spine surgeries. J Neurosurg Spine. 2013;19(3):279-287.
14. Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(8):1178-1184.
15. Gore DR, Sepic SB, Gardner GM. Roentgenographic findings of the cervical spine in asymptomatic people. Spine. 1986;11(6):521-524.
16. Law MD Jr, Bernhardt M, White AA 3rd. Cervical spondylotic myelopathy: a review of surgical indications and decision making. Yale J Biol Med. 1993;66(3):165-177.
17. Kelly JC, Groarke PJ, Butler JS, Poynton AR, O’Byrne JM. The natural history and clinical syndromes of degenerative cervical spondylosis. Adv Orthop. 2012;(2012):393642.
18. Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007;60(1 suppl 1):S35-S41.
19. Kadanka Z, Mares M, Bednarik J, et al. Approaches to spondylotic cervical myelopathy: conservative versus surgical results in a 3-year follow-up study. Spine. 2002;27(20):2205-2210.
20. Sampath P, Bendebba M, Davis JD, Ducker TB. Outcome of patients treated for cervical myelopathy. A prospective, multicenter study with independent clinical review. Spine. 2000;25(6):670-676.
21. Scardino FB, Rocha LP, Barcelos AC, Rotta JM, Botelho RV. Is there a benefit to operating on patients (bedridden or in wheelchairs) with advanced stage cervical spondylotic myelopathy? Eur Spine J. 2010;19(5):699-705.
22. Edwards CC 2nd, Riew KD, Anderson PA, Hilibrand AS, Vaccaro AF. Cervical myelopathy. Current diagnostic and treatment strategies. Spine J. 2003;3(1):68-81.
23. Herkowitz HN. A comparison of anterior cervical fusion, cervical laminectomy, and cervical laminoplasty for the surgical management of multiple level spondylotic radiculopathy. Spine. 1988;13(7):774-780.
24. Hukuda S, Mochizuki T, Ogata M, Shichikawa K, Shimomura Y. Operations for cervical spondylotic myelopathy. A comparison of the results of anterior and posterior procedures. J Bone Joint Surg Br. 1985;67(4):609-615.
25. Fernyhough JC, White JI, LaRocca H. Fusion rates in multilevel cervical spondylosis comparing allograft fibula with autograft fibula in 126 patients. Spine. 1991;16(10 suppl):S561-S564.
26. Macdonald RL, Fehlings MG, Tator CH, et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86(6):990-997.
27. Mayr MT, Subach BR, Comey CH, Rodts GE, Haid RW Jr. Cervical spinal stenosis: outcome after anterior corpectomy, allograft reconstruction, and instrumentation. J Neurosurg. 2002;96(1 suppl):10-16.
28. Swank ML, Lowery GL, Bhat AL, McDonough RF. Anterior cervical allograft arthrodesis and instrumentation: multilevel interbody grafting or strut graft reconstruction. Eur Spine J. 1997;6(2):138-143.
29. Böhm H, Harms J, Donk R, Zielke K. Correction and stabilization of angular kyphosis. Clin Orthop Relat Res. 1990;(258):56-61.
30. Spencer DL, DeWald RL. Simultaneous anterior and posterior surgical approach to the thoracic and lumbar spine. Spine. 1979;4(1):29-36.
31. Whitesides TE Jr, Shah SGA. On the management of unstable fractures of the thoracolumbar spine: rationale for use of anterior decompression and fusion and posterior stabilization. Spine. 1976;1(2):99-107.
32. Gok B, Sciubba DM, McLoughlin GS, et al. Surgical treatment of cervical spondylotic myelopathy with anterior compression: a review of 67 cases. J Neurosurg Spine. 2008;9(2):152-157.
33. Song KJ, Johnson JS, Choi BR, Wang JC, Lee KB. Anterior fusion alone compared with combined anterior and posterior fusion for the treatment of degenerative cervical kyphosis. J Bone Joint Surg Br. 2010;92(11):1548-1552.
34. McAfee PC, Bohlman HH, Ducker TB, Zeidman SM, Goldstein JA. One-stage anterior cervical decompression and posterior stabilization. A study of one hundred patients with a minimum of two years of follow-up. J Bone Joint Surg Am. 1995;77(12):1791-1800.
35. Schultz KD Jr, McLaughlin MR, Haid RW Jr, Comey CH, Rodts GE Jr, Alexander J. Single-stage anterior-posterior decompression and stabilization for complex cervical spine disorders. J Neurosurg. 2000;93(2 suppl):214-221.
36. Konya D, Ozgen S, Gercek A, Pamir MN. Outcomes for combined anterior and posterior surgical approaches for patients with multisegmental cervical spondylotic myelopathy. J Clin Neurosci. 2009;16(3):404-409.
37. Shufflebarger HL, Grimm JO, Bui V, Thomson JD. Anterior and posterior spinal fusion. Staged versus same-day surgery. Spine. 1991;16(8):930-933.
38. Ozturk C, Aydinli U, Vural R, Sehirlioglu A, Mutlu M. Simultaneous versus sequential one-stage combined anterior and posterior spinal surgery for spinal infections (outcomes and complications). Int Orthop. 2007;31(3):363-366.
39. Aryan HE, Sanchez-Mejia RO, Ben-Haim S, Ames CP. Successful treatment of cervical myelopathy with minimal morbidity by circumferential decompression and fusion. Eur Spine J. 2007;16(9):1401-1409.
40. Steinmetz MP, Miller J, Warbel A, Krishnaney AA, Bingaman W, Benzel EC. Regional instability following cervicothoracic junction surgery. J Neurosurg Spine. 2006;4(4):278-284.
Molecular Profiles Guide Colorectal Cancer Treatment
Colorectal cancer (CRC) is the third leading cause of cancer-related death in veterans, despite significant advances in treatment options.1,2 Over the past 20 years, the median survival of patients with metastatic CRC (mCRC), has improved with the most recent clinical trials demonstrating a median overall survival (OS) of up to 29 months.3
In addition to standard chemotherapeutic regimens using 5-fluorouracil, oxaliplatin, and irinotecan, biologic therapies have resulted in improved OS for patients with mCRC. These therapies include the human vascular endothelial growth factor (VEGF) monoclonal antibody bevacizumab and the epidermal growth factor receptor (EGFR) monoclonal antibodies cetuximab and panitumumab. Additional agents, including aflibercept, ramucirumab, regorafenib, and TAS-102, also have been FDA approved for mCRC, though the OS benefit for these agents as part of the series of standard-of-care treatments is less clear.
Investigators continue to determine subtypes of CRC to further advance treatment options. The histologic classification of colon cancers is actually a collection of multiple cancer subtypes. Each subtype possesses a unique biology largely dependent on the mutations present within the cancer. Recent data, reviewed below, indicate predictive and prognostic benefits to understanding the unique mutational profile of mCRC. Here, the authors present a brief updated summary of these biomarkers and a discussion of treatment strategies.
Resistance to Anti-EGFR Therapies
KRAS and NRAS are members of the RAS family of oncogenes. Activating mutations in these genes results in the propagation of growth factor signals independent of EGFR. The most common KRAS mutations are found in exon 2 (codon 12 or 13). Numerous studies over the past 10 years have confirmed that KRAS mutations at exon 2 predict resistance to cetuximab and panitumumab.4-11 Since at least 2009, restricting use of cetuximab and panitumumab has been the standard of care for patients with KRAS exon 2 wild-type cancers.12
Recent investigations have indicated a predictive role for extended-spectrum KRAS and NRAS mutations (KRAS mutations at exons 2, 3, and 4 and NRAS mutations at exons 2, 3, and 4). In the OPUS clinical trial, patients whose cancers possessed extended-spectrum RAS mutations received no benefit with the addition of cetuximab to standard chemotherapy in response rate (RR), progression-free survival (PFS), or OS compared with standard chemotherapy alone.13 Interestingly, median OS was shorter for those treated with cetuximab when a RAS mutation was present, though the difference was not statistically significant. Additional studies also have confirmed similar benefits in different settings.8,14-18
The CALGB/SWOG 80405 phase 3 clinical trial investigated the first-line use of biologic therapies in combination with standard chemotherapy. The extendedspectrum RAS testing from this study now has been presented.3,19 In the RAS wild-type population, the median OS was 31.2 months in the chemotherapy plus bevacizumab arm and 32.0 months in the chemotherapy plus cetuximab arm (no significant difference). No difference in PFS was observed. A significant improvement in the RR was seen in the cetuximab arm for the RAS wild-type population.
Predictive Biomarkers
BRAF is an oncogene in the RAF gene family that encodes a serine-threonine protein kinase found in the Ras-Raf-MAPK cascade. About 10% of CRC harbor a BRAF mutation.20,21 The most significant and prevalent mutation occurs at the kinase domain from the single substitution V600E. Numerous clinical studies have suggested the presence of this mutation as a predictor of resistance to anti-EGFR therapies and a marker of poor prognosis.6,17,22-25 In a retrospective analysis of RAS and BRAF mutation status of PRIME study data, patients without RAS and BRAF mutations showed significantly better OS and PFS when treated with FOLFOX4 (5-fluorouracil, oxaliplatin, and leucovorin) plus panitumumab, compared with FOLFOX4 alone.8 The presence of BRAF mutations in RAS wild-type patients resulted in a worse outcome. Treatment with anti-EGFR therapy did not significantly improve median PFS or OS.
PIK3CA mutations. Phosphoinositide 3-kinase (PI3K) is a lipid kinase important for multiple cellular processes including cell growth, proliferation, survival, and apoptosis. PIK3CA encodes the catalytic subunit and is mutant in about 20% of mCRC.26 The PI3K is downstream of EGFR signaling; activation of this pathway in the setting of an oncogenic mutation might lead to resistance to anti-EGFR therapies. Sartore-Bianchi and colleagues examined 110 patients with mCRC treated with either panitumumab or cetuximab.27 Of these, 15 patient cancers featured PIK3CA mutations, and none of these responded to anti-EGFR therapies. In addition, preclinical studies have demonstrated that targeting CRC downstream of PI3K might result in significant treatment benefit.28,29
Human epidermal growth factor receptor 2 (HER2) amplification. A subpopulation of CRC with amplification of HER2, a growth factor receptor commonly used in selecting treatment options in breast cancer, has recently been described. The HERACLES phase 2 study evaluated dual HER2 targeting with lapatinib and trastuzumab in therapy-refractory mCRC with HER2 amplification.30 A RR of 35% was observed in this treatment-refractory population.
BRAF mutations. In addition to predicting a poor prognosis and resistance to EGFR-directed therapies, BRAF mutations might be predictive of treatment response using combination regimens containing RAF inhibitors. A recent phase 1B study of a combination therapy using the BRAF inhibitor vemurafenib with irinotecan and cetuximab observed a partial RR of 35%.31 This is being investigated further in the Southwest Oncology Group 1406 phase 2 trial.
Mismatch repair deficiency. Detection of microsatellite instability or the presence of mismatch repair deficiency has become standard-of-care testing for CRC. This is important for the detection of Lynch syndrome and predicting potential resistance to adjuvant 5-fluorouracil in the adjuvant setting.32,33 A recent clinical trial has demonstrated benefits for the use of programmed death 1 (PD-1) inhibitors in the setting of mismatch repair deficiency, including a RR of 40% and PFS of 5.4 months.34
Discussion
Metastatic CRC is now better understood as a collection of multiple cancer subtypes based on mutational profile. This improved understanding of the biology of CRC is altering treatment strategies to a precision medicine-based approach. It is now the standard of care for all patients with mCRC to have the cancers assayed for mutations in KRAS (exons 2, 3, and 4), NRAS (exons 2, 3, and 4), and BRAF. Anti-EGFR therapies should not be used for patients with RAS or BRAF mutations outside of a clinical trial because of a demonstrated lack of benefit in all lines of therapy. Currently, there is no evidence that these mutations significantly alter the response to the approved anti-angiogenic agents bevacizumab, aflibercept, ramucirumab, and regorafenib.
The timing of EGFR-directed therapies for patients with wild-type KRAS, NRAS, and BRAF is still being debated. According to the available data, first-line treatment with anti-EGFR agents in combination with FOLFOX or FOLFIRI (5-fluorouracil, irinotecan, and leucovorin) should be considered for all patients with KRAS, NRAS, and BRAF wild-type mCRCs. The toxicities of anti-EGFR therapies also should be considered for this setting, as some patients find that the acneiform rash, fatigue, nausea, and diarrhea that occur with these agents can have a negative impact on quality of life. As there is no improvement in OS with first-line anti-EGFR therapies for these patients, the increased toxicity from these agents limits their use. In addition, patients with mCRC with known PIK3CA mutation should consider use of EGFR-directed therapies only in the later line setting.
Research is focused on how to best use the mutational profile of the tumor to tailor therapies for mCRC. High-quality, large-volume data sets will become more important as molecular subtypes of cancer become more narrowly defined and less common. Further investigations are needed to look for other markers of resistance and to identify biomarkers predictive of treatment sensitivity.
This is an exciting time in the treatment of many cancers, especially mCRC, which significantly impacts the veteran population, because routine DNA sequencing of patient samples has allowed for rapid advances in the realization of precision medicine. This allows for improved patient selection to reduce costs and toxicities while increasing the benefit for veterans.
Acknowledgments
This work was supported by the National Institutes of Health (P30 CA014520, Core Grant, University of Wisconsin Carbone Cancer Center).
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. Landrum MB, Keating NL, Lamont EB, et al. Survival of older patients with cancer in the Veterans Health Administration versus fee-for-service Medicare. J Clin Oncol. 2012;30(10):1072-1079.
2. Hynes DM, Tarlov E, Durazo-Arvizu R, et al. Surgery and adjuvant chemotherapy use among veterans with colon cancer: insights from a California study. J Clin Oncol. 2010;28(15):2571-2576.
3. Venook AP, Niedzwiecki D, Lenz H-J, et al; Cancer and Leukemia Group B (Alliance), SWOG, and ECOG. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol. 2014;32(suppl 18):Abstract LBA3.
4. Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67(6):2643-2648.
5. Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22(7):1535-1546.
6. De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11(8):753-7562.
7. Di Fiore F, Blanchard F, Charbonnier F, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by cetuximab plus chemotherapy. Br J Cancer. 2007;96(8):1166-1169.
8. Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023-1034.
9. Lièvre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26(3):374-379.
10. Lièvre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8):3992-3995.
11. Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408-1417.
12. Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27(12):2091-2096.
13. Tejpar S, Lenz HJ, Kohne CH, et al. Effect of KRAS and NRAS mutations on treatment outcomes in patients with metastatic colorectal cancer (mCRC) treated first-line with cetuximab plus FOLFOX4: new results from the OPUS study. J Clin Oncol. 2014;32(suppl 3):LBA444.
14. Abad A, Massuti B, Gravalos C, et al. Panitumumab plus FOLFOX4 or panitumumab plus FOLFIRI in subjects with wild-type KRAS (exon 2) colorectal cancer and multiple or unresectable liver-limited metastases: data from the randomized, phase II planet study. Ann Oncol. 2014;25(suppl 2):ii7-ii18.
15. Schwartzberg LS, Rivera F, Karthaus M, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32(21):2240-2247.
16. Peeters M, Oliner K, Price T, et al. KRAS/NRAS and BRAF mutations in the 20050181 study of panitumumab plus FOLFIRI for the 2nd-line treatment of metastatic colorectal cancer: updated analysis. Ann Oncol. 2014;25(suppl 2):ii5.
17. Bokemeyer C, Van Cutsem E, Rougier P, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48(10):1466-1475.
18. Van Cutsem E, Lenz HJ, Köhne CH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33(7):692-700.
19. ESMO. ESMO 2014: results from the CALGB/SWOG 80405 and FIRE-3 (AIO KRK-0306) studies in all RAS wild type population. ESMO website. http://www.esmo.org/Conferences/Past-Conferences/ESMO-2014-Congress/News-Articles/Results-From-the-CALGB-SWOG-80405-and-FIRE-3-AIO-KRK -0306-Studies-In-All-RAS-Wild-Type-Population. Updated September 29, 2014. Accessed April 6, 2016.
20. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949-954.
21. Samowitz WS, Sweeney C, Herrick J, Albertsen H, Levin TR, Murtaugh MA, Wolff RK, Slattery ML. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65(14):6063-6069.
22. Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26(35):5705-5712.
23. Laurent-Puig P, Cayre A, Manceau G, et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27(35):5924-5930.
24. Richman SD, Seymour MT, Chambers P, et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27(35):5931-5937.
25. Tveit KM, Guren T, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: The NORDIC-VII study. J Clin Oncol. 2012;30(15):1755-1762.
26. Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554.
27. Sartore-Bianchi A, Martini M, Molinari F, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69(5):1851-1857.
28. Deming DA, Leystra AA, Farhoud M, et al. mTOR inhibition elicits a dramatic response in PI3K-dependent colon cancers. PLoS One. 2013;8(4):e60709.
29. Yueh AE, Payne SN, Leystra AA, et al. Colon cancer tumorigenesis initiated by the H1047R mutant PI3K. PLoS One. 2016;11(2):e0148730.
30. Siena S, Sartore-Bianchi A, Lonardi S, et al. Trastuzumab and lapatinib in HER2-amplified metastatic colorectal cancer patients (mCRC): the HERACLES trial. J Clin Oncol. 2015;33(suppl 15):3508.
31. Hong DS, Morris VK, El Osta BE, et al. Phase Ib study of vemurafenib in combination with irinotecan and cetuximab in patients with BRAF-mutated metastatic colorectal cancer and advanced cancers. J Clin Oncol. 2015;33(suppl 15):3511.
32. Benatti P, Gafà R, Barana D, et al. Microsatellite instability and colorectal cancer prognosis. Clin Cancer Res. 2005;11(23):8332-8340.
33. Funkhouser WK, Jr, Lubin IM, Monzon FA, et al. Relevance, pathogenesis, and testing algorithm for mismatch repair-defective colorectal carcinomas: a report of the association for molecular pathology. J Mol Diagn. 2012;14(2):91-103.
34. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair
deficiency. N Engl J Med. 2015;372(26):2509-2520.
Colorectal cancer (CRC) is the third leading cause of cancer-related death in veterans, despite significant advances in treatment options.1,2 Over the past 20 years, the median survival of patients with metastatic CRC (mCRC), has improved with the most recent clinical trials demonstrating a median overall survival (OS) of up to 29 months.3
In addition to standard chemotherapeutic regimens using 5-fluorouracil, oxaliplatin, and irinotecan, biologic therapies have resulted in improved OS for patients with mCRC. These therapies include the human vascular endothelial growth factor (VEGF) monoclonal antibody bevacizumab and the epidermal growth factor receptor (EGFR) monoclonal antibodies cetuximab and panitumumab. Additional agents, including aflibercept, ramucirumab, regorafenib, and TAS-102, also have been FDA approved for mCRC, though the OS benefit for these agents as part of the series of standard-of-care treatments is less clear.
Investigators continue to determine subtypes of CRC to further advance treatment options. The histologic classification of colon cancers is actually a collection of multiple cancer subtypes. Each subtype possesses a unique biology largely dependent on the mutations present within the cancer. Recent data, reviewed below, indicate predictive and prognostic benefits to understanding the unique mutational profile of mCRC. Here, the authors present a brief updated summary of these biomarkers and a discussion of treatment strategies.
Resistance to Anti-EGFR Therapies
KRAS and NRAS are members of the RAS family of oncogenes. Activating mutations in these genes results in the propagation of growth factor signals independent of EGFR. The most common KRAS mutations are found in exon 2 (codon 12 or 13). Numerous studies over the past 10 years have confirmed that KRAS mutations at exon 2 predict resistance to cetuximab and panitumumab.4-11 Since at least 2009, restricting use of cetuximab and panitumumab has been the standard of care for patients with KRAS exon 2 wild-type cancers.12
Recent investigations have indicated a predictive role for extended-spectrum KRAS and NRAS mutations (KRAS mutations at exons 2, 3, and 4 and NRAS mutations at exons 2, 3, and 4). In the OPUS clinical trial, patients whose cancers possessed extended-spectrum RAS mutations received no benefit with the addition of cetuximab to standard chemotherapy in response rate (RR), progression-free survival (PFS), or OS compared with standard chemotherapy alone.13 Interestingly, median OS was shorter for those treated with cetuximab when a RAS mutation was present, though the difference was not statistically significant. Additional studies also have confirmed similar benefits in different settings.8,14-18
The CALGB/SWOG 80405 phase 3 clinical trial investigated the first-line use of biologic therapies in combination with standard chemotherapy. The extendedspectrum RAS testing from this study now has been presented.3,19 In the RAS wild-type population, the median OS was 31.2 months in the chemotherapy plus bevacizumab arm and 32.0 months in the chemotherapy plus cetuximab arm (no significant difference). No difference in PFS was observed. A significant improvement in the RR was seen in the cetuximab arm for the RAS wild-type population.
Predictive Biomarkers
BRAF is an oncogene in the RAF gene family that encodes a serine-threonine protein kinase found in the Ras-Raf-MAPK cascade. About 10% of CRC harbor a BRAF mutation.20,21 The most significant and prevalent mutation occurs at the kinase domain from the single substitution V600E. Numerous clinical studies have suggested the presence of this mutation as a predictor of resistance to anti-EGFR therapies and a marker of poor prognosis.6,17,22-25 In a retrospective analysis of RAS and BRAF mutation status of PRIME study data, patients without RAS and BRAF mutations showed significantly better OS and PFS when treated with FOLFOX4 (5-fluorouracil, oxaliplatin, and leucovorin) plus panitumumab, compared with FOLFOX4 alone.8 The presence of BRAF mutations in RAS wild-type patients resulted in a worse outcome. Treatment with anti-EGFR therapy did not significantly improve median PFS or OS.
PIK3CA mutations. Phosphoinositide 3-kinase (PI3K) is a lipid kinase important for multiple cellular processes including cell growth, proliferation, survival, and apoptosis. PIK3CA encodes the catalytic subunit and is mutant in about 20% of mCRC.26 The PI3K is downstream of EGFR signaling; activation of this pathway in the setting of an oncogenic mutation might lead to resistance to anti-EGFR therapies. Sartore-Bianchi and colleagues examined 110 patients with mCRC treated with either panitumumab or cetuximab.27 Of these, 15 patient cancers featured PIK3CA mutations, and none of these responded to anti-EGFR therapies. In addition, preclinical studies have demonstrated that targeting CRC downstream of PI3K might result in significant treatment benefit.28,29
Human epidermal growth factor receptor 2 (HER2) amplification. A subpopulation of CRC with amplification of HER2, a growth factor receptor commonly used in selecting treatment options in breast cancer, has recently been described. The HERACLES phase 2 study evaluated dual HER2 targeting with lapatinib and trastuzumab in therapy-refractory mCRC with HER2 amplification.30 A RR of 35% was observed in this treatment-refractory population.
BRAF mutations. In addition to predicting a poor prognosis and resistance to EGFR-directed therapies, BRAF mutations might be predictive of treatment response using combination regimens containing RAF inhibitors. A recent phase 1B study of a combination therapy using the BRAF inhibitor vemurafenib with irinotecan and cetuximab observed a partial RR of 35%.31 This is being investigated further in the Southwest Oncology Group 1406 phase 2 trial.
Mismatch repair deficiency. Detection of microsatellite instability or the presence of mismatch repair deficiency has become standard-of-care testing for CRC. This is important for the detection of Lynch syndrome and predicting potential resistance to adjuvant 5-fluorouracil in the adjuvant setting.32,33 A recent clinical trial has demonstrated benefits for the use of programmed death 1 (PD-1) inhibitors in the setting of mismatch repair deficiency, including a RR of 40% and PFS of 5.4 months.34
Discussion
Metastatic CRC is now better understood as a collection of multiple cancer subtypes based on mutational profile. This improved understanding of the biology of CRC is altering treatment strategies to a precision medicine-based approach. It is now the standard of care for all patients with mCRC to have the cancers assayed for mutations in KRAS (exons 2, 3, and 4), NRAS (exons 2, 3, and 4), and BRAF. Anti-EGFR therapies should not be used for patients with RAS or BRAF mutations outside of a clinical trial because of a demonstrated lack of benefit in all lines of therapy. Currently, there is no evidence that these mutations significantly alter the response to the approved anti-angiogenic agents bevacizumab, aflibercept, ramucirumab, and regorafenib.
The timing of EGFR-directed therapies for patients with wild-type KRAS, NRAS, and BRAF is still being debated. According to the available data, first-line treatment with anti-EGFR agents in combination with FOLFOX or FOLFIRI (5-fluorouracil, irinotecan, and leucovorin) should be considered for all patients with KRAS, NRAS, and BRAF wild-type mCRCs. The toxicities of anti-EGFR therapies also should be considered for this setting, as some patients find that the acneiform rash, fatigue, nausea, and diarrhea that occur with these agents can have a negative impact on quality of life. As there is no improvement in OS with first-line anti-EGFR therapies for these patients, the increased toxicity from these agents limits their use. In addition, patients with mCRC with known PIK3CA mutation should consider use of EGFR-directed therapies only in the later line setting.
Research is focused on how to best use the mutational profile of the tumor to tailor therapies for mCRC. High-quality, large-volume data sets will become more important as molecular subtypes of cancer become more narrowly defined and less common. Further investigations are needed to look for other markers of resistance and to identify biomarkers predictive of treatment sensitivity.
This is an exciting time in the treatment of many cancers, especially mCRC, which significantly impacts the veteran population, because routine DNA sequencing of patient samples has allowed for rapid advances in the realization of precision medicine. This allows for improved patient selection to reduce costs and toxicities while increasing the benefit for veterans.
Acknowledgments
This work was supported by the National Institutes of Health (P30 CA014520, Core Grant, University of Wisconsin Carbone Cancer Center).
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
Colorectal cancer (CRC) is the third leading cause of cancer-related death in veterans, despite significant advances in treatment options.1,2 Over the past 20 years, the median survival of patients with metastatic CRC (mCRC), has improved with the most recent clinical trials demonstrating a median overall survival (OS) of up to 29 months.3
In addition to standard chemotherapeutic regimens using 5-fluorouracil, oxaliplatin, and irinotecan, biologic therapies have resulted in improved OS for patients with mCRC. These therapies include the human vascular endothelial growth factor (VEGF) monoclonal antibody bevacizumab and the epidermal growth factor receptor (EGFR) monoclonal antibodies cetuximab and panitumumab. Additional agents, including aflibercept, ramucirumab, regorafenib, and TAS-102, also have been FDA approved for mCRC, though the OS benefit for these agents as part of the series of standard-of-care treatments is less clear.
Investigators continue to determine subtypes of CRC to further advance treatment options. The histologic classification of colon cancers is actually a collection of multiple cancer subtypes. Each subtype possesses a unique biology largely dependent on the mutations present within the cancer. Recent data, reviewed below, indicate predictive and prognostic benefits to understanding the unique mutational profile of mCRC. Here, the authors present a brief updated summary of these biomarkers and a discussion of treatment strategies.
Resistance to Anti-EGFR Therapies
KRAS and NRAS are members of the RAS family of oncogenes. Activating mutations in these genes results in the propagation of growth factor signals independent of EGFR. The most common KRAS mutations are found in exon 2 (codon 12 or 13). Numerous studies over the past 10 years have confirmed that KRAS mutations at exon 2 predict resistance to cetuximab and panitumumab.4-11 Since at least 2009, restricting use of cetuximab and panitumumab has been the standard of care for patients with KRAS exon 2 wild-type cancers.12
Recent investigations have indicated a predictive role for extended-spectrum KRAS and NRAS mutations (KRAS mutations at exons 2, 3, and 4 and NRAS mutations at exons 2, 3, and 4). In the OPUS clinical trial, patients whose cancers possessed extended-spectrum RAS mutations received no benefit with the addition of cetuximab to standard chemotherapy in response rate (RR), progression-free survival (PFS), or OS compared with standard chemotherapy alone.13 Interestingly, median OS was shorter for those treated with cetuximab when a RAS mutation was present, though the difference was not statistically significant. Additional studies also have confirmed similar benefits in different settings.8,14-18
The CALGB/SWOG 80405 phase 3 clinical trial investigated the first-line use of biologic therapies in combination with standard chemotherapy. The extendedspectrum RAS testing from this study now has been presented.3,19 In the RAS wild-type population, the median OS was 31.2 months in the chemotherapy plus bevacizumab arm and 32.0 months in the chemotherapy plus cetuximab arm (no significant difference). No difference in PFS was observed. A significant improvement in the RR was seen in the cetuximab arm for the RAS wild-type population.
Predictive Biomarkers
BRAF is an oncogene in the RAF gene family that encodes a serine-threonine protein kinase found in the Ras-Raf-MAPK cascade. About 10% of CRC harbor a BRAF mutation.20,21 The most significant and prevalent mutation occurs at the kinase domain from the single substitution V600E. Numerous clinical studies have suggested the presence of this mutation as a predictor of resistance to anti-EGFR therapies and a marker of poor prognosis.6,17,22-25 In a retrospective analysis of RAS and BRAF mutation status of PRIME study data, patients without RAS and BRAF mutations showed significantly better OS and PFS when treated with FOLFOX4 (5-fluorouracil, oxaliplatin, and leucovorin) plus panitumumab, compared with FOLFOX4 alone.8 The presence of BRAF mutations in RAS wild-type patients resulted in a worse outcome. Treatment with anti-EGFR therapy did not significantly improve median PFS or OS.
PIK3CA mutations. Phosphoinositide 3-kinase (PI3K) is a lipid kinase important for multiple cellular processes including cell growth, proliferation, survival, and apoptosis. PIK3CA encodes the catalytic subunit and is mutant in about 20% of mCRC.26 The PI3K is downstream of EGFR signaling; activation of this pathway in the setting of an oncogenic mutation might lead to resistance to anti-EGFR therapies. Sartore-Bianchi and colleagues examined 110 patients with mCRC treated with either panitumumab or cetuximab.27 Of these, 15 patient cancers featured PIK3CA mutations, and none of these responded to anti-EGFR therapies. In addition, preclinical studies have demonstrated that targeting CRC downstream of PI3K might result in significant treatment benefit.28,29
Human epidermal growth factor receptor 2 (HER2) amplification. A subpopulation of CRC with amplification of HER2, a growth factor receptor commonly used in selecting treatment options in breast cancer, has recently been described. The HERACLES phase 2 study evaluated dual HER2 targeting with lapatinib and trastuzumab in therapy-refractory mCRC with HER2 amplification.30 A RR of 35% was observed in this treatment-refractory population.
BRAF mutations. In addition to predicting a poor prognosis and resistance to EGFR-directed therapies, BRAF mutations might be predictive of treatment response using combination regimens containing RAF inhibitors. A recent phase 1B study of a combination therapy using the BRAF inhibitor vemurafenib with irinotecan and cetuximab observed a partial RR of 35%.31 This is being investigated further in the Southwest Oncology Group 1406 phase 2 trial.
Mismatch repair deficiency. Detection of microsatellite instability or the presence of mismatch repair deficiency has become standard-of-care testing for CRC. This is important for the detection of Lynch syndrome and predicting potential resistance to adjuvant 5-fluorouracil in the adjuvant setting.32,33 A recent clinical trial has demonstrated benefits for the use of programmed death 1 (PD-1) inhibitors in the setting of mismatch repair deficiency, including a RR of 40% and PFS of 5.4 months.34
Discussion
Metastatic CRC is now better understood as a collection of multiple cancer subtypes based on mutational profile. This improved understanding of the biology of CRC is altering treatment strategies to a precision medicine-based approach. It is now the standard of care for all patients with mCRC to have the cancers assayed for mutations in KRAS (exons 2, 3, and 4), NRAS (exons 2, 3, and 4), and BRAF. Anti-EGFR therapies should not be used for patients with RAS or BRAF mutations outside of a clinical trial because of a demonstrated lack of benefit in all lines of therapy. Currently, there is no evidence that these mutations significantly alter the response to the approved anti-angiogenic agents bevacizumab, aflibercept, ramucirumab, and regorafenib.
The timing of EGFR-directed therapies for patients with wild-type KRAS, NRAS, and BRAF is still being debated. According to the available data, first-line treatment with anti-EGFR agents in combination with FOLFOX or FOLFIRI (5-fluorouracil, irinotecan, and leucovorin) should be considered for all patients with KRAS, NRAS, and BRAF wild-type mCRCs. The toxicities of anti-EGFR therapies also should be considered for this setting, as some patients find that the acneiform rash, fatigue, nausea, and diarrhea that occur with these agents can have a negative impact on quality of life. As there is no improvement in OS with first-line anti-EGFR therapies for these patients, the increased toxicity from these agents limits their use. In addition, patients with mCRC with known PIK3CA mutation should consider use of EGFR-directed therapies only in the later line setting.
Research is focused on how to best use the mutational profile of the tumor to tailor therapies for mCRC. High-quality, large-volume data sets will become more important as molecular subtypes of cancer become more narrowly defined and less common. Further investigations are needed to look for other markers of resistance and to identify biomarkers predictive of treatment sensitivity.
This is an exciting time in the treatment of many cancers, especially mCRC, which significantly impacts the veteran population, because routine DNA sequencing of patient samples has allowed for rapid advances in the realization of precision medicine. This allows for improved patient selection to reduce costs and toxicities while increasing the benefit for veterans.
Acknowledgments
This work was supported by the National Institutes of Health (P30 CA014520, Core Grant, University of Wisconsin Carbone Cancer Center).
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. Landrum MB, Keating NL, Lamont EB, et al. Survival of older patients with cancer in the Veterans Health Administration versus fee-for-service Medicare. J Clin Oncol. 2012;30(10):1072-1079.
2. Hynes DM, Tarlov E, Durazo-Arvizu R, et al. Surgery and adjuvant chemotherapy use among veterans with colon cancer: insights from a California study. J Clin Oncol. 2010;28(15):2571-2576.
3. Venook AP, Niedzwiecki D, Lenz H-J, et al; Cancer and Leukemia Group B (Alliance), SWOG, and ECOG. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol. 2014;32(suppl 18):Abstract LBA3.
4. Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67(6):2643-2648.
5. Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22(7):1535-1546.
6. De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11(8):753-7562.
7. Di Fiore F, Blanchard F, Charbonnier F, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by cetuximab plus chemotherapy. Br J Cancer. 2007;96(8):1166-1169.
8. Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023-1034.
9. Lièvre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26(3):374-379.
10. Lièvre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8):3992-3995.
11. Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408-1417.
12. Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27(12):2091-2096.
13. Tejpar S, Lenz HJ, Kohne CH, et al. Effect of KRAS and NRAS mutations on treatment outcomes in patients with metastatic colorectal cancer (mCRC) treated first-line with cetuximab plus FOLFOX4: new results from the OPUS study. J Clin Oncol. 2014;32(suppl 3):LBA444.
14. Abad A, Massuti B, Gravalos C, et al. Panitumumab plus FOLFOX4 or panitumumab plus FOLFIRI in subjects with wild-type KRAS (exon 2) colorectal cancer and multiple or unresectable liver-limited metastases: data from the randomized, phase II planet study. Ann Oncol. 2014;25(suppl 2):ii7-ii18.
15. Schwartzberg LS, Rivera F, Karthaus M, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32(21):2240-2247.
16. Peeters M, Oliner K, Price T, et al. KRAS/NRAS and BRAF mutations in the 20050181 study of panitumumab plus FOLFIRI for the 2nd-line treatment of metastatic colorectal cancer: updated analysis. Ann Oncol. 2014;25(suppl 2):ii5.
17. Bokemeyer C, Van Cutsem E, Rougier P, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48(10):1466-1475.
18. Van Cutsem E, Lenz HJ, Köhne CH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33(7):692-700.
19. ESMO. ESMO 2014: results from the CALGB/SWOG 80405 and FIRE-3 (AIO KRK-0306) studies in all RAS wild type population. ESMO website. http://www.esmo.org/Conferences/Past-Conferences/ESMO-2014-Congress/News-Articles/Results-From-the-CALGB-SWOG-80405-and-FIRE-3-AIO-KRK -0306-Studies-In-All-RAS-Wild-Type-Population. Updated September 29, 2014. Accessed April 6, 2016.
20. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949-954.
21. Samowitz WS, Sweeney C, Herrick J, Albertsen H, Levin TR, Murtaugh MA, Wolff RK, Slattery ML. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65(14):6063-6069.
22. Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26(35):5705-5712.
23. Laurent-Puig P, Cayre A, Manceau G, et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27(35):5924-5930.
24. Richman SD, Seymour MT, Chambers P, et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27(35):5931-5937.
25. Tveit KM, Guren T, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: The NORDIC-VII study. J Clin Oncol. 2012;30(15):1755-1762.
26. Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554.
27. Sartore-Bianchi A, Martini M, Molinari F, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69(5):1851-1857.
28. Deming DA, Leystra AA, Farhoud M, et al. mTOR inhibition elicits a dramatic response in PI3K-dependent colon cancers. PLoS One. 2013;8(4):e60709.
29. Yueh AE, Payne SN, Leystra AA, et al. Colon cancer tumorigenesis initiated by the H1047R mutant PI3K. PLoS One. 2016;11(2):e0148730.
30. Siena S, Sartore-Bianchi A, Lonardi S, et al. Trastuzumab and lapatinib in HER2-amplified metastatic colorectal cancer patients (mCRC): the HERACLES trial. J Clin Oncol. 2015;33(suppl 15):3508.
31. Hong DS, Morris VK, El Osta BE, et al. Phase Ib study of vemurafenib in combination with irinotecan and cetuximab in patients with BRAF-mutated metastatic colorectal cancer and advanced cancers. J Clin Oncol. 2015;33(suppl 15):3511.
32. Benatti P, Gafà R, Barana D, et al. Microsatellite instability and colorectal cancer prognosis. Clin Cancer Res. 2005;11(23):8332-8340.
33. Funkhouser WK, Jr, Lubin IM, Monzon FA, et al. Relevance, pathogenesis, and testing algorithm for mismatch repair-defective colorectal carcinomas: a report of the association for molecular pathology. J Mol Diagn. 2012;14(2):91-103.
34. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair
deficiency. N Engl J Med. 2015;372(26):2509-2520.
1. Landrum MB, Keating NL, Lamont EB, et al. Survival of older patients with cancer in the Veterans Health Administration versus fee-for-service Medicare. J Clin Oncol. 2012;30(10):1072-1079.
2. Hynes DM, Tarlov E, Durazo-Arvizu R, et al. Surgery and adjuvant chemotherapy use among veterans with colon cancer: insights from a California study. J Clin Oncol. 2010;28(15):2571-2576.
3. Venook AP, Niedzwiecki D, Lenz H-J, et al; Cancer and Leukemia Group B (Alliance), SWOG, and ECOG. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol. 2014;32(suppl 18):Abstract LBA3.
4. Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67(6):2643-2648.
5. Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22(7):1535-1546.
6. De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11(8):753-7562.
7. Di Fiore F, Blanchard F, Charbonnier F, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by cetuximab plus chemotherapy. Br J Cancer. 2007;96(8):1166-1169.
8. Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023-1034.
9. Lièvre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26(3):374-379.
10. Lièvre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8):3992-3995.
11. Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408-1417.
12. Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27(12):2091-2096.
13. Tejpar S, Lenz HJ, Kohne CH, et al. Effect of KRAS and NRAS mutations on treatment outcomes in patients with metastatic colorectal cancer (mCRC) treated first-line with cetuximab plus FOLFOX4: new results from the OPUS study. J Clin Oncol. 2014;32(suppl 3):LBA444.
14. Abad A, Massuti B, Gravalos C, et al. Panitumumab plus FOLFOX4 or panitumumab plus FOLFIRI in subjects with wild-type KRAS (exon 2) colorectal cancer and multiple or unresectable liver-limited metastases: data from the randomized, phase II planet study. Ann Oncol. 2014;25(suppl 2):ii7-ii18.
15. Schwartzberg LS, Rivera F, Karthaus M, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32(21):2240-2247.
16. Peeters M, Oliner K, Price T, et al. KRAS/NRAS and BRAF mutations in the 20050181 study of panitumumab plus FOLFIRI for the 2nd-line treatment of metastatic colorectal cancer: updated analysis. Ann Oncol. 2014;25(suppl 2):ii5.
17. Bokemeyer C, Van Cutsem E, Rougier P, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48(10):1466-1475.
18. Van Cutsem E, Lenz HJ, Köhne CH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33(7):692-700.
19. ESMO. ESMO 2014: results from the CALGB/SWOG 80405 and FIRE-3 (AIO KRK-0306) studies in all RAS wild type population. ESMO website. http://www.esmo.org/Conferences/Past-Conferences/ESMO-2014-Congress/News-Articles/Results-From-the-CALGB-SWOG-80405-and-FIRE-3-AIO-KRK -0306-Studies-In-All-RAS-Wild-Type-Population. Updated September 29, 2014. Accessed April 6, 2016.
20. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949-954.
21. Samowitz WS, Sweeney C, Herrick J, Albertsen H, Levin TR, Murtaugh MA, Wolff RK, Slattery ML. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65(14):6063-6069.
22. Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26(35):5705-5712.
23. Laurent-Puig P, Cayre A, Manceau G, et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27(35):5924-5930.
24. Richman SD, Seymour MT, Chambers P, et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27(35):5931-5937.
25. Tveit KM, Guren T, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: The NORDIC-VII study. J Clin Oncol. 2012;30(15):1755-1762.
26. Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554.
27. Sartore-Bianchi A, Martini M, Molinari F, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69(5):1851-1857.
28. Deming DA, Leystra AA, Farhoud M, et al. mTOR inhibition elicits a dramatic response in PI3K-dependent colon cancers. PLoS One. 2013;8(4):e60709.
29. Yueh AE, Payne SN, Leystra AA, et al. Colon cancer tumorigenesis initiated by the H1047R mutant PI3K. PLoS One. 2016;11(2):e0148730.
30. Siena S, Sartore-Bianchi A, Lonardi S, et al. Trastuzumab and lapatinib in HER2-amplified metastatic colorectal cancer patients (mCRC): the HERACLES trial. J Clin Oncol. 2015;33(suppl 15):3508.
31. Hong DS, Morris VK, El Osta BE, et al. Phase Ib study of vemurafenib in combination with irinotecan and cetuximab in patients with BRAF-mutated metastatic colorectal cancer and advanced cancers. J Clin Oncol. 2015;33(suppl 15):3511.
32. Benatti P, Gafà R, Barana D, et al. Microsatellite instability and colorectal cancer prognosis. Clin Cancer Res. 2005;11(23):8332-8340.
33. Funkhouser WK, Jr, Lubin IM, Monzon FA, et al. Relevance, pathogenesis, and testing algorithm for mismatch repair-defective colorectal carcinomas: a report of the association for molecular pathology. J Mol Diagn. 2012;14(2):91-103.
34. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair
deficiency. N Engl J Med. 2015;372(26):2509-2520.
Prevalence of Hypogonadism in Low-Risk Prostate Cancer Survivors
Hypogonadism is characterized by low testosterone levels that can result in symptoms such as reduced libido, erectile dysfunction (ED), fatigue, anemia, decreased bone density, decreased lean body mass, and increased body fat.1,2 The Endocrine Society defines male hypogonadism as serum total testosterone level (T) < 300 ng/dL, a threshold at which the likelihood of most symptoms associated with hypogonadism increases.1
Testosterone replacement therapy (TRT) is recommended for treatment of androgen deficiency in symptomatic men with unequivocally low serum T levels. Although current guidelines recommend against using TRT in men with a history of prostate cancer (PCa),1,3 and the FDA has a black box warning against prescribing TRT for these men, little evidence suggests that TRT stimulates tumor growth in patients treated for low-risk PCa.4-6 Retrospective studies and case series suggest that TRT can be safe in patients with low-risk PCa treated with radical prostatectomy, brachytherapy, or external beam radiation.7,8 Despite observed increases in prostate specific antigen (PSA), TRT does not seem to increase PCa recurrence rates when used cautiously, even in men with high-risk disease.9
Almost 3 million men living in the U.S. have been diagnosed with PCa. While 1 in 7 men will be diagnosed with PCa during their lifetime, most men diagnosed with PCa do not die of it.10 More than 90% of patients with PCa have localized or low-grade disease that does not result in PCa-related mortality.11 Active surveillance, brachytherapy, external beam radiation, and radical prostatectomy are considered appropriate monotherapy modalities for low-risk PCa.12 With successful treatment of early PCa, primary care providers may increasingly encounter PCa survivors with or without symptomatic hypogonadism. Surprisingly, the prevalence of hypogonadism in men with low-risk PCa has not been reported.
The primary objective of this study was to estimate the prevalence of hypogonadism in low-risk PCa survivors who received curative treatment. A second objective was to examine the presence of hypogonadism among subgroups of patients. The authors hypothesized that the prevalence in this population may be high enough to support prospective trials designed to determine the safety of TRT in selected hypogonadal men with a history of PCa.
Methods
This is a cross-sectional study conducted at the Edward Hines, Jr. VA Hospital (EHJVA) that included a convenience sample of 52 veterans aged 25 to 100 years who had been treated for low-risk PCa more than 12 months previously and were currently receiving medical care at EHJVA. Low-risk PCa was defined as tumors with a Gleason score ≤ 6 and PSA at diagnosis < 10 or a (clinical or pathologic) American Joint Committee on Cancer (AJCC) stage I or II. Patients were excluded if they had any of the following: Gleason score > 7, PSA at diagnosis > 10, prior TRT or androgen deprivation therapy, recurrent or active PCa, incomplete treatment of PCa, or history of breast cancer. The study was approved by the institutional review board at EHJVA.
Participant Identification and Recruitment
The EHJVA cancer registry provided a roster of about 600 patients who were diagnosed with AJCC stage I or II PCa after 2002 and had completed treatment by 2011. About 50% of the patients were excluded because they no longer were receiving care at EHJVA. More than 150 patients were excluded because they did not have upcoming appointments at EHJVA, they followed up at community-based outpatient clinics, or they did not meet the remaining parameters of the inclusion criteria.
Between April 2013 and August 2014, the authors approached 75 potentially eligible patients who had upcoming outpatient care appointments at EHJVA and invited them to participate in the study. After explaining the risks and benefits, 15 patients declined to participate. Although 60 eligible patients signed an informed consent, 6 later refused to consent to the medical record review. After the medical record review, 2 patients were excluded because their eligibility could not be confirmed (eg, missing tumor stage or PSA information). A total of 52 men were included in this study.
Data Collection
The study participants were asked to complete a brief validated Androgen Deficiency in the Aging Male (ADAM) questionnaire and provide a blood sample. The standard ADAM questionnaire consists of 10 yes/no questions concerning symptoms of androgen deficiency. A positive questionnaire was defined as a “yes” to any 3 questions.13 A blood sample was obtained between 7AM and 9AM for total and free testosterone level, follicle stimulating hormone, and luteinizing hormone (LH) to determine whether the hypogonadism was primary or secondary to a hypothalamicpituitary process. Additional data were collected, including age, height, weight, race, presence of diabetes mellitus (DM), tumor characteristics at diagnosis (ie, PSA, Gleason score, AJCC stage), and first course of cancer treatment.
Main Outcomes and Measures
The primary outcome was hypogonadism. Patients with serum T < 250 ng/dL were identified as having hypogonadism (the lower limit of the reference range in the EHJVA laboratory assay 250-1,100 ng/dL). The type of hypogonadism was further categorized as primary (LH > 10.6 IU/L) or secondary (LH < 10.6 IU/L). Patients with a body mass index (BMI) > 30 kg/m2 were considered obese.
The prevalence of hypogonadism was assessed overall and for subgroups of patients. The 95% confidence interval (CI) was calculated for each estimate. The correlation between BMI and serum T was also determined. Analyses were carried out using SAS 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
The mean age was 69.1 years and patients were primarily white (Table 1). Half the patients were obese, and 40% had DM. The majority of patients had been diagnosed with stage I PCa, had received radiation as their primary treatment, and had completed the PCa treatment more than 5 years ago.
The prevalence of hypogonadism did not differ by race, DM, or tumor stage (Table 3). The oldest age group had the highest prevalence, but it was not statistically significant. However, obese patients were significantly more likely to have hypogonadism than were nonobese patients (57.6% vs 7.6%, P < .001). The negative association between BMI and serum T was more apparent when the correlation (r = -0.37, P < .01) between these 2 factors were examined (Figure). Patients with a higher ADAM score (> 3) were more likely to have hypogonadism than patients with a lower score (40.6% vs 20.0%, P = .14; Table 3). The prevalence of hypogonadism was higher in patients who were treated with radiation compared with patients who had surgery (41.6% vs 13.3%, P = .05). However, obese patients were more likely to have been treated with radiation (Table 4). After stratifying by obesity status, there was no difference in hypogonadism by treatment type.
Discussion
To the authors’ knowledge, this is the first study to assess the prevalence of hypogonadism in treated low-risk PCa survivors. In this sample of 52 men in an outpatient setting, about 1 in 3 had low testosterone levels. Three patients had primary hypogonadism, and 14 had secondary
(hypogonadotrophic) hypogonadism. Obese patients were significantly more likely to have hypogonadism than were nonobese patients. There was no evidence of an associationbetween PCa treatment and hypogonadism.
Estimates of prevalence of hypogonadism in the general population vary in the literature based on different patient demographics, study designs, and geography. A recent review found that the prevalence of hypogonadism in adult men ≥ 18 years had a range of 2.1% to 31.2% since the authors included studies that were population-based, community-based, and primary care or screening-based.14 The prevalence of hypogonadism was 15.0% to 78.8% in obese people and 21.4% to 33% in men with newly diagnosed PCa.14
Mulligan and colleagues estimated that 38.7% of men aged ≥ 45 years who visited primary care clinics in the U.S. had hypogonadism.15 They also found that the prevalence of hypogonadism, defined as serum T < 300 ng/dL, was significantly higher in men with obesity, DM, hypertension, hyperlipidemia, asthma or chronic obstructive pulmonary disease, and prostate disease. The prevalence of hypogonadism in this study was slightly lower (32.6%), but the authors used a different serum T level (< 250 ng/dL).15 Using the Mulligan and colleagues’ definition of serum T level, there was a prevalence of 40.3% in this study’s population of men with treated low-risk PCa.
Other patient characteristics were examined to determine whether the prevalence of hypogonadism differed by age, tumor stage, or mode of treatment. Testosterone levels drop in all men, 1% per year on average after age 30 years.15 Hypogonadism has been consistently found to increase with age and certain comorbidities. Zarotsky and colleagues found a 17% increase in the risk for hypogonadism with every 10-year increase in age.14 In this study, the authors did not find a significant difference in the prevalence of hypogonadism in this group by age or DM.
In the Prostate Cancer Outcomes Study (PCOS), the prevalence of erectile dysfunction was almost 90% in the prostatectomy and radiotherapy group after 15 years. It was unclear whether this was due to PCa and its treatment, the normal aging process, or a combination of factors, but no significant differences were observed between the treatment groups. However, it is unclear whether the PCOS men had hypogonadism, because testosterone levels were not reported.16
In the current study, the association between obesity and hypogonadism is consistent with epidemiologic evidence that suggests complex multidirectional interactions between testosterone, sex hormone-binding globulin, obesity, metabolic syndrome, and type 2 DM (T2DM), mediated by cytokines and adipokines. Several studies show that obesity adversely affects testicular function and is associated with a reduction in sex hormone-binding globulin, serum T, and free testosterone levels.17,18 In addition, secondary hypogonadism has been shown to be higher in men who are overweight (25-29 kg/m2 BMI) and obese (≥ 30 kg/ m2 BMI) compared with normal weight men.19,20 Hypogonadism has also been shown to increase insulin resistance, thereby increasing the risk for developing metabolic syndrome, which is a precursor for cardiovascular disease (CVD) and T2DM.21 Furthermore, low testosterone concentration may be associated with increased insulin resistance, incidence of CVD events, anemia, and low bone density.19,22,23
The relationship between obesity, metabolic syndrome, and androgen deficiency remains unclear because of the complex mechanisms involved in this association. Impairment of hypothalamic-pituitary function by decreased LH pulse amplitude, inhibitory effects of estrogen at the
hypothalamus and pituitary, and the effects of leptin, ghrelin, and resistin, both centrally and on testicular Leydig cells, may explain lower testosterone level in obese males.24,25 Recent studies have suggested a possible increased risk of CV events among groups of men prescribed TRT.26,27 However, other studies demonstrate beneficial effects of TRT on CVD risk factors, and research over several decades suggests a strong beneficial relationship between normal T and CV health.26 The evidence to suggest that TRT increases CV morbidity and mortality risks is poor,28 but FDA is investigating the link between TRT and adverse CV outcomes.29
Testosterone replacement therapy is recommended for symptomatic men with androgen deficiency to induce or maintain secondary sex characteristics and improve their sexual function, sense of well-being, muscle mass, strength, and bone mineral density. In a recent study supported by the National Institutes of Health (NIH) in men aged 65 years, increasing serum testosterone levels to mid-normal range for 1 year was associated with a moderate benefit in sexual function and improved mood, but there was no significant benefit in vitality (as measured by a fatigue scale) or walking distance.28 In the NIH study, 4 men in the testosterone group and 1 in the placebo group received a diagnosis of PCa during or within the subsequent year of treatment, but the sample size was inadequate to reliably assess the effect of testosterone
on the risk of PCa.28
With increasing direct-to-consumer marketing of branded pharmaceutical products in the U.S., there is widening interest in testosterone levels and hypogonadism symptoms in middle-aged and older men. Indeed, 2.3 million patients received a prescription for testosterone in 2013, up from 1.3 million in 2010.29,30 This change has become important because whereas TRT is standard therapy in symptomatic hypogonadal men, it has long been considered taboo for men with a history of PCa, regardless of disease status. Transdermal testosterone is contraindicated in men with carcinoma of the breast or known or suspected carcinoma of the prostate, according to the package insert.31 Androgens are contraindicated in men with known or suspected carcinoma of the prostate or breast, according to the testosterone cypionate injection package insert.32 Although TRT may increase serum PSA levels in some men, it often remains within clinically acceptable ranges and has not been shown to increase the risk for PCa. A recent observation from 3 registries of more than 1,000 hypogonadal men receiving TRT for up to 17 years concluded that TRT does not increase the risk for PCa.33
Prostate cancer encompasses a heterogeneous collection of androgen-dependent and independent cells. Androgens have been known to play an important role in PCa biology, but this relationship is more complex than the traditional view that androgens stimulate PCa growth. More than 7 decades ago, Huggins and colleagues showed that disseminated PCa was inhibited by eliminating androgens by castration and activated by androgen injections.34 Recently, the androgen hypothesis and the relationship of testosterone to PCa has been more clearly defined. Although it has been established that effective suppression of serum T levels with surgical or chemical castration remains an essential strategy in the management of advanced PCa, the assertion that testosterone causes growth of PCa has been challenged.35,36
Recent studies have shown that there may be a more complex relationship between serum T and PCa risk than was previously established.37 Although PCa cells have been shown to become androgen-independent as they progress into the castrate-resistant phase,9 several studies have indicated that low-serum T is associated with greater PCa risk and more worrisome features of PCa.38,39 Hence, a saturation model has been proposed: Changes in serum T concentrations below the point of maximal androgen-androgen receptor (AR) binding will elicit substantial changes in PCa growth, as seen with castration or with serum T administration to castrated men.40 However, once maximal androgen-AR binding is reached, the presence of additional androgen produces little further effect, suggesting that there is a limit to the ability of androgens to stimulate growth of PCa.40
A meta-analysis of 45 articles studying the relationship between serum T and PCa risk has reported conflicting results.4 Eugonadal testosterone levels, whether physiologically or pharmacologically replaced, do not seem to promote PCa growth. It is unclear whether the timing of sex hormone exposure affects PCa or whether the cancer may influence blood levels of sex hormones. Since 2004, there have been case series totaling almost 150 men treated with prostatectomy, brachytherapy, or external beam radiation who have been safely treated with TRT.6-9 These case studies suggest that after a thorough discussion of risks and benefits, TRT may be safer than previously thought for men who have been successfully treated for PCa, are deemed low risk for recurrence, and are monitored closely. However, no randomized controlled trials are available, and published guidelines recommend against starting TRT in patients with a history of breast or PCa.1
Limitations
This study has limitations. The sample size is small and, therefore, it may not have had enough power to show differences in prevalence by subgroups. In addition, the population may not be representative of all male veterans who seek care in the VA or of men in other health care settings, because this was a convenience sample of VA patients who received most of their care in the VA. However, the prevalence of hypogonadism, in this outpatient population is very similar to that found among men seen in primary care clinics.14 Finally, because pretreatment serum T levels were lacking, the authors were unable to assess whether hypogonadism was present before surgery or radiation therapy, and unable to determine whether treatment had any effect on serum T levels.
The study has several strengths. First, to the authors’ knowledge, this is the first study to assess the prevalence of hypogonadism after patients with low-risk PCa have received treatment with curative intent. Second, the authors assessed hypogonadism in every patient by measuring serum T levels in the early morning when levels are known to be at their highest. Third, differentiating primary and secondary hypogonadism helped provide insight into the possible etiology of the low serum T levels. Fourth, this study was performed at a single institution that uses electronic medical records, with almost complete data on patient demographics, PCa treatment, and receipt of TRT or androgen deprivation therapy. Fifth, because this study had the participation of endocrinologists and urologists, the study design helped answer questions pertinent to both medical and surgical specialties.
Conclusion
As life expectancy increases, many survivors of treated PCa present with symptoms of hypogonadism associated with low serum T levels and request TRT. The prevalence of hypogonadism before or after treatment for PCa in this population is not known. This study suggests that many low-risk PCa survivors have hypogonadism. Because hypogonadism negatively impacts quality of life by increasing the risk for sexual dysfunction, mood disturbances, bone fracture, development of metabolic syndrome, frailty, and decline in the feeling of general well-being and may have significant deleterious effects on other body systems, consideration for treatment is warranted.
Patients with PCa may be untreated because the safety of TRT in this population is unknown. Clinical practice guidelines caution against using TRT in this population, and recent literature questions the benefits and risks associated with the long-term safety of TRT, particularly in older men.28,41 Although further studies are necessary before definitive conclusions can be drawn, increasing evidence, albeit small, suggests that TRT can be cautiously considered in selected hypogonadal men treated with curative intent for PCa and without evidence of active disease. However, because obese patients are at higher risk for aggressive PCa and mortality, it is unclear whether obese PCa survivors have an additional risk in regard to TRT.42,43 To help clinicians provide information and care for their patients, appropriately designed prospective randomized studies using a collaborative approach and long-term follow-up are urgently needed to determine the safety of TRT in hypogonadal men with a history of low-risk PCa.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Edward Hines Jr. VA Hospital. Dr. Silva’s work was carried out while she was a postdoctoral fellow supported by the VA Office of Academic Affiliations (TPP 42-013). The authors thank Ahmer V. Farooq, DO, Department of Surgery, Division of Urology, for his help in planning the design of the study. Dr. Agrawal affirms that all coauthors contributed significantly to the work and had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559.
2. Shortridge EF, Polzer P, Donga P, et al. Experiences and treatment patterns of hypogonadal
men in a U.S. health system. Int J Clin Pract. 2014;68(10):1257-1263.
3. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): prostate cancer. National Comprehensive Cancer Network website. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated February 8, 2016. Accessed April 21, 2016.
4. Klap J, Schmid M, Loughlin KR. The relationship between total testosterone levels and prostate cancer: a review of the continuing controversy. J Urol. 2015;193(2):403-414.
5. Landau D, Tsakok T, Aylwin S, Hughes S. Should testosterone replacement be offered to hypogonadal men treated previously for prostatic carcinoma? Clin Endocrinol (Oxf). 2012;76(2):179-181.
6. Dupree JM, Langille GM, Khera M, Lipshultz LI. The safety of testosterone supplementation therapy in prostate cancer. Nat Rev Urol. 2014;11(9):526-530.
7. Pastuszak AW, Pearlman AM, Godoy G, Miles BJ, Lipshultz LI, Khera M. Testosterone replacement therapy in the setting of prostate cancer treated with radiation. Int J Impot Res. 2013;25(1):24-28.
8. Pastuszak AW, Pearlman AM, Lai WS, et al. Testosterone replacement therapy in patients with prostate cancer after radical prostatectomy. J Urol. 2013;190(2):639-644.
9. Morgentaler A. Testosterone therapy in men with prostate cancer: scientific and ethical considerations. J Urol. 2013;189(1)(suppl):s26-s33.
10. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
11. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):104-117.
12. Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106-2131.
13. Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000;49(9):1239-1242.
14. Zarotsky V, Huang M-Y, Carman W, et al. Systematic literature review of the epidemiology of nongenetic forms of hypogonadism in adult males. J Hormones. 2014;2014:190347.
15. Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60(7):762-769.
16. Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368(5):436-445.
17. Wu FC, Tajar A, Pye SR, et al; European Male Aging Study Group. Hypothalamicpituitary- testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
18. MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16(3):293-311.
19. Dandona P, Dhindsa S. Update: hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab. 2011;96(9):2643-2651.
20. Hofstra J, Loves S, van Wageningen B, Ruinemans-Koerts J, Jansen I, de Boer H. High prevalence of hypogonadotropic hypogonadism in men referred for obesity treatment. Neth J Med. 2008;66(3):103-109.
21. Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006;91(3):843-850.
22. Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93(1):68-75.
23. Orwoll ES, Klein RF. Osteoporosis in men. Endocrine Rev. 1995;16(1):87-116.
24. Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85(5):1319-1340.
25. Mah PM, Wittert GA. Obesity and testicular function. Mol Cell Endocrinol. 2010;316(2):180-186.
26. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
27. Morgentaler A, Miner MM, Caliber M, Guay AT, Khera M, Traish AM. Testosterone
therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc. 2015;90(2):224-251.
28. Snyder PJ, Bhasin S, Cunningham GR, et al; Testosterone Trials Investigators. Effects
of testosterone treatment in older men. N Engl J Med. 2016;374(7):611-624.
29. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA evaluating risk of stroke, heart attack and death with FDA-approved testosterone products. U.S. Food and Drug Administration website. http://www.fda.gov/Drugs/DrugSafety/ucm436259.htm. Updated January 14, 2016. Accessed April 12, 2016.
30. Handelsman DJ. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548-551.
31. AndroGel [package insert]. North Chicago, IL: AbbVie; 2015.
32. Depo-Testosterone [package insert]. New York, NY: Pfizer; 2015.
33. Haider A, Zitzmann M, Doros G, Isbarn H, Hammerer P, Yassin A. Incidence of prostate cancer in hypogonadal men receiving testosterone therapy: observations from 5-year median followup of 3 registries. J Urol. 2015;193(1):80-86.
34. Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22(4):232-240.
35. Gomella LG. Effective testosterone suppression for prostate cancer: is there a best castration therapy? Rev Urol. 2009;11(2):52-60.
36. Khera M, Crawford D, Morales A, Salonia A, Morgentaler A. A new era of testosterone and prostate cancer: from physiology to clinical implications. Eur Urol. 2014;65(1):115-123.
37. Morgentaler A. Testosterone and prostate cancer: an historical perspective on a modern myth. Eur Urol. 2006;50(5):935-939.
38. Morgentaler A, Rhoden EL. Prevalence of prostate cancer among hypogonadal men with prostate-specific antigen levels of 4.0 ng/mL or less. Urology. 2006;68(6):1263-1267.
39. Schatzl G, Madersbacher S, Thurridl T, et al. High-grade prostate cancer is associated with low serum testosterone levels. Prostate. 2001;47(1):52-58.
40. Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol. 2009;55(2):310-320.
41. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109-122.
42. Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63(5):800-809.
43. Buschemeyer WC 3rd, Freedland SJ. Obesity and prostate cancer: epidemiology and clinical implications. Eur Urol. 2007;52(2):331-343.
Hypogonadism is characterized by low testosterone levels that can result in symptoms such as reduced libido, erectile dysfunction (ED), fatigue, anemia, decreased bone density, decreased lean body mass, and increased body fat.1,2 The Endocrine Society defines male hypogonadism as serum total testosterone level (T) < 300 ng/dL, a threshold at which the likelihood of most symptoms associated with hypogonadism increases.1
Testosterone replacement therapy (TRT) is recommended for treatment of androgen deficiency in symptomatic men with unequivocally low serum T levels. Although current guidelines recommend against using TRT in men with a history of prostate cancer (PCa),1,3 and the FDA has a black box warning against prescribing TRT for these men, little evidence suggests that TRT stimulates tumor growth in patients treated for low-risk PCa.4-6 Retrospective studies and case series suggest that TRT can be safe in patients with low-risk PCa treated with radical prostatectomy, brachytherapy, or external beam radiation.7,8 Despite observed increases in prostate specific antigen (PSA), TRT does not seem to increase PCa recurrence rates when used cautiously, even in men with high-risk disease.9
Almost 3 million men living in the U.S. have been diagnosed with PCa. While 1 in 7 men will be diagnosed with PCa during their lifetime, most men diagnosed with PCa do not die of it.10 More than 90% of patients with PCa have localized or low-grade disease that does not result in PCa-related mortality.11 Active surveillance, brachytherapy, external beam radiation, and radical prostatectomy are considered appropriate monotherapy modalities for low-risk PCa.12 With successful treatment of early PCa, primary care providers may increasingly encounter PCa survivors with or without symptomatic hypogonadism. Surprisingly, the prevalence of hypogonadism in men with low-risk PCa has not been reported.
The primary objective of this study was to estimate the prevalence of hypogonadism in low-risk PCa survivors who received curative treatment. A second objective was to examine the presence of hypogonadism among subgroups of patients. The authors hypothesized that the prevalence in this population may be high enough to support prospective trials designed to determine the safety of TRT in selected hypogonadal men with a history of PCa.
Methods
This is a cross-sectional study conducted at the Edward Hines, Jr. VA Hospital (EHJVA) that included a convenience sample of 52 veterans aged 25 to 100 years who had been treated for low-risk PCa more than 12 months previously and were currently receiving medical care at EHJVA. Low-risk PCa was defined as tumors with a Gleason score ≤ 6 and PSA at diagnosis < 10 or a (clinical or pathologic) American Joint Committee on Cancer (AJCC) stage I or II. Patients were excluded if they had any of the following: Gleason score > 7, PSA at diagnosis > 10, prior TRT or androgen deprivation therapy, recurrent or active PCa, incomplete treatment of PCa, or history of breast cancer. The study was approved by the institutional review board at EHJVA.
Participant Identification and Recruitment
The EHJVA cancer registry provided a roster of about 600 patients who were diagnosed with AJCC stage I or II PCa after 2002 and had completed treatment by 2011. About 50% of the patients were excluded because they no longer were receiving care at EHJVA. More than 150 patients were excluded because they did not have upcoming appointments at EHJVA, they followed up at community-based outpatient clinics, or they did not meet the remaining parameters of the inclusion criteria.
Between April 2013 and August 2014, the authors approached 75 potentially eligible patients who had upcoming outpatient care appointments at EHJVA and invited them to participate in the study. After explaining the risks and benefits, 15 patients declined to participate. Although 60 eligible patients signed an informed consent, 6 later refused to consent to the medical record review. After the medical record review, 2 patients were excluded because their eligibility could not be confirmed (eg, missing tumor stage or PSA information). A total of 52 men were included in this study.
Data Collection
The study participants were asked to complete a brief validated Androgen Deficiency in the Aging Male (ADAM) questionnaire and provide a blood sample. The standard ADAM questionnaire consists of 10 yes/no questions concerning symptoms of androgen deficiency. A positive questionnaire was defined as a “yes” to any 3 questions.13 A blood sample was obtained between 7AM and 9AM for total and free testosterone level, follicle stimulating hormone, and luteinizing hormone (LH) to determine whether the hypogonadism was primary or secondary to a hypothalamicpituitary process. Additional data were collected, including age, height, weight, race, presence of diabetes mellitus (DM), tumor characteristics at diagnosis (ie, PSA, Gleason score, AJCC stage), and first course of cancer treatment.
Main Outcomes and Measures
The primary outcome was hypogonadism. Patients with serum T < 250 ng/dL were identified as having hypogonadism (the lower limit of the reference range in the EHJVA laboratory assay 250-1,100 ng/dL). The type of hypogonadism was further categorized as primary (LH > 10.6 IU/L) or secondary (LH < 10.6 IU/L). Patients with a body mass index (BMI) > 30 kg/m2 were considered obese.
The prevalence of hypogonadism was assessed overall and for subgroups of patients. The 95% confidence interval (CI) was calculated for each estimate. The correlation between BMI and serum T was also determined. Analyses were carried out using SAS 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
The mean age was 69.1 years and patients were primarily white (Table 1). Half the patients were obese, and 40% had DM. The majority of patients had been diagnosed with stage I PCa, had received radiation as their primary treatment, and had completed the PCa treatment more than 5 years ago.
The prevalence of hypogonadism did not differ by race, DM, or tumor stage (Table 3). The oldest age group had the highest prevalence, but it was not statistically significant. However, obese patients were significantly more likely to have hypogonadism than were nonobese patients (57.6% vs 7.6%, P < .001). The negative association between BMI and serum T was more apparent when the correlation (r = -0.37, P < .01) between these 2 factors were examined (Figure). Patients with a higher ADAM score (> 3) were more likely to have hypogonadism than patients with a lower score (40.6% vs 20.0%, P = .14; Table 3). The prevalence of hypogonadism was higher in patients who were treated with radiation compared with patients who had surgery (41.6% vs 13.3%, P = .05). However, obese patients were more likely to have been treated with radiation (Table 4). After stratifying by obesity status, there was no difference in hypogonadism by treatment type.
Discussion
To the authors’ knowledge, this is the first study to assess the prevalence of hypogonadism in treated low-risk PCa survivors. In this sample of 52 men in an outpatient setting, about 1 in 3 had low testosterone levels. Three patients had primary hypogonadism, and 14 had secondary
(hypogonadotrophic) hypogonadism. Obese patients were significantly more likely to have hypogonadism than were nonobese patients. There was no evidence of an associationbetween PCa treatment and hypogonadism.
Estimates of prevalence of hypogonadism in the general population vary in the literature based on different patient demographics, study designs, and geography. A recent review found that the prevalence of hypogonadism in adult men ≥ 18 years had a range of 2.1% to 31.2% since the authors included studies that were population-based, community-based, and primary care or screening-based.14 The prevalence of hypogonadism was 15.0% to 78.8% in obese people and 21.4% to 33% in men with newly diagnosed PCa.14
Mulligan and colleagues estimated that 38.7% of men aged ≥ 45 years who visited primary care clinics in the U.S. had hypogonadism.15 They also found that the prevalence of hypogonadism, defined as serum T < 300 ng/dL, was significantly higher in men with obesity, DM, hypertension, hyperlipidemia, asthma or chronic obstructive pulmonary disease, and prostate disease. The prevalence of hypogonadism in this study was slightly lower (32.6%), but the authors used a different serum T level (< 250 ng/dL).15 Using the Mulligan and colleagues’ definition of serum T level, there was a prevalence of 40.3% in this study’s population of men with treated low-risk PCa.
Other patient characteristics were examined to determine whether the prevalence of hypogonadism differed by age, tumor stage, or mode of treatment. Testosterone levels drop in all men, 1% per year on average after age 30 years.15 Hypogonadism has been consistently found to increase with age and certain comorbidities. Zarotsky and colleagues found a 17% increase in the risk for hypogonadism with every 10-year increase in age.14 In this study, the authors did not find a significant difference in the prevalence of hypogonadism in this group by age or DM.
In the Prostate Cancer Outcomes Study (PCOS), the prevalence of erectile dysfunction was almost 90% in the prostatectomy and radiotherapy group after 15 years. It was unclear whether this was due to PCa and its treatment, the normal aging process, or a combination of factors, but no significant differences were observed between the treatment groups. However, it is unclear whether the PCOS men had hypogonadism, because testosterone levels were not reported.16
In the current study, the association between obesity and hypogonadism is consistent with epidemiologic evidence that suggests complex multidirectional interactions between testosterone, sex hormone-binding globulin, obesity, metabolic syndrome, and type 2 DM (T2DM), mediated by cytokines and adipokines. Several studies show that obesity adversely affects testicular function and is associated with a reduction in sex hormone-binding globulin, serum T, and free testosterone levels.17,18 In addition, secondary hypogonadism has been shown to be higher in men who are overweight (25-29 kg/m2 BMI) and obese (≥ 30 kg/ m2 BMI) compared with normal weight men.19,20 Hypogonadism has also been shown to increase insulin resistance, thereby increasing the risk for developing metabolic syndrome, which is a precursor for cardiovascular disease (CVD) and T2DM.21 Furthermore, low testosterone concentration may be associated with increased insulin resistance, incidence of CVD events, anemia, and low bone density.19,22,23
The relationship between obesity, metabolic syndrome, and androgen deficiency remains unclear because of the complex mechanisms involved in this association. Impairment of hypothalamic-pituitary function by decreased LH pulse amplitude, inhibitory effects of estrogen at the
hypothalamus and pituitary, and the effects of leptin, ghrelin, and resistin, both centrally and on testicular Leydig cells, may explain lower testosterone level in obese males.24,25 Recent studies have suggested a possible increased risk of CV events among groups of men prescribed TRT.26,27 However, other studies demonstrate beneficial effects of TRT on CVD risk factors, and research over several decades suggests a strong beneficial relationship between normal T and CV health.26 The evidence to suggest that TRT increases CV morbidity and mortality risks is poor,28 but FDA is investigating the link between TRT and adverse CV outcomes.29
Testosterone replacement therapy is recommended for symptomatic men with androgen deficiency to induce or maintain secondary sex characteristics and improve their sexual function, sense of well-being, muscle mass, strength, and bone mineral density. In a recent study supported by the National Institutes of Health (NIH) in men aged 65 years, increasing serum testosterone levels to mid-normal range for 1 year was associated with a moderate benefit in sexual function and improved mood, but there was no significant benefit in vitality (as measured by a fatigue scale) or walking distance.28 In the NIH study, 4 men in the testosterone group and 1 in the placebo group received a diagnosis of PCa during or within the subsequent year of treatment, but the sample size was inadequate to reliably assess the effect of testosterone
on the risk of PCa.28
With increasing direct-to-consumer marketing of branded pharmaceutical products in the U.S., there is widening interest in testosterone levels and hypogonadism symptoms in middle-aged and older men. Indeed, 2.3 million patients received a prescription for testosterone in 2013, up from 1.3 million in 2010.29,30 This change has become important because whereas TRT is standard therapy in symptomatic hypogonadal men, it has long been considered taboo for men with a history of PCa, regardless of disease status. Transdermal testosterone is contraindicated in men with carcinoma of the breast or known or suspected carcinoma of the prostate, according to the package insert.31 Androgens are contraindicated in men with known or suspected carcinoma of the prostate or breast, according to the testosterone cypionate injection package insert.32 Although TRT may increase serum PSA levels in some men, it often remains within clinically acceptable ranges and has not been shown to increase the risk for PCa. A recent observation from 3 registries of more than 1,000 hypogonadal men receiving TRT for up to 17 years concluded that TRT does not increase the risk for PCa.33
Prostate cancer encompasses a heterogeneous collection of androgen-dependent and independent cells. Androgens have been known to play an important role in PCa biology, but this relationship is more complex than the traditional view that androgens stimulate PCa growth. More than 7 decades ago, Huggins and colleagues showed that disseminated PCa was inhibited by eliminating androgens by castration and activated by androgen injections.34 Recently, the androgen hypothesis and the relationship of testosterone to PCa has been more clearly defined. Although it has been established that effective suppression of serum T levels with surgical or chemical castration remains an essential strategy in the management of advanced PCa, the assertion that testosterone causes growth of PCa has been challenged.35,36
Recent studies have shown that there may be a more complex relationship between serum T and PCa risk than was previously established.37 Although PCa cells have been shown to become androgen-independent as they progress into the castrate-resistant phase,9 several studies have indicated that low-serum T is associated with greater PCa risk and more worrisome features of PCa.38,39 Hence, a saturation model has been proposed: Changes in serum T concentrations below the point of maximal androgen-androgen receptor (AR) binding will elicit substantial changes in PCa growth, as seen with castration or with serum T administration to castrated men.40 However, once maximal androgen-AR binding is reached, the presence of additional androgen produces little further effect, suggesting that there is a limit to the ability of androgens to stimulate growth of PCa.40
A meta-analysis of 45 articles studying the relationship between serum T and PCa risk has reported conflicting results.4 Eugonadal testosterone levels, whether physiologically or pharmacologically replaced, do not seem to promote PCa growth. It is unclear whether the timing of sex hormone exposure affects PCa or whether the cancer may influence blood levels of sex hormones. Since 2004, there have been case series totaling almost 150 men treated with prostatectomy, brachytherapy, or external beam radiation who have been safely treated with TRT.6-9 These case studies suggest that after a thorough discussion of risks and benefits, TRT may be safer than previously thought for men who have been successfully treated for PCa, are deemed low risk for recurrence, and are monitored closely. However, no randomized controlled trials are available, and published guidelines recommend against starting TRT in patients with a history of breast or PCa.1
Limitations
This study has limitations. The sample size is small and, therefore, it may not have had enough power to show differences in prevalence by subgroups. In addition, the population may not be representative of all male veterans who seek care in the VA or of men in other health care settings, because this was a convenience sample of VA patients who received most of their care in the VA. However, the prevalence of hypogonadism, in this outpatient population is very similar to that found among men seen in primary care clinics.14 Finally, because pretreatment serum T levels were lacking, the authors were unable to assess whether hypogonadism was present before surgery or radiation therapy, and unable to determine whether treatment had any effect on serum T levels.
The study has several strengths. First, to the authors’ knowledge, this is the first study to assess the prevalence of hypogonadism after patients with low-risk PCa have received treatment with curative intent. Second, the authors assessed hypogonadism in every patient by measuring serum T levels in the early morning when levels are known to be at their highest. Third, differentiating primary and secondary hypogonadism helped provide insight into the possible etiology of the low serum T levels. Fourth, this study was performed at a single institution that uses electronic medical records, with almost complete data on patient demographics, PCa treatment, and receipt of TRT or androgen deprivation therapy. Fifth, because this study had the participation of endocrinologists and urologists, the study design helped answer questions pertinent to both medical and surgical specialties.
Conclusion
As life expectancy increases, many survivors of treated PCa present with symptoms of hypogonadism associated with low serum T levels and request TRT. The prevalence of hypogonadism before or after treatment for PCa in this population is not known. This study suggests that many low-risk PCa survivors have hypogonadism. Because hypogonadism negatively impacts quality of life by increasing the risk for sexual dysfunction, mood disturbances, bone fracture, development of metabolic syndrome, frailty, and decline in the feeling of general well-being and may have significant deleterious effects on other body systems, consideration for treatment is warranted.
Patients with PCa may be untreated because the safety of TRT in this population is unknown. Clinical practice guidelines caution against using TRT in this population, and recent literature questions the benefits and risks associated with the long-term safety of TRT, particularly in older men.28,41 Although further studies are necessary before definitive conclusions can be drawn, increasing evidence, albeit small, suggests that TRT can be cautiously considered in selected hypogonadal men treated with curative intent for PCa and without evidence of active disease. However, because obese patients are at higher risk for aggressive PCa and mortality, it is unclear whether obese PCa survivors have an additional risk in regard to TRT.42,43 To help clinicians provide information and care for their patients, appropriately designed prospective randomized studies using a collaborative approach and long-term follow-up are urgently needed to determine the safety of TRT in hypogonadal men with a history of low-risk PCa.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Edward Hines Jr. VA Hospital. Dr. Silva’s work was carried out while she was a postdoctoral fellow supported by the VA Office of Academic Affiliations (TPP 42-013). The authors thank Ahmer V. Farooq, DO, Department of Surgery, Division of Urology, for his help in planning the design of the study. Dr. Agrawal affirms that all coauthors contributed significantly to the work and had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Hypogonadism is characterized by low testosterone levels that can result in symptoms such as reduced libido, erectile dysfunction (ED), fatigue, anemia, decreased bone density, decreased lean body mass, and increased body fat.1,2 The Endocrine Society defines male hypogonadism as serum total testosterone level (T) < 300 ng/dL, a threshold at which the likelihood of most symptoms associated with hypogonadism increases.1
Testosterone replacement therapy (TRT) is recommended for treatment of androgen deficiency in symptomatic men with unequivocally low serum T levels. Although current guidelines recommend against using TRT in men with a history of prostate cancer (PCa),1,3 and the FDA has a black box warning against prescribing TRT for these men, little evidence suggests that TRT stimulates tumor growth in patients treated for low-risk PCa.4-6 Retrospective studies and case series suggest that TRT can be safe in patients with low-risk PCa treated with radical prostatectomy, brachytherapy, or external beam radiation.7,8 Despite observed increases in prostate specific antigen (PSA), TRT does not seem to increase PCa recurrence rates when used cautiously, even in men with high-risk disease.9
Almost 3 million men living in the U.S. have been diagnosed with PCa. While 1 in 7 men will be diagnosed with PCa during their lifetime, most men diagnosed with PCa do not die of it.10 More than 90% of patients with PCa have localized or low-grade disease that does not result in PCa-related mortality.11 Active surveillance, brachytherapy, external beam radiation, and radical prostatectomy are considered appropriate monotherapy modalities for low-risk PCa.12 With successful treatment of early PCa, primary care providers may increasingly encounter PCa survivors with or without symptomatic hypogonadism. Surprisingly, the prevalence of hypogonadism in men with low-risk PCa has not been reported.
The primary objective of this study was to estimate the prevalence of hypogonadism in low-risk PCa survivors who received curative treatment. A second objective was to examine the presence of hypogonadism among subgroups of patients. The authors hypothesized that the prevalence in this population may be high enough to support prospective trials designed to determine the safety of TRT in selected hypogonadal men with a history of PCa.
Methods
This is a cross-sectional study conducted at the Edward Hines, Jr. VA Hospital (EHJVA) that included a convenience sample of 52 veterans aged 25 to 100 years who had been treated for low-risk PCa more than 12 months previously and were currently receiving medical care at EHJVA. Low-risk PCa was defined as tumors with a Gleason score ≤ 6 and PSA at diagnosis < 10 or a (clinical or pathologic) American Joint Committee on Cancer (AJCC) stage I or II. Patients were excluded if they had any of the following: Gleason score > 7, PSA at diagnosis > 10, prior TRT or androgen deprivation therapy, recurrent or active PCa, incomplete treatment of PCa, or history of breast cancer. The study was approved by the institutional review board at EHJVA.
Participant Identification and Recruitment
The EHJVA cancer registry provided a roster of about 600 patients who were diagnosed with AJCC stage I or II PCa after 2002 and had completed treatment by 2011. About 50% of the patients were excluded because they no longer were receiving care at EHJVA. More than 150 patients were excluded because they did not have upcoming appointments at EHJVA, they followed up at community-based outpatient clinics, or they did not meet the remaining parameters of the inclusion criteria.
Between April 2013 and August 2014, the authors approached 75 potentially eligible patients who had upcoming outpatient care appointments at EHJVA and invited them to participate in the study. After explaining the risks and benefits, 15 patients declined to participate. Although 60 eligible patients signed an informed consent, 6 later refused to consent to the medical record review. After the medical record review, 2 patients were excluded because their eligibility could not be confirmed (eg, missing tumor stage or PSA information). A total of 52 men were included in this study.
Data Collection
The study participants were asked to complete a brief validated Androgen Deficiency in the Aging Male (ADAM) questionnaire and provide a blood sample. The standard ADAM questionnaire consists of 10 yes/no questions concerning symptoms of androgen deficiency. A positive questionnaire was defined as a “yes” to any 3 questions.13 A blood sample was obtained between 7AM and 9AM for total and free testosterone level, follicle stimulating hormone, and luteinizing hormone (LH) to determine whether the hypogonadism was primary or secondary to a hypothalamicpituitary process. Additional data were collected, including age, height, weight, race, presence of diabetes mellitus (DM), tumor characteristics at diagnosis (ie, PSA, Gleason score, AJCC stage), and first course of cancer treatment.
Main Outcomes and Measures
The primary outcome was hypogonadism. Patients with serum T < 250 ng/dL were identified as having hypogonadism (the lower limit of the reference range in the EHJVA laboratory assay 250-1,100 ng/dL). The type of hypogonadism was further categorized as primary (LH > 10.6 IU/L) or secondary (LH < 10.6 IU/L). Patients with a body mass index (BMI) > 30 kg/m2 were considered obese.
The prevalence of hypogonadism was assessed overall and for subgroups of patients. The 95% confidence interval (CI) was calculated for each estimate. The correlation between BMI and serum T was also determined. Analyses were carried out using SAS 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
The mean age was 69.1 years and patients were primarily white (Table 1). Half the patients were obese, and 40% had DM. The majority of patients had been diagnosed with stage I PCa, had received radiation as their primary treatment, and had completed the PCa treatment more than 5 years ago.
The prevalence of hypogonadism did not differ by race, DM, or tumor stage (Table 3). The oldest age group had the highest prevalence, but it was not statistically significant. However, obese patients were significantly more likely to have hypogonadism than were nonobese patients (57.6% vs 7.6%, P < .001). The negative association between BMI and serum T was more apparent when the correlation (r = -0.37, P < .01) between these 2 factors were examined (Figure). Patients with a higher ADAM score (> 3) were more likely to have hypogonadism than patients with a lower score (40.6% vs 20.0%, P = .14; Table 3). The prevalence of hypogonadism was higher in patients who were treated with radiation compared with patients who had surgery (41.6% vs 13.3%, P = .05). However, obese patients were more likely to have been treated with radiation (Table 4). After stratifying by obesity status, there was no difference in hypogonadism by treatment type.
Discussion
To the authors’ knowledge, this is the first study to assess the prevalence of hypogonadism in treated low-risk PCa survivors. In this sample of 52 men in an outpatient setting, about 1 in 3 had low testosterone levels. Three patients had primary hypogonadism, and 14 had secondary
(hypogonadotrophic) hypogonadism. Obese patients were significantly more likely to have hypogonadism than were nonobese patients. There was no evidence of an associationbetween PCa treatment and hypogonadism.
Estimates of prevalence of hypogonadism in the general population vary in the literature based on different patient demographics, study designs, and geography. A recent review found that the prevalence of hypogonadism in adult men ≥ 18 years had a range of 2.1% to 31.2% since the authors included studies that were population-based, community-based, and primary care or screening-based.14 The prevalence of hypogonadism was 15.0% to 78.8% in obese people and 21.4% to 33% in men with newly diagnosed PCa.14
Mulligan and colleagues estimated that 38.7% of men aged ≥ 45 years who visited primary care clinics in the U.S. had hypogonadism.15 They also found that the prevalence of hypogonadism, defined as serum T < 300 ng/dL, was significantly higher in men with obesity, DM, hypertension, hyperlipidemia, asthma or chronic obstructive pulmonary disease, and prostate disease. The prevalence of hypogonadism in this study was slightly lower (32.6%), but the authors used a different serum T level (< 250 ng/dL).15 Using the Mulligan and colleagues’ definition of serum T level, there was a prevalence of 40.3% in this study’s population of men with treated low-risk PCa.
Other patient characteristics were examined to determine whether the prevalence of hypogonadism differed by age, tumor stage, or mode of treatment. Testosterone levels drop in all men, 1% per year on average after age 30 years.15 Hypogonadism has been consistently found to increase with age and certain comorbidities. Zarotsky and colleagues found a 17% increase in the risk for hypogonadism with every 10-year increase in age.14 In this study, the authors did not find a significant difference in the prevalence of hypogonadism in this group by age or DM.
In the Prostate Cancer Outcomes Study (PCOS), the prevalence of erectile dysfunction was almost 90% in the prostatectomy and radiotherapy group after 15 years. It was unclear whether this was due to PCa and its treatment, the normal aging process, or a combination of factors, but no significant differences were observed between the treatment groups. However, it is unclear whether the PCOS men had hypogonadism, because testosterone levels were not reported.16
In the current study, the association between obesity and hypogonadism is consistent with epidemiologic evidence that suggests complex multidirectional interactions between testosterone, sex hormone-binding globulin, obesity, metabolic syndrome, and type 2 DM (T2DM), mediated by cytokines and adipokines. Several studies show that obesity adversely affects testicular function and is associated with a reduction in sex hormone-binding globulin, serum T, and free testosterone levels.17,18 In addition, secondary hypogonadism has been shown to be higher in men who are overweight (25-29 kg/m2 BMI) and obese (≥ 30 kg/ m2 BMI) compared with normal weight men.19,20 Hypogonadism has also been shown to increase insulin resistance, thereby increasing the risk for developing metabolic syndrome, which is a precursor for cardiovascular disease (CVD) and T2DM.21 Furthermore, low testosterone concentration may be associated with increased insulin resistance, incidence of CVD events, anemia, and low bone density.19,22,23
The relationship between obesity, metabolic syndrome, and androgen deficiency remains unclear because of the complex mechanisms involved in this association. Impairment of hypothalamic-pituitary function by decreased LH pulse amplitude, inhibitory effects of estrogen at the
hypothalamus and pituitary, and the effects of leptin, ghrelin, and resistin, both centrally and on testicular Leydig cells, may explain lower testosterone level in obese males.24,25 Recent studies have suggested a possible increased risk of CV events among groups of men prescribed TRT.26,27 However, other studies demonstrate beneficial effects of TRT on CVD risk factors, and research over several decades suggests a strong beneficial relationship between normal T and CV health.26 The evidence to suggest that TRT increases CV morbidity and mortality risks is poor,28 but FDA is investigating the link between TRT and adverse CV outcomes.29
Testosterone replacement therapy is recommended for symptomatic men with androgen deficiency to induce or maintain secondary sex characteristics and improve their sexual function, sense of well-being, muscle mass, strength, and bone mineral density. In a recent study supported by the National Institutes of Health (NIH) in men aged 65 years, increasing serum testosterone levels to mid-normal range for 1 year was associated with a moderate benefit in sexual function and improved mood, but there was no significant benefit in vitality (as measured by a fatigue scale) or walking distance.28 In the NIH study, 4 men in the testosterone group and 1 in the placebo group received a diagnosis of PCa during or within the subsequent year of treatment, but the sample size was inadequate to reliably assess the effect of testosterone
on the risk of PCa.28
With increasing direct-to-consumer marketing of branded pharmaceutical products in the U.S., there is widening interest in testosterone levels and hypogonadism symptoms in middle-aged and older men. Indeed, 2.3 million patients received a prescription for testosterone in 2013, up from 1.3 million in 2010.29,30 This change has become important because whereas TRT is standard therapy in symptomatic hypogonadal men, it has long been considered taboo for men with a history of PCa, regardless of disease status. Transdermal testosterone is contraindicated in men with carcinoma of the breast or known or suspected carcinoma of the prostate, according to the package insert.31 Androgens are contraindicated in men with known or suspected carcinoma of the prostate or breast, according to the testosterone cypionate injection package insert.32 Although TRT may increase serum PSA levels in some men, it often remains within clinically acceptable ranges and has not been shown to increase the risk for PCa. A recent observation from 3 registries of more than 1,000 hypogonadal men receiving TRT for up to 17 years concluded that TRT does not increase the risk for PCa.33
Prostate cancer encompasses a heterogeneous collection of androgen-dependent and independent cells. Androgens have been known to play an important role in PCa biology, but this relationship is more complex than the traditional view that androgens stimulate PCa growth. More than 7 decades ago, Huggins and colleagues showed that disseminated PCa was inhibited by eliminating androgens by castration and activated by androgen injections.34 Recently, the androgen hypothesis and the relationship of testosterone to PCa has been more clearly defined. Although it has been established that effective suppression of serum T levels with surgical or chemical castration remains an essential strategy in the management of advanced PCa, the assertion that testosterone causes growth of PCa has been challenged.35,36
Recent studies have shown that there may be a more complex relationship between serum T and PCa risk than was previously established.37 Although PCa cells have been shown to become androgen-independent as they progress into the castrate-resistant phase,9 several studies have indicated that low-serum T is associated with greater PCa risk and more worrisome features of PCa.38,39 Hence, a saturation model has been proposed: Changes in serum T concentrations below the point of maximal androgen-androgen receptor (AR) binding will elicit substantial changes in PCa growth, as seen with castration or with serum T administration to castrated men.40 However, once maximal androgen-AR binding is reached, the presence of additional androgen produces little further effect, suggesting that there is a limit to the ability of androgens to stimulate growth of PCa.40
A meta-analysis of 45 articles studying the relationship between serum T and PCa risk has reported conflicting results.4 Eugonadal testosterone levels, whether physiologically or pharmacologically replaced, do not seem to promote PCa growth. It is unclear whether the timing of sex hormone exposure affects PCa or whether the cancer may influence blood levels of sex hormones. Since 2004, there have been case series totaling almost 150 men treated with prostatectomy, brachytherapy, or external beam radiation who have been safely treated with TRT.6-9 These case studies suggest that after a thorough discussion of risks and benefits, TRT may be safer than previously thought for men who have been successfully treated for PCa, are deemed low risk for recurrence, and are monitored closely. However, no randomized controlled trials are available, and published guidelines recommend against starting TRT in patients with a history of breast or PCa.1
Limitations
This study has limitations. The sample size is small and, therefore, it may not have had enough power to show differences in prevalence by subgroups. In addition, the population may not be representative of all male veterans who seek care in the VA or of men in other health care settings, because this was a convenience sample of VA patients who received most of their care in the VA. However, the prevalence of hypogonadism, in this outpatient population is very similar to that found among men seen in primary care clinics.14 Finally, because pretreatment serum T levels were lacking, the authors were unable to assess whether hypogonadism was present before surgery or radiation therapy, and unable to determine whether treatment had any effect on serum T levels.
The study has several strengths. First, to the authors’ knowledge, this is the first study to assess the prevalence of hypogonadism after patients with low-risk PCa have received treatment with curative intent. Second, the authors assessed hypogonadism in every patient by measuring serum T levels in the early morning when levels are known to be at their highest. Third, differentiating primary and secondary hypogonadism helped provide insight into the possible etiology of the low serum T levels. Fourth, this study was performed at a single institution that uses electronic medical records, with almost complete data on patient demographics, PCa treatment, and receipt of TRT or androgen deprivation therapy. Fifth, because this study had the participation of endocrinologists and urologists, the study design helped answer questions pertinent to both medical and surgical specialties.
Conclusion
As life expectancy increases, many survivors of treated PCa present with symptoms of hypogonadism associated with low serum T levels and request TRT. The prevalence of hypogonadism before or after treatment for PCa in this population is not known. This study suggests that many low-risk PCa survivors have hypogonadism. Because hypogonadism negatively impacts quality of life by increasing the risk for sexual dysfunction, mood disturbances, bone fracture, development of metabolic syndrome, frailty, and decline in the feeling of general well-being and may have significant deleterious effects on other body systems, consideration for treatment is warranted.
Patients with PCa may be untreated because the safety of TRT in this population is unknown. Clinical practice guidelines caution against using TRT in this population, and recent literature questions the benefits and risks associated with the long-term safety of TRT, particularly in older men.28,41 Although further studies are necessary before definitive conclusions can be drawn, increasing evidence, albeit small, suggests that TRT can be cautiously considered in selected hypogonadal men treated with curative intent for PCa and without evidence of active disease. However, because obese patients are at higher risk for aggressive PCa and mortality, it is unclear whether obese PCa survivors have an additional risk in regard to TRT.42,43 To help clinicians provide information and care for their patients, appropriately designed prospective randomized studies using a collaborative approach and long-term follow-up are urgently needed to determine the safety of TRT in hypogonadal men with a history of low-risk PCa.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Edward Hines Jr. VA Hospital. Dr. Silva’s work was carried out while she was a postdoctoral fellow supported by the VA Office of Academic Affiliations (TPP 42-013). The authors thank Ahmer V. Farooq, DO, Department of Surgery, Division of Urology, for his help in planning the design of the study. Dr. Agrawal affirms that all coauthors contributed significantly to the work and had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559.
2. Shortridge EF, Polzer P, Donga P, et al. Experiences and treatment patterns of hypogonadal
men in a U.S. health system. Int J Clin Pract. 2014;68(10):1257-1263.
3. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): prostate cancer. National Comprehensive Cancer Network website. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated February 8, 2016. Accessed April 21, 2016.
4. Klap J, Schmid M, Loughlin KR. The relationship between total testosterone levels and prostate cancer: a review of the continuing controversy. J Urol. 2015;193(2):403-414.
5. Landau D, Tsakok T, Aylwin S, Hughes S. Should testosterone replacement be offered to hypogonadal men treated previously for prostatic carcinoma? Clin Endocrinol (Oxf). 2012;76(2):179-181.
6. Dupree JM, Langille GM, Khera M, Lipshultz LI. The safety of testosterone supplementation therapy in prostate cancer. Nat Rev Urol. 2014;11(9):526-530.
7. Pastuszak AW, Pearlman AM, Godoy G, Miles BJ, Lipshultz LI, Khera M. Testosterone replacement therapy in the setting of prostate cancer treated with radiation. Int J Impot Res. 2013;25(1):24-28.
8. Pastuszak AW, Pearlman AM, Lai WS, et al. Testosterone replacement therapy in patients with prostate cancer after radical prostatectomy. J Urol. 2013;190(2):639-644.
9. Morgentaler A. Testosterone therapy in men with prostate cancer: scientific and ethical considerations. J Urol. 2013;189(1)(suppl):s26-s33.
10. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
11. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):104-117.
12. Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106-2131.
13. Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000;49(9):1239-1242.
14. Zarotsky V, Huang M-Y, Carman W, et al. Systematic literature review of the epidemiology of nongenetic forms of hypogonadism in adult males. J Hormones. 2014;2014:190347.
15. Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60(7):762-769.
16. Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368(5):436-445.
17. Wu FC, Tajar A, Pye SR, et al; European Male Aging Study Group. Hypothalamicpituitary- testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
18. MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16(3):293-311.
19. Dandona P, Dhindsa S. Update: hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab. 2011;96(9):2643-2651.
20. Hofstra J, Loves S, van Wageningen B, Ruinemans-Koerts J, Jansen I, de Boer H. High prevalence of hypogonadotropic hypogonadism in men referred for obesity treatment. Neth J Med. 2008;66(3):103-109.
21. Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006;91(3):843-850.
22. Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93(1):68-75.
23. Orwoll ES, Klein RF. Osteoporosis in men. Endocrine Rev. 1995;16(1):87-116.
24. Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85(5):1319-1340.
25. Mah PM, Wittert GA. Obesity and testicular function. Mol Cell Endocrinol. 2010;316(2):180-186.
26. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
27. Morgentaler A, Miner MM, Caliber M, Guay AT, Khera M, Traish AM. Testosterone
therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc. 2015;90(2):224-251.
28. Snyder PJ, Bhasin S, Cunningham GR, et al; Testosterone Trials Investigators. Effects
of testosterone treatment in older men. N Engl J Med. 2016;374(7):611-624.
29. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA evaluating risk of stroke, heart attack and death with FDA-approved testosterone products. U.S. Food and Drug Administration website. http://www.fda.gov/Drugs/DrugSafety/ucm436259.htm. Updated January 14, 2016. Accessed April 12, 2016.
30. Handelsman DJ. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548-551.
31. AndroGel [package insert]. North Chicago, IL: AbbVie; 2015.
32. Depo-Testosterone [package insert]. New York, NY: Pfizer; 2015.
33. Haider A, Zitzmann M, Doros G, Isbarn H, Hammerer P, Yassin A. Incidence of prostate cancer in hypogonadal men receiving testosterone therapy: observations from 5-year median followup of 3 registries. J Urol. 2015;193(1):80-86.
34. Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22(4):232-240.
35. Gomella LG. Effective testosterone suppression for prostate cancer: is there a best castration therapy? Rev Urol. 2009;11(2):52-60.
36. Khera M, Crawford D, Morales A, Salonia A, Morgentaler A. A new era of testosterone and prostate cancer: from physiology to clinical implications. Eur Urol. 2014;65(1):115-123.
37. Morgentaler A. Testosterone and prostate cancer: an historical perspective on a modern myth. Eur Urol. 2006;50(5):935-939.
38. Morgentaler A, Rhoden EL. Prevalence of prostate cancer among hypogonadal men with prostate-specific antigen levels of 4.0 ng/mL or less. Urology. 2006;68(6):1263-1267.
39. Schatzl G, Madersbacher S, Thurridl T, et al. High-grade prostate cancer is associated with low serum testosterone levels. Prostate. 2001;47(1):52-58.
40. Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol. 2009;55(2):310-320.
41. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109-122.
42. Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63(5):800-809.
43. Buschemeyer WC 3rd, Freedland SJ. Obesity and prostate cancer: epidemiology and clinical implications. Eur Urol. 2007;52(2):331-343.
1. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559.
2. Shortridge EF, Polzer P, Donga P, et al. Experiences and treatment patterns of hypogonadal
men in a U.S. health system. Int J Clin Pract. 2014;68(10):1257-1263.
3. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): prostate cancer. National Comprehensive Cancer Network website. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated February 8, 2016. Accessed April 21, 2016.
4. Klap J, Schmid M, Loughlin KR. The relationship between total testosterone levels and prostate cancer: a review of the continuing controversy. J Urol. 2015;193(2):403-414.
5. Landau D, Tsakok T, Aylwin S, Hughes S. Should testosterone replacement be offered to hypogonadal men treated previously for prostatic carcinoma? Clin Endocrinol (Oxf). 2012;76(2):179-181.
6. Dupree JM, Langille GM, Khera M, Lipshultz LI. The safety of testosterone supplementation therapy in prostate cancer. Nat Rev Urol. 2014;11(9):526-530.
7. Pastuszak AW, Pearlman AM, Godoy G, Miles BJ, Lipshultz LI, Khera M. Testosterone replacement therapy in the setting of prostate cancer treated with radiation. Int J Impot Res. 2013;25(1):24-28.
8. Pastuszak AW, Pearlman AM, Lai WS, et al. Testosterone replacement therapy in patients with prostate cancer after radical prostatectomy. J Urol. 2013;190(2):639-644.
9. Morgentaler A. Testosterone therapy in men with prostate cancer: scientific and ethical considerations. J Urol. 2013;189(1)(suppl):s26-s33.
10. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
11. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):104-117.
12. Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106-2131.
13. Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000;49(9):1239-1242.
14. Zarotsky V, Huang M-Y, Carman W, et al. Systematic literature review of the epidemiology of nongenetic forms of hypogonadism in adult males. J Hormones. 2014;2014:190347.
15. Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60(7):762-769.
16. Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368(5):436-445.
17. Wu FC, Tajar A, Pye SR, et al; European Male Aging Study Group. Hypothalamicpituitary- testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
18. MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16(3):293-311.
19. Dandona P, Dhindsa S. Update: hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab. 2011;96(9):2643-2651.
20. Hofstra J, Loves S, van Wageningen B, Ruinemans-Koerts J, Jansen I, de Boer H. High prevalence of hypogonadotropic hypogonadism in men referred for obesity treatment. Neth J Med. 2008;66(3):103-109.
21. Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006;91(3):843-850.
22. Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93(1):68-75.
23. Orwoll ES, Klein RF. Osteoporosis in men. Endocrine Rev. 1995;16(1):87-116.
24. Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85(5):1319-1340.
25. Mah PM, Wittert GA. Obesity and testicular function. Mol Cell Endocrinol. 2010;316(2):180-186.
26. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
27. Morgentaler A, Miner MM, Caliber M, Guay AT, Khera M, Traish AM. Testosterone
therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc. 2015;90(2):224-251.
28. Snyder PJ, Bhasin S, Cunningham GR, et al; Testosterone Trials Investigators. Effects
of testosterone treatment in older men. N Engl J Med. 2016;374(7):611-624.
29. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA evaluating risk of stroke, heart attack and death with FDA-approved testosterone products. U.S. Food and Drug Administration website. http://www.fda.gov/Drugs/DrugSafety/ucm436259.htm. Updated January 14, 2016. Accessed April 12, 2016.
30. Handelsman DJ. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548-551.
31. AndroGel [package insert]. North Chicago, IL: AbbVie; 2015.
32. Depo-Testosterone [package insert]. New York, NY: Pfizer; 2015.
33. Haider A, Zitzmann M, Doros G, Isbarn H, Hammerer P, Yassin A. Incidence of prostate cancer in hypogonadal men receiving testosterone therapy: observations from 5-year median followup of 3 registries. J Urol. 2015;193(1):80-86.
34. Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22(4):232-240.
35. Gomella LG. Effective testosterone suppression for prostate cancer: is there a best castration therapy? Rev Urol. 2009;11(2):52-60.
36. Khera M, Crawford D, Morales A, Salonia A, Morgentaler A. A new era of testosterone and prostate cancer: from physiology to clinical implications. Eur Urol. 2014;65(1):115-123.
37. Morgentaler A. Testosterone and prostate cancer: an historical perspective on a modern myth. Eur Urol. 2006;50(5):935-939.
38. Morgentaler A, Rhoden EL. Prevalence of prostate cancer among hypogonadal men with prostate-specific antigen levels of 4.0 ng/mL or less. Urology. 2006;68(6):1263-1267.
39. Schatzl G, Madersbacher S, Thurridl T, et al. High-grade prostate cancer is associated with low serum testosterone levels. Prostate. 2001;47(1):52-58.
40. Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol. 2009;55(2):310-320.
41. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109-122.
42. Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63(5):800-809.
43. Buschemeyer WC 3rd, Freedland SJ. Obesity and prostate cancer: epidemiology and clinical implications. Eur Urol. 2007;52(2):331-343.
Use of Fluorodeoxyglucose-Positron Emission Tomography in the Diagnosis of Intravascular Diffuse Large B-Cell Lymphoma
Patient 1
A white man aged 67 years, with diastolic heart failure and chronic obstructive pulmonary disease, presented to the emergency department (ED) with shortness of breath. The initial laboratory results were significant for a newly elevated creatinine level of 2.06 mg/dL and a brain natriuretic peptide level of 648 pg/mL.
Imaging studies included a chest radiograph, a ventilation/perfusion scan, and an echocardiogram, as well as a right heart catheterization. All were nondiagnostic.
The patient's shortness of breath persisted despite treatment with diuretics, antibiotics, and steroids. Further laboratory workup revealed an elevated lactate dehydrogenase (LDH) level of 1,338 IU/L. A bone marrow biopsy performed because of concern about malignancy was unremarkable. Flow cytometry of the bone marrow aspirate did not reveal clonal B- or T-cell populations. Immunohistochemical staining was not performed. During this hospitalization for shortness of breath, the patient's mental staus began to decline, and his oxygen requirements increased. The patient was intubated but expired 48 hours after mechanical ventilation was initiated.
Patient 2
A white woman aged 67 years presented to the ED with generalized weakness, fatigue, and nausea. The patient’s medical history was significant for a diagnosis of stage IIIa ovarian cancer. She was treated with surgical resection and completed 6 cycles of adjuvant carboplatin and paclitaxel 3 months prior to this presentation. She had good response to treatment with normalization of CA-125.
After completion of chemotherapy, the patient was found to have persistent anemia and thrombocytopenia. Admission laboratory results were significant for a hemoglobin level of 8.4 g/dL, a platelet count of 20,000/μL, and an LDH level of 1,220 IU/L.
Chest, abdomen, and pelvis CT scans showed mesenteric adenopathy and splenomegaly (Figures 3A, 3B, and 3C) compared with prior imaging. Bone marrow biopsy revealed large lymphoid cells with scant cytoplasm and irregular nuclei, primarily within blood vessels and sinusoids consistent with IVLBCL (Figure 4). Flow cytometry of the bone marrow specimen showed an abnormal B-cell population with expression CD20, CD19, FMC-7, and dim κ light chain restriction. The cells were negative for CD5 and CD10. Immunohistochemical staining was positive for CD20, CD79a, PAX5, BCL-2 , and MUM1.
The patient was treated with 4 cycles of cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab, plus intrathecal methotrexate. The chemotherapy dose was reduced in the final cycle because of neuropathy in the hands and feet. The patient had undergone autologous stem-cell transplantation to allow high-dose chemotherapy. She was doing well more than 5 months after her transplant without evidence of recurrent disease.
Patient 3
A white man aged 76 years presented to the ED with cutaneous nodules, weight loss, fatigue, fevers, and epigastric pain. The patient’s medical history was significant for asymptomatic lymphoplasmacytic lymphoma diagnosed 2 months earlier, which had not required treatment. Laboratory results on admission revealed transaminitis, mild anemia with a hemoglobin level of 11 g/dL, and LDH level of 497 IU/L.
Chest, abdomen, and pelvis CT scans showed a 1.7cm hepatic lesion and mesenteric adenopathy. A bone marrow biopsy was unchanged from prior studies and showed minimal involvement (5%) of marrow space by low grade B-cell lymphoma.
Fluorodeoxyglucose-positron emission tomography (FDG-PET) scans showed multiple areas of uptake in the neck, chest, abdomen, and pelvis (Figures 5 and 6). No increased uptake in the subcutaneous nodules was noted on examination. Laparoscopic biopsy of FDG-avid mesenteric nodes showed clusters of atypical large lymphoid cells resulting in distention of the vascular lumina, resulting in the diagnosis of IVLBCL (Figure 7).
Immunohistochemical stains showed that the intravascular lymphocytes were strongly positive for CD20 and BCL-2 and negative for CD5 and CD10. Flow cytometry on the sample was limited by a low cell count and could not be assessed for clonality. The patient completed 6 cycles of rituximab as well as intrathecal methotrexate. Restaging studies showed a complete remission.
Two months later, the patient developed a skin nodule on the right shoulder. A repeat FDG-PET scan showed increased uptake, and fine-needle biopsy confirmed recurrent disease. The patient is undergoing treatment with ifosfamide, carboplatin, etoposide, and rituximab, as well as workup for autologous stem-cell transplant.
Discussion
Intravascular large B-cell lymphoma, a subtype of diffuse large B-cell lymphoma, is unique because it is primarily extranodal and typically without significant tumor burden.1-4 Standard imaging modalities, therefore, are often nonspecific and do not aid clinicians in establishing a diagnosis. Fluorodeoxyglucose-positron emission tomography has a known role in the assessment of diffuse large B-cell lymphoma, both at time of diagnosis and in monitoring response to treatment.5 However, the use of FDGPET in the diagnosis and management of IVLBCL has not been clearly established.
In a review of the literature, 26 English-language case reports and small case series reporting individual centers’ experience with the use of this imaging modality in the diagnosis of IVLBCL were identified. Two cases were eliminated from review because they did not discuss the use of FDG-PET in relationship to diagnosis. Of the remaining 24 cases, 21 underwent initial imaging with 1 or more of the following imaging modalities: CT, magnetic resonance, ultrasound, bone scan, and gallium scintigraphy, all of which were nonspecific and did not lead to a definitive diagnosis.3,6-25 Each of the 21 cases was followed up by FDG-PET; in 19, the FDG-PET scan was positive and resulted in a diagnosis of IVLBCL. In 2 cases, the FDG-PET scan was nonrevealing and was not considered helpful in diagnosis.11,18 In 3 of the 21 cases, the FDG-PET scan was the primary imaging modality.6,14,25
In this review, all 3 patients had initial imaging with CT scans of anatomic locations that were largely unrevealing, although later histologic examination showed them to be locations of active disease either by biopsy or on autopsy. One patient who underwent early FDG-PET was found to have increased uptake in the mesenteric lymph nodes, which were later biopsied, as well as uptake in the bilateral adrenal glands, lungs, and bone.
Several characteristic FDG-PET findings that have been described in the literature have been identified in patients with IVLBCL, including diffuse accumulation in bilateral lung fields, accumulation in the renal cortex or adrenal glands, diffuse bony involvement, and hypometabolism in the brain.7,10,12,13,17,23,25 These findings show that organs with the richest blood supply, specifically the lungs and kidneys, often are affected. The brain, an obligate glucose metabolizer, would be expected to have high uptake; however, with tumor thrombi occluding small intracranial vessels, micro infarcts ensue and are evidenced by areas of low uptake on FDG-PET scans in patients with IVLBCL.7 These characteristic patterns seen on FDG-PET scans can help to support a diagnosis of IVLBCL when clinical suspicion is high. Further, clinicians may be able to use imaging results to guide an appropriate site for biopsy to confirm diagnosis.
Conclusion
Intravascular large B-cell lymphoma remains a diagnostic challenge for clinicians. Prognosis is generally poor and likely related to frequent delays in diagnosis.1 Clinicians continue to work toward improving their ability to diagnose this disease in its early stages. New diagnostic algorithms and the use of random skin biopsies have shown some promise in improving diagnostic efficiency.26-28 Based on the authors’ experience and review of the literature, FDG-PET may be another promising tool to aid early diagnosis. Characteristic FDG-PET findings have been well described and may help to support the diagnosis of IVLBCL and guide an appropriate biopsy site when clinical suspicion for IVLBCL exists.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Patient 1
A white man aged 67 years, with diastolic heart failure and chronic obstructive pulmonary disease, presented to the emergency department (ED) with shortness of breath. The initial laboratory results were significant for a newly elevated creatinine level of 2.06 mg/dL and a brain natriuretic peptide level of 648 pg/mL.
Imaging studies included a chest radiograph, a ventilation/perfusion scan, and an echocardiogram, as well as a right heart catheterization. All were nondiagnostic.
The patient's shortness of breath persisted despite treatment with diuretics, antibiotics, and steroids. Further laboratory workup revealed an elevated lactate dehydrogenase (LDH) level of 1,338 IU/L. A bone marrow biopsy performed because of concern about malignancy was unremarkable. Flow cytometry of the bone marrow aspirate did not reveal clonal B- or T-cell populations. Immunohistochemical staining was not performed. During this hospitalization for shortness of breath, the patient's mental staus began to decline, and his oxygen requirements increased. The patient was intubated but expired 48 hours after mechanical ventilation was initiated.
Patient 2
A white woman aged 67 years presented to the ED with generalized weakness, fatigue, and nausea. The patient’s medical history was significant for a diagnosis of stage IIIa ovarian cancer. She was treated with surgical resection and completed 6 cycles of adjuvant carboplatin and paclitaxel 3 months prior to this presentation. She had good response to treatment with normalization of CA-125.
After completion of chemotherapy, the patient was found to have persistent anemia and thrombocytopenia. Admission laboratory results were significant for a hemoglobin level of 8.4 g/dL, a platelet count of 20,000/μL, and an LDH level of 1,220 IU/L.
Chest, abdomen, and pelvis CT scans showed mesenteric adenopathy and splenomegaly (Figures 3A, 3B, and 3C) compared with prior imaging. Bone marrow biopsy revealed large lymphoid cells with scant cytoplasm and irregular nuclei, primarily within blood vessels and sinusoids consistent with IVLBCL (Figure 4). Flow cytometry of the bone marrow specimen showed an abnormal B-cell population with expression CD20, CD19, FMC-7, and dim κ light chain restriction. The cells were negative for CD5 and CD10. Immunohistochemical staining was positive for CD20, CD79a, PAX5, BCL-2 , and MUM1.
The patient was treated with 4 cycles of cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab, plus intrathecal methotrexate. The chemotherapy dose was reduced in the final cycle because of neuropathy in the hands and feet. The patient had undergone autologous stem-cell transplantation to allow high-dose chemotherapy. She was doing well more than 5 months after her transplant without evidence of recurrent disease.
Patient 3
A white man aged 76 years presented to the ED with cutaneous nodules, weight loss, fatigue, fevers, and epigastric pain. The patient’s medical history was significant for asymptomatic lymphoplasmacytic lymphoma diagnosed 2 months earlier, which had not required treatment. Laboratory results on admission revealed transaminitis, mild anemia with a hemoglobin level of 11 g/dL, and LDH level of 497 IU/L.
Chest, abdomen, and pelvis CT scans showed a 1.7cm hepatic lesion and mesenteric adenopathy. A bone marrow biopsy was unchanged from prior studies and showed minimal involvement (5%) of marrow space by low grade B-cell lymphoma.
Fluorodeoxyglucose-positron emission tomography (FDG-PET) scans showed multiple areas of uptake in the neck, chest, abdomen, and pelvis (Figures 5 and 6). No increased uptake in the subcutaneous nodules was noted on examination. Laparoscopic biopsy of FDG-avid mesenteric nodes showed clusters of atypical large lymphoid cells resulting in distention of the vascular lumina, resulting in the diagnosis of IVLBCL (Figure 7).
Immunohistochemical stains showed that the intravascular lymphocytes were strongly positive for CD20 and BCL-2 and negative for CD5 and CD10. Flow cytometry on the sample was limited by a low cell count and could not be assessed for clonality. The patient completed 6 cycles of rituximab as well as intrathecal methotrexate. Restaging studies showed a complete remission.
Two months later, the patient developed a skin nodule on the right shoulder. A repeat FDG-PET scan showed increased uptake, and fine-needle biopsy confirmed recurrent disease. The patient is undergoing treatment with ifosfamide, carboplatin, etoposide, and rituximab, as well as workup for autologous stem-cell transplant.
Discussion
Intravascular large B-cell lymphoma, a subtype of diffuse large B-cell lymphoma, is unique because it is primarily extranodal and typically without significant tumor burden.1-4 Standard imaging modalities, therefore, are often nonspecific and do not aid clinicians in establishing a diagnosis. Fluorodeoxyglucose-positron emission tomography has a known role in the assessment of diffuse large B-cell lymphoma, both at time of diagnosis and in monitoring response to treatment.5 However, the use of FDGPET in the diagnosis and management of IVLBCL has not been clearly established.
In a review of the literature, 26 English-language case reports and small case series reporting individual centers’ experience with the use of this imaging modality in the diagnosis of IVLBCL were identified. Two cases were eliminated from review because they did not discuss the use of FDG-PET in relationship to diagnosis. Of the remaining 24 cases, 21 underwent initial imaging with 1 or more of the following imaging modalities: CT, magnetic resonance, ultrasound, bone scan, and gallium scintigraphy, all of which were nonspecific and did not lead to a definitive diagnosis.3,6-25 Each of the 21 cases was followed up by FDG-PET; in 19, the FDG-PET scan was positive and resulted in a diagnosis of IVLBCL. In 2 cases, the FDG-PET scan was nonrevealing and was not considered helpful in diagnosis.11,18 In 3 of the 21 cases, the FDG-PET scan was the primary imaging modality.6,14,25
In this review, all 3 patients had initial imaging with CT scans of anatomic locations that were largely unrevealing, although later histologic examination showed them to be locations of active disease either by biopsy or on autopsy. One patient who underwent early FDG-PET was found to have increased uptake in the mesenteric lymph nodes, which were later biopsied, as well as uptake in the bilateral adrenal glands, lungs, and bone.
Several characteristic FDG-PET findings that have been described in the literature have been identified in patients with IVLBCL, including diffuse accumulation in bilateral lung fields, accumulation in the renal cortex or adrenal glands, diffuse bony involvement, and hypometabolism in the brain.7,10,12,13,17,23,25 These findings show that organs with the richest blood supply, specifically the lungs and kidneys, often are affected. The brain, an obligate glucose metabolizer, would be expected to have high uptake; however, with tumor thrombi occluding small intracranial vessels, micro infarcts ensue and are evidenced by areas of low uptake on FDG-PET scans in patients with IVLBCL.7 These characteristic patterns seen on FDG-PET scans can help to support a diagnosis of IVLBCL when clinical suspicion is high. Further, clinicians may be able to use imaging results to guide an appropriate site for biopsy to confirm diagnosis.
Conclusion
Intravascular large B-cell lymphoma remains a diagnostic challenge for clinicians. Prognosis is generally poor and likely related to frequent delays in diagnosis.1 Clinicians continue to work toward improving their ability to diagnose this disease in its early stages. New diagnostic algorithms and the use of random skin biopsies have shown some promise in improving diagnostic efficiency.26-28 Based on the authors’ experience and review of the literature, FDG-PET may be another promising tool to aid early diagnosis. Characteristic FDG-PET findings have been well described and may help to support the diagnosis of IVLBCL and guide an appropriate biopsy site when clinical suspicion for IVLBCL exists.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Patient 1
A white man aged 67 years, with diastolic heart failure and chronic obstructive pulmonary disease, presented to the emergency department (ED) with shortness of breath. The initial laboratory results were significant for a newly elevated creatinine level of 2.06 mg/dL and a brain natriuretic peptide level of 648 pg/mL.
Imaging studies included a chest radiograph, a ventilation/perfusion scan, and an echocardiogram, as well as a right heart catheterization. All were nondiagnostic.
The patient's shortness of breath persisted despite treatment with diuretics, antibiotics, and steroids. Further laboratory workup revealed an elevated lactate dehydrogenase (LDH) level of 1,338 IU/L. A bone marrow biopsy performed because of concern about malignancy was unremarkable. Flow cytometry of the bone marrow aspirate did not reveal clonal B- or T-cell populations. Immunohistochemical staining was not performed. During this hospitalization for shortness of breath, the patient's mental staus began to decline, and his oxygen requirements increased. The patient was intubated but expired 48 hours after mechanical ventilation was initiated.
Patient 2
A white woman aged 67 years presented to the ED with generalized weakness, fatigue, and nausea. The patient’s medical history was significant for a diagnosis of stage IIIa ovarian cancer. She was treated with surgical resection and completed 6 cycles of adjuvant carboplatin and paclitaxel 3 months prior to this presentation. She had good response to treatment with normalization of CA-125.
After completion of chemotherapy, the patient was found to have persistent anemia and thrombocytopenia. Admission laboratory results were significant for a hemoglobin level of 8.4 g/dL, a platelet count of 20,000/μL, and an LDH level of 1,220 IU/L.
Chest, abdomen, and pelvis CT scans showed mesenteric adenopathy and splenomegaly (Figures 3A, 3B, and 3C) compared with prior imaging. Bone marrow biopsy revealed large lymphoid cells with scant cytoplasm and irregular nuclei, primarily within blood vessels and sinusoids consistent with IVLBCL (Figure 4). Flow cytometry of the bone marrow specimen showed an abnormal B-cell population with expression CD20, CD19, FMC-7, and dim κ light chain restriction. The cells were negative for CD5 and CD10. Immunohistochemical staining was positive for CD20, CD79a, PAX5, BCL-2 , and MUM1.
The patient was treated with 4 cycles of cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab, plus intrathecal methotrexate. The chemotherapy dose was reduced in the final cycle because of neuropathy in the hands and feet. The patient had undergone autologous stem-cell transplantation to allow high-dose chemotherapy. She was doing well more than 5 months after her transplant without evidence of recurrent disease.
Patient 3
A white man aged 76 years presented to the ED with cutaneous nodules, weight loss, fatigue, fevers, and epigastric pain. The patient’s medical history was significant for asymptomatic lymphoplasmacytic lymphoma diagnosed 2 months earlier, which had not required treatment. Laboratory results on admission revealed transaminitis, mild anemia with a hemoglobin level of 11 g/dL, and LDH level of 497 IU/L.
Chest, abdomen, and pelvis CT scans showed a 1.7cm hepatic lesion and mesenteric adenopathy. A bone marrow biopsy was unchanged from prior studies and showed minimal involvement (5%) of marrow space by low grade B-cell lymphoma.
Fluorodeoxyglucose-positron emission tomography (FDG-PET) scans showed multiple areas of uptake in the neck, chest, abdomen, and pelvis (Figures 5 and 6). No increased uptake in the subcutaneous nodules was noted on examination. Laparoscopic biopsy of FDG-avid mesenteric nodes showed clusters of atypical large lymphoid cells resulting in distention of the vascular lumina, resulting in the diagnosis of IVLBCL (Figure 7).
Immunohistochemical stains showed that the intravascular lymphocytes were strongly positive for CD20 and BCL-2 and negative for CD5 and CD10. Flow cytometry on the sample was limited by a low cell count and could not be assessed for clonality. The patient completed 6 cycles of rituximab as well as intrathecal methotrexate. Restaging studies showed a complete remission.
Two months later, the patient developed a skin nodule on the right shoulder. A repeat FDG-PET scan showed increased uptake, and fine-needle biopsy confirmed recurrent disease. The patient is undergoing treatment with ifosfamide, carboplatin, etoposide, and rituximab, as well as workup for autologous stem-cell transplant.
Discussion
Intravascular large B-cell lymphoma, a subtype of diffuse large B-cell lymphoma, is unique because it is primarily extranodal and typically without significant tumor burden.1-4 Standard imaging modalities, therefore, are often nonspecific and do not aid clinicians in establishing a diagnosis. Fluorodeoxyglucose-positron emission tomography has a known role in the assessment of diffuse large B-cell lymphoma, both at time of diagnosis and in monitoring response to treatment.5 However, the use of FDGPET in the diagnosis and management of IVLBCL has not been clearly established.
In a review of the literature, 26 English-language case reports and small case series reporting individual centers’ experience with the use of this imaging modality in the diagnosis of IVLBCL were identified. Two cases were eliminated from review because they did not discuss the use of FDG-PET in relationship to diagnosis. Of the remaining 24 cases, 21 underwent initial imaging with 1 or more of the following imaging modalities: CT, magnetic resonance, ultrasound, bone scan, and gallium scintigraphy, all of which were nonspecific and did not lead to a definitive diagnosis.3,6-25 Each of the 21 cases was followed up by FDG-PET; in 19, the FDG-PET scan was positive and resulted in a diagnosis of IVLBCL. In 2 cases, the FDG-PET scan was nonrevealing and was not considered helpful in diagnosis.11,18 In 3 of the 21 cases, the FDG-PET scan was the primary imaging modality.6,14,25
In this review, all 3 patients had initial imaging with CT scans of anatomic locations that were largely unrevealing, although later histologic examination showed them to be locations of active disease either by biopsy or on autopsy. One patient who underwent early FDG-PET was found to have increased uptake in the mesenteric lymph nodes, which were later biopsied, as well as uptake in the bilateral adrenal glands, lungs, and bone.
Several characteristic FDG-PET findings that have been described in the literature have been identified in patients with IVLBCL, including diffuse accumulation in bilateral lung fields, accumulation in the renal cortex or adrenal glands, diffuse bony involvement, and hypometabolism in the brain.7,10,12,13,17,23,25 These findings show that organs with the richest blood supply, specifically the lungs and kidneys, often are affected. The brain, an obligate glucose metabolizer, would be expected to have high uptake; however, with tumor thrombi occluding small intracranial vessels, micro infarcts ensue and are evidenced by areas of low uptake on FDG-PET scans in patients with IVLBCL.7 These characteristic patterns seen on FDG-PET scans can help to support a diagnosis of IVLBCL when clinical suspicion is high. Further, clinicians may be able to use imaging results to guide an appropriate site for biopsy to confirm diagnosis.
Conclusion
Intravascular large B-cell lymphoma remains a diagnostic challenge for clinicians. Prognosis is generally poor and likely related to frequent delays in diagnosis.1 Clinicians continue to work toward improving their ability to diagnose this disease in its early stages. New diagnostic algorithms and the use of random skin biopsies have shown some promise in improving diagnostic efficiency.26-28 Based on the authors’ experience and review of the literature, FDG-PET may be another promising tool to aid early diagnosis. Characteristic FDG-PET findings have been well described and may help to support the diagnosis of IVLBCL and guide an appropriate biopsy site when clinical suspicion for IVLBCL exists.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
γ-δ T-Cell Lymphoma With Disseminated Intravascular Coagulation and Autoimmune Hemolytic Anemia
Gamma-delta (γ-δ) T-cell lymphomas (GDTCL) are rare and aggressive cancers with specific morphologic, phenotypic, and functional properties. When discovered in 1984, the T-cell receptor (TCR) was characterized as an alpha-beta (α-β) heterodimer. The γ-δ heterodimer was discovered later, when a third rearranging gene was recognized.1
Gaulard and colleagues described the first case of peripheral neoplasm with the γ-δ TCR.2 Now the present authors report the case of a patient with an autoimmune hemolytic anemia (AIHA) with both cold and warm antibodies—an atypical presentation of this rare form of TCL. Such a case has not been previously reported.
Clinical History
A 77-year-old woman with a past medical history of osteoarthritis, gout, mitral stenosis, bioprosthetic aortic valve replacement, and obesity presented to the emergency department (ED) reporting progressive weakness, confusion, and jaundice. She had been recently discharged from
another hospital after an 18-day stay for gangrenous cholecystitis and shingles. Her home medications were metronidazole and acyclovir. In the ED, she was febrile at 100.5°. Laboratory test results revealed anemia with a hemoglobin level of 50 g/L (83 g/L in clinic 2 weeks earlier) and neutropenia with an absolute neutrophilic count of 500 cells/μL (normal range 1,520-6,370 cells/μL). She also was thrombocytopenic with a platelet count of 71x109/L (normal range 150-450×109/L).
On admission, the hematology service was consulted for pancytopenia. The pertinent workup included a lactate dehydrogenase level of 31.16 μkat/L (normal range 1.7-3.4 μkat/L), a haptoglobin level of < 1,500 mg/L (normal range 260-1,850 mg/L), and a direct bilirubin level of 13.68 μmol/L (normal range 1.7-5.1 μmol/L). A peripheral blood smear was negative for schistocytes. Fibrin split products were 40 mg/L (normal < 10 mg/L), fibrinogen level was 6.94 μmol/L (normal range 5.8-11.8 μmol/L), prothrombin time was 14.6 seconds (normal range 10-14 sec), and international normalized ratio was 1.3 (normal < 1). The concomitant decrease in fibrinogen level and increase in fibrin split product titers were consistent with the diagnosis of acute disseminated intravascular coagulation. Iron studies were consistent with anemia of chronic disease (low reticulocyte count of 0.4%) and vitamin B12 deficiency (level 195). Coombs test results were positive for both cold and warm antibodies, with cold being more prominent. Abdominal ultrasonography revealed hepatosplenomegaly (HSM).
The patient was diagnosed with AIHA with no initial obvious underlying etiology. The differential diagnosis included autoimmune disorder, lymphoproliferative disease, and drug-induced process. She also was diagnosed with sepsis, which was thought to be contributing to the pancytopenia.
Broad-spectrum antibiotics (cefepime, metronidazole) and vitamin B12 supplements were started. After a blood transfusion, the patient developed fever and hypoxia, which required transfer to the medical intensive care unit. The differentials at this time included a transfusion reaction and/or transfusion-associated circulatory overload. Intravenous immunoglobulin was started at 1 g/kg to help with cold agglutinins. Prednisone 1 mg/kg was started as well. Peripheral blood flow cytometry results were positive for an abnormal T-cell population likely consistent with T-cell lineage lymphoma. Bone marrow biopsy results were consistent with GDTCL. Computed tomography (CT) of chest/abdomen/pelvis showed bilateral lung nodules < 1 cm, HSM with multiple spleen infarcts, and a 4.7-cm right adnexal soft-tissue lesion. Liver biopsy results were consistent with GDTCL. Results of a workup for cytomegalovirus and Epstein-Barr virus were negative, as was a mycoplasma screen. The patient was diagnosed with GDTCL with hepatic involvement, and CHOP (cyclophosphamide, hydroxydaunorubicin [doxorubicin], Oncovin [vincristine], prednisone) therapy was started.
Discussion
Peripheral TCL (PTCL) are a rare, typically extranodal group of malignancies. They are aggressive and generally have a poor outcome, with most patients dying of lymphoma within 2 years.3 T-cell lymphomas most commonly express the γ-δ TCR. About 2% to 4% of TCLs express the γ-δ TCR.4 In 2008, the World Health Organization recognized 2 distinct GDTCL subgroups: hepatosplenic GDTCL (HSGDTCL) and primary cutaneous GDTCL.5 As the patient presented with hepatic involvement, this discussion focused on HSGDTCL.
Hepatosplenic GDTCL are rare types of PTCL. First described as a separate TCL subgroup in the 1990 REAL (Revised European-American Lymphoma) classification,6 they are estimated to represent about 1.4% of all TCL, with about 100 cases reported in the literature.4
The GDTCL cells tend to live in mucosa, lymphoid tissue, epithelial-rich tissues (skin, gastrointestinal tract), and red pulp of spleen.7 They develop from thymic precursors in bone marrow and are CD4-/CD8- and thus known as double negative cells.8 They mimic natural killer cells, behave as cytotoxic cells, and are capable of TCR rearrangement as well as phagocytosis.9
Hepatosplenic GDTCL are usually phenotypically CD2+, CD3+, CD4-, CD5-, CD7+, CD8-, and TCR γ-δ+.10 They are rarely associated with Epstein-Barr virus infection; reported cases seem more common in Asia.11 Peak incidence is in young men (median age 20-25 years; male:female ratio 10:1). At-risk populations include the chronically immunosuppressed, including solid organ transplanted patients and patients under prolonged antigenic stimulation.12
The most common clinical features of HSGDTCL include B symptoms (fever of unknown origin, night sweats, loss of > 10% of body weight), marked HSM, and lack of lymphadenopathy. Patients often present with fever, weakness, and abdominal pain. Laboratory test results
typically show abnormal liver function and abnormal lactate dehydrogenase levels. Bone marrow is almost always involved, with possible trilineage cytopenia. Anemia and thrombocytopenia are reported in 75% and 85% of cases, respectively.13
Warm (70%) and cold auto-antibodies are the 2 classifications of AIHA.14 The AIHA can be primary, idiopathic, or a manifestation of underlying disease conditions, including non-Hodgkin lymphomas, systemic autoimmune diseases, chronic infections, postorgan transplantation, and solid tumors. It has also been reported as a complication of treatment with nucleoside analogues.15
Lacking specific symptoms, HSGDTCL is usually diagnosed late. The diagnosis should be suspected in young men who present with the aforementioned symptoms. However, not everyone with HSGDTCL falls in that group—the present patient was a 77-year-old woman.
Hepatosplenic GDTCL staging is similar to staging of other non-Hodgkin lymphomas. Total-body CT with contrast, bone marrow aspiration/biopsy, and direct lesion biopsy are required. Although positron emission tomography is generally thought to be as useful in TCL as in B-cell lymphomas, there is not enough evidence to support its use specifically in HSGDTCL.16 The staging classification follows the Ann Arbor system, with the majority of cases classified as stage IV.
Hepatosplenic GDTCL are aggressive tumors with a strong tendency to rapidly progress, and they are highly resistant to primary chemotherapy agents. Remission is rarely complete with use of conventional chemotherapy agents. Most patients die of the disease within 2 years of
diagnosis.12 Although the rarity of HSGDTCL has made it difficult to identify any clear prognostic factors, a correlation between thrombocytopenia severity and disease progression has been found in many studies.17 There is no standard treatment regimen. Proposed therapies
include splenectomy (for diagnosis or thrombocytopenia management), corticosteroids, alkylating agents, purine analogue, anthracycline-containing regimens, and cytarabine/cisplatin combinations. The anthracycline-based regimen most commonly used as first-line therapy is CHOP, or CHOP derivatives, with complete remission rates between 30% and 45%. However, long-term results remain disappointing (median relapse time 4 months).10 In 3 reviews, median survival was 16 months, 11 months, and 9.5 months.10,17,18 In the International T-Cell Lymphoma Project study, the 5-year failure-free survival rate was 0%, and the overall survival rate was 7%.4 In these studies, the majority of patients received some variation of CHOP-based therapy, and although positive responses were appreciated in many of the cases, they were generally short-lived.
These results have been disappointing, and other modalities have been tried—including high-dose cytarabine regimens, 2'-deoxycoformycin (pentostatin), and anti-CD52 monoclonal antibodies (alemtuzumab).19 In an HSGDTCL study, 2 of 21 patients treated with platinum/cytarabine-based induction regimens were still in remission at 42 and 52 months.17 Another study examined a variety of induction regimens used to treat HSGDTCL in 15 patients.18 Responses tended to be more durable in patients who received a dose-intense Hyper-CVIDDoxil regimen (fractionated cyclophosphamide, liposomal doxorubicin, vincristine, dexamethasone) alternated with methotrexate and cytarabine. Complete response was 50%, and median duration of complete response was 8 months. Over the past 10 years, a few case reports have described successful treatment with autologous or allogeneic stem cell transplantation.20
Conclusion
The present case represents a unique HSGDTCL presentation. To the authors’ knowledge, this is the first report of HSGDTCL presenting with acute disseminated intravascular coagulation and AIHA with both cold and warm antibodies.
Hepatosplenic GDTCL is a rare, novel disease. To understand more about this pathology, investigators need to better characterize the disease process and the manifestations. The hope is that more information will contribute to the development of more effective therapies. The unique presentation reported here may help in further characterizing and understanding this uncommon disease.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. Saito H, Kranz DM, Takagaki Y, Hayday AC, Eisen HN, Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984;312(5989):36-40.
2. Gaulard P, Zafrani ES, Mavier P, et al. Peripheral T-cell lymphoma presenting as predominant liver disease: a report of three cases. Hepatology. 1986;6(5):864-868.
3. Gaulard P, de Leval L. Pathology of peripheral T-cell lymphomas: where do we stand? Semin Hematol. 2014;51(1):5-16.
4. Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-4130.
5. The International Agency for Research on Cancer. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Vol 2. 4th ed. Lyon, France: IARC Press; 2008.
6. Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasm: a proposal from the International Lymphoma Study Group. Blood. 1994;84(5):1361-1392.
7. Farcet JP, Gaulard P, Marolleau JP, et al. Hepatosplenic T-cell lymphoma: sinusal/sinusoidal localization of malignant cells expressing the T-cell receptor gamma delta. Blood. 1990;75(11):2213-2219.
8. Bluestone JA, Khattri R, Sciammas R, Sperling AI. TCR gamma delta cells: a specialized T-cell subset in the immune system. Annu Rev Cell Dev Biol. 1995;11:307-353.
9. Holtmeier W, Kabelitz D. Gamma delta T cells link innate and adaptive immune responses. Chem Immunol Allergy. 2005;86:151-183.
10. Weidmann E. Hepatosplenic T cell lymphoma. A review on 45 cases since the first report describing the disease as a distinct lymphoma entity in 1990. Leukemia. 2000;14(6):991-997.
11. Yu WW, Hsieh PP, Chuang SS. Cutaneous EBV-positive γδ T-cell lymphoma vs. extranodal NK/T-cell lymphoma: a case report and literature review. J Cutan Pathol. 2013;40(3):310-316.
12. Tripodo C, Iannitto E, Florena AM, et al. Gamma-delta T-cell lymphomas. Nat Rev Clin Oncol. 2009;6(12):707-717.
13. Foppoli M, Ferreri AJM. Gamma-delta T-cell lymphomas. Eur J Haematol. 2015;94(3):206-218.
14. Hoffbrand AV, Catovsky D, Tuddenham EGD, Green AR, eds. Postgraduate Haematology. 6th ed. Oxford, England: Wiley-Blackwell; 2011.
15. Valent P, Lechner K. Diagnosis and treatment of autoimmune haemolytic anaemias in adults: a clinical review. Wien Klin Wochenschr. 2008;120(5-6):136-151.
16. Khong PL, Pang CB, Liang R, Kwong YL, Au WY. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008;87(8):613-621.
17. Belhadj K, Reyes F, Farcet JP, et al. Hepatosplenic gammadelta T-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood. 2003;102(13):4261-4269.
18. Falchook GS, Vega F, Dang NH, et al. Hepatosplenic gamma-delta T-cell lymphoma: clinicopathological features and treatment. Ann Oncol. 2009;20(6):1080-1085.
19. Konuma T, Ooi J, Takahashi S, et al. Allogeneic stem cell transplantation for hepatosplenic
gammadelta T-cell lymphoma. Leuk Lymphoma. 2007;48(3):630-632.
20. Ferreri AJ, Govi S, Pileri SA. Hepatosplenic gamma-delta T-cell lymphoma. Crit
Rev Oncol Hematol. 2012;83(2):283-292.
Gamma-delta (γ-δ) T-cell lymphomas (GDTCL) are rare and aggressive cancers with specific morphologic, phenotypic, and functional properties. When discovered in 1984, the T-cell receptor (TCR) was characterized as an alpha-beta (α-β) heterodimer. The γ-δ heterodimer was discovered later, when a third rearranging gene was recognized.1
Gaulard and colleagues described the first case of peripheral neoplasm with the γ-δ TCR.2 Now the present authors report the case of a patient with an autoimmune hemolytic anemia (AIHA) with both cold and warm antibodies—an atypical presentation of this rare form of TCL. Such a case has not been previously reported.
Clinical History
A 77-year-old woman with a past medical history of osteoarthritis, gout, mitral stenosis, bioprosthetic aortic valve replacement, and obesity presented to the emergency department (ED) reporting progressive weakness, confusion, and jaundice. She had been recently discharged from
another hospital after an 18-day stay for gangrenous cholecystitis and shingles. Her home medications were metronidazole and acyclovir. In the ED, she was febrile at 100.5°. Laboratory test results revealed anemia with a hemoglobin level of 50 g/L (83 g/L in clinic 2 weeks earlier) and neutropenia with an absolute neutrophilic count of 500 cells/μL (normal range 1,520-6,370 cells/μL). She also was thrombocytopenic with a platelet count of 71x109/L (normal range 150-450×109/L).
On admission, the hematology service was consulted for pancytopenia. The pertinent workup included a lactate dehydrogenase level of 31.16 μkat/L (normal range 1.7-3.4 μkat/L), a haptoglobin level of < 1,500 mg/L (normal range 260-1,850 mg/L), and a direct bilirubin level of 13.68 μmol/L (normal range 1.7-5.1 μmol/L). A peripheral blood smear was negative for schistocytes. Fibrin split products were 40 mg/L (normal < 10 mg/L), fibrinogen level was 6.94 μmol/L (normal range 5.8-11.8 μmol/L), prothrombin time was 14.6 seconds (normal range 10-14 sec), and international normalized ratio was 1.3 (normal < 1). The concomitant decrease in fibrinogen level and increase in fibrin split product titers were consistent with the diagnosis of acute disseminated intravascular coagulation. Iron studies were consistent with anemia of chronic disease (low reticulocyte count of 0.4%) and vitamin B12 deficiency (level 195). Coombs test results were positive for both cold and warm antibodies, with cold being more prominent. Abdominal ultrasonography revealed hepatosplenomegaly (HSM).
The patient was diagnosed with AIHA with no initial obvious underlying etiology. The differential diagnosis included autoimmune disorder, lymphoproliferative disease, and drug-induced process. She also was diagnosed with sepsis, which was thought to be contributing to the pancytopenia.
Broad-spectrum antibiotics (cefepime, metronidazole) and vitamin B12 supplements were started. After a blood transfusion, the patient developed fever and hypoxia, which required transfer to the medical intensive care unit. The differentials at this time included a transfusion reaction and/or transfusion-associated circulatory overload. Intravenous immunoglobulin was started at 1 g/kg to help with cold agglutinins. Prednisone 1 mg/kg was started as well. Peripheral blood flow cytometry results were positive for an abnormal T-cell population likely consistent with T-cell lineage lymphoma. Bone marrow biopsy results were consistent with GDTCL. Computed tomography (CT) of chest/abdomen/pelvis showed bilateral lung nodules < 1 cm, HSM with multiple spleen infarcts, and a 4.7-cm right adnexal soft-tissue lesion. Liver biopsy results were consistent with GDTCL. Results of a workup for cytomegalovirus and Epstein-Barr virus were negative, as was a mycoplasma screen. The patient was diagnosed with GDTCL with hepatic involvement, and CHOP (cyclophosphamide, hydroxydaunorubicin [doxorubicin], Oncovin [vincristine], prednisone) therapy was started.
Discussion
Peripheral TCL (PTCL) are a rare, typically extranodal group of malignancies. They are aggressive and generally have a poor outcome, with most patients dying of lymphoma within 2 years.3 T-cell lymphomas most commonly express the γ-δ TCR. About 2% to 4% of TCLs express the γ-δ TCR.4 In 2008, the World Health Organization recognized 2 distinct GDTCL subgroups: hepatosplenic GDTCL (HSGDTCL) and primary cutaneous GDTCL.5 As the patient presented with hepatic involvement, this discussion focused on HSGDTCL.
Hepatosplenic GDTCL are rare types of PTCL. First described as a separate TCL subgroup in the 1990 REAL (Revised European-American Lymphoma) classification,6 they are estimated to represent about 1.4% of all TCL, with about 100 cases reported in the literature.4
The GDTCL cells tend to live in mucosa, lymphoid tissue, epithelial-rich tissues (skin, gastrointestinal tract), and red pulp of spleen.7 They develop from thymic precursors in bone marrow and are CD4-/CD8- and thus known as double negative cells.8 They mimic natural killer cells, behave as cytotoxic cells, and are capable of TCR rearrangement as well as phagocytosis.9
Hepatosplenic GDTCL are usually phenotypically CD2+, CD3+, CD4-, CD5-, CD7+, CD8-, and TCR γ-δ+.10 They are rarely associated with Epstein-Barr virus infection; reported cases seem more common in Asia.11 Peak incidence is in young men (median age 20-25 years; male:female ratio 10:1). At-risk populations include the chronically immunosuppressed, including solid organ transplanted patients and patients under prolonged antigenic stimulation.12
The most common clinical features of HSGDTCL include B symptoms (fever of unknown origin, night sweats, loss of > 10% of body weight), marked HSM, and lack of lymphadenopathy. Patients often present with fever, weakness, and abdominal pain. Laboratory test results
typically show abnormal liver function and abnormal lactate dehydrogenase levels. Bone marrow is almost always involved, with possible trilineage cytopenia. Anemia and thrombocytopenia are reported in 75% and 85% of cases, respectively.13
Warm (70%) and cold auto-antibodies are the 2 classifications of AIHA.14 The AIHA can be primary, idiopathic, or a manifestation of underlying disease conditions, including non-Hodgkin lymphomas, systemic autoimmune diseases, chronic infections, postorgan transplantation, and solid tumors. It has also been reported as a complication of treatment with nucleoside analogues.15
Lacking specific symptoms, HSGDTCL is usually diagnosed late. The diagnosis should be suspected in young men who present with the aforementioned symptoms. However, not everyone with HSGDTCL falls in that group—the present patient was a 77-year-old woman.
Hepatosplenic GDTCL staging is similar to staging of other non-Hodgkin lymphomas. Total-body CT with contrast, bone marrow aspiration/biopsy, and direct lesion biopsy are required. Although positron emission tomography is generally thought to be as useful in TCL as in B-cell lymphomas, there is not enough evidence to support its use specifically in HSGDTCL.16 The staging classification follows the Ann Arbor system, with the majority of cases classified as stage IV.
Hepatosplenic GDTCL are aggressive tumors with a strong tendency to rapidly progress, and they are highly resistant to primary chemotherapy agents. Remission is rarely complete with use of conventional chemotherapy agents. Most patients die of the disease within 2 years of
diagnosis.12 Although the rarity of HSGDTCL has made it difficult to identify any clear prognostic factors, a correlation between thrombocytopenia severity and disease progression has been found in many studies.17 There is no standard treatment regimen. Proposed therapies
include splenectomy (for diagnosis or thrombocytopenia management), corticosteroids, alkylating agents, purine analogue, anthracycline-containing regimens, and cytarabine/cisplatin combinations. The anthracycline-based regimen most commonly used as first-line therapy is CHOP, or CHOP derivatives, with complete remission rates between 30% and 45%. However, long-term results remain disappointing (median relapse time 4 months).10 In 3 reviews, median survival was 16 months, 11 months, and 9.5 months.10,17,18 In the International T-Cell Lymphoma Project study, the 5-year failure-free survival rate was 0%, and the overall survival rate was 7%.4 In these studies, the majority of patients received some variation of CHOP-based therapy, and although positive responses were appreciated in many of the cases, they were generally short-lived.
These results have been disappointing, and other modalities have been tried—including high-dose cytarabine regimens, 2'-deoxycoformycin (pentostatin), and anti-CD52 monoclonal antibodies (alemtuzumab).19 In an HSGDTCL study, 2 of 21 patients treated with platinum/cytarabine-based induction regimens were still in remission at 42 and 52 months.17 Another study examined a variety of induction regimens used to treat HSGDTCL in 15 patients.18 Responses tended to be more durable in patients who received a dose-intense Hyper-CVIDDoxil regimen (fractionated cyclophosphamide, liposomal doxorubicin, vincristine, dexamethasone) alternated with methotrexate and cytarabine. Complete response was 50%, and median duration of complete response was 8 months. Over the past 10 years, a few case reports have described successful treatment with autologous or allogeneic stem cell transplantation.20
Conclusion
The present case represents a unique HSGDTCL presentation. To the authors’ knowledge, this is the first report of HSGDTCL presenting with acute disseminated intravascular coagulation and AIHA with both cold and warm antibodies.
Hepatosplenic GDTCL is a rare, novel disease. To understand more about this pathology, investigators need to better characterize the disease process and the manifestations. The hope is that more information will contribute to the development of more effective therapies. The unique presentation reported here may help in further characterizing and understanding this uncommon disease.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
Gamma-delta (γ-δ) T-cell lymphomas (GDTCL) are rare and aggressive cancers with specific morphologic, phenotypic, and functional properties. When discovered in 1984, the T-cell receptor (TCR) was characterized as an alpha-beta (α-β) heterodimer. The γ-δ heterodimer was discovered later, when a third rearranging gene was recognized.1
Gaulard and colleagues described the first case of peripheral neoplasm with the γ-δ TCR.2 Now the present authors report the case of a patient with an autoimmune hemolytic anemia (AIHA) with both cold and warm antibodies—an atypical presentation of this rare form of TCL. Such a case has not been previously reported.
Clinical History
A 77-year-old woman with a past medical history of osteoarthritis, gout, mitral stenosis, bioprosthetic aortic valve replacement, and obesity presented to the emergency department (ED) reporting progressive weakness, confusion, and jaundice. She had been recently discharged from
another hospital after an 18-day stay for gangrenous cholecystitis and shingles. Her home medications were metronidazole and acyclovir. In the ED, she was febrile at 100.5°. Laboratory test results revealed anemia with a hemoglobin level of 50 g/L (83 g/L in clinic 2 weeks earlier) and neutropenia with an absolute neutrophilic count of 500 cells/μL (normal range 1,520-6,370 cells/μL). She also was thrombocytopenic with a platelet count of 71x109/L (normal range 150-450×109/L).
On admission, the hematology service was consulted for pancytopenia. The pertinent workup included a lactate dehydrogenase level of 31.16 μkat/L (normal range 1.7-3.4 μkat/L), a haptoglobin level of < 1,500 mg/L (normal range 260-1,850 mg/L), and a direct bilirubin level of 13.68 μmol/L (normal range 1.7-5.1 μmol/L). A peripheral blood smear was negative for schistocytes. Fibrin split products were 40 mg/L (normal < 10 mg/L), fibrinogen level was 6.94 μmol/L (normal range 5.8-11.8 μmol/L), prothrombin time was 14.6 seconds (normal range 10-14 sec), and international normalized ratio was 1.3 (normal < 1). The concomitant decrease in fibrinogen level and increase in fibrin split product titers were consistent with the diagnosis of acute disseminated intravascular coagulation. Iron studies were consistent with anemia of chronic disease (low reticulocyte count of 0.4%) and vitamin B12 deficiency (level 195). Coombs test results were positive for both cold and warm antibodies, with cold being more prominent. Abdominal ultrasonography revealed hepatosplenomegaly (HSM).
The patient was diagnosed with AIHA with no initial obvious underlying etiology. The differential diagnosis included autoimmune disorder, lymphoproliferative disease, and drug-induced process. She also was diagnosed with sepsis, which was thought to be contributing to the pancytopenia.
Broad-spectrum antibiotics (cefepime, metronidazole) and vitamin B12 supplements were started. After a blood transfusion, the patient developed fever and hypoxia, which required transfer to the medical intensive care unit. The differentials at this time included a transfusion reaction and/or transfusion-associated circulatory overload. Intravenous immunoglobulin was started at 1 g/kg to help with cold agglutinins. Prednisone 1 mg/kg was started as well. Peripheral blood flow cytometry results were positive for an abnormal T-cell population likely consistent with T-cell lineage lymphoma. Bone marrow biopsy results were consistent with GDTCL. Computed tomography (CT) of chest/abdomen/pelvis showed bilateral lung nodules < 1 cm, HSM with multiple spleen infarcts, and a 4.7-cm right adnexal soft-tissue lesion. Liver biopsy results were consistent with GDTCL. Results of a workup for cytomegalovirus and Epstein-Barr virus were negative, as was a mycoplasma screen. The patient was diagnosed with GDTCL with hepatic involvement, and CHOP (cyclophosphamide, hydroxydaunorubicin [doxorubicin], Oncovin [vincristine], prednisone) therapy was started.
Discussion
Peripheral TCL (PTCL) are a rare, typically extranodal group of malignancies. They are aggressive and generally have a poor outcome, with most patients dying of lymphoma within 2 years.3 T-cell lymphomas most commonly express the γ-δ TCR. About 2% to 4% of TCLs express the γ-δ TCR.4 In 2008, the World Health Organization recognized 2 distinct GDTCL subgroups: hepatosplenic GDTCL (HSGDTCL) and primary cutaneous GDTCL.5 As the patient presented with hepatic involvement, this discussion focused on HSGDTCL.
Hepatosplenic GDTCL are rare types of PTCL. First described as a separate TCL subgroup in the 1990 REAL (Revised European-American Lymphoma) classification,6 they are estimated to represent about 1.4% of all TCL, with about 100 cases reported in the literature.4
The GDTCL cells tend to live in mucosa, lymphoid tissue, epithelial-rich tissues (skin, gastrointestinal tract), and red pulp of spleen.7 They develop from thymic precursors in bone marrow and are CD4-/CD8- and thus known as double negative cells.8 They mimic natural killer cells, behave as cytotoxic cells, and are capable of TCR rearrangement as well as phagocytosis.9
Hepatosplenic GDTCL are usually phenotypically CD2+, CD3+, CD4-, CD5-, CD7+, CD8-, and TCR γ-δ+.10 They are rarely associated with Epstein-Barr virus infection; reported cases seem more common in Asia.11 Peak incidence is in young men (median age 20-25 years; male:female ratio 10:1). At-risk populations include the chronically immunosuppressed, including solid organ transplanted patients and patients under prolonged antigenic stimulation.12
The most common clinical features of HSGDTCL include B symptoms (fever of unknown origin, night sweats, loss of > 10% of body weight), marked HSM, and lack of lymphadenopathy. Patients often present with fever, weakness, and abdominal pain. Laboratory test results
typically show abnormal liver function and abnormal lactate dehydrogenase levels. Bone marrow is almost always involved, with possible trilineage cytopenia. Anemia and thrombocytopenia are reported in 75% and 85% of cases, respectively.13
Warm (70%) and cold auto-antibodies are the 2 classifications of AIHA.14 The AIHA can be primary, idiopathic, or a manifestation of underlying disease conditions, including non-Hodgkin lymphomas, systemic autoimmune diseases, chronic infections, postorgan transplantation, and solid tumors. It has also been reported as a complication of treatment with nucleoside analogues.15
Lacking specific symptoms, HSGDTCL is usually diagnosed late. The diagnosis should be suspected in young men who present with the aforementioned symptoms. However, not everyone with HSGDTCL falls in that group—the present patient was a 77-year-old woman.
Hepatosplenic GDTCL staging is similar to staging of other non-Hodgkin lymphomas. Total-body CT with contrast, bone marrow aspiration/biopsy, and direct lesion biopsy are required. Although positron emission tomography is generally thought to be as useful in TCL as in B-cell lymphomas, there is not enough evidence to support its use specifically in HSGDTCL.16 The staging classification follows the Ann Arbor system, with the majority of cases classified as stage IV.
Hepatosplenic GDTCL are aggressive tumors with a strong tendency to rapidly progress, and they are highly resistant to primary chemotherapy agents. Remission is rarely complete with use of conventional chemotherapy agents. Most patients die of the disease within 2 years of
diagnosis.12 Although the rarity of HSGDTCL has made it difficult to identify any clear prognostic factors, a correlation between thrombocytopenia severity and disease progression has been found in many studies.17 There is no standard treatment regimen. Proposed therapies
include splenectomy (for diagnosis or thrombocytopenia management), corticosteroids, alkylating agents, purine analogue, anthracycline-containing regimens, and cytarabine/cisplatin combinations. The anthracycline-based regimen most commonly used as first-line therapy is CHOP, or CHOP derivatives, with complete remission rates between 30% and 45%. However, long-term results remain disappointing (median relapse time 4 months).10 In 3 reviews, median survival was 16 months, 11 months, and 9.5 months.10,17,18 In the International T-Cell Lymphoma Project study, the 5-year failure-free survival rate was 0%, and the overall survival rate was 7%.4 In these studies, the majority of patients received some variation of CHOP-based therapy, and although positive responses were appreciated in many of the cases, they were generally short-lived.
These results have been disappointing, and other modalities have been tried—including high-dose cytarabine regimens, 2'-deoxycoformycin (pentostatin), and anti-CD52 monoclonal antibodies (alemtuzumab).19 In an HSGDTCL study, 2 of 21 patients treated with platinum/cytarabine-based induction regimens were still in remission at 42 and 52 months.17 Another study examined a variety of induction regimens used to treat HSGDTCL in 15 patients.18 Responses tended to be more durable in patients who received a dose-intense Hyper-CVIDDoxil regimen (fractionated cyclophosphamide, liposomal doxorubicin, vincristine, dexamethasone) alternated with methotrexate and cytarabine. Complete response was 50%, and median duration of complete response was 8 months. Over the past 10 years, a few case reports have described successful treatment with autologous or allogeneic stem cell transplantation.20
Conclusion
The present case represents a unique HSGDTCL presentation. To the authors’ knowledge, this is the first report of HSGDTCL presenting with acute disseminated intravascular coagulation and AIHA with both cold and warm antibodies.
Hepatosplenic GDTCL is a rare, novel disease. To understand more about this pathology, investigators need to better characterize the disease process and the manifestations. The hope is that more information will contribute to the development of more effective therapies. The unique presentation reported here may help in further characterizing and understanding this uncommon disease.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. Saito H, Kranz DM, Takagaki Y, Hayday AC, Eisen HN, Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984;312(5989):36-40.
2. Gaulard P, Zafrani ES, Mavier P, et al. Peripheral T-cell lymphoma presenting as predominant liver disease: a report of three cases. Hepatology. 1986;6(5):864-868.
3. Gaulard P, de Leval L. Pathology of peripheral T-cell lymphomas: where do we stand? Semin Hematol. 2014;51(1):5-16.
4. Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-4130.
5. The International Agency for Research on Cancer. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Vol 2. 4th ed. Lyon, France: IARC Press; 2008.
6. Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasm: a proposal from the International Lymphoma Study Group. Blood. 1994;84(5):1361-1392.
7. Farcet JP, Gaulard P, Marolleau JP, et al. Hepatosplenic T-cell lymphoma: sinusal/sinusoidal localization of malignant cells expressing the T-cell receptor gamma delta. Blood. 1990;75(11):2213-2219.
8. Bluestone JA, Khattri R, Sciammas R, Sperling AI. TCR gamma delta cells: a specialized T-cell subset in the immune system. Annu Rev Cell Dev Biol. 1995;11:307-353.
9. Holtmeier W, Kabelitz D. Gamma delta T cells link innate and adaptive immune responses. Chem Immunol Allergy. 2005;86:151-183.
10. Weidmann E. Hepatosplenic T cell lymphoma. A review on 45 cases since the first report describing the disease as a distinct lymphoma entity in 1990. Leukemia. 2000;14(6):991-997.
11. Yu WW, Hsieh PP, Chuang SS. Cutaneous EBV-positive γδ T-cell lymphoma vs. extranodal NK/T-cell lymphoma: a case report and literature review. J Cutan Pathol. 2013;40(3):310-316.
12. Tripodo C, Iannitto E, Florena AM, et al. Gamma-delta T-cell lymphomas. Nat Rev Clin Oncol. 2009;6(12):707-717.
13. Foppoli M, Ferreri AJM. Gamma-delta T-cell lymphomas. Eur J Haematol. 2015;94(3):206-218.
14. Hoffbrand AV, Catovsky D, Tuddenham EGD, Green AR, eds. Postgraduate Haematology. 6th ed. Oxford, England: Wiley-Blackwell; 2011.
15. Valent P, Lechner K. Diagnosis and treatment of autoimmune haemolytic anaemias in adults: a clinical review. Wien Klin Wochenschr. 2008;120(5-6):136-151.
16. Khong PL, Pang CB, Liang R, Kwong YL, Au WY. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008;87(8):613-621.
17. Belhadj K, Reyes F, Farcet JP, et al. Hepatosplenic gammadelta T-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood. 2003;102(13):4261-4269.
18. Falchook GS, Vega F, Dang NH, et al. Hepatosplenic gamma-delta T-cell lymphoma: clinicopathological features and treatment. Ann Oncol. 2009;20(6):1080-1085.
19. Konuma T, Ooi J, Takahashi S, et al. Allogeneic stem cell transplantation for hepatosplenic
gammadelta T-cell lymphoma. Leuk Lymphoma. 2007;48(3):630-632.
20. Ferreri AJ, Govi S, Pileri SA. Hepatosplenic gamma-delta T-cell lymphoma. Crit
Rev Oncol Hematol. 2012;83(2):283-292.
1. Saito H, Kranz DM, Takagaki Y, Hayday AC, Eisen HN, Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984;312(5989):36-40.
2. Gaulard P, Zafrani ES, Mavier P, et al. Peripheral T-cell lymphoma presenting as predominant liver disease: a report of three cases. Hepatology. 1986;6(5):864-868.
3. Gaulard P, de Leval L. Pathology of peripheral T-cell lymphomas: where do we stand? Semin Hematol. 2014;51(1):5-16.
4. Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-4130.
5. The International Agency for Research on Cancer. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Vol 2. 4th ed. Lyon, France: IARC Press; 2008.
6. Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasm: a proposal from the International Lymphoma Study Group. Blood. 1994;84(5):1361-1392.
7. Farcet JP, Gaulard P, Marolleau JP, et al. Hepatosplenic T-cell lymphoma: sinusal/sinusoidal localization of malignant cells expressing the T-cell receptor gamma delta. Blood. 1990;75(11):2213-2219.
8. Bluestone JA, Khattri R, Sciammas R, Sperling AI. TCR gamma delta cells: a specialized T-cell subset in the immune system. Annu Rev Cell Dev Biol. 1995;11:307-353.
9. Holtmeier W, Kabelitz D. Gamma delta T cells link innate and adaptive immune responses. Chem Immunol Allergy. 2005;86:151-183.
10. Weidmann E. Hepatosplenic T cell lymphoma. A review on 45 cases since the first report describing the disease as a distinct lymphoma entity in 1990. Leukemia. 2000;14(6):991-997.
11. Yu WW, Hsieh PP, Chuang SS. Cutaneous EBV-positive γδ T-cell lymphoma vs. extranodal NK/T-cell lymphoma: a case report and literature review. J Cutan Pathol. 2013;40(3):310-316.
12. Tripodo C, Iannitto E, Florena AM, et al. Gamma-delta T-cell lymphomas. Nat Rev Clin Oncol. 2009;6(12):707-717.
13. Foppoli M, Ferreri AJM. Gamma-delta T-cell lymphomas. Eur J Haematol. 2015;94(3):206-218.
14. Hoffbrand AV, Catovsky D, Tuddenham EGD, Green AR, eds. Postgraduate Haematology. 6th ed. Oxford, England: Wiley-Blackwell; 2011.
15. Valent P, Lechner K. Diagnosis and treatment of autoimmune haemolytic anaemias in adults: a clinical review. Wien Klin Wochenschr. 2008;120(5-6):136-151.
16. Khong PL, Pang CB, Liang R, Kwong YL, Au WY. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008;87(8):613-621.
17. Belhadj K, Reyes F, Farcet JP, et al. Hepatosplenic gammadelta T-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood. 2003;102(13):4261-4269.
18. Falchook GS, Vega F, Dang NH, et al. Hepatosplenic gamma-delta T-cell lymphoma: clinicopathological features and treatment. Ann Oncol. 2009;20(6):1080-1085.
19. Konuma T, Ooi J, Takahashi S, et al. Allogeneic stem cell transplantation for hepatosplenic
gammadelta T-cell lymphoma. Leuk Lymphoma. 2007;48(3):630-632.
20. Ferreri AJ, Govi S, Pileri SA. Hepatosplenic gamma-delta T-cell lymphoma. Crit
Rev Oncol Hematol. 2012;83(2):283-292.
Consensus Statement Supporting the Recommendation for Single-Fraction Palliative Radiotherapy for Uncomplicated, Painful Bone Metastases
The authors would like to acknowledge Tony Quang, MD, JD, for the advice given on this project.
Palliative radiotherapy for bone metastases is typically delivered either as a short course of 1 to 5 fractions or protracted over longer courses of up to 20 treatments. These longer courses can be burdensome and discourage its utilization, despite a 50% to 80% likelihood of meaningful pain relief from only a single fraction of radiation therapy. Meanwhile, there are multiple randomized studies that have demonstrated that shorter course(s) are equivalent for pain control.
Although the VHA currently has 143 medical facilities that have cancer diagnostic and treatment capabilities, only 40 have radiation oncology services on-site.1 Thus, access to palliative radiotherapy may be limited for veterans who do not live close by, and many may seek care outside the VHA. At VHA radiation oncology centers, single-fraction radiation therapy (SFRT) is routinely offered by the majority of radiation oncologists.2,3 However, the longer course is commonly preferred outside the VA, and a recent SEER-Medicare analysis of more than 3,000 patients demonstrated that the majority of patients treated outside the VA actually receive more than 10 treatments.4 For this reason, the VA National Palliative Radiotherapy Task Force prepared this document to provide guidance for clinicians within and outside the VA to increase awareness of the appropriateness, effectiveness, and convenience of SFRT as opposed to longer courses of treatment that increase the burden of care at the end of life and often are unnecessary.
Veterans, Cancer, and Metastases
Within the VA, an estimated 40,000 new cancer cases are diagnosed each year, and 175,000 veterans undergo cancer care within the VHA annually.1 Unfortunately, the majority will develop bone metastases with postmortem examinations, suggesting that the rate can be as high as 90% at the end of life.5-7 For many, including veterans with cancer, pain control can be difficult, and access to palliative radiotherapy is critical.8
Single-Fraction Palliatiev Radiation Therapy
Historically, patients with painful bone metastases have been treated with courses of palliative radiotherapy ranging between 2 and 4 weeks of daily treatments. However, several large randomized clinical trials comparing a single treatment with multiple treatments have established that SFRT provides equivalent rates of pain relief even when it may be required for a second time.9-12 Recommendations based on these trials have been incorporated into various treatment guidelines that widely acknowledge the efficacy of SFRT.13-15
For this reason, SFRT is often preferred at many centers because it is substantially more convenient for patients with cancer. It reduces travel time for daily radiation clinic visits, which allows for more time with loved ones outside the medical establishment. Furthermore, SFRT improves patient access to radiotherapy and reduces costs. The benefits can be direct as well as indirect to those who have to take time for numerous visits.
Longer courses of palliative radiotherapy can be burdensome for patients and primary care providers. Unnecessarily protracted courses of palliative radiotherapy also delay the receipt of systemic therapies because they are typically considered unsafe to administer concurrently. Moreover, when SFRT is unavailable, the burden of long-course palliation is known to discourage health care providers from referring patients since opioid therapy is more convenient, even though it exchanges lucidity for analgesia.16,17
For this reason, the authors believe that it is in the best interest for veterans with terminal cancers and their providers to be aware of the shorter SFRT for effective, convenient pain relief. This treatment option is particularly relevant for patients with a poor performance status, patients already in hospice care, or patient who travel long distances.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
2. Moghanaki D, Cheuk AV, Fosmire H, et al; U.S. Veterans Healthcare Administration National Palliative Radiotherapy Taskforce. Availability of single fraction palliative radiotherapy for cancer patients receiving end-of-life care within the Veterans Healthcare Administration. J Palliat Med. 2014;17(11):1221-1225.
3. Dawson GA, Glushko I, Hagan MP. A cross-sectional view of radiation dose fractionation schemes used for painful bone metastases cases within Veterans Health Administration Radiation Oncology Centers. J Clin Oncol. 2015;33(29 suppl):abstract 177.
4. Bekelman JE, Epstein AJ, Emanuel EJ. Single- vs multiple-fraction radiotherapy for bone metastases from prostate cancer. JAMA. 2013;310(14):1501-1502.
5. Galasko CSB. The anatomy and pathways of skeletal metastases. In: Weiss L, Gilbert AH, eds. Bone Metastasis. Boston, MA: GK Hall; 1981:49-63.
6. Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns in prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578-583.
7. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20, pt 2):6243s-6249s.
8. Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee, Veterans Health Administration. Pain as the 5th Vital Sign Toolkit. Rev. ed. Washington, DC: National Pain Management Coordinating Committee; 2000.
9. Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798-804.
10. Chow E, Hoskins PJ, Wu J, et al. A phase III international randomised trial comparing single with multiple fractions for re-irradiation of painful bone metastases: National Cancer Institute of Canada Clinical Trials Group (NCTC CTG) SC 20. Clin Oncol (R Coll Radiol). 2006;18(2):125-128.
11. Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys. 2009;75(5):1501-1510.
12. Chow E, van der Linden YM, Roos D, et al. Single fraction versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol. 2014;15(2):164-171.
13. Lutz ST, Berk L, Chang E, et al; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidencebased guideline. Int J Radiat Oncol, Biol, Phys. 2011;79(4):965-976.
14. Expert Panel on Radiation Oncology-Bone Metastases, Lo SS, Lutz ST, Chang EL, et al. ACR Appropriateness Criteria® spinal bone metastases. J Palliat Med. 2013;16(1):9-19.
15. Expert Panel on Radiation Oncology-Bone Metastases, Lutz ST, Lo SS, Chang EL, et al. ACR Appropriateness Criteria® non-spinal bone metastases. J Palliative Med. 2012;15(5):521-526.
16. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buchholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31(1):80-87.
17. Schuster J, Han T, Anscher M, Moghanaki D. Hospice providers awareness of the benefits and availability of single-fraction palliative radiotherapy. J Hospice Palliat Care Nurs. 2014;16(2):67-72.
18. Cheon PM, Wong E, Thavarajah N, et al. A definition of “uncomplicated bone metastases” based on previous bone metastases trials comparing single-fraction and multi-fraction radiation therapy. J Bone Oncol. 2015;4(1):13-17.
The authors would like to acknowledge Tony Quang, MD, JD, for the advice given on this project.
Palliative radiotherapy for bone metastases is typically delivered either as a short course of 1 to 5 fractions or protracted over longer courses of up to 20 treatments. These longer courses can be burdensome and discourage its utilization, despite a 50% to 80% likelihood of meaningful pain relief from only a single fraction of radiation therapy. Meanwhile, there are multiple randomized studies that have demonstrated that shorter course(s) are equivalent for pain control.
Although the VHA currently has 143 medical facilities that have cancer diagnostic and treatment capabilities, only 40 have radiation oncology services on-site.1 Thus, access to palliative radiotherapy may be limited for veterans who do not live close by, and many may seek care outside the VHA. At VHA radiation oncology centers, single-fraction radiation therapy (SFRT) is routinely offered by the majority of radiation oncologists.2,3 However, the longer course is commonly preferred outside the VA, and a recent SEER-Medicare analysis of more than 3,000 patients demonstrated that the majority of patients treated outside the VA actually receive more than 10 treatments.4 For this reason, the VA National Palliative Radiotherapy Task Force prepared this document to provide guidance for clinicians within and outside the VA to increase awareness of the appropriateness, effectiveness, and convenience of SFRT as opposed to longer courses of treatment that increase the burden of care at the end of life and often are unnecessary.
Veterans, Cancer, and Metastases
Within the VA, an estimated 40,000 new cancer cases are diagnosed each year, and 175,000 veterans undergo cancer care within the VHA annually.1 Unfortunately, the majority will develop bone metastases with postmortem examinations, suggesting that the rate can be as high as 90% at the end of life.5-7 For many, including veterans with cancer, pain control can be difficult, and access to palliative radiotherapy is critical.8
Single-Fraction Palliatiev Radiation Therapy
Historically, patients with painful bone metastases have been treated with courses of palliative radiotherapy ranging between 2 and 4 weeks of daily treatments. However, several large randomized clinical trials comparing a single treatment with multiple treatments have established that SFRT provides equivalent rates of pain relief even when it may be required for a second time.9-12 Recommendations based on these trials have been incorporated into various treatment guidelines that widely acknowledge the efficacy of SFRT.13-15
For this reason, SFRT is often preferred at many centers because it is substantially more convenient for patients with cancer. It reduces travel time for daily radiation clinic visits, which allows for more time with loved ones outside the medical establishment. Furthermore, SFRT improves patient access to radiotherapy and reduces costs. The benefits can be direct as well as indirect to those who have to take time for numerous visits.
Longer courses of palliative radiotherapy can be burdensome for patients and primary care providers. Unnecessarily protracted courses of palliative radiotherapy also delay the receipt of systemic therapies because they are typically considered unsafe to administer concurrently. Moreover, when SFRT is unavailable, the burden of long-course palliation is known to discourage health care providers from referring patients since opioid therapy is more convenient, even though it exchanges lucidity for analgesia.16,17
For this reason, the authors believe that it is in the best interest for veterans with terminal cancers and their providers to be aware of the shorter SFRT for effective, convenient pain relief. This treatment option is particularly relevant for patients with a poor performance status, patients already in hospice care, or patient who travel long distances.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
The authors would like to acknowledge Tony Quang, MD, JD, for the advice given on this project.
Palliative radiotherapy for bone metastases is typically delivered either as a short course of 1 to 5 fractions or protracted over longer courses of up to 20 treatments. These longer courses can be burdensome and discourage its utilization, despite a 50% to 80% likelihood of meaningful pain relief from only a single fraction of radiation therapy. Meanwhile, there are multiple randomized studies that have demonstrated that shorter course(s) are equivalent for pain control.
Although the VHA currently has 143 medical facilities that have cancer diagnostic and treatment capabilities, only 40 have radiation oncology services on-site.1 Thus, access to palliative radiotherapy may be limited for veterans who do not live close by, and many may seek care outside the VHA. At VHA radiation oncology centers, single-fraction radiation therapy (SFRT) is routinely offered by the majority of radiation oncologists.2,3 However, the longer course is commonly preferred outside the VA, and a recent SEER-Medicare analysis of more than 3,000 patients demonstrated that the majority of patients treated outside the VA actually receive more than 10 treatments.4 For this reason, the VA National Palliative Radiotherapy Task Force prepared this document to provide guidance for clinicians within and outside the VA to increase awareness of the appropriateness, effectiveness, and convenience of SFRT as opposed to longer courses of treatment that increase the burden of care at the end of life and often are unnecessary.
Veterans, Cancer, and Metastases
Within the VA, an estimated 40,000 new cancer cases are diagnosed each year, and 175,000 veterans undergo cancer care within the VHA annually.1 Unfortunately, the majority will develop bone metastases with postmortem examinations, suggesting that the rate can be as high as 90% at the end of life.5-7 For many, including veterans with cancer, pain control can be difficult, and access to palliative radiotherapy is critical.8
Single-Fraction Palliatiev Radiation Therapy
Historically, patients with painful bone metastases have been treated with courses of palliative radiotherapy ranging between 2 and 4 weeks of daily treatments. However, several large randomized clinical trials comparing a single treatment with multiple treatments have established that SFRT provides equivalent rates of pain relief even when it may be required for a second time.9-12 Recommendations based on these trials have been incorporated into various treatment guidelines that widely acknowledge the efficacy of SFRT.13-15
For this reason, SFRT is often preferred at many centers because it is substantially more convenient for patients with cancer. It reduces travel time for daily radiation clinic visits, which allows for more time with loved ones outside the medical establishment. Furthermore, SFRT improves patient access to radiotherapy and reduces costs. The benefits can be direct as well as indirect to those who have to take time for numerous visits.
Longer courses of palliative radiotherapy can be burdensome for patients and primary care providers. Unnecessarily protracted courses of palliative radiotherapy also delay the receipt of systemic therapies because they are typically considered unsafe to administer concurrently. Moreover, when SFRT is unavailable, the burden of long-course palliation is known to discourage health care providers from referring patients since opioid therapy is more convenient, even though it exchanges lucidity for analgesia.16,17
For this reason, the authors believe that it is in the best interest for veterans with terminal cancers and their providers to be aware of the shorter SFRT for effective, convenient pain relief. This treatment option is particularly relevant for patients with a poor performance status, patients already in hospice care, or patient who travel long distances.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
2. Moghanaki D, Cheuk AV, Fosmire H, et al; U.S. Veterans Healthcare Administration National Palliative Radiotherapy Taskforce. Availability of single fraction palliative radiotherapy for cancer patients receiving end-of-life care within the Veterans Healthcare Administration. J Palliat Med. 2014;17(11):1221-1225.
3. Dawson GA, Glushko I, Hagan MP. A cross-sectional view of radiation dose fractionation schemes used for painful bone metastases cases within Veterans Health Administration Radiation Oncology Centers. J Clin Oncol. 2015;33(29 suppl):abstract 177.
4. Bekelman JE, Epstein AJ, Emanuel EJ. Single- vs multiple-fraction radiotherapy for bone metastases from prostate cancer. JAMA. 2013;310(14):1501-1502.
5. Galasko CSB. The anatomy and pathways of skeletal metastases. In: Weiss L, Gilbert AH, eds. Bone Metastasis. Boston, MA: GK Hall; 1981:49-63.
6. Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns in prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578-583.
7. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20, pt 2):6243s-6249s.
8. Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee, Veterans Health Administration. Pain as the 5th Vital Sign Toolkit. Rev. ed. Washington, DC: National Pain Management Coordinating Committee; 2000.
9. Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798-804.
10. Chow E, Hoskins PJ, Wu J, et al. A phase III international randomised trial comparing single with multiple fractions for re-irradiation of painful bone metastases: National Cancer Institute of Canada Clinical Trials Group (NCTC CTG) SC 20. Clin Oncol (R Coll Radiol). 2006;18(2):125-128.
11. Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys. 2009;75(5):1501-1510.
12. Chow E, van der Linden YM, Roos D, et al. Single fraction versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol. 2014;15(2):164-171.
13. Lutz ST, Berk L, Chang E, et al; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidencebased guideline. Int J Radiat Oncol, Biol, Phys. 2011;79(4):965-976.
14. Expert Panel on Radiation Oncology-Bone Metastases, Lo SS, Lutz ST, Chang EL, et al. ACR Appropriateness Criteria® spinal bone metastases. J Palliat Med. 2013;16(1):9-19.
15. Expert Panel on Radiation Oncology-Bone Metastases, Lutz ST, Lo SS, Chang EL, et al. ACR Appropriateness Criteria® non-spinal bone metastases. J Palliative Med. 2012;15(5):521-526.
16. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buchholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31(1):80-87.
17. Schuster J, Han T, Anscher M, Moghanaki D. Hospice providers awareness of the benefits and availability of single-fraction palliative radiotherapy. J Hospice Palliat Care Nurs. 2014;16(2):67-72.
18. Cheon PM, Wong E, Thavarajah N, et al. A definition of “uncomplicated bone metastases” based on previous bone metastases trials comparing single-fraction and multi-fraction radiation therapy. J Bone Oncol. 2015;4(1):13-17.
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
2. Moghanaki D, Cheuk AV, Fosmire H, et al; U.S. Veterans Healthcare Administration National Palliative Radiotherapy Taskforce. Availability of single fraction palliative radiotherapy for cancer patients receiving end-of-life care within the Veterans Healthcare Administration. J Palliat Med. 2014;17(11):1221-1225.
3. Dawson GA, Glushko I, Hagan MP. A cross-sectional view of radiation dose fractionation schemes used for painful bone metastases cases within Veterans Health Administration Radiation Oncology Centers. J Clin Oncol. 2015;33(29 suppl):abstract 177.
4. Bekelman JE, Epstein AJ, Emanuel EJ. Single- vs multiple-fraction radiotherapy for bone metastases from prostate cancer. JAMA. 2013;310(14):1501-1502.
5. Galasko CSB. The anatomy and pathways of skeletal metastases. In: Weiss L, Gilbert AH, eds. Bone Metastasis. Boston, MA: GK Hall; 1981:49-63.
6. Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns in prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578-583.
7. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20, pt 2):6243s-6249s.
8. Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee, Veterans Health Administration. Pain as the 5th Vital Sign Toolkit. Rev. ed. Washington, DC: National Pain Management Coordinating Committee; 2000.
9. Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798-804.
10. Chow E, Hoskins PJ, Wu J, et al. A phase III international randomised trial comparing single with multiple fractions for re-irradiation of painful bone metastases: National Cancer Institute of Canada Clinical Trials Group (NCTC CTG) SC 20. Clin Oncol (R Coll Radiol). 2006;18(2):125-128.
11. Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys. 2009;75(5):1501-1510.
12. Chow E, van der Linden YM, Roos D, et al. Single fraction versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol. 2014;15(2):164-171.
13. Lutz ST, Berk L, Chang E, et al; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidencebased guideline. Int J Radiat Oncol, Biol, Phys. 2011;79(4):965-976.
14. Expert Panel on Radiation Oncology-Bone Metastases, Lo SS, Lutz ST, Chang EL, et al. ACR Appropriateness Criteria® spinal bone metastases. J Palliat Med. 2013;16(1):9-19.
15. Expert Panel on Radiation Oncology-Bone Metastases, Lutz ST, Lo SS, Chang EL, et al. ACR Appropriateness Criteria® non-spinal bone metastases. J Palliative Med. 2012;15(5):521-526.
16. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buchholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31(1):80-87.
17. Schuster J, Han T, Anscher M, Moghanaki D. Hospice providers awareness of the benefits and availability of single-fraction palliative radiotherapy. J Hospice Palliat Care Nurs. 2014;16(2):67-72.
18. Cheon PM, Wong E, Thavarajah N, et al. A definition of “uncomplicated bone metastases” based on previous bone metastases trials comparing single-fraction and multi-fraction radiation therapy. J Bone Oncol. 2015;4(1):13-17.