User login
Risk Factors for Discharge to Rehabilitation Among Hip Fracture Patients
Length of stay (LOS) is a significant driver of costs after hip fracture surgery.1-3 Multiple studies have identified factors associated with increased LOS in hip fracture patients. These factors include admission time, delay to surgery, presence of comorbidities, and older age.4-9
One significant and potentially modifiable factor affecting LOS is delayed transfer to a rehabilitation center after surgery.8-11 Although patients after orthopedic surgeries require additional rehabilitation services or subacute care directly attributable to their injuries, specialized rehabilitation centers may not always have beds readily available.6-11 Studies have shown that delays in transfer to skilled nursing facilities or rehabilitation centers are highly common among orthopedic patients.8 It is therefore imperative that orthopedists have a mechanism for predicting and identifying which patients require rehabilitation services early in the postoperative period. Identifying risk factors and stratifying patients who are most likely to require rehabilitation would facilitate the early transfer of these patients and thereby directly decrease LOS and hospitalization-related costs.
In this article, we report results from prospective, national, multicenter data to identify commonly measured risk factors for discharge to rehabilitation facilities for hip fracture patients. Through multivariate analysis of ACS-NSQIP (American College of Surgeons National Surgical Quality Improvement Program) data, we determined which risk factors significantly predispose patients to discharge to rehabilitation centers versus discharge home. Knowledge of these risk factors allows the practicing orthopedist to be better equipped to identify patients who require additional rehabilitation early in the postoperative course. By mobilizing case managers and social workers to help avoid delays in the transfers of these identified patients, LOS-associated costs may ultimately decrease.
Materials and Methods
After obtaining institutional review board approval for this study from the Office of Research at Vanderbilt University, we prospectively collected 2011 discharge data from the ACS-NSQIP database (these data are unavailable for earlier years). All patients who underwent hip fracture surgery in 2011 were identified by CPT (Current Procedural Terminology) codes. Cases of patients with unknown discharge information and of those who died during their hospitalizations were excluded from analysis. For the remaining patients, discharge information as categorized by ACS-NSQIP included skilled care (eg, subacute hospital, skilled nursing home), unskilled facility (eg, nursing home, assisted facility), separate acute care, and rehabilitation. All other patients were discharged home without additional assistance or to the previous home where they received chronic care, assisted living, or unskilled aid. Patients were dichotomized according to whether they were discharged home or to one of the rehabilitation facilities mentioned.
To determine which risk factors significantly contributed to a patient’s discharge to rehabilitation, we ran univariate analyses using Fisher exact tests for categorical variables and Student t tests for continuous variables on multiple patient factors, including demographics, preoperative comorbidities, and operative factors. Demographics included age and sex. Preoperative comorbidities included 32 conditions: diabetes mellitus, active smoking status, current alcohol use, dyspnea, history of chronic obstructive pulmonary disease, history of congestive heart failure, hypertension requiring medication, history of esophageal varices, history of myocardial infarction, current renal failure, current dialysis dependence, steroid use, recent weight loss, existing bleeding disorder, transfusion before discharge, presence of central nervous system tumor, recent chemotherapy, recent radiation therapy, previous percutaneous coronary intervention, previous percutaneous coronary stenting, history of angina, peripheral vascular disease, cerebrovascular accidents, recent surgery (within 30 days), rest pain, impaired sensorium, history of transient ischemic attacks, current hemiplegia status, current paraplegia status, current quadriplegia status, current ascites, hypertension, and disseminated cancer. Operative factors included wound infection, DNR (do not resuscitate) status, ventilator support, anesthesia type, wound class, ASA (American Society of Anesthesiologists) class, and operative time.
For the univariate analyses, significance was set at P < .05. Demographics, preoperative comorbidities, and operative factors that were significantly associated with discharge to a rehabilitation facility in the univariate analysis were selected as covariates for a multivariate analysis. We incorporated a binary logistic regression to analyze which of these significant risk factors are correlated with a patient’s discharge to a rehabilitation facility after hip fracture surgery.
Results
A total of 4974 patients undergoing surgery for hip fractures in 2011 were identified. Of these patients, 4815 had complete information on discharge location and were included in the analysis.
Table 1 lists the results of the univariate analysis comparing demographics, preoperative comorbidities, and operative factors between the home and rehabilitation groups. Both age (P < .001) and sex (P = .012) were significantly different between groups; the rehabilitation group was older by about 10 years and included significantly more females. In addition to demographic factors, 16 preoperative comorbidities, and 5 surgical factors were significantly associated with discharge to rehabilitation.
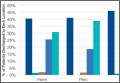
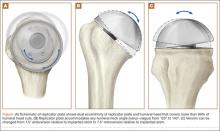
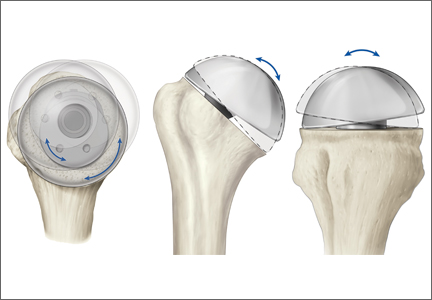
Surgery type significantly affected discharge to rehabilitation (Figure). Patients who were undergoing open plating of a femoral neck fracture or intramedullary nailing of an intertrochanteric, peritrochanteric, or subtrochanteric femoral fracture constituted 30% of all patients discharged to rehabilitation centers. In contrast, patients undergoing percutaneous skeletal fixation of a proximal femoral fracture constituted only 5.5% of all patients discharged to rehabilitation. Based on surgery type, we broke down discharge location further, into categories of skilled nursing facility, unskilled facility (not patient’s previous home), separate acute-care facility, dedicated rehabilitation center, and home. Of all 4815 patients combined, 2102 (43.6%) were discharged to a skilled nursing facility, 31 (0.6%) to an unskilled facility (not home), 106 (2.2%) to separate acute care, 1312 (27.2%) to a dedicated rehabilitation center, and 950 (19.7%) home.
Table 2 lists the significant results from the multivariate logistical analysis comparing discharge to a rehabilitation center and discharge home after controlling for the significant risk factors (Table 1). Current diabetes, history of dyspnea, previous myocardial infarction, history of ischemic attacks, current bleeding disorder, transfusion during hospitalization, previous percutaneous cardiac stenting, chemotherapy, past cerebrovascular accident, presence of cancer, surgery type based on CPT code, history of chronic obstructive pulmonary disease or congestive heart failure, current smoking status, and operative time longer than 90 minutes were not significantly correlated with discharge to rehabilitation in the multivariate analysis. All significant factors were associated with higher odds of discharge to rehabilitation except for DNR status. DNR patients were 2.04 times more likely (95% CI, 1.49-2.78; P < .001) to be discharged home than to rehabilitation centers.
Applying these adjusted odds ratios, we see that an elderly woman (age, >65 years) who underwent general anesthesia with an ASA class higher than 2 was 17.63 times more likely than a patient without these risk factors to be discharged to rehabilitation. If this patient were also dialysis-dependent, she would be 61.52 times more likely than a similar patient without dialysis needs to be discharged to rehabilitation.
Even when controlling for all significant and nonsignificant variables in multivariate logistical analysis, age over 65 years (β = 1.05; P < .001), female sex (β = 1.76; P = .004), dialysis dependence (β = 12.98; P = .036), hypertension requiring medication (β = 1.53; P = .032), and ASA class higher than 2 (β = 1.98; P = .001) were found to be significant risk factors for discharge to rehabilitation.
Discussion
This study was the first to investigate the issue of which patient risk factors allow the practicing orthopedist to identify patients who require rehabilitation after hip fracture surgery. Through our multivariate analysis, which controlled for demographics, comorbidities, and operative factors, we found that older age, female sex, history of percutaneous coronary intervention, dialysis dependence, general anesthesia, and ASA class higher than 2 significantly increased the odds of discharge to a rehabilitation center versus home.
Using our study’s results, we can create a risk stratification model for patients and thereby a means of targeting patients who need rehabilitation and starting the process of finding a rehabilitation bed early in the postoperative course. Our study’s variables are easily measured metrics that may be collected in any hospital setting. Especially for hip fracture patients, early planning and discharge to the appropriate rehabilitation center are important in decreasing LOS and associated hospitalization costs. According to one report,3 about 85% of all hip fracture costs are directly related to LOS, given the unnecessarily long rehabilitation periods in hospitals. Hollingworth and colleagues2 compared costs for patients who remained in the hospital with costs for those discharged with rehabilitation services. Overall costs were significantly lower for patients discharged home with rehabilitation. The authors concluded that 40% of hip fracture patients may be suitable for early discharge.2 In an analysis of Medicare payments for hip fracture treatment, hospital costs including LOS accounted for 60% of all payments.12 The results of these 2 studies suggest that the overall driver of hip fracture costs is prolonged LOS and that, if patients are discharged to rehabilitation, then overall costs may be lowered through a direct reduction in hospital LOS. Given that hip fractures account for almost 350,000 hospital admissions in the United States each year, and using our institution’s average hospital charge per day ($4500), about $1.6 billion may be saved if each patient’s LOS decreased by 1 day.13 Although multiple factors affect LOS, discharge planning is under orthopedists’ direct control. Therefore, early identification of patients who will require rehabilitation may help reduce LOS-associated costs in our health care system.
The patient variables that were significantly associated with discharge to rehabilitation are also associated with increased morbidity and mortality in hip fracture patients, according to the literature,14-20 which provides some external validation of using these risk factors as predictors for rehabilitation. A patient with one of these risk factors may require rehabilitation, given that rehabilitation services are specifically linked to lower morbidity and mortality rates among hip fracture patients. For example, patients with dialysis needs were 3.49 times more likely to be discharged to a rehabilitation center in our study. In a 2000 study by Coco and Rush,16 hip fracture patients on dialysis had a 1-year mortality rate 2.5 times higher than that of patients who were not dialysis-dependent. In 2010, Cameron and colleagues17 found that cardiovascular disease was associated with a 2.68 times higher risk of mortality in hip fracture patients. Similarly in our study, both hypertension and history of percutaneous coronary intervention were associated with discharge to rehabilitation. We found higher odds of discharge to rehabilitation with higher ASA classes, which mirror results from a study by Michel and colleagues,15 who found that higher (vs lower) preoperative ASA classes were associated with higher 1-year mortality in hip fracture patients. Interestingly, DNR status was associated with higher odds of discharge home, which may reflect patients’ desires to forgo noninvasive or lifesaving procedures that may be performed at rehabilitation facilities. Although general anesthesia predisposed patients to discharge to a rehabilitation center, multiple studies have found no association between anesthesia type and postoperative mortality rates for hip fracture patients.18,19 Last, Marcantonio and colleagues20 found delirium specifically had a higher odds ratio for discharge, but our univariate analysis did not find a significant association between impaired sensorium and discharge location. Given the correlation of our risk factors with increased morbidity and mortality in the literature, our study’s results provide the initial groundwork for creating a risk calculator that orthopedists can use to predict discharge to rehabilitation.
Our study had some limitations. Although we analyzed a large number of demographics, preoperative comorbidities, and surgical factors, our univariate analysis was limited to information in the ACS-NSQIP database. We did not incorporate other clinically relevant factors (eg, social factors, including patients’ support networks) that may influence discharge decisions. Furthermore, ACS-NSQIP records patient data only up to 30 days after surgery. Discharge information for the time after that was missing for a subset of hip fracture patients, and these patients had to be excluded, potentially skewing our data. ACS-NSQIP also does not collect cost data for patients based on hospitalization or LOS, so we could not determine whether patients discharged to rehabilitation incurred higher costs because of longer hospitalizations.
Nevertheless, our study identified significant patient and operative variables that are associated with discharge to a rehabilitation center. By identifying hip fracture patients with these risk factors early and mobilizing the appropriate resources, practicing orthopedists should be better equipped to help facilitate the discharge of patients to the appropriate location after surgery. Validation of these risk factors should be prospectively determined with an analysis of LOS and cost implications. Use of a risk calculator may in fact result in decreased LOS and hospital-related costs. Furthermore, using these risk factors in a prospective patient cohort would help validate their use and determine whether there is clinical correlation. The orthopedists in our institution are becoming more aware of these risk factors, but validation is necessary.
1. Garcia AE, Bonnaig JV, Yoneda ZT, et al. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
2. Hollingworth W, Todd C, Parker M, Roberts JA, Williams R. Cost analysis of early discharge after hip fracture. BMJ. 1993;307(6909):903-906.
3. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
4. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
5. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
6. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927.
7. Clague JE, Craddock E, Andrew G, Horan MA, Pendleton N. Predictors of outcome following hip fracture. Admission time predicts length of stay and in-hospital mortality. Injury. 2002;33(1):1-6.
8. Parker MJ, Todd CJ, Palmer CR, et al. Inter-hospital variations in length of hospital stay following hip fracture. Age Ageing. 1998;27(31):333-337.
9. Brasel KJ, Rasmussen J, Cauley C, Weigelt JA. Reasons for delayed discharge of trauma patients. J Surg Res. 2002;107(2):223-226.
10. Bonar SK, Tinetti ME, Speechley M, Cooney LM. Factors associated with short- versus long-term skilled nursing facility placement among community-living hip fracture patients. J Am Geriatr Soc. 1990;38(10):1139-1144.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290-1299.
12. Birkmeyer JD, Gust C, Baser O, Dimick JB, Sutherland JM, Skinner JS. Medicare payments for common inpatient procedures: implications for episode-based payment bundling. Health Serv Res. 2010;45(6 pt 1):1783-1795.
13. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
14. Maciejewski ML, Radcliff A, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276.
15. Michel JP, Klopfenstein C, Hoffmeyer P, Stern R, Grab B. Hip fracture surgery: is the pre-operative American Society of Anesthesiologists (ASA) score a predictor of functional outcome? Aging Clin Exp Res. 2002;14(5):389-394.
16. Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36(6):1115-1121.
17. Cameron ID, Chen JS, March LM, et al. Hip fracture causes excess mortality owing to cardiovascular and infectious disease in institutionalized older people: a prospective 5-year study. J Bone Miner Res. 2010;25(4):866-872.
18. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
19. Le-Wendling L, Bihorac A, Baslanti TO, et al. Regional anesthesia as compared with general anesthesia for surgery in geriatric patients with hip fracture: does it decrease morbidity, mortality, and health care costs? Results of a single-centered study. Pain Med. 2012;13(7):948-956.
20. Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48(6):618-624.
Length of stay (LOS) is a significant driver of costs after hip fracture surgery.1-3 Multiple studies have identified factors associated with increased LOS in hip fracture patients. These factors include admission time, delay to surgery, presence of comorbidities, and older age.4-9
One significant and potentially modifiable factor affecting LOS is delayed transfer to a rehabilitation center after surgery.8-11 Although patients after orthopedic surgeries require additional rehabilitation services or subacute care directly attributable to their injuries, specialized rehabilitation centers may not always have beds readily available.6-11 Studies have shown that delays in transfer to skilled nursing facilities or rehabilitation centers are highly common among orthopedic patients.8 It is therefore imperative that orthopedists have a mechanism for predicting and identifying which patients require rehabilitation services early in the postoperative period. Identifying risk factors and stratifying patients who are most likely to require rehabilitation would facilitate the early transfer of these patients and thereby directly decrease LOS and hospitalization-related costs.
In this article, we report results from prospective, national, multicenter data to identify commonly measured risk factors for discharge to rehabilitation facilities for hip fracture patients. Through multivariate analysis of ACS-NSQIP (American College of Surgeons National Surgical Quality Improvement Program) data, we determined which risk factors significantly predispose patients to discharge to rehabilitation centers versus discharge home. Knowledge of these risk factors allows the practicing orthopedist to be better equipped to identify patients who require additional rehabilitation early in the postoperative course. By mobilizing case managers and social workers to help avoid delays in the transfers of these identified patients, LOS-associated costs may ultimately decrease.
Materials and Methods
After obtaining institutional review board approval for this study from the Office of Research at Vanderbilt University, we prospectively collected 2011 discharge data from the ACS-NSQIP database (these data are unavailable for earlier years). All patients who underwent hip fracture surgery in 2011 were identified by CPT (Current Procedural Terminology) codes. Cases of patients with unknown discharge information and of those who died during their hospitalizations were excluded from analysis. For the remaining patients, discharge information as categorized by ACS-NSQIP included skilled care (eg, subacute hospital, skilled nursing home), unskilled facility (eg, nursing home, assisted facility), separate acute care, and rehabilitation. All other patients were discharged home without additional assistance or to the previous home where they received chronic care, assisted living, or unskilled aid. Patients were dichotomized according to whether they were discharged home or to one of the rehabilitation facilities mentioned.
To determine which risk factors significantly contributed to a patient’s discharge to rehabilitation, we ran univariate analyses using Fisher exact tests for categorical variables and Student t tests for continuous variables on multiple patient factors, including demographics, preoperative comorbidities, and operative factors. Demographics included age and sex. Preoperative comorbidities included 32 conditions: diabetes mellitus, active smoking status, current alcohol use, dyspnea, history of chronic obstructive pulmonary disease, history of congestive heart failure, hypertension requiring medication, history of esophageal varices, history of myocardial infarction, current renal failure, current dialysis dependence, steroid use, recent weight loss, existing bleeding disorder, transfusion before discharge, presence of central nervous system tumor, recent chemotherapy, recent radiation therapy, previous percutaneous coronary intervention, previous percutaneous coronary stenting, history of angina, peripheral vascular disease, cerebrovascular accidents, recent surgery (within 30 days), rest pain, impaired sensorium, history of transient ischemic attacks, current hemiplegia status, current paraplegia status, current quadriplegia status, current ascites, hypertension, and disseminated cancer. Operative factors included wound infection, DNR (do not resuscitate) status, ventilator support, anesthesia type, wound class, ASA (American Society of Anesthesiologists) class, and operative time.
For the univariate analyses, significance was set at P < .05. Demographics, preoperative comorbidities, and operative factors that were significantly associated with discharge to a rehabilitation facility in the univariate analysis were selected as covariates for a multivariate analysis. We incorporated a binary logistic regression to analyze which of these significant risk factors are correlated with a patient’s discharge to a rehabilitation facility after hip fracture surgery.
Results
A total of 4974 patients undergoing surgery for hip fractures in 2011 were identified. Of these patients, 4815 had complete information on discharge location and were included in the analysis.
Table 1 lists the results of the univariate analysis comparing demographics, preoperative comorbidities, and operative factors between the home and rehabilitation groups. Both age (P < .001) and sex (P = .012) were significantly different between groups; the rehabilitation group was older by about 10 years and included significantly more females. In addition to demographic factors, 16 preoperative comorbidities, and 5 surgical factors were significantly associated with discharge to rehabilitation.
Surgery type significantly affected discharge to rehabilitation (Figure). Patients who were undergoing open plating of a femoral neck fracture or intramedullary nailing of an intertrochanteric, peritrochanteric, or subtrochanteric femoral fracture constituted 30% of all patients discharged to rehabilitation centers. In contrast, patients undergoing percutaneous skeletal fixation of a proximal femoral fracture constituted only 5.5% of all patients discharged to rehabilitation. Based on surgery type, we broke down discharge location further, into categories of skilled nursing facility, unskilled facility (not patient’s previous home), separate acute-care facility, dedicated rehabilitation center, and home. Of all 4815 patients combined, 2102 (43.6%) were discharged to a skilled nursing facility, 31 (0.6%) to an unskilled facility (not home), 106 (2.2%) to separate acute care, 1312 (27.2%) to a dedicated rehabilitation center, and 950 (19.7%) home.
Table 2 lists the significant results from the multivariate logistical analysis comparing discharge to a rehabilitation center and discharge home after controlling for the significant risk factors (Table 1). Current diabetes, history of dyspnea, previous myocardial infarction, history of ischemic attacks, current bleeding disorder, transfusion during hospitalization, previous percutaneous cardiac stenting, chemotherapy, past cerebrovascular accident, presence of cancer, surgery type based on CPT code, history of chronic obstructive pulmonary disease or congestive heart failure, current smoking status, and operative time longer than 90 minutes were not significantly correlated with discharge to rehabilitation in the multivariate analysis. All significant factors were associated with higher odds of discharge to rehabilitation except for DNR status. DNR patients were 2.04 times more likely (95% CI, 1.49-2.78; P < .001) to be discharged home than to rehabilitation centers.
Applying these adjusted odds ratios, we see that an elderly woman (age, >65 years) who underwent general anesthesia with an ASA class higher than 2 was 17.63 times more likely than a patient without these risk factors to be discharged to rehabilitation. If this patient were also dialysis-dependent, she would be 61.52 times more likely than a similar patient without dialysis needs to be discharged to rehabilitation.
Even when controlling for all significant and nonsignificant variables in multivariate logistical analysis, age over 65 years (β = 1.05; P < .001), female sex (β = 1.76; P = .004), dialysis dependence (β = 12.98; P = .036), hypertension requiring medication (β = 1.53; P = .032), and ASA class higher than 2 (β = 1.98; P = .001) were found to be significant risk factors for discharge to rehabilitation.
Discussion
This study was the first to investigate the issue of which patient risk factors allow the practicing orthopedist to identify patients who require rehabilitation after hip fracture surgery. Through our multivariate analysis, which controlled for demographics, comorbidities, and operative factors, we found that older age, female sex, history of percutaneous coronary intervention, dialysis dependence, general anesthesia, and ASA class higher than 2 significantly increased the odds of discharge to a rehabilitation center versus home.
Using our study’s results, we can create a risk stratification model for patients and thereby a means of targeting patients who need rehabilitation and starting the process of finding a rehabilitation bed early in the postoperative course. Our study’s variables are easily measured metrics that may be collected in any hospital setting. Especially for hip fracture patients, early planning and discharge to the appropriate rehabilitation center are important in decreasing LOS and associated hospitalization costs. According to one report,3 about 85% of all hip fracture costs are directly related to LOS, given the unnecessarily long rehabilitation periods in hospitals. Hollingworth and colleagues2 compared costs for patients who remained in the hospital with costs for those discharged with rehabilitation services. Overall costs were significantly lower for patients discharged home with rehabilitation. The authors concluded that 40% of hip fracture patients may be suitable for early discharge.2 In an analysis of Medicare payments for hip fracture treatment, hospital costs including LOS accounted for 60% of all payments.12 The results of these 2 studies suggest that the overall driver of hip fracture costs is prolonged LOS and that, if patients are discharged to rehabilitation, then overall costs may be lowered through a direct reduction in hospital LOS. Given that hip fractures account for almost 350,000 hospital admissions in the United States each year, and using our institution’s average hospital charge per day ($4500), about $1.6 billion may be saved if each patient’s LOS decreased by 1 day.13 Although multiple factors affect LOS, discharge planning is under orthopedists’ direct control. Therefore, early identification of patients who will require rehabilitation may help reduce LOS-associated costs in our health care system.
The patient variables that were significantly associated with discharge to rehabilitation are also associated with increased morbidity and mortality in hip fracture patients, according to the literature,14-20 which provides some external validation of using these risk factors as predictors for rehabilitation. A patient with one of these risk factors may require rehabilitation, given that rehabilitation services are specifically linked to lower morbidity and mortality rates among hip fracture patients. For example, patients with dialysis needs were 3.49 times more likely to be discharged to a rehabilitation center in our study. In a 2000 study by Coco and Rush,16 hip fracture patients on dialysis had a 1-year mortality rate 2.5 times higher than that of patients who were not dialysis-dependent. In 2010, Cameron and colleagues17 found that cardiovascular disease was associated with a 2.68 times higher risk of mortality in hip fracture patients. Similarly in our study, both hypertension and history of percutaneous coronary intervention were associated with discharge to rehabilitation. We found higher odds of discharge to rehabilitation with higher ASA classes, which mirror results from a study by Michel and colleagues,15 who found that higher (vs lower) preoperative ASA classes were associated with higher 1-year mortality in hip fracture patients. Interestingly, DNR status was associated with higher odds of discharge home, which may reflect patients’ desires to forgo noninvasive or lifesaving procedures that may be performed at rehabilitation facilities. Although general anesthesia predisposed patients to discharge to a rehabilitation center, multiple studies have found no association between anesthesia type and postoperative mortality rates for hip fracture patients.18,19 Last, Marcantonio and colleagues20 found delirium specifically had a higher odds ratio for discharge, but our univariate analysis did not find a significant association between impaired sensorium and discharge location. Given the correlation of our risk factors with increased morbidity and mortality in the literature, our study’s results provide the initial groundwork for creating a risk calculator that orthopedists can use to predict discharge to rehabilitation.
Our study had some limitations. Although we analyzed a large number of demographics, preoperative comorbidities, and surgical factors, our univariate analysis was limited to information in the ACS-NSQIP database. We did not incorporate other clinically relevant factors (eg, social factors, including patients’ support networks) that may influence discharge decisions. Furthermore, ACS-NSQIP records patient data only up to 30 days after surgery. Discharge information for the time after that was missing for a subset of hip fracture patients, and these patients had to be excluded, potentially skewing our data. ACS-NSQIP also does not collect cost data for patients based on hospitalization or LOS, so we could not determine whether patients discharged to rehabilitation incurred higher costs because of longer hospitalizations.
Nevertheless, our study identified significant patient and operative variables that are associated with discharge to a rehabilitation center. By identifying hip fracture patients with these risk factors early and mobilizing the appropriate resources, practicing orthopedists should be better equipped to help facilitate the discharge of patients to the appropriate location after surgery. Validation of these risk factors should be prospectively determined with an analysis of LOS and cost implications. Use of a risk calculator may in fact result in decreased LOS and hospital-related costs. Furthermore, using these risk factors in a prospective patient cohort would help validate their use and determine whether there is clinical correlation. The orthopedists in our institution are becoming more aware of these risk factors, but validation is necessary.
Length of stay (LOS) is a significant driver of costs after hip fracture surgery.1-3 Multiple studies have identified factors associated with increased LOS in hip fracture patients. These factors include admission time, delay to surgery, presence of comorbidities, and older age.4-9
One significant and potentially modifiable factor affecting LOS is delayed transfer to a rehabilitation center after surgery.8-11 Although patients after orthopedic surgeries require additional rehabilitation services or subacute care directly attributable to their injuries, specialized rehabilitation centers may not always have beds readily available.6-11 Studies have shown that delays in transfer to skilled nursing facilities or rehabilitation centers are highly common among orthopedic patients.8 It is therefore imperative that orthopedists have a mechanism for predicting and identifying which patients require rehabilitation services early in the postoperative period. Identifying risk factors and stratifying patients who are most likely to require rehabilitation would facilitate the early transfer of these patients and thereby directly decrease LOS and hospitalization-related costs.
In this article, we report results from prospective, national, multicenter data to identify commonly measured risk factors for discharge to rehabilitation facilities for hip fracture patients. Through multivariate analysis of ACS-NSQIP (American College of Surgeons National Surgical Quality Improvement Program) data, we determined which risk factors significantly predispose patients to discharge to rehabilitation centers versus discharge home. Knowledge of these risk factors allows the practicing orthopedist to be better equipped to identify patients who require additional rehabilitation early in the postoperative course. By mobilizing case managers and social workers to help avoid delays in the transfers of these identified patients, LOS-associated costs may ultimately decrease.
Materials and Methods
After obtaining institutional review board approval for this study from the Office of Research at Vanderbilt University, we prospectively collected 2011 discharge data from the ACS-NSQIP database (these data are unavailable for earlier years). All patients who underwent hip fracture surgery in 2011 were identified by CPT (Current Procedural Terminology) codes. Cases of patients with unknown discharge information and of those who died during their hospitalizations were excluded from analysis. For the remaining patients, discharge information as categorized by ACS-NSQIP included skilled care (eg, subacute hospital, skilled nursing home), unskilled facility (eg, nursing home, assisted facility), separate acute care, and rehabilitation. All other patients were discharged home without additional assistance or to the previous home where they received chronic care, assisted living, or unskilled aid. Patients were dichotomized according to whether they were discharged home or to one of the rehabilitation facilities mentioned.
To determine which risk factors significantly contributed to a patient’s discharge to rehabilitation, we ran univariate analyses using Fisher exact tests for categorical variables and Student t tests for continuous variables on multiple patient factors, including demographics, preoperative comorbidities, and operative factors. Demographics included age and sex. Preoperative comorbidities included 32 conditions: diabetes mellitus, active smoking status, current alcohol use, dyspnea, history of chronic obstructive pulmonary disease, history of congestive heart failure, hypertension requiring medication, history of esophageal varices, history of myocardial infarction, current renal failure, current dialysis dependence, steroid use, recent weight loss, existing bleeding disorder, transfusion before discharge, presence of central nervous system tumor, recent chemotherapy, recent radiation therapy, previous percutaneous coronary intervention, previous percutaneous coronary stenting, history of angina, peripheral vascular disease, cerebrovascular accidents, recent surgery (within 30 days), rest pain, impaired sensorium, history of transient ischemic attacks, current hemiplegia status, current paraplegia status, current quadriplegia status, current ascites, hypertension, and disseminated cancer. Operative factors included wound infection, DNR (do not resuscitate) status, ventilator support, anesthesia type, wound class, ASA (American Society of Anesthesiologists) class, and operative time.
For the univariate analyses, significance was set at P < .05. Demographics, preoperative comorbidities, and operative factors that were significantly associated with discharge to a rehabilitation facility in the univariate analysis were selected as covariates for a multivariate analysis. We incorporated a binary logistic regression to analyze which of these significant risk factors are correlated with a patient’s discharge to a rehabilitation facility after hip fracture surgery.
Results
A total of 4974 patients undergoing surgery for hip fractures in 2011 were identified. Of these patients, 4815 had complete information on discharge location and were included in the analysis.
Table 1 lists the results of the univariate analysis comparing demographics, preoperative comorbidities, and operative factors between the home and rehabilitation groups. Both age (P < .001) and sex (P = .012) were significantly different between groups; the rehabilitation group was older by about 10 years and included significantly more females. In addition to demographic factors, 16 preoperative comorbidities, and 5 surgical factors were significantly associated with discharge to rehabilitation.
Surgery type significantly affected discharge to rehabilitation (Figure). Patients who were undergoing open plating of a femoral neck fracture or intramedullary nailing of an intertrochanteric, peritrochanteric, or subtrochanteric femoral fracture constituted 30% of all patients discharged to rehabilitation centers. In contrast, patients undergoing percutaneous skeletal fixation of a proximal femoral fracture constituted only 5.5% of all patients discharged to rehabilitation. Based on surgery type, we broke down discharge location further, into categories of skilled nursing facility, unskilled facility (not patient’s previous home), separate acute-care facility, dedicated rehabilitation center, and home. Of all 4815 patients combined, 2102 (43.6%) were discharged to a skilled nursing facility, 31 (0.6%) to an unskilled facility (not home), 106 (2.2%) to separate acute care, 1312 (27.2%) to a dedicated rehabilitation center, and 950 (19.7%) home.
Table 2 lists the significant results from the multivariate logistical analysis comparing discharge to a rehabilitation center and discharge home after controlling for the significant risk factors (Table 1). Current diabetes, history of dyspnea, previous myocardial infarction, history of ischemic attacks, current bleeding disorder, transfusion during hospitalization, previous percutaneous cardiac stenting, chemotherapy, past cerebrovascular accident, presence of cancer, surgery type based on CPT code, history of chronic obstructive pulmonary disease or congestive heart failure, current smoking status, and operative time longer than 90 minutes were not significantly correlated with discharge to rehabilitation in the multivariate analysis. All significant factors were associated with higher odds of discharge to rehabilitation except for DNR status. DNR patients were 2.04 times more likely (95% CI, 1.49-2.78; P < .001) to be discharged home than to rehabilitation centers.
Applying these adjusted odds ratios, we see that an elderly woman (age, >65 years) who underwent general anesthesia with an ASA class higher than 2 was 17.63 times more likely than a patient without these risk factors to be discharged to rehabilitation. If this patient were also dialysis-dependent, she would be 61.52 times more likely than a similar patient without dialysis needs to be discharged to rehabilitation.
Even when controlling for all significant and nonsignificant variables in multivariate logistical analysis, age over 65 years (β = 1.05; P < .001), female sex (β = 1.76; P = .004), dialysis dependence (β = 12.98; P = .036), hypertension requiring medication (β = 1.53; P = .032), and ASA class higher than 2 (β = 1.98; P = .001) were found to be significant risk factors for discharge to rehabilitation.
Discussion
This study was the first to investigate the issue of which patient risk factors allow the practicing orthopedist to identify patients who require rehabilitation after hip fracture surgery. Through our multivariate analysis, which controlled for demographics, comorbidities, and operative factors, we found that older age, female sex, history of percutaneous coronary intervention, dialysis dependence, general anesthesia, and ASA class higher than 2 significantly increased the odds of discharge to a rehabilitation center versus home.
Using our study’s results, we can create a risk stratification model for patients and thereby a means of targeting patients who need rehabilitation and starting the process of finding a rehabilitation bed early in the postoperative course. Our study’s variables are easily measured metrics that may be collected in any hospital setting. Especially for hip fracture patients, early planning and discharge to the appropriate rehabilitation center are important in decreasing LOS and associated hospitalization costs. According to one report,3 about 85% of all hip fracture costs are directly related to LOS, given the unnecessarily long rehabilitation periods in hospitals. Hollingworth and colleagues2 compared costs for patients who remained in the hospital with costs for those discharged with rehabilitation services. Overall costs were significantly lower for patients discharged home with rehabilitation. The authors concluded that 40% of hip fracture patients may be suitable for early discharge.2 In an analysis of Medicare payments for hip fracture treatment, hospital costs including LOS accounted for 60% of all payments.12 The results of these 2 studies suggest that the overall driver of hip fracture costs is prolonged LOS and that, if patients are discharged to rehabilitation, then overall costs may be lowered through a direct reduction in hospital LOS. Given that hip fractures account for almost 350,000 hospital admissions in the United States each year, and using our institution’s average hospital charge per day ($4500), about $1.6 billion may be saved if each patient’s LOS decreased by 1 day.13 Although multiple factors affect LOS, discharge planning is under orthopedists’ direct control. Therefore, early identification of patients who will require rehabilitation may help reduce LOS-associated costs in our health care system.
The patient variables that were significantly associated with discharge to rehabilitation are also associated with increased morbidity and mortality in hip fracture patients, according to the literature,14-20 which provides some external validation of using these risk factors as predictors for rehabilitation. A patient with one of these risk factors may require rehabilitation, given that rehabilitation services are specifically linked to lower morbidity and mortality rates among hip fracture patients. For example, patients with dialysis needs were 3.49 times more likely to be discharged to a rehabilitation center in our study. In a 2000 study by Coco and Rush,16 hip fracture patients on dialysis had a 1-year mortality rate 2.5 times higher than that of patients who were not dialysis-dependent. In 2010, Cameron and colleagues17 found that cardiovascular disease was associated with a 2.68 times higher risk of mortality in hip fracture patients. Similarly in our study, both hypertension and history of percutaneous coronary intervention were associated with discharge to rehabilitation. We found higher odds of discharge to rehabilitation with higher ASA classes, which mirror results from a study by Michel and colleagues,15 who found that higher (vs lower) preoperative ASA classes were associated with higher 1-year mortality in hip fracture patients. Interestingly, DNR status was associated with higher odds of discharge home, which may reflect patients’ desires to forgo noninvasive or lifesaving procedures that may be performed at rehabilitation facilities. Although general anesthesia predisposed patients to discharge to a rehabilitation center, multiple studies have found no association between anesthesia type and postoperative mortality rates for hip fracture patients.18,19 Last, Marcantonio and colleagues20 found delirium specifically had a higher odds ratio for discharge, but our univariate analysis did not find a significant association between impaired sensorium and discharge location. Given the correlation of our risk factors with increased morbidity and mortality in the literature, our study’s results provide the initial groundwork for creating a risk calculator that orthopedists can use to predict discharge to rehabilitation.
Our study had some limitations. Although we analyzed a large number of demographics, preoperative comorbidities, and surgical factors, our univariate analysis was limited to information in the ACS-NSQIP database. We did not incorporate other clinically relevant factors (eg, social factors, including patients’ support networks) that may influence discharge decisions. Furthermore, ACS-NSQIP records patient data only up to 30 days after surgery. Discharge information for the time after that was missing for a subset of hip fracture patients, and these patients had to be excluded, potentially skewing our data. ACS-NSQIP also does not collect cost data for patients based on hospitalization or LOS, so we could not determine whether patients discharged to rehabilitation incurred higher costs because of longer hospitalizations.
Nevertheless, our study identified significant patient and operative variables that are associated with discharge to a rehabilitation center. By identifying hip fracture patients with these risk factors early and mobilizing the appropriate resources, practicing orthopedists should be better equipped to help facilitate the discharge of patients to the appropriate location after surgery. Validation of these risk factors should be prospectively determined with an analysis of LOS and cost implications. Use of a risk calculator may in fact result in decreased LOS and hospital-related costs. Furthermore, using these risk factors in a prospective patient cohort would help validate their use and determine whether there is clinical correlation. The orthopedists in our institution are becoming more aware of these risk factors, but validation is necessary.
1. Garcia AE, Bonnaig JV, Yoneda ZT, et al. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
2. Hollingworth W, Todd C, Parker M, Roberts JA, Williams R. Cost analysis of early discharge after hip fracture. BMJ. 1993;307(6909):903-906.
3. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
4. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
5. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
6. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927.
7. Clague JE, Craddock E, Andrew G, Horan MA, Pendleton N. Predictors of outcome following hip fracture. Admission time predicts length of stay and in-hospital mortality. Injury. 2002;33(1):1-6.
8. Parker MJ, Todd CJ, Palmer CR, et al. Inter-hospital variations in length of hospital stay following hip fracture. Age Ageing. 1998;27(31):333-337.
9. Brasel KJ, Rasmussen J, Cauley C, Weigelt JA. Reasons for delayed discharge of trauma patients. J Surg Res. 2002;107(2):223-226.
10. Bonar SK, Tinetti ME, Speechley M, Cooney LM. Factors associated with short- versus long-term skilled nursing facility placement among community-living hip fracture patients. J Am Geriatr Soc. 1990;38(10):1139-1144.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290-1299.
12. Birkmeyer JD, Gust C, Baser O, Dimick JB, Sutherland JM, Skinner JS. Medicare payments for common inpatient procedures: implications for episode-based payment bundling. Health Serv Res. 2010;45(6 pt 1):1783-1795.
13. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
14. Maciejewski ML, Radcliff A, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276.
15. Michel JP, Klopfenstein C, Hoffmeyer P, Stern R, Grab B. Hip fracture surgery: is the pre-operative American Society of Anesthesiologists (ASA) score a predictor of functional outcome? Aging Clin Exp Res. 2002;14(5):389-394.
16. Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36(6):1115-1121.
17. Cameron ID, Chen JS, March LM, et al. Hip fracture causes excess mortality owing to cardiovascular and infectious disease in institutionalized older people: a prospective 5-year study. J Bone Miner Res. 2010;25(4):866-872.
18. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
19. Le-Wendling L, Bihorac A, Baslanti TO, et al. Regional anesthesia as compared with general anesthesia for surgery in geriatric patients with hip fracture: does it decrease morbidity, mortality, and health care costs? Results of a single-centered study. Pain Med. 2012;13(7):948-956.
20. Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48(6):618-624.
1. Garcia AE, Bonnaig JV, Yoneda ZT, et al. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
2. Hollingworth W, Todd C, Parker M, Roberts JA, Williams R. Cost analysis of early discharge after hip fracture. BMJ. 1993;307(6909):903-906.
3. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
4. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
5. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
6. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927.
7. Clague JE, Craddock E, Andrew G, Horan MA, Pendleton N. Predictors of outcome following hip fracture. Admission time predicts length of stay and in-hospital mortality. Injury. 2002;33(1):1-6.
8. Parker MJ, Todd CJ, Palmer CR, et al. Inter-hospital variations in length of hospital stay following hip fracture. Age Ageing. 1998;27(31):333-337.
9. Brasel KJ, Rasmussen J, Cauley C, Weigelt JA. Reasons for delayed discharge of trauma patients. J Surg Res. 2002;107(2):223-226.
10. Bonar SK, Tinetti ME, Speechley M, Cooney LM. Factors associated with short- versus long-term skilled nursing facility placement among community-living hip fracture patients. J Am Geriatr Soc. 1990;38(10):1139-1144.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290-1299.
12. Birkmeyer JD, Gust C, Baser O, Dimick JB, Sutherland JM, Skinner JS. Medicare payments for common inpatient procedures: implications for episode-based payment bundling. Health Serv Res. 2010;45(6 pt 1):1783-1795.
13. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
14. Maciejewski ML, Radcliff A, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276.
15. Michel JP, Klopfenstein C, Hoffmeyer P, Stern R, Grab B. Hip fracture surgery: is the pre-operative American Society of Anesthesiologists (ASA) score a predictor of functional outcome? Aging Clin Exp Res. 2002;14(5):389-394.
16. Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36(6):1115-1121.
17. Cameron ID, Chen JS, March LM, et al. Hip fracture causes excess mortality owing to cardiovascular and infectious disease in institutionalized older people: a prospective 5-year study. J Bone Miner Res. 2010;25(4):866-872.
18. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
19. Le-Wendling L, Bihorac A, Baslanti TO, et al. Regional anesthesia as compared with general anesthesia for surgery in geriatric patients with hip fracture: does it decrease morbidity, mortality, and health care costs? Results of a single-centered study. Pain Med. 2012;13(7):948-956.
20. Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48(6):618-624.
Incidence and Functional Outcomes of Malunion of Nonoperatively Treated Humeral Shaft Fractures
Humeral shaft fractures account for about 1% of all fractures.1 With the exception of the few absolute indications for surgical intervention, such as the presence of an open fracture, the current teaching on treatment of these fractures is that the majority can be successfully managed nonoperatively.1-3 These conservative measures consist of bandages, abduction splints, U-casts, hanging arm casts, and, most commonly, functional bracing, which is considered the gold standard for treatment of humeral shaft fractures by many authors.1-3 One of the most often cited disadvantages of nonoperative management over surgical treatment is the higher incidence of residual deformity, the most common of which is varus angulation.4
The incidence of malunion (>20° of angulation in any plane or shortening of ≥2.5 cm) after nonoperative treatment varies in the literature from 0% to 13%,2,4-9 with a recent literature review documenting a mean incidence of 4.4% within the frontal plane and 2% within the sagittal plane across all studies.2 As reported initially by Sarmiento and colleagues3,9 and echoed by other authors,2,5,8 angular deformity of less than 20° is thought to be both cosmetically and functionally acceptable. Whether angular deformities or malunion of more than 20° actually leads to functional limitations is unknown. Although some observational reports suggest that the degree of radiographic malalignment does not necessarily correlate with functional outcome,8 no studies have specifically evaluated patient outcomes of humeral shaft fracture malunions.
We conducted a study to determine the overall incidence and long-term clinical and functional outcomes of patients with malunion after nonoperative management of humeral shaft fractures. Long-term outcomes were assessed with current symptoms, physical examination findings, need for subsequent operative intervention, DASH (Disabilities of the Arm, Shoulder, and Hand) scores, and a self-reported questionnaire. We hypothesized that patients who develop a malunion after nonoperative treatment of a closed humeral shaft fracture will have satisfactory functional outcomes based on subjective reports, physical examination findings, and DASH scores.
Methods
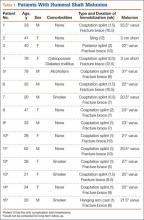
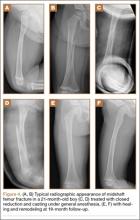
After obtaining institutional review board approval for the study, we selected patients from a retrospective medical record review of all those 18 years or older with a humeral shaft fracture managed nonoperatively at our institution between January 1, 2001, and June 30, 2012, with a minimum 1-year follow-up. We identified 156 patients with nonoperatively managed midshaft humerus fractures. Study exclusion criteria included fracture associated with a tumor (3 patients), ipsilateral upper extremity injury (9), open/ballistic injury (18), nonunion (9), underlying cognitive disability or psychiatric illness (4), and insufficient follow-up to clinical or radiographic healing (22). Ninety-one patients were eligible for study inclusion. Radiographs at time of final clinical visit were reviewed to assess for evidence of malunion at the fracture site, as defined by previously reported criteria3 (>20° angulation in anterior/posterior or varus/valgus plane of motion or shortening of ≥2.5 cm). Fifteen patients met all the inclusion criteria for further evaluation.
Medical records were retrospectively reviewed for information on age at injury, sex, comorbidities (eg, diabetes, osteoporosis, smoking), body mass index, type and duration of immobilization, complications, return to work, cosmetic perception, time to final clinical follow-up, and symptoms at final clinical follow-up. Incidence of potential risk factors associated with malunion—obesity, noncompliance, and comorbidities such as smoking and diabetes—was compared between the 15 patients with malunion and the other study patients, who healed without malunion.
For long-term postoperative follow-up, patients were contacted to be seen in clinic to complete an updated physical examination, self-reported questionnaire, and the DASH form. Physical examination included measurements of range of motion (ROM) and strength involving the shoulder, elbow, and forearm, with ROM reported as the difference between the injured and contralateral upper extremities. Neurovascular status and focal tenderness to palpation were also assessed on examination. When in-person examination was not possible, the questionnaire and DASH form were completed over the telephone. The self-reported questionnaire asked for information on smoking status, pain, functional limitations, cosmetic perception, satisfaction, and whether or not the patient would still opt for nonoperative management if presented with the same injury again. Pain and satisfaction were measured on numerical scales: Pain scores ranged from 0 (no pain) to 10 (worst possible pain), and satisfaction scores ranged from 1 (not satisfied) to 5 (very satisfied). Data are presented as mean values.
Results
Of the 91 study-eligible patients, 15 (16%) met the radiographic criteria for the diagnosis of malunion. Retrospective data were available for all 15 patients from time of injury to final clinical follow-up (mean, 19 weeks; range, 7-53 weeks). Mean age at injury was 39 years (range, 20-79 years). Additional demographics are listed in Table 1. Incidence of potential risk factors, such as body mass index (26.5 vs 25.4), smoking (33% vs 33%), and diabetes (0% vs 8%), was not significantly different between the malunion and healed-without-malunion groups, respectively. Furthermore, all malunion patients were compliant with their treatment protocol.
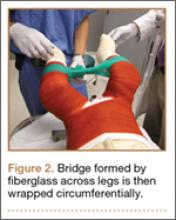
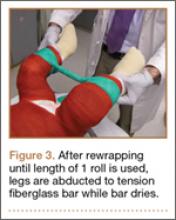
Radiographs were assessed at time of final follow-up to confirm healing and to document malunion. Varus malunion was found in 13 patients (mean, 24°; range, 20.5°-35.5°), and shortening was documented in the other 2 patients (mean, 4 cm; range, 3-5 cm). Patients were immobilized a mean of 10 weeks (range, 6-13 weeks). Initial fracture management consisted of coaptation splinting for 1 to 2 weeks (12 patients), hanging arm cast for 1 week (1 patient), and posterior splint for 1 week (1 patient). Patients were then transitioned to Sarmiento fracture bracing for the duration of their treatment (range, 5-12 months). One patient, followed initially at an outside institution, was managed in a sling throughout the duration of treatment (12 weeks) (Table 1). All 15 patients were neurovascularly intact at time of final clinical examination, with return of full upper extremity ROM in all but 3 patients. Only 1 of these 3 patients reported residual pain and functional limitations 4 months after injury (Table 2). Twelve patients were evaluated for return to work, with all successfully returning to work without restrictions at time of final follow-up. The 1 minor complication noted during the treatment period involved medial-sided elbow skin breakdown from brace wear, which resolved with local wound care. No patient required or requested surgical intervention for their residual malunion.
Of the 15 patients, 8 (53%) were reached for in-person examination (6 patients) or telephone interview (2 patients) for follow-up assessment by means of DASH form and self-reported questionnaire a mean of 47 months (range, 12-99 months) after initial injury. The 6 patients who had a physical examination were neurovascularly intact, lacked focal tenderness to palpation, and demonstrated full (5/5) strength within the deltoid, biceps, triceps, pronator, and supinator musculature. Each patient had equal ROM compared with the contralateral uninjured extremity on shoulder forward flexion and abduction, elbow flexion and extension, and forearm pronation and supination. Three patients (50%) had mild residual loss of ROM, with 2 demonstrating decreased shoulder external rotation of 10° and 15°, respectively, and 1 demonstrating decreased shoulder internal rotation of 10°.
Mean DASH score was 10.4 (range, 0-49.2). Evaluation of the self-reported questionnaire revealed a mean pain score of 1.1 (range, 0-7), with only 2 patients reporting any ongoing pain. In addition, 2 patients reported functional limitations, both related to overhead activities. However, 6 (75%) of the 8 patients reported noticeable cosmetic deformity, most commonly varus angulation (4 patients), as well as palpable bony prominence (2) and muscle atrophy (1). The majority of patients were satisfied with the outcome of their treatment (mean, 4; range, 2-5), with 6 patients reporting being satisfied or very satisfied, and all 6 indicating they would undergo nonoperative management again if presented with the same injury. Two patients reported being dissatisfied with their outcome, 1 because of cosmetic appearance and 1 because of cosmetic appearance and functional limitations. Both patients indicated they would choose operative management if presented with the same injury. There was no apparent relationship between outcome and degree of residual deformity, as both patients with varus angulation of more than 30° reported no residual pain or functional limitation and were very satisfied with the outcome of their treatment (Table 2).
Of the 7 patients who could not be reached for final follow-up, 2 on initial contact expressed overall satisfaction with their outcome and denied functional limitations. However, both asked to complete the study at a later date. Subsequently, these 2 patients could not be reached to complete the formal follow-up.
Discussion
Humeral shaft fractures are usually managed nonoperatively. One of the most commonly cited disadvantages of nonoperative management is its higher incidence of residual angular deformity, up to 13% in previous studies.4 Our study found a slightly higher incidence, 16%, on review of 91 nonoperatively managed humeral shaft fractures treated over an 11.5 year period. Although previous studies have reported acceptable functional and cosmetic outcomes with residual angular deformity of less than 20°,2,3,5,8,9 only observational reports have suggested acceptable function in patients with a documented malunion.8
To our knowledge, ours is the first study to correlate malunion with functional parameters and subjective patient-reported outcomes. We found that malunion was not associated with significant pain or functional limitation after nonoperative management of humeral shaft fractures. Furthermore, 75% of patients were satisfied or very satisfied with the outcome of their treatment and indicated they would undergo nonoperative management if presented with the same injury again. However, 75% of patients reported a noticeable cosmetic deformity, and one-third of these patients cited it as a major reason for dissatisfaction with their overall outcome. Regarding function, all patients returned to full strength and ROM of the affected extremity, aside from small losses of internal or external shoulder rotation on the magnitude of 10° to 15° in 50% of those patients tested. In addition, 75% of patients returned to regular activity without functional limitations; the other 25% reported trouble with overhead activities. There were no significant complications during the treatment or follow-up period, once the fracture had healed.
The major limitation of this study was its small patient population. (Obtaining a larger series of patients with malunion after nonoperative treatment of humeral shaft fractures likely would require a multicenter study.) Some of our study findings, such as lack of correlation between degree of malunion and subsequent functional or subjective outcomes, would require a larger sample size for verification and more definitive conclusions. Another limitation is that the study was not designed to evaluate the cause of malunion. Therefore, we cannot draw any definitive conclusions regarding what may have contributed to the development of malunion in our study population. However, all our malunion patients were compliant with their treatment protocol, and they showed no significant difference in incidence of potential risk factors (eg, obesity, comorbidities) compared with the patients who healed without malunion.
Conclusion
Malunion after nonoperative management of humeral shaft fractures does not appear to result in significant pain, dissatisfaction, or functional limitation as measured on physical examination and with validated objective outcome measures in the majority of patients. Furthermore, no patients in this study required surgical intervention for any residual limitations or complications after malunion. The majority of patients reported a noticeable cosmetic deformity, which left a small subset of patients dissatisfied. Overall, our study findings can be used to help counsel patients before and during nonoperative management—particularly patients who appear to be healing with some malunion. Our findings suggest that operative intervention to prevent malunion is not necessary, as it likely would not result in any overall improvement in patient function or satisfaction, but patients should be counseled regarding the high likelihood of cosmetic deformity, which may or may not be bothersome.
1. Rockwood CA, Green DP, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
2. Papasoulis E, Drosos GI, Ververidis AN, Verettas DA. Functional bracing of humeral shaft fractures. A review of clinical studies. Injury. 2010;41(7):e21-e27.
3. Sarmiento A, Latta LL. Functional fracture bracing. J Am Acad Orthop Surg. 1999;7(1):66-75.
4. Denard A Jr, Richards JE, Obremskey WT, Tucker MC, Floyd M, Herzog GA. Outcome of nonoperative vs operative treatment of humeral shaft fractures: a retrospective study of 213 patients. Orthopedics. 2010;33(8).
5. Fjalestad T, Strømsøe K, Salvesen P, Rostad B. Functional results of braced humeral diaphyseal fractures: why do 38% lose external rotation of the shoulder? Arch Orthop Trauma Surg. 2000;120(5-6):281-285.
6. Koch PP, Gross DF, Gerber C. The results of functional (Sarmiento) bracing of humeral shaft fractures. J Shoulder Elbow Surg. 2002;11(2):143-150.
7. Ozkurt B, Altay M, Aktekin CN, Toprak A, Tabak Y. The role of functional bracing in the treatment of humeral shaft fractures [in Turkish]. Acta Orthop Traumatol Turc. 2007;41(1):15-20.
8. Rutgers M, Ring D. Treatment of diaphyseal fractures of the humerus using a functional brace. J Orthop Trauma. 2006;20(9):597-601.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
Humeral shaft fractures account for about 1% of all fractures.1 With the exception of the few absolute indications for surgical intervention, such as the presence of an open fracture, the current teaching on treatment of these fractures is that the majority can be successfully managed nonoperatively.1-3 These conservative measures consist of bandages, abduction splints, U-casts, hanging arm casts, and, most commonly, functional bracing, which is considered the gold standard for treatment of humeral shaft fractures by many authors.1-3 One of the most often cited disadvantages of nonoperative management over surgical treatment is the higher incidence of residual deformity, the most common of which is varus angulation.4
The incidence of malunion (>20° of angulation in any plane or shortening of ≥2.5 cm) after nonoperative treatment varies in the literature from 0% to 13%,2,4-9 with a recent literature review documenting a mean incidence of 4.4% within the frontal plane and 2% within the sagittal plane across all studies.2 As reported initially by Sarmiento and colleagues3,9 and echoed by other authors,2,5,8 angular deformity of less than 20° is thought to be both cosmetically and functionally acceptable. Whether angular deformities or malunion of more than 20° actually leads to functional limitations is unknown. Although some observational reports suggest that the degree of radiographic malalignment does not necessarily correlate with functional outcome,8 no studies have specifically evaluated patient outcomes of humeral shaft fracture malunions.
We conducted a study to determine the overall incidence and long-term clinical and functional outcomes of patients with malunion after nonoperative management of humeral shaft fractures. Long-term outcomes were assessed with current symptoms, physical examination findings, need for subsequent operative intervention, DASH (Disabilities of the Arm, Shoulder, and Hand) scores, and a self-reported questionnaire. We hypothesized that patients who develop a malunion after nonoperative treatment of a closed humeral shaft fracture will have satisfactory functional outcomes based on subjective reports, physical examination findings, and DASH scores.
Methods
After obtaining institutional review board approval for the study, we selected patients from a retrospective medical record review of all those 18 years or older with a humeral shaft fracture managed nonoperatively at our institution between January 1, 2001, and June 30, 2012, with a minimum 1-year follow-up. We identified 156 patients with nonoperatively managed midshaft humerus fractures. Study exclusion criteria included fracture associated with a tumor (3 patients), ipsilateral upper extremity injury (9), open/ballistic injury (18), nonunion (9), underlying cognitive disability or psychiatric illness (4), and insufficient follow-up to clinical or radiographic healing (22). Ninety-one patients were eligible for study inclusion. Radiographs at time of final clinical visit were reviewed to assess for evidence of malunion at the fracture site, as defined by previously reported criteria3 (>20° angulation in anterior/posterior or varus/valgus plane of motion or shortening of ≥2.5 cm). Fifteen patients met all the inclusion criteria for further evaluation.
Medical records were retrospectively reviewed for information on age at injury, sex, comorbidities (eg, diabetes, osteoporosis, smoking), body mass index, type and duration of immobilization, complications, return to work, cosmetic perception, time to final clinical follow-up, and symptoms at final clinical follow-up. Incidence of potential risk factors associated with malunion—obesity, noncompliance, and comorbidities such as smoking and diabetes—was compared between the 15 patients with malunion and the other study patients, who healed without malunion.
For long-term postoperative follow-up, patients were contacted to be seen in clinic to complete an updated physical examination, self-reported questionnaire, and the DASH form. Physical examination included measurements of range of motion (ROM) and strength involving the shoulder, elbow, and forearm, with ROM reported as the difference between the injured and contralateral upper extremities. Neurovascular status and focal tenderness to palpation were also assessed on examination. When in-person examination was not possible, the questionnaire and DASH form were completed over the telephone. The self-reported questionnaire asked for information on smoking status, pain, functional limitations, cosmetic perception, satisfaction, and whether or not the patient would still opt for nonoperative management if presented with the same injury again. Pain and satisfaction were measured on numerical scales: Pain scores ranged from 0 (no pain) to 10 (worst possible pain), and satisfaction scores ranged from 1 (not satisfied) to 5 (very satisfied). Data are presented as mean values.
Results
Of the 91 study-eligible patients, 15 (16%) met the radiographic criteria for the diagnosis of malunion. Retrospective data were available for all 15 patients from time of injury to final clinical follow-up (mean, 19 weeks; range, 7-53 weeks). Mean age at injury was 39 years (range, 20-79 years). Additional demographics are listed in Table 1. Incidence of potential risk factors, such as body mass index (26.5 vs 25.4), smoking (33% vs 33%), and diabetes (0% vs 8%), was not significantly different between the malunion and healed-without-malunion groups, respectively. Furthermore, all malunion patients were compliant with their treatment protocol.
Radiographs were assessed at time of final follow-up to confirm healing and to document malunion. Varus malunion was found in 13 patients (mean, 24°; range, 20.5°-35.5°), and shortening was documented in the other 2 patients (mean, 4 cm; range, 3-5 cm). Patients were immobilized a mean of 10 weeks (range, 6-13 weeks). Initial fracture management consisted of coaptation splinting for 1 to 2 weeks (12 patients), hanging arm cast for 1 week (1 patient), and posterior splint for 1 week (1 patient). Patients were then transitioned to Sarmiento fracture bracing for the duration of their treatment (range, 5-12 months). One patient, followed initially at an outside institution, was managed in a sling throughout the duration of treatment (12 weeks) (Table 1). All 15 patients were neurovascularly intact at time of final clinical examination, with return of full upper extremity ROM in all but 3 patients. Only 1 of these 3 patients reported residual pain and functional limitations 4 months after injury (Table 2). Twelve patients were evaluated for return to work, with all successfully returning to work without restrictions at time of final follow-up. The 1 minor complication noted during the treatment period involved medial-sided elbow skin breakdown from brace wear, which resolved with local wound care. No patient required or requested surgical intervention for their residual malunion.
Of the 15 patients, 8 (53%) were reached for in-person examination (6 patients) or telephone interview (2 patients) for follow-up assessment by means of DASH form and self-reported questionnaire a mean of 47 months (range, 12-99 months) after initial injury. The 6 patients who had a physical examination were neurovascularly intact, lacked focal tenderness to palpation, and demonstrated full (5/5) strength within the deltoid, biceps, triceps, pronator, and supinator musculature. Each patient had equal ROM compared with the contralateral uninjured extremity on shoulder forward flexion and abduction, elbow flexion and extension, and forearm pronation and supination. Three patients (50%) had mild residual loss of ROM, with 2 demonstrating decreased shoulder external rotation of 10° and 15°, respectively, and 1 demonstrating decreased shoulder internal rotation of 10°.
Mean DASH score was 10.4 (range, 0-49.2). Evaluation of the self-reported questionnaire revealed a mean pain score of 1.1 (range, 0-7), with only 2 patients reporting any ongoing pain. In addition, 2 patients reported functional limitations, both related to overhead activities. However, 6 (75%) of the 8 patients reported noticeable cosmetic deformity, most commonly varus angulation (4 patients), as well as palpable bony prominence (2) and muscle atrophy (1). The majority of patients were satisfied with the outcome of their treatment (mean, 4; range, 2-5), with 6 patients reporting being satisfied or very satisfied, and all 6 indicating they would undergo nonoperative management again if presented with the same injury. Two patients reported being dissatisfied with their outcome, 1 because of cosmetic appearance and 1 because of cosmetic appearance and functional limitations. Both patients indicated they would choose operative management if presented with the same injury. There was no apparent relationship between outcome and degree of residual deformity, as both patients with varus angulation of more than 30° reported no residual pain or functional limitation and were very satisfied with the outcome of their treatment (Table 2).
Of the 7 patients who could not be reached for final follow-up, 2 on initial contact expressed overall satisfaction with their outcome and denied functional limitations. However, both asked to complete the study at a later date. Subsequently, these 2 patients could not be reached to complete the formal follow-up.
Discussion
Humeral shaft fractures are usually managed nonoperatively. One of the most commonly cited disadvantages of nonoperative management is its higher incidence of residual angular deformity, up to 13% in previous studies.4 Our study found a slightly higher incidence, 16%, on review of 91 nonoperatively managed humeral shaft fractures treated over an 11.5 year period. Although previous studies have reported acceptable functional and cosmetic outcomes with residual angular deformity of less than 20°,2,3,5,8,9 only observational reports have suggested acceptable function in patients with a documented malunion.8
To our knowledge, ours is the first study to correlate malunion with functional parameters and subjective patient-reported outcomes. We found that malunion was not associated with significant pain or functional limitation after nonoperative management of humeral shaft fractures. Furthermore, 75% of patients were satisfied or very satisfied with the outcome of their treatment and indicated they would undergo nonoperative management if presented with the same injury again. However, 75% of patients reported a noticeable cosmetic deformity, and one-third of these patients cited it as a major reason for dissatisfaction with their overall outcome. Regarding function, all patients returned to full strength and ROM of the affected extremity, aside from small losses of internal or external shoulder rotation on the magnitude of 10° to 15° in 50% of those patients tested. In addition, 75% of patients returned to regular activity without functional limitations; the other 25% reported trouble with overhead activities. There were no significant complications during the treatment or follow-up period, once the fracture had healed.
The major limitation of this study was its small patient population. (Obtaining a larger series of patients with malunion after nonoperative treatment of humeral shaft fractures likely would require a multicenter study.) Some of our study findings, such as lack of correlation between degree of malunion and subsequent functional or subjective outcomes, would require a larger sample size for verification and more definitive conclusions. Another limitation is that the study was not designed to evaluate the cause of malunion. Therefore, we cannot draw any definitive conclusions regarding what may have contributed to the development of malunion in our study population. However, all our malunion patients were compliant with their treatment protocol, and they showed no significant difference in incidence of potential risk factors (eg, obesity, comorbidities) compared with the patients who healed without malunion.
Conclusion
Malunion after nonoperative management of humeral shaft fractures does not appear to result in significant pain, dissatisfaction, or functional limitation as measured on physical examination and with validated objective outcome measures in the majority of patients. Furthermore, no patients in this study required surgical intervention for any residual limitations or complications after malunion. The majority of patients reported a noticeable cosmetic deformity, which left a small subset of patients dissatisfied. Overall, our study findings can be used to help counsel patients before and during nonoperative management—particularly patients who appear to be healing with some malunion. Our findings suggest that operative intervention to prevent malunion is not necessary, as it likely would not result in any overall improvement in patient function or satisfaction, but patients should be counseled regarding the high likelihood of cosmetic deformity, which may or may not be bothersome.
Humeral shaft fractures account for about 1% of all fractures.1 With the exception of the few absolute indications for surgical intervention, such as the presence of an open fracture, the current teaching on treatment of these fractures is that the majority can be successfully managed nonoperatively.1-3 These conservative measures consist of bandages, abduction splints, U-casts, hanging arm casts, and, most commonly, functional bracing, which is considered the gold standard for treatment of humeral shaft fractures by many authors.1-3 One of the most often cited disadvantages of nonoperative management over surgical treatment is the higher incidence of residual deformity, the most common of which is varus angulation.4
The incidence of malunion (>20° of angulation in any plane or shortening of ≥2.5 cm) after nonoperative treatment varies in the literature from 0% to 13%,2,4-9 with a recent literature review documenting a mean incidence of 4.4% within the frontal plane and 2% within the sagittal plane across all studies.2 As reported initially by Sarmiento and colleagues3,9 and echoed by other authors,2,5,8 angular deformity of less than 20° is thought to be both cosmetically and functionally acceptable. Whether angular deformities or malunion of more than 20° actually leads to functional limitations is unknown. Although some observational reports suggest that the degree of radiographic malalignment does not necessarily correlate with functional outcome,8 no studies have specifically evaluated patient outcomes of humeral shaft fracture malunions.
We conducted a study to determine the overall incidence and long-term clinical and functional outcomes of patients with malunion after nonoperative management of humeral shaft fractures. Long-term outcomes were assessed with current symptoms, physical examination findings, need for subsequent operative intervention, DASH (Disabilities of the Arm, Shoulder, and Hand) scores, and a self-reported questionnaire. We hypothesized that patients who develop a malunion after nonoperative treatment of a closed humeral shaft fracture will have satisfactory functional outcomes based on subjective reports, physical examination findings, and DASH scores.
Methods
After obtaining institutional review board approval for the study, we selected patients from a retrospective medical record review of all those 18 years or older with a humeral shaft fracture managed nonoperatively at our institution between January 1, 2001, and June 30, 2012, with a minimum 1-year follow-up. We identified 156 patients with nonoperatively managed midshaft humerus fractures. Study exclusion criteria included fracture associated with a tumor (3 patients), ipsilateral upper extremity injury (9), open/ballistic injury (18), nonunion (9), underlying cognitive disability or psychiatric illness (4), and insufficient follow-up to clinical or radiographic healing (22). Ninety-one patients were eligible for study inclusion. Radiographs at time of final clinical visit were reviewed to assess for evidence of malunion at the fracture site, as defined by previously reported criteria3 (>20° angulation in anterior/posterior or varus/valgus plane of motion or shortening of ≥2.5 cm). Fifteen patients met all the inclusion criteria for further evaluation.
Medical records were retrospectively reviewed for information on age at injury, sex, comorbidities (eg, diabetes, osteoporosis, smoking), body mass index, type and duration of immobilization, complications, return to work, cosmetic perception, time to final clinical follow-up, and symptoms at final clinical follow-up. Incidence of potential risk factors associated with malunion—obesity, noncompliance, and comorbidities such as smoking and diabetes—was compared between the 15 patients with malunion and the other study patients, who healed without malunion.
For long-term postoperative follow-up, patients were contacted to be seen in clinic to complete an updated physical examination, self-reported questionnaire, and the DASH form. Physical examination included measurements of range of motion (ROM) and strength involving the shoulder, elbow, and forearm, with ROM reported as the difference between the injured and contralateral upper extremities. Neurovascular status and focal tenderness to palpation were also assessed on examination. When in-person examination was not possible, the questionnaire and DASH form were completed over the telephone. The self-reported questionnaire asked for information on smoking status, pain, functional limitations, cosmetic perception, satisfaction, and whether or not the patient would still opt for nonoperative management if presented with the same injury again. Pain and satisfaction were measured on numerical scales: Pain scores ranged from 0 (no pain) to 10 (worst possible pain), and satisfaction scores ranged from 1 (not satisfied) to 5 (very satisfied). Data are presented as mean values.
Results
Of the 91 study-eligible patients, 15 (16%) met the radiographic criteria for the diagnosis of malunion. Retrospective data were available for all 15 patients from time of injury to final clinical follow-up (mean, 19 weeks; range, 7-53 weeks). Mean age at injury was 39 years (range, 20-79 years). Additional demographics are listed in Table 1. Incidence of potential risk factors, such as body mass index (26.5 vs 25.4), smoking (33% vs 33%), and diabetes (0% vs 8%), was not significantly different between the malunion and healed-without-malunion groups, respectively. Furthermore, all malunion patients were compliant with their treatment protocol.
Radiographs were assessed at time of final follow-up to confirm healing and to document malunion. Varus malunion was found in 13 patients (mean, 24°; range, 20.5°-35.5°), and shortening was documented in the other 2 patients (mean, 4 cm; range, 3-5 cm). Patients were immobilized a mean of 10 weeks (range, 6-13 weeks). Initial fracture management consisted of coaptation splinting for 1 to 2 weeks (12 patients), hanging arm cast for 1 week (1 patient), and posterior splint for 1 week (1 patient). Patients were then transitioned to Sarmiento fracture bracing for the duration of their treatment (range, 5-12 months). One patient, followed initially at an outside institution, was managed in a sling throughout the duration of treatment (12 weeks) (Table 1). All 15 patients were neurovascularly intact at time of final clinical examination, with return of full upper extremity ROM in all but 3 patients. Only 1 of these 3 patients reported residual pain and functional limitations 4 months after injury (Table 2). Twelve patients were evaluated for return to work, with all successfully returning to work without restrictions at time of final follow-up. The 1 minor complication noted during the treatment period involved medial-sided elbow skin breakdown from brace wear, which resolved with local wound care. No patient required or requested surgical intervention for their residual malunion.
Of the 15 patients, 8 (53%) were reached for in-person examination (6 patients) or telephone interview (2 patients) for follow-up assessment by means of DASH form and self-reported questionnaire a mean of 47 months (range, 12-99 months) after initial injury. The 6 patients who had a physical examination were neurovascularly intact, lacked focal tenderness to palpation, and demonstrated full (5/5) strength within the deltoid, biceps, triceps, pronator, and supinator musculature. Each patient had equal ROM compared with the contralateral uninjured extremity on shoulder forward flexion and abduction, elbow flexion and extension, and forearm pronation and supination. Three patients (50%) had mild residual loss of ROM, with 2 demonstrating decreased shoulder external rotation of 10° and 15°, respectively, and 1 demonstrating decreased shoulder internal rotation of 10°.
Mean DASH score was 10.4 (range, 0-49.2). Evaluation of the self-reported questionnaire revealed a mean pain score of 1.1 (range, 0-7), with only 2 patients reporting any ongoing pain. In addition, 2 patients reported functional limitations, both related to overhead activities. However, 6 (75%) of the 8 patients reported noticeable cosmetic deformity, most commonly varus angulation (4 patients), as well as palpable bony prominence (2) and muscle atrophy (1). The majority of patients were satisfied with the outcome of their treatment (mean, 4; range, 2-5), with 6 patients reporting being satisfied or very satisfied, and all 6 indicating they would undergo nonoperative management again if presented with the same injury. Two patients reported being dissatisfied with their outcome, 1 because of cosmetic appearance and 1 because of cosmetic appearance and functional limitations. Both patients indicated they would choose operative management if presented with the same injury. There was no apparent relationship between outcome and degree of residual deformity, as both patients with varus angulation of more than 30° reported no residual pain or functional limitation and were very satisfied with the outcome of their treatment (Table 2).
Of the 7 patients who could not be reached for final follow-up, 2 on initial contact expressed overall satisfaction with their outcome and denied functional limitations. However, both asked to complete the study at a later date. Subsequently, these 2 patients could not be reached to complete the formal follow-up.
Discussion
Humeral shaft fractures are usually managed nonoperatively. One of the most commonly cited disadvantages of nonoperative management is its higher incidence of residual angular deformity, up to 13% in previous studies.4 Our study found a slightly higher incidence, 16%, on review of 91 nonoperatively managed humeral shaft fractures treated over an 11.5 year period. Although previous studies have reported acceptable functional and cosmetic outcomes with residual angular deformity of less than 20°,2,3,5,8,9 only observational reports have suggested acceptable function in patients with a documented malunion.8
To our knowledge, ours is the first study to correlate malunion with functional parameters and subjective patient-reported outcomes. We found that malunion was not associated with significant pain or functional limitation after nonoperative management of humeral shaft fractures. Furthermore, 75% of patients were satisfied or very satisfied with the outcome of their treatment and indicated they would undergo nonoperative management if presented with the same injury again. However, 75% of patients reported a noticeable cosmetic deformity, and one-third of these patients cited it as a major reason for dissatisfaction with their overall outcome. Regarding function, all patients returned to full strength and ROM of the affected extremity, aside from small losses of internal or external shoulder rotation on the magnitude of 10° to 15° in 50% of those patients tested. In addition, 75% of patients returned to regular activity without functional limitations; the other 25% reported trouble with overhead activities. There were no significant complications during the treatment or follow-up period, once the fracture had healed.
The major limitation of this study was its small patient population. (Obtaining a larger series of patients with malunion after nonoperative treatment of humeral shaft fractures likely would require a multicenter study.) Some of our study findings, such as lack of correlation between degree of malunion and subsequent functional or subjective outcomes, would require a larger sample size for verification and more definitive conclusions. Another limitation is that the study was not designed to evaluate the cause of malunion. Therefore, we cannot draw any definitive conclusions regarding what may have contributed to the development of malunion in our study population. However, all our malunion patients were compliant with their treatment protocol, and they showed no significant difference in incidence of potential risk factors (eg, obesity, comorbidities) compared with the patients who healed without malunion.
Conclusion
Malunion after nonoperative management of humeral shaft fractures does not appear to result in significant pain, dissatisfaction, or functional limitation as measured on physical examination and with validated objective outcome measures in the majority of patients. Furthermore, no patients in this study required surgical intervention for any residual limitations or complications after malunion. The majority of patients reported a noticeable cosmetic deformity, which left a small subset of patients dissatisfied. Overall, our study findings can be used to help counsel patients before and during nonoperative management—particularly patients who appear to be healing with some malunion. Our findings suggest that operative intervention to prevent malunion is not necessary, as it likely would not result in any overall improvement in patient function or satisfaction, but patients should be counseled regarding the high likelihood of cosmetic deformity, which may or may not be bothersome.
1. Rockwood CA, Green DP, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
2. Papasoulis E, Drosos GI, Ververidis AN, Verettas DA. Functional bracing of humeral shaft fractures. A review of clinical studies. Injury. 2010;41(7):e21-e27.
3. Sarmiento A, Latta LL. Functional fracture bracing. J Am Acad Orthop Surg. 1999;7(1):66-75.
4. Denard A Jr, Richards JE, Obremskey WT, Tucker MC, Floyd M, Herzog GA. Outcome of nonoperative vs operative treatment of humeral shaft fractures: a retrospective study of 213 patients. Orthopedics. 2010;33(8).
5. Fjalestad T, Strømsøe K, Salvesen P, Rostad B. Functional results of braced humeral diaphyseal fractures: why do 38% lose external rotation of the shoulder? Arch Orthop Trauma Surg. 2000;120(5-6):281-285.
6. Koch PP, Gross DF, Gerber C. The results of functional (Sarmiento) bracing of humeral shaft fractures. J Shoulder Elbow Surg. 2002;11(2):143-150.
7. Ozkurt B, Altay M, Aktekin CN, Toprak A, Tabak Y. The role of functional bracing in the treatment of humeral shaft fractures [in Turkish]. Acta Orthop Traumatol Turc. 2007;41(1):15-20.
8. Rutgers M, Ring D. Treatment of diaphyseal fractures of the humerus using a functional brace. J Orthop Trauma. 2006;20(9):597-601.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
1. Rockwood CA, Green DP, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
2. Papasoulis E, Drosos GI, Ververidis AN, Verettas DA. Functional bracing of humeral shaft fractures. A review of clinical studies. Injury. 2010;41(7):e21-e27.
3. Sarmiento A, Latta LL. Functional fracture bracing. J Am Acad Orthop Surg. 1999;7(1):66-75.
4. Denard A Jr, Richards JE, Obremskey WT, Tucker MC, Floyd M, Herzog GA. Outcome of nonoperative vs operative treatment of humeral shaft fractures: a retrospective study of 213 patients. Orthopedics. 2010;33(8).
5. Fjalestad T, Strømsøe K, Salvesen P, Rostad B. Functional results of braced humeral diaphyseal fractures: why do 38% lose external rotation of the shoulder? Arch Orthop Trauma Surg. 2000;120(5-6):281-285.
6. Koch PP, Gross DF, Gerber C. The results of functional (Sarmiento) bracing of humeral shaft fractures. J Shoulder Elbow Surg. 2002;11(2):143-150.
7. Ozkurt B, Altay M, Aktekin CN, Toprak A, Tabak Y. The role of functional bracing in the treatment of humeral shaft fractures [in Turkish]. Acta Orthop Traumatol Turc. 2007;41(1):15-20.
8. Rutgers M, Ring D. Treatment of diaphyseal fractures of the humerus using a functional brace. J Orthop Trauma. 2006;20(9):597-601.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
Long-Term Elastic Durability of Polymer Matrix Composite Materials After Repeated Steam Sterilization
Polymer matrix composite materials have been widely promoted for orthopedic use in a variety of settings, including surgical instruments, medical devices, implants, and bone models.1-13 These types of composites are engineered from 2 or more constituent materials with significantly different physical or chemical properties; these materials remain separate and distinct on a macroscopic level within the finished composite structure. As a result of ongoing biomaterial research, polymer matrix composite materials can be engineered with a wide range of physical, mechanical, and surface properties, tailored to their application. Given their advantages (eg, high strength-to-weight ratio, radiolucency), these polymer matrix composite materials have gained popularity over traditional metallic materials.
Sterilization is an essential day-to-day procedure in the health care sector, both for single- and multiple-use devices or instruments, and thus a composite material used in medical components should remain unaffected by the process. The type of sterilization most commonly performed is steam sterilization, which achieves microbiological death by moist heat and pressure. Steam is created in an autoclave at a temperature of 132°C (270°F) in typical hospital settings. Steam sterilization cycles last 5 to 14 minutes based on specific manufacturer recommendations. Most medical-grade plastics used in health care have been designed and formulated to withstand the required sterilization cycles without sacrificing key properties. The structure integrity and overall performance of polymer matrix composites may be strongly influenced by the stability of the fiber/polymer interfacial region in terms of physical, chemical, and mechanical characteristics of the material at different scales.14 Absorption of moisture causes dilatational expansion and induces stresses, which are associated with the moisture-induced expansion resulting in degradation of structure stability.15 Thus, steam sterilization could affect the properties of the polymer matrix composite materials by excessive absorption of moisture by the polymer.
To our knowledge, no one has studied whether polymer matrix material properties degrade from long-term, repeated steam sterilization followed by mechanical loading. We conducted a study to evaluate the structural properties (short-beam strength, SBS) of several composite materials exposed to repeated sterilization as compared with traditional metal materials: SS-316L (stainless steel 316L) and Al-7075-T6 (aluminum 7075-T6).
Materials and Methods
We evaluated 3 types of composite materials: Tepex (Tepex Dynalite 201; HiPer Technology Inc.), CFR-PPS (carbon-fiber–reinforced polyphenylene sulfide, Cetex PPS; TenCate Advanced Composites USA Inc.), and HTN-53 (Zytel HTN53G50HSLR NC010; HiPer Technology Inc.) (Figure 1). Tepex is being used for orthopedic applications (knee braces, orthoses, insoles) and sporting goods applications. The performance of this material is superior to that of unreinforced thermoplastics. CFR-PPS represented the state of the art in composite materials for aerospace applications (eg, airframe structures, engine nacelles, fan casings, floorboards, interior parts). This is a high-performance material with exceptional high temperature and aggressive chemical resistance characteristics. CFR-PPS is also used to make filter fabric for coal boilers, papermaking felts, electrical insulation, specialty membranes, gaskets, and packing. It is not solubilized by any known solvents, even in long-term exposure, at temperatures up to 200°C. In addition, it exhibits exceptional resistance to organic and inorganic solutions, acids and alkali solutions, and a wide array of miscellaneous chemicals. HTN-53 is a 50% glass-reinforced, lubricated, high-performance polyamide resin with improved flow, developed for applications requiring excellent surface appearance with water-heated molds. This material has specifically shown survivability in hot, cold, chemically aggressive, and load-bearing environments. In addition, it has shown superior moisture and temperature resistance. These 3 composite materials were compared with SS-316L and Al-7075-T6. SS-316L is commonly used for implants in orthopedics, and Al-7075-T6 is a relatively radiolucent alternative for medical applications. Two different tests were performed to evaluate and validate these composite materials: (1) radiographic density evaluation and (2) structural property tests (short-beam load-to-failure [LTF] test, short-beam cyclic compression loading [CCL] test) before and after sterilization cycling.
Radiographic Density Evaluation
The radiographic density of the 5 materials was evaluated with radiographic images of a cadaveric knee specimen (Figure 2). Radiographic image intensification is the gold standard for repeated radiographic imaging in the operating room. Six different radiographic images were obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). Image-Pro Plus software (Media Cybernetics) was used to measure the radiographic density of the materials from the grayscale of the images.
Structural Properties Testing Before and After Sterilization Cycling
We used a standard SBS testing method to determine whether any degradation of structural properties resulted from standard repeated sterilization. The material geometries of the test specimens were 18.96×6.50×3.37 mm (length × width × thickness). Standard sterilization procedures were performed with steam sterilization using an autoclave at a temperature of 132°C (270°F) for at least 5 minutes (range, 5-14 minutes). Sample interval testing ran at 0, 200, and 400 sterilization cycles for structural properties in terms of SBS and moisture retention, with the structural properties at the 0th sterilization cycle (material before sterilization was performed) used as a baseline for comparison. Materials were subjected to 400 sterilization cycles, which is representative of the number of sterilization cycles per year an instrument or device would be subjected to.
Three structural tests were performed for each sample interval: moisture retention, LTF, and CCL. Moisture retention was investigated before and after repeated sterilization by measuring the weight of the test materials, as steam sterilization is known to affect the amount of moisture that is absorbed by a material. Twelve specimens of each proposed material were weighed at each sample interval, with the structural weight at the 0th sterilization cycle (material before sterilization is performed) serving as a baseline for comparison.
SBS testing was based on the ASTM (American Society for Testing and Materials) D2344 standard16 for LTF and CCL tests (Figure 3). Six samples of material were used for each test at every sample interval, yielding 180 samples. Seven servohydraulic material testing system instruments (1 MTS 810 and 6 MTS 858 Mini Bionix) were used to test the SBS of each material. For LTF testing, each specimen was loaded in compression from 30 N to complete structural failure at a constant displacement rate of 1.0 mm/min (0.05 in/min). Testing was initiated with 5 preconditioning loading cycles from 30 to 100 N at 1 Hz. The load was then applied continuously until failure occurred; force and displacement data were collected every 0.02 second. This procedure was performed for 6 replicates for each sample interval for each test material.
The calculation for SBS, Fsbs (MPa), for the constant loading rate until structural failure is:
Fsbs = 0.75 × Pm
b × h
where Pm (N) is the maximum applied load observed during the test, b is the measured specimen width (mm), and h is the measured specimen thickness (mm).
CCL testing consisted of each test material axially loaded with 100 to 500 N at a frequency of 1 Hz for 100,000 cycles. The maximum load of 500 N was chosen as a standard based on 80% of the minimum ultimate failure load from previous LTF tests. Displacement and force data were collected every 5 cycles at the maximum compressive load. Degradation of the material was calculated using the difference between the deflection of the initial cycle and the deflection of the final cycle (50th cycle and 100,000th cycle). This procedure was performed for 6 replicates for each sample interval for each test material.
Statistical Analysis
LTF and CCL testing data were analyzed for any differences among the test materials using 1-way analysis of variance with the least significant difference multiple comparisons post hoc test method using SPSS Version 16.0, with P < .05 denoting significance. These analyses were used to determine the statistical relevance of the difference between the SBS (LTF and CCL) of each test material. Means and standard deviations were calculated for all tests.
Results
Radiographic Density Evaluation
Overall, all the tested composite materials were significantly more radiolucent than either SS-316L or Al-7075-T6. Figure 4 shows the 6 different radiographic images obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). SS-316L can be considered radiopaque, and Al-7075-T6 has been used as a relatively radiolucent alternative. Tepex was statistically more radiolucent than the other 2 tested composite materials (Table 1). Even with 2 pieces placed anterior to the subject and 2 placed posterior, the radiodensity compared to the cortical bone was still lower than 1 piece of Al-7075-T6 either anterior or posterior to the subject.
Structural Properties Testing
Moisture Retention. Moisture retention was evaluated by weighing the test materials before and after repeated sterilization. There was no significant difference in moisture retention, as weight differences for all the tested materials were less than 0.5 weight percentage compared to the 0th sterilization cycle (Table 2). Therefore, the results of this study showed that all the tested materials exhibited good moisture/temperature resistance after 400 sterilization cycles.
Load to Failure. In the LTF test, significant differences were detected in SBS between all 5 tested materials (P < .05). Figure 5 shows the comparison of the structural properties in terms of SBS between the 5 tested materials, and Figure 6 shows the failure modes for the tested materials. There was no SBS for SS-316L, as the material did not exhibit complete structural failure even after 400 sterilization cycles; however, SS-316L was observed in inelastic deformation failure (Figure 6D). Al-7075-T6 had much higher SBS compared with the other composite materials, and it also resulted in an inelastic deformation failure mode only after 400 sterilization cycles; otherwise, flexure failure modes were observed. Tepex and CFR-PPS exhibited interlaminar shear failure, and HTN-53 exhibited complete structural failure.
Every composite material tested using the short-beam test for LTF showed a decrease in SBS with increased sterilization cycles (Figure 5). This decrease ranged from 17% to 57% compared with the 0th sterilization cycle. SBS was higher for CFR-PPS than for the other 2 composites. No statistically significant difference was found between CFR-PPS and Tepex except at the 200th sterilization cycle. HTN-53 was brittle at the 0th sterilization cycle but performed more like a ductile material at the 200th cycle. In addition, HTN-53 had the lowest SBS in terms of LTF testing when compared with the other 2 composites.
During the complete structural failure test, the failure modes for Tepex and CFR-PPS were visually identified as interlaminar shear failure (Figures 6A, 6B), whereas HTN-53 visually exhibited pure flexure failure (Figure 6C). As for the metals, SS-316L exhibited plastic deformation, and Al-7075-T6 exhibited flexure failure (Figures 6D, 6E).
Cyclic Compression Loading. Tepex was the only material to pass the 100,000 loading cycle without failure (Table 3). HTN-53 had the poorest performance of all: Its failure rates were 33% (2/6 samples) before sterilization (average cycle, 22,213; range, 21,500-22,925), 83% (5/6 samples) at the 200th sterilization cycle (average cycle, 4,210; range, 0-14,360), and 100% after 400 sterilization cycles (average cycle, 12,725; range, 1,190-21,900). CFR-PPS had no failures before the 400th sterilization cycle, and its failure rate after 400 sterilization cycles (average cycle, 50,735; range, 50,270-51,200) was 33% (2/6 samples).
Discussion
The success of a reusable composite material for use in orthopedic surgery depends not only on radiographic density, fabrication methods, and design but also on the ability to withstand repeated sterilization. Over the past 3 decades, investigators have explored several high-performance polymer matrix composite materials for use in orthopedics, especially in trauma, hip stems, and spinal implants.1,3,4,17-34 According to Evans and Gregson,35 composite materials have been widely promoted as possible orthopedic biomaterials but to date have found few successful commercial applications, because of the many challenging problems encountered in fabrication and testing. One of the most important factors in the mechanical properties of many composite materials is the influence of the cooling and loading rates on fiber-matrix interface adhesion.36-38 Our results tended to agree with the findings of Evans and Gregson,35 as some of these composite materials did not withstand repeated sterilizations well.
Guan and colleagues39 evaluated the influence of sterilization treatment on continuous carbon-fiber–reinforced polyolefin composite. Their 3-point bending test results showed that the levels of maximum load of all the specimens undergoing sterilization by autoclave were lower than those of the control group. For these composites, they concluded that autoclave sterilization and Co-60 gamma ray irradiation sterilization should be avoided and that ethylene oxide is the best method. Our results support their findings with a different set of composites.
Although HTN-53 has shown promise in other orthopedic applications because of its superior moisture and temperature resistance, we found that its performance after repeated sterilization was relatively poor. Tepex showed the greatest potential for durability after repeated sterilization; its mechanical properties were stable after 200 steam sterilization cycles.
Clinical Applications
The composite materials investigated in the present study have potential for use in either instrumentation or long-term implantation applications because of their versatility, mechanical strength, fatigue resistance, and biocompatibility. Akay and Aslan40 stated that carbon-fiber–filled composite implants can be designed with more appropriate modulus, strength, toughness, or stiffness by the arrangement of reinforcing fiber volume and orientation, and can provide better fatigue resistance. A notable advantage of using a composite plate with metal screws is that the potential for corrosion of metallic components is eliminated. Another major advantage of composite medical implants (eg, DiPhos-RM) is radiolucency, which allows direct visualization of osseous callus formation as well as monitoring of fracture healing, thereby improving clinical assessment and accuracy.
Numerous studies have documented the successful clinical performance of composite materials in orthopedic, trauma, and spinal surgery applications.41-45 Bagheri and colleagues41 developed a new carbon fiber–flax–epoxy composite plate and biomechanically compared it with a standard clinical metal plate. Their results confirmed that the carbon fiber–flax–epoxy material represents a potential candidate for bone fracture plate applications, as it can simultaneously provide similar mechanical stiffness and lower stress shielding (higher bone stress) compared with a standard clinical metal bone plate. Tarallo and colleagues45 evaluated the clinical results of 40 cases at 12-month follow-up using a new plate made of carbon-fiber–reinforced polyetheretherketon (DiPhos-RM, Lima Corporate) for the treatment of distal radius fractures. They reported good clinical results for this device at early follow-up, and its use allowed maintenance of reduction in complex AO (Arbeitsgemeinschaft für Osteosynthesefragen) fractures.
The main advantage in using composites for surgical instruments is their radiolucency. These materials do not obscure images or radiographs during fluoroscopic visualization. Surgery often requires fluoroscopic visualization of internal organs or bones, which may require temporary removal of radiopaque devices (eg, retractors, clamps, forceps, hooks, distractors). Aside from being inconvenient, removal and subsequent reinsertion consume valuable time and interfere with the smooth flow of an operation.
The shortcomings of using composite materials for surgical instruments involve detectability and sterilization. A significant issue in surgery is the accidental leaving behind of instruments in patients, which can cause serious problems ranging from organ perforation and blood infection to death. Although instrument counting and other safety protocols can reduce the risk of overlooking an instrument, mistakes are bound to happen. The other shortcoming is the influence of repeated sterilization on the mechanical properties of the composite materials, as sterilization is mandatory for surgical instruments used in the operation room. The structural integrity and overall performance of the polymer composite materials—especially the stability of the interface and the interphase zones—are strongly influenced by repeated sterilization.
On the other hand, composite materials have potential advantages that may support their introduction into long-term medical implant applications, as sterilization commonly is performed only once, during packaging. The effects of sterilization by radiation or steam are much less pronounced on composite implants than on composite surgical instruments. However, composite implants require careful consideration with respect to the bioactivity of wear particles that may be produced from articulation. Further, carbon-fiber–reinforced polymer implants are still substantially more difficult to manufacture and more costly than their metallic counterparts.46
Limitations
This study has some limitations. Most important, studies of this nature do not account for biological factors such as corrosion, biological wear, and the soft-tissue attachment effects on structural properties for potential in vivo use. Another limitation was that the study tested only the mechanical properties in terms of SBS and provided no information about other mechanical properties, such as tensile, compression, and torsion strengths. We think SBS testing adequately evaluates challenging scenarios like thin and narrow instruments/devices that are anticipated in application, and information regarding other modes of failure and mechanical properties (compression, tension, torsion) would be a further area of research. An additional limitation was that our model used a relatively small number of samples. A larger study with more samples and varying layout patterns and layers of the carbon fibers may more clearly demonstrate the effect of steam sterilization on composite materials.
Conclusion
This study provided new information on 3 selected composite materials and their structural properties after repeated steam sterilization. We discovered that these composites were similar in radiographic density and water retention but behaved very differently in terms of mechanical durability after repeated steam sterilization. All selected composites demonstrated deterioration of mechanical properties after repeated steam sterilization. Knowing these results could aid in making decisions about the design and manufacturing of operative instruments and orthopedic biomaterials. Although our preliminary findings are intriguing, further study is warranted to seek specific applications for these composite materials in orthopedic surgery.
1. Ali MS, French TA, Hastings GW, et al. Carbon fibre composite bone plates. Development, evaluation and early clinical experience. J Bone Joint Surg Br. 1990;72(4):586-591.
2. Brooks RA, Jones E, Storer A, Rushton N. Biological evaluation of carbon-fibre–reinforced polybutyleneterephthalate (CFRPBT) employed in a novel acetabular cup. Biomaterials. 2004;25(17):3429-3438.
3. Brown SA, Hastings RS, Mason JJ, Moet A. Characterization of short-fibre reinforced thermoplastics for fracture fixation devices. Biomaterials. 1990;11(8):541-547.
4. Skinner HB. Composite technology for total hip arthroplasty. Clin Orthop Relat Res. 1988;(235):224-236.
5. Field RE, Jones E, Nuijten P, Storer A, Cronin M, Rushton N. Pre-clinical evaluation of the Cambridge acetabular cup. J Mater Sci Mater Med. 2008;19(8):2791-2798.
6. Han N, Ahmed I, Parsons AJ, et al. Influence of screw holes and gamma sterilization on properties of phosphate glass fiber–reinforced composite bone plates. J Biomater Appl. 2013;27(8):990-1002.
7. Losi P, Munaò A, Spiller D, et al. Evaluation of a new composite prosthesis for the repair of abdominal wall defects. J Mater Sci Mater Med. 2007;18(10):1939-1944.
8. Pait TG, Kaufman HH, Voelker JL, McAllister HP, Willison C. Use of a carbon composite radiolucent anterior cervical retractor system. Neurosurgery. 1993;33(5):941-942.
9. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
10. Gardner MP, Chong AC, Pollock AG, Wooley PH. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng. 2010;38(3):613-620.
11. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
12. Dunlap JT, Chong AC, Lucas GL, Cooke FW. Structural properties of a novel design of composite analogue humeri models. Ann Biomed Eng. 2008;36(11):1922-1926.
13. Grover P, Albert C, Wang M, Harris GF. Mechanical characterization of fourth generation composite humerus. Proc Inst Mech Eng H. 2011;225(12):1169-1176.
14. Zheng Q, Morgan RJ. Synergistic thermal-moisture damage mechanisms of epoxies and their carbon fiber composites. J Compos Mater. 1993;27(15):1465-1478.
15. Ray BC. Temperature effect during humid ageing on interfaces of glass and carbon fibers reinforced epoxy composites. J Colloid Interface Sci. 2006;298(1):111-117.
16. Standard test method for short-beam strength of polymer matrix composite materials and their laminates [ASTM specification D2344/D2344M-00]. In: Annual Book of ASTM Standards. Vol 15.03. West Conshohocken, PA: American Society for Testing and Materials; 2006.
17. Bradley JS, Hastings GW, Johnson-Nurse C. Carbon fibre reinforced epoxy as a high strength, low modulus material for internal fixation plates. Biomaterials. 1980;1(1):38-40.
18. McKenna GB, Bradley GW, Dunn HK, Statton WO. Mechanical properties of some fibre reinforced polymer composites after implantation as fracture fixation plates. Biomaterials. 1980;1(4):189-192.
19. Tayton K, Johnson-Nurse C, McKibbin B, Bradley J, Hastings G. The use of semi-rigid carbon-fibre–reinforced plastic plates for fixation of human fractures. Results of preliminary trials. J Bone Joint Surg Br. 1982;64(1):105-111.
20. Tayton K, Bradley J. How stiff should semi-rigid fixation of the human tibia be? A clue to the answer. J Bone Joint Surg Br. 1983;65(3):312-315.
21. Tayton K. Corrosive effect of carbon-fibre reinforced plastic on stainless-steel screws during implantation into man. J Med Eng Technol. 1983;7(1):24-26.
22. Howard CB, Tayton KJ, Gibbs A. The response of human tissues to carbon reinforced epoxy resin. J Bone Joint Surg Br. 1985;67(4):656-658.
23. Skirving AP, Day R, Macdonald W, McLaren R. Carbon fiber reinforced plastic (CFRP) plates versus stainless steel dynamic compression plates in the treatment of fractures of the tibiae in dogs. Clin Orthop Relat Res. 1987;(224):117-124.
24. Prakash R, Marwah S, Goel SC, Tuli SM. Carbon fibre reinforced epoxy implants for bridging large osteoperiosteal gaps. Biomaterials. 1988;9(2):198-202.
25. Pemberton DJ, McKibbin B, Savage R, Tayton K, Stuart D. Carbon-fibre reinforced plates for problem fractures. J Bone Joint Surg Br. 1992;74(1):88-92.
26. Pemberton DJ, Evans PD, Grant A, McKibbin B. Fractures of the distal femur in the elderly treated with a carbon fibre supracondylar plate. Injury. 1994;25(5):317-321.
27. Kelsey DJ, Springer GS, Goodman SB. Composite implant for bone replacement. J Compos Mater. 1997;31(16):1593-1632.
28. Corvelli AA, Biermann PJ, Roberts JC. Design, analysis and fabrication of a composite segmental bone replacement implant. J Adv Mater. 1997;28:2-8.
29. Glassman AH, Crowninshield RD, Schenck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001;(393):128-136.
30. Williams D. New horizons for thermoplastic polymers. Med Device Technol. 2001;12(4):8-9.
31. Al-Shawi AK, Smith SP, Anderson GH. The use of a carbon fiber plate for periprosthetic supracondylar femoral fractures. J Arthroplasty. 2002;17(3):320-324.
32. Baker D, Kadambande SS, Alderman PM. Carbon fibre plates in the treatment of femoral periprosthetic fractures. Injury. 2004;35(6):596-598.
33. Akhavan S, Matthiesen MM, Schulte L, et al. Clinical and histologic results related to a low-modulus composite total hip replacement stem. J Bone Joint Surg Am. 2006;88(6):1308-1314.
34. Toth JM, Wang M, Estes BT, Scifert JL, Seim HB 3rd, Turner AS. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials. 2006;27(3):324-334.
35. Evans SL, Gregson PJ. Composite technology in load-bearing orthopaedic implants. Biomaterials. 1998;19(15):1329-1342.
36. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part I. Crystallinity and interface adhesion. Composites Part A. 2000;31(6):517-530.
37. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part II. Interlaminar fracture toughness. Composites Part A. 2001;32(6):763-774.
38. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part III. Impact damage performance. Composites Part A. 2001;32(6):775-785.
39. Guan SB, Hou CL, Chen AM, Zhang W, Wang JE. Influence of sterilization treatments on continuous carbon-fiber reinforced polyolefin composite. Zhonghua Yi Xue Za Zhi. 2007;87(31):2228-2231.
40. Akay M, Aslan N. An estimation of fatigue life for a carbon fibre/poly ether ether ketone hip joint prosthesis. Proc Inst Mech Eng H. 1995;209(2):93-103.
41. Bagheri ZS, Tavakkoli Awal P, Bougherara H, Aziz MS, Schemitsch EH, Zdero R. Biomechanical analysis of a new carbon fiber/flax/epoxy bone fracture plate shows less stress shielding compared to a standard clinical metal plate. J Biomech Eng. 2014;136(9):091002.
42. Rhee PC, Shin AY. The rate of successful four-corner arthrodesis with a locking, dorsal circular polyether-ether-ketone (PEEK-Optima) plate. J Hand Surg Eur Vol. 2013;38(7):767-773.
43. Nakahara I, Takao M, Bandoh S, Bertollo N, Walsh WR, Sugano N. In vivo implant fixation of carbon fiber–reinforced PEEK hip prostheses in an ovine model. J Orthop Res. 2013;31(3):485-492.
44. Kasliwal MK, O’Toole JE. Clinical experience using polyetheretherketone (PEEK) intervertebral structural cage for anterior cervical corpectomy and fusion. J Clin Neurosci. 2014;21(2):217-220.
45. Tarallo L, Mugnai R, Adani R, Zambianchi F, Catani F. A new volar plate made of carbon-fiber–reinforced polyetheretherketon for distal radius fracture: analysis of 40 cases. J Orthop Traumatol. 2014;15(4):277-283.
46. Cordey J, Perren SM, Steinemann SG. Stress protection due to plates: myth or reality? A parametric analysis made using the composite beam theory. Injury. 2000;31(suppl 3):C1-C13.
Polymer matrix composite materials have been widely promoted for orthopedic use in a variety of settings, including surgical instruments, medical devices, implants, and bone models.1-13 These types of composites are engineered from 2 or more constituent materials with significantly different physical or chemical properties; these materials remain separate and distinct on a macroscopic level within the finished composite structure. As a result of ongoing biomaterial research, polymer matrix composite materials can be engineered with a wide range of physical, mechanical, and surface properties, tailored to their application. Given their advantages (eg, high strength-to-weight ratio, radiolucency), these polymer matrix composite materials have gained popularity over traditional metallic materials.
Sterilization is an essential day-to-day procedure in the health care sector, both for single- and multiple-use devices or instruments, and thus a composite material used in medical components should remain unaffected by the process. The type of sterilization most commonly performed is steam sterilization, which achieves microbiological death by moist heat and pressure. Steam is created in an autoclave at a temperature of 132°C (270°F) in typical hospital settings. Steam sterilization cycles last 5 to 14 minutes based on specific manufacturer recommendations. Most medical-grade plastics used in health care have been designed and formulated to withstand the required sterilization cycles without sacrificing key properties. The structure integrity and overall performance of polymer matrix composites may be strongly influenced by the stability of the fiber/polymer interfacial region in terms of physical, chemical, and mechanical characteristics of the material at different scales.14 Absorption of moisture causes dilatational expansion and induces stresses, which are associated with the moisture-induced expansion resulting in degradation of structure stability.15 Thus, steam sterilization could affect the properties of the polymer matrix composite materials by excessive absorption of moisture by the polymer.
To our knowledge, no one has studied whether polymer matrix material properties degrade from long-term, repeated steam sterilization followed by mechanical loading. We conducted a study to evaluate the structural properties (short-beam strength, SBS) of several composite materials exposed to repeated sterilization as compared with traditional metal materials: SS-316L (stainless steel 316L) and Al-7075-T6 (aluminum 7075-T6).
Materials and Methods
We evaluated 3 types of composite materials: Tepex (Tepex Dynalite 201; HiPer Technology Inc.), CFR-PPS (carbon-fiber–reinforced polyphenylene sulfide, Cetex PPS; TenCate Advanced Composites USA Inc.), and HTN-53 (Zytel HTN53G50HSLR NC010; HiPer Technology Inc.) (Figure 1). Tepex is being used for orthopedic applications (knee braces, orthoses, insoles) and sporting goods applications. The performance of this material is superior to that of unreinforced thermoplastics. CFR-PPS represented the state of the art in composite materials for aerospace applications (eg, airframe structures, engine nacelles, fan casings, floorboards, interior parts). This is a high-performance material with exceptional high temperature and aggressive chemical resistance characteristics. CFR-PPS is also used to make filter fabric for coal boilers, papermaking felts, electrical insulation, specialty membranes, gaskets, and packing. It is not solubilized by any known solvents, even in long-term exposure, at temperatures up to 200°C. In addition, it exhibits exceptional resistance to organic and inorganic solutions, acids and alkali solutions, and a wide array of miscellaneous chemicals. HTN-53 is a 50% glass-reinforced, lubricated, high-performance polyamide resin with improved flow, developed for applications requiring excellent surface appearance with water-heated molds. This material has specifically shown survivability in hot, cold, chemically aggressive, and load-bearing environments. In addition, it has shown superior moisture and temperature resistance. These 3 composite materials were compared with SS-316L and Al-7075-T6. SS-316L is commonly used for implants in orthopedics, and Al-7075-T6 is a relatively radiolucent alternative for medical applications. Two different tests were performed to evaluate and validate these composite materials: (1) radiographic density evaluation and (2) structural property tests (short-beam load-to-failure [LTF] test, short-beam cyclic compression loading [CCL] test) before and after sterilization cycling.
Radiographic Density Evaluation
The radiographic density of the 5 materials was evaluated with radiographic images of a cadaveric knee specimen (Figure 2). Radiographic image intensification is the gold standard for repeated radiographic imaging in the operating room. Six different radiographic images were obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). Image-Pro Plus software (Media Cybernetics) was used to measure the radiographic density of the materials from the grayscale of the images.
Structural Properties Testing Before and After Sterilization Cycling
We used a standard SBS testing method to determine whether any degradation of structural properties resulted from standard repeated sterilization. The material geometries of the test specimens were 18.96×6.50×3.37 mm (length × width × thickness). Standard sterilization procedures were performed with steam sterilization using an autoclave at a temperature of 132°C (270°F) for at least 5 minutes (range, 5-14 minutes). Sample interval testing ran at 0, 200, and 400 sterilization cycles for structural properties in terms of SBS and moisture retention, with the structural properties at the 0th sterilization cycle (material before sterilization was performed) used as a baseline for comparison. Materials were subjected to 400 sterilization cycles, which is representative of the number of sterilization cycles per year an instrument or device would be subjected to.
Three structural tests were performed for each sample interval: moisture retention, LTF, and CCL. Moisture retention was investigated before and after repeated sterilization by measuring the weight of the test materials, as steam sterilization is known to affect the amount of moisture that is absorbed by a material. Twelve specimens of each proposed material were weighed at each sample interval, with the structural weight at the 0th sterilization cycle (material before sterilization is performed) serving as a baseline for comparison.
SBS testing was based on the ASTM (American Society for Testing and Materials) D2344 standard16 for LTF and CCL tests (Figure 3). Six samples of material were used for each test at every sample interval, yielding 180 samples. Seven servohydraulic material testing system instruments (1 MTS 810 and 6 MTS 858 Mini Bionix) were used to test the SBS of each material. For LTF testing, each specimen was loaded in compression from 30 N to complete structural failure at a constant displacement rate of 1.0 mm/min (0.05 in/min). Testing was initiated with 5 preconditioning loading cycles from 30 to 100 N at 1 Hz. The load was then applied continuously until failure occurred; force and displacement data were collected every 0.02 second. This procedure was performed for 6 replicates for each sample interval for each test material.
The calculation for SBS, Fsbs (MPa), for the constant loading rate until structural failure is:
Fsbs = 0.75 × Pm
b × h
where Pm (N) is the maximum applied load observed during the test, b is the measured specimen width (mm), and h is the measured specimen thickness (mm).
CCL testing consisted of each test material axially loaded with 100 to 500 N at a frequency of 1 Hz for 100,000 cycles. The maximum load of 500 N was chosen as a standard based on 80% of the minimum ultimate failure load from previous LTF tests. Displacement and force data were collected every 5 cycles at the maximum compressive load. Degradation of the material was calculated using the difference between the deflection of the initial cycle and the deflection of the final cycle (50th cycle and 100,000th cycle). This procedure was performed for 6 replicates for each sample interval for each test material.
Statistical Analysis
LTF and CCL testing data were analyzed for any differences among the test materials using 1-way analysis of variance with the least significant difference multiple comparisons post hoc test method using SPSS Version 16.0, with P < .05 denoting significance. These analyses were used to determine the statistical relevance of the difference between the SBS (LTF and CCL) of each test material. Means and standard deviations were calculated for all tests.
Results
Radiographic Density Evaluation
Overall, all the tested composite materials were significantly more radiolucent than either SS-316L or Al-7075-T6. Figure 4 shows the 6 different radiographic images obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). SS-316L can be considered radiopaque, and Al-7075-T6 has been used as a relatively radiolucent alternative. Tepex was statistically more radiolucent than the other 2 tested composite materials (Table 1). Even with 2 pieces placed anterior to the subject and 2 placed posterior, the radiodensity compared to the cortical bone was still lower than 1 piece of Al-7075-T6 either anterior or posterior to the subject.
Structural Properties Testing
Moisture Retention. Moisture retention was evaluated by weighing the test materials before and after repeated sterilization. There was no significant difference in moisture retention, as weight differences for all the tested materials were less than 0.5 weight percentage compared to the 0th sterilization cycle (Table 2). Therefore, the results of this study showed that all the tested materials exhibited good moisture/temperature resistance after 400 sterilization cycles.
Load to Failure. In the LTF test, significant differences were detected in SBS between all 5 tested materials (P < .05). Figure 5 shows the comparison of the structural properties in terms of SBS between the 5 tested materials, and Figure 6 shows the failure modes for the tested materials. There was no SBS for SS-316L, as the material did not exhibit complete structural failure even after 400 sterilization cycles; however, SS-316L was observed in inelastic deformation failure (Figure 6D). Al-7075-T6 had much higher SBS compared with the other composite materials, and it also resulted in an inelastic deformation failure mode only after 400 sterilization cycles; otherwise, flexure failure modes were observed. Tepex and CFR-PPS exhibited interlaminar shear failure, and HTN-53 exhibited complete structural failure.
Every composite material tested using the short-beam test for LTF showed a decrease in SBS with increased sterilization cycles (Figure 5). This decrease ranged from 17% to 57% compared with the 0th sterilization cycle. SBS was higher for CFR-PPS than for the other 2 composites. No statistically significant difference was found between CFR-PPS and Tepex except at the 200th sterilization cycle. HTN-53 was brittle at the 0th sterilization cycle but performed more like a ductile material at the 200th cycle. In addition, HTN-53 had the lowest SBS in terms of LTF testing when compared with the other 2 composites.
During the complete structural failure test, the failure modes for Tepex and CFR-PPS were visually identified as interlaminar shear failure (Figures 6A, 6B), whereas HTN-53 visually exhibited pure flexure failure (Figure 6C). As for the metals, SS-316L exhibited plastic deformation, and Al-7075-T6 exhibited flexure failure (Figures 6D, 6E).
Cyclic Compression Loading. Tepex was the only material to pass the 100,000 loading cycle without failure (Table 3). HTN-53 had the poorest performance of all: Its failure rates were 33% (2/6 samples) before sterilization (average cycle, 22,213; range, 21,500-22,925), 83% (5/6 samples) at the 200th sterilization cycle (average cycle, 4,210; range, 0-14,360), and 100% after 400 sterilization cycles (average cycle, 12,725; range, 1,190-21,900). CFR-PPS had no failures before the 400th sterilization cycle, and its failure rate after 400 sterilization cycles (average cycle, 50,735; range, 50,270-51,200) was 33% (2/6 samples).
Discussion
The success of a reusable composite material for use in orthopedic surgery depends not only on radiographic density, fabrication methods, and design but also on the ability to withstand repeated sterilization. Over the past 3 decades, investigators have explored several high-performance polymer matrix composite materials for use in orthopedics, especially in trauma, hip stems, and spinal implants.1,3,4,17-34 According to Evans and Gregson,35 composite materials have been widely promoted as possible orthopedic biomaterials but to date have found few successful commercial applications, because of the many challenging problems encountered in fabrication and testing. One of the most important factors in the mechanical properties of many composite materials is the influence of the cooling and loading rates on fiber-matrix interface adhesion.36-38 Our results tended to agree with the findings of Evans and Gregson,35 as some of these composite materials did not withstand repeated sterilizations well.
Guan and colleagues39 evaluated the influence of sterilization treatment on continuous carbon-fiber–reinforced polyolefin composite. Their 3-point bending test results showed that the levels of maximum load of all the specimens undergoing sterilization by autoclave were lower than those of the control group. For these composites, they concluded that autoclave sterilization and Co-60 gamma ray irradiation sterilization should be avoided and that ethylene oxide is the best method. Our results support their findings with a different set of composites.
Although HTN-53 has shown promise in other orthopedic applications because of its superior moisture and temperature resistance, we found that its performance after repeated sterilization was relatively poor. Tepex showed the greatest potential for durability after repeated sterilization; its mechanical properties were stable after 200 steam sterilization cycles.
Clinical Applications
The composite materials investigated in the present study have potential for use in either instrumentation or long-term implantation applications because of their versatility, mechanical strength, fatigue resistance, and biocompatibility. Akay and Aslan40 stated that carbon-fiber–filled composite implants can be designed with more appropriate modulus, strength, toughness, or stiffness by the arrangement of reinforcing fiber volume and orientation, and can provide better fatigue resistance. A notable advantage of using a composite plate with metal screws is that the potential for corrosion of metallic components is eliminated. Another major advantage of composite medical implants (eg, DiPhos-RM) is radiolucency, which allows direct visualization of osseous callus formation as well as monitoring of fracture healing, thereby improving clinical assessment and accuracy.
Numerous studies have documented the successful clinical performance of composite materials in orthopedic, trauma, and spinal surgery applications.41-45 Bagheri and colleagues41 developed a new carbon fiber–flax–epoxy composite plate and biomechanically compared it with a standard clinical metal plate. Their results confirmed that the carbon fiber–flax–epoxy material represents a potential candidate for bone fracture plate applications, as it can simultaneously provide similar mechanical stiffness and lower stress shielding (higher bone stress) compared with a standard clinical metal bone plate. Tarallo and colleagues45 evaluated the clinical results of 40 cases at 12-month follow-up using a new plate made of carbon-fiber–reinforced polyetheretherketon (DiPhos-RM, Lima Corporate) for the treatment of distal radius fractures. They reported good clinical results for this device at early follow-up, and its use allowed maintenance of reduction in complex AO (Arbeitsgemeinschaft für Osteosynthesefragen) fractures.
The main advantage in using composites for surgical instruments is their radiolucency. These materials do not obscure images or radiographs during fluoroscopic visualization. Surgery often requires fluoroscopic visualization of internal organs or bones, which may require temporary removal of radiopaque devices (eg, retractors, clamps, forceps, hooks, distractors). Aside from being inconvenient, removal and subsequent reinsertion consume valuable time and interfere with the smooth flow of an operation.
The shortcomings of using composite materials for surgical instruments involve detectability and sterilization. A significant issue in surgery is the accidental leaving behind of instruments in patients, which can cause serious problems ranging from organ perforation and blood infection to death. Although instrument counting and other safety protocols can reduce the risk of overlooking an instrument, mistakes are bound to happen. The other shortcoming is the influence of repeated sterilization on the mechanical properties of the composite materials, as sterilization is mandatory for surgical instruments used in the operation room. The structural integrity and overall performance of the polymer composite materials—especially the stability of the interface and the interphase zones—are strongly influenced by repeated sterilization.
On the other hand, composite materials have potential advantages that may support their introduction into long-term medical implant applications, as sterilization commonly is performed only once, during packaging. The effects of sterilization by radiation or steam are much less pronounced on composite implants than on composite surgical instruments. However, composite implants require careful consideration with respect to the bioactivity of wear particles that may be produced from articulation. Further, carbon-fiber–reinforced polymer implants are still substantially more difficult to manufacture and more costly than their metallic counterparts.46
Limitations
This study has some limitations. Most important, studies of this nature do not account for biological factors such as corrosion, biological wear, and the soft-tissue attachment effects on structural properties for potential in vivo use. Another limitation was that the study tested only the mechanical properties in terms of SBS and provided no information about other mechanical properties, such as tensile, compression, and torsion strengths. We think SBS testing adequately evaluates challenging scenarios like thin and narrow instruments/devices that are anticipated in application, and information regarding other modes of failure and mechanical properties (compression, tension, torsion) would be a further area of research. An additional limitation was that our model used a relatively small number of samples. A larger study with more samples and varying layout patterns and layers of the carbon fibers may more clearly demonstrate the effect of steam sterilization on composite materials.
Conclusion
This study provided new information on 3 selected composite materials and their structural properties after repeated steam sterilization. We discovered that these composites were similar in radiographic density and water retention but behaved very differently in terms of mechanical durability after repeated steam sterilization. All selected composites demonstrated deterioration of mechanical properties after repeated steam sterilization. Knowing these results could aid in making decisions about the design and manufacturing of operative instruments and orthopedic biomaterials. Although our preliminary findings are intriguing, further study is warranted to seek specific applications for these composite materials in orthopedic surgery.
Polymer matrix composite materials have been widely promoted for orthopedic use in a variety of settings, including surgical instruments, medical devices, implants, and bone models.1-13 These types of composites are engineered from 2 or more constituent materials with significantly different physical or chemical properties; these materials remain separate and distinct on a macroscopic level within the finished composite structure. As a result of ongoing biomaterial research, polymer matrix composite materials can be engineered with a wide range of physical, mechanical, and surface properties, tailored to their application. Given their advantages (eg, high strength-to-weight ratio, radiolucency), these polymer matrix composite materials have gained popularity over traditional metallic materials.
Sterilization is an essential day-to-day procedure in the health care sector, both for single- and multiple-use devices or instruments, and thus a composite material used in medical components should remain unaffected by the process. The type of sterilization most commonly performed is steam sterilization, which achieves microbiological death by moist heat and pressure. Steam is created in an autoclave at a temperature of 132°C (270°F) in typical hospital settings. Steam sterilization cycles last 5 to 14 minutes based on specific manufacturer recommendations. Most medical-grade plastics used in health care have been designed and formulated to withstand the required sterilization cycles without sacrificing key properties. The structure integrity and overall performance of polymer matrix composites may be strongly influenced by the stability of the fiber/polymer interfacial region in terms of physical, chemical, and mechanical characteristics of the material at different scales.14 Absorption of moisture causes dilatational expansion and induces stresses, which are associated with the moisture-induced expansion resulting in degradation of structure stability.15 Thus, steam sterilization could affect the properties of the polymer matrix composite materials by excessive absorption of moisture by the polymer.
To our knowledge, no one has studied whether polymer matrix material properties degrade from long-term, repeated steam sterilization followed by mechanical loading. We conducted a study to evaluate the structural properties (short-beam strength, SBS) of several composite materials exposed to repeated sterilization as compared with traditional metal materials: SS-316L (stainless steel 316L) and Al-7075-T6 (aluminum 7075-T6).
Materials and Methods
We evaluated 3 types of composite materials: Tepex (Tepex Dynalite 201; HiPer Technology Inc.), CFR-PPS (carbon-fiber–reinforced polyphenylene sulfide, Cetex PPS; TenCate Advanced Composites USA Inc.), and HTN-53 (Zytel HTN53G50HSLR NC010; HiPer Technology Inc.) (Figure 1). Tepex is being used for orthopedic applications (knee braces, orthoses, insoles) and sporting goods applications. The performance of this material is superior to that of unreinforced thermoplastics. CFR-PPS represented the state of the art in composite materials for aerospace applications (eg, airframe structures, engine nacelles, fan casings, floorboards, interior parts). This is a high-performance material with exceptional high temperature and aggressive chemical resistance characteristics. CFR-PPS is also used to make filter fabric for coal boilers, papermaking felts, electrical insulation, specialty membranes, gaskets, and packing. It is not solubilized by any known solvents, even in long-term exposure, at temperatures up to 200°C. In addition, it exhibits exceptional resistance to organic and inorganic solutions, acids and alkali solutions, and a wide array of miscellaneous chemicals. HTN-53 is a 50% glass-reinforced, lubricated, high-performance polyamide resin with improved flow, developed for applications requiring excellent surface appearance with water-heated molds. This material has specifically shown survivability in hot, cold, chemically aggressive, and load-bearing environments. In addition, it has shown superior moisture and temperature resistance. These 3 composite materials were compared with SS-316L and Al-7075-T6. SS-316L is commonly used for implants in orthopedics, and Al-7075-T6 is a relatively radiolucent alternative for medical applications. Two different tests were performed to evaluate and validate these composite materials: (1) radiographic density evaluation and (2) structural property tests (short-beam load-to-failure [LTF] test, short-beam cyclic compression loading [CCL] test) before and after sterilization cycling.
Radiographic Density Evaluation
The radiographic density of the 5 materials was evaluated with radiographic images of a cadaveric knee specimen (Figure 2). Radiographic image intensification is the gold standard for repeated radiographic imaging in the operating room. Six different radiographic images were obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). Image-Pro Plus software (Media Cybernetics) was used to measure the radiographic density of the materials from the grayscale of the images.
Structural Properties Testing Before and After Sterilization Cycling
We used a standard SBS testing method to determine whether any degradation of structural properties resulted from standard repeated sterilization. The material geometries of the test specimens were 18.96×6.50×3.37 mm (length × width × thickness). Standard sterilization procedures were performed with steam sterilization using an autoclave at a temperature of 132°C (270°F) for at least 5 minutes (range, 5-14 minutes). Sample interval testing ran at 0, 200, and 400 sterilization cycles for structural properties in terms of SBS and moisture retention, with the structural properties at the 0th sterilization cycle (material before sterilization was performed) used as a baseline for comparison. Materials were subjected to 400 sterilization cycles, which is representative of the number of sterilization cycles per year an instrument or device would be subjected to.
Three structural tests were performed for each sample interval: moisture retention, LTF, and CCL. Moisture retention was investigated before and after repeated sterilization by measuring the weight of the test materials, as steam sterilization is known to affect the amount of moisture that is absorbed by a material. Twelve specimens of each proposed material were weighed at each sample interval, with the structural weight at the 0th sterilization cycle (material before sterilization is performed) serving as a baseline for comparison.
SBS testing was based on the ASTM (American Society for Testing and Materials) D2344 standard16 for LTF and CCL tests (Figure 3). Six samples of material were used for each test at every sample interval, yielding 180 samples. Seven servohydraulic material testing system instruments (1 MTS 810 and 6 MTS 858 Mini Bionix) were used to test the SBS of each material. For LTF testing, each specimen was loaded in compression from 30 N to complete structural failure at a constant displacement rate of 1.0 mm/min (0.05 in/min). Testing was initiated with 5 preconditioning loading cycles from 30 to 100 N at 1 Hz. The load was then applied continuously until failure occurred; force and displacement data were collected every 0.02 second. This procedure was performed for 6 replicates for each sample interval for each test material.
The calculation for SBS, Fsbs (MPa), for the constant loading rate until structural failure is:
Fsbs = 0.75 × Pm
b × h
where Pm (N) is the maximum applied load observed during the test, b is the measured specimen width (mm), and h is the measured specimen thickness (mm).
CCL testing consisted of each test material axially loaded with 100 to 500 N at a frequency of 1 Hz for 100,000 cycles. The maximum load of 500 N was chosen as a standard based on 80% of the minimum ultimate failure load from previous LTF tests. Displacement and force data were collected every 5 cycles at the maximum compressive load. Degradation of the material was calculated using the difference between the deflection of the initial cycle and the deflection of the final cycle (50th cycle and 100,000th cycle). This procedure was performed for 6 replicates for each sample interval for each test material.
Statistical Analysis
LTF and CCL testing data were analyzed for any differences among the test materials using 1-way analysis of variance with the least significant difference multiple comparisons post hoc test method using SPSS Version 16.0, with P < .05 denoting significance. These analyses were used to determine the statistical relevance of the difference between the SBS (LTF and CCL) of each test material. Means and standard deviations were calculated for all tests.
Results
Radiographic Density Evaluation
Overall, all the tested composite materials were significantly more radiolucent than either SS-316L or Al-7075-T6. Figure 4 shows the 6 different radiographic images obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). SS-316L can be considered radiopaque, and Al-7075-T6 has been used as a relatively radiolucent alternative. Tepex was statistically more radiolucent than the other 2 tested composite materials (Table 1). Even with 2 pieces placed anterior to the subject and 2 placed posterior, the radiodensity compared to the cortical bone was still lower than 1 piece of Al-7075-T6 either anterior or posterior to the subject.
Structural Properties Testing
Moisture Retention. Moisture retention was evaluated by weighing the test materials before and after repeated sterilization. There was no significant difference in moisture retention, as weight differences for all the tested materials were less than 0.5 weight percentage compared to the 0th sterilization cycle (Table 2). Therefore, the results of this study showed that all the tested materials exhibited good moisture/temperature resistance after 400 sterilization cycles.
Load to Failure. In the LTF test, significant differences were detected in SBS between all 5 tested materials (P < .05). Figure 5 shows the comparison of the structural properties in terms of SBS between the 5 tested materials, and Figure 6 shows the failure modes for the tested materials. There was no SBS for SS-316L, as the material did not exhibit complete structural failure even after 400 sterilization cycles; however, SS-316L was observed in inelastic deformation failure (Figure 6D). Al-7075-T6 had much higher SBS compared with the other composite materials, and it also resulted in an inelastic deformation failure mode only after 400 sterilization cycles; otherwise, flexure failure modes were observed. Tepex and CFR-PPS exhibited interlaminar shear failure, and HTN-53 exhibited complete structural failure.
Every composite material tested using the short-beam test for LTF showed a decrease in SBS with increased sterilization cycles (Figure 5). This decrease ranged from 17% to 57% compared with the 0th sterilization cycle. SBS was higher for CFR-PPS than for the other 2 composites. No statistically significant difference was found between CFR-PPS and Tepex except at the 200th sterilization cycle. HTN-53 was brittle at the 0th sterilization cycle but performed more like a ductile material at the 200th cycle. In addition, HTN-53 had the lowest SBS in terms of LTF testing when compared with the other 2 composites.
During the complete structural failure test, the failure modes for Tepex and CFR-PPS were visually identified as interlaminar shear failure (Figures 6A, 6B), whereas HTN-53 visually exhibited pure flexure failure (Figure 6C). As for the metals, SS-316L exhibited plastic deformation, and Al-7075-T6 exhibited flexure failure (Figures 6D, 6E).
Cyclic Compression Loading. Tepex was the only material to pass the 100,000 loading cycle without failure (Table 3). HTN-53 had the poorest performance of all: Its failure rates were 33% (2/6 samples) before sterilization (average cycle, 22,213; range, 21,500-22,925), 83% (5/6 samples) at the 200th sterilization cycle (average cycle, 4,210; range, 0-14,360), and 100% after 400 sterilization cycles (average cycle, 12,725; range, 1,190-21,900). CFR-PPS had no failures before the 400th sterilization cycle, and its failure rate after 400 sterilization cycles (average cycle, 50,735; range, 50,270-51,200) was 33% (2/6 samples).
Discussion
The success of a reusable composite material for use in orthopedic surgery depends not only on radiographic density, fabrication methods, and design but also on the ability to withstand repeated sterilization. Over the past 3 decades, investigators have explored several high-performance polymer matrix composite materials for use in orthopedics, especially in trauma, hip stems, and spinal implants.1,3,4,17-34 According to Evans and Gregson,35 composite materials have been widely promoted as possible orthopedic biomaterials but to date have found few successful commercial applications, because of the many challenging problems encountered in fabrication and testing. One of the most important factors in the mechanical properties of many composite materials is the influence of the cooling and loading rates on fiber-matrix interface adhesion.36-38 Our results tended to agree with the findings of Evans and Gregson,35 as some of these composite materials did not withstand repeated sterilizations well.
Guan and colleagues39 evaluated the influence of sterilization treatment on continuous carbon-fiber–reinforced polyolefin composite. Their 3-point bending test results showed that the levels of maximum load of all the specimens undergoing sterilization by autoclave were lower than those of the control group. For these composites, they concluded that autoclave sterilization and Co-60 gamma ray irradiation sterilization should be avoided and that ethylene oxide is the best method. Our results support their findings with a different set of composites.
Although HTN-53 has shown promise in other orthopedic applications because of its superior moisture and temperature resistance, we found that its performance after repeated sterilization was relatively poor. Tepex showed the greatest potential for durability after repeated sterilization; its mechanical properties were stable after 200 steam sterilization cycles.
Clinical Applications
The composite materials investigated in the present study have potential for use in either instrumentation or long-term implantation applications because of their versatility, mechanical strength, fatigue resistance, and biocompatibility. Akay and Aslan40 stated that carbon-fiber–filled composite implants can be designed with more appropriate modulus, strength, toughness, or stiffness by the arrangement of reinforcing fiber volume and orientation, and can provide better fatigue resistance. A notable advantage of using a composite plate with metal screws is that the potential for corrosion of metallic components is eliminated. Another major advantage of composite medical implants (eg, DiPhos-RM) is radiolucency, which allows direct visualization of osseous callus formation as well as monitoring of fracture healing, thereby improving clinical assessment and accuracy.
Numerous studies have documented the successful clinical performance of composite materials in orthopedic, trauma, and spinal surgery applications.41-45 Bagheri and colleagues41 developed a new carbon fiber–flax–epoxy composite plate and biomechanically compared it with a standard clinical metal plate. Their results confirmed that the carbon fiber–flax–epoxy material represents a potential candidate for bone fracture plate applications, as it can simultaneously provide similar mechanical stiffness and lower stress shielding (higher bone stress) compared with a standard clinical metal bone plate. Tarallo and colleagues45 evaluated the clinical results of 40 cases at 12-month follow-up using a new plate made of carbon-fiber–reinforced polyetheretherketon (DiPhos-RM, Lima Corporate) for the treatment of distal radius fractures. They reported good clinical results for this device at early follow-up, and its use allowed maintenance of reduction in complex AO (Arbeitsgemeinschaft für Osteosynthesefragen) fractures.
The main advantage in using composites for surgical instruments is their radiolucency. These materials do not obscure images or radiographs during fluoroscopic visualization. Surgery often requires fluoroscopic visualization of internal organs or bones, which may require temporary removal of radiopaque devices (eg, retractors, clamps, forceps, hooks, distractors). Aside from being inconvenient, removal and subsequent reinsertion consume valuable time and interfere with the smooth flow of an operation.
The shortcomings of using composite materials for surgical instruments involve detectability and sterilization. A significant issue in surgery is the accidental leaving behind of instruments in patients, which can cause serious problems ranging from organ perforation and blood infection to death. Although instrument counting and other safety protocols can reduce the risk of overlooking an instrument, mistakes are bound to happen. The other shortcoming is the influence of repeated sterilization on the mechanical properties of the composite materials, as sterilization is mandatory for surgical instruments used in the operation room. The structural integrity and overall performance of the polymer composite materials—especially the stability of the interface and the interphase zones—are strongly influenced by repeated sterilization.
On the other hand, composite materials have potential advantages that may support their introduction into long-term medical implant applications, as sterilization commonly is performed only once, during packaging. The effects of sterilization by radiation or steam are much less pronounced on composite implants than on composite surgical instruments. However, composite implants require careful consideration with respect to the bioactivity of wear particles that may be produced from articulation. Further, carbon-fiber–reinforced polymer implants are still substantially more difficult to manufacture and more costly than their metallic counterparts.46
Limitations
This study has some limitations. Most important, studies of this nature do not account for biological factors such as corrosion, biological wear, and the soft-tissue attachment effects on structural properties for potential in vivo use. Another limitation was that the study tested only the mechanical properties in terms of SBS and provided no information about other mechanical properties, such as tensile, compression, and torsion strengths. We think SBS testing adequately evaluates challenging scenarios like thin and narrow instruments/devices that are anticipated in application, and information regarding other modes of failure and mechanical properties (compression, tension, torsion) would be a further area of research. An additional limitation was that our model used a relatively small number of samples. A larger study with more samples and varying layout patterns and layers of the carbon fibers may more clearly demonstrate the effect of steam sterilization on composite materials.
Conclusion
This study provided new information on 3 selected composite materials and their structural properties after repeated steam sterilization. We discovered that these composites were similar in radiographic density and water retention but behaved very differently in terms of mechanical durability after repeated steam sterilization. All selected composites demonstrated deterioration of mechanical properties after repeated steam sterilization. Knowing these results could aid in making decisions about the design and manufacturing of operative instruments and orthopedic biomaterials. Although our preliminary findings are intriguing, further study is warranted to seek specific applications for these composite materials in orthopedic surgery.
1. Ali MS, French TA, Hastings GW, et al. Carbon fibre composite bone plates. Development, evaluation and early clinical experience. J Bone Joint Surg Br. 1990;72(4):586-591.
2. Brooks RA, Jones E, Storer A, Rushton N. Biological evaluation of carbon-fibre–reinforced polybutyleneterephthalate (CFRPBT) employed in a novel acetabular cup. Biomaterials. 2004;25(17):3429-3438.
3. Brown SA, Hastings RS, Mason JJ, Moet A. Characterization of short-fibre reinforced thermoplastics for fracture fixation devices. Biomaterials. 1990;11(8):541-547.
4. Skinner HB. Composite technology for total hip arthroplasty. Clin Orthop Relat Res. 1988;(235):224-236.
5. Field RE, Jones E, Nuijten P, Storer A, Cronin M, Rushton N. Pre-clinical evaluation of the Cambridge acetabular cup. J Mater Sci Mater Med. 2008;19(8):2791-2798.
6. Han N, Ahmed I, Parsons AJ, et al. Influence of screw holes and gamma sterilization on properties of phosphate glass fiber–reinforced composite bone plates. J Biomater Appl. 2013;27(8):990-1002.
7. Losi P, Munaò A, Spiller D, et al. Evaluation of a new composite prosthesis for the repair of abdominal wall defects. J Mater Sci Mater Med. 2007;18(10):1939-1944.
8. Pait TG, Kaufman HH, Voelker JL, McAllister HP, Willison C. Use of a carbon composite radiolucent anterior cervical retractor system. Neurosurgery. 1993;33(5):941-942.
9. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
10. Gardner MP, Chong AC, Pollock AG, Wooley PH. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng. 2010;38(3):613-620.
11. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
12. Dunlap JT, Chong AC, Lucas GL, Cooke FW. Structural properties of a novel design of composite analogue humeri models. Ann Biomed Eng. 2008;36(11):1922-1926.
13. Grover P, Albert C, Wang M, Harris GF. Mechanical characterization of fourth generation composite humerus. Proc Inst Mech Eng H. 2011;225(12):1169-1176.
14. Zheng Q, Morgan RJ. Synergistic thermal-moisture damage mechanisms of epoxies and their carbon fiber composites. J Compos Mater. 1993;27(15):1465-1478.
15. Ray BC. Temperature effect during humid ageing on interfaces of glass and carbon fibers reinforced epoxy composites. J Colloid Interface Sci. 2006;298(1):111-117.
16. Standard test method for short-beam strength of polymer matrix composite materials and their laminates [ASTM specification D2344/D2344M-00]. In: Annual Book of ASTM Standards. Vol 15.03. West Conshohocken, PA: American Society for Testing and Materials; 2006.
17. Bradley JS, Hastings GW, Johnson-Nurse C. Carbon fibre reinforced epoxy as a high strength, low modulus material for internal fixation plates. Biomaterials. 1980;1(1):38-40.
18. McKenna GB, Bradley GW, Dunn HK, Statton WO. Mechanical properties of some fibre reinforced polymer composites after implantation as fracture fixation plates. Biomaterials. 1980;1(4):189-192.
19. Tayton K, Johnson-Nurse C, McKibbin B, Bradley J, Hastings G. The use of semi-rigid carbon-fibre–reinforced plastic plates for fixation of human fractures. Results of preliminary trials. J Bone Joint Surg Br. 1982;64(1):105-111.
20. Tayton K, Bradley J. How stiff should semi-rigid fixation of the human tibia be? A clue to the answer. J Bone Joint Surg Br. 1983;65(3):312-315.
21. Tayton K. Corrosive effect of carbon-fibre reinforced plastic on stainless-steel screws during implantation into man. J Med Eng Technol. 1983;7(1):24-26.
22. Howard CB, Tayton KJ, Gibbs A. The response of human tissues to carbon reinforced epoxy resin. J Bone Joint Surg Br. 1985;67(4):656-658.
23. Skirving AP, Day R, Macdonald W, McLaren R. Carbon fiber reinforced plastic (CFRP) plates versus stainless steel dynamic compression plates in the treatment of fractures of the tibiae in dogs. Clin Orthop Relat Res. 1987;(224):117-124.
24. Prakash R, Marwah S, Goel SC, Tuli SM. Carbon fibre reinforced epoxy implants for bridging large osteoperiosteal gaps. Biomaterials. 1988;9(2):198-202.
25. Pemberton DJ, McKibbin B, Savage R, Tayton K, Stuart D. Carbon-fibre reinforced plates for problem fractures. J Bone Joint Surg Br. 1992;74(1):88-92.
26. Pemberton DJ, Evans PD, Grant A, McKibbin B. Fractures of the distal femur in the elderly treated with a carbon fibre supracondylar plate. Injury. 1994;25(5):317-321.
27. Kelsey DJ, Springer GS, Goodman SB. Composite implant for bone replacement. J Compos Mater. 1997;31(16):1593-1632.
28. Corvelli AA, Biermann PJ, Roberts JC. Design, analysis and fabrication of a composite segmental bone replacement implant. J Adv Mater. 1997;28:2-8.
29. Glassman AH, Crowninshield RD, Schenck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001;(393):128-136.
30. Williams D. New horizons for thermoplastic polymers. Med Device Technol. 2001;12(4):8-9.
31. Al-Shawi AK, Smith SP, Anderson GH. The use of a carbon fiber plate for periprosthetic supracondylar femoral fractures. J Arthroplasty. 2002;17(3):320-324.
32. Baker D, Kadambande SS, Alderman PM. Carbon fibre plates in the treatment of femoral periprosthetic fractures. Injury. 2004;35(6):596-598.
33. Akhavan S, Matthiesen MM, Schulte L, et al. Clinical and histologic results related to a low-modulus composite total hip replacement stem. J Bone Joint Surg Am. 2006;88(6):1308-1314.
34. Toth JM, Wang M, Estes BT, Scifert JL, Seim HB 3rd, Turner AS. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials. 2006;27(3):324-334.
35. Evans SL, Gregson PJ. Composite technology in load-bearing orthopaedic implants. Biomaterials. 1998;19(15):1329-1342.
36. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part I. Crystallinity and interface adhesion. Composites Part A. 2000;31(6):517-530.
37. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part II. Interlaminar fracture toughness. Composites Part A. 2001;32(6):763-774.
38. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part III. Impact damage performance. Composites Part A. 2001;32(6):775-785.
39. Guan SB, Hou CL, Chen AM, Zhang W, Wang JE. Influence of sterilization treatments on continuous carbon-fiber reinforced polyolefin composite. Zhonghua Yi Xue Za Zhi. 2007;87(31):2228-2231.
40. Akay M, Aslan N. An estimation of fatigue life for a carbon fibre/poly ether ether ketone hip joint prosthesis. Proc Inst Mech Eng H. 1995;209(2):93-103.
41. Bagheri ZS, Tavakkoli Awal P, Bougherara H, Aziz MS, Schemitsch EH, Zdero R. Biomechanical analysis of a new carbon fiber/flax/epoxy bone fracture plate shows less stress shielding compared to a standard clinical metal plate. J Biomech Eng. 2014;136(9):091002.
42. Rhee PC, Shin AY. The rate of successful four-corner arthrodesis with a locking, dorsal circular polyether-ether-ketone (PEEK-Optima) plate. J Hand Surg Eur Vol. 2013;38(7):767-773.
43. Nakahara I, Takao M, Bandoh S, Bertollo N, Walsh WR, Sugano N. In vivo implant fixation of carbon fiber–reinforced PEEK hip prostheses in an ovine model. J Orthop Res. 2013;31(3):485-492.
44. Kasliwal MK, O’Toole JE. Clinical experience using polyetheretherketone (PEEK) intervertebral structural cage for anterior cervical corpectomy and fusion. J Clin Neurosci. 2014;21(2):217-220.
45. Tarallo L, Mugnai R, Adani R, Zambianchi F, Catani F. A new volar plate made of carbon-fiber–reinforced polyetheretherketon for distal radius fracture: analysis of 40 cases. J Orthop Traumatol. 2014;15(4):277-283.
46. Cordey J, Perren SM, Steinemann SG. Stress protection due to plates: myth or reality? A parametric analysis made using the composite beam theory. Injury. 2000;31(suppl 3):C1-C13.
1. Ali MS, French TA, Hastings GW, et al. Carbon fibre composite bone plates. Development, evaluation and early clinical experience. J Bone Joint Surg Br. 1990;72(4):586-591.
2. Brooks RA, Jones E, Storer A, Rushton N. Biological evaluation of carbon-fibre–reinforced polybutyleneterephthalate (CFRPBT) employed in a novel acetabular cup. Biomaterials. 2004;25(17):3429-3438.
3. Brown SA, Hastings RS, Mason JJ, Moet A. Characterization of short-fibre reinforced thermoplastics for fracture fixation devices. Biomaterials. 1990;11(8):541-547.
4. Skinner HB. Composite technology for total hip arthroplasty. Clin Orthop Relat Res. 1988;(235):224-236.
5. Field RE, Jones E, Nuijten P, Storer A, Cronin M, Rushton N. Pre-clinical evaluation of the Cambridge acetabular cup. J Mater Sci Mater Med. 2008;19(8):2791-2798.
6. Han N, Ahmed I, Parsons AJ, et al. Influence of screw holes and gamma sterilization on properties of phosphate glass fiber–reinforced composite bone plates. J Biomater Appl. 2013;27(8):990-1002.
7. Losi P, Munaò A, Spiller D, et al. Evaluation of a new composite prosthesis for the repair of abdominal wall defects. J Mater Sci Mater Med. 2007;18(10):1939-1944.
8. Pait TG, Kaufman HH, Voelker JL, McAllister HP, Willison C. Use of a carbon composite radiolucent anterior cervical retractor system. Neurosurgery. 1993;33(5):941-942.
9. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
10. Gardner MP, Chong AC, Pollock AG, Wooley PH. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng. 2010;38(3):613-620.
11. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
12. Dunlap JT, Chong AC, Lucas GL, Cooke FW. Structural properties of a novel design of composite analogue humeri models. Ann Biomed Eng. 2008;36(11):1922-1926.
13. Grover P, Albert C, Wang M, Harris GF. Mechanical characterization of fourth generation composite humerus. Proc Inst Mech Eng H. 2011;225(12):1169-1176.
14. Zheng Q, Morgan RJ. Synergistic thermal-moisture damage mechanisms of epoxies and their carbon fiber composites. J Compos Mater. 1993;27(15):1465-1478.
15. Ray BC. Temperature effect during humid ageing on interfaces of glass and carbon fibers reinforced epoxy composites. J Colloid Interface Sci. 2006;298(1):111-117.
16. Standard test method for short-beam strength of polymer matrix composite materials and their laminates [ASTM specification D2344/D2344M-00]. In: Annual Book of ASTM Standards. Vol 15.03. West Conshohocken, PA: American Society for Testing and Materials; 2006.
17. Bradley JS, Hastings GW, Johnson-Nurse C. Carbon fibre reinforced epoxy as a high strength, low modulus material for internal fixation plates. Biomaterials. 1980;1(1):38-40.
18. McKenna GB, Bradley GW, Dunn HK, Statton WO. Mechanical properties of some fibre reinforced polymer composites after implantation as fracture fixation plates. Biomaterials. 1980;1(4):189-192.
19. Tayton K, Johnson-Nurse C, McKibbin B, Bradley J, Hastings G. The use of semi-rigid carbon-fibre–reinforced plastic plates for fixation of human fractures. Results of preliminary trials. J Bone Joint Surg Br. 1982;64(1):105-111.
20. Tayton K, Bradley J. How stiff should semi-rigid fixation of the human tibia be? A clue to the answer. J Bone Joint Surg Br. 1983;65(3):312-315.
21. Tayton K. Corrosive effect of carbon-fibre reinforced plastic on stainless-steel screws during implantation into man. J Med Eng Technol. 1983;7(1):24-26.
22. Howard CB, Tayton KJ, Gibbs A. The response of human tissues to carbon reinforced epoxy resin. J Bone Joint Surg Br. 1985;67(4):656-658.
23. Skirving AP, Day R, Macdonald W, McLaren R. Carbon fiber reinforced plastic (CFRP) plates versus stainless steel dynamic compression plates in the treatment of fractures of the tibiae in dogs. Clin Orthop Relat Res. 1987;(224):117-124.
24. Prakash R, Marwah S, Goel SC, Tuli SM. Carbon fibre reinforced epoxy implants for bridging large osteoperiosteal gaps. Biomaterials. 1988;9(2):198-202.
25. Pemberton DJ, McKibbin B, Savage R, Tayton K, Stuart D. Carbon-fibre reinforced plates for problem fractures. J Bone Joint Surg Br. 1992;74(1):88-92.
26. Pemberton DJ, Evans PD, Grant A, McKibbin B. Fractures of the distal femur in the elderly treated with a carbon fibre supracondylar plate. Injury. 1994;25(5):317-321.
27. Kelsey DJ, Springer GS, Goodman SB. Composite implant for bone replacement. J Compos Mater. 1997;31(16):1593-1632.
28. Corvelli AA, Biermann PJ, Roberts JC. Design, analysis and fabrication of a composite segmental bone replacement implant. J Adv Mater. 1997;28:2-8.
29. Glassman AH, Crowninshield RD, Schenck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001;(393):128-136.
30. Williams D. New horizons for thermoplastic polymers. Med Device Technol. 2001;12(4):8-9.
31. Al-Shawi AK, Smith SP, Anderson GH. The use of a carbon fiber plate for periprosthetic supracondylar femoral fractures. J Arthroplasty. 2002;17(3):320-324.
32. Baker D, Kadambande SS, Alderman PM. Carbon fibre plates in the treatment of femoral periprosthetic fractures. Injury. 2004;35(6):596-598.
33. Akhavan S, Matthiesen MM, Schulte L, et al. Clinical and histologic results related to a low-modulus composite total hip replacement stem. J Bone Joint Surg Am. 2006;88(6):1308-1314.
34. Toth JM, Wang M, Estes BT, Scifert JL, Seim HB 3rd, Turner AS. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials. 2006;27(3):324-334.
35. Evans SL, Gregson PJ. Composite technology in load-bearing orthopaedic implants. Biomaterials. 1998;19(15):1329-1342.
36. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part I. Crystallinity and interface adhesion. Composites Part A. 2000;31(6):517-530.
37. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part II. Interlaminar fracture toughness. Composites Part A. 2001;32(6):763-774.
38. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part III. Impact damage performance. Composites Part A. 2001;32(6):775-785.
39. Guan SB, Hou CL, Chen AM, Zhang W, Wang JE. Influence of sterilization treatments on continuous carbon-fiber reinforced polyolefin composite. Zhonghua Yi Xue Za Zhi. 2007;87(31):2228-2231.
40. Akay M, Aslan N. An estimation of fatigue life for a carbon fibre/poly ether ether ketone hip joint prosthesis. Proc Inst Mech Eng H. 1995;209(2):93-103.
41. Bagheri ZS, Tavakkoli Awal P, Bougherara H, Aziz MS, Schemitsch EH, Zdero R. Biomechanical analysis of a new carbon fiber/flax/epoxy bone fracture plate shows less stress shielding compared to a standard clinical metal plate. J Biomech Eng. 2014;136(9):091002.
42. Rhee PC, Shin AY. The rate of successful four-corner arthrodesis with a locking, dorsal circular polyether-ether-ketone (PEEK-Optima) plate. J Hand Surg Eur Vol. 2013;38(7):767-773.
43. Nakahara I, Takao M, Bandoh S, Bertollo N, Walsh WR, Sugano N. In vivo implant fixation of carbon fiber–reinforced PEEK hip prostheses in an ovine model. J Orthop Res. 2013;31(3):485-492.
44. Kasliwal MK, O’Toole JE. Clinical experience using polyetheretherketone (PEEK) intervertebral structural cage for anterior cervical corpectomy and fusion. J Clin Neurosci. 2014;21(2):217-220.
45. Tarallo L, Mugnai R, Adani R, Zambianchi F, Catani F. A new volar plate made of carbon-fiber–reinforced polyetheretherketon for distal radius fracture: analysis of 40 cases. J Orthop Traumatol. 2014;15(4):277-283.
46. Cordey J, Perren SM, Steinemann SG. Stress protection due to plates: myth or reality? A parametric analysis made using the composite beam theory. Injury. 2000;31(suppl 3):C1-C13.
Reinforcing a Spica Cast With a Fiberglass Bar
Femur fractures (Orthopaedic Trauma Association classes 31, 32, 33)1 are common childhood injuries, occurring at a rate of 19 per 100,000 children in the United States.2 Peak occurrence is bimodal at ages 2 and 17 years. The most common mechanism of injury in children under 6 years is a fall, and hip spica casting is the preferred treatment modality in this group.3-5
A bar connecting the legs of the spica cast has been shown to facilitate patient transport5 and significantly decrease mechanical failure of the spica cast.6 This bar often consists of a broom handle or pipe that must be cut to size during the case and subsequently incorporated into the cast—tasks that are often inconvenient and time-consuming for on-call or emergency department staff unfamiliar with orthopedic tools.
In this article, we review a spica cast application that incorporates a low-cost, lightweight technique for fabricating a connecting bar from existing fiberglass casting material. The Institutional Review Board at Connecticut Children’s Medical Center approved this work.
Technique of Double-Leg Spica Casting With Fiberglass Bar
A spica casting table (Orthopedic Systems) with a well-padded post is placed on the operating room table and adjusted to the length of the patient from perineum to just below the shoulders. With the patient under general anesthesia, folded towels are used to provide 2 to 4 cm of padding on the anterior torso, atop which a waterproof pantaloon is applied. The patient is transferred to the spica table, and the patient’s arms are gently secured to the casting table with cast padding or tape in an abducted position at the shoulders. A surgeon controls the legs by holding the feet with the long fingers just above the heels, the index fingers on the anterior ankle, and the thumbs on the soles of the feet. Cast padding is wrapped from the nipple line to the supramalleolar region on each leg. The bony prominences of the malleoli, patella, fibular head, femoral condyles, iliac crests, and coccyx are well padded.
Fiberglass is then rolled without compression onto the patient, beginning with the torso and perineal areas. The injured leg is wrapped to its final length above the malleoli while the uninjured leg is kept free. Maintaining the position of the injured leg with simultaneous molding at the fracture site, typically to promote valgus, allows fracture reduction. The fracture position is then checked under image intensification. For femur fractures, hip abduction and flexion are set to 45° and 90°, respectively, while knee flexion is between 50° and 90°. The uninjured leg is then wrapped with fiberglass. Additional strips of fiberglass can be used to reinforce weak junctional regions between the torso and the legs, posteriorly over the “intern’s triangle” and anteriorly along the hip crease.
A connecting fiberglass bar is then created using a fiberglass roll once the cast is hardened. A 2-inch fiberglass roll is wrapped around one leg to secure its position (Figure 1A) and then rolled around the second limb (Figure 1B). Fiberglass is then pulled taut and rolled around the bridge that has been created in order to thicken the bar (Figure 2). The roll is again brought around the closest limb, wrapped back across the bridge to the other limb, and rolled out to its full length. Last, the legs are abducted 1 to 2 cm to tension the bar (Figure 3). Although this does not produce enough movement to cause a crease and a resultant ulcer, careful inspection of common pressure points (eg, popliteal fossa) should be performed after the cast is complete.
The chest towels are removed, and the final cast is inspected clinically and fluoroscopically at the fracture site before extubation. The cast is trimmed as needed to ensure room for perineal care, as well as full ankle flexion and extension without impingement. Cast edges are further petaled with plastic tape (Hy-Tape International) to provide padding and prevent the waterproof lining from tearing.
Postoperative care involves overnight observation and caregiver practice in perineal care. Frequent rotation from supine to prone is encouraged. Nurses confirm car-seat fit before discharge. If needed, radiographs are obtained 7 to 10 days later to help with wedging adjustment. The cast is removed in the clinic when adequate callus is appreciated on subsequent radiographs.
Case Series
Our experience with this technique in 16 unilateral femur fractures has been favorable (Table). Patient age ranged from 5 months to 3 years. Mean pretreatment angulation was 13° varus and 11° procurvatum. The majority of fractures were femoral shaft fractures; 1 was proximal, 2 distal.
All fractures united without cast revision. Mean cast time was 4.5 weeks (range, 16 days–6 weeks). Immediate postoperative alignment was 2.5° varus (range, 11° valgus to 16° varus) and 7° procurvatum (range, 1° recurvatum to 22° procurvatum). Mean shortening was 1.5 cm (range, 0-2.7 cm). Final alignment was 1° valgus (range, 9° valgus to 12° varus) and 5° procurvatum (range, 0° to 22°). Mean follow-up was 8 months. There were no cases of skin maceration or cast failure. No casts precluded use of a spica car-seat. Figure 4 shows a typical case with a midshaft fracture treated with closed reduction and casting for 4 weeks with good remodeling at final follow-up, 19 months after injury.
Discussion
Although single-leg walking spica casts have been shown to safely treat low-energy femur fractures in children 1 to 6 years old,7 length-unstable femur fractures, bilateral femur fractures, and patients with hip dysplasia continue to be managed with a double-leg hip spica construct. Cast integrity remains fundamental to the control of most fractures and prevention of cast-related complications, such as skin maceration and ulceration. Surgeons typically use spica cast reinforcement schemes—such as cast augments of the torso–limb junction, with multiple layers of casting material or incorporation of a connecting bar between the legs, typically constructed by overwrapping a wooden dowel in casting material—to improve the mechanical stability of casts.6 The present technique of creating a connecting bar from fiberglass casting material significantly simplifies the standard wooden dowel approach and provided excellent results in our treatment group in terms of cast integrity and fracture alignment. In addition, at our institution, a roll of fiberglass costs $2.10, whereas a wooden dowel costs $3 to $10 and can be difficult to locate if not frequently used. Other tube-shaped materials, such as the disposable material used to package implants and tubes, carry an even lower cost. However, we have found that a single fiberglass roll is most readily available and easiest to apply.
Although proper spica cast application remains important in managing pediatric trauma, it lacks a good technical description in the literature. In this technical report, we have presented our standard spica cast application method, which minimizes the range of cast complications that have been reported, from minor skin irritation to superior mesenteric artery syndrome. Two salient technical highlights are use of waterproof pantaloon liners and cast petaling, which we have found almost eliminate the morbidity of potential skin complications, reported to occur at a rate of 28%.8 In addition, we forgo applying the cast on the injured leg in segments. Application of a short-leg cast on the injured leg to allow traction on the leg during cast application is of dubious utility and may be potentially harmful, with described complications of peroneal nerve palsy and compartment syndrome.9-11 Further, it is important to use an abdominal spacer (eg, a stack of towels) under the cast padding to create room for abdominal expansion and minimize pressure thought to induce superior mesenteric artery syndrome. Plastic or rubber abdominal spacers have also been described.12,13 Last, leg position is important for reduction and maintenance of the fracture, as well as patient care. Literature advocates minimizing hip abduction to just that needed for perineal care and maximizing hip flexion and knee extension to optimize car-seat fit and safety.14
Conclusion
Construction of a spica cast lower limb connecting bar from readily available fiberglass casting material allows a facile and rapid addition to the mechanical stability of a spica cast in the treatment of pediatric femur fractures. The technique is low-cost and obviates the need for additional extraneous materials.
1. Slongo TF, Audigé L; AO Pediatric Classification Group. Fracture and dislocation classification compendium for children: the AO Pediatric Comprehensive Classification of Long Bone Fractures (PCCF). J Orthop Trauma. 2007;21(10):S135-S160.
2. Hinton RY, Lincoln A, Crockett MM, Sponseller P, Smith G. Fractures of the femoral shaft in children. Incidence, mechanisms, and sociodemographic risk factors. J Bone Joint Surg Am. 1999;81(4):500-509.
3. Campbell WC, Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby Elsevier; 2008.
4. Lovell WW, Winter RB, Morrissy RT, Weinstein SL. Lovell and Winter’s Pediatric Orthopaedics. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
5. Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2009.
6. Hosalkar HS, Jones S, Chowdhury M, Chatoo M, Hill RA. Connecting bar for hip spica reinforcement: does it help? J Pediatr Orthop B. 2003;12(2):100-102.
7. Flynn JM, Garner MR, Jones KJ, et al. The treatment of low-energy femoral shaft fractures: a prospective study comparing the “walking spica” with the traditional spica cast. J Bone Joint Surg Am. 2011;93(23):2196-2202.
8. DiFazio R, Vessey J, Zurakowski D, Hresko MT, Matheney T. Incidence of skin complications and associated charges in children treated with hip spica casts for femur fractures. J Pediatr Orthop. 2011;31(1):17-22.
9. Weiss AP, Schenck RC Jr, Sponseller PD, Thompson JD. Peroneal nerve palsy after early cast application for femoral fractures in children. J Pediatr Orthop. 1992;12(1):25-28.
10. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
11. Large TM, Frick SL. Compartment syndrome of the leg after treatment of a femoral fracture with an early sitting spica cast. A report of two cases. J Bone Joint Surg Am. 2003;85(11):2207-2210.
12. Sharma S, Azzopardi T. Reduction of abdominal pressure for prophylaxis of the mesenteric artery syndrome (cast syndrome) in a hip spica—a simple technique. Ann R Coll Surg Engl. 2006;88(3):317.
13. Kiter E, Demirkan F, Kiliç BA, Erkula G. A new technique for creating an abdominal window in a hip spica cast. J Orthop Trauma. 2003;17(6):442-443.
14. Zielinski J, Oliver G, Sybesma J, Walter N, Atkinson P. Casting technique and restraint choice influence child safety during transport of body casted children subjected to a simulated frontal MVA. J Trauma. 2009;66(6):1653-1665.
Femur fractures (Orthopaedic Trauma Association classes 31, 32, 33)1 are common childhood injuries, occurring at a rate of 19 per 100,000 children in the United States.2 Peak occurrence is bimodal at ages 2 and 17 years. The most common mechanism of injury in children under 6 years is a fall, and hip spica casting is the preferred treatment modality in this group.3-5
A bar connecting the legs of the spica cast has been shown to facilitate patient transport5 and significantly decrease mechanical failure of the spica cast.6 This bar often consists of a broom handle or pipe that must be cut to size during the case and subsequently incorporated into the cast—tasks that are often inconvenient and time-consuming for on-call or emergency department staff unfamiliar with orthopedic tools.
In this article, we review a spica cast application that incorporates a low-cost, lightweight technique for fabricating a connecting bar from existing fiberglass casting material. The Institutional Review Board at Connecticut Children’s Medical Center approved this work.
Technique of Double-Leg Spica Casting With Fiberglass Bar
A spica casting table (Orthopedic Systems) with a well-padded post is placed on the operating room table and adjusted to the length of the patient from perineum to just below the shoulders. With the patient under general anesthesia, folded towels are used to provide 2 to 4 cm of padding on the anterior torso, atop which a waterproof pantaloon is applied. The patient is transferred to the spica table, and the patient’s arms are gently secured to the casting table with cast padding or tape in an abducted position at the shoulders. A surgeon controls the legs by holding the feet with the long fingers just above the heels, the index fingers on the anterior ankle, and the thumbs on the soles of the feet. Cast padding is wrapped from the nipple line to the supramalleolar region on each leg. The bony prominences of the malleoli, patella, fibular head, femoral condyles, iliac crests, and coccyx are well padded.
Fiberglass is then rolled without compression onto the patient, beginning with the torso and perineal areas. The injured leg is wrapped to its final length above the malleoli while the uninjured leg is kept free. Maintaining the position of the injured leg with simultaneous molding at the fracture site, typically to promote valgus, allows fracture reduction. The fracture position is then checked under image intensification. For femur fractures, hip abduction and flexion are set to 45° and 90°, respectively, while knee flexion is between 50° and 90°. The uninjured leg is then wrapped with fiberglass. Additional strips of fiberglass can be used to reinforce weak junctional regions between the torso and the legs, posteriorly over the “intern’s triangle” and anteriorly along the hip crease.
A connecting fiberglass bar is then created using a fiberglass roll once the cast is hardened. A 2-inch fiberglass roll is wrapped around one leg to secure its position (Figure 1A) and then rolled around the second limb (Figure 1B). Fiberglass is then pulled taut and rolled around the bridge that has been created in order to thicken the bar (Figure 2). The roll is again brought around the closest limb, wrapped back across the bridge to the other limb, and rolled out to its full length. Last, the legs are abducted 1 to 2 cm to tension the bar (Figure 3). Although this does not produce enough movement to cause a crease and a resultant ulcer, careful inspection of common pressure points (eg, popliteal fossa) should be performed after the cast is complete.
The chest towels are removed, and the final cast is inspected clinically and fluoroscopically at the fracture site before extubation. The cast is trimmed as needed to ensure room for perineal care, as well as full ankle flexion and extension without impingement. Cast edges are further petaled with plastic tape (Hy-Tape International) to provide padding and prevent the waterproof lining from tearing.
Postoperative care involves overnight observation and caregiver practice in perineal care. Frequent rotation from supine to prone is encouraged. Nurses confirm car-seat fit before discharge. If needed, radiographs are obtained 7 to 10 days later to help with wedging adjustment. The cast is removed in the clinic when adequate callus is appreciated on subsequent radiographs.
Case Series
Our experience with this technique in 16 unilateral femur fractures has been favorable (Table). Patient age ranged from 5 months to 3 years. Mean pretreatment angulation was 13° varus and 11° procurvatum. The majority of fractures were femoral shaft fractures; 1 was proximal, 2 distal.
All fractures united without cast revision. Mean cast time was 4.5 weeks (range, 16 days–6 weeks). Immediate postoperative alignment was 2.5° varus (range, 11° valgus to 16° varus) and 7° procurvatum (range, 1° recurvatum to 22° procurvatum). Mean shortening was 1.5 cm (range, 0-2.7 cm). Final alignment was 1° valgus (range, 9° valgus to 12° varus) and 5° procurvatum (range, 0° to 22°). Mean follow-up was 8 months. There were no cases of skin maceration or cast failure. No casts precluded use of a spica car-seat. Figure 4 shows a typical case with a midshaft fracture treated with closed reduction and casting for 4 weeks with good remodeling at final follow-up, 19 months after injury.
Discussion
Although single-leg walking spica casts have been shown to safely treat low-energy femur fractures in children 1 to 6 years old,7 length-unstable femur fractures, bilateral femur fractures, and patients with hip dysplasia continue to be managed with a double-leg hip spica construct. Cast integrity remains fundamental to the control of most fractures and prevention of cast-related complications, such as skin maceration and ulceration. Surgeons typically use spica cast reinforcement schemes—such as cast augments of the torso–limb junction, with multiple layers of casting material or incorporation of a connecting bar between the legs, typically constructed by overwrapping a wooden dowel in casting material—to improve the mechanical stability of casts.6 The present technique of creating a connecting bar from fiberglass casting material significantly simplifies the standard wooden dowel approach and provided excellent results in our treatment group in terms of cast integrity and fracture alignment. In addition, at our institution, a roll of fiberglass costs $2.10, whereas a wooden dowel costs $3 to $10 and can be difficult to locate if not frequently used. Other tube-shaped materials, such as the disposable material used to package implants and tubes, carry an even lower cost. However, we have found that a single fiberglass roll is most readily available and easiest to apply.
Although proper spica cast application remains important in managing pediatric trauma, it lacks a good technical description in the literature. In this technical report, we have presented our standard spica cast application method, which minimizes the range of cast complications that have been reported, from minor skin irritation to superior mesenteric artery syndrome. Two salient technical highlights are use of waterproof pantaloon liners and cast petaling, which we have found almost eliminate the morbidity of potential skin complications, reported to occur at a rate of 28%.8 In addition, we forgo applying the cast on the injured leg in segments. Application of a short-leg cast on the injured leg to allow traction on the leg during cast application is of dubious utility and may be potentially harmful, with described complications of peroneal nerve palsy and compartment syndrome.9-11 Further, it is important to use an abdominal spacer (eg, a stack of towels) under the cast padding to create room for abdominal expansion and minimize pressure thought to induce superior mesenteric artery syndrome. Plastic or rubber abdominal spacers have also been described.12,13 Last, leg position is important for reduction and maintenance of the fracture, as well as patient care. Literature advocates minimizing hip abduction to just that needed for perineal care and maximizing hip flexion and knee extension to optimize car-seat fit and safety.14
Conclusion
Construction of a spica cast lower limb connecting bar from readily available fiberglass casting material allows a facile and rapid addition to the mechanical stability of a spica cast in the treatment of pediatric femur fractures. The technique is low-cost and obviates the need for additional extraneous materials.
Femur fractures (Orthopaedic Trauma Association classes 31, 32, 33)1 are common childhood injuries, occurring at a rate of 19 per 100,000 children in the United States.2 Peak occurrence is bimodal at ages 2 and 17 years. The most common mechanism of injury in children under 6 years is a fall, and hip spica casting is the preferred treatment modality in this group.3-5
A bar connecting the legs of the spica cast has been shown to facilitate patient transport5 and significantly decrease mechanical failure of the spica cast.6 This bar often consists of a broom handle or pipe that must be cut to size during the case and subsequently incorporated into the cast—tasks that are often inconvenient and time-consuming for on-call or emergency department staff unfamiliar with orthopedic tools.
In this article, we review a spica cast application that incorporates a low-cost, lightweight technique for fabricating a connecting bar from existing fiberglass casting material. The Institutional Review Board at Connecticut Children’s Medical Center approved this work.
Technique of Double-Leg Spica Casting With Fiberglass Bar
A spica casting table (Orthopedic Systems) with a well-padded post is placed on the operating room table and adjusted to the length of the patient from perineum to just below the shoulders. With the patient under general anesthesia, folded towels are used to provide 2 to 4 cm of padding on the anterior torso, atop which a waterproof pantaloon is applied. The patient is transferred to the spica table, and the patient’s arms are gently secured to the casting table with cast padding or tape in an abducted position at the shoulders. A surgeon controls the legs by holding the feet with the long fingers just above the heels, the index fingers on the anterior ankle, and the thumbs on the soles of the feet. Cast padding is wrapped from the nipple line to the supramalleolar region on each leg. The bony prominences of the malleoli, patella, fibular head, femoral condyles, iliac crests, and coccyx are well padded.
Fiberglass is then rolled without compression onto the patient, beginning with the torso and perineal areas. The injured leg is wrapped to its final length above the malleoli while the uninjured leg is kept free. Maintaining the position of the injured leg with simultaneous molding at the fracture site, typically to promote valgus, allows fracture reduction. The fracture position is then checked under image intensification. For femur fractures, hip abduction and flexion are set to 45° and 90°, respectively, while knee flexion is between 50° and 90°. The uninjured leg is then wrapped with fiberglass. Additional strips of fiberglass can be used to reinforce weak junctional regions between the torso and the legs, posteriorly over the “intern’s triangle” and anteriorly along the hip crease.
A connecting fiberglass bar is then created using a fiberglass roll once the cast is hardened. A 2-inch fiberglass roll is wrapped around one leg to secure its position (Figure 1A) and then rolled around the second limb (Figure 1B). Fiberglass is then pulled taut and rolled around the bridge that has been created in order to thicken the bar (Figure 2). The roll is again brought around the closest limb, wrapped back across the bridge to the other limb, and rolled out to its full length. Last, the legs are abducted 1 to 2 cm to tension the bar (Figure 3). Although this does not produce enough movement to cause a crease and a resultant ulcer, careful inspection of common pressure points (eg, popliteal fossa) should be performed after the cast is complete.
The chest towels are removed, and the final cast is inspected clinically and fluoroscopically at the fracture site before extubation. The cast is trimmed as needed to ensure room for perineal care, as well as full ankle flexion and extension without impingement. Cast edges are further petaled with plastic tape (Hy-Tape International) to provide padding and prevent the waterproof lining from tearing.
Postoperative care involves overnight observation and caregiver practice in perineal care. Frequent rotation from supine to prone is encouraged. Nurses confirm car-seat fit before discharge. If needed, radiographs are obtained 7 to 10 days later to help with wedging adjustment. The cast is removed in the clinic when adequate callus is appreciated on subsequent radiographs.
Case Series
Our experience with this technique in 16 unilateral femur fractures has been favorable (Table). Patient age ranged from 5 months to 3 years. Mean pretreatment angulation was 13° varus and 11° procurvatum. The majority of fractures were femoral shaft fractures; 1 was proximal, 2 distal.
All fractures united without cast revision. Mean cast time was 4.5 weeks (range, 16 days–6 weeks). Immediate postoperative alignment was 2.5° varus (range, 11° valgus to 16° varus) and 7° procurvatum (range, 1° recurvatum to 22° procurvatum). Mean shortening was 1.5 cm (range, 0-2.7 cm). Final alignment was 1° valgus (range, 9° valgus to 12° varus) and 5° procurvatum (range, 0° to 22°). Mean follow-up was 8 months. There were no cases of skin maceration or cast failure. No casts precluded use of a spica car-seat. Figure 4 shows a typical case with a midshaft fracture treated with closed reduction and casting for 4 weeks with good remodeling at final follow-up, 19 months after injury.
Discussion
Although single-leg walking spica casts have been shown to safely treat low-energy femur fractures in children 1 to 6 years old,7 length-unstable femur fractures, bilateral femur fractures, and patients with hip dysplasia continue to be managed with a double-leg hip spica construct. Cast integrity remains fundamental to the control of most fractures and prevention of cast-related complications, such as skin maceration and ulceration. Surgeons typically use spica cast reinforcement schemes—such as cast augments of the torso–limb junction, with multiple layers of casting material or incorporation of a connecting bar between the legs, typically constructed by overwrapping a wooden dowel in casting material—to improve the mechanical stability of casts.6 The present technique of creating a connecting bar from fiberglass casting material significantly simplifies the standard wooden dowel approach and provided excellent results in our treatment group in terms of cast integrity and fracture alignment. In addition, at our institution, a roll of fiberglass costs $2.10, whereas a wooden dowel costs $3 to $10 and can be difficult to locate if not frequently used. Other tube-shaped materials, such as the disposable material used to package implants and tubes, carry an even lower cost. However, we have found that a single fiberglass roll is most readily available and easiest to apply.
Although proper spica cast application remains important in managing pediatric trauma, it lacks a good technical description in the literature. In this technical report, we have presented our standard spica cast application method, which minimizes the range of cast complications that have been reported, from minor skin irritation to superior mesenteric artery syndrome. Two salient technical highlights are use of waterproof pantaloon liners and cast petaling, which we have found almost eliminate the morbidity of potential skin complications, reported to occur at a rate of 28%.8 In addition, we forgo applying the cast on the injured leg in segments. Application of a short-leg cast on the injured leg to allow traction on the leg during cast application is of dubious utility and may be potentially harmful, with described complications of peroneal nerve palsy and compartment syndrome.9-11 Further, it is important to use an abdominal spacer (eg, a stack of towels) under the cast padding to create room for abdominal expansion and minimize pressure thought to induce superior mesenteric artery syndrome. Plastic or rubber abdominal spacers have also been described.12,13 Last, leg position is important for reduction and maintenance of the fracture, as well as patient care. Literature advocates minimizing hip abduction to just that needed for perineal care and maximizing hip flexion and knee extension to optimize car-seat fit and safety.14
Conclusion
Construction of a spica cast lower limb connecting bar from readily available fiberglass casting material allows a facile and rapid addition to the mechanical stability of a spica cast in the treatment of pediatric femur fractures. The technique is low-cost and obviates the need for additional extraneous materials.
1. Slongo TF, Audigé L; AO Pediatric Classification Group. Fracture and dislocation classification compendium for children: the AO Pediatric Comprehensive Classification of Long Bone Fractures (PCCF). J Orthop Trauma. 2007;21(10):S135-S160.
2. Hinton RY, Lincoln A, Crockett MM, Sponseller P, Smith G. Fractures of the femoral shaft in children. Incidence, mechanisms, and sociodemographic risk factors. J Bone Joint Surg Am. 1999;81(4):500-509.
3. Campbell WC, Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby Elsevier; 2008.
4. Lovell WW, Winter RB, Morrissy RT, Weinstein SL. Lovell and Winter’s Pediatric Orthopaedics. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
5. Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2009.
6. Hosalkar HS, Jones S, Chowdhury M, Chatoo M, Hill RA. Connecting bar for hip spica reinforcement: does it help? J Pediatr Orthop B. 2003;12(2):100-102.
7. Flynn JM, Garner MR, Jones KJ, et al. The treatment of low-energy femoral shaft fractures: a prospective study comparing the “walking spica” with the traditional spica cast. J Bone Joint Surg Am. 2011;93(23):2196-2202.
8. DiFazio R, Vessey J, Zurakowski D, Hresko MT, Matheney T. Incidence of skin complications and associated charges in children treated with hip spica casts for femur fractures. J Pediatr Orthop. 2011;31(1):17-22.
9. Weiss AP, Schenck RC Jr, Sponseller PD, Thompson JD. Peroneal nerve palsy after early cast application for femoral fractures in children. J Pediatr Orthop. 1992;12(1):25-28.
10. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
11. Large TM, Frick SL. Compartment syndrome of the leg after treatment of a femoral fracture with an early sitting spica cast. A report of two cases. J Bone Joint Surg Am. 2003;85(11):2207-2210.
12. Sharma S, Azzopardi T. Reduction of abdominal pressure for prophylaxis of the mesenteric artery syndrome (cast syndrome) in a hip spica—a simple technique. Ann R Coll Surg Engl. 2006;88(3):317.
13. Kiter E, Demirkan F, Kiliç BA, Erkula G. A new technique for creating an abdominal window in a hip spica cast. J Orthop Trauma. 2003;17(6):442-443.
14. Zielinski J, Oliver G, Sybesma J, Walter N, Atkinson P. Casting technique and restraint choice influence child safety during transport of body casted children subjected to a simulated frontal MVA. J Trauma. 2009;66(6):1653-1665.
1. Slongo TF, Audigé L; AO Pediatric Classification Group. Fracture and dislocation classification compendium for children: the AO Pediatric Comprehensive Classification of Long Bone Fractures (PCCF). J Orthop Trauma. 2007;21(10):S135-S160.
2. Hinton RY, Lincoln A, Crockett MM, Sponseller P, Smith G. Fractures of the femoral shaft in children. Incidence, mechanisms, and sociodemographic risk factors. J Bone Joint Surg Am. 1999;81(4):500-509.
3. Campbell WC, Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby Elsevier; 2008.
4. Lovell WW, Winter RB, Morrissy RT, Weinstein SL. Lovell and Winter’s Pediatric Orthopaedics. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
5. Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2009.
6. Hosalkar HS, Jones S, Chowdhury M, Chatoo M, Hill RA. Connecting bar for hip spica reinforcement: does it help? J Pediatr Orthop B. 2003;12(2):100-102.
7. Flynn JM, Garner MR, Jones KJ, et al. The treatment of low-energy femoral shaft fractures: a prospective study comparing the “walking spica” with the traditional spica cast. J Bone Joint Surg Am. 2011;93(23):2196-2202.
8. DiFazio R, Vessey J, Zurakowski D, Hresko MT, Matheney T. Incidence of skin complications and associated charges in children treated with hip spica casts for femur fractures. J Pediatr Orthop. 2011;31(1):17-22.
9. Weiss AP, Schenck RC Jr, Sponseller PD, Thompson JD. Peroneal nerve palsy after early cast application for femoral fractures in children. J Pediatr Orthop. 1992;12(1):25-28.
10. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
11. Large TM, Frick SL. Compartment syndrome of the leg after treatment of a femoral fracture with an early sitting spica cast. A report of two cases. J Bone Joint Surg Am. 2003;85(11):2207-2210.
12. Sharma S, Azzopardi T. Reduction of abdominal pressure for prophylaxis of the mesenteric artery syndrome (cast syndrome) in a hip spica—a simple technique. Ann R Coll Surg Engl. 2006;88(3):317.
13. Kiter E, Demirkan F, Kiliç BA, Erkula G. A new technique for creating an abdominal window in a hip spica cast. J Orthop Trauma. 2003;17(6):442-443.
14. Zielinski J, Oliver G, Sybesma J, Walter N, Atkinson P. Casting technique and restraint choice influence child safety during transport of body casted children subjected to a simulated frontal MVA. J Trauma. 2009;66(6):1653-1665.
Total Shoulder Arthroplasty Outcome for Treatment of Osteoarthritis: A Multicenter Study Using a Contemporary Implant
Anatomical total shoulder arthroplasty (TSA) is an effective treatment for advanced osteoarthritis (OA) of the glenohumeral joint.1-4 Over the past 40 years, since the early reports appeared, the implants have evolved from the early monoblock humeral component to modular components, variable neck angled components with eccentric heads, and components that can provide variable neck angles, version angles, and dual eccentricity to match the anatomy of the proximal humerus. The goal of the new implants is to replicate the individual patient’s native anatomy using a combination of modularity, multiple neck and version angles, and dual eccentricity of the neck and head. The flexibility of the implant system is made possible by a replicator plate. There are few reports on outcomes of using these new implants for OA.
In this article, we report outcomes of using a dual eccentric, variable neck angle, variable version angle implant with a replicator plate for the treatment of OA of the shoulder at 4 centers.
Materials and Methods
The Western Institutional Review Board approved this study, and consent was prospectively obtained and retrospectively reviewed.
The data banks of a 4-center consortium were queried. Only primary TSA patients treated for OA with a fourth-generation Exactech Equinoxe implant (Exactech, Inc.) were included. For the center to be included, it had to have an 80% patient follow-up rate at a minimum of 2 years. Four centers qualified for inclusion: University of Florida, Medical College of Georgia, New York University, and Bordeaux-Merignac Clinic. Data were obtained on surgeries sequentially performed between August 1, 2006, and December 31, 2010. All data were obtained prospectively using a common data collection format.
The Equinoxe anatomical TSA allows for independent adaptation of neck angle and humeral version and provides 2 variable offset times (1 on replicator plate, 1 on humeral head) for matching the native anatomy in more than 99% of cases5 (Figure). The replicator plate is eccentric and can be angled 7.5° in any direction and rotated 360° to provide humeral head coverage. Once its optimal position is obtained, the plate is permanently fixed to the humeral stem using a breakaway screw. Some contemporary implants have similar features.
There were 218 primary shoulder arthroplasties performed on 201 patients (98 male, 103 female). Mean age at time of surgery was 67 years (range, 31-87 years), and mean follow-up was 36 months (range, 24-72 months). The collective follow-up rate at the 3-year mean follow-up and 2-year minimal follow-up was 81%. Eleven shoulders had a cemented stem, and 207 had an uncemented stem. Forty-eight shoulders used the 1.5-mm replicator plate, and 170 used the 4.5-mm offset replicator plate. The patients in this study were typically not very healthy: mean American Society of Anesthesiologists (ASA) score was 2.57 (range, 1-3).
Five outcome scores were calculated from the prospectively obtained data: Constant normalized, Shoulder Pain and Disability Index (SPADI), Simple Shoulder Test (SST), UCLA Shoulder Rating Scale (UCLA), and American Shoulder and Elbow Surgeons Shoulder Assessment (ASES). Before initiating data collection, we developed the Metric Form6 so we could calculate multiple scores while asking the minimal possible number of questions. This could be done for all 5 outcome scores, as their questions have significant overlap.
Objective outcomes included active external rotation, active scaption, active abduction, and active internal rotation. Complications, including revisions, were noted and analyzed. We focus on functional outcomes and do not present radiographic outcomes.
Results
A 2-tailed unpaired t test was used to compare preoperative values with final outcome values (P < .05). Four objective outcomes were significantly improved over preoperative levels: active external rotation (preoperative, 15°; postoperative, 42°), active scaption (pre, 92°; post, 137°), active abduction (pre, 80°; post, 121°), and active internal rotation (pre, S3; post, L2). The functional outcome scores that were significantly (P < .05) improved at final follow-up were Constant normalized (pre, 39; post, 79), SPADI (pre, 86; post, 20), SST (pre, 3.3; post, 10), UCLA (pre, 13; post, 31), and ASES (pre, 33; post, 85).
The outcome improvements at latest follow-up were active external rotation (+28), active scaption (+45), active abduction (+42), active internal rotation (+6 anatomical segments), Constant normalized (+40), SPADI (–66), SST (+6.7), UCLA (+18), and ASES (+52).
There were 32 complications in 25 shoulders. There were no bilateral complications. Seven shoulders had multiple complications, of which many were not independent events. For example, rotator cuff deficiency was associated with instability, and infection was associated with glenoid loosening. One patient had 2 procedures, the first an arthroscopic release and the second a revision shoulder arthroplasty for glenoid loosening. The most common postoperative complication was rotator cuff failure (RCF) or suspected RCF (13 shoulders, including 8 treated with revision arthroplasty). RCF occurred most commonly at the rotator cuff interval, followed by the subscapularis and the supraspinatus. RCF location was based on computed tomography scan or intraoperative observation. The few subscapularis failures occurred with both subscapularis tendon repair and osteotomy. The high RCF rate may derive from scrutinizing postoperative radiographs and was not necessarily confirmed with repeat surgery. We think this represents a more realistic estimate of true postoperative rotator cuff dysfunction, rather than including only reoperated cases. The second most common complication was infection (6 shoulders, 1 with a superficial suture abscess and 5 with deep infections). Other complications were instability (4, with 2 caused by rotator cuff insufficiency), glenoid loosening (4, with 2 caused by infection), stiffness (3), nerve issue (1), and hematoma evacuation (1).
In 21 shoulders, these complications were treated with revision shoulder arthroplasty (16 shoulders), arthroscopic capsular release (3), evacuation of postoperative hematoma (1), and débridement of suture abscess (1). The 16 revision shoulder arthroplasties performed were conversion to reverse shoulder arthroplasty (11 shoulders) and placement of an antibiotic spacer for infection (5). The stem was left in place for all revisions, excluding those for infection. This is a significant advantage of the modular platform stem. Details of the complications and treatments are listed in the Table. There was no difference in health status between patients with a complication (ASA, 2.57) and those without one (ASA, 2.56).
Discussion
The implant described in this article consists of a metaphyseal press-fit stem, a replicator plate, multiple eccentric humeral heads, and a glenoid of multiple sizes with 2 radii of curvatures used to match the patient’s native anatomy and still maintain the appropriate radius of curvature mismatch between the humeral head and the glenoid. Between the eccentricity in the replicator plate and the eccentricity in the humeral head, almost any humeral head cut can be covered, more than 99% of the time.1 However, it remains to be seen if a versatile implant that comes close to matching the patient’s native anatomy will make a difference clinically.
The objective and functional outcomes in this study compare well with those of other, large TSA studies using older prostheses.1-4 There are few reports on contemporary implants with sufficient follow-up numbers for the single diagnosis of OA. Norris and Iannotti2 reported on a multicenter study of 176 patients with a Depuy Global TSA. The design of their study comes closest to that of our clinical outcome study. Nineteen surgeons were involved in their study. The follow-up rate is not clear. Their outcomes (with ours in parentheses for comparison) were active external rotation of 45° (42°), active elevation of 138° (137°), ASES of 84 (85), and SST of 9.2 (10). Norris and Iannotti2 noted an overall complication rate of 13% (12% in our series). Their most common postoperative complications were RCF and glenoid loosening; ours were RCF and infection. Another multicenter study with short-term results using a contemporary prosthesis included 268 shoulders followed for a minimum of 12 months.1 At final follow-up, Constant score was 97, active elevation was 145°, and the complication rate was 8.6%. Godenèche and colleagues1 also noted a glenoid lucent-line rate of 58% and reported that rotator cuff pathology adversely affected outcome.
Although the overall clinical outcome results are encouraging and the complication rate is in the reported range, we believe that a focus on the major complication categories may have a significant positive impact on our patients. The present article places significant importance on reporting complications prospectively, which is more accurate than retrospective reporting. The rates of both RCF and infection, the most common complications in our study, need to be decreased. Aldinger and colleagues7 reported a 12% complication rate in 485 primary shoulder arthroplasties—a rate identical to ours here. In their study, nerve injuries and humeral fractures were both more common than rotator cuff tears. We think that rotator cuff deficiency after TSA is underreported because it is often based on revision surgery alone. It is also interesting that the majority of the cuff deficiencies were through the upper subscapularis rotator interval and were not a complete failure of the subscapularis repair. Not all these patients will undergo revision surgery. In the future, the RCF rate may drop with the increasingly common use of reverse shoulder arthroplasty for substandard rotator cuffs.
Use of this contemporary variable neck angle, variable version angle, dual eccentric shoulder arthroplasty with a replicator plate provides satisfying short-term clinical outcomes. Patients with less than optimal health (mean ASA, 2.57) seem to tolerate the procedure well. Continued focus on RCF and infection will have the greatest impact on the overall complication rate.
1. Godenèche A, Boileau P, Favard L, et al. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11(1):11-18.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
3. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
4. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
5. Irlenbusch U, Rott O, Gebhardt K, Werner A. Reconstruction of the rotational centre of the humeral head with double eccentric adaptable shoulder prosthesis [abstract]. In: Proceedings of the European Federation of National Associations of Orthopaedics and Traumatology (EFORT); May 29-June 1, 2008; Nice, France.
6. Flurin PH, Roche CP, Wright TW, Zuckerman J, Johnson D, Christensen M. A correlation of five commonly used clinical metrics to measure outcomes in shoulder arthroplasty. In: Transactions of the 58th Annual Meeting of the Orthopaedic Research Society (ORS); February 4-7, 2012; San Francisco, CA.
7. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
Anatomical total shoulder arthroplasty (TSA) is an effective treatment for advanced osteoarthritis (OA) of the glenohumeral joint.1-4 Over the past 40 years, since the early reports appeared, the implants have evolved from the early monoblock humeral component to modular components, variable neck angled components with eccentric heads, and components that can provide variable neck angles, version angles, and dual eccentricity to match the anatomy of the proximal humerus. The goal of the new implants is to replicate the individual patient’s native anatomy using a combination of modularity, multiple neck and version angles, and dual eccentricity of the neck and head. The flexibility of the implant system is made possible by a replicator plate. There are few reports on outcomes of using these new implants for OA.
In this article, we report outcomes of using a dual eccentric, variable neck angle, variable version angle implant with a replicator plate for the treatment of OA of the shoulder at 4 centers.
Materials and Methods
The Western Institutional Review Board approved this study, and consent was prospectively obtained and retrospectively reviewed.
The data banks of a 4-center consortium were queried. Only primary TSA patients treated for OA with a fourth-generation Exactech Equinoxe implant (Exactech, Inc.) were included. For the center to be included, it had to have an 80% patient follow-up rate at a minimum of 2 years. Four centers qualified for inclusion: University of Florida, Medical College of Georgia, New York University, and Bordeaux-Merignac Clinic. Data were obtained on surgeries sequentially performed between August 1, 2006, and December 31, 2010. All data were obtained prospectively using a common data collection format.
The Equinoxe anatomical TSA allows for independent adaptation of neck angle and humeral version and provides 2 variable offset times (1 on replicator plate, 1 on humeral head) for matching the native anatomy in more than 99% of cases5 (Figure). The replicator plate is eccentric and can be angled 7.5° in any direction and rotated 360° to provide humeral head coverage. Once its optimal position is obtained, the plate is permanently fixed to the humeral stem using a breakaway screw. Some contemporary implants have similar features.
There were 218 primary shoulder arthroplasties performed on 201 patients (98 male, 103 female). Mean age at time of surgery was 67 years (range, 31-87 years), and mean follow-up was 36 months (range, 24-72 months). The collective follow-up rate at the 3-year mean follow-up and 2-year minimal follow-up was 81%. Eleven shoulders had a cemented stem, and 207 had an uncemented stem. Forty-eight shoulders used the 1.5-mm replicator plate, and 170 used the 4.5-mm offset replicator plate. The patients in this study were typically not very healthy: mean American Society of Anesthesiologists (ASA) score was 2.57 (range, 1-3).
Five outcome scores were calculated from the prospectively obtained data: Constant normalized, Shoulder Pain and Disability Index (SPADI), Simple Shoulder Test (SST), UCLA Shoulder Rating Scale (UCLA), and American Shoulder and Elbow Surgeons Shoulder Assessment (ASES). Before initiating data collection, we developed the Metric Form6 so we could calculate multiple scores while asking the minimal possible number of questions. This could be done for all 5 outcome scores, as their questions have significant overlap.
Objective outcomes included active external rotation, active scaption, active abduction, and active internal rotation. Complications, including revisions, were noted and analyzed. We focus on functional outcomes and do not present radiographic outcomes.
Results
A 2-tailed unpaired t test was used to compare preoperative values with final outcome values (P < .05). Four objective outcomes were significantly improved over preoperative levels: active external rotation (preoperative, 15°; postoperative, 42°), active scaption (pre, 92°; post, 137°), active abduction (pre, 80°; post, 121°), and active internal rotation (pre, S3; post, L2). The functional outcome scores that were significantly (P < .05) improved at final follow-up were Constant normalized (pre, 39; post, 79), SPADI (pre, 86; post, 20), SST (pre, 3.3; post, 10), UCLA (pre, 13; post, 31), and ASES (pre, 33; post, 85).
The outcome improvements at latest follow-up were active external rotation (+28), active scaption (+45), active abduction (+42), active internal rotation (+6 anatomical segments), Constant normalized (+40), SPADI (–66), SST (+6.7), UCLA (+18), and ASES (+52).
There were 32 complications in 25 shoulders. There were no bilateral complications. Seven shoulders had multiple complications, of which many were not independent events. For example, rotator cuff deficiency was associated with instability, and infection was associated with glenoid loosening. One patient had 2 procedures, the first an arthroscopic release and the second a revision shoulder arthroplasty for glenoid loosening. The most common postoperative complication was rotator cuff failure (RCF) or suspected RCF (13 shoulders, including 8 treated with revision arthroplasty). RCF occurred most commonly at the rotator cuff interval, followed by the subscapularis and the supraspinatus. RCF location was based on computed tomography scan or intraoperative observation. The few subscapularis failures occurred with both subscapularis tendon repair and osteotomy. The high RCF rate may derive from scrutinizing postoperative radiographs and was not necessarily confirmed with repeat surgery. We think this represents a more realistic estimate of true postoperative rotator cuff dysfunction, rather than including only reoperated cases. The second most common complication was infection (6 shoulders, 1 with a superficial suture abscess and 5 with deep infections). Other complications were instability (4, with 2 caused by rotator cuff insufficiency), glenoid loosening (4, with 2 caused by infection), stiffness (3), nerve issue (1), and hematoma evacuation (1).
In 21 shoulders, these complications were treated with revision shoulder arthroplasty (16 shoulders), arthroscopic capsular release (3), evacuation of postoperative hematoma (1), and débridement of suture abscess (1). The 16 revision shoulder arthroplasties performed were conversion to reverse shoulder arthroplasty (11 shoulders) and placement of an antibiotic spacer for infection (5). The stem was left in place for all revisions, excluding those for infection. This is a significant advantage of the modular platform stem. Details of the complications and treatments are listed in the Table. There was no difference in health status between patients with a complication (ASA, 2.57) and those without one (ASA, 2.56).
Discussion
The implant described in this article consists of a metaphyseal press-fit stem, a replicator plate, multiple eccentric humeral heads, and a glenoid of multiple sizes with 2 radii of curvatures used to match the patient’s native anatomy and still maintain the appropriate radius of curvature mismatch between the humeral head and the glenoid. Between the eccentricity in the replicator plate and the eccentricity in the humeral head, almost any humeral head cut can be covered, more than 99% of the time.1 However, it remains to be seen if a versatile implant that comes close to matching the patient’s native anatomy will make a difference clinically.
The objective and functional outcomes in this study compare well with those of other, large TSA studies using older prostheses.1-4 There are few reports on contemporary implants with sufficient follow-up numbers for the single diagnosis of OA. Norris and Iannotti2 reported on a multicenter study of 176 patients with a Depuy Global TSA. The design of their study comes closest to that of our clinical outcome study. Nineteen surgeons were involved in their study. The follow-up rate is not clear. Their outcomes (with ours in parentheses for comparison) were active external rotation of 45° (42°), active elevation of 138° (137°), ASES of 84 (85), and SST of 9.2 (10). Norris and Iannotti2 noted an overall complication rate of 13% (12% in our series). Their most common postoperative complications were RCF and glenoid loosening; ours were RCF and infection. Another multicenter study with short-term results using a contemporary prosthesis included 268 shoulders followed for a minimum of 12 months.1 At final follow-up, Constant score was 97, active elevation was 145°, and the complication rate was 8.6%. Godenèche and colleagues1 also noted a glenoid lucent-line rate of 58% and reported that rotator cuff pathology adversely affected outcome.
Although the overall clinical outcome results are encouraging and the complication rate is in the reported range, we believe that a focus on the major complication categories may have a significant positive impact on our patients. The present article places significant importance on reporting complications prospectively, which is more accurate than retrospective reporting. The rates of both RCF and infection, the most common complications in our study, need to be decreased. Aldinger and colleagues7 reported a 12% complication rate in 485 primary shoulder arthroplasties—a rate identical to ours here. In their study, nerve injuries and humeral fractures were both more common than rotator cuff tears. We think that rotator cuff deficiency after TSA is underreported because it is often based on revision surgery alone. It is also interesting that the majority of the cuff deficiencies were through the upper subscapularis rotator interval and were not a complete failure of the subscapularis repair. Not all these patients will undergo revision surgery. In the future, the RCF rate may drop with the increasingly common use of reverse shoulder arthroplasty for substandard rotator cuffs.
Use of this contemporary variable neck angle, variable version angle, dual eccentric shoulder arthroplasty with a replicator plate provides satisfying short-term clinical outcomes. Patients with less than optimal health (mean ASA, 2.57) seem to tolerate the procedure well. Continued focus on RCF and infection will have the greatest impact on the overall complication rate.
Anatomical total shoulder arthroplasty (TSA) is an effective treatment for advanced osteoarthritis (OA) of the glenohumeral joint.1-4 Over the past 40 years, since the early reports appeared, the implants have evolved from the early monoblock humeral component to modular components, variable neck angled components with eccentric heads, and components that can provide variable neck angles, version angles, and dual eccentricity to match the anatomy of the proximal humerus. The goal of the new implants is to replicate the individual patient’s native anatomy using a combination of modularity, multiple neck and version angles, and dual eccentricity of the neck and head. The flexibility of the implant system is made possible by a replicator plate. There are few reports on outcomes of using these new implants for OA.
In this article, we report outcomes of using a dual eccentric, variable neck angle, variable version angle implant with a replicator plate for the treatment of OA of the shoulder at 4 centers.
Materials and Methods
The Western Institutional Review Board approved this study, and consent was prospectively obtained and retrospectively reviewed.
The data banks of a 4-center consortium were queried. Only primary TSA patients treated for OA with a fourth-generation Exactech Equinoxe implant (Exactech, Inc.) were included. For the center to be included, it had to have an 80% patient follow-up rate at a minimum of 2 years. Four centers qualified for inclusion: University of Florida, Medical College of Georgia, New York University, and Bordeaux-Merignac Clinic. Data were obtained on surgeries sequentially performed between August 1, 2006, and December 31, 2010. All data were obtained prospectively using a common data collection format.
The Equinoxe anatomical TSA allows for independent adaptation of neck angle and humeral version and provides 2 variable offset times (1 on replicator plate, 1 on humeral head) for matching the native anatomy in more than 99% of cases5 (Figure). The replicator plate is eccentric and can be angled 7.5° in any direction and rotated 360° to provide humeral head coverage. Once its optimal position is obtained, the plate is permanently fixed to the humeral stem using a breakaway screw. Some contemporary implants have similar features.
There were 218 primary shoulder arthroplasties performed on 201 patients (98 male, 103 female). Mean age at time of surgery was 67 years (range, 31-87 years), and mean follow-up was 36 months (range, 24-72 months). The collective follow-up rate at the 3-year mean follow-up and 2-year minimal follow-up was 81%. Eleven shoulders had a cemented stem, and 207 had an uncemented stem. Forty-eight shoulders used the 1.5-mm replicator plate, and 170 used the 4.5-mm offset replicator plate. The patients in this study were typically not very healthy: mean American Society of Anesthesiologists (ASA) score was 2.57 (range, 1-3).
Five outcome scores were calculated from the prospectively obtained data: Constant normalized, Shoulder Pain and Disability Index (SPADI), Simple Shoulder Test (SST), UCLA Shoulder Rating Scale (UCLA), and American Shoulder and Elbow Surgeons Shoulder Assessment (ASES). Before initiating data collection, we developed the Metric Form6 so we could calculate multiple scores while asking the minimal possible number of questions. This could be done for all 5 outcome scores, as their questions have significant overlap.
Objective outcomes included active external rotation, active scaption, active abduction, and active internal rotation. Complications, including revisions, were noted and analyzed. We focus on functional outcomes and do not present radiographic outcomes.
Results
A 2-tailed unpaired t test was used to compare preoperative values with final outcome values (P < .05). Four objective outcomes were significantly improved over preoperative levels: active external rotation (preoperative, 15°; postoperative, 42°), active scaption (pre, 92°; post, 137°), active abduction (pre, 80°; post, 121°), and active internal rotation (pre, S3; post, L2). The functional outcome scores that were significantly (P < .05) improved at final follow-up were Constant normalized (pre, 39; post, 79), SPADI (pre, 86; post, 20), SST (pre, 3.3; post, 10), UCLA (pre, 13; post, 31), and ASES (pre, 33; post, 85).
The outcome improvements at latest follow-up were active external rotation (+28), active scaption (+45), active abduction (+42), active internal rotation (+6 anatomical segments), Constant normalized (+40), SPADI (–66), SST (+6.7), UCLA (+18), and ASES (+52).
There were 32 complications in 25 shoulders. There were no bilateral complications. Seven shoulders had multiple complications, of which many were not independent events. For example, rotator cuff deficiency was associated with instability, and infection was associated with glenoid loosening. One patient had 2 procedures, the first an arthroscopic release and the second a revision shoulder arthroplasty for glenoid loosening. The most common postoperative complication was rotator cuff failure (RCF) or suspected RCF (13 shoulders, including 8 treated with revision arthroplasty). RCF occurred most commonly at the rotator cuff interval, followed by the subscapularis and the supraspinatus. RCF location was based on computed tomography scan or intraoperative observation. The few subscapularis failures occurred with both subscapularis tendon repair and osteotomy. The high RCF rate may derive from scrutinizing postoperative radiographs and was not necessarily confirmed with repeat surgery. We think this represents a more realistic estimate of true postoperative rotator cuff dysfunction, rather than including only reoperated cases. The second most common complication was infection (6 shoulders, 1 with a superficial suture abscess and 5 with deep infections). Other complications were instability (4, with 2 caused by rotator cuff insufficiency), glenoid loosening (4, with 2 caused by infection), stiffness (3), nerve issue (1), and hematoma evacuation (1).
In 21 shoulders, these complications were treated with revision shoulder arthroplasty (16 shoulders), arthroscopic capsular release (3), evacuation of postoperative hematoma (1), and débridement of suture abscess (1). The 16 revision shoulder arthroplasties performed were conversion to reverse shoulder arthroplasty (11 shoulders) and placement of an antibiotic spacer for infection (5). The stem was left in place for all revisions, excluding those for infection. This is a significant advantage of the modular platform stem. Details of the complications and treatments are listed in the Table. There was no difference in health status between patients with a complication (ASA, 2.57) and those without one (ASA, 2.56).
Discussion
The implant described in this article consists of a metaphyseal press-fit stem, a replicator plate, multiple eccentric humeral heads, and a glenoid of multiple sizes with 2 radii of curvatures used to match the patient’s native anatomy and still maintain the appropriate radius of curvature mismatch between the humeral head and the glenoid. Between the eccentricity in the replicator plate and the eccentricity in the humeral head, almost any humeral head cut can be covered, more than 99% of the time.1 However, it remains to be seen if a versatile implant that comes close to matching the patient’s native anatomy will make a difference clinically.
The objective and functional outcomes in this study compare well with those of other, large TSA studies using older prostheses.1-4 There are few reports on contemporary implants with sufficient follow-up numbers for the single diagnosis of OA. Norris and Iannotti2 reported on a multicenter study of 176 patients with a Depuy Global TSA. The design of their study comes closest to that of our clinical outcome study. Nineteen surgeons were involved in their study. The follow-up rate is not clear. Their outcomes (with ours in parentheses for comparison) were active external rotation of 45° (42°), active elevation of 138° (137°), ASES of 84 (85), and SST of 9.2 (10). Norris and Iannotti2 noted an overall complication rate of 13% (12% in our series). Their most common postoperative complications were RCF and glenoid loosening; ours were RCF and infection. Another multicenter study with short-term results using a contemporary prosthesis included 268 shoulders followed for a minimum of 12 months.1 At final follow-up, Constant score was 97, active elevation was 145°, and the complication rate was 8.6%. Godenèche and colleagues1 also noted a glenoid lucent-line rate of 58% and reported that rotator cuff pathology adversely affected outcome.
Although the overall clinical outcome results are encouraging and the complication rate is in the reported range, we believe that a focus on the major complication categories may have a significant positive impact on our patients. The present article places significant importance on reporting complications prospectively, which is more accurate than retrospective reporting. The rates of both RCF and infection, the most common complications in our study, need to be decreased. Aldinger and colleagues7 reported a 12% complication rate in 485 primary shoulder arthroplasties—a rate identical to ours here. In their study, nerve injuries and humeral fractures were both more common than rotator cuff tears. We think that rotator cuff deficiency after TSA is underreported because it is often based on revision surgery alone. It is also interesting that the majority of the cuff deficiencies were through the upper subscapularis rotator interval and were not a complete failure of the subscapularis repair. Not all these patients will undergo revision surgery. In the future, the RCF rate may drop with the increasingly common use of reverse shoulder arthroplasty for substandard rotator cuffs.
Use of this contemporary variable neck angle, variable version angle, dual eccentric shoulder arthroplasty with a replicator plate provides satisfying short-term clinical outcomes. Patients with less than optimal health (mean ASA, 2.57) seem to tolerate the procedure well. Continued focus on RCF and infection will have the greatest impact on the overall complication rate.
1. Godenèche A, Boileau P, Favard L, et al. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11(1):11-18.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
3. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
4. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
5. Irlenbusch U, Rott O, Gebhardt K, Werner A. Reconstruction of the rotational centre of the humeral head with double eccentric adaptable shoulder prosthesis [abstract]. In: Proceedings of the European Federation of National Associations of Orthopaedics and Traumatology (EFORT); May 29-June 1, 2008; Nice, France.
6. Flurin PH, Roche CP, Wright TW, Zuckerman J, Johnson D, Christensen M. A correlation of five commonly used clinical metrics to measure outcomes in shoulder arthroplasty. In: Transactions of the 58th Annual Meeting of the Orthopaedic Research Society (ORS); February 4-7, 2012; San Francisco, CA.
7. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
1. Godenèche A, Boileau P, Favard L, et al. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11(1):11-18.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
3. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
4. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
5. Irlenbusch U, Rott O, Gebhardt K, Werner A. Reconstruction of the rotational centre of the humeral head with double eccentric adaptable shoulder prosthesis [abstract]. In: Proceedings of the European Federation of National Associations of Orthopaedics and Traumatology (EFORT); May 29-June 1, 2008; Nice, France.
6. Flurin PH, Roche CP, Wright TW, Zuckerman J, Johnson D, Christensen M. A correlation of five commonly used clinical metrics to measure outcomes in shoulder arthroplasty. In: Transactions of the 58th Annual Meeting of the Orthopaedic Research Society (ORS); February 4-7, 2012; San Francisco, CA.
7. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
Collagenase Enzymatic Fasciotomy for Dupuytren Contracture in Patients on Chronic Immunosuppression
The incidence of Dupuytren disease increases with advancing age,1 as do the medical comorbidities of patients seeking treatment for disabling hand contractures. For patients with significant comorbidities, open surgical fasciectomy, the current standard of treatment for Dupuytren disease,2,3 may be associated with increased perioperative risks.
Collagenase enzymatic fasciotomy has become an accepted nonsurgical treatment alternative to traditional fasciectomy or surgical fasciotomy for significant digital contractures caused by Dupuytren disease.4-6 Clostridium histolyticum collagenase (CHC) is a foreign protein, made up of 2 collagenases isolated from the bacteria C histolyticum.7 The collagenases are zinc-dependent matrix metalloproteinases that cleave the triple helical structure of collagen molecules.8 Also known as Xiaflex (Auxilium Pharmaceuticals), CHC was approved by the US Food and Drug Administration (FDA) in February 2010 for use in patients with Dupuytren contractures.
Enzymatic rupture is safe and efficacious at midterm follow-up and offers the theoretical advantage of avoiding palmar and digital fasciectomy and the associated risks of surgical-site infection and wound-healing complications.6 The risks of surgical wound complications are magnified in immunosuppressed patients, particularly those on chronic steroid therapy; wound-healing complication rates may be increased 2 to 5 times compared with controls.9 In a pooled literature review, wound-healing complications were reported after 22.9% of open primary fasciectomies, with infection occurring in 2.4%.10 A nonsurgical alternative is therefore particularly appealing for a patient cohort that may be at higher risk for a frequently described complication of surgery for Dupuytren contracture.
The exclusion criteria in the trials for FDA approval were extensive and included breast-feeding, pregnancy, bleeding disorder, recent stroke, use of tetracycline derivative within 14 days before start of study, use of anticoagulant within 7 days before start of study, allergy to collagenase, and chronic muscular, neurologic, or neuromuscular disorder affecting the hands.6 Safety and efficacy of collagenase in patients requiring chronic immunosuppressive therapy for medical comorbidities have not been previously documented. Furthermore, although skin tears were reported in 11% of patients after manual cord rupture in the CORD (Collagenase Option for the Reduction of Dupuytren’s) I trial,6 the likelihood of deep and superficial infection and delayed wound healing has not been quantitated.
In this article, we report on outcomes of 13 collagenase enzymatic fasciotomies performed in 8 patients who were on chronic immunosuppressive therapy.
Methods
Institutional review board approval was obtained at both academic hand surgery institutions. We retrospectively reviewed prospectively collected clinical data within our 2 centers’ databases of patients with Dupuytren disease. Eight patients on chronic immunosuppressive therapies treated with collagenase for metacarpophalangeal (MP) or proximal interphalangeal (PIP) joint contractures between February 2010 and December 2011 were identified. Three of these patients received collagenase injections into 2 or more separate Dupuytren cords at different encounters, resulting in a total of 13 individual collagenase enzymatic fasciotomies.
Collagenase injections were administered following CORD I trial protocol,6 except we injected Dupuytren cords crossing the PIP joint using a lateral approach to minimize risk of flexor tendon rupture. Manipulation of the treated joint was performed between 24 and 48 hours after collagenase injection under local anesthesia with 3 mL of 1% mepivacaine or lidocaine without epinephrine. After manipulation and cord rupture, patients were placed in a hand-based extension splint to wear at night for up to 3 months. Patients were followed at 1 and 12 months.
Results
Patients’ baseline characteristics are summarized in Table 1. Four patients were maintained on chronic prednisone therapy, 3 on methotrexate, and 1 on azathioprine. Therapy duration, medication dose, and diagnoses requiring immunosuppressant therapy varied among patients.
Outcomes and adverse events are summarized in Table 2. Mean number of joint contractures per hand treated was 2.8 (MP, 1.4; PIP, 1.4). However, not all joints met the intervention criteria. Of the 13 joints treated, 7 were MP joints, and 6 were PIP joints. Mean preinjection contracture of the treated joints was 53.0° (range, 20°-90°). Twelve of the 13 joint contractures improved. At mean follow-up of 6.7 months (range, 1-22 months), mean magnitude of contracture improved to 12.9° (range, 0°-45°). Mean MP joint contracture improved from 42.0° to 4.2° (range, 0°-10°), and mean PIP joint contracture improved from 65.8° to 21.7° (range, 0°-45°).
All 13 collagenase injections were well tolerated, and there were no systemic reactions. Injection-site pain was common. Mild injection-site bruising and edema were reported in all cases. Enzymatic fasciotomy was performed in all patients, and immediate improvement in contracture after manipulation 24 to 48 hours after injection was recorded.
Three of the 13 injections were complicated by skin tears during manipulation and cord rupture. All 3 skin tears were treated with local wound care, which included use of povidone-iodine and wet-to-dry dressings. There was no evidence of subsequent superficial or deep, local or regional infection. In 2 cases, the wound healed within 1 week; in the third case, wound healing was present by 2 weeks. Once the wounds showed early re-epithelialization, hand-based extension splinting in a position of comfort was used at night for up to 3 months after injection. Two of the 13 injections were complicated by small blood blisters. These were treated with observation and resolved spontaneously.
Discussion
Collagenase enzymatic fasciotomy appeared to be a safe and efficacious alternative to surgical treatment of Dupuytren contractures in this cohort of patients maintained on chronic immunosuppressive agents. MP contractures responded more substantially than PIP contractures did, as expected.6 No previously undescribed adverse outcomes were noted in these 8 patients on chronic immunosuppressive therapy beyond those reported in the CORD I trial. Three (23%) of the 13 collagenase injections in our series were complicated by skin tears after manipulation. Skins tears were reported in 22 (11%) of 204 patients after manual cord rupture in the CORD I trial.6 Given the limited numbers in this series, it remains unclear if chronic immunosuppression truly increases the risk of skin tears in this subset of patients. Other common treatment-related adverse events seen in the CORD I trial—injection-site hemorrhage (37%), pruritis (11%) and lymphadenopathy (10%)—were not seen after the 13 injections in our case series. We are prospectively following all patients with Dupuytren disease, and this is an area of ongoing research at our centers.
The immunosuppressive actions of prednisone, azathioprine, and methotrexate are well documented. Prednisone is a glucocorticoid, converted in the liver to prednisolone, which suppresses inflammation and immune responses by regulation of gene expression. Its immunosuppressive actions are multifactorial, relating to inhibition of lymphocytes, neutrophils, and monocytes. These effects are dose- and time-dependent11 and may become evident in patients receiving low doses over prolonged periods. Skin atrophy12 and delayed wound healing9 are side effects of long-term prednisone use. Skin atrophy may make the prednisone-treated patient more susceptible to skin tears after collagenase injection and manipulation. Azathioprine inhibits purine synthesis, which is especially important in the proliferation of immune cells.13 It has been shown to inhibit both cellular immunity at low doses and humoral immunity at higher doses.14 Methotrexate inhibits lymphocyte folic acid metabolism. The immunosuppressive properties of low-dose methotrexate have been linked to the induction of apoptosis in activated T cells.15
A more complex process in immunosuppressed patients is the immunogenicity of injected collagenase. As CHC in current use is a mixture of 2 foreign proteins, an immunologic response is expected in the host after injection. It has been shown that, after 3 injections of CHC into Dupuytren cords, 100% of patients developed antibodies to both enzymes in their serum.6 More than 85% demonstrated anti-CHC antibodies after a single injection. However, no patients showed signs of anaphylaxis or allergic reaction, and there was no correlation between serum levels of anti-CHC and adverse events. It has been hypothesized that there is a potential for cross-reactivity of the anti-CHC antibodies with human matrix metalloproteinases, causing enzymatic dysfunction within the host.16 This has yet to be reported clinically, and Xiaflex is currently under postmarketing surveillance. Immunocompromised people, with suppressed humoral and cellular immune responses, may produce less of an antibody response to the foreign CHC proteins. Whether this conclusively leads to a change in the side effect profile of the medication in these individuals is beyond the scope of this article. However, we identified no new side effects in this small but higher risk cohort. The issue should be continually monitored as collagenase is used in wider clinical settings.
Collagenase enzymatic fasciotomy is a new nonsurgical therapeutic option for Dupuytren disease. Indications and guidelines for use continue to evolve. This case series highlights the use of collagenase in 8 patients who were on long-term immunosuppressive therapy. This study has the limitations inherent to retrospective analyses. It is difficult to generalize results across broader immunosuppressed populations. A larger cohort, with long-term follow-up assessing recurrence of contracture, is needed to make definitive conclusions about use of collagenase in this challenging subset of patients. Based on our observations in this limited cohort, it appears appropriate to pursue further studies on use of collagenase enzymatic fasciotomy. A randomized, prospective or case–control series comparing surgical fasciectomy with enzymatic fasciotomy would yield further meaningful data. As more patients seek nonsurgical treatment for Dupuytren disease, its safety and efficacy in select cohorts of patients should continue to be evaluated.
1. Loos B, Puschkin V, Horch RE. 50 years experience with Dupuytren’s contracture in the Erlangen University Hospital—a retrospective analysis of 2919 operated hands from 1956 to 2006. BMC Musculoskelet Disord. 2007;8:60.
2. Coert JH, Nérin JP, Meek MF. Results of partial fasciectomy for Dupuytren disease in 261 consecutive patients. Ann Plast Surg. 2006;57(1):13-17.
3. Sennwald GR. Fasciectomy for treatment of Dupuytren’s disease and early complications. J Hand Surg Am. 1990;15(5):755-761.
4. Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg Am. 2000;25(4):629-636.
5. Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: nonoperative treatment of Dupuytren’s disease. J Hand Surg Am. 2002;27(5):788-798.
6. Hurst LC, Badalamente MA, Hentz VR, et al; CORD I Study Group. Injectable collagenase Clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968-979.
7. Mookhtiar KA, Van Wart HE. Clostridium histolyticum collagenases: a new look at some old enzymes. Matrix Suppl. 1992;1:116-126.
8. Watanabe K. Collagenolytic proteases from bacteria. Appl Microbiol Biotechnol. 2004;63(5):520-526.
9. Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg. 2013;206(3):410-417.
10. Denkler K. Surgical complications associated with fasciectomy for Dupuytren’s disease: a 20-year review of the English literature. Eplasty. 2010;10:e15.
11. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954-963.
12. Oikarinen A, Autio P. New aspects of the mechanism of corticosteroid-induced dermal atrophy. Clin Exp Dermatol. 1991;16(6):416-419.
13. Makinodan T, Santos GW, Quinn RP. Immunosuppressive drugs. Pharmacol Rev. 1970;22(2):189-247.
14. Röllinghoff M, Schrader J, Wagner H. Effect of azathioprine and cytosine arabinoside on humoral and cellular immunity in vitro. Clin Exp Immunol. 1973;15(2):261-269.
15. Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102(2):322-328.
16. Desai SS, Hentz VR. Collagenase Clostridium histolyticum for Dupuytren’s contracture. Expert Opin Biol Ther. 2010;10(9):1395-1404.
The incidence of Dupuytren disease increases with advancing age,1 as do the medical comorbidities of patients seeking treatment for disabling hand contractures. For patients with significant comorbidities, open surgical fasciectomy, the current standard of treatment for Dupuytren disease,2,3 may be associated with increased perioperative risks.
Collagenase enzymatic fasciotomy has become an accepted nonsurgical treatment alternative to traditional fasciectomy or surgical fasciotomy for significant digital contractures caused by Dupuytren disease.4-6 Clostridium histolyticum collagenase (CHC) is a foreign protein, made up of 2 collagenases isolated from the bacteria C histolyticum.7 The collagenases are zinc-dependent matrix metalloproteinases that cleave the triple helical structure of collagen molecules.8 Also known as Xiaflex (Auxilium Pharmaceuticals), CHC was approved by the US Food and Drug Administration (FDA) in February 2010 for use in patients with Dupuytren contractures.
Enzymatic rupture is safe and efficacious at midterm follow-up and offers the theoretical advantage of avoiding palmar and digital fasciectomy and the associated risks of surgical-site infection and wound-healing complications.6 The risks of surgical wound complications are magnified in immunosuppressed patients, particularly those on chronic steroid therapy; wound-healing complication rates may be increased 2 to 5 times compared with controls.9 In a pooled literature review, wound-healing complications were reported after 22.9% of open primary fasciectomies, with infection occurring in 2.4%.10 A nonsurgical alternative is therefore particularly appealing for a patient cohort that may be at higher risk for a frequently described complication of surgery for Dupuytren contracture.
The exclusion criteria in the trials for FDA approval were extensive and included breast-feeding, pregnancy, bleeding disorder, recent stroke, use of tetracycline derivative within 14 days before start of study, use of anticoagulant within 7 days before start of study, allergy to collagenase, and chronic muscular, neurologic, or neuromuscular disorder affecting the hands.6 Safety and efficacy of collagenase in patients requiring chronic immunosuppressive therapy for medical comorbidities have not been previously documented. Furthermore, although skin tears were reported in 11% of patients after manual cord rupture in the CORD (Collagenase Option for the Reduction of Dupuytren’s) I trial,6 the likelihood of deep and superficial infection and delayed wound healing has not been quantitated.
In this article, we report on outcomes of 13 collagenase enzymatic fasciotomies performed in 8 patients who were on chronic immunosuppressive therapy.
Methods
Institutional review board approval was obtained at both academic hand surgery institutions. We retrospectively reviewed prospectively collected clinical data within our 2 centers’ databases of patients with Dupuytren disease. Eight patients on chronic immunosuppressive therapies treated with collagenase for metacarpophalangeal (MP) or proximal interphalangeal (PIP) joint contractures between February 2010 and December 2011 were identified. Three of these patients received collagenase injections into 2 or more separate Dupuytren cords at different encounters, resulting in a total of 13 individual collagenase enzymatic fasciotomies.
Collagenase injections were administered following CORD I trial protocol,6 except we injected Dupuytren cords crossing the PIP joint using a lateral approach to minimize risk of flexor tendon rupture. Manipulation of the treated joint was performed between 24 and 48 hours after collagenase injection under local anesthesia with 3 mL of 1% mepivacaine or lidocaine without epinephrine. After manipulation and cord rupture, patients were placed in a hand-based extension splint to wear at night for up to 3 months. Patients were followed at 1 and 12 months.
Results
Patients’ baseline characteristics are summarized in Table 1. Four patients were maintained on chronic prednisone therapy, 3 on methotrexate, and 1 on azathioprine. Therapy duration, medication dose, and diagnoses requiring immunosuppressant therapy varied among patients.
Outcomes and adverse events are summarized in Table 2. Mean number of joint contractures per hand treated was 2.8 (MP, 1.4; PIP, 1.4). However, not all joints met the intervention criteria. Of the 13 joints treated, 7 were MP joints, and 6 were PIP joints. Mean preinjection contracture of the treated joints was 53.0° (range, 20°-90°). Twelve of the 13 joint contractures improved. At mean follow-up of 6.7 months (range, 1-22 months), mean magnitude of contracture improved to 12.9° (range, 0°-45°). Mean MP joint contracture improved from 42.0° to 4.2° (range, 0°-10°), and mean PIP joint contracture improved from 65.8° to 21.7° (range, 0°-45°).
All 13 collagenase injections were well tolerated, and there were no systemic reactions. Injection-site pain was common. Mild injection-site bruising and edema were reported in all cases. Enzymatic fasciotomy was performed in all patients, and immediate improvement in contracture after manipulation 24 to 48 hours after injection was recorded.
Three of the 13 injections were complicated by skin tears during manipulation and cord rupture. All 3 skin tears were treated with local wound care, which included use of povidone-iodine and wet-to-dry dressings. There was no evidence of subsequent superficial or deep, local or regional infection. In 2 cases, the wound healed within 1 week; in the third case, wound healing was present by 2 weeks. Once the wounds showed early re-epithelialization, hand-based extension splinting in a position of comfort was used at night for up to 3 months after injection. Two of the 13 injections were complicated by small blood blisters. These were treated with observation and resolved spontaneously.
Discussion
Collagenase enzymatic fasciotomy appeared to be a safe and efficacious alternative to surgical treatment of Dupuytren contractures in this cohort of patients maintained on chronic immunosuppressive agents. MP contractures responded more substantially than PIP contractures did, as expected.6 No previously undescribed adverse outcomes were noted in these 8 patients on chronic immunosuppressive therapy beyond those reported in the CORD I trial. Three (23%) of the 13 collagenase injections in our series were complicated by skin tears after manipulation. Skins tears were reported in 22 (11%) of 204 patients after manual cord rupture in the CORD I trial.6 Given the limited numbers in this series, it remains unclear if chronic immunosuppression truly increases the risk of skin tears in this subset of patients. Other common treatment-related adverse events seen in the CORD I trial—injection-site hemorrhage (37%), pruritis (11%) and lymphadenopathy (10%)—were not seen after the 13 injections in our case series. We are prospectively following all patients with Dupuytren disease, and this is an area of ongoing research at our centers.
The immunosuppressive actions of prednisone, azathioprine, and methotrexate are well documented. Prednisone is a glucocorticoid, converted in the liver to prednisolone, which suppresses inflammation and immune responses by regulation of gene expression. Its immunosuppressive actions are multifactorial, relating to inhibition of lymphocytes, neutrophils, and monocytes. These effects are dose- and time-dependent11 and may become evident in patients receiving low doses over prolonged periods. Skin atrophy12 and delayed wound healing9 are side effects of long-term prednisone use. Skin atrophy may make the prednisone-treated patient more susceptible to skin tears after collagenase injection and manipulation. Azathioprine inhibits purine synthesis, which is especially important in the proliferation of immune cells.13 It has been shown to inhibit both cellular immunity at low doses and humoral immunity at higher doses.14 Methotrexate inhibits lymphocyte folic acid metabolism. The immunosuppressive properties of low-dose methotrexate have been linked to the induction of apoptosis in activated T cells.15
A more complex process in immunosuppressed patients is the immunogenicity of injected collagenase. As CHC in current use is a mixture of 2 foreign proteins, an immunologic response is expected in the host after injection. It has been shown that, after 3 injections of CHC into Dupuytren cords, 100% of patients developed antibodies to both enzymes in their serum.6 More than 85% demonstrated anti-CHC antibodies after a single injection. However, no patients showed signs of anaphylaxis or allergic reaction, and there was no correlation between serum levels of anti-CHC and adverse events. It has been hypothesized that there is a potential for cross-reactivity of the anti-CHC antibodies with human matrix metalloproteinases, causing enzymatic dysfunction within the host.16 This has yet to be reported clinically, and Xiaflex is currently under postmarketing surveillance. Immunocompromised people, with suppressed humoral and cellular immune responses, may produce less of an antibody response to the foreign CHC proteins. Whether this conclusively leads to a change in the side effect profile of the medication in these individuals is beyond the scope of this article. However, we identified no new side effects in this small but higher risk cohort. The issue should be continually monitored as collagenase is used in wider clinical settings.
Collagenase enzymatic fasciotomy is a new nonsurgical therapeutic option for Dupuytren disease. Indications and guidelines for use continue to evolve. This case series highlights the use of collagenase in 8 patients who were on long-term immunosuppressive therapy. This study has the limitations inherent to retrospective analyses. It is difficult to generalize results across broader immunosuppressed populations. A larger cohort, with long-term follow-up assessing recurrence of contracture, is needed to make definitive conclusions about use of collagenase in this challenging subset of patients. Based on our observations in this limited cohort, it appears appropriate to pursue further studies on use of collagenase enzymatic fasciotomy. A randomized, prospective or case–control series comparing surgical fasciectomy with enzymatic fasciotomy would yield further meaningful data. As more patients seek nonsurgical treatment for Dupuytren disease, its safety and efficacy in select cohorts of patients should continue to be evaluated.
The incidence of Dupuytren disease increases with advancing age,1 as do the medical comorbidities of patients seeking treatment for disabling hand contractures. For patients with significant comorbidities, open surgical fasciectomy, the current standard of treatment for Dupuytren disease,2,3 may be associated with increased perioperative risks.
Collagenase enzymatic fasciotomy has become an accepted nonsurgical treatment alternative to traditional fasciectomy or surgical fasciotomy for significant digital contractures caused by Dupuytren disease.4-6 Clostridium histolyticum collagenase (CHC) is a foreign protein, made up of 2 collagenases isolated from the bacteria C histolyticum.7 The collagenases are zinc-dependent matrix metalloproteinases that cleave the triple helical structure of collagen molecules.8 Also known as Xiaflex (Auxilium Pharmaceuticals), CHC was approved by the US Food and Drug Administration (FDA) in February 2010 for use in patients with Dupuytren contractures.
Enzymatic rupture is safe and efficacious at midterm follow-up and offers the theoretical advantage of avoiding palmar and digital fasciectomy and the associated risks of surgical-site infection and wound-healing complications.6 The risks of surgical wound complications are magnified in immunosuppressed patients, particularly those on chronic steroid therapy; wound-healing complication rates may be increased 2 to 5 times compared with controls.9 In a pooled literature review, wound-healing complications were reported after 22.9% of open primary fasciectomies, with infection occurring in 2.4%.10 A nonsurgical alternative is therefore particularly appealing for a patient cohort that may be at higher risk for a frequently described complication of surgery for Dupuytren contracture.
The exclusion criteria in the trials for FDA approval were extensive and included breast-feeding, pregnancy, bleeding disorder, recent stroke, use of tetracycline derivative within 14 days before start of study, use of anticoagulant within 7 days before start of study, allergy to collagenase, and chronic muscular, neurologic, or neuromuscular disorder affecting the hands.6 Safety and efficacy of collagenase in patients requiring chronic immunosuppressive therapy for medical comorbidities have not been previously documented. Furthermore, although skin tears were reported in 11% of patients after manual cord rupture in the CORD (Collagenase Option for the Reduction of Dupuytren’s) I trial,6 the likelihood of deep and superficial infection and delayed wound healing has not been quantitated.
In this article, we report on outcomes of 13 collagenase enzymatic fasciotomies performed in 8 patients who were on chronic immunosuppressive therapy.
Methods
Institutional review board approval was obtained at both academic hand surgery institutions. We retrospectively reviewed prospectively collected clinical data within our 2 centers’ databases of patients with Dupuytren disease. Eight patients on chronic immunosuppressive therapies treated with collagenase for metacarpophalangeal (MP) or proximal interphalangeal (PIP) joint contractures between February 2010 and December 2011 were identified. Three of these patients received collagenase injections into 2 or more separate Dupuytren cords at different encounters, resulting in a total of 13 individual collagenase enzymatic fasciotomies.
Collagenase injections were administered following CORD I trial protocol,6 except we injected Dupuytren cords crossing the PIP joint using a lateral approach to minimize risk of flexor tendon rupture. Manipulation of the treated joint was performed between 24 and 48 hours after collagenase injection under local anesthesia with 3 mL of 1% mepivacaine or lidocaine without epinephrine. After manipulation and cord rupture, patients were placed in a hand-based extension splint to wear at night for up to 3 months. Patients were followed at 1 and 12 months.
Results
Patients’ baseline characteristics are summarized in Table 1. Four patients were maintained on chronic prednisone therapy, 3 on methotrexate, and 1 on azathioprine. Therapy duration, medication dose, and diagnoses requiring immunosuppressant therapy varied among patients.
Outcomes and adverse events are summarized in Table 2. Mean number of joint contractures per hand treated was 2.8 (MP, 1.4; PIP, 1.4). However, not all joints met the intervention criteria. Of the 13 joints treated, 7 were MP joints, and 6 were PIP joints. Mean preinjection contracture of the treated joints was 53.0° (range, 20°-90°). Twelve of the 13 joint contractures improved. At mean follow-up of 6.7 months (range, 1-22 months), mean magnitude of contracture improved to 12.9° (range, 0°-45°). Mean MP joint contracture improved from 42.0° to 4.2° (range, 0°-10°), and mean PIP joint contracture improved from 65.8° to 21.7° (range, 0°-45°).
All 13 collagenase injections were well tolerated, and there were no systemic reactions. Injection-site pain was common. Mild injection-site bruising and edema were reported in all cases. Enzymatic fasciotomy was performed in all patients, and immediate improvement in contracture after manipulation 24 to 48 hours after injection was recorded.
Three of the 13 injections were complicated by skin tears during manipulation and cord rupture. All 3 skin tears were treated with local wound care, which included use of povidone-iodine and wet-to-dry dressings. There was no evidence of subsequent superficial or deep, local or regional infection. In 2 cases, the wound healed within 1 week; in the third case, wound healing was present by 2 weeks. Once the wounds showed early re-epithelialization, hand-based extension splinting in a position of comfort was used at night for up to 3 months after injection. Two of the 13 injections were complicated by small blood blisters. These were treated with observation and resolved spontaneously.
Discussion
Collagenase enzymatic fasciotomy appeared to be a safe and efficacious alternative to surgical treatment of Dupuytren contractures in this cohort of patients maintained on chronic immunosuppressive agents. MP contractures responded more substantially than PIP contractures did, as expected.6 No previously undescribed adverse outcomes were noted in these 8 patients on chronic immunosuppressive therapy beyond those reported in the CORD I trial. Three (23%) of the 13 collagenase injections in our series were complicated by skin tears after manipulation. Skins tears were reported in 22 (11%) of 204 patients after manual cord rupture in the CORD I trial.6 Given the limited numbers in this series, it remains unclear if chronic immunosuppression truly increases the risk of skin tears in this subset of patients. Other common treatment-related adverse events seen in the CORD I trial—injection-site hemorrhage (37%), pruritis (11%) and lymphadenopathy (10%)—were not seen after the 13 injections in our case series. We are prospectively following all patients with Dupuytren disease, and this is an area of ongoing research at our centers.
The immunosuppressive actions of prednisone, azathioprine, and methotrexate are well documented. Prednisone is a glucocorticoid, converted in the liver to prednisolone, which suppresses inflammation and immune responses by regulation of gene expression. Its immunosuppressive actions are multifactorial, relating to inhibition of lymphocytes, neutrophils, and monocytes. These effects are dose- and time-dependent11 and may become evident in patients receiving low doses over prolonged periods. Skin atrophy12 and delayed wound healing9 are side effects of long-term prednisone use. Skin atrophy may make the prednisone-treated patient more susceptible to skin tears after collagenase injection and manipulation. Azathioprine inhibits purine synthesis, which is especially important in the proliferation of immune cells.13 It has been shown to inhibit both cellular immunity at low doses and humoral immunity at higher doses.14 Methotrexate inhibits lymphocyte folic acid metabolism. The immunosuppressive properties of low-dose methotrexate have been linked to the induction of apoptosis in activated T cells.15
A more complex process in immunosuppressed patients is the immunogenicity of injected collagenase. As CHC in current use is a mixture of 2 foreign proteins, an immunologic response is expected in the host after injection. It has been shown that, after 3 injections of CHC into Dupuytren cords, 100% of patients developed antibodies to both enzymes in their serum.6 More than 85% demonstrated anti-CHC antibodies after a single injection. However, no patients showed signs of anaphylaxis or allergic reaction, and there was no correlation between serum levels of anti-CHC and adverse events. It has been hypothesized that there is a potential for cross-reactivity of the anti-CHC antibodies with human matrix metalloproteinases, causing enzymatic dysfunction within the host.16 This has yet to be reported clinically, and Xiaflex is currently under postmarketing surveillance. Immunocompromised people, with suppressed humoral and cellular immune responses, may produce less of an antibody response to the foreign CHC proteins. Whether this conclusively leads to a change in the side effect profile of the medication in these individuals is beyond the scope of this article. However, we identified no new side effects in this small but higher risk cohort. The issue should be continually monitored as collagenase is used in wider clinical settings.
Collagenase enzymatic fasciotomy is a new nonsurgical therapeutic option for Dupuytren disease. Indications and guidelines for use continue to evolve. This case series highlights the use of collagenase in 8 patients who were on long-term immunosuppressive therapy. This study has the limitations inherent to retrospective analyses. It is difficult to generalize results across broader immunosuppressed populations. A larger cohort, with long-term follow-up assessing recurrence of contracture, is needed to make definitive conclusions about use of collagenase in this challenging subset of patients. Based on our observations in this limited cohort, it appears appropriate to pursue further studies on use of collagenase enzymatic fasciotomy. A randomized, prospective or case–control series comparing surgical fasciectomy with enzymatic fasciotomy would yield further meaningful data. As more patients seek nonsurgical treatment for Dupuytren disease, its safety and efficacy in select cohorts of patients should continue to be evaluated.
1. Loos B, Puschkin V, Horch RE. 50 years experience with Dupuytren’s contracture in the Erlangen University Hospital—a retrospective analysis of 2919 operated hands from 1956 to 2006. BMC Musculoskelet Disord. 2007;8:60.
2. Coert JH, Nérin JP, Meek MF. Results of partial fasciectomy for Dupuytren disease in 261 consecutive patients. Ann Plast Surg. 2006;57(1):13-17.
3. Sennwald GR. Fasciectomy for treatment of Dupuytren’s disease and early complications. J Hand Surg Am. 1990;15(5):755-761.
4. Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg Am. 2000;25(4):629-636.
5. Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: nonoperative treatment of Dupuytren’s disease. J Hand Surg Am. 2002;27(5):788-798.
6. Hurst LC, Badalamente MA, Hentz VR, et al; CORD I Study Group. Injectable collagenase Clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968-979.
7. Mookhtiar KA, Van Wart HE. Clostridium histolyticum collagenases: a new look at some old enzymes. Matrix Suppl. 1992;1:116-126.
8. Watanabe K. Collagenolytic proteases from bacteria. Appl Microbiol Biotechnol. 2004;63(5):520-526.
9. Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg. 2013;206(3):410-417.
10. Denkler K. Surgical complications associated with fasciectomy for Dupuytren’s disease: a 20-year review of the English literature. Eplasty. 2010;10:e15.
11. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954-963.
12. Oikarinen A, Autio P. New aspects of the mechanism of corticosteroid-induced dermal atrophy. Clin Exp Dermatol. 1991;16(6):416-419.
13. Makinodan T, Santos GW, Quinn RP. Immunosuppressive drugs. Pharmacol Rev. 1970;22(2):189-247.
14. Röllinghoff M, Schrader J, Wagner H. Effect of azathioprine and cytosine arabinoside on humoral and cellular immunity in vitro. Clin Exp Immunol. 1973;15(2):261-269.
15. Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102(2):322-328.
16. Desai SS, Hentz VR. Collagenase Clostridium histolyticum for Dupuytren’s contracture. Expert Opin Biol Ther. 2010;10(9):1395-1404.
1. Loos B, Puschkin V, Horch RE. 50 years experience with Dupuytren’s contracture in the Erlangen University Hospital—a retrospective analysis of 2919 operated hands from 1956 to 2006. BMC Musculoskelet Disord. 2007;8:60.
2. Coert JH, Nérin JP, Meek MF. Results of partial fasciectomy for Dupuytren disease in 261 consecutive patients. Ann Plast Surg. 2006;57(1):13-17.
3. Sennwald GR. Fasciectomy for treatment of Dupuytren’s disease and early complications. J Hand Surg Am. 1990;15(5):755-761.
4. Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg Am. 2000;25(4):629-636.
5. Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: nonoperative treatment of Dupuytren’s disease. J Hand Surg Am. 2002;27(5):788-798.
6. Hurst LC, Badalamente MA, Hentz VR, et al; CORD I Study Group. Injectable collagenase Clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968-979.
7. Mookhtiar KA, Van Wart HE. Clostridium histolyticum collagenases: a new look at some old enzymes. Matrix Suppl. 1992;1:116-126.
8. Watanabe K. Collagenolytic proteases from bacteria. Appl Microbiol Biotechnol. 2004;63(5):520-526.
9. Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg. 2013;206(3):410-417.
10. Denkler K. Surgical complications associated with fasciectomy for Dupuytren’s disease: a 20-year review of the English literature. Eplasty. 2010;10:e15.
11. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954-963.
12. Oikarinen A, Autio P. New aspects of the mechanism of corticosteroid-induced dermal atrophy. Clin Exp Dermatol. 1991;16(6):416-419.
13. Makinodan T, Santos GW, Quinn RP. Immunosuppressive drugs. Pharmacol Rev. 1970;22(2):189-247.
14. Röllinghoff M, Schrader J, Wagner H. Effect of azathioprine and cytosine arabinoside on humoral and cellular immunity in vitro. Clin Exp Immunol. 1973;15(2):261-269.
15. Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102(2):322-328.
16. Desai SS, Hentz VR. Collagenase Clostridium histolyticum for Dupuytren’s contracture. Expert Opin Biol Ther. 2010;10(9):1395-1404.
Open Carpal Tunnel Release With Use of a Nasal Turbinate Speculum
Carpal tunnel syndrome (CTS) is a disorder characterized by entrapment of the median nerve at the wrist, which may lead to symptoms of pain, paresthesia, and, ultimately, thenar muscle atrophy. Surgical intervention is indicated with persistent or progressive symptoms despite nonoperative management. Timely surgical decompression aims to halt progression of this disorder and prevent permanent peripheral nerve injury.
Carpal tunnel release (CTR) is the most common hand and wrist surgery in the United States, with about 400,000 operations performed annually.1,2 Several methods of decompressing the carpal tunnel have been described.3 These include standard open CTR (OCTR), mini-open approaches, and various endoscopic techniques. OCTR was initially described by Sir James Learmonth in 1933,4 and it remains the gold-standard surgical treatment for patients with symptomatic CTS. Uniform excellent results with high patient satisfaction and low complication rates have been reported in several series.5-9 Common to all techniques is complete proximal-to-distal division of the transverse carpal ligament (TCL). Magnetic resonance imaging studies have shown that TCL transection and the resulting diastasis between the radial and ulnar leaflets cause a significant increase in the volume of the carpal tunnel, leading to decreased pressure.10,11
Endoscopic CTR (ECTR) techniques were developed in an effort to reduce complications, scar sensitivity, and pillar pain and facilitate more rapid return to work.12-17 Outcome studies have demonstrated that both open and endoscopic releases yield patient-reported subjective improvements over preoperative symptoms.18-22 A randomized, controlled trial by Trumble and colleagues23 in 2002 found that ECTR led to improved patient outcomes in the early postoperative period (first 3 months), though differences in outcomes were reduced at final follow-up. More recently (2007), a Cochrane review of 33 trials concluded there was no strong evidence favoring use of alternative techniques over OCTR.3 Further, OCTR has been found to be technically less demanding and associated with decreased complications and costs.24
Indications
The benefit of median nerve decompression at the wrist for CTS is clear.6,7 Indications for surgery in patients with CTS include persistent symptoms despite nonoperative treatment, objective sensory disturbance or motor weakness, and thenar atrophy. Symptomatic response to corticosteroid injection is predictive of success after carpal tunnel surgery.25 More than 87% of patients who gain symptomatic relief from corticosteroid injection have an excellent surgical outcome.
Technique
OCTR allows direct visualization of the TCL and the distal volar forearm fascia (DVFF) and evaluation for the presence of anomalous branching patterns of the median nerve. OCTR traditionally was performed through a 4- to 5-cm longitudinal incision extending from the wrist crease proximally to the Kaplan cardinal line distally. The mini-open technique is identical with the exception of incision length. We routinely use a 2.5- to 3-cm incision. Regardless of incision length, each OCTR should proceed through the same reproducible steps.
We perform OCTR under tourniquet control. Choice of anesthesia is surgeon and patient preference. We prefer local anesthesia with conscious sedation. After conscious sedation is administered, we infiltrate the carpal tunnel and surrounding subcutaneous tissue with 10 mL of a 50:50 mixture of 0.5% bupivacaine and 1% lidocaine without epinephrine.
A 2.5- to 3-cm longitudinal incision is made along the axis of the radial border of the ring finger from the Kaplan cardinal line26 and extending about 3 cm proximally toward the wrist flexion crease ulnar to the palmaris longus if present (Figure 1).
After the skin is incised longitudinally, the subcutaneous fat is mobilized and cutaneous sensory branches identified and protected. The underlying superficial palmar fascia is incised in line with the skin incision. The underlying midportion of the TCL is now visualized.
Transverse Carpal Ligament Release
Occasionally, the investing fascia along the ulnar edge of the thenar musculature is mobilized radialward (if the thenar musculature is well developed) to visualize the proximal limb of the TCL. Injury to any anomalous motor branch of the median nerve is avoided by directly visualizing and then incising the TCL (Figure 2). The TCL is incised along its ulnar border just radial to the hook of hamate from distal to proximal in line with the radial border of the ring finger. Staying near the ulnar attachment of the TCL keeps the plane of ligament division farther away from the median nerve and its recurrent motor branches. Although the ulnar neurovascular bundle typically resides ulnar to the hook of hamate in the canal of Guyon, the surgeon must be aware that it can be located radial to the hook in some instances.27,28 In the elderly, the ulnar artery may be tortuous and enter the field and require retraction. The TCL is incised distally until the sentinel fat pad, which marks the superficial palmar arterial arch, is visualized. This bed of adipose tissue marks the distal edge of the TCL.29
Proximally, subcutaneous tissues above the proximal limb of the TCL and DVFF are mobilized to about 2 cm proximal to the wrist flexion crease to create a plane for the fine long nasal turbinate speculum. The nasal turbinate speculum is then inserted into this plane above the proximal limb of the TCL and DVFF (Figure 3). Once inserted to the level of the confluence of the TCL and the DVFF, the speculum is opened.
Topside visualization is now encountered with the ulnar neurovascular bundle protected by the ulnar blade of the speculum. A long-handle scalpel is used to incise the TCL and the DVFF under direct visualization from proximal to distal in line with the previously completed distal release (Figure 4). As the nasal turbinate speculum is stretching the TCL and putting it under tension, the TCL can be heard splitting as it is being incised. Once the TCL and the DVFF are divided, the speculum is slowly closed and removed. Wide diastasis of the radial and ulnar leaflets of the TCL and the DVFF is directly visualized. Complete decompression of the median nerve from the distal forearm fascia to the superficial palmar arch is confirmed.
Adhesions between the undersurface of the radial leaflet and the flexor tendons and median nerve are mobilized. The median nerve is assessed for “hourglass” morphology or atrophy. The flexor tendons can be swept radialward with a free elevator to inspect the floor of the carpal tunnel. Flexor tenosynovectomy is not routinely performed. The incision is closed with interrupted simple sutures using 4-0 nylon.
Study Results
This study was conducted at Hand Surgery PC, Newton-Wellesley Hospital, Tufts University School of Medicine. Over a 10-month interval, 101 consecutive mini-OCTRs (63 right hands, 38 left hands) were performed with this proximal release modification in 88 patients (51 females, 37 males) by Dr. Ruchelsman and Dr. Belsky (Table). CTRs performed in the setting of wrist and/or carpal trauma were excluded. Mean age was 62.8 years. Mean follow-up was 11.3 weeks (~3 months). For isolated cases of CTR, mean tourniquet time was 16 minutes. CTS symptoms were relieved in all patients with a high degree of satisfaction as measured with history and examination findings at follow-up visits. There were no major complications (eg, infection, neural or vascular damage, severe residual pain). Four patients reported minor residual numbness in the fingers at latest follow-up but nevertheless had major improvement over preoperative baseline. These 4 patients had preoperative electromyograms or nerve conduction studies documenting the extent of their disease. There was 1 case of minor wound complication. Three weeks after surgery, the patient had a 1-cm wound opening, which closed with local wound care. The patient did not develop any drainage, infection, bleeding, or neurologic symptoms.
Discussion
Open release of the TCL—the gold standard of surgical treatment for CTS—produces reliable symptom relief in the vast majority of patients.25,30 Given that the most common complication of carpal tunnel surgery is incomplete release of the TCL,31,32 this technique, which uses a nasal turbinate speculum to better visualize the median nerve, could potentially reduce the reoperation rate. The nasal turbinate speculum allows the surgeon to see the confluence of the TCL and the DVFF. In addition, as the complete release can be visualized, there is minimal chance of injury.
The 2007 Cochrane review3 found no strong evidence supporting replacing OCTR with endoscopic techniques. Previous investigators have questioned the utility of ECTR given that it is higher in cost and more resource-intensive than OCTR1,33,34 and is associated with higher rates of certain complications.5,22,35-37 A 2004 meta-analysis of 13 randomized, controlled trials found a higher rate of reversible nerve damage with an odds ratio of 3.1 for ECTR versus OCTR.35 A more recent (2006) review of more than 80 studies found transient neurapraxias in 1.45% of ECTR cases and 0.25% of OCTR cases.5 The same study reported overall complication rates (reversible and major neurovascular structural injuries) of 0.74% for OCTR and 1.63% for ECTR (P < .005). Another limitation of ECTR is that endoscopic techniques require a higher degree of surgical skill, which makes teaching residents and fellows more challenging.
The novel nasal turbinate speculum technique presented here is easily reproducible and allows first-time surgeons to visualize all important structures. Given that this technique does not require an endoscope or an endoscope-viewing tower, it is likely more cost-effective and requires less time for turnover between cases. Patients obtain good relief of their CTS symptoms with this technique, and most return to their daily activities within weeks after operation.
1. Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. Int J Gen Med. 2010;3(4):255-261.
2. Concannon MJ, Brownfield ML, Puckett CL. The incidence of recurrence after endoscopic carpal tunnel release. Plast Reconstr Surg. 2000;105(5):1662-1665.
3. Scholten RJ, Mink van der Molen A, Uitdehaag BM, Bouter LM, de Vet HC. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905.
4. In memoriam Sir James Learmonth, K.C.V.O., C.B.E., hon. F.R.C.S. (1895-1967). Ann R Coll Surg Engl. 1967;41(5):438-439.
5. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
6. Jarvik JG, Comstock BA, Kliot M, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet. 2009;374(9695):1074-1081.
7. Verdugo RJ, Salinas RA, Castillo JL, et al. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;(4):CD001552.
8. Garland H, Langworth EP, Taverner D, et al. Surgical treatment for the carpal tunnel syndrome. Lancet. 1964;1(7343):1129-1130.
9. Gerritsen AA, de Vet HC, Scholten RJ, et al. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA. 2002;288(10):1245-1251.
10. Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63(3):380-383.
11. Sucher BM. Myofascial manipulative release of carpal tunnel syndrome: documentation with magnetic resonance imaging. J Am Osteopath Assoc. 1993;93(12):1273-1278.
12. Pereira EE, Miranda DA, Sere I, et al. Endoscopic release of the carpal tunnel: a 2-portal-modified technique. Tech Hand Up Extrem Surg. 2010;14(4):263-265.
13. Louis DS, Greene TL, Noellert RC. Complications of carpal tunnel surgery. J Neurosurg. 1985;62(3):352-356.
14. Mirza MA, King ET Jr, Tanveer S. Palmar uniportal extrabursal endoscopic carpal tunnel release. Arthroscopy. 1995;11(1):82-90.
15. Brown MG, Keyser B, Rothenberg ES. Endoscopic carpal tunnel release. J Hand Surg Am. 1992;17(6):1009-1011.
16. Agee JM, McCarroll HR Jr, Tortosa RD, et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17(6):987-995.
17. Okutsu I, Ninomiya S, Takatori Y, et al. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
18. Ghaly RF, Saban KL, Haley DA, et al. Endoscopic carpal tunnel release surgery: report of patient satisfaction. Neurol Res. 2000;22(6):551-555.
19. Lee WP, Plancher KD, Strickland JW. Carpal tunnel release with a small palmar incision. Hand Clin. 1996;12(2):271-284.
20. Biyani A, Downes EM. An open twin incision technique of carpal tunnel decompression with reduced incidence of scar tenderness. J Hand Surg Br. 1993;18(3):331-334.
21. Brown RA, Gelberman RH, Seiler JG 3rd, et al. Carpal tunnel release. A prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75(9):1265-1275.
22. Chow JC. Endoscopic release of the carpal ligament for carpal tunnel syndrome: 22-month clinical result. Arthroscopy. 1990;6(4):288-296.
23. Trumble TE, Diao E, Abrams RA, et al. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84(7):1107-1115.
24. Gerritsen AA, Uitdehaag BM, van Geldere D, et al. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
25. Edgell SE, McCabe SJ, Breidenbach WC, et al. Predicting the outcome of carpal tunnel release. J Hand Surg Am. 2003;28(2):255-261.
26. Vella JC, Hartigan BJ, Stern PJ. Kaplan’s cardinal line. J Hand Surg Am. 2006;31(6):912-918.
27. Kwon JY, Kim JY, Hong JT, et al. Position change of the neurovascular structures around the carpal tunnel with dynamic wrist motion. J Korean Neurosurg Soc. 2011;50(4):377-380.
28. Netscher D, Polsen C, Thornby J, et al. Anatomic delineation of the ulnar nerve and ulnar artery in relation to the carpal tunnel by axial magnetic resonance imaging scanning. J Hand Surg Am. 1996;21(2):273-276.
29. Madhav TJ, To P, Stern PJ. The palmar fat pad is a reliable intraoperative landmark during carpal tunnel release. J Hand Surg Am. 2009;34(7):1204-1209.
30. Kulick MI, Gordillo G, Javidi T, et al. Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am. 1986;11(1):59-66.
31. Bland JD. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007;36(2):167-171.
32. MacDonald RI, Lichtman DM, Hanlon JJ, et al. Complications of surgical release for carpal tunnel syndrome. J Hand Surg Am. 1978;3(1):70-76.
33. Atroshi I, Larsson GU, Ornstein E, Hofer M, Johnsson R, Ranstam J. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ. 2006;332(7556):1473.
34. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002;84(3):375-379.
35. Thoma A, Veltri K, Haines T, et al. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
36. Murphy RX Jr, Jennings JF, Wukich DK. Major neurovascular complications of endoscopic carpal tunnel release. J Hand Surg Am. 1994;19(1):114-118.
37. Palmer DH, Paulson JC, Lane-Larsen CL, et al. Endoscopic carpal tunnel release: a comparison of two techniques with open release. Arthroscopy. 1993;9(5):498-508.
Carpal tunnel syndrome (CTS) is a disorder characterized by entrapment of the median nerve at the wrist, which may lead to symptoms of pain, paresthesia, and, ultimately, thenar muscle atrophy. Surgical intervention is indicated with persistent or progressive symptoms despite nonoperative management. Timely surgical decompression aims to halt progression of this disorder and prevent permanent peripheral nerve injury.
Carpal tunnel release (CTR) is the most common hand and wrist surgery in the United States, with about 400,000 operations performed annually.1,2 Several methods of decompressing the carpal tunnel have been described.3 These include standard open CTR (OCTR), mini-open approaches, and various endoscopic techniques. OCTR was initially described by Sir James Learmonth in 1933,4 and it remains the gold-standard surgical treatment for patients with symptomatic CTS. Uniform excellent results with high patient satisfaction and low complication rates have been reported in several series.5-9 Common to all techniques is complete proximal-to-distal division of the transverse carpal ligament (TCL). Magnetic resonance imaging studies have shown that TCL transection and the resulting diastasis between the radial and ulnar leaflets cause a significant increase in the volume of the carpal tunnel, leading to decreased pressure.10,11
Endoscopic CTR (ECTR) techniques were developed in an effort to reduce complications, scar sensitivity, and pillar pain and facilitate more rapid return to work.12-17 Outcome studies have demonstrated that both open and endoscopic releases yield patient-reported subjective improvements over preoperative symptoms.18-22 A randomized, controlled trial by Trumble and colleagues23 in 2002 found that ECTR led to improved patient outcomes in the early postoperative period (first 3 months), though differences in outcomes were reduced at final follow-up. More recently (2007), a Cochrane review of 33 trials concluded there was no strong evidence favoring use of alternative techniques over OCTR.3 Further, OCTR has been found to be technically less demanding and associated with decreased complications and costs.24
Indications
The benefit of median nerve decompression at the wrist for CTS is clear.6,7 Indications for surgery in patients with CTS include persistent symptoms despite nonoperative treatment, objective sensory disturbance or motor weakness, and thenar atrophy. Symptomatic response to corticosteroid injection is predictive of success after carpal tunnel surgery.25 More than 87% of patients who gain symptomatic relief from corticosteroid injection have an excellent surgical outcome.
Technique
OCTR allows direct visualization of the TCL and the distal volar forearm fascia (DVFF) and evaluation for the presence of anomalous branching patterns of the median nerve. OCTR traditionally was performed through a 4- to 5-cm longitudinal incision extending from the wrist crease proximally to the Kaplan cardinal line distally. The mini-open technique is identical with the exception of incision length. We routinely use a 2.5- to 3-cm incision. Regardless of incision length, each OCTR should proceed through the same reproducible steps.
We perform OCTR under tourniquet control. Choice of anesthesia is surgeon and patient preference. We prefer local anesthesia with conscious sedation. After conscious sedation is administered, we infiltrate the carpal tunnel and surrounding subcutaneous tissue with 10 mL of a 50:50 mixture of 0.5% bupivacaine and 1% lidocaine without epinephrine.
A 2.5- to 3-cm longitudinal incision is made along the axis of the radial border of the ring finger from the Kaplan cardinal line26 and extending about 3 cm proximally toward the wrist flexion crease ulnar to the palmaris longus if present (Figure 1).
After the skin is incised longitudinally, the subcutaneous fat is mobilized and cutaneous sensory branches identified and protected. The underlying superficial palmar fascia is incised in line with the skin incision. The underlying midportion of the TCL is now visualized.
Transverse Carpal Ligament Release
Occasionally, the investing fascia along the ulnar edge of the thenar musculature is mobilized radialward (if the thenar musculature is well developed) to visualize the proximal limb of the TCL. Injury to any anomalous motor branch of the median nerve is avoided by directly visualizing and then incising the TCL (Figure 2). The TCL is incised along its ulnar border just radial to the hook of hamate from distal to proximal in line with the radial border of the ring finger. Staying near the ulnar attachment of the TCL keeps the plane of ligament division farther away from the median nerve and its recurrent motor branches. Although the ulnar neurovascular bundle typically resides ulnar to the hook of hamate in the canal of Guyon, the surgeon must be aware that it can be located radial to the hook in some instances.27,28 In the elderly, the ulnar artery may be tortuous and enter the field and require retraction. The TCL is incised distally until the sentinel fat pad, which marks the superficial palmar arterial arch, is visualized. This bed of adipose tissue marks the distal edge of the TCL.29
Proximally, subcutaneous tissues above the proximal limb of the TCL and DVFF are mobilized to about 2 cm proximal to the wrist flexion crease to create a plane for the fine long nasal turbinate speculum. The nasal turbinate speculum is then inserted into this plane above the proximal limb of the TCL and DVFF (Figure 3). Once inserted to the level of the confluence of the TCL and the DVFF, the speculum is opened.
Topside visualization is now encountered with the ulnar neurovascular bundle protected by the ulnar blade of the speculum. A long-handle scalpel is used to incise the TCL and the DVFF under direct visualization from proximal to distal in line with the previously completed distal release (Figure 4). As the nasal turbinate speculum is stretching the TCL and putting it under tension, the TCL can be heard splitting as it is being incised. Once the TCL and the DVFF are divided, the speculum is slowly closed and removed. Wide diastasis of the radial and ulnar leaflets of the TCL and the DVFF is directly visualized. Complete decompression of the median nerve from the distal forearm fascia to the superficial palmar arch is confirmed.
Adhesions between the undersurface of the radial leaflet and the flexor tendons and median nerve are mobilized. The median nerve is assessed for “hourglass” morphology or atrophy. The flexor tendons can be swept radialward with a free elevator to inspect the floor of the carpal tunnel. Flexor tenosynovectomy is not routinely performed. The incision is closed with interrupted simple sutures using 4-0 nylon.
Study Results
This study was conducted at Hand Surgery PC, Newton-Wellesley Hospital, Tufts University School of Medicine. Over a 10-month interval, 101 consecutive mini-OCTRs (63 right hands, 38 left hands) were performed with this proximal release modification in 88 patients (51 females, 37 males) by Dr. Ruchelsman and Dr. Belsky (Table). CTRs performed in the setting of wrist and/or carpal trauma were excluded. Mean age was 62.8 years. Mean follow-up was 11.3 weeks (~3 months). For isolated cases of CTR, mean tourniquet time was 16 minutes. CTS symptoms were relieved in all patients with a high degree of satisfaction as measured with history and examination findings at follow-up visits. There were no major complications (eg, infection, neural or vascular damage, severe residual pain). Four patients reported minor residual numbness in the fingers at latest follow-up but nevertheless had major improvement over preoperative baseline. These 4 patients had preoperative electromyograms or nerve conduction studies documenting the extent of their disease. There was 1 case of minor wound complication. Three weeks after surgery, the patient had a 1-cm wound opening, which closed with local wound care. The patient did not develop any drainage, infection, bleeding, or neurologic symptoms.
Discussion
Open release of the TCL—the gold standard of surgical treatment for CTS—produces reliable symptom relief in the vast majority of patients.25,30 Given that the most common complication of carpal tunnel surgery is incomplete release of the TCL,31,32 this technique, which uses a nasal turbinate speculum to better visualize the median nerve, could potentially reduce the reoperation rate. The nasal turbinate speculum allows the surgeon to see the confluence of the TCL and the DVFF. In addition, as the complete release can be visualized, there is minimal chance of injury.
The 2007 Cochrane review3 found no strong evidence supporting replacing OCTR with endoscopic techniques. Previous investigators have questioned the utility of ECTR given that it is higher in cost and more resource-intensive than OCTR1,33,34 and is associated with higher rates of certain complications.5,22,35-37 A 2004 meta-analysis of 13 randomized, controlled trials found a higher rate of reversible nerve damage with an odds ratio of 3.1 for ECTR versus OCTR.35 A more recent (2006) review of more than 80 studies found transient neurapraxias in 1.45% of ECTR cases and 0.25% of OCTR cases.5 The same study reported overall complication rates (reversible and major neurovascular structural injuries) of 0.74% for OCTR and 1.63% for ECTR (P < .005). Another limitation of ECTR is that endoscopic techniques require a higher degree of surgical skill, which makes teaching residents and fellows more challenging.
The novel nasal turbinate speculum technique presented here is easily reproducible and allows first-time surgeons to visualize all important structures. Given that this technique does not require an endoscope or an endoscope-viewing tower, it is likely more cost-effective and requires less time for turnover between cases. Patients obtain good relief of their CTS symptoms with this technique, and most return to their daily activities within weeks after operation.
Carpal tunnel syndrome (CTS) is a disorder characterized by entrapment of the median nerve at the wrist, which may lead to symptoms of pain, paresthesia, and, ultimately, thenar muscle atrophy. Surgical intervention is indicated with persistent or progressive symptoms despite nonoperative management. Timely surgical decompression aims to halt progression of this disorder and prevent permanent peripheral nerve injury.
Carpal tunnel release (CTR) is the most common hand and wrist surgery in the United States, with about 400,000 operations performed annually.1,2 Several methods of decompressing the carpal tunnel have been described.3 These include standard open CTR (OCTR), mini-open approaches, and various endoscopic techniques. OCTR was initially described by Sir James Learmonth in 1933,4 and it remains the gold-standard surgical treatment for patients with symptomatic CTS. Uniform excellent results with high patient satisfaction and low complication rates have been reported in several series.5-9 Common to all techniques is complete proximal-to-distal division of the transverse carpal ligament (TCL). Magnetic resonance imaging studies have shown that TCL transection and the resulting diastasis between the radial and ulnar leaflets cause a significant increase in the volume of the carpal tunnel, leading to decreased pressure.10,11
Endoscopic CTR (ECTR) techniques were developed in an effort to reduce complications, scar sensitivity, and pillar pain and facilitate more rapid return to work.12-17 Outcome studies have demonstrated that both open and endoscopic releases yield patient-reported subjective improvements over preoperative symptoms.18-22 A randomized, controlled trial by Trumble and colleagues23 in 2002 found that ECTR led to improved patient outcomes in the early postoperative period (first 3 months), though differences in outcomes were reduced at final follow-up. More recently (2007), a Cochrane review of 33 trials concluded there was no strong evidence favoring use of alternative techniques over OCTR.3 Further, OCTR has been found to be technically less demanding and associated with decreased complications and costs.24
Indications
The benefit of median nerve decompression at the wrist for CTS is clear.6,7 Indications for surgery in patients with CTS include persistent symptoms despite nonoperative treatment, objective sensory disturbance or motor weakness, and thenar atrophy. Symptomatic response to corticosteroid injection is predictive of success after carpal tunnel surgery.25 More than 87% of patients who gain symptomatic relief from corticosteroid injection have an excellent surgical outcome.
Technique
OCTR allows direct visualization of the TCL and the distal volar forearm fascia (DVFF) and evaluation for the presence of anomalous branching patterns of the median nerve. OCTR traditionally was performed through a 4- to 5-cm longitudinal incision extending from the wrist crease proximally to the Kaplan cardinal line distally. The mini-open technique is identical with the exception of incision length. We routinely use a 2.5- to 3-cm incision. Regardless of incision length, each OCTR should proceed through the same reproducible steps.
We perform OCTR under tourniquet control. Choice of anesthesia is surgeon and patient preference. We prefer local anesthesia with conscious sedation. After conscious sedation is administered, we infiltrate the carpal tunnel and surrounding subcutaneous tissue with 10 mL of a 50:50 mixture of 0.5% bupivacaine and 1% lidocaine without epinephrine.
A 2.5- to 3-cm longitudinal incision is made along the axis of the radial border of the ring finger from the Kaplan cardinal line26 and extending about 3 cm proximally toward the wrist flexion crease ulnar to the palmaris longus if present (Figure 1).
After the skin is incised longitudinally, the subcutaneous fat is mobilized and cutaneous sensory branches identified and protected. The underlying superficial palmar fascia is incised in line with the skin incision. The underlying midportion of the TCL is now visualized.
Transverse Carpal Ligament Release
Occasionally, the investing fascia along the ulnar edge of the thenar musculature is mobilized radialward (if the thenar musculature is well developed) to visualize the proximal limb of the TCL. Injury to any anomalous motor branch of the median nerve is avoided by directly visualizing and then incising the TCL (Figure 2). The TCL is incised along its ulnar border just radial to the hook of hamate from distal to proximal in line with the radial border of the ring finger. Staying near the ulnar attachment of the TCL keeps the plane of ligament division farther away from the median nerve and its recurrent motor branches. Although the ulnar neurovascular bundle typically resides ulnar to the hook of hamate in the canal of Guyon, the surgeon must be aware that it can be located radial to the hook in some instances.27,28 In the elderly, the ulnar artery may be tortuous and enter the field and require retraction. The TCL is incised distally until the sentinel fat pad, which marks the superficial palmar arterial arch, is visualized. This bed of adipose tissue marks the distal edge of the TCL.29
Proximally, subcutaneous tissues above the proximal limb of the TCL and DVFF are mobilized to about 2 cm proximal to the wrist flexion crease to create a plane for the fine long nasal turbinate speculum. The nasal turbinate speculum is then inserted into this plane above the proximal limb of the TCL and DVFF (Figure 3). Once inserted to the level of the confluence of the TCL and the DVFF, the speculum is opened.
Topside visualization is now encountered with the ulnar neurovascular bundle protected by the ulnar blade of the speculum. A long-handle scalpel is used to incise the TCL and the DVFF under direct visualization from proximal to distal in line with the previously completed distal release (Figure 4). As the nasal turbinate speculum is stretching the TCL and putting it under tension, the TCL can be heard splitting as it is being incised. Once the TCL and the DVFF are divided, the speculum is slowly closed and removed. Wide diastasis of the radial and ulnar leaflets of the TCL and the DVFF is directly visualized. Complete decompression of the median nerve from the distal forearm fascia to the superficial palmar arch is confirmed.
Adhesions between the undersurface of the radial leaflet and the flexor tendons and median nerve are mobilized. The median nerve is assessed for “hourglass” morphology or atrophy. The flexor tendons can be swept radialward with a free elevator to inspect the floor of the carpal tunnel. Flexor tenosynovectomy is not routinely performed. The incision is closed with interrupted simple sutures using 4-0 nylon.
Study Results
This study was conducted at Hand Surgery PC, Newton-Wellesley Hospital, Tufts University School of Medicine. Over a 10-month interval, 101 consecutive mini-OCTRs (63 right hands, 38 left hands) were performed with this proximal release modification in 88 patients (51 females, 37 males) by Dr. Ruchelsman and Dr. Belsky (Table). CTRs performed in the setting of wrist and/or carpal trauma were excluded. Mean age was 62.8 years. Mean follow-up was 11.3 weeks (~3 months). For isolated cases of CTR, mean tourniquet time was 16 minutes. CTS symptoms were relieved in all patients with a high degree of satisfaction as measured with history and examination findings at follow-up visits. There were no major complications (eg, infection, neural or vascular damage, severe residual pain). Four patients reported minor residual numbness in the fingers at latest follow-up but nevertheless had major improvement over preoperative baseline. These 4 patients had preoperative electromyograms or nerve conduction studies documenting the extent of their disease. There was 1 case of minor wound complication. Three weeks after surgery, the patient had a 1-cm wound opening, which closed with local wound care. The patient did not develop any drainage, infection, bleeding, or neurologic symptoms.
Discussion
Open release of the TCL—the gold standard of surgical treatment for CTS—produces reliable symptom relief in the vast majority of patients.25,30 Given that the most common complication of carpal tunnel surgery is incomplete release of the TCL,31,32 this technique, which uses a nasal turbinate speculum to better visualize the median nerve, could potentially reduce the reoperation rate. The nasal turbinate speculum allows the surgeon to see the confluence of the TCL and the DVFF. In addition, as the complete release can be visualized, there is minimal chance of injury.
The 2007 Cochrane review3 found no strong evidence supporting replacing OCTR with endoscopic techniques. Previous investigators have questioned the utility of ECTR given that it is higher in cost and more resource-intensive than OCTR1,33,34 and is associated with higher rates of certain complications.5,22,35-37 A 2004 meta-analysis of 13 randomized, controlled trials found a higher rate of reversible nerve damage with an odds ratio of 3.1 for ECTR versus OCTR.35 A more recent (2006) review of more than 80 studies found transient neurapraxias in 1.45% of ECTR cases and 0.25% of OCTR cases.5 The same study reported overall complication rates (reversible and major neurovascular structural injuries) of 0.74% for OCTR and 1.63% for ECTR (P < .005). Another limitation of ECTR is that endoscopic techniques require a higher degree of surgical skill, which makes teaching residents and fellows more challenging.
The novel nasal turbinate speculum technique presented here is easily reproducible and allows first-time surgeons to visualize all important structures. Given that this technique does not require an endoscope or an endoscope-viewing tower, it is likely more cost-effective and requires less time for turnover between cases. Patients obtain good relief of their CTS symptoms with this technique, and most return to their daily activities within weeks after operation.
1. Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. Int J Gen Med. 2010;3(4):255-261.
2. Concannon MJ, Brownfield ML, Puckett CL. The incidence of recurrence after endoscopic carpal tunnel release. Plast Reconstr Surg. 2000;105(5):1662-1665.
3. Scholten RJ, Mink van der Molen A, Uitdehaag BM, Bouter LM, de Vet HC. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905.
4. In memoriam Sir James Learmonth, K.C.V.O., C.B.E., hon. F.R.C.S. (1895-1967). Ann R Coll Surg Engl. 1967;41(5):438-439.
5. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
6. Jarvik JG, Comstock BA, Kliot M, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet. 2009;374(9695):1074-1081.
7. Verdugo RJ, Salinas RA, Castillo JL, et al. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;(4):CD001552.
8. Garland H, Langworth EP, Taverner D, et al. Surgical treatment for the carpal tunnel syndrome. Lancet. 1964;1(7343):1129-1130.
9. Gerritsen AA, de Vet HC, Scholten RJ, et al. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA. 2002;288(10):1245-1251.
10. Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63(3):380-383.
11. Sucher BM. Myofascial manipulative release of carpal tunnel syndrome: documentation with magnetic resonance imaging. J Am Osteopath Assoc. 1993;93(12):1273-1278.
12. Pereira EE, Miranda DA, Sere I, et al. Endoscopic release of the carpal tunnel: a 2-portal-modified technique. Tech Hand Up Extrem Surg. 2010;14(4):263-265.
13. Louis DS, Greene TL, Noellert RC. Complications of carpal tunnel surgery. J Neurosurg. 1985;62(3):352-356.
14. Mirza MA, King ET Jr, Tanveer S. Palmar uniportal extrabursal endoscopic carpal tunnel release. Arthroscopy. 1995;11(1):82-90.
15. Brown MG, Keyser B, Rothenberg ES. Endoscopic carpal tunnel release. J Hand Surg Am. 1992;17(6):1009-1011.
16. Agee JM, McCarroll HR Jr, Tortosa RD, et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17(6):987-995.
17. Okutsu I, Ninomiya S, Takatori Y, et al. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
18. Ghaly RF, Saban KL, Haley DA, et al. Endoscopic carpal tunnel release surgery: report of patient satisfaction. Neurol Res. 2000;22(6):551-555.
19. Lee WP, Plancher KD, Strickland JW. Carpal tunnel release with a small palmar incision. Hand Clin. 1996;12(2):271-284.
20. Biyani A, Downes EM. An open twin incision technique of carpal tunnel decompression with reduced incidence of scar tenderness. J Hand Surg Br. 1993;18(3):331-334.
21. Brown RA, Gelberman RH, Seiler JG 3rd, et al. Carpal tunnel release. A prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75(9):1265-1275.
22. Chow JC. Endoscopic release of the carpal ligament for carpal tunnel syndrome: 22-month clinical result. Arthroscopy. 1990;6(4):288-296.
23. Trumble TE, Diao E, Abrams RA, et al. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84(7):1107-1115.
24. Gerritsen AA, Uitdehaag BM, van Geldere D, et al. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
25. Edgell SE, McCabe SJ, Breidenbach WC, et al. Predicting the outcome of carpal tunnel release. J Hand Surg Am. 2003;28(2):255-261.
26. Vella JC, Hartigan BJ, Stern PJ. Kaplan’s cardinal line. J Hand Surg Am. 2006;31(6):912-918.
27. Kwon JY, Kim JY, Hong JT, et al. Position change of the neurovascular structures around the carpal tunnel with dynamic wrist motion. J Korean Neurosurg Soc. 2011;50(4):377-380.
28. Netscher D, Polsen C, Thornby J, et al. Anatomic delineation of the ulnar nerve and ulnar artery in relation to the carpal tunnel by axial magnetic resonance imaging scanning. J Hand Surg Am. 1996;21(2):273-276.
29. Madhav TJ, To P, Stern PJ. The palmar fat pad is a reliable intraoperative landmark during carpal tunnel release. J Hand Surg Am. 2009;34(7):1204-1209.
30. Kulick MI, Gordillo G, Javidi T, et al. Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am. 1986;11(1):59-66.
31. Bland JD. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007;36(2):167-171.
32. MacDonald RI, Lichtman DM, Hanlon JJ, et al. Complications of surgical release for carpal tunnel syndrome. J Hand Surg Am. 1978;3(1):70-76.
33. Atroshi I, Larsson GU, Ornstein E, Hofer M, Johnsson R, Ranstam J. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ. 2006;332(7556):1473.
34. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002;84(3):375-379.
35. Thoma A, Veltri K, Haines T, et al. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
36. Murphy RX Jr, Jennings JF, Wukich DK. Major neurovascular complications of endoscopic carpal tunnel release. J Hand Surg Am. 1994;19(1):114-118.
37. Palmer DH, Paulson JC, Lane-Larsen CL, et al. Endoscopic carpal tunnel release: a comparison of two techniques with open release. Arthroscopy. 1993;9(5):498-508.
1. Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. Int J Gen Med. 2010;3(4):255-261.
2. Concannon MJ, Brownfield ML, Puckett CL. The incidence of recurrence after endoscopic carpal tunnel release. Plast Reconstr Surg. 2000;105(5):1662-1665.
3. Scholten RJ, Mink van der Molen A, Uitdehaag BM, Bouter LM, de Vet HC. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905.
4. In memoriam Sir James Learmonth, K.C.V.O., C.B.E., hon. F.R.C.S. (1895-1967). Ann R Coll Surg Engl. 1967;41(5):438-439.
5. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
6. Jarvik JG, Comstock BA, Kliot M, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet. 2009;374(9695):1074-1081.
7. Verdugo RJ, Salinas RA, Castillo JL, et al. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;(4):CD001552.
8. Garland H, Langworth EP, Taverner D, et al. Surgical treatment for the carpal tunnel syndrome. Lancet. 1964;1(7343):1129-1130.
9. Gerritsen AA, de Vet HC, Scholten RJ, et al. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA. 2002;288(10):1245-1251.
10. Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63(3):380-383.
11. Sucher BM. Myofascial manipulative release of carpal tunnel syndrome: documentation with magnetic resonance imaging. J Am Osteopath Assoc. 1993;93(12):1273-1278.
12. Pereira EE, Miranda DA, Sere I, et al. Endoscopic release of the carpal tunnel: a 2-portal-modified technique. Tech Hand Up Extrem Surg. 2010;14(4):263-265.
13. Louis DS, Greene TL, Noellert RC. Complications of carpal tunnel surgery. J Neurosurg. 1985;62(3):352-356.
14. Mirza MA, King ET Jr, Tanveer S. Palmar uniportal extrabursal endoscopic carpal tunnel release. Arthroscopy. 1995;11(1):82-90.
15. Brown MG, Keyser B, Rothenberg ES. Endoscopic carpal tunnel release. J Hand Surg Am. 1992;17(6):1009-1011.
16. Agee JM, McCarroll HR Jr, Tortosa RD, et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17(6):987-995.
17. Okutsu I, Ninomiya S, Takatori Y, et al. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
18. Ghaly RF, Saban KL, Haley DA, et al. Endoscopic carpal tunnel release surgery: report of patient satisfaction. Neurol Res. 2000;22(6):551-555.
19. Lee WP, Plancher KD, Strickland JW. Carpal tunnel release with a small palmar incision. Hand Clin. 1996;12(2):271-284.
20. Biyani A, Downes EM. An open twin incision technique of carpal tunnel decompression with reduced incidence of scar tenderness. J Hand Surg Br. 1993;18(3):331-334.
21. Brown RA, Gelberman RH, Seiler JG 3rd, et al. Carpal tunnel release. A prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75(9):1265-1275.
22. Chow JC. Endoscopic release of the carpal ligament for carpal tunnel syndrome: 22-month clinical result. Arthroscopy. 1990;6(4):288-296.
23. Trumble TE, Diao E, Abrams RA, et al. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84(7):1107-1115.
24. Gerritsen AA, Uitdehaag BM, van Geldere D, et al. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
25. Edgell SE, McCabe SJ, Breidenbach WC, et al. Predicting the outcome of carpal tunnel release. J Hand Surg Am. 2003;28(2):255-261.
26. Vella JC, Hartigan BJ, Stern PJ. Kaplan’s cardinal line. J Hand Surg Am. 2006;31(6):912-918.
27. Kwon JY, Kim JY, Hong JT, et al. Position change of the neurovascular structures around the carpal tunnel with dynamic wrist motion. J Korean Neurosurg Soc. 2011;50(4):377-380.
28. Netscher D, Polsen C, Thornby J, et al. Anatomic delineation of the ulnar nerve and ulnar artery in relation to the carpal tunnel by axial magnetic resonance imaging scanning. J Hand Surg Am. 1996;21(2):273-276.
29. Madhav TJ, To P, Stern PJ. The palmar fat pad is a reliable intraoperative landmark during carpal tunnel release. J Hand Surg Am. 2009;34(7):1204-1209.
30. Kulick MI, Gordillo G, Javidi T, et al. Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am. 1986;11(1):59-66.
31. Bland JD. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007;36(2):167-171.
32. MacDonald RI, Lichtman DM, Hanlon JJ, et al. Complications of surgical release for carpal tunnel syndrome. J Hand Surg Am. 1978;3(1):70-76.
33. Atroshi I, Larsson GU, Ornstein E, Hofer M, Johnsson R, Ranstam J. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ. 2006;332(7556):1473.
34. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002;84(3):375-379.
35. Thoma A, Veltri K, Haines T, et al. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
36. Murphy RX Jr, Jennings JF, Wukich DK. Major neurovascular complications of endoscopic carpal tunnel release. J Hand Surg Am. 1994;19(1):114-118.
37. Palmer DH, Paulson JC, Lane-Larsen CL, et al. Endoscopic carpal tunnel release: a comparison of two techniques with open release. Arthroscopy. 1993;9(5):498-508.
Excision of Symptomatic Spinous Process Nonunion in Adolescent Athletes
Fractures of the spinous process of the lower cervical spine or upper thoracic spine are frequently referred to as clay-shoveler’s fractures. Originally reported by Hall1 in 1940, these fractures were described in workers in Australia who dug drains in clay soil and threw the clay overhead with long shovels. Occasionally, the mud would not release from the shovel, causing excess force to be transmitted to the supraspinous ligaments and resulting in a forceful avulsion fracture of one or multiple spinous processes. The few reports following the earliest description in the literature frequently describe the mechanism of injury as being athletic in nature.2-4 The forceful contraction of the paraspinal and trapezius muscles on the supraspinous ligaments and the resultant attachment to the spinous processes make this a not uncommon injury during athletics, especially with a flexed position of the neck and shoulders. The resultant fracture or apophyseal avulsion is painful and often necessitates a visit to the physician, with plain films, computed tomography (CT) scans, or magnetic resonance imaging (MRI) confirming the diagnosis.5
Treatment of these fractures has not been well described, but frequently a period of rest followed by physical therapy will allow a return to activity. We present a series of adolescent athletes who developed nonunion of the fracture of the T1 spinous process with continued symptoms, despite rest and conservative therapy, and who underwent surgical excision of the ununited fragment.
Materials and Methods
We obtained institutional review board permission for this study and searched the surgical database between 2006 and 2013 for patients who had undergone resection of a spinous process nonunion. We collected demographic data on the patients, evaluated the radiographic studies, and reviewed operative reports and follow-up patient data.
Results
Dr. Hedequist operated on 3 patients with a spinous process nonunion over the study time period. The average age of the patients was 14 years; the location of the spinous process fracture was the T1 vertebra in all patients. Two patients sustained the injury while playing hockey and 1 during wrestling. The average duration of symptoms prior to operation was 10 months; all patients had seen physicians without a diagnosis prior to evaluation at out institution. All patients had a trial of physical therapy before surgery, and all had been unable to return to sport after injury secondary to pain.
Examination of all patients revealed pain directly over the fracture site and accentuated by forward flexion of the neck and shoulders. Evaluation of injury plain films revealed a fracture fragment in 2 patients (Figure 1). All 3 patients underwent MRI and CT scans confirming the diagnosis. MRI confirmed areas of increased signal at the tip of the T1 spinous process, with inflammation in the supraspinous ligament directly at that region (Figure 2). The CT scans confirmed the presence of a bony fragment correlating with the tip of the T1 spinous process (Figure 3).
Surgery was performed under general endotracheal anesthesia via a midline incision over the affected area down to the spinous process. The supraspinous ligament was opened revealing an easily identified and definable ununited ossicle, which was removed without taking down the interspinous ligament. All 3 nonunions were noted to be atrophic with no evidence of surrounding inflammatory tissue or bursa. The residual end of the spinous process was smoothed down with a rongeur. Standard closure was performed. There were no surgical complications.
All patients had complete relief of pain at follow-up; 1 patient returned to full sports activity at 6 weeks and the other 2 returned to full sports activity at 3 months. There was no loss of cervical motion or trapezial strength at follow-up. All patients voiced satisfaction with the decision for surgical intervention.
Discussion
Clay-shoveler’s fracture is an injury well known to orthopedists. This fracture is thought to be caused by a forceful contraction of the thoracic paraspinal and trapezial muscles, causing an avulsion fracture with pain and frequently a “pop” experienced by the patient.1 Usually considered self-limiting injuries, treatment involves a period of rest and activity modification with occasional physical therapy. Return to sports has been reported with occasional pain but with patient satisfaction.3,5,6
Our series of patients represent a group of adolescent athletes who sustained spinous process fractures of the T1 vertebra and, despite a significant period of rest and activity modification, were unable to return to sports given their pain. The examination of these patients revealed focal tenderness at the tip of the spinous process. The diagnosis is made clinically, with radiographic studies confirming the diagnosis. In our series of patients, MRI was the original modality used to confirm injury to the area, with hyperintensity seen in the area of the supraspinous ligament and tip of the spinous process. CT confirmed the nonunion and presence of an ossicle in all patients. Surgical exposure of that area easily exposed the ununited ossicle, which was removed in all patients.
To our knowledge, this is the first report in the literature describing surgical excision of an ununited spinous process fracture in adolescent athletes. The original descriptive case series by Hall1 states “in the minds of surgeons who have seen many of these cases that early operative removal of the fragments is the proper routine treatment.” Since that original series, we have not found articles in the literature that support surgical removal; however, persistent symptoms after fracture are described.5 It is not surprising that these patients developed pain at the site of the fracture given the forces acting in that area. The trapezial and paraspinal muscles acting on that area are forceful and repetitive during activities, especially sports. All our patients had pain with attempts at activity and all had had a significant period of rest. In a recent article, this injury was described in adolescents without the patients having clear relief of symptoms despite a period of inactivity.5 While physical therapy is therapeutic in some patients experiencing pain, it can be a source of aggravation due to neck and shoulder motion and muscle contraction. It is not surprising that therapy would not help in most cases, as neck and shoulder motion and muscle contraction are the sources of continuing discomfort.
Clinical practice suggests that most patients with spinous process fractures will become pain-free; however, that is not universal. This series demonstrates that a small subset of patients with this injury will continue to have significant symptoms despite a period of rest. In those patients who desire a pain-free return to sports, we recommend consideration of surgical excision after confirmation of nonunion with radiographic studies. The inherent risks of surgical treatment are minimal with this procedure, and the benefits include return to pain-free sports activity, with the resultant physical and psychosocial benefits for adolescent athletes.
1. Hall RDM. Clay-shoveler’s fracture. J Bone Joint Surg Am. 1940;22(1):63-75.
2. Herrick RT. Clay-shoveler’s fracture in power-lifting. A case report. Am J Sports Med. 1981;9(1):29-30.
3. Hetsroni I, Mann G, Dolev E, Morgenstern D, Nyska M. Clay shoveler’s fracture in a volleyball player. Phys Sportsmed. 2005;33(7):38-42.
4. Kaloostian PE, Kim JE, Calabresi PA, Bydon A, Witham T. Clay-shoveler’s fracture during indoor rock climbing. Orthopedics. 2013;36(3):e381-e383.
5. Yamaguchi KT Jr, Myung KS, Alonso MA, Skaggs DL. Clay-shoveler’s fracture equivalent in children. Spine. 2012;37(26):e1672-e1675.
6. Kang DH, Lee SH. Multiple spinous process fractures of the thoracic vertebrae (clay-shoveler’s fracture) in a beginning golfer: a case report. Spine. 2009;34(15):e534-e537.
Fractures of the spinous process of the lower cervical spine or upper thoracic spine are frequently referred to as clay-shoveler’s fractures. Originally reported by Hall1 in 1940, these fractures were described in workers in Australia who dug drains in clay soil and threw the clay overhead with long shovels. Occasionally, the mud would not release from the shovel, causing excess force to be transmitted to the supraspinous ligaments and resulting in a forceful avulsion fracture of one or multiple spinous processes. The few reports following the earliest description in the literature frequently describe the mechanism of injury as being athletic in nature.2-4 The forceful contraction of the paraspinal and trapezius muscles on the supraspinous ligaments and the resultant attachment to the spinous processes make this a not uncommon injury during athletics, especially with a flexed position of the neck and shoulders. The resultant fracture or apophyseal avulsion is painful and often necessitates a visit to the physician, with plain films, computed tomography (CT) scans, or magnetic resonance imaging (MRI) confirming the diagnosis.5
Treatment of these fractures has not been well described, but frequently a period of rest followed by physical therapy will allow a return to activity. We present a series of adolescent athletes who developed nonunion of the fracture of the T1 spinous process with continued symptoms, despite rest and conservative therapy, and who underwent surgical excision of the ununited fragment.
Materials and Methods
We obtained institutional review board permission for this study and searched the surgical database between 2006 and 2013 for patients who had undergone resection of a spinous process nonunion. We collected demographic data on the patients, evaluated the radiographic studies, and reviewed operative reports and follow-up patient data.
Results
Dr. Hedequist operated on 3 patients with a spinous process nonunion over the study time period. The average age of the patients was 14 years; the location of the spinous process fracture was the T1 vertebra in all patients. Two patients sustained the injury while playing hockey and 1 during wrestling. The average duration of symptoms prior to operation was 10 months; all patients had seen physicians without a diagnosis prior to evaluation at out institution. All patients had a trial of physical therapy before surgery, and all had been unable to return to sport after injury secondary to pain.
Examination of all patients revealed pain directly over the fracture site and accentuated by forward flexion of the neck and shoulders. Evaluation of injury plain films revealed a fracture fragment in 2 patients (Figure 1). All 3 patients underwent MRI and CT scans confirming the diagnosis. MRI confirmed areas of increased signal at the tip of the T1 spinous process, with inflammation in the supraspinous ligament directly at that region (Figure 2). The CT scans confirmed the presence of a bony fragment correlating with the tip of the T1 spinous process (Figure 3).
Surgery was performed under general endotracheal anesthesia via a midline incision over the affected area down to the spinous process. The supraspinous ligament was opened revealing an easily identified and definable ununited ossicle, which was removed without taking down the interspinous ligament. All 3 nonunions were noted to be atrophic with no evidence of surrounding inflammatory tissue or bursa. The residual end of the spinous process was smoothed down with a rongeur. Standard closure was performed. There were no surgical complications.
All patients had complete relief of pain at follow-up; 1 patient returned to full sports activity at 6 weeks and the other 2 returned to full sports activity at 3 months. There was no loss of cervical motion or trapezial strength at follow-up. All patients voiced satisfaction with the decision for surgical intervention.
Discussion
Clay-shoveler’s fracture is an injury well known to orthopedists. This fracture is thought to be caused by a forceful contraction of the thoracic paraspinal and trapezial muscles, causing an avulsion fracture with pain and frequently a “pop” experienced by the patient.1 Usually considered self-limiting injuries, treatment involves a period of rest and activity modification with occasional physical therapy. Return to sports has been reported with occasional pain but with patient satisfaction.3,5,6
Our series of patients represent a group of adolescent athletes who sustained spinous process fractures of the T1 vertebra and, despite a significant period of rest and activity modification, were unable to return to sports given their pain. The examination of these patients revealed focal tenderness at the tip of the spinous process. The diagnosis is made clinically, with radiographic studies confirming the diagnosis. In our series of patients, MRI was the original modality used to confirm injury to the area, with hyperintensity seen in the area of the supraspinous ligament and tip of the spinous process. CT confirmed the nonunion and presence of an ossicle in all patients. Surgical exposure of that area easily exposed the ununited ossicle, which was removed in all patients.
To our knowledge, this is the first report in the literature describing surgical excision of an ununited spinous process fracture in adolescent athletes. The original descriptive case series by Hall1 states “in the minds of surgeons who have seen many of these cases that early operative removal of the fragments is the proper routine treatment.” Since that original series, we have not found articles in the literature that support surgical removal; however, persistent symptoms after fracture are described.5 It is not surprising that these patients developed pain at the site of the fracture given the forces acting in that area. The trapezial and paraspinal muscles acting on that area are forceful and repetitive during activities, especially sports. All our patients had pain with attempts at activity and all had had a significant period of rest. In a recent article, this injury was described in adolescents without the patients having clear relief of symptoms despite a period of inactivity.5 While physical therapy is therapeutic in some patients experiencing pain, it can be a source of aggravation due to neck and shoulder motion and muscle contraction. It is not surprising that therapy would not help in most cases, as neck and shoulder motion and muscle contraction are the sources of continuing discomfort.
Clinical practice suggests that most patients with spinous process fractures will become pain-free; however, that is not universal. This series demonstrates that a small subset of patients with this injury will continue to have significant symptoms despite a period of rest. In those patients who desire a pain-free return to sports, we recommend consideration of surgical excision after confirmation of nonunion with radiographic studies. The inherent risks of surgical treatment are minimal with this procedure, and the benefits include return to pain-free sports activity, with the resultant physical and psychosocial benefits for adolescent athletes.
Fractures of the spinous process of the lower cervical spine or upper thoracic spine are frequently referred to as clay-shoveler’s fractures. Originally reported by Hall1 in 1940, these fractures were described in workers in Australia who dug drains in clay soil and threw the clay overhead with long shovels. Occasionally, the mud would not release from the shovel, causing excess force to be transmitted to the supraspinous ligaments and resulting in a forceful avulsion fracture of one or multiple spinous processes. The few reports following the earliest description in the literature frequently describe the mechanism of injury as being athletic in nature.2-4 The forceful contraction of the paraspinal and trapezius muscles on the supraspinous ligaments and the resultant attachment to the spinous processes make this a not uncommon injury during athletics, especially with a flexed position of the neck and shoulders. The resultant fracture or apophyseal avulsion is painful and often necessitates a visit to the physician, with plain films, computed tomography (CT) scans, or magnetic resonance imaging (MRI) confirming the diagnosis.5
Treatment of these fractures has not been well described, but frequently a period of rest followed by physical therapy will allow a return to activity. We present a series of adolescent athletes who developed nonunion of the fracture of the T1 spinous process with continued symptoms, despite rest and conservative therapy, and who underwent surgical excision of the ununited fragment.
Materials and Methods
We obtained institutional review board permission for this study and searched the surgical database between 2006 and 2013 for patients who had undergone resection of a spinous process nonunion. We collected demographic data on the patients, evaluated the radiographic studies, and reviewed operative reports and follow-up patient data.
Results
Dr. Hedequist operated on 3 patients with a spinous process nonunion over the study time period. The average age of the patients was 14 years; the location of the spinous process fracture was the T1 vertebra in all patients. Two patients sustained the injury while playing hockey and 1 during wrestling. The average duration of symptoms prior to operation was 10 months; all patients had seen physicians without a diagnosis prior to evaluation at out institution. All patients had a trial of physical therapy before surgery, and all had been unable to return to sport after injury secondary to pain.
Examination of all patients revealed pain directly over the fracture site and accentuated by forward flexion of the neck and shoulders. Evaluation of injury plain films revealed a fracture fragment in 2 patients (Figure 1). All 3 patients underwent MRI and CT scans confirming the diagnosis. MRI confirmed areas of increased signal at the tip of the T1 spinous process, with inflammation in the supraspinous ligament directly at that region (Figure 2). The CT scans confirmed the presence of a bony fragment correlating with the tip of the T1 spinous process (Figure 3).
Surgery was performed under general endotracheal anesthesia via a midline incision over the affected area down to the spinous process. The supraspinous ligament was opened revealing an easily identified and definable ununited ossicle, which was removed without taking down the interspinous ligament. All 3 nonunions were noted to be atrophic with no evidence of surrounding inflammatory tissue or bursa. The residual end of the spinous process was smoothed down with a rongeur. Standard closure was performed. There were no surgical complications.
All patients had complete relief of pain at follow-up; 1 patient returned to full sports activity at 6 weeks and the other 2 returned to full sports activity at 3 months. There was no loss of cervical motion or trapezial strength at follow-up. All patients voiced satisfaction with the decision for surgical intervention.
Discussion
Clay-shoveler’s fracture is an injury well known to orthopedists. This fracture is thought to be caused by a forceful contraction of the thoracic paraspinal and trapezial muscles, causing an avulsion fracture with pain and frequently a “pop” experienced by the patient.1 Usually considered self-limiting injuries, treatment involves a period of rest and activity modification with occasional physical therapy. Return to sports has been reported with occasional pain but with patient satisfaction.3,5,6
Our series of patients represent a group of adolescent athletes who sustained spinous process fractures of the T1 vertebra and, despite a significant period of rest and activity modification, were unable to return to sports given their pain. The examination of these patients revealed focal tenderness at the tip of the spinous process. The diagnosis is made clinically, with radiographic studies confirming the diagnosis. In our series of patients, MRI was the original modality used to confirm injury to the area, with hyperintensity seen in the area of the supraspinous ligament and tip of the spinous process. CT confirmed the nonunion and presence of an ossicle in all patients. Surgical exposure of that area easily exposed the ununited ossicle, which was removed in all patients.
To our knowledge, this is the first report in the literature describing surgical excision of an ununited spinous process fracture in adolescent athletes. The original descriptive case series by Hall1 states “in the minds of surgeons who have seen many of these cases that early operative removal of the fragments is the proper routine treatment.” Since that original series, we have not found articles in the literature that support surgical removal; however, persistent symptoms after fracture are described.5 It is not surprising that these patients developed pain at the site of the fracture given the forces acting in that area. The trapezial and paraspinal muscles acting on that area are forceful and repetitive during activities, especially sports. All our patients had pain with attempts at activity and all had had a significant period of rest. In a recent article, this injury was described in adolescents without the patients having clear relief of symptoms despite a period of inactivity.5 While physical therapy is therapeutic in some patients experiencing pain, it can be a source of aggravation due to neck and shoulder motion and muscle contraction. It is not surprising that therapy would not help in most cases, as neck and shoulder motion and muscle contraction are the sources of continuing discomfort.
Clinical practice suggests that most patients with spinous process fractures will become pain-free; however, that is not universal. This series demonstrates that a small subset of patients with this injury will continue to have significant symptoms despite a period of rest. In those patients who desire a pain-free return to sports, we recommend consideration of surgical excision after confirmation of nonunion with radiographic studies. The inherent risks of surgical treatment are minimal with this procedure, and the benefits include return to pain-free sports activity, with the resultant physical and psychosocial benefits for adolescent athletes.
1. Hall RDM. Clay-shoveler’s fracture. J Bone Joint Surg Am. 1940;22(1):63-75.
2. Herrick RT. Clay-shoveler’s fracture in power-lifting. A case report. Am J Sports Med. 1981;9(1):29-30.
3. Hetsroni I, Mann G, Dolev E, Morgenstern D, Nyska M. Clay shoveler’s fracture in a volleyball player. Phys Sportsmed. 2005;33(7):38-42.
4. Kaloostian PE, Kim JE, Calabresi PA, Bydon A, Witham T. Clay-shoveler’s fracture during indoor rock climbing. Orthopedics. 2013;36(3):e381-e383.
5. Yamaguchi KT Jr, Myung KS, Alonso MA, Skaggs DL. Clay-shoveler’s fracture equivalent in children. Spine. 2012;37(26):e1672-e1675.
6. Kang DH, Lee SH. Multiple spinous process fractures of the thoracic vertebrae (clay-shoveler’s fracture) in a beginning golfer: a case report. Spine. 2009;34(15):e534-e537.
1. Hall RDM. Clay-shoveler’s fracture. J Bone Joint Surg Am. 1940;22(1):63-75.
2. Herrick RT. Clay-shoveler’s fracture in power-lifting. A case report. Am J Sports Med. 1981;9(1):29-30.
3. Hetsroni I, Mann G, Dolev E, Morgenstern D, Nyska M. Clay shoveler’s fracture in a volleyball player. Phys Sportsmed. 2005;33(7):38-42.
4. Kaloostian PE, Kim JE, Calabresi PA, Bydon A, Witham T. Clay-shoveler’s fracture during indoor rock climbing. Orthopedics. 2013;36(3):e381-e383.
5. Yamaguchi KT Jr, Myung KS, Alonso MA, Skaggs DL. Clay-shoveler’s fracture equivalent in children. Spine. 2012;37(26):e1672-e1675.
6. Kang DH, Lee SH. Multiple spinous process fractures of the thoracic vertebrae (clay-shoveler’s fracture) in a beginning golfer: a case report. Spine. 2009;34(15):e534-e537.
Academic Characteristics of Orthopedic Team Physicians Affiliated With High School, Collegiate, and Professional Teams
The responsibilities of team physicians have increased dramatically since the early 19th century, when these physicians first appeared on the sidelines during football games.1 Although the primary role of the team physician is to care for the athlete, other responsibilities include administrative and legal duties, equipment- and environment-related duties, teaching, and communication with parents, coaches, and other physicians.2-4 These responsibilities differ greatly by the level of the athlete and the team being covered. For example, compared with high school and collegiate sport physicians, physicians caring for professional athletes may have increased interaction with the media.5
Despite the increasing demands and responsibilities of team physicians, it is important that they continue to advance the field of sports medicine through teaching and research.3,6 Team physicians have direct access to athletes at multiple levels of competition, from novice to professional, and therefore have a unique understanding of the injuries that commonly affect these athletes. Efforts to both teach and study the prevention, diagnosis, and treatment of these injuries have dramatically advanced the field of sports medicine. In fact, several advancements in sports medicine have come from team physicians, including advancements in anterior cruciate ligament reconstruction,7,8 shoulder arthroscopy,9 and “Tommy John” surgery,10 to name a few.
Given the important role of team physicians (particularly orthopedic team physicians) in advancing sports medicine, it is important to understand the degree to which team physicians at all levels of sport contribute to teaching and research.
We conducted a study to determine the overall academic involvement of orthopedic team physicians at all levels of sport, including the degree to which these physicians are affiliated with academic medical centers (by level of sport and by professional sport) and the quantity and impact of these physicians’ scientific publications. We hypothesized that orthopedic physician academic involvement would be higher at the professional level of sport than at the collegiate or high school level and that the degree of physician academic involvement would differ between professional sporting leagues.
Materials and Methods
In August 2012, we performed a comprehensive telephone- and Internet-based search to identify a sample of team physicians caring for athletes at the high school, collegiate, and professional levels of sport. Data were collected on all team physicians, regardless of medical specialty. We defined a physician as any person listed as having either a doctor of medicine (MD) or a doctor of osteopathic medicine (DO) degree. A physician listed as a team physician at 2 different levels of competition (high school, college, professional) was included in both cohorts. A physician listed as a team physician in 2 different professional sports leagues was included independently for both leagues. All other medical personnel, including athletic trainers, therapists, and nursing staff, were excluded. Data on our sample population were collected as follows:
1. High school. Performing a comprehensive database search through the US Department of Education, we generated a list of all 20,989 US schools that include grades 9 to 12.11 We then used a random number generator (random.org) to randomly select a sample of 120 high schools. These schools were contacted by telephone and asked to identify the team physician(s) for their sports teams. Twenty of these schools reported not having an athletic team, so we randomly generated a list of 20 additional high schools. High schools that had an athletic team but denied having a team physician were included in the analysis.
2. College. We used the National Collegiate Athletic Association (NCAA) website (ncaa.org) to generate a list of all colleges affiliated with the NCAA. Of these colleges, 347 were Division I, 316 were Division II, and 443 were Division III. The random.org random number generator was used to generate a list of 40 schools for each division, for a total of 120 schools. An Internet-based search was then performed to identify any and all team physicians caring for athletes at that particular school. In select cases, telephone calls were made to determine all the team physicians involved in the care of athletes at that institution.
3. Professional. Team physician data were collected for 4 of the most popular professional sporting leagues12: Major League Baseball (MLB), National Basketball Association (NBA), National Football League (NFL), and National Hockey League (NHL). Each team’s official website was identified through its league website (mlb.com, nba.com, nfl.com, nhl.com), and the roster or directory listing of all team physicians was recorded. In 2 cases, the team’s medical personnel listing could not be retrieved through the Internet, and a telephone call had to be made to identify all team physicians. Team physicians were identified for 122 professional teams: 30 MLB, 30 NBA, 32 NFL, and 30 NHL.
For this study, all physicians were classified as either orthopedic or nonorthopedic. Orthopedic surgeons—the focus of this study—were defined as those who completed residency training in orthopedic surgery. Median number of orthopedic and nonorthopedic surgeons per team was calculated at the high school, collegiate, and professional levels.
After identifying all orthopedic team physicians, we performed additional Internet searches to determine any affiliation between each physician and an applicable academic medical center. Physicians were placed in 1 of 3 different categories based on “level” of academic affiliation. Orthopedists with no identifiable connection to an academic medical center were listed under none. The first 100 search results were studied before this determination was made. Orthopedists with any academic affiliation below the level of full professorship were placed in the category associate/assistant/adjunct professor, which included any physician who was an associate professor, adjunct professor, clinical instructor, or volunteer instructor at an academic medical center. Last, orthopedists listed as full professors were placed in the professor category.
Number of publications written by each orthopedic team physician was then calculated using SciVerse Scopus (scopus.com), a comprehensive abstract and citation database of research literature that offers complete coverage of the Medline and Embase databases.13 Scopus offers a Scopus Author Identifier, which assigns each author in Scopus a unique identification number.14 This number is based on an “algorithm that matches author names based on their affiliation, address, subject area, source title, dates of publication citations, and co-authors.”14 Authors whose names did not appear in Scopus were assumed to have no publications, and this was reported after cross-referencing with Medline to ensure no documents were missed. This study included all publications: original research articles, reviews, letters, and commentaries. Any level of authorship (first, second, etc) was included. All publications were scanned, and duplicate listings were not included. Median number of publications per orthopedic team physician was calculated at the high school, college, and professional levels.
We also determined the h-index for each orthopedic team physician. The h-index is used to measure the impact of the published work of a scholar: “A scientist has index h if h of his/her papers have at least h citations each, and the other papers have no more than h citations each.”15 For example, an h-index of 12 means that, out of an author’s total number of publications, 12 have been cited at least 12 times, and all of his or her other publications have been cited fewer than 12 times. All authors in Scopus are automatically assigned h-indexes, and we collected these numbers.16 Of note, citations for articles published before 1996 are not included in the h-index calculation. Median h-index score per orthopedic team physician was calculated at the high school, college, and professional levels.
Analysis of variance was used to compare continuous data (eg, number of publications per surgeon) across different groups (eg, physicians from respective sports). Chi-square tests were used to detect whole-number differences between groups (eg, difference in number of physicians per team across the various professional sports leagues). Statistical significance was set at P < .05.
Results
We identified 1054 team physicians among the 362 total high schools, colleges, and professional sports teams included in this study. Of the 1054 physicians, 678 (64%) were orthopedic surgeons (Table 1). Seventy-two (60%) of the 120 high schools did not have a team physician, whereas all the colleges and professional teams did. Number of orthopedic surgeons per team was higher at the collegiate level (2.29; range, 0-11) and professional level (2.21; range, 1-9) than at the high school level (1.11; range, 0-24) (Table 1). Median number of nonorthopedic surgeons was highest in professional sports (1.88; range, 0-9) followed by college sports (1.06; range, 0-9) and high school sports (0.16; range, 0-2) (Table 1).
Of the 678 orthopedic team physicians, 298 (44%) were officially affiliated with an academic medical center, either as clinical instructor, associate/adjunct professor, or full professor. Percentage of orthopedists affiliated with an academic medical center was highest in professional sports (173/270, 64%) followed by collegiate sports (98/275, 36%) and high school sports (27/133, 20%) (P < .001, Table 2). Percentage of orthopedists identified as full professors was highest at the professional level (42/270, 16%) followed by the collegiate level (14/275, 5.1%) and the high school level (3/133, 2.3%) (P < .001, Table 2).
We found 12,036 publications written by the 678 orthopedic team physicians included in this study. Median number of publications per orthopedist was significantly higher in professional sports (30.6; range, 0-460) than in collegiate sports (10.7; range, 0-581) and high school sports (6.0; range, 0-220) (P < .001). Number of authors with more than 25 publications was highest at the professional level (82) followed by the collegiate level (27) and the high school level (7) (Table 3). Median number of publications per orthopedist was also higher at the professional level (12) than at the collegiate level (2) and high school level (1). Median h-index was higher among orthopedists in professional sports (7.1; range, 0-50) than at colleges (2.7; range, 0-63) and high schools (1.8; range, 0-32) (P < .001). Median h-index was also significantly higher at the professional level (5) than at the collegiate level (1) and high school level (0).
At the professional level of sports, we identified 499 team physicians (270 orthopedic, 54%; 229 nonorthopedic, 46%). Median number of orthopedic team physicians varied by sport, with MLB (2.8; range, 1-8) and the NFL (2.4; range, 1-4) having relatively more of these physicians than the NHL (2.0; range, 1-6) and the NBA (1.7; range, 1-9) (Table 4). Percentage of orthopedic team physicians affiliated with academic medical centers was highest in MLB (58/83, 69.9%) followed by the NFL (47/76, 61.8%), the NHL (37/60, 61.7%), and the NBA (31/51, 60.8%) (Table 5). Median number of publications by orthopedists also varied by sport, with the highest number in MLB (37.9; range, 0-225) followed by the NBA (32.0; range, 0-227) and the NFL (30.4; range, 0-460), with the lowest number in the NHL (20.7; range, 0-144) (Table 6). Median number of publications was the same (17.5) in MLB and the NFL and lower in the NBA (11) and the NHL (7.5). Median h-index was highest in the NFL (8.2; range, 0-50) and MLB (7.9; range, 0-32) followed by the NBA (6.6; range, 0-35) and the NHL (4.9; range, 0-20) (Table 7) Median h-index was the same (6) in MLB and the NFL and lower (3) in the NBA and the NHL.
Discussion
To our knowledge, this is the first study of academic involvement and the research activities of orthopedic team physicians at the high school, college, and professional levels of sport. We found that, on average, there were almost twice as many orthopedists at the collegiate and professional levels than at the high school level—likely because 72 of the 120 high schools randomly selected did not have a team physician, despite having sports teams. We can attribute this to the organizational structure of teams in a high school setting, where it is fairly common that no medically educated health care provider is readily available for the student athletes.5 Although the median number of orthopedists was similar at the collegiate and professional levels, the number of nonorthopedic team physicians was higher at the professional level than at the collegiate level. Although most collegiate and professional teams have an internist and an orthopedist on staff, medical staff at the professional level may also include several subspecialists from a variety of medical fields (eg, dental medicine, ophthalmology, neurology).17
We found that a significantly larger proportion of orthopedists at the professional level (64%) were affiliated with academic medical centers as associate/adjunct professors and full professors compared with orthopedists at the collegiate level (36%) and high school level (20%). The academic relationship with collegiate teams was much lower than expected. Regarding professional sports, however, this finding confirmed our hypothesis, and the explanation is likely multifactorial and historical. Moreover, the median number of publications was higher for orthopedists at the professional level (30.8) than at the collegiate level (10.7) and high school level (6). In the late 1940s and early 1950s, many orthopedic team physicians entered into contracts with major universities.4 For many physicians, this contractual relationship increased their prestige, and some orthopedic groups were alleged to have endorsed scholarships at those schools.4 Given the high level of publicity and scrutiny surrounding medical decisions at the professional level of sports, it is possible that professional sports teams specifically seek orthopedists who are well respected within academia. Moreover, contracts between universities/academic medical centers and professional teams may mandate that a faculty member from that organization provide the orthopedic/medical care for the team. This may also increase the likelihood of professional teams being paired with academic orthopedic physicians. However, such contractual agreements are made between professional teams and large private medical groups as well.
In addition to measuring quantity of publications, we used the h-index to measure their quality. Following the same pattern as the publication rate, median h-index per orthopedic team physician was significantly higher at the professional level (7.1) than at the collegiate level (2.7) and high school level (1.8). As with publication volume, this is not entirely surprising, as h-index has been shown to correlate with academic rank in other surgical specialties,18 and there was a higher percentage of academic physicians at the professional level than at the collegiate and high school levels.
At the professional level of sports, 56% of all team physicians were orthopedic surgeons. Orthopedists caring for MLB teams had the highest median number of publications (37.9), followed by the NBA (32.0), the NFL (30.4), and the NHL (20.7). One likely explanation is the higher percentage of MLB physicians affiliated with academic medical centers. Regarding the h-index, MLB and NFL physicians had the highest values (7.9 and 8.2, respectively).
Our study had several limitations. First, we may not have captured data on all the team physicians at the high school, college, and professional levels. By following a detailed protocol in identifying surgeons, however, we tried to minimize the impact of any such omissions. In addition, teams may have had many unofficial consultants acting as team physicians, whether orthopedic or nonorthopedic, and, if these physicians were not listed in an official capacity, they may have been omitted from this study. We further realize that a true measure of academic productivity should also include book chapters and books published, research grants awarded, and patents registered. By including only peer-reviewed articles, we omitted these other criteria.
To our knowledge, the data presented here represent the first attempt to quantify the academic involvement and research productivity of orthopedic team physicians at the high school, college, and professional levels of sport. These data help us understand how research productivity varies by orthopedic team physicians at different levels of sport and may be useful to those considering a career as a team physician, as they can better evaluate their own productivity in the context of team physicians across different levels of competition.
1. Thorndike A. Athletic Injuries: Prevention, Diagnosis, and Treatment. Philadelphia, PA: Lea & Febiger; 1956.
2. The team physician. A statement of the Committee on the Medical Aspects of Sports of the American Medical Association, September 1967. J School Health. 1967;37(10):510-514.
3. Team physician consensus statement. Am J Sports Med. 2000;28(3):440-441.
4. Whiteside J, Andrews JR. Trends for the future as a team physician: Herodicus to hereafter. Clin Sports Med. 2007;26(2):285-304.
5. Goforth M, Almquist J, Matney M, et al. Understanding organization structures of the college, university, high school, clinical, and professional settings. Clin Sports Med. 2007;26(2):201-226.
6. Hughston JC. Want to be in sports medicine? Get involved. Am J Sports Med. 1979;7(2):79-80.
7. Marshall JL, Warren RF, Wickiewicz TL, Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;(143):97-106.
8. Clancy WG Jr, Nelson DA, Reider B, Narechania RG. Anterior cruciate ligament reconstruction using one-third of the patellar ligament, augmented by extra-articular tendon transfers. J Bone Joint Surg Am. 1982;64(3):352-359.
9. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
10. Indelicato PA, Jobe FW, Kerlan RK, Carter VS, Shields CL, Lombardo SJ. Correctable elbow lesions in professional baseball players: a review of 25 cases. Am J Sports Med. 1979;7(1):72-75.
11. Elementary/Secondary Information System (EISi). National Center for Education Statistics, Institute of Education Sciences, US Department of Education website. http://nces.ed.gov/ccd/elsi/. Accessed September 21, 2015.
12. Corso RA; Harris Interactive. Football is America’s favorite sport as lead over baseball continues to grow; college football and auto racing come next. Harris Interactive website. http://www.harrisinteractive.com/vault/Harris Poll 9 - Favorite sport_1.25.12.pdf. Harris Poll 9, January 25, 2012. Accessed September 21, 2015.
13. [Scopus content]. Elsevier website. http://www.elsevier.com/solutions/scopus/content. Accessed September 21, 2015.
14. Scopus Author Identifier. Scopus website. http://help.scopus.com/Content/h_autsrch_intro.htm. Accessed October 5, 2015.
15. Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572.
16. Author Evaluator h Index Tab. Scopus website. http://help.scopus.com/Content/h_auteval_hindex.htm. Accessed October 5, 2015.
17. Boyd JL. Understanding the politics of being a team physician. Clin Sports Med. 2007;26(2):161-172.
18. Lee J, Kraus KL, Couldwell WT. Use of the h index in neurosurgery. Clinical article. J Neurosurg. 2009;111(2):387-
The responsibilities of team physicians have increased dramatically since the early 19th century, when these physicians first appeared on the sidelines during football games.1 Although the primary role of the team physician is to care for the athlete, other responsibilities include administrative and legal duties, equipment- and environment-related duties, teaching, and communication with parents, coaches, and other physicians.2-4 These responsibilities differ greatly by the level of the athlete and the team being covered. For example, compared with high school and collegiate sport physicians, physicians caring for professional athletes may have increased interaction with the media.5
Despite the increasing demands and responsibilities of team physicians, it is important that they continue to advance the field of sports medicine through teaching and research.3,6 Team physicians have direct access to athletes at multiple levels of competition, from novice to professional, and therefore have a unique understanding of the injuries that commonly affect these athletes. Efforts to both teach and study the prevention, diagnosis, and treatment of these injuries have dramatically advanced the field of sports medicine. In fact, several advancements in sports medicine have come from team physicians, including advancements in anterior cruciate ligament reconstruction,7,8 shoulder arthroscopy,9 and “Tommy John” surgery,10 to name a few.
Given the important role of team physicians (particularly orthopedic team physicians) in advancing sports medicine, it is important to understand the degree to which team physicians at all levels of sport contribute to teaching and research.
We conducted a study to determine the overall academic involvement of orthopedic team physicians at all levels of sport, including the degree to which these physicians are affiliated with academic medical centers (by level of sport and by professional sport) and the quantity and impact of these physicians’ scientific publications. We hypothesized that orthopedic physician academic involvement would be higher at the professional level of sport than at the collegiate or high school level and that the degree of physician academic involvement would differ between professional sporting leagues.
Materials and Methods
In August 2012, we performed a comprehensive telephone- and Internet-based search to identify a sample of team physicians caring for athletes at the high school, collegiate, and professional levels of sport. Data were collected on all team physicians, regardless of medical specialty. We defined a physician as any person listed as having either a doctor of medicine (MD) or a doctor of osteopathic medicine (DO) degree. A physician listed as a team physician at 2 different levels of competition (high school, college, professional) was included in both cohorts. A physician listed as a team physician in 2 different professional sports leagues was included independently for both leagues. All other medical personnel, including athletic trainers, therapists, and nursing staff, were excluded. Data on our sample population were collected as follows:
1. High school. Performing a comprehensive database search through the US Department of Education, we generated a list of all 20,989 US schools that include grades 9 to 12.11 We then used a random number generator (random.org) to randomly select a sample of 120 high schools. These schools were contacted by telephone and asked to identify the team physician(s) for their sports teams. Twenty of these schools reported not having an athletic team, so we randomly generated a list of 20 additional high schools. High schools that had an athletic team but denied having a team physician were included in the analysis.
2. College. We used the National Collegiate Athletic Association (NCAA) website (ncaa.org) to generate a list of all colleges affiliated with the NCAA. Of these colleges, 347 were Division I, 316 were Division II, and 443 were Division III. The random.org random number generator was used to generate a list of 40 schools for each division, for a total of 120 schools. An Internet-based search was then performed to identify any and all team physicians caring for athletes at that particular school. In select cases, telephone calls were made to determine all the team physicians involved in the care of athletes at that institution.
3. Professional. Team physician data were collected for 4 of the most popular professional sporting leagues12: Major League Baseball (MLB), National Basketball Association (NBA), National Football League (NFL), and National Hockey League (NHL). Each team’s official website was identified through its league website (mlb.com, nba.com, nfl.com, nhl.com), and the roster or directory listing of all team physicians was recorded. In 2 cases, the team’s medical personnel listing could not be retrieved through the Internet, and a telephone call had to be made to identify all team physicians. Team physicians were identified for 122 professional teams: 30 MLB, 30 NBA, 32 NFL, and 30 NHL.
For this study, all physicians were classified as either orthopedic or nonorthopedic. Orthopedic surgeons—the focus of this study—were defined as those who completed residency training in orthopedic surgery. Median number of orthopedic and nonorthopedic surgeons per team was calculated at the high school, collegiate, and professional levels.
After identifying all orthopedic team physicians, we performed additional Internet searches to determine any affiliation between each physician and an applicable academic medical center. Physicians were placed in 1 of 3 different categories based on “level” of academic affiliation. Orthopedists with no identifiable connection to an academic medical center were listed under none. The first 100 search results were studied before this determination was made. Orthopedists with any academic affiliation below the level of full professorship were placed in the category associate/assistant/adjunct professor, which included any physician who was an associate professor, adjunct professor, clinical instructor, or volunteer instructor at an academic medical center. Last, orthopedists listed as full professors were placed in the professor category.
Number of publications written by each orthopedic team physician was then calculated using SciVerse Scopus (scopus.com), a comprehensive abstract and citation database of research literature that offers complete coverage of the Medline and Embase databases.13 Scopus offers a Scopus Author Identifier, which assigns each author in Scopus a unique identification number.14 This number is based on an “algorithm that matches author names based on their affiliation, address, subject area, source title, dates of publication citations, and co-authors.”14 Authors whose names did not appear in Scopus were assumed to have no publications, and this was reported after cross-referencing with Medline to ensure no documents were missed. This study included all publications: original research articles, reviews, letters, and commentaries. Any level of authorship (first, second, etc) was included. All publications were scanned, and duplicate listings were not included. Median number of publications per orthopedic team physician was calculated at the high school, college, and professional levels.
We also determined the h-index for each orthopedic team physician. The h-index is used to measure the impact of the published work of a scholar: “A scientist has index h if h of his/her papers have at least h citations each, and the other papers have no more than h citations each.”15 For example, an h-index of 12 means that, out of an author’s total number of publications, 12 have been cited at least 12 times, and all of his or her other publications have been cited fewer than 12 times. All authors in Scopus are automatically assigned h-indexes, and we collected these numbers.16 Of note, citations for articles published before 1996 are not included in the h-index calculation. Median h-index score per orthopedic team physician was calculated at the high school, college, and professional levels.
Analysis of variance was used to compare continuous data (eg, number of publications per surgeon) across different groups (eg, physicians from respective sports). Chi-square tests were used to detect whole-number differences between groups (eg, difference in number of physicians per team across the various professional sports leagues). Statistical significance was set at P < .05.
Results
We identified 1054 team physicians among the 362 total high schools, colleges, and professional sports teams included in this study. Of the 1054 physicians, 678 (64%) were orthopedic surgeons (Table 1). Seventy-two (60%) of the 120 high schools did not have a team physician, whereas all the colleges and professional teams did. Number of orthopedic surgeons per team was higher at the collegiate level (2.29; range, 0-11) and professional level (2.21; range, 1-9) than at the high school level (1.11; range, 0-24) (Table 1). Median number of nonorthopedic surgeons was highest in professional sports (1.88; range, 0-9) followed by college sports (1.06; range, 0-9) and high school sports (0.16; range, 0-2) (Table 1).
Of the 678 orthopedic team physicians, 298 (44%) were officially affiliated with an academic medical center, either as clinical instructor, associate/adjunct professor, or full professor. Percentage of orthopedists affiliated with an academic medical center was highest in professional sports (173/270, 64%) followed by collegiate sports (98/275, 36%) and high school sports (27/133, 20%) (P < .001, Table 2). Percentage of orthopedists identified as full professors was highest at the professional level (42/270, 16%) followed by the collegiate level (14/275, 5.1%) and the high school level (3/133, 2.3%) (P < .001, Table 2).
We found 12,036 publications written by the 678 orthopedic team physicians included in this study. Median number of publications per orthopedist was significantly higher in professional sports (30.6; range, 0-460) than in collegiate sports (10.7; range, 0-581) and high school sports (6.0; range, 0-220) (P < .001). Number of authors with more than 25 publications was highest at the professional level (82) followed by the collegiate level (27) and the high school level (7) (Table 3). Median number of publications per orthopedist was also higher at the professional level (12) than at the collegiate level (2) and high school level (1). Median h-index was higher among orthopedists in professional sports (7.1; range, 0-50) than at colleges (2.7; range, 0-63) and high schools (1.8; range, 0-32) (P < .001). Median h-index was also significantly higher at the professional level (5) than at the collegiate level (1) and high school level (0).
At the professional level of sports, we identified 499 team physicians (270 orthopedic, 54%; 229 nonorthopedic, 46%). Median number of orthopedic team physicians varied by sport, with MLB (2.8; range, 1-8) and the NFL (2.4; range, 1-4) having relatively more of these physicians than the NHL (2.0; range, 1-6) and the NBA (1.7; range, 1-9) (Table 4). Percentage of orthopedic team physicians affiliated with academic medical centers was highest in MLB (58/83, 69.9%) followed by the NFL (47/76, 61.8%), the NHL (37/60, 61.7%), and the NBA (31/51, 60.8%) (Table 5). Median number of publications by orthopedists also varied by sport, with the highest number in MLB (37.9; range, 0-225) followed by the NBA (32.0; range, 0-227) and the NFL (30.4; range, 0-460), with the lowest number in the NHL (20.7; range, 0-144) (Table 6). Median number of publications was the same (17.5) in MLB and the NFL and lower in the NBA (11) and the NHL (7.5). Median h-index was highest in the NFL (8.2; range, 0-50) and MLB (7.9; range, 0-32) followed by the NBA (6.6; range, 0-35) and the NHL (4.9; range, 0-20) (Table 7) Median h-index was the same (6) in MLB and the NFL and lower (3) in the NBA and the NHL.
Discussion
To our knowledge, this is the first study of academic involvement and the research activities of orthopedic team physicians at the high school, college, and professional levels of sport. We found that, on average, there were almost twice as many orthopedists at the collegiate and professional levels than at the high school level—likely because 72 of the 120 high schools randomly selected did not have a team physician, despite having sports teams. We can attribute this to the organizational structure of teams in a high school setting, where it is fairly common that no medically educated health care provider is readily available for the student athletes.5 Although the median number of orthopedists was similar at the collegiate and professional levels, the number of nonorthopedic team physicians was higher at the professional level than at the collegiate level. Although most collegiate and professional teams have an internist and an orthopedist on staff, medical staff at the professional level may also include several subspecialists from a variety of medical fields (eg, dental medicine, ophthalmology, neurology).17
We found that a significantly larger proportion of orthopedists at the professional level (64%) were affiliated with academic medical centers as associate/adjunct professors and full professors compared with orthopedists at the collegiate level (36%) and high school level (20%). The academic relationship with collegiate teams was much lower than expected. Regarding professional sports, however, this finding confirmed our hypothesis, and the explanation is likely multifactorial and historical. Moreover, the median number of publications was higher for orthopedists at the professional level (30.8) than at the collegiate level (10.7) and high school level (6). In the late 1940s and early 1950s, many orthopedic team physicians entered into contracts with major universities.4 For many physicians, this contractual relationship increased their prestige, and some orthopedic groups were alleged to have endorsed scholarships at those schools.4 Given the high level of publicity and scrutiny surrounding medical decisions at the professional level of sports, it is possible that professional sports teams specifically seek orthopedists who are well respected within academia. Moreover, contracts between universities/academic medical centers and professional teams may mandate that a faculty member from that organization provide the orthopedic/medical care for the team. This may also increase the likelihood of professional teams being paired with academic orthopedic physicians. However, such contractual agreements are made between professional teams and large private medical groups as well.
In addition to measuring quantity of publications, we used the h-index to measure their quality. Following the same pattern as the publication rate, median h-index per orthopedic team physician was significantly higher at the professional level (7.1) than at the collegiate level (2.7) and high school level (1.8). As with publication volume, this is not entirely surprising, as h-index has been shown to correlate with academic rank in other surgical specialties,18 and there was a higher percentage of academic physicians at the professional level than at the collegiate and high school levels.
At the professional level of sports, 56% of all team physicians were orthopedic surgeons. Orthopedists caring for MLB teams had the highest median number of publications (37.9), followed by the NBA (32.0), the NFL (30.4), and the NHL (20.7). One likely explanation is the higher percentage of MLB physicians affiliated with academic medical centers. Regarding the h-index, MLB and NFL physicians had the highest values (7.9 and 8.2, respectively).
Our study had several limitations. First, we may not have captured data on all the team physicians at the high school, college, and professional levels. By following a detailed protocol in identifying surgeons, however, we tried to minimize the impact of any such omissions. In addition, teams may have had many unofficial consultants acting as team physicians, whether orthopedic or nonorthopedic, and, if these physicians were not listed in an official capacity, they may have been omitted from this study. We further realize that a true measure of academic productivity should also include book chapters and books published, research grants awarded, and patents registered. By including only peer-reviewed articles, we omitted these other criteria.
To our knowledge, the data presented here represent the first attempt to quantify the academic involvement and research productivity of orthopedic team physicians at the high school, college, and professional levels of sport. These data help us understand how research productivity varies by orthopedic team physicians at different levels of sport and may be useful to those considering a career as a team physician, as they can better evaluate their own productivity in the context of team physicians across different levels of competition.
The responsibilities of team physicians have increased dramatically since the early 19th century, when these physicians first appeared on the sidelines during football games.1 Although the primary role of the team physician is to care for the athlete, other responsibilities include administrative and legal duties, equipment- and environment-related duties, teaching, and communication with parents, coaches, and other physicians.2-4 These responsibilities differ greatly by the level of the athlete and the team being covered. For example, compared with high school and collegiate sport physicians, physicians caring for professional athletes may have increased interaction with the media.5
Despite the increasing demands and responsibilities of team physicians, it is important that they continue to advance the field of sports medicine through teaching and research.3,6 Team physicians have direct access to athletes at multiple levels of competition, from novice to professional, and therefore have a unique understanding of the injuries that commonly affect these athletes. Efforts to both teach and study the prevention, diagnosis, and treatment of these injuries have dramatically advanced the field of sports medicine. In fact, several advancements in sports medicine have come from team physicians, including advancements in anterior cruciate ligament reconstruction,7,8 shoulder arthroscopy,9 and “Tommy John” surgery,10 to name a few.
Given the important role of team physicians (particularly orthopedic team physicians) in advancing sports medicine, it is important to understand the degree to which team physicians at all levels of sport contribute to teaching and research.
We conducted a study to determine the overall academic involvement of orthopedic team physicians at all levels of sport, including the degree to which these physicians are affiliated with academic medical centers (by level of sport and by professional sport) and the quantity and impact of these physicians’ scientific publications. We hypothesized that orthopedic physician academic involvement would be higher at the professional level of sport than at the collegiate or high school level and that the degree of physician academic involvement would differ between professional sporting leagues.
Materials and Methods
In August 2012, we performed a comprehensive telephone- and Internet-based search to identify a sample of team physicians caring for athletes at the high school, collegiate, and professional levels of sport. Data were collected on all team physicians, regardless of medical specialty. We defined a physician as any person listed as having either a doctor of medicine (MD) or a doctor of osteopathic medicine (DO) degree. A physician listed as a team physician at 2 different levels of competition (high school, college, professional) was included in both cohorts. A physician listed as a team physician in 2 different professional sports leagues was included independently for both leagues. All other medical personnel, including athletic trainers, therapists, and nursing staff, were excluded. Data on our sample population were collected as follows:
1. High school. Performing a comprehensive database search through the US Department of Education, we generated a list of all 20,989 US schools that include grades 9 to 12.11 We then used a random number generator (random.org) to randomly select a sample of 120 high schools. These schools were contacted by telephone and asked to identify the team physician(s) for their sports teams. Twenty of these schools reported not having an athletic team, so we randomly generated a list of 20 additional high schools. High schools that had an athletic team but denied having a team physician were included in the analysis.
2. College. We used the National Collegiate Athletic Association (NCAA) website (ncaa.org) to generate a list of all colleges affiliated with the NCAA. Of these colleges, 347 were Division I, 316 were Division II, and 443 were Division III. The random.org random number generator was used to generate a list of 40 schools for each division, for a total of 120 schools. An Internet-based search was then performed to identify any and all team physicians caring for athletes at that particular school. In select cases, telephone calls were made to determine all the team physicians involved in the care of athletes at that institution.
3. Professional. Team physician data were collected for 4 of the most popular professional sporting leagues12: Major League Baseball (MLB), National Basketball Association (NBA), National Football League (NFL), and National Hockey League (NHL). Each team’s official website was identified through its league website (mlb.com, nba.com, nfl.com, nhl.com), and the roster or directory listing of all team physicians was recorded. In 2 cases, the team’s medical personnel listing could not be retrieved through the Internet, and a telephone call had to be made to identify all team physicians. Team physicians were identified for 122 professional teams: 30 MLB, 30 NBA, 32 NFL, and 30 NHL.
For this study, all physicians were classified as either orthopedic or nonorthopedic. Orthopedic surgeons—the focus of this study—were defined as those who completed residency training in orthopedic surgery. Median number of orthopedic and nonorthopedic surgeons per team was calculated at the high school, collegiate, and professional levels.
After identifying all orthopedic team physicians, we performed additional Internet searches to determine any affiliation between each physician and an applicable academic medical center. Physicians were placed in 1 of 3 different categories based on “level” of academic affiliation. Orthopedists with no identifiable connection to an academic medical center were listed under none. The first 100 search results were studied before this determination was made. Orthopedists with any academic affiliation below the level of full professorship were placed in the category associate/assistant/adjunct professor, which included any physician who was an associate professor, adjunct professor, clinical instructor, or volunteer instructor at an academic medical center. Last, orthopedists listed as full professors were placed in the professor category.
Number of publications written by each orthopedic team physician was then calculated using SciVerse Scopus (scopus.com), a comprehensive abstract and citation database of research literature that offers complete coverage of the Medline and Embase databases.13 Scopus offers a Scopus Author Identifier, which assigns each author in Scopus a unique identification number.14 This number is based on an “algorithm that matches author names based on their affiliation, address, subject area, source title, dates of publication citations, and co-authors.”14 Authors whose names did not appear in Scopus were assumed to have no publications, and this was reported after cross-referencing with Medline to ensure no documents were missed. This study included all publications: original research articles, reviews, letters, and commentaries. Any level of authorship (first, second, etc) was included. All publications were scanned, and duplicate listings were not included. Median number of publications per orthopedic team physician was calculated at the high school, college, and professional levels.
We also determined the h-index for each orthopedic team physician. The h-index is used to measure the impact of the published work of a scholar: “A scientist has index h if h of his/her papers have at least h citations each, and the other papers have no more than h citations each.”15 For example, an h-index of 12 means that, out of an author’s total number of publications, 12 have been cited at least 12 times, and all of his or her other publications have been cited fewer than 12 times. All authors in Scopus are automatically assigned h-indexes, and we collected these numbers.16 Of note, citations for articles published before 1996 are not included in the h-index calculation. Median h-index score per orthopedic team physician was calculated at the high school, college, and professional levels.
Analysis of variance was used to compare continuous data (eg, number of publications per surgeon) across different groups (eg, physicians from respective sports). Chi-square tests were used to detect whole-number differences between groups (eg, difference in number of physicians per team across the various professional sports leagues). Statistical significance was set at P < .05.
Results
We identified 1054 team physicians among the 362 total high schools, colleges, and professional sports teams included in this study. Of the 1054 physicians, 678 (64%) were orthopedic surgeons (Table 1). Seventy-two (60%) of the 120 high schools did not have a team physician, whereas all the colleges and professional teams did. Number of orthopedic surgeons per team was higher at the collegiate level (2.29; range, 0-11) and professional level (2.21; range, 1-9) than at the high school level (1.11; range, 0-24) (Table 1). Median number of nonorthopedic surgeons was highest in professional sports (1.88; range, 0-9) followed by college sports (1.06; range, 0-9) and high school sports (0.16; range, 0-2) (Table 1).
Of the 678 orthopedic team physicians, 298 (44%) were officially affiliated with an academic medical center, either as clinical instructor, associate/adjunct professor, or full professor. Percentage of orthopedists affiliated with an academic medical center was highest in professional sports (173/270, 64%) followed by collegiate sports (98/275, 36%) and high school sports (27/133, 20%) (P < .001, Table 2). Percentage of orthopedists identified as full professors was highest at the professional level (42/270, 16%) followed by the collegiate level (14/275, 5.1%) and the high school level (3/133, 2.3%) (P < .001, Table 2).
We found 12,036 publications written by the 678 orthopedic team physicians included in this study. Median number of publications per orthopedist was significantly higher in professional sports (30.6; range, 0-460) than in collegiate sports (10.7; range, 0-581) and high school sports (6.0; range, 0-220) (P < .001). Number of authors with more than 25 publications was highest at the professional level (82) followed by the collegiate level (27) and the high school level (7) (Table 3). Median number of publications per orthopedist was also higher at the professional level (12) than at the collegiate level (2) and high school level (1). Median h-index was higher among orthopedists in professional sports (7.1; range, 0-50) than at colleges (2.7; range, 0-63) and high schools (1.8; range, 0-32) (P < .001). Median h-index was also significantly higher at the professional level (5) than at the collegiate level (1) and high school level (0).
At the professional level of sports, we identified 499 team physicians (270 orthopedic, 54%; 229 nonorthopedic, 46%). Median number of orthopedic team physicians varied by sport, with MLB (2.8; range, 1-8) and the NFL (2.4; range, 1-4) having relatively more of these physicians than the NHL (2.0; range, 1-6) and the NBA (1.7; range, 1-9) (Table 4). Percentage of orthopedic team physicians affiliated with academic medical centers was highest in MLB (58/83, 69.9%) followed by the NFL (47/76, 61.8%), the NHL (37/60, 61.7%), and the NBA (31/51, 60.8%) (Table 5). Median number of publications by orthopedists also varied by sport, with the highest number in MLB (37.9; range, 0-225) followed by the NBA (32.0; range, 0-227) and the NFL (30.4; range, 0-460), with the lowest number in the NHL (20.7; range, 0-144) (Table 6). Median number of publications was the same (17.5) in MLB and the NFL and lower in the NBA (11) and the NHL (7.5). Median h-index was highest in the NFL (8.2; range, 0-50) and MLB (7.9; range, 0-32) followed by the NBA (6.6; range, 0-35) and the NHL (4.9; range, 0-20) (Table 7) Median h-index was the same (6) in MLB and the NFL and lower (3) in the NBA and the NHL.
Discussion
To our knowledge, this is the first study of academic involvement and the research activities of orthopedic team physicians at the high school, college, and professional levels of sport. We found that, on average, there were almost twice as many orthopedists at the collegiate and professional levels than at the high school level—likely because 72 of the 120 high schools randomly selected did not have a team physician, despite having sports teams. We can attribute this to the organizational structure of teams in a high school setting, where it is fairly common that no medically educated health care provider is readily available for the student athletes.5 Although the median number of orthopedists was similar at the collegiate and professional levels, the number of nonorthopedic team physicians was higher at the professional level than at the collegiate level. Although most collegiate and professional teams have an internist and an orthopedist on staff, medical staff at the professional level may also include several subspecialists from a variety of medical fields (eg, dental medicine, ophthalmology, neurology).17
We found that a significantly larger proportion of orthopedists at the professional level (64%) were affiliated with academic medical centers as associate/adjunct professors and full professors compared with orthopedists at the collegiate level (36%) and high school level (20%). The academic relationship with collegiate teams was much lower than expected. Regarding professional sports, however, this finding confirmed our hypothesis, and the explanation is likely multifactorial and historical. Moreover, the median number of publications was higher for orthopedists at the professional level (30.8) than at the collegiate level (10.7) and high school level (6). In the late 1940s and early 1950s, many orthopedic team physicians entered into contracts with major universities.4 For many physicians, this contractual relationship increased their prestige, and some orthopedic groups were alleged to have endorsed scholarships at those schools.4 Given the high level of publicity and scrutiny surrounding medical decisions at the professional level of sports, it is possible that professional sports teams specifically seek orthopedists who are well respected within academia. Moreover, contracts between universities/academic medical centers and professional teams may mandate that a faculty member from that organization provide the orthopedic/medical care for the team. This may also increase the likelihood of professional teams being paired with academic orthopedic physicians. However, such contractual agreements are made between professional teams and large private medical groups as well.
In addition to measuring quantity of publications, we used the h-index to measure their quality. Following the same pattern as the publication rate, median h-index per orthopedic team physician was significantly higher at the professional level (7.1) than at the collegiate level (2.7) and high school level (1.8). As with publication volume, this is not entirely surprising, as h-index has been shown to correlate with academic rank in other surgical specialties,18 and there was a higher percentage of academic physicians at the professional level than at the collegiate and high school levels.
At the professional level of sports, 56% of all team physicians were orthopedic surgeons. Orthopedists caring for MLB teams had the highest median number of publications (37.9), followed by the NBA (32.0), the NFL (30.4), and the NHL (20.7). One likely explanation is the higher percentage of MLB physicians affiliated with academic medical centers. Regarding the h-index, MLB and NFL physicians had the highest values (7.9 and 8.2, respectively).
Our study had several limitations. First, we may not have captured data on all the team physicians at the high school, college, and professional levels. By following a detailed protocol in identifying surgeons, however, we tried to minimize the impact of any such omissions. In addition, teams may have had many unofficial consultants acting as team physicians, whether orthopedic or nonorthopedic, and, if these physicians were not listed in an official capacity, they may have been omitted from this study. We further realize that a true measure of academic productivity should also include book chapters and books published, research grants awarded, and patents registered. By including only peer-reviewed articles, we omitted these other criteria.
To our knowledge, the data presented here represent the first attempt to quantify the academic involvement and research productivity of orthopedic team physicians at the high school, college, and professional levels of sport. These data help us understand how research productivity varies by orthopedic team physicians at different levels of sport and may be useful to those considering a career as a team physician, as they can better evaluate their own productivity in the context of team physicians across different levels of competition.
1. Thorndike A. Athletic Injuries: Prevention, Diagnosis, and Treatment. Philadelphia, PA: Lea & Febiger; 1956.
2. The team physician. A statement of the Committee on the Medical Aspects of Sports of the American Medical Association, September 1967. J School Health. 1967;37(10):510-514.
3. Team physician consensus statement. Am J Sports Med. 2000;28(3):440-441.
4. Whiteside J, Andrews JR. Trends for the future as a team physician: Herodicus to hereafter. Clin Sports Med. 2007;26(2):285-304.
5. Goforth M, Almquist J, Matney M, et al. Understanding organization structures of the college, university, high school, clinical, and professional settings. Clin Sports Med. 2007;26(2):201-226.
6. Hughston JC. Want to be in sports medicine? Get involved. Am J Sports Med. 1979;7(2):79-80.
7. Marshall JL, Warren RF, Wickiewicz TL, Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;(143):97-106.
8. Clancy WG Jr, Nelson DA, Reider B, Narechania RG. Anterior cruciate ligament reconstruction using one-third of the patellar ligament, augmented by extra-articular tendon transfers. J Bone Joint Surg Am. 1982;64(3):352-359.
9. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
10. Indelicato PA, Jobe FW, Kerlan RK, Carter VS, Shields CL, Lombardo SJ. Correctable elbow lesions in professional baseball players: a review of 25 cases. Am J Sports Med. 1979;7(1):72-75.
11. Elementary/Secondary Information System (EISi). National Center for Education Statistics, Institute of Education Sciences, US Department of Education website. http://nces.ed.gov/ccd/elsi/. Accessed September 21, 2015.
12. Corso RA; Harris Interactive. Football is America’s favorite sport as lead over baseball continues to grow; college football and auto racing come next. Harris Interactive website. http://www.harrisinteractive.com/vault/Harris Poll 9 - Favorite sport_1.25.12.pdf. Harris Poll 9, January 25, 2012. Accessed September 21, 2015.
13. [Scopus content]. Elsevier website. http://www.elsevier.com/solutions/scopus/content. Accessed September 21, 2015.
14. Scopus Author Identifier. Scopus website. http://help.scopus.com/Content/h_autsrch_intro.htm. Accessed October 5, 2015.
15. Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572.
16. Author Evaluator h Index Tab. Scopus website. http://help.scopus.com/Content/h_auteval_hindex.htm. Accessed October 5, 2015.
17. Boyd JL. Understanding the politics of being a team physician. Clin Sports Med. 2007;26(2):161-172.
18. Lee J, Kraus KL, Couldwell WT. Use of the h index in neurosurgery. Clinical article. J Neurosurg. 2009;111(2):387-
1. Thorndike A. Athletic Injuries: Prevention, Diagnosis, and Treatment. Philadelphia, PA: Lea & Febiger; 1956.
2. The team physician. A statement of the Committee on the Medical Aspects of Sports of the American Medical Association, September 1967. J School Health. 1967;37(10):510-514.
3. Team physician consensus statement. Am J Sports Med. 2000;28(3):440-441.
4. Whiteside J, Andrews JR. Trends for the future as a team physician: Herodicus to hereafter. Clin Sports Med. 2007;26(2):285-304.
5. Goforth M, Almquist J, Matney M, et al. Understanding organization structures of the college, university, high school, clinical, and professional settings. Clin Sports Med. 2007;26(2):201-226.
6. Hughston JC. Want to be in sports medicine? Get involved. Am J Sports Med. 1979;7(2):79-80.
7. Marshall JL, Warren RF, Wickiewicz TL, Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;(143):97-106.
8. Clancy WG Jr, Nelson DA, Reider B, Narechania RG. Anterior cruciate ligament reconstruction using one-third of the patellar ligament, augmented by extra-articular tendon transfers. J Bone Joint Surg Am. 1982;64(3):352-359.
9. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
10. Indelicato PA, Jobe FW, Kerlan RK, Carter VS, Shields CL, Lombardo SJ. Correctable elbow lesions in professional baseball players: a review of 25 cases. Am J Sports Med. 1979;7(1):72-75.
11. Elementary/Secondary Information System (EISi). National Center for Education Statistics, Institute of Education Sciences, US Department of Education website. http://nces.ed.gov/ccd/elsi/. Accessed September 21, 2015.
12. Corso RA; Harris Interactive. Football is America’s favorite sport as lead over baseball continues to grow; college football and auto racing come next. Harris Interactive website. http://www.harrisinteractive.com/vault/Harris Poll 9 - Favorite sport_1.25.12.pdf. Harris Poll 9, January 25, 2012. Accessed September 21, 2015.
13. [Scopus content]. Elsevier website. http://www.elsevier.com/solutions/scopus/content. Accessed September 21, 2015.
14. Scopus Author Identifier. Scopus website. http://help.scopus.com/Content/h_autsrch_intro.htm. Accessed October 5, 2015.
15. Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572.
16. Author Evaluator h Index Tab. Scopus website. http://help.scopus.com/Content/h_auteval_hindex.htm. Accessed October 5, 2015.
17. Boyd JL. Understanding the politics of being a team physician. Clin Sports Med. 2007;26(2):161-172.
18. Lee J, Kraus KL, Couldwell WT. Use of the h index in neurosurgery. Clinical article. J Neurosurg. 2009;111(2):387-
Conflict of Interest in Sports Medicine: Does It Affect Our Judgment?
As defined by the American Academy of Orthopaedic Surgeons (AAOS) in 1996, conflict of interest (COI) is the “circumstance that exists when, because of personal financial gain, an individual has the potential to be less than objective when called on to reach a judgment or interpret a result.”1 In medical research, COIs often occur in relationships between physician-researchers and pharmaceutical, medical device, and biotechnology companies. These relationships usually take the form of research grants but can also arise when the researcher has a financial interest in the product being tested or in the company that manufactures the product.
Although constructive collaboration between academic medicine and industry has worked to improve health care and ultimately benefit patients, potential drawbacks of such relationships include sequestration and suppression of results that may be disadvantageous to the industry sponsor,2 increased likelihood of reporting positive results (pro-industry),3-7 and biased study designs.8 The nature of such relationships may threaten the integrity of scientific studies, the objectivity of medical education, the quality of patient care, and the public’s trust in medicine.9
Financial relationships and affiliations are increasing as we seek to answer a growing number of clinical questions—with funding often being a limiting factor. At national scientific meetings, the number of presentations reporting COIs reflects this trend. Paper and poster presentations accepted for annual meetings of the Orthopaedic Trauma Association (OTA) and reporting a COI increased from 7.6% in 1993 to 12.6% in 2002 (P = .0129).2
Medical subspecialties outside of orthopedics are experiencing similar trends. Most notable is the American Psychiatric Association (APA). After the APA published a mandatory financial COI disclosure policy in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), the percentage of task force members reporting industry relationships increased by 12%.10 Analysis of the DSM-5 panels demonstrated that the panels with the largest percentage of reported COIs are those for which pharmacological treatment is the first-line intervention, including the panels for mood disorders (67%), psychotic disorders (83%) and sleep/wake disorders (100%).10 Moreover, the industry ties reported are to the pharmaceutical companies that manufacture the medications used to treat these disorders or to companies that service the pharmaceutical industry.10
The degree to which financial COIs affect the interpretation of the orthopedic literature has never been quantified. Although it is clear that COIs can confound the results and reporting of data, how the medical community uses disclosures when interpreting the literature and when formulating opinions that may or may not affect their practice patterns is largely unknown.
We conducted a study to evaluate how a hypothetical financial COI disclosure would influence the interpretation of data by orthopedic clinicians. We also wanted to determine the reliability of the data as perceived in association with different study designs, levels of evidence, research institutional settings, and reporting of positive or negative results.
Methods
We asked members of the Arthroscopy Association of North America (AANA) and the American Orthopaedic Society for Sports Medicine (AOSSM) to complete a multiple-choice situational questionnaire (Table). The questionnaire assesses the degree to which respondents use COI disclosures when interpreting the literature. It further explores the perceived clinical value of a study with a given reported COI, assuming variations in study design, research institutional setting, and significance of results. The fictional research team disclosed the project was funded by a pharmaceutical company and all team members received consulting compensation. The survey and study were reviewed and approved by our institutional review board. The survey consisted of 14 multiple-choice questions that allowed for only 1 answer selection per person and allowed survey takers to skip questions they did not wish to answer. The survey questions and associated response options appear in edited form in the Table. A link to the questionnaire (https://www.surveymonkey.com/s/MPCCLCX) was sent with a message explaining the study. The responses to the questionnaire constituted the data.
Results
We sent a request to participate in the survey to 750 physicians and received 522 responses (overall response rate, 70%). The response rate for each question equaled or exceeded 98%.
The majority of respondents (95.6%) were male. Ninety-nine percent of respondents were orthopedic surgeons. The Northeast (US) was the most common geographical practice location of respondents (32%), followed by the Midwest (19.1%) and the Southeast (16.6%). Most respondents (40%) had been in practice for more than 20 years; 67% had been in practice a minimum of 10 years. The majority (68.8%) were employed by private practice groups, either single specialty (57.8%) or multispecialty (11%).
Eighty percent of respondents strongly agreed that COI disclosure is important when interpreting study results, 62% reported always reading the disclosure slide during academy or other meeting presentations, and 41% reported always using this information when deciding how to interpret scientific data.
Seventy-five percent of respondents thought the study—an academic-center case series with significant results in favor of the pharmaceutical company funding the study—was biased (42% indicated biased with merit, 33% biased without merit). Twenty-three percent thought the study was possibly biased, but likely trustworthy given the academic institutional affiliation. When the study setting was changed to community hospital, 95% thought the study was biased (51% biased with merit, 44% biased without merit). With the same study performed at an academic center, and no statistically significant results (not in favor of the pharmaceutical company funding the study), 88% thought the study had merit (46% biased with merit, 42% unbiased with merit).
When the study design was changed to a randomized controlled trial (level I evidence) conducted at an academic center with negative results, an overwhelming 95% of respondents thought the study had merit (33% biased with merit, 62% unbiased with merit). Given the same study design at an academic center, with positive results, 78% still thought the study had merit (39% biased with merit, 39% unbiased with merit). An additional 18% thought the study was biased, but still likely trustworthy given the academic institutional affiliation. Finally, given a randomized controlled trial and positive results, but with the research setting a small community practice, 90% thought the study had merit (51% biased with merit, 39% unbiased with merit). The percentage of respondents who found the study biased and likely without merit increased from 3.7% to 9.5% when the institutional affiliation changed from academic to community.
Discussion
As governmental funding sources become increasingly limited, the role of industry sponsorship of orthopedic research has grown. Potential drawbacks and biases of such research support have been well described—most notably, increased positive result reporting, suppression of results that may be disadvantageous to the industry sponsor, and biased study designs.2-8 However, the extent to which financial COIs affect the orthopedic medical community’s interpretation of the literature has never been quantified. To our knowledge, the present study is the first to quantify the impact of reported COI on study interpretation.
Our goal was to examine how reported financial COIs influence the interpretation of the literature by the orthopedic medical community. Moreover, we wanted to determine the perceived reliability of the data when variables (study design, institutional affiliation, positive vs negative results) were changed. The results of our survey indicate that, when a financial COI is reported, study reliability is perceived as highest when negative results were found.
Our survey noted a discrepancy between the documented importance of the hypothetical research team’s COI disclosure and the use of such disclosures when interpreting study results. Eighty percent of respondents agreed that COI disclosure is important when interpreting study results, but only 62% reported always reading disclosures, and even fewer (41%) reported always using the information when interpreting results. It is unclear exactly why this trend exists, as one would expect the percentages to be more similar. These particular survey questions were formed around using COI disclosures when interpreting study results during academic presentations at national meetings and not during the review of published literature. It is possible that positioning the COI disclosure at the beginning of a presentation has an effect, but only 3.7% of respondents indicated they seldom remembered the disclosure by the end of the presentation. The results of our survey may have varied if the questions had targeted reading and interpreting the literature.
Interestingly, the results of these survey questions tended to be more consistent with rates of reported financial COI by presenters at national orthopedic meetings. A study published in the New England Journal of Medicine found that less than 80% of orthopedic surgeons reported their disclosures at a large annual meeting (AAOS), even when the disclosure involved payments pertinent to the research they were presenting.5 When the payments were indirectly related to the research, the percentage of surgeons reporting disclosures was 50%, almost the same as the disclosure rate for unrelated payments.5
When the study was changed to a level I randomized controlled trial, more survey respondents found it to be less biased and have more merit. Although it would seem intuitive for a study with a higher level of evidence to carry more clinical value during interpretation, this may not hold true in the setting of industry-sponsored clinical trials. Several studies have documented a significant association between the reporting of positive results and industry-sponsored randomized clinical trials. In 2008, Khan and colleagues3 examined 100 orthopedic randomized clinical trials reported in 5 major orthopedic subspecialty journals over a 2-year period. The association between industry funding and favorable outcome in all original randomized clinical trials was strong and significant (P < .001). This is not surprising, given the amount of time and money required for a well-designed clinical study. Commercial products with preclinical promise are pushed to testing in a clinical trial, whereas resources would not be wasted on products lacking preclinical merit.
The most important variable affecting interpretation of study merit by survey respondents was the reporting of negative results. As more researchers are developing COIs, many studies are discovering a relationship between COIs and outcomes of research studies. Reviewing the adult total joint literature, Ezzet8 found an industry funding rate of 50%. Positive results were reported in 93% of cases in commercially funded studies versus 37% of cases in independently funded studies. Furthermore, no negative results were reported by investigators who were receiving royalties from the respective companies.
Studies across the medical literature have also found this association between industry sponsorship and reporting of positive results. One such study, reported by Valachis and colleagues7 in the Journal of Clinical Oncology, examined more than 80 economic analyses of targeted oncologic therapies and found the studies funded by pharmaceutical companies were more likely to report favorable qualitative cost estimates. In addition, when studies with a COI disclosure were examined, those reporting any financial relationship with a manufacturer (eg, author affiliation, funding) were more likely than those without such a relationship to report favorable results.
Our study had several limitations. First, as most of the survey respondents were orthopedic surgeons, extrapolating their data to the medical community at large may not be appropriate, as each specialty may view industry affiliations differently. In addition, respondents were asked to base their interpretations of a study on conclusions we predetermined—no direct visualization of the data set or statistical testing methods. It is possible that these responses may have been different had the respondents had the opportunity to further evaluate the study in question. In a recent study, Altwairgi and colleagues11 found that 10% of randomized clinical trials involving lung cancer treatment were reported with different conclusions in their full manuscripts relative to their abstracts. We think our survey design perhaps best mimics an annual meeting environment in which participants have very limited ability to interpret studies and may rely more heavily on the factors we investigated—study design, significance of findings, and setting, all similar to information presented in an abstract—when making informed decisions. Although our response rate was only 70%, this is comparable to or better than the rates in similar survey studies that used email-based questionnaires.12,13
Another limitation was that our survey may have forced respondents into answers they did not entirely agree with, given the limited options of the multiple-choice response format and the specific wording of the questions. Our conclusions may have been more dramatic when we were evaluating whether the study was deemed meritorious or not. However, there is no adopted standard for evaluating the extent of bias perceived by a clinician. We thought it was important to include answer options indicating a study had merit despite obvious bias in design and execution. That a study had merit can mean different things. It may change clinical practice, may require further study and reproducibility, or may not be significant enough to matter. Asking follow-up questions to evaluate this perception among the respondents could have provided validity to the term merit. Further studies in this field are needed to determine how studies are interpreted and translated into clinical practice by various clinicians.
Conclusion
Although the present study is not a quantitative analysis of the determination of bias in the orthopedic community, it is the first to evaluate orthopedic surgeons’ perceptions on the basis of key fundamentals of orthopedic research relative to COI. It is clear from our study results that introducing levels of evidence to the orthopedic milieu has had a significant impact both on the quality of research and on the foundational use of deductive reasoning when interpreting the literature. Reporting negative outcomes is perhaps the most important factor in eliminating the perception of bias among orthopedic surgeons. To what extent a perceived COI plays into medical decision-making and the ultimate treatment of patients is still relatively unknown.
1. Lubahn JD, Mankin CJ, Mankin HJ, Kuhn PJ. Orthopaedics, ethics, and industry. Appropriateness of gifts, grants, and awards. Clin Orthop Relat Res. 2000;(371):256-263.
2. Kubiak EN, Park SS, Egol K, Zuckerman JD, Koval KJ. Increasingly conflicted: an analysis of conflicts of interest reported at the annual meetings of the Orthopaedic Trauma Association. Bull Hosp Jt Dis. 2006;63(3-4):83-87.
3. Khan SN, Mermer MJ, Myers E, Sandhu HS. The roles of funding source, clinical trial outcome, and quality of reporting in orthopedic surgery literature. Am J Orthop. 2008;37(12):E205-E212.
4. Okike K, Kocher MS, Mehlman CT, Bhandari M. Conflict of interest in orthopaedic research. An association between findings and funding in scientific presentations. J Bone Joint Surg Am. 2007;89(3):608-613.
5. Okike K, Kocher MS, Wei EX, Mehlman CT, Bhandari M. Accuracy of conflict-of-interest disclosures reported by physicians. N Engl J Med. 2009;361(15):1466-1474.
6. Shah RV, Albert TJ, Bruegel-Sanchez V, Vaccaro AR, Hilibrand AS, Grauer JN. Industry support and correlation to study outcome for papers published in Spine. Spine. 2005;30(9):1099-1104.
7. Valachis A, Polyzos NP, Nearchou A, Lind P, Mauri D. Financial relationships in economic analyses of targeted therapies in oncology. J Clin Oncol. 2012;30(12):1316-1320.
8. Ezzet KA. The prevalence of corporate funding in adult lower extremity research and its correlation with reported results. J Arthroplasty. 2003;18(7 suppl 1):138-145.
9. Lo B, Field MJ, eds; Institute of Medicine, Committee on Conflict of Interest in Medical Research, Education, and Practice, Board on Health Sciences Policy. Conflict of Interest in Medical Research, Education, and Practice. Washington, DC: National Academies Press; 2009. http://www.ncbi.nlm.nih.gov/books/NBK22942. Accessed September 29, 2015.
10. Cosgrove L, Krimsky S. A comparison of DSM-IV and DSM-5 panel members’ financial associations with industry: a pernicious problem persists. PLoS Med. 2012;9(3):e1001190.
11. Altwairgi AK, Booth CM, Hopman WM, Baetz TD. Discordance between conclusions stated in the abstract and conclusions in the article: analysis of published randomized controlled trials of systemic therapy in lung cancer. J Clin Oncol. 2012;30(28):3552-3557.
12. Decoster LC, Vailas JC, Swartz WG. Functional ACL bracing. A survey of current opinion and practice. Am J Orthop. 1995;24(11):838-843.
13. Mann BJ, Grana WA, Indelicato PA, O’Neill DF, George SZ. A survey of sports medicine physicians regarding psychological issues in patient-athletes. Am J Sports Med. 2007;35(12):2140-2147.
As defined by the American Academy of Orthopaedic Surgeons (AAOS) in 1996, conflict of interest (COI) is the “circumstance that exists when, because of personal financial gain, an individual has the potential to be less than objective when called on to reach a judgment or interpret a result.”1 In medical research, COIs often occur in relationships between physician-researchers and pharmaceutical, medical device, and biotechnology companies. These relationships usually take the form of research grants but can also arise when the researcher has a financial interest in the product being tested or in the company that manufactures the product.
Although constructive collaboration between academic medicine and industry has worked to improve health care and ultimately benefit patients, potential drawbacks of such relationships include sequestration and suppression of results that may be disadvantageous to the industry sponsor,2 increased likelihood of reporting positive results (pro-industry),3-7 and biased study designs.8 The nature of such relationships may threaten the integrity of scientific studies, the objectivity of medical education, the quality of patient care, and the public’s trust in medicine.9
Financial relationships and affiliations are increasing as we seek to answer a growing number of clinical questions—with funding often being a limiting factor. At national scientific meetings, the number of presentations reporting COIs reflects this trend. Paper and poster presentations accepted for annual meetings of the Orthopaedic Trauma Association (OTA) and reporting a COI increased from 7.6% in 1993 to 12.6% in 2002 (P = .0129).2
Medical subspecialties outside of orthopedics are experiencing similar trends. Most notable is the American Psychiatric Association (APA). After the APA published a mandatory financial COI disclosure policy in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), the percentage of task force members reporting industry relationships increased by 12%.10 Analysis of the DSM-5 panels demonstrated that the panels with the largest percentage of reported COIs are those for which pharmacological treatment is the first-line intervention, including the panels for mood disorders (67%), psychotic disorders (83%) and sleep/wake disorders (100%).10 Moreover, the industry ties reported are to the pharmaceutical companies that manufacture the medications used to treat these disorders or to companies that service the pharmaceutical industry.10
The degree to which financial COIs affect the interpretation of the orthopedic literature has never been quantified. Although it is clear that COIs can confound the results and reporting of data, how the medical community uses disclosures when interpreting the literature and when formulating opinions that may or may not affect their practice patterns is largely unknown.
We conducted a study to evaluate how a hypothetical financial COI disclosure would influence the interpretation of data by orthopedic clinicians. We also wanted to determine the reliability of the data as perceived in association with different study designs, levels of evidence, research institutional settings, and reporting of positive or negative results.
Methods
We asked members of the Arthroscopy Association of North America (AANA) and the American Orthopaedic Society for Sports Medicine (AOSSM) to complete a multiple-choice situational questionnaire (Table). The questionnaire assesses the degree to which respondents use COI disclosures when interpreting the literature. It further explores the perceived clinical value of a study with a given reported COI, assuming variations in study design, research institutional setting, and significance of results. The fictional research team disclosed the project was funded by a pharmaceutical company and all team members received consulting compensation. The survey and study were reviewed and approved by our institutional review board. The survey consisted of 14 multiple-choice questions that allowed for only 1 answer selection per person and allowed survey takers to skip questions they did not wish to answer. The survey questions and associated response options appear in edited form in the Table. A link to the questionnaire (https://www.surveymonkey.com/s/MPCCLCX) was sent with a message explaining the study. The responses to the questionnaire constituted the data.
Results
We sent a request to participate in the survey to 750 physicians and received 522 responses (overall response rate, 70%). The response rate for each question equaled or exceeded 98%.
The majority of respondents (95.6%) were male. Ninety-nine percent of respondents were orthopedic surgeons. The Northeast (US) was the most common geographical practice location of respondents (32%), followed by the Midwest (19.1%) and the Southeast (16.6%). Most respondents (40%) had been in practice for more than 20 years; 67% had been in practice a minimum of 10 years. The majority (68.8%) were employed by private practice groups, either single specialty (57.8%) or multispecialty (11%).
Eighty percent of respondents strongly agreed that COI disclosure is important when interpreting study results, 62% reported always reading the disclosure slide during academy or other meeting presentations, and 41% reported always using this information when deciding how to interpret scientific data.
Seventy-five percent of respondents thought the study—an academic-center case series with significant results in favor of the pharmaceutical company funding the study—was biased (42% indicated biased with merit, 33% biased without merit). Twenty-three percent thought the study was possibly biased, but likely trustworthy given the academic institutional affiliation. When the study setting was changed to community hospital, 95% thought the study was biased (51% biased with merit, 44% biased without merit). With the same study performed at an academic center, and no statistically significant results (not in favor of the pharmaceutical company funding the study), 88% thought the study had merit (46% biased with merit, 42% unbiased with merit).
When the study design was changed to a randomized controlled trial (level I evidence) conducted at an academic center with negative results, an overwhelming 95% of respondents thought the study had merit (33% biased with merit, 62% unbiased with merit). Given the same study design at an academic center, with positive results, 78% still thought the study had merit (39% biased with merit, 39% unbiased with merit). An additional 18% thought the study was biased, but still likely trustworthy given the academic institutional affiliation. Finally, given a randomized controlled trial and positive results, but with the research setting a small community practice, 90% thought the study had merit (51% biased with merit, 39% unbiased with merit). The percentage of respondents who found the study biased and likely without merit increased from 3.7% to 9.5% when the institutional affiliation changed from academic to community.
Discussion
As governmental funding sources become increasingly limited, the role of industry sponsorship of orthopedic research has grown. Potential drawbacks and biases of such research support have been well described—most notably, increased positive result reporting, suppression of results that may be disadvantageous to the industry sponsor, and biased study designs.2-8 However, the extent to which financial COIs affect the orthopedic medical community’s interpretation of the literature has never been quantified. To our knowledge, the present study is the first to quantify the impact of reported COI on study interpretation.
Our goal was to examine how reported financial COIs influence the interpretation of the literature by the orthopedic medical community. Moreover, we wanted to determine the perceived reliability of the data when variables (study design, institutional affiliation, positive vs negative results) were changed. The results of our survey indicate that, when a financial COI is reported, study reliability is perceived as highest when negative results were found.
Our survey noted a discrepancy between the documented importance of the hypothetical research team’s COI disclosure and the use of such disclosures when interpreting study results. Eighty percent of respondents agreed that COI disclosure is important when interpreting study results, but only 62% reported always reading disclosures, and even fewer (41%) reported always using the information when interpreting results. It is unclear exactly why this trend exists, as one would expect the percentages to be more similar. These particular survey questions were formed around using COI disclosures when interpreting study results during academic presentations at national meetings and not during the review of published literature. It is possible that positioning the COI disclosure at the beginning of a presentation has an effect, but only 3.7% of respondents indicated they seldom remembered the disclosure by the end of the presentation. The results of our survey may have varied if the questions had targeted reading and interpreting the literature.
Interestingly, the results of these survey questions tended to be more consistent with rates of reported financial COI by presenters at national orthopedic meetings. A study published in the New England Journal of Medicine found that less than 80% of orthopedic surgeons reported their disclosures at a large annual meeting (AAOS), even when the disclosure involved payments pertinent to the research they were presenting.5 When the payments were indirectly related to the research, the percentage of surgeons reporting disclosures was 50%, almost the same as the disclosure rate for unrelated payments.5
When the study was changed to a level I randomized controlled trial, more survey respondents found it to be less biased and have more merit. Although it would seem intuitive for a study with a higher level of evidence to carry more clinical value during interpretation, this may not hold true in the setting of industry-sponsored clinical trials. Several studies have documented a significant association between the reporting of positive results and industry-sponsored randomized clinical trials. In 2008, Khan and colleagues3 examined 100 orthopedic randomized clinical trials reported in 5 major orthopedic subspecialty journals over a 2-year period. The association between industry funding and favorable outcome in all original randomized clinical trials was strong and significant (P < .001). This is not surprising, given the amount of time and money required for a well-designed clinical study. Commercial products with preclinical promise are pushed to testing in a clinical trial, whereas resources would not be wasted on products lacking preclinical merit.
The most important variable affecting interpretation of study merit by survey respondents was the reporting of negative results. As more researchers are developing COIs, many studies are discovering a relationship between COIs and outcomes of research studies. Reviewing the adult total joint literature, Ezzet8 found an industry funding rate of 50%. Positive results were reported in 93% of cases in commercially funded studies versus 37% of cases in independently funded studies. Furthermore, no negative results were reported by investigators who were receiving royalties from the respective companies.
Studies across the medical literature have also found this association between industry sponsorship and reporting of positive results. One such study, reported by Valachis and colleagues7 in the Journal of Clinical Oncology, examined more than 80 economic analyses of targeted oncologic therapies and found the studies funded by pharmaceutical companies were more likely to report favorable qualitative cost estimates. In addition, when studies with a COI disclosure were examined, those reporting any financial relationship with a manufacturer (eg, author affiliation, funding) were more likely than those without such a relationship to report favorable results.
Our study had several limitations. First, as most of the survey respondents were orthopedic surgeons, extrapolating their data to the medical community at large may not be appropriate, as each specialty may view industry affiliations differently. In addition, respondents were asked to base their interpretations of a study on conclusions we predetermined—no direct visualization of the data set or statistical testing methods. It is possible that these responses may have been different had the respondents had the opportunity to further evaluate the study in question. In a recent study, Altwairgi and colleagues11 found that 10% of randomized clinical trials involving lung cancer treatment were reported with different conclusions in their full manuscripts relative to their abstracts. We think our survey design perhaps best mimics an annual meeting environment in which participants have very limited ability to interpret studies and may rely more heavily on the factors we investigated—study design, significance of findings, and setting, all similar to information presented in an abstract—when making informed decisions. Although our response rate was only 70%, this is comparable to or better than the rates in similar survey studies that used email-based questionnaires.12,13
Another limitation was that our survey may have forced respondents into answers they did not entirely agree with, given the limited options of the multiple-choice response format and the specific wording of the questions. Our conclusions may have been more dramatic when we were evaluating whether the study was deemed meritorious or not. However, there is no adopted standard for evaluating the extent of bias perceived by a clinician. We thought it was important to include answer options indicating a study had merit despite obvious bias in design and execution. That a study had merit can mean different things. It may change clinical practice, may require further study and reproducibility, or may not be significant enough to matter. Asking follow-up questions to evaluate this perception among the respondents could have provided validity to the term merit. Further studies in this field are needed to determine how studies are interpreted and translated into clinical practice by various clinicians.
Conclusion
Although the present study is not a quantitative analysis of the determination of bias in the orthopedic community, it is the first to evaluate orthopedic surgeons’ perceptions on the basis of key fundamentals of orthopedic research relative to COI. It is clear from our study results that introducing levels of evidence to the orthopedic milieu has had a significant impact both on the quality of research and on the foundational use of deductive reasoning when interpreting the literature. Reporting negative outcomes is perhaps the most important factor in eliminating the perception of bias among orthopedic surgeons. To what extent a perceived COI plays into medical decision-making and the ultimate treatment of patients is still relatively unknown.
As defined by the American Academy of Orthopaedic Surgeons (AAOS) in 1996, conflict of interest (COI) is the “circumstance that exists when, because of personal financial gain, an individual has the potential to be less than objective when called on to reach a judgment or interpret a result.”1 In medical research, COIs often occur in relationships between physician-researchers and pharmaceutical, medical device, and biotechnology companies. These relationships usually take the form of research grants but can also arise when the researcher has a financial interest in the product being tested or in the company that manufactures the product.
Although constructive collaboration between academic medicine and industry has worked to improve health care and ultimately benefit patients, potential drawbacks of such relationships include sequestration and suppression of results that may be disadvantageous to the industry sponsor,2 increased likelihood of reporting positive results (pro-industry),3-7 and biased study designs.8 The nature of such relationships may threaten the integrity of scientific studies, the objectivity of medical education, the quality of patient care, and the public’s trust in medicine.9
Financial relationships and affiliations are increasing as we seek to answer a growing number of clinical questions—with funding often being a limiting factor. At national scientific meetings, the number of presentations reporting COIs reflects this trend. Paper and poster presentations accepted for annual meetings of the Orthopaedic Trauma Association (OTA) and reporting a COI increased from 7.6% in 1993 to 12.6% in 2002 (P = .0129).2
Medical subspecialties outside of orthopedics are experiencing similar trends. Most notable is the American Psychiatric Association (APA). After the APA published a mandatory financial COI disclosure policy in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), the percentage of task force members reporting industry relationships increased by 12%.10 Analysis of the DSM-5 panels demonstrated that the panels with the largest percentage of reported COIs are those for which pharmacological treatment is the first-line intervention, including the panels for mood disorders (67%), psychotic disorders (83%) and sleep/wake disorders (100%).10 Moreover, the industry ties reported are to the pharmaceutical companies that manufacture the medications used to treat these disorders or to companies that service the pharmaceutical industry.10
The degree to which financial COIs affect the interpretation of the orthopedic literature has never been quantified. Although it is clear that COIs can confound the results and reporting of data, how the medical community uses disclosures when interpreting the literature and when formulating opinions that may or may not affect their practice patterns is largely unknown.
We conducted a study to evaluate how a hypothetical financial COI disclosure would influence the interpretation of data by orthopedic clinicians. We also wanted to determine the reliability of the data as perceived in association with different study designs, levels of evidence, research institutional settings, and reporting of positive or negative results.
Methods
We asked members of the Arthroscopy Association of North America (AANA) and the American Orthopaedic Society for Sports Medicine (AOSSM) to complete a multiple-choice situational questionnaire (Table). The questionnaire assesses the degree to which respondents use COI disclosures when interpreting the literature. It further explores the perceived clinical value of a study with a given reported COI, assuming variations in study design, research institutional setting, and significance of results. The fictional research team disclosed the project was funded by a pharmaceutical company and all team members received consulting compensation. The survey and study were reviewed and approved by our institutional review board. The survey consisted of 14 multiple-choice questions that allowed for only 1 answer selection per person and allowed survey takers to skip questions they did not wish to answer. The survey questions and associated response options appear in edited form in the Table. A link to the questionnaire (https://www.surveymonkey.com/s/MPCCLCX) was sent with a message explaining the study. The responses to the questionnaire constituted the data.
Results
We sent a request to participate in the survey to 750 physicians and received 522 responses (overall response rate, 70%). The response rate for each question equaled or exceeded 98%.
The majority of respondents (95.6%) were male. Ninety-nine percent of respondents were orthopedic surgeons. The Northeast (US) was the most common geographical practice location of respondents (32%), followed by the Midwest (19.1%) and the Southeast (16.6%). Most respondents (40%) had been in practice for more than 20 years; 67% had been in practice a minimum of 10 years. The majority (68.8%) were employed by private practice groups, either single specialty (57.8%) or multispecialty (11%).
Eighty percent of respondents strongly agreed that COI disclosure is important when interpreting study results, 62% reported always reading the disclosure slide during academy or other meeting presentations, and 41% reported always using this information when deciding how to interpret scientific data.
Seventy-five percent of respondents thought the study—an academic-center case series with significant results in favor of the pharmaceutical company funding the study—was biased (42% indicated biased with merit, 33% biased without merit). Twenty-three percent thought the study was possibly biased, but likely trustworthy given the academic institutional affiliation. When the study setting was changed to community hospital, 95% thought the study was biased (51% biased with merit, 44% biased without merit). With the same study performed at an academic center, and no statistically significant results (not in favor of the pharmaceutical company funding the study), 88% thought the study had merit (46% biased with merit, 42% unbiased with merit).
When the study design was changed to a randomized controlled trial (level I evidence) conducted at an academic center with negative results, an overwhelming 95% of respondents thought the study had merit (33% biased with merit, 62% unbiased with merit). Given the same study design at an academic center, with positive results, 78% still thought the study had merit (39% biased with merit, 39% unbiased with merit). An additional 18% thought the study was biased, but still likely trustworthy given the academic institutional affiliation. Finally, given a randomized controlled trial and positive results, but with the research setting a small community practice, 90% thought the study had merit (51% biased with merit, 39% unbiased with merit). The percentage of respondents who found the study biased and likely without merit increased from 3.7% to 9.5% when the institutional affiliation changed from academic to community.
Discussion
As governmental funding sources become increasingly limited, the role of industry sponsorship of orthopedic research has grown. Potential drawbacks and biases of such research support have been well described—most notably, increased positive result reporting, suppression of results that may be disadvantageous to the industry sponsor, and biased study designs.2-8 However, the extent to which financial COIs affect the orthopedic medical community’s interpretation of the literature has never been quantified. To our knowledge, the present study is the first to quantify the impact of reported COI on study interpretation.
Our goal was to examine how reported financial COIs influence the interpretation of the literature by the orthopedic medical community. Moreover, we wanted to determine the perceived reliability of the data when variables (study design, institutional affiliation, positive vs negative results) were changed. The results of our survey indicate that, when a financial COI is reported, study reliability is perceived as highest when negative results were found.
Our survey noted a discrepancy between the documented importance of the hypothetical research team’s COI disclosure and the use of such disclosures when interpreting study results. Eighty percent of respondents agreed that COI disclosure is important when interpreting study results, but only 62% reported always reading disclosures, and even fewer (41%) reported always using the information when interpreting results. It is unclear exactly why this trend exists, as one would expect the percentages to be more similar. These particular survey questions were formed around using COI disclosures when interpreting study results during academic presentations at national meetings and not during the review of published literature. It is possible that positioning the COI disclosure at the beginning of a presentation has an effect, but only 3.7% of respondents indicated they seldom remembered the disclosure by the end of the presentation. The results of our survey may have varied if the questions had targeted reading and interpreting the literature.
Interestingly, the results of these survey questions tended to be more consistent with rates of reported financial COI by presenters at national orthopedic meetings. A study published in the New England Journal of Medicine found that less than 80% of orthopedic surgeons reported their disclosures at a large annual meeting (AAOS), even when the disclosure involved payments pertinent to the research they were presenting.5 When the payments were indirectly related to the research, the percentage of surgeons reporting disclosures was 50%, almost the same as the disclosure rate for unrelated payments.5
When the study was changed to a level I randomized controlled trial, more survey respondents found it to be less biased and have more merit. Although it would seem intuitive for a study with a higher level of evidence to carry more clinical value during interpretation, this may not hold true in the setting of industry-sponsored clinical trials. Several studies have documented a significant association between the reporting of positive results and industry-sponsored randomized clinical trials. In 2008, Khan and colleagues3 examined 100 orthopedic randomized clinical trials reported in 5 major orthopedic subspecialty journals over a 2-year period. The association between industry funding and favorable outcome in all original randomized clinical trials was strong and significant (P < .001). This is not surprising, given the amount of time and money required for a well-designed clinical study. Commercial products with preclinical promise are pushed to testing in a clinical trial, whereas resources would not be wasted on products lacking preclinical merit.
The most important variable affecting interpretation of study merit by survey respondents was the reporting of negative results. As more researchers are developing COIs, many studies are discovering a relationship between COIs and outcomes of research studies. Reviewing the adult total joint literature, Ezzet8 found an industry funding rate of 50%. Positive results were reported in 93% of cases in commercially funded studies versus 37% of cases in independently funded studies. Furthermore, no negative results were reported by investigators who were receiving royalties from the respective companies.
Studies across the medical literature have also found this association between industry sponsorship and reporting of positive results. One such study, reported by Valachis and colleagues7 in the Journal of Clinical Oncology, examined more than 80 economic analyses of targeted oncologic therapies and found the studies funded by pharmaceutical companies were more likely to report favorable qualitative cost estimates. In addition, when studies with a COI disclosure were examined, those reporting any financial relationship with a manufacturer (eg, author affiliation, funding) were more likely than those without such a relationship to report favorable results.
Our study had several limitations. First, as most of the survey respondents were orthopedic surgeons, extrapolating their data to the medical community at large may not be appropriate, as each specialty may view industry affiliations differently. In addition, respondents were asked to base their interpretations of a study on conclusions we predetermined—no direct visualization of the data set or statistical testing methods. It is possible that these responses may have been different had the respondents had the opportunity to further evaluate the study in question. In a recent study, Altwairgi and colleagues11 found that 10% of randomized clinical trials involving lung cancer treatment were reported with different conclusions in their full manuscripts relative to their abstracts. We think our survey design perhaps best mimics an annual meeting environment in which participants have very limited ability to interpret studies and may rely more heavily on the factors we investigated—study design, significance of findings, and setting, all similar to information presented in an abstract—when making informed decisions. Although our response rate was only 70%, this is comparable to or better than the rates in similar survey studies that used email-based questionnaires.12,13
Another limitation was that our survey may have forced respondents into answers they did not entirely agree with, given the limited options of the multiple-choice response format and the specific wording of the questions. Our conclusions may have been more dramatic when we were evaluating whether the study was deemed meritorious or not. However, there is no adopted standard for evaluating the extent of bias perceived by a clinician. We thought it was important to include answer options indicating a study had merit despite obvious bias in design and execution. That a study had merit can mean different things. It may change clinical practice, may require further study and reproducibility, or may not be significant enough to matter. Asking follow-up questions to evaluate this perception among the respondents could have provided validity to the term merit. Further studies in this field are needed to determine how studies are interpreted and translated into clinical practice by various clinicians.
Conclusion
Although the present study is not a quantitative analysis of the determination of bias in the orthopedic community, it is the first to evaluate orthopedic surgeons’ perceptions on the basis of key fundamentals of orthopedic research relative to COI. It is clear from our study results that introducing levels of evidence to the orthopedic milieu has had a significant impact both on the quality of research and on the foundational use of deductive reasoning when interpreting the literature. Reporting negative outcomes is perhaps the most important factor in eliminating the perception of bias among orthopedic surgeons. To what extent a perceived COI plays into medical decision-making and the ultimate treatment of patients is still relatively unknown.
1. Lubahn JD, Mankin CJ, Mankin HJ, Kuhn PJ. Orthopaedics, ethics, and industry. Appropriateness of gifts, grants, and awards. Clin Orthop Relat Res. 2000;(371):256-263.
2. Kubiak EN, Park SS, Egol K, Zuckerman JD, Koval KJ. Increasingly conflicted: an analysis of conflicts of interest reported at the annual meetings of the Orthopaedic Trauma Association. Bull Hosp Jt Dis. 2006;63(3-4):83-87.
3. Khan SN, Mermer MJ, Myers E, Sandhu HS. The roles of funding source, clinical trial outcome, and quality of reporting in orthopedic surgery literature. Am J Orthop. 2008;37(12):E205-E212.
4. Okike K, Kocher MS, Mehlman CT, Bhandari M. Conflict of interest in orthopaedic research. An association between findings and funding in scientific presentations. J Bone Joint Surg Am. 2007;89(3):608-613.
5. Okike K, Kocher MS, Wei EX, Mehlman CT, Bhandari M. Accuracy of conflict-of-interest disclosures reported by physicians. N Engl J Med. 2009;361(15):1466-1474.
6. Shah RV, Albert TJ, Bruegel-Sanchez V, Vaccaro AR, Hilibrand AS, Grauer JN. Industry support and correlation to study outcome for papers published in Spine. Spine. 2005;30(9):1099-1104.
7. Valachis A, Polyzos NP, Nearchou A, Lind P, Mauri D. Financial relationships in economic analyses of targeted therapies in oncology. J Clin Oncol. 2012;30(12):1316-1320.
8. Ezzet KA. The prevalence of corporate funding in adult lower extremity research and its correlation with reported results. J Arthroplasty. 2003;18(7 suppl 1):138-145.
9. Lo B, Field MJ, eds; Institute of Medicine, Committee on Conflict of Interest in Medical Research, Education, and Practice, Board on Health Sciences Policy. Conflict of Interest in Medical Research, Education, and Practice. Washington, DC: National Academies Press; 2009. http://www.ncbi.nlm.nih.gov/books/NBK22942. Accessed September 29, 2015.
10. Cosgrove L, Krimsky S. A comparison of DSM-IV and DSM-5 panel members’ financial associations with industry: a pernicious problem persists. PLoS Med. 2012;9(3):e1001190.
11. Altwairgi AK, Booth CM, Hopman WM, Baetz TD. Discordance between conclusions stated in the abstract and conclusions in the article: analysis of published randomized controlled trials of systemic therapy in lung cancer. J Clin Oncol. 2012;30(28):3552-3557.
12. Decoster LC, Vailas JC, Swartz WG. Functional ACL bracing. A survey of current opinion and practice. Am J Orthop. 1995;24(11):838-843.
13. Mann BJ, Grana WA, Indelicato PA, O’Neill DF, George SZ. A survey of sports medicine physicians regarding psychological issues in patient-athletes. Am J Sports Med. 2007;35(12):2140-2147.
1. Lubahn JD, Mankin CJ, Mankin HJ, Kuhn PJ. Orthopaedics, ethics, and industry. Appropriateness of gifts, grants, and awards. Clin Orthop Relat Res. 2000;(371):256-263.
2. Kubiak EN, Park SS, Egol K, Zuckerman JD, Koval KJ. Increasingly conflicted: an analysis of conflicts of interest reported at the annual meetings of the Orthopaedic Trauma Association. Bull Hosp Jt Dis. 2006;63(3-4):83-87.
3. Khan SN, Mermer MJ, Myers E, Sandhu HS. The roles of funding source, clinical trial outcome, and quality of reporting in orthopedic surgery literature. Am J Orthop. 2008;37(12):E205-E212.
4. Okike K, Kocher MS, Mehlman CT, Bhandari M. Conflict of interest in orthopaedic research. An association between findings and funding in scientific presentations. J Bone Joint Surg Am. 2007;89(3):608-613.
5. Okike K, Kocher MS, Wei EX, Mehlman CT, Bhandari M. Accuracy of conflict-of-interest disclosures reported by physicians. N Engl J Med. 2009;361(15):1466-1474.
6. Shah RV, Albert TJ, Bruegel-Sanchez V, Vaccaro AR, Hilibrand AS, Grauer JN. Industry support and correlation to study outcome for papers published in Spine. Spine. 2005;30(9):1099-1104.
7. Valachis A, Polyzos NP, Nearchou A, Lind P, Mauri D. Financial relationships in economic analyses of targeted therapies in oncology. J Clin Oncol. 2012;30(12):1316-1320.
8. Ezzet KA. The prevalence of corporate funding in adult lower extremity research and its correlation with reported results. J Arthroplasty. 2003;18(7 suppl 1):138-145.
9. Lo B, Field MJ, eds; Institute of Medicine, Committee on Conflict of Interest in Medical Research, Education, and Practice, Board on Health Sciences Policy. Conflict of Interest in Medical Research, Education, and Practice. Washington, DC: National Academies Press; 2009. http://www.ncbi.nlm.nih.gov/books/NBK22942. Accessed September 29, 2015.
10. Cosgrove L, Krimsky S. A comparison of DSM-IV and DSM-5 panel members’ financial associations with industry: a pernicious problem persists. PLoS Med. 2012;9(3):e1001190.
11. Altwairgi AK, Booth CM, Hopman WM, Baetz TD. Discordance between conclusions stated in the abstract and conclusions in the article: analysis of published randomized controlled trials of systemic therapy in lung cancer. J Clin Oncol. 2012;30(28):3552-3557.
12. Decoster LC, Vailas JC, Swartz WG. Functional ACL bracing. A survey of current opinion and practice. Am J Orthop. 1995;24(11):838-843.
13. Mann BJ, Grana WA, Indelicato PA, O’Neill DF, George SZ. A survey of sports medicine physicians regarding psychological issues in patient-athletes. Am J Sports Med. 2007;35(12):2140-2147.