User login
Screening Tool to Reduce Anticoagulant Clinic Encounters
Metrics from 2017 at the Fayetteville Veterans Affairs Heath Care Center (FVAHCC) Anticoagulation Clinic indicate that 43% of patients with atrial fibrillation (AF) who are prescribed warfarin have difficulty maintaining a therapeutic international normalized ratio (INR). These patients require frequent clinic appointments to adjust their regimens to ensure anticoagulation efficacy. FVAHCC policy requires a patient to return to the clinic for repeat INR evaluation within 5 to 14 days of the visit where INR was outside of the established therapeutic range.1 These frequent INR monitoring appointments increase patient and health care provider burden.
Direct oral anticoagulants (DOACs) are an alternative to warfarin for patients with AF who require anticoagulation. DOACs, which do not require regular efficacy monitoring, can be beneficial to patients who struggle to maintain a therapeutic INR when taking warfarin. FVAHCC policy regarding warfarin therapy monitoring allows for a maximum of 6 weeks between appointments. This period is often extended to 3 to 6 months for patients on DOACs.1
At FVAHCC, patients prescribed warfarin are managed in a centralized Anticoagulation Clinic led by a clinical pharmacy specialist (CPS). When a patient reports for an appointment, a clinical pharmacy technician performs point-of-care INR testing and asks standardized questions regarding therapy, including an assessment of adherence. The CPS then evaluates the patient’s INR test results, adjusts the dosage of warfarin as indicated, and determines appropriate follow-up.
A patient who is prescribed a DOAC is monitored by a CPS who works within a patient aligned care team (PACT). The PACT, a multidisciplinary team providing health care to veterans, includes physicians, nurses, pharmacists, dieticians, and mental health providers. Each CPS covers 3 or 4 PACTs. These pharmacists monitor all aspects of DOAC therapy at regular intervals, including renal and hepatic function, complete blood counts, medication adherence, and adverse effects.
Clinic and patient INR data are tracked using a time in therapeutic range (TTR) report generated by the US Department of Veterans Affairs (VA). The TTR report provides clinical information to enhance patient anticoagulation care.2 The TTR report identifies patients with an active order for warfarin and a diagnosis of AF or venous thromboembolism (VTE) whose INR is within therapeutic range (between 2 and 3) < 60% of the time over the previous 160 days.2 The patient must have had at least 3 INR levels drawn within that time frame for a TTR report calculation.2 The report excludes patients who were first prescribed warfarin within the previous 42 days and those with mechanical heart valves. The TTR report is used by the VA to see concrete facility-level results for quality improvement efforts.2
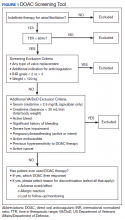
A quality improvement screening tool was developed to identify patients with AF being treated with warfarin who may appropriately transition to DOAC therapy. Anticoagulation Clinic patients were eligible for further evaluation if they had a TTR report level of < 60% and were prescribed indefinite warfarin therapy for AF.
The national VA goal is to have patient TTR report levels read > 60%. Therefore, the primary objective of this project was to improve Anticoagulation Clinic TTR metrics by targeting patients with TTR levels below the national goal.2
Patients who were successfully converted from warfarin to a DOAC were no longer included in Anticoagulation Clinic metrics and instead were followed by a PACT CPS. Thus, it was hypothesized that the average number of monthly Anticoagulation Clinic encounters would decrease on successful implementation of the screening tool. A secondary endpoint of the study evaluated the change in the total number of encounters of those who converted from warfarin to a DOAC.
Fewer clinic encounters could increase time available for the CPS to incorporate other initiatives into workflow and could increase clinic availability for newly referred veterans.
Methods
As this undertaking was considered to be a quality improvement project, institutional review board approval was not required. During an 8-week screening period (August to September 2018), the DOAC screening tool was implemented into the Anticoagulation Clinic workflow. This screening tool (Figure 1) was established based on VA Pharmacy Benefit Management (PBM) Service’s Criteria for Use for Stroke Prevention in Nonvalvular Atrial Fibrillation, a national set of standards used to determine appropriate candidates for DOAC therapy.3
Exclusion criteria included patients with INR goals < 2 or > 3, patients with a diagnosis of VTE, and patients with weight > 120 kg. Patients with a diagnosis of VTE were excluded due to the variability in therapy duration. Weight cutoffs were based on recommendations by the International Society on Thrombosis and Haemostasis. Due to a lack of available data, it was suggested that clinical judgment be used in patients whose weight was > 120 kg.4
During the screening period, weekly TTR reports identified patients in the clinic who had TTR < 60%. When a patient with a TTR report results of < 60% also had a scheduled appointment within a week, a CPS then further reviewed patient eligibility using the DOAC screening tool. On arrival for an appointment, the eligible patient was counseled on DOAC medications and the differences between warfarin and DOACs, including monitoring requirements. Patients had the option to switch to DOAC therapy or remain on warfarin.
The change in the average number of monthly Anticoagulation Clinic encounters for 3 months prior to the screening period (May to July 2018) and 2 months following screening (October to November 2018) was evaluated to measure the impact of the DOAC screening tool. The total number of encounters in the clinic was assessed using the monthly VA reports and were averaged for each period. Then data from the 2 periods were compared.
The monthly encounter reports, a data tool that monitors the number of unique visits per veteran each calendar month, also were used to generate a secondary endpoint showing the number of encounters in the Anticoagulation Clinic associated with patients who switched to a DOAC, including visits prior to changing therapy, and before and after the screening period.
Student’s t test was used to compare the change in encounter frequency before and after screening tool implementation for both primary and secondary endpoints. α was defined as .05 a priori. Continuous data were presented as means and standard deviations. Data were calculated with Microsoft Excel 2016.
Results
For the 3 months before the 8-week screening period, an average of 476 Anticoagulation Clinic encounters per month were documented. Two months of data following the screening period averaged 546 encounters per month. There were an average of 70 additional encounters per month after screening tool implementation (P = .15), reflecting the study’s primary objective.
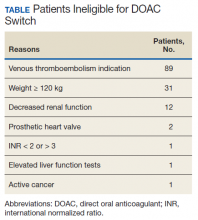
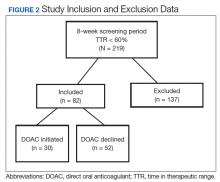
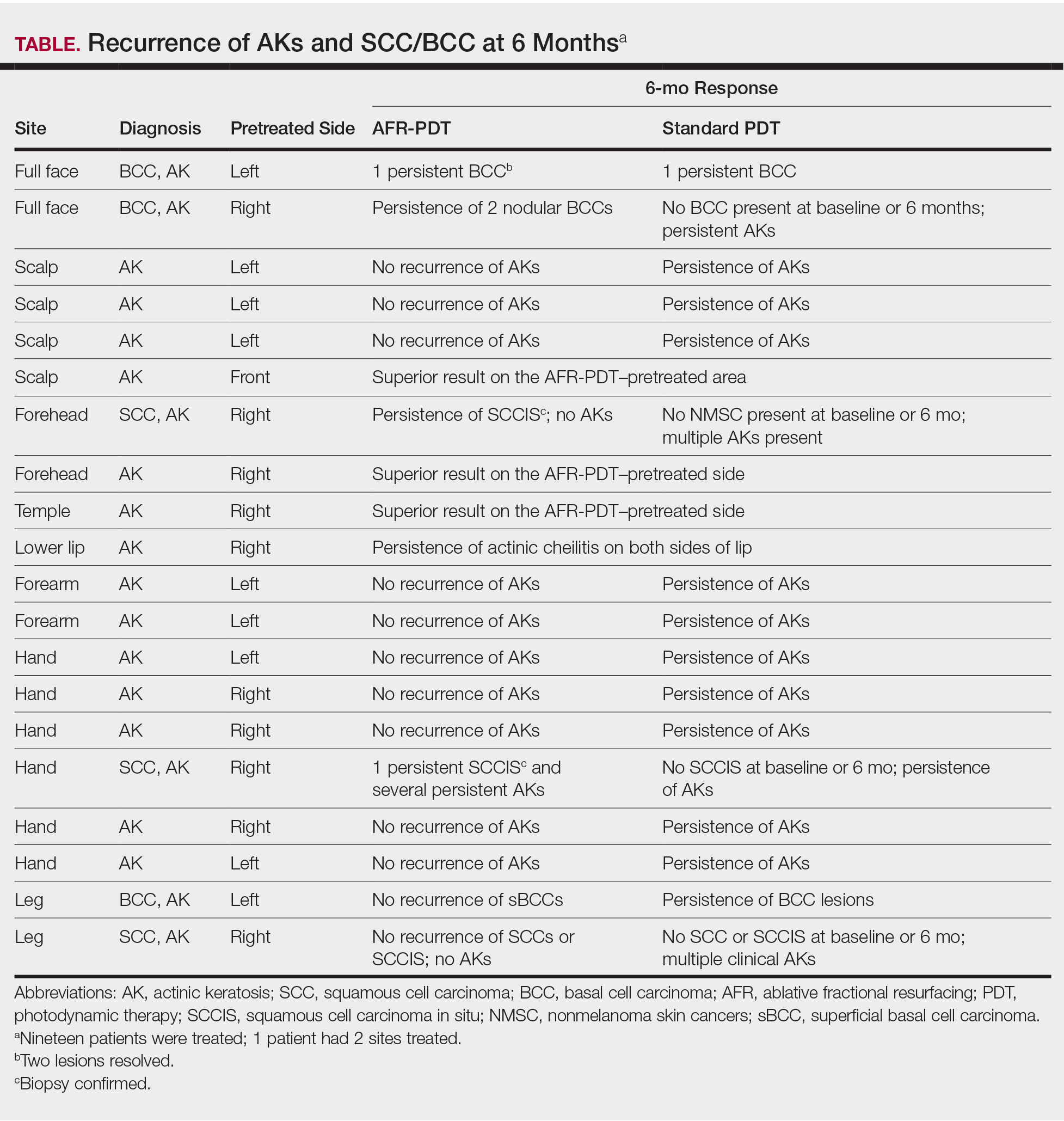
A total of 219 patients in the Anticoagulation Clinic were identified as having a TTR report results of < 60% during the 8-week screening period (Figure 2). Eighty-two of those patients (37.4%) were considered eligible to switch from warfarin to DOAC therapy. Thirty of those eligible patients (13.7%) switched to a DOAC. A total of 107 clinic encounters (22.5%) was associated with these 30 patients prior to screening and 32 associated encounters (5.9%) following screening (P = .01). Of the remaining 137 patients (62.6%) who were ineligible for DOAC therapy, the most common reason for disqualification was a diagnosis of VTE (Table).
Discussion
The general results of this quality improvement project showed that implementation of a screening tool designed to identify patients eligible for DOAC therapy did not decrease the average number of Anticoagulation Clinic encounters. Thirty of 82 eligible patients (36.6%) decided to switch to DOAC therapy during the study period. For those 30 patients, there was a statistically significant decrease in the number of individual clinic encounters. This suggests that the screening tool may positively impact Anticoagulation Clinic metrics when evaluating individual patients, potentially increasing clinic appointment availability.
Confounding Factors
Multiple confounding factors may have affected this project’s results. First, Class I recall for point-of-care test strips used by the clinic was mandated by the US Food and Drug Administration on November 1, 2018.5 Before the recall, investigators found that many nontherapeutic INRs using point-of-care testing later showed results that were within the therapeutic INR range using same-day venous blood collection. This may have led to increases in falsely recorded nontherapeutic INRs and lowered TTR report results. Initially, the project was designed to collect monthly clinic encounter data for 3 months following the 8-week screening period; however, data collection was stopped after 2 months because of the test strip recall.
In addition, in early December 2018, all patients were moved from the Anticoagulation Clinic to the Anticoagulation Telephone Clinic that uses venous blood draws and telephone appointments. Data from venous blood draw results had previously been excluded from this project because results were not available on the same day. Patients in this program are contacted by telephone rather than being offered a face-to-face appointment, thus reducing in-clinic encounters.
Another confounding factor was a FVAHCC policy change in August 2018 requiring that any patient initiated on a DOAC make a onetime visit to the Anticoagulation Clinic prior to establishing care with a PACT CPS. Investigators were unable to exclude these patients from monthly encounter data. Some patients transitioning from warfarin to DOAC therapy were required to continue receiving anticoagulation monitoring from the clinic because of limited PACT CPS clinic availability, thus further increasing postscreening encounters.
Health care providers outside of the Anticoagulation Clinic and uninvolved with the quality improvement project also were switching patients from warfarin to DOAC therapies. Although this may have affected encounter data positively, investigators cannot guarantee these patients would have met criteria outlined by the screening tool.
In September 2018 Hurricane Florence disrupted health care delivery during the 8-week screening period. This event disrupted numerous clinic appointments. Although screening of patients was completed during the 8-week screening period, some patients did not switch to DOAC therapies until November 2018.
Secondary Endpoint Results
Promising results can be seen by specifically looking at the secondary endpoint: the number of encounters associated with patients who chose DOAC therapy. There were 107 encounters associated with the 30 patients who switched to a DOAC prior to screening and only 32 associated encounters after screening, a reduction of 70.1%. This suggests that multiple appointment slots were freed when the screening tool led to successful conversion from warfarin to a DOAC. Further assessment is warranted.
Future Project Development
Future areas for quality improvement project development include expanding project criteria to include patients taking warfarin for VTE. Eighty-nine of 137 patients (65%) who were deemed ineligible to switch to DOAC therapy were excluded due to a diagnosis of VTE. There are existing VA/Department of Defense Criteria for Use for DOAC use in VTE recommendations. Straightforward modification of the screening tool could include this patient group and may be especially useful for patients on indefinite warfarin therapy for recurrent VTE who have poor TTR report results.6
Given the number of confounding factors caused by unforeseen changes to the Anticoagulation Clinic workflow, use of the DOAC screening tool was placed on hold at the conclusion of data collection. This limited the ability to analyze encounter data in the months following project conclusion. Future plans include reimplementation of the screening tool with minor adjustments to include patients on warfarin for VTE and patients with a TTR report results above 60%.
Conclusion
This quality improvement project sought to determine the impact of a screening tool on effecting Anticoagulation Clinic encounter metrics. Results of this project show that the screening tool was unsuccessful in reducing the number of overall clinic encounters. Some promise was shown when evaluating clinic encounters for patients who switched anticoagulation therapies. Numerous confounding factors may have contributed to these results.
1. US Department of Veterans Affairs, Fayetteville Veterans Affairs Health Care Center. MCM 11-188 Anticoagulation Management Program. Revised July 11, 2017. [Source not verified.]
2. US Department of Veterans Affairs, Pharmacy Benefits Management Clinical Pharmacy Practice Office. Anticoagulation percent time in therapeutic range reports. https://spsites.cdw.va.gov/sites/PBM_CPPO/Pages/AnticoagulationTTR.aspx. Revised May 24, 2017. Accessed April 20, 2020. [Source not verified.]
3. US Department of Veterans Affairs, VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs). Dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis) and edoxaban (SAVAYSA) criteria for use for stroke prevention in nonvalvular atrial fibrillation (AF). https://www.pbm.va.gov/apps/VANationalFormulary/. Updated December 2017. Accessed April 30, 2020.
4. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308-1313.
5. US Food and Drug Administration. Roche Diagnostics recalls CoaguChek XS PT Test Strips due to naccurate INR test results. https://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm624822.htm. Published November 1, 2018. Accessed April 16, 2019.
6. US Department of Veterans Affairs, VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs) (formerly called TSOACs) dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban(Savaysa) criteria for use for *treatment of venous thromboembolism (VTE)* https://www.pbm.va.gov/apps/VANationalFormulary/. Updated December 2017. Accessed April 30, 2020.
Metrics from 2017 at the Fayetteville Veterans Affairs Heath Care Center (FVAHCC) Anticoagulation Clinic indicate that 43% of patients with atrial fibrillation (AF) who are prescribed warfarin have difficulty maintaining a therapeutic international normalized ratio (INR). These patients require frequent clinic appointments to adjust their regimens to ensure anticoagulation efficacy. FVAHCC policy requires a patient to return to the clinic for repeat INR evaluation within 5 to 14 days of the visit where INR was outside of the established therapeutic range.1 These frequent INR monitoring appointments increase patient and health care provider burden.
Direct oral anticoagulants (DOACs) are an alternative to warfarin for patients with AF who require anticoagulation. DOACs, which do not require regular efficacy monitoring, can be beneficial to patients who struggle to maintain a therapeutic INR when taking warfarin. FVAHCC policy regarding warfarin therapy monitoring allows for a maximum of 6 weeks between appointments. This period is often extended to 3 to 6 months for patients on DOACs.1
At FVAHCC, patients prescribed warfarin are managed in a centralized Anticoagulation Clinic led by a clinical pharmacy specialist (CPS). When a patient reports for an appointment, a clinical pharmacy technician performs point-of-care INR testing and asks standardized questions regarding therapy, including an assessment of adherence. The CPS then evaluates the patient’s INR test results, adjusts the dosage of warfarin as indicated, and determines appropriate follow-up.
A patient who is prescribed a DOAC is monitored by a CPS who works within a patient aligned care team (PACT). The PACT, a multidisciplinary team providing health care to veterans, includes physicians, nurses, pharmacists, dieticians, and mental health providers. Each CPS covers 3 or 4 PACTs. These pharmacists monitor all aspects of DOAC therapy at regular intervals, including renal and hepatic function, complete blood counts, medication adherence, and adverse effects.
Clinic and patient INR data are tracked using a time in therapeutic range (TTR) report generated by the US Department of Veterans Affairs (VA). The TTR report provides clinical information to enhance patient anticoagulation care.2 The TTR report identifies patients with an active order for warfarin and a diagnosis of AF or venous thromboembolism (VTE) whose INR is within therapeutic range (between 2 and 3) < 60% of the time over the previous 160 days.2 The patient must have had at least 3 INR levels drawn within that time frame for a TTR report calculation.2 The report excludes patients who were first prescribed warfarin within the previous 42 days and those with mechanical heart valves. The TTR report is used by the VA to see concrete facility-level results for quality improvement efforts.2
A quality improvement screening tool was developed to identify patients with AF being treated with warfarin who may appropriately transition to DOAC therapy. Anticoagulation Clinic patients were eligible for further evaluation if they had a TTR report level of < 60% and were prescribed indefinite warfarin therapy for AF.
The national VA goal is to have patient TTR report levels read > 60%. Therefore, the primary objective of this project was to improve Anticoagulation Clinic TTR metrics by targeting patients with TTR levels below the national goal.2
Patients who were successfully converted from warfarin to a DOAC were no longer included in Anticoagulation Clinic metrics and instead were followed by a PACT CPS. Thus, it was hypothesized that the average number of monthly Anticoagulation Clinic encounters would decrease on successful implementation of the screening tool. A secondary endpoint of the study evaluated the change in the total number of encounters of those who converted from warfarin to a DOAC.
Fewer clinic encounters could increase time available for the CPS to incorporate other initiatives into workflow and could increase clinic availability for newly referred veterans.
Methods
As this undertaking was considered to be a quality improvement project, institutional review board approval was not required. During an 8-week screening period (August to September 2018), the DOAC screening tool was implemented into the Anticoagulation Clinic workflow. This screening tool (Figure 1) was established based on VA Pharmacy Benefit Management (PBM) Service’s Criteria for Use for Stroke Prevention in Nonvalvular Atrial Fibrillation, a national set of standards used to determine appropriate candidates for DOAC therapy.3
Exclusion criteria included patients with INR goals < 2 or > 3, patients with a diagnosis of VTE, and patients with weight > 120 kg. Patients with a diagnosis of VTE were excluded due to the variability in therapy duration. Weight cutoffs were based on recommendations by the International Society on Thrombosis and Haemostasis. Due to a lack of available data, it was suggested that clinical judgment be used in patients whose weight was > 120 kg.4
During the screening period, weekly TTR reports identified patients in the clinic who had TTR < 60%. When a patient with a TTR report results of < 60% also had a scheduled appointment within a week, a CPS then further reviewed patient eligibility using the DOAC screening tool. On arrival for an appointment, the eligible patient was counseled on DOAC medications and the differences between warfarin and DOACs, including monitoring requirements. Patients had the option to switch to DOAC therapy or remain on warfarin.
The change in the average number of monthly Anticoagulation Clinic encounters for 3 months prior to the screening period (May to July 2018) and 2 months following screening (October to November 2018) was evaluated to measure the impact of the DOAC screening tool. The total number of encounters in the clinic was assessed using the monthly VA reports and were averaged for each period. Then data from the 2 periods were compared.
The monthly encounter reports, a data tool that monitors the number of unique visits per veteran each calendar month, also were used to generate a secondary endpoint showing the number of encounters in the Anticoagulation Clinic associated with patients who switched to a DOAC, including visits prior to changing therapy, and before and after the screening period.
Student’s t test was used to compare the change in encounter frequency before and after screening tool implementation for both primary and secondary endpoints. α was defined as .05 a priori. Continuous data were presented as means and standard deviations. Data were calculated with Microsoft Excel 2016.
Results
For the 3 months before the 8-week screening period, an average of 476 Anticoagulation Clinic encounters per month were documented. Two months of data following the screening period averaged 546 encounters per month. There were an average of 70 additional encounters per month after screening tool implementation (P = .15), reflecting the study’s primary objective.
A total of 219 patients in the Anticoagulation Clinic were identified as having a TTR report results of < 60% during the 8-week screening period (Figure 2). Eighty-two of those patients (37.4%) were considered eligible to switch from warfarin to DOAC therapy. Thirty of those eligible patients (13.7%) switched to a DOAC. A total of 107 clinic encounters (22.5%) was associated with these 30 patients prior to screening and 32 associated encounters (5.9%) following screening (P = .01). Of the remaining 137 patients (62.6%) who were ineligible for DOAC therapy, the most common reason for disqualification was a diagnosis of VTE (Table).
Discussion
The general results of this quality improvement project showed that implementation of a screening tool designed to identify patients eligible for DOAC therapy did not decrease the average number of Anticoagulation Clinic encounters. Thirty of 82 eligible patients (36.6%) decided to switch to DOAC therapy during the study period. For those 30 patients, there was a statistically significant decrease in the number of individual clinic encounters. This suggests that the screening tool may positively impact Anticoagulation Clinic metrics when evaluating individual patients, potentially increasing clinic appointment availability.
Confounding Factors
Multiple confounding factors may have affected this project’s results. First, Class I recall for point-of-care test strips used by the clinic was mandated by the US Food and Drug Administration on November 1, 2018.5 Before the recall, investigators found that many nontherapeutic INRs using point-of-care testing later showed results that were within the therapeutic INR range using same-day venous blood collection. This may have led to increases in falsely recorded nontherapeutic INRs and lowered TTR report results. Initially, the project was designed to collect monthly clinic encounter data for 3 months following the 8-week screening period; however, data collection was stopped after 2 months because of the test strip recall.
In addition, in early December 2018, all patients were moved from the Anticoagulation Clinic to the Anticoagulation Telephone Clinic that uses venous blood draws and telephone appointments. Data from venous blood draw results had previously been excluded from this project because results were not available on the same day. Patients in this program are contacted by telephone rather than being offered a face-to-face appointment, thus reducing in-clinic encounters.
Another confounding factor was a FVAHCC policy change in August 2018 requiring that any patient initiated on a DOAC make a onetime visit to the Anticoagulation Clinic prior to establishing care with a PACT CPS. Investigators were unable to exclude these patients from monthly encounter data. Some patients transitioning from warfarin to DOAC therapy were required to continue receiving anticoagulation monitoring from the clinic because of limited PACT CPS clinic availability, thus further increasing postscreening encounters.
Health care providers outside of the Anticoagulation Clinic and uninvolved with the quality improvement project also were switching patients from warfarin to DOAC therapies. Although this may have affected encounter data positively, investigators cannot guarantee these patients would have met criteria outlined by the screening tool.
In September 2018 Hurricane Florence disrupted health care delivery during the 8-week screening period. This event disrupted numerous clinic appointments. Although screening of patients was completed during the 8-week screening period, some patients did not switch to DOAC therapies until November 2018.
Secondary Endpoint Results
Promising results can be seen by specifically looking at the secondary endpoint: the number of encounters associated with patients who chose DOAC therapy. There were 107 encounters associated with the 30 patients who switched to a DOAC prior to screening and only 32 associated encounters after screening, a reduction of 70.1%. This suggests that multiple appointment slots were freed when the screening tool led to successful conversion from warfarin to a DOAC. Further assessment is warranted.
Future Project Development
Future areas for quality improvement project development include expanding project criteria to include patients taking warfarin for VTE. Eighty-nine of 137 patients (65%) who were deemed ineligible to switch to DOAC therapy were excluded due to a diagnosis of VTE. There are existing VA/Department of Defense Criteria for Use for DOAC use in VTE recommendations. Straightforward modification of the screening tool could include this patient group and may be especially useful for patients on indefinite warfarin therapy for recurrent VTE who have poor TTR report results.6
Given the number of confounding factors caused by unforeseen changes to the Anticoagulation Clinic workflow, use of the DOAC screening tool was placed on hold at the conclusion of data collection. This limited the ability to analyze encounter data in the months following project conclusion. Future plans include reimplementation of the screening tool with minor adjustments to include patients on warfarin for VTE and patients with a TTR report results above 60%.
Conclusion
This quality improvement project sought to determine the impact of a screening tool on effecting Anticoagulation Clinic encounter metrics. Results of this project show that the screening tool was unsuccessful in reducing the number of overall clinic encounters. Some promise was shown when evaluating clinic encounters for patients who switched anticoagulation therapies. Numerous confounding factors may have contributed to these results.
Metrics from 2017 at the Fayetteville Veterans Affairs Heath Care Center (FVAHCC) Anticoagulation Clinic indicate that 43% of patients with atrial fibrillation (AF) who are prescribed warfarin have difficulty maintaining a therapeutic international normalized ratio (INR). These patients require frequent clinic appointments to adjust their regimens to ensure anticoagulation efficacy. FVAHCC policy requires a patient to return to the clinic for repeat INR evaluation within 5 to 14 days of the visit where INR was outside of the established therapeutic range.1 These frequent INR monitoring appointments increase patient and health care provider burden.
Direct oral anticoagulants (DOACs) are an alternative to warfarin for patients with AF who require anticoagulation. DOACs, which do not require regular efficacy monitoring, can be beneficial to patients who struggle to maintain a therapeutic INR when taking warfarin. FVAHCC policy regarding warfarin therapy monitoring allows for a maximum of 6 weeks between appointments. This period is often extended to 3 to 6 months for patients on DOACs.1
At FVAHCC, patients prescribed warfarin are managed in a centralized Anticoagulation Clinic led by a clinical pharmacy specialist (CPS). When a patient reports for an appointment, a clinical pharmacy technician performs point-of-care INR testing and asks standardized questions regarding therapy, including an assessment of adherence. The CPS then evaluates the patient’s INR test results, adjusts the dosage of warfarin as indicated, and determines appropriate follow-up.
A patient who is prescribed a DOAC is monitored by a CPS who works within a patient aligned care team (PACT). The PACT, a multidisciplinary team providing health care to veterans, includes physicians, nurses, pharmacists, dieticians, and mental health providers. Each CPS covers 3 or 4 PACTs. These pharmacists monitor all aspects of DOAC therapy at regular intervals, including renal and hepatic function, complete blood counts, medication adherence, and adverse effects.
Clinic and patient INR data are tracked using a time in therapeutic range (TTR) report generated by the US Department of Veterans Affairs (VA). The TTR report provides clinical information to enhance patient anticoagulation care.2 The TTR report identifies patients with an active order for warfarin and a diagnosis of AF or venous thromboembolism (VTE) whose INR is within therapeutic range (between 2 and 3) < 60% of the time over the previous 160 days.2 The patient must have had at least 3 INR levels drawn within that time frame for a TTR report calculation.2 The report excludes patients who were first prescribed warfarin within the previous 42 days and those with mechanical heart valves. The TTR report is used by the VA to see concrete facility-level results for quality improvement efforts.2
A quality improvement screening tool was developed to identify patients with AF being treated with warfarin who may appropriately transition to DOAC therapy. Anticoagulation Clinic patients were eligible for further evaluation if they had a TTR report level of < 60% and were prescribed indefinite warfarin therapy for AF.
The national VA goal is to have patient TTR report levels read > 60%. Therefore, the primary objective of this project was to improve Anticoagulation Clinic TTR metrics by targeting patients with TTR levels below the national goal.2
Patients who were successfully converted from warfarin to a DOAC were no longer included in Anticoagulation Clinic metrics and instead were followed by a PACT CPS. Thus, it was hypothesized that the average number of monthly Anticoagulation Clinic encounters would decrease on successful implementation of the screening tool. A secondary endpoint of the study evaluated the change in the total number of encounters of those who converted from warfarin to a DOAC.
Fewer clinic encounters could increase time available for the CPS to incorporate other initiatives into workflow and could increase clinic availability for newly referred veterans.
Methods
As this undertaking was considered to be a quality improvement project, institutional review board approval was not required. During an 8-week screening period (August to September 2018), the DOAC screening tool was implemented into the Anticoagulation Clinic workflow. This screening tool (Figure 1) was established based on VA Pharmacy Benefit Management (PBM) Service’s Criteria for Use for Stroke Prevention in Nonvalvular Atrial Fibrillation, a national set of standards used to determine appropriate candidates for DOAC therapy.3
Exclusion criteria included patients with INR goals < 2 or > 3, patients with a diagnosis of VTE, and patients with weight > 120 kg. Patients with a diagnosis of VTE were excluded due to the variability in therapy duration. Weight cutoffs were based on recommendations by the International Society on Thrombosis and Haemostasis. Due to a lack of available data, it was suggested that clinical judgment be used in patients whose weight was > 120 kg.4
During the screening period, weekly TTR reports identified patients in the clinic who had TTR < 60%. When a patient with a TTR report results of < 60% also had a scheduled appointment within a week, a CPS then further reviewed patient eligibility using the DOAC screening tool. On arrival for an appointment, the eligible patient was counseled on DOAC medications and the differences between warfarin and DOACs, including monitoring requirements. Patients had the option to switch to DOAC therapy or remain on warfarin.
The change in the average number of monthly Anticoagulation Clinic encounters for 3 months prior to the screening period (May to July 2018) and 2 months following screening (October to November 2018) was evaluated to measure the impact of the DOAC screening tool. The total number of encounters in the clinic was assessed using the monthly VA reports and were averaged for each period. Then data from the 2 periods were compared.
The monthly encounter reports, a data tool that monitors the number of unique visits per veteran each calendar month, also were used to generate a secondary endpoint showing the number of encounters in the Anticoagulation Clinic associated with patients who switched to a DOAC, including visits prior to changing therapy, and before and after the screening period.
Student’s t test was used to compare the change in encounter frequency before and after screening tool implementation for both primary and secondary endpoints. α was defined as .05 a priori. Continuous data were presented as means and standard deviations. Data were calculated with Microsoft Excel 2016.
Results
For the 3 months before the 8-week screening period, an average of 476 Anticoagulation Clinic encounters per month were documented. Two months of data following the screening period averaged 546 encounters per month. There were an average of 70 additional encounters per month after screening tool implementation (P = .15), reflecting the study’s primary objective.
A total of 219 patients in the Anticoagulation Clinic were identified as having a TTR report results of < 60% during the 8-week screening period (Figure 2). Eighty-two of those patients (37.4%) were considered eligible to switch from warfarin to DOAC therapy. Thirty of those eligible patients (13.7%) switched to a DOAC. A total of 107 clinic encounters (22.5%) was associated with these 30 patients prior to screening and 32 associated encounters (5.9%) following screening (P = .01). Of the remaining 137 patients (62.6%) who were ineligible for DOAC therapy, the most common reason for disqualification was a diagnosis of VTE (Table).
Discussion
The general results of this quality improvement project showed that implementation of a screening tool designed to identify patients eligible for DOAC therapy did not decrease the average number of Anticoagulation Clinic encounters. Thirty of 82 eligible patients (36.6%) decided to switch to DOAC therapy during the study period. For those 30 patients, there was a statistically significant decrease in the number of individual clinic encounters. This suggests that the screening tool may positively impact Anticoagulation Clinic metrics when evaluating individual patients, potentially increasing clinic appointment availability.
Confounding Factors
Multiple confounding factors may have affected this project’s results. First, Class I recall for point-of-care test strips used by the clinic was mandated by the US Food and Drug Administration on November 1, 2018.5 Before the recall, investigators found that many nontherapeutic INRs using point-of-care testing later showed results that were within the therapeutic INR range using same-day venous blood collection. This may have led to increases in falsely recorded nontherapeutic INRs and lowered TTR report results. Initially, the project was designed to collect monthly clinic encounter data for 3 months following the 8-week screening period; however, data collection was stopped after 2 months because of the test strip recall.
In addition, in early December 2018, all patients were moved from the Anticoagulation Clinic to the Anticoagulation Telephone Clinic that uses venous blood draws and telephone appointments. Data from venous blood draw results had previously been excluded from this project because results were not available on the same day. Patients in this program are contacted by telephone rather than being offered a face-to-face appointment, thus reducing in-clinic encounters.
Another confounding factor was a FVAHCC policy change in August 2018 requiring that any patient initiated on a DOAC make a onetime visit to the Anticoagulation Clinic prior to establishing care with a PACT CPS. Investigators were unable to exclude these patients from monthly encounter data. Some patients transitioning from warfarin to DOAC therapy were required to continue receiving anticoagulation monitoring from the clinic because of limited PACT CPS clinic availability, thus further increasing postscreening encounters.
Health care providers outside of the Anticoagulation Clinic and uninvolved with the quality improvement project also were switching patients from warfarin to DOAC therapies. Although this may have affected encounter data positively, investigators cannot guarantee these patients would have met criteria outlined by the screening tool.
In September 2018 Hurricane Florence disrupted health care delivery during the 8-week screening period. This event disrupted numerous clinic appointments. Although screening of patients was completed during the 8-week screening period, some patients did not switch to DOAC therapies until November 2018.
Secondary Endpoint Results
Promising results can be seen by specifically looking at the secondary endpoint: the number of encounters associated with patients who chose DOAC therapy. There were 107 encounters associated with the 30 patients who switched to a DOAC prior to screening and only 32 associated encounters after screening, a reduction of 70.1%. This suggests that multiple appointment slots were freed when the screening tool led to successful conversion from warfarin to a DOAC. Further assessment is warranted.
Future Project Development
Future areas for quality improvement project development include expanding project criteria to include patients taking warfarin for VTE. Eighty-nine of 137 patients (65%) who were deemed ineligible to switch to DOAC therapy were excluded due to a diagnosis of VTE. There are existing VA/Department of Defense Criteria for Use for DOAC use in VTE recommendations. Straightforward modification of the screening tool could include this patient group and may be especially useful for patients on indefinite warfarin therapy for recurrent VTE who have poor TTR report results.6
Given the number of confounding factors caused by unforeseen changes to the Anticoagulation Clinic workflow, use of the DOAC screening tool was placed on hold at the conclusion of data collection. This limited the ability to analyze encounter data in the months following project conclusion. Future plans include reimplementation of the screening tool with minor adjustments to include patients on warfarin for VTE and patients with a TTR report results above 60%.
Conclusion
This quality improvement project sought to determine the impact of a screening tool on effecting Anticoagulation Clinic encounter metrics. Results of this project show that the screening tool was unsuccessful in reducing the number of overall clinic encounters. Some promise was shown when evaluating clinic encounters for patients who switched anticoagulation therapies. Numerous confounding factors may have contributed to these results.
1. US Department of Veterans Affairs, Fayetteville Veterans Affairs Health Care Center. MCM 11-188 Anticoagulation Management Program. Revised July 11, 2017. [Source not verified.]
2. US Department of Veterans Affairs, Pharmacy Benefits Management Clinical Pharmacy Practice Office. Anticoagulation percent time in therapeutic range reports. https://spsites.cdw.va.gov/sites/PBM_CPPO/Pages/AnticoagulationTTR.aspx. Revised May 24, 2017. Accessed April 20, 2020. [Source not verified.]
3. US Department of Veterans Affairs, VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs). Dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis) and edoxaban (SAVAYSA) criteria for use for stroke prevention in nonvalvular atrial fibrillation (AF). https://www.pbm.va.gov/apps/VANationalFormulary/. Updated December 2017. Accessed April 30, 2020.
4. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308-1313.
5. US Food and Drug Administration. Roche Diagnostics recalls CoaguChek XS PT Test Strips due to naccurate INR test results. https://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm624822.htm. Published November 1, 2018. Accessed April 16, 2019.
6. US Department of Veterans Affairs, VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs) (formerly called TSOACs) dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban(Savaysa) criteria for use for *treatment of venous thromboembolism (VTE)* https://www.pbm.va.gov/apps/VANationalFormulary/. Updated December 2017. Accessed April 30, 2020.
1. US Department of Veterans Affairs, Fayetteville Veterans Affairs Health Care Center. MCM 11-188 Anticoagulation Management Program. Revised July 11, 2017. [Source not verified.]
2. US Department of Veterans Affairs, Pharmacy Benefits Management Clinical Pharmacy Practice Office. Anticoagulation percent time in therapeutic range reports. https://spsites.cdw.va.gov/sites/PBM_CPPO/Pages/AnticoagulationTTR.aspx. Revised May 24, 2017. Accessed April 20, 2020. [Source not verified.]
3. US Department of Veterans Affairs, VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs). Dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis) and edoxaban (SAVAYSA) criteria for use for stroke prevention in nonvalvular atrial fibrillation (AF). https://www.pbm.va.gov/apps/VANationalFormulary/. Updated December 2017. Accessed April 30, 2020.
4. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308-1313.
5. US Food and Drug Administration. Roche Diagnostics recalls CoaguChek XS PT Test Strips due to naccurate INR test results. https://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm624822.htm. Published November 1, 2018. Accessed April 16, 2019.
6. US Department of Veterans Affairs, VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs) (formerly called TSOACs) dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban(Savaysa) criteria for use for *treatment of venous thromboembolism (VTE)* https://www.pbm.va.gov/apps/VANationalFormulary/. Updated December 2017. Accessed April 30, 2020.
Performance of the Veterans Choice Program for Improving Access to Colonoscopy at a Tertiary VA Facility
In April 2014, amid concerns for long wait times for care within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA), the Veterans Access, Choice, and Accountability Act was signed into law. This included the Veterans Choice Program (VCP), which included a provision for veterans to be referred outside of the VA to the community for care if their nearest VHA facility could not provide the requested care within 30 days of the clinically indicated date.1 Since implementation of the VCP, both media outlets and policy researchers have raised concerns about both the timeliness and quality of care provided through this program.2-4
Specifically for colonoscopy, referral outside of the VA in the pre-VCP era resulted in lower adenoma detection rate (ADR) and decreased adherence to surveillance guidelines when compared with matched VA control colonoscopies, raising concerns about quality assurance.5 Colorectal cancer (CRC) screening and timely colonoscopy is a VA priority; however, the performance of the VCP for colonoscopy timelines and quality has not been examined in detail.
Methods
We identified 3,855 veterans at the VA Pittsburgh Healthcare System (VAPHS) who were referred for colonoscopy in the community by using VCP from June 2015 through May 2017, using a query for colonoscopy procedure orders within the VA Corporate Data Warehouse. A total of 190 patients had a colonoscopy completed in the community by utilizing the VCP during this time frame.
At VAPHS, veterans who are referred for colonoscopy are contacted by a scheduler. The scheduler contacts the patient and offers the first available colonoscopy date at VAPHS and schedules the procedure for this date. However, if this date is > 30 days from the procedure order date, the scheduler gives the veteran the option of being contacted by VCP to schedule a colonoscopy within the community (Figure 1). We measured the time interval from the date of the initially scheduled first available colonoscopy at VAPHS to the date the colonoscopy was actually performed through VCP.
Quality assurance also was assessed by checking for the availability of records of colonoscopies performed through the VCP in the VA electronic health record (EHR) system. Colonoscopy procedure reports also were reviewed to assess for documentation of established colonoscopy quality metrics for examinations performed through the VCP. Additionally, we reviewed records scanned into the VA EHR pertaining to the VCP colonoscopy, including pathology information and pre- or postvisit records if available.
Data extraction was initiated in November 2017 to allow for at least 6 months of lead time for outside health records from the community to be received and scanned into the EHR for the veteran at VAPHS. For colonoscopy quality metrics, we chose 3 metrics that are universally documented for all colonoscopy procedures performed at VAPHS: quality of bowel preparation, cecal withdrawal time, and performance of retroflexion in the rectum. Documentation of these quality metrics is recommended in gastroenterology practice guidelinesand/or required by VA national policy.6,7
We separately reviewed a sample of 350 of the 3,855 patients referred for colonoscopy through VCP at VAPHS during the same time period to investigate overall VCP utilization. This sample was representative at a 95% CI with 5% margin of error of the total and sampled from 2 high-volume referral months (October and November 2015) and 3 low-volume months (January, February, and March 2017). Detailed data were collected regarding the colonoscopy scheduling and VCP referral process, including dates of colonoscopy procedure request; scheduling within the VAPHS; scheduling through the VCP; and ultimately if, when, and where (VAPHS vs community) a veteran had a colonoscopy performed. Wait times for colonoscopy procedures performed at the VAPHS and those performed through the VCP were compared.
The institutional review board at VAPHS reviewed and approved this quality improvement study.
Statistical Analysis
For the 190 veterans who had a colonoscopy performed through VCP, a 1-sample Wilcoxon signed rank test was used with a null hypothesis that the median difference in days between first available VAPHS colonoscopy and community colonoscopy dates was 0. For the utilization sample of 350 veterans, an independent samples median test was used to compare the median wait times for colonoscopy procedures performed at the VA and those performed through VCP. IBM SPSS Version 25 was used for all statistical analysis.
Results
Of the 190 identified colonoscopies completed in the community utilizing VCP, scanned records could not be found for 29 procedures (15.3%) (Table). VCP procedures were performed a median 2 days earlier than the first available VAPHS procedure, but this difference was not statistically significant (P = .62) (Figure 2). Although 52% of colonoscopies occurred sooner through VCP than the initially scheduled VAPHS date, 44% were performed later, and there was wide variability in the difference between these dates, ranging from 49 days sooner to 165 days later.
Pathology results from VCP procedures for which tissue samples were obtained were absent in 11.9% (14 of 118) of procedures. There were no clear follow-up recommendations to referring VA health care providers in the 18% (29 of 161) of available procedure reports. In VCP procedures, documentation of selected quality metrics: bowel preparation, cecal withdrawal time, and rectal retroflexion, were deficient in 27.3%, 70.2%, and 32.9%, respectively (Figure 3).
The utilization dataset sample included 350 veterans who were offered a VCP colonoscopy because the first available VAPHS procedure could not be scheduled for > 30 days. Of these patients, 231 (66%) ultimately had their colonoscopy performed at VAPHS. An additional 26.6% of the patients in the utilization sample were lost in the scheduling process (ie, could not be contacted, cancelled and could not be rescheduled, or were a “no show” their scheduled VAPHS procedure). An unknown number of these patients may have had a procedure outside of the VA, but there are no records to confirm or exclude this possibility. Ultimately, there were only 26 (7.4%) confirmed VCP colonoscopy procedures within the utilization sample (Figure 4). The median actual wait time for colonoscopy was 61 days for VA procedures and 66 days for procedures referred through the VCP, which was not statistically significant (P = .15).
Discussion
This is the first study to evaluate the performance of the VCP for colonoscopy referrals. Consistent with recently reported data in other specialties, colonoscopy referrals through VCP did not lead to more timely procedures overall, although there was wide variation.8 The use of VCP for veteran referral to the community for colonoscopy led to fragmentation of care—with 15% of records for VCP colonoscopies unavailable in the VA EHR 6 months after the procedure. In addition, there were 45 pre- or postprocedure visits in the community, which is not standard practice at VAPHS, and therefore may add to the cost of care for veterans.
Documentation of selected colonoscopy quality metrics were deficient in 27.3% to 70.2% of available VCP procedure reports. Although many veterans were eligible for VCP referral for colonoscopy, only 7.4% had a documented procedure through VCP, and two-thirds of veterans eligible for VCP participation had their colonoscopy performed at the VAPHS, reflecting overall low utilization of the program.
The national average wait time for VCP referrals for multiple specialties was estimated to be 51 days in a 2018 Government Accountability Office (GAO) report, which is similar to our findings.9 The GAO report also concluded that the VCP does not have timeliness standards and notes missed opportunities to develop a mechanism for record transfer between the community and the VA. Our finding of missing colonoscopy procedure and pathology reports within the VA EHR is consistent with this claim. Our analysis revealed that widely accepted quality standards for colonoscopy, those that are required at the VA and monitored for quality assurance at the VAPHS, are not being consistently reported for veterans who undergo procedures in the community. Last, the overall low utilization rate, combined with overall similar wait times for colonoscopies referred through the VCP vs those done at the VA, should lead to reconsideration of offering community care referral to all veterans based solely on static wait time cutoffs.
Limitations
There are several limitations to our analysis. First, all data were extracted via chart review by one author; therefore, some scanned procedure or pathology reports or pre- and postprocedure records may have been missed. Second, these data are representative of a single VA medical center and may not reflect trends nationwide. Third, there are many factors that can influence veteran decision making regarding when and where colonoscopy procedures are performed, which could be related to the VCP community care referral process or independent of this. Finally, colonoscopies performed through the VCP are grouped and may not reflect variability in the performance of community practices that veterans were referred to though the VCP.
Adenoma detection rates (ADR) were not included in the assessment for 2 reasons. First, there was an insufficient number of screening colonoscopies to use for the ADR calculation. Second, a composite non-VA ADR of multiple community endoscopists in different practices would likely be inaccurate and not clinically meaningful. Of note, the VAPHS does calculate and maintain ADR information as a practice for its endoscopists.
Conclusions
Our findings are particularly important as the VA expands access to care in the community through the VA Mission Act, which replaces the VCP but continues to include a static wait time threshold of 28 days for referral to community-based care.10 Especially for colonoscopies with the indication of screening or surveillance, wait times > 28 days are likely not clinically significant. Additionally, this study demonstrates that there also are delays in access to colonoscopy by community-based care providers, and potentially reflects widespread colonoscopy access issues that are not unique to the VA.
Our findings are similar to other published results and reports and raise similar concerns about the pitfalls of veteran referral into the community, including (1) similar wait times for the community and the VA; (2) the risk of fragmented care; (3) unevenquality of care; and (4) low overall utilization of VCP for colonoscopy.11 We agree with the GAO’s recommendations, which include establishing clinically meaningful wait time thresholds, systemic monitoring of the timeliness of care, and additional mechanisms for seamless transfer of complete records of care into the VA system. If a referral is placed for community-based care, this should come with an expectation that the care will be offered and can be delivered sooner than would be possible at the VA. We additionally recommend that standards for reporting quality metrics, including ADR, also be required of community colonoscopy providers contracted to provide care for veterans through the VA Mission Act. Importantly, we recommend that data for comparative wait times and quality metrics for VA and the community should be publicly available for veterans so that they may make more informed choices about where they receive health care.
Acknowledgments
The authors thank Kaneen Allen, PhD, for her administrative assistance and guidance.
1. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
2. Farmer CM, Hosek SD. Did we improve veterans health care? It’s unclear. https://www.rand.org/blog/2016/05/did-we-improve-veterans-health-care-its-unclear.html. Published May 24, 2016. Accessed April 20, 2020.
3. Farmer CM, Hosek SD, Adamson DM. balancing demand and supply for veterans’ health care: a summary of three RAND assessments conducted under the Veterans Choice Act. Rand Health Q. 2016;6(1):12.
4. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: a qualitative examination of rapid policy implementation in the Department of Veterans Affairs. Med Care. 2017;55(suppl 7)(suppl 1):S71-S75.
5. Bartel MJ, Robertson DJ, Pohl H. Colonoscopy practice for veterans within and outside the Veterans Affairs setting: a matched cohort study. Gastrointest Endosc. 2016;84(2):272-278.
6. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110(1):72-90.
7. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1015, colorectal cancer screening. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3068.Published December 30, 2014. Accessed April 12, 2020.
8. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
9. US Government Accountability Office. Veterans Choice Program: improvements needed to address access-related challenges as VA plans consolidation of its community care programs. https://www.gao.gov/assets/700/692271.pdf. Published June 4, 2018. Accessed April 12, 2020.
10. VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018. 38 USC §1703 (2018).
11. Barnett PG, Hong JS, Carey E, Grunwald GK, Joynt Maddox K, Maddox TM. Comparison of accessibility, cost, and quality of elective coronary revascularization between Veterans Affairs and community care hospitals. JAMA Cardiol. 2018;3(2):133-141.
In April 2014, amid concerns for long wait times for care within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA), the Veterans Access, Choice, and Accountability Act was signed into law. This included the Veterans Choice Program (VCP), which included a provision for veterans to be referred outside of the VA to the community for care if their nearest VHA facility could not provide the requested care within 30 days of the clinically indicated date.1 Since implementation of the VCP, both media outlets and policy researchers have raised concerns about both the timeliness and quality of care provided through this program.2-4
Specifically for colonoscopy, referral outside of the VA in the pre-VCP era resulted in lower adenoma detection rate (ADR) and decreased adherence to surveillance guidelines when compared with matched VA control colonoscopies, raising concerns about quality assurance.5 Colorectal cancer (CRC) screening and timely colonoscopy is a VA priority; however, the performance of the VCP for colonoscopy timelines and quality has not been examined in detail.
Methods
We identified 3,855 veterans at the VA Pittsburgh Healthcare System (VAPHS) who were referred for colonoscopy in the community by using VCP from June 2015 through May 2017, using a query for colonoscopy procedure orders within the VA Corporate Data Warehouse. A total of 190 patients had a colonoscopy completed in the community by utilizing the VCP during this time frame.
At VAPHS, veterans who are referred for colonoscopy are contacted by a scheduler. The scheduler contacts the patient and offers the first available colonoscopy date at VAPHS and schedules the procedure for this date. However, if this date is > 30 days from the procedure order date, the scheduler gives the veteran the option of being contacted by VCP to schedule a colonoscopy within the community (Figure 1). We measured the time interval from the date of the initially scheduled first available colonoscopy at VAPHS to the date the colonoscopy was actually performed through VCP.
Quality assurance also was assessed by checking for the availability of records of colonoscopies performed through the VCP in the VA electronic health record (EHR) system. Colonoscopy procedure reports also were reviewed to assess for documentation of established colonoscopy quality metrics for examinations performed through the VCP. Additionally, we reviewed records scanned into the VA EHR pertaining to the VCP colonoscopy, including pathology information and pre- or postvisit records if available.
Data extraction was initiated in November 2017 to allow for at least 6 months of lead time for outside health records from the community to be received and scanned into the EHR for the veteran at VAPHS. For colonoscopy quality metrics, we chose 3 metrics that are universally documented for all colonoscopy procedures performed at VAPHS: quality of bowel preparation, cecal withdrawal time, and performance of retroflexion in the rectum. Documentation of these quality metrics is recommended in gastroenterology practice guidelinesand/or required by VA national policy.6,7
We separately reviewed a sample of 350 of the 3,855 patients referred for colonoscopy through VCP at VAPHS during the same time period to investigate overall VCP utilization. This sample was representative at a 95% CI with 5% margin of error of the total and sampled from 2 high-volume referral months (October and November 2015) and 3 low-volume months (January, February, and March 2017). Detailed data were collected regarding the colonoscopy scheduling and VCP referral process, including dates of colonoscopy procedure request; scheduling within the VAPHS; scheduling through the VCP; and ultimately if, when, and where (VAPHS vs community) a veteran had a colonoscopy performed. Wait times for colonoscopy procedures performed at the VAPHS and those performed through the VCP were compared.
The institutional review board at VAPHS reviewed and approved this quality improvement study.
Statistical Analysis
For the 190 veterans who had a colonoscopy performed through VCP, a 1-sample Wilcoxon signed rank test was used with a null hypothesis that the median difference in days between first available VAPHS colonoscopy and community colonoscopy dates was 0. For the utilization sample of 350 veterans, an independent samples median test was used to compare the median wait times for colonoscopy procedures performed at the VA and those performed through VCP. IBM SPSS Version 25 was used for all statistical analysis.
Results
Of the 190 identified colonoscopies completed in the community utilizing VCP, scanned records could not be found for 29 procedures (15.3%) (Table). VCP procedures were performed a median 2 days earlier than the first available VAPHS procedure, but this difference was not statistically significant (P = .62) (Figure 2). Although 52% of colonoscopies occurred sooner through VCP than the initially scheduled VAPHS date, 44% were performed later, and there was wide variability in the difference between these dates, ranging from 49 days sooner to 165 days later.
Pathology results from VCP procedures for which tissue samples were obtained were absent in 11.9% (14 of 118) of procedures. There were no clear follow-up recommendations to referring VA health care providers in the 18% (29 of 161) of available procedure reports. In VCP procedures, documentation of selected quality metrics: bowel preparation, cecal withdrawal time, and rectal retroflexion, were deficient in 27.3%, 70.2%, and 32.9%, respectively (Figure 3).
The utilization dataset sample included 350 veterans who were offered a VCP colonoscopy because the first available VAPHS procedure could not be scheduled for > 30 days. Of these patients, 231 (66%) ultimately had their colonoscopy performed at VAPHS. An additional 26.6% of the patients in the utilization sample were lost in the scheduling process (ie, could not be contacted, cancelled and could not be rescheduled, or were a “no show” their scheduled VAPHS procedure). An unknown number of these patients may have had a procedure outside of the VA, but there are no records to confirm or exclude this possibility. Ultimately, there were only 26 (7.4%) confirmed VCP colonoscopy procedures within the utilization sample (Figure 4). The median actual wait time for colonoscopy was 61 days for VA procedures and 66 days for procedures referred through the VCP, which was not statistically significant (P = .15).
Discussion
This is the first study to evaluate the performance of the VCP for colonoscopy referrals. Consistent with recently reported data in other specialties, colonoscopy referrals through VCP did not lead to more timely procedures overall, although there was wide variation.8 The use of VCP for veteran referral to the community for colonoscopy led to fragmentation of care—with 15% of records for VCP colonoscopies unavailable in the VA EHR 6 months after the procedure. In addition, there were 45 pre- or postprocedure visits in the community, which is not standard practice at VAPHS, and therefore may add to the cost of care for veterans.
Documentation of selected colonoscopy quality metrics were deficient in 27.3% to 70.2% of available VCP procedure reports. Although many veterans were eligible for VCP referral for colonoscopy, only 7.4% had a documented procedure through VCP, and two-thirds of veterans eligible for VCP participation had their colonoscopy performed at the VAPHS, reflecting overall low utilization of the program.
The national average wait time for VCP referrals for multiple specialties was estimated to be 51 days in a 2018 Government Accountability Office (GAO) report, which is similar to our findings.9 The GAO report also concluded that the VCP does not have timeliness standards and notes missed opportunities to develop a mechanism for record transfer between the community and the VA. Our finding of missing colonoscopy procedure and pathology reports within the VA EHR is consistent with this claim. Our analysis revealed that widely accepted quality standards for colonoscopy, those that are required at the VA and monitored for quality assurance at the VAPHS, are not being consistently reported for veterans who undergo procedures in the community. Last, the overall low utilization rate, combined with overall similar wait times for colonoscopies referred through the VCP vs those done at the VA, should lead to reconsideration of offering community care referral to all veterans based solely on static wait time cutoffs.
Limitations
There are several limitations to our analysis. First, all data were extracted via chart review by one author; therefore, some scanned procedure or pathology reports or pre- and postprocedure records may have been missed. Second, these data are representative of a single VA medical center and may not reflect trends nationwide. Third, there are many factors that can influence veteran decision making regarding when and where colonoscopy procedures are performed, which could be related to the VCP community care referral process or independent of this. Finally, colonoscopies performed through the VCP are grouped and may not reflect variability in the performance of community practices that veterans were referred to though the VCP.
Adenoma detection rates (ADR) were not included in the assessment for 2 reasons. First, there was an insufficient number of screening colonoscopies to use for the ADR calculation. Second, a composite non-VA ADR of multiple community endoscopists in different practices would likely be inaccurate and not clinically meaningful. Of note, the VAPHS does calculate and maintain ADR information as a practice for its endoscopists.
Conclusions
Our findings are particularly important as the VA expands access to care in the community through the VA Mission Act, which replaces the VCP but continues to include a static wait time threshold of 28 days for referral to community-based care.10 Especially for colonoscopies with the indication of screening or surveillance, wait times > 28 days are likely not clinically significant. Additionally, this study demonstrates that there also are delays in access to colonoscopy by community-based care providers, and potentially reflects widespread colonoscopy access issues that are not unique to the VA.
Our findings are similar to other published results and reports and raise similar concerns about the pitfalls of veteran referral into the community, including (1) similar wait times for the community and the VA; (2) the risk of fragmented care; (3) unevenquality of care; and (4) low overall utilization of VCP for colonoscopy.11 We agree with the GAO’s recommendations, which include establishing clinically meaningful wait time thresholds, systemic monitoring of the timeliness of care, and additional mechanisms for seamless transfer of complete records of care into the VA system. If a referral is placed for community-based care, this should come with an expectation that the care will be offered and can be delivered sooner than would be possible at the VA. We additionally recommend that standards for reporting quality metrics, including ADR, also be required of community colonoscopy providers contracted to provide care for veterans through the VA Mission Act. Importantly, we recommend that data for comparative wait times and quality metrics for VA and the community should be publicly available for veterans so that they may make more informed choices about where they receive health care.
Acknowledgments
The authors thank Kaneen Allen, PhD, for her administrative assistance and guidance.
In April 2014, amid concerns for long wait times for care within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA), the Veterans Access, Choice, and Accountability Act was signed into law. This included the Veterans Choice Program (VCP), which included a provision for veterans to be referred outside of the VA to the community for care if their nearest VHA facility could not provide the requested care within 30 days of the clinically indicated date.1 Since implementation of the VCP, both media outlets and policy researchers have raised concerns about both the timeliness and quality of care provided through this program.2-4
Specifically for colonoscopy, referral outside of the VA in the pre-VCP era resulted in lower adenoma detection rate (ADR) and decreased adherence to surveillance guidelines when compared with matched VA control colonoscopies, raising concerns about quality assurance.5 Colorectal cancer (CRC) screening and timely colonoscopy is a VA priority; however, the performance of the VCP for colonoscopy timelines and quality has not been examined in detail.
Methods
We identified 3,855 veterans at the VA Pittsburgh Healthcare System (VAPHS) who were referred for colonoscopy in the community by using VCP from June 2015 through May 2017, using a query for colonoscopy procedure orders within the VA Corporate Data Warehouse. A total of 190 patients had a colonoscopy completed in the community by utilizing the VCP during this time frame.
At VAPHS, veterans who are referred for colonoscopy are contacted by a scheduler. The scheduler contacts the patient and offers the first available colonoscopy date at VAPHS and schedules the procedure for this date. However, if this date is > 30 days from the procedure order date, the scheduler gives the veteran the option of being contacted by VCP to schedule a colonoscopy within the community (Figure 1). We measured the time interval from the date of the initially scheduled first available colonoscopy at VAPHS to the date the colonoscopy was actually performed through VCP.
Quality assurance also was assessed by checking for the availability of records of colonoscopies performed through the VCP in the VA electronic health record (EHR) system. Colonoscopy procedure reports also were reviewed to assess for documentation of established colonoscopy quality metrics for examinations performed through the VCP. Additionally, we reviewed records scanned into the VA EHR pertaining to the VCP colonoscopy, including pathology information and pre- or postvisit records if available.
Data extraction was initiated in November 2017 to allow for at least 6 months of lead time for outside health records from the community to be received and scanned into the EHR for the veteran at VAPHS. For colonoscopy quality metrics, we chose 3 metrics that are universally documented for all colonoscopy procedures performed at VAPHS: quality of bowel preparation, cecal withdrawal time, and performance of retroflexion in the rectum. Documentation of these quality metrics is recommended in gastroenterology practice guidelinesand/or required by VA national policy.6,7
We separately reviewed a sample of 350 of the 3,855 patients referred for colonoscopy through VCP at VAPHS during the same time period to investigate overall VCP utilization. This sample was representative at a 95% CI with 5% margin of error of the total and sampled from 2 high-volume referral months (October and November 2015) and 3 low-volume months (January, February, and March 2017). Detailed data were collected regarding the colonoscopy scheduling and VCP referral process, including dates of colonoscopy procedure request; scheduling within the VAPHS; scheduling through the VCP; and ultimately if, when, and where (VAPHS vs community) a veteran had a colonoscopy performed. Wait times for colonoscopy procedures performed at the VAPHS and those performed through the VCP were compared.
The institutional review board at VAPHS reviewed and approved this quality improvement study.
Statistical Analysis
For the 190 veterans who had a colonoscopy performed through VCP, a 1-sample Wilcoxon signed rank test was used with a null hypothesis that the median difference in days between first available VAPHS colonoscopy and community colonoscopy dates was 0. For the utilization sample of 350 veterans, an independent samples median test was used to compare the median wait times for colonoscopy procedures performed at the VA and those performed through VCP. IBM SPSS Version 25 was used for all statistical analysis.
Results
Of the 190 identified colonoscopies completed in the community utilizing VCP, scanned records could not be found for 29 procedures (15.3%) (Table). VCP procedures were performed a median 2 days earlier than the first available VAPHS procedure, but this difference was not statistically significant (P = .62) (Figure 2). Although 52% of colonoscopies occurred sooner through VCP than the initially scheduled VAPHS date, 44% were performed later, and there was wide variability in the difference between these dates, ranging from 49 days sooner to 165 days later.
Pathology results from VCP procedures for which tissue samples were obtained were absent in 11.9% (14 of 118) of procedures. There were no clear follow-up recommendations to referring VA health care providers in the 18% (29 of 161) of available procedure reports. In VCP procedures, documentation of selected quality metrics: bowel preparation, cecal withdrawal time, and rectal retroflexion, were deficient in 27.3%, 70.2%, and 32.9%, respectively (Figure 3).
The utilization dataset sample included 350 veterans who were offered a VCP colonoscopy because the first available VAPHS procedure could not be scheduled for > 30 days. Of these patients, 231 (66%) ultimately had their colonoscopy performed at VAPHS. An additional 26.6% of the patients in the utilization sample were lost in the scheduling process (ie, could not be contacted, cancelled and could not be rescheduled, or were a “no show” their scheduled VAPHS procedure). An unknown number of these patients may have had a procedure outside of the VA, but there are no records to confirm or exclude this possibility. Ultimately, there were only 26 (7.4%) confirmed VCP colonoscopy procedures within the utilization sample (Figure 4). The median actual wait time for colonoscopy was 61 days for VA procedures and 66 days for procedures referred through the VCP, which was not statistically significant (P = .15).
Discussion
This is the first study to evaluate the performance of the VCP for colonoscopy referrals. Consistent with recently reported data in other specialties, colonoscopy referrals through VCP did not lead to more timely procedures overall, although there was wide variation.8 The use of VCP for veteran referral to the community for colonoscopy led to fragmentation of care—with 15% of records for VCP colonoscopies unavailable in the VA EHR 6 months after the procedure. In addition, there were 45 pre- or postprocedure visits in the community, which is not standard practice at VAPHS, and therefore may add to the cost of care for veterans.
Documentation of selected colonoscopy quality metrics were deficient in 27.3% to 70.2% of available VCP procedure reports. Although many veterans were eligible for VCP referral for colonoscopy, only 7.4% had a documented procedure through VCP, and two-thirds of veterans eligible for VCP participation had their colonoscopy performed at the VAPHS, reflecting overall low utilization of the program.
The national average wait time for VCP referrals for multiple specialties was estimated to be 51 days in a 2018 Government Accountability Office (GAO) report, which is similar to our findings.9 The GAO report also concluded that the VCP does not have timeliness standards and notes missed opportunities to develop a mechanism for record transfer between the community and the VA. Our finding of missing colonoscopy procedure and pathology reports within the VA EHR is consistent with this claim. Our analysis revealed that widely accepted quality standards for colonoscopy, those that are required at the VA and monitored for quality assurance at the VAPHS, are not being consistently reported for veterans who undergo procedures in the community. Last, the overall low utilization rate, combined with overall similar wait times for colonoscopies referred through the VCP vs those done at the VA, should lead to reconsideration of offering community care referral to all veterans based solely on static wait time cutoffs.
Limitations
There are several limitations to our analysis. First, all data were extracted via chart review by one author; therefore, some scanned procedure or pathology reports or pre- and postprocedure records may have been missed. Second, these data are representative of a single VA medical center and may not reflect trends nationwide. Third, there are many factors that can influence veteran decision making regarding when and where colonoscopy procedures are performed, which could be related to the VCP community care referral process or independent of this. Finally, colonoscopies performed through the VCP are grouped and may not reflect variability in the performance of community practices that veterans were referred to though the VCP.
Adenoma detection rates (ADR) were not included in the assessment for 2 reasons. First, there was an insufficient number of screening colonoscopies to use for the ADR calculation. Second, a composite non-VA ADR of multiple community endoscopists in different practices would likely be inaccurate and not clinically meaningful. Of note, the VAPHS does calculate and maintain ADR information as a practice for its endoscopists.
Conclusions
Our findings are particularly important as the VA expands access to care in the community through the VA Mission Act, which replaces the VCP but continues to include a static wait time threshold of 28 days for referral to community-based care.10 Especially for colonoscopies with the indication of screening or surveillance, wait times > 28 days are likely not clinically significant. Additionally, this study demonstrates that there also are delays in access to colonoscopy by community-based care providers, and potentially reflects widespread colonoscopy access issues that are not unique to the VA.
Our findings are similar to other published results and reports and raise similar concerns about the pitfalls of veteran referral into the community, including (1) similar wait times for the community and the VA; (2) the risk of fragmented care; (3) unevenquality of care; and (4) low overall utilization of VCP for colonoscopy.11 We agree with the GAO’s recommendations, which include establishing clinically meaningful wait time thresholds, systemic monitoring of the timeliness of care, and additional mechanisms for seamless transfer of complete records of care into the VA system. If a referral is placed for community-based care, this should come with an expectation that the care will be offered and can be delivered sooner than would be possible at the VA. We additionally recommend that standards for reporting quality metrics, including ADR, also be required of community colonoscopy providers contracted to provide care for veterans through the VA Mission Act. Importantly, we recommend that data for comparative wait times and quality metrics for VA and the community should be publicly available for veterans so that they may make more informed choices about where they receive health care.
Acknowledgments
The authors thank Kaneen Allen, PhD, for her administrative assistance and guidance.
1. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
2. Farmer CM, Hosek SD. Did we improve veterans health care? It’s unclear. https://www.rand.org/blog/2016/05/did-we-improve-veterans-health-care-its-unclear.html. Published May 24, 2016. Accessed April 20, 2020.
3. Farmer CM, Hosek SD, Adamson DM. balancing demand and supply for veterans’ health care: a summary of three RAND assessments conducted under the Veterans Choice Act. Rand Health Q. 2016;6(1):12.
4. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: a qualitative examination of rapid policy implementation in the Department of Veterans Affairs. Med Care. 2017;55(suppl 7)(suppl 1):S71-S75.
5. Bartel MJ, Robertson DJ, Pohl H. Colonoscopy practice for veterans within and outside the Veterans Affairs setting: a matched cohort study. Gastrointest Endosc. 2016;84(2):272-278.
6. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110(1):72-90.
7. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1015, colorectal cancer screening. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3068.Published December 30, 2014. Accessed April 12, 2020.
8. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
9. US Government Accountability Office. Veterans Choice Program: improvements needed to address access-related challenges as VA plans consolidation of its community care programs. https://www.gao.gov/assets/700/692271.pdf. Published June 4, 2018. Accessed April 12, 2020.
10. VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018. 38 USC §1703 (2018).
11. Barnett PG, Hong JS, Carey E, Grunwald GK, Joynt Maddox K, Maddox TM. Comparison of accessibility, cost, and quality of elective coronary revascularization between Veterans Affairs and community care hospitals. JAMA Cardiol. 2018;3(2):133-141.
1. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
2. Farmer CM, Hosek SD. Did we improve veterans health care? It’s unclear. https://www.rand.org/blog/2016/05/did-we-improve-veterans-health-care-its-unclear.html. Published May 24, 2016. Accessed April 20, 2020.
3. Farmer CM, Hosek SD, Adamson DM. balancing demand and supply for veterans’ health care: a summary of three RAND assessments conducted under the Veterans Choice Act. Rand Health Q. 2016;6(1):12.
4. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: a qualitative examination of rapid policy implementation in the Department of Veterans Affairs. Med Care. 2017;55(suppl 7)(suppl 1):S71-S75.
5. Bartel MJ, Robertson DJ, Pohl H. Colonoscopy practice for veterans within and outside the Veterans Affairs setting: a matched cohort study. Gastrointest Endosc. 2016;84(2):272-278.
6. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110(1):72-90.
7. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1015, colorectal cancer screening. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3068.Published December 30, 2014. Accessed April 12, 2020.
8. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
9. US Government Accountability Office. Veterans Choice Program: improvements needed to address access-related challenges as VA plans consolidation of its community care programs. https://www.gao.gov/assets/700/692271.pdf. Published June 4, 2018. Accessed April 12, 2020.
10. VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018. 38 USC §1703 (2018).
11. Barnett PG, Hong JS, Carey E, Grunwald GK, Joynt Maddox K, Maddox TM. Comparison of accessibility, cost, and quality of elective coronary revascularization between Veterans Affairs and community care hospitals. JAMA Cardiol. 2018;3(2):133-141.
Urgent and Emergent Eye Care Strategies to Protect Against COVID-19
COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and its symptoms range from mild to severe respiratory illness, fever, cough, fatigue, and shortness of breath.1 Diarrhea is common early on with infection and loss of taste and smell have also been reported.1 Follicular conjunctivitis has also been reported, either as an early sign of infection or during hospitalization for severe COVID-19 disease.2-4 The incubation period of COVID-19 falls within 2 to 14 days according to the CDC.5
It has been confirmed that COVID-19 is transmitted through both respiratory droplets and direct contact. Another possible route of viral transmission is entry through aerosolized droplets into the tears, which then pass through the nasolacrimal ducts and into the respiratory tract.6
Preparations Prior to Office Visit
It is essential for the eye care provider to prioritize patient care in order of absolute necessity, such as sudden vision loss, sudden onset flashes and floaters, and eye trauma. In cases of potentially sight threatening pathology, it is in the best interest of the patient to conduct a face-to-face appointment. Therefore, it is important to implement new guidelines and protocols as we continue to see these patients (Figure 1).
Prior to the patient entering the medical facility, measures should be implemented to minimize exposure risk. This can be done over the telephone or at vehicle entrance screening stations. The triage technician answering the telephone should have a script of questions to ask. The patient should be instructed to come into the office alone unless, for physical or mental reasons, a caregiver is required.
SARS-CoV-2 Screening Questions
Preparedness through risk mitigation strategies are recommended with a targeted questionnaire and noncontact temperature check at the clinic or hospital entrance. Below are some general questions to further triage patients exposed to SARS-CoV-2.
- Do you have fever or any respiratory symptoms?
- Do you have new or worsening cough or shortness of breath?
- Do you have flulike symptoms?
- Have you been in close contact with someone, including health care workers, confirmed to have the COVID-19?
If the patient answers yes to any of the above questions, the CDC urges health care providers to immediately notify both infection control personnel at your health care facility and your local or state health department.1,2 In regions currently managing significant outbreaks of COVID-19, the AAO recommends that eye care providers assume that any patient could be infected with SARS-CoV-2 and to proceed accordingly.2 If urgent eye care is needed, a referral call should be made to a hospital or center equipped to deal with COVID-19 and urgent eye conditions. When calling the referral center, ensure adequate staffing and space and relay all pertinent information along with receiving approval from the treating physician.
Face-to-Face Office Visits
Once it has been determined that it is in the best interest of the patient to be seen in a face-to-face visit, the patient should be instructed to call the office when they arrive in the parking lot. The CDC recommends limiting points of entry upon arrival and during the visit.1 As soon as an examination lane is ready, the patient can then be messaged to come into the office and escorted into the examination room.
An urgent or emergent ophthalmic examination for a patient with no respiratory symptoms, no fever, and no COVID-19 risk factors should include proper hand hygiene, use of personal protective equipment (PPE), and proper disinfection. Several studies have documented SARS-CoV-2 infection in asymptomatic and presymptomatic patients, making PPE of the up most importance.2,7,8 PPE should include mask, face shield, and gloves. Currently, there are national and international shortages on PPE and a heightened topic of discussion concerning mask use, effectiveness with extended wear, and reuse. Please refer to the CDC and AAO websites for up-to-date guidelines (Table).1,2 According to the CDC, N95 respirators are restricted to those performing or present for an aerosol-generating procedure.9
It is recommended that the eye care provider should only perform necessary tests and procedures. Noncontact tonometry should be avoided, as this might cause aerosolization of virus particles. The close proximity between eye care providers and their patients during slit-lamp examination may require further precautions to lower the risk of transmission via droplets or through hand to eye contact. The patient should be advised not to speak during the examination portion and the AAO also recommends a surgical mask or cloth face covering for the patient.2 An additional protective device that may be used during the slit-lamp exam is a breath shield or a barrier shield (Figures 2 and 3).2 Some manufacturers are offering clinicians free slit-lamp breath shields online.
Infection Prevention and Control Measures
Last, once the patient leaves the examination room, it should be properly disinfected. A disinfection checklist may be made to ensure uniform systematic cleaning. Alcohol and bleach-based disinfectants commonly used in health care settings are likely very effective against virus particles that cause COVID-19.10 During the disinfection process, gloves should be worn and careful attention paid to the contact time. Contact time is the amount of time the surface should appear visibly wet for proper disinfection. For example, Metrex CaviWipes have a recommended contact time of 3 minutes; however, this varies depending on type of virus and formulation, check labels or manufacturers’ websites for further directions.10 Also, the US Environmental Protection Agency has a database search available for disinfectants that meet their criteria for use against SARS-CoV-2.11
In an ever-changing environment, we offer this article to help equip providers to deliver the best possible patient care when face-to-face encounters are necessary. Currently nonurgent eye care follow-up visits are being conducted by telephone or video clinics. It is our goal to inform fellow practitioners on options and strategies to elevate the safety of staff and patients while minimizing the risk of exposure.
1. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): for healthcare professionals. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html. Updated April 7, 2020. Accessed April 13, 2020.
2. American Academy of Ophthalmology. Important coronavirus context for ophthalmologists. https://www.aao.org/headline/alert-important-coronavirus-context. Updated April 12, 2020. Accessed April 13, 2020.
3. Zhou Y, Zeng Y, Tong Y, Chen CZ. Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva [preprint]. https://doi.org/10.1101/2020.02.11.20021956. Published February 12, 2020. Accessed April 13, 2020.
4. Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020; 395(10224):e39.
5. Centers for Disease Control and Prevention. Symptoms of coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Updated March 20, 2020. Accessed April 13, 2020.
6. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;NEJMc2004973. [Published online ahead of print, March 17, 2020].
7. Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and Presymptomatic SARS-CoV-Infections in Residents of a Long-Term Care Skilled Nursing Facility - King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):377-381.
8. Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) [published online ahead of print, 2020 Mar 16]. Science. 2020; eabb3221.
9. Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Updated April 9, 2020. Accessed April 13, 2020.
10. Centers for Disease Control and Prevention. Cleaning and disinfection for households interim recommendations for U.S. households with suspected or confirmed coronavirus disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cleaning-disinfection.html. Updated March 28, 2020. Accessed April 13, 2020.
11. US Environmental Protection Agency. Pesticide registration: List N: disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Updated April 10, 2020. Accessed April 13, 2020.
COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and its symptoms range from mild to severe respiratory illness, fever, cough, fatigue, and shortness of breath.1 Diarrhea is common early on with infection and loss of taste and smell have also been reported.1 Follicular conjunctivitis has also been reported, either as an early sign of infection or during hospitalization for severe COVID-19 disease.2-4 The incubation period of COVID-19 falls within 2 to 14 days according to the CDC.5
It has been confirmed that COVID-19 is transmitted through both respiratory droplets and direct contact. Another possible route of viral transmission is entry through aerosolized droplets into the tears, which then pass through the nasolacrimal ducts and into the respiratory tract.6
Preparations Prior to Office Visit
It is essential for the eye care provider to prioritize patient care in order of absolute necessity, such as sudden vision loss, sudden onset flashes and floaters, and eye trauma. In cases of potentially sight threatening pathology, it is in the best interest of the patient to conduct a face-to-face appointment. Therefore, it is important to implement new guidelines and protocols as we continue to see these patients (Figure 1).
Prior to the patient entering the medical facility, measures should be implemented to minimize exposure risk. This can be done over the telephone or at vehicle entrance screening stations. The triage technician answering the telephone should have a script of questions to ask. The patient should be instructed to come into the office alone unless, for physical or mental reasons, a caregiver is required.
SARS-CoV-2 Screening Questions
Preparedness through risk mitigation strategies are recommended with a targeted questionnaire and noncontact temperature check at the clinic or hospital entrance. Below are some general questions to further triage patients exposed to SARS-CoV-2.
- Do you have fever or any respiratory symptoms?
- Do you have new or worsening cough or shortness of breath?
- Do you have flulike symptoms?
- Have you been in close contact with someone, including health care workers, confirmed to have the COVID-19?
If the patient answers yes to any of the above questions, the CDC urges health care providers to immediately notify both infection control personnel at your health care facility and your local or state health department.1,2 In regions currently managing significant outbreaks of COVID-19, the AAO recommends that eye care providers assume that any patient could be infected with SARS-CoV-2 and to proceed accordingly.2 If urgent eye care is needed, a referral call should be made to a hospital or center equipped to deal with COVID-19 and urgent eye conditions. When calling the referral center, ensure adequate staffing and space and relay all pertinent information along with receiving approval from the treating physician.
Face-to-Face Office Visits
Once it has been determined that it is in the best interest of the patient to be seen in a face-to-face visit, the patient should be instructed to call the office when they arrive in the parking lot. The CDC recommends limiting points of entry upon arrival and during the visit.1 As soon as an examination lane is ready, the patient can then be messaged to come into the office and escorted into the examination room.
An urgent or emergent ophthalmic examination for a patient with no respiratory symptoms, no fever, and no COVID-19 risk factors should include proper hand hygiene, use of personal protective equipment (PPE), and proper disinfection. Several studies have documented SARS-CoV-2 infection in asymptomatic and presymptomatic patients, making PPE of the up most importance.2,7,8 PPE should include mask, face shield, and gloves. Currently, there are national and international shortages on PPE and a heightened topic of discussion concerning mask use, effectiveness with extended wear, and reuse. Please refer to the CDC and AAO websites for up-to-date guidelines (Table).1,2 According to the CDC, N95 respirators are restricted to those performing or present for an aerosol-generating procedure.9
It is recommended that the eye care provider should only perform necessary tests and procedures. Noncontact tonometry should be avoided, as this might cause aerosolization of virus particles. The close proximity between eye care providers and their patients during slit-lamp examination may require further precautions to lower the risk of transmission via droplets or through hand to eye contact. The patient should be advised not to speak during the examination portion and the AAO also recommends a surgical mask or cloth face covering for the patient.2 An additional protective device that may be used during the slit-lamp exam is a breath shield or a barrier shield (Figures 2 and 3).2 Some manufacturers are offering clinicians free slit-lamp breath shields online.
Infection Prevention and Control Measures
Last, once the patient leaves the examination room, it should be properly disinfected. A disinfection checklist may be made to ensure uniform systematic cleaning. Alcohol and bleach-based disinfectants commonly used in health care settings are likely very effective against virus particles that cause COVID-19.10 During the disinfection process, gloves should be worn and careful attention paid to the contact time. Contact time is the amount of time the surface should appear visibly wet for proper disinfection. For example, Metrex CaviWipes have a recommended contact time of 3 minutes; however, this varies depending on type of virus and formulation, check labels or manufacturers’ websites for further directions.10 Also, the US Environmental Protection Agency has a database search available for disinfectants that meet their criteria for use against SARS-CoV-2.11
In an ever-changing environment, we offer this article to help equip providers to deliver the best possible patient care when face-to-face encounters are necessary. Currently nonurgent eye care follow-up visits are being conducted by telephone or video clinics. It is our goal to inform fellow practitioners on options and strategies to elevate the safety of staff and patients while minimizing the risk of exposure.
COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and its symptoms range from mild to severe respiratory illness, fever, cough, fatigue, and shortness of breath.1 Diarrhea is common early on with infection and loss of taste and smell have also been reported.1 Follicular conjunctivitis has also been reported, either as an early sign of infection or during hospitalization for severe COVID-19 disease.2-4 The incubation period of COVID-19 falls within 2 to 14 days according to the CDC.5
It has been confirmed that COVID-19 is transmitted through both respiratory droplets and direct contact. Another possible route of viral transmission is entry through aerosolized droplets into the tears, which then pass through the nasolacrimal ducts and into the respiratory tract.6
Preparations Prior to Office Visit
It is essential for the eye care provider to prioritize patient care in order of absolute necessity, such as sudden vision loss, sudden onset flashes and floaters, and eye trauma. In cases of potentially sight threatening pathology, it is in the best interest of the patient to conduct a face-to-face appointment. Therefore, it is important to implement new guidelines and protocols as we continue to see these patients (Figure 1).
Prior to the patient entering the medical facility, measures should be implemented to minimize exposure risk. This can be done over the telephone or at vehicle entrance screening stations. The triage technician answering the telephone should have a script of questions to ask. The patient should be instructed to come into the office alone unless, for physical or mental reasons, a caregiver is required.
SARS-CoV-2 Screening Questions
Preparedness through risk mitigation strategies are recommended with a targeted questionnaire and noncontact temperature check at the clinic or hospital entrance. Below are some general questions to further triage patients exposed to SARS-CoV-2.
- Do you have fever or any respiratory symptoms?
- Do you have new or worsening cough or shortness of breath?
- Do you have flulike symptoms?
- Have you been in close contact with someone, including health care workers, confirmed to have the COVID-19?
If the patient answers yes to any of the above questions, the CDC urges health care providers to immediately notify both infection control personnel at your health care facility and your local or state health department.1,2 In regions currently managing significant outbreaks of COVID-19, the AAO recommends that eye care providers assume that any patient could be infected with SARS-CoV-2 and to proceed accordingly.2 If urgent eye care is needed, a referral call should be made to a hospital or center equipped to deal with COVID-19 and urgent eye conditions. When calling the referral center, ensure adequate staffing and space and relay all pertinent information along with receiving approval from the treating physician.
Face-to-Face Office Visits
Once it has been determined that it is in the best interest of the patient to be seen in a face-to-face visit, the patient should be instructed to call the office when they arrive in the parking lot. The CDC recommends limiting points of entry upon arrival and during the visit.1 As soon as an examination lane is ready, the patient can then be messaged to come into the office and escorted into the examination room.
An urgent or emergent ophthalmic examination for a patient with no respiratory symptoms, no fever, and no COVID-19 risk factors should include proper hand hygiene, use of personal protective equipment (PPE), and proper disinfection. Several studies have documented SARS-CoV-2 infection in asymptomatic and presymptomatic patients, making PPE of the up most importance.2,7,8 PPE should include mask, face shield, and gloves. Currently, there are national and international shortages on PPE and a heightened topic of discussion concerning mask use, effectiveness with extended wear, and reuse. Please refer to the CDC and AAO websites for up-to-date guidelines (Table).1,2 According to the CDC, N95 respirators are restricted to those performing or present for an aerosol-generating procedure.9
It is recommended that the eye care provider should only perform necessary tests and procedures. Noncontact tonometry should be avoided, as this might cause aerosolization of virus particles. The close proximity between eye care providers and their patients during slit-lamp examination may require further precautions to lower the risk of transmission via droplets or through hand to eye contact. The patient should be advised not to speak during the examination portion and the AAO also recommends a surgical mask or cloth face covering for the patient.2 An additional protective device that may be used during the slit-lamp exam is a breath shield or a barrier shield (Figures 2 and 3).2 Some manufacturers are offering clinicians free slit-lamp breath shields online.
Infection Prevention and Control Measures
Last, once the patient leaves the examination room, it should be properly disinfected. A disinfection checklist may be made to ensure uniform systematic cleaning. Alcohol and bleach-based disinfectants commonly used in health care settings are likely very effective against virus particles that cause COVID-19.10 During the disinfection process, gloves should be worn and careful attention paid to the contact time. Contact time is the amount of time the surface should appear visibly wet for proper disinfection. For example, Metrex CaviWipes have a recommended contact time of 3 minutes; however, this varies depending on type of virus and formulation, check labels or manufacturers’ websites for further directions.10 Also, the US Environmental Protection Agency has a database search available for disinfectants that meet their criteria for use against SARS-CoV-2.11
In an ever-changing environment, we offer this article to help equip providers to deliver the best possible patient care when face-to-face encounters are necessary. Currently nonurgent eye care follow-up visits are being conducted by telephone or video clinics. It is our goal to inform fellow practitioners on options and strategies to elevate the safety of staff and patients while minimizing the risk of exposure.
1. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): for healthcare professionals. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html. Updated April 7, 2020. Accessed April 13, 2020.
2. American Academy of Ophthalmology. Important coronavirus context for ophthalmologists. https://www.aao.org/headline/alert-important-coronavirus-context. Updated April 12, 2020. Accessed April 13, 2020.
3. Zhou Y, Zeng Y, Tong Y, Chen CZ. Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva [preprint]. https://doi.org/10.1101/2020.02.11.20021956. Published February 12, 2020. Accessed April 13, 2020.
4. Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020; 395(10224):e39.
5. Centers for Disease Control and Prevention. Symptoms of coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Updated March 20, 2020. Accessed April 13, 2020.
6. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;NEJMc2004973. [Published online ahead of print, March 17, 2020].
7. Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and Presymptomatic SARS-CoV-Infections in Residents of a Long-Term Care Skilled Nursing Facility - King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):377-381.
8. Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) [published online ahead of print, 2020 Mar 16]. Science. 2020; eabb3221.
9. Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Updated April 9, 2020. Accessed April 13, 2020.
10. Centers for Disease Control and Prevention. Cleaning and disinfection for households interim recommendations for U.S. households with suspected or confirmed coronavirus disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cleaning-disinfection.html. Updated March 28, 2020. Accessed April 13, 2020.
11. US Environmental Protection Agency. Pesticide registration: List N: disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Updated April 10, 2020. Accessed April 13, 2020.
1. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): for healthcare professionals. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html. Updated April 7, 2020. Accessed April 13, 2020.
2. American Academy of Ophthalmology. Important coronavirus context for ophthalmologists. https://www.aao.org/headline/alert-important-coronavirus-context. Updated April 12, 2020. Accessed April 13, 2020.
3. Zhou Y, Zeng Y, Tong Y, Chen CZ. Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva [preprint]. https://doi.org/10.1101/2020.02.11.20021956. Published February 12, 2020. Accessed April 13, 2020.
4. Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020; 395(10224):e39.
5. Centers for Disease Control and Prevention. Symptoms of coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Updated March 20, 2020. Accessed April 13, 2020.
6. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;NEJMc2004973. [Published online ahead of print, March 17, 2020].
7. Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and Presymptomatic SARS-CoV-Infections in Residents of a Long-Term Care Skilled Nursing Facility - King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):377-381.
8. Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) [published online ahead of print, 2020 Mar 16]. Science. 2020; eabb3221.
9. Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Updated April 9, 2020. Accessed April 13, 2020.
10. Centers for Disease Control and Prevention. Cleaning and disinfection for households interim recommendations for U.S. households with suspected or confirmed coronavirus disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cleaning-disinfection.html. Updated March 28, 2020. Accessed April 13, 2020.
11. US Environmental Protection Agency. Pesticide registration: List N: disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Updated April 10, 2020. Accessed April 13, 2020.
Analysis of Education on Nail Conditions at the American Academy of Dermatology Annual Meetings
To the Editor:
The diagnosis and treatment of nail conditions are necessary competencies for board-certified dermatologists, but appropriate education often is lacking.1 The American Academy of Dermatology (AAD) annual meeting is one of the largest and most highly attended dermatology educational conferences worldwide. We sought to determine the number of hours dedicated to nail-related topics at the AAD annual meetings from 2013 to 2019.
We accessed programs from the AAD annual meetings archive online (https://www.aad.org/meetings/previous-meetings-archive), and we used hair and psoriasis content for comparison. Event titles and descriptions were searched for nail-related content (using search terms nail, onychia, and onycho), hair-related content (hair, alopecia, trichosis, hirsutism), and psoriasis content (psoriasis). Data acquired for each event included the date, hours, title, and event type (eg, forum, course, focus session, symposium, discussion group, workshop, plenary session).
The number of hours dedicated to nail education consistently lagged behind those related to hair and psoriasis content during the study period (Figure 1). According to the AAD, the conference runs Friday to Tuesday with higher attendance Friday to Sunday (Tim Moses, personal communication, July 9, 2019). Lectures during the weekend are likely to have a broader reach than lectures on Monday and Tuesday. The proportion of nail content during weekend prime time slots was similar to that of hair and psoriasis (Figure 2). Plenary sessions often are presented by renowned experts on hot topics in dermatology. Notably, hair (2014-2015) and psoriasis (2015-2017) content were represented in the plenary sessions during the study period, while nail content was not featured.

Our study shows that nail-related education was underrepresented at the AAD annual meetings from 2013 to 2019 compared to hair- and psoriasis-related content. Educational gaps in the diagnosis of fignail conditions previously have been delineated, and prioritization of instruction on nail disease pathology and diagnostic procedures has been recommended to improve patient care.1 The majority of nail unit melanomas are diagnosed at late stages, which has been attributed to deficiencies in clinical knowledge and failure to perform or inadequate biopsy techniques.2 Notably, a survey of third-year dermatology residents (N=240) assessing experience in procedural dermatology showed that 58% performed 10 or fewer nail procedures and 30% did not feel competent in performing nail surgery.3 Furthermore, a survey examining the management of longitudinal melanonychia among attending and resident dermatologists (N=402) found that 62% of residents and 28% of total respondents were not confident in managing melanonychia.4
A limitation of this study was the lack of online data available for AAD annual meetings before 2013, so we were unable to characterize any long-term trends. Furthermore, we were unable to assess the educational reach of these sessions, as data on attendance are lacking.
This study demonstrates a paucity of nail-related content at the AAD annual meetings. The introduction of the “Hands-on: Nail Surgery” in 2015 is an important step forward to diminish the knowledge gap in the diagnosis of various nail diseases and malignancies. We recommend increasing the number of hours and overall content of didactic nail sessions at the AAD annual meeting to further the knowledge and procedural skills of dermatologists in caring for patients with nail disorders.
- Hare AQ, R ich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
To the Editor:
The diagnosis and treatment of nail conditions are necessary competencies for board-certified dermatologists, but appropriate education often is lacking.1 The American Academy of Dermatology (AAD) annual meeting is one of the largest and most highly attended dermatology educational conferences worldwide. We sought to determine the number of hours dedicated to nail-related topics at the AAD annual meetings from 2013 to 2019.
We accessed programs from the AAD annual meetings archive online (https://www.aad.org/meetings/previous-meetings-archive), and we used hair and psoriasis content for comparison. Event titles and descriptions were searched for nail-related content (using search terms nail, onychia, and onycho), hair-related content (hair, alopecia, trichosis, hirsutism), and psoriasis content (psoriasis). Data acquired for each event included the date, hours, title, and event type (eg, forum, course, focus session, symposium, discussion group, workshop, plenary session).
The number of hours dedicated to nail education consistently lagged behind those related to hair and psoriasis content during the study period (Figure 1). According to the AAD, the conference runs Friday to Tuesday with higher attendance Friday to Sunday (Tim Moses, personal communication, July 9, 2019). Lectures during the weekend are likely to have a broader reach than lectures on Monday and Tuesday. The proportion of nail content during weekend prime time slots was similar to that of hair and psoriasis (Figure 2). Plenary sessions often are presented by renowned experts on hot topics in dermatology. Notably, hair (2014-2015) and psoriasis (2015-2017) content were represented in the plenary sessions during the study period, while nail content was not featured.


Our study shows that nail-related education was underrepresented at the AAD annual meetings from 2013 to 2019 compared to hair- and psoriasis-related content. Educational gaps in the diagnosis of fignail conditions previously have been delineated, and prioritization of instruction on nail disease pathology and diagnostic procedures has been recommended to improve patient care.1 The majority of nail unit melanomas are diagnosed at late stages, which has been attributed to deficiencies in clinical knowledge and failure to perform or inadequate biopsy techniques.2 Notably, a survey of third-year dermatology residents (N=240) assessing experience in procedural dermatology showed that 58% performed 10 or fewer nail procedures and 30% did not feel competent in performing nail surgery.3 Furthermore, a survey examining the management of longitudinal melanonychia among attending and resident dermatologists (N=402) found that 62% of residents and 28% of total respondents were not confident in managing melanonychia.4
A limitation of this study was the lack of online data available for AAD annual meetings before 2013, so we were unable to characterize any long-term trends. Furthermore, we were unable to assess the educational reach of these sessions, as data on attendance are lacking.
This study demonstrates a paucity of nail-related content at the AAD annual meetings. The introduction of the “Hands-on: Nail Surgery” in 2015 is an important step forward to diminish the knowledge gap in the diagnosis of various nail diseases and malignancies. We recommend increasing the number of hours and overall content of didactic nail sessions at the AAD annual meeting to further the knowledge and procedural skills of dermatologists in caring for patients with nail disorders.
To the Editor:
The diagnosis and treatment of nail conditions are necessary competencies for board-certified dermatologists, but appropriate education often is lacking.1 The American Academy of Dermatology (AAD) annual meeting is one of the largest and most highly attended dermatology educational conferences worldwide. We sought to determine the number of hours dedicated to nail-related topics at the AAD annual meetings from 2013 to 2019.
We accessed programs from the AAD annual meetings archive online (https://www.aad.org/meetings/previous-meetings-archive), and we used hair and psoriasis content for comparison. Event titles and descriptions were searched for nail-related content (using search terms nail, onychia, and onycho), hair-related content (hair, alopecia, trichosis, hirsutism), and psoriasis content (psoriasis). Data acquired for each event included the date, hours, title, and event type (eg, forum, course, focus session, symposium, discussion group, workshop, plenary session).
The number of hours dedicated to nail education consistently lagged behind those related to hair and psoriasis content during the study period (Figure 1). According to the AAD, the conference runs Friday to Tuesday with higher attendance Friday to Sunday (Tim Moses, personal communication, July 9, 2019). Lectures during the weekend are likely to have a broader reach than lectures on Monday and Tuesday. The proportion of nail content during weekend prime time slots was similar to that of hair and psoriasis (Figure 2). Plenary sessions often are presented by renowned experts on hot topics in dermatology. Notably, hair (2014-2015) and psoriasis (2015-2017) content were represented in the plenary sessions during the study period, while nail content was not featured.


Our study shows that nail-related education was underrepresented at the AAD annual meetings from 2013 to 2019 compared to hair- and psoriasis-related content. Educational gaps in the diagnosis of fignail conditions previously have been delineated, and prioritization of instruction on nail disease pathology and diagnostic procedures has been recommended to improve patient care.1 The majority of nail unit melanomas are diagnosed at late stages, which has been attributed to deficiencies in clinical knowledge and failure to perform or inadequate biopsy techniques.2 Notably, a survey of third-year dermatology residents (N=240) assessing experience in procedural dermatology showed that 58% performed 10 or fewer nail procedures and 30% did not feel competent in performing nail surgery.3 Furthermore, a survey examining the management of longitudinal melanonychia among attending and resident dermatologists (N=402) found that 62% of residents and 28% of total respondents were not confident in managing melanonychia.4
A limitation of this study was the lack of online data available for AAD annual meetings before 2013, so we were unable to characterize any long-term trends. Furthermore, we were unable to assess the educational reach of these sessions, as data on attendance are lacking.
This study demonstrates a paucity of nail-related content at the AAD annual meetings. The introduction of the “Hands-on: Nail Surgery” in 2015 is an important step forward to diminish the knowledge gap in the diagnosis of various nail diseases and malignancies. We recommend increasing the number of hours and overall content of didactic nail sessions at the AAD annual meeting to further the knowledge and procedural skills of dermatologists in caring for patients with nail disorders.
- Hare AQ, R ich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
- Hare AQ, R ich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
Practice Points
- Diagnosis and treatment of nail conditions are necessary competencies for board-certified dermatologists, but appropriate education often is lacking.
- We recommend increasing the number of hours and overall content of didactic nail sessions at the American Academy of Dermatology annual meeting to further the knowledge and procedural skills of dermatologists caring for patients with nail disorders.
Facial Malignancies in Patients Referred for Mohs Micrographic Surgery: A Retrospective Review of the Impact of Hair Growth on Tumor and Defect Size
Male facial hair trends are continuously changing and are influenced by culture, geography, religion, and ethnicity.1 Although the natural pattern of these hairs is largely androgen dependent, the phenotypic presentation often is a result of contemporary grooming practices that reflect prevailing trends.2 Beards are common throughout adulthood, and thus, preserving this facial hair pattern is considered with reconstructive techniques.3,4 Male facial skin physiology and beard hair biology are a dynamic interplay between both internal (eg, hormonal) and external (eg, shaving) variables. The density of beard hair follicles varies within different subunits, ranging between 20 and 80 follicles/cm2. Macroscopically, hairs vary in length, diameter, color, and growth rate across individuals and ethnicities.1,5
There is a paucity of literature assessing if male facial hair offers a protective role for external insults. One study utilized dosimetry to examine the effectiveness of facial hair on mannequins with varying lengths of hair in protecting against erythemal UV radiation (UVR). The authors concluded that, although facial hair provides protection from UVR, it is not significant.6 In a study of 200 male patients with
We sought to determine if facial hair growth is implicated in the diagnosis and treatment of cutaneous malignancies. Specifically, we hypothesized that the presence of facial hair leads to a delay in diagnosis with increased subclinical growth given that tumors may be camouflaged and go undetected. Although there is a lack of literature, our anecdotal evidence suggests that male patients with facial hair have larger tumors compared to patients who do not regularly maintain any facial hair.
Methods
We performed a retrospective chart review following approval from the institutional review board at The University of North Carolina at Chapel Hill. We identified all male patients with a cutaneous malignancy located on the face who were treated from January 2015 to December 2018. Photographs were reviewed and patients with tumors located within the following facial hair-bearing anatomic subunits were included: lip, melolabial fold, chin, mandible, preauricular cheek, buccal cheek, and parotid-masseteric cheek. Tumors located within the medial cheek were excluded.
Facial hair growth was determined via image review. Because biopsy photographs were not uploaded into the health record for patients who were referred externally, we reviewed all historical photographs for patients who had undergone prior Mohs micrographic surgery at The University of North Carolina at Chapel Hill, preoperative photographs, and follow-up photographs as a proxy to determine facial hair status. Postoperative photographs taken within 2 weeks following surgery were not reviewed, as any facial hair growth was likely due to disinclination on behalf of the patient to shave near or over the incision. Age, number of days from biopsy to surgery, pathology, preoperative tumor size, number of Mohs layers, and defect size also were extrapolated from our chart review.
Statistical Analysis
Summary statistics were applied to describe demographic and clinical characteristics. An unpaired 2-tailed t test was utilized to test the null hypothesis that the mean difference was zero. The χ2 test was used for categorical variables. Results achieving P<.05 were considered statistically significant.
Results
We reviewed medical records for 171 patients with facial hair and 336 patients without facial hair. The primary outcomes for this study assessed tumor and defect size in patients with facial hair compared to patients with no facial hair (Table 1). On average, patients who had facial hair were younger (67.5 years vs 74.0 years, P<.001). The median number of days from biopsy to surgery (43.0 vs 44.0 days) was comparable across both groups. The majority of patients (47%) exhibited a beard, while 30% had a mustache and 23% had a goatee. The most common tumor location was the preauricular cheek for both groups (29% and 28%, respectively). The mean preoperative tumor size in the facial hair cohort was 1.40 cm compared to 1.22 cm in the group with no facial hair (P=.03). The mean number of Mohs layers in the facial hair cohort was 1.53 compared to 1.33 in the group with no facial hair (P=.03). The facial hair cohort also had a larger mean postoperative defect size (2.18 cm) compared to the group with no facial hair (1.98 cm); however, this finding was not significant (P=.05).
We then stratified our data to analyze only lip tumors in patients with and without a mustache (Table 2). The mean preoperative tumor size in the mustache cohort was 1.10 cm compared to 0.82 cm in the group with no mustaches (P=.046). The mean number of Mohs layers in the mustache cohort was 1.57 compared to 1.42 in the group with no mustaches (P=.43). The mustache cohort also had a larger mean postoperative defect size (1.63 cm) compared to the group with no facial hair (1.33 cm), though this finding also did not reach significance (P=.13).
Comment
Our findings support anecdotal observations that tumors in men with facial hair are larger, require more Mohs layers, and result in larger defects compared with patients who are clean shaven. Similarly, in lip tumors, men with a mustache had a larger preoperative tumor size. Although these patients also required more Mohs layers to clear and a larger defect size, these parameters did not reach significance. These outcomes may, in part, be explained by a delay in diagnosis, as patients with facial hair may not notice any new suspicious lesions within the underlying skin as easily as patients with glabrous skin.
Although facial hair may shield skin from UVR, we agree with Parisi et al6 that this protection is marginal at best and that early persistent exposure to UVR plays a much more notable role in cutaneous carcinogenesis. As more men continue to grow facial hairstyles that emulate historical or contemporary trends, dermatologists should emphasize the risk for cutaneous malignancies within these sun-exposed areas of the face. Although some facial hair practices may reflect cultural or ethnic settings, the majority reflect a desired appearance that is achieved with grooming or otherwise.
Skin cancer screening in men with facial hair, particularly those with a strong history of UVR exposure and/or family history, should be discussed and encouraged to diagnose cutaneous tumors earlier. We encourage men with facial hair to be cognizant that cutaneous malignancies can arise within hair-bearing skin and to incorporate self–skin checks into grooming routines, which is particularly important in men with dense facial hair who forego regular self-care grooming or trim intermittently. Furthermore, we urge dermatologists to continue to thoroughly examine the underlying skin, especially in patients with full beards, during skin examinations. Diagnosing and treating cutaneous malignancies early is imperative to maximize ideal functional and cosmetic outcomes, particularly within perioral and lip subunits, where marginal millimeters can impact reconstructive complexity.
Conclusion
Men with facial hair who had cutaneous tumors in our study exhibited larger tumors, required more Mohs layers, and had a larger defect size compared to men without any facial hair growth. Similar findings also were noted when we stratified and compared lip tumors in patients with and without mustaches. Given these observations, patients and dermatologists should continue to have a high index of suspicion for any concerning lesion located within skin underlying facial hair. Regular screening in men with facial hair should be discussed and encouraged to diagnose and treat potential cutaneous tumors earlier.
- Wu Y, Konduru R, Deng D. Skin characteristics of Chinese men and their beard removal habits. Br J Dermatol. 2012;166:17-21.
- Janif ZJ, Brooks RC, Dixson BJ. Negative frequency-dependent preferences and variation in male facial hair. Biol Lett. 2014;10:20130958.
- Benjegerdes KE, Jamerson J, Housewright CD. Repair of a large submental defect. Dermatol Surg. 2019;45:141-143.
- Ninkovic M, Heidekruegger PI, Ehri D, et al. Beard reconstruction: a surgical algorithm. J Plast Reconstr Aesthet Surg. 2016;69:E111-E118.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin–challenges for shaving. Int J Cosmet Sci. 2016;38(suppl 1):3-9.
- Parisi AV, Turnbull DJ, Downs N, et al. Dosimetric investigation of the solar erythemal UV radiation protection provided by beards and moustaches. Radiat Prot Dosimetry. 2012;150:278-282.
- Liu DY, Gul MI, Wick J, et al. Long-term sheltering mustaches reduce incidence of lower lip actinic keratosis. J Am Acad Dermatol. 2019;80:1757-1758.e1.
Male facial hair trends are continuously changing and are influenced by culture, geography, religion, and ethnicity.1 Although the natural pattern of these hairs is largely androgen dependent, the phenotypic presentation often is a result of contemporary grooming practices that reflect prevailing trends.2 Beards are common throughout adulthood, and thus, preserving this facial hair pattern is considered with reconstructive techniques.3,4 Male facial skin physiology and beard hair biology are a dynamic interplay between both internal (eg, hormonal) and external (eg, shaving) variables. The density of beard hair follicles varies within different subunits, ranging between 20 and 80 follicles/cm2. Macroscopically, hairs vary in length, diameter, color, and growth rate across individuals and ethnicities.1,5
There is a paucity of literature assessing if male facial hair offers a protective role for external insults. One study utilized dosimetry to examine the effectiveness of facial hair on mannequins with varying lengths of hair in protecting against erythemal UV radiation (UVR). The authors concluded that, although facial hair provides protection from UVR, it is not significant.6 In a study of 200 male patients with
We sought to determine if facial hair growth is implicated in the diagnosis and treatment of cutaneous malignancies. Specifically, we hypothesized that the presence of facial hair leads to a delay in diagnosis with increased subclinical growth given that tumors may be camouflaged and go undetected. Although there is a lack of literature, our anecdotal evidence suggests that male patients with facial hair have larger tumors compared to patients who do not regularly maintain any facial hair.
Methods
We performed a retrospective chart review following approval from the institutional review board at The University of North Carolina at Chapel Hill. We identified all male patients with a cutaneous malignancy located on the face who were treated from January 2015 to December 2018. Photographs were reviewed and patients with tumors located within the following facial hair-bearing anatomic subunits were included: lip, melolabial fold, chin, mandible, preauricular cheek, buccal cheek, and parotid-masseteric cheek. Tumors located within the medial cheek were excluded.
Facial hair growth was determined via image review. Because biopsy photographs were not uploaded into the health record for patients who were referred externally, we reviewed all historical photographs for patients who had undergone prior Mohs micrographic surgery at The University of North Carolina at Chapel Hill, preoperative photographs, and follow-up photographs as a proxy to determine facial hair status. Postoperative photographs taken within 2 weeks following surgery were not reviewed, as any facial hair growth was likely due to disinclination on behalf of the patient to shave near or over the incision. Age, number of days from biopsy to surgery, pathology, preoperative tumor size, number of Mohs layers, and defect size also were extrapolated from our chart review.
Statistical Analysis
Summary statistics were applied to describe demographic and clinical characteristics. An unpaired 2-tailed t test was utilized to test the null hypothesis that the mean difference was zero. The χ2 test was used for categorical variables. Results achieving P<.05 were considered statistically significant.
Results
We reviewed medical records for 171 patients with facial hair and 336 patients without facial hair. The primary outcomes for this study assessed tumor and defect size in patients with facial hair compared to patients with no facial hair (Table 1). On average, patients who had facial hair were younger (67.5 years vs 74.0 years, P<.001). The median number of days from biopsy to surgery (43.0 vs 44.0 days) was comparable across both groups. The majority of patients (47%) exhibited a beard, while 30% had a mustache and 23% had a goatee. The most common tumor location was the preauricular cheek for both groups (29% and 28%, respectively). The mean preoperative tumor size in the facial hair cohort was 1.40 cm compared to 1.22 cm in the group with no facial hair (P=.03). The mean number of Mohs layers in the facial hair cohort was 1.53 compared to 1.33 in the group with no facial hair (P=.03). The facial hair cohort also had a larger mean postoperative defect size (2.18 cm) compared to the group with no facial hair (1.98 cm); however, this finding was not significant (P=.05).
We then stratified our data to analyze only lip tumors in patients with and without a mustache (Table 2). The mean preoperative tumor size in the mustache cohort was 1.10 cm compared to 0.82 cm in the group with no mustaches (P=.046). The mean number of Mohs layers in the mustache cohort was 1.57 compared to 1.42 in the group with no mustaches (P=.43). The mustache cohort also had a larger mean postoperative defect size (1.63 cm) compared to the group with no facial hair (1.33 cm), though this finding also did not reach significance (P=.13).
Comment
Our findings support anecdotal observations that tumors in men with facial hair are larger, require more Mohs layers, and result in larger defects compared with patients who are clean shaven. Similarly, in lip tumors, men with a mustache had a larger preoperative tumor size. Although these patients also required more Mohs layers to clear and a larger defect size, these parameters did not reach significance. These outcomes may, in part, be explained by a delay in diagnosis, as patients with facial hair may not notice any new suspicious lesions within the underlying skin as easily as patients with glabrous skin.
Although facial hair may shield skin from UVR, we agree with Parisi et al6 that this protection is marginal at best and that early persistent exposure to UVR plays a much more notable role in cutaneous carcinogenesis. As more men continue to grow facial hairstyles that emulate historical or contemporary trends, dermatologists should emphasize the risk for cutaneous malignancies within these sun-exposed areas of the face. Although some facial hair practices may reflect cultural or ethnic settings, the majority reflect a desired appearance that is achieved with grooming or otherwise.
Skin cancer screening in men with facial hair, particularly those with a strong history of UVR exposure and/or family history, should be discussed and encouraged to diagnose cutaneous tumors earlier. We encourage men with facial hair to be cognizant that cutaneous malignancies can arise within hair-bearing skin and to incorporate self–skin checks into grooming routines, which is particularly important in men with dense facial hair who forego regular self-care grooming or trim intermittently. Furthermore, we urge dermatologists to continue to thoroughly examine the underlying skin, especially in patients with full beards, during skin examinations. Diagnosing and treating cutaneous malignancies early is imperative to maximize ideal functional and cosmetic outcomes, particularly within perioral and lip subunits, where marginal millimeters can impact reconstructive complexity.
Conclusion
Men with facial hair who had cutaneous tumors in our study exhibited larger tumors, required more Mohs layers, and had a larger defect size compared to men without any facial hair growth. Similar findings also were noted when we stratified and compared lip tumors in patients with and without mustaches. Given these observations, patients and dermatologists should continue to have a high index of suspicion for any concerning lesion located within skin underlying facial hair. Regular screening in men with facial hair should be discussed and encouraged to diagnose and treat potential cutaneous tumors earlier.
Male facial hair trends are continuously changing and are influenced by culture, geography, religion, and ethnicity.1 Although the natural pattern of these hairs is largely androgen dependent, the phenotypic presentation often is a result of contemporary grooming practices that reflect prevailing trends.2 Beards are common throughout adulthood, and thus, preserving this facial hair pattern is considered with reconstructive techniques.3,4 Male facial skin physiology and beard hair biology are a dynamic interplay between both internal (eg, hormonal) and external (eg, shaving) variables. The density of beard hair follicles varies within different subunits, ranging between 20 and 80 follicles/cm2. Macroscopically, hairs vary in length, diameter, color, and growth rate across individuals and ethnicities.1,5
There is a paucity of literature assessing if male facial hair offers a protective role for external insults. One study utilized dosimetry to examine the effectiveness of facial hair on mannequins with varying lengths of hair in protecting against erythemal UV radiation (UVR). The authors concluded that, although facial hair provides protection from UVR, it is not significant.6 In a study of 200 male patients with
We sought to determine if facial hair growth is implicated in the diagnosis and treatment of cutaneous malignancies. Specifically, we hypothesized that the presence of facial hair leads to a delay in diagnosis with increased subclinical growth given that tumors may be camouflaged and go undetected. Although there is a lack of literature, our anecdotal evidence suggests that male patients with facial hair have larger tumors compared to patients who do not regularly maintain any facial hair.
Methods
We performed a retrospective chart review following approval from the institutional review board at The University of North Carolina at Chapel Hill. We identified all male patients with a cutaneous malignancy located on the face who were treated from January 2015 to December 2018. Photographs were reviewed and patients with tumors located within the following facial hair-bearing anatomic subunits were included: lip, melolabial fold, chin, mandible, preauricular cheek, buccal cheek, and parotid-masseteric cheek. Tumors located within the medial cheek were excluded.
Facial hair growth was determined via image review. Because biopsy photographs were not uploaded into the health record for patients who were referred externally, we reviewed all historical photographs for patients who had undergone prior Mohs micrographic surgery at The University of North Carolina at Chapel Hill, preoperative photographs, and follow-up photographs as a proxy to determine facial hair status. Postoperative photographs taken within 2 weeks following surgery were not reviewed, as any facial hair growth was likely due to disinclination on behalf of the patient to shave near or over the incision. Age, number of days from biopsy to surgery, pathology, preoperative tumor size, number of Mohs layers, and defect size also were extrapolated from our chart review.
Statistical Analysis
Summary statistics were applied to describe demographic and clinical characteristics. An unpaired 2-tailed t test was utilized to test the null hypothesis that the mean difference was zero. The χ2 test was used for categorical variables. Results achieving P<.05 were considered statistically significant.
Results
We reviewed medical records for 171 patients with facial hair and 336 patients without facial hair. The primary outcomes for this study assessed tumor and defect size in patients with facial hair compared to patients with no facial hair (Table 1). On average, patients who had facial hair were younger (67.5 years vs 74.0 years, P<.001). The median number of days from biopsy to surgery (43.0 vs 44.0 days) was comparable across both groups. The majority of patients (47%) exhibited a beard, while 30% had a mustache and 23% had a goatee. The most common tumor location was the preauricular cheek for both groups (29% and 28%, respectively). The mean preoperative tumor size in the facial hair cohort was 1.40 cm compared to 1.22 cm in the group with no facial hair (P=.03). The mean number of Mohs layers in the facial hair cohort was 1.53 compared to 1.33 in the group with no facial hair (P=.03). The facial hair cohort also had a larger mean postoperative defect size (2.18 cm) compared to the group with no facial hair (1.98 cm); however, this finding was not significant (P=.05).
We then stratified our data to analyze only lip tumors in patients with and without a mustache (Table 2). The mean preoperative tumor size in the mustache cohort was 1.10 cm compared to 0.82 cm in the group with no mustaches (P=.046). The mean number of Mohs layers in the mustache cohort was 1.57 compared to 1.42 in the group with no mustaches (P=.43). The mustache cohort also had a larger mean postoperative defect size (1.63 cm) compared to the group with no facial hair (1.33 cm), though this finding also did not reach significance (P=.13).
Comment
Our findings support anecdotal observations that tumors in men with facial hair are larger, require more Mohs layers, and result in larger defects compared with patients who are clean shaven. Similarly, in lip tumors, men with a mustache had a larger preoperative tumor size. Although these patients also required more Mohs layers to clear and a larger defect size, these parameters did not reach significance. These outcomes may, in part, be explained by a delay in diagnosis, as patients with facial hair may not notice any new suspicious lesions within the underlying skin as easily as patients with glabrous skin.
Although facial hair may shield skin from UVR, we agree with Parisi et al6 that this protection is marginal at best and that early persistent exposure to UVR plays a much more notable role in cutaneous carcinogenesis. As more men continue to grow facial hairstyles that emulate historical or contemporary trends, dermatologists should emphasize the risk for cutaneous malignancies within these sun-exposed areas of the face. Although some facial hair practices may reflect cultural or ethnic settings, the majority reflect a desired appearance that is achieved with grooming or otherwise.
Skin cancer screening in men with facial hair, particularly those with a strong history of UVR exposure and/or family history, should be discussed and encouraged to diagnose cutaneous tumors earlier. We encourage men with facial hair to be cognizant that cutaneous malignancies can arise within hair-bearing skin and to incorporate self–skin checks into grooming routines, which is particularly important in men with dense facial hair who forego regular self-care grooming or trim intermittently. Furthermore, we urge dermatologists to continue to thoroughly examine the underlying skin, especially in patients with full beards, during skin examinations. Diagnosing and treating cutaneous malignancies early is imperative to maximize ideal functional and cosmetic outcomes, particularly within perioral and lip subunits, where marginal millimeters can impact reconstructive complexity.
Conclusion
Men with facial hair who had cutaneous tumors in our study exhibited larger tumors, required more Mohs layers, and had a larger defect size compared to men without any facial hair growth. Similar findings also were noted when we stratified and compared lip tumors in patients with and without mustaches. Given these observations, patients and dermatologists should continue to have a high index of suspicion for any concerning lesion located within skin underlying facial hair. Regular screening in men with facial hair should be discussed and encouraged to diagnose and treat potential cutaneous tumors earlier.
- Wu Y, Konduru R, Deng D. Skin characteristics of Chinese men and their beard removal habits. Br J Dermatol. 2012;166:17-21.
- Janif ZJ, Brooks RC, Dixson BJ. Negative frequency-dependent preferences and variation in male facial hair. Biol Lett. 2014;10:20130958.
- Benjegerdes KE, Jamerson J, Housewright CD. Repair of a large submental defect. Dermatol Surg. 2019;45:141-143.
- Ninkovic M, Heidekruegger PI, Ehri D, et al. Beard reconstruction: a surgical algorithm. J Plast Reconstr Aesthet Surg. 2016;69:E111-E118.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin–challenges for shaving. Int J Cosmet Sci. 2016;38(suppl 1):3-9.
- Parisi AV, Turnbull DJ, Downs N, et al. Dosimetric investigation of the solar erythemal UV radiation protection provided by beards and moustaches. Radiat Prot Dosimetry. 2012;150:278-282.
- Liu DY, Gul MI, Wick J, et al. Long-term sheltering mustaches reduce incidence of lower lip actinic keratosis. J Am Acad Dermatol. 2019;80:1757-1758.e1.
- Wu Y, Konduru R, Deng D. Skin characteristics of Chinese men and their beard removal habits. Br J Dermatol. 2012;166:17-21.
- Janif ZJ, Brooks RC, Dixson BJ. Negative frequency-dependent preferences and variation in male facial hair. Biol Lett. 2014;10:20130958.
- Benjegerdes KE, Jamerson J, Housewright CD. Repair of a large submental defect. Dermatol Surg. 2019;45:141-143.
- Ninkovic M, Heidekruegger PI, Ehri D, et al. Beard reconstruction: a surgical algorithm. J Plast Reconstr Aesthet Surg. 2016;69:E111-E118.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin–challenges for shaving. Int J Cosmet Sci. 2016;38(suppl 1):3-9.
- Parisi AV, Turnbull DJ, Downs N, et al. Dosimetric investigation of the solar erythemal UV radiation protection provided by beards and moustaches. Radiat Prot Dosimetry. 2012;150:278-282.
- Liu DY, Gul MI, Wick J, et al. Long-term sheltering mustaches reduce incidence of lower lip actinic keratosis. J Am Acad Dermatol. 2019;80:1757-1758.e1.
Practice Points
- In our study, men with cutaneous tumors who had facial hair exhibited larger tumors, required more Mohs layers, and had a larger defect size compared to men who do not have any facial hair growth.
- Both patients and dermatologists should have a high index of suspicion for any concerning lesion contained within skin underlying facial hair to ensure prompt diagnosis and treatment of cutaneous tumors.
Patient Questionnaire to Reduce Anxiety Prior to Full-Body Skin Examination
To the Editor:
A thorough full-body skin examination (FBSE) is an integral component of a dermatologic encounter and helps identify potentially malignant and high-risk lesions, particularly in areas that are difficult for the patient to visualize.1 Despite these benefits, many patients experience discomfort and anxiety about this examination because it involves sensitive anatomical areas. The true psychological impact of an FBSE is not clearly understood; however, research into improving patient comfort in these circumstances can have a broad positive impact.2 The purpose of this pilot study was to establish patients’ willingness to complete a pre-encounter questionnaire that defines their FBSE preferences as well as to identify the anatomical areas that are of most concern.
This study was approved by the University of Kansas institutional review board as nonhuman subjects research. A pre-encounter questionnaire that included information about the benefits of FBSEs was administered to 34 patients, allowing them to identify anatomic locations that they wanted to exclude from the FBSE.
Following the patient visit (in which the identified anatomical locations were excluded), patients were given a brief exit survey that asked about (1) their preference for a pre-encounter FBSE questionnaire and (2) the impact of the questionnaire on their anxiety level throughout the encounter. Preference for asking was surveyed using a 10-point scale (10=strong preference for the pre-encounter survey; 1=strong preference against the pre-encounter survey). Change in anxiety was surveyed using a 10-point scale (10=strong reduction in anxiety after the pre-encounter survey; 1=strong increase in anxiety after the pre-encounter survey). Statistical analysis was performed using 2-tailed unpaired t tests, with P<.05 considered statistically significant.
Twenty female and 14 male patients were enrolled (mean age, 53 years)(Table). The most commonly excluded anatomical location on the pre-encounter survey was the genitals, followed by the buttocks, breasts/chest, legs, feet, and abdomen (Table); 10 (71%) male and 13 (65%) female respondents did not exclude any component of the FBSE.
After the provider visit, females had a higher preference for the pre-encounter survey (mean score, 9.0) compared to males (mean score, 7.2; P=.021). Similarly, females had reduced anxiety about the office visit after survey administration compared to males (mean score, 8.3 vs 6.0; P=.001)(Table).
The results of our pilot study showed that a brief pre-encounter questionnaire may reduce the distress associated with an FBSE. Our survey took less than 1 minute to complete and served as a useful guide to direct the provider during the FBSE. Moreover, recognizing that patients do not want certain anatomic locations examined can serve as an opportunity for the dermatologist to provide helpful home skin check instructions and recommendations.
The small sample size was a limitation of this study. Future studies can assess with greater precision the clear benefits of a pre-encounter survey as well as the benefits or drawbacks of a survey compared to other modalities that are aimed at reducing patient anxiety about the FBSE, such as having the physician directly ask the patient about areas to avoid during the examination.
A pre-encounter survey about the FBSE can serve as an efficient means of determining patient preference and reducing self-reported anxiety about the visit.
- Hoorens I, Vossaert K, Pil L, et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016;152:27-34.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
To the Editor:
A thorough full-body skin examination (FBSE) is an integral component of a dermatologic encounter and helps identify potentially malignant and high-risk lesions, particularly in areas that are difficult for the patient to visualize.1 Despite these benefits, many patients experience discomfort and anxiety about this examination because it involves sensitive anatomical areas. The true psychological impact of an FBSE is not clearly understood; however, research into improving patient comfort in these circumstances can have a broad positive impact.2 The purpose of this pilot study was to establish patients’ willingness to complete a pre-encounter questionnaire that defines their FBSE preferences as well as to identify the anatomical areas that are of most concern.
This study was approved by the University of Kansas institutional review board as nonhuman subjects research. A pre-encounter questionnaire that included information about the benefits of FBSEs was administered to 34 patients, allowing them to identify anatomic locations that they wanted to exclude from the FBSE.
Following the patient visit (in which the identified anatomical locations were excluded), patients were given a brief exit survey that asked about (1) their preference for a pre-encounter FBSE questionnaire and (2) the impact of the questionnaire on their anxiety level throughout the encounter. Preference for asking was surveyed using a 10-point scale (10=strong preference for the pre-encounter survey; 1=strong preference against the pre-encounter survey). Change in anxiety was surveyed using a 10-point scale (10=strong reduction in anxiety after the pre-encounter survey; 1=strong increase in anxiety after the pre-encounter survey). Statistical analysis was performed using 2-tailed unpaired t tests, with P<.05 considered statistically significant.
Twenty female and 14 male patients were enrolled (mean age, 53 years)(Table). The most commonly excluded anatomical location on the pre-encounter survey was the genitals, followed by the buttocks, breasts/chest, legs, feet, and abdomen (Table); 10 (71%) male and 13 (65%) female respondents did not exclude any component of the FBSE.
After the provider visit, females had a higher preference for the pre-encounter survey (mean score, 9.0) compared to males (mean score, 7.2; P=.021). Similarly, females had reduced anxiety about the office visit after survey administration compared to males (mean score, 8.3 vs 6.0; P=.001)(Table).
The results of our pilot study showed that a brief pre-encounter questionnaire may reduce the distress associated with an FBSE. Our survey took less than 1 minute to complete and served as a useful guide to direct the provider during the FBSE. Moreover, recognizing that patients do not want certain anatomic locations examined can serve as an opportunity for the dermatologist to provide helpful home skin check instructions and recommendations.
The small sample size was a limitation of this study. Future studies can assess with greater precision the clear benefits of a pre-encounter survey as well as the benefits or drawbacks of a survey compared to other modalities that are aimed at reducing patient anxiety about the FBSE, such as having the physician directly ask the patient about areas to avoid during the examination.
A pre-encounter survey about the FBSE can serve as an efficient means of determining patient preference and reducing self-reported anxiety about the visit.
To the Editor:
A thorough full-body skin examination (FBSE) is an integral component of a dermatologic encounter and helps identify potentially malignant and high-risk lesions, particularly in areas that are difficult for the patient to visualize.1 Despite these benefits, many patients experience discomfort and anxiety about this examination because it involves sensitive anatomical areas. The true psychological impact of an FBSE is not clearly understood; however, research into improving patient comfort in these circumstances can have a broad positive impact.2 The purpose of this pilot study was to establish patients’ willingness to complete a pre-encounter questionnaire that defines their FBSE preferences as well as to identify the anatomical areas that are of most concern.
This study was approved by the University of Kansas institutional review board as nonhuman subjects research. A pre-encounter questionnaire that included information about the benefits of FBSEs was administered to 34 patients, allowing them to identify anatomic locations that they wanted to exclude from the FBSE.
Following the patient visit (in which the identified anatomical locations were excluded), patients were given a brief exit survey that asked about (1) their preference for a pre-encounter FBSE questionnaire and (2) the impact of the questionnaire on their anxiety level throughout the encounter. Preference for asking was surveyed using a 10-point scale (10=strong preference for the pre-encounter survey; 1=strong preference against the pre-encounter survey). Change in anxiety was surveyed using a 10-point scale (10=strong reduction in anxiety after the pre-encounter survey; 1=strong increase in anxiety after the pre-encounter survey). Statistical analysis was performed using 2-tailed unpaired t tests, with P<.05 considered statistically significant.
Twenty female and 14 male patients were enrolled (mean age, 53 years)(Table). The most commonly excluded anatomical location on the pre-encounter survey was the genitals, followed by the buttocks, breasts/chest, legs, feet, and abdomen (Table); 10 (71%) male and 13 (65%) female respondents did not exclude any component of the FBSE.
After the provider visit, females had a higher preference for the pre-encounter survey (mean score, 9.0) compared to males (mean score, 7.2; P=.021). Similarly, females had reduced anxiety about the office visit after survey administration compared to males (mean score, 8.3 vs 6.0; P=.001)(Table).
The results of our pilot study showed that a brief pre-encounter questionnaire may reduce the distress associated with an FBSE. Our survey took less than 1 minute to complete and served as a useful guide to direct the provider during the FBSE. Moreover, recognizing that patients do not want certain anatomic locations examined can serve as an opportunity for the dermatologist to provide helpful home skin check instructions and recommendations.
The small sample size was a limitation of this study. Future studies can assess with greater precision the clear benefits of a pre-encounter survey as well as the benefits or drawbacks of a survey compared to other modalities that are aimed at reducing patient anxiety about the FBSE, such as having the physician directly ask the patient about areas to avoid during the examination.
A pre-encounter survey about the FBSE can serve as an efficient means of determining patient preference and reducing self-reported anxiety about the visit.
- Hoorens I, Vossaert K, Pil L, et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016;152:27-34.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
- Hoorens I, Vossaert K, Pil L, et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016;152:27-34.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
Practice Points
- Full-body skin examination (FBSE) is an assessment that requires examination of sensitive body areas, any of which can be seen as intrusive by certain patients.
- A pre-encounter survey on the FBSE can offer an efficient means by which to determine patient preference and reduce visit-associated anxiety.
CO2 Laser Ablative Fractional Resurfacing Photodynamic Therapy for Actinic Keratosis and Nonmelanoma Skin Cancer: A Randomized Split-Side Study
Actinic keratosis (AK) is the most common cutaneous lesion and is regarded as a precursor to nonmelanoma skin cancer (NMSC), particularly squamous cell carcinoma (SCC).1 Field cancerization refers to broad areas of chronically sun-exposed skin that show cumulative sun damage in the form of clinical and subclinical lesions. It is not feasible to treat large areas with multiple overt and subclinical lesions using surgical methods, and photodynamic therapy (PDT) has become a preferred method for treatment of field cancerization.2 Topical PDT uses the heme biosynthesis pathway precursors aminolevulinic acid (ALA) or methyl ALA (MAL), which localizes in the treatment area and is metabolized to protoporphyrin IX.3 After an incubation period, activation by a light source results in the formation of cytotoxic oxygen species,4 with reports of efficacy over large areas and excellent cosmetic outcomes.2
Laser ablative fractional resurfacing (AFR) also has been investigated as a treatment of AKs; CO2 laser AFR treatment resulted in a short-term reduction in the number of AK lesions and appeared to reduce the development of new lesions.5 However, case reports and small studies have indicated that pretreatment with laser AFR can increase the efficacy of PDT by creating microscopic vertical channels facilitating deeper penetration and uptake of the ALA.6 The use of erbium:YAG lasers in combination with PDT has demonstrated notable clinical and aesthetic improvements in treating basal cell carcinomas (BCCs)7 and AKs,8 with enhanced efficacy in moderate to thick AKs in particular. Hædersdal et al6 reported that CO2 laser AFR facilitated delivery of MAL into porcine skin, with AFR appearing to bypass the stratum corneum and deliver the treatment to the deep dermis.
The combination of CO2 laser AFR and PDT has shown statistically significant increases in efficacy for treatment of AKs compared to PDT alone (P<.001).9 In a small study, Alexiades10 reported a statistically significant improvement in AKs at 4 and 8 weeks posttreatment for 10 patients receiving CO2 laser AFR-PDT vs conventional PDT (P<.05). Studies of organ transplant recipients—who are at higher risk for AK and NMSC development—demonstrated favorable results for combined CO2 laser AFR and PDT vs either laser treatment11 or PDT9,12 alone, with significant reductions in the number of AKs (P=.002). Results were maintained for 3 to 4 months after treatment. Additional studies have shown that combining CO2 laser AFR and PDT may reduce the PDT incubation time or number of treatments required to achieve a response over conventional PDT.13,14
Our proof-of-concept study was designed to assess efficacy of CO2 laser AFR to enhance an approved drug delivery system in the treatment of AK and NMSC. The objective was to compare effect and durability of AFR-PDT vs standard ALA-PDT in the treatment of AK and NMSCs in a split-sided study of various body locations.
Methods
This randomized, split-sided study compared CO2 laser AFR-PDT to standard ALA-PDT for the treatment of AK and NMSC conducted at 1 site in Los Gatos, California. Patients who had a skin cancer screening and received a biopsy diagnosis of AK or NMSC were invited to attend an enrollment visit. Key inclusion criteria for enrollment were male or female patients aged 40 to 85 years with notable symmetrically comparable photodamage (at least 1 AK per square centimeter) in 1 or more skin areas—scalp, face, or distal extremities—with presence of clinically identifiable NMSCs proven by biopsy. Key exclusion criteria were patients who were pregnant; patients with epilepsy, seizures, or a photosensitive disorder; those taking photosensitizing medication (eg, doxycycline, hydrochlorothiazide); or immunocompromised patients. The study was approved by an institutional review board (Salus IRB [Austin, Texas]), and each participant underwent a complete and informed consent process.
Laterality for pretreatment with AFR followed by ALA-PDT vs ALA-PDT alone was determined at the time of treatment using a computer-based random number generator; even numbers resulted in pretreatment of the right side, and odd numbers resulted in pretreatment of the left side. Because of the difference in pretreatment methods for the 2 sides, it was not possible to perform the procedure under blinded conditions.
The treatment area was prepared by defatting the entire site with 70% isopropyl alcohol, followed by benzalkonium chloride antibacterial cleansing for the AFR pretreatment side. A 7% lidocaine/7% tetracaine ointment was applied under polyethylene wrap occlusion to the AFR pretreatment side for 20 minutes. Additionally, nerve blocks and field blocks with a mixture of 1.1% lidocaine with epinephrine/0.5% bupivacaine with epinephrine were performed wherever feasible. After 20 minutes, the lidocaine-tetracaine ointment was removed with isopropanol, and AFR treatment commenced immediately with the SmartXide DOT laser (DEKA)(1 pass of 25 W, 1200-microsecond duration at 500-µm spacing, 200-µm spot size, achieving 12% surface area ablation). Hyperkeratotic treated areas were debrided with saline and received a second pass with the laser. Aminolevulinic acid solution 20% (Levulan Kerastick; DUSA Pharmaceuticals, Inc)15 was applied to both sides of the treatment area and allowed to absorb for a 1-hour incubation period, which was followed by blue-light exposure at a power density of 10 mW/cm2 for 16 minutes and 40 seconds using the BLU-U Photodynamic Therapy Illuminator (DUSA Pharmaceuticals, Inc). Areas treated with AFR were then covered with a layer of Aquaphor ointment (Beiersdorf, Inc) and an absorptive hydrogel dressing for48 to 96 hours, with continued application of the ointment until resolution of all crusting. After treatment, patients were instructed to avoid direct sun exposure, wear a hat or visor for the first 2 weeks posttreatment when outdoors, and apply sunscreen with a sun protection factor greater than 30 once skin had healed.
Follow-up was conducted at 1 week, 1 month, 3 months, and 6 months after the PDT procedure. The primary end points were clinical clearance of NMSC lesions at 1, 3, and 6 months posttreatment and histological clearance at 6 months. Secondary end points assessed quality of life and functional improvements.
Results
Twenty-four potential participants experiencing AKs and/or NMSCs were screened for the study, with 19 meeting inclusion criteria. All participants were white, non-Hispanic, and had Fitzpatrick skin types I or II. Treated areas for all participants had field cancerization defined as at least 1 AK per square centimeter. All 19 participants enrolled in the study completed the posttreatment evaluations up to 6 months. All AFR-pretreated sites showed superior results in reduction in number, size, or hyperkeratosis of AKs at all follow-up visits, with a complete absence of new AK formation at the 6-month follow-up (Table). Conversely, sites treated with standard PDT only showed some recurrence of AKs at 6 months. Of the 3 participants who had biopsy-confirmed BCCs on the AFR-pretreated side, there were 3 persistent lesions after treatment at the 6-month visit. Two participants experienced persistence of a confirmed SCC in situ that was on the laser-pretreated side only (1 on the forehead and 1 on the hand), whereas 1 participant with an SCC on the leg at baseline had no recurrence at 6 months. A participant who received treatment on the lower lip had persistence of actinic cheilitis on both the AFR- and non–AFR-treated sides of the lip.
Scalp and facial sites healed fully in an average of 7 days, whereas upper extremities—forearm and hands—took approximately 14 days to heal completely. Lower extremity AFR-pretreated sites exhibited substantial weeping, resulting in prolonged healing of approximately 21 days for resolution of all scabbing.
Comment
In this split-sided study in patients with field cancerization, the use of CO2 laser AFR before treatment with PDT increased AK lesion clearance compared to ALA-PDT alone. Prior studies of fractional laser–assisted drug delivery on porcine skin using topical MAL showed that laser channels approximately 3-mm apart were able to distribute protoporphyrin through the entire skin.6 The ablative nature of AFR theoretically provides deeper and more effusive penetration of the ALA solution than using conventional PDT or erbium:YAG lasers with PDT.7,8 Helsing et al11 applied CO2 laser AFR MAL-PDT to AKs in organ transplant recipients and obtained complete responses in 73% of patients compared to a complete response of 31% for AFR alone. The results reported in our study are consistent with Helsing et al,11 showing a complete clinical response for 14 of 19 patients (74%), of whom 4 (21%) had no recurrence of NMSC and 10 (53%) had no recurrence of AK on the AFR-PDT–treated side.
The pretreatment process required for the laser AFR added time to the initial visit compared to conventional PDT, which is balanced by a reduced PDT incubation time (1 hour vs the approved indication of 14–18 hours for face/scalp or 3 hours for upper extremities under occlusion). The use of microneedling as an alternative pretreatment procedure before PDT also has been investigated, with the aim of decreasing the optimum ALA absorption time. The mean reduction in AKs (89.3%) was significantly greater than for PDT alone (69.5%; P<.05) in a small study by Spencer and Freeman.18 Although microneedling is less time-intensive and labor-intensive than laser AFR, the photocoagulative effect and subsequent microhemorrhages resulting from AFR should result in much deeper penetration of ALA solution than for microneedling.
The limitations of this proof-of-concept study arose from the small sample size of 19 participants and the short follow-up period of 6 months. Furthermore, the unblinded nature of the study could create selection, detection, or reporting bias. Further follow-up appointments would aid in determining the longevity of results, which may encourage future use of this technique, despite the time-consuming preparation. A larger study with follow-up greater than 1 year would be beneficial, particularly for monitoring remission from SCCs and BCCs.
Conclusion
Pretreatment with CO2 laser AFR before ALA-PDT provided superior clearance of AKs and thin NMSCs at 6 months compared to ALA-PDT alone (Figure). Additionally, the incubation period for ALA absorption can be reduced before PDT, leading to a shorter treatment time overall. The benefits of AFR pretreatment on AK clearance demonstrated in this study warrant further investigation in a larger trial with a longer follow-up period to monitor maintenance of response.
Acknowledgments
The authors thank the patients who participated in this study. Editorial assistance was provided by Louise Gildea, PhD, of JK Associates Inc, part of the Fishawack Group of Companies (Fishawack, United Kingdom), funded by Sun Pharmaceutical Industries, Inc.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-1266.
- Casas A, Fukuda H, Di Venosa G, et al. Photosensitization and mechanism of cytotoxicity induced by the use of ALA derivatives in photodynamic therapy. Br J Cancer. 2001;85:279-284.
- Klotz LO, Fritsch C, Briviba K, et al. Activation of JNK and p38 but not ERK MAP kinases in human skin cells by 5-aminolevulinate-photodynamic therapy. Cancer Res. 1998;58:4297-4300.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Hædersdal M, Sakamoto FH, Farinelli WA, et al. Fractional CO(2) laser-assisted drug delivery. Lasers Surg Med. 2010;42:113-122.
- Šmucler R, Vlk M. Combination of Er:YAG laser and photodynamic therapy in the treatment of nodular basal cell carcinoma. Lasers Surg Med. 2008;40:153-158.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Alexiades M. Randomized, controlled trial of fractional carbon dioxide laser resurfacing followed by ultrashort incubation aminolevulinic acid blue light photodynamic therapy for actinic keratosis. Dermatol Surg. 2017;43:1053-1064.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Jang YH, Lee DJ, Shin J, et al. Photodynamic therapy with ablative carbon dioxide fractional laser in treatment of actinic keratosis. Ann Dermatol. 2013;25:417-422.
- Song HS, Jung SE, Jang YH, et al. Fractional carbon dioxide laser-assisted photodynamic therapy for patients with actinic keratosis. Photodermatol Photoimmunol Photomed. 2015;31:296-301.
- ALA Kerastick (aminolevulinic acid HCl) for topical solution, 20% [package insert]. Wilmington, MA: DUSA Pharmaceuticals; 2010.
- Data on file. Wilmington, MA: DUSA Pharmaceuticals; 2020.
- Campbell TM, Goldman MP. Adverse events of fractionated carbon dioxide laser: review of 373 treatments. Dermatol Surg. 2010;36:1645-1650.
- Spencer JM, Freeman SA. Microneedling prior to Levulan PDT for the treatment of actinic keratoses: a split-face, blinded trial. J Drugs Dermatol. 2016;15:1072-1074.
Actinic keratosis (AK) is the most common cutaneous lesion and is regarded as a precursor to nonmelanoma skin cancer (NMSC), particularly squamous cell carcinoma (SCC).1 Field cancerization refers to broad areas of chronically sun-exposed skin that show cumulative sun damage in the form of clinical and subclinical lesions. It is not feasible to treat large areas with multiple overt and subclinical lesions using surgical methods, and photodynamic therapy (PDT) has become a preferred method for treatment of field cancerization.2 Topical PDT uses the heme biosynthesis pathway precursors aminolevulinic acid (ALA) or methyl ALA (MAL), which localizes in the treatment area and is metabolized to protoporphyrin IX.3 After an incubation period, activation by a light source results in the formation of cytotoxic oxygen species,4 with reports of efficacy over large areas and excellent cosmetic outcomes.2
Laser ablative fractional resurfacing (AFR) also has been investigated as a treatment of AKs; CO2 laser AFR treatment resulted in a short-term reduction in the number of AK lesions and appeared to reduce the development of new lesions.5 However, case reports and small studies have indicated that pretreatment with laser AFR can increase the efficacy of PDT by creating microscopic vertical channels facilitating deeper penetration and uptake of the ALA.6 The use of erbium:YAG lasers in combination with PDT has demonstrated notable clinical and aesthetic improvements in treating basal cell carcinomas (BCCs)7 and AKs,8 with enhanced efficacy in moderate to thick AKs in particular. Hædersdal et al6 reported that CO2 laser AFR facilitated delivery of MAL into porcine skin, with AFR appearing to bypass the stratum corneum and deliver the treatment to the deep dermis.
The combination of CO2 laser AFR and PDT has shown statistically significant increases in efficacy for treatment of AKs compared to PDT alone (P<.001).9 In a small study, Alexiades10 reported a statistically significant improvement in AKs at 4 and 8 weeks posttreatment for 10 patients receiving CO2 laser AFR-PDT vs conventional PDT (P<.05). Studies of organ transplant recipients—who are at higher risk for AK and NMSC development—demonstrated favorable results for combined CO2 laser AFR and PDT vs either laser treatment11 or PDT9,12 alone, with significant reductions in the number of AKs (P=.002). Results were maintained for 3 to 4 months after treatment. Additional studies have shown that combining CO2 laser AFR and PDT may reduce the PDT incubation time or number of treatments required to achieve a response over conventional PDT.13,14
Our proof-of-concept study was designed to assess efficacy of CO2 laser AFR to enhance an approved drug delivery system in the treatment of AK and NMSC. The objective was to compare effect and durability of AFR-PDT vs standard ALA-PDT in the treatment of AK and NMSCs in a split-sided study of various body locations.
Methods
This randomized, split-sided study compared CO2 laser AFR-PDT to standard ALA-PDT for the treatment of AK and NMSC conducted at 1 site in Los Gatos, California. Patients who had a skin cancer screening and received a biopsy diagnosis of AK or NMSC were invited to attend an enrollment visit. Key inclusion criteria for enrollment were male or female patients aged 40 to 85 years with notable symmetrically comparable photodamage (at least 1 AK per square centimeter) in 1 or more skin areas—scalp, face, or distal extremities—with presence of clinically identifiable NMSCs proven by biopsy. Key exclusion criteria were patients who were pregnant; patients with epilepsy, seizures, or a photosensitive disorder; those taking photosensitizing medication (eg, doxycycline, hydrochlorothiazide); or immunocompromised patients. The study was approved by an institutional review board (Salus IRB [Austin, Texas]), and each participant underwent a complete and informed consent process.
Laterality for pretreatment with AFR followed by ALA-PDT vs ALA-PDT alone was determined at the time of treatment using a computer-based random number generator; even numbers resulted in pretreatment of the right side, and odd numbers resulted in pretreatment of the left side. Because of the difference in pretreatment methods for the 2 sides, it was not possible to perform the procedure under blinded conditions.
The treatment area was prepared by defatting the entire site with 70% isopropyl alcohol, followed by benzalkonium chloride antibacterial cleansing for the AFR pretreatment side. A 7% lidocaine/7% tetracaine ointment was applied under polyethylene wrap occlusion to the AFR pretreatment side for 20 minutes. Additionally, nerve blocks and field blocks with a mixture of 1.1% lidocaine with epinephrine/0.5% bupivacaine with epinephrine were performed wherever feasible. After 20 minutes, the lidocaine-tetracaine ointment was removed with isopropanol, and AFR treatment commenced immediately with the SmartXide DOT laser (DEKA)(1 pass of 25 W, 1200-microsecond duration at 500-µm spacing, 200-µm spot size, achieving 12% surface area ablation). Hyperkeratotic treated areas were debrided with saline and received a second pass with the laser. Aminolevulinic acid solution 20% (Levulan Kerastick; DUSA Pharmaceuticals, Inc)15 was applied to both sides of the treatment area and allowed to absorb for a 1-hour incubation period, which was followed by blue-light exposure at a power density of 10 mW/cm2 for 16 minutes and 40 seconds using the BLU-U Photodynamic Therapy Illuminator (DUSA Pharmaceuticals, Inc). Areas treated with AFR were then covered with a layer of Aquaphor ointment (Beiersdorf, Inc) and an absorptive hydrogel dressing for48 to 96 hours, with continued application of the ointment until resolution of all crusting. After treatment, patients were instructed to avoid direct sun exposure, wear a hat or visor for the first 2 weeks posttreatment when outdoors, and apply sunscreen with a sun protection factor greater than 30 once skin had healed.
Follow-up was conducted at 1 week, 1 month, 3 months, and 6 months after the PDT procedure. The primary end points were clinical clearance of NMSC lesions at 1, 3, and 6 months posttreatment and histological clearance at 6 months. Secondary end points assessed quality of life and functional improvements.
Results
Twenty-four potential participants experiencing AKs and/or NMSCs were screened for the study, with 19 meeting inclusion criteria. All participants were white, non-Hispanic, and had Fitzpatrick skin types I or II. Treated areas for all participants had field cancerization defined as at least 1 AK per square centimeter. All 19 participants enrolled in the study completed the posttreatment evaluations up to 6 months. All AFR-pretreated sites showed superior results in reduction in number, size, or hyperkeratosis of AKs at all follow-up visits, with a complete absence of new AK formation at the 6-month follow-up (Table). Conversely, sites treated with standard PDT only showed some recurrence of AKs at 6 months. Of the 3 participants who had biopsy-confirmed BCCs on the AFR-pretreated side, there were 3 persistent lesions after treatment at the 6-month visit. Two participants experienced persistence of a confirmed SCC in situ that was on the laser-pretreated side only (1 on the forehead and 1 on the hand), whereas 1 participant with an SCC on the leg at baseline had no recurrence at 6 months. A participant who received treatment on the lower lip had persistence of actinic cheilitis on both the AFR- and non–AFR-treated sides of the lip.
Scalp and facial sites healed fully in an average of 7 days, whereas upper extremities—forearm and hands—took approximately 14 days to heal completely. Lower extremity AFR-pretreated sites exhibited substantial weeping, resulting in prolonged healing of approximately 21 days for resolution of all scabbing.
Comment
In this split-sided study in patients with field cancerization, the use of CO2 laser AFR before treatment with PDT increased AK lesion clearance compared to ALA-PDT alone. Prior studies of fractional laser–assisted drug delivery on porcine skin using topical MAL showed that laser channels approximately 3-mm apart were able to distribute protoporphyrin through the entire skin.6 The ablative nature of AFR theoretically provides deeper and more effusive penetration of the ALA solution than using conventional PDT or erbium:YAG lasers with PDT.7,8 Helsing et al11 applied CO2 laser AFR MAL-PDT to AKs in organ transplant recipients and obtained complete responses in 73% of patients compared to a complete response of 31% for AFR alone. The results reported in our study are consistent with Helsing et al,11 showing a complete clinical response for 14 of 19 patients (74%), of whom 4 (21%) had no recurrence of NMSC and 10 (53%) had no recurrence of AK on the AFR-PDT–treated side.
The pretreatment process required for the laser AFR added time to the initial visit compared to conventional PDT, which is balanced by a reduced PDT incubation time (1 hour vs the approved indication of 14–18 hours for face/scalp or 3 hours for upper extremities under occlusion). The use of microneedling as an alternative pretreatment procedure before PDT also has been investigated, with the aim of decreasing the optimum ALA absorption time. The mean reduction in AKs (89.3%) was significantly greater than for PDT alone (69.5%; P<.05) in a small study by Spencer and Freeman.18 Although microneedling is less time-intensive and labor-intensive than laser AFR, the photocoagulative effect and subsequent microhemorrhages resulting from AFR should result in much deeper penetration of ALA solution than for microneedling.
The limitations of this proof-of-concept study arose from the small sample size of 19 participants and the short follow-up period of 6 months. Furthermore, the unblinded nature of the study could create selection, detection, or reporting bias. Further follow-up appointments would aid in determining the longevity of results, which may encourage future use of this technique, despite the time-consuming preparation. A larger study with follow-up greater than 1 year would be beneficial, particularly for monitoring remission from SCCs and BCCs.
Conclusion
Pretreatment with CO2 laser AFR before ALA-PDT provided superior clearance of AKs and thin NMSCs at 6 months compared to ALA-PDT alone (Figure). Additionally, the incubation period for ALA absorption can be reduced before PDT, leading to a shorter treatment time overall. The benefits of AFR pretreatment on AK clearance demonstrated in this study warrant further investigation in a larger trial with a longer follow-up period to monitor maintenance of response.
Acknowledgments
The authors thank the patients who participated in this study. Editorial assistance was provided by Louise Gildea, PhD, of JK Associates Inc, part of the Fishawack Group of Companies (Fishawack, United Kingdom), funded by Sun Pharmaceutical Industries, Inc.
Actinic keratosis (AK) is the most common cutaneous lesion and is regarded as a precursor to nonmelanoma skin cancer (NMSC), particularly squamous cell carcinoma (SCC).1 Field cancerization refers to broad areas of chronically sun-exposed skin that show cumulative sun damage in the form of clinical and subclinical lesions. It is not feasible to treat large areas with multiple overt and subclinical lesions using surgical methods, and photodynamic therapy (PDT) has become a preferred method for treatment of field cancerization.2 Topical PDT uses the heme biosynthesis pathway precursors aminolevulinic acid (ALA) or methyl ALA (MAL), which localizes in the treatment area and is metabolized to protoporphyrin IX.3 After an incubation period, activation by a light source results in the formation of cytotoxic oxygen species,4 with reports of efficacy over large areas and excellent cosmetic outcomes.2
Laser ablative fractional resurfacing (AFR) also has been investigated as a treatment of AKs; CO2 laser AFR treatment resulted in a short-term reduction in the number of AK lesions and appeared to reduce the development of new lesions.5 However, case reports and small studies have indicated that pretreatment with laser AFR can increase the efficacy of PDT by creating microscopic vertical channels facilitating deeper penetration and uptake of the ALA.6 The use of erbium:YAG lasers in combination with PDT has demonstrated notable clinical and aesthetic improvements in treating basal cell carcinomas (BCCs)7 and AKs,8 with enhanced efficacy in moderate to thick AKs in particular. Hædersdal et al6 reported that CO2 laser AFR facilitated delivery of MAL into porcine skin, with AFR appearing to bypass the stratum corneum and deliver the treatment to the deep dermis.
The combination of CO2 laser AFR and PDT has shown statistically significant increases in efficacy for treatment of AKs compared to PDT alone (P<.001).9 In a small study, Alexiades10 reported a statistically significant improvement in AKs at 4 and 8 weeks posttreatment for 10 patients receiving CO2 laser AFR-PDT vs conventional PDT (P<.05). Studies of organ transplant recipients—who are at higher risk for AK and NMSC development—demonstrated favorable results for combined CO2 laser AFR and PDT vs either laser treatment11 or PDT9,12 alone, with significant reductions in the number of AKs (P=.002). Results were maintained for 3 to 4 months after treatment. Additional studies have shown that combining CO2 laser AFR and PDT may reduce the PDT incubation time or number of treatments required to achieve a response over conventional PDT.13,14
Our proof-of-concept study was designed to assess efficacy of CO2 laser AFR to enhance an approved drug delivery system in the treatment of AK and NMSC. The objective was to compare effect and durability of AFR-PDT vs standard ALA-PDT in the treatment of AK and NMSCs in a split-sided study of various body locations.
Methods
This randomized, split-sided study compared CO2 laser AFR-PDT to standard ALA-PDT for the treatment of AK and NMSC conducted at 1 site in Los Gatos, California. Patients who had a skin cancer screening and received a biopsy diagnosis of AK or NMSC were invited to attend an enrollment visit. Key inclusion criteria for enrollment were male or female patients aged 40 to 85 years with notable symmetrically comparable photodamage (at least 1 AK per square centimeter) in 1 or more skin areas—scalp, face, or distal extremities—with presence of clinically identifiable NMSCs proven by biopsy. Key exclusion criteria were patients who were pregnant; patients with epilepsy, seizures, or a photosensitive disorder; those taking photosensitizing medication (eg, doxycycline, hydrochlorothiazide); or immunocompromised patients. The study was approved by an institutional review board (Salus IRB [Austin, Texas]), and each participant underwent a complete and informed consent process.
Laterality for pretreatment with AFR followed by ALA-PDT vs ALA-PDT alone was determined at the time of treatment using a computer-based random number generator; even numbers resulted in pretreatment of the right side, and odd numbers resulted in pretreatment of the left side. Because of the difference in pretreatment methods for the 2 sides, it was not possible to perform the procedure under blinded conditions.
The treatment area was prepared by defatting the entire site with 70% isopropyl alcohol, followed by benzalkonium chloride antibacterial cleansing for the AFR pretreatment side. A 7% lidocaine/7% tetracaine ointment was applied under polyethylene wrap occlusion to the AFR pretreatment side for 20 minutes. Additionally, nerve blocks and field blocks with a mixture of 1.1% lidocaine with epinephrine/0.5% bupivacaine with epinephrine were performed wherever feasible. After 20 minutes, the lidocaine-tetracaine ointment was removed with isopropanol, and AFR treatment commenced immediately with the SmartXide DOT laser (DEKA)(1 pass of 25 W, 1200-microsecond duration at 500-µm spacing, 200-µm spot size, achieving 12% surface area ablation). Hyperkeratotic treated areas were debrided with saline and received a second pass with the laser. Aminolevulinic acid solution 20% (Levulan Kerastick; DUSA Pharmaceuticals, Inc)15 was applied to both sides of the treatment area and allowed to absorb for a 1-hour incubation period, which was followed by blue-light exposure at a power density of 10 mW/cm2 for 16 minutes and 40 seconds using the BLU-U Photodynamic Therapy Illuminator (DUSA Pharmaceuticals, Inc). Areas treated with AFR were then covered with a layer of Aquaphor ointment (Beiersdorf, Inc) and an absorptive hydrogel dressing for48 to 96 hours, with continued application of the ointment until resolution of all crusting. After treatment, patients were instructed to avoid direct sun exposure, wear a hat or visor for the first 2 weeks posttreatment when outdoors, and apply sunscreen with a sun protection factor greater than 30 once skin had healed.
Follow-up was conducted at 1 week, 1 month, 3 months, and 6 months after the PDT procedure. The primary end points were clinical clearance of NMSC lesions at 1, 3, and 6 months posttreatment and histological clearance at 6 months. Secondary end points assessed quality of life and functional improvements.
Results
Twenty-four potential participants experiencing AKs and/or NMSCs were screened for the study, with 19 meeting inclusion criteria. All participants were white, non-Hispanic, and had Fitzpatrick skin types I or II. Treated areas for all participants had field cancerization defined as at least 1 AK per square centimeter. All 19 participants enrolled in the study completed the posttreatment evaluations up to 6 months. All AFR-pretreated sites showed superior results in reduction in number, size, or hyperkeratosis of AKs at all follow-up visits, with a complete absence of new AK formation at the 6-month follow-up (Table). Conversely, sites treated with standard PDT only showed some recurrence of AKs at 6 months. Of the 3 participants who had biopsy-confirmed BCCs on the AFR-pretreated side, there were 3 persistent lesions after treatment at the 6-month visit. Two participants experienced persistence of a confirmed SCC in situ that was on the laser-pretreated side only (1 on the forehead and 1 on the hand), whereas 1 participant with an SCC on the leg at baseline had no recurrence at 6 months. A participant who received treatment on the lower lip had persistence of actinic cheilitis on both the AFR- and non–AFR-treated sides of the lip.
Scalp and facial sites healed fully in an average of 7 days, whereas upper extremities—forearm and hands—took approximately 14 days to heal completely. Lower extremity AFR-pretreated sites exhibited substantial weeping, resulting in prolonged healing of approximately 21 days for resolution of all scabbing.
Comment
In this split-sided study in patients with field cancerization, the use of CO2 laser AFR before treatment with PDT increased AK lesion clearance compared to ALA-PDT alone. Prior studies of fractional laser–assisted drug delivery on porcine skin using topical MAL showed that laser channels approximately 3-mm apart were able to distribute protoporphyrin through the entire skin.6 The ablative nature of AFR theoretically provides deeper and more effusive penetration of the ALA solution than using conventional PDT or erbium:YAG lasers with PDT.7,8 Helsing et al11 applied CO2 laser AFR MAL-PDT to AKs in organ transplant recipients and obtained complete responses in 73% of patients compared to a complete response of 31% for AFR alone. The results reported in our study are consistent with Helsing et al,11 showing a complete clinical response for 14 of 19 patients (74%), of whom 4 (21%) had no recurrence of NMSC and 10 (53%) had no recurrence of AK on the AFR-PDT–treated side.
The pretreatment process required for the laser AFR added time to the initial visit compared to conventional PDT, which is balanced by a reduced PDT incubation time (1 hour vs the approved indication of 14–18 hours for face/scalp or 3 hours for upper extremities under occlusion). The use of microneedling as an alternative pretreatment procedure before PDT also has been investigated, with the aim of decreasing the optimum ALA absorption time. The mean reduction in AKs (89.3%) was significantly greater than for PDT alone (69.5%; P<.05) in a small study by Spencer and Freeman.18 Although microneedling is less time-intensive and labor-intensive than laser AFR, the photocoagulative effect and subsequent microhemorrhages resulting from AFR should result in much deeper penetration of ALA solution than for microneedling.
The limitations of this proof-of-concept study arose from the small sample size of 19 participants and the short follow-up period of 6 months. Furthermore, the unblinded nature of the study could create selection, detection, or reporting bias. Further follow-up appointments would aid in determining the longevity of results, which may encourage future use of this technique, despite the time-consuming preparation. A larger study with follow-up greater than 1 year would be beneficial, particularly for monitoring remission from SCCs and BCCs.
Conclusion
Pretreatment with CO2 laser AFR before ALA-PDT provided superior clearance of AKs and thin NMSCs at 6 months compared to ALA-PDT alone (Figure). Additionally, the incubation period for ALA absorption can be reduced before PDT, leading to a shorter treatment time overall. The benefits of AFR pretreatment on AK clearance demonstrated in this study warrant further investigation in a larger trial with a longer follow-up period to monitor maintenance of response.
Acknowledgments
The authors thank the patients who participated in this study. Editorial assistance was provided by Louise Gildea, PhD, of JK Associates Inc, part of the Fishawack Group of Companies (Fishawack, United Kingdom), funded by Sun Pharmaceutical Industries, Inc.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-1266.
- Casas A, Fukuda H, Di Venosa G, et al. Photosensitization and mechanism of cytotoxicity induced by the use of ALA derivatives in photodynamic therapy. Br J Cancer. 2001;85:279-284.
- Klotz LO, Fritsch C, Briviba K, et al. Activation of JNK and p38 but not ERK MAP kinases in human skin cells by 5-aminolevulinate-photodynamic therapy. Cancer Res. 1998;58:4297-4300.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Hædersdal M, Sakamoto FH, Farinelli WA, et al. Fractional CO(2) laser-assisted drug delivery. Lasers Surg Med. 2010;42:113-122.
- Šmucler R, Vlk M. Combination of Er:YAG laser and photodynamic therapy in the treatment of nodular basal cell carcinoma. Lasers Surg Med. 2008;40:153-158.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Alexiades M. Randomized, controlled trial of fractional carbon dioxide laser resurfacing followed by ultrashort incubation aminolevulinic acid blue light photodynamic therapy for actinic keratosis. Dermatol Surg. 2017;43:1053-1064.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Jang YH, Lee DJ, Shin J, et al. Photodynamic therapy with ablative carbon dioxide fractional laser in treatment of actinic keratosis. Ann Dermatol. 2013;25:417-422.
- Song HS, Jung SE, Jang YH, et al. Fractional carbon dioxide laser-assisted photodynamic therapy for patients with actinic keratosis. Photodermatol Photoimmunol Photomed. 2015;31:296-301.
- ALA Kerastick (aminolevulinic acid HCl) for topical solution, 20% [package insert]. Wilmington, MA: DUSA Pharmaceuticals; 2010.
- Data on file. Wilmington, MA: DUSA Pharmaceuticals; 2020.
- Campbell TM, Goldman MP. Adverse events of fractionated carbon dioxide laser: review of 373 treatments. Dermatol Surg. 2010;36:1645-1650.
- Spencer JM, Freeman SA. Microneedling prior to Levulan PDT for the treatment of actinic keratoses: a split-face, blinded trial. J Drugs Dermatol. 2016;15:1072-1074.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-1266.
- Casas A, Fukuda H, Di Venosa G, et al. Photosensitization and mechanism of cytotoxicity induced by the use of ALA derivatives in photodynamic therapy. Br J Cancer. 2001;85:279-284.
- Klotz LO, Fritsch C, Briviba K, et al. Activation of JNK and p38 but not ERK MAP kinases in human skin cells by 5-aminolevulinate-photodynamic therapy. Cancer Res. 1998;58:4297-4300.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Hædersdal M, Sakamoto FH, Farinelli WA, et al. Fractional CO(2) laser-assisted drug delivery. Lasers Surg Med. 2010;42:113-122.
- Šmucler R, Vlk M. Combination of Er:YAG laser and photodynamic therapy in the treatment of nodular basal cell carcinoma. Lasers Surg Med. 2008;40:153-158.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Alexiades M. Randomized, controlled trial of fractional carbon dioxide laser resurfacing followed by ultrashort incubation aminolevulinic acid blue light photodynamic therapy for actinic keratosis. Dermatol Surg. 2017;43:1053-1064.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Jang YH, Lee DJ, Shin J, et al. Photodynamic therapy with ablative carbon dioxide fractional laser in treatment of actinic keratosis. Ann Dermatol. 2013;25:417-422.
- Song HS, Jung SE, Jang YH, et al. Fractional carbon dioxide laser-assisted photodynamic therapy for patients with actinic keratosis. Photodermatol Photoimmunol Photomed. 2015;31:296-301.
- ALA Kerastick (aminolevulinic acid HCl) for topical solution, 20% [package insert]. Wilmington, MA: DUSA Pharmaceuticals; 2010.
- Data on file. Wilmington, MA: DUSA Pharmaceuticals; 2020.
- Campbell TM, Goldman MP. Adverse events of fractionated carbon dioxide laser: review of 373 treatments. Dermatol Surg. 2010;36:1645-1650.
- Spencer JM, Freeman SA. Microneedling prior to Levulan PDT for the treatment of actinic keratoses: a split-face, blinded trial. J Drugs Dermatol. 2016;15:1072-1074.
Practice Points
- Pretreatment with CO2 laser ablative fractional resurfacing (AFR) before photodynamic therapy (PDT) provided efficient clearance of actinic keratosis (AK).
- Superior clearance of lesions was seen at 6 months for AK and thin nonmelanoma skin cancers (NMSCs) on pretreated sites compared to PDT alone, with no novel adverse events reported.
- A reduced incubation period for aminolevulinic acid (ALA) absorption before PDT was used, leading to a shorter overall treatment time.
Group Clinic for Chemoprevention of Squamous Cell Carcinoma: A Pilot Study
Squamous cell carcinoma (SCC) has an estimated incidence of more than 2.5 million cases per year in the United States.1 Its precursor lesion, actinic keratosis (AK), had an estimated prevalence of 39.5 million cases in the United States in 2004.2 The dermatology clinic at the Providence VA Medical Center in Rhode Island exerts consistent efforts to treat both SCC and AK by prescribing topical 5-fluorouracil (5-FU) and lifestyle changes that include avoiding sun exposure, wearing protective clothing, and using effective sunscreen.3 A single course of topical 5-FU in veterans has been shown to decrease the risk for SCC by 74% during the year after treatment and also improve AK clearance rates.4,5
Effectiveness of 5-FU for secondary prevention can be decreased by patient misunderstandings, such as applying 5-FU for too short a time or using the corticosteroid cream prematurely, as well as patient nonadherence due to expected adverse skin reactions to 5-FU.6 Education and reassurance before and during therapy maximize patient compliance but can be difficult to accomplish in clinics when time is in short supply. During standard 5-FU treatment at the Providence VA Medical Center, the provider prescribes 5-FU and posttherapy corticosteroid cream at a clinic visit after an informed consent process that includes reviewing with the patient a color handout depicting the expected adverse skin reaction. Patients who later experience severe inflammation and anxiety call the clinic and are overbooked as needed.
To address the practical obstacles to the patient experience with topical 5-FU therapy, we developed a group chemoprevention clinic based on the shared medical appointment (SMA) model. Shared medical appointments, during which multiple patients are scheduled at the same visit with 1 or more health care providers, promote patient risk reduction and guideline adherence in complex diseases, such as chronic heart failure and diabetes mellitus, through efficient resource use, improvement of access to care, and promotion of behavioral changes through group support.7-13 To increase efficiency in the group chemoprevention clinic, we integrated dermatology nurses and nurse practitioners from the chronic care model into the group medical visits, which ran from September 2016 through March 2017. Because veterans could interact with peers undergoing the same treatment, we hypothesized that use of the cream in a group setting would provide positive reinforcement during the course of therapy, resulting in a positive treatment experience. We conducted a retrospective review of medical records of the patients involved in this pilot study to evaluate this model.
Methods
Institutional review board approval was obtained from the Providence VA Medical Center. Informed consent was waived because this study was a retrospective review of medical records.
Study Population
We offered participation in a group chemoprevention clinic based on the SMA model for patients of the dermatology clinic at the Providence VA Medical Center who were planning to start 5-FU in the fall of 2016. Patients were asked if they were interested in participating in a group clinic to receive their 5-FU treatment. Patients who were established dermatology patients within the Veterans Affairs system and had scheduled annual full-body skin examinations were included; patients were not excluded if they had a prior diagnosis of AK but had not been previously treated with 5-FU.
Design
Each SMA group consisted of 3 to 4 patients who met initially to receive the 5-FU medication and attend a 10-minute live presentation that included information on the dangers and causes of SCC and AK, treatment options, directions for using 5-FU, expected spectrum of side effects, and how to minimize the discomfort of treatment side effects. Patients had field treatment limited to areas with clinically apparent AKs on the face and ears. They were prescribed 5-FU cream 5% twice daily.
One physician, one nurse practitioner, and one registered nurse were present at each 1-hour clinic. Patients arrived and were checked in individually by the providers. At check-in, the provider handed the patient a printout of his/her current medication list and a pen to make any necessary corrections. This list was reviewed privately with the patient so the provider could reconcile the medication list and review the patient’s medical history and so the patient could provide informed consent. After, the patient had the opportunity to select a seat from chairs arranged in a circle. There was a live PowerPoint presentation given at the beginning of the clinic with a question-and-answer session immediately following that contained information about the disease and medication process. Clinicians assisted the patients with the initial application of 5-FU in the large group room, and each patient received a handout with information about AKs and a 40-g tube of the 5-FU cream.
This same group then met again 2 weeks later, at which time most patients were experiencing expected adverse skin reactions. At that time, there was a 10-minute live presentation that congratulated the patients on their success in the treatment process, reviewed what to expect in the following weeks, and reinforced the importance of future sun-protective practices. At each visit, photographs and feedback about the group setting were obtained in the large group room. After photographing and rating each patient’s skin reaction severity, the clinicians advised each patient either to continue the 5-FU medication for another week or to discontinue it and apply the triamcinolone cream 0.1% up to 4 times daily as needed for up to 7 days. Each patient received the prescription corticosteroid cream and a gift, courtesy of the VA Voluntary Service Program, of a 360-degree brimmed hat and sunscreen. Time for questions or concerns was available at both sessions.
Data Collection
We reviewed medical records via the Computerized Patient Record System, a nationally accessible electronic health record system, for all patients who participated in the SMA visits from September 2016 through March 2017. Any patient who attended the initial visit but declined therapy at that time was excluded.
Outcomes included attendance at both appointments, stated completion of 14 days of 5-FU treatment, and evidence of 5-FU use according to a validated numeric scale of skin reaction severity.14 We recorded telephone calls and other dermatology clinic and teledermatology appointments during the 3 weeks after the first appointment and the number of dermatology clinic appointments 6 months before and after the SMA for side effects related to 5-FU treatment. Feedback about treatment in the group setting was obtained at both visits.
Results
A total of 16 male patients attended the SMAs, and 14 attended both sessions. Of the 2 patients who were excluded from the study, 1 declined to be scheduled for the second group appointment, and the other was scheduled and confirmed but did not come for his appointment. The mean age was 72 years.
Of the 14 study patients who attended both sessions of the group clinic, 10 stated that they completed 2 weeks of 5-FU therapy, and the other 4 stated that they completed at least 11 days. Results of the validated scale used by clinicians during the second visit to grade the patients’ 5-FU reactions showed that all 14 patients demonstrated at least some expected adverse reactions (eTable). Eleven of 14 patients showed crusting and erosion; 13 showed grade 2 or higher erythema severity. One patient who stopped treatment after 11 days telephoned the dermatology clinic within 1 week of his second SMA. Another patient who stopped treatment after 11 days had a separate dermatology surgery clinic appointment within the 3-week period after starting 5-FU for a recent basal cell carcinoma excision. None of the 14 patients had a dermatology appointment scheduled within 6 months before or after for a 5-FU adverse reaction. One patient who completed the 14-day course was referred to teledermatology for insect bites within that period.
None of the patients were prophylaxed for herpes simplex virus during the treatment period, and none developed a herpes simplex virus eruption during this study. None of the patients required antibiotics for secondary impetiginization of the treatment site.
The verbal feedback about the group setting from patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers. At the conclusion of the second appointment, all of the patients reported an increased understanding of their condition and the importance of future sun-protective behaviors.
Comment
Shared medical appointments promote treatment adherence in patients with chronic heart failure and diabetes mellitus through efficient resource use, improvement of access to care, and promotion of behavioral change through group support.7-13 Within the dermatology literature, SMAs are more profitable than regular clinic appointments.15 In SMAs designed to improve patient education for preoperative consultations for Mohs micrographic surgery, patient satisfaction reported in postvisit surveys was high, with 84.7% of 149 patients reporting they found the session useful, highlighting how SMAs have potential as practical alternatives to regular medical appointments.16 Similarly, the feedback about the group setting from our patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers.
The group setting—where patients were interacting with peers undergoing the same treatment—provided an encouraging environment during the course of 5-FU therapy, resulting in a positive treatment experience. Additionally, at the conclusion of the second visit, patients reported an increased understanding of their condition and the importance of future sun-protective behaviors, further demonstrating the impact of this pilot initiative.
The Veterans Affairs’ Current Procedural Terminology code for a group clinic is 99078. Veterans Affairs medical centers and private practices have different approaches to billing and compensation. As more accountable care organizations are formed, there may be a different mixture of ways for handling these SMAs.
Limitations
Our study is limited by the small sample size, selection bias, and self-reported measure of adherence. Adherence to 5-FU is excellent without group support, and without a control group, it is unclear how beneficial the group setting was for adherence.17 The presence of the expected skin reactions at the 2-week return visit cannot account for adherence during the interval between the visits, and this close follow-up may be responsible for the high adherence in this group setting. The major side effects with 5-FU are short-term. Nonetheless, longer-term follow-up would be helpful and a worthy future endeavor.
Veterans share a common bond of military service that may not be shared in a typical private practice setting, which may have facilitated success of this pilot study. We recommend group clinics be evaluated independently in private practices and other systems. However, despite these limitations, the patients in the SMAs demonstrated positive reactions to 5-FU therapy, suggesting the potential for utilizing group clinics as a practical alternative to regular medical appointments.
Conclusion
Our pilot group clinics for AK treatment and chemoprevention of SCC with 5-FU suggest that this model is well received. The group format, which demonstrated uniformly positive reactions to 5-FU therapy, shows promise in battling an epidemic of skin cancer that demands cost-effective interventions.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Siegel JA, Korgavkar K, Weinstock MA. Current perspective on actinic keratosis: a review. Br J Dermatol. 2017;177:350-358.
- Weinstock MA, Thwin SS, Siegel JA, et al. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
- Pomerantz H, Hogan D, Eilers D, et al. Long-term efficacy of topical fluorouracil cream, 5%, for treating actinic keratosis: a randomized clinical trial. JAMA Dermatol. 2015;151:952-960.
- Foley P, Stockfleth E, Peris K, et al. Adherence to topical therapies in actinic keratosis: a literature review. J Dermatolog Treat. 2016;27:538-545.
- Desouza CV, Rentschler L, Haynatzki G. The effect of group clinics in the control of diabetes. Prim Care Diabetes. 2010;4:251-254.
- Edelman D, McDuffie JR, Oddone E, et al. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review. Washington, DC: Department of Veterans Affairs; 2012.
- Edelman D, Gierisch JM, McDuffie JR, et al. Shared medical appointments for patients with diabetes mellitus: a systematic review. J Gen Intern Med. 2015;30:99-106.
- Trento M, Passera P, Tomalino M, et al. Group visits improve metabolic control in type 2 diabetes: a 2-year follow-up. Diabetes Care. 2001;24:995-1000.
- Wagner EH, Grothaus LC, Sandhu N, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care. 2001;24:695-700.
- Harris MD, Kirsh S, Higgins PA. Shared medical appointments: impact on clinical and quality outcomes in veterans with diabetes. Qual Manag Health Care. 2016;25:176-180.
- Kirsh S, Watts S, Pascuzzi K, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16:349-353.
- Pomerantz H, Korgavkar K, Lee KC, et al. Validation of photograph-based toxicity score for topical 5-fluorouracil cream application. J Cutan Med Surg. 2016;20:458-466.
- Sidorsky T, Huang Z, Dinulos JG. A business case for shared medical appointments in dermatology: improving access and the bottom line. Arch Dermatol. 2010;146:374-381.
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344.
- Yentzer B, Hick J, Williams L, et al. Adherence to a topical regimen of 5-fluorouracil, 0.5%, cream for the treatment of actinic keratoses. JAMA Dermatol. 2009;145:203-205.
Squamous cell carcinoma (SCC) has an estimated incidence of more than 2.5 million cases per year in the United States.1 Its precursor lesion, actinic keratosis (AK), had an estimated prevalence of 39.5 million cases in the United States in 2004.2 The dermatology clinic at the Providence VA Medical Center in Rhode Island exerts consistent efforts to treat both SCC and AK by prescribing topical 5-fluorouracil (5-FU) and lifestyle changes that include avoiding sun exposure, wearing protective clothing, and using effective sunscreen.3 A single course of topical 5-FU in veterans has been shown to decrease the risk for SCC by 74% during the year after treatment and also improve AK clearance rates.4,5
Effectiveness of 5-FU for secondary prevention can be decreased by patient misunderstandings, such as applying 5-FU for too short a time or using the corticosteroid cream prematurely, as well as patient nonadherence due to expected adverse skin reactions to 5-FU.6 Education and reassurance before and during therapy maximize patient compliance but can be difficult to accomplish in clinics when time is in short supply. During standard 5-FU treatment at the Providence VA Medical Center, the provider prescribes 5-FU and posttherapy corticosteroid cream at a clinic visit after an informed consent process that includes reviewing with the patient a color handout depicting the expected adverse skin reaction. Patients who later experience severe inflammation and anxiety call the clinic and are overbooked as needed.
To address the practical obstacles to the patient experience with topical 5-FU therapy, we developed a group chemoprevention clinic based on the shared medical appointment (SMA) model. Shared medical appointments, during which multiple patients are scheduled at the same visit with 1 or more health care providers, promote patient risk reduction and guideline adherence in complex diseases, such as chronic heart failure and diabetes mellitus, through efficient resource use, improvement of access to care, and promotion of behavioral changes through group support.7-13 To increase efficiency in the group chemoprevention clinic, we integrated dermatology nurses and nurse practitioners from the chronic care model into the group medical visits, which ran from September 2016 through March 2017. Because veterans could interact with peers undergoing the same treatment, we hypothesized that use of the cream in a group setting would provide positive reinforcement during the course of therapy, resulting in a positive treatment experience. We conducted a retrospective review of medical records of the patients involved in this pilot study to evaluate this model.
Methods
Institutional review board approval was obtained from the Providence VA Medical Center. Informed consent was waived because this study was a retrospective review of medical records.
Study Population
We offered participation in a group chemoprevention clinic based on the SMA model for patients of the dermatology clinic at the Providence VA Medical Center who were planning to start 5-FU in the fall of 2016. Patients were asked if they were interested in participating in a group clinic to receive their 5-FU treatment. Patients who were established dermatology patients within the Veterans Affairs system and had scheduled annual full-body skin examinations were included; patients were not excluded if they had a prior diagnosis of AK but had not been previously treated with 5-FU.
Design
Each SMA group consisted of 3 to 4 patients who met initially to receive the 5-FU medication and attend a 10-minute live presentation that included information on the dangers and causes of SCC and AK, treatment options, directions for using 5-FU, expected spectrum of side effects, and how to minimize the discomfort of treatment side effects. Patients had field treatment limited to areas with clinically apparent AKs on the face and ears. They were prescribed 5-FU cream 5% twice daily.
One physician, one nurse practitioner, and one registered nurse were present at each 1-hour clinic. Patients arrived and were checked in individually by the providers. At check-in, the provider handed the patient a printout of his/her current medication list and a pen to make any necessary corrections. This list was reviewed privately with the patient so the provider could reconcile the medication list and review the patient’s medical history and so the patient could provide informed consent. After, the patient had the opportunity to select a seat from chairs arranged in a circle. There was a live PowerPoint presentation given at the beginning of the clinic with a question-and-answer session immediately following that contained information about the disease and medication process. Clinicians assisted the patients with the initial application of 5-FU in the large group room, and each patient received a handout with information about AKs and a 40-g tube of the 5-FU cream.
This same group then met again 2 weeks later, at which time most patients were experiencing expected adverse skin reactions. At that time, there was a 10-minute live presentation that congratulated the patients on their success in the treatment process, reviewed what to expect in the following weeks, and reinforced the importance of future sun-protective practices. At each visit, photographs and feedback about the group setting were obtained in the large group room. After photographing and rating each patient’s skin reaction severity, the clinicians advised each patient either to continue the 5-FU medication for another week or to discontinue it and apply the triamcinolone cream 0.1% up to 4 times daily as needed for up to 7 days. Each patient received the prescription corticosteroid cream and a gift, courtesy of the VA Voluntary Service Program, of a 360-degree brimmed hat and sunscreen. Time for questions or concerns was available at both sessions.
Data Collection
We reviewed medical records via the Computerized Patient Record System, a nationally accessible electronic health record system, for all patients who participated in the SMA visits from September 2016 through March 2017. Any patient who attended the initial visit but declined therapy at that time was excluded.
Outcomes included attendance at both appointments, stated completion of 14 days of 5-FU treatment, and evidence of 5-FU use according to a validated numeric scale of skin reaction severity.14 We recorded telephone calls and other dermatology clinic and teledermatology appointments during the 3 weeks after the first appointment and the number of dermatology clinic appointments 6 months before and after the SMA for side effects related to 5-FU treatment. Feedback about treatment in the group setting was obtained at both visits.
Results
A total of 16 male patients attended the SMAs, and 14 attended both sessions. Of the 2 patients who were excluded from the study, 1 declined to be scheduled for the second group appointment, and the other was scheduled and confirmed but did not come for his appointment. The mean age was 72 years.
Of the 14 study patients who attended both sessions of the group clinic, 10 stated that they completed 2 weeks of 5-FU therapy, and the other 4 stated that they completed at least 11 days. Results of the validated scale used by clinicians during the second visit to grade the patients’ 5-FU reactions showed that all 14 patients demonstrated at least some expected adverse reactions (eTable). Eleven of 14 patients showed crusting and erosion; 13 showed grade 2 or higher erythema severity. One patient who stopped treatment after 11 days telephoned the dermatology clinic within 1 week of his second SMA. Another patient who stopped treatment after 11 days had a separate dermatology surgery clinic appointment within the 3-week period after starting 5-FU for a recent basal cell carcinoma excision. None of the 14 patients had a dermatology appointment scheduled within 6 months before or after for a 5-FU adverse reaction. One patient who completed the 14-day course was referred to teledermatology for insect bites within that period.
None of the patients were prophylaxed for herpes simplex virus during the treatment period, and none developed a herpes simplex virus eruption during this study. None of the patients required antibiotics for secondary impetiginization of the treatment site.
The verbal feedback about the group setting from patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers. At the conclusion of the second appointment, all of the patients reported an increased understanding of their condition and the importance of future sun-protective behaviors.
Comment
Shared medical appointments promote treatment adherence in patients with chronic heart failure and diabetes mellitus through efficient resource use, improvement of access to care, and promotion of behavioral change through group support.7-13 Within the dermatology literature, SMAs are more profitable than regular clinic appointments.15 In SMAs designed to improve patient education for preoperative consultations for Mohs micrographic surgery, patient satisfaction reported in postvisit surveys was high, with 84.7% of 149 patients reporting they found the session useful, highlighting how SMAs have potential as practical alternatives to regular medical appointments.16 Similarly, the feedback about the group setting from our patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers.
The group setting—where patients were interacting with peers undergoing the same treatment—provided an encouraging environment during the course of 5-FU therapy, resulting in a positive treatment experience. Additionally, at the conclusion of the second visit, patients reported an increased understanding of their condition and the importance of future sun-protective behaviors, further demonstrating the impact of this pilot initiative.
The Veterans Affairs’ Current Procedural Terminology code for a group clinic is 99078. Veterans Affairs medical centers and private practices have different approaches to billing and compensation. As more accountable care organizations are formed, there may be a different mixture of ways for handling these SMAs.
Limitations
Our study is limited by the small sample size, selection bias, and self-reported measure of adherence. Adherence to 5-FU is excellent without group support, and without a control group, it is unclear how beneficial the group setting was for adherence.17 The presence of the expected skin reactions at the 2-week return visit cannot account for adherence during the interval between the visits, and this close follow-up may be responsible for the high adherence in this group setting. The major side effects with 5-FU are short-term. Nonetheless, longer-term follow-up would be helpful and a worthy future endeavor.
Veterans share a common bond of military service that may not be shared in a typical private practice setting, which may have facilitated success of this pilot study. We recommend group clinics be evaluated independently in private practices and other systems. However, despite these limitations, the patients in the SMAs demonstrated positive reactions to 5-FU therapy, suggesting the potential for utilizing group clinics as a practical alternative to regular medical appointments.
Conclusion
Our pilot group clinics for AK treatment and chemoprevention of SCC with 5-FU suggest that this model is well received. The group format, which demonstrated uniformly positive reactions to 5-FU therapy, shows promise in battling an epidemic of skin cancer that demands cost-effective interventions.
Squamous cell carcinoma (SCC) has an estimated incidence of more than 2.5 million cases per year in the United States.1 Its precursor lesion, actinic keratosis (AK), had an estimated prevalence of 39.5 million cases in the United States in 2004.2 The dermatology clinic at the Providence VA Medical Center in Rhode Island exerts consistent efforts to treat both SCC and AK by prescribing topical 5-fluorouracil (5-FU) and lifestyle changes that include avoiding sun exposure, wearing protective clothing, and using effective sunscreen.3 A single course of topical 5-FU in veterans has been shown to decrease the risk for SCC by 74% during the year after treatment and also improve AK clearance rates.4,5
Effectiveness of 5-FU for secondary prevention can be decreased by patient misunderstandings, such as applying 5-FU for too short a time or using the corticosteroid cream prematurely, as well as patient nonadherence due to expected adverse skin reactions to 5-FU.6 Education and reassurance before and during therapy maximize patient compliance but can be difficult to accomplish in clinics when time is in short supply. During standard 5-FU treatment at the Providence VA Medical Center, the provider prescribes 5-FU and posttherapy corticosteroid cream at a clinic visit after an informed consent process that includes reviewing with the patient a color handout depicting the expected adverse skin reaction. Patients who later experience severe inflammation and anxiety call the clinic and are overbooked as needed.
To address the practical obstacles to the patient experience with topical 5-FU therapy, we developed a group chemoprevention clinic based on the shared medical appointment (SMA) model. Shared medical appointments, during which multiple patients are scheduled at the same visit with 1 or more health care providers, promote patient risk reduction and guideline adherence in complex diseases, such as chronic heart failure and diabetes mellitus, through efficient resource use, improvement of access to care, and promotion of behavioral changes through group support.7-13 To increase efficiency in the group chemoprevention clinic, we integrated dermatology nurses and nurse practitioners from the chronic care model into the group medical visits, which ran from September 2016 through March 2017. Because veterans could interact with peers undergoing the same treatment, we hypothesized that use of the cream in a group setting would provide positive reinforcement during the course of therapy, resulting in a positive treatment experience. We conducted a retrospective review of medical records of the patients involved in this pilot study to evaluate this model.
Methods
Institutional review board approval was obtained from the Providence VA Medical Center. Informed consent was waived because this study was a retrospective review of medical records.
Study Population
We offered participation in a group chemoprevention clinic based on the SMA model for patients of the dermatology clinic at the Providence VA Medical Center who were planning to start 5-FU in the fall of 2016. Patients were asked if they were interested in participating in a group clinic to receive their 5-FU treatment. Patients who were established dermatology patients within the Veterans Affairs system and had scheduled annual full-body skin examinations were included; patients were not excluded if they had a prior diagnosis of AK but had not been previously treated with 5-FU.
Design
Each SMA group consisted of 3 to 4 patients who met initially to receive the 5-FU medication and attend a 10-minute live presentation that included information on the dangers and causes of SCC and AK, treatment options, directions for using 5-FU, expected spectrum of side effects, and how to minimize the discomfort of treatment side effects. Patients had field treatment limited to areas with clinically apparent AKs on the face and ears. They were prescribed 5-FU cream 5% twice daily.
One physician, one nurse practitioner, and one registered nurse were present at each 1-hour clinic. Patients arrived and were checked in individually by the providers. At check-in, the provider handed the patient a printout of his/her current medication list and a pen to make any necessary corrections. This list was reviewed privately with the patient so the provider could reconcile the medication list and review the patient’s medical history and so the patient could provide informed consent. After, the patient had the opportunity to select a seat from chairs arranged in a circle. There was a live PowerPoint presentation given at the beginning of the clinic with a question-and-answer session immediately following that contained information about the disease and medication process. Clinicians assisted the patients with the initial application of 5-FU in the large group room, and each patient received a handout with information about AKs and a 40-g tube of the 5-FU cream.
This same group then met again 2 weeks later, at which time most patients were experiencing expected adverse skin reactions. At that time, there was a 10-minute live presentation that congratulated the patients on their success in the treatment process, reviewed what to expect in the following weeks, and reinforced the importance of future sun-protective practices. At each visit, photographs and feedback about the group setting were obtained in the large group room. After photographing and rating each patient’s skin reaction severity, the clinicians advised each patient either to continue the 5-FU medication for another week or to discontinue it and apply the triamcinolone cream 0.1% up to 4 times daily as needed for up to 7 days. Each patient received the prescription corticosteroid cream and a gift, courtesy of the VA Voluntary Service Program, of a 360-degree brimmed hat and sunscreen. Time for questions or concerns was available at both sessions.
Data Collection
We reviewed medical records via the Computerized Patient Record System, a nationally accessible electronic health record system, for all patients who participated in the SMA visits from September 2016 through March 2017. Any patient who attended the initial visit but declined therapy at that time was excluded.
Outcomes included attendance at both appointments, stated completion of 14 days of 5-FU treatment, and evidence of 5-FU use according to a validated numeric scale of skin reaction severity.14 We recorded telephone calls and other dermatology clinic and teledermatology appointments during the 3 weeks after the first appointment and the number of dermatology clinic appointments 6 months before and after the SMA for side effects related to 5-FU treatment. Feedback about treatment in the group setting was obtained at both visits.
Results
A total of 16 male patients attended the SMAs, and 14 attended both sessions. Of the 2 patients who were excluded from the study, 1 declined to be scheduled for the second group appointment, and the other was scheduled and confirmed but did not come for his appointment. The mean age was 72 years.
Of the 14 study patients who attended both sessions of the group clinic, 10 stated that they completed 2 weeks of 5-FU therapy, and the other 4 stated that they completed at least 11 days. Results of the validated scale used by clinicians during the second visit to grade the patients’ 5-FU reactions showed that all 14 patients demonstrated at least some expected adverse reactions (eTable). Eleven of 14 patients showed crusting and erosion; 13 showed grade 2 or higher erythema severity. One patient who stopped treatment after 11 days telephoned the dermatology clinic within 1 week of his second SMA. Another patient who stopped treatment after 11 days had a separate dermatology surgery clinic appointment within the 3-week period after starting 5-FU for a recent basal cell carcinoma excision. None of the 14 patients had a dermatology appointment scheduled within 6 months before or after for a 5-FU adverse reaction. One patient who completed the 14-day course was referred to teledermatology for insect bites within that period.
None of the patients were prophylaxed for herpes simplex virus during the treatment period, and none developed a herpes simplex virus eruption during this study. None of the patients required antibiotics for secondary impetiginization of the treatment site.
The verbal feedback about the group setting from patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers. At the conclusion of the second appointment, all of the patients reported an increased understanding of their condition and the importance of future sun-protective behaviors.
Comment
Shared medical appointments promote treatment adherence in patients with chronic heart failure and diabetes mellitus through efficient resource use, improvement of access to care, and promotion of behavioral change through group support.7-13 Within the dermatology literature, SMAs are more profitable than regular clinic appointments.15 In SMAs designed to improve patient education for preoperative consultations for Mohs micrographic surgery, patient satisfaction reported in postvisit surveys was high, with 84.7% of 149 patients reporting they found the session useful, highlighting how SMAs have potential as practical alternatives to regular medical appointments.16 Similarly, the feedback about the group setting from our patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers.
The group setting—where patients were interacting with peers undergoing the same treatment—provided an encouraging environment during the course of 5-FU therapy, resulting in a positive treatment experience. Additionally, at the conclusion of the second visit, patients reported an increased understanding of their condition and the importance of future sun-protective behaviors, further demonstrating the impact of this pilot initiative.
The Veterans Affairs’ Current Procedural Terminology code for a group clinic is 99078. Veterans Affairs medical centers and private practices have different approaches to billing and compensation. As more accountable care organizations are formed, there may be a different mixture of ways for handling these SMAs.
Limitations
Our study is limited by the small sample size, selection bias, and self-reported measure of adherence. Adherence to 5-FU is excellent without group support, and without a control group, it is unclear how beneficial the group setting was for adherence.17 The presence of the expected skin reactions at the 2-week return visit cannot account for adherence during the interval between the visits, and this close follow-up may be responsible for the high adherence in this group setting. The major side effects with 5-FU are short-term. Nonetheless, longer-term follow-up would be helpful and a worthy future endeavor.
Veterans share a common bond of military service that may not be shared in a typical private practice setting, which may have facilitated success of this pilot study. We recommend group clinics be evaluated independently in private practices and other systems. However, despite these limitations, the patients in the SMAs demonstrated positive reactions to 5-FU therapy, suggesting the potential for utilizing group clinics as a practical alternative to regular medical appointments.
Conclusion
Our pilot group clinics for AK treatment and chemoprevention of SCC with 5-FU suggest that this model is well received. The group format, which demonstrated uniformly positive reactions to 5-FU therapy, shows promise in battling an epidemic of skin cancer that demands cost-effective interventions.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Siegel JA, Korgavkar K, Weinstock MA. Current perspective on actinic keratosis: a review. Br J Dermatol. 2017;177:350-358.
- Weinstock MA, Thwin SS, Siegel JA, et al. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
- Pomerantz H, Hogan D, Eilers D, et al. Long-term efficacy of topical fluorouracil cream, 5%, for treating actinic keratosis: a randomized clinical trial. JAMA Dermatol. 2015;151:952-960.
- Foley P, Stockfleth E, Peris K, et al. Adherence to topical therapies in actinic keratosis: a literature review. J Dermatolog Treat. 2016;27:538-545.
- Desouza CV, Rentschler L, Haynatzki G. The effect of group clinics in the control of diabetes. Prim Care Diabetes. 2010;4:251-254.
- Edelman D, McDuffie JR, Oddone E, et al. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review. Washington, DC: Department of Veterans Affairs; 2012.
- Edelman D, Gierisch JM, McDuffie JR, et al. Shared medical appointments for patients with diabetes mellitus: a systematic review. J Gen Intern Med. 2015;30:99-106.
- Trento M, Passera P, Tomalino M, et al. Group visits improve metabolic control in type 2 diabetes: a 2-year follow-up. Diabetes Care. 2001;24:995-1000.
- Wagner EH, Grothaus LC, Sandhu N, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care. 2001;24:695-700.
- Harris MD, Kirsh S, Higgins PA. Shared medical appointments: impact on clinical and quality outcomes in veterans with diabetes. Qual Manag Health Care. 2016;25:176-180.
- Kirsh S, Watts S, Pascuzzi K, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16:349-353.
- Pomerantz H, Korgavkar K, Lee KC, et al. Validation of photograph-based toxicity score for topical 5-fluorouracil cream application. J Cutan Med Surg. 2016;20:458-466.
- Sidorsky T, Huang Z, Dinulos JG. A business case for shared medical appointments in dermatology: improving access and the bottom line. Arch Dermatol. 2010;146:374-381.
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344.
- Yentzer B, Hick J, Williams L, et al. Adherence to a topical regimen of 5-fluorouracil, 0.5%, cream for the treatment of actinic keratoses. JAMA Dermatol. 2009;145:203-205.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Siegel JA, Korgavkar K, Weinstock MA. Current perspective on actinic keratosis: a review. Br J Dermatol. 2017;177:350-358.
- Weinstock MA, Thwin SS, Siegel JA, et al. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
- Pomerantz H, Hogan D, Eilers D, et al. Long-term efficacy of topical fluorouracil cream, 5%, for treating actinic keratosis: a randomized clinical trial. JAMA Dermatol. 2015;151:952-960.
- Foley P, Stockfleth E, Peris K, et al. Adherence to topical therapies in actinic keratosis: a literature review. J Dermatolog Treat. 2016;27:538-545.
- Desouza CV, Rentschler L, Haynatzki G. The effect of group clinics in the control of diabetes. Prim Care Diabetes. 2010;4:251-254.
- Edelman D, McDuffie JR, Oddone E, et al. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review. Washington, DC: Department of Veterans Affairs; 2012.
- Edelman D, Gierisch JM, McDuffie JR, et al. Shared medical appointments for patients with diabetes mellitus: a systematic review. J Gen Intern Med. 2015;30:99-106.
- Trento M, Passera P, Tomalino M, et al. Group visits improve metabolic control in type 2 diabetes: a 2-year follow-up. Diabetes Care. 2001;24:995-1000.
- Wagner EH, Grothaus LC, Sandhu N, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care. 2001;24:695-700.
- Harris MD, Kirsh S, Higgins PA. Shared medical appointments: impact on clinical and quality outcomes in veterans with diabetes. Qual Manag Health Care. 2016;25:176-180.
- Kirsh S, Watts S, Pascuzzi K, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16:349-353.
- Pomerantz H, Korgavkar K, Lee KC, et al. Validation of photograph-based toxicity score for topical 5-fluorouracil cream application. J Cutan Med Surg. 2016;20:458-466.
- Sidorsky T, Huang Z, Dinulos JG. A business case for shared medical appointments in dermatology: improving access and the bottom line. Arch Dermatol. 2010;146:374-381.
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344.
- Yentzer B, Hick J, Williams L, et al. Adherence to a topical regimen of 5-fluorouracil, 0.5%, cream for the treatment of actinic keratoses. JAMA Dermatol. 2009;145:203-205.
Practice Points
- Shared medical appointments (SMAs) enhance patient experience with topical 5-fluorouracil (5-FU) treatment of actinic keratosis (AK).
- Dermatologists should consider utilizing the SMA model for their patients being treated with 5-FU, as patients demonstrated a positive emotional response to 5-FU therapy in the group clinic setting.
You Need a Plan: A Stepwise Protocol for Operating Room Preparedness During an Infectious Pandemic
The worldwide spread of SARS-CoV-2, the coronavirus that causes the syndrome designated COVID-19 by the World Health Organization (WHO), presents a challenge for emergency operative care in a global pandemic setting that is novel for modern surgical practice. The virulence of this new pathogen has raised concern for how to protect operating room (OR) staff and their environs in the event that an infected patient requires urgent surgical care. Because coronaviridae spread mainly through contact with contaminated respiratory droplets or aerosolized virion-containing particles, personal protective equipment (PPE) is vital to personnel involved in these cases, and proper utilization of these scarce resources poses an additional challenge. Establishment of a clear protocol that adheres to rigorous infection control measures while providing a safe system for intrafacility transport and operative care is an essential component of a successful surgical pandemic response.
The first case of COVID-19 disease identified in the US was diagnosed in Everett, Washington, on January 21, 2020.1 In the succeeding months, the Seattle region became an early epicenter of the epidemic in the US, with Washington State becoming the first state to see in excess of 1,000 cases by mid-March 2020. As hospitalizations for COVID-19 increased, emergency surge preparations were enacted at medical centers across the region. Recommendations for how to manage infected patients evolved rapidly. Anticipating the need to provide surgical services during this pandemic, starting in early March 2020, the perioperative services staff at the US Department of Veterans Affairs (VA) Puget Sound Health Care System (PSHCS) convened to develop the protocol described here through a process of literature review, multidisciplinary discussion, and practical trial runs and drills. VAPSHCS is an urban academic medical center affiliated with the University of Washington, Seattle. The result of this collaboration is a detailed, step-by-step protocol that establishes the roles and responsibilities of the various personnel who intersect in the OR and recruits their teamwork to prevent environmental contamination and health care worker transmission of SARS-CoV-2.
The protocol is divided into discrete practice recommendations for the preoperative, intraoperative, and postoperative management of patients with confirmed or suspected COVID-19 infection, with a focus on maintaining Centers of Disease Control and Prevention-defined respiratory droplet and airborne precautions throughout the period of patient contact and mitigating infectious contamination of the operating suite.2 It is acknowledged that no written protocol can encompass all the possible considerations that attend the vast diversity of surgical scenarios which can transpire in the operative setting. Patient acuity must sometimes mandate modifications to even the most thoroughly laid plans; for instance, the exsanguinating patient requiring emergent surgery for hemorrhage control will undoubtedly require an urgent appraisal of the relative risks and benefits of certain elements of the practices here described. Nevertheless, we believe that this protocol provides a useful framework for mitigating the infection and contamination risks of operative care in an epidemic environment, and should be readily adaptable to any facility that may perform surgery in patients infected with a high-risk contagious pathogen.
Preoperative Management
In addition to introducing the risk of viral transmission, surgery in the patient with COVID-19 also imposes a large consumption of vital PPE, supplies and can become dangerously low in health care centers coping with an influx of infected patients. Early in the pandemic, to reduce exposure, conserve the medical workforce and lessen the resource strain on the overall health care infrastructure, the American College of Surgeons (ACS), American College of Gastroenterology, and other professional societies recommended cancellation of elective procedures, confining operations to urgent or emergent procedures for high-acuity diseases that would negatively impact morbidity or mortality if delayed.3,4 In each case, physicians from the surgical and anesthesia services should discuss the rationale for the operation and secure agreement to commit resources to the endeavor prior to reserving the OR. These considerations should be shared with the patient prior to obtaining informed consent.
Preoperatively, the surgical team, consisting of surgeon, anesthesiologist, OR nurse, surgical technician, and assistants to the surgeon, anesthesiologist and nurse, convene for a preoperative “team huddle.” While assistants will aid in patient transport and supplying equipment to the team during the procedure, they should not be in the OR during the case, to minimize personnel exposure and PPE consumption. All members of the surgical team remove their personal effects, including wallets, phones, badges, and jewelry; any pagers are handed to other members of the care team for the duration of the surgery. During this preoperative team huddle, proper setup and accounting of the surgical equipment is confirmed, as well as the availability of all necessary anesthesia equipment and medications.
A specific OR with versatile characteristics was chosen to be the designated OR for procedures in patients with confirmed or suspected COVID-19. The COVID OR is on standby when no such cases are active, and it is not used for surgeries in noninfected patients. This is in accord with published recommendations of anesthesiologists who, throughout the COVID-19 epidemic in China, maintained designated ORs and anesthesia machines for only infected patients.5 Strong consideration should be given to performing procedures for which endotracheal intubation is not required in the patient’s own respiratory isolation room, rather than the OR to avoid room contamination and excessive use of PPE.5,6
The availability of adequate PPE is confirmed during the preoperative team huddle. At a minimum, powered air purifying respirator devices (PAPRs) with hoods must be available for the anesthesia provider, surgeon and surgical technician, recognizing the Anesthesia Patient Safety Foundation (APSF) recommendation that these devices confer superior protection for those with the highest risk and most proximate exposure to the patient throughout the case.7,8 An N95 respirator, at minimum, must be available for the circulating OR nurse. Patient condition, need for critical care transport, anesthetic plan (monitored anesthesia care or general anesthesia), and availability of negative pressure isolation rooms in the ward vs in the operating suite should help decide patient transport strategies and help determine the most suitable location to secure the airway. In case of an inadvertent tube disconnection, transporting intubated patients carries the risk of disseminating virus laden aerosols into the environment. Risks of pre-OR intubation should be balanced with the potential benefit of securing the airway prior to transport and decreased gross OR contamination with intubation in the operating suite. Airway manipulation and intubation are among the highest risk procedures for nosocomial transmission and performance of these procedures should utilize precautions described in current APSF recommendations.3,9,10
For patients not requiring critical care transport, and when conditions favor intubation in the OR, patients should be transported in a gurney while wearing a surgical mask. Verification of the operative site, surgical plan, and other components of the WHO universal surgical safety checklist or time out are performed in the OR prior to induction of anesthesia, and a conscious patient can be an active participant.
If critical care transport is deemed necessary and/or a decision is made to intubate the patient outside the OR, preferably in a negative airflow respiratory isolation room, the perioperative team will confirm the availability of the following equipment needed for patient transport:
- Portable transport monitor;
- Video laryngoscope;
- Airway supplies and medications for induction of general anesthesia;
- Self-inflating bag-mask apparatus attached to an oxygen source;
- High-quality HMEF (heat and moisture exchanging filter) rated to remove at least 99.97% of airborne particles ≥ 0.3 microns to filter out viral particles attached to the expiratory outlet; and
- PPE including impermeable disposable gowns, gloves, and shoe covers.
While the surgical technician remains in the OR, the rest of the team will proceed to the patient’s location with these supplies, along with the necessary number of PAPRs and N95 respirators.
Outside the patient room, the team consisting of surgeon, anesthesia provider, OR nurse, and the assistant to each of these health care providers, gathers for the first time out, confirming the patient’s identification, intended procedure, surgical site, laterality, and informed consent. If the patient is verbal and has decision-making capacity, they confirm their identification, understanding of the planned procedure, and consent with the team over the phone from the confines of their room. If a patient lacks decision making capacity standard organization policies should be adhered to, most of which do not require direct patient contact and do not pose any unique infection control challenges. The anesthesia provider and surgeon don their PPE including PAPR devices with the aid of their assistants. Using a PPE checklist, the surgical team member dons with the assistance of a PPE partner, who is charged with reading the instructions on the checklist to the surgical team member step by step and inspecting the adequacy of the full PPE attire (Figure 1). A similar secondary check of appropriate PPE by an assistant during high risk encounters has also been advocated by other authors.6
Consideration should be given to intubating the patient prior to transport to the OR particularly if the patient originates in a respiratory isolation room with negative pressure airflow, being mindful that most operating suites are ventilated with positive airflow that could help disperse virus laden aerosols in the procedure area. It may also be beneficial to have a secure airway in a patient who is actively coughing, sneezing, and dispersing respiratory droplets to the surrounding environment prior to leaving respiratory isolation. When intubation prior to OR transport is chosen, the fully attired anesthesiologist enters the patient room first, with a video laryngoscope, medication, and other supplies needed to successfully induce general endotracheal tube anesthesia. The anesthesia and surgery assistants don droplet precaution PPE and remain outside the patient room. Whenever possible, a rapid sequence induction is performed with minimization of bag-mask ventilation. Video laryngoscopy is preferred over direct laryngoscopy in patients with COVID-19 as it enables a greater distance between the health care provider and the airway.5,6 The surgeon and OR nurse then enter the room, wearing PPE including PAPR, and assist with attaching the transport monitor and moving the patient bed out of the room. The OR nurse wipes the front face shield and PAPR hood of the anesthesia provider after intubation, to clean these presumably contaminated components prior to exiting the room. A second, clean disposable gown covers the one worn during intubation to minimize environmental contamination during transport.11,12
The patient is intubated, anesthetized, and, transported to the OR, with a self-inflating bag mask apparatus attached to an oxygen source and a second high-quality HMEF rated to remove at least 99.97% of airborne particles ≥ 0.3 microns is attached to the expiratory outlet, or a transport ventilator with HEPA filter attached to the expiratory limb. In the OR, the anesthesia provider, surgical technician, and OR nurse assist with moving the patient to the operating gurney and attaching the monitor. The surgeon remains outside the room in order to doff the gown and gloves worn during transport, disinfect their hands (preoperative scrubbing), and don sterile attire, all while continuing to wear the same PAPR and hood.
Intraoperative Management
Advance planning can help to ensure a safer intraoperative period when a COVID-19 patient is brought to the OR. Patient room airflow patterns and ventilation capacity should be considered when developing measures to prevent aerosol transmission of airborne infectious agents. Although negative pressure rooms are ideal for aerosol generating procedures such as intubation, most ORs are generally maintained at a positive pressure when compared with the surrounding areas. The feasibility of rapidly converting ORs into negative pressure rooms should be in facility planning for COVID-19; portable high-efficiency particulate air (HEPA) machines, for instance, can be set up to create negative pressure areas around the OR.13 We established a negative pressure anteroom outside our OR to be used for doffing and as an airlock, for use by staff who need to enter midcase or pass supplies or specimens into and out of the procedure room (Figure 2). By adding 2 portable HEPA filters and directing the HEPA-filtered exhaust into the OR ventilation return columns, we were able to establish negative pressure airflow in the OR (Figure 3).
The protocol was devised with the current pandemic-associated shortage of PPE taken into consideration. We decided to minimize staffing across disciplines by excluding all nonessential personal from entering the OR. This includes observers, researchers, and medical students. Residents and fellows may participate if their presence is deemed vital to the patient’s intraoperative care. To further prevent resource consumption, equipment in the designated COVID OR was reduced to essential elements such as the anesthesia machine, a minimized anesthesia drug cart and general supply cabinet, all of which were covered with disposable transparent covers (Figure 4).14 After transfer of the patient to the OR table, the patient stretcher is kept in the OR (space permitting) to minimize contamination of areas immediately outside the OR.
Prior to incision a second time out is performed to confirm the previously verified operative site and plan. During the case, the assistants to the OR nurse and anesthesia provider act as facilitators or “runners” for equipment retrieval and communication with the outside OR staff. These roles are assigned to personnel who are familiar with the layout and day-to-day functioning of the ORs, such as anesthesia technicians and OR circulating nurses. All staff agreed on a strategy of no breaks or alternations whenever possible to conserve PPEs.15 Near the conclusion of the surgical procedure, the receiving intensive care unit (ICU) is given a verbal report on patient status over the phone.
Postperative Management
Similar to intubation, extubation poses a risk of generating aerosolization of infectious airborne microbes.10 It is helpful for OR personnel to be aware of the airflow pattern in their ORs, whether it is positive, negative, or neutral. As the PSHCS ORs were originally engineered as positive pressure rooms, we elected to have to postoperative patients with COVID-19 transported intubated to a reverse airflow or negative pressure room in the ICU. Extubation is performed when the intensive care team has determined the patient meets extubation criteria and has passed a spontaneous breathing trial. When a negative pressure room in the ICU is not available for recovery, extubation may be performed in the OR.
In that circumstance, the patient remains in the OR for 30 minutes after extubation to allow for turnover of air in the room prior to the doors opening for patient transport to the ICU.16 A surgical mask is placed over the patient’s oxygenating face mask to reduce droplet spread during transport. Patients who are not intubated for the anesthetic may be first recovered in the operating room or transported under droplet precautions directly back to a negative pressure isolation room.
Prior to transport, the patient’s gurney is thoroughly cleaned with Environmental Protection Agency-approved disinfectant wipes, and a clean sheet is placed over the patient’s body below the head.17 The front face shield of the surgeon’s and anesthesiologist’s PAPR hood should be wiped down with an alcohol-based disinfectant. Both health care providers don a clean disposable gown as an outer layer to minimize contamination by their used attire during transport. Once the patient is transported out of the OR, all disposable items are discarded. Reusable medical equipment are cleaned and disinfected according to a thorough application of local environmental services standard operating procedures.18 The surgeon and anesthesia providers aid in transporting the patient to the ICU, along with their outside OR assistants. All personnel remaining in the OR exit and doff their PPE according to the doffing protocol, which is similar to the donning protocol, utilizes a PPE partner tasked with providing instructions to the surgical team member step by step (Figure 5).
After leaving the OR, terminal cleaning must be performed by environmental services (EVS) personnel, but they should delay entry into the room until a sufficient amount of time has elapsed after the last aerosol-generating procedure in the OR. Time determination will depend on the air change per hour (ACH) in the OR that will achieve 99.9% removal of airborne contaminates. For example, ventilation in our operating rooms operate at approximately 15 to 20 ACH, which should attain that level of air clearance in 21 to 28 minutes.16 Once the stipulated time has elapsed EVS personnel may enter the room but should wear a gown and gloves when performing terminal cleaning. A face mask and eye protection should be added if splashes or sprays during cleaning and disinfection activities are anticipated, or otherwise required based on the selected cleaning products. Anesthesia technicians can now also enter the room to disinfect the anesthesia machines and set up all disposable supplies for any potential following case.
Conclusions
The outbreak of COVID-19 has resulted in an unprecedented modern health care crisis across the globe. Perioperative management of patients with COVID-19 pose unique challenges to all personnel working in the OR, where the risk of nosocomial transmission of infection is ever present. It is essential that hospitals consider their local resources, infrastructure and capabilities when devising policies to respond to the COVID-19 emergency. In all perioperative situations, meticulous attention should be given to both donning and doffing of PPE, crucial for the safety of everyone involved in the care of patients with COVID-19.
Our experience also highlighted the importance of treating a new protocol as an evolving document, which can be modified and improved through the conduct of training and simulation exercises with providers across disciplines (Figure 6). In gathering nurses, anesthesia staff, and surgeons to perform drills and simulate their roles in an imaginary scenario, we gained new insights, and made corrections and additions that ultimately generated the presently described process. Modifications to any protocol may be necessary depending on the unique circumstances of individual health care systems and hospitals, the characteristics of the patient population they cater to, and the resources and expertise they have available. As the pandemic continues, we are bound to learn more about the epidemiology and modes of transmission of SARS-CoV-2, which may demand further changes to our practice. It is crucial to remember that while emergency policies must be rapidly developed, they should be collaboratively improved and incorporate new knowledge when it becomes available. This is essential to ensure the ultimate protocol is useful, up-to-date, easy to follow and tailored to the unique local environment of each health care setting.
After the initial apprehensions and struggles that attended our confrontation with the crisis, it is our hope that the experience we share will be helpful to surgical staff at other institutions grappling with the challenges of operative care in the pandemic environment. While this protocol focuses on the current COVID-19 pandemic, these recommendations serve as a template for surgical preparedness that can be readily adapted to the next infectious disease crisis that will inevitably emerge.
1. Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929-936.
2. Siegel S RE, Jackson M, Chiarello L. Healthcare Infection Control Practices Advisory Committee; Guideline for Isolation Precautions. Centers For Disease Control and Prevention. https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html. Published 2007. Accessed March 28, 2020.
3. American College of Surgeons: COVID-19: Guidance for Triage of Non-Emergent Surgical Procedures. American College of Surgeons. https://www.facs.org/covid-19/clinical-guidance/triage. Published March 17, 2020. Accessed April 19, 2020.
4. American College of Gastroenterology. Gastroenterology professional society Guidance on endoscopic procedures During the covid-19 pandemic. American College of Gastroenterology. https://webfiles.gi.org/links/media/Joint_GI_Society_Guidance_on_Endoscopic_Procedure_During_COVID19_FINAL_impending_3312020.pdf. Published March 31, 2020. Accessed April 19, 2020.
5. Chen X, Liu Y, Gong Y, et al. Perioperative management of patients infected with the novel coronavirus: recommendation from the Joint Task Force of the Chinese Society of Anesthesiology and the Chinese Association of Anesthesiologists [published online ahead of print, 2020 Mar 26]. Anesthesiology. 2020;10.1097/ALN.0000000000003301.
6. Zhang HF, Bo L, Lin Y, et al. Response of Chinese anesthesiologists to the COVID-19 outbreak [published online ahead of print, 2020 Mar 30]. Anesthesiology. 2020;10.1097/ALN.0000000000003300.
7. Kamming D, Gardam M, Chung F. Anaesthesia and SARS. Br J Anaesth. 2003;90(6):715-718.
8. Zucco L LN, Ketchandji D, Aziz M, Ramachandran SK. Perioperative considerations for the 2019 novel coronavirus (COVID-19). https://www.apsf.org/news-updatesperioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published Feb 12, 2020. Accessed March 30, 2020.
9. Caputo KM, Byrick R, Chapman MG, Orser BJ, Orser BA. Intubation of SARS patients: infection and perspectives of healthcare workers. Can J Anaesth. 2006;53(2):122-129.
10. Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 2019;11(10):940.
11. Peng PWH, Ho PL, Hota SS. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anaesth. 2020;124(5):497‐501.
12. Ti LK, Ang LS, Foong TW, Ng BSW. What we do when a COVID-19 patient needs an operation: operating room preparation and guidance [published online ahead of print, 2020 Mar 6]. Can J Anaesth. 2020;1‐3.
13. Chow TT, Kwan A, Lin Z, Bai W. Conversion of operating theatre from positive to negative pressure environment. J Hosp Infect. 2006;64(4):371-378.
14. Clark C, Taenzer A, Charette K, Whitty M. Decreasing contamination of the anesthesia environment. Am J Infect Control. 2014;42(11):1223-1225.
15. Dexter F, Parra MC, Brown JR, Loftus RW. Perioperative COVID-19 defense: an evidence-based approach for optimization of infection control and operating room management [published online ahead of print, 2020 Mar 26]. Anesth Analg. 2020;10.1213/ANE.0000000000004829.
16. Jensen PA, Lambert LA, Iademarco MF, Ridzon R, CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54(RR-17):1-141.
17. US Environmental Protection Agency. List N: disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Updated April 16, 2020. Accessed April 19, 2020.
18. Munoz-Price LS, Bowdle A, Johnston BL, et al. Infection prevention in the operating room anesthesia work area [published correction appears in Infect Control Hosp Epidemiol. 2019 Apr;40(4):500]. Infect Control Hosp Epidemiol. 2018;1‐17.
The worldwide spread of SARS-CoV-2, the coronavirus that causes the syndrome designated COVID-19 by the World Health Organization (WHO), presents a challenge for emergency operative care in a global pandemic setting that is novel for modern surgical practice. The virulence of this new pathogen has raised concern for how to protect operating room (OR) staff and their environs in the event that an infected patient requires urgent surgical care. Because coronaviridae spread mainly through contact with contaminated respiratory droplets or aerosolized virion-containing particles, personal protective equipment (PPE) is vital to personnel involved in these cases, and proper utilization of these scarce resources poses an additional challenge. Establishment of a clear protocol that adheres to rigorous infection control measures while providing a safe system for intrafacility transport and operative care is an essential component of a successful surgical pandemic response.
The first case of COVID-19 disease identified in the US was diagnosed in Everett, Washington, on January 21, 2020.1 In the succeeding months, the Seattle region became an early epicenter of the epidemic in the US, with Washington State becoming the first state to see in excess of 1,000 cases by mid-March 2020. As hospitalizations for COVID-19 increased, emergency surge preparations were enacted at medical centers across the region. Recommendations for how to manage infected patients evolved rapidly. Anticipating the need to provide surgical services during this pandemic, starting in early March 2020, the perioperative services staff at the US Department of Veterans Affairs (VA) Puget Sound Health Care System (PSHCS) convened to develop the protocol described here through a process of literature review, multidisciplinary discussion, and practical trial runs and drills. VAPSHCS is an urban academic medical center affiliated with the University of Washington, Seattle. The result of this collaboration is a detailed, step-by-step protocol that establishes the roles and responsibilities of the various personnel who intersect in the OR and recruits their teamwork to prevent environmental contamination and health care worker transmission of SARS-CoV-2.
The protocol is divided into discrete practice recommendations for the preoperative, intraoperative, and postoperative management of patients with confirmed or suspected COVID-19 infection, with a focus on maintaining Centers of Disease Control and Prevention-defined respiratory droplet and airborne precautions throughout the period of patient contact and mitigating infectious contamination of the operating suite.2 It is acknowledged that no written protocol can encompass all the possible considerations that attend the vast diversity of surgical scenarios which can transpire in the operative setting. Patient acuity must sometimes mandate modifications to even the most thoroughly laid plans; for instance, the exsanguinating patient requiring emergent surgery for hemorrhage control will undoubtedly require an urgent appraisal of the relative risks and benefits of certain elements of the practices here described. Nevertheless, we believe that this protocol provides a useful framework for mitigating the infection and contamination risks of operative care in an epidemic environment, and should be readily adaptable to any facility that may perform surgery in patients infected with a high-risk contagious pathogen.
Preoperative Management
In addition to introducing the risk of viral transmission, surgery in the patient with COVID-19 also imposes a large consumption of vital PPE, supplies and can become dangerously low in health care centers coping with an influx of infected patients. Early in the pandemic, to reduce exposure, conserve the medical workforce and lessen the resource strain on the overall health care infrastructure, the American College of Surgeons (ACS), American College of Gastroenterology, and other professional societies recommended cancellation of elective procedures, confining operations to urgent or emergent procedures for high-acuity diseases that would negatively impact morbidity or mortality if delayed.3,4 In each case, physicians from the surgical and anesthesia services should discuss the rationale for the operation and secure agreement to commit resources to the endeavor prior to reserving the OR. These considerations should be shared with the patient prior to obtaining informed consent.
Preoperatively, the surgical team, consisting of surgeon, anesthesiologist, OR nurse, surgical technician, and assistants to the surgeon, anesthesiologist and nurse, convene for a preoperative “team huddle.” While assistants will aid in patient transport and supplying equipment to the team during the procedure, they should not be in the OR during the case, to minimize personnel exposure and PPE consumption. All members of the surgical team remove their personal effects, including wallets, phones, badges, and jewelry; any pagers are handed to other members of the care team for the duration of the surgery. During this preoperative team huddle, proper setup and accounting of the surgical equipment is confirmed, as well as the availability of all necessary anesthesia equipment and medications.
A specific OR with versatile characteristics was chosen to be the designated OR for procedures in patients with confirmed or suspected COVID-19. The COVID OR is on standby when no such cases are active, and it is not used for surgeries in noninfected patients. This is in accord with published recommendations of anesthesiologists who, throughout the COVID-19 epidemic in China, maintained designated ORs and anesthesia machines for only infected patients.5 Strong consideration should be given to performing procedures for which endotracheal intubation is not required in the patient’s own respiratory isolation room, rather than the OR to avoid room contamination and excessive use of PPE.5,6
The availability of adequate PPE is confirmed during the preoperative team huddle. At a minimum, powered air purifying respirator devices (PAPRs) with hoods must be available for the anesthesia provider, surgeon and surgical technician, recognizing the Anesthesia Patient Safety Foundation (APSF) recommendation that these devices confer superior protection for those with the highest risk and most proximate exposure to the patient throughout the case.7,8 An N95 respirator, at minimum, must be available for the circulating OR nurse. Patient condition, need for critical care transport, anesthetic plan (monitored anesthesia care or general anesthesia), and availability of negative pressure isolation rooms in the ward vs in the operating suite should help decide patient transport strategies and help determine the most suitable location to secure the airway. In case of an inadvertent tube disconnection, transporting intubated patients carries the risk of disseminating virus laden aerosols into the environment. Risks of pre-OR intubation should be balanced with the potential benefit of securing the airway prior to transport and decreased gross OR contamination with intubation in the operating suite. Airway manipulation and intubation are among the highest risk procedures for nosocomial transmission and performance of these procedures should utilize precautions described in current APSF recommendations.3,9,10
For patients not requiring critical care transport, and when conditions favor intubation in the OR, patients should be transported in a gurney while wearing a surgical mask. Verification of the operative site, surgical plan, and other components of the WHO universal surgical safety checklist or time out are performed in the OR prior to induction of anesthesia, and a conscious patient can be an active participant.
If critical care transport is deemed necessary and/or a decision is made to intubate the patient outside the OR, preferably in a negative airflow respiratory isolation room, the perioperative team will confirm the availability of the following equipment needed for patient transport:
- Portable transport monitor;
- Video laryngoscope;
- Airway supplies and medications for induction of general anesthesia;
- Self-inflating bag-mask apparatus attached to an oxygen source;
- High-quality HMEF (heat and moisture exchanging filter) rated to remove at least 99.97% of airborne particles ≥ 0.3 microns to filter out viral particles attached to the expiratory outlet; and
- PPE including impermeable disposable gowns, gloves, and shoe covers.
While the surgical technician remains in the OR, the rest of the team will proceed to the patient’s location with these supplies, along with the necessary number of PAPRs and N95 respirators.
Outside the patient room, the team consisting of surgeon, anesthesia provider, OR nurse, and the assistant to each of these health care providers, gathers for the first time out, confirming the patient’s identification, intended procedure, surgical site, laterality, and informed consent. If the patient is verbal and has decision-making capacity, they confirm their identification, understanding of the planned procedure, and consent with the team over the phone from the confines of their room. If a patient lacks decision making capacity standard organization policies should be adhered to, most of which do not require direct patient contact and do not pose any unique infection control challenges. The anesthesia provider and surgeon don their PPE including PAPR devices with the aid of their assistants. Using a PPE checklist, the surgical team member dons with the assistance of a PPE partner, who is charged with reading the instructions on the checklist to the surgical team member step by step and inspecting the adequacy of the full PPE attire (Figure 1). A similar secondary check of appropriate PPE by an assistant during high risk encounters has also been advocated by other authors.6
Consideration should be given to intubating the patient prior to transport to the OR particularly if the patient originates in a respiratory isolation room with negative pressure airflow, being mindful that most operating suites are ventilated with positive airflow that could help disperse virus laden aerosols in the procedure area. It may also be beneficial to have a secure airway in a patient who is actively coughing, sneezing, and dispersing respiratory droplets to the surrounding environment prior to leaving respiratory isolation. When intubation prior to OR transport is chosen, the fully attired anesthesiologist enters the patient room first, with a video laryngoscope, medication, and other supplies needed to successfully induce general endotracheal tube anesthesia. The anesthesia and surgery assistants don droplet precaution PPE and remain outside the patient room. Whenever possible, a rapid sequence induction is performed with minimization of bag-mask ventilation. Video laryngoscopy is preferred over direct laryngoscopy in patients with COVID-19 as it enables a greater distance between the health care provider and the airway.5,6 The surgeon and OR nurse then enter the room, wearing PPE including PAPR, and assist with attaching the transport monitor and moving the patient bed out of the room. The OR nurse wipes the front face shield and PAPR hood of the anesthesia provider after intubation, to clean these presumably contaminated components prior to exiting the room. A second, clean disposable gown covers the one worn during intubation to minimize environmental contamination during transport.11,12
The patient is intubated, anesthetized, and, transported to the OR, with a self-inflating bag mask apparatus attached to an oxygen source and a second high-quality HMEF rated to remove at least 99.97% of airborne particles ≥ 0.3 microns is attached to the expiratory outlet, or a transport ventilator with HEPA filter attached to the expiratory limb. In the OR, the anesthesia provider, surgical technician, and OR nurse assist with moving the patient to the operating gurney and attaching the monitor. The surgeon remains outside the room in order to doff the gown and gloves worn during transport, disinfect their hands (preoperative scrubbing), and don sterile attire, all while continuing to wear the same PAPR and hood.
Intraoperative Management
Advance planning can help to ensure a safer intraoperative period when a COVID-19 patient is brought to the OR. Patient room airflow patterns and ventilation capacity should be considered when developing measures to prevent aerosol transmission of airborne infectious agents. Although negative pressure rooms are ideal for aerosol generating procedures such as intubation, most ORs are generally maintained at a positive pressure when compared with the surrounding areas. The feasibility of rapidly converting ORs into negative pressure rooms should be in facility planning for COVID-19; portable high-efficiency particulate air (HEPA) machines, for instance, can be set up to create negative pressure areas around the OR.13 We established a negative pressure anteroom outside our OR to be used for doffing and as an airlock, for use by staff who need to enter midcase or pass supplies or specimens into and out of the procedure room (Figure 2). By adding 2 portable HEPA filters and directing the HEPA-filtered exhaust into the OR ventilation return columns, we were able to establish negative pressure airflow in the OR (Figure 3).
The protocol was devised with the current pandemic-associated shortage of PPE taken into consideration. We decided to minimize staffing across disciplines by excluding all nonessential personal from entering the OR. This includes observers, researchers, and medical students. Residents and fellows may participate if their presence is deemed vital to the patient’s intraoperative care. To further prevent resource consumption, equipment in the designated COVID OR was reduced to essential elements such as the anesthesia machine, a minimized anesthesia drug cart and general supply cabinet, all of which were covered with disposable transparent covers (Figure 4).14 After transfer of the patient to the OR table, the patient stretcher is kept in the OR (space permitting) to minimize contamination of areas immediately outside the OR.
Prior to incision a second time out is performed to confirm the previously verified operative site and plan. During the case, the assistants to the OR nurse and anesthesia provider act as facilitators or “runners” for equipment retrieval and communication with the outside OR staff. These roles are assigned to personnel who are familiar with the layout and day-to-day functioning of the ORs, such as anesthesia technicians and OR circulating nurses. All staff agreed on a strategy of no breaks or alternations whenever possible to conserve PPEs.15 Near the conclusion of the surgical procedure, the receiving intensive care unit (ICU) is given a verbal report on patient status over the phone.
Postperative Management
Similar to intubation, extubation poses a risk of generating aerosolization of infectious airborne microbes.10 It is helpful for OR personnel to be aware of the airflow pattern in their ORs, whether it is positive, negative, or neutral. As the PSHCS ORs were originally engineered as positive pressure rooms, we elected to have to postoperative patients with COVID-19 transported intubated to a reverse airflow or negative pressure room in the ICU. Extubation is performed when the intensive care team has determined the patient meets extubation criteria and has passed a spontaneous breathing trial. When a negative pressure room in the ICU is not available for recovery, extubation may be performed in the OR.
In that circumstance, the patient remains in the OR for 30 minutes after extubation to allow for turnover of air in the room prior to the doors opening for patient transport to the ICU.16 A surgical mask is placed over the patient’s oxygenating face mask to reduce droplet spread during transport. Patients who are not intubated for the anesthetic may be first recovered in the operating room or transported under droplet precautions directly back to a negative pressure isolation room.
Prior to transport, the patient’s gurney is thoroughly cleaned with Environmental Protection Agency-approved disinfectant wipes, and a clean sheet is placed over the patient’s body below the head.17 The front face shield of the surgeon’s and anesthesiologist’s PAPR hood should be wiped down with an alcohol-based disinfectant. Both health care providers don a clean disposable gown as an outer layer to minimize contamination by their used attire during transport. Once the patient is transported out of the OR, all disposable items are discarded. Reusable medical equipment are cleaned and disinfected according to a thorough application of local environmental services standard operating procedures.18 The surgeon and anesthesia providers aid in transporting the patient to the ICU, along with their outside OR assistants. All personnel remaining in the OR exit and doff their PPE according to the doffing protocol, which is similar to the donning protocol, utilizes a PPE partner tasked with providing instructions to the surgical team member step by step (Figure 5).
After leaving the OR, terminal cleaning must be performed by environmental services (EVS) personnel, but they should delay entry into the room until a sufficient amount of time has elapsed after the last aerosol-generating procedure in the OR. Time determination will depend on the air change per hour (ACH) in the OR that will achieve 99.9% removal of airborne contaminates. For example, ventilation in our operating rooms operate at approximately 15 to 20 ACH, which should attain that level of air clearance in 21 to 28 minutes.16 Once the stipulated time has elapsed EVS personnel may enter the room but should wear a gown and gloves when performing terminal cleaning. A face mask and eye protection should be added if splashes or sprays during cleaning and disinfection activities are anticipated, or otherwise required based on the selected cleaning products. Anesthesia technicians can now also enter the room to disinfect the anesthesia machines and set up all disposable supplies for any potential following case.
Conclusions
The outbreak of COVID-19 has resulted in an unprecedented modern health care crisis across the globe. Perioperative management of patients with COVID-19 pose unique challenges to all personnel working in the OR, where the risk of nosocomial transmission of infection is ever present. It is essential that hospitals consider their local resources, infrastructure and capabilities when devising policies to respond to the COVID-19 emergency. In all perioperative situations, meticulous attention should be given to both donning and doffing of PPE, crucial for the safety of everyone involved in the care of patients with COVID-19.
Our experience also highlighted the importance of treating a new protocol as an evolving document, which can be modified and improved through the conduct of training and simulation exercises with providers across disciplines (Figure 6). In gathering nurses, anesthesia staff, and surgeons to perform drills and simulate their roles in an imaginary scenario, we gained new insights, and made corrections and additions that ultimately generated the presently described process. Modifications to any protocol may be necessary depending on the unique circumstances of individual health care systems and hospitals, the characteristics of the patient population they cater to, and the resources and expertise they have available. As the pandemic continues, we are bound to learn more about the epidemiology and modes of transmission of SARS-CoV-2, which may demand further changes to our practice. It is crucial to remember that while emergency policies must be rapidly developed, they should be collaboratively improved and incorporate new knowledge when it becomes available. This is essential to ensure the ultimate protocol is useful, up-to-date, easy to follow and tailored to the unique local environment of each health care setting.
After the initial apprehensions and struggles that attended our confrontation with the crisis, it is our hope that the experience we share will be helpful to surgical staff at other institutions grappling with the challenges of operative care in the pandemic environment. While this protocol focuses on the current COVID-19 pandemic, these recommendations serve as a template for surgical preparedness that can be readily adapted to the next infectious disease crisis that will inevitably emerge.
The worldwide spread of SARS-CoV-2, the coronavirus that causes the syndrome designated COVID-19 by the World Health Organization (WHO), presents a challenge for emergency operative care in a global pandemic setting that is novel for modern surgical practice. The virulence of this new pathogen has raised concern for how to protect operating room (OR) staff and their environs in the event that an infected patient requires urgent surgical care. Because coronaviridae spread mainly through contact with contaminated respiratory droplets or aerosolized virion-containing particles, personal protective equipment (PPE) is vital to personnel involved in these cases, and proper utilization of these scarce resources poses an additional challenge. Establishment of a clear protocol that adheres to rigorous infection control measures while providing a safe system for intrafacility transport and operative care is an essential component of a successful surgical pandemic response.
The first case of COVID-19 disease identified in the US was diagnosed in Everett, Washington, on January 21, 2020.1 In the succeeding months, the Seattle region became an early epicenter of the epidemic in the US, with Washington State becoming the first state to see in excess of 1,000 cases by mid-March 2020. As hospitalizations for COVID-19 increased, emergency surge preparations were enacted at medical centers across the region. Recommendations for how to manage infected patients evolved rapidly. Anticipating the need to provide surgical services during this pandemic, starting in early March 2020, the perioperative services staff at the US Department of Veterans Affairs (VA) Puget Sound Health Care System (PSHCS) convened to develop the protocol described here through a process of literature review, multidisciplinary discussion, and practical trial runs and drills. VAPSHCS is an urban academic medical center affiliated with the University of Washington, Seattle. The result of this collaboration is a detailed, step-by-step protocol that establishes the roles and responsibilities of the various personnel who intersect in the OR and recruits their teamwork to prevent environmental contamination and health care worker transmission of SARS-CoV-2.
The protocol is divided into discrete practice recommendations for the preoperative, intraoperative, and postoperative management of patients with confirmed or suspected COVID-19 infection, with a focus on maintaining Centers of Disease Control and Prevention-defined respiratory droplet and airborne precautions throughout the period of patient contact and mitigating infectious contamination of the operating suite.2 It is acknowledged that no written protocol can encompass all the possible considerations that attend the vast diversity of surgical scenarios which can transpire in the operative setting. Patient acuity must sometimes mandate modifications to even the most thoroughly laid plans; for instance, the exsanguinating patient requiring emergent surgery for hemorrhage control will undoubtedly require an urgent appraisal of the relative risks and benefits of certain elements of the practices here described. Nevertheless, we believe that this protocol provides a useful framework for mitigating the infection and contamination risks of operative care in an epidemic environment, and should be readily adaptable to any facility that may perform surgery in patients infected with a high-risk contagious pathogen.
Preoperative Management
In addition to introducing the risk of viral transmission, surgery in the patient with COVID-19 also imposes a large consumption of vital PPE, supplies and can become dangerously low in health care centers coping with an influx of infected patients. Early in the pandemic, to reduce exposure, conserve the medical workforce and lessen the resource strain on the overall health care infrastructure, the American College of Surgeons (ACS), American College of Gastroenterology, and other professional societies recommended cancellation of elective procedures, confining operations to urgent or emergent procedures for high-acuity diseases that would negatively impact morbidity or mortality if delayed.3,4 In each case, physicians from the surgical and anesthesia services should discuss the rationale for the operation and secure agreement to commit resources to the endeavor prior to reserving the OR. These considerations should be shared with the patient prior to obtaining informed consent.
Preoperatively, the surgical team, consisting of surgeon, anesthesiologist, OR nurse, surgical technician, and assistants to the surgeon, anesthesiologist and nurse, convene for a preoperative “team huddle.” While assistants will aid in patient transport and supplying equipment to the team during the procedure, they should not be in the OR during the case, to minimize personnel exposure and PPE consumption. All members of the surgical team remove their personal effects, including wallets, phones, badges, and jewelry; any pagers are handed to other members of the care team for the duration of the surgery. During this preoperative team huddle, proper setup and accounting of the surgical equipment is confirmed, as well as the availability of all necessary anesthesia equipment and medications.
A specific OR with versatile characteristics was chosen to be the designated OR for procedures in patients with confirmed or suspected COVID-19. The COVID OR is on standby when no such cases are active, and it is not used for surgeries in noninfected patients. This is in accord with published recommendations of anesthesiologists who, throughout the COVID-19 epidemic in China, maintained designated ORs and anesthesia machines for only infected patients.5 Strong consideration should be given to performing procedures for which endotracheal intubation is not required in the patient’s own respiratory isolation room, rather than the OR to avoid room contamination and excessive use of PPE.5,6
The availability of adequate PPE is confirmed during the preoperative team huddle. At a minimum, powered air purifying respirator devices (PAPRs) with hoods must be available for the anesthesia provider, surgeon and surgical technician, recognizing the Anesthesia Patient Safety Foundation (APSF) recommendation that these devices confer superior protection for those with the highest risk and most proximate exposure to the patient throughout the case.7,8 An N95 respirator, at minimum, must be available for the circulating OR nurse. Patient condition, need for critical care transport, anesthetic plan (monitored anesthesia care or general anesthesia), and availability of negative pressure isolation rooms in the ward vs in the operating suite should help decide patient transport strategies and help determine the most suitable location to secure the airway. In case of an inadvertent tube disconnection, transporting intubated patients carries the risk of disseminating virus laden aerosols into the environment. Risks of pre-OR intubation should be balanced with the potential benefit of securing the airway prior to transport and decreased gross OR contamination with intubation in the operating suite. Airway manipulation and intubation are among the highest risk procedures for nosocomial transmission and performance of these procedures should utilize precautions described in current APSF recommendations.3,9,10
For patients not requiring critical care transport, and when conditions favor intubation in the OR, patients should be transported in a gurney while wearing a surgical mask. Verification of the operative site, surgical plan, and other components of the WHO universal surgical safety checklist or time out are performed in the OR prior to induction of anesthesia, and a conscious patient can be an active participant.
If critical care transport is deemed necessary and/or a decision is made to intubate the patient outside the OR, preferably in a negative airflow respiratory isolation room, the perioperative team will confirm the availability of the following equipment needed for patient transport:
- Portable transport monitor;
- Video laryngoscope;
- Airway supplies and medications for induction of general anesthesia;
- Self-inflating bag-mask apparatus attached to an oxygen source;
- High-quality HMEF (heat and moisture exchanging filter) rated to remove at least 99.97% of airborne particles ≥ 0.3 microns to filter out viral particles attached to the expiratory outlet; and
- PPE including impermeable disposable gowns, gloves, and shoe covers.
While the surgical technician remains in the OR, the rest of the team will proceed to the patient’s location with these supplies, along with the necessary number of PAPRs and N95 respirators.
Outside the patient room, the team consisting of surgeon, anesthesia provider, OR nurse, and the assistant to each of these health care providers, gathers for the first time out, confirming the patient’s identification, intended procedure, surgical site, laterality, and informed consent. If the patient is verbal and has decision-making capacity, they confirm their identification, understanding of the planned procedure, and consent with the team over the phone from the confines of their room. If a patient lacks decision making capacity standard organization policies should be adhered to, most of which do not require direct patient contact and do not pose any unique infection control challenges. The anesthesia provider and surgeon don their PPE including PAPR devices with the aid of their assistants. Using a PPE checklist, the surgical team member dons with the assistance of a PPE partner, who is charged with reading the instructions on the checklist to the surgical team member step by step and inspecting the adequacy of the full PPE attire (Figure 1). A similar secondary check of appropriate PPE by an assistant during high risk encounters has also been advocated by other authors.6
Consideration should be given to intubating the patient prior to transport to the OR particularly if the patient originates in a respiratory isolation room with negative pressure airflow, being mindful that most operating suites are ventilated with positive airflow that could help disperse virus laden aerosols in the procedure area. It may also be beneficial to have a secure airway in a patient who is actively coughing, sneezing, and dispersing respiratory droplets to the surrounding environment prior to leaving respiratory isolation. When intubation prior to OR transport is chosen, the fully attired anesthesiologist enters the patient room first, with a video laryngoscope, medication, and other supplies needed to successfully induce general endotracheal tube anesthesia. The anesthesia and surgery assistants don droplet precaution PPE and remain outside the patient room. Whenever possible, a rapid sequence induction is performed with minimization of bag-mask ventilation. Video laryngoscopy is preferred over direct laryngoscopy in patients with COVID-19 as it enables a greater distance between the health care provider and the airway.5,6 The surgeon and OR nurse then enter the room, wearing PPE including PAPR, and assist with attaching the transport monitor and moving the patient bed out of the room. The OR nurse wipes the front face shield and PAPR hood of the anesthesia provider after intubation, to clean these presumably contaminated components prior to exiting the room. A second, clean disposable gown covers the one worn during intubation to minimize environmental contamination during transport.11,12
The patient is intubated, anesthetized, and, transported to the OR, with a self-inflating bag mask apparatus attached to an oxygen source and a second high-quality HMEF rated to remove at least 99.97% of airborne particles ≥ 0.3 microns is attached to the expiratory outlet, or a transport ventilator with HEPA filter attached to the expiratory limb. In the OR, the anesthesia provider, surgical technician, and OR nurse assist with moving the patient to the operating gurney and attaching the monitor. The surgeon remains outside the room in order to doff the gown and gloves worn during transport, disinfect their hands (preoperative scrubbing), and don sterile attire, all while continuing to wear the same PAPR and hood.
Intraoperative Management
Advance planning can help to ensure a safer intraoperative period when a COVID-19 patient is brought to the OR. Patient room airflow patterns and ventilation capacity should be considered when developing measures to prevent aerosol transmission of airborne infectious agents. Although negative pressure rooms are ideal for aerosol generating procedures such as intubation, most ORs are generally maintained at a positive pressure when compared with the surrounding areas. The feasibility of rapidly converting ORs into negative pressure rooms should be in facility planning for COVID-19; portable high-efficiency particulate air (HEPA) machines, for instance, can be set up to create negative pressure areas around the OR.13 We established a negative pressure anteroom outside our OR to be used for doffing and as an airlock, for use by staff who need to enter midcase or pass supplies or specimens into and out of the procedure room (Figure 2). By adding 2 portable HEPA filters and directing the HEPA-filtered exhaust into the OR ventilation return columns, we were able to establish negative pressure airflow in the OR (Figure 3).
The protocol was devised with the current pandemic-associated shortage of PPE taken into consideration. We decided to minimize staffing across disciplines by excluding all nonessential personal from entering the OR. This includes observers, researchers, and medical students. Residents and fellows may participate if their presence is deemed vital to the patient’s intraoperative care. To further prevent resource consumption, equipment in the designated COVID OR was reduced to essential elements such as the anesthesia machine, a minimized anesthesia drug cart and general supply cabinet, all of which were covered with disposable transparent covers (Figure 4).14 After transfer of the patient to the OR table, the patient stretcher is kept in the OR (space permitting) to minimize contamination of areas immediately outside the OR.
Prior to incision a second time out is performed to confirm the previously verified operative site and plan. During the case, the assistants to the OR nurse and anesthesia provider act as facilitators or “runners” for equipment retrieval and communication with the outside OR staff. These roles are assigned to personnel who are familiar with the layout and day-to-day functioning of the ORs, such as anesthesia technicians and OR circulating nurses. All staff agreed on a strategy of no breaks or alternations whenever possible to conserve PPEs.15 Near the conclusion of the surgical procedure, the receiving intensive care unit (ICU) is given a verbal report on patient status over the phone.
Postperative Management
Similar to intubation, extubation poses a risk of generating aerosolization of infectious airborne microbes.10 It is helpful for OR personnel to be aware of the airflow pattern in their ORs, whether it is positive, negative, or neutral. As the PSHCS ORs were originally engineered as positive pressure rooms, we elected to have to postoperative patients with COVID-19 transported intubated to a reverse airflow or negative pressure room in the ICU. Extubation is performed when the intensive care team has determined the patient meets extubation criteria and has passed a spontaneous breathing trial. When a negative pressure room in the ICU is not available for recovery, extubation may be performed in the OR.
In that circumstance, the patient remains in the OR for 30 minutes after extubation to allow for turnover of air in the room prior to the doors opening for patient transport to the ICU.16 A surgical mask is placed over the patient’s oxygenating face mask to reduce droplet spread during transport. Patients who are not intubated for the anesthetic may be first recovered in the operating room or transported under droplet precautions directly back to a negative pressure isolation room.
Prior to transport, the patient’s gurney is thoroughly cleaned with Environmental Protection Agency-approved disinfectant wipes, and a clean sheet is placed over the patient’s body below the head.17 The front face shield of the surgeon’s and anesthesiologist’s PAPR hood should be wiped down with an alcohol-based disinfectant. Both health care providers don a clean disposable gown as an outer layer to minimize contamination by their used attire during transport. Once the patient is transported out of the OR, all disposable items are discarded. Reusable medical equipment are cleaned and disinfected according to a thorough application of local environmental services standard operating procedures.18 The surgeon and anesthesia providers aid in transporting the patient to the ICU, along with their outside OR assistants. All personnel remaining in the OR exit and doff their PPE according to the doffing protocol, which is similar to the donning protocol, utilizes a PPE partner tasked with providing instructions to the surgical team member step by step (Figure 5).
After leaving the OR, terminal cleaning must be performed by environmental services (EVS) personnel, but they should delay entry into the room until a sufficient amount of time has elapsed after the last aerosol-generating procedure in the OR. Time determination will depend on the air change per hour (ACH) in the OR that will achieve 99.9% removal of airborne contaminates. For example, ventilation in our operating rooms operate at approximately 15 to 20 ACH, which should attain that level of air clearance in 21 to 28 minutes.16 Once the stipulated time has elapsed EVS personnel may enter the room but should wear a gown and gloves when performing terminal cleaning. A face mask and eye protection should be added if splashes or sprays during cleaning and disinfection activities are anticipated, or otherwise required based on the selected cleaning products. Anesthesia technicians can now also enter the room to disinfect the anesthesia machines and set up all disposable supplies for any potential following case.
Conclusions
The outbreak of COVID-19 has resulted in an unprecedented modern health care crisis across the globe. Perioperative management of patients with COVID-19 pose unique challenges to all personnel working in the OR, where the risk of nosocomial transmission of infection is ever present. It is essential that hospitals consider their local resources, infrastructure and capabilities when devising policies to respond to the COVID-19 emergency. In all perioperative situations, meticulous attention should be given to both donning and doffing of PPE, crucial for the safety of everyone involved in the care of patients with COVID-19.
Our experience also highlighted the importance of treating a new protocol as an evolving document, which can be modified and improved through the conduct of training and simulation exercises with providers across disciplines (Figure 6). In gathering nurses, anesthesia staff, and surgeons to perform drills and simulate their roles in an imaginary scenario, we gained new insights, and made corrections and additions that ultimately generated the presently described process. Modifications to any protocol may be necessary depending on the unique circumstances of individual health care systems and hospitals, the characteristics of the patient population they cater to, and the resources and expertise they have available. As the pandemic continues, we are bound to learn more about the epidemiology and modes of transmission of SARS-CoV-2, which may demand further changes to our practice. It is crucial to remember that while emergency policies must be rapidly developed, they should be collaboratively improved and incorporate new knowledge when it becomes available. This is essential to ensure the ultimate protocol is useful, up-to-date, easy to follow and tailored to the unique local environment of each health care setting.
After the initial apprehensions and struggles that attended our confrontation with the crisis, it is our hope that the experience we share will be helpful to surgical staff at other institutions grappling with the challenges of operative care in the pandemic environment. While this protocol focuses on the current COVID-19 pandemic, these recommendations serve as a template for surgical preparedness that can be readily adapted to the next infectious disease crisis that will inevitably emerge.
1. Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929-936.
2. Siegel S RE, Jackson M, Chiarello L. Healthcare Infection Control Practices Advisory Committee; Guideline for Isolation Precautions. Centers For Disease Control and Prevention. https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html. Published 2007. Accessed March 28, 2020.
3. American College of Surgeons: COVID-19: Guidance for Triage of Non-Emergent Surgical Procedures. American College of Surgeons. https://www.facs.org/covid-19/clinical-guidance/triage. Published March 17, 2020. Accessed April 19, 2020.
4. American College of Gastroenterology. Gastroenterology professional society Guidance on endoscopic procedures During the covid-19 pandemic. American College of Gastroenterology. https://webfiles.gi.org/links/media/Joint_GI_Society_Guidance_on_Endoscopic_Procedure_During_COVID19_FINAL_impending_3312020.pdf. Published March 31, 2020. Accessed April 19, 2020.
5. Chen X, Liu Y, Gong Y, et al. Perioperative management of patients infected with the novel coronavirus: recommendation from the Joint Task Force of the Chinese Society of Anesthesiology and the Chinese Association of Anesthesiologists [published online ahead of print, 2020 Mar 26]. Anesthesiology. 2020;10.1097/ALN.0000000000003301.
6. Zhang HF, Bo L, Lin Y, et al. Response of Chinese anesthesiologists to the COVID-19 outbreak [published online ahead of print, 2020 Mar 30]. Anesthesiology. 2020;10.1097/ALN.0000000000003300.
7. Kamming D, Gardam M, Chung F. Anaesthesia and SARS. Br J Anaesth. 2003;90(6):715-718.
8. Zucco L LN, Ketchandji D, Aziz M, Ramachandran SK. Perioperative considerations for the 2019 novel coronavirus (COVID-19). https://www.apsf.org/news-updatesperioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published Feb 12, 2020. Accessed March 30, 2020.
9. Caputo KM, Byrick R, Chapman MG, Orser BJ, Orser BA. Intubation of SARS patients: infection and perspectives of healthcare workers. Can J Anaesth. 2006;53(2):122-129.
10. Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 2019;11(10):940.
11. Peng PWH, Ho PL, Hota SS. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anaesth. 2020;124(5):497‐501.
12. Ti LK, Ang LS, Foong TW, Ng BSW. What we do when a COVID-19 patient needs an operation: operating room preparation and guidance [published online ahead of print, 2020 Mar 6]. Can J Anaesth. 2020;1‐3.
13. Chow TT, Kwan A, Lin Z, Bai W. Conversion of operating theatre from positive to negative pressure environment. J Hosp Infect. 2006;64(4):371-378.
14. Clark C, Taenzer A, Charette K, Whitty M. Decreasing contamination of the anesthesia environment. Am J Infect Control. 2014;42(11):1223-1225.
15. Dexter F, Parra MC, Brown JR, Loftus RW. Perioperative COVID-19 defense: an evidence-based approach for optimization of infection control and operating room management [published online ahead of print, 2020 Mar 26]. Anesth Analg. 2020;10.1213/ANE.0000000000004829.
16. Jensen PA, Lambert LA, Iademarco MF, Ridzon R, CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54(RR-17):1-141.
17. US Environmental Protection Agency. List N: disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Updated April 16, 2020. Accessed April 19, 2020.
18. Munoz-Price LS, Bowdle A, Johnston BL, et al. Infection prevention in the operating room anesthesia work area [published correction appears in Infect Control Hosp Epidemiol. 2019 Apr;40(4):500]. Infect Control Hosp Epidemiol. 2018;1‐17.
1. Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929-936.
2. Siegel S RE, Jackson M, Chiarello L. Healthcare Infection Control Practices Advisory Committee; Guideline for Isolation Precautions. Centers For Disease Control and Prevention. https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html. Published 2007. Accessed March 28, 2020.
3. American College of Surgeons: COVID-19: Guidance for Triage of Non-Emergent Surgical Procedures. American College of Surgeons. https://www.facs.org/covid-19/clinical-guidance/triage. Published March 17, 2020. Accessed April 19, 2020.
4. American College of Gastroenterology. Gastroenterology professional society Guidance on endoscopic procedures During the covid-19 pandemic. American College of Gastroenterology. https://webfiles.gi.org/links/media/Joint_GI_Society_Guidance_on_Endoscopic_Procedure_During_COVID19_FINAL_impending_3312020.pdf. Published March 31, 2020. Accessed April 19, 2020.
5. Chen X, Liu Y, Gong Y, et al. Perioperative management of patients infected with the novel coronavirus: recommendation from the Joint Task Force of the Chinese Society of Anesthesiology and the Chinese Association of Anesthesiologists [published online ahead of print, 2020 Mar 26]. Anesthesiology. 2020;10.1097/ALN.0000000000003301.
6. Zhang HF, Bo L, Lin Y, et al. Response of Chinese anesthesiologists to the COVID-19 outbreak [published online ahead of print, 2020 Mar 30]. Anesthesiology. 2020;10.1097/ALN.0000000000003300.
7. Kamming D, Gardam M, Chung F. Anaesthesia and SARS. Br J Anaesth. 2003;90(6):715-718.
8. Zucco L LN, Ketchandji D, Aziz M, Ramachandran SK. Perioperative considerations for the 2019 novel coronavirus (COVID-19). https://www.apsf.org/news-updatesperioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published Feb 12, 2020. Accessed March 30, 2020.
9. Caputo KM, Byrick R, Chapman MG, Orser BJ, Orser BA. Intubation of SARS patients: infection and perspectives of healthcare workers. Can J Anaesth. 2006;53(2):122-129.
10. Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 2019;11(10):940.
11. Peng PWH, Ho PL, Hota SS. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anaesth. 2020;124(5):497‐501.
12. Ti LK, Ang LS, Foong TW, Ng BSW. What we do when a COVID-19 patient needs an operation: operating room preparation and guidance [published online ahead of print, 2020 Mar 6]. Can J Anaesth. 2020;1‐3.
13. Chow TT, Kwan A, Lin Z, Bai W. Conversion of operating theatre from positive to negative pressure environment. J Hosp Infect. 2006;64(4):371-378.
14. Clark C, Taenzer A, Charette K, Whitty M. Decreasing contamination of the anesthesia environment. Am J Infect Control. 2014;42(11):1223-1225.
15. Dexter F, Parra MC, Brown JR, Loftus RW. Perioperative COVID-19 defense: an evidence-based approach for optimization of infection control and operating room management [published online ahead of print, 2020 Mar 26]. Anesth Analg. 2020;10.1213/ANE.0000000000004829.
16. Jensen PA, Lambert LA, Iademarco MF, Ridzon R, CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54(RR-17):1-141.
17. US Environmental Protection Agency. List N: disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Updated April 16, 2020. Accessed April 19, 2020.
18. Munoz-Price LS, Bowdle A, Johnston BL, et al. Infection prevention in the operating room anesthesia work area [published correction appears in Infect Control Hosp Epidemiol. 2019 Apr;40(4):500]. Infect Control Hosp Epidemiol. 2018;1‐17.