User login
VIDEO: Sexuality, fertility are focus of cancer education website
MIAMI BEACH – Often people living with cancer hesitate to ask their providers about sensitive and important issues surrounding sexuality and fertility. At the same time, some clinicians remain uncomfortable raising questions regarding sexual function or simply lack the time to appropriately address the issues during a patient encounter.
A new online resource aims to solve both problems simultaneously, giving both patients and providers the tools to meaningfully address sexuality and fertility issues, Leslie R. Schover, PhD, said in a video interview at the annual Miami Breast Cancer Conference, held by Physicians’ Education Resource.
The Will2love.com site features first-person patient account videos from women and men who faced similar concerns, said Dr. Schover, founder of the Will2Love digital health company based in Houston. In addition, vignettes with actors inform patients and also model how oncologists, oncology nurses, and other staff could effectively communicate with concerned patients. A professional portal offers online skills training for clinicians.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MIAMI BEACH – Often people living with cancer hesitate to ask their providers about sensitive and important issues surrounding sexuality and fertility. At the same time, some clinicians remain uncomfortable raising questions regarding sexual function or simply lack the time to appropriately address the issues during a patient encounter.
A new online resource aims to solve both problems simultaneously, giving both patients and providers the tools to meaningfully address sexuality and fertility issues, Leslie R. Schover, PhD, said in a video interview at the annual Miami Breast Cancer Conference, held by Physicians’ Education Resource.
The Will2love.com site features first-person patient account videos from women and men who faced similar concerns, said Dr. Schover, founder of the Will2Love digital health company based in Houston. In addition, vignettes with actors inform patients and also model how oncologists, oncology nurses, and other staff could effectively communicate with concerned patients. A professional portal offers online skills training for clinicians.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MIAMI BEACH – Often people living with cancer hesitate to ask their providers about sensitive and important issues surrounding sexuality and fertility. At the same time, some clinicians remain uncomfortable raising questions regarding sexual function or simply lack the time to appropriately address the issues during a patient encounter.
A new online resource aims to solve both problems simultaneously, giving both patients and providers the tools to meaningfully address sexuality and fertility issues, Leslie R. Schover, PhD, said in a video interview at the annual Miami Breast Cancer Conference, held by Physicians’ Education Resource.
The Will2love.com site features first-person patient account videos from women and men who faced similar concerns, said Dr. Schover, founder of the Will2Love digital health company based in Houston. In addition, vignettes with actors inform patients and also model how oncologists, oncology nurses, and other staff could effectively communicate with concerned patients. A professional portal offers online skills training for clinicians.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
David Hafler, MD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIDEO: Don’t overlook psychosocial concerns of vitiligo patients
ORLANDO – There are ways to assess the psychosocial needs of your patients with vitiligo, even if you don’t believe you have the necessary skills to do a complete mental health work-up, according to Seemal R. Desai, MD.
In an interview recorded at this year’s annual meeting of the American Academy of Dermatology, Dr. Desai, an assistant clinical professor of dermatology at the University of Texas, Dallas, shares his ideas for how to have casual conversations with patients that can help reveal important clues to psychosocial stress patients with this serious medical skin condition might be facing.
“There are subtle clues to look for to know that these patients are uncomfortable with others seeing their skin,” says Dr. Desai.
In the video interview, he also covers taking a multidisciplinary approach to caring for these patients, what treatments are available to those who are suffering psychosocial stress after having failed several interventions, and how patients from many Asian and African counties are especially at risk for ostracization.
“It’s important to let your patients know that you understand this is really affecting them psychosocially, and that you care,” says Dr. Desai.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
wmcknight@frontlinemedcom.com
On Twitter @whitneymcknight
ORLANDO – There are ways to assess the psychosocial needs of your patients with vitiligo, even if you don’t believe you have the necessary skills to do a complete mental health work-up, according to Seemal R. Desai, MD.
In an interview recorded at this year’s annual meeting of the American Academy of Dermatology, Dr. Desai, an assistant clinical professor of dermatology at the University of Texas, Dallas, shares his ideas for how to have casual conversations with patients that can help reveal important clues to psychosocial stress patients with this serious medical skin condition might be facing.
“There are subtle clues to look for to know that these patients are uncomfortable with others seeing their skin,” says Dr. Desai.
In the video interview, he also covers taking a multidisciplinary approach to caring for these patients, what treatments are available to those who are suffering psychosocial stress after having failed several interventions, and how patients from many Asian and African counties are especially at risk for ostracization.
“It’s important to let your patients know that you understand this is really affecting them psychosocially, and that you care,” says Dr. Desai.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
wmcknight@frontlinemedcom.com
On Twitter @whitneymcknight
ORLANDO – There are ways to assess the psychosocial needs of your patients with vitiligo, even if you don’t believe you have the necessary skills to do a complete mental health work-up, according to Seemal R. Desai, MD.
In an interview recorded at this year’s annual meeting of the American Academy of Dermatology, Dr. Desai, an assistant clinical professor of dermatology at the University of Texas, Dallas, shares his ideas for how to have casual conversations with patients that can help reveal important clues to psychosocial stress patients with this serious medical skin condition might be facing.
“There are subtle clues to look for to know that these patients are uncomfortable with others seeing their skin,” says Dr. Desai.
In the video interview, he also covers taking a multidisciplinary approach to caring for these patients, what treatments are available to those who are suffering psychosocial stress after having failed several interventions, and how patients from many Asian and African counties are especially at risk for ostracization.
“It’s important to let your patients know that you understand this is really affecting them psychosocially, and that you care,” says Dr. Desai.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
wmcknight@frontlinemedcom.com
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM AAD 17
VIDEO: Gold nanoparticles target laser acne therapy
ORLANDO – There may soon be a new gold standard in acne therapy – gold nanoparticles.
Evidence is mounting that the combination of a laser and a topical suspension of gold- or silver-coated silica nanoparticles can ablate the inner lining of the pilosebaceous unit, dramatically reducing or eliminating acne.
The system is still investigational in the United States, but shows great promise, Gilly Munavalli, MD, said in a video interview at the annual meeting of the American Academy of Dermatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Munavalli, medical director and founder of Dermatology, Laser, and Vein Specialists of the Carolinas, Charlotte, N.C., described how the system works, and some of the evidence supporting it.
“Basically what we’re doing is creating a chromophore for the laser to target,” he said. Just as a laser won’t work on light hair, it can’t target the colorless sebum inside the gland. The colored particles absorb the laser energy, vibrate, and create the locally destructive heat.
The particles are about 150 nm in diameter. They are designed to absorb infrared and near-infrared irradiation. A nanoparticle solution is applied to the affected area. Dr. Munavalli uses an ultrasound paddle to drive the particles into the sebaceous gland, and then removes any solution left on the skin.
He passes a hand-held, 800-nm laser over the skin. The laser activates the particles, generating heat that disrupts the lining of the pilosebaceous unit.
In 2014, Sebacia (Duluth, Ga.), which is developing the technology, reported positive results from two small European trials enrolling a total of 97 patients with acne. The patients were randomized to the gold nanoparticles or to a control group of, in the first study, an over-the-counter face wash and later, the vehicle solution minus the gold nanoparticles.
At 12 weeks’ follow-up, there was a significant reduction in inflammatory lesions and in the Investigator’s Global Assessment score. Treatment was well tolerated and results were durable at 6 months. At that time, the inflammatory lesion count was still 60% below baseline. Side effects were transient erythema and mild edema.
Dr. Munavalli serves as a scientific advisor for Sebacia.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
ORLANDO – There may soon be a new gold standard in acne therapy – gold nanoparticles.
Evidence is mounting that the combination of a laser and a topical suspension of gold- or silver-coated silica nanoparticles can ablate the inner lining of the pilosebaceous unit, dramatically reducing or eliminating acne.
The system is still investigational in the United States, but shows great promise, Gilly Munavalli, MD, said in a video interview at the annual meeting of the American Academy of Dermatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Munavalli, medical director and founder of Dermatology, Laser, and Vein Specialists of the Carolinas, Charlotte, N.C., described how the system works, and some of the evidence supporting it.
“Basically what we’re doing is creating a chromophore for the laser to target,” he said. Just as a laser won’t work on light hair, it can’t target the colorless sebum inside the gland. The colored particles absorb the laser energy, vibrate, and create the locally destructive heat.
The particles are about 150 nm in diameter. They are designed to absorb infrared and near-infrared irradiation. A nanoparticle solution is applied to the affected area. Dr. Munavalli uses an ultrasound paddle to drive the particles into the sebaceous gland, and then removes any solution left on the skin.
He passes a hand-held, 800-nm laser over the skin. The laser activates the particles, generating heat that disrupts the lining of the pilosebaceous unit.
In 2014, Sebacia (Duluth, Ga.), which is developing the technology, reported positive results from two small European trials enrolling a total of 97 patients with acne. The patients were randomized to the gold nanoparticles or to a control group of, in the first study, an over-the-counter face wash and later, the vehicle solution minus the gold nanoparticles.
At 12 weeks’ follow-up, there was a significant reduction in inflammatory lesions and in the Investigator’s Global Assessment score. Treatment was well tolerated and results were durable at 6 months. At that time, the inflammatory lesion count was still 60% below baseline. Side effects were transient erythema and mild edema.
Dr. Munavalli serves as a scientific advisor for Sebacia.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
ORLANDO – There may soon be a new gold standard in acne therapy – gold nanoparticles.
Evidence is mounting that the combination of a laser and a topical suspension of gold- or silver-coated silica nanoparticles can ablate the inner lining of the pilosebaceous unit, dramatically reducing or eliminating acne.
The system is still investigational in the United States, but shows great promise, Gilly Munavalli, MD, said in a video interview at the annual meeting of the American Academy of Dermatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Munavalli, medical director and founder of Dermatology, Laser, and Vein Specialists of the Carolinas, Charlotte, N.C., described how the system works, and some of the evidence supporting it.
“Basically what we’re doing is creating a chromophore for the laser to target,” he said. Just as a laser won’t work on light hair, it can’t target the colorless sebum inside the gland. The colored particles absorb the laser energy, vibrate, and create the locally destructive heat.
The particles are about 150 nm in diameter. They are designed to absorb infrared and near-infrared irradiation. A nanoparticle solution is applied to the affected area. Dr. Munavalli uses an ultrasound paddle to drive the particles into the sebaceous gland, and then removes any solution left on the skin.
He passes a hand-held, 800-nm laser over the skin. The laser activates the particles, generating heat that disrupts the lining of the pilosebaceous unit.
In 2014, Sebacia (Duluth, Ga.), which is developing the technology, reported positive results from two small European trials enrolling a total of 97 patients with acne. The patients were randomized to the gold nanoparticles or to a control group of, in the first study, an over-the-counter face wash and later, the vehicle solution minus the gold nanoparticles.
At 12 weeks’ follow-up, there was a significant reduction in inflammatory lesions and in the Investigator’s Global Assessment score. Treatment was well tolerated and results were durable at 6 months. At that time, the inflammatory lesion count was still 60% below baseline. Side effects were transient erythema and mild edema.
Dr. Munavalli serves as a scientific advisor for Sebacia.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
AT AAD 17
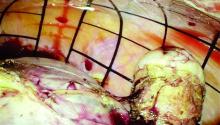
VIDEO: Tips for performing contained power morcellation
Experience with electromechanical power morcellation in a bag has advanced in the last several years in an effort to achieve safe tissue removal for minimally invasive procedures such as myomectomy, laparoscopic supracervical hysterectomy, or total hysterectomy of a large uterus.
Tissue extraction using contained power morcellation has become favored over contained morcellation using a scalpel – not only because the latter approach is cumbersome but because of the risk of bag puncture and subsequent organ injury. Surgeons have experimented with various sizes and types of retrieval bags and with various techniques for contained power morcellation.
The PneumoLiner carries the same restrictions as do other laparoscopic power morcellation systems – namely that it should not be used in surgery in which the tissue to be morcellated is known or suspected to contain malignancy, and that it should not be used in women who are peri- or postmenopausal. Moreover, to further enhance safety, physicians must have successfully completed the FDA-required validated training program run by Advanced Surgical Concepts and Olympus in order to use the device.
The FDA reviewed the PneumoLiner through a regulatory process known as the de novo classification process. This regulatory process is for first of its kind, low- to moderate-risk medical devices. The PneumoLiner was tested in laboratory conditions to ensure that it could withstand stress force in excess of the normal forces of surgery, and was found to be impervious to substances similar in molecular size to tissues, cells, and body fluids. There could be no cellular migration or leakage.
As surgeons were advancing the idea of inflated bag morcellation, one promising adaptation was to puncture the inflated bag to place accessory ports. However, recent research has shown that contained morcellation involving intentional bag puncture with a trocar may result in tissue or fluid leakage.
Spillage was noted in 7 of 76 cases (9.2%) in a multicenter prospective cohort of women who underwent hysterectomy or myomectomy using a contained power morcellation technique that involved perforation of the containment bag with a balloon-tipped lateral trocar. Investigators had injected blue dye into the bag prior to morcellation and examined the abdomen and pelvis after removing the bag for signs of spillage of dye, fluid, or tissue. In all cases, the containment bags were intact (Am J Obstet Gynecol. 2016 Feb;214[2]:257.e1-6).
The authors prematurely closed this study and recommended against this puncture technique. For complete containment, it appears to be important that we morcellate using a bag that has a single opening and is not punctured with accessory trocars.
The technique
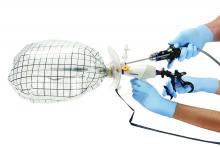
The PneumoLiner comes loaded in an insertion tube for placement. It has a plunger to deploy the device and a retrieval lanyard that closes the bag around the specimen, enabling retrieval of the neck of the bag outside the abdomen.
Included with the PneumoLiner is a multi-instrument port that can be used during the laparoscopic procedure and then converted to the active port for morcellation. The port has an opening for the laparoscope (either a 5-mm 30-degree straight or a 5-mm articulating laparoscope) and an opening for the morcellator, as well as two small openings for insufflation and for smoke exhaustion.
Surgery may be performed using this single-port or a multiport laparoscopic or robotic approach. For morcellation, the approach converts to a single-site technique that involves only one entry point for all instruments and no perforation of the bag.
At the beginning of the procedure (or at the end of the case if preferred), a 25-mm incision is made in the umbilicus and the system’s port is inserted and trimmed. The port cap is placed, the abdomen is insufflated, and the laparoscope is inserted. If placed at the beginning of the case, this port can be used as a camera or accessory port.
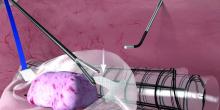
Before deployment of the PneumoLiner, the uterus or target tissue is placed out of the way; I recommend the upper right quadrant. The PneumoLiner is then inserted with its directional tab pointing upward, and the system’s plunger is depressed while the sleeve is pulled back. In essence, the PneumoLiner is advanced while the sleeve is simultaneously withdrawn, laying it flat in the pelvis.
With an atraumatic grasper, the uterus is placed within the opening of the bag, and the bag is grasped at the collar and elevated up and around the specimen. When full containment of the specimen is visualized, the retrieval lanyard is withdrawn until an opening ring partially protrudes outside the port. All lateral trocars must have been withdrawn prior to inflation of the bag to prevent it from being damaged.
At this point, the port cap is removed and the PneumoLiner neck is withdrawn until a black grid pattern on the bag is visible. The surgeon should then ensure there are no twists in the bag before replacing the port cap and insufflating the bag to a pressure of 15 mm Hg.*
The bag must be correctly in place and fully insufflated before the laparoscope is inserted. The laparoscope must be inserted prior to the morcellator. When the morcellator is inserted, care must be taken to ensure that the morcellator probe is in place.
Once the morcellator is placed, the probe is withdrawn and a closed tenaculum is placed. With the closed tenaculum, the surgeon can manipulate tissue and gauge depth and bearings without inadvertently grabbing the bag. The black grid pattern on the bag assists with estimation of tissue fragment size; morcellation proceeds under direct vision until the tissue fragments are smaller than four printed grids.
Instrumentation is removed in a set order, with the morcellator first and the laparoscope last. The port cap is detached and the PneumoLiner is removed while allowing fumes to escape. The morcellator, camera, tenaculum, and port cap are considered contaminated at this point and should not re-enter the field.
Pearls for morcellation
- The single-site nature of the procedure can sometimes be challenging. If you’ve placed your laparoscope and are having difficulty locating the morcellator, bring your laparoscope and morcellator shaft in parallel to each other, and you’ll be able to better orient yourself.
- To enlarge your field of view after you’ve inflated the PneumoLiner and captured the tissue within the bag, level the patient a bit and move the tissue further away from the laparoscope.
- If the morcellator tube is limiting visualization of the tenaculum tip, slide the morcellator back while leaving the tenaculum in a fixed position.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy Dr. Tony Shibley and Olympus
Dr. Shibley is an ob.gyn. in private practice in the Minneapolis area. He receives royalties from Advanced Surgical Concepts and serves as a consultant for Olympus.
*Correction 3/8/17: An earlier version of this article misstated the name of the Pneumoliner device in a photo caption. The pressure of the morcellation bag also was misstated.
Experience with electromechanical power morcellation in a bag has advanced in the last several years in an effort to achieve safe tissue removal for minimally invasive procedures such as myomectomy, laparoscopic supracervical hysterectomy, or total hysterectomy of a large uterus.
Tissue extraction using contained power morcellation has become favored over contained morcellation using a scalpel – not only because the latter approach is cumbersome but because of the risk of bag puncture and subsequent organ injury. Surgeons have experimented with various sizes and types of retrieval bags and with various techniques for contained power morcellation.
The PneumoLiner carries the same restrictions as do other laparoscopic power morcellation systems – namely that it should not be used in surgery in which the tissue to be morcellated is known or suspected to contain malignancy, and that it should not be used in women who are peri- or postmenopausal. Moreover, to further enhance safety, physicians must have successfully completed the FDA-required validated training program run by Advanced Surgical Concepts and Olympus in order to use the device.
The FDA reviewed the PneumoLiner through a regulatory process known as the de novo classification process. This regulatory process is for first of its kind, low- to moderate-risk medical devices. The PneumoLiner was tested in laboratory conditions to ensure that it could withstand stress force in excess of the normal forces of surgery, and was found to be impervious to substances similar in molecular size to tissues, cells, and body fluids. There could be no cellular migration or leakage.
As surgeons were advancing the idea of inflated bag morcellation, one promising adaptation was to puncture the inflated bag to place accessory ports. However, recent research has shown that contained morcellation involving intentional bag puncture with a trocar may result in tissue or fluid leakage.
Spillage was noted in 7 of 76 cases (9.2%) in a multicenter prospective cohort of women who underwent hysterectomy or myomectomy using a contained power morcellation technique that involved perforation of the containment bag with a balloon-tipped lateral trocar. Investigators had injected blue dye into the bag prior to morcellation and examined the abdomen and pelvis after removing the bag for signs of spillage of dye, fluid, or tissue. In all cases, the containment bags were intact (Am J Obstet Gynecol. 2016 Feb;214[2]:257.e1-6).
The authors prematurely closed this study and recommended against this puncture technique. For complete containment, it appears to be important that we morcellate using a bag that has a single opening and is not punctured with accessory trocars.
The technique
The PneumoLiner comes loaded in an insertion tube for placement. It has a plunger to deploy the device and a retrieval lanyard that closes the bag around the specimen, enabling retrieval of the neck of the bag outside the abdomen.
Included with the PneumoLiner is a multi-instrument port that can be used during the laparoscopic procedure and then converted to the active port for morcellation. The port has an opening for the laparoscope (either a 5-mm 30-degree straight or a 5-mm articulating laparoscope) and an opening for the morcellator, as well as two small openings for insufflation and for smoke exhaustion.
Surgery may be performed using this single-port or a multiport laparoscopic or robotic approach. For morcellation, the approach converts to a single-site technique that involves only one entry point for all instruments and no perforation of the bag.
At the beginning of the procedure (or at the end of the case if preferred), a 25-mm incision is made in the umbilicus and the system’s port is inserted and trimmed. The port cap is placed, the abdomen is insufflated, and the laparoscope is inserted. If placed at the beginning of the case, this port can be used as a camera or accessory port.
Before deployment of the PneumoLiner, the uterus or target tissue is placed out of the way; I recommend the upper right quadrant. The PneumoLiner is then inserted with its directional tab pointing upward, and the system’s plunger is depressed while the sleeve is pulled back. In essence, the PneumoLiner is advanced while the sleeve is simultaneously withdrawn, laying it flat in the pelvis.
With an atraumatic grasper, the uterus is placed within the opening of the bag, and the bag is grasped at the collar and elevated up and around the specimen. When full containment of the specimen is visualized, the retrieval lanyard is withdrawn until an opening ring partially protrudes outside the port. All lateral trocars must have been withdrawn prior to inflation of the bag to prevent it from being damaged.
At this point, the port cap is removed and the PneumoLiner neck is withdrawn until a black grid pattern on the bag is visible. The surgeon should then ensure there are no twists in the bag before replacing the port cap and insufflating the bag to a pressure of 15 mm Hg.*
The bag must be correctly in place and fully insufflated before the laparoscope is inserted. The laparoscope must be inserted prior to the morcellator. When the morcellator is inserted, care must be taken to ensure that the morcellator probe is in place.
Once the morcellator is placed, the probe is withdrawn and a closed tenaculum is placed. With the closed tenaculum, the surgeon can manipulate tissue and gauge depth and bearings without inadvertently grabbing the bag. The black grid pattern on the bag assists with estimation of tissue fragment size; morcellation proceeds under direct vision until the tissue fragments are smaller than four printed grids.
Instrumentation is removed in a set order, with the morcellator first and the laparoscope last. The port cap is detached and the PneumoLiner is removed while allowing fumes to escape. The morcellator, camera, tenaculum, and port cap are considered contaminated at this point and should not re-enter the field.
Pearls for morcellation
- The single-site nature of the procedure can sometimes be challenging. If you’ve placed your laparoscope and are having difficulty locating the morcellator, bring your laparoscope and morcellator shaft in parallel to each other, and you’ll be able to better orient yourself.
- To enlarge your field of view after you’ve inflated the PneumoLiner and captured the tissue within the bag, level the patient a bit and move the tissue further away from the laparoscope.
- If the morcellator tube is limiting visualization of the tenaculum tip, slide the morcellator back while leaving the tenaculum in a fixed position.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy Dr. Tony Shibley and Olympus
Dr. Shibley is an ob.gyn. in private practice in the Minneapolis area. He receives royalties from Advanced Surgical Concepts and serves as a consultant for Olympus.
*Correction 3/8/17: An earlier version of this article misstated the name of the Pneumoliner device in a photo caption. The pressure of the morcellation bag also was misstated.
Experience with electromechanical power morcellation in a bag has advanced in the last several years in an effort to achieve safe tissue removal for minimally invasive procedures such as myomectomy, laparoscopic supracervical hysterectomy, or total hysterectomy of a large uterus.
Tissue extraction using contained power morcellation has become favored over contained morcellation using a scalpel – not only because the latter approach is cumbersome but because of the risk of bag puncture and subsequent organ injury. Surgeons have experimented with various sizes and types of retrieval bags and with various techniques for contained power morcellation.
The PneumoLiner carries the same restrictions as do other laparoscopic power morcellation systems – namely that it should not be used in surgery in which the tissue to be morcellated is known or suspected to contain malignancy, and that it should not be used in women who are peri- or postmenopausal. Moreover, to further enhance safety, physicians must have successfully completed the FDA-required validated training program run by Advanced Surgical Concepts and Olympus in order to use the device.
The FDA reviewed the PneumoLiner through a regulatory process known as the de novo classification process. This regulatory process is for first of its kind, low- to moderate-risk medical devices. The PneumoLiner was tested in laboratory conditions to ensure that it could withstand stress force in excess of the normal forces of surgery, and was found to be impervious to substances similar in molecular size to tissues, cells, and body fluids. There could be no cellular migration or leakage.
As surgeons were advancing the idea of inflated bag morcellation, one promising adaptation was to puncture the inflated bag to place accessory ports. However, recent research has shown that contained morcellation involving intentional bag puncture with a trocar may result in tissue or fluid leakage.
Spillage was noted in 7 of 76 cases (9.2%) in a multicenter prospective cohort of women who underwent hysterectomy or myomectomy using a contained power morcellation technique that involved perforation of the containment bag with a balloon-tipped lateral trocar. Investigators had injected blue dye into the bag prior to morcellation and examined the abdomen and pelvis after removing the bag for signs of spillage of dye, fluid, or tissue. In all cases, the containment bags were intact (Am J Obstet Gynecol. 2016 Feb;214[2]:257.e1-6).
The authors prematurely closed this study and recommended against this puncture technique. For complete containment, it appears to be important that we morcellate using a bag that has a single opening and is not punctured with accessory trocars.
The technique
The PneumoLiner comes loaded in an insertion tube for placement. It has a plunger to deploy the device and a retrieval lanyard that closes the bag around the specimen, enabling retrieval of the neck of the bag outside the abdomen.
Included with the PneumoLiner is a multi-instrument port that can be used during the laparoscopic procedure and then converted to the active port for morcellation. The port has an opening for the laparoscope (either a 5-mm 30-degree straight or a 5-mm articulating laparoscope) and an opening for the morcellator, as well as two small openings for insufflation and for smoke exhaustion.
Surgery may be performed using this single-port or a multiport laparoscopic or robotic approach. For morcellation, the approach converts to a single-site technique that involves only one entry point for all instruments and no perforation of the bag.
At the beginning of the procedure (or at the end of the case if preferred), a 25-mm incision is made in the umbilicus and the system’s port is inserted and trimmed. The port cap is placed, the abdomen is insufflated, and the laparoscope is inserted. If placed at the beginning of the case, this port can be used as a camera or accessory port.
Before deployment of the PneumoLiner, the uterus or target tissue is placed out of the way; I recommend the upper right quadrant. The PneumoLiner is then inserted with its directional tab pointing upward, and the system’s plunger is depressed while the sleeve is pulled back. In essence, the PneumoLiner is advanced while the sleeve is simultaneously withdrawn, laying it flat in the pelvis.
With an atraumatic grasper, the uterus is placed within the opening of the bag, and the bag is grasped at the collar and elevated up and around the specimen. When full containment of the specimen is visualized, the retrieval lanyard is withdrawn until an opening ring partially protrudes outside the port. All lateral trocars must have been withdrawn prior to inflation of the bag to prevent it from being damaged.
At this point, the port cap is removed and the PneumoLiner neck is withdrawn until a black grid pattern on the bag is visible. The surgeon should then ensure there are no twists in the bag before replacing the port cap and insufflating the bag to a pressure of 15 mm Hg.*
The bag must be correctly in place and fully insufflated before the laparoscope is inserted. The laparoscope must be inserted prior to the morcellator. When the morcellator is inserted, care must be taken to ensure that the morcellator probe is in place.
Once the morcellator is placed, the probe is withdrawn and a closed tenaculum is placed. With the closed tenaculum, the surgeon can manipulate tissue and gauge depth and bearings without inadvertently grabbing the bag. The black grid pattern on the bag assists with estimation of tissue fragment size; morcellation proceeds under direct vision until the tissue fragments are smaller than four printed grids.
Instrumentation is removed in a set order, with the morcellator first and the laparoscope last. The port cap is detached and the PneumoLiner is removed while allowing fumes to escape. The morcellator, camera, tenaculum, and port cap are considered contaminated at this point and should not re-enter the field.
Pearls for morcellation
- The single-site nature of the procedure can sometimes be challenging. If you’ve placed your laparoscope and are having difficulty locating the morcellator, bring your laparoscope and morcellator shaft in parallel to each other, and you’ll be able to better orient yourself.
- To enlarge your field of view after you’ve inflated the PneumoLiner and captured the tissue within the bag, level the patient a bit and move the tissue further away from the laparoscope.
- If the morcellator tube is limiting visualization of the tenaculum tip, slide the morcellator back while leaving the tenaculum in a fixed position.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy Dr. Tony Shibley and Olympus
Dr. Shibley is an ob.gyn. in private practice in the Minneapolis area. He receives royalties from Advanced Surgical Concepts and serves as a consultant for Olympus.
*Correction 3/8/17: An earlier version of this article misstated the name of the Pneumoliner device in a photo caption. The pressure of the morcellation bag also was misstated.
VIDEO: Point-of-care assay caught acetaminophen toxicity
A rapid point-of-care assay for acetaminophen-related liver toxicity had a sensitivity of 100% and a specificity of 86%, compared with etiologic diagnosis, based on the results of a multicenter study published in the April issue of Clinical Gastroenterology and Hepatology.
The test might help guide treatment decisions for these patients in the emergency department and intensive care unit, said Dean W. Roberts, PhD, of the University of Arkansas, Little Rock, and his associates.
About 45% of acute liver failure cases in the United States stem from acetaminophen toxicity, but the diagnosis can be hard to confirm because the drug has a short half-life and patients often cannot or will not report an overdose, which also may consist of multiple exposures, limiting the interpretability of the Rumack nonogram. High-pressure liquid chromatography with electrochemical detection (HPLC-EC) accurately detects acetaminophen-protein adducts (3-[cysteine-S-yl] acetaminophen) released by lysed hepatocytes into the peripheral circulation, but this test requires specialized equipment and skilled personnel, the researchers noted (Clin Gastroenterol Hepatol. 2016 Sep 15. doi: 10.1016/j.cgh.2016.09.007).
The point-of-care assay was positive in all 33 patients diagnosed with acetaminophen toxicity, for a test sensitivity of 100%, the researchers reported. The median band amplitude for cases was 584 (range, 222-1,027), significantly lower than that for patients with nonacetaminophen acute liver failure (3,678; range, 394-8,289; P less than .001) or for controls (8,971; range, 5,151-11,108; P less than .001). Band amplitude correlated inversely with adduct levels because AcetaSTAT is a competitive immunoassay – the presence of adducts decreases reactions at the test band, the investigators reported.
AcetaSTAT results were negative for 25 of 29 patients who were initially diagnosed with nonacetaminophen liver failure, for a test specificity of 86%, a positive predictive value of 89%, and a negative predictive value of 100%. Among the remaining four “false positives,” three tested near or above the toxicity threshold on HPLC-EC and were considered positive after further review, the investigators said. The fourth false-positive case was HPLC-EC–negative autoimmune hepatitis.
AcetaSTAT might not catch cases very early after acetaminophen overdose or that have only mild toxicity, the researchers noted. Nonetheless, it can help guide treatment decisions “at the point of clinical care,” they said. “Because the survival rate of acetaminophen acute liver failure is more favorable than that of other causes of acute live failure, assay results could impact future physician referral patterns and reduce medical costs associated with additional tests to determine the etiology of liver injury.”
The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. Roberts and two coinvestigators are part owners of Acetaminophen Toxicity Diagnostics and have submitted a patent application for the AcetaSTAT serum assay used in this study. There were no other disclosures.
Source: American Gastroenterological Association
A rapid point-of-care assay for acetaminophen-related liver toxicity had a sensitivity of 100% and a specificity of 86%, compared with etiologic diagnosis, based on the results of a multicenter study published in the April issue of Clinical Gastroenterology and Hepatology.
The test might help guide treatment decisions for these patients in the emergency department and intensive care unit, said Dean W. Roberts, PhD, of the University of Arkansas, Little Rock, and his associates.
About 45% of acute liver failure cases in the United States stem from acetaminophen toxicity, but the diagnosis can be hard to confirm because the drug has a short half-life and patients often cannot or will not report an overdose, which also may consist of multiple exposures, limiting the interpretability of the Rumack nonogram. High-pressure liquid chromatography with electrochemical detection (HPLC-EC) accurately detects acetaminophen-protein adducts (3-[cysteine-S-yl] acetaminophen) released by lysed hepatocytes into the peripheral circulation, but this test requires specialized equipment and skilled personnel, the researchers noted (Clin Gastroenterol Hepatol. 2016 Sep 15. doi: 10.1016/j.cgh.2016.09.007).
The point-of-care assay was positive in all 33 patients diagnosed with acetaminophen toxicity, for a test sensitivity of 100%, the researchers reported. The median band amplitude for cases was 584 (range, 222-1,027), significantly lower than that for patients with nonacetaminophen acute liver failure (3,678; range, 394-8,289; P less than .001) or for controls (8,971; range, 5,151-11,108; P less than .001). Band amplitude correlated inversely with adduct levels because AcetaSTAT is a competitive immunoassay – the presence of adducts decreases reactions at the test band, the investigators reported.
AcetaSTAT results were negative for 25 of 29 patients who were initially diagnosed with nonacetaminophen liver failure, for a test specificity of 86%, a positive predictive value of 89%, and a negative predictive value of 100%. Among the remaining four “false positives,” three tested near or above the toxicity threshold on HPLC-EC and were considered positive after further review, the investigators said. The fourth false-positive case was HPLC-EC–negative autoimmune hepatitis.
AcetaSTAT might not catch cases very early after acetaminophen overdose or that have only mild toxicity, the researchers noted. Nonetheless, it can help guide treatment decisions “at the point of clinical care,” they said. “Because the survival rate of acetaminophen acute liver failure is more favorable than that of other causes of acute live failure, assay results could impact future physician referral patterns and reduce medical costs associated with additional tests to determine the etiology of liver injury.”
The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. Roberts and two coinvestigators are part owners of Acetaminophen Toxicity Diagnostics and have submitted a patent application for the AcetaSTAT serum assay used in this study. There were no other disclosures.
Source: American Gastroenterological Association
A rapid point-of-care assay for acetaminophen-related liver toxicity had a sensitivity of 100% and a specificity of 86%, compared with etiologic diagnosis, based on the results of a multicenter study published in the April issue of Clinical Gastroenterology and Hepatology.
The test might help guide treatment decisions for these patients in the emergency department and intensive care unit, said Dean W. Roberts, PhD, of the University of Arkansas, Little Rock, and his associates.
About 45% of acute liver failure cases in the United States stem from acetaminophen toxicity, but the diagnosis can be hard to confirm because the drug has a short half-life and patients often cannot or will not report an overdose, which also may consist of multiple exposures, limiting the interpretability of the Rumack nonogram. High-pressure liquid chromatography with electrochemical detection (HPLC-EC) accurately detects acetaminophen-protein adducts (3-[cysteine-S-yl] acetaminophen) released by lysed hepatocytes into the peripheral circulation, but this test requires specialized equipment and skilled personnel, the researchers noted (Clin Gastroenterol Hepatol. 2016 Sep 15. doi: 10.1016/j.cgh.2016.09.007).
The point-of-care assay was positive in all 33 patients diagnosed with acetaminophen toxicity, for a test sensitivity of 100%, the researchers reported. The median band amplitude for cases was 584 (range, 222-1,027), significantly lower than that for patients with nonacetaminophen acute liver failure (3,678; range, 394-8,289; P less than .001) or for controls (8,971; range, 5,151-11,108; P less than .001). Band amplitude correlated inversely with adduct levels because AcetaSTAT is a competitive immunoassay – the presence of adducts decreases reactions at the test band, the investigators reported.
AcetaSTAT results were negative for 25 of 29 patients who were initially diagnosed with nonacetaminophen liver failure, for a test specificity of 86%, a positive predictive value of 89%, and a negative predictive value of 100%. Among the remaining four “false positives,” three tested near or above the toxicity threshold on HPLC-EC and were considered positive after further review, the investigators said. The fourth false-positive case was HPLC-EC–negative autoimmune hepatitis.
AcetaSTAT might not catch cases very early after acetaminophen overdose or that have only mild toxicity, the researchers noted. Nonetheless, it can help guide treatment decisions “at the point of clinical care,” they said. “Because the survival rate of acetaminophen acute liver failure is more favorable than that of other causes of acute live failure, assay results could impact future physician referral patterns and reduce medical costs associated with additional tests to determine the etiology of liver injury.”
The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. Roberts and two coinvestigators are part owners of Acetaminophen Toxicity Diagnostics and have submitted a patent application for the AcetaSTAT serum assay used in this study. There were no other disclosures.
Source: American Gastroenterological Association
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point:
Major finding: Compared with etiologic diagnosis, its sensitivity was 100%, specificity was 86%, positive predictive value was 89%, and negative predictive value was 100%.
Data source: Competitive immunoassays of serum samples from 19 healthy controls, 29 patients with nonacetaminophen acute liver failure, and 33 patients with acetaminophen-induced acute liver failure.
Disclosures: The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. Roberts and two coinvestigators are part owners of Acetaminophen Toxicity Diagnostics and have submitted a patent application for the AcetaSTAT serum assay used in this study. There were no other disclosures.
Minimizing use of antipsychotics
Alessandro Biffi, MD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Rebecca Gottesman, MD, PhD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIDEO: Bacterial DNA predicted infections associated with prednisolone in severe alcoholic hepatitis
High baseline levels of circulating bacterial DNA increased the odds of serious infections by nearly fivefold in patients receiving prednisolone for severe alcoholic hepatitis, even after controlling for MELD score and white blood cell count, investigators reported in the April issue of Gastroenterology (2016 Dec 31. doi: 10.1053/j.gastro.2016.08.029).
“Patients with severe alcoholic hepatitis given prednisolone are at greater risk for developing serious infections and infections after treatment than patients not given prednisolone, which may offset its therapeutic benefit,” Nikhil Vergis, MD, and his associates wrote in Gastroenterology. “Level of circulating bacterial DNA before treatment could identify patients at high risk of infection if given prednisolone, which could be used to select therapies for patients with severe alcoholic hepatitis.”
To further explore rates and predictors of infections in STOPAH, the researchers analyzed longitudinal data on incident infections for 1,092 trial participants who received either prednisolone (40 mg daily) or pentoxifylline (400 mg three times daily). For 731 patients, they also examined whether baseline circulating levels of 16s ribosomal bacterial DNA were associated with infections.
A total of 135 patients (12%) had an infection at baseline, 251 (23%) developed infections during treatment, and 89 (8%) developed infections after treatment, the investigators reported. Prednisolone therapy was not associated with infections during treatment, but was associated with a nearly 30% rise in the odds of serious posttreatment infections compared with pentoxifylline (odds ratio, 1.27; 95% confidence interval, 1.27-2.92; P = .002). Prednisolone recipients who developed infections were significantly more likely to die within 90 days than those who did not, even after controlling for end-stage liver disease or Lille score (OR, 2.5; 95% CI, 1.4-4.3; P = .002). Antibiotic therapy appeared to significantly reduce the risk of mortality among infected prednisolone recipients (13% vs. 52%; OR, 0.13; 95% CI 0.04-0.47; P = .002).
There was “a striking association between bacterial DNA and the development of infection within 7 days in patients treated with prednisolone,” the researchers reported. These patients had a median baseline circulating DNA level of 20.9 pg/mL, while prednisolone recipients who did not develop infections had a median baseline bacterial DNA level of 8.3 pg/mL (P = .004). Bacterial DNA predicted infections with an area under receiver operating characteristic curve of 0.70 (95% CI, 0.58-0.83; P = .003), which substantially exceeded the curve for white blood cell count (0.58).
A cut-off value of 18.5 pg/mL was 80% specific for predicting infection within 7 days of prednisolone therapy, the investigators also reported. Bacterial DNA level did not, however, predict infections within 7 days of pentoxifylline therapy, and pentoxifylline was not linked with infections that were serious, infections during treatment, or infections after treatment. (P =.08).
Using bacterial DNA levels to guide prednisolone prescription also appeared to reduce 90-day mortality in this patient population, although the effect achieved borderline statistical significance, the researchers said. “Larger prospective randomized studies are needed to definitely report whether bacterial DNA-guided therapy can [have an] impact on mortality in severe alcoholic hepatitis, and perhaps in other acute inflammatory conditions” in which immunosuppression is required, they added.
The National Institute for Health Research and Wellcome Trust and Medical Research Council provided funding. Dr. Vergis and 10 coinvestigators disclosed no conflicts of interest. Senior author Dr. Mark Thursz and one coinvestigator disclosed ties to Gilead, Bristol-Myers Squibb, AbbVie, Abbott, and Norgine.
Source: American Gastroenterological Association
High baseline levels of circulating bacterial DNA increased the odds of serious infections by nearly fivefold in patients receiving prednisolone for severe alcoholic hepatitis, even after controlling for MELD score and white blood cell count, investigators reported in the April issue of Gastroenterology (2016 Dec 31. doi: 10.1053/j.gastro.2016.08.029).
“Patients with severe alcoholic hepatitis given prednisolone are at greater risk for developing serious infections and infections after treatment than patients not given prednisolone, which may offset its therapeutic benefit,” Nikhil Vergis, MD, and his associates wrote in Gastroenterology. “Level of circulating bacterial DNA before treatment could identify patients at high risk of infection if given prednisolone, which could be used to select therapies for patients with severe alcoholic hepatitis.”
To further explore rates and predictors of infections in STOPAH, the researchers analyzed longitudinal data on incident infections for 1,092 trial participants who received either prednisolone (40 mg daily) or pentoxifylline (400 mg three times daily). For 731 patients, they also examined whether baseline circulating levels of 16s ribosomal bacterial DNA were associated with infections.
A total of 135 patients (12%) had an infection at baseline, 251 (23%) developed infections during treatment, and 89 (8%) developed infections after treatment, the investigators reported. Prednisolone therapy was not associated with infections during treatment, but was associated with a nearly 30% rise in the odds of serious posttreatment infections compared with pentoxifylline (odds ratio, 1.27; 95% confidence interval, 1.27-2.92; P = .002). Prednisolone recipients who developed infections were significantly more likely to die within 90 days than those who did not, even after controlling for end-stage liver disease or Lille score (OR, 2.5; 95% CI, 1.4-4.3; P = .002). Antibiotic therapy appeared to significantly reduce the risk of mortality among infected prednisolone recipients (13% vs. 52%; OR, 0.13; 95% CI 0.04-0.47; P = .002).
There was “a striking association between bacterial DNA and the development of infection within 7 days in patients treated with prednisolone,” the researchers reported. These patients had a median baseline circulating DNA level of 20.9 pg/mL, while prednisolone recipients who did not develop infections had a median baseline bacterial DNA level of 8.3 pg/mL (P = .004). Bacterial DNA predicted infections with an area under receiver operating characteristic curve of 0.70 (95% CI, 0.58-0.83; P = .003), which substantially exceeded the curve for white blood cell count (0.58).
A cut-off value of 18.5 pg/mL was 80% specific for predicting infection within 7 days of prednisolone therapy, the investigators also reported. Bacterial DNA level did not, however, predict infections within 7 days of pentoxifylline therapy, and pentoxifylline was not linked with infections that were serious, infections during treatment, or infections after treatment. (P =.08).
Using bacterial DNA levels to guide prednisolone prescription also appeared to reduce 90-day mortality in this patient population, although the effect achieved borderline statistical significance, the researchers said. “Larger prospective randomized studies are needed to definitely report whether bacterial DNA-guided therapy can [have an] impact on mortality in severe alcoholic hepatitis, and perhaps in other acute inflammatory conditions” in which immunosuppression is required, they added.
The National Institute for Health Research and Wellcome Trust and Medical Research Council provided funding. Dr. Vergis and 10 coinvestigators disclosed no conflicts of interest. Senior author Dr. Mark Thursz and one coinvestigator disclosed ties to Gilead, Bristol-Myers Squibb, AbbVie, Abbott, and Norgine.
Source: American Gastroenterological Association
High baseline levels of circulating bacterial DNA increased the odds of serious infections by nearly fivefold in patients receiving prednisolone for severe alcoholic hepatitis, even after controlling for MELD score and white blood cell count, investigators reported in the April issue of Gastroenterology (2016 Dec 31. doi: 10.1053/j.gastro.2016.08.029).
“Patients with severe alcoholic hepatitis given prednisolone are at greater risk for developing serious infections and infections after treatment than patients not given prednisolone, which may offset its therapeutic benefit,” Nikhil Vergis, MD, and his associates wrote in Gastroenterology. “Level of circulating bacterial DNA before treatment could identify patients at high risk of infection if given prednisolone, which could be used to select therapies for patients with severe alcoholic hepatitis.”
To further explore rates and predictors of infections in STOPAH, the researchers analyzed longitudinal data on incident infections for 1,092 trial participants who received either prednisolone (40 mg daily) or pentoxifylline (400 mg three times daily). For 731 patients, they also examined whether baseline circulating levels of 16s ribosomal bacterial DNA were associated with infections.
A total of 135 patients (12%) had an infection at baseline, 251 (23%) developed infections during treatment, and 89 (8%) developed infections after treatment, the investigators reported. Prednisolone therapy was not associated with infections during treatment, but was associated with a nearly 30% rise in the odds of serious posttreatment infections compared with pentoxifylline (odds ratio, 1.27; 95% confidence interval, 1.27-2.92; P = .002). Prednisolone recipients who developed infections were significantly more likely to die within 90 days than those who did not, even after controlling for end-stage liver disease or Lille score (OR, 2.5; 95% CI, 1.4-4.3; P = .002). Antibiotic therapy appeared to significantly reduce the risk of mortality among infected prednisolone recipients (13% vs. 52%; OR, 0.13; 95% CI 0.04-0.47; P = .002).
There was “a striking association between bacterial DNA and the development of infection within 7 days in patients treated with prednisolone,” the researchers reported. These patients had a median baseline circulating DNA level of 20.9 pg/mL, while prednisolone recipients who did not develop infections had a median baseline bacterial DNA level of 8.3 pg/mL (P = .004). Bacterial DNA predicted infections with an area under receiver operating characteristic curve of 0.70 (95% CI, 0.58-0.83; P = .003), which substantially exceeded the curve for white blood cell count (0.58).
A cut-off value of 18.5 pg/mL was 80% specific for predicting infection within 7 days of prednisolone therapy, the investigators also reported. Bacterial DNA level did not, however, predict infections within 7 days of pentoxifylline therapy, and pentoxifylline was not linked with infections that were serious, infections during treatment, or infections after treatment. (P =.08).
Using bacterial DNA levels to guide prednisolone prescription also appeared to reduce 90-day mortality in this patient population, although the effect achieved borderline statistical significance, the researchers said. “Larger prospective randomized studies are needed to definitely report whether bacterial DNA-guided therapy can [have an] impact on mortality in severe alcoholic hepatitis, and perhaps in other acute inflammatory conditions” in which immunosuppression is required, they added.
The National Institute for Health Research and Wellcome Trust and Medical Research Council provided funding. Dr. Vergis and 10 coinvestigators disclosed no conflicts of interest. Senior author Dr. Mark Thursz and one coinvestigator disclosed ties to Gilead, Bristol-Myers Squibb, AbbVie, Abbott, and Norgine.
Source: American Gastroenterological Association
FROM GASTROENTEROLOGY
Key clinical point: High baseline levels of circulating bacterial DNA predicted serious infections in patients receiving prednisolone for severe alcoholic hepatitis.
Major finding: The odds of serious posttreatment infections were significantly higher for prednisolone compared with pentoxifylline (OR, 1.27; P = .002). High baseline levels of circulating bacterial DNA predicted infection within 7 days of prednisolone therapy (adjusted OR, 4.68; P = .001).
Data source: An analysis of 1,092 patients with severe alcoholic hepatitis from the randomized, double-blind STOPAH trial.
Disclosures: The National Institute for Health Research and Wellcome Trust and Medical Research Council provided funding. Dr. Vergis and 10 coinvestigators reported having no competing interests. Senior author Mark Thursz and one coinvestigator disclosed ties to Gilead, Bristol-Myers Squibb, AbbVie, Abbott, and Norgine.