User login
Hypertension, white matter hyperintensities, and dementia: What’s the link?
LOS ANGELES – While uncontrolled hypertension is an established risk factor for dementias including Alzheimer’s disease, the pathways by which it might lead to dementia remain poorly understood.
In research presented at the Alzheimer’s Association International Conference, Jérémie Lespinasse, PharmD, an investigator with the University of Bordeaux and INSERM U1219 in Bordeaux, France, presented data showing that hypertension-linked increases in the brain’s load of white matter hyperintensities – an imaging biomarker linked to small-vessel disease – was associated with cognitive decline independent of amyloid cascade biomarkers.
“We know that hypertension is related to cognitive decline and dementia, including Alzheimer’s dementia,” Carole Dufouil, PhD, the study’s last author and the research director at INSERM U1219, said in an interview. “But we didn’t know the pathway. It could go through what’s more typical of vascular pathology or it could go through what’s more typical of AD – and that’s what we wanted to test.”
The researchers found that the impact of hypertension on cognition doesn’t “go through amyloid,” Dr. Dufouil said, but rather a typically vascular pathway of white matter hyperintensities and neurodegeneration. “This is a big difference from other brain markers for which you know they exist, but you don’t know how to treat them,” she said. “This one is treatable.”
For their research, Dr. Lespinasse, Dr. Dufouil, and colleagues used a cross-sectional sample of data from the MEMENTO study, a 5-year observational cohort of 2,323 patients recruited at 26 memory centers in France between 2011 and 2014. Of the patients in MEMENTO, 62% were women, and the mean age was 71. All patients were deemed free of dementia and had isolated cognitive complaints or mild cognitive impairment at baseline. A total of 60% had hypertension, and 17% had uncontrolled hypertension defined as above 140/90 mm Hg despite treatment. Cognitive testing and MRI was conducted on all patients, while 60% also had 18F-fluorodeoxyglucose PET scanning and a minority, 18%, had cerebrospinal fluid samples.
The investigators found in using a structural equation model that the uncontrolled hypertension subjects had significantly lower cognition when compared against those without (P = .001). About half of the harmful effect of uncontrolled hypertension on brain functions was mediated by white matter hyperintensities load (P = .021) and neurodegeneration (P = .024) but not by cerebrospinal fluid biomarkers for amyloid-beta 42/40 ratio or tau.
The study’s main limitation was its use of cross-sectional data, the investigators said, while its strength was in a multifactorial model that allowed for a more integrative look at the relationships among hypertension, Alzheimer’s biomarkers, white matter hyperintensities, and cognition.
The investigators stressed the importance of controlling hypertension generally – and for all clinicians to be more aware of its cognitive impacts. Dr. Dufouil said that memory clinics should make blood pressure monitoring a key part of their workups, and should ensure that people are well controlled. “Up until recently it hasn’t been obvious” that control of hypertension has a key role in dementia prevention.
“It’s now obvious,” she said.
Dr. Lespinasse and Dr. Dufouil disclosed no industry relationships.
LOS ANGELES – While uncontrolled hypertension is an established risk factor for dementias including Alzheimer’s disease, the pathways by which it might lead to dementia remain poorly understood.
In research presented at the Alzheimer’s Association International Conference, Jérémie Lespinasse, PharmD, an investigator with the University of Bordeaux and INSERM U1219 in Bordeaux, France, presented data showing that hypertension-linked increases in the brain’s load of white matter hyperintensities – an imaging biomarker linked to small-vessel disease – was associated with cognitive decline independent of amyloid cascade biomarkers.
“We know that hypertension is related to cognitive decline and dementia, including Alzheimer’s dementia,” Carole Dufouil, PhD, the study’s last author and the research director at INSERM U1219, said in an interview. “But we didn’t know the pathway. It could go through what’s more typical of vascular pathology or it could go through what’s more typical of AD – and that’s what we wanted to test.”
The researchers found that the impact of hypertension on cognition doesn’t “go through amyloid,” Dr. Dufouil said, but rather a typically vascular pathway of white matter hyperintensities and neurodegeneration. “This is a big difference from other brain markers for which you know they exist, but you don’t know how to treat them,” she said. “This one is treatable.”
For their research, Dr. Lespinasse, Dr. Dufouil, and colleagues used a cross-sectional sample of data from the MEMENTO study, a 5-year observational cohort of 2,323 patients recruited at 26 memory centers in France between 2011 and 2014. Of the patients in MEMENTO, 62% were women, and the mean age was 71. All patients were deemed free of dementia and had isolated cognitive complaints or mild cognitive impairment at baseline. A total of 60% had hypertension, and 17% had uncontrolled hypertension defined as above 140/90 mm Hg despite treatment. Cognitive testing and MRI was conducted on all patients, while 60% also had 18F-fluorodeoxyglucose PET scanning and a minority, 18%, had cerebrospinal fluid samples.
The investigators found in using a structural equation model that the uncontrolled hypertension subjects had significantly lower cognition when compared against those without (P = .001). About half of the harmful effect of uncontrolled hypertension on brain functions was mediated by white matter hyperintensities load (P = .021) and neurodegeneration (P = .024) but not by cerebrospinal fluid biomarkers for amyloid-beta 42/40 ratio or tau.
The study’s main limitation was its use of cross-sectional data, the investigators said, while its strength was in a multifactorial model that allowed for a more integrative look at the relationships among hypertension, Alzheimer’s biomarkers, white matter hyperintensities, and cognition.
The investigators stressed the importance of controlling hypertension generally – and for all clinicians to be more aware of its cognitive impacts. Dr. Dufouil said that memory clinics should make blood pressure monitoring a key part of their workups, and should ensure that people are well controlled. “Up until recently it hasn’t been obvious” that control of hypertension has a key role in dementia prevention.
“It’s now obvious,” she said.
Dr. Lespinasse and Dr. Dufouil disclosed no industry relationships.
LOS ANGELES – While uncontrolled hypertension is an established risk factor for dementias including Alzheimer’s disease, the pathways by which it might lead to dementia remain poorly understood.
In research presented at the Alzheimer’s Association International Conference, Jérémie Lespinasse, PharmD, an investigator with the University of Bordeaux and INSERM U1219 in Bordeaux, France, presented data showing that hypertension-linked increases in the brain’s load of white matter hyperintensities – an imaging biomarker linked to small-vessel disease – was associated with cognitive decline independent of amyloid cascade biomarkers.
“We know that hypertension is related to cognitive decline and dementia, including Alzheimer’s dementia,” Carole Dufouil, PhD, the study’s last author and the research director at INSERM U1219, said in an interview. “But we didn’t know the pathway. It could go through what’s more typical of vascular pathology or it could go through what’s more typical of AD – and that’s what we wanted to test.”
The researchers found that the impact of hypertension on cognition doesn’t “go through amyloid,” Dr. Dufouil said, but rather a typically vascular pathway of white matter hyperintensities and neurodegeneration. “This is a big difference from other brain markers for which you know they exist, but you don’t know how to treat them,” she said. “This one is treatable.”
For their research, Dr. Lespinasse, Dr. Dufouil, and colleagues used a cross-sectional sample of data from the MEMENTO study, a 5-year observational cohort of 2,323 patients recruited at 26 memory centers in France between 2011 and 2014. Of the patients in MEMENTO, 62% were women, and the mean age was 71. All patients were deemed free of dementia and had isolated cognitive complaints or mild cognitive impairment at baseline. A total of 60% had hypertension, and 17% had uncontrolled hypertension defined as above 140/90 mm Hg despite treatment. Cognitive testing and MRI was conducted on all patients, while 60% also had 18F-fluorodeoxyglucose PET scanning and a minority, 18%, had cerebrospinal fluid samples.
The investigators found in using a structural equation model that the uncontrolled hypertension subjects had significantly lower cognition when compared against those without (P = .001). About half of the harmful effect of uncontrolled hypertension on brain functions was mediated by white matter hyperintensities load (P = .021) and neurodegeneration (P = .024) but not by cerebrospinal fluid biomarkers for amyloid-beta 42/40 ratio or tau.
The study’s main limitation was its use of cross-sectional data, the investigators said, while its strength was in a multifactorial model that allowed for a more integrative look at the relationships among hypertension, Alzheimer’s biomarkers, white matter hyperintensities, and cognition.
The investigators stressed the importance of controlling hypertension generally – and for all clinicians to be more aware of its cognitive impacts. Dr. Dufouil said that memory clinics should make blood pressure monitoring a key part of their workups, and should ensure that people are well controlled. “Up until recently it hasn’t been obvious” that control of hypertension has a key role in dementia prevention.
“It’s now obvious,” she said.
Dr. Lespinasse and Dr. Dufouil disclosed no industry relationships.
REPORTING FROM AAIC 2019
Alzheimer’s disease raises risk for recurrent seizures
LOS ANGELES – Seizures are not uncommon among patients with Alzheimer’s disease – particularly as patients live longer with the disease – and are often associated with worse cognitive and functional performance, according to research findings presented at the Alzheimer’s Association International Conference.
Jonathan Vöglein, MD, of the German Center for Neurodegenerative Diseases and Ludwig-Maximilian University in Munich presented results from a cohort of 9,127 patients with Alzheimer’s disease (AD), of whom 287 had experienced a seizure, and more than 10,000 non-AD control subjects recruited at clinics during 2005-2016.
Dr. Vöglein and colleagues found that seizure risk increased with duration of disease, from 1.5% of patients at 4.8 years with the disease to 5.4% at 11 years, with likelihood of a seizure increasing steadily over time.
Moreover, 70% of AD patients who experienced a seizure had a second one within 7.5 months. People who had seizures fared worse on cognitive and functional tests: a mean 16.6 on the Mini Mental State Examination, compared with 19.6 for patients without seizures. On a severity rating scale, the Clinical Dementia Rating Sum of Boxes, patients with seizures also fared worse, with scores of 9.3, compared with 6.8 for patients without seizures (P less than .0001 for all, with results adjusted for age and disease duration).
“The data of our study show that there’s an association of seizures with worse cognitive and functional performance,” Dr. Vöglein said in an interview.
“It’s important for clinicians to know that Alzheimer’s patients are at an increased risk for seizures,” Dr. Vöglein said. “In my clinical care experience, seizures are rarely the main complaint of patients with Alzheimer’s disease.” Detailed interviews with the patient and a proxy are important, he added, because patients with Alzheimer’s disease may not always remember events that could be a seizure.
Dr. Vöglein noted that, to his knowledge, there are no reliable data showing that treating seizures with antiepileptic drugs slows cognitive decline. “The results of our study suggest that an antiepileptic treatment after a first seizure in patients with Alzheimer’s dementia may be considered,” he said.
Also at the conference, researcher Ruby Castilla-Puentes, MD, DrPH, of Janssen Pharmaceuticals in Hopewell, N.J., along with Miguel Habeych, MD, MPH, of the University of Cincinnati presented findings on dementia and seizure risk from a large U.S. national managed care database of nearly 3 million people aged 60 years and older, of whom 56% were women.
The researchers analyzed this cohort during 2005-2014 and identified 80,000 people (2.8% of the cohort) as having any dementia diagnosis. The overall incidence of new-onset seizures in patients with dementia was 12.3% per year. In general, all subtypes of seizures and epileptic disorders (partial, generalized, or undifferentiated) occurred more frequently in patients with dementia, compared against patients without dementia (P less than .0001).
People with dementia had more than six times greater risk for experiencing recurring epileptic seizures than did people without dementia (95% confidence interval, 4.4-9.5). They were at six times higher risk for partial seizures (95% CI, 5.5-6.6); fivefold higher risk for generalized (95% CI, 4.9-5.5) and undifferentiated epilepsy (95% CI, 4.8-5.2); and 4.75 times higher risk for generalized seizures (95% CI, 4.5-5.0) and partial epilepsy (95% CI, 4.4-5.1).
“Although there are limitations with the use of administrative claims databases to calculate incidence rates, this analysis suggests that patients of 60 years of age or older have higher risks of new-onset seizures associated with a dementia diagnosis,” Dr. Castilla-Puentes commented.
The findings, she said, reinforce the need for clinicians to monitor for seizures to ensure that patients with dementia receive appropriate treatment.
Dr. Vöglein disclosed no financial conflicts of interest. Dr. Castilla-Puentes disclosed being an employee of Janssen, which funded her study.
LOS ANGELES – Seizures are not uncommon among patients with Alzheimer’s disease – particularly as patients live longer with the disease – and are often associated with worse cognitive and functional performance, according to research findings presented at the Alzheimer’s Association International Conference.
Jonathan Vöglein, MD, of the German Center for Neurodegenerative Diseases and Ludwig-Maximilian University in Munich presented results from a cohort of 9,127 patients with Alzheimer’s disease (AD), of whom 287 had experienced a seizure, and more than 10,000 non-AD control subjects recruited at clinics during 2005-2016.
Dr. Vöglein and colleagues found that seizure risk increased with duration of disease, from 1.5% of patients at 4.8 years with the disease to 5.4% at 11 years, with likelihood of a seizure increasing steadily over time.
Moreover, 70% of AD patients who experienced a seizure had a second one within 7.5 months. People who had seizures fared worse on cognitive and functional tests: a mean 16.6 on the Mini Mental State Examination, compared with 19.6 for patients without seizures. On a severity rating scale, the Clinical Dementia Rating Sum of Boxes, patients with seizures also fared worse, with scores of 9.3, compared with 6.8 for patients without seizures (P less than .0001 for all, with results adjusted for age and disease duration).
“The data of our study show that there’s an association of seizures with worse cognitive and functional performance,” Dr. Vöglein said in an interview.
“It’s important for clinicians to know that Alzheimer’s patients are at an increased risk for seizures,” Dr. Vöglein said. “In my clinical care experience, seizures are rarely the main complaint of patients with Alzheimer’s disease.” Detailed interviews with the patient and a proxy are important, he added, because patients with Alzheimer’s disease may not always remember events that could be a seizure.
Dr. Vöglein noted that, to his knowledge, there are no reliable data showing that treating seizures with antiepileptic drugs slows cognitive decline. “The results of our study suggest that an antiepileptic treatment after a first seizure in patients with Alzheimer’s dementia may be considered,” he said.
Also at the conference, researcher Ruby Castilla-Puentes, MD, DrPH, of Janssen Pharmaceuticals in Hopewell, N.J., along with Miguel Habeych, MD, MPH, of the University of Cincinnati presented findings on dementia and seizure risk from a large U.S. national managed care database of nearly 3 million people aged 60 years and older, of whom 56% were women.
The researchers analyzed this cohort during 2005-2014 and identified 80,000 people (2.8% of the cohort) as having any dementia diagnosis. The overall incidence of new-onset seizures in patients with dementia was 12.3% per year. In general, all subtypes of seizures and epileptic disorders (partial, generalized, or undifferentiated) occurred more frequently in patients with dementia, compared against patients without dementia (P less than .0001).
People with dementia had more than six times greater risk for experiencing recurring epileptic seizures than did people without dementia (95% confidence interval, 4.4-9.5). They were at six times higher risk for partial seizures (95% CI, 5.5-6.6); fivefold higher risk for generalized (95% CI, 4.9-5.5) and undifferentiated epilepsy (95% CI, 4.8-5.2); and 4.75 times higher risk for generalized seizures (95% CI, 4.5-5.0) and partial epilepsy (95% CI, 4.4-5.1).
“Although there are limitations with the use of administrative claims databases to calculate incidence rates, this analysis suggests that patients of 60 years of age or older have higher risks of new-onset seizures associated with a dementia diagnosis,” Dr. Castilla-Puentes commented.
The findings, she said, reinforce the need for clinicians to monitor for seizures to ensure that patients with dementia receive appropriate treatment.
Dr. Vöglein disclosed no financial conflicts of interest. Dr. Castilla-Puentes disclosed being an employee of Janssen, which funded her study.
LOS ANGELES – Seizures are not uncommon among patients with Alzheimer’s disease – particularly as patients live longer with the disease – and are often associated with worse cognitive and functional performance, according to research findings presented at the Alzheimer’s Association International Conference.
Jonathan Vöglein, MD, of the German Center for Neurodegenerative Diseases and Ludwig-Maximilian University in Munich presented results from a cohort of 9,127 patients with Alzheimer’s disease (AD), of whom 287 had experienced a seizure, and more than 10,000 non-AD control subjects recruited at clinics during 2005-2016.
Dr. Vöglein and colleagues found that seizure risk increased with duration of disease, from 1.5% of patients at 4.8 years with the disease to 5.4% at 11 years, with likelihood of a seizure increasing steadily over time.
Moreover, 70% of AD patients who experienced a seizure had a second one within 7.5 months. People who had seizures fared worse on cognitive and functional tests: a mean 16.6 on the Mini Mental State Examination, compared with 19.6 for patients without seizures. On a severity rating scale, the Clinical Dementia Rating Sum of Boxes, patients with seizures also fared worse, with scores of 9.3, compared with 6.8 for patients without seizures (P less than .0001 for all, with results adjusted for age and disease duration).
“The data of our study show that there’s an association of seizures with worse cognitive and functional performance,” Dr. Vöglein said in an interview.
“It’s important for clinicians to know that Alzheimer’s patients are at an increased risk for seizures,” Dr. Vöglein said. “In my clinical care experience, seizures are rarely the main complaint of patients with Alzheimer’s disease.” Detailed interviews with the patient and a proxy are important, he added, because patients with Alzheimer’s disease may not always remember events that could be a seizure.
Dr. Vöglein noted that, to his knowledge, there are no reliable data showing that treating seizures with antiepileptic drugs slows cognitive decline. “The results of our study suggest that an antiepileptic treatment after a first seizure in patients with Alzheimer’s dementia may be considered,” he said.
Also at the conference, researcher Ruby Castilla-Puentes, MD, DrPH, of Janssen Pharmaceuticals in Hopewell, N.J., along with Miguel Habeych, MD, MPH, of the University of Cincinnati presented findings on dementia and seizure risk from a large U.S. national managed care database of nearly 3 million people aged 60 years and older, of whom 56% were women.
The researchers analyzed this cohort during 2005-2014 and identified 80,000 people (2.8% of the cohort) as having any dementia diagnosis. The overall incidence of new-onset seizures in patients with dementia was 12.3% per year. In general, all subtypes of seizures and epileptic disorders (partial, generalized, or undifferentiated) occurred more frequently in patients with dementia, compared against patients without dementia (P less than .0001).
People with dementia had more than six times greater risk for experiencing recurring epileptic seizures than did people without dementia (95% confidence interval, 4.4-9.5). They were at six times higher risk for partial seizures (95% CI, 5.5-6.6); fivefold higher risk for generalized (95% CI, 4.9-5.5) and undifferentiated epilepsy (95% CI, 4.8-5.2); and 4.75 times higher risk for generalized seizures (95% CI, 4.5-5.0) and partial epilepsy (95% CI, 4.4-5.1).
“Although there are limitations with the use of administrative claims databases to calculate incidence rates, this analysis suggests that patients of 60 years of age or older have higher risks of new-onset seizures associated with a dementia diagnosis,” Dr. Castilla-Puentes commented.
The findings, she said, reinforce the need for clinicians to monitor for seizures to ensure that patients with dementia receive appropriate treatment.
Dr. Vöglein disclosed no financial conflicts of interest. Dr. Castilla-Puentes disclosed being an employee of Janssen, which funded her study.
REPORTING FROM AAIC 2019
Changes in sleep-wake timing accompany cerebral glucose hypometabolism and cognitive function
LOS ANGELES – Dysregulated sleep-wake cycles may be linked to cerebral glucose hypometabolism and subtle cognitive changes, both of which are early signs of Alzheimer’s disease–like neurodegeneration, according to a 2-year study of older Korean adults.
The association was particularly strong in subjects who experienced delayed acrophase, the peak of the normal sleep-wake cycle, So-Yeon Jeon, MD, said at the Alzheimer’s Association International Conference. It’s not yet clear whether the changes are a risk factor for dementia or a prodromal sign of neurodegeneration, but even without full elucidation, the findings could have value as a signal of impending neurodegeneration, said Dr. Jeon of Seoul (South Korea) National University.
“Our findings suggest that delayed acrophase may be used as a predictor for the progression of Alzheimer’s-type neurodegeneration and cognitive decline in the near future in old individuals with diverse cognitive status,” she said. “But the relationship between circadian phases and neurodegeneration is complex and not yet well understood.”
The 24-month study comprised 215 elderly adults enrolled in the Korean Brain Aging Study for the Early Diagnosis and Prediction of Alzheimer’s Disease (KBASE). They were a mean of 70 years old at baseline; 143 were cognitively normal, 40 had mild cognitive impairment, and 32 had Alzheimer’s dementia. Both at baseline and 2 years, everyone underwent a comprehensive neuropsychological assessment, amyloid PET brain imaging with Pittsburgh compound B, and an [18F]-fluorodeoxyglucose PET scan to determine brain glucose metabolic rate.
Before each assessment, the investigators measured sleep and circadian rhythms with 8 days of actigraphy. This assessed sleep variables (total sleep time, sleep latency, sleep efficiency, and wakefulness after sleep); rest-activity rhythm variables (midline estimated statistic of rhythm, amplitude, and acrophase), and some nonparametric values including interdaily stability, intradaily variability, and relative amplitude of sleep cycles. Subjects also completed sleep diaries during these periods.
The study’s main outcomes were 2-year changes in the Mini Mental State Exam (MMSE) score and in Alzheimer’s imaging biomarkers, including glucose metabolism and amyloid deposition. All analyses controlled for age, sex, Clinical Dementia Rating score, apolipoprotein E allele status, and baseline cognition.
At baseline, lower total sleep time was significantly associated with hypometabolism in areas associated with Alzheimer’s pathology as well as lower mean MMSE scores. Circadian variables showed no significant associations with these characteristics. However, the relative amplitude of circadian rhythm was significantly associated with hypometabolism and with lower MMSE score. There were no associations with brain amyloid load.
At 2 years, acrophase was associated with declines in cerebral glucose metabolism and further changes in the MMSE, even after the researchers controlled for the potential confounders. Delayed acrophase, although not associated with either metabolic rate or cognition at baseline, did significantly influence both at 2 years, suggesting a rapidly eroding clinical picture.
“Neurodegeneration over 2 years means the disease is progressing rapidly and subjects are likely to have tauopathies or other proteinopathy,” Dr. Jeon said. “These pathologies may either be resulting in delayed acrophase followed by neurodegeneration, or they may be prodromal symptoms of impending neurodegeneration. Whether they are early symptoms or early risk factors is not currently known, however. Two years is too short of a follow-up to determine these questions.”
Dr. Jeon had no financial declarations.
SOURCE: Jeon SY et al. AAIC 2019, abstract 33543.
LOS ANGELES – Dysregulated sleep-wake cycles may be linked to cerebral glucose hypometabolism and subtle cognitive changes, both of which are early signs of Alzheimer’s disease–like neurodegeneration, according to a 2-year study of older Korean adults.
The association was particularly strong in subjects who experienced delayed acrophase, the peak of the normal sleep-wake cycle, So-Yeon Jeon, MD, said at the Alzheimer’s Association International Conference. It’s not yet clear whether the changes are a risk factor for dementia or a prodromal sign of neurodegeneration, but even without full elucidation, the findings could have value as a signal of impending neurodegeneration, said Dr. Jeon of Seoul (South Korea) National University.
“Our findings suggest that delayed acrophase may be used as a predictor for the progression of Alzheimer’s-type neurodegeneration and cognitive decline in the near future in old individuals with diverse cognitive status,” she said. “But the relationship between circadian phases and neurodegeneration is complex and not yet well understood.”
The 24-month study comprised 215 elderly adults enrolled in the Korean Brain Aging Study for the Early Diagnosis and Prediction of Alzheimer’s Disease (KBASE). They were a mean of 70 years old at baseline; 143 were cognitively normal, 40 had mild cognitive impairment, and 32 had Alzheimer’s dementia. Both at baseline and 2 years, everyone underwent a comprehensive neuropsychological assessment, amyloid PET brain imaging with Pittsburgh compound B, and an [18F]-fluorodeoxyglucose PET scan to determine brain glucose metabolic rate.
Before each assessment, the investigators measured sleep and circadian rhythms with 8 days of actigraphy. This assessed sleep variables (total sleep time, sleep latency, sleep efficiency, and wakefulness after sleep); rest-activity rhythm variables (midline estimated statistic of rhythm, amplitude, and acrophase), and some nonparametric values including interdaily stability, intradaily variability, and relative amplitude of sleep cycles. Subjects also completed sleep diaries during these periods.
The study’s main outcomes were 2-year changes in the Mini Mental State Exam (MMSE) score and in Alzheimer’s imaging biomarkers, including glucose metabolism and amyloid deposition. All analyses controlled for age, sex, Clinical Dementia Rating score, apolipoprotein E allele status, and baseline cognition.
At baseline, lower total sleep time was significantly associated with hypometabolism in areas associated with Alzheimer’s pathology as well as lower mean MMSE scores. Circadian variables showed no significant associations with these characteristics. However, the relative amplitude of circadian rhythm was significantly associated with hypometabolism and with lower MMSE score. There were no associations with brain amyloid load.
At 2 years, acrophase was associated with declines in cerebral glucose metabolism and further changes in the MMSE, even after the researchers controlled for the potential confounders. Delayed acrophase, although not associated with either metabolic rate or cognition at baseline, did significantly influence both at 2 years, suggesting a rapidly eroding clinical picture.
“Neurodegeneration over 2 years means the disease is progressing rapidly and subjects are likely to have tauopathies or other proteinopathy,” Dr. Jeon said. “These pathologies may either be resulting in delayed acrophase followed by neurodegeneration, or they may be prodromal symptoms of impending neurodegeneration. Whether they are early symptoms or early risk factors is not currently known, however. Two years is too short of a follow-up to determine these questions.”
Dr. Jeon had no financial declarations.
SOURCE: Jeon SY et al. AAIC 2019, abstract 33543.
LOS ANGELES – Dysregulated sleep-wake cycles may be linked to cerebral glucose hypometabolism and subtle cognitive changes, both of which are early signs of Alzheimer’s disease–like neurodegeneration, according to a 2-year study of older Korean adults.
The association was particularly strong in subjects who experienced delayed acrophase, the peak of the normal sleep-wake cycle, So-Yeon Jeon, MD, said at the Alzheimer’s Association International Conference. It’s not yet clear whether the changes are a risk factor for dementia or a prodromal sign of neurodegeneration, but even without full elucidation, the findings could have value as a signal of impending neurodegeneration, said Dr. Jeon of Seoul (South Korea) National University.
“Our findings suggest that delayed acrophase may be used as a predictor for the progression of Alzheimer’s-type neurodegeneration and cognitive decline in the near future in old individuals with diverse cognitive status,” she said. “But the relationship between circadian phases and neurodegeneration is complex and not yet well understood.”
The 24-month study comprised 215 elderly adults enrolled in the Korean Brain Aging Study for the Early Diagnosis and Prediction of Alzheimer’s Disease (KBASE). They were a mean of 70 years old at baseline; 143 were cognitively normal, 40 had mild cognitive impairment, and 32 had Alzheimer’s dementia. Both at baseline and 2 years, everyone underwent a comprehensive neuropsychological assessment, amyloid PET brain imaging with Pittsburgh compound B, and an [18F]-fluorodeoxyglucose PET scan to determine brain glucose metabolic rate.
Before each assessment, the investigators measured sleep and circadian rhythms with 8 days of actigraphy. This assessed sleep variables (total sleep time, sleep latency, sleep efficiency, and wakefulness after sleep); rest-activity rhythm variables (midline estimated statistic of rhythm, amplitude, and acrophase), and some nonparametric values including interdaily stability, intradaily variability, and relative amplitude of sleep cycles. Subjects also completed sleep diaries during these periods.
The study’s main outcomes were 2-year changes in the Mini Mental State Exam (MMSE) score and in Alzheimer’s imaging biomarkers, including glucose metabolism and amyloid deposition. All analyses controlled for age, sex, Clinical Dementia Rating score, apolipoprotein E allele status, and baseline cognition.
At baseline, lower total sleep time was significantly associated with hypometabolism in areas associated with Alzheimer’s pathology as well as lower mean MMSE scores. Circadian variables showed no significant associations with these characteristics. However, the relative amplitude of circadian rhythm was significantly associated with hypometabolism and with lower MMSE score. There were no associations with brain amyloid load.
At 2 years, acrophase was associated with declines in cerebral glucose metabolism and further changes in the MMSE, even after the researchers controlled for the potential confounders. Delayed acrophase, although not associated with either metabolic rate or cognition at baseline, did significantly influence both at 2 years, suggesting a rapidly eroding clinical picture.
“Neurodegeneration over 2 years means the disease is progressing rapidly and subjects are likely to have tauopathies or other proteinopathy,” Dr. Jeon said. “These pathologies may either be resulting in delayed acrophase followed by neurodegeneration, or they may be prodromal symptoms of impending neurodegeneration. Whether they are early symptoms or early risk factors is not currently known, however. Two years is too short of a follow-up to determine these questions.”
Dr. Jeon had no financial declarations.
SOURCE: Jeon SY et al. AAIC 2019, abstract 33543.
REPORTING FROM AAIC 2019
Metformin linked to lower dementia risk in black patients
Black individuals who develop type 2 diabetes are more likely than their white counterparts to develop dementia. Now, findings from a new study point to a possible preventive strategy: Putting older patients on metformin when they are diagnosed could reduce their risk for dementia by as much as 40%, whereas sulfonylureas do not seem to have such an effect.
The researchers did not examine cause and effect, so their findings are not conclusive, and very few women were included in the study. Still, the authors said that their data showing a 29% lower risk of dementia associated with metformin use in black patients aged 65-74 years, and a 40% lower risk in those aged 50-64 years, suggested that “this inexpensive, widely available treatment could be broadly prescribed to substantially reduce the risk of dementia in younger [black] patients with [type 2 diabetes]” (Ann Fam Med. 2019;17:352-62).
Previous findings have suggested that black patients with type 2 diabetes face a 10%-18% higher risk of dementia, compared with white patients (Diabetes Care. 2014; 37[4]:1009-15). Another study linked type 2 diabetes in middle-aged black patients to a 41% decrease in cognition per test results over 14 years. There was no such decrease in white patients (Neuroepidemiology. 2014;43[3-4]: 220-7).
For the new study, researchers led by Jeffrey F. Scherrer, PhD, of Saint Louis University tracked 73,761 patients aged 50 years or older from 2000-2001 (when they were free of dementia and not taking diabetes) to 2015. Among the patients, 86% were white and 14% were black. In the white and black groups, 97% and 95% were men, respectively, and 61% and 55% were obese, respectively.
All participants began metformin (76%) or sulfonylurea (24%) monotherapy after the baseline period. Guidelines recommend metformin as a first-line treatment for type 2 diabetes, whereas sulfonylureas are considered second-line drugs that should be added to metformin.
After adjustment for confounders such as socioeconomic status and other medical conditions, the researchers found a significantly lower risk of dementia in black patients who took metformin, compared with those taking a sulfonylurea (hazard ratio, 0.73; 95% confidence interval, 0.6-0.89). There was no difference between the drugs among white patients (HR, 0.96; 95% CI, 0.9-1.03, both P = .008)
The results were not statistically significant among age groups, but there were trends. In black patients, the dementia-lowering benefit was largest among those aged 50-64 years (HR, 0.6; 95% CI, 0.45-0.81), followed by those aged 65-74 years (HR, 0.71; 95% CI, 0.53-0.94), and there was no benefit among those aged at least 75 (HR, 1.17; 95% CI, 0.73-1.85) all P = .055. There was a slight benefit among white patients in one of the age groups – 65-74 years (HR, 0.9; 95% CI, 0.82-0.99; P = .315).
The authors suggested that the findings could have been the result of an effect of metformin to reduce vascular disease and chronic inflammation in black patients.
They also noted that further research is needed to identify the demographic and clinical subgroups in which metformin is most strongly associated with a reduction in the risk of dementia. In addition, they emphasized that clinical trials are needed to confirm the study findings.
The National Institutes of Health funded the study. The authors report no relevant disclosures.
SOURCE: Scherrer JF et al. Ann Fam Med. 2019;17:352-62.
Black individuals who develop type 2 diabetes are more likely than their white counterparts to develop dementia. Now, findings from a new study point to a possible preventive strategy: Putting older patients on metformin when they are diagnosed could reduce their risk for dementia by as much as 40%, whereas sulfonylureas do not seem to have such an effect.
The researchers did not examine cause and effect, so their findings are not conclusive, and very few women were included in the study. Still, the authors said that their data showing a 29% lower risk of dementia associated with metformin use in black patients aged 65-74 years, and a 40% lower risk in those aged 50-64 years, suggested that “this inexpensive, widely available treatment could be broadly prescribed to substantially reduce the risk of dementia in younger [black] patients with [type 2 diabetes]” (Ann Fam Med. 2019;17:352-62).
Previous findings have suggested that black patients with type 2 diabetes face a 10%-18% higher risk of dementia, compared with white patients (Diabetes Care. 2014; 37[4]:1009-15). Another study linked type 2 diabetes in middle-aged black patients to a 41% decrease in cognition per test results over 14 years. There was no such decrease in white patients (Neuroepidemiology. 2014;43[3-4]: 220-7).
For the new study, researchers led by Jeffrey F. Scherrer, PhD, of Saint Louis University tracked 73,761 patients aged 50 years or older from 2000-2001 (when they were free of dementia and not taking diabetes) to 2015. Among the patients, 86% were white and 14% were black. In the white and black groups, 97% and 95% were men, respectively, and 61% and 55% were obese, respectively.
All participants began metformin (76%) or sulfonylurea (24%) monotherapy after the baseline period. Guidelines recommend metformin as a first-line treatment for type 2 diabetes, whereas sulfonylureas are considered second-line drugs that should be added to metformin.
After adjustment for confounders such as socioeconomic status and other medical conditions, the researchers found a significantly lower risk of dementia in black patients who took metformin, compared with those taking a sulfonylurea (hazard ratio, 0.73; 95% confidence interval, 0.6-0.89). There was no difference between the drugs among white patients (HR, 0.96; 95% CI, 0.9-1.03, both P = .008)
The results were not statistically significant among age groups, but there were trends. In black patients, the dementia-lowering benefit was largest among those aged 50-64 years (HR, 0.6; 95% CI, 0.45-0.81), followed by those aged 65-74 years (HR, 0.71; 95% CI, 0.53-0.94), and there was no benefit among those aged at least 75 (HR, 1.17; 95% CI, 0.73-1.85) all P = .055. There was a slight benefit among white patients in one of the age groups – 65-74 years (HR, 0.9; 95% CI, 0.82-0.99; P = .315).
The authors suggested that the findings could have been the result of an effect of metformin to reduce vascular disease and chronic inflammation in black patients.
They also noted that further research is needed to identify the demographic and clinical subgroups in which metformin is most strongly associated with a reduction in the risk of dementia. In addition, they emphasized that clinical trials are needed to confirm the study findings.
The National Institutes of Health funded the study. The authors report no relevant disclosures.
SOURCE: Scherrer JF et al. Ann Fam Med. 2019;17:352-62.
Black individuals who develop type 2 diabetes are more likely than their white counterparts to develop dementia. Now, findings from a new study point to a possible preventive strategy: Putting older patients on metformin when they are diagnosed could reduce their risk for dementia by as much as 40%, whereas sulfonylureas do not seem to have such an effect.
The researchers did not examine cause and effect, so their findings are not conclusive, and very few women were included in the study. Still, the authors said that their data showing a 29% lower risk of dementia associated with metformin use in black patients aged 65-74 years, and a 40% lower risk in those aged 50-64 years, suggested that “this inexpensive, widely available treatment could be broadly prescribed to substantially reduce the risk of dementia in younger [black] patients with [type 2 diabetes]” (Ann Fam Med. 2019;17:352-62).
Previous findings have suggested that black patients with type 2 diabetes face a 10%-18% higher risk of dementia, compared with white patients (Diabetes Care. 2014; 37[4]:1009-15). Another study linked type 2 diabetes in middle-aged black patients to a 41% decrease in cognition per test results over 14 years. There was no such decrease in white patients (Neuroepidemiology. 2014;43[3-4]: 220-7).
For the new study, researchers led by Jeffrey F. Scherrer, PhD, of Saint Louis University tracked 73,761 patients aged 50 years or older from 2000-2001 (when they were free of dementia and not taking diabetes) to 2015. Among the patients, 86% were white and 14% were black. In the white and black groups, 97% and 95% were men, respectively, and 61% and 55% were obese, respectively.
All participants began metformin (76%) or sulfonylurea (24%) monotherapy after the baseline period. Guidelines recommend metformin as a first-line treatment for type 2 diabetes, whereas sulfonylureas are considered second-line drugs that should be added to metformin.
After adjustment for confounders such as socioeconomic status and other medical conditions, the researchers found a significantly lower risk of dementia in black patients who took metformin, compared with those taking a sulfonylurea (hazard ratio, 0.73; 95% confidence interval, 0.6-0.89). There was no difference between the drugs among white patients (HR, 0.96; 95% CI, 0.9-1.03, both P = .008)
The results were not statistically significant among age groups, but there were trends. In black patients, the dementia-lowering benefit was largest among those aged 50-64 years (HR, 0.6; 95% CI, 0.45-0.81), followed by those aged 65-74 years (HR, 0.71; 95% CI, 0.53-0.94), and there was no benefit among those aged at least 75 (HR, 1.17; 95% CI, 0.73-1.85) all P = .055. There was a slight benefit among white patients in one of the age groups – 65-74 years (HR, 0.9; 95% CI, 0.82-0.99; P = .315).
The authors suggested that the findings could have been the result of an effect of metformin to reduce vascular disease and chronic inflammation in black patients.
They also noted that further research is needed to identify the demographic and clinical subgroups in which metformin is most strongly associated with a reduction in the risk of dementia. In addition, they emphasized that clinical trials are needed to confirm the study findings.
The National Institutes of Health funded the study. The authors report no relevant disclosures.
SOURCE: Scherrer JF et al. Ann Fam Med. 2019;17:352-62.
FROM ANNALS OF FAMILY MEDICINE
Key clinical point:
Major finding: Metformin monotherapy, compared with sulfonylurea monotherapy, was linked to a significantly lower risk for dementia in black patients (HR, 0.73; 95% CI, 0.6-0.89), but not in white patients (HR, 0.96; 95% CI, 0.9-1.03; P = .008).
Study details: Retrospective analysis of 73,761 patients aged 50 years or older in the Veterans Health Administration system who were tracked from 2000-2001 to 2015 and began metformin or sulfonylurea monotherapy after baseline.
Disclosures: The National Institutes of Health funded the study. The authors report no relevant disclosures.
Source: Scherrer JF et al. Ann Fam Med. 2019;17:352-62.
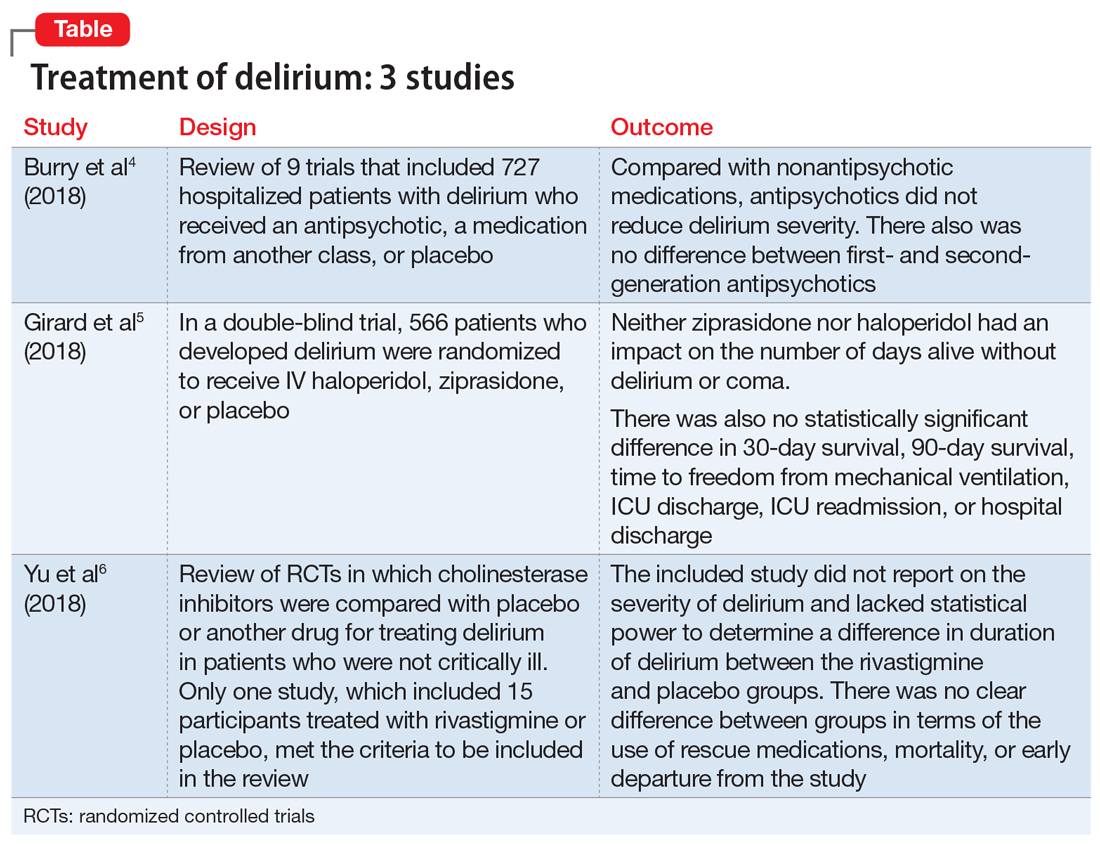
Treatment of delirium: A review of 3 studies
Delirium is defined as a disturbance in attention, awareness, and cognition that develops over hours to days as a direct physiological consequence of an underlying medical condition and is not better explained by another neurocognitive disorder.1 This condition is found in up to 31% of general medical patients and up to 87% of critically ill medical patients. Delirium is commonly seen in patients who have undergone surgery, those who are in palliative care, and patients with cancer.2 It is associated with increased morbidity and mortality. Compared with those who do not develop delirium, patients who are hospitalized who develop delirium have a higher risk of longer hospital stays, post-hospitalization nursing facility placement, persistent cognitive dysfunction, and death.3
Thus far, the management and treatment of delirium have been complicated by an incomplete understanding of the pathophysiology of this condition. However, prevailing theories suggest a dysregulation of neurotransmitter synthesis, function, or availability.2 Recent literature reflects this theory; researchers have investigated agents that target dopamine or acetylcholine. Below we review some of this recent literature on treating delirium; these studies are summarized in the Table.4-6
1. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594.
An extensive literature review identified randomized or quasi-randomized trials on the treatment of delirium among non-critically ill hospitalized patients in which antipsychotics were compared with nonantipsychotic medications or placebo, or in which a first-generation antipsychotic (FGA) was compared with a second-generation antipsychotic (SGA).4
Study design
- Researchers conducted a literature review of 9 trials that included 727 hospitalized but not critically ill patients (ie, they were not in an ICU) who developed delirium.
- Four trials compared an antipsychotic with a medication from another drug class or with placebo.
- Seven trials compared a FGA with an SGA.
Outcomes
- Although the intended primary outcome was the duration of delirium, none of the included studies reported on duration of delirium. Secondary outcomes were delirium severity and resolution, mortality, hospital length of stay, discharge disposition, health-related quality of life, and adverse effects.
- Among the secondary outcomes, no statistical difference was observed between delirium severity, delirium resolution, or mortality.
- None of the included studies reported on hospital length of stay, discharge disposition, or health-related quality of life.
- Evidence related to adverse effects was determined to be very low quality due to potential bias, inconsistency, and imprecision.
Conclusion
- A review of 9 randomized trials did not find any evidence supporting the use of antipsychotics for treating delirium. However, most of the studies included were of lower quality because they were single-center trials with insufficient sample sizes, heterogeneous study populations, and risk of bias.
Continue to: 2...
2. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
Study design
- Researchers used the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) to assess 1,183 patients with acute respiratory failure or shock in 16 medical centers in the United States.5
- Overall, 566 patients developed delirium and were randomized in a double-blind fashion to receive IV haloperidol, ziprasidone, or placebo.
- Haloperidol was started at 2.5 mg (age <70) or 1.25 mg (age ≥70) every 12 hours and titrated to a maximum dose of 20 mg/d as tolerated.
- Ziprasidone was started at 5 mg (age <70) or 2.5 mg (age ≥70) every 12 hours and titrated to a maximum dose of 40 mg/d as tolerated.
Outcomes
- The primary endpoint was days alive without delirium or coma. Secondary endpoints included duration of delirium, time to freedom from mechanical ventilation, time to final successful ICU discharge, time to ICU readmission, time to successful hospital discharge, 30-day survival, and 90-day survival.
- Neither ziprasidone nor haloperidol had an impact on number of days alive without delirium or coma.
- There was also no statistically significant difference in 30-day survival, 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Conclusion
- This study found no evidence supporting haloperidol or ziprasidone for the treatment of delirium. Because all patients in this study were critically ill, it is unclear if these results would be generalizable to other hospitalized patient populations.
3. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Study design
- A literature review identified published and unpublished randomized controlled trials in English and Chinese in which cholinesterase inhibitors were compared with placebo or another drug for treating delirium in non-critically ill patients.6
- Only one study met the criteria to be included in the review. It included 15 participants treated with rivastigmine or placebo.
Outcomes
- The intended primary outcomes were severity of delirium and duration of delirium. However, the included study did not report on the severity of delirium. It also lacked statistical power to determine a difference in duration of delirium between the rivastigmine and placebo groups.
- Secondary outcomes included use of a rescue medication, persistent cognitive impairment, length of hospitalization, institutionalization, mortality, cost of intervention, early departure from the study, and quality of life.
- There was no clear difference between the rivastigmine group and the placebo group in terms of the use of rescue medications, mortality, or early departure from the study. The included study did not report on persistent cognitive impairment, length of hospitalization, institutionalization, cost of intervention, or quality of life.
Conclusion
- This literature review did not find any evidence to support the use of cholinesterase inhibitors for treating delirium. However, because this review included only a single small study, limited conclusions can be drawn from this research.
In summary, delirium is common, especially among patients who are acutely medically ill, and it is associated with poor physical and cognitive clinical outcomes. Because of these poor outcomes, it is important to identify delirium early and intervene aggressively. Clearly, there is a need for further research into short- and long-term treatments for delirium.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
3. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466.
4. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594. doi: 10.1002/14651858.CD005594.pub3.
5. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
6. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Delirium is defined as a disturbance in attention, awareness, and cognition that develops over hours to days as a direct physiological consequence of an underlying medical condition and is not better explained by another neurocognitive disorder.1 This condition is found in up to 31% of general medical patients and up to 87% of critically ill medical patients. Delirium is commonly seen in patients who have undergone surgery, those who are in palliative care, and patients with cancer.2 It is associated with increased morbidity and mortality. Compared with those who do not develop delirium, patients who are hospitalized who develop delirium have a higher risk of longer hospital stays, post-hospitalization nursing facility placement, persistent cognitive dysfunction, and death.3
Thus far, the management and treatment of delirium have been complicated by an incomplete understanding of the pathophysiology of this condition. However, prevailing theories suggest a dysregulation of neurotransmitter synthesis, function, or availability.2 Recent literature reflects this theory; researchers have investigated agents that target dopamine or acetylcholine. Below we review some of this recent literature on treating delirium; these studies are summarized in the Table.4-6
1. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594.
An extensive literature review identified randomized or quasi-randomized trials on the treatment of delirium among non-critically ill hospitalized patients in which antipsychotics were compared with nonantipsychotic medications or placebo, or in which a first-generation antipsychotic (FGA) was compared with a second-generation antipsychotic (SGA).4
Study design
- Researchers conducted a literature review of 9 trials that included 727 hospitalized but not critically ill patients (ie, they were not in an ICU) who developed delirium.
- Four trials compared an antipsychotic with a medication from another drug class or with placebo.
- Seven trials compared a FGA with an SGA.
Outcomes
- Although the intended primary outcome was the duration of delirium, none of the included studies reported on duration of delirium. Secondary outcomes were delirium severity and resolution, mortality, hospital length of stay, discharge disposition, health-related quality of life, and adverse effects.
- Among the secondary outcomes, no statistical difference was observed between delirium severity, delirium resolution, or mortality.
- None of the included studies reported on hospital length of stay, discharge disposition, or health-related quality of life.
- Evidence related to adverse effects was determined to be very low quality due to potential bias, inconsistency, and imprecision.
Conclusion
- A review of 9 randomized trials did not find any evidence supporting the use of antipsychotics for treating delirium. However, most of the studies included were of lower quality because they were single-center trials with insufficient sample sizes, heterogeneous study populations, and risk of bias.
Continue to: 2...
2. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
Study design
- Researchers used the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) to assess 1,183 patients with acute respiratory failure or shock in 16 medical centers in the United States.5
- Overall, 566 patients developed delirium and were randomized in a double-blind fashion to receive IV haloperidol, ziprasidone, or placebo.
- Haloperidol was started at 2.5 mg (age <70) or 1.25 mg (age ≥70) every 12 hours and titrated to a maximum dose of 20 mg/d as tolerated.
- Ziprasidone was started at 5 mg (age <70) or 2.5 mg (age ≥70) every 12 hours and titrated to a maximum dose of 40 mg/d as tolerated.
Outcomes
- The primary endpoint was days alive without delirium or coma. Secondary endpoints included duration of delirium, time to freedom from mechanical ventilation, time to final successful ICU discharge, time to ICU readmission, time to successful hospital discharge, 30-day survival, and 90-day survival.
- Neither ziprasidone nor haloperidol had an impact on number of days alive without delirium or coma.
- There was also no statistically significant difference in 30-day survival, 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Conclusion
- This study found no evidence supporting haloperidol or ziprasidone for the treatment of delirium. Because all patients in this study were critically ill, it is unclear if these results would be generalizable to other hospitalized patient populations.
3. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Study design
- A literature review identified published and unpublished randomized controlled trials in English and Chinese in which cholinesterase inhibitors were compared with placebo or another drug for treating delirium in non-critically ill patients.6
- Only one study met the criteria to be included in the review. It included 15 participants treated with rivastigmine or placebo.
Outcomes
- The intended primary outcomes were severity of delirium and duration of delirium. However, the included study did not report on the severity of delirium. It also lacked statistical power to determine a difference in duration of delirium between the rivastigmine and placebo groups.
- Secondary outcomes included use of a rescue medication, persistent cognitive impairment, length of hospitalization, institutionalization, mortality, cost of intervention, early departure from the study, and quality of life.
- There was no clear difference between the rivastigmine group and the placebo group in terms of the use of rescue medications, mortality, or early departure from the study. The included study did not report on persistent cognitive impairment, length of hospitalization, institutionalization, cost of intervention, or quality of life.
Conclusion
- This literature review did not find any evidence to support the use of cholinesterase inhibitors for treating delirium. However, because this review included only a single small study, limited conclusions can be drawn from this research.
In summary, delirium is common, especially among patients who are acutely medically ill, and it is associated with poor physical and cognitive clinical outcomes. Because of these poor outcomes, it is important to identify delirium early and intervene aggressively. Clearly, there is a need for further research into short- and long-term treatments for delirium.
Delirium is defined as a disturbance in attention, awareness, and cognition that develops over hours to days as a direct physiological consequence of an underlying medical condition and is not better explained by another neurocognitive disorder.1 This condition is found in up to 31% of general medical patients and up to 87% of critically ill medical patients. Delirium is commonly seen in patients who have undergone surgery, those who are in palliative care, and patients with cancer.2 It is associated with increased morbidity and mortality. Compared with those who do not develop delirium, patients who are hospitalized who develop delirium have a higher risk of longer hospital stays, post-hospitalization nursing facility placement, persistent cognitive dysfunction, and death.3
Thus far, the management and treatment of delirium have been complicated by an incomplete understanding of the pathophysiology of this condition. However, prevailing theories suggest a dysregulation of neurotransmitter synthesis, function, or availability.2 Recent literature reflects this theory; researchers have investigated agents that target dopamine or acetylcholine. Below we review some of this recent literature on treating delirium; these studies are summarized in the Table.4-6
1. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594.
An extensive literature review identified randomized or quasi-randomized trials on the treatment of delirium among non-critically ill hospitalized patients in which antipsychotics were compared with nonantipsychotic medications or placebo, or in which a first-generation antipsychotic (FGA) was compared with a second-generation antipsychotic (SGA).4
Study design
- Researchers conducted a literature review of 9 trials that included 727 hospitalized but not critically ill patients (ie, they were not in an ICU) who developed delirium.
- Four trials compared an antipsychotic with a medication from another drug class or with placebo.
- Seven trials compared a FGA with an SGA.
Outcomes
- Although the intended primary outcome was the duration of delirium, none of the included studies reported on duration of delirium. Secondary outcomes were delirium severity and resolution, mortality, hospital length of stay, discharge disposition, health-related quality of life, and adverse effects.
- Among the secondary outcomes, no statistical difference was observed between delirium severity, delirium resolution, or mortality.
- None of the included studies reported on hospital length of stay, discharge disposition, or health-related quality of life.
- Evidence related to adverse effects was determined to be very low quality due to potential bias, inconsistency, and imprecision.
Conclusion
- A review of 9 randomized trials did not find any evidence supporting the use of antipsychotics for treating delirium. However, most of the studies included were of lower quality because they were single-center trials with insufficient sample sizes, heterogeneous study populations, and risk of bias.
Continue to: 2...
2. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
Study design
- Researchers used the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) to assess 1,183 patients with acute respiratory failure or shock in 16 medical centers in the United States.5
- Overall, 566 patients developed delirium and were randomized in a double-blind fashion to receive IV haloperidol, ziprasidone, or placebo.
- Haloperidol was started at 2.5 mg (age <70) or 1.25 mg (age ≥70) every 12 hours and titrated to a maximum dose of 20 mg/d as tolerated.
- Ziprasidone was started at 5 mg (age <70) or 2.5 mg (age ≥70) every 12 hours and titrated to a maximum dose of 40 mg/d as tolerated.
Outcomes
- The primary endpoint was days alive without delirium or coma. Secondary endpoints included duration of delirium, time to freedom from mechanical ventilation, time to final successful ICU discharge, time to ICU readmission, time to successful hospital discharge, 30-day survival, and 90-day survival.
- Neither ziprasidone nor haloperidol had an impact on number of days alive without delirium or coma.
- There was also no statistically significant difference in 30-day survival, 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Conclusion
- This study found no evidence supporting haloperidol or ziprasidone for the treatment of delirium. Because all patients in this study were critically ill, it is unclear if these results would be generalizable to other hospitalized patient populations.
3. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Study design
- A literature review identified published and unpublished randomized controlled trials in English and Chinese in which cholinesterase inhibitors were compared with placebo or another drug for treating delirium in non-critically ill patients.6
- Only one study met the criteria to be included in the review. It included 15 participants treated with rivastigmine or placebo.
Outcomes
- The intended primary outcomes were severity of delirium and duration of delirium. However, the included study did not report on the severity of delirium. It also lacked statistical power to determine a difference in duration of delirium between the rivastigmine and placebo groups.
- Secondary outcomes included use of a rescue medication, persistent cognitive impairment, length of hospitalization, institutionalization, mortality, cost of intervention, early departure from the study, and quality of life.
- There was no clear difference between the rivastigmine group and the placebo group in terms of the use of rescue medications, mortality, or early departure from the study. The included study did not report on persistent cognitive impairment, length of hospitalization, institutionalization, cost of intervention, or quality of life.
Conclusion
- This literature review did not find any evidence to support the use of cholinesterase inhibitors for treating delirium. However, because this review included only a single small study, limited conclusions can be drawn from this research.
In summary, delirium is common, especially among patients who are acutely medically ill, and it is associated with poor physical and cognitive clinical outcomes. Because of these poor outcomes, it is important to identify delirium early and intervene aggressively. Clearly, there is a need for further research into short- and long-term treatments for delirium.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
3. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466.
4. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594. doi: 10.1002/14651858.CD005594.pub3.
5. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
6. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
3. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466.
4. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594. doi: 10.1002/14651858.CD005594.pub3.
5. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
6. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Stigma in dementia: It’s time to talk about it
Dementia is a family of disorders characterized by a decline in multiple cognitive abilities that significantly interferes with an individual’s functioning. An estimated 50 million people are living with a dementia worldwide.1 Alzheimer’s disease (AD) is the leading cause of dementia, accounting for approximately two-thirds of dementia cases.1 These numbers are expected to increase dramatically in the upcoming decades.
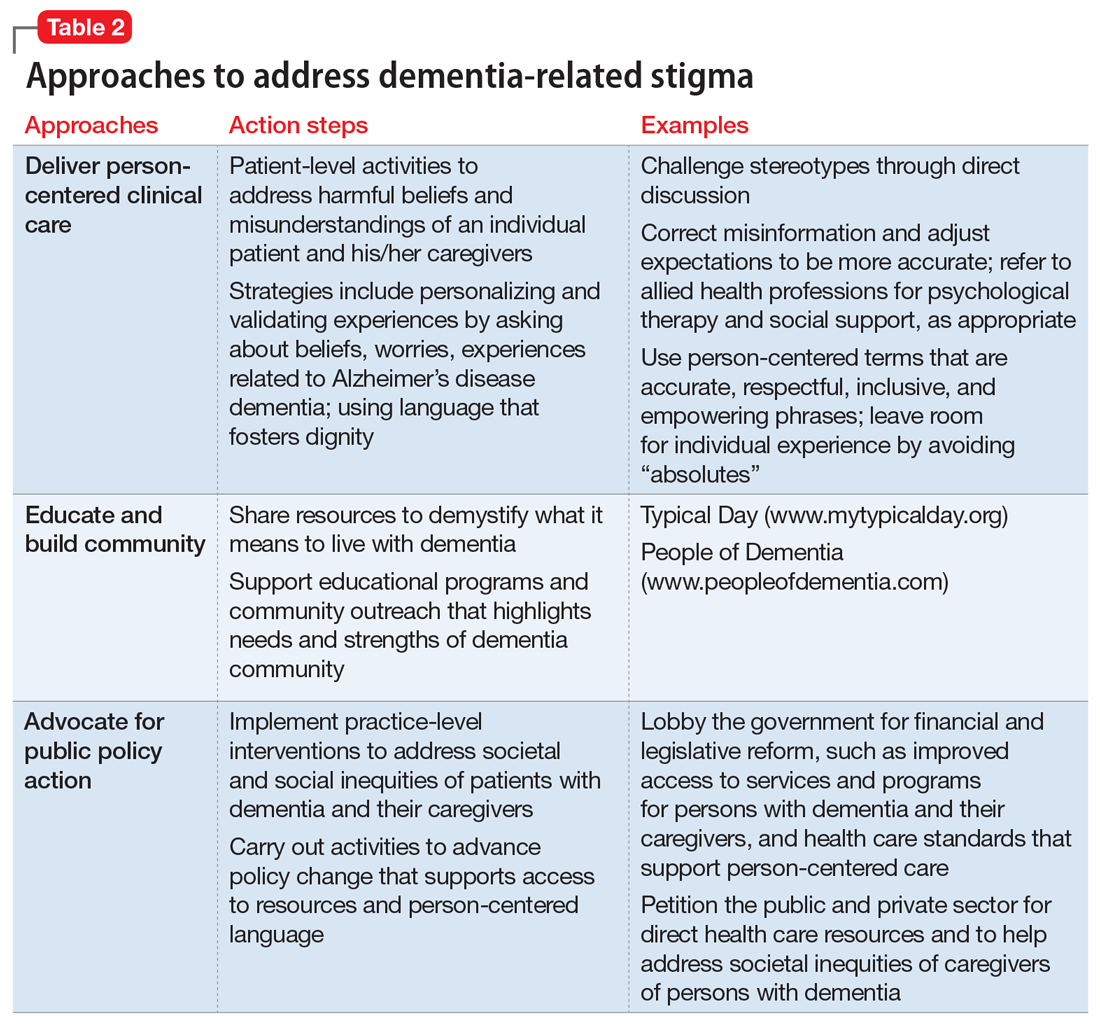
Sociologist Erving Goffman defined stigma as “an attribute, behaviour, or reputation which is socially discrediting in a particular way: it causes an individual to be mentally classified by others in an undesirable, rejected stereotype rather than in an accepted, normal one.”2 Goffman2 defined 3 broad categories of stigma: public, self, and courtesy (Table 12).
Considerable evidence shows that the combined impact of having dementia and the negative response to the diagnosis significantly undermines an individual’s psychosocial well-being and quality of life.3 Persons with dementia (PwD) commonly report a loss of identity and self-worth, and stigma appears to deepen this distress.3 Stigma also negatively affects individuals associated with PwD, including family members and professionals. In this article, we discuss the impact of dementia-related stigma, and steps you can take to address it, including implementing person-centered clinical practices, promoting anti-stigma messaging campaigns, and advocating for public policy action to improve the lives of PwD and their families.
A pervasive problem
Although the Alzheimer’s Society International and the World Health Organization acknowledge that stigma has a central role in defining the experience of AD, how stigma may present, how clinicians and researchers can recognize and measure stigma, and how to best combat it have been understudied.3-5 A recent systematic literature review examined worldwide evidence on dementia-related stigma over the past decade.6 Hermann et al6 found that health care providers and the general public may hold stigmatizing attitudes toward PwD, and that stigma may be particularly harsh among racial and ethnic minorities, although the literature is scarce in this area. Cultural factors may also worsen stigma, and stigma may be associated with reduced awareness of dementia services and reduced help-seeking among minority groups.7,8 Studies show that stigmatizing attitudes are more pronounced in people with limited knowledge of dementia, in those with little contact with PwD, in men, in younger individuals, and in the context of cultural interpretations of dementia.6 Health care providers can also sometimes contribute to the perpetuation of stigma.6
In terms of standardized scales or instruments for evaluating dementia-related stigma, there is no uniformly accepted “gold standard” measure, which makes it difficult to compare studies.6 In order to effectively study efforts to reduce stigma, researchers need to identify and establish a consensus on rating scales for evaluating stigma among PwD, caregivers, and the general public. Three instruments that may be used for this purpose are the Family Stigma in Alzheimer’s Disease Scale (FS-ADS),9 the Stigma Scale for Chronic Illness (SSCI),10 and the Perceptions Regarding Investigational Screening for Memory in Primary Care (PRISM-PC).11
The detrimental effects of stigma
Burgener et al12 reported that personal stigma impacted functioning and quality of life in PwD. Higher levels of stigma were associated with higher anxiety, depression, and behavioral symptoms and lower self-esteem, social support, participation in activities, personal control, and physical health.12 Personal characteristics that may affect stigma include gender, location (rural vs urban), ethnicity, education level, and living arrangements (alone vs with family).12
In a subset of PwD with early-stage memory loss (n = 22), Burgener and Buckwalter13 found that 42% of participants were reluctant to reveal their diagnosis to others, with some fearing they would no longer be allowed to live alone and would be “sent to a facility.” In addition, 46% indicated they did not want “to be talked about like they were not there.” More than 50% of participants reported changes in their social network after receiving the diagnosis, including reducing activities and limiting types of contacts (ie, telephone only) or interacting only when “people come to me.” Participants were most comfortable with good friends “who understand” and persons within their faith communities. When asked about how they were treated by family members, >50% of participants described being treated differently, including loss of financial independence, more limited contact, and being “treated like a baby” by their children, who in general were uncomfortable talking about the diagnosis.
Continue to: In a recent study...
In a recent study by Harper et al,14 stigma was prevalent in the experience of PwD. One participant disclosed:
“I think there is [are] people I know who don’t ask me to go places or do things ’cause I have a dementia…I think lots of people don’t know what dementia is and I think it scares them ’cause they think of it as crazy. It hurts…”
Another participant said:
“I have had friends for over thirty years. They have turned their backs on me…we used to go for walks and they would phone me and go for coffee. Now I don’t hear from any of them…those aren’t true friends…true friends will stand behind you, not in front of you. That’s why I am not happy.”
Overall, quantitative and qualitative findings indicate multiple, detrimental effects of personal stigma on PwD. These effects fit well with measures of self-stigma, including social rejection (eg, being treated differently, participating in fewer activities, and having fewer friends), internalized shame (eg, being treated like a child, having fewer responsibilities, others acting as if dementia is “contagious”), and social isolation (eg, being less outgoing, feeling more comfortable in small groups, having limited social contacts).15
Continue to: Receiving a diagnosis of dementia...
Receiving a diagnosis of dementia presents patients and their families with psychological and social challenges.16 Many of these challenges are the consequence of stigma. A broad range of efforts are underway worldwide to reduce dementia-related stigma. These efforts include programs to promote public awareness and education, campaigns to develop inclusive social policies, and skills-based training initiatives to promote delivery of patient-centered care by clinicians and educators.3,17,18 Many of these efforts share a common focus on promoting the “dignity” and “personhood” of PwD in order to disrupt stereotypes or fixed, oversimplified beliefs associated with dementia.
Implementing person-centered clinical care
In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Health care communications that call attention to stereotypes may allow PwD to identify stereotypes as well as inaccuracies in those stereotypes. Interventions that validate the value of diversity can help PwD accept the ways in which they may not conform to social norms. This could include language such as “There is no one way to have Alzheimer’s disease. A person’s experience can differ from what others might experience or expect, and that’s okay.” In addition, the use of language that is accurate, respectful, inclusive, and empowering can support PwD and their caregivers.19,20 For example, referring to PwD as “individuals living with dementia” rather than “those who are demented” conveys respect and appreciation for personhood. Other clinicians have provided additional practical suggestions.21
Anti-stigma messaging campaigns
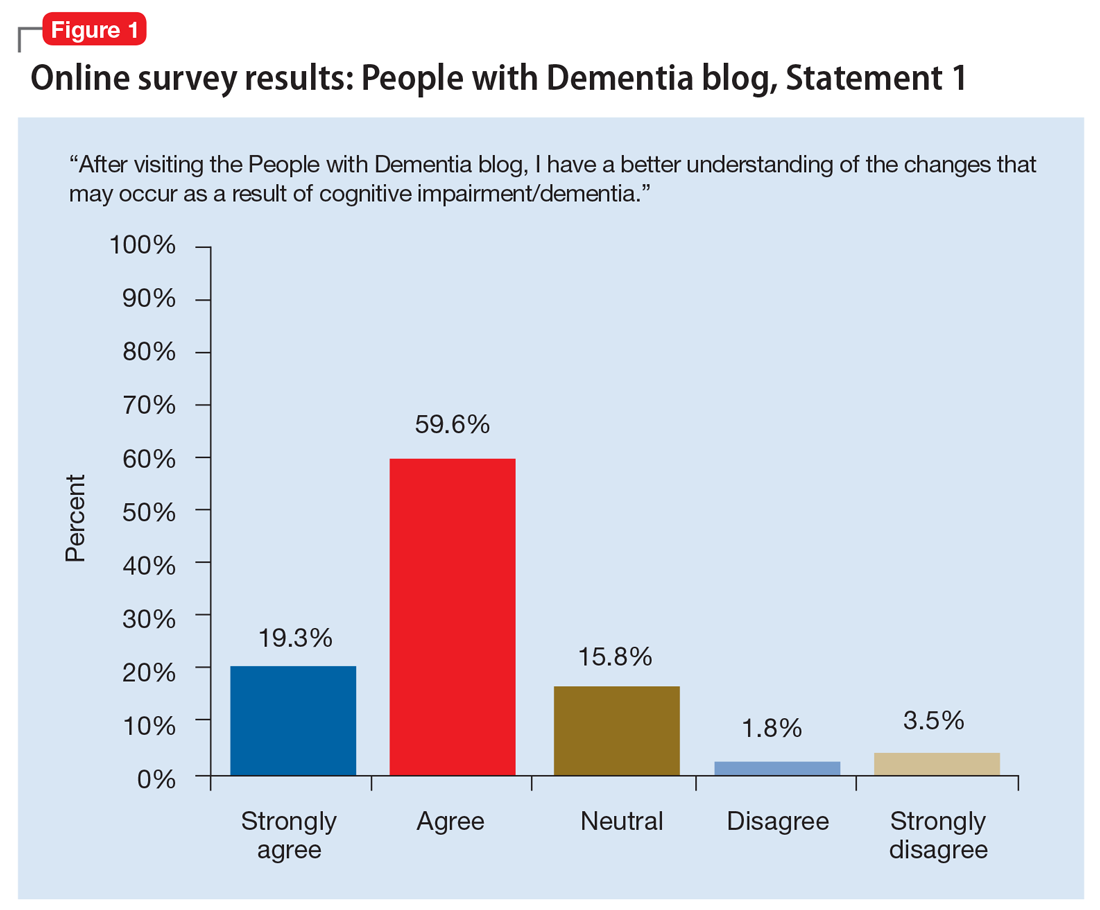
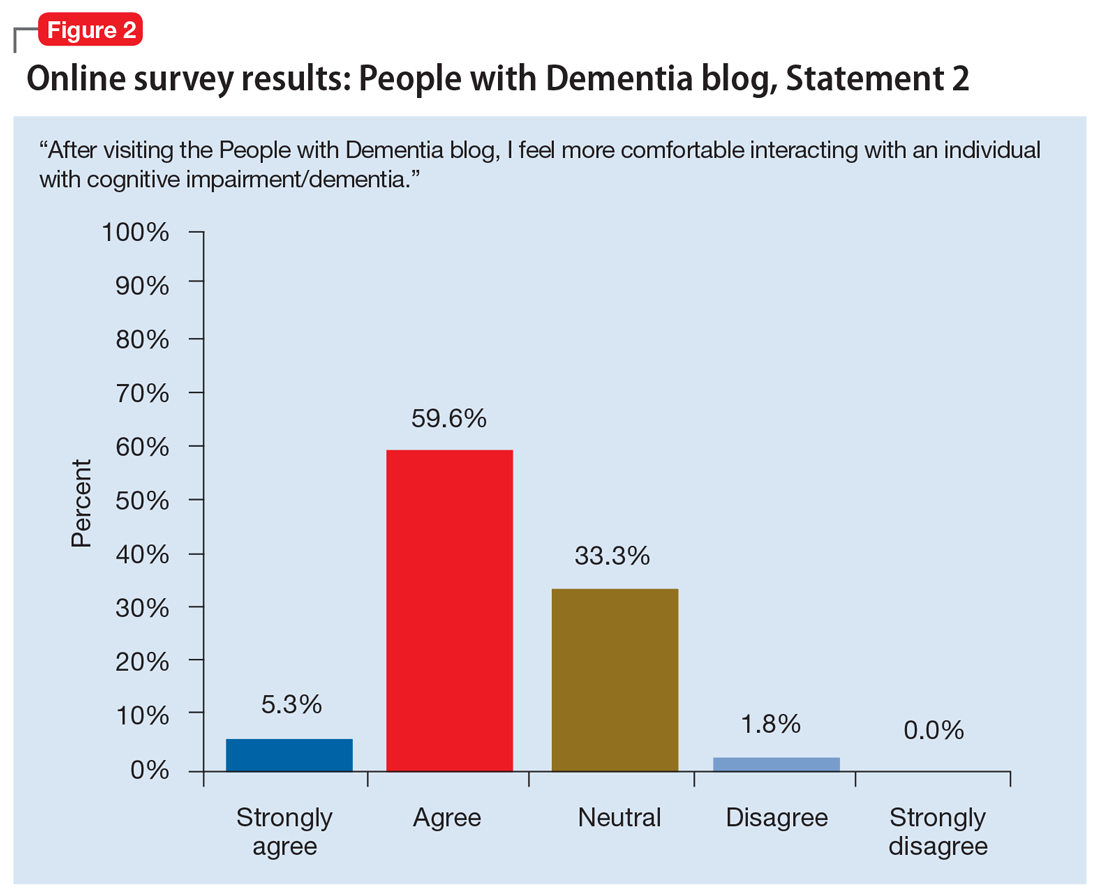
The mass media is a common source of stereotypes about AD and other dementias. They typically present a “worst-case” scenario that promotes ageism, gerontophobia, and negative emotions, which may worsen stigma and discrimination towards PwD and the people who care for them. However, public messaging campaigns are emerging to counter negative messages and stereotypes in the mass media. Projects such as Typical Day, People with Dementia, and other online anti-stigma messaging campaigns allow a broad audience to gain a more nuanced understanding of the lives of PwD and their caregivers. These projects are rich resources that offer education and personal stories that can counter common stereotypes about dementia.
Typical Day is a photography project developed and maintained by clinicians and researchers at the University of Pennsylvania. Since early 2017, the project has provided a forum for individuals with mild cognitive impairment or dementia to document their lives and show what it means to them to live with dementia. Participants in the project photo-document the people, places, and objects that define their daily lives. They review and explain these photos with researchers at Penn Memory Center, who help them tell their stories. The participants’ stories, the photos they capture, and their portraits are available at www.mytypicalday.org.
People of Dementia. Storytelling is a powerful way to raise awareness of and reduce the stigma associated with dementia. For PwD, telling their stories can be an effective and therapeutic way to communicate their emotions and deliver an important message. In the blog People of Dementia (www.peopleofdementia.com),22,23 PwD highlight who they were before the disease and how things have changed, with family members highlighting the challenges of caring for a person with dementia.
Continue to: The common thread is...
The common thread is the enduring “person” behind the exterior that is obscured by dementia. By allowing the audience to form a connection with who the individual was prior to the disease, and understanding the changes that have come as a result of dementia to both PwD and their support network, readers gain a greater appreciation of those affected by dementia. Between May 1, 2017 and May 31, 2019, the blog had more than 3,860 visitors. In an accompanying online survey (N = 57), 79% of respondents agreed/strongly agreed that after visiting the People of Dementia blog, they had a better understanding of the changes that occur as a result of cognitive impairment/dementia (Figure 1). Almost two-thirds of respondents (65%) agreed/strongly agreed that they felt more comfortable interacting with PwD (Figure 2). Additionally, 60% of respondents agreed/strongly agreed that they were more encouraged to work with PwD, and 90% agreed/strongly agreed that they had a greater appreciation of the challenges of being a caregiver for PwD. Overall, these findings suggest that the People of Dementia blog is useful for engaging the public and promoting a better understanding of dementia.
Work for policy changes
Clinicians can support public policy through education and advocacy both in the delivery of care and as spokespersons and stakeholders in their local communities. Public policies are important for providing access to medical and social services to meet the needs of PwD and their caregivers. The absence—real or perceived—of sufficient resources exacerbates dementia-related stigma. In addition to facilitating access to resources, national dementia strategies or legal frameworks, such as the National Alzheimer’s Project Act in the United States, include policy initiatives to identify and promote communication approaches that are effective and sensitive with respect to people living with dementia and their caregivers.
State and local legislators and patient advocates are leading policy efforts to reduce dementia-related stigma. For example, Colorado recently changed statutory references from being specific to diseases that cause dementia to the broader, more inclusive phrase “dementia diseases and related disabilities.”18 In addition to making funds available to support caregiving services for PwD, this legislative change added training for first responders to better meet the needs of missing PwD, and shifted the terminology used to diagnose and communicate about diseases causing dementia. The shift in language added new terminology that was chosen for being more person-centered to replace prior references to “senior senility,” “senility,” and other terms with pejorative meanings.
In Canada, a National Dementia Strategy will commit the Canadian government to action with definitive timelines, targets, reporting structures, and measurable outcomes.24
Table 2 summarizes approaches to a
Continue to: An open discussion
An open discussion
Larger studies and testing of diverse approaches are needed to better understand whether intergenerational initiatives or other approaches can genuinely modify stigmatizing attitudes in various dementia populations, especially considering language, health literacy, cultural preferences, and other needs. The identified effects on physical and mental health, quality of life, self-esteem, and behavioral symptoms further support the extensive, negative effects of self-stigma on PwD, and emphasize the need to develop and test interventions to ameliorate these effects.
We presented at a Stigma Symposium at the 2018 Gerontological Society of America Annual Scientific Meeting in Boston, Massachusetts.25 Attendees of this conference shared our concerns about the detrimental effects of stigma. The main question we were asked was “What can we do to reduce stigma?” Perhaps the most immediate response is that in order to move the stigma dial, clinicians need to recognize that stigma has multiple, broad-reaching, and negative effects on PwD and their families.6 Bringing the discussion into the open and targeting stigma at multiple levels needs to be addressed by clinicians, researchers, administrators, and society at large.
Bottom Line
Stigma has multiple, broad-reaching, and negative effects on persons with dementia and their families. In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Anti-stigma messaging campaigns and public policy changes also can be used to address societal and social inequities of patients with dementia and their caregivers.
Related Resources
- Khoury R, Shach R, Nair A, et al. Can lifestyle modifications delay or prevent Alzheimer’s disease? Current Psychiatry. 2019;18(1):29-36,38.
- Burke AD, Burke WJ. Antipsychotics for patients with dementia: The road less traveled. Current Psychiatry. 2018;17(10):26-32,35-37.
1. World Health Organization. Towards a dementia plan: a WHO guide. https://www.who.int/mental_health/neurology/dementia/policy_guidance/en/. Published 2018. Accessed May 28, 2019.
2. Goffman E. Stigma. New York, NY: Prentice-Hall; 1963:1-123.
3. Alzheimer’s Disease International. World Alzheimer Report 2012: overcoming the stigma of dementia. https://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf. Published 2012. Accessed May 28, 2019.
4. Blay SL, Peluso ETP. Public stigma: the community’s tolerance of Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):163-171.
5. Piver LC, Nubukpo P, Faure A, et al. Describing perceived stigma against Alzheimer’s disease in a general population in France: the STIG-MA survey. Int J Geriatr Psychiatry. 2013;28(9):933-938.
6. Herrmann LK, Welter E, Leverenz J, et al. A systematic review of dementia-related stigma research: can we move the stigma dial? Am J Geriatr Psychiatry. 2018;26(3):316-331.
7. Eng KJ, Woo BKP. Knowledge of dementia community resources and stigma among Chinese American immigrants. Gen Hosp Psychiatry. 2015;37(1):e3-e4. doi:10.1016/j.genhosppsych.2014.11.003.
8. Jang Y, Kim G, Chiriboga D. Knowledge of Alzheimer’s disease, feelings of shame, and awareness of services among Korean American elders. J Aging Health. 2010;22(4):419-433.
9. Werner P, Goldstein D, Heinik J. Development and validity of the Family Stigma in Alzheimer’s disease scale (FS-ADS). Alzheimer Disease & Associated Disorders. 2011;25(1):42-48.
10. Rao D, Choi SW, Victorson D, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Qual Life Res. 2009;18(5):585-595.
11. Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry. 2008;23(8):812-820.
12. Burgener SC, Buckwalter K, Perkounkova Y, et al. Perceived stigma in persons with early-stage dementia: longitudinal findings: Part 2. Dementia. 2015;14(5):609-632.
13. Burgener SC, Buckwalter K. The effects of perceived stigma on persons with dementia and their family caregivers. In: Symposium on Stigma: It’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
14. Harper L, Dobbs B, Royan H, et al. The experience of stigma in care partners of people with dementia – results from an exploratory study. In Symposium on stigma: it’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
15. Burgener S, Berger B. Measuring perceived stigma in persons with progressive neurological disease: Alzheimer’s dementia and Parkinson disease. Dementia. 2008;7(1):31-53.
16. Stites SD, Milne R, Karlawish J. Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;10;285-300.
17. Anderson LA, Egge R. Expanding efforts to address Alzheimer’s disease: the Healthy Brain Initiative. Alzheimer’s Dement. 2014;10(50):S453-S456.
18. Alzheimer’s Association National Plan Milestone Workgroup. Report on the milestones for the US National plan to address Alzheimer’s disease. Alzheimer’s Dementia. 2014;10(Suppl 5);S430-S452. doi:10.1016/j/jalz.2014.08.103.
19. Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australasian Journal on Ageing. 2006;25(2):74-79.
20. Swaffer, K. Dementia: stigma, language, and dementia-friendly. Dementia. 2014;13(6):709-716.
21. Stites SD, Karlawish J. Stigma of Alzheimer’s disease dementia: considerations for practice. Practical Neurology. https://practicalneurology.com/articles/2018-june/stigma-of-alzheimers-disease-dementia. Published June 2018. Accessed May 28, 2019.
22. Jamieson J, Dobbs B, Charles L, et al. Forgetful, but not forgotten people of dementia: a novel, technology focused project with a humanistic touch. Geriatric Grand Rounds; October 10, 2017. Edmonton, Alberta, Canada.
23. Dobbs B, Charles L, Chan K, et al. People of Dementia. CGS 37th Annual Scientific Meeting: Integrating Care, Making an Impact. Can Geriatr J. 2017;20(3):220.
24. Government of Canada. Conference report: National Dementia Conference. https://www.canada.ca/en/services/health/publications/diseases-conditions/national-dementia-conference-report.html. Government of Canada. Published August 2018. Accessed May 28, 2019.
25. The Gerontological Society of America. Program Abstracts from the GSA 2018 Annual Scientific Meeting “The Purposes of Longer Lives.” Innovation in Aging. 2018;2(Suppl 1):143.
Dementia is a family of disorders characterized by a decline in multiple cognitive abilities that significantly interferes with an individual’s functioning. An estimated 50 million people are living with a dementia worldwide.1 Alzheimer’s disease (AD) is the leading cause of dementia, accounting for approximately two-thirds of dementia cases.1 These numbers are expected to increase dramatically in the upcoming decades.
Sociologist Erving Goffman defined stigma as “an attribute, behaviour, or reputation which is socially discrediting in a particular way: it causes an individual to be mentally classified by others in an undesirable, rejected stereotype rather than in an accepted, normal one.”2 Goffman2 defined 3 broad categories of stigma: public, self, and courtesy (Table 12).
Considerable evidence shows that the combined impact of having dementia and the negative response to the diagnosis significantly undermines an individual’s psychosocial well-being and quality of life.3 Persons with dementia (PwD) commonly report a loss of identity and self-worth, and stigma appears to deepen this distress.3 Stigma also negatively affects individuals associated with PwD, including family members and professionals. In this article, we discuss the impact of dementia-related stigma, and steps you can take to address it, including implementing person-centered clinical practices, promoting anti-stigma messaging campaigns, and advocating for public policy action to improve the lives of PwD and their families.
A pervasive problem
Although the Alzheimer’s Society International and the World Health Organization acknowledge that stigma has a central role in defining the experience of AD, how stigma may present, how clinicians and researchers can recognize and measure stigma, and how to best combat it have been understudied.3-5 A recent systematic literature review examined worldwide evidence on dementia-related stigma over the past decade.6 Hermann et al6 found that health care providers and the general public may hold stigmatizing attitudes toward PwD, and that stigma may be particularly harsh among racial and ethnic minorities, although the literature is scarce in this area. Cultural factors may also worsen stigma, and stigma may be associated with reduced awareness of dementia services and reduced help-seeking among minority groups.7,8 Studies show that stigmatizing attitudes are more pronounced in people with limited knowledge of dementia, in those with little contact with PwD, in men, in younger individuals, and in the context of cultural interpretations of dementia.6 Health care providers can also sometimes contribute to the perpetuation of stigma.6
In terms of standardized scales or instruments for evaluating dementia-related stigma, there is no uniformly accepted “gold standard” measure, which makes it difficult to compare studies.6 In order to effectively study efforts to reduce stigma, researchers need to identify and establish a consensus on rating scales for evaluating stigma among PwD, caregivers, and the general public. Three instruments that may be used for this purpose are the Family Stigma in Alzheimer’s Disease Scale (FS-ADS),9 the Stigma Scale for Chronic Illness (SSCI),10 and the Perceptions Regarding Investigational Screening for Memory in Primary Care (PRISM-PC).11
The detrimental effects of stigma
Burgener et al12 reported that personal stigma impacted functioning and quality of life in PwD. Higher levels of stigma were associated with higher anxiety, depression, and behavioral symptoms and lower self-esteem, social support, participation in activities, personal control, and physical health.12 Personal characteristics that may affect stigma include gender, location (rural vs urban), ethnicity, education level, and living arrangements (alone vs with family).12
In a subset of PwD with early-stage memory loss (n = 22), Burgener and Buckwalter13 found that 42% of participants were reluctant to reveal their diagnosis to others, with some fearing they would no longer be allowed to live alone and would be “sent to a facility.” In addition, 46% indicated they did not want “to be talked about like they were not there.” More than 50% of participants reported changes in their social network after receiving the diagnosis, including reducing activities and limiting types of contacts (ie, telephone only) or interacting only when “people come to me.” Participants were most comfortable with good friends “who understand” and persons within their faith communities. When asked about how they were treated by family members, >50% of participants described being treated differently, including loss of financial independence, more limited contact, and being “treated like a baby” by their children, who in general were uncomfortable talking about the diagnosis.
Continue to: In a recent study...
In a recent study by Harper et al,14 stigma was prevalent in the experience of PwD. One participant disclosed:
“I think there is [are] people I know who don’t ask me to go places or do things ’cause I have a dementia…I think lots of people don’t know what dementia is and I think it scares them ’cause they think of it as crazy. It hurts…”
Another participant said:
“I have had friends for over thirty years. They have turned their backs on me…we used to go for walks and they would phone me and go for coffee. Now I don’t hear from any of them…those aren’t true friends…true friends will stand behind you, not in front of you. That’s why I am not happy.”
Overall, quantitative and qualitative findings indicate multiple, detrimental effects of personal stigma on PwD. These effects fit well with measures of self-stigma, including social rejection (eg, being treated differently, participating in fewer activities, and having fewer friends), internalized shame (eg, being treated like a child, having fewer responsibilities, others acting as if dementia is “contagious”), and social isolation (eg, being less outgoing, feeling more comfortable in small groups, having limited social contacts).15
Continue to: Receiving a diagnosis of dementia...
Receiving a diagnosis of dementia presents patients and their families with psychological and social challenges.16 Many of these challenges are the consequence of stigma. A broad range of efforts are underway worldwide to reduce dementia-related stigma. These efforts include programs to promote public awareness and education, campaigns to develop inclusive social policies, and skills-based training initiatives to promote delivery of patient-centered care by clinicians and educators.3,17,18 Many of these efforts share a common focus on promoting the “dignity” and “personhood” of PwD in order to disrupt stereotypes or fixed, oversimplified beliefs associated with dementia.
Implementing person-centered clinical care
In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Health care communications that call attention to stereotypes may allow PwD to identify stereotypes as well as inaccuracies in those stereotypes. Interventions that validate the value of diversity can help PwD accept the ways in which they may not conform to social norms. This could include language such as “There is no one way to have Alzheimer’s disease. A person’s experience can differ from what others might experience or expect, and that’s okay.” In addition, the use of language that is accurate, respectful, inclusive, and empowering can support PwD and their caregivers.19,20 For example, referring to PwD as “individuals living with dementia” rather than “those who are demented” conveys respect and appreciation for personhood. Other clinicians have provided additional practical suggestions.21
Anti-stigma messaging campaigns
The mass media is a common source of stereotypes about AD and other dementias. They typically present a “worst-case” scenario that promotes ageism, gerontophobia, and negative emotions, which may worsen stigma and discrimination towards PwD and the people who care for them. However, public messaging campaigns are emerging to counter negative messages and stereotypes in the mass media. Projects such as Typical Day, People with Dementia, and other online anti-stigma messaging campaigns allow a broad audience to gain a more nuanced understanding of the lives of PwD and their caregivers. These projects are rich resources that offer education and personal stories that can counter common stereotypes about dementia.
Typical Day is a photography project developed and maintained by clinicians and researchers at the University of Pennsylvania. Since early 2017, the project has provided a forum for individuals with mild cognitive impairment or dementia to document their lives and show what it means to them to live with dementia. Participants in the project photo-document the people, places, and objects that define their daily lives. They review and explain these photos with researchers at Penn Memory Center, who help them tell their stories. The participants’ stories, the photos they capture, and their portraits are available at www.mytypicalday.org.
People of Dementia. Storytelling is a powerful way to raise awareness of and reduce the stigma associated with dementia. For PwD, telling their stories can be an effective and therapeutic way to communicate their emotions and deliver an important message. In the blog People of Dementia (www.peopleofdementia.com),22,23 PwD highlight who they were before the disease and how things have changed, with family members highlighting the challenges of caring for a person with dementia.
Continue to: The common thread is...
The common thread is the enduring “person” behind the exterior that is obscured by dementia. By allowing the audience to form a connection with who the individual was prior to the disease, and understanding the changes that have come as a result of dementia to both PwD and their support network, readers gain a greater appreciation of those affected by dementia. Between May 1, 2017 and May 31, 2019, the blog had more than 3,860 visitors. In an accompanying online survey (N = 57), 79% of respondents agreed/strongly agreed that after visiting the People of Dementia blog, they had a better understanding of the changes that occur as a result of cognitive impairment/dementia (Figure 1). Almost two-thirds of respondents (65%) agreed/strongly agreed that they felt more comfortable interacting with PwD (Figure 2). Additionally, 60% of respondents agreed/strongly agreed that they were more encouraged to work with PwD, and 90% agreed/strongly agreed that they had a greater appreciation of the challenges of being a caregiver for PwD. Overall, these findings suggest that the People of Dementia blog is useful for engaging the public and promoting a better understanding of dementia.
Work for policy changes
Clinicians can support public policy through education and advocacy both in the delivery of care and as spokespersons and stakeholders in their local communities. Public policies are important for providing access to medical and social services to meet the needs of PwD and their caregivers. The absence—real or perceived—of sufficient resources exacerbates dementia-related stigma. In addition to facilitating access to resources, national dementia strategies or legal frameworks, such as the National Alzheimer’s Project Act in the United States, include policy initiatives to identify and promote communication approaches that are effective and sensitive with respect to people living with dementia and their caregivers.
State and local legislators and patient advocates are leading policy efforts to reduce dementia-related stigma. For example, Colorado recently changed statutory references from being specific to diseases that cause dementia to the broader, more inclusive phrase “dementia diseases and related disabilities.”18 In addition to making funds available to support caregiving services for PwD, this legislative change added training for first responders to better meet the needs of missing PwD, and shifted the terminology used to diagnose and communicate about diseases causing dementia. The shift in language added new terminology that was chosen for being more person-centered to replace prior references to “senior senility,” “senility,” and other terms with pejorative meanings.
In Canada, a National Dementia Strategy will commit the Canadian government to action with definitive timelines, targets, reporting structures, and measurable outcomes.24
Table 2 summarizes approaches to a
Continue to: An open discussion
An open discussion
Larger studies and testing of diverse approaches are needed to better understand whether intergenerational initiatives or other approaches can genuinely modify stigmatizing attitudes in various dementia populations, especially considering language, health literacy, cultural preferences, and other needs. The identified effects on physical and mental health, quality of life, self-esteem, and behavioral symptoms further support the extensive, negative effects of self-stigma on PwD, and emphasize the need to develop and test interventions to ameliorate these effects.
We presented at a Stigma Symposium at the 2018 Gerontological Society of America Annual Scientific Meeting in Boston, Massachusetts.25 Attendees of this conference shared our concerns about the detrimental effects of stigma. The main question we were asked was “What can we do to reduce stigma?” Perhaps the most immediate response is that in order to move the stigma dial, clinicians need to recognize that stigma has multiple, broad-reaching, and negative effects on PwD and their families.6 Bringing the discussion into the open and targeting stigma at multiple levels needs to be addressed by clinicians, researchers, administrators, and society at large.
Bottom Line
Stigma has multiple, broad-reaching, and negative effects on persons with dementia and their families. In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Anti-stigma messaging campaigns and public policy changes also can be used to address societal and social inequities of patients with dementia and their caregivers.
Related Resources
- Khoury R, Shach R, Nair A, et al. Can lifestyle modifications delay or prevent Alzheimer’s disease? Current Psychiatry. 2019;18(1):29-36,38.
- Burke AD, Burke WJ. Antipsychotics for patients with dementia: The road less traveled. Current Psychiatry. 2018;17(10):26-32,35-37.
Dementia is a family of disorders characterized by a decline in multiple cognitive abilities that significantly interferes with an individual’s functioning. An estimated 50 million people are living with a dementia worldwide.1 Alzheimer’s disease (AD) is the leading cause of dementia, accounting for approximately two-thirds of dementia cases.1 These numbers are expected to increase dramatically in the upcoming decades.
Sociologist Erving Goffman defined stigma as “an attribute, behaviour, or reputation which is socially discrediting in a particular way: it causes an individual to be mentally classified by others in an undesirable, rejected stereotype rather than in an accepted, normal one.”2 Goffman2 defined 3 broad categories of stigma: public, self, and courtesy (Table 12).
Considerable evidence shows that the combined impact of having dementia and the negative response to the diagnosis significantly undermines an individual’s psychosocial well-being and quality of life.3 Persons with dementia (PwD) commonly report a loss of identity and self-worth, and stigma appears to deepen this distress.3 Stigma also negatively affects individuals associated with PwD, including family members and professionals. In this article, we discuss the impact of dementia-related stigma, and steps you can take to address it, including implementing person-centered clinical practices, promoting anti-stigma messaging campaigns, and advocating for public policy action to improve the lives of PwD and their families.
A pervasive problem
Although the Alzheimer’s Society International and the World Health Organization acknowledge that stigma has a central role in defining the experience of AD, how stigma may present, how clinicians and researchers can recognize and measure stigma, and how to best combat it have been understudied.3-5 A recent systematic literature review examined worldwide evidence on dementia-related stigma over the past decade.6 Hermann et al6 found that health care providers and the general public may hold stigmatizing attitudes toward PwD, and that stigma may be particularly harsh among racial and ethnic minorities, although the literature is scarce in this area. Cultural factors may also worsen stigma, and stigma may be associated with reduced awareness of dementia services and reduced help-seeking among minority groups.7,8 Studies show that stigmatizing attitudes are more pronounced in people with limited knowledge of dementia, in those with little contact with PwD, in men, in younger individuals, and in the context of cultural interpretations of dementia.6 Health care providers can also sometimes contribute to the perpetuation of stigma.6
In terms of standardized scales or instruments for evaluating dementia-related stigma, there is no uniformly accepted “gold standard” measure, which makes it difficult to compare studies.6 In order to effectively study efforts to reduce stigma, researchers need to identify and establish a consensus on rating scales for evaluating stigma among PwD, caregivers, and the general public. Three instruments that may be used for this purpose are the Family Stigma in Alzheimer’s Disease Scale (FS-ADS),9 the Stigma Scale for Chronic Illness (SSCI),10 and the Perceptions Regarding Investigational Screening for Memory in Primary Care (PRISM-PC).11
The detrimental effects of stigma
Burgener et al12 reported that personal stigma impacted functioning and quality of life in PwD. Higher levels of stigma were associated with higher anxiety, depression, and behavioral symptoms and lower self-esteem, social support, participation in activities, personal control, and physical health.12 Personal characteristics that may affect stigma include gender, location (rural vs urban), ethnicity, education level, and living arrangements (alone vs with family).12
In a subset of PwD with early-stage memory loss (n = 22), Burgener and Buckwalter13 found that 42% of participants were reluctant to reveal their diagnosis to others, with some fearing they would no longer be allowed to live alone and would be “sent to a facility.” In addition, 46% indicated they did not want “to be talked about like they were not there.” More than 50% of participants reported changes in their social network after receiving the diagnosis, including reducing activities and limiting types of contacts (ie, telephone only) or interacting only when “people come to me.” Participants were most comfortable with good friends “who understand” and persons within their faith communities. When asked about how they were treated by family members, >50% of participants described being treated differently, including loss of financial independence, more limited contact, and being “treated like a baby” by their children, who in general were uncomfortable talking about the diagnosis.
Continue to: In a recent study...
In a recent study by Harper et al,14 stigma was prevalent in the experience of PwD. One participant disclosed:
“I think there is [are] people I know who don’t ask me to go places or do things ’cause I have a dementia…I think lots of people don’t know what dementia is and I think it scares them ’cause they think of it as crazy. It hurts…”
Another participant said:
“I have had friends for over thirty years. They have turned their backs on me…we used to go for walks and they would phone me and go for coffee. Now I don’t hear from any of them…those aren’t true friends…true friends will stand behind you, not in front of you. That’s why I am not happy.”
Overall, quantitative and qualitative findings indicate multiple, detrimental effects of personal stigma on PwD. These effects fit well with measures of self-stigma, including social rejection (eg, being treated differently, participating in fewer activities, and having fewer friends), internalized shame (eg, being treated like a child, having fewer responsibilities, others acting as if dementia is “contagious”), and social isolation (eg, being less outgoing, feeling more comfortable in small groups, having limited social contacts).15
Continue to: Receiving a diagnosis of dementia...
Receiving a diagnosis of dementia presents patients and their families with psychological and social challenges.16 Many of these challenges are the consequence of stigma. A broad range of efforts are underway worldwide to reduce dementia-related stigma. These efforts include programs to promote public awareness and education, campaigns to develop inclusive social policies, and skills-based training initiatives to promote delivery of patient-centered care by clinicians and educators.3,17,18 Many of these efforts share a common focus on promoting the “dignity” and “personhood” of PwD in order to disrupt stereotypes or fixed, oversimplified beliefs associated with dementia.
Implementing person-centered clinical care
In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Health care communications that call attention to stereotypes may allow PwD to identify stereotypes as well as inaccuracies in those stereotypes. Interventions that validate the value of diversity can help PwD accept the ways in which they may not conform to social norms. This could include language such as “There is no one way to have Alzheimer’s disease. A person’s experience can differ from what others might experience or expect, and that’s okay.” In addition, the use of language that is accurate, respectful, inclusive, and empowering can support PwD and their caregivers.19,20 For example, referring to PwD as “individuals living with dementia” rather than “those who are demented” conveys respect and appreciation for personhood. Other clinicians have provided additional practical suggestions.21
Anti-stigma messaging campaigns
The mass media is a common source of stereotypes about AD and other dementias. They typically present a “worst-case” scenario that promotes ageism, gerontophobia, and negative emotions, which may worsen stigma and discrimination towards PwD and the people who care for them. However, public messaging campaigns are emerging to counter negative messages and stereotypes in the mass media. Projects such as Typical Day, People with Dementia, and other online anti-stigma messaging campaigns allow a broad audience to gain a more nuanced understanding of the lives of PwD and their caregivers. These projects are rich resources that offer education and personal stories that can counter common stereotypes about dementia.
Typical Day is a photography project developed and maintained by clinicians and researchers at the University of Pennsylvania. Since early 2017, the project has provided a forum for individuals with mild cognitive impairment or dementia to document their lives and show what it means to them to live with dementia. Participants in the project photo-document the people, places, and objects that define their daily lives. They review and explain these photos with researchers at Penn Memory Center, who help them tell their stories. The participants’ stories, the photos they capture, and their portraits are available at www.mytypicalday.org.
People of Dementia. Storytelling is a powerful way to raise awareness of and reduce the stigma associated with dementia. For PwD, telling their stories can be an effective and therapeutic way to communicate their emotions and deliver an important message. In the blog People of Dementia (www.peopleofdementia.com),22,23 PwD highlight who they were before the disease and how things have changed, with family members highlighting the challenges of caring for a person with dementia.
Continue to: The common thread is...
The common thread is the enduring “person” behind the exterior that is obscured by dementia. By allowing the audience to form a connection with who the individual was prior to the disease, and understanding the changes that have come as a result of dementia to both PwD and their support network, readers gain a greater appreciation of those affected by dementia. Between May 1, 2017 and May 31, 2019, the blog had more than 3,860 visitors. In an accompanying online survey (N = 57), 79% of respondents agreed/strongly agreed that after visiting the People of Dementia blog, they had a better understanding of the changes that occur as a result of cognitive impairment/dementia (Figure 1). Almost two-thirds of respondents (65%) agreed/strongly agreed that they felt more comfortable interacting with PwD (Figure 2). Additionally, 60% of respondents agreed/strongly agreed that they were more encouraged to work with PwD, and 90% agreed/strongly agreed that they had a greater appreciation of the challenges of being a caregiver for PwD. Overall, these findings suggest that the People of Dementia blog is useful for engaging the public and promoting a better understanding of dementia.
Work for policy changes
Clinicians can support public policy through education and advocacy both in the delivery of care and as spokespersons and stakeholders in their local communities. Public policies are important for providing access to medical and social services to meet the needs of PwD and their caregivers. The absence—real or perceived—of sufficient resources exacerbates dementia-related stigma. In addition to facilitating access to resources, national dementia strategies or legal frameworks, such as the National Alzheimer’s Project Act in the United States, include policy initiatives to identify and promote communication approaches that are effective and sensitive with respect to people living with dementia and their caregivers.
State and local legislators and patient advocates are leading policy efforts to reduce dementia-related stigma. For example, Colorado recently changed statutory references from being specific to diseases that cause dementia to the broader, more inclusive phrase “dementia diseases and related disabilities.”18 In addition to making funds available to support caregiving services for PwD, this legislative change added training for first responders to better meet the needs of missing PwD, and shifted the terminology used to diagnose and communicate about diseases causing dementia. The shift in language added new terminology that was chosen for being more person-centered to replace prior references to “senior senility,” “senility,” and other terms with pejorative meanings.
In Canada, a National Dementia Strategy will commit the Canadian government to action with definitive timelines, targets, reporting structures, and measurable outcomes.24
Table 2 summarizes approaches to a
Continue to: An open discussion
An open discussion
Larger studies and testing of diverse approaches are needed to better understand whether intergenerational initiatives or other approaches can genuinely modify stigmatizing attitudes in various dementia populations, especially considering language, health literacy, cultural preferences, and other needs. The identified effects on physical and mental health, quality of life, self-esteem, and behavioral symptoms further support the extensive, negative effects of self-stigma on PwD, and emphasize the need to develop and test interventions to ameliorate these effects.
We presented at a Stigma Symposium at the 2018 Gerontological Society of America Annual Scientific Meeting in Boston, Massachusetts.25 Attendees of this conference shared our concerns about the detrimental effects of stigma. The main question we were asked was “What can we do to reduce stigma?” Perhaps the most immediate response is that in order to move the stigma dial, clinicians need to recognize that stigma has multiple, broad-reaching, and negative effects on PwD and their families.6 Bringing the discussion into the open and targeting stigma at multiple levels needs to be addressed by clinicians, researchers, administrators, and society at large.
Bottom Line
Stigma has multiple, broad-reaching, and negative effects on persons with dementia and their families. In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Anti-stigma messaging campaigns and public policy changes also can be used to address societal and social inequities of patients with dementia and their caregivers.
Related Resources
- Khoury R, Shach R, Nair A, et al. Can lifestyle modifications delay or prevent Alzheimer’s disease? Current Psychiatry. 2019;18(1):29-36,38.
- Burke AD, Burke WJ. Antipsychotics for patients with dementia: The road less traveled. Current Psychiatry. 2018;17(10):26-32,35-37.
1. World Health Organization. Towards a dementia plan: a WHO guide. https://www.who.int/mental_health/neurology/dementia/policy_guidance/en/. Published 2018. Accessed May 28, 2019.
2. Goffman E. Stigma. New York, NY: Prentice-Hall; 1963:1-123.
3. Alzheimer’s Disease International. World Alzheimer Report 2012: overcoming the stigma of dementia. https://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf. Published 2012. Accessed May 28, 2019.
4. Blay SL, Peluso ETP. Public stigma: the community’s tolerance of Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):163-171.
5. Piver LC, Nubukpo P, Faure A, et al. Describing perceived stigma against Alzheimer’s disease in a general population in France: the STIG-MA survey. Int J Geriatr Psychiatry. 2013;28(9):933-938.
6. Herrmann LK, Welter E, Leverenz J, et al. A systematic review of dementia-related stigma research: can we move the stigma dial? Am J Geriatr Psychiatry. 2018;26(3):316-331.
7. Eng KJ, Woo BKP. Knowledge of dementia community resources and stigma among Chinese American immigrants. Gen Hosp Psychiatry. 2015;37(1):e3-e4. doi:10.1016/j.genhosppsych.2014.11.003.
8. Jang Y, Kim G, Chiriboga D. Knowledge of Alzheimer’s disease, feelings of shame, and awareness of services among Korean American elders. J Aging Health. 2010;22(4):419-433.
9. Werner P, Goldstein D, Heinik J. Development and validity of the Family Stigma in Alzheimer’s disease scale (FS-ADS). Alzheimer Disease & Associated Disorders. 2011;25(1):42-48.
10. Rao D, Choi SW, Victorson D, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Qual Life Res. 2009;18(5):585-595.
11. Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry. 2008;23(8):812-820.
12. Burgener SC, Buckwalter K, Perkounkova Y, et al. Perceived stigma in persons with early-stage dementia: longitudinal findings: Part 2. Dementia. 2015;14(5):609-632.
13. Burgener SC, Buckwalter K. The effects of perceived stigma on persons with dementia and their family caregivers. In: Symposium on Stigma: It’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
14. Harper L, Dobbs B, Royan H, et al. The experience of stigma in care partners of people with dementia – results from an exploratory study. In Symposium on stigma: it’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
15. Burgener S, Berger B. Measuring perceived stigma in persons with progressive neurological disease: Alzheimer’s dementia and Parkinson disease. Dementia. 2008;7(1):31-53.
16. Stites SD, Milne R, Karlawish J. Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;10;285-300.
17. Anderson LA, Egge R. Expanding efforts to address Alzheimer’s disease: the Healthy Brain Initiative. Alzheimer’s Dement. 2014;10(50):S453-S456.
18. Alzheimer’s Association National Plan Milestone Workgroup. Report on the milestones for the US National plan to address Alzheimer’s disease. Alzheimer’s Dementia. 2014;10(Suppl 5);S430-S452. doi:10.1016/j/jalz.2014.08.103.
19. Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australasian Journal on Ageing. 2006;25(2):74-79.
20. Swaffer, K. Dementia: stigma, language, and dementia-friendly. Dementia. 2014;13(6):709-716.
21. Stites SD, Karlawish J. Stigma of Alzheimer’s disease dementia: considerations for practice. Practical Neurology. https://practicalneurology.com/articles/2018-june/stigma-of-alzheimers-disease-dementia. Published June 2018. Accessed May 28, 2019.
22. Jamieson J, Dobbs B, Charles L, et al. Forgetful, but not forgotten people of dementia: a novel, technology focused project with a humanistic touch. Geriatric Grand Rounds; October 10, 2017. Edmonton, Alberta, Canada.
23. Dobbs B, Charles L, Chan K, et al. People of Dementia. CGS 37th Annual Scientific Meeting: Integrating Care, Making an Impact. Can Geriatr J. 2017;20(3):220.
24. Government of Canada. Conference report: National Dementia Conference. https://www.canada.ca/en/services/health/publications/diseases-conditions/national-dementia-conference-report.html. Government of Canada. Published August 2018. Accessed May 28, 2019.
25. The Gerontological Society of America. Program Abstracts from the GSA 2018 Annual Scientific Meeting “The Purposes of Longer Lives.” Innovation in Aging. 2018;2(Suppl 1):143.
1. World Health Organization. Towards a dementia plan: a WHO guide. https://www.who.int/mental_health/neurology/dementia/policy_guidance/en/. Published 2018. Accessed May 28, 2019.
2. Goffman E. Stigma. New York, NY: Prentice-Hall; 1963:1-123.
3. Alzheimer’s Disease International. World Alzheimer Report 2012: overcoming the stigma of dementia. https://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf. Published 2012. Accessed May 28, 2019.
4. Blay SL, Peluso ETP. Public stigma: the community’s tolerance of Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):163-171.
5. Piver LC, Nubukpo P, Faure A, et al. Describing perceived stigma against Alzheimer’s disease in a general population in France: the STIG-MA survey. Int J Geriatr Psychiatry. 2013;28(9):933-938.
6. Herrmann LK, Welter E, Leverenz J, et al. A systematic review of dementia-related stigma research: can we move the stigma dial? Am J Geriatr Psychiatry. 2018;26(3):316-331.
7. Eng KJ, Woo BKP. Knowledge of dementia community resources and stigma among Chinese American immigrants. Gen Hosp Psychiatry. 2015;37(1):e3-e4. doi:10.1016/j.genhosppsych.2014.11.003.
8. Jang Y, Kim G, Chiriboga D. Knowledge of Alzheimer’s disease, feelings of shame, and awareness of services among Korean American elders. J Aging Health. 2010;22(4):419-433.
9. Werner P, Goldstein D, Heinik J. Development and validity of the Family Stigma in Alzheimer’s disease scale (FS-ADS). Alzheimer Disease & Associated Disorders. 2011;25(1):42-48.
10. Rao D, Choi SW, Victorson D, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Qual Life Res. 2009;18(5):585-595.
11. Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry. 2008;23(8):812-820.
12. Burgener SC, Buckwalter K, Perkounkova Y, et al. Perceived stigma in persons with early-stage dementia: longitudinal findings: Part 2. Dementia. 2015;14(5):609-632.
13. Burgener SC, Buckwalter K. The effects of perceived stigma on persons with dementia and their family caregivers. In: Symposium on Stigma: It’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
14. Harper L, Dobbs B, Royan H, et al. The experience of stigma in care partners of people with dementia – results from an exploratory study. In Symposium on stigma: it’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
15. Burgener S, Berger B. Measuring perceived stigma in persons with progressive neurological disease: Alzheimer’s dementia and Parkinson disease. Dementia. 2008;7(1):31-53.
16. Stites SD, Milne R, Karlawish J. Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;10;285-300.
17. Anderson LA, Egge R. Expanding efforts to address Alzheimer’s disease: the Healthy Brain Initiative. Alzheimer’s Dement. 2014;10(50):S453-S456.
18. Alzheimer’s Association National Plan Milestone Workgroup. Report on the milestones for the US National plan to address Alzheimer’s disease. Alzheimer’s Dementia. 2014;10(Suppl 5);S430-S452. doi:10.1016/j/jalz.2014.08.103.
19. Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australasian Journal on Ageing. 2006;25(2):74-79.
20. Swaffer, K. Dementia: stigma, language, and dementia-friendly. Dementia. 2014;13(6):709-716.
21. Stites SD, Karlawish J. Stigma of Alzheimer’s disease dementia: considerations for practice. Practical Neurology. https://practicalneurology.com/articles/2018-june/stigma-of-alzheimers-disease-dementia. Published June 2018. Accessed May 28, 2019.
22. Jamieson J, Dobbs B, Charles L, et al. Forgetful, but not forgotten people of dementia: a novel, technology focused project with a humanistic touch. Geriatric Grand Rounds; October 10, 2017. Edmonton, Alberta, Canada.
23. Dobbs B, Charles L, Chan K, et al. People of Dementia. CGS 37th Annual Scientific Meeting: Integrating Care, Making an Impact. Can Geriatr J. 2017;20(3):220.
24. Government of Canada. Conference report: National Dementia Conference. https://www.canada.ca/en/services/health/publications/diseases-conditions/national-dementia-conference-report.html. Government of Canada. Published August 2018. Accessed May 28, 2019.
25. The Gerontological Society of America. Program Abstracts from the GSA 2018 Annual Scientific Meeting “The Purposes of Longer Lives.” Innovation in Aging. 2018;2(Suppl 1):143.
Automated measurements of plasma predict amyloid status
, according to research published online ahead of print June 24 in JAMA Neurology. Analyzing APOE genotype in addition to these biomarkers increases the accuracy of the prediction. This blood test thus could allow neurologists to identify patients at risk of amyloid-beta positivity who should undergo further assessment, said the authors. It also could be used to enroll amyloid-beta–positive participants in clinical trials.
In vivo PET imaging and analysis of cerebrospinal fluid (CSF) can detect amyloid-beta, but these procedures are expensive, and their availability is limited. Clinicians need readily available methods for detecting amyloid-beta, and research has indicated that blood-based biomarkers correlate with those in CSF. Fully automated immunoassays, such as the Elecsys test developed by Roche Diagnostics, have recently demonstrated high reliability and precision for CSF amyloid-beta. Using the Elecsys assay, Sebastian Palmqvist, MD, PhD, a neurologist at Skåne University Hospital in Malmö, Sweden, and colleagues sought to examine the accuracy of plasma amyloid-beta and tau, together with other blood-based biomarkers, at detecting cerebral amyloid-beta.
Testing the immunoassay in two cohorts
Dr. Palmqvist and colleagues examined participants in the prospective Swedish BioFINDER Study, which enrolled patients between July 6, 2009, and February 11, 2015. This cohort included 513 cognitively unimpaired (CU) participants, 265 participants with mild cognitive impairment (MCI), and 64 participants with Alzheimer’s disease dementia. Investigators collected blood and CSF samples at the same time from all participants. Participants’ amyloid-beta status was ascertained using the Elecsys CSF amyloid-beta 42/amyloid-beta 40 ratio. The researchers defined amyloid-beta positivity with an unbiased cutoff of less than 0.059.
Dr. Palmqvist and colleagues also examined a validation cohort that included 237 participants who had been enrolled between January 29, 2000, and October 11, 2006, in Ulm and Hannover, Germany. This group included 34 CU participants, 109 participants with MCI, and 94 participants with mild Alzheimer’s disease dementia. The investigators applied the same cutoff of CSF amyloid-beta 42/amyloid-beta 40 to define amyloid-beta positivity in this cohort as they applied to the BioFINDER cohort.
Automated immunoassay had high predictive accuracy
The mean age of the BioFINDER cohort was 72 years, and 52.5% of participants were female. Overall, 44% of this cohort was amyloid-beta positive, including 29% of CU participants, 60% of participants with MCI, and 100% of participants with Alzheimer’s dementia. The investigators found statistically significant positive correlations between all plasma and corresponding CSF biomarkers in this cohort.
Plasma amyloid-beta 42 and amyloid-beta 40 levels predicted amyloid-beta status with an area under the receiver operating characteristic curve (AUC) of 0.80. When the researchers added APOE to the model, the AUC increased significantly to 0.85. Accuracy improved slightly when the researchers added plasma tau (AUC, 0.86) or tau and neurofilament light (AUC, 0.87) to amyloid-beta 42, amyloid-beta 40, and APOE. The results were similar in CU and cognitively impaired participants, and in younger and older participants.
In the validation cohort, the mean age was 66 years, and 50.6% of participants were female. When Dr. Palmqvist and colleagues applied the plasma amyloid-beta 42 and amyloid-beta 40 model from the BioFINDER cohort to this population, they obtained a slightly higher AUC (0.86), but plasma tau did not increase predictive accuracy.
The investigators performed a cost-benefit analysis using a scenario in which 1,000 amyloid-positive participants are included in a trial and given a cost of $4,000 per participant for amyloid PET. Using plasma amyloid-beta 42, amyloid-beta 40, and APOE in this scenario reduced PET costs by as much as 30%-50%, depending on the cutoff.
Validation cohort was small
Dr. Palmqvist and colleagues acknowledged that a lack of data about APOE was a limitation of their validation analysis. Other limitations that they acknowledged were the small population size, which precluded subpopulation analysis, and the lack of improvement in predictive ability when they replicated the model that included plasma tau.
“Overall, the accuracies of the amyloid-beta 42 and amyloid-beta 40 assays are not sufficient to be used on their own as a clinical test of amyloid-beta positivity,” said Dr. Palmqvist and colleagues. “Additional assay development is needed before this can be recommended, possibly together with other blood biomarkers and screening tools in diagnostic algorithms.”
Even though additional validation studies are necessary, the present findings indicate “the potential usefulness blood assays might have, especially considering the ongoing great need to recruit large cohorts for Alzheimer’s disease drug trials in preclinical and prodromal stages,” the authors concluded.
This investigation was funded by foundations including the European Research Council, the Swedish Research Council, and the Knut and Alice Wallenberg foundation. Several authors are employees of the Roche Group. One author served on a scientific advisory board for Roche Diagnostics, and another received institutional research support from that company.
SOURCE: Palmqvist S et al. JAMA Neurol. 2019 Jun 24. doi: 10.1001/jamaneurol.2019.1632.
The investigation by Palmqvist et al. “makes several significant advancements in the field,” said Sid E. O’Bryant, PhD, professor of pharmacology and neuroscience at the University of North Texas Health Science Center in Fort Worth, in an accompanying editorial. The study’s protocol design clears the ground for a context of use of a blood screen for amyloid positivity. Also, the fully automated immunoassay “yields performance measurements that are superior to [those of] many earlier nonautomated procedures,” said Dr. O’Bryant. When Dr. Palmqvist and colleagues applied their discovery findings from a training cohort directly to a test cohort, it produced strong results. “This study suggests that the field is one step closer to the actual application of blood-based biomarkers with specific contexts of use in Alzheimer’s disease.”
The main concern about the plasma biomarkers, however, is the scalability of the methods used to measure them. “If primary care physicians are to use such a technology, the technology must have the capacity to conduct hundreds of millions of assays annually around the globe,” said Dr. O’Bryant. “A blood test for primary care must fit into the existing protocols and parameters in clinical laboratory settings. The blood collection and processing procedures are not applicable to standard clinical lab practice and will cause substantial barriers to clinical application.”
In addition, the study authors emphasize the utility of the immunoassay for primary care, but the study was designed to test for amyloid positivity, which is more appropriate for clinical trials. “No currently available drugs for patient use target amyloid,” said Dr. O’Bryant. “Therefore, this specific context of use is geared more toward clinical trial application than primary care physicians who currently need a test for the presence or absence of Alzheimer’s disease so currently available treatments and support can be put in place for patients and family members.”
Nevertheless, Dr. Palmqvist and associates have presented promising data, Dr. O’Bryant continued. The question in the field is ceasing to be whether blood biomarkers can be used in Alzheimer’s disease, and becoming how they can be used.
The investigation by Palmqvist et al. “makes several significant advancements in the field,” said Sid E. O’Bryant, PhD, professor of pharmacology and neuroscience at the University of North Texas Health Science Center in Fort Worth, in an accompanying editorial. The study’s protocol design clears the ground for a context of use of a blood screen for amyloid positivity. Also, the fully automated immunoassay “yields performance measurements that are superior to [those of] many earlier nonautomated procedures,” said Dr. O’Bryant. When Dr. Palmqvist and colleagues applied their discovery findings from a training cohort directly to a test cohort, it produced strong results. “This study suggests that the field is one step closer to the actual application of blood-based biomarkers with specific contexts of use in Alzheimer’s disease.”
The main concern about the plasma biomarkers, however, is the scalability of the methods used to measure them. “If primary care physicians are to use such a technology, the technology must have the capacity to conduct hundreds of millions of assays annually around the globe,” said Dr. O’Bryant. “A blood test for primary care must fit into the existing protocols and parameters in clinical laboratory settings. The blood collection and processing procedures are not applicable to standard clinical lab practice and will cause substantial barriers to clinical application.”
In addition, the study authors emphasize the utility of the immunoassay for primary care, but the study was designed to test for amyloid positivity, which is more appropriate for clinical trials. “No currently available drugs for patient use target amyloid,” said Dr. O’Bryant. “Therefore, this specific context of use is geared more toward clinical trial application than primary care physicians who currently need a test for the presence or absence of Alzheimer’s disease so currently available treatments and support can be put in place for patients and family members.”
Nevertheless, Dr. Palmqvist and associates have presented promising data, Dr. O’Bryant continued. The question in the field is ceasing to be whether blood biomarkers can be used in Alzheimer’s disease, and becoming how they can be used.
The investigation by Palmqvist et al. “makes several significant advancements in the field,” said Sid E. O’Bryant, PhD, professor of pharmacology and neuroscience at the University of North Texas Health Science Center in Fort Worth, in an accompanying editorial. The study’s protocol design clears the ground for a context of use of a blood screen for amyloid positivity. Also, the fully automated immunoassay “yields performance measurements that are superior to [those of] many earlier nonautomated procedures,” said Dr. O’Bryant. When Dr. Palmqvist and colleagues applied their discovery findings from a training cohort directly to a test cohort, it produced strong results. “This study suggests that the field is one step closer to the actual application of blood-based biomarkers with specific contexts of use in Alzheimer’s disease.”
The main concern about the plasma biomarkers, however, is the scalability of the methods used to measure them. “If primary care physicians are to use such a technology, the technology must have the capacity to conduct hundreds of millions of assays annually around the globe,” said Dr. O’Bryant. “A blood test for primary care must fit into the existing protocols and parameters in clinical laboratory settings. The blood collection and processing procedures are not applicable to standard clinical lab practice and will cause substantial barriers to clinical application.”
In addition, the study authors emphasize the utility of the immunoassay for primary care, but the study was designed to test for amyloid positivity, which is more appropriate for clinical trials. “No currently available drugs for patient use target amyloid,” said Dr. O’Bryant. “Therefore, this specific context of use is geared more toward clinical trial application than primary care physicians who currently need a test for the presence or absence of Alzheimer’s disease so currently available treatments and support can be put in place for patients and family members.”
Nevertheless, Dr. Palmqvist and associates have presented promising data, Dr. O’Bryant continued. The question in the field is ceasing to be whether blood biomarkers can be used in Alzheimer’s disease, and becoming how they can be used.
, according to research published online ahead of print June 24 in JAMA Neurology. Analyzing APOE genotype in addition to these biomarkers increases the accuracy of the prediction. This blood test thus could allow neurologists to identify patients at risk of amyloid-beta positivity who should undergo further assessment, said the authors. It also could be used to enroll amyloid-beta–positive participants in clinical trials.
In vivo PET imaging and analysis of cerebrospinal fluid (CSF) can detect amyloid-beta, but these procedures are expensive, and their availability is limited. Clinicians need readily available methods for detecting amyloid-beta, and research has indicated that blood-based biomarkers correlate with those in CSF. Fully automated immunoassays, such as the Elecsys test developed by Roche Diagnostics, have recently demonstrated high reliability and precision for CSF amyloid-beta. Using the Elecsys assay, Sebastian Palmqvist, MD, PhD, a neurologist at Skåne University Hospital in Malmö, Sweden, and colleagues sought to examine the accuracy of plasma amyloid-beta and tau, together with other blood-based biomarkers, at detecting cerebral amyloid-beta.
Testing the immunoassay in two cohorts
Dr. Palmqvist and colleagues examined participants in the prospective Swedish BioFINDER Study, which enrolled patients between July 6, 2009, and February 11, 2015. This cohort included 513 cognitively unimpaired (CU) participants, 265 participants with mild cognitive impairment (MCI), and 64 participants with Alzheimer’s disease dementia. Investigators collected blood and CSF samples at the same time from all participants. Participants’ amyloid-beta status was ascertained using the Elecsys CSF amyloid-beta 42/amyloid-beta 40 ratio. The researchers defined amyloid-beta positivity with an unbiased cutoff of less than 0.059.
Dr. Palmqvist and colleagues also examined a validation cohort that included 237 participants who had been enrolled between January 29, 2000, and October 11, 2006, in Ulm and Hannover, Germany. This group included 34 CU participants, 109 participants with MCI, and 94 participants with mild Alzheimer’s disease dementia. The investigators applied the same cutoff of CSF amyloid-beta 42/amyloid-beta 40 to define amyloid-beta positivity in this cohort as they applied to the BioFINDER cohort.
Automated immunoassay had high predictive accuracy
The mean age of the BioFINDER cohort was 72 years, and 52.5% of participants were female. Overall, 44% of this cohort was amyloid-beta positive, including 29% of CU participants, 60% of participants with MCI, and 100% of participants with Alzheimer’s dementia. The investigators found statistically significant positive correlations between all plasma and corresponding CSF biomarkers in this cohort.
Plasma amyloid-beta 42 and amyloid-beta 40 levels predicted amyloid-beta status with an area under the receiver operating characteristic curve (AUC) of 0.80. When the researchers added APOE to the model, the AUC increased significantly to 0.85. Accuracy improved slightly when the researchers added plasma tau (AUC, 0.86) or tau and neurofilament light (AUC, 0.87) to amyloid-beta 42, amyloid-beta 40, and APOE. The results were similar in CU and cognitively impaired participants, and in younger and older participants.
In the validation cohort, the mean age was 66 years, and 50.6% of participants were female. When Dr. Palmqvist and colleagues applied the plasma amyloid-beta 42 and amyloid-beta 40 model from the BioFINDER cohort to this population, they obtained a slightly higher AUC (0.86), but plasma tau did not increase predictive accuracy.
The investigators performed a cost-benefit analysis using a scenario in which 1,000 amyloid-positive participants are included in a trial and given a cost of $4,000 per participant for amyloid PET. Using plasma amyloid-beta 42, amyloid-beta 40, and APOE in this scenario reduced PET costs by as much as 30%-50%, depending on the cutoff.
Validation cohort was small
Dr. Palmqvist and colleagues acknowledged that a lack of data about APOE was a limitation of their validation analysis. Other limitations that they acknowledged were the small population size, which precluded subpopulation analysis, and the lack of improvement in predictive ability when they replicated the model that included plasma tau.
“Overall, the accuracies of the amyloid-beta 42 and amyloid-beta 40 assays are not sufficient to be used on their own as a clinical test of amyloid-beta positivity,” said Dr. Palmqvist and colleagues. “Additional assay development is needed before this can be recommended, possibly together with other blood biomarkers and screening tools in diagnostic algorithms.”
Even though additional validation studies are necessary, the present findings indicate “the potential usefulness blood assays might have, especially considering the ongoing great need to recruit large cohorts for Alzheimer’s disease drug trials in preclinical and prodromal stages,” the authors concluded.
This investigation was funded by foundations including the European Research Council, the Swedish Research Council, and the Knut and Alice Wallenberg foundation. Several authors are employees of the Roche Group. One author served on a scientific advisory board for Roche Diagnostics, and another received institutional research support from that company.
SOURCE: Palmqvist S et al. JAMA Neurol. 2019 Jun 24. doi: 10.1001/jamaneurol.2019.1632.
, according to research published online ahead of print June 24 in JAMA Neurology. Analyzing APOE genotype in addition to these biomarkers increases the accuracy of the prediction. This blood test thus could allow neurologists to identify patients at risk of amyloid-beta positivity who should undergo further assessment, said the authors. It also could be used to enroll amyloid-beta–positive participants in clinical trials.
In vivo PET imaging and analysis of cerebrospinal fluid (CSF) can detect amyloid-beta, but these procedures are expensive, and their availability is limited. Clinicians need readily available methods for detecting amyloid-beta, and research has indicated that blood-based biomarkers correlate with those in CSF. Fully automated immunoassays, such as the Elecsys test developed by Roche Diagnostics, have recently demonstrated high reliability and precision for CSF amyloid-beta. Using the Elecsys assay, Sebastian Palmqvist, MD, PhD, a neurologist at Skåne University Hospital in Malmö, Sweden, and colleagues sought to examine the accuracy of plasma amyloid-beta and tau, together with other blood-based biomarkers, at detecting cerebral amyloid-beta.
Testing the immunoassay in two cohorts
Dr. Palmqvist and colleagues examined participants in the prospective Swedish BioFINDER Study, which enrolled patients between July 6, 2009, and February 11, 2015. This cohort included 513 cognitively unimpaired (CU) participants, 265 participants with mild cognitive impairment (MCI), and 64 participants with Alzheimer’s disease dementia. Investigators collected blood and CSF samples at the same time from all participants. Participants’ amyloid-beta status was ascertained using the Elecsys CSF amyloid-beta 42/amyloid-beta 40 ratio. The researchers defined amyloid-beta positivity with an unbiased cutoff of less than 0.059.
Dr. Palmqvist and colleagues also examined a validation cohort that included 237 participants who had been enrolled between January 29, 2000, and October 11, 2006, in Ulm and Hannover, Germany. This group included 34 CU participants, 109 participants with MCI, and 94 participants with mild Alzheimer’s disease dementia. The investigators applied the same cutoff of CSF amyloid-beta 42/amyloid-beta 40 to define amyloid-beta positivity in this cohort as they applied to the BioFINDER cohort.
Automated immunoassay had high predictive accuracy
The mean age of the BioFINDER cohort was 72 years, and 52.5% of participants were female. Overall, 44% of this cohort was amyloid-beta positive, including 29% of CU participants, 60% of participants with MCI, and 100% of participants with Alzheimer’s dementia. The investigators found statistically significant positive correlations between all plasma and corresponding CSF biomarkers in this cohort.
Plasma amyloid-beta 42 and amyloid-beta 40 levels predicted amyloid-beta status with an area under the receiver operating characteristic curve (AUC) of 0.80. When the researchers added APOE to the model, the AUC increased significantly to 0.85. Accuracy improved slightly when the researchers added plasma tau (AUC, 0.86) or tau and neurofilament light (AUC, 0.87) to amyloid-beta 42, amyloid-beta 40, and APOE. The results were similar in CU and cognitively impaired participants, and in younger and older participants.
In the validation cohort, the mean age was 66 years, and 50.6% of participants were female. When Dr. Palmqvist and colleagues applied the plasma amyloid-beta 42 and amyloid-beta 40 model from the BioFINDER cohort to this population, they obtained a slightly higher AUC (0.86), but plasma tau did not increase predictive accuracy.
The investigators performed a cost-benefit analysis using a scenario in which 1,000 amyloid-positive participants are included in a trial and given a cost of $4,000 per participant for amyloid PET. Using plasma amyloid-beta 42, amyloid-beta 40, and APOE in this scenario reduced PET costs by as much as 30%-50%, depending on the cutoff.
Validation cohort was small
Dr. Palmqvist and colleagues acknowledged that a lack of data about APOE was a limitation of their validation analysis. Other limitations that they acknowledged were the small population size, which precluded subpopulation analysis, and the lack of improvement in predictive ability when they replicated the model that included plasma tau.
“Overall, the accuracies of the amyloid-beta 42 and amyloid-beta 40 assays are not sufficient to be used on their own as a clinical test of amyloid-beta positivity,” said Dr. Palmqvist and colleagues. “Additional assay development is needed before this can be recommended, possibly together with other blood biomarkers and screening tools in diagnostic algorithms.”
Even though additional validation studies are necessary, the present findings indicate “the potential usefulness blood assays might have, especially considering the ongoing great need to recruit large cohorts for Alzheimer’s disease drug trials in preclinical and prodromal stages,” the authors concluded.
This investigation was funded by foundations including the European Research Council, the Swedish Research Council, and the Knut and Alice Wallenberg foundation. Several authors are employees of the Roche Group. One author served on a scientific advisory board for Roche Diagnostics, and another received institutional research support from that company.
SOURCE: Palmqvist S et al. JAMA Neurol. 2019 Jun 24. doi: 10.1001/jamaneurol.2019.1632.
FROM JAMA NEUROLOGY
Anticholinergic drugs linked to dementia in older populations
Exposures to various types of anticholinergic medications were associated with a significantly increased risk of dementia in people aged 55 years or older in a large pharmacoepidemiologic study.
“This study was designed to assess the association between cumulative anticholinergic drug use and risk of dementia in a large, representative British population,” wrote Carol A. C. Coupland, PhD, of the division of primary care at the University of Nottingham (England), and colleagues. The findings were published in JAMA Internal Medicine.
The researchers conducted a large nested case-control study that included 58,769 patients with dementia and 225,574 matched controls from the QResearch database in England. Each study participant was matched to five controls based on various characteristics, including sex, age, and calendar time, among others.
Prescription data related to 56 different drugs with strong anticholinergic properties, including antipsychotics, bladder antimuscarinics, antiepileptics, antiparkinson agents, and antidepressants were used to measure drug exposure. The study data were analyzed from 2016 to 2018.
“The primary exposure was the total standardized daily doses (TSDDs) of anticholinergic drugs prescribed in the 1 to 11 years prior to the date of diagnosis of dementia or equivalent date in matched controls,” Dr. Coupland and colleagues wrote.
After analysis, the researchers found that exposure to antipsychotics (adjusted odds ratio, 1.70), bladder antimuscarinics (aOR, 1.65), antiepileptics (aOR, 1.39), antiparkinson agents (aOR, 1.52), and anticholinergic antidepressants (aOR, 1.29) was associated with an increased risk of dementia after adjustment for confounding factors.
“Associations were stronger in [dementia] cases diagnosed before the age of 80 years,” the researchers noted.
However, antihistamine, antivertigo/antiemetic, skeletal muscle relaxant, gastrointestinal antispasmodic, antiarrhythmic, and antimuscarinic bronchodilator anticholinergic agents were not associated with any increased risk of dementia.
One key limitation of the study was the absence of medication compliance assessment, which could result in exposure misclassification. Dr. Coupland and colleagues acknowledged this could underestimate some associations with medication exposure.
The stronger risk of dementia found among people who had dementia before age 80 “indicates that anticholinergic drugs should be prescribed with caution in middle-aged and older people,” they concluded.
One question that remains from the current study is whether anticholinergic drugs are a definite modifiable risk factor for Alzheimer’s disease and related dementias, Noll L. Campbell, PharmD, of Purdue University, West Lafayette, Ind., and colleagues wrote in an editorial accompanying the study by Dr. Coupland and associates (JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0676).
While a pharmacologic basis for this association has been proposed, causation has yet to be established by means of prospective randomized studies. The current supposition is that deprescribing anticholinergic medications has the potential to positively effect cholinergic neurotransmission in certain regions of the brain, which could lead to improved cognitive functioning, and lower the likelihood of developing Alzheimer’s disease and related dementias, they wrote in the editorial.
However, the discontinuation of some anticholinergic agents may pose other risks, such as worsening pain or depressive symptoms, in addition to increasing the utilization of acute care facilities. As a result, high-quality, well-designed, randomized trials are needed to better understand the long-term effects of deprescribing anticholinergic medications. These trials would help inform clinicians, patients, and policymakers about the risks and benefits of deprescribing interventions, Dr. Campbell and coauthors said.
The study was supported by the National Institute for Health Research and the University of Nottingham. The authors reported financial affiliations with ClinRisk Ltd. The authors of the editorial reported receiving support from the National Institute on Aging and the Agency for Healthcare Research and Quality. Dr. Campbell reported receiving personal fees from Astellas Pharma US.
SOURCE: Coupland C et al. JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0677
Exposures to various types of anticholinergic medications were associated with a significantly increased risk of dementia in people aged 55 years or older in a large pharmacoepidemiologic study.
“This study was designed to assess the association between cumulative anticholinergic drug use and risk of dementia in a large, representative British population,” wrote Carol A. C. Coupland, PhD, of the division of primary care at the University of Nottingham (England), and colleagues. The findings were published in JAMA Internal Medicine.
The researchers conducted a large nested case-control study that included 58,769 patients with dementia and 225,574 matched controls from the QResearch database in England. Each study participant was matched to five controls based on various characteristics, including sex, age, and calendar time, among others.
Prescription data related to 56 different drugs with strong anticholinergic properties, including antipsychotics, bladder antimuscarinics, antiepileptics, antiparkinson agents, and antidepressants were used to measure drug exposure. The study data were analyzed from 2016 to 2018.
“The primary exposure was the total standardized daily doses (TSDDs) of anticholinergic drugs prescribed in the 1 to 11 years prior to the date of diagnosis of dementia or equivalent date in matched controls,” Dr. Coupland and colleagues wrote.
After analysis, the researchers found that exposure to antipsychotics (adjusted odds ratio, 1.70), bladder antimuscarinics (aOR, 1.65), antiepileptics (aOR, 1.39), antiparkinson agents (aOR, 1.52), and anticholinergic antidepressants (aOR, 1.29) was associated with an increased risk of dementia after adjustment for confounding factors.
“Associations were stronger in [dementia] cases diagnosed before the age of 80 years,” the researchers noted.
However, antihistamine, antivertigo/antiemetic, skeletal muscle relaxant, gastrointestinal antispasmodic, antiarrhythmic, and antimuscarinic bronchodilator anticholinergic agents were not associated with any increased risk of dementia.
One key limitation of the study was the absence of medication compliance assessment, which could result in exposure misclassification. Dr. Coupland and colleagues acknowledged this could underestimate some associations with medication exposure.
The stronger risk of dementia found among people who had dementia before age 80 “indicates that anticholinergic drugs should be prescribed with caution in middle-aged and older people,” they concluded.
One question that remains from the current study is whether anticholinergic drugs are a definite modifiable risk factor for Alzheimer’s disease and related dementias, Noll L. Campbell, PharmD, of Purdue University, West Lafayette, Ind., and colleagues wrote in an editorial accompanying the study by Dr. Coupland and associates (JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0676).
While a pharmacologic basis for this association has been proposed, causation has yet to be established by means of prospective randomized studies. The current supposition is that deprescribing anticholinergic medications has the potential to positively effect cholinergic neurotransmission in certain regions of the brain, which could lead to improved cognitive functioning, and lower the likelihood of developing Alzheimer’s disease and related dementias, they wrote in the editorial.
However, the discontinuation of some anticholinergic agents may pose other risks, such as worsening pain or depressive symptoms, in addition to increasing the utilization of acute care facilities. As a result, high-quality, well-designed, randomized trials are needed to better understand the long-term effects of deprescribing anticholinergic medications. These trials would help inform clinicians, patients, and policymakers about the risks and benefits of deprescribing interventions, Dr. Campbell and coauthors said.
The study was supported by the National Institute for Health Research and the University of Nottingham. The authors reported financial affiliations with ClinRisk Ltd. The authors of the editorial reported receiving support from the National Institute on Aging and the Agency for Healthcare Research and Quality. Dr. Campbell reported receiving personal fees from Astellas Pharma US.
SOURCE: Coupland C et al. JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0677
Exposures to various types of anticholinergic medications were associated with a significantly increased risk of dementia in people aged 55 years or older in a large pharmacoepidemiologic study.
“This study was designed to assess the association between cumulative anticholinergic drug use and risk of dementia in a large, representative British population,” wrote Carol A. C. Coupland, PhD, of the division of primary care at the University of Nottingham (England), and colleagues. The findings were published in JAMA Internal Medicine.
The researchers conducted a large nested case-control study that included 58,769 patients with dementia and 225,574 matched controls from the QResearch database in England. Each study participant was matched to five controls based on various characteristics, including sex, age, and calendar time, among others.
Prescription data related to 56 different drugs with strong anticholinergic properties, including antipsychotics, bladder antimuscarinics, antiepileptics, antiparkinson agents, and antidepressants were used to measure drug exposure. The study data were analyzed from 2016 to 2018.
“The primary exposure was the total standardized daily doses (TSDDs) of anticholinergic drugs prescribed in the 1 to 11 years prior to the date of diagnosis of dementia or equivalent date in matched controls,” Dr. Coupland and colleagues wrote.
After analysis, the researchers found that exposure to antipsychotics (adjusted odds ratio, 1.70), bladder antimuscarinics (aOR, 1.65), antiepileptics (aOR, 1.39), antiparkinson agents (aOR, 1.52), and anticholinergic antidepressants (aOR, 1.29) was associated with an increased risk of dementia after adjustment for confounding factors.
“Associations were stronger in [dementia] cases diagnosed before the age of 80 years,” the researchers noted.
However, antihistamine, antivertigo/antiemetic, skeletal muscle relaxant, gastrointestinal antispasmodic, antiarrhythmic, and antimuscarinic bronchodilator anticholinergic agents were not associated with any increased risk of dementia.
One key limitation of the study was the absence of medication compliance assessment, which could result in exposure misclassification. Dr. Coupland and colleagues acknowledged this could underestimate some associations with medication exposure.
The stronger risk of dementia found among people who had dementia before age 80 “indicates that anticholinergic drugs should be prescribed with caution in middle-aged and older people,” they concluded.
One question that remains from the current study is whether anticholinergic drugs are a definite modifiable risk factor for Alzheimer’s disease and related dementias, Noll L. Campbell, PharmD, of Purdue University, West Lafayette, Ind., and colleagues wrote in an editorial accompanying the study by Dr. Coupland and associates (JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0676).
While a pharmacologic basis for this association has been proposed, causation has yet to be established by means of prospective randomized studies. The current supposition is that deprescribing anticholinergic medications has the potential to positively effect cholinergic neurotransmission in certain regions of the brain, which could lead to improved cognitive functioning, and lower the likelihood of developing Alzheimer’s disease and related dementias, they wrote in the editorial.
However, the discontinuation of some anticholinergic agents may pose other risks, such as worsening pain or depressive symptoms, in addition to increasing the utilization of acute care facilities. As a result, high-quality, well-designed, randomized trials are needed to better understand the long-term effects of deprescribing anticholinergic medications. These trials would help inform clinicians, patients, and policymakers about the risks and benefits of deprescribing interventions, Dr. Campbell and coauthors said.
The study was supported by the National Institute for Health Research and the University of Nottingham. The authors reported financial affiliations with ClinRisk Ltd. The authors of the editorial reported receiving support from the National Institute on Aging and the Agency for Healthcare Research and Quality. Dr. Campbell reported receiving personal fees from Astellas Pharma US.
SOURCE: Coupland C et al. JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0677
FROM JAMA INTERNAL MEDICINE
Hippocampal cerebral blood flow upped with antihypertensive use in Alzheimer’s
according to a new study.
Cerebral blood flow in other regions of the brain did not significantly change in patients who took the antihypertensive drug nilvadipine, according to a report on the trial published in Hypertension. Reduced cerebral blood flow is an early marker of Alzheimer’s disease, and the SPRINT MIND study suggests that intensive blood pressure control may reduce the risk of cognitive impairment.
“These findings [of the new study] not only indicate preserved cerebral autoregulation in Alzheimer’s disease but also point toward beneficial cerebrovascular effects of antihypertensive treatment,” said Jurgen A.H.R. Claassen, MD, PhD of Radboud University Medical Center in Nijmegen, the Netherlands, and coauthors. “An important question is whether this observed increase in [cerebral blood flow] translates to clinical benefits. Unfortunately, sample sizes were too small and follow-up time too short to reliably study the effects ... on structural brain measures and cognitive measures.”
Nilvadipine is a dihydropyridine calcium antagonist used to treat hypertension. In the NILVAD trial, investigators assessed the effects of nilvadipine versus placebo in approximately 500 patients with Alzheimer’s disease. The 18-month trial found no beneficial effects of nilvadipine on cognitive function, but subgroup analyses suggested a potential benefit among patients in the early stages of the disease (PLoS Med. 2018 Sep 24;15[9]:e1002660.).
The cerebral blood flow analysis was a preplanned substudy of NILVAD designed to assess how 6 months of treatment with the drug affects cerebral blood flow as measured using MRI arterial spin labeling. The researchers looked at cerebral blood flow in whole-brain gray matter and in specific regions such as the hippocampus.
The substudy analysis included 22 patients who received nilvadipine and 22 who received placebo during the randomized, double-blind study. Participants had a mean age of 72.8 years and a mean Mini-Mental State Examination score of 20.4.
At 6 months, nilvadipine lowered systolic BP by 11.5 mm Hg, and whole-brain gray matter cerebral blood flow remained stable. Blood flow to the hippocampus increased by approximately 20% among patients treated with nilvadipine – by 24.4 mL/100 g per minute to the left hippocampus and by 20.1 mL/100 g per minute to the right hippocampus.
The increased hippocampal cerebral blood flow could be related to nilvadipine’s antihypertensive effects or its effects on amyloid-beta, the authors noted.
“These findings indicate that the known decrease in [cerebral blood flow] in patients with [Alzheimer’s disease] can in some regions be reversed,” they wrote.
“Even though no medical treatment is without risk, getting treatment for high blood pressure could be important to maintain brain health in patients with Alzheimer’s disease,” Dr. Claassen said in a statement. “In the future, we need to find out whether the improvement in blood flow, especially in the hippocampus, can be used as a supportive treatment to slow down progression of Alzheimer’s disease, especially in earlier states of disease.”
The researchers wrote they lacked biomarkers to confirm Alzheimer’s disease pathology. Most of the study participants were white Europeans, which “limits extrapolation [of the findings] to other populations,” they added.
The Alzheimer’s Drug Discovery Foundation and the Dutch Alzheimer Society funded the NILVAD cerebral blood flow substudy. NILVAD was funded by the European Commission Framework 7 Program Health Theme. Dr. Claassen had no disclosures; one coauthor disclosed a pending patent for nilvadipine.
SOURCE: Claassen JAHR et al. Hypertension. 2019 Jun 17. doi: 10.1161/HYPERTENSIONAHA.119.12892.
according to a new study.
Cerebral blood flow in other regions of the brain did not significantly change in patients who took the antihypertensive drug nilvadipine, according to a report on the trial published in Hypertension. Reduced cerebral blood flow is an early marker of Alzheimer’s disease, and the SPRINT MIND study suggests that intensive blood pressure control may reduce the risk of cognitive impairment.
“These findings [of the new study] not only indicate preserved cerebral autoregulation in Alzheimer’s disease but also point toward beneficial cerebrovascular effects of antihypertensive treatment,” said Jurgen A.H.R. Claassen, MD, PhD of Radboud University Medical Center in Nijmegen, the Netherlands, and coauthors. “An important question is whether this observed increase in [cerebral blood flow] translates to clinical benefits. Unfortunately, sample sizes were too small and follow-up time too short to reliably study the effects ... on structural brain measures and cognitive measures.”
Nilvadipine is a dihydropyridine calcium antagonist used to treat hypertension. In the NILVAD trial, investigators assessed the effects of nilvadipine versus placebo in approximately 500 patients with Alzheimer’s disease. The 18-month trial found no beneficial effects of nilvadipine on cognitive function, but subgroup analyses suggested a potential benefit among patients in the early stages of the disease (PLoS Med. 2018 Sep 24;15[9]:e1002660.).
The cerebral blood flow analysis was a preplanned substudy of NILVAD designed to assess how 6 months of treatment with the drug affects cerebral blood flow as measured using MRI arterial spin labeling. The researchers looked at cerebral blood flow in whole-brain gray matter and in specific regions such as the hippocampus.
The substudy analysis included 22 patients who received nilvadipine and 22 who received placebo during the randomized, double-blind study. Participants had a mean age of 72.8 years and a mean Mini-Mental State Examination score of 20.4.
At 6 months, nilvadipine lowered systolic BP by 11.5 mm Hg, and whole-brain gray matter cerebral blood flow remained stable. Blood flow to the hippocampus increased by approximately 20% among patients treated with nilvadipine – by 24.4 mL/100 g per minute to the left hippocampus and by 20.1 mL/100 g per minute to the right hippocampus.
The increased hippocampal cerebral blood flow could be related to nilvadipine’s antihypertensive effects or its effects on amyloid-beta, the authors noted.
“These findings indicate that the known decrease in [cerebral blood flow] in patients with [Alzheimer’s disease] can in some regions be reversed,” they wrote.
“Even though no medical treatment is without risk, getting treatment for high blood pressure could be important to maintain brain health in patients with Alzheimer’s disease,” Dr. Claassen said in a statement. “In the future, we need to find out whether the improvement in blood flow, especially in the hippocampus, can be used as a supportive treatment to slow down progression of Alzheimer’s disease, especially in earlier states of disease.”
The researchers wrote they lacked biomarkers to confirm Alzheimer’s disease pathology. Most of the study participants were white Europeans, which “limits extrapolation [of the findings] to other populations,” they added.
The Alzheimer’s Drug Discovery Foundation and the Dutch Alzheimer Society funded the NILVAD cerebral blood flow substudy. NILVAD was funded by the European Commission Framework 7 Program Health Theme. Dr. Claassen had no disclosures; one coauthor disclosed a pending patent for nilvadipine.
SOURCE: Claassen JAHR et al. Hypertension. 2019 Jun 17. doi: 10.1161/HYPERTENSIONAHA.119.12892.
according to a new study.
Cerebral blood flow in other regions of the brain did not significantly change in patients who took the antihypertensive drug nilvadipine, according to a report on the trial published in Hypertension. Reduced cerebral blood flow is an early marker of Alzheimer’s disease, and the SPRINT MIND study suggests that intensive blood pressure control may reduce the risk of cognitive impairment.
“These findings [of the new study] not only indicate preserved cerebral autoregulation in Alzheimer’s disease but also point toward beneficial cerebrovascular effects of antihypertensive treatment,” said Jurgen A.H.R. Claassen, MD, PhD of Radboud University Medical Center in Nijmegen, the Netherlands, and coauthors. “An important question is whether this observed increase in [cerebral blood flow] translates to clinical benefits. Unfortunately, sample sizes were too small and follow-up time too short to reliably study the effects ... on structural brain measures and cognitive measures.”
Nilvadipine is a dihydropyridine calcium antagonist used to treat hypertension. In the NILVAD trial, investigators assessed the effects of nilvadipine versus placebo in approximately 500 patients with Alzheimer’s disease. The 18-month trial found no beneficial effects of nilvadipine on cognitive function, but subgroup analyses suggested a potential benefit among patients in the early stages of the disease (PLoS Med. 2018 Sep 24;15[9]:e1002660.).
The cerebral blood flow analysis was a preplanned substudy of NILVAD designed to assess how 6 months of treatment with the drug affects cerebral blood flow as measured using MRI arterial spin labeling. The researchers looked at cerebral blood flow in whole-brain gray matter and in specific regions such as the hippocampus.
The substudy analysis included 22 patients who received nilvadipine and 22 who received placebo during the randomized, double-blind study. Participants had a mean age of 72.8 years and a mean Mini-Mental State Examination score of 20.4.
At 6 months, nilvadipine lowered systolic BP by 11.5 mm Hg, and whole-brain gray matter cerebral blood flow remained stable. Blood flow to the hippocampus increased by approximately 20% among patients treated with nilvadipine – by 24.4 mL/100 g per minute to the left hippocampus and by 20.1 mL/100 g per minute to the right hippocampus.
The increased hippocampal cerebral blood flow could be related to nilvadipine’s antihypertensive effects or its effects on amyloid-beta, the authors noted.
“These findings indicate that the known decrease in [cerebral blood flow] in patients with [Alzheimer’s disease] can in some regions be reversed,” they wrote.
“Even though no medical treatment is without risk, getting treatment for high blood pressure could be important to maintain brain health in patients with Alzheimer’s disease,” Dr. Claassen said in a statement. “In the future, we need to find out whether the improvement in blood flow, especially in the hippocampus, can be used as a supportive treatment to slow down progression of Alzheimer’s disease, especially in earlier states of disease.”
The researchers wrote they lacked biomarkers to confirm Alzheimer’s disease pathology. Most of the study participants were white Europeans, which “limits extrapolation [of the findings] to other populations,” they added.
The Alzheimer’s Drug Discovery Foundation and the Dutch Alzheimer Society funded the NILVAD cerebral blood flow substudy. NILVAD was funded by the European Commission Framework 7 Program Health Theme. Dr. Claassen had no disclosures; one coauthor disclosed a pending patent for nilvadipine.
SOURCE: Claassen JAHR et al. Hypertension. 2019 Jun 17. doi: 10.1161/HYPERTENSIONAHA.119.12892.
FROM HYPERTENSION
A/T/N system predicts cognitive decline
Adding the amyloid/tau/neurodegeneration (A/T/N) model of dementia to a clinical model may give an incremental but still significantly increased ability to predict cognitive decline over nearly 5 years, according to findings from a longitudinal cohort study of patients without dementia at baseline.
Although the A/T/N model is still intended only for research purposes, the study came to another important conclusion: About 50% of the memory change associated with normal aging was, in fact, caused by changes associated with Alzheimer’s disease, Clifford R. Jack Jr., MD, and colleagues wrote in JAMA.
The three groups with the fastest rates of memory decline all had abnormal amyloid and either abnormal tau and/or imaging signs of neurodegeneration. “This illustrated a dominant association of memory decline with amyloidosis but only when present in combination with tauopathy, neurodegeneration, or both,” Dr. Jack of the Mayo Clinic, Rochester, Minn., and coauthors wrote.
A/T/N, also known as the National Institute on Aging and Alzheimer’s Association Research Framework, is based on objective amyloid and tau biomarkers and imaging markers of neurodegeneration and is intended to more accurately differentiate Alzheimer’s from other dementias and, potentially, to stage the disease and predict and track decline. It generates eight clinical profiles that can identify Alzheimer’s, rule it out, or include it as a possible diagnosis.
The study comprised 480 elderly individuals enrolled in the Mayo Clinic Study on Aging. Median age of the participants ranged from 67 years in one of the eight clinical profiles (A–/T–/N–) to 83 years in another (A+/T+/N+). Most (92%) were cognitively normal; the remainder had mild cognitive impairment (MCI). They were followed for a median of 4.8 years.
Both amyloid and tau were measured with PET imaging; neuropathology was represented by MRI scans of cortical thickness. Most (n = 140) were negative for all biomarkers (A–/T–/N–). The group positive for all markers (A+/T+/N+) had the largest proportion of MCI subjects (30%). The apolipoprotein E epsilon 4 (APOE4) genotype was more common among the A+ groups than it was among the A– groups (40% vs. 21%).
The individual cognitive decline trajectories varied considerably by age and within each classification group. Only 7% of the A–/T–/N– group were 80 years or older, and only 2% of the A+/N+/T+ group were younger than 70 years.
In a clinical model, age and APOE4 status were significantly associated with faster rates of memory decline. Sex, education, and a cardiovascular/metabolic model were not, however.
“The estimated rate of memory decline in a 75-year-old individual who was an APOE4 noncarrier was –0.04 z-score units per year,” the authors wrote. “An 85-year-old individual who was also an APOE4 noncarrier could be expected to have a decline of –0.08 units per year, while a 75-year-old E4 carrier could be expected to have a decline of –0.08 units per year.”
Every 10 years of additional age was associated with a significant median worsening of 0.4 on z score for memory. A 4-year difference in education was associated with a 0.6-unit higher memory score, while APOE4 carriers had a 0.3-unit lower memory score.
The addition of the A/T/N model significantly improved the prediction of cognitive decline and memory score, although the rates of decline were still considerably variable. All of the A+ groups had the fastest decline rates.
“To place the predictive utility of biomarkers in clinical context, the decline in rates of memory for A+/T+/N–, A+/T–/N+, A+/T+/N+ [abnormal amyloid plus tau or neurodegeneration] were of similar magnitude to a 20-year increase in age and were twice that associated with APOE4 carriership,” they wrote.
A total of 88 participants had a second imaging visit at a median of 15 months. Most (n = 72) had no change in the A/T/N classification. A and T classifications were more stable (98% and 97%, respectively) than was N classification (84%).
A secondary analysis compared this model with generally accepted clinical and biomarker characteristics. Prior research has shown that prevalence of abnormal A/T/N biomarker groups increased with age in the Mayo Clinic Study on Aging. The mean annual memory z-score in this cohort at 60 years was 0.02, which dropped to 0.11 by age 90.
“Forty-six percent of this increase in decline rate [–0.06] was partitioned to the increasing prevalence of abnormal A/T/N profiles, while the remaining decline [–0.07] was partitioned to age,” the investigators reported.
While A+ subjects were most likely to decline, the A+/T–/N+ group presents a conundrum, the team wrote. “A possible explanation is that these individuals have early Alzheimer’s disease [denoted by A+T–] plus neurodegeneration due to comorbid non–Alzheimer’s disease neuropathic changes.”
This is an important point because the cognitive decline of Alzheimer’s is thought to be largely associated with tauopathy, not amyloidosis. “One possible explanation is an effect of subthreshold tau in A+/T–/N+ individuals, but this is speculative. Clearer understanding of the neuropathologic bases for the A+/T–/N+ group, as well as other A/T/N groups, awaits future biomarker-autopsy correlation studies.”
SOURCE: Jack CR et al. JAMA 2019;321:2316-25.
The findings reported by Jack et al. most immediately affect research cohorts, but they raise an interesting suggestion: Only in the presence of concomitant tau, neuropathology, or both does amyloidosis appear related to an increased rate of cognitive decline when compared with non-Alzheimer’s groups.
Prevention studies lasting only a few years may be more likely to find treatment effects on disease progression in actively treated groups of those patients.
An interesting finding in the study is that A+/T–/N+ subjects showed faster rates of cognitive decline than did the A–/T–/N+ groups even though, in both cases, neurodegeneration is thought to be driven by non-Alzheimer’s pathology. What is causing disease in the A–/T–/N+ group will be unclear until the framework is enriched with other important contributors to age-related cognitive decline.
Currently, A/T/N classification – based on neuroimaging – is costly and impractical on a large scale, and so far lacks data on the added value of each specific A/T/N measure and generalizability to more diverse patient populations.
Despite these concerns, the study by Jack et al. represents an important contribution in conceptualizing Alzheimer’s disease and testing the research framework in a relatively large sample of participants.
David Wolk, MD, of the University of Pennsylvania Memory Center, Philadelphia, and colleagues’ comments here are paraphrased from an accompanying editorial (JAMA. 2019;321[23]:2289-91). Dr. Wolk reported receiving grants and personal fees from Avid/Eli Lilly and Merck; personal fees from Janssen, GE Healthcare, and Neuronix; and grants from Biogen and Functional Neuromodulation.
The findings reported by Jack et al. most immediately affect research cohorts, but they raise an interesting suggestion: Only in the presence of concomitant tau, neuropathology, or both does amyloidosis appear related to an increased rate of cognitive decline when compared with non-Alzheimer’s groups.
Prevention studies lasting only a few years may be more likely to find treatment effects on disease progression in actively treated groups of those patients.
An interesting finding in the study is that A+/T–/N+ subjects showed faster rates of cognitive decline than did the A–/T–/N+ groups even though, in both cases, neurodegeneration is thought to be driven by non-Alzheimer’s pathology. What is causing disease in the A–/T–/N+ group will be unclear until the framework is enriched with other important contributors to age-related cognitive decline.
Currently, A/T/N classification – based on neuroimaging – is costly and impractical on a large scale, and so far lacks data on the added value of each specific A/T/N measure and generalizability to more diverse patient populations.
Despite these concerns, the study by Jack et al. represents an important contribution in conceptualizing Alzheimer’s disease and testing the research framework in a relatively large sample of participants.
David Wolk, MD, of the University of Pennsylvania Memory Center, Philadelphia, and colleagues’ comments here are paraphrased from an accompanying editorial (JAMA. 2019;321[23]:2289-91). Dr. Wolk reported receiving grants and personal fees from Avid/Eli Lilly and Merck; personal fees from Janssen, GE Healthcare, and Neuronix; and grants from Biogen and Functional Neuromodulation.
The findings reported by Jack et al. most immediately affect research cohorts, but they raise an interesting suggestion: Only in the presence of concomitant tau, neuropathology, or both does amyloidosis appear related to an increased rate of cognitive decline when compared with non-Alzheimer’s groups.
Prevention studies lasting only a few years may be more likely to find treatment effects on disease progression in actively treated groups of those patients.
An interesting finding in the study is that A+/T–/N+ subjects showed faster rates of cognitive decline than did the A–/T–/N+ groups even though, in both cases, neurodegeneration is thought to be driven by non-Alzheimer’s pathology. What is causing disease in the A–/T–/N+ group will be unclear until the framework is enriched with other important contributors to age-related cognitive decline.
Currently, A/T/N classification – based on neuroimaging – is costly and impractical on a large scale, and so far lacks data on the added value of each specific A/T/N measure and generalizability to more diverse patient populations.
Despite these concerns, the study by Jack et al. represents an important contribution in conceptualizing Alzheimer’s disease and testing the research framework in a relatively large sample of participants.
David Wolk, MD, of the University of Pennsylvania Memory Center, Philadelphia, and colleagues’ comments here are paraphrased from an accompanying editorial (JAMA. 2019;321[23]:2289-91). Dr. Wolk reported receiving grants and personal fees from Avid/Eli Lilly and Merck; personal fees from Janssen, GE Healthcare, and Neuronix; and grants from Biogen and Functional Neuromodulation.
Adding the amyloid/tau/neurodegeneration (A/T/N) model of dementia to a clinical model may give an incremental but still significantly increased ability to predict cognitive decline over nearly 5 years, according to findings from a longitudinal cohort study of patients without dementia at baseline.
Although the A/T/N model is still intended only for research purposes, the study came to another important conclusion: About 50% of the memory change associated with normal aging was, in fact, caused by changes associated with Alzheimer’s disease, Clifford R. Jack Jr., MD, and colleagues wrote in JAMA.
The three groups with the fastest rates of memory decline all had abnormal amyloid and either abnormal tau and/or imaging signs of neurodegeneration. “This illustrated a dominant association of memory decline with amyloidosis but only when present in combination with tauopathy, neurodegeneration, or both,” Dr. Jack of the Mayo Clinic, Rochester, Minn., and coauthors wrote.
A/T/N, also known as the National Institute on Aging and Alzheimer’s Association Research Framework, is based on objective amyloid and tau biomarkers and imaging markers of neurodegeneration and is intended to more accurately differentiate Alzheimer’s from other dementias and, potentially, to stage the disease and predict and track decline. It generates eight clinical profiles that can identify Alzheimer’s, rule it out, or include it as a possible diagnosis.
The study comprised 480 elderly individuals enrolled in the Mayo Clinic Study on Aging. Median age of the participants ranged from 67 years in one of the eight clinical profiles (A–/T–/N–) to 83 years in another (A+/T+/N+). Most (92%) were cognitively normal; the remainder had mild cognitive impairment (MCI). They were followed for a median of 4.8 years.
Both amyloid and tau were measured with PET imaging; neuropathology was represented by MRI scans of cortical thickness. Most (n = 140) were negative for all biomarkers (A–/T–/N–). The group positive for all markers (A+/T+/N+) had the largest proportion of MCI subjects (30%). The apolipoprotein E epsilon 4 (APOE4) genotype was more common among the A+ groups than it was among the A– groups (40% vs. 21%).
The individual cognitive decline trajectories varied considerably by age and within each classification group. Only 7% of the A–/T–/N– group were 80 years or older, and only 2% of the A+/N+/T+ group were younger than 70 years.
In a clinical model, age and APOE4 status were significantly associated with faster rates of memory decline. Sex, education, and a cardiovascular/metabolic model were not, however.
“The estimated rate of memory decline in a 75-year-old individual who was an APOE4 noncarrier was –0.04 z-score units per year,” the authors wrote. “An 85-year-old individual who was also an APOE4 noncarrier could be expected to have a decline of –0.08 units per year, while a 75-year-old E4 carrier could be expected to have a decline of –0.08 units per year.”
Every 10 years of additional age was associated with a significant median worsening of 0.4 on z score for memory. A 4-year difference in education was associated with a 0.6-unit higher memory score, while APOE4 carriers had a 0.3-unit lower memory score.
The addition of the A/T/N model significantly improved the prediction of cognitive decline and memory score, although the rates of decline were still considerably variable. All of the A+ groups had the fastest decline rates.
“To place the predictive utility of biomarkers in clinical context, the decline in rates of memory for A+/T+/N–, A+/T–/N+, A+/T+/N+ [abnormal amyloid plus tau or neurodegeneration] were of similar magnitude to a 20-year increase in age and were twice that associated with APOE4 carriership,” they wrote.
A total of 88 participants had a second imaging visit at a median of 15 months. Most (n = 72) had no change in the A/T/N classification. A and T classifications were more stable (98% and 97%, respectively) than was N classification (84%).
A secondary analysis compared this model with generally accepted clinical and biomarker characteristics. Prior research has shown that prevalence of abnormal A/T/N biomarker groups increased with age in the Mayo Clinic Study on Aging. The mean annual memory z-score in this cohort at 60 years was 0.02, which dropped to 0.11 by age 90.
“Forty-six percent of this increase in decline rate [–0.06] was partitioned to the increasing prevalence of abnormal A/T/N profiles, while the remaining decline [–0.07] was partitioned to age,” the investigators reported.
While A+ subjects were most likely to decline, the A+/T–/N+ group presents a conundrum, the team wrote. “A possible explanation is that these individuals have early Alzheimer’s disease [denoted by A+T–] plus neurodegeneration due to comorbid non–Alzheimer’s disease neuropathic changes.”
This is an important point because the cognitive decline of Alzheimer’s is thought to be largely associated with tauopathy, not amyloidosis. “One possible explanation is an effect of subthreshold tau in A+/T–/N+ individuals, but this is speculative. Clearer understanding of the neuropathologic bases for the A+/T–/N+ group, as well as other A/T/N groups, awaits future biomarker-autopsy correlation studies.”
SOURCE: Jack CR et al. JAMA 2019;321:2316-25.
Adding the amyloid/tau/neurodegeneration (A/T/N) model of dementia to a clinical model may give an incremental but still significantly increased ability to predict cognitive decline over nearly 5 years, according to findings from a longitudinal cohort study of patients without dementia at baseline.
Although the A/T/N model is still intended only for research purposes, the study came to another important conclusion: About 50% of the memory change associated with normal aging was, in fact, caused by changes associated with Alzheimer’s disease, Clifford R. Jack Jr., MD, and colleagues wrote in JAMA.
The three groups with the fastest rates of memory decline all had abnormal amyloid and either abnormal tau and/or imaging signs of neurodegeneration. “This illustrated a dominant association of memory decline with amyloidosis but only when present in combination with tauopathy, neurodegeneration, or both,” Dr. Jack of the Mayo Clinic, Rochester, Minn., and coauthors wrote.
A/T/N, also known as the National Institute on Aging and Alzheimer’s Association Research Framework, is based on objective amyloid and tau biomarkers and imaging markers of neurodegeneration and is intended to more accurately differentiate Alzheimer’s from other dementias and, potentially, to stage the disease and predict and track decline. It generates eight clinical profiles that can identify Alzheimer’s, rule it out, or include it as a possible diagnosis.
The study comprised 480 elderly individuals enrolled in the Mayo Clinic Study on Aging. Median age of the participants ranged from 67 years in one of the eight clinical profiles (A–/T–/N–) to 83 years in another (A+/T+/N+). Most (92%) were cognitively normal; the remainder had mild cognitive impairment (MCI). They were followed for a median of 4.8 years.
Both amyloid and tau were measured with PET imaging; neuropathology was represented by MRI scans of cortical thickness. Most (n = 140) were negative for all biomarkers (A–/T–/N–). The group positive for all markers (A+/T+/N+) had the largest proportion of MCI subjects (30%). The apolipoprotein E epsilon 4 (APOE4) genotype was more common among the A+ groups than it was among the A– groups (40% vs. 21%).
The individual cognitive decline trajectories varied considerably by age and within each classification group. Only 7% of the A–/T–/N– group were 80 years or older, and only 2% of the A+/N+/T+ group were younger than 70 years.
In a clinical model, age and APOE4 status were significantly associated with faster rates of memory decline. Sex, education, and a cardiovascular/metabolic model were not, however.
“The estimated rate of memory decline in a 75-year-old individual who was an APOE4 noncarrier was –0.04 z-score units per year,” the authors wrote. “An 85-year-old individual who was also an APOE4 noncarrier could be expected to have a decline of –0.08 units per year, while a 75-year-old E4 carrier could be expected to have a decline of –0.08 units per year.”
Every 10 years of additional age was associated with a significant median worsening of 0.4 on z score for memory. A 4-year difference in education was associated with a 0.6-unit higher memory score, while APOE4 carriers had a 0.3-unit lower memory score.
The addition of the A/T/N model significantly improved the prediction of cognitive decline and memory score, although the rates of decline were still considerably variable. All of the A+ groups had the fastest decline rates.
“To place the predictive utility of biomarkers in clinical context, the decline in rates of memory for A+/T+/N–, A+/T–/N+, A+/T+/N+ [abnormal amyloid plus tau or neurodegeneration] were of similar magnitude to a 20-year increase in age and were twice that associated with APOE4 carriership,” they wrote.
A total of 88 participants had a second imaging visit at a median of 15 months. Most (n = 72) had no change in the A/T/N classification. A and T classifications were more stable (98% and 97%, respectively) than was N classification (84%).
A secondary analysis compared this model with generally accepted clinical and biomarker characteristics. Prior research has shown that prevalence of abnormal A/T/N biomarker groups increased with age in the Mayo Clinic Study on Aging. The mean annual memory z-score in this cohort at 60 years was 0.02, which dropped to 0.11 by age 90.
“Forty-six percent of this increase in decline rate [–0.06] was partitioned to the increasing prevalence of abnormal A/T/N profiles, while the remaining decline [–0.07] was partitioned to age,” the investigators reported.
While A+ subjects were most likely to decline, the A+/T–/N+ group presents a conundrum, the team wrote. “A possible explanation is that these individuals have early Alzheimer’s disease [denoted by A+T–] plus neurodegeneration due to comorbid non–Alzheimer’s disease neuropathic changes.”
This is an important point because the cognitive decline of Alzheimer’s is thought to be largely associated with tauopathy, not amyloidosis. “One possible explanation is an effect of subthreshold tau in A+/T–/N+ individuals, but this is speculative. Clearer understanding of the neuropathologic bases for the A+/T–/N+ group, as well as other A/T/N groups, awaits future biomarker-autopsy correlation studies.”
SOURCE: Jack CR et al. JAMA 2019;321:2316-25.
FROM JAMA