User login
Consider cyclosporine a go-to for refractory atopic dermatitis in kids
AUSTIN, TEX. – For
Cyclosporine “works quickly, and it’s very reliable,” Dr. Lio said at the annual meeting of the Society for Pediatric Dermatology. “In my experience, more than 90% of patients will see significant improvement, but there are real risks, including hypertension, kidney damage, monthly blood work, tremor, hypertrichosis, gum hypertrophy, and cancer/infection risk.”
To mitigate those risks, Dr. Lio, of the departments of dermatology and pediatrics at Northwestern University, Chicago, prescribes cyclosporine for 3-6 months at a dose of 5 mg/kg per day with a cap of 300 mg per day to “to cool things down.” He then transitions patients to phototherapy or mycophenolate right away. “Those are my two favorites,” he said. “Methotrexate can also be used, but I rarely use azathioprine.
“If you do this, you avoid most of the major risks and you can put people in a remission. More than half of the time, maybe two-thirds of the time, I get them into at least a relative remission,” Dr. Lio said.
While patients are on cyclosporine, blood pressure should be monitored each week for 4 weeks, and then monthly, he said. Draws for complete blood count, liver function tests, comprehensive metabolic panel, uric acid, and lipids should be performed monthly for 3 months, then every 8 weeks, he advised. Dr. Lio typically maintains the cyclosporine for 3 months, then tapers patients off the drug.
Patients who continue to struggle for relief might try taking cyclosporine on weekends only, a concept reported by Spanish investigators in 2015 (Pediatr Dermatol 2015 32[4]:551-2). “This involves twice-daily full dosing just on Saturdays and Sundays,” Dr. Lio said. “I now have quite a few patients doing well with this approach.”
Dr. Lio disclosed having financial ties to numerous pharmaceutical companies but none related to cyclosporine.
AUSTIN, TEX. – For
Cyclosporine “works quickly, and it’s very reliable,” Dr. Lio said at the annual meeting of the Society for Pediatric Dermatology. “In my experience, more than 90% of patients will see significant improvement, but there are real risks, including hypertension, kidney damage, monthly blood work, tremor, hypertrichosis, gum hypertrophy, and cancer/infection risk.”
To mitigate those risks, Dr. Lio, of the departments of dermatology and pediatrics at Northwestern University, Chicago, prescribes cyclosporine for 3-6 months at a dose of 5 mg/kg per day with a cap of 300 mg per day to “to cool things down.” He then transitions patients to phototherapy or mycophenolate right away. “Those are my two favorites,” he said. “Methotrexate can also be used, but I rarely use azathioprine.
“If you do this, you avoid most of the major risks and you can put people in a remission. More than half of the time, maybe two-thirds of the time, I get them into at least a relative remission,” Dr. Lio said.
While patients are on cyclosporine, blood pressure should be monitored each week for 4 weeks, and then monthly, he said. Draws for complete blood count, liver function tests, comprehensive metabolic panel, uric acid, and lipids should be performed monthly for 3 months, then every 8 weeks, he advised. Dr. Lio typically maintains the cyclosporine for 3 months, then tapers patients off the drug.
Patients who continue to struggle for relief might try taking cyclosporine on weekends only, a concept reported by Spanish investigators in 2015 (Pediatr Dermatol 2015 32[4]:551-2). “This involves twice-daily full dosing just on Saturdays and Sundays,” Dr. Lio said. “I now have quite a few patients doing well with this approach.”
Dr. Lio disclosed having financial ties to numerous pharmaceutical companies but none related to cyclosporine.
AUSTIN, TEX. – For
Cyclosporine “works quickly, and it’s very reliable,” Dr. Lio said at the annual meeting of the Society for Pediatric Dermatology. “In my experience, more than 90% of patients will see significant improvement, but there are real risks, including hypertension, kidney damage, monthly blood work, tremor, hypertrichosis, gum hypertrophy, and cancer/infection risk.”
To mitigate those risks, Dr. Lio, of the departments of dermatology and pediatrics at Northwestern University, Chicago, prescribes cyclosporine for 3-6 months at a dose of 5 mg/kg per day with a cap of 300 mg per day to “to cool things down.” He then transitions patients to phototherapy or mycophenolate right away. “Those are my two favorites,” he said. “Methotrexate can also be used, but I rarely use azathioprine.
“If you do this, you avoid most of the major risks and you can put people in a remission. More than half of the time, maybe two-thirds of the time, I get them into at least a relative remission,” Dr. Lio said.
While patients are on cyclosporine, blood pressure should be monitored each week for 4 weeks, and then monthly, he said. Draws for complete blood count, liver function tests, comprehensive metabolic panel, uric acid, and lipids should be performed monthly for 3 months, then every 8 weeks, he advised. Dr. Lio typically maintains the cyclosporine for 3 months, then tapers patients off the drug.
Patients who continue to struggle for relief might try taking cyclosporine on weekends only, a concept reported by Spanish investigators in 2015 (Pediatr Dermatol 2015 32[4]:551-2). “This involves twice-daily full dosing just on Saturdays and Sundays,” Dr. Lio said. “I now have quite a few patients doing well with this approach.”
Dr. Lio disclosed having financial ties to numerous pharmaceutical companies but none related to cyclosporine.
REPORTING FROM SPD 2019
Dupilumab found effective for adolescents with moderate to severe AD
AUSTIN, TEX. – according to results of a phase 3 study.
“Dupilumab works as effectively in adolescents as in adults,” Randy Prescilla, MD, one of the study authors, said in an interview at the annual meeting of the Society for Pediatric Dermatology. “It gives us promise that we could go into other age groups with the same optimism. We are enrolling patients in even younger age groups.”
The double-blind, placebo-controlled study analyzed the efficacy and safety of dupilumab monotherapy in patients between the ages of 12 and 17 years with moderate to severe atopic dermatitis (AD) inadequately controlled with topical therapies. In the United States, dupilumab is approved for those aged 12 years and older with moderate to severe disease inadequately controlled by topical prescription treatments or when those therapies are not advisable.
For the 16-week study, Dr. Prescilla, global medical affairs director of pediatric dermatology for Sanofi Genzyme, and colleagues randomized 251 patients to one of three groups: dupilumab every 2 weeks (200 mg if baseline weight was less than 60 kg; 300 mg if that weight was 60 kg or more); 300 mg dupilumab every 4 weeks; or placebo every 2 weeks.
At week 16, a significantly higher proportion of patients in the two drug treatment groups had Investigator’s Global Assessment scores of 0/1, compared with those in the placebo group (24.4%, 17.9%, and 2.4%) as well as a significantly higher percentage of patients who achieved at least a 75% improvement in the Eczema Area and Severity Index (EASI-75) score (41.5%, 38.1%, and 8.2%).
In addition, patients in the two drug treatment groups experienced improved percent change in least square-means on the EASI from baseline to week 16, compared with those in the placebo group (–65.9%, –64.8%, and –23.6%), the Peak Pruritus Numerical Rating Scale (–47.9%, –45.5%, and –19.0%), body surface area affected by AD (–30.1%, –33.4%, and –11.7%), and in the SCORing AD clinical tool (P less than .001 for all comparisons).
Between baseline and week 16, scores on the Children’s Dermatology Life Quality Index and Patient-Oriented Eczema Measure improved significantly in the two dupilumab groups, compared with the placebo group. The rate of skin infection was higher in the placebo group (20%), compared with 11% in the group that received dupilumab every 2 weeks and 13.3% in the group receiving the drug every 4 weeks.
Conjunctivitis occurred more frequently with dupilumab treatment (9.8% in the every-2-weeks dupilumab group, 10.8% in the every-4-weeks dupilumab group, and 4.7% in the placebo group) as did injection site reactions (8.5%, 6.0%, and 3.5%). Two adverse events, one of which was serious, occurred in the placebo group.
Dr. Prescilla acknowledged certain limitations of the study, including its small sample size and the fact that it was limited to 16 weeks. “However, smaller sample size and duration are typical for this type of study and in line with the study design of the SOLO 1 and SOLO 2 studies in adults,” he said.
On Aug. 6, the European Commission extended the marketing authorization for dupilumab in the European Union to include adolescents 12-17 years of age with moderate to severe atopic dermatitis who are candidates for systemic therapy. On the same day, Sanofi Genzyme and Regeneron announced positive topline results in a phase 3 trial in children aged 6-11 years with severe AD.
The study’s principal investigator was Amy S. Paller, MD. The study was funded by Sanofi Genzyme and Regeneron. Dr. Prescilla is an employee of Sanofi Genzyme.
AUSTIN, TEX. – according to results of a phase 3 study.
“Dupilumab works as effectively in adolescents as in adults,” Randy Prescilla, MD, one of the study authors, said in an interview at the annual meeting of the Society for Pediatric Dermatology. “It gives us promise that we could go into other age groups with the same optimism. We are enrolling patients in even younger age groups.”
The double-blind, placebo-controlled study analyzed the efficacy and safety of dupilumab monotherapy in patients between the ages of 12 and 17 years with moderate to severe atopic dermatitis (AD) inadequately controlled with topical therapies. In the United States, dupilumab is approved for those aged 12 years and older with moderate to severe disease inadequately controlled by topical prescription treatments or when those therapies are not advisable.
For the 16-week study, Dr. Prescilla, global medical affairs director of pediatric dermatology for Sanofi Genzyme, and colleagues randomized 251 patients to one of three groups: dupilumab every 2 weeks (200 mg if baseline weight was less than 60 kg; 300 mg if that weight was 60 kg or more); 300 mg dupilumab every 4 weeks; or placebo every 2 weeks.
At week 16, a significantly higher proportion of patients in the two drug treatment groups had Investigator’s Global Assessment scores of 0/1, compared with those in the placebo group (24.4%, 17.9%, and 2.4%) as well as a significantly higher percentage of patients who achieved at least a 75% improvement in the Eczema Area and Severity Index (EASI-75) score (41.5%, 38.1%, and 8.2%).
In addition, patients in the two drug treatment groups experienced improved percent change in least square-means on the EASI from baseline to week 16, compared with those in the placebo group (–65.9%, –64.8%, and –23.6%), the Peak Pruritus Numerical Rating Scale (–47.9%, –45.5%, and –19.0%), body surface area affected by AD (–30.1%, –33.4%, and –11.7%), and in the SCORing AD clinical tool (P less than .001 for all comparisons).
Between baseline and week 16, scores on the Children’s Dermatology Life Quality Index and Patient-Oriented Eczema Measure improved significantly in the two dupilumab groups, compared with the placebo group. The rate of skin infection was higher in the placebo group (20%), compared with 11% in the group that received dupilumab every 2 weeks and 13.3% in the group receiving the drug every 4 weeks.
Conjunctivitis occurred more frequently with dupilumab treatment (9.8% in the every-2-weeks dupilumab group, 10.8% in the every-4-weeks dupilumab group, and 4.7% in the placebo group) as did injection site reactions (8.5%, 6.0%, and 3.5%). Two adverse events, one of which was serious, occurred in the placebo group.
Dr. Prescilla acknowledged certain limitations of the study, including its small sample size and the fact that it was limited to 16 weeks. “However, smaller sample size and duration are typical for this type of study and in line with the study design of the SOLO 1 and SOLO 2 studies in adults,” he said.
On Aug. 6, the European Commission extended the marketing authorization for dupilumab in the European Union to include adolescents 12-17 years of age with moderate to severe atopic dermatitis who are candidates for systemic therapy. On the same day, Sanofi Genzyme and Regeneron announced positive topline results in a phase 3 trial in children aged 6-11 years with severe AD.
The study’s principal investigator was Amy S. Paller, MD. The study was funded by Sanofi Genzyme and Regeneron. Dr. Prescilla is an employee of Sanofi Genzyme.
AUSTIN, TEX. – according to results of a phase 3 study.
“Dupilumab works as effectively in adolescents as in adults,” Randy Prescilla, MD, one of the study authors, said in an interview at the annual meeting of the Society for Pediatric Dermatology. “It gives us promise that we could go into other age groups with the same optimism. We are enrolling patients in even younger age groups.”
The double-blind, placebo-controlled study analyzed the efficacy and safety of dupilumab monotherapy in patients between the ages of 12 and 17 years with moderate to severe atopic dermatitis (AD) inadequately controlled with topical therapies. In the United States, dupilumab is approved for those aged 12 years and older with moderate to severe disease inadequately controlled by topical prescription treatments or when those therapies are not advisable.
For the 16-week study, Dr. Prescilla, global medical affairs director of pediatric dermatology for Sanofi Genzyme, and colleagues randomized 251 patients to one of three groups: dupilumab every 2 weeks (200 mg if baseline weight was less than 60 kg; 300 mg if that weight was 60 kg or more); 300 mg dupilumab every 4 weeks; or placebo every 2 weeks.
At week 16, a significantly higher proportion of patients in the two drug treatment groups had Investigator’s Global Assessment scores of 0/1, compared with those in the placebo group (24.4%, 17.9%, and 2.4%) as well as a significantly higher percentage of patients who achieved at least a 75% improvement in the Eczema Area and Severity Index (EASI-75) score (41.5%, 38.1%, and 8.2%).
In addition, patients in the two drug treatment groups experienced improved percent change in least square-means on the EASI from baseline to week 16, compared with those in the placebo group (–65.9%, –64.8%, and –23.6%), the Peak Pruritus Numerical Rating Scale (–47.9%, –45.5%, and –19.0%), body surface area affected by AD (–30.1%, –33.4%, and –11.7%), and in the SCORing AD clinical tool (P less than .001 for all comparisons).
Between baseline and week 16, scores on the Children’s Dermatology Life Quality Index and Patient-Oriented Eczema Measure improved significantly in the two dupilumab groups, compared with the placebo group. The rate of skin infection was higher in the placebo group (20%), compared with 11% in the group that received dupilumab every 2 weeks and 13.3% in the group receiving the drug every 4 weeks.
Conjunctivitis occurred more frequently with dupilumab treatment (9.8% in the every-2-weeks dupilumab group, 10.8% in the every-4-weeks dupilumab group, and 4.7% in the placebo group) as did injection site reactions (8.5%, 6.0%, and 3.5%). Two adverse events, one of which was serious, occurred in the placebo group.
Dr. Prescilla acknowledged certain limitations of the study, including its small sample size and the fact that it was limited to 16 weeks. “However, smaller sample size and duration are typical for this type of study and in line with the study design of the SOLO 1 and SOLO 2 studies in adults,” he said.
On Aug. 6, the European Commission extended the marketing authorization for dupilumab in the European Union to include adolescents 12-17 years of age with moderate to severe atopic dermatitis who are candidates for systemic therapy. On the same day, Sanofi Genzyme and Regeneron announced positive topline results in a phase 3 trial in children aged 6-11 years with severe AD.
The study’s principal investigator was Amy S. Paller, MD. The study was funded by Sanofi Genzyme and Regeneron. Dr. Prescilla is an employee of Sanofi Genzyme.
REPORTING FROM SPD 2019
Rescue fantasies
In Walter Mitty moments, many of us daydream of glory: We’ll make that big discovery, score that disruptive app, homer in the bottom of the ninth to win the series. Then we wake up.
Those of us in the helping professions have fantasies, too, though fewer as times goes by. But the temptation to dream, day or night, never quite goes away. ...
Curtis is 45. He’s had eczema forever. It covers half his body. Topical steroids and courses of prednisone have failed him for decades. Maybe he’ll respond to dupilumab. Maybe his insurer will let him try.
The insurer rejects my Prior Authorization request; guidelines won’t authorize dupilumab unless the patient has failed on pimecrolimus.
Pimecrolimus?!!!
I figure – what the heck – I’ll dash off a stem-winder of a letter to the insurer’s medical director.
Esteemed Director,
Like every doctor, I spend my days filling out Prior Authorization forms. These are tedious but at least make some sense on their own terms. But your rejection of dupilumab is so silly that I must object.
My patient is 6-feet tall. Half his body has been covered with eczema for a long time. No expert could possibly have told you that someone who failed oral and topical steroids would respond to pimecrolimus. Besides, how many gallons of pimecrolimus would it take to smear all over a man this size in a useless effort to show it doesn’t work?
Cordially,
Two days later they approved dupilumab. Triumph! Excited, I call Curtis to tell him the news.
Curtis does not respond.
My staff calls three times. He doesn’t call back.
I write Curtis a letter. Nothing.
Maybe the Prior Authorization form chased away his eczema.
Not long after Curtis, Warren comes by. In his mid-50s, Warren is miserable. “I had a responsible job,” he says. “Now I feel as though my brain is disintegrating. For the last month, I’ve had worms crawling out of my pores. ...”
I don’t know about you, dear colleagues, but nothing stirs within me a deeper sense of futility than a patient with parasitical delusions.
“Here,” says Warren, on cue, “I brought some worms in,” handing me the requisite rumpled tissue filled with squiggles of mucus.
“Look, Warren,” I say, “you’re not going to like hearing this, but there are no worms coming out of you.”
“There aren’t?”
“You think you have them, but you need help realizing you don’t. You should see a psychiatrist.”
“Really?” says Warren. “If you think it would help, that would be wonderful. Could you help me find one?”
In all my years, no patient with parasitic delusions has ever responded positively to my suggesting a psychiatric referral. Maybe I can actually help this man!
A shrink I know refers me to a colleague at TweedleDum Medical Empire, who is most cordial. “Yes,” he says, “we work closely with dermatology and handle such patients all the time. Recent symptom onset does suggest an organic cause. Have him call my appointment coordinator.”
Which I do, with great excitement. Warren is enthused too. His emails express optimism and deep gratitude, catnip to a rescue fantasist.
What follows is – not much. Warren calls me. His insurer has balked, because his primary care is at TweedleDee Medical Empire. Courtney at TweedleDum should straighten it out, but she is away. For a very long time. And so forth.
Days go by. Weeks. Emails fly back and forth. Warren wavers between hope and despair. He is waiting for Courtney. I am waiting for Godot.
I put my staff on it. Three hours later they find Courtney. In person. It’s all set!
I let Warren know. And then ...
Nothing.
Warren stops answering my emails. I write the cordial psychiatrist at TweedleDum.
No response.
My batting average with delusional parasitosis remains an immaculate 0.000.
Rescuing people is tricky. You need to know a bit. You need to persevere. You need contacts. You need luck.
And the patient needs to want to be rescued.
Not for nothing do they call them Rescue Fantasies.
Now I can go back to work on that disruptive app. Just wait, my friends – it’s going to disrupt the world and change everything!
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. He had no disclosures relevant to this column. Write to him at dermnews@mdedge.com.
In Walter Mitty moments, many of us daydream of glory: We’ll make that big discovery, score that disruptive app, homer in the bottom of the ninth to win the series. Then we wake up.
Those of us in the helping professions have fantasies, too, though fewer as times goes by. But the temptation to dream, day or night, never quite goes away. ...
Curtis is 45. He’s had eczema forever. It covers half his body. Topical steroids and courses of prednisone have failed him for decades. Maybe he’ll respond to dupilumab. Maybe his insurer will let him try.
The insurer rejects my Prior Authorization request; guidelines won’t authorize dupilumab unless the patient has failed on pimecrolimus.
Pimecrolimus?!!!
I figure – what the heck – I’ll dash off a stem-winder of a letter to the insurer’s medical director.
Esteemed Director,
Like every doctor, I spend my days filling out Prior Authorization forms. These are tedious but at least make some sense on their own terms. But your rejection of dupilumab is so silly that I must object.
My patient is 6-feet tall. Half his body has been covered with eczema for a long time. No expert could possibly have told you that someone who failed oral and topical steroids would respond to pimecrolimus. Besides, how many gallons of pimecrolimus would it take to smear all over a man this size in a useless effort to show it doesn’t work?
Cordially,
Two days later they approved dupilumab. Triumph! Excited, I call Curtis to tell him the news.
Curtis does not respond.
My staff calls three times. He doesn’t call back.
I write Curtis a letter. Nothing.
Maybe the Prior Authorization form chased away his eczema.
Not long after Curtis, Warren comes by. In his mid-50s, Warren is miserable. “I had a responsible job,” he says. “Now I feel as though my brain is disintegrating. For the last month, I’ve had worms crawling out of my pores. ...”
I don’t know about you, dear colleagues, but nothing stirs within me a deeper sense of futility than a patient with parasitical delusions.
“Here,” says Warren, on cue, “I brought some worms in,” handing me the requisite rumpled tissue filled with squiggles of mucus.
“Look, Warren,” I say, “you’re not going to like hearing this, but there are no worms coming out of you.”
“There aren’t?”
“You think you have them, but you need help realizing you don’t. You should see a psychiatrist.”
“Really?” says Warren. “If you think it would help, that would be wonderful. Could you help me find one?”
In all my years, no patient with parasitic delusions has ever responded positively to my suggesting a psychiatric referral. Maybe I can actually help this man!
A shrink I know refers me to a colleague at TweedleDum Medical Empire, who is most cordial. “Yes,” he says, “we work closely with dermatology and handle such patients all the time. Recent symptom onset does suggest an organic cause. Have him call my appointment coordinator.”
Which I do, with great excitement. Warren is enthused too. His emails express optimism and deep gratitude, catnip to a rescue fantasist.
What follows is – not much. Warren calls me. His insurer has balked, because his primary care is at TweedleDee Medical Empire. Courtney at TweedleDum should straighten it out, but she is away. For a very long time. And so forth.
Days go by. Weeks. Emails fly back and forth. Warren wavers between hope and despair. He is waiting for Courtney. I am waiting for Godot.
I put my staff on it. Three hours later they find Courtney. In person. It’s all set!
I let Warren know. And then ...
Nothing.
Warren stops answering my emails. I write the cordial psychiatrist at TweedleDum.
No response.
My batting average with delusional parasitosis remains an immaculate 0.000.
Rescuing people is tricky. You need to know a bit. You need to persevere. You need contacts. You need luck.
And the patient needs to want to be rescued.
Not for nothing do they call them Rescue Fantasies.
Now I can go back to work on that disruptive app. Just wait, my friends – it’s going to disrupt the world and change everything!
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. He had no disclosures relevant to this column. Write to him at dermnews@mdedge.com.
In Walter Mitty moments, many of us daydream of glory: We’ll make that big discovery, score that disruptive app, homer in the bottom of the ninth to win the series. Then we wake up.
Those of us in the helping professions have fantasies, too, though fewer as times goes by. But the temptation to dream, day or night, never quite goes away. ...
Curtis is 45. He’s had eczema forever. It covers half his body. Topical steroids and courses of prednisone have failed him for decades. Maybe he’ll respond to dupilumab. Maybe his insurer will let him try.
The insurer rejects my Prior Authorization request; guidelines won’t authorize dupilumab unless the patient has failed on pimecrolimus.
Pimecrolimus?!!!
I figure – what the heck – I’ll dash off a stem-winder of a letter to the insurer’s medical director.
Esteemed Director,
Like every doctor, I spend my days filling out Prior Authorization forms. These are tedious but at least make some sense on their own terms. But your rejection of dupilumab is so silly that I must object.
My patient is 6-feet tall. Half his body has been covered with eczema for a long time. No expert could possibly have told you that someone who failed oral and topical steroids would respond to pimecrolimus. Besides, how many gallons of pimecrolimus would it take to smear all over a man this size in a useless effort to show it doesn’t work?
Cordially,
Two days later they approved dupilumab. Triumph! Excited, I call Curtis to tell him the news.
Curtis does not respond.
My staff calls three times. He doesn’t call back.
I write Curtis a letter. Nothing.
Maybe the Prior Authorization form chased away his eczema.
Not long after Curtis, Warren comes by. In his mid-50s, Warren is miserable. “I had a responsible job,” he says. “Now I feel as though my brain is disintegrating. For the last month, I’ve had worms crawling out of my pores. ...”
I don’t know about you, dear colleagues, but nothing stirs within me a deeper sense of futility than a patient with parasitical delusions.
“Here,” says Warren, on cue, “I brought some worms in,” handing me the requisite rumpled tissue filled with squiggles of mucus.
“Look, Warren,” I say, “you’re not going to like hearing this, but there are no worms coming out of you.”
“There aren’t?”
“You think you have them, but you need help realizing you don’t. You should see a psychiatrist.”
“Really?” says Warren. “If you think it would help, that would be wonderful. Could you help me find one?”
In all my years, no patient with parasitic delusions has ever responded positively to my suggesting a psychiatric referral. Maybe I can actually help this man!
A shrink I know refers me to a colleague at TweedleDum Medical Empire, who is most cordial. “Yes,” he says, “we work closely with dermatology and handle such patients all the time. Recent symptom onset does suggest an organic cause. Have him call my appointment coordinator.”
Which I do, with great excitement. Warren is enthused too. His emails express optimism and deep gratitude, catnip to a rescue fantasist.
What follows is – not much. Warren calls me. His insurer has balked, because his primary care is at TweedleDee Medical Empire. Courtney at TweedleDum should straighten it out, but she is away. For a very long time. And so forth.
Days go by. Weeks. Emails fly back and forth. Warren wavers between hope and despair. He is waiting for Courtney. I am waiting for Godot.
I put my staff on it. Three hours later they find Courtney. In person. It’s all set!
I let Warren know. And then ...
Nothing.
Warren stops answering my emails. I write the cordial psychiatrist at TweedleDum.
No response.
My batting average with delusional parasitosis remains an immaculate 0.000.
Rescuing people is tricky. You need to know a bit. You need to persevere. You need contacts. You need luck.
And the patient needs to want to be rescued.
Not for nothing do they call them Rescue Fantasies.
Now I can go back to work on that disruptive app. Just wait, my friends – it’s going to disrupt the world and change everything!
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. He had no disclosures relevant to this column. Write to him at dermnews@mdedge.com.
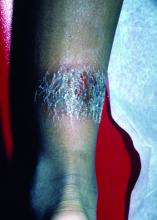
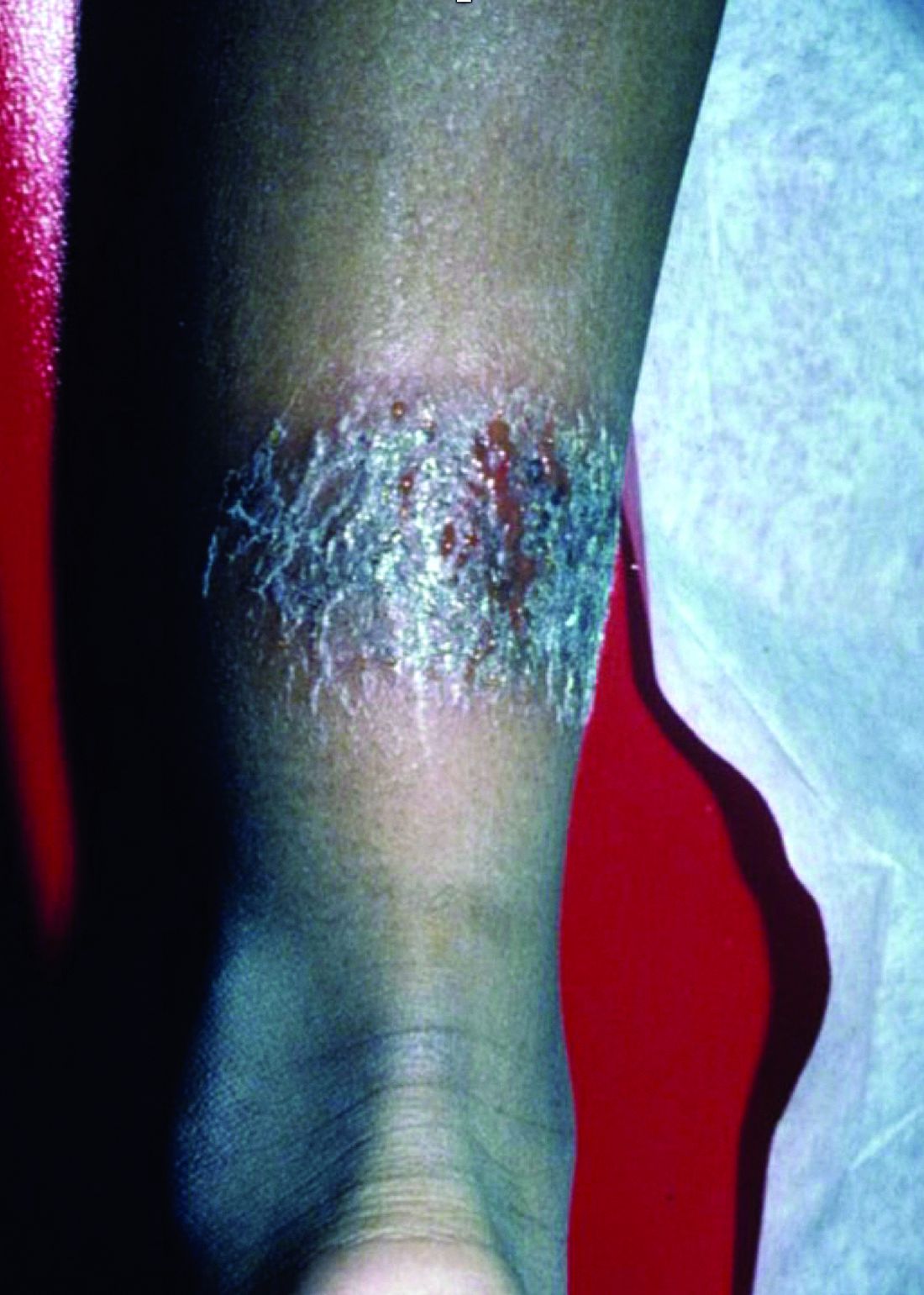
A 3-year-old is brought to the clinic for evaluation of a localized, scaling inflamed lesion on the left leg
Nummular dermatitis, or nummular eczema, is an inflammatory skin condition that is considered to be a distinctive form of idiopathic eczema, while the term also is used to describe lesional morphology associated with other conditions.
The term nummular derives from the Latin word for “coin,” as lesions are commonly annular plaques. Lesions of nummular dermatitis can be single or multiple. The typical distribution involves the extremities and, although less common, it can affect the trunk as well.
Nummular dermatitis may be associated with atopic dermatitis, or it can be an isolated condition.1 While the pathogenesis is uncertain, instigating factors include xerotic skin, insect bites, or scratches or scrapes.1Staphylococcus infection or colonization, contact allergies to metals such as nickel and less commonly mercury, sensitivity to formaldehyde or medicines such as neomycin, and sensitization to an environmental aeroallergen (such as Candida albicans, dust mites) are considered risk factors.2
The diagnosis of nummular dermatitis is clinical. Laboratory testing and/or biopsy generally are not necessary, although a bacterial culture can be considered in patients with exudative and/or crusted lesions to rule out impetigo as a primary process of secondary infection. In some cases, patch testing for allergic contact dermatitis may be useful.
The differential diagnosis of nummular dermatitis includes tinea corporis (ringworm), atopic dermatitis, allergic contact dermatitis, impetigo, and psoriasis. Tinea corporis usually presents as annular lesions with a distinct peripheral scaling, rather than the diffuse induration of nummular dermatitis. Potassium hydroxide preparation or fungal culture can identify tinea species. Nummular dermatitis may be seen in patients with atopic dermatitis, who should have typical history, morphology, and course consistent with standard diagnostic criteria. Allergic contact dermatitis can present with regional, localized eczematous plaques in areas exposed to contact allergens. Patterns of lesions in areas of contact and worsening with repeat exposures can be clues to this diagnosis. Impetigo can present with honey-colored crusted lesions and/or superficial erosions, or purulent pyoderma. Lesions can be single or multiple and generally appear less inflammatory than nummular dermatitis. Psoriasis lesions may be annular, are more common on extensor surfaces, and usually have more prominent overlying pinkish, silvery white or micaceous scale.
Management of nummular dermatitis requires strong anti-inflammatory medications, usually mid-potency or higher topical corticosteroids, along with moisturizers and limiting exposure to skin irritants. “Wet wraps,” with application of topical corticosteroids to wet skin with occlusive wet dressings can enhance response. Transition from higher strength topical corticosteroids to lower strength agents used intermittently can help achieve remission or cure. Management practices include less frequent bathing with lukewarm water, using hypoallergenic cleansers and detergents, and applying moisturizers frequently. If plaques do recur, they tend to do so in the same location and in some patients resolution may result in hyper or hypopigmentation. Refractory disease may be managed with intralesional steroid injections, or systemic medications such as methotrexate.3
Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital–San Diego and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Tracy nor Dr. Eichenfield have any relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. Pediatr Dermatol. 2012 Sep-Oct;29(5):580-3.
2. American Academy of Dermatology. Nummular Dermatitis Overview
3. Pediatr Dermatol. 2018 Sep;35(5):611-5.
Nummular dermatitis, or nummular eczema, is an inflammatory skin condition that is considered to be a distinctive form of idiopathic eczema, while the term also is used to describe lesional morphology associated with other conditions.
The term nummular derives from the Latin word for “coin,” as lesions are commonly annular plaques. Lesions of nummular dermatitis can be single or multiple. The typical distribution involves the extremities and, although less common, it can affect the trunk as well.
Nummular dermatitis may be associated with atopic dermatitis, or it can be an isolated condition.1 While the pathogenesis is uncertain, instigating factors include xerotic skin, insect bites, or scratches or scrapes.1Staphylococcus infection or colonization, contact allergies to metals such as nickel and less commonly mercury, sensitivity to formaldehyde or medicines such as neomycin, and sensitization to an environmental aeroallergen (such as Candida albicans, dust mites) are considered risk factors.2
The diagnosis of nummular dermatitis is clinical. Laboratory testing and/or biopsy generally are not necessary, although a bacterial culture can be considered in patients with exudative and/or crusted lesions to rule out impetigo as a primary process of secondary infection. In some cases, patch testing for allergic contact dermatitis may be useful.
The differential diagnosis of nummular dermatitis includes tinea corporis (ringworm), atopic dermatitis, allergic contact dermatitis, impetigo, and psoriasis. Tinea corporis usually presents as annular lesions with a distinct peripheral scaling, rather than the diffuse induration of nummular dermatitis. Potassium hydroxide preparation or fungal culture can identify tinea species. Nummular dermatitis may be seen in patients with atopic dermatitis, who should have typical history, morphology, and course consistent with standard diagnostic criteria. Allergic contact dermatitis can present with regional, localized eczematous plaques in areas exposed to contact allergens. Patterns of lesions in areas of contact and worsening with repeat exposures can be clues to this diagnosis. Impetigo can present with honey-colored crusted lesions and/or superficial erosions, or purulent pyoderma. Lesions can be single or multiple and generally appear less inflammatory than nummular dermatitis. Psoriasis lesions may be annular, are more common on extensor surfaces, and usually have more prominent overlying pinkish, silvery white or micaceous scale.
Management of nummular dermatitis requires strong anti-inflammatory medications, usually mid-potency or higher topical corticosteroids, along with moisturizers and limiting exposure to skin irritants. “Wet wraps,” with application of topical corticosteroids to wet skin with occlusive wet dressings can enhance response. Transition from higher strength topical corticosteroids to lower strength agents used intermittently can help achieve remission or cure. Management practices include less frequent bathing with lukewarm water, using hypoallergenic cleansers and detergents, and applying moisturizers frequently. If plaques do recur, they tend to do so in the same location and in some patients resolution may result in hyper or hypopigmentation. Refractory disease may be managed with intralesional steroid injections, or systemic medications such as methotrexate.3
Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital–San Diego and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Tracy nor Dr. Eichenfield have any relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. Pediatr Dermatol. 2012 Sep-Oct;29(5):580-3.
2. American Academy of Dermatology. Nummular Dermatitis Overview
3. Pediatr Dermatol. 2018 Sep;35(5):611-5.
Nummular dermatitis, or nummular eczema, is an inflammatory skin condition that is considered to be a distinctive form of idiopathic eczema, while the term also is used to describe lesional morphology associated with other conditions.
The term nummular derives from the Latin word for “coin,” as lesions are commonly annular plaques. Lesions of nummular dermatitis can be single or multiple. The typical distribution involves the extremities and, although less common, it can affect the trunk as well.
Nummular dermatitis may be associated with atopic dermatitis, or it can be an isolated condition.1 While the pathogenesis is uncertain, instigating factors include xerotic skin, insect bites, or scratches or scrapes.1Staphylococcus infection or colonization, contact allergies to metals such as nickel and less commonly mercury, sensitivity to formaldehyde or medicines such as neomycin, and sensitization to an environmental aeroallergen (such as Candida albicans, dust mites) are considered risk factors.2
The diagnosis of nummular dermatitis is clinical. Laboratory testing and/or biopsy generally are not necessary, although a bacterial culture can be considered in patients with exudative and/or crusted lesions to rule out impetigo as a primary process of secondary infection. In some cases, patch testing for allergic contact dermatitis may be useful.
The differential diagnosis of nummular dermatitis includes tinea corporis (ringworm), atopic dermatitis, allergic contact dermatitis, impetigo, and psoriasis. Tinea corporis usually presents as annular lesions with a distinct peripheral scaling, rather than the diffuse induration of nummular dermatitis. Potassium hydroxide preparation or fungal culture can identify tinea species. Nummular dermatitis may be seen in patients with atopic dermatitis, who should have typical history, morphology, and course consistent with standard diagnostic criteria. Allergic contact dermatitis can present with regional, localized eczematous plaques in areas exposed to contact allergens. Patterns of lesions in areas of contact and worsening with repeat exposures can be clues to this diagnosis. Impetigo can present with honey-colored crusted lesions and/or superficial erosions, or purulent pyoderma. Lesions can be single or multiple and generally appear less inflammatory than nummular dermatitis. Psoriasis lesions may be annular, are more common on extensor surfaces, and usually have more prominent overlying pinkish, silvery white or micaceous scale.
Management of nummular dermatitis requires strong anti-inflammatory medications, usually mid-potency or higher topical corticosteroids, along with moisturizers and limiting exposure to skin irritants. “Wet wraps,” with application of topical corticosteroids to wet skin with occlusive wet dressings can enhance response. Transition from higher strength topical corticosteroids to lower strength agents used intermittently can help achieve remission or cure. Management practices include less frequent bathing with lukewarm water, using hypoallergenic cleansers and detergents, and applying moisturizers frequently. If plaques do recur, they tend to do so in the same location and in some patients resolution may result in hyper or hypopigmentation. Refractory disease may be managed with intralesional steroid injections, or systemic medications such as methotrexate.3
Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital–San Diego and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Tracy nor Dr. Eichenfield have any relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. Pediatr Dermatol. 2012 Sep-Oct;29(5):580-3.
2. American Academy of Dermatology. Nummular Dermatitis Overview
3. Pediatr Dermatol. 2018 Sep;35(5):611-5.
On physical exam, he is noted to have a localized eczematous plaque with erythema and edema. Also, he is noted to have diffuse, fine xerosis of the bilateral lower extremities. His skin is otherwise nonremarkable.
Topical calcineurin inhibitors are an effective treatment option for pediatric periorificial dermatitis
AUSTIN, TEX. – , results from a retrospective cohort study showed.
The mainstays of treatment for POD include topical and oral antibiotics. In an interview prior to the annual meeting of the Society for Pediatric Dermatology, Ayelet Ollech, MD, said that the most common systemic agents used include erythromycin, azithromycin, and, in patients older than 8-10 years of age, minocycline or doxycycline. Topical agents, which are often used as monotherapy in mild disease, include metronidazole, clindamycin, erythromycin, sodium sulfacetamide, and, less often, azelaic acid, topical retinoids, and ivermectin. “TCIs (pimecrolimus 1% cream and tacrolimus 0.03% or 0.1% ointment) are a good steroid sparing option for POD,” said Dr. Ollech, a pediatric dermatology fellow at Ann & Robert H. Lurie Children’s Hospital of Chicago. “In the adult population, two randomized controlled studies of pimecrolimus 1% cream showed good results. In the pediatric population, there are only a few case series and case reports of TCIs for the treatment of POD.”
In what is believed to be the largest study of its kind, Dr. Ollech, Anthony J. Mancini, MD, and colleagues assessed the clinical utility of TCI in 132 pediatric patients with POD who were treated in the division of dermatology at Children’s Hospital of Chicago between 2008 and 2018. The researchers made note of epidemiologic variables, personal and family medical histories, possible triggers, duration of illness, previous treatments, distribution (periocular, perinasal, perioral, extra facial regions), severity of POD, treatment(s) prescribed, duration of therapy, clinical response, recurrences, and side effects. In an effort to capture missing data, the researchers performed follow-up via telephone for all patients who lacked appropriate follow-up documentation in the medical record.
Of the 132 patients, the female: male ratio was 1.2:1 and the median age at diagnosis was 4.2 years. About one-third of patients (33%) had involvement of one region, 38% had involvement of two regions, 26% had involvement of three regions, and 3% patients had involvement of all regions. The most common disorders on medical history were atopic dermatitis and asthma (in 29% and 17% of patients, respectively).
Dr. Ollech reported that 72 of the 132 patients (55%) had evaluable follow up data via either medical record documentation or the phone questionnaire. Of these, 67% were treated with TCI alone, 19% were treated with a combination of TCI and topical metronidazole, and 10% were treated with a combination of TCI and a systemic antibiotic. The median duration of treatment was 60 days. The researchers observed complete response in 65% of patients treated with TCI alone, in 64% of those treated with TCI and metronidazole, and in 70% of those treated with TCI and a systemic antibiotic. Adverse events attributed to TCI were rare and mild in severity.
“We were surprised that there were almost no reported side effects from the usage of TCIs as it is known that these agents can cause a burning or stinging sensation,” Dr. Ollech said. “Only one case described this side effect. We found 30% of the patients to have associated atopic dermatitis (AD) as well as a few patients with irritant dermatitis. We were also surprised how convenient the TCI treatment was for a patient who had POD and concomitant facial AD or even irritant dermatitis as an agent that can treat both. This can be very helpful for the parents that apply the medication to have a single solution to more than one rash.”
The researchers noted recurrence of POD in 14% of patients overall, including 6% of patients treated with TCI alone, 29% of patients treated with TCI and metronidazole, and 30% of patients treated with TCI and a systemic antibiotic.
Dr. Ollech acknowledged certain limitations of the study, including its retrospective design and lack of a control group. She and her colleagues reported having no financial disclosures.
SOURCE: Ollech A et al. SPD 2019, poster 23.
AUSTIN, TEX. – , results from a retrospective cohort study showed.
The mainstays of treatment for POD include topical and oral antibiotics. In an interview prior to the annual meeting of the Society for Pediatric Dermatology, Ayelet Ollech, MD, said that the most common systemic agents used include erythromycin, azithromycin, and, in patients older than 8-10 years of age, minocycline or doxycycline. Topical agents, which are often used as monotherapy in mild disease, include metronidazole, clindamycin, erythromycin, sodium sulfacetamide, and, less often, azelaic acid, topical retinoids, and ivermectin. “TCIs (pimecrolimus 1% cream and tacrolimus 0.03% or 0.1% ointment) are a good steroid sparing option for POD,” said Dr. Ollech, a pediatric dermatology fellow at Ann & Robert H. Lurie Children’s Hospital of Chicago. “In the adult population, two randomized controlled studies of pimecrolimus 1% cream showed good results. In the pediatric population, there are only a few case series and case reports of TCIs for the treatment of POD.”
In what is believed to be the largest study of its kind, Dr. Ollech, Anthony J. Mancini, MD, and colleagues assessed the clinical utility of TCI in 132 pediatric patients with POD who were treated in the division of dermatology at Children’s Hospital of Chicago between 2008 and 2018. The researchers made note of epidemiologic variables, personal and family medical histories, possible triggers, duration of illness, previous treatments, distribution (periocular, perinasal, perioral, extra facial regions), severity of POD, treatment(s) prescribed, duration of therapy, clinical response, recurrences, and side effects. In an effort to capture missing data, the researchers performed follow-up via telephone for all patients who lacked appropriate follow-up documentation in the medical record.
Of the 132 patients, the female: male ratio was 1.2:1 and the median age at diagnosis was 4.2 years. About one-third of patients (33%) had involvement of one region, 38% had involvement of two regions, 26% had involvement of three regions, and 3% patients had involvement of all regions. The most common disorders on medical history were atopic dermatitis and asthma (in 29% and 17% of patients, respectively).
Dr. Ollech reported that 72 of the 132 patients (55%) had evaluable follow up data via either medical record documentation or the phone questionnaire. Of these, 67% were treated with TCI alone, 19% were treated with a combination of TCI and topical metronidazole, and 10% were treated with a combination of TCI and a systemic antibiotic. The median duration of treatment was 60 days. The researchers observed complete response in 65% of patients treated with TCI alone, in 64% of those treated with TCI and metronidazole, and in 70% of those treated with TCI and a systemic antibiotic. Adverse events attributed to TCI were rare and mild in severity.
“We were surprised that there were almost no reported side effects from the usage of TCIs as it is known that these agents can cause a burning or stinging sensation,” Dr. Ollech said. “Only one case described this side effect. We found 30% of the patients to have associated atopic dermatitis (AD) as well as a few patients with irritant dermatitis. We were also surprised how convenient the TCI treatment was for a patient who had POD and concomitant facial AD or even irritant dermatitis as an agent that can treat both. This can be very helpful for the parents that apply the medication to have a single solution to more than one rash.”
The researchers noted recurrence of POD in 14% of patients overall, including 6% of patients treated with TCI alone, 29% of patients treated with TCI and metronidazole, and 30% of patients treated with TCI and a systemic antibiotic.
Dr. Ollech acknowledged certain limitations of the study, including its retrospective design and lack of a control group. She and her colleagues reported having no financial disclosures.
SOURCE: Ollech A et al. SPD 2019, poster 23.
AUSTIN, TEX. – , results from a retrospective cohort study showed.
The mainstays of treatment for POD include topical and oral antibiotics. In an interview prior to the annual meeting of the Society for Pediatric Dermatology, Ayelet Ollech, MD, said that the most common systemic agents used include erythromycin, azithromycin, and, in patients older than 8-10 years of age, minocycline or doxycycline. Topical agents, which are often used as monotherapy in mild disease, include metronidazole, clindamycin, erythromycin, sodium sulfacetamide, and, less often, azelaic acid, topical retinoids, and ivermectin. “TCIs (pimecrolimus 1% cream and tacrolimus 0.03% or 0.1% ointment) are a good steroid sparing option for POD,” said Dr. Ollech, a pediatric dermatology fellow at Ann & Robert H. Lurie Children’s Hospital of Chicago. “In the adult population, two randomized controlled studies of pimecrolimus 1% cream showed good results. In the pediatric population, there are only a few case series and case reports of TCIs for the treatment of POD.”
In what is believed to be the largest study of its kind, Dr. Ollech, Anthony J. Mancini, MD, and colleagues assessed the clinical utility of TCI in 132 pediatric patients with POD who were treated in the division of dermatology at Children’s Hospital of Chicago between 2008 and 2018. The researchers made note of epidemiologic variables, personal and family medical histories, possible triggers, duration of illness, previous treatments, distribution (periocular, perinasal, perioral, extra facial regions), severity of POD, treatment(s) prescribed, duration of therapy, clinical response, recurrences, and side effects. In an effort to capture missing data, the researchers performed follow-up via telephone for all patients who lacked appropriate follow-up documentation in the medical record.
Of the 132 patients, the female: male ratio was 1.2:1 and the median age at diagnosis was 4.2 years. About one-third of patients (33%) had involvement of one region, 38% had involvement of two regions, 26% had involvement of three regions, and 3% patients had involvement of all regions. The most common disorders on medical history were atopic dermatitis and asthma (in 29% and 17% of patients, respectively).
Dr. Ollech reported that 72 of the 132 patients (55%) had evaluable follow up data via either medical record documentation or the phone questionnaire. Of these, 67% were treated with TCI alone, 19% were treated with a combination of TCI and topical metronidazole, and 10% were treated with a combination of TCI and a systemic antibiotic. The median duration of treatment was 60 days. The researchers observed complete response in 65% of patients treated with TCI alone, in 64% of those treated with TCI and metronidazole, and in 70% of those treated with TCI and a systemic antibiotic. Adverse events attributed to TCI were rare and mild in severity.
“We were surprised that there were almost no reported side effects from the usage of TCIs as it is known that these agents can cause a burning or stinging sensation,” Dr. Ollech said. “Only one case described this side effect. We found 30% of the patients to have associated atopic dermatitis (AD) as well as a few patients with irritant dermatitis. We were also surprised how convenient the TCI treatment was for a patient who had POD and concomitant facial AD or even irritant dermatitis as an agent that can treat both. This can be very helpful for the parents that apply the medication to have a single solution to more than one rash.”
The researchers noted recurrence of POD in 14% of patients overall, including 6% of patients treated with TCI alone, 29% of patients treated with TCI and metronidazole, and 30% of patients treated with TCI and a systemic antibiotic.
Dr. Ollech acknowledged certain limitations of the study, including its retrospective design and lack of a control group. She and her colleagues reported having no financial disclosures.
SOURCE: Ollech A et al. SPD 2019, poster 23.
REPORTING FROM SPD 2019
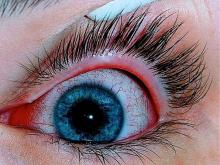
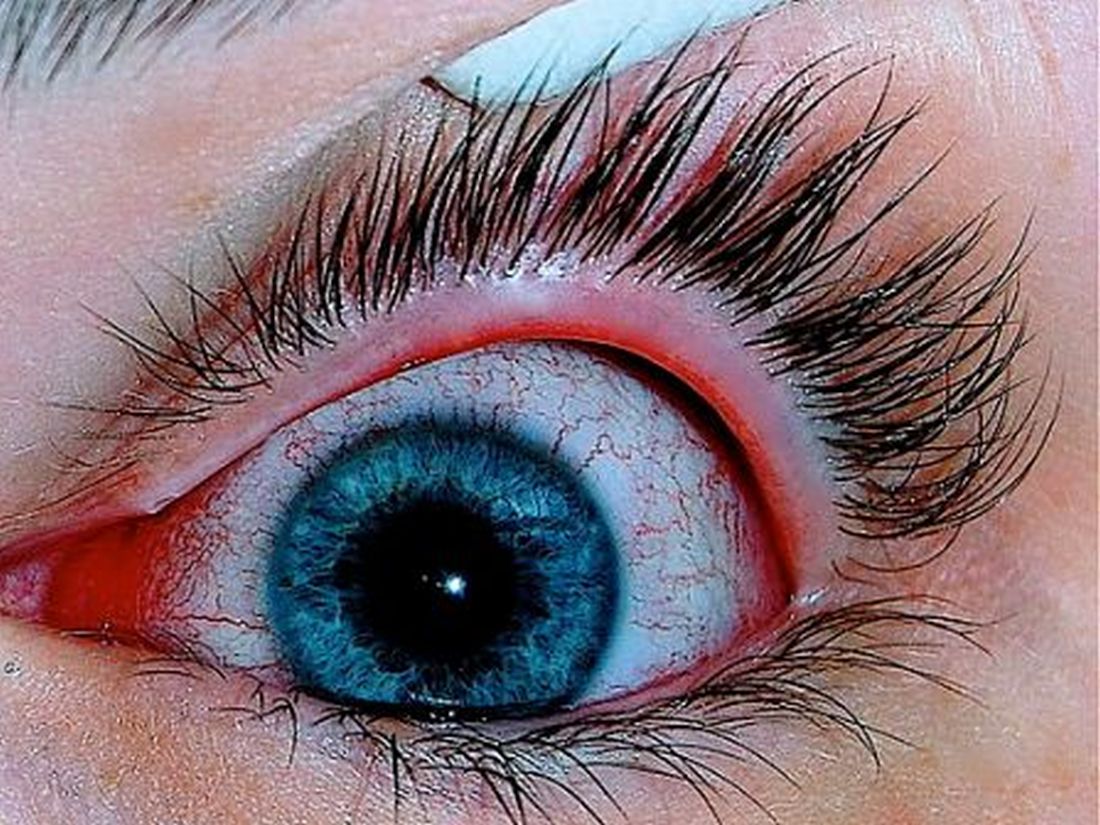
Patients with AD should routinely be asked about conjunctivitis
according to a recent position statement from the International Eczema Council.
Patients with AD who develop conjunctivitis during dupilumab treatment may experience “bilateral inflammation of the anterior conjunctiva and hyperaemia of the limbus, which may cause nodular swelling.”according to the statement, which pertains to conjunctivitis in AD patients, “with and without dupilumab therapy.” (J Eur Acad Dermatol Venereol. 2019 May 6. doi: 10.1111/jdv.15608). Currently, there are no predictive factors of conjunctivitis and no guidance in the literature on how to manage conjunctivitis associated with dupilumab, which in some cases can make it necessary to stop treatment, the authors wrote.
The International Eczema Council (IEC) polled 86 dermatologists in 22 countries who are experts in AD; 46 members responded from 19 countries, including dermatologists from Australia, Canada, Denmark, France, Germany, Japan, Korea, the Netherlands, the United Kingdom, and the United States. The questions centered on the diagnosis and management of conjunctivitis in AD patients, and whether to refer cases to an ophthalmologist. Consensus was achieved if less than 30% of participants disagreed with a statement. IEC members then met in person at a European Academy of Dermatology and Venereology meeting in Paris to discuss the results of the survey. Survey respondents noted they had seen dupilumab-associated conjunctivitis in both pediatric and adult patients.
The IEC members recommended that:
- Patients should be informed about the risks of conjunctivitis before being prescribed dupilumab.
- AD patients should be asked “routinely” about ocular complaints or symptoms.
- AD patients with conjunctivitis should be referred to an ophthalmologist for diagnosis and treatment.
- AD patients with new-onset conjunctivitis during dupilumab treatment always should be referred to an ophthalmologist, especially in more severe cases such as when they do not respond to treatment with antihistamine or artificial tears.
- Dermatologists also should rule out keratoconjunctivitis before treating with dupilumab, as it may cause keratitis and blindness.
- Patients who have had keratoconjunctivitis in the past should not be precluded from treatment with dupilumab, and those who develop conjunctivitis during treatment should be referred to an ophthalmologist – but should stay on treatment while waiting for the consult.
“It was stressed that at this moment there are also no reliable data on the course of atopic keratoconjunctivitis and vernal keratoconjunctivitis during dupilumab treatment,” according to Jacob P. Thyssen, MD, PhD, Herlev and Gentofte Hospital, Hellerup, Denmark, and coauthors. These patients “should be carefully monitored by an ophthalmologist before and during treatment with dupilumab.”
The recommendations also centered around which treatments should be initiated by dermatologists, and which should be referred to ophthalmologists. Those patients with conjunctivitis should receive eye drops, eye ointment, or oral antihistamines from their dermatologists before an ophthalmology referral, the IEC members said. Dermatologists also should perform, or refer patients for, skin prick testing or specific IgE testing for aeroallergens in patients with AD who have conjunctivitis, and patch testing with an “ophthalmologic series, and native eye drops/ointments to diagnose possible delayed type hypersensitivity reactions to topical ingredients,” they added.
Among the treatments for conjunctivitis best left to ophthalmologists are cyclosporine, tacrolimus, or corticosteroid eye drops.
“Despite the more limited experience with eye drops by dermatologists, rapid access to ophthalmological service may be difficult, sometimes warranting a short course of corticosteroid eye drops without ophthalmological consultations,” Dr. Thyssen and associates said. “However, persistent or recurrent conjunctivitis requiring repeated or prolonged use of corticosteroid, tacrolimus, and ciclosporin-containing eye drops, must be managed by an ophthalmologist, given the risk of glaucoma, cataract, and infections.”
“The AD severity, conjunctivitis severity, possible contraindications, possible effect of dupilumab therapy on concomitant asthma or other comorbidities, as well as other treatment options, should be considered on an individual patient basis,” the authors concluded.
The IEC survey was limited by the small survey response and reliance on expert opinion.
The authors reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speakers bureau positions, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. No funding was obtained for the study.
This article was updated 7/17/19.
according to a recent position statement from the International Eczema Council.
Patients with AD who develop conjunctivitis during dupilumab treatment may experience “bilateral inflammation of the anterior conjunctiva and hyperaemia of the limbus, which may cause nodular swelling.”according to the statement, which pertains to conjunctivitis in AD patients, “with and without dupilumab therapy.” (J Eur Acad Dermatol Venereol. 2019 May 6. doi: 10.1111/jdv.15608). Currently, there are no predictive factors of conjunctivitis and no guidance in the literature on how to manage conjunctivitis associated with dupilumab, which in some cases can make it necessary to stop treatment, the authors wrote.
The International Eczema Council (IEC) polled 86 dermatologists in 22 countries who are experts in AD; 46 members responded from 19 countries, including dermatologists from Australia, Canada, Denmark, France, Germany, Japan, Korea, the Netherlands, the United Kingdom, and the United States. The questions centered on the diagnosis and management of conjunctivitis in AD patients, and whether to refer cases to an ophthalmologist. Consensus was achieved if less than 30% of participants disagreed with a statement. IEC members then met in person at a European Academy of Dermatology and Venereology meeting in Paris to discuss the results of the survey. Survey respondents noted they had seen dupilumab-associated conjunctivitis in both pediatric and adult patients.
The IEC members recommended that:
- Patients should be informed about the risks of conjunctivitis before being prescribed dupilumab.
- AD patients should be asked “routinely” about ocular complaints or symptoms.
- AD patients with conjunctivitis should be referred to an ophthalmologist for diagnosis and treatment.
- AD patients with new-onset conjunctivitis during dupilumab treatment always should be referred to an ophthalmologist, especially in more severe cases such as when they do not respond to treatment with antihistamine or artificial tears.
- Dermatologists also should rule out keratoconjunctivitis before treating with dupilumab, as it may cause keratitis and blindness.
- Patients who have had keratoconjunctivitis in the past should not be precluded from treatment with dupilumab, and those who develop conjunctivitis during treatment should be referred to an ophthalmologist – but should stay on treatment while waiting for the consult.
“It was stressed that at this moment there are also no reliable data on the course of atopic keratoconjunctivitis and vernal keratoconjunctivitis during dupilumab treatment,” according to Jacob P. Thyssen, MD, PhD, Herlev and Gentofte Hospital, Hellerup, Denmark, and coauthors. These patients “should be carefully monitored by an ophthalmologist before and during treatment with dupilumab.”
The recommendations also centered around which treatments should be initiated by dermatologists, and which should be referred to ophthalmologists. Those patients with conjunctivitis should receive eye drops, eye ointment, or oral antihistamines from their dermatologists before an ophthalmology referral, the IEC members said. Dermatologists also should perform, or refer patients for, skin prick testing or specific IgE testing for aeroallergens in patients with AD who have conjunctivitis, and patch testing with an “ophthalmologic series, and native eye drops/ointments to diagnose possible delayed type hypersensitivity reactions to topical ingredients,” they added.
Among the treatments for conjunctivitis best left to ophthalmologists are cyclosporine, tacrolimus, or corticosteroid eye drops.
“Despite the more limited experience with eye drops by dermatologists, rapid access to ophthalmological service may be difficult, sometimes warranting a short course of corticosteroid eye drops without ophthalmological consultations,” Dr. Thyssen and associates said. “However, persistent or recurrent conjunctivitis requiring repeated or prolonged use of corticosteroid, tacrolimus, and ciclosporin-containing eye drops, must be managed by an ophthalmologist, given the risk of glaucoma, cataract, and infections.”
“The AD severity, conjunctivitis severity, possible contraindications, possible effect of dupilumab therapy on concomitant asthma or other comorbidities, as well as other treatment options, should be considered on an individual patient basis,” the authors concluded.
The IEC survey was limited by the small survey response and reliance on expert opinion.
The authors reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speakers bureau positions, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. No funding was obtained for the study.
This article was updated 7/17/19.
according to a recent position statement from the International Eczema Council.
Patients with AD who develop conjunctivitis during dupilumab treatment may experience “bilateral inflammation of the anterior conjunctiva and hyperaemia of the limbus, which may cause nodular swelling.”according to the statement, which pertains to conjunctivitis in AD patients, “with and without dupilumab therapy.” (J Eur Acad Dermatol Venereol. 2019 May 6. doi: 10.1111/jdv.15608). Currently, there are no predictive factors of conjunctivitis and no guidance in the literature on how to manage conjunctivitis associated with dupilumab, which in some cases can make it necessary to stop treatment, the authors wrote.
The International Eczema Council (IEC) polled 86 dermatologists in 22 countries who are experts in AD; 46 members responded from 19 countries, including dermatologists from Australia, Canada, Denmark, France, Germany, Japan, Korea, the Netherlands, the United Kingdom, and the United States. The questions centered on the diagnosis and management of conjunctivitis in AD patients, and whether to refer cases to an ophthalmologist. Consensus was achieved if less than 30% of participants disagreed with a statement. IEC members then met in person at a European Academy of Dermatology and Venereology meeting in Paris to discuss the results of the survey. Survey respondents noted they had seen dupilumab-associated conjunctivitis in both pediatric and adult patients.
The IEC members recommended that:
- Patients should be informed about the risks of conjunctivitis before being prescribed dupilumab.
- AD patients should be asked “routinely” about ocular complaints or symptoms.
- AD patients with conjunctivitis should be referred to an ophthalmologist for diagnosis and treatment.
- AD patients with new-onset conjunctivitis during dupilumab treatment always should be referred to an ophthalmologist, especially in more severe cases such as when they do not respond to treatment with antihistamine or artificial tears.
- Dermatologists also should rule out keratoconjunctivitis before treating with dupilumab, as it may cause keratitis and blindness.
- Patients who have had keratoconjunctivitis in the past should not be precluded from treatment with dupilumab, and those who develop conjunctivitis during treatment should be referred to an ophthalmologist – but should stay on treatment while waiting for the consult.
“It was stressed that at this moment there are also no reliable data on the course of atopic keratoconjunctivitis and vernal keratoconjunctivitis during dupilumab treatment,” according to Jacob P. Thyssen, MD, PhD, Herlev and Gentofte Hospital, Hellerup, Denmark, and coauthors. These patients “should be carefully monitored by an ophthalmologist before and during treatment with dupilumab.”
The recommendations also centered around which treatments should be initiated by dermatologists, and which should be referred to ophthalmologists. Those patients with conjunctivitis should receive eye drops, eye ointment, or oral antihistamines from their dermatologists before an ophthalmology referral, the IEC members said. Dermatologists also should perform, or refer patients for, skin prick testing or specific IgE testing for aeroallergens in patients with AD who have conjunctivitis, and patch testing with an “ophthalmologic series, and native eye drops/ointments to diagnose possible delayed type hypersensitivity reactions to topical ingredients,” they added.
Among the treatments for conjunctivitis best left to ophthalmologists are cyclosporine, tacrolimus, or corticosteroid eye drops.
“Despite the more limited experience with eye drops by dermatologists, rapid access to ophthalmological service may be difficult, sometimes warranting a short course of corticosteroid eye drops without ophthalmological consultations,” Dr. Thyssen and associates said. “However, persistent or recurrent conjunctivitis requiring repeated or prolonged use of corticosteroid, tacrolimus, and ciclosporin-containing eye drops, must be managed by an ophthalmologist, given the risk of glaucoma, cataract, and infections.”
“The AD severity, conjunctivitis severity, possible contraindications, possible effect of dupilumab therapy on concomitant asthma or other comorbidities, as well as other treatment options, should be considered on an individual patient basis,” the authors concluded.
The IEC survey was limited by the small survey response and reliance on expert opinion.
The authors reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speakers bureau positions, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. No funding was obtained for the study.
This article was updated 7/17/19.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Neonatal ICU stay found ‘protective’ against risk for developing atopic dermatitis
AUSTIN – The
“While more time in the NICU is associated with a lesser risk of developing atopic dermatitis, we certainly do not want to keep infants in the NICU longer in order to lower their risk of atopic dermatitis,” the study’s first author, Jennifer J. Schoch, MD, said in an interview prior to the annual meeting of the Society for Pediatric Dermatology. “Instead, we need to work on understanding the mechanisms behind this relationship. For example, are there certain exposures in the NICU that influence the cutaneous immunity to ultimately reduce the risk of atopic dermatitis?”
According to Dr. Schoch, a pediatric dermatologist at the University of Florida, Gainesville, the medical literature has been conflicted regarding the relationship between prematurity and eczema. A recent meta-analysis of 18 studies found an association between very preterm birth and a decreased risk of eczema, yet the risk became insignificant among children born moderately preterm (J Am Acad Dermatol. 2018;78[6]:1142-8). However, the factors contributing to this relationship are not well understood.
In an effort to explore the infant, maternal, and environmental factors of infants who developed AD, compared with infants who did not, Dr. Schoch and colleagues evaluated infants who were born at University of Florida Health from June 1, 2011, to April 30, 2017; had at least two well-child visits; and had at least one visit at 300 days old or later. The researchers included 4,016 mother-infant dyads in the study. Atopic dermatitis was diagnosed in 26.5% of the infants. Factors significantly associated with the incidence of AD were delivery mode (P = .0127), NICU stay (P = .0001), gestational age (P = .0006), and birth weight (P = .0020). Specifically, infants had a higher risk of developing AD if they were delivered vaginally, did not stay in the NICU, had a higher gestational age, or had a higher birth weight. Extremely preterm (less than 28 weeks’ gestation) and very preterm (28 to less than 32 weeks’ gestation) infants had the lowest rates of AD, at 10.9% and 19%, respectively.
When the researchers adjusted for other variables to their model, only length of stay in the NICU was related to the development of AD. Specifically, infants who spent more time in the NICU had a lower risk of developing atopic dermatitis (P = .0039).
“We were surprised to find that the length of stay in the neonatal intensive care unit was the strongest protective factor against the future development of eczema,” Dr. Schoch said. “Instead of this relationship being mediated by gestational age or birth weight, it was how much time the infants spent in the NICU that seemed to ‘protect’ from future eczema.”
She acknowledged certain limitations of the study, including its retrospective design with data gathered from electronic medical records. Also, “diagnosis was determined by ICD-9 or ICD-10 code, and not confirmed by dermatologists,” she said.
In their abstract, the researchers wrote that the finding highlights “the importance of early life interactions between the microbiome, developing cutaneous immunity, and the evolving skin barrier of the preterm infant. The skin microbiome of premature infants differs from full-term infants, in that the premature infant cutaneous microbiome is dominated by Staphylococcus species” (Microbiome. 2018;6[1]:98). They added that “the early presence of Staphylococcus on the skin may confer protection.”
Dr. Schoch reported having no relevant financial disclosures.
SOURCE: Schoch J et al. SPD 2019, Poster 2.
AUSTIN – The
“While more time in the NICU is associated with a lesser risk of developing atopic dermatitis, we certainly do not want to keep infants in the NICU longer in order to lower their risk of atopic dermatitis,” the study’s first author, Jennifer J. Schoch, MD, said in an interview prior to the annual meeting of the Society for Pediatric Dermatology. “Instead, we need to work on understanding the mechanisms behind this relationship. For example, are there certain exposures in the NICU that influence the cutaneous immunity to ultimately reduce the risk of atopic dermatitis?”
According to Dr. Schoch, a pediatric dermatologist at the University of Florida, Gainesville, the medical literature has been conflicted regarding the relationship between prematurity and eczema. A recent meta-analysis of 18 studies found an association between very preterm birth and a decreased risk of eczema, yet the risk became insignificant among children born moderately preterm (J Am Acad Dermatol. 2018;78[6]:1142-8). However, the factors contributing to this relationship are not well understood.
In an effort to explore the infant, maternal, and environmental factors of infants who developed AD, compared with infants who did not, Dr. Schoch and colleagues evaluated infants who were born at University of Florida Health from June 1, 2011, to April 30, 2017; had at least two well-child visits; and had at least one visit at 300 days old or later. The researchers included 4,016 mother-infant dyads in the study. Atopic dermatitis was diagnosed in 26.5% of the infants. Factors significantly associated with the incidence of AD were delivery mode (P = .0127), NICU stay (P = .0001), gestational age (P = .0006), and birth weight (P = .0020). Specifically, infants had a higher risk of developing AD if they were delivered vaginally, did not stay in the NICU, had a higher gestational age, or had a higher birth weight. Extremely preterm (less than 28 weeks’ gestation) and very preterm (28 to less than 32 weeks’ gestation) infants had the lowest rates of AD, at 10.9% and 19%, respectively.
When the researchers adjusted for other variables to their model, only length of stay in the NICU was related to the development of AD. Specifically, infants who spent more time in the NICU had a lower risk of developing atopic dermatitis (P = .0039).
“We were surprised to find that the length of stay in the neonatal intensive care unit was the strongest protective factor against the future development of eczema,” Dr. Schoch said. “Instead of this relationship being mediated by gestational age or birth weight, it was how much time the infants spent in the NICU that seemed to ‘protect’ from future eczema.”
She acknowledged certain limitations of the study, including its retrospective design with data gathered from electronic medical records. Also, “diagnosis was determined by ICD-9 or ICD-10 code, and not confirmed by dermatologists,” she said.
In their abstract, the researchers wrote that the finding highlights “the importance of early life interactions between the microbiome, developing cutaneous immunity, and the evolving skin barrier of the preterm infant. The skin microbiome of premature infants differs from full-term infants, in that the premature infant cutaneous microbiome is dominated by Staphylococcus species” (Microbiome. 2018;6[1]:98). They added that “the early presence of Staphylococcus on the skin may confer protection.”
Dr. Schoch reported having no relevant financial disclosures.
SOURCE: Schoch J et al. SPD 2019, Poster 2.
AUSTIN – The
“While more time in the NICU is associated with a lesser risk of developing atopic dermatitis, we certainly do not want to keep infants in the NICU longer in order to lower their risk of atopic dermatitis,” the study’s first author, Jennifer J. Schoch, MD, said in an interview prior to the annual meeting of the Society for Pediatric Dermatology. “Instead, we need to work on understanding the mechanisms behind this relationship. For example, are there certain exposures in the NICU that influence the cutaneous immunity to ultimately reduce the risk of atopic dermatitis?”
According to Dr. Schoch, a pediatric dermatologist at the University of Florida, Gainesville, the medical literature has been conflicted regarding the relationship between prematurity and eczema. A recent meta-analysis of 18 studies found an association between very preterm birth and a decreased risk of eczema, yet the risk became insignificant among children born moderately preterm (J Am Acad Dermatol. 2018;78[6]:1142-8). However, the factors contributing to this relationship are not well understood.
In an effort to explore the infant, maternal, and environmental factors of infants who developed AD, compared with infants who did not, Dr. Schoch and colleagues evaluated infants who were born at University of Florida Health from June 1, 2011, to April 30, 2017; had at least two well-child visits; and had at least one visit at 300 days old or later. The researchers included 4,016 mother-infant dyads in the study. Atopic dermatitis was diagnosed in 26.5% of the infants. Factors significantly associated with the incidence of AD were delivery mode (P = .0127), NICU stay (P = .0001), gestational age (P = .0006), and birth weight (P = .0020). Specifically, infants had a higher risk of developing AD if they were delivered vaginally, did not stay in the NICU, had a higher gestational age, or had a higher birth weight. Extremely preterm (less than 28 weeks’ gestation) and very preterm (28 to less than 32 weeks’ gestation) infants had the lowest rates of AD, at 10.9% and 19%, respectively.
When the researchers adjusted for other variables to their model, only length of stay in the NICU was related to the development of AD. Specifically, infants who spent more time in the NICU had a lower risk of developing atopic dermatitis (P = .0039).
“We were surprised to find that the length of stay in the neonatal intensive care unit was the strongest protective factor against the future development of eczema,” Dr. Schoch said. “Instead of this relationship being mediated by gestational age or birth weight, it was how much time the infants spent in the NICU that seemed to ‘protect’ from future eczema.”
She acknowledged certain limitations of the study, including its retrospective design with data gathered from electronic medical records. Also, “diagnosis was determined by ICD-9 or ICD-10 code, and not confirmed by dermatologists,” she said.
In their abstract, the researchers wrote that the finding highlights “the importance of early life interactions between the microbiome, developing cutaneous immunity, and the evolving skin barrier of the preterm infant. The skin microbiome of premature infants differs from full-term infants, in that the premature infant cutaneous microbiome is dominated by Staphylococcus species” (Microbiome. 2018;6[1]:98). They added that “the early presence of Staphylococcus on the skin may confer protection.”
Dr. Schoch reported having no relevant financial disclosures.
SOURCE: Schoch J et al. SPD 2019, Poster 2.
REPORTING FROM SPD 2019
Key clinical point: Preterm infants develop atopic dermatitis less often than full term infants.
Major finding: Infants that spent more time in the neonatal ICU had a lower risk of developing atopic dermatitis (P = .0039).
Study details: A single-center study of 4,016 mother-infant dyads.
Disclosures: Dr. Schoch reported having no relevant financial disclosures.
Source: Schoch J et al. SPD 2019, Poster 2.
Round 1: Biologics, JAK inhibitors training up for atopic dermatitis ‘boxing match’
MILAN – Get ready for a “boxing match” (AD), Thomas Bieber, MD, PhD, said in an oral presentation at the World Congress of Dermatology.
“In one corner of the ring you will have the biologics, and in the other corner of the ring, you will have the Janus kinase (JAK) inhibitors and some other small molecules,” said Dr. Bieber, medical director of dermatology and allergy, at University Hospital Bonn, Germany.
There will be no winner-take-all scenario, since AD is a heterogeneous disease for which a variety of treatments will be needed, Dr. Bieber noted. However, he said, there are clear differences in mode of action, safety, mode of application, price, and more that will be important as dermatologists weigh the choice of biologics versus JAK inhibitors for a specific patient.
“Currently, dupilumab is the only one that is on the market (in the European Union) and this molecule is really a revolution for us,” Dr. Bieber said at the meeting. “But we will see in the next years, many, many other molecules coming up.”
Beyond dupilumab (Dupixent), the interleukin-4 (IL-4) receptor alpha antagonist approved by the Food and Drug Administration in 2017, biologics under development include tralokinumab, lebrikizumab, and nemolizumab, among others. Development on the small molecule side includes agents such as ZPL-389, a histamine H4 receptor agonist, but is dominated by JAK inhibitors such as baricitinib(Olumiant), approved by the FDA in 2018 for rheumatoid arthritis; upadacitinib; and abrocitinib, according to Dr. Bieber.
There are advantages and disadvantages to each class of molecule, he said. For example, JAK inhibitors act very rapidly to control itch and improve exacerbations, while biologics are “much slower” and provide delayed control of inflammation, he said. In terms of safety, by contrast, biologics probably offer a “better” benefit-to-risk ratio, with no risk for drug-drug interactions and no monitoring needed, while JAK inhibitors appear to have a “narrow” therapeutic window, with a risk for drug-drug interactions and a need for some monitoring, he said.
JAK inhibitors come in a tablet formulation, which, from the patient perspective, may be more convenient and acceptable compared with repeated injections of biologics every 2 or 4 weeks, Dr. Bieber noted. Another point potentially in favor of JAK inhibitors is price: “I think the current situation tells us clearly that the biologics are the more expensive drugs, while the small molecules typically are much less expensive,” he said.
One theoretical advantage of biologics, he said, is the potential for disease modification, while the disease-modifying potential of JAK inhibitors is unclear, as of yet. “We have not had enough experience with all these molecules in order to understand how we can do that particular job, in terms of switching off the disease,” he said.
In terms of the need for patient stratification in the future, it’s a draw between biologics and JAK inhibitors, Dr. Bieber said. “You will not see any of these drugs doing 100 percent of the job in 100 percent of the patients, so to me, currently from my understanding of this disease, there is no one-size-fits-all molecule.”
Toward that end, machine learning and artificial intelligence may aid in evaluating large amounts of data to better understand AD phenotypes. “That’s really something that is a particular kind of challenge in the next years, in order to improve at-the-end drug development, and the management of our patients,” he concluded.
Dr. Bieber reported disclosures related to LEO Pharmaceuticals, Regeneron, and Sanofi.
MILAN – Get ready for a “boxing match” (AD), Thomas Bieber, MD, PhD, said in an oral presentation at the World Congress of Dermatology.
“In one corner of the ring you will have the biologics, and in the other corner of the ring, you will have the Janus kinase (JAK) inhibitors and some other small molecules,” said Dr. Bieber, medical director of dermatology and allergy, at University Hospital Bonn, Germany.
There will be no winner-take-all scenario, since AD is a heterogeneous disease for which a variety of treatments will be needed, Dr. Bieber noted. However, he said, there are clear differences in mode of action, safety, mode of application, price, and more that will be important as dermatologists weigh the choice of biologics versus JAK inhibitors for a specific patient.
“Currently, dupilumab is the only one that is on the market (in the European Union) and this molecule is really a revolution for us,” Dr. Bieber said at the meeting. “But we will see in the next years, many, many other molecules coming up.”
Beyond dupilumab (Dupixent), the interleukin-4 (IL-4) receptor alpha antagonist approved by the Food and Drug Administration in 2017, biologics under development include tralokinumab, lebrikizumab, and nemolizumab, among others. Development on the small molecule side includes agents such as ZPL-389, a histamine H4 receptor agonist, but is dominated by JAK inhibitors such as baricitinib(Olumiant), approved by the FDA in 2018 for rheumatoid arthritis; upadacitinib; and abrocitinib, according to Dr. Bieber.
There are advantages and disadvantages to each class of molecule, he said. For example, JAK inhibitors act very rapidly to control itch and improve exacerbations, while biologics are “much slower” and provide delayed control of inflammation, he said. In terms of safety, by contrast, biologics probably offer a “better” benefit-to-risk ratio, with no risk for drug-drug interactions and no monitoring needed, while JAK inhibitors appear to have a “narrow” therapeutic window, with a risk for drug-drug interactions and a need for some monitoring, he said.
JAK inhibitors come in a tablet formulation, which, from the patient perspective, may be more convenient and acceptable compared with repeated injections of biologics every 2 or 4 weeks, Dr. Bieber noted. Another point potentially in favor of JAK inhibitors is price: “I think the current situation tells us clearly that the biologics are the more expensive drugs, while the small molecules typically are much less expensive,” he said.
One theoretical advantage of biologics, he said, is the potential for disease modification, while the disease-modifying potential of JAK inhibitors is unclear, as of yet. “We have not had enough experience with all these molecules in order to understand how we can do that particular job, in terms of switching off the disease,” he said.
In terms of the need for patient stratification in the future, it’s a draw between biologics and JAK inhibitors, Dr. Bieber said. “You will not see any of these drugs doing 100 percent of the job in 100 percent of the patients, so to me, currently from my understanding of this disease, there is no one-size-fits-all molecule.”
Toward that end, machine learning and artificial intelligence may aid in evaluating large amounts of data to better understand AD phenotypes. “That’s really something that is a particular kind of challenge in the next years, in order to improve at-the-end drug development, and the management of our patients,” he concluded.
Dr. Bieber reported disclosures related to LEO Pharmaceuticals, Regeneron, and Sanofi.
MILAN – Get ready for a “boxing match” (AD), Thomas Bieber, MD, PhD, said in an oral presentation at the World Congress of Dermatology.
“In one corner of the ring you will have the biologics, and in the other corner of the ring, you will have the Janus kinase (JAK) inhibitors and some other small molecules,” said Dr. Bieber, medical director of dermatology and allergy, at University Hospital Bonn, Germany.
There will be no winner-take-all scenario, since AD is a heterogeneous disease for which a variety of treatments will be needed, Dr. Bieber noted. However, he said, there are clear differences in mode of action, safety, mode of application, price, and more that will be important as dermatologists weigh the choice of biologics versus JAK inhibitors for a specific patient.
“Currently, dupilumab is the only one that is on the market (in the European Union) and this molecule is really a revolution for us,” Dr. Bieber said at the meeting. “But we will see in the next years, many, many other molecules coming up.”
Beyond dupilumab (Dupixent), the interleukin-4 (IL-4) receptor alpha antagonist approved by the Food and Drug Administration in 2017, biologics under development include tralokinumab, lebrikizumab, and nemolizumab, among others. Development on the small molecule side includes agents such as ZPL-389, a histamine H4 receptor agonist, but is dominated by JAK inhibitors such as baricitinib(Olumiant), approved by the FDA in 2018 for rheumatoid arthritis; upadacitinib; and abrocitinib, according to Dr. Bieber.
There are advantages and disadvantages to each class of molecule, he said. For example, JAK inhibitors act very rapidly to control itch and improve exacerbations, while biologics are “much slower” and provide delayed control of inflammation, he said. In terms of safety, by contrast, biologics probably offer a “better” benefit-to-risk ratio, with no risk for drug-drug interactions and no monitoring needed, while JAK inhibitors appear to have a “narrow” therapeutic window, with a risk for drug-drug interactions and a need for some monitoring, he said.
JAK inhibitors come in a tablet formulation, which, from the patient perspective, may be more convenient and acceptable compared with repeated injections of biologics every 2 or 4 weeks, Dr. Bieber noted. Another point potentially in favor of JAK inhibitors is price: “I think the current situation tells us clearly that the biologics are the more expensive drugs, while the small molecules typically are much less expensive,” he said.
One theoretical advantage of biologics, he said, is the potential for disease modification, while the disease-modifying potential of JAK inhibitors is unclear, as of yet. “We have not had enough experience with all these molecules in order to understand how we can do that particular job, in terms of switching off the disease,” he said.
In terms of the need for patient stratification in the future, it’s a draw between biologics and JAK inhibitors, Dr. Bieber said. “You will not see any of these drugs doing 100 percent of the job in 100 percent of the patients, so to me, currently from my understanding of this disease, there is no one-size-fits-all molecule.”
Toward that end, machine learning and artificial intelligence may aid in evaluating large amounts of data to better understand AD phenotypes. “That’s really something that is a particular kind of challenge in the next years, in order to improve at-the-end drug development, and the management of our patients,” he concluded.
Dr. Bieber reported disclosures related to LEO Pharmaceuticals, Regeneron, and Sanofi.
EXPERT ANALYSIS FROM WCD2019
Real-world experience with dupilumab in AD mirrors clinical trial efficacy
MILAN – A retrospective, multicenter Maria Fargnoli, MD, reported at the World Congress of Dermatology.
By the end of 4 weeks of treatment, participants’ mean Eczema Area and Severity Index (EASI) score had dropped from 33.3 to 15.3, a 54.2% reduction. At 16 weeks, the mean EASI score was 9.2, a reduction of 72.5% from baseline (P less than .001 for both time points, compared with baseline). At 16 weeks, 87.2% of patients achieved EASI 50, 60.6% achieved EASI 75, and 32.4% achieved EASI 90.
“In a real-life context, dupilumab significantly improved disease severity, pruritus, sleep loss, and quality of life in adult moderate to severe atopic dermatitis patients,” said Dr. Fargnoli, presenting results of the study during a late-breaking abstract session at the meeting. “All measures improved at 4 weeks, and a further decline was seen at 16 weeks. … These results confirm data from clinical trials, and from other real-life experiences with dupilumab.”
The study, conducted in Italy, tracked outcomes for 109 patients treated for moderate to severe atopic dermatitis at 39 centers from June 2018 to February 2019. Adult patients with EASI scores of at least 24 with contraindications, failure, or intolerance of corticosteroid therapy were included and followed for at least 16 weeks. Those who had concomitant systemic anti-inflammatory or immunomodulator use were excluded, as were those with missing data, said Dr. Fargnoli, chair of the department of dermatology at the University of L’Aquila (Italy).
Patients were given a loading dose of two 300-mg subcutaneous injections of dupilumab, followed by 300-mg injections at 2-week intervals.
Patients were assessed at baseline and after 4 and 16 weeks of treatment. In addition to EASI score, itch and sleep were measured via numeric rating scales; mean itch scores dropped from 8.4 at baseline to 4.1 after 4 weeks, and to 2.5 at 16 weeks (P less than .001 for both time points, compared with baseline).
Sleep scores also improved, from a mean 6.9 at baseline to 3.3 at four weeks, and 1.9 at 16 weeks (P less than .001 for both time points, compared with baseline).
Patients also completed the Dermatology Life Quality Index. At the 4-week mark, patients saw a reduction to 8.3 points from the baseline score of 17.6 points (out of a possible 30, with higher scores indicating worse quality of life); scores dropped to 5.4 by week 16 (P less than .001 for both time points, compared with baseline).
Dupilumab was generally well tolerated, with conjunctivitis – seen in 11% of patients – being the most commonly reported adverse event. This falls in line with other recently published real-world studies of dupilumab, Dr. Fargnoli noted.
Efficacy, as measured by EASI reduction and improvement in itch and sleep, were also comparable between the Italian cohort and clinical trial results, as well as other real-life studies in Denmark, France, the Netherlands, and Spain, she said.
Patients, about one-third female, had a mean body mass index of about 24 kg/m2. Mean age was about 38 years (range, 19-80 years). The mean age of disease onset was about 14 years (range, 0-77 years).
Atopic dermatitis was characterized by phenotype for each patient; groupings included classic adult type (73%), nummular dermatitis (7%), prurigo (8%), and erythrodermic dermatitis (12%). About three in four patients (76.1%) had facial involvement; 61.5% had hand involvement, and 22.9% had genital involvement.
Allergic comorbidities were reported by many patients; 44.9% had rhinitis, 38.5% had asthma, 33% had conjunctivitis, and 15.6% reported food allergies. Other notable comorbidities included psychiatric or psychological conditions, present in 11% of patients, and hypertension or other cardiovascular disorders, seen in 9.1% of patients.
Most patients had tried treatment with both cyclosporine A and corticosteroids (88.9% and 88.1%, respectively). Almost half (45.8%) had tried UV-light therapy, and about a quarter had tried methotrexate.
“The results give real-life data on patterns of treatment response according to heterogeneous atopic dermatitis phenotypes, and on long-term efficacy and safety,” said Dr. Fargnoli.
The study was not funded by any company, according to Dr. Fargnoli. She has served on the advisory board for and has received honoraria for lectures and research grants from Sanofi-Genzyme.
MILAN – A retrospective, multicenter Maria Fargnoli, MD, reported at the World Congress of Dermatology.
By the end of 4 weeks of treatment, participants’ mean Eczema Area and Severity Index (EASI) score had dropped from 33.3 to 15.3, a 54.2% reduction. At 16 weeks, the mean EASI score was 9.2, a reduction of 72.5% from baseline (P less than .001 for both time points, compared with baseline). At 16 weeks, 87.2% of patients achieved EASI 50, 60.6% achieved EASI 75, and 32.4% achieved EASI 90.
“In a real-life context, dupilumab significantly improved disease severity, pruritus, sleep loss, and quality of life in adult moderate to severe atopic dermatitis patients,” said Dr. Fargnoli, presenting results of the study during a late-breaking abstract session at the meeting. “All measures improved at 4 weeks, and a further decline was seen at 16 weeks. … These results confirm data from clinical trials, and from other real-life experiences with dupilumab.”
The study, conducted in Italy, tracked outcomes for 109 patients treated for moderate to severe atopic dermatitis at 39 centers from June 2018 to February 2019. Adult patients with EASI scores of at least 24 with contraindications, failure, or intolerance of corticosteroid therapy were included and followed for at least 16 weeks. Those who had concomitant systemic anti-inflammatory or immunomodulator use were excluded, as were those with missing data, said Dr. Fargnoli, chair of the department of dermatology at the University of L’Aquila (Italy).
Patients were given a loading dose of two 300-mg subcutaneous injections of dupilumab, followed by 300-mg injections at 2-week intervals.
Patients were assessed at baseline and after 4 and 16 weeks of treatment. In addition to EASI score, itch and sleep were measured via numeric rating scales; mean itch scores dropped from 8.4 at baseline to 4.1 after 4 weeks, and to 2.5 at 16 weeks (P less than .001 for both time points, compared with baseline).
Sleep scores also improved, from a mean 6.9 at baseline to 3.3 at four weeks, and 1.9 at 16 weeks (P less than .001 for both time points, compared with baseline).
Patients also completed the Dermatology Life Quality Index. At the 4-week mark, patients saw a reduction to 8.3 points from the baseline score of 17.6 points (out of a possible 30, with higher scores indicating worse quality of life); scores dropped to 5.4 by week 16 (P less than .001 for both time points, compared with baseline).
Dupilumab was generally well tolerated, with conjunctivitis – seen in 11% of patients – being the most commonly reported adverse event. This falls in line with other recently published real-world studies of dupilumab, Dr. Fargnoli noted.
Efficacy, as measured by EASI reduction and improvement in itch and sleep, were also comparable between the Italian cohort and clinical trial results, as well as other real-life studies in Denmark, France, the Netherlands, and Spain, she said.
Patients, about one-third female, had a mean body mass index of about 24 kg/m2. Mean age was about 38 years (range, 19-80 years). The mean age of disease onset was about 14 years (range, 0-77 years).
Atopic dermatitis was characterized by phenotype for each patient; groupings included classic adult type (73%), nummular dermatitis (7%), prurigo (8%), and erythrodermic dermatitis (12%). About three in four patients (76.1%) had facial involvement; 61.5% had hand involvement, and 22.9% had genital involvement.
Allergic comorbidities were reported by many patients; 44.9% had rhinitis, 38.5% had asthma, 33% had conjunctivitis, and 15.6% reported food allergies. Other notable comorbidities included psychiatric or psychological conditions, present in 11% of patients, and hypertension or other cardiovascular disorders, seen in 9.1% of patients.
Most patients had tried treatment with both cyclosporine A and corticosteroids (88.9% and 88.1%, respectively). Almost half (45.8%) had tried UV-light therapy, and about a quarter had tried methotrexate.
“The results give real-life data on patterns of treatment response according to heterogeneous atopic dermatitis phenotypes, and on long-term efficacy and safety,” said Dr. Fargnoli.
The study was not funded by any company, according to Dr. Fargnoli. She has served on the advisory board for and has received honoraria for lectures and research grants from Sanofi-Genzyme.
MILAN – A retrospective, multicenter Maria Fargnoli, MD, reported at the World Congress of Dermatology.
By the end of 4 weeks of treatment, participants’ mean Eczema Area and Severity Index (EASI) score had dropped from 33.3 to 15.3, a 54.2% reduction. At 16 weeks, the mean EASI score was 9.2, a reduction of 72.5% from baseline (P less than .001 for both time points, compared with baseline). At 16 weeks, 87.2% of patients achieved EASI 50, 60.6% achieved EASI 75, and 32.4% achieved EASI 90.
“In a real-life context, dupilumab significantly improved disease severity, pruritus, sleep loss, and quality of life in adult moderate to severe atopic dermatitis patients,” said Dr. Fargnoli, presenting results of the study during a late-breaking abstract session at the meeting. “All measures improved at 4 weeks, and a further decline was seen at 16 weeks. … These results confirm data from clinical trials, and from other real-life experiences with dupilumab.”
The study, conducted in Italy, tracked outcomes for 109 patients treated for moderate to severe atopic dermatitis at 39 centers from June 2018 to February 2019. Adult patients with EASI scores of at least 24 with contraindications, failure, or intolerance of corticosteroid therapy were included and followed for at least 16 weeks. Those who had concomitant systemic anti-inflammatory or immunomodulator use were excluded, as were those with missing data, said Dr. Fargnoli, chair of the department of dermatology at the University of L’Aquila (Italy).
Patients were given a loading dose of two 300-mg subcutaneous injections of dupilumab, followed by 300-mg injections at 2-week intervals.
Patients were assessed at baseline and after 4 and 16 weeks of treatment. In addition to EASI score, itch and sleep were measured via numeric rating scales; mean itch scores dropped from 8.4 at baseline to 4.1 after 4 weeks, and to 2.5 at 16 weeks (P less than .001 for both time points, compared with baseline).
Sleep scores also improved, from a mean 6.9 at baseline to 3.3 at four weeks, and 1.9 at 16 weeks (P less than .001 for both time points, compared with baseline).
Patients also completed the Dermatology Life Quality Index. At the 4-week mark, patients saw a reduction to 8.3 points from the baseline score of 17.6 points (out of a possible 30, with higher scores indicating worse quality of life); scores dropped to 5.4 by week 16 (P less than .001 for both time points, compared with baseline).
Dupilumab was generally well tolerated, with conjunctivitis – seen in 11% of patients – being the most commonly reported adverse event. This falls in line with other recently published real-world studies of dupilumab, Dr. Fargnoli noted.
Efficacy, as measured by EASI reduction and improvement in itch and sleep, were also comparable between the Italian cohort and clinical trial results, as well as other real-life studies in Denmark, France, the Netherlands, and Spain, she said.
Patients, about one-third female, had a mean body mass index of about 24 kg/m2. Mean age was about 38 years (range, 19-80 years). The mean age of disease onset was about 14 years (range, 0-77 years).
Atopic dermatitis was characterized by phenotype for each patient; groupings included classic adult type (73%), nummular dermatitis (7%), prurigo (8%), and erythrodermic dermatitis (12%). About three in four patients (76.1%) had facial involvement; 61.5% had hand involvement, and 22.9% had genital involvement.
Allergic comorbidities were reported by many patients; 44.9% had rhinitis, 38.5% had asthma, 33% had conjunctivitis, and 15.6% reported food allergies. Other notable comorbidities included psychiatric or psychological conditions, present in 11% of patients, and hypertension or other cardiovascular disorders, seen in 9.1% of patients.
Most patients had tried treatment with both cyclosporine A and corticosteroids (88.9% and 88.1%, respectively). Almost half (45.8%) had tried UV-light therapy, and about a quarter had tried methotrexate.
“The results give real-life data on patterns of treatment response according to heterogeneous atopic dermatitis phenotypes, and on long-term efficacy and safety,” said Dr. Fargnoli.
The study was not funded by any company, according to Dr. Fargnoli. She has served on the advisory board for and has received honoraria for lectures and research grants from Sanofi-Genzyme.
REPORTING FROM WCD2019
Cyclosporine, methotrexate have lowest 6-month infection risk for AD patients
(AD) receiving systemic therapy in a real-world setting, according to a recently published population-based study.
When compared with methotrexate, there was a significant reduction in risk of serious infection at 6 months for patients with AD receiving cyclosporine. Prednisone, azathioprine, and mycophenolate carried higher risks of serious infections at 6 months than methotrexate or cyclosporine, researchers said in the study, which appeared in the Journal of the American Academy of Dermatology.
“Among non-biologic systemic agents, cyclosporine and methotrexate appear to have better safety profiles than mycophenolate, azathioprine, and systemic prednisone with regard to serious infections,” they concluded. “These findings may help inform clinicians in their selection of medications for patients requiring systemic therapy for atopic dermatitis,” Maria C. Schneeweiss, MD, from the departments of dermatology and medicine and Division of Pharmacoepidemiology and Pharmacoeconomics at Brigham and Women’s Hospital, Boston, and colleagues wrote in their study.
Using population-based claims data, the researchers evaluated rates of serious infection requiring hospitalization in 232,611 patients between January 2003 and January 2017 who received methotrexate, cyclosporine, azathioprine, prednisone or mycophenolate for treatment of AD. Patients first received the same level of corticosteroids before moving to systemic therapy or phototherapy. They also compared results with 23,908 patients in a second cohort who were new users of dupilumab (391 patients) or non-biologic systemic immunomodulators (23,517).
Overall, the rate of serious infections was 7.53 per 1,000 for patients receiving systemic non-biologic therapy at 6 months compared with 7.38 per 1,000 for patients receiving phototherapy, and 2.6 per 1,000 for patients receiving dupilumab.
When matching using propensity scores, the researchers found a significantly reduced risk at 6 months of serious infections from cyclosporine compared with methotrexate (relative risk, 0.87; 95% confidence interval, 0.59-1.28). Compared with methotrexate, there was an increased risk of serious infection at 6 months for azathioprine (RR, 1.78; 95% CI, 0.98-3.25), prednisone (RR, 1.89; 95% CI, 1.05-3.42) and mycophenolate (RR, 3.31; 95% CI, 1.94-5.64).
According to preliminary data, when compared with patients who received non-biologic systemic therapy, there was no increased risk for patients receiving dupilumab (RR, 0.33; 95% CI, 0.03-3.20). Dupilumab was approved in March 2017, and “with one year of data resulting in one event among 391 patients, this analysis is limited but does not show an obvious signal for increased risk” for dupilumab, they wrote.
Dr. Schneeweiss and colleagues noted some of their analyses had wide confidence intervals, they did not account for dosing schemes or cumulative dose exposure over the study period, and the data on dupilumab showing no increase were preliminary and not conclusive.
“Our findings on systemic non-biologics are highly plausible, given the known risk of systemic immunomodulators in patients treated for other indications, the meaningful effect size, and the methodologically robust approach with a new-user active-comparator design and propensity score matching,” the researchers said.
This study was funded in part by Brigham and Women’s Hospital in Boston. One author reported being a consultant for multiple pharmaceutical companies. The other authors report no relevant conflicts of interest.
SOURCE: Schneeweiss M, et al. J Am Acad Dermatol. 2019. doi:10.1016/j.jaad.2019.05.073.
(AD) receiving systemic therapy in a real-world setting, according to a recently published population-based study.
When compared with methotrexate, there was a significant reduction in risk of serious infection at 6 months for patients with AD receiving cyclosporine. Prednisone, azathioprine, and mycophenolate carried higher risks of serious infections at 6 months than methotrexate or cyclosporine, researchers said in the study, which appeared in the Journal of the American Academy of Dermatology.
“Among non-biologic systemic agents, cyclosporine and methotrexate appear to have better safety profiles than mycophenolate, azathioprine, and systemic prednisone with regard to serious infections,” they concluded. “These findings may help inform clinicians in their selection of medications for patients requiring systemic therapy for atopic dermatitis,” Maria C. Schneeweiss, MD, from the departments of dermatology and medicine and Division of Pharmacoepidemiology and Pharmacoeconomics at Brigham and Women’s Hospital, Boston, and colleagues wrote in their study.
Using population-based claims data, the researchers evaluated rates of serious infection requiring hospitalization in 232,611 patients between January 2003 and January 2017 who received methotrexate, cyclosporine, azathioprine, prednisone or mycophenolate for treatment of AD. Patients first received the same level of corticosteroids before moving to systemic therapy or phototherapy. They also compared results with 23,908 patients in a second cohort who were new users of dupilumab (391 patients) or non-biologic systemic immunomodulators (23,517).
Overall, the rate of serious infections was 7.53 per 1,000 for patients receiving systemic non-biologic therapy at 6 months compared with 7.38 per 1,000 for patients receiving phototherapy, and 2.6 per 1,000 for patients receiving dupilumab.
When matching using propensity scores, the researchers found a significantly reduced risk at 6 months of serious infections from cyclosporine compared with methotrexate (relative risk, 0.87; 95% confidence interval, 0.59-1.28). Compared with methotrexate, there was an increased risk of serious infection at 6 months for azathioprine (RR, 1.78; 95% CI, 0.98-3.25), prednisone (RR, 1.89; 95% CI, 1.05-3.42) and mycophenolate (RR, 3.31; 95% CI, 1.94-5.64).
According to preliminary data, when compared with patients who received non-biologic systemic therapy, there was no increased risk for patients receiving dupilumab (RR, 0.33; 95% CI, 0.03-3.20). Dupilumab was approved in March 2017, and “with one year of data resulting in one event among 391 patients, this analysis is limited but does not show an obvious signal for increased risk” for dupilumab, they wrote.
Dr. Schneeweiss and colleagues noted some of their analyses had wide confidence intervals, they did not account for dosing schemes or cumulative dose exposure over the study period, and the data on dupilumab showing no increase were preliminary and not conclusive.
“Our findings on systemic non-biologics are highly plausible, given the known risk of systemic immunomodulators in patients treated for other indications, the meaningful effect size, and the methodologically robust approach with a new-user active-comparator design and propensity score matching,” the researchers said.
This study was funded in part by Brigham and Women’s Hospital in Boston. One author reported being a consultant for multiple pharmaceutical companies. The other authors report no relevant conflicts of interest.
SOURCE: Schneeweiss M, et al. J Am Acad Dermatol. 2019. doi:10.1016/j.jaad.2019.05.073.
(AD) receiving systemic therapy in a real-world setting, according to a recently published population-based study.
When compared with methotrexate, there was a significant reduction in risk of serious infection at 6 months for patients with AD receiving cyclosporine. Prednisone, azathioprine, and mycophenolate carried higher risks of serious infections at 6 months than methotrexate or cyclosporine, researchers said in the study, which appeared in the Journal of the American Academy of Dermatology.
“Among non-biologic systemic agents, cyclosporine and methotrexate appear to have better safety profiles than mycophenolate, azathioprine, and systemic prednisone with regard to serious infections,” they concluded. “These findings may help inform clinicians in their selection of medications for patients requiring systemic therapy for atopic dermatitis,” Maria C. Schneeweiss, MD, from the departments of dermatology and medicine and Division of Pharmacoepidemiology and Pharmacoeconomics at Brigham and Women’s Hospital, Boston, and colleagues wrote in their study.
Using population-based claims data, the researchers evaluated rates of serious infection requiring hospitalization in 232,611 patients between January 2003 and January 2017 who received methotrexate, cyclosporine, azathioprine, prednisone or mycophenolate for treatment of AD. Patients first received the same level of corticosteroids before moving to systemic therapy or phototherapy. They also compared results with 23,908 patients in a second cohort who were new users of dupilumab (391 patients) or non-biologic systemic immunomodulators (23,517).
Overall, the rate of serious infections was 7.53 per 1,000 for patients receiving systemic non-biologic therapy at 6 months compared with 7.38 per 1,000 for patients receiving phototherapy, and 2.6 per 1,000 for patients receiving dupilumab.
When matching using propensity scores, the researchers found a significantly reduced risk at 6 months of serious infections from cyclosporine compared with methotrexate (relative risk, 0.87; 95% confidence interval, 0.59-1.28). Compared with methotrexate, there was an increased risk of serious infection at 6 months for azathioprine (RR, 1.78; 95% CI, 0.98-3.25), prednisone (RR, 1.89; 95% CI, 1.05-3.42) and mycophenolate (RR, 3.31; 95% CI, 1.94-5.64).
According to preliminary data, when compared with patients who received non-biologic systemic therapy, there was no increased risk for patients receiving dupilumab (RR, 0.33; 95% CI, 0.03-3.20). Dupilumab was approved in March 2017, and “with one year of data resulting in one event among 391 patients, this analysis is limited but does not show an obvious signal for increased risk” for dupilumab, they wrote.
Dr. Schneeweiss and colleagues noted some of their analyses had wide confidence intervals, they did not account for dosing schemes or cumulative dose exposure over the study period, and the data on dupilumab showing no increase were preliminary and not conclusive.
“Our findings on systemic non-biologics are highly plausible, given the known risk of systemic immunomodulators in patients treated for other indications, the meaningful effect size, and the methodologically robust approach with a new-user active-comparator design and propensity score matching,” the researchers said.
This study was funded in part by Brigham and Women’s Hospital in Boston. One author reported being a consultant for multiple pharmaceutical companies. The other authors report no relevant conflicts of interest.
SOURCE: Schneeweiss M, et al. J Am Acad Dermatol. 2019. doi:10.1016/j.jaad.2019.05.073.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY