User login
One-year data support dupilumab’s efficacy and safety in adolescents with AD
A study of and continued evidence of efficacy for up to 52 weeks, reported the authors of the study, published online Oct. 9 in the British Journal of Dermatology.
The phase 2a open-label, ascending-dose cohort study of dupilumab in 40 adolescents with moderate to severe AD was followed by a 48-week phase 3 open-label extension study in 36 of those participants. Dupilumab is a monoclonal antibody that inhibits signaling of interleukin (IL)-4 and IL-13.
In the phase 2a study, participants were treated with a single subcutaneous dose of dupilumab – either 2 mg/kg or 4 mg/kg – and had 8 weeks of pharmacokinetic sampling. They subsequently received that same dose weekly for 4 weeks, with an 8-week-long safety follow-up period. Those who participated in the open-label extension continued their weekly dose to a maximum of 300 mg. per kg
The most common treatment-emergent adverse events (a primary endpoint) seen in both the phase 2a and phase 3 studies were nasopharyngitis and exacerbation of AD – in the phase 2a study, exacerbations were seen in the period when patients weren’t taking the treatment. In the 2-mg and 4-mg groups, the incidence of skin infections was 29% and 42%, respectively, and the incidence of injection site reactions – which were mostly mild – were 18% and 11%, respectively. Researchers also noted conjunctivitis in 18% and 16% of the patients in the 2-mg and 4-mg groups, respectively, but none of the cases were considered serious and all resolved over the course of the study. In the phase 2a study, 50% of patients on the 2-mg/kg dose and 65% of those on the 4-mg/kg dose experienced an adverse event, while in the open-label extension all reported at least one adverse event.
There was one case of suicidal behavior and one case of systemic or severe hypersensitivity reported in the 2-mg/kg groups, both of which were considered adverse events of special interest. There were no deaths.
However none of the serious adverse events – which included infected AD, palpitations, patent ductus arteriosus, and food allergy – were linked to the study treatment, and no adverse events led to study discontinuation, the authors reported.
By week 12, 70% of participants in the 2-mg/kg group and 75% in the 4-mg/kg group had achieved a 50% or greater improvement in their Eczema Area and Severity Index (EASI) scores, which was a secondary outcome. By week 52, that had increased to 100% and 89% respectively.
More than half the patients (55%) in the 2-mg/kg group, and 40% of those in the 4-mg/kg group achieved a 75% or more improvement in their EASI scores by week 12, which increased to 88% and 78%, respectively, by week 52 in the open label phase.
“The results from these studies support use of dupilumab for the long-term management of moderate to severe AD in adolescents,” wrote Michael J. Cork, MD, professor of dermatology, University of Sheffield, England, and coauthors. No new safety signals were identified, “compared with the known safety profile of dupilumab in adults with moderate to severe AD,” and “the PK profile was characterized by nonlinear, target-mediated kinetics, consistent with the profile in adults with moderate to severe AD,” they added.
Dupilumab was approved in the United States in March 2019 for adolescents with moderate to severe AD whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
The study was sponsored by dupilumab manufacturers Sanofi and Regeneron Pharmaceuticals, which market dupilumab as Dupixent in the United States. Dr. Cork disclosures included those related to Sanofi Genzyme and Regeneron; other authors included employees of the companies.
SOURCE: Cork M et al. Br J Dermatol. 2019 Oct 9. doi: 10.1111/bjd.18476.
A study of and continued evidence of efficacy for up to 52 weeks, reported the authors of the study, published online Oct. 9 in the British Journal of Dermatology.
The phase 2a open-label, ascending-dose cohort study of dupilumab in 40 adolescents with moderate to severe AD was followed by a 48-week phase 3 open-label extension study in 36 of those participants. Dupilumab is a monoclonal antibody that inhibits signaling of interleukin (IL)-4 and IL-13.
In the phase 2a study, participants were treated with a single subcutaneous dose of dupilumab – either 2 mg/kg or 4 mg/kg – and had 8 weeks of pharmacokinetic sampling. They subsequently received that same dose weekly for 4 weeks, with an 8-week-long safety follow-up period. Those who participated in the open-label extension continued their weekly dose to a maximum of 300 mg. per kg
The most common treatment-emergent adverse events (a primary endpoint) seen in both the phase 2a and phase 3 studies were nasopharyngitis and exacerbation of AD – in the phase 2a study, exacerbations were seen in the period when patients weren’t taking the treatment. In the 2-mg and 4-mg groups, the incidence of skin infections was 29% and 42%, respectively, and the incidence of injection site reactions – which were mostly mild – were 18% and 11%, respectively. Researchers also noted conjunctivitis in 18% and 16% of the patients in the 2-mg and 4-mg groups, respectively, but none of the cases were considered serious and all resolved over the course of the study. In the phase 2a study, 50% of patients on the 2-mg/kg dose and 65% of those on the 4-mg/kg dose experienced an adverse event, while in the open-label extension all reported at least one adverse event.
There was one case of suicidal behavior and one case of systemic or severe hypersensitivity reported in the 2-mg/kg groups, both of which were considered adverse events of special interest. There were no deaths.
However none of the serious adverse events – which included infected AD, palpitations, patent ductus arteriosus, and food allergy – were linked to the study treatment, and no adverse events led to study discontinuation, the authors reported.
By week 12, 70% of participants in the 2-mg/kg group and 75% in the 4-mg/kg group had achieved a 50% or greater improvement in their Eczema Area and Severity Index (EASI) scores, which was a secondary outcome. By week 52, that had increased to 100% and 89% respectively.
More than half the patients (55%) in the 2-mg/kg group, and 40% of those in the 4-mg/kg group achieved a 75% or more improvement in their EASI scores by week 12, which increased to 88% and 78%, respectively, by week 52 in the open label phase.
“The results from these studies support use of dupilumab for the long-term management of moderate to severe AD in adolescents,” wrote Michael J. Cork, MD, professor of dermatology, University of Sheffield, England, and coauthors. No new safety signals were identified, “compared with the known safety profile of dupilumab in adults with moderate to severe AD,” and “the PK profile was characterized by nonlinear, target-mediated kinetics, consistent with the profile in adults with moderate to severe AD,” they added.
Dupilumab was approved in the United States in March 2019 for adolescents with moderate to severe AD whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
The study was sponsored by dupilumab manufacturers Sanofi and Regeneron Pharmaceuticals, which market dupilumab as Dupixent in the United States. Dr. Cork disclosures included those related to Sanofi Genzyme and Regeneron; other authors included employees of the companies.
SOURCE: Cork M et al. Br J Dermatol. 2019 Oct 9. doi: 10.1111/bjd.18476.
A study of and continued evidence of efficacy for up to 52 weeks, reported the authors of the study, published online Oct. 9 in the British Journal of Dermatology.
The phase 2a open-label, ascending-dose cohort study of dupilumab in 40 adolescents with moderate to severe AD was followed by a 48-week phase 3 open-label extension study in 36 of those participants. Dupilumab is a monoclonal antibody that inhibits signaling of interleukin (IL)-4 and IL-13.
In the phase 2a study, participants were treated with a single subcutaneous dose of dupilumab – either 2 mg/kg or 4 mg/kg – and had 8 weeks of pharmacokinetic sampling. They subsequently received that same dose weekly for 4 weeks, with an 8-week-long safety follow-up period. Those who participated in the open-label extension continued their weekly dose to a maximum of 300 mg. per kg
The most common treatment-emergent adverse events (a primary endpoint) seen in both the phase 2a and phase 3 studies were nasopharyngitis and exacerbation of AD – in the phase 2a study, exacerbations were seen in the period when patients weren’t taking the treatment. In the 2-mg and 4-mg groups, the incidence of skin infections was 29% and 42%, respectively, and the incidence of injection site reactions – which were mostly mild – were 18% and 11%, respectively. Researchers also noted conjunctivitis in 18% and 16% of the patients in the 2-mg and 4-mg groups, respectively, but none of the cases were considered serious and all resolved over the course of the study. In the phase 2a study, 50% of patients on the 2-mg/kg dose and 65% of those on the 4-mg/kg dose experienced an adverse event, while in the open-label extension all reported at least one adverse event.
There was one case of suicidal behavior and one case of systemic or severe hypersensitivity reported in the 2-mg/kg groups, both of which were considered adverse events of special interest. There were no deaths.
However none of the serious adverse events – which included infected AD, palpitations, patent ductus arteriosus, and food allergy – were linked to the study treatment, and no adverse events led to study discontinuation, the authors reported.
By week 12, 70% of participants in the 2-mg/kg group and 75% in the 4-mg/kg group had achieved a 50% or greater improvement in their Eczema Area and Severity Index (EASI) scores, which was a secondary outcome. By week 52, that had increased to 100% and 89% respectively.
More than half the patients (55%) in the 2-mg/kg group, and 40% of those in the 4-mg/kg group achieved a 75% or more improvement in their EASI scores by week 12, which increased to 88% and 78%, respectively, by week 52 in the open label phase.
“The results from these studies support use of dupilumab for the long-term management of moderate to severe AD in adolescents,” wrote Michael J. Cork, MD, professor of dermatology, University of Sheffield, England, and coauthors. No new safety signals were identified, “compared with the known safety profile of dupilumab in adults with moderate to severe AD,” and “the PK profile was characterized by nonlinear, target-mediated kinetics, consistent with the profile in adults with moderate to severe AD,” they added.
Dupilumab was approved in the United States in March 2019 for adolescents with moderate to severe AD whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
The study was sponsored by dupilumab manufacturers Sanofi and Regeneron Pharmaceuticals, which market dupilumab as Dupixent in the United States. Dr. Cork disclosures included those related to Sanofi Genzyme and Regeneron; other authors included employees of the companies.
SOURCE: Cork M et al. Br J Dermatol. 2019 Oct 9. doi: 10.1111/bjd.18476.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Apple cider vinegar soaks fall short in atopic dermatitis
, in a pilot split-arm study.
The aim of the study was to evaluate the effects of diluted apple cider vinegar application on transepidermal water loss (TEWL) and pH on skin affected by AD and on healthy skin, according to Lydia A. Luu of the department of dermatology at University of Virginia, Charlottesville, and colleagues. “Acetic acid, particularly apple cider vinegar, is prominent among emerging natural remedies used in AD. Therefore, determining the safety of this commonly used product is crucial,” they wrote in the study, published in Pediatric Dermatology.
In total, 11 patients with AD and 11 healthy controls were included; most of those with AD were considered mild (36.4%) or moderate (45.5%). Participants had not used systemic or topical antimicrobial treatments in the month preceding the study, and they were aged 12 years and older (mean ages were 20.6 years in the AD group and 28.8 years among controls). Those with AD had significantly elevated TEWL at baseline, compared with controls.
For 14 days, study participants soaked one forearm in dilute apple cider vinegar (0.5% acetic acid) and the other in tap water for 10 minutes daily. Changes in pH and TEWL before and after application were measured.
The researchers found that TEWL significantly increased immediately post treatment (at 0 and 15 minutes) in both groups, dropping to baseline at 30 minutes among those with AD and at 60 minutes among controls.
Skin pH was similar in both groups at baseline (4.86-4.88). After the cider vinegar soak, there was a transient reduction in skin pH among AD patients that lasted for 15 minutes among those with AD and 60 minutes in controls. This finding “suggests temporary acidification of the skin that has theoretical benefit of correcting disrupted skin pH in AD,” the authors wrote, noting that increased TEWL and alkaline skin pH is common among people with AD because of skin barrier dysfunction.
With respect to safety, 72.7% (16) of the participants experienced skin discomfort, mostly described as mild, limited to the vinegar-treated arm. After discontinuation, the majority of skin irritation resolved quickly, with no additional therapy.
The authors acknowledged two key limitations of the study were the homogeneous patient population and small sample size. “Although epidermal acidification would theoretically be beneficial in treating AD, our study shows that acidification by way of topical bathing in a 0.5% [apple cider vinegar] solution as performed in this study is not useful in AD treatment,” they wrote. “Further studies in a more diverse population will be necessary to fully characterize the risk/benefit profile of topical dilute apple cider vinegar treatments.”
The study was funded by the University of Virginia. The authors did not provide information on financial disclosures.
SOURCE: Luu LA et al. Pediatr Dermatol. 2019 Jul 22. doi: 10.1111/pde.13888.
, in a pilot split-arm study.
The aim of the study was to evaluate the effects of diluted apple cider vinegar application on transepidermal water loss (TEWL) and pH on skin affected by AD and on healthy skin, according to Lydia A. Luu of the department of dermatology at University of Virginia, Charlottesville, and colleagues. “Acetic acid, particularly apple cider vinegar, is prominent among emerging natural remedies used in AD. Therefore, determining the safety of this commonly used product is crucial,” they wrote in the study, published in Pediatric Dermatology.
In total, 11 patients with AD and 11 healthy controls were included; most of those with AD were considered mild (36.4%) or moderate (45.5%). Participants had not used systemic or topical antimicrobial treatments in the month preceding the study, and they were aged 12 years and older (mean ages were 20.6 years in the AD group and 28.8 years among controls). Those with AD had significantly elevated TEWL at baseline, compared with controls.
For 14 days, study participants soaked one forearm in dilute apple cider vinegar (0.5% acetic acid) and the other in tap water for 10 minutes daily. Changes in pH and TEWL before and after application were measured.
The researchers found that TEWL significantly increased immediately post treatment (at 0 and 15 minutes) in both groups, dropping to baseline at 30 minutes among those with AD and at 60 minutes among controls.
Skin pH was similar in both groups at baseline (4.86-4.88). After the cider vinegar soak, there was a transient reduction in skin pH among AD patients that lasted for 15 minutes among those with AD and 60 minutes in controls. This finding “suggests temporary acidification of the skin that has theoretical benefit of correcting disrupted skin pH in AD,” the authors wrote, noting that increased TEWL and alkaline skin pH is common among people with AD because of skin barrier dysfunction.
With respect to safety, 72.7% (16) of the participants experienced skin discomfort, mostly described as mild, limited to the vinegar-treated arm. After discontinuation, the majority of skin irritation resolved quickly, with no additional therapy.
The authors acknowledged two key limitations of the study were the homogeneous patient population and small sample size. “Although epidermal acidification would theoretically be beneficial in treating AD, our study shows that acidification by way of topical bathing in a 0.5% [apple cider vinegar] solution as performed in this study is not useful in AD treatment,” they wrote. “Further studies in a more diverse population will be necessary to fully characterize the risk/benefit profile of topical dilute apple cider vinegar treatments.”
The study was funded by the University of Virginia. The authors did not provide information on financial disclosures.
SOURCE: Luu LA et al. Pediatr Dermatol. 2019 Jul 22. doi: 10.1111/pde.13888.
, in a pilot split-arm study.
The aim of the study was to evaluate the effects of diluted apple cider vinegar application on transepidermal water loss (TEWL) and pH on skin affected by AD and on healthy skin, according to Lydia A. Luu of the department of dermatology at University of Virginia, Charlottesville, and colleagues. “Acetic acid, particularly apple cider vinegar, is prominent among emerging natural remedies used in AD. Therefore, determining the safety of this commonly used product is crucial,” they wrote in the study, published in Pediatric Dermatology.
In total, 11 patients with AD and 11 healthy controls were included; most of those with AD were considered mild (36.4%) or moderate (45.5%). Participants had not used systemic or topical antimicrobial treatments in the month preceding the study, and they were aged 12 years and older (mean ages were 20.6 years in the AD group and 28.8 years among controls). Those with AD had significantly elevated TEWL at baseline, compared with controls.
For 14 days, study participants soaked one forearm in dilute apple cider vinegar (0.5% acetic acid) and the other in tap water for 10 minutes daily. Changes in pH and TEWL before and after application were measured.
The researchers found that TEWL significantly increased immediately post treatment (at 0 and 15 minutes) in both groups, dropping to baseline at 30 minutes among those with AD and at 60 minutes among controls.
Skin pH was similar in both groups at baseline (4.86-4.88). After the cider vinegar soak, there was a transient reduction in skin pH among AD patients that lasted for 15 minutes among those with AD and 60 minutes in controls. This finding “suggests temporary acidification of the skin that has theoretical benefit of correcting disrupted skin pH in AD,” the authors wrote, noting that increased TEWL and alkaline skin pH is common among people with AD because of skin barrier dysfunction.
With respect to safety, 72.7% (16) of the participants experienced skin discomfort, mostly described as mild, limited to the vinegar-treated arm. After discontinuation, the majority of skin irritation resolved quickly, with no additional therapy.
The authors acknowledged two key limitations of the study were the homogeneous patient population and small sample size. “Although epidermal acidification would theoretically be beneficial in treating AD, our study shows that acidification by way of topical bathing in a 0.5% [apple cider vinegar] solution as performed in this study is not useful in AD treatment,” they wrote. “Further studies in a more diverse population will be necessary to fully characterize the risk/benefit profile of topical dilute apple cider vinegar treatments.”
The study was funded by the University of Virginia. The authors did not provide information on financial disclosures.
SOURCE: Luu LA et al. Pediatr Dermatol. 2019 Jul 22. doi: 10.1111/pde.13888.
FROM PEDIATRIC DERMATOLOGY
Parent survey sheds light on suboptimal compliance with eczema medications
of children with AD.
Perceived effectiveness was the main driver of this variation, Alan Schwartz PhD, and Korey Capozza, MPH, wrote in the study, published in Pediatric Dermatology.
“Responses suggest parents may be willing to use therapies with concerning side effects if they can see a clear benefit for their child’s eczema, but when anticipated improvements fail to materialize, they may change their usage, usually in the direction of using less medication or stopping,” observed Dr. Schwartz, of the University of Illinois, Chicago, and Ms. Capozza, of Global Parents for Eczema Research.
“Addressing expectations related to effectiveness, rather than concerns about medication use, may thus be more likely to lead to taking medication as directed.”
The researchers posted a 15-question survey on the Facebook page of Global Parents for Eczema Research, an international coalition of parents of children with AD. During the month that the survey was posted, 86 parents completed it; questions pertained to adherence to medications and reasons for changing treatments. The mean age of their children was 6 years, most (about 83%) had moderate or severe eczema, and about half lived in the United States.
More than half (55%) reported using the AD medications as directed. But 30% said they took or applied less than prescribed, 13% had stopped the prescribed medication altogether, and 2% took or applied more (or more often) than prescribed.
There were several reasons stated for this variance. Concern over side effects was the most common (46%) reason for not using medications as directed. The next most common reasons were that the child’s symptoms went away (28%); or the “medication was not helping or was not helping as much,” in 23%.
A lack of physician trust or not agreeing with the physician’s recommendations accounted for 18% of the concerns. The remainder thought it wasn’t important to take the medication as prescribed, it was inconvenient or too time consuming, that they forgot, it was too expensive, or they were confused about the directions.
To the question asking “What would have made you more likely to use the medication as prescribed?” the most common answer was a clearer indication of effectiveness (56%). The next most common was “access to research or evidence about benefit and side effect profile” (14%).
A good relationship between the physician and patient was associated with taking medication as directed
“Improvement in adherence to topical treatments among children with AD could yield large gains in quality-of-life improvements and reduce exposure to costlier and potentially more toxic systemic agents,” the authors noted. “Given the large, documented gains in disease improvement, and even remission, achieved with interventions that address adherence among patients with other chronic diseases, strategies that address the underlying causes for poor adherence among parents of children with atopic dermatitis stand to provide a significant, untapped benefit.”
No financial disclosures were noted.
SOURCE: Pediatr Dermatol. 2019 Aug 28. doi: 10.1111/pde.13991.
of children with AD.
Perceived effectiveness was the main driver of this variation, Alan Schwartz PhD, and Korey Capozza, MPH, wrote in the study, published in Pediatric Dermatology.
“Responses suggest parents may be willing to use therapies with concerning side effects if they can see a clear benefit for their child’s eczema, but when anticipated improvements fail to materialize, they may change their usage, usually in the direction of using less medication or stopping,” observed Dr. Schwartz, of the University of Illinois, Chicago, and Ms. Capozza, of Global Parents for Eczema Research.
“Addressing expectations related to effectiveness, rather than concerns about medication use, may thus be more likely to lead to taking medication as directed.”
The researchers posted a 15-question survey on the Facebook page of Global Parents for Eczema Research, an international coalition of parents of children with AD. During the month that the survey was posted, 86 parents completed it; questions pertained to adherence to medications and reasons for changing treatments. The mean age of their children was 6 years, most (about 83%) had moderate or severe eczema, and about half lived in the United States.
More than half (55%) reported using the AD medications as directed. But 30% said they took or applied less than prescribed, 13% had stopped the prescribed medication altogether, and 2% took or applied more (or more often) than prescribed.
There were several reasons stated for this variance. Concern over side effects was the most common (46%) reason for not using medications as directed. The next most common reasons were that the child’s symptoms went away (28%); or the “medication was not helping or was not helping as much,” in 23%.
A lack of physician trust or not agreeing with the physician’s recommendations accounted for 18% of the concerns. The remainder thought it wasn’t important to take the medication as prescribed, it was inconvenient or too time consuming, that they forgot, it was too expensive, or they were confused about the directions.
To the question asking “What would have made you more likely to use the medication as prescribed?” the most common answer was a clearer indication of effectiveness (56%). The next most common was “access to research or evidence about benefit and side effect profile” (14%).
A good relationship between the physician and patient was associated with taking medication as directed
“Improvement in adherence to topical treatments among children with AD could yield large gains in quality-of-life improvements and reduce exposure to costlier and potentially more toxic systemic agents,” the authors noted. “Given the large, documented gains in disease improvement, and even remission, achieved with interventions that address adherence among patients with other chronic diseases, strategies that address the underlying causes for poor adherence among parents of children with atopic dermatitis stand to provide a significant, untapped benefit.”
No financial disclosures were noted.
SOURCE: Pediatr Dermatol. 2019 Aug 28. doi: 10.1111/pde.13991.
of children with AD.
Perceived effectiveness was the main driver of this variation, Alan Schwartz PhD, and Korey Capozza, MPH, wrote in the study, published in Pediatric Dermatology.
“Responses suggest parents may be willing to use therapies with concerning side effects if they can see a clear benefit for their child’s eczema, but when anticipated improvements fail to materialize, they may change their usage, usually in the direction of using less medication or stopping,” observed Dr. Schwartz, of the University of Illinois, Chicago, and Ms. Capozza, of Global Parents for Eczema Research.
“Addressing expectations related to effectiveness, rather than concerns about medication use, may thus be more likely to lead to taking medication as directed.”
The researchers posted a 15-question survey on the Facebook page of Global Parents for Eczema Research, an international coalition of parents of children with AD. During the month that the survey was posted, 86 parents completed it; questions pertained to adherence to medications and reasons for changing treatments. The mean age of their children was 6 years, most (about 83%) had moderate or severe eczema, and about half lived in the United States.
More than half (55%) reported using the AD medications as directed. But 30% said they took or applied less than prescribed, 13% had stopped the prescribed medication altogether, and 2% took or applied more (or more often) than prescribed.
There were several reasons stated for this variance. Concern over side effects was the most common (46%) reason for not using medications as directed. The next most common reasons were that the child’s symptoms went away (28%); or the “medication was not helping or was not helping as much,” in 23%.
A lack of physician trust or not agreeing with the physician’s recommendations accounted for 18% of the concerns. The remainder thought it wasn’t important to take the medication as prescribed, it was inconvenient or too time consuming, that they forgot, it was too expensive, or they were confused about the directions.
To the question asking “What would have made you more likely to use the medication as prescribed?” the most common answer was a clearer indication of effectiveness (56%). The next most common was “access to research or evidence about benefit and side effect profile” (14%).
A good relationship between the physician and patient was associated with taking medication as directed
“Improvement in adherence to topical treatments among children with AD could yield large gains in quality-of-life improvements and reduce exposure to costlier and potentially more toxic systemic agents,” the authors noted. “Given the large, documented gains in disease improvement, and even remission, achieved with interventions that address adherence among patients with other chronic diseases, strategies that address the underlying causes for poor adherence among parents of children with atopic dermatitis stand to provide a significant, untapped benefit.”
No financial disclosures were noted.
SOURCE: Pediatr Dermatol. 2019 Aug 28. doi: 10.1111/pde.13991.
FROM PEDIATRIC DERMATOLOGY
Crossword: Atopic Dermatitis
Ocular Complications of Atopic Dermatitis
Atopic dermatitis (AD) is a chronic inflammatory skin condition with a lifetime prevalence of 15% to 20% in industrialized countries.1 It affects both children and adults and is predominantly characterized by a waxing and waning course of eczematous skin lesions and pruritus. In recent years, there is increasing recognition that AD can present with extracutaneous findings. Large-scale epidemiologic studies have reported a notably higher prevalence of ophthalmic complications in the AD population compared to the general population, in a severity-dependent manner.2,3 Potential complications include blepharitis, keratoconjunctivitis, keratoconus, glaucoma, cataracts, retinal detachment, ophthalmic herpes simplex virus infections, and dupilumab-associated ocular complications.
The etiology of each ocular complication in the context of AD is complex and likely multifactorial. Intrinsic immune dysregulation, physical trauma from eye rubbing, AD medication side effects, and genetics all have been speculated to play a role.2 Some of these ocular complications have a chronic course, while others present with sudden onset of symptoms; many of them can result in visual impairment if undiagnosed or left untreated. This article reviews several of the most common ocular comorbidities associated with AD. We discuss the clinical presentation, pathophysiology, and management strategies for each condition.
Blepharitis
Blepharitis, an inflammatory condition of the eyelids, is estimated to affect more than 6% of patients with AD compared to less than 1% of the general population.2 Blepharitis can be classified as anterior or posterior, based on the anatomic location of the affected region relative to the lash margin. Affected individuals may experience pruritus and irritation of the eyelids, tearing, a foreign body or burning sensation, crusting of the eyelids, and photophobia.4 Anterior blepharitis commonly is due to staphylococcal disease, and posterior blepharitis is secondary to structural changes and obstruction of meibomian gland orifices.
Although the pathophysiology is not well defined, xerosis in atopic patients is accompanied by barrier disruption and transepidermal water loss, which promote eyelid skin inflammation.
The mainstay of therapy for atopic blepharitis consists of conventional lid hygiene regimens, such as warm compresses and gentle scrubbing of the lid margins to remove crust and debris, which can be done with nonprescription cleansers, pads, and baby shampoos. Acute exacerbations may require topical antibiotics (ie, erythromycin or bacitracin applied to the lid margins once daily), topical calcineurin inhibitors (ie, cyclosporine ophthalmic emulsion 0.05%), or low-potency topical corticosteroids (ie, fluorometholone 0.1% or loteprednol etabonate 0.5% ophthalmic suspensions).5 Due to potential side effects of medications, especially topical corticosteroids, patients should be referred to ophthalmologists for definitive diagnosis and treatment.
Keratoconjunctivitis
Atopic keratoconjunctivitis (AKC) is a noninfectious inflammatory condition of the cornea and conjunctiva that occurs in an estimated 25% to 42% of patients with AD.6,7 It frequently presents in late adolescence and has a peak incidence between 30 and 50 years of age.8 The symptoms of AKC include ocular pruritus, redness, ropy mucoid discharge, burning discomfort, photophobia, and blurring of vision. Corneal involvement can progress to corneal neovascularization and punctate or macroepithelial erosions and ulcerations, which increase the risk for corneal scarring and visual impairment.7
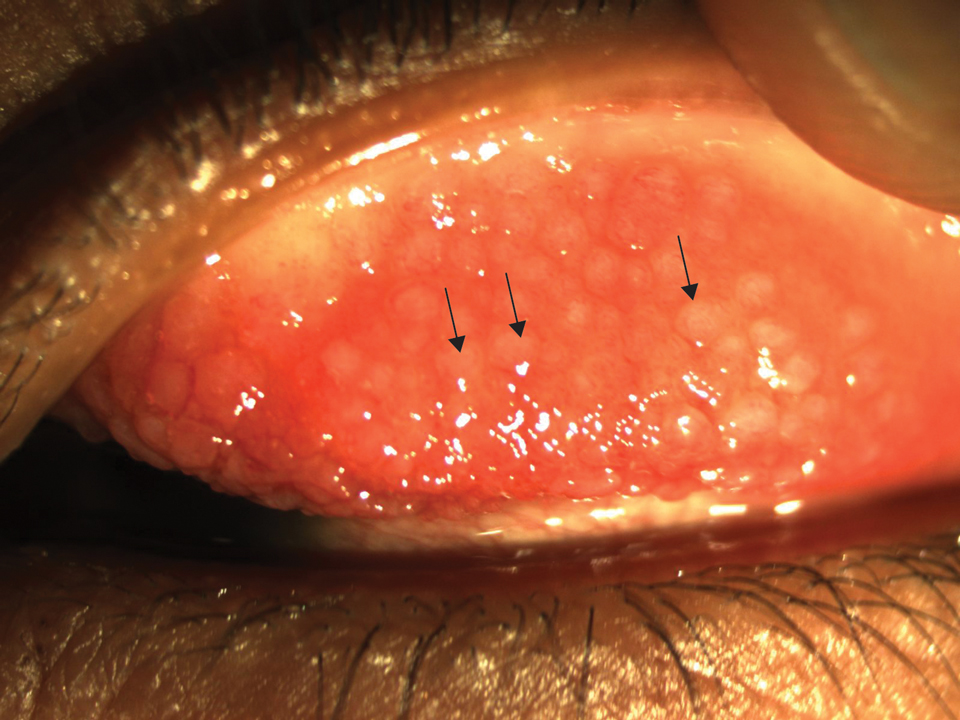
Keratoconjunctivitis is a complex inflammatory disease characterized by infiltration of the conjunctival epithelium by eosinophils, mast cells, and lymphocytes. On examination, patients frequently are found to have concurrent AD of the periorbital skin as well as papillary hypertrophy of the tarsal conjunctiva with accompanying fibrosis, which can lead to entropion (turning inward of the lid margins and lashes) in severe cases.7 Ophthalmic evaluation is strongly recommended for patients with AKC to control symptoms, to limit exacerbations, and to prevent sight-threatening inflammation leading to vision loss. Treatment can be challenging given the chronicity of the condition and may require multiple treatment arms. Conservative measures include cool compresses and treatment with ophthalmic eye drops containing antihistamines (ie, ketotifen 0.025% [available over-the-counter]) and mast cell stabilizers (ie, olopatadine ophthalmic solution 0.1%).8 Atopic keratoconjunctivitis exacerbations may require short-term use of topical steroids or calcineurin inhibitors, or systemic equivalents for refractory cases.6 Long-term maintenance therapy typically consists of proper eye hygiene and steroid-sparing agents that reduce ocular inflammation, such as topical cyclosporine and tacrolimus, neither of which are associated with increased intraocular pressure (IOP)(Figure 1).8 Cornea disease resulting from chronic conjunctival/lid microtrauma can be managed with soft or scleral contact lenses.
Keratoconus
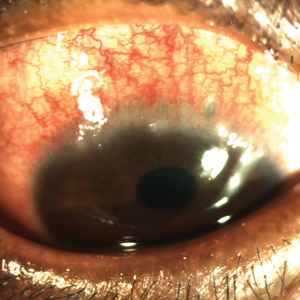
Keratoconus is a noninflammatory ocular disorder characterized by progressive thinning and conelike protrusion of the cornea. The corneal topographic changes result in high irregular astigmatism and reduced visual acuity, which can manifest as image blurring or distortion (Figure 2).2,9 Multiple case series and controlled studies have reported a positive association between keratoconus and a history of atopic disease.10,11
The precise etiology of keratoconus in the context of AD is unclear and likely is multifactorial. Habitual eye rubbing from periocular pruritus and discomfort has been reported to be a notable contributor to keratoconus.12 In addition, intrinsic inflammation and imbalance of cytokines and proteases also may contribute to development of keratoconus.13
Keratoconus is a progressive condition that can severely impact vision, making it critical to diagnose patients before irreversible vision loss occurs. Individuals with risk factors, such as AD of the eyelids, history of eye rubbing, or family history of keratoconus, should be advised to receive routine vision screening for worsening astigmatism, especially during the first few decades of life when keratoconus progresses rapidly.
The conservative management for early keratoconus includes glasses and gas-permeable contact lenses for correction of visual acuity and astigmatism. For advanced keratoconus, scleral lenses often are prescribed. These large-diameter, gas-permeable lenses are designed to rest on the sclera and arch over the entire cornea.9 Alternatively, corneal collagen cross-linking is a newer technique that utilizes riboflavin and UVA irradiation to strengthen the corneal tissue. It has proven to be safe and effective in slowing or stopping the progression of keratoconus, particularly when treated at the early stage, and received US Food and Drug Administration approval in 2016.9
Glaucoma
Glaucoma is a well-known complication of AD and can lead to irreversible ocular hypertension and optic nerve damage. Corticosteroid use is a major risk factor for glaucoma, and the rise in IOP is thought to be due to increased aqueous outflow resistance.14
Multiple case reports have linked glaucoma to long-term use of potent topical corticosteroids in the facial and palpebral regions, which has been attributed to direct steroid contact and absorption by ocular tissues, as glaucoma rarely occurs with topical steroid application elsewhere on the body.15-17 Systemic steroids (ie, prednisolone) taken for more than 8 weeks also have been associated with a marked rise in IOP.18
Certain risk factors may predispose a steroid user to increased IOP, including existing open-angle glaucoma, diabetes mellitus, collagen disease, and high myopia.15,19 Steroid responders and younger individuals also demonstrate increased sensitivity to steroids.20
Given that glaucoma often is asymptomatic until advanced stages, early detection is the key for proper intervention. Periodic glaucoma screening by an ophthalmologist would be appropriate for known steroid responders, as well as patients with a prolonged history of topical steroid application in the palpebral region and systemic steroid use, family history of glaucoma, or known ocular pathology.21 Furthermore, patients with concurrent glaucoma and AD should be jointly managed by dermatology and ophthalmology, and systemic and topical corticosteroid use should be minimized in favor of alterative agents such as calcineurin inhibitors.22
In addition to steroid-induced glaucoma, intrinsic atopic glaucoma recently has been proposed as a clinical entity and is characterized by increased inflammatory cytokines—IL-8 and CCL2—in the aqueous humor and abnormal accumulation of fibers in corneoscleral meshwork.23
Cataracts
Cataracts are estimated to affect 8% to 25% of patients with AD.21,24 Unlike age-related cataracts, cataracts associated with AD are observed in adolescents and young adults in addition to the older population. The progression of lenticular opacity can rapidly occur and has been reported to coincide with AD flares.25,26
Patients with AD typically present with anterior or posterior subcapsular cataracts instead of nuclear and cortical cataracts, which are more common in the general population.27,28 Anterior subcapsular cataracts are more specific to AD, whereas posterior subcapsular cataracts are associated with both prolonged corticosteroid use and AD.26 Children generally are more sensitive to steroids than adults and may develop cataracts more rapidly and at lower concentrations.29
The pathophysiology of cataract formation and progression in the context of AD is multifactorial. Cataract patients with AD have compromised blood-retinal barrier integrity as well as increased oxidative damage in the lens.30,31 Genetics and blunt trauma from eye rubbing are thought to play a role, and the latter has been associated with faster progression of cataracts.28 In contrast, corticosteroid-induced cataracts likely are caused by transcriptional changes and disrupted osmotic balance in the lens fibers, which can lead to fiber rupture and lens opacification.26,32 Systemic corticosteroids show the strongest association with cataract development, but inhaled and topical steroids also have been implicated.26
Although cataracts can be surgically corrected, prevention is critical. Patients with early-onset periorbital AD, prolonged use of topical or systemic corticosteroids, and family history of cataracts should be routinely screened. Anterior and posterior subcapsular cataracts are diagnosed with red reflex examinations that can be readily performed by the primary care physician or ophthalmologist.33 Atopic dermatitis patients with cataracts should be advised to use calcineurin inhibitors and alternative treatments in place of corticosteroids.
Retinal Detachment
Retinal detachment (RD) is a serious complication of AD that can present in individuals younger than 35 years. The incidence of RD in patients with AD has been estimated to be 4% to 8%.34 Retinal detachment manifests with visual disturbances such as flashing lights, shadows, visual field defect, and blurring of vision, but also may occur in the absence of vision changes.35,36
Across multiple case series, patients who developed RD were consistently found to have AD in the facial or periorbital region and a history of chronic eye rubbing. Multiple patients also presented with concurrent proliferative vitreoretinopathy, lens subluxation, and/or cataracts.35,37 The mechanism for RD has been attributed to ocular contusion from vigorous eye rubbing, as fundus findings between traumatic and AD-associated RD are similarly characterized by tractional breaks in the retina at vitreous base borders.37
Avoidance of eye rubbing and optimized treatment of facial AD may help prevent RD in patients with AD. Furthermore, all patients with symptoms of RD should be immediately referred to ophthalmology for surgical repair.
Herpetic Ocular Disease
Ocular herpes simplex virus infections cause ocular pain and are associated with notable visual morbidity, as recurrences can result in irreversible corneal scarring and neovascularization. Two retrospective case-control studies independently reported that individuals with a history of AD are at greater risk for herpetic ocular disease compared to age-matched controls.38,39 Furthermore, atopic disease is associated with higher recurrence rates and slower regeneration of the corneal epithelium.40
These findings suggest that AD patients with a history of recurrent herpetic ocular diseases should be closely monitored and treated with antiviral prophylaxis and/or topical corticosteroids, depending on the type of keratitis (epithelial or stromal).40 Furthermore, active ocular herpetic infections warrant urgent referral to an ophthalmologist.
Dupilumab-Associated Ocular Complications
Dupilumab, a monoclonal antibody that blocks IL-4 and IL-13 signaling, is the first biologic therapy to be approved for treatment of moderate to severe AD. Prior clinical trials have described a higher incidence of anterior conjunctivitis in dupilumab-treated AD patients (5%–28%) compared to placebo (2%–11%).41 Of note, the incidence may be as high as 70%, as reported in a recent case series.42 Interestingly, independent trials assessing dupilumab treatment in asthma, nasal polyposis, and eosinophilic esophagitis patients did not observe a higher incidence of conjunctivitis in dupilumab-treated patients compared to placebo, suggesting an AD-specific mechanism.43
Prominent features of dupilumab-associated conjunctivitis include hyperemia of the conjunctiva and limbus, in addition to ocular symptoms such as tearing, burning, and bilateral decrease in visual acuity. Marked reduction of conjunctival goblet cells has been reported.44 In addition to conjunctivitis, blepharitis also has been reported during dupilumab treatment.45
Standardized treatment guidelines for dupilumab-associated ocular complications have not yet been established. Surprisingly, antihistamine eye drops appear to be inefficacious in the treatment of dupilumab-associated conjunctivitis.41 However, the condition has been successfully managed with topical steroids (fluorometholone ophthalmic suspension 0.1%) and tacrolimus ointment 0.03%.41 Lifitegrast, an anti-inflammatory agent approved for chronic dry eye, also has been suggested as a treatment option for patients refractory to topical steroids.45 Alternatively, cessation of dupilumab could be considered in AD patients who experience severe ocular complications. Atopic dermatitis patients taking dupilumab who have any concerning signs for ocular complications should be referred to an ophthalmologist for further diagnosis and management.
Conclusion
Practicing dermatologists likely will encounter patients with concurrent AD and ocular complications. Although eye examinations are not routinely performed in the care of AD patients, dermatologists can proactively inquire about ocular symptoms and monitor patients longitudinally. Early diagnosis and treatment of these ocular conditions can prevent vision loss in these patients. Furthermore, symptomatic control of AD and careful consideration of the side-effect profiles of medications can potentially reduce the incidence of ocular complications in individuals with AD.
Patients with visual concerns or risk factors, such as a history of vigorous eye rubbing or chronic corticosteroid use, should be jointly managed with an ophthalmologist for optimized care. Moreover, acute exacerbations of ocular symptoms and visual deterioration warrant urgent referral to ophthalmology.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1.
- Govind K, Whang K, Khanna R, et al. Atopic dermatitis is associated with increased prevalence of multiple ocular comorbidities. J Allergy Clin Immunol Pract. 2019;7:298-299.
- Thyssen JP, Toft PB, Halling-Overgaard AS, et al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.e281.
- Putnam CM. Diagnosis and management of blepharitis: an optometrist’s perspective. Clin Optom (Auckl). 2016;8:71-78.
- Amescua G, Akpek EK, Farid M, et al. Blepharitis Preferred Practice Pattern®. Ophthalmology. 2019;126:P56-P93.
- Bielory B, Bielory L. Atopic dermatitis and keratoconjunctivitis. Immunol Allergy Clin North Am. 2010;30:323-336.
- Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2010;10:478-485.
- Chen JJ, Applebaum DS, Sun GS, et al. Atopic keratoconjunctivitis: a review. J Am Acad Dermatol. 2014;70:569-575.
- Andreanos KD, Hashemi K, Petrelli M, et al. Keratoconus treatment algorithm. Ophthalmol Ther. 2017;6:245-262.
- Rahi A, Davies P, Ruben M, et al. Keratoconus and coexisting atopic disease. Br J Ophthalmol. 1977;61:761-764.
- Gasset AR, Hinson WA, Frias JL. Keratoconus and atopic diseases. Ann Ophthalmol. 1978;10:991-994.
- Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84:834-836.
- Galvis V, Sherwin T, Tello A, et al. Keratoconus: an inflammatory disorder? Eye (Lond). 2015;29:843-859.
- Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88:752-759.
- Daniel BS, Orchard D. Ocular side-effects of topical corticosteroids: what a dermatologist needs to know. Australas J Dermatol. 2015;56:164-169.
- Garrott HM, Walland MJ. Glaucoma from topical corticosteroids to the eyelids. Clin Exp Ophthalmol. 2004;32:224-226.
- Aggarwal RK, Potamitis T, Chong NH, et al. Extensive visual loss with topical facial steroids. Eye (Lond). 1993;7(pt 5):664-666.
- Mandapati JS, Metta AK. Intraocular pressure variation in patients on long-term corticosteroids. Indian Dermatol Online J. 2011;2:67-69.
- Jones R 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163-167.
- Tamagawa-Mineoka R, Yasuoka N, Ueta M, et al. Influence of topical steroids on intraocular pressure in patients with atopic dermatitis. Allergol Int. 2018;67:388-391.
- Bercovitch L. Screening for ocular complications in atopic dermatitis. Arch Dermatol. 2011;147:588-589.
- Abramovits W, Hung P, Tong KB. Efficacy and economics of topical calcineurin inhibitors for the treatment of atopic dermatitis. Am J Clin Dermatol. 2006;7:213-222.
- Takakuwa K, Hamanaka T, Mori K, et al. Atopic glaucoma: clinical and pathophysiological analysis. J Glaucoma. 2015;24:662-668.
- Haeck IM, Rouwen TJ, Timmer-de Mik L, et al. Topical corticosteroids in atopic dermatitis and the risk of glaucoma and cataracts. J Am Acad Dermatol. 2011;64:275-281.
- Amemiya T, Matsuda H, Uehara M. Ocular findings in atopic dermatitis with special reference to the clinical features of atopic cataract. Ophthalmologica. 1980;180:129-132.
- Tatham A. Atopic dermatitis, cutaneous steroids and cataracts in children: two case reports. J Med Case Rep. 2008;2:124.
- Chew M, Chiang PP, Zheng Y, et al. The impact of cataract, cataract types, and cataract grades on vision-specific functioning using Rasch analysis. Am J Ophthalmol. 2012;154:29-38.
- Nagaki Y, Hayasaka S, Kadoi C. Cataract progression in patients with atopic dermatitis. J Cataract Refract Surg. 1999;25:96-99.
- Kaye LD, Kalenak JW, Price RL, et al. Ocular implications of long-term prednisone therapy in children. J Pediatr Ophthalmol Strabismus. 1993;30:142-144.
- Matsuo T, Saito H, Matsuo N. Cataract and aqueous flare levels in patients with atopic dermatitis. Am J Ophthalmol. 1997;124:36-39.
- Namazi MR, Handjani F, Amirahmadi M. Increased oxidative activity from hydrogen peroxide may be the cause of the predisposition to cataracts among patients with atopic dermatitis. Med Hypotheses. 2006;66:863-864.
- James ER. The etiology of steroid cataract. J Ocul Pharmacol Ther. 2007;23:403-420.
- Lambert SR, Teng JMC. Assessing whether the cataracts associated with atopic dermatitis are associated with steroids or inflammatory factors. JAMA Ophthalmol. 2018;136:918-919.
- Sasoh M, Mizutani H, Matsubara H, et al. Incidence of retinal detachment associated with atopic dermatitis in Japan: review of cases from 1992 to 2011. Clin Ophthalmol. 2015;9:1129-1134.
- Yoneda K, Okamoto H, Wada Y, et al. Atopic retinal detachment. report of four cases and a review of the literature. Br J Dermatol. 1995;133:586-591.
- Gnana Jothi V, McGimpsey S, Sharkey JA, et al. Retinal detachment repair and cataract surgery in patients with atopic dermatitis. Eye (Lond). 2017;31:1296-1301.
- Oka C, Ideta H, Nagasaki H, et al. Retinal detachment with atopic dermatitis similar to traumatic retinal detachment. Ophthalmology. 1994;101:1050-1054.
- Prabriputaloong T, Margolis TP, Lietman TM, et al. Atopic disease and herpes simplex eye disease: a population-based case-control study. Am J Ophthalmol. 2006;142:745-749.
- Borkar DS, Gonzales JA, Tham VM, et al. Association between atopy and herpetic eye disease: results from the pacific ocular inflammation study. JAMA Ophthalmol. 2014;132:326-331.
- Rezende RA, Hammersmith K, Bisol T, et al. Comparative study of ocular herpes simplex virus in patients with and without self-reported atopy. Am J Ophthalmol. 2006;141:1120-1125.
- Wollenberg A, Ariens L, Thurau S, et al. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J Allergy Clin Immunol Pract. 2018;6:1778-1780.e1.
- Ivert LU, Wahlgren CF, Ivert L, et al. Eye complications during dupilumab treatment for severe atopic dermatitis. Acta Derm Venereol. 2019;99:375-378.
- Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials [published online March 9, 2019]. Br J Dermatol. doi:10.1111/bjd.17869.
- Bakker DS, Ariens LFM, van Luijk C, et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. 2019;180:1248-1249.
- Zirwas MJ, Wulff K, Beckman K. Lifitegrast add-on treatment for dupilumab-induced ocular surface disease (DIOSD): a novel case report. JAAD Case Rep. 2019;5:34-36.
Atopic dermatitis (AD) is a chronic inflammatory skin condition with a lifetime prevalence of 15% to 20% in industrialized countries.1 It affects both children and adults and is predominantly characterized by a waxing and waning course of eczematous skin lesions and pruritus. In recent years, there is increasing recognition that AD can present with extracutaneous findings. Large-scale epidemiologic studies have reported a notably higher prevalence of ophthalmic complications in the AD population compared to the general population, in a severity-dependent manner.2,3 Potential complications include blepharitis, keratoconjunctivitis, keratoconus, glaucoma, cataracts, retinal detachment, ophthalmic herpes simplex virus infections, and dupilumab-associated ocular complications.
The etiology of each ocular complication in the context of AD is complex and likely multifactorial. Intrinsic immune dysregulation, physical trauma from eye rubbing, AD medication side effects, and genetics all have been speculated to play a role.2 Some of these ocular complications have a chronic course, while others present with sudden onset of symptoms; many of them can result in visual impairment if undiagnosed or left untreated. This article reviews several of the most common ocular comorbidities associated with AD. We discuss the clinical presentation, pathophysiology, and management strategies for each condition.
Blepharitis
Blepharitis, an inflammatory condition of the eyelids, is estimated to affect more than 6% of patients with AD compared to less than 1% of the general population.2 Blepharitis can be classified as anterior or posterior, based on the anatomic location of the affected region relative to the lash margin. Affected individuals may experience pruritus and irritation of the eyelids, tearing, a foreign body or burning sensation, crusting of the eyelids, and photophobia.4 Anterior blepharitis commonly is due to staphylococcal disease, and posterior blepharitis is secondary to structural changes and obstruction of meibomian gland orifices.
Although the pathophysiology is not well defined, xerosis in atopic patients is accompanied by barrier disruption and transepidermal water loss, which promote eyelid skin inflammation.
The mainstay of therapy for atopic blepharitis consists of conventional lid hygiene regimens, such as warm compresses and gentle scrubbing of the lid margins to remove crust and debris, which can be done with nonprescription cleansers, pads, and baby shampoos. Acute exacerbations may require topical antibiotics (ie, erythromycin or bacitracin applied to the lid margins once daily), topical calcineurin inhibitors (ie, cyclosporine ophthalmic emulsion 0.05%), or low-potency topical corticosteroids (ie, fluorometholone 0.1% or loteprednol etabonate 0.5% ophthalmic suspensions).5 Due to potential side effects of medications, especially topical corticosteroids, patients should be referred to ophthalmologists for definitive diagnosis and treatment.
Keratoconjunctivitis
Atopic keratoconjunctivitis (AKC) is a noninfectious inflammatory condition of the cornea and conjunctiva that occurs in an estimated 25% to 42% of patients with AD.6,7 It frequently presents in late adolescence and has a peak incidence between 30 and 50 years of age.8 The symptoms of AKC include ocular pruritus, redness, ropy mucoid discharge, burning discomfort, photophobia, and blurring of vision. Corneal involvement can progress to corneal neovascularization and punctate or macroepithelial erosions and ulcerations, which increase the risk for corneal scarring and visual impairment.7
Keratoconjunctivitis is a complex inflammatory disease characterized by infiltration of the conjunctival epithelium by eosinophils, mast cells, and lymphocytes. On examination, patients frequently are found to have concurrent AD of the periorbital skin as well as papillary hypertrophy of the tarsal conjunctiva with accompanying fibrosis, which can lead to entropion (turning inward of the lid margins and lashes) in severe cases.7 Ophthalmic evaluation is strongly recommended for patients with AKC to control symptoms, to limit exacerbations, and to prevent sight-threatening inflammation leading to vision loss. Treatment can be challenging given the chronicity of the condition and may require multiple treatment arms. Conservative measures include cool compresses and treatment with ophthalmic eye drops containing antihistamines (ie, ketotifen 0.025% [available over-the-counter]) and mast cell stabilizers (ie, olopatadine ophthalmic solution 0.1%).8 Atopic keratoconjunctivitis exacerbations may require short-term use of topical steroids or calcineurin inhibitors, or systemic equivalents for refractory cases.6 Long-term maintenance therapy typically consists of proper eye hygiene and steroid-sparing agents that reduce ocular inflammation, such as topical cyclosporine and tacrolimus, neither of which are associated with increased intraocular pressure (IOP)(Figure 1).8 Cornea disease resulting from chronic conjunctival/lid microtrauma can be managed with soft or scleral contact lenses.
Keratoconus
Keratoconus is a noninflammatory ocular disorder characterized by progressive thinning and conelike protrusion of the cornea. The corneal topographic changes result in high irregular astigmatism and reduced visual acuity, which can manifest as image blurring or distortion (Figure 2).2,9 Multiple case series and controlled studies have reported a positive association between keratoconus and a history of atopic disease.10,11
The precise etiology of keratoconus in the context of AD is unclear and likely is multifactorial. Habitual eye rubbing from periocular pruritus and discomfort has been reported to be a notable contributor to keratoconus.12 In addition, intrinsic inflammation and imbalance of cytokines and proteases also may contribute to development of keratoconus.13
Keratoconus is a progressive condition that can severely impact vision, making it critical to diagnose patients before irreversible vision loss occurs. Individuals with risk factors, such as AD of the eyelids, history of eye rubbing, or family history of keratoconus, should be advised to receive routine vision screening for worsening astigmatism, especially during the first few decades of life when keratoconus progresses rapidly.
The conservative management for early keratoconus includes glasses and gas-permeable contact lenses for correction of visual acuity and astigmatism. For advanced keratoconus, scleral lenses often are prescribed. These large-diameter, gas-permeable lenses are designed to rest on the sclera and arch over the entire cornea.9 Alternatively, corneal collagen cross-linking is a newer technique that utilizes riboflavin and UVA irradiation to strengthen the corneal tissue. It has proven to be safe and effective in slowing or stopping the progression of keratoconus, particularly when treated at the early stage, and received US Food and Drug Administration approval in 2016.9
Glaucoma
Glaucoma is a well-known complication of AD and can lead to irreversible ocular hypertension and optic nerve damage. Corticosteroid use is a major risk factor for glaucoma, and the rise in IOP is thought to be due to increased aqueous outflow resistance.14
Multiple case reports have linked glaucoma to long-term use of potent topical corticosteroids in the facial and palpebral regions, which has been attributed to direct steroid contact and absorption by ocular tissues, as glaucoma rarely occurs with topical steroid application elsewhere on the body.15-17 Systemic steroids (ie, prednisolone) taken for more than 8 weeks also have been associated with a marked rise in IOP.18
Certain risk factors may predispose a steroid user to increased IOP, including existing open-angle glaucoma, diabetes mellitus, collagen disease, and high myopia.15,19 Steroid responders and younger individuals also demonstrate increased sensitivity to steroids.20
Given that glaucoma often is asymptomatic until advanced stages, early detection is the key for proper intervention. Periodic glaucoma screening by an ophthalmologist would be appropriate for known steroid responders, as well as patients with a prolonged history of topical steroid application in the palpebral region and systemic steroid use, family history of glaucoma, or known ocular pathology.21 Furthermore, patients with concurrent glaucoma and AD should be jointly managed by dermatology and ophthalmology, and systemic and topical corticosteroid use should be minimized in favor of alterative agents such as calcineurin inhibitors.22
In addition to steroid-induced glaucoma, intrinsic atopic glaucoma recently has been proposed as a clinical entity and is characterized by increased inflammatory cytokines—IL-8 and CCL2—in the aqueous humor and abnormal accumulation of fibers in corneoscleral meshwork.23
Cataracts
Cataracts are estimated to affect 8% to 25% of patients with AD.21,24 Unlike age-related cataracts, cataracts associated with AD are observed in adolescents and young adults in addition to the older population. The progression of lenticular opacity can rapidly occur and has been reported to coincide with AD flares.25,26
Patients with AD typically present with anterior or posterior subcapsular cataracts instead of nuclear and cortical cataracts, which are more common in the general population.27,28 Anterior subcapsular cataracts are more specific to AD, whereas posterior subcapsular cataracts are associated with both prolonged corticosteroid use and AD.26 Children generally are more sensitive to steroids than adults and may develop cataracts more rapidly and at lower concentrations.29
The pathophysiology of cataract formation and progression in the context of AD is multifactorial. Cataract patients with AD have compromised blood-retinal barrier integrity as well as increased oxidative damage in the lens.30,31 Genetics and blunt trauma from eye rubbing are thought to play a role, and the latter has been associated with faster progression of cataracts.28 In contrast, corticosteroid-induced cataracts likely are caused by transcriptional changes and disrupted osmotic balance in the lens fibers, which can lead to fiber rupture and lens opacification.26,32 Systemic corticosteroids show the strongest association with cataract development, but inhaled and topical steroids also have been implicated.26
Although cataracts can be surgically corrected, prevention is critical. Patients with early-onset periorbital AD, prolonged use of topical or systemic corticosteroids, and family history of cataracts should be routinely screened. Anterior and posterior subcapsular cataracts are diagnosed with red reflex examinations that can be readily performed by the primary care physician or ophthalmologist.33 Atopic dermatitis patients with cataracts should be advised to use calcineurin inhibitors and alternative treatments in place of corticosteroids.
Retinal Detachment
Retinal detachment (RD) is a serious complication of AD that can present in individuals younger than 35 years. The incidence of RD in patients with AD has been estimated to be 4% to 8%.34 Retinal detachment manifests with visual disturbances such as flashing lights, shadows, visual field defect, and blurring of vision, but also may occur in the absence of vision changes.35,36
Across multiple case series, patients who developed RD were consistently found to have AD in the facial or periorbital region and a history of chronic eye rubbing. Multiple patients also presented with concurrent proliferative vitreoretinopathy, lens subluxation, and/or cataracts.35,37 The mechanism for RD has been attributed to ocular contusion from vigorous eye rubbing, as fundus findings between traumatic and AD-associated RD are similarly characterized by tractional breaks in the retina at vitreous base borders.37
Avoidance of eye rubbing and optimized treatment of facial AD may help prevent RD in patients with AD. Furthermore, all patients with symptoms of RD should be immediately referred to ophthalmology for surgical repair.
Herpetic Ocular Disease
Ocular herpes simplex virus infections cause ocular pain and are associated with notable visual morbidity, as recurrences can result in irreversible corneal scarring and neovascularization. Two retrospective case-control studies independently reported that individuals with a history of AD are at greater risk for herpetic ocular disease compared to age-matched controls.38,39 Furthermore, atopic disease is associated with higher recurrence rates and slower regeneration of the corneal epithelium.40
These findings suggest that AD patients with a history of recurrent herpetic ocular diseases should be closely monitored and treated with antiviral prophylaxis and/or topical corticosteroids, depending on the type of keratitis (epithelial or stromal).40 Furthermore, active ocular herpetic infections warrant urgent referral to an ophthalmologist.
Dupilumab-Associated Ocular Complications
Dupilumab, a monoclonal antibody that blocks IL-4 and IL-13 signaling, is the first biologic therapy to be approved for treatment of moderate to severe AD. Prior clinical trials have described a higher incidence of anterior conjunctivitis in dupilumab-treated AD patients (5%–28%) compared to placebo (2%–11%).41 Of note, the incidence may be as high as 70%, as reported in a recent case series.42 Interestingly, independent trials assessing dupilumab treatment in asthma, nasal polyposis, and eosinophilic esophagitis patients did not observe a higher incidence of conjunctivitis in dupilumab-treated patients compared to placebo, suggesting an AD-specific mechanism.43
Prominent features of dupilumab-associated conjunctivitis include hyperemia of the conjunctiva and limbus, in addition to ocular symptoms such as tearing, burning, and bilateral decrease in visual acuity. Marked reduction of conjunctival goblet cells has been reported.44 In addition to conjunctivitis, blepharitis also has been reported during dupilumab treatment.45
Standardized treatment guidelines for dupilumab-associated ocular complications have not yet been established. Surprisingly, antihistamine eye drops appear to be inefficacious in the treatment of dupilumab-associated conjunctivitis.41 However, the condition has been successfully managed with topical steroids (fluorometholone ophthalmic suspension 0.1%) and tacrolimus ointment 0.03%.41 Lifitegrast, an anti-inflammatory agent approved for chronic dry eye, also has been suggested as a treatment option for patients refractory to topical steroids.45 Alternatively, cessation of dupilumab could be considered in AD patients who experience severe ocular complications. Atopic dermatitis patients taking dupilumab who have any concerning signs for ocular complications should be referred to an ophthalmologist for further diagnosis and management.
Conclusion
Practicing dermatologists likely will encounter patients with concurrent AD and ocular complications. Although eye examinations are not routinely performed in the care of AD patients, dermatologists can proactively inquire about ocular symptoms and monitor patients longitudinally. Early diagnosis and treatment of these ocular conditions can prevent vision loss in these patients. Furthermore, symptomatic control of AD and careful consideration of the side-effect profiles of medications can potentially reduce the incidence of ocular complications in individuals with AD.
Patients with visual concerns or risk factors, such as a history of vigorous eye rubbing or chronic corticosteroid use, should be jointly managed with an ophthalmologist for optimized care. Moreover, acute exacerbations of ocular symptoms and visual deterioration warrant urgent referral to ophthalmology.
Atopic dermatitis (AD) is a chronic inflammatory skin condition with a lifetime prevalence of 15% to 20% in industrialized countries.1 It affects both children and adults and is predominantly characterized by a waxing and waning course of eczematous skin lesions and pruritus. In recent years, there is increasing recognition that AD can present with extracutaneous findings. Large-scale epidemiologic studies have reported a notably higher prevalence of ophthalmic complications in the AD population compared to the general population, in a severity-dependent manner.2,3 Potential complications include blepharitis, keratoconjunctivitis, keratoconus, glaucoma, cataracts, retinal detachment, ophthalmic herpes simplex virus infections, and dupilumab-associated ocular complications.
The etiology of each ocular complication in the context of AD is complex and likely multifactorial. Intrinsic immune dysregulation, physical trauma from eye rubbing, AD medication side effects, and genetics all have been speculated to play a role.2 Some of these ocular complications have a chronic course, while others present with sudden onset of symptoms; many of them can result in visual impairment if undiagnosed or left untreated. This article reviews several of the most common ocular comorbidities associated with AD. We discuss the clinical presentation, pathophysiology, and management strategies for each condition.
Blepharitis
Blepharitis, an inflammatory condition of the eyelids, is estimated to affect more than 6% of patients with AD compared to less than 1% of the general population.2 Blepharitis can be classified as anterior or posterior, based on the anatomic location of the affected region relative to the lash margin. Affected individuals may experience pruritus and irritation of the eyelids, tearing, a foreign body or burning sensation, crusting of the eyelids, and photophobia.4 Anterior blepharitis commonly is due to staphylococcal disease, and posterior blepharitis is secondary to structural changes and obstruction of meibomian gland orifices.
Although the pathophysiology is not well defined, xerosis in atopic patients is accompanied by barrier disruption and transepidermal water loss, which promote eyelid skin inflammation.
The mainstay of therapy for atopic blepharitis consists of conventional lid hygiene regimens, such as warm compresses and gentle scrubbing of the lid margins to remove crust and debris, which can be done with nonprescription cleansers, pads, and baby shampoos. Acute exacerbations may require topical antibiotics (ie, erythromycin or bacitracin applied to the lid margins once daily), topical calcineurin inhibitors (ie, cyclosporine ophthalmic emulsion 0.05%), or low-potency topical corticosteroids (ie, fluorometholone 0.1% or loteprednol etabonate 0.5% ophthalmic suspensions).5 Due to potential side effects of medications, especially topical corticosteroids, patients should be referred to ophthalmologists for definitive diagnosis and treatment.
Keratoconjunctivitis
Atopic keratoconjunctivitis (AKC) is a noninfectious inflammatory condition of the cornea and conjunctiva that occurs in an estimated 25% to 42% of patients with AD.6,7 It frequently presents in late adolescence and has a peak incidence between 30 and 50 years of age.8 The symptoms of AKC include ocular pruritus, redness, ropy mucoid discharge, burning discomfort, photophobia, and blurring of vision. Corneal involvement can progress to corneal neovascularization and punctate or macroepithelial erosions and ulcerations, which increase the risk for corneal scarring and visual impairment.7
Keratoconjunctivitis is a complex inflammatory disease characterized by infiltration of the conjunctival epithelium by eosinophils, mast cells, and lymphocytes. On examination, patients frequently are found to have concurrent AD of the periorbital skin as well as papillary hypertrophy of the tarsal conjunctiva with accompanying fibrosis, which can lead to entropion (turning inward of the lid margins and lashes) in severe cases.7 Ophthalmic evaluation is strongly recommended for patients with AKC to control symptoms, to limit exacerbations, and to prevent sight-threatening inflammation leading to vision loss. Treatment can be challenging given the chronicity of the condition and may require multiple treatment arms. Conservative measures include cool compresses and treatment with ophthalmic eye drops containing antihistamines (ie, ketotifen 0.025% [available over-the-counter]) and mast cell stabilizers (ie, olopatadine ophthalmic solution 0.1%).8 Atopic keratoconjunctivitis exacerbations may require short-term use of topical steroids or calcineurin inhibitors, or systemic equivalents for refractory cases.6 Long-term maintenance therapy typically consists of proper eye hygiene and steroid-sparing agents that reduce ocular inflammation, such as topical cyclosporine and tacrolimus, neither of which are associated with increased intraocular pressure (IOP)(Figure 1).8 Cornea disease resulting from chronic conjunctival/lid microtrauma can be managed with soft or scleral contact lenses.
Keratoconus
Keratoconus is a noninflammatory ocular disorder characterized by progressive thinning and conelike protrusion of the cornea. The corneal topographic changes result in high irregular astigmatism and reduced visual acuity, which can manifest as image blurring or distortion (Figure 2).2,9 Multiple case series and controlled studies have reported a positive association between keratoconus and a history of atopic disease.10,11
The precise etiology of keratoconus in the context of AD is unclear and likely is multifactorial. Habitual eye rubbing from periocular pruritus and discomfort has been reported to be a notable contributor to keratoconus.12 In addition, intrinsic inflammation and imbalance of cytokines and proteases also may contribute to development of keratoconus.13
Keratoconus is a progressive condition that can severely impact vision, making it critical to diagnose patients before irreversible vision loss occurs. Individuals with risk factors, such as AD of the eyelids, history of eye rubbing, or family history of keratoconus, should be advised to receive routine vision screening for worsening astigmatism, especially during the first few decades of life when keratoconus progresses rapidly.
The conservative management for early keratoconus includes glasses and gas-permeable contact lenses for correction of visual acuity and astigmatism. For advanced keratoconus, scleral lenses often are prescribed. These large-diameter, gas-permeable lenses are designed to rest on the sclera and arch over the entire cornea.9 Alternatively, corneal collagen cross-linking is a newer technique that utilizes riboflavin and UVA irradiation to strengthen the corneal tissue. It has proven to be safe and effective in slowing or stopping the progression of keratoconus, particularly when treated at the early stage, and received US Food and Drug Administration approval in 2016.9
Glaucoma
Glaucoma is a well-known complication of AD and can lead to irreversible ocular hypertension and optic nerve damage. Corticosteroid use is a major risk factor for glaucoma, and the rise in IOP is thought to be due to increased aqueous outflow resistance.14
Multiple case reports have linked glaucoma to long-term use of potent topical corticosteroids in the facial and palpebral regions, which has been attributed to direct steroid contact and absorption by ocular tissues, as glaucoma rarely occurs with topical steroid application elsewhere on the body.15-17 Systemic steroids (ie, prednisolone) taken for more than 8 weeks also have been associated with a marked rise in IOP.18
Certain risk factors may predispose a steroid user to increased IOP, including existing open-angle glaucoma, diabetes mellitus, collagen disease, and high myopia.15,19 Steroid responders and younger individuals also demonstrate increased sensitivity to steroids.20
Given that glaucoma often is asymptomatic until advanced stages, early detection is the key for proper intervention. Periodic glaucoma screening by an ophthalmologist would be appropriate for known steroid responders, as well as patients with a prolonged history of topical steroid application in the palpebral region and systemic steroid use, family history of glaucoma, or known ocular pathology.21 Furthermore, patients with concurrent glaucoma and AD should be jointly managed by dermatology and ophthalmology, and systemic and topical corticosteroid use should be minimized in favor of alterative agents such as calcineurin inhibitors.22
In addition to steroid-induced glaucoma, intrinsic atopic glaucoma recently has been proposed as a clinical entity and is characterized by increased inflammatory cytokines—IL-8 and CCL2—in the aqueous humor and abnormal accumulation of fibers in corneoscleral meshwork.23
Cataracts
Cataracts are estimated to affect 8% to 25% of patients with AD.21,24 Unlike age-related cataracts, cataracts associated with AD are observed in adolescents and young adults in addition to the older population. The progression of lenticular opacity can rapidly occur and has been reported to coincide with AD flares.25,26
Patients with AD typically present with anterior or posterior subcapsular cataracts instead of nuclear and cortical cataracts, which are more common in the general population.27,28 Anterior subcapsular cataracts are more specific to AD, whereas posterior subcapsular cataracts are associated with both prolonged corticosteroid use and AD.26 Children generally are more sensitive to steroids than adults and may develop cataracts more rapidly and at lower concentrations.29
The pathophysiology of cataract formation and progression in the context of AD is multifactorial. Cataract patients with AD have compromised blood-retinal barrier integrity as well as increased oxidative damage in the lens.30,31 Genetics and blunt trauma from eye rubbing are thought to play a role, and the latter has been associated with faster progression of cataracts.28 In contrast, corticosteroid-induced cataracts likely are caused by transcriptional changes and disrupted osmotic balance in the lens fibers, which can lead to fiber rupture and lens opacification.26,32 Systemic corticosteroids show the strongest association with cataract development, but inhaled and topical steroids also have been implicated.26
Although cataracts can be surgically corrected, prevention is critical. Patients with early-onset periorbital AD, prolonged use of topical or systemic corticosteroids, and family history of cataracts should be routinely screened. Anterior and posterior subcapsular cataracts are diagnosed with red reflex examinations that can be readily performed by the primary care physician or ophthalmologist.33 Atopic dermatitis patients with cataracts should be advised to use calcineurin inhibitors and alternative treatments in place of corticosteroids.
Retinal Detachment
Retinal detachment (RD) is a serious complication of AD that can present in individuals younger than 35 years. The incidence of RD in patients with AD has been estimated to be 4% to 8%.34 Retinal detachment manifests with visual disturbances such as flashing lights, shadows, visual field defect, and blurring of vision, but also may occur in the absence of vision changes.35,36
Across multiple case series, patients who developed RD were consistently found to have AD in the facial or periorbital region and a history of chronic eye rubbing. Multiple patients also presented with concurrent proliferative vitreoretinopathy, lens subluxation, and/or cataracts.35,37 The mechanism for RD has been attributed to ocular contusion from vigorous eye rubbing, as fundus findings between traumatic and AD-associated RD are similarly characterized by tractional breaks in the retina at vitreous base borders.37
Avoidance of eye rubbing and optimized treatment of facial AD may help prevent RD in patients with AD. Furthermore, all patients with symptoms of RD should be immediately referred to ophthalmology for surgical repair.
Herpetic Ocular Disease
Ocular herpes simplex virus infections cause ocular pain and are associated with notable visual morbidity, as recurrences can result in irreversible corneal scarring and neovascularization. Two retrospective case-control studies independently reported that individuals with a history of AD are at greater risk for herpetic ocular disease compared to age-matched controls.38,39 Furthermore, atopic disease is associated with higher recurrence rates and slower regeneration of the corneal epithelium.40
These findings suggest that AD patients with a history of recurrent herpetic ocular diseases should be closely monitored and treated with antiviral prophylaxis and/or topical corticosteroids, depending on the type of keratitis (epithelial or stromal).40 Furthermore, active ocular herpetic infections warrant urgent referral to an ophthalmologist.
Dupilumab-Associated Ocular Complications
Dupilumab, a monoclonal antibody that blocks IL-4 and IL-13 signaling, is the first biologic therapy to be approved for treatment of moderate to severe AD. Prior clinical trials have described a higher incidence of anterior conjunctivitis in dupilumab-treated AD patients (5%–28%) compared to placebo (2%–11%).41 Of note, the incidence may be as high as 70%, as reported in a recent case series.42 Interestingly, independent trials assessing dupilumab treatment in asthma, nasal polyposis, and eosinophilic esophagitis patients did not observe a higher incidence of conjunctivitis in dupilumab-treated patients compared to placebo, suggesting an AD-specific mechanism.43
Prominent features of dupilumab-associated conjunctivitis include hyperemia of the conjunctiva and limbus, in addition to ocular symptoms such as tearing, burning, and bilateral decrease in visual acuity. Marked reduction of conjunctival goblet cells has been reported.44 In addition to conjunctivitis, blepharitis also has been reported during dupilumab treatment.45
Standardized treatment guidelines for dupilumab-associated ocular complications have not yet been established. Surprisingly, antihistamine eye drops appear to be inefficacious in the treatment of dupilumab-associated conjunctivitis.41 However, the condition has been successfully managed with topical steroids (fluorometholone ophthalmic suspension 0.1%) and tacrolimus ointment 0.03%.41 Lifitegrast, an anti-inflammatory agent approved for chronic dry eye, also has been suggested as a treatment option for patients refractory to topical steroids.45 Alternatively, cessation of dupilumab could be considered in AD patients who experience severe ocular complications. Atopic dermatitis patients taking dupilumab who have any concerning signs for ocular complications should be referred to an ophthalmologist for further diagnosis and management.
Conclusion
Practicing dermatologists likely will encounter patients with concurrent AD and ocular complications. Although eye examinations are not routinely performed in the care of AD patients, dermatologists can proactively inquire about ocular symptoms and monitor patients longitudinally. Early diagnosis and treatment of these ocular conditions can prevent vision loss in these patients. Furthermore, symptomatic control of AD and careful consideration of the side-effect profiles of medications can potentially reduce the incidence of ocular complications in individuals with AD.
Patients with visual concerns or risk factors, such as a history of vigorous eye rubbing or chronic corticosteroid use, should be jointly managed with an ophthalmologist for optimized care. Moreover, acute exacerbations of ocular symptoms and visual deterioration warrant urgent referral to ophthalmology.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1.
- Govind K, Whang K, Khanna R, et al. Atopic dermatitis is associated with increased prevalence of multiple ocular comorbidities. J Allergy Clin Immunol Pract. 2019;7:298-299.
- Thyssen JP, Toft PB, Halling-Overgaard AS, et al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.e281.
- Putnam CM. Diagnosis and management of blepharitis: an optometrist’s perspective. Clin Optom (Auckl). 2016;8:71-78.
- Amescua G, Akpek EK, Farid M, et al. Blepharitis Preferred Practice Pattern®. Ophthalmology. 2019;126:P56-P93.
- Bielory B, Bielory L. Atopic dermatitis and keratoconjunctivitis. Immunol Allergy Clin North Am. 2010;30:323-336.
- Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2010;10:478-485.
- Chen JJ, Applebaum DS, Sun GS, et al. Atopic keratoconjunctivitis: a review. J Am Acad Dermatol. 2014;70:569-575.
- Andreanos KD, Hashemi K, Petrelli M, et al. Keratoconus treatment algorithm. Ophthalmol Ther. 2017;6:245-262.
- Rahi A, Davies P, Ruben M, et al. Keratoconus and coexisting atopic disease. Br J Ophthalmol. 1977;61:761-764.
- Gasset AR, Hinson WA, Frias JL. Keratoconus and atopic diseases. Ann Ophthalmol. 1978;10:991-994.
- Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84:834-836.
- Galvis V, Sherwin T, Tello A, et al. Keratoconus: an inflammatory disorder? Eye (Lond). 2015;29:843-859.
- Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88:752-759.
- Daniel BS, Orchard D. Ocular side-effects of topical corticosteroids: what a dermatologist needs to know. Australas J Dermatol. 2015;56:164-169.
- Garrott HM, Walland MJ. Glaucoma from topical corticosteroids to the eyelids. Clin Exp Ophthalmol. 2004;32:224-226.
- Aggarwal RK, Potamitis T, Chong NH, et al. Extensive visual loss with topical facial steroids. Eye (Lond). 1993;7(pt 5):664-666.
- Mandapati JS, Metta AK. Intraocular pressure variation in patients on long-term corticosteroids. Indian Dermatol Online J. 2011;2:67-69.
- Jones R 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163-167.
- Tamagawa-Mineoka R, Yasuoka N, Ueta M, et al. Influence of topical steroids on intraocular pressure in patients with atopic dermatitis. Allergol Int. 2018;67:388-391.
- Bercovitch L. Screening for ocular complications in atopic dermatitis. Arch Dermatol. 2011;147:588-589.
- Abramovits W, Hung P, Tong KB. Efficacy and economics of topical calcineurin inhibitors for the treatment of atopic dermatitis. Am J Clin Dermatol. 2006;7:213-222.
- Takakuwa K, Hamanaka T, Mori K, et al. Atopic glaucoma: clinical and pathophysiological analysis. J Glaucoma. 2015;24:662-668.
- Haeck IM, Rouwen TJ, Timmer-de Mik L, et al. Topical corticosteroids in atopic dermatitis and the risk of glaucoma and cataracts. J Am Acad Dermatol. 2011;64:275-281.
- Amemiya T, Matsuda H, Uehara M. Ocular findings in atopic dermatitis with special reference to the clinical features of atopic cataract. Ophthalmologica. 1980;180:129-132.
- Tatham A. Atopic dermatitis, cutaneous steroids and cataracts in children: two case reports. J Med Case Rep. 2008;2:124.
- Chew M, Chiang PP, Zheng Y, et al. The impact of cataract, cataract types, and cataract grades on vision-specific functioning using Rasch analysis. Am J Ophthalmol. 2012;154:29-38.
- Nagaki Y, Hayasaka S, Kadoi C. Cataract progression in patients with atopic dermatitis. J Cataract Refract Surg. 1999;25:96-99.
- Kaye LD, Kalenak JW, Price RL, et al. Ocular implications of long-term prednisone therapy in children. J Pediatr Ophthalmol Strabismus. 1993;30:142-144.
- Matsuo T, Saito H, Matsuo N. Cataract and aqueous flare levels in patients with atopic dermatitis. Am J Ophthalmol. 1997;124:36-39.
- Namazi MR, Handjani F, Amirahmadi M. Increased oxidative activity from hydrogen peroxide may be the cause of the predisposition to cataracts among patients with atopic dermatitis. Med Hypotheses. 2006;66:863-864.
- James ER. The etiology of steroid cataract. J Ocul Pharmacol Ther. 2007;23:403-420.
- Lambert SR, Teng JMC. Assessing whether the cataracts associated with atopic dermatitis are associated with steroids or inflammatory factors. JAMA Ophthalmol. 2018;136:918-919.
- Sasoh M, Mizutani H, Matsubara H, et al. Incidence of retinal detachment associated with atopic dermatitis in Japan: review of cases from 1992 to 2011. Clin Ophthalmol. 2015;9:1129-1134.
- Yoneda K, Okamoto H, Wada Y, et al. Atopic retinal detachment. report of four cases and a review of the literature. Br J Dermatol. 1995;133:586-591.
- Gnana Jothi V, McGimpsey S, Sharkey JA, et al. Retinal detachment repair and cataract surgery in patients with atopic dermatitis. Eye (Lond). 2017;31:1296-1301.
- Oka C, Ideta H, Nagasaki H, et al. Retinal detachment with atopic dermatitis similar to traumatic retinal detachment. Ophthalmology. 1994;101:1050-1054.
- Prabriputaloong T, Margolis TP, Lietman TM, et al. Atopic disease and herpes simplex eye disease: a population-based case-control study. Am J Ophthalmol. 2006;142:745-749.
- Borkar DS, Gonzales JA, Tham VM, et al. Association between atopy and herpetic eye disease: results from the pacific ocular inflammation study. JAMA Ophthalmol. 2014;132:326-331.
- Rezende RA, Hammersmith K, Bisol T, et al. Comparative study of ocular herpes simplex virus in patients with and without self-reported atopy. Am J Ophthalmol. 2006;141:1120-1125.
- Wollenberg A, Ariens L, Thurau S, et al. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J Allergy Clin Immunol Pract. 2018;6:1778-1780.e1.
- Ivert LU, Wahlgren CF, Ivert L, et al. Eye complications during dupilumab treatment for severe atopic dermatitis. Acta Derm Venereol. 2019;99:375-378.
- Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials [published online March 9, 2019]. Br J Dermatol. doi:10.1111/bjd.17869.
- Bakker DS, Ariens LFM, van Luijk C, et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. 2019;180:1248-1249.
- Zirwas MJ, Wulff K, Beckman K. Lifitegrast add-on treatment for dupilumab-induced ocular surface disease (DIOSD): a novel case report. JAAD Case Rep. 2019;5:34-36.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1.
- Govind K, Whang K, Khanna R, et al. Atopic dermatitis is associated with increased prevalence of multiple ocular comorbidities. J Allergy Clin Immunol Pract. 2019;7:298-299.
- Thyssen JP, Toft PB, Halling-Overgaard AS, et al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.e281.
- Putnam CM. Diagnosis and management of blepharitis: an optometrist’s perspective. Clin Optom (Auckl). 2016;8:71-78.
- Amescua G, Akpek EK, Farid M, et al. Blepharitis Preferred Practice Pattern®. Ophthalmology. 2019;126:P56-P93.
- Bielory B, Bielory L. Atopic dermatitis and keratoconjunctivitis. Immunol Allergy Clin North Am. 2010;30:323-336.
- Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2010;10:478-485.
- Chen JJ, Applebaum DS, Sun GS, et al. Atopic keratoconjunctivitis: a review. J Am Acad Dermatol. 2014;70:569-575.
- Andreanos KD, Hashemi K, Petrelli M, et al. Keratoconus treatment algorithm. Ophthalmol Ther. 2017;6:245-262.
- Rahi A, Davies P, Ruben M, et al. Keratoconus and coexisting atopic disease. Br J Ophthalmol. 1977;61:761-764.
- Gasset AR, Hinson WA, Frias JL. Keratoconus and atopic diseases. Ann Ophthalmol. 1978;10:991-994.
- Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84:834-836.
- Galvis V, Sherwin T, Tello A, et al. Keratoconus: an inflammatory disorder? Eye (Lond). 2015;29:843-859.
- Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88:752-759.
- Daniel BS, Orchard D. Ocular side-effects of topical corticosteroids: what a dermatologist needs to know. Australas J Dermatol. 2015;56:164-169.
- Garrott HM, Walland MJ. Glaucoma from topical corticosteroids to the eyelids. Clin Exp Ophthalmol. 2004;32:224-226.
- Aggarwal RK, Potamitis T, Chong NH, et al. Extensive visual loss with topical facial steroids. Eye (Lond). 1993;7(pt 5):664-666.
- Mandapati JS, Metta AK. Intraocular pressure variation in patients on long-term corticosteroids. Indian Dermatol Online J. 2011;2:67-69.
- Jones R 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163-167.
- Tamagawa-Mineoka R, Yasuoka N, Ueta M, et al. Influence of topical steroids on intraocular pressure in patients with atopic dermatitis. Allergol Int. 2018;67:388-391.
- Bercovitch L. Screening for ocular complications in atopic dermatitis. Arch Dermatol. 2011;147:588-589.
- Abramovits W, Hung P, Tong KB. Efficacy and economics of topical calcineurin inhibitors for the treatment of atopic dermatitis. Am J Clin Dermatol. 2006;7:213-222.
- Takakuwa K, Hamanaka T, Mori K, et al. Atopic glaucoma: clinical and pathophysiological analysis. J Glaucoma. 2015;24:662-668.
- Haeck IM, Rouwen TJ, Timmer-de Mik L, et al. Topical corticosteroids in atopic dermatitis and the risk of glaucoma and cataracts. J Am Acad Dermatol. 2011;64:275-281.
- Amemiya T, Matsuda H, Uehara M. Ocular findings in atopic dermatitis with special reference to the clinical features of atopic cataract. Ophthalmologica. 1980;180:129-132.
- Tatham A. Atopic dermatitis, cutaneous steroids and cataracts in children: two case reports. J Med Case Rep. 2008;2:124.
- Chew M, Chiang PP, Zheng Y, et al. The impact of cataract, cataract types, and cataract grades on vision-specific functioning using Rasch analysis. Am J Ophthalmol. 2012;154:29-38.
- Nagaki Y, Hayasaka S, Kadoi C. Cataract progression in patients with atopic dermatitis. J Cataract Refract Surg. 1999;25:96-99.
- Kaye LD, Kalenak JW, Price RL, et al. Ocular implications of long-term prednisone therapy in children. J Pediatr Ophthalmol Strabismus. 1993;30:142-144.
- Matsuo T, Saito H, Matsuo N. Cataract and aqueous flare levels in patients with atopic dermatitis. Am J Ophthalmol. 1997;124:36-39.
- Namazi MR, Handjani F, Amirahmadi M. Increased oxidative activity from hydrogen peroxide may be the cause of the predisposition to cataracts among patients with atopic dermatitis. Med Hypotheses. 2006;66:863-864.
- James ER. The etiology of steroid cataract. J Ocul Pharmacol Ther. 2007;23:403-420.
- Lambert SR, Teng JMC. Assessing whether the cataracts associated with atopic dermatitis are associated with steroids or inflammatory factors. JAMA Ophthalmol. 2018;136:918-919.
- Sasoh M, Mizutani H, Matsubara H, et al. Incidence of retinal detachment associated with atopic dermatitis in Japan: review of cases from 1992 to 2011. Clin Ophthalmol. 2015;9:1129-1134.
- Yoneda K, Okamoto H, Wada Y, et al. Atopic retinal detachment. report of four cases and a review of the literature. Br J Dermatol. 1995;133:586-591.
- Gnana Jothi V, McGimpsey S, Sharkey JA, et al. Retinal detachment repair and cataract surgery in patients with atopic dermatitis. Eye (Lond). 2017;31:1296-1301.
- Oka C, Ideta H, Nagasaki H, et al. Retinal detachment with atopic dermatitis similar to traumatic retinal detachment. Ophthalmology. 1994;101:1050-1054.
- Prabriputaloong T, Margolis TP, Lietman TM, et al. Atopic disease and herpes simplex eye disease: a population-based case-control study. Am J Ophthalmol. 2006;142:745-749.
- Borkar DS, Gonzales JA, Tham VM, et al. Association between atopy and herpetic eye disease: results from the pacific ocular inflammation study. JAMA Ophthalmol. 2014;132:326-331.
- Rezende RA, Hammersmith K, Bisol T, et al. Comparative study of ocular herpes simplex virus in patients with and without self-reported atopy. Am J Ophthalmol. 2006;141:1120-1125.
- Wollenberg A, Ariens L, Thurau S, et al. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J Allergy Clin Immunol Pract. 2018;6:1778-1780.e1.
- Ivert LU, Wahlgren CF, Ivert L, et al. Eye complications during dupilumab treatment for severe atopic dermatitis. Acta Derm Venereol. 2019;99:375-378.
- Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials [published online March 9, 2019]. Br J Dermatol. doi:10.1111/bjd.17869.
- Bakker DS, Ariens LFM, van Luijk C, et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. 2019;180:1248-1249.
- Zirwas MJ, Wulff K, Beckman K. Lifitegrast add-on treatment for dupilumab-induced ocular surface disease (DIOSD): a novel case report. JAAD Case Rep. 2019;5:34-36.
Practice Points
- Atopic dermatitis (AD) is associated with various ocular comorbidities that can result in permanent vision loss if untreated.
- Timely recognition of ocular complications in AD patients is critical, and dermatologists should proactively inquire about ocular symptoms in the review of systems.
- Patients with ocular symptoms should be jointly managed with ophthalmology.
Quality of Life in Patients With Atopic Dermatitis
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease typically with childhood onset. In some cases, the condition persists, but AD usually resolves by the time a child reaches adulthood. Prevalence is difficult to estimate but, in developed countries, is approximately 15% to 30% among children and 2% to 10% among adults.1
Atopic dermatitis is characterized by chronically itchy dry skin, weeping erythematous papules and plaques, and lichenification. Furthermore, AD often is associated with other atopic diseases, such as food allergy, allergic rhinitis, and bronchial asthma.
In this article, we review the literature on the quality of life (QOL) of patients with AD. Our goals are to discuss the most common methods for measuring QOL in AD and how to use them; highlight specific alterations of QOL in AD; and review data about QOL of children with AD, which is underrepresented in the medical literature, as studies tend to focus on adults. In addition, we address the importance of assessing QOL in patients with AD due to the psychological burden of the disease.
Quality of Life
The harmful effects of AD can include a range of areas, including
Because QOL is an important instrument used in many AD studies, we call attention to the work of the Harmonising Outcome Measures for Eczema (HOME) initiative, which established a core outcome set for all AD clinical trials to enable comparison of results of individual studies.2 Quality of life was identified in HOME as one of 4 basic outcome measures that should be included in every AD trial (the others are clinician-reported signs, patient-reported symptoms, and long-term control).3 According to the recent agreement, the following QOL instruments should be used: Dermatology Life Quality Index (DLQI) for adults, Children’s Dermatology Life Quality Index (CDLQI) for children, and Infants’ Dermatitis Quality of Life Index (IDQOL) for infants.4
In dermatology, these instruments can be divided into 3 basic categories: generic, dermatology specific, and disease specific.5 Generic QOL questionnaires are beneficial when comparing the QOL of an AD patient to patients with other conditions or to healthy individuals. On the other hand, dermatology-specific and AD-specific methods are more effective instruments for detecting impairments linked directly to the disease and, therefore, are more sensitive to changes in QOL.5 Some of the most frequently used QOL measures5,6 for AD along with their key attributes are
Given that AD is a chronic disease that requires constant care, parents/guardians or the partner of the patient usually are affected as well. To detect this effect, the Family Dermatology Life Quality Index (FDLQI), a dermatology-specific instrument, measures the QOL in family members of dermatology patients.7 The Dermatitis Family Impact (DFI)8 is a disease-specific method for assessing how having a child with AD can impact the QOL of family members; it is a 10-item questionnaire completed by an adult family member. The FDLQI7 and DFI8 both help to understand the secondary impact of the disease.
In contrast, several other methods that also are administered by a parent/guardian assess how the parent perceives the QOL of their child with AD; these methods are essential for small children and infants who cannot answer questions themselves. The IDQOL9 was designed to assess the QOL of patients younger than 4 years using a parent-completed questionnaire. For older children and adolescents aged 4 to 16 years, the CDLQI10 is a widely used instrument; the questionnaire is completed by the child and is available in a cartoon format.10
For patients older than 16 years, 2 important instruments are the DLQI, a generic dermatology instrument, and the Quality of Life Index for Atopic Dermatitis (QoLIAD).11
Clearly it can be troublesome for researchers and clinicians to find the most suitable instrument to evaluate QOL in AD patients. To make this task easier, the European Academy of Dermatology and Venereology Task Force released a position paper with the following recommendations: (1) only validated instruments should be used, and (2) their use should be based on the age of the patients for which the instruments were designed. It is reommended that researchers use a combination of a generic and a dermatology-specific or AD-specific instrument, whereas clinicians should apply a dermatology-specific or AD-specific method, or both.5
Alterations of QOL in AD
Sleep Disturbance in AD
Sleep disorders observed in AD include difficulty falling asleep, frequent waking episodes, shorter sleep duration, and feelings of inadequate sleep, which often result in impairment of daily activity.12,13 Correlation has been found between sleep quality and QOL in both children and adults.14 Approximately 60% of children affected by AD experience a sleep disturbance,15 which seems to correlate well with disease severity.16 A US study found that adults with AD are more likely to experience a sleep disturbance, which often affects daytime functioning and work productivity.13
Financial Aspects and Impact on Work
The financial burden of AD is extensive.17 There are direct medical costs, including medication, visits to the physician, alternative therapies, and nonprescription products. Patients tend to spend relevant money on such items as moisturizers, bath products, antihistamines, topical steroids, and topical antibiotics.18,19 However, it seems that most of the cost of AD is due to indirect and nonmedical costs, including transportation to medical visits; loss of work days; extra childcare; and expenditures associated with lifestyle changes,19,20 such as modifying diet, wearing special clothes, using special bed linens, and purchasing special household items (eg, anti–dust mite vacuum cleaner, humidifier, new carpeting).17,19
Absenteeism from work often is a consequence of physician appointments; in addition, parents/guardians of a child with AD often miss work due to medical care. Even at work, patients (or parents/guardians) often experience decreased work productivity (so-called presenteeism) due to loss of sleep and anxiety.21 In addressing the effects of AD on work life, a systematic literature review found that AD strongly affects sick leave and might have an impact on job choice and change or loss of job.22
Furthermore, according to Su et al,23 the costs of AD are related to disease severity. Moreover, their data suggest that among chronic childhood diseases, the financial burden of AD is greater than the cost of asthma and similar to the cost of diabetes mellitus.23
Association Between QOL and Disease Severity
A large observational study found that improvement in AD severity was followed by an increase in QOL.24 A positive correlation between disease severity and QOL has been found in other studies,25,26 though no correlation or only moderate correlation also has been reported.27 Apparently, in addition to QOL, disease severity scores are substantial parameters in the evaluation of distress caused by AD; the HOME initiative has identified clinician-reported signs and patient-reported symptoms as 2 of 4 core outcomes domains to include in all future AD clinical trials.3 For measuring symptoms, the Patient-Oriented Eczema Measure (POEM) is the recommended instrument.28 Regarding clinical signs, the HOME group named the Eczema Area and Severity Index (EASI) as the preferred instrument.29
Psychological Burden
Stress is a triggering factor for AD, but the connection between skin and mind appears bidirectional. The biological reaction to stress probably lowers the itch threshold and disrupts the skin barrier.30 The Global Burden of Disease Study showed that skin diseases are the fourth leading cause of nonfatal disease burden.31 There are several factors—pruritus, scratch, and pain—that can all lead to sleep deprivation and daytime fatigue. Based on our experience, if lesions develop on visible areas, patients can feel stigmatized, which restricts their social life.
The most common psychological comorbidities of AD are anxiety and depression. In a cross-sectional, multicenter study, there was a significantly higher prevalence of depression (P<.001) and anxiety disorder (P=.02) among patients with common skin diseases compared to a control group.32 In a study that assessed AD patients, researchers found a higher risk of depression and anxiety.33 Suicidal ideation also is more common in the population with AD32,34; a study showed that the risk of suicidal ideation in adolescents was nearly 4-fold in patients with itching skin lesions compared to those without itch.34
According to Linnet and Jemec,35 mental and psychological comorbidities of AD are associated with lower QOL, not with clinical severity. As a result, to improve QOL in AD, one should take care of both dermatological and psychological problems. It has been demonstrated that psychological interventions, such as autogenic training, cognitive-behavioral therapy, relaxation techniques, habit reversal training,36 and hypnotherapy37 might be helpful in individual cases; educational interventions also are recommended.36 With these adjuvant therapies, psychological status, unpleasant clinical symptoms, and QOL could be improved, though further studies are needed to confirm these benefits.
Conclusion
Atopic dermatitis places a notable burden on patients and their families. The degree of burden is probably related to disease severity. For measuring QOL, researchers and clinicians should use validated methods suited to the age of the patients for which they were designed. More studies are needed to assess the effects of different treatments on QOL. Besides pharmacotherapy, psychotherapy and educational programs might be beneficial for improving QOL, another important area to be studied.
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483-1494.
- Schmitt J, Williams H; HOME Development Group. Harmonising Outcome Measures for Eczema (HOME). report from the First International Consensus Meeting (HOME 1), 24 July 2010, Munich, Germany. Br J Dermatol. 2010;163:1166-1168.
- Schmitt J, Spuls P, Boers M, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy. 2012;67:1111-1117.
- Quality of Life (QoL). Harmonising Outcome Measures for Eczema (HOME) website. http://www.homeforeczema.org/research/quality-of-life.aspx. Accessed August 18, 2019.
Chernyshov PV, Tomas-Aragones L, Manolache L, et al; EADV Quality of Life Task Force. Quality of life measurement in atopic dermatitis. Position paper of the European Academy of Dermatology and Venereology (EADV) Task Force on quality of life. J Eur Acad Dermatol Venereol. 2017;31:576-593. - Hill MK, Kheirandish Pishkenari A, Braunberger TL, et al. Recent trends in disease severity and quality of life instruments for patients with atopic dermatitis: a systematic review. J Am Acad Dermatol. 2016;75:906-917.
- Basra MK, Sue-Ho R, Finlay AY. The Family Dermatology Life Quality Index: measuring the secondary impact of skin disease. Br J Dermatol. 2007;156:528-538.
- Dodington SR, Basra MK, Finlay AY, et al. The Dermatitis Family Impact questionnaire: a review of its measurement properties and clinical application. Br J Dermatol. 2013;169:31-46.
- Lewis-Jones MS, Finlay AY, Dykes PJ. The Infants’ Dermatitis Quality of Life Index. Br J Dermatol. 2001;144:104-110.
- Holme SA, Man I, Sharpe JL, et al. The Children’s Dermatology Life Quality Index: validation of the cartoon version. Br J Dermatol. 2003;148:285-290.
- Whalley D, McKenna SP, Dewar AL, et al. A new instrument for assessing quality of life in atopic dermatitis: international development of the Quality of Life Index for Atopic Dermatitis (QoLIAD). Br J Dermatol. 2004;150:274-283.
- Jeon C, Yan D, Nakamura M, et al. Frequency and management of sleep disturbance in adults with atopic dermatitis: a systematic review. Dermatol Ther (Heidelb). 2017;7:349-364.
- Yu SH, Attarian H, Zee P, et al. Burden of sleep and fatigue in US adults with atopic dermatitis. Dermatitis. 2016;27:50-58.
- Kong TS, Han TY, Lee JH, et al. Correlation between severity of atopic dermatitis and sleep quality in children and adults. Ann Dermatol. 2016;28:321-326.
- Fishbein AB, Mueller K, Kruse L, et al. Sleep disturbance in children with moderate/severe atopic dermatitis: a case-control study. J Am Acad Dermatol. 2018;78:336-341.
- Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159:745-750.
- Emerson RM, Williams HC, Allen BR. What is the cost of atopic dermatitis in preschool children? Br J Dermatol. 2001;144:514-522.
- Filanovsky MG, Pootongkam S, Tamburro JE, et al. The financial and emotional impact of atopic dermatitis on children and their families. J Pediatr. 2016;169:284-290.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Drucker AM, Wang AR, Qureshi AA. Research gaps in quality of life and economic burden of atopic dermatitis: the National Eczema Association Burden of Disease Audit. JAMA Dermatol. 2016;152:873-874.
- Nørreslet LB, Ebbehøj NE, Ellekilde Bonde JP, et al. The impact of atopic dermatitis on work life—a systematic review. J Eur Acad Dermatol Venereol. 2018;32:23-38.
- Su JC, Kemp AS, Varigos GA, et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76:159-162.
- Coutanceau C, Stalder JF. Analysis of correlations between patient-oriented SCORAD (PO-SCORAD) and other assessment scores of atopic dermatitis severity and quality of life. Dermatology. 2014;229:248-255.
- Ben-Gashir MA, Seed PT, Hay RJ. Quality of life and disease severity are correlated in children with atopic dermatitis. Br J Dermatol. 2004;150:284-290.
- van Valburg RW, Willemsen MG, Dirven-Meijer PC, et al. Quality of life measurement and its relationship to disease severity in children with atopic dermatitis in general practice. Acta Derm Venereol. 2011;91:147-151.
- Haeck IM, ten Berge O, van Velsen SG, et al. Moderate correlation between quality of life and disease activity in adult patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2012;26:236-241.
- Spuls PI, Gerbens LAA, Simpson E, et al; HOME initiative collaborators. Patient-Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: a Harmonising Outcome Measures for Eczema (HOME) statement. Br J Dermatol. 2017;176:979-984.
- Schmitt J, Spuls PI, Thomas KS, et al; HOME initiative collaborators. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol. 2014;134:800-807.
- Oh SH, Bae BG, Park CO, et al. Association of stress with symptoms of atopic dermatitis. Acta Derm Venereol. 2010;90:582-588.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Cheng CM, Hsu JW, Huang KL, et al. Risk of developing major depressive disorder and anxiety disorders among adolescents and adults with atopic dermatitis: a nationwide longitudinal study. J Affect Disord. 2015;178:60-65.
Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854. - Linnet J, Jemec GB. An assessment of anxiety and dermatology life quality in patients with atopic dermatitis. Br J Dermatol. 1999;140:268-272.
- Ring J, Alomar A, Bieber T, et al; European Dermatology Forum; European Academy of Dermatology and Venereology; European Task Force on Atopic Dermatitis; European Federation of Allergy; European Society of Pediatric Dermatology; Global Allergy and Asthma European Network. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J Eur Acad Dermatol Venereol. 2012;26:1176-1193.
- Perczel K, Gál J. Hypnotherapy of atopic dermatitis in an adult. case report. Orv Hetil. 2016;157:111-115.
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease typically with childhood onset. In some cases, the condition persists, but AD usually resolves by the time a child reaches adulthood. Prevalence is difficult to estimate but, in developed countries, is approximately 15% to 30% among children and 2% to 10% among adults.1
Atopic dermatitis is characterized by chronically itchy dry skin, weeping erythematous papules and plaques, and lichenification. Furthermore, AD often is associated with other atopic diseases, such as food allergy, allergic rhinitis, and bronchial asthma.
In this article, we review the literature on the quality of life (QOL) of patients with AD. Our goals are to discuss the most common methods for measuring QOL in AD and how to use them; highlight specific alterations of QOL in AD; and review data about QOL of children with AD, which is underrepresented in the medical literature, as studies tend to focus on adults. In addition, we address the importance of assessing QOL in patients with AD due to the psychological burden of the disease.
Quality of Life
The harmful effects of AD can include a range of areas, including
Because QOL is an important instrument used in many AD studies, we call attention to the work of the Harmonising Outcome Measures for Eczema (HOME) initiative, which established a core outcome set for all AD clinical trials to enable comparison of results of individual studies.2 Quality of life was identified in HOME as one of 4 basic outcome measures that should be included in every AD trial (the others are clinician-reported signs, patient-reported symptoms, and long-term control).3 According to the recent agreement, the following QOL instruments should be used: Dermatology Life Quality Index (DLQI) for adults, Children’s Dermatology Life Quality Index (CDLQI) for children, and Infants’ Dermatitis Quality of Life Index (IDQOL) for infants.4
In dermatology, these instruments can be divided into 3 basic categories: generic, dermatology specific, and disease specific.5 Generic QOL questionnaires are beneficial when comparing the QOL of an AD patient to patients with other conditions or to healthy individuals. On the other hand, dermatology-specific and AD-specific methods are more effective instruments for detecting impairments linked directly to the disease and, therefore, are more sensitive to changes in QOL.5 Some of the most frequently used QOL measures5,6 for AD along with their key attributes are
Given that AD is a chronic disease that requires constant care, parents/guardians or the partner of the patient usually are affected as well. To detect this effect, the Family Dermatology Life Quality Index (FDLQI), a dermatology-specific instrument, measures the QOL in family members of dermatology patients.7 The Dermatitis Family Impact (DFI)8 is a disease-specific method for assessing how having a child with AD can impact the QOL of family members; it is a 10-item questionnaire completed by an adult family member. The FDLQI7 and DFI8 both help to understand the secondary impact of the disease.
In contrast, several other methods that also are administered by a parent/guardian assess how the parent perceives the QOL of their child with AD; these methods are essential for small children and infants who cannot answer questions themselves. The IDQOL9 was designed to assess the QOL of patients younger than 4 years using a parent-completed questionnaire. For older children and adolescents aged 4 to 16 years, the CDLQI10 is a widely used instrument; the questionnaire is completed by the child and is available in a cartoon format.10
For patients older than 16 years, 2 important instruments are the DLQI, a generic dermatology instrument, and the Quality of Life Index for Atopic Dermatitis (QoLIAD).11
Clearly it can be troublesome for researchers and clinicians to find the most suitable instrument to evaluate QOL in AD patients. To make this task easier, the European Academy of Dermatology and Venereology Task Force released a position paper with the following recommendations: (1) only validated instruments should be used, and (2) their use should be based on the age of the patients for which the instruments were designed. It is reommended that researchers use a combination of a generic and a dermatology-specific or AD-specific instrument, whereas clinicians should apply a dermatology-specific or AD-specific method, or both.5
Alterations of QOL in AD
Sleep Disturbance in AD
Sleep disorders observed in AD include difficulty falling asleep, frequent waking episodes, shorter sleep duration, and feelings of inadequate sleep, which often result in impairment of daily activity.12,13 Correlation has been found between sleep quality and QOL in both children and adults.14 Approximately 60% of children affected by AD experience a sleep disturbance,15 which seems to correlate well with disease severity.16 A US study found that adults with AD are more likely to experience a sleep disturbance, which often affects daytime functioning and work productivity.13
Financial Aspects and Impact on Work
The financial burden of AD is extensive.17 There are direct medical costs, including medication, visits to the physician, alternative therapies, and nonprescription products. Patients tend to spend relevant money on such items as moisturizers, bath products, antihistamines, topical steroids, and topical antibiotics.18,19 However, it seems that most of the cost of AD is due to indirect and nonmedical costs, including transportation to medical visits; loss of work days; extra childcare; and expenditures associated with lifestyle changes,19,20 such as modifying diet, wearing special clothes, using special bed linens, and purchasing special household items (eg, anti–dust mite vacuum cleaner, humidifier, new carpeting).17,19
Absenteeism from work often is a consequence of physician appointments; in addition, parents/guardians of a child with AD often miss work due to medical care. Even at work, patients (or parents/guardians) often experience decreased work productivity (so-called presenteeism) due to loss of sleep and anxiety.21 In addressing the effects of AD on work life, a systematic literature review found that AD strongly affects sick leave and might have an impact on job choice and change or loss of job.22
Furthermore, according to Su et al,23 the costs of AD are related to disease severity. Moreover, their data suggest that among chronic childhood diseases, the financial burden of AD is greater than the cost of asthma and similar to the cost of diabetes mellitus.23
Association Between QOL and Disease Severity
A large observational study found that improvement in AD severity was followed by an increase in QOL.24 A positive correlation between disease severity and QOL has been found in other studies,25,26 though no correlation or only moderate correlation also has been reported.27 Apparently, in addition to QOL, disease severity scores are substantial parameters in the evaluation of distress caused by AD; the HOME initiative has identified clinician-reported signs and patient-reported symptoms as 2 of 4 core outcomes domains to include in all future AD clinical trials.3 For measuring symptoms, the Patient-Oriented Eczema Measure (POEM) is the recommended instrument.28 Regarding clinical signs, the HOME group named the Eczema Area and Severity Index (EASI) as the preferred instrument.29
Psychological Burden
Stress is a triggering factor for AD, but the connection between skin and mind appears bidirectional. The biological reaction to stress probably lowers the itch threshold and disrupts the skin barrier.30 The Global Burden of Disease Study showed that skin diseases are the fourth leading cause of nonfatal disease burden.31 There are several factors—pruritus, scratch, and pain—that can all lead to sleep deprivation and daytime fatigue. Based on our experience, if lesions develop on visible areas, patients can feel stigmatized, which restricts their social life.
The most common psychological comorbidities of AD are anxiety and depression. In a cross-sectional, multicenter study, there was a significantly higher prevalence of depression (P<.001) and anxiety disorder (P=.02) among patients with common skin diseases compared to a control group.32 In a study that assessed AD patients, researchers found a higher risk of depression and anxiety.33 Suicidal ideation also is more common in the population with AD32,34; a study showed that the risk of suicidal ideation in adolescents was nearly 4-fold in patients with itching skin lesions compared to those without itch.34
According to Linnet and Jemec,35 mental and psychological comorbidities of AD are associated with lower QOL, not with clinical severity. As a result, to improve QOL in AD, one should take care of both dermatological and psychological problems. It has been demonstrated that psychological interventions, such as autogenic training, cognitive-behavioral therapy, relaxation techniques, habit reversal training,36 and hypnotherapy37 might be helpful in individual cases; educational interventions also are recommended.36 With these adjuvant therapies, psychological status, unpleasant clinical symptoms, and QOL could be improved, though further studies are needed to confirm these benefits.
Conclusion
Atopic dermatitis places a notable burden on patients and their families. The degree of burden is probably related to disease severity. For measuring QOL, researchers and clinicians should use validated methods suited to the age of the patients for which they were designed. More studies are needed to assess the effects of different treatments on QOL. Besides pharmacotherapy, psychotherapy and educational programs might be beneficial for improving QOL, another important area to be studied.
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease typically with childhood onset. In some cases, the condition persists, but AD usually resolves by the time a child reaches adulthood. Prevalence is difficult to estimate but, in developed countries, is approximately 15% to 30% among children and 2% to 10% among adults.1
Atopic dermatitis is characterized by chronically itchy dry skin, weeping erythematous papules and plaques, and lichenification. Furthermore, AD often is associated with other atopic diseases, such as food allergy, allergic rhinitis, and bronchial asthma.
In this article, we review the literature on the quality of life (QOL) of patients with AD. Our goals are to discuss the most common methods for measuring QOL in AD and how to use them; highlight specific alterations of QOL in AD; and review data about QOL of children with AD, which is underrepresented in the medical literature, as studies tend to focus on adults. In addition, we address the importance of assessing QOL in patients with AD due to the psychological burden of the disease.
Quality of Life
The harmful effects of AD can include a range of areas, including
Because QOL is an important instrument used in many AD studies, we call attention to the work of the Harmonising Outcome Measures for Eczema (HOME) initiative, which established a core outcome set for all AD clinical trials to enable comparison of results of individual studies.2 Quality of life was identified in HOME as one of 4 basic outcome measures that should be included in every AD trial (the others are clinician-reported signs, patient-reported symptoms, and long-term control).3 According to the recent agreement, the following QOL instruments should be used: Dermatology Life Quality Index (DLQI) for adults, Children’s Dermatology Life Quality Index (CDLQI) for children, and Infants’ Dermatitis Quality of Life Index (IDQOL) for infants.4
In dermatology, these instruments can be divided into 3 basic categories: generic, dermatology specific, and disease specific.5 Generic QOL questionnaires are beneficial when comparing the QOL of an AD patient to patients with other conditions or to healthy individuals. On the other hand, dermatology-specific and AD-specific methods are more effective instruments for detecting impairments linked directly to the disease and, therefore, are more sensitive to changes in QOL.5 Some of the most frequently used QOL measures5,6 for AD along with their key attributes are
Given that AD is a chronic disease that requires constant care, parents/guardians or the partner of the patient usually are affected as well. To detect this effect, the Family Dermatology Life Quality Index (FDLQI), a dermatology-specific instrument, measures the QOL in family members of dermatology patients.7 The Dermatitis Family Impact (DFI)8 is a disease-specific method for assessing how having a child with AD can impact the QOL of family members; it is a 10-item questionnaire completed by an adult family member. The FDLQI7 and DFI8 both help to understand the secondary impact of the disease.
In contrast, several other methods that also are administered by a parent/guardian assess how the parent perceives the QOL of their child with AD; these methods are essential for small children and infants who cannot answer questions themselves. The IDQOL9 was designed to assess the QOL of patients younger than 4 years using a parent-completed questionnaire. For older children and adolescents aged 4 to 16 years, the CDLQI10 is a widely used instrument; the questionnaire is completed by the child and is available in a cartoon format.10
For patients older than 16 years, 2 important instruments are the DLQI, a generic dermatology instrument, and the Quality of Life Index for Atopic Dermatitis (QoLIAD).11
Clearly it can be troublesome for researchers and clinicians to find the most suitable instrument to evaluate QOL in AD patients. To make this task easier, the European Academy of Dermatology and Venereology Task Force released a position paper with the following recommendations: (1) only validated instruments should be used, and (2) their use should be based on the age of the patients for which the instruments were designed. It is reommended that researchers use a combination of a generic and a dermatology-specific or AD-specific instrument, whereas clinicians should apply a dermatology-specific or AD-specific method, or both.5
Alterations of QOL in AD
Sleep Disturbance in AD
Sleep disorders observed in AD include difficulty falling asleep, frequent waking episodes, shorter sleep duration, and feelings of inadequate sleep, which often result in impairment of daily activity.12,13 Correlation has been found between sleep quality and QOL in both children and adults.14 Approximately 60% of children affected by AD experience a sleep disturbance,15 which seems to correlate well with disease severity.16 A US study found that adults with AD are more likely to experience a sleep disturbance, which often affects daytime functioning and work productivity.13
Financial Aspects and Impact on Work
The financial burden of AD is extensive.17 There are direct medical costs, including medication, visits to the physician, alternative therapies, and nonprescription products. Patients tend to spend relevant money on such items as moisturizers, bath products, antihistamines, topical steroids, and topical antibiotics.18,19 However, it seems that most of the cost of AD is due to indirect and nonmedical costs, including transportation to medical visits; loss of work days; extra childcare; and expenditures associated with lifestyle changes,19,20 such as modifying diet, wearing special clothes, using special bed linens, and purchasing special household items (eg, anti–dust mite vacuum cleaner, humidifier, new carpeting).17,19
Absenteeism from work often is a consequence of physician appointments; in addition, parents/guardians of a child with AD often miss work due to medical care. Even at work, patients (or parents/guardians) often experience decreased work productivity (so-called presenteeism) due to loss of sleep and anxiety.21 In addressing the effects of AD on work life, a systematic literature review found that AD strongly affects sick leave and might have an impact on job choice and change or loss of job.22
Furthermore, according to Su et al,23 the costs of AD are related to disease severity. Moreover, their data suggest that among chronic childhood diseases, the financial burden of AD is greater than the cost of asthma and similar to the cost of diabetes mellitus.23
Association Between QOL and Disease Severity
A large observational study found that improvement in AD severity was followed by an increase in QOL.24 A positive correlation between disease severity and QOL has been found in other studies,25,26 though no correlation or only moderate correlation also has been reported.27 Apparently, in addition to QOL, disease severity scores are substantial parameters in the evaluation of distress caused by AD; the HOME initiative has identified clinician-reported signs and patient-reported symptoms as 2 of 4 core outcomes domains to include in all future AD clinical trials.3 For measuring symptoms, the Patient-Oriented Eczema Measure (POEM) is the recommended instrument.28 Regarding clinical signs, the HOME group named the Eczema Area and Severity Index (EASI) as the preferred instrument.29
Psychological Burden
Stress is a triggering factor for AD, but the connection between skin and mind appears bidirectional. The biological reaction to stress probably lowers the itch threshold and disrupts the skin barrier.30 The Global Burden of Disease Study showed that skin diseases are the fourth leading cause of nonfatal disease burden.31 There are several factors—pruritus, scratch, and pain—that can all lead to sleep deprivation and daytime fatigue. Based on our experience, if lesions develop on visible areas, patients can feel stigmatized, which restricts their social life.
The most common psychological comorbidities of AD are anxiety and depression. In a cross-sectional, multicenter study, there was a significantly higher prevalence of depression (P<.001) and anxiety disorder (P=.02) among patients with common skin diseases compared to a control group.32 In a study that assessed AD patients, researchers found a higher risk of depression and anxiety.33 Suicidal ideation also is more common in the population with AD32,34; a study showed that the risk of suicidal ideation in adolescents was nearly 4-fold in patients with itching skin lesions compared to those without itch.34
According to Linnet and Jemec,35 mental and psychological comorbidities of AD are associated with lower QOL, not with clinical severity. As a result, to improve QOL in AD, one should take care of both dermatological and psychological problems. It has been demonstrated that psychological interventions, such as autogenic training, cognitive-behavioral therapy, relaxation techniques, habit reversal training,36 and hypnotherapy37 might be helpful in individual cases; educational interventions also are recommended.36 With these adjuvant therapies, psychological status, unpleasant clinical symptoms, and QOL could be improved, though further studies are needed to confirm these benefits.
Conclusion
Atopic dermatitis places a notable burden on patients and their families. The degree of burden is probably related to disease severity. For measuring QOL, researchers and clinicians should use validated methods suited to the age of the patients for which they were designed. More studies are needed to assess the effects of different treatments on QOL. Besides pharmacotherapy, psychotherapy and educational programs might be beneficial for improving QOL, another important area to be studied.
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483-1494.
- Schmitt J, Williams H; HOME Development Group. Harmonising Outcome Measures for Eczema (HOME). report from the First International Consensus Meeting (HOME 1), 24 July 2010, Munich, Germany. Br J Dermatol. 2010;163:1166-1168.
- Schmitt J, Spuls P, Boers M, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy. 2012;67:1111-1117.
- Quality of Life (QoL). Harmonising Outcome Measures for Eczema (HOME) website. http://www.homeforeczema.org/research/quality-of-life.aspx. Accessed August 18, 2019.
Chernyshov PV, Tomas-Aragones L, Manolache L, et al; EADV Quality of Life Task Force. Quality of life measurement in atopic dermatitis. Position paper of the European Academy of Dermatology and Venereology (EADV) Task Force on quality of life. J Eur Acad Dermatol Venereol. 2017;31:576-593. - Hill MK, Kheirandish Pishkenari A, Braunberger TL, et al. Recent trends in disease severity and quality of life instruments for patients with atopic dermatitis: a systematic review. J Am Acad Dermatol. 2016;75:906-917.
- Basra MK, Sue-Ho R, Finlay AY. The Family Dermatology Life Quality Index: measuring the secondary impact of skin disease. Br J Dermatol. 2007;156:528-538.
- Dodington SR, Basra MK, Finlay AY, et al. The Dermatitis Family Impact questionnaire: a review of its measurement properties and clinical application. Br J Dermatol. 2013;169:31-46.
- Lewis-Jones MS, Finlay AY, Dykes PJ. The Infants’ Dermatitis Quality of Life Index. Br J Dermatol. 2001;144:104-110.
- Holme SA, Man I, Sharpe JL, et al. The Children’s Dermatology Life Quality Index: validation of the cartoon version. Br J Dermatol. 2003;148:285-290.
- Whalley D, McKenna SP, Dewar AL, et al. A new instrument for assessing quality of life in atopic dermatitis: international development of the Quality of Life Index for Atopic Dermatitis (QoLIAD). Br J Dermatol. 2004;150:274-283.
- Jeon C, Yan D, Nakamura M, et al. Frequency and management of sleep disturbance in adults with atopic dermatitis: a systematic review. Dermatol Ther (Heidelb). 2017;7:349-364.
- Yu SH, Attarian H, Zee P, et al. Burden of sleep and fatigue in US adults with atopic dermatitis. Dermatitis. 2016;27:50-58.
- Kong TS, Han TY, Lee JH, et al. Correlation between severity of atopic dermatitis and sleep quality in children and adults. Ann Dermatol. 2016;28:321-326.
- Fishbein AB, Mueller K, Kruse L, et al. Sleep disturbance in children with moderate/severe atopic dermatitis: a case-control study. J Am Acad Dermatol. 2018;78:336-341.
- Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159:745-750.
- Emerson RM, Williams HC, Allen BR. What is the cost of atopic dermatitis in preschool children? Br J Dermatol. 2001;144:514-522.
- Filanovsky MG, Pootongkam S, Tamburro JE, et al. The financial and emotional impact of atopic dermatitis on children and their families. J Pediatr. 2016;169:284-290.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Drucker AM, Wang AR, Qureshi AA. Research gaps in quality of life and economic burden of atopic dermatitis: the National Eczema Association Burden of Disease Audit. JAMA Dermatol. 2016;152:873-874.
- Nørreslet LB, Ebbehøj NE, Ellekilde Bonde JP, et al. The impact of atopic dermatitis on work life—a systematic review. J Eur Acad Dermatol Venereol. 2018;32:23-38.
- Su JC, Kemp AS, Varigos GA, et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76:159-162.
- Coutanceau C, Stalder JF. Analysis of correlations between patient-oriented SCORAD (PO-SCORAD) and other assessment scores of atopic dermatitis severity and quality of life. Dermatology. 2014;229:248-255.
- Ben-Gashir MA, Seed PT, Hay RJ. Quality of life and disease severity are correlated in children with atopic dermatitis. Br J Dermatol. 2004;150:284-290.
- van Valburg RW, Willemsen MG, Dirven-Meijer PC, et al. Quality of life measurement and its relationship to disease severity in children with atopic dermatitis in general practice. Acta Derm Venereol. 2011;91:147-151.
- Haeck IM, ten Berge O, van Velsen SG, et al. Moderate correlation between quality of life and disease activity in adult patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2012;26:236-241.
- Spuls PI, Gerbens LAA, Simpson E, et al; HOME initiative collaborators. Patient-Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: a Harmonising Outcome Measures for Eczema (HOME) statement. Br J Dermatol. 2017;176:979-984.
- Schmitt J, Spuls PI, Thomas KS, et al; HOME initiative collaborators. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol. 2014;134:800-807.
- Oh SH, Bae BG, Park CO, et al. Association of stress with symptoms of atopic dermatitis. Acta Derm Venereol. 2010;90:582-588.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Cheng CM, Hsu JW, Huang KL, et al. Risk of developing major depressive disorder and anxiety disorders among adolescents and adults with atopic dermatitis: a nationwide longitudinal study. J Affect Disord. 2015;178:60-65.
Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854. - Linnet J, Jemec GB. An assessment of anxiety and dermatology life quality in patients with atopic dermatitis. Br J Dermatol. 1999;140:268-272.
- Ring J, Alomar A, Bieber T, et al; European Dermatology Forum; European Academy of Dermatology and Venereology; European Task Force on Atopic Dermatitis; European Federation of Allergy; European Society of Pediatric Dermatology; Global Allergy and Asthma European Network. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J Eur Acad Dermatol Venereol. 2012;26:1176-1193.
- Perczel K, Gál J. Hypnotherapy of atopic dermatitis in an adult. case report. Orv Hetil. 2016;157:111-115.
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483-1494.
- Schmitt J, Williams H; HOME Development Group. Harmonising Outcome Measures for Eczema (HOME). report from the First International Consensus Meeting (HOME 1), 24 July 2010, Munich, Germany. Br J Dermatol. 2010;163:1166-1168.
- Schmitt J, Spuls P, Boers M, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy. 2012;67:1111-1117.
- Quality of Life (QoL). Harmonising Outcome Measures for Eczema (HOME) website. http://www.homeforeczema.org/research/quality-of-life.aspx. Accessed August 18, 2019.
Chernyshov PV, Tomas-Aragones L, Manolache L, et al; EADV Quality of Life Task Force. Quality of life measurement in atopic dermatitis. Position paper of the European Academy of Dermatology and Venereology (EADV) Task Force on quality of life. J Eur Acad Dermatol Venereol. 2017;31:576-593. - Hill MK, Kheirandish Pishkenari A, Braunberger TL, et al. Recent trends in disease severity and quality of life instruments for patients with atopic dermatitis: a systematic review. J Am Acad Dermatol. 2016;75:906-917.
- Basra MK, Sue-Ho R, Finlay AY. The Family Dermatology Life Quality Index: measuring the secondary impact of skin disease. Br J Dermatol. 2007;156:528-538.
- Dodington SR, Basra MK, Finlay AY, et al. The Dermatitis Family Impact questionnaire: a review of its measurement properties and clinical application. Br J Dermatol. 2013;169:31-46.
- Lewis-Jones MS, Finlay AY, Dykes PJ. The Infants’ Dermatitis Quality of Life Index. Br J Dermatol. 2001;144:104-110.
- Holme SA, Man I, Sharpe JL, et al. The Children’s Dermatology Life Quality Index: validation of the cartoon version. Br J Dermatol. 2003;148:285-290.
- Whalley D, McKenna SP, Dewar AL, et al. A new instrument for assessing quality of life in atopic dermatitis: international development of the Quality of Life Index for Atopic Dermatitis (QoLIAD). Br J Dermatol. 2004;150:274-283.
- Jeon C, Yan D, Nakamura M, et al. Frequency and management of sleep disturbance in adults with atopic dermatitis: a systematic review. Dermatol Ther (Heidelb). 2017;7:349-364.
- Yu SH, Attarian H, Zee P, et al. Burden of sleep and fatigue in US adults with atopic dermatitis. Dermatitis. 2016;27:50-58.
- Kong TS, Han TY, Lee JH, et al. Correlation between severity of atopic dermatitis and sleep quality in children and adults. Ann Dermatol. 2016;28:321-326.
- Fishbein AB, Mueller K, Kruse L, et al. Sleep disturbance in children with moderate/severe atopic dermatitis: a case-control study. J Am Acad Dermatol. 2018;78:336-341.
- Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159:745-750.
- Emerson RM, Williams HC, Allen BR. What is the cost of atopic dermatitis in preschool children? Br J Dermatol. 2001;144:514-522.
- Filanovsky MG, Pootongkam S, Tamburro JE, et al. The financial and emotional impact of atopic dermatitis on children and their families. J Pediatr. 2016;169:284-290.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Drucker AM, Wang AR, Qureshi AA. Research gaps in quality of life and economic burden of atopic dermatitis: the National Eczema Association Burden of Disease Audit. JAMA Dermatol. 2016;152:873-874.
- Nørreslet LB, Ebbehøj NE, Ellekilde Bonde JP, et al. The impact of atopic dermatitis on work life—a systematic review. J Eur Acad Dermatol Venereol. 2018;32:23-38.
- Su JC, Kemp AS, Varigos GA, et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76:159-162.
- Coutanceau C, Stalder JF. Analysis of correlations between patient-oriented SCORAD (PO-SCORAD) and other assessment scores of atopic dermatitis severity and quality of life. Dermatology. 2014;229:248-255.
- Ben-Gashir MA, Seed PT, Hay RJ. Quality of life and disease severity are correlated in children with atopic dermatitis. Br J Dermatol. 2004;150:284-290.
- van Valburg RW, Willemsen MG, Dirven-Meijer PC, et al. Quality of life measurement and its relationship to disease severity in children with atopic dermatitis in general practice. Acta Derm Venereol. 2011;91:147-151.
- Haeck IM, ten Berge O, van Velsen SG, et al. Moderate correlation between quality of life and disease activity in adult patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2012;26:236-241.
- Spuls PI, Gerbens LAA, Simpson E, et al; HOME initiative collaborators. Patient-Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: a Harmonising Outcome Measures for Eczema (HOME) statement. Br J Dermatol. 2017;176:979-984.
- Schmitt J, Spuls PI, Thomas KS, et al; HOME initiative collaborators. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol. 2014;134:800-807.
- Oh SH, Bae BG, Park CO, et al. Association of stress with symptoms of atopic dermatitis. Acta Derm Venereol. 2010;90:582-588.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Cheng CM, Hsu JW, Huang KL, et al. Risk of developing major depressive disorder and anxiety disorders among adolescents and adults with atopic dermatitis: a nationwide longitudinal study. J Affect Disord. 2015;178:60-65.
Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854. - Linnet J, Jemec GB. An assessment of anxiety and dermatology life quality in patients with atopic dermatitis. Br J Dermatol. 1999;140:268-272.
- Ring J, Alomar A, Bieber T, et al; European Dermatology Forum; European Academy of Dermatology and Venereology; European Task Force on Atopic Dermatitis; European Federation of Allergy; European Society of Pediatric Dermatology; Global Allergy and Asthma European Network. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J Eur Acad Dermatol Venereol. 2012;26:1176-1193.
- Perczel K, Gál J. Hypnotherapy of atopic dermatitis in an adult. case report. Orv Hetil. 2016;157:111-115.
Practice Points
- For assessing quality of life (QOL) in atopic dermatitis (AD), it is recommended that researchers use a combination of a generic and a dermatology-specific or AD-specific instrument, whereas clinicians should apply a dermatology-specific or an AD-specific method or both.
- Anxiety and depression are common comorbidities in AD; patients also may need psychological support.
- Patient education is key for improving QOL in AD.
- Financial aspects of the treatment of AD should be taken into consideration because AD requires constant care, which puts a financial burden on patients.
Atopic Dermatitis in Adolescents With Skin of Color
Data are limited on the management of atopic dermatitis (AD) in adolescents, particularly in patients with skin of color, making it important to identify factors that may improve AD management in this population. Comorbid conditions (eg, acne, postinflammatory hyperpigmentation [PIH]), extracurricular activities (eg, athletics), and experimentation with cosmetics in adolescents, all of which can undermine treatment efficacy and medication adherence, make it particularly challenging to devise a therapeutic regimen in this patient population. We review the management of AD in black adolescents, with special consideration of concomitant treatment of acne vulgaris (AV) as well as lifestyle and social choices (Table).
Prevalence and Epidemiology
Atopic dermatitis affects 13% to 25% of children and 2% to 10% of adults.1,2 Population‐based studies in the United States show a higher prevalence of AD in black children (19.3%) compared to European American (EA) children (16.1%).3,4
AD in Black Adolescents
Atopic dermatitis is a common skin condition that is defined as a chronic, pruritic, inflammatory dermatosis with recurrent scaling, papules, and plaques (Figure) that usually develop during infancy and early childhood.3 Although AD severity improves for some patients in adolescence, it can be a lifelong issue affecting performance in academic and occupational settings.5 One US study of 8015 children found that there are racial and ethnic disparities in school absences among children (age range, 2–17 years) with AD, with children with skin of color being absent more often than white children.6 The same study noted that black children had a 1.5-fold higher chance of being absent 6 days over a 6-month school period compared to white children. It is postulated that AD has a greater impact on quality of life (QOL) in children with skin of color, resulting in the increased number of school absences in this population.6
The origin of AD currently is thought to be complex and can involve skin barrier dysfunction, environmental factors, microbiome effects, genetic predisposition, and immune dysregulation.1,4 Atopic dermatitis is a heterogeneous disease with variations in the prevalence, genetic background, and immune activation patterns across racial groups.4 It is now understood to be an immune-mediated disease with multiple inflammatory pathways, with type 2–associated inflammation being a primary pathway. Patients with AD have strong helper T cell (TH2) activation, and black patients with AD have higher IgE serum levels as well as absent TH17/TH1 activation.4
Atopic dermatitis currently is seen as a defect of the epidermal barrier, with variable clinical manifestations and expressivity.7 Filaggrin is an epidermal barrier protein, encoded by the FLG gene, and plays a major role in barrier function by regulating pH and promoting hydration of the skin.4 Loss of function of the FLG gene is the most well-studied genetic risk factor for developing AD, and this mutation is seen in patients with more severe and persistent AD in addition to patients with more skin infections and allergic sensitizations.3,4 However, in the skin of color population, FLG mutations are 6 times less common than in the EA population, despite the fact that AD is more prevalent in patients of African descent.4 Therefore, the role of the FLG loss-of-function mutation and AD is not as well defined in black patients, and some researchers have found no association.3 The FLG loss-of-function mutation seems to play a smaller role in black patients than in EA patients, and other genes may be involved in skin barrier dysfunction.3,4 In a small study of patients with mild AD compared to nonaffected patients, those with AD had lower total ceramide levels in the stratum corneum of affected sites than normal skin sites in healthy individuals.8
Particular disturbances in the gut microbiome have the possibility of impacting the development of AD.9 Additionally, the development of AD may be influenced by the skin microbiome, which can change depending on body site, with fungal organisms thought to make up a large proportion of the microbiome of patients with AD. In patients with AD, there is a lack of microbial diversity and an overgrowth of Staphylococcus aureus.9
Diagnosis
Clinicians diagnose AD based on clinical characteristics, and the lack of objective criteria can hinder diagnosis.1 Thus, diagnosing AD in children with dark skin can pose a particular challenge given the varied clinical presentation of AD across skin types. Severe cases of AD may not be diagnosed or treated adequately in deeply pigmented children because erythema, a defining characteristic of AD, may be hard to identify in darker skin types.10 Furthermore, clinical erythema scores among black children may be “strongly” underestimated using scoring systems such as Eczema Area and Severity Index and SCORing Atopic Dermatitis.4 It is estimated that the risk for severe AD may be 6 times higher in black children compared to white children.10 Additionally, patients with skin of color can present with more treatment-resistant AD.4
Treatment of AD
Current treatment is focused on restoring epidermal barrier function, often with topical agents, such as moisturizers containing different amounts of emollients, occlusives, and humectants; corticosteroids; calcineurin inhibitors; and antimicrobials. Emollients such as glycol stearate, glyceryl stearate, and soy sterols function as lubricants, softening the skin. Occlusive agents include petrolatum, dimethicone, and mineral oil; they act by forming a layer to slow evaporation of water. Humectants including glycerol, lactic acid, and urea function by promoting water retention.11 For acute flares, mid- to high-potency topical corticosteroids are recommended. Also, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used alone or in combination with topical steroids. Finally, bleach baths and topical mupirocin applied to the nares also have proved helpful in moderate to severe AD with secondary bacterial infections.11 Phototherapy can be used in adult and pediatric patients with acute and chronic AD if traditional treatments have failed.2
Systemic agents are indicated and recommended for the subset of adult and pediatric patients in whom optimized topical regimens and/or phototherapy do not adequately provide disease control or when QOL is substantially impacted. The systemic agents effective in the pediatric population include cyclosporine, azathioprine, mycophenolate mofetil, and possibly methotrexate.11 Dupilumab recently was approved by the US Food and Drug Administration for patients 12 years and older with moderate to severe AD whose disease is not well controlled with topical medications.
Patients with AD are predisposed to secondary bacterial and viral infections because of their dysfunctional skin barrier; these infections most commonly are caused by S aureus and herpes simplex virus, respectively.2 Systemic antibiotics are only recommended for patients with AD when there is clinical evidence of bacterial infection. In patients with evidence of eczema herpeticum, systemic antiviral agents should be used to treat the underlying herpes simplex virus infection.2 Atopic dermatitis typically has been studied in white patients; however, patients with skin of color have higher frequencies of treatment-resistant AD. Further research on treatment efficacy for AD in this patient population is needed, as data are limited.4
Treatment of AV in Patients With AD
Two of the most prevalent skin diseases affecting the pediatric population are AD and AV, and both can remarkably impact QOL.12 Acne is one of the most common reasons for adolescent patients to seek dermatologic care, including patients with skin of color (Fitzpatrick skin types IV to VI).13 Thus, it is to be expected that many black adolescents with AD also will have AV. For mild to moderate acne in patients with skin of color, topical retinoids and benzoyl peroxide typically are first line.13 These medications can be problematic for patients with AD, as retinoids and many other acne treatments can cause dryness, which may exacerbate AD.
Moisturizers containing ceramide can be a helpful adjunctive therapy in treating acne,14 especially in patients with AD. Modifications to application of acne medications, such as using topical retinoids every other night or mixing them with moisturizers to minimize dryness, may be beneficial to these patients. Dapsone gel 7.5% used daily also may be an option for adolescents with AD and AV. A double-blind, vehicle-controlled study demonstrated that dapsone is safe and effective for patients 12 years and older with moderate acne, and patients with Fitzpatrick skin types IV to VI rated local scaling, erythema, dryness, and stinging/burning as “none” in the study.15 Another potentially helpful topical agent in patients with AD and AV is sulfacetamide, as it is not likely to cause dryness of the skin. In a small study, sodium sulfacetamide 10% and sulfur 5% in an emollient foam vehicle showed no residual film or sulfur smell and resulted in acne reduction of 50%.16
Patients with skin of color often experience PIH in AD and acne or hypopigmentation from inflammatory dermatoses including AD.17,18 In addition to the dryness from AD and topical retinoid use, patients with skin of color may develop irritant contact dermatitis, thus leading to PIH.13 Dryness and irritant contact dermatitis also can be seen with the use of benzoyl peroxide in black patients. Because of these effects, gentle moisturizers are recommended, and both benzoyl peroxide and retinoids should be initiated at lower doses in patients with skin of color.13
For patients with severe nodulocystic acne, isotretinoin is the treatment of choice in patients with skin of color,13 but there is a dearth of clinical studies addressing complications seen in black adolescents on this treatment, especially with respect to those with AD. Of note, systemic antibiotics typically are initiated before isotretinoin; however, this strategy is falling out of favor due to concern for antibiotic resistance with long-term use.19
Impact of Athletics on AD in Black Adolescents
Because of the exacerbating effects of perspiration and heat causing itch and irritation in patients with AD, it is frequently advised that pediatric patients limit their participation in athletics because of the exacerbating effects of strenuous physical exercise on their disease.12 In one study, 429 pediatric patients or their parents/guardians completed QOL questionnaires; 89% of patients 15 years and younger with severe AD reported that their disease was impacted by athletics and outdoor activities, and 86% of these pediatric patients with severe AD responded that their social lives and leisure activities were impacted.20 Because adolescents often are involved in athletics or have mandatory physical education classes, AD may be isolating and may have a severe impact on self-esteem.
Aggressive treatment of AD with topical and systemic medications may be helpful in adolescents who may be reluctant to participate in sports because of teasing, bullying, or worsening of symptoms with heat or sweating.21 Now that dupilumab is available for adolescents, there is a chance that patients with severe and/or recalcitrant disease managed on this medication can achieve better control of their symptoms without the laboratory requirement of methotrexate and the difficulties of topical medication application, allowing them to engage in mandatory athletic classes as well as desired organized sports.
Use of Cosmetics for AD
Many adolescents experiment with cosmetics, and those with AD may use cosmetic products to cover hyperpigmented or hypopigmented lesions.18 In patients with active AD or increased sensitivity to allergens in cosmetic products, use of makeup can be a contributing factor for AD flares. Acne associated with cosmetics is especially important to consider in darker-skinned patients who may use makeup that is opaque and contains oil to conceal acne or PIH.
Allergens can be present in both cosmetics and pharmaceutical topical agents, and a Brazilian study found that approximately 89% of 813 prescription and nonprescription products (eg, topical drugs, sunscreens, moisturizers, soaps, cleansing lotions, shampoos, cosmeceuticals) contained allergens.22 Patients with AD have a higher prevalence of contact sensitization to fragrances, including balsam of Peru.23 Some AD treatments that contain fragrances have caused further skin issues in a few patients. In one case series, 3 pediatric patients developed allergic contact dermatitis to Myroxylon pereirae (balsam of Peru) when using topical treatments for their AD, and their symptoms of scalp inflammation and alopecia resolved with discontinuation.23
In a Dutch study, sensitization to Fragrance Mix I and M pereirae as well as other ingredients (eg, lanolin alcohol, Amerchol™ L 101 [a lanolin product]) was notably more common in pediatric patients with AD than in patients without AD; however, no data on patients with skin of color were included in this study.24
Because of the increased risk of sensitization to fragrances and other ingredients in patients with AD as well as the high percentage of allergens in prescription and nonprescription products, it is important to discuss all personal care products that patients may be using, not just their cosmetic products. Also, patch testing may be helpful in determining true allergens in some patients. Patch testing is recommended for patients with treatment-resistant AD, and a recent study suggested it should be done prior to long-term use of immunosuppressive agents.25 Increased steroid phobia and a push toward alternative medicines are leading both patients with AD and guardians of children with AD to look for other forms of moisturization, such as olive oil, coconut oil, sunflower seed oil, and shea butter, to decrease transepidermal water loss.26,27 An important factor in AD treatment efficacy is patient acceptability in using what is recommended.27 One study showed there was no difference in efficacy or acceptability in using a cream containing shea butter extract vs the ceramide-precursor product.27 Current data show olive oil may exacerbate dry skin and AD,26 and recommendation of any over-the-counter oils and butters in patients with AD should be made with great caution, as many of these products contain fragrances and other potential allergens.
Alternative Therapies for AD
Patients with AD often seek alternative or integrative treatment options, including dietary modifications and holistic remedies. Studies investigating the role of vitamins and supplements in treating AD are limited by sample size.28 However, there is some evidence that may support supplementation with vitamins D and E in addressing AD symptoms. The use of probiotics in treating AD is controversial, but there are studies suggesting that the use of probiotics may prove beneficial in preventing infantile AD.28 Additionally, findings from an ex vivo and in vitro study show that some conditions, including AD and acne, may benefit from the same probiotics, despite the differences in these two diseases. Both AD and acne have inflammatory and skin dysbiosis characteristics, which may be the common thread leading to both conditions potentially responding to treatment with probiotics.29
Preliminary evidence indicates that supplements containing fatty acids such as docosahexaenoic acid, sea buckthorn oil, and hemp seed oil may decrease the severity of AD.28 In a 20-week, randomized, single-blind, crossover study published in 2005, dietary hemp seed oil showed an improvement of clinical symptoms, including dry skin and itchiness, in patients with AD.30
In light of recent legalization in several states, patients may turn to use of cannabinoid products to manage AD. In a systematic review, cannabinoid use was reportedly a therapeutic option in the treatment of AD and AV; however, the data are based on preclinical work, and there are no randomized, placebo-controlled studies to support the use of cannabinoids.31 Furthermore, there is great concern that use of these products in adolescents is an even larger unknown.
Final Thoughts
Eighty percent of children diagnosed with AD experience symptom improvement before their early teens32; for those with AD during their preteen and teenage years, there can be psychological ramifications, as teenagers with AD report having fewer friends, are less socially involved, participate in fewer sports, and are absent from classes more often than their peers.5 In black patients with AD, school absences are even more common.6 Given the social and emotional impact of AD on patients with skin of color, it is imperative to treat the condition appropriately.33 There are areas of opportunity for further research on alternate dosing of existing treatments for AV in patients with AD, further recommendations for adolescent athletes with AD, and which cosmetic and alternative medicine products may be beneficial for this population to improve their QOL.
Providers should discuss medical management in a broader context considering patients’ extracurricular activities, treatment vehicle preferences, expectations, and personal care habits. It also is important to address the many possible factors that may influence treatment adherence early on, particularly in adolescents, as these could be barriers to treatment. This article highlights considerations for treating AD and comorbid conditions that may further complicate treatment in adolescent patients with skin of color. The information provided should serve as a guide in initial counseling and management of AD in adolescents with skin of color.
- Feldman SR, Cox LS, Strowd LC, et al. The challenge of managing atopic dermatitis in the United States. Am Health Drug Benefits. 2019;12:83-93.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357.
- Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122:449-455.
- Vivar KL, Kruse L. The impact of pediatric skin disease on self-esteem. Int J Womens Dermatol. 2018;4:27-31.
- Wan J, Margolis DJ, Mitra N, et al. Racial and ethnic differences in atopic dermatitis–related school absences among US children [published online May 22, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.0597.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109-1122.
- Ishikawa J, Narita H, Kondo N, et al. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130:2511-2514.
- Chernikova D, Yuan I, Shaker M. Prevention of allergy with diverse and healthy microbiota: an update. Curr Opin Pediatr. 2019;31:418-425.
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Nguyen CM, Koo J, Cordoro KM. Psychodermatologic effects of atopic dermatitis and acne: a review on self-esteem and identity. Pediatr Dermatol. 2016;33:129-135.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Lynde CW, Andriessen A, Barankin B, et al. Moisturizers and ceramide-containing moisturizers may offer concomitant therapy with benefits. J Clin Aesthet Dermatol. 2014;7:18-26.
- Taylor SC, Cook-Bolden FE, McMichael A, et al. Efficacy, safety, and tolerability of topical dapsone gel, 7.5% for treatment of acne vulgaris by Fitzpatrick skin phototype. J Drugs Dermatol. 2018;17:160-167.
- Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-236.
- Vachiramon V, Tey HL, Thompson AE, et al. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29:395-402.
- Heath CR. Managing postinflammatory hyperpigmentation in pediatric patients with skin of color. Cutis. 2018;102:71-73.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Paller AS, McAlister RO, Doyle JJ, et al. Perceptions of physicians and pediatric patients about atopic dermatitis, its impact, and its treatment. Clin Pediatr. 2002;41:323-332.
- Sibbald C, Drucker AM. Patient burden of atopic dermatitis. Dermatol Clin. 2017;35:303-316.
- Rocha VB, Machado CJ, Bittencourt FV. Presence of allergens in the vehicles of Brazilian dermatological products. Contact Dermatitis. 2017;76:126-128.
- Admani S, Goldenberg A, Jacob SE. Contact alopecia: improvement of alopecia with discontinuation of fluocinolone oil in individuals allergic to balsam fragrance. Pediatr Dermatol. 2017;34:e57-e60.
- Uter W, Werfel T, White IR, et al. Contact allergy: a review of current problems from a clinical perspective. Int J Environ Res Public Health. 2018;15:E1108.
- López-Jiménez EC, Marrero-Alemán G, Borrego L. One-third of patients with therapy-resistant atopic dermatitis may benefit after patch testing [published online May 13, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15672.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15.
- Hon KL, Tsang YC, Pong NH, et al. Patient acceptability, efficacy, and skin biophysiology of a cream and cleanser containing lipid complex with shea butter extract versus a ceramide product for eczema. Hong Kong Med J. 2015;21:417-425.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review [published online March 20, 2019]. Int J Dermatol. doi:10.1111/ijd.14404.
- Mottin VHM, Suyenaga ES. An approach on the potential use of probiotics in the treatment of skin conditions: acne and atopic dermatitis. Int J Dermatol. 2018;57:1425-1432.
- Callaway J, Schwab U, Harvima I, et al. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J Dermatol Treat. 2005;16:87-94.
- Eagleston LRM, Kalani NK, Patel RR, et al. Cannabinoids in dermatology: a scoping review [published June 15, 2018]. Dermatol Online J. 2018;24.
- Kim JP, Chao LX, Simpson EL, et al. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:681-687.e611.
- de María Díaz Granados L, Quijano MA, Ramírez PA, et al. Quality assessment of atopic dermatitis clinical practice guidelines in ≤ 18 years. Arch Dermatol Res. 2018;310:29-37.
Data are limited on the management of atopic dermatitis (AD) in adolescents, particularly in patients with skin of color, making it important to identify factors that may improve AD management in this population. Comorbid conditions (eg, acne, postinflammatory hyperpigmentation [PIH]), extracurricular activities (eg, athletics), and experimentation with cosmetics in adolescents, all of which can undermine treatment efficacy and medication adherence, make it particularly challenging to devise a therapeutic regimen in this patient population. We review the management of AD in black adolescents, with special consideration of concomitant treatment of acne vulgaris (AV) as well as lifestyle and social choices (Table).
Prevalence and Epidemiology
Atopic dermatitis affects 13% to 25% of children and 2% to 10% of adults.1,2 Population‐based studies in the United States show a higher prevalence of AD in black children (19.3%) compared to European American (EA) children (16.1%).3,4
AD in Black Adolescents
Atopic dermatitis is a common skin condition that is defined as a chronic, pruritic, inflammatory dermatosis with recurrent scaling, papules, and plaques (Figure) that usually develop during infancy and early childhood.3 Although AD severity improves for some patients in adolescence, it can be a lifelong issue affecting performance in academic and occupational settings.5 One US study of 8015 children found that there are racial and ethnic disparities in school absences among children (age range, 2–17 years) with AD, with children with skin of color being absent more often than white children.6 The same study noted that black children had a 1.5-fold higher chance of being absent 6 days over a 6-month school period compared to white children. It is postulated that AD has a greater impact on quality of life (QOL) in children with skin of color, resulting in the increased number of school absences in this population.6
The origin of AD currently is thought to be complex and can involve skin barrier dysfunction, environmental factors, microbiome effects, genetic predisposition, and immune dysregulation.1,4 Atopic dermatitis is a heterogeneous disease with variations in the prevalence, genetic background, and immune activation patterns across racial groups.4 It is now understood to be an immune-mediated disease with multiple inflammatory pathways, with type 2–associated inflammation being a primary pathway. Patients with AD have strong helper T cell (TH2) activation, and black patients with AD have higher IgE serum levels as well as absent TH17/TH1 activation.4
Atopic dermatitis currently is seen as a defect of the epidermal barrier, with variable clinical manifestations and expressivity.7 Filaggrin is an epidermal barrier protein, encoded by the FLG gene, and plays a major role in barrier function by regulating pH and promoting hydration of the skin.4 Loss of function of the FLG gene is the most well-studied genetic risk factor for developing AD, and this mutation is seen in patients with more severe and persistent AD in addition to patients with more skin infections and allergic sensitizations.3,4 However, in the skin of color population, FLG mutations are 6 times less common than in the EA population, despite the fact that AD is more prevalent in patients of African descent.4 Therefore, the role of the FLG loss-of-function mutation and AD is not as well defined in black patients, and some researchers have found no association.3 The FLG loss-of-function mutation seems to play a smaller role in black patients than in EA patients, and other genes may be involved in skin barrier dysfunction.3,4 In a small study of patients with mild AD compared to nonaffected patients, those with AD had lower total ceramide levels in the stratum corneum of affected sites than normal skin sites in healthy individuals.8
Particular disturbances in the gut microbiome have the possibility of impacting the development of AD.9 Additionally, the development of AD may be influenced by the skin microbiome, which can change depending on body site, with fungal organisms thought to make up a large proportion of the microbiome of patients with AD. In patients with AD, there is a lack of microbial diversity and an overgrowth of Staphylococcus aureus.9
Diagnosis
Clinicians diagnose AD based on clinical characteristics, and the lack of objective criteria can hinder diagnosis.1 Thus, diagnosing AD in children with dark skin can pose a particular challenge given the varied clinical presentation of AD across skin types. Severe cases of AD may not be diagnosed or treated adequately in deeply pigmented children because erythema, a defining characteristic of AD, may be hard to identify in darker skin types.10 Furthermore, clinical erythema scores among black children may be “strongly” underestimated using scoring systems such as Eczema Area and Severity Index and SCORing Atopic Dermatitis.4 It is estimated that the risk for severe AD may be 6 times higher in black children compared to white children.10 Additionally, patients with skin of color can present with more treatment-resistant AD.4
Treatment of AD
Current treatment is focused on restoring epidermal barrier function, often with topical agents, such as moisturizers containing different amounts of emollients, occlusives, and humectants; corticosteroids; calcineurin inhibitors; and antimicrobials. Emollients such as glycol stearate, glyceryl stearate, and soy sterols function as lubricants, softening the skin. Occlusive agents include petrolatum, dimethicone, and mineral oil; they act by forming a layer to slow evaporation of water. Humectants including glycerol, lactic acid, and urea function by promoting water retention.11 For acute flares, mid- to high-potency topical corticosteroids are recommended. Also, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used alone or in combination with topical steroids. Finally, bleach baths and topical mupirocin applied to the nares also have proved helpful in moderate to severe AD with secondary bacterial infections.11 Phototherapy can be used in adult and pediatric patients with acute and chronic AD if traditional treatments have failed.2
Systemic agents are indicated and recommended for the subset of adult and pediatric patients in whom optimized topical regimens and/or phototherapy do not adequately provide disease control or when QOL is substantially impacted. The systemic agents effective in the pediatric population include cyclosporine, azathioprine, mycophenolate mofetil, and possibly methotrexate.11 Dupilumab recently was approved by the US Food and Drug Administration for patients 12 years and older with moderate to severe AD whose disease is not well controlled with topical medications.
Patients with AD are predisposed to secondary bacterial and viral infections because of their dysfunctional skin barrier; these infections most commonly are caused by S aureus and herpes simplex virus, respectively.2 Systemic antibiotics are only recommended for patients with AD when there is clinical evidence of bacterial infection. In patients with evidence of eczema herpeticum, systemic antiviral agents should be used to treat the underlying herpes simplex virus infection.2 Atopic dermatitis typically has been studied in white patients; however, patients with skin of color have higher frequencies of treatment-resistant AD. Further research on treatment efficacy for AD in this patient population is needed, as data are limited.4
Treatment of AV in Patients With AD
Two of the most prevalent skin diseases affecting the pediatric population are AD and AV, and both can remarkably impact QOL.12 Acne is one of the most common reasons for adolescent patients to seek dermatologic care, including patients with skin of color (Fitzpatrick skin types IV to VI).13 Thus, it is to be expected that many black adolescents with AD also will have AV. For mild to moderate acne in patients with skin of color, topical retinoids and benzoyl peroxide typically are first line.13 These medications can be problematic for patients with AD, as retinoids and many other acne treatments can cause dryness, which may exacerbate AD.
Moisturizers containing ceramide can be a helpful adjunctive therapy in treating acne,14 especially in patients with AD. Modifications to application of acne medications, such as using topical retinoids every other night or mixing them with moisturizers to minimize dryness, may be beneficial to these patients. Dapsone gel 7.5% used daily also may be an option for adolescents with AD and AV. A double-blind, vehicle-controlled study demonstrated that dapsone is safe and effective for patients 12 years and older with moderate acne, and patients with Fitzpatrick skin types IV to VI rated local scaling, erythema, dryness, and stinging/burning as “none” in the study.15 Another potentially helpful topical agent in patients with AD and AV is sulfacetamide, as it is not likely to cause dryness of the skin. In a small study, sodium sulfacetamide 10% and sulfur 5% in an emollient foam vehicle showed no residual film or sulfur smell and resulted in acne reduction of 50%.16
Patients with skin of color often experience PIH in AD and acne or hypopigmentation from inflammatory dermatoses including AD.17,18 In addition to the dryness from AD and topical retinoid use, patients with skin of color may develop irritant contact dermatitis, thus leading to PIH.13 Dryness and irritant contact dermatitis also can be seen with the use of benzoyl peroxide in black patients. Because of these effects, gentle moisturizers are recommended, and both benzoyl peroxide and retinoids should be initiated at lower doses in patients with skin of color.13
For patients with severe nodulocystic acne, isotretinoin is the treatment of choice in patients with skin of color,13 but there is a dearth of clinical studies addressing complications seen in black adolescents on this treatment, especially with respect to those with AD. Of note, systemic antibiotics typically are initiated before isotretinoin; however, this strategy is falling out of favor due to concern for antibiotic resistance with long-term use.19
Impact of Athletics on AD in Black Adolescents
Because of the exacerbating effects of perspiration and heat causing itch and irritation in patients with AD, it is frequently advised that pediatric patients limit their participation in athletics because of the exacerbating effects of strenuous physical exercise on their disease.12 In one study, 429 pediatric patients or their parents/guardians completed QOL questionnaires; 89% of patients 15 years and younger with severe AD reported that their disease was impacted by athletics and outdoor activities, and 86% of these pediatric patients with severe AD responded that their social lives and leisure activities were impacted.20 Because adolescents often are involved in athletics or have mandatory physical education classes, AD may be isolating and may have a severe impact on self-esteem.
Aggressive treatment of AD with topical and systemic medications may be helpful in adolescents who may be reluctant to participate in sports because of teasing, bullying, or worsening of symptoms with heat or sweating.21 Now that dupilumab is available for adolescents, there is a chance that patients with severe and/or recalcitrant disease managed on this medication can achieve better control of their symptoms without the laboratory requirement of methotrexate and the difficulties of topical medication application, allowing them to engage in mandatory athletic classes as well as desired organized sports.
Use of Cosmetics for AD
Many adolescents experiment with cosmetics, and those with AD may use cosmetic products to cover hyperpigmented or hypopigmented lesions.18 In patients with active AD or increased sensitivity to allergens in cosmetic products, use of makeup can be a contributing factor for AD flares. Acne associated with cosmetics is especially important to consider in darker-skinned patients who may use makeup that is opaque and contains oil to conceal acne or PIH.
Allergens can be present in both cosmetics and pharmaceutical topical agents, and a Brazilian study found that approximately 89% of 813 prescription and nonprescription products (eg, topical drugs, sunscreens, moisturizers, soaps, cleansing lotions, shampoos, cosmeceuticals) contained allergens.22 Patients with AD have a higher prevalence of contact sensitization to fragrances, including balsam of Peru.23 Some AD treatments that contain fragrances have caused further skin issues in a few patients. In one case series, 3 pediatric patients developed allergic contact dermatitis to Myroxylon pereirae (balsam of Peru) when using topical treatments for their AD, and their symptoms of scalp inflammation and alopecia resolved with discontinuation.23
In a Dutch study, sensitization to Fragrance Mix I and M pereirae as well as other ingredients (eg, lanolin alcohol, Amerchol™ L 101 [a lanolin product]) was notably more common in pediatric patients with AD than in patients without AD; however, no data on patients with skin of color were included in this study.24
Because of the increased risk of sensitization to fragrances and other ingredients in patients with AD as well as the high percentage of allergens in prescription and nonprescription products, it is important to discuss all personal care products that patients may be using, not just their cosmetic products. Also, patch testing may be helpful in determining true allergens in some patients. Patch testing is recommended for patients with treatment-resistant AD, and a recent study suggested it should be done prior to long-term use of immunosuppressive agents.25 Increased steroid phobia and a push toward alternative medicines are leading both patients with AD and guardians of children with AD to look for other forms of moisturization, such as olive oil, coconut oil, sunflower seed oil, and shea butter, to decrease transepidermal water loss.26,27 An important factor in AD treatment efficacy is patient acceptability in using what is recommended.27 One study showed there was no difference in efficacy or acceptability in using a cream containing shea butter extract vs the ceramide-precursor product.27 Current data show olive oil may exacerbate dry skin and AD,26 and recommendation of any over-the-counter oils and butters in patients with AD should be made with great caution, as many of these products contain fragrances and other potential allergens.
Alternative Therapies for AD
Patients with AD often seek alternative or integrative treatment options, including dietary modifications and holistic remedies. Studies investigating the role of vitamins and supplements in treating AD are limited by sample size.28 However, there is some evidence that may support supplementation with vitamins D and E in addressing AD symptoms. The use of probiotics in treating AD is controversial, but there are studies suggesting that the use of probiotics may prove beneficial in preventing infantile AD.28 Additionally, findings from an ex vivo and in vitro study show that some conditions, including AD and acne, may benefit from the same probiotics, despite the differences in these two diseases. Both AD and acne have inflammatory and skin dysbiosis characteristics, which may be the common thread leading to both conditions potentially responding to treatment with probiotics.29
Preliminary evidence indicates that supplements containing fatty acids such as docosahexaenoic acid, sea buckthorn oil, and hemp seed oil may decrease the severity of AD.28 In a 20-week, randomized, single-blind, crossover study published in 2005, dietary hemp seed oil showed an improvement of clinical symptoms, including dry skin and itchiness, in patients with AD.30
In light of recent legalization in several states, patients may turn to use of cannabinoid products to manage AD. In a systematic review, cannabinoid use was reportedly a therapeutic option in the treatment of AD and AV; however, the data are based on preclinical work, and there are no randomized, placebo-controlled studies to support the use of cannabinoids.31 Furthermore, there is great concern that use of these products in adolescents is an even larger unknown.
Final Thoughts
Eighty percent of children diagnosed with AD experience symptom improvement before their early teens32; for those with AD during their preteen and teenage years, there can be psychological ramifications, as teenagers with AD report having fewer friends, are less socially involved, participate in fewer sports, and are absent from classes more often than their peers.5 In black patients with AD, school absences are even more common.6 Given the social and emotional impact of AD on patients with skin of color, it is imperative to treat the condition appropriately.33 There are areas of opportunity for further research on alternate dosing of existing treatments for AV in patients with AD, further recommendations for adolescent athletes with AD, and which cosmetic and alternative medicine products may be beneficial for this population to improve their QOL.
Providers should discuss medical management in a broader context considering patients’ extracurricular activities, treatment vehicle preferences, expectations, and personal care habits. It also is important to address the many possible factors that may influence treatment adherence early on, particularly in adolescents, as these could be barriers to treatment. This article highlights considerations for treating AD and comorbid conditions that may further complicate treatment in adolescent patients with skin of color. The information provided should serve as a guide in initial counseling and management of AD in adolescents with skin of color.
Data are limited on the management of atopic dermatitis (AD) in adolescents, particularly in patients with skin of color, making it important to identify factors that may improve AD management in this population. Comorbid conditions (eg, acne, postinflammatory hyperpigmentation [PIH]), extracurricular activities (eg, athletics), and experimentation with cosmetics in adolescents, all of which can undermine treatment efficacy and medication adherence, make it particularly challenging to devise a therapeutic regimen in this patient population. We review the management of AD in black adolescents, with special consideration of concomitant treatment of acne vulgaris (AV) as well as lifestyle and social choices (Table).
Prevalence and Epidemiology
Atopic dermatitis affects 13% to 25% of children and 2% to 10% of adults.1,2 Population‐based studies in the United States show a higher prevalence of AD in black children (19.3%) compared to European American (EA) children (16.1%).3,4
AD in Black Adolescents
Atopic dermatitis is a common skin condition that is defined as a chronic, pruritic, inflammatory dermatosis with recurrent scaling, papules, and plaques (Figure) that usually develop during infancy and early childhood.3 Although AD severity improves for some patients in adolescence, it can be a lifelong issue affecting performance in academic and occupational settings.5 One US study of 8015 children found that there are racial and ethnic disparities in school absences among children (age range, 2–17 years) with AD, with children with skin of color being absent more often than white children.6 The same study noted that black children had a 1.5-fold higher chance of being absent 6 days over a 6-month school period compared to white children. It is postulated that AD has a greater impact on quality of life (QOL) in children with skin of color, resulting in the increased number of school absences in this population.6
The origin of AD currently is thought to be complex and can involve skin barrier dysfunction, environmental factors, microbiome effects, genetic predisposition, and immune dysregulation.1,4 Atopic dermatitis is a heterogeneous disease with variations in the prevalence, genetic background, and immune activation patterns across racial groups.4 It is now understood to be an immune-mediated disease with multiple inflammatory pathways, with type 2–associated inflammation being a primary pathway. Patients with AD have strong helper T cell (TH2) activation, and black patients with AD have higher IgE serum levels as well as absent TH17/TH1 activation.4
Atopic dermatitis currently is seen as a defect of the epidermal barrier, with variable clinical manifestations and expressivity.7 Filaggrin is an epidermal barrier protein, encoded by the FLG gene, and plays a major role in barrier function by regulating pH and promoting hydration of the skin.4 Loss of function of the FLG gene is the most well-studied genetic risk factor for developing AD, and this mutation is seen in patients with more severe and persistent AD in addition to patients with more skin infections and allergic sensitizations.3,4 However, in the skin of color population, FLG mutations are 6 times less common than in the EA population, despite the fact that AD is more prevalent in patients of African descent.4 Therefore, the role of the FLG loss-of-function mutation and AD is not as well defined in black patients, and some researchers have found no association.3 The FLG loss-of-function mutation seems to play a smaller role in black patients than in EA patients, and other genes may be involved in skin barrier dysfunction.3,4 In a small study of patients with mild AD compared to nonaffected patients, those with AD had lower total ceramide levels in the stratum corneum of affected sites than normal skin sites in healthy individuals.8
Particular disturbances in the gut microbiome have the possibility of impacting the development of AD.9 Additionally, the development of AD may be influenced by the skin microbiome, which can change depending on body site, with fungal organisms thought to make up a large proportion of the microbiome of patients with AD. In patients with AD, there is a lack of microbial diversity and an overgrowth of Staphylococcus aureus.9
Diagnosis
Clinicians diagnose AD based on clinical characteristics, and the lack of objective criteria can hinder diagnosis.1 Thus, diagnosing AD in children with dark skin can pose a particular challenge given the varied clinical presentation of AD across skin types. Severe cases of AD may not be diagnosed or treated adequately in deeply pigmented children because erythema, a defining characteristic of AD, may be hard to identify in darker skin types.10 Furthermore, clinical erythema scores among black children may be “strongly” underestimated using scoring systems such as Eczema Area and Severity Index and SCORing Atopic Dermatitis.4 It is estimated that the risk for severe AD may be 6 times higher in black children compared to white children.10 Additionally, patients with skin of color can present with more treatment-resistant AD.4
Treatment of AD
Current treatment is focused on restoring epidermal barrier function, often with topical agents, such as moisturizers containing different amounts of emollients, occlusives, and humectants; corticosteroids; calcineurin inhibitors; and antimicrobials. Emollients such as glycol stearate, glyceryl stearate, and soy sterols function as lubricants, softening the skin. Occlusive agents include petrolatum, dimethicone, and mineral oil; they act by forming a layer to slow evaporation of water. Humectants including glycerol, lactic acid, and urea function by promoting water retention.11 For acute flares, mid- to high-potency topical corticosteroids are recommended. Also, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used alone or in combination with topical steroids. Finally, bleach baths and topical mupirocin applied to the nares also have proved helpful in moderate to severe AD with secondary bacterial infections.11 Phototherapy can be used in adult and pediatric patients with acute and chronic AD if traditional treatments have failed.2
Systemic agents are indicated and recommended for the subset of adult and pediatric patients in whom optimized topical regimens and/or phototherapy do not adequately provide disease control or when QOL is substantially impacted. The systemic agents effective in the pediatric population include cyclosporine, azathioprine, mycophenolate mofetil, and possibly methotrexate.11 Dupilumab recently was approved by the US Food and Drug Administration for patients 12 years and older with moderate to severe AD whose disease is not well controlled with topical medications.
Patients with AD are predisposed to secondary bacterial and viral infections because of their dysfunctional skin barrier; these infections most commonly are caused by S aureus and herpes simplex virus, respectively.2 Systemic antibiotics are only recommended for patients with AD when there is clinical evidence of bacterial infection. In patients with evidence of eczema herpeticum, systemic antiviral agents should be used to treat the underlying herpes simplex virus infection.2 Atopic dermatitis typically has been studied in white patients; however, patients with skin of color have higher frequencies of treatment-resistant AD. Further research on treatment efficacy for AD in this patient population is needed, as data are limited.4
Treatment of AV in Patients With AD
Two of the most prevalent skin diseases affecting the pediatric population are AD and AV, and both can remarkably impact QOL.12 Acne is one of the most common reasons for adolescent patients to seek dermatologic care, including patients with skin of color (Fitzpatrick skin types IV to VI).13 Thus, it is to be expected that many black adolescents with AD also will have AV. For mild to moderate acne in patients with skin of color, topical retinoids and benzoyl peroxide typically are first line.13 These medications can be problematic for patients with AD, as retinoids and many other acne treatments can cause dryness, which may exacerbate AD.
Moisturizers containing ceramide can be a helpful adjunctive therapy in treating acne,14 especially in patients with AD. Modifications to application of acne medications, such as using topical retinoids every other night or mixing them with moisturizers to minimize dryness, may be beneficial to these patients. Dapsone gel 7.5% used daily also may be an option for adolescents with AD and AV. A double-blind, vehicle-controlled study demonstrated that dapsone is safe and effective for patients 12 years and older with moderate acne, and patients with Fitzpatrick skin types IV to VI rated local scaling, erythema, dryness, and stinging/burning as “none” in the study.15 Another potentially helpful topical agent in patients with AD and AV is sulfacetamide, as it is not likely to cause dryness of the skin. In a small study, sodium sulfacetamide 10% and sulfur 5% in an emollient foam vehicle showed no residual film or sulfur smell and resulted in acne reduction of 50%.16
Patients with skin of color often experience PIH in AD and acne or hypopigmentation from inflammatory dermatoses including AD.17,18 In addition to the dryness from AD and topical retinoid use, patients with skin of color may develop irritant contact dermatitis, thus leading to PIH.13 Dryness and irritant contact dermatitis also can be seen with the use of benzoyl peroxide in black patients. Because of these effects, gentle moisturizers are recommended, and both benzoyl peroxide and retinoids should be initiated at lower doses in patients with skin of color.13
For patients with severe nodulocystic acne, isotretinoin is the treatment of choice in patients with skin of color,13 but there is a dearth of clinical studies addressing complications seen in black adolescents on this treatment, especially with respect to those with AD. Of note, systemic antibiotics typically are initiated before isotretinoin; however, this strategy is falling out of favor due to concern for antibiotic resistance with long-term use.19
Impact of Athletics on AD in Black Adolescents
Because of the exacerbating effects of perspiration and heat causing itch and irritation in patients with AD, it is frequently advised that pediatric patients limit their participation in athletics because of the exacerbating effects of strenuous physical exercise on their disease.12 In one study, 429 pediatric patients or their parents/guardians completed QOL questionnaires; 89% of patients 15 years and younger with severe AD reported that their disease was impacted by athletics and outdoor activities, and 86% of these pediatric patients with severe AD responded that their social lives and leisure activities were impacted.20 Because adolescents often are involved in athletics or have mandatory physical education classes, AD may be isolating and may have a severe impact on self-esteem.
Aggressive treatment of AD with topical and systemic medications may be helpful in adolescents who may be reluctant to participate in sports because of teasing, bullying, or worsening of symptoms with heat or sweating.21 Now that dupilumab is available for adolescents, there is a chance that patients with severe and/or recalcitrant disease managed on this medication can achieve better control of their symptoms without the laboratory requirement of methotrexate and the difficulties of topical medication application, allowing them to engage in mandatory athletic classes as well as desired organized sports.
Use of Cosmetics for AD
Many adolescents experiment with cosmetics, and those with AD may use cosmetic products to cover hyperpigmented or hypopigmented lesions.18 In patients with active AD or increased sensitivity to allergens in cosmetic products, use of makeup can be a contributing factor for AD flares. Acne associated with cosmetics is especially important to consider in darker-skinned patients who may use makeup that is opaque and contains oil to conceal acne or PIH.
Allergens can be present in both cosmetics and pharmaceutical topical agents, and a Brazilian study found that approximately 89% of 813 prescription and nonprescription products (eg, topical drugs, sunscreens, moisturizers, soaps, cleansing lotions, shampoos, cosmeceuticals) contained allergens.22 Patients with AD have a higher prevalence of contact sensitization to fragrances, including balsam of Peru.23 Some AD treatments that contain fragrances have caused further skin issues in a few patients. In one case series, 3 pediatric patients developed allergic contact dermatitis to Myroxylon pereirae (balsam of Peru) when using topical treatments for their AD, and their symptoms of scalp inflammation and alopecia resolved with discontinuation.23
In a Dutch study, sensitization to Fragrance Mix I and M pereirae as well as other ingredients (eg, lanolin alcohol, Amerchol™ L 101 [a lanolin product]) was notably more common in pediatric patients with AD than in patients without AD; however, no data on patients with skin of color were included in this study.24
Because of the increased risk of sensitization to fragrances and other ingredients in patients with AD as well as the high percentage of allergens in prescription and nonprescription products, it is important to discuss all personal care products that patients may be using, not just their cosmetic products. Also, patch testing may be helpful in determining true allergens in some patients. Patch testing is recommended for patients with treatment-resistant AD, and a recent study suggested it should be done prior to long-term use of immunosuppressive agents.25 Increased steroid phobia and a push toward alternative medicines are leading both patients with AD and guardians of children with AD to look for other forms of moisturization, such as olive oil, coconut oil, sunflower seed oil, and shea butter, to decrease transepidermal water loss.26,27 An important factor in AD treatment efficacy is patient acceptability in using what is recommended.27 One study showed there was no difference in efficacy or acceptability in using a cream containing shea butter extract vs the ceramide-precursor product.27 Current data show olive oil may exacerbate dry skin and AD,26 and recommendation of any over-the-counter oils and butters in patients with AD should be made with great caution, as many of these products contain fragrances and other potential allergens.
Alternative Therapies for AD
Patients with AD often seek alternative or integrative treatment options, including dietary modifications and holistic remedies. Studies investigating the role of vitamins and supplements in treating AD are limited by sample size.28 However, there is some evidence that may support supplementation with vitamins D and E in addressing AD symptoms. The use of probiotics in treating AD is controversial, but there are studies suggesting that the use of probiotics may prove beneficial in preventing infantile AD.28 Additionally, findings from an ex vivo and in vitro study show that some conditions, including AD and acne, may benefit from the same probiotics, despite the differences in these two diseases. Both AD and acne have inflammatory and skin dysbiosis characteristics, which may be the common thread leading to both conditions potentially responding to treatment with probiotics.29
Preliminary evidence indicates that supplements containing fatty acids such as docosahexaenoic acid, sea buckthorn oil, and hemp seed oil may decrease the severity of AD.28 In a 20-week, randomized, single-blind, crossover study published in 2005, dietary hemp seed oil showed an improvement of clinical symptoms, including dry skin and itchiness, in patients with AD.30
In light of recent legalization in several states, patients may turn to use of cannabinoid products to manage AD. In a systematic review, cannabinoid use was reportedly a therapeutic option in the treatment of AD and AV; however, the data are based on preclinical work, and there are no randomized, placebo-controlled studies to support the use of cannabinoids.31 Furthermore, there is great concern that use of these products in adolescents is an even larger unknown.
Final Thoughts
Eighty percent of children diagnosed with AD experience symptom improvement before their early teens32; for those with AD during their preteen and teenage years, there can be psychological ramifications, as teenagers with AD report having fewer friends, are less socially involved, participate in fewer sports, and are absent from classes more often than their peers.5 In black patients with AD, school absences are even more common.6 Given the social and emotional impact of AD on patients with skin of color, it is imperative to treat the condition appropriately.33 There are areas of opportunity for further research on alternate dosing of existing treatments for AV in patients with AD, further recommendations for adolescent athletes with AD, and which cosmetic and alternative medicine products may be beneficial for this population to improve their QOL.
Providers should discuss medical management in a broader context considering patients’ extracurricular activities, treatment vehicle preferences, expectations, and personal care habits. It also is important to address the many possible factors that may influence treatment adherence early on, particularly in adolescents, as these could be barriers to treatment. This article highlights considerations for treating AD and comorbid conditions that may further complicate treatment in adolescent patients with skin of color. The information provided should serve as a guide in initial counseling and management of AD in adolescents with skin of color.
- Feldman SR, Cox LS, Strowd LC, et al. The challenge of managing atopic dermatitis in the United States. Am Health Drug Benefits. 2019;12:83-93.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357.
- Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122:449-455.
- Vivar KL, Kruse L. The impact of pediatric skin disease on self-esteem. Int J Womens Dermatol. 2018;4:27-31.
- Wan J, Margolis DJ, Mitra N, et al. Racial and ethnic differences in atopic dermatitis–related school absences among US children [published online May 22, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.0597.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109-1122.
- Ishikawa J, Narita H, Kondo N, et al. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130:2511-2514.
- Chernikova D, Yuan I, Shaker M. Prevention of allergy with diverse and healthy microbiota: an update. Curr Opin Pediatr. 2019;31:418-425.
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Nguyen CM, Koo J, Cordoro KM. Psychodermatologic effects of atopic dermatitis and acne: a review on self-esteem and identity. Pediatr Dermatol. 2016;33:129-135.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Lynde CW, Andriessen A, Barankin B, et al. Moisturizers and ceramide-containing moisturizers may offer concomitant therapy with benefits. J Clin Aesthet Dermatol. 2014;7:18-26.
- Taylor SC, Cook-Bolden FE, McMichael A, et al. Efficacy, safety, and tolerability of topical dapsone gel, 7.5% for treatment of acne vulgaris by Fitzpatrick skin phototype. J Drugs Dermatol. 2018;17:160-167.
- Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-236.
- Vachiramon V, Tey HL, Thompson AE, et al. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29:395-402.
- Heath CR. Managing postinflammatory hyperpigmentation in pediatric patients with skin of color. Cutis. 2018;102:71-73.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Paller AS, McAlister RO, Doyle JJ, et al. Perceptions of physicians and pediatric patients about atopic dermatitis, its impact, and its treatment. Clin Pediatr. 2002;41:323-332.
- Sibbald C, Drucker AM. Patient burden of atopic dermatitis. Dermatol Clin. 2017;35:303-316.
- Rocha VB, Machado CJ, Bittencourt FV. Presence of allergens in the vehicles of Brazilian dermatological products. Contact Dermatitis. 2017;76:126-128.
- Admani S, Goldenberg A, Jacob SE. Contact alopecia: improvement of alopecia with discontinuation of fluocinolone oil in individuals allergic to balsam fragrance. Pediatr Dermatol. 2017;34:e57-e60.
- Uter W, Werfel T, White IR, et al. Contact allergy: a review of current problems from a clinical perspective. Int J Environ Res Public Health. 2018;15:E1108.
- López-Jiménez EC, Marrero-Alemán G, Borrego L. One-third of patients with therapy-resistant atopic dermatitis may benefit after patch testing [published online May 13, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15672.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15.
- Hon KL, Tsang YC, Pong NH, et al. Patient acceptability, efficacy, and skin biophysiology of a cream and cleanser containing lipid complex with shea butter extract versus a ceramide product for eczema. Hong Kong Med J. 2015;21:417-425.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review [published online March 20, 2019]. Int J Dermatol. doi:10.1111/ijd.14404.
- Mottin VHM, Suyenaga ES. An approach on the potential use of probiotics in the treatment of skin conditions: acne and atopic dermatitis. Int J Dermatol. 2018;57:1425-1432.
- Callaway J, Schwab U, Harvima I, et al. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J Dermatol Treat. 2005;16:87-94.
- Eagleston LRM, Kalani NK, Patel RR, et al. Cannabinoids in dermatology: a scoping review [published June 15, 2018]. Dermatol Online J. 2018;24.
- Kim JP, Chao LX, Simpson EL, et al. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:681-687.e611.
- de María Díaz Granados L, Quijano MA, Ramírez PA, et al. Quality assessment of atopic dermatitis clinical practice guidelines in ≤ 18 years. Arch Dermatol Res. 2018;310:29-37.
- Feldman SR, Cox LS, Strowd LC, et al. The challenge of managing atopic dermatitis in the United States. Am Health Drug Benefits. 2019;12:83-93.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357.
- Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122:449-455.
- Vivar KL, Kruse L. The impact of pediatric skin disease on self-esteem. Int J Womens Dermatol. 2018;4:27-31.
- Wan J, Margolis DJ, Mitra N, et al. Racial and ethnic differences in atopic dermatitis–related school absences among US children [published online May 22, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.0597.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109-1122.
- Ishikawa J, Narita H, Kondo N, et al. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130:2511-2514.
- Chernikova D, Yuan I, Shaker M. Prevention of allergy with diverse and healthy microbiota: an update. Curr Opin Pediatr. 2019;31:418-425.
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Nguyen CM, Koo J, Cordoro KM. Psychodermatologic effects of atopic dermatitis and acne: a review on self-esteem and identity. Pediatr Dermatol. 2016;33:129-135.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Lynde CW, Andriessen A, Barankin B, et al. Moisturizers and ceramide-containing moisturizers may offer concomitant therapy with benefits. J Clin Aesthet Dermatol. 2014;7:18-26.
- Taylor SC, Cook-Bolden FE, McMichael A, et al. Efficacy, safety, and tolerability of topical dapsone gel, 7.5% for treatment of acne vulgaris by Fitzpatrick skin phototype. J Drugs Dermatol. 2018;17:160-167.
- Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-236.
- Vachiramon V, Tey HL, Thompson AE, et al. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29:395-402.
- Heath CR. Managing postinflammatory hyperpigmentation in pediatric patients with skin of color. Cutis. 2018;102:71-73.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Paller AS, McAlister RO, Doyle JJ, et al. Perceptions of physicians and pediatric patients about atopic dermatitis, its impact, and its treatment. Clin Pediatr. 2002;41:323-332.
- Sibbald C, Drucker AM. Patient burden of atopic dermatitis. Dermatol Clin. 2017;35:303-316.
- Rocha VB, Machado CJ, Bittencourt FV. Presence of allergens in the vehicles of Brazilian dermatological products. Contact Dermatitis. 2017;76:126-128.
- Admani S, Goldenberg A, Jacob SE. Contact alopecia: improvement of alopecia with discontinuation of fluocinolone oil in individuals allergic to balsam fragrance. Pediatr Dermatol. 2017;34:e57-e60.
- Uter W, Werfel T, White IR, et al. Contact allergy: a review of current problems from a clinical perspective. Int J Environ Res Public Health. 2018;15:E1108.
- López-Jiménez EC, Marrero-Alemán G, Borrego L. One-third of patients with therapy-resistant atopic dermatitis may benefit after patch testing [published online May 13, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15672.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15.
- Hon KL, Tsang YC, Pong NH, et al. Patient acceptability, efficacy, and skin biophysiology of a cream and cleanser containing lipid complex with shea butter extract versus a ceramide product for eczema. Hong Kong Med J. 2015;21:417-425.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review [published online March 20, 2019]. Int J Dermatol. doi:10.1111/ijd.14404.
- Mottin VHM, Suyenaga ES. An approach on the potential use of probiotics in the treatment of skin conditions: acne and atopic dermatitis. Int J Dermatol. 2018;57:1425-1432.
- Callaway J, Schwab U, Harvima I, et al. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J Dermatol Treat. 2005;16:87-94.
- Eagleston LRM, Kalani NK, Patel RR, et al. Cannabinoids in dermatology: a scoping review [published June 15, 2018]. Dermatol Online J. 2018;24.
- Kim JP, Chao LX, Simpson EL, et al. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:681-687.e611.
- de María Díaz Granados L, Quijano MA, Ramírez PA, et al. Quality assessment of atopic dermatitis clinical practice guidelines in ≤ 18 years. Arch Dermatol Res. 2018;310:29-37.
Practice Points
- Atopic dermatitis (AD) can be a lifelong issue that affects academic and occupational performance, with higher rates of absenteeism seen in black patients.
- The FLG loss-of-function mutation seems to play a smaller role in black patients, and other genes may be involved in skin barrier dysfunction, which could be why there is a higher rate of skin of color patients with treatment-resistant AD.
- Diagnosing AD in skin of color patients can pose a particular challenge, and severe cases of AD may not be diagnosed or treated adequately in deeply pigmented children because erythema, a defining characteristic of AD, may be hard to identify in darker skin tones.
- There are several areas of opportunity for further research to better treat AD in this patient population and improve
quality of life.
Atopic Dermatitis in the US Military
Dermatologic conditions historically have affected military members’ ability to serve during times of peace and conflict. These conditions range from chronic dermatologic diseases to environment- or occupation-related dermatologic diseases. Mild to moderate atopic dermatitis (AD) typically is a manageable skin condition. However, in a deployed setting, a flare of AD can result in the inability of a member to perform their military duty, which directly compromises mission safety and effectiveness. The military developed and updates medical standards for entry and retention of service members. These standards are designed to ensure the greatest potential for a military member to successfully serve at home station and during combat operations.
Impact of Injuries in Military
Historically, disease and nonbattle injuries have resulted in notably more hospitalizations and time lost than injuries sustained on the battlefield.1 A review of major conflicts dating from World War II shows approximately 10% of all dermatologic concerns were related to eczematous dermatitis, with 2% specifically related to AD. These numbers varied remarkably depending on the location and environment of the conflict, with eczema accounting for 25% of dermatologic concerns during the Gulf War.2 During the initial phases of Operation Iraqi Freedom, approximately 75% of hospitalizations were from disease and nonbattle injuries, of which dermatologic disease accounted for 3%.1 From 2003 to 2006 in Iraq, 35 service members were evacuated from combat zones specifically for uncontrolled AD.3 In a deployed environment, each member is critical to the unit’s success in completing their mission. A single member of a unit often is the only person qualified to perform a function for that team. There are rarely extra people with similar skills to replace a member unable to complete his/her duties. The loss of a single member compromises the effectiveness and safety of the team and can lead to mission failure. Therefore, AD can have a profound impact on military operations in a deployed environment.
Military Medical Standards for Accession and Retention
There are 2 main goals of the military medical standards. First, the individual health of the applicant or military member is of utmost importance. Applicants with medical conditions that will be exacerbated by military service or that limit the ability for successful military operations are not accepted for military service. Once an active-duty member is diagnosed with a medical condition, the military determines if limitations are needed for military assignments and deployments based on available medical care in those locations. Second, mission accomplishment in combat operations requires that healthy military members are able to complete their jobs in extreme environments and under notable stress. If an applicant has a medical condition unsuitable for military service, it is in the best interest of the applicant and the military to deny entry.
The Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03) lists conditions that are disqualifying for military service.4 Section 5.21 lists the following as disqualifying for military service in relation to eczematous dermatitis:
d. History of AD or eczema after the 12th birthday. History of residual or recurrent lesions in characteristic areas (face, neck, antecubital or popliteal fossae, occasionally wrists and hands).
e. History of recurrent or chronic nonspecific dermatitis within the past 2 years to include contact (irritant or allergic) or dyshidrotic dermatitis requiring more than treatment with topical corticosteroid.4
Although cases of incorrect diagnosis or very mild AD can be considered for a waiver, the process can be laborious and consideration or approval is not guaranteed. For current military members with new chronic eczematous dermatitis, each service has a process for evaluation and treatment. Some special operational jobs, such as aircrew, missile operators, and divers, have more restrictive medical requirements that are monitored by physicians with special training in these populations.
Atopic dermatitis affects 25% of children and 2% to 3% of adults.5 Approximately 60% of patients with AD will develop their first eruption by 1 year of age, and 90% by 5 years of age. Although the majority of patients will have resolution of their disease during childhood, 10% to 30% will have persistent disease into adulthood.5 Because the majority of AD resolves in childhood, it is understandable that asymptomatic individuals with a history of AD before 12 years of age meet military entrance medical standards.
Provoking Factors
The US Military maintains stringent medical standards because of the nature of the dynamic, rapidly changing military environment and its demands. Whether training for readiness in an austere location, deploying to extreme climates, or being stationed overseas, service members must be prepared to encounter a myriad of environmental extremes, physical stress, and psychological stressors. Environmental factors commonly experienced in the military can provoke or exacerbate symptoms of AD (Figures 1 and 2). Ideally, an individual with AD lives in a stable climate, has access to moisturizers and topical steroids, bathes regularly to remove dust and debris, wears 100% cotton garments to avoid irritation, and avoids using gear that would cause exacerbations. Service members rarely have such accommodations in deployed settings. A recent article in Military Medicine explained quite well, “If someone wanted to design an experience with the explicit goal to flare a person with otherwise well controlled atopic dermatitis it would probably look like a military deployment.”3
The United States has a military presence in countries with extreme temperature and humidity variations all over the world. Uniforms are standardized, and members are required to wear prescribed clothing with no alternatives. Uniforms are made of durable sturdy material. If uniforms can be laundered, they often are grouped together, and sensitive detergent cannot be specified. Bathing is challenging in deployed locations, with troops often going weeks using baby wipes for self-hygiene. These conditions increase risk for development of contact allergens, and little access to proper hygiene practices also increases risk for secondary infections in members with AD.
In addition to environmental challenges, the military gear and equipment used can flare AD. Service members must wear protective gear such as body armor. These heavy hard pieces of material are bulky; difficult to wash; and cause friction, sweating, and irritation. The military prepares for operations in chemical, biological, radiological, or nuclear environments, which requires wearing a rubber mask, multiple layers of boots and gloves, and thick charcoal impregnated over garments for many hours. Such conditions may flare AD or make it intolerable.
Although stress is a part of any deployment experience, excessive or prolonged stress can lead to combat operational stress reactions that inhibit a service member’s ability to function.6 Stressors during deployment can accumulate and may be caused by the operational environment, loss of fellow service members to injury or death, illness, leadership demands, personal choices, issues on the home front, interpersonal conflicts, and sleep loss.7 Atopic dermatitis can be exacerbated by such stress, leading to increased pruritus and scratching.7-9 Symptomatic AD also can play a role in worsening combat stress. Although severe pruritus may affect attentiveness to job duties during the day, these symptoms, if uncontrolled, also can negatively affect sleep. As many as 60% of patients with AD at baseline and 83% of patients with exacerbations experience sleep disturbance due to their disease.5 These stressors experienced by deployed military personnel can contribute to combat stress reactions, which may vary from simple inattentiveness to more serious behaviors such as suicidal ideation.6 Combat stress reactions inhibit a military member’s ability to function properly in the deployed environment and can lead to notable safety concerns and potential mission failure.
Vaccinations
Military members deploying overseas are required to receive specific vaccinations, including the smallpox vaccine. Although the virus was eradicated in 1980, the concern for smallpox to be used as a biological weapon in certain areas of the world necessitates continued vaccination of military populations. According to the Centers for Disease Control and Prevention, the only known reservoir for the virus is humans, and the disease has a mortality rate of 30%.10 A history of or present AD is a contraindication for primary smallpox vaccination and revaccination for nonemergency use because of the risk for eczema vaccinatum.11 The risk also applies to close contacts of vaccinated members. For 30 days after vaccination, service members must avoid skin-to-skin contact with anyone who has active AD.12 Eczema vaccinatum in vaccinated individuals is typically self-limited; however, eczema vaccinatum in nonvaccinated contacts can be severe. One case report described a 28-month-old child with refractory AD who developed severe eczema vaccinatum after contact with her recently vaccinated military parent. The child required a 48-day admission to the intensive care unit and multiple skin grafts; fortunately, the child did not develop any apparent long-term sequelae.13 This case highlights the importance of understanding the risks associated with smallpox vaccination in military members with AD and the responsibility of health care providers to properly screen and counsel individuals prior to administering smallpox vaccines.
Treatment
Treatment of mild to moderate AD is relatively straightforward in developed countries with good access to medical care. The most recent American Academy of Dermatology clinical guidelines for AD focus on minimizing irritants and triggers, regularly using moisturizers soon after bathing, and using topical steroids as needed.5 Military members face specific challenges regarding treatment of AD, particularly when deployed to remote locations without access to treatment facilities or medications. Military members are required to carry all necessary personal medications with them for at least 6 months and preferably the duration of the deployment, sometimes up to 1 year. Military members carry a large amount of gear for deployments, and it is not feasible to pack an additional 10 to 20 lb worth of emollients and topical steroids to last the entire deployment. Routine laboratory monitoring is limited or completely unavailable. Refrigeration typically is not available, making use of systemic medications nearly impossible during deployments. In the event of complications such as eczema herpeticum or secondary bacterial infection, service members could require evacuation from the deployed location to a larger field hospital or to the United States, which is costly and also removes a valuable team member from the deployed unit. These limitations in access to care, medications, and treatment options make AD a difficult condition to treat in the deployed setting.
Nonmilitary Medical Providers
Civilian providers play an important role in diagnosing and treating AD. It is vital to completely and accurately document treatment of all skin diseases; however, it is especially important for those who desire to or currently serve in the military. Military primary care providers or military dermatologists must review the information from civilian providers to aid in determining suitability for entry or retention in the military. Clearly documenting the morphology, extent of disease involvement (eg, body surface area), treatment plan, response to treatment, and exacerbating factors will aid in ensuring the patient’s medical record accurately reflects their skin disease. Ultimately, this record often is the only information available to make health determinations regarding military service.
Conclusion
A career in the military is challenging and rewarding for those who volunteer to serve. Because of the demanding and unpredictable lifestyle inherent with military service, the Department of Defense maintains strict medical standards for entrance and retention. These standards ensure members are capable of safely completing training and deploying anywhere in the world. Although AD is a relatively common and treatable skin disease in locations with well-established medical care, it can pose a notable problem for service members while deployed to austere locations with variable environments around the world. Environmental factors and gear requirements, coupled with limited access to treatment facilities and medications, render AD a potentially serious issue. Atopic dermatitis in military members can affect individual medical readiness and unit success. It is important that all providers understand the myriad effects that AD can have on an individual who wishes to join or continue service in the military.
- Zouris JM, Wade AL, Magno CP. Injury and illness casualty distributions among U.S. Army and Marine Corps personnel during Operation Iraqi Freedom. Mil Med. 2008;173:247-252.
- Gelman, AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:177-182.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 8, 2019.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Force Health Protection (Army Techniques Publication No. 4-02.8). Washington, DC: Department of the Army; March 2016. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/atp4_02x8.pdf. Accessed August 19, 2019.
- Judkins JL, Bradley DL. A review of the effectiveness of a combat and operational stress control restoration center in Afghanistan. Mil Med. 2017;182:1755-1762.
- Suarez AL, Feramisco JD, Koo J, et al. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Dermatol Venereol. 2012;92:7-15.
- Mochizuki H, Lavery MJ, Nattkemper LA, et al. Impact of acute stress on itch sensation and scratching behaviour in patients with atopic dermatitis and healthy controls. Br J Dermatol. 2019;180:821-827.
- Centers for Disease Control and Prevention. Smallpox: contraindications to vaccination. https://www.cdc.gov/smallpox/clinicians/vaccination-contraindications1.html. Updated December 5, 2016. Accessed August 19, 2019.
- Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;5:84-90.
- Reed JL, Scott DE, Bray M. Eczema vaccinatum. Clin Infect Dis. 2012;54:832-840.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccine. Clin Infect Dis. 2008;46:1555-1561.
Dermatologic conditions historically have affected military members’ ability to serve during times of peace and conflict. These conditions range from chronic dermatologic diseases to environment- or occupation-related dermatologic diseases. Mild to moderate atopic dermatitis (AD) typically is a manageable skin condition. However, in a deployed setting, a flare of AD can result in the inability of a member to perform their military duty, which directly compromises mission safety and effectiveness. The military developed and updates medical standards for entry and retention of service members. These standards are designed to ensure the greatest potential for a military member to successfully serve at home station and during combat operations.
Impact of Injuries in Military
Historically, disease and nonbattle injuries have resulted in notably more hospitalizations and time lost than injuries sustained on the battlefield.1 A review of major conflicts dating from World War II shows approximately 10% of all dermatologic concerns were related to eczematous dermatitis, with 2% specifically related to AD. These numbers varied remarkably depending on the location and environment of the conflict, with eczema accounting for 25% of dermatologic concerns during the Gulf War.2 During the initial phases of Operation Iraqi Freedom, approximately 75% of hospitalizations were from disease and nonbattle injuries, of which dermatologic disease accounted for 3%.1 From 2003 to 2006 in Iraq, 35 service members were evacuated from combat zones specifically for uncontrolled AD.3 In a deployed environment, each member is critical to the unit’s success in completing their mission. A single member of a unit often is the only person qualified to perform a function for that team. There are rarely extra people with similar skills to replace a member unable to complete his/her duties. The loss of a single member compromises the effectiveness and safety of the team and can lead to mission failure. Therefore, AD can have a profound impact on military operations in a deployed environment.
Military Medical Standards for Accession and Retention
There are 2 main goals of the military medical standards. First, the individual health of the applicant or military member is of utmost importance. Applicants with medical conditions that will be exacerbated by military service or that limit the ability for successful military operations are not accepted for military service. Once an active-duty member is diagnosed with a medical condition, the military determines if limitations are needed for military assignments and deployments based on available medical care in those locations. Second, mission accomplishment in combat operations requires that healthy military members are able to complete their jobs in extreme environments and under notable stress. If an applicant has a medical condition unsuitable for military service, it is in the best interest of the applicant and the military to deny entry.
The Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03) lists conditions that are disqualifying for military service.4 Section 5.21 lists the following as disqualifying for military service in relation to eczematous dermatitis:
d. History of AD or eczema after the 12th birthday. History of residual or recurrent lesions in characteristic areas (face, neck, antecubital or popliteal fossae, occasionally wrists and hands).
e. History of recurrent or chronic nonspecific dermatitis within the past 2 years to include contact (irritant or allergic) or dyshidrotic dermatitis requiring more than treatment with topical corticosteroid.4
Although cases of incorrect diagnosis or very mild AD can be considered for a waiver, the process can be laborious and consideration or approval is not guaranteed. For current military members with new chronic eczematous dermatitis, each service has a process for evaluation and treatment. Some special operational jobs, such as aircrew, missile operators, and divers, have more restrictive medical requirements that are monitored by physicians with special training in these populations.
Atopic dermatitis affects 25% of children and 2% to 3% of adults.5 Approximately 60% of patients with AD will develop their first eruption by 1 year of age, and 90% by 5 years of age. Although the majority of patients will have resolution of their disease during childhood, 10% to 30% will have persistent disease into adulthood.5 Because the majority of AD resolves in childhood, it is understandable that asymptomatic individuals with a history of AD before 12 years of age meet military entrance medical standards.
Provoking Factors
The US Military maintains stringent medical standards because of the nature of the dynamic, rapidly changing military environment and its demands. Whether training for readiness in an austere location, deploying to extreme climates, or being stationed overseas, service members must be prepared to encounter a myriad of environmental extremes, physical stress, and psychological stressors. Environmental factors commonly experienced in the military can provoke or exacerbate symptoms of AD (Figures 1 and 2). Ideally, an individual with AD lives in a stable climate, has access to moisturizers and topical steroids, bathes regularly to remove dust and debris, wears 100% cotton garments to avoid irritation, and avoids using gear that would cause exacerbations. Service members rarely have such accommodations in deployed settings. A recent article in Military Medicine explained quite well, “If someone wanted to design an experience with the explicit goal to flare a person with otherwise well controlled atopic dermatitis it would probably look like a military deployment.”3
The United States has a military presence in countries with extreme temperature and humidity variations all over the world. Uniforms are standardized, and members are required to wear prescribed clothing with no alternatives. Uniforms are made of durable sturdy material. If uniforms can be laundered, they often are grouped together, and sensitive detergent cannot be specified. Bathing is challenging in deployed locations, with troops often going weeks using baby wipes for self-hygiene. These conditions increase risk for development of contact allergens, and little access to proper hygiene practices also increases risk for secondary infections in members with AD.
In addition to environmental challenges, the military gear and equipment used can flare AD. Service members must wear protective gear such as body armor. These heavy hard pieces of material are bulky; difficult to wash; and cause friction, sweating, and irritation. The military prepares for operations in chemical, biological, radiological, or nuclear environments, which requires wearing a rubber mask, multiple layers of boots and gloves, and thick charcoal impregnated over garments for many hours. Such conditions may flare AD or make it intolerable.
Although stress is a part of any deployment experience, excessive or prolonged stress can lead to combat operational stress reactions that inhibit a service member’s ability to function.6 Stressors during deployment can accumulate and may be caused by the operational environment, loss of fellow service members to injury or death, illness, leadership demands, personal choices, issues on the home front, interpersonal conflicts, and sleep loss.7 Atopic dermatitis can be exacerbated by such stress, leading to increased pruritus and scratching.7-9 Symptomatic AD also can play a role in worsening combat stress. Although severe pruritus may affect attentiveness to job duties during the day, these symptoms, if uncontrolled, also can negatively affect sleep. As many as 60% of patients with AD at baseline and 83% of patients with exacerbations experience sleep disturbance due to their disease.5 These stressors experienced by deployed military personnel can contribute to combat stress reactions, which may vary from simple inattentiveness to more serious behaviors such as suicidal ideation.6 Combat stress reactions inhibit a military member’s ability to function properly in the deployed environment and can lead to notable safety concerns and potential mission failure.
Vaccinations
Military members deploying overseas are required to receive specific vaccinations, including the smallpox vaccine. Although the virus was eradicated in 1980, the concern for smallpox to be used as a biological weapon in certain areas of the world necessitates continued vaccination of military populations. According to the Centers for Disease Control and Prevention, the only known reservoir for the virus is humans, and the disease has a mortality rate of 30%.10 A history of or present AD is a contraindication for primary smallpox vaccination and revaccination for nonemergency use because of the risk for eczema vaccinatum.11 The risk also applies to close contacts of vaccinated members. For 30 days after vaccination, service members must avoid skin-to-skin contact with anyone who has active AD.12 Eczema vaccinatum in vaccinated individuals is typically self-limited; however, eczema vaccinatum in nonvaccinated contacts can be severe. One case report described a 28-month-old child with refractory AD who developed severe eczema vaccinatum after contact with her recently vaccinated military parent. The child required a 48-day admission to the intensive care unit and multiple skin grafts; fortunately, the child did not develop any apparent long-term sequelae.13 This case highlights the importance of understanding the risks associated with smallpox vaccination in military members with AD and the responsibility of health care providers to properly screen and counsel individuals prior to administering smallpox vaccines.
Treatment
Treatment of mild to moderate AD is relatively straightforward in developed countries with good access to medical care. The most recent American Academy of Dermatology clinical guidelines for AD focus on minimizing irritants and triggers, regularly using moisturizers soon after bathing, and using topical steroids as needed.5 Military members face specific challenges regarding treatment of AD, particularly when deployed to remote locations without access to treatment facilities or medications. Military members are required to carry all necessary personal medications with them for at least 6 months and preferably the duration of the deployment, sometimes up to 1 year. Military members carry a large amount of gear for deployments, and it is not feasible to pack an additional 10 to 20 lb worth of emollients and topical steroids to last the entire deployment. Routine laboratory monitoring is limited or completely unavailable. Refrigeration typically is not available, making use of systemic medications nearly impossible during deployments. In the event of complications such as eczema herpeticum or secondary bacterial infection, service members could require evacuation from the deployed location to a larger field hospital or to the United States, which is costly and also removes a valuable team member from the deployed unit. These limitations in access to care, medications, and treatment options make AD a difficult condition to treat in the deployed setting.
Nonmilitary Medical Providers
Civilian providers play an important role in diagnosing and treating AD. It is vital to completely and accurately document treatment of all skin diseases; however, it is especially important for those who desire to or currently serve in the military. Military primary care providers or military dermatologists must review the information from civilian providers to aid in determining suitability for entry or retention in the military. Clearly documenting the morphology, extent of disease involvement (eg, body surface area), treatment plan, response to treatment, and exacerbating factors will aid in ensuring the patient’s medical record accurately reflects their skin disease. Ultimately, this record often is the only information available to make health determinations regarding military service.
Conclusion
A career in the military is challenging and rewarding for those who volunteer to serve. Because of the demanding and unpredictable lifestyle inherent with military service, the Department of Defense maintains strict medical standards for entrance and retention. These standards ensure members are capable of safely completing training and deploying anywhere in the world. Although AD is a relatively common and treatable skin disease in locations with well-established medical care, it can pose a notable problem for service members while deployed to austere locations with variable environments around the world. Environmental factors and gear requirements, coupled with limited access to treatment facilities and medications, render AD a potentially serious issue. Atopic dermatitis in military members can affect individual medical readiness and unit success. It is important that all providers understand the myriad effects that AD can have on an individual who wishes to join or continue service in the military.
Dermatologic conditions historically have affected military members’ ability to serve during times of peace and conflict. These conditions range from chronic dermatologic diseases to environment- or occupation-related dermatologic diseases. Mild to moderate atopic dermatitis (AD) typically is a manageable skin condition. However, in a deployed setting, a flare of AD can result in the inability of a member to perform their military duty, which directly compromises mission safety and effectiveness. The military developed and updates medical standards for entry and retention of service members. These standards are designed to ensure the greatest potential for a military member to successfully serve at home station and during combat operations.
Impact of Injuries in Military
Historically, disease and nonbattle injuries have resulted in notably more hospitalizations and time lost than injuries sustained on the battlefield.1 A review of major conflicts dating from World War II shows approximately 10% of all dermatologic concerns were related to eczematous dermatitis, with 2% specifically related to AD. These numbers varied remarkably depending on the location and environment of the conflict, with eczema accounting for 25% of dermatologic concerns during the Gulf War.2 During the initial phases of Operation Iraqi Freedom, approximately 75% of hospitalizations were from disease and nonbattle injuries, of which dermatologic disease accounted for 3%.1 From 2003 to 2006 in Iraq, 35 service members were evacuated from combat zones specifically for uncontrolled AD.3 In a deployed environment, each member is critical to the unit’s success in completing their mission. A single member of a unit often is the only person qualified to perform a function for that team. There are rarely extra people with similar skills to replace a member unable to complete his/her duties. The loss of a single member compromises the effectiveness and safety of the team and can lead to mission failure. Therefore, AD can have a profound impact on military operations in a deployed environment.
Military Medical Standards for Accession and Retention
There are 2 main goals of the military medical standards. First, the individual health of the applicant or military member is of utmost importance. Applicants with medical conditions that will be exacerbated by military service or that limit the ability for successful military operations are not accepted for military service. Once an active-duty member is diagnosed with a medical condition, the military determines if limitations are needed for military assignments and deployments based on available medical care in those locations. Second, mission accomplishment in combat operations requires that healthy military members are able to complete their jobs in extreme environments and under notable stress. If an applicant has a medical condition unsuitable for military service, it is in the best interest of the applicant and the military to deny entry.
The Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03) lists conditions that are disqualifying for military service.4 Section 5.21 lists the following as disqualifying for military service in relation to eczematous dermatitis:
d. History of AD or eczema after the 12th birthday. History of residual or recurrent lesions in characteristic areas (face, neck, antecubital or popliteal fossae, occasionally wrists and hands).
e. History of recurrent or chronic nonspecific dermatitis within the past 2 years to include contact (irritant or allergic) or dyshidrotic dermatitis requiring more than treatment with topical corticosteroid.4
Although cases of incorrect diagnosis or very mild AD can be considered for a waiver, the process can be laborious and consideration or approval is not guaranteed. For current military members with new chronic eczematous dermatitis, each service has a process for evaluation and treatment. Some special operational jobs, such as aircrew, missile operators, and divers, have more restrictive medical requirements that are monitored by physicians with special training in these populations.
Atopic dermatitis affects 25% of children and 2% to 3% of adults.5 Approximately 60% of patients with AD will develop their first eruption by 1 year of age, and 90% by 5 years of age. Although the majority of patients will have resolution of their disease during childhood, 10% to 30% will have persistent disease into adulthood.5 Because the majority of AD resolves in childhood, it is understandable that asymptomatic individuals with a history of AD before 12 years of age meet military entrance medical standards.
Provoking Factors
The US Military maintains stringent medical standards because of the nature of the dynamic, rapidly changing military environment and its demands. Whether training for readiness in an austere location, deploying to extreme climates, or being stationed overseas, service members must be prepared to encounter a myriad of environmental extremes, physical stress, and psychological stressors. Environmental factors commonly experienced in the military can provoke or exacerbate symptoms of AD (Figures 1 and 2). Ideally, an individual with AD lives in a stable climate, has access to moisturizers and topical steroids, bathes regularly to remove dust and debris, wears 100% cotton garments to avoid irritation, and avoids using gear that would cause exacerbations. Service members rarely have such accommodations in deployed settings. A recent article in Military Medicine explained quite well, “If someone wanted to design an experience with the explicit goal to flare a person with otherwise well controlled atopic dermatitis it would probably look like a military deployment.”3
The United States has a military presence in countries with extreme temperature and humidity variations all over the world. Uniforms are standardized, and members are required to wear prescribed clothing with no alternatives. Uniforms are made of durable sturdy material. If uniforms can be laundered, they often are grouped together, and sensitive detergent cannot be specified. Bathing is challenging in deployed locations, with troops often going weeks using baby wipes for self-hygiene. These conditions increase risk for development of contact allergens, and little access to proper hygiene practices also increases risk for secondary infections in members with AD.
In addition to environmental challenges, the military gear and equipment used can flare AD. Service members must wear protective gear such as body armor. These heavy hard pieces of material are bulky; difficult to wash; and cause friction, sweating, and irritation. The military prepares for operations in chemical, biological, radiological, or nuclear environments, which requires wearing a rubber mask, multiple layers of boots and gloves, and thick charcoal impregnated over garments for many hours. Such conditions may flare AD or make it intolerable.
Although stress is a part of any deployment experience, excessive or prolonged stress can lead to combat operational stress reactions that inhibit a service member’s ability to function.6 Stressors during deployment can accumulate and may be caused by the operational environment, loss of fellow service members to injury or death, illness, leadership demands, personal choices, issues on the home front, interpersonal conflicts, and sleep loss.7 Atopic dermatitis can be exacerbated by such stress, leading to increased pruritus and scratching.7-9 Symptomatic AD also can play a role in worsening combat stress. Although severe pruritus may affect attentiveness to job duties during the day, these symptoms, if uncontrolled, also can negatively affect sleep. As many as 60% of patients with AD at baseline and 83% of patients with exacerbations experience sleep disturbance due to their disease.5 These stressors experienced by deployed military personnel can contribute to combat stress reactions, which may vary from simple inattentiveness to more serious behaviors such as suicidal ideation.6 Combat stress reactions inhibit a military member’s ability to function properly in the deployed environment and can lead to notable safety concerns and potential mission failure.
Vaccinations
Military members deploying overseas are required to receive specific vaccinations, including the smallpox vaccine. Although the virus was eradicated in 1980, the concern for smallpox to be used as a biological weapon in certain areas of the world necessitates continued vaccination of military populations. According to the Centers for Disease Control and Prevention, the only known reservoir for the virus is humans, and the disease has a mortality rate of 30%.10 A history of or present AD is a contraindication for primary smallpox vaccination and revaccination for nonemergency use because of the risk for eczema vaccinatum.11 The risk also applies to close contacts of vaccinated members. For 30 days after vaccination, service members must avoid skin-to-skin contact with anyone who has active AD.12 Eczema vaccinatum in vaccinated individuals is typically self-limited; however, eczema vaccinatum in nonvaccinated contacts can be severe. One case report described a 28-month-old child with refractory AD who developed severe eczema vaccinatum after contact with her recently vaccinated military parent. The child required a 48-day admission to the intensive care unit and multiple skin grafts; fortunately, the child did not develop any apparent long-term sequelae.13 This case highlights the importance of understanding the risks associated with smallpox vaccination in military members with AD and the responsibility of health care providers to properly screen and counsel individuals prior to administering smallpox vaccines.
Treatment
Treatment of mild to moderate AD is relatively straightforward in developed countries with good access to medical care. The most recent American Academy of Dermatology clinical guidelines for AD focus on minimizing irritants and triggers, regularly using moisturizers soon after bathing, and using topical steroids as needed.5 Military members face specific challenges regarding treatment of AD, particularly when deployed to remote locations without access to treatment facilities or medications. Military members are required to carry all necessary personal medications with them for at least 6 months and preferably the duration of the deployment, sometimes up to 1 year. Military members carry a large amount of gear for deployments, and it is not feasible to pack an additional 10 to 20 lb worth of emollients and topical steroids to last the entire deployment. Routine laboratory monitoring is limited or completely unavailable. Refrigeration typically is not available, making use of systemic medications nearly impossible during deployments. In the event of complications such as eczema herpeticum or secondary bacterial infection, service members could require evacuation from the deployed location to a larger field hospital or to the United States, which is costly and also removes a valuable team member from the deployed unit. These limitations in access to care, medications, and treatment options make AD a difficult condition to treat in the deployed setting.
Nonmilitary Medical Providers
Civilian providers play an important role in diagnosing and treating AD. It is vital to completely and accurately document treatment of all skin diseases; however, it is especially important for those who desire to or currently serve in the military. Military primary care providers or military dermatologists must review the information from civilian providers to aid in determining suitability for entry or retention in the military. Clearly documenting the morphology, extent of disease involvement (eg, body surface area), treatment plan, response to treatment, and exacerbating factors will aid in ensuring the patient’s medical record accurately reflects their skin disease. Ultimately, this record often is the only information available to make health determinations regarding military service.
Conclusion
A career in the military is challenging and rewarding for those who volunteer to serve. Because of the demanding and unpredictable lifestyle inherent with military service, the Department of Defense maintains strict medical standards for entrance and retention. These standards ensure members are capable of safely completing training and deploying anywhere in the world. Although AD is a relatively common and treatable skin disease in locations with well-established medical care, it can pose a notable problem for service members while deployed to austere locations with variable environments around the world. Environmental factors and gear requirements, coupled with limited access to treatment facilities and medications, render AD a potentially serious issue. Atopic dermatitis in military members can affect individual medical readiness and unit success. It is important that all providers understand the myriad effects that AD can have on an individual who wishes to join or continue service in the military.
- Zouris JM, Wade AL, Magno CP. Injury and illness casualty distributions among U.S. Army and Marine Corps personnel during Operation Iraqi Freedom. Mil Med. 2008;173:247-252.
- Gelman, AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:177-182.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 8, 2019.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Force Health Protection (Army Techniques Publication No. 4-02.8). Washington, DC: Department of the Army; March 2016. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/atp4_02x8.pdf. Accessed August 19, 2019.
- Judkins JL, Bradley DL. A review of the effectiveness of a combat and operational stress control restoration center in Afghanistan. Mil Med. 2017;182:1755-1762.
- Suarez AL, Feramisco JD, Koo J, et al. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Dermatol Venereol. 2012;92:7-15.
- Mochizuki H, Lavery MJ, Nattkemper LA, et al. Impact of acute stress on itch sensation and scratching behaviour in patients with atopic dermatitis and healthy controls. Br J Dermatol. 2019;180:821-827.
- Centers for Disease Control and Prevention. Smallpox: contraindications to vaccination. https://www.cdc.gov/smallpox/clinicians/vaccination-contraindications1.html. Updated December 5, 2016. Accessed August 19, 2019.
- Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;5:84-90.
- Reed JL, Scott DE, Bray M. Eczema vaccinatum. Clin Infect Dis. 2012;54:832-840.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccine. Clin Infect Dis. 2008;46:1555-1561.
- Zouris JM, Wade AL, Magno CP. Injury and illness casualty distributions among U.S. Army and Marine Corps personnel during Operation Iraqi Freedom. Mil Med. 2008;173:247-252.
- Gelman, AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:177-182.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 8, 2019.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Force Health Protection (Army Techniques Publication No. 4-02.8). Washington, DC: Department of the Army; March 2016. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/atp4_02x8.pdf. Accessed August 19, 2019.
- Judkins JL, Bradley DL. A review of the effectiveness of a combat and operational stress control restoration center in Afghanistan. Mil Med. 2017;182:1755-1762.
- Suarez AL, Feramisco JD, Koo J, et al. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Dermatol Venereol. 2012;92:7-15.
- Mochizuki H, Lavery MJ, Nattkemper LA, et al. Impact of acute stress on itch sensation and scratching behaviour in patients with atopic dermatitis and healthy controls. Br J Dermatol. 2019;180:821-827.
- Centers for Disease Control and Prevention. Smallpox: contraindications to vaccination. https://www.cdc.gov/smallpox/clinicians/vaccination-contraindications1.html. Updated December 5, 2016. Accessed August 19, 2019.
- Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;5:84-90.
- Reed JL, Scott DE, Bray M. Eczema vaccinatum. Clin Infect Dis. 2012;54:832-840.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccine. Clin Infect Dis. 2008;46:1555-1561.
Practice Points
- The US Military follows strict medical eligibility requirements for enlistment and retention. Atopic dermatitis (AD) and chronic eczematous conditions after 12 years of age is disqualifying for military service, but waivers may be possible for mild cases.
- Unpredictable and rigorous environmental and occupational stressors associated with military service as well as limited access to medical care make AD a challenging condition to manage for service members, particularly during military deployment.
- Accurate diagnosis and documentation of AD in childhood and adolescence by nonmilitary providers are essential, as they will aid in appropriately determining an applicant’s potential to successfully serve in the military.
- For current service members, nonmilitary providers play a vital role in diagnosis and management where military dermatologists are not readily available.
Revolutionizing Atopic Dermatitis
Impressive progress has been made in recent years in the management and treatment of atopic dermatitis (AD) and its comorbidities; however, there is a major need for state-of-the-art, evidence-based, multidisciplinary education for AD management. To address this need, the first Revolutionizing Atopic Dermatitis (RAD) Conference was held in April 2019 in Chicago, Illinois, featuring cutting-edge research presented by globally recognized experts in dermatology, allergy and immunology, sleep medicine, ophthalmology, and nursing care. The following is a recap of the latest topics in AD research presented at the conference.
Diagnosis and Assessment of AD: Jonathan I. Silverberg, MD, PhD, MPH
Although diagnosis of AD typically is straightforward in children, it can be challenging in adults, even for expert clinicians. These challenges stem from the different lesional distribution and morphology of AD in adults vs children.1,2 Additionally, the conditions included in the differential diagnosis of AD (eg, allergic contact dermatitis, cutaneous T-cell lymphoma, psoriasis) are far more common in adults than in children. Formal diagnostic criteria can be useful to improve the diagnosis of AD in clinical practice.3 It is important to note that flexural lesions and early disease onset are diagnostic criteria in AD; nevertheless, neither are essential nor sufficient on their own to make the diagnosis.
Patch Testing: Jacob P. Thyssen, MD, PhD, DmSci, and Noreen Heer Nicol, PhD, RN, FNP, NEA-BC
Patch testing can be used in AD patients to rule out contact dermatitis as an alternative or comorbid diagnosis.4-6 Because contact dermatitis can mimic AD, patch testing is recommended for all patients with adolescent and adult-onset AD.5 Additionally, refractory cases of AD across all ages, especially prior to initiation of systemic therapy, warrant patch testing. The unique challenges of patch testing in AD patients were reviewed.
Patient Panel
Atopic dermatitis can be a considerable disease burden on both patients and society in general. At the 2019 RAD Conference, a panel of patients bravely shared their AD journeys. Their eye-opening stories highlighted opportunities for improving real-world assessment and management of AD. Some key takeaways included the importance of adequately assessing the symptom burden of AD and not merely relying on visual inspection of the skin. The need for long-term treatment approaches beyond quick fixes with steroids also was discussed.
Pathogenesis of AD: Mark Boguniewicz, MD
There have been many advances in our understanding of the complex pathogenesis of AD,7-11 which is characterized by an altered skin barrier and immune dysregulation. Filaggrin deficiency in the skin has structural and biophysical consequences. A subset of patients with AD has filaggrin loss-of-function genetic polymorphisms inherited in an autosomal-semidominant pattern; however, many other genetic polymorphisms have been identified that affect different components of the skin architecture and immune system. Many cytokine pathways have been found to be upregulated in AD lesions, including IL-13, IL-4, IL-31, and IL-5 in acute and chronic lesions, and IFN-γ and other helper T cell (TH1) cytokines in chronic lesions. IL-4 and IL-13 (TH2 cytokines) have been shown to decrease epidermal expression of filaggrin and lead to lipid abnormalities in the skin of patients with AD. Even normal-appearing, nonlesional skin has substantial immune activation and barrier abnormalities in patients with moderate to severe AD. Activation of different immune pathways may contribute to the heterogeneous clinical presentation of AD. There also is an increasingly recognized role of superantigen-producing Staphylococcus aureus and decreased microbial diversity in AD.
Therapies for AD
The advances in our understanding of AD pathophysiology have led to the development of 2 recently approved therapeutic agents.7-10 Crisaborole ointment 2% is a topical phosphodiesterase 4 inhibitor that was approved by the US Food and Drug Administration in 2016 for treatment of mild to moderate AD. Treatment with crisaborole ointment 2% demonstrated improvement in lesion severity, itch, and quality of life in children and adults with AD. Dupilumab, an injectable biologic therapy that inhibits IL-4 and IL-13 signaling, was approved by the US Food and Drug Administration in 2017 for adults and in 2019 for adolescents aged 12 to 17 years with moderate to severe AD. The expert panel of speakers at the 2019 RAD Conference discussed many practical clinical pearls regarding patient education, optimization of both short- and long-term efficacy, and prevention and management of treatment-related adverse events. The discussion included evidence-based guidelines for bathing practices and topical therapy in AD, as well as practical pearls for patient and provider education in AD, reviewed by Dr. Nicol. Evidence-based guidelines for use of phototherapy and systemic and biologic therapy for AD also were highlighted by Dr. Silverberg.
After decades of limited therapeutic options, there is a large therapeutic pipeline of topical, oral, and biologic agents in development for the treatment AD.7-9 Dr. Boguniewicz reviewed the state-of-the-art treatments that are the furthest advanced in development. Many of these agents may be approved within the next couple of years and look promising in terms of their potential to improve the care of patients with AD.
Comorbidities of AD
The impact of AD is not just skin deep. Atopic dermatitis is associated with myriad comorbid health conditions.12-16 Dr. Boguniewicz reviewed the relationship between AD and atopic comorbidities, including asthma, hay fever, and food allergies, which are common across all AD patients. In addition, a subset of children with AD demonstrated the atopic march, in which AD first appears early in life followed by the development of other atopic comorbidities in later childhood or adulthood. In particular, children with filaggrin null mutations were found to be at increased risk of early-onset, severe, persistent AD with asthma and allergic sensitization.17 More recently, eosinophilic esophagitis was demonstrated to be a late-onset comorbidity of the atopic march.18 The allergy guidelines for which patients are appropriate candidates for food and/or aeroallergen testing were discussed,19 and it was emphasized that patients with AD should not routinely receive this testing.
Atopic dermatitis is associated with many other comorbidities, including sleep disturbances. Phyllis C. Zee, MD, PhD, provided a brilliant review of circadian regulation of physiology and the immune system. Sleep is one of the most important determinants of patients’ health and well-being. Atopic dermatitis is associated with disturbances of sleep and circadian rhythms. Sleep disturbances are gaining recognition as an important end point to assess for improvement in clinical practice and trials.
Patients with AD have long been recognized to have increased ophthalmic comorbidities, including allergic conjunctivitis, atopic keratoconjunctivitis, and cataracts. More recently, conjunctivitis has emerged as an important adverse event with dupilumab treatment.20 Jeanine Baqai, MD, reviewed the various ophthalmic comorbidities and shared numerous clinical signs of ophthalmic comorbidities that dermatologists can assess with the naked eye (no slit-lamp examination needed). Pearls to manage dupilumab-related conjunctivitis shared by Dr. Baqai and the speaker panel included elimination of eye rubbing, cold compresses, avoidance of exacerbating factors, artificial tears, and timely referral to an ophthalmologist. Medications discussed were mast cell stabilizers, antihistamines, and corticosteroids and calcineurin inhibitors.
Final Thoughts
There has been an explosion of new research that has increased our understanding of all aspects of AD, and the standard of care is truly being revolutionized. Clinicians should stay tuned to a wealth of new evidence-based recommendations coming down the pike.
- Vakharia PP, Silverberg JI. Adult-onset atopic dermatitis: characteristics and management [published online May 28, 2019]. Am J Clin Dermatol. doi:10.1007/s40257-019-00453-7.
- Silverberg JI. Adult-onset atopic dermatitis. J Allergy Clin Immunol Pract. 2019;7:28-33.
- Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh). 1980;92(suppl):44-47.
- Hamann CR, Hamann D, Egeberg A, et al. Association between atopic dermatitis and contact sensitization: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:70-78.
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302.
- Rastogi S, Patel KR, Singam V, et al. Allergic contact dermatitis to personal care products and topical medications in adults with atopic dermatitis [published online July 25, 2018]. J Am Acad Dermatol. 2018;79:1028-1033.e6.
- Vakharia PP, Silverberg JI. New and emerging therapies for paediatric atopic dermatitis. Lancet Child Adolesc Health. 2019;3:343-353.
- Vakharia PP, Silverberg JI. New therapies for atopic dermatitis: additional treatment classes [published online December 14, 2017]. J Am Acad Dermatol. 2018;78(3 suppl 1):S76-S83.
- Silverberg JI. Atopic dermatitis treatment: current state of the art and emerging therapies. Allergy Asthma Proc. 2017;38:243-249.
- Vakharia PP, Silverberg JI. Monoclonal antibodies for atopic dermatitis: progress and potential. BioDrugs. 2017;31:409-422.
- Silverberg NB, Silverberg JI. Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis. 2015;96:359-361.
- Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123:144-151.
- Brunner PM, Silverberg JI, Guttman-Yassky E, et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol. 2017;137:18-25.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Silverberg JI. Selected comorbidities of atopic dermatitis: atopy, neuropsychiatric, and musculoskeletal disorders. Clin Dermatol. 2017;35:360-366.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Association of atopic dermatitis with allergic, autoimmune, and cardiovascular comorbidities in US adults. Ann Allergy Asthma Immunol. 2018;121:604-612.e603.
- Henderson J, Northstone K, Lee SP, et al. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol. 2008;121:872-877.e879.
- Hill DA, Grundmeier RW, Ramos M, et al. Eosinophilic esophagitis is a late manifestation of the allergic march. J Allergy Clin Immunol. 2018;6:1528-1533.
- Boyce JA, Assa’ad A, Burks AW, et al; NIAID-Sponsored Expert Panel. Guidelines for th
e diagnosis and management of food allergy in the United States. J Allergy Clin Immunol. 2010;126:1105-1118. - Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials [published online March 9, 2019]. Br J Dermatol. doi:10.1111/bjd.17869.
Impressive progress has been made in recent years in the management and treatment of atopic dermatitis (AD) and its comorbidities; however, there is a major need for state-of-the-art, evidence-based, multidisciplinary education for AD management. To address this need, the first Revolutionizing Atopic Dermatitis (RAD) Conference was held in April 2019 in Chicago, Illinois, featuring cutting-edge research presented by globally recognized experts in dermatology, allergy and immunology, sleep medicine, ophthalmology, and nursing care. The following is a recap of the latest topics in AD research presented at the conference.
Diagnosis and Assessment of AD: Jonathan I. Silverberg, MD, PhD, MPH
Although diagnosis of AD typically is straightforward in children, it can be challenging in adults, even for expert clinicians. These challenges stem from the different lesional distribution and morphology of AD in adults vs children.1,2 Additionally, the conditions included in the differential diagnosis of AD (eg, allergic contact dermatitis, cutaneous T-cell lymphoma, psoriasis) are far more common in adults than in children. Formal diagnostic criteria can be useful to improve the diagnosis of AD in clinical practice.3 It is important to note that flexural lesions and early disease onset are diagnostic criteria in AD; nevertheless, neither are essential nor sufficient on their own to make the diagnosis.
Patch Testing: Jacob P. Thyssen, MD, PhD, DmSci, and Noreen Heer Nicol, PhD, RN, FNP, NEA-BC
Patch testing can be used in AD patients to rule out contact dermatitis as an alternative or comorbid diagnosis.4-6 Because contact dermatitis can mimic AD, patch testing is recommended for all patients with adolescent and adult-onset AD.5 Additionally, refractory cases of AD across all ages, especially prior to initiation of systemic therapy, warrant patch testing. The unique challenges of patch testing in AD patients were reviewed.
Patient Panel
Atopic dermatitis can be a considerable disease burden on both patients and society in general. At the 2019 RAD Conference, a panel of patients bravely shared their AD journeys. Their eye-opening stories highlighted opportunities for improving real-world assessment and management of AD. Some key takeaways included the importance of adequately assessing the symptom burden of AD and not merely relying on visual inspection of the skin. The need for long-term treatment approaches beyond quick fixes with steroids also was discussed.
Pathogenesis of AD: Mark Boguniewicz, MD
There have been many advances in our understanding of the complex pathogenesis of AD,7-11 which is characterized by an altered skin barrier and immune dysregulation. Filaggrin deficiency in the skin has structural and biophysical consequences. A subset of patients with AD has filaggrin loss-of-function genetic polymorphisms inherited in an autosomal-semidominant pattern; however, many other genetic polymorphisms have been identified that affect different components of the skin architecture and immune system. Many cytokine pathways have been found to be upregulated in AD lesions, including IL-13, IL-4, IL-31, and IL-5 in acute and chronic lesions, and IFN-γ and other helper T cell (TH1) cytokines in chronic lesions. IL-4 and IL-13 (TH2 cytokines) have been shown to decrease epidermal expression of filaggrin and lead to lipid abnormalities in the skin of patients with AD. Even normal-appearing, nonlesional skin has substantial immune activation and barrier abnormalities in patients with moderate to severe AD. Activation of different immune pathways may contribute to the heterogeneous clinical presentation of AD. There also is an increasingly recognized role of superantigen-producing Staphylococcus aureus and decreased microbial diversity in AD.
Therapies for AD
The advances in our understanding of AD pathophysiology have led to the development of 2 recently approved therapeutic agents.7-10 Crisaborole ointment 2% is a topical phosphodiesterase 4 inhibitor that was approved by the US Food and Drug Administration in 2016 for treatment of mild to moderate AD. Treatment with crisaborole ointment 2% demonstrated improvement in lesion severity, itch, and quality of life in children and adults with AD. Dupilumab, an injectable biologic therapy that inhibits IL-4 and IL-13 signaling, was approved by the US Food and Drug Administration in 2017 for adults and in 2019 for adolescents aged 12 to 17 years with moderate to severe AD. The expert panel of speakers at the 2019 RAD Conference discussed many practical clinical pearls regarding patient education, optimization of both short- and long-term efficacy, and prevention and management of treatment-related adverse events. The discussion included evidence-based guidelines for bathing practices and topical therapy in AD, as well as practical pearls for patient and provider education in AD, reviewed by Dr. Nicol. Evidence-based guidelines for use of phototherapy and systemic and biologic therapy for AD also were highlighted by Dr. Silverberg.
After decades of limited therapeutic options, there is a large therapeutic pipeline of topical, oral, and biologic agents in development for the treatment AD.7-9 Dr. Boguniewicz reviewed the state-of-the-art treatments that are the furthest advanced in development. Many of these agents may be approved within the next couple of years and look promising in terms of their potential to improve the care of patients with AD.
Comorbidities of AD
The impact of AD is not just skin deep. Atopic dermatitis is associated with myriad comorbid health conditions.12-16 Dr. Boguniewicz reviewed the relationship between AD and atopic comorbidities, including asthma, hay fever, and food allergies, which are common across all AD patients. In addition, a subset of children with AD demonstrated the atopic march, in which AD first appears early in life followed by the development of other atopic comorbidities in later childhood or adulthood. In particular, children with filaggrin null mutations were found to be at increased risk of early-onset, severe, persistent AD with asthma and allergic sensitization.17 More recently, eosinophilic esophagitis was demonstrated to be a late-onset comorbidity of the atopic march.18 The allergy guidelines for which patients are appropriate candidates for food and/or aeroallergen testing were discussed,19 and it was emphasized that patients with AD should not routinely receive this testing.
Atopic dermatitis is associated with many other comorbidities, including sleep disturbances. Phyllis C. Zee, MD, PhD, provided a brilliant review of circadian regulation of physiology and the immune system. Sleep is one of the most important determinants of patients’ health and well-being. Atopic dermatitis is associated with disturbances of sleep and circadian rhythms. Sleep disturbances are gaining recognition as an important end point to assess for improvement in clinical practice and trials.
Patients with AD have long been recognized to have increased ophthalmic comorbidities, including allergic conjunctivitis, atopic keratoconjunctivitis, and cataracts. More recently, conjunctivitis has emerged as an important adverse event with dupilumab treatment.20 Jeanine Baqai, MD, reviewed the various ophthalmic comorbidities and shared numerous clinical signs of ophthalmic comorbidities that dermatologists can assess with the naked eye (no slit-lamp examination needed). Pearls to manage dupilumab-related conjunctivitis shared by Dr. Baqai and the speaker panel included elimination of eye rubbing, cold compresses, avoidance of exacerbating factors, artificial tears, and timely referral to an ophthalmologist. Medications discussed were mast cell stabilizers, antihistamines, and corticosteroids and calcineurin inhibitors.
Final Thoughts
There has been an explosion of new research that has increased our understanding of all aspects of AD, and the standard of care is truly being revolutionized. Clinicians should stay tuned to a wealth of new evidence-based recommendations coming down the pike.
Impressive progress has been made in recent years in the management and treatment of atopic dermatitis (AD) and its comorbidities; however, there is a major need for state-of-the-art, evidence-based, multidisciplinary education for AD management. To address this need, the first Revolutionizing Atopic Dermatitis (RAD) Conference was held in April 2019 in Chicago, Illinois, featuring cutting-edge research presented by globally recognized experts in dermatology, allergy and immunology, sleep medicine, ophthalmology, and nursing care. The following is a recap of the latest topics in AD research presented at the conference.
Diagnosis and Assessment of AD: Jonathan I. Silverberg, MD, PhD, MPH
Although diagnosis of AD typically is straightforward in children, it can be challenging in adults, even for expert clinicians. These challenges stem from the different lesional distribution and morphology of AD in adults vs children.1,2 Additionally, the conditions included in the differential diagnosis of AD (eg, allergic contact dermatitis, cutaneous T-cell lymphoma, psoriasis) are far more common in adults than in children. Formal diagnostic criteria can be useful to improve the diagnosis of AD in clinical practice.3 It is important to note that flexural lesions and early disease onset are diagnostic criteria in AD; nevertheless, neither are essential nor sufficient on their own to make the diagnosis.
Patch Testing: Jacob P. Thyssen, MD, PhD, DmSci, and Noreen Heer Nicol, PhD, RN, FNP, NEA-BC
Patch testing can be used in AD patients to rule out contact dermatitis as an alternative or comorbid diagnosis.4-6 Because contact dermatitis can mimic AD, patch testing is recommended for all patients with adolescent and adult-onset AD.5 Additionally, refractory cases of AD across all ages, especially prior to initiation of systemic therapy, warrant patch testing. The unique challenges of patch testing in AD patients were reviewed.
Patient Panel
Atopic dermatitis can be a considerable disease burden on both patients and society in general. At the 2019 RAD Conference, a panel of patients bravely shared their AD journeys. Their eye-opening stories highlighted opportunities for improving real-world assessment and management of AD. Some key takeaways included the importance of adequately assessing the symptom burden of AD and not merely relying on visual inspection of the skin. The need for long-term treatment approaches beyond quick fixes with steroids also was discussed.
Pathogenesis of AD: Mark Boguniewicz, MD
There have been many advances in our understanding of the complex pathogenesis of AD,7-11 which is characterized by an altered skin barrier and immune dysregulation. Filaggrin deficiency in the skin has structural and biophysical consequences. A subset of patients with AD has filaggrin loss-of-function genetic polymorphisms inherited in an autosomal-semidominant pattern; however, many other genetic polymorphisms have been identified that affect different components of the skin architecture and immune system. Many cytokine pathways have been found to be upregulated in AD lesions, including IL-13, IL-4, IL-31, and IL-5 in acute and chronic lesions, and IFN-γ and other helper T cell (TH1) cytokines in chronic lesions. IL-4 and IL-13 (TH2 cytokines) have been shown to decrease epidermal expression of filaggrin and lead to lipid abnormalities in the skin of patients with AD. Even normal-appearing, nonlesional skin has substantial immune activation and barrier abnormalities in patients with moderate to severe AD. Activation of different immune pathways may contribute to the heterogeneous clinical presentation of AD. There also is an increasingly recognized role of superantigen-producing Staphylococcus aureus and decreased microbial diversity in AD.
Therapies for AD
The advances in our understanding of AD pathophysiology have led to the development of 2 recently approved therapeutic agents.7-10 Crisaborole ointment 2% is a topical phosphodiesterase 4 inhibitor that was approved by the US Food and Drug Administration in 2016 for treatment of mild to moderate AD. Treatment with crisaborole ointment 2% demonstrated improvement in lesion severity, itch, and quality of life in children and adults with AD. Dupilumab, an injectable biologic therapy that inhibits IL-4 and IL-13 signaling, was approved by the US Food and Drug Administration in 2017 for adults and in 2019 for adolescents aged 12 to 17 years with moderate to severe AD. The expert panel of speakers at the 2019 RAD Conference discussed many practical clinical pearls regarding patient education, optimization of both short- and long-term efficacy, and prevention and management of treatment-related adverse events. The discussion included evidence-based guidelines for bathing practices and topical therapy in AD, as well as practical pearls for patient and provider education in AD, reviewed by Dr. Nicol. Evidence-based guidelines for use of phototherapy and systemic and biologic therapy for AD also were highlighted by Dr. Silverberg.
After decades of limited therapeutic options, there is a large therapeutic pipeline of topical, oral, and biologic agents in development for the treatment AD.7-9 Dr. Boguniewicz reviewed the state-of-the-art treatments that are the furthest advanced in development. Many of these agents may be approved within the next couple of years and look promising in terms of their potential to improve the care of patients with AD.
Comorbidities of AD
The impact of AD is not just skin deep. Atopic dermatitis is associated with myriad comorbid health conditions.12-16 Dr. Boguniewicz reviewed the relationship between AD and atopic comorbidities, including asthma, hay fever, and food allergies, which are common across all AD patients. In addition, a subset of children with AD demonstrated the atopic march, in which AD first appears early in life followed by the development of other atopic comorbidities in later childhood or adulthood. In particular, children with filaggrin null mutations were found to be at increased risk of early-onset, severe, persistent AD with asthma and allergic sensitization.17 More recently, eosinophilic esophagitis was demonstrated to be a late-onset comorbidity of the atopic march.18 The allergy guidelines for which patients are appropriate candidates for food and/or aeroallergen testing were discussed,19 and it was emphasized that patients with AD should not routinely receive this testing.
Atopic dermatitis is associated with many other comorbidities, including sleep disturbances. Phyllis C. Zee, MD, PhD, provided a brilliant review of circadian regulation of physiology and the immune system. Sleep is one of the most important determinants of patients’ health and well-being. Atopic dermatitis is associated with disturbances of sleep and circadian rhythms. Sleep disturbances are gaining recognition as an important end point to assess for improvement in clinical practice and trials.
Patients with AD have long been recognized to have increased ophthalmic comorbidities, including allergic conjunctivitis, atopic keratoconjunctivitis, and cataracts. More recently, conjunctivitis has emerged as an important adverse event with dupilumab treatment.20 Jeanine Baqai, MD, reviewed the various ophthalmic comorbidities and shared numerous clinical signs of ophthalmic comorbidities that dermatologists can assess with the naked eye (no slit-lamp examination needed). Pearls to manage dupilumab-related conjunctivitis shared by Dr. Baqai and the speaker panel included elimination of eye rubbing, cold compresses, avoidance of exacerbating factors, artificial tears, and timely referral to an ophthalmologist. Medications discussed were mast cell stabilizers, antihistamines, and corticosteroids and calcineurin inhibitors.
Final Thoughts
There has been an explosion of new research that has increased our understanding of all aspects of AD, and the standard of care is truly being revolutionized. Clinicians should stay tuned to a wealth of new evidence-based recommendations coming down the pike.
- Vakharia PP, Silverberg JI. Adult-onset atopic dermatitis: characteristics and management [published online May 28, 2019]. Am J Clin Dermatol. doi:10.1007/s40257-019-00453-7.
- Silverberg JI. Adult-onset atopic dermatitis. J Allergy Clin Immunol Pract. 2019;7:28-33.
- Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh). 1980;92(suppl):44-47.
- Hamann CR, Hamann D, Egeberg A, et al. Association between atopic dermatitis and contact sensitization: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:70-78.
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302.
- Rastogi S, Patel KR, Singam V, et al. Allergic contact dermatitis to personal care products and topical medications in adults with atopic dermatitis [published online July 25, 2018]. J Am Acad Dermatol. 2018;79:1028-1033.e6.
- Vakharia PP, Silverberg JI. New and emerging therapies for paediatric atopic dermatitis. Lancet Child Adolesc Health. 2019;3:343-353.
- Vakharia PP, Silverberg JI. New therapies for atopic dermatitis: additional treatment classes [published online December 14, 2017]. J Am Acad Dermatol. 2018;78(3 suppl 1):S76-S83.
- Silverberg JI. Atopic dermatitis treatment: current state of the art and emerging therapies. Allergy Asthma Proc. 2017;38:243-249.
- Vakharia PP, Silverberg JI. Monoclonal antibodies for atopic dermatitis: progress and potential. BioDrugs. 2017;31:409-422.
- Silverberg NB, Silverberg JI. Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis. 2015;96:359-361.
- Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123:144-151.
- Brunner PM, Silverberg JI, Guttman-Yassky E, et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol. 2017;137:18-25.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Silverberg JI. Selected comorbidities of atopic dermatitis: atopy, neuropsychiatric, and musculoskeletal disorders. Clin Dermatol. 2017;35:360-366.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Association of atopic dermatitis with allergic, autoimmune, and cardiovascular comorbidities in US adults. Ann Allergy Asthma Immunol. 2018;121:604-612.e603.
- Henderson J, Northstone K, Lee SP, et al. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol. 2008;121:872-877.e879.
- Hill DA, Grundmeier RW, Ramos M, et al. Eosinophilic esophagitis is a late manifestation of the allergic march. J Allergy Clin Immunol. 2018;6:1528-1533.
- Boyce JA, Assa’ad A, Burks AW, et al; NIAID-Sponsored Expert Panel. Guidelines for th
e diagnosis and management of food allergy in the United States. J Allergy Clin Immunol. 2010;126:1105-1118. - Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials [published online March 9, 2019]. Br J Dermatol. doi:10.1111/bjd.17869.
- Vakharia PP, Silverberg JI. Adult-onset atopic dermatitis: characteristics and management [published online May 28, 2019]. Am J Clin Dermatol. doi:10.1007/s40257-019-00453-7.
- Silverberg JI. Adult-onset atopic dermatitis. J Allergy Clin Immunol Pract. 2019;7:28-33.
- Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh). 1980;92(suppl):44-47.
- Hamann CR, Hamann D, Egeberg A, et al. Association between atopic dermatitis and contact sensitization: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:70-78.
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302.
- Rastogi S, Patel KR, Singam V, et al. Allergic contact dermatitis to personal care products and topical medications in adults with atopic dermatitis [published online July 25, 2018]. J Am Acad Dermatol. 2018;79:1028-1033.e6.
- Vakharia PP, Silverberg JI. New and emerging therapies for paediatric atopic dermatitis. Lancet Child Adolesc Health. 2019;3:343-353.
- Vakharia PP, Silverberg JI. New therapies for atopic dermatitis: additional treatment classes [published online December 14, 2017]. J Am Acad Dermatol. 2018;78(3 suppl 1):S76-S83.
- Silverberg JI. Atopic dermatitis treatment: current state of the art and emerging therapies. Allergy Asthma Proc. 2017;38:243-249.
- Vakharia PP, Silverberg JI. Monoclonal antibodies for atopic dermatitis: progress and potential. BioDrugs. 2017;31:409-422.
- Silverberg NB, Silverberg JI. Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis. 2015;96:359-361.
- Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123:144-151.
- Brunner PM, Silverberg JI, Guttman-Yassky E, et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol. 2017;137:18-25.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Silverberg JI. Selected comorbidities of atopic dermatitis: atopy, neuropsychiatric, and musculoskeletal disorders. Clin Dermatol. 2017;35:360-366.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Association of atopic dermatitis with allergic, autoimmune, and cardiovascular comorbidities in US adults. Ann Allergy Asthma Immunol. 2018;121:604-612.e603.
- Henderson J, Northstone K, Lee SP, et al. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol. 2008;121:872-877.e879.
- Hill DA, Grundmeier RW, Ramos M, et al. Eosinophilic esophagitis is a late manifestation of the allergic march. J Allergy Clin Immunol. 2018;6:1528-1533.
- Boyce JA, Assa’ad A, Burks AW, et al; NIAID-Sponsored Expert Panel. Guidelines for th
e diagnosis and management of food allergy in the United States. J Allergy Clin Immunol. 2010;126:1105-1118. - Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials [published online March 9, 2019]. Br J Dermatol. doi:10.1111/bjd.17869.
Cephalosporins remain empiric therapy for skin infections in pediatric AD
“Clindamycin, tetracyclines, or TMP‐SMX can be considered in patients suspected to have, or with a history of, MRSA [methicillin‐resistant S. aureus] infection,” wrote Cristopher C. Briscoe, MD, of the Washington University School of Medicine in St. Louis, Missouri, and his coauthors. The study was published in Pediatric Dermatology.
To determine the optimal empiric antibiotic for pediatric AD patients with skin infections, the researchers analyzed skin cultures from 106 patients seen at Saint Louis Children’s Hospital (SLCH). The results were also compared to cultures from pediatric patients who presented at the SLCH emergency department (ED) with S. aureus skin abscesses.
Of the 170 cultures that grew S. aureus, 130 (77.8%) grew MSSA, and 37 (22.2%) grew MRSA. Three of the cultures grew both. The prevalence of MRSA in the cohort differed from the prevalence in the ED patients (44%). The prevalence of either infection did not differ significantly by age, sex or race, though the average number of cultures in African American patients topped the average for Caucasian patients (1.8 vs. 1.2, P less than .003).
All patients with MSSA – in both the cohort and the ED – proved 100% susceptible to cefazolin. Cohort patients with MSSA saw lower susceptibility to doxycycline compared to the ED patients (89.4% vs. 97%), as did MRSA cohort patients to trimethoprim‐sulfamethoxazole (92% vs. 98%).
“When a patient with AD walks into your office and looks like they have an infection of their eczema, your go-to antibiotic is going to be one that targets MSSA,” said coauthor Carrie Coughlin, MD, of the Washington University School of Medicine in an interview. “You’ll still do a culture to prove or disprove that assumption, but it gives you a guide to help make that patient better in the short term while you work things up.”
“Also, remember that MSSA is not ‘better’ to have than MRSA,” she added. “You can now see some of the virulence factors from MRSA strains in MSSA strains, so treating both of them is important.”
The authors acknowledged their study’s limitations, including the limited generalizability of a single-center design and a lack of information as to the body sites from which the cultures were obtained. They were also unable to reliably determine prior antibiotic exposure, noting that “future work could examine whether prior exposure differed significantly in the MRSA and MSSA groups.”
The study was funded by grants from the Agency for Healthcare Research and Quality. The authors reported no conflicts of interest.
SOURCE: Briscoe CC et al. Pediatr Dermatol. 2019 May 24. doi: 10.1111/pde.13867.
“Clindamycin, tetracyclines, or TMP‐SMX can be considered in patients suspected to have, or with a history of, MRSA [methicillin‐resistant S. aureus] infection,” wrote Cristopher C. Briscoe, MD, of the Washington University School of Medicine in St. Louis, Missouri, and his coauthors. The study was published in Pediatric Dermatology.
To determine the optimal empiric antibiotic for pediatric AD patients with skin infections, the researchers analyzed skin cultures from 106 patients seen at Saint Louis Children’s Hospital (SLCH). The results were also compared to cultures from pediatric patients who presented at the SLCH emergency department (ED) with S. aureus skin abscesses.
Of the 170 cultures that grew S. aureus, 130 (77.8%) grew MSSA, and 37 (22.2%) grew MRSA. Three of the cultures grew both. The prevalence of MRSA in the cohort differed from the prevalence in the ED patients (44%). The prevalence of either infection did not differ significantly by age, sex or race, though the average number of cultures in African American patients topped the average for Caucasian patients (1.8 vs. 1.2, P less than .003).
All patients with MSSA – in both the cohort and the ED – proved 100% susceptible to cefazolin. Cohort patients with MSSA saw lower susceptibility to doxycycline compared to the ED patients (89.4% vs. 97%), as did MRSA cohort patients to trimethoprim‐sulfamethoxazole (92% vs. 98%).
“When a patient with AD walks into your office and looks like they have an infection of their eczema, your go-to antibiotic is going to be one that targets MSSA,” said coauthor Carrie Coughlin, MD, of the Washington University School of Medicine in an interview. “You’ll still do a culture to prove or disprove that assumption, but it gives you a guide to help make that patient better in the short term while you work things up.”
“Also, remember that MSSA is not ‘better’ to have than MRSA,” she added. “You can now see some of the virulence factors from MRSA strains in MSSA strains, so treating both of them is important.”
The authors acknowledged their study’s limitations, including the limited generalizability of a single-center design and a lack of information as to the body sites from which the cultures were obtained. They were also unable to reliably determine prior antibiotic exposure, noting that “future work could examine whether prior exposure differed significantly in the MRSA and MSSA groups.”
The study was funded by grants from the Agency for Healthcare Research and Quality. The authors reported no conflicts of interest.
SOURCE: Briscoe CC et al. Pediatr Dermatol. 2019 May 24. doi: 10.1111/pde.13867.
“Clindamycin, tetracyclines, or TMP‐SMX can be considered in patients suspected to have, or with a history of, MRSA [methicillin‐resistant S. aureus] infection,” wrote Cristopher C. Briscoe, MD, of the Washington University School of Medicine in St. Louis, Missouri, and his coauthors. The study was published in Pediatric Dermatology.
To determine the optimal empiric antibiotic for pediatric AD patients with skin infections, the researchers analyzed skin cultures from 106 patients seen at Saint Louis Children’s Hospital (SLCH). The results were also compared to cultures from pediatric patients who presented at the SLCH emergency department (ED) with S. aureus skin abscesses.
Of the 170 cultures that grew S. aureus, 130 (77.8%) grew MSSA, and 37 (22.2%) grew MRSA. Three of the cultures grew both. The prevalence of MRSA in the cohort differed from the prevalence in the ED patients (44%). The prevalence of either infection did not differ significantly by age, sex or race, though the average number of cultures in African American patients topped the average for Caucasian patients (1.8 vs. 1.2, P less than .003).
All patients with MSSA – in both the cohort and the ED – proved 100% susceptible to cefazolin. Cohort patients with MSSA saw lower susceptibility to doxycycline compared to the ED patients (89.4% vs. 97%), as did MRSA cohort patients to trimethoprim‐sulfamethoxazole (92% vs. 98%).
“When a patient with AD walks into your office and looks like they have an infection of their eczema, your go-to antibiotic is going to be one that targets MSSA,” said coauthor Carrie Coughlin, MD, of the Washington University School of Medicine in an interview. “You’ll still do a culture to prove or disprove that assumption, but it gives you a guide to help make that patient better in the short term while you work things up.”
“Also, remember that MSSA is not ‘better’ to have than MRSA,” she added. “You can now see some of the virulence factors from MRSA strains in MSSA strains, so treating both of them is important.”
The authors acknowledged their study’s limitations, including the limited generalizability of a single-center design and a lack of information as to the body sites from which the cultures were obtained. They were also unable to reliably determine prior antibiotic exposure, noting that “future work could examine whether prior exposure differed significantly in the MRSA and MSSA groups.”
The study was funded by grants from the Agency for Healthcare Research and Quality. The authors reported no conflicts of interest.
SOURCE: Briscoe CC et al. Pediatr Dermatol. 2019 May 24. doi: 10.1111/pde.13867.
FROM PEDIATRIC DERMATOLOGY