User login
Breast cancer: 10-year treatment extension with aromatase inhibitors yields no benefit
Key clinical point: A 10-year vs 7-year treatment with aromatase inhibitors (anastrozole) in patients with hormone receptor (HR)-positive breast cancer does not yield survival benefit but increases the risk for bone fracture.
Major finding: Anastrozole treatment for 10 years vs. 7 years was not associated with a significant difference in disease-free survival (P = 0.90). The risk of clinical bone fracture was higher with 10-year treatment (hazard ratio, 1.35; 95% confidence interval, 1.00-1.84).
Study details: The phase 3 Secondary Adjuvant Long Term Study With Arimidex (SALSA) trial studied 3,484 postmenopausal women with HR-positive breast cancer who had received anastrozole for 5 years and were randomly assigned to therapy extension by 2 years (for a total of 7 years) or 5 years (for a total of 10 years).
Disclosures: This study was supported by AstraZeneca and the Austrian Breast and Colorectal Cancer Study Group. The authors received grants, honoraria, personal/lecture/advisory/consulting/speaker fees, and/or travel/accommodation/expenses outside this work.
Source: Gnant M et al. New Engl J Med. 2021;385:395-405. doi: 10.1056/NEJMoa2104162.
Key clinical point: A 10-year vs 7-year treatment with aromatase inhibitors (anastrozole) in patients with hormone receptor (HR)-positive breast cancer does not yield survival benefit but increases the risk for bone fracture.
Major finding: Anastrozole treatment for 10 years vs. 7 years was not associated with a significant difference in disease-free survival (P = 0.90). The risk of clinical bone fracture was higher with 10-year treatment (hazard ratio, 1.35; 95% confidence interval, 1.00-1.84).
Study details: The phase 3 Secondary Adjuvant Long Term Study With Arimidex (SALSA) trial studied 3,484 postmenopausal women with HR-positive breast cancer who had received anastrozole for 5 years and were randomly assigned to therapy extension by 2 years (for a total of 7 years) or 5 years (for a total of 10 years).
Disclosures: This study was supported by AstraZeneca and the Austrian Breast and Colorectal Cancer Study Group. The authors received grants, honoraria, personal/lecture/advisory/consulting/speaker fees, and/or travel/accommodation/expenses outside this work.
Source: Gnant M et al. New Engl J Med. 2021;385:395-405. doi: 10.1056/NEJMoa2104162.
Key clinical point: A 10-year vs 7-year treatment with aromatase inhibitors (anastrozole) in patients with hormone receptor (HR)-positive breast cancer does not yield survival benefit but increases the risk for bone fracture.
Major finding: Anastrozole treatment for 10 years vs. 7 years was not associated with a significant difference in disease-free survival (P = 0.90). The risk of clinical bone fracture was higher with 10-year treatment (hazard ratio, 1.35; 95% confidence interval, 1.00-1.84).
Study details: The phase 3 Secondary Adjuvant Long Term Study With Arimidex (SALSA) trial studied 3,484 postmenopausal women with HR-positive breast cancer who had received anastrozole for 5 years and were randomly assigned to therapy extension by 2 years (for a total of 7 years) or 5 years (for a total of 10 years).
Disclosures: This study was supported by AstraZeneca and the Austrian Breast and Colorectal Cancer Study Group. The authors received grants, honoraria, personal/lecture/advisory/consulting/speaker fees, and/or travel/accommodation/expenses outside this work.
Source: Gnant M et al. New Engl J Med. 2021;385:395-405. doi: 10.1056/NEJMoa2104162.
Pandemic-related drops in breast cancer screening hit hardest among medically underserved
Breast cancer screening rates at community health centers (CHCs) in the United States declined during the pandemic, particularly among Black and uninsured individuals, based on a retrospective look at 32 sites.
Still, drops in screening were less dramatic than national declines previously reported, possibly because of the American Cancer Society–directed CHANGE program, which was simultaneously underway at the CHCs involved, reported lead author Stacey A. Fedewa, PhD, senior principal scientist at the ACS in Atlanta, and colleagues.
“This is one of the first studies to examine breast cancer screening rates during the pandemic specifically among clinics providing care to communities of color and lower income populations, a group with lower utilization of and greater barriers to [breast cancer] screening,” the investigators wrote in Cancer. “This is important because these populations have longstanding barriers to accessing care, lower breast screening rates, higher breast cancer mortality rates, and are especially vulnerable to health care disruptions.”
According to a previous analysis of electronic health records by Mast and Munoz del Rio, breast cancer screening rates in the United States dropped 94% in March/April 2020, when the COVID-19 pandemic was declared a national emergency. Although a recent follow-up report showed a rebound in breast cancer screening, the estimated rate remains 13% below average.
The present study evaluated data from 32 out of 1,385 CHCs in the United States. All centers were involved in the ACS-run CHANGE grant program, which funded the clinics for 2 years, during which time they implemented at least three evidence-based provider and client interventions, such as patient navigation or electronic medical record enhancements. The clinics reported breast cancer screening rates on a routine basis throughout the 2-year period, beginning August 2018.
Breast cancer screening rate was defined as the percentage of women aged 50-74 years who had a screening mammogram within the past 27 months, out of a total pool of women who had a medical visit within the past year. For 2018, 2019, and 2020, respectively, 142,207; 142,003; and 150,630 women had a medical visit. Screening rates were compared across years in either June or July. Findings were further characterized by demographic characteristics, urban/rural status, and clinic region.
From 2018 to 2019 breast cancer screening rates rose 18%, from 45.8% to 53.9%. This increase was followed by an 8% decline during the 2019-2020 period, from 53.9% to 49.6%.
The investigators estimated the number of missed mammograms and breast cancer diagnoses for two comparative, hypothetical scenarios: first, if the rising trend from 2018 to 2019 had continued through 2020, and second, if the rate had plateaued at 53.9%.
The rising trend model suggested that 47,517 fewer mammograms than normal were conducted during 2019-2020, resulting in 242 missed breast cancer diagnoses, of which 166 were invasive and 76 were ductal carcinoma in situ. The plateau model suggested that 6,477 fewer mammograms were conducted, leading to 33 missed diagnoses.
Compared with the 8% decline in screening overall, the rate among Black patients dropped 12%, while rates at clinics with a lower proportion of uninsured patients dropped an average of 15%. In contrast, clinics in the South did not have a significant reduction in screening, “possibly reflecting lower baseline rates or impact of stay-at-home orders,” the investigators wrote.
Dr. Fedewa and colleagues also noted that their findings were less dramatic than those reported by Mast and Munoz del Rio. They suggested that the CHANGE program may have softened the blow dealt by the pandemic.
“The CHANGE program–funded interventions – that were established before and continued through 2020 – may have mitigated the pandemic’s effects on breast cancer screening services among the 32 CHCs that were studied,” they wrote. “Further investigation of breast cancer screening rates among additional CHCs will further inform where targeted interventions (e.g., client reminders, education on return to screening) are most needed.”
According to Madeline Sutton, MD, assistant professor of obstetrics and gynecology at Morehouse School of Medicine, Atlanta, “Progress seen with the CHANGE program should be duplicated in other clinical venues based on improvements seen in numbers of mammograms and breast cancers detected.”
Still, Dr. Sutton noted that the racial/ethnic disparities remain cause for concern.
“This study has implications for persons served at CHCs, especially if breast cancer racial/ethnic disparities are unintentionally widened during this pandemic,” Dr. Sutton said in a written comment. “Policy-level changes that decrease BCSR [breast cancer screen rate] gaps for women are warranted.”
Ana Velázquez Mañana, MD, a medical oncology fellow at the University of California, San Francisco, suggested that the effects of the pandemic may have been even more pronounced among medically underserved patients in whom interventions to increase screening were not being conducted, as they were through the CHANGE program.
“One must wonder to what degree these interventions reduced the decline in screening mammography rates observed during the pandemic and to what degree could disparities in screening be magnified in community health centers with less resources,” Dr. Velázquez said in a written comment. “Therefore, understanding barriers to breast cancer screening among our specific health care systems is key to guide resource allocation and the development of evidence-based multilevel interventions that can address these barriers, and ultimately increase screening rates.”
Dr. Velázquez also noted that the study by Dr. Fedewa and colleagues may have missed drops in screening among vulnerable populations that occurred later in the pandemic and in geographic hotspots. In a recent JAMA Network Open study, Dr. Velázquez reported a 41% drop in breast cancer screening at a safety-net hospital in San Francisco during the first stay-at-home order, which lasted from Feb. 1, 2020 to May 31, 2020.
The Breast Health Equity CHANGE grant was funded by the National Football League in partnership with the American Cancer Society. The investigators reported employment by the American Cancer Society. Dr. Wehling and Dr. Wysocki disclosed grants from Pfizer unrelated to this research. Dr. Sutton and Dr. Velázquez disclosed no conflicts of interest.
Breast cancer screening rates at community health centers (CHCs) in the United States declined during the pandemic, particularly among Black and uninsured individuals, based on a retrospective look at 32 sites.
Still, drops in screening were less dramatic than national declines previously reported, possibly because of the American Cancer Society–directed CHANGE program, which was simultaneously underway at the CHCs involved, reported lead author Stacey A. Fedewa, PhD, senior principal scientist at the ACS in Atlanta, and colleagues.
“This is one of the first studies to examine breast cancer screening rates during the pandemic specifically among clinics providing care to communities of color and lower income populations, a group with lower utilization of and greater barriers to [breast cancer] screening,” the investigators wrote in Cancer. “This is important because these populations have longstanding barriers to accessing care, lower breast screening rates, higher breast cancer mortality rates, and are especially vulnerable to health care disruptions.”
According to a previous analysis of electronic health records by Mast and Munoz del Rio, breast cancer screening rates in the United States dropped 94% in March/April 2020, when the COVID-19 pandemic was declared a national emergency. Although a recent follow-up report showed a rebound in breast cancer screening, the estimated rate remains 13% below average.
The present study evaluated data from 32 out of 1,385 CHCs in the United States. All centers were involved in the ACS-run CHANGE grant program, which funded the clinics for 2 years, during which time they implemented at least three evidence-based provider and client interventions, such as patient navigation or electronic medical record enhancements. The clinics reported breast cancer screening rates on a routine basis throughout the 2-year period, beginning August 2018.
Breast cancer screening rate was defined as the percentage of women aged 50-74 years who had a screening mammogram within the past 27 months, out of a total pool of women who had a medical visit within the past year. For 2018, 2019, and 2020, respectively, 142,207; 142,003; and 150,630 women had a medical visit. Screening rates were compared across years in either June or July. Findings were further characterized by demographic characteristics, urban/rural status, and clinic region.
From 2018 to 2019 breast cancer screening rates rose 18%, from 45.8% to 53.9%. This increase was followed by an 8% decline during the 2019-2020 period, from 53.9% to 49.6%.
The investigators estimated the number of missed mammograms and breast cancer diagnoses for two comparative, hypothetical scenarios: first, if the rising trend from 2018 to 2019 had continued through 2020, and second, if the rate had plateaued at 53.9%.
The rising trend model suggested that 47,517 fewer mammograms than normal were conducted during 2019-2020, resulting in 242 missed breast cancer diagnoses, of which 166 were invasive and 76 were ductal carcinoma in situ. The plateau model suggested that 6,477 fewer mammograms were conducted, leading to 33 missed diagnoses.
Compared with the 8% decline in screening overall, the rate among Black patients dropped 12%, while rates at clinics with a lower proportion of uninsured patients dropped an average of 15%. In contrast, clinics in the South did not have a significant reduction in screening, “possibly reflecting lower baseline rates or impact of stay-at-home orders,” the investigators wrote.
Dr. Fedewa and colleagues also noted that their findings were less dramatic than those reported by Mast and Munoz del Rio. They suggested that the CHANGE program may have softened the blow dealt by the pandemic.
“The CHANGE program–funded interventions – that were established before and continued through 2020 – may have mitigated the pandemic’s effects on breast cancer screening services among the 32 CHCs that were studied,” they wrote. “Further investigation of breast cancer screening rates among additional CHCs will further inform where targeted interventions (e.g., client reminders, education on return to screening) are most needed.”
According to Madeline Sutton, MD, assistant professor of obstetrics and gynecology at Morehouse School of Medicine, Atlanta, “Progress seen with the CHANGE program should be duplicated in other clinical venues based on improvements seen in numbers of mammograms and breast cancers detected.”
Still, Dr. Sutton noted that the racial/ethnic disparities remain cause for concern.
“This study has implications for persons served at CHCs, especially if breast cancer racial/ethnic disparities are unintentionally widened during this pandemic,” Dr. Sutton said in a written comment. “Policy-level changes that decrease BCSR [breast cancer screen rate] gaps for women are warranted.”
Ana Velázquez Mañana, MD, a medical oncology fellow at the University of California, San Francisco, suggested that the effects of the pandemic may have been even more pronounced among medically underserved patients in whom interventions to increase screening were not being conducted, as they were through the CHANGE program.
“One must wonder to what degree these interventions reduced the decline in screening mammography rates observed during the pandemic and to what degree could disparities in screening be magnified in community health centers with less resources,” Dr. Velázquez said in a written comment. “Therefore, understanding barriers to breast cancer screening among our specific health care systems is key to guide resource allocation and the development of evidence-based multilevel interventions that can address these barriers, and ultimately increase screening rates.”
Dr. Velázquez also noted that the study by Dr. Fedewa and colleagues may have missed drops in screening among vulnerable populations that occurred later in the pandemic and in geographic hotspots. In a recent JAMA Network Open study, Dr. Velázquez reported a 41% drop in breast cancer screening at a safety-net hospital in San Francisco during the first stay-at-home order, which lasted from Feb. 1, 2020 to May 31, 2020.
The Breast Health Equity CHANGE grant was funded by the National Football League in partnership with the American Cancer Society. The investigators reported employment by the American Cancer Society. Dr. Wehling and Dr. Wysocki disclosed grants from Pfizer unrelated to this research. Dr. Sutton and Dr. Velázquez disclosed no conflicts of interest.
Breast cancer screening rates at community health centers (CHCs) in the United States declined during the pandemic, particularly among Black and uninsured individuals, based on a retrospective look at 32 sites.
Still, drops in screening were less dramatic than national declines previously reported, possibly because of the American Cancer Society–directed CHANGE program, which was simultaneously underway at the CHCs involved, reported lead author Stacey A. Fedewa, PhD, senior principal scientist at the ACS in Atlanta, and colleagues.
“This is one of the first studies to examine breast cancer screening rates during the pandemic specifically among clinics providing care to communities of color and lower income populations, a group with lower utilization of and greater barriers to [breast cancer] screening,” the investigators wrote in Cancer. “This is important because these populations have longstanding barriers to accessing care, lower breast screening rates, higher breast cancer mortality rates, and are especially vulnerable to health care disruptions.”
According to a previous analysis of electronic health records by Mast and Munoz del Rio, breast cancer screening rates in the United States dropped 94% in March/April 2020, when the COVID-19 pandemic was declared a national emergency. Although a recent follow-up report showed a rebound in breast cancer screening, the estimated rate remains 13% below average.
The present study evaluated data from 32 out of 1,385 CHCs in the United States. All centers were involved in the ACS-run CHANGE grant program, which funded the clinics for 2 years, during which time they implemented at least three evidence-based provider and client interventions, such as patient navigation or electronic medical record enhancements. The clinics reported breast cancer screening rates on a routine basis throughout the 2-year period, beginning August 2018.
Breast cancer screening rate was defined as the percentage of women aged 50-74 years who had a screening mammogram within the past 27 months, out of a total pool of women who had a medical visit within the past year. For 2018, 2019, and 2020, respectively, 142,207; 142,003; and 150,630 women had a medical visit. Screening rates were compared across years in either June or July. Findings were further characterized by demographic characteristics, urban/rural status, and clinic region.
From 2018 to 2019 breast cancer screening rates rose 18%, from 45.8% to 53.9%. This increase was followed by an 8% decline during the 2019-2020 period, from 53.9% to 49.6%.
The investigators estimated the number of missed mammograms and breast cancer diagnoses for two comparative, hypothetical scenarios: first, if the rising trend from 2018 to 2019 had continued through 2020, and second, if the rate had plateaued at 53.9%.
The rising trend model suggested that 47,517 fewer mammograms than normal were conducted during 2019-2020, resulting in 242 missed breast cancer diagnoses, of which 166 were invasive and 76 were ductal carcinoma in situ. The plateau model suggested that 6,477 fewer mammograms were conducted, leading to 33 missed diagnoses.
Compared with the 8% decline in screening overall, the rate among Black patients dropped 12%, while rates at clinics with a lower proportion of uninsured patients dropped an average of 15%. In contrast, clinics in the South did not have a significant reduction in screening, “possibly reflecting lower baseline rates or impact of stay-at-home orders,” the investigators wrote.
Dr. Fedewa and colleagues also noted that their findings were less dramatic than those reported by Mast and Munoz del Rio. They suggested that the CHANGE program may have softened the blow dealt by the pandemic.
“The CHANGE program–funded interventions – that were established before and continued through 2020 – may have mitigated the pandemic’s effects on breast cancer screening services among the 32 CHCs that were studied,” they wrote. “Further investigation of breast cancer screening rates among additional CHCs will further inform where targeted interventions (e.g., client reminders, education on return to screening) are most needed.”
According to Madeline Sutton, MD, assistant professor of obstetrics and gynecology at Morehouse School of Medicine, Atlanta, “Progress seen with the CHANGE program should be duplicated in other clinical venues based on improvements seen in numbers of mammograms and breast cancers detected.”
Still, Dr. Sutton noted that the racial/ethnic disparities remain cause for concern.
“This study has implications for persons served at CHCs, especially if breast cancer racial/ethnic disparities are unintentionally widened during this pandemic,” Dr. Sutton said in a written comment. “Policy-level changes that decrease BCSR [breast cancer screen rate] gaps for women are warranted.”
Ana Velázquez Mañana, MD, a medical oncology fellow at the University of California, San Francisco, suggested that the effects of the pandemic may have been even more pronounced among medically underserved patients in whom interventions to increase screening were not being conducted, as they were through the CHANGE program.
“One must wonder to what degree these interventions reduced the decline in screening mammography rates observed during the pandemic and to what degree could disparities in screening be magnified in community health centers with less resources,” Dr. Velázquez said in a written comment. “Therefore, understanding barriers to breast cancer screening among our specific health care systems is key to guide resource allocation and the development of evidence-based multilevel interventions that can address these barriers, and ultimately increase screening rates.”
Dr. Velázquez also noted that the study by Dr. Fedewa and colleagues may have missed drops in screening among vulnerable populations that occurred later in the pandemic and in geographic hotspots. In a recent JAMA Network Open study, Dr. Velázquez reported a 41% drop in breast cancer screening at a safety-net hospital in San Francisco during the first stay-at-home order, which lasted from Feb. 1, 2020 to May 31, 2020.
The Breast Health Equity CHANGE grant was funded by the National Football League in partnership with the American Cancer Society. The investigators reported employment by the American Cancer Society. Dr. Wehling and Dr. Wysocki disclosed grants from Pfizer unrelated to this research. Dr. Sutton and Dr. Velázquez disclosed no conflicts of interest.
FROM CANCER
Gender-affirming mastectomy and breast cancer screening in transmasculine patients
Since the reversal of the Medicare exclusion in 2014, the rates of gender-affirming surgery have increased markedly in the United States.1 Gender-affirming mastectomy, otherwise known as “top surgery,” is one of the more commonly performed procedures; with 97% of patients having either undergone or expressed desire for the surgery.2 The goals of this procedure are to remove all visible breast tissue and reconstruct the chest wall so it is more masculine in appearance. For transmasculine and nonbinary patients, this procedure is associated with significant improvements in mental health and quality of life.3,4 While the mastectomy procedure is often performed by plastic surgeons, patients will see an ob.gyn. in the preoperative or postoperative period. Ob.gyns. should have a general understanding of the procedure, but most importantly know how to screen for breast cancer in patients who have undergone a gender-affirming mastectomy.
Providers will likely encounter transmasculine or nonbinary patients during annual screening examinations or for a preoperative exam. If a patient is seeking a preoperative risk assessment prior to undergoing a gender-affirming mastectomy, assessing a patient’s risk status for breast cancer is paramount. While testosterone therapy is no longer a prerequisite for gender-affirming mastectomies, documenting hormone use, age at initiation, and dosage is important.5 The overall effects of testosterone on breast tissue are inconsistent. However, studies have demonstrated that patients taking testosterone are not at an increased risk of breast cancer secondary to testosterone use.5-7 Patients should be asked about a personal of family history of breast cancer, breast surgery, history of prior breast biopsies, parity, age at menarche, smoking status, and breastfeeding history if applicable. Patients with high-risk mutations or a strong family history of breast cancer should be referred to genetic counselors, surgical oncologists, and possibly undergo genetic testing.8 Before an examination, providers should counsel patients about the nature of the examination and use gender-neutral language such as “chest” to avoid exacerbating gender dysphoria.
It is important to educate transmasculine patients about their risk for the development of breast cancer after mastectomy. Larger-scale, population-based studies of breast cancer in the transgender population have reported an incidence of 5.9 per 100,000 patients-years and an overall incidence comparable to cisgender men in age-standardized national samples.5-7 Unfortunately, data on the rates of breast cancer in transmasculine patients after gender-affirming mastectomy are limited, which makes defining postoperative guidelines challenging. Additionally, the amount of residual breast tissue remaining varies based on the surgeon and technique.
Several techniques are described for mastectomy procedures with differences that can affect the amount of residual breast tissue. The most common type of gender-affirming mastectomy is the double incision. With this procedure, the nipple-areolar complex is reduced in size, removed, and thinned to improve graft take. Dissection is then carried to the level of the breast capsule and the breast tissue and axillary tail are removed en bloc.5 During the dissection, the subcutaneous fat is left on the skin flap to provide appropriate contour and to avoid creating a concave-appearing chest wall. Prior to closure, the superior and inferior flaps are inspected for any visible residual breast tissue, which is removed if needed. In a circumareolar mastectomy, the nipple-areolar complex is also reduced but is preserved on a 1- to 1.5-cm-thick pedicle to maintain perfusion.5 The mastectomy is performed through an inferior periareolar incision and all visible breast tissue and the axillary tail are removed. Breast tissue specimens are sent for pathologic evaluation at the end of the procedure.
Following gender-affirming mastectomy, there is limited evidence to guide screening. During the patient visit, the provider should obtain a thorough history regarding mastectomy type, and if unknown, attempt to acquire the operative report detailing the procedure. For low-risk patients who undergo a subcutaneous mastectomy such as the double incision or circumareolar technique, screening mammography is not indicated nor is it technically feasible.9 For patients with a high-risk genetic mutation or a strong family history of breast cancer, monitoring with alternative modalities such as breast ultrasound or breast MRI may be beneficial, although there is no evidence to currently support this suggestion. Given the variety of surgical techniques of breast tissue removal, it is difficult to develop strong evidence-based guidelines. Annual chest wall examinations have been suggested as a screening modality; however, the clinical utility of clinical breast and chest exams has been debated and is no longer recommended as a screening method in cisgender patients.9 Clinicians can promote chest self-awareness and discuss the possibility of breast cancer in postmastectomy patients at annual examination visits. As research continues to resolve some of these unknowns, it is important that patients are informed of these areas of ambiguity and updated regarding any changes in screening recommendations.10
Dr. Brandt is an ob.gyn. and fellowship-trained gender affirming surgeon in West Reading, Pa.
References
1. American Society of Plastic Surgeons. 2018 plastic surgery statistics report. https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic-surgery-statistics-full-report-2018.pdf. Accessed Aug. 20, 2021.
2. James SE et al. The report of the 2015 U.S. Transgender survey. Washington, D.C.: National Center for Transgender Equality, 2016.
3. Agarwal CA et al. J Plast Reconstr Aesthet Surg. 2018;71:651-7.
4. Poudrier G et al. Plast Reconstr Surg. 2018;80:679-83.
5. Salibian AA et al. Plast Reconstr Surg. 2020;147:213e-21e.
6. Gooren LJ et al. J Sex Med. 2013;10:3129-34.
7. Brown GR and Jones KT. Breast Cancer Res Treat. 2015;149:191-8.
8. Deutsch MF et al. Semin Reprod Med. 2017;35:434-41.
9. Phillips J et al. AJR Am J Roentgenol. 2014;202:1149-59.
10. Smith RA et al. CA Cancer J Clin. 2018;68:297-316.
Since the reversal of the Medicare exclusion in 2014, the rates of gender-affirming surgery have increased markedly in the United States.1 Gender-affirming mastectomy, otherwise known as “top surgery,” is one of the more commonly performed procedures; with 97% of patients having either undergone or expressed desire for the surgery.2 The goals of this procedure are to remove all visible breast tissue and reconstruct the chest wall so it is more masculine in appearance. For transmasculine and nonbinary patients, this procedure is associated with significant improvements in mental health and quality of life.3,4 While the mastectomy procedure is often performed by plastic surgeons, patients will see an ob.gyn. in the preoperative or postoperative period. Ob.gyns. should have a general understanding of the procedure, but most importantly know how to screen for breast cancer in patients who have undergone a gender-affirming mastectomy.
Providers will likely encounter transmasculine or nonbinary patients during annual screening examinations or for a preoperative exam. If a patient is seeking a preoperative risk assessment prior to undergoing a gender-affirming mastectomy, assessing a patient’s risk status for breast cancer is paramount. While testosterone therapy is no longer a prerequisite for gender-affirming mastectomies, documenting hormone use, age at initiation, and dosage is important.5 The overall effects of testosterone on breast tissue are inconsistent. However, studies have demonstrated that patients taking testosterone are not at an increased risk of breast cancer secondary to testosterone use.5-7 Patients should be asked about a personal of family history of breast cancer, breast surgery, history of prior breast biopsies, parity, age at menarche, smoking status, and breastfeeding history if applicable. Patients with high-risk mutations or a strong family history of breast cancer should be referred to genetic counselors, surgical oncologists, and possibly undergo genetic testing.8 Before an examination, providers should counsel patients about the nature of the examination and use gender-neutral language such as “chest” to avoid exacerbating gender dysphoria.
It is important to educate transmasculine patients about their risk for the development of breast cancer after mastectomy. Larger-scale, population-based studies of breast cancer in the transgender population have reported an incidence of 5.9 per 100,000 patients-years and an overall incidence comparable to cisgender men in age-standardized national samples.5-7 Unfortunately, data on the rates of breast cancer in transmasculine patients after gender-affirming mastectomy are limited, which makes defining postoperative guidelines challenging. Additionally, the amount of residual breast tissue remaining varies based on the surgeon and technique.
Several techniques are described for mastectomy procedures with differences that can affect the amount of residual breast tissue. The most common type of gender-affirming mastectomy is the double incision. With this procedure, the nipple-areolar complex is reduced in size, removed, and thinned to improve graft take. Dissection is then carried to the level of the breast capsule and the breast tissue and axillary tail are removed en bloc.5 During the dissection, the subcutaneous fat is left on the skin flap to provide appropriate contour and to avoid creating a concave-appearing chest wall. Prior to closure, the superior and inferior flaps are inspected for any visible residual breast tissue, which is removed if needed. In a circumareolar mastectomy, the nipple-areolar complex is also reduced but is preserved on a 1- to 1.5-cm-thick pedicle to maintain perfusion.5 The mastectomy is performed through an inferior periareolar incision and all visible breast tissue and the axillary tail are removed. Breast tissue specimens are sent for pathologic evaluation at the end of the procedure.
Following gender-affirming mastectomy, there is limited evidence to guide screening. During the patient visit, the provider should obtain a thorough history regarding mastectomy type, and if unknown, attempt to acquire the operative report detailing the procedure. For low-risk patients who undergo a subcutaneous mastectomy such as the double incision or circumareolar technique, screening mammography is not indicated nor is it technically feasible.9 For patients with a high-risk genetic mutation or a strong family history of breast cancer, monitoring with alternative modalities such as breast ultrasound or breast MRI may be beneficial, although there is no evidence to currently support this suggestion. Given the variety of surgical techniques of breast tissue removal, it is difficult to develop strong evidence-based guidelines. Annual chest wall examinations have been suggested as a screening modality; however, the clinical utility of clinical breast and chest exams has been debated and is no longer recommended as a screening method in cisgender patients.9 Clinicians can promote chest self-awareness and discuss the possibility of breast cancer in postmastectomy patients at annual examination visits. As research continues to resolve some of these unknowns, it is important that patients are informed of these areas of ambiguity and updated regarding any changes in screening recommendations.10
Dr. Brandt is an ob.gyn. and fellowship-trained gender affirming surgeon in West Reading, Pa.
References
1. American Society of Plastic Surgeons. 2018 plastic surgery statistics report. https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic-surgery-statistics-full-report-2018.pdf. Accessed Aug. 20, 2021.
2. James SE et al. The report of the 2015 U.S. Transgender survey. Washington, D.C.: National Center for Transgender Equality, 2016.
3. Agarwal CA et al. J Plast Reconstr Aesthet Surg. 2018;71:651-7.
4. Poudrier G et al. Plast Reconstr Surg. 2018;80:679-83.
5. Salibian AA et al. Plast Reconstr Surg. 2020;147:213e-21e.
6. Gooren LJ et al. J Sex Med. 2013;10:3129-34.
7. Brown GR and Jones KT. Breast Cancer Res Treat. 2015;149:191-8.
8. Deutsch MF et al. Semin Reprod Med. 2017;35:434-41.
9. Phillips J et al. AJR Am J Roentgenol. 2014;202:1149-59.
10. Smith RA et al. CA Cancer J Clin. 2018;68:297-316.
Since the reversal of the Medicare exclusion in 2014, the rates of gender-affirming surgery have increased markedly in the United States.1 Gender-affirming mastectomy, otherwise known as “top surgery,” is one of the more commonly performed procedures; with 97% of patients having either undergone or expressed desire for the surgery.2 The goals of this procedure are to remove all visible breast tissue and reconstruct the chest wall so it is more masculine in appearance. For transmasculine and nonbinary patients, this procedure is associated with significant improvements in mental health and quality of life.3,4 While the mastectomy procedure is often performed by plastic surgeons, patients will see an ob.gyn. in the preoperative or postoperative period. Ob.gyns. should have a general understanding of the procedure, but most importantly know how to screen for breast cancer in patients who have undergone a gender-affirming mastectomy.
Providers will likely encounter transmasculine or nonbinary patients during annual screening examinations or for a preoperative exam. If a patient is seeking a preoperative risk assessment prior to undergoing a gender-affirming mastectomy, assessing a patient’s risk status for breast cancer is paramount. While testosterone therapy is no longer a prerequisite for gender-affirming mastectomies, documenting hormone use, age at initiation, and dosage is important.5 The overall effects of testosterone on breast tissue are inconsistent. However, studies have demonstrated that patients taking testosterone are not at an increased risk of breast cancer secondary to testosterone use.5-7 Patients should be asked about a personal of family history of breast cancer, breast surgery, history of prior breast biopsies, parity, age at menarche, smoking status, and breastfeeding history if applicable. Patients with high-risk mutations or a strong family history of breast cancer should be referred to genetic counselors, surgical oncologists, and possibly undergo genetic testing.8 Before an examination, providers should counsel patients about the nature of the examination and use gender-neutral language such as “chest” to avoid exacerbating gender dysphoria.
It is important to educate transmasculine patients about their risk for the development of breast cancer after mastectomy. Larger-scale, population-based studies of breast cancer in the transgender population have reported an incidence of 5.9 per 100,000 patients-years and an overall incidence comparable to cisgender men in age-standardized national samples.5-7 Unfortunately, data on the rates of breast cancer in transmasculine patients after gender-affirming mastectomy are limited, which makes defining postoperative guidelines challenging. Additionally, the amount of residual breast tissue remaining varies based on the surgeon and technique.
Several techniques are described for mastectomy procedures with differences that can affect the amount of residual breast tissue. The most common type of gender-affirming mastectomy is the double incision. With this procedure, the nipple-areolar complex is reduced in size, removed, and thinned to improve graft take. Dissection is then carried to the level of the breast capsule and the breast tissue and axillary tail are removed en bloc.5 During the dissection, the subcutaneous fat is left on the skin flap to provide appropriate contour and to avoid creating a concave-appearing chest wall. Prior to closure, the superior and inferior flaps are inspected for any visible residual breast tissue, which is removed if needed. In a circumareolar mastectomy, the nipple-areolar complex is also reduced but is preserved on a 1- to 1.5-cm-thick pedicle to maintain perfusion.5 The mastectomy is performed through an inferior periareolar incision and all visible breast tissue and the axillary tail are removed. Breast tissue specimens are sent for pathologic evaluation at the end of the procedure.
Following gender-affirming mastectomy, there is limited evidence to guide screening. During the patient visit, the provider should obtain a thorough history regarding mastectomy type, and if unknown, attempt to acquire the operative report detailing the procedure. For low-risk patients who undergo a subcutaneous mastectomy such as the double incision or circumareolar technique, screening mammography is not indicated nor is it technically feasible.9 For patients with a high-risk genetic mutation or a strong family history of breast cancer, monitoring with alternative modalities such as breast ultrasound or breast MRI may be beneficial, although there is no evidence to currently support this suggestion. Given the variety of surgical techniques of breast tissue removal, it is difficult to develop strong evidence-based guidelines. Annual chest wall examinations have been suggested as a screening modality; however, the clinical utility of clinical breast and chest exams has been debated and is no longer recommended as a screening method in cisgender patients.9 Clinicians can promote chest self-awareness and discuss the possibility of breast cancer in postmastectomy patients at annual examination visits. As research continues to resolve some of these unknowns, it is important that patients are informed of these areas of ambiguity and updated regarding any changes in screening recommendations.10
Dr. Brandt is an ob.gyn. and fellowship-trained gender affirming surgeon in West Reading, Pa.
References
1. American Society of Plastic Surgeons. 2018 plastic surgery statistics report. https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic-surgery-statistics-full-report-2018.pdf. Accessed Aug. 20, 2021.
2. James SE et al. The report of the 2015 U.S. Transgender survey. Washington, D.C.: National Center for Transgender Equality, 2016.
3. Agarwal CA et al. J Plast Reconstr Aesthet Surg. 2018;71:651-7.
4. Poudrier G et al. Plast Reconstr Surg. 2018;80:679-83.
5. Salibian AA et al. Plast Reconstr Surg. 2020;147:213e-21e.
6. Gooren LJ et al. J Sex Med. 2013;10:3129-34.
7. Brown GR and Jones KT. Breast Cancer Res Treat. 2015;149:191-8.
8. Deutsch MF et al. Semin Reprod Med. 2017;35:434-41.
9. Phillips J et al. AJR Am J Roentgenol. 2014;202:1149-59.
10. Smith RA et al. CA Cancer J Clin. 2018;68:297-316.
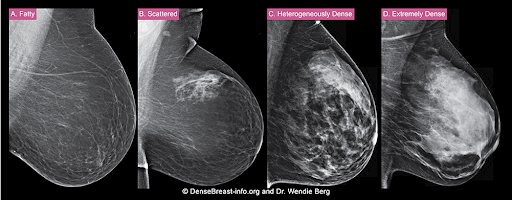
How is a woman determined to have dense breast tissue?
Breasts that are heterogeneously dense or extremely dense on mammography are considered “dense breasts.” Breast density matters for 2 reasons: Dense tissue can mask cancer on a mammogram, and having dense breasts increases the risk of developing breast cancer.
Breast density measurement
A woman’s breast density is usually determined during her breast cancer screening with mammography by her radiologist through visual evaluation of the images taken. Breast density also can be measured from individual mammograms by computer software, and it can be estimated on computed tomography (CT) scan and magnetic resonance imaging (MRI). In the United States, information about breast density is usually included in a report sent from the radiologist to the referring clinician after a mammogram is taken, and may also be included in the patient letter following up screening mammography. In Europe, national reporting guidelines for physicians vary.
The density of a woman’s breast tissue is described using one of four BI-RADS® breast composition categories1 as shown in the FIGURE.
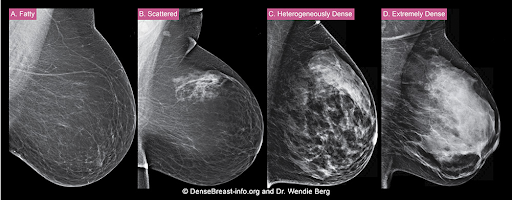
A. ALMOST ENTIRELY FATTY – On a mammogram, most of the tissue appears dark gray or black, while small amounts of dense (or fibroglandular) tissue display as light gray or white. About 13% of women aged 40 to 74 have breasts considered to be “fatty.”2
B. SCATTERED FIBROGLANDULAR DENSITY – There are scattered areas of dense (fibroglandular) tissue mixed with fat. Even in breasts with scattered areas of breast tissue, cancers can sometimes be missed when they look like areas of normal tissue or are within an area of denser tissue. About 43% of women aged 40 to 74 have breasts with scattered fibroglandular tissue.2
C. HETEROGENEOUSLY DENSE – There are large portions of the breast where dense (fibroglandular) tissue could hide small masses. About 36% of all women aged 40 to 74 have heterogeneously dense breasts.2
D. EXTREMELY DENSE – Most of the breast appears to consist of dense (fibroglandular) tissue, creating a “white out” situation and making it extremely difficult to see through and lowering the sensitivity of mammography. About 7% of all women aged 40 to 74 have extremely dense breasts.2
Factors that may impact breast density
Age. Breasts tend to become less dense as women get older, especially after menopause (as the glandular tissue atrophies and the breasts may appear more fatty-replaced).
Postmenopausal hormone therapy. An increase in mammographic density is more common among women taking continuous combined hormonal therapy than for those using oral low-dose estrogen or transdermal estrogen therapy.
Lactation. Breast density increases with lactation.
Weight changes. Weight gain can increase the amount of fat relative to dense tissue, resulting in slightly lower density as a proportion of breast tissue overall. Similarly, weight loss can decrease the amount of fat in the breasts, making breast density appear greater overall. Importantly, there is no change in the amount of glandular tissue; only the relative proportions change.
Tamoxifen or aromatase inhibitors. These medications can slightly reduce breast density.
Because breast density may change with age and other factors, it should be assessed every year.
For more information, visit medically sourced DenseBreast-info.org.
Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
1. Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS Mammography. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013.
2. Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jnci/dju255.
Breasts that are heterogeneously dense or extremely dense on mammography are considered “dense breasts.” Breast density matters for 2 reasons: Dense tissue can mask cancer on a mammogram, and having dense breasts increases the risk of developing breast cancer.
Breast density measurement
A woman’s breast density is usually determined during her breast cancer screening with mammography by her radiologist through visual evaluation of the images taken. Breast density also can be measured from individual mammograms by computer software, and it can be estimated on computed tomography (CT) scan and magnetic resonance imaging (MRI). In the United States, information about breast density is usually included in a report sent from the radiologist to the referring clinician after a mammogram is taken, and may also be included in the patient letter following up screening mammography. In Europe, national reporting guidelines for physicians vary.
The density of a woman’s breast tissue is described using one of four BI-RADS® breast composition categories1 as shown in the FIGURE.

A. ALMOST ENTIRELY FATTY – On a mammogram, most of the tissue appears dark gray or black, while small amounts of dense (or fibroglandular) tissue display as light gray or white. About 13% of women aged 40 to 74 have breasts considered to be “fatty.”2
B. SCATTERED FIBROGLANDULAR DENSITY – There are scattered areas of dense (fibroglandular) tissue mixed with fat. Even in breasts with scattered areas of breast tissue, cancers can sometimes be missed when they look like areas of normal tissue or are within an area of denser tissue. About 43% of women aged 40 to 74 have breasts with scattered fibroglandular tissue.2
C. HETEROGENEOUSLY DENSE – There are large portions of the breast where dense (fibroglandular) tissue could hide small masses. About 36% of all women aged 40 to 74 have heterogeneously dense breasts.2
D. EXTREMELY DENSE – Most of the breast appears to consist of dense (fibroglandular) tissue, creating a “white out” situation and making it extremely difficult to see through and lowering the sensitivity of mammography. About 7% of all women aged 40 to 74 have extremely dense breasts.2
Factors that may impact breast density
Age. Breasts tend to become less dense as women get older, especially after menopause (as the glandular tissue atrophies and the breasts may appear more fatty-replaced).
Postmenopausal hormone therapy. An increase in mammographic density is more common among women taking continuous combined hormonal therapy than for those using oral low-dose estrogen or transdermal estrogen therapy.
Lactation. Breast density increases with lactation.
Weight changes. Weight gain can increase the amount of fat relative to dense tissue, resulting in slightly lower density as a proportion of breast tissue overall. Similarly, weight loss can decrease the amount of fat in the breasts, making breast density appear greater overall. Importantly, there is no change in the amount of glandular tissue; only the relative proportions change.
Tamoxifen or aromatase inhibitors. These medications can slightly reduce breast density.
Because breast density may change with age and other factors, it should be assessed every year.
For more information, visit medically sourced DenseBreast-info.org.
Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Breasts that are heterogeneously dense or extremely dense on mammography are considered “dense breasts.” Breast density matters for 2 reasons: Dense tissue can mask cancer on a mammogram, and having dense breasts increases the risk of developing breast cancer.
Breast density measurement
A woman’s breast density is usually determined during her breast cancer screening with mammography by her radiologist through visual evaluation of the images taken. Breast density also can be measured from individual mammograms by computer software, and it can be estimated on computed tomography (CT) scan and magnetic resonance imaging (MRI). In the United States, information about breast density is usually included in a report sent from the radiologist to the referring clinician after a mammogram is taken, and may also be included in the patient letter following up screening mammography. In Europe, national reporting guidelines for physicians vary.
The density of a woman’s breast tissue is described using one of four BI-RADS® breast composition categories1 as shown in the FIGURE.

A. ALMOST ENTIRELY FATTY – On a mammogram, most of the tissue appears dark gray or black, while small amounts of dense (or fibroglandular) tissue display as light gray or white. About 13% of women aged 40 to 74 have breasts considered to be “fatty.”2
B. SCATTERED FIBROGLANDULAR DENSITY – There are scattered areas of dense (fibroglandular) tissue mixed with fat. Even in breasts with scattered areas of breast tissue, cancers can sometimes be missed when they look like areas of normal tissue or are within an area of denser tissue. About 43% of women aged 40 to 74 have breasts with scattered fibroglandular tissue.2
C. HETEROGENEOUSLY DENSE – There are large portions of the breast where dense (fibroglandular) tissue could hide small masses. About 36% of all women aged 40 to 74 have heterogeneously dense breasts.2
D. EXTREMELY DENSE – Most of the breast appears to consist of dense (fibroglandular) tissue, creating a “white out” situation and making it extremely difficult to see through and lowering the sensitivity of mammography. About 7% of all women aged 40 to 74 have extremely dense breasts.2
Factors that may impact breast density
Age. Breasts tend to become less dense as women get older, especially after menopause (as the glandular tissue atrophies and the breasts may appear more fatty-replaced).
Postmenopausal hormone therapy. An increase in mammographic density is more common among women taking continuous combined hormonal therapy than for those using oral low-dose estrogen or transdermal estrogen therapy.
Lactation. Breast density increases with lactation.
Weight changes. Weight gain can increase the amount of fat relative to dense tissue, resulting in slightly lower density as a proportion of breast tissue overall. Similarly, weight loss can decrease the amount of fat in the breasts, making breast density appear greater overall. Importantly, there is no change in the amount of glandular tissue; only the relative proportions change.
Tamoxifen or aromatase inhibitors. These medications can slightly reduce breast density.
Because breast density may change with age and other factors, it should be assessed every year.
For more information, visit medically sourced DenseBreast-info.org.
Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
1. Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS Mammography. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013.
2. Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jnci/dju255.
1. Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS Mammography. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013.
2. Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jnci/dju255.
Polygenic breast cancer risk scores strive to overcome racial bias
The potential of polygenic risk scores (PRSs) to become key components in the assessment of individual risk for disease in the clinical setting is inching closer to fruition; however, the technology is plagued by one glaring omission of most existing PRSs – the lack of applicability to those of non-European ancestry.
Polygenic risk scores predict an individual’s risk of disease based on common genetic variants identified in large genomewide association studies (GWASs). They have gained ground in research, as well as in the unregulated realm of the direct-to-consumer market where they are sold as add-ons to DNA ancestry kits such as 23andMe and MyHeritage.com.
While the risk scores show strong validation in estimating risk among people of European descent, their striking caveat is the lack of applicability to other ancestries, particularly African, and their use in practice outside of clinical trials is discouraged in National Comprehensive Cancer Network guidelines.
Study underscores need for ethnically diverse datasets
In a recent study published in JAMA Network Open, researchers evaluated the use of polygenic risk scores’ models in a clinical setting. Researchers tested 7 PRSs models for breast cancer risk against the medical records data of 39,591 women of European, African, and Latinx ancestry.
The PRSs models – all used only for research purposes – included three models involving European ancestry cohorts, two from Latinx cohorts, and two from women African descent.
After adjusting for factors including age, breast cancer family history, and ancestry, the PRSs from women with European ancestry highly corresponded to breast cancer risk, with a mean odds ratio of 1.46 per standard deviation increase in the score.
PRSs were also generalized relatively well among women of Latinx ancestry with a mean OR of 1.31. The authors noted that association is likely caused by Latinx individuals in the United States having a greater proportion of European ancestry than individuals with African ancestry. Importantly, however, the effect size was lower for women of African ancestry with a highest OR of 1.19 per standard deviation.
In the highest percentiles of breast cancer risk, women of European descent had odds ratio as high as 2.19-2.48, suggesting a statistically significant association with overall breast cancer risk. No statistically significant associations were found among women of Latinx and African-ancestry.
The PRSs models were smaller for women of non-European ancestry and included fewer genetic variants for women of non-European ancestry were notably smaller and hence reflected fewer genetic variants. Of the two risk scores involving African ancestry, the Women’s Health Initiative for Women with African ancestry risk score had just 75 variants, while the African diaspora study (ROOT) had 34 variants, compared with 3,820 and 5,218 in the two largest European ancestry PRSs, the Breast Cancer Association Consortium and the UK Biobank, respectively.
“These results highlight the need to improve representation of diverse population groups, particularly women with African ancestry, in genomic research cohorts,” the authors wrote.
First author, Cong Liu, PhD, of Columbia University Irving Medical Center, New York, said that efforts are underway to improve the inclusivity in the Electronic Medical Records and Genomics network data set used in this study.
“Until well-developed and validated PRSs for women with non-European ancestry become available, the current PRSs based on cohorts with European ancestry could be adapted for Latinx women, but not women with African ancestry until additional data sets become available in this important and high-risk group,” Dr. Liu and colleagues wrote.
In a commentary published with the study, Payal D. Shah, MD, of the Basser Center for BRCA at the University of Pennsylvania, Philadelphia, said that PRSs are “disproportionately applicable to patients with European ancestry and are insufficiently vetted and developed in other populations. If an instrument exists that has clinical utility in informing effective cancer risk mitigation strategies, then we must strive to ensure that it is available and applicable to all.”
Higher morality among African American women
While American Cancer Society data shows women with African ancestry generally have incidence rates of breast cancer similar to White women, they have significantly higher mortality from the disease in part because of later-stage diagnosis and health care barriers.
Anne Marie McCarthy, PhD, of the University of Pennsylvania, and Katrina Armstrong, MD, of Harvard Medical School, Boston, wrote in the Journal of the National Cancer Institute that African American women “have 42% higher breast cancer mortality than white women, despite having lower disease incidence, and are more likely to be diagnosed with triple-negative breast cancer, which has poorer prognosis than other molecular subtypes.”
Dr. McCarthy and Dr. Armstrong wrote that African American women are chronically underrepresented in breast cancer studies. And as such, it is impossible to know the extent of the prevalence of mutations and risk.
Failing to address the lack of diversity in genomic studies may worsen health disparities for women with African ancestry, Dr. Liu and colleagues wrote. The higher mortality “underscores the urgent need to increase diversity in genomic studies so that future clinical applications of the PRS do not exacerbate existing health disparities. These results highlight the need to improve representation of diverse population groups, particularly women with African ancestry, in genomic research cohorts.”
Potential PRS benefits underscore need to eliminate bias
The potentially important benefits of PRSs as risk prediction tools used in combination with family history, reproductive history and other factors, should provide strong incentive to push for improvement, Dr. Shah wrote.
For instance, if an individual is estrogen receptor positive and shows elevations in breast cancer risk on a reliable PRS, “this may inform antiestrogen chemoprevention strategies,” she wrote.
A risk score could furthermore influence the age at which breast cancer screening should begin or factor into whether a patient should also receive surveillance breast MRI.
Importantly, PRSs could also add to other risk factors to provide more precise risk estimates and inform management of women with a pathogenic variant in a breast cancer risk predisposition gene, Dr. Shah wrote.
Confluence project
Among the most promising developments in research is the National Cancer Institute’s Confluence Project, a large research resource aiming to include approximately 300,000 breast cancer cases and 300,000 controls of different races/ethnicities, utilizing the confluence of existing GWAS and new genomewide genotyping data.
Having started enrollment in 2018, the project is approaching implementation, said Montserrat García-Closas, MD, MPH, DrPH, deputy director of cancer epidemiology and genetics with the National Cancer Institute.
“We expect genotyping to be completed by the end of 2022 and for the data to be made available to the research community soon after that,” she said.
Among the project’s key objectives are the development of PRSs to be integrated with known risk factors to provide a personalized risk assessment for breast cancer, overall and by ancestral subtype.
“We plan to apply novel methods to derive multiancestry PRS that will account for differences and similarities in genetic architecture across ethnic/racial groups to develop breast cancer PRSs that can be applied in multiethnic/racial populations,” she said.
NCI is working with investigators in Africa, Central and South America, and Asia, and reaching out to non-European organizations such as AORTIC for studies of African populations.
Direct-to-consumer global PRS
In the commercial PRS market, efforts to address diversity shortcomings are also gaining momentum, with Myriad Genetics touting a first-of-its kind “global PRS.”
The PRS, a recalibrated version the company’s riskScore PRS, sold as part of its Myriad myRisk Hereditary Cancer test, will reportedly apply to all ethnicities in estimating an individual’s 5-year and lifetime risk of breast cancer.
A study presented in June at the American Society of Clinical Oncology meeting, describes the development of the model with the use of three large ancestry-specific PRSs based on African American, Asian, and European cohorts, with the system including a total of 149 single-nucleotide polymorphisms, including 93 well established for breast cancer and 56 that are ancestry specific.
In validation of the data in an independent cohort of 62,707 individuals, the global PRS was strongly associated with breast cancer in the full combined validation cohort as well as in all three of the ancestry subcohorts.
However, the effect size among women with African ancestry was still the lowest of all of the groups, with a mean OR of 1.24 per standard deviation, versus the highest rate of mixed ancestry (OR, 1.59).
According to senior author Holly Pederson, MD, director of medical breast services at the Cleveland Clinic, the applicability of the PRS to women with African ancestry is expected to further improve as additional data become available.
“The discriminatory power in women of African descent was significantly improved but still suboptimal,” she said. “The need for more data, particularly in Black women, is challenging not only because there is likely more diversity in the genomic landscape of women of African descent, but also because the barriers created by historical, cultural, institutional and interpersonal dynamics result in the paucity of this data.”
“We must be committed to ending bias resulting in health care disparities,” Dr. Pederson said. She noted that the global PRS is nevertheless “still clinically useful in Black women,” and recommended that clinicians be up front with patients on the status of the research challenges.
“As with any clinical shared decision-making conversation between a patient and her provider, it is important for Black women to know that data is limited in the African American population, particularly given the vast genomic diversity of the African continent,” she said. “This model, as models that have gone before it, will improve with additional data, particularly in this population.”
Commercial PRSs may benefit research
While the commercial marketing of PRSs in a direct-to-consumer fashion have raised some concerns, such as how individuals respond to their risk scores, there could be important benefits as well, commented Megan C. Roberts, PhD.
“There may be an opportunity to learn from these companies about how to engage diverse communities in genomic testing,” said Dr. Roberts, an assistant professor and director of implementation science in precision health and society at the University of North Carolina at Chapel Hill. “Moreover, the data they collect from their customers often can be used for research purposes as well.”
In a recent perspective, Dr. Roberts and colleagues addressed the role of health disparities in PRSs. She’ll be joining international precision public health researchers in October in hosting a free virtual conference at UNC on the topic.
“There is a huge need to improve racial and ethnic diversity in our genomic datasets,” Dr. Roberts said. “Without this, we will not be able to return on the promise of precision medicine and prevention for improving the health of our whole population.”
Dr. Pederson disclosed that she is a consultant for Myriad Genetics.
The potential of polygenic risk scores (PRSs) to become key components in the assessment of individual risk for disease in the clinical setting is inching closer to fruition; however, the technology is plagued by one glaring omission of most existing PRSs – the lack of applicability to those of non-European ancestry.
Polygenic risk scores predict an individual’s risk of disease based on common genetic variants identified in large genomewide association studies (GWASs). They have gained ground in research, as well as in the unregulated realm of the direct-to-consumer market where they are sold as add-ons to DNA ancestry kits such as 23andMe and MyHeritage.com.
While the risk scores show strong validation in estimating risk among people of European descent, their striking caveat is the lack of applicability to other ancestries, particularly African, and their use in practice outside of clinical trials is discouraged in National Comprehensive Cancer Network guidelines.
Study underscores need for ethnically diverse datasets
In a recent study published in JAMA Network Open, researchers evaluated the use of polygenic risk scores’ models in a clinical setting. Researchers tested 7 PRSs models for breast cancer risk against the medical records data of 39,591 women of European, African, and Latinx ancestry.
The PRSs models – all used only for research purposes – included three models involving European ancestry cohorts, two from Latinx cohorts, and two from women African descent.
After adjusting for factors including age, breast cancer family history, and ancestry, the PRSs from women with European ancestry highly corresponded to breast cancer risk, with a mean odds ratio of 1.46 per standard deviation increase in the score.
PRSs were also generalized relatively well among women of Latinx ancestry with a mean OR of 1.31. The authors noted that association is likely caused by Latinx individuals in the United States having a greater proportion of European ancestry than individuals with African ancestry. Importantly, however, the effect size was lower for women of African ancestry with a highest OR of 1.19 per standard deviation.
In the highest percentiles of breast cancer risk, women of European descent had odds ratio as high as 2.19-2.48, suggesting a statistically significant association with overall breast cancer risk. No statistically significant associations were found among women of Latinx and African-ancestry.
The PRSs models were smaller for women of non-European ancestry and included fewer genetic variants for women of non-European ancestry were notably smaller and hence reflected fewer genetic variants. Of the two risk scores involving African ancestry, the Women’s Health Initiative for Women with African ancestry risk score had just 75 variants, while the African diaspora study (ROOT) had 34 variants, compared with 3,820 and 5,218 in the two largest European ancestry PRSs, the Breast Cancer Association Consortium and the UK Biobank, respectively.
“These results highlight the need to improve representation of diverse population groups, particularly women with African ancestry, in genomic research cohorts,” the authors wrote.
First author, Cong Liu, PhD, of Columbia University Irving Medical Center, New York, said that efforts are underway to improve the inclusivity in the Electronic Medical Records and Genomics network data set used in this study.
“Until well-developed and validated PRSs for women with non-European ancestry become available, the current PRSs based on cohorts with European ancestry could be adapted for Latinx women, but not women with African ancestry until additional data sets become available in this important and high-risk group,” Dr. Liu and colleagues wrote.
In a commentary published with the study, Payal D. Shah, MD, of the Basser Center for BRCA at the University of Pennsylvania, Philadelphia, said that PRSs are “disproportionately applicable to patients with European ancestry and are insufficiently vetted and developed in other populations. If an instrument exists that has clinical utility in informing effective cancer risk mitigation strategies, then we must strive to ensure that it is available and applicable to all.”
Higher morality among African American women
While American Cancer Society data shows women with African ancestry generally have incidence rates of breast cancer similar to White women, they have significantly higher mortality from the disease in part because of later-stage diagnosis and health care barriers.
Anne Marie McCarthy, PhD, of the University of Pennsylvania, and Katrina Armstrong, MD, of Harvard Medical School, Boston, wrote in the Journal of the National Cancer Institute that African American women “have 42% higher breast cancer mortality than white women, despite having lower disease incidence, and are more likely to be diagnosed with triple-negative breast cancer, which has poorer prognosis than other molecular subtypes.”
Dr. McCarthy and Dr. Armstrong wrote that African American women are chronically underrepresented in breast cancer studies. And as such, it is impossible to know the extent of the prevalence of mutations and risk.
Failing to address the lack of diversity in genomic studies may worsen health disparities for women with African ancestry, Dr. Liu and colleagues wrote. The higher mortality “underscores the urgent need to increase diversity in genomic studies so that future clinical applications of the PRS do not exacerbate existing health disparities. These results highlight the need to improve representation of diverse population groups, particularly women with African ancestry, in genomic research cohorts.”
Potential PRS benefits underscore need to eliminate bias
The potentially important benefits of PRSs as risk prediction tools used in combination with family history, reproductive history and other factors, should provide strong incentive to push for improvement, Dr. Shah wrote.
For instance, if an individual is estrogen receptor positive and shows elevations in breast cancer risk on a reliable PRS, “this may inform antiestrogen chemoprevention strategies,” she wrote.
A risk score could furthermore influence the age at which breast cancer screening should begin or factor into whether a patient should also receive surveillance breast MRI.
Importantly, PRSs could also add to other risk factors to provide more precise risk estimates and inform management of women with a pathogenic variant in a breast cancer risk predisposition gene, Dr. Shah wrote.
Confluence project
Among the most promising developments in research is the National Cancer Institute’s Confluence Project, a large research resource aiming to include approximately 300,000 breast cancer cases and 300,000 controls of different races/ethnicities, utilizing the confluence of existing GWAS and new genomewide genotyping data.
Having started enrollment in 2018, the project is approaching implementation, said Montserrat García-Closas, MD, MPH, DrPH, deputy director of cancer epidemiology and genetics with the National Cancer Institute.
“We expect genotyping to be completed by the end of 2022 and for the data to be made available to the research community soon after that,” she said.
Among the project’s key objectives are the development of PRSs to be integrated with known risk factors to provide a personalized risk assessment for breast cancer, overall and by ancestral subtype.
“We plan to apply novel methods to derive multiancestry PRS that will account for differences and similarities in genetic architecture across ethnic/racial groups to develop breast cancer PRSs that can be applied in multiethnic/racial populations,” she said.
NCI is working with investigators in Africa, Central and South America, and Asia, and reaching out to non-European organizations such as AORTIC for studies of African populations.
Direct-to-consumer global PRS
In the commercial PRS market, efforts to address diversity shortcomings are also gaining momentum, with Myriad Genetics touting a first-of-its kind “global PRS.”
The PRS, a recalibrated version the company’s riskScore PRS, sold as part of its Myriad myRisk Hereditary Cancer test, will reportedly apply to all ethnicities in estimating an individual’s 5-year and lifetime risk of breast cancer.
A study presented in June at the American Society of Clinical Oncology meeting, describes the development of the model with the use of three large ancestry-specific PRSs based on African American, Asian, and European cohorts, with the system including a total of 149 single-nucleotide polymorphisms, including 93 well established for breast cancer and 56 that are ancestry specific.
In validation of the data in an independent cohort of 62,707 individuals, the global PRS was strongly associated with breast cancer in the full combined validation cohort as well as in all three of the ancestry subcohorts.
However, the effect size among women with African ancestry was still the lowest of all of the groups, with a mean OR of 1.24 per standard deviation, versus the highest rate of mixed ancestry (OR, 1.59).
According to senior author Holly Pederson, MD, director of medical breast services at the Cleveland Clinic, the applicability of the PRS to women with African ancestry is expected to further improve as additional data become available.
“The discriminatory power in women of African descent was significantly improved but still suboptimal,” she said. “The need for more data, particularly in Black women, is challenging not only because there is likely more diversity in the genomic landscape of women of African descent, but also because the barriers created by historical, cultural, institutional and interpersonal dynamics result in the paucity of this data.”
“We must be committed to ending bias resulting in health care disparities,” Dr. Pederson said. She noted that the global PRS is nevertheless “still clinically useful in Black women,” and recommended that clinicians be up front with patients on the status of the research challenges.
“As with any clinical shared decision-making conversation between a patient and her provider, it is important for Black women to know that data is limited in the African American population, particularly given the vast genomic diversity of the African continent,” she said. “This model, as models that have gone before it, will improve with additional data, particularly in this population.”
Commercial PRSs may benefit research
While the commercial marketing of PRSs in a direct-to-consumer fashion have raised some concerns, such as how individuals respond to their risk scores, there could be important benefits as well, commented Megan C. Roberts, PhD.
“There may be an opportunity to learn from these companies about how to engage diverse communities in genomic testing,” said Dr. Roberts, an assistant professor and director of implementation science in precision health and society at the University of North Carolina at Chapel Hill. “Moreover, the data they collect from their customers often can be used for research purposes as well.”
In a recent perspective, Dr. Roberts and colleagues addressed the role of health disparities in PRSs. She’ll be joining international precision public health researchers in October in hosting a free virtual conference at UNC on the topic.
“There is a huge need to improve racial and ethnic diversity in our genomic datasets,” Dr. Roberts said. “Without this, we will not be able to return on the promise of precision medicine and prevention for improving the health of our whole population.”
Dr. Pederson disclosed that she is a consultant for Myriad Genetics.
The potential of polygenic risk scores (PRSs) to become key components in the assessment of individual risk for disease in the clinical setting is inching closer to fruition; however, the technology is plagued by one glaring omission of most existing PRSs – the lack of applicability to those of non-European ancestry.
Polygenic risk scores predict an individual’s risk of disease based on common genetic variants identified in large genomewide association studies (GWASs). They have gained ground in research, as well as in the unregulated realm of the direct-to-consumer market where they are sold as add-ons to DNA ancestry kits such as 23andMe and MyHeritage.com.
While the risk scores show strong validation in estimating risk among people of European descent, their striking caveat is the lack of applicability to other ancestries, particularly African, and their use in practice outside of clinical trials is discouraged in National Comprehensive Cancer Network guidelines.
Study underscores need for ethnically diverse datasets
In a recent study published in JAMA Network Open, researchers evaluated the use of polygenic risk scores’ models in a clinical setting. Researchers tested 7 PRSs models for breast cancer risk against the medical records data of 39,591 women of European, African, and Latinx ancestry.
The PRSs models – all used only for research purposes – included three models involving European ancestry cohorts, two from Latinx cohorts, and two from women African descent.
After adjusting for factors including age, breast cancer family history, and ancestry, the PRSs from women with European ancestry highly corresponded to breast cancer risk, with a mean odds ratio of 1.46 per standard deviation increase in the score.
PRSs were also generalized relatively well among women of Latinx ancestry with a mean OR of 1.31. The authors noted that association is likely caused by Latinx individuals in the United States having a greater proportion of European ancestry than individuals with African ancestry. Importantly, however, the effect size was lower for women of African ancestry with a highest OR of 1.19 per standard deviation.
In the highest percentiles of breast cancer risk, women of European descent had odds ratio as high as 2.19-2.48, suggesting a statistically significant association with overall breast cancer risk. No statistically significant associations were found among women of Latinx and African-ancestry.
The PRSs models were smaller for women of non-European ancestry and included fewer genetic variants for women of non-European ancestry were notably smaller and hence reflected fewer genetic variants. Of the two risk scores involving African ancestry, the Women’s Health Initiative for Women with African ancestry risk score had just 75 variants, while the African diaspora study (ROOT) had 34 variants, compared with 3,820 and 5,218 in the two largest European ancestry PRSs, the Breast Cancer Association Consortium and the UK Biobank, respectively.
“These results highlight the need to improve representation of diverse population groups, particularly women with African ancestry, in genomic research cohorts,” the authors wrote.
First author, Cong Liu, PhD, of Columbia University Irving Medical Center, New York, said that efforts are underway to improve the inclusivity in the Electronic Medical Records and Genomics network data set used in this study.
“Until well-developed and validated PRSs for women with non-European ancestry become available, the current PRSs based on cohorts with European ancestry could be adapted for Latinx women, but not women with African ancestry until additional data sets become available in this important and high-risk group,” Dr. Liu and colleagues wrote.
In a commentary published with the study, Payal D. Shah, MD, of the Basser Center for BRCA at the University of Pennsylvania, Philadelphia, said that PRSs are “disproportionately applicable to patients with European ancestry and are insufficiently vetted and developed in other populations. If an instrument exists that has clinical utility in informing effective cancer risk mitigation strategies, then we must strive to ensure that it is available and applicable to all.”
Higher morality among African American women
While American Cancer Society data shows women with African ancestry generally have incidence rates of breast cancer similar to White women, they have significantly higher mortality from the disease in part because of later-stage diagnosis and health care barriers.
Anne Marie McCarthy, PhD, of the University of Pennsylvania, and Katrina Armstrong, MD, of Harvard Medical School, Boston, wrote in the Journal of the National Cancer Institute that African American women “have 42% higher breast cancer mortality than white women, despite having lower disease incidence, and are more likely to be diagnosed with triple-negative breast cancer, which has poorer prognosis than other molecular subtypes.”
Dr. McCarthy and Dr. Armstrong wrote that African American women are chronically underrepresented in breast cancer studies. And as such, it is impossible to know the extent of the prevalence of mutations and risk.
Failing to address the lack of diversity in genomic studies may worsen health disparities for women with African ancestry, Dr. Liu and colleagues wrote. The higher mortality “underscores the urgent need to increase diversity in genomic studies so that future clinical applications of the PRS do not exacerbate existing health disparities. These results highlight the need to improve representation of diverse population groups, particularly women with African ancestry, in genomic research cohorts.”
Potential PRS benefits underscore need to eliminate bias
The potentially important benefits of PRSs as risk prediction tools used in combination with family history, reproductive history and other factors, should provide strong incentive to push for improvement, Dr. Shah wrote.
For instance, if an individual is estrogen receptor positive and shows elevations in breast cancer risk on a reliable PRS, “this may inform antiestrogen chemoprevention strategies,” she wrote.
A risk score could furthermore influence the age at which breast cancer screening should begin or factor into whether a patient should also receive surveillance breast MRI.
Importantly, PRSs could also add to other risk factors to provide more precise risk estimates and inform management of women with a pathogenic variant in a breast cancer risk predisposition gene, Dr. Shah wrote.
Confluence project
Among the most promising developments in research is the National Cancer Institute’s Confluence Project, a large research resource aiming to include approximately 300,000 breast cancer cases and 300,000 controls of different races/ethnicities, utilizing the confluence of existing GWAS and new genomewide genotyping data.
Having started enrollment in 2018, the project is approaching implementation, said Montserrat García-Closas, MD, MPH, DrPH, deputy director of cancer epidemiology and genetics with the National Cancer Institute.
“We expect genotyping to be completed by the end of 2022 and for the data to be made available to the research community soon after that,” she said.
Among the project’s key objectives are the development of PRSs to be integrated with known risk factors to provide a personalized risk assessment for breast cancer, overall and by ancestral subtype.
“We plan to apply novel methods to derive multiancestry PRS that will account for differences and similarities in genetic architecture across ethnic/racial groups to develop breast cancer PRSs that can be applied in multiethnic/racial populations,” she said.
NCI is working with investigators in Africa, Central and South America, and Asia, and reaching out to non-European organizations such as AORTIC for studies of African populations.
Direct-to-consumer global PRS
In the commercial PRS market, efforts to address diversity shortcomings are also gaining momentum, with Myriad Genetics touting a first-of-its kind “global PRS.”
The PRS, a recalibrated version the company’s riskScore PRS, sold as part of its Myriad myRisk Hereditary Cancer test, will reportedly apply to all ethnicities in estimating an individual’s 5-year and lifetime risk of breast cancer.
A study presented in June at the American Society of Clinical Oncology meeting, describes the development of the model with the use of three large ancestry-specific PRSs based on African American, Asian, and European cohorts, with the system including a total of 149 single-nucleotide polymorphisms, including 93 well established for breast cancer and 56 that are ancestry specific.
In validation of the data in an independent cohort of 62,707 individuals, the global PRS was strongly associated with breast cancer in the full combined validation cohort as well as in all three of the ancestry subcohorts.
However, the effect size among women with African ancestry was still the lowest of all of the groups, with a mean OR of 1.24 per standard deviation, versus the highest rate of mixed ancestry (OR, 1.59).
According to senior author Holly Pederson, MD, director of medical breast services at the Cleveland Clinic, the applicability of the PRS to women with African ancestry is expected to further improve as additional data become available.
“The discriminatory power in women of African descent was significantly improved but still suboptimal,” she said. “The need for more data, particularly in Black women, is challenging not only because there is likely more diversity in the genomic landscape of women of African descent, but also because the barriers created by historical, cultural, institutional and interpersonal dynamics result in the paucity of this data.”
“We must be committed to ending bias resulting in health care disparities,” Dr. Pederson said. She noted that the global PRS is nevertheless “still clinically useful in Black women,” and recommended that clinicians be up front with patients on the status of the research challenges.
“As with any clinical shared decision-making conversation between a patient and her provider, it is important for Black women to know that data is limited in the African American population, particularly given the vast genomic diversity of the African continent,” she said. “This model, as models that have gone before it, will improve with additional data, particularly in this population.”
Commercial PRSs may benefit research
While the commercial marketing of PRSs in a direct-to-consumer fashion have raised some concerns, such as how individuals respond to their risk scores, there could be important benefits as well, commented Megan C. Roberts, PhD.
“There may be an opportunity to learn from these companies about how to engage diverse communities in genomic testing,” said Dr. Roberts, an assistant professor and director of implementation science in precision health and society at the University of North Carolina at Chapel Hill. “Moreover, the data they collect from their customers often can be used for research purposes as well.”
In a recent perspective, Dr. Roberts and colleagues addressed the role of health disparities in PRSs. She’ll be joining international precision public health researchers in October in hosting a free virtual conference at UNC on the topic.
“There is a huge need to improve racial and ethnic diversity in our genomic datasets,” Dr. Roberts said. “Without this, we will not be able to return on the promise of precision medicine and prevention for improving the health of our whole population.”
Dr. Pederson disclosed that she is a consultant for Myriad Genetics.
FROM JAMA NETWORK OPEN
Internal mammary lymph node radiation safe over the long term
After a median follow-up of 15.7 years among almost 4,000 women, for half of patients who received postoperative internal mammary and medial supraclavicular (IM-MS) lymph node irradiation, the “absolute rates and differences” of heart and lung complications “were very low, with no increased non–breast cancer related mortality, even before introducing heart-sparing techniques,” say investigators.
The findings come from the European Organization for Research and Treatment of Cancer (EORTC) trial. The investigators were led by Philip Poortmans, MD, PhD, a radiation oncologist at the University of Antwerp, Belgium.
The team had previously reported lower breast cancer mortality and breast cancer recurrence rates in the radiation group.
Women in the trial were treated from 1996 to 2004. “We expect that with contemporary volume-based radiation therapy outcomes will be even better, by improved coverage of target volumes, more homogeneous dose delivery, and decreased doses to non-target tissues,” the team says.
In the end, “our findings ... have important – reassuring – consequences for decision-making concerning elective lymph node treatment in breast cancer,” the researchers comment.
The study was published online on July 28 in the Journal of the National Cancer Institute.
Resolving the debate
There’s been debate for decades on whether the long-term risk associated with nodal irradiation, particularly collateral heart and lung damage from internal mammary irradiation, outweighs the benefits of better disease control, noted Julia White, MD, a radiation oncologist at the Ohio State University Breast Center, Columbus, in an accompanying editorial.
Concerns stem originally from trials conducted from the 1950s to the 1970s. In those trials, higher doses of radiation were delivered to the internal mammary node with far less precision than today. Subsequent studies have not laid the worry to rest, and protocols vary across institutions, Dr. White explains. Some treat IM nodes in high-risk patients, but others only treat the axilla and the medial supraclavicular lymph nodes.
Dr. White says the new EORTC trial “moves us one step closer to resolving the debate about the value of internal mammary nodal (IMN) radiation.”
She notes that since 2014, advances in the field have led to an almost 50% reduction in cardiac radiation exposure during breast cancer treatment. Current guidelines recommend that internal mammary nodes “should generally be treated” as part of postmastectomy radiotherapy, but cardiopulmonary complications are still possible even with improved techniques, she writes.
Mostly grade 1 morbidity
Women in the study had stage I-III breast cancer with axillary node involvement and/or medially located primary tumors. The median age at study entry was 54 years. The patients were treated at 46 centers in 13 countries.
The group that received IM-MS irradiation after surgery received 50 Gy in 25 fractions over 5 weeks.
The cumulative 15-year incidence of lung fibrosis was 5.7% among treated women, versus 2.9% among control patients. The incidence of cardiac fibrosis was 1.9% with treatment, versus 1.1% without.
The incidence of any cardiac disease was 11.1% in the radiation arm, versus 9.4% in the control group.
Complications were mostly of grade 1. The only statistically significant difference in rates of events of grade 2 or higher was in the incidence of pulmonary morbidity, which was 0.8% with radiation versus 0.1% without. There were no differences in the incidence of second malignancies, contralateral breast cancer cases, or cardiovascular deaths with IMN irradiation.
The authors note that their results conflict with a 2013 study that found a relative increase in major coronary events of 7.4% per Gy mean heart dose. The women in that trial were treated in Sweden and Denmark between 1958 and 2001.
Dr. Poortmans and collegues note, however, that this 2013 study and others found a proportional and not an absolute increase in risk. With a baseline risk of 10%, for instance, a 7% increase per 1 Gy translates to a total risk of 10.07%.
Also, no increased risk has been reported in more recently published trials, and a meta-analysis found no increase in non–breast cancer related mortality with trials that began after 1988.
Still, “it seems logical to take the pre-existing cardiac comorbidity of patients into consideration,” the investigators conclude. For patients with higher baseline cardiopulmonary risk factors, lower mean heart doses should be used, and such patients should undergo longer-term follow-up, they write.
The study was funded by La Ligue Nationale Contre Le Cancer and the KWF Kanker Bestrijding from the Netherlands. The investigators and Dr. White have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After a median follow-up of 15.7 years among almost 4,000 women, for half of patients who received postoperative internal mammary and medial supraclavicular (IM-MS) lymph node irradiation, the “absolute rates and differences” of heart and lung complications “were very low, with no increased non–breast cancer related mortality, even before introducing heart-sparing techniques,” say investigators.
The findings come from the European Organization for Research and Treatment of Cancer (EORTC) trial. The investigators were led by Philip Poortmans, MD, PhD, a radiation oncologist at the University of Antwerp, Belgium.
The team had previously reported lower breast cancer mortality and breast cancer recurrence rates in the radiation group.
Women in the trial were treated from 1996 to 2004. “We expect that with contemporary volume-based radiation therapy outcomes will be even better, by improved coverage of target volumes, more homogeneous dose delivery, and decreased doses to non-target tissues,” the team says.
In the end, “our findings ... have important – reassuring – consequences for decision-making concerning elective lymph node treatment in breast cancer,” the researchers comment.
The study was published online on July 28 in the Journal of the National Cancer Institute.
Resolving the debate
There’s been debate for decades on whether the long-term risk associated with nodal irradiation, particularly collateral heart and lung damage from internal mammary irradiation, outweighs the benefits of better disease control, noted Julia White, MD, a radiation oncologist at the Ohio State University Breast Center, Columbus, in an accompanying editorial.
Concerns stem originally from trials conducted from the 1950s to the 1970s. In those trials, higher doses of radiation were delivered to the internal mammary node with far less precision than today. Subsequent studies have not laid the worry to rest, and protocols vary across institutions, Dr. White explains. Some treat IM nodes in high-risk patients, but others only treat the axilla and the medial supraclavicular lymph nodes.
Dr. White says the new EORTC trial “moves us one step closer to resolving the debate about the value of internal mammary nodal (IMN) radiation.”
She notes that since 2014, advances in the field have led to an almost 50% reduction in cardiac radiation exposure during breast cancer treatment. Current guidelines recommend that internal mammary nodes “should generally be treated” as part of postmastectomy radiotherapy, but cardiopulmonary complications are still possible even with improved techniques, she writes.
Mostly grade 1 morbidity
Women in the study had stage I-III breast cancer with axillary node involvement and/or medially located primary tumors. The median age at study entry was 54 years. The patients were treated at 46 centers in 13 countries.
The group that received IM-MS irradiation after surgery received 50 Gy in 25 fractions over 5 weeks.
The cumulative 15-year incidence of lung fibrosis was 5.7% among treated women, versus 2.9% among control patients. The incidence of cardiac fibrosis was 1.9% with treatment, versus 1.1% without.
The incidence of any cardiac disease was 11.1% in the radiation arm, versus 9.4% in the control group.
Complications were mostly of grade 1. The only statistically significant difference in rates of events of grade 2 or higher was in the incidence of pulmonary morbidity, which was 0.8% with radiation versus 0.1% without. There were no differences in the incidence of second malignancies, contralateral breast cancer cases, or cardiovascular deaths with IMN irradiation.
The authors note that their results conflict with a 2013 study that found a relative increase in major coronary events of 7.4% per Gy mean heart dose. The women in that trial were treated in Sweden and Denmark between 1958 and 2001.
Dr. Poortmans and collegues note, however, that this 2013 study and others found a proportional and not an absolute increase in risk. With a baseline risk of 10%, for instance, a 7% increase per 1 Gy translates to a total risk of 10.07%.
Also, no increased risk has been reported in more recently published trials, and a meta-analysis found no increase in non–breast cancer related mortality with trials that began after 1988.
Still, “it seems logical to take the pre-existing cardiac comorbidity of patients into consideration,” the investigators conclude. For patients with higher baseline cardiopulmonary risk factors, lower mean heart doses should be used, and such patients should undergo longer-term follow-up, they write.
The study was funded by La Ligue Nationale Contre Le Cancer and the KWF Kanker Bestrijding from the Netherlands. The investigators and Dr. White have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After a median follow-up of 15.7 years among almost 4,000 women, for half of patients who received postoperative internal mammary and medial supraclavicular (IM-MS) lymph node irradiation, the “absolute rates and differences” of heart and lung complications “were very low, with no increased non–breast cancer related mortality, even before introducing heart-sparing techniques,” say investigators.
The findings come from the European Organization for Research and Treatment of Cancer (EORTC) trial. The investigators were led by Philip Poortmans, MD, PhD, a radiation oncologist at the University of Antwerp, Belgium.
The team had previously reported lower breast cancer mortality and breast cancer recurrence rates in the radiation group.
Women in the trial were treated from 1996 to 2004. “We expect that with contemporary volume-based radiation therapy outcomes will be even better, by improved coverage of target volumes, more homogeneous dose delivery, and decreased doses to non-target tissues,” the team says.
In the end, “our findings ... have important – reassuring – consequences for decision-making concerning elective lymph node treatment in breast cancer,” the researchers comment.
The study was published online on July 28 in the Journal of the National Cancer Institute.
Resolving the debate
There’s been debate for decades on whether the long-term risk associated with nodal irradiation, particularly collateral heart and lung damage from internal mammary irradiation, outweighs the benefits of better disease control, noted Julia White, MD, a radiation oncologist at the Ohio State University Breast Center, Columbus, in an accompanying editorial.
Concerns stem originally from trials conducted from the 1950s to the 1970s. In those trials, higher doses of radiation were delivered to the internal mammary node with far less precision than today. Subsequent studies have not laid the worry to rest, and protocols vary across institutions, Dr. White explains. Some treat IM nodes in high-risk patients, but others only treat the axilla and the medial supraclavicular lymph nodes.
Dr. White says the new EORTC trial “moves us one step closer to resolving the debate about the value of internal mammary nodal (IMN) radiation.”
She notes that since 2014, advances in the field have led to an almost 50% reduction in cardiac radiation exposure during breast cancer treatment. Current guidelines recommend that internal mammary nodes “should generally be treated” as part of postmastectomy radiotherapy, but cardiopulmonary complications are still possible even with improved techniques, she writes.
Mostly grade 1 morbidity
Women in the study had stage I-III breast cancer with axillary node involvement and/or medially located primary tumors. The median age at study entry was 54 years. The patients were treated at 46 centers in 13 countries.
The group that received IM-MS irradiation after surgery received 50 Gy in 25 fractions over 5 weeks.
The cumulative 15-year incidence of lung fibrosis was 5.7% among treated women, versus 2.9% among control patients. The incidence of cardiac fibrosis was 1.9% with treatment, versus 1.1% without.
The incidence of any cardiac disease was 11.1% in the radiation arm, versus 9.4% in the control group.
Complications were mostly of grade 1. The only statistically significant difference in rates of events of grade 2 or higher was in the incidence of pulmonary morbidity, which was 0.8% with radiation versus 0.1% without. There were no differences in the incidence of second malignancies, contralateral breast cancer cases, or cardiovascular deaths with IMN irradiation.
The authors note that their results conflict with a 2013 study that found a relative increase in major coronary events of 7.4% per Gy mean heart dose. The women in that trial were treated in Sweden and Denmark between 1958 and 2001.
Dr. Poortmans and collegues note, however, that this 2013 study and others found a proportional and not an absolute increase in risk. With a baseline risk of 10%, for instance, a 7% increase per 1 Gy translates to a total risk of 10.07%.
Also, no increased risk has been reported in more recently published trials, and a meta-analysis found no increase in non–breast cancer related mortality with trials that began after 1988.
Still, “it seems logical to take the pre-existing cardiac comorbidity of patients into consideration,” the investigators conclude. For patients with higher baseline cardiopulmonary risk factors, lower mean heart doses should be used, and such patients should undergo longer-term follow-up, they write.
The study was funded by La Ligue Nationale Contre Le Cancer and the KWF Kanker Bestrijding from the Netherlands. The investigators and Dr. White have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Internal mammary lymph node radiation safe over the long term
After a median follow-up 15.7 years among almost 4,000 women, for half of patients who received postoperative internal mammary and medial supraclavicular (IM-MS) lymph node irradiation, the “absolute rates and differences” of heart and lung complications “were very low, with no increased non–breast cancer–related mortality, even before introducing heart-sparing techniques,” said the investigators.
The findings come from the European Organization for Research and Treatment of Cancer (EORTC) trial. The investigators were led by Philip Poortmans, MD, PhD, a radiation oncologist at the University of Antwerp (Belgium).
The team had previously reported lower breast cancer mortality and breast cancer recurrence rates in the radiation group.
Women in the trial were treated from 1996 to 2004. “We expect that with contemporary volume-based radiation therapy outcomes will be even better, by improved coverage of target volumes, more homogeneous dose delivery and decreased doses to nontarget tissues,” the team wrote.
In the end, “our findings ... have important – reassuring – consequences for decision-making concerning elective lymph node treatment in breast cancer,” the researchers commented.
The study was published online on July 28, 2021, in the Journal of the National Cancer Institute.
Resolving the debate
There’s been debate for decades on whether the long-term risk associated with nodal irradiation, particularly collateral heart and lung damage from internal mammary irradiation, outweigh the benefits of better disease control, Julia White, MD, a radiation oncologist at the Ohio State University Breast Center, Columbus, noted in an accompanying editorial.
Concerns stem originally from trials conducted from the 1950s to the 1970s. In those trials, higher doses of radiation were delivered to the internal mammary node with far less precision than today. Subsequent studies have not laid the worry to rest, and protocols vary across institutions, Dr. White explained. Some treat IM nodes in high-risk patients, but others only treat the axilla and the medial supraclavicular lymph nodes.
Dr. White said the new EORTC trial “moves us one step closer to resolving the debate about the value of internal mammary nodal [IMN] radiation.”
She noted that, since 2014, advances in the field have led to an almost 50% reduction in cardiac radiation exposure during breast cancer treatment. Current guidelines recommend that internal mammary nodes “should generally be treated” as part of postmastectomy radiotherapy but that cardiopulmonary complications are still possible even with improved techniques, she wrote.
Mostly grade 1 morbidity
Women in the study had stage I-III breast cancer with axillary node involvement and/or medially located primary tumors. The median age at study entry was 54 years. The patients were treated at 46 centers in 13 countries.
The group that received IM-MS irradiation after surgery received 50 Gy in 25 fractions over 5 weeks.
The cumulative 15-year incidence of lung fibrosis was 5.7% among treated women versus 2.9% among control patients. The incidence of cardiac fibrosis was 1.9% with treatment, versus 1.1%.
The incidence of any cardiac disease was 11.1% in the radiation arm versus 9.4% in the control group.
Complications were mostly of grade 1. The only statistically significant difference in rates of events of grade 2 or higher was in the incidence of pulmonary morbidity, which was 0.8% with radiation versus 0.1% without. There were no differences in the incidence of second malignancies, contralateral breast cancer cases, or cardiovascular deaths with IMN irradiation.
The authors noted that their results conflict with a 2013 study that found a relative increase in major coronary events of 7.4% per Gy mean heart dose. The women in that trial were treated in Sweden and Denmark between 1958 and 2001.
Dr. Poortmans and colleagues noted, however, that this 2013 study and others found a proportional and not an absolute increase in risk. With a baseline risk of 10%, for instance, a 7% increase per 1 Gy translates to a total risk of 10.07%.
Also, no increased risk has been reported in more recently published trials, and a meta-analysis found no increase in non–breast cancer–related mortality with trials that began after 1988.
Still, “it seems logical to take the preexisting cardiac comorbidity of patients into consideration,” the investigators concluded. For patients with higher baseline cardiopulmonary risk factors, lower mean heart doses should be used, and such patients should undergo longer-term follow-up.
The study was funded by La Ligue Nationale Contre Le Cancer and the KWF Kanker Bestrijding from the Netherlands. The investigators and Dr. White disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After a median follow-up 15.7 years among almost 4,000 women, for half of patients who received postoperative internal mammary and medial supraclavicular (IM-MS) lymph node irradiation, the “absolute rates and differences” of heart and lung complications “were very low, with no increased non–breast cancer–related mortality, even before introducing heart-sparing techniques,” said the investigators.
The findings come from the European Organization for Research and Treatment of Cancer (EORTC) trial. The investigators were led by Philip Poortmans, MD, PhD, a radiation oncologist at the University of Antwerp (Belgium).
The team had previously reported lower breast cancer mortality and breast cancer recurrence rates in the radiation group.
Women in the trial were treated from 1996 to 2004. “We expect that with contemporary volume-based radiation therapy outcomes will be even better, by improved coverage of target volumes, more homogeneous dose delivery and decreased doses to nontarget tissues,” the team wrote.
In the end, “our findings ... have important – reassuring – consequences for decision-making concerning elective lymph node treatment in breast cancer,” the researchers commented.
The study was published online on July 28, 2021, in the Journal of the National Cancer Institute.
Resolving the debate
There’s been debate for decades on whether the long-term risk associated with nodal irradiation, particularly collateral heart and lung damage from internal mammary irradiation, outweigh the benefits of better disease control, Julia White, MD, a radiation oncologist at the Ohio State University Breast Center, Columbus, noted in an accompanying editorial.
Concerns stem originally from trials conducted from the 1950s to the 1970s. In those trials, higher doses of radiation were delivered to the internal mammary node with far less precision than today. Subsequent studies have not laid the worry to rest, and protocols vary across institutions, Dr. White explained. Some treat IM nodes in high-risk patients, but others only treat the axilla and the medial supraclavicular lymph nodes.
Dr. White said the new EORTC trial “moves us one step closer to resolving the debate about the value of internal mammary nodal [IMN] radiation.”
She noted that, since 2014, advances in the field have led to an almost 50% reduction in cardiac radiation exposure during breast cancer treatment. Current guidelines recommend that internal mammary nodes “should generally be treated” as part of postmastectomy radiotherapy but that cardiopulmonary complications are still possible even with improved techniques, she wrote.
Mostly grade 1 morbidity
Women in the study had stage I-III breast cancer with axillary node involvement and/or medially located primary tumors. The median age at study entry was 54 years. The patients were treated at 46 centers in 13 countries.
The group that received IM-MS irradiation after surgery received 50 Gy in 25 fractions over 5 weeks.
The cumulative 15-year incidence of lung fibrosis was 5.7% among treated women versus 2.9% among control patients. The incidence of cardiac fibrosis was 1.9% with treatment, versus 1.1%.
The incidence of any cardiac disease was 11.1% in the radiation arm versus 9.4% in the control group.
Complications were mostly of grade 1. The only statistically significant difference in rates of events of grade 2 or higher was in the incidence of pulmonary morbidity, which was 0.8% with radiation versus 0.1% without. There were no differences in the incidence of second malignancies, contralateral breast cancer cases, or cardiovascular deaths with IMN irradiation.
The authors noted that their results conflict with a 2013 study that found a relative increase in major coronary events of 7.4% per Gy mean heart dose. The women in that trial were treated in Sweden and Denmark between 1958 and 2001.
Dr. Poortmans and colleagues noted, however, that this 2013 study and others found a proportional and not an absolute increase in risk. With a baseline risk of 10%, for instance, a 7% increase per 1 Gy translates to a total risk of 10.07%.
Also, no increased risk has been reported in more recently published trials, and a meta-analysis found no increase in non–breast cancer–related mortality with trials that began after 1988.
Still, “it seems logical to take the preexisting cardiac comorbidity of patients into consideration,” the investigators concluded. For patients with higher baseline cardiopulmonary risk factors, lower mean heart doses should be used, and such patients should undergo longer-term follow-up.
The study was funded by La Ligue Nationale Contre Le Cancer and the KWF Kanker Bestrijding from the Netherlands. The investigators and Dr. White disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After a median follow-up 15.7 years among almost 4,000 women, for half of patients who received postoperative internal mammary and medial supraclavicular (IM-MS) lymph node irradiation, the “absolute rates and differences” of heart and lung complications “were very low, with no increased non–breast cancer–related mortality, even before introducing heart-sparing techniques,” said the investigators.
The findings come from the European Organization for Research and Treatment of Cancer (EORTC) trial. The investigators were led by Philip Poortmans, MD, PhD, a radiation oncologist at the University of Antwerp (Belgium).
The team had previously reported lower breast cancer mortality and breast cancer recurrence rates in the radiation group.
Women in the trial were treated from 1996 to 2004. “We expect that with contemporary volume-based radiation therapy outcomes will be even better, by improved coverage of target volumes, more homogeneous dose delivery and decreased doses to nontarget tissues,” the team wrote.
In the end, “our findings ... have important – reassuring – consequences for decision-making concerning elective lymph node treatment in breast cancer,” the researchers commented.
The study was published online on July 28, 2021, in the Journal of the National Cancer Institute.
Resolving the debate
There’s been debate for decades on whether the long-term risk associated with nodal irradiation, particularly collateral heart and lung damage from internal mammary irradiation, outweigh the benefits of better disease control, Julia White, MD, a radiation oncologist at the Ohio State University Breast Center, Columbus, noted in an accompanying editorial.
Concerns stem originally from trials conducted from the 1950s to the 1970s. In those trials, higher doses of radiation were delivered to the internal mammary node with far less precision than today. Subsequent studies have not laid the worry to rest, and protocols vary across institutions, Dr. White explained. Some treat IM nodes in high-risk patients, but others only treat the axilla and the medial supraclavicular lymph nodes.
Dr. White said the new EORTC trial “moves us one step closer to resolving the debate about the value of internal mammary nodal [IMN] radiation.”
She noted that, since 2014, advances in the field have led to an almost 50% reduction in cardiac radiation exposure during breast cancer treatment. Current guidelines recommend that internal mammary nodes “should generally be treated” as part of postmastectomy radiotherapy but that cardiopulmonary complications are still possible even with improved techniques, she wrote.
Mostly grade 1 morbidity
Women in the study had stage I-III breast cancer with axillary node involvement and/or medially located primary tumors. The median age at study entry was 54 years. The patients were treated at 46 centers in 13 countries.
The group that received IM-MS irradiation after surgery received 50 Gy in 25 fractions over 5 weeks.
The cumulative 15-year incidence of lung fibrosis was 5.7% among treated women versus 2.9% among control patients. The incidence of cardiac fibrosis was 1.9% with treatment, versus 1.1%.
The incidence of any cardiac disease was 11.1% in the radiation arm versus 9.4% in the control group.
Complications were mostly of grade 1. The only statistically significant difference in rates of events of grade 2 or higher was in the incidence of pulmonary morbidity, which was 0.8% with radiation versus 0.1% without. There were no differences in the incidence of second malignancies, contralateral breast cancer cases, or cardiovascular deaths with IMN irradiation.
The authors noted that their results conflict with a 2013 study that found a relative increase in major coronary events of 7.4% per Gy mean heart dose. The women in that trial were treated in Sweden and Denmark between 1958 and 2001.
Dr. Poortmans and colleagues noted, however, that this 2013 study and others found a proportional and not an absolute increase in risk. With a baseline risk of 10%, for instance, a 7% increase per 1 Gy translates to a total risk of 10.07%.
Also, no increased risk has been reported in more recently published trials, and a meta-analysis found no increase in non–breast cancer–related mortality with trials that began after 1988.
Still, “it seems logical to take the preexisting cardiac comorbidity of patients into consideration,” the investigators concluded. For patients with higher baseline cardiopulmonary risk factors, lower mean heart doses should be used, and such patients should undergo longer-term follow-up.
The study was funded by La Ligue Nationale Contre Le Cancer and the KWF Kanker Bestrijding from the Netherlands. The investigators and Dr. White disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
How many years of aromatase inhibitor therapy in breast cancer?
That’s a question that has been vexing oncologists for more than a decade, but final results from a large study now suggest that, after an initial 5 years of adjuvant endocrine therapy with tamoxifen and/or an AI, an additional 5 years of AI therapy offer no clinical benefits, compared with an additional 2 years of therapy.
What’s more, 5 years of additional treatment were associated with a significantly increased risk for fractures, compared with 2 years of therapy, Michael Gnant, MD, from the Medical University of Vienna, and colleagues reported in the phase 3 Austrian Breast and Colorectal Cancer Study Group Trial 16 (ABCSG-16).
“Thus, in postmenopausal women with hormone receptor–positive breast cancer who had received 5 years of adjuvant endocrine therapy, the extension of aromatase inhibitor therapy for 2 years rather than 5 years was sufficient to maximize the benefits of such therapy in most patients without extending the exposure to toxic effects,” they concluded.
The study was published online on July 28, 2021, in the New England Journal of Medicine.
The new results “in patients with hormone-receptor–positive breast cancer who are at low or average risk underscore the importance of avoiding iatrogenic complications when making these decisions,” Pamela J. Goodwin, MD, from the Lunenfeld–Tanenbaum Research Institute at the University of Toronto, wrote in an accompanying editorial.
She noted that these latest results echo those of two earlier studies: the DATA trial and the optimistically named IDEAL trial, both of which showed no benefit of short-term versus longer-term adjuvant endocrine therapy with regard to either disease-free survival (DFS) or overall survival (OS).
“These results provide strong evidence against the routine use of more than 2 years of extended aromatase inhibitor therapy in women who are at low or average risk, similar to those who were included in this trial,” Dr. Goodwin commented.
ABCSG-16 details
Dr. Gnant and colleagues enrolled 3,484 women aged 80 years or younger with HR-positive stage I, II, or III breast cancer for whom there was no evidence of recurrence at baseline. The patients had all received adjuvant tamoxifen or an AI for 5 years; for some patients, the two drugs were administered in sequence until 12 months before randomization.
The patients were randomly assigned to receive oral anastrozole 1 mg daily for either 2 or 5 additional years.
Among all patients, 3,208 had experienced no recurrence 2 years after randomization. These patients were included in the primary DFS analysis. Of this group, 1,635 (51%) had received tamoxifen alone for 5 years, 235 (7.3%) had received an AI alone, and 41.7% had received sequential AIs and tamoxifen.
At a median follow-up of 118 months after randomization, disease progression or death occurred in 670 women – 335 in each trial arm.
The rate of DFS 10 years after randomization was 73.6% in the 2-year additional-therapy group and 73.9% in the 5-year group. The hazard ratio for disease recurrence or death was 0.99 and was not significant. After adjustment for potential confounding factors, the HR remained essentially unchanged (HR, 1.0).
Overall survival at 8 years, a secondary endpoint, was virtually identical between the groups, at 87.5% in the 2-year group and 87.3% in the 5-year group. The HR for death from any cause was 1.02 and was not significant.
The HR for contralateral breast cancer was 1.15, and the HR for a second primary cancer was 1.06; neither was statistically significant.
Although the use of bone-targeted medication was similar between the groups, among patients in the 2-year group, the incidence of clinical bone fractures 5 years after randomization was lower, at 4.7% versus 6.3%, which translates to an HR for fracture with 5 additional years of AI therapy of 1.35 (95% confidence interval, 1.00-1.84).
Adverse events with anastrozole were consistent with its known toxicity profile. At least one serious adverse event occurred in 26.5% of patients in the 2-year group and in 40.2% in the 5-year group. Investigator-assessed serious adverse events that were judged to be related to anastrozole occurred in 2.3% and 4.0% of patients, respectively.
Osteoarthritis, the most frequently reported adverse event, was documented in 1.7% of patients in the 2-year group and in 4.3% in the 5-year group.
AI duration still unclear
“We did not investigate the value of extending adjuvant endocrine therapy per se, since the benefit of extending aromatase inhibitors after 5 years of adjuvant tamoxifen has been well established. In contrast, the most effective duration of adjuvant aromatase inhibitor therapy remains unclear in randomized trials,” Dr. Gnant and colleagues commented.
In her editorial, Dr. Goodwin summarized potential approaches for improving late outcomes for patients with hormone receptor–positive breast cancers, including the use of biomarkers to detect minimal residual disease and therapies to treat it.
She noted that the detection of circulating tumor cells in patients with HR-positive breast cancer 5 years after diagnosis is associated with a 13-fold increase in risk for recurrence and a median time to clinical recurrence of 2.8 years.
“This interval may be sufficiently long that therapeutic intervention can prevent the development of incurable clinical metastases. Continued improvement in these assays and the development of new therapies that target the unique biologic features of dormant cells will no doubt be required for a major reduction in late recurrence risk,” she wrote.
The study was supported by AstraZeneca and the Austrian Breast and Colorectal Cancer Study Group. Dr. Gnant has received lecture fees from AstraZeneca and others. Dr. Goodwin has received institutional research funding from the Breast Cancer Research Foundation and EPIC Sciences.
A version of this article first appeared on Medscape.com.
That’s a question that has been vexing oncologists for more than a decade, but final results from a large study now suggest that, after an initial 5 years of adjuvant endocrine therapy with tamoxifen and/or an AI, an additional 5 years of AI therapy offer no clinical benefits, compared with an additional 2 years of therapy.
What’s more, 5 years of additional treatment were associated with a significantly increased risk for fractures, compared with 2 years of therapy, Michael Gnant, MD, from the Medical University of Vienna, and colleagues reported in the phase 3 Austrian Breast and Colorectal Cancer Study Group Trial 16 (ABCSG-16).
“Thus, in postmenopausal women with hormone receptor–positive breast cancer who had received 5 years of adjuvant endocrine therapy, the extension of aromatase inhibitor therapy for 2 years rather than 5 years was sufficient to maximize the benefits of such therapy in most patients without extending the exposure to toxic effects,” they concluded.
The study was published online on July 28, 2021, in the New England Journal of Medicine.
The new results “in patients with hormone-receptor–positive breast cancer who are at low or average risk underscore the importance of avoiding iatrogenic complications when making these decisions,” Pamela J. Goodwin, MD, from the Lunenfeld–Tanenbaum Research Institute at the University of Toronto, wrote in an accompanying editorial.
She noted that these latest results echo those of two earlier studies: the DATA trial and the optimistically named IDEAL trial, both of which showed no benefit of short-term versus longer-term adjuvant endocrine therapy with regard to either disease-free survival (DFS) or overall survival (OS).
“These results provide strong evidence against the routine use of more than 2 years of extended aromatase inhibitor therapy in women who are at low or average risk, similar to those who were included in this trial,” Dr. Goodwin commented.
ABCSG-16 details
Dr. Gnant and colleagues enrolled 3,484 women aged 80 years or younger with HR-positive stage I, II, or III breast cancer for whom there was no evidence of recurrence at baseline. The patients had all received adjuvant tamoxifen or an AI for 5 years; for some patients, the two drugs were administered in sequence until 12 months before randomization.
The patients were randomly assigned to receive oral anastrozole 1 mg daily for either 2 or 5 additional years.
Among all patients, 3,208 had experienced no recurrence 2 years after randomization. These patients were included in the primary DFS analysis. Of this group, 1,635 (51%) had received tamoxifen alone for 5 years, 235 (7.3%) had received an AI alone, and 41.7% had received sequential AIs and tamoxifen.
At a median follow-up of 118 months after randomization, disease progression or death occurred in 670 women – 335 in each trial arm.
The rate of DFS 10 years after randomization was 73.6% in the 2-year additional-therapy group and 73.9% in the 5-year group. The hazard ratio for disease recurrence or death was 0.99 and was not significant. After adjustment for potential confounding factors, the HR remained essentially unchanged (HR, 1.0).
Overall survival at 8 years, a secondary endpoint, was virtually identical between the groups, at 87.5% in the 2-year group and 87.3% in the 5-year group. The HR for death from any cause was 1.02 and was not significant.
The HR for contralateral breast cancer was 1.15, and the HR for a second primary cancer was 1.06; neither was statistically significant.
Although the use of bone-targeted medication was similar between the groups, among patients in the 2-year group, the incidence of clinical bone fractures 5 years after randomization was lower, at 4.7% versus 6.3%, which translates to an HR for fracture with 5 additional years of AI therapy of 1.35 (95% confidence interval, 1.00-1.84).
Adverse events with anastrozole were consistent with its known toxicity profile. At least one serious adverse event occurred in 26.5% of patients in the 2-year group and in 40.2% in the 5-year group. Investigator-assessed serious adverse events that were judged to be related to anastrozole occurred in 2.3% and 4.0% of patients, respectively.
Osteoarthritis, the most frequently reported adverse event, was documented in 1.7% of patients in the 2-year group and in 4.3% in the 5-year group.
AI duration still unclear
“We did not investigate the value of extending adjuvant endocrine therapy per se, since the benefit of extending aromatase inhibitors after 5 years of adjuvant tamoxifen has been well established. In contrast, the most effective duration of adjuvant aromatase inhibitor therapy remains unclear in randomized trials,” Dr. Gnant and colleagues commented.
In her editorial, Dr. Goodwin summarized potential approaches for improving late outcomes for patients with hormone receptor–positive breast cancers, including the use of biomarkers to detect minimal residual disease and therapies to treat it.
She noted that the detection of circulating tumor cells in patients with HR-positive breast cancer 5 years after diagnosis is associated with a 13-fold increase in risk for recurrence and a median time to clinical recurrence of 2.8 years.
“This interval may be sufficiently long that therapeutic intervention can prevent the development of incurable clinical metastases. Continued improvement in these assays and the development of new therapies that target the unique biologic features of dormant cells will no doubt be required for a major reduction in late recurrence risk,” she wrote.
The study was supported by AstraZeneca and the Austrian Breast and Colorectal Cancer Study Group. Dr. Gnant has received lecture fees from AstraZeneca and others. Dr. Goodwin has received institutional research funding from the Breast Cancer Research Foundation and EPIC Sciences.
A version of this article first appeared on Medscape.com.
That’s a question that has been vexing oncologists for more than a decade, but final results from a large study now suggest that, after an initial 5 years of adjuvant endocrine therapy with tamoxifen and/or an AI, an additional 5 years of AI therapy offer no clinical benefits, compared with an additional 2 years of therapy.
What’s more, 5 years of additional treatment were associated with a significantly increased risk for fractures, compared with 2 years of therapy, Michael Gnant, MD, from the Medical University of Vienna, and colleagues reported in the phase 3 Austrian Breast and Colorectal Cancer Study Group Trial 16 (ABCSG-16).
“Thus, in postmenopausal women with hormone receptor–positive breast cancer who had received 5 years of adjuvant endocrine therapy, the extension of aromatase inhibitor therapy for 2 years rather than 5 years was sufficient to maximize the benefits of such therapy in most patients without extending the exposure to toxic effects,” they concluded.
The study was published online on July 28, 2021, in the New England Journal of Medicine.
The new results “in patients with hormone-receptor–positive breast cancer who are at low or average risk underscore the importance of avoiding iatrogenic complications when making these decisions,” Pamela J. Goodwin, MD, from the Lunenfeld–Tanenbaum Research Institute at the University of Toronto, wrote in an accompanying editorial.
She noted that these latest results echo those of two earlier studies: the DATA trial and the optimistically named IDEAL trial, both of which showed no benefit of short-term versus longer-term adjuvant endocrine therapy with regard to either disease-free survival (DFS) or overall survival (OS).
“These results provide strong evidence against the routine use of more than 2 years of extended aromatase inhibitor therapy in women who are at low or average risk, similar to those who were included in this trial,” Dr. Goodwin commented.
ABCSG-16 details
Dr. Gnant and colleagues enrolled 3,484 women aged 80 years or younger with HR-positive stage I, II, or III breast cancer for whom there was no evidence of recurrence at baseline. The patients had all received adjuvant tamoxifen or an AI for 5 years; for some patients, the two drugs were administered in sequence until 12 months before randomization.
The patients were randomly assigned to receive oral anastrozole 1 mg daily for either 2 or 5 additional years.
Among all patients, 3,208 had experienced no recurrence 2 years after randomization. These patients were included in the primary DFS analysis. Of this group, 1,635 (51%) had received tamoxifen alone for 5 years, 235 (7.3%) had received an AI alone, and 41.7% had received sequential AIs and tamoxifen.
At a median follow-up of 118 months after randomization, disease progression or death occurred in 670 women – 335 in each trial arm.
The rate of DFS 10 years after randomization was 73.6% in the 2-year additional-therapy group and 73.9% in the 5-year group. The hazard ratio for disease recurrence or death was 0.99 and was not significant. After adjustment for potential confounding factors, the HR remained essentially unchanged (HR, 1.0).
Overall survival at 8 years, a secondary endpoint, was virtually identical between the groups, at 87.5% in the 2-year group and 87.3% in the 5-year group. The HR for death from any cause was 1.02 and was not significant.
The HR for contralateral breast cancer was 1.15, and the HR for a second primary cancer was 1.06; neither was statistically significant.
Although the use of bone-targeted medication was similar between the groups, among patients in the 2-year group, the incidence of clinical bone fractures 5 years after randomization was lower, at 4.7% versus 6.3%, which translates to an HR for fracture with 5 additional years of AI therapy of 1.35 (95% confidence interval, 1.00-1.84).
Adverse events with anastrozole were consistent with its known toxicity profile. At least one serious adverse event occurred in 26.5% of patients in the 2-year group and in 40.2% in the 5-year group. Investigator-assessed serious adverse events that were judged to be related to anastrozole occurred in 2.3% and 4.0% of patients, respectively.
Osteoarthritis, the most frequently reported adverse event, was documented in 1.7% of patients in the 2-year group and in 4.3% in the 5-year group.
AI duration still unclear
“We did not investigate the value of extending adjuvant endocrine therapy per se, since the benefit of extending aromatase inhibitors after 5 years of adjuvant tamoxifen has been well established. In contrast, the most effective duration of adjuvant aromatase inhibitor therapy remains unclear in randomized trials,” Dr. Gnant and colleagues commented.
In her editorial, Dr. Goodwin summarized potential approaches for improving late outcomes for patients with hormone receptor–positive breast cancers, including the use of biomarkers to detect minimal residual disease and therapies to treat it.
She noted that the detection of circulating tumor cells in patients with HR-positive breast cancer 5 years after diagnosis is associated with a 13-fold increase in risk for recurrence and a median time to clinical recurrence of 2.8 years.
“This interval may be sufficiently long that therapeutic intervention can prevent the development of incurable clinical metastases. Continued improvement in these assays and the development of new therapies that target the unique biologic features of dormant cells will no doubt be required for a major reduction in late recurrence risk,” she wrote.
The study was supported by AstraZeneca and the Austrian Breast and Colorectal Cancer Study Group. Dr. Gnant has received lecture fees from AstraZeneca and others. Dr. Goodwin has received institutional research funding from the Breast Cancer Research Foundation and EPIC Sciences.
A version of this article first appeared on Medscape.com.
One in three cancer articles on social media has wrong info
Of the 200 most popular articles (50 each for prostate, lung, breast, and colorectal cancer), about a third (32.5%, n = 65) contained misinformation.
Among these articles containing misinformation, 76.9% (50/65) contained harmful information.
“The Internet is a leading source of health misinformation,” the study authors wrote. This is “particularly true for social media, where false information spreads faster and more broadly than fact-checked information,” they said, citing other research.
“We need to address these issues head on,” said lead author Skyler Johnson, MD, of the University of Utah’s Huntsman Cancer Institute in Salt Lake City.
“As a medical community, we can’t ignore the problem of cancer misinformation on social media or ask our patients to ignore it. We must empathize with our patients and help them when they encounter this type of information,” he said in a statement. “My goal is to help answer their questions, and provide cancer patients with accurate information that will give them the best chance for the best outcome.”
The study was published online July 22 in the Journal of the National Cancer Institute.
The study period ran from 2018 to 2019, and looked at articles posted on social media platforms Facebook, Reddit, Twitter, or Pinterest. Popularity was measured by engagement with readers, such as upvotes, comments, reactions, and shares.
Some of the articles came from long-established news entities such as CBS News, The New York Times, and medical journals, while others came from fleeting crowdfunding web pages and fledging nontraditional news sites.
One example of popular and harmful misinformation highlighted by Dr. Johnson in an interview was titled, “44-Year-Old Mother Claims CBD Oil Cured Her of Breast Cancer within 5 Months.” Posted on truththeory.com in February 2018, the article is tagged as “opinion” by the publisher and in turn links to another news story about the same woman in the UK’s Daily Mail newspaper.
The ideas and claims in such articles can be very influential, Jennifer L. Lycette, MD, suggested in a recent blog post.
“After 18 years as a cancer doctor, it sadly doesn’t come as a surprise anymore when a patient declines treatment recommendations and instead opts for ‘alternative’ treatment,” she wrote.
Sometimes, misinformation is not sensational but is still effective via clever wording and presentation, observed Brian G. Southwell, PhD, of Duke University, Durham, N.C., who has studied patients and misinformation.
“It isn’t the falsehood that is somehow magically attractive, per se, but the way that misinformation is often framed that can make it attractive,” he said in an interview.
Dr. Southwell recommends that clinicians be proactive about medical misinformation.
“Rather than expect patients to raise concerns without prompting, health care providers should invite conversations about potential misinformation with their patients,” he wrote in a recent essay in the American Journal of Public Health.
In short, ask patients what they know about the treatment of their cancer, he suggests.
“Patients don’t typically know that the misinformation they are encountering is misinformation,” said Dr. Southwell. “Approaching patients with compassion and empathy is a good first step.”
Study details
For the study, reported by Johnson et al., two National Comprehensive Cancer Network panel members were selected as content experts for each of the four cancers and were tasked with reviewing the primary medical claims in each article. The experts then completed a set of ratings to arrive at the proportion of misinformation and potential for harm in each article.
Of the 200 articles, 41.5% were from nontraditional news (digital only), 37.5% were from traditional news sources (online versions of print and/or broadcast media), 17% were from medical journals, 3% were from a crowdfunding site, and 1% were from personal blogs.
This expert review concluded that nearly one-third of the articles contained misinformation, as noted above. The misinformation was described as misleading (title not supported by text or statistics/data do not support conclusion, 28.8%), strength of the evidence mischaracterized (weak evidence portrayed as strong or vice versa, 27.7%) and unproven therapies (not studied or insufficient evidence, 26.7%).
Notably, the median number of engagements, such as likes on Twitter, for articles with misinformation was greater than that of factual articles (median, 2,300 vs. 1,600; P = .05).
In total, 30.5% of all 200 articles contained harmful information. This was described as harmful inaction (could lead to delay or not seeking medical attention for treatable/curable condition, 31.0%), economic harm (out-of-pocket financial costs associated with treatment/travel, 27.7%), harmful action (potentially toxic effects of the suggested test/treatment, 17.0%), and harmful interactions (known/unknown medical interactions with curative therapies, 16.2%).
The median number of engagements for articles with harmful information was statistically significantly greater than that of articles with correct information (median, 2,300 vs. 1,500; P = .007).
A limitation of the study is that it included only the most popular English language cancer articles.
This study was funded in part by the Huntsman Cancer Institute. Dr. Johnson, Dr. Lycette, and Dr. Southwell have disclosed no relevant financial relationships. Some study authors have ties to the pharmaceutical industry.
A version of this article first appeared on Medscape.com.
Of the 200 most popular articles (50 each for prostate, lung, breast, and colorectal cancer), about a third (32.5%, n = 65) contained misinformation.
Among these articles containing misinformation, 76.9% (50/65) contained harmful information.
“The Internet is a leading source of health misinformation,” the study authors wrote. This is “particularly true for social media, where false information spreads faster and more broadly than fact-checked information,” they said, citing other research.
“We need to address these issues head on,” said lead author Skyler Johnson, MD, of the University of Utah’s Huntsman Cancer Institute in Salt Lake City.
“As a medical community, we can’t ignore the problem of cancer misinformation on social media or ask our patients to ignore it. We must empathize with our patients and help them when they encounter this type of information,” he said in a statement. “My goal is to help answer their questions, and provide cancer patients with accurate information that will give them the best chance for the best outcome.”
The study was published online July 22 in the Journal of the National Cancer Institute.
The study period ran from 2018 to 2019, and looked at articles posted on social media platforms Facebook, Reddit, Twitter, or Pinterest. Popularity was measured by engagement with readers, such as upvotes, comments, reactions, and shares.
Some of the articles came from long-established news entities such as CBS News, The New York Times, and medical journals, while others came from fleeting crowdfunding web pages and fledging nontraditional news sites.
One example of popular and harmful misinformation highlighted by Dr. Johnson in an interview was titled, “44-Year-Old Mother Claims CBD Oil Cured Her of Breast Cancer within 5 Months.” Posted on truththeory.com in February 2018, the article is tagged as “opinion” by the publisher and in turn links to another news story about the same woman in the UK’s Daily Mail newspaper.
The ideas and claims in such articles can be very influential, Jennifer L. Lycette, MD, suggested in a recent blog post.
“After 18 years as a cancer doctor, it sadly doesn’t come as a surprise anymore when a patient declines treatment recommendations and instead opts for ‘alternative’ treatment,” she wrote.
Sometimes, misinformation is not sensational but is still effective via clever wording and presentation, observed Brian G. Southwell, PhD, of Duke University, Durham, N.C., who has studied patients and misinformation.
“It isn’t the falsehood that is somehow magically attractive, per se, but the way that misinformation is often framed that can make it attractive,” he said in an interview.
Dr. Southwell recommends that clinicians be proactive about medical misinformation.
“Rather than expect patients to raise concerns without prompting, health care providers should invite conversations about potential misinformation with their patients,” he wrote in a recent essay in the American Journal of Public Health.
In short, ask patients what they know about the treatment of their cancer, he suggests.
“Patients don’t typically know that the misinformation they are encountering is misinformation,” said Dr. Southwell. “Approaching patients with compassion and empathy is a good first step.”
Study details
For the study, reported by Johnson et al., two National Comprehensive Cancer Network panel members were selected as content experts for each of the four cancers and were tasked with reviewing the primary medical claims in each article. The experts then completed a set of ratings to arrive at the proportion of misinformation and potential for harm in each article.
Of the 200 articles, 41.5% were from nontraditional news (digital only), 37.5% were from traditional news sources (online versions of print and/or broadcast media), 17% were from medical journals, 3% were from a crowdfunding site, and 1% were from personal blogs.
This expert review concluded that nearly one-third of the articles contained misinformation, as noted above. The misinformation was described as misleading (title not supported by text or statistics/data do not support conclusion, 28.8%), strength of the evidence mischaracterized (weak evidence portrayed as strong or vice versa, 27.7%) and unproven therapies (not studied or insufficient evidence, 26.7%).
Notably, the median number of engagements, such as likes on Twitter, for articles with misinformation was greater than that of factual articles (median, 2,300 vs. 1,600; P = .05).
In total, 30.5% of all 200 articles contained harmful information. This was described as harmful inaction (could lead to delay or not seeking medical attention for treatable/curable condition, 31.0%), economic harm (out-of-pocket financial costs associated with treatment/travel, 27.7%), harmful action (potentially toxic effects of the suggested test/treatment, 17.0%), and harmful interactions (known/unknown medical interactions with curative therapies, 16.2%).
The median number of engagements for articles with harmful information was statistically significantly greater than that of articles with correct information (median, 2,300 vs. 1,500; P = .007).
A limitation of the study is that it included only the most popular English language cancer articles.
This study was funded in part by the Huntsman Cancer Institute. Dr. Johnson, Dr. Lycette, and Dr. Southwell have disclosed no relevant financial relationships. Some study authors have ties to the pharmaceutical industry.
A version of this article first appeared on Medscape.com.
Of the 200 most popular articles (50 each for prostate, lung, breast, and colorectal cancer), about a third (32.5%, n = 65) contained misinformation.
Among these articles containing misinformation, 76.9% (50/65) contained harmful information.
“The Internet is a leading source of health misinformation,” the study authors wrote. This is “particularly true for social media, where false information spreads faster and more broadly than fact-checked information,” they said, citing other research.
“We need to address these issues head on,” said lead author Skyler Johnson, MD, of the University of Utah’s Huntsman Cancer Institute in Salt Lake City.
“As a medical community, we can’t ignore the problem of cancer misinformation on social media or ask our patients to ignore it. We must empathize with our patients and help them when they encounter this type of information,” he said in a statement. “My goal is to help answer their questions, and provide cancer patients with accurate information that will give them the best chance for the best outcome.”
The study was published online July 22 in the Journal of the National Cancer Institute.
The study period ran from 2018 to 2019, and looked at articles posted on social media platforms Facebook, Reddit, Twitter, or Pinterest. Popularity was measured by engagement with readers, such as upvotes, comments, reactions, and shares.
Some of the articles came from long-established news entities such as CBS News, The New York Times, and medical journals, while others came from fleeting crowdfunding web pages and fledging nontraditional news sites.
One example of popular and harmful misinformation highlighted by Dr. Johnson in an interview was titled, “44-Year-Old Mother Claims CBD Oil Cured Her of Breast Cancer within 5 Months.” Posted on truththeory.com in February 2018, the article is tagged as “opinion” by the publisher and in turn links to another news story about the same woman in the UK’s Daily Mail newspaper.
The ideas and claims in such articles can be very influential, Jennifer L. Lycette, MD, suggested in a recent blog post.
“After 18 years as a cancer doctor, it sadly doesn’t come as a surprise anymore when a patient declines treatment recommendations and instead opts for ‘alternative’ treatment,” she wrote.
Sometimes, misinformation is not sensational but is still effective via clever wording and presentation, observed Brian G. Southwell, PhD, of Duke University, Durham, N.C., who has studied patients and misinformation.
“It isn’t the falsehood that is somehow magically attractive, per se, but the way that misinformation is often framed that can make it attractive,” he said in an interview.
Dr. Southwell recommends that clinicians be proactive about medical misinformation.
“Rather than expect patients to raise concerns without prompting, health care providers should invite conversations about potential misinformation with their patients,” he wrote in a recent essay in the American Journal of Public Health.
In short, ask patients what they know about the treatment of their cancer, he suggests.
“Patients don’t typically know that the misinformation they are encountering is misinformation,” said Dr. Southwell. “Approaching patients with compassion and empathy is a good first step.”
Study details
For the study, reported by Johnson et al., two National Comprehensive Cancer Network panel members were selected as content experts for each of the four cancers and were tasked with reviewing the primary medical claims in each article. The experts then completed a set of ratings to arrive at the proportion of misinformation and potential for harm in each article.
Of the 200 articles, 41.5% were from nontraditional news (digital only), 37.5% were from traditional news sources (online versions of print and/or broadcast media), 17% were from medical journals, 3% were from a crowdfunding site, and 1% were from personal blogs.
This expert review concluded that nearly one-third of the articles contained misinformation, as noted above. The misinformation was described as misleading (title not supported by text or statistics/data do not support conclusion, 28.8%), strength of the evidence mischaracterized (weak evidence portrayed as strong or vice versa, 27.7%) and unproven therapies (not studied or insufficient evidence, 26.7%).
Notably, the median number of engagements, such as likes on Twitter, for articles with misinformation was greater than that of factual articles (median, 2,300 vs. 1,600; P = .05).
In total, 30.5% of all 200 articles contained harmful information. This was described as harmful inaction (could lead to delay or not seeking medical attention for treatable/curable condition, 31.0%), economic harm (out-of-pocket financial costs associated with treatment/travel, 27.7%), harmful action (potentially toxic effects of the suggested test/treatment, 17.0%), and harmful interactions (known/unknown medical interactions with curative therapies, 16.2%).
The median number of engagements for articles with harmful information was statistically significantly greater than that of articles with correct information (median, 2,300 vs. 1,500; P = .007).
A limitation of the study is that it included only the most popular English language cancer articles.
This study was funded in part by the Huntsman Cancer Institute. Dr. Johnson, Dr. Lycette, and Dr. Southwell have disclosed no relevant financial relationships. Some study authors have ties to the pharmaceutical industry.
A version of this article first appeared on Medscape.com.
FDA approves neoadjuvant pembro for triple-negative breast cancer
This approval is based on findings from the randomized, phase 3 KEYNOTE-522 trial, which showed significantly prolonged event-free survival with the pembrolizumab regimen versus neoadjuvant chemotherapy alone for previously untreated stage II-III TNBC.
This is the 30th indication for pembrolizumab in the United States.
The immunotherapy received accelerated approval in November 2020 for adjuvant use in locally recurrent unresectable or metastatic TNBC for patients whose tumors express programmed death–ligand-1, as determined by an FDA-approved test. That accelerated approval was based on results from the phase 3 KEYNOTE-355 trial. The approval has now been converted to a full approval on the basis of confirmatory data from the KEYNOTE-522, notes a statement from the manufacturer, Merck.
“Triple-negative is a difficult-to-treat type of breast cancer that unfortunately is more common in the U.S. in younger women and in Black women,” commented Vicki Goodman, MD, vice president of clinical research, Merck Research Laboratories. “We are proud to offer a new treatment option for patients faced with this challenging cancer. This neoadjuvant and adjuvant combination with pembrolizumab is the first immunotherapy regimen to be approved in high-risk early-stage TNBC, marking a meaningful milestone for the breast cancer community.”
In KEYNOTE-522, participants were randomly assigned to receive either placebo or pembrolizumab plus chemotherapy with carboplatin and paclitaxel, followed by doxorubicin or epirubicin and cyclophosphamide before surgery, as well as placebo or pembrolizumab as single-agent therapy after surgery.
The results from this trial, first reported in 2019 at the annual meeting of the European Society of Medical Oncology, showed that, for patients in the pembrolizumab arm of the trial, the pathological complete response rate was nearly 65% versus 51% among the patients who received placebo. The benefit was seen both in those whose tumors were positive and those whose tumors were negative for PD-L1 expression.
Among patients in the pembrolizumab arm, there was a 37% reduction in the risk for disease progression that precluded definitive surgery, a local/distant recurrence, a second primary cancer, or death from any cause (hazard ratio, 0.63).
Pembrolizumab can be associated with immune-mediated adverse reactions that may be severe or fatal, Merck noted.
These events “can occur in any organ system or tissue and can affect more than one body system simultaneously. Immune-mediated adverse reactions can occur at any time during or after treatment,” Merck warned. The company states: “Early identification and management of immune-mediated adverse reactions are essential.”
Treatment may need to be withheld or permanently discontinued, and corticosteroids may be needed, depending on the severity of the adverse reaction, according to the statement.
Infusion-related reactions can also occur. Because of its mechanism of action, pembrolizumab can cause fetal harm when administered to women during pregnancy.
A version of this article first appeared on Medscape.com.
This approval is based on findings from the randomized, phase 3 KEYNOTE-522 trial, which showed significantly prolonged event-free survival with the pembrolizumab regimen versus neoadjuvant chemotherapy alone for previously untreated stage II-III TNBC.
This is the 30th indication for pembrolizumab in the United States.
The immunotherapy received accelerated approval in November 2020 for adjuvant use in locally recurrent unresectable or metastatic TNBC for patients whose tumors express programmed death–ligand-1, as determined by an FDA-approved test. That accelerated approval was based on results from the phase 3 KEYNOTE-355 trial. The approval has now been converted to a full approval on the basis of confirmatory data from the KEYNOTE-522, notes a statement from the manufacturer, Merck.
“Triple-negative is a difficult-to-treat type of breast cancer that unfortunately is more common in the U.S. in younger women and in Black women,” commented Vicki Goodman, MD, vice president of clinical research, Merck Research Laboratories. “We are proud to offer a new treatment option for patients faced with this challenging cancer. This neoadjuvant and adjuvant combination with pembrolizumab is the first immunotherapy regimen to be approved in high-risk early-stage TNBC, marking a meaningful milestone for the breast cancer community.”
In KEYNOTE-522, participants were randomly assigned to receive either placebo or pembrolizumab plus chemotherapy with carboplatin and paclitaxel, followed by doxorubicin or epirubicin and cyclophosphamide before surgery, as well as placebo or pembrolizumab as single-agent therapy after surgery.
The results from this trial, first reported in 2019 at the annual meeting of the European Society of Medical Oncology, showed that, for patients in the pembrolizumab arm of the trial, the pathological complete response rate was nearly 65% versus 51% among the patients who received placebo. The benefit was seen both in those whose tumors were positive and those whose tumors were negative for PD-L1 expression.
Among patients in the pembrolizumab arm, there was a 37% reduction in the risk for disease progression that precluded definitive surgery, a local/distant recurrence, a second primary cancer, or death from any cause (hazard ratio, 0.63).
Pembrolizumab can be associated with immune-mediated adverse reactions that may be severe or fatal, Merck noted.
These events “can occur in any organ system or tissue and can affect more than one body system simultaneously. Immune-mediated adverse reactions can occur at any time during or after treatment,” Merck warned. The company states: “Early identification and management of immune-mediated adverse reactions are essential.”
Treatment may need to be withheld or permanently discontinued, and corticosteroids may be needed, depending on the severity of the adverse reaction, according to the statement.
Infusion-related reactions can also occur. Because of its mechanism of action, pembrolizumab can cause fetal harm when administered to women during pregnancy.
A version of this article first appeared on Medscape.com.
This approval is based on findings from the randomized, phase 3 KEYNOTE-522 trial, which showed significantly prolonged event-free survival with the pembrolizumab regimen versus neoadjuvant chemotherapy alone for previously untreated stage II-III TNBC.
This is the 30th indication for pembrolizumab in the United States.
The immunotherapy received accelerated approval in November 2020 for adjuvant use in locally recurrent unresectable or metastatic TNBC for patients whose tumors express programmed death–ligand-1, as determined by an FDA-approved test. That accelerated approval was based on results from the phase 3 KEYNOTE-355 trial. The approval has now been converted to a full approval on the basis of confirmatory data from the KEYNOTE-522, notes a statement from the manufacturer, Merck.
“Triple-negative is a difficult-to-treat type of breast cancer that unfortunately is more common in the U.S. in younger women and in Black women,” commented Vicki Goodman, MD, vice president of clinical research, Merck Research Laboratories. “We are proud to offer a new treatment option for patients faced with this challenging cancer. This neoadjuvant and adjuvant combination with pembrolizumab is the first immunotherapy regimen to be approved in high-risk early-stage TNBC, marking a meaningful milestone for the breast cancer community.”
In KEYNOTE-522, participants were randomly assigned to receive either placebo or pembrolizumab plus chemotherapy with carboplatin and paclitaxel, followed by doxorubicin or epirubicin and cyclophosphamide before surgery, as well as placebo or pembrolizumab as single-agent therapy after surgery.
The results from this trial, first reported in 2019 at the annual meeting of the European Society of Medical Oncology, showed that, for patients in the pembrolizumab arm of the trial, the pathological complete response rate was nearly 65% versus 51% among the patients who received placebo. The benefit was seen both in those whose tumors were positive and those whose tumors were negative for PD-L1 expression.
Among patients in the pembrolizumab arm, there was a 37% reduction in the risk for disease progression that precluded definitive surgery, a local/distant recurrence, a second primary cancer, or death from any cause (hazard ratio, 0.63).
Pembrolizumab can be associated with immune-mediated adverse reactions that may be severe or fatal, Merck noted.
These events “can occur in any organ system or tissue and can affect more than one body system simultaneously. Immune-mediated adverse reactions can occur at any time during or after treatment,” Merck warned. The company states: “Early identification and management of immune-mediated adverse reactions are essential.”
Treatment may need to be withheld or permanently discontinued, and corticosteroids may be needed, depending on the severity of the adverse reaction, according to the statement.
Infusion-related reactions can also occur. Because of its mechanism of action, pembrolizumab can cause fetal harm when administered to women during pregnancy.
A version of this article first appeared on Medscape.com.