User login
Most breast cancer screening centers not following guidelines
, say researchers reporting on a new analysis.
They assessed 606 centers and report that, among the centers that recommended a starting age for screening mammography, nearly 90% advised women to begin screening at age 40 years and to continue annually.
This contrasts with the current recommendations from the U.S. Preventive Services Task Force (USPSTF) on mammography screening, which stipulate starting at age 50 years and continuing every 2 years.
The new analysis was published online in JAMA Internal Medicine.
This may be doing “more harm than good,” warn the authors of an accompanying editorial.
“The recommendation for annual mammography in women younger than 50 years is, at best, confusing for patients and is likely to conflict with advice from their primary care physicians, which can create tension,” write Anand R. Habib, MD, MPhil; Deborah Grady, MD; and Rita F. Redberg, MD, all from the University of California, San Francisco.
“This recommendation can also produce unnecessary testing, invasive procedures, overdiagnosis, and anxiety among women who receive screening,” they write.
“Breast cancer centers with clear financial benefits from increased mammography rates may wish to reconsider offering recommendations that create greater referral volume but conflict with unbiased evidence-based USPSTF guidelines and have the potential to increase harms among women,” the editorialists add.
The age at which to start mammography screening has long been a contentious issue, with some experts and medical societies arguing that it should begin at 40.
The American College of Radiology, the Society of Breast Imaging, and the American Society of Breast Surgeons recommend that women start annual mammography screening at age 40.
The American Cancer Society’s guidelines recommend an initial screening mammogram between ages 45 and 55 and after that, screening every 1-2 years.
One expert who argues for starting at 40 years is Laurie Margolies, MD, chief of breast imaging, Mount Sinai Health System, and professor of radiology, Icahn School of Medicine at Mount Sinai, New York.
In a statement, she noted that 17% of all breast cancers are diagnosed in women in their 40s and that the majority of these women are not considered to be at high risk of developing the disease.
“Our own Mount Sinai research has shown that women with screen-detected breast cancers are less likely to need a mastectomy and are less likely to require chemotherapy or axillary node dissection,” Dr. Margolies said.
“Additionally, women who get regular breast cancer screening have a 47% lower risk of breast cancer death within 20 years of diagnosis than those not regularly screened. Skipping a mammogram can have lethal consequences,” she said.
Details of the analysis
The analysis of recommendations by breast cancer centers regarding screening mammography was carried out by Jennifer L. Marti, MD, from Weill Cornell Medicine, New York, and colleagues.
They examined 606 centers and found that 487 (80.4%) offered screening recommendations on their public websites.
Of 431 centers that recommended a starting age, 376 centers (87.2%) advised women to begin screening at age 40 years; 35 centers (8.1%) recommended beginning at age 45 years; and the remaining 20 centers (4.6%) recommended that screening begin at age 50 years.
A total of 429 centers recommended both a starting age and a screening interval. Of this group, 347 centers (80.9%) advised that annual screening begin at age 40 years. Only 16 centers (3.3%) recommended biennial mammography (as per the USPSTF guidelines). Almost three-quarters (72.7%, n = 354) recommended annual screening; 59 centers (12.1%) recommended annual or biennial screening; and 58 centers (11.9%) recommended a discussion with a physician.
The authors note that there were differences between centers according to National Cancer Institute designation, but these differences did not reach statistical significance.
Dr. Marti and coauthors, Dr. Habib and coauthors, and Dr. Margolies have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, say researchers reporting on a new analysis.
They assessed 606 centers and report that, among the centers that recommended a starting age for screening mammography, nearly 90% advised women to begin screening at age 40 years and to continue annually.
This contrasts with the current recommendations from the U.S. Preventive Services Task Force (USPSTF) on mammography screening, which stipulate starting at age 50 years and continuing every 2 years.
The new analysis was published online in JAMA Internal Medicine.
This may be doing “more harm than good,” warn the authors of an accompanying editorial.
“The recommendation for annual mammography in women younger than 50 years is, at best, confusing for patients and is likely to conflict with advice from their primary care physicians, which can create tension,” write Anand R. Habib, MD, MPhil; Deborah Grady, MD; and Rita F. Redberg, MD, all from the University of California, San Francisco.
“This recommendation can also produce unnecessary testing, invasive procedures, overdiagnosis, and anxiety among women who receive screening,” they write.
“Breast cancer centers with clear financial benefits from increased mammography rates may wish to reconsider offering recommendations that create greater referral volume but conflict with unbiased evidence-based USPSTF guidelines and have the potential to increase harms among women,” the editorialists add.
The age at which to start mammography screening has long been a contentious issue, with some experts and medical societies arguing that it should begin at 40.
The American College of Radiology, the Society of Breast Imaging, and the American Society of Breast Surgeons recommend that women start annual mammography screening at age 40.
The American Cancer Society’s guidelines recommend an initial screening mammogram between ages 45 and 55 and after that, screening every 1-2 years.
One expert who argues for starting at 40 years is Laurie Margolies, MD, chief of breast imaging, Mount Sinai Health System, and professor of radiology, Icahn School of Medicine at Mount Sinai, New York.
In a statement, she noted that 17% of all breast cancers are diagnosed in women in their 40s and that the majority of these women are not considered to be at high risk of developing the disease.
“Our own Mount Sinai research has shown that women with screen-detected breast cancers are less likely to need a mastectomy and are less likely to require chemotherapy or axillary node dissection,” Dr. Margolies said.
“Additionally, women who get regular breast cancer screening have a 47% lower risk of breast cancer death within 20 years of diagnosis than those not regularly screened. Skipping a mammogram can have lethal consequences,” she said.
Details of the analysis
The analysis of recommendations by breast cancer centers regarding screening mammography was carried out by Jennifer L. Marti, MD, from Weill Cornell Medicine, New York, and colleagues.
They examined 606 centers and found that 487 (80.4%) offered screening recommendations on their public websites.
Of 431 centers that recommended a starting age, 376 centers (87.2%) advised women to begin screening at age 40 years; 35 centers (8.1%) recommended beginning at age 45 years; and the remaining 20 centers (4.6%) recommended that screening begin at age 50 years.
A total of 429 centers recommended both a starting age and a screening interval. Of this group, 347 centers (80.9%) advised that annual screening begin at age 40 years. Only 16 centers (3.3%) recommended biennial mammography (as per the USPSTF guidelines). Almost three-quarters (72.7%, n = 354) recommended annual screening; 59 centers (12.1%) recommended annual or biennial screening; and 58 centers (11.9%) recommended a discussion with a physician.
The authors note that there were differences between centers according to National Cancer Institute designation, but these differences did not reach statistical significance.
Dr. Marti and coauthors, Dr. Habib and coauthors, and Dr. Margolies have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, say researchers reporting on a new analysis.
They assessed 606 centers and report that, among the centers that recommended a starting age for screening mammography, nearly 90% advised women to begin screening at age 40 years and to continue annually.
This contrasts with the current recommendations from the U.S. Preventive Services Task Force (USPSTF) on mammography screening, which stipulate starting at age 50 years and continuing every 2 years.
The new analysis was published online in JAMA Internal Medicine.
This may be doing “more harm than good,” warn the authors of an accompanying editorial.
“The recommendation for annual mammography in women younger than 50 years is, at best, confusing for patients and is likely to conflict with advice from their primary care physicians, which can create tension,” write Anand R. Habib, MD, MPhil; Deborah Grady, MD; and Rita F. Redberg, MD, all from the University of California, San Francisco.
“This recommendation can also produce unnecessary testing, invasive procedures, overdiagnosis, and anxiety among women who receive screening,” they write.
“Breast cancer centers with clear financial benefits from increased mammography rates may wish to reconsider offering recommendations that create greater referral volume but conflict with unbiased evidence-based USPSTF guidelines and have the potential to increase harms among women,” the editorialists add.
The age at which to start mammography screening has long been a contentious issue, with some experts and medical societies arguing that it should begin at 40.
The American College of Radiology, the Society of Breast Imaging, and the American Society of Breast Surgeons recommend that women start annual mammography screening at age 40.
The American Cancer Society’s guidelines recommend an initial screening mammogram between ages 45 and 55 and after that, screening every 1-2 years.
One expert who argues for starting at 40 years is Laurie Margolies, MD, chief of breast imaging, Mount Sinai Health System, and professor of radiology, Icahn School of Medicine at Mount Sinai, New York.
In a statement, she noted that 17% of all breast cancers are diagnosed in women in their 40s and that the majority of these women are not considered to be at high risk of developing the disease.
“Our own Mount Sinai research has shown that women with screen-detected breast cancers are less likely to need a mastectomy and are less likely to require chemotherapy or axillary node dissection,” Dr. Margolies said.
“Additionally, women who get regular breast cancer screening have a 47% lower risk of breast cancer death within 20 years of diagnosis than those not regularly screened. Skipping a mammogram can have lethal consequences,” she said.
Details of the analysis
The analysis of recommendations by breast cancer centers regarding screening mammography was carried out by Jennifer L. Marti, MD, from Weill Cornell Medicine, New York, and colleagues.
They examined 606 centers and found that 487 (80.4%) offered screening recommendations on their public websites.
Of 431 centers that recommended a starting age, 376 centers (87.2%) advised women to begin screening at age 40 years; 35 centers (8.1%) recommended beginning at age 45 years; and the remaining 20 centers (4.6%) recommended that screening begin at age 50 years.
A total of 429 centers recommended both a starting age and a screening interval. Of this group, 347 centers (80.9%) advised that annual screening begin at age 40 years. Only 16 centers (3.3%) recommended biennial mammography (as per the USPSTF guidelines). Almost three-quarters (72.7%, n = 354) recommended annual screening; 59 centers (12.1%) recommended annual or biennial screening; and 58 centers (11.9%) recommended a discussion with a physician.
The authors note that there were differences between centers according to National Cancer Institute designation, but these differences did not reach statistical significance.
Dr. Marti and coauthors, Dr. Habib and coauthors, and Dr. Margolies have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA scrutinizes cancer therapies granted accelerated approval
U.S. regulators are stepping up scrutiny of therapies that were granted an accelerated approval to treat cancers on the basis of surrogate endpoints but have failed to show clinical or survival benefits upon more extensive testing.
At issue are a number of cancer indications for immunotherapies. Four have already been withdrawn (voluntarily by the manufacturer), and six more will be reviewed at an upcoming meeting.
In recent years, the US Food and Drug Administration has granted accelerated approvals to oncology medicines on the basis of evidence that suggests a benefit for patients. Examples of such evidence relate to response rates and estimates of tumor shrinkage. But these approvals are granted on the condition that the manufacturer conducts larger clinical trials that show clinical benefit, including benefit in overall survival.
Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, has argued that the point of these conditional approvals is to find acceptable surrogate markers to allow people with “desperate illnesses” to have access to potentially helpful drugs while work continues to determine the drug’s actual benefit to patients.
Oncologists are now questioning whether the FDA has become too lenient in its approach, Daniel A. Goldstein, MD, a senior physician in medical oncology and internal medicine at the Rabin Medical Center, Petah Tikva, Israel, told this news organization.
“The main two things you want from a cancer drug is to live longer and live a higher quality of life,” said Goldstein. “But these endpoints that they’ve been using over the past few years are not really giving us confidence that these drugs are actually going to help to live longer or better.”
Dr. Pazdur said the FDA will consider withdrawing its accelerated approvals when results of further studies do not confirm expected benefit for patients.
“This is like the pendulum has swung as far as it was going to swing and now is on the backswing,” said Dr. Goldstein, also of the department of health policy and management at the University of North Carolina at Chapel Hill. “You could call this a watershed moment.”
Although there’s near universal interest in allowing people with advanced cancer access to promising medicines, there’s also rising concern about exposing patients needlessly to costly drugs with potentially tough side effects. That may prompt a shift in the standards U.S. regulators apply to cancer medicines, Dr. Goldstein said.
Indications withdrawn and under review
In a meeting scheduled for April 27-29, the FDA’s Oncologic Drugs Advisory Committee will review indications granted through the accelerated approval process for three immunotherapies: pembrolizumab (Keytruda), atezolizumab (Tecentriq), and nivolumab (Opdivo).
It is part of an industry-wide evaluation of accelerated approvals for cancer indications in which confirmatory trials did not confirm clinical benefit, the FDA noted.
The process has already led to voluntary withdrawals of four cancer indications by the manufacturers, including one indication each for pembrolizumab, atezolizumab, and nivolumab, and one for durvalumab (Imfinzi).
All of these immunotherapies are approved for numerous cancer indications, and they all remain on the market. It is only the U.S. approvals for particular cancer indications that have been withdrawn.
In the past, olaratumab (Lartruvo) was withdrawn from the market altogether. The FDA granted accelerated approval of the drug for soft tissue sarcoma, but clinical benefit was not confirmed in a phase 3 trial.
Issue highlighted by Dr. Prasad and Dr. Gyawali
In recent years, much of the attention on accelerated approvals was spurred by the work of a few researchers, particularly Vinay Prasad, MD, MPH, associate professor in the department of epidemiology and biostatistics, University of California, San Francisco, and Bishal Gyawali, MD, PhD, from Queen’s University Cancer Research Institute, Kingston, Ont. (Both are regular contributors to the oncology section of this news organization.)
Dr. Goldstein made this point in a tweet about the FDA’s announcement of the April ODAC meetings:
“Well done to @oncology_bg and @VPrasadMDMPH among others for highlighting in their papers that the FDA wasn’t properly evaluating accelerated approval drugs.
FDA have listened.
And I thought that the impact of academia was limited!”
Dr. Prasad has made the case for closer scrutiny of accelerated approvals in a number of journal articles and in his 2020 book, “Malignant: How Bad Policy and Bad Evidence Harm People with Cancer,” published by Johns Hopkins University Press.
The book includes highlights of a 2016 article published in Mayo Clinic Proceedings that focused on surrogate endpoints used for FDA approvals. In the article, Dr. Prasad and his coauthor report that they did not find formal analyses of the strength of the surrogate-survival correlation in 14 of 25 cases of accelerated approvals (56%) and in 11 of 30 traditional approvals (37%).
“Our results were concerning. They imply that many surrogates are based on little more than a gut feeling. You might rationalize that and argue a gut feeling is the same as ‘reasonably likely to predict,’ but no reasonable person could think a gut feeling means established,” Dr. Prasad writes in his book. “Our result suggests the FDA is using surrogate endpoints far beyond what may be fair or reasonable.”
Dr. Gyawali has argued that the process by which the FDA assesses cancer drugs for approvals has undergone a profound shift. He has most recently remarked on this in an October 2020 commentary on Medscape.
“Until the recent floodgate of approvals based on response rates from single-arm trials, the majority of cancer therapy decisions were supported by evidence generated from randomized controlled trials (RCTs),” Dr. Gyawali wrote. “The evidence base to support clinical decisions in managing therapeutic side effects has been comparatively sparse.”
Accelerated approval to improve access
The FDA has struggled for about 2 decades with questions of where to set the bar on evidence for promising cancer drugs.
The agency’s accelerated approval program for drugs began in 1992. During the first decade, the focus was largely on medicines related to HIV.
In the early 2000s, oncology drugs began to dominate the program.
Dr. Pazdur has presided over the FDA’s marked changes regarding the use of surrogate markers when weighing whether to allow sales of cancer medicines. Formerly a professor at the University of Texas MD Anderson Cancer Center, Houston, Dr. Pazdur joined the FDA as director of the Division of Oncology Drug Products in 1999.
Soon after his appointment, he had to field inquiries from pharmaceutical companies about how much evidence they needed to receive accelerated approvals.
Early on, he publicly expressed impatience about the drugmakers’ approach. “The purpose of accelerated approval was not accelerated drug company profits,” Dr. Padzur said at a 2004 ODAC meeting.
Rather, the point is to allow access to potentially helpful drugs while work continues to determine their actual benefit to patients, he explained.
“It wasn’t a license to do less, less, less, and less to a point now that we may be getting companies that are coming in” intent on determining the minimum evidence the FDA will take, Dr. Pazdur said. “It shouldn’t be what is the lowest. It is what is a sufficient amount to give patients and physicians a real understanding of what their drug will do.”
In a 2016 interview with The New York Times, Dr. Pazdur said that his views on cancer drug approvals have evolved with time. He described himself as being “on a jihad to streamline the review process and get things out the door faster.”
“I have evolved from regulator to regulator-advocate,” Dr. Pazdur told the newspaper.
His attitude reflected his personal experience in losing his wife to ovarian cancer in 2015, as well as shifts in science and law. In 2012, Congress passed a law that gave the FDA new resources to speed medicines for life-threatening diseases to market. In addition, advances in genetics appeared to be making some medications more effective and easier to test, Dr. Pazdur said in The New York Times interview.
Withdrawals seen as sign of success
Since the program’s inception, only 6% of accelerated approvals for oncology indications have been withdrawn, the FDA said.
It would be a sign that the program is working if the April meetings lead to further withdrawals of indications that have been granted accelerated approval, Julie R. Gralow, MD, chief medical officer of the American Society of Clinical Oncology, said in an interview with this news organization.
“It shouldn’t be seen as a failure,” Dr. Gralow said.
In her own practice at the Fred Hutchinson Cancer Research Center, Seattle, she has seen the value of emerging therapies for patients fighting advanced cancers. During her 25 years of clinical practice in an academic setting, she has gained access to drugs through single-patient investigative new drug applications.
However, this path is not an option for many patients who undergo treatment in facilities other than academic centers, she commented. She noted that the accelerated approval process is a way to expand access to emerging medicines, but she sees a need for caution in the use of drugs that have been given only this conditional approval. She emphasizes that such drugs may be suitable only for certain patients.
“I would say that, for metastatic patients, patients with incurable disease, we are willing to take some risk,” Dr. Gralow said. “We don’t have other options. They can’t wait the years that it would take to get a drug approved.”
One such patient is David Mitchell, who serves as the consumer representative on ODAC. He told this news organization that he is taking three drugs for multiple myeloma that received accelerated approvals: pomalidomide, bortezomib, and daratumumab.
“I want the FDA to have the option to approve drugs in an accelerated pathway, because as a patient taking three drugs granted accelerated approval, I’m benefiting – I’ve lived the benefit,” Mr. Mitchell said, “and I want other patients to have the opportunity to have that benefit.”
He believes that the FDA’s approach regarding accelerated approvals serves to get potentially beneficial medicines to patients who have few options and also fulfills the FDA’s mandate to protect the public from treatments that have little benefit but can cause harm.
Accelerated approval also offers needed flexibility to drugmakers as they develop more specifically targeted drugs for diseases that affect relatively few people, such as multiple myeloma, he said. “As the targeting of your therapies gets tighter and for smaller groups of patients, you have a harder time following the traditional model,” such as conducting large, double-blind, placebo-controlled trials that may indicate increased overall survival, he said.
“To me, this is the way the FDA intended it to work,” he added. “It’s going to offer the accelerated approval based on a surrogate endpoint for a safe drug, but it’s going to require the confirmatory trial, and if the confirmatory trial fails, it will pull the drug off the market.”
Some medicines that have received accelerated approvals may ultimately be found not to benefit patients, Mr. Mitchell acknowledged. But people in his situation, whose disease has progressed despite treatments, may want to take that risk, he added.
Four cancer indications recently withdrawn voluntarily by the manufacturer
- December 2020: Nivolumab for the treatment of patients with metastatic small cell lung cancer with progression after platinum-based chemotherapy and at least one other line of therapy (Bristol Myers Squibb).
- February 2021: Durvalumab for the treatment of patients with locally advanced or metastatic urothelial carcinoma whose disease has progressed during or following platinum-based chemotherapy or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy (AstraZeneca).
- March 2021: Pembrolizumab for the treatment of patients with metastatic small cell lung cancer with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy (Merck).
- March 2021: Atezolizumab for treatment of patients with locally advanced or metastatic urothelial carcinoma who experience disease progression during or following platinum-containing atezolizumab chemotherapy or disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy (Genentech).
Six cancer indications under review at the April 2021 ODAC meeting
- Atezolizumab indicated in combination with protein-bound for the treatment of adults with unresectable locally advanced or metastatic triple-negative whose tumors express PD-L1 (PD-L1 stained tumor-infiltrating immune cells of any intensity covering ≥1% of the tumor area), as determined by an FDA-approved test.
- Atezolizumab indicated for patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1 (Combined Positive Score ≥1), as determined by an FDA-approved test, with disease progression on or after two or more prior lines of therapy including fluoropyrimidine- and platinum-containing chemotherapy and if appropriate, HER2/neu-targeted therapy.
- Pembrolizumab indicated for the treatment of patients with who have been previously treated with .
- Nivolumab indicated as a single agent for the treatment of patients with hepatocellular carcinoma who have been previously treated with sorafenib.
A version of this article first appeared on Medscape.com.
U.S. regulators are stepping up scrutiny of therapies that were granted an accelerated approval to treat cancers on the basis of surrogate endpoints but have failed to show clinical or survival benefits upon more extensive testing.
At issue are a number of cancer indications for immunotherapies. Four have already been withdrawn (voluntarily by the manufacturer), and six more will be reviewed at an upcoming meeting.
In recent years, the US Food and Drug Administration has granted accelerated approvals to oncology medicines on the basis of evidence that suggests a benefit for patients. Examples of such evidence relate to response rates and estimates of tumor shrinkage. But these approvals are granted on the condition that the manufacturer conducts larger clinical trials that show clinical benefit, including benefit in overall survival.
Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, has argued that the point of these conditional approvals is to find acceptable surrogate markers to allow people with “desperate illnesses” to have access to potentially helpful drugs while work continues to determine the drug’s actual benefit to patients.
Oncologists are now questioning whether the FDA has become too lenient in its approach, Daniel A. Goldstein, MD, a senior physician in medical oncology and internal medicine at the Rabin Medical Center, Petah Tikva, Israel, told this news organization.
“The main two things you want from a cancer drug is to live longer and live a higher quality of life,” said Goldstein. “But these endpoints that they’ve been using over the past few years are not really giving us confidence that these drugs are actually going to help to live longer or better.”
Dr. Pazdur said the FDA will consider withdrawing its accelerated approvals when results of further studies do not confirm expected benefit for patients.
“This is like the pendulum has swung as far as it was going to swing and now is on the backswing,” said Dr. Goldstein, also of the department of health policy and management at the University of North Carolina at Chapel Hill. “You could call this a watershed moment.”
Although there’s near universal interest in allowing people with advanced cancer access to promising medicines, there’s also rising concern about exposing patients needlessly to costly drugs with potentially tough side effects. That may prompt a shift in the standards U.S. regulators apply to cancer medicines, Dr. Goldstein said.
Indications withdrawn and under review
In a meeting scheduled for April 27-29, the FDA’s Oncologic Drugs Advisory Committee will review indications granted through the accelerated approval process for three immunotherapies: pembrolizumab (Keytruda), atezolizumab (Tecentriq), and nivolumab (Opdivo).
It is part of an industry-wide evaluation of accelerated approvals for cancer indications in which confirmatory trials did not confirm clinical benefit, the FDA noted.
The process has already led to voluntary withdrawals of four cancer indications by the manufacturers, including one indication each for pembrolizumab, atezolizumab, and nivolumab, and one for durvalumab (Imfinzi).
All of these immunotherapies are approved for numerous cancer indications, and they all remain on the market. It is only the U.S. approvals for particular cancer indications that have been withdrawn.
In the past, olaratumab (Lartruvo) was withdrawn from the market altogether. The FDA granted accelerated approval of the drug for soft tissue sarcoma, but clinical benefit was not confirmed in a phase 3 trial.
Issue highlighted by Dr. Prasad and Dr. Gyawali
In recent years, much of the attention on accelerated approvals was spurred by the work of a few researchers, particularly Vinay Prasad, MD, MPH, associate professor in the department of epidemiology and biostatistics, University of California, San Francisco, and Bishal Gyawali, MD, PhD, from Queen’s University Cancer Research Institute, Kingston, Ont. (Both are regular contributors to the oncology section of this news organization.)
Dr. Goldstein made this point in a tweet about the FDA’s announcement of the April ODAC meetings:
“Well done to @oncology_bg and @VPrasadMDMPH among others for highlighting in their papers that the FDA wasn’t properly evaluating accelerated approval drugs.
FDA have listened.
And I thought that the impact of academia was limited!”
Dr. Prasad has made the case for closer scrutiny of accelerated approvals in a number of journal articles and in his 2020 book, “Malignant: How Bad Policy and Bad Evidence Harm People with Cancer,” published by Johns Hopkins University Press.
The book includes highlights of a 2016 article published in Mayo Clinic Proceedings that focused on surrogate endpoints used for FDA approvals. In the article, Dr. Prasad and his coauthor report that they did not find formal analyses of the strength of the surrogate-survival correlation in 14 of 25 cases of accelerated approvals (56%) and in 11 of 30 traditional approvals (37%).
“Our results were concerning. They imply that many surrogates are based on little more than a gut feeling. You might rationalize that and argue a gut feeling is the same as ‘reasonably likely to predict,’ but no reasonable person could think a gut feeling means established,” Dr. Prasad writes in his book. “Our result suggests the FDA is using surrogate endpoints far beyond what may be fair or reasonable.”
Dr. Gyawali has argued that the process by which the FDA assesses cancer drugs for approvals has undergone a profound shift. He has most recently remarked on this in an October 2020 commentary on Medscape.
“Until the recent floodgate of approvals based on response rates from single-arm trials, the majority of cancer therapy decisions were supported by evidence generated from randomized controlled trials (RCTs),” Dr. Gyawali wrote. “The evidence base to support clinical decisions in managing therapeutic side effects has been comparatively sparse.”
Accelerated approval to improve access
The FDA has struggled for about 2 decades with questions of where to set the bar on evidence for promising cancer drugs.
The agency’s accelerated approval program for drugs began in 1992. During the first decade, the focus was largely on medicines related to HIV.
In the early 2000s, oncology drugs began to dominate the program.
Dr. Pazdur has presided over the FDA’s marked changes regarding the use of surrogate markers when weighing whether to allow sales of cancer medicines. Formerly a professor at the University of Texas MD Anderson Cancer Center, Houston, Dr. Pazdur joined the FDA as director of the Division of Oncology Drug Products in 1999.
Soon after his appointment, he had to field inquiries from pharmaceutical companies about how much evidence they needed to receive accelerated approvals.
Early on, he publicly expressed impatience about the drugmakers’ approach. “The purpose of accelerated approval was not accelerated drug company profits,” Dr. Padzur said at a 2004 ODAC meeting.
Rather, the point is to allow access to potentially helpful drugs while work continues to determine their actual benefit to patients, he explained.
“It wasn’t a license to do less, less, less, and less to a point now that we may be getting companies that are coming in” intent on determining the minimum evidence the FDA will take, Dr. Pazdur said. “It shouldn’t be what is the lowest. It is what is a sufficient amount to give patients and physicians a real understanding of what their drug will do.”
In a 2016 interview with The New York Times, Dr. Pazdur said that his views on cancer drug approvals have evolved with time. He described himself as being “on a jihad to streamline the review process and get things out the door faster.”
“I have evolved from regulator to regulator-advocate,” Dr. Pazdur told the newspaper.
His attitude reflected his personal experience in losing his wife to ovarian cancer in 2015, as well as shifts in science and law. In 2012, Congress passed a law that gave the FDA new resources to speed medicines for life-threatening diseases to market. In addition, advances in genetics appeared to be making some medications more effective and easier to test, Dr. Pazdur said in The New York Times interview.
Withdrawals seen as sign of success
Since the program’s inception, only 6% of accelerated approvals for oncology indications have been withdrawn, the FDA said.
It would be a sign that the program is working if the April meetings lead to further withdrawals of indications that have been granted accelerated approval, Julie R. Gralow, MD, chief medical officer of the American Society of Clinical Oncology, said in an interview with this news organization.
“It shouldn’t be seen as a failure,” Dr. Gralow said.
In her own practice at the Fred Hutchinson Cancer Research Center, Seattle, she has seen the value of emerging therapies for patients fighting advanced cancers. During her 25 years of clinical practice in an academic setting, she has gained access to drugs through single-patient investigative new drug applications.
However, this path is not an option for many patients who undergo treatment in facilities other than academic centers, she commented. She noted that the accelerated approval process is a way to expand access to emerging medicines, but she sees a need for caution in the use of drugs that have been given only this conditional approval. She emphasizes that such drugs may be suitable only for certain patients.
“I would say that, for metastatic patients, patients with incurable disease, we are willing to take some risk,” Dr. Gralow said. “We don’t have other options. They can’t wait the years that it would take to get a drug approved.”
One such patient is David Mitchell, who serves as the consumer representative on ODAC. He told this news organization that he is taking three drugs for multiple myeloma that received accelerated approvals: pomalidomide, bortezomib, and daratumumab.
“I want the FDA to have the option to approve drugs in an accelerated pathway, because as a patient taking three drugs granted accelerated approval, I’m benefiting – I’ve lived the benefit,” Mr. Mitchell said, “and I want other patients to have the opportunity to have that benefit.”
He believes that the FDA’s approach regarding accelerated approvals serves to get potentially beneficial medicines to patients who have few options and also fulfills the FDA’s mandate to protect the public from treatments that have little benefit but can cause harm.
Accelerated approval also offers needed flexibility to drugmakers as they develop more specifically targeted drugs for diseases that affect relatively few people, such as multiple myeloma, he said. “As the targeting of your therapies gets tighter and for smaller groups of patients, you have a harder time following the traditional model,” such as conducting large, double-blind, placebo-controlled trials that may indicate increased overall survival, he said.
“To me, this is the way the FDA intended it to work,” he added. “It’s going to offer the accelerated approval based on a surrogate endpoint for a safe drug, but it’s going to require the confirmatory trial, and if the confirmatory trial fails, it will pull the drug off the market.”
Some medicines that have received accelerated approvals may ultimately be found not to benefit patients, Mr. Mitchell acknowledged. But people in his situation, whose disease has progressed despite treatments, may want to take that risk, he added.
Four cancer indications recently withdrawn voluntarily by the manufacturer
- December 2020: Nivolumab for the treatment of patients with metastatic small cell lung cancer with progression after platinum-based chemotherapy and at least one other line of therapy (Bristol Myers Squibb).
- February 2021: Durvalumab for the treatment of patients with locally advanced or metastatic urothelial carcinoma whose disease has progressed during or following platinum-based chemotherapy or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy (AstraZeneca).
- March 2021: Pembrolizumab for the treatment of patients with metastatic small cell lung cancer with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy (Merck).
- March 2021: Atezolizumab for treatment of patients with locally advanced or metastatic urothelial carcinoma who experience disease progression during or following platinum-containing atezolizumab chemotherapy or disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy (Genentech).
Six cancer indications under review at the April 2021 ODAC meeting
- Atezolizumab indicated in combination with protein-bound for the treatment of adults with unresectable locally advanced or metastatic triple-negative whose tumors express PD-L1 (PD-L1 stained tumor-infiltrating immune cells of any intensity covering ≥1% of the tumor area), as determined by an FDA-approved test.
- Atezolizumab indicated for patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1 (Combined Positive Score ≥1), as determined by an FDA-approved test, with disease progression on or after two or more prior lines of therapy including fluoropyrimidine- and platinum-containing chemotherapy and if appropriate, HER2/neu-targeted therapy.
- Pembrolizumab indicated for the treatment of patients with who have been previously treated with .
- Nivolumab indicated as a single agent for the treatment of patients with hepatocellular carcinoma who have been previously treated with sorafenib.
A version of this article first appeared on Medscape.com.
U.S. regulators are stepping up scrutiny of therapies that were granted an accelerated approval to treat cancers on the basis of surrogate endpoints but have failed to show clinical or survival benefits upon more extensive testing.
At issue are a number of cancer indications for immunotherapies. Four have already been withdrawn (voluntarily by the manufacturer), and six more will be reviewed at an upcoming meeting.
In recent years, the US Food and Drug Administration has granted accelerated approvals to oncology medicines on the basis of evidence that suggests a benefit for patients. Examples of such evidence relate to response rates and estimates of tumor shrinkage. But these approvals are granted on the condition that the manufacturer conducts larger clinical trials that show clinical benefit, including benefit in overall survival.
Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, has argued that the point of these conditional approvals is to find acceptable surrogate markers to allow people with “desperate illnesses” to have access to potentially helpful drugs while work continues to determine the drug’s actual benefit to patients.
Oncologists are now questioning whether the FDA has become too lenient in its approach, Daniel A. Goldstein, MD, a senior physician in medical oncology and internal medicine at the Rabin Medical Center, Petah Tikva, Israel, told this news organization.
“The main two things you want from a cancer drug is to live longer and live a higher quality of life,” said Goldstein. “But these endpoints that they’ve been using over the past few years are not really giving us confidence that these drugs are actually going to help to live longer or better.”
Dr. Pazdur said the FDA will consider withdrawing its accelerated approvals when results of further studies do not confirm expected benefit for patients.
“This is like the pendulum has swung as far as it was going to swing and now is on the backswing,” said Dr. Goldstein, also of the department of health policy and management at the University of North Carolina at Chapel Hill. “You could call this a watershed moment.”
Although there’s near universal interest in allowing people with advanced cancer access to promising medicines, there’s also rising concern about exposing patients needlessly to costly drugs with potentially tough side effects. That may prompt a shift in the standards U.S. regulators apply to cancer medicines, Dr. Goldstein said.
Indications withdrawn and under review
In a meeting scheduled for April 27-29, the FDA’s Oncologic Drugs Advisory Committee will review indications granted through the accelerated approval process for three immunotherapies: pembrolizumab (Keytruda), atezolizumab (Tecentriq), and nivolumab (Opdivo).
It is part of an industry-wide evaluation of accelerated approvals for cancer indications in which confirmatory trials did not confirm clinical benefit, the FDA noted.
The process has already led to voluntary withdrawals of four cancer indications by the manufacturers, including one indication each for pembrolizumab, atezolizumab, and nivolumab, and one for durvalumab (Imfinzi).
All of these immunotherapies are approved for numerous cancer indications, and they all remain on the market. It is only the U.S. approvals for particular cancer indications that have been withdrawn.
In the past, olaratumab (Lartruvo) was withdrawn from the market altogether. The FDA granted accelerated approval of the drug for soft tissue sarcoma, but clinical benefit was not confirmed in a phase 3 trial.
Issue highlighted by Dr. Prasad and Dr. Gyawali
In recent years, much of the attention on accelerated approvals was spurred by the work of a few researchers, particularly Vinay Prasad, MD, MPH, associate professor in the department of epidemiology and biostatistics, University of California, San Francisco, and Bishal Gyawali, MD, PhD, from Queen’s University Cancer Research Institute, Kingston, Ont. (Both are regular contributors to the oncology section of this news organization.)
Dr. Goldstein made this point in a tweet about the FDA’s announcement of the April ODAC meetings:
“Well done to @oncology_bg and @VPrasadMDMPH among others for highlighting in their papers that the FDA wasn’t properly evaluating accelerated approval drugs.
FDA have listened.
And I thought that the impact of academia was limited!”
Dr. Prasad has made the case for closer scrutiny of accelerated approvals in a number of journal articles and in his 2020 book, “Malignant: How Bad Policy and Bad Evidence Harm People with Cancer,” published by Johns Hopkins University Press.
The book includes highlights of a 2016 article published in Mayo Clinic Proceedings that focused on surrogate endpoints used for FDA approvals. In the article, Dr. Prasad and his coauthor report that they did not find formal analyses of the strength of the surrogate-survival correlation in 14 of 25 cases of accelerated approvals (56%) and in 11 of 30 traditional approvals (37%).
“Our results were concerning. They imply that many surrogates are based on little more than a gut feeling. You might rationalize that and argue a gut feeling is the same as ‘reasonably likely to predict,’ but no reasonable person could think a gut feeling means established,” Dr. Prasad writes in his book. “Our result suggests the FDA is using surrogate endpoints far beyond what may be fair or reasonable.”
Dr. Gyawali has argued that the process by which the FDA assesses cancer drugs for approvals has undergone a profound shift. He has most recently remarked on this in an October 2020 commentary on Medscape.
“Until the recent floodgate of approvals based on response rates from single-arm trials, the majority of cancer therapy decisions were supported by evidence generated from randomized controlled trials (RCTs),” Dr. Gyawali wrote. “The evidence base to support clinical decisions in managing therapeutic side effects has been comparatively sparse.”
Accelerated approval to improve access
The FDA has struggled for about 2 decades with questions of where to set the bar on evidence for promising cancer drugs.
The agency’s accelerated approval program for drugs began in 1992. During the first decade, the focus was largely on medicines related to HIV.
In the early 2000s, oncology drugs began to dominate the program.
Dr. Pazdur has presided over the FDA’s marked changes regarding the use of surrogate markers when weighing whether to allow sales of cancer medicines. Formerly a professor at the University of Texas MD Anderson Cancer Center, Houston, Dr. Pazdur joined the FDA as director of the Division of Oncology Drug Products in 1999.
Soon after his appointment, he had to field inquiries from pharmaceutical companies about how much evidence they needed to receive accelerated approvals.
Early on, he publicly expressed impatience about the drugmakers’ approach. “The purpose of accelerated approval was not accelerated drug company profits,” Dr. Padzur said at a 2004 ODAC meeting.
Rather, the point is to allow access to potentially helpful drugs while work continues to determine their actual benefit to patients, he explained.
“It wasn’t a license to do less, less, less, and less to a point now that we may be getting companies that are coming in” intent on determining the minimum evidence the FDA will take, Dr. Pazdur said. “It shouldn’t be what is the lowest. It is what is a sufficient amount to give patients and physicians a real understanding of what their drug will do.”
In a 2016 interview with The New York Times, Dr. Pazdur said that his views on cancer drug approvals have evolved with time. He described himself as being “on a jihad to streamline the review process and get things out the door faster.”
“I have evolved from regulator to regulator-advocate,” Dr. Pazdur told the newspaper.
His attitude reflected his personal experience in losing his wife to ovarian cancer in 2015, as well as shifts in science and law. In 2012, Congress passed a law that gave the FDA new resources to speed medicines for life-threatening diseases to market. In addition, advances in genetics appeared to be making some medications more effective and easier to test, Dr. Pazdur said in The New York Times interview.
Withdrawals seen as sign of success
Since the program’s inception, only 6% of accelerated approvals for oncology indications have been withdrawn, the FDA said.
It would be a sign that the program is working if the April meetings lead to further withdrawals of indications that have been granted accelerated approval, Julie R. Gralow, MD, chief medical officer of the American Society of Clinical Oncology, said in an interview with this news organization.
“It shouldn’t be seen as a failure,” Dr. Gralow said.
In her own practice at the Fred Hutchinson Cancer Research Center, Seattle, she has seen the value of emerging therapies for patients fighting advanced cancers. During her 25 years of clinical practice in an academic setting, she has gained access to drugs through single-patient investigative new drug applications.
However, this path is not an option for many patients who undergo treatment in facilities other than academic centers, she commented. She noted that the accelerated approval process is a way to expand access to emerging medicines, but she sees a need for caution in the use of drugs that have been given only this conditional approval. She emphasizes that such drugs may be suitable only for certain patients.
“I would say that, for metastatic patients, patients with incurable disease, we are willing to take some risk,” Dr. Gralow said. “We don’t have other options. They can’t wait the years that it would take to get a drug approved.”
One such patient is David Mitchell, who serves as the consumer representative on ODAC. He told this news organization that he is taking three drugs for multiple myeloma that received accelerated approvals: pomalidomide, bortezomib, and daratumumab.
“I want the FDA to have the option to approve drugs in an accelerated pathway, because as a patient taking three drugs granted accelerated approval, I’m benefiting – I’ve lived the benefit,” Mr. Mitchell said, “and I want other patients to have the opportunity to have that benefit.”
He believes that the FDA’s approach regarding accelerated approvals serves to get potentially beneficial medicines to patients who have few options and also fulfills the FDA’s mandate to protect the public from treatments that have little benefit but can cause harm.
Accelerated approval also offers needed flexibility to drugmakers as they develop more specifically targeted drugs for diseases that affect relatively few people, such as multiple myeloma, he said. “As the targeting of your therapies gets tighter and for smaller groups of patients, you have a harder time following the traditional model,” such as conducting large, double-blind, placebo-controlled trials that may indicate increased overall survival, he said.
“To me, this is the way the FDA intended it to work,” he added. “It’s going to offer the accelerated approval based on a surrogate endpoint for a safe drug, but it’s going to require the confirmatory trial, and if the confirmatory trial fails, it will pull the drug off the market.”
Some medicines that have received accelerated approvals may ultimately be found not to benefit patients, Mr. Mitchell acknowledged. But people in his situation, whose disease has progressed despite treatments, may want to take that risk, he added.
Four cancer indications recently withdrawn voluntarily by the manufacturer
- December 2020: Nivolumab for the treatment of patients with metastatic small cell lung cancer with progression after platinum-based chemotherapy and at least one other line of therapy (Bristol Myers Squibb).
- February 2021: Durvalumab for the treatment of patients with locally advanced or metastatic urothelial carcinoma whose disease has progressed during or following platinum-based chemotherapy or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy (AstraZeneca).
- March 2021: Pembrolizumab for the treatment of patients with metastatic small cell lung cancer with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy (Merck).
- March 2021: Atezolizumab for treatment of patients with locally advanced or metastatic urothelial carcinoma who experience disease progression during or following platinum-containing atezolizumab chemotherapy or disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy (Genentech).
Six cancer indications under review at the April 2021 ODAC meeting
- Atezolizumab indicated in combination with protein-bound for the treatment of adults with unresectable locally advanced or metastatic triple-negative whose tumors express PD-L1 (PD-L1 stained tumor-infiltrating immune cells of any intensity covering ≥1% of the tumor area), as determined by an FDA-approved test.
- Atezolizumab indicated for patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1 (Combined Positive Score ≥1), as determined by an FDA-approved test, with disease progression on or after two or more prior lines of therapy including fluoropyrimidine- and platinum-containing chemotherapy and if appropriate, HER2/neu-targeted therapy.
- Pembrolizumab indicated for the treatment of patients with who have been previously treated with .
- Nivolumab indicated as a single agent for the treatment of patients with hepatocellular carcinoma who have been previously treated with sorafenib.
A version of this article first appeared on Medscape.com.
SNP chips deemed ‘extremely unreliable’ for identifying rare variants
In fact, SNP chips are “extremely unreliable for genotyping very rare pathogenic variants,” and a positive result for such a variant “is more likely to be wrong than right,” researchers reported in the BMJ.
The authors explained that SNP chips are “DNA microarrays that test genetic variation at many hundreds of thousands of specific locations across the genome.” Although SNP chips have proven accurate in identifying common variants, past reports have suggested that SNP chips perform poorly for genotyping rare variants.
To gain more insight, Caroline Wright, PhD, of the University of Exeter (England) and colleagues conducted a large study.
The researchers analyzed data on 49,908 people from the UK Biobank who had SNP chip and next-generation sequencing results, as well as an additional 21 people who purchased consumer genetic tests and shared their data online via the Personal Genome Project.
The researchers compared the SNP chip and sequencing results. They also selected rare pathogenic variants in BRCA1 and BRCA2 for detailed analysis of clinically actionable variants in the UK Biobank, and they assessed BRCA-related cancers in participants using cancer registry data.
Largest evaluation of SNP chips
SNP chips performed well for common variants, the researchers found. Sensitivity, specificity, positive-predictive value, and negative-predictive value all exceeded 99% for 108,574 common variants.
For rare variants, SNP chips performed poorly, with a positive-predictive value of 16% for variants with a frequency below 0.001% in the UK Biobank.
“The study provides the largest evaluation of the performance of SNP chips for genotyping genetic variants at different frequencies in the population, particularly focusing on very rare variants,” Dr. Wright said. “The biggest surprise was how poorly the SNP chips we evaluated performed for rare variants.”
Dr. Wright noted that there is an inherent problem built into using SNP chip technology to genotype very rare variants.
“The SNP chip technology relies on clustering data from multiple individuals in order to determine what genotype each individual has at a specific position in their genome,” Dr. Wright explained. “Although this method works very well for common variants, the rarer the variant, the harder it is to distinguish from experimental noise.”
False positives and cancer: ‘Don’t trust the results’
The researchers found that, for rare BRCA variants (frequency below 0.01%), SNP chips had a sensitivity of 34.6%, specificity of 98.3%, negative-predictive value of 99.9%, and positive-predictive value of 4.2%.
Rates of BRCA-related cancers in patients with positive SNP chip results were similar to rates in age-matched control subjects because “the vast majority of variants were false positives,” the researchers noted.
“If these variants are incorrectly genotyped – that is, false positives detected – a woman could be offered screening or even prophylactic surgery inappropriately when she is more likely to be at population background risk [for BRCA-related cancers],” Dr. Wright said.
“For very-rare-disease–causing genetic variants, don’t trust the results from SNP chips; for example, those from direct-to-consumer genetic tests. Never use them to guide clinical action without diagnostic validation,” she added.
Heather Hampel, a genetic counselor and researcher at the Ohio State University Comprehensive Cancer Center in Columbus, agreed.
“Positive results on SNP-based tests need to be confirmed by medical-grade genetic testing using a sequencing technology,” she said. “Negative results on an SNP- based test cannot be considered to rule out mutations in BRCA1/2 or other cancer-susceptibility genes, so individuals with strong personal and family histories of cancer should be seen by a genetic counselor to consider medical-grade genetic testing using a sequencing technology.”
Practicing oncologists can trust patients’ prior germline genetic test results if the testing was performed in a cancer genetics clinic, which uses sequencing-based technologies, Ms. Hampel noted.
“If the test was performed before 2013, there are likely new genes that have been discovered for which their patient was not tested, and repeat testing may be warranted,” Ms. Hampel said. “A referral to a cancer genetic counselor would be appropriate.”
Ms. Hampel disclosed relationships with Genome Medical, GI OnDemand, Invitae Genetics, and Promega. Dr. Wright and her coauthors disclosed no conflicts of interest. The group’s research was conducted using the UK Biobank and the University of Exeter High-Performance Computing, with funding from the Wellcome Trust and the National Institute for Health Research.
In fact, SNP chips are “extremely unreliable for genotyping very rare pathogenic variants,” and a positive result for such a variant “is more likely to be wrong than right,” researchers reported in the BMJ.
The authors explained that SNP chips are “DNA microarrays that test genetic variation at many hundreds of thousands of specific locations across the genome.” Although SNP chips have proven accurate in identifying common variants, past reports have suggested that SNP chips perform poorly for genotyping rare variants.
To gain more insight, Caroline Wright, PhD, of the University of Exeter (England) and colleagues conducted a large study.
The researchers analyzed data on 49,908 people from the UK Biobank who had SNP chip and next-generation sequencing results, as well as an additional 21 people who purchased consumer genetic tests and shared their data online via the Personal Genome Project.
The researchers compared the SNP chip and sequencing results. They also selected rare pathogenic variants in BRCA1 and BRCA2 for detailed analysis of clinically actionable variants in the UK Biobank, and they assessed BRCA-related cancers in participants using cancer registry data.
Largest evaluation of SNP chips
SNP chips performed well for common variants, the researchers found. Sensitivity, specificity, positive-predictive value, and negative-predictive value all exceeded 99% for 108,574 common variants.
For rare variants, SNP chips performed poorly, with a positive-predictive value of 16% for variants with a frequency below 0.001% in the UK Biobank.
“The study provides the largest evaluation of the performance of SNP chips for genotyping genetic variants at different frequencies in the population, particularly focusing on very rare variants,” Dr. Wright said. “The biggest surprise was how poorly the SNP chips we evaluated performed for rare variants.”
Dr. Wright noted that there is an inherent problem built into using SNP chip technology to genotype very rare variants.
“The SNP chip technology relies on clustering data from multiple individuals in order to determine what genotype each individual has at a specific position in their genome,” Dr. Wright explained. “Although this method works very well for common variants, the rarer the variant, the harder it is to distinguish from experimental noise.”
False positives and cancer: ‘Don’t trust the results’
The researchers found that, for rare BRCA variants (frequency below 0.01%), SNP chips had a sensitivity of 34.6%, specificity of 98.3%, negative-predictive value of 99.9%, and positive-predictive value of 4.2%.
Rates of BRCA-related cancers in patients with positive SNP chip results were similar to rates in age-matched control subjects because “the vast majority of variants were false positives,” the researchers noted.
“If these variants are incorrectly genotyped – that is, false positives detected – a woman could be offered screening or even prophylactic surgery inappropriately when she is more likely to be at population background risk [for BRCA-related cancers],” Dr. Wright said.
“For very-rare-disease–causing genetic variants, don’t trust the results from SNP chips; for example, those from direct-to-consumer genetic tests. Never use them to guide clinical action without diagnostic validation,” she added.
Heather Hampel, a genetic counselor and researcher at the Ohio State University Comprehensive Cancer Center in Columbus, agreed.
“Positive results on SNP-based tests need to be confirmed by medical-grade genetic testing using a sequencing technology,” she said. “Negative results on an SNP- based test cannot be considered to rule out mutations in BRCA1/2 or other cancer-susceptibility genes, so individuals with strong personal and family histories of cancer should be seen by a genetic counselor to consider medical-grade genetic testing using a sequencing technology.”
Practicing oncologists can trust patients’ prior germline genetic test results if the testing was performed in a cancer genetics clinic, which uses sequencing-based technologies, Ms. Hampel noted.
“If the test was performed before 2013, there are likely new genes that have been discovered for which their patient was not tested, and repeat testing may be warranted,” Ms. Hampel said. “A referral to a cancer genetic counselor would be appropriate.”
Ms. Hampel disclosed relationships with Genome Medical, GI OnDemand, Invitae Genetics, and Promega. Dr. Wright and her coauthors disclosed no conflicts of interest. The group’s research was conducted using the UK Biobank and the University of Exeter High-Performance Computing, with funding from the Wellcome Trust and the National Institute for Health Research.
In fact, SNP chips are “extremely unreliable for genotyping very rare pathogenic variants,” and a positive result for such a variant “is more likely to be wrong than right,” researchers reported in the BMJ.
The authors explained that SNP chips are “DNA microarrays that test genetic variation at many hundreds of thousands of specific locations across the genome.” Although SNP chips have proven accurate in identifying common variants, past reports have suggested that SNP chips perform poorly for genotyping rare variants.
To gain more insight, Caroline Wright, PhD, of the University of Exeter (England) and colleagues conducted a large study.
The researchers analyzed data on 49,908 people from the UK Biobank who had SNP chip and next-generation sequencing results, as well as an additional 21 people who purchased consumer genetic tests and shared their data online via the Personal Genome Project.
The researchers compared the SNP chip and sequencing results. They also selected rare pathogenic variants in BRCA1 and BRCA2 for detailed analysis of clinically actionable variants in the UK Biobank, and they assessed BRCA-related cancers in participants using cancer registry data.
Largest evaluation of SNP chips
SNP chips performed well for common variants, the researchers found. Sensitivity, specificity, positive-predictive value, and negative-predictive value all exceeded 99% for 108,574 common variants.
For rare variants, SNP chips performed poorly, with a positive-predictive value of 16% for variants with a frequency below 0.001% in the UK Biobank.
“The study provides the largest evaluation of the performance of SNP chips for genotyping genetic variants at different frequencies in the population, particularly focusing on very rare variants,” Dr. Wright said. “The biggest surprise was how poorly the SNP chips we evaluated performed for rare variants.”
Dr. Wright noted that there is an inherent problem built into using SNP chip technology to genotype very rare variants.
“The SNP chip technology relies on clustering data from multiple individuals in order to determine what genotype each individual has at a specific position in their genome,” Dr. Wright explained. “Although this method works very well for common variants, the rarer the variant, the harder it is to distinguish from experimental noise.”
False positives and cancer: ‘Don’t trust the results’
The researchers found that, for rare BRCA variants (frequency below 0.01%), SNP chips had a sensitivity of 34.6%, specificity of 98.3%, negative-predictive value of 99.9%, and positive-predictive value of 4.2%.
Rates of BRCA-related cancers in patients with positive SNP chip results were similar to rates in age-matched control subjects because “the vast majority of variants were false positives,” the researchers noted.
“If these variants are incorrectly genotyped – that is, false positives detected – a woman could be offered screening or even prophylactic surgery inappropriately when she is more likely to be at population background risk [for BRCA-related cancers],” Dr. Wright said.
“For very-rare-disease–causing genetic variants, don’t trust the results from SNP chips; for example, those from direct-to-consumer genetic tests. Never use them to guide clinical action without diagnostic validation,” she added.
Heather Hampel, a genetic counselor and researcher at the Ohio State University Comprehensive Cancer Center in Columbus, agreed.
“Positive results on SNP-based tests need to be confirmed by medical-grade genetic testing using a sequencing technology,” she said. “Negative results on an SNP- based test cannot be considered to rule out mutations in BRCA1/2 or other cancer-susceptibility genes, so individuals with strong personal and family histories of cancer should be seen by a genetic counselor to consider medical-grade genetic testing using a sequencing technology.”
Practicing oncologists can trust patients’ prior germline genetic test results if the testing was performed in a cancer genetics clinic, which uses sequencing-based technologies, Ms. Hampel noted.
“If the test was performed before 2013, there are likely new genes that have been discovered for which their patient was not tested, and repeat testing may be warranted,” Ms. Hampel said. “A referral to a cancer genetic counselor would be appropriate.”
Ms. Hampel disclosed relationships with Genome Medical, GI OnDemand, Invitae Genetics, and Promega. Dr. Wright and her coauthors disclosed no conflicts of interest. The group’s research was conducted using the UK Biobank and the University of Exeter High-Performance Computing, with funding from the Wellcome Trust and the National Institute for Health Research.
FROM BMJ
Don’t delay: Cancer patients need both doses of COVID vaccine
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
mCODE: Improving data sharing to enhance cancer care
An initiative designed to improve sharing of patient data may provide “tremendous benefits” in cancer care and research, according to authors of a review article.
The goals of the initiative, called Minimal Common Oncology Data Elements (mCODE), were to identify the data elements in electronic health records that are “essential” for making treatment decisions and create “a standardized computable data format” that would improve the exchange of data across EHRs, according to the mCODE website.
Travis J. Osterman, DO, of Vanderbilt University Medical Center in Nashville, Tenn., and colleagues described the mCODE initiative in a review published in JCO Clinical Cancer Informatics.
At present, commercially available EHRs are poorly designed to support modern oncology workflow, requiring laborious data entry and lacking a common library of oncology-specific discrete data elements. As an example, most EHRs poorly support the needs of precision oncology and clinical genetics, since next-generation sequencing and genetic test results are almost universally reported in PDF files.
In addition, basic, operational oncology data (e.g., cancer staging, adverse event documentation, response to treatment, etc.) are captured in EHRs primarily as an unstructured narrative.
Computable, analytical data are found for only the small percentage of patients in clinical trials. Even then, some degree of manual data abstraction is regularly required.
Interoperability of EHRs between practices and health care institutions is often so poor that the transfer of basic cancer-related information as analyzable data is difficult or even impossible.
Making progress: The 21st Century Cures Act
The American Society of Clinical Oncology has a more than 15-year history of developing oncology data standards. Unfortunately, progress in implementing these standards has been glacially slow. Impediments have included:
- A lack of conformance with clinical workflows.
- Failure to test standards on specific-use cases during pilot testing.
- A focus on data exchange, rather than the practical impediments to data entry.
- Poor engagement with EHR vendors in distributing clinical information modules with an oncology-specific focus
- Instability of data interoperability technologies.
The 21st Century Cures Act, which became law in December 2016, mandated improvement in the interoperability of health information through the development of data standards and application programming interfaces.
In early 2020, final rules for implementation required technology vendors to employ application programming interfaces using a single interoperability resource. In addition, payers were required to use the United States Core Data for Interoperability Standard for data exchange. These requirements were intended to provide patients with access to their own health care data “without special effort.”
As a fortunate byproduct, since EHR vendors are required to implement application program interfaces using the Health Level Seven International (HL7) Fast Healthcare Interoperability Resource (FHIR) Specification, the final rules could enable systems like mCODE to be more easily integrated with existing EHRs.
Lessons from CancerLinQ
ASCO created the health technology platform CancerLinQ in 2014, envisioning that it could become an oncology-focused learning health system – a system in which internal data and experience are systematically integrated with external evidence, allowing knowledge to be put into practice.
CancerLinQ extracts data from EHRs and other sources via direct software connections. CancerLinQ then aggregates, harmonizes, and normalizes the data in a cloud-based environment.
The data are available to participating practices for quality improvement in patient care and secondary research. In 2020, records of cancer patients in the CancerLinQ database surpassed 2 million.
CancerLinQ has been successful. However, because of the nature of the EHR ecosystem and the scope and variability of data capture by clinicians, supporting a true learning health system has proven to be a formidable task. Postprocessing manual review using trained human curators is laborious and unsustainable.
The CancerLinQ experience illustrated that basic cancer-pertinent data should be standardized in the EHR and collected prospectively.
The mCODE model
The mCODE initiative seeks to facilitate progress in care quality, clinical research, and health care policy by developing and maintaining a standard, computable, interoperable data format.
Guiding principles that were adopted early in mCODE’s development included:
- A collaborative, noncommercial, use case–driven developmental model.
- Iterative processes.
- User-driven development, refinement, and maintenance.
- Low ongoing maintenance requirements.
A foundational moment in mCODE’s development involved achieving consensus among stakeholders that the project would fail if EHR vendors required additional data entry by users.
After pilot work, a real-world endpoints project, working-group deliberation, public comment, and refinement, the final data standard included six primary domains: patient, disease, laboratory data/vital signs, genomics, treatment, and outcome.
Each domain is further divided into several concepts with specific associated data elements. The data elements are modeled into value sets that specify the possible values for the data element.
To test mCODE, eight organizations representing oncology EHR vendors, standards developers, and research organizations participated in a cancer interoperability track. The comments helped refine mCODE version 1.0, which was released in March 2020 and is accessible via the mCODE website.
Additions will likely be reviewed by a technical review group after external piloting of new use cases.
Innovation, not regulation
Every interaction between a patient and care provider yields information that could lead to improved safety and better outcomes. To be successful, the information must be collected in a computable format so it can be aggregated with data from other patients, analyzed without manual curation, and shared through interoperable systems. Those data should also be secure enough to protect the privacy of individual patients.
mCODE is a consensus data standard for oncology that provides an infrastructure to share patient data between oncology practices and health care systems while promising little to no additional data entry on the part of clinicians. Adoption by sites will be critical, however.
Publishing the standard through the HL7 FHIR technology demonstrated to EHR vendors and regulatory agencies the stability of HL7, an essential requirement for its incorporation into software.
EHR vendors and others are engaged in the CodeX HL7 FHIR Accelerator to design projects to expand and/or modify mCODE. Their creativity and innovativeness via the external advisory mCODE council and/or CodeX will be encouraged to help mCODE reach its full potential.
As part of CodeX, the Community of Practice, an open forum for end users, was established to provide regular updates about mCODE-related initiatives and use cases to solicit in-progress input, according to Robert S. Miller, MD, medical director of CancerLinQ and an author of the mCODE review.
For mCODE to be embraced by all stakeholders, there should be no additional regulations. By engaging stakeholders in an enterprise that supports innovation and collaboration – without additional regulation – mCODE could maximize the potential of EHRs that, until now, have assisted us only marginally in accomplishing those goals.
mCODE is a joint venture of ASCO/CancerLinQ, the Alliance for Clinical Trials in Oncology Foundation, the MITRE Corporation, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Osterman disclosed a grant from the National Cancer Institute and relationships with Infostratix, eHealth, AstraZeneca, Outcomes Insights, Biodesix, MD Outlook, GenomOncology, Cota Healthcare, GE Healthcare, and Microsoft. Dr. Miller and the third review author disclosed no conflicts of interest.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
An initiative designed to improve sharing of patient data may provide “tremendous benefits” in cancer care and research, according to authors of a review article.
The goals of the initiative, called Minimal Common Oncology Data Elements (mCODE), were to identify the data elements in electronic health records that are “essential” for making treatment decisions and create “a standardized computable data format” that would improve the exchange of data across EHRs, according to the mCODE website.
Travis J. Osterman, DO, of Vanderbilt University Medical Center in Nashville, Tenn., and colleagues described the mCODE initiative in a review published in JCO Clinical Cancer Informatics.
At present, commercially available EHRs are poorly designed to support modern oncology workflow, requiring laborious data entry and lacking a common library of oncology-specific discrete data elements. As an example, most EHRs poorly support the needs of precision oncology and clinical genetics, since next-generation sequencing and genetic test results are almost universally reported in PDF files.
In addition, basic, operational oncology data (e.g., cancer staging, adverse event documentation, response to treatment, etc.) are captured in EHRs primarily as an unstructured narrative.
Computable, analytical data are found for only the small percentage of patients in clinical trials. Even then, some degree of manual data abstraction is regularly required.
Interoperability of EHRs between practices and health care institutions is often so poor that the transfer of basic cancer-related information as analyzable data is difficult or even impossible.
Making progress: The 21st Century Cures Act
The American Society of Clinical Oncology has a more than 15-year history of developing oncology data standards. Unfortunately, progress in implementing these standards has been glacially slow. Impediments have included:
- A lack of conformance with clinical workflows.
- Failure to test standards on specific-use cases during pilot testing.
- A focus on data exchange, rather than the practical impediments to data entry.
- Poor engagement with EHR vendors in distributing clinical information modules with an oncology-specific focus
- Instability of data interoperability technologies.
The 21st Century Cures Act, which became law in December 2016, mandated improvement in the interoperability of health information through the development of data standards and application programming interfaces.
In early 2020, final rules for implementation required technology vendors to employ application programming interfaces using a single interoperability resource. In addition, payers were required to use the United States Core Data for Interoperability Standard for data exchange. These requirements were intended to provide patients with access to their own health care data “without special effort.”
As a fortunate byproduct, since EHR vendors are required to implement application program interfaces using the Health Level Seven International (HL7) Fast Healthcare Interoperability Resource (FHIR) Specification, the final rules could enable systems like mCODE to be more easily integrated with existing EHRs.
Lessons from CancerLinQ
ASCO created the health technology platform CancerLinQ in 2014, envisioning that it could become an oncology-focused learning health system – a system in which internal data and experience are systematically integrated with external evidence, allowing knowledge to be put into practice.
CancerLinQ extracts data from EHRs and other sources via direct software connections. CancerLinQ then aggregates, harmonizes, and normalizes the data in a cloud-based environment.
The data are available to participating practices for quality improvement in patient care and secondary research. In 2020, records of cancer patients in the CancerLinQ database surpassed 2 million.
CancerLinQ has been successful. However, because of the nature of the EHR ecosystem and the scope and variability of data capture by clinicians, supporting a true learning health system has proven to be a formidable task. Postprocessing manual review using trained human curators is laborious and unsustainable.
The CancerLinQ experience illustrated that basic cancer-pertinent data should be standardized in the EHR and collected prospectively.
The mCODE model
The mCODE initiative seeks to facilitate progress in care quality, clinical research, and health care policy by developing and maintaining a standard, computable, interoperable data format.
Guiding principles that were adopted early in mCODE’s development included:
- A collaborative, noncommercial, use case–driven developmental model.
- Iterative processes.
- User-driven development, refinement, and maintenance.
- Low ongoing maintenance requirements.
A foundational moment in mCODE’s development involved achieving consensus among stakeholders that the project would fail if EHR vendors required additional data entry by users.
After pilot work, a real-world endpoints project, working-group deliberation, public comment, and refinement, the final data standard included six primary domains: patient, disease, laboratory data/vital signs, genomics, treatment, and outcome.
Each domain is further divided into several concepts with specific associated data elements. The data elements are modeled into value sets that specify the possible values for the data element.
To test mCODE, eight organizations representing oncology EHR vendors, standards developers, and research organizations participated in a cancer interoperability track. The comments helped refine mCODE version 1.0, which was released in March 2020 and is accessible via the mCODE website.
Additions will likely be reviewed by a technical review group after external piloting of new use cases.
Innovation, not regulation
Every interaction between a patient and care provider yields information that could lead to improved safety and better outcomes. To be successful, the information must be collected in a computable format so it can be aggregated with data from other patients, analyzed without manual curation, and shared through interoperable systems. Those data should also be secure enough to protect the privacy of individual patients.
mCODE is a consensus data standard for oncology that provides an infrastructure to share patient data between oncology practices and health care systems while promising little to no additional data entry on the part of clinicians. Adoption by sites will be critical, however.
Publishing the standard through the HL7 FHIR technology demonstrated to EHR vendors and regulatory agencies the stability of HL7, an essential requirement for its incorporation into software.
EHR vendors and others are engaged in the CodeX HL7 FHIR Accelerator to design projects to expand and/or modify mCODE. Their creativity and innovativeness via the external advisory mCODE council and/or CodeX will be encouraged to help mCODE reach its full potential.
As part of CodeX, the Community of Practice, an open forum for end users, was established to provide regular updates about mCODE-related initiatives and use cases to solicit in-progress input, according to Robert S. Miller, MD, medical director of CancerLinQ and an author of the mCODE review.
For mCODE to be embraced by all stakeholders, there should be no additional regulations. By engaging stakeholders in an enterprise that supports innovation and collaboration – without additional regulation – mCODE could maximize the potential of EHRs that, until now, have assisted us only marginally in accomplishing those goals.
mCODE is a joint venture of ASCO/CancerLinQ, the Alliance for Clinical Trials in Oncology Foundation, the MITRE Corporation, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Osterman disclosed a grant from the National Cancer Institute and relationships with Infostratix, eHealth, AstraZeneca, Outcomes Insights, Biodesix, MD Outlook, GenomOncology, Cota Healthcare, GE Healthcare, and Microsoft. Dr. Miller and the third review author disclosed no conflicts of interest.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
An initiative designed to improve sharing of patient data may provide “tremendous benefits” in cancer care and research, according to authors of a review article.
The goals of the initiative, called Minimal Common Oncology Data Elements (mCODE), were to identify the data elements in electronic health records that are “essential” for making treatment decisions and create “a standardized computable data format” that would improve the exchange of data across EHRs, according to the mCODE website.
Travis J. Osterman, DO, of Vanderbilt University Medical Center in Nashville, Tenn., and colleagues described the mCODE initiative in a review published in JCO Clinical Cancer Informatics.
At present, commercially available EHRs are poorly designed to support modern oncology workflow, requiring laborious data entry and lacking a common library of oncology-specific discrete data elements. As an example, most EHRs poorly support the needs of precision oncology and clinical genetics, since next-generation sequencing and genetic test results are almost universally reported in PDF files.
In addition, basic, operational oncology data (e.g., cancer staging, adverse event documentation, response to treatment, etc.) are captured in EHRs primarily as an unstructured narrative.
Computable, analytical data are found for only the small percentage of patients in clinical trials. Even then, some degree of manual data abstraction is regularly required.
Interoperability of EHRs between practices and health care institutions is often so poor that the transfer of basic cancer-related information as analyzable data is difficult or even impossible.
Making progress: The 21st Century Cures Act
The American Society of Clinical Oncology has a more than 15-year history of developing oncology data standards. Unfortunately, progress in implementing these standards has been glacially slow. Impediments have included:
- A lack of conformance with clinical workflows.
- Failure to test standards on specific-use cases during pilot testing.
- A focus on data exchange, rather than the practical impediments to data entry.
- Poor engagement with EHR vendors in distributing clinical information modules with an oncology-specific focus
- Instability of data interoperability technologies.
The 21st Century Cures Act, which became law in December 2016, mandated improvement in the interoperability of health information through the development of data standards and application programming interfaces.
In early 2020, final rules for implementation required technology vendors to employ application programming interfaces using a single interoperability resource. In addition, payers were required to use the United States Core Data for Interoperability Standard for data exchange. These requirements were intended to provide patients with access to their own health care data “without special effort.”
As a fortunate byproduct, since EHR vendors are required to implement application program interfaces using the Health Level Seven International (HL7) Fast Healthcare Interoperability Resource (FHIR) Specification, the final rules could enable systems like mCODE to be more easily integrated with existing EHRs.
Lessons from CancerLinQ
ASCO created the health technology platform CancerLinQ in 2014, envisioning that it could become an oncology-focused learning health system – a system in which internal data and experience are systematically integrated with external evidence, allowing knowledge to be put into practice.
CancerLinQ extracts data from EHRs and other sources via direct software connections. CancerLinQ then aggregates, harmonizes, and normalizes the data in a cloud-based environment.
The data are available to participating practices for quality improvement in patient care and secondary research. In 2020, records of cancer patients in the CancerLinQ database surpassed 2 million.
CancerLinQ has been successful. However, because of the nature of the EHR ecosystem and the scope and variability of data capture by clinicians, supporting a true learning health system has proven to be a formidable task. Postprocessing manual review using trained human curators is laborious and unsustainable.
The CancerLinQ experience illustrated that basic cancer-pertinent data should be standardized in the EHR and collected prospectively.
The mCODE model
The mCODE initiative seeks to facilitate progress in care quality, clinical research, and health care policy by developing and maintaining a standard, computable, interoperable data format.
Guiding principles that were adopted early in mCODE’s development included:
- A collaborative, noncommercial, use case–driven developmental model.
- Iterative processes.
- User-driven development, refinement, and maintenance.
- Low ongoing maintenance requirements.
A foundational moment in mCODE’s development involved achieving consensus among stakeholders that the project would fail if EHR vendors required additional data entry by users.
After pilot work, a real-world endpoints project, working-group deliberation, public comment, and refinement, the final data standard included six primary domains: patient, disease, laboratory data/vital signs, genomics, treatment, and outcome.
Each domain is further divided into several concepts with specific associated data elements. The data elements are modeled into value sets that specify the possible values for the data element.
To test mCODE, eight organizations representing oncology EHR vendors, standards developers, and research organizations participated in a cancer interoperability track. The comments helped refine mCODE version 1.0, which was released in March 2020 and is accessible via the mCODE website.
Additions will likely be reviewed by a technical review group after external piloting of new use cases.
Innovation, not regulation
Every interaction between a patient and care provider yields information that could lead to improved safety and better outcomes. To be successful, the information must be collected in a computable format so it can be aggregated with data from other patients, analyzed without manual curation, and shared through interoperable systems. Those data should also be secure enough to protect the privacy of individual patients.
mCODE is a consensus data standard for oncology that provides an infrastructure to share patient data between oncology practices and health care systems while promising little to no additional data entry on the part of clinicians. Adoption by sites will be critical, however.
Publishing the standard through the HL7 FHIR technology demonstrated to EHR vendors and regulatory agencies the stability of HL7, an essential requirement for its incorporation into software.
EHR vendors and others are engaged in the CodeX HL7 FHIR Accelerator to design projects to expand and/or modify mCODE. Their creativity and innovativeness via the external advisory mCODE council and/or CodeX will be encouraged to help mCODE reach its full potential.
As part of CodeX, the Community of Practice, an open forum for end users, was established to provide regular updates about mCODE-related initiatives and use cases to solicit in-progress input, according to Robert S. Miller, MD, medical director of CancerLinQ and an author of the mCODE review.
For mCODE to be embraced by all stakeholders, there should be no additional regulations. By engaging stakeholders in an enterprise that supports innovation and collaboration – without additional regulation – mCODE could maximize the potential of EHRs that, until now, have assisted us only marginally in accomplishing those goals.
mCODE is a joint venture of ASCO/CancerLinQ, the Alliance for Clinical Trials in Oncology Foundation, the MITRE Corporation, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Osterman disclosed a grant from the National Cancer Institute and relationships with Infostratix, eHealth, AstraZeneca, Outcomes Insights, Biodesix, MD Outlook, GenomOncology, Cota Healthcare, GE Healthcare, and Microsoft. Dr. Miller and the third review author disclosed no conflicts of interest.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM JCO CLINICAL CANCER INFORMATICS
Is there liability if you don’t test for BRCA?
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6
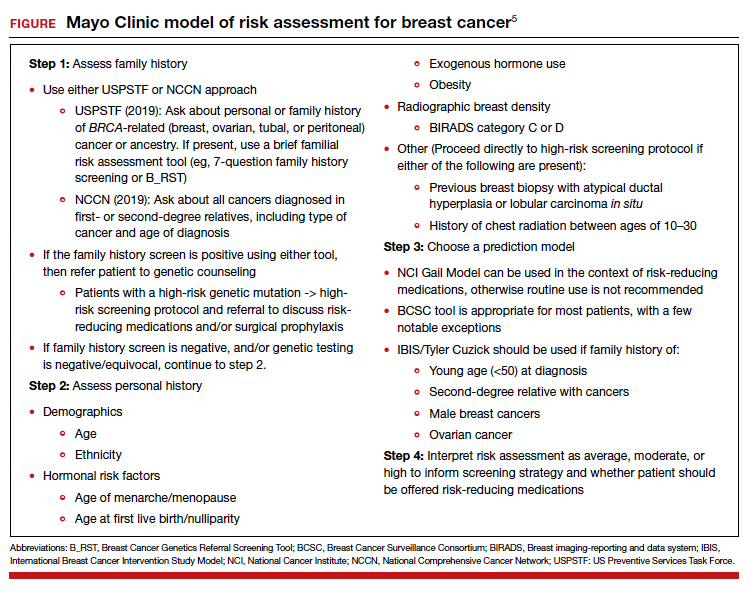
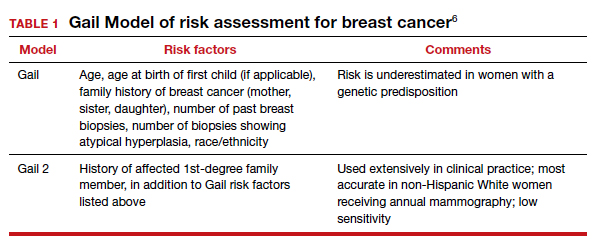
Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7
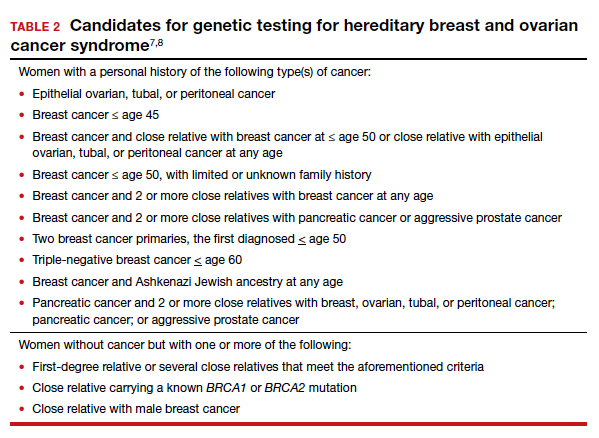
The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6


Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7

The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6


Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7

The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
Clinical Edge Journal Scan Commentary: Breast Cancer March 2021
Peripheral neuropathy is a well-recognized complication of taxane therapy that can impact functioning and quality of life. Dose-reductions are applied in an effort to continue treatment and minimize risk of worsening neuropathy. In a prospective analysis of breast cancer patients receiving weekly paclitaxel, Timmins et al showed neuropathy symptoms affected 85% with severe symptoms in 38%, and about half of the cohort had persistent symptoms up to 12 months post-chemotherapy. Patients who received dose reductions reported worse neuropathy and symptom burden compared to those who received full dose paclitaxel chemotherapy. It is challenging to predict with certainty which patients may experience significant neuropathy, and important to acknowledge individual patients factors such as age and other medical co-morbidities. Additional research is warranted to refine individual risk assessment as well as prevention and management strategies.
The treatment landscape for metastatic HER2-positive breast cancer is evolving at a rapid pace. Margetuximab is a chimeric antibody with similar epitope specificity to trastuzumab, but with an engineered Fc region that enhances immune activation. The phase 3 SOPHIA trial included 536 patients with pretreated HER2-positive advanced breast cancer and demonstrated modest improvement in progression free-survival with margetuximab plus chemotherapy compared to trastuzumab plus chemotherapy (median PFS 5.8 versus 4.9 months; HR 0.76, p=0.03). The introduction of other therapies in this space (tucatinib, trastuzumab deruxtecan, neratinib) provides patients with many options, but simultaneously creates a complex treatment algorithm when it comes to therapy selection. Toxicity profiles and sites of metastases should be taken into consideration when deciding on best therapy for an individual patient.
Given the impressive outcomes seen with endocrine therapy plus CDK 4/6 inhibitors in the advanced HR+/HER2- population, these combinations are being studied in the curative setting. The phase 3 PALLAS study randomized 5,760 patients with stage I-III HR+/HER2- breast cancer to ongoing endocrine therapy with or without palbociclib for 2 years. Data from the second interim analysis of this trial showed similar invasive disease-free survival rates for the two arms (3 years iDFS 88.2% for palbociclib plus endocrine therapy versus 88.5% for endocrine therapy alone; HR 0.93, p=0.51). In contrast, the phase 3 monarchE trial showed improvement in iDFS with abemaciclib for 2 years with ongoing endocrine therapy compared to endocrine therapy alone (2 year iDFS rate of 92.3% versus 89.3%; HR 0.713, p=0.0009). Differences in study populations, mechanism of action of various CDK 4/6 inhibitors, dosing and drug exposure, may possibly impact results. Long-term follow-up and biomarker studies are desired to further delineate the role of CDK 4/6 inhibitors in this setting.
References:
Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA, Hurria A, Marks LB, LaMonte SJ, Warner E, Lyman GH, Ganz PA. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol. 2016;34:611-35.
Ghoreishi Z, Keshavarz S, Asghari Jafarabadi M, Fathifar Z, Goodman KA, Esfahani A. Risk factors for paclitaxel-induced peripheral neuropathy in patients with breast cancer. BMC Cancer. 2018;18:958.
O'Shaughnessy JA, Johnston S, Harbeck N, Toi M, Im Y-H, Reinisch M, Shao Z, Kellokumpu Lehtinen PL, Huang C-S, Tryakin A, Goetz M, Rugo HS, Senkus E, Testa L, Andersson M, Tamura K, Steger GG, Del Mastro L, Cox J, Forrester T, Sherwood S, Li X, Wei R, Martin M, Rastogi P. Primary outcome analysis of invasive disease-free survival for monarchE: abemaciclib combined with adjuvant endocrine therapy for high risk early breast cancer. Presented at: 2020 Virtual San Antonio Breast Cancer Symposium; December 8-11, 2020. Abstract GS1-01.
Peripheral neuropathy is a well-recognized complication of taxane therapy that can impact functioning and quality of life. Dose-reductions are applied in an effort to continue treatment and minimize risk of worsening neuropathy. In a prospective analysis of breast cancer patients receiving weekly paclitaxel, Timmins et al showed neuropathy symptoms affected 85% with severe symptoms in 38%, and about half of the cohort had persistent symptoms up to 12 months post-chemotherapy. Patients who received dose reductions reported worse neuropathy and symptom burden compared to those who received full dose paclitaxel chemotherapy. It is challenging to predict with certainty which patients may experience significant neuropathy, and important to acknowledge individual patients factors such as age and other medical co-morbidities. Additional research is warranted to refine individual risk assessment as well as prevention and management strategies.
The treatment landscape for metastatic HER2-positive breast cancer is evolving at a rapid pace. Margetuximab is a chimeric antibody with similar epitope specificity to trastuzumab, but with an engineered Fc region that enhances immune activation. The phase 3 SOPHIA trial included 536 patients with pretreated HER2-positive advanced breast cancer and demonstrated modest improvement in progression free-survival with margetuximab plus chemotherapy compared to trastuzumab plus chemotherapy (median PFS 5.8 versus 4.9 months; HR 0.76, p=0.03). The introduction of other therapies in this space (tucatinib, trastuzumab deruxtecan, neratinib) provides patients with many options, but simultaneously creates a complex treatment algorithm when it comes to therapy selection. Toxicity profiles and sites of metastases should be taken into consideration when deciding on best therapy for an individual patient.
Given the impressive outcomes seen with endocrine therapy plus CDK 4/6 inhibitors in the advanced HR+/HER2- population, these combinations are being studied in the curative setting. The phase 3 PALLAS study randomized 5,760 patients with stage I-III HR+/HER2- breast cancer to ongoing endocrine therapy with or without palbociclib for 2 years. Data from the second interim analysis of this trial showed similar invasive disease-free survival rates for the two arms (3 years iDFS 88.2% for palbociclib plus endocrine therapy versus 88.5% for endocrine therapy alone; HR 0.93, p=0.51). In contrast, the phase 3 monarchE trial showed improvement in iDFS with abemaciclib for 2 years with ongoing endocrine therapy compared to endocrine therapy alone (2 year iDFS rate of 92.3% versus 89.3%; HR 0.713, p=0.0009). Differences in study populations, mechanism of action of various CDK 4/6 inhibitors, dosing and drug exposure, may possibly impact results. Long-term follow-up and biomarker studies are desired to further delineate the role of CDK 4/6 inhibitors in this setting.
References:
Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA, Hurria A, Marks LB, LaMonte SJ, Warner E, Lyman GH, Ganz PA. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol. 2016;34:611-35.
Ghoreishi Z, Keshavarz S, Asghari Jafarabadi M, Fathifar Z, Goodman KA, Esfahani A. Risk factors for paclitaxel-induced peripheral neuropathy in patients with breast cancer. BMC Cancer. 2018;18:958.
O'Shaughnessy JA, Johnston S, Harbeck N, Toi M, Im Y-H, Reinisch M, Shao Z, Kellokumpu Lehtinen PL, Huang C-S, Tryakin A, Goetz M, Rugo HS, Senkus E, Testa L, Andersson M, Tamura K, Steger GG, Del Mastro L, Cox J, Forrester T, Sherwood S, Li X, Wei R, Martin M, Rastogi P. Primary outcome analysis of invasive disease-free survival for monarchE: abemaciclib combined with adjuvant endocrine therapy for high risk early breast cancer. Presented at: 2020 Virtual San Antonio Breast Cancer Symposium; December 8-11, 2020. Abstract GS1-01.
Peripheral neuropathy is a well-recognized complication of taxane therapy that can impact functioning and quality of life. Dose-reductions are applied in an effort to continue treatment and minimize risk of worsening neuropathy. In a prospective analysis of breast cancer patients receiving weekly paclitaxel, Timmins et al showed neuropathy symptoms affected 85% with severe symptoms in 38%, and about half of the cohort had persistent symptoms up to 12 months post-chemotherapy. Patients who received dose reductions reported worse neuropathy and symptom burden compared to those who received full dose paclitaxel chemotherapy. It is challenging to predict with certainty which patients may experience significant neuropathy, and important to acknowledge individual patients factors such as age and other medical co-morbidities. Additional research is warranted to refine individual risk assessment as well as prevention and management strategies.
The treatment landscape for metastatic HER2-positive breast cancer is evolving at a rapid pace. Margetuximab is a chimeric antibody with similar epitope specificity to trastuzumab, but with an engineered Fc region that enhances immune activation. The phase 3 SOPHIA trial included 536 patients with pretreated HER2-positive advanced breast cancer and demonstrated modest improvement in progression free-survival with margetuximab plus chemotherapy compared to trastuzumab plus chemotherapy (median PFS 5.8 versus 4.9 months; HR 0.76, p=0.03). The introduction of other therapies in this space (tucatinib, trastuzumab deruxtecan, neratinib) provides patients with many options, but simultaneously creates a complex treatment algorithm when it comes to therapy selection. Toxicity profiles and sites of metastases should be taken into consideration when deciding on best therapy for an individual patient.
Given the impressive outcomes seen with endocrine therapy plus CDK 4/6 inhibitors in the advanced HR+/HER2- population, these combinations are being studied in the curative setting. The phase 3 PALLAS study randomized 5,760 patients with stage I-III HR+/HER2- breast cancer to ongoing endocrine therapy with or without palbociclib for 2 years. Data from the second interim analysis of this trial showed similar invasive disease-free survival rates for the two arms (3 years iDFS 88.2% for palbociclib plus endocrine therapy versus 88.5% for endocrine therapy alone; HR 0.93, p=0.51). In contrast, the phase 3 monarchE trial showed improvement in iDFS with abemaciclib for 2 years with ongoing endocrine therapy compared to endocrine therapy alone (2 year iDFS rate of 92.3% versus 89.3%; HR 0.713, p=0.0009). Differences in study populations, mechanism of action of various CDK 4/6 inhibitors, dosing and drug exposure, may possibly impact results. Long-term follow-up and biomarker studies are desired to further delineate the role of CDK 4/6 inhibitors in this setting.
References:
Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA, Hurria A, Marks LB, LaMonte SJ, Warner E, Lyman GH, Ganz PA. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol. 2016;34:611-35.
Ghoreishi Z, Keshavarz S, Asghari Jafarabadi M, Fathifar Z, Goodman KA, Esfahani A. Risk factors for paclitaxel-induced peripheral neuropathy in patients with breast cancer. BMC Cancer. 2018;18:958.
O'Shaughnessy JA, Johnston S, Harbeck N, Toi M, Im Y-H, Reinisch M, Shao Z, Kellokumpu Lehtinen PL, Huang C-S, Tryakin A, Goetz M, Rugo HS, Senkus E, Testa L, Andersson M, Tamura K, Steger GG, Del Mastro L, Cox J, Forrester T, Sherwood S, Li X, Wei R, Martin M, Rastogi P. Primary outcome analysis of invasive disease-free survival for monarchE: abemaciclib combined with adjuvant endocrine therapy for high risk early breast cancer. Presented at: 2020 Virtual San Antonio Breast Cancer Symposium; December 8-11, 2020. Abstract GS1-01.
De-escalated radiation and endocrine therapy strategies in older women with breast cancer
Key clinical point: Adjuvant radiation therapy (RT) alone or in combination with endocrine therapy (ET) was associated with a lower risk for recurrence than ET alone in older women with early node-negative, human receptor-positive (HR+) breast cancer (BC). In addition, most older women with stage I HR+ breast cancers continue to receive radiation, at higher rates than patients with node-negative stage II tumors.
Major finding: Compared with ET alone, use of RT+ET (hazard ratio [HR], 0.62; P less than .0001) and RT alone (HR, 0.75; P less than .0001) was associated with a lower risk for recurrence at a median follow-up of 48 months. RT was received by 65.5% of patients, with no decrease over time. However, patients with T2 vs. T1 tumors remained less likely to receive RT (odds ratio, 0.83; P = .0024).
Study details: This study evaluated the use of adjuvant RT (n=2,046), ET (n=2,407), or RT+ET (n=4,643) after breast-conserving therapeutic surgery in older women (age at diagnosis, 66 years or more) with T1-2 node-negative, HR+ BC.
Disclosures: This study was supported by grants from the Cancer Information and Population Health Resource, UNC Lineberger Comprehensive Cancer Center, and the American Society for Radiation Oncology. Some of the study investigators reported employment and ownership in various pharmaceutical companies.
Source: Reeder-Hayes KE et al. J Geriatr Oncol. 2021 Feb 4. doi: 10.1016/j.jgo.2021.01.003.
Key clinical point: Adjuvant radiation therapy (RT) alone or in combination with endocrine therapy (ET) was associated with a lower risk for recurrence than ET alone in older women with early node-negative, human receptor-positive (HR+) breast cancer (BC). In addition, most older women with stage I HR+ breast cancers continue to receive radiation, at higher rates than patients with node-negative stage II tumors.
Major finding: Compared with ET alone, use of RT+ET (hazard ratio [HR], 0.62; P less than .0001) and RT alone (HR, 0.75; P less than .0001) was associated with a lower risk for recurrence at a median follow-up of 48 months. RT was received by 65.5% of patients, with no decrease over time. However, patients with T2 vs. T1 tumors remained less likely to receive RT (odds ratio, 0.83; P = .0024).
Study details: This study evaluated the use of adjuvant RT (n=2,046), ET (n=2,407), or RT+ET (n=4,643) after breast-conserving therapeutic surgery in older women (age at diagnosis, 66 years or more) with T1-2 node-negative, HR+ BC.
Disclosures: This study was supported by grants from the Cancer Information and Population Health Resource, UNC Lineberger Comprehensive Cancer Center, and the American Society for Radiation Oncology. Some of the study investigators reported employment and ownership in various pharmaceutical companies.
Source: Reeder-Hayes KE et al. J Geriatr Oncol. 2021 Feb 4. doi: 10.1016/j.jgo.2021.01.003.
Key clinical point: Adjuvant radiation therapy (RT) alone or in combination with endocrine therapy (ET) was associated with a lower risk for recurrence than ET alone in older women with early node-negative, human receptor-positive (HR+) breast cancer (BC). In addition, most older women with stage I HR+ breast cancers continue to receive radiation, at higher rates than patients with node-negative stage II tumors.
Major finding: Compared with ET alone, use of RT+ET (hazard ratio [HR], 0.62; P less than .0001) and RT alone (HR, 0.75; P less than .0001) was associated with a lower risk for recurrence at a median follow-up of 48 months. RT was received by 65.5% of patients, with no decrease over time. However, patients with T2 vs. T1 tumors remained less likely to receive RT (odds ratio, 0.83; P = .0024).
Study details: This study evaluated the use of adjuvant RT (n=2,046), ET (n=2,407), or RT+ET (n=4,643) after breast-conserving therapeutic surgery in older women (age at diagnosis, 66 years or more) with T1-2 node-negative, HR+ BC.
Disclosures: This study was supported by grants from the Cancer Information and Population Health Resource, UNC Lineberger Comprehensive Cancer Center, and the American Society for Radiation Oncology. Some of the study investigators reported employment and ownership in various pharmaceutical companies.
Source: Reeder-Hayes KE et al. J Geriatr Oncol. 2021 Feb 4. doi: 10.1016/j.jgo.2021.01.003.
Locoregional surgery improves PFS in de novo stage IV breast cancer
Key clinical point: Locoregional surgery of the primary tumor vs. no surgery significantly improved locoregional progression-free survival (PFS) in patients with de novo stage IV breast cancer.
Major finding: Locoregional PFS was significantly longer with locoregional surgery vs. no surgery (hazard ratio, 0.23; P less than .001).
Study details: Findings are from a meta-analysis of 1,110 patients from 6 prospective clinical trials and 353 patients from a cohort study that assessed effects of locoregional surgery vs. no surgery in de novo stage IV breast cancer.
Disclosures: This study was supported by grants from the National Science and Technology Major Project, Sun Yat-Sen Memorial Hospital, the National Natural Science Foundation of Guangdong Province, Guangzhou Science and Technology Major Program, the Guangdong Science and Technology Department, Sun Yat-Sen University Clinical Research 5010 Program, and Sun Yat-Sen Clinical Research Cultivating Program. The authors declared no conflicts of interest.
Source: Yu Y et al. Ann Surg Oncol. 2021 Feb 3. doi: 10.1245/s10434-021-09650-3.
Key clinical point: Locoregional surgery of the primary tumor vs. no surgery significantly improved locoregional progression-free survival (PFS) in patients with de novo stage IV breast cancer.
Major finding: Locoregional PFS was significantly longer with locoregional surgery vs. no surgery (hazard ratio, 0.23; P less than .001).
Study details: Findings are from a meta-analysis of 1,110 patients from 6 prospective clinical trials and 353 patients from a cohort study that assessed effects of locoregional surgery vs. no surgery in de novo stage IV breast cancer.
Disclosures: This study was supported by grants from the National Science and Technology Major Project, Sun Yat-Sen Memorial Hospital, the National Natural Science Foundation of Guangdong Province, Guangzhou Science and Technology Major Program, the Guangdong Science and Technology Department, Sun Yat-Sen University Clinical Research 5010 Program, and Sun Yat-Sen Clinical Research Cultivating Program. The authors declared no conflicts of interest.
Source: Yu Y et al. Ann Surg Oncol. 2021 Feb 3. doi: 10.1245/s10434-021-09650-3.
Key clinical point: Locoregional surgery of the primary tumor vs. no surgery significantly improved locoregional progression-free survival (PFS) in patients with de novo stage IV breast cancer.
Major finding: Locoregional PFS was significantly longer with locoregional surgery vs. no surgery (hazard ratio, 0.23; P less than .001).
Study details: Findings are from a meta-analysis of 1,110 patients from 6 prospective clinical trials and 353 patients from a cohort study that assessed effects of locoregional surgery vs. no surgery in de novo stage IV breast cancer.
Disclosures: This study was supported by grants from the National Science and Technology Major Project, Sun Yat-Sen Memorial Hospital, the National Natural Science Foundation of Guangdong Province, Guangzhou Science and Technology Major Program, the Guangdong Science and Technology Department, Sun Yat-Sen University Clinical Research 5010 Program, and Sun Yat-Sen Clinical Research Cultivating Program. The authors declared no conflicts of interest.
Source: Yu Y et al. Ann Surg Oncol. 2021 Feb 3. doi: 10.1245/s10434-021-09650-3.




