User login
Two cases of possible remission in metastatic triple-negative breast cancer
Triple-negative breast cancer (TNBC) has been shown to generally have a poor prognosis. Within the first 3-5 years of diagnosis, the mortality rate is the highest of all the subtypes of breast cancer, although late relapses are less common.1,2 TNBC is markedly heterogeneous tumor, and the individual prognosis can vary widely.1,3 Metastatic TNBC is generally considered a noncurable disease. The median time from recurrence to death for metastatic disease is about 9 months, compared with 20 months for patients with other subtypes of breast cancers.4,5 The median survival time for patients with metastatic TNBC is about 13 months.3
New targeted therapies are emerging for breast cancer, but there are currently no effective targeted therapies for patients with TNBC. In addition, few reports in the literature that discuss long-term complete remissions in patients who have metastatic TNBC. Here, we describe two cases in which patients with metastatic TNBC achieved sustained complete response on conventional chemotherapy regimens.
Case presentations and summaries
Case 1
A 59-year-old woman (age in 2015) had been diagnosed on biopsy in February 2005 with locally advanced right breast cancer (stage T2N2bM0). She underwent lumpectomy, and the results of her pathology tests revealed a triple-negative invasive ductal carcinoma. She was started on 4 cycles of neoadjuvant doxorubicin (60 mg/m2 IV) and cyclophosphamide (600 mg/m2 IV)
In November 2007, the patient was found to have right chest wall metastasis confirmed by ultrasound-guided needle biopsy, and underwent right-side chest wall and partial sternum resection. In May 2008, she had recurrence in the left axilla, and biopsy results showed that she had TNBC disease. She was started on weekly paclitaxel (90 mg/m2) and bevacizumab (10 mg/kg every 2 weeks) continued until July 2008. Chemotherapy was stopped in July 2008 because of a methicillin-resistant Staphylococcus aureus (MRSA) infection of the chest wall and was not resumed after the infection had resolved.
A follow-up positron-emission tomography– computed tomography (PET-CT) scan in June 2009, showed no evidence of disease and the scan was negative for disease in her left axilla. Another PET scan about a year later, in September 2010, was also negative for any disease recurrence.
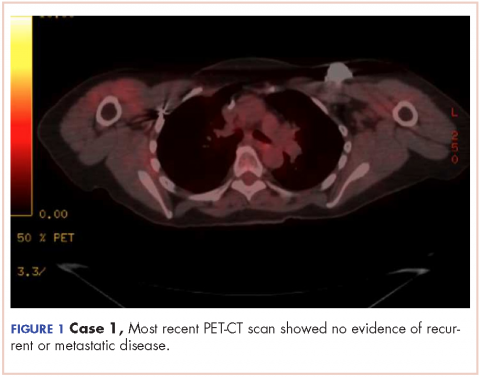
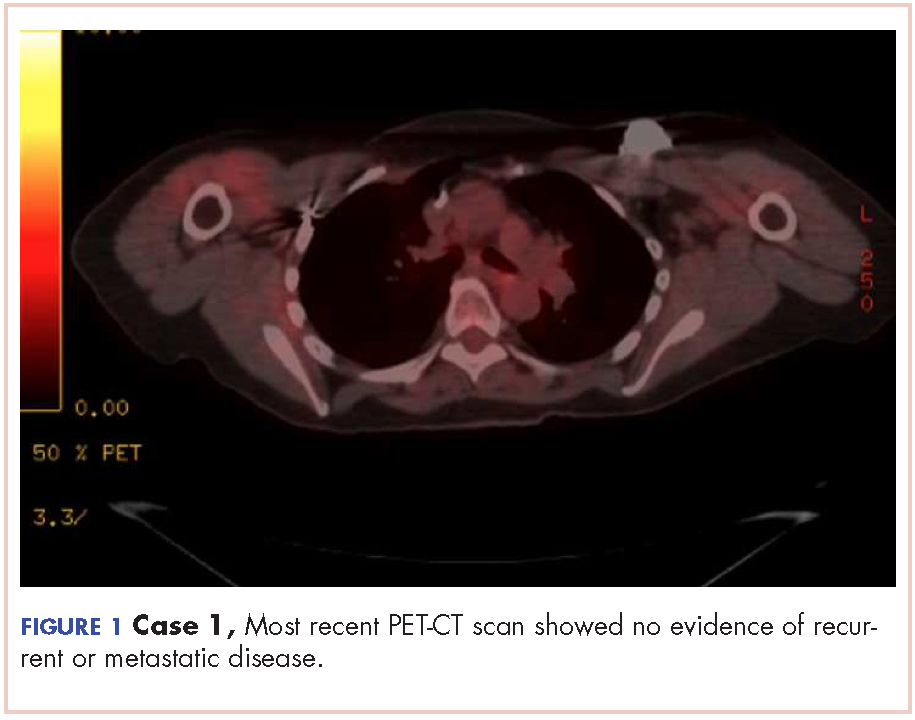
The patient has continued her follow-up with physical examinations and imaging scans. A CT scan of the abdomen and pelvis (December 2010), an MRI of the breasts (February 2011, August 2015), and a PET-CT scan (April 2015, Figure 1) were all negative for any evidence of disease. In September 2011, she had a CT-guided biopsy of a medial right clavicle and costal junction lesion; and in November 2011 and January 2013, surgical biopsies of the right chest wall and first rib lesions, all negative for any evidence for malignancy. At her last follow-up in January 2017, the patient remained in remission.
Case 2
A 68-year old woman (age in 2015) had been diagnosed in Russia in 2004 with infiltrating ductal carcinoma of the right breast (T4N1M0; receptor status unknown at that time). She underwent a right modified radical mastectomy and received adjuvant chemotherapy with 4 cycles of cyclophosphamide (100 mg/m2 day 1 to day 14), methotrexate (40 mg/m2 IV day 1 and day 8), and fluorouracil (600 mg/m2 IV, day 1 and day 8) followed by 2 cycles of docetaxel (75 mg/m2 IV) and anthracycline adriyamycin (50 mg/m2 IV). The patient later received radiation therapy (radiation dose not known, treatment was received in Russia), and completed her treatment in November 2004.
The patient moved to the United States and was started on 25 mg daily exemestane in February 2005. In March 2009, she was diagnosed by biopsy to have recurrence in her internal mammary and hilar lymph nodes and sternum. The cancer was found to be ER- and PR-negative and HER2-neu–negative. The patient was treated with radiation therapy (37.5 Gy in 15 fractions) to sternum and hilar and internal mammary lymph nodes with improvement in pain and shrinkage of lymph nodes size. In May 2009, she was started on 1,500 mg oral twice a day capecitabine (3 cycles). The therapy was started after completion of radiation treatment due to progression of disease. She developed hand-and-foot syndrome as side effect of the capecitabine, so the dose was reduced. She was switched to gemcitabine (1,000 mg/m2 on days 1, 8, and 15, every 28-day cycle) as a single-agent therapy and completed 3 cycles. A follow-up PET-CT scan in February 2010 showed no evidence of disease.
In May 2010, the patient had a recurrence in the same metastatic foci as before, and she was again started on gemcitabine (1,000 mg/m2 on days 1, 8, and 15, every 28-day cycle). She continued gemcitabine until there was evidence of disease progression on a PET-CT scan in October 2010, which showed new areas of disease in the left parasternal region, left sternum, prevascular mediastinal nodes, and left supraclavicular, hilar and axillary adenopathy, and fourth thoracic vertebra. Gemcitabine was discontinued and patient was started on weekly paclitaxel (90 mg/m2) for 6 cycles. Paclitaxel was discontinued after 6 weeks because she developed a drug-related rash. A follow-up PET-CT scan in December 2010 again showed complete resolution of disease in terms of response.
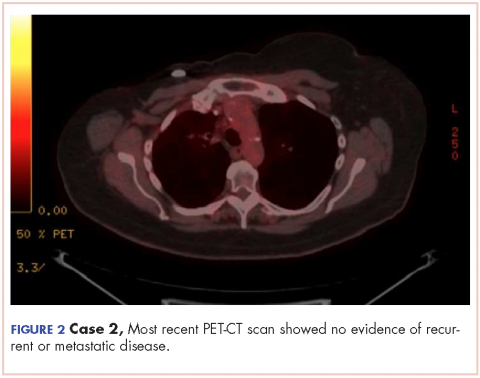
In March 2011, PET imaging showed progression of disease in the left chest wall and axillary lymph nodes, so the patient was started on eribulin therapy (1.4 mg/m2 on days 1 and 8 every 21-day cycle) and completed 3 cycles. In May 2011, PET imaging showed complete response to treatment with no evidence of recurrent or metastatic disease. The patient has not had chemotherapy since November 2011, and surveillance PET imaging has not demonstrated any recurrence of disease (Figure 2). Following her last follow-up in November 2016, the patient remains in remission.
Discussion
Triple-negative breast cancers (TNBCs) are defined as tumors that lack expression of estrogen receptor (ER), progesterone receptor (PR), and HER2, and represent about 12%-17% of breast cancer cases.1,6 TNBCs tend to be larger in size at diagnosis than are other subtypes, are usually high-grade (poorly differentiated), and are more likely to be invasive ductal carcinomas.1,7 TNBC and the basal-like breast cancers as a group are associated with an adverse prognosis.1,7 There is no standard preferred chemotherapy and no biologic therapy available for TNBC.1,6-7 A sharp decline in survival outcome during the first 3-5 years after diagnosis initial is observed in TNBC, although the distant relapses after this time are less common.1 Beyond 10 years from diagnosis, the relapses are seen more common among patients with ER-positive cancers than among those with ER-negative subtype cancers. Therefore, although TNBCs are biologically aggressive, many are possibly curable, and this reflects their interesting characteristic heterogeneity.1,6
Chemotherapy is currently the mainstay of systemic medical treatment. Although patients with TNBC have a worse outcome after chemotherapy than patients with breast cancers of other subtypes, it still improves their outcome to a greater extent than in patients with ER-positive subtypes.1,6,7 Considering the heterogeneity of TNBC, it is difficult to predict which patients will benefit more from chemotherapy. The same has been observed in previous studies when subgroups of women with TNBC were extremely sensitive to chemotherapy, whereas in others it was of uncertain benefit.1
Currently, there is no preferred standard form of chemotherapy for TNBC. There are few case reports that demonstrate long-term survival and complete remission in metastatic TNBC. Shakir has reported on a significant clinical response to nab-paclitaxel monotherapy in a patient with triple-negative BRCA1-positive breast cancer, although patient survived a little more than 5 years and died with central nervous system recurrence.8 Montero and Gluck have described a patient with metastatic TNBC who was treated with nab-paclitaxel, gemcitabine, and bevacizumab and who also survived for 5 years after diagnosis.9 Different retrospective analyses have suggested that the addition of docetaxel or paclitaxel to anthracycline-containing adjuvant regimens may be of greater benefit for the treatment of TNBC than for ER-positive tumors.10 A meta-analysis of trials comparing the effects of cyclophosphamide, methotrexate, and fluorouracil (CMF, which was used in Case 2) with anthracycline-containing regimens has suggested that the latter therapy regimen is more effective against TNBC,11 although another retrospective analysis of a separate trial suggested the opposite for basal-like breast cancers. 12 The authors of the latter analysis concluded that anthracycline-containing adjuvant chemotherapy regimens are inferior to adjuvant CMF in women with basal breast cancer.12
Miller and colleagues have shown that the addition of bevacizumab (angiogenesis inhibitor) to paclitaxel (used in Case 1) improved progression-free survival (median PFS, 11.8 vs 5.9 months; hazard ratio [HR] for progression, 0.60; P < .001) in women with TNBC as it did in the overall study group (HR, 0.53 and 0.60, respectively), although the overall survival rate was similar in the two groups (median OS, 26.7 vs 25.2 months; HR, 0.88; P = .16).13
An interesting clinical target in TNBC is the enzyme poly (adenosine diphosphate– ribose) polymerase (PARP), which is involved in base-excision repair after DNA damage. PARP inhibitors have shown encouraging clinical activity in trials of tumors arising in BRCA mutation carriers and in sporadic TNBC cancers.14 Similarly, the use of an oral PARP inhibitor, olaparib, resulted in tumor regression in up to 41% of patients carrying BRCA mutations, most of whom had TNBC.15
Conclusion
TNBC and basal-like breast cancers show aggressive clinical behavior, but a subgroup of these cancers may be markedly sensitive to chemotherapy and associated with a good prognosis when treated with conventional chemotherapy regimens. The two cases presented here show that some patients can get a prolonged disease control from chemotherapy, even after progressing on multiple previous chemotherapy regimens and that after, 5 years or so, these rare patients could be in true long-term remission. Novel approaches, for example PARP inhibitors, have shown encouraging clinical activity in trials of tumors arising in BRCA mutation carriers and as well as sporadic TNBC.
1. Foulkes WD, Smith IE, Reis-Filho JS, Triple-negative breast cancer. N Engl J Med. 2010;363:1938-1948.
2. Pogoda K, Niwińska A, Murawska M, Pieńkowski T. Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Med Oncol. 2013;30(1):388.
3. Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009;9(1):29-33.
4. Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist. 2010;15(suppl 5):39-48.
5. Rakha EA, Chan S. Metastatic triple-negative breast cancer. Clin Oncol (R Coll Radiol). 2011;23(9):587-600.
6. Williams N, Harris L. Triple-negative breast cancer in the post-genomic era. Oncology (Williston Park). 2013;27(9):859-860, 864.
7. Randhawa SK, Venur VA, Kawsar H, et al. A retrospective comparison of the characteristics and recurrence outcome of triple-negative and triple-positive breast cancer. J Clin Oncol. 2013;31(suppl; abstr 1038).
8. Shakir AR. Strong and sustained response to treatment with carboplatin plus nab-paclitaxel in a patient with metastatic, triple-negative, BRCA1-positive breast cancer. Case Rep Oncol. 2014;7(1)252-259.
9. Montero A, Glück S. Long-term complete remission with nab-paclitaxel, bevacizumab, and gemcitabine combination therapy in a patient with triple-negative metastatic breast cancer. Case Rep Oncol. 2012;5(3):687-692.
10. Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496-1506.
11. Di Leo A, Isola J, Piette F, et al. A meta- analysis of phase III trials evaluating the predictive value of HER2 and topoisomerase alpha in early breast cancer patients treated with CMF or anthracycline-based adjuvant therapy [SABCS, abstract 705]. http://cancerres.aacrjournals.org/content/69/2_Supplement/705. Published 2008. Accessed May 4, 2017.
12. Cheang M, Chia SK, Tu D, et al. Anthracycline in basal breast cancer: the NCIC-CTG trial MA5 comparing adjuvant CMF to CEF [ASCO; abstract 519]. http://meetinglibrary.asco.org/content/35150-65. Published 2009. Accessed May 4, 2017.
13. Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666-2676.
14. Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123-134.
15. Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235-244.
Triple-negative breast cancer (TNBC) has been shown to generally have a poor prognosis. Within the first 3-5 years of diagnosis, the mortality rate is the highest of all the subtypes of breast cancer, although late relapses are less common.1,2 TNBC is markedly heterogeneous tumor, and the individual prognosis can vary widely.1,3 Metastatic TNBC is generally considered a noncurable disease. The median time from recurrence to death for metastatic disease is about 9 months, compared with 20 months for patients with other subtypes of breast cancers.4,5 The median survival time for patients with metastatic TNBC is about 13 months.3
New targeted therapies are emerging for breast cancer, but there are currently no effective targeted therapies for patients with TNBC. In addition, few reports in the literature that discuss long-term complete remissions in patients who have metastatic TNBC. Here, we describe two cases in which patients with metastatic TNBC achieved sustained complete response on conventional chemotherapy regimens.
Case presentations and summaries
Case 1
A 59-year-old woman (age in 2015) had been diagnosed on biopsy in February 2005 with locally advanced right breast cancer (stage T2N2bM0). She underwent lumpectomy, and the results of her pathology tests revealed a triple-negative invasive ductal carcinoma. She was started on 4 cycles of neoadjuvant doxorubicin (60 mg/m2 IV) and cyclophosphamide (600 mg/m2 IV)
In November 2007, the patient was found to have right chest wall metastasis confirmed by ultrasound-guided needle biopsy, and underwent right-side chest wall and partial sternum resection. In May 2008, she had recurrence in the left axilla, and biopsy results showed that she had TNBC disease. She was started on weekly paclitaxel (90 mg/m2) and bevacizumab (10 mg/kg every 2 weeks) continued until July 2008. Chemotherapy was stopped in July 2008 because of a methicillin-resistant Staphylococcus aureus (MRSA) infection of the chest wall and was not resumed after the infection had resolved.
A follow-up positron-emission tomography– computed tomography (PET-CT) scan in June 2009, showed no evidence of disease and the scan was negative for disease in her left axilla. Another PET scan about a year later, in September 2010, was also negative for any disease recurrence.
The patient has continued her follow-up with physical examinations and imaging scans. A CT scan of the abdomen and pelvis (December 2010), an MRI of the breasts (February 2011, August 2015), and a PET-CT scan (April 2015, Figure 1) were all negative for any evidence of disease. In September 2011, she had a CT-guided biopsy of a medial right clavicle and costal junction lesion; and in November 2011 and January 2013, surgical biopsies of the right chest wall and first rib lesions, all negative for any evidence for malignancy. At her last follow-up in January 2017, the patient remained in remission.
Case 2
A 68-year old woman (age in 2015) had been diagnosed in Russia in 2004 with infiltrating ductal carcinoma of the right breast (T4N1M0; receptor status unknown at that time). She underwent a right modified radical mastectomy and received adjuvant chemotherapy with 4 cycles of cyclophosphamide (100 mg/m2 day 1 to day 14), methotrexate (40 mg/m2 IV day 1 and day 8), and fluorouracil (600 mg/m2 IV, day 1 and day 8) followed by 2 cycles of docetaxel (75 mg/m2 IV) and anthracycline adriyamycin (50 mg/m2 IV). The patient later received radiation therapy (radiation dose not known, treatment was received in Russia), and completed her treatment in November 2004.
The patient moved to the United States and was started on 25 mg daily exemestane in February 2005. In March 2009, she was diagnosed by biopsy to have recurrence in her internal mammary and hilar lymph nodes and sternum. The cancer was found to be ER- and PR-negative and HER2-neu–negative. The patient was treated with radiation therapy (37.5 Gy in 15 fractions) to sternum and hilar and internal mammary lymph nodes with improvement in pain and shrinkage of lymph nodes size. In May 2009, she was started on 1,500 mg oral twice a day capecitabine (3 cycles). The therapy was started after completion of radiation treatment due to progression of disease. She developed hand-and-foot syndrome as side effect of the capecitabine, so the dose was reduced. She was switched to gemcitabine (1,000 mg/m2 on days 1, 8, and 15, every 28-day cycle) as a single-agent therapy and completed 3 cycles. A follow-up PET-CT scan in February 2010 showed no evidence of disease.
In May 2010, the patient had a recurrence in the same metastatic foci as before, and she was again started on gemcitabine (1,000 mg/m2 on days 1, 8, and 15, every 28-day cycle). She continued gemcitabine until there was evidence of disease progression on a PET-CT scan in October 2010, which showed new areas of disease in the left parasternal region, left sternum, prevascular mediastinal nodes, and left supraclavicular, hilar and axillary adenopathy, and fourth thoracic vertebra. Gemcitabine was discontinued and patient was started on weekly paclitaxel (90 mg/m2) for 6 cycles. Paclitaxel was discontinued after 6 weeks because she developed a drug-related rash. A follow-up PET-CT scan in December 2010 again showed complete resolution of disease in terms of response.
In March 2011, PET imaging showed progression of disease in the left chest wall and axillary lymph nodes, so the patient was started on eribulin therapy (1.4 mg/m2 on days 1 and 8 every 21-day cycle) and completed 3 cycles. In May 2011, PET imaging showed complete response to treatment with no evidence of recurrent or metastatic disease. The patient has not had chemotherapy since November 2011, and surveillance PET imaging has not demonstrated any recurrence of disease (Figure 2). Following her last follow-up in November 2016, the patient remains in remission.
Discussion
Triple-negative breast cancers (TNBCs) are defined as tumors that lack expression of estrogen receptor (ER), progesterone receptor (PR), and HER2, and represent about 12%-17% of breast cancer cases.1,6 TNBCs tend to be larger in size at diagnosis than are other subtypes, are usually high-grade (poorly differentiated), and are more likely to be invasive ductal carcinomas.1,7 TNBC and the basal-like breast cancers as a group are associated with an adverse prognosis.1,7 There is no standard preferred chemotherapy and no biologic therapy available for TNBC.1,6-7 A sharp decline in survival outcome during the first 3-5 years after diagnosis initial is observed in TNBC, although the distant relapses after this time are less common.1 Beyond 10 years from diagnosis, the relapses are seen more common among patients with ER-positive cancers than among those with ER-negative subtype cancers. Therefore, although TNBCs are biologically aggressive, many are possibly curable, and this reflects their interesting characteristic heterogeneity.1,6
Chemotherapy is currently the mainstay of systemic medical treatment. Although patients with TNBC have a worse outcome after chemotherapy than patients with breast cancers of other subtypes, it still improves their outcome to a greater extent than in patients with ER-positive subtypes.1,6,7 Considering the heterogeneity of TNBC, it is difficult to predict which patients will benefit more from chemotherapy. The same has been observed in previous studies when subgroups of women with TNBC were extremely sensitive to chemotherapy, whereas in others it was of uncertain benefit.1
Currently, there is no preferred standard form of chemotherapy for TNBC. There are few case reports that demonstrate long-term survival and complete remission in metastatic TNBC. Shakir has reported on a significant clinical response to nab-paclitaxel monotherapy in a patient with triple-negative BRCA1-positive breast cancer, although patient survived a little more than 5 years and died with central nervous system recurrence.8 Montero and Gluck have described a patient with metastatic TNBC who was treated with nab-paclitaxel, gemcitabine, and bevacizumab and who also survived for 5 years after diagnosis.9 Different retrospective analyses have suggested that the addition of docetaxel or paclitaxel to anthracycline-containing adjuvant regimens may be of greater benefit for the treatment of TNBC than for ER-positive tumors.10 A meta-analysis of trials comparing the effects of cyclophosphamide, methotrexate, and fluorouracil (CMF, which was used in Case 2) with anthracycline-containing regimens has suggested that the latter therapy regimen is more effective against TNBC,11 although another retrospective analysis of a separate trial suggested the opposite for basal-like breast cancers. 12 The authors of the latter analysis concluded that anthracycline-containing adjuvant chemotherapy regimens are inferior to adjuvant CMF in women with basal breast cancer.12
Miller and colleagues have shown that the addition of bevacizumab (angiogenesis inhibitor) to paclitaxel (used in Case 1) improved progression-free survival (median PFS, 11.8 vs 5.9 months; hazard ratio [HR] for progression, 0.60; P < .001) in women with TNBC as it did in the overall study group (HR, 0.53 and 0.60, respectively), although the overall survival rate was similar in the two groups (median OS, 26.7 vs 25.2 months; HR, 0.88; P = .16).13
An interesting clinical target in TNBC is the enzyme poly (adenosine diphosphate– ribose) polymerase (PARP), which is involved in base-excision repair after DNA damage. PARP inhibitors have shown encouraging clinical activity in trials of tumors arising in BRCA mutation carriers and in sporadic TNBC cancers.14 Similarly, the use of an oral PARP inhibitor, olaparib, resulted in tumor regression in up to 41% of patients carrying BRCA mutations, most of whom had TNBC.15
Conclusion
TNBC and basal-like breast cancers show aggressive clinical behavior, but a subgroup of these cancers may be markedly sensitive to chemotherapy and associated with a good prognosis when treated with conventional chemotherapy regimens. The two cases presented here show that some patients can get a prolonged disease control from chemotherapy, even after progressing on multiple previous chemotherapy regimens and that after, 5 years or so, these rare patients could be in true long-term remission. Novel approaches, for example PARP inhibitors, have shown encouraging clinical activity in trials of tumors arising in BRCA mutation carriers and as well as sporadic TNBC.
Triple-negative breast cancer (TNBC) has been shown to generally have a poor prognosis. Within the first 3-5 years of diagnosis, the mortality rate is the highest of all the subtypes of breast cancer, although late relapses are less common.1,2 TNBC is markedly heterogeneous tumor, and the individual prognosis can vary widely.1,3 Metastatic TNBC is generally considered a noncurable disease. The median time from recurrence to death for metastatic disease is about 9 months, compared with 20 months for patients with other subtypes of breast cancers.4,5 The median survival time for patients with metastatic TNBC is about 13 months.3
New targeted therapies are emerging for breast cancer, but there are currently no effective targeted therapies for patients with TNBC. In addition, few reports in the literature that discuss long-term complete remissions in patients who have metastatic TNBC. Here, we describe two cases in which patients with metastatic TNBC achieved sustained complete response on conventional chemotherapy regimens.
Case presentations and summaries
Case 1
A 59-year-old woman (age in 2015) had been diagnosed on biopsy in February 2005 with locally advanced right breast cancer (stage T2N2bM0). She underwent lumpectomy, and the results of her pathology tests revealed a triple-negative invasive ductal carcinoma. She was started on 4 cycles of neoadjuvant doxorubicin (60 mg/m2 IV) and cyclophosphamide (600 mg/m2 IV)
In November 2007, the patient was found to have right chest wall metastasis confirmed by ultrasound-guided needle biopsy, and underwent right-side chest wall and partial sternum resection. In May 2008, she had recurrence in the left axilla, and biopsy results showed that she had TNBC disease. She was started on weekly paclitaxel (90 mg/m2) and bevacizumab (10 mg/kg every 2 weeks) continued until July 2008. Chemotherapy was stopped in July 2008 because of a methicillin-resistant Staphylococcus aureus (MRSA) infection of the chest wall and was not resumed after the infection had resolved.
A follow-up positron-emission tomography– computed tomography (PET-CT) scan in June 2009, showed no evidence of disease and the scan was negative for disease in her left axilla. Another PET scan about a year later, in September 2010, was also negative for any disease recurrence.
The patient has continued her follow-up with physical examinations and imaging scans. A CT scan of the abdomen and pelvis (December 2010), an MRI of the breasts (February 2011, August 2015), and a PET-CT scan (April 2015, Figure 1) were all negative for any evidence of disease. In September 2011, she had a CT-guided biopsy of a medial right clavicle and costal junction lesion; and in November 2011 and January 2013, surgical biopsies of the right chest wall and first rib lesions, all negative for any evidence for malignancy. At her last follow-up in January 2017, the patient remained in remission.
Case 2
A 68-year old woman (age in 2015) had been diagnosed in Russia in 2004 with infiltrating ductal carcinoma of the right breast (T4N1M0; receptor status unknown at that time). She underwent a right modified radical mastectomy and received adjuvant chemotherapy with 4 cycles of cyclophosphamide (100 mg/m2 day 1 to day 14), methotrexate (40 mg/m2 IV day 1 and day 8), and fluorouracil (600 mg/m2 IV, day 1 and day 8) followed by 2 cycles of docetaxel (75 mg/m2 IV) and anthracycline adriyamycin (50 mg/m2 IV). The patient later received radiation therapy (radiation dose not known, treatment was received in Russia), and completed her treatment in November 2004.
The patient moved to the United States and was started on 25 mg daily exemestane in February 2005. In March 2009, she was diagnosed by biopsy to have recurrence in her internal mammary and hilar lymph nodes and sternum. The cancer was found to be ER- and PR-negative and HER2-neu–negative. The patient was treated with radiation therapy (37.5 Gy in 15 fractions) to sternum and hilar and internal mammary lymph nodes with improvement in pain and shrinkage of lymph nodes size. In May 2009, she was started on 1,500 mg oral twice a day capecitabine (3 cycles). The therapy was started after completion of radiation treatment due to progression of disease. She developed hand-and-foot syndrome as side effect of the capecitabine, so the dose was reduced. She was switched to gemcitabine (1,000 mg/m2 on days 1, 8, and 15, every 28-day cycle) as a single-agent therapy and completed 3 cycles. A follow-up PET-CT scan in February 2010 showed no evidence of disease.
In May 2010, the patient had a recurrence in the same metastatic foci as before, and she was again started on gemcitabine (1,000 mg/m2 on days 1, 8, and 15, every 28-day cycle). She continued gemcitabine until there was evidence of disease progression on a PET-CT scan in October 2010, which showed new areas of disease in the left parasternal region, left sternum, prevascular mediastinal nodes, and left supraclavicular, hilar and axillary adenopathy, and fourth thoracic vertebra. Gemcitabine was discontinued and patient was started on weekly paclitaxel (90 mg/m2) for 6 cycles. Paclitaxel was discontinued after 6 weeks because she developed a drug-related rash. A follow-up PET-CT scan in December 2010 again showed complete resolution of disease in terms of response.
In March 2011, PET imaging showed progression of disease in the left chest wall and axillary lymph nodes, so the patient was started on eribulin therapy (1.4 mg/m2 on days 1 and 8 every 21-day cycle) and completed 3 cycles. In May 2011, PET imaging showed complete response to treatment with no evidence of recurrent or metastatic disease. The patient has not had chemotherapy since November 2011, and surveillance PET imaging has not demonstrated any recurrence of disease (Figure 2). Following her last follow-up in November 2016, the patient remains in remission.
Discussion
Triple-negative breast cancers (TNBCs) are defined as tumors that lack expression of estrogen receptor (ER), progesterone receptor (PR), and HER2, and represent about 12%-17% of breast cancer cases.1,6 TNBCs tend to be larger in size at diagnosis than are other subtypes, are usually high-grade (poorly differentiated), and are more likely to be invasive ductal carcinomas.1,7 TNBC and the basal-like breast cancers as a group are associated with an adverse prognosis.1,7 There is no standard preferred chemotherapy and no biologic therapy available for TNBC.1,6-7 A sharp decline in survival outcome during the first 3-5 years after diagnosis initial is observed in TNBC, although the distant relapses after this time are less common.1 Beyond 10 years from diagnosis, the relapses are seen more common among patients with ER-positive cancers than among those with ER-negative subtype cancers. Therefore, although TNBCs are biologically aggressive, many are possibly curable, and this reflects their interesting characteristic heterogeneity.1,6
Chemotherapy is currently the mainstay of systemic medical treatment. Although patients with TNBC have a worse outcome after chemotherapy than patients with breast cancers of other subtypes, it still improves their outcome to a greater extent than in patients with ER-positive subtypes.1,6,7 Considering the heterogeneity of TNBC, it is difficult to predict which patients will benefit more from chemotherapy. The same has been observed in previous studies when subgroups of women with TNBC were extremely sensitive to chemotherapy, whereas in others it was of uncertain benefit.1
Currently, there is no preferred standard form of chemotherapy for TNBC. There are few case reports that demonstrate long-term survival and complete remission in metastatic TNBC. Shakir has reported on a significant clinical response to nab-paclitaxel monotherapy in a patient with triple-negative BRCA1-positive breast cancer, although patient survived a little more than 5 years and died with central nervous system recurrence.8 Montero and Gluck have described a patient with metastatic TNBC who was treated with nab-paclitaxel, gemcitabine, and bevacizumab and who also survived for 5 years after diagnosis.9 Different retrospective analyses have suggested that the addition of docetaxel or paclitaxel to anthracycline-containing adjuvant regimens may be of greater benefit for the treatment of TNBC than for ER-positive tumors.10 A meta-analysis of trials comparing the effects of cyclophosphamide, methotrexate, and fluorouracil (CMF, which was used in Case 2) with anthracycline-containing regimens has suggested that the latter therapy regimen is more effective against TNBC,11 although another retrospective analysis of a separate trial suggested the opposite for basal-like breast cancers. 12 The authors of the latter analysis concluded that anthracycline-containing adjuvant chemotherapy regimens are inferior to adjuvant CMF in women with basal breast cancer.12
Miller and colleagues have shown that the addition of bevacizumab (angiogenesis inhibitor) to paclitaxel (used in Case 1) improved progression-free survival (median PFS, 11.8 vs 5.9 months; hazard ratio [HR] for progression, 0.60; P < .001) in women with TNBC as it did in the overall study group (HR, 0.53 and 0.60, respectively), although the overall survival rate was similar in the two groups (median OS, 26.7 vs 25.2 months; HR, 0.88; P = .16).13
An interesting clinical target in TNBC is the enzyme poly (adenosine diphosphate– ribose) polymerase (PARP), which is involved in base-excision repair after DNA damage. PARP inhibitors have shown encouraging clinical activity in trials of tumors arising in BRCA mutation carriers and in sporadic TNBC cancers.14 Similarly, the use of an oral PARP inhibitor, olaparib, resulted in tumor regression in up to 41% of patients carrying BRCA mutations, most of whom had TNBC.15
Conclusion
TNBC and basal-like breast cancers show aggressive clinical behavior, but a subgroup of these cancers may be markedly sensitive to chemotherapy and associated with a good prognosis when treated with conventional chemotherapy regimens. The two cases presented here show that some patients can get a prolonged disease control from chemotherapy, even after progressing on multiple previous chemotherapy regimens and that after, 5 years or so, these rare patients could be in true long-term remission. Novel approaches, for example PARP inhibitors, have shown encouraging clinical activity in trials of tumors arising in BRCA mutation carriers and as well as sporadic TNBC.
1. Foulkes WD, Smith IE, Reis-Filho JS, Triple-negative breast cancer. N Engl J Med. 2010;363:1938-1948.
2. Pogoda K, Niwińska A, Murawska M, Pieńkowski T. Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Med Oncol. 2013;30(1):388.
3. Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009;9(1):29-33.
4. Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist. 2010;15(suppl 5):39-48.
5. Rakha EA, Chan S. Metastatic triple-negative breast cancer. Clin Oncol (R Coll Radiol). 2011;23(9):587-600.
6. Williams N, Harris L. Triple-negative breast cancer in the post-genomic era. Oncology (Williston Park). 2013;27(9):859-860, 864.
7. Randhawa SK, Venur VA, Kawsar H, et al. A retrospective comparison of the characteristics and recurrence outcome of triple-negative and triple-positive breast cancer. J Clin Oncol. 2013;31(suppl; abstr 1038).
8. Shakir AR. Strong and sustained response to treatment with carboplatin plus nab-paclitaxel in a patient with metastatic, triple-negative, BRCA1-positive breast cancer. Case Rep Oncol. 2014;7(1)252-259.
9. Montero A, Glück S. Long-term complete remission with nab-paclitaxel, bevacizumab, and gemcitabine combination therapy in a patient with triple-negative metastatic breast cancer. Case Rep Oncol. 2012;5(3):687-692.
10. Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496-1506.
11. Di Leo A, Isola J, Piette F, et al. A meta- analysis of phase III trials evaluating the predictive value of HER2 and topoisomerase alpha in early breast cancer patients treated with CMF or anthracycline-based adjuvant therapy [SABCS, abstract 705]. http://cancerres.aacrjournals.org/content/69/2_Supplement/705. Published 2008. Accessed May 4, 2017.
12. Cheang M, Chia SK, Tu D, et al. Anthracycline in basal breast cancer: the NCIC-CTG trial MA5 comparing adjuvant CMF to CEF [ASCO; abstract 519]. http://meetinglibrary.asco.org/content/35150-65. Published 2009. Accessed May 4, 2017.
13. Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666-2676.
14. Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123-134.
15. Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235-244.
1. Foulkes WD, Smith IE, Reis-Filho JS, Triple-negative breast cancer. N Engl J Med. 2010;363:1938-1948.
2. Pogoda K, Niwińska A, Murawska M, Pieńkowski T. Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Med Oncol. 2013;30(1):388.
3. Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009;9(1):29-33.
4. Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist. 2010;15(suppl 5):39-48.
5. Rakha EA, Chan S. Metastatic triple-negative breast cancer. Clin Oncol (R Coll Radiol). 2011;23(9):587-600.
6. Williams N, Harris L. Triple-negative breast cancer in the post-genomic era. Oncology (Williston Park). 2013;27(9):859-860, 864.
7. Randhawa SK, Venur VA, Kawsar H, et al. A retrospective comparison of the characteristics and recurrence outcome of triple-negative and triple-positive breast cancer. J Clin Oncol. 2013;31(suppl; abstr 1038).
8. Shakir AR. Strong and sustained response to treatment with carboplatin plus nab-paclitaxel in a patient with metastatic, triple-negative, BRCA1-positive breast cancer. Case Rep Oncol. 2014;7(1)252-259.
9. Montero A, Glück S. Long-term complete remission with nab-paclitaxel, bevacizumab, and gemcitabine combination therapy in a patient with triple-negative metastatic breast cancer. Case Rep Oncol. 2012;5(3):687-692.
10. Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496-1506.
11. Di Leo A, Isola J, Piette F, et al. A meta- analysis of phase III trials evaluating the predictive value of HER2 and topoisomerase alpha in early breast cancer patients treated with CMF or anthracycline-based adjuvant therapy [SABCS, abstract 705]. http://cancerres.aacrjournals.org/content/69/2_Supplement/705. Published 2008. Accessed May 4, 2017.
12. Cheang M, Chia SK, Tu D, et al. Anthracycline in basal breast cancer: the NCIC-CTG trial MA5 comparing adjuvant CMF to CEF [ASCO; abstract 519]. http://meetinglibrary.asco.org/content/35150-65. Published 2009. Accessed May 4, 2017.
13. Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666-2676.
14. Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123-134.
15. Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235-244.
Breast density and optimal screening for breast cancer
MY STORY: Prologue
My aunt received a breast cancer diagnosis at age 40, and she died at age 60, in 1970. Then, in 1975, my mother’s breast cancer was found at age 55, but only after she was examined for nipple retraction; on mammography, the cancer had been obscured by dense breast tissue. Mom had 2 metastatic nodes but participated in the earliest clinical trials of chemotherapy and lived free of breast cancer for another 41 years. Naturally I thought that, were I to develop this disease, I would want it found earlier. Ironically, it was, but only because I had spent my career trying to understand the optimal screening approaches for women with dense breasts—women like me.
Cancers are masked on mammography in dense breasts
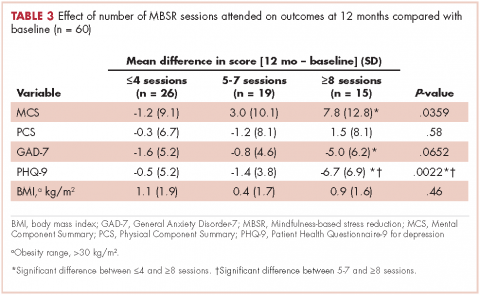
For women, screening mammography is an important step in reducing the risk of dying from breast cancer. The greatest benefits are realized by those who start annual screening at age 40, or 45 at the latest.1 As it takes 9 to 10 years to see a benefit from breast cancer screening at the population level, it is not logical to continue this testing when life expectancy is less than 10 years, as is the case with women age 85 or older, even those in the healthiest quartile.2–4 However, despite recent advances, the development of 3D mammography (tomosynthesis) (FIGURE 1) in particular, cancers can still be masked by dense breast tissue. Both 2D and 3D mammograms are x-rays; both dense tissue and cancers absorb x-rays and appear white.
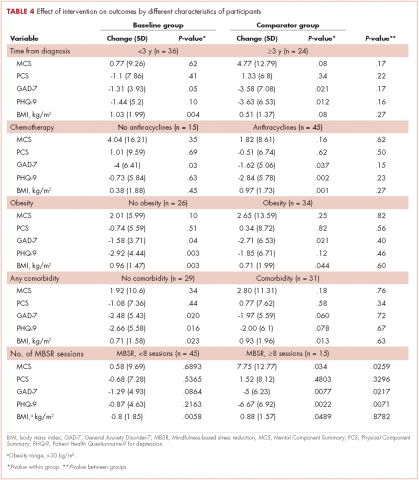
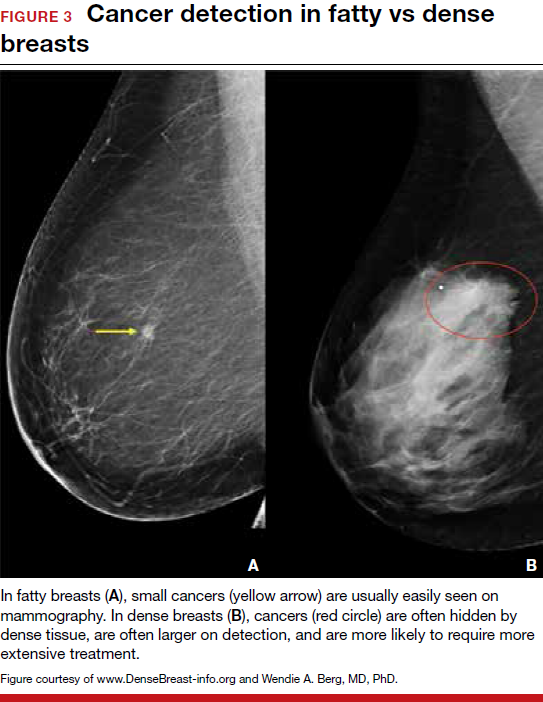
Breast density is determined on mammography and is categorized as fatty, scattered fibroglandular, heterogeneously dense, or extremely dense (FIGURE 2).5 Tissue in the heterogeneous and extreme categories is considered dense. More than half of women in their 40s have dense breasts; with some fatty involution occurring around menopause, the proportion drops to 25% for women in their 60s.6 About half of breast cancers have calcifications, which on mammography are usually easily visible even in dense breasts. The problem is with noncalcified invasive cancers that can be hidden by dense tissue (FIGURE 3).
3D mammography improves cancer detection but is of minimal benefit in extremely dense breasts
Although 3D mammography improves cancer detection in most women, any benefit is minimal in women with extremely dense breasts, as there is no inherent soft-tissue contrast.7 Masked cancers are often only discovered because of a lump after a normal screening mammogram, as so-called “interval cancers.” Compared with screen-detected cancers, interval cancers tend to be more biologically aggressive, to have spread to lymph nodes, and to have worse prognoses. However, even some small screen-detected cancers are biologically aggressive and can spread to lymph nodes quickly, and no screening test or combination of screening tests can prevent this occurrence completely, regardless of breast density.
Related article:
Get smart about dense breasts
MRI provides early detection across all breast densities
In all tissue densities, contrast-enhanced magnetic resonance imaging (MRI) is far better than mammography in detecting breast cancer.8 Women at high risk for breast cancer caused by mutations in BRCA1, BRCA2, p53, and other genes have poor outcomes with screening mammography alone—up to 50% of cancers are interval cancers. Annual screening MRI reduces this percentage significantly, to 11% in women with pathogenic BRCA1 mutations and to 4% in women with BRCA2 mutations.9 Warner and colleagues found a decrease in late-stage cancers in high-risk women who underwent annual MRI screenings compared to high-risk women unable to have MRI.10
The use of MRI for screening is limited by availability, patient tolerance,11 and high cost. Research is being conducted to further validate approaches using shortened screening MRI times (so-called “abbreviated” or “fast” MRI) and, thereby, improve access, tolerance, and reduce associated costs; several investigators already have reported promising results, and a few centers offer this modality directly to patients willing to pay $300 to $350 out of pocket.12,13 Even in normal-risk women, MRI significantly increases detection of early breast cancer after a normal mammogram and ultrasound, and the cancer detection benefit of MRI is seen across all breast densities.14
Most health insurance plans cover screening MRI only for women who meet defined risk criteria, including women who have a known disease-causing mutation—or are suspected of having one, given a family history of breast cancer with higher than 20% to 25% lifetime risk by a model that predicts mutation carrier status—as well as women who had chest radiation therapy before age 30, typically for Hodgkin lymphoma, and at least 8 years earlier.15 In addition, MRI can be considered in women with atypical breast biopsy results or a personal history of lobular carcinoma in situ (LCIS).16
Screening MRI should start by age 25 in women with disease-causing mutations, or at the time of atypical or LCIS biopsy results, and should be performed annually unless the woman is pregnant or has a metallic implant, renal insufficiency, or another contraindication to MRI. MRI can be beneficial in women with a personal history of cancer, although annual mammography remains the standard of care.17–19
MRI and mammography can be performed at the same time or on an alternating 6-month basis, with mammography usually starting only after age 30 because of the small risk that radiation poses for younger women. There are a few other impediments to having breast MRI: The woman must lie on her stomach within a confined space (tunnel), the contrast that is injected may not be well tolerated, and insurance does not cover the test for women who do not meet the defined risk criteria.11
Read why mammography supplemented by US is best for women with dense breasts.
Ultrasonography supplements mammography
Mammography supplemented with ultrasonography (US) has been studied as a “Goldilocks” or best-fit solution for the screening of women with dense breasts, as detection of invasive cancers is improved with the 2 modalities over mammography alone, and US is less invasive, better tolerated, and lower in cost than the more sensitive MRI.
In women with dense breasts, US has been found to improve cancer detection over mammography alone, and early results suggest a larger cancer detection benefit from US than from 3D mammography, although research is ongoing.20 Adding US reduces the interval cancer rate in women with dense breasts to less than 10% of all cancers found—similar to results for women with fatty breasts.17,21,22
US can be performed by a trained technologist or a physician using a small transducer, which usually provides diagnostic images (so that most callbacks would be for a true finding), or a larger transducer and an automated system can be used to create more than a thousand images for radiologist review.23,24 Use of a hybrid system, a small transducer with an automated arm, has been validated as well.25 Screening US is not available universally, and with all these approaches optimal performance requires trained personnel. Supplemental screening US usually is covered by insurance but is nearly always subject to a deductible/copay.
Related article:
Educate patients about dense breasts and cancer risk
Reducing false-positives, callbacks, and additional testing
Mammography carries a risk of false-positives. On average, 11% to 12% of women are called back for additional testing after a screening mammogram, and in more than 95% of women brought back for extra testing, no cancer is found.26 Women with dense breasts are more likely than those with less dense breasts to be called back.27 US and MRI improve cancer detection and therefore yield additional positive, but also false-positive, findings. Notably, callbacks decrease after the first round of screening with any modality or combination of tests, as long as prior examinations are available for comparison.
One advantage of 3D over 2D mammography is a decrease in extra testing for areas of asymmetry, which are often recognizable on 3D mammography as representing normal superimposed tissue.28–30 Architectural distortion, which is better seen on 3D mammography and usually represents either cancer or a benign radial scar, can lead to false-positive biopsies, although the average biopsy rate is no higher for 3D than for 2D alone.31 Typically, the 3D and 2D examinations are performed together (slightly more than doubling the radiation dose), or synthetic 2D images can be created from the 3D slices (resulting in a total radiation dose almost the same as standard 2D alone).
Most additional cancers seen on 3D mammography or US are lower-grade invasive cancers with good prognoses. Some aggressive high-grade breast cancers go undetected even when mammography is supplemented with US, either because they are too small to be seen or because they resemble common benign masses and may not be recognized. MRI is particularly effective in depicting high-grade cancers, even small ones.
The TABLE summarizes the relative rates of cancer detection and additional testing by various breast screening tests or combinations of tests. Neither clinical breast examination by a physician or other health care professional nor routine breast self-examination reduces the number of deaths caused by breast cancer. Nevertheless, women should monitor any changes in their breasts and report these changes to their clinician. A new lump, skin or nipple retraction, or a spontaneous clear or bloody nipple discharge merits diagnostic breast imaging even if a recent screening mammogram was normal.
FIGURE 4 is an updated decision support tool that suggests strategies for optimizingcancer detection with widely available screening methods.
Read how to take advantage of today’s technology for breast density screening
MY STORY: Epilogue
My annual 3D mammograms were normal, even the year my cancer was present. In 2014, I entered my family history into the IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model of breast cancer risk) (http://www.ems-trials.org/riskevaluator/) and calculated my lifetime risk at 19.7%. That is when I decided to have a screening MRI. My invasive breast cancer was easily seen on MRI and then on US. The cancer was node-negative, easily confirmed with needle biopsy, and treated with lumpectomy and radiation. There was no need for chemotherapy.
My personal experience prompted me to join JoAnn Pushkin and Cindy Henke-Sarmento, RT(R)(M), BA, in developing a website, www.DenseBreast-info.org, to give women and their physicians easy access to information on making decisions about screening in dense breasts.
My colleagues and I are often asked what is the best way to order supplemental imaging for a patient who may have dense breasts. Even in cases in which a mammogram does not exist or is unavailable, the following prescription can be implemented easily at centers that offer US: “2D plus 3D mammogram if available; if dense, perform ultrasound as needed.”
Related article:
DenseBreast-info.org: What this resource can offer you, and your patients
Breast density screening: Take advantage of today’s technology
Breast screening and diagnostic imaging have improved significantly since the 1970s, when many of the randomized trials of mammography were conducted. Breast density is one of the most common and important risk factors for development of breast cancer and is now incorporated into the Breast Cancer Surveillance Consortium model (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm) and the Tyrer-Cuzick model (see also http://densebreast-info.org/explanation-of-dense-breast-risk-models.aspx).32 Although we continue to validate newer approaches, women should take advantage of the improved methods of early cancer detection, particularly if they have dense breasts or are at high risk for breast cancer.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361(9367):1405–1410.
- Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
- Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS mammography. In: D’Orsi CJ, Sickles EA, Mendelson EB, et al, eds. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315(16):1784–1786.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening—MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1458–1468.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254(1):79–87.
- Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–2310.
- Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat. 2017;162(2):283–295.
- Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283(2):361–370.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- National Comprehensive Cancer Network. NCCN guidelines for detection, prevention, and risk reduction: breast cancer screening and diagnosis. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- Berg WA, Zhang Z, Lehrer D, et al; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010;195(2):510–516.
- Lehman CD, Lee JM, DeMartini WB, et al. Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst. 2016;108(3).
- Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial [published online ahead of print March 9, 2016]. J Clin Oncol. JCO634147.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7):1021–1026.
- Ohuchi N, Suzuki A, Sobue T, et al; J-START Investigator Groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-Cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387(10016):341–348.
- Berg WA, Mendelson EB. Technologist-performed handheld screening breast US imaging: how is it performed and what are the outcomes to date? Radiology. 2014;272(1):12–27.
- Brem RF, Tabár L, Duffy SW, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight study. Radiology. 2015;274(3):663–673.
- Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010;20(3):734–742.
- Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49–58.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173(9):807–816.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583–589.
- Bahl M, Lamb LR, Lehman CD. Pathologic outcomes of architectural distortion on digital 2D versus tomosynthesis mammography [published online ahead of print August 23, 2017]. AJR Am J Roentgenol. doi:10.2214/AJR.17.17979.
- Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K; Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer [published online ahead of print February 2, 2017]. JAMA Oncol. doi:10.1001/jamaoncol.2016.6326.
MY STORY: Prologue
My aunt received a breast cancer diagnosis at age 40, and she died at age 60, in 1970. Then, in 1975, my mother’s breast cancer was found at age 55, but only after she was examined for nipple retraction; on mammography, the cancer had been obscured by dense breast tissue. Mom had 2 metastatic nodes but participated in the earliest clinical trials of chemotherapy and lived free of breast cancer for another 41 years. Naturally I thought that, were I to develop this disease, I would want it found earlier. Ironically, it was, but only because I had spent my career trying to understand the optimal screening approaches for women with dense breasts—women like me.
Cancers are masked on mammography in dense breasts
For women, screening mammography is an important step in reducing the risk of dying from breast cancer. The greatest benefits are realized by those who start annual screening at age 40, or 45 at the latest.1 As it takes 9 to 10 years to see a benefit from breast cancer screening at the population level, it is not logical to continue this testing when life expectancy is less than 10 years, as is the case with women age 85 or older, even those in the healthiest quartile.2–4 However, despite recent advances, the development of 3D mammography (tomosynthesis) (FIGURE 1) in particular, cancers can still be masked by dense breast tissue. Both 2D and 3D mammograms are x-rays; both dense tissue and cancers absorb x-rays and appear white.

Breast density is determined on mammography and is categorized as fatty, scattered fibroglandular, heterogeneously dense, or extremely dense (FIGURE 2).5 Tissue in the heterogeneous and extreme categories is considered dense. More than half of women in their 40s have dense breasts; with some fatty involution occurring around menopause, the proportion drops to 25% for women in their 60s.6 About half of breast cancers have calcifications, which on mammography are usually easily visible even in dense breasts. The problem is with noncalcified invasive cancers that can be hidden by dense tissue (FIGURE 3).


3D mammography improves cancer detection but is of minimal benefit in extremely dense breasts
Although 3D mammography improves cancer detection in most women, any benefit is minimal in women with extremely dense breasts, as there is no inherent soft-tissue contrast.7 Masked cancers are often only discovered because of a lump after a normal screening mammogram, as so-called “interval cancers.” Compared with screen-detected cancers, interval cancers tend to be more biologically aggressive, to have spread to lymph nodes, and to have worse prognoses. However, even some small screen-detected cancers are biologically aggressive and can spread to lymph nodes quickly, and no screening test or combination of screening tests can prevent this occurrence completely, regardless of breast density.
Related article:
Get smart about dense breasts

MRI provides early detection across all breast densities
In all tissue densities, contrast-enhanced magnetic resonance imaging (MRI) is far better than mammography in detecting breast cancer.8 Women at high risk for breast cancer caused by mutations in BRCA1, BRCA2, p53, and other genes have poor outcomes with screening mammography alone—up to 50% of cancers are interval cancers. Annual screening MRI reduces this percentage significantly, to 11% in women with pathogenic BRCA1 mutations and to 4% in women with BRCA2 mutations.9 Warner and colleagues found a decrease in late-stage cancers in high-risk women who underwent annual MRI screenings compared to high-risk women unable to have MRI.10
The use of MRI for screening is limited by availability, patient tolerance,11 and high cost. Research is being conducted to further validate approaches using shortened screening MRI times (so-called “abbreviated” or “fast” MRI) and, thereby, improve access, tolerance, and reduce associated costs; several investigators already have reported promising results, and a few centers offer this modality directly to patients willing to pay $300 to $350 out of pocket.12,13 Even in normal-risk women, MRI significantly increases detection of early breast cancer after a normal mammogram and ultrasound, and the cancer detection benefit of MRI is seen across all breast densities.14
Most health insurance plans cover screening MRI only for women who meet defined risk criteria, including women who have a known disease-causing mutation—or are suspected of having one, given a family history of breast cancer with higher than 20% to 25% lifetime risk by a model that predicts mutation carrier status—as well as women who had chest radiation therapy before age 30, typically for Hodgkin lymphoma, and at least 8 years earlier.15 In addition, MRI can be considered in women with atypical breast biopsy results or a personal history of lobular carcinoma in situ (LCIS).16
Screening MRI should start by age 25 in women with disease-causing mutations, or at the time of atypical or LCIS biopsy results, and should be performed annually unless the woman is pregnant or has a metallic implant, renal insufficiency, or another contraindication to MRI. MRI can be beneficial in women with a personal history of cancer, although annual mammography remains the standard of care.17–19
MRI and mammography can be performed at the same time or on an alternating 6-month basis, with mammography usually starting only after age 30 because of the small risk that radiation poses for younger women. There are a few other impediments to having breast MRI: The woman must lie on her stomach within a confined space (tunnel), the contrast that is injected may not be well tolerated, and insurance does not cover the test for women who do not meet the defined risk criteria.11
Read why mammography supplemented by US is best for women with dense breasts.
Ultrasonography supplements mammography
Mammography supplemented with ultrasonography (US) has been studied as a “Goldilocks” or best-fit solution for the screening of women with dense breasts, as detection of invasive cancers is improved with the 2 modalities over mammography alone, and US is less invasive, better tolerated, and lower in cost than the more sensitive MRI.
In women with dense breasts, US has been found to improve cancer detection over mammography alone, and early results suggest a larger cancer detection benefit from US than from 3D mammography, although research is ongoing.20 Adding US reduces the interval cancer rate in women with dense breasts to less than 10% of all cancers found—similar to results for women with fatty breasts.17,21,22
US can be performed by a trained technologist or a physician using a small transducer, which usually provides diagnostic images (so that most callbacks would be for a true finding), or a larger transducer and an automated system can be used to create more than a thousand images for radiologist review.23,24 Use of a hybrid system, a small transducer with an automated arm, has been validated as well.25 Screening US is not available universally, and with all these approaches optimal performance requires trained personnel. Supplemental screening US usually is covered by insurance but is nearly always subject to a deductible/copay.
Related article:
Educate patients about dense breasts and cancer risk
Reducing false-positives, callbacks, and additional testing
Mammography carries a risk of false-positives. On average, 11% to 12% of women are called back for additional testing after a screening mammogram, and in more than 95% of women brought back for extra testing, no cancer is found.26 Women with dense breasts are more likely than those with less dense breasts to be called back.27 US and MRI improve cancer detection and therefore yield additional positive, but also false-positive, findings. Notably, callbacks decrease after the first round of screening with any modality or combination of tests, as long as prior examinations are available for comparison.
One advantage of 3D over 2D mammography is a decrease in extra testing for areas of asymmetry, which are often recognizable on 3D mammography as representing normal superimposed tissue.28–30 Architectural distortion, which is better seen on 3D mammography and usually represents either cancer or a benign radial scar, can lead to false-positive biopsies, although the average biopsy rate is no higher for 3D than for 2D alone.31 Typically, the 3D and 2D examinations are performed together (slightly more than doubling the radiation dose), or synthetic 2D images can be created from the 3D slices (resulting in a total radiation dose almost the same as standard 2D alone).
Most additional cancers seen on 3D mammography or US are lower-grade invasive cancers with good prognoses. Some aggressive high-grade breast cancers go undetected even when mammography is supplemented with US, either because they are too small to be seen or because they resemble common benign masses and may not be recognized. MRI is particularly effective in depicting high-grade cancers, even small ones.
The TABLE summarizes the relative rates of cancer detection and additional testing by various breast screening tests or combinations of tests. Neither clinical breast examination by a physician or other health care professional nor routine breast self-examination reduces the number of deaths caused by breast cancer. Nevertheless, women should monitor any changes in their breasts and report these changes to their clinician. A new lump, skin or nipple retraction, or a spontaneous clear or bloody nipple discharge merits diagnostic breast imaging even if a recent screening mammogram was normal.
FIGURE 4 is an updated decision support tool that suggests strategies for optimizingcancer detection with widely available screening methods.
Read how to take advantage of today’s technology for breast density screening
MY STORY: Epilogue
My annual 3D mammograms were normal, even the year my cancer was present. In 2014, I entered my family history into the IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model of breast cancer risk) (http://www.ems-trials.org/riskevaluator/) and calculated my lifetime risk at 19.7%. That is when I decided to have a screening MRI. My invasive breast cancer was easily seen on MRI and then on US. The cancer was node-negative, easily confirmed with needle biopsy, and treated with lumpectomy and radiation. There was no need for chemotherapy.
My personal experience prompted me to join JoAnn Pushkin and Cindy Henke-Sarmento, RT(R)(M), BA, in developing a website, www.DenseBreast-info.org, to give women and their physicians easy access to information on making decisions about screening in dense breasts.
My colleagues and I are often asked what is the best way to order supplemental imaging for a patient who may have dense breasts. Even in cases in which a mammogram does not exist or is unavailable, the following prescription can be implemented easily at centers that offer US: “2D plus 3D mammogram if available; if dense, perform ultrasound as needed.”
Related article:
DenseBreast-info.org: What this resource can offer you, and your patients
Breast density screening: Take advantage of today’s technology
Breast screening and diagnostic imaging have improved significantly since the 1970s, when many of the randomized trials of mammography were conducted. Breast density is one of the most common and important risk factors for development of breast cancer and is now incorporated into the Breast Cancer Surveillance Consortium model (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm) and the Tyrer-Cuzick model (see also http://densebreast-info.org/explanation-of-dense-breast-risk-models.aspx).32 Although we continue to validate newer approaches, women should take advantage of the improved methods of early cancer detection, particularly if they have dense breasts or are at high risk for breast cancer.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
MY STORY: Prologue
My aunt received a breast cancer diagnosis at age 40, and she died at age 60, in 1970. Then, in 1975, my mother’s breast cancer was found at age 55, but only after she was examined for nipple retraction; on mammography, the cancer had been obscured by dense breast tissue. Mom had 2 metastatic nodes but participated in the earliest clinical trials of chemotherapy and lived free of breast cancer for another 41 years. Naturally I thought that, were I to develop this disease, I would want it found earlier. Ironically, it was, but only because I had spent my career trying to understand the optimal screening approaches for women with dense breasts—women like me.
Cancers are masked on mammography in dense breasts
For women, screening mammography is an important step in reducing the risk of dying from breast cancer. The greatest benefits are realized by those who start annual screening at age 40, or 45 at the latest.1 As it takes 9 to 10 years to see a benefit from breast cancer screening at the population level, it is not logical to continue this testing when life expectancy is less than 10 years, as is the case with women age 85 or older, even those in the healthiest quartile.2–4 However, despite recent advances, the development of 3D mammography (tomosynthesis) (FIGURE 1) in particular, cancers can still be masked by dense breast tissue. Both 2D and 3D mammograms are x-rays; both dense tissue and cancers absorb x-rays and appear white.

Breast density is determined on mammography and is categorized as fatty, scattered fibroglandular, heterogeneously dense, or extremely dense (FIGURE 2).5 Tissue in the heterogeneous and extreme categories is considered dense. More than half of women in their 40s have dense breasts; with some fatty involution occurring around menopause, the proportion drops to 25% for women in their 60s.6 About half of breast cancers have calcifications, which on mammography are usually easily visible even in dense breasts. The problem is with noncalcified invasive cancers that can be hidden by dense tissue (FIGURE 3).


3D mammography improves cancer detection but is of minimal benefit in extremely dense breasts
Although 3D mammography improves cancer detection in most women, any benefit is minimal in women with extremely dense breasts, as there is no inherent soft-tissue contrast.7 Masked cancers are often only discovered because of a lump after a normal screening mammogram, as so-called “interval cancers.” Compared with screen-detected cancers, interval cancers tend to be more biologically aggressive, to have spread to lymph nodes, and to have worse prognoses. However, even some small screen-detected cancers are biologically aggressive and can spread to lymph nodes quickly, and no screening test or combination of screening tests can prevent this occurrence completely, regardless of breast density.
Related article:
Get smart about dense breasts

MRI provides early detection across all breast densities
In all tissue densities, contrast-enhanced magnetic resonance imaging (MRI) is far better than mammography in detecting breast cancer.8 Women at high risk for breast cancer caused by mutations in BRCA1, BRCA2, p53, and other genes have poor outcomes with screening mammography alone—up to 50% of cancers are interval cancers. Annual screening MRI reduces this percentage significantly, to 11% in women with pathogenic BRCA1 mutations and to 4% in women with BRCA2 mutations.9 Warner and colleagues found a decrease in late-stage cancers in high-risk women who underwent annual MRI screenings compared to high-risk women unable to have MRI.10
The use of MRI for screening is limited by availability, patient tolerance,11 and high cost. Research is being conducted to further validate approaches using shortened screening MRI times (so-called “abbreviated” or “fast” MRI) and, thereby, improve access, tolerance, and reduce associated costs; several investigators already have reported promising results, and a few centers offer this modality directly to patients willing to pay $300 to $350 out of pocket.12,13 Even in normal-risk women, MRI significantly increases detection of early breast cancer after a normal mammogram and ultrasound, and the cancer detection benefit of MRI is seen across all breast densities.14
Most health insurance plans cover screening MRI only for women who meet defined risk criteria, including women who have a known disease-causing mutation—or are suspected of having one, given a family history of breast cancer with higher than 20% to 25% lifetime risk by a model that predicts mutation carrier status—as well as women who had chest radiation therapy before age 30, typically for Hodgkin lymphoma, and at least 8 years earlier.15 In addition, MRI can be considered in women with atypical breast biopsy results or a personal history of lobular carcinoma in situ (LCIS).16
Screening MRI should start by age 25 in women with disease-causing mutations, or at the time of atypical or LCIS biopsy results, and should be performed annually unless the woman is pregnant or has a metallic implant, renal insufficiency, or another contraindication to MRI. MRI can be beneficial in women with a personal history of cancer, although annual mammography remains the standard of care.17–19
MRI and mammography can be performed at the same time or on an alternating 6-month basis, with mammography usually starting only after age 30 because of the small risk that radiation poses for younger women. There are a few other impediments to having breast MRI: The woman must lie on her stomach within a confined space (tunnel), the contrast that is injected may not be well tolerated, and insurance does not cover the test for women who do not meet the defined risk criteria.11
Read why mammography supplemented by US is best for women with dense breasts.
Ultrasonography supplements mammography
Mammography supplemented with ultrasonography (US) has been studied as a “Goldilocks” or best-fit solution for the screening of women with dense breasts, as detection of invasive cancers is improved with the 2 modalities over mammography alone, and US is less invasive, better tolerated, and lower in cost than the more sensitive MRI.
In women with dense breasts, US has been found to improve cancer detection over mammography alone, and early results suggest a larger cancer detection benefit from US than from 3D mammography, although research is ongoing.20 Adding US reduces the interval cancer rate in women with dense breasts to less than 10% of all cancers found—similar to results for women with fatty breasts.17,21,22
US can be performed by a trained technologist or a physician using a small transducer, which usually provides diagnostic images (so that most callbacks would be for a true finding), or a larger transducer and an automated system can be used to create more than a thousand images for radiologist review.23,24 Use of a hybrid system, a small transducer with an automated arm, has been validated as well.25 Screening US is not available universally, and with all these approaches optimal performance requires trained personnel. Supplemental screening US usually is covered by insurance but is nearly always subject to a deductible/copay.
Related article:
Educate patients about dense breasts and cancer risk
Reducing false-positives, callbacks, and additional testing
Mammography carries a risk of false-positives. On average, 11% to 12% of women are called back for additional testing after a screening mammogram, and in more than 95% of women brought back for extra testing, no cancer is found.26 Women with dense breasts are more likely than those with less dense breasts to be called back.27 US and MRI improve cancer detection and therefore yield additional positive, but also false-positive, findings. Notably, callbacks decrease after the first round of screening with any modality or combination of tests, as long as prior examinations are available for comparison.
One advantage of 3D over 2D mammography is a decrease in extra testing for areas of asymmetry, which are often recognizable on 3D mammography as representing normal superimposed tissue.28–30 Architectural distortion, which is better seen on 3D mammography and usually represents either cancer or a benign radial scar, can lead to false-positive biopsies, although the average biopsy rate is no higher for 3D than for 2D alone.31 Typically, the 3D and 2D examinations are performed together (slightly more than doubling the radiation dose), or synthetic 2D images can be created from the 3D slices (resulting in a total radiation dose almost the same as standard 2D alone).
Most additional cancers seen on 3D mammography or US are lower-grade invasive cancers with good prognoses. Some aggressive high-grade breast cancers go undetected even when mammography is supplemented with US, either because they are too small to be seen or because they resemble common benign masses and may not be recognized. MRI is particularly effective in depicting high-grade cancers, even small ones.
The TABLE summarizes the relative rates of cancer detection and additional testing by various breast screening tests or combinations of tests. Neither clinical breast examination by a physician or other health care professional nor routine breast self-examination reduces the number of deaths caused by breast cancer. Nevertheless, women should monitor any changes in their breasts and report these changes to their clinician. A new lump, skin or nipple retraction, or a spontaneous clear or bloody nipple discharge merits diagnostic breast imaging even if a recent screening mammogram was normal.
FIGURE 4 is an updated decision support tool that suggests strategies for optimizingcancer detection with widely available screening methods.
Read how to take advantage of today’s technology for breast density screening
MY STORY: Epilogue
My annual 3D mammograms were normal, even the year my cancer was present. In 2014, I entered my family history into the IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model of breast cancer risk) (http://www.ems-trials.org/riskevaluator/) and calculated my lifetime risk at 19.7%. That is when I decided to have a screening MRI. My invasive breast cancer was easily seen on MRI and then on US. The cancer was node-negative, easily confirmed with needle biopsy, and treated with lumpectomy and radiation. There was no need for chemotherapy.
My personal experience prompted me to join JoAnn Pushkin and Cindy Henke-Sarmento, RT(R)(M), BA, in developing a website, www.DenseBreast-info.org, to give women and their physicians easy access to information on making decisions about screening in dense breasts.
My colleagues and I are often asked what is the best way to order supplemental imaging for a patient who may have dense breasts. Even in cases in which a mammogram does not exist or is unavailable, the following prescription can be implemented easily at centers that offer US: “2D plus 3D mammogram if available; if dense, perform ultrasound as needed.”
Related article:
DenseBreast-info.org: What this resource can offer you, and your patients
Breast density screening: Take advantage of today’s technology
Breast screening and diagnostic imaging have improved significantly since the 1970s, when many of the randomized trials of mammography were conducted. Breast density is one of the most common and important risk factors for development of breast cancer and is now incorporated into the Breast Cancer Surveillance Consortium model (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm) and the Tyrer-Cuzick model (see also http://densebreast-info.org/explanation-of-dense-breast-risk-models.aspx).32 Although we continue to validate newer approaches, women should take advantage of the improved methods of early cancer detection, particularly if they have dense breasts or are at high risk for breast cancer.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361(9367):1405–1410.
- Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
- Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS mammography. In: D’Orsi CJ, Sickles EA, Mendelson EB, et al, eds. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315(16):1784–1786.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening—MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1458–1468.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254(1):79–87.
- Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–2310.
- Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat. 2017;162(2):283–295.
- Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283(2):361–370.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- National Comprehensive Cancer Network. NCCN guidelines for detection, prevention, and risk reduction: breast cancer screening and diagnosis. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- Berg WA, Zhang Z, Lehrer D, et al; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010;195(2):510–516.
- Lehman CD, Lee JM, DeMartini WB, et al. Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst. 2016;108(3).
- Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial [published online ahead of print March 9, 2016]. J Clin Oncol. JCO634147.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7):1021–1026.
- Ohuchi N, Suzuki A, Sobue T, et al; J-START Investigator Groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-Cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387(10016):341–348.
- Berg WA, Mendelson EB. Technologist-performed handheld screening breast US imaging: how is it performed and what are the outcomes to date? Radiology. 2014;272(1):12–27.
- Brem RF, Tabár L, Duffy SW, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight study. Radiology. 2015;274(3):663–673.
- Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010;20(3):734–742.
- Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49–58.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173(9):807–816.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583–589.
- Bahl M, Lamb LR, Lehman CD. Pathologic outcomes of architectural distortion on digital 2D versus tomosynthesis mammography [published online ahead of print August 23, 2017]. AJR Am J Roentgenol. doi:10.2214/AJR.17.17979.
- Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K; Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer [published online ahead of print February 2, 2017]. JAMA Oncol. doi:10.1001/jamaoncol.2016.6326.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361(9367):1405–1410.
- Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
- Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS mammography. In: D’Orsi CJ, Sickles EA, Mendelson EB, et al, eds. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315(16):1784–1786.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening—MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1458–1468.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254(1):79–87.
- Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–2310.
- Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat. 2017;162(2):283–295.
- Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283(2):361–370.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- National Comprehensive Cancer Network. NCCN guidelines for detection, prevention, and risk reduction: breast cancer screening and diagnosis. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- Berg WA, Zhang Z, Lehrer D, et al; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010;195(2):510–516.
- Lehman CD, Lee JM, DeMartini WB, et al. Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst. 2016;108(3).
- Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial [published online ahead of print March 9, 2016]. J Clin Oncol. JCO634147.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7):1021–1026.
- Ohuchi N, Suzuki A, Sobue T, et al; J-START Investigator Groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-Cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387(10016):341–348.
- Berg WA, Mendelson EB. Technologist-performed handheld screening breast US imaging: how is it performed and what are the outcomes to date? Radiology. 2014;272(1):12–27.
- Brem RF, Tabár L, Duffy SW, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight study. Radiology. 2015;274(3):663–673.
- Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010;20(3):734–742.
- Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49–58.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173(9):807–816.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583–589.
- Bahl M, Lamb LR, Lehman CD. Pathologic outcomes of architectural distortion on digital 2D versus tomosynthesis mammography [published online ahead of print August 23, 2017]. AJR Am J Roentgenol. doi:10.2214/AJR.17.17979.
- Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K; Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer [published online ahead of print February 2, 2017]. JAMA Oncol. doi:10.1001/jamaoncol.2016.6326.
GEICAM/2006-10: Adjuvant fulvestrant/anastrozole role in early breast cancer uncertain
MADRID – Until funders pulled the financial rug out from under them, investigators in the GEICAM/2006-10 trial thought they were going to find out whether adding fulvestrant (Faslodex) to anastrozole (Femara) could improve disease-free survival for postmenopausal women with early-stage hormone receptor–positive/human epidermal growth factor receptor 2–negative (HR+/HER2–) breast cancer.
But, when the results of the FACT trial were published, showing no advantage for fulvestrant and anastrozole over anastrozole alone as first-line therapy for postmenopausal women with HR+ breast cancer, recruitment in the GEICAM 2006-10 trial was halted midstream after only 872 of the planned 2852 patients were enrolled.
“Fulvestrant at the current recommended dose of 500 mg merits further testing as adjuvant endocrine therapy, either alone, in sequence, or in combination with aromatase inhibitors,” he said.
Invited discussant Nadia Harbeck, MD, PhD, from the University of Munich, agreed.
“I just want to urge you that you don’t take this as [evidence of] nonefficacy of a SERD in the adjuvant setting, but it’s a trial with an underdosed drug, and there could be better development in future with a novel compound to come or even with fulvestrant at a better dose,” she said.
Approximately 15% of patients with HR+ breast cancer treated with endocrine therapy have a relapse within the first 5 years of therapy, which led investigators to speculate whether incomplete suppression of estrogen receptors could lead to resistance to aromatase inhibitors (AIs), such as anastrozole.
The GEICAM/2006-10 trial was designed to see whether achieving a complete estrogen blockade with an AI, minimizing serum estradiol levels, and using the SERD fulvestrant to prevent activation of tumor estrogen receptors could prove a more effective treatment strategy than endocrine therapy with an AI alone, Dr. Ruíz-Borrego explained.
The investigators enrolled postmenopausal women with early-stage breast cancer who had undergone surgery with or without neoadjuvant or adjuvant chemotherapy, and – after stratification for number of lymph nodes, for chemotherapy, and for hormone receptor status (positive for estrogen and/or progesterone receptors) – randomly assigned them to oral anastrozole 1 mg daily or oral anastrozole 1mg daily plus fulvestrant delivered intramuscularly 500 mg on the first day of treatment, 250 mg on days 14 and 28, then 250 mg every 28 days thereafter for 3 years.
As noted, only 872 of the initial target of 2,825 patients were enrolled and randomized to both fulvestrant and anastrozole (435 patients) or to anastrozole alone (437).
After 5 years of follow-up, there were no significant differences in either the primary endpoint of disease-free survival between patients treated with the combination and those treated with anastrozole alone (90.97% vs. 90.76%, respectively). Similarly, there were no differences in the secondary endpoints of breast cancer–specific survival (93.17% vs. 92.39%) or overall survival (94.81% vs. 95.34%).
The trial results “reflect on the checkered history of the development of this drug [fulvestrant], which probably missed out on a great potential for patients to get access to a drug like this in the early breast cancer setting,” Dr. Harbeck remarked. The trial results were muddied by the abrupt closure of accrual and by the use of a fulvestrant dose half that of the currently recommended dose, she noted, adding that there is preclinical evidence to suggest that fulvestrant and anastrozole combined may be less effective than if the drugs were used in sequence.
“So maybe, for the further development of SERDs, we may want to go into more sequencing than combination strategies,” she said.
The trial was funded by AstraZeneca, although funding was withdrawn before full recruitment was completed. Dr. Ruíz-Borrego and Dr. Harbeck reported having no relevant disclosures.
MADRID – Until funders pulled the financial rug out from under them, investigators in the GEICAM/2006-10 trial thought they were going to find out whether adding fulvestrant (Faslodex) to anastrozole (Femara) could improve disease-free survival for postmenopausal women with early-stage hormone receptor–positive/human epidermal growth factor receptor 2–negative (HR+/HER2–) breast cancer.
But, when the results of the FACT trial were published, showing no advantage for fulvestrant and anastrozole over anastrozole alone as first-line therapy for postmenopausal women with HR+ breast cancer, recruitment in the GEICAM 2006-10 trial was halted midstream after only 872 of the planned 2852 patients were enrolled.
“Fulvestrant at the current recommended dose of 500 mg merits further testing as adjuvant endocrine therapy, either alone, in sequence, or in combination with aromatase inhibitors,” he said.
Invited discussant Nadia Harbeck, MD, PhD, from the University of Munich, agreed.
“I just want to urge you that you don’t take this as [evidence of] nonefficacy of a SERD in the adjuvant setting, but it’s a trial with an underdosed drug, and there could be better development in future with a novel compound to come or even with fulvestrant at a better dose,” she said.
Approximately 15% of patients with HR+ breast cancer treated with endocrine therapy have a relapse within the first 5 years of therapy, which led investigators to speculate whether incomplete suppression of estrogen receptors could lead to resistance to aromatase inhibitors (AIs), such as anastrozole.
The GEICAM/2006-10 trial was designed to see whether achieving a complete estrogen blockade with an AI, minimizing serum estradiol levels, and using the SERD fulvestrant to prevent activation of tumor estrogen receptors could prove a more effective treatment strategy than endocrine therapy with an AI alone, Dr. Ruíz-Borrego explained.
The investigators enrolled postmenopausal women with early-stage breast cancer who had undergone surgery with or without neoadjuvant or adjuvant chemotherapy, and – after stratification for number of lymph nodes, for chemotherapy, and for hormone receptor status (positive for estrogen and/or progesterone receptors) – randomly assigned them to oral anastrozole 1 mg daily or oral anastrozole 1mg daily plus fulvestrant delivered intramuscularly 500 mg on the first day of treatment, 250 mg on days 14 and 28, then 250 mg every 28 days thereafter for 3 years.
As noted, only 872 of the initial target of 2,825 patients were enrolled and randomized to both fulvestrant and anastrozole (435 patients) or to anastrozole alone (437).
After 5 years of follow-up, there were no significant differences in either the primary endpoint of disease-free survival between patients treated with the combination and those treated with anastrozole alone (90.97% vs. 90.76%, respectively). Similarly, there were no differences in the secondary endpoints of breast cancer–specific survival (93.17% vs. 92.39%) or overall survival (94.81% vs. 95.34%).
The trial results “reflect on the checkered history of the development of this drug [fulvestrant], which probably missed out on a great potential for patients to get access to a drug like this in the early breast cancer setting,” Dr. Harbeck remarked. The trial results were muddied by the abrupt closure of accrual and by the use of a fulvestrant dose half that of the currently recommended dose, she noted, adding that there is preclinical evidence to suggest that fulvestrant and anastrozole combined may be less effective than if the drugs were used in sequence.
“So maybe, for the further development of SERDs, we may want to go into more sequencing than combination strategies,” she said.
The trial was funded by AstraZeneca, although funding was withdrawn before full recruitment was completed. Dr. Ruíz-Borrego and Dr. Harbeck reported having no relevant disclosures.
MADRID – Until funders pulled the financial rug out from under them, investigators in the GEICAM/2006-10 trial thought they were going to find out whether adding fulvestrant (Faslodex) to anastrozole (Femara) could improve disease-free survival for postmenopausal women with early-stage hormone receptor–positive/human epidermal growth factor receptor 2–negative (HR+/HER2–) breast cancer.
But, when the results of the FACT trial were published, showing no advantage for fulvestrant and anastrozole over anastrozole alone as first-line therapy for postmenopausal women with HR+ breast cancer, recruitment in the GEICAM 2006-10 trial was halted midstream after only 872 of the planned 2852 patients were enrolled.
“Fulvestrant at the current recommended dose of 500 mg merits further testing as adjuvant endocrine therapy, either alone, in sequence, or in combination with aromatase inhibitors,” he said.
Invited discussant Nadia Harbeck, MD, PhD, from the University of Munich, agreed.
“I just want to urge you that you don’t take this as [evidence of] nonefficacy of a SERD in the adjuvant setting, but it’s a trial with an underdosed drug, and there could be better development in future with a novel compound to come or even with fulvestrant at a better dose,” she said.
Approximately 15% of patients with HR+ breast cancer treated with endocrine therapy have a relapse within the first 5 years of therapy, which led investigators to speculate whether incomplete suppression of estrogen receptors could lead to resistance to aromatase inhibitors (AIs), such as anastrozole.
The GEICAM/2006-10 trial was designed to see whether achieving a complete estrogen blockade with an AI, minimizing serum estradiol levels, and using the SERD fulvestrant to prevent activation of tumor estrogen receptors could prove a more effective treatment strategy than endocrine therapy with an AI alone, Dr. Ruíz-Borrego explained.
The investigators enrolled postmenopausal women with early-stage breast cancer who had undergone surgery with or without neoadjuvant or adjuvant chemotherapy, and – after stratification for number of lymph nodes, for chemotherapy, and for hormone receptor status (positive for estrogen and/or progesterone receptors) – randomly assigned them to oral anastrozole 1 mg daily or oral anastrozole 1mg daily plus fulvestrant delivered intramuscularly 500 mg on the first day of treatment, 250 mg on days 14 and 28, then 250 mg every 28 days thereafter for 3 years.
As noted, only 872 of the initial target of 2,825 patients were enrolled and randomized to both fulvestrant and anastrozole (435 patients) or to anastrozole alone (437).
After 5 years of follow-up, there were no significant differences in either the primary endpoint of disease-free survival between patients treated with the combination and those treated with anastrozole alone (90.97% vs. 90.76%, respectively). Similarly, there were no differences in the secondary endpoints of breast cancer–specific survival (93.17% vs. 92.39%) or overall survival (94.81% vs. 95.34%).
The trial results “reflect on the checkered history of the development of this drug [fulvestrant], which probably missed out on a great potential for patients to get access to a drug like this in the early breast cancer setting,” Dr. Harbeck remarked. The trial results were muddied by the abrupt closure of accrual and by the use of a fulvestrant dose half that of the currently recommended dose, she noted, adding that there is preclinical evidence to suggest that fulvestrant and anastrozole combined may be less effective than if the drugs were used in sequence.
“So maybe, for the further development of SERDs, we may want to go into more sequencing than combination strategies,” she said.
The trial was funded by AstraZeneca, although funding was withdrawn before full recruitment was completed. Dr. Ruíz-Borrego and Dr. Harbeck reported having no relevant disclosures.
AT ESMO 2017
Key clinical point: Adding fulvestrant to anastrozole did not improve outcomes in women with HR+/HER2– early-stage breast cancer, but the trial was hampered by early closure.
Major finding: There were no significant differences in disease-free, breast cancer–specific, or overall survival with fulvestrant/anastrozole vs. anastrozole alone.
Data source: Randomized phase 3 trial in 872 of 2,582 planned patients.
Disclosures: The trial was funded by AstraZeneca, although funding was withdrawn before full recruitment was completed. Dr. Ruíz-Borrego and Dr. Harbeck reported having no relevant disclosures.
‘Very daring study’ of neoadjuvant AI/CDKi combo in early BC is hypothesis generating
MADRID – For women with luminal breast cancer who are not initially candidates for breast-conserving surgery, neoadjuvant therapy with an aromatase inhibitor and a cyclin-dependent kinases 4/6 (CDK4/6) inhibitor offered a slightly higher residual cancer burden prior to surgery, but a significantly better safety profile than conventional chemotherapy with similar near-term safety outcomes, results of a phase 2 parallel group, noncomparative trial suggested.
Among 60 patients evaluable for response in an interim analysis of the UNICANCER NeoPAL trial, one patient (3.3%) treated with a combination of letrozole (Femara) and palbociclib (Ibrance) had a residual cancer burden (RCB) score of 0 (equivalent to a pathologic complete response; pCR), whereas three patients (10%) treated with FEC 100 chemotherapy (5-fluorouracil, epirubicin, and cyclophosphamide) had RCB 0 or I, reported Paul-Henri Cottu, MD, of the Institut Curie in Paris.
Following the interim analysis, the independent data monitoring committee for the NeoPAL trial recommended halting accrual; accrual was stopped in November 2016, after 106 patients had been randomized.
The IDMC also recommended that patients in the letrozole/palbociclib arm who did not have an RCB of 0 or I be offered adjuvant chemotherapy.
“Please note that 70% of those patients refused adjuvant chemotherapy,” Dr. Cottu said.
The investigators set out to test whether letrozole and palbociclib, which have been shown to have synergistic antiproliferative activity against advanced luminal breast cancer, could have similar benefits in the neoadjuvant setting.
They screened for women with luminal breast cancer who had newly diagnosed stage II or III breast cancer with biopsy-proven endocrine receptor–positive, human epidermal growth factor receptor 2–negative tumors, using the Prosigna test, based on the PAM50 gene signature assay. Women with node-positive luminal A or luminal B disease were enrolled and randomized to receive either letrozole 2.5 mg and palbociclib 125 mg daily for 3 out of every 4 weeks over 19 weeks, or three cycles of FEC 100, followed by three cycles of docetaxel 100 mg/m2 every 3 weeks, followed by surgery.
An interim analysis was planned after 30 patients were evaluable for RCB in the experimental arm, and, as prespecified, the trial was stopped for futility when fewer than five patients had an RCB of 0 or I.
The safety analysis, conducted with all 106 patients randomized, showed that letrozole/palbocilib was associated with more frequent grade 3 neutropenia (23% vs. 10% of patients with FEC), but less grade 4 neutropenia (1% vs. 11%, respectively), and no febrile neutropenia vs. 6% in the chemotherapy arm.
There were 2 serious adverse events with the AI/CDK-inhibitor combination vs. 17 with chemotherapy. Dose reductions or interruptions were less frequent with letrozole/palbociclib (10 and 16), and only two patients in the experimental arm required premature cessation of therapy vs. seven in the chemotherapy arm.
The final response analysis in 103 patients showed that the rate of RCB 0 or I was 7.7% with letrozole/palbociclib and 15.7% with chemotherapy. Respective rates of RCB II-III were 92.3% and 84.3%.
Clinical response rates were similar in each study arm, with approximately 30% complete responses and 44% partial responses.
In each arm, slightly less than one-third of patients underwent mastectomy, and a little more than two-thirds were able to have breast-conserving surgery after neoadjuvant therapy.
The patients will be followed out to at least 3 years to see whether those patients in the letrozole/palbociclib arm who turned down subsequent chemotherapy will have worse survival than patients who decided to undergo it, Dr. Cottu said.
Invited discussant Nadia Harbeck, MD, PhD, of the University of Munich called the NeoPAL trial “a very daring study.”
“This is not a practice-changing trial, but it’s a very, very interesting hypothesis-generating trial,” she said.
She said that the choice of RCB was probably not the best endpoint in a trial of endocrine-based therapy vs. chemotherapy.
“I think the challenge remains to identify those patients with luminal early breast cancer for whom an endocrine-based approach – not endocrine, but endocrine-based – will improve outcome, either replacing chemotherapy in the intermediate-risk setting or as an add-on in high-risk disease,” she said.
The study was funded by Pfizer and Nanostring. Dr. Cottu disclosed advisory board participation and travel support from Pfizer and others, and research support from Roche, Novartis, and AstraZeneca. Dr. Harbeck disclosed advising and consulting fees from Pfizer, Nanostring, and other companies.
MADRID – For women with luminal breast cancer who are not initially candidates for breast-conserving surgery, neoadjuvant therapy with an aromatase inhibitor and a cyclin-dependent kinases 4/6 (CDK4/6) inhibitor offered a slightly higher residual cancer burden prior to surgery, but a significantly better safety profile than conventional chemotherapy with similar near-term safety outcomes, results of a phase 2 parallel group, noncomparative trial suggested.
Among 60 patients evaluable for response in an interim analysis of the UNICANCER NeoPAL trial, one patient (3.3%) treated with a combination of letrozole (Femara) and palbociclib (Ibrance) had a residual cancer burden (RCB) score of 0 (equivalent to a pathologic complete response; pCR), whereas three patients (10%) treated with FEC 100 chemotherapy (5-fluorouracil, epirubicin, and cyclophosphamide) had RCB 0 or I, reported Paul-Henri Cottu, MD, of the Institut Curie in Paris.
Following the interim analysis, the independent data monitoring committee for the NeoPAL trial recommended halting accrual; accrual was stopped in November 2016, after 106 patients had been randomized.
The IDMC also recommended that patients in the letrozole/palbociclib arm who did not have an RCB of 0 or I be offered adjuvant chemotherapy.
“Please note that 70% of those patients refused adjuvant chemotherapy,” Dr. Cottu said.
The investigators set out to test whether letrozole and palbociclib, which have been shown to have synergistic antiproliferative activity against advanced luminal breast cancer, could have similar benefits in the neoadjuvant setting.
They screened for women with luminal breast cancer who had newly diagnosed stage II or III breast cancer with biopsy-proven endocrine receptor–positive, human epidermal growth factor receptor 2–negative tumors, using the Prosigna test, based on the PAM50 gene signature assay. Women with node-positive luminal A or luminal B disease were enrolled and randomized to receive either letrozole 2.5 mg and palbociclib 125 mg daily for 3 out of every 4 weeks over 19 weeks, or three cycles of FEC 100, followed by three cycles of docetaxel 100 mg/m2 every 3 weeks, followed by surgery.
An interim analysis was planned after 30 patients were evaluable for RCB in the experimental arm, and, as prespecified, the trial was stopped for futility when fewer than five patients had an RCB of 0 or I.
The safety analysis, conducted with all 106 patients randomized, showed that letrozole/palbocilib was associated with more frequent grade 3 neutropenia (23% vs. 10% of patients with FEC), but less grade 4 neutropenia (1% vs. 11%, respectively), and no febrile neutropenia vs. 6% in the chemotherapy arm.
There were 2 serious adverse events with the AI/CDK-inhibitor combination vs. 17 with chemotherapy. Dose reductions or interruptions were less frequent with letrozole/palbociclib (10 and 16), and only two patients in the experimental arm required premature cessation of therapy vs. seven in the chemotherapy arm.
The final response analysis in 103 patients showed that the rate of RCB 0 or I was 7.7% with letrozole/palbociclib and 15.7% with chemotherapy. Respective rates of RCB II-III were 92.3% and 84.3%.
Clinical response rates were similar in each study arm, with approximately 30% complete responses and 44% partial responses.
In each arm, slightly less than one-third of patients underwent mastectomy, and a little more than two-thirds were able to have breast-conserving surgery after neoadjuvant therapy.
The patients will be followed out to at least 3 years to see whether those patients in the letrozole/palbociclib arm who turned down subsequent chemotherapy will have worse survival than patients who decided to undergo it, Dr. Cottu said.
Invited discussant Nadia Harbeck, MD, PhD, of the University of Munich called the NeoPAL trial “a very daring study.”
“This is not a practice-changing trial, but it’s a very, very interesting hypothesis-generating trial,” she said.
She said that the choice of RCB was probably not the best endpoint in a trial of endocrine-based therapy vs. chemotherapy.
“I think the challenge remains to identify those patients with luminal early breast cancer for whom an endocrine-based approach – not endocrine, but endocrine-based – will improve outcome, either replacing chemotherapy in the intermediate-risk setting or as an add-on in high-risk disease,” she said.
The study was funded by Pfizer and Nanostring. Dr. Cottu disclosed advisory board participation and travel support from Pfizer and others, and research support from Roche, Novartis, and AstraZeneca. Dr. Harbeck disclosed advising and consulting fees from Pfizer, Nanostring, and other companies.
MADRID – For women with luminal breast cancer who are not initially candidates for breast-conserving surgery, neoadjuvant therapy with an aromatase inhibitor and a cyclin-dependent kinases 4/6 (CDK4/6) inhibitor offered a slightly higher residual cancer burden prior to surgery, but a significantly better safety profile than conventional chemotherapy with similar near-term safety outcomes, results of a phase 2 parallel group, noncomparative trial suggested.
Among 60 patients evaluable for response in an interim analysis of the UNICANCER NeoPAL trial, one patient (3.3%) treated with a combination of letrozole (Femara) and palbociclib (Ibrance) had a residual cancer burden (RCB) score of 0 (equivalent to a pathologic complete response; pCR), whereas three patients (10%) treated with FEC 100 chemotherapy (5-fluorouracil, epirubicin, and cyclophosphamide) had RCB 0 or I, reported Paul-Henri Cottu, MD, of the Institut Curie in Paris.
Following the interim analysis, the independent data monitoring committee for the NeoPAL trial recommended halting accrual; accrual was stopped in November 2016, after 106 patients had been randomized.
The IDMC also recommended that patients in the letrozole/palbociclib arm who did not have an RCB of 0 or I be offered adjuvant chemotherapy.
“Please note that 70% of those patients refused adjuvant chemotherapy,” Dr. Cottu said.
The investigators set out to test whether letrozole and palbociclib, which have been shown to have synergistic antiproliferative activity against advanced luminal breast cancer, could have similar benefits in the neoadjuvant setting.
They screened for women with luminal breast cancer who had newly diagnosed stage II or III breast cancer with biopsy-proven endocrine receptor–positive, human epidermal growth factor receptor 2–negative tumors, using the Prosigna test, based on the PAM50 gene signature assay. Women with node-positive luminal A or luminal B disease were enrolled and randomized to receive either letrozole 2.5 mg and palbociclib 125 mg daily for 3 out of every 4 weeks over 19 weeks, or three cycles of FEC 100, followed by three cycles of docetaxel 100 mg/m2 every 3 weeks, followed by surgery.
An interim analysis was planned after 30 patients were evaluable for RCB in the experimental arm, and, as prespecified, the trial was stopped for futility when fewer than five patients had an RCB of 0 or I.
The safety analysis, conducted with all 106 patients randomized, showed that letrozole/palbocilib was associated with more frequent grade 3 neutropenia (23% vs. 10% of patients with FEC), but less grade 4 neutropenia (1% vs. 11%, respectively), and no febrile neutropenia vs. 6% in the chemotherapy arm.
There were 2 serious adverse events with the AI/CDK-inhibitor combination vs. 17 with chemotherapy. Dose reductions or interruptions were less frequent with letrozole/palbociclib (10 and 16), and only two patients in the experimental arm required premature cessation of therapy vs. seven in the chemotherapy arm.
The final response analysis in 103 patients showed that the rate of RCB 0 or I was 7.7% with letrozole/palbociclib and 15.7% with chemotherapy. Respective rates of RCB II-III were 92.3% and 84.3%.
Clinical response rates were similar in each study arm, with approximately 30% complete responses and 44% partial responses.
In each arm, slightly less than one-third of patients underwent mastectomy, and a little more than two-thirds were able to have breast-conserving surgery after neoadjuvant therapy.
The patients will be followed out to at least 3 years to see whether those patients in the letrozole/palbociclib arm who turned down subsequent chemotherapy will have worse survival than patients who decided to undergo it, Dr. Cottu said.
Invited discussant Nadia Harbeck, MD, PhD, of the University of Munich called the NeoPAL trial “a very daring study.”
“This is not a practice-changing trial, but it’s a very, very interesting hypothesis-generating trial,” she said.
She said that the choice of RCB was probably not the best endpoint in a trial of endocrine-based therapy vs. chemotherapy.
“I think the challenge remains to identify those patients with luminal early breast cancer for whom an endocrine-based approach – not endocrine, but endocrine-based – will improve outcome, either replacing chemotherapy in the intermediate-risk setting or as an add-on in high-risk disease,” she said.
The study was funded by Pfizer and Nanostring. Dr. Cottu disclosed advisory board participation and travel support from Pfizer and others, and research support from Roche, Novartis, and AstraZeneca. Dr. Harbeck disclosed advising and consulting fees from Pfizer, Nanostring, and other companies.
AT ESMO 2017
Key clinical point:
Major finding: One patient (3.3%) assigned to letrozole/palbociclib had a residual cancer burden score of 0 or I, compared with three patients (10%) assigned to chemotherapy.
Data source: Interim analysis of a phase 2 parallel group trial with 60 patients evaluable for response and 106 evaluable for safety.
Disclosures: The study was funded by Pfizer and Nanostring. Dr. Cottu disclosed advisory board participation and travel support from Pfizer and others and research support from Roche, Novartis, and AstraZeneca. Dr. Harbeck disclosed advising and consulting fees from Pfizer, Nanostring, and other companies.
MONARCH 3: Abemaciclib plus AI boosts PFS in HR+/HER2- breast cancer
Madrid – A combination of the investigational cyclin-dependent kinase 4/6 (CDK4/6) agent abemaciclib and a nonsteroidal aromatase inhibitor (AI) was associated with a near doubling of progression-free survival in postmenopausal women with previously untreated hormone-receptor positive, human epidermal growth factor receptor 2–negative (HR+/HER2-) advanced breast cancer.
At a planned 18-month interim analysis of the MONARCH 3 trial, the median investigator-assessed progression free survival (PFS), the primary endpoint, had not been reached for 328 patients assigned to receive abemaciclib with either anastrozole (Arimidex) or letrozole (Femara). In contrast, the median PFS for 165 patients assigned to an AI and a placebo was 14.7 months, translating into a hazard ratio (HR) of 0.543 (P = .000021), reported Angelo Di Leo, MD, of Hospital of Prato, Istituto Toscano Tumori, Prato, Italy.
“Abemaciclib in combination with a nonsteroidal aromatase inhibitor is superior to a nonsteroidal aromatase inhibitor alone in terms of progression-free survival, but also in terms of the objective response rate as the initial treatment of HER2-negative, endocrine sensitive advanced breast cancer,” he said at a briefing prior to his presentation of the data in a presidential symposium at the European Society for Medical Oncology Congress.
The efficacy of abemaciclib was consistently seen across all subgroups.
“However, we have observed that the patients deriving the largest benefit from abemaciclib are those who have adverse prognostic factors such as, for instance, the presence of liver metastases, or the fact the disease has relapsed only after a few years from the end of adjuvant endocrine therapy,” he added.
The study was stopped for efficacy at the interim analysis.
Abemaciclib has previously been shown to be active as a monotherapy in treatment-refractory HR+/HER2- breast cancer, and in combination with fulvestrant (Faslodex) in patients who had disease progression on endocrine therapy.
Dr. Di Leo and his colleagues enrolled 493 postmenopausal women with metastatic or locally recurrent HR+/HER2- breast cancer who had not received systemic therapy in this setting. Patients who had prior neoadjuvant or adjuvant endocrine therapy were allowed if they had a disease-free interval of more than 1 year since completing endocrine therapy, The patients also had to have good performance status (Eastern Cooperative Oncology Group PS score 1 or less).
They were randomly assigned on a 2:1 basis to receive abemaciclib 150 mg b.i.d. on a continuous schedule plus either anastrozole 1 mg or letrozole 2.5 mg daily until disease progression, or to placebo plus either of the two AIs.
In addition to the superior PFS with abemaciclib added to an AI, as noted before, the CDK4/6 inhibitor was associated with a significantly better objective response rate (ORR), at 48.2% compared with 34.5% for placebo (P = .002). Among patients with measurable disease at baseline, the respective ORRs were 59.2% and 43.8% (P = .004). The clinical benefit rate in this subgroup was also better with abemaciclib, at 79.3% vs. 69.2% (P = .024).
In exploratory subgroup analyses, the investigators found that patients who had indicators of poor prognosis seemed to derive “substantial” benefit from the addition of abemaciclib. However, in an exploratory analysis in patients with disease only in bone, the investigators found that adding abemaciclib did not appear to improve PFS, suggesting that this subgroup could be treated effectively with endocrine therapy alone. Dr. Di Leo cautioned against overinterpreting this finding however, as only 109 patients had bone-only disease.
The safety analysis showed that patients were able to tolerate the combination fairly well. The incidence of grade 3 or 4 neutropenia was 21.1% with the combination compared with 1.2% with placebo, and grade 3 diarrhea occurred in 9.5% vs. 1.2% (no grade 4 diarrhea in either arm). The diarrhea tended to occur early in therapy and could be managed with dose adjustments and antidiarrheal medications, Dr. Di Leo said.
“What we would like to ask is, is this a practice-changing study? Do the results change standard first-line endocrine-based therapy, and then do these results change who we give endocrine therapy to?,” said invited discussant Nicholas Turner, PhD, of The Royal Marsden Hospital in London.
“The study stopped at the reported interim analysis, so at the moment the abemaciclib arm hasn’t reached the median PFS, but we can anticipate that with further follow-up we will see approximately a year improvement in median PFS by the addition of abemaciclib, which is really a substantial improvement in PFS for these patients. And importantly, this benefit was confirmed by a blinded independent central review of the investigator PFS,” he said.
Eli Lilly funded MONARCH 3. Dr. Di Leo and Dr. Turner reported receiving honoraria from the company.
Madrid – A combination of the investigational cyclin-dependent kinase 4/6 (CDK4/6) agent abemaciclib and a nonsteroidal aromatase inhibitor (AI) was associated with a near doubling of progression-free survival in postmenopausal women with previously untreated hormone-receptor positive, human epidermal growth factor receptor 2–negative (HR+/HER2-) advanced breast cancer.
At a planned 18-month interim analysis of the MONARCH 3 trial, the median investigator-assessed progression free survival (PFS), the primary endpoint, had not been reached for 328 patients assigned to receive abemaciclib with either anastrozole (Arimidex) or letrozole (Femara). In contrast, the median PFS for 165 patients assigned to an AI and a placebo was 14.7 months, translating into a hazard ratio (HR) of 0.543 (P = .000021), reported Angelo Di Leo, MD, of Hospital of Prato, Istituto Toscano Tumori, Prato, Italy.
“Abemaciclib in combination with a nonsteroidal aromatase inhibitor is superior to a nonsteroidal aromatase inhibitor alone in terms of progression-free survival, but also in terms of the objective response rate as the initial treatment of HER2-negative, endocrine sensitive advanced breast cancer,” he said at a briefing prior to his presentation of the data in a presidential symposium at the European Society for Medical Oncology Congress.
The efficacy of abemaciclib was consistently seen across all subgroups.
“However, we have observed that the patients deriving the largest benefit from abemaciclib are those who have adverse prognostic factors such as, for instance, the presence of liver metastases, or the fact the disease has relapsed only after a few years from the end of adjuvant endocrine therapy,” he added.
The study was stopped for efficacy at the interim analysis.
Abemaciclib has previously been shown to be active as a monotherapy in treatment-refractory HR+/HER2- breast cancer, and in combination with fulvestrant (Faslodex) in patients who had disease progression on endocrine therapy.
Dr. Di Leo and his colleagues enrolled 493 postmenopausal women with metastatic or locally recurrent HR+/HER2- breast cancer who had not received systemic therapy in this setting. Patients who had prior neoadjuvant or adjuvant endocrine therapy were allowed if they had a disease-free interval of more than 1 year since completing endocrine therapy, The patients also had to have good performance status (Eastern Cooperative Oncology Group PS score 1 or less).
They were randomly assigned on a 2:1 basis to receive abemaciclib 150 mg b.i.d. on a continuous schedule plus either anastrozole 1 mg or letrozole 2.5 mg daily until disease progression, or to placebo plus either of the two AIs.
In addition to the superior PFS with abemaciclib added to an AI, as noted before, the CDK4/6 inhibitor was associated with a significantly better objective response rate (ORR), at 48.2% compared with 34.5% for placebo (P = .002). Among patients with measurable disease at baseline, the respective ORRs were 59.2% and 43.8% (P = .004). The clinical benefit rate in this subgroup was also better with abemaciclib, at 79.3% vs. 69.2% (P = .024).
In exploratory subgroup analyses, the investigators found that patients who had indicators of poor prognosis seemed to derive “substantial” benefit from the addition of abemaciclib. However, in an exploratory analysis in patients with disease only in bone, the investigators found that adding abemaciclib did not appear to improve PFS, suggesting that this subgroup could be treated effectively with endocrine therapy alone. Dr. Di Leo cautioned against overinterpreting this finding however, as only 109 patients had bone-only disease.
The safety analysis showed that patients were able to tolerate the combination fairly well. The incidence of grade 3 or 4 neutropenia was 21.1% with the combination compared with 1.2% with placebo, and grade 3 diarrhea occurred in 9.5% vs. 1.2% (no grade 4 diarrhea in either arm). The diarrhea tended to occur early in therapy and could be managed with dose adjustments and antidiarrheal medications, Dr. Di Leo said.
“What we would like to ask is, is this a practice-changing study? Do the results change standard first-line endocrine-based therapy, and then do these results change who we give endocrine therapy to?,” said invited discussant Nicholas Turner, PhD, of The Royal Marsden Hospital in London.
“The study stopped at the reported interim analysis, so at the moment the abemaciclib arm hasn’t reached the median PFS, but we can anticipate that with further follow-up we will see approximately a year improvement in median PFS by the addition of abemaciclib, which is really a substantial improvement in PFS for these patients. And importantly, this benefit was confirmed by a blinded independent central review of the investigator PFS,” he said.
Eli Lilly funded MONARCH 3. Dr. Di Leo and Dr. Turner reported receiving honoraria from the company.
Madrid – A combination of the investigational cyclin-dependent kinase 4/6 (CDK4/6) agent abemaciclib and a nonsteroidal aromatase inhibitor (AI) was associated with a near doubling of progression-free survival in postmenopausal women with previously untreated hormone-receptor positive, human epidermal growth factor receptor 2–negative (HR+/HER2-) advanced breast cancer.
At a planned 18-month interim analysis of the MONARCH 3 trial, the median investigator-assessed progression free survival (PFS), the primary endpoint, had not been reached for 328 patients assigned to receive abemaciclib with either anastrozole (Arimidex) or letrozole (Femara). In contrast, the median PFS for 165 patients assigned to an AI and a placebo was 14.7 months, translating into a hazard ratio (HR) of 0.543 (P = .000021), reported Angelo Di Leo, MD, of Hospital of Prato, Istituto Toscano Tumori, Prato, Italy.
“Abemaciclib in combination with a nonsteroidal aromatase inhibitor is superior to a nonsteroidal aromatase inhibitor alone in terms of progression-free survival, but also in terms of the objective response rate as the initial treatment of HER2-negative, endocrine sensitive advanced breast cancer,” he said at a briefing prior to his presentation of the data in a presidential symposium at the European Society for Medical Oncology Congress.
The efficacy of abemaciclib was consistently seen across all subgroups.
“However, we have observed that the patients deriving the largest benefit from abemaciclib are those who have adverse prognostic factors such as, for instance, the presence of liver metastases, or the fact the disease has relapsed only after a few years from the end of adjuvant endocrine therapy,” he added.
The study was stopped for efficacy at the interim analysis.
Abemaciclib has previously been shown to be active as a monotherapy in treatment-refractory HR+/HER2- breast cancer, and in combination with fulvestrant (Faslodex) in patients who had disease progression on endocrine therapy.
Dr. Di Leo and his colleagues enrolled 493 postmenopausal women with metastatic or locally recurrent HR+/HER2- breast cancer who had not received systemic therapy in this setting. Patients who had prior neoadjuvant or adjuvant endocrine therapy were allowed if they had a disease-free interval of more than 1 year since completing endocrine therapy, The patients also had to have good performance status (Eastern Cooperative Oncology Group PS score 1 or less).
They were randomly assigned on a 2:1 basis to receive abemaciclib 150 mg b.i.d. on a continuous schedule plus either anastrozole 1 mg or letrozole 2.5 mg daily until disease progression, or to placebo plus either of the two AIs.
In addition to the superior PFS with abemaciclib added to an AI, as noted before, the CDK4/6 inhibitor was associated with a significantly better objective response rate (ORR), at 48.2% compared with 34.5% for placebo (P = .002). Among patients with measurable disease at baseline, the respective ORRs were 59.2% and 43.8% (P = .004). The clinical benefit rate in this subgroup was also better with abemaciclib, at 79.3% vs. 69.2% (P = .024).
In exploratory subgroup analyses, the investigators found that patients who had indicators of poor prognosis seemed to derive “substantial” benefit from the addition of abemaciclib. However, in an exploratory analysis in patients with disease only in bone, the investigators found that adding abemaciclib did not appear to improve PFS, suggesting that this subgroup could be treated effectively with endocrine therapy alone. Dr. Di Leo cautioned against overinterpreting this finding however, as only 109 patients had bone-only disease.
The safety analysis showed that patients were able to tolerate the combination fairly well. The incidence of grade 3 or 4 neutropenia was 21.1% with the combination compared with 1.2% with placebo, and grade 3 diarrhea occurred in 9.5% vs. 1.2% (no grade 4 diarrhea in either arm). The diarrhea tended to occur early in therapy and could be managed with dose adjustments and antidiarrheal medications, Dr. Di Leo said.
“What we would like to ask is, is this a practice-changing study? Do the results change standard first-line endocrine-based therapy, and then do these results change who we give endocrine therapy to?,” said invited discussant Nicholas Turner, PhD, of The Royal Marsden Hospital in London.
“The study stopped at the reported interim analysis, so at the moment the abemaciclib arm hasn’t reached the median PFS, but we can anticipate that with further follow-up we will see approximately a year improvement in median PFS by the addition of abemaciclib, which is really a substantial improvement in PFS for these patients. And importantly, this benefit was confirmed by a blinded independent central review of the investigator PFS,” he said.
Eli Lilly funded MONARCH 3. Dr. Di Leo and Dr. Turner reported receiving honoraria from the company.
AT ESMO 2017
Key clinical point: Adding the CDK4/6 inhibitor abemaciclib to an aromatase inhibitor significantly improved progression-free survival in the frontline for postmenopausal women with HR+/HER2- breast cancer.
Major finding: Median PFS was not reached with abemaciclib and letrozole or anastrozole, vs. 14.7 months for a placebo plus aromatase inhibitor.
Data source: Randomized phase 3 trial of 493 postmenopausal women with metastatic or locally recurrent HR+/HER2- breast cancer.
Disclosures: Eli Lilly funded MONARCH 3. Dr. Di Leo and Dr. Turner reported receiving honoraria from the company.
LORELEI sings praises of neoadjuvant taselisib/letrozole in ER+/HER2– breast cancer
MADRID – Adding the investigational PI3K inhibitor taselisib to letrozole (Femara) significantly improved overall response rates, compared with neoadjuvant letrozole alone, in postmenopausal women with early estrogen receptor–positive, human epidermal growth factor receptor 2–negative (ER+/HER2–) breast cancer, results of the LORELEI trial show.
In this phase 2 trial, the overall response rate (ORR), the primary endpoint, was 50% for 166 patients assigned to letrozole plus taselisib, compared with 39.3% for 168 patients assigned to letrozole plus placebo, reported Cristina Saura, MD, of Vall d’Hebron University Hospital in Barcelona.
Although the pathologic complete rate (pCR) was low in each study arm, this was not unexpected, due to the short course (4 months) of endocrine-based therapy, she said.
Taselisib is a selective inhibitor of phosphatidylinositol 3-kinase (PI3K), with enhanced activity against the mutation isoform of the protein, labeled p110a. The PI3K alpha isoform, Taselisib degrades p110a by a mechanism of action that is different from other PI3K inhibitors, Dr. Saura said.
As monotherapy and in combination with endocrine therapy agents in phase 1 trials, taselisib elicited partial responses in patients with PIK3CA-mutated metastatic breast cancer, she noted.
In LORELEI, 334 postmenopausal women with previously untreated ER+/HER2– stage I-III operable breast cancer with tumors of 2 cm or greater on MRI were enrolled. The patients were stratified by tumor size and nodal status and then randomly assigned to letrozole 2.5 mg daily plus taselisib 4 mg taken in 2 mg doses twice daily for 5 days each week for 16 weeks, or letrozole plus placebo. Patients then went on to surgery, were followed for safety for 30 days, and then had adjuvant therapy consisting of endocrine therapy and/or chemotherapy and/or radiation therapy.
As noted, the ORR for patients assigned to the combination was 50%, consisting of 4.8% CR, and 45.2% partial responses (PR). Additionally, 40.4% of patients assigned to the taselisib arm had stable disease. The corresponding response rates for patients assigned to the letrozole/placebo arm were 1.8%, 37.5%, and 51.2%.
The odds ratio favoring the combination was 1.55 (P = .049).
For the subset of patients with PIK3CA-mutated tumors, the ORR was 56.2% for 73 patients in the taselisib arm, vs. 38% for 79 patients in the placebo arm (OR, 2.03; P = .033).
The pCRs in the overall population were 1.8% with taselisib vs. 0.6% with placebo. The corresponding pCRs in the PIK3CA-mutated population were 1.4% and 0%. None of the differences were statistically significant.
In all, 11.4% of patients assigned to taselisib required dose reductions, and 10.8% had to discontinue the drug.
The incidence of adverse events of any grade was 91% with letrozole/taselisib, and 83.2% with letrozole placebo.
Taselisib was associated with higher rates of gastrointestinal side effects, skin and subcutaneous disorders, metabolic and nutritional side effects and respiratory, thoracic, and mediastinal disorders. Patients in the taselisib arm had a lower incidence of musculoskeletal/connective tissue problems, vascular disorders, and psychiatric complaints.
The toxicities were generally manageable and consistent with that of other PI3K inhibitors, Dr. Saura said.
She said that although taselisib is more selective than other PI3K inhibitors and has a somewhat lower incidence of GI toxicities, “it’s still difficult to manage as a drug in the clinic, but I think if we can tell a patient there is a selection criterion which is a PIK3CA mutation, then patients are more willing and we as therapists are more willing to give those drugs.”
LORELEI provides the first clinical proof of efficacy of a specific PI3K inhibitor in early breast cancer, and “results of confirmatory phase 3 trials in metastatic breast cancer are eagerly awaited,” Dr. Harbeck added.
The study was supported by Genentech/F. Hoffman–La Roche. Dr. Saura disclosed consulting fees and institutional funding from Roche. Dr. Harbeck reported no disclosures relevant to the study.
MADRID – Adding the investigational PI3K inhibitor taselisib to letrozole (Femara) significantly improved overall response rates, compared with neoadjuvant letrozole alone, in postmenopausal women with early estrogen receptor–positive, human epidermal growth factor receptor 2–negative (ER+/HER2–) breast cancer, results of the LORELEI trial show.
In this phase 2 trial, the overall response rate (ORR), the primary endpoint, was 50% for 166 patients assigned to letrozole plus taselisib, compared with 39.3% for 168 patients assigned to letrozole plus placebo, reported Cristina Saura, MD, of Vall d’Hebron University Hospital in Barcelona.
Although the pathologic complete rate (pCR) was low in each study arm, this was not unexpected, due to the short course (4 months) of endocrine-based therapy, she said.
Taselisib is a selective inhibitor of phosphatidylinositol 3-kinase (PI3K), with enhanced activity against the mutation isoform of the protein, labeled p110a. The PI3K alpha isoform, Taselisib degrades p110a by a mechanism of action that is different from other PI3K inhibitors, Dr. Saura said.
As monotherapy and in combination with endocrine therapy agents in phase 1 trials, taselisib elicited partial responses in patients with PIK3CA-mutated metastatic breast cancer, she noted.
In LORELEI, 334 postmenopausal women with previously untreated ER+/HER2– stage I-III operable breast cancer with tumors of 2 cm or greater on MRI were enrolled. The patients were stratified by tumor size and nodal status and then randomly assigned to letrozole 2.5 mg daily plus taselisib 4 mg taken in 2 mg doses twice daily for 5 days each week for 16 weeks, or letrozole plus placebo. Patients then went on to surgery, were followed for safety for 30 days, and then had adjuvant therapy consisting of endocrine therapy and/or chemotherapy and/or radiation therapy.
As noted, the ORR for patients assigned to the combination was 50%, consisting of 4.8% CR, and 45.2% partial responses (PR). Additionally, 40.4% of patients assigned to the taselisib arm had stable disease. The corresponding response rates for patients assigned to the letrozole/placebo arm were 1.8%, 37.5%, and 51.2%.
The odds ratio favoring the combination was 1.55 (P = .049).
For the subset of patients with PIK3CA-mutated tumors, the ORR was 56.2% for 73 patients in the taselisib arm, vs. 38% for 79 patients in the placebo arm (OR, 2.03; P = .033).
The pCRs in the overall population were 1.8% with taselisib vs. 0.6% with placebo. The corresponding pCRs in the PIK3CA-mutated population were 1.4% and 0%. None of the differences were statistically significant.
In all, 11.4% of patients assigned to taselisib required dose reductions, and 10.8% had to discontinue the drug.
The incidence of adverse events of any grade was 91% with letrozole/taselisib, and 83.2% with letrozole placebo.
Taselisib was associated with higher rates of gastrointestinal side effects, skin and subcutaneous disorders, metabolic and nutritional side effects and respiratory, thoracic, and mediastinal disorders. Patients in the taselisib arm had a lower incidence of musculoskeletal/connective tissue problems, vascular disorders, and psychiatric complaints.
The toxicities were generally manageable and consistent with that of other PI3K inhibitors, Dr. Saura said.
She said that although taselisib is more selective than other PI3K inhibitors and has a somewhat lower incidence of GI toxicities, “it’s still difficult to manage as a drug in the clinic, but I think if we can tell a patient there is a selection criterion which is a PIK3CA mutation, then patients are more willing and we as therapists are more willing to give those drugs.”
LORELEI provides the first clinical proof of efficacy of a specific PI3K inhibitor in early breast cancer, and “results of confirmatory phase 3 trials in metastatic breast cancer are eagerly awaited,” Dr. Harbeck added.
The study was supported by Genentech/F. Hoffman–La Roche. Dr. Saura disclosed consulting fees and institutional funding from Roche. Dr. Harbeck reported no disclosures relevant to the study.
MADRID – Adding the investigational PI3K inhibitor taselisib to letrozole (Femara) significantly improved overall response rates, compared with neoadjuvant letrozole alone, in postmenopausal women with early estrogen receptor–positive, human epidermal growth factor receptor 2–negative (ER+/HER2–) breast cancer, results of the LORELEI trial show.
In this phase 2 trial, the overall response rate (ORR), the primary endpoint, was 50% for 166 patients assigned to letrozole plus taselisib, compared with 39.3% for 168 patients assigned to letrozole plus placebo, reported Cristina Saura, MD, of Vall d’Hebron University Hospital in Barcelona.
Although the pathologic complete rate (pCR) was low in each study arm, this was not unexpected, due to the short course (4 months) of endocrine-based therapy, she said.
Taselisib is a selective inhibitor of phosphatidylinositol 3-kinase (PI3K), with enhanced activity against the mutation isoform of the protein, labeled p110a. The PI3K alpha isoform, Taselisib degrades p110a by a mechanism of action that is different from other PI3K inhibitors, Dr. Saura said.
As monotherapy and in combination with endocrine therapy agents in phase 1 trials, taselisib elicited partial responses in patients with PIK3CA-mutated metastatic breast cancer, she noted.
In LORELEI, 334 postmenopausal women with previously untreated ER+/HER2– stage I-III operable breast cancer with tumors of 2 cm or greater on MRI were enrolled. The patients were stratified by tumor size and nodal status and then randomly assigned to letrozole 2.5 mg daily plus taselisib 4 mg taken in 2 mg doses twice daily for 5 days each week for 16 weeks, or letrozole plus placebo. Patients then went on to surgery, were followed for safety for 30 days, and then had adjuvant therapy consisting of endocrine therapy and/or chemotherapy and/or radiation therapy.
As noted, the ORR for patients assigned to the combination was 50%, consisting of 4.8% CR, and 45.2% partial responses (PR). Additionally, 40.4% of patients assigned to the taselisib arm had stable disease. The corresponding response rates for patients assigned to the letrozole/placebo arm were 1.8%, 37.5%, and 51.2%.
The odds ratio favoring the combination was 1.55 (P = .049).
For the subset of patients with PIK3CA-mutated tumors, the ORR was 56.2% for 73 patients in the taselisib arm, vs. 38% for 79 patients in the placebo arm (OR, 2.03; P = .033).
The pCRs in the overall population were 1.8% with taselisib vs. 0.6% with placebo. The corresponding pCRs in the PIK3CA-mutated population were 1.4% and 0%. None of the differences were statistically significant.
In all, 11.4% of patients assigned to taselisib required dose reductions, and 10.8% had to discontinue the drug.
The incidence of adverse events of any grade was 91% with letrozole/taselisib, and 83.2% with letrozole placebo.
Taselisib was associated with higher rates of gastrointestinal side effects, skin and subcutaneous disorders, metabolic and nutritional side effects and respiratory, thoracic, and mediastinal disorders. Patients in the taselisib arm had a lower incidence of musculoskeletal/connective tissue problems, vascular disorders, and psychiatric complaints.
The toxicities were generally manageable and consistent with that of other PI3K inhibitors, Dr. Saura said.
She said that although taselisib is more selective than other PI3K inhibitors and has a somewhat lower incidence of GI toxicities, “it’s still difficult to manage as a drug in the clinic, but I think if we can tell a patient there is a selection criterion which is a PIK3CA mutation, then patients are more willing and we as therapists are more willing to give those drugs.”
LORELEI provides the first clinical proof of efficacy of a specific PI3K inhibitor in early breast cancer, and “results of confirmatory phase 3 trials in metastatic breast cancer are eagerly awaited,” Dr. Harbeck added.
The study was supported by Genentech/F. Hoffman–La Roche. Dr. Saura disclosed consulting fees and institutional funding from Roche. Dr. Harbeck reported no disclosures relevant to the study.
AT ESMO 2017
Key clinical point: Adding the PI3K inhibitor taselisib to letrozole improved response rates over letrozole alone in postmenopausal women with ER+/HER2– early breast cancer.
Major finding: The ORR was 50% for letrozole/taselisib, vs. 39.3% for letrozole/placebo (P = .049).
Data source: Randomized, double-blind phase 2 trial in 334 postmenopausal women.
Disclosures: The study was supported by Genentech/F. Hoffman–La Roche. Dr. Saura disclosed consulting fees and institutional funding from Roche. Dr. Harbeck reported no disclosures relevant to the study.
Surgeons strongly influenced chances of contralateral prophylactic mastectomy
Surgeons, not clinical factors, accounted for 20% of variation in rates of contralateral prophylactic mastectomy (CPM), according to the results of a large survey study.
Only 4% of patients elected CPM when their surgeons were among those who least favored it overall and most preferred breast-conserving treatment, according to Steven J. Katz, MD, MPH, of the University of Michigan, Ann Arbor, and his associates. But 34% of patients chose CPM when their surgeons least favored BCT and were most willing to perform CPM, the researchers found. “Attending surgeons exert strong influence on the likelihood of receipt of CPM after diagnosis of breast cancer,” highlighting “the need to help surgeons address this growing clinical conundrum in the examination room,” they wrote (JAMA Surg. 2017 Sep 13. doi: 10.1001/jamasurg.2017.3415).
Rates of CPM have risen markedly in the United States although it has not been shown to confer a survival advantage for average-risk women. To examine how surgeons themselves affected rates of CPM, the investigators sent surveys to 7,810 women treated for stage 0 to II breast cancer from 2013 to 2015 and included in the Surveillance, Epidemiology, and End Results (SEER) registries of Georgia and Los Angeles County. (Among the 7,810 women, 507 were ineligible.) The researchers also surveyed 488 attending surgeons of these patients.
Response rates were high – 70% among patients (5,080 of 7,303) and 77% (377 of 488) among surgeons, the investigators reported. The average age of the patients was 62 years; 28% had an elevated risk of second primary cancer, and 16% underwent CPM. Patients whose surgeons’ rates of CPM exceeded the mean by at least one standard deviation had nearly threefold greater odds of undergoing CPM themselves (odds ratio, 2.8; 95% confidence interval, 2.1-3.4) regardless of age, date of diagnosis, BRCA mutation status, or risk of second primary cancer.
“One quarter of the surgeon influence was explained by attending attitudes about initial recommendations for surgery and responses to patient requests for CPM,” the researchers wrote. Additional predictors of CPM included elevated risk of second primary breast cancer, BRCA mutation, and younger age.
“We observed a range of reasons why a surgeon would be willing to perform CPM if asked: give peace of mind, yield better cosmetic outcomes, avoid conflict with patient, reduce need for surveillance, improve long-term quality of life, reduce recurrence of invasive disease, avoid losing patient to another surgeon, or improve survival (in order of endorsement),” the researchers wrote. “Our findings reinforce the need to address better ways to communicate with patients with regard to their beliefs about the benefits of more extensive surgery and their reactions to the management plan including surgeon training and deployment of decision aids.”
The National Cancer Institute provided funding. The researchers reported having no conflicts of interest.
Patients who are provided education tools regarding the decision between [breast conserving therapy] and mastectomy are more likely to opt for BCT. However, this discussion is arduous and time consuming. We offer decision-making autonomy to patients, but, in creating that autonomy, we have resigned to overtreatment, motivated by the desire to avoid creating conflict in our relationship with the patient.
How do we overcome this hurdle? Consensus statements reinforce that contralateral prophylactic mastectomy should be discouraged in average-risk patients, but it is time to move beyond consensus statements and create communication tools that guide the surgeon and patient through a stepwise informed discussion. We are participating in a multi-institutional randomized trial to develop such an aid, and we believe this will effect real change in the way surgeons counsel patients. The goal is to standardize the methods and information patients receive to ensure that their decisions are based on facts, not fear.
Julie A. Margenthaler, MD, and Amy E. Cyr, MD, are in the department of surgery, Washington University, St. Louis. They reported no conflicts of interest. These comments are from their editorial (JAMA Surg. 2017 Sep 13. doi: 10.1001/jamasurg.2017.3435).
Patients who are provided education tools regarding the decision between [breast conserving therapy] and mastectomy are more likely to opt for BCT. However, this discussion is arduous and time consuming. We offer decision-making autonomy to patients, but, in creating that autonomy, we have resigned to overtreatment, motivated by the desire to avoid creating conflict in our relationship with the patient.
How do we overcome this hurdle? Consensus statements reinforce that contralateral prophylactic mastectomy should be discouraged in average-risk patients, but it is time to move beyond consensus statements and create communication tools that guide the surgeon and patient through a stepwise informed discussion. We are participating in a multi-institutional randomized trial to develop such an aid, and we believe this will effect real change in the way surgeons counsel patients. The goal is to standardize the methods and information patients receive to ensure that their decisions are based on facts, not fear.
Julie A. Margenthaler, MD, and Amy E. Cyr, MD, are in the department of surgery, Washington University, St. Louis. They reported no conflicts of interest. These comments are from their editorial (JAMA Surg. 2017 Sep 13. doi: 10.1001/jamasurg.2017.3435).
Patients who are provided education tools regarding the decision between [breast conserving therapy] and mastectomy are more likely to opt for BCT. However, this discussion is arduous and time consuming. We offer decision-making autonomy to patients, but, in creating that autonomy, we have resigned to overtreatment, motivated by the desire to avoid creating conflict in our relationship with the patient.
How do we overcome this hurdle? Consensus statements reinforce that contralateral prophylactic mastectomy should be discouraged in average-risk patients, but it is time to move beyond consensus statements and create communication tools that guide the surgeon and patient through a stepwise informed discussion. We are participating in a multi-institutional randomized trial to develop such an aid, and we believe this will effect real change in the way surgeons counsel patients. The goal is to standardize the methods and information patients receive to ensure that their decisions are based on facts, not fear.
Julie A. Margenthaler, MD, and Amy E. Cyr, MD, are in the department of surgery, Washington University, St. Louis. They reported no conflicts of interest. These comments are from their editorial (JAMA Surg. 2017 Sep 13. doi: 10.1001/jamasurg.2017.3435).
Surgeons, not clinical factors, accounted for 20% of variation in rates of contralateral prophylactic mastectomy (CPM), according to the results of a large survey study.
Only 4% of patients elected CPM when their surgeons were among those who least favored it overall and most preferred breast-conserving treatment, according to Steven J. Katz, MD, MPH, of the University of Michigan, Ann Arbor, and his associates. But 34% of patients chose CPM when their surgeons least favored BCT and were most willing to perform CPM, the researchers found. “Attending surgeons exert strong influence on the likelihood of receipt of CPM after diagnosis of breast cancer,” highlighting “the need to help surgeons address this growing clinical conundrum in the examination room,” they wrote (JAMA Surg. 2017 Sep 13. doi: 10.1001/jamasurg.2017.3415).
Rates of CPM have risen markedly in the United States although it has not been shown to confer a survival advantage for average-risk women. To examine how surgeons themselves affected rates of CPM, the investigators sent surveys to 7,810 women treated for stage 0 to II breast cancer from 2013 to 2015 and included in the Surveillance, Epidemiology, and End Results (SEER) registries of Georgia and Los Angeles County. (Among the 7,810 women, 507 were ineligible.) The researchers also surveyed 488 attending surgeons of these patients.
Response rates were high – 70% among patients (5,080 of 7,303) and 77% (377 of 488) among surgeons, the investigators reported. The average age of the patients was 62 years; 28% had an elevated risk of second primary cancer, and 16% underwent CPM. Patients whose surgeons’ rates of CPM exceeded the mean by at least one standard deviation had nearly threefold greater odds of undergoing CPM themselves (odds ratio, 2.8; 95% confidence interval, 2.1-3.4) regardless of age, date of diagnosis, BRCA mutation status, or risk of second primary cancer.
“One quarter of the surgeon influence was explained by attending attitudes about initial recommendations for surgery and responses to patient requests for CPM,” the researchers wrote. Additional predictors of CPM included elevated risk of second primary breast cancer, BRCA mutation, and younger age.
“We observed a range of reasons why a surgeon would be willing to perform CPM if asked: give peace of mind, yield better cosmetic outcomes, avoid conflict with patient, reduce need for surveillance, improve long-term quality of life, reduce recurrence of invasive disease, avoid losing patient to another surgeon, or improve survival (in order of endorsement),” the researchers wrote. “Our findings reinforce the need to address better ways to communicate with patients with regard to their beliefs about the benefits of more extensive surgery and their reactions to the management plan including surgeon training and deployment of decision aids.”
The National Cancer Institute provided funding. The researchers reported having no conflicts of interest.
Surgeons, not clinical factors, accounted for 20% of variation in rates of contralateral prophylactic mastectomy (CPM), according to the results of a large survey study.
Only 4% of patients elected CPM when their surgeons were among those who least favored it overall and most preferred breast-conserving treatment, according to Steven J. Katz, MD, MPH, of the University of Michigan, Ann Arbor, and his associates. But 34% of patients chose CPM when their surgeons least favored BCT and were most willing to perform CPM, the researchers found. “Attending surgeons exert strong influence on the likelihood of receipt of CPM after diagnosis of breast cancer,” highlighting “the need to help surgeons address this growing clinical conundrum in the examination room,” they wrote (JAMA Surg. 2017 Sep 13. doi: 10.1001/jamasurg.2017.3415).
Rates of CPM have risen markedly in the United States although it has not been shown to confer a survival advantage for average-risk women. To examine how surgeons themselves affected rates of CPM, the investigators sent surveys to 7,810 women treated for stage 0 to II breast cancer from 2013 to 2015 and included in the Surveillance, Epidemiology, and End Results (SEER) registries of Georgia and Los Angeles County. (Among the 7,810 women, 507 were ineligible.) The researchers also surveyed 488 attending surgeons of these patients.
Response rates were high – 70% among patients (5,080 of 7,303) and 77% (377 of 488) among surgeons, the investigators reported. The average age of the patients was 62 years; 28% had an elevated risk of second primary cancer, and 16% underwent CPM. Patients whose surgeons’ rates of CPM exceeded the mean by at least one standard deviation had nearly threefold greater odds of undergoing CPM themselves (odds ratio, 2.8; 95% confidence interval, 2.1-3.4) regardless of age, date of diagnosis, BRCA mutation status, or risk of second primary cancer.
“One quarter of the surgeon influence was explained by attending attitudes about initial recommendations for surgery and responses to patient requests for CPM,” the researchers wrote. Additional predictors of CPM included elevated risk of second primary breast cancer, BRCA mutation, and younger age.
“We observed a range of reasons why a surgeon would be willing to perform CPM if asked: give peace of mind, yield better cosmetic outcomes, avoid conflict with patient, reduce need for surveillance, improve long-term quality of life, reduce recurrence of invasive disease, avoid losing patient to another surgeon, or improve survival (in order of endorsement),” the researchers wrote. “Our findings reinforce the need to address better ways to communicate with patients with regard to their beliefs about the benefits of more extensive surgery and their reactions to the management plan including surgeon training and deployment of decision aids.”
The National Cancer Institute provided funding. The researchers reported having no conflicts of interest.
FROM JAMA SURGERY
Key clinical point: Attending surgeons explained 20% of variation in rates of contralateral prophylactic mastectomy.
Major finding: Only 4% of patients elected CPM when their surgeons were among those who least favored it and most preferred breast-conserving treatment (BCT). However, 34% of patients chose CPM when their surgeons least favored initial BCT and were most willing to perform CPM.
Data source: Surveys of 5,080 patients with stage 0-II breast cancer and 339 attending surgeons.
Disclosures: The National Cancer Institute provided funding. The researchers reported having no conflicts of interest.
Ten-year outcomes support skipping axillary lymph node dissection with positive sentinel nodes
A follow-up to a study showing the noninferiority of sentinel lymph node dissection to axillary lymph node dissection for breast cancer in overall and disease-free survival at a median of 6.3 years found similar noninferiority in overall survival at 10 years.
Axillary lymph node dissection has a risk of complications including lymphedema, numbness, axillary web syndrome, and decreased upper-extremity range of motion. The American College of Surgeons Oncology Group Z0011 trial sought to determine if the procedure could be avoided without inferior survival outcomes.
Criticism of the study focused on the potential for later recurrence, particularly in patients with hormone receptor–positive breast cancer. All enrolled patients had one or two sentinel nodes with metastases. At randomization, 436 received sentinel lymph node dissection alone, and 420 received the additional axillary lymph node dissection. The patients were assessed every 6 months for the first 3 years, then annually.
After a median of 9.3 years, 110 of the patients had died of any cause – 51 in the sentinel lymph node dissection group and 59 in the axillary lymph node dissection group – a 10-year overall survival rate of 86.3% and 83.6%, respectively. This met the study’s primary endpoint of showing noninferior overall survival without the riskier procedure. In the study’s secondary endpoint, disease-free survival, there was not a significant difference either (80.2% vs. 78.2%).
“Axillary dissections are associated with considerable morbidity, and the results of this trial demonstrated that this morbidity can be avoided without decreasing cancer control. … These findings do not support routine use of axillary lymph node dissection in this patient population based on 10-year outcomes,” wrote Armando E. Guiliano, MD, of Cedars-Sinai Medical, Los Angeles and his coauthors (JAMA. 2017;318[10]:918-26. doi: 10.1001/jama.2017.11470).
A follow-up to a study showing the noninferiority of sentinel lymph node dissection to axillary lymph node dissection for breast cancer in overall and disease-free survival at a median of 6.3 years found similar noninferiority in overall survival at 10 years.
Axillary lymph node dissection has a risk of complications including lymphedema, numbness, axillary web syndrome, and decreased upper-extremity range of motion. The American College of Surgeons Oncology Group Z0011 trial sought to determine if the procedure could be avoided without inferior survival outcomes.
Criticism of the study focused on the potential for later recurrence, particularly in patients with hormone receptor–positive breast cancer. All enrolled patients had one or two sentinel nodes with metastases. At randomization, 436 received sentinel lymph node dissection alone, and 420 received the additional axillary lymph node dissection. The patients were assessed every 6 months for the first 3 years, then annually.
After a median of 9.3 years, 110 of the patients had died of any cause – 51 in the sentinel lymph node dissection group and 59 in the axillary lymph node dissection group – a 10-year overall survival rate of 86.3% and 83.6%, respectively. This met the study’s primary endpoint of showing noninferior overall survival without the riskier procedure. In the study’s secondary endpoint, disease-free survival, there was not a significant difference either (80.2% vs. 78.2%).
“Axillary dissections are associated with considerable morbidity, and the results of this trial demonstrated that this morbidity can be avoided without decreasing cancer control. … These findings do not support routine use of axillary lymph node dissection in this patient population based on 10-year outcomes,” wrote Armando E. Guiliano, MD, of Cedars-Sinai Medical, Los Angeles and his coauthors (JAMA. 2017;318[10]:918-26. doi: 10.1001/jama.2017.11470).
A follow-up to a study showing the noninferiority of sentinel lymph node dissection to axillary lymph node dissection for breast cancer in overall and disease-free survival at a median of 6.3 years found similar noninferiority in overall survival at 10 years.
Axillary lymph node dissection has a risk of complications including lymphedema, numbness, axillary web syndrome, and decreased upper-extremity range of motion. The American College of Surgeons Oncology Group Z0011 trial sought to determine if the procedure could be avoided without inferior survival outcomes.
Criticism of the study focused on the potential for later recurrence, particularly in patients with hormone receptor–positive breast cancer. All enrolled patients had one or two sentinel nodes with metastases. At randomization, 436 received sentinel lymph node dissection alone, and 420 received the additional axillary lymph node dissection. The patients were assessed every 6 months for the first 3 years, then annually.
After a median of 9.3 years, 110 of the patients had died of any cause – 51 in the sentinel lymph node dissection group and 59 in the axillary lymph node dissection group – a 10-year overall survival rate of 86.3% and 83.6%, respectively. This met the study’s primary endpoint of showing noninferior overall survival without the riskier procedure. In the study’s secondary endpoint, disease-free survival, there was not a significant difference either (80.2% vs. 78.2%).
“Axillary dissections are associated with considerable morbidity, and the results of this trial demonstrated that this morbidity can be avoided without decreasing cancer control. … These findings do not support routine use of axillary lymph node dissection in this patient population based on 10-year outcomes,” wrote Armando E. Guiliano, MD, of Cedars-Sinai Medical, Los Angeles and his coauthors (JAMA. 2017;318[10]:918-26. doi: 10.1001/jama.2017.11470).
FROM JAMA
Assessing a multidisciplinary survivorship program in a group of predominantly Hispanic women with breast cancer
Breast cancer survivors comprise the most prevalent cancer survivor population in the United States.1 The number of breast cancer survivors is increasing because of early detection and diagnosis, and advances in treatment have resulted in increased life expectancy. Therefore, greater attention is needed to improve the long-term quality of life of these survivors and to help them re-adjust to normal life. For many women, although the medical treatment may have been completed, the recovery process may have not.2 The prevalence of long-term mental and physical illness is significant among many breast cancer survivors. Long-term mental consequences may include memory problems, anxiety, depression, and fear of recurrence3, and long-term physical consequences may include pain, fatigue, and lymphedema, among others.4
El Paso, Texas, is the fourth most populous city in Texas and has a Hispanic majority. This provides an opportunity to conduct clinical research targeting participants of Hispanic descent. Several studies have noted the influence of race/ethnicity on the psychosocial function of breast cancer survivors.5,6 We have previously reported that Hispanic breast cancer survivors might experience decreased mental and physical health-related quality of life (QoL) which limit their normal social functioning.6Other studies have similarly reported poor outcomes of breast cancer survivors and higher rates of fatigue and depression among Hispanic patients.7 However, there is a paucity of research addressing specific interventions needed to improve these outcomes and provide better QoL for breast cancer survivors.8,9 In addition, a few survivorship care interventions have focused on minorities. We sought to assess whether a multidisciplinary cancer survivorship program in a primarily Hispanic populated area would lead to improved QoL and reduce anxiety and depressive symptoms among breast cancer survivors.
Methods
After obtaining Institutional Review Board approval, we recruited consecutive patients who were treated at our institution during October 2013-October 2014 and obtained informed consent from them. The participants were within the first 5 years after diagnosis with stages I-III breast cancer and had completed surgery, chemotherapy, and/or radiation therapy. We sought to determine whether breast cancer survivors would benefit from this intervention as determined by improvement of performance at 12 months compared with baseline based on the following self-reported validated questionnaires: Patient Health Questionnaire-9 (PHQ-9) for depression; General Anxiety Disorder-7 (GAD-7); and Short-Form Health Survey-36 (SF-36, version 2) for patient quality of life. The participants were enrolled in a comprehensive survivorship program staffed by an oncologist, an oncology nurse practitioner, a nutritionist, and a certified clinical psychologist who had trained in mindfulness-based stress reduction (MBSR).
Interventions
The participants received a one-on-one individual psychological consultation visit every 3 months for 20-45 minutes during which the psychologist addressed each patient’s emotional and psychological issues in depth, discussed relaxation techniques, and provided psychosocial counselling. In addition, all participants were asked to attend an 8-week-course (in Spanish or English) using MBSR, an interventional program in which participants receive training to promote reduction of stress by self-regulating mindfulness practice.3,10 Our institution’s MBSR program consists of a weekly 2-hour class for 8 sessions or more. The program is provided 3 times a year, in English and Spanish. It includes the following components:
- Learning various mindfulness meditation techniques (eg, body scans, awareness of breathing, sitting/walking meditations);
- Practicing the mindfulness techniques in class; and
- Practicing techniques at home through audiorecordings of mindfulness meditation exercises and daily diary writing.
Participants were provided with a workbook on MBSR in their preferred language.11 In addition to the psychological component, they were also provided with oncologic evaluations by an oncology nurse practitioner. The nurse practitioner met with participants every 3 months and provided each one with a personalized summary of all the treatments received and routine oncology follow-up care in consultation with the patients’ regular oncologists. This care also addresses the long-term sequelae of treatment, including arthritis and osteoporosis, referrals to receive screening for other cancers (eg, cervical and colon cancer), and genetic counselling as appropriate. In addition, a nutritionist provided general dietary advice in individual and group sessions every 3 months.
The self-administered questionnaires, PHQ-9, GAD-7, and SF-36, were completed at baseline, and every 3 months for 12 months. The scores were reviewed by the psychologist and the oncologist. The PHQ-9 was used to initially screen survivors for depression and monitor their improvement after the intervention. The PHQ-9 is a reliable and validated self-administered depression module.12 The PQH-9 exclusively focuses on the 9 diagnostic criteria for DSM-IV depression disorder and it can be used as a useful measure for monitoring outcomes of depression therapy. A score of 5-14 suggests mild-moderate depression, and a score of >15 suggests severe depression
The survivors were screened for anxiety using the GAD-7, a brief 7-item self-report scale to identify probable cases of anxiety disorder that has been shown to be an efficient tool for screening and assessing the severity of anxiety.13 For GAD-7, a score of 5 or higher is suggestive of anxiety. Scores of 5, 10, and 15 represent cut-off points for mild, moderate, and severe anxiety, respectively.
Survivor QoL was evaluated using the SF-36 questionnaire, a multipurpose survey containing 36 questions. It ranges from 0-100 and a score that is <50.0 is considered low. The lower the score, the worse the mental or physical function.14 The SF-36 yields a patient profile of 8 health domains – vitality, physical functioning, bodily pain, general health perceptions, physical, emotional, and social role functioning; and mental health.15,16 A score of 50.0 on either the Physical Component Summary (PCS – vitality, physical functioning, bodily pain, general health perceptions, physical role functioning) or Mental Component Summary (MCS – emotional and social role functioning, and mental health) is consistent with the US norm.
Statistical analysis
In this study, the primary objective was to use the MBSR survivorship program to improve the survivors’ outcomes at 12 months compared with baseline using the following measures: PHQ-9 for depression, GAD-7 for anxiety, and SF-36 for QoL using the PCS and MCS. Quantitative data were described using the mean and standard deviation, and categorical data were described using frequency and percentage. The outcome measures were compared between patients who completed 12-month follow-up and those who did not, using unpaired t test. The change in outcome measures at 12 months from baseline was evaluated using paired t test. The effect of intervention was summarized using relative percentage change. The “dose” of the intervention was categorized the number of MBSR sessions – ≤4 sessions, 5-7 sessions, or ≤8 sessions. The change in outcome measures were compared among three groups using 1-way analysis of variance (ANOVA) followed by post hoc multiple comparison using the Bonferroni adjustment. In addition, the effect of intervention on each outcome was evaluated by important baseline characteristics of patients. In each subgroup, the changes were compared with baseline measures using the paired t test, whereas changes in outcome between groups were compared using the unpaired t test. Statistical analyses were conducted using SAS 9.3. P-values less than 5% were considered to be significant.
Results
A total of 94 survivors of breast cancer were included in this study and 60 (63.8%) completed the 12 months of follow-up. The average age of the participants was 54.4 years (SD, 8.7), and 90.4% were Hispanic (Table 1). Tumor characteristics were as follows: invasive ductal carcinoma (84.04%), estrogen receptor–positive (ER-, 71.28%), progesterone receptor–positive (PR-, 58.51%), and HER2-neu–positive (20%). In regard to therapy received, 48% of the participants had received anthracycline- and taxane-based adjuvant chemotherapy and 23%, nonanthracycline-based chemotherapy; 71% had received anti-estrogen (hormonal) therapy and 80%, radiation therapy. In regard to surgery, half of the participants had a lumpectomy, and half, a mastectomy. The trends in the outcome measures over the follow-up period are show in the Figure 1.
The effect of survivorship program intervention on SF-36 (PCS and MCS), anxiety (GAD-7), and (PHQ-9) at 12 months are shown in Table 2, which also includes the 12-month effects on the body-mass index (BMI). The P-values correspond to the comparison of mean change in scores between baseline and 12-month follow-up. Significant improvement from baseline was observed for PHQ-9 (P = .0031) and GAD-7 (P = .0027). There was a significant trend toward improvement (14%) relative to baseline in the SF-36 MCS at 12 months (P = .097). Although the SF-36 PCS improved numerically, it did not reach to a statistical significance level (P = .896). The BMI at 12 months was found to be statistically significantly increased compared with baseline (P = .0007).
The effect of the number of MBSR sessions attended on the outcome measures is summarized in Table 3. There were significant improvements in the 12-month MCS scores for patients who completed 5-7 sessions of MBSR or ≥8 sessions, compared with patients who completed ≤4 sessions of MBSR. There was an improvement observed in PCS scores only among patients who received at least 8 sessions of MBSR. There was a marked improvement observed in GAD-7 and PHQ-9 among patients who received ≥8 sessions. There was no statistically significant change in the GAD-7 or PHQ-9 scores between patients who received ≤4 sessions and 5-7 sessions. No significant association was obtained between number of MBSR sessions attended and BMI.
The effect of survivorship program intervention on all outcomes according to important baseline cofactors is shown in Table 4. As such, there were no significant differences in changes in the outcome measures after intervention according to any considered baseline characteristics. However, the effect of survivorship program intervention was more pronounced in patients who were ≥3 years away from their initial diagnosis and who had attended a minimum of 80% of the 3-monthly visits and received a minimum of 8 MBSR sessions.
The mean baseline PCS and MCS scores of the SF-36 were 43.7 and 45.8, respectively, indicating that the participants’ scores were significantly less than half the standard deviation below the US norm (50.0; SD, 10). The SF-36 health-related QoL categories showed that, on an average, scores improved by more than 4 units for emotional and physical role functions, vitality, and mental health compared with baseline. In addition, scores improved by about 2 units for general health and social functioning compared with baseline data. In all, 65% of survivors had difficulty preforming work at baseline, but that dropped to 55% after enrollment in the program; and 60% had originally reduced the amount of time spent on work, but that increased to 50% after the intervention. Also of note is that 70% of survivors reported accomplishing less than they would like to have (role physical) before the intervention, but that was reduced to 57% after the intervention. Similarly, 77% of survivors felt worn out at baseline, compared with 65% at the 12-month follow-up; and 88% felt tired at baseline, but that percentage was reduced to 68% after the intervention. Before the intervention, 60% of the participants reported that they had been very nervous, and 45% said they had been so down in the dumps that nothing could cheer them up, but those percentages were reduced to 43% and 32%, respectively, after intervention. Before intervention, 63% of the women said they felt depressed and that was reduced to 50% after the intervention.
Discussion
In this study, we showed that a group of predominately Hispanic breast cancer survivors benefited from participating in a multidisciplinary cancer survivorship program that emphasized in-depth psychological care and MBSR. They also benefited from an education effort that included providing survivors with personalized summaries of their treatment and oncology survivorship care, addressing potential long-term side effects of treatment, referral for genetic counselling and screening for other cancers as appropriate, as well dietary advice. We found significant improvement compared with baseline in both mental and physical determinants of the patient-reported outcomes, including anxiety (GAD-7), depression (PHQ-9), and HR-QoL (PCS) and (MCS). Survivors demonstrated significant improvement on the MCS and PHQ-9 if they attended 5 or more sessions of the 8-week MBSR course, and attending 8 sessions was associated with significant improvement in GAD-7 and PCS. This might suggest that survivors who are more motivated do benefit the most from such program.
To our knowledge, this study is the first to address the benefit of the MBSR intervention in Hispanic breast cancer survivors. In a randomized controlled trial that included breast cancer survivors with stages 0-III breast cancer who completed surgery, adjunctive radiation, and/or chemotherapy, MBSR was shown to reduce the symptoms of depression and anxiety and increase energy and physical functioning compared with participants who received “usual care”.3 Furthermore, Bower and colleagues have reported improvements in sleep, fatigue, and pro-inflammatory signaling in younger survivors of breast cancer.17 A similar standardized MBSR program was tested on Danish women who had been treated for stage I-III breast cancer18 and the results showed reduced levels of anxiety and depression at the 12-month follow-up. A similar study by Hoffman and colleagues19 reported improved mood, breast- and endocrine-related quality of life, and well-being with MBSR compared with standard care in women with stage 0-III breast cancer.
Several theories have been suggested to explain how MBSR reduces symptoms of depression, anxiety, and fear of recurrence in breast cancer survivors, one of which is that it provides supportive interaction between group members to practice meditation and apply mindfulness in daily situations.3 In addition, evidence is beginning to emerge that stress-reducing interventions such as MBSR may improve telomere length (TL) and telomerase activity (TA), the markers for cellular aging, psychological stress, and disease risk.20-24 Lengacher and colleagues conducted a randomized controlled study to investigate the effects of MBSR on TL and TA in women with breast cancer, and suggested that MBSR increases telomere length and telomerase activity.25 The 142 patients with stages 0-III breast cancer had completed adjuvant treatment with radiation and/or chemotherapy at least 2 weeks before enrollment and within 2 years of completion of treatment with lumpectomy and/or mastectomy. They were randomly assigned to either a 6-week MBSR for breast cancer program or usual care.25 Assessments of TA and TL were obtained along with psychological measurements at baseline, 6 weeks, and 12 weeks after the patients had completed the MBSR program. The mean age of the participants was 55.3 years; 72% were non-Hispanic white; 78% had stage I or II cancer; and 36% received both chemotherapy and radiation. In analyses adjusted for baseline TA and psychological status, TA increased steadily by about 17% over 12 weeks in the MBSR group, compared with about 3% (P < .01) in the control group. No difference was observed for TL (P = .92). The authors concluded that the data provide preliminary evidence that MBSR increases TA in peripheral blood mononuclear cells from breast cancer patients and have implications for understanding how MBSR may extend cell longevity at the cellular level.
In another study among healthy volunteers who were randomly assigned to a 3-month meditation retreat or a control group, the 30 participants in the meditation group had higher TA compared with controls.20 In a nonrandomized study among prostate cancer patients, TA increased and psychological stress decreased following a stress-reducing, lifestyle-modification program.21 The results of another intervention study among overweight women showed improvement in distress, eating behavior, and metabolic health in women participating in a MBSR program, all of which correlated with increases in TA.22 Most recently, researchers explored the impact on TA of a Kirtan Kriya yogic meditation intervention compared with exposure to relaxing music in 39 dementia family caregivers. The yogic-meditation intervention group had a 43% increase in TA after the 8-week intervention period compared with 3.7% the music group (P < .05).23 Finally, among 22 patients with cervical cancer who were randomized to a psychosocial telephone counseling intervention,24 investigators found a significant association between increased TL and changes in psychological distress.20 Findings from other studies have assessed interventions to improve outcome of breast cancer survivors, such as the Taking CHARGE self-management intervention that is designed to facilitate the transition to survivorship after breast cancer treatment.8 Another intervention using home-based physical activity was shown in a randomized controlled trial to improve self-reported physical activity, body-mass index, and health-related QoL.9 Findings from another study suggested that a combined exercise and psychological counselling program might improve QoL more than a single entity intervention.26 As noted previously, these studies did not focus on minority breast cancer survivors’ population, and it is not clear if they are generalizable to Hispanics.
In addition to the MBSR component, our program has also included one-on-one psychological assessment for long-term treatment complications and provided participants with appropriate care and follow-up plans, adding the benefits of self-awareness and self-attention for the survivors, which can effectively reduce the fear of recurrence.3 Furthermore, we included dietary consults based on general cancer survivor guidelines recommending a high fruit and vegetable diet that is low in fat and sugar.27 Healthier dietary lifestyle has been reported to improve breast cancer prognosis, metabolic disease, and cardiovascular outcomes among Hispanic breast cancer survivors.28
Our study has some limitations, including a relatively small sample size. It did not include an exercise program, which would have been helpful in addressing the issue of overweight and obesity we encountered in the most of the Hispanic breast cancer survivors (baseline average BMI, 31.32 kg/m2; obesity range, >30 kg/m2). Because of the small sample size and nonrandomized design of the study, it is hard to evaluate the confounding effect of time on intervention effect. However, a subgroup analysis by MBSR number of sessions showed that the survivors who completed the full course of MBSR sessions (8 sessions) achieved superior benefit, compared with those who did not complete the full course, which indicates that the intervention did weigh in regardless of time. Despite these limitations, the participants in this interventional program showed improved outcomes, including less anxiety and depression and improved MCS score of the SF-36. A larger and longer follow-up prospective, randomized study is needed to validate the findings of this study. Implementing cancer survivorship as an integral component of cancer care during and after treatment is essential to improve the quality of life of cancer survivors and empower them in their transition from cancer treatment to survivorship.
1. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012 [published correction in CA Cancer J Clin. 2012;62(5):348].CA Cancer J Clin. 2012;62(4):220-241.
2. Williams F, Jeanetta SC. Lived experiences of breast cancer survivors after diagnosis, treatment and beyond: qualitative study. Health Expect. 2016;19(3):631-642.
3. Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18(12):1261-1272.
4. Feiten S, Dünnebacke J, Friesenhahn V, et al. Follow-up reality for breast cancer patients - standardised survey of patients and physicians and analysis of treatment data. Geburtshilfe Frauenheilkd. 2016;76(5):557-563.
5. Bowen DJ, Alfano CM, McGregor BA, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007;106(1):85-95.
6. Nahleh ZA, Dwivedi A, Khang T, et al. Decreased health related quality of life among hispanic breast cancer survivors. http://medcraveonline.com/MOJWH/MOJWH-01-00016.php. Published January 28, 2016. Accessed July 25, 2017.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Post-treatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-254.
8. Cimprich B, Janz NK, Northouse L, Wren PA, Given B, Given CW. Taking CHARGE: a self-management program for women following breast cancer treatment. Psychooncology. 2005;14(9):704-717.
9. Lahart IM, Metsios GS, Nevill AM, Kitas GD, Carmichael AR. Randomised controlled trial of a home-based physical activity intervention in breast cancer survivors. https://bmccancer.biomedcentral.com/articles/10.1186/s12885-016-2258-5. Published 2016. Accessed July 25, 2017.
10. Huang J, Shi L. The effectiveness of mindfulness-based stress reduction (MBSR) for survivors of breast cancer: study protocol for a randomized controlled trial. Trials. 2016;17(1):209.
11. Stahl B and Goldstein E, A mindfulness-based stress reduction workbook. 2010: New Harbinger Publications.
12. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
13. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
14. Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 Suppl):AS264-279.
15. Gandek B, Sinclair SJ, Kosinski M, Ware JE Jr. Psychometric evaluation of the SF-36 health survey in Medicare managed care. Health Care Financ Rev. 2004;25(4):5-25.
16. Ruta D, Garratt A, Abdalla M, Buckingham K, Russell I. The SF-36 health survey questionnaire. A valid measure of health status. BMJ. 1993;307(6901):448-449.
17. Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121(8):1231-1240.
18. Würtzen H, Dalton SO, Elsass P, et al. Mindfulness significantly reduces self-reported levels of anxiety and depression: results of a randomised controlled trial among 336 Danish women treated for stage I-III breast cancer. Eur J Cancer. 2013;49(6):1365-1373.
19. Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol. 2012;30(12):1335-1342.
20. Jacobs TL, Epel ES, Lin J, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36(5):664-681.
21. Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048-1057.
22. Daubenmier J, Lin J, Blackburn E, et al. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology. 2012;37(7):917-928.
23. Lavretsky H, Epel ES, Siddarth P, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28(1):57-65.
24. Biegler KA, Anderson AK, Wenzel LB, Osann K, Nelson EL. Longitudinal change in telomere length and the chronic stress response in a randomized pilot biobehavioral clinical study: implications for cancer prevention. Cancer Prev Res (Phila). 2012;5(10):1173-1182.
25. Lengacher CA, Reich RR, Kip KE. Influence of mindfulness-based stress reduction (MBSR) on telomerase activity in women with breast cancer (BC). Biol Res Nurs. 2014;16(4):438-447.
26. Naumann F, Martin E, Philpott M, Smith C, Groff D, Battaglini C. Can counseling add value to an exercise intervention for improving quality of life in breast cancer survivors? A feasibility study. J Support Oncol. 2012;10(5):188-194.
27. Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30-67.
28. Greenlee H, Gaffney AO, Aycinena AC, et al. Cocinar para su salud!: randomized controlled trial of a culturally based dietary intervention among Hispanic breast cancer survivors. J Acad Nutr Diet. 2015;115(5):709-723.e3.
Breast cancer survivors comprise the most prevalent cancer survivor population in the United States.1 The number of breast cancer survivors is increasing because of early detection and diagnosis, and advances in treatment have resulted in increased life expectancy. Therefore, greater attention is needed to improve the long-term quality of life of these survivors and to help them re-adjust to normal life. For many women, although the medical treatment may have been completed, the recovery process may have not.2 The prevalence of long-term mental and physical illness is significant among many breast cancer survivors. Long-term mental consequences may include memory problems, anxiety, depression, and fear of recurrence3, and long-term physical consequences may include pain, fatigue, and lymphedema, among others.4
El Paso, Texas, is the fourth most populous city in Texas and has a Hispanic majority. This provides an opportunity to conduct clinical research targeting participants of Hispanic descent. Several studies have noted the influence of race/ethnicity on the psychosocial function of breast cancer survivors.5,6 We have previously reported that Hispanic breast cancer survivors might experience decreased mental and physical health-related quality of life (QoL) which limit their normal social functioning.6Other studies have similarly reported poor outcomes of breast cancer survivors and higher rates of fatigue and depression among Hispanic patients.7 However, there is a paucity of research addressing specific interventions needed to improve these outcomes and provide better QoL for breast cancer survivors.8,9 In addition, a few survivorship care interventions have focused on minorities. We sought to assess whether a multidisciplinary cancer survivorship program in a primarily Hispanic populated area would lead to improved QoL and reduce anxiety and depressive symptoms among breast cancer survivors.
Methods
After obtaining Institutional Review Board approval, we recruited consecutive patients who were treated at our institution during October 2013-October 2014 and obtained informed consent from them. The participants were within the first 5 years after diagnosis with stages I-III breast cancer and had completed surgery, chemotherapy, and/or radiation therapy. We sought to determine whether breast cancer survivors would benefit from this intervention as determined by improvement of performance at 12 months compared with baseline based on the following self-reported validated questionnaires: Patient Health Questionnaire-9 (PHQ-9) for depression; General Anxiety Disorder-7 (GAD-7); and Short-Form Health Survey-36 (SF-36, version 2) for patient quality of life. The participants were enrolled in a comprehensive survivorship program staffed by an oncologist, an oncology nurse practitioner, a nutritionist, and a certified clinical psychologist who had trained in mindfulness-based stress reduction (MBSR).
Interventions
The participants received a one-on-one individual psychological consultation visit every 3 months for 20-45 minutes during which the psychologist addressed each patient’s emotional and psychological issues in depth, discussed relaxation techniques, and provided psychosocial counselling. In addition, all participants were asked to attend an 8-week-course (in Spanish or English) using MBSR, an interventional program in which participants receive training to promote reduction of stress by self-regulating mindfulness practice.3,10 Our institution’s MBSR program consists of a weekly 2-hour class for 8 sessions or more. The program is provided 3 times a year, in English and Spanish. It includes the following components:
- Learning various mindfulness meditation techniques (eg, body scans, awareness of breathing, sitting/walking meditations);
- Practicing the mindfulness techniques in class; and
- Practicing techniques at home through audiorecordings of mindfulness meditation exercises and daily diary writing.
Participants were provided with a workbook on MBSR in their preferred language.11 In addition to the psychological component, they were also provided with oncologic evaluations by an oncology nurse practitioner. The nurse practitioner met with participants every 3 months and provided each one with a personalized summary of all the treatments received and routine oncology follow-up care in consultation with the patients’ regular oncologists. This care also addresses the long-term sequelae of treatment, including arthritis and osteoporosis, referrals to receive screening for other cancers (eg, cervical and colon cancer), and genetic counselling as appropriate. In addition, a nutritionist provided general dietary advice in individual and group sessions every 3 months.
The self-administered questionnaires, PHQ-9, GAD-7, and SF-36, were completed at baseline, and every 3 months for 12 months. The scores were reviewed by the psychologist and the oncologist. The PHQ-9 was used to initially screen survivors for depression and monitor their improvement after the intervention. The PHQ-9 is a reliable and validated self-administered depression module.12 The PQH-9 exclusively focuses on the 9 diagnostic criteria for DSM-IV depression disorder and it can be used as a useful measure for monitoring outcomes of depression therapy. A score of 5-14 suggests mild-moderate depression, and a score of >15 suggests severe depression
The survivors were screened for anxiety using the GAD-7, a brief 7-item self-report scale to identify probable cases of anxiety disorder that has been shown to be an efficient tool for screening and assessing the severity of anxiety.13 For GAD-7, a score of 5 or higher is suggestive of anxiety. Scores of 5, 10, and 15 represent cut-off points for mild, moderate, and severe anxiety, respectively.
Survivor QoL was evaluated using the SF-36 questionnaire, a multipurpose survey containing 36 questions. It ranges from 0-100 and a score that is <50.0 is considered low. The lower the score, the worse the mental or physical function.14 The SF-36 yields a patient profile of 8 health domains – vitality, physical functioning, bodily pain, general health perceptions, physical, emotional, and social role functioning; and mental health.15,16 A score of 50.0 on either the Physical Component Summary (PCS – vitality, physical functioning, bodily pain, general health perceptions, physical role functioning) or Mental Component Summary (MCS – emotional and social role functioning, and mental health) is consistent with the US norm.
Statistical analysis
In this study, the primary objective was to use the MBSR survivorship program to improve the survivors’ outcomes at 12 months compared with baseline using the following measures: PHQ-9 for depression, GAD-7 for anxiety, and SF-36 for QoL using the PCS and MCS. Quantitative data were described using the mean and standard deviation, and categorical data were described using frequency and percentage. The outcome measures were compared between patients who completed 12-month follow-up and those who did not, using unpaired t test. The change in outcome measures at 12 months from baseline was evaluated using paired t test. The effect of intervention was summarized using relative percentage change. The “dose” of the intervention was categorized the number of MBSR sessions – ≤4 sessions, 5-7 sessions, or ≤8 sessions. The change in outcome measures were compared among three groups using 1-way analysis of variance (ANOVA) followed by post hoc multiple comparison using the Bonferroni adjustment. In addition, the effect of intervention on each outcome was evaluated by important baseline characteristics of patients. In each subgroup, the changes were compared with baseline measures using the paired t test, whereas changes in outcome between groups were compared using the unpaired t test. Statistical analyses were conducted using SAS 9.3. P-values less than 5% were considered to be significant.
Results
A total of 94 survivors of breast cancer were included in this study and 60 (63.8%) completed the 12 months of follow-up. The average age of the participants was 54.4 years (SD, 8.7), and 90.4% were Hispanic (Table 1). Tumor characteristics were as follows: invasive ductal carcinoma (84.04%), estrogen receptor–positive (ER-, 71.28%), progesterone receptor–positive (PR-, 58.51%), and HER2-neu–positive (20%). In regard to therapy received, 48% of the participants had received anthracycline- and taxane-based adjuvant chemotherapy and 23%, nonanthracycline-based chemotherapy; 71% had received anti-estrogen (hormonal) therapy and 80%, radiation therapy. In regard to surgery, half of the participants had a lumpectomy, and half, a mastectomy. The trends in the outcome measures over the follow-up period are show in the Figure 1.
The effect of survivorship program intervention on SF-36 (PCS and MCS), anxiety (GAD-7), and (PHQ-9) at 12 months are shown in Table 2, which also includes the 12-month effects on the body-mass index (BMI). The P-values correspond to the comparison of mean change in scores between baseline and 12-month follow-up. Significant improvement from baseline was observed for PHQ-9 (P = .0031) and GAD-7 (P = .0027). There was a significant trend toward improvement (14%) relative to baseline in the SF-36 MCS at 12 months (P = .097). Although the SF-36 PCS improved numerically, it did not reach to a statistical significance level (P = .896). The BMI at 12 months was found to be statistically significantly increased compared with baseline (P = .0007).
The effect of the number of MBSR sessions attended on the outcome measures is summarized in Table 3. There were significant improvements in the 12-month MCS scores for patients who completed 5-7 sessions of MBSR or ≥8 sessions, compared with patients who completed ≤4 sessions of MBSR. There was an improvement observed in PCS scores only among patients who received at least 8 sessions of MBSR. There was a marked improvement observed in GAD-7 and PHQ-9 among patients who received ≥8 sessions. There was no statistically significant change in the GAD-7 or PHQ-9 scores between patients who received ≤4 sessions and 5-7 sessions. No significant association was obtained between number of MBSR sessions attended and BMI.
The effect of survivorship program intervention on all outcomes according to important baseline cofactors is shown in Table 4. As such, there were no significant differences in changes in the outcome measures after intervention according to any considered baseline characteristics. However, the effect of survivorship program intervention was more pronounced in patients who were ≥3 years away from their initial diagnosis and who had attended a minimum of 80% of the 3-monthly visits and received a minimum of 8 MBSR sessions.
The mean baseline PCS and MCS scores of the SF-36 were 43.7 and 45.8, respectively, indicating that the participants’ scores were significantly less than half the standard deviation below the US norm (50.0; SD, 10). The SF-36 health-related QoL categories showed that, on an average, scores improved by more than 4 units for emotional and physical role functions, vitality, and mental health compared with baseline. In addition, scores improved by about 2 units for general health and social functioning compared with baseline data. In all, 65% of survivors had difficulty preforming work at baseline, but that dropped to 55% after enrollment in the program; and 60% had originally reduced the amount of time spent on work, but that increased to 50% after the intervention. Also of note is that 70% of survivors reported accomplishing less than they would like to have (role physical) before the intervention, but that was reduced to 57% after the intervention. Similarly, 77% of survivors felt worn out at baseline, compared with 65% at the 12-month follow-up; and 88% felt tired at baseline, but that percentage was reduced to 68% after the intervention. Before the intervention, 60% of the participants reported that they had been very nervous, and 45% said they had been so down in the dumps that nothing could cheer them up, but those percentages were reduced to 43% and 32%, respectively, after intervention. Before intervention, 63% of the women said they felt depressed and that was reduced to 50% after the intervention.
Discussion
In this study, we showed that a group of predominately Hispanic breast cancer survivors benefited from participating in a multidisciplinary cancer survivorship program that emphasized in-depth psychological care and MBSR. They also benefited from an education effort that included providing survivors with personalized summaries of their treatment and oncology survivorship care, addressing potential long-term side effects of treatment, referral for genetic counselling and screening for other cancers as appropriate, as well dietary advice. We found significant improvement compared with baseline in both mental and physical determinants of the patient-reported outcomes, including anxiety (GAD-7), depression (PHQ-9), and HR-QoL (PCS) and (MCS). Survivors demonstrated significant improvement on the MCS and PHQ-9 if they attended 5 or more sessions of the 8-week MBSR course, and attending 8 sessions was associated with significant improvement in GAD-7 and PCS. This might suggest that survivors who are more motivated do benefit the most from such program.
To our knowledge, this study is the first to address the benefit of the MBSR intervention in Hispanic breast cancer survivors. In a randomized controlled trial that included breast cancer survivors with stages 0-III breast cancer who completed surgery, adjunctive radiation, and/or chemotherapy, MBSR was shown to reduce the symptoms of depression and anxiety and increase energy and physical functioning compared with participants who received “usual care”.3 Furthermore, Bower and colleagues have reported improvements in sleep, fatigue, and pro-inflammatory signaling in younger survivors of breast cancer.17 A similar standardized MBSR program was tested on Danish women who had been treated for stage I-III breast cancer18 and the results showed reduced levels of anxiety and depression at the 12-month follow-up. A similar study by Hoffman and colleagues19 reported improved mood, breast- and endocrine-related quality of life, and well-being with MBSR compared with standard care in women with stage 0-III breast cancer.
Several theories have been suggested to explain how MBSR reduces symptoms of depression, anxiety, and fear of recurrence in breast cancer survivors, one of which is that it provides supportive interaction between group members to practice meditation and apply mindfulness in daily situations.3 In addition, evidence is beginning to emerge that stress-reducing interventions such as MBSR may improve telomere length (TL) and telomerase activity (TA), the markers for cellular aging, psychological stress, and disease risk.20-24 Lengacher and colleagues conducted a randomized controlled study to investigate the effects of MBSR on TL and TA in women with breast cancer, and suggested that MBSR increases telomere length and telomerase activity.25 The 142 patients with stages 0-III breast cancer had completed adjuvant treatment with radiation and/or chemotherapy at least 2 weeks before enrollment and within 2 years of completion of treatment with lumpectomy and/or mastectomy. They were randomly assigned to either a 6-week MBSR for breast cancer program or usual care.25 Assessments of TA and TL were obtained along with psychological measurements at baseline, 6 weeks, and 12 weeks after the patients had completed the MBSR program. The mean age of the participants was 55.3 years; 72% were non-Hispanic white; 78% had stage I or II cancer; and 36% received both chemotherapy and radiation. In analyses adjusted for baseline TA and psychological status, TA increased steadily by about 17% over 12 weeks in the MBSR group, compared with about 3% (P < .01) in the control group. No difference was observed for TL (P = .92). The authors concluded that the data provide preliminary evidence that MBSR increases TA in peripheral blood mononuclear cells from breast cancer patients and have implications for understanding how MBSR may extend cell longevity at the cellular level.
In another study among healthy volunteers who were randomly assigned to a 3-month meditation retreat or a control group, the 30 participants in the meditation group had higher TA compared with controls.20 In a nonrandomized study among prostate cancer patients, TA increased and psychological stress decreased following a stress-reducing, lifestyle-modification program.21 The results of another intervention study among overweight women showed improvement in distress, eating behavior, and metabolic health in women participating in a MBSR program, all of which correlated with increases in TA.22 Most recently, researchers explored the impact on TA of a Kirtan Kriya yogic meditation intervention compared with exposure to relaxing music in 39 dementia family caregivers. The yogic-meditation intervention group had a 43% increase in TA after the 8-week intervention period compared with 3.7% the music group (P < .05).23 Finally, among 22 patients with cervical cancer who were randomized to a psychosocial telephone counseling intervention,24 investigators found a significant association between increased TL and changes in psychological distress.20 Findings from other studies have assessed interventions to improve outcome of breast cancer survivors, such as the Taking CHARGE self-management intervention that is designed to facilitate the transition to survivorship after breast cancer treatment.8 Another intervention using home-based physical activity was shown in a randomized controlled trial to improve self-reported physical activity, body-mass index, and health-related QoL.9 Findings from another study suggested that a combined exercise and psychological counselling program might improve QoL more than a single entity intervention.26 As noted previously, these studies did not focus on minority breast cancer survivors’ population, and it is not clear if they are generalizable to Hispanics.
In addition to the MBSR component, our program has also included one-on-one psychological assessment for long-term treatment complications and provided participants with appropriate care and follow-up plans, adding the benefits of self-awareness and self-attention for the survivors, which can effectively reduce the fear of recurrence.3 Furthermore, we included dietary consults based on general cancer survivor guidelines recommending a high fruit and vegetable diet that is low in fat and sugar.27 Healthier dietary lifestyle has been reported to improve breast cancer prognosis, metabolic disease, and cardiovascular outcomes among Hispanic breast cancer survivors.28
Our study has some limitations, including a relatively small sample size. It did not include an exercise program, which would have been helpful in addressing the issue of overweight and obesity we encountered in the most of the Hispanic breast cancer survivors (baseline average BMI, 31.32 kg/m2; obesity range, >30 kg/m2). Because of the small sample size and nonrandomized design of the study, it is hard to evaluate the confounding effect of time on intervention effect. However, a subgroup analysis by MBSR number of sessions showed that the survivors who completed the full course of MBSR sessions (8 sessions) achieved superior benefit, compared with those who did not complete the full course, which indicates that the intervention did weigh in regardless of time. Despite these limitations, the participants in this interventional program showed improved outcomes, including less anxiety and depression and improved MCS score of the SF-36. A larger and longer follow-up prospective, randomized study is needed to validate the findings of this study. Implementing cancer survivorship as an integral component of cancer care during and after treatment is essential to improve the quality of life of cancer survivors and empower them in their transition from cancer treatment to survivorship.
Breast cancer survivors comprise the most prevalent cancer survivor population in the United States.1 The number of breast cancer survivors is increasing because of early detection and diagnosis, and advances in treatment have resulted in increased life expectancy. Therefore, greater attention is needed to improve the long-term quality of life of these survivors and to help them re-adjust to normal life. For many women, although the medical treatment may have been completed, the recovery process may have not.2 The prevalence of long-term mental and physical illness is significant among many breast cancer survivors. Long-term mental consequences may include memory problems, anxiety, depression, and fear of recurrence3, and long-term physical consequences may include pain, fatigue, and lymphedema, among others.4
El Paso, Texas, is the fourth most populous city in Texas and has a Hispanic majority. This provides an opportunity to conduct clinical research targeting participants of Hispanic descent. Several studies have noted the influence of race/ethnicity on the psychosocial function of breast cancer survivors.5,6 We have previously reported that Hispanic breast cancer survivors might experience decreased mental and physical health-related quality of life (QoL) which limit their normal social functioning.6Other studies have similarly reported poor outcomes of breast cancer survivors and higher rates of fatigue and depression among Hispanic patients.7 However, there is a paucity of research addressing specific interventions needed to improve these outcomes and provide better QoL for breast cancer survivors.8,9 In addition, a few survivorship care interventions have focused on minorities. We sought to assess whether a multidisciplinary cancer survivorship program in a primarily Hispanic populated area would lead to improved QoL and reduce anxiety and depressive symptoms among breast cancer survivors.
Methods
After obtaining Institutional Review Board approval, we recruited consecutive patients who were treated at our institution during October 2013-October 2014 and obtained informed consent from them. The participants were within the first 5 years after diagnosis with stages I-III breast cancer and had completed surgery, chemotherapy, and/or radiation therapy. We sought to determine whether breast cancer survivors would benefit from this intervention as determined by improvement of performance at 12 months compared with baseline based on the following self-reported validated questionnaires: Patient Health Questionnaire-9 (PHQ-9) for depression; General Anxiety Disorder-7 (GAD-7); and Short-Form Health Survey-36 (SF-36, version 2) for patient quality of life. The participants were enrolled in a comprehensive survivorship program staffed by an oncologist, an oncology nurse practitioner, a nutritionist, and a certified clinical psychologist who had trained in mindfulness-based stress reduction (MBSR).
Interventions
The participants received a one-on-one individual psychological consultation visit every 3 months for 20-45 minutes during which the psychologist addressed each patient’s emotional and psychological issues in depth, discussed relaxation techniques, and provided psychosocial counselling. In addition, all participants were asked to attend an 8-week-course (in Spanish or English) using MBSR, an interventional program in which participants receive training to promote reduction of stress by self-regulating mindfulness practice.3,10 Our institution’s MBSR program consists of a weekly 2-hour class for 8 sessions or more. The program is provided 3 times a year, in English and Spanish. It includes the following components:
- Learning various mindfulness meditation techniques (eg, body scans, awareness of breathing, sitting/walking meditations);
- Practicing the mindfulness techniques in class; and
- Practicing techniques at home through audiorecordings of mindfulness meditation exercises and daily diary writing.
Participants were provided with a workbook on MBSR in their preferred language.11 In addition to the psychological component, they were also provided with oncologic evaluations by an oncology nurse practitioner. The nurse practitioner met with participants every 3 months and provided each one with a personalized summary of all the treatments received and routine oncology follow-up care in consultation with the patients’ regular oncologists. This care also addresses the long-term sequelae of treatment, including arthritis and osteoporosis, referrals to receive screening for other cancers (eg, cervical and colon cancer), and genetic counselling as appropriate. In addition, a nutritionist provided general dietary advice in individual and group sessions every 3 months.
The self-administered questionnaires, PHQ-9, GAD-7, and SF-36, were completed at baseline, and every 3 months for 12 months. The scores were reviewed by the psychologist and the oncologist. The PHQ-9 was used to initially screen survivors for depression and monitor their improvement after the intervention. The PHQ-9 is a reliable and validated self-administered depression module.12 The PQH-9 exclusively focuses on the 9 diagnostic criteria for DSM-IV depression disorder and it can be used as a useful measure for monitoring outcomes of depression therapy. A score of 5-14 suggests mild-moderate depression, and a score of >15 suggests severe depression
The survivors were screened for anxiety using the GAD-7, a brief 7-item self-report scale to identify probable cases of anxiety disorder that has been shown to be an efficient tool for screening and assessing the severity of anxiety.13 For GAD-7, a score of 5 or higher is suggestive of anxiety. Scores of 5, 10, and 15 represent cut-off points for mild, moderate, and severe anxiety, respectively.
Survivor QoL was evaluated using the SF-36 questionnaire, a multipurpose survey containing 36 questions. It ranges from 0-100 and a score that is <50.0 is considered low. The lower the score, the worse the mental or physical function.14 The SF-36 yields a patient profile of 8 health domains – vitality, physical functioning, bodily pain, general health perceptions, physical, emotional, and social role functioning; and mental health.15,16 A score of 50.0 on either the Physical Component Summary (PCS – vitality, physical functioning, bodily pain, general health perceptions, physical role functioning) or Mental Component Summary (MCS – emotional and social role functioning, and mental health) is consistent with the US norm.
Statistical analysis
In this study, the primary objective was to use the MBSR survivorship program to improve the survivors’ outcomes at 12 months compared with baseline using the following measures: PHQ-9 for depression, GAD-7 for anxiety, and SF-36 for QoL using the PCS and MCS. Quantitative data were described using the mean and standard deviation, and categorical data were described using frequency and percentage. The outcome measures were compared between patients who completed 12-month follow-up and those who did not, using unpaired t test. The change in outcome measures at 12 months from baseline was evaluated using paired t test. The effect of intervention was summarized using relative percentage change. The “dose” of the intervention was categorized the number of MBSR sessions – ≤4 sessions, 5-7 sessions, or ≤8 sessions. The change in outcome measures were compared among three groups using 1-way analysis of variance (ANOVA) followed by post hoc multiple comparison using the Bonferroni adjustment. In addition, the effect of intervention on each outcome was evaluated by important baseline characteristics of patients. In each subgroup, the changes were compared with baseline measures using the paired t test, whereas changes in outcome between groups were compared using the unpaired t test. Statistical analyses were conducted using SAS 9.3. P-values less than 5% were considered to be significant.
Results
A total of 94 survivors of breast cancer were included in this study and 60 (63.8%) completed the 12 months of follow-up. The average age of the participants was 54.4 years (SD, 8.7), and 90.4% were Hispanic (Table 1). Tumor characteristics were as follows: invasive ductal carcinoma (84.04%), estrogen receptor–positive (ER-, 71.28%), progesterone receptor–positive (PR-, 58.51%), and HER2-neu–positive (20%). In regard to therapy received, 48% of the participants had received anthracycline- and taxane-based adjuvant chemotherapy and 23%, nonanthracycline-based chemotherapy; 71% had received anti-estrogen (hormonal) therapy and 80%, radiation therapy. In regard to surgery, half of the participants had a lumpectomy, and half, a mastectomy. The trends in the outcome measures over the follow-up period are show in the Figure 1.
The effect of survivorship program intervention on SF-36 (PCS and MCS), anxiety (GAD-7), and (PHQ-9) at 12 months are shown in Table 2, which also includes the 12-month effects on the body-mass index (BMI). The P-values correspond to the comparison of mean change in scores between baseline and 12-month follow-up. Significant improvement from baseline was observed for PHQ-9 (P = .0031) and GAD-7 (P = .0027). There was a significant trend toward improvement (14%) relative to baseline in the SF-36 MCS at 12 months (P = .097). Although the SF-36 PCS improved numerically, it did not reach to a statistical significance level (P = .896). The BMI at 12 months was found to be statistically significantly increased compared with baseline (P = .0007).
The effect of the number of MBSR sessions attended on the outcome measures is summarized in Table 3. There were significant improvements in the 12-month MCS scores for patients who completed 5-7 sessions of MBSR or ≥8 sessions, compared with patients who completed ≤4 sessions of MBSR. There was an improvement observed in PCS scores only among patients who received at least 8 sessions of MBSR. There was a marked improvement observed in GAD-7 and PHQ-9 among patients who received ≥8 sessions. There was no statistically significant change in the GAD-7 or PHQ-9 scores between patients who received ≤4 sessions and 5-7 sessions. No significant association was obtained between number of MBSR sessions attended and BMI.
The effect of survivorship program intervention on all outcomes according to important baseline cofactors is shown in Table 4. As such, there were no significant differences in changes in the outcome measures after intervention according to any considered baseline characteristics. However, the effect of survivorship program intervention was more pronounced in patients who were ≥3 years away from their initial diagnosis and who had attended a minimum of 80% of the 3-monthly visits and received a minimum of 8 MBSR sessions.
The mean baseline PCS and MCS scores of the SF-36 were 43.7 and 45.8, respectively, indicating that the participants’ scores were significantly less than half the standard deviation below the US norm (50.0; SD, 10). The SF-36 health-related QoL categories showed that, on an average, scores improved by more than 4 units for emotional and physical role functions, vitality, and mental health compared with baseline. In addition, scores improved by about 2 units for general health and social functioning compared with baseline data. In all, 65% of survivors had difficulty preforming work at baseline, but that dropped to 55% after enrollment in the program; and 60% had originally reduced the amount of time spent on work, but that increased to 50% after the intervention. Also of note is that 70% of survivors reported accomplishing less than they would like to have (role physical) before the intervention, but that was reduced to 57% after the intervention. Similarly, 77% of survivors felt worn out at baseline, compared with 65% at the 12-month follow-up; and 88% felt tired at baseline, but that percentage was reduced to 68% after the intervention. Before the intervention, 60% of the participants reported that they had been very nervous, and 45% said they had been so down in the dumps that nothing could cheer them up, but those percentages were reduced to 43% and 32%, respectively, after intervention. Before intervention, 63% of the women said they felt depressed and that was reduced to 50% after the intervention.
Discussion
In this study, we showed that a group of predominately Hispanic breast cancer survivors benefited from participating in a multidisciplinary cancer survivorship program that emphasized in-depth psychological care and MBSR. They also benefited from an education effort that included providing survivors with personalized summaries of their treatment and oncology survivorship care, addressing potential long-term side effects of treatment, referral for genetic counselling and screening for other cancers as appropriate, as well dietary advice. We found significant improvement compared with baseline in both mental and physical determinants of the patient-reported outcomes, including anxiety (GAD-7), depression (PHQ-9), and HR-QoL (PCS) and (MCS). Survivors demonstrated significant improvement on the MCS and PHQ-9 if they attended 5 or more sessions of the 8-week MBSR course, and attending 8 sessions was associated with significant improvement in GAD-7 and PCS. This might suggest that survivors who are more motivated do benefit the most from such program.
To our knowledge, this study is the first to address the benefit of the MBSR intervention in Hispanic breast cancer survivors. In a randomized controlled trial that included breast cancer survivors with stages 0-III breast cancer who completed surgery, adjunctive radiation, and/or chemotherapy, MBSR was shown to reduce the symptoms of depression and anxiety and increase energy and physical functioning compared with participants who received “usual care”.3 Furthermore, Bower and colleagues have reported improvements in sleep, fatigue, and pro-inflammatory signaling in younger survivors of breast cancer.17 A similar standardized MBSR program was tested on Danish women who had been treated for stage I-III breast cancer18 and the results showed reduced levels of anxiety and depression at the 12-month follow-up. A similar study by Hoffman and colleagues19 reported improved mood, breast- and endocrine-related quality of life, and well-being with MBSR compared with standard care in women with stage 0-III breast cancer.
Several theories have been suggested to explain how MBSR reduces symptoms of depression, anxiety, and fear of recurrence in breast cancer survivors, one of which is that it provides supportive interaction between group members to practice meditation and apply mindfulness in daily situations.3 In addition, evidence is beginning to emerge that stress-reducing interventions such as MBSR may improve telomere length (TL) and telomerase activity (TA), the markers for cellular aging, psychological stress, and disease risk.20-24 Lengacher and colleagues conducted a randomized controlled study to investigate the effects of MBSR on TL and TA in women with breast cancer, and suggested that MBSR increases telomere length and telomerase activity.25 The 142 patients with stages 0-III breast cancer had completed adjuvant treatment with radiation and/or chemotherapy at least 2 weeks before enrollment and within 2 years of completion of treatment with lumpectomy and/or mastectomy. They were randomly assigned to either a 6-week MBSR for breast cancer program or usual care.25 Assessments of TA and TL were obtained along with psychological measurements at baseline, 6 weeks, and 12 weeks after the patients had completed the MBSR program. The mean age of the participants was 55.3 years; 72% were non-Hispanic white; 78% had stage I or II cancer; and 36% received both chemotherapy and radiation. In analyses adjusted for baseline TA and psychological status, TA increased steadily by about 17% over 12 weeks in the MBSR group, compared with about 3% (P < .01) in the control group. No difference was observed for TL (P = .92). The authors concluded that the data provide preliminary evidence that MBSR increases TA in peripheral blood mononuclear cells from breast cancer patients and have implications for understanding how MBSR may extend cell longevity at the cellular level.
In another study among healthy volunteers who were randomly assigned to a 3-month meditation retreat or a control group, the 30 participants in the meditation group had higher TA compared with controls.20 In a nonrandomized study among prostate cancer patients, TA increased and psychological stress decreased following a stress-reducing, lifestyle-modification program.21 The results of another intervention study among overweight women showed improvement in distress, eating behavior, and metabolic health in women participating in a MBSR program, all of which correlated with increases in TA.22 Most recently, researchers explored the impact on TA of a Kirtan Kriya yogic meditation intervention compared with exposure to relaxing music in 39 dementia family caregivers. The yogic-meditation intervention group had a 43% increase in TA after the 8-week intervention period compared with 3.7% the music group (P < .05).23 Finally, among 22 patients with cervical cancer who were randomized to a psychosocial telephone counseling intervention,24 investigators found a significant association between increased TL and changes in psychological distress.20 Findings from other studies have assessed interventions to improve outcome of breast cancer survivors, such as the Taking CHARGE self-management intervention that is designed to facilitate the transition to survivorship after breast cancer treatment.8 Another intervention using home-based physical activity was shown in a randomized controlled trial to improve self-reported physical activity, body-mass index, and health-related QoL.9 Findings from another study suggested that a combined exercise and psychological counselling program might improve QoL more than a single entity intervention.26 As noted previously, these studies did not focus on minority breast cancer survivors’ population, and it is not clear if they are generalizable to Hispanics.
In addition to the MBSR component, our program has also included one-on-one psychological assessment for long-term treatment complications and provided participants with appropriate care and follow-up plans, adding the benefits of self-awareness and self-attention for the survivors, which can effectively reduce the fear of recurrence.3 Furthermore, we included dietary consults based on general cancer survivor guidelines recommending a high fruit and vegetable diet that is low in fat and sugar.27 Healthier dietary lifestyle has been reported to improve breast cancer prognosis, metabolic disease, and cardiovascular outcomes among Hispanic breast cancer survivors.28
Our study has some limitations, including a relatively small sample size. It did not include an exercise program, which would have been helpful in addressing the issue of overweight and obesity we encountered in the most of the Hispanic breast cancer survivors (baseline average BMI, 31.32 kg/m2; obesity range, >30 kg/m2). Because of the small sample size and nonrandomized design of the study, it is hard to evaluate the confounding effect of time on intervention effect. However, a subgroup analysis by MBSR number of sessions showed that the survivors who completed the full course of MBSR sessions (8 sessions) achieved superior benefit, compared with those who did not complete the full course, which indicates that the intervention did weigh in regardless of time. Despite these limitations, the participants in this interventional program showed improved outcomes, including less anxiety and depression and improved MCS score of the SF-36. A larger and longer follow-up prospective, randomized study is needed to validate the findings of this study. Implementing cancer survivorship as an integral component of cancer care during and after treatment is essential to improve the quality of life of cancer survivors and empower them in their transition from cancer treatment to survivorship.
1. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012 [published correction in CA Cancer J Clin. 2012;62(5):348].CA Cancer J Clin. 2012;62(4):220-241.
2. Williams F, Jeanetta SC. Lived experiences of breast cancer survivors after diagnosis, treatment and beyond: qualitative study. Health Expect. 2016;19(3):631-642.
3. Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18(12):1261-1272.
4. Feiten S, Dünnebacke J, Friesenhahn V, et al. Follow-up reality for breast cancer patients - standardised survey of patients and physicians and analysis of treatment data. Geburtshilfe Frauenheilkd. 2016;76(5):557-563.
5. Bowen DJ, Alfano CM, McGregor BA, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007;106(1):85-95.
6. Nahleh ZA, Dwivedi A, Khang T, et al. Decreased health related quality of life among hispanic breast cancer survivors. http://medcraveonline.com/MOJWH/MOJWH-01-00016.php. Published January 28, 2016. Accessed July 25, 2017.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Post-treatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-254.
8. Cimprich B, Janz NK, Northouse L, Wren PA, Given B, Given CW. Taking CHARGE: a self-management program for women following breast cancer treatment. Psychooncology. 2005;14(9):704-717.
9. Lahart IM, Metsios GS, Nevill AM, Kitas GD, Carmichael AR. Randomised controlled trial of a home-based physical activity intervention in breast cancer survivors. https://bmccancer.biomedcentral.com/articles/10.1186/s12885-016-2258-5. Published 2016. Accessed July 25, 2017.
10. Huang J, Shi L. The effectiveness of mindfulness-based stress reduction (MBSR) for survivors of breast cancer: study protocol for a randomized controlled trial. Trials. 2016;17(1):209.
11. Stahl B and Goldstein E, A mindfulness-based stress reduction workbook. 2010: New Harbinger Publications.
12. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
13. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
14. Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 Suppl):AS264-279.
15. Gandek B, Sinclair SJ, Kosinski M, Ware JE Jr. Psychometric evaluation of the SF-36 health survey in Medicare managed care. Health Care Financ Rev. 2004;25(4):5-25.
16. Ruta D, Garratt A, Abdalla M, Buckingham K, Russell I. The SF-36 health survey questionnaire. A valid measure of health status. BMJ. 1993;307(6901):448-449.
17. Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121(8):1231-1240.
18. Würtzen H, Dalton SO, Elsass P, et al. Mindfulness significantly reduces self-reported levels of anxiety and depression: results of a randomised controlled trial among 336 Danish women treated for stage I-III breast cancer. Eur J Cancer. 2013;49(6):1365-1373.
19. Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol. 2012;30(12):1335-1342.
20. Jacobs TL, Epel ES, Lin J, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36(5):664-681.
21. Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048-1057.
22. Daubenmier J, Lin J, Blackburn E, et al. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology. 2012;37(7):917-928.
23. Lavretsky H, Epel ES, Siddarth P, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28(1):57-65.
24. Biegler KA, Anderson AK, Wenzel LB, Osann K, Nelson EL. Longitudinal change in telomere length and the chronic stress response in a randomized pilot biobehavioral clinical study: implications for cancer prevention. Cancer Prev Res (Phila). 2012;5(10):1173-1182.
25. Lengacher CA, Reich RR, Kip KE. Influence of mindfulness-based stress reduction (MBSR) on telomerase activity in women with breast cancer (BC). Biol Res Nurs. 2014;16(4):438-447.
26. Naumann F, Martin E, Philpott M, Smith C, Groff D, Battaglini C. Can counseling add value to an exercise intervention for improving quality of life in breast cancer survivors? A feasibility study. J Support Oncol. 2012;10(5):188-194.
27. Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30-67.
28. Greenlee H, Gaffney AO, Aycinena AC, et al. Cocinar para su salud!: randomized controlled trial of a culturally based dietary intervention among Hispanic breast cancer survivors. J Acad Nutr Diet. 2015;115(5):709-723.e3.
1. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012 [published correction in CA Cancer J Clin. 2012;62(5):348].CA Cancer J Clin. 2012;62(4):220-241.
2. Williams F, Jeanetta SC. Lived experiences of breast cancer survivors after diagnosis, treatment and beyond: qualitative study. Health Expect. 2016;19(3):631-642.
3. Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18(12):1261-1272.
4. Feiten S, Dünnebacke J, Friesenhahn V, et al. Follow-up reality for breast cancer patients - standardised survey of patients and physicians and analysis of treatment data. Geburtshilfe Frauenheilkd. 2016;76(5):557-563.
5. Bowen DJ, Alfano CM, McGregor BA, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007;106(1):85-95.
6. Nahleh ZA, Dwivedi A, Khang T, et al. Decreased health related quality of life among hispanic breast cancer survivors. http://medcraveonline.com/MOJWH/MOJWH-01-00016.php. Published January 28, 2016. Accessed July 25, 2017.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Post-treatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-254.
8. Cimprich B, Janz NK, Northouse L, Wren PA, Given B, Given CW. Taking CHARGE: a self-management program for women following breast cancer treatment. Psychooncology. 2005;14(9):704-717.
9. Lahart IM, Metsios GS, Nevill AM, Kitas GD, Carmichael AR. Randomised controlled trial of a home-based physical activity intervention in breast cancer survivors. https://bmccancer.biomedcentral.com/articles/10.1186/s12885-016-2258-5. Published 2016. Accessed July 25, 2017.
10. Huang J, Shi L. The effectiveness of mindfulness-based stress reduction (MBSR) for survivors of breast cancer: study protocol for a randomized controlled trial. Trials. 2016;17(1):209.
11. Stahl B and Goldstein E, A mindfulness-based stress reduction workbook. 2010: New Harbinger Publications.
12. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
13. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
14. Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 Suppl):AS264-279.
15. Gandek B, Sinclair SJ, Kosinski M, Ware JE Jr. Psychometric evaluation of the SF-36 health survey in Medicare managed care. Health Care Financ Rev. 2004;25(4):5-25.
16. Ruta D, Garratt A, Abdalla M, Buckingham K, Russell I. The SF-36 health survey questionnaire. A valid measure of health status. BMJ. 1993;307(6901):448-449.
17. Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121(8):1231-1240.
18. Würtzen H, Dalton SO, Elsass P, et al. Mindfulness significantly reduces self-reported levels of anxiety and depression: results of a randomised controlled trial among 336 Danish women treated for stage I-III breast cancer. Eur J Cancer. 2013;49(6):1365-1373.
19. Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol. 2012;30(12):1335-1342.
20. Jacobs TL, Epel ES, Lin J, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36(5):664-681.
21. Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048-1057.
22. Daubenmier J, Lin J, Blackburn E, et al. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology. 2012;37(7):917-928.
23. Lavretsky H, Epel ES, Siddarth P, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28(1):57-65.
24. Biegler KA, Anderson AK, Wenzel LB, Osann K, Nelson EL. Longitudinal change in telomere length and the chronic stress response in a randomized pilot biobehavioral clinical study: implications for cancer prevention. Cancer Prev Res (Phila). 2012;5(10):1173-1182.
25. Lengacher CA, Reich RR, Kip KE. Influence of mindfulness-based stress reduction (MBSR) on telomerase activity in women with breast cancer (BC). Biol Res Nurs. 2014;16(4):438-447.
26. Naumann F, Martin E, Philpott M, Smith C, Groff D, Battaglini C. Can counseling add value to an exercise intervention for improving quality of life in breast cancer survivors? A feasibility study. J Support Oncol. 2012;10(5):188-194.
27. Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30-67.
28. Greenlee H, Gaffney AO, Aycinena AC, et al. Cocinar para su salud!: randomized controlled trial of a culturally based dietary intervention among Hispanic breast cancer survivors. J Acad Nutr Diet. 2015;115(5):709-723.e3.
An ASCO 2017 recap: significant advances continue
As we head into vacation season and the dog days of summer, let’s reflect for a few minutes on some of the very important advances we heard about at this year’s annual meeting of the American Society of Clinical Oncology in Chicago. Nearly 40,000 individuals registered for the conference, an indication of both the interest and the excitement around the new agents and the emerging clinical trial data. Scientific sessions dedicated to the use of combination immunotherapy, the role of antibody drug conjugates, and targeting molecular aberrations with small molecules were among the most popular (p. e236).
In the setting of metastatic breast cancer, several trials produced highly significant results that will positively affect the duration and quality of life for our patients. The use of PARP inhibitors in BRCA-mutated cancers has been shown to be effective in a few areas, particularly advanced ovarian cancer. The OlympiAD study evaluated olaparib monotherapy and a physician’s choice arm (capecitabine, eribulin, or vinorelbine) in BRCA-mutated, HER2-negative metastatic breast cancer. The 2:1 design enrolled 302 patients and demonstrated a 3-month improvement in progression-free survival (PFS) for olaparib compared with the control arm (7.0 vs 4.2 months, respectively; P = .0009). The patient population for this BRCA-mutated trial was relatively young, with a median age of 45 years, and 50% of the women were hormone positive and 30%, platinum resistant.
The CDK4/6 inhibitors continue to be impressive, with the recently reported results from the MONARCH 2 trial showing encouraging PFS and overall response rate results with the addition of the CDK4/6 inhibitor abemaciclib to fulvestrant, a selective estrogen-receptor degrader. In this study, hormone-positive, HER2-negative women who had progressed on previous endocrine therapy were randomized 2:1 to abemaciclib plus fulvestrant or placebo plus fulvestrant. A total of 669 patients were accrued, and after a median follow-up of 19 months, a highly significant PFS difference of 7 months between the abemaciclib–fulvestrant and fulvestrant–only groups was observed (16.4 vs 9.3 months, respectively; P < .0000001) along with an overall response rate of 48.1 months, compared with 21.3 months. Previous findings have demonstrated monotherapy activity for abemaciclib, and the comparisons with palbociclib and ribociclib will be forthcoming, although no comparative trials are underway. These agents will be extensively assessed in a variety of settings, including adjuvantly.
The results of the much anticipated APHINITY study, which evaluated the addition of pertuzumab to trastuzumab in the adjuvant HER2-positive setting, were met with mixed reviews. Patients were included if they had node-positive invasive breast cancer or node-negative tumors of >1.0 cm. A total of 4,804 patients (37% node negative) were enrolled in the study. The intent-to-treat primary endpoint of invasive disease-free survival (DFS) was statistically positive (P = .045), although the 3-year absolute percentages for the pertuzumab–trastuzumab and trastuzumab-only groups were 94.1% and 93.2%, respectively. It should be noted that the planned statistical assumption was for a delta of 2.6% – 91.8% and 89.2%, respectively. Thus, both arms actually did better than had been planned, which was based on historical comparisons, and the node-positive and hormone-negative subgroups trended toward a greater benefit with the addition of pertuzumab. There was, and will continue to be, much debate around the cost–benefit ratio and which patients should be offered the combination. The outstanding results with the addition of pertuzumab in the neoadjuvant setting will continue to be the setting in which the greatest absolute clinical benefit will be seen. It is unusual in this era to see trials this large planned to identify a small difference, and it is likely that resource constraints will make such studies a thing of the past.
The very active hormonal therapies, abiraterone and enzalutimide, for castrate-resistant prostate cancer remain of high interest in the area of clinical trials. The LATITUDE study evaluated a straightforward design that compared abiraterone with placebo in patients who were newly diagnosed with high-risk, metastatic hormone-naïve prostate cancer. Patients in both arms received androgen-deprivation therapy and high risk was defined by having 2 of 3 criteria: a Gleason score of ≥8; 3 or more bone lesions; or visceral disease. Of note is that 1,199 patients were enrolled before publication of the CHAARTED or STAMPEDE results, which established docetaxel as a standard for these patients. The median age in the LATITUDE trial was 68 years, with 17% of patients having visceral disease and 48% having nodal disease, making it a similar patient population to those in the docetaxel studies. The results favoring abiraterone were strikingly positive, with a 38% reduction in the risk of death (P < .0001) and a 53% reduction in the risk of radiographic progression or death (P < .0001). The regimen was well tolerated overall, and it is clear that this option will be widely considered by physicians and their patients.
Two studies addressing the importance of managing symptoms and improving outcomes were also part of the plenary session. The IDEA Collaboration conducted a prospective pooled analysis of 6 phase 3 studies that assessed 3 and 6 months of oxaliplatin-based regimens for stage 3 colon cancer. FOLFOX and CAPOX given to 12,834 patients in 6 studies from the United States, European Union, Canada, Australia, New Zealand, and Japan were evaluated for DFS, treatment compliance, and adverse events. As would be anticipated, fewer side effects, particularly neurotoxicity, and greater compliance were observed in the 3-month group. Although DFS noninferiority for 3 months of therapy was not established statistically, the overall data led the investigators to issue a consensus statement advocating for a risk-based approach in deciding the duration of therapy and recommending 3 months of therapy for patients with stage 3, T1-3N1 disease, and consideration of 6 months therapy for T4 and/ or N2 disease. The investigators also acknowledged the leader and creator of IDEA, the late Daniel Sargent, PhD, of the Mayo Clinic, who passed away far too young after a brief illness last fall (1970-2016).
The second symptom-based study was performed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and designed by a group of investigators from the Dana-Farber Cancer Institute in Boston; the Mayo Clinic in Rochester, Minnesota; the University of North Carolina in Chapel Hill; and MSKCC (p. e236). The hypothesis was simply that proactive symptom monitoring during chemotherapy would improve symptom management and lead to better outcomes. For the study, 766 patients with advanced solid tumors who were receiving outpatient chemotherapy were randomized to a control arm with standard follow-up or to the intervention arm, on which patients self-reported on 12 common symptoms before and between visits using a web-based tool and received weekly e-mail reminders and nursing alerts. At 6 months, and compared with baseline, the self-reporting patients in the intervention arm experienced an improved quality of life (P < .001). In addition, 7% fewer of the self-reporting patients visited the emergency department (P = .02), and they experienced longer survival by 5 months compared with the standard follow-up group (31.2 vs 26.0 months, respectively; P = .03). Although there are limitations to such a study, the growth in technological advances should create the opportunity to expand on this strategy in further trials and in practice. With such an emphasis in the Medicare Oncology Home Model on decreasing hospital admissions and visits to the emergency department, there should great motivation for all involved to consider incorporating self-reporting into their patterns of care.
A continued emphasis on molecular profiling, personalized and/or precision medicine, and identifying or matching the patient to the best possible therapy or the most appropriate clinical trial remains vital to improving outcomes. Just before the ASCO meeting, the US Food and Drug Administration approved pembrolizumab for the treatment of patients with high-level microsatellite instability (MSI-H) and mismatch-repair deficient (dMMR) cancers, regardless of the site of origin. The approval was based on data from 149 patients with MSI-H or dMMR cancers, which showed a 40% response rate in this group of patients, two-thirds of whom had previously treated colon cancer. This landmark approval of a cancer therapy for a specific molecular profile and not the site of the disease, will certainly shape the future of oncology drug development. One of the highlighted stories at ASCO was the success of the larotrectinib (LOXO 101) tropomyosin receptor kinase inhibitor in patients with the TRK fusion mutations (p. e237). The data, including waterfall charts, swimmer plots, and computed-tomography scans, were impressive in this targeted population with a 76% response rate and a 91% duration of response at 6 months with a mild side effect profile.
In summary, across a variety of cancers, with treatment strategies of an equally diverse nature, we saw practice-changing data from the ASCO meeting that will benefit our patients. Continuing to seek out clinical trial options for patients will be critical in answering the many questions that have emerged and the substantial number of studies that are ongoing with combination immunotherapies, targeted small molecules, and a growing armamentarium of monoclonal antibodies.
As we head into vacation season and the dog days of summer, let’s reflect for a few minutes on some of the very important advances we heard about at this year’s annual meeting of the American Society of Clinical Oncology in Chicago. Nearly 40,000 individuals registered for the conference, an indication of both the interest and the excitement around the new agents and the emerging clinical trial data. Scientific sessions dedicated to the use of combination immunotherapy, the role of antibody drug conjugates, and targeting molecular aberrations with small molecules were among the most popular (p. e236).
In the setting of metastatic breast cancer, several trials produced highly significant results that will positively affect the duration and quality of life for our patients. The use of PARP inhibitors in BRCA-mutated cancers has been shown to be effective in a few areas, particularly advanced ovarian cancer. The OlympiAD study evaluated olaparib monotherapy and a physician’s choice arm (capecitabine, eribulin, or vinorelbine) in BRCA-mutated, HER2-negative metastatic breast cancer. The 2:1 design enrolled 302 patients and demonstrated a 3-month improvement in progression-free survival (PFS) for olaparib compared with the control arm (7.0 vs 4.2 months, respectively; P = .0009). The patient population for this BRCA-mutated trial was relatively young, with a median age of 45 years, and 50% of the women were hormone positive and 30%, platinum resistant.
The CDK4/6 inhibitors continue to be impressive, with the recently reported results from the MONARCH 2 trial showing encouraging PFS and overall response rate results with the addition of the CDK4/6 inhibitor abemaciclib to fulvestrant, a selective estrogen-receptor degrader. In this study, hormone-positive, HER2-negative women who had progressed on previous endocrine therapy were randomized 2:1 to abemaciclib plus fulvestrant or placebo plus fulvestrant. A total of 669 patients were accrued, and after a median follow-up of 19 months, a highly significant PFS difference of 7 months between the abemaciclib–fulvestrant and fulvestrant–only groups was observed (16.4 vs 9.3 months, respectively; P < .0000001) along with an overall response rate of 48.1 months, compared with 21.3 months. Previous findings have demonstrated monotherapy activity for abemaciclib, and the comparisons with palbociclib and ribociclib will be forthcoming, although no comparative trials are underway. These agents will be extensively assessed in a variety of settings, including adjuvantly.
The results of the much anticipated APHINITY study, which evaluated the addition of pertuzumab to trastuzumab in the adjuvant HER2-positive setting, were met with mixed reviews. Patients were included if they had node-positive invasive breast cancer or node-negative tumors of >1.0 cm. A total of 4,804 patients (37% node negative) were enrolled in the study. The intent-to-treat primary endpoint of invasive disease-free survival (DFS) was statistically positive (P = .045), although the 3-year absolute percentages for the pertuzumab–trastuzumab and trastuzumab-only groups were 94.1% and 93.2%, respectively. It should be noted that the planned statistical assumption was for a delta of 2.6% – 91.8% and 89.2%, respectively. Thus, both arms actually did better than had been planned, which was based on historical comparisons, and the node-positive and hormone-negative subgroups trended toward a greater benefit with the addition of pertuzumab. There was, and will continue to be, much debate around the cost–benefit ratio and which patients should be offered the combination. The outstanding results with the addition of pertuzumab in the neoadjuvant setting will continue to be the setting in which the greatest absolute clinical benefit will be seen. It is unusual in this era to see trials this large planned to identify a small difference, and it is likely that resource constraints will make such studies a thing of the past.
The very active hormonal therapies, abiraterone and enzalutimide, for castrate-resistant prostate cancer remain of high interest in the area of clinical trials. The LATITUDE study evaluated a straightforward design that compared abiraterone with placebo in patients who were newly diagnosed with high-risk, metastatic hormone-naïve prostate cancer. Patients in both arms received androgen-deprivation therapy and high risk was defined by having 2 of 3 criteria: a Gleason score of ≥8; 3 or more bone lesions; or visceral disease. Of note is that 1,199 patients were enrolled before publication of the CHAARTED or STAMPEDE results, which established docetaxel as a standard for these patients. The median age in the LATITUDE trial was 68 years, with 17% of patients having visceral disease and 48% having nodal disease, making it a similar patient population to those in the docetaxel studies. The results favoring abiraterone were strikingly positive, with a 38% reduction in the risk of death (P < .0001) and a 53% reduction in the risk of radiographic progression or death (P < .0001). The regimen was well tolerated overall, and it is clear that this option will be widely considered by physicians and their patients.
Two studies addressing the importance of managing symptoms and improving outcomes were also part of the plenary session. The IDEA Collaboration conducted a prospective pooled analysis of 6 phase 3 studies that assessed 3 and 6 months of oxaliplatin-based regimens for stage 3 colon cancer. FOLFOX and CAPOX given to 12,834 patients in 6 studies from the United States, European Union, Canada, Australia, New Zealand, and Japan were evaluated for DFS, treatment compliance, and adverse events. As would be anticipated, fewer side effects, particularly neurotoxicity, and greater compliance were observed in the 3-month group. Although DFS noninferiority for 3 months of therapy was not established statistically, the overall data led the investigators to issue a consensus statement advocating for a risk-based approach in deciding the duration of therapy and recommending 3 months of therapy for patients with stage 3, T1-3N1 disease, and consideration of 6 months therapy for T4 and/ or N2 disease. The investigators also acknowledged the leader and creator of IDEA, the late Daniel Sargent, PhD, of the Mayo Clinic, who passed away far too young after a brief illness last fall (1970-2016).
The second symptom-based study was performed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and designed by a group of investigators from the Dana-Farber Cancer Institute in Boston; the Mayo Clinic in Rochester, Minnesota; the University of North Carolina in Chapel Hill; and MSKCC (p. e236). The hypothesis was simply that proactive symptom monitoring during chemotherapy would improve symptom management and lead to better outcomes. For the study, 766 patients with advanced solid tumors who were receiving outpatient chemotherapy were randomized to a control arm with standard follow-up or to the intervention arm, on which patients self-reported on 12 common symptoms before and between visits using a web-based tool and received weekly e-mail reminders and nursing alerts. At 6 months, and compared with baseline, the self-reporting patients in the intervention arm experienced an improved quality of life (P < .001). In addition, 7% fewer of the self-reporting patients visited the emergency department (P = .02), and they experienced longer survival by 5 months compared with the standard follow-up group (31.2 vs 26.0 months, respectively; P = .03). Although there are limitations to such a study, the growth in technological advances should create the opportunity to expand on this strategy in further trials and in practice. With such an emphasis in the Medicare Oncology Home Model on decreasing hospital admissions and visits to the emergency department, there should great motivation for all involved to consider incorporating self-reporting into their patterns of care.
A continued emphasis on molecular profiling, personalized and/or precision medicine, and identifying or matching the patient to the best possible therapy or the most appropriate clinical trial remains vital to improving outcomes. Just before the ASCO meeting, the US Food and Drug Administration approved pembrolizumab for the treatment of patients with high-level microsatellite instability (MSI-H) and mismatch-repair deficient (dMMR) cancers, regardless of the site of origin. The approval was based on data from 149 patients with MSI-H or dMMR cancers, which showed a 40% response rate in this group of patients, two-thirds of whom had previously treated colon cancer. This landmark approval of a cancer therapy for a specific molecular profile and not the site of the disease, will certainly shape the future of oncology drug development. One of the highlighted stories at ASCO was the success of the larotrectinib (LOXO 101) tropomyosin receptor kinase inhibitor in patients with the TRK fusion mutations (p. e237). The data, including waterfall charts, swimmer plots, and computed-tomography scans, were impressive in this targeted population with a 76% response rate and a 91% duration of response at 6 months with a mild side effect profile.
In summary, across a variety of cancers, with treatment strategies of an equally diverse nature, we saw practice-changing data from the ASCO meeting that will benefit our patients. Continuing to seek out clinical trial options for patients will be critical in answering the many questions that have emerged and the substantial number of studies that are ongoing with combination immunotherapies, targeted small molecules, and a growing armamentarium of monoclonal antibodies.
As we head into vacation season and the dog days of summer, let’s reflect for a few minutes on some of the very important advances we heard about at this year’s annual meeting of the American Society of Clinical Oncology in Chicago. Nearly 40,000 individuals registered for the conference, an indication of both the interest and the excitement around the new agents and the emerging clinical trial data. Scientific sessions dedicated to the use of combination immunotherapy, the role of antibody drug conjugates, and targeting molecular aberrations with small molecules were among the most popular (p. e236).
In the setting of metastatic breast cancer, several trials produced highly significant results that will positively affect the duration and quality of life for our patients. The use of PARP inhibitors in BRCA-mutated cancers has been shown to be effective in a few areas, particularly advanced ovarian cancer. The OlympiAD study evaluated olaparib monotherapy and a physician’s choice arm (capecitabine, eribulin, or vinorelbine) in BRCA-mutated, HER2-negative metastatic breast cancer. The 2:1 design enrolled 302 patients and demonstrated a 3-month improvement in progression-free survival (PFS) for olaparib compared with the control arm (7.0 vs 4.2 months, respectively; P = .0009). The patient population for this BRCA-mutated trial was relatively young, with a median age of 45 years, and 50% of the women were hormone positive and 30%, platinum resistant.
The CDK4/6 inhibitors continue to be impressive, with the recently reported results from the MONARCH 2 trial showing encouraging PFS and overall response rate results with the addition of the CDK4/6 inhibitor abemaciclib to fulvestrant, a selective estrogen-receptor degrader. In this study, hormone-positive, HER2-negative women who had progressed on previous endocrine therapy were randomized 2:1 to abemaciclib plus fulvestrant or placebo plus fulvestrant. A total of 669 patients were accrued, and after a median follow-up of 19 months, a highly significant PFS difference of 7 months between the abemaciclib–fulvestrant and fulvestrant–only groups was observed (16.4 vs 9.3 months, respectively; P < .0000001) along with an overall response rate of 48.1 months, compared with 21.3 months. Previous findings have demonstrated monotherapy activity for abemaciclib, and the comparisons with palbociclib and ribociclib will be forthcoming, although no comparative trials are underway. These agents will be extensively assessed in a variety of settings, including adjuvantly.
The results of the much anticipated APHINITY study, which evaluated the addition of pertuzumab to trastuzumab in the adjuvant HER2-positive setting, were met with mixed reviews. Patients were included if they had node-positive invasive breast cancer or node-negative tumors of >1.0 cm. A total of 4,804 patients (37% node negative) were enrolled in the study. The intent-to-treat primary endpoint of invasive disease-free survival (DFS) was statistically positive (P = .045), although the 3-year absolute percentages for the pertuzumab–trastuzumab and trastuzumab-only groups were 94.1% and 93.2%, respectively. It should be noted that the planned statistical assumption was for a delta of 2.6% – 91.8% and 89.2%, respectively. Thus, both arms actually did better than had been planned, which was based on historical comparisons, and the node-positive and hormone-negative subgroups trended toward a greater benefit with the addition of pertuzumab. There was, and will continue to be, much debate around the cost–benefit ratio and which patients should be offered the combination. The outstanding results with the addition of pertuzumab in the neoadjuvant setting will continue to be the setting in which the greatest absolute clinical benefit will be seen. It is unusual in this era to see trials this large planned to identify a small difference, and it is likely that resource constraints will make such studies a thing of the past.
The very active hormonal therapies, abiraterone and enzalutimide, for castrate-resistant prostate cancer remain of high interest in the area of clinical trials. The LATITUDE study evaluated a straightforward design that compared abiraterone with placebo in patients who were newly diagnosed with high-risk, metastatic hormone-naïve prostate cancer. Patients in both arms received androgen-deprivation therapy and high risk was defined by having 2 of 3 criteria: a Gleason score of ≥8; 3 or more bone lesions; or visceral disease. Of note is that 1,199 patients were enrolled before publication of the CHAARTED or STAMPEDE results, which established docetaxel as a standard for these patients. The median age in the LATITUDE trial was 68 years, with 17% of patients having visceral disease and 48% having nodal disease, making it a similar patient population to those in the docetaxel studies. The results favoring abiraterone were strikingly positive, with a 38% reduction in the risk of death (P < .0001) and a 53% reduction in the risk of radiographic progression or death (P < .0001). The regimen was well tolerated overall, and it is clear that this option will be widely considered by physicians and their patients.
Two studies addressing the importance of managing symptoms and improving outcomes were also part of the plenary session. The IDEA Collaboration conducted a prospective pooled analysis of 6 phase 3 studies that assessed 3 and 6 months of oxaliplatin-based regimens for stage 3 colon cancer. FOLFOX and CAPOX given to 12,834 patients in 6 studies from the United States, European Union, Canada, Australia, New Zealand, and Japan were evaluated for DFS, treatment compliance, and adverse events. As would be anticipated, fewer side effects, particularly neurotoxicity, and greater compliance were observed in the 3-month group. Although DFS noninferiority for 3 months of therapy was not established statistically, the overall data led the investigators to issue a consensus statement advocating for a risk-based approach in deciding the duration of therapy and recommending 3 months of therapy for patients with stage 3, T1-3N1 disease, and consideration of 6 months therapy for T4 and/ or N2 disease. The investigators also acknowledged the leader and creator of IDEA, the late Daniel Sargent, PhD, of the Mayo Clinic, who passed away far too young after a brief illness last fall (1970-2016).
The second symptom-based study was performed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and designed by a group of investigators from the Dana-Farber Cancer Institute in Boston; the Mayo Clinic in Rochester, Minnesota; the University of North Carolina in Chapel Hill; and MSKCC (p. e236). The hypothesis was simply that proactive symptom monitoring during chemotherapy would improve symptom management and lead to better outcomes. For the study, 766 patients with advanced solid tumors who were receiving outpatient chemotherapy were randomized to a control arm with standard follow-up or to the intervention arm, on which patients self-reported on 12 common symptoms before and between visits using a web-based tool and received weekly e-mail reminders and nursing alerts. At 6 months, and compared with baseline, the self-reporting patients in the intervention arm experienced an improved quality of life (P < .001). In addition, 7% fewer of the self-reporting patients visited the emergency department (P = .02), and they experienced longer survival by 5 months compared with the standard follow-up group (31.2 vs 26.0 months, respectively; P = .03). Although there are limitations to such a study, the growth in technological advances should create the opportunity to expand on this strategy in further trials and in practice. With such an emphasis in the Medicare Oncology Home Model on decreasing hospital admissions and visits to the emergency department, there should great motivation for all involved to consider incorporating self-reporting into their patterns of care.
A continued emphasis on molecular profiling, personalized and/or precision medicine, and identifying or matching the patient to the best possible therapy or the most appropriate clinical trial remains vital to improving outcomes. Just before the ASCO meeting, the US Food and Drug Administration approved pembrolizumab for the treatment of patients with high-level microsatellite instability (MSI-H) and mismatch-repair deficient (dMMR) cancers, regardless of the site of origin. The approval was based on data from 149 patients with MSI-H or dMMR cancers, which showed a 40% response rate in this group of patients, two-thirds of whom had previously treated colon cancer. This landmark approval of a cancer therapy for a specific molecular profile and not the site of the disease, will certainly shape the future of oncology drug development. One of the highlighted stories at ASCO was the success of the larotrectinib (LOXO 101) tropomyosin receptor kinase inhibitor in patients with the TRK fusion mutations (p. e237). The data, including waterfall charts, swimmer plots, and computed-tomography scans, were impressive in this targeted population with a 76% response rate and a 91% duration of response at 6 months with a mild side effect profile.
In summary, across a variety of cancers, with treatment strategies of an equally diverse nature, we saw practice-changing data from the ASCO meeting that will benefit our patients. Continuing to seek out clinical trial options for patients will be critical in answering the many questions that have emerged and the substantial number of studies that are ongoing with combination immunotherapies, targeted small molecules, and a growing armamentarium of monoclonal antibodies.