User login
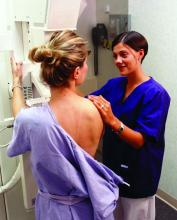
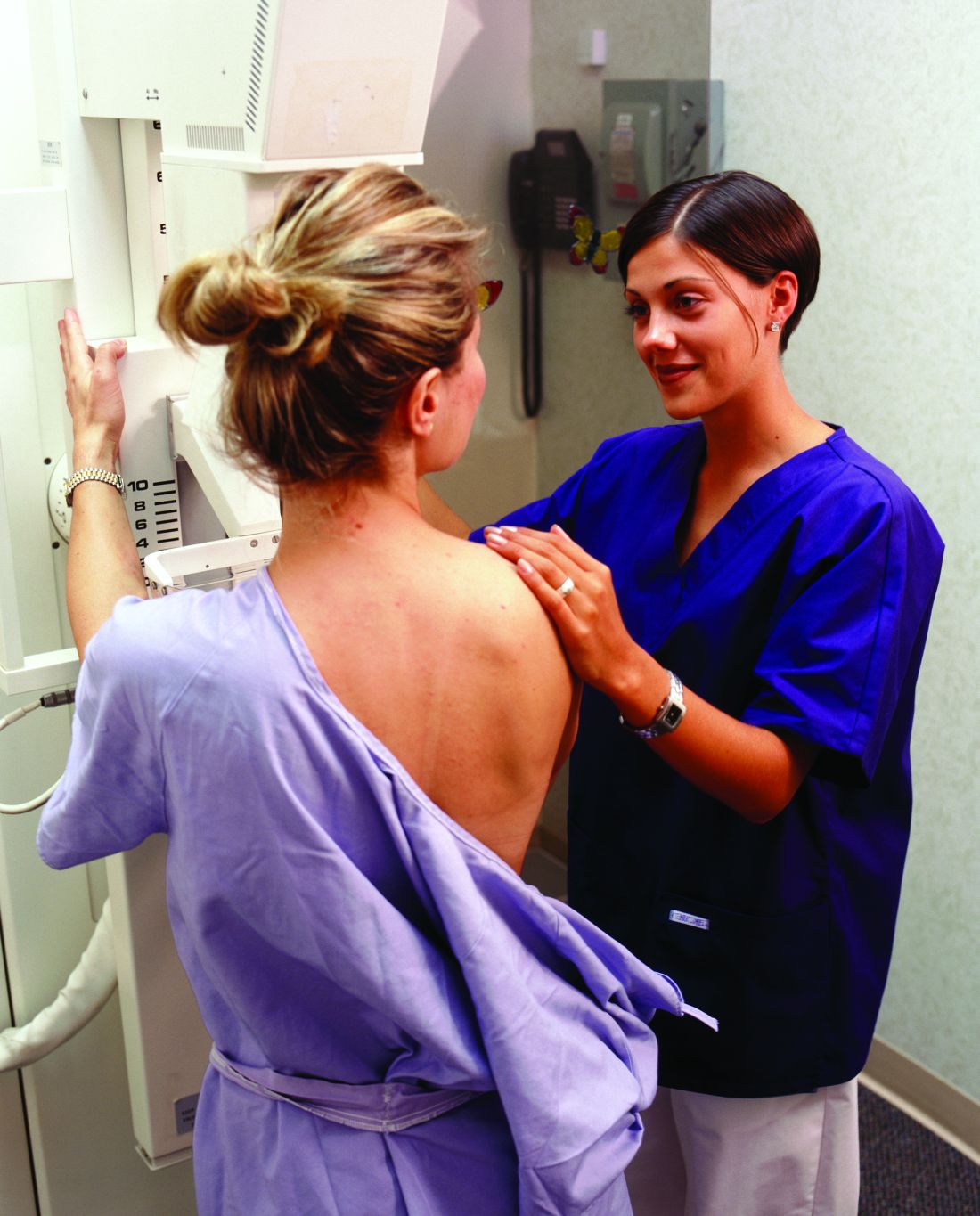
FDA clears first 2D mammography device with patient-controlled compression
The Food and Drug Administration has granted premarket clearance to the first 2D digital mammography system that allows patients to control the amount of compression applied to their own breasts before the mammogram x-ray is taken.
Senographe Pristina with Self-Compression uses a handheld wireless remote control that allows women to adjust the compression force after breast positioning and during a mammography exam. The technologist positions the patient and initiates compression, then guides the patient to gradually increase compression using the remote control until adequate compression is reached. The technologist checks the applied compression and breast positioning and makes the final decision on whether the compression is adequate or needs to be adjusted, according to the FDA’s Sept. 1 announcement.
“Regular mammograms are an important tool in detecting breast cancer,” Alberto Gutierrez, PhD, director of the FDA’s Office of In Vitro Diagnostics and Radiological Health, said in a statement. “However, some patients may experience anxiety or stress about the discomfort from the compression during the mammogram. This device allows patients some control over the amount of compression for their exam.”
Read more details of the clearance on the FDA’s website.
The Food and Drug Administration has granted premarket clearance to the first 2D digital mammography system that allows patients to control the amount of compression applied to their own breasts before the mammogram x-ray is taken.
Senographe Pristina with Self-Compression uses a handheld wireless remote control that allows women to adjust the compression force after breast positioning and during a mammography exam. The technologist positions the patient and initiates compression, then guides the patient to gradually increase compression using the remote control until adequate compression is reached. The technologist checks the applied compression and breast positioning and makes the final decision on whether the compression is adequate or needs to be adjusted, according to the FDA’s Sept. 1 announcement.
“Regular mammograms are an important tool in detecting breast cancer,” Alberto Gutierrez, PhD, director of the FDA’s Office of In Vitro Diagnostics and Radiological Health, said in a statement. “However, some patients may experience anxiety or stress about the discomfort from the compression during the mammogram. This device allows patients some control over the amount of compression for their exam.”
Read more details of the clearance on the FDA’s website.
The Food and Drug Administration has granted premarket clearance to the first 2D digital mammography system that allows patients to control the amount of compression applied to their own breasts before the mammogram x-ray is taken.
Senographe Pristina with Self-Compression uses a handheld wireless remote control that allows women to adjust the compression force after breast positioning and during a mammography exam. The technologist positions the patient and initiates compression, then guides the patient to gradually increase compression using the remote control until adequate compression is reached. The technologist checks the applied compression and breast positioning and makes the final decision on whether the compression is adequate or needs to be adjusted, according to the FDA’s Sept. 1 announcement.
“Regular mammograms are an important tool in detecting breast cancer,” Alberto Gutierrez, PhD, director of the FDA’s Office of In Vitro Diagnostics and Radiological Health, said in a statement. “However, some patients may experience anxiety or stress about the discomfort from the compression during the mammogram. This device allows patients some control over the amount of compression for their exam.”
Read more details of the clearance on the FDA’s website.
2017 Update on female sexual dysfunction
Sexual function is a complex, multifaceted process mediated by neurologic functions, hormonal regulation, and ps
As it turns out, quite a lot. Female sexual dysfunction is a common, vastly undertreated sexual health problem that can have wide-reaching effects on a woman’s life. These effects may include impaired body image, self-confidence, and self-worth. Sexual dysfunction also can contribute to relationship dissatisfaction and leave one feeling less connected with her partner.1,2 Studies have shown women with sexual dysfunction have higher health care expenditures3 and that depression and fatigue are common comorbidities, as is frequently seen in other chronic conditions such as diabetes and back pain.4
Understanding the pathogenesis of female sexual dysfunction helps to guide our approach to its management. Indeed, increased understanding of its pathology has helped to usher in new and emerging treatment options, as well as a personalized, biopsychosocial approach to its management.
Related article:
2016 Update on female sexual dysfunction
In this Update, I discuss the interplay of physiologic and psychological factors that affect female sexual function as well as the latest options for its management. I have also assembled a panel of experts to discuss 2 cases representative of sexual dysfunction that you may encounter in your clinical practice and how prescribing decisions are made for their management.
Read about factors that impact sexual function and agents to help manage dysfunction.
Multiple transmitters in the brain can increase or decrease sexual desire and function
Neurotransmitters involved in sexual excitation include brain dopamine, melanocortin, oxytocin, vasopressin, and norepinephrine, whereas brain opioids, serotonin, prolactin, and endocannabinoids function as sexual inhibitors. Inhibitory transmitters are activated normally during sexual refractoriness but also from primary aversion or secondary avoidance disorders.1 Drugs or conditions that reduce brain dopamine levels, increase the action of brain serotonin, or enhance brain opioid pathways have been shown to inhibit sexual desire, while those that increase hypothalamic and mesolimbic dopamine or decrease serotonin release have been shown to stimulate sexual desire.1
Estradiol and progesterone can impact sexual function and desire
In addition to the neurotransmitters, hormones are important modulators of female sexual function. Decreasing levels of circulating estrogen after menopause lead to physiologic, biologic, and clinical changes in the urogenital tissues, such as decreased elastin, thinning of the epithelium, reduced vaginal blood flow, diminished lubrication, and decreased flexibility and elasticity. These changes result in the symptoms of genitourinary syndrome of menopause (GSM), which affects as many as half of all menopausal women.5,6 In clinical trials, dyspareunia and vaginal dryness are the most bothersome GSM symptoms reported.7
The role of hormonal regulation in sexual dysfunction among premenopausal women is not yet fully understood, but we do know that estradiol has been shown to improve sexual desire, progesterone tends to dampen sexual desire, and that testosterone at physiological levels has been shown in most studies to have a neutral effect on sexual desire in a well-estrogenized patient.8
Related article:
Focus on treating genital atrophy symptoms
Experience and behavior modulate or reinforce sexual dysfunction
The most common psychological factors that trigger or amplify female sexual dysfunction are depression, anxiety, distraction, negative body image, sexual abuse, and emotional neglect.9 Contextual or sociocultural factors, such as relationship discord, life-stage stressors (the empty nest syndrome or anxiety and sleep deprivation from a new baby), as well as cultural or religious values that suppress sexuality, also should be considered.9 Experience-based neuroplasticity (changes in brain pathways that become solidified by negative or positive experiences) may elucidate how a multimodal approach, utilizing medical and psychological treatment, can be beneficial for patients, particularly those with hypoactive sexual desire disorder (HSDD).1
New and emerging approaches to managing female sexual dysfunction
Three agents, one of which has been available for prescription for some time, one that is newly available, and one in the pipeline, are or may soon be in the gynecologist's armamentarium.
Flibanserin
Medications that target excitatory pathways or blunt inhibitory pathways are in development, and one, flibanserin (Addyi), has been US Food and Drug Administration (FDA)-approved for the treatment of acquired, generalized HSDD in premenopausal women.1,10 Flibanserin is a nonhormonal, centrally acting, postsynaptic serotonin 1A receptor agonist and a serotonin 2A receptor antagonist that is taken daily at bedtime (100 mg); several weeks are usually needed before any effects are noted.1 It is not approved for postmenopausal women and has a boxed warning about the risks of hypotension and syncope; its use is contraindicated in women who drink alcohol, in those who have hepatic impairment, and with the use of moderate or strong CYP3A4 inhibitors.11
Also keep in mind that flibanserin is only available through a Risk Evaluation and Mitigation Strategy program, so clinicians who wish to prescribe it must enroll in and complete training to become certified providers.9
Related article:
What you need to know (and do) to prescribe the new drug flibanserin
Prasterone
Prasterone (Intrarosa), a once-daily intravaginal dehydroepiandrosterone (DHEA) product, is a prohormone that increases local estrogen and testosterone and has the advantage of improved sexual function, desire, arousal, lubrication, orgasm, satisfaction, as well as pain at sexual activity.12 It was approved by the FDA in November 2016 to treat moderate to severe dyspareunia and has been available for prescribing since July 2017. Its cost is comparable to topical estrogen products, with a $25 copay program.
Because prasterone is not an estrogen, it does not have the boxed warning that all estrogen products are mandated by the FDA to have. This may make it more acceptable to patients, who often decline to use an estrogen product after seeing the boxed warning on the package. The Centers for Medicare and Medicaid Services (CMS) does not have prasterone on its list of potentially hazardous drugs for the elderly. However, keep in mind that because its label is for dyspareunia and not specifically for GSM, CMS considers it a drug of choice--in other words, like sildenafil (Viagra), a lifestyle choice and not for treatment of a medical condition. As such, at the present time, Medicare does not cover it.
Bremelanotide
Late-stage trials of bremelanotide, a melanocortin receptor agonist, are underway. Its mechanism of action is somewhat like that of flibanserin in that both drugs increase dopamine and norepinephrine levels. The advantage of bremelanotide is that it is used as needed. It is dosed subcutaneously (1.75 mg) and it can be used as often as a woman would like to use it. The FDA is expected to consider it for approval in about a year. Unpublished data from poster sessions at recent meetings show that, in a phase 3 study of 1,247 premenopausal women with HSDD (who had already been screened for depression and were found to have a physiologic condition), improvements in desire, arousal, lubrication, and orgasm were shown with bremelanotide. About 18% of women stopped using the drug because of adverse effects (nausea, vomiting, flushing, or headache) versus 2% for placebo. Like flibanserin, it is expected to be approved for premenopausal women only.
Read how 3 experts would manage differing GSM symptoms.
What would you prescribe for these patients?
CASE Genitourinary syndrome of menopause (GSM) in a 55-year-old woman
A 55-year-old widow is beginning a new relationship. She has not had partnered sexual activity for several years, but she recently has begun a relationship. She describes pain with attempted penetration with her new partner. Her last menstrual period was 3 years ago and she has experienced very minor menopausal symptoms, which are not bothersome. On examination, the vulva and vagina are pale, with thin epithelium and absent rugae. The tissue lacks elasticity. A virginal speculum is needed to visualize the cervix.
How would you go about deciding which of the many options for management of GSM you will recommend for this patient? What do you weigh as you consider DHEA versus estrogen and topical versus oral therapy?
JoAnn V. Pinkerton, MD: Vulvovaginal atrophy (VVA), part of GSM, is associated with postmenopausal estrogen deficiency and includes the signs and symptoms seen on this patient's physical exam: vaginal narrowing, pallor, loss of elasticity, as well as pain with intercourse.6 Estrogen therapy is the most effective treatment for vaginal atrophy.13 Since she does not have significant menopausal symptoms, low-dose vaginal estrogen preparations are effective and generally safe treatments for VVA; these include creams, tablets containing estradiol or conjugated equine estrogen (CEE), and a low-dose vaginal estradiol ring--all available at doses that result in minimal systemic absorption.
Choice is usually made based on patient desire and likely adherence. If the patient prefers nonestrogen therapies that improve VVA and have been approved for relief of dyspareunia in postmenopausal women, I would discuss with the patient the oral selective estrogen receptor modulator ospemifene,14 and the new intravaginal DHEA suppositories, prasterone.15 Ospemifene is taken daily as an oral tablet, has a small risk of blood clots, and is my choice for women who do not need systemic hormone therapy and prefer to avoid vaginal therapy.
Andrew M. Kaunitz, MD: GSM is prevalent in menopausal women and, if not treated, causes progressive vaginal dryness and sexual discomfort. When the main indication for hormonal management in a menopausal woman is GSM (as opposed to treatment of vasomotor symptoms or prevention of osteoporosis), the treatment of choice is low-dose local vaginal estrogen, ospemifene, or prasterone (DHEA). Prasterone is a vaginally administered nonestrogen steroid that was approved by the FDA to treat dyspareunia associated with GSM. DHEA is an endogenous inactive steroid that is converted locally into androgens and estrogens; one vaginal insert is placed nightly.16,17
This 55-year-old widow has not been sexually active for some time. The facts that attempted penetration was painful and only an ultrathin (virginal) speculum could be used for examination indicate that contraction of the pelvic floor muscles is likely present. Simply starting medical management may not lead to comfortable/successful penetrative sex for this woman. In addition to medical management, she would likely benefit from referral for physical therapy. Using dilators and other strategies, along with the positive impact that medical management will have on the vaginal mucosa, a woman's physical therapist can work with this patient to help the pelvic floor muscles relax and facilitate comfortable penetrative sex.
James A. Simon, MD: With only minor vasomotor symptoms, I would assess the other potential benefits of a systemic therapy. These might include cardiovascular risk reduction (systemic estrogens or estrogens/progesterone in some), breast cancer risk reduction (some data suggesting ospemifene can accomplish this), osteoporosis prevention (systemic estrogens and estrogen/androgens), etc. If there is an option for a treatment to address more than one symptom, in this case GSM, assessing the risks/benefits of each of these therapies should be estimated for this specific patient.
If there are no systemic benefits to be had, then any of the local treatments should be helpful. As there are no head-to-head comparisons available, local estrogen cream, tablets, rings, local DHEA, or systemic ospemifene each should be considered possible treatments. I also feel this patient may benefit from supplementary self-dilation and/or physical therapy.
Related article:
2017 Update on menopause
CASE Dyspareunia and vasomotor symptoms in a 42-year-old breast cancer survivor
A 42-year-old woman with a BRCA1 mutation has undergone prophylactic mastectomies as well as hysterectomy with bilateral salpingo-oophorectomy. She reports mild to moderate hot flashes and bothersome vaginal dryness and dyspareunia. Examination confirms GSM.
Would you advise systemic hormone therapy for this patient? What would your recommendation be for management of her GSM symptoms?
Dr. Simon: While one's gut reaction would be to avoid systemic estrogen therapy in a patient with a BRCA1 mutation, the scientific information confirming this fear is lacking.18 Such patients may benefit significantly from systemic estrogen therapy (reduced risk of cardiovascular disease and cognitive decline, etc.), and with both breasts and both ovaries removed, estrogen's breast cancer risks, if any in this population, are largely avoided. The patient also may benefit from additional local therapy with either estrogens or DHEA.
Dr. Kaunitz: Due to her high lifetime risk of breast and ovarian cancer, this woman has proceeded with risk-reducing breast and gynecologic surgery. As more BRCA mutation carriers are being identified and undergo risk-reducing bilateral mastectomy (usually with reconstruction) and salpingo-oophorectomy, clinicians and mutation carriers more frequently face decisions regarding use of systemic hormone therapy.
Mutation carriers who have undergone bilateral risk-reducing mastectomy experience a very low baseline future risk for breast cancer; accordingly, concerns regarding this disease should not prevent use of systemic hormone therapy. Furthermore, without hormone replacement, induced menopause in women this age is associated with an elevated risk of osteoporosis, persistent vasomotor symptoms, cardiovascular disease, stroke, mood changes, dementia, Parkinson disease, and overall mortality. Recognizing the safety of estrogen therapy in this setting, this 42-year-old BRCA1 mutation carrier can initiate estrogen therapy. Standard dose estrogen therapy refers to oral estradiol 1.0 mg, conjugated equine estrogen 0.625 mg,or transdermal estradiol 0.05 mg. In younger women like this 42-year-old with surgically induced menopause, higher than standard replacement doses of estrogen are often appropriate.17
Due to concerns the hormone therapy might further increase future risk of breast cancer, some mutation carriers may delay or avoid risk-reducing bilateral salpingo-oophorectomy, a potentially lifesaving surgery which reduces not only future risk of ovarian cancer but also future risk for breast cancer.
Among mutation carriers with intact breasts, several studies address risk of breast cancer with use of systemic hormone therapy. Although limited in numbers of participants and years of follow-up, in aggregate, these studies provide reassurance that short-term use of systemic hormone therapy does not increase breast cancer risk in women with BRCA1 or BRCA2 mutations and intact breasts.19
Dr. Pinkerton: For this woman with early surgical menopause and hysterectomy, estrogen therapy could improve her vasomotor symptoms and decrease her risk of bone loss and GSM.17 In the Women's Health Initiative trial, there were 7 fewer breast cancers per 10,000 women-years in the estrogen-onlyarm.20 Observational studies suggest that hormone therapy, when given to the average age of menopause, decreases the risks of heart disease, Parkinson disease, and dementia.21 Limited observational evidence suggests that hormone therapy use does not further increase risk of breast cancer in women following oophorectomy for BRCA1 or BRCA2 gene mutation.22
The absolute risks observed with hormone therapy tended to be small, especially in younger, healthy women. Systemic hormone therapy could treat her hot flashes and her GSM symptoms and potentially decrease health risks associated with premature estrogen deficiency. Nonestrogen therapies for hot flashes include low-dose antidepressants, gabapentin, and mind-body options, such as cognitive behavioral therapy and hypnosis, but these would not decrease her health risks or treat her GSM.
If she only requests treatment of her GSM symptoms, she would be a candidate for low-dose vaginal estrogen therapy, given as a cream, tablet, or ring depending on her choice. I would not choose ospemifene as my first choice as she is having hot flashes, and there are no data yet on the drug's health benefits in early menopause. If she prefers nonestrogen vaginal therapy, the new intravaginal DHEA might be a good choice as both estrogen and testosterone are increased locally in the vagina while hormone levels remain in the postmenopausal range. There is no boxed warning on the patient insert, although safety in women with breast cancer or in those with elevated risk of breast cancer has not been tested.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive Sexual Desire Disorder: International Society for the Study of Women’s Sexual Health (ISSWSH) Expert Consensus Panel Review. Mayo Clin Proc. 2017;92(1):114–128.
- Kingsberg SA. Attitudinal survey of women living with low sexual desire. J Womens Health (Larchmt). 2014;23(10):817–823.
- Foley K, Foley D, Johnson BH. Healthcare resource utilization and expenditures of women diagnosed with hypoactive sexual desire disorder. J Med Econ. 2010;13(4):583–590.
- Biddle AK, West SL, D’Aloisio AA, Wheeler SB, Borisov NN, Thorp J. Hypoactive sexual desire disorder in postmenopausal women: quality of life and health burden. Value Health. 2009;12(5):763–772.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Ettinger B, Hait H, Reape KZ, Shu H. Measuring symptom relief in studies of vaginal and vulvar atrophy: the most bothersome symptom approach. Menopause. 2008;15(5):885–889.
- Dennerstein L, Randolph J, Taffe J, Dudley E, Burger H. Hormones, mood, sexuality, and the menopausal transition. Fertil Steril. 2002;77(suppl 4):S42–S48.
- Brotto LA, Bitzer J, Laan E, Leiblum S, Luria M. Women’s sexual desire and arousal disorders [published correction appears in J Sex Med. 2010;7(2 pt 1):856]. J Sex Med. 2010;7(1 pt 2):586–614.
- US Food and Drug Administration website. FDA approves first treatment for sexual desire disorder. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm458734.htm. Accessed August 14, 2017.
- Addyi (flibanserin) [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America, LLC; 2016.
- Labrie F, Derogatis L, Archer DF, et al; Members of the VVA Prasterone Research Group. Effect of intravaginal prasterone on sexual dysfunction in postmenopausal women with vulvovaginal atrophy. J Sex Med. 2015;12(12):2401–2412.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016;8:CD001500.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20(6):623–630.
- Labrie F, Archer DF, Koltun, W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243–256.
- Kaunitz AM. Focus on treating genital atrophy symptoms. OBG Manag. 2017;29(1):14, 16–17.
- The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. August 14, 2017. doi:10.1097/GME.0000000000000956.
- Domchek S, Kaunitz AM. Use of systemic hormone therapy in BRCA mutation carriers. Menopause. 2016;23(9):1026–1027.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Gabriel CA, Tigges-Cardwell J, Stopfer J, Erlichman J, Nathanson K, Domchek SM. Use of total abdominal hysterectomy and hormone replacement therapy in BRCA1 and BRCA2 mutation carriers undergoing risk-reducing salpingo-oophorectomy. Fam Cancer. 2009;8(1):23-28.
Sexual function is a complex, multifaceted process mediated by neurologic functions, hormonal regulation, and ps
As it turns out, quite a lot. Female sexual dysfunction is a common, vastly undertreated sexual health problem that can have wide-reaching effects on a woman’s life. These effects may include impaired body image, self-confidence, and self-worth. Sexual dysfunction also can contribute to relationship dissatisfaction and leave one feeling less connected with her partner.1,2 Studies have shown women with sexual dysfunction have higher health care expenditures3 and that depression and fatigue are common comorbidities, as is frequently seen in other chronic conditions such as diabetes and back pain.4
Understanding the pathogenesis of female sexual dysfunction helps to guide our approach to its management. Indeed, increased understanding of its pathology has helped to usher in new and emerging treatment options, as well as a personalized, biopsychosocial approach to its management.
Related article:
2016 Update on female sexual dysfunction
In this Update, I discuss the interplay of physiologic and psychological factors that affect female sexual function as well as the latest options for its management. I have also assembled a panel of experts to discuss 2 cases representative of sexual dysfunction that you may encounter in your clinical practice and how prescribing decisions are made for their management.
Read about factors that impact sexual function and agents to help manage dysfunction.
Multiple transmitters in the brain can increase or decrease sexual desire and function
Neurotransmitters involved in sexual excitation include brain dopamine, melanocortin, oxytocin, vasopressin, and norepinephrine, whereas brain opioids, serotonin, prolactin, and endocannabinoids function as sexual inhibitors. Inhibitory transmitters are activated normally during sexual refractoriness but also from primary aversion or secondary avoidance disorders.1 Drugs or conditions that reduce brain dopamine levels, increase the action of brain serotonin, or enhance brain opioid pathways have been shown to inhibit sexual desire, while those that increase hypothalamic and mesolimbic dopamine or decrease serotonin release have been shown to stimulate sexual desire.1
Estradiol and progesterone can impact sexual function and desire
In addition to the neurotransmitters, hormones are important modulators of female sexual function. Decreasing levels of circulating estrogen after menopause lead to physiologic, biologic, and clinical changes in the urogenital tissues, such as decreased elastin, thinning of the epithelium, reduced vaginal blood flow, diminished lubrication, and decreased flexibility and elasticity. These changes result in the symptoms of genitourinary syndrome of menopause (GSM), which affects as many as half of all menopausal women.5,6 In clinical trials, dyspareunia and vaginal dryness are the most bothersome GSM symptoms reported.7
The role of hormonal regulation in sexual dysfunction among premenopausal women is not yet fully understood, but we do know that estradiol has been shown to improve sexual desire, progesterone tends to dampen sexual desire, and that testosterone at physiological levels has been shown in most studies to have a neutral effect on sexual desire in a well-estrogenized patient.8
Related article:
Focus on treating genital atrophy symptoms
Experience and behavior modulate or reinforce sexual dysfunction
The most common psychological factors that trigger or amplify female sexual dysfunction are depression, anxiety, distraction, negative body image, sexual abuse, and emotional neglect.9 Contextual or sociocultural factors, such as relationship discord, life-stage stressors (the empty nest syndrome or anxiety and sleep deprivation from a new baby), as well as cultural or religious values that suppress sexuality, also should be considered.9 Experience-based neuroplasticity (changes in brain pathways that become solidified by negative or positive experiences) may elucidate how a multimodal approach, utilizing medical and psychological treatment, can be beneficial for patients, particularly those with hypoactive sexual desire disorder (HSDD).1
New and emerging approaches to managing female sexual dysfunction
Three agents, one of which has been available for prescription for some time, one that is newly available, and one in the pipeline, are or may soon be in the gynecologist's armamentarium.
Flibanserin
Medications that target excitatory pathways or blunt inhibitory pathways are in development, and one, flibanserin (Addyi), has been US Food and Drug Administration (FDA)-approved for the treatment of acquired, generalized HSDD in premenopausal women.1,10 Flibanserin is a nonhormonal, centrally acting, postsynaptic serotonin 1A receptor agonist and a serotonin 2A receptor antagonist that is taken daily at bedtime (100 mg); several weeks are usually needed before any effects are noted.1 It is not approved for postmenopausal women and has a boxed warning about the risks of hypotension and syncope; its use is contraindicated in women who drink alcohol, in those who have hepatic impairment, and with the use of moderate or strong CYP3A4 inhibitors.11
Also keep in mind that flibanserin is only available through a Risk Evaluation and Mitigation Strategy program, so clinicians who wish to prescribe it must enroll in and complete training to become certified providers.9
Related article:
What you need to know (and do) to prescribe the new drug flibanserin
Prasterone
Prasterone (Intrarosa), a once-daily intravaginal dehydroepiandrosterone (DHEA) product, is a prohormone that increases local estrogen and testosterone and has the advantage of improved sexual function, desire, arousal, lubrication, orgasm, satisfaction, as well as pain at sexual activity.12 It was approved by the FDA in November 2016 to treat moderate to severe dyspareunia and has been available for prescribing since July 2017. Its cost is comparable to topical estrogen products, with a $25 copay program.
Because prasterone is not an estrogen, it does not have the boxed warning that all estrogen products are mandated by the FDA to have. This may make it more acceptable to patients, who often decline to use an estrogen product after seeing the boxed warning on the package. The Centers for Medicare and Medicaid Services (CMS) does not have prasterone on its list of potentially hazardous drugs for the elderly. However, keep in mind that because its label is for dyspareunia and not specifically for GSM, CMS considers it a drug of choice--in other words, like sildenafil (Viagra), a lifestyle choice and not for treatment of a medical condition. As such, at the present time, Medicare does not cover it.
Bremelanotide
Late-stage trials of bremelanotide, a melanocortin receptor agonist, are underway. Its mechanism of action is somewhat like that of flibanserin in that both drugs increase dopamine and norepinephrine levels. The advantage of bremelanotide is that it is used as needed. It is dosed subcutaneously (1.75 mg) and it can be used as often as a woman would like to use it. The FDA is expected to consider it for approval in about a year. Unpublished data from poster sessions at recent meetings show that, in a phase 3 study of 1,247 premenopausal women with HSDD (who had already been screened for depression and were found to have a physiologic condition), improvements in desire, arousal, lubrication, and orgasm were shown with bremelanotide. About 18% of women stopped using the drug because of adverse effects (nausea, vomiting, flushing, or headache) versus 2% for placebo. Like flibanserin, it is expected to be approved for premenopausal women only.
Read how 3 experts would manage differing GSM symptoms.
What would you prescribe for these patients?
CASE Genitourinary syndrome of menopause (GSM) in a 55-year-old woman
A 55-year-old widow is beginning a new relationship. She has not had partnered sexual activity for several years, but she recently has begun a relationship. She describes pain with attempted penetration with her new partner. Her last menstrual period was 3 years ago and she has experienced very minor menopausal symptoms, which are not bothersome. On examination, the vulva and vagina are pale, with thin epithelium and absent rugae. The tissue lacks elasticity. A virginal speculum is needed to visualize the cervix.
How would you go about deciding which of the many options for management of GSM you will recommend for this patient? What do you weigh as you consider DHEA versus estrogen and topical versus oral therapy?
JoAnn V. Pinkerton, MD: Vulvovaginal atrophy (VVA), part of GSM, is associated with postmenopausal estrogen deficiency and includes the signs and symptoms seen on this patient's physical exam: vaginal narrowing, pallor, loss of elasticity, as well as pain with intercourse.6 Estrogen therapy is the most effective treatment for vaginal atrophy.13 Since she does not have significant menopausal symptoms, low-dose vaginal estrogen preparations are effective and generally safe treatments for VVA; these include creams, tablets containing estradiol or conjugated equine estrogen (CEE), and a low-dose vaginal estradiol ring--all available at doses that result in minimal systemic absorption.
Choice is usually made based on patient desire and likely adherence. If the patient prefers nonestrogen therapies that improve VVA and have been approved for relief of dyspareunia in postmenopausal women, I would discuss with the patient the oral selective estrogen receptor modulator ospemifene,14 and the new intravaginal DHEA suppositories, prasterone.15 Ospemifene is taken daily as an oral tablet, has a small risk of blood clots, and is my choice for women who do not need systemic hormone therapy and prefer to avoid vaginal therapy.
Andrew M. Kaunitz, MD: GSM is prevalent in menopausal women and, if not treated, causes progressive vaginal dryness and sexual discomfort. When the main indication for hormonal management in a menopausal woman is GSM (as opposed to treatment of vasomotor symptoms or prevention of osteoporosis), the treatment of choice is low-dose local vaginal estrogen, ospemifene, or prasterone (DHEA). Prasterone is a vaginally administered nonestrogen steroid that was approved by the FDA to treat dyspareunia associated with GSM. DHEA is an endogenous inactive steroid that is converted locally into androgens and estrogens; one vaginal insert is placed nightly.16,17
This 55-year-old widow has not been sexually active for some time. The facts that attempted penetration was painful and only an ultrathin (virginal) speculum could be used for examination indicate that contraction of the pelvic floor muscles is likely present. Simply starting medical management may not lead to comfortable/successful penetrative sex for this woman. In addition to medical management, she would likely benefit from referral for physical therapy. Using dilators and other strategies, along with the positive impact that medical management will have on the vaginal mucosa, a woman's physical therapist can work with this patient to help the pelvic floor muscles relax and facilitate comfortable penetrative sex.
James A. Simon, MD: With only minor vasomotor symptoms, I would assess the other potential benefits of a systemic therapy. These might include cardiovascular risk reduction (systemic estrogens or estrogens/progesterone in some), breast cancer risk reduction (some data suggesting ospemifene can accomplish this), osteoporosis prevention (systemic estrogens and estrogen/androgens), etc. If there is an option for a treatment to address more than one symptom, in this case GSM, assessing the risks/benefits of each of these therapies should be estimated for this specific patient.
If there are no systemic benefits to be had, then any of the local treatments should be helpful. As there are no head-to-head comparisons available, local estrogen cream, tablets, rings, local DHEA, or systemic ospemifene each should be considered possible treatments. I also feel this patient may benefit from supplementary self-dilation and/or physical therapy.
Related article:
2017 Update on menopause
CASE Dyspareunia and vasomotor symptoms in a 42-year-old breast cancer survivor
A 42-year-old woman with a BRCA1 mutation has undergone prophylactic mastectomies as well as hysterectomy with bilateral salpingo-oophorectomy. She reports mild to moderate hot flashes and bothersome vaginal dryness and dyspareunia. Examination confirms GSM.
Would you advise systemic hormone therapy for this patient? What would your recommendation be for management of her GSM symptoms?
Dr. Simon: While one's gut reaction would be to avoid systemic estrogen therapy in a patient with a BRCA1 mutation, the scientific information confirming this fear is lacking.18 Such patients may benefit significantly from systemic estrogen therapy (reduced risk of cardiovascular disease and cognitive decline, etc.), and with both breasts and both ovaries removed, estrogen's breast cancer risks, if any in this population, are largely avoided. The patient also may benefit from additional local therapy with either estrogens or DHEA.
Dr. Kaunitz: Due to her high lifetime risk of breast and ovarian cancer, this woman has proceeded with risk-reducing breast and gynecologic surgery. As more BRCA mutation carriers are being identified and undergo risk-reducing bilateral mastectomy (usually with reconstruction) and salpingo-oophorectomy, clinicians and mutation carriers more frequently face decisions regarding use of systemic hormone therapy.
Mutation carriers who have undergone bilateral risk-reducing mastectomy experience a very low baseline future risk for breast cancer; accordingly, concerns regarding this disease should not prevent use of systemic hormone therapy. Furthermore, without hormone replacement, induced menopause in women this age is associated with an elevated risk of osteoporosis, persistent vasomotor symptoms, cardiovascular disease, stroke, mood changes, dementia, Parkinson disease, and overall mortality. Recognizing the safety of estrogen therapy in this setting, this 42-year-old BRCA1 mutation carrier can initiate estrogen therapy. Standard dose estrogen therapy refers to oral estradiol 1.0 mg, conjugated equine estrogen 0.625 mg,or transdermal estradiol 0.05 mg. In younger women like this 42-year-old with surgically induced menopause, higher than standard replacement doses of estrogen are often appropriate.17
Due to concerns the hormone therapy might further increase future risk of breast cancer, some mutation carriers may delay or avoid risk-reducing bilateral salpingo-oophorectomy, a potentially lifesaving surgery which reduces not only future risk of ovarian cancer but also future risk for breast cancer.
Among mutation carriers with intact breasts, several studies address risk of breast cancer with use of systemic hormone therapy. Although limited in numbers of participants and years of follow-up, in aggregate, these studies provide reassurance that short-term use of systemic hormone therapy does not increase breast cancer risk in women with BRCA1 or BRCA2 mutations and intact breasts.19
Dr. Pinkerton: For this woman with early surgical menopause and hysterectomy, estrogen therapy could improve her vasomotor symptoms and decrease her risk of bone loss and GSM.17 In the Women's Health Initiative trial, there were 7 fewer breast cancers per 10,000 women-years in the estrogen-onlyarm.20 Observational studies suggest that hormone therapy, when given to the average age of menopause, decreases the risks of heart disease, Parkinson disease, and dementia.21 Limited observational evidence suggests that hormone therapy use does not further increase risk of breast cancer in women following oophorectomy for BRCA1 or BRCA2 gene mutation.22
The absolute risks observed with hormone therapy tended to be small, especially in younger, healthy women. Systemic hormone therapy could treat her hot flashes and her GSM symptoms and potentially decrease health risks associated with premature estrogen deficiency. Nonestrogen therapies for hot flashes include low-dose antidepressants, gabapentin, and mind-body options, such as cognitive behavioral therapy and hypnosis, but these would not decrease her health risks or treat her GSM.
If she only requests treatment of her GSM symptoms, she would be a candidate for low-dose vaginal estrogen therapy, given as a cream, tablet, or ring depending on her choice. I would not choose ospemifene as my first choice as she is having hot flashes, and there are no data yet on the drug's health benefits in early menopause. If she prefers nonestrogen vaginal therapy, the new intravaginal DHEA might be a good choice as both estrogen and testosterone are increased locally in the vagina while hormone levels remain in the postmenopausal range. There is no boxed warning on the patient insert, although safety in women with breast cancer or in those with elevated risk of breast cancer has not been tested.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Sexual function is a complex, multifaceted process mediated by neurologic functions, hormonal regulation, and ps
As it turns out, quite a lot. Female sexual dysfunction is a common, vastly undertreated sexual health problem that can have wide-reaching effects on a woman’s life. These effects may include impaired body image, self-confidence, and self-worth. Sexual dysfunction also can contribute to relationship dissatisfaction and leave one feeling less connected with her partner.1,2 Studies have shown women with sexual dysfunction have higher health care expenditures3 and that depression and fatigue are common comorbidities, as is frequently seen in other chronic conditions such as diabetes and back pain.4
Understanding the pathogenesis of female sexual dysfunction helps to guide our approach to its management. Indeed, increased understanding of its pathology has helped to usher in new and emerging treatment options, as well as a personalized, biopsychosocial approach to its management.
Related article:
2016 Update on female sexual dysfunction
In this Update, I discuss the interplay of physiologic and psychological factors that affect female sexual function as well as the latest options for its management. I have also assembled a panel of experts to discuss 2 cases representative of sexual dysfunction that you may encounter in your clinical practice and how prescribing decisions are made for their management.
Read about factors that impact sexual function and agents to help manage dysfunction.
Multiple transmitters in the brain can increase or decrease sexual desire and function
Neurotransmitters involved in sexual excitation include brain dopamine, melanocortin, oxytocin, vasopressin, and norepinephrine, whereas brain opioids, serotonin, prolactin, and endocannabinoids function as sexual inhibitors. Inhibitory transmitters are activated normally during sexual refractoriness but also from primary aversion or secondary avoidance disorders.1 Drugs or conditions that reduce brain dopamine levels, increase the action of brain serotonin, or enhance brain opioid pathways have been shown to inhibit sexual desire, while those that increase hypothalamic and mesolimbic dopamine or decrease serotonin release have been shown to stimulate sexual desire.1
Estradiol and progesterone can impact sexual function and desire
In addition to the neurotransmitters, hormones are important modulators of female sexual function. Decreasing levels of circulating estrogen after menopause lead to physiologic, biologic, and clinical changes in the urogenital tissues, such as decreased elastin, thinning of the epithelium, reduced vaginal blood flow, diminished lubrication, and decreased flexibility and elasticity. These changes result in the symptoms of genitourinary syndrome of menopause (GSM), which affects as many as half of all menopausal women.5,6 In clinical trials, dyspareunia and vaginal dryness are the most bothersome GSM symptoms reported.7
The role of hormonal regulation in sexual dysfunction among premenopausal women is not yet fully understood, but we do know that estradiol has been shown to improve sexual desire, progesterone tends to dampen sexual desire, and that testosterone at physiological levels has been shown in most studies to have a neutral effect on sexual desire in a well-estrogenized patient.8
Related article:
Focus on treating genital atrophy symptoms
Experience and behavior modulate or reinforce sexual dysfunction
The most common psychological factors that trigger or amplify female sexual dysfunction are depression, anxiety, distraction, negative body image, sexual abuse, and emotional neglect.9 Contextual or sociocultural factors, such as relationship discord, life-stage stressors (the empty nest syndrome or anxiety and sleep deprivation from a new baby), as well as cultural or religious values that suppress sexuality, also should be considered.9 Experience-based neuroplasticity (changes in brain pathways that become solidified by negative or positive experiences) may elucidate how a multimodal approach, utilizing medical and psychological treatment, can be beneficial for patients, particularly those with hypoactive sexual desire disorder (HSDD).1
New and emerging approaches to managing female sexual dysfunction
Three agents, one of which has been available for prescription for some time, one that is newly available, and one in the pipeline, are or may soon be in the gynecologist's armamentarium.
Flibanserin
Medications that target excitatory pathways or blunt inhibitory pathways are in development, and one, flibanserin (Addyi), has been US Food and Drug Administration (FDA)-approved for the treatment of acquired, generalized HSDD in premenopausal women.1,10 Flibanserin is a nonhormonal, centrally acting, postsynaptic serotonin 1A receptor agonist and a serotonin 2A receptor antagonist that is taken daily at bedtime (100 mg); several weeks are usually needed before any effects are noted.1 It is not approved for postmenopausal women and has a boxed warning about the risks of hypotension and syncope; its use is contraindicated in women who drink alcohol, in those who have hepatic impairment, and with the use of moderate or strong CYP3A4 inhibitors.11
Also keep in mind that flibanserin is only available through a Risk Evaluation and Mitigation Strategy program, so clinicians who wish to prescribe it must enroll in and complete training to become certified providers.9
Related article:
What you need to know (and do) to prescribe the new drug flibanserin
Prasterone
Prasterone (Intrarosa), a once-daily intravaginal dehydroepiandrosterone (DHEA) product, is a prohormone that increases local estrogen and testosterone and has the advantage of improved sexual function, desire, arousal, lubrication, orgasm, satisfaction, as well as pain at sexual activity.12 It was approved by the FDA in November 2016 to treat moderate to severe dyspareunia and has been available for prescribing since July 2017. Its cost is comparable to topical estrogen products, with a $25 copay program.
Because prasterone is not an estrogen, it does not have the boxed warning that all estrogen products are mandated by the FDA to have. This may make it more acceptable to patients, who often decline to use an estrogen product after seeing the boxed warning on the package. The Centers for Medicare and Medicaid Services (CMS) does not have prasterone on its list of potentially hazardous drugs for the elderly. However, keep in mind that because its label is for dyspareunia and not specifically for GSM, CMS considers it a drug of choice--in other words, like sildenafil (Viagra), a lifestyle choice and not for treatment of a medical condition. As such, at the present time, Medicare does not cover it.
Bremelanotide
Late-stage trials of bremelanotide, a melanocortin receptor agonist, are underway. Its mechanism of action is somewhat like that of flibanserin in that both drugs increase dopamine and norepinephrine levels. The advantage of bremelanotide is that it is used as needed. It is dosed subcutaneously (1.75 mg) and it can be used as often as a woman would like to use it. The FDA is expected to consider it for approval in about a year. Unpublished data from poster sessions at recent meetings show that, in a phase 3 study of 1,247 premenopausal women with HSDD (who had already been screened for depression and were found to have a physiologic condition), improvements in desire, arousal, lubrication, and orgasm were shown with bremelanotide. About 18% of women stopped using the drug because of adverse effects (nausea, vomiting, flushing, or headache) versus 2% for placebo. Like flibanserin, it is expected to be approved for premenopausal women only.
Read how 3 experts would manage differing GSM symptoms.
What would you prescribe for these patients?
CASE Genitourinary syndrome of menopause (GSM) in a 55-year-old woman
A 55-year-old widow is beginning a new relationship. She has not had partnered sexual activity for several years, but she recently has begun a relationship. She describes pain with attempted penetration with her new partner. Her last menstrual period was 3 years ago and she has experienced very minor menopausal symptoms, which are not bothersome. On examination, the vulva and vagina are pale, with thin epithelium and absent rugae. The tissue lacks elasticity. A virginal speculum is needed to visualize the cervix.
How would you go about deciding which of the many options for management of GSM you will recommend for this patient? What do you weigh as you consider DHEA versus estrogen and topical versus oral therapy?
JoAnn V. Pinkerton, MD: Vulvovaginal atrophy (VVA), part of GSM, is associated with postmenopausal estrogen deficiency and includes the signs and symptoms seen on this patient's physical exam: vaginal narrowing, pallor, loss of elasticity, as well as pain with intercourse.6 Estrogen therapy is the most effective treatment for vaginal atrophy.13 Since she does not have significant menopausal symptoms, low-dose vaginal estrogen preparations are effective and generally safe treatments for VVA; these include creams, tablets containing estradiol or conjugated equine estrogen (CEE), and a low-dose vaginal estradiol ring--all available at doses that result in minimal systemic absorption.
Choice is usually made based on patient desire and likely adherence. If the patient prefers nonestrogen therapies that improve VVA and have been approved for relief of dyspareunia in postmenopausal women, I would discuss with the patient the oral selective estrogen receptor modulator ospemifene,14 and the new intravaginal DHEA suppositories, prasterone.15 Ospemifene is taken daily as an oral tablet, has a small risk of blood clots, and is my choice for women who do not need systemic hormone therapy and prefer to avoid vaginal therapy.
Andrew M. Kaunitz, MD: GSM is prevalent in menopausal women and, if not treated, causes progressive vaginal dryness and sexual discomfort. When the main indication for hormonal management in a menopausal woman is GSM (as opposed to treatment of vasomotor symptoms or prevention of osteoporosis), the treatment of choice is low-dose local vaginal estrogen, ospemifene, or prasterone (DHEA). Prasterone is a vaginally administered nonestrogen steroid that was approved by the FDA to treat dyspareunia associated with GSM. DHEA is an endogenous inactive steroid that is converted locally into androgens and estrogens; one vaginal insert is placed nightly.16,17
This 55-year-old widow has not been sexually active for some time. The facts that attempted penetration was painful and only an ultrathin (virginal) speculum could be used for examination indicate that contraction of the pelvic floor muscles is likely present. Simply starting medical management may not lead to comfortable/successful penetrative sex for this woman. In addition to medical management, she would likely benefit from referral for physical therapy. Using dilators and other strategies, along with the positive impact that medical management will have on the vaginal mucosa, a woman's physical therapist can work with this patient to help the pelvic floor muscles relax and facilitate comfortable penetrative sex.
James A. Simon, MD: With only minor vasomotor symptoms, I would assess the other potential benefits of a systemic therapy. These might include cardiovascular risk reduction (systemic estrogens or estrogens/progesterone in some), breast cancer risk reduction (some data suggesting ospemifene can accomplish this), osteoporosis prevention (systemic estrogens and estrogen/androgens), etc. If there is an option for a treatment to address more than one symptom, in this case GSM, assessing the risks/benefits of each of these therapies should be estimated for this specific patient.
If there are no systemic benefits to be had, then any of the local treatments should be helpful. As there are no head-to-head comparisons available, local estrogen cream, tablets, rings, local DHEA, or systemic ospemifene each should be considered possible treatments. I also feel this patient may benefit from supplementary self-dilation and/or physical therapy.
Related article:
2017 Update on menopause
CASE Dyspareunia and vasomotor symptoms in a 42-year-old breast cancer survivor
A 42-year-old woman with a BRCA1 mutation has undergone prophylactic mastectomies as well as hysterectomy with bilateral salpingo-oophorectomy. She reports mild to moderate hot flashes and bothersome vaginal dryness and dyspareunia. Examination confirms GSM.
Would you advise systemic hormone therapy for this patient? What would your recommendation be for management of her GSM symptoms?
Dr. Simon: While one's gut reaction would be to avoid systemic estrogen therapy in a patient with a BRCA1 mutation, the scientific information confirming this fear is lacking.18 Such patients may benefit significantly from systemic estrogen therapy (reduced risk of cardiovascular disease and cognitive decline, etc.), and with both breasts and both ovaries removed, estrogen's breast cancer risks, if any in this population, are largely avoided. The patient also may benefit from additional local therapy with either estrogens or DHEA.
Dr. Kaunitz: Due to her high lifetime risk of breast and ovarian cancer, this woman has proceeded with risk-reducing breast and gynecologic surgery. As more BRCA mutation carriers are being identified and undergo risk-reducing bilateral mastectomy (usually with reconstruction) and salpingo-oophorectomy, clinicians and mutation carriers more frequently face decisions regarding use of systemic hormone therapy.
Mutation carriers who have undergone bilateral risk-reducing mastectomy experience a very low baseline future risk for breast cancer; accordingly, concerns regarding this disease should not prevent use of systemic hormone therapy. Furthermore, without hormone replacement, induced menopause in women this age is associated with an elevated risk of osteoporosis, persistent vasomotor symptoms, cardiovascular disease, stroke, mood changes, dementia, Parkinson disease, and overall mortality. Recognizing the safety of estrogen therapy in this setting, this 42-year-old BRCA1 mutation carrier can initiate estrogen therapy. Standard dose estrogen therapy refers to oral estradiol 1.0 mg, conjugated equine estrogen 0.625 mg,or transdermal estradiol 0.05 mg. In younger women like this 42-year-old with surgically induced menopause, higher than standard replacement doses of estrogen are often appropriate.17
Due to concerns the hormone therapy might further increase future risk of breast cancer, some mutation carriers may delay or avoid risk-reducing bilateral salpingo-oophorectomy, a potentially lifesaving surgery which reduces not only future risk of ovarian cancer but also future risk for breast cancer.
Among mutation carriers with intact breasts, several studies address risk of breast cancer with use of systemic hormone therapy. Although limited in numbers of participants and years of follow-up, in aggregate, these studies provide reassurance that short-term use of systemic hormone therapy does not increase breast cancer risk in women with BRCA1 or BRCA2 mutations and intact breasts.19
Dr. Pinkerton: For this woman with early surgical menopause and hysterectomy, estrogen therapy could improve her vasomotor symptoms and decrease her risk of bone loss and GSM.17 In the Women's Health Initiative trial, there were 7 fewer breast cancers per 10,000 women-years in the estrogen-onlyarm.20 Observational studies suggest that hormone therapy, when given to the average age of menopause, decreases the risks of heart disease, Parkinson disease, and dementia.21 Limited observational evidence suggests that hormone therapy use does not further increase risk of breast cancer in women following oophorectomy for BRCA1 or BRCA2 gene mutation.22
The absolute risks observed with hormone therapy tended to be small, especially in younger, healthy women. Systemic hormone therapy could treat her hot flashes and her GSM symptoms and potentially decrease health risks associated with premature estrogen deficiency. Nonestrogen therapies for hot flashes include low-dose antidepressants, gabapentin, and mind-body options, such as cognitive behavioral therapy and hypnosis, but these would not decrease her health risks or treat her GSM.
If she only requests treatment of her GSM symptoms, she would be a candidate for low-dose vaginal estrogen therapy, given as a cream, tablet, or ring depending on her choice. I would not choose ospemifene as my first choice as she is having hot flashes, and there are no data yet on the drug's health benefits in early menopause. If she prefers nonestrogen vaginal therapy, the new intravaginal DHEA might be a good choice as both estrogen and testosterone are increased locally in the vagina while hormone levels remain in the postmenopausal range. There is no boxed warning on the patient insert, although safety in women with breast cancer or in those with elevated risk of breast cancer has not been tested.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive Sexual Desire Disorder: International Society for the Study of Women’s Sexual Health (ISSWSH) Expert Consensus Panel Review. Mayo Clin Proc. 2017;92(1):114–128.
- Kingsberg SA. Attitudinal survey of women living with low sexual desire. J Womens Health (Larchmt). 2014;23(10):817–823.
- Foley K, Foley D, Johnson BH. Healthcare resource utilization and expenditures of women diagnosed with hypoactive sexual desire disorder. J Med Econ. 2010;13(4):583–590.
- Biddle AK, West SL, D’Aloisio AA, Wheeler SB, Borisov NN, Thorp J. Hypoactive sexual desire disorder in postmenopausal women: quality of life and health burden. Value Health. 2009;12(5):763–772.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Ettinger B, Hait H, Reape KZ, Shu H. Measuring symptom relief in studies of vaginal and vulvar atrophy: the most bothersome symptom approach. Menopause. 2008;15(5):885–889.
- Dennerstein L, Randolph J, Taffe J, Dudley E, Burger H. Hormones, mood, sexuality, and the menopausal transition. Fertil Steril. 2002;77(suppl 4):S42–S48.
- Brotto LA, Bitzer J, Laan E, Leiblum S, Luria M. Women’s sexual desire and arousal disorders [published correction appears in J Sex Med. 2010;7(2 pt 1):856]. J Sex Med. 2010;7(1 pt 2):586–614.
- US Food and Drug Administration website. FDA approves first treatment for sexual desire disorder. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm458734.htm. Accessed August 14, 2017.
- Addyi (flibanserin) [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America, LLC; 2016.
- Labrie F, Derogatis L, Archer DF, et al; Members of the VVA Prasterone Research Group. Effect of intravaginal prasterone on sexual dysfunction in postmenopausal women with vulvovaginal atrophy. J Sex Med. 2015;12(12):2401–2412.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016;8:CD001500.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20(6):623–630.
- Labrie F, Archer DF, Koltun, W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243–256.
- Kaunitz AM. Focus on treating genital atrophy symptoms. OBG Manag. 2017;29(1):14, 16–17.
- The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. August 14, 2017. doi:10.1097/GME.0000000000000956.
- Domchek S, Kaunitz AM. Use of systemic hormone therapy in BRCA mutation carriers. Menopause. 2016;23(9):1026–1027.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Gabriel CA, Tigges-Cardwell J, Stopfer J, Erlichman J, Nathanson K, Domchek SM. Use of total abdominal hysterectomy and hormone replacement therapy in BRCA1 and BRCA2 mutation carriers undergoing risk-reducing salpingo-oophorectomy. Fam Cancer. 2009;8(1):23-28.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive Sexual Desire Disorder: International Society for the Study of Women’s Sexual Health (ISSWSH) Expert Consensus Panel Review. Mayo Clin Proc. 2017;92(1):114–128.
- Kingsberg SA. Attitudinal survey of women living with low sexual desire. J Womens Health (Larchmt). 2014;23(10):817–823.
- Foley K, Foley D, Johnson BH. Healthcare resource utilization and expenditures of women diagnosed with hypoactive sexual desire disorder. J Med Econ. 2010;13(4):583–590.
- Biddle AK, West SL, D’Aloisio AA, Wheeler SB, Borisov NN, Thorp J. Hypoactive sexual desire disorder in postmenopausal women: quality of life and health burden. Value Health. 2009;12(5):763–772.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Ettinger B, Hait H, Reape KZ, Shu H. Measuring symptom relief in studies of vaginal and vulvar atrophy: the most bothersome symptom approach. Menopause. 2008;15(5):885–889.
- Dennerstein L, Randolph J, Taffe J, Dudley E, Burger H. Hormones, mood, sexuality, and the menopausal transition. Fertil Steril. 2002;77(suppl 4):S42–S48.
- Brotto LA, Bitzer J, Laan E, Leiblum S, Luria M. Women’s sexual desire and arousal disorders [published correction appears in J Sex Med. 2010;7(2 pt 1):856]. J Sex Med. 2010;7(1 pt 2):586–614.
- US Food and Drug Administration website. FDA approves first treatment for sexual desire disorder. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm458734.htm. Accessed August 14, 2017.
- Addyi (flibanserin) [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America, LLC; 2016.
- Labrie F, Derogatis L, Archer DF, et al; Members of the VVA Prasterone Research Group. Effect of intravaginal prasterone on sexual dysfunction in postmenopausal women with vulvovaginal atrophy. J Sex Med. 2015;12(12):2401–2412.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016;8:CD001500.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20(6):623–630.
- Labrie F, Archer DF, Koltun, W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243–256.
- Kaunitz AM. Focus on treating genital atrophy symptoms. OBG Manag. 2017;29(1):14, 16–17.
- The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. August 14, 2017. doi:10.1097/GME.0000000000000956.
- Domchek S, Kaunitz AM. Use of systemic hormone therapy in BRCA mutation carriers. Menopause. 2016;23(9):1026–1027.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Gabriel CA, Tigges-Cardwell J, Stopfer J, Erlichman J, Nathanson K, Domchek SM. Use of total abdominal hysterectomy and hormone replacement therapy in BRCA1 and BRCA2 mutation carriers undergoing risk-reducing salpingo-oophorectomy. Fam Cancer. 2009;8(1):23-28.
Fast Tracks
- Although not fully understood how, estradiol can improve sexual desire, progesterone tends to dampen sexual desire, and testosterone has a neutral effect in premenopausal women
- Newly available since July 2017, prasterone is a once-daily intravaginal agent that treats moderate to severe dyspareunia and has costs similar to topical estrogens
- Estrogen therapy may be considered in a breast cancer mutation carrier who has undergone prophylactic mastectomies and bilateral salpingo-oophorectomy
Focus on lifestyle to manage menopause symptoms after breast cancer
Lifestyle modification, rather than hormone therapy, should form the basis for managing estrogen-depletion symptoms and associated clinical problems in breast cancer survivors, according to a review of available evidence.
The review, conducted by the writing group for the Endocrine Society’s guidelines on management of menopausal symptoms, was prompted by the paucity of both randomized controlled trials in breast cancer survivors with estrogen deficiency issues and guidelines that sufficiently focus on treatment of this subgroup of women.
“A large proportion of women experience menopausal symptoms or clinical manifestations of estrogen deficiency during treatment of their breast cancer or after completion of therapy. The specific symptoms and clinical challenges differ based on menopausal status prior to initiation of cancer treatment and therapeutic agents used,” the researchers wrote in a report published in the Journal of Clinical Endocrinology & Metabolism (2017 Aug 2. doi: 10.1210/jc.2017-01138).
For instance, among premenopausal women treated with chemotherapy, ovarian insufficiency, severe menopausal symptoms, and infertility can result. Postmenopasual women treated with aromatase inhibitors may experience arthralgia, accelerated bone loss, and osteoporotic fractures, as well as severe vulvovaginal atrophy, they explained, noting that both premenopausal and postmenopausal survivors can experience moderate-to-severe vasomotor symptoms and sleep disturbance with related fatigue, depressive symptoms, and mood changes.
“Less common problems include weight gain, symptomatic osteoarthritis and intervertebral disk degeneration, degenerative skin changes, radiation and chemotherapy-related cardiovascular disease, and reduced quality of life,” the researchers wrote.
Based on a review of randomized controlled clinical trials, observational studies, evidence-based guidelines, and expert opinion from professional societies, the writing group concluded that individualized lifestyle modifications and nonpharmacologic therapies are recommended for the treatment of these symptoms.
Specifically, the writing group recommended smoking cessation, weight loss when indicated, limited alcohol intake, maintenance of adequate vitamin D and calcium levels, a healthy diet, and regular physical activity for all women with prior breast cancer.
They also recommended nonpharmacologic therapies for vasomotor symptoms, and noted that cognitive behavioral therapy, hypnosis, and acupuncture are among the approaches that may be helpful.
Vaginal lubricants and moisturizers can also be helpful for mild vulvovaginal atrophy, they wrote. For women with more severe symptoms or signs of estrogen deficiency, pharmacologic agents are available to relieve vasomotor symptoms and vulvovaginal atrophy, and to prevent and treat fractures, they wrote, adding that “therapy must be individualized based on each woman’s needs and goals for therapy.”
Among emerging approaches to treatment of symptoms are selective estrogen receptor modulators (SERMs), tissue selective estrogen complex (TSEC) therapy, estetrol, and neurokinin B inhibitors, which show promise for expanding options for symptom relief with less breast cancer risk. However, these have not yet been tested in women with prior breast cancer, the researchers noted.
Dr. Santen reported receiving research funding from Panterhei Bioscience. Other authors received research funding from Therapeutics MD and Lawley Pharmaceuticals, and honoraria from Abbott, Besins Health Care, and Pfizer.
Lifestyle modification, rather than hormone therapy, should form the basis for managing estrogen-depletion symptoms and associated clinical problems in breast cancer survivors, according to a review of available evidence.
The review, conducted by the writing group for the Endocrine Society’s guidelines on management of menopausal symptoms, was prompted by the paucity of both randomized controlled trials in breast cancer survivors with estrogen deficiency issues and guidelines that sufficiently focus on treatment of this subgroup of women.
“A large proportion of women experience menopausal symptoms or clinical manifestations of estrogen deficiency during treatment of their breast cancer or after completion of therapy. The specific symptoms and clinical challenges differ based on menopausal status prior to initiation of cancer treatment and therapeutic agents used,” the researchers wrote in a report published in the Journal of Clinical Endocrinology & Metabolism (2017 Aug 2. doi: 10.1210/jc.2017-01138).
For instance, among premenopausal women treated with chemotherapy, ovarian insufficiency, severe menopausal symptoms, and infertility can result. Postmenopasual women treated with aromatase inhibitors may experience arthralgia, accelerated bone loss, and osteoporotic fractures, as well as severe vulvovaginal atrophy, they explained, noting that both premenopausal and postmenopausal survivors can experience moderate-to-severe vasomotor symptoms and sleep disturbance with related fatigue, depressive symptoms, and mood changes.
“Less common problems include weight gain, symptomatic osteoarthritis and intervertebral disk degeneration, degenerative skin changes, radiation and chemotherapy-related cardiovascular disease, and reduced quality of life,” the researchers wrote.
Based on a review of randomized controlled clinical trials, observational studies, evidence-based guidelines, and expert opinion from professional societies, the writing group concluded that individualized lifestyle modifications and nonpharmacologic therapies are recommended for the treatment of these symptoms.
Specifically, the writing group recommended smoking cessation, weight loss when indicated, limited alcohol intake, maintenance of adequate vitamin D and calcium levels, a healthy diet, and regular physical activity for all women with prior breast cancer.
They also recommended nonpharmacologic therapies for vasomotor symptoms, and noted that cognitive behavioral therapy, hypnosis, and acupuncture are among the approaches that may be helpful.
Vaginal lubricants and moisturizers can also be helpful for mild vulvovaginal atrophy, they wrote. For women with more severe symptoms or signs of estrogen deficiency, pharmacologic agents are available to relieve vasomotor symptoms and vulvovaginal atrophy, and to prevent and treat fractures, they wrote, adding that “therapy must be individualized based on each woman’s needs and goals for therapy.”
Among emerging approaches to treatment of symptoms are selective estrogen receptor modulators (SERMs), tissue selective estrogen complex (TSEC) therapy, estetrol, and neurokinin B inhibitors, which show promise for expanding options for symptom relief with less breast cancer risk. However, these have not yet been tested in women with prior breast cancer, the researchers noted.
Dr. Santen reported receiving research funding from Panterhei Bioscience. Other authors received research funding from Therapeutics MD and Lawley Pharmaceuticals, and honoraria from Abbott, Besins Health Care, and Pfizer.
Lifestyle modification, rather than hormone therapy, should form the basis for managing estrogen-depletion symptoms and associated clinical problems in breast cancer survivors, according to a review of available evidence.
The review, conducted by the writing group for the Endocrine Society’s guidelines on management of menopausal symptoms, was prompted by the paucity of both randomized controlled trials in breast cancer survivors with estrogen deficiency issues and guidelines that sufficiently focus on treatment of this subgroup of women.
“A large proportion of women experience menopausal symptoms or clinical manifestations of estrogen deficiency during treatment of their breast cancer or after completion of therapy. The specific symptoms and clinical challenges differ based on menopausal status prior to initiation of cancer treatment and therapeutic agents used,” the researchers wrote in a report published in the Journal of Clinical Endocrinology & Metabolism (2017 Aug 2. doi: 10.1210/jc.2017-01138).
For instance, among premenopausal women treated with chemotherapy, ovarian insufficiency, severe menopausal symptoms, and infertility can result. Postmenopasual women treated with aromatase inhibitors may experience arthralgia, accelerated bone loss, and osteoporotic fractures, as well as severe vulvovaginal atrophy, they explained, noting that both premenopausal and postmenopausal survivors can experience moderate-to-severe vasomotor symptoms and sleep disturbance with related fatigue, depressive symptoms, and mood changes.
“Less common problems include weight gain, symptomatic osteoarthritis and intervertebral disk degeneration, degenerative skin changes, radiation and chemotherapy-related cardiovascular disease, and reduced quality of life,” the researchers wrote.
Based on a review of randomized controlled clinical trials, observational studies, evidence-based guidelines, and expert opinion from professional societies, the writing group concluded that individualized lifestyle modifications and nonpharmacologic therapies are recommended for the treatment of these symptoms.
Specifically, the writing group recommended smoking cessation, weight loss when indicated, limited alcohol intake, maintenance of adequate vitamin D and calcium levels, a healthy diet, and regular physical activity for all women with prior breast cancer.
They also recommended nonpharmacologic therapies for vasomotor symptoms, and noted that cognitive behavioral therapy, hypnosis, and acupuncture are among the approaches that may be helpful.
Vaginal lubricants and moisturizers can also be helpful for mild vulvovaginal atrophy, they wrote. For women with more severe symptoms or signs of estrogen deficiency, pharmacologic agents are available to relieve vasomotor symptoms and vulvovaginal atrophy, and to prevent and treat fractures, they wrote, adding that “therapy must be individualized based on each woman’s needs and goals for therapy.”
Among emerging approaches to treatment of symptoms are selective estrogen receptor modulators (SERMs), tissue selective estrogen complex (TSEC) therapy, estetrol, and neurokinin B inhibitors, which show promise for expanding options for symptom relief with less breast cancer risk. However, these have not yet been tested in women with prior breast cancer, the researchers noted.
Dr. Santen reported receiving research funding from Panterhei Bioscience. Other authors received research funding from Therapeutics MD and Lawley Pharmaceuticals, and honoraria from Abbott, Besins Health Care, and Pfizer.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
FDA expands approval of fulvestrant for HR+/HER2– advanced breast cancer
The Food and Drug Administration has approved fulvestrant (Faslodex) as monotherapy for women with hormone receptor–positive, human epidermal growth factor receptor 2–negative (HR+/HER2–) advanced breast cancer who are postmenopausal and previously untreated.
Approval was based on improved progression-free survival (PFS) in the phase 3 FALCON trial of 462 postmenopausal women with HR+/HER2– metastatic or locally advanced breast cancer who had not previously received hormonal therapy, drug maker AstraZeneca said in a press release.
The most common adverse events were arthralgia (16.7% of patients on fulvestrant vs. 10.3% on anastrozole), and hot flushes/flashes (11.4% vs. 10.3%).
Grade 3 or greater adverse events occurred in 22.4% vs. 17.7%. Deaths from adverse events occurred in six patients on fulvestrant vs. seven on anastrozole, according to results published in The Lancet.
The Food and Drug Administration has approved fulvestrant (Faslodex) as monotherapy for women with hormone receptor–positive, human epidermal growth factor receptor 2–negative (HR+/HER2–) advanced breast cancer who are postmenopausal and previously untreated.
Approval was based on improved progression-free survival (PFS) in the phase 3 FALCON trial of 462 postmenopausal women with HR+/HER2– metastatic or locally advanced breast cancer who had not previously received hormonal therapy, drug maker AstraZeneca said in a press release.
The most common adverse events were arthralgia (16.7% of patients on fulvestrant vs. 10.3% on anastrozole), and hot flushes/flashes (11.4% vs. 10.3%).
Grade 3 or greater adverse events occurred in 22.4% vs. 17.7%. Deaths from adverse events occurred in six patients on fulvestrant vs. seven on anastrozole, according to results published in The Lancet.
The Food and Drug Administration has approved fulvestrant (Faslodex) as monotherapy for women with hormone receptor–positive, human epidermal growth factor receptor 2–negative (HR+/HER2–) advanced breast cancer who are postmenopausal and previously untreated.
Approval was based on improved progression-free survival (PFS) in the phase 3 FALCON trial of 462 postmenopausal women with HR+/HER2– metastatic or locally advanced breast cancer who had not previously received hormonal therapy, drug maker AstraZeneca said in a press release.
The most common adverse events were arthralgia (16.7% of patients on fulvestrant vs. 10.3% on anastrozole), and hot flushes/flashes (11.4% vs. 10.3%).
Grade 3 or greater adverse events occurred in 22.4% vs. 17.7%. Deaths from adverse events occurred in six patients on fulvestrant vs. seven on anastrozole, according to results published in The Lancet.
Annual mammograms from age 40 linked with greatest reductions in mortality
Annual mammograms starting at age 40 and continuing until age 84 will achieve the greatest reductions in breast cancer deaths, according to a modeling study published in Cancer.
Researchers used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer models to compare three current mammography-based screening approaches for women at average risk of breast cancer. The first approach is for annual screening from ages 40-84 years, the second is a hybrid approach with annual screening from ages 45 to 54 then biennial screening from ages 55 to 79, and the third approach involves biennial mammograms from ages 50 to 74.
The modeling suggested that the largest mean reduction in mortality – 36.9% – was associated with annual screening starting at age 40. The second largest reduction of 30.8% occurred with the hybrid screening approach starting at age 45, while the smallest reduction in mortality – 23.2% – occurred with biennial screening starting at age 50 (Cancer 2017 Aug 21. doi: 10.1002/cncr.30842).
Annual screening also averted the most deaths from breast cancer – 11.9 per 1,000 women screened – and gained the most life-years (189 per 1,000 women screened, compared with 149 for the hybrid approach and 110 for biennial screening), reported Elizabeth K. Arleo, MD, of Weill Cornell Imaging at New York–Presbyterian, New York, and her coauthors.
However, the annual screening approach was also associated with the largest number of benign recalls and benign biopsies. The model suggested that a woman starting annual screening from age 40 could expect on average to be recalled for a benign diagnostic work-up every 13 years and undergo a benign biopsy every 187 years.
Overall, using a single-year cohort of women born in 1960 who were 100% compliant with screening, the model predicted that 44,671 would still die of breast cancer with annual screening, 51,211 would die under the hybrid screening model, and 56,887 would die under the biennial screening model.
“If the goal is to avert the most breast cancer deaths and gain the most life-years, CISNET modeling shows that the optimal age of initiation for screening mammography is 40 years, the optimal screening frequency is annual, and the optimal stopping age is when a woman’s life expectancy is less than 5-7 years,” the researchers wrote. “Individual women should continue to have the choice to reduce their risk of dying from breast cancer as much as possible, and as CISNET models show, annual mammography starting at age 40 years is the best way to do so.”
Two of the researchers reported consulting or institutional support from GE Healthcare. No other conflicts of interest were reported.
Annual mammograms starting at age 40 and continuing until age 84 will achieve the greatest reductions in breast cancer deaths, according to a modeling study published in Cancer.
Researchers used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer models to compare three current mammography-based screening approaches for women at average risk of breast cancer. The first approach is for annual screening from ages 40-84 years, the second is a hybrid approach with annual screening from ages 45 to 54 then biennial screening from ages 55 to 79, and the third approach involves biennial mammograms from ages 50 to 74.
The modeling suggested that the largest mean reduction in mortality – 36.9% – was associated with annual screening starting at age 40. The second largest reduction of 30.8% occurred with the hybrid screening approach starting at age 45, while the smallest reduction in mortality – 23.2% – occurred with biennial screening starting at age 50 (Cancer 2017 Aug 21. doi: 10.1002/cncr.30842).
Annual screening also averted the most deaths from breast cancer – 11.9 per 1,000 women screened – and gained the most life-years (189 per 1,000 women screened, compared with 149 for the hybrid approach and 110 for biennial screening), reported Elizabeth K. Arleo, MD, of Weill Cornell Imaging at New York–Presbyterian, New York, and her coauthors.
However, the annual screening approach was also associated with the largest number of benign recalls and benign biopsies. The model suggested that a woman starting annual screening from age 40 could expect on average to be recalled for a benign diagnostic work-up every 13 years and undergo a benign biopsy every 187 years.
Overall, using a single-year cohort of women born in 1960 who were 100% compliant with screening, the model predicted that 44,671 would still die of breast cancer with annual screening, 51,211 would die under the hybrid screening model, and 56,887 would die under the biennial screening model.
“If the goal is to avert the most breast cancer deaths and gain the most life-years, CISNET modeling shows that the optimal age of initiation for screening mammography is 40 years, the optimal screening frequency is annual, and the optimal stopping age is when a woman’s life expectancy is less than 5-7 years,” the researchers wrote. “Individual women should continue to have the choice to reduce their risk of dying from breast cancer as much as possible, and as CISNET models show, annual mammography starting at age 40 years is the best way to do so.”
Two of the researchers reported consulting or institutional support from GE Healthcare. No other conflicts of interest were reported.
Annual mammograms starting at age 40 and continuing until age 84 will achieve the greatest reductions in breast cancer deaths, according to a modeling study published in Cancer.
Researchers used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer models to compare three current mammography-based screening approaches for women at average risk of breast cancer. The first approach is for annual screening from ages 40-84 years, the second is a hybrid approach with annual screening from ages 45 to 54 then biennial screening from ages 55 to 79, and the third approach involves biennial mammograms from ages 50 to 74.
The modeling suggested that the largest mean reduction in mortality – 36.9% – was associated with annual screening starting at age 40. The second largest reduction of 30.8% occurred with the hybrid screening approach starting at age 45, while the smallest reduction in mortality – 23.2% – occurred with biennial screening starting at age 50 (Cancer 2017 Aug 21. doi: 10.1002/cncr.30842).
Annual screening also averted the most deaths from breast cancer – 11.9 per 1,000 women screened – and gained the most life-years (189 per 1,000 women screened, compared with 149 for the hybrid approach and 110 for biennial screening), reported Elizabeth K. Arleo, MD, of Weill Cornell Imaging at New York–Presbyterian, New York, and her coauthors.
However, the annual screening approach was also associated with the largest number of benign recalls and benign biopsies. The model suggested that a woman starting annual screening from age 40 could expect on average to be recalled for a benign diagnostic work-up every 13 years and undergo a benign biopsy every 187 years.
Overall, using a single-year cohort of women born in 1960 who were 100% compliant with screening, the model predicted that 44,671 would still die of breast cancer with annual screening, 51,211 would die under the hybrid screening model, and 56,887 would die under the biennial screening model.
“If the goal is to avert the most breast cancer deaths and gain the most life-years, CISNET modeling shows that the optimal age of initiation for screening mammography is 40 years, the optimal screening frequency is annual, and the optimal stopping age is when a woman’s life expectancy is less than 5-7 years,” the researchers wrote. “Individual women should continue to have the choice to reduce their risk of dying from breast cancer as much as possible, and as CISNET models show, annual mammography starting at age 40 years is the best way to do so.”
Two of the researchers reported consulting or institutional support from GE Healthcare. No other conflicts of interest were reported.
FROM CANCER
Key clinical point:
Major finding: Annual mammograms from age 40 are associated with a 36.9% mean reduction in mortality, compared with a 23.2% reduction with biennial screening from age 50.
Data source: A study using Cancer Intervention and Surveillance Modeling Network models.
Disclosures: Two of the researchers reported consulting or institutional support from GE Healthcare. No other conflicts of interest were reported.
Ribociclib: another CDK inhibitor hits the mark in breast cancer
This spring, the US Food and Drug Administration approved a second cyclin-dependent kinase (CDK) inhibitor for the treatment of postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced/metastatic breast cancer in combination with aromatase inhibitors (AIs).1 The drug, ribociclib, joins palbociclib as the second drug in this class, which targets key regulators of the mammalian cell cycle and can help to overcome resistance to endocrine therapy–like AIs, a standard front-line treatment option in this group of patients. Palbociclib (Ibrance) was approved last year in combination with the AI letrozole, which was recently expanded to include its use in combination with all AIs, the same indication for which ribociclib received approval.
The ribociclib approval was based on the results of a phase 3, randomized, double-blind, placebo-controlled, international clinical trial called MONALEESA-2.2 The trial, conducted in 29 countries, compared the effects of ribociclib plus letrozole with letrozole plus placebo in 668 postmenopausal women with locally confirmed, HR-positive, HER2-negative, recurrent or metastatic breast cancer.
Patients had not received previous systemic therapy for advanced disease, had measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), had an Eastern Cooperative Oncology Group performance status of 0 or 1 (range, 1-5; 0, fully active and 5, dead), and had adequate bone marrow and organ function.
Patients were excluded if they had received previous CDK4/6 therapy, any previous systemic chemotherapy, endocrine therapy for advanced disease, previous neoadjuvant or adjuvant therapy with any nonsteroidal AI (unless they had been disease free for more than 12 months), and had inflammatory breast cancer, central nervous system metastases, history of cardiac disease or dysfunction, or impaired gastrointestinal function that alters drug absorption.
Patients were treated with ribociclib at a dose of 600 mg daily on a 3-weeks-on, 1-week-off schedule in 28-day cycles or placebo, which were combined with letrozole at a dose of 2.5 mg a day on a continuous schedule. Randomization was stratified according to the presence or absence of liver or lung metastases and treatment was continued until disease progression, unacceptable toxicity, death or discontinuation of treatment. Dose reductions of ribociclib were allowed, to manage adverse events (AEs), but treatment crossover was not permitted.
Tumor assessments were performed at screening, every 8 weeks during the first 18 months, every 12 weeks thereafter until disease progression, and at the end of treatment, and were assessed by an independent review committee. The baseline characteristics of the patient population were well balanced; patients had a median age of 62 years, all were HR positive except 1 patient who was HER2 positive.
The trial was ended prematurely after an initial interim analysis demonstrated a significant benefit in favor of ribociclib in the primary endpoint, progression-free survival (PFS). Over a median duration of follow-up of 15.3 months, the median PFS was not yet reached in the ribociclib arm, compared with 14.7 months in the placebo arm (hazard ratio, 0.556; P < .0001). In a subsequent analysis with 11 months of additional follow-up, the median PFS was 25.3 months in the combination arm, compared with 16 months in the placebo arm, which translated into a 44% reduction in the risk of disease progression or death. The PFS benefit with ribociclib was observed across all preplanned subgroup analyses. The objective response rates were 52.7% in the ribociclib arm, compared with 37.1% in the placebo arm, but overall survival data were immature.
The frequency and severity of AEs were increased in the combination arm; most common were neutropenia, nausea, fatigue, diarrhea, leukopenia, alopecia, vomiting, constipation, headache, and back pain. The most common grade 3 or 4 AEs experienced with ribociclib were neutropenia, leukopenia, abnormal liver function tests, lymphopenia, and vomiting.
Ribociclib is accompanied by warnings and precautions about QT interval prolongation, hepatobiliary toxicity, and neutropenia. Clinicians are advised to monitor electrocardiograms and electrolytes before the start of ribociclib therapy and to begin treatment only in patients with QTcF values <450 ms and in whom electrolyte abnormalities have been corrected. ECG should be repeated at around day 14 of the first cycle, the beginning of the second cycle, and as deemed clinically necessary.
Liver function tests should be performed before starting treatment, every 2 weeks for the first 2 cycles, at the beginning of each of the subsequent 4 cycles, and as clinically indicated. For aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) levels greater than 3-5 times the upper limit of normal (ULN, grade 2), ribociclib should be interrupted until recovery to baseline or lower. For levels >5-20 times the ULN (grade 3) or recurring grade 2 increases, treatment should be interrupted until recovery to baseline or lower and then resumed at the next lowest dose level. Treatment with ribociclib should be discontinued in the event of recurring grade 3 elevations or for AST/ALT elevations >3 times ULN in combination with total bilirubin >2 times ULN.
Complete blood counts should be performed before starting treatment and monitored every 2 weeks for the first 2 cycles, at the beginning of each of the 4 subsequent cycles, and as clinically needed. If absolute neutrophil counts are 500-1,000 mm3 (grade 3), treatment should be discontinued until recovery to grade 2 or lower. If grade 3 neutropenia recurs or for grade 3 febrile neutropenia or grade 4 neutropenia, treatment should resume at a lower dose level upon recovery to grade 2 or lower.
Pregnant women and those of reproductive age should be warned of the risk of fetal harm and the need for effective contraception during treatment and for at least 3 weeks after the last dose. Ribociclib is marketed as Kisqali by Novartis.
1. Ribociclib (Kisqali). US Food and Drug Administration website. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm546438.htm. Last updated March 14, 2017. Accessed April 3, 2017.
2. Kisqali (ribociclib) tables, for oral use. Prescribing information. Novartis Pharmaceuticals Corp. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/kisqali.pdf. March 2017. Accessed April 3, 2017.
3. Horobagyi GN, Stemmer SN, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738-1748.
This spring, the US Food and Drug Administration approved a second cyclin-dependent kinase (CDK) inhibitor for the treatment of postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced/metastatic breast cancer in combination with aromatase inhibitors (AIs).1 The drug, ribociclib, joins palbociclib as the second drug in this class, which targets key regulators of the mammalian cell cycle and can help to overcome resistance to endocrine therapy–like AIs, a standard front-line treatment option in this group of patients. Palbociclib (Ibrance) was approved last year in combination with the AI letrozole, which was recently expanded to include its use in combination with all AIs, the same indication for which ribociclib received approval.
The ribociclib approval was based on the results of a phase 3, randomized, double-blind, placebo-controlled, international clinical trial called MONALEESA-2.2 The trial, conducted in 29 countries, compared the effects of ribociclib plus letrozole with letrozole plus placebo in 668 postmenopausal women with locally confirmed, HR-positive, HER2-negative, recurrent or metastatic breast cancer.
Patients had not received previous systemic therapy for advanced disease, had measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), had an Eastern Cooperative Oncology Group performance status of 0 or 1 (range, 1-5; 0, fully active and 5, dead), and had adequate bone marrow and organ function.
Patients were excluded if they had received previous CDK4/6 therapy, any previous systemic chemotherapy, endocrine therapy for advanced disease, previous neoadjuvant or adjuvant therapy with any nonsteroidal AI (unless they had been disease free for more than 12 months), and had inflammatory breast cancer, central nervous system metastases, history of cardiac disease or dysfunction, or impaired gastrointestinal function that alters drug absorption.
Patients were treated with ribociclib at a dose of 600 mg daily on a 3-weeks-on, 1-week-off schedule in 28-day cycles or placebo, which were combined with letrozole at a dose of 2.5 mg a day on a continuous schedule. Randomization was stratified according to the presence or absence of liver or lung metastases and treatment was continued until disease progression, unacceptable toxicity, death or discontinuation of treatment. Dose reductions of ribociclib were allowed, to manage adverse events (AEs), but treatment crossover was not permitted.
Tumor assessments were performed at screening, every 8 weeks during the first 18 months, every 12 weeks thereafter until disease progression, and at the end of treatment, and were assessed by an independent review committee. The baseline characteristics of the patient population were well balanced; patients had a median age of 62 years, all were HR positive except 1 patient who was HER2 positive.
The trial was ended prematurely after an initial interim analysis demonstrated a significant benefit in favor of ribociclib in the primary endpoint, progression-free survival (PFS). Over a median duration of follow-up of 15.3 months, the median PFS was not yet reached in the ribociclib arm, compared with 14.7 months in the placebo arm (hazard ratio, 0.556; P < .0001). In a subsequent analysis with 11 months of additional follow-up, the median PFS was 25.3 months in the combination arm, compared with 16 months in the placebo arm, which translated into a 44% reduction in the risk of disease progression or death. The PFS benefit with ribociclib was observed across all preplanned subgroup analyses. The objective response rates were 52.7% in the ribociclib arm, compared with 37.1% in the placebo arm, but overall survival data were immature.
The frequency and severity of AEs were increased in the combination arm; most common were neutropenia, nausea, fatigue, diarrhea, leukopenia, alopecia, vomiting, constipation, headache, and back pain. The most common grade 3 or 4 AEs experienced with ribociclib were neutropenia, leukopenia, abnormal liver function tests, lymphopenia, and vomiting.
Ribociclib is accompanied by warnings and precautions about QT interval prolongation, hepatobiliary toxicity, and neutropenia. Clinicians are advised to monitor electrocardiograms and electrolytes before the start of ribociclib therapy and to begin treatment only in patients with QTcF values <450 ms and in whom electrolyte abnormalities have been corrected. ECG should be repeated at around day 14 of the first cycle, the beginning of the second cycle, and as deemed clinically necessary.
Liver function tests should be performed before starting treatment, every 2 weeks for the first 2 cycles, at the beginning of each of the subsequent 4 cycles, and as clinically indicated. For aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) levels greater than 3-5 times the upper limit of normal (ULN, grade 2), ribociclib should be interrupted until recovery to baseline or lower. For levels >5-20 times the ULN (grade 3) or recurring grade 2 increases, treatment should be interrupted until recovery to baseline or lower and then resumed at the next lowest dose level. Treatment with ribociclib should be discontinued in the event of recurring grade 3 elevations or for AST/ALT elevations >3 times ULN in combination with total bilirubin >2 times ULN.
Complete blood counts should be performed before starting treatment and monitored every 2 weeks for the first 2 cycles, at the beginning of each of the 4 subsequent cycles, and as clinically needed. If absolute neutrophil counts are 500-1,000 mm3 (grade 3), treatment should be discontinued until recovery to grade 2 or lower. If grade 3 neutropenia recurs or for grade 3 febrile neutropenia or grade 4 neutropenia, treatment should resume at a lower dose level upon recovery to grade 2 or lower.
Pregnant women and those of reproductive age should be warned of the risk of fetal harm and the need for effective contraception during treatment and for at least 3 weeks after the last dose. Ribociclib is marketed as Kisqali by Novartis.
This spring, the US Food and Drug Administration approved a second cyclin-dependent kinase (CDK) inhibitor for the treatment of postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced/metastatic breast cancer in combination with aromatase inhibitors (AIs).1 The drug, ribociclib, joins palbociclib as the second drug in this class, which targets key regulators of the mammalian cell cycle and can help to overcome resistance to endocrine therapy–like AIs, a standard front-line treatment option in this group of patients. Palbociclib (Ibrance) was approved last year in combination with the AI letrozole, which was recently expanded to include its use in combination with all AIs, the same indication for which ribociclib received approval.
The ribociclib approval was based on the results of a phase 3, randomized, double-blind, placebo-controlled, international clinical trial called MONALEESA-2.2 The trial, conducted in 29 countries, compared the effects of ribociclib plus letrozole with letrozole plus placebo in 668 postmenopausal women with locally confirmed, HR-positive, HER2-negative, recurrent or metastatic breast cancer.
Patients had not received previous systemic therapy for advanced disease, had measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), had an Eastern Cooperative Oncology Group performance status of 0 or 1 (range, 1-5; 0, fully active and 5, dead), and had adequate bone marrow and organ function.
Patients were excluded if they had received previous CDK4/6 therapy, any previous systemic chemotherapy, endocrine therapy for advanced disease, previous neoadjuvant or adjuvant therapy with any nonsteroidal AI (unless they had been disease free for more than 12 months), and had inflammatory breast cancer, central nervous system metastases, history of cardiac disease or dysfunction, or impaired gastrointestinal function that alters drug absorption.
Patients were treated with ribociclib at a dose of 600 mg daily on a 3-weeks-on, 1-week-off schedule in 28-day cycles or placebo, which were combined with letrozole at a dose of 2.5 mg a day on a continuous schedule. Randomization was stratified according to the presence or absence of liver or lung metastases and treatment was continued until disease progression, unacceptable toxicity, death or discontinuation of treatment. Dose reductions of ribociclib were allowed, to manage adverse events (AEs), but treatment crossover was not permitted.
Tumor assessments were performed at screening, every 8 weeks during the first 18 months, every 12 weeks thereafter until disease progression, and at the end of treatment, and were assessed by an independent review committee. The baseline characteristics of the patient population were well balanced; patients had a median age of 62 years, all were HR positive except 1 patient who was HER2 positive.
The trial was ended prematurely after an initial interim analysis demonstrated a significant benefit in favor of ribociclib in the primary endpoint, progression-free survival (PFS). Over a median duration of follow-up of 15.3 months, the median PFS was not yet reached in the ribociclib arm, compared with 14.7 months in the placebo arm (hazard ratio, 0.556; P < .0001). In a subsequent analysis with 11 months of additional follow-up, the median PFS was 25.3 months in the combination arm, compared with 16 months in the placebo arm, which translated into a 44% reduction in the risk of disease progression or death. The PFS benefit with ribociclib was observed across all preplanned subgroup analyses. The objective response rates were 52.7% in the ribociclib arm, compared with 37.1% in the placebo arm, but overall survival data were immature.
The frequency and severity of AEs were increased in the combination arm; most common were neutropenia, nausea, fatigue, diarrhea, leukopenia, alopecia, vomiting, constipation, headache, and back pain. The most common grade 3 or 4 AEs experienced with ribociclib were neutropenia, leukopenia, abnormal liver function tests, lymphopenia, and vomiting.
Ribociclib is accompanied by warnings and precautions about QT interval prolongation, hepatobiliary toxicity, and neutropenia. Clinicians are advised to monitor electrocardiograms and electrolytes before the start of ribociclib therapy and to begin treatment only in patients with QTcF values <450 ms and in whom electrolyte abnormalities have been corrected. ECG should be repeated at around day 14 of the first cycle, the beginning of the second cycle, and as deemed clinically necessary.
Liver function tests should be performed before starting treatment, every 2 weeks for the first 2 cycles, at the beginning of each of the subsequent 4 cycles, and as clinically indicated. For aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) levels greater than 3-5 times the upper limit of normal (ULN, grade 2), ribociclib should be interrupted until recovery to baseline or lower. For levels >5-20 times the ULN (grade 3) or recurring grade 2 increases, treatment should be interrupted until recovery to baseline or lower and then resumed at the next lowest dose level. Treatment with ribociclib should be discontinued in the event of recurring grade 3 elevations or for AST/ALT elevations >3 times ULN in combination with total bilirubin >2 times ULN.
Complete blood counts should be performed before starting treatment and monitored every 2 weeks for the first 2 cycles, at the beginning of each of the 4 subsequent cycles, and as clinically needed. If absolute neutrophil counts are 500-1,000 mm3 (grade 3), treatment should be discontinued until recovery to grade 2 or lower. If grade 3 neutropenia recurs or for grade 3 febrile neutropenia or grade 4 neutropenia, treatment should resume at a lower dose level upon recovery to grade 2 or lower.
Pregnant women and those of reproductive age should be warned of the risk of fetal harm and the need for effective contraception during treatment and for at least 3 weeks after the last dose. Ribociclib is marketed as Kisqali by Novartis.
1. Ribociclib (Kisqali). US Food and Drug Administration website. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm546438.htm. Last updated March 14, 2017. Accessed April 3, 2017.
2. Kisqali (ribociclib) tables, for oral use. Prescribing information. Novartis Pharmaceuticals Corp. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/kisqali.pdf. March 2017. Accessed April 3, 2017.
3. Horobagyi GN, Stemmer SN, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738-1748.
1. Ribociclib (Kisqali). US Food and Drug Administration website. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm546438.htm. Last updated March 14, 2017. Accessed April 3, 2017.
2. Kisqali (ribociclib) tables, for oral use. Prescribing information. Novartis Pharmaceuticals Corp. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/kisqali.pdf. March 2017. Accessed April 3, 2017.
3. Horobagyi GN, Stemmer SN, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738-1748.
LIFSCREEN data support broader cancer screening in Li-Fraumeni syndrome
Broader cancer screening of individuals with Li-Fraumeni syndrome (LFS), with or without whole-body magnetic resonance imaging, has a good diagnostic yield and identifies a wide range of cancers, according to a preliminary analysis of the ongoing LIFSCREEN phase 3, randomized, controlled trial.
Investigators led by Olivier Caron, MD, chair of the oncogenetics committee, department of medical oncology, at the Gustave Roussy University Hospital in Villejuif, France, enrolled in the trial 107 individuals from 75 families carrying a TP53 mutation, a genetic aberration commonly present in LFS that confers heightened risk of a variety of malignancies.
Participants had a median age at baseline of 32.9 years, with a range from 5 to 67 years. Fully 98% had a family history of cancer, and 48% had a personal history of cancer.
The participants were assigned to 5 years of standard screening – annual clinical examination, abdomen and pelvis ultrasound, brain MRI, complete blood cell count, and, for women older than 20 years, breast ultrasound and MRI – or intensive screening, entailing the addition of diffusion whole-body MRI.
At the time of the preliminary analysis, 15 patients had undergone only one round of screening; 35, two rounds; 19, three rounds; 24, four rounds; and 7, five rounds, Dr. Caron and associates reported in a research letter (JAMA Oncol. 2017; Aug 3 doi: 10.1001/jamaoncol.2017.1358).
Collectively, this amounted to 226.4 person-years of follow-up.
Screening with either trial strategy (with or without whole-body MRI) led to diagnosis of 23 new primary cancers in 20 patients. Nearly half of the total (12 cancers) were detected at the first round. Patients had a median age of 39.8 at the new cancer diagnosis, with a range from 6 to 70 years.
Of the new cancers, 10 belonged to the core LFS spectrum of breast cancer, sarcoma, and brain tumors. However, the other 13 were outside that spectrum, for example, lung adenocarcinomas, all seen in never or light smokers, and leukemias. Screening also detected three relapses of previous cancers.
Analyses further showed that prior cancer diagnosis was not a reliable marker for risk of new primaries. Although 12 of the patients with a screening-detected new primary had a personal cancer history, 8 did not (P = .22).
“The proportion and diversity of off–core LFS spectrum cancers detected in TP53 mutation carriers as reported by others give growing evidence of a broader LFS spectrum, in agreement with the permissive role of TP53 mutations,” write Dr. Caron and colleagues, who report having no relevant disclosures. “Our observations seem to support recent moves toward broader cancer screening in TP53 mutation carriers.”
The investigators continue to collect data in LIFSCREEN and plan to undertake main analysis later this year. “Our final analysis will help to determine the benefits and drawbacks (mostly related to false-positive test results) of whole-body MRI in TP53 mutation carrier surveillance,” they conclude. “Studies focused on TP53 mutation penetrance, using methods limiting selection bias, are required to refine cancer risks to improve TP53 mutation carrier management.”
Broader cancer screening of individuals with Li-Fraumeni syndrome (LFS), with or without whole-body magnetic resonance imaging, has a good diagnostic yield and identifies a wide range of cancers, according to a preliminary analysis of the ongoing LIFSCREEN phase 3, randomized, controlled trial.
Investigators led by Olivier Caron, MD, chair of the oncogenetics committee, department of medical oncology, at the Gustave Roussy University Hospital in Villejuif, France, enrolled in the trial 107 individuals from 75 families carrying a TP53 mutation, a genetic aberration commonly present in LFS that confers heightened risk of a variety of malignancies.
Participants had a median age at baseline of 32.9 years, with a range from 5 to 67 years. Fully 98% had a family history of cancer, and 48% had a personal history of cancer.
The participants were assigned to 5 years of standard screening – annual clinical examination, abdomen and pelvis ultrasound, brain MRI, complete blood cell count, and, for women older than 20 years, breast ultrasound and MRI – or intensive screening, entailing the addition of diffusion whole-body MRI.
At the time of the preliminary analysis, 15 patients had undergone only one round of screening; 35, two rounds; 19, three rounds; 24, four rounds; and 7, five rounds, Dr. Caron and associates reported in a research letter (JAMA Oncol. 2017; Aug 3 doi: 10.1001/jamaoncol.2017.1358).
Collectively, this amounted to 226.4 person-years of follow-up.
Screening with either trial strategy (with or without whole-body MRI) led to diagnosis of 23 new primary cancers in 20 patients. Nearly half of the total (12 cancers) were detected at the first round. Patients had a median age of 39.8 at the new cancer diagnosis, with a range from 6 to 70 years.
Of the new cancers, 10 belonged to the core LFS spectrum of breast cancer, sarcoma, and brain tumors. However, the other 13 were outside that spectrum, for example, lung adenocarcinomas, all seen in never or light smokers, and leukemias. Screening also detected three relapses of previous cancers.
Analyses further showed that prior cancer diagnosis was not a reliable marker for risk of new primaries. Although 12 of the patients with a screening-detected new primary had a personal cancer history, 8 did not (P = .22).
“The proportion and diversity of off–core LFS spectrum cancers detected in TP53 mutation carriers as reported by others give growing evidence of a broader LFS spectrum, in agreement with the permissive role of TP53 mutations,” write Dr. Caron and colleagues, who report having no relevant disclosures. “Our observations seem to support recent moves toward broader cancer screening in TP53 mutation carriers.”
The investigators continue to collect data in LIFSCREEN and plan to undertake main analysis later this year. “Our final analysis will help to determine the benefits and drawbacks (mostly related to false-positive test results) of whole-body MRI in TP53 mutation carrier surveillance,” they conclude. “Studies focused on TP53 mutation penetrance, using methods limiting selection bias, are required to refine cancer risks to improve TP53 mutation carrier management.”
Broader cancer screening of individuals with Li-Fraumeni syndrome (LFS), with or without whole-body magnetic resonance imaging, has a good diagnostic yield and identifies a wide range of cancers, according to a preliminary analysis of the ongoing LIFSCREEN phase 3, randomized, controlled trial.
Investigators led by Olivier Caron, MD, chair of the oncogenetics committee, department of medical oncology, at the Gustave Roussy University Hospital in Villejuif, France, enrolled in the trial 107 individuals from 75 families carrying a TP53 mutation, a genetic aberration commonly present in LFS that confers heightened risk of a variety of malignancies.
Participants had a median age at baseline of 32.9 years, with a range from 5 to 67 years. Fully 98% had a family history of cancer, and 48% had a personal history of cancer.
The participants were assigned to 5 years of standard screening – annual clinical examination, abdomen and pelvis ultrasound, brain MRI, complete blood cell count, and, for women older than 20 years, breast ultrasound and MRI – or intensive screening, entailing the addition of diffusion whole-body MRI.
At the time of the preliminary analysis, 15 patients had undergone only one round of screening; 35, two rounds; 19, three rounds; 24, four rounds; and 7, five rounds, Dr. Caron and associates reported in a research letter (JAMA Oncol. 2017; Aug 3 doi: 10.1001/jamaoncol.2017.1358).
Collectively, this amounted to 226.4 person-years of follow-up.
Screening with either trial strategy (with or without whole-body MRI) led to diagnosis of 23 new primary cancers in 20 patients. Nearly half of the total (12 cancers) were detected at the first round. Patients had a median age of 39.8 at the new cancer diagnosis, with a range from 6 to 70 years.
Of the new cancers, 10 belonged to the core LFS spectrum of breast cancer, sarcoma, and brain tumors. However, the other 13 were outside that spectrum, for example, lung adenocarcinomas, all seen in never or light smokers, and leukemias. Screening also detected three relapses of previous cancers.
Analyses further showed that prior cancer diagnosis was not a reliable marker for risk of new primaries. Although 12 of the patients with a screening-detected new primary had a personal cancer history, 8 did not (P = .22).
“The proportion and diversity of off–core LFS spectrum cancers detected in TP53 mutation carriers as reported by others give growing evidence of a broader LFS spectrum, in agreement with the permissive role of TP53 mutations,” write Dr. Caron and colleagues, who report having no relevant disclosures. “Our observations seem to support recent moves toward broader cancer screening in TP53 mutation carriers.”
The investigators continue to collect data in LIFSCREEN and plan to undertake main analysis later this year. “Our final analysis will help to determine the benefits and drawbacks (mostly related to false-positive test results) of whole-body MRI in TP53 mutation carrier surveillance,” they conclude. “Studies focused on TP53 mutation penetrance, using methods limiting selection bias, are required to refine cancer risks to improve TP53 mutation carrier management.”
FROM JAMA ONCOLOGY
Key clinical point:
Major finding: A total of 23 new primary cancers were diagnosed in 20 patients; more than half were outside the core spectrum of Li-Fraumeni syndrome.
Data source: A preliminary analysis of a phase 3, randomized, controlled trial comparing standard and intensive screening among 107 individuals with Li-Fraumeni syndrome carrying a TP53 mutation (LIFSCREEN trial).
Disclosures: The investigators report having no relevant disclosures. The trial was funded by the French Ligue Contre le Cancer.
Breast Cancer Risk Assessment and Chemoprevention Use Among VA Primary Care
Background: Despite recommended guidelines and available medications to reduce breast cancer risk by up to 50-65%, < 5% of the 10 million eligible women are offered chemoprevention in the U.S. The comfort level, practice patterns, and barriers to breast cancer risk assessment and chemoprevention use within the VA have not been reported.
Methods: We assessed VA primary care providers using a REDcap survey. We obtained provider demographics, use and comfort level with breast cancer risk models and chemoprevention, and knowledge about chemoprevention. Data was analyzed with Fisher exact or chi-square tests.
Results: Of the 200 survey respondents, 167 were included for analysis. Overall, 30% used the Gail model monthly or more often, and 1.5% prescribed chemoprevention in the past 2 years. Fewer than 30% correctly answered chemoprevention knowledge questions. Designated women’s
health providers were more comfortable with risk assessment and chemoprevention (P < .046, P < .004) and used risk models more often (P < .045). 63% expressed interest in education about breast cancer prevention.
Conclusions: Breast cancer risk assessment and chemoprevention use by VA primary care is limited by lack of comfort and familiarity. Women‘s health providers are more comfortable and knowledgeable about breast cancer risk models and chemoprevention, offering an opportunity for partnership with high-risk oncologists to improve breast cancer risk assessment and chemoprevention use among female Veterans.
Background: Despite recommended guidelines and available medications to reduce breast cancer risk by up to 50-65%, < 5% of the 10 million eligible women are offered chemoprevention in the U.S. The comfort level, practice patterns, and barriers to breast cancer risk assessment and chemoprevention use within the VA have not been reported.
Methods: We assessed VA primary care providers using a REDcap survey. We obtained provider demographics, use and comfort level with breast cancer risk models and chemoprevention, and knowledge about chemoprevention. Data was analyzed with Fisher exact or chi-square tests.
Results: Of the 200 survey respondents, 167 were included for analysis. Overall, 30% used the Gail model monthly or more often, and 1.5% prescribed chemoprevention in the past 2 years. Fewer than 30% correctly answered chemoprevention knowledge questions. Designated women’s
health providers were more comfortable with risk assessment and chemoprevention (P < .046, P < .004) and used risk models more often (P < .045). 63% expressed interest in education about breast cancer prevention.
Conclusions: Breast cancer risk assessment and chemoprevention use by VA primary care is limited by lack of comfort and familiarity. Women‘s health providers are more comfortable and knowledgeable about breast cancer risk models and chemoprevention, offering an opportunity for partnership with high-risk oncologists to improve breast cancer risk assessment and chemoprevention use among female Veterans.
Background: Despite recommended guidelines and available medications to reduce breast cancer risk by up to 50-65%, < 5% of the 10 million eligible women are offered chemoprevention in the U.S. The comfort level, practice patterns, and barriers to breast cancer risk assessment and chemoprevention use within the VA have not been reported.
Methods: We assessed VA primary care providers using a REDcap survey. We obtained provider demographics, use and comfort level with breast cancer risk models and chemoprevention, and knowledge about chemoprevention. Data was analyzed with Fisher exact or chi-square tests.
Results: Of the 200 survey respondents, 167 were included for analysis. Overall, 30% used the Gail model monthly or more often, and 1.5% prescribed chemoprevention in the past 2 years. Fewer than 30% correctly answered chemoprevention knowledge questions. Designated women’s
health providers were more comfortable with risk assessment and chemoprevention (P < .046, P < .004) and used risk models more often (P < .045). 63% expressed interest in education about breast cancer prevention.
Conclusions: Breast cancer risk assessment and chemoprevention use by VA primary care is limited by lack of comfort and familiarity. Women‘s health providers are more comfortable and knowledgeable about breast cancer risk models and chemoprevention, offering an opportunity for partnership with high-risk oncologists to improve breast cancer risk assessment and chemoprevention use among female Veterans.
Women Living Longer With Metastatic Breast Cancer
More women are living longer with distant metastatic breast cancer (MBC), according to a National Cancer Institute study. Between 1992-1994 and 2005-2012, 5-year relative survival among women who were diagnosed with MBC at ages 15 to 49 doubled, from 18% to 36%.
Researchers also found that relative survival time increased from 22.3 months to 38.7 months for women diagnosed aged 15 -49 years, and from 19.1 months to 29.7 months for those aged 50 – 64 years.
Moreover, a “small but meaningful” number of women are living years after an initial diagnosis of MBC, the study found. More than 11% of women diagnosed between 2000-2004 aged < 64 years survived ≥ 10 years. Although nearly half of women with MBC have had it for ≤ 2 , one third have lived with it for ≥ 5 years.
The study findings “make clear that the majority of MBC patients, those who are diagnosed with non-metastatic cancer but progress to distant disease, have never been properly documented,” said Angela Mariotto, PhD, chief of the NCI Data Analytics Branch of the Division of Cancer Control and Population Sciences. By including women with recurrence, the study provides a more accurate number of women in the U.S. living with MBC, which can help with health care planning.
More women are living longer with distant metastatic breast cancer (MBC), according to a National Cancer Institute study. Between 1992-1994 and 2005-2012, 5-year relative survival among women who were diagnosed with MBC at ages 15 to 49 doubled, from 18% to 36%.
Researchers also found that relative survival time increased from 22.3 months to 38.7 months for women diagnosed aged 15 -49 years, and from 19.1 months to 29.7 months for those aged 50 – 64 years.
Moreover, a “small but meaningful” number of women are living years after an initial diagnosis of MBC, the study found. More than 11% of women diagnosed between 2000-2004 aged < 64 years survived ≥ 10 years. Although nearly half of women with MBC have had it for ≤ 2 , one third have lived with it for ≥ 5 years.
The study findings “make clear that the majority of MBC patients, those who are diagnosed with non-metastatic cancer but progress to distant disease, have never been properly documented,” said Angela Mariotto, PhD, chief of the NCI Data Analytics Branch of the Division of Cancer Control and Population Sciences. By including women with recurrence, the study provides a more accurate number of women in the U.S. living with MBC, which can help with health care planning.
More women are living longer with distant metastatic breast cancer (MBC), according to a National Cancer Institute study. Between 1992-1994 and 2005-2012, 5-year relative survival among women who were diagnosed with MBC at ages 15 to 49 doubled, from 18% to 36%.
Researchers also found that relative survival time increased from 22.3 months to 38.7 months for women diagnosed aged 15 -49 years, and from 19.1 months to 29.7 months for those aged 50 – 64 years.
Moreover, a “small but meaningful” number of women are living years after an initial diagnosis of MBC, the study found. More than 11% of women diagnosed between 2000-2004 aged < 64 years survived ≥ 10 years. Although nearly half of women with MBC have had it for ≤ 2 , one third have lived with it for ≥ 5 years.
The study findings “make clear that the majority of MBC patients, those who are diagnosed with non-metastatic cancer but progress to distant disease, have never been properly documented,” said Angela Mariotto, PhD, chief of the NCI Data Analytics Branch of the Division of Cancer Control and Population Sciences. By including women with recurrence, the study provides a more accurate number of women in the U.S. living with MBC, which can help with health care planning.
FDA approves neratinib for extended adjuvant treatment of HER2+ breast cancer
The Food and Drug Administration has approved neratinib, an oral tyrosine kinase inhibitor, for the extended adjuvant treatment of patients with early-stage, HER2-positive breast cancer who have previously been treated with trastuzumab.
Approval was based on improved invasive disease-free survival (iDFS) in the phase 3 ExteNET trial of 2,840 women with early-stage HER2-positive breast cancer who were within 2 years of completing adjuvant trastuzumab. Patients were randomized to receive either neratinib or placebo daily for 1 year. After 2 years of follow-up, iDFS was 94.2% in patients treated with neratinib, compared with 91.9% in those receiving placebo (hazard ratio, 0.66; 95% confidence interval, 0.49, 0.90; P = .008), according to the FDA statement.
The most common adverse reactions to neratinib in ExteNET were diarrhea, nausea, abdominal pain, fatigue, vomiting, rash, stomatitis, decreased appetite, muscle spasms, dyspepsia, AST or ALT increase, nail disorder, dry skin, abdominal distention, weight loss, and urinary tract infection. Diarrhea was observed in 16.8% of neratinib-treated patients.
The recommended dose of of neratinib is 240 mg (six 40 mg tablets) given orally once daily with food, continuously, for 1 year. Patients should be given antidiarrheal prophylaxis for the first 56 days of treatment with neratinib and as needed thereafter to help manage diarrhea, the FDA said.
The Food and Drug Administration has approved neratinib, an oral tyrosine kinase inhibitor, for the extended adjuvant treatment of patients with early-stage, HER2-positive breast cancer who have previously been treated with trastuzumab.
Approval was based on improved invasive disease-free survival (iDFS) in the phase 3 ExteNET trial of 2,840 women with early-stage HER2-positive breast cancer who were within 2 years of completing adjuvant trastuzumab. Patients were randomized to receive either neratinib or placebo daily for 1 year. After 2 years of follow-up, iDFS was 94.2% in patients treated with neratinib, compared with 91.9% in those receiving placebo (hazard ratio, 0.66; 95% confidence interval, 0.49, 0.90; P = .008), according to the FDA statement.
The most common adverse reactions to neratinib in ExteNET were diarrhea, nausea, abdominal pain, fatigue, vomiting, rash, stomatitis, decreased appetite, muscle spasms, dyspepsia, AST or ALT increase, nail disorder, dry skin, abdominal distention, weight loss, and urinary tract infection. Diarrhea was observed in 16.8% of neratinib-treated patients.
The recommended dose of of neratinib is 240 mg (six 40 mg tablets) given orally once daily with food, continuously, for 1 year. Patients should be given antidiarrheal prophylaxis for the first 56 days of treatment with neratinib and as needed thereafter to help manage diarrhea, the FDA said.
The Food and Drug Administration has approved neratinib, an oral tyrosine kinase inhibitor, for the extended adjuvant treatment of patients with early-stage, HER2-positive breast cancer who have previously been treated with trastuzumab.
Approval was based on improved invasive disease-free survival (iDFS) in the phase 3 ExteNET trial of 2,840 women with early-stage HER2-positive breast cancer who were within 2 years of completing adjuvant trastuzumab. Patients were randomized to receive either neratinib or placebo daily for 1 year. After 2 years of follow-up, iDFS was 94.2% in patients treated with neratinib, compared with 91.9% in those receiving placebo (hazard ratio, 0.66; 95% confidence interval, 0.49, 0.90; P = .008), according to the FDA statement.
The most common adverse reactions to neratinib in ExteNET were diarrhea, nausea, abdominal pain, fatigue, vomiting, rash, stomatitis, decreased appetite, muscle spasms, dyspepsia, AST or ALT increase, nail disorder, dry skin, abdominal distention, weight loss, and urinary tract infection. Diarrhea was observed in 16.8% of neratinib-treated patients.
The recommended dose of of neratinib is 240 mg (six 40 mg tablets) given orally once daily with food, continuously, for 1 year. Patients should be given antidiarrheal prophylaxis for the first 56 days of treatment with neratinib and as needed thereafter to help manage diarrhea, the FDA said.