User login
Novel calculator predicts cancer risk in patients with CVD
Individualized 10-year and lifetime risks of cancer can now for the first time be estimated in patients with established cardiovascular disease, Cilie C. van ’t Klooster, MD, reported at the virtual annual congress of the European Society of Cardiology.
She and her coinvestigators have developed an easy-to-use predictive model that generates individualized risk estimates for total cancer, lung cancer, and colorectal cancer. The tool relies on nine readily available clinical variables: age, sex, smoking, weight, height, alcohol use, diabetes, antiplatelet drug use, and C-reactive protein level. The cancer risk calculator factors in an individual’s competing risk of death because of cardiovascular disease (CVD).
The risk calculator was developed using data on 7,280 patients with established CVD enrolled in the ongoing long-term Dutch UCC-SMART (Utrecht Cardiovascular Cohort – Second Manifestations of Arterial Disease) study, then independently validated in 9,322 patients in the double-blind CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes) trial, explained Dr. van ’t Klooster of Utrecht (the Netherlands) University.
Several other prediction models estimate the risk of a specific type of cancer, most commonly breast cancer or lung cancer. But the new Utrecht prediction tool is the first one to estimate total cancer risk. It’s also the first to apply specifically to patients with known CVD, thus filling an unmet need, because patients with established CVD are known to be on average at 19% increased risk of total cancer and 56% greater risk for lung cancer, compared with the general population. This is thought to be caused mainly by shared risk factors, including smoking, obesity, and low-grade systemic inflammation.
As the Utrecht/CANTOS analysis shows, however, that 19% increased relative risk for cancer in patients with CVD doesn’t tell the whole story. While the median lifetime and 10-year risks of total cancer in CANTOS were 26% and 10%, respectively, the individual patient risks for total cancer estimated using the Dutch prediction model ranged from 1% to 52% for lifetime and from 1% to 31% for 10-year risk. The same was true for lung cancer risk: median 5% lifetime and 2% 10-year risks, with individual patient risks ranging from 0% to 37% and from 0% to 24%. Likewise for colorectal cancer: a median 4% lifetime risk, ranging from 0% to 6%, and a median 2% risk over the next 10 years, with personalized risks ranging as high as 13% for lifetime risk and 6% for 10-year colorectal cancer risk.
The risk calculator performed “reasonably well,” according to Dr. van ’t Klooster. She pointed to a C-statistic of 0.74 for lung cancer, 0.63 for total cancer, and 0.64 for colorectal cancer. It’s possible the risk predictor’s performance could be further enhanced by incorporation of several potentially important factors that weren’t available in the UCC-SMART derivation cohort, including race, education level, and socioeconomic status, she added.
Potential applications for the risk calculator in clinical practice require further study, but include using the lifetime risk prediction for cancer as a motivational aid in conversations with patients about the importance of behavioral change in support of a healthier lifestyle. Also, a high predicted 10-year lung cancer risk could potentially be used to lower the threshold for a screening chest CT, resulting in earlier detection and treatment of lung cancer, Dr. van ’t Klooster noted.
In an interview, Bonnie Ky, MD, MSCE, praised the risk prediction study as rigorously executed, topical, and clinically significant.
“This paper signifies the overlap between our two disciplines of cancer and cardiovascular disease in terms of the risks that we face together when we care for this patient population,” said Dr. Ky, a cardiologist at the University of Pennsylvania, Philadelphia.
“Many of us in medicine believe in the importance of risk prediction: identifying who’s at high risk and doing everything we can to mitigate that risk. This paper speaks to that and moves us one step closer to accomplishing that aim,” added Dr. Ky, who is editor in chief of JACC: CardioOncology, which published the study simultaneously with Dr. van ’t Klooster’s presentation at ESC 2020. The paper provides direct access to the risk calculator.
Dr. van ’t Klooster reported having no financial conflicts regarding her study. UCC-SMART is funded by a Utrecht University grant, and CANTOS was funded by Novartis.
SOURCE: van ’t Klooster CC. ESC 2020 and JACC CardioOncol. 2020 Aug. doi: 10.1016/j.jaccao.2020.07.001.
Individualized 10-year and lifetime risks of cancer can now for the first time be estimated in patients with established cardiovascular disease, Cilie C. van ’t Klooster, MD, reported at the virtual annual congress of the European Society of Cardiology.
She and her coinvestigators have developed an easy-to-use predictive model that generates individualized risk estimates for total cancer, lung cancer, and colorectal cancer. The tool relies on nine readily available clinical variables: age, sex, smoking, weight, height, alcohol use, diabetes, antiplatelet drug use, and C-reactive protein level. The cancer risk calculator factors in an individual’s competing risk of death because of cardiovascular disease (CVD).
The risk calculator was developed using data on 7,280 patients with established CVD enrolled in the ongoing long-term Dutch UCC-SMART (Utrecht Cardiovascular Cohort – Second Manifestations of Arterial Disease) study, then independently validated in 9,322 patients in the double-blind CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes) trial, explained Dr. van ’t Klooster of Utrecht (the Netherlands) University.
Several other prediction models estimate the risk of a specific type of cancer, most commonly breast cancer or lung cancer. But the new Utrecht prediction tool is the first one to estimate total cancer risk. It’s also the first to apply specifically to patients with known CVD, thus filling an unmet need, because patients with established CVD are known to be on average at 19% increased risk of total cancer and 56% greater risk for lung cancer, compared with the general population. This is thought to be caused mainly by shared risk factors, including smoking, obesity, and low-grade systemic inflammation.
As the Utrecht/CANTOS analysis shows, however, that 19% increased relative risk for cancer in patients with CVD doesn’t tell the whole story. While the median lifetime and 10-year risks of total cancer in CANTOS were 26% and 10%, respectively, the individual patient risks for total cancer estimated using the Dutch prediction model ranged from 1% to 52% for lifetime and from 1% to 31% for 10-year risk. The same was true for lung cancer risk: median 5% lifetime and 2% 10-year risks, with individual patient risks ranging from 0% to 37% and from 0% to 24%. Likewise for colorectal cancer: a median 4% lifetime risk, ranging from 0% to 6%, and a median 2% risk over the next 10 years, with personalized risks ranging as high as 13% for lifetime risk and 6% for 10-year colorectal cancer risk.
The risk calculator performed “reasonably well,” according to Dr. van ’t Klooster. She pointed to a C-statistic of 0.74 for lung cancer, 0.63 for total cancer, and 0.64 for colorectal cancer. It’s possible the risk predictor’s performance could be further enhanced by incorporation of several potentially important factors that weren’t available in the UCC-SMART derivation cohort, including race, education level, and socioeconomic status, she added.
Potential applications for the risk calculator in clinical practice require further study, but include using the lifetime risk prediction for cancer as a motivational aid in conversations with patients about the importance of behavioral change in support of a healthier lifestyle. Also, a high predicted 10-year lung cancer risk could potentially be used to lower the threshold for a screening chest CT, resulting in earlier detection and treatment of lung cancer, Dr. van ’t Klooster noted.
In an interview, Bonnie Ky, MD, MSCE, praised the risk prediction study as rigorously executed, topical, and clinically significant.
“This paper signifies the overlap between our two disciplines of cancer and cardiovascular disease in terms of the risks that we face together when we care for this patient population,” said Dr. Ky, a cardiologist at the University of Pennsylvania, Philadelphia.
“Many of us in medicine believe in the importance of risk prediction: identifying who’s at high risk and doing everything we can to mitigate that risk. This paper speaks to that and moves us one step closer to accomplishing that aim,” added Dr. Ky, who is editor in chief of JACC: CardioOncology, which published the study simultaneously with Dr. van ’t Klooster’s presentation at ESC 2020. The paper provides direct access to the risk calculator.
Dr. van ’t Klooster reported having no financial conflicts regarding her study. UCC-SMART is funded by a Utrecht University grant, and CANTOS was funded by Novartis.
SOURCE: van ’t Klooster CC. ESC 2020 and JACC CardioOncol. 2020 Aug. doi: 10.1016/j.jaccao.2020.07.001.
Individualized 10-year and lifetime risks of cancer can now for the first time be estimated in patients with established cardiovascular disease, Cilie C. van ’t Klooster, MD, reported at the virtual annual congress of the European Society of Cardiology.
She and her coinvestigators have developed an easy-to-use predictive model that generates individualized risk estimates for total cancer, lung cancer, and colorectal cancer. The tool relies on nine readily available clinical variables: age, sex, smoking, weight, height, alcohol use, diabetes, antiplatelet drug use, and C-reactive protein level. The cancer risk calculator factors in an individual’s competing risk of death because of cardiovascular disease (CVD).
The risk calculator was developed using data on 7,280 patients with established CVD enrolled in the ongoing long-term Dutch UCC-SMART (Utrecht Cardiovascular Cohort – Second Manifestations of Arterial Disease) study, then independently validated in 9,322 patients in the double-blind CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes) trial, explained Dr. van ’t Klooster of Utrecht (the Netherlands) University.
Several other prediction models estimate the risk of a specific type of cancer, most commonly breast cancer or lung cancer. But the new Utrecht prediction tool is the first one to estimate total cancer risk. It’s also the first to apply specifically to patients with known CVD, thus filling an unmet need, because patients with established CVD are known to be on average at 19% increased risk of total cancer and 56% greater risk for lung cancer, compared with the general population. This is thought to be caused mainly by shared risk factors, including smoking, obesity, and low-grade systemic inflammation.
As the Utrecht/CANTOS analysis shows, however, that 19% increased relative risk for cancer in patients with CVD doesn’t tell the whole story. While the median lifetime and 10-year risks of total cancer in CANTOS were 26% and 10%, respectively, the individual patient risks for total cancer estimated using the Dutch prediction model ranged from 1% to 52% for lifetime and from 1% to 31% for 10-year risk. The same was true for lung cancer risk: median 5% lifetime and 2% 10-year risks, with individual patient risks ranging from 0% to 37% and from 0% to 24%. Likewise for colorectal cancer: a median 4% lifetime risk, ranging from 0% to 6%, and a median 2% risk over the next 10 years, with personalized risks ranging as high as 13% for lifetime risk and 6% for 10-year colorectal cancer risk.
The risk calculator performed “reasonably well,” according to Dr. van ’t Klooster. She pointed to a C-statistic of 0.74 for lung cancer, 0.63 for total cancer, and 0.64 for colorectal cancer. It’s possible the risk predictor’s performance could be further enhanced by incorporation of several potentially important factors that weren’t available in the UCC-SMART derivation cohort, including race, education level, and socioeconomic status, she added.
Potential applications for the risk calculator in clinical practice require further study, but include using the lifetime risk prediction for cancer as a motivational aid in conversations with patients about the importance of behavioral change in support of a healthier lifestyle. Also, a high predicted 10-year lung cancer risk could potentially be used to lower the threshold for a screening chest CT, resulting in earlier detection and treatment of lung cancer, Dr. van ’t Klooster noted.
In an interview, Bonnie Ky, MD, MSCE, praised the risk prediction study as rigorously executed, topical, and clinically significant.
“This paper signifies the overlap between our two disciplines of cancer and cardiovascular disease in terms of the risks that we face together when we care for this patient population,” said Dr. Ky, a cardiologist at the University of Pennsylvania, Philadelphia.
“Many of us in medicine believe in the importance of risk prediction: identifying who’s at high risk and doing everything we can to mitigate that risk. This paper speaks to that and moves us one step closer to accomplishing that aim,” added Dr. Ky, who is editor in chief of JACC: CardioOncology, which published the study simultaneously with Dr. van ’t Klooster’s presentation at ESC 2020. The paper provides direct access to the risk calculator.
Dr. van ’t Klooster reported having no financial conflicts regarding her study. UCC-SMART is funded by a Utrecht University grant, and CANTOS was funded by Novartis.
SOURCE: van ’t Klooster CC. ESC 2020 and JACC CardioOncol. 2020 Aug. doi: 10.1016/j.jaccao.2020.07.001.
FROM ESC CONGRESS 2020
The earlier the better for colchicine post-MI: COLCOT
The earlier the anti-inflammatory drug colchicine is initiated after a myocardial infarction (MI) the greater the benefit, a new COLCOT analysis suggests.
The parent trial was conducted in patients with a recent MI because of the intense inflammation present at that time, and added colchicine 0.5 mg daily to standard care within 30 days following MI.
As previously reported, colchicine significantly reduced the risk of the primary end point – a composite of cardiovascular (CV) death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring revascularization – by 23% compared with placebo.
This new analysis shows the risk was reduced by 48% in patients receiving colchicine within 3 days of an MI (4.3% vs. 8.3%; adjusted hazard ratio, 0.52; 95% confidence interval, 0.32-0.84, P = .007).
Risk of a secondary efficacy end point – CV death, resuscitated cardiac arrest, MI, or stroke – was reduced by 45% over an average follow up of 22.7 months (3.3% vs 6.1%; adjusted HR, 0.55; 95% CI, 0.32-0.95, P = .031).
“We believe that our results support an early, in-hospital initiation of adjunctive colchicine for post-MI prevention,” Nadia Bouabdallaoui, MD, Montreal Heart Institute, Quebec, Canada, said during an online session devoted to colchicine at the European Society of Cardiology Congress 2020.
Session moderator Massimo Imazio, MD, professor of cardiology at the University of Turin, Italy, said the improved outcomes suggest that earlier treatment is better – a finding that parallels his own experience using colchicine in patients with pericarditis.
“This substudy is very important because this is probably also the year in cardiovascular applications [that] early use of the drug could improve outcomes,” he said.
Positive data have been accumulating for colchicine from COLCOT, LoDoCo, and, most recently, the LoDoCo2 trial, even as another anti-inflammatory drug, methotrexate, flamed out as secondary prevention in the CIRT trial.
The new COLCOT substudy included 4,661 of the 4,745 original patients and examined treatment initiation using three strata: within 0-3 days (n = 1,193), 4-7 days (n = 720), and 8-30 days (n = 2,748). Patients who received treatment within 3 days were slightly younger, more likely to be smokers, and to have a shorter time from MI to randomization (2.1 days vs 5.1 days vs. 20.8 days, respectively).
In the subset receiving treatment within 3 days, those assigned to colchicine had the same number of cardiac deaths as those given placebo (2 vs. 2) but fewer resuscitated cardiac arrests (1 vs. 3), MIs (17 vs. 29), strokes (1 vs. 5), and urgent hospitalizations for angina requiring revascularization (6 vs. 17).
“A larger trial might have allowed for a better assessment of individual endpoints and subgroups,” observed Bouabdallaoui.
Although there is growing support for colchicine, experts caution that the drug many not be for everyone. In COLCOT, 1 in 10 patients were unable to tolerate the drug, largely because of gastrointestinal (GI) issues.
Pharmacogenomics substudy
A second COLCOT substudy aimed to identify genetic markers predictive of colchicine response and to gain insights into the mechanisms behind this response. It included 767 patients treated with colchicine and another 755 treated with placebo – or about one-third the patients in the original trial.
A genome-wide association study did not find a significant association for the primary CV endpoint, although a prespecified subgroup analysis in men identified an interesting region on chromosome 9 (variant: rs10811106), which just missed reaching genomewide significance, said Marie-Pierre Dubé, PhD, director of the Université de Montréal Beaulieu-Saucier Pharmacogenomics Centre at the Montreal Heart Institute.
In addition, the genomewide analysis found two significant regions for GI events: one on chromosome 6 (variant: rs6916345) and one on chromosome 10 (variant: rs74795203).
For each of the identified regions, the researchers then tested the effect of the allele in the placebo group and the interaction between the genetic variant and treatment with colchicine. For the chromosome 9 region in males, there was no effect in the placebo group and a significant interaction in the colchicine group.
For the significant GI event findings, there was a small effect for the chromosome 6 region in the placebo group and a very significant interaction with colchicine, Dubé said. Similarly, there was no effect for the chromosome 10 region in the placebo group and a significant interaction with colchicine.
Additional analyses in stratified patient populations showed that males with the protective allele (CC) for the chromosome 9 region represented 83% of the population. The primary CV endpoint occurred in 3.2% of these men treated with colchicine and 6.3% treated with placebo (HR, 0.46; 95% CI, 0.24 - 0.86).
For the gastrointestinal events, 25% of patients carried the risk allele (AA) for the chromosome 6 region and 36.9% of these had GI events when treated with colchicine versus 18.6% when treated with placebo (HR, 2.42; 95% CI, 1.57-3.72).
Similarly, 13% of individuals carried one or two copies of the risk allele (AG+GG) for the chromosome 10 region and the risk of GI events in these was nearly four times higher with colchicine (47.1% vs. 18.9%; HR, 3.98; 95% CI 2.24-7.07).
Functional genomic analyses of the identified regions were also performed and showed that the chromosome 9 locus overlaps with the SAXO1 gene, a stabilizer of axonemal microtubules 1.
“The leading variant at this locus (rs10811106 C allele) correlated with the expression of the HAUS6 gene, which is involved in microtubule generation from existing microtubules, and may interact with the effect of colchicine, which is known to inhibit microtubule formation,” observed Dubé.
Also, the chromosome 6 locus associated with gastrointestinal events was colocalizing with the Crohn’s disease locus, adding further support for this region.
“The results support potential personalized approaches to inflammation reduction for cardiovascular prevention,” Dubé said.
This is a post hoc subgroup analysis, however, and replication is necessary, ideally in prospective randomized trials, she noted.
The substudy is important because it provides further insights into the link between colchicine and microtubule polymerization, affecting the activation of the inflammasome, session moderator Imazio said.
“Second, it is important because pharmacogenomics can help us to better understand the optimal responder to colchicine and colchicine resistance,” he said. “So it can be useful for personalized medicine, leading to the proper use of the drug for the proper patient.”
COLCOT was supported by the government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Bouabdallaoui has disclosed no relevant financial relationships. Dubé reported grants from the government of Quebec; personal fees from DalCor and GlaxoSmithKline; research support from AstraZeneca, Pfizer, Servier, Sanofi; and minor equity interest in DalCor. Dubé is also coauthor of patents on pharmacogenomics-guided CETP inhibition, and pharmacogenomics markers of response to colchicine.
This article first appeared on Medscape.com.
The earlier the anti-inflammatory drug colchicine is initiated after a myocardial infarction (MI) the greater the benefit, a new COLCOT analysis suggests.
The parent trial was conducted in patients with a recent MI because of the intense inflammation present at that time, and added colchicine 0.5 mg daily to standard care within 30 days following MI.
As previously reported, colchicine significantly reduced the risk of the primary end point – a composite of cardiovascular (CV) death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring revascularization – by 23% compared with placebo.
This new analysis shows the risk was reduced by 48% in patients receiving colchicine within 3 days of an MI (4.3% vs. 8.3%; adjusted hazard ratio, 0.52; 95% confidence interval, 0.32-0.84, P = .007).
Risk of a secondary efficacy end point – CV death, resuscitated cardiac arrest, MI, or stroke – was reduced by 45% over an average follow up of 22.7 months (3.3% vs 6.1%; adjusted HR, 0.55; 95% CI, 0.32-0.95, P = .031).
“We believe that our results support an early, in-hospital initiation of adjunctive colchicine for post-MI prevention,” Nadia Bouabdallaoui, MD, Montreal Heart Institute, Quebec, Canada, said during an online session devoted to colchicine at the European Society of Cardiology Congress 2020.
Session moderator Massimo Imazio, MD, professor of cardiology at the University of Turin, Italy, said the improved outcomes suggest that earlier treatment is better – a finding that parallels his own experience using colchicine in patients with pericarditis.
“This substudy is very important because this is probably also the year in cardiovascular applications [that] early use of the drug could improve outcomes,” he said.
Positive data have been accumulating for colchicine from COLCOT, LoDoCo, and, most recently, the LoDoCo2 trial, even as another anti-inflammatory drug, methotrexate, flamed out as secondary prevention in the CIRT trial.
The new COLCOT substudy included 4,661 of the 4,745 original patients and examined treatment initiation using three strata: within 0-3 days (n = 1,193), 4-7 days (n = 720), and 8-30 days (n = 2,748). Patients who received treatment within 3 days were slightly younger, more likely to be smokers, and to have a shorter time from MI to randomization (2.1 days vs 5.1 days vs. 20.8 days, respectively).
In the subset receiving treatment within 3 days, those assigned to colchicine had the same number of cardiac deaths as those given placebo (2 vs. 2) but fewer resuscitated cardiac arrests (1 vs. 3), MIs (17 vs. 29), strokes (1 vs. 5), and urgent hospitalizations for angina requiring revascularization (6 vs. 17).
“A larger trial might have allowed for a better assessment of individual endpoints and subgroups,” observed Bouabdallaoui.
Although there is growing support for colchicine, experts caution that the drug many not be for everyone. In COLCOT, 1 in 10 patients were unable to tolerate the drug, largely because of gastrointestinal (GI) issues.
Pharmacogenomics substudy
A second COLCOT substudy aimed to identify genetic markers predictive of colchicine response and to gain insights into the mechanisms behind this response. It included 767 patients treated with colchicine and another 755 treated with placebo – or about one-third the patients in the original trial.
A genome-wide association study did not find a significant association for the primary CV endpoint, although a prespecified subgroup analysis in men identified an interesting region on chromosome 9 (variant: rs10811106), which just missed reaching genomewide significance, said Marie-Pierre Dubé, PhD, director of the Université de Montréal Beaulieu-Saucier Pharmacogenomics Centre at the Montreal Heart Institute.
In addition, the genomewide analysis found two significant regions for GI events: one on chromosome 6 (variant: rs6916345) and one on chromosome 10 (variant: rs74795203).
For each of the identified regions, the researchers then tested the effect of the allele in the placebo group and the interaction between the genetic variant and treatment with colchicine. For the chromosome 9 region in males, there was no effect in the placebo group and a significant interaction in the colchicine group.
For the significant GI event findings, there was a small effect for the chromosome 6 region in the placebo group and a very significant interaction with colchicine, Dubé said. Similarly, there was no effect for the chromosome 10 region in the placebo group and a significant interaction with colchicine.
Additional analyses in stratified patient populations showed that males with the protective allele (CC) for the chromosome 9 region represented 83% of the population. The primary CV endpoint occurred in 3.2% of these men treated with colchicine and 6.3% treated with placebo (HR, 0.46; 95% CI, 0.24 - 0.86).
For the gastrointestinal events, 25% of patients carried the risk allele (AA) for the chromosome 6 region and 36.9% of these had GI events when treated with colchicine versus 18.6% when treated with placebo (HR, 2.42; 95% CI, 1.57-3.72).
Similarly, 13% of individuals carried one or two copies of the risk allele (AG+GG) for the chromosome 10 region and the risk of GI events in these was nearly four times higher with colchicine (47.1% vs. 18.9%; HR, 3.98; 95% CI 2.24-7.07).
Functional genomic analyses of the identified regions were also performed and showed that the chromosome 9 locus overlaps with the SAXO1 gene, a stabilizer of axonemal microtubules 1.
“The leading variant at this locus (rs10811106 C allele) correlated with the expression of the HAUS6 gene, which is involved in microtubule generation from existing microtubules, and may interact with the effect of colchicine, which is known to inhibit microtubule formation,” observed Dubé.
Also, the chromosome 6 locus associated with gastrointestinal events was colocalizing with the Crohn’s disease locus, adding further support for this region.
“The results support potential personalized approaches to inflammation reduction for cardiovascular prevention,” Dubé said.
This is a post hoc subgroup analysis, however, and replication is necessary, ideally in prospective randomized trials, she noted.
The substudy is important because it provides further insights into the link between colchicine and microtubule polymerization, affecting the activation of the inflammasome, session moderator Imazio said.
“Second, it is important because pharmacogenomics can help us to better understand the optimal responder to colchicine and colchicine resistance,” he said. “So it can be useful for personalized medicine, leading to the proper use of the drug for the proper patient.”
COLCOT was supported by the government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Bouabdallaoui has disclosed no relevant financial relationships. Dubé reported grants from the government of Quebec; personal fees from DalCor and GlaxoSmithKline; research support from AstraZeneca, Pfizer, Servier, Sanofi; and minor equity interest in DalCor. Dubé is also coauthor of patents on pharmacogenomics-guided CETP inhibition, and pharmacogenomics markers of response to colchicine.
This article first appeared on Medscape.com.
The earlier the anti-inflammatory drug colchicine is initiated after a myocardial infarction (MI) the greater the benefit, a new COLCOT analysis suggests.
The parent trial was conducted in patients with a recent MI because of the intense inflammation present at that time, and added colchicine 0.5 mg daily to standard care within 30 days following MI.
As previously reported, colchicine significantly reduced the risk of the primary end point – a composite of cardiovascular (CV) death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring revascularization – by 23% compared with placebo.
This new analysis shows the risk was reduced by 48% in patients receiving colchicine within 3 days of an MI (4.3% vs. 8.3%; adjusted hazard ratio, 0.52; 95% confidence interval, 0.32-0.84, P = .007).
Risk of a secondary efficacy end point – CV death, resuscitated cardiac arrest, MI, or stroke – was reduced by 45% over an average follow up of 22.7 months (3.3% vs 6.1%; adjusted HR, 0.55; 95% CI, 0.32-0.95, P = .031).
“We believe that our results support an early, in-hospital initiation of adjunctive colchicine for post-MI prevention,” Nadia Bouabdallaoui, MD, Montreal Heart Institute, Quebec, Canada, said during an online session devoted to colchicine at the European Society of Cardiology Congress 2020.
Session moderator Massimo Imazio, MD, professor of cardiology at the University of Turin, Italy, said the improved outcomes suggest that earlier treatment is better – a finding that parallels his own experience using colchicine in patients with pericarditis.
“This substudy is very important because this is probably also the year in cardiovascular applications [that] early use of the drug could improve outcomes,” he said.
Positive data have been accumulating for colchicine from COLCOT, LoDoCo, and, most recently, the LoDoCo2 trial, even as another anti-inflammatory drug, methotrexate, flamed out as secondary prevention in the CIRT trial.
The new COLCOT substudy included 4,661 of the 4,745 original patients and examined treatment initiation using three strata: within 0-3 days (n = 1,193), 4-7 days (n = 720), and 8-30 days (n = 2,748). Patients who received treatment within 3 days were slightly younger, more likely to be smokers, and to have a shorter time from MI to randomization (2.1 days vs 5.1 days vs. 20.8 days, respectively).
In the subset receiving treatment within 3 days, those assigned to colchicine had the same number of cardiac deaths as those given placebo (2 vs. 2) but fewer resuscitated cardiac arrests (1 vs. 3), MIs (17 vs. 29), strokes (1 vs. 5), and urgent hospitalizations for angina requiring revascularization (6 vs. 17).
“A larger trial might have allowed for a better assessment of individual endpoints and subgroups,” observed Bouabdallaoui.
Although there is growing support for colchicine, experts caution that the drug many not be for everyone. In COLCOT, 1 in 10 patients were unable to tolerate the drug, largely because of gastrointestinal (GI) issues.
Pharmacogenomics substudy
A second COLCOT substudy aimed to identify genetic markers predictive of colchicine response and to gain insights into the mechanisms behind this response. It included 767 patients treated with colchicine and another 755 treated with placebo – or about one-third the patients in the original trial.
A genome-wide association study did not find a significant association for the primary CV endpoint, although a prespecified subgroup analysis in men identified an interesting region on chromosome 9 (variant: rs10811106), which just missed reaching genomewide significance, said Marie-Pierre Dubé, PhD, director of the Université de Montréal Beaulieu-Saucier Pharmacogenomics Centre at the Montreal Heart Institute.
In addition, the genomewide analysis found two significant regions for GI events: one on chromosome 6 (variant: rs6916345) and one on chromosome 10 (variant: rs74795203).
For each of the identified regions, the researchers then tested the effect of the allele in the placebo group and the interaction between the genetic variant and treatment with colchicine. For the chromosome 9 region in males, there was no effect in the placebo group and a significant interaction in the colchicine group.
For the significant GI event findings, there was a small effect for the chromosome 6 region in the placebo group and a very significant interaction with colchicine, Dubé said. Similarly, there was no effect for the chromosome 10 region in the placebo group and a significant interaction with colchicine.
Additional analyses in stratified patient populations showed that males with the protective allele (CC) for the chromosome 9 region represented 83% of the population. The primary CV endpoint occurred in 3.2% of these men treated with colchicine and 6.3% treated with placebo (HR, 0.46; 95% CI, 0.24 - 0.86).
For the gastrointestinal events, 25% of patients carried the risk allele (AA) for the chromosome 6 region and 36.9% of these had GI events when treated with colchicine versus 18.6% when treated with placebo (HR, 2.42; 95% CI, 1.57-3.72).
Similarly, 13% of individuals carried one or two copies of the risk allele (AG+GG) for the chromosome 10 region and the risk of GI events in these was nearly four times higher with colchicine (47.1% vs. 18.9%; HR, 3.98; 95% CI 2.24-7.07).
Functional genomic analyses of the identified regions were also performed and showed that the chromosome 9 locus overlaps with the SAXO1 gene, a stabilizer of axonemal microtubules 1.
“The leading variant at this locus (rs10811106 C allele) correlated with the expression of the HAUS6 gene, which is involved in microtubule generation from existing microtubules, and may interact with the effect of colchicine, which is known to inhibit microtubule formation,” observed Dubé.
Also, the chromosome 6 locus associated with gastrointestinal events was colocalizing with the Crohn’s disease locus, adding further support for this region.
“The results support potential personalized approaches to inflammation reduction for cardiovascular prevention,” Dubé said.
This is a post hoc subgroup analysis, however, and replication is necessary, ideally in prospective randomized trials, she noted.
The substudy is important because it provides further insights into the link between colchicine and microtubule polymerization, affecting the activation of the inflammasome, session moderator Imazio said.
“Second, it is important because pharmacogenomics can help us to better understand the optimal responder to colchicine and colchicine resistance,” he said. “So it can be useful for personalized medicine, leading to the proper use of the drug for the proper patient.”
COLCOT was supported by the government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Bouabdallaoui has disclosed no relevant financial relationships. Dubé reported grants from the government of Quebec; personal fees from DalCor and GlaxoSmithKline; research support from AstraZeneca, Pfizer, Servier, Sanofi; and minor equity interest in DalCor. Dubé is also coauthor of patents on pharmacogenomics-guided CETP inhibition, and pharmacogenomics markers of response to colchicine.
This article first appeared on Medscape.com.
Does evidence support the use of supplements to aid in BP control?
EVIDENCE SUMMARY
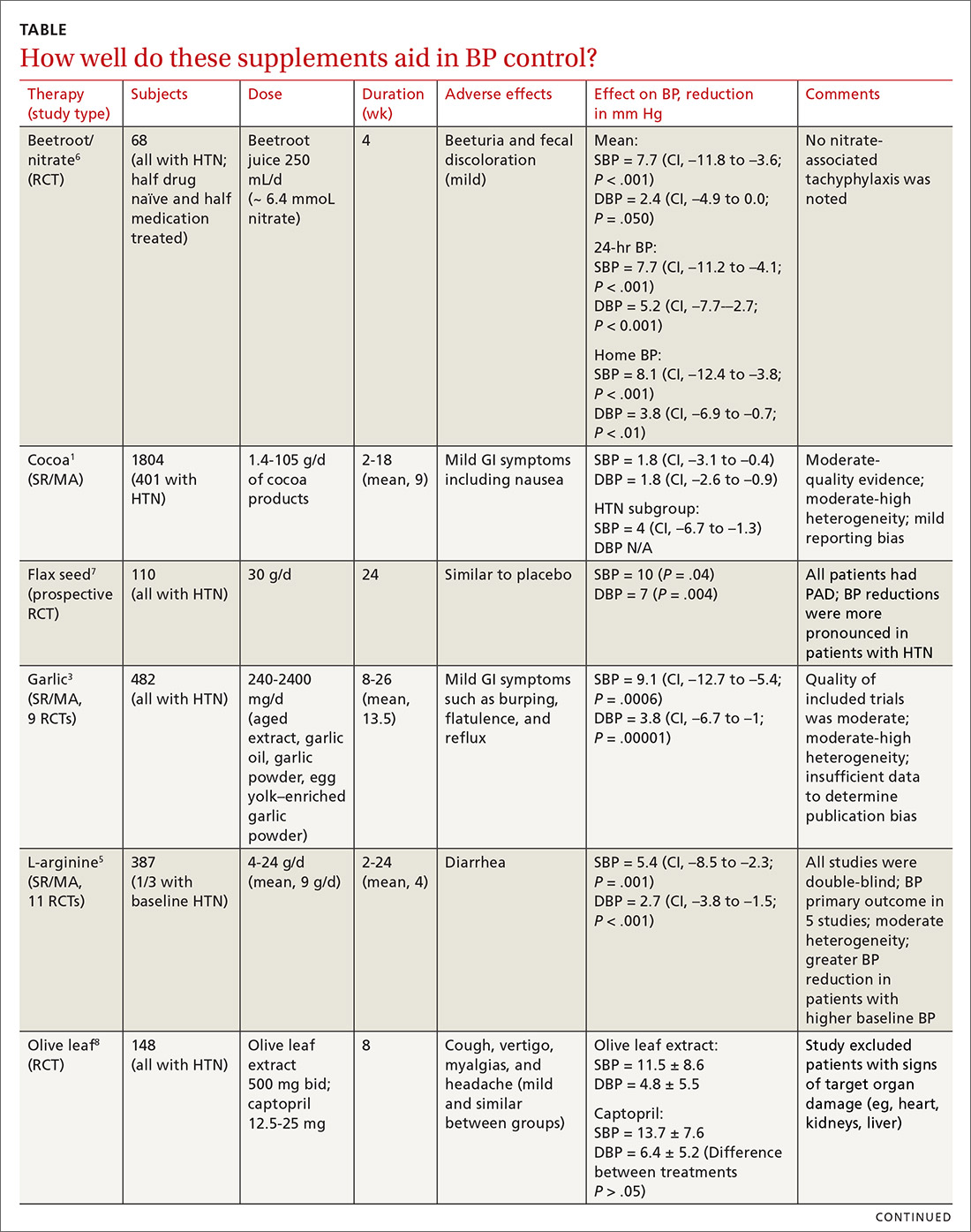
Cocoa. A 2017 Cochrane review evaluated data from more than 1800 patients (401 in hypertension studies) to determine the effect of cocoa on BP.1 Compared with placebo (in flavanol-free or low-flavanol controls), cocoa lowered systolic BP by 1.8 mm Hg (confidence interval [CI], –3.1 to –0.4) and diastolic BP by 1.8 mm Hg (CI, –2.6 to –0.9). Further analysis of patients with hypertension (only) showed a reduction in systolic BP of 4 mm Hg (CI, –6.7 to –1.3).
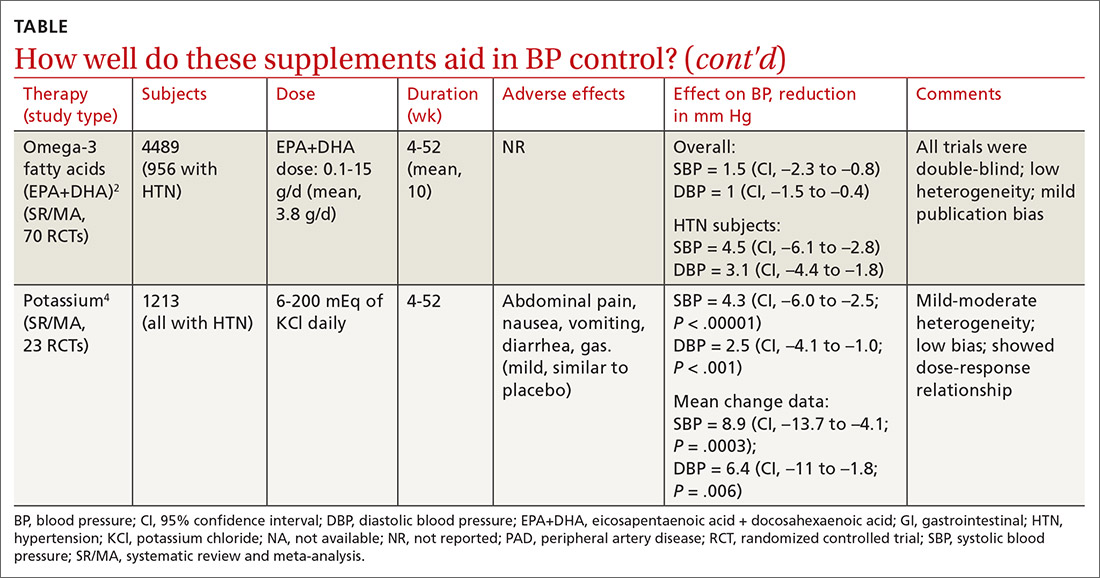
Omega-3 fatty acids. Similarly, a 2014 meta-analysis investigating omega-3 fatty acids (eicosapentaenoic acid [EPA] + docosahexaenoic acid [DHA]) included data from 4489 patients (956 with hypertension) and showed reductions in systolic BP of 1.5 mm Hg (CI, –2.3 to –0.8) and diastolic BP of 1 mm Hg (CI, –1.5 to –0.4), compared with placebo.2 Again, subgroup analysis of patients with hypertension (only) at baseline revealed a greater decrease in systolic and diastolic BP: 4.5 mm Hg (CI, –6.1 to –2.8) and 3.1 mm Hg (CI, –4.4 to –1.8), respectively.
Garlic and potassium chloride. Separate meta-analyses that included only patients with hypertension found that both garlic and potassium significantly lowered BP.3,4 A 2015 meta-analysis comparing a variety of garlic preparations with placebo in patients with hypertension showed decreases in systolic BP of 9.1 mm Hg (CI, –12.7 to –5.4) and in diastolic BP of 3.8 mm Hg (CI, –6.7 to –1).3 Meanwhile, a meta-analysis in 2017 comparing different doses of potassium chloride with placebo demonstrated reductions in systolic BP of 4.3 mm Hg (CI, –6 to –2.5) and diastolic BP of 2.5 mm Hg (CI, –4.1 to –1).4
L-arginine. Another meta-analysis of randomized controlled trials reported evidence that oral L-arginine, compared with placebo, significantly reduced systolic BP by 5.4 mm Hg (CI, –8.5 to –2.3) and diastolic BP by 2.7 mm Hg (CI, –3.8 to –1.5).5 Close to one-third of patients had hypertension at baseline.
Beetroot juice. A double-blind, placebo-controlled study showed that consumption of beetroot juice (with nitrate) once daily reduced BP in 3 different settings (clinic, 24-hour ambulatory, and home readings) when compared with placebo (nitrate-free beetroot juice).6 Study participants were mostly British women, overweight, without significant cardiovascular or renal disease, and with uncontrolled ambulatory BP (> 135/85 mm Hg).
Flax seed. A prospective, double-blind trial of patients with peripheral artery disease compared the antihypertensive effects of flax seed with placebo in patients with and without hypertension and found marked decreases in systolic and diastolic BP.7 Study participants were all older than 40 years without other major cardiac or renal disease, and the majority of enrolled patients with hypertension were concurrently taking medications to treat hypertension during the study.
Olive leaf extract. A double-blind, parallel, and active-control clinical trial in Indonesia compared the BP-lowering effect of olive leaf extract (Olea europaea) to captopril as monotherapies in patients with stage 1 hypertension.8 After a 4-week period of dietary intervention, individuals who were still hypertensive (range, 140/90 to 159/99 mm Hg) were treated with either olive leaf extract or captopril. After 8 weeks of treatment, both groups saw comparable reductions in BP.
Continue to: Editor's takeaway
Editor’s takeaway
Many studies have demonstrated BP benefits from a variety of natural supplements. Although the studies’ durations are short, the effects sometimes modest, and the outcomes disease-oriented rather than patient-oriented, the findings can provide a useful complement to our efforts to manage this most common chronic disease.
1. Ried K, Fakler P, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2017;(4):CD008893.
2. Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens. 2014;27:885-896.
3. Rohner A, Ried K, Sobenin IA, et al. A systematic review and meta-analysis on the effects of garlic preparations on blood pressure in individuals with hypertension. Am J Hypertens. 2015;28:414-423.
4. Poorolajal J, Zeraati F, Soltanian AR, et al. Oral potassium supplementation for management of essential hypertension: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0174967.
5. Dong JY, Qin LQ, Zhang Z, et al. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162:959-965.
6. Kapil V, Khambata RS, Robertson A, et al. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320-327.
7. Rodriguez-Leyva D, Weighell W, Edel AL, et al. Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension. 2013;62:1081-1089.
8. Susalit E, Agus N, Effendi I, et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with captopril. Phytomedicine. 2011;18:251-258.
EVIDENCE SUMMARY
Cocoa. A 2017 Cochrane review evaluated data from more than 1800 patients (401 in hypertension studies) to determine the effect of cocoa on BP.1 Compared with placebo (in flavanol-free or low-flavanol controls), cocoa lowered systolic BP by 1.8 mm Hg (confidence interval [CI], –3.1 to –0.4) and diastolic BP by 1.8 mm Hg (CI, –2.6 to –0.9). Further analysis of patients with hypertension (only) showed a reduction in systolic BP of 4 mm Hg (CI, –6.7 to –1.3).
Omega-3 fatty acids. Similarly, a 2014 meta-analysis investigating omega-3 fatty acids (eicosapentaenoic acid [EPA] + docosahexaenoic acid [DHA]) included data from 4489 patients (956 with hypertension) and showed reductions in systolic BP of 1.5 mm Hg (CI, –2.3 to –0.8) and diastolic BP of 1 mm Hg (CI, –1.5 to –0.4), compared with placebo.2 Again, subgroup analysis of patients with hypertension (only) at baseline revealed a greater decrease in systolic and diastolic BP: 4.5 mm Hg (CI, –6.1 to –2.8) and 3.1 mm Hg (CI, –4.4 to –1.8), respectively.
Garlic and potassium chloride. Separate meta-analyses that included only patients with hypertension found that both garlic and potassium significantly lowered BP.3,4 A 2015 meta-analysis comparing a variety of garlic preparations with placebo in patients with hypertension showed decreases in systolic BP of 9.1 mm Hg (CI, –12.7 to –5.4) and in diastolic BP of 3.8 mm Hg (CI, –6.7 to –1).3 Meanwhile, a meta-analysis in 2017 comparing different doses of potassium chloride with placebo demonstrated reductions in systolic BP of 4.3 mm Hg (CI, –6 to –2.5) and diastolic BP of 2.5 mm Hg (CI, –4.1 to –1).4
L-arginine. Another meta-analysis of randomized controlled trials reported evidence that oral L-arginine, compared with placebo, significantly reduced systolic BP by 5.4 mm Hg (CI, –8.5 to –2.3) and diastolic BP by 2.7 mm Hg (CI, –3.8 to –1.5).5 Close to one-third of patients had hypertension at baseline.
Beetroot juice. A double-blind, placebo-controlled study showed that consumption of beetroot juice (with nitrate) once daily reduced BP in 3 different settings (clinic, 24-hour ambulatory, and home readings) when compared with placebo (nitrate-free beetroot juice).6 Study participants were mostly British women, overweight, without significant cardiovascular or renal disease, and with uncontrolled ambulatory BP (> 135/85 mm Hg).
Flax seed. A prospective, double-blind trial of patients with peripheral artery disease compared the antihypertensive effects of flax seed with placebo in patients with and without hypertension and found marked decreases in systolic and diastolic BP.7 Study participants were all older than 40 years without other major cardiac or renal disease, and the majority of enrolled patients with hypertension were concurrently taking medications to treat hypertension during the study.
Olive leaf extract. A double-blind, parallel, and active-control clinical trial in Indonesia compared the BP-lowering effect of olive leaf extract (Olea europaea) to captopril as monotherapies in patients with stage 1 hypertension.8 After a 4-week period of dietary intervention, individuals who were still hypertensive (range, 140/90 to 159/99 mm Hg) were treated with either olive leaf extract or captopril. After 8 weeks of treatment, both groups saw comparable reductions in BP.
Continue to: Editor's takeaway
Editor’s takeaway
Many studies have demonstrated BP benefits from a variety of natural supplements. Although the studies’ durations are short, the effects sometimes modest, and the outcomes disease-oriented rather than patient-oriented, the findings can provide a useful complement to our efforts to manage this most common chronic disease.
EVIDENCE SUMMARY
Cocoa. A 2017 Cochrane review evaluated data from more than 1800 patients (401 in hypertension studies) to determine the effect of cocoa on BP.1 Compared with placebo (in flavanol-free or low-flavanol controls), cocoa lowered systolic BP by 1.8 mm Hg (confidence interval [CI], –3.1 to –0.4) and diastolic BP by 1.8 mm Hg (CI, –2.6 to –0.9). Further analysis of patients with hypertension (only) showed a reduction in systolic BP of 4 mm Hg (CI, –6.7 to –1.3).
Omega-3 fatty acids. Similarly, a 2014 meta-analysis investigating omega-3 fatty acids (eicosapentaenoic acid [EPA] + docosahexaenoic acid [DHA]) included data from 4489 patients (956 with hypertension) and showed reductions in systolic BP of 1.5 mm Hg (CI, –2.3 to –0.8) and diastolic BP of 1 mm Hg (CI, –1.5 to –0.4), compared with placebo.2 Again, subgroup analysis of patients with hypertension (only) at baseline revealed a greater decrease in systolic and diastolic BP: 4.5 mm Hg (CI, –6.1 to –2.8) and 3.1 mm Hg (CI, –4.4 to –1.8), respectively.
Garlic and potassium chloride. Separate meta-analyses that included only patients with hypertension found that both garlic and potassium significantly lowered BP.3,4 A 2015 meta-analysis comparing a variety of garlic preparations with placebo in patients with hypertension showed decreases in systolic BP of 9.1 mm Hg (CI, –12.7 to –5.4) and in diastolic BP of 3.8 mm Hg (CI, –6.7 to –1).3 Meanwhile, a meta-analysis in 2017 comparing different doses of potassium chloride with placebo demonstrated reductions in systolic BP of 4.3 mm Hg (CI, –6 to –2.5) and diastolic BP of 2.5 mm Hg (CI, –4.1 to –1).4
L-arginine. Another meta-analysis of randomized controlled trials reported evidence that oral L-arginine, compared with placebo, significantly reduced systolic BP by 5.4 mm Hg (CI, –8.5 to –2.3) and diastolic BP by 2.7 mm Hg (CI, –3.8 to –1.5).5 Close to one-third of patients had hypertension at baseline.
Beetroot juice. A double-blind, placebo-controlled study showed that consumption of beetroot juice (with nitrate) once daily reduced BP in 3 different settings (clinic, 24-hour ambulatory, and home readings) when compared with placebo (nitrate-free beetroot juice).6 Study participants were mostly British women, overweight, without significant cardiovascular or renal disease, and with uncontrolled ambulatory BP (> 135/85 mm Hg).
Flax seed. A prospective, double-blind trial of patients with peripheral artery disease compared the antihypertensive effects of flax seed with placebo in patients with and without hypertension and found marked decreases in systolic and diastolic BP.7 Study participants were all older than 40 years without other major cardiac or renal disease, and the majority of enrolled patients with hypertension were concurrently taking medications to treat hypertension during the study.
Olive leaf extract. A double-blind, parallel, and active-control clinical trial in Indonesia compared the BP-lowering effect of olive leaf extract (Olea europaea) to captopril as monotherapies in patients with stage 1 hypertension.8 After a 4-week period of dietary intervention, individuals who were still hypertensive (range, 140/90 to 159/99 mm Hg) were treated with either olive leaf extract or captopril. After 8 weeks of treatment, both groups saw comparable reductions in BP.
Continue to: Editor's takeaway
Editor’s takeaway
Many studies have demonstrated BP benefits from a variety of natural supplements. Although the studies’ durations are short, the effects sometimes modest, and the outcomes disease-oriented rather than patient-oriented, the findings can provide a useful complement to our efforts to manage this most common chronic disease.
1. Ried K, Fakler P, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2017;(4):CD008893.
2. Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens. 2014;27:885-896.
3. Rohner A, Ried K, Sobenin IA, et al. A systematic review and meta-analysis on the effects of garlic preparations on blood pressure in individuals with hypertension. Am J Hypertens. 2015;28:414-423.
4. Poorolajal J, Zeraati F, Soltanian AR, et al. Oral potassium supplementation for management of essential hypertension: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0174967.
5. Dong JY, Qin LQ, Zhang Z, et al. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162:959-965.
6. Kapil V, Khambata RS, Robertson A, et al. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320-327.
7. Rodriguez-Leyva D, Weighell W, Edel AL, et al. Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension. 2013;62:1081-1089.
8. Susalit E, Agus N, Effendi I, et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with captopril. Phytomedicine. 2011;18:251-258.
1. Ried K, Fakler P, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2017;(4):CD008893.
2. Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens. 2014;27:885-896.
3. Rohner A, Ried K, Sobenin IA, et al. A systematic review and meta-analysis on the effects of garlic preparations on blood pressure in individuals with hypertension. Am J Hypertens. 2015;28:414-423.
4. Poorolajal J, Zeraati F, Soltanian AR, et al. Oral potassium supplementation for management of essential hypertension: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0174967.
5. Dong JY, Qin LQ, Zhang Z, et al. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162:959-965.
6. Kapil V, Khambata RS, Robertson A, et al. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320-327.
7. Rodriguez-Leyva D, Weighell W, Edel AL, et al. Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension. 2013;62:1081-1089.
8. Susalit E, Agus N, Effendi I, et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with captopril. Phytomedicine. 2011;18:251-258.
EVIDENCE-BASED ANSWER:
Yes. A number of well-tolerated natural therapies have been shown to reduce systolic and diastolic blood pressure (BP). (See Table1-8 for summary.) However, the studies don’t provide direct evidence of whether the decrease in BP is linked to patient-oriented outcomes. Nor do they allow definitive conclusions concerning the lasting nature of the reductions, because most studies were fewer than 6 months in duration (strength of recommendation: C, disease-oriented evidence).
It's time to change when BP meds are taken
In this issue of JFP, there is an extraordinarily valuable PURL (Priority Updates from the Research Literature) for you. PURLs® are written by academic family physicians who comb through volumes of research to identify and then summarize for JFP important studies we believe should change your practice. After reading a PURL, you may find that you have already implemented this new evidence into your practice. In that case, the PURL confirms that you are doing the right thing.
Here is the good news from this month’s PURL: Having patients take their blood pressure (BP) medication in the evening, rather than in the morning, leads not only to better BP control but also to a reduction in cardiovascular events.1 How large is this effect? This simple intervention nearly cut in half the number of major cardiovascular events—including myocardial infarction, stroke, and congestive heart failure—and the risk for death from a cardiovascular event was reduced 56%. The number needed to treat to prevent 1 major cardiovascular event over the course of 6.3 years was 20. That means this intervention is more powerful than taking a statin!
The investigators, who call this intervention “chronotherapy” since it takes into account the body’s circadian rhythms, have been studying the effect of this simple intervention for many years, and this large randomized trial provides very strong evidence that it’s effective. Despite the excellent trial design and execution, some cardiovascular researchers have questioned the integrity of the trial and believe patients should continue to take their antihypertensives in the morning.2 The main investigator of the study, however, has provided a very strong rebuttal in print.3
I am delighted to see the positive results of this definitive trial of chronotherapy for hypertension. When these investigators published their first randomized trial of chronotherapy in 2010,4 which demonstrated a significant BP reduction with evening dosing of antihypertensives, I began telling all of my patients to take at least 1 of their antihypertensive meds in the evening. Maybe I was jumping the gun at that time, but we should all tell our patients to take their BP medication in the evening from now on. What could be an easier way to reduce cardiovascular morbidity and mortality?
1. Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial [published online ahead of print October 22, 2019]. Eur Heart J. 2019;ehz754. doi:10.1093/eurheartj/ehz754
2. Kreutz R, Kjeldsen SE, Burnier M, et al. Blood pressure medication should not be routinely dosed at bedtime. We must disregard the data from the HYGIA project [editorial]. Blood Press. 2020;29:135-136.
3. Crespo JJ, Domínguez-Sardiña M, Otero A, et. al. Bedtime hypertension chronotherapy best reduces cardiovascular disease risk as corroborated by the Hygia Chronotherapy Trial. Rebuttal to European Society of Hypertension officials. Chronobiol Int. 2020;37:771-780.
4. Hermida RC, Ayala DE, Mojón A, Fernández JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629-1651.
In this issue of JFP, there is an extraordinarily valuable PURL (Priority Updates from the Research Literature) for you. PURLs® are written by academic family physicians who comb through volumes of research to identify and then summarize for JFP important studies we believe should change your practice. After reading a PURL, you may find that you have already implemented this new evidence into your practice. In that case, the PURL confirms that you are doing the right thing.
Here is the good news from this month’s PURL: Having patients take their blood pressure (BP) medication in the evening, rather than in the morning, leads not only to better BP control but also to a reduction in cardiovascular events.1 How large is this effect? This simple intervention nearly cut in half the number of major cardiovascular events—including myocardial infarction, stroke, and congestive heart failure—and the risk for death from a cardiovascular event was reduced 56%. The number needed to treat to prevent 1 major cardiovascular event over the course of 6.3 years was 20. That means this intervention is more powerful than taking a statin!
The investigators, who call this intervention “chronotherapy” since it takes into account the body’s circadian rhythms, have been studying the effect of this simple intervention for many years, and this large randomized trial provides very strong evidence that it’s effective. Despite the excellent trial design and execution, some cardiovascular researchers have questioned the integrity of the trial and believe patients should continue to take their antihypertensives in the morning.2 The main investigator of the study, however, has provided a very strong rebuttal in print.3
I am delighted to see the positive results of this definitive trial of chronotherapy for hypertension. When these investigators published their first randomized trial of chronotherapy in 2010,4 which demonstrated a significant BP reduction with evening dosing of antihypertensives, I began telling all of my patients to take at least 1 of their antihypertensive meds in the evening. Maybe I was jumping the gun at that time, but we should all tell our patients to take their BP medication in the evening from now on. What could be an easier way to reduce cardiovascular morbidity and mortality?
In this issue of JFP, there is an extraordinarily valuable PURL (Priority Updates from the Research Literature) for you. PURLs® are written by academic family physicians who comb through volumes of research to identify and then summarize for JFP important studies we believe should change your practice. After reading a PURL, you may find that you have already implemented this new evidence into your practice. In that case, the PURL confirms that you are doing the right thing.
Here is the good news from this month’s PURL: Having patients take their blood pressure (BP) medication in the evening, rather than in the morning, leads not only to better BP control but also to a reduction in cardiovascular events.1 How large is this effect? This simple intervention nearly cut in half the number of major cardiovascular events—including myocardial infarction, stroke, and congestive heart failure—and the risk for death from a cardiovascular event was reduced 56%. The number needed to treat to prevent 1 major cardiovascular event over the course of 6.3 years was 20. That means this intervention is more powerful than taking a statin!
The investigators, who call this intervention “chronotherapy” since it takes into account the body’s circadian rhythms, have been studying the effect of this simple intervention for many years, and this large randomized trial provides very strong evidence that it’s effective. Despite the excellent trial design and execution, some cardiovascular researchers have questioned the integrity of the trial and believe patients should continue to take their antihypertensives in the morning.2 The main investigator of the study, however, has provided a very strong rebuttal in print.3
I am delighted to see the positive results of this definitive trial of chronotherapy for hypertension. When these investigators published their first randomized trial of chronotherapy in 2010,4 which demonstrated a significant BP reduction with evening dosing of antihypertensives, I began telling all of my patients to take at least 1 of their antihypertensive meds in the evening. Maybe I was jumping the gun at that time, but we should all tell our patients to take their BP medication in the evening from now on. What could be an easier way to reduce cardiovascular morbidity and mortality?
1. Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial [published online ahead of print October 22, 2019]. Eur Heart J. 2019;ehz754. doi:10.1093/eurheartj/ehz754
2. Kreutz R, Kjeldsen SE, Burnier M, et al. Blood pressure medication should not be routinely dosed at bedtime. We must disregard the data from the HYGIA project [editorial]. Blood Press. 2020;29:135-136.
3. Crespo JJ, Domínguez-Sardiña M, Otero A, et. al. Bedtime hypertension chronotherapy best reduces cardiovascular disease risk as corroborated by the Hygia Chronotherapy Trial. Rebuttal to European Society of Hypertension officials. Chronobiol Int. 2020;37:771-780.
4. Hermida RC, Ayala DE, Mojón A, Fernández JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629-1651.
1. Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial [published online ahead of print October 22, 2019]. Eur Heart J. 2019;ehz754. doi:10.1093/eurheartj/ehz754
2. Kreutz R, Kjeldsen SE, Burnier M, et al. Blood pressure medication should not be routinely dosed at bedtime. We must disregard the data from the HYGIA project [editorial]. Blood Press. 2020;29:135-136.
3. Crespo JJ, Domínguez-Sardiña M, Otero A, et. al. Bedtime hypertension chronotherapy best reduces cardiovascular disease risk as corroborated by the Hygia Chronotherapy Trial. Rebuttal to European Society of Hypertension officials. Chronobiol Int. 2020;37:771-780.
4. Hermida RC, Ayala DE, Mojón A, Fernández JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629-1651.
Is it better to take that antihypertensive at night?
ILLUSTRATIVE CASE
A 54-year-old White woman presents to your office with new-onset hypertension. As you are discussing options for treatment, she mentions she would prefer once-daily dosing to help her remember to take her medication. She also wants to know what the best time of day is to take her medication to reduce her risk of cardiovascular disease (CVD). What do you advise?
The burden of hypertension is significant and growing in the United States. The 2017 American College of Cardiology/American Heart Association (ACC/AHA) guidelines reported that more than 108 million people were affected in 2015-2016—up from 87 million in 1999-2000.2 Yet control of hypertension is improving among those receiving antihypertension pharmacotherapy. As reported in the ACC/AHA guidelines, data from the 2016 National Health and Nutrition Examination Survey (NHANES) indicate an increase of controlled hypertension among those receiving treatment from 25.6% (1999-2000) to 43.5% (2015-2016).2
Chronotherapy involves the administration of medication in coordination with the body’s circadian rhythms to maximize therapeutic effectiveness and/or minimize adverse effects. It is not a new concept as it applies to hypertension. Circadian rhythm–dependent mechanisms influence the natural rise and fall of blood pressure (BP).1 The renin-angiotensin-aldosterone system, known to be most active at night, is a target mechanism for BP control.1 Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are more effective (alone or in combination with other agents) at reducing BP during sleep and wakefulness when they are taken at night.3,4 Additional prospective clinical trials and systematic reviews have documented improved BP during sleep and on 24-hour ambulatory monitoring when antihypertensives are taken at bedtime.3-5
However, there have been few long-term studies assessing the effects of bedtime administration of antihypertensive medication on CVD risk reduction with patient-oriented outcomes.6,7 Additionally, no studies have evaluated morning vs bedtime administration of antihypertensive medication for CVD risk reduction in a primary care setting. The 2019 ACC/AHA guideline on the primary prevention of CVD offers no recommendation regarding when to take antihypertensive medication.8 Timing of medication administration also is not addressed in the NHANES study of hypertension awareness, treatment, and control in US adults.9
This study sought to determine in a primary care setting whether taking antihypertensives at bedtime, as opposed to upon waking, more effectively reduces CVD risk.
STUDY SUMMARY
PM vs AM antihypertensive dosing reduces CV events
This prospective, randomized, open-label, blinded endpoint trial of antihypertensive medication administration timing was part of a large, multicenter Spanish study investigating ambulatory BP monitoring (ABPM) as a routine diagnostic tool.
Study participants were randomly assigned in a 1:1 ratio to 2 treatment arms; participants either took all of their BP medications in the morning upon waking (n = 9532) or right before bedtime (n = 9552). The study was conducted in a primary care clinical setting. It included adult participants (age ≥ 18 years) with hypertension (defined as having at least 1 of the following benchmarks: awake systolic BP [SBP] mean ≥ 135 mm Hg, awake diastolic BP (DBP) mean ≥ 85 mm Hg, asleep SBP mean ≥ 120 mm Hg, asleep DBP mean ≥ 70 mm Hg as corroborated by 48-hour ABPM) who were taking at least 1 antihypertensive medication.
Continue to: Any antihypertension medication...
Any antihypertension medication included in the Spanish national formulary was allowed (exact agents were not delineated, but the following classes were included: ARB, ACE inhibitor, calcium channel blocker [CCB], beta-blocker, and/or diuretic). All BP medications had to be dosed once daily for inclusion. Exclusion criteria included pregnancy, night or rotating-shift work, alcohol or other substance dependence, acquired immunodeficiency syndrome, preexisting CVD (unstable angina, heart failure, arrhythmia, kidney failure, and retinopathy), inability to tolerate ABPM, and inability to comply with required 1-year follow-up.
Upon enrollment and at every subsequent clinic visit (scheduled at least annually), participants underwent 48-hour ABPM. Those with uncontrolled BP or elevated CVD risk had scheduled follow-up and ABPM more frequently. The primary outcome was a composite of CVD events including new-onset myocardial infarction, coronary revascularization, heart failure, ischemic stroke, hemorrhagic stroke, and CVD death. Secondary endpoints were individually analyzed primary outcomes of CVD events. The typical patient at baseline was 60.5 years of age with a body mass index of 29.7, an almost 9-year duration of hypertension, and a baseline office BP of 149/86 mm Hg. The patient break-out by antihypertensive class (awakening vs bedtime groups) was as follows: ARB (53% vs 53%), ACE inhibitor (25% vs 23%), CCB (33% vs 37%), beta-blocker (22% vs 18%), and diuretic (47% vs 40%).
During the median 6.3-year patient follow-up period, 1752 participants experienced a total of 2454 CVD events. Patients in the bedtime administration group, compared with those in the morning group, showed significantly lower risk for a CVD event (hazard ratio [HR] = 0.55; 95% confidence interval [CI], 0.50-0.61; P < .001). Also, there was a lower risk for individual CVD events in the bedtime administration group: CVD death (HR = 0.44; 95% CI, 0.34-0.56), myocardial infarction (HR = 0.66; 95% CI, 0.52-0.84), coronary revascularization (HR = 0.60; 95% CI, 0.47-0.75), heart failure (HR = 0.58; 95% CI, 0.49-0.70), and stroke (HR = 0.51; 95% CI, 0.41-0.63). This difference remained after correction for multiple potential confounders. There were no differences in adverse events, such as sleep-time hypotension, between groups.
WHAT’S NEW
First RCT in primary care to show dosing time change reduces CV risk
This is the first randomized controlled trial (RCT) performed in a primary care setting to compare before-bedtime to upon-waking administration of antihypertensive medications using clinically significant endpoints. The study demonstrates that a simple change in administration time has the potential to significantly improve the lives of our patients by reducing the risk for cardiovascular events and their medication burden.
CAVEATS
Homogenous population and exclusions limit generalizability
Because the study population consisted of white Spanish men and women, the results may not be generalizable beyond that ethnic group. In addition, the study exclusions limit interpretation in night/rotating-shift employees, patients with secondary hypertension, and those with CVD, chronic kidney disease, or severe retinopathy looking to reduce their risk.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Nighttime urination could lead to nonadherence
Taking diuretics at bedtime may result in unwanted nighttime awakenings for visits to the bathroom, which could lead to nonadherence in some patients.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial [published online ahead of print October 22, 2019]. Eur Heart J. 2019;ehz754. doi:10.1093/eurheartj/ehz754.
2. Dorans KS, Mills KT, Liu Y, et al. Trends in prevalence and control of hypertension according to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline. J Am Heart Assoc. 2018;7:e008888.
3. Hermida RC, Ayala DE, Smolensky MH, et al. Chronotherapy with conventional blood pressure medications improves management of hypertension and reduces cardiovascular and stroke risks. Hypertens Res. 2016;39:277-292.
4. Bowles NP, Thosar SS, Herzig MX, et al. Chronotherapy for hypertension. Curr Hypertens Rep. 2018;20:97.
5. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011:CD004184.
6. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients: the Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145-153.
7. Black HR, Elliott WJ, Grandits G, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289:2073-2082.
8. Arnette DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:e177-e232.
9. Foti K, Wang D, Appel LJ, et al. Hypertension awareness, treatment, and control in US adults: trends in the hypertensive control cascade by population subgroup (National Health and Nutrition Examination Survey, 1999-2016). Am J Epidemiol. 2019;188:2165-2174.
ILLUSTRATIVE CASE
A 54-year-old White woman presents to your office with new-onset hypertension. As you are discussing options for treatment, she mentions she would prefer once-daily dosing to help her remember to take her medication. She also wants to know what the best time of day is to take her medication to reduce her risk of cardiovascular disease (CVD). What do you advise?
The burden of hypertension is significant and growing in the United States. The 2017 American College of Cardiology/American Heart Association (ACC/AHA) guidelines reported that more than 108 million people were affected in 2015-2016—up from 87 million in 1999-2000.2 Yet control of hypertension is improving among those receiving antihypertension pharmacotherapy. As reported in the ACC/AHA guidelines, data from the 2016 National Health and Nutrition Examination Survey (NHANES) indicate an increase of controlled hypertension among those receiving treatment from 25.6% (1999-2000) to 43.5% (2015-2016).2
Chronotherapy involves the administration of medication in coordination with the body’s circadian rhythms to maximize therapeutic effectiveness and/or minimize adverse effects. It is not a new concept as it applies to hypertension. Circadian rhythm–dependent mechanisms influence the natural rise and fall of blood pressure (BP).1 The renin-angiotensin-aldosterone system, known to be most active at night, is a target mechanism for BP control.1 Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are more effective (alone or in combination with other agents) at reducing BP during sleep and wakefulness when they are taken at night.3,4 Additional prospective clinical trials and systematic reviews have documented improved BP during sleep and on 24-hour ambulatory monitoring when antihypertensives are taken at bedtime.3-5
However, there have been few long-term studies assessing the effects of bedtime administration of antihypertensive medication on CVD risk reduction with patient-oriented outcomes.6,7 Additionally, no studies have evaluated morning vs bedtime administration of antihypertensive medication for CVD risk reduction in a primary care setting. The 2019 ACC/AHA guideline on the primary prevention of CVD offers no recommendation regarding when to take antihypertensive medication.8 Timing of medication administration also is not addressed in the NHANES study of hypertension awareness, treatment, and control in US adults.9
This study sought to determine in a primary care setting whether taking antihypertensives at bedtime, as opposed to upon waking, more effectively reduces CVD risk.
STUDY SUMMARY
PM vs AM antihypertensive dosing reduces CV events
This prospective, randomized, open-label, blinded endpoint trial of antihypertensive medication administration timing was part of a large, multicenter Spanish study investigating ambulatory BP monitoring (ABPM) as a routine diagnostic tool.
Study participants were randomly assigned in a 1:1 ratio to 2 treatment arms; participants either took all of their BP medications in the morning upon waking (n = 9532) or right before bedtime (n = 9552). The study was conducted in a primary care clinical setting. It included adult participants (age ≥ 18 years) with hypertension (defined as having at least 1 of the following benchmarks: awake systolic BP [SBP] mean ≥ 135 mm Hg, awake diastolic BP (DBP) mean ≥ 85 mm Hg, asleep SBP mean ≥ 120 mm Hg, asleep DBP mean ≥ 70 mm Hg as corroborated by 48-hour ABPM) who were taking at least 1 antihypertensive medication.
Continue to: Any antihypertension medication...
Any antihypertension medication included in the Spanish national formulary was allowed (exact agents were not delineated, but the following classes were included: ARB, ACE inhibitor, calcium channel blocker [CCB], beta-blocker, and/or diuretic). All BP medications had to be dosed once daily for inclusion. Exclusion criteria included pregnancy, night or rotating-shift work, alcohol or other substance dependence, acquired immunodeficiency syndrome, preexisting CVD (unstable angina, heart failure, arrhythmia, kidney failure, and retinopathy), inability to tolerate ABPM, and inability to comply with required 1-year follow-up.
Upon enrollment and at every subsequent clinic visit (scheduled at least annually), participants underwent 48-hour ABPM. Those with uncontrolled BP or elevated CVD risk had scheduled follow-up and ABPM more frequently. The primary outcome was a composite of CVD events including new-onset myocardial infarction, coronary revascularization, heart failure, ischemic stroke, hemorrhagic stroke, and CVD death. Secondary endpoints were individually analyzed primary outcomes of CVD events. The typical patient at baseline was 60.5 years of age with a body mass index of 29.7, an almost 9-year duration of hypertension, and a baseline office BP of 149/86 mm Hg. The patient break-out by antihypertensive class (awakening vs bedtime groups) was as follows: ARB (53% vs 53%), ACE inhibitor (25% vs 23%), CCB (33% vs 37%), beta-blocker (22% vs 18%), and diuretic (47% vs 40%).
During the median 6.3-year patient follow-up period, 1752 participants experienced a total of 2454 CVD events. Patients in the bedtime administration group, compared with those in the morning group, showed significantly lower risk for a CVD event (hazard ratio [HR] = 0.55; 95% confidence interval [CI], 0.50-0.61; P < .001). Also, there was a lower risk for individual CVD events in the bedtime administration group: CVD death (HR = 0.44; 95% CI, 0.34-0.56), myocardial infarction (HR = 0.66; 95% CI, 0.52-0.84), coronary revascularization (HR = 0.60; 95% CI, 0.47-0.75), heart failure (HR = 0.58; 95% CI, 0.49-0.70), and stroke (HR = 0.51; 95% CI, 0.41-0.63). This difference remained after correction for multiple potential confounders. There were no differences in adverse events, such as sleep-time hypotension, between groups.
WHAT’S NEW
First RCT in primary care to show dosing time change reduces CV risk
This is the first randomized controlled trial (RCT) performed in a primary care setting to compare before-bedtime to upon-waking administration of antihypertensive medications using clinically significant endpoints. The study demonstrates that a simple change in administration time has the potential to significantly improve the lives of our patients by reducing the risk for cardiovascular events and their medication burden.
CAVEATS
Homogenous population and exclusions limit generalizability
Because the study population consisted of white Spanish men and women, the results may not be generalizable beyond that ethnic group. In addition, the study exclusions limit interpretation in night/rotating-shift employees, patients with secondary hypertension, and those with CVD, chronic kidney disease, or severe retinopathy looking to reduce their risk.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Nighttime urination could lead to nonadherence
Taking diuretics at bedtime may result in unwanted nighttime awakenings for visits to the bathroom, which could lead to nonadherence in some patients.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 54-year-old White woman presents to your office with new-onset hypertension. As you are discussing options for treatment, she mentions she would prefer once-daily dosing to help her remember to take her medication. She also wants to know what the best time of day is to take her medication to reduce her risk of cardiovascular disease (CVD). What do you advise?
The burden of hypertension is significant and growing in the United States. The 2017 American College of Cardiology/American Heart Association (ACC/AHA) guidelines reported that more than 108 million people were affected in 2015-2016—up from 87 million in 1999-2000.2 Yet control of hypertension is improving among those receiving antihypertension pharmacotherapy. As reported in the ACC/AHA guidelines, data from the 2016 National Health and Nutrition Examination Survey (NHANES) indicate an increase of controlled hypertension among those receiving treatment from 25.6% (1999-2000) to 43.5% (2015-2016).2
Chronotherapy involves the administration of medication in coordination with the body’s circadian rhythms to maximize therapeutic effectiveness and/or minimize adverse effects. It is not a new concept as it applies to hypertension. Circadian rhythm–dependent mechanisms influence the natural rise and fall of blood pressure (BP).1 The renin-angiotensin-aldosterone system, known to be most active at night, is a target mechanism for BP control.1 Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are more effective (alone or in combination with other agents) at reducing BP during sleep and wakefulness when they are taken at night.3,4 Additional prospective clinical trials and systematic reviews have documented improved BP during sleep and on 24-hour ambulatory monitoring when antihypertensives are taken at bedtime.3-5
However, there have been few long-term studies assessing the effects of bedtime administration of antihypertensive medication on CVD risk reduction with patient-oriented outcomes.6,7 Additionally, no studies have evaluated morning vs bedtime administration of antihypertensive medication for CVD risk reduction in a primary care setting. The 2019 ACC/AHA guideline on the primary prevention of CVD offers no recommendation regarding when to take antihypertensive medication.8 Timing of medication administration also is not addressed in the NHANES study of hypertension awareness, treatment, and control in US adults.9
This study sought to determine in a primary care setting whether taking antihypertensives at bedtime, as opposed to upon waking, more effectively reduces CVD risk.
STUDY SUMMARY
PM vs AM antihypertensive dosing reduces CV events
This prospective, randomized, open-label, blinded endpoint trial of antihypertensive medication administration timing was part of a large, multicenter Spanish study investigating ambulatory BP monitoring (ABPM) as a routine diagnostic tool.
Study participants were randomly assigned in a 1:1 ratio to 2 treatment arms; participants either took all of their BP medications in the morning upon waking (n = 9532) or right before bedtime (n = 9552). The study was conducted in a primary care clinical setting. It included adult participants (age ≥ 18 years) with hypertension (defined as having at least 1 of the following benchmarks: awake systolic BP [SBP] mean ≥ 135 mm Hg, awake diastolic BP (DBP) mean ≥ 85 mm Hg, asleep SBP mean ≥ 120 mm Hg, asleep DBP mean ≥ 70 mm Hg as corroborated by 48-hour ABPM) who were taking at least 1 antihypertensive medication.
Continue to: Any antihypertension medication...
Any antihypertension medication included in the Spanish national formulary was allowed (exact agents were not delineated, but the following classes were included: ARB, ACE inhibitor, calcium channel blocker [CCB], beta-blocker, and/or diuretic). All BP medications had to be dosed once daily for inclusion. Exclusion criteria included pregnancy, night or rotating-shift work, alcohol or other substance dependence, acquired immunodeficiency syndrome, preexisting CVD (unstable angina, heart failure, arrhythmia, kidney failure, and retinopathy), inability to tolerate ABPM, and inability to comply with required 1-year follow-up.
Upon enrollment and at every subsequent clinic visit (scheduled at least annually), participants underwent 48-hour ABPM. Those with uncontrolled BP or elevated CVD risk had scheduled follow-up and ABPM more frequently. The primary outcome was a composite of CVD events including new-onset myocardial infarction, coronary revascularization, heart failure, ischemic stroke, hemorrhagic stroke, and CVD death. Secondary endpoints were individually analyzed primary outcomes of CVD events. The typical patient at baseline was 60.5 years of age with a body mass index of 29.7, an almost 9-year duration of hypertension, and a baseline office BP of 149/86 mm Hg. The patient break-out by antihypertensive class (awakening vs bedtime groups) was as follows: ARB (53% vs 53%), ACE inhibitor (25% vs 23%), CCB (33% vs 37%), beta-blocker (22% vs 18%), and diuretic (47% vs 40%).
During the median 6.3-year patient follow-up period, 1752 participants experienced a total of 2454 CVD events. Patients in the bedtime administration group, compared with those in the morning group, showed significantly lower risk for a CVD event (hazard ratio [HR] = 0.55; 95% confidence interval [CI], 0.50-0.61; P < .001). Also, there was a lower risk for individual CVD events in the bedtime administration group: CVD death (HR = 0.44; 95% CI, 0.34-0.56), myocardial infarction (HR = 0.66; 95% CI, 0.52-0.84), coronary revascularization (HR = 0.60; 95% CI, 0.47-0.75), heart failure (HR = 0.58; 95% CI, 0.49-0.70), and stroke (HR = 0.51; 95% CI, 0.41-0.63). This difference remained after correction for multiple potential confounders. There were no differences in adverse events, such as sleep-time hypotension, between groups.
WHAT’S NEW
First RCT in primary care to show dosing time change reduces CV risk
This is the first randomized controlled trial (RCT) performed in a primary care setting to compare before-bedtime to upon-waking administration of antihypertensive medications using clinically significant endpoints. The study demonstrates that a simple change in administration time has the potential to significantly improve the lives of our patients by reducing the risk for cardiovascular events and their medication burden.
CAVEATS
Homogenous population and exclusions limit generalizability
Because the study population consisted of white Spanish men and women, the results may not be generalizable beyond that ethnic group. In addition, the study exclusions limit interpretation in night/rotating-shift employees, patients with secondary hypertension, and those with CVD, chronic kidney disease, or severe retinopathy looking to reduce their risk.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Nighttime urination could lead to nonadherence
Taking diuretics at bedtime may result in unwanted nighttime awakenings for visits to the bathroom, which could lead to nonadherence in some patients.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial [published online ahead of print October 22, 2019]. Eur Heart J. 2019;ehz754. doi:10.1093/eurheartj/ehz754.
2. Dorans KS, Mills KT, Liu Y, et al. Trends in prevalence and control of hypertension according to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline. J Am Heart Assoc. 2018;7:e008888.
3. Hermida RC, Ayala DE, Smolensky MH, et al. Chronotherapy with conventional blood pressure medications improves management of hypertension and reduces cardiovascular and stroke risks. Hypertens Res. 2016;39:277-292.
4. Bowles NP, Thosar SS, Herzig MX, et al. Chronotherapy for hypertension. Curr Hypertens Rep. 2018;20:97.
5. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011:CD004184.
6. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients: the Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145-153.
7. Black HR, Elliott WJ, Grandits G, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289:2073-2082.
8. Arnette DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:e177-e232.
9. Foti K, Wang D, Appel LJ, et al. Hypertension awareness, treatment, and control in US adults: trends in the hypertensive control cascade by population subgroup (National Health and Nutrition Examination Survey, 1999-2016). Am J Epidemiol. 2019;188:2165-2174.
1. Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial [published online ahead of print October 22, 2019]. Eur Heart J. 2019;ehz754. doi:10.1093/eurheartj/ehz754.
2. Dorans KS, Mills KT, Liu Y, et al. Trends in prevalence and control of hypertension according to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline. J Am Heart Assoc. 2018;7:e008888.
3. Hermida RC, Ayala DE, Smolensky MH, et al. Chronotherapy with conventional blood pressure medications improves management of hypertension and reduces cardiovascular and stroke risks. Hypertens Res. 2016;39:277-292.
4. Bowles NP, Thosar SS, Herzig MX, et al. Chronotherapy for hypertension. Curr Hypertens Rep. 2018;20:97.
5. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011:CD004184.
6. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients: the Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145-153.
7. Black HR, Elliott WJ, Grandits G, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289:2073-2082.
8. Arnette DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:e177-e232.
9. Foti K, Wang D, Appel LJ, et al. Hypertension awareness, treatment, and control in US adults: trends in the hypertensive control cascade by population subgroup (National Health and Nutrition Examination Survey, 1999-2016). Am J Epidemiol. 2019;188:2165-2174.
PRACTICE CHANGER
Advise patients to take blood pressure (BP) medication at bedtime rather than upon waking because it results in a decrease in major cardiovascular disease events.
STRENGTH OF RECOMMENDATION
B: Based on a single, good-quality, multicenter trial.
Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial [published online ahead of print October 22, 2019]. Eur Heart J. 2019;ehz754. doi:10.1093/eurheartj/ehz754.1
MIS-C cardiac evaluation requires more than EF
Patients with multisystem inflammatory syndrome caused by COVID-19 typically seem to avoid coronary artery dilation early on, but they may be prone to cardiac injury and dysfunction longer term that requires a more discerning diagnostic approach to sort out.
The findings were revealed in a study of 28 children with COVID-19–related multisystem inflammatory syndrome (MIS-C) at Children’s Hospital of Philadelphia. The study reported that cardiac injury and dysfunction are common in these patients – even those who have preserved ejection fraction – and that diastolic dysfunction is persistent. For comparison, the study also included 20 healthy controls and 20 patients with classic Kawasaki disease (KD).
The study analyzed echocardiography findings in the patients, reporting left ventricular (LV) systolic and diastolic function were worse than in classic Kawasaki disease (KD), which MIS-C mimics. Lead author Daisuke Matsubara, MD, PhD, and colleagues reported that four markers – LV global longitudinal strain, LV circumferential strain rate, right ventricular strain, and left atrial strain – were the strongest predictors of myocardial injury in these patients. After the acute phase, systolic function tended to recover, but diastolic dysfunction persisted.
‘Strain’ measurement boosts accuracy
While echocardiography has been reported to be valuable in evaluating coronary artery function in MIS-C patients, Dr. Matsubara of the division of cardiology at CHOP, said in an interview that study is the first to use the newer echocardiography indexes, known as “strain,” to assess heart function.
“Strain is a more sensitive tool than more conventional indexes and can detect subtle decrease in heart function, even when ejection fraction is preserved,” he said. “Numerous publications have reached conclusions that strain improves the prognostic and diagnostic accuracy of echocardiography in a wide variety of cardiac pathologies causing LV dysfunction.”
Dr. Matsubara noted that the coronary arteries were mostly unaffected in the acute stage of MIS-C, as only one patient in their MIS-C cohort had coronary artery involvement, which normalized during early follow-up. “On the other hand, 20% of our classic KD patients had coronary abnormalities, including two with aneurysms.”
By using positive troponin I or elevated brain natriuretic peptide (BNP) to assess cardiac injury, they found a “high” (60%) incidence of myocardial injury in their MIS-C cohort. During early follow-up, most of the MIS-C patients showed normalization of systolic function, although diastolic dysfunction persisted.
When compared with the classic KD group, MIS-C patients had higher rates of mitral regurgitation (46% vs. 15%, P = .06), more pericardial effusion (32% vs. 15%, P = 0.46), and more pleural effusion (39% vs. 0%, P = .004). MIS-C patients with suspected myocardial injury show these findings more frequently than those with actual myocardial injury.
Compared with the healthy controls, the MIS-C patients showed both LV systolic and diastolic dysfunction as well as significantly lower left atrium (LA) strain and peak right ventricle (RV) free-wall longitudinal strain.
“In addition to the left ventricle, two other chambers of the heart, the LA and the RV that are often labeled as the ‘forgotten chambers’ of the heart, were also affected by MIS-C,” Dr. Matsubara said. “Both LA and RV strains were markedly reduced in MIS-C patients, compared to normal and KD patients.”
The study also indicates that elevated troponin I levels may not be as dire in children as they are in adults. Dr. Matsubara cited a study of more than 2,700 adult COVID-19 patients that found that even mild increases in troponin I level were associated with increased death during hospitalization (J Am Coll Cardiol. 2020;76:533-46).
However, most of the patients in the CHOP study, even those with elevated troponin I levels, recovered systolic function quickly. “We speculate that the elevation in cardiac troponins may have less dire implications in children, likely due to a more transient type of cardiac injury and less comorbidities in children,” he said. “Clearly further studies are needed before a definitive statement can be made.”
Dr. Matsubara added that recovered COVID-19 patients may be able to participate in sports as some schools reopen. “We are not saying restrict sport participation, but we are merely urging caution.”
Comprehensive LV evaluation needed
The findings reinforce that myocardial involvement is more frequent and sometimes more severe in MIS-C than previously thought, said Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital. “We are underestimating it by using just traditional measures like ejection fraction. It requires a comprehensive evaluation of left ventricular function; it really affects all aspects of the ventricle, both the systolic function and the diastolic function.”
This study supports that MIS-C patients should have a more detailed analysis than EF on echocardiography, including strain imaging. “Probably these patients should all be followed at centers where they can evaluate a more detailed analysis of the LV and RV function,” he said. Patients with ongoing CA enlargement and LV dysfunction should have follow-up cardiac care indefinitely. Patients who have no cardiac symptoms during the acute phase probably don’t need long-term follow-up.
“We’re just trying to learn more about this disease, and it’s certainly concerning that so many kids are having cardiac involvement,” Dr. Friedman said. “Fortunately they’re getting better; we’re just trying to find out what this means for the long term.”
Dr. Matsubara and Dr. Friedman have no relevant financial disclosures.
SOURCE: Matsubara D et al. J Am Coll Cardiol. 2020 Sep 2. doi: 10.1016/j.jacc.2020.08.056.
Patients with multisystem inflammatory syndrome caused by COVID-19 typically seem to avoid coronary artery dilation early on, but they may be prone to cardiac injury and dysfunction longer term that requires a more discerning diagnostic approach to sort out.
The findings were revealed in a study of 28 children with COVID-19–related multisystem inflammatory syndrome (MIS-C) at Children’s Hospital of Philadelphia. The study reported that cardiac injury and dysfunction are common in these patients – even those who have preserved ejection fraction – and that diastolic dysfunction is persistent. For comparison, the study also included 20 healthy controls and 20 patients with classic Kawasaki disease (KD).
The study analyzed echocardiography findings in the patients, reporting left ventricular (LV) systolic and diastolic function were worse than in classic Kawasaki disease (KD), which MIS-C mimics. Lead author Daisuke Matsubara, MD, PhD, and colleagues reported that four markers – LV global longitudinal strain, LV circumferential strain rate, right ventricular strain, and left atrial strain – were the strongest predictors of myocardial injury in these patients. After the acute phase, systolic function tended to recover, but diastolic dysfunction persisted.
‘Strain’ measurement boosts accuracy
While echocardiography has been reported to be valuable in evaluating coronary artery function in MIS-C patients, Dr. Matsubara of the division of cardiology at CHOP, said in an interview that study is the first to use the newer echocardiography indexes, known as “strain,” to assess heart function.
“Strain is a more sensitive tool than more conventional indexes and can detect subtle decrease in heart function, even when ejection fraction is preserved,” he said. “Numerous publications have reached conclusions that strain improves the prognostic and diagnostic accuracy of echocardiography in a wide variety of cardiac pathologies causing LV dysfunction.”
Dr. Matsubara noted that the coronary arteries were mostly unaffected in the acute stage of MIS-C, as only one patient in their MIS-C cohort had coronary artery involvement, which normalized during early follow-up. “On the other hand, 20% of our classic KD patients had coronary abnormalities, including two with aneurysms.”
By using positive troponin I or elevated brain natriuretic peptide (BNP) to assess cardiac injury, they found a “high” (60%) incidence of myocardial injury in their MIS-C cohort. During early follow-up, most of the MIS-C patients showed normalization of systolic function, although diastolic dysfunction persisted.
When compared with the classic KD group, MIS-C patients had higher rates of mitral regurgitation (46% vs. 15%, P = .06), more pericardial effusion (32% vs. 15%, P = 0.46), and more pleural effusion (39% vs. 0%, P = .004). MIS-C patients with suspected myocardial injury show these findings more frequently than those with actual myocardial injury.
Compared with the healthy controls, the MIS-C patients showed both LV systolic and diastolic dysfunction as well as significantly lower left atrium (LA) strain and peak right ventricle (RV) free-wall longitudinal strain.
“In addition to the left ventricle, two other chambers of the heart, the LA and the RV that are often labeled as the ‘forgotten chambers’ of the heart, were also affected by MIS-C,” Dr. Matsubara said. “Both LA and RV strains were markedly reduced in MIS-C patients, compared to normal and KD patients.”
The study also indicates that elevated troponin I levels may not be as dire in children as they are in adults. Dr. Matsubara cited a study of more than 2,700 adult COVID-19 patients that found that even mild increases in troponin I level were associated with increased death during hospitalization (J Am Coll Cardiol. 2020;76:533-46).
However, most of the patients in the CHOP study, even those with elevated troponin I levels, recovered systolic function quickly. “We speculate that the elevation in cardiac troponins may have less dire implications in children, likely due to a more transient type of cardiac injury and less comorbidities in children,” he said. “Clearly further studies are needed before a definitive statement can be made.”
Dr. Matsubara added that recovered COVID-19 patients may be able to participate in sports as some schools reopen. “We are not saying restrict sport participation, but we are merely urging caution.”
Comprehensive LV evaluation needed
The findings reinforce that myocardial involvement is more frequent and sometimes more severe in MIS-C than previously thought, said Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital. “We are underestimating it by using just traditional measures like ejection fraction. It requires a comprehensive evaluation of left ventricular function; it really affects all aspects of the ventricle, both the systolic function and the diastolic function.”
This study supports that MIS-C patients should have a more detailed analysis than EF on echocardiography, including strain imaging. “Probably these patients should all be followed at centers where they can evaluate a more detailed analysis of the LV and RV function,” he said. Patients with ongoing CA enlargement and LV dysfunction should have follow-up cardiac care indefinitely. Patients who have no cardiac symptoms during the acute phase probably don’t need long-term follow-up.
“We’re just trying to learn more about this disease, and it’s certainly concerning that so many kids are having cardiac involvement,” Dr. Friedman said. “Fortunately they’re getting better; we’re just trying to find out what this means for the long term.”
Dr. Matsubara and Dr. Friedman have no relevant financial disclosures.
SOURCE: Matsubara D et al. J Am Coll Cardiol. 2020 Sep 2. doi: 10.1016/j.jacc.2020.08.056.
Patients with multisystem inflammatory syndrome caused by COVID-19 typically seem to avoid coronary artery dilation early on, but they may be prone to cardiac injury and dysfunction longer term that requires a more discerning diagnostic approach to sort out.
The findings were revealed in a study of 28 children with COVID-19–related multisystem inflammatory syndrome (MIS-C) at Children’s Hospital of Philadelphia. The study reported that cardiac injury and dysfunction are common in these patients – even those who have preserved ejection fraction – and that diastolic dysfunction is persistent. For comparison, the study also included 20 healthy controls and 20 patients with classic Kawasaki disease (KD).
The study analyzed echocardiography findings in the patients, reporting left ventricular (LV) systolic and diastolic function were worse than in classic Kawasaki disease (KD), which MIS-C mimics. Lead author Daisuke Matsubara, MD, PhD, and colleagues reported that four markers – LV global longitudinal strain, LV circumferential strain rate, right ventricular strain, and left atrial strain – were the strongest predictors of myocardial injury in these patients. After the acute phase, systolic function tended to recover, but diastolic dysfunction persisted.
‘Strain’ measurement boosts accuracy
While echocardiography has been reported to be valuable in evaluating coronary artery function in MIS-C patients, Dr. Matsubara of the division of cardiology at CHOP, said in an interview that study is the first to use the newer echocardiography indexes, known as “strain,” to assess heart function.
“Strain is a more sensitive tool than more conventional indexes and can detect subtle decrease in heart function, even when ejection fraction is preserved,” he said. “Numerous publications have reached conclusions that strain improves the prognostic and diagnostic accuracy of echocardiography in a wide variety of cardiac pathologies causing LV dysfunction.”
Dr. Matsubara noted that the coronary arteries were mostly unaffected in the acute stage of MIS-C, as only one patient in their MIS-C cohort had coronary artery involvement, which normalized during early follow-up. “On the other hand, 20% of our classic KD patients had coronary abnormalities, including two with aneurysms.”
By using positive troponin I or elevated brain natriuretic peptide (BNP) to assess cardiac injury, they found a “high” (60%) incidence of myocardial injury in their MIS-C cohort. During early follow-up, most of the MIS-C patients showed normalization of systolic function, although diastolic dysfunction persisted.
When compared with the classic KD group, MIS-C patients had higher rates of mitral regurgitation (46% vs. 15%, P = .06), more pericardial effusion (32% vs. 15%, P = 0.46), and more pleural effusion (39% vs. 0%, P = .004). MIS-C patients with suspected myocardial injury show these findings more frequently than those with actual myocardial injury.
Compared with the healthy controls, the MIS-C patients showed both LV systolic and diastolic dysfunction as well as significantly lower left atrium (LA) strain and peak right ventricle (RV) free-wall longitudinal strain.
“In addition to the left ventricle, two other chambers of the heart, the LA and the RV that are often labeled as the ‘forgotten chambers’ of the heart, were also affected by MIS-C,” Dr. Matsubara said. “Both LA and RV strains were markedly reduced in MIS-C patients, compared to normal and KD patients.”
The study also indicates that elevated troponin I levels may not be as dire in children as they are in adults. Dr. Matsubara cited a study of more than 2,700 adult COVID-19 patients that found that even mild increases in troponin I level were associated with increased death during hospitalization (J Am Coll Cardiol. 2020;76:533-46).
However, most of the patients in the CHOP study, even those with elevated troponin I levels, recovered systolic function quickly. “We speculate that the elevation in cardiac troponins may have less dire implications in children, likely due to a more transient type of cardiac injury and less comorbidities in children,” he said. “Clearly further studies are needed before a definitive statement can be made.”
Dr. Matsubara added that recovered COVID-19 patients may be able to participate in sports as some schools reopen. “We are not saying restrict sport participation, but we are merely urging caution.”
Comprehensive LV evaluation needed
The findings reinforce that myocardial involvement is more frequent and sometimes more severe in MIS-C than previously thought, said Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital. “We are underestimating it by using just traditional measures like ejection fraction. It requires a comprehensive evaluation of left ventricular function; it really affects all aspects of the ventricle, both the systolic function and the diastolic function.”
This study supports that MIS-C patients should have a more detailed analysis than EF on echocardiography, including strain imaging. “Probably these patients should all be followed at centers where they can evaluate a more detailed analysis of the LV and RV function,” he said. Patients with ongoing CA enlargement and LV dysfunction should have follow-up cardiac care indefinitely. Patients who have no cardiac symptoms during the acute phase probably don’t need long-term follow-up.
“We’re just trying to learn more about this disease, and it’s certainly concerning that so many kids are having cardiac involvement,” Dr. Friedman said. “Fortunately they’re getting better; we’re just trying to find out what this means for the long term.”
Dr. Matsubara and Dr. Friedman have no relevant financial disclosures.
SOURCE: Matsubara D et al. J Am Coll Cardiol. 2020 Sep 2. doi: 10.1016/j.jacc.2020.08.056.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Final EVAPORATE results for Vascepa raise eyebrows
Final 18-month results of the EVAPORATE trial suggest icosapent ethyl (Vascepa) provides even greater slowing of coronary plaque progression when added to statins for patients with high triglyceride levels, but not all cardiologists are convinced.
The study was designed to explore a potential mechanism behind the cardiovascular event reduction in REDUCE-IT. Previously reported interim results showed that, after 9 months, the pharmaceutical-grade omega-3 fatty acid formation significantly slowed the progression of several plaque types but not the primary endpoint of change in low-attenuation plaque volume on multidetector CT.
From baseline to 18-month follow-up, however, the primary endpoint was significantly reduced by 17% in the icosapent ethyl group, whereas low-attenuation plaque volumes increased by 109% in the placebo group (P = .0061).
Significant declines were also seen with icosapent ethyl 4 g/day versus the mineral oil placebo for all other plaque types except dense calcium after adjustment for age, sex, diabetes, hypertension, and triglyceride levels at baseline:
- Dense calcium: –1% versus 15% (P = .0531).
- Fibro-fatty: –34% versus 32% (P = .0002).
- Fibrous: –20% versus 1% (P = .0028).
- Noncalcified: –19% versus 9% (P = .0005).
- Total plaque: –9% versus 11% (P = .0019).
The results parallel nicely with recent clinical data from REDUCE-IT REVASC, in which icosapent ethyl 4 g/day provided a very early benefit on first revascularization events that reached statistical significance after only 11 months (hazard ratio, 0.66), principal investigator Matthew Budoff, MD, director of cardiac CT at Harbor–University of California, Los Angeles, Medical Center in Torrance, Calif., said during the virtual European Society of Cardiology Congress 2020.
The findings were also published simultaneously in the European Heart Journal and quickly prompted a flurry of comments on social media.
Some were supportive. Christopher Cannon, MD, of Harvard Medical School, Boston; Dan Soffer, MD, a lipidologist at the University of Pennsylvania, Philadelphia; and Viet Le, MPAS, PA, a researcher at the Intermountain Heart Institute, Murray, Utah, took to Twitter to praise Dr. Budoff and the final results of the mechanistic study. Dr. Soffer called the study “elegant,” while Dr. Cannon said the results provide “important mechanistic data on plaque character.”
Others were highly critical, including a poll questioning whether the article should be retracted or revised.
Ibrahim H. Tanboga, MD, PhD, a cardiology professor and biostatistician at Hisar Intercontinental Hospital in Istanbul, questioned how the longitudinal change in low-attenuation plaque was possible clinically; his plot of the data showed these lesions getting worse in both arms before getting better in both arms.
A more volatile exchange concerned whether there were differences in the baseline characteristics between the two groups and whether the data might have been unblinded.
“I am sympathetic to the boss of a big laboratory [who] might not know how every step of the process was done and therefore might not be aware of opportunities for accidental bias. This can easily happen in a large and active department,” Darrel Francis, MD, professor of cardiology at the National Heart and Lung Institute, Imperial College, London, said in an interview.
An alternative explanation proffered on Twitter was that the interim analysis found no significant differences in baseline measures because it used nonparametric tests, whereas log transformation was applied to the final data. In any event, the tweets prompted a sharp rebuke from Dr. Budoff.
Dr. Francis raised another point of contention on Twitter regarding the degree of plaque progression in the placebo group.
In an interview, Dr. Francis pointed out that the final data represent the percentage change in the logarithm, not the actual percentage change in atheroma. So the increase in total atheroma volume in the placebo arm is not 11% but rather a scaling-up by 100.4 or 2.51, in other words, 151%.
He also offered a “less subtle feature of possible erroneous data,” in that the abstract reported low-attenuation plaque “more than doubles” in 18 months, which he described as a “ghastly supercharged version of Moore’s law for atheroma, instead of microchips.”
So “either it’s a mistake in the measurement or the placebo is harmful, because I can’t see how this is sustainable,” he said. “Why isn’t everyone dead from coronary disease?”
Concerns were raised previously over the possibility that the mineral oil placebo used in both EVAPORATE and REDUCE-IT could be having ill effects, notably, by increasing LDL cholesterol and C-reactive protein levels.
In an interview, Steven Nissen, MD, who is chair of cardiovascular medicine at the Cleveland Clinic and has been among the critics of the mineral oil placebo, also questioned the plaque progression over the 18 months.
“I’ve published more than dozen regression/progression trials, and we have never seen anything like this in a placebo group, ever,” he said. “If this was a clean placebo, why would this happen in a short amount of time?
“I’m concerned this is all about an increase, in the case of REDUCE-IT, in morbidity and mortality in the placebo group, and in the EVAPORATE trial, an increase in plaque in the placebo group,” Dr. Nissen said. “So this raises serious doubts about whether there is any benefit to icosapent ethyl.”
Asked about the 109% increase, Dr. Budoff said in an interview that low-attenuation plaque represents a much smaller quantity of overall plaque volume. “So the percentages might be exaggerated if you look at just percentage change because they;re small volumes.”
He also noted that previous trials that evaluated atherosclerosis progression used intravascular ultrasound (IVUS), whereas EVAPORATE is the first to make the transition to CT angiography-based analysis of plaque progression.
“I would point out that Dr. Nissen has only worked on intravascular ultrasound, which, while it’s parallel in its ability to measure plaque, measures different volumes and measures it in a totally different way,” said Dr. Budoff. “So I don’t think we can directly compare the results of CT angiography to Dr. Nissen’s examples of IVUS.”
During his presentation, Dr. Budoff highlighted their recent data showing a similar rate of plaque progression between the mineral oil placebo in EVAPORATE and a cellulose-based placebo in the Garlic5 study. “So we have high confidence that the benefits seen in this trial with icosapent ethyl represent icosapent ethyl’s beneficial effects on atherosclerosis and not harm of mineral oil,” he said.
Exactly how icosapent ethyl is slowing atherosclerosis, however, is not fully known, Dr. Budoff said in an interview. “It might be inflammation and oxidation; those have both been shown to be better with icosapent ethyl, but I don’t think we fully understand the implications of these results.”
Dr. Budoff dismissed tweets that suggest the data might have been unblinded as unprofessional and said they are requesting that Imperial College have Francis cease and desist.
“He doesn’t have the actual data, so there is no way to do statistics without the dataset. The whole thing is inappropriate,” Dr. Budoff said.
Amarin Pharma provided funding and drug for the trial. Dr. Budoff has received research funding from and has served as a speaker for Amarin Pharma, Amgen, AstraZeneca, Boehringer Ingelheim, Novo Nordisk, and Pfizer and has served as a speaker for Bristol-Myers Squibb. Dr. Francis has disclosed no relevant financial relationships..
A version of this article originally appeared on Medscape.com.
Final 18-month results of the EVAPORATE trial suggest icosapent ethyl (Vascepa) provides even greater slowing of coronary plaque progression when added to statins for patients with high triglyceride levels, but not all cardiologists are convinced.
The study was designed to explore a potential mechanism behind the cardiovascular event reduction in REDUCE-IT. Previously reported interim results showed that, after 9 months, the pharmaceutical-grade omega-3 fatty acid formation significantly slowed the progression of several plaque types but not the primary endpoint of change in low-attenuation plaque volume on multidetector CT.
From baseline to 18-month follow-up, however, the primary endpoint was significantly reduced by 17% in the icosapent ethyl group, whereas low-attenuation plaque volumes increased by 109% in the placebo group (P = .0061).
Significant declines were also seen with icosapent ethyl 4 g/day versus the mineral oil placebo for all other plaque types except dense calcium after adjustment for age, sex, diabetes, hypertension, and triglyceride levels at baseline:
- Dense calcium: –1% versus 15% (P = .0531).
- Fibro-fatty: –34% versus 32% (P = .0002).
- Fibrous: –20% versus 1% (P = .0028).
- Noncalcified: –19% versus 9% (P = .0005).
- Total plaque: –9% versus 11% (P = .0019).
The results parallel nicely with recent clinical data from REDUCE-IT REVASC, in which icosapent ethyl 4 g/day provided a very early benefit on first revascularization events that reached statistical significance after only 11 months (hazard ratio, 0.66), principal investigator Matthew Budoff, MD, director of cardiac CT at Harbor–University of California, Los Angeles, Medical Center in Torrance, Calif., said during the virtual European Society of Cardiology Congress 2020.
The findings were also published simultaneously in the European Heart Journal and quickly prompted a flurry of comments on social media.
Some were supportive. Christopher Cannon, MD, of Harvard Medical School, Boston; Dan Soffer, MD, a lipidologist at the University of Pennsylvania, Philadelphia; and Viet Le, MPAS, PA, a researcher at the Intermountain Heart Institute, Murray, Utah, took to Twitter to praise Dr. Budoff and the final results of the mechanistic study. Dr. Soffer called the study “elegant,” while Dr. Cannon said the results provide “important mechanistic data on plaque character.”
Others were highly critical, including a poll questioning whether the article should be retracted or revised.
Ibrahim H. Tanboga, MD, PhD, a cardiology professor and biostatistician at Hisar Intercontinental Hospital in Istanbul, questioned how the longitudinal change in low-attenuation plaque was possible clinically; his plot of the data showed these lesions getting worse in both arms before getting better in both arms.
A more volatile exchange concerned whether there were differences in the baseline characteristics between the two groups and whether the data might have been unblinded.
“I am sympathetic to the boss of a big laboratory [who] might not know how every step of the process was done and therefore might not be aware of opportunities for accidental bias. This can easily happen in a large and active department,” Darrel Francis, MD, professor of cardiology at the National Heart and Lung Institute, Imperial College, London, said in an interview.
An alternative explanation proffered on Twitter was that the interim analysis found no significant differences in baseline measures because it used nonparametric tests, whereas log transformation was applied to the final data. In any event, the tweets prompted a sharp rebuke from Dr. Budoff.
Dr. Francis raised another point of contention on Twitter regarding the degree of plaque progression in the placebo group.
In an interview, Dr. Francis pointed out that the final data represent the percentage change in the logarithm, not the actual percentage change in atheroma. So the increase in total atheroma volume in the placebo arm is not 11% but rather a scaling-up by 100.4 or 2.51, in other words, 151%.
He also offered a “less subtle feature of possible erroneous data,” in that the abstract reported low-attenuation plaque “more than doubles” in 18 months, which he described as a “ghastly supercharged version of Moore’s law for atheroma, instead of microchips.”
So “either it’s a mistake in the measurement or the placebo is harmful, because I can’t see how this is sustainable,” he said. “Why isn’t everyone dead from coronary disease?”
Concerns were raised previously over the possibility that the mineral oil placebo used in both EVAPORATE and REDUCE-IT could be having ill effects, notably, by increasing LDL cholesterol and C-reactive protein levels.
In an interview, Steven Nissen, MD, who is chair of cardiovascular medicine at the Cleveland Clinic and has been among the critics of the mineral oil placebo, also questioned the plaque progression over the 18 months.
“I’ve published more than dozen regression/progression trials, and we have never seen anything like this in a placebo group, ever,” he said. “If this was a clean placebo, why would this happen in a short amount of time?
“I’m concerned this is all about an increase, in the case of REDUCE-IT, in morbidity and mortality in the placebo group, and in the EVAPORATE trial, an increase in plaque in the placebo group,” Dr. Nissen said. “So this raises serious doubts about whether there is any benefit to icosapent ethyl.”
Asked about the 109% increase, Dr. Budoff said in an interview that low-attenuation plaque represents a much smaller quantity of overall plaque volume. “So the percentages might be exaggerated if you look at just percentage change because they;re small volumes.”
He also noted that previous trials that evaluated atherosclerosis progression used intravascular ultrasound (IVUS), whereas EVAPORATE is the first to make the transition to CT angiography-based analysis of plaque progression.
“I would point out that Dr. Nissen has only worked on intravascular ultrasound, which, while it’s parallel in its ability to measure plaque, measures different volumes and measures it in a totally different way,” said Dr. Budoff. “So I don’t think we can directly compare the results of CT angiography to Dr. Nissen’s examples of IVUS.”
During his presentation, Dr. Budoff highlighted their recent data showing a similar rate of plaque progression between the mineral oil placebo in EVAPORATE and a cellulose-based placebo in the Garlic5 study. “So we have high confidence that the benefits seen in this trial with icosapent ethyl represent icosapent ethyl’s beneficial effects on atherosclerosis and not harm of mineral oil,” he said.
Exactly how icosapent ethyl is slowing atherosclerosis, however, is not fully known, Dr. Budoff said in an interview. “It might be inflammation and oxidation; those have both been shown to be better with icosapent ethyl, but I don’t think we fully understand the implications of these results.”
Dr. Budoff dismissed tweets that suggest the data might have been unblinded as unprofessional and said they are requesting that Imperial College have Francis cease and desist.
“He doesn’t have the actual data, so there is no way to do statistics without the dataset. The whole thing is inappropriate,” Dr. Budoff said.
Amarin Pharma provided funding and drug for the trial. Dr. Budoff has received research funding from and has served as a speaker for Amarin Pharma, Amgen, AstraZeneca, Boehringer Ingelheim, Novo Nordisk, and Pfizer and has served as a speaker for Bristol-Myers Squibb. Dr. Francis has disclosed no relevant financial relationships..
A version of this article originally appeared on Medscape.com.
Final 18-month results of the EVAPORATE trial suggest icosapent ethyl (Vascepa) provides even greater slowing of coronary plaque progression when added to statins for patients with high triglyceride levels, but not all cardiologists are convinced.
The study was designed to explore a potential mechanism behind the cardiovascular event reduction in REDUCE-IT. Previously reported interim results showed that, after 9 months, the pharmaceutical-grade omega-3 fatty acid formation significantly slowed the progression of several plaque types but not the primary endpoint of change in low-attenuation plaque volume on multidetector CT.
From baseline to 18-month follow-up, however, the primary endpoint was significantly reduced by 17% in the icosapent ethyl group, whereas low-attenuation plaque volumes increased by 109% in the placebo group (P = .0061).
Significant declines were also seen with icosapent ethyl 4 g/day versus the mineral oil placebo for all other plaque types except dense calcium after adjustment for age, sex, diabetes, hypertension, and triglyceride levels at baseline:
- Dense calcium: –1% versus 15% (P = .0531).
- Fibro-fatty: –34% versus 32% (P = .0002).
- Fibrous: –20% versus 1% (P = .0028).
- Noncalcified: –19% versus 9% (P = .0005).
- Total plaque: –9% versus 11% (P = .0019).
The results parallel nicely with recent clinical data from REDUCE-IT REVASC, in which icosapent ethyl 4 g/day provided a very early benefit on first revascularization events that reached statistical significance after only 11 months (hazard ratio, 0.66), principal investigator Matthew Budoff, MD, director of cardiac CT at Harbor–University of California, Los Angeles, Medical Center in Torrance, Calif., said during the virtual European Society of Cardiology Congress 2020.
The findings were also published simultaneously in the European Heart Journal and quickly prompted a flurry of comments on social media.
Some were supportive. Christopher Cannon, MD, of Harvard Medical School, Boston; Dan Soffer, MD, a lipidologist at the University of Pennsylvania, Philadelphia; and Viet Le, MPAS, PA, a researcher at the Intermountain Heart Institute, Murray, Utah, took to Twitter to praise Dr. Budoff and the final results of the mechanistic study. Dr. Soffer called the study “elegant,” while Dr. Cannon said the results provide “important mechanistic data on plaque character.”
Others were highly critical, including a poll questioning whether the article should be retracted or revised.
Ibrahim H. Tanboga, MD, PhD, a cardiology professor and biostatistician at Hisar Intercontinental Hospital in Istanbul, questioned how the longitudinal change in low-attenuation plaque was possible clinically; his plot of the data showed these lesions getting worse in both arms before getting better in both arms.
A more volatile exchange concerned whether there were differences in the baseline characteristics between the two groups and whether the data might have been unblinded.
“I am sympathetic to the boss of a big laboratory [who] might not know how every step of the process was done and therefore might not be aware of opportunities for accidental bias. This can easily happen in a large and active department,” Darrel Francis, MD, professor of cardiology at the National Heart and Lung Institute, Imperial College, London, said in an interview.
An alternative explanation proffered on Twitter was that the interim analysis found no significant differences in baseline measures because it used nonparametric tests, whereas log transformation was applied to the final data. In any event, the tweets prompted a sharp rebuke from Dr. Budoff.
Dr. Francis raised another point of contention on Twitter regarding the degree of plaque progression in the placebo group.
In an interview, Dr. Francis pointed out that the final data represent the percentage change in the logarithm, not the actual percentage change in atheroma. So the increase in total atheroma volume in the placebo arm is not 11% but rather a scaling-up by 100.4 or 2.51, in other words, 151%.
He also offered a “less subtle feature of possible erroneous data,” in that the abstract reported low-attenuation plaque “more than doubles” in 18 months, which he described as a “ghastly supercharged version of Moore’s law for atheroma, instead of microchips.”
So “either it’s a mistake in the measurement or the placebo is harmful, because I can’t see how this is sustainable,” he said. “Why isn’t everyone dead from coronary disease?”
Concerns were raised previously over the possibility that the mineral oil placebo used in both EVAPORATE and REDUCE-IT could be having ill effects, notably, by increasing LDL cholesterol and C-reactive protein levels.
In an interview, Steven Nissen, MD, who is chair of cardiovascular medicine at the Cleveland Clinic and has been among the critics of the mineral oil placebo, also questioned the plaque progression over the 18 months.
“I’ve published more than dozen regression/progression trials, and we have never seen anything like this in a placebo group, ever,” he said. “If this was a clean placebo, why would this happen in a short amount of time?
“I’m concerned this is all about an increase, in the case of REDUCE-IT, in morbidity and mortality in the placebo group, and in the EVAPORATE trial, an increase in plaque in the placebo group,” Dr. Nissen said. “So this raises serious doubts about whether there is any benefit to icosapent ethyl.”
Asked about the 109% increase, Dr. Budoff said in an interview that low-attenuation plaque represents a much smaller quantity of overall plaque volume. “So the percentages might be exaggerated if you look at just percentage change because they;re small volumes.”
He also noted that previous trials that evaluated atherosclerosis progression used intravascular ultrasound (IVUS), whereas EVAPORATE is the first to make the transition to CT angiography-based analysis of plaque progression.
“I would point out that Dr. Nissen has only worked on intravascular ultrasound, which, while it’s parallel in its ability to measure plaque, measures different volumes and measures it in a totally different way,” said Dr. Budoff. “So I don’t think we can directly compare the results of CT angiography to Dr. Nissen’s examples of IVUS.”
During his presentation, Dr. Budoff highlighted their recent data showing a similar rate of plaque progression between the mineral oil placebo in EVAPORATE and a cellulose-based placebo in the Garlic5 study. “So we have high confidence that the benefits seen in this trial with icosapent ethyl represent icosapent ethyl’s beneficial effects on atherosclerosis and not harm of mineral oil,” he said.
Exactly how icosapent ethyl is slowing atherosclerosis, however, is not fully known, Dr. Budoff said in an interview. “It might be inflammation and oxidation; those have both been shown to be better with icosapent ethyl, but I don’t think we fully understand the implications of these results.”
Dr. Budoff dismissed tweets that suggest the data might have been unblinded as unprofessional and said they are requesting that Imperial College have Francis cease and desist.
“He doesn’t have the actual data, so there is no way to do statistics without the dataset. The whole thing is inappropriate,” Dr. Budoff said.
Amarin Pharma provided funding and drug for the trial. Dr. Budoff has received research funding from and has served as a speaker for Amarin Pharma, Amgen, AstraZeneca, Boehringer Ingelheim, Novo Nordisk, and Pfizer and has served as a speaker for Bristol-Myers Squibb. Dr. Francis has disclosed no relevant financial relationships..
A version of this article originally appeared on Medscape.com.
Early evolocumab quickly lowers LDL cholesterol after primary PCI
Early administration of evolocumab significantly reduced levels of LDL cholesterol in patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention, according to data from an open-label randomized trial of 102 adults in Japan.

Data from previous studies have shown that proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can reduce LDL cholesterol in acute coronary syndrome patients, wrote Tomoaki Okada, MD, of Kagawa (Japan) Prefectural Central Hospital and colleagues.
In particular, “The EVOPACS trial [J Am Coll Cardiol 2019; 74:2452-62] reported that evolocumab therapy initiated at an early phase of ACS showed [LDL cholesterol] level reduction by 4-8 weeks,” they said.
“However, the 4-week efficacy of PCSK9 inhibitor therapy combined with a statin remains unknown,” they said.
In a study presented at the virtual annual congress of the European Society of Cardiology and published simultaneously in JACC: Cardiovascular Interventions, the researchers randomized 52 patients to receive 140 mg of evolocumab subcutaneously within 24 hours of indexed percutaneous coronary intervention and again after 2 weeks. A group of 50 controls received evolocumab after PCI only, but no additional dose after 2 weeks.
The average age of the patients was 65 years, 88% were men, and 26% had a history of statin treatment.
A total of 49 patients in each group were included in the final analysis, with a primary outcome of change in LDL cholesterol levels from baseline to 4 weeks.
Baseline LCL cholesterol levels were 120.8 mg/dL and 124.7 mg/dL in the evolocumab and control groups, respectively. Changes from baseline were significantly greater in the evolocumab group, compared with controls, at –76% and –33%, respectively.
All patients in the evolocumab group and 27% of patients in the control groups achieved LDL cholesterol levels of less than 70 mg/dL at 4 weeks. In addition, 92% and 96% of evolocumab patients achieved LDL cholesterol levels less than 55 mg/dL at 2 weeks and 4 weeks, respectively.
Overall changes in non-HDL cholesterol, HDL cholesterol, and small dense LDL in the evolocumab and control groups were –66.2% and –26.0%; 2.8% and –0.7%; and –67% and –13.8%, respectively. Of these, changes in non-HDL cholesterol and small dense LDL were significantly different between the groups.
In addition, patients in the evolocumab group showed a 3% decrease in lipoprotein, compared with an 82% increase in the control group. This finding suggests the additional benefit of including evolocumab for managing residual risk in patients with high lipoprotein(a) levels” after acute MI, the researchers noted.
Adverse events and serious adverse events were similar between the groups.
‘Early and strong’ LDL cholesterol lowering best for preventing repeat events
“By using the PCSK9 inhibitors, we have the opportunity to lower LDL cholesterol [LDL-C]” both quickly and dramatically, said Heinz Drexel, MD, in an interview.
“This Japanese study shows that very low LDL-C levels can be obtained as fast as within 4 weeks,” he said. “This fits into the concept that risk for future infarctions and strokes is best reduced by early and strong LDL-C lowering,” he explained.
Dr. Drexel said that he was not surprised by the magnitude of the decrease in LDL cholesterol in study findings in light of the EVOPACS study and other research, as well as his own clinical experience.
“The primary message for doctors is that it is now possible to achieve these low levels of LDL-C in a short time,” he said.
“Additional research must prove that this low LDL-C translates to reduction of MIs and strokes, and there is increasing evidence that this will happen,” Dr. Drexel noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Drexel had no financial conflicts to disclose.
SOURCE: Okada T et al. ESC 2020. JACC Cardiovascular Interventions. 2020 Aug 28. doi: 10.1016/j.jcin.2020.08.026.
Early administration of evolocumab significantly reduced levels of LDL cholesterol in patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention, according to data from an open-label randomized trial of 102 adults in Japan.

Data from previous studies have shown that proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can reduce LDL cholesterol in acute coronary syndrome patients, wrote Tomoaki Okada, MD, of Kagawa (Japan) Prefectural Central Hospital and colleagues.
In particular, “The EVOPACS trial [J Am Coll Cardiol 2019; 74:2452-62] reported that evolocumab therapy initiated at an early phase of ACS showed [LDL cholesterol] level reduction by 4-8 weeks,” they said.
“However, the 4-week efficacy of PCSK9 inhibitor therapy combined with a statin remains unknown,” they said.
In a study presented at the virtual annual congress of the European Society of Cardiology and published simultaneously in JACC: Cardiovascular Interventions, the researchers randomized 52 patients to receive 140 mg of evolocumab subcutaneously within 24 hours of indexed percutaneous coronary intervention and again after 2 weeks. A group of 50 controls received evolocumab after PCI only, but no additional dose after 2 weeks.
The average age of the patients was 65 years, 88% were men, and 26% had a history of statin treatment.
A total of 49 patients in each group were included in the final analysis, with a primary outcome of change in LDL cholesterol levels from baseline to 4 weeks.
Baseline LCL cholesterol levels were 120.8 mg/dL and 124.7 mg/dL in the evolocumab and control groups, respectively. Changes from baseline were significantly greater in the evolocumab group, compared with controls, at –76% and –33%, respectively.
All patients in the evolocumab group and 27% of patients in the control groups achieved LDL cholesterol levels of less than 70 mg/dL at 4 weeks. In addition, 92% and 96% of evolocumab patients achieved LDL cholesterol levels less than 55 mg/dL at 2 weeks and 4 weeks, respectively.
Overall changes in non-HDL cholesterol, HDL cholesterol, and small dense LDL in the evolocumab and control groups were –66.2% and –26.0%; 2.8% and –0.7%; and –67% and –13.8%, respectively. Of these, changes in non-HDL cholesterol and small dense LDL were significantly different between the groups.
In addition, patients in the evolocumab group showed a 3% decrease in lipoprotein, compared with an 82% increase in the control group. This finding suggests the additional benefit of including evolocumab for managing residual risk in patients with high lipoprotein(a) levels” after acute MI, the researchers noted.
Adverse events and serious adverse events were similar between the groups.
‘Early and strong’ LDL cholesterol lowering best for preventing repeat events
“By using the PCSK9 inhibitors, we have the opportunity to lower LDL cholesterol [LDL-C]” both quickly and dramatically, said Heinz Drexel, MD, in an interview.
“This Japanese study shows that very low LDL-C levels can be obtained as fast as within 4 weeks,” he said. “This fits into the concept that risk for future infarctions and strokes is best reduced by early and strong LDL-C lowering,” he explained.
Dr. Drexel said that he was not surprised by the magnitude of the decrease in LDL cholesterol in study findings in light of the EVOPACS study and other research, as well as his own clinical experience.
“The primary message for doctors is that it is now possible to achieve these low levels of LDL-C in a short time,” he said.
“Additional research must prove that this low LDL-C translates to reduction of MIs and strokes, and there is increasing evidence that this will happen,” Dr. Drexel noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Drexel had no financial conflicts to disclose.
SOURCE: Okada T et al. ESC 2020. JACC Cardiovascular Interventions. 2020 Aug 28. doi: 10.1016/j.jcin.2020.08.026.
Early administration of evolocumab significantly reduced levels of LDL cholesterol in patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention, according to data from an open-label randomized trial of 102 adults in Japan.

Data from previous studies have shown that proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can reduce LDL cholesterol in acute coronary syndrome patients, wrote Tomoaki Okada, MD, of Kagawa (Japan) Prefectural Central Hospital and colleagues.
In particular, “The EVOPACS trial [J Am Coll Cardiol 2019; 74:2452-62] reported that evolocumab therapy initiated at an early phase of ACS showed [LDL cholesterol] level reduction by 4-8 weeks,” they said.
“However, the 4-week efficacy of PCSK9 inhibitor therapy combined with a statin remains unknown,” they said.
In a study presented at the virtual annual congress of the European Society of Cardiology and published simultaneously in JACC: Cardiovascular Interventions, the researchers randomized 52 patients to receive 140 mg of evolocumab subcutaneously within 24 hours of indexed percutaneous coronary intervention and again after 2 weeks. A group of 50 controls received evolocumab after PCI only, but no additional dose after 2 weeks.
The average age of the patients was 65 years, 88% were men, and 26% had a history of statin treatment.
A total of 49 patients in each group were included in the final analysis, with a primary outcome of change in LDL cholesterol levels from baseline to 4 weeks.
Baseline LCL cholesterol levels were 120.8 mg/dL and 124.7 mg/dL in the evolocumab and control groups, respectively. Changes from baseline were significantly greater in the evolocumab group, compared with controls, at –76% and –33%, respectively.
All patients in the evolocumab group and 27% of patients in the control groups achieved LDL cholesterol levels of less than 70 mg/dL at 4 weeks. In addition, 92% and 96% of evolocumab patients achieved LDL cholesterol levels less than 55 mg/dL at 2 weeks and 4 weeks, respectively.
Overall changes in non-HDL cholesterol, HDL cholesterol, and small dense LDL in the evolocumab and control groups were –66.2% and –26.0%; 2.8% and –0.7%; and –67% and –13.8%, respectively. Of these, changes in non-HDL cholesterol and small dense LDL were significantly different between the groups.
In addition, patients in the evolocumab group showed a 3% decrease in lipoprotein, compared with an 82% increase in the control group. This finding suggests the additional benefit of including evolocumab for managing residual risk in patients with high lipoprotein(a) levels” after acute MI, the researchers noted.
Adverse events and serious adverse events were similar between the groups.
‘Early and strong’ LDL cholesterol lowering best for preventing repeat events
“By using the PCSK9 inhibitors, we have the opportunity to lower LDL cholesterol [LDL-C]” both quickly and dramatically, said Heinz Drexel, MD, in an interview.
“This Japanese study shows that very low LDL-C levels can be obtained as fast as within 4 weeks,” he said. “This fits into the concept that risk for future infarctions and strokes is best reduced by early and strong LDL-C lowering,” he explained.
Dr. Drexel said that he was not surprised by the magnitude of the decrease in LDL cholesterol in study findings in light of the EVOPACS study and other research, as well as his own clinical experience.
“The primary message for doctors is that it is now possible to achieve these low levels of LDL-C in a short time,” he said.
“Additional research must prove that this low LDL-C translates to reduction of MIs and strokes, and there is increasing evidence that this will happen,” Dr. Drexel noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Drexel had no financial conflicts to disclose.
SOURCE: Okada T et al. ESC 2020. JACC Cardiovascular Interventions. 2020 Aug 28. doi: 10.1016/j.jcin.2020.08.026.
FROM ESC CONGRESS 2020
Sleep disorders may be undetected precursors for cardiometabolic disease in U.S. Latinos
Sleep disorders may be silent precursors of cardiometabolic disease among U.S. Latinos, said authors of a newly published study.
Xiaoyu Li, ScD, and Susan Redline, MD, MPH, of Harvard Medical School and Brigham and Women’s Hospital, Boston, and coauthors conducted a study of people who self-identified as Latino, who had baseline sleeping disorders, and who developed hypertension and diabetes over time. The study was published in the American Journal of Respiratory and Critical Care Medicine.
The findings suggested that sleep disorders preceded the development of hypertension and diabetes. Examining records from a major multiyear federal project, the Hispanic Community Health Study/Study of Latinos, Dr. Li, Dr. Redline, and coauthors found sleep-disordered breathing (SDB) was associated with a 1.54 higher adjusted odds of incident hypertension (95% confidence interval [CI], 1.18-2.00) and 1.33 higher odds of incident diabetes (95% CI, 1.05-1.67), compared with no SDB. Insomnia was associated with incident hypertension (odds ratio, 1.37; 95% CI, 1.11-1.69), but not diabetes. The association between insomnia and incident hypertension was stronger among men than women, they reported.
“We now need large-scale rigorous trials to evaluate the impact of early treatment of sleep disordered breathing and insomnia on preventing the development of hypertension and diabetes,” Dr. Redline said in an interview. “Clinicians should consider screening their patients at risk for hypertension and diabetes for both sleep-disordered breathing and insomnia.”
Implications for public health strategies
The study results may have implications for health strategies and policies aimed at addressing health differentials among ethnic and economic groups in the United States.
Suboptimal sleep health may be an important fundamental but understudied contributor to health disparities, Chandra L. Jackson, PhD, MS, of the National Institute of Environmental Health Sciences, Research Triangle, N.C., said in an interview. Dr. Jackson is the lead author for a report published in August on a 2018 National Institutes of Health workshop regarding the importance of studying sleep health disparities (Sleep 2020 Mar 10. doi: 10.1093/sleep/zsaa037). The NIH workshop emphasized how little research has been done on the prevalence, incidence, morbidity, or mortality of sleep deficiencies of racial and ethnic minority populations, even though members of these groups are generally more likely to experience sleep disorders. The report urged “a nuanced integration between health disparity causal pathways and sleep and circadian-related mechanisms” tailored for these groups, with attention paid to sociocultural context.
Dr. Jackson said the study by Dr. Li and colleagues fits nicely with the strategies recommended in this report. She added: “Prospective design is particularly important for establishing temporality or that the SDB and insomnia symptoms occurred before the outcomes of hypertension and diabetes.”
In commenting on the Xi/Redline paper, Krishna M. Sundar, MD, FCCP, medical director of the Sleep-Wake Center at the University of Utah, Salt Lake City, commended the study and noted that one of the challenges in sleep research is the long time period over which the effects of disordered breathing become clear, he said.
“Things don’t happen immediately. It takes months, years for the effects to develop,” Dr. Sundar said. “To try to piece together the relationships, you need very well planned studies.”
Study design: Participants and exclusions
Latinos currently make up 17.8%, or 57.5 million, of the U.S. population, and this group is expected to double within the next 4 decades, the investigators wrote. A few prior studies on the roles of sleep disorders in the cardiometabolic health of Latinos, though suggestive, were limited by cross-sectional designs, relatively small samples, and underrepresentation of various Latino heritage groups.
The investigators on this new study worked with data from the federal Hispanic Community Health Study/Study of Latinos (HCHS/SOL) in which more than 16,000 people participated.
This multiyear federal study drew people who self-identified with different heritage groups, including Cuban, Dominican, Mexican, Central American, South American, and Puerto Rican. Participants initially aged 18-74 years underwent a first round of exams and assessments between 2008 and 2011 to determine what risk factors they had at the start of the study. In the second phase, which took place from 2013 to 2018, participants had a second set of exams. The National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases funded the HCHS/SOL.
The investigators initially had a potential data pool of 11,623 participants in the HCHS/SOL. About 1 of 8 in this group, or 1,424 participants (12.3%), did not undergo a sleep study or did not have sufficient sleep data for analyses. Another 93 (0.8%) participants were excluded for missing data on covariates.
For incident hypertension analyses, participants who had prevalent hypertension at the first screening in the HCHS/SOL (n = 3,139) or had missing data on hypertension (n = 2) were excluded. That resulted in an analytic sample of 6,965 for hypertension questions.
For incident diabetes analyses, participants who had prevalent diabetes at the first screening (n = 2,062) or had missing data on diabetes (n = 21) were excluded, yielding an analytic sample of 8,023.
Incident hypertension was defined as participants not having hypertension at baseline and having hypertension, defined as a systolic blood pressure 140 mm Hg or greater, diastolic blood pressure 90 mm Hg or greater, or receiving antihypertensive medication within 4 weeks, at the second round of screening.
Cardiometabolic disease definitions
The researchers did not discriminate between type 1 and type 2 diabetes. They used the American Diabetes Association definition as a fasting plasma glucose 126 mg/dL or greater, 2-hour, postload plasma glucose 200 mg/dL or greater, or hemoglobin A1c 6.5% or greater, with an additional criterion on self-reported use of antidiabetic medication within 4 weeks before the visit.
In line with previous research, the investigators controlled for potential confounders measured at baseline including sociodemographic factors, health behaviors, and adiposity, which are considered important risk factors for both sleep disorders and incident metabolic diseases. These factors include education level, age, gender, and body mass index and whether participants had ever been smokers or users of alcohol.
Study limitations
Limitations of the study include use of a home sleep apnea test device that did not allow evaluation of arousal or sleep architecture. The researchers said this may have led to an underestimation of disease severity both due to overestimation of sleep time and underrecognition of hypopneas unassociated with desaturation. In addition, prior research has suggested that minority populations might underreport sleep disturbances, possibly “due to social desirability (a tendency not to encode a negative event), stress, stereotype threat, acculturation, attitudes, etc.” The participants were recruited mostly from urban areas, and the results might not be generalized to rural populations. In addition, 41% of study participants were of Mexican heritage, compared with 63% of the Hispanic population being of Mexican heritage in the United States.
Another researcher in the field of health disparities, Julia Roncoroni, PhD, assistant professor of psychology at the University of Denver, also noted this slight underrepresentation of Hispanics of Mexican origin and an overrepresentation of urban individuals in the HCHS/SOL.
“However, using data from HCHS/SOL, which is the largest multicenter epidemiological study of cardiovascular risk factors and sleep traits in U.S. Hispanics/Latinx, allows researchers to answer a high-impact question that would otherwise be prohibitively expensive and time consuming,” wrote Dr. Roncoroni.
Conclusions
The study offers “the first prospective evidence on the associations between SDB and insomnia with incident hypertension and diabetes in US Hispanics/Latinos.” The investigators concluded: “Given the fact that sleep disorders are largely undiagnosed and undertreated, they might represent modifiable targets for disease prevention and reduction among US Hispanics/Latinos. Culturally informed interventions focusing on detecting and treating sleep disorders might improve cardiometabolic health among US Hispanics/Latinos.”
Dr. Redline was partially supported by NIH grant R35 HL135818. This study drew from the Hispanic Community Health Study/Study of Latinos, which has been supported by contracts from the National Heart, Lung, and Blood Institute.
SOURCE: Li X et al. Am J Respir Crit Care Med. 2020 Aug 6. doi: 10.1164/rccm.201912-2330OC.
Sleep disorders may be silent precursors of cardiometabolic disease among U.S. Latinos, said authors of a newly published study.
Xiaoyu Li, ScD, and Susan Redline, MD, MPH, of Harvard Medical School and Brigham and Women’s Hospital, Boston, and coauthors conducted a study of people who self-identified as Latino, who had baseline sleeping disorders, and who developed hypertension and diabetes over time. The study was published in the American Journal of Respiratory and Critical Care Medicine.
The findings suggested that sleep disorders preceded the development of hypertension and diabetes. Examining records from a major multiyear federal project, the Hispanic Community Health Study/Study of Latinos, Dr. Li, Dr. Redline, and coauthors found sleep-disordered breathing (SDB) was associated with a 1.54 higher adjusted odds of incident hypertension (95% confidence interval [CI], 1.18-2.00) and 1.33 higher odds of incident diabetes (95% CI, 1.05-1.67), compared with no SDB. Insomnia was associated with incident hypertension (odds ratio, 1.37; 95% CI, 1.11-1.69), but not diabetes. The association between insomnia and incident hypertension was stronger among men than women, they reported.
“We now need large-scale rigorous trials to evaluate the impact of early treatment of sleep disordered breathing and insomnia on preventing the development of hypertension and diabetes,” Dr. Redline said in an interview. “Clinicians should consider screening their patients at risk for hypertension and diabetes for both sleep-disordered breathing and insomnia.”
Implications for public health strategies
The study results may have implications for health strategies and policies aimed at addressing health differentials among ethnic and economic groups in the United States.
Suboptimal sleep health may be an important fundamental but understudied contributor to health disparities, Chandra L. Jackson, PhD, MS, of the National Institute of Environmental Health Sciences, Research Triangle, N.C., said in an interview. Dr. Jackson is the lead author for a report published in August on a 2018 National Institutes of Health workshop regarding the importance of studying sleep health disparities (Sleep 2020 Mar 10. doi: 10.1093/sleep/zsaa037). The NIH workshop emphasized how little research has been done on the prevalence, incidence, morbidity, or mortality of sleep deficiencies of racial and ethnic minority populations, even though members of these groups are generally more likely to experience sleep disorders. The report urged “a nuanced integration between health disparity causal pathways and sleep and circadian-related mechanisms” tailored for these groups, with attention paid to sociocultural context.
Dr. Jackson said the study by Dr. Li and colleagues fits nicely with the strategies recommended in this report. She added: “Prospective design is particularly important for establishing temporality or that the SDB and insomnia symptoms occurred before the outcomes of hypertension and diabetes.”
In commenting on the Xi/Redline paper, Krishna M. Sundar, MD, FCCP, medical director of the Sleep-Wake Center at the University of Utah, Salt Lake City, commended the study and noted that one of the challenges in sleep research is the long time period over which the effects of disordered breathing become clear, he said.
“Things don’t happen immediately. It takes months, years for the effects to develop,” Dr. Sundar said. “To try to piece together the relationships, you need very well planned studies.”
Study design: Participants and exclusions
Latinos currently make up 17.8%, or 57.5 million, of the U.S. population, and this group is expected to double within the next 4 decades, the investigators wrote. A few prior studies on the roles of sleep disorders in the cardiometabolic health of Latinos, though suggestive, were limited by cross-sectional designs, relatively small samples, and underrepresentation of various Latino heritage groups.
The investigators on this new study worked with data from the federal Hispanic Community Health Study/Study of Latinos (HCHS/SOL) in which more than 16,000 people participated.
This multiyear federal study drew people who self-identified with different heritage groups, including Cuban, Dominican, Mexican, Central American, South American, and Puerto Rican. Participants initially aged 18-74 years underwent a first round of exams and assessments between 2008 and 2011 to determine what risk factors they had at the start of the study. In the second phase, which took place from 2013 to 2018, participants had a second set of exams. The National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases funded the HCHS/SOL.
The investigators initially had a potential data pool of 11,623 participants in the HCHS/SOL. About 1 of 8 in this group, or 1,424 participants (12.3%), did not undergo a sleep study or did not have sufficient sleep data for analyses. Another 93 (0.8%) participants were excluded for missing data on covariates.
For incident hypertension analyses, participants who had prevalent hypertension at the first screening in the HCHS/SOL (n = 3,139) or had missing data on hypertension (n = 2) were excluded. That resulted in an analytic sample of 6,965 for hypertension questions.
For incident diabetes analyses, participants who had prevalent diabetes at the first screening (n = 2,062) or had missing data on diabetes (n = 21) were excluded, yielding an analytic sample of 8,023.
Incident hypertension was defined as participants not having hypertension at baseline and having hypertension, defined as a systolic blood pressure 140 mm Hg or greater, diastolic blood pressure 90 mm Hg or greater, or receiving antihypertensive medication within 4 weeks, at the second round of screening.
Cardiometabolic disease definitions
The researchers did not discriminate between type 1 and type 2 diabetes. They used the American Diabetes Association definition as a fasting plasma glucose 126 mg/dL or greater, 2-hour, postload plasma glucose 200 mg/dL or greater, or hemoglobin A1c 6.5% or greater, with an additional criterion on self-reported use of antidiabetic medication within 4 weeks before the visit.
In line with previous research, the investigators controlled for potential confounders measured at baseline including sociodemographic factors, health behaviors, and adiposity, which are considered important risk factors for both sleep disorders and incident metabolic diseases. These factors include education level, age, gender, and body mass index and whether participants had ever been smokers or users of alcohol.
Study limitations
Limitations of the study include use of a home sleep apnea test device that did not allow evaluation of arousal or sleep architecture. The researchers said this may have led to an underestimation of disease severity both due to overestimation of sleep time and underrecognition of hypopneas unassociated with desaturation. In addition, prior research has suggested that minority populations might underreport sleep disturbances, possibly “due to social desirability (a tendency not to encode a negative event), stress, stereotype threat, acculturation, attitudes, etc.” The participants were recruited mostly from urban areas, and the results might not be generalized to rural populations. In addition, 41% of study participants were of Mexican heritage, compared with 63% of the Hispanic population being of Mexican heritage in the United States.
Another researcher in the field of health disparities, Julia Roncoroni, PhD, assistant professor of psychology at the University of Denver, also noted this slight underrepresentation of Hispanics of Mexican origin and an overrepresentation of urban individuals in the HCHS/SOL.
“However, using data from HCHS/SOL, which is the largest multicenter epidemiological study of cardiovascular risk factors and sleep traits in U.S. Hispanics/Latinx, allows researchers to answer a high-impact question that would otherwise be prohibitively expensive and time consuming,” wrote Dr. Roncoroni.
Conclusions
The study offers “the first prospective evidence on the associations between SDB and insomnia with incident hypertension and diabetes in US Hispanics/Latinos.” The investigators concluded: “Given the fact that sleep disorders are largely undiagnosed and undertreated, they might represent modifiable targets for disease prevention and reduction among US Hispanics/Latinos. Culturally informed interventions focusing on detecting and treating sleep disorders might improve cardiometabolic health among US Hispanics/Latinos.”
Dr. Redline was partially supported by NIH grant R35 HL135818. This study drew from the Hispanic Community Health Study/Study of Latinos, which has been supported by contracts from the National Heart, Lung, and Blood Institute.
SOURCE: Li X et al. Am J Respir Crit Care Med. 2020 Aug 6. doi: 10.1164/rccm.201912-2330OC.
Sleep disorders may be silent precursors of cardiometabolic disease among U.S. Latinos, said authors of a newly published study.
Xiaoyu Li, ScD, and Susan Redline, MD, MPH, of Harvard Medical School and Brigham and Women’s Hospital, Boston, and coauthors conducted a study of people who self-identified as Latino, who had baseline sleeping disorders, and who developed hypertension and diabetes over time. The study was published in the American Journal of Respiratory and Critical Care Medicine.
The findings suggested that sleep disorders preceded the development of hypertension and diabetes. Examining records from a major multiyear federal project, the Hispanic Community Health Study/Study of Latinos, Dr. Li, Dr. Redline, and coauthors found sleep-disordered breathing (SDB) was associated with a 1.54 higher adjusted odds of incident hypertension (95% confidence interval [CI], 1.18-2.00) and 1.33 higher odds of incident diabetes (95% CI, 1.05-1.67), compared with no SDB. Insomnia was associated with incident hypertension (odds ratio, 1.37; 95% CI, 1.11-1.69), but not diabetes. The association between insomnia and incident hypertension was stronger among men than women, they reported.
“We now need large-scale rigorous trials to evaluate the impact of early treatment of sleep disordered breathing and insomnia on preventing the development of hypertension and diabetes,” Dr. Redline said in an interview. “Clinicians should consider screening their patients at risk for hypertension and diabetes for both sleep-disordered breathing and insomnia.”
Implications for public health strategies
The study results may have implications for health strategies and policies aimed at addressing health differentials among ethnic and economic groups in the United States.
Suboptimal sleep health may be an important fundamental but understudied contributor to health disparities, Chandra L. Jackson, PhD, MS, of the National Institute of Environmental Health Sciences, Research Triangle, N.C., said in an interview. Dr. Jackson is the lead author for a report published in August on a 2018 National Institutes of Health workshop regarding the importance of studying sleep health disparities (Sleep 2020 Mar 10. doi: 10.1093/sleep/zsaa037). The NIH workshop emphasized how little research has been done on the prevalence, incidence, morbidity, or mortality of sleep deficiencies of racial and ethnic minority populations, even though members of these groups are generally more likely to experience sleep disorders. The report urged “a nuanced integration between health disparity causal pathways and sleep and circadian-related mechanisms” tailored for these groups, with attention paid to sociocultural context.
Dr. Jackson said the study by Dr. Li and colleagues fits nicely with the strategies recommended in this report. She added: “Prospective design is particularly important for establishing temporality or that the SDB and insomnia symptoms occurred before the outcomes of hypertension and diabetes.”
In commenting on the Xi/Redline paper, Krishna M. Sundar, MD, FCCP, medical director of the Sleep-Wake Center at the University of Utah, Salt Lake City, commended the study and noted that one of the challenges in sleep research is the long time period over which the effects of disordered breathing become clear, he said.
“Things don’t happen immediately. It takes months, years for the effects to develop,” Dr. Sundar said. “To try to piece together the relationships, you need very well planned studies.”
Study design: Participants and exclusions
Latinos currently make up 17.8%, or 57.5 million, of the U.S. population, and this group is expected to double within the next 4 decades, the investigators wrote. A few prior studies on the roles of sleep disorders in the cardiometabolic health of Latinos, though suggestive, were limited by cross-sectional designs, relatively small samples, and underrepresentation of various Latino heritage groups.
The investigators on this new study worked with data from the federal Hispanic Community Health Study/Study of Latinos (HCHS/SOL) in which more than 16,000 people participated.
This multiyear federal study drew people who self-identified with different heritage groups, including Cuban, Dominican, Mexican, Central American, South American, and Puerto Rican. Participants initially aged 18-74 years underwent a first round of exams and assessments between 2008 and 2011 to determine what risk factors they had at the start of the study. In the second phase, which took place from 2013 to 2018, participants had a second set of exams. The National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases funded the HCHS/SOL.
The investigators initially had a potential data pool of 11,623 participants in the HCHS/SOL. About 1 of 8 in this group, or 1,424 participants (12.3%), did not undergo a sleep study or did not have sufficient sleep data for analyses. Another 93 (0.8%) participants were excluded for missing data on covariates.
For incident hypertension analyses, participants who had prevalent hypertension at the first screening in the HCHS/SOL (n = 3,139) or had missing data on hypertension (n = 2) were excluded. That resulted in an analytic sample of 6,965 for hypertension questions.
For incident diabetes analyses, participants who had prevalent diabetes at the first screening (n = 2,062) or had missing data on diabetes (n = 21) were excluded, yielding an analytic sample of 8,023.
Incident hypertension was defined as participants not having hypertension at baseline and having hypertension, defined as a systolic blood pressure 140 mm Hg or greater, diastolic blood pressure 90 mm Hg or greater, or receiving antihypertensive medication within 4 weeks, at the second round of screening.
Cardiometabolic disease definitions
The researchers did not discriminate between type 1 and type 2 diabetes. They used the American Diabetes Association definition as a fasting plasma glucose 126 mg/dL or greater, 2-hour, postload plasma glucose 200 mg/dL or greater, or hemoglobin A1c 6.5% or greater, with an additional criterion on self-reported use of antidiabetic medication within 4 weeks before the visit.
In line with previous research, the investigators controlled for potential confounders measured at baseline including sociodemographic factors, health behaviors, and adiposity, which are considered important risk factors for both sleep disorders and incident metabolic diseases. These factors include education level, age, gender, and body mass index and whether participants had ever been smokers or users of alcohol.
Study limitations
Limitations of the study include use of a home sleep apnea test device that did not allow evaluation of arousal or sleep architecture. The researchers said this may have led to an underestimation of disease severity both due to overestimation of sleep time and underrecognition of hypopneas unassociated with desaturation. In addition, prior research has suggested that minority populations might underreport sleep disturbances, possibly “due to social desirability (a tendency not to encode a negative event), stress, stereotype threat, acculturation, attitudes, etc.” The participants were recruited mostly from urban areas, and the results might not be generalized to rural populations. In addition, 41% of study participants were of Mexican heritage, compared with 63% of the Hispanic population being of Mexican heritage in the United States.
Another researcher in the field of health disparities, Julia Roncoroni, PhD, assistant professor of psychology at the University of Denver, also noted this slight underrepresentation of Hispanics of Mexican origin and an overrepresentation of urban individuals in the HCHS/SOL.
“However, using data from HCHS/SOL, which is the largest multicenter epidemiological study of cardiovascular risk factors and sleep traits in U.S. Hispanics/Latinx, allows researchers to answer a high-impact question that would otherwise be prohibitively expensive and time consuming,” wrote Dr. Roncoroni.
Conclusions
The study offers “the first prospective evidence on the associations between SDB and insomnia with incident hypertension and diabetes in US Hispanics/Latinos.” The investigators concluded: “Given the fact that sleep disorders are largely undiagnosed and undertreated, they might represent modifiable targets for disease prevention and reduction among US Hispanics/Latinos. Culturally informed interventions focusing on detecting and treating sleep disorders might improve cardiometabolic health among US Hispanics/Latinos.”
Dr. Redline was partially supported by NIH grant R35 HL135818. This study drew from the Hispanic Community Health Study/Study of Latinos, which has been supported by contracts from the National Heart, Lung, and Blood Institute.
SOURCE: Li X et al. Am J Respir Crit Care Med. 2020 Aug 6. doi: 10.1164/rccm.201912-2330OC.
FROM THE AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
LoDoCo2: Added steam for colchicine as secondary prevention
The anti-inflammatory drug colchicine picked up new support as secondary prevention in chronic coronary disease, cutting the risk of cardiovascular events by one-third when added to standard prevention therapies in the double-blind LoDoCo2 study.
Across a median follow up of 29 months in more than 5,000 patients, almost 1 in 10 patients assigned to placebo experienced the primary endpoint of cardiovascular death, myocardial infarction (MI), ischemic stroke, or ischemia-driven coronary revascularization. That risk was 31% lower and resulted in 77 fewer events in those assigned to colchicine (hazard ratio, 0.69; 95% confidence interval, 0.57-0.83).
The beneficial effect of low-dose colchicine 0.5 mg daily was seen early on and accrued over time, extending to five of the eight secondary end points, including a near 30% reduction in the composite of major adverse cardiac events, as well as reductions in the individual endpoints of MI and ischemia-driven revascularization.
“It did that with broadly consistent effects across a range of clinical subgroups, which together speak to the strength of the effect of colchicine on cardiovascular outcomes in the sort of patients we routinely see in our clinics,” primary investigator Mark Nidorf, MD, MBBS, GenesisCare Western Australia, Perth, said at the virtual annual congress of the European Society of Cardiology.
The results were published simultaneously in the New England Journal of Medicine (2020 Aug 31. doi: 10.1056/NEJMoa2021372).
“The totality of evidence from the big three double-blind placebo controlled trials – CANTOS, COLCOT, and LoDoCo2 – are highly consistent and should be practice changing,” Paul Ridker, MD, MPH, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital in Boston, said in an interview.
Massimo Imazio, MD, the formal discussant for the study and professor of cardiology at the University of Turin, Italy, also called for repurposing the inexpensive gout medication for cardiovascular patients.
“I would like to congratulate the authors for a well-designed, large, randomized trial that in my view provides convincing evidence that colchicine is safe and efficacious for secondary prevention in chronic coronary syndrome, of course if tolerated,” he said.
Dr. Imazio noted that colchicine demonstrated similar benefits in the smaller, open-label LoDoCo trial, but that 1 in 10 patients couldn’t tolerate the drug, largely because of gastrointestinal issues. The LoCoDo2 investigators very wisely opted for a 30-day run-in period for tolerance without a loading dose, and 90% of patients in each arm continued study medication while 3.4% stopped because of perceived effects.
Clinicians should bear in mind the potential for side effects and interactions with other medications, particularly statins, observed Dr. Imazio. “So monitoring of repeat blood tests is indicated, especially blood cell count, transaminase, and [creatine kinase] CK.”
Colchicine can be problematic in patients with chronic kidney disease because it is renally excreted, particularly if patients also take some common antibiotics such as clarithromycin, said Dr. Ridker, who led the landmark CANTOS trial. “So while these data are exciting and confirm the importance of inflammation inhibition in stable coronary disease, colchicine is not for all patients.”
During the discussion of the results, Dr. Nidorf said: “We were very concerned at the outset that there would be an interaction because there is certainly literature there, particularly in renal patients. But as the data showed, the incidence of myotoxicity was decidedly rare.”
Further, myotoxic episodes were independently assessed by a blinded reviewer, and although there was one case of mild rhabdomyolysis in the treatment group, it was considered not primarily caused by colchicine, he said. “So we’re fairly comfortable that you can use colchicine at a low dose quite comfortably with full-dose statins.”
Notably, 94% of patients in both groups were taking statins, and two-thirds were on moderate- or high-dose statins. About one-quarter were on dual-antiplatelet therapy, and 12% were on an anticoagulant.
In all, 5,522 patients aged 35-82 years (mean, 66 years) were randomly assigned to colchicine 0.5 mg once daily or placebo on top of proven secondary prevention therapies, and all but one was available for analysis.
Most were male (85%), one-half had hypertension, 18% had diabetes, and 84% had a history of acute coronary syndrome, with an equal number having undergone revascularization. Patients with advanced renal disease, severe heart failure, or severe valvular heart disease were excluded.
Colchicine, when compared with placebo, was associated with significantly lower incidence rates of the top five ranked secondary endpoints:
- Cardiovascular death, MI, or ischemic stroke (4.2% vs. 5.7%; HR, 0.72).
- MI or ischemia-driven revascularization (5.6% vs. 8.1%; HR, 0.67).
- Cardiovascular death or MI (3.6% vs. 5.0%; HR, 0.71).
- Ischemia-driven revascularization (4.9% vs. 6.4%; HR, 0.75).
- MI (3.0% vs. 4.2%; HR, 0.70).
The incidence rates were similar among the remaining three secondary outcomes: ischemic stroke (0.6% vs. 0.9%), all-cause death (2.6% vs. 2.2%), and CV death (0.7% vs. 0.9%), Dr. Nidorf reported.
The effect of colchicine was consistent in 13 subgroups, including those with and without hypertension, diabetes, or prior acute coronary syndrome. Patients in Australia appeared to do better with colchicine than did those in the Netherlands, which was a bit unexpected but likely caused by the play of chance, Dr. Nidorf said.
“Importantly, the effect when we looked at the predictors of outcome of our patients in this trial, they related to factors such as age and diabetes, which were included in both populations. So we believe the effect of therapy to be universal,” he added.
Session moderator Stephan Achenbach, MD, chair of cardiology at the University of Erlangen (Germany), however, noted that event rates were about 3% per year and many patients had undergone coronary revascularizations for acute coronary syndromes, suggesting this may be a preselected, somewhat higher-risk cohort. “Do you think we can transfer these findings to the just-average patient who comes in with chest pain and gets an elective [percutaneous coronary intervention]?” he asked.
Dr. Nidorf replied that, unlike the patients in COLCOT, who were randomized to colchicine within 30 days of an MI, acute events occurred more than 24 months before randomization in most (68.2%) patients. As such, patients were quite stable, and major adverse cardiac event and cardiovascular death rates were also exceedingly low.
“We did not see them as a particularly high-risk group, which I think is one of the beauties of this study,” Dr. Nidorf said. “It looks at people that are very similar to those who come and meet us in our clinics for regular review and follow-up.”
“And in that regard, I think the next time we’re faced with patients in our rooms, we have to ask the question: Are we doing enough for this patient beyond aspirin and statins? Should we be considering treating the inflammatory axis? And now we have an opportunity to do that,” he said.
Serious adverse effects were similar in the colchicine and placebo groups, including hospitalizations for infection (5.0% vs. 5.2%), pneumonia (1.7% vs. 2.0%), or gastrointestinal reasons (1.9% vs. 1.8%). Myotoxicity occurred in four and three patients, respectively.
Although the signal for increased risk of infection observed in CANTOS and COLCOT was not borne out, Dr. Nidorf observed that chest infections can occur frequently in these patients and echoed cautions about a potential unfavorable interaction between clarithromycin and colchicine.
“If we are to use this drug widely, clinicians will need to learn how to use this drug and what drugs to avoid, and that’s an important teaching point,” he said.
Limitations of the study are the small number of women and lack of routine measurement of C-reactive protein or other inflammatory markers at baseline.
The study was supported by the National Health Medical Research Council of Australia, a grant from the Sir Charles Gairdner Research Advisory Committee, the Withering Foundation the Netherlands, the Netherlands Heart Foundation, the Netherlands Organization for Health Research and Development, and a consortium of Teva, Disphar, and Tiofarma in the Netherlands. The authors’ disclosures are listed in the article.
A version of this article originally appeared on Medscape.com.
The anti-inflammatory drug colchicine picked up new support as secondary prevention in chronic coronary disease, cutting the risk of cardiovascular events by one-third when added to standard prevention therapies in the double-blind LoDoCo2 study.
Across a median follow up of 29 months in more than 5,000 patients, almost 1 in 10 patients assigned to placebo experienced the primary endpoint of cardiovascular death, myocardial infarction (MI), ischemic stroke, or ischemia-driven coronary revascularization. That risk was 31% lower and resulted in 77 fewer events in those assigned to colchicine (hazard ratio, 0.69; 95% confidence interval, 0.57-0.83).
The beneficial effect of low-dose colchicine 0.5 mg daily was seen early on and accrued over time, extending to five of the eight secondary end points, including a near 30% reduction in the composite of major adverse cardiac events, as well as reductions in the individual endpoints of MI and ischemia-driven revascularization.
“It did that with broadly consistent effects across a range of clinical subgroups, which together speak to the strength of the effect of colchicine on cardiovascular outcomes in the sort of patients we routinely see in our clinics,” primary investigator Mark Nidorf, MD, MBBS, GenesisCare Western Australia, Perth, said at the virtual annual congress of the European Society of Cardiology.
The results were published simultaneously in the New England Journal of Medicine (2020 Aug 31. doi: 10.1056/NEJMoa2021372).
“The totality of evidence from the big three double-blind placebo controlled trials – CANTOS, COLCOT, and LoDoCo2 – are highly consistent and should be practice changing,” Paul Ridker, MD, MPH, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital in Boston, said in an interview.
Massimo Imazio, MD, the formal discussant for the study and professor of cardiology at the University of Turin, Italy, also called for repurposing the inexpensive gout medication for cardiovascular patients.
“I would like to congratulate the authors for a well-designed, large, randomized trial that in my view provides convincing evidence that colchicine is safe and efficacious for secondary prevention in chronic coronary syndrome, of course if tolerated,” he said.
Dr. Imazio noted that colchicine demonstrated similar benefits in the smaller, open-label LoDoCo trial, but that 1 in 10 patients couldn’t tolerate the drug, largely because of gastrointestinal issues. The LoCoDo2 investigators very wisely opted for a 30-day run-in period for tolerance without a loading dose, and 90% of patients in each arm continued study medication while 3.4% stopped because of perceived effects.
Clinicians should bear in mind the potential for side effects and interactions with other medications, particularly statins, observed Dr. Imazio. “So monitoring of repeat blood tests is indicated, especially blood cell count, transaminase, and [creatine kinase] CK.”
Colchicine can be problematic in patients with chronic kidney disease because it is renally excreted, particularly if patients also take some common antibiotics such as clarithromycin, said Dr. Ridker, who led the landmark CANTOS trial. “So while these data are exciting and confirm the importance of inflammation inhibition in stable coronary disease, colchicine is not for all patients.”
During the discussion of the results, Dr. Nidorf said: “We were very concerned at the outset that there would be an interaction because there is certainly literature there, particularly in renal patients. But as the data showed, the incidence of myotoxicity was decidedly rare.”
Further, myotoxic episodes were independently assessed by a blinded reviewer, and although there was one case of mild rhabdomyolysis in the treatment group, it was considered not primarily caused by colchicine, he said. “So we’re fairly comfortable that you can use colchicine at a low dose quite comfortably with full-dose statins.”
Notably, 94% of patients in both groups were taking statins, and two-thirds were on moderate- or high-dose statins. About one-quarter were on dual-antiplatelet therapy, and 12% were on an anticoagulant.
In all, 5,522 patients aged 35-82 years (mean, 66 years) were randomly assigned to colchicine 0.5 mg once daily or placebo on top of proven secondary prevention therapies, and all but one was available for analysis.
Most were male (85%), one-half had hypertension, 18% had diabetes, and 84% had a history of acute coronary syndrome, with an equal number having undergone revascularization. Patients with advanced renal disease, severe heart failure, or severe valvular heart disease were excluded.
Colchicine, when compared with placebo, was associated with significantly lower incidence rates of the top five ranked secondary endpoints:
- Cardiovascular death, MI, or ischemic stroke (4.2% vs. 5.7%; HR, 0.72).
- MI or ischemia-driven revascularization (5.6% vs. 8.1%; HR, 0.67).
- Cardiovascular death or MI (3.6% vs. 5.0%; HR, 0.71).
- Ischemia-driven revascularization (4.9% vs. 6.4%; HR, 0.75).
- MI (3.0% vs. 4.2%; HR, 0.70).
The incidence rates were similar among the remaining three secondary outcomes: ischemic stroke (0.6% vs. 0.9%), all-cause death (2.6% vs. 2.2%), and CV death (0.7% vs. 0.9%), Dr. Nidorf reported.
The effect of colchicine was consistent in 13 subgroups, including those with and without hypertension, diabetes, or prior acute coronary syndrome. Patients in Australia appeared to do better with colchicine than did those in the Netherlands, which was a bit unexpected but likely caused by the play of chance, Dr. Nidorf said.
“Importantly, the effect when we looked at the predictors of outcome of our patients in this trial, they related to factors such as age and diabetes, which were included in both populations. So we believe the effect of therapy to be universal,” he added.
Session moderator Stephan Achenbach, MD, chair of cardiology at the University of Erlangen (Germany), however, noted that event rates were about 3% per year and many patients had undergone coronary revascularizations for acute coronary syndromes, suggesting this may be a preselected, somewhat higher-risk cohort. “Do you think we can transfer these findings to the just-average patient who comes in with chest pain and gets an elective [percutaneous coronary intervention]?” he asked.
Dr. Nidorf replied that, unlike the patients in COLCOT, who were randomized to colchicine within 30 days of an MI, acute events occurred more than 24 months before randomization in most (68.2%) patients. As such, patients were quite stable, and major adverse cardiac event and cardiovascular death rates were also exceedingly low.
“We did not see them as a particularly high-risk group, which I think is one of the beauties of this study,” Dr. Nidorf said. “It looks at people that are very similar to those who come and meet us in our clinics for regular review and follow-up.”
“And in that regard, I think the next time we’re faced with patients in our rooms, we have to ask the question: Are we doing enough for this patient beyond aspirin and statins? Should we be considering treating the inflammatory axis? And now we have an opportunity to do that,” he said.
Serious adverse effects were similar in the colchicine and placebo groups, including hospitalizations for infection (5.0% vs. 5.2%), pneumonia (1.7% vs. 2.0%), or gastrointestinal reasons (1.9% vs. 1.8%). Myotoxicity occurred in four and three patients, respectively.
Although the signal for increased risk of infection observed in CANTOS and COLCOT was not borne out, Dr. Nidorf observed that chest infections can occur frequently in these patients and echoed cautions about a potential unfavorable interaction between clarithromycin and colchicine.
“If we are to use this drug widely, clinicians will need to learn how to use this drug and what drugs to avoid, and that’s an important teaching point,” he said.
Limitations of the study are the small number of women and lack of routine measurement of C-reactive protein or other inflammatory markers at baseline.
The study was supported by the National Health Medical Research Council of Australia, a grant from the Sir Charles Gairdner Research Advisory Committee, the Withering Foundation the Netherlands, the Netherlands Heart Foundation, the Netherlands Organization for Health Research and Development, and a consortium of Teva, Disphar, and Tiofarma in the Netherlands. The authors’ disclosures are listed in the article.
A version of this article originally appeared on Medscape.com.
The anti-inflammatory drug colchicine picked up new support as secondary prevention in chronic coronary disease, cutting the risk of cardiovascular events by one-third when added to standard prevention therapies in the double-blind LoDoCo2 study.
Across a median follow up of 29 months in more than 5,000 patients, almost 1 in 10 patients assigned to placebo experienced the primary endpoint of cardiovascular death, myocardial infarction (MI), ischemic stroke, or ischemia-driven coronary revascularization. That risk was 31% lower and resulted in 77 fewer events in those assigned to colchicine (hazard ratio, 0.69; 95% confidence interval, 0.57-0.83).
The beneficial effect of low-dose colchicine 0.5 mg daily was seen early on and accrued over time, extending to five of the eight secondary end points, including a near 30% reduction in the composite of major adverse cardiac events, as well as reductions in the individual endpoints of MI and ischemia-driven revascularization.
“It did that with broadly consistent effects across a range of clinical subgroups, which together speak to the strength of the effect of colchicine on cardiovascular outcomes in the sort of patients we routinely see in our clinics,” primary investigator Mark Nidorf, MD, MBBS, GenesisCare Western Australia, Perth, said at the virtual annual congress of the European Society of Cardiology.
The results were published simultaneously in the New England Journal of Medicine (2020 Aug 31. doi: 10.1056/NEJMoa2021372).
“The totality of evidence from the big three double-blind placebo controlled trials – CANTOS, COLCOT, and LoDoCo2 – are highly consistent and should be practice changing,” Paul Ridker, MD, MPH, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital in Boston, said in an interview.
Massimo Imazio, MD, the formal discussant for the study and professor of cardiology at the University of Turin, Italy, also called for repurposing the inexpensive gout medication for cardiovascular patients.
“I would like to congratulate the authors for a well-designed, large, randomized trial that in my view provides convincing evidence that colchicine is safe and efficacious for secondary prevention in chronic coronary syndrome, of course if tolerated,” he said.
Dr. Imazio noted that colchicine demonstrated similar benefits in the smaller, open-label LoDoCo trial, but that 1 in 10 patients couldn’t tolerate the drug, largely because of gastrointestinal issues. The LoCoDo2 investigators very wisely opted for a 30-day run-in period for tolerance without a loading dose, and 90% of patients in each arm continued study medication while 3.4% stopped because of perceived effects.
Clinicians should bear in mind the potential for side effects and interactions with other medications, particularly statins, observed Dr. Imazio. “So monitoring of repeat blood tests is indicated, especially blood cell count, transaminase, and [creatine kinase] CK.”
Colchicine can be problematic in patients with chronic kidney disease because it is renally excreted, particularly if patients also take some common antibiotics such as clarithromycin, said Dr. Ridker, who led the landmark CANTOS trial. “So while these data are exciting and confirm the importance of inflammation inhibition in stable coronary disease, colchicine is not for all patients.”
During the discussion of the results, Dr. Nidorf said: “We were very concerned at the outset that there would be an interaction because there is certainly literature there, particularly in renal patients. But as the data showed, the incidence of myotoxicity was decidedly rare.”
Further, myotoxic episodes were independently assessed by a blinded reviewer, and although there was one case of mild rhabdomyolysis in the treatment group, it was considered not primarily caused by colchicine, he said. “So we’re fairly comfortable that you can use colchicine at a low dose quite comfortably with full-dose statins.”
Notably, 94% of patients in both groups were taking statins, and two-thirds were on moderate- or high-dose statins. About one-quarter were on dual-antiplatelet therapy, and 12% were on an anticoagulant.
In all, 5,522 patients aged 35-82 years (mean, 66 years) were randomly assigned to colchicine 0.5 mg once daily or placebo on top of proven secondary prevention therapies, and all but one was available for analysis.
Most were male (85%), one-half had hypertension, 18% had diabetes, and 84% had a history of acute coronary syndrome, with an equal number having undergone revascularization. Patients with advanced renal disease, severe heart failure, or severe valvular heart disease were excluded.
Colchicine, when compared with placebo, was associated with significantly lower incidence rates of the top five ranked secondary endpoints:
- Cardiovascular death, MI, or ischemic stroke (4.2% vs. 5.7%; HR, 0.72).
- MI or ischemia-driven revascularization (5.6% vs. 8.1%; HR, 0.67).
- Cardiovascular death or MI (3.6% vs. 5.0%; HR, 0.71).
- Ischemia-driven revascularization (4.9% vs. 6.4%; HR, 0.75).
- MI (3.0% vs. 4.2%; HR, 0.70).
The incidence rates were similar among the remaining three secondary outcomes: ischemic stroke (0.6% vs. 0.9%), all-cause death (2.6% vs. 2.2%), and CV death (0.7% vs. 0.9%), Dr. Nidorf reported.
The effect of colchicine was consistent in 13 subgroups, including those with and without hypertension, diabetes, or prior acute coronary syndrome. Patients in Australia appeared to do better with colchicine than did those in the Netherlands, which was a bit unexpected but likely caused by the play of chance, Dr. Nidorf said.
“Importantly, the effect when we looked at the predictors of outcome of our patients in this trial, they related to factors such as age and diabetes, which were included in both populations. So we believe the effect of therapy to be universal,” he added.
Session moderator Stephan Achenbach, MD, chair of cardiology at the University of Erlangen (Germany), however, noted that event rates were about 3% per year and many patients had undergone coronary revascularizations for acute coronary syndromes, suggesting this may be a preselected, somewhat higher-risk cohort. “Do you think we can transfer these findings to the just-average patient who comes in with chest pain and gets an elective [percutaneous coronary intervention]?” he asked.
Dr. Nidorf replied that, unlike the patients in COLCOT, who were randomized to colchicine within 30 days of an MI, acute events occurred more than 24 months before randomization in most (68.2%) patients. As such, patients were quite stable, and major adverse cardiac event and cardiovascular death rates were also exceedingly low.
“We did not see them as a particularly high-risk group, which I think is one of the beauties of this study,” Dr. Nidorf said. “It looks at people that are very similar to those who come and meet us in our clinics for regular review and follow-up.”
“And in that regard, I think the next time we’re faced with patients in our rooms, we have to ask the question: Are we doing enough for this patient beyond aspirin and statins? Should we be considering treating the inflammatory axis? And now we have an opportunity to do that,” he said.
Serious adverse effects were similar in the colchicine and placebo groups, including hospitalizations for infection (5.0% vs. 5.2%), pneumonia (1.7% vs. 2.0%), or gastrointestinal reasons (1.9% vs. 1.8%). Myotoxicity occurred in four and three patients, respectively.
Although the signal for increased risk of infection observed in CANTOS and COLCOT was not borne out, Dr. Nidorf observed that chest infections can occur frequently in these patients and echoed cautions about a potential unfavorable interaction between clarithromycin and colchicine.
“If we are to use this drug widely, clinicians will need to learn how to use this drug and what drugs to avoid, and that’s an important teaching point,” he said.
Limitations of the study are the small number of women and lack of routine measurement of C-reactive protein or other inflammatory markers at baseline.
The study was supported by the National Health Medical Research Council of Australia, a grant from the Sir Charles Gairdner Research Advisory Committee, the Withering Foundation the Netherlands, the Netherlands Heart Foundation, the Netherlands Organization for Health Research and Development, and a consortium of Teva, Disphar, and Tiofarma in the Netherlands. The authors’ disclosures are listed in the article.
A version of this article originally appeared on Medscape.com.