User login
Dapagliflozin’s cardiovascular benefits bloom in T2D with prior MI
NEW ORLEANS – Dapagliflozin markedly reduces the risks of both major adverse cardiovascular events and heart failure hospitalization in the subset of patients with type 2 diabetes (T2D) and prior MI, according to a new subanalysis of the landmark DECLARE-TIMI 58 trial.
The effectiveness of dapagliflozin (Farxiga), an oral sodium glucose transporter-2 inhibitor (SGLT-2i), was particularly striking with regard to prevention of recurrent MI, Remo H.M. Furtado, MD, reported at the annual meeting of the American College of Cardiology.
“The 22% relative risk reduction in recurrent MI with dapagliflozin is comparable to other established therapies used in secondary prevention after MI, like DAPT [dual-antiplatelet therapy] and intensive lipid lowering,” observed Dr. Furtado of Brigham and Women’s Hospital, Boston.
Not bad for a drug developed as a glucose-lowering agent.
The new DECLARE-TIMI 58 subanalysis provides information that’s relevant to ACC guidelines issued in late 2018. The guidelines, in the form of an “expert consensus decision pathway,” emphatically recommend that all patients with T2D and known atherosclerotic cardiovascular disease (ASCVD) should have metformin as their first-line glucose-lowering agent, while at the same time giving serious consideration to the addition of either an oral SGLT-2i or a subcutaneously injected glucagonlike peptide–1 receptor agonist (GLP-1RA) with demonstrated cardiovascular benefit as a second glucose-lowering agent (J Am Coll Cardiol. 2018 Dec 18;72[24]:3200-23). The DECLARE-TIMI 58 subanalysis bolsters that guidance and shows, more specifically, that the cardioprotective benefits of dapagliflozin are significantly greater in T2D with prior MI than in those with known ASCVD but no history of MI, the cardiologist explained.
The main results of DECLARE-TIMI 58 have been published. The trial included 17,160 patients with T2D, 6,974 of whom had established ASCVD, while the remainder had multiple ASCVD risk factors. Participants were randomized to oral dapagliflozin at 10 mg/day or placebo on top of background guideline-directed medical therapy and followed for a median of 4.2 years. The dapagliflozin group had a 27% reduction in heart failure hospitalizations, compared with controls, but there were no significant between-group differences in the composite MACE (major adverse cardiovascular events) endpoint of cardiovascular death, MI, or ischemic stroke (N Engl J Med. 2019 Jan 24;380[4]:347-57).
Dr. Furtado presented a prespecified subgroup analysis focused on the 3,584 study participants with prior MI. Their rate of the composite endpoint of cardiovascular death, MI, or ischemic stroke was 15.2%, compared with 17.8% in controls, for a statistically significant and clinically meaningful 16% relative risk reduction and an absolute 2.6% risk reduction. Of note, the risk of recurrent MI was reduced by 22%. In contrast, there was no difference in MACE risk between the dapagliflozin and placebo groups in patients with no prior MI, even if they had established ASCVD.
A noteworthy finding was that the benefit in MACE reduction in patients with prior MI was greater in those who were closer in time to their most recent MI at enrollment in the study. Those who started on dapagliflozin within 12 months of their last MI had a 34% relative risk reduction in MACE, compared with placebo, while those who enrolled 12-24 months after their last MI enjoyed an even more robust 58% relative risk reduction on dapagliflozin. In contrast, patients who enrolled 24-36 months post MI had only a 17% relative risk reduction, and the 2,400 patients who enrolled more than 36 months after their last MI had a subsequent MACE rate no different from controls.
Session cochair Nadia R. Sutton, MD, a cardiologist at the University of Michigan, Ann Arbor, commented that she found this time-dependent benefit fascinating.
“Do you think this has anything to do with the escalation of other therapies, such as Plavix [clopidogrel]?” she asked.
Dr. Furtado replied, “This is a finding that we should interpret with a little bit of caution.” For one thing, patients in the acute phase of an MI were excluded from participation in the trial, so nothing is known about how they would fare on dapagliflozin. For another, only 844 of the 3,584 patients with T2D and prior MI had their most recent MI within 24 months of enrollment, so even though the differences were statistically significant, the confidence intervals are fairly wide.
That being said, the finding does underscore a truism about cardiovascular prevention: The higher the risk, the greater the benefit of effective therapy – and, of course, the initial months following an MI are a particularly high-risk period.
“Also, this finding is a caution to the clinicians to avoid clinical inertia in prescribing an SGLT-2i, because maybe you can get an early benefit if you prescribe the drug closer to the acute phase and not wait until some months after the patient has tried diet and exercise and so on,” he added.
With regard to the second coprimary endpoint comprising cardiovascular death or heart failure hospitalization in T2D patients with prior MI, the rate in the dapagliflozin group was 8.6%, a 19% relative risk reduction and absolute risk reduction of 1.9%, compared with the 10.5% rate with placebo.
Dr. Furtado noted that the main results of DECLARE TIMI-58 are consistent with a recent systematic review and meta-analysis of three randomized cardiovascular outcome trials of SGLT-2is for primary and secondary prevention of cardiovascular and renal outcomes in T2D. The meta-analysis included more than 34,000 patients, roughly half drawn from DECLARE-TIMI 58. SGLT-2i therapy reduced MACE by 14% in patients with established ASCVD but not significantly in those without. And the agents reduced the risk of cardiovascular death/heart failure hospitalization by 23%, regardless of whether or not patients had known ASCVD or a history of heart failure (Lancet. 2019 Jan 5;393[10166]:31-9).
Dr. Furtado reported serving as a consultant to AstraZeneca, which funded DECLARE-TIMI 58, as well as receiving direct institutional research grants from half a dozen other pharmaceutical companies.
Simultaneous with his presentation at ACC 2019 in New Orleans, the subanalysis results were published online (Circulation. 2019 Mar 18. doi: 10.1161/CIRCULATIONAHA.119.039996. [Epub ahead of print]).
NEW ORLEANS – Dapagliflozin markedly reduces the risks of both major adverse cardiovascular events and heart failure hospitalization in the subset of patients with type 2 diabetes (T2D) and prior MI, according to a new subanalysis of the landmark DECLARE-TIMI 58 trial.
The effectiveness of dapagliflozin (Farxiga), an oral sodium glucose transporter-2 inhibitor (SGLT-2i), was particularly striking with regard to prevention of recurrent MI, Remo H.M. Furtado, MD, reported at the annual meeting of the American College of Cardiology.
“The 22% relative risk reduction in recurrent MI with dapagliflozin is comparable to other established therapies used in secondary prevention after MI, like DAPT [dual-antiplatelet therapy] and intensive lipid lowering,” observed Dr. Furtado of Brigham and Women’s Hospital, Boston.
Not bad for a drug developed as a glucose-lowering agent.
The new DECLARE-TIMI 58 subanalysis provides information that’s relevant to ACC guidelines issued in late 2018. The guidelines, in the form of an “expert consensus decision pathway,” emphatically recommend that all patients with T2D and known atherosclerotic cardiovascular disease (ASCVD) should have metformin as their first-line glucose-lowering agent, while at the same time giving serious consideration to the addition of either an oral SGLT-2i or a subcutaneously injected glucagonlike peptide–1 receptor agonist (GLP-1RA) with demonstrated cardiovascular benefit as a second glucose-lowering agent (J Am Coll Cardiol. 2018 Dec 18;72[24]:3200-23). The DECLARE-TIMI 58 subanalysis bolsters that guidance and shows, more specifically, that the cardioprotective benefits of dapagliflozin are significantly greater in T2D with prior MI than in those with known ASCVD but no history of MI, the cardiologist explained.
The main results of DECLARE-TIMI 58 have been published. The trial included 17,160 patients with T2D, 6,974 of whom had established ASCVD, while the remainder had multiple ASCVD risk factors. Participants were randomized to oral dapagliflozin at 10 mg/day or placebo on top of background guideline-directed medical therapy and followed for a median of 4.2 years. The dapagliflozin group had a 27% reduction in heart failure hospitalizations, compared with controls, but there were no significant between-group differences in the composite MACE (major adverse cardiovascular events) endpoint of cardiovascular death, MI, or ischemic stroke (N Engl J Med. 2019 Jan 24;380[4]:347-57).
Dr. Furtado presented a prespecified subgroup analysis focused on the 3,584 study participants with prior MI. Their rate of the composite endpoint of cardiovascular death, MI, or ischemic stroke was 15.2%, compared with 17.8% in controls, for a statistically significant and clinically meaningful 16% relative risk reduction and an absolute 2.6% risk reduction. Of note, the risk of recurrent MI was reduced by 22%. In contrast, there was no difference in MACE risk between the dapagliflozin and placebo groups in patients with no prior MI, even if they had established ASCVD.
A noteworthy finding was that the benefit in MACE reduction in patients with prior MI was greater in those who were closer in time to their most recent MI at enrollment in the study. Those who started on dapagliflozin within 12 months of their last MI had a 34% relative risk reduction in MACE, compared with placebo, while those who enrolled 12-24 months after their last MI enjoyed an even more robust 58% relative risk reduction on dapagliflozin. In contrast, patients who enrolled 24-36 months post MI had only a 17% relative risk reduction, and the 2,400 patients who enrolled more than 36 months after their last MI had a subsequent MACE rate no different from controls.
Session cochair Nadia R. Sutton, MD, a cardiologist at the University of Michigan, Ann Arbor, commented that she found this time-dependent benefit fascinating.
“Do you think this has anything to do with the escalation of other therapies, such as Plavix [clopidogrel]?” she asked.
Dr. Furtado replied, “This is a finding that we should interpret with a little bit of caution.” For one thing, patients in the acute phase of an MI were excluded from participation in the trial, so nothing is known about how they would fare on dapagliflozin. For another, only 844 of the 3,584 patients with T2D and prior MI had their most recent MI within 24 months of enrollment, so even though the differences were statistically significant, the confidence intervals are fairly wide.
That being said, the finding does underscore a truism about cardiovascular prevention: The higher the risk, the greater the benefit of effective therapy – and, of course, the initial months following an MI are a particularly high-risk period.
“Also, this finding is a caution to the clinicians to avoid clinical inertia in prescribing an SGLT-2i, because maybe you can get an early benefit if you prescribe the drug closer to the acute phase and not wait until some months after the patient has tried diet and exercise and so on,” he added.
With regard to the second coprimary endpoint comprising cardiovascular death or heart failure hospitalization in T2D patients with prior MI, the rate in the dapagliflozin group was 8.6%, a 19% relative risk reduction and absolute risk reduction of 1.9%, compared with the 10.5% rate with placebo.
Dr. Furtado noted that the main results of DECLARE TIMI-58 are consistent with a recent systematic review and meta-analysis of three randomized cardiovascular outcome trials of SGLT-2is for primary and secondary prevention of cardiovascular and renal outcomes in T2D. The meta-analysis included more than 34,000 patients, roughly half drawn from DECLARE-TIMI 58. SGLT-2i therapy reduced MACE by 14% in patients with established ASCVD but not significantly in those without. And the agents reduced the risk of cardiovascular death/heart failure hospitalization by 23%, regardless of whether or not patients had known ASCVD or a history of heart failure (Lancet. 2019 Jan 5;393[10166]:31-9).
Dr. Furtado reported serving as a consultant to AstraZeneca, which funded DECLARE-TIMI 58, as well as receiving direct institutional research grants from half a dozen other pharmaceutical companies.
Simultaneous with his presentation at ACC 2019 in New Orleans, the subanalysis results were published online (Circulation. 2019 Mar 18. doi: 10.1161/CIRCULATIONAHA.119.039996. [Epub ahead of print]).
NEW ORLEANS – Dapagliflozin markedly reduces the risks of both major adverse cardiovascular events and heart failure hospitalization in the subset of patients with type 2 diabetes (T2D) and prior MI, according to a new subanalysis of the landmark DECLARE-TIMI 58 trial.
The effectiveness of dapagliflozin (Farxiga), an oral sodium glucose transporter-2 inhibitor (SGLT-2i), was particularly striking with regard to prevention of recurrent MI, Remo H.M. Furtado, MD, reported at the annual meeting of the American College of Cardiology.
“The 22% relative risk reduction in recurrent MI with dapagliflozin is comparable to other established therapies used in secondary prevention after MI, like DAPT [dual-antiplatelet therapy] and intensive lipid lowering,” observed Dr. Furtado of Brigham and Women’s Hospital, Boston.
Not bad for a drug developed as a glucose-lowering agent.
The new DECLARE-TIMI 58 subanalysis provides information that’s relevant to ACC guidelines issued in late 2018. The guidelines, in the form of an “expert consensus decision pathway,” emphatically recommend that all patients with T2D and known atherosclerotic cardiovascular disease (ASCVD) should have metformin as their first-line glucose-lowering agent, while at the same time giving serious consideration to the addition of either an oral SGLT-2i or a subcutaneously injected glucagonlike peptide–1 receptor agonist (GLP-1RA) with demonstrated cardiovascular benefit as a second glucose-lowering agent (J Am Coll Cardiol. 2018 Dec 18;72[24]:3200-23). The DECLARE-TIMI 58 subanalysis bolsters that guidance and shows, more specifically, that the cardioprotective benefits of dapagliflozin are significantly greater in T2D with prior MI than in those with known ASCVD but no history of MI, the cardiologist explained.
The main results of DECLARE-TIMI 58 have been published. The trial included 17,160 patients with T2D, 6,974 of whom had established ASCVD, while the remainder had multiple ASCVD risk factors. Participants were randomized to oral dapagliflozin at 10 mg/day or placebo on top of background guideline-directed medical therapy and followed for a median of 4.2 years. The dapagliflozin group had a 27% reduction in heart failure hospitalizations, compared with controls, but there were no significant between-group differences in the composite MACE (major adverse cardiovascular events) endpoint of cardiovascular death, MI, or ischemic stroke (N Engl J Med. 2019 Jan 24;380[4]:347-57).
Dr. Furtado presented a prespecified subgroup analysis focused on the 3,584 study participants with prior MI. Their rate of the composite endpoint of cardiovascular death, MI, or ischemic stroke was 15.2%, compared with 17.8% in controls, for a statistically significant and clinically meaningful 16% relative risk reduction and an absolute 2.6% risk reduction. Of note, the risk of recurrent MI was reduced by 22%. In contrast, there was no difference in MACE risk between the dapagliflozin and placebo groups in patients with no prior MI, even if they had established ASCVD.
A noteworthy finding was that the benefit in MACE reduction in patients with prior MI was greater in those who were closer in time to their most recent MI at enrollment in the study. Those who started on dapagliflozin within 12 months of their last MI had a 34% relative risk reduction in MACE, compared with placebo, while those who enrolled 12-24 months after their last MI enjoyed an even more robust 58% relative risk reduction on dapagliflozin. In contrast, patients who enrolled 24-36 months post MI had only a 17% relative risk reduction, and the 2,400 patients who enrolled more than 36 months after their last MI had a subsequent MACE rate no different from controls.
Session cochair Nadia R. Sutton, MD, a cardiologist at the University of Michigan, Ann Arbor, commented that she found this time-dependent benefit fascinating.
“Do you think this has anything to do with the escalation of other therapies, such as Plavix [clopidogrel]?” she asked.
Dr. Furtado replied, “This is a finding that we should interpret with a little bit of caution.” For one thing, patients in the acute phase of an MI were excluded from participation in the trial, so nothing is known about how they would fare on dapagliflozin. For another, only 844 of the 3,584 patients with T2D and prior MI had their most recent MI within 24 months of enrollment, so even though the differences were statistically significant, the confidence intervals are fairly wide.
That being said, the finding does underscore a truism about cardiovascular prevention: The higher the risk, the greater the benefit of effective therapy – and, of course, the initial months following an MI are a particularly high-risk period.
“Also, this finding is a caution to the clinicians to avoid clinical inertia in prescribing an SGLT-2i, because maybe you can get an early benefit if you prescribe the drug closer to the acute phase and not wait until some months after the patient has tried diet and exercise and so on,” he added.
With regard to the second coprimary endpoint comprising cardiovascular death or heart failure hospitalization in T2D patients with prior MI, the rate in the dapagliflozin group was 8.6%, a 19% relative risk reduction and absolute risk reduction of 1.9%, compared with the 10.5% rate with placebo.
Dr. Furtado noted that the main results of DECLARE TIMI-58 are consistent with a recent systematic review and meta-analysis of three randomized cardiovascular outcome trials of SGLT-2is for primary and secondary prevention of cardiovascular and renal outcomes in T2D. The meta-analysis included more than 34,000 patients, roughly half drawn from DECLARE-TIMI 58. SGLT-2i therapy reduced MACE by 14% in patients with established ASCVD but not significantly in those without. And the agents reduced the risk of cardiovascular death/heart failure hospitalization by 23%, regardless of whether or not patients had known ASCVD or a history of heart failure (Lancet. 2019 Jan 5;393[10166]:31-9).
Dr. Furtado reported serving as a consultant to AstraZeneca, which funded DECLARE-TIMI 58, as well as receiving direct institutional research grants from half a dozen other pharmaceutical companies.
Simultaneous with his presentation at ACC 2019 in New Orleans, the subanalysis results were published online (Circulation. 2019 Mar 18. doi: 10.1161/CIRCULATIONAHA.119.039996. [Epub ahead of print]).
REPORTING FROM ACC 19
Alirocumab reduces both type 1 and 2 MIs
NEW ORLEANS – Lowering LDL cholesterol with alirocumab to levels below what’s achievable with intensive statin therapy appears to be an important strategy for prevention of type 1 MI – and perhaps even more impressively, type 2 MI – following acute coronary syndrome, Harvey D. White, MD, reported at the annual meeting of the American College of Cardiology.
What’s so important about the 23% reduction in risk of type 2 MI achieved with alirocumab (Praluent) relative to placebo documented in a prespecified secondary analysis from the ODYSSEY Outcomes trial?
“For type 2 MI, this is the first data indicating that a lipid-lowering therapy can attenuate risk,” according to Dr. White, a cardiologist at Auckland (N.Z.) City Hospital.
The ODYSSEY Outcomes trial compared the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor alirocumab to placebo in 18,924 patients with a recent acute coronary syndrome and an LDL cholesterol level of at least 70 mg/dL despite intensive statin therapy. At 4 months, the PCSK9 inhibitor plus statin therapy reduced participants’ mean LDL by 54%, from 93 to 48 mg/dL, while the LDL level actually drifted upward in the control group on placebo plus statin therapy. In the previously reported primary results of this landmark randomized clinical trial, alirocumab on top of background intensive statin therapy reduced the primary composite endpoint of death attributable to coronary heart disease, ischemic stroke, MI, or unstable angina requiring hospitalization by 15%, compared with controls (N Engl J Med. 2018 Nov 29;379[22]:2097-107).
During a median 2.8 years of prospective follow-up, there were 1,860 new MIs in study participants. A blinded clinical events committee evaluated the myocardial infarctions according to the Third Universal Definition and determined 66% were type 1 MIs, 21% were type 2, and 13% were type 4, with lesser numbers of types 3 and 5 MI.
Alirocumab reduced the risk of any MI by 15%, with a 6.8% incidence during follow-up, compared with 7.9% on placebo. The risk of type 1 MI, typically attributable to plaque rupture, was reduced by 13%, with an incidence of 4.9% with alirocumab and 5.6% with placebo. The risk reduction conferred by the PCSK9 inhibitor was even more robust for type 2 MI, the type caused by an oxygen supply/demand imbalance most commonly attributable to coronary artery spasm, coronary embolism, arrhythmias, anemia, hypertension, or hypotension: a 23% relative risk reduction as reflected in a 1.3% incidence in the alirocumab group, compared with a 1.7% rate in controls.
In contrast, alirocumab had no impact on the incidence of type 4 MI, a category that includes peri–percutaneous coronary intervention MIs as well as those attributable to stent thrombosis or restenosis.
The beneficial effect of alirocumab on MI risk mostly involved a reduction in larger MIs – those with a biomarker peak greater than three times the upper limit of normal.
An emphatic difference was found in the risk of death following type 1 as opposed to type 2 MI. Patients who experienced a type 1 MI during the study had an 11.9% mortality rate during an average of 1.6 years of post-MI follow-up, as compared with a 25.4% rate during 1.3 years of follow-up after a type 2 MI.
Alirocumab significantly reduced the risk of mortality following a type 1 MI, with a 10.2% rate as compared to 13.4% with placebo; that’s a 31% relative risk reduction. Yet the PCSK9 inhibitor had no impact on the risk of death after a type 2 MI: 24.8% in the alirocumab group and 25.9% in controls.
Asked for his thoughts as to possible explanatory mechanistic pathways for the benefit of alirocumab in preventing type 2 MI, Dr. White noted that in a Scottish study of the PCSK9 inhibitor evolocumab (Repatha), over the course of 72 months the drug appeared to reduce atherosclerotic progression and induce plaque stabilization and perhaps even regression.
“I think that’s the probable mechanism. And we also know that statins improve endothelial function,” he said.
He reported receiving research grant support and consultant fees from Sanofi and Regeneron, funders of the ODYSSEY Outcomes trial.
NEW ORLEANS – Lowering LDL cholesterol with alirocumab to levels below what’s achievable with intensive statin therapy appears to be an important strategy for prevention of type 1 MI – and perhaps even more impressively, type 2 MI – following acute coronary syndrome, Harvey D. White, MD, reported at the annual meeting of the American College of Cardiology.
What’s so important about the 23% reduction in risk of type 2 MI achieved with alirocumab (Praluent) relative to placebo documented in a prespecified secondary analysis from the ODYSSEY Outcomes trial?
“For type 2 MI, this is the first data indicating that a lipid-lowering therapy can attenuate risk,” according to Dr. White, a cardiologist at Auckland (N.Z.) City Hospital.
The ODYSSEY Outcomes trial compared the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor alirocumab to placebo in 18,924 patients with a recent acute coronary syndrome and an LDL cholesterol level of at least 70 mg/dL despite intensive statin therapy. At 4 months, the PCSK9 inhibitor plus statin therapy reduced participants’ mean LDL by 54%, from 93 to 48 mg/dL, while the LDL level actually drifted upward in the control group on placebo plus statin therapy. In the previously reported primary results of this landmark randomized clinical trial, alirocumab on top of background intensive statin therapy reduced the primary composite endpoint of death attributable to coronary heart disease, ischemic stroke, MI, or unstable angina requiring hospitalization by 15%, compared with controls (N Engl J Med. 2018 Nov 29;379[22]:2097-107).
During a median 2.8 years of prospective follow-up, there were 1,860 new MIs in study participants. A blinded clinical events committee evaluated the myocardial infarctions according to the Third Universal Definition and determined 66% were type 1 MIs, 21% were type 2, and 13% were type 4, with lesser numbers of types 3 and 5 MI.
Alirocumab reduced the risk of any MI by 15%, with a 6.8% incidence during follow-up, compared with 7.9% on placebo. The risk of type 1 MI, typically attributable to plaque rupture, was reduced by 13%, with an incidence of 4.9% with alirocumab and 5.6% with placebo. The risk reduction conferred by the PCSK9 inhibitor was even more robust for type 2 MI, the type caused by an oxygen supply/demand imbalance most commonly attributable to coronary artery spasm, coronary embolism, arrhythmias, anemia, hypertension, or hypotension: a 23% relative risk reduction as reflected in a 1.3% incidence in the alirocumab group, compared with a 1.7% rate in controls.
In contrast, alirocumab had no impact on the incidence of type 4 MI, a category that includes peri–percutaneous coronary intervention MIs as well as those attributable to stent thrombosis or restenosis.
The beneficial effect of alirocumab on MI risk mostly involved a reduction in larger MIs – those with a biomarker peak greater than three times the upper limit of normal.
An emphatic difference was found in the risk of death following type 1 as opposed to type 2 MI. Patients who experienced a type 1 MI during the study had an 11.9% mortality rate during an average of 1.6 years of post-MI follow-up, as compared with a 25.4% rate during 1.3 years of follow-up after a type 2 MI.
Alirocumab significantly reduced the risk of mortality following a type 1 MI, with a 10.2% rate as compared to 13.4% with placebo; that’s a 31% relative risk reduction. Yet the PCSK9 inhibitor had no impact on the risk of death after a type 2 MI: 24.8% in the alirocumab group and 25.9% in controls.
Asked for his thoughts as to possible explanatory mechanistic pathways for the benefit of alirocumab in preventing type 2 MI, Dr. White noted that in a Scottish study of the PCSK9 inhibitor evolocumab (Repatha), over the course of 72 months the drug appeared to reduce atherosclerotic progression and induce plaque stabilization and perhaps even regression.
“I think that’s the probable mechanism. And we also know that statins improve endothelial function,” he said.
He reported receiving research grant support and consultant fees from Sanofi and Regeneron, funders of the ODYSSEY Outcomes trial.
NEW ORLEANS – Lowering LDL cholesterol with alirocumab to levels below what’s achievable with intensive statin therapy appears to be an important strategy for prevention of type 1 MI – and perhaps even more impressively, type 2 MI – following acute coronary syndrome, Harvey D. White, MD, reported at the annual meeting of the American College of Cardiology.
What’s so important about the 23% reduction in risk of type 2 MI achieved with alirocumab (Praluent) relative to placebo documented in a prespecified secondary analysis from the ODYSSEY Outcomes trial?
“For type 2 MI, this is the first data indicating that a lipid-lowering therapy can attenuate risk,” according to Dr. White, a cardiologist at Auckland (N.Z.) City Hospital.
The ODYSSEY Outcomes trial compared the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor alirocumab to placebo in 18,924 patients with a recent acute coronary syndrome and an LDL cholesterol level of at least 70 mg/dL despite intensive statin therapy. At 4 months, the PCSK9 inhibitor plus statin therapy reduced participants’ mean LDL by 54%, from 93 to 48 mg/dL, while the LDL level actually drifted upward in the control group on placebo plus statin therapy. In the previously reported primary results of this landmark randomized clinical trial, alirocumab on top of background intensive statin therapy reduced the primary composite endpoint of death attributable to coronary heart disease, ischemic stroke, MI, or unstable angina requiring hospitalization by 15%, compared with controls (N Engl J Med. 2018 Nov 29;379[22]:2097-107).
During a median 2.8 years of prospective follow-up, there were 1,860 new MIs in study participants. A blinded clinical events committee evaluated the myocardial infarctions according to the Third Universal Definition and determined 66% were type 1 MIs, 21% were type 2, and 13% were type 4, with lesser numbers of types 3 and 5 MI.
Alirocumab reduced the risk of any MI by 15%, with a 6.8% incidence during follow-up, compared with 7.9% on placebo. The risk of type 1 MI, typically attributable to plaque rupture, was reduced by 13%, with an incidence of 4.9% with alirocumab and 5.6% with placebo. The risk reduction conferred by the PCSK9 inhibitor was even more robust for type 2 MI, the type caused by an oxygen supply/demand imbalance most commonly attributable to coronary artery spasm, coronary embolism, arrhythmias, anemia, hypertension, or hypotension: a 23% relative risk reduction as reflected in a 1.3% incidence in the alirocumab group, compared with a 1.7% rate in controls.
In contrast, alirocumab had no impact on the incidence of type 4 MI, a category that includes peri–percutaneous coronary intervention MIs as well as those attributable to stent thrombosis or restenosis.
The beneficial effect of alirocumab on MI risk mostly involved a reduction in larger MIs – those with a biomarker peak greater than three times the upper limit of normal.
An emphatic difference was found in the risk of death following type 1 as opposed to type 2 MI. Patients who experienced a type 1 MI during the study had an 11.9% mortality rate during an average of 1.6 years of post-MI follow-up, as compared with a 25.4% rate during 1.3 years of follow-up after a type 2 MI.
Alirocumab significantly reduced the risk of mortality following a type 1 MI, with a 10.2% rate as compared to 13.4% with placebo; that’s a 31% relative risk reduction. Yet the PCSK9 inhibitor had no impact on the risk of death after a type 2 MI: 24.8% in the alirocumab group and 25.9% in controls.
Asked for his thoughts as to possible explanatory mechanistic pathways for the benefit of alirocumab in preventing type 2 MI, Dr. White noted that in a Scottish study of the PCSK9 inhibitor evolocumab (Repatha), over the course of 72 months the drug appeared to reduce atherosclerotic progression and induce plaque stabilization and perhaps even regression.
“I think that’s the probable mechanism. And we also know that statins improve endothelial function,” he said.
He reported receiving research grant support and consultant fees from Sanofi and Regeneron, funders of the ODYSSEY Outcomes trial.
REPORTING FROM ACC 19
Look for alcohol septal ablation in the next HOCM guideline
SNOWMASS, COLO. – Recent data on long-term outcomes of alcohol septal ablation for hypertrophic obstructive cardiomyopathy are “quite favorable” and will be considered in the deliberations of the task force charged with revising the 2011 American College of Cardiology/American Heart Association guidelines.
Paul Sorajja, MD, a member of the task force and director of the Center of Valve and Structural Heart Disease at the Minneapolis Heart Institute, explained that the 2011 ACC/AHA guidelines on hypertrophic cardiomyopathy took an appropriately cautious stance regarding alcohol septal ablation (ASA) in light of a 2010 Dutch report warning of an increased risk of sudden cardiac death following the procedure (Circ Heart Fail. 2010 May;3[3]:362-9) and a dearth of evidence to the contrary.
The 2011 guidelines recommend surgical myectomy performed in an experienced center as the class I treatment of choice for patients with severely symptomatic, drug-refractory hypertrophic obstructive cardiomyopathy (HOCM). ASA gets a class IIa recommendation for patients at high surgical risk, and is class III – meaning don’t do it – for patients under age 40 years if myectomy is a viable option (J Am Coll Cardiol. 2011 Dec 13;58[25]:e212-60), Dr. Sorajja noted at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
However, the cautionary Dutch study that influenced the 2011 guidelines is considered controversial, he explained. It was small – just 91 patients – and the operators used twice the normal volume of alcohol, with a resultant much larger, potentially arrhythmogenic myocardial ablation scar. So, many experts have been eagerly awaiting additional long-term studies. And that long-sought data has recently been piling up. Since the 2011 guidelines, six long-term studies have been published, including one led by Dr. Sorajja (Circulation. 2012 Nov 13;126[20]:2374-80). The results have been consistently favorable, with 5-year survival rates of 87%-96%, in line with rates in the general population.
The largest of these studies included 1,197 patients who underwent ASA at seven centers in four European countries. The 30-day mortality and pacemaker implantation rates were significantly lower in patients aged up to 50 years, compared with those aged 65 and up. The annual mortality rate during a mean follow-up of 5.4 years was 1% in patients age 50 years and younger, 2.1% in those aged 51-64, and 5.1% in the oldest group. Arrhythmic events occurred at a rate of about 1% per year in all three age groups. And 95% of patients in the youngest group were in New York Heart Association class I or II at last follow-up (JACC Cardiovasc Interv. 2017 Jun 12;10[11]:1134-43).
In an accompanying editorial, Michael A. Fifer, MD, of Massachusetts General Hospital, Boston, commented that “high-volume surgical myectomy centers are few and far between” and there is “a clear inverse relation between [surgical] procedure volume and outcomes.”
The study “provides the most robust data to date regarding the outcomes of ASA in younger patients, precisely the type of data that were missing at the time of writing of the ACCF/AHA and European Society of Cardiology guidelines. Given the favorable outcomes of ASA in this age group, and the unavailability of high-volume myectomy programs in many geographic regions, the time has come to liberalize the indication for ASA in younger patients,” declared Dr. Fifer (JACC Cardiovasc Interv. 2017 Jun 12;10[11]:1144-6).
The second-largest long-term study of ASA was a recent report on 952 German patients with a minimum 6-year follow-up. The estimated 5-, 10-, and 15-year survival rates were 95.8%, 88.3%, and 79.7%, respectively. Estimated survival free of cardiac events was 98.9% at 5 years, 97.0% at 10 years, and 96.5% at 15 years. About 5% of patients received an implantable cardioverter defibrillator.
The investigators concluded, “In this study, PTSMA [percutaneous transluminal septal myocardial ablation] could be proofed as a safe procedure with ongoing symptomatic improvement and excellent long-term survival. Therefore, PTSMA is a reasonable alternative to surgical myectomy in HOCM.” (J Am Coll Cardiol. 2018 Dec 18;72[24]:3087-94) It’s way too early in the ACC/AHA guideline revision process to say what the new recommendations will be, according to Dr. Sorajja.
One unsettled issue, in his view, is whether ASA outcomes are significantly better in high-volume centers. A study of all 11,248 patients who underwent surgical myectomy of ASA during 2003-2011 in a large U.S. inpatient database concluded that undergoing surgical myectomy in a bottom-tertile-volume hospital was independently associated with an adjusted 210% increased risk of inpatient all-cause mortality and a 280% increased risk of bleeding, but that being in the lowest tertile of ASA hospital volume wasn’t independently associated with increased risk after adjustment for potential confounders (JAMA Cardiol. 2016 Jun 1;1:[3]:324-32).
However, Dr. Sorajja indicated he didn’t find the statistically adjusted results in the ASA cohort persuasive.
“I will tell you that the favorable results in the long-term studies came from hospitals in the highest-volume tertile,” the cardiologist said.
At present, he considers surgical myectomy the gold standard therapy. With well-selected patients for ASA – that is, those for whom imaging has identified an appropriate septal artery for delivery of the alcohol, along with no more than 24 mm of septal hypertrophy so the alcohol dose can be limited to a maximum of 20-25 cc – it’s reasonable to expect gradient relief in more than 90% of patients, surgical-like results with optimal relief of left ventricular outflow tract obstruction and a residual gradient of less than 10 mm Hg in about 75%, and a procedural mortality of about 1%, he said.
Dr. Sorajja reported receiving research funding from Abbott Structural, Boston Scientific, Edwards Lifesciences, and Medtronic, and serving as a consultant to those companies and several others.
SNOWMASS, COLO. – Recent data on long-term outcomes of alcohol septal ablation for hypertrophic obstructive cardiomyopathy are “quite favorable” and will be considered in the deliberations of the task force charged with revising the 2011 American College of Cardiology/American Heart Association guidelines.
Paul Sorajja, MD, a member of the task force and director of the Center of Valve and Structural Heart Disease at the Minneapolis Heart Institute, explained that the 2011 ACC/AHA guidelines on hypertrophic cardiomyopathy took an appropriately cautious stance regarding alcohol septal ablation (ASA) in light of a 2010 Dutch report warning of an increased risk of sudden cardiac death following the procedure (Circ Heart Fail. 2010 May;3[3]:362-9) and a dearth of evidence to the contrary.
The 2011 guidelines recommend surgical myectomy performed in an experienced center as the class I treatment of choice for patients with severely symptomatic, drug-refractory hypertrophic obstructive cardiomyopathy (HOCM). ASA gets a class IIa recommendation for patients at high surgical risk, and is class III – meaning don’t do it – for patients under age 40 years if myectomy is a viable option (J Am Coll Cardiol. 2011 Dec 13;58[25]:e212-60), Dr. Sorajja noted at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
However, the cautionary Dutch study that influenced the 2011 guidelines is considered controversial, he explained. It was small – just 91 patients – and the operators used twice the normal volume of alcohol, with a resultant much larger, potentially arrhythmogenic myocardial ablation scar. So, many experts have been eagerly awaiting additional long-term studies. And that long-sought data has recently been piling up. Since the 2011 guidelines, six long-term studies have been published, including one led by Dr. Sorajja (Circulation. 2012 Nov 13;126[20]:2374-80). The results have been consistently favorable, with 5-year survival rates of 87%-96%, in line with rates in the general population.
The largest of these studies included 1,197 patients who underwent ASA at seven centers in four European countries. The 30-day mortality and pacemaker implantation rates were significantly lower in patients aged up to 50 years, compared with those aged 65 and up. The annual mortality rate during a mean follow-up of 5.4 years was 1% in patients age 50 years and younger, 2.1% in those aged 51-64, and 5.1% in the oldest group. Arrhythmic events occurred at a rate of about 1% per year in all three age groups. And 95% of patients in the youngest group were in New York Heart Association class I or II at last follow-up (JACC Cardiovasc Interv. 2017 Jun 12;10[11]:1134-43).
In an accompanying editorial, Michael A. Fifer, MD, of Massachusetts General Hospital, Boston, commented that “high-volume surgical myectomy centers are few and far between” and there is “a clear inverse relation between [surgical] procedure volume and outcomes.”
The study “provides the most robust data to date regarding the outcomes of ASA in younger patients, precisely the type of data that were missing at the time of writing of the ACCF/AHA and European Society of Cardiology guidelines. Given the favorable outcomes of ASA in this age group, and the unavailability of high-volume myectomy programs in many geographic regions, the time has come to liberalize the indication for ASA in younger patients,” declared Dr. Fifer (JACC Cardiovasc Interv. 2017 Jun 12;10[11]:1144-6).
The second-largest long-term study of ASA was a recent report on 952 German patients with a minimum 6-year follow-up. The estimated 5-, 10-, and 15-year survival rates were 95.8%, 88.3%, and 79.7%, respectively. Estimated survival free of cardiac events was 98.9% at 5 years, 97.0% at 10 years, and 96.5% at 15 years. About 5% of patients received an implantable cardioverter defibrillator.
The investigators concluded, “In this study, PTSMA [percutaneous transluminal septal myocardial ablation] could be proofed as a safe procedure with ongoing symptomatic improvement and excellent long-term survival. Therefore, PTSMA is a reasonable alternative to surgical myectomy in HOCM.” (J Am Coll Cardiol. 2018 Dec 18;72[24]:3087-94) It’s way too early in the ACC/AHA guideline revision process to say what the new recommendations will be, according to Dr. Sorajja.
One unsettled issue, in his view, is whether ASA outcomes are significantly better in high-volume centers. A study of all 11,248 patients who underwent surgical myectomy of ASA during 2003-2011 in a large U.S. inpatient database concluded that undergoing surgical myectomy in a bottom-tertile-volume hospital was independently associated with an adjusted 210% increased risk of inpatient all-cause mortality and a 280% increased risk of bleeding, but that being in the lowest tertile of ASA hospital volume wasn’t independently associated with increased risk after adjustment for potential confounders (JAMA Cardiol. 2016 Jun 1;1:[3]:324-32).
However, Dr. Sorajja indicated he didn’t find the statistically adjusted results in the ASA cohort persuasive.
“I will tell you that the favorable results in the long-term studies came from hospitals in the highest-volume tertile,” the cardiologist said.
At present, he considers surgical myectomy the gold standard therapy. With well-selected patients for ASA – that is, those for whom imaging has identified an appropriate septal artery for delivery of the alcohol, along with no more than 24 mm of septal hypertrophy so the alcohol dose can be limited to a maximum of 20-25 cc – it’s reasonable to expect gradient relief in more than 90% of patients, surgical-like results with optimal relief of left ventricular outflow tract obstruction and a residual gradient of less than 10 mm Hg in about 75%, and a procedural mortality of about 1%, he said.
Dr. Sorajja reported receiving research funding from Abbott Structural, Boston Scientific, Edwards Lifesciences, and Medtronic, and serving as a consultant to those companies and several others.
SNOWMASS, COLO. – Recent data on long-term outcomes of alcohol septal ablation for hypertrophic obstructive cardiomyopathy are “quite favorable” and will be considered in the deliberations of the task force charged with revising the 2011 American College of Cardiology/American Heart Association guidelines.
Paul Sorajja, MD, a member of the task force and director of the Center of Valve and Structural Heart Disease at the Minneapolis Heart Institute, explained that the 2011 ACC/AHA guidelines on hypertrophic cardiomyopathy took an appropriately cautious stance regarding alcohol septal ablation (ASA) in light of a 2010 Dutch report warning of an increased risk of sudden cardiac death following the procedure (Circ Heart Fail. 2010 May;3[3]:362-9) and a dearth of evidence to the contrary.
The 2011 guidelines recommend surgical myectomy performed in an experienced center as the class I treatment of choice for patients with severely symptomatic, drug-refractory hypertrophic obstructive cardiomyopathy (HOCM). ASA gets a class IIa recommendation for patients at high surgical risk, and is class III – meaning don’t do it – for patients under age 40 years if myectomy is a viable option (J Am Coll Cardiol. 2011 Dec 13;58[25]:e212-60), Dr. Sorajja noted at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
However, the cautionary Dutch study that influenced the 2011 guidelines is considered controversial, he explained. It was small – just 91 patients – and the operators used twice the normal volume of alcohol, with a resultant much larger, potentially arrhythmogenic myocardial ablation scar. So, many experts have been eagerly awaiting additional long-term studies. And that long-sought data has recently been piling up. Since the 2011 guidelines, six long-term studies have been published, including one led by Dr. Sorajja (Circulation. 2012 Nov 13;126[20]:2374-80). The results have been consistently favorable, with 5-year survival rates of 87%-96%, in line with rates in the general population.
The largest of these studies included 1,197 patients who underwent ASA at seven centers in four European countries. The 30-day mortality and pacemaker implantation rates were significantly lower in patients aged up to 50 years, compared with those aged 65 and up. The annual mortality rate during a mean follow-up of 5.4 years was 1% in patients age 50 years and younger, 2.1% in those aged 51-64, and 5.1% in the oldest group. Arrhythmic events occurred at a rate of about 1% per year in all three age groups. And 95% of patients in the youngest group were in New York Heart Association class I or II at last follow-up (JACC Cardiovasc Interv. 2017 Jun 12;10[11]:1134-43).
In an accompanying editorial, Michael A. Fifer, MD, of Massachusetts General Hospital, Boston, commented that “high-volume surgical myectomy centers are few and far between” and there is “a clear inverse relation between [surgical] procedure volume and outcomes.”
The study “provides the most robust data to date regarding the outcomes of ASA in younger patients, precisely the type of data that were missing at the time of writing of the ACCF/AHA and European Society of Cardiology guidelines. Given the favorable outcomes of ASA in this age group, and the unavailability of high-volume myectomy programs in many geographic regions, the time has come to liberalize the indication for ASA in younger patients,” declared Dr. Fifer (JACC Cardiovasc Interv. 2017 Jun 12;10[11]:1144-6).
The second-largest long-term study of ASA was a recent report on 952 German patients with a minimum 6-year follow-up. The estimated 5-, 10-, and 15-year survival rates were 95.8%, 88.3%, and 79.7%, respectively. Estimated survival free of cardiac events was 98.9% at 5 years, 97.0% at 10 years, and 96.5% at 15 years. About 5% of patients received an implantable cardioverter defibrillator.
The investigators concluded, “In this study, PTSMA [percutaneous transluminal septal myocardial ablation] could be proofed as a safe procedure with ongoing symptomatic improvement and excellent long-term survival. Therefore, PTSMA is a reasonable alternative to surgical myectomy in HOCM.” (J Am Coll Cardiol. 2018 Dec 18;72[24]:3087-94) It’s way too early in the ACC/AHA guideline revision process to say what the new recommendations will be, according to Dr. Sorajja.
One unsettled issue, in his view, is whether ASA outcomes are significantly better in high-volume centers. A study of all 11,248 patients who underwent surgical myectomy of ASA during 2003-2011 in a large U.S. inpatient database concluded that undergoing surgical myectomy in a bottom-tertile-volume hospital was independently associated with an adjusted 210% increased risk of inpatient all-cause mortality and a 280% increased risk of bleeding, but that being in the lowest tertile of ASA hospital volume wasn’t independently associated with increased risk after adjustment for potential confounders (JAMA Cardiol. 2016 Jun 1;1:[3]:324-32).
However, Dr. Sorajja indicated he didn’t find the statistically adjusted results in the ASA cohort persuasive.
“I will tell you that the favorable results in the long-term studies came from hospitals in the highest-volume tertile,” the cardiologist said.
At present, he considers surgical myectomy the gold standard therapy. With well-selected patients for ASA – that is, those for whom imaging has identified an appropriate septal artery for delivery of the alcohol, along with no more than 24 mm of septal hypertrophy so the alcohol dose can be limited to a maximum of 20-25 cc – it’s reasonable to expect gradient relief in more than 90% of patients, surgical-like results with optimal relief of left ventricular outflow tract obstruction and a residual gradient of less than 10 mm Hg in about 75%, and a procedural mortality of about 1%, he said.
Dr. Sorajja reported receiving research funding from Abbott Structural, Boston Scientific, Edwards Lifesciences, and Medtronic, and serving as a consultant to those companies and several others.
EXPERT ANALYSIS FROM ACC SNOWMASS 2019
Effects of Process Improvement on Guideline-Concordant Cardiac Enzyme Testing
In recent years, driven by accelerating health care costs and desire for improved health care value, major specialty group guidelines have incorporated resource utilization and value calculations into their recommendations. High-value care has the characteristics of enhancing outcomes, safety, and patient satisfaction at a reasonable cost. As one example, the American College of Cardiology (ACC) recently published a consensus statement on its clinical practice guidelines with a specific focus on cost and value.1 This guideline acknowledges the difficulty in incorporating value into clinical decision making but stresses a need for increased transparency and consistency to boost value in everyday practice.
Chest pain and related symptoms were listed as the second leading principle reasons for emergency department visits in the US in 2011 with 14% of patients undergoing cardiac enzyme testing.2 The ACC guidelines advocate use of troponin as the preferred laboratory test for the initial evaluation of acute coronary syndrome (ACS). Fractionated creatine kinase (CK-MB) is an acceptable alternative only when a cardiac troponin test is not available.3 Furthermore, troponins should be obtained no more than 3 times for the initial evaluation of a single event, and further trending provides no additional benefit or prognostic information.
A recent study from an academic hospital showed that process improvement interventions focused on eliminating unnecessary cardiac enzyme testing led to a 1-year cost savings of $1.25 million while increasing the rate of ACS diagnosis.4 Common clinical practice at Naval Medical Center Portsmouth (NMCP) in Virginia still routinely includes both troponin as well as a CK panel comprised of CK, CK-MB, and a calculated CK-MB/CK index. Our study focuses on the implementation of quality improvement efforts described by Larochelle and colleagues at NMCP.4 The study aimed to determine the impact of implementing interventions designed to improve the ordering practices and reduce the cost of cardiac enzyme testing.
Methods
The primary focus of the intervention was on ordering practices of the emergency medicine department (EMD), internal medicine (IM) inpatient services, and cardiology inpatient services. Specific interventions were: (1) removal of the CK panel from the chest pain order set in the EMD electronic health record (EHR); (2) removal of the CK panel from the inpatient cardiology order set; (3) education of staff on the changes in CK panel utility via direct communication during IM academic seminars; (4) education of nursing staff ordering laboratory results on behalf of physicians on the cardiology service at the morning and evening huddles; and (5) addition of “max of 3 tests indicated” comment to the inpatient EHR ordering page of the troponin test
Data Source
The process improvement interventions were considered exempt from institutional review board (IRB) approval; however, we obtained expedited IRB approval with waiver of consent for the research aspect of the project. We obtained clinical administrative data from the Military Health System Data Repository (MDR). We identified all adult patients aged ≥ 18 years who had a troponin test, CK-MB, or both drawn at NMCP on the following services: the EMD, IM, and cardiology. A troponin or CK-MB test was defined using Current Procedural Terminology (CPT) codes and unique Logical Observation Identifiers Names and Codes (LOINC).
Measures
The study was divided into 3 periods: the preintervention period from August 1, 2013 to July 31, 2014; the intervention period from August 1, 2014 to January 31, 2015; and the postintervention period February 1, 2015 to January 31, 2016.
The primary outcomes measured were the frequency of guideline concordance and total costs for tests ordered per month using the Centers for Medicare and Medicaid Services (CMS) clinical laboratory fee schedule of $13.40 for troponin and $16.17 for CK-MB.5Concordance was defined as ≤ 3 troponin tests and no CK-MB tests ordered during 1 encounter for a patient without an ACS diagnosis in the preceding 7 days. Due to faster cellular release kinetics of CK-MB compared with that of troponin, this test has utility in evaluating new or worsening chest pain in the setting of a recent myocardial infarction (MI). Therefore, we excluded any patient who had a MI within the preceding 7 days of an order for either CK-MB or troponin tests. Additionally, the number of tests, both CK-MB and troponin, ordered per patient encounter (hereafter referred to as an episode) were measured. Finally, we measured the monthly prevalence of ACS diagnosis and percentage of visits having that diagnosis.
Data Analysis
Descriptive statistics were used to calculate population demographics of age group, sex, beneficiary category, sponsor service, and clinical setting. Monthly data were grouped into the preintervention and postintervention periods. The analysis was performed using t tests to compare mean values and CIs before and after the intervention. Simple linear regression with attention to correlation was used to create best fit lines with confidence bands before and after the intervention. Interrupted time series (ITS) regression was used to describe all data points throughout the study. Consistency between these various methods was verified. Mean values and CIs were reported from the t tests. Statistical significance was reported when appropriate. Equations and confidence predictions on the simple linear regressions were produced and reported. These were used to identify values at the start, midpoint, and end of the pre- and postintervention periods.
Results
There were a total of 6,281 patients in the study population. More patients were seen during the postintervention period than in the preintervention period. The mean age of patients was slightly higher during the preintervention period (Table 1).
Guideline Concordance
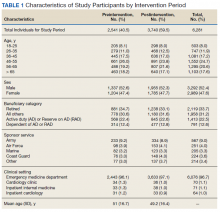
To determine whether ordering practices for cardiac enzyme testing improved, we assessed the changes in the frequency of guideline concordance during the pre- and postintervention period. On average during the preintervention year, the percentage of tests ordered that met guideline concordance was 10.1% (95% CI, 7.4%-12.9%), increasing by 0.80% (95% CI, 0.17%-1.42%) each month.
Costs
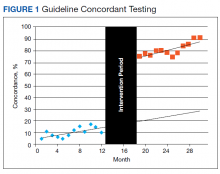
We assessed changes in total dollars spent on cardiac enzyme testing during the pre- and postintervention periods. During the preintervention year, $9,400 (95% CI, $8,700-$10,100) was spent on average each month, which did not change significantly throughout the period. During the postintervention year, the cost was stable at $5,000 (95% CI, $4,600-$5,300) on average each month, a reduction of $4,400 (95% CI, $3,700-$5,100) (Figure 2).
CK-MB and Troponin Tests per Patient
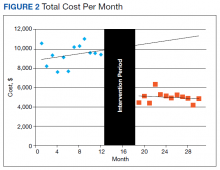
To further assess ordering practices for cardiac enzyme testing, we compared the changes in the monthly number of tests and the average number of CK-MB and troponin tests ordered per episode pre- and postintervention. On average during the preintervention year, 297 tests (95% CI, 278-315) were run per month, with an average of 1.21 CK tests (95% CI, 1.15-1.27) per episode (Table 2, Figure 3).
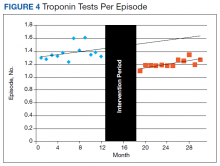
The changes in troponin testing were not as dramatic. The counts of tests each month remained similar, with a preintervention year average of 341 (95% CI, 306-377) and postintervention year average of 310 (95% CI, 287-332), which were not statistically different. However, there was a statistically significant decrease in the number of tests per episode. During the preintervention year, 1.38 troponin tests (95% CI, 1.31-1.45) were ordered per patient on average. This dropped by 0.17 (95% CI, 0.09-0.24) to the postintervention average of 1.21 (95% CI, 1.17-1.25) (Table 2, Figure 4).
ACS Prevalence
To determine whether there was an impact on ACS diagnoses, we looked at the numbers of ACS diagnoses and their prevalence among visits before and after the intervention. During the preintervention year, the average monthly number of diagnoses was 29.7 (95% CI, 26.1-33.2), and prevalence of ACS was 0.56% (95% CI, 0.48%-0.63%) of all episodes. Although the monthly rate was statistically decreasing by 0.022% (95% CI, 0.003-0.41), this has little meaning since the level of correlation (r2 = 0.2522, not displayed) was poor due to the essentially nonexistent correlation in number of visits each month (r2 = 0.0112, not displayed). During the postintervention year, the average number of diagnoses was 32.2 (95% CI, 27.9-36.6), and the prevalence of ACS was 0.62% (95% CI, 0.54-0.65). Neither of these values changed significantly between the pre- and postintervention period. All ICD-9 and ICD-10 diagnosis codes used for the analysis are available upon request from the authors.
Discussion
Our data demonstrate the ability of simple process improvement interventions to decrease unnecessary testing in the workup of ACS, increasing the rate of guideline concordant testing by > 70% at a single military treatment facility (MTF). In particular, with the now widespread use of EHR, the order set presents a high-yield target for process improvement in an easily implemented, durable fashion. We had expected to see some decrease in the efficacy of the intervention at a time of staff turnover in the summer of 2015 because ongoing dedicated teaching sessions were not performed. Despite that, the intervention remained effective without further dedicated teaching sessions. This outcome was certainly attributable to the hardwired interventions made (mainly via order sets), but possibly indicates an institutional memory that can take hold after an initial concerted effort is made.
We reduced the estimated preintervention annual cost of $113,000 by $53,000 (95% CI, $42,000-$64,000). Although on a much smaller scale than the study by Larochelle, our study represents a nearly 50% reduction in the total cost of initial testing for possible ACS and a > 80% reduction in unnecessary CK-MB testing.4 This result was achieved with no statistical change in the prevalence of ACS. The cost reduction does not account for the labor costs to clinically follow-up and address additional unnecessary lab results. The estimated cost of intervention was limited to the time required to educate residents, interns, and nursing staff as well as the implementation of the automated, reflexive laboratory results ordering process.
Unique to our study, we also demonstrated an intervention that satisfied all the major stakeholders in the ordering of these laboratory results. By instituting the reflexive ordering of CK-MB tests for positive troponins, we obtained the support of the facility’s interventional cardiology department, which finds value in that data. Appreciating the time-sensitive nature of an ACS diagnosis, the reflexive ordering minimized the delay in receiving these data while still greatly reducing the number of tests performed. That being said, if the current trend away from CK-MB in favor of exclusively testing troponin continues, removing the reflexive ordering for positive laboratory results protocol would be an easy follow-on intervention.
Limitations
Our study presented several limitations. First, reporting errors due to improper or insufficient medical coding as well as data entry errors may exist within the MDR; therefore, the results of this analysis may be over- or underestimated. Specifically, CPT codes for troponin and CK-MB were available only in 1 of the 2 data sets used for this study, which primarily contains outpatient patient encounters. For this reason, most of the laboratory testing comes from the EMD rather than from inpatient services. However, because we excluded all patients who eventually had an ACS diagnosis (patients who likely had more inpatient time and better indication for repeat troponin), we feel that our intervention was still thoroughly investigated. Second, the number of tests drawn per patient was significantly < 2, the expected minimum number of tests to rule out ACS in patients with appropriate symptoms.
This study was not designed to answer the source of variation from guidelines. Many patients had only 1 test, which we feel represents an opportunity for future study to identify other ways cardiac enzyme testing is being used clinically. These tests might be used for patients without convincing symptoms and signs of coronary syndromes or for patients with other primary problems. Third, by using the ITS analysis, we assumed that the outcome during each intervention period follows a linear pattern. However, changes may follow a nonlinear pattern over a long period. Finally, our intervention was limited to only a single MTF, which may limit generalizability to other facilities across military medicine. However, we feel this study should serve as a guide for other MTFs as well as US Department of Veterans Affairs facilities that could institute similar process improvements.
Conclusion
We made easily implemented and durable process improvement interventions that changed institution-wide ordering practices. These changes dramatically increased the rate of guideline-concordant testing, decreasing cost and furthering the goal of high-value medical care.
1. Anderson JL, Heidenreich PA, Barnett PG, et al; ACC/AHA Task Force on Performance Measures; ACC/AHA Task Force on Practice Guidelines. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129(22):2329-2345.
2. Centers for Disease Control and Prevention, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2010_ed_web_tables.pdf. Accessed March 15, 2019.
3. Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13):e356-e375.
4. Larochelle MR, Knight AM, Pantle H, Riedel S, Trost JC. Reducing excess cardiac biomarker testing at an academic medical center. J Gen Intern Med. 2014;29(11):1468-1474.
5. Centers for Medicare and Medicaid Services. 2016 clinical laboratory fee schedule. https://www.cms.gov/Medicare/Medicare-Fee -for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files-Items/16CLAB.html?DLPage=1&DLEntries=10&DLSort=2&DLSortDir=descending. Accessed March 15, 2019.
In recent years, driven by accelerating health care costs and desire for improved health care value, major specialty group guidelines have incorporated resource utilization and value calculations into their recommendations. High-value care has the characteristics of enhancing outcomes, safety, and patient satisfaction at a reasonable cost. As one example, the American College of Cardiology (ACC) recently published a consensus statement on its clinical practice guidelines with a specific focus on cost and value.1 This guideline acknowledges the difficulty in incorporating value into clinical decision making but stresses a need for increased transparency and consistency to boost value in everyday practice.
Chest pain and related symptoms were listed as the second leading principle reasons for emergency department visits in the US in 2011 with 14% of patients undergoing cardiac enzyme testing.2 The ACC guidelines advocate use of troponin as the preferred laboratory test for the initial evaluation of acute coronary syndrome (ACS). Fractionated creatine kinase (CK-MB) is an acceptable alternative only when a cardiac troponin test is not available.3 Furthermore, troponins should be obtained no more than 3 times for the initial evaluation of a single event, and further trending provides no additional benefit or prognostic information.
A recent study from an academic hospital showed that process improvement interventions focused on eliminating unnecessary cardiac enzyme testing led to a 1-year cost savings of $1.25 million while increasing the rate of ACS diagnosis.4 Common clinical practice at Naval Medical Center Portsmouth (NMCP) in Virginia still routinely includes both troponin as well as a CK panel comprised of CK, CK-MB, and a calculated CK-MB/CK index. Our study focuses on the implementation of quality improvement efforts described by Larochelle and colleagues at NMCP.4 The study aimed to determine the impact of implementing interventions designed to improve the ordering practices and reduce the cost of cardiac enzyme testing.
Methods
The primary focus of the intervention was on ordering practices of the emergency medicine department (EMD), internal medicine (IM) inpatient services, and cardiology inpatient services. Specific interventions were: (1) removal of the CK panel from the chest pain order set in the EMD electronic health record (EHR); (2) removal of the CK panel from the inpatient cardiology order set; (3) education of staff on the changes in CK panel utility via direct communication during IM academic seminars; (4) education of nursing staff ordering laboratory results on behalf of physicians on the cardiology service at the morning and evening huddles; and (5) addition of “max of 3 tests indicated” comment to the inpatient EHR ordering page of the troponin test
Data Source
The process improvement interventions were considered exempt from institutional review board (IRB) approval; however, we obtained expedited IRB approval with waiver of consent for the research aspect of the project. We obtained clinical administrative data from the Military Health System Data Repository (MDR). We identified all adult patients aged ≥ 18 years who had a troponin test, CK-MB, or both drawn at NMCP on the following services: the EMD, IM, and cardiology. A troponin or CK-MB test was defined using Current Procedural Terminology (CPT) codes and unique Logical Observation Identifiers Names and Codes (LOINC).
Measures
The study was divided into 3 periods: the preintervention period from August 1, 2013 to July 31, 2014; the intervention period from August 1, 2014 to January 31, 2015; and the postintervention period February 1, 2015 to January 31, 2016.
The primary outcomes measured were the frequency of guideline concordance and total costs for tests ordered per month using the Centers for Medicare and Medicaid Services (CMS) clinical laboratory fee schedule of $13.40 for troponin and $16.17 for CK-MB.5Concordance was defined as ≤ 3 troponin tests and no CK-MB tests ordered during 1 encounter for a patient without an ACS diagnosis in the preceding 7 days. Due to faster cellular release kinetics of CK-MB compared with that of troponin, this test has utility in evaluating new or worsening chest pain in the setting of a recent myocardial infarction (MI). Therefore, we excluded any patient who had a MI within the preceding 7 days of an order for either CK-MB or troponin tests. Additionally, the number of tests, both CK-MB and troponin, ordered per patient encounter (hereafter referred to as an episode) were measured. Finally, we measured the monthly prevalence of ACS diagnosis and percentage of visits having that diagnosis.
Data Analysis
Descriptive statistics were used to calculate population demographics of age group, sex, beneficiary category, sponsor service, and clinical setting. Monthly data were grouped into the preintervention and postintervention periods. The analysis was performed using t tests to compare mean values and CIs before and after the intervention. Simple linear regression with attention to correlation was used to create best fit lines with confidence bands before and after the intervention. Interrupted time series (ITS) regression was used to describe all data points throughout the study. Consistency between these various methods was verified. Mean values and CIs were reported from the t tests. Statistical significance was reported when appropriate. Equations and confidence predictions on the simple linear regressions were produced and reported. These were used to identify values at the start, midpoint, and end of the pre- and postintervention periods.
Results
There were a total of 6,281 patients in the study population. More patients were seen during the postintervention period than in the preintervention period. The mean age of patients was slightly higher during the preintervention period (Table 1).
Guideline Concordance
To determine whether ordering practices for cardiac enzyme testing improved, we assessed the changes in the frequency of guideline concordance during the pre- and postintervention period. On average during the preintervention year, the percentage of tests ordered that met guideline concordance was 10.1% (95% CI, 7.4%-12.9%), increasing by 0.80% (95% CI, 0.17%-1.42%) each month.
Costs
We assessed changes in total dollars spent on cardiac enzyme testing during the pre- and postintervention periods. During the preintervention year, $9,400 (95% CI, $8,700-$10,100) was spent on average each month, which did not change significantly throughout the period. During the postintervention year, the cost was stable at $5,000 (95% CI, $4,600-$5,300) on average each month, a reduction of $4,400 (95% CI, $3,700-$5,100) (Figure 2).
CK-MB and Troponin Tests per Patient
To further assess ordering practices for cardiac enzyme testing, we compared the changes in the monthly number of tests and the average number of CK-MB and troponin tests ordered per episode pre- and postintervention. On average during the preintervention year, 297 tests (95% CI, 278-315) were run per month, with an average of 1.21 CK tests (95% CI, 1.15-1.27) per episode (Table 2, Figure 3).
The changes in troponin testing were not as dramatic. The counts of tests each month remained similar, with a preintervention year average of 341 (95% CI, 306-377) and postintervention year average of 310 (95% CI, 287-332), which were not statistically different. However, there was a statistically significant decrease in the number of tests per episode. During the preintervention year, 1.38 troponin tests (95% CI, 1.31-1.45) were ordered per patient on average. This dropped by 0.17 (95% CI, 0.09-0.24) to the postintervention average of 1.21 (95% CI, 1.17-1.25) (Table 2, Figure 4).
ACS Prevalence
To determine whether there was an impact on ACS diagnoses, we looked at the numbers of ACS diagnoses and their prevalence among visits before and after the intervention. During the preintervention year, the average monthly number of diagnoses was 29.7 (95% CI, 26.1-33.2), and prevalence of ACS was 0.56% (95% CI, 0.48%-0.63%) of all episodes. Although the monthly rate was statistically decreasing by 0.022% (95% CI, 0.003-0.41), this has little meaning since the level of correlation (r2 = 0.2522, not displayed) was poor due to the essentially nonexistent correlation in number of visits each month (r2 = 0.0112, not displayed). During the postintervention year, the average number of diagnoses was 32.2 (95% CI, 27.9-36.6), and the prevalence of ACS was 0.62% (95% CI, 0.54-0.65). Neither of these values changed significantly between the pre- and postintervention period. All ICD-9 and ICD-10 diagnosis codes used for the analysis are available upon request from the authors.
Discussion
Our data demonstrate the ability of simple process improvement interventions to decrease unnecessary testing in the workup of ACS, increasing the rate of guideline concordant testing by > 70% at a single military treatment facility (MTF). In particular, with the now widespread use of EHR, the order set presents a high-yield target for process improvement in an easily implemented, durable fashion. We had expected to see some decrease in the efficacy of the intervention at a time of staff turnover in the summer of 2015 because ongoing dedicated teaching sessions were not performed. Despite that, the intervention remained effective without further dedicated teaching sessions. This outcome was certainly attributable to the hardwired interventions made (mainly via order sets), but possibly indicates an institutional memory that can take hold after an initial concerted effort is made.
We reduced the estimated preintervention annual cost of $113,000 by $53,000 (95% CI, $42,000-$64,000). Although on a much smaller scale than the study by Larochelle, our study represents a nearly 50% reduction in the total cost of initial testing for possible ACS and a > 80% reduction in unnecessary CK-MB testing.4 This result was achieved with no statistical change in the prevalence of ACS. The cost reduction does not account for the labor costs to clinically follow-up and address additional unnecessary lab results. The estimated cost of intervention was limited to the time required to educate residents, interns, and nursing staff as well as the implementation of the automated, reflexive laboratory results ordering process.
Unique to our study, we also demonstrated an intervention that satisfied all the major stakeholders in the ordering of these laboratory results. By instituting the reflexive ordering of CK-MB tests for positive troponins, we obtained the support of the facility’s interventional cardiology department, which finds value in that data. Appreciating the time-sensitive nature of an ACS diagnosis, the reflexive ordering minimized the delay in receiving these data while still greatly reducing the number of tests performed. That being said, if the current trend away from CK-MB in favor of exclusively testing troponin continues, removing the reflexive ordering for positive laboratory results protocol would be an easy follow-on intervention.
Limitations
Our study presented several limitations. First, reporting errors due to improper or insufficient medical coding as well as data entry errors may exist within the MDR; therefore, the results of this analysis may be over- or underestimated. Specifically, CPT codes for troponin and CK-MB were available only in 1 of the 2 data sets used for this study, which primarily contains outpatient patient encounters. For this reason, most of the laboratory testing comes from the EMD rather than from inpatient services. However, because we excluded all patients who eventually had an ACS diagnosis (patients who likely had more inpatient time and better indication for repeat troponin), we feel that our intervention was still thoroughly investigated. Second, the number of tests drawn per patient was significantly < 2, the expected minimum number of tests to rule out ACS in patients with appropriate symptoms.
This study was not designed to answer the source of variation from guidelines. Many patients had only 1 test, which we feel represents an opportunity for future study to identify other ways cardiac enzyme testing is being used clinically. These tests might be used for patients without convincing symptoms and signs of coronary syndromes or for patients with other primary problems. Third, by using the ITS analysis, we assumed that the outcome during each intervention period follows a linear pattern. However, changes may follow a nonlinear pattern over a long period. Finally, our intervention was limited to only a single MTF, which may limit generalizability to other facilities across military medicine. However, we feel this study should serve as a guide for other MTFs as well as US Department of Veterans Affairs facilities that could institute similar process improvements.
Conclusion
We made easily implemented and durable process improvement interventions that changed institution-wide ordering practices. These changes dramatically increased the rate of guideline-concordant testing, decreasing cost and furthering the goal of high-value medical care.
In recent years, driven by accelerating health care costs and desire for improved health care value, major specialty group guidelines have incorporated resource utilization and value calculations into their recommendations. High-value care has the characteristics of enhancing outcomes, safety, and patient satisfaction at a reasonable cost. As one example, the American College of Cardiology (ACC) recently published a consensus statement on its clinical practice guidelines with a specific focus on cost and value.1 This guideline acknowledges the difficulty in incorporating value into clinical decision making but stresses a need for increased transparency and consistency to boost value in everyday practice.
Chest pain and related symptoms were listed as the second leading principle reasons for emergency department visits in the US in 2011 with 14% of patients undergoing cardiac enzyme testing.2 The ACC guidelines advocate use of troponin as the preferred laboratory test for the initial evaluation of acute coronary syndrome (ACS). Fractionated creatine kinase (CK-MB) is an acceptable alternative only when a cardiac troponin test is not available.3 Furthermore, troponins should be obtained no more than 3 times for the initial evaluation of a single event, and further trending provides no additional benefit or prognostic information.
A recent study from an academic hospital showed that process improvement interventions focused on eliminating unnecessary cardiac enzyme testing led to a 1-year cost savings of $1.25 million while increasing the rate of ACS diagnosis.4 Common clinical practice at Naval Medical Center Portsmouth (NMCP) in Virginia still routinely includes both troponin as well as a CK panel comprised of CK, CK-MB, and a calculated CK-MB/CK index. Our study focuses on the implementation of quality improvement efforts described by Larochelle and colleagues at NMCP.4 The study aimed to determine the impact of implementing interventions designed to improve the ordering practices and reduce the cost of cardiac enzyme testing.
Methods
The primary focus of the intervention was on ordering practices of the emergency medicine department (EMD), internal medicine (IM) inpatient services, and cardiology inpatient services. Specific interventions were: (1) removal of the CK panel from the chest pain order set in the EMD electronic health record (EHR); (2) removal of the CK panel from the inpatient cardiology order set; (3) education of staff on the changes in CK panel utility via direct communication during IM academic seminars; (4) education of nursing staff ordering laboratory results on behalf of physicians on the cardiology service at the morning and evening huddles; and (5) addition of “max of 3 tests indicated” comment to the inpatient EHR ordering page of the troponin test
Data Source
The process improvement interventions were considered exempt from institutional review board (IRB) approval; however, we obtained expedited IRB approval with waiver of consent for the research aspect of the project. We obtained clinical administrative data from the Military Health System Data Repository (MDR). We identified all adult patients aged ≥ 18 years who had a troponin test, CK-MB, or both drawn at NMCP on the following services: the EMD, IM, and cardiology. A troponin or CK-MB test was defined using Current Procedural Terminology (CPT) codes and unique Logical Observation Identifiers Names and Codes (LOINC).
Measures
The study was divided into 3 periods: the preintervention period from August 1, 2013 to July 31, 2014; the intervention period from August 1, 2014 to January 31, 2015; and the postintervention period February 1, 2015 to January 31, 2016.
The primary outcomes measured were the frequency of guideline concordance and total costs for tests ordered per month using the Centers for Medicare and Medicaid Services (CMS) clinical laboratory fee schedule of $13.40 for troponin and $16.17 for CK-MB.5Concordance was defined as ≤ 3 troponin tests and no CK-MB tests ordered during 1 encounter for a patient without an ACS diagnosis in the preceding 7 days. Due to faster cellular release kinetics of CK-MB compared with that of troponin, this test has utility in evaluating new or worsening chest pain in the setting of a recent myocardial infarction (MI). Therefore, we excluded any patient who had a MI within the preceding 7 days of an order for either CK-MB or troponin tests. Additionally, the number of tests, both CK-MB and troponin, ordered per patient encounter (hereafter referred to as an episode) were measured. Finally, we measured the monthly prevalence of ACS diagnosis and percentage of visits having that diagnosis.
Data Analysis
Descriptive statistics were used to calculate population demographics of age group, sex, beneficiary category, sponsor service, and clinical setting. Monthly data were grouped into the preintervention and postintervention periods. The analysis was performed using t tests to compare mean values and CIs before and after the intervention. Simple linear regression with attention to correlation was used to create best fit lines with confidence bands before and after the intervention. Interrupted time series (ITS) regression was used to describe all data points throughout the study. Consistency between these various methods was verified. Mean values and CIs were reported from the t tests. Statistical significance was reported when appropriate. Equations and confidence predictions on the simple linear regressions were produced and reported. These were used to identify values at the start, midpoint, and end of the pre- and postintervention periods.
Results
There were a total of 6,281 patients in the study population. More patients were seen during the postintervention period than in the preintervention period. The mean age of patients was slightly higher during the preintervention period (Table 1).
Guideline Concordance
To determine whether ordering practices for cardiac enzyme testing improved, we assessed the changes in the frequency of guideline concordance during the pre- and postintervention period. On average during the preintervention year, the percentage of tests ordered that met guideline concordance was 10.1% (95% CI, 7.4%-12.9%), increasing by 0.80% (95% CI, 0.17%-1.42%) each month.
Costs
We assessed changes in total dollars spent on cardiac enzyme testing during the pre- and postintervention periods. During the preintervention year, $9,400 (95% CI, $8,700-$10,100) was spent on average each month, which did not change significantly throughout the period. During the postintervention year, the cost was stable at $5,000 (95% CI, $4,600-$5,300) on average each month, a reduction of $4,400 (95% CI, $3,700-$5,100) (Figure 2).
CK-MB and Troponin Tests per Patient
To further assess ordering practices for cardiac enzyme testing, we compared the changes in the monthly number of tests and the average number of CK-MB and troponin tests ordered per episode pre- and postintervention. On average during the preintervention year, 297 tests (95% CI, 278-315) were run per month, with an average of 1.21 CK tests (95% CI, 1.15-1.27) per episode (Table 2, Figure 3).
The changes in troponin testing were not as dramatic. The counts of tests each month remained similar, with a preintervention year average of 341 (95% CI, 306-377) and postintervention year average of 310 (95% CI, 287-332), which were not statistically different. However, there was a statistically significant decrease in the number of tests per episode. During the preintervention year, 1.38 troponin tests (95% CI, 1.31-1.45) were ordered per patient on average. This dropped by 0.17 (95% CI, 0.09-0.24) to the postintervention average of 1.21 (95% CI, 1.17-1.25) (Table 2, Figure 4).
ACS Prevalence
To determine whether there was an impact on ACS diagnoses, we looked at the numbers of ACS diagnoses and their prevalence among visits before and after the intervention. During the preintervention year, the average monthly number of diagnoses was 29.7 (95% CI, 26.1-33.2), and prevalence of ACS was 0.56% (95% CI, 0.48%-0.63%) of all episodes. Although the monthly rate was statistically decreasing by 0.022% (95% CI, 0.003-0.41), this has little meaning since the level of correlation (r2 = 0.2522, not displayed) was poor due to the essentially nonexistent correlation in number of visits each month (r2 = 0.0112, not displayed). During the postintervention year, the average number of diagnoses was 32.2 (95% CI, 27.9-36.6), and the prevalence of ACS was 0.62% (95% CI, 0.54-0.65). Neither of these values changed significantly between the pre- and postintervention period. All ICD-9 and ICD-10 diagnosis codes used for the analysis are available upon request from the authors.
Discussion
Our data demonstrate the ability of simple process improvement interventions to decrease unnecessary testing in the workup of ACS, increasing the rate of guideline concordant testing by > 70% at a single military treatment facility (MTF). In particular, with the now widespread use of EHR, the order set presents a high-yield target for process improvement in an easily implemented, durable fashion. We had expected to see some decrease in the efficacy of the intervention at a time of staff turnover in the summer of 2015 because ongoing dedicated teaching sessions were not performed. Despite that, the intervention remained effective without further dedicated teaching sessions. This outcome was certainly attributable to the hardwired interventions made (mainly via order sets), but possibly indicates an institutional memory that can take hold after an initial concerted effort is made.
We reduced the estimated preintervention annual cost of $113,000 by $53,000 (95% CI, $42,000-$64,000). Although on a much smaller scale than the study by Larochelle, our study represents a nearly 50% reduction in the total cost of initial testing for possible ACS and a > 80% reduction in unnecessary CK-MB testing.4 This result was achieved with no statistical change in the prevalence of ACS. The cost reduction does not account for the labor costs to clinically follow-up and address additional unnecessary lab results. The estimated cost of intervention was limited to the time required to educate residents, interns, and nursing staff as well as the implementation of the automated, reflexive laboratory results ordering process.
Unique to our study, we also demonstrated an intervention that satisfied all the major stakeholders in the ordering of these laboratory results. By instituting the reflexive ordering of CK-MB tests for positive troponins, we obtained the support of the facility’s interventional cardiology department, which finds value in that data. Appreciating the time-sensitive nature of an ACS diagnosis, the reflexive ordering minimized the delay in receiving these data while still greatly reducing the number of tests performed. That being said, if the current trend away from CK-MB in favor of exclusively testing troponin continues, removing the reflexive ordering for positive laboratory results protocol would be an easy follow-on intervention.
Limitations
Our study presented several limitations. First, reporting errors due to improper or insufficient medical coding as well as data entry errors may exist within the MDR; therefore, the results of this analysis may be over- or underestimated. Specifically, CPT codes for troponin and CK-MB were available only in 1 of the 2 data sets used for this study, which primarily contains outpatient patient encounters. For this reason, most of the laboratory testing comes from the EMD rather than from inpatient services. However, because we excluded all patients who eventually had an ACS diagnosis (patients who likely had more inpatient time and better indication for repeat troponin), we feel that our intervention was still thoroughly investigated. Second, the number of tests drawn per patient was significantly < 2, the expected minimum number of tests to rule out ACS in patients with appropriate symptoms.
This study was not designed to answer the source of variation from guidelines. Many patients had only 1 test, which we feel represents an opportunity for future study to identify other ways cardiac enzyme testing is being used clinically. These tests might be used for patients without convincing symptoms and signs of coronary syndromes or for patients with other primary problems. Third, by using the ITS analysis, we assumed that the outcome during each intervention period follows a linear pattern. However, changes may follow a nonlinear pattern over a long period. Finally, our intervention was limited to only a single MTF, which may limit generalizability to other facilities across military medicine. However, we feel this study should serve as a guide for other MTFs as well as US Department of Veterans Affairs facilities that could institute similar process improvements.
Conclusion
We made easily implemented and durable process improvement interventions that changed institution-wide ordering practices. These changes dramatically increased the rate of guideline-concordant testing, decreasing cost and furthering the goal of high-value medical care.
1. Anderson JL, Heidenreich PA, Barnett PG, et al; ACC/AHA Task Force on Performance Measures; ACC/AHA Task Force on Practice Guidelines. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129(22):2329-2345.
2. Centers for Disease Control and Prevention, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2010_ed_web_tables.pdf. Accessed March 15, 2019.
3. Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13):e356-e375.
4. Larochelle MR, Knight AM, Pantle H, Riedel S, Trost JC. Reducing excess cardiac biomarker testing at an academic medical center. J Gen Intern Med. 2014;29(11):1468-1474.
5. Centers for Medicare and Medicaid Services. 2016 clinical laboratory fee schedule. https://www.cms.gov/Medicare/Medicare-Fee -for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files-Items/16CLAB.html?DLPage=1&DLEntries=10&DLSort=2&DLSortDir=descending. Accessed March 15, 2019.
1. Anderson JL, Heidenreich PA, Barnett PG, et al; ACC/AHA Task Force on Performance Measures; ACC/AHA Task Force on Practice Guidelines. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129(22):2329-2345.
2. Centers for Disease Control and Prevention, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2010_ed_web_tables.pdf. Accessed March 15, 2019.
3. Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13):e356-e375.
4. Larochelle MR, Knight AM, Pantle H, Riedel S, Trost JC. Reducing excess cardiac biomarker testing at an academic medical center. J Gen Intern Med. 2014;29(11):1468-1474.
5. Centers for Medicare and Medicaid Services. 2016 clinical laboratory fee schedule. https://www.cms.gov/Medicare/Medicare-Fee -for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files-Items/16CLAB.html?DLPage=1&DLEntries=10&DLSort=2&DLSortDir=descending. Accessed March 15, 2019.
CV disease and mortality risk higher with younger age of type 2 diabetes diagnosis
Individuals who are younger when diagnosed with type 2 diabetes are at greater risk of cardiovascular disease and death, compared with those diagnosed at an older age, according to a retrospective study involving almost 2 million people.
People diagnosed with type 2 diabetes at age 40 or younger were at greatest risk of most outcomes, reported lead author Naveed Sattar, MD, PhD, professor of metabolic medicine, University of Glasgow, Scotland, and his colleagues. “Treatment target recommendations in regards to the risk factor control may need to be more aggressive in people developing diabetes at younger ages,” they wrote in Circulation.
In contrast, developing type 2 diabetes over the age of 80 years had little impact on risks.
“[R]eassessment of treatment goals in elderly might be useful,” the investigators wrote. “Diabetes screening needs for the elderly (above 80) should also be reevaluated.”
The study involved 318,083 patients with type 2 diabetes registered in the Swedish National Diabetes Registry between 1998 and 2012. Each patient was matched with 5 individuals from the general population based on sex, age, and country of residence, providing a control population of 1,575,108. Outcomes assessed included non-cardiovascular mortality, cardiovascular mortality, all causemortality, hospitalization for heart failure, coronary heart disease, stroke, atrial fibrillation, and acute myocardial infarction. Patients were followed for cardiovascular outcomes from 1998 to December 2013, while mortality surveillance continued through 2014.
In comparison with controls, patients 40 years or less had the highest excess risk of the most outcomes. *Excess risk of heart failure was elevated almost 5-fold (hazard ratio (HR), R 4.77), and risk of coronary heart disease wasn’t far behind (HR, 4.33). Risks of acute MI (HR, 3.41), stroke (HR, 3.58), and atrial fibrillation (HR, 1.95) were also elevated. Cardiovascular-related mortality was increased almost 3-fold (HR, 2.72), while total mortality (HR, 2.05) and non-cardiovascular mortality (HR, 1.95) were raised to a lesser degree.
“Thereafter, incremental risks generally declined with each higher decade age at diagnosis” of type 2 diabetes,” the investigators wrote.
After 80 years of age, all relative mortality risk factors dropped to less than 1, indicating lower risk than controls. Although non-fatal outcomes were still greater than 1 in this age group, these risks were “substantially attenuated compared with relative incremental risks in those diagnosed with T2DM at younger ages,” the investigators wrote.
The study was funded by the Swedish Association of Local Authorities Regions, the Swedish Heart and Lung Foundation, and the Swedish Research Council.
The investigators disclosed financial relationships with Amgen, AstraZeneca, Eli Lilly, and other pharmaceutical companies.
SOURCE: Sattar et al. Circulation. 2019 Apr 8. doi:10.1161/CIRCULATIONAHA.118.037885.
Individuals who are younger when diagnosed with type 2 diabetes are at greater risk of cardiovascular disease and death, compared with those diagnosed at an older age, according to a retrospective study involving almost 2 million people.
People diagnosed with type 2 diabetes at age 40 or younger were at greatest risk of most outcomes, reported lead author Naveed Sattar, MD, PhD, professor of metabolic medicine, University of Glasgow, Scotland, and his colleagues. “Treatment target recommendations in regards to the risk factor control may need to be more aggressive in people developing diabetes at younger ages,” they wrote in Circulation.
In contrast, developing type 2 diabetes over the age of 80 years had little impact on risks.
“[R]eassessment of treatment goals in elderly might be useful,” the investigators wrote. “Diabetes screening needs for the elderly (above 80) should also be reevaluated.”
The study involved 318,083 patients with type 2 diabetes registered in the Swedish National Diabetes Registry between 1998 and 2012. Each patient was matched with 5 individuals from the general population based on sex, age, and country of residence, providing a control population of 1,575,108. Outcomes assessed included non-cardiovascular mortality, cardiovascular mortality, all causemortality, hospitalization for heart failure, coronary heart disease, stroke, atrial fibrillation, and acute myocardial infarction. Patients were followed for cardiovascular outcomes from 1998 to December 2013, while mortality surveillance continued through 2014.
In comparison with controls, patients 40 years or less had the highest excess risk of the most outcomes. *Excess risk of heart failure was elevated almost 5-fold (hazard ratio (HR), R 4.77), and risk of coronary heart disease wasn’t far behind (HR, 4.33). Risks of acute MI (HR, 3.41), stroke (HR, 3.58), and atrial fibrillation (HR, 1.95) were also elevated. Cardiovascular-related mortality was increased almost 3-fold (HR, 2.72), while total mortality (HR, 2.05) and non-cardiovascular mortality (HR, 1.95) were raised to a lesser degree.
“Thereafter, incremental risks generally declined with each higher decade age at diagnosis” of type 2 diabetes,” the investigators wrote.
After 80 years of age, all relative mortality risk factors dropped to less than 1, indicating lower risk than controls. Although non-fatal outcomes were still greater than 1 in this age group, these risks were “substantially attenuated compared with relative incremental risks in those diagnosed with T2DM at younger ages,” the investigators wrote.
The study was funded by the Swedish Association of Local Authorities Regions, the Swedish Heart and Lung Foundation, and the Swedish Research Council.
The investigators disclosed financial relationships with Amgen, AstraZeneca, Eli Lilly, and other pharmaceutical companies.
SOURCE: Sattar et al. Circulation. 2019 Apr 8. doi:10.1161/CIRCULATIONAHA.118.037885.
Individuals who are younger when diagnosed with type 2 diabetes are at greater risk of cardiovascular disease and death, compared with those diagnosed at an older age, according to a retrospective study involving almost 2 million people.
People diagnosed with type 2 diabetes at age 40 or younger were at greatest risk of most outcomes, reported lead author Naveed Sattar, MD, PhD, professor of metabolic medicine, University of Glasgow, Scotland, and his colleagues. “Treatment target recommendations in regards to the risk factor control may need to be more aggressive in people developing diabetes at younger ages,” they wrote in Circulation.
In contrast, developing type 2 diabetes over the age of 80 years had little impact on risks.
“[R]eassessment of treatment goals in elderly might be useful,” the investigators wrote. “Diabetes screening needs for the elderly (above 80) should also be reevaluated.”
The study involved 318,083 patients with type 2 diabetes registered in the Swedish National Diabetes Registry between 1998 and 2012. Each patient was matched with 5 individuals from the general population based on sex, age, and country of residence, providing a control population of 1,575,108. Outcomes assessed included non-cardiovascular mortality, cardiovascular mortality, all causemortality, hospitalization for heart failure, coronary heart disease, stroke, atrial fibrillation, and acute myocardial infarction. Patients were followed for cardiovascular outcomes from 1998 to December 2013, while mortality surveillance continued through 2014.
In comparison with controls, patients 40 years or less had the highest excess risk of the most outcomes. *Excess risk of heart failure was elevated almost 5-fold (hazard ratio (HR), R 4.77), and risk of coronary heart disease wasn’t far behind (HR, 4.33). Risks of acute MI (HR, 3.41), stroke (HR, 3.58), and atrial fibrillation (HR, 1.95) were also elevated. Cardiovascular-related mortality was increased almost 3-fold (HR, 2.72), while total mortality (HR, 2.05) and non-cardiovascular mortality (HR, 1.95) were raised to a lesser degree.
“Thereafter, incremental risks generally declined with each higher decade age at diagnosis” of type 2 diabetes,” the investigators wrote.
After 80 years of age, all relative mortality risk factors dropped to less than 1, indicating lower risk than controls. Although non-fatal outcomes were still greater than 1 in this age group, these risks were “substantially attenuated compared with relative incremental risks in those diagnosed with T2DM at younger ages,” the investigators wrote.
The study was funded by the Swedish Association of Local Authorities Regions, the Swedish Heart and Lung Foundation, and the Swedish Research Council.
The investigators disclosed financial relationships with Amgen, AstraZeneca, Eli Lilly, and other pharmaceutical companies.
SOURCE: Sattar et al. Circulation. 2019 Apr 8. doi:10.1161/CIRCULATIONAHA.118.037885.
FROM CIRCULATION
Key clinical point: Patients who are younger when diagnosed with type 2 diabetes mellitus (T2DM) are at greater risk of cardiovascular disease and death than patients diagnosed at an older age.
Major finding: Patients diagnosed with T2DM at age 40 or younger had twice the risk of death from any cause, compared with age-matched controls (hazard ratio, 2.05).
Study details: A retrospective analysis of type 2 diabetes and associations with cardiovascular and mortality risks, using data from 318,083 patients in the Swedish National Diabetes Registry.
Disclosures: The study was funded by the Swedish Association of Local Authorities Regions, the Swedish Heart and Lung Foundation, and the Swedish Research Council. The investigators disclosed financial relationships with Amgen, Astra-Zeneca, Eli Lilly, and others.
Source: Sattar et al. Circulation. 2019 Apr 8. doi:10.1161/CIRCULATIONAHA.118.037885.
FDA names 40 ARBs that are free of nitrosamines
The Food and Drug Administration has identified 40 angiotensin II receptor blockers (ARBs) that do not contain the environmental contaminants, nitrosamines.
since impurities in these antihypertensive drugs were discovered last summer, according to a statement from the regulatory agency.
Among the drugs on this list are Accord Healthcare’s amlodipine and olmesartan medoxomil, Alembic Pharmaceuticals’ valsartan and hydrochlorothiazide, and Hisun Pharmaceuticals USA’s telmisartan.
Despite the FDA’s findings, the agency recommends patients continue taking the ARBs they have been prescribed until their pharmacists or physicians change their prescriptions to a safe replacement or different treatment option.
“We want to reassure patients that we strongly believe the risks, such as stroke, of abruptly discontinuing these important medications far outweighs the low risk associated with continuing the medications with these impurities,” says the statement.
The FDA noted that it is “continuing to work with manufacturers to swiftly remove medications from the market if they contain a nitrosamine impurity at levels higher than the interim acceptable intake limits,” and that this effort has resulted in shortages of valsartan products. In anticipation of more shortages, the FDA “is not objecting to temporary distribution” of specific lots of losartan containing impurities at levels exceeding the regulatory agency’s aforementioned standards.
The FDA’s scientists said that using ARBs with impurity levels above the interim acceptable intake limits over the time it should take to get impurity-free losartan to market will not result in an increased risk for cancer.
More information, including the full statement, is available on the FDA’s website.
cpalmer@mdedge.com
The Food and Drug Administration has identified 40 angiotensin II receptor blockers (ARBs) that do not contain the environmental contaminants, nitrosamines.
since impurities in these antihypertensive drugs were discovered last summer, according to a statement from the regulatory agency.
Among the drugs on this list are Accord Healthcare’s amlodipine and olmesartan medoxomil, Alembic Pharmaceuticals’ valsartan and hydrochlorothiazide, and Hisun Pharmaceuticals USA’s telmisartan.
Despite the FDA’s findings, the agency recommends patients continue taking the ARBs they have been prescribed until their pharmacists or physicians change their prescriptions to a safe replacement or different treatment option.
“We want to reassure patients that we strongly believe the risks, such as stroke, of abruptly discontinuing these important medications far outweighs the low risk associated with continuing the medications with these impurities,” says the statement.
The FDA noted that it is “continuing to work with manufacturers to swiftly remove medications from the market if they contain a nitrosamine impurity at levels higher than the interim acceptable intake limits,” and that this effort has resulted in shortages of valsartan products. In anticipation of more shortages, the FDA “is not objecting to temporary distribution” of specific lots of losartan containing impurities at levels exceeding the regulatory agency’s aforementioned standards.
The FDA’s scientists said that using ARBs with impurity levels above the interim acceptable intake limits over the time it should take to get impurity-free losartan to market will not result in an increased risk for cancer.
More information, including the full statement, is available on the FDA’s website.
cpalmer@mdedge.com
The Food and Drug Administration has identified 40 angiotensin II receptor blockers (ARBs) that do not contain the environmental contaminants, nitrosamines.
since impurities in these antihypertensive drugs were discovered last summer, according to a statement from the regulatory agency.
Among the drugs on this list are Accord Healthcare’s amlodipine and olmesartan medoxomil, Alembic Pharmaceuticals’ valsartan and hydrochlorothiazide, and Hisun Pharmaceuticals USA’s telmisartan.
Despite the FDA’s findings, the agency recommends patients continue taking the ARBs they have been prescribed until their pharmacists or physicians change their prescriptions to a safe replacement or different treatment option.
“We want to reassure patients that we strongly believe the risks, such as stroke, of abruptly discontinuing these important medications far outweighs the low risk associated with continuing the medications with these impurities,” says the statement.
The FDA noted that it is “continuing to work with manufacturers to swiftly remove medications from the market if they contain a nitrosamine impurity at levels higher than the interim acceptable intake limits,” and that this effort has resulted in shortages of valsartan products. In anticipation of more shortages, the FDA “is not objecting to temporary distribution” of specific lots of losartan containing impurities at levels exceeding the regulatory agency’s aforementioned standards.
The FDA’s scientists said that using ARBs with impurity levels above the interim acceptable intake limits over the time it should take to get impurity-free losartan to market will not result in an increased risk for cancer.
More information, including the full statement, is available on the FDA’s website.
cpalmer@mdedge.com
One-dose-fits-all aspirin administration strategy may not be advisable
Clinical question: Are the same doses of aspirin equally effective in preventing cardiovascular (CV) events and long-term colorectal risk reduction in patients of various body sizes?
Background: Strong evidence for the one-dose-fits-all approach to use of aspirin in long-term prevention of CV events is lacking. Aspirin effect may be dependent on patient’s body size. Excess dosing of aspirin in patients of small body size might negatively affect their outcomes.
Study design: Meta-analysis.
Setting: Trials from the Antithrombotic Trialists’ Collaboration, other systematic reviews of trials of aspirin, and from the Cochrane Database of Systematic Reviews.
Synopsis: The authors included 10 trials (117,279 participants altogether) and analyzed the association of body weight with the effectiveness of aspirin doses on CV event and colon cancer prevention. The greatest benefit of low-dose aspirin (75-100 mg) in reducing CV events was seen in patients weighing 50-69 kg (hazard ratio, 0.75; 95% confidence interval, 0.65-0.85; P less than .0001), with CV events increasing with increasing weights (P interaction = .0072). There was an increased rate of fatality with low-dose aspirin among patients at body weights greater than 70 kg (HR, 1.33; 95% CI, 1.08-1.64; P = .0082) or less than 50 kg (HR, 1.52; 95% CI, 1.04-2.21; P = .031). Higher doses of aspirin were more effective at higher body weights (P interaction = .017). Similar weight-dependent effects were seen in the 20-year risk of colorectal cancer.
While findings are consistent across trials looking at dose-dependent aspirin effects in patients of various body sizes, limitations included lack of generalizability of the results in secondary prevention trials, inclusion of older trials, variability of participants’ characteristics, and aspirin compliance across trials.
Bottom line: Weight-based aspirin dosing may be required for prevention of CV events, sudden cardiac death, and cancer. Based on the results of this meta-analysis, one-dose-fits-all aspirin administration strategy may not be advisable.
Citation: Rothwell PM et al. Effects of aspirin on risks of vascular events and cancer according to body weight and dose: Analysis of individual patient data from randomized trials. Lancet. 2018;392:387-99.
Dr. Burklin is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Clinical question: Are the same doses of aspirin equally effective in preventing cardiovascular (CV) events and long-term colorectal risk reduction in patients of various body sizes?
Background: Strong evidence for the one-dose-fits-all approach to use of aspirin in long-term prevention of CV events is lacking. Aspirin effect may be dependent on patient’s body size. Excess dosing of aspirin in patients of small body size might negatively affect their outcomes.
Study design: Meta-analysis.
Setting: Trials from the Antithrombotic Trialists’ Collaboration, other systematic reviews of trials of aspirin, and from the Cochrane Database of Systematic Reviews.
Synopsis: The authors included 10 trials (117,279 participants altogether) and analyzed the association of body weight with the effectiveness of aspirin doses on CV event and colon cancer prevention. The greatest benefit of low-dose aspirin (75-100 mg) in reducing CV events was seen in patients weighing 50-69 kg (hazard ratio, 0.75; 95% confidence interval, 0.65-0.85; P less than .0001), with CV events increasing with increasing weights (P interaction = .0072). There was an increased rate of fatality with low-dose aspirin among patients at body weights greater than 70 kg (HR, 1.33; 95% CI, 1.08-1.64; P = .0082) or less than 50 kg (HR, 1.52; 95% CI, 1.04-2.21; P = .031). Higher doses of aspirin were more effective at higher body weights (P interaction = .017). Similar weight-dependent effects were seen in the 20-year risk of colorectal cancer.
While findings are consistent across trials looking at dose-dependent aspirin effects in patients of various body sizes, limitations included lack of generalizability of the results in secondary prevention trials, inclusion of older trials, variability of participants’ characteristics, and aspirin compliance across trials.
Bottom line: Weight-based aspirin dosing may be required for prevention of CV events, sudden cardiac death, and cancer. Based on the results of this meta-analysis, one-dose-fits-all aspirin administration strategy may not be advisable.
Citation: Rothwell PM et al. Effects of aspirin on risks of vascular events and cancer according to body weight and dose: Analysis of individual patient data from randomized trials. Lancet. 2018;392:387-99.
Dr. Burklin is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Clinical question: Are the same doses of aspirin equally effective in preventing cardiovascular (CV) events and long-term colorectal risk reduction in patients of various body sizes?
Background: Strong evidence for the one-dose-fits-all approach to use of aspirin in long-term prevention of CV events is lacking. Aspirin effect may be dependent on patient’s body size. Excess dosing of aspirin in patients of small body size might negatively affect their outcomes.
Study design: Meta-analysis.
Setting: Trials from the Antithrombotic Trialists’ Collaboration, other systematic reviews of trials of aspirin, and from the Cochrane Database of Systematic Reviews.
Synopsis: The authors included 10 trials (117,279 participants altogether) and analyzed the association of body weight with the effectiveness of aspirin doses on CV event and colon cancer prevention. The greatest benefit of low-dose aspirin (75-100 mg) in reducing CV events was seen in patients weighing 50-69 kg (hazard ratio, 0.75; 95% confidence interval, 0.65-0.85; P less than .0001), with CV events increasing with increasing weights (P interaction = .0072). There was an increased rate of fatality with low-dose aspirin among patients at body weights greater than 70 kg (HR, 1.33; 95% CI, 1.08-1.64; P = .0082) or less than 50 kg (HR, 1.52; 95% CI, 1.04-2.21; P = .031). Higher doses of aspirin were more effective at higher body weights (P interaction = .017). Similar weight-dependent effects were seen in the 20-year risk of colorectal cancer.
While findings are consistent across trials looking at dose-dependent aspirin effects in patients of various body sizes, limitations included lack of generalizability of the results in secondary prevention trials, inclusion of older trials, variability of participants’ characteristics, and aspirin compliance across trials.
Bottom line: Weight-based aspirin dosing may be required for prevention of CV events, sudden cardiac death, and cancer. Based on the results of this meta-analysis, one-dose-fits-all aspirin administration strategy may not be advisable.
Citation: Rothwell PM et al. Effects of aspirin on risks of vascular events and cancer according to body weight and dose: Analysis of individual patient data from randomized trials. Lancet. 2018;392:387-99.
Dr. Burklin is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Despite failed primary endpoint, MI alert device has predictive value
WASHINGTON –Although an implantable device for detecting myocardial infarction missed the primary composite outcome endpoint in a controlled trial, a newly completed extended analysis associated the device with a higher positive predictive value and a lower false positive rate when compared to sham control, according to data presented at CRT 2019, sponsored by MedStar Heart & Vascular Institute.
“Among high risk patients, this system may be beneficial in the identification of both symptomatic and asymptomatic coronary events,” reported C. Michael Gibson, MD, chief of clinical research in the cardiology division at Beth Israel Deaconess Hospital, Boston.
The implantable device (AngelMed Guardian System), which received Food and Drug Administration approval in April 2018, is designed to identify MI through detection of ST-segment elevations in the absence of an elevated heart rate. When the system detects an event during continuous monitoring, it sends a signal (internal vibration and auditory signal to an external monitor) designed to tell the patient to seek medical care.
The previously published multicenter and randomized ALERTS (AngelMed for Early Recognition and Treatment of STEMI) trial that tested this device was negative for primary composite endpoint of cardiac or unexplained death, new Q-wave MI, or presentation at the emergency department (ED) more than 2 hours after symptom onset (J Am Coll Cardiol. 2019 Feb 25. pii: S0735-1097[19]30237-2). In that trial 907 patients were fitted with the device and then randomized to having the device switched on or left off.
At 7 days, a primary endpoint was reached by 3.8% of those in the device-on group versus 4.9% of those in the device-off group, which was not significantly different.
Although the primary endpoint was not met, there were promising results. For example, in those who did have an occlusive event, patients in the device-on group had better preserved left ventricular function when evaluated after the event, a result consistent with earlier presentation in the ED and earlier treatment. In fact, 85% of patients with an MI in the device-on group presented to a hospital within 2 hours, compared with just 5% of those in the device-off control group during the initial study period.
More evidence of a potential clinical role for the device has now been generated in a new extended analysis. This analysis was made possible because patients in both of the randomized groups continue to wear the device, including those in the device-off group who had their devices activated after 6 months. There are now 3 more years of data of follow-up from those initially in the device-on group and those switched from the device-off group.
“So we started the clock over with a new statistical analysis plan and new endpoints,” Dr. Gibson explained. The FDA was consulted in selecting endpoints, particularly regarding evidence that the device did not increase false-positive ED visits.
There were numerous encouraging findings. One was that 42 silent MIs, which would otherwise have been missed, were detected over the extended follow-up. Another was that the annualized false-positive rate was lower in those with an activated device (0.164/year) when compared to the original device-off group (0.678/year; P less than .001). Lastly, the positive predictive value of an alarm during the extended follow-up was higher than that of symptoms alone among the original device-off group (25.8% vs. 18.2%).
The device was found safe. The rate of system-related complications was under 4%, which Dr. Gibson said is noninferior to that associated with pacemakers.
One of the potential explanations for the failure of the device to achieve the primary endpoint in the original trial was an unexpectedly low event rate, according to Dr. Gibson.
Even before this extended analysis, the FDA had accepted the potential benefits of this device as demonstrated in the approval last year. In the labeling, the device is called “a more accurate predictor of acute coronary syndrome events when compared to patient recognized symptoms alone and demonstrates a reduced rate over time of patient presentations without ACS events.”
“About 50% of patients wait more than 3 hours after the onset of symptoms before reaching an emergency room,” observed Dr. Gibson. Emphasizing the evidence that delay is an important predictor of adverse outcomes, he suggested the alarm device might be useful in accelerating care in some high risk groups.
WASHINGTON –Although an implantable device for detecting myocardial infarction missed the primary composite outcome endpoint in a controlled trial, a newly completed extended analysis associated the device with a higher positive predictive value and a lower false positive rate when compared to sham control, according to data presented at CRT 2019, sponsored by MedStar Heart & Vascular Institute.
“Among high risk patients, this system may be beneficial in the identification of both symptomatic and asymptomatic coronary events,” reported C. Michael Gibson, MD, chief of clinical research in the cardiology division at Beth Israel Deaconess Hospital, Boston.
The implantable device (AngelMed Guardian System), which received Food and Drug Administration approval in April 2018, is designed to identify MI through detection of ST-segment elevations in the absence of an elevated heart rate. When the system detects an event during continuous monitoring, it sends a signal (internal vibration and auditory signal to an external monitor) designed to tell the patient to seek medical care.
The previously published multicenter and randomized ALERTS (AngelMed for Early Recognition and Treatment of STEMI) trial that tested this device was negative for primary composite endpoint of cardiac or unexplained death, new Q-wave MI, or presentation at the emergency department (ED) more than 2 hours after symptom onset (J Am Coll Cardiol. 2019 Feb 25. pii: S0735-1097[19]30237-2). In that trial 907 patients were fitted with the device and then randomized to having the device switched on or left off.
At 7 days, a primary endpoint was reached by 3.8% of those in the device-on group versus 4.9% of those in the device-off group, which was not significantly different.
Although the primary endpoint was not met, there were promising results. For example, in those who did have an occlusive event, patients in the device-on group had better preserved left ventricular function when evaluated after the event, a result consistent with earlier presentation in the ED and earlier treatment. In fact, 85% of patients with an MI in the device-on group presented to a hospital within 2 hours, compared with just 5% of those in the device-off control group during the initial study period.
More evidence of a potential clinical role for the device has now been generated in a new extended analysis. This analysis was made possible because patients in both of the randomized groups continue to wear the device, including those in the device-off group who had their devices activated after 6 months. There are now 3 more years of data of follow-up from those initially in the device-on group and those switched from the device-off group.
“So we started the clock over with a new statistical analysis plan and new endpoints,” Dr. Gibson explained. The FDA was consulted in selecting endpoints, particularly regarding evidence that the device did not increase false-positive ED visits.
There were numerous encouraging findings. One was that 42 silent MIs, which would otherwise have been missed, were detected over the extended follow-up. Another was that the annualized false-positive rate was lower in those with an activated device (0.164/year) when compared to the original device-off group (0.678/year; P less than .001). Lastly, the positive predictive value of an alarm during the extended follow-up was higher than that of symptoms alone among the original device-off group (25.8% vs. 18.2%).
The device was found safe. The rate of system-related complications was under 4%, which Dr. Gibson said is noninferior to that associated with pacemakers.
One of the potential explanations for the failure of the device to achieve the primary endpoint in the original trial was an unexpectedly low event rate, according to Dr. Gibson.
Even before this extended analysis, the FDA had accepted the potential benefits of this device as demonstrated in the approval last year. In the labeling, the device is called “a more accurate predictor of acute coronary syndrome events when compared to patient recognized symptoms alone and demonstrates a reduced rate over time of patient presentations without ACS events.”
“About 50% of patients wait more than 3 hours after the onset of symptoms before reaching an emergency room,” observed Dr. Gibson. Emphasizing the evidence that delay is an important predictor of adverse outcomes, he suggested the alarm device might be useful in accelerating care in some high risk groups.
WASHINGTON –Although an implantable device for detecting myocardial infarction missed the primary composite outcome endpoint in a controlled trial, a newly completed extended analysis associated the device with a higher positive predictive value and a lower false positive rate when compared to sham control, according to data presented at CRT 2019, sponsored by MedStar Heart & Vascular Institute.
“Among high risk patients, this system may be beneficial in the identification of both symptomatic and asymptomatic coronary events,” reported C. Michael Gibson, MD, chief of clinical research in the cardiology division at Beth Israel Deaconess Hospital, Boston.
The implantable device (AngelMed Guardian System), which received Food and Drug Administration approval in April 2018, is designed to identify MI through detection of ST-segment elevations in the absence of an elevated heart rate. When the system detects an event during continuous monitoring, it sends a signal (internal vibration and auditory signal to an external monitor) designed to tell the patient to seek medical care.
The previously published multicenter and randomized ALERTS (AngelMed for Early Recognition and Treatment of STEMI) trial that tested this device was negative for primary composite endpoint of cardiac or unexplained death, new Q-wave MI, or presentation at the emergency department (ED) more than 2 hours after symptom onset (J Am Coll Cardiol. 2019 Feb 25. pii: S0735-1097[19]30237-2). In that trial 907 patients were fitted with the device and then randomized to having the device switched on or left off.
At 7 days, a primary endpoint was reached by 3.8% of those in the device-on group versus 4.9% of those in the device-off group, which was not significantly different.
Although the primary endpoint was not met, there were promising results. For example, in those who did have an occlusive event, patients in the device-on group had better preserved left ventricular function when evaluated after the event, a result consistent with earlier presentation in the ED and earlier treatment. In fact, 85% of patients with an MI in the device-on group presented to a hospital within 2 hours, compared with just 5% of those in the device-off control group during the initial study period.
More evidence of a potential clinical role for the device has now been generated in a new extended analysis. This analysis was made possible because patients in both of the randomized groups continue to wear the device, including those in the device-off group who had their devices activated after 6 months. There are now 3 more years of data of follow-up from those initially in the device-on group and those switched from the device-off group.
“So we started the clock over with a new statistical analysis plan and new endpoints,” Dr. Gibson explained. The FDA was consulted in selecting endpoints, particularly regarding evidence that the device did not increase false-positive ED visits.
There were numerous encouraging findings. One was that 42 silent MIs, which would otherwise have been missed, were detected over the extended follow-up. Another was that the annualized false-positive rate was lower in those with an activated device (0.164/year) when compared to the original device-off group (0.678/year; P less than .001). Lastly, the positive predictive value of an alarm during the extended follow-up was higher than that of symptoms alone among the original device-off group (25.8% vs. 18.2%).
The device was found safe. The rate of system-related complications was under 4%, which Dr. Gibson said is noninferior to that associated with pacemakers.
One of the potential explanations for the failure of the device to achieve the primary endpoint in the original trial was an unexpectedly low event rate, according to Dr. Gibson.
Even before this extended analysis, the FDA had accepted the potential benefits of this device as demonstrated in the approval last year. In the labeling, the device is called “a more accurate predictor of acute coronary syndrome events when compared to patient recognized symptoms alone and demonstrates a reduced rate over time of patient presentations without ACS events.”
“About 50% of patients wait more than 3 hours after the onset of symptoms before reaching an emergency room,” observed Dr. Gibson. Emphasizing the evidence that delay is an important predictor of adverse outcomes, he suggested the alarm device might be useful in accelerating care in some high risk groups.
REPORTING FROM CRT 2019
What are the risks of long-term PPI use for GERD symptoms in patients > 65 years?
EVIDENCE SUMMARY
A 2017 meta-analysis of 16 RCTs examined the risk of cardiovascular events in 7540 adult patients taking PPIs for GERD (mean ages 45-55 years).1 The primary outcome was cardiovascular events—including acute myocardial infarction, myocardial ischemia, angina pectoris, cardiac failure, and coronary artery stenosis—and cardiac disorders.
Analysis of pooled data found that PPI use was associated with a 70% increase in cardiovascular risk (relative risk [RR] = 1.7; 95% confidence interval [CI], 1.13-2.56; number needed to harm [NNH] = 241) when compared with controls (placebo, H2 blocker, or surgery). A subgroup analysis found that PPI use for longer than 8 weeks was associated with an even higher risk of adverse cardiovascular events (6 trials, 2296 patients; RR = 2.33; 95% CI, 1.33-4.08; NNH = 67) when compared with controls. The meta-analysis wasn’t limited by heterogeneity (I2 = 0).
C difficile infection risk is higherfor PPI users
A 2016 meta-analysis of 23 observational studies (19 case-control, 4 retrospective cohort; 186,033 patients) examined the risk of hospital-acquired C difficile infections in adults prescribed PPI for any indication.2 PPI exposure varied from use at time of diagnosis or hospitalization to any use within 90 days. Of the 23 studies, 16 reported sufficient data to calculate the mean age for the patients which was 69.9 years.
The risk of C difficile infection was found to be higher with PPI use than no use (pooled odds ratio [OR] = 1.81; 95% CI, 1.52-2.14). Although a significant association was found across a large group, the results were limited by considerable heterogeneity (I2 = 82%).
Risk of community-acquired pneumonia also increases with PPI use
A 2015 systematic review and meta-analysis of 33 trials (18 case-control, 10 cohort, 4 RCTs, and 1 case-crossover study) examined the risk of CAP in adult patients prescribed PPI for any indication for durations ranging from less than 1 month to > 6 months.3 The systematic review was distilled to 26 studies because of overlapping study populations. These 26 studies included 226,769 cases of CAP among 6,351,656 patients. The primary outcome was development of CAP, the secondary outcome was hospitalization for CAP.
PPI use, compared with no use, was associated with an increased risk of developing CAP (pooled OR = 1.49; 95% CI, 1.16-1.92) and an increased risk of hospitalization for CAP (pooled OR = 1.61; 95% CI, 1.12-2.31).
In a subgroup analysis for age, patients older than 65 years were also found to have an increased risk of developing CAP with PPI use (11 trials, total number of patients not provided; OR = 1.33; 95% CI, 1.13-1.58). Despite the significant associations of PPI use with risk revealed in the primary, secondary, and subgroup analyses, the results were limited by marked heterogeneity, with an I2 > 99%.
Continue to: Hip and vertebral fracture risks associated with PPIs
Hip and vertebral fracture riskis associated with PPIs
A 2011 systematic review and meta-analysis investigated the risk of fracture in adult patients taking PPIs for any indication.4 The analysis included 10 observational studies (4 cohort, 6 case-control) with a total of 223,210 fracture cases. The authors examined the incidence of hip, vertebral, and wrist or forearm fractures.
No significant association was found between PPI use and wrist or forearm fracture (3 studies; pooled OR = 1.09; 95% CI, 0.95-1.24). A modest association was noted between PPI use and both hip fractures (9 trials; OR = 1.25; 95% CI, 1.14-1.37) and vertebral fractures (4 trials; OR = 1.5; 95% CI, 1.32-1.72).
Subgroup analysis didn’t reveal evidence of an effect of duration of PPI use on fracture. Investigators didn’t conduct subgroup analysis of different patient ages. Final results were limited by significant heterogeneity with an I2 of 86%.
RECOMMENDATIONS
A 2015 American Geriatrics Society Beers Criteria update recommends limiting PPI use because of increased risk of C difficile infections and fractures. It also recommends against using PPIs for longer than 8 weeks except for high-risk patients (such as patients taking oral corticosteroids or chronic nonsteroidal anti-inflammatory drug users), patients with Barrett’s esophagitis, or patients who need maintenance after failure of a drug discontinuation trial or H2 blockers (quality of evidence, high; SOR, strong).5
Editor’s take
1. Sun S, Cui Z, Zhou M, et al. Proton pump inhibitor monotherapy and the risk of cardiovascular events in patients with gastro-esophageal reflux disease: a meta-analysis. Neurogastroenterol Motil. 2017;29:e12926.
2. Arriola V, Tischendorf J, Musuuza J, et al. Assessing the risk of hospital-acquired clostridium difficile infection with proton pump inhibitor use: a meta-analysis. Infect Control Hosp Epidemiol. 2016;37:1408-1417.
3. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10:e0128004.
4. Ngamruengphong S, Leontiadis GI, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106:1209-1218.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
EVIDENCE SUMMARY
A 2017 meta-analysis of 16 RCTs examined the risk of cardiovascular events in 7540 adult patients taking PPIs for GERD (mean ages 45-55 years).1 The primary outcome was cardiovascular events—including acute myocardial infarction, myocardial ischemia, angina pectoris, cardiac failure, and coronary artery stenosis—and cardiac disorders.
Analysis of pooled data found that PPI use was associated with a 70% increase in cardiovascular risk (relative risk [RR] = 1.7; 95% confidence interval [CI], 1.13-2.56; number needed to harm [NNH] = 241) when compared with controls (placebo, H2 blocker, or surgery). A subgroup analysis found that PPI use for longer than 8 weeks was associated with an even higher risk of adverse cardiovascular events (6 trials, 2296 patients; RR = 2.33; 95% CI, 1.33-4.08; NNH = 67) when compared with controls. The meta-analysis wasn’t limited by heterogeneity (I2 = 0).
C difficile infection risk is higherfor PPI users
A 2016 meta-analysis of 23 observational studies (19 case-control, 4 retrospective cohort; 186,033 patients) examined the risk of hospital-acquired C difficile infections in adults prescribed PPI for any indication.2 PPI exposure varied from use at time of diagnosis or hospitalization to any use within 90 days. Of the 23 studies, 16 reported sufficient data to calculate the mean age for the patients which was 69.9 years.
The risk of C difficile infection was found to be higher with PPI use than no use (pooled odds ratio [OR] = 1.81; 95% CI, 1.52-2.14). Although a significant association was found across a large group, the results were limited by considerable heterogeneity (I2 = 82%).
Risk of community-acquired pneumonia also increases with PPI use
A 2015 systematic review and meta-analysis of 33 trials (18 case-control, 10 cohort, 4 RCTs, and 1 case-crossover study) examined the risk of CAP in adult patients prescribed PPI for any indication for durations ranging from less than 1 month to > 6 months.3 The systematic review was distilled to 26 studies because of overlapping study populations. These 26 studies included 226,769 cases of CAP among 6,351,656 patients. The primary outcome was development of CAP, the secondary outcome was hospitalization for CAP.
PPI use, compared with no use, was associated with an increased risk of developing CAP (pooled OR = 1.49; 95% CI, 1.16-1.92) and an increased risk of hospitalization for CAP (pooled OR = 1.61; 95% CI, 1.12-2.31).
In a subgroup analysis for age, patients older than 65 years were also found to have an increased risk of developing CAP with PPI use (11 trials, total number of patients not provided; OR = 1.33; 95% CI, 1.13-1.58). Despite the significant associations of PPI use with risk revealed in the primary, secondary, and subgroup analyses, the results were limited by marked heterogeneity, with an I2 > 99%.
Continue to: Hip and vertebral fracture risks associated with PPIs
Hip and vertebral fracture riskis associated with PPIs
A 2011 systematic review and meta-analysis investigated the risk of fracture in adult patients taking PPIs for any indication.4 The analysis included 10 observational studies (4 cohort, 6 case-control) with a total of 223,210 fracture cases. The authors examined the incidence of hip, vertebral, and wrist or forearm fractures.
No significant association was found between PPI use and wrist or forearm fracture (3 studies; pooled OR = 1.09; 95% CI, 0.95-1.24). A modest association was noted between PPI use and both hip fractures (9 trials; OR = 1.25; 95% CI, 1.14-1.37) and vertebral fractures (4 trials; OR = 1.5; 95% CI, 1.32-1.72).
Subgroup analysis didn’t reveal evidence of an effect of duration of PPI use on fracture. Investigators didn’t conduct subgroup analysis of different patient ages. Final results were limited by significant heterogeneity with an I2 of 86%.
RECOMMENDATIONS
A 2015 American Geriatrics Society Beers Criteria update recommends limiting PPI use because of increased risk of C difficile infections and fractures. It also recommends against using PPIs for longer than 8 weeks except for high-risk patients (such as patients taking oral corticosteroids or chronic nonsteroidal anti-inflammatory drug users), patients with Barrett’s esophagitis, or patients who need maintenance after failure of a drug discontinuation trial or H2 blockers (quality of evidence, high; SOR, strong).5
Editor’s take
EVIDENCE SUMMARY
A 2017 meta-analysis of 16 RCTs examined the risk of cardiovascular events in 7540 adult patients taking PPIs for GERD (mean ages 45-55 years).1 The primary outcome was cardiovascular events—including acute myocardial infarction, myocardial ischemia, angina pectoris, cardiac failure, and coronary artery stenosis—and cardiac disorders.
Analysis of pooled data found that PPI use was associated with a 70% increase in cardiovascular risk (relative risk [RR] = 1.7; 95% confidence interval [CI], 1.13-2.56; number needed to harm [NNH] = 241) when compared with controls (placebo, H2 blocker, or surgery). A subgroup analysis found that PPI use for longer than 8 weeks was associated with an even higher risk of adverse cardiovascular events (6 trials, 2296 patients; RR = 2.33; 95% CI, 1.33-4.08; NNH = 67) when compared with controls. The meta-analysis wasn’t limited by heterogeneity (I2 = 0).
C difficile infection risk is higherfor PPI users
A 2016 meta-analysis of 23 observational studies (19 case-control, 4 retrospective cohort; 186,033 patients) examined the risk of hospital-acquired C difficile infections in adults prescribed PPI for any indication.2 PPI exposure varied from use at time of diagnosis or hospitalization to any use within 90 days. Of the 23 studies, 16 reported sufficient data to calculate the mean age for the patients which was 69.9 years.
The risk of C difficile infection was found to be higher with PPI use than no use (pooled odds ratio [OR] = 1.81; 95% CI, 1.52-2.14). Although a significant association was found across a large group, the results were limited by considerable heterogeneity (I2 = 82%).
Risk of community-acquired pneumonia also increases with PPI use
A 2015 systematic review and meta-analysis of 33 trials (18 case-control, 10 cohort, 4 RCTs, and 1 case-crossover study) examined the risk of CAP in adult patients prescribed PPI for any indication for durations ranging from less than 1 month to > 6 months.3 The systematic review was distilled to 26 studies because of overlapping study populations. These 26 studies included 226,769 cases of CAP among 6,351,656 patients. The primary outcome was development of CAP, the secondary outcome was hospitalization for CAP.
PPI use, compared with no use, was associated with an increased risk of developing CAP (pooled OR = 1.49; 95% CI, 1.16-1.92) and an increased risk of hospitalization for CAP (pooled OR = 1.61; 95% CI, 1.12-2.31).
In a subgroup analysis for age, patients older than 65 years were also found to have an increased risk of developing CAP with PPI use (11 trials, total number of patients not provided; OR = 1.33; 95% CI, 1.13-1.58). Despite the significant associations of PPI use with risk revealed in the primary, secondary, and subgroup analyses, the results were limited by marked heterogeneity, with an I2 > 99%.
Continue to: Hip and vertebral fracture risks associated with PPIs
Hip and vertebral fracture riskis associated with PPIs
A 2011 systematic review and meta-analysis investigated the risk of fracture in adult patients taking PPIs for any indication.4 The analysis included 10 observational studies (4 cohort, 6 case-control) with a total of 223,210 fracture cases. The authors examined the incidence of hip, vertebral, and wrist or forearm fractures.
No significant association was found between PPI use and wrist or forearm fracture (3 studies; pooled OR = 1.09; 95% CI, 0.95-1.24). A modest association was noted between PPI use and both hip fractures (9 trials; OR = 1.25; 95% CI, 1.14-1.37) and vertebral fractures (4 trials; OR = 1.5; 95% CI, 1.32-1.72).
Subgroup analysis didn’t reveal evidence of an effect of duration of PPI use on fracture. Investigators didn’t conduct subgroup analysis of different patient ages. Final results were limited by significant heterogeneity with an I2 of 86%.
RECOMMENDATIONS
A 2015 American Geriatrics Society Beers Criteria update recommends limiting PPI use because of increased risk of C difficile infections and fractures. It also recommends against using PPIs for longer than 8 weeks except for high-risk patients (such as patients taking oral corticosteroids or chronic nonsteroidal anti-inflammatory drug users), patients with Barrett’s esophagitis, or patients who need maintenance after failure of a drug discontinuation trial or H2 blockers (quality of evidence, high; SOR, strong).5
Editor’s take
1. Sun S, Cui Z, Zhou M, et al. Proton pump inhibitor monotherapy and the risk of cardiovascular events in patients with gastro-esophageal reflux disease: a meta-analysis. Neurogastroenterol Motil. 2017;29:e12926.
2. Arriola V, Tischendorf J, Musuuza J, et al. Assessing the risk of hospital-acquired clostridium difficile infection with proton pump inhibitor use: a meta-analysis. Infect Control Hosp Epidemiol. 2016;37:1408-1417.
3. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10:e0128004.
4. Ngamruengphong S, Leontiadis GI, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106:1209-1218.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
1. Sun S, Cui Z, Zhou M, et al. Proton pump inhibitor monotherapy and the risk of cardiovascular events in patients with gastro-esophageal reflux disease: a meta-analysis. Neurogastroenterol Motil. 2017;29:e12926.
2. Arriola V, Tischendorf J, Musuuza J, et al. Assessing the risk of hospital-acquired clostridium difficile infection with proton pump inhibitor use: a meta-analysis. Infect Control Hosp Epidemiol. 2016;37:1408-1417.
3. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10:e0128004.
4. Ngamruengphong S, Leontiadis GI, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106:1209-1218.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
EVIDENCE-BASED ANSWER:
The use of proton pump inhibitors (PPIs) to control gastroesophageal reflux disease (GERD) is significantly associated with an increased risk of cardiovascular events such as acute myocardial infarction and myocardial ischemia, especially with treatment longer than 8 weeks (strength of recommendation [SOR]: A, systematic review of randomized, controlled trials [RCTs]). This summary is based on data extrapolated from studies on all adults because there is limited evidence that specifically addresses patients older than 65 years.
Adults taking PPIs also appear to be at increased risk of Clostridium difficile infection, community-acquired pneumonia (CAP; with use for < 30 days), and fracture (SOR: B, systematic reviews of heterogeneous prospective and retrospective observational studies).
Aspirin for primary prevention: It depends
Acetylsalicylic acid has been around for nearly 200 years. It traces its history back to a French chemist (Charles Frederic Gerhardt) and 2 German chemists (Felix Hoffmann and Arthur Eichengrün) who worked at Bayer, the company that launched the pain reliever under the name “aspirin” in 1899. It is now one of the most commonly used medications in the world.
With aspirin's anti-inflammatory properties in mind, researchers conducted randomized trials for secondary prevention of heart attacks in the 1970s; low-dose aspirin was proven effective in reducing risk for a second myocardial infarction. These trials led to speculation that aspirin might be effective for primary prevention as well. Indeed, in the 1980s the large Physicians' Health Study found aspirin reduced the incidence of first heart attack in healthy physicians by 44%.1 Unfortunately, there was no reduction in mortality from heart disease and it was only effective for those older than 50.
The downside of aspirin was a slight increase in the incidence of hemorrhagic stroke and bleeding requiring transfusion. Nonetheless, many healthy adults started taking daily aspirin hoping to prevent a heart attack.
In this issue of JFP, Smith and colleagues summarize the 2016 recommendations of the US Preventive Services Task Force (USPSTF) regarding aspirin for primary prevention, as well as the 4 large aspirin prevention trials published in 2018 subsequent to the USPSTF recommendations. The USPSTF recommended aspirin for adults ages 50 to 59 with a 10-year cardiovascular risk of at least 10% (B recommendation). For those ages 60-69, the USPSTF recommendation for aspirin as primary prevention has a “C” rating, meaning that patient preference is important to consider in balancing benefit and harms. For those 70 and older, the USPSTF gave aspirin an “I” (insufficient evidence) rating because of increased risk for bleeding. It is important to note that the positive B recommendation for those ages 50-59 is based not only on cardiovascular risk reduction but also on a slight risk reduction for colon cancer for those taking aspirin for at least 10 years.
The 4 new, large randomized trials published in 2018, however, cast doubt on the USPSTF recommendations because the results of these trials were negative for the most part. The bottom line is that daily aspirin for prevention is definitely not for everyone and perhaps not for anyone except those who have established vascular disease or are at high risk for vascular disease and low risk for bleeding.
No wonder patients are confused!
Smith recommends that, before prescribing aspirin to healthy adults for prevention, we assess each individual’s personal cardiovascular and bleeding risk using an online decision tool called Aspirin-Guide (www.aspiringuide.com). I agree.
1. Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129-135.
Acetylsalicylic acid has been around for nearly 200 years. It traces its history back to a French chemist (Charles Frederic Gerhardt) and 2 German chemists (Felix Hoffmann and Arthur Eichengrün) who worked at Bayer, the company that launched the pain reliever under the name “aspirin” in 1899. It is now one of the most commonly used medications in the world.
With aspirin's anti-inflammatory properties in mind, researchers conducted randomized trials for secondary prevention of heart attacks in the 1970s; low-dose aspirin was proven effective in reducing risk for a second myocardial infarction. These trials led to speculation that aspirin might be effective for primary prevention as well. Indeed, in the 1980s the large Physicians' Health Study found aspirin reduced the incidence of first heart attack in healthy physicians by 44%.1 Unfortunately, there was no reduction in mortality from heart disease and it was only effective for those older than 50.
The downside of aspirin was a slight increase in the incidence of hemorrhagic stroke and bleeding requiring transfusion. Nonetheless, many healthy adults started taking daily aspirin hoping to prevent a heart attack.
In this issue of JFP, Smith and colleagues summarize the 2016 recommendations of the US Preventive Services Task Force (USPSTF) regarding aspirin for primary prevention, as well as the 4 large aspirin prevention trials published in 2018 subsequent to the USPSTF recommendations. The USPSTF recommended aspirin for adults ages 50 to 59 with a 10-year cardiovascular risk of at least 10% (B recommendation). For those ages 60-69, the USPSTF recommendation for aspirin as primary prevention has a “C” rating, meaning that patient preference is important to consider in balancing benefit and harms. For those 70 and older, the USPSTF gave aspirin an “I” (insufficient evidence) rating because of increased risk for bleeding. It is important to note that the positive B recommendation for those ages 50-59 is based not only on cardiovascular risk reduction but also on a slight risk reduction for colon cancer for those taking aspirin for at least 10 years.
The 4 new, large randomized trials published in 2018, however, cast doubt on the USPSTF recommendations because the results of these trials were negative for the most part. The bottom line is that daily aspirin for prevention is definitely not for everyone and perhaps not for anyone except those who have established vascular disease or are at high risk for vascular disease and low risk for bleeding.
No wonder patients are confused!
Smith recommends that, before prescribing aspirin to healthy adults for prevention, we assess each individual’s personal cardiovascular and bleeding risk using an online decision tool called Aspirin-Guide (www.aspiringuide.com). I agree.
Acetylsalicylic acid has been around for nearly 200 years. It traces its history back to a French chemist (Charles Frederic Gerhardt) and 2 German chemists (Felix Hoffmann and Arthur Eichengrün) who worked at Bayer, the company that launched the pain reliever under the name “aspirin” in 1899. It is now one of the most commonly used medications in the world.
With aspirin's anti-inflammatory properties in mind, researchers conducted randomized trials for secondary prevention of heart attacks in the 1970s; low-dose aspirin was proven effective in reducing risk for a second myocardial infarction. These trials led to speculation that aspirin might be effective for primary prevention as well. Indeed, in the 1980s the large Physicians' Health Study found aspirin reduced the incidence of first heart attack in healthy physicians by 44%.1 Unfortunately, there was no reduction in mortality from heart disease and it was only effective for those older than 50.
The downside of aspirin was a slight increase in the incidence of hemorrhagic stroke and bleeding requiring transfusion. Nonetheless, many healthy adults started taking daily aspirin hoping to prevent a heart attack.
In this issue of JFP, Smith and colleagues summarize the 2016 recommendations of the US Preventive Services Task Force (USPSTF) regarding aspirin for primary prevention, as well as the 4 large aspirin prevention trials published in 2018 subsequent to the USPSTF recommendations. The USPSTF recommended aspirin for adults ages 50 to 59 with a 10-year cardiovascular risk of at least 10% (B recommendation). For those ages 60-69, the USPSTF recommendation for aspirin as primary prevention has a “C” rating, meaning that patient preference is important to consider in balancing benefit and harms. For those 70 and older, the USPSTF gave aspirin an “I” (insufficient evidence) rating because of increased risk for bleeding. It is important to note that the positive B recommendation for those ages 50-59 is based not only on cardiovascular risk reduction but also on a slight risk reduction for colon cancer for those taking aspirin for at least 10 years.
The 4 new, large randomized trials published in 2018, however, cast doubt on the USPSTF recommendations because the results of these trials were negative for the most part. The bottom line is that daily aspirin for prevention is definitely not for everyone and perhaps not for anyone except those who have established vascular disease or are at high risk for vascular disease and low risk for bleeding.
No wonder patients are confused!
Smith recommends that, before prescribing aspirin to healthy adults for prevention, we assess each individual’s personal cardiovascular and bleeding risk using an online decision tool called Aspirin-Guide (www.aspiringuide.com). I agree.
1. Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129-135.
1. Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129-135.