User login
Aspirin for primary prevention: It depends
Acetylsalicylic acid has been around for nearly 200 years. It traces its history back to a French chemist (Charles Frederic Gerhardt) and 2 German chemists (Felix Hoffmann and Arthur Eichengrün) who worked at Bayer, the company that launched the pain reliever under the name “aspirin” in 1899. It is now one of the most commonly used medications in the world.
With aspirin's anti-inflammatory properties in mind, researchers conducted randomized trials for secondary prevention of heart attacks in the 1970s; low-dose aspirin was proven effective in reducing risk for a second myocardial infarction. These trials led to speculation that aspirin might be effective for primary prevention as well. Indeed, in the 1980s the large Physicians' Health Study found aspirin reduced the incidence of first heart attack in healthy physicians by 44%.1 Unfortunately, there was no reduction in mortality from heart disease and it was only effective for those older than 50.
The downside of aspirin was a slight increase in the incidence of hemorrhagic stroke and bleeding requiring transfusion. Nonetheless, many healthy adults started taking daily aspirin hoping to prevent a heart attack.
In this issue of JFP, Smith and colleagues summarize the 2016 recommendations of the US Preventive Services Task Force (USPSTF) regarding aspirin for primary prevention, as well as the 4 large aspirin prevention trials published in 2018 subsequent to the USPSTF recommendations. The USPSTF recommended aspirin for adults ages 50 to 59 with a 10-year cardiovascular risk of at least 10% (B recommendation). For those ages 60-69, the USPSTF recommendation for aspirin as primary prevention has a “C” rating, meaning that patient preference is important to consider in balancing benefit and harms. For those 70 and older, the USPSTF gave aspirin an “I” (insufficient evidence) rating because of increased risk for bleeding. It is important to note that the positive B recommendation for those ages 50-59 is based not only on cardiovascular risk reduction but also on a slight risk reduction for colon cancer for those taking aspirin for at least 10 years.
The 4 new, large randomized trials published in 2018, however, cast doubt on the USPSTF recommendations because the results of these trials were negative for the most part. The bottom line is that daily aspirin for prevention is definitely not for everyone and perhaps not for anyone except those who have established vascular disease or are at high risk for vascular disease and low risk for bleeding.
No wonder patients are confused!
Smith recommends that, before prescribing aspirin to healthy adults for prevention, we assess each individual’s personal cardiovascular and bleeding risk using an online decision tool called Aspirin-Guide (www.aspiringuide.com). I agree.
1. Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129-135.
Acetylsalicylic acid has been around for nearly 200 years. It traces its history back to a French chemist (Charles Frederic Gerhardt) and 2 German chemists (Felix Hoffmann and Arthur Eichengrün) who worked at Bayer, the company that launched the pain reliever under the name “aspirin” in 1899. It is now one of the most commonly used medications in the world.
With aspirin's anti-inflammatory properties in mind, researchers conducted randomized trials for secondary prevention of heart attacks in the 1970s; low-dose aspirin was proven effective in reducing risk for a second myocardial infarction. These trials led to speculation that aspirin might be effective for primary prevention as well. Indeed, in the 1980s the large Physicians' Health Study found aspirin reduced the incidence of first heart attack in healthy physicians by 44%.1 Unfortunately, there was no reduction in mortality from heart disease and it was only effective for those older than 50.
The downside of aspirin was a slight increase in the incidence of hemorrhagic stroke and bleeding requiring transfusion. Nonetheless, many healthy adults started taking daily aspirin hoping to prevent a heart attack.
In this issue of JFP, Smith and colleagues summarize the 2016 recommendations of the US Preventive Services Task Force (USPSTF) regarding aspirin for primary prevention, as well as the 4 large aspirin prevention trials published in 2018 subsequent to the USPSTF recommendations. The USPSTF recommended aspirin for adults ages 50 to 59 with a 10-year cardiovascular risk of at least 10% (B recommendation). For those ages 60-69, the USPSTF recommendation for aspirin as primary prevention has a “C” rating, meaning that patient preference is important to consider in balancing benefit and harms. For those 70 and older, the USPSTF gave aspirin an “I” (insufficient evidence) rating because of increased risk for bleeding. It is important to note that the positive B recommendation for those ages 50-59 is based not only on cardiovascular risk reduction but also on a slight risk reduction for colon cancer for those taking aspirin for at least 10 years.
The 4 new, large randomized trials published in 2018, however, cast doubt on the USPSTF recommendations because the results of these trials were negative for the most part. The bottom line is that daily aspirin for prevention is definitely not for everyone and perhaps not for anyone except those who have established vascular disease or are at high risk for vascular disease and low risk for bleeding.
No wonder patients are confused!
Smith recommends that, before prescribing aspirin to healthy adults for prevention, we assess each individual’s personal cardiovascular and bleeding risk using an online decision tool called Aspirin-Guide (www.aspiringuide.com). I agree.
Acetylsalicylic acid has been around for nearly 200 years. It traces its history back to a French chemist (Charles Frederic Gerhardt) and 2 German chemists (Felix Hoffmann and Arthur Eichengrün) who worked at Bayer, the company that launched the pain reliever under the name “aspirin” in 1899. It is now one of the most commonly used medications in the world.
With aspirin's anti-inflammatory properties in mind, researchers conducted randomized trials for secondary prevention of heart attacks in the 1970s; low-dose aspirin was proven effective in reducing risk for a second myocardial infarction. These trials led to speculation that aspirin might be effective for primary prevention as well. Indeed, in the 1980s the large Physicians' Health Study found aspirin reduced the incidence of first heart attack in healthy physicians by 44%.1 Unfortunately, there was no reduction in mortality from heart disease and it was only effective for those older than 50.
The downside of aspirin was a slight increase in the incidence of hemorrhagic stroke and bleeding requiring transfusion. Nonetheless, many healthy adults started taking daily aspirin hoping to prevent a heart attack.
In this issue of JFP, Smith and colleagues summarize the 2016 recommendations of the US Preventive Services Task Force (USPSTF) regarding aspirin for primary prevention, as well as the 4 large aspirin prevention trials published in 2018 subsequent to the USPSTF recommendations. The USPSTF recommended aspirin for adults ages 50 to 59 with a 10-year cardiovascular risk of at least 10% (B recommendation). For those ages 60-69, the USPSTF recommendation for aspirin as primary prevention has a “C” rating, meaning that patient preference is important to consider in balancing benefit and harms. For those 70 and older, the USPSTF gave aspirin an “I” (insufficient evidence) rating because of increased risk for bleeding. It is important to note that the positive B recommendation for those ages 50-59 is based not only on cardiovascular risk reduction but also on a slight risk reduction for colon cancer for those taking aspirin for at least 10 years.
The 4 new, large randomized trials published in 2018, however, cast doubt on the USPSTF recommendations because the results of these trials were negative for the most part. The bottom line is that daily aspirin for prevention is definitely not for everyone and perhaps not for anyone except those who have established vascular disease or are at high risk for vascular disease and low risk for bleeding.
No wonder patients are confused!
Smith recommends that, before prescribing aspirin to healthy adults for prevention, we assess each individual’s personal cardiovascular and bleeding risk using an online decision tool called Aspirin-Guide (www.aspiringuide.com). I agree.
1. Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129-135.
1. Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129-135.
Should you switch the DAPT agent one month after ACS?
ILLUSTRATIVE CASE
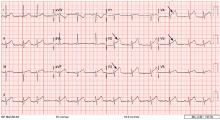
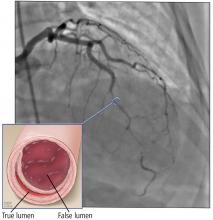
A 60-year-old man is seen in your clinic 30 days after he was hospitalized for acute coronary syndrome (ACS) due to ST-elevation myocardial infarction (STEMI). He underwent percutaneous coronary intervention (PCI) with placement of one stent. He received aspirin and a loading dose of ticagrelor for antiplatelet therapy. He was discharged on dual antiplatelet therapy (DAPT) consisting of daily aspirin and ticagrelor. He asks about the risk of bleeding associated with these medications. Should you recommend any changes?
Platelet inhibition during and after ACS to prevent recurrent ischemic events is a cornerstone of treatment for patients after myocardial infarction (MI).2 Current American Cardiology Association and European Society of Cardiology guidelines recommend patients with coronary artery disease who have had a recent MI continue DAPT with aspirin and a P2Y12 blocker (ie, clopidogrel, ticlopidine, ticagrelor, prasugrel, or cangrelor) for 12 months following ACSto reduce recurrent ischemia.2-4
Studies have shown that using the newer P2Y12 inhibitors (ie, prasugrel and ticagrelor) after PCI leads to a significant reduction in recurrent ischemic events when compared to clopidogrel.5-7 These data led to a guideline change recommending the use of the newer agents over clopidogrel for 12 months following PCI.2 Follow-up studies evaluating the newer P2Y12 agents continue to show strong evidence for their use in the first month following PCI, while also demonstrating an increased bleeding risk in the maintenance phase (from 30 days to 12 months post-PCI).6,7 This increased risk is the basis for the current study, which tested switching from a newer P2Y12 agent to clopidogrel after the initial 30-day period following PCI.
STUDY SUMMARY
Switched DAPT is superior to unchanged DAPT
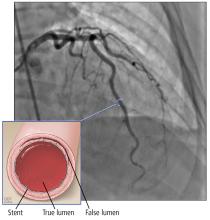
This open-label RCT (N = 646) examined changing DAPT from aspirin plus a newer P2Y12 blocker (prasugrel or ticagrelor) to a combination of aspirin and clopidogrel after the first month of DAPT post-ACS.1 Prior to PCI, all patients received a loading dose of ticagrelor 180 mg or prasugrel 60 mg. Subsequently, all patients in the trial took aspirin (75 mg/d) and one of the newer P2Y12 inhibitors (prasugrel 10 mg/d or ticagrelor 90 mg BID) for 1 month. For those enrollees who had no adverse events after 30 days, half were randomly switched to aspirin and clopidogrel 75 mg/d and the other half remained on aspirin and their newer P2Y12 blocker in a 1:1 ratio. For the next year, researchers examined the composite outcome of cardiovascular death, urgent revascularization, stroke, and major bleeding (as defined by the Bleeding Academic Research Consortium [BARC] classification ≥ Type 2 at 1 year post-ACS).
The average age of the participants was 60 years; 40% had experienced a STEMI and 60% had a non–STEMI. Overall, 43% of patients were prescribed ticagrelor and 57% prasugrel. At 1 year, 86% of the switched DAPT group and 75% of the unchanged DAPT group were still taking their medication. At the 1-year follow-up, the composite outcome was lower in the switched group, compared with the unchanged group (13% vs 26%; hazard ratio [HR] = 0.48; 95% confidence interval [CI], 0.34-0.68; number needed to treat [NNT] = 8).
All bleeding events (ranging from minimal to fatal) were lower in the switched group (9% vs 24%; HR = 0.39; 95% CI, 0.27-0.57; NNT = 7), and bleeding events identified as BARC ≥ Type 2 (defined as needing medical treatment) were also lower in the switched group (4% vs 15%; HR = 0.30, 95% CI, 0.18-0.50; NNT = 9). There were no significant differences in reported recurrent cardiovascular ischemic events (9.3% vs 11.5%; HR = 0.80, 95% CI, 0.50-1.29).
WHAT’S NEW
Fewer bleeding events without an increase in ischemic events
Cardiology guidelines recommend the newer P2Y12 blockers as part of DAPT after ACS, but this trial showed switching to clopidogrel for DAPT after 30 days of treatment lowers bleeding events with no difference in recurrent ischemic events.2-4
Continue to: CAVEATS
CAVEATS
Less-than-ideal study methods
This trial was an open-label, unblinded study. The investigators who adjudicated critical events were blinded to the treatment allocation, but some events, such as minor bleeding and medication discontinuation, could be self-reported by patients. In addition, the investigators used a less-than-ideal method (opaque envelopes) to conceal allocation at enrollment.
CHALLENGES TO IMPLEMENTATION
Implementation may require changing a cardiologist’s prescription
Implementing this practice change is facilitated by the fact that clopidogrel is currently less expensive than the newer P2Y12 blockers. However, after ACS and PCI treatment, cardiologists usually initiate antiplatelet therapy and may continue to manage patients after discharge. So the family physician (FP) may not be responsible for the DAPT switch initially. Further, switching may necessitate coordination with the cardiologist, as FPs may be hesitant to change cardiologists’ prescriptions. Lastly, guidelines currently recommend using the newer P2Y12 blockers for 12 months.2
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070-3078.
2. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082-1115.
3. Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619.
4. Roffi M, Patrono C, Collet J-P, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2015;37:267-315.
5. Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2008;51:2028-2033.
6. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-1057.
7. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015.
ILLUSTRATIVE CASE
A 60-year-old man is seen in your clinic 30 days after he was hospitalized for acute coronary syndrome (ACS) due to ST-elevation myocardial infarction (STEMI). He underwent percutaneous coronary intervention (PCI) with placement of one stent. He received aspirin and a loading dose of ticagrelor for antiplatelet therapy. He was discharged on dual antiplatelet therapy (DAPT) consisting of daily aspirin and ticagrelor. He asks about the risk of bleeding associated with these medications. Should you recommend any changes?
Platelet inhibition during and after ACS to prevent recurrent ischemic events is a cornerstone of treatment for patients after myocardial infarction (MI).2 Current American Cardiology Association and European Society of Cardiology guidelines recommend patients with coronary artery disease who have had a recent MI continue DAPT with aspirin and a P2Y12 blocker (ie, clopidogrel, ticlopidine, ticagrelor, prasugrel, or cangrelor) for 12 months following ACSto reduce recurrent ischemia.2-4
Studies have shown that using the newer P2Y12 inhibitors (ie, prasugrel and ticagrelor) after PCI leads to a significant reduction in recurrent ischemic events when compared to clopidogrel.5-7 These data led to a guideline change recommending the use of the newer agents over clopidogrel for 12 months following PCI.2 Follow-up studies evaluating the newer P2Y12 agents continue to show strong evidence for their use in the first month following PCI, while also demonstrating an increased bleeding risk in the maintenance phase (from 30 days to 12 months post-PCI).6,7 This increased risk is the basis for the current study, which tested switching from a newer P2Y12 agent to clopidogrel after the initial 30-day period following PCI.
STUDY SUMMARY
Switched DAPT is superior to unchanged DAPT
This open-label RCT (N = 646) examined changing DAPT from aspirin plus a newer P2Y12 blocker (prasugrel or ticagrelor) to a combination of aspirin and clopidogrel after the first month of DAPT post-ACS.1 Prior to PCI, all patients received a loading dose of ticagrelor 180 mg or prasugrel 60 mg. Subsequently, all patients in the trial took aspirin (75 mg/d) and one of the newer P2Y12 inhibitors (prasugrel 10 mg/d or ticagrelor 90 mg BID) for 1 month. For those enrollees who had no adverse events after 30 days, half were randomly switched to aspirin and clopidogrel 75 mg/d and the other half remained on aspirin and their newer P2Y12 blocker in a 1:1 ratio. For the next year, researchers examined the composite outcome of cardiovascular death, urgent revascularization, stroke, and major bleeding (as defined by the Bleeding Academic Research Consortium [BARC] classification ≥ Type 2 at 1 year post-ACS).
The average age of the participants was 60 years; 40% had experienced a STEMI and 60% had a non–STEMI. Overall, 43% of patients were prescribed ticagrelor and 57% prasugrel. At 1 year, 86% of the switched DAPT group and 75% of the unchanged DAPT group were still taking their medication. At the 1-year follow-up, the composite outcome was lower in the switched group, compared with the unchanged group (13% vs 26%; hazard ratio [HR] = 0.48; 95% confidence interval [CI], 0.34-0.68; number needed to treat [NNT] = 8).
All bleeding events (ranging from minimal to fatal) were lower in the switched group (9% vs 24%; HR = 0.39; 95% CI, 0.27-0.57; NNT = 7), and bleeding events identified as BARC ≥ Type 2 (defined as needing medical treatment) were also lower in the switched group (4% vs 15%; HR = 0.30, 95% CI, 0.18-0.50; NNT = 9). There were no significant differences in reported recurrent cardiovascular ischemic events (9.3% vs 11.5%; HR = 0.80, 95% CI, 0.50-1.29).
WHAT’S NEW
Fewer bleeding events without an increase in ischemic events
Cardiology guidelines recommend the newer P2Y12 blockers as part of DAPT after ACS, but this trial showed switching to clopidogrel for DAPT after 30 days of treatment lowers bleeding events with no difference in recurrent ischemic events.2-4
Continue to: CAVEATS
CAVEATS
Less-than-ideal study methods
This trial was an open-label, unblinded study. The investigators who adjudicated critical events were blinded to the treatment allocation, but some events, such as minor bleeding and medication discontinuation, could be self-reported by patients. In addition, the investigators used a less-than-ideal method (opaque envelopes) to conceal allocation at enrollment.
CHALLENGES TO IMPLEMENTATION
Implementation may require changing a cardiologist’s prescription
Implementing this practice change is facilitated by the fact that clopidogrel is currently less expensive than the newer P2Y12 blockers. However, after ACS and PCI treatment, cardiologists usually initiate antiplatelet therapy and may continue to manage patients after discharge. So the family physician (FP) may not be responsible for the DAPT switch initially. Further, switching may necessitate coordination with the cardiologist, as FPs may be hesitant to change cardiologists’ prescriptions. Lastly, guidelines currently recommend using the newer P2Y12 blockers for 12 months.2
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 60-year-old man is seen in your clinic 30 days after he was hospitalized for acute coronary syndrome (ACS) due to ST-elevation myocardial infarction (STEMI). He underwent percutaneous coronary intervention (PCI) with placement of one stent. He received aspirin and a loading dose of ticagrelor for antiplatelet therapy. He was discharged on dual antiplatelet therapy (DAPT) consisting of daily aspirin and ticagrelor. He asks about the risk of bleeding associated with these medications. Should you recommend any changes?
Platelet inhibition during and after ACS to prevent recurrent ischemic events is a cornerstone of treatment for patients after myocardial infarction (MI).2 Current American Cardiology Association and European Society of Cardiology guidelines recommend patients with coronary artery disease who have had a recent MI continue DAPT with aspirin and a P2Y12 blocker (ie, clopidogrel, ticlopidine, ticagrelor, prasugrel, or cangrelor) for 12 months following ACSto reduce recurrent ischemia.2-4
Studies have shown that using the newer P2Y12 inhibitors (ie, prasugrel and ticagrelor) after PCI leads to a significant reduction in recurrent ischemic events when compared to clopidogrel.5-7 These data led to a guideline change recommending the use of the newer agents over clopidogrel for 12 months following PCI.2 Follow-up studies evaluating the newer P2Y12 agents continue to show strong evidence for their use in the first month following PCI, while also demonstrating an increased bleeding risk in the maintenance phase (from 30 days to 12 months post-PCI).6,7 This increased risk is the basis for the current study, which tested switching from a newer P2Y12 agent to clopidogrel after the initial 30-day period following PCI.
STUDY SUMMARY
Switched DAPT is superior to unchanged DAPT
This open-label RCT (N = 646) examined changing DAPT from aspirin plus a newer P2Y12 blocker (prasugrel or ticagrelor) to a combination of aspirin and clopidogrel after the first month of DAPT post-ACS.1 Prior to PCI, all patients received a loading dose of ticagrelor 180 mg or prasugrel 60 mg. Subsequently, all patients in the trial took aspirin (75 mg/d) and one of the newer P2Y12 inhibitors (prasugrel 10 mg/d or ticagrelor 90 mg BID) for 1 month. For those enrollees who had no adverse events after 30 days, half were randomly switched to aspirin and clopidogrel 75 mg/d and the other half remained on aspirin and their newer P2Y12 blocker in a 1:1 ratio. For the next year, researchers examined the composite outcome of cardiovascular death, urgent revascularization, stroke, and major bleeding (as defined by the Bleeding Academic Research Consortium [BARC] classification ≥ Type 2 at 1 year post-ACS).
The average age of the participants was 60 years; 40% had experienced a STEMI and 60% had a non–STEMI. Overall, 43% of patients were prescribed ticagrelor and 57% prasugrel. At 1 year, 86% of the switched DAPT group and 75% of the unchanged DAPT group were still taking their medication. At the 1-year follow-up, the composite outcome was lower in the switched group, compared with the unchanged group (13% vs 26%; hazard ratio [HR] = 0.48; 95% confidence interval [CI], 0.34-0.68; number needed to treat [NNT] = 8).
All bleeding events (ranging from minimal to fatal) were lower in the switched group (9% vs 24%; HR = 0.39; 95% CI, 0.27-0.57; NNT = 7), and bleeding events identified as BARC ≥ Type 2 (defined as needing medical treatment) were also lower in the switched group (4% vs 15%; HR = 0.30, 95% CI, 0.18-0.50; NNT = 9). There were no significant differences in reported recurrent cardiovascular ischemic events (9.3% vs 11.5%; HR = 0.80, 95% CI, 0.50-1.29).
WHAT’S NEW
Fewer bleeding events without an increase in ischemic events
Cardiology guidelines recommend the newer P2Y12 blockers as part of DAPT after ACS, but this trial showed switching to clopidogrel for DAPT after 30 days of treatment lowers bleeding events with no difference in recurrent ischemic events.2-4
Continue to: CAVEATS
CAVEATS
Less-than-ideal study methods
This trial was an open-label, unblinded study. The investigators who adjudicated critical events were blinded to the treatment allocation, but some events, such as minor bleeding and medication discontinuation, could be self-reported by patients. In addition, the investigators used a less-than-ideal method (opaque envelopes) to conceal allocation at enrollment.
CHALLENGES TO IMPLEMENTATION
Implementation may require changing a cardiologist’s prescription
Implementing this practice change is facilitated by the fact that clopidogrel is currently less expensive than the newer P2Y12 blockers. However, after ACS and PCI treatment, cardiologists usually initiate antiplatelet therapy and may continue to manage patients after discharge. So the family physician (FP) may not be responsible for the DAPT switch initially. Further, switching may necessitate coordination with the cardiologist, as FPs may be hesitant to change cardiologists’ prescriptions. Lastly, guidelines currently recommend using the newer P2Y12 blockers for 12 months.2
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070-3078.
2. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082-1115.
3. Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619.
4. Roffi M, Patrono C, Collet J-P, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2015;37:267-315.
5. Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2008;51:2028-2033.
6. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-1057.
7. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015.
1. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070-3078.
2. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082-1115.
3. Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619.
4. Roffi M, Patrono C, Collet J-P, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2015;37:267-315.
5. Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2008;51:2028-2033.
6. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-1057.
7. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015.
PRACTICE CHANGER
Switch to clopidogrel from one of the newer P2Y12 blockers 1 month after an acute coronary event, while continuing aspirin, to decrease bleeding events without increasing the risk of ischemic events.1
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial (RCT).
Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070-3078.
Hospital TAVR volume matters to patient survival
Hospitals that performed more transcatheter aortic valve replacements continued to outperform low-volume centers for 30-day postprocedure survival, in data collected from more than 113,000 transcatheter aortic valves replaced during 2015-2017.
During that time, 113,662 transcatheter aortic valve replacement (TAVR) procedures occurred in the United States and were entered into a registry maintained by the Society of Thoracic Surgeons and American College of Cardiology. The new analysis focused on the more than 96,000 valve placements done via a transfemoral approach. The analysis divided these patients into quartiles based on total annual TAVR volume at each of the 554 centers where the procedures occurred, and this showed that 30-day mortality, after adjustment for 39 demographic and clinical variables, was 3.19% among patients treated at centers in the lowest-volume quartile and 2.66% in patients treated at centers in the highest-volume quartile. This translated to a 21% relative risk increase in 30-day mortality at the lowest volume centers that was statistically significant, Sreekanth Vemulapalli, MD, and his associates reported in an article published online on April 3 in the New England Journal of Medicine.
The mean annual volume among centers in the lowest-volume quartile during the 3 years studied was 27 procedures/year, while the average volume among the 25% highest-volume centers was 143 TAVRs each year, reported Dr. Vemulapalli, an interventional cardiologist at Duke University in Durham, N.C., and his associates. After excluding the first 12 months of TAVR performance for each center during the study period, the adjusted 30-day mortality averaged 3.10% in the lowest-volume tertile and 2.61% in centers in the highest-volume tertile. That meant the lowest-volume centers saw a 19% relative increase in mortality that was statistically significant.
This is not the first study to show a significant link between TAVR procedure volumes at individual centers and patient outcomes, and since 2012 the Centers for Medicare & Medicaid Services has stipulated that eligibility for Medicare coverage of TAVR requires that it be done at a center that performs at least 20 TAVR procedures annually or at least 40 during the most recent 2 years. A prior report showing a similar volume-outcome link looked at U.S. TAVR cases during 2011-2015 (J Am Coll Cardiol. 2017 July;70[1]:29-41), and reports of volume-outcome relationships have also come out for other catheter-based intravascular procedures.
“Our results suggest that raising the minimum volume requirements for TAVR centers may improve the quality of outcomes. However, this potential improvement in quality needs to be balanced against access to care in general, and for underserved and underrepresented populations in particular,” Dr. Vemulapalli said in an interview. The data suggested that a significant number of patients from underserved populations are treated at lower-volume TAVR centers. It’s unclear what impact raising the threshold volume [by CMS] might have on these underserved populations,” he explained.
Dr. Vemulapalli conceded that his analysis may have been affected by several potential confounding variables that did not receive adjustment in the analyses he and his associates ran. The variables of hospital size and teaching status both showed an association with TAVR volume. Hospitals with a greater number of beds and those that were teaching hospitals were also the places where TAVR volumes were highest, while lower-volume centers tended to be smaller, nonteaching institutions. But the variables of size and teaching status did not receive adjustment. Both are “difficult to tease apart from TAVR volume,” he noted.
The CMS mandated Transcatheter Valve Therapy Registry also functions as a quality-improvement mechanism in which U.S. TAVR sites receive quarterly feedback on their performance and are benchmarked against other programs in a risk-adjusted way. The registry also disseminates best practices as part of the quality improvement process, Dr. Vemulapalli said.
Results from two TAVR trials reported at the American College of Cardiology’s annual meeting in March, PARTNER 3 and Evolut Low Risk, documented the efficacy and safety of TAVR compared with surgery in low-risk patients, findings that will soon substantially increase the volume of TAVR cases performed (N Engl J Med. 2019 Mar 16. doi: 10.1056/NEJMoa1814052 and doi: 10.1056/NEJMoa1816885).
When the impact of TAVR moving to low-risk patients starts to kick in, “the findings from our analysis will become even more relevant,” Dr. Vemulapalli predicted. “As TAVR moves to low-risk, healthier patients, and more patients undergo the procedure, a firm commitment to measuring and ensuring quality while balancing access to care will be pivotal. The data in our study regarding the association between TAVR volume and outcomes and the characteristics of low- and high-volume hospitals and the patients they treat are fundamental to striking this balance.”
SOURCE: Vemulapalli S. et al. N Engl J Med. 2019 Apr 3.doi: 10.1056/NEJMsa1901109.
Hospitals that performed more transcatheter aortic valve replacements continued to outperform low-volume centers for 30-day postprocedure survival, in data collected from more than 113,000 transcatheter aortic valves replaced during 2015-2017.
During that time, 113,662 transcatheter aortic valve replacement (TAVR) procedures occurred in the United States and were entered into a registry maintained by the Society of Thoracic Surgeons and American College of Cardiology. The new analysis focused on the more than 96,000 valve placements done via a transfemoral approach. The analysis divided these patients into quartiles based on total annual TAVR volume at each of the 554 centers where the procedures occurred, and this showed that 30-day mortality, after adjustment for 39 demographic and clinical variables, was 3.19% among patients treated at centers in the lowest-volume quartile and 2.66% in patients treated at centers in the highest-volume quartile. This translated to a 21% relative risk increase in 30-day mortality at the lowest volume centers that was statistically significant, Sreekanth Vemulapalli, MD, and his associates reported in an article published online on April 3 in the New England Journal of Medicine.
The mean annual volume among centers in the lowest-volume quartile during the 3 years studied was 27 procedures/year, while the average volume among the 25% highest-volume centers was 143 TAVRs each year, reported Dr. Vemulapalli, an interventional cardiologist at Duke University in Durham, N.C., and his associates. After excluding the first 12 months of TAVR performance for each center during the study period, the adjusted 30-day mortality averaged 3.10% in the lowest-volume tertile and 2.61% in centers in the highest-volume tertile. That meant the lowest-volume centers saw a 19% relative increase in mortality that was statistically significant.
This is not the first study to show a significant link between TAVR procedure volumes at individual centers and patient outcomes, and since 2012 the Centers for Medicare & Medicaid Services has stipulated that eligibility for Medicare coverage of TAVR requires that it be done at a center that performs at least 20 TAVR procedures annually or at least 40 during the most recent 2 years. A prior report showing a similar volume-outcome link looked at U.S. TAVR cases during 2011-2015 (J Am Coll Cardiol. 2017 July;70[1]:29-41), and reports of volume-outcome relationships have also come out for other catheter-based intravascular procedures.
“Our results suggest that raising the minimum volume requirements for TAVR centers may improve the quality of outcomes. However, this potential improvement in quality needs to be balanced against access to care in general, and for underserved and underrepresented populations in particular,” Dr. Vemulapalli said in an interview. The data suggested that a significant number of patients from underserved populations are treated at lower-volume TAVR centers. It’s unclear what impact raising the threshold volume [by CMS] might have on these underserved populations,” he explained.
Dr. Vemulapalli conceded that his analysis may have been affected by several potential confounding variables that did not receive adjustment in the analyses he and his associates ran. The variables of hospital size and teaching status both showed an association with TAVR volume. Hospitals with a greater number of beds and those that were teaching hospitals were also the places where TAVR volumes were highest, while lower-volume centers tended to be smaller, nonteaching institutions. But the variables of size and teaching status did not receive adjustment. Both are “difficult to tease apart from TAVR volume,” he noted.
The CMS mandated Transcatheter Valve Therapy Registry also functions as a quality-improvement mechanism in which U.S. TAVR sites receive quarterly feedback on their performance and are benchmarked against other programs in a risk-adjusted way. The registry also disseminates best practices as part of the quality improvement process, Dr. Vemulapalli said.
Results from two TAVR trials reported at the American College of Cardiology’s annual meeting in March, PARTNER 3 and Evolut Low Risk, documented the efficacy and safety of TAVR compared with surgery in low-risk patients, findings that will soon substantially increase the volume of TAVR cases performed (N Engl J Med. 2019 Mar 16. doi: 10.1056/NEJMoa1814052 and doi: 10.1056/NEJMoa1816885).
When the impact of TAVR moving to low-risk patients starts to kick in, “the findings from our analysis will become even more relevant,” Dr. Vemulapalli predicted. “As TAVR moves to low-risk, healthier patients, and more patients undergo the procedure, a firm commitment to measuring and ensuring quality while balancing access to care will be pivotal. The data in our study regarding the association between TAVR volume and outcomes and the characteristics of low- and high-volume hospitals and the patients they treat are fundamental to striking this balance.”
SOURCE: Vemulapalli S. et al. N Engl J Med. 2019 Apr 3.doi: 10.1056/NEJMsa1901109.
Hospitals that performed more transcatheter aortic valve replacements continued to outperform low-volume centers for 30-day postprocedure survival, in data collected from more than 113,000 transcatheter aortic valves replaced during 2015-2017.
During that time, 113,662 transcatheter aortic valve replacement (TAVR) procedures occurred in the United States and were entered into a registry maintained by the Society of Thoracic Surgeons and American College of Cardiology. The new analysis focused on the more than 96,000 valve placements done via a transfemoral approach. The analysis divided these patients into quartiles based on total annual TAVR volume at each of the 554 centers where the procedures occurred, and this showed that 30-day mortality, after adjustment for 39 demographic and clinical variables, was 3.19% among patients treated at centers in the lowest-volume quartile and 2.66% in patients treated at centers in the highest-volume quartile. This translated to a 21% relative risk increase in 30-day mortality at the lowest volume centers that was statistically significant, Sreekanth Vemulapalli, MD, and his associates reported in an article published online on April 3 in the New England Journal of Medicine.
The mean annual volume among centers in the lowest-volume quartile during the 3 years studied was 27 procedures/year, while the average volume among the 25% highest-volume centers was 143 TAVRs each year, reported Dr. Vemulapalli, an interventional cardiologist at Duke University in Durham, N.C., and his associates. After excluding the first 12 months of TAVR performance for each center during the study period, the adjusted 30-day mortality averaged 3.10% in the lowest-volume tertile and 2.61% in centers in the highest-volume tertile. That meant the lowest-volume centers saw a 19% relative increase in mortality that was statistically significant.
This is not the first study to show a significant link between TAVR procedure volumes at individual centers and patient outcomes, and since 2012 the Centers for Medicare & Medicaid Services has stipulated that eligibility for Medicare coverage of TAVR requires that it be done at a center that performs at least 20 TAVR procedures annually or at least 40 during the most recent 2 years. A prior report showing a similar volume-outcome link looked at U.S. TAVR cases during 2011-2015 (J Am Coll Cardiol. 2017 July;70[1]:29-41), and reports of volume-outcome relationships have also come out for other catheter-based intravascular procedures.
“Our results suggest that raising the minimum volume requirements for TAVR centers may improve the quality of outcomes. However, this potential improvement in quality needs to be balanced against access to care in general, and for underserved and underrepresented populations in particular,” Dr. Vemulapalli said in an interview. The data suggested that a significant number of patients from underserved populations are treated at lower-volume TAVR centers. It’s unclear what impact raising the threshold volume [by CMS] might have on these underserved populations,” he explained.
Dr. Vemulapalli conceded that his analysis may have been affected by several potential confounding variables that did not receive adjustment in the analyses he and his associates ran. The variables of hospital size and teaching status both showed an association with TAVR volume. Hospitals with a greater number of beds and those that were teaching hospitals were also the places where TAVR volumes were highest, while lower-volume centers tended to be smaller, nonteaching institutions. But the variables of size and teaching status did not receive adjustment. Both are “difficult to tease apart from TAVR volume,” he noted.
The CMS mandated Transcatheter Valve Therapy Registry also functions as a quality-improvement mechanism in which U.S. TAVR sites receive quarterly feedback on their performance and are benchmarked against other programs in a risk-adjusted way. The registry also disseminates best practices as part of the quality improvement process, Dr. Vemulapalli said.
Results from two TAVR trials reported at the American College of Cardiology’s annual meeting in March, PARTNER 3 and Evolut Low Risk, documented the efficacy and safety of TAVR compared with surgery in low-risk patients, findings that will soon substantially increase the volume of TAVR cases performed (N Engl J Med. 2019 Mar 16. doi: 10.1056/NEJMoa1814052 and doi: 10.1056/NEJMoa1816885).
When the impact of TAVR moving to low-risk patients starts to kick in, “the findings from our analysis will become even more relevant,” Dr. Vemulapalli predicted. “As TAVR moves to low-risk, healthier patients, and more patients undergo the procedure, a firm commitment to measuring and ensuring quality while balancing access to care will be pivotal. The data in our study regarding the association between TAVR volume and outcomes and the characteristics of low- and high-volume hospitals and the patients they treat are fundamental to striking this balance.”
SOURCE: Vemulapalli S. et al. N Engl J Med. 2019 Apr 3.doi: 10.1056/NEJMsa1901109.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: U.S. centers that performed the most TAVR procedures had the best rates of 30-day patient survival.
Major finding: .
Study details: Analysis of data from 113,622 TAVR procedures done at U.S. hospitals during 2015-2017.
Disclosures: Dr. Vemulapalli has received personal fees from Boston Scientific, Janssen, Novella, Premiere, and Zafgen, and he has received research funding from Abbott Vascular and Boston Scientific.
Source: Vemulapalli S et al. N Engl J Med. 2019 Apr 3. doi: 10.1056/NEJMsa1901109.
Aspirin for primary prevention: USPSTF recommendations for CVD and colorectal cancer
Which patients are likely to benefit from using aspirin for primary prevention? In this article, we review the evidence to date, summarized for primary care settings in guidelines issued by the US Preventive Services Task Force (USPSTF). We supplement this summary with a rundown of the risks associated with aspirin use. And then we wrap up by identifying a clinical decision tool that is available to help make personalized decisions in a busy clinic setting, where determining an individual’s potential cardiovascular benefits and bleeding risk can be challenging.
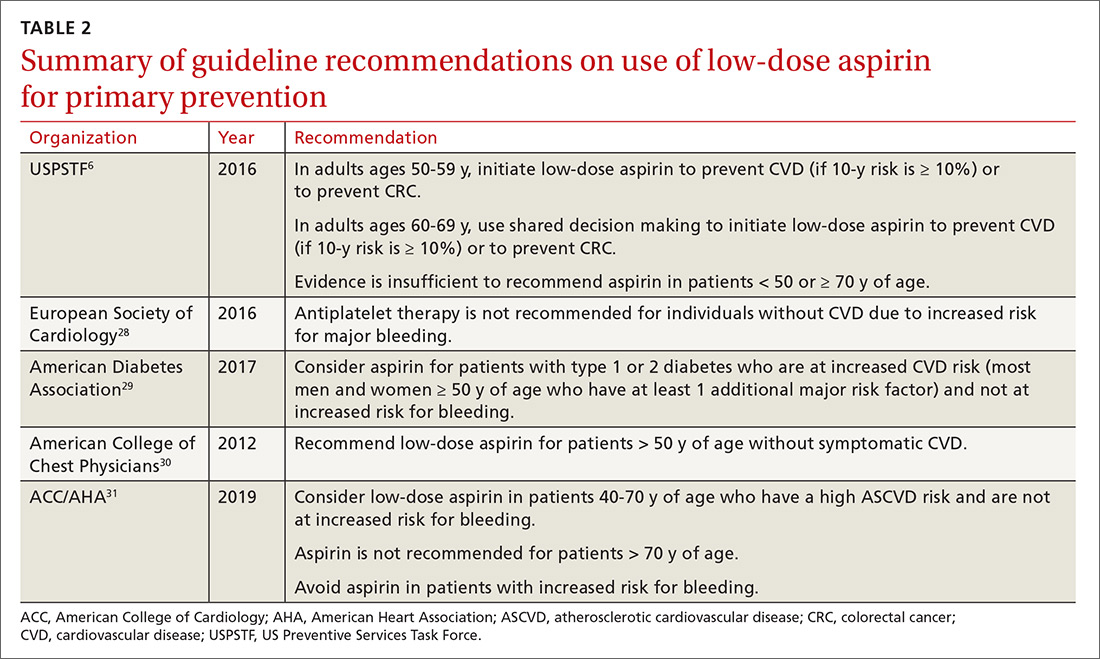
The “roadmap” from the guidelines. In 2014, after performing a review of the literature, the US Food and Drug Administration recommended against the routine use of aspirin for primary prevention of cardiovascular disease (CVD).1 In 2016, the USPSTF published 4 separate systematic reviews along with a decision analysis using a microsimulation model, which informed their position statement on aspirin for primary prevention.2-6 These USPSTF reviews and recommendations incorporated both CVD and colorectal cancer (CRC) benefits with the bleeding risks from aspirin. Generally, for individuals 50 to 59 years old, the benefits are deemed to outweigh the harms; shared decision making is advised with those 60 to 69 years of age. For patients younger than 50 or 70 and older, evidence is inconclusive.
The benefits of primary prevention with aspirin
Cardiovascular disease
The Antithrombotic Trialists’ (ATT) Collaboration was one of the first meta-analyses that addressed the benefit-to-harm balance and called into question the routine use of aspirin for primary prevention.7 The USPSTF systematic review included the studies from the ATT Collaboration as well as trials performed after its publication, bringing the total number of eligible randomized controlled trials reviewed to 11.2
The benefit of aspirin for primary prevention of nonfatal myocardial infarction (MI) has been shown in multiple randomized controlled trials. The USPSTF systematic review showed a statistically significant relative risk reduction of 17% in patients taking low-dose aspirin (≤ 100 mg; relative risk [RR] = 0.83; confidence interval [95% CI], 0.74-0.94), although the heterogeneity of the studies was high. The same low dose of aspirin showed a statistically significant reduction in nonfatal stroke (RR = 0.86; 95% CI, 0.76-0.98), although the same benefit was not observed when all doses of aspirin were included. Cardiovascular disease mortality and all-cause mortality were not statistically different for patients taking low-dose aspirin when compared with placebo (RR = 0.97; 95% CI, 0.85-1.10 for CVD mortality; RR = 0.95; 95% CI, 0.89-1.01 for all-cause mortality).2
One study of more than 14,000 older (≥ 60 years) Japanese patients showed a statistically significant reduction in nonfatal MI (hazard ratio [HR] = 0.53; 95% CI, 0.31-0.91, P = .02) and nonfatal strokes (HR = 0.57; 95% CI, 0.32-0.99; P = .04). The study was stopped early because at 5 years of follow-up there was no statistically significant difference in a composite primary outcome, which included death from cardiovascular causes, nonfatal MI, and nonfatal stroke (HR = 0.94; 95% CI, 0.77-1.15; P = .54).8
Several recent landmark studies have called into question the benefit of aspirin for cardiovascular primary prevention, especially in obese individuals, patients with diabetes, and the elderly. A meta-analysis of 10 trials showed that the effectiveness of aspirin doses between 75 mg and 100 mg for primary prevention decreased as weight increased; patients weighing 70 kg or more received no benefit.9 The ASCEND (A Study of Cardiovascular Events in Diabetes) trial included more than 15,000 patients with diabetes but no cardiovascular disease. Patients randomized to receive the low-dose aspirin did have fewer serious vascular events (incidence rate ratio [IRR] = 0.88; 95% CI, 0.79-0.97; P = .01), but they also had high risk of major bleeding events (IRR = 1.29; 95% CI, 1.09-1.52; P = .003).10 The ASPREE (Aspirin in Reducing Events in the Elderly) trial included more than 19,000 patients ages 70 years and older with no cardiovascular disease and compared low-dose aspirin to placebo. There was no statistically significant cardiovascular benefit, although there was an increase of major hemorrhage (HR = 1.38; 95% CI, 1.18-1.62; P < .001).11 The ARRIVE (A Randomized Trial of Induction Versus Expectant Management) trial included 12,546 moderate atherosclerotic CVD (ASCVD) risk patients. Although a per-protocol analysis showed a decrease in rates of fatal and nonfatal MI (HR = 0.53; 95% CI, 0.36-0.79; P = .0014), the more reliable intention-to-treat analysis showed no improvement for any outcomes.12
[polldaddy:10286821]
Colorectal cancer
The literature base on prevention of cancer has been growing rapidly. However, the deluge of findings over the past 2 decades of trials and analyses has also introduced ambiguity and, often, conflicting results. The first journal article suggesting aspirin for primary prevention of cancer, published in 1988, was a case-control study wherein a population with CRC was matched to controls to look for potential protective factors.13 The most notable finding was the CRC risk reduction for those taking aspirin or aspirin-containing medications. Since then numerous studies and analyses have explored aspirin’s potential in primary prevention of many types of cancer, with overall unclear findings as denoted in the 2016 USPSTF systemic reviews and recommendations.
Continue to: One major limiting factor...
One major limiting factor is that most data come from CVD prevention trials, and only a limited number of trials have focused specifically on cancer prevention. For the USPSTF, these data showed no statistically significant risk reduction in overall cancer mortality (RR = 0.96; 95% CI, 0.87-1.06) or in total cancer incidence (RR = 0.98; 95% CI, 0.93-1.04).4 Other ongoing trials may yield more definitive data.14
The particular interest in CRC was due to it being the first cancer found to be preventable with aspirin therapy. The USPSTF, while acknowledging the homogeneous nature of supporting studies, noted that their significant number and resulting evidence made CRC the only cancer warranting evaluation. Population studies have now shown more benefit than the few randomized control trials. The Women’s Health Study and the Physicians’ Health Study were both limited by their duration. But such studies conducted over a longer period revealed notable benefits in the second decade of use, with a statistically significant lower CRC incidence (RR = 0.60; 95% CI, 0.47-0.76). Additionally, CRC mortality at 20 years was decreased in patients taking aspirin regularly (RR = 0.67; 95% CI, 0.52-0.86).4 Multiple studies are in progress to better establish aspirin’s CRC benefit.
While not directly applicable to the general population, use of aspirin for patients with Lynch syndrome to prevent CRC has strong supporting evidence.15 Beyond CRC, there is nascent evidence from limited observational studies that aspirin may have a preventive effect on melanoma and ovarian and pancreatic cancers.16-18 Further studies or compilations of data would be needed to draw more significant conclusions on other types of cancers. Larger studies would prove more difficult to do, given the smaller incidences of these cancers.
Interestingly, a recent study showed that for individuals 70 years and older, aspirin might increase the risk for all-cause mortality, primarily due to increased cancer mortality across all types.19 Although this result was unexpected, caution should be used when prescribing aspirin particularly for patients 70 or older with active cancer.
A look at the harms associated with aspirin use
Aspirin has long been known to cause clinically significant bleeding. Aspirin inhibits platelet-derived cyclooxygenase-1 (COX-1), a potent vasoconstrictor, and thereby decreases platelet aggregation, reducing thromboembolic potential and prolonging bleeding time. These effects can confer health benefits but also carry the potential for risks. A decision to initiate aspirin therapy for primary prevention relies on an understanding of the benefit-to-harm balance.
Continue to: Initial aspirin studies...
Initial aspirin studies did not show a statistically significant increase in bleeding, likely due to too few events and inadequate powering. Subsequent meta-analyses from multiple evaluations have consistently shown bleeding to be a risk.3,7 The risk for bleeding with aspirin has also been examined in multiple cohort studies, which has helped elucidate the risk in greater detail.
Gastrointestinal bleeding
Epidemiologic data show that among patients who do not use nonsteroidal anti-inflammatory drugs (NSAIDs), the rate of upper gastrointestinal (GI) complications is 1 case per 1000 patient-years.20 Multiple studies have consistently shown that aspirin use increases the rate of significant upper GI bleeding over baseline risk (odds ratio [OR] = 1.54-1.58).3,21,22 Interestingly, these increases seem not to be influenced by other factors, such as comorbidities that increase the risk for ASCVD. Analysis of cancer prevention studies showed similar epidemiologic trends, with aspirin use exceeding a baseline bleeding risk of 0.7 cases of upper GI complications per 1000 patient-years (
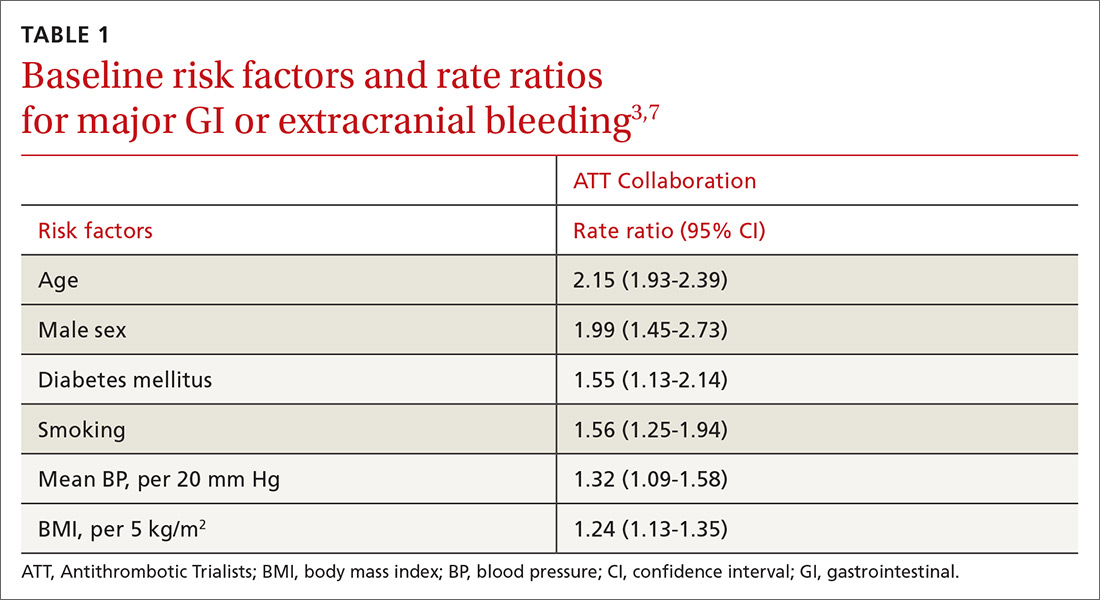
Other risk factors. Evaluation of risk factors for bleeding primarily comes from 2 studies.3,7 Most data concern the impact of individual factors on significant GI bleeding, with fewer data available for evaluating risk for intracerebral hemorrhage (ICH). Initial analysis of individual prospective studies showed little or no correlation between risk for bleeding and such factors as gender, age, or history of hypertension or ASCVD.21 Subsequent analysis of meta-data and large cohorts did show statistically significant impact on rates of bleeding across several factors (TABLE 13,7).
Of note is a large heterogeneous cohort study conducted in Spain. Data showed significant increases in baseline risk for GI bleeding in older men with a history of GI bleeding and NSAID use. The absolute risk for GI bleed in this group was potentially as high as 150 cases per 1000 patient-years, well above the risk level assumed for the average patient.24 A seemingly small OR of 1.5 could dramatically increase the absolute risk for bleeding in such patients, and it suggests that a generalized risk for bleeding probably shouldn’t be applied to all patients. Individuals may be better served by a baseline risk calculation reflecting multiple factors.
Intracerebral hemorrhage
Due to the comparatively uncommon nature of ICH, fewer data are available to support definitive conclusions about its increased risk with aspirin use. Aspirin use appeared to increase the risk for ICH with ratios between 1.27 and 1.32 in meta-analyses (measured as an OR or as an RR),3,7,21 with an IRR of 1.54 in a cohort study.22 The only statistically significant factors suspected to increase the risk of ICH at baseline were smoking (RR = 2.18) and mean BP > 20 mm Hg over normal (OR = 2.18). Age, gender, and diabetes all showed a nonsignificant trend toward risk increase.7
Continue to: Risk based on dose and formulation
Risk based on dose and formulation
The effect of aspirin dose and formulation on bleeding risk is uncertain. Some studies have shown an increased risk for bleeding with daily doses of aspirin ≥ 300 mg, while others have shown no significant increase in rates for bleeding with differing doses.21,25 Enteric coating does appear to lower the rates of gastric mucosal injury, although there are few data on the effect toward reducing clinically significant bleeding.26 Currently, several prospective studies are underway to help clarify the evidence.27
Putting it all together
For the general population, the evidence shows that the benefits and harms of aspirin for primary prevention are relatively even. The USPSTF guidelines are the first to recommend aspirin for both CVD and cancer prevention while taking into account the bleeding risk. According to the findings of the USPSTF, the balance of benefits and harms of aspirin use is contingent on 4 factors: age, baseline CVD risk, risk for bleeding, and preferences about taking aspirin.6 The complete recommendations from the USPSTF, along with other leading organizations, are outlined in TABLE 2.6,28-31
Applying the evidence and varying guidelines in practice can feel daunting. Some practical tools have been developed to help clinicians understand patients’ bleeding risk and potential benefits with aspirin use. One such tool is highlighted below. Others are also available, and each has its own strengths and weaknesses.
Aspirin-Guide (www.aspiringuide.com) is a Web-based clinical decision support tool with an associated mobile application. It uses internal calculators (including the pooled cohort calculator prepared jointly by the American College of Cardiology and the American Heart Association) to assess CVD risk as well as bleeding risk. This tool gives clinicians patient-specific numbers-needed-to-treat and numbers-needed-to-harm when considering starting aspirin for primary prevention. It gives specific recommendations for aspirin use based on the data entered, and it also gives providers information to help guide shared decision-making with patients.32 Unfortunately, this decision support tool and others do not take into account the data from the most recent trials, so they should be used with caution.
CORRESPONDENCE
LCDR Dustin K. Smith, DO, Naval Branch Clinic Diego Garcia, PSC 466, Box 301, FPO, AP 96595; dustinksmith@yahoo.com.
1. FDA. Use of aspirin for primary prevention of heart attack and stroke. https://www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm390574.htm. Accessed March 22, 2019.
2. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
3. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:826-835.
4. Chubak J, Whitlock EP, Williams SB, et al. Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:814-825.
5. Dehmer SP, Maciosek MV, Flottemesch TJ, et al. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:777-786.
6. Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836-845.
7. Baigent C, Blackwell L, Colins R, et al; Antithrombotic Trialists (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participation data from randomised trials. Lancet. 2009:373:1849-1860.
8. Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312:2510-2520.
9. Rothwell PM, Cook NR, Gaziano JM, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet. 2018;392:387-399.
10. Bowman L, Mafham M, Wallendszus K, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539.
11. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.
12. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046.
13. Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illness, operations, and medications: case control results from Melbourne Colorectal Cancer Study. Cancer Res. 1988;48:4399-4404.
14. Sutcliffe P, Connock M, Gurung T, et al. Aspirin for prophylactic use in the primary prevention of cardiovascular disease and cancer: a systematic review and overview of reviews. Health Technol Assess. 2013;17:1-253.
15. Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081-2087.
16. Gamba CA, Swetter SM, Stefanick ML, et al. Aspirin is associated with lower melanoma risk among postmenopausal Caucasian women: the Women’s Health Initiative. Cancer. 2013;119:1562-1569.
17. Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431.
18. Risch H, Lu L, Streicher SA, et al. Aspirin use and reduced risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2016;26:68-74.
19. McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-1528.
20. Hernández-Díaz S, Rodríguez LA. Incidence of serious upper gastrointestinal bleeding/perforation in the general population: review of epidemiologic studies. J Clin Epidemiol. 2002;55:157-163.
21. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis no 131. Rockville, MD: Agency for Healthcare Research and Quality; 2015. https://www.ncbi.nlm.nih.gov/books/NBK321623/. Accessed March 22, 2019.
22. De Berardis G, Lucisano G, D’Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286-2294.
23. Thorat MA, Cuzick J. Prophylactic use of aspirin: systematic review of harms and approaches to mitigation in the general population. Eur J Epidemiol. 2015;30:5-18.
24. Hernández-Díaz S, García Rodríguez LA. Cardioprotective aspirin users and their excess risk of upper gastrointestinal complications. BMC Med. 2006;4:22.
25. Huang ES, Strate LL, Ho WW, et al. Long term use of aspirin and the risk of gastrointestinal bleeding. Am J Med. 2011:124;426-433.
26. Walker J, Robinson J, Stewart J, et al. Does enteric-coated aspirin result in a lower incidence of gastrointestinal complications compared to normal aspirin? Interact Cardiovasc Thorac Surg. 2007:6;519-522.
27. NIH. Aspirin dosing: a patient-centric trial assessing benefits and long-term effectiveness (ADAPTABLE). https://clinicaltrials.gov/ct2/show/NCT02697916. Accessed March 22, 2019.
28. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2016;37:2315-2381.
29. ADA. Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40(suppl 1). http://care.diabetesjournals.org/content/diacare/suppl/2016/12/15/40.Supplement_1.DC1/DC_40_S1_final.pdf. Accessed March 22, 2019.
30. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
31. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. J Am Col Cardiol. 2019. doi: https://doi.org/10.1016/j.jacc.2019.03.010. Accessed March 22, 2019.
32. Mora S, Manson JE. Aspirin for primary prevention of atherosclerotic cardiovascular disease: advances in diagnosis and treatment. JAMA Intern Med. 2016;176:1195-1204.
Which patients are likely to benefit from using aspirin for primary prevention? In this article, we review the evidence to date, summarized for primary care settings in guidelines issued by the US Preventive Services Task Force (USPSTF). We supplement this summary with a rundown of the risks associated with aspirin use. And then we wrap up by identifying a clinical decision tool that is available to help make personalized decisions in a busy clinic setting, where determining an individual’s potential cardiovascular benefits and bleeding risk can be challenging.
The “roadmap” from the guidelines. In 2014, after performing a review of the literature, the US Food and Drug Administration recommended against the routine use of aspirin for primary prevention of cardiovascular disease (CVD).1 In 2016, the USPSTF published 4 separate systematic reviews along with a decision analysis using a microsimulation model, which informed their position statement on aspirin for primary prevention.2-6 These USPSTF reviews and recommendations incorporated both CVD and colorectal cancer (CRC) benefits with the bleeding risks from aspirin. Generally, for individuals 50 to 59 years old, the benefits are deemed to outweigh the harms; shared decision making is advised with those 60 to 69 years of age. For patients younger than 50 or 70 and older, evidence is inconclusive.
The benefits of primary prevention with aspirin
Cardiovascular disease
The Antithrombotic Trialists’ (ATT) Collaboration was one of the first meta-analyses that addressed the benefit-to-harm balance and called into question the routine use of aspirin for primary prevention.7 The USPSTF systematic review included the studies from the ATT Collaboration as well as trials performed after its publication, bringing the total number of eligible randomized controlled trials reviewed to 11.2
The benefit of aspirin for primary prevention of nonfatal myocardial infarction (MI) has been shown in multiple randomized controlled trials. The USPSTF systematic review showed a statistically significant relative risk reduction of 17% in patients taking low-dose aspirin (≤ 100 mg; relative risk [RR] = 0.83; confidence interval [95% CI], 0.74-0.94), although the heterogeneity of the studies was high. The same low dose of aspirin showed a statistically significant reduction in nonfatal stroke (RR = 0.86; 95% CI, 0.76-0.98), although the same benefit was not observed when all doses of aspirin were included. Cardiovascular disease mortality and all-cause mortality were not statistically different for patients taking low-dose aspirin when compared with placebo (RR = 0.97; 95% CI, 0.85-1.10 for CVD mortality; RR = 0.95; 95% CI, 0.89-1.01 for all-cause mortality).2
One study of more than 14,000 older (≥ 60 years) Japanese patients showed a statistically significant reduction in nonfatal MI (hazard ratio [HR] = 0.53; 95% CI, 0.31-0.91, P = .02) and nonfatal strokes (HR = 0.57; 95% CI, 0.32-0.99; P = .04). The study was stopped early because at 5 years of follow-up there was no statistically significant difference in a composite primary outcome, which included death from cardiovascular causes, nonfatal MI, and nonfatal stroke (HR = 0.94; 95% CI, 0.77-1.15; P = .54).8
Several recent landmark studies have called into question the benefit of aspirin for cardiovascular primary prevention, especially in obese individuals, patients with diabetes, and the elderly. A meta-analysis of 10 trials showed that the effectiveness of aspirin doses between 75 mg and 100 mg for primary prevention decreased as weight increased; patients weighing 70 kg or more received no benefit.9 The ASCEND (A Study of Cardiovascular Events in Diabetes) trial included more than 15,000 patients with diabetes but no cardiovascular disease. Patients randomized to receive the low-dose aspirin did have fewer serious vascular events (incidence rate ratio [IRR] = 0.88; 95% CI, 0.79-0.97; P = .01), but they also had high risk of major bleeding events (IRR = 1.29; 95% CI, 1.09-1.52; P = .003).10 The ASPREE (Aspirin in Reducing Events in the Elderly) trial included more than 19,000 patients ages 70 years and older with no cardiovascular disease and compared low-dose aspirin to placebo. There was no statistically significant cardiovascular benefit, although there was an increase of major hemorrhage (HR = 1.38; 95% CI, 1.18-1.62; P < .001).11 The ARRIVE (A Randomized Trial of Induction Versus Expectant Management) trial included 12,546 moderate atherosclerotic CVD (ASCVD) risk patients. Although a per-protocol analysis showed a decrease in rates of fatal and nonfatal MI (HR = 0.53; 95% CI, 0.36-0.79; P = .0014), the more reliable intention-to-treat analysis showed no improvement for any outcomes.12
[polldaddy:10286821]
Colorectal cancer
The literature base on prevention of cancer has been growing rapidly. However, the deluge of findings over the past 2 decades of trials and analyses has also introduced ambiguity and, often, conflicting results. The first journal article suggesting aspirin for primary prevention of cancer, published in 1988, was a case-control study wherein a population with CRC was matched to controls to look for potential protective factors.13 The most notable finding was the CRC risk reduction for those taking aspirin or aspirin-containing medications. Since then numerous studies and analyses have explored aspirin’s potential in primary prevention of many types of cancer, with overall unclear findings as denoted in the 2016 USPSTF systemic reviews and recommendations.
Continue to: One major limiting factor...
One major limiting factor is that most data come from CVD prevention trials, and only a limited number of trials have focused specifically on cancer prevention. For the USPSTF, these data showed no statistically significant risk reduction in overall cancer mortality (RR = 0.96; 95% CI, 0.87-1.06) or in total cancer incidence (RR = 0.98; 95% CI, 0.93-1.04).4 Other ongoing trials may yield more definitive data.14
The particular interest in CRC was due to it being the first cancer found to be preventable with aspirin therapy. The USPSTF, while acknowledging the homogeneous nature of supporting studies, noted that their significant number and resulting evidence made CRC the only cancer warranting evaluation. Population studies have now shown more benefit than the few randomized control trials. The Women’s Health Study and the Physicians’ Health Study were both limited by their duration. But such studies conducted over a longer period revealed notable benefits in the second decade of use, with a statistically significant lower CRC incidence (RR = 0.60; 95% CI, 0.47-0.76). Additionally, CRC mortality at 20 years was decreased in patients taking aspirin regularly (RR = 0.67; 95% CI, 0.52-0.86).4 Multiple studies are in progress to better establish aspirin’s CRC benefit.
While not directly applicable to the general population, use of aspirin for patients with Lynch syndrome to prevent CRC has strong supporting evidence.15 Beyond CRC, there is nascent evidence from limited observational studies that aspirin may have a preventive effect on melanoma and ovarian and pancreatic cancers.16-18 Further studies or compilations of data would be needed to draw more significant conclusions on other types of cancers. Larger studies would prove more difficult to do, given the smaller incidences of these cancers.
Interestingly, a recent study showed that for individuals 70 years and older, aspirin might increase the risk for all-cause mortality, primarily due to increased cancer mortality across all types.19 Although this result was unexpected, caution should be used when prescribing aspirin particularly for patients 70 or older with active cancer.
A look at the harms associated with aspirin use
Aspirin has long been known to cause clinically significant bleeding. Aspirin inhibits platelet-derived cyclooxygenase-1 (COX-1), a potent vasoconstrictor, and thereby decreases platelet aggregation, reducing thromboembolic potential and prolonging bleeding time. These effects can confer health benefits but also carry the potential for risks. A decision to initiate aspirin therapy for primary prevention relies on an understanding of the benefit-to-harm balance.
Continue to: Initial aspirin studies...
Initial aspirin studies did not show a statistically significant increase in bleeding, likely due to too few events and inadequate powering. Subsequent meta-analyses from multiple evaluations have consistently shown bleeding to be a risk.3,7 The risk for bleeding with aspirin has also been examined in multiple cohort studies, which has helped elucidate the risk in greater detail.
Gastrointestinal bleeding
Epidemiologic data show that among patients who do not use nonsteroidal anti-inflammatory drugs (NSAIDs), the rate of upper gastrointestinal (GI) complications is 1 case per 1000 patient-years.20 Multiple studies have consistently shown that aspirin use increases the rate of significant upper GI bleeding over baseline risk (odds ratio [OR] = 1.54-1.58).3,21,22 Interestingly, these increases seem not to be influenced by other factors, such as comorbidities that increase the risk for ASCVD. Analysis of cancer prevention studies showed similar epidemiologic trends, with aspirin use exceeding a baseline bleeding risk of 0.7 cases of upper GI complications per 1000 patient-years (
Other risk factors. Evaluation of risk factors for bleeding primarily comes from 2 studies.3,7 Most data concern the impact of individual factors on significant GI bleeding, with fewer data available for evaluating risk for intracerebral hemorrhage (ICH). Initial analysis of individual prospective studies showed little or no correlation between risk for bleeding and such factors as gender, age, or history of hypertension or ASCVD.21 Subsequent analysis of meta-data and large cohorts did show statistically significant impact on rates of bleeding across several factors (TABLE 13,7).
Of note is a large heterogeneous cohort study conducted in Spain. Data showed significant increases in baseline risk for GI bleeding in older men with a history of GI bleeding and NSAID use. The absolute risk for GI bleed in this group was potentially as high as 150 cases per 1000 patient-years, well above the risk level assumed for the average patient.24 A seemingly small OR of 1.5 could dramatically increase the absolute risk for bleeding in such patients, and it suggests that a generalized risk for bleeding probably shouldn’t be applied to all patients. Individuals may be better served by a baseline risk calculation reflecting multiple factors.
Intracerebral hemorrhage
Due to the comparatively uncommon nature of ICH, fewer data are available to support definitive conclusions about its increased risk with aspirin use. Aspirin use appeared to increase the risk for ICH with ratios between 1.27 and 1.32 in meta-analyses (measured as an OR or as an RR),3,7,21 with an IRR of 1.54 in a cohort study.22 The only statistically significant factors suspected to increase the risk of ICH at baseline were smoking (RR = 2.18) and mean BP > 20 mm Hg over normal (OR = 2.18). Age, gender, and diabetes all showed a nonsignificant trend toward risk increase.7
Continue to: Risk based on dose and formulation
Risk based on dose and formulation
The effect of aspirin dose and formulation on bleeding risk is uncertain. Some studies have shown an increased risk for bleeding with daily doses of aspirin ≥ 300 mg, while others have shown no significant increase in rates for bleeding with differing doses.21,25 Enteric coating does appear to lower the rates of gastric mucosal injury, although there are few data on the effect toward reducing clinically significant bleeding.26 Currently, several prospective studies are underway to help clarify the evidence.27
Putting it all together
For the general population, the evidence shows that the benefits and harms of aspirin for primary prevention are relatively even. The USPSTF guidelines are the first to recommend aspirin for both CVD and cancer prevention while taking into account the bleeding risk. According to the findings of the USPSTF, the balance of benefits and harms of aspirin use is contingent on 4 factors: age, baseline CVD risk, risk for bleeding, and preferences about taking aspirin.6 The complete recommendations from the USPSTF, along with other leading organizations, are outlined in TABLE 2.6,28-31
Applying the evidence and varying guidelines in practice can feel daunting. Some practical tools have been developed to help clinicians understand patients’ bleeding risk and potential benefits with aspirin use. One such tool is highlighted below. Others are also available, and each has its own strengths and weaknesses.
Aspirin-Guide (www.aspiringuide.com) is a Web-based clinical decision support tool with an associated mobile application. It uses internal calculators (including the pooled cohort calculator prepared jointly by the American College of Cardiology and the American Heart Association) to assess CVD risk as well as bleeding risk. This tool gives clinicians patient-specific numbers-needed-to-treat and numbers-needed-to-harm when considering starting aspirin for primary prevention. It gives specific recommendations for aspirin use based on the data entered, and it also gives providers information to help guide shared decision-making with patients.32 Unfortunately, this decision support tool and others do not take into account the data from the most recent trials, so they should be used with caution.
CORRESPONDENCE
LCDR Dustin K. Smith, DO, Naval Branch Clinic Diego Garcia, PSC 466, Box 301, FPO, AP 96595; dustinksmith@yahoo.com.
Which patients are likely to benefit from using aspirin for primary prevention? In this article, we review the evidence to date, summarized for primary care settings in guidelines issued by the US Preventive Services Task Force (USPSTF). We supplement this summary with a rundown of the risks associated with aspirin use. And then we wrap up by identifying a clinical decision tool that is available to help make personalized decisions in a busy clinic setting, where determining an individual’s potential cardiovascular benefits and bleeding risk can be challenging.
The “roadmap” from the guidelines. In 2014, after performing a review of the literature, the US Food and Drug Administration recommended against the routine use of aspirin for primary prevention of cardiovascular disease (CVD).1 In 2016, the USPSTF published 4 separate systematic reviews along with a decision analysis using a microsimulation model, which informed their position statement on aspirin for primary prevention.2-6 These USPSTF reviews and recommendations incorporated both CVD and colorectal cancer (CRC) benefits with the bleeding risks from aspirin. Generally, for individuals 50 to 59 years old, the benefits are deemed to outweigh the harms; shared decision making is advised with those 60 to 69 years of age. For patients younger than 50 or 70 and older, evidence is inconclusive.
The benefits of primary prevention with aspirin
Cardiovascular disease
The Antithrombotic Trialists’ (ATT) Collaboration was one of the first meta-analyses that addressed the benefit-to-harm balance and called into question the routine use of aspirin for primary prevention.7 The USPSTF systematic review included the studies from the ATT Collaboration as well as trials performed after its publication, bringing the total number of eligible randomized controlled trials reviewed to 11.2
The benefit of aspirin for primary prevention of nonfatal myocardial infarction (MI) has been shown in multiple randomized controlled trials. The USPSTF systematic review showed a statistically significant relative risk reduction of 17% in patients taking low-dose aspirin (≤ 100 mg; relative risk [RR] = 0.83; confidence interval [95% CI], 0.74-0.94), although the heterogeneity of the studies was high. The same low dose of aspirin showed a statistically significant reduction in nonfatal stroke (RR = 0.86; 95% CI, 0.76-0.98), although the same benefit was not observed when all doses of aspirin were included. Cardiovascular disease mortality and all-cause mortality were not statistically different for patients taking low-dose aspirin when compared with placebo (RR = 0.97; 95% CI, 0.85-1.10 for CVD mortality; RR = 0.95; 95% CI, 0.89-1.01 for all-cause mortality).2
One study of more than 14,000 older (≥ 60 years) Japanese patients showed a statistically significant reduction in nonfatal MI (hazard ratio [HR] = 0.53; 95% CI, 0.31-0.91, P = .02) and nonfatal strokes (HR = 0.57; 95% CI, 0.32-0.99; P = .04). The study was stopped early because at 5 years of follow-up there was no statistically significant difference in a composite primary outcome, which included death from cardiovascular causes, nonfatal MI, and nonfatal stroke (HR = 0.94; 95% CI, 0.77-1.15; P = .54).8
Several recent landmark studies have called into question the benefit of aspirin for cardiovascular primary prevention, especially in obese individuals, patients with diabetes, and the elderly. A meta-analysis of 10 trials showed that the effectiveness of aspirin doses between 75 mg and 100 mg for primary prevention decreased as weight increased; patients weighing 70 kg or more received no benefit.9 The ASCEND (A Study of Cardiovascular Events in Diabetes) trial included more than 15,000 patients with diabetes but no cardiovascular disease. Patients randomized to receive the low-dose aspirin did have fewer serious vascular events (incidence rate ratio [IRR] = 0.88; 95% CI, 0.79-0.97; P = .01), but they also had high risk of major bleeding events (IRR = 1.29; 95% CI, 1.09-1.52; P = .003).10 The ASPREE (Aspirin in Reducing Events in the Elderly) trial included more than 19,000 patients ages 70 years and older with no cardiovascular disease and compared low-dose aspirin to placebo. There was no statistically significant cardiovascular benefit, although there was an increase of major hemorrhage (HR = 1.38; 95% CI, 1.18-1.62; P < .001).11 The ARRIVE (A Randomized Trial of Induction Versus Expectant Management) trial included 12,546 moderate atherosclerotic CVD (ASCVD) risk patients. Although a per-protocol analysis showed a decrease in rates of fatal and nonfatal MI (HR = 0.53; 95% CI, 0.36-0.79; P = .0014), the more reliable intention-to-treat analysis showed no improvement for any outcomes.12
[polldaddy:10286821]
Colorectal cancer
The literature base on prevention of cancer has been growing rapidly. However, the deluge of findings over the past 2 decades of trials and analyses has also introduced ambiguity and, often, conflicting results. The first journal article suggesting aspirin for primary prevention of cancer, published in 1988, was a case-control study wherein a population with CRC was matched to controls to look for potential protective factors.13 The most notable finding was the CRC risk reduction for those taking aspirin or aspirin-containing medications. Since then numerous studies and analyses have explored aspirin’s potential in primary prevention of many types of cancer, with overall unclear findings as denoted in the 2016 USPSTF systemic reviews and recommendations.
Continue to: One major limiting factor...
One major limiting factor is that most data come from CVD prevention trials, and only a limited number of trials have focused specifically on cancer prevention. For the USPSTF, these data showed no statistically significant risk reduction in overall cancer mortality (RR = 0.96; 95% CI, 0.87-1.06) or in total cancer incidence (RR = 0.98; 95% CI, 0.93-1.04).4 Other ongoing trials may yield more definitive data.14
The particular interest in CRC was due to it being the first cancer found to be preventable with aspirin therapy. The USPSTF, while acknowledging the homogeneous nature of supporting studies, noted that their significant number and resulting evidence made CRC the only cancer warranting evaluation. Population studies have now shown more benefit than the few randomized control trials. The Women’s Health Study and the Physicians’ Health Study were both limited by their duration. But such studies conducted over a longer period revealed notable benefits in the second decade of use, with a statistically significant lower CRC incidence (RR = 0.60; 95% CI, 0.47-0.76). Additionally, CRC mortality at 20 years was decreased in patients taking aspirin regularly (RR = 0.67; 95% CI, 0.52-0.86).4 Multiple studies are in progress to better establish aspirin’s CRC benefit.
While not directly applicable to the general population, use of aspirin for patients with Lynch syndrome to prevent CRC has strong supporting evidence.15 Beyond CRC, there is nascent evidence from limited observational studies that aspirin may have a preventive effect on melanoma and ovarian and pancreatic cancers.16-18 Further studies or compilations of data would be needed to draw more significant conclusions on other types of cancers. Larger studies would prove more difficult to do, given the smaller incidences of these cancers.
Interestingly, a recent study showed that for individuals 70 years and older, aspirin might increase the risk for all-cause mortality, primarily due to increased cancer mortality across all types.19 Although this result was unexpected, caution should be used when prescribing aspirin particularly for patients 70 or older with active cancer.
A look at the harms associated with aspirin use
Aspirin has long been known to cause clinically significant bleeding. Aspirin inhibits platelet-derived cyclooxygenase-1 (COX-1), a potent vasoconstrictor, and thereby decreases platelet aggregation, reducing thromboembolic potential and prolonging bleeding time. These effects can confer health benefits but also carry the potential for risks. A decision to initiate aspirin therapy for primary prevention relies on an understanding of the benefit-to-harm balance.
Continue to: Initial aspirin studies...
Initial aspirin studies did not show a statistically significant increase in bleeding, likely due to too few events and inadequate powering. Subsequent meta-analyses from multiple evaluations have consistently shown bleeding to be a risk.3,7 The risk for bleeding with aspirin has also been examined in multiple cohort studies, which has helped elucidate the risk in greater detail.
Gastrointestinal bleeding
Epidemiologic data show that among patients who do not use nonsteroidal anti-inflammatory drugs (NSAIDs), the rate of upper gastrointestinal (GI) complications is 1 case per 1000 patient-years.20 Multiple studies have consistently shown that aspirin use increases the rate of significant upper GI bleeding over baseline risk (odds ratio [OR] = 1.54-1.58).3,21,22 Interestingly, these increases seem not to be influenced by other factors, such as comorbidities that increase the risk for ASCVD. Analysis of cancer prevention studies showed similar epidemiologic trends, with aspirin use exceeding a baseline bleeding risk of 0.7 cases of upper GI complications per 1000 patient-years (
Other risk factors. Evaluation of risk factors for bleeding primarily comes from 2 studies.3,7 Most data concern the impact of individual factors on significant GI bleeding, with fewer data available for evaluating risk for intracerebral hemorrhage (ICH). Initial analysis of individual prospective studies showed little or no correlation between risk for bleeding and such factors as gender, age, or history of hypertension or ASCVD.21 Subsequent analysis of meta-data and large cohorts did show statistically significant impact on rates of bleeding across several factors (TABLE 13,7).
Of note is a large heterogeneous cohort study conducted in Spain. Data showed significant increases in baseline risk for GI bleeding in older men with a history of GI bleeding and NSAID use. The absolute risk for GI bleed in this group was potentially as high as 150 cases per 1000 patient-years, well above the risk level assumed for the average patient.24 A seemingly small OR of 1.5 could dramatically increase the absolute risk for bleeding in such patients, and it suggests that a generalized risk for bleeding probably shouldn’t be applied to all patients. Individuals may be better served by a baseline risk calculation reflecting multiple factors.
Intracerebral hemorrhage
Due to the comparatively uncommon nature of ICH, fewer data are available to support definitive conclusions about its increased risk with aspirin use. Aspirin use appeared to increase the risk for ICH with ratios between 1.27 and 1.32 in meta-analyses (measured as an OR or as an RR),3,7,21 with an IRR of 1.54 in a cohort study.22 The only statistically significant factors suspected to increase the risk of ICH at baseline were smoking (RR = 2.18) and mean BP > 20 mm Hg over normal (OR = 2.18). Age, gender, and diabetes all showed a nonsignificant trend toward risk increase.7
Continue to: Risk based on dose and formulation
Risk based on dose and formulation
The effect of aspirin dose and formulation on bleeding risk is uncertain. Some studies have shown an increased risk for bleeding with daily doses of aspirin ≥ 300 mg, while others have shown no significant increase in rates for bleeding with differing doses.21,25 Enteric coating does appear to lower the rates of gastric mucosal injury, although there are few data on the effect toward reducing clinically significant bleeding.26 Currently, several prospective studies are underway to help clarify the evidence.27
Putting it all together
For the general population, the evidence shows that the benefits and harms of aspirin for primary prevention are relatively even. The USPSTF guidelines are the first to recommend aspirin for both CVD and cancer prevention while taking into account the bleeding risk. According to the findings of the USPSTF, the balance of benefits and harms of aspirin use is contingent on 4 factors: age, baseline CVD risk, risk for bleeding, and preferences about taking aspirin.6 The complete recommendations from the USPSTF, along with other leading organizations, are outlined in TABLE 2.6,28-31
Applying the evidence and varying guidelines in practice can feel daunting. Some practical tools have been developed to help clinicians understand patients’ bleeding risk and potential benefits with aspirin use. One such tool is highlighted below. Others are also available, and each has its own strengths and weaknesses.
Aspirin-Guide (www.aspiringuide.com) is a Web-based clinical decision support tool with an associated mobile application. It uses internal calculators (including the pooled cohort calculator prepared jointly by the American College of Cardiology and the American Heart Association) to assess CVD risk as well as bleeding risk. This tool gives clinicians patient-specific numbers-needed-to-treat and numbers-needed-to-harm when considering starting aspirin for primary prevention. It gives specific recommendations for aspirin use based on the data entered, and it also gives providers information to help guide shared decision-making with patients.32 Unfortunately, this decision support tool and others do not take into account the data from the most recent trials, so they should be used with caution.
CORRESPONDENCE
LCDR Dustin K. Smith, DO, Naval Branch Clinic Diego Garcia, PSC 466, Box 301, FPO, AP 96595; dustinksmith@yahoo.com.
1. FDA. Use of aspirin for primary prevention of heart attack and stroke. https://www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm390574.htm. Accessed March 22, 2019.
2. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
3. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:826-835.
4. Chubak J, Whitlock EP, Williams SB, et al. Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:814-825.
5. Dehmer SP, Maciosek MV, Flottemesch TJ, et al. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:777-786.
6. Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836-845.
7. Baigent C, Blackwell L, Colins R, et al; Antithrombotic Trialists (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participation data from randomised trials. Lancet. 2009:373:1849-1860.
8. Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312:2510-2520.
9. Rothwell PM, Cook NR, Gaziano JM, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet. 2018;392:387-399.
10. Bowman L, Mafham M, Wallendszus K, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539.
11. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.
12. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046.
13. Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illness, operations, and medications: case control results from Melbourne Colorectal Cancer Study. Cancer Res. 1988;48:4399-4404.
14. Sutcliffe P, Connock M, Gurung T, et al. Aspirin for prophylactic use in the primary prevention of cardiovascular disease and cancer: a systematic review and overview of reviews. Health Technol Assess. 2013;17:1-253.
15. Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081-2087.
16. Gamba CA, Swetter SM, Stefanick ML, et al. Aspirin is associated with lower melanoma risk among postmenopausal Caucasian women: the Women’s Health Initiative. Cancer. 2013;119:1562-1569.
17. Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431.
18. Risch H, Lu L, Streicher SA, et al. Aspirin use and reduced risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2016;26:68-74.
19. McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-1528.
20. Hernández-Díaz S, Rodríguez LA. Incidence of serious upper gastrointestinal bleeding/perforation in the general population: review of epidemiologic studies. J Clin Epidemiol. 2002;55:157-163.
21. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis no 131. Rockville, MD: Agency for Healthcare Research and Quality; 2015. https://www.ncbi.nlm.nih.gov/books/NBK321623/. Accessed March 22, 2019.
22. De Berardis G, Lucisano G, D’Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286-2294.
23. Thorat MA, Cuzick J. Prophylactic use of aspirin: systematic review of harms and approaches to mitigation in the general population. Eur J Epidemiol. 2015;30:5-18.
24. Hernández-Díaz S, García Rodríguez LA. Cardioprotective aspirin users and their excess risk of upper gastrointestinal complications. BMC Med. 2006;4:22.
25. Huang ES, Strate LL, Ho WW, et al. Long term use of aspirin and the risk of gastrointestinal bleeding. Am J Med. 2011:124;426-433.
26. Walker J, Robinson J, Stewart J, et al. Does enteric-coated aspirin result in a lower incidence of gastrointestinal complications compared to normal aspirin? Interact Cardiovasc Thorac Surg. 2007:6;519-522.
27. NIH. Aspirin dosing: a patient-centric trial assessing benefits and long-term effectiveness (ADAPTABLE). https://clinicaltrials.gov/ct2/show/NCT02697916. Accessed March 22, 2019.
28. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2016;37:2315-2381.
29. ADA. Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40(suppl 1). http://care.diabetesjournals.org/content/diacare/suppl/2016/12/15/40.Supplement_1.DC1/DC_40_S1_final.pdf. Accessed March 22, 2019.
30. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
31. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. J Am Col Cardiol. 2019. doi: https://doi.org/10.1016/j.jacc.2019.03.010. Accessed March 22, 2019.
32. Mora S, Manson JE. Aspirin for primary prevention of atherosclerotic cardiovascular disease: advances in diagnosis and treatment. JAMA Intern Med. 2016;176:1195-1204.
1. FDA. Use of aspirin for primary prevention of heart attack and stroke. https://www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm390574.htm. Accessed March 22, 2019.
2. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
3. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:826-835.
4. Chubak J, Whitlock EP, Williams SB, et al. Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:814-825.
5. Dehmer SP, Maciosek MV, Flottemesch TJ, et al. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:777-786.
6. Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836-845.
7. Baigent C, Blackwell L, Colins R, et al; Antithrombotic Trialists (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participation data from randomised trials. Lancet. 2009:373:1849-1860.
8. Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312:2510-2520.
9. Rothwell PM, Cook NR, Gaziano JM, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet. 2018;392:387-399.
10. Bowman L, Mafham M, Wallendszus K, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539.
11. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.
12. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046.
13. Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illness, operations, and medications: case control results from Melbourne Colorectal Cancer Study. Cancer Res. 1988;48:4399-4404.
14. Sutcliffe P, Connock M, Gurung T, et al. Aspirin for prophylactic use in the primary prevention of cardiovascular disease and cancer: a systematic review and overview of reviews. Health Technol Assess. 2013;17:1-253.
15. Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081-2087.
16. Gamba CA, Swetter SM, Stefanick ML, et al. Aspirin is associated with lower melanoma risk among postmenopausal Caucasian women: the Women’s Health Initiative. Cancer. 2013;119:1562-1569.
17. Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431.
18. Risch H, Lu L, Streicher SA, et al. Aspirin use and reduced risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2016;26:68-74.
19. McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-1528.
20. Hernández-Díaz S, Rodríguez LA. Incidence of serious upper gastrointestinal bleeding/perforation in the general population: review of epidemiologic studies. J Clin Epidemiol. 2002;55:157-163.
21. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis no 131. Rockville, MD: Agency for Healthcare Research and Quality; 2015. https://www.ncbi.nlm.nih.gov/books/NBK321623/. Accessed March 22, 2019.
22. De Berardis G, Lucisano G, D’Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286-2294.
23. Thorat MA, Cuzick J. Prophylactic use of aspirin: systematic review of harms and approaches to mitigation in the general population. Eur J Epidemiol. 2015;30:5-18.
24. Hernández-Díaz S, García Rodríguez LA. Cardioprotective aspirin users and their excess risk of upper gastrointestinal complications. BMC Med. 2006;4:22.
25. Huang ES, Strate LL, Ho WW, et al. Long term use of aspirin and the risk of gastrointestinal bleeding. Am J Med. 2011:124;426-433.
26. Walker J, Robinson J, Stewart J, et al. Does enteric-coated aspirin result in a lower incidence of gastrointestinal complications compared to normal aspirin? Interact Cardiovasc Thorac Surg. 2007:6;519-522.
27. NIH. Aspirin dosing: a patient-centric trial assessing benefits and long-term effectiveness (ADAPTABLE). https://clinicaltrials.gov/ct2/show/NCT02697916. Accessed March 22, 2019.
28. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2016;37:2315-2381.
29. ADA. Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40(suppl 1). http://care.diabetesjournals.org/content/diacare/suppl/2016/12/15/40.Supplement_1.DC1/DC_40_S1_final.pdf. Accessed March 22, 2019.
30. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
31. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. J Am Col Cardiol. 2019. doi: https://doi.org/10.1016/j.jacc.2019.03.010. Accessed March 22, 2019.
32. Mora S, Manson JE. Aspirin for primary prevention of atherosclerotic cardiovascular disease: advances in diagnosis and treatment. JAMA Intern Med. 2016;176:1195-1204.
PRACTICE RECOMMENDATIONS
› Consider aspirin for patients 50 to 59 years of age who have a 10-year cardiovascular disease (CVD) risk of ≥ 10% and low bleeding risk. C
› Discuss prophylactic aspirin (using a shared decision-making model) with patients 60 to 69 years of age who have a 10-year CVD risk of ≥ 10% and low bleeding risk. C
› Avoid using aspirin for primary prevention in patients ≥ 70 years of age. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Genetic variant increases stroke risk in childhood cancer survivors
ATLANTA – Adult survivors of childhood cancers are at significantly greater risk than the general population for late-term complications related to therapy, including secondary cancers, cardiovascular disease, and cerebrovascular complications, including ischemic and hemorrhagic strokes.
In particular, childhood cancer survivors have an approximately eightfold higher risk for stroke, compared with their siblings, with a history of cranial irradiation being a strong, dose-dependent risk factor for stroke.
Researchers at St. Jude Children’s Research Hospital in Memphis, Tenn., are conducting a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of childhood cancer survivors who are 5 or more years out of therapy.
The study includes publicly available, whole-genome sequencing data on 4,500 participants. Sifting through these data, Yadav Sapkota, PhD, a clinical research scientist at St. Jude, and his colleagues have identified a genetic variant strongly associated with stroke risk in survivors of European ancestry, and they have replicated the finding in survivors of African ancestry.
In a video interview at the annual meeting of the American Association for Cancer Research, Dr. Sapkota describes his group’s findings and potential research and clinical implications.
The study was sponsored by the National Cancer Institute and ALSAC, the fundraising and awareness organization of St. Jude. Dr. Sapkota declared no conflict of interest.
ATLANTA – Adult survivors of childhood cancers are at significantly greater risk than the general population for late-term complications related to therapy, including secondary cancers, cardiovascular disease, and cerebrovascular complications, including ischemic and hemorrhagic strokes.
In particular, childhood cancer survivors have an approximately eightfold higher risk for stroke, compared with their siblings, with a history of cranial irradiation being a strong, dose-dependent risk factor for stroke.
Researchers at St. Jude Children’s Research Hospital in Memphis, Tenn., are conducting a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of childhood cancer survivors who are 5 or more years out of therapy.
The study includes publicly available, whole-genome sequencing data on 4,500 participants. Sifting through these data, Yadav Sapkota, PhD, a clinical research scientist at St. Jude, and his colleagues have identified a genetic variant strongly associated with stroke risk in survivors of European ancestry, and they have replicated the finding in survivors of African ancestry.
In a video interview at the annual meeting of the American Association for Cancer Research, Dr. Sapkota describes his group’s findings and potential research and clinical implications.
The study was sponsored by the National Cancer Institute and ALSAC, the fundraising and awareness organization of St. Jude. Dr. Sapkota declared no conflict of interest.
ATLANTA – Adult survivors of childhood cancers are at significantly greater risk than the general population for late-term complications related to therapy, including secondary cancers, cardiovascular disease, and cerebrovascular complications, including ischemic and hemorrhagic strokes.
In particular, childhood cancer survivors have an approximately eightfold higher risk for stroke, compared with their siblings, with a history of cranial irradiation being a strong, dose-dependent risk factor for stroke.
Researchers at St. Jude Children’s Research Hospital in Memphis, Tenn., are conducting a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of childhood cancer survivors who are 5 or more years out of therapy.
The study includes publicly available, whole-genome sequencing data on 4,500 participants. Sifting through these data, Yadav Sapkota, PhD, a clinical research scientist at St. Jude, and his colleagues have identified a genetic variant strongly associated with stroke risk in survivors of European ancestry, and they have replicated the finding in survivors of African ancestry.
In a video interview at the annual meeting of the American Association for Cancer Research, Dr. Sapkota describes his group’s findings and potential research and clinical implications.
The study was sponsored by the National Cancer Institute and ALSAC, the fundraising and awareness organization of St. Jude. Dr. Sapkota declared no conflict of interest.
REPORTING FROM AACR 2019
30-day readmissions after STEMI with cardiogenic shock 13%
WASHINGTON – and remain in hospital for an average of 6 days, according to an analysis from the National Readmission Database presented at CRT 2019 sponsored by MedStar Heart & Vascular Institute.
“About one in four of the readmissions was for heart failure,” reported Karan Sud, MD, a cardiology resident at the Mount Sinai St. Luke’s West Hospital, New York.
Despite gains in acute survival among STEMI patients in cardiogenic shock, little attention has been paid to the risk of readmissions, according to Dr. Sud. According to data collected from the National Readmissions Database for 2010-2014, these rates are high enough to deserve attention, he said.
“Our goal is now to develop a scoring system based on our predictive model to identify patients at the index admission who are at risk for readmission,” Dr. Sud reported. On the basis of these predictors, it might be possible to implement strategies to optimize management and improve access to care.
In the years studied, there were 94,991 patients with STEMI and cardiogenic shock captured in the National Readmissions Database, of whom 43,205 survived and were followed for readmission. Of the 5,503 readmissions within 30 days, 12% were considered unplanned.
Half of the readmissions were for noncardiovascular causes, including sepsis, respiratory failure, and major bleeding. Of those related to cardiovascular disease, about half, or nearly 25% of the total, were for heart failure.
The predictors of readmission included female sex, age older than 75 years, average length of stay longer than 10 days, and more than three comorbidities, such as diabetes or chronic kidney disease, according to Dr. Sud.
“Those sent home from the index admission were more likely than those discharged to an extended care facility to be readmitted,” he added. He also noted that lower socioeconomic status was a risk factor for readmission, a phenomenon that he attributed to access issues regarding follow-up care.
“We are now conducting a prospective study to look at readmissions at 6 months,” reported Dr. Sud, who believes that efforts to understand the risk of readmission following STEMI complicated by cardiogenic shock might uncover opportunities for better management.
WASHINGTON – and remain in hospital for an average of 6 days, according to an analysis from the National Readmission Database presented at CRT 2019 sponsored by MedStar Heart & Vascular Institute.
“About one in four of the readmissions was for heart failure,” reported Karan Sud, MD, a cardiology resident at the Mount Sinai St. Luke’s West Hospital, New York.
Despite gains in acute survival among STEMI patients in cardiogenic shock, little attention has been paid to the risk of readmissions, according to Dr. Sud. According to data collected from the National Readmissions Database for 2010-2014, these rates are high enough to deserve attention, he said.
“Our goal is now to develop a scoring system based on our predictive model to identify patients at the index admission who are at risk for readmission,” Dr. Sud reported. On the basis of these predictors, it might be possible to implement strategies to optimize management and improve access to care.
In the years studied, there were 94,991 patients with STEMI and cardiogenic shock captured in the National Readmissions Database, of whom 43,205 survived and were followed for readmission. Of the 5,503 readmissions within 30 days, 12% were considered unplanned.
Half of the readmissions were for noncardiovascular causes, including sepsis, respiratory failure, and major bleeding. Of those related to cardiovascular disease, about half, or nearly 25% of the total, were for heart failure.
The predictors of readmission included female sex, age older than 75 years, average length of stay longer than 10 days, and more than three comorbidities, such as diabetes or chronic kidney disease, according to Dr. Sud.
“Those sent home from the index admission were more likely than those discharged to an extended care facility to be readmitted,” he added. He also noted that lower socioeconomic status was a risk factor for readmission, a phenomenon that he attributed to access issues regarding follow-up care.
“We are now conducting a prospective study to look at readmissions at 6 months,” reported Dr. Sud, who believes that efforts to understand the risk of readmission following STEMI complicated by cardiogenic shock might uncover opportunities for better management.
WASHINGTON – and remain in hospital for an average of 6 days, according to an analysis from the National Readmission Database presented at CRT 2019 sponsored by MedStar Heart & Vascular Institute.
“About one in four of the readmissions was for heart failure,” reported Karan Sud, MD, a cardiology resident at the Mount Sinai St. Luke’s West Hospital, New York.
Despite gains in acute survival among STEMI patients in cardiogenic shock, little attention has been paid to the risk of readmissions, according to Dr. Sud. According to data collected from the National Readmissions Database for 2010-2014, these rates are high enough to deserve attention, he said.
“Our goal is now to develop a scoring system based on our predictive model to identify patients at the index admission who are at risk for readmission,” Dr. Sud reported. On the basis of these predictors, it might be possible to implement strategies to optimize management and improve access to care.
In the years studied, there were 94,991 patients with STEMI and cardiogenic shock captured in the National Readmissions Database, of whom 43,205 survived and were followed for readmission. Of the 5,503 readmissions within 30 days, 12% were considered unplanned.
Half of the readmissions were for noncardiovascular causes, including sepsis, respiratory failure, and major bleeding. Of those related to cardiovascular disease, about half, or nearly 25% of the total, were for heart failure.
The predictors of readmission included female sex, age older than 75 years, average length of stay longer than 10 days, and more than three comorbidities, such as diabetes or chronic kidney disease, according to Dr. Sud.
“Those sent home from the index admission were more likely than those discharged to an extended care facility to be readmitted,” he added. He also noted that lower socioeconomic status was a risk factor for readmission, a phenomenon that he attributed to access issues regarding follow-up care.
“We are now conducting a prospective study to look at readmissions at 6 months,” reported Dr. Sud, who believes that efforts to understand the risk of readmission following STEMI complicated by cardiogenic shock might uncover opportunities for better management.
REPORTING FROM CRT 2019
Studies link TMAO to microbiome, reveal new heart disease target
MIAMI – Researchers are one step closer to developing “drugs for bugs” – agents that target the gut microbiome to prevent and treat cardiometabolic diseases, Stanley L. Hazen, MD, PhD, said at the 2019 Gut Microbiota for Health World Summit.
“Each person experiences a meal differently through the filter of their gut microbiome, which helps explain individual differences in susceptibility to disease,” said Dr. Hazen of Cleveland Clinic. “In the future, our medicine cabinets will have drugs in them that not only affect us, but also target the microbial enzymes that affect levels of metabolites like TMAO.”
Trimethylamine N-oxide (TMAO) is produced by gut bacteria. High levels (in one study, approximately 6.2 micromolar) significantly increase the risk of major adverse cardiovascular events even after controlling for traditional demographic and clinical risk factors. Studies indicate that TMAO alters cholesterol and bile acid metabolism, upregulates inflammatory pathways, and promotes foam cell formation, all of which worsen atherosclerosis. In addition, TMAO increases clotting risk by enhancing platelet reactivity.
“Reducing the amount of animal products in one’s diet helps reduce TMAO levels,” said Dr. Hazen. Certain fish – mainly those found in deep, cold water, such as cod – are high in TMAO. However, a bigger culprit in the United States is red meat, which contains two major TMAO precursors – choline and carnitine. In a recent study, Dr. Hazen and his associates gave 113 healthy volunteers three isocaloric diets in random order based on red meat, white meat, or plant-based protein. After 4 weeks, eating the daily equivalent of 8 ounces of steak or two quarter-pound beef patties nearly tripled plasma TMAO levels (P less than .05) from baseline. The white meat and vegetarian diets showed no such effect.
Crucially, the effect of red meat was reversible – TMAO levels fell significantly within 4 weeks after participants stopped consuming red meat. Eating red meat low in saturated fat did not prevent TMAO levels from rising, Dr. Hazen noted at the meeting at the meeting sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
In a second study, Dr. Hazen and his associates identified a two-step process by which gut bacteria metabolize carnitine to TMAO. The second step was greatly enhanced in individuals who eat red meat, suggesting a possible therapeutic target. In a third study, they found that high TMAO levels in mice fell significantly with a single oral dose of a second-generation inhibitor of trimethylamine lyase, the enzyme used by gut bacteria to convert choline to TMAO. The inhibitory effect was irreversible, did not reduce the viability of commensal microorganisms, and significantly lowered platelet hyperreactivity and clot formation.
Such results are exciting, but “drugs for bugs” will exhibit varying effects depending on which gut species are present at baseline, Dr. Hazen explained. Investigators will need to understand and account for these differences before therapies for the microbiome can enter the clinic. For now, a blood test for TMAO is available and can help clinicians tailor their suggestions on what to eat.
Dr. Hazen disclosed a consulting relationship with Proctor & Gamble, royalties for patents from Proctor & Gamble, Cleveland Heart Lab, and Quest Diagnostics, and research support from AstraZeneca, Pfizer, Roche Diagnostics, and Proctor & Gamble.
MIAMI – Researchers are one step closer to developing “drugs for bugs” – agents that target the gut microbiome to prevent and treat cardiometabolic diseases, Stanley L. Hazen, MD, PhD, said at the 2019 Gut Microbiota for Health World Summit.
“Each person experiences a meal differently through the filter of their gut microbiome, which helps explain individual differences in susceptibility to disease,” said Dr. Hazen of Cleveland Clinic. “In the future, our medicine cabinets will have drugs in them that not only affect us, but also target the microbial enzymes that affect levels of metabolites like TMAO.”
Trimethylamine N-oxide (TMAO) is produced by gut bacteria. High levels (in one study, approximately 6.2 micromolar) significantly increase the risk of major adverse cardiovascular events even after controlling for traditional demographic and clinical risk factors. Studies indicate that TMAO alters cholesterol and bile acid metabolism, upregulates inflammatory pathways, and promotes foam cell formation, all of which worsen atherosclerosis. In addition, TMAO increases clotting risk by enhancing platelet reactivity.
“Reducing the amount of animal products in one’s diet helps reduce TMAO levels,” said Dr. Hazen. Certain fish – mainly those found in deep, cold water, such as cod – are high in TMAO. However, a bigger culprit in the United States is red meat, which contains two major TMAO precursors – choline and carnitine. In a recent study, Dr. Hazen and his associates gave 113 healthy volunteers three isocaloric diets in random order based on red meat, white meat, or plant-based protein. After 4 weeks, eating the daily equivalent of 8 ounces of steak or two quarter-pound beef patties nearly tripled plasma TMAO levels (P less than .05) from baseline. The white meat and vegetarian diets showed no such effect.
Crucially, the effect of red meat was reversible – TMAO levels fell significantly within 4 weeks after participants stopped consuming red meat. Eating red meat low in saturated fat did not prevent TMAO levels from rising, Dr. Hazen noted at the meeting at the meeting sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
In a second study, Dr. Hazen and his associates identified a two-step process by which gut bacteria metabolize carnitine to TMAO. The second step was greatly enhanced in individuals who eat red meat, suggesting a possible therapeutic target. In a third study, they found that high TMAO levels in mice fell significantly with a single oral dose of a second-generation inhibitor of trimethylamine lyase, the enzyme used by gut bacteria to convert choline to TMAO. The inhibitory effect was irreversible, did not reduce the viability of commensal microorganisms, and significantly lowered platelet hyperreactivity and clot formation.
Such results are exciting, but “drugs for bugs” will exhibit varying effects depending on which gut species are present at baseline, Dr. Hazen explained. Investigators will need to understand and account for these differences before therapies for the microbiome can enter the clinic. For now, a blood test for TMAO is available and can help clinicians tailor their suggestions on what to eat.
Dr. Hazen disclosed a consulting relationship with Proctor & Gamble, royalties for patents from Proctor & Gamble, Cleveland Heart Lab, and Quest Diagnostics, and research support from AstraZeneca, Pfizer, Roche Diagnostics, and Proctor & Gamble.
MIAMI – Researchers are one step closer to developing “drugs for bugs” – agents that target the gut microbiome to prevent and treat cardiometabolic diseases, Stanley L. Hazen, MD, PhD, said at the 2019 Gut Microbiota for Health World Summit.
“Each person experiences a meal differently through the filter of their gut microbiome, which helps explain individual differences in susceptibility to disease,” said Dr. Hazen of Cleveland Clinic. “In the future, our medicine cabinets will have drugs in them that not only affect us, but also target the microbial enzymes that affect levels of metabolites like TMAO.”
Trimethylamine N-oxide (TMAO) is produced by gut bacteria. High levels (in one study, approximately 6.2 micromolar) significantly increase the risk of major adverse cardiovascular events even after controlling for traditional demographic and clinical risk factors. Studies indicate that TMAO alters cholesterol and bile acid metabolism, upregulates inflammatory pathways, and promotes foam cell formation, all of which worsen atherosclerosis. In addition, TMAO increases clotting risk by enhancing platelet reactivity.
“Reducing the amount of animal products in one’s diet helps reduce TMAO levels,” said Dr. Hazen. Certain fish – mainly those found in deep, cold water, such as cod – are high in TMAO. However, a bigger culprit in the United States is red meat, which contains two major TMAO precursors – choline and carnitine. In a recent study, Dr. Hazen and his associates gave 113 healthy volunteers three isocaloric diets in random order based on red meat, white meat, or plant-based protein. After 4 weeks, eating the daily equivalent of 8 ounces of steak or two quarter-pound beef patties nearly tripled plasma TMAO levels (P less than .05) from baseline. The white meat and vegetarian diets showed no such effect.
Crucially, the effect of red meat was reversible – TMAO levels fell significantly within 4 weeks after participants stopped consuming red meat. Eating red meat low in saturated fat did not prevent TMAO levels from rising, Dr. Hazen noted at the meeting at the meeting sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
In a second study, Dr. Hazen and his associates identified a two-step process by which gut bacteria metabolize carnitine to TMAO. The second step was greatly enhanced in individuals who eat red meat, suggesting a possible therapeutic target. In a third study, they found that high TMAO levels in mice fell significantly with a single oral dose of a second-generation inhibitor of trimethylamine lyase, the enzyme used by gut bacteria to convert choline to TMAO. The inhibitory effect was irreversible, did not reduce the viability of commensal microorganisms, and significantly lowered platelet hyperreactivity and clot formation.
Such results are exciting, but “drugs for bugs” will exhibit varying effects depending on which gut species are present at baseline, Dr. Hazen explained. Investigators will need to understand and account for these differences before therapies for the microbiome can enter the clinic. For now, a blood test for TMAO is available and can help clinicians tailor their suggestions on what to eat.
Dr. Hazen disclosed a consulting relationship with Proctor & Gamble, royalties for patents from Proctor & Gamble, Cleveland Heart Lab, and Quest Diagnostics, and research support from AstraZeneca, Pfizer, Roche Diagnostics, and Proctor & Gamble.
REPORTING FROM GMFH 2019
Renal denervation reduced BP in sham-controlled trials, meta-analysis shows
although previous investigations of the procedure have had conflicting results.
Renal sympathetic denervation (RSD) was associated with statistically significant reductions in blood pressure assessed by 24-hour ambulatory, daytime ambulatory, and office measurements in the analysis of six trials including a total of 977 participants.
However, the benefit was particularly pronounced in more recent randomized trials that had few patients with isolated systolic hypertension, had highly experienced operators; used more complete techniques of radiofrequency ablation, used novel approaches such as endovascular renal denervation, and used efficacy endpoints such as clinical outcomes, according to investigator Partha Sardar, MD, of Brown University, Providence, R.I., and his colleagues.
“Altogether, the present study affirms the safety and efficacy of renal denervation for blood pressure reduction, and highlights the importance of incorporating the previously described modifications in trial design,” wrote Dr. Sardar and his coauthors. The report is in the Journal of the American College of Cardiology.
While initial trials of catheter-based denervation of renal arteries were positive, three blinded randomized, controlled trials showed no difference in blood pressure between the procedure and a sham procedure, the investigators said. Those findings led to several small, sham-controlled trials that incorporated the aforementioned changes.
For the six trials combined in the meta-analysis, reductions in 24-hour ambulatory systolic blood pressure were significantly lower for RSD, with a weighted mean difference of –3.65 mm Hg (P less than .001), Dr. Sardar and his colleagues reported.
For the earlier trials, the average reductions in 24-hour ambulatory systolic and diastolic blood pressure were 2.23 and 0.66 for RSD and sham patients, respectively.
By contrast, in the second-generation trials, those blood pressure reductions were 4.85 for RSD and 2.98 mm Hg for sham, they said in the report, adding that the reduction in daytime ambulatory systolic blood pressure with RSD was significantly greater for the second-generation studies.
The second-generation studies excluded patients with isolated systolic hypertension, based in part on observations that RSD has a more pronounced impact on blood pressure with combined systolic and diastolic hypertension, according to the authors.
Moreover, the second-generation studies required that very experienced operators perform the procedures, incorporated advanced catheter and ablation techniques, less often used modified medication regimens, and set ambulatory blood pressure as the primary end point, they added.
“These results should inform the design and powering of larger, pivotal trials to evaluate the long-term efficacy and safety of RSD in patients with uncontrolled and resistant hypertension,” Dr. Sardar and his coauthors said.
Dr. Sardar reported no relevant financial disclosures, as did most of the coauthors. Three coauthors provided disclosures related to Regado Biosciences, Abbott Vascular, Amgen, Bristol-Myers Squibb, Lilly, Medtronic, and ReCor Medical, among others.
SOURCE: Sardar P et al. J Am Coll Cardiol. 2019;73(13):1633-42.
While questions remain about the future of renal sympathetic denervation for treatment of hypertension, the present meta-analysis provides “interesting” findings that confirm a benefit of the procedure, particularly in the more recent randomized trials, editorialists said.
“The evidence is now there to conclude that RSD does lower blood pressure in hypertensive patients,” Sverre E. Kjeldsen, MD, PhD, Fadl E.M. Fadl Elmula, MD, PhD, and Alexandre Persu, MD, PhD, wrote in their editorial. That conclusion makes sense in light of knowledge that sympathetic overactivity is a known contributor to hypertension pathogenesis.
Although the blood pressure benefits of RSD in the second-generation trials still seem “relatively modest” and equate roughly to the effect of one antihypertensive drug, the aggregate results mask a wide variation in individual patient response, with up to 30% of patients experiencing dramatic improvements after the procedure, they said.
Accordingly, one key research priority is to figure out what patient characteristics might be used to single out patients who are extreme responders to the therapy.
That kind of optimized patient selection, in tandem with technical improvements in the procedure, they said, may help break the “glass ceiling” in blood pressure reduction reported in randomized trials to date.
“Research on RSD still has good days to come, and patients may eventually benefit from this research effort,” Dr. Kjeldsen, Dr. Fadl Elmula, and Dr. Persu concluded.
Dr. Kjeldsen and Dr. Fadl Elmula are at Oslo University Hospital, Ullevaal, and the University of Oslo; Dr. Persu is at the Université Catholique de Louvain, Brussels. The comments summarize an editorial accompanying the article by Sardar et al. (J Am Coll Cardiol. 2019. doi: 10.1016/j.jacc.2019.02.008). Dr. Kjeldsen reported disclosures related to Merck KGaA, Merck Sharp and Dohme, Sanofi, and Takeda.
While questions remain about the future of renal sympathetic denervation for treatment of hypertension, the present meta-analysis provides “interesting” findings that confirm a benefit of the procedure, particularly in the more recent randomized trials, editorialists said.
“The evidence is now there to conclude that RSD does lower blood pressure in hypertensive patients,” Sverre E. Kjeldsen, MD, PhD, Fadl E.M. Fadl Elmula, MD, PhD, and Alexandre Persu, MD, PhD, wrote in their editorial. That conclusion makes sense in light of knowledge that sympathetic overactivity is a known contributor to hypertension pathogenesis.
Although the blood pressure benefits of RSD in the second-generation trials still seem “relatively modest” and equate roughly to the effect of one antihypertensive drug, the aggregate results mask a wide variation in individual patient response, with up to 30% of patients experiencing dramatic improvements after the procedure, they said.
Accordingly, one key research priority is to figure out what patient characteristics might be used to single out patients who are extreme responders to the therapy.
That kind of optimized patient selection, in tandem with technical improvements in the procedure, they said, may help break the “glass ceiling” in blood pressure reduction reported in randomized trials to date.
“Research on RSD still has good days to come, and patients may eventually benefit from this research effort,” Dr. Kjeldsen, Dr. Fadl Elmula, and Dr. Persu concluded.
Dr. Kjeldsen and Dr. Fadl Elmula are at Oslo University Hospital, Ullevaal, and the University of Oslo; Dr. Persu is at the Université Catholique de Louvain, Brussels. The comments summarize an editorial accompanying the article by Sardar et al. (J Am Coll Cardiol. 2019. doi: 10.1016/j.jacc.2019.02.008). Dr. Kjeldsen reported disclosures related to Merck KGaA, Merck Sharp and Dohme, Sanofi, and Takeda.
While questions remain about the future of renal sympathetic denervation for treatment of hypertension, the present meta-analysis provides “interesting” findings that confirm a benefit of the procedure, particularly in the more recent randomized trials, editorialists said.
“The evidence is now there to conclude that RSD does lower blood pressure in hypertensive patients,” Sverre E. Kjeldsen, MD, PhD, Fadl E.M. Fadl Elmula, MD, PhD, and Alexandre Persu, MD, PhD, wrote in their editorial. That conclusion makes sense in light of knowledge that sympathetic overactivity is a known contributor to hypertension pathogenesis.
Although the blood pressure benefits of RSD in the second-generation trials still seem “relatively modest” and equate roughly to the effect of one antihypertensive drug, the aggregate results mask a wide variation in individual patient response, with up to 30% of patients experiencing dramatic improvements after the procedure, they said.
Accordingly, one key research priority is to figure out what patient characteristics might be used to single out patients who are extreme responders to the therapy.
That kind of optimized patient selection, in tandem with technical improvements in the procedure, they said, may help break the “glass ceiling” in blood pressure reduction reported in randomized trials to date.
“Research on RSD still has good days to come, and patients may eventually benefit from this research effort,” Dr. Kjeldsen, Dr. Fadl Elmula, and Dr. Persu concluded.
Dr. Kjeldsen and Dr. Fadl Elmula are at Oslo University Hospital, Ullevaal, and the University of Oslo; Dr. Persu is at the Université Catholique de Louvain, Brussels. The comments summarize an editorial accompanying the article by Sardar et al. (J Am Coll Cardiol. 2019. doi: 10.1016/j.jacc.2019.02.008). Dr. Kjeldsen reported disclosures related to Merck KGaA, Merck Sharp and Dohme, Sanofi, and Takeda.
although previous investigations of the procedure have had conflicting results.
Renal sympathetic denervation (RSD) was associated with statistically significant reductions in blood pressure assessed by 24-hour ambulatory, daytime ambulatory, and office measurements in the analysis of six trials including a total of 977 participants.
However, the benefit was particularly pronounced in more recent randomized trials that had few patients with isolated systolic hypertension, had highly experienced operators; used more complete techniques of radiofrequency ablation, used novel approaches such as endovascular renal denervation, and used efficacy endpoints such as clinical outcomes, according to investigator Partha Sardar, MD, of Brown University, Providence, R.I., and his colleagues.
“Altogether, the present study affirms the safety and efficacy of renal denervation for blood pressure reduction, and highlights the importance of incorporating the previously described modifications in trial design,” wrote Dr. Sardar and his coauthors. The report is in the Journal of the American College of Cardiology.
While initial trials of catheter-based denervation of renal arteries were positive, three blinded randomized, controlled trials showed no difference in blood pressure between the procedure and a sham procedure, the investigators said. Those findings led to several small, sham-controlled trials that incorporated the aforementioned changes.
For the six trials combined in the meta-analysis, reductions in 24-hour ambulatory systolic blood pressure were significantly lower for RSD, with a weighted mean difference of –3.65 mm Hg (P less than .001), Dr. Sardar and his colleagues reported.
For the earlier trials, the average reductions in 24-hour ambulatory systolic and diastolic blood pressure were 2.23 and 0.66 for RSD and sham patients, respectively.
By contrast, in the second-generation trials, those blood pressure reductions were 4.85 for RSD and 2.98 mm Hg for sham, they said in the report, adding that the reduction in daytime ambulatory systolic blood pressure with RSD was significantly greater for the second-generation studies.
The second-generation studies excluded patients with isolated systolic hypertension, based in part on observations that RSD has a more pronounced impact on blood pressure with combined systolic and diastolic hypertension, according to the authors.
Moreover, the second-generation studies required that very experienced operators perform the procedures, incorporated advanced catheter and ablation techniques, less often used modified medication regimens, and set ambulatory blood pressure as the primary end point, they added.
“These results should inform the design and powering of larger, pivotal trials to evaluate the long-term efficacy and safety of RSD in patients with uncontrolled and resistant hypertension,” Dr. Sardar and his coauthors said.
Dr. Sardar reported no relevant financial disclosures, as did most of the coauthors. Three coauthors provided disclosures related to Regado Biosciences, Abbott Vascular, Amgen, Bristol-Myers Squibb, Lilly, Medtronic, and ReCor Medical, among others.
SOURCE: Sardar P et al. J Am Coll Cardiol. 2019;73(13):1633-42.
although previous investigations of the procedure have had conflicting results.
Renal sympathetic denervation (RSD) was associated with statistically significant reductions in blood pressure assessed by 24-hour ambulatory, daytime ambulatory, and office measurements in the analysis of six trials including a total of 977 participants.
However, the benefit was particularly pronounced in more recent randomized trials that had few patients with isolated systolic hypertension, had highly experienced operators; used more complete techniques of radiofrequency ablation, used novel approaches such as endovascular renal denervation, and used efficacy endpoints such as clinical outcomes, according to investigator Partha Sardar, MD, of Brown University, Providence, R.I., and his colleagues.
“Altogether, the present study affirms the safety and efficacy of renal denervation for blood pressure reduction, and highlights the importance of incorporating the previously described modifications in trial design,” wrote Dr. Sardar and his coauthors. The report is in the Journal of the American College of Cardiology.
While initial trials of catheter-based denervation of renal arteries were positive, three blinded randomized, controlled trials showed no difference in blood pressure between the procedure and a sham procedure, the investigators said. Those findings led to several small, sham-controlled trials that incorporated the aforementioned changes.
For the six trials combined in the meta-analysis, reductions in 24-hour ambulatory systolic blood pressure were significantly lower for RSD, with a weighted mean difference of –3.65 mm Hg (P less than .001), Dr. Sardar and his colleagues reported.
For the earlier trials, the average reductions in 24-hour ambulatory systolic and diastolic blood pressure were 2.23 and 0.66 for RSD and sham patients, respectively.
By contrast, in the second-generation trials, those blood pressure reductions were 4.85 for RSD and 2.98 mm Hg for sham, they said in the report, adding that the reduction in daytime ambulatory systolic blood pressure with RSD was significantly greater for the second-generation studies.
The second-generation studies excluded patients with isolated systolic hypertension, based in part on observations that RSD has a more pronounced impact on blood pressure with combined systolic and diastolic hypertension, according to the authors.
Moreover, the second-generation studies required that very experienced operators perform the procedures, incorporated advanced catheter and ablation techniques, less often used modified medication regimens, and set ambulatory blood pressure as the primary end point, they added.
“These results should inform the design and powering of larger, pivotal trials to evaluate the long-term efficacy and safety of RSD in patients with uncontrolled and resistant hypertension,” Dr. Sardar and his coauthors said.
Dr. Sardar reported no relevant financial disclosures, as did most of the coauthors. Three coauthors provided disclosures related to Regado Biosciences, Abbott Vascular, Amgen, Bristol-Myers Squibb, Lilly, Medtronic, and ReCor Medical, among others.
SOURCE: Sardar P et al. J Am Coll Cardiol. 2019;73(13):1633-42.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Renal sympathetic denervation significantly reduced blood pressure in randomized, sham-controlled trials.
Major finding: In second-generation trials of renal sympathetic denervation for hypertension therapy, 24-hour ambulatory blood pressure reductions were 4.85 for RSD and 2.98 mm Hg for sham.
Study details: Renal sympathetic denervation for treating hypertension was tested in six randomized, sham-controlled trials of 24-hour ambulatory, daytime ambulatory, and blood pressure office measurements including a total of 977 participants.
Disclosures: Dr. Sardar reported no relevant financial disclosures, as did most of the coauthors. Three coauthors provided disclosures related to Regado Biosciences, Abbott Vascular, Amgen, Bristol-Myers Squibb, Lilly, Medtronic, and ReCor Medical, among others.
Source: Sardar P et al. J Am Coll Cardiol. 2019;73(13):1633-42.
FDA approves multiple ambrisentan generics for patients with PAH
The Food and Drug Administration has approved multiple generics for ambrisentan (Letairis) tablets, as well as their associated risk evaluation and mitigation strategies (REMS), for patients with pulmonary arterial hypertension.
A total of four generics were approved, licensed to Mylan Pharmaceuticals, Sun Pharma Global, Watson Laboratories, and Zydus Pharmaceuticals. All four were approved at 5-mg and 10-mg doses. According to the label, the most common adverse events associated with ambrisentan include peripheral edema, nasal congestion, sinusitis, and flushing.
In addition to the generics, the FDA also approved two shared system REMS programs for ambrisentan. The first, Ambrisentan REMS, includes the brand sponsor and three abbreviated new drug applications. The FDA also approved two shared system REMS programs for ambrisentan. The first, Ambrisentan REMS, is composed of the brand sponsor and three abbreviated new drug applications. The second, PS-Ambrisentan REMS, is a parallel system and currently is constituted by one abbreviated new drug application.
“With the approval of these first generics ... patients will now have access to additional products (brand-name and generic) and additional types of pharmacies to fill their prescriptions,” the FDA said.
Find the full press release on the FDA website.
The Food and Drug Administration has approved multiple generics for ambrisentan (Letairis) tablets, as well as their associated risk evaluation and mitigation strategies (REMS), for patients with pulmonary arterial hypertension.
A total of four generics were approved, licensed to Mylan Pharmaceuticals, Sun Pharma Global, Watson Laboratories, and Zydus Pharmaceuticals. All four were approved at 5-mg and 10-mg doses. According to the label, the most common adverse events associated with ambrisentan include peripheral edema, nasal congestion, sinusitis, and flushing.
In addition to the generics, the FDA also approved two shared system REMS programs for ambrisentan. The first, Ambrisentan REMS, includes the brand sponsor and three abbreviated new drug applications. The FDA also approved two shared system REMS programs for ambrisentan. The first, Ambrisentan REMS, is composed of the brand sponsor and three abbreviated new drug applications. The second, PS-Ambrisentan REMS, is a parallel system and currently is constituted by one abbreviated new drug application.
“With the approval of these first generics ... patients will now have access to additional products (brand-name and generic) and additional types of pharmacies to fill their prescriptions,” the FDA said.
Find the full press release on the FDA website.
The Food and Drug Administration has approved multiple generics for ambrisentan (Letairis) tablets, as well as their associated risk evaluation and mitigation strategies (REMS), for patients with pulmonary arterial hypertension.
A total of four generics were approved, licensed to Mylan Pharmaceuticals, Sun Pharma Global, Watson Laboratories, and Zydus Pharmaceuticals. All four were approved at 5-mg and 10-mg doses. According to the label, the most common adverse events associated with ambrisentan include peripheral edema, nasal congestion, sinusitis, and flushing.
In addition to the generics, the FDA also approved two shared system REMS programs for ambrisentan. The first, Ambrisentan REMS, includes the brand sponsor and three abbreviated new drug applications. The FDA also approved two shared system REMS programs for ambrisentan. The first, Ambrisentan REMS, is composed of the brand sponsor and three abbreviated new drug applications. The second, PS-Ambrisentan REMS, is a parallel system and currently is constituted by one abbreviated new drug application.
“With the approval of these first generics ... patients will now have access to additional products (brand-name and generic) and additional types of pharmacies to fill their prescriptions,” the FDA said.
Find the full press release on the FDA website.
Spontaneous coronary artery dissection: An often unrecognized cause of acute coronary syndrome
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
KEY POINTS
- SCAD often presents with symptoms of acute coronary syndrome but can be asymptomatic or cause sudden death.
- Management is generally conservative, but a left main or severe proximal 2-vessel dissection, hemodynamic instability, or ongoing ischemic symptoms may warrant revascularization.
- All patients with SCAD should be screened for other vascular problems, especially fibromuscular dysplasia.
- Long-term aspirin therapy and 1 year of clopidogrel are recommended after an episode of SCAD.