User login
During Flu Season Risk of Heart Failure Rises
A study of > 450,000 adults has “confirmed the long-held notion” that flu and heart failure are connected. The Atherosclerosis Risk in Communities study (ARIC), led by a VA researcher, found influenza significantly increased the risk of hospitalization for heart failure.
Every flu season about 36,000 people die, and > 200,000 are hospitalized due to flu, which is known to be associated with a higher risk of cardiovascular events. Several mechanisms likely contribute: Some form of immunocompromise is thought to be a key link. But few studies, the researchers note, have explored the temporal association between influenza activity and hospitalizations, particularly those caused by heart failure.
In ARIC, the researchers analyzed hospitalization data for adults aged 35 to 84 years between 2010 and 2014 in geographically diverse communities in Mississippi, Minnesota, North Carolina, and Maryland. They correlated those data with reports of influenza activity from the CDC Surveillance Network.
A 5% monthly increase in influenza activity was associated with a 24% relative increase in heart failure hospitalization rates. Myocardial infarction hospitalizations did not rise significantly. The most pneumonia and influenza-associated deaths were during the 2012-2013 season, when influenza-like illness (ILI) activity was highest, and the fewest deaths occurred during 2011-2012, when ILI activity was lowest. The model suggests that in a month with high influenza activity, about 19% of hospitalizations could be attributable to influenza, the researchers say.
“The study’s findings support VA’s aggressive effort every year to provide veterans with influenza vaccine,” said VA Secretary Robert Wilkie. Although the flu season is winding down, he added, it is not too late for veterans—and others—to get vaccinated.
A study of > 450,000 adults has “confirmed the long-held notion” that flu and heart failure are connected. The Atherosclerosis Risk in Communities study (ARIC), led by a VA researcher, found influenza significantly increased the risk of hospitalization for heart failure.
Every flu season about 36,000 people die, and > 200,000 are hospitalized due to flu, which is known to be associated with a higher risk of cardiovascular events. Several mechanisms likely contribute: Some form of immunocompromise is thought to be a key link. But few studies, the researchers note, have explored the temporal association between influenza activity and hospitalizations, particularly those caused by heart failure.
In ARIC, the researchers analyzed hospitalization data for adults aged 35 to 84 years between 2010 and 2014 in geographically diverse communities in Mississippi, Minnesota, North Carolina, and Maryland. They correlated those data with reports of influenza activity from the CDC Surveillance Network.
A 5% monthly increase in influenza activity was associated with a 24% relative increase in heart failure hospitalization rates. Myocardial infarction hospitalizations did not rise significantly. The most pneumonia and influenza-associated deaths were during the 2012-2013 season, when influenza-like illness (ILI) activity was highest, and the fewest deaths occurred during 2011-2012, when ILI activity was lowest. The model suggests that in a month with high influenza activity, about 19% of hospitalizations could be attributable to influenza, the researchers say.
“The study’s findings support VA’s aggressive effort every year to provide veterans with influenza vaccine,” said VA Secretary Robert Wilkie. Although the flu season is winding down, he added, it is not too late for veterans—and others—to get vaccinated.
A study of > 450,000 adults has “confirmed the long-held notion” that flu and heart failure are connected. The Atherosclerosis Risk in Communities study (ARIC), led by a VA researcher, found influenza significantly increased the risk of hospitalization for heart failure.
Every flu season about 36,000 people die, and > 200,000 are hospitalized due to flu, which is known to be associated with a higher risk of cardiovascular events. Several mechanisms likely contribute: Some form of immunocompromise is thought to be a key link. But few studies, the researchers note, have explored the temporal association between influenza activity and hospitalizations, particularly those caused by heart failure.
In ARIC, the researchers analyzed hospitalization data for adults aged 35 to 84 years between 2010 and 2014 in geographically diverse communities in Mississippi, Minnesota, North Carolina, and Maryland. They correlated those data with reports of influenza activity from the CDC Surveillance Network.
A 5% monthly increase in influenza activity was associated with a 24% relative increase in heart failure hospitalization rates. Myocardial infarction hospitalizations did not rise significantly. The most pneumonia and influenza-associated deaths were during the 2012-2013 season, when influenza-like illness (ILI) activity was highest, and the fewest deaths occurred during 2011-2012, when ILI activity was lowest. The model suggests that in a month with high influenza activity, about 19% of hospitalizations could be attributable to influenza, the researchers say.
“The study’s findings support VA’s aggressive effort every year to provide veterans with influenza vaccine,” said VA Secretary Robert Wilkie. Although the flu season is winding down, he added, it is not too late for veterans—and others—to get vaccinated.
Coughing Won’t Pay the Bills
ANSWER
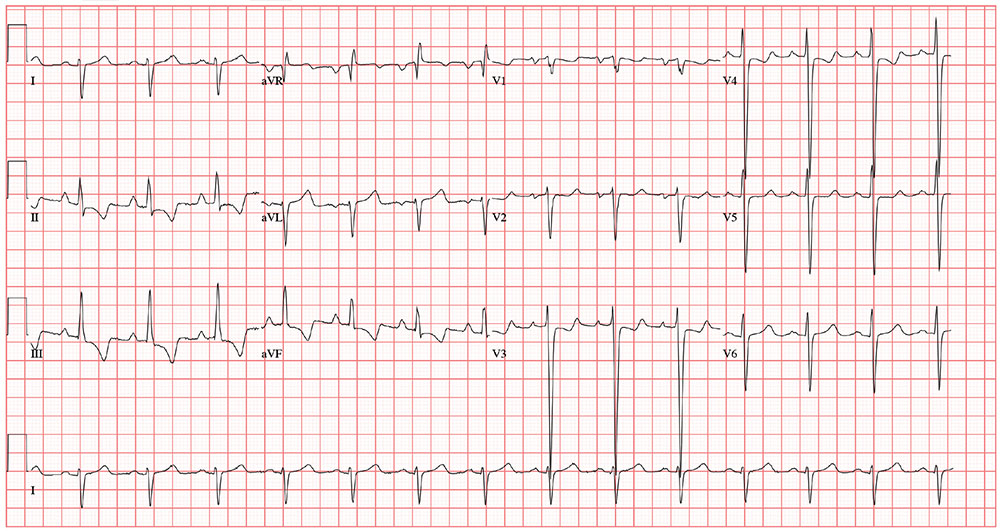
The correct interpretation includes normal sinus rhythm, biatrial enlargement, and right-axis deviation. Other findings include a prolonged QT interval and ST-T wave abnormalities in inferior and anterior distributions.
A P wave
A normal QTc interval is generally 350 to 440 ms; the patient’s QTc interval (477 ms) meets the criteria for prolonged QT. There are ST- and T-wave inversions in leads II, III, and aVF, consistent with inferior ischemia, with ST depressions in leads V3 and V4, which could suggest anterior ischemia. Such findings are also seen in a pulmonary disease pattern.
Further workup included a chest x-ray, laboratory testing, and an echocardiogram. The last revealed right lower lobe consolidation and a diffuse, dilated cardiomyopathy with mild mitral and tricuspid regurgitation.
ANSWER
The correct interpretation includes normal sinus rhythm, biatrial enlargement, and right-axis deviation. Other findings include a prolonged QT interval and ST-T wave abnormalities in inferior and anterior distributions.
A P wave
A normal QTc interval is generally 350 to 440 ms; the patient’s QTc interval (477 ms) meets the criteria for prolonged QT. There are ST- and T-wave inversions in leads II, III, and aVF, consistent with inferior ischemia, with ST depressions in leads V3 and V4, which could suggest anterior ischemia. Such findings are also seen in a pulmonary disease pattern.
Further workup included a chest x-ray, laboratory testing, and an echocardiogram. The last revealed right lower lobe consolidation and a diffuse, dilated cardiomyopathy with mild mitral and tricuspid regurgitation.
ANSWER
The correct interpretation includes normal sinus rhythm, biatrial enlargement, and right-axis deviation. Other findings include a prolonged QT interval and ST-T wave abnormalities in inferior and anterior distributions.
A P wave
A normal QTc interval is generally 350 to 440 ms; the patient’s QTc interval (477 ms) meets the criteria for prolonged QT. There are ST- and T-wave inversions in leads II, III, and aVF, consistent with inferior ischemia, with ST depressions in leads V3 and V4, which could suggest anterior ischemia. Such findings are also seen in a pulmonary disease pattern.
Further workup included a chest x-ray, laboratory testing, and an echocardiogram. The last revealed right lower lobe consolidation and a diffuse, dilated cardiomyopathy with mild mitral and tricuspid regurgitation.
For the past 5 days, a 57-year-old man with “the flu” hasn’t been able to work. This morning, he awoke with chest tightness but denies chest pain. Because work has been busy lately, he just wants an antibiotic to help get him back on the job.
His primary symptom is a persistent, nonproductive cough that prevents him from getting a good night’s sleep. Associated symptoms include rhinorrhea, myalgias and arthralgias, and (for the first 3 days of illness) a low-grade fever. He’s been coughing so hard and for so long that his chest has begun to hurt. His cough is aggravated when lying down and somewhat relieved when sitting upright. He denies wheezing. He presents with specific requests: a chest x-ray to rule out pneumonia and prescriptions for azithromycin and codeine/acetaminophen (the latter for cough suppression).
Past medical history is remarkable for chronic obstructive pulmonary disease (COPD) and chronic bronchitis. Six months ago, a pulmonary function test revealed an FEV1 of 76% and an FEV1/FVC of 60%. He has chronic, mild to moderate shortness of breath with exertion. He has never had pneumonia or any anginal symptoms. Past surgical history is remarkable for an open reduction and internal fixation repair of his right tibia and an appendectomy. Current medications include acetaminophen for fever. He has used an albuterol inhaler in the past but has not had one for more than a year. He has no known drug allergies.
The patient is a crane operator for a large construction business. He is divorced and has an adult son with whom he has no contact. He’s been smoking 1.5 to 2 packs a day since he was 16. He used recreational marijuana until his employer began conducting random drug screenings 3 years ago. He drinks about 2 six-packs of beer a week—most of it on the weekends.
Family history is positive for COPD (mother), acute abdominal aortic dissection (father), and myocardial infarction (paternal grandfather).
The review of systems is positive for myalgias and arthralgias, which have been exacerbated by his recent illness. He denies a productive cough and any changes in heart rhythm and bowel or bladder function. He has no neurologic symptoms.
His vital signs include a blood pressure of 164/96 mm Hg; pulse, 80 beats/min; respiratory rate, 18 breaths/min-1; O2 saturation, 92% on room air; and temperature, 99.8°F.
Physical exam reveals an anxious, tired man in mild distress. His weight is 164 lb and his height, 70 in. The HEENT exam reveals ill-fitting corrective contact lenses, disheveled hair, and a nicotine-stained beard and mustache. His teeth are in poor repair; however, none are loose or missing. The neck is supple, and there is no thyromegaly. There is jugular venous distention, but not to the angle of the jaw. His respirations are shallow, requiring the use of accessory muscles. Deep inhalation causes uncontrollable coughing. Although there is anterior-posterior chest wall enlargement, it is insufficient to consider the patient barrel chested. Auscultation reveals diffuse, coarse rhonchi and end-expiratory wheezing.
The cardiac exam reveals a regular rate and rhythm of 80 beats/min, with a soft diastolic murmur (grade II/VI) that is best heard along the left sternal border. There are no extra heart sounds or rubs. The abdomen is tender to deep palpation in the right upper quadrant, with no evidence of rebound to suggest peritonitis. There are no abdominal or femoral arterial bruits. The extremities demonstrate 2+ pulses bilaterally with 1+ pitting edema in the lower legs. The neurologic exam is grossly intact.
The previously undocumented murmur prompts you to order an ECG. It shows a ventricular rate of 83 beats/min; PR interval, 178 ms; QRS duration, 110 ms; QT/QTc interval, 406/477 ms; P axis, 67°; R axis, 126°; and T axis, –57°. What is your interpretation of this ECG?
Epinephrine linked with more refractory cardiogenic shock after acute MI
Background: Norepinephrine and epinephrine are the most commonly used vasopressors in clinical practice and in septic shock have been found to be equivalent in effectiveness. Their different physiological effects may influence their effectiveness in cardiogenic shock, and previous retrospective studies have suggested that epinephrine may have worse clinical outcomes in this setting.
Study design: A multicenter, prospective, randomized, double-blind study.
Setting: ICUs in nine French hospitals.
Synopsis: Adults (older than 18 years old) who suffered cardiogenic shock following successful revascularization after AMI were enrolled. Fifty-seven patients were randomly assigned to receive either norepinephrine or epinephrine with patients, nurses, and physicians unaware of which study drug was being used. The primary outcome variable was change in cardiac index within the first 72 hours, and refractory cardiogenic shock served as the main safety endpoint. This study was stopped early because of the higher risk of refractory cardiogenic shock noted in the epinephrine group, compared with that seen in the norepinephrine group (10 of 27 vs. 2 of 30; P = .011). There was no difference in evolution of cardiac index (P = .43) between the two groups. Potentially harmful metabolic and physiologic changes were noted in the epinephrine group including greater lactic acidosis and increased heart rate.
This study was underpowered for clinical endpoints because of the study’s early termination. It also did not include patients in cardiogenic shock from other causes, such as myositis or postcardiopulmonary bypass.
Bottom line: For patients in cardiogenic shock after AMI with successful reperfusion, epinephrine use was associated with increased refractory cardiogenic shock, compared with norepinephrine use.
Citation: Levy B et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018 Jul 10;72(2):173-82.
Dr. Witt is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Background: Norepinephrine and epinephrine are the most commonly used vasopressors in clinical practice and in septic shock have been found to be equivalent in effectiveness. Their different physiological effects may influence their effectiveness in cardiogenic shock, and previous retrospective studies have suggested that epinephrine may have worse clinical outcomes in this setting.
Study design: A multicenter, prospective, randomized, double-blind study.
Setting: ICUs in nine French hospitals.
Synopsis: Adults (older than 18 years old) who suffered cardiogenic shock following successful revascularization after AMI were enrolled. Fifty-seven patients were randomly assigned to receive either norepinephrine or epinephrine with patients, nurses, and physicians unaware of which study drug was being used. The primary outcome variable was change in cardiac index within the first 72 hours, and refractory cardiogenic shock served as the main safety endpoint. This study was stopped early because of the higher risk of refractory cardiogenic shock noted in the epinephrine group, compared with that seen in the norepinephrine group (10 of 27 vs. 2 of 30; P = .011). There was no difference in evolution of cardiac index (P = .43) between the two groups. Potentially harmful metabolic and physiologic changes were noted in the epinephrine group including greater lactic acidosis and increased heart rate.
This study was underpowered for clinical endpoints because of the study’s early termination. It also did not include patients in cardiogenic shock from other causes, such as myositis or postcardiopulmonary bypass.
Bottom line: For patients in cardiogenic shock after AMI with successful reperfusion, epinephrine use was associated with increased refractory cardiogenic shock, compared with norepinephrine use.
Citation: Levy B et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018 Jul 10;72(2):173-82.
Dr. Witt is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Background: Norepinephrine and epinephrine are the most commonly used vasopressors in clinical practice and in septic shock have been found to be equivalent in effectiveness. Their different physiological effects may influence their effectiveness in cardiogenic shock, and previous retrospective studies have suggested that epinephrine may have worse clinical outcomes in this setting.
Study design: A multicenter, prospective, randomized, double-blind study.
Setting: ICUs in nine French hospitals.
Synopsis: Adults (older than 18 years old) who suffered cardiogenic shock following successful revascularization after AMI were enrolled. Fifty-seven patients were randomly assigned to receive either norepinephrine or epinephrine with patients, nurses, and physicians unaware of which study drug was being used. The primary outcome variable was change in cardiac index within the first 72 hours, and refractory cardiogenic shock served as the main safety endpoint. This study was stopped early because of the higher risk of refractory cardiogenic shock noted in the epinephrine group, compared with that seen in the norepinephrine group (10 of 27 vs. 2 of 30; P = .011). There was no difference in evolution of cardiac index (P = .43) between the two groups. Potentially harmful metabolic and physiologic changes were noted in the epinephrine group including greater lactic acidosis and increased heart rate.
This study was underpowered for clinical endpoints because of the study’s early termination. It also did not include patients in cardiogenic shock from other causes, such as myositis or postcardiopulmonary bypass.
Bottom line: For patients in cardiogenic shock after AMI with successful reperfusion, epinephrine use was associated with increased refractory cardiogenic shock, compared with norepinephrine use.
Citation: Levy B et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018 Jul 10;72(2):173-82.
Dr. Witt is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Poor response to statins hikes risk of cardiovascular events
About half of patients taking statins for hyperlipidemia don’t adequately respond, leaving them at a 22% increased risk of cardiovascular disease, compared with optimal responders.
Over 6 years, there were about 2,000 more cardiovascular events among those who failed to experience the national treatment target of at least a 40% reduction in LDL cholesterol, according to Stephen F. Weng, MD, and his colleagues. The report is in Heart.
Physicians’ choice of initial statin weighed heavily in the outcomes. Patients who ended up with an optimal response were more likely to get a more potent statin right off, while those with a poorer response were more likely to get a less-potent statin.
“This study provides ‘real world evidence’ that 50% of patients started on statins do not derive the intended therapeutic benefit from them, significantly increasing their risk of future cardiovascular disease,” wrote Dr. Weng of the University of Nottingham, England, and his colleagues. “These findings contribute to the debate on the effectiveness of statin therapy and highlight the need for personalized medicine in lipid management for patients.”
The study comprised 165,411 primary care patients who had hypercholesterolemia but were free of cardiovascular disease at baseline. Statins were prescribed with the goal of at least a 40% reduction in baseline LDL within 24 months of the start of therapy.
Patients had a mean age of 62 years, with a mean baseline LDL of 4.1 mmol/L (158 mg/dL). About 49% were women.
The primary endpoints were the number of patients who did not achieve the 40% or higher reduction in baseline LDL and the between-group risk differences in cardiovascular events (coronary heart disease, stroke or transient ischemic attack, peripheral vascular disease, cardiovascular death).
After 24 months, 51.2% of patients experienced a suboptimal LDL response, with a mean reduction of 2.1 mmol/L (81 mg/dL) compared with 3.1 mmol/L (120 mg/dL). Compared with optimal responders, these patients were significantly more likely to have received a low-potency statin (29% vs. 18%).
Incident cardiovascular events occurred in 14% of the overall group (coronary artery disease, 8%; stroke/TIA, 3%; peripheral vascular disease 1.9%; cardiovascular death, 1%). All of these outcomes were significantly more common among suboptimal responders than optimal responders.
During a mean of 6 years of follow-up, there were 22,798 cardiovascular disease events overall, with significantly more occurring in suboptimal than optimal responders (12,142 vs. 10,656). This translated to a cardiovascular disease rate of 22.6 and 19.7 per 1,000 person-years, respectively.
In a multivariate analysis controlling for age and baseline LDL level, suboptimal responders were 22% more likely to have a cardiovascular disease incident than were optimal responders.
Among suboptimal responders, every unit decrease of 1 mmol/L (39 mg/dL) conferred a significant 6% risk reduction in cardiovascular disease (odds ratio, 0.94).
The benefit was not universal, the authors pointed out. “In this group, the decreased risk remained significant for only stroke/TIA and was not significant for other constituent cardiovascular disease outcomes. However, in patients with an optimal response, an even greater protective effect of LDL reduction and future cardiovascular disease was seen [13%; OR, 0.87],” and this reduction was significant for all of the individual outcomes.
“The study also highlights the benefit of reducing LDL to optimal values, which would lead to better cardiovascular disease outcomes for patients currently on statins,” the authors concluded.
None of the authors had any relevant financial disclosures.
SOURCE: Weng S. et al. Heart 2019 Apr. doi: 10.1136/heartjnl-2018-314253.
Guidelines always look good on paper, but they’re only as good as their implementation, Márcio S. Bittencourt, MD, wrote in an accompanying editorial.
In the United Kingdom, the National Institute for Health and Care Excellence (NICE) guideline pinned effective statin therapy as a lowering of LDL cholesterol by at least 40%. This target aligns well with data accumulated in randomized controlled studies, but it doesn’t benefit patients unless it can be put into practice.
“An important step after a guideline publication is the assessment of its uptake among health practitioners and patients in the real world, as well as of the impact of its adherence on clinical outcomes. These analyses may not only verify its appropriateness, providing feedback for continuous improvement of recommendations, but also identify targets to optimize delivery of health to the society.”
To understand suboptimal statin response, we must understand the many possible reasons behind it – on the part of both physicians and patients.
Physicians may prefer to prescribe low-potency statins for several reasons, including unawareness of guideline recommendations, doubtfulness of better outcomes with higher potent statins or when a lower LDL is attained, and fear of adverse reactions or drug interactions, Dr. Bittencourt noted. “Moreover, doctors may be reluctant to up-titrate drugs when the treatment goals are not achieved, the so-called therapeutic inertia.”
In this study, for example, optimal responders were more likely to initially receive moderately potent statins. Suboptimal responders, on the other hand, were more likely to receive low-potency statins.
“This probably explains why baseline LDL was higher in optimal responders, indicating that higher LDL motivates the physician to be more aggressive upfront.”
Patients bring their own issues to the treatment table.
“Although an inter-individual response to statins may occur according to the genetic background, most cases where LDL response is less than expected are probably due to lack of adherence or persistence to the treatment. ... Of note, poor adherence to lipid-lowering therapy, together with low-intensity therapy, as opposed to high-intensity treatment, is associated with higher cardiovascular risk.”
Effective implementation of guidelines “has been a challenge for a long time. Both physicians and patients should be targets for approaches aiming at improving adherence to guidelines.”
For clinicians, these could include continuing medical education and simplified treatment algorithms. Patients, too, would benefit from some teaching.
“Patients and society should be educated on the scientific evidence documenting the benefits of lipid-lowering therapy, and antistatin propaganda based on pseudoscience should be strongly disavowed and demystified by health authorities.”
Dr. Bittencourt is an internist at the University Hospital San Paolo, Brazil.
Guidelines always look good on paper, but they’re only as good as their implementation, Márcio S. Bittencourt, MD, wrote in an accompanying editorial.
In the United Kingdom, the National Institute for Health and Care Excellence (NICE) guideline pinned effective statin therapy as a lowering of LDL cholesterol by at least 40%. This target aligns well with data accumulated in randomized controlled studies, but it doesn’t benefit patients unless it can be put into practice.
“An important step after a guideline publication is the assessment of its uptake among health practitioners and patients in the real world, as well as of the impact of its adherence on clinical outcomes. These analyses may not only verify its appropriateness, providing feedback for continuous improvement of recommendations, but also identify targets to optimize delivery of health to the society.”
To understand suboptimal statin response, we must understand the many possible reasons behind it – on the part of both physicians and patients.
Physicians may prefer to prescribe low-potency statins for several reasons, including unawareness of guideline recommendations, doubtfulness of better outcomes with higher potent statins or when a lower LDL is attained, and fear of adverse reactions or drug interactions, Dr. Bittencourt noted. “Moreover, doctors may be reluctant to up-titrate drugs when the treatment goals are not achieved, the so-called therapeutic inertia.”
In this study, for example, optimal responders were more likely to initially receive moderately potent statins. Suboptimal responders, on the other hand, were more likely to receive low-potency statins.
“This probably explains why baseline LDL was higher in optimal responders, indicating that higher LDL motivates the physician to be more aggressive upfront.”
Patients bring their own issues to the treatment table.
“Although an inter-individual response to statins may occur according to the genetic background, most cases where LDL response is less than expected are probably due to lack of adherence or persistence to the treatment. ... Of note, poor adherence to lipid-lowering therapy, together with low-intensity therapy, as opposed to high-intensity treatment, is associated with higher cardiovascular risk.”
Effective implementation of guidelines “has been a challenge for a long time. Both physicians and patients should be targets for approaches aiming at improving adherence to guidelines.”
For clinicians, these could include continuing medical education and simplified treatment algorithms. Patients, too, would benefit from some teaching.
“Patients and society should be educated on the scientific evidence documenting the benefits of lipid-lowering therapy, and antistatin propaganda based on pseudoscience should be strongly disavowed and demystified by health authorities.”
Dr. Bittencourt is an internist at the University Hospital San Paolo, Brazil.
Guidelines always look good on paper, but they’re only as good as their implementation, Márcio S. Bittencourt, MD, wrote in an accompanying editorial.
In the United Kingdom, the National Institute for Health and Care Excellence (NICE) guideline pinned effective statin therapy as a lowering of LDL cholesterol by at least 40%. This target aligns well with data accumulated in randomized controlled studies, but it doesn’t benefit patients unless it can be put into practice.
“An important step after a guideline publication is the assessment of its uptake among health practitioners and patients in the real world, as well as of the impact of its adherence on clinical outcomes. These analyses may not only verify its appropriateness, providing feedback for continuous improvement of recommendations, but also identify targets to optimize delivery of health to the society.”
To understand suboptimal statin response, we must understand the many possible reasons behind it – on the part of both physicians and patients.
Physicians may prefer to prescribe low-potency statins for several reasons, including unawareness of guideline recommendations, doubtfulness of better outcomes with higher potent statins or when a lower LDL is attained, and fear of adverse reactions or drug interactions, Dr. Bittencourt noted. “Moreover, doctors may be reluctant to up-titrate drugs when the treatment goals are not achieved, the so-called therapeutic inertia.”
In this study, for example, optimal responders were more likely to initially receive moderately potent statins. Suboptimal responders, on the other hand, were more likely to receive low-potency statins.
“This probably explains why baseline LDL was higher in optimal responders, indicating that higher LDL motivates the physician to be more aggressive upfront.”
Patients bring their own issues to the treatment table.
“Although an inter-individual response to statins may occur according to the genetic background, most cases where LDL response is less than expected are probably due to lack of adherence or persistence to the treatment. ... Of note, poor adherence to lipid-lowering therapy, together with low-intensity therapy, as opposed to high-intensity treatment, is associated with higher cardiovascular risk.”
Effective implementation of guidelines “has been a challenge for a long time. Both physicians and patients should be targets for approaches aiming at improving adherence to guidelines.”
For clinicians, these could include continuing medical education and simplified treatment algorithms. Patients, too, would benefit from some teaching.
“Patients and society should be educated on the scientific evidence documenting the benefits of lipid-lowering therapy, and antistatin propaganda based on pseudoscience should be strongly disavowed and demystified by health authorities.”
Dr. Bittencourt is an internist at the University Hospital San Paolo, Brazil.
About half of patients taking statins for hyperlipidemia don’t adequately respond, leaving them at a 22% increased risk of cardiovascular disease, compared with optimal responders.
Over 6 years, there were about 2,000 more cardiovascular events among those who failed to experience the national treatment target of at least a 40% reduction in LDL cholesterol, according to Stephen F. Weng, MD, and his colleagues. The report is in Heart.
Physicians’ choice of initial statin weighed heavily in the outcomes. Patients who ended up with an optimal response were more likely to get a more potent statin right off, while those with a poorer response were more likely to get a less-potent statin.
“This study provides ‘real world evidence’ that 50% of patients started on statins do not derive the intended therapeutic benefit from them, significantly increasing their risk of future cardiovascular disease,” wrote Dr. Weng of the University of Nottingham, England, and his colleagues. “These findings contribute to the debate on the effectiveness of statin therapy and highlight the need for personalized medicine in lipid management for patients.”
The study comprised 165,411 primary care patients who had hypercholesterolemia but were free of cardiovascular disease at baseline. Statins were prescribed with the goal of at least a 40% reduction in baseline LDL within 24 months of the start of therapy.
Patients had a mean age of 62 years, with a mean baseline LDL of 4.1 mmol/L (158 mg/dL). About 49% were women.
The primary endpoints were the number of patients who did not achieve the 40% or higher reduction in baseline LDL and the between-group risk differences in cardiovascular events (coronary heart disease, stroke or transient ischemic attack, peripheral vascular disease, cardiovascular death).
After 24 months, 51.2% of patients experienced a suboptimal LDL response, with a mean reduction of 2.1 mmol/L (81 mg/dL) compared with 3.1 mmol/L (120 mg/dL). Compared with optimal responders, these patients were significantly more likely to have received a low-potency statin (29% vs. 18%).
Incident cardiovascular events occurred in 14% of the overall group (coronary artery disease, 8%; stroke/TIA, 3%; peripheral vascular disease 1.9%; cardiovascular death, 1%). All of these outcomes were significantly more common among suboptimal responders than optimal responders.
During a mean of 6 years of follow-up, there were 22,798 cardiovascular disease events overall, with significantly more occurring in suboptimal than optimal responders (12,142 vs. 10,656). This translated to a cardiovascular disease rate of 22.6 and 19.7 per 1,000 person-years, respectively.
In a multivariate analysis controlling for age and baseline LDL level, suboptimal responders were 22% more likely to have a cardiovascular disease incident than were optimal responders.
Among suboptimal responders, every unit decrease of 1 mmol/L (39 mg/dL) conferred a significant 6% risk reduction in cardiovascular disease (odds ratio, 0.94).
The benefit was not universal, the authors pointed out. “In this group, the decreased risk remained significant for only stroke/TIA and was not significant for other constituent cardiovascular disease outcomes. However, in patients with an optimal response, an even greater protective effect of LDL reduction and future cardiovascular disease was seen [13%; OR, 0.87],” and this reduction was significant for all of the individual outcomes.
“The study also highlights the benefit of reducing LDL to optimal values, which would lead to better cardiovascular disease outcomes for patients currently on statins,” the authors concluded.
None of the authors had any relevant financial disclosures.
SOURCE: Weng S. et al. Heart 2019 Apr. doi: 10.1136/heartjnl-2018-314253.
About half of patients taking statins for hyperlipidemia don’t adequately respond, leaving them at a 22% increased risk of cardiovascular disease, compared with optimal responders.
Over 6 years, there were about 2,000 more cardiovascular events among those who failed to experience the national treatment target of at least a 40% reduction in LDL cholesterol, according to Stephen F. Weng, MD, and his colleagues. The report is in Heart.
Physicians’ choice of initial statin weighed heavily in the outcomes. Patients who ended up with an optimal response were more likely to get a more potent statin right off, while those with a poorer response were more likely to get a less-potent statin.
“This study provides ‘real world evidence’ that 50% of patients started on statins do not derive the intended therapeutic benefit from them, significantly increasing their risk of future cardiovascular disease,” wrote Dr. Weng of the University of Nottingham, England, and his colleagues. “These findings contribute to the debate on the effectiveness of statin therapy and highlight the need for personalized medicine in lipid management for patients.”
The study comprised 165,411 primary care patients who had hypercholesterolemia but were free of cardiovascular disease at baseline. Statins were prescribed with the goal of at least a 40% reduction in baseline LDL within 24 months of the start of therapy.
Patients had a mean age of 62 years, with a mean baseline LDL of 4.1 mmol/L (158 mg/dL). About 49% were women.
The primary endpoints were the number of patients who did not achieve the 40% or higher reduction in baseline LDL and the between-group risk differences in cardiovascular events (coronary heart disease, stroke or transient ischemic attack, peripheral vascular disease, cardiovascular death).
After 24 months, 51.2% of patients experienced a suboptimal LDL response, with a mean reduction of 2.1 mmol/L (81 mg/dL) compared with 3.1 mmol/L (120 mg/dL). Compared with optimal responders, these patients were significantly more likely to have received a low-potency statin (29% vs. 18%).
Incident cardiovascular events occurred in 14% of the overall group (coronary artery disease, 8%; stroke/TIA, 3%; peripheral vascular disease 1.9%; cardiovascular death, 1%). All of these outcomes were significantly more common among suboptimal responders than optimal responders.
During a mean of 6 years of follow-up, there were 22,798 cardiovascular disease events overall, with significantly more occurring in suboptimal than optimal responders (12,142 vs. 10,656). This translated to a cardiovascular disease rate of 22.6 and 19.7 per 1,000 person-years, respectively.
In a multivariate analysis controlling for age and baseline LDL level, suboptimal responders were 22% more likely to have a cardiovascular disease incident than were optimal responders.
Among suboptimal responders, every unit decrease of 1 mmol/L (39 mg/dL) conferred a significant 6% risk reduction in cardiovascular disease (odds ratio, 0.94).
The benefit was not universal, the authors pointed out. “In this group, the decreased risk remained significant for only stroke/TIA and was not significant for other constituent cardiovascular disease outcomes. However, in patients with an optimal response, an even greater protective effect of LDL reduction and future cardiovascular disease was seen [13%; OR, 0.87],” and this reduction was significant for all of the individual outcomes.
“The study also highlights the benefit of reducing LDL to optimal values, which would lead to better cardiovascular disease outcomes for patients currently on statins,” the authors concluded.
None of the authors had any relevant financial disclosures.
SOURCE: Weng S. et al. Heart 2019 Apr. doi: 10.1136/heartjnl-2018-314253.
FROM HEART
Statin exposure associated with idiopathic inflammatory myositis
Clinical question: What is the association between exposure to statin medications and histologically confirmed idiopathic inflammatory myositis?
Background: More than 200 million people worldwide use statin therapy, mostly for cardiovascular risk reduction. There is mounting evidence of an infrequent side effect known as idiopathic inflammatory myositis (IIM), that requires immunosuppressive therapy rather than just discontinuation of the medication. While there is a recently described association of statin use with an immune-mediated necrotizing myositis through the formation of an autoantibody against HMG-CoA Reductase, this epidemiological study aimed to look at the incidence of statin use against all confirmed cases of IIM.
Study design: Retrospective, population-based, case-control study.
Setting: Northwest Adelaide Health Study in Adelaide, Australia.
Synopsis: A retrospective, population-based, case-control study was conducted that compared the incidence of histologically confirmed IIM identified from the South Australian Myositis Database in patients 40 years or older with known statin exposure (n = 221) against population-based controls obtained from the North West Adelaide Health Study. The unadjusted and adjusted odds ratios and 95% confidence intervals were calculated using the conditional logistic regression analysis for the risk of statin exposure associated with IIM. There was an almost twofold (79%) increased likelihood of statin exposure in patients with IIM by comparison with controls (adjusted OR, 1.79; 95% CI, 1.23-2.60; P = .001). This study’s results indicate that patients with histologically confirmed IIM had a significantly increased likelihood of statin exposure, compared with population-based matched controls. Results were similar even when excluding necrotizing myositis, which already has a known association with statin use, which suggests that statin use could be associated with all types of IIM.
Bottom line: There was a statistically significant association between statin use and the incidence of idiopathic inflammatory myositis, which suggests that this condition is a potential serious side effect of statin therapy.
Citation: Caughey GE et al. Association of statin exposure with histologically confirmed idiopathic inflammatory myositis in an Australian population. JAMA Intern Med. 2018 Jul 30. doi: 10.1001/jamainternmed.2018.2859.
Dr. Nave is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Clinical question: What is the association between exposure to statin medications and histologically confirmed idiopathic inflammatory myositis?
Background: More than 200 million people worldwide use statin therapy, mostly for cardiovascular risk reduction. There is mounting evidence of an infrequent side effect known as idiopathic inflammatory myositis (IIM), that requires immunosuppressive therapy rather than just discontinuation of the medication. While there is a recently described association of statin use with an immune-mediated necrotizing myositis through the formation of an autoantibody against HMG-CoA Reductase, this epidemiological study aimed to look at the incidence of statin use against all confirmed cases of IIM.
Study design: Retrospective, population-based, case-control study.
Setting: Northwest Adelaide Health Study in Adelaide, Australia.
Synopsis: A retrospective, population-based, case-control study was conducted that compared the incidence of histologically confirmed IIM identified from the South Australian Myositis Database in patients 40 years or older with known statin exposure (n = 221) against population-based controls obtained from the North West Adelaide Health Study. The unadjusted and adjusted odds ratios and 95% confidence intervals were calculated using the conditional logistic regression analysis for the risk of statin exposure associated with IIM. There was an almost twofold (79%) increased likelihood of statin exposure in patients with IIM by comparison with controls (adjusted OR, 1.79; 95% CI, 1.23-2.60; P = .001). This study’s results indicate that patients with histologically confirmed IIM had a significantly increased likelihood of statin exposure, compared with population-based matched controls. Results were similar even when excluding necrotizing myositis, which already has a known association with statin use, which suggests that statin use could be associated with all types of IIM.
Bottom line: There was a statistically significant association between statin use and the incidence of idiopathic inflammatory myositis, which suggests that this condition is a potential serious side effect of statin therapy.
Citation: Caughey GE et al. Association of statin exposure with histologically confirmed idiopathic inflammatory myositis in an Australian population. JAMA Intern Med. 2018 Jul 30. doi: 10.1001/jamainternmed.2018.2859.
Dr. Nave is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Clinical question: What is the association between exposure to statin medications and histologically confirmed idiopathic inflammatory myositis?
Background: More than 200 million people worldwide use statin therapy, mostly for cardiovascular risk reduction. There is mounting evidence of an infrequent side effect known as idiopathic inflammatory myositis (IIM), that requires immunosuppressive therapy rather than just discontinuation of the medication. While there is a recently described association of statin use with an immune-mediated necrotizing myositis through the formation of an autoantibody against HMG-CoA Reductase, this epidemiological study aimed to look at the incidence of statin use against all confirmed cases of IIM.
Study design: Retrospective, population-based, case-control study.
Setting: Northwest Adelaide Health Study in Adelaide, Australia.
Synopsis: A retrospective, population-based, case-control study was conducted that compared the incidence of histologically confirmed IIM identified from the South Australian Myositis Database in patients 40 years or older with known statin exposure (n = 221) against population-based controls obtained from the North West Adelaide Health Study. The unadjusted and adjusted odds ratios and 95% confidence intervals were calculated using the conditional logistic regression analysis for the risk of statin exposure associated with IIM. There was an almost twofold (79%) increased likelihood of statin exposure in patients with IIM by comparison with controls (adjusted OR, 1.79; 95% CI, 1.23-2.60; P = .001). This study’s results indicate that patients with histologically confirmed IIM had a significantly increased likelihood of statin exposure, compared with population-based matched controls. Results were similar even when excluding necrotizing myositis, which already has a known association with statin use, which suggests that statin use could be associated with all types of IIM.
Bottom line: There was a statistically significant association between statin use and the incidence of idiopathic inflammatory myositis, which suggests that this condition is a potential serious side effect of statin therapy.
Citation: Caughey GE et al. Association of statin exposure with histologically confirmed idiopathic inflammatory myositis in an Australian population. JAMA Intern Med. 2018 Jul 30. doi: 10.1001/jamainternmed.2018.2859.
Dr. Nave is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Canagliflozin lowers kidney failure risk in T2D: CREDENCE
Patients with type 2 diabetes and chronic kidney disease (CKD) show significantly lower incidence of kidney failure and cardiovascular events after treatment with the sodium-glucose cotransporter 2 inhibitor canagliflozin, in the CREDENCE trial.
CREDENCE (Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy) is a double-blind, placebo-controlled trial involving 4,401 patients with type 2 diabetes and albuminuric CKD, who were randomized to either 100 mg of canagliflozin daily or placebo.
After a median follow-up of 2.62 years, there was a significant 30% lower risk of the primary outcome, which was a composite of end-stage kidney disease, a doubling of serum creatinine, or death from renal or cardiovascular causes, a highly significant difference at P = .00001.
Separately, there was a 32% lower risk of end-stage kidney disease, a 20% lower risk of cardiovascular death, MI, or stroke, and a 39% lower risk of hospitalization for heart failure, both significant differences. Patients treated with canagliflozin also had a 40% lower risk of a doubling of serum creatinine, and a 28% lower risk of dialysis, kidney transplantation, or renal death.
“These findings were observed despite very modest between-group differences in blood glucose level, weight, and blood pressure, and in contrast to previous concern about the initial acute reduction in the estimated GFR [glomerular filtration rate] observed with SGLT2 inhibitors,” wrote Vlado Perkovic, MD, from the George Institute for Global Health, University of New South Wales Sydney, and his coauthors. “This suggests that the mechanism of benefit is likely to be independent of glucose levels and may possibly stem from a reduction in intraglomerular pressure, with other possible mechanisms presently being studied.”
The trial was stopped early after reaching the prespecified efficacy criteria for early cessation. The authors estimated that 21.2 patients would need to be treated with canagliflozin to prevent one primary outcome.
There were no significant differences between the two groups in the rate of adverse and serious adverse events, including the risk of lower limb amputation and fracture.
The study was supported by Janssen Research and Development. Eighteen authors declared steering committee, support and consultancies with Janssen, and thirteen also declared personal fees from other pharmaceutical and private industry. Five authors were employees of Janssen.
SOURCE: Perkovic V et al. N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMoa1811744.
Sodium-glucose cotransporter 2 inhibitors are the most promising of a number of diabetes medications that have shown potential in renoprotection through a mechanism other than glucose homeostasis.
The study suggests canagliflozin’s effects are felt both in the renal system and systemically. The initial decrease in glomerular filtration rate in the first few weeks of treatment could be the result of decreases in glomerular perfusion and intraglomerular pressure, but this effect does stabilize. Levels of angiotensin II and atrial natriuretic peptide decrease, and there is also a decrease in inflammation and an increase in intrarenal oxygenation.
These findings are good news for patients with diabetes and chronic kidney disease, and their importance cannot be overstated.
Julie R. Ingelfinger, MD, is from the Tufts University in Boston, and Clifford J. Rosen, MD, is from the Center for Clinical and Translational Research, Maine Medical Center Research Institute, Scarborough. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMe1904740).
Sodium-glucose cotransporter 2 inhibitors are the most promising of a number of diabetes medications that have shown potential in renoprotection through a mechanism other than glucose homeostasis.
The study suggests canagliflozin’s effects are felt both in the renal system and systemically. The initial decrease in glomerular filtration rate in the first few weeks of treatment could be the result of decreases in glomerular perfusion and intraglomerular pressure, but this effect does stabilize. Levels of angiotensin II and atrial natriuretic peptide decrease, and there is also a decrease in inflammation and an increase in intrarenal oxygenation.
These findings are good news for patients with diabetes and chronic kidney disease, and their importance cannot be overstated.
Julie R. Ingelfinger, MD, is from the Tufts University in Boston, and Clifford J. Rosen, MD, is from the Center for Clinical and Translational Research, Maine Medical Center Research Institute, Scarborough. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMe1904740).
Sodium-glucose cotransporter 2 inhibitors are the most promising of a number of diabetes medications that have shown potential in renoprotection through a mechanism other than glucose homeostasis.
The study suggests canagliflozin’s effects are felt both in the renal system and systemically. The initial decrease in glomerular filtration rate in the first few weeks of treatment could be the result of decreases in glomerular perfusion and intraglomerular pressure, but this effect does stabilize. Levels of angiotensin II and atrial natriuretic peptide decrease, and there is also a decrease in inflammation and an increase in intrarenal oxygenation.
These findings are good news for patients with diabetes and chronic kidney disease, and their importance cannot be overstated.
Julie R. Ingelfinger, MD, is from the Tufts University in Boston, and Clifford J. Rosen, MD, is from the Center for Clinical and Translational Research, Maine Medical Center Research Institute, Scarborough. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMe1904740).
Patients with type 2 diabetes and chronic kidney disease (CKD) show significantly lower incidence of kidney failure and cardiovascular events after treatment with the sodium-glucose cotransporter 2 inhibitor canagliflozin, in the CREDENCE trial.
CREDENCE (Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy) is a double-blind, placebo-controlled trial involving 4,401 patients with type 2 diabetes and albuminuric CKD, who were randomized to either 100 mg of canagliflozin daily or placebo.
After a median follow-up of 2.62 years, there was a significant 30% lower risk of the primary outcome, which was a composite of end-stage kidney disease, a doubling of serum creatinine, or death from renal or cardiovascular causes, a highly significant difference at P = .00001.
Separately, there was a 32% lower risk of end-stage kidney disease, a 20% lower risk of cardiovascular death, MI, or stroke, and a 39% lower risk of hospitalization for heart failure, both significant differences. Patients treated with canagliflozin also had a 40% lower risk of a doubling of serum creatinine, and a 28% lower risk of dialysis, kidney transplantation, or renal death.
“These findings were observed despite very modest between-group differences in blood glucose level, weight, and blood pressure, and in contrast to previous concern about the initial acute reduction in the estimated GFR [glomerular filtration rate] observed with SGLT2 inhibitors,” wrote Vlado Perkovic, MD, from the George Institute for Global Health, University of New South Wales Sydney, and his coauthors. “This suggests that the mechanism of benefit is likely to be independent of glucose levels and may possibly stem from a reduction in intraglomerular pressure, with other possible mechanisms presently being studied.”
The trial was stopped early after reaching the prespecified efficacy criteria for early cessation. The authors estimated that 21.2 patients would need to be treated with canagliflozin to prevent one primary outcome.
There were no significant differences between the two groups in the rate of adverse and serious adverse events, including the risk of lower limb amputation and fracture.
The study was supported by Janssen Research and Development. Eighteen authors declared steering committee, support and consultancies with Janssen, and thirteen also declared personal fees from other pharmaceutical and private industry. Five authors were employees of Janssen.
SOURCE: Perkovic V et al. N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMoa1811744.
Patients with type 2 diabetes and chronic kidney disease (CKD) show significantly lower incidence of kidney failure and cardiovascular events after treatment with the sodium-glucose cotransporter 2 inhibitor canagliflozin, in the CREDENCE trial.
CREDENCE (Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy) is a double-blind, placebo-controlled trial involving 4,401 patients with type 2 diabetes and albuminuric CKD, who were randomized to either 100 mg of canagliflozin daily or placebo.
After a median follow-up of 2.62 years, there was a significant 30% lower risk of the primary outcome, which was a composite of end-stage kidney disease, a doubling of serum creatinine, or death from renal or cardiovascular causes, a highly significant difference at P = .00001.
Separately, there was a 32% lower risk of end-stage kidney disease, a 20% lower risk of cardiovascular death, MI, or stroke, and a 39% lower risk of hospitalization for heart failure, both significant differences. Patients treated with canagliflozin also had a 40% lower risk of a doubling of serum creatinine, and a 28% lower risk of dialysis, kidney transplantation, or renal death.
“These findings were observed despite very modest between-group differences in blood glucose level, weight, and blood pressure, and in contrast to previous concern about the initial acute reduction in the estimated GFR [glomerular filtration rate] observed with SGLT2 inhibitors,” wrote Vlado Perkovic, MD, from the George Institute for Global Health, University of New South Wales Sydney, and his coauthors. “This suggests that the mechanism of benefit is likely to be independent of glucose levels and may possibly stem from a reduction in intraglomerular pressure, with other possible mechanisms presently being studied.”
The trial was stopped early after reaching the prespecified efficacy criteria for early cessation. The authors estimated that 21.2 patients would need to be treated with canagliflozin to prevent one primary outcome.
There were no significant differences between the two groups in the rate of adverse and serious adverse events, including the risk of lower limb amputation and fracture.
The study was supported by Janssen Research and Development. Eighteen authors declared steering committee, support and consultancies with Janssen, and thirteen also declared personal fees from other pharmaceutical and private industry. Five authors were employees of Janssen.
SOURCE: Perkovic V et al. N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMoa1811744.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
PIONEER-HF Extension: Don’t stall starting sacubitril/valsartan
NEW ORLEANS – Waiting a few months after a patient has been hospitalized for acute decompensated heart failure before launching a switch from enalapril to sacubitril/valsartan imposes a steep price in terms of extra major cardiovascular events, compared with starting the angiotensin-neprilysin inhibitor during the initial hospitalization, according to the open-label extension of the PIONEER-HF trial.
“We think these data have important clinical implications: While sacubitril/valsartan decreases NT-proBNP compared with enalapril regardless of when it is initiated, the early improvement in postdischarge outcomes supports the in-hospital initiation of sacubitril/valsartan in stabilized patients with acute decompensated heart failure,” Adam D. DeVore, MD, declared in presenting the PIONEER-HF Extension results at the annual meeting of the American College of Cardiology.
PIONEER-HF was a landmark, practice-changing, double-blind clinical trial in which 881 patients were randomized to initiation of sacubitril/valsartan (Entresto) or enalapril during hospitalization for acute decompensated heart failure. In the previously reported main outcome, 8 weeks after discharge the sacubitril/valsartan group had a 29% greater reduction in NT-proBNP (the N-terminal prohormone of brain natriuretic peptide) and a 42% lower rate of the composite clinical endpoint of cardiovascular death or heart failure rehospitalization than the enalapril group (N Engl J Med. 2019 Feb 7;380[6]:539-48).
The 4-week open-label extension of PIONEER-HF began at week 8, when participants initially randomized to enalapril during the double-blind phase were switched to sacubitril/valsartan, while those assigned to in-hospital initiation of the angiotensin-neprilysin inhibitor (ARNI) stayed the course.
At week 12, after 4 weeks of open-label treatment, patients on sacubitril/valsartan from the start experienced an additional 18.5% drop in NT-proBNP from their week-8 baseline of 1,218 pg/mL. Meanwhile, the NT-proBNP level in the switch group plunged by 35.8% from a week-8 baseline of 1,630 pg/mL. As a result, both groups ended up at the same much-improved biomarker level at week 12, observed Dr. DeVore, a cardiologist at Duke University in Durham, N.C.
Clinical event rates, however, were another story altogether. The clinical event gap between the two study arms documented at week 8 in the double-blind phase of the trial didn’t close significantly in the 4 weeks after the enalapril group crossed over to open-label sacubitril/valsartan. Indeed, the relative risk of the composite endpoint of cardiovascular death, heart failure rehospitalization, or left ventricular assist device implantation during the 4-week extension phase was 33% lower in the continuous sacubitril/valsartan group than in the switchers. The absolute risk reduction was 5.6%, with a favorable number needed to treat of 18.
This difference was driven mainly by less rehospitalization for heart failure. Few cardiovascular deaths or LVAD implantations occurred during the relatively brief 4-week extension phase of the trial.
“But this is an important thing as we think about what we’re trying to accomplish in heart failure: trying to find tools that improve rehospitalization rates after people leave the hospital is extremely important,” Dr. DeVore said. “We do know that the really vulnerable period for rehospitalization is early on, so my sus```picion – though I can’t prove it – is that’s the important part. That’s when we need to have patients on the best therapy.”
He was asked how practical it is to initiate sacubitril/valsartan during hospitalization for acute decompensated heart failure in real-world clinical practice, given that it can be done only after patients achieve hemodynamic stability.
“I think the definition of hemodynamic stability we used in the trial was a fairly straightforward one, very clinical, and one we can translate to the bedside,” Dr. DeVore replied. “Patients had to have a systolic blood pressure of 100 mm Hg or greater for 6 hours, which is easily documented in the hospital, no changes in IV diuretics or IV vasodilators for 6 hours, and no IV inotropes for the last 24 hours. That’s how we defined hemodynamic stability. I think we should be able to find these patients.”
Average length of stay in the index hospitalization in PIONEER-HF was just over 5 days, but the study protocol actually resulted in longer-than-needed hospitalization because it required that patients had to receive three double-blind doses of their study medication before discharge. In routine practice, it’s unlikely that in-hospital initiation of sacubitril/valsartan will result in a length of stay greater than the national average of about 4.5 days, according the cardiologist.
Current ACC/American Heart Association/Heart Failure Society of American guidelines on management of heart failure include a Class Ia recommendation to switch patients from an ACE inhibitor or angiotensin inhibitor to sacubitril/valsartan (Circulation. 2017 Aug 8;136[6]:e137-61).
However, heart failure specialists are concerned by national data showing that sacubitril/valsartan remains widely underprescribed.
Dr. DeVore reported serving as a consultant to Novartis and receiving research grants from a half dozen pharmaceutical companies as well as the American Heart Association, National Heart, Lung, and Blood Institute, and the Patient-Centered Outcomes Research Institute .
NEW ORLEANS – Waiting a few months after a patient has been hospitalized for acute decompensated heart failure before launching a switch from enalapril to sacubitril/valsartan imposes a steep price in terms of extra major cardiovascular events, compared with starting the angiotensin-neprilysin inhibitor during the initial hospitalization, according to the open-label extension of the PIONEER-HF trial.
“We think these data have important clinical implications: While sacubitril/valsartan decreases NT-proBNP compared with enalapril regardless of when it is initiated, the early improvement in postdischarge outcomes supports the in-hospital initiation of sacubitril/valsartan in stabilized patients with acute decompensated heart failure,” Adam D. DeVore, MD, declared in presenting the PIONEER-HF Extension results at the annual meeting of the American College of Cardiology.
PIONEER-HF was a landmark, practice-changing, double-blind clinical trial in which 881 patients were randomized to initiation of sacubitril/valsartan (Entresto) or enalapril during hospitalization for acute decompensated heart failure. In the previously reported main outcome, 8 weeks after discharge the sacubitril/valsartan group had a 29% greater reduction in NT-proBNP (the N-terminal prohormone of brain natriuretic peptide) and a 42% lower rate of the composite clinical endpoint of cardiovascular death or heart failure rehospitalization than the enalapril group (N Engl J Med. 2019 Feb 7;380[6]:539-48).
The 4-week open-label extension of PIONEER-HF began at week 8, when participants initially randomized to enalapril during the double-blind phase were switched to sacubitril/valsartan, while those assigned to in-hospital initiation of the angiotensin-neprilysin inhibitor (ARNI) stayed the course.
At week 12, after 4 weeks of open-label treatment, patients on sacubitril/valsartan from the start experienced an additional 18.5% drop in NT-proBNP from their week-8 baseline of 1,218 pg/mL. Meanwhile, the NT-proBNP level in the switch group plunged by 35.8% from a week-8 baseline of 1,630 pg/mL. As a result, both groups ended up at the same much-improved biomarker level at week 12, observed Dr. DeVore, a cardiologist at Duke University in Durham, N.C.
Clinical event rates, however, were another story altogether. The clinical event gap between the two study arms documented at week 8 in the double-blind phase of the trial didn’t close significantly in the 4 weeks after the enalapril group crossed over to open-label sacubitril/valsartan. Indeed, the relative risk of the composite endpoint of cardiovascular death, heart failure rehospitalization, or left ventricular assist device implantation during the 4-week extension phase was 33% lower in the continuous sacubitril/valsartan group than in the switchers. The absolute risk reduction was 5.6%, with a favorable number needed to treat of 18.
This difference was driven mainly by less rehospitalization for heart failure. Few cardiovascular deaths or LVAD implantations occurred during the relatively brief 4-week extension phase of the trial.
“But this is an important thing as we think about what we’re trying to accomplish in heart failure: trying to find tools that improve rehospitalization rates after people leave the hospital is extremely important,” Dr. DeVore said. “We do know that the really vulnerable period for rehospitalization is early on, so my sus```picion – though I can’t prove it – is that’s the important part. That’s when we need to have patients on the best therapy.”
He was asked how practical it is to initiate sacubitril/valsartan during hospitalization for acute decompensated heart failure in real-world clinical practice, given that it can be done only after patients achieve hemodynamic stability.
“I think the definition of hemodynamic stability we used in the trial was a fairly straightforward one, very clinical, and one we can translate to the bedside,” Dr. DeVore replied. “Patients had to have a systolic blood pressure of 100 mm Hg or greater for 6 hours, which is easily documented in the hospital, no changes in IV diuretics or IV vasodilators for 6 hours, and no IV inotropes for the last 24 hours. That’s how we defined hemodynamic stability. I think we should be able to find these patients.”
Average length of stay in the index hospitalization in PIONEER-HF was just over 5 days, but the study protocol actually resulted in longer-than-needed hospitalization because it required that patients had to receive three double-blind doses of their study medication before discharge. In routine practice, it’s unlikely that in-hospital initiation of sacubitril/valsartan will result in a length of stay greater than the national average of about 4.5 days, according the cardiologist.
Current ACC/American Heart Association/Heart Failure Society of American guidelines on management of heart failure include a Class Ia recommendation to switch patients from an ACE inhibitor or angiotensin inhibitor to sacubitril/valsartan (Circulation. 2017 Aug 8;136[6]:e137-61).
However, heart failure specialists are concerned by national data showing that sacubitril/valsartan remains widely underprescribed.
Dr. DeVore reported serving as a consultant to Novartis and receiving research grants from a half dozen pharmaceutical companies as well as the American Heart Association, National Heart, Lung, and Blood Institute, and the Patient-Centered Outcomes Research Institute .
NEW ORLEANS – Waiting a few months after a patient has been hospitalized for acute decompensated heart failure before launching a switch from enalapril to sacubitril/valsartan imposes a steep price in terms of extra major cardiovascular events, compared with starting the angiotensin-neprilysin inhibitor during the initial hospitalization, according to the open-label extension of the PIONEER-HF trial.
“We think these data have important clinical implications: While sacubitril/valsartan decreases NT-proBNP compared with enalapril regardless of when it is initiated, the early improvement in postdischarge outcomes supports the in-hospital initiation of sacubitril/valsartan in stabilized patients with acute decompensated heart failure,” Adam D. DeVore, MD, declared in presenting the PIONEER-HF Extension results at the annual meeting of the American College of Cardiology.
PIONEER-HF was a landmark, practice-changing, double-blind clinical trial in which 881 patients were randomized to initiation of sacubitril/valsartan (Entresto) or enalapril during hospitalization for acute decompensated heart failure. In the previously reported main outcome, 8 weeks after discharge the sacubitril/valsartan group had a 29% greater reduction in NT-proBNP (the N-terminal prohormone of brain natriuretic peptide) and a 42% lower rate of the composite clinical endpoint of cardiovascular death or heart failure rehospitalization than the enalapril group (N Engl J Med. 2019 Feb 7;380[6]:539-48).
The 4-week open-label extension of PIONEER-HF began at week 8, when participants initially randomized to enalapril during the double-blind phase were switched to sacubitril/valsartan, while those assigned to in-hospital initiation of the angiotensin-neprilysin inhibitor (ARNI) stayed the course.
At week 12, after 4 weeks of open-label treatment, patients on sacubitril/valsartan from the start experienced an additional 18.5% drop in NT-proBNP from their week-8 baseline of 1,218 pg/mL. Meanwhile, the NT-proBNP level in the switch group plunged by 35.8% from a week-8 baseline of 1,630 pg/mL. As a result, both groups ended up at the same much-improved biomarker level at week 12, observed Dr. DeVore, a cardiologist at Duke University in Durham, N.C.
Clinical event rates, however, were another story altogether. The clinical event gap between the two study arms documented at week 8 in the double-blind phase of the trial didn’t close significantly in the 4 weeks after the enalapril group crossed over to open-label sacubitril/valsartan. Indeed, the relative risk of the composite endpoint of cardiovascular death, heart failure rehospitalization, or left ventricular assist device implantation during the 4-week extension phase was 33% lower in the continuous sacubitril/valsartan group than in the switchers. The absolute risk reduction was 5.6%, with a favorable number needed to treat of 18.
This difference was driven mainly by less rehospitalization for heart failure. Few cardiovascular deaths or LVAD implantations occurred during the relatively brief 4-week extension phase of the trial.
“But this is an important thing as we think about what we’re trying to accomplish in heart failure: trying to find tools that improve rehospitalization rates after people leave the hospital is extremely important,” Dr. DeVore said. “We do know that the really vulnerable period for rehospitalization is early on, so my sus```picion – though I can’t prove it – is that’s the important part. That’s when we need to have patients on the best therapy.”
He was asked how practical it is to initiate sacubitril/valsartan during hospitalization for acute decompensated heart failure in real-world clinical practice, given that it can be done only after patients achieve hemodynamic stability.
“I think the definition of hemodynamic stability we used in the trial was a fairly straightforward one, very clinical, and one we can translate to the bedside,” Dr. DeVore replied. “Patients had to have a systolic blood pressure of 100 mm Hg or greater for 6 hours, which is easily documented in the hospital, no changes in IV diuretics or IV vasodilators for 6 hours, and no IV inotropes for the last 24 hours. That’s how we defined hemodynamic stability. I think we should be able to find these patients.”
Average length of stay in the index hospitalization in PIONEER-HF was just over 5 days, but the study protocol actually resulted in longer-than-needed hospitalization because it required that patients had to receive three double-blind doses of their study medication before discharge. In routine practice, it’s unlikely that in-hospital initiation of sacubitril/valsartan will result in a length of stay greater than the national average of about 4.5 days, according the cardiologist.
Current ACC/American Heart Association/Heart Failure Society of American guidelines on management of heart failure include a Class Ia recommendation to switch patients from an ACE inhibitor or angiotensin inhibitor to sacubitril/valsartan (Circulation. 2017 Aug 8;136[6]:e137-61).
However, heart failure specialists are concerned by national data showing that sacubitril/valsartan remains widely underprescribed.
Dr. DeVore reported serving as a consultant to Novartis and receiving research grants from a half dozen pharmaceutical companies as well as the American Heart Association, National Heart, Lung, and Blood Institute, and the Patient-Centered Outcomes Research Institute .
REPORTING FROM ACC 19
Key clinical point: over the next 12 weeks, compared with initiation of enalapril followed by a delayed switch to sacubitril/valsartan at 8 weeks.
Major finding: The number needed to treat with in-hospital initiation of sacubitril/valsartan instead of enalapril to avoid one cardiovascular death, heart failure rehospitalization, or implantation of a left ventricular assist device was 18.
Study details: The PIONEER-HF Extension study included 881 heart failure patients, all on open-label sacubitril/valsartan during the 4-week extension phase.
Disclosures: The study was sponsored by AstraZeneca. The presenter reported receiving research grants from and serving as a consultant to the company.
Highlights from the ‘Updates in ACS’ session (VIDEO)
Hospital Medicine 2019 attendees outlined their key takeaways from the Updates in Acute Coronary Syndrome session, presented by Jeffrey Trost, MD, of Johns Hopkins University, Baltimore.

Dr. Trost’s discussion focused on the relationship between dual antiplatelet therapy, in-stent thrombosis, and in-stent restenosis. He also explored the diagnostic role of fractional flow reserve, and he outlined effective approaches to PCSK9 inhibitor use.
Hospital Medicine 2019 attendees outlined their key takeaways from the Updates in Acute Coronary Syndrome session, presented by Jeffrey Trost, MD, of Johns Hopkins University, Baltimore.

Dr. Trost’s discussion focused on the relationship between dual antiplatelet therapy, in-stent thrombosis, and in-stent restenosis. He also explored the diagnostic role of fractional flow reserve, and he outlined effective approaches to PCSK9 inhibitor use.
Hospital Medicine 2019 attendees outlined their key takeaways from the Updates in Acute Coronary Syndrome session, presented by Jeffrey Trost, MD, of Johns Hopkins University, Baltimore.

Dr. Trost’s discussion focused on the relationship between dual antiplatelet therapy, in-stent thrombosis, and in-stent restenosis. He also explored the diagnostic role of fractional flow reserve, and he outlined effective approaches to PCSK9 inhibitor use.
REPORTING FROM HM19
Low LDL cholesterol may increase women’s risk of hemorrhagic stroke
, according to research published in Neurology.
“Women with very low LDL cholesterol or low triglycerides should be monitored by their doctors for other stroke risk factors that can be modified, like high blood pressure and smoking, in order to reduce their risk of hemorrhagic stroke,” said Pamela M. Rist, ScD, instructor in epidemiology at Harvard Medical School, Boston. “Additional research is needed to determine how to lower the risk of hemorrhagic stroke in women with very low LDL and low triglycerides.”
Several meta-analyses have indicated that LDL cholesterol levels are inversely associated with the risk of hemorrhagic stroke. Because lipid-lowering treatments are used to prevent cardiovascular disease, this potential association has implications for clinical practice. Most of the studies included in these meta-analyses had low numbers of events among women, which prevented researchers from stratifying their results by sex. Because women are at greater risk of stroke than men, Dr. Rist and her colleagues sought to evaluate the association between lipid levels and risk of hemorrhagic stroke.
An analysis of the Women’s Health Study
The investigators examined data from the Women’s Health Study, a randomized, double-blind, placebo-controlled trial of low-dose aspirin and vitamin E for the primary prevention of cardiovascular disease and cancer among female American health professionals aged 45 years or older. The study ended in March 2004, but follow-up is ongoing. At regular intervals, the women complete a questionnaire about disease outcomes, including stroke. Some participants agreed to provide a fasting venous blood sample before randomization. With the subjects’ permission, a committee of physicians examined medical records for women who reported a stroke on a follow-up questionnaire.
Dr. Rist and her colleagues analyzed 27,937 samples for levels of LDL cholesterol, HDL cholesterol, total cholesterol, and triglycerides. They assigned each sample to one of five cholesterol level categories that were based on Adult Treatment Panel III guidelines. Cox proportional hazards models enabled the researchers to calculate the hazard ratio of incident hemorrhagic stroke events. They adjusted their results for covariates such as age, smoking status, menopausal status, body mass index, and alcohol consumption.
A U-shaped association
Women in the lowest category of LDL cholesterol level (less than 70 mg/dL) were younger, less likely to have a history of hypertension, and less likely to use cholesterol-lowering drugs than women in the reference group (100.0-129.9 mg/dL). Women with the lowest LDL cholesterol level were more likely to consume alcohol, have a normal weight, engage in physical activity, and be premenopausal than women in the reference group. The investigators confirmed 137 incident hemorrhagic stroke events during a mean 19.3 years of follow-up.
After data adjustment, the researchers found that women with the lowest level of LDL cholesterol had 2.17 times the risk of hemorrhagic stroke, compared with participants in the reference group. They found a trend toward increased risk among women with an LDL cholesterol level of 160 mg/dL or higher, but the result was not statistically significant. The highest risk for intracerebral hemorrhage (ICH) was among women with an LDL cholesterol level of less than 70 mg/dL (relative risk, 2.32), followed by women with a level of 160 mg/dL or higher (RR, 1.71).
In addition, after multivariable adjustment, women in the lowest quartile of triglycerides (less than or equal to 74 mg/dL for fasting and less than or equal to 85 mg/dL for nonfasting) had a significantly increased risk of hemorrhagic stroke, compared with women in the highest quartile (RR, 2.00). Low triglyceride levels were associated with an increased risk of subarachnoid hemorrhage, but not with an increased risk of ICH. Neither HDL cholesterol nor total cholesterol was associated with risk of hemorrhagic stroke, the researchers wrote.
Mechanism of increased risk unclear
The researchers do not yet know how low triglyceride and LDL cholesterol levels increase the risk of hemorrhagic stroke. One hypothesis is that low cholesterol promotes necrosis of the arterial medial layer’s smooth muscle cells. This impaired endothelium might be more susceptible to microaneurysms, which are common in patients with ICH, said the researchers.
The prospective design and the large sample size were two of the study’s strengths, but the study had important weaknesses as well, the researchers wrote. For example, few women were premenopausal at baseline, so the investigators could not evaluate whether menopausal status modifies the association between lipid levels and risk of hemorrhagic stroke. In addition, lipid levels were measured only at baseline, which prevented an analysis of whether change in lipid levels over time modifies the risk of hemorrhagic stroke.
Dr. Rist reported receiving a grant from the National Institutes of Health.
SOURCE: Rist PM et al. Neurology. 2019 April 10. doi: 10.1212/WNL.0000000000007454.
, according to research published in Neurology.
“Women with very low LDL cholesterol or low triglycerides should be monitored by their doctors for other stroke risk factors that can be modified, like high blood pressure and smoking, in order to reduce their risk of hemorrhagic stroke,” said Pamela M. Rist, ScD, instructor in epidemiology at Harvard Medical School, Boston. “Additional research is needed to determine how to lower the risk of hemorrhagic stroke in women with very low LDL and low triglycerides.”
Several meta-analyses have indicated that LDL cholesterol levels are inversely associated with the risk of hemorrhagic stroke. Because lipid-lowering treatments are used to prevent cardiovascular disease, this potential association has implications for clinical practice. Most of the studies included in these meta-analyses had low numbers of events among women, which prevented researchers from stratifying their results by sex. Because women are at greater risk of stroke than men, Dr. Rist and her colleagues sought to evaluate the association between lipid levels and risk of hemorrhagic stroke.
An analysis of the Women’s Health Study
The investigators examined data from the Women’s Health Study, a randomized, double-blind, placebo-controlled trial of low-dose aspirin and vitamin E for the primary prevention of cardiovascular disease and cancer among female American health professionals aged 45 years or older. The study ended in March 2004, but follow-up is ongoing. At regular intervals, the women complete a questionnaire about disease outcomes, including stroke. Some participants agreed to provide a fasting venous blood sample before randomization. With the subjects’ permission, a committee of physicians examined medical records for women who reported a stroke on a follow-up questionnaire.
Dr. Rist and her colleagues analyzed 27,937 samples for levels of LDL cholesterol, HDL cholesterol, total cholesterol, and triglycerides. They assigned each sample to one of five cholesterol level categories that were based on Adult Treatment Panel III guidelines. Cox proportional hazards models enabled the researchers to calculate the hazard ratio of incident hemorrhagic stroke events. They adjusted their results for covariates such as age, smoking status, menopausal status, body mass index, and alcohol consumption.
A U-shaped association
Women in the lowest category of LDL cholesterol level (less than 70 mg/dL) were younger, less likely to have a history of hypertension, and less likely to use cholesterol-lowering drugs than women in the reference group (100.0-129.9 mg/dL). Women with the lowest LDL cholesterol level were more likely to consume alcohol, have a normal weight, engage in physical activity, and be premenopausal than women in the reference group. The investigators confirmed 137 incident hemorrhagic stroke events during a mean 19.3 years of follow-up.
After data adjustment, the researchers found that women with the lowest level of LDL cholesterol had 2.17 times the risk of hemorrhagic stroke, compared with participants in the reference group. They found a trend toward increased risk among women with an LDL cholesterol level of 160 mg/dL or higher, but the result was not statistically significant. The highest risk for intracerebral hemorrhage (ICH) was among women with an LDL cholesterol level of less than 70 mg/dL (relative risk, 2.32), followed by women with a level of 160 mg/dL or higher (RR, 1.71).
In addition, after multivariable adjustment, women in the lowest quartile of triglycerides (less than or equal to 74 mg/dL for fasting and less than or equal to 85 mg/dL for nonfasting) had a significantly increased risk of hemorrhagic stroke, compared with women in the highest quartile (RR, 2.00). Low triglyceride levels were associated with an increased risk of subarachnoid hemorrhage, but not with an increased risk of ICH. Neither HDL cholesterol nor total cholesterol was associated with risk of hemorrhagic stroke, the researchers wrote.
Mechanism of increased risk unclear
The researchers do not yet know how low triglyceride and LDL cholesterol levels increase the risk of hemorrhagic stroke. One hypothesis is that low cholesterol promotes necrosis of the arterial medial layer’s smooth muscle cells. This impaired endothelium might be more susceptible to microaneurysms, which are common in patients with ICH, said the researchers.
The prospective design and the large sample size were two of the study’s strengths, but the study had important weaknesses as well, the researchers wrote. For example, few women were premenopausal at baseline, so the investigators could not evaluate whether menopausal status modifies the association between lipid levels and risk of hemorrhagic stroke. In addition, lipid levels were measured only at baseline, which prevented an analysis of whether change in lipid levels over time modifies the risk of hemorrhagic stroke.
Dr. Rist reported receiving a grant from the National Institutes of Health.
SOURCE: Rist PM et al. Neurology. 2019 April 10. doi: 10.1212/WNL.0000000000007454.
, according to research published in Neurology.
“Women with very low LDL cholesterol or low triglycerides should be monitored by their doctors for other stroke risk factors that can be modified, like high blood pressure and smoking, in order to reduce their risk of hemorrhagic stroke,” said Pamela M. Rist, ScD, instructor in epidemiology at Harvard Medical School, Boston. “Additional research is needed to determine how to lower the risk of hemorrhagic stroke in women with very low LDL and low triglycerides.”
Several meta-analyses have indicated that LDL cholesterol levels are inversely associated with the risk of hemorrhagic stroke. Because lipid-lowering treatments are used to prevent cardiovascular disease, this potential association has implications for clinical practice. Most of the studies included in these meta-analyses had low numbers of events among women, which prevented researchers from stratifying their results by sex. Because women are at greater risk of stroke than men, Dr. Rist and her colleagues sought to evaluate the association between lipid levels and risk of hemorrhagic stroke.
An analysis of the Women’s Health Study
The investigators examined data from the Women’s Health Study, a randomized, double-blind, placebo-controlled trial of low-dose aspirin and vitamin E for the primary prevention of cardiovascular disease and cancer among female American health professionals aged 45 years or older. The study ended in March 2004, but follow-up is ongoing. At regular intervals, the women complete a questionnaire about disease outcomes, including stroke. Some participants agreed to provide a fasting venous blood sample before randomization. With the subjects’ permission, a committee of physicians examined medical records for women who reported a stroke on a follow-up questionnaire.
Dr. Rist and her colleagues analyzed 27,937 samples for levels of LDL cholesterol, HDL cholesterol, total cholesterol, and triglycerides. They assigned each sample to one of five cholesterol level categories that were based on Adult Treatment Panel III guidelines. Cox proportional hazards models enabled the researchers to calculate the hazard ratio of incident hemorrhagic stroke events. They adjusted their results for covariates such as age, smoking status, menopausal status, body mass index, and alcohol consumption.
A U-shaped association
Women in the lowest category of LDL cholesterol level (less than 70 mg/dL) were younger, less likely to have a history of hypertension, and less likely to use cholesterol-lowering drugs than women in the reference group (100.0-129.9 mg/dL). Women with the lowest LDL cholesterol level were more likely to consume alcohol, have a normal weight, engage in physical activity, and be premenopausal than women in the reference group. The investigators confirmed 137 incident hemorrhagic stroke events during a mean 19.3 years of follow-up.
After data adjustment, the researchers found that women with the lowest level of LDL cholesterol had 2.17 times the risk of hemorrhagic stroke, compared with participants in the reference group. They found a trend toward increased risk among women with an LDL cholesterol level of 160 mg/dL or higher, but the result was not statistically significant. The highest risk for intracerebral hemorrhage (ICH) was among women with an LDL cholesterol level of less than 70 mg/dL (relative risk, 2.32), followed by women with a level of 160 mg/dL or higher (RR, 1.71).
In addition, after multivariable adjustment, women in the lowest quartile of triglycerides (less than or equal to 74 mg/dL for fasting and less than or equal to 85 mg/dL for nonfasting) had a significantly increased risk of hemorrhagic stroke, compared with women in the highest quartile (RR, 2.00). Low triglyceride levels were associated with an increased risk of subarachnoid hemorrhage, but not with an increased risk of ICH. Neither HDL cholesterol nor total cholesterol was associated with risk of hemorrhagic stroke, the researchers wrote.
Mechanism of increased risk unclear
The researchers do not yet know how low triglyceride and LDL cholesterol levels increase the risk of hemorrhagic stroke. One hypothesis is that low cholesterol promotes necrosis of the arterial medial layer’s smooth muscle cells. This impaired endothelium might be more susceptible to microaneurysms, which are common in patients with ICH, said the researchers.
The prospective design and the large sample size were two of the study’s strengths, but the study had important weaknesses as well, the researchers wrote. For example, few women were premenopausal at baseline, so the investigators could not evaluate whether menopausal status modifies the association between lipid levels and risk of hemorrhagic stroke. In addition, lipid levels were measured only at baseline, which prevented an analysis of whether change in lipid levels over time modifies the risk of hemorrhagic stroke.
Dr. Rist reported receiving a grant from the National Institutes of Health.
SOURCE: Rist PM et al. Neurology. 2019 April 10. doi: 10.1212/WNL.0000000000007454.
FROM NEUROLOGY
New data further suggest that stress does a number on the CV system
Stress disorders triggered by life events or trauma have a marked deleterious impact on the cardiovascular system, especially in the short term, suggests a Swedish population-based cohort study of more than 1.6 million people.
Previous studies have had limited power to detect associations and have seldom explored conditions other than PTSD, noted the investigators, led by Huan Song, PhD. In addition, little is known about how genetic predisposition to cardiovascular disease may influence the association.
Results of the new study, reported online in the BMJ, showed that with a median follow-up of about 6.5 years, relative to unaffected siblings, patients who had PTSD, acute stress reaction, adjustment disorder, or another stress reaction had a 64% higher risk of developing a cardiovascular disease during the first year post diagnosis, and a 29% higher risk thereafter.
In the first year, risk was most elevated for heart failure – nearly seven times higher for the patients than for their siblings. In addition, overall risk was increased to a significantly greater extent for cardiovascular diseases with early onset, starting before age 50 years, than for those with later onset, according to Dr. Song of the Center of Public Health Sciences at the University of Iceland, Reykjavík, and the Karolinska Institutet in Stockholm. Findings were essentially the same when patients were instead compared with age- and sex-matched individuals from the general population.
“Stress-related disorders are robustly associated with multiple types of cardiovascular disease, independently of familial background, history of somatic/psychiatric diseases, and psychiatric comorbidity,” Dr. Song and her coinvestigators wrote. “These findings call for enhanced clinical awareness and, if verified, monitoring or early intervention among patients with recently diagnosed stress related disorders.”
Study details
In their population-based cohort study of the Swedish National Patient Register, the investigators identified 136,637 patients with new-onset, stress-related disorders diagnosed between 1987 and 2013. They compared these patients with 171,314 unaffected full siblings and with 1,366,370 unaffected matched people from the general population. Median follow-up was 6.2 years, 6.9 years, and 6.5 years, respectively.
Results showed that the crude incidence rate of any cardiovascular disease was 10.5 per 1,000 person years among the patients with stress-related disorders, 8.4 per 1,000 person years among their unaffected siblings, and 6.9 per 1,000 person years among the matched unaffected individuals from the general population.
Compared with unaffected siblings, patients with stress-related disorders had a 60% elevated risk for developing any cardiovascular disease during the first year after diagnosis. Risk in this window was most elevated for heart failure (hazard ratio, 6.95); cerebrovascular disease other than ischemic and hemorrhagic stroke and arachnoidal bleeding (HR, 5.64), conduction disorders (HR, 5.00), and cardiac arrest (HR, 3.37).
Risk of any cardiovascular disease associated with stress-related disorders was still elevated but to a lesser extent after the first year post diagnosis (HR, 1.29). During this period, risk was most elevated for arterial thrombosis/embolus (HR, 2.02), hemorrhagic stroke (HR, 1.56), and fatal cerebrovascular events (HR, 1.56).
In analyses looking at age of onset of the cardiovascular disease, stress-related disorders showed stronger association with early-onset disease (occurring before age 50 years) than with later-onset disease (HR, 1.40 vs. 1.24; P = .002).
Fatal cardiovascular disease was the only category for which the associations were modified by presence of psychiatric comorbidity. Here, presence of such comorbidity amplified the risk conferred by the stress-related disorder.
Findings were much the same when patients were compared with matched individuals from the general population. Risk for any cardiovascular disease was again elevated substantially in the first year post diagnosis by 71%, and to 36% thereafter.
The authors reported that they had no relevant conflicts of interest. The study was supported by the Icelandic Research Fund, an ERC Consolidator Grant, the Karolinska Institutet, and the Swedish Research Council.
SOURCE: Song H et al. BMJ. 2019 Apr 10. doi: 10.1136/bmj.l1255.
The current study cannot rule out the possibility of reverse causation, whereby cardiovascular disease, which typically manifests slowly, may predispose individuals to stress-related disorders.
In fact, the association may be bidirectional.
“[T]he ultimate test of an underlying unidirectional relation between acute stress induced psychiatric disorders and cardiovascular disease will be through intervention studies to treat these disorders,” Simon L. Bacon, PhD, maintained in an editorial. A reduction in cardiovascular risk after effective treatment of the stress disorders would provide confirmation.
“Psychiatric intervention studies have so far been disappointing in relation to reductions in cardiovascular disease events ... although some people suggest that the null findings had more to do with the limitations of the interventions than a true failure of the hypothesis,” Dr. Bacon observed. “In the future, well-designed studies evaluating more appropriate interventions ... will be critical not only to confirm the inferences of the new study but also to provide real benefits to patients.”
Dr. Bacon is a professor at the Montreal Behavioural Medicine Centre, and department of health, kinesiology, and applied physiology at Concordia University, also in Montreal.
The current study cannot rule out the possibility of reverse causation, whereby cardiovascular disease, which typically manifests slowly, may predispose individuals to stress-related disorders.
In fact, the association may be bidirectional.
“[T]he ultimate test of an underlying unidirectional relation between acute stress induced psychiatric disorders and cardiovascular disease will be through intervention studies to treat these disorders,” Simon L. Bacon, PhD, maintained in an editorial. A reduction in cardiovascular risk after effective treatment of the stress disorders would provide confirmation.
“Psychiatric intervention studies have so far been disappointing in relation to reductions in cardiovascular disease events ... although some people suggest that the null findings had more to do with the limitations of the interventions than a true failure of the hypothesis,” Dr. Bacon observed. “In the future, well-designed studies evaluating more appropriate interventions ... will be critical not only to confirm the inferences of the new study but also to provide real benefits to patients.”
Dr. Bacon is a professor at the Montreal Behavioural Medicine Centre, and department of health, kinesiology, and applied physiology at Concordia University, also in Montreal.
The current study cannot rule out the possibility of reverse causation, whereby cardiovascular disease, which typically manifests slowly, may predispose individuals to stress-related disorders.
In fact, the association may be bidirectional.
“[T]he ultimate test of an underlying unidirectional relation between acute stress induced psychiatric disorders and cardiovascular disease will be through intervention studies to treat these disorders,” Simon L. Bacon, PhD, maintained in an editorial. A reduction in cardiovascular risk after effective treatment of the stress disorders would provide confirmation.
“Psychiatric intervention studies have so far been disappointing in relation to reductions in cardiovascular disease events ... although some people suggest that the null findings had more to do with the limitations of the interventions than a true failure of the hypothesis,” Dr. Bacon observed. “In the future, well-designed studies evaluating more appropriate interventions ... will be critical not only to confirm the inferences of the new study but also to provide real benefits to patients.”
Dr. Bacon is a professor at the Montreal Behavioural Medicine Centre, and department of health, kinesiology, and applied physiology at Concordia University, also in Montreal.
Stress disorders triggered by life events or trauma have a marked deleterious impact on the cardiovascular system, especially in the short term, suggests a Swedish population-based cohort study of more than 1.6 million people.
Previous studies have had limited power to detect associations and have seldom explored conditions other than PTSD, noted the investigators, led by Huan Song, PhD. In addition, little is known about how genetic predisposition to cardiovascular disease may influence the association.
Results of the new study, reported online in the BMJ, showed that with a median follow-up of about 6.5 years, relative to unaffected siblings, patients who had PTSD, acute stress reaction, adjustment disorder, or another stress reaction had a 64% higher risk of developing a cardiovascular disease during the first year post diagnosis, and a 29% higher risk thereafter.
In the first year, risk was most elevated for heart failure – nearly seven times higher for the patients than for their siblings. In addition, overall risk was increased to a significantly greater extent for cardiovascular diseases with early onset, starting before age 50 years, than for those with later onset, according to Dr. Song of the Center of Public Health Sciences at the University of Iceland, Reykjavík, and the Karolinska Institutet in Stockholm. Findings were essentially the same when patients were instead compared with age- and sex-matched individuals from the general population.
“Stress-related disorders are robustly associated with multiple types of cardiovascular disease, independently of familial background, history of somatic/psychiatric diseases, and psychiatric comorbidity,” Dr. Song and her coinvestigators wrote. “These findings call for enhanced clinical awareness and, if verified, monitoring or early intervention among patients with recently diagnosed stress related disorders.”
Study details
In their population-based cohort study of the Swedish National Patient Register, the investigators identified 136,637 patients with new-onset, stress-related disorders diagnosed between 1987 and 2013. They compared these patients with 171,314 unaffected full siblings and with 1,366,370 unaffected matched people from the general population. Median follow-up was 6.2 years, 6.9 years, and 6.5 years, respectively.
Results showed that the crude incidence rate of any cardiovascular disease was 10.5 per 1,000 person years among the patients with stress-related disorders, 8.4 per 1,000 person years among their unaffected siblings, and 6.9 per 1,000 person years among the matched unaffected individuals from the general population.
Compared with unaffected siblings, patients with stress-related disorders had a 60% elevated risk for developing any cardiovascular disease during the first year after diagnosis. Risk in this window was most elevated for heart failure (hazard ratio, 6.95); cerebrovascular disease other than ischemic and hemorrhagic stroke and arachnoidal bleeding (HR, 5.64), conduction disorders (HR, 5.00), and cardiac arrest (HR, 3.37).
Risk of any cardiovascular disease associated with stress-related disorders was still elevated but to a lesser extent after the first year post diagnosis (HR, 1.29). During this period, risk was most elevated for arterial thrombosis/embolus (HR, 2.02), hemorrhagic stroke (HR, 1.56), and fatal cerebrovascular events (HR, 1.56).
In analyses looking at age of onset of the cardiovascular disease, stress-related disorders showed stronger association with early-onset disease (occurring before age 50 years) than with later-onset disease (HR, 1.40 vs. 1.24; P = .002).
Fatal cardiovascular disease was the only category for which the associations were modified by presence of psychiatric comorbidity. Here, presence of such comorbidity amplified the risk conferred by the stress-related disorder.
Findings were much the same when patients were compared with matched individuals from the general population. Risk for any cardiovascular disease was again elevated substantially in the first year post diagnosis by 71%, and to 36% thereafter.
The authors reported that they had no relevant conflicts of interest. The study was supported by the Icelandic Research Fund, an ERC Consolidator Grant, the Karolinska Institutet, and the Swedish Research Council.
SOURCE: Song H et al. BMJ. 2019 Apr 10. doi: 10.1136/bmj.l1255.
Stress disorders triggered by life events or trauma have a marked deleterious impact on the cardiovascular system, especially in the short term, suggests a Swedish population-based cohort study of more than 1.6 million people.
Previous studies have had limited power to detect associations and have seldom explored conditions other than PTSD, noted the investigators, led by Huan Song, PhD. In addition, little is known about how genetic predisposition to cardiovascular disease may influence the association.
Results of the new study, reported online in the BMJ, showed that with a median follow-up of about 6.5 years, relative to unaffected siblings, patients who had PTSD, acute stress reaction, adjustment disorder, or another stress reaction had a 64% higher risk of developing a cardiovascular disease during the first year post diagnosis, and a 29% higher risk thereafter.
In the first year, risk was most elevated for heart failure – nearly seven times higher for the patients than for their siblings. In addition, overall risk was increased to a significantly greater extent for cardiovascular diseases with early onset, starting before age 50 years, than for those with later onset, according to Dr. Song of the Center of Public Health Sciences at the University of Iceland, Reykjavík, and the Karolinska Institutet in Stockholm. Findings were essentially the same when patients were instead compared with age- and sex-matched individuals from the general population.
“Stress-related disorders are robustly associated with multiple types of cardiovascular disease, independently of familial background, history of somatic/psychiatric diseases, and psychiatric comorbidity,” Dr. Song and her coinvestigators wrote. “These findings call for enhanced clinical awareness and, if verified, monitoring or early intervention among patients with recently diagnosed stress related disorders.”
Study details
In their population-based cohort study of the Swedish National Patient Register, the investigators identified 136,637 patients with new-onset, stress-related disorders diagnosed between 1987 and 2013. They compared these patients with 171,314 unaffected full siblings and with 1,366,370 unaffected matched people from the general population. Median follow-up was 6.2 years, 6.9 years, and 6.5 years, respectively.
Results showed that the crude incidence rate of any cardiovascular disease was 10.5 per 1,000 person years among the patients with stress-related disorders, 8.4 per 1,000 person years among their unaffected siblings, and 6.9 per 1,000 person years among the matched unaffected individuals from the general population.
Compared with unaffected siblings, patients with stress-related disorders had a 60% elevated risk for developing any cardiovascular disease during the first year after diagnosis. Risk in this window was most elevated for heart failure (hazard ratio, 6.95); cerebrovascular disease other than ischemic and hemorrhagic stroke and arachnoidal bleeding (HR, 5.64), conduction disorders (HR, 5.00), and cardiac arrest (HR, 3.37).
Risk of any cardiovascular disease associated with stress-related disorders was still elevated but to a lesser extent after the first year post diagnosis (HR, 1.29). During this period, risk was most elevated for arterial thrombosis/embolus (HR, 2.02), hemorrhagic stroke (HR, 1.56), and fatal cerebrovascular events (HR, 1.56).
In analyses looking at age of onset of the cardiovascular disease, stress-related disorders showed stronger association with early-onset disease (occurring before age 50 years) than with later-onset disease (HR, 1.40 vs. 1.24; P = .002).
Fatal cardiovascular disease was the only category for which the associations were modified by presence of psychiatric comorbidity. Here, presence of such comorbidity amplified the risk conferred by the stress-related disorder.
Findings were much the same when patients were compared with matched individuals from the general population. Risk for any cardiovascular disease was again elevated substantially in the first year post diagnosis by 71%, and to 36% thereafter.
The authors reported that they had no relevant conflicts of interest. The study was supported by the Icelandic Research Fund, an ERC Consolidator Grant, the Karolinska Institutet, and the Swedish Research Council.
SOURCE: Song H et al. BMJ. 2019 Apr 10. doi: 10.1136/bmj.l1255.
FROM BMJ