User login
GLP-1s May Improve Colon Cancer Outcomes
Treatment with a GLP-1 receptor agonist (RA) may offer a survival advantage in patients with colon cancer and obesity.
In a real-world analysis of nearly 7000 patients with colon cancer, those taking a GLP-1 RA were less than half as likely to die within 5 years compared with those who weren’t on a GLP-1 drug.
The association between GLP-1 exposure and lower 5–year mortality in colon cancer was “robust” and appeared to be concentrated in patients with severe obesity (BMI ≥ 35), lead investigator Raphael E. Cuomo, PhD, with University of California San Diego, told this news organization.
The apparent protective effect “persisted after controlling for differences in disease severity and demographics, as well as differences in circulating carcinoembryonic antigen, a biomarker of disease aggressiveness,” Cuomo said.
The study was published online in Cancer Investigation.
Effects Beyond Glucose-Lowering
Colon cancer remains a major global cause of cancer-related deaths, and obesity is both a risk factor and a driver of worse outcomes.
Beyond regulating blood sugar, GLP-1 drugs reduce systemic inflammation, improve insulin sensitivity, and promote weight loss. Prior preclinical work has also suggested they may prevent cancer cell growth, trigger cancer cell death, and reshape the tumor microenvironment.
To investigate further, Cuomo analyzed electronic health records of 6871 patients diagnosed with primary colon cancer before 2019 — of which 103 had at least 1 documented prescription for a GLP-1 drug within 5 years of diagnosis.
Five–year mortality was significantly lower in GLP-1 RA users than in nonusers (15.5% vs 37.1%; P < .001). A significant reduction in 5–year mortality among GLP-1 RA users was evident in an unadjusted model (odds ratio [OR], 0.38; P < .001) and persisted in fully adjusted models (OR, 0.28; P < .001).
When stratified by BMI, the odds of 5-year mortality with GLP-1 use was reduced only in patients with Class II obesity (BMI ≥ 35: fully adjusted hazard ratio [HR], 0.051; P = .004). In this group, fully adjusted hazard ratios suggested markedly lower risk for death (HR, 0.07; P = .009).
Beyond mortality, GLP-1 users also experienced fewer late cardiovascular events and had fewer markers of advanced colon cancer progression in the final months of follow-up, “which suggests that GLP-1 drugs exert benefits through both oncologic and cardiometabolic pathways,” Cuomo told this news organization.
Intriguing and Promising — but Further Studies Needed
“To further study the potential of GLP-1 therapy as an adjunct to standard care in colon cancer, randomized trials should be conducted with stratification by BMI, diabetes status, and disease severity, with endpoints spanning overall and cancerspecific survival and major cardiovascular events,” Cuomo said.
“We also need prospective translational studies integrating dosing/timing, adherence, tumor genomics, and serial biomarkers (including ctDNA and metabolic panels) to elucidate mechanisms, assess the role of adiposity and insulin resistance, and identify the patient subgroups most likely to benefit,” he noted.
For now, GLP1 medications are an option in “eligible colon cancer patients with severe obesity or diabetes who meet standard metabolic indications,” Cuomo told this news organization.
Commenting on this study for this news organization, David Greenwald, MD, director of Clinical Gastroenterology and Endoscopy at Icahn School of Medicine at Mount Sinai Hospital in New York City, noted “other studies have showed a lower risk of developing colorectal cancer in the first place and then improved survival.”
Greenwald cited a recent study that found people with diabetes who took GLP-1 RAs had a 44% lower risk of developing colorectal cancer than those who took insulin, and a 25% lower risk than those who took metformin.
The effects of GLP-1s in colon cancer are “very intriguing and very promising but more research is needed to confirm whether this is really true and the mechanisms behind it,” said Greenwald.
In terms of the lowering risk of developing colorectal cancer, “probably first and foremost is that the drugs are really effective in promoting weight loss. And if you can reduce obesity in the population, you do all sorts of good things — reduce diabetes, reduce heart disease, and maybe reduce colorectal cancer,” Greenwald said.
This study had no specific funding. Cuomo and Greenwald had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Treatment with a GLP-1 receptor agonist (RA) may offer a survival advantage in patients with colon cancer and obesity.
In a real-world analysis of nearly 7000 patients with colon cancer, those taking a GLP-1 RA were less than half as likely to die within 5 years compared with those who weren’t on a GLP-1 drug.
The association between GLP-1 exposure and lower 5–year mortality in colon cancer was “robust” and appeared to be concentrated in patients with severe obesity (BMI ≥ 35), lead investigator Raphael E. Cuomo, PhD, with University of California San Diego, told this news organization.
The apparent protective effect “persisted after controlling for differences in disease severity and demographics, as well as differences in circulating carcinoembryonic antigen, a biomarker of disease aggressiveness,” Cuomo said.
The study was published online in Cancer Investigation.
Effects Beyond Glucose-Lowering
Colon cancer remains a major global cause of cancer-related deaths, and obesity is both a risk factor and a driver of worse outcomes.
Beyond regulating blood sugar, GLP-1 drugs reduce systemic inflammation, improve insulin sensitivity, and promote weight loss. Prior preclinical work has also suggested they may prevent cancer cell growth, trigger cancer cell death, and reshape the tumor microenvironment.
To investigate further, Cuomo analyzed electronic health records of 6871 patients diagnosed with primary colon cancer before 2019 — of which 103 had at least 1 documented prescription for a GLP-1 drug within 5 years of diagnosis.
Five–year mortality was significantly lower in GLP-1 RA users than in nonusers (15.5% vs 37.1%; P < .001). A significant reduction in 5–year mortality among GLP-1 RA users was evident in an unadjusted model (odds ratio [OR], 0.38; P < .001) and persisted in fully adjusted models (OR, 0.28; P < .001).
When stratified by BMI, the odds of 5-year mortality with GLP-1 use was reduced only in patients with Class II obesity (BMI ≥ 35: fully adjusted hazard ratio [HR], 0.051; P = .004). In this group, fully adjusted hazard ratios suggested markedly lower risk for death (HR, 0.07; P = .009).
Beyond mortality, GLP-1 users also experienced fewer late cardiovascular events and had fewer markers of advanced colon cancer progression in the final months of follow-up, “which suggests that GLP-1 drugs exert benefits through both oncologic and cardiometabolic pathways,” Cuomo told this news organization.
Intriguing and Promising — but Further Studies Needed
“To further study the potential of GLP-1 therapy as an adjunct to standard care in colon cancer, randomized trials should be conducted with stratification by BMI, diabetes status, and disease severity, with endpoints spanning overall and cancerspecific survival and major cardiovascular events,” Cuomo said.
“We also need prospective translational studies integrating dosing/timing, adherence, tumor genomics, and serial biomarkers (including ctDNA and metabolic panels) to elucidate mechanisms, assess the role of adiposity and insulin resistance, and identify the patient subgroups most likely to benefit,” he noted.
For now, GLP1 medications are an option in “eligible colon cancer patients with severe obesity or diabetes who meet standard metabolic indications,” Cuomo told this news organization.
Commenting on this study for this news organization, David Greenwald, MD, director of Clinical Gastroenterology and Endoscopy at Icahn School of Medicine at Mount Sinai Hospital in New York City, noted “other studies have showed a lower risk of developing colorectal cancer in the first place and then improved survival.”
Greenwald cited a recent study that found people with diabetes who took GLP-1 RAs had a 44% lower risk of developing colorectal cancer than those who took insulin, and a 25% lower risk than those who took metformin.
The effects of GLP-1s in colon cancer are “very intriguing and very promising but more research is needed to confirm whether this is really true and the mechanisms behind it,” said Greenwald.
In terms of the lowering risk of developing colorectal cancer, “probably first and foremost is that the drugs are really effective in promoting weight loss. And if you can reduce obesity in the population, you do all sorts of good things — reduce diabetes, reduce heart disease, and maybe reduce colorectal cancer,” Greenwald said.
This study had no specific funding. Cuomo and Greenwald had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Treatment with a GLP-1 receptor agonist (RA) may offer a survival advantage in patients with colon cancer and obesity.
In a real-world analysis of nearly 7000 patients with colon cancer, those taking a GLP-1 RA were less than half as likely to die within 5 years compared with those who weren’t on a GLP-1 drug.
The association between GLP-1 exposure and lower 5–year mortality in colon cancer was “robust” and appeared to be concentrated in patients with severe obesity (BMI ≥ 35), lead investigator Raphael E. Cuomo, PhD, with University of California San Diego, told this news organization.
The apparent protective effect “persisted after controlling for differences in disease severity and demographics, as well as differences in circulating carcinoembryonic antigen, a biomarker of disease aggressiveness,” Cuomo said.
The study was published online in Cancer Investigation.
Effects Beyond Glucose-Lowering
Colon cancer remains a major global cause of cancer-related deaths, and obesity is both a risk factor and a driver of worse outcomes.
Beyond regulating blood sugar, GLP-1 drugs reduce systemic inflammation, improve insulin sensitivity, and promote weight loss. Prior preclinical work has also suggested they may prevent cancer cell growth, trigger cancer cell death, and reshape the tumor microenvironment.
To investigate further, Cuomo analyzed electronic health records of 6871 patients diagnosed with primary colon cancer before 2019 — of which 103 had at least 1 documented prescription for a GLP-1 drug within 5 years of diagnosis.
Five–year mortality was significantly lower in GLP-1 RA users than in nonusers (15.5% vs 37.1%; P < .001). A significant reduction in 5–year mortality among GLP-1 RA users was evident in an unadjusted model (odds ratio [OR], 0.38; P < .001) and persisted in fully adjusted models (OR, 0.28; P < .001).
When stratified by BMI, the odds of 5-year mortality with GLP-1 use was reduced only in patients with Class II obesity (BMI ≥ 35: fully adjusted hazard ratio [HR], 0.051; P = .004). In this group, fully adjusted hazard ratios suggested markedly lower risk for death (HR, 0.07; P = .009).
Beyond mortality, GLP-1 users also experienced fewer late cardiovascular events and had fewer markers of advanced colon cancer progression in the final months of follow-up, “which suggests that GLP-1 drugs exert benefits through both oncologic and cardiometabolic pathways,” Cuomo told this news organization.
Intriguing and Promising — but Further Studies Needed
“To further study the potential of GLP-1 therapy as an adjunct to standard care in colon cancer, randomized trials should be conducted with stratification by BMI, diabetes status, and disease severity, with endpoints spanning overall and cancerspecific survival and major cardiovascular events,” Cuomo said.
“We also need prospective translational studies integrating dosing/timing, adherence, tumor genomics, and serial biomarkers (including ctDNA and metabolic panels) to elucidate mechanisms, assess the role of adiposity and insulin resistance, and identify the patient subgroups most likely to benefit,” he noted.
For now, GLP1 medications are an option in “eligible colon cancer patients with severe obesity or diabetes who meet standard metabolic indications,” Cuomo told this news organization.
Commenting on this study for this news organization, David Greenwald, MD, director of Clinical Gastroenterology and Endoscopy at Icahn School of Medicine at Mount Sinai Hospital in New York City, noted “other studies have showed a lower risk of developing colorectal cancer in the first place and then improved survival.”
Greenwald cited a recent study that found people with diabetes who took GLP-1 RAs had a 44% lower risk of developing colorectal cancer than those who took insulin, and a 25% lower risk than those who took metformin.
The effects of GLP-1s in colon cancer are “very intriguing and very promising but more research is needed to confirm whether this is really true and the mechanisms behind it,” said Greenwald.
In terms of the lowering risk of developing colorectal cancer, “probably first and foremost is that the drugs are really effective in promoting weight loss. And if you can reduce obesity in the population, you do all sorts of good things — reduce diabetes, reduce heart disease, and maybe reduce colorectal cancer,” Greenwald said.
This study had no specific funding. Cuomo and Greenwald had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM CANCER INVESTIGATION
Does This Bacterial Toxin Drive Early CRC Risk?
Recent studies have cited an alarming increase in early-onset colorectal cancer (CRC) rates, raising concern among gastroenterologists, public health experts, and patients alike. Approximately 10% of CRC cases now occur in those under age 50, and that proportion continues to grow. Between 2000 and 2016, colon cancer rose by 13% and rectal cancer by 16% among those aged 40–49.
According to recently published data from the Surveillance, Epidemiology and End Results Program, between 2019 and 2022, CRC incidence among patients aged 45–49 rose by approximately 12% per year.
A Potential Bacterial Connection
What accounts for this disturbing spike? A research group from the University of California, San Diego, may have uncovered part of the answer.
In their study of 981 CRC genomes, most carried mutations suggestive of prior exposure to colibactin, a toxin produced by certain Escherichia coli (E coli) strains. Patients with extremely early-onset CRC (aged < 40 years) were 3 times more likely to have colibactin-suggestive mutations than patients older than 70. Crucially, colonic exposure to colibactin was linked to an adenomatous polyposis coli driver mutation.
These findings suggest that colibactin-induced injury in the gut microbiome may accelerate cancer development in some individuals. Environmental factors may contribute to the rise in early-onset CRC as well, such as consuming red meats, carcinogens from grilling, and processed meats and other highly processed foods; low fiber intake; lack of fruits and vegetables; drinking alcohol; lack of exercise; obesity; and colibactin exposure.
In this video, we will take a closer look at how E coli and colibactin may increase CRC risk.
Bacteria’s Cancer-Causing Properties
The idea that bacteria has cancer-causing properties isn’t new. In the 1970s, researchers linked Streptococcus bovis type 1 (now called Streptococcus gallolyticus) to CRC in a subset of patients with bacterial endocarditis stemming from right-sided colon cancer. Similarly, Helicobacter pylori infection has long been associated with increased gastric cancer risk.
Today, E coli infection is emerging as another possible contributor to CRC, especially via certain pathogenic strains containing the polyketide synthase (pks) genomic island, which encodes the colibactin and is sometimes present in the colon mucosa of patients with CRC.
Colibactin and DNA Damage
Colibactin-producing pks+ E coli strains can cause DNA double-strand breaks, one pathway to carcinogenesis. In animal studies, pks+ E coli strains have been linked to both increased risk for CRC and CRC progression.
In an important study published in Nature, Pleguezuelos-Manzano and colleagues repeatedly exposed intestinal organoids to pks+ E coli over 5 months and then performed whole genome sequencing. The result was a concerning potential for short insertions and deletions and single–base substitutions.
The authors concluded that their “study describes the distinct mutational signature in colorectal cancer and implies that the underlying mutational process results directly from past exposure to bacteria carrying the colibactin-producing pks pathogenicity island.”
Other E coli virulence factors may also contribute. For example, alpha-hemolysin may downregulate DNA mismatch repair proteins. In other words, E coli is probably just a contributing factor for the development of CRC, not the sole cause.
Biofilms and Inflammation
Previous studies have associated dense bacterial biofilms, particularly antibiotic-resistant strains, with CRC. This raises the possibility that widespread antibiotic overuse could predispose certain individuals to CRC development.
Biofilms normally separate the colon mucosal epithelium from bacteria and are essential for protecting against inflammation. In a 2018 study in Science, Dejea and colleagues concluded that “tumor-prone mice colonized with E coli (expressing colibactin), and enterotoxigenic B fragilis showed increased interleukin-17 in the colon and DNA damage in colonic epithelium with faster tumor onset and greater mortality, compared to mice with either bacteria strain alone. These data suggest an unexpected link between early neoplasia of the colon and tumorigenic bacteria.”
Additional research revealed that E coli can create a pro-carcinogenic environment by stimulating mucosal inflammation, hindering DNA and mismatch repair mechanisms, and altering immune responses.
Dysbiosis and Diet
Colibactin can also drive dysbiosis and imbalance in bacteria in the colon, which fuels inflammation and disrupts mucosal barrier integrity. This creates a vicious cycle in which chronic inflammation can further drive additional mucus deterioration and dysbiosis.
In mouse models where the colon mucosal barrier is damaged with dextrin sulfate sodium (DSS), pks+ E coli gains better access to colon epithelium, causes injury, and can even lead to chronic colitis. Colibactin can also hinder epithelial recovery after DSS treatment.
Diet plays a central role in this process. Low fiber consumption can disrupt the barrier between the colon mucus layer and the colon’s exterior layer where bacteria live. A traditional Western diet may bolster bacteria that degrade the mucus layer when the bacteria consume the glycosylated portion as an energy source.
Fortunately, diet is modifiable. High–fiber diets (ideally 25-30 g/d) boost short–chain fatty acids in the colon. This is important because short-chain fatty acids can decrease intercellular pH and impede Enterobacteriaceae replication, yet another reason why we should encourage patients to eat a diet high in vegetables, fruits, and [green] salads.
Two Types of Bacterial Drivers
There appear to be two broad types of bacteria associated with CRC development. It’s been hypothesized that there are “driver” bacteria that might initiate the development of CRC, possibly by creating oxidative stress and causing DNA breaks. Several potential pathogenic bacteria have been identified, including E coli, Enterococcus faecalis, and Bacteroides fragilis. Unfortunately, there are also bacteria such as Fusobacterium species and Streptococcus gallolyticus with the potential to alter intestinal permeability, resulting in downstream effects that can allow colon cancers to expand. Fusobacterium species and Streptococcus gallolyticus have the potential to cause DNA double–strand breaks in the intestine, which can produce chromosomal precariousness.
These secondary bacteria can also lead to DNA epigenetic changes and gene mutations. However, it should be emphasized that “the direct causation of imprinted DNA changes resulting from a direct interaction between bacteria and host cells is not so far established.”
E coli produces compounds called cyclomodulins, which can cause DNA breaks and potentially trigger cell cycle arrest and even cell death through activation of the DNA damage checkpoint pathway. The DNA damage checkpoint pathway is a cellular signaling network that helps detect DNA lesions and allows for genetic stability by stopping growth to allow for repair and simulating cell survival or apoptosis. A key cyclomodulin that E coli makes is colibactin, produced by the pks locus. Other cyclomodulins include cytolethal distending toxin, cytotoxic necrotizing factor, and cycle-inhibiting factor.
Previous research has shown that E coli is the only culturable bacteria found near CRC. A groundbreaking 1998 study employing PCR technology found E coli in 60% of colon polyp adenomas and an alarming 77% of CRC biopsies.
E coli’s capability to downregulate essential DNA mismatch repair proteins has been implicated in colorectal carcinogenesis. Interestingly, when the genetic region responsible for producing colibactin is deleted in animals, the bacteria aren’t able to promote cancer.
Mechanistically, colibactin causes double-stranded DNA breaks, eukaryotic cell cycle arrest, and chromosome abnormalities. It also alkylates DNA. This occurs when the cyclopropane ring of colibactin interacts with the N3 position of adenine in DNA, forming a covalent bond and creating a DNA adduct. DNA adducts occur when a chemical moiety from an environmental or dietary source binds to DNA base. Colibactin can cause DNA interstrand cross-links to form via alkalization of adenine residues on opposing DNA strands, a crucial step in DNA damage. DNA adducts can occur through carcinogens in N-nitroso compounds, such as in processed meats and in polycyclic aromatic hydrocarbons found in cigarette smoke. Colibactin-induced damage may also stimulate the senescence–associated secretory phenotype pathway, increasing proinflammatory cytokines.
E coli and Inflammatory Bowel Disease
E coli, the primary colibactin producer in the human intestinal microbiome, is found at higher bacterial percentages in the microbiomes of patients with inflammatory bowel disease (IBD). In a study by Dubinsky and colleagues, “the medium relative levels of colibactin–encoding E. coli were about threefold higher in IBD.”
Researchers have also postulated that antibiotics and microbiome dysbiosis may create conditions that allow colibactin–producing bacteria to overpopulate.
Future Directions
Not every patient with CRC carries a colorectal mutational signature, but these findings underscore the need for continued vigilance and prevention.
From a public health standpoint, our advice remains consistent: Promote high-fiber diets with more vegetables and less red meat; avoid highly processed foods; avoid alcohol; encourage exercise; and address overweight and obesity. Our goal is to create the best possible colon environment to prevent DNA damage from bacterial and environmental carcinogens.
In the future, we need more research to clarify exactly how E coli and colibactin increase early–onset CRC risk and whether antibiotics and dysbiosis facilitate their ability to damage the DNA of colon mucosa. It’s still unclear why younger patients are at greater risk. In time, we may be able to screen for colibactin–producing bacteria such as E coli and manipulate the fecal microbiome to prevent damage.
A recent mouse study in Nature by Jans and colleagues suggests it might be possible to block bacterial adhesion and hopefully mitigate damage caused by colibactin. With continued work, colibactin–targeted strategies could become a part of CRC prevention.
Benjamin H. Levy III, MD, is a gastroenterologist at the University of Chicago. In 2017, Levy, a previous Fulbright Fellow in France, also started a gastroenterology clinic for refugees resettling in Chicago. His clinical projects focus on the development of colorectal cancer screening campaigns. Levy, who gave a TEDx Talk about building health education campaigns using music and concerts, organizes "Tune It Up: A Concert To Raise Colorectal Cancer Awareness" with the American College of Gastroenterology (ACG). He frequently publishes on a variety of gastroenterology topics and serves on ACG’s Public Relations Committee and FDA-Related Matters Committee.
A version of this article first appeared on Medscape.com.
Recent studies have cited an alarming increase in early-onset colorectal cancer (CRC) rates, raising concern among gastroenterologists, public health experts, and patients alike. Approximately 10% of CRC cases now occur in those under age 50, and that proportion continues to grow. Between 2000 and 2016, colon cancer rose by 13% and rectal cancer by 16% among those aged 40–49.
According to recently published data from the Surveillance, Epidemiology and End Results Program, between 2019 and 2022, CRC incidence among patients aged 45–49 rose by approximately 12% per year.
A Potential Bacterial Connection
What accounts for this disturbing spike? A research group from the University of California, San Diego, may have uncovered part of the answer.
In their study of 981 CRC genomes, most carried mutations suggestive of prior exposure to colibactin, a toxin produced by certain Escherichia coli (E coli) strains. Patients with extremely early-onset CRC (aged < 40 years) were 3 times more likely to have colibactin-suggestive mutations than patients older than 70. Crucially, colonic exposure to colibactin was linked to an adenomatous polyposis coli driver mutation.
These findings suggest that colibactin-induced injury in the gut microbiome may accelerate cancer development in some individuals. Environmental factors may contribute to the rise in early-onset CRC as well, such as consuming red meats, carcinogens from grilling, and processed meats and other highly processed foods; low fiber intake; lack of fruits and vegetables; drinking alcohol; lack of exercise; obesity; and colibactin exposure.
In this video, we will take a closer look at how E coli and colibactin may increase CRC risk.
Bacteria’s Cancer-Causing Properties
The idea that bacteria has cancer-causing properties isn’t new. In the 1970s, researchers linked Streptococcus bovis type 1 (now called Streptococcus gallolyticus) to CRC in a subset of patients with bacterial endocarditis stemming from right-sided colon cancer. Similarly, Helicobacter pylori infection has long been associated with increased gastric cancer risk.
Today, E coli infection is emerging as another possible contributor to CRC, especially via certain pathogenic strains containing the polyketide synthase (pks) genomic island, which encodes the colibactin and is sometimes present in the colon mucosa of patients with CRC.
Colibactin and DNA Damage
Colibactin-producing pks+ E coli strains can cause DNA double-strand breaks, one pathway to carcinogenesis. In animal studies, pks+ E coli strains have been linked to both increased risk for CRC and CRC progression.
In an important study published in Nature, Pleguezuelos-Manzano and colleagues repeatedly exposed intestinal organoids to pks+ E coli over 5 months and then performed whole genome sequencing. The result was a concerning potential for short insertions and deletions and single–base substitutions.
The authors concluded that their “study describes the distinct mutational signature in colorectal cancer and implies that the underlying mutational process results directly from past exposure to bacteria carrying the colibactin-producing pks pathogenicity island.”
Other E coli virulence factors may also contribute. For example, alpha-hemolysin may downregulate DNA mismatch repair proteins. In other words, E coli is probably just a contributing factor for the development of CRC, not the sole cause.
Biofilms and Inflammation
Previous studies have associated dense bacterial biofilms, particularly antibiotic-resistant strains, with CRC. This raises the possibility that widespread antibiotic overuse could predispose certain individuals to CRC development.
Biofilms normally separate the colon mucosal epithelium from bacteria and are essential for protecting against inflammation. In a 2018 study in Science, Dejea and colleagues concluded that “tumor-prone mice colonized with E coli (expressing colibactin), and enterotoxigenic B fragilis showed increased interleukin-17 in the colon and DNA damage in colonic epithelium with faster tumor onset and greater mortality, compared to mice with either bacteria strain alone. These data suggest an unexpected link between early neoplasia of the colon and tumorigenic bacteria.”
Additional research revealed that E coli can create a pro-carcinogenic environment by stimulating mucosal inflammation, hindering DNA and mismatch repair mechanisms, and altering immune responses.
Dysbiosis and Diet
Colibactin can also drive dysbiosis and imbalance in bacteria in the colon, which fuels inflammation and disrupts mucosal barrier integrity. This creates a vicious cycle in which chronic inflammation can further drive additional mucus deterioration and dysbiosis.
In mouse models where the colon mucosal barrier is damaged with dextrin sulfate sodium (DSS), pks+ E coli gains better access to colon epithelium, causes injury, and can even lead to chronic colitis. Colibactin can also hinder epithelial recovery after DSS treatment.
Diet plays a central role in this process. Low fiber consumption can disrupt the barrier between the colon mucus layer and the colon’s exterior layer where bacteria live. A traditional Western diet may bolster bacteria that degrade the mucus layer when the bacteria consume the glycosylated portion as an energy source.
Fortunately, diet is modifiable. High–fiber diets (ideally 25-30 g/d) boost short–chain fatty acids in the colon. This is important because short-chain fatty acids can decrease intercellular pH and impede Enterobacteriaceae replication, yet another reason why we should encourage patients to eat a diet high in vegetables, fruits, and [green] salads.
Two Types of Bacterial Drivers
There appear to be two broad types of bacteria associated with CRC development. It’s been hypothesized that there are “driver” bacteria that might initiate the development of CRC, possibly by creating oxidative stress and causing DNA breaks. Several potential pathogenic bacteria have been identified, including E coli, Enterococcus faecalis, and Bacteroides fragilis. Unfortunately, there are also bacteria such as Fusobacterium species and Streptococcus gallolyticus with the potential to alter intestinal permeability, resulting in downstream effects that can allow colon cancers to expand. Fusobacterium species and Streptococcus gallolyticus have the potential to cause DNA double–strand breaks in the intestine, which can produce chromosomal precariousness.
These secondary bacteria can also lead to DNA epigenetic changes and gene mutations. However, it should be emphasized that “the direct causation of imprinted DNA changes resulting from a direct interaction between bacteria and host cells is not so far established.”
E coli produces compounds called cyclomodulins, which can cause DNA breaks and potentially trigger cell cycle arrest and even cell death through activation of the DNA damage checkpoint pathway. The DNA damage checkpoint pathway is a cellular signaling network that helps detect DNA lesions and allows for genetic stability by stopping growth to allow for repair and simulating cell survival or apoptosis. A key cyclomodulin that E coli makes is colibactin, produced by the pks locus. Other cyclomodulins include cytolethal distending toxin, cytotoxic necrotizing factor, and cycle-inhibiting factor.
Previous research has shown that E coli is the only culturable bacteria found near CRC. A groundbreaking 1998 study employing PCR technology found E coli in 60% of colon polyp adenomas and an alarming 77% of CRC biopsies.
E coli’s capability to downregulate essential DNA mismatch repair proteins has been implicated in colorectal carcinogenesis. Interestingly, when the genetic region responsible for producing colibactin is deleted in animals, the bacteria aren’t able to promote cancer.
Mechanistically, colibactin causes double-stranded DNA breaks, eukaryotic cell cycle arrest, and chromosome abnormalities. It also alkylates DNA. This occurs when the cyclopropane ring of colibactin interacts with the N3 position of adenine in DNA, forming a covalent bond and creating a DNA adduct. DNA adducts occur when a chemical moiety from an environmental or dietary source binds to DNA base. Colibactin can cause DNA interstrand cross-links to form via alkalization of adenine residues on opposing DNA strands, a crucial step in DNA damage. DNA adducts can occur through carcinogens in N-nitroso compounds, such as in processed meats and in polycyclic aromatic hydrocarbons found in cigarette smoke. Colibactin-induced damage may also stimulate the senescence–associated secretory phenotype pathway, increasing proinflammatory cytokines.
E coli and Inflammatory Bowel Disease
E coli, the primary colibactin producer in the human intestinal microbiome, is found at higher bacterial percentages in the microbiomes of patients with inflammatory bowel disease (IBD). In a study by Dubinsky and colleagues, “the medium relative levels of colibactin–encoding E. coli were about threefold higher in IBD.”
Researchers have also postulated that antibiotics and microbiome dysbiosis may create conditions that allow colibactin–producing bacteria to overpopulate.
Future Directions
Not every patient with CRC carries a colorectal mutational signature, but these findings underscore the need for continued vigilance and prevention.
From a public health standpoint, our advice remains consistent: Promote high-fiber diets with more vegetables and less red meat; avoid highly processed foods; avoid alcohol; encourage exercise; and address overweight and obesity. Our goal is to create the best possible colon environment to prevent DNA damage from bacterial and environmental carcinogens.
In the future, we need more research to clarify exactly how E coli and colibactin increase early–onset CRC risk and whether antibiotics and dysbiosis facilitate their ability to damage the DNA of colon mucosa. It’s still unclear why younger patients are at greater risk. In time, we may be able to screen for colibactin–producing bacteria such as E coli and manipulate the fecal microbiome to prevent damage.
A recent mouse study in Nature by Jans and colleagues suggests it might be possible to block bacterial adhesion and hopefully mitigate damage caused by colibactin. With continued work, colibactin–targeted strategies could become a part of CRC prevention.
Benjamin H. Levy III, MD, is a gastroenterologist at the University of Chicago. In 2017, Levy, a previous Fulbright Fellow in France, also started a gastroenterology clinic for refugees resettling in Chicago. His clinical projects focus on the development of colorectal cancer screening campaigns. Levy, who gave a TEDx Talk about building health education campaigns using music and concerts, organizes "Tune It Up: A Concert To Raise Colorectal Cancer Awareness" with the American College of Gastroenterology (ACG). He frequently publishes on a variety of gastroenterology topics and serves on ACG’s Public Relations Committee and FDA-Related Matters Committee.
A version of this article first appeared on Medscape.com.
Recent studies have cited an alarming increase in early-onset colorectal cancer (CRC) rates, raising concern among gastroenterologists, public health experts, and patients alike. Approximately 10% of CRC cases now occur in those under age 50, and that proportion continues to grow. Between 2000 and 2016, colon cancer rose by 13% and rectal cancer by 16% among those aged 40–49.
According to recently published data from the Surveillance, Epidemiology and End Results Program, between 2019 and 2022, CRC incidence among patients aged 45–49 rose by approximately 12% per year.
A Potential Bacterial Connection
What accounts for this disturbing spike? A research group from the University of California, San Diego, may have uncovered part of the answer.
In their study of 981 CRC genomes, most carried mutations suggestive of prior exposure to colibactin, a toxin produced by certain Escherichia coli (E coli) strains. Patients with extremely early-onset CRC (aged < 40 years) were 3 times more likely to have colibactin-suggestive mutations than patients older than 70. Crucially, colonic exposure to colibactin was linked to an adenomatous polyposis coli driver mutation.
These findings suggest that colibactin-induced injury in the gut microbiome may accelerate cancer development in some individuals. Environmental factors may contribute to the rise in early-onset CRC as well, such as consuming red meats, carcinogens from grilling, and processed meats and other highly processed foods; low fiber intake; lack of fruits and vegetables; drinking alcohol; lack of exercise; obesity; and colibactin exposure.
In this video, we will take a closer look at how E coli and colibactin may increase CRC risk.
Bacteria’s Cancer-Causing Properties
The idea that bacteria has cancer-causing properties isn’t new. In the 1970s, researchers linked Streptococcus bovis type 1 (now called Streptococcus gallolyticus) to CRC in a subset of patients with bacterial endocarditis stemming from right-sided colon cancer. Similarly, Helicobacter pylori infection has long been associated with increased gastric cancer risk.
Today, E coli infection is emerging as another possible contributor to CRC, especially via certain pathogenic strains containing the polyketide synthase (pks) genomic island, which encodes the colibactin and is sometimes present in the colon mucosa of patients with CRC.
Colibactin and DNA Damage
Colibactin-producing pks+ E coli strains can cause DNA double-strand breaks, one pathway to carcinogenesis. In animal studies, pks+ E coli strains have been linked to both increased risk for CRC and CRC progression.
In an important study published in Nature, Pleguezuelos-Manzano and colleagues repeatedly exposed intestinal organoids to pks+ E coli over 5 months and then performed whole genome sequencing. The result was a concerning potential for short insertions and deletions and single–base substitutions.
The authors concluded that their “study describes the distinct mutational signature in colorectal cancer and implies that the underlying mutational process results directly from past exposure to bacteria carrying the colibactin-producing pks pathogenicity island.”
Other E coli virulence factors may also contribute. For example, alpha-hemolysin may downregulate DNA mismatch repair proteins. In other words, E coli is probably just a contributing factor for the development of CRC, not the sole cause.
Biofilms and Inflammation
Previous studies have associated dense bacterial biofilms, particularly antibiotic-resistant strains, with CRC. This raises the possibility that widespread antibiotic overuse could predispose certain individuals to CRC development.
Biofilms normally separate the colon mucosal epithelium from bacteria and are essential for protecting against inflammation. In a 2018 study in Science, Dejea and colleagues concluded that “tumor-prone mice colonized with E coli (expressing colibactin), and enterotoxigenic B fragilis showed increased interleukin-17 in the colon and DNA damage in colonic epithelium with faster tumor onset and greater mortality, compared to mice with either bacteria strain alone. These data suggest an unexpected link between early neoplasia of the colon and tumorigenic bacteria.”
Additional research revealed that E coli can create a pro-carcinogenic environment by stimulating mucosal inflammation, hindering DNA and mismatch repair mechanisms, and altering immune responses.
Dysbiosis and Diet
Colibactin can also drive dysbiosis and imbalance in bacteria in the colon, which fuels inflammation and disrupts mucosal barrier integrity. This creates a vicious cycle in which chronic inflammation can further drive additional mucus deterioration and dysbiosis.
In mouse models where the colon mucosal barrier is damaged with dextrin sulfate sodium (DSS), pks+ E coli gains better access to colon epithelium, causes injury, and can even lead to chronic colitis. Colibactin can also hinder epithelial recovery after DSS treatment.
Diet plays a central role in this process. Low fiber consumption can disrupt the barrier between the colon mucus layer and the colon’s exterior layer where bacteria live. A traditional Western diet may bolster bacteria that degrade the mucus layer when the bacteria consume the glycosylated portion as an energy source.
Fortunately, diet is modifiable. High–fiber diets (ideally 25-30 g/d) boost short–chain fatty acids in the colon. This is important because short-chain fatty acids can decrease intercellular pH and impede Enterobacteriaceae replication, yet another reason why we should encourage patients to eat a diet high in vegetables, fruits, and [green] salads.
Two Types of Bacterial Drivers
There appear to be two broad types of bacteria associated with CRC development. It’s been hypothesized that there are “driver” bacteria that might initiate the development of CRC, possibly by creating oxidative stress and causing DNA breaks. Several potential pathogenic bacteria have been identified, including E coli, Enterococcus faecalis, and Bacteroides fragilis. Unfortunately, there are also bacteria such as Fusobacterium species and Streptococcus gallolyticus with the potential to alter intestinal permeability, resulting in downstream effects that can allow colon cancers to expand. Fusobacterium species and Streptococcus gallolyticus have the potential to cause DNA double–strand breaks in the intestine, which can produce chromosomal precariousness.
These secondary bacteria can also lead to DNA epigenetic changes and gene mutations. However, it should be emphasized that “the direct causation of imprinted DNA changes resulting from a direct interaction between bacteria and host cells is not so far established.”
E coli produces compounds called cyclomodulins, which can cause DNA breaks and potentially trigger cell cycle arrest and even cell death through activation of the DNA damage checkpoint pathway. The DNA damage checkpoint pathway is a cellular signaling network that helps detect DNA lesions and allows for genetic stability by stopping growth to allow for repair and simulating cell survival or apoptosis. A key cyclomodulin that E coli makes is colibactin, produced by the pks locus. Other cyclomodulins include cytolethal distending toxin, cytotoxic necrotizing factor, and cycle-inhibiting factor.
Previous research has shown that E coli is the only culturable bacteria found near CRC. A groundbreaking 1998 study employing PCR technology found E coli in 60% of colon polyp adenomas and an alarming 77% of CRC biopsies.
E coli’s capability to downregulate essential DNA mismatch repair proteins has been implicated in colorectal carcinogenesis. Interestingly, when the genetic region responsible for producing colibactin is deleted in animals, the bacteria aren’t able to promote cancer.
Mechanistically, colibactin causes double-stranded DNA breaks, eukaryotic cell cycle arrest, and chromosome abnormalities. It also alkylates DNA. This occurs when the cyclopropane ring of colibactin interacts with the N3 position of adenine in DNA, forming a covalent bond and creating a DNA adduct. DNA adducts occur when a chemical moiety from an environmental or dietary source binds to DNA base. Colibactin can cause DNA interstrand cross-links to form via alkalization of adenine residues on opposing DNA strands, a crucial step in DNA damage. DNA adducts can occur through carcinogens in N-nitroso compounds, such as in processed meats and in polycyclic aromatic hydrocarbons found in cigarette smoke. Colibactin-induced damage may also stimulate the senescence–associated secretory phenotype pathway, increasing proinflammatory cytokines.
E coli and Inflammatory Bowel Disease
E coli, the primary colibactin producer in the human intestinal microbiome, is found at higher bacterial percentages in the microbiomes of patients with inflammatory bowel disease (IBD). In a study by Dubinsky and colleagues, “the medium relative levels of colibactin–encoding E. coli were about threefold higher in IBD.”
Researchers have also postulated that antibiotics and microbiome dysbiosis may create conditions that allow colibactin–producing bacteria to overpopulate.
Future Directions
Not every patient with CRC carries a colorectal mutational signature, but these findings underscore the need for continued vigilance and prevention.
From a public health standpoint, our advice remains consistent: Promote high-fiber diets with more vegetables and less red meat; avoid highly processed foods; avoid alcohol; encourage exercise; and address overweight and obesity. Our goal is to create the best possible colon environment to prevent DNA damage from bacterial and environmental carcinogens.
In the future, we need more research to clarify exactly how E coli and colibactin increase early–onset CRC risk and whether antibiotics and dysbiosis facilitate their ability to damage the DNA of colon mucosa. It’s still unclear why younger patients are at greater risk. In time, we may be able to screen for colibactin–producing bacteria such as E coli and manipulate the fecal microbiome to prevent damage.
A recent mouse study in Nature by Jans and colleagues suggests it might be possible to block bacterial adhesion and hopefully mitigate damage caused by colibactin. With continued work, colibactin–targeted strategies could become a part of CRC prevention.
Benjamin H. Levy III, MD, is a gastroenterologist at the University of Chicago. In 2017, Levy, a previous Fulbright Fellow in France, also started a gastroenterology clinic for refugees resettling in Chicago. His clinical projects focus on the development of colorectal cancer screening campaigns. Levy, who gave a TEDx Talk about building health education campaigns using music and concerts, organizes "Tune It Up: A Concert To Raise Colorectal Cancer Awareness" with the American College of Gastroenterology (ACG). He frequently publishes on a variety of gastroenterology topics and serves on ACG’s Public Relations Committee and FDA-Related Matters Committee.
A version of this article first appeared on Medscape.com.
Is There Really a Cancer Epidemic in Younger Adults?
A global analysis challenged the notion that a rise in cancer is disproportionately affecting younger adults, finding instead that several cancer types previously seen rising in younger adults are also increasing in older adults.
More specifically, the analysis found that incidence rates for thyroid cancer, breast cancer, kidney cancer, endometrial cancer, and leukemia increased similarly in both younger and older adults in most countries over a 15-year period. Colorectal cancer (CRC) was the exception, where incidence rates increased in younger adults in most countries but only increased slightly in older adults in about half and decreased in about one quarter.
“Our findings suggest that whatever is triggering the rise in these cancers is more likely to be common across all age groups, rather than specific to cancers in the under 50s, since there were similar increases in younger and older adults,” Amy Berrington de González, DPhil, The Institute of Cancer Research, London, England, who led the study, said in a statement.
The authors of an editorial agreed, adding that the growing “concern about increasing cancer rates should recognize that this increase is not restricted to young adults but affects all generations.”
The study and editorial were published recently in Annals of Internal Medicine.
Data Defy Early-Onset Cancer Epidemic Narrative
A growing body of evidence suggests that cancer incidence rates are increasing among younger adults in many countries. However, studies tracking international trends have largely evaluated cancer incidence in younger adults without comparing these trends in older adults or analyses have focused the age comparison in individual countries, Berrington de González and colleagues explained.
To better understand cancer incidence trends across countries and age groups, the researchers evaluated cancer trends in 42 countries between 2003 and 2017, focusing on 13 cancer types previously reported to be climbing in adults younger than age 50 years.
The researchers found that incidence rates for six of the 13 cancer types increased among younger adults (aged 20-49 years) in more than three quarters of the countries studied.
The largest increase was in thyroid cancer (median average annual percentage change [AAPC], 3.57%), followed by kidney cancer (median AAPC, 2.21%), endometrial cancer (median AAPC, 1.66%), CRC (median AAPC, 1.45%), breast cancer (median AAPC, 0.89%), and leukemia (median AAPC, 0.78%).
But with the exception of CRC, incidence rates for these cancers increased to a similar degree in adults aged 50 years or older — with median AAPCs of 3% (vs 3.57%) for thyroid cancer, 1.65% (vs 2.21%) for kidney cancer, 1.20% (vs 1.66%) for endometrial cancer, 0.86% (vs 0.89%) for breast cancer, and 0.61% (vs 0.78%) for leukemia.
In older adults, CRC incidence rates only increased in about half the countries (median AAPC, 0.37%), and the annual percentage change was much greater in younger than older adults in nearly 70% of countries. CRC incidence rates in older individuals also decreased in nearly 25% of countries.
Why is CRC an apparent outlier?
“Bowel cancer screening not only helps detect cancer at earlier stages but also helps prevent cancer through the removal of premalignant lesions,” Berrington de González said. “This could be why bowel cancer cases seem to be rising faster in younger adults — we’re getting better at preventing them developing in older adults.”
The incidence of certain cancers also declined in younger adults. Specifically, rates of liver, oral, esophageal, and stomach cancers decreased in younger adults in more than half of countries assessed, with median AAPCs of -0.14% for liver, -0.42% for oral, -0.92% for esophageal, and -1.62% for stomach cancers.
Over half of countries also saw declining rates of stomach (median AAPC, -2.05%) and esophageal (median AAPC, -0.25%) cancers among older adults, while rates of liver and oral cancers increased in older individuals (median AAPC, 2.17% and 0.49%, respectively).
For gallbladder, pancreatic, and prostate cancers — three other cancers previously found to be increasing in younger adults — the researchers reported that incidence rates increased in younger adults in just over half of countries (median AAPCs, 3.2% for prostate cancer, 0.49% for gallbladder cancer, and 1% for pancreatic cancer). Incidence rates also often increased in older adults but to a lesser extent (median AAPCs, 0.75% for prostate cancer, -0.10% for gallbladder, and 0.96% for pancreatic cancer).
True Rise or Increased Scrutiny?
Why are cancer rates increasing?
“Understanding factors that contribute to the increase in incidence across the age spectrum was beyond the scope of the study,” editorialists Christopher Cann, MD, Fox Chase Cancer Center, and Efrat Dotan, MD, University of Pennsylvania Health System, both in Philadelphia, wrote.
Several studies have suggested that rising rates of obesity could help explain increasing cancer incidence, particularly in younger adults. In fact, “the cancers that we identified as increasing are all obesity-related cancers, including endometrial and kidney cancer,” Berrington de González said. However, so far, the evidence on this link remains unclear, she acknowledged.
Weighing in on the study, Gilbert Welch, MD, Brigham and Women’s Hospital, Boston, told this news organization that it’s “critically important” to distinguish between two explanations for rising cancer incidence.
There may be an increase in the true occurrence of clinically meaningful cancer, which “warrants investigation into biologic explanations, better treatment, and perhaps more testing,” Welch said.
But it may instead reflect changes in diagnostic scrutiny. “Simply put, whenever we doctors look harder for cancer, we find more,” Welch said. “And there are lots of ways to look harder: testing more people, testing people more frequently, using tests with increasing ability to detect small irregularities, and using lower diagnostic thresholds for labeling these as cancer.”
If increased incidence is the result of greater diagnostic scrutiny, searching for biologic causes is bound to be unproductive and more testing will only aggravate the problem, he explained.
Welch pointed out that the fastest rising cancer in both younger and older adults was thyroid cancer (AAPC, ≥ 3%), which is “exquisitely sensitive” to diagnostic scrutiny.
Take what happened in South Korea. Around 2000, the government of South Korea started a national screening program for breast, colon, and stomach cancers. Doctors and hospitals often added on ultrasound scans for thyroid cancer for a small additional fee.
“A decade later the rate of thyroid cancer diagnosis had increased 15-fold, turning what was once a rare cancer into the most common cancer in Korea,” Welch said. “But the death rate from thyroid cancer did not change. This was not an epidemic of disease; this was an epidemic of diagnosis.”
Welch also noted that the study authors and editorialists put the finding in perspective by explaining that, despite the rising rates of certain cancers in younger adults, cancer remains rare in these adults.
Welch highlighted that, for younger adults in the US, cancer death rates in young adults have cut in half over the last 30 years. “Cancer accounts for only 10% of deaths in young people in the US — and that number is falling,” Welch said.
The study was funded by the Institute of Cancer Research and the National Institutes of Health Intramural Research Program. Disclosures for authors and editorial writers are available with the original articles. Welch reported receiving royalties from three books including “Should I be tested for cancer?”
A version of this article first appeared on Medscape.com.
A global analysis challenged the notion that a rise in cancer is disproportionately affecting younger adults, finding instead that several cancer types previously seen rising in younger adults are also increasing in older adults.
More specifically, the analysis found that incidence rates for thyroid cancer, breast cancer, kidney cancer, endometrial cancer, and leukemia increased similarly in both younger and older adults in most countries over a 15-year period. Colorectal cancer (CRC) was the exception, where incidence rates increased in younger adults in most countries but only increased slightly in older adults in about half and decreased in about one quarter.
“Our findings suggest that whatever is triggering the rise in these cancers is more likely to be common across all age groups, rather than specific to cancers in the under 50s, since there were similar increases in younger and older adults,” Amy Berrington de González, DPhil, The Institute of Cancer Research, London, England, who led the study, said in a statement.
The authors of an editorial agreed, adding that the growing “concern about increasing cancer rates should recognize that this increase is not restricted to young adults but affects all generations.”
The study and editorial were published recently in Annals of Internal Medicine.
Data Defy Early-Onset Cancer Epidemic Narrative
A growing body of evidence suggests that cancer incidence rates are increasing among younger adults in many countries. However, studies tracking international trends have largely evaluated cancer incidence in younger adults without comparing these trends in older adults or analyses have focused the age comparison in individual countries, Berrington de González and colleagues explained.
To better understand cancer incidence trends across countries and age groups, the researchers evaluated cancer trends in 42 countries between 2003 and 2017, focusing on 13 cancer types previously reported to be climbing in adults younger than age 50 years.
The researchers found that incidence rates for six of the 13 cancer types increased among younger adults (aged 20-49 years) in more than three quarters of the countries studied.
The largest increase was in thyroid cancer (median average annual percentage change [AAPC], 3.57%), followed by kidney cancer (median AAPC, 2.21%), endometrial cancer (median AAPC, 1.66%), CRC (median AAPC, 1.45%), breast cancer (median AAPC, 0.89%), and leukemia (median AAPC, 0.78%).
But with the exception of CRC, incidence rates for these cancers increased to a similar degree in adults aged 50 years or older — with median AAPCs of 3% (vs 3.57%) for thyroid cancer, 1.65% (vs 2.21%) for kidney cancer, 1.20% (vs 1.66%) for endometrial cancer, 0.86% (vs 0.89%) for breast cancer, and 0.61% (vs 0.78%) for leukemia.
In older adults, CRC incidence rates only increased in about half the countries (median AAPC, 0.37%), and the annual percentage change was much greater in younger than older adults in nearly 70% of countries. CRC incidence rates in older individuals also decreased in nearly 25% of countries.
Why is CRC an apparent outlier?
“Bowel cancer screening not only helps detect cancer at earlier stages but also helps prevent cancer through the removal of premalignant lesions,” Berrington de González said. “This could be why bowel cancer cases seem to be rising faster in younger adults — we’re getting better at preventing them developing in older adults.”
The incidence of certain cancers also declined in younger adults. Specifically, rates of liver, oral, esophageal, and stomach cancers decreased in younger adults in more than half of countries assessed, with median AAPCs of -0.14% for liver, -0.42% for oral, -0.92% for esophageal, and -1.62% for stomach cancers.
Over half of countries also saw declining rates of stomach (median AAPC, -2.05%) and esophageal (median AAPC, -0.25%) cancers among older adults, while rates of liver and oral cancers increased in older individuals (median AAPC, 2.17% and 0.49%, respectively).
For gallbladder, pancreatic, and prostate cancers — three other cancers previously found to be increasing in younger adults — the researchers reported that incidence rates increased in younger adults in just over half of countries (median AAPCs, 3.2% for prostate cancer, 0.49% for gallbladder cancer, and 1% for pancreatic cancer). Incidence rates also often increased in older adults but to a lesser extent (median AAPCs, 0.75% for prostate cancer, -0.10% for gallbladder, and 0.96% for pancreatic cancer).
True Rise or Increased Scrutiny?
Why are cancer rates increasing?
“Understanding factors that contribute to the increase in incidence across the age spectrum was beyond the scope of the study,” editorialists Christopher Cann, MD, Fox Chase Cancer Center, and Efrat Dotan, MD, University of Pennsylvania Health System, both in Philadelphia, wrote.
Several studies have suggested that rising rates of obesity could help explain increasing cancer incidence, particularly in younger adults. In fact, “the cancers that we identified as increasing are all obesity-related cancers, including endometrial and kidney cancer,” Berrington de González said. However, so far, the evidence on this link remains unclear, she acknowledged.
Weighing in on the study, Gilbert Welch, MD, Brigham and Women’s Hospital, Boston, told this news organization that it’s “critically important” to distinguish between two explanations for rising cancer incidence.
There may be an increase in the true occurrence of clinically meaningful cancer, which “warrants investigation into biologic explanations, better treatment, and perhaps more testing,” Welch said.
But it may instead reflect changes in diagnostic scrutiny. “Simply put, whenever we doctors look harder for cancer, we find more,” Welch said. “And there are lots of ways to look harder: testing more people, testing people more frequently, using tests with increasing ability to detect small irregularities, and using lower diagnostic thresholds for labeling these as cancer.”
If increased incidence is the result of greater diagnostic scrutiny, searching for biologic causes is bound to be unproductive and more testing will only aggravate the problem, he explained.
Welch pointed out that the fastest rising cancer in both younger and older adults was thyroid cancer (AAPC, ≥ 3%), which is “exquisitely sensitive” to diagnostic scrutiny.
Take what happened in South Korea. Around 2000, the government of South Korea started a national screening program for breast, colon, and stomach cancers. Doctors and hospitals often added on ultrasound scans for thyroid cancer for a small additional fee.
“A decade later the rate of thyroid cancer diagnosis had increased 15-fold, turning what was once a rare cancer into the most common cancer in Korea,” Welch said. “But the death rate from thyroid cancer did not change. This was not an epidemic of disease; this was an epidemic of diagnosis.”
Welch also noted that the study authors and editorialists put the finding in perspective by explaining that, despite the rising rates of certain cancers in younger adults, cancer remains rare in these adults.
Welch highlighted that, for younger adults in the US, cancer death rates in young adults have cut in half over the last 30 years. “Cancer accounts for only 10% of deaths in young people in the US — and that number is falling,” Welch said.
The study was funded by the Institute of Cancer Research and the National Institutes of Health Intramural Research Program. Disclosures for authors and editorial writers are available with the original articles. Welch reported receiving royalties from three books including “Should I be tested for cancer?”
A version of this article first appeared on Medscape.com.
A global analysis challenged the notion that a rise in cancer is disproportionately affecting younger adults, finding instead that several cancer types previously seen rising in younger adults are also increasing in older adults.
More specifically, the analysis found that incidence rates for thyroid cancer, breast cancer, kidney cancer, endometrial cancer, and leukemia increased similarly in both younger and older adults in most countries over a 15-year period. Colorectal cancer (CRC) was the exception, where incidence rates increased in younger adults in most countries but only increased slightly in older adults in about half and decreased in about one quarter.
“Our findings suggest that whatever is triggering the rise in these cancers is more likely to be common across all age groups, rather than specific to cancers in the under 50s, since there were similar increases in younger and older adults,” Amy Berrington de González, DPhil, The Institute of Cancer Research, London, England, who led the study, said in a statement.
The authors of an editorial agreed, adding that the growing “concern about increasing cancer rates should recognize that this increase is not restricted to young adults but affects all generations.”
The study and editorial were published recently in Annals of Internal Medicine.
Data Defy Early-Onset Cancer Epidemic Narrative
A growing body of evidence suggests that cancer incidence rates are increasing among younger adults in many countries. However, studies tracking international trends have largely evaluated cancer incidence in younger adults without comparing these trends in older adults or analyses have focused the age comparison in individual countries, Berrington de González and colleagues explained.
To better understand cancer incidence trends across countries and age groups, the researchers evaluated cancer trends in 42 countries between 2003 and 2017, focusing on 13 cancer types previously reported to be climbing in adults younger than age 50 years.
The researchers found that incidence rates for six of the 13 cancer types increased among younger adults (aged 20-49 years) in more than three quarters of the countries studied.
The largest increase was in thyroid cancer (median average annual percentage change [AAPC], 3.57%), followed by kidney cancer (median AAPC, 2.21%), endometrial cancer (median AAPC, 1.66%), CRC (median AAPC, 1.45%), breast cancer (median AAPC, 0.89%), and leukemia (median AAPC, 0.78%).
But with the exception of CRC, incidence rates for these cancers increased to a similar degree in adults aged 50 years or older — with median AAPCs of 3% (vs 3.57%) for thyroid cancer, 1.65% (vs 2.21%) for kidney cancer, 1.20% (vs 1.66%) for endometrial cancer, 0.86% (vs 0.89%) for breast cancer, and 0.61% (vs 0.78%) for leukemia.
In older adults, CRC incidence rates only increased in about half the countries (median AAPC, 0.37%), and the annual percentage change was much greater in younger than older adults in nearly 70% of countries. CRC incidence rates in older individuals also decreased in nearly 25% of countries.
Why is CRC an apparent outlier?
“Bowel cancer screening not only helps detect cancer at earlier stages but also helps prevent cancer through the removal of premalignant lesions,” Berrington de González said. “This could be why bowel cancer cases seem to be rising faster in younger adults — we’re getting better at preventing them developing in older adults.”
The incidence of certain cancers also declined in younger adults. Specifically, rates of liver, oral, esophageal, and stomach cancers decreased in younger adults in more than half of countries assessed, with median AAPCs of -0.14% for liver, -0.42% for oral, -0.92% for esophageal, and -1.62% for stomach cancers.
Over half of countries also saw declining rates of stomach (median AAPC, -2.05%) and esophageal (median AAPC, -0.25%) cancers among older adults, while rates of liver and oral cancers increased in older individuals (median AAPC, 2.17% and 0.49%, respectively).
For gallbladder, pancreatic, and prostate cancers — three other cancers previously found to be increasing in younger adults — the researchers reported that incidence rates increased in younger adults in just over half of countries (median AAPCs, 3.2% for prostate cancer, 0.49% for gallbladder cancer, and 1% for pancreatic cancer). Incidence rates also often increased in older adults but to a lesser extent (median AAPCs, 0.75% for prostate cancer, -0.10% for gallbladder, and 0.96% for pancreatic cancer).
True Rise or Increased Scrutiny?
Why are cancer rates increasing?
“Understanding factors that contribute to the increase in incidence across the age spectrum was beyond the scope of the study,” editorialists Christopher Cann, MD, Fox Chase Cancer Center, and Efrat Dotan, MD, University of Pennsylvania Health System, both in Philadelphia, wrote.
Several studies have suggested that rising rates of obesity could help explain increasing cancer incidence, particularly in younger adults. In fact, “the cancers that we identified as increasing are all obesity-related cancers, including endometrial and kidney cancer,” Berrington de González said. However, so far, the evidence on this link remains unclear, she acknowledged.
Weighing in on the study, Gilbert Welch, MD, Brigham and Women’s Hospital, Boston, told this news organization that it’s “critically important” to distinguish between two explanations for rising cancer incidence.
There may be an increase in the true occurrence of clinically meaningful cancer, which “warrants investigation into biologic explanations, better treatment, and perhaps more testing,” Welch said.
But it may instead reflect changes in diagnostic scrutiny. “Simply put, whenever we doctors look harder for cancer, we find more,” Welch said. “And there are lots of ways to look harder: testing more people, testing people more frequently, using tests with increasing ability to detect small irregularities, and using lower diagnostic thresholds for labeling these as cancer.”
If increased incidence is the result of greater diagnostic scrutiny, searching for biologic causes is bound to be unproductive and more testing will only aggravate the problem, he explained.
Welch pointed out that the fastest rising cancer in both younger and older adults was thyroid cancer (AAPC, ≥ 3%), which is “exquisitely sensitive” to diagnostic scrutiny.
Take what happened in South Korea. Around 2000, the government of South Korea started a national screening program for breast, colon, and stomach cancers. Doctors and hospitals often added on ultrasound scans for thyroid cancer for a small additional fee.
“A decade later the rate of thyroid cancer diagnosis had increased 15-fold, turning what was once a rare cancer into the most common cancer in Korea,” Welch said. “But the death rate from thyroid cancer did not change. This was not an epidemic of disease; this was an epidemic of diagnosis.”
Welch also noted that the study authors and editorialists put the finding in perspective by explaining that, despite the rising rates of certain cancers in younger adults, cancer remains rare in these adults.
Welch highlighted that, for younger adults in the US, cancer death rates in young adults have cut in half over the last 30 years. “Cancer accounts for only 10% of deaths in young people in the US — and that number is falling,” Welch said.
The study was funded by the Institute of Cancer Research and the National Institutes of Health Intramural Research Program. Disclosures for authors and editorial writers are available with the original articles. Welch reported receiving royalties from three books including “Should I be tested for cancer?”
A version of this article first appeared on Medscape.com.
LLMs Show High Accuracy in Extracting CRC Data From VA Health Records
TOPLINE: Large Language Models (LLMs) achieve more than 95% accuracy in extracting colorectal cancer and dysplasia diagnoses from Veterans Health Administration (VHA) pathology reports, including patients with Million Veteran Program (MVP) genomic data. The validated approach using publicly available LLMs demonstrates excellent performance across both Inflammatory Bowel Disease (IBD) and non-IBD populations.
METHODOLOGY:
Researchers analyzed 116,373 pathology reports generated in the VHA between 1999 and 2024, utilizing search term filtering followed by simple yes/no question prompts for identifying colorectal dysplasia, high-grade dysplasia and/or colorectal adenocarcinoma, and invasive colorectal cancer.
Results were compared to blinded manual chart review of 200 to 300 pathology reports for each patient cohort and diagnostic task, totaling 3,816 reviewed reports, to validate the LLM approach.
Validation was performed independently in IBD and non-IBD populations using Gemma-2 and Llama-3 LLMs without any task-specific training or fine-tuning.
Performance metrics included F1 scores, positive predictive value, negative predictive value, sensitivity, specificity, and Matthew's correlation coefficient to evaluate accuracy across different tasks.
TAKEAWAY:
In patients with IBD in the MVP, the LLM achieved (F1-score, 96.9%; 95% confidence interval [CI], 94.0%-99.6%) for identifying dysplasia, (F1-score, 93.7%; 95% CI, 88.2%-98.4%) for identifying high-grade dysplasia/colorectal cancer, and (F1-score, 98%; 95% CI, 96.3%-99.4%) for identifying colorectal cancer.
In non-IBD MVP patients, the LLM demonstrated (F1-score, 99.2%; 95% CI, 98.2%-100%) for identifying colorectal dysplasia, (F1-score, 96.5%; 95% CI, 93.0%-99.2%) for high-grade dysplasia/colorectal cancer, and (F1-score, 95%; 95% CI, 92.8%-97.2%) for identifying colorectal cancer.
Agreement between reviewers was excellent across tasks, with (Cohen's kappa, 89%-97%) for main tasks, and (Cohen's kappa, 78.1%-93.1%) for indefinite for dysplasia in IBD cohort.
The LLM approach maintained high accuracy when applied to full pathology reports, with (F1-score, 97.1%; 95% CI, 93.5%-100%) for dysplasia detection in IBD patients.
IN PRACTICE: “We have shown that LLMs are powerful, potentially generalizable tools for accurately extracting important information from clinical semistructured and unstructured text and which require little human-led development.” the authors of the study wrote
SOURCE: The study was based on data from the Million Veteran Program and supported by the Office of Research and Development, Veterans Health Administration, and the US Department of Veterans Affairs Biomedical Laboratory. It was published online in BMJ Open Gastroenterology.
LIMITATIONS: According to the authors, this research may be specific to the VHA system and the LLM models used. The authors did not test larger models. The authors acknowledge that without long-term access to graphics processing units, they could not feasibly test larger models, which may overcome some of the shortcomings seen in smaller models. Additionally, the researchers could not rule out overlap between Million Veteran Program and Corporate Data Warehouse reports, though they state that results in either cohort alone are sufficient validation compared with previously published work.
DISCLOSURES: The study was supported by Merit Review Award from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service, AGA Research Foundation, National Institutes of Health grants, and the National Library of Medicine Training Grant. Kit Curtius reported receiving an investigator-led research grant from Phathom Pharmaceuticals. Shailja C Shah disclosed being a paid consultant for RedHill Biopharma and Phathom Pharmaceuticals, and an unpaid scientific advisory board member for Ilico Genetics, Inc.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: Large Language Models (LLMs) achieve more than 95% accuracy in extracting colorectal cancer and dysplasia diagnoses from Veterans Health Administration (VHA) pathology reports, including patients with Million Veteran Program (MVP) genomic data. The validated approach using publicly available LLMs demonstrates excellent performance across both Inflammatory Bowel Disease (IBD) and non-IBD populations.
METHODOLOGY:
Researchers analyzed 116,373 pathology reports generated in the VHA between 1999 and 2024, utilizing search term filtering followed by simple yes/no question prompts for identifying colorectal dysplasia, high-grade dysplasia and/or colorectal adenocarcinoma, and invasive colorectal cancer.
Results were compared to blinded manual chart review of 200 to 300 pathology reports for each patient cohort and diagnostic task, totaling 3,816 reviewed reports, to validate the LLM approach.
Validation was performed independently in IBD and non-IBD populations using Gemma-2 and Llama-3 LLMs without any task-specific training or fine-tuning.
Performance metrics included F1 scores, positive predictive value, negative predictive value, sensitivity, specificity, and Matthew's correlation coefficient to evaluate accuracy across different tasks.
TAKEAWAY:
In patients with IBD in the MVP, the LLM achieved (F1-score, 96.9%; 95% confidence interval [CI], 94.0%-99.6%) for identifying dysplasia, (F1-score, 93.7%; 95% CI, 88.2%-98.4%) for identifying high-grade dysplasia/colorectal cancer, and (F1-score, 98%; 95% CI, 96.3%-99.4%) for identifying colorectal cancer.
In non-IBD MVP patients, the LLM demonstrated (F1-score, 99.2%; 95% CI, 98.2%-100%) for identifying colorectal dysplasia, (F1-score, 96.5%; 95% CI, 93.0%-99.2%) for high-grade dysplasia/colorectal cancer, and (F1-score, 95%; 95% CI, 92.8%-97.2%) for identifying colorectal cancer.
Agreement between reviewers was excellent across tasks, with (Cohen's kappa, 89%-97%) for main tasks, and (Cohen's kappa, 78.1%-93.1%) for indefinite for dysplasia in IBD cohort.
The LLM approach maintained high accuracy when applied to full pathology reports, with (F1-score, 97.1%; 95% CI, 93.5%-100%) for dysplasia detection in IBD patients.
IN PRACTICE: “We have shown that LLMs are powerful, potentially generalizable tools for accurately extracting important information from clinical semistructured and unstructured text and which require little human-led development.” the authors of the study wrote
SOURCE: The study was based on data from the Million Veteran Program and supported by the Office of Research and Development, Veterans Health Administration, and the US Department of Veterans Affairs Biomedical Laboratory. It was published online in BMJ Open Gastroenterology.
LIMITATIONS: According to the authors, this research may be specific to the VHA system and the LLM models used. The authors did not test larger models. The authors acknowledge that without long-term access to graphics processing units, they could not feasibly test larger models, which may overcome some of the shortcomings seen in smaller models. Additionally, the researchers could not rule out overlap between Million Veteran Program and Corporate Data Warehouse reports, though they state that results in either cohort alone are sufficient validation compared with previously published work.
DISCLOSURES: The study was supported by Merit Review Award from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service, AGA Research Foundation, National Institutes of Health grants, and the National Library of Medicine Training Grant. Kit Curtius reported receiving an investigator-led research grant from Phathom Pharmaceuticals. Shailja C Shah disclosed being a paid consultant for RedHill Biopharma and Phathom Pharmaceuticals, and an unpaid scientific advisory board member for Ilico Genetics, Inc.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: Large Language Models (LLMs) achieve more than 95% accuracy in extracting colorectal cancer and dysplasia diagnoses from Veterans Health Administration (VHA) pathology reports, including patients with Million Veteran Program (MVP) genomic data. The validated approach using publicly available LLMs demonstrates excellent performance across both Inflammatory Bowel Disease (IBD) and non-IBD populations.
METHODOLOGY:
Researchers analyzed 116,373 pathology reports generated in the VHA between 1999 and 2024, utilizing search term filtering followed by simple yes/no question prompts for identifying colorectal dysplasia, high-grade dysplasia and/or colorectal adenocarcinoma, and invasive colorectal cancer.
Results were compared to blinded manual chart review of 200 to 300 pathology reports for each patient cohort and diagnostic task, totaling 3,816 reviewed reports, to validate the LLM approach.
Validation was performed independently in IBD and non-IBD populations using Gemma-2 and Llama-3 LLMs without any task-specific training or fine-tuning.
Performance metrics included F1 scores, positive predictive value, negative predictive value, sensitivity, specificity, and Matthew's correlation coefficient to evaluate accuracy across different tasks.
TAKEAWAY:
In patients with IBD in the MVP, the LLM achieved (F1-score, 96.9%; 95% confidence interval [CI], 94.0%-99.6%) for identifying dysplasia, (F1-score, 93.7%; 95% CI, 88.2%-98.4%) for identifying high-grade dysplasia/colorectal cancer, and (F1-score, 98%; 95% CI, 96.3%-99.4%) for identifying colorectal cancer.
In non-IBD MVP patients, the LLM demonstrated (F1-score, 99.2%; 95% CI, 98.2%-100%) for identifying colorectal dysplasia, (F1-score, 96.5%; 95% CI, 93.0%-99.2%) for high-grade dysplasia/colorectal cancer, and (F1-score, 95%; 95% CI, 92.8%-97.2%) for identifying colorectal cancer.
Agreement between reviewers was excellent across tasks, with (Cohen's kappa, 89%-97%) for main tasks, and (Cohen's kappa, 78.1%-93.1%) for indefinite for dysplasia in IBD cohort.
The LLM approach maintained high accuracy when applied to full pathology reports, with (F1-score, 97.1%; 95% CI, 93.5%-100%) for dysplasia detection in IBD patients.
IN PRACTICE: “We have shown that LLMs are powerful, potentially generalizable tools for accurately extracting important information from clinical semistructured and unstructured text and which require little human-led development.” the authors of the study wrote
SOURCE: The study was based on data from the Million Veteran Program and supported by the Office of Research and Development, Veterans Health Administration, and the US Department of Veterans Affairs Biomedical Laboratory. It was published online in BMJ Open Gastroenterology.
LIMITATIONS: According to the authors, this research may be specific to the VHA system and the LLM models used. The authors did not test larger models. The authors acknowledge that without long-term access to graphics processing units, they could not feasibly test larger models, which may overcome some of the shortcomings seen in smaller models. Additionally, the researchers could not rule out overlap between Million Veteran Program and Corporate Data Warehouse reports, though they state that results in either cohort alone are sufficient validation compared with previously published work.
DISCLOSURES: The study was supported by Merit Review Award from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service, AGA Research Foundation, National Institutes of Health grants, and the National Library of Medicine Training Grant. Kit Curtius reported receiving an investigator-led research grant from Phathom Pharmaceuticals. Shailja C Shah disclosed being a paid consultant for RedHill Biopharma and Phathom Pharmaceuticals, and an unpaid scientific advisory board member for Ilico Genetics, Inc.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Patients With a Positive FIT Fail to Get Follow-Up Colonoscopies
Patients With a Positive FIT Fail to Get Follow-Up Colonoscopies
PHOENIX -- Patients with or without polyp removal in an index colonoscopy commonly receive follow-up surveillance with a fecal immunochemical test (FIT), yet many of these patients do not receive a recommended colonoscopy after a positive FIT.
"In this large US study, we found interval FITs are frequently performed in patients with and without prior polypectomy," said first author Natalie J. Wilson, MD, of the University of Minnesota in Minneapolis, while presenting the findings this week at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
"These findings reinforce the importance of colonoscopy following positive interval FIT, given the high risk of advanced neoplasia and colorectal cancer, regardless of polypectomy history," Wilson said.
Guideline recommendations stress the need for follow-up surveillance with a colonoscopy, particularly in patients who have had a prior polypectomy, due to the higher risk.
Reasons patients may instead turn to FIT include cost or other factors.
To determine just how often that happens, how having a previous polypectomy affects FIT results, and how adherent patients are to follow up if a FIT result is positive, Wilson and her colleagues evaluated data from nearly 4.8 million individuals in the Veterans Health Administration Corporate Data Warehouse who underwent colonoscopy between 2000 and 2004.
Of the patients, 10.9% were found to have subsequently received interval FIT within 10 years of the index colonoscopy, and of those patients, nearly half (49.9%) had received a polypectomy at the index colonoscopy.
The average time from the colonoscopy/polypectomy to the interval FIT was 5.9 years (5.6 years in the polypectomy group vs 6.2 years in the nonpolypectomy group).
Among the FIT screenings, results were positive in 17.2% of postpolypectomy patients and 14.1% of patients who no prior polypectomy, indicating a history of polypectomy to be predictive of positive interval FIT (odds ratio [OR], 1.12; P < .0001).
Notably, while a follow-up colonoscopy is considered essential following a positive FIT result -- and having a previous polypectomy should add further emergency to the matter -- the study showed only 50.4% of those who had an earlier polypectomy went on to receive the recommended follow-up colonoscopy after a positive follow-up FIT, and the rate was 49.3% among those who had not received a polypectomy (P = .001).
For those who did receive a follow-up colonoscopy after a positive FIT, the duration of time to receiving the colonoscopy was longer among those who had a prior polypectomy, at 2.9 months compared with 2.5 months in the nonpolypectomy group (P < .001).
Colonoscopy results following a positive FIT showed higher rates of detections among patients who had prior polypectomies than among those with no prior polypectomy, including tubular adenomas (54.7% vs 45.8%), tubulovillous adenomas (5.6% vs 4.7%), adenomas with high-grade dysplasia (0.8% vs 0.7%), sessile serrated lesions (3.52% vs 2.4%), advanced colorectal neoplasia (9.2% vs 7.9%), and colorectal cancer (3.3% vs 3.0%).
However, a prior polypectomy was not independently predictive of colorectal cancer (OR, 0.96; P = .65) or advanced colorectal neoplasia (OR, 0.97; P = .57) in the postcolonoscopy interval FIT.
The findings underscore that "positive results carried a high risk of advanced neoplasia or cancer, irrespective or prior polypectomy history," Wilson said.
Commenting on the study, William D. Chey, MD, chief of the Division of Gastroenterology & Hepatology at the University of Michigan in Ann Arbor, Michigan, noted that the study "addresses one of the biggest challenges we face as a profession, which is making sure that patients who have a positive stool test get a colonoscopy."
He noted that the low rate of just 50% of recipients of positive FITs going on to receive a colonoscopy is consistent with what is observed in other trials.
"Other data suggest that the rate might even be significantly higher -- at 70% to 80%, depending upon the population and the test," Chey told Medscape Medical News.
Reasons for the failure to receive the follow-up testing range from income restrictions (due to the high cost of a colonoscopy, especially if not covered by insurance), education, speaking a foreign language, and other factors, he said.
The relatively high rates of colon cancers detected by FIT in the study, in those with and without a prior polypectomy, along with findings from other studies "should raise questions about whether there might be a role for FIT testing in addition to colonoscopy." However, much stronger evidence would be needed, Chey noted.
In the meantime, a key issue is "how do we do a better job of making sure that individuals who have a positive FIT test get a colonoscopy," he said.
"I think a lot of this is going to come down to how it's down at the primary care level."
Chey added that in that, and any other setting, "the main message that needs to get out to people who are undergoing stool-based screening is that the stool test is only the first part of the screening process, and if it's positive, a follow-up colonoscopy must be performed.
"Otherwise, the stool-based test is of no value."
Wilson had no disclosures to report. Chey's disclosures include consulting and/or other relationships with Ardelyx, Atmo, Biomerica, Commonwealth Diagnostics International, Corprata, Dieta, Evinature, Food Marble, Gemelli, Kiwi BioScience, Modify Health, Nestle, Phathom, Redhill, Salix/Valean, Takeda, and Vibrant.
A version of this article first appeared on Medscape.com.
PHOENIX -- Patients with or without polyp removal in an index colonoscopy commonly receive follow-up surveillance with a fecal immunochemical test (FIT), yet many of these patients do not receive a recommended colonoscopy after a positive FIT.
"In this large US study, we found interval FITs are frequently performed in patients with and without prior polypectomy," said first author Natalie J. Wilson, MD, of the University of Minnesota in Minneapolis, while presenting the findings this week at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
"These findings reinforce the importance of colonoscopy following positive interval FIT, given the high risk of advanced neoplasia and colorectal cancer, regardless of polypectomy history," Wilson said.
Guideline recommendations stress the need for follow-up surveillance with a colonoscopy, particularly in patients who have had a prior polypectomy, due to the higher risk.
Reasons patients may instead turn to FIT include cost or other factors.
To determine just how often that happens, how having a previous polypectomy affects FIT results, and how adherent patients are to follow up if a FIT result is positive, Wilson and her colleagues evaluated data from nearly 4.8 million individuals in the Veterans Health Administration Corporate Data Warehouse who underwent colonoscopy between 2000 and 2004.
Of the patients, 10.9% were found to have subsequently received interval FIT within 10 years of the index colonoscopy, and of those patients, nearly half (49.9%) had received a polypectomy at the index colonoscopy.
The average time from the colonoscopy/polypectomy to the interval FIT was 5.9 years (5.6 years in the polypectomy group vs 6.2 years in the nonpolypectomy group).
Among the FIT screenings, results were positive in 17.2% of postpolypectomy patients and 14.1% of patients who no prior polypectomy, indicating a history of polypectomy to be predictive of positive interval FIT (odds ratio [OR], 1.12; P < .0001).
Notably, while a follow-up colonoscopy is considered essential following a positive FIT result -- and having a previous polypectomy should add further emergency to the matter -- the study showed only 50.4% of those who had an earlier polypectomy went on to receive the recommended follow-up colonoscopy after a positive follow-up FIT, and the rate was 49.3% among those who had not received a polypectomy (P = .001).
For those who did receive a follow-up colonoscopy after a positive FIT, the duration of time to receiving the colonoscopy was longer among those who had a prior polypectomy, at 2.9 months compared with 2.5 months in the nonpolypectomy group (P < .001).
Colonoscopy results following a positive FIT showed higher rates of detections among patients who had prior polypectomies than among those with no prior polypectomy, including tubular adenomas (54.7% vs 45.8%), tubulovillous adenomas (5.6% vs 4.7%), adenomas with high-grade dysplasia (0.8% vs 0.7%), sessile serrated lesions (3.52% vs 2.4%), advanced colorectal neoplasia (9.2% vs 7.9%), and colorectal cancer (3.3% vs 3.0%).
However, a prior polypectomy was not independently predictive of colorectal cancer (OR, 0.96; P = .65) or advanced colorectal neoplasia (OR, 0.97; P = .57) in the postcolonoscopy interval FIT.
The findings underscore that "positive results carried a high risk of advanced neoplasia or cancer, irrespective or prior polypectomy history," Wilson said.
Commenting on the study, William D. Chey, MD, chief of the Division of Gastroenterology & Hepatology at the University of Michigan in Ann Arbor, Michigan, noted that the study "addresses one of the biggest challenges we face as a profession, which is making sure that patients who have a positive stool test get a colonoscopy."
He noted that the low rate of just 50% of recipients of positive FITs going on to receive a colonoscopy is consistent with what is observed in other trials.
"Other data suggest that the rate might even be significantly higher -- at 70% to 80%, depending upon the population and the test," Chey told Medscape Medical News.
Reasons for the failure to receive the follow-up testing range from income restrictions (due to the high cost of a colonoscopy, especially if not covered by insurance), education, speaking a foreign language, and other factors, he said.
The relatively high rates of colon cancers detected by FIT in the study, in those with and without a prior polypectomy, along with findings from other studies "should raise questions about whether there might be a role for FIT testing in addition to colonoscopy." However, much stronger evidence would be needed, Chey noted.
In the meantime, a key issue is "how do we do a better job of making sure that individuals who have a positive FIT test get a colonoscopy," he said.
"I think a lot of this is going to come down to how it's down at the primary care level."
Chey added that in that, and any other setting, "the main message that needs to get out to people who are undergoing stool-based screening is that the stool test is only the first part of the screening process, and if it's positive, a follow-up colonoscopy must be performed.
"Otherwise, the stool-based test is of no value."
Wilson had no disclosures to report. Chey's disclosures include consulting and/or other relationships with Ardelyx, Atmo, Biomerica, Commonwealth Diagnostics International, Corprata, Dieta, Evinature, Food Marble, Gemelli, Kiwi BioScience, Modify Health, Nestle, Phathom, Redhill, Salix/Valean, Takeda, and Vibrant.
A version of this article first appeared on Medscape.com.
PHOENIX -- Patients with or without polyp removal in an index colonoscopy commonly receive follow-up surveillance with a fecal immunochemical test (FIT), yet many of these patients do not receive a recommended colonoscopy after a positive FIT.
"In this large US study, we found interval FITs are frequently performed in patients with and without prior polypectomy," said first author Natalie J. Wilson, MD, of the University of Minnesota in Minneapolis, while presenting the findings this week at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
"These findings reinforce the importance of colonoscopy following positive interval FIT, given the high risk of advanced neoplasia and colorectal cancer, regardless of polypectomy history," Wilson said.
Guideline recommendations stress the need for follow-up surveillance with a colonoscopy, particularly in patients who have had a prior polypectomy, due to the higher risk.
Reasons patients may instead turn to FIT include cost or other factors.
To determine just how often that happens, how having a previous polypectomy affects FIT results, and how adherent patients are to follow up if a FIT result is positive, Wilson and her colleagues evaluated data from nearly 4.8 million individuals in the Veterans Health Administration Corporate Data Warehouse who underwent colonoscopy between 2000 and 2004.
Of the patients, 10.9% were found to have subsequently received interval FIT within 10 years of the index colonoscopy, and of those patients, nearly half (49.9%) had received a polypectomy at the index colonoscopy.
The average time from the colonoscopy/polypectomy to the interval FIT was 5.9 years (5.6 years in the polypectomy group vs 6.2 years in the nonpolypectomy group).
Among the FIT screenings, results were positive in 17.2% of postpolypectomy patients and 14.1% of patients who no prior polypectomy, indicating a history of polypectomy to be predictive of positive interval FIT (odds ratio [OR], 1.12; P < .0001).
Notably, while a follow-up colonoscopy is considered essential following a positive FIT result -- and having a previous polypectomy should add further emergency to the matter -- the study showed only 50.4% of those who had an earlier polypectomy went on to receive the recommended follow-up colonoscopy after a positive follow-up FIT, and the rate was 49.3% among those who had not received a polypectomy (P = .001).
For those who did receive a follow-up colonoscopy after a positive FIT, the duration of time to receiving the colonoscopy was longer among those who had a prior polypectomy, at 2.9 months compared with 2.5 months in the nonpolypectomy group (P < .001).
Colonoscopy results following a positive FIT showed higher rates of detections among patients who had prior polypectomies than among those with no prior polypectomy, including tubular adenomas (54.7% vs 45.8%), tubulovillous adenomas (5.6% vs 4.7%), adenomas with high-grade dysplasia (0.8% vs 0.7%), sessile serrated lesions (3.52% vs 2.4%), advanced colorectal neoplasia (9.2% vs 7.9%), and colorectal cancer (3.3% vs 3.0%).
However, a prior polypectomy was not independently predictive of colorectal cancer (OR, 0.96; P = .65) or advanced colorectal neoplasia (OR, 0.97; P = .57) in the postcolonoscopy interval FIT.
The findings underscore that "positive results carried a high risk of advanced neoplasia or cancer, irrespective or prior polypectomy history," Wilson said.
Commenting on the study, William D. Chey, MD, chief of the Division of Gastroenterology & Hepatology at the University of Michigan in Ann Arbor, Michigan, noted that the study "addresses one of the biggest challenges we face as a profession, which is making sure that patients who have a positive stool test get a colonoscopy."
He noted that the low rate of just 50% of recipients of positive FITs going on to receive a colonoscopy is consistent with what is observed in other trials.
"Other data suggest that the rate might even be significantly higher -- at 70% to 80%, depending upon the population and the test," Chey told Medscape Medical News.
Reasons for the failure to receive the follow-up testing range from income restrictions (due to the high cost of a colonoscopy, especially if not covered by insurance), education, speaking a foreign language, and other factors, he said.
The relatively high rates of colon cancers detected by FIT in the study, in those with and without a prior polypectomy, along with findings from other studies "should raise questions about whether there might be a role for FIT testing in addition to colonoscopy." However, much stronger evidence would be needed, Chey noted.
In the meantime, a key issue is "how do we do a better job of making sure that individuals who have a positive FIT test get a colonoscopy," he said.
"I think a lot of this is going to come down to how it's down at the primary care level."
Chey added that in that, and any other setting, "the main message that needs to get out to people who are undergoing stool-based screening is that the stool test is only the first part of the screening process, and if it's positive, a follow-up colonoscopy must be performed.
"Otherwise, the stool-based test is of no value."
Wilson had no disclosures to report. Chey's disclosures include consulting and/or other relationships with Ardelyx, Atmo, Biomerica, Commonwealth Diagnostics International, Corprata, Dieta, Evinature, Food Marble, Gemelli, Kiwi BioScience, Modify Health, Nestle, Phathom, Redhill, Salix/Valean, Takeda, and Vibrant.
A version of this article first appeared on Medscape.com.
Patients With a Positive FIT Fail to Get Follow-Up Colonoscopies
Patients With a Positive FIT Fail to Get Follow-Up Colonoscopies
When in the Treatment Sequence Should Metastatic CRC Be Retreated With an Anti-EGFR?
BERLIN — Re-treatment with an antiepidermal growth factor receptor (EGFR) agent is effective in patients with chemorefractory metastatic colorectal cancer (mCRC) with RAS and BRAF wild-type tumors confirmed on circulating tumor DNA (ctDNA), although the sequencing of therapy does not seem to matter, suggest overall survival results from the crossover trial PARERE.
The findings nevertheless indicate that anti-EGFR rechallenge with panitumumab may prolong progression-free survival (PFS) over the multiple kinase inhibitor regorafenib. This suggests that “the most pragmatic choice” would be to give the anti-EGFR before regorafenib, said study presenter Marco Maria Germani, MD, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy.
The caveat, however, is in patients who have an anti-EGFR interval since previously receiving the drugs of < 6 months. Those patients appeared to do better if they had regorafenib first and then anti-EGFR rechallenge.
Overall, Germani said that “since [trifluridine/tipiracil] plus bevacizumab is today the third-line standard of care” in this patient population, “anti-EGFR re-treatment might be considered after progression” on that combination.
Germani presented the research on October 18 at the European Society for Medical Oncology (ESMO) Annual Meeting 2025, which was simultaneously published in the Annals of Oncology.
Michel P. Ducreux, MD, PhD, head of the Digestive Cancer Committee at Gustave Roussy, Villejuif, France, and invited discussant for the results, said, despite the study being negative, it is “very important to continue to perform this kind of trial to evaluate the [ideal] sequence in the treatment of our patients.”
He continued that the secondary endpoints in the trial of PFS and objective response and disease control rates were “fairly in favor of the use of rechallenge before regorafenib, and in my opinion, this is really quite convincing.”
Ducreux, who was not involved in PARERE trail, also pointed to the sex difference seen in the study, which suggested that women responded much better to having anti-EGFR retreatment before regorafenib than did men.
Similar findings have been reported in a number of other trials, and previous work has suggested that there are sex differences in the pharmacokinetics of several anticancer drugs. However, while this is “very important,” he said that “we never consider it, because we are not able to really explain [it].”
Overall, he concluded that, on the basis of these results, he would agree with the notion that it is better to propose a rechallenge with anti-EGFR treatment as the fourth-line therapy in this patient population, before administering regorafenib.
Ducreux explained that, after a partial response, tumors acquire resistance to EGFR inhibitors through alterations and mutations that occur during treatment, via nongenetic mechanisms, and through treatment-induced selection for preexisting mutations.
Previous work has shown that mutations, such as in the RAS gene, are detectable early during EGFR inhibitor therapy, but that they then decay exponentially once the drugs are stopped, with the potential that tumors regain their sensitivity to them.
Germani said that this means that ctDNA-guided retreatment with anti-EGFR therapies is a “promising approach” in pretreated patients with RAS and BRAF wild-type mCRC, and that the sequencing of the drugs may be important. Indeed, the REVERCE trial showed that giving regorafenib followed by the anti-EGFR drug cetuzximab was associated with longer overall survival than the other way around in anti-EGFR medication-naive patients.
Methods and Results
For PARERE, the researchers enrolled patients aged at least 18 years with RAS and BRAF wild-type mCRC who were previously treated with a first-line anti-EGFR-containing regimen and had at least a partial response or stable disease for at least 6 months.
The patients were also required to have had at least one intervening anti-EGFR-free line of therapy, and to have previously received treatment with fluoropyrimidine, oxaliplatin, irinotecan, and anti-angiogenics. At least 4 months were required to have passed between the end of anti-EGFR administration and screening for the study.
In all, 428 patients were screened between December 2020 and December 2024, with 213 patients with RAS and BRAF wild-type mCRC, as detected on ctDNA, enrolled. They were randomized to panitumumab or regorafenib until first progression, followed by regorafenib, if they started on panitumumab, or panitumumab, if they started on regorafenib, until second progression.
The median age of the patients was 61 years among those who started on panitumumab and 64 years among those initially given regorafenib in the trial, and 63% and 57%, respectively, were male. The median number of prior lines of therapy was two in both groups, and 65% and 69%, respectively, had received pantitumumab as their first-line anti-EGFR.
Initial findings from the study presented at the 2025 ASCO Annual Meeting indicated that, after a median follow-up of 23.5 months, there was no significant difference in the median first PFS between the two treatment arms.
However, patients who started with panitumumab had a significant improvement in both the objective response and disease control rates (P < .001), as well as a signal for a potentially longer median second PFS, than those who started with regorafenib, particularly on the per-protocol analysis.
Presenting the overall survival results, Germani said that there was no significant difference between the groups on the intention-to-treat analysis, at a stratified hazard ratio of 1.13 (P = .440), or on the per-protocol analysis, at a hazard ratio of 1.07 (P = .730).
“We then ran a subgroup analysis,” he continued, “and we found out that an anti-EGFR-free interval before liquid biopsy shorter than 6 months was associated with less benefit from a panitumumab [first] sequence, which is biologically sound.”
It was also observed that women did significantly better when having panitumumab first, whereas men did not, for which “we do not have a clear biological explanation,” Germani added.
Confining the analysis to so-called “hyperselected” patients, who not only were RAS and BRAF wild type but also had no pathogenic mutations associated with anti-EGFR resistance, did not reveal any significant overall survival differences between the treatment groups.
However, Ducreux took issue with the way in which hyperselection, which is turning up more and more regularly in trials, is defined, as the choice of which mutations to include varies widely. He suggested that a consensus group be assembled to resolve this issue.
Looking more broadly, the researchers were able to show that, in this updated analysis, anti-EGFR re-treatment was superior to regorafenib regardless of the treatment sequence in terms of PFS, at 4.2 months vs 2.4 months (P = .103) when given first in the trial, and 3.9 months vs 2.7 months (P = .019) when given second in the trial, as well as in terms of objective response and disease control rates.
Adverse Events
In terms of safety, the results showed that, as expected, acneiform rash, fatigue, and hypomagnesemia were the most common adverse events associated with panitumumb, while those with regorafenib were fatigue, hand-foot skin reactions, and hypertension.
There were no notable differences in the number of patients receiving a post-study treatment nor in the post-study therapeutic choices, between the study arms.
The study was sponsored by GONO Foundation and partially supported by Amgen and Bayer. Germani declared having relationships with MSD and Amgen. Ducreux declared having relationships with Amgen, Bayer, BeiGene, Incyte, Jazz, Merck KGaA, Merck Serono, Merck Sharp & Dohme, Pierre Fabre, Roche, Servier, Keocyt, AbbVie, Abcely, Arcus, Bayer, BMS, Boehringer, GlaxoSmithKline, Sanofi, Scandion, and Zymeworks.
A version of this article first appeared on Medscape.com.
BERLIN — Re-treatment with an antiepidermal growth factor receptor (EGFR) agent is effective in patients with chemorefractory metastatic colorectal cancer (mCRC) with RAS and BRAF wild-type tumors confirmed on circulating tumor DNA (ctDNA), although the sequencing of therapy does not seem to matter, suggest overall survival results from the crossover trial PARERE.
The findings nevertheless indicate that anti-EGFR rechallenge with panitumumab may prolong progression-free survival (PFS) over the multiple kinase inhibitor regorafenib. This suggests that “the most pragmatic choice” would be to give the anti-EGFR before regorafenib, said study presenter Marco Maria Germani, MD, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy.
The caveat, however, is in patients who have an anti-EGFR interval since previously receiving the drugs of < 6 months. Those patients appeared to do better if they had regorafenib first and then anti-EGFR rechallenge.
Overall, Germani said that “since [trifluridine/tipiracil] plus bevacizumab is today the third-line standard of care” in this patient population, “anti-EGFR re-treatment might be considered after progression” on that combination.
Germani presented the research on October 18 at the European Society for Medical Oncology (ESMO) Annual Meeting 2025, which was simultaneously published in the Annals of Oncology.
Michel P. Ducreux, MD, PhD, head of the Digestive Cancer Committee at Gustave Roussy, Villejuif, France, and invited discussant for the results, said, despite the study being negative, it is “very important to continue to perform this kind of trial to evaluate the [ideal] sequence in the treatment of our patients.”
He continued that the secondary endpoints in the trial of PFS and objective response and disease control rates were “fairly in favor of the use of rechallenge before regorafenib, and in my opinion, this is really quite convincing.”
Ducreux, who was not involved in PARERE trail, also pointed to the sex difference seen in the study, which suggested that women responded much better to having anti-EGFR retreatment before regorafenib than did men.
Similar findings have been reported in a number of other trials, and previous work has suggested that there are sex differences in the pharmacokinetics of several anticancer drugs. However, while this is “very important,” he said that “we never consider it, because we are not able to really explain [it].”
Overall, he concluded that, on the basis of these results, he would agree with the notion that it is better to propose a rechallenge with anti-EGFR treatment as the fourth-line therapy in this patient population, before administering regorafenib.
Ducreux explained that, after a partial response, tumors acquire resistance to EGFR inhibitors through alterations and mutations that occur during treatment, via nongenetic mechanisms, and through treatment-induced selection for preexisting mutations.
Previous work has shown that mutations, such as in the RAS gene, are detectable early during EGFR inhibitor therapy, but that they then decay exponentially once the drugs are stopped, with the potential that tumors regain their sensitivity to them.
Germani said that this means that ctDNA-guided retreatment with anti-EGFR therapies is a “promising approach” in pretreated patients with RAS and BRAF wild-type mCRC, and that the sequencing of the drugs may be important. Indeed, the REVERCE trial showed that giving regorafenib followed by the anti-EGFR drug cetuzximab was associated with longer overall survival than the other way around in anti-EGFR medication-naive patients.
Methods and Results
For PARERE, the researchers enrolled patients aged at least 18 years with RAS and BRAF wild-type mCRC who were previously treated with a first-line anti-EGFR-containing regimen and had at least a partial response or stable disease for at least 6 months.
The patients were also required to have had at least one intervening anti-EGFR-free line of therapy, and to have previously received treatment with fluoropyrimidine, oxaliplatin, irinotecan, and anti-angiogenics. At least 4 months were required to have passed between the end of anti-EGFR administration and screening for the study.
In all, 428 patients were screened between December 2020 and December 2024, with 213 patients with RAS and BRAF wild-type mCRC, as detected on ctDNA, enrolled. They were randomized to panitumumab or regorafenib until first progression, followed by regorafenib, if they started on panitumumab, or panitumumab, if they started on regorafenib, until second progression.
The median age of the patients was 61 years among those who started on panitumumab and 64 years among those initially given regorafenib in the trial, and 63% and 57%, respectively, were male. The median number of prior lines of therapy was two in both groups, and 65% and 69%, respectively, had received pantitumumab as their first-line anti-EGFR.
Initial findings from the study presented at the 2025 ASCO Annual Meeting indicated that, after a median follow-up of 23.5 months, there was no significant difference in the median first PFS between the two treatment arms.
However, patients who started with panitumumab had a significant improvement in both the objective response and disease control rates (P < .001), as well as a signal for a potentially longer median second PFS, than those who started with regorafenib, particularly on the per-protocol analysis.
Presenting the overall survival results, Germani said that there was no significant difference between the groups on the intention-to-treat analysis, at a stratified hazard ratio of 1.13 (P = .440), or on the per-protocol analysis, at a hazard ratio of 1.07 (P = .730).
“We then ran a subgroup analysis,” he continued, “and we found out that an anti-EGFR-free interval before liquid biopsy shorter than 6 months was associated with less benefit from a panitumumab [first] sequence, which is biologically sound.”
It was also observed that women did significantly better when having panitumumab first, whereas men did not, for which “we do not have a clear biological explanation,” Germani added.
Confining the analysis to so-called “hyperselected” patients, who not only were RAS and BRAF wild type but also had no pathogenic mutations associated with anti-EGFR resistance, did not reveal any significant overall survival differences between the treatment groups.
However, Ducreux took issue with the way in which hyperselection, which is turning up more and more regularly in trials, is defined, as the choice of which mutations to include varies widely. He suggested that a consensus group be assembled to resolve this issue.
Looking more broadly, the researchers were able to show that, in this updated analysis, anti-EGFR re-treatment was superior to regorafenib regardless of the treatment sequence in terms of PFS, at 4.2 months vs 2.4 months (P = .103) when given first in the trial, and 3.9 months vs 2.7 months (P = .019) when given second in the trial, as well as in terms of objective response and disease control rates.
Adverse Events
In terms of safety, the results showed that, as expected, acneiform rash, fatigue, and hypomagnesemia were the most common adverse events associated with panitumumb, while those with regorafenib were fatigue, hand-foot skin reactions, and hypertension.
There were no notable differences in the number of patients receiving a post-study treatment nor in the post-study therapeutic choices, between the study arms.
The study was sponsored by GONO Foundation and partially supported by Amgen and Bayer. Germani declared having relationships with MSD and Amgen. Ducreux declared having relationships with Amgen, Bayer, BeiGene, Incyte, Jazz, Merck KGaA, Merck Serono, Merck Sharp & Dohme, Pierre Fabre, Roche, Servier, Keocyt, AbbVie, Abcely, Arcus, Bayer, BMS, Boehringer, GlaxoSmithKline, Sanofi, Scandion, and Zymeworks.
A version of this article first appeared on Medscape.com.
BERLIN — Re-treatment with an antiepidermal growth factor receptor (EGFR) agent is effective in patients with chemorefractory metastatic colorectal cancer (mCRC) with RAS and BRAF wild-type tumors confirmed on circulating tumor DNA (ctDNA), although the sequencing of therapy does not seem to matter, suggest overall survival results from the crossover trial PARERE.
The findings nevertheless indicate that anti-EGFR rechallenge with panitumumab may prolong progression-free survival (PFS) over the multiple kinase inhibitor regorafenib. This suggests that “the most pragmatic choice” would be to give the anti-EGFR before regorafenib, said study presenter Marco Maria Germani, MD, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy.
The caveat, however, is in patients who have an anti-EGFR interval since previously receiving the drugs of < 6 months. Those patients appeared to do better if they had regorafenib first and then anti-EGFR rechallenge.
Overall, Germani said that “since [trifluridine/tipiracil] plus bevacizumab is today the third-line standard of care” in this patient population, “anti-EGFR re-treatment might be considered after progression” on that combination.
Germani presented the research on October 18 at the European Society for Medical Oncology (ESMO) Annual Meeting 2025, which was simultaneously published in the Annals of Oncology.
Michel P. Ducreux, MD, PhD, head of the Digestive Cancer Committee at Gustave Roussy, Villejuif, France, and invited discussant for the results, said, despite the study being negative, it is “very important to continue to perform this kind of trial to evaluate the [ideal] sequence in the treatment of our patients.”
He continued that the secondary endpoints in the trial of PFS and objective response and disease control rates were “fairly in favor of the use of rechallenge before regorafenib, and in my opinion, this is really quite convincing.”
Ducreux, who was not involved in PARERE trail, also pointed to the sex difference seen in the study, which suggested that women responded much better to having anti-EGFR retreatment before regorafenib than did men.
Similar findings have been reported in a number of other trials, and previous work has suggested that there are sex differences in the pharmacokinetics of several anticancer drugs. However, while this is “very important,” he said that “we never consider it, because we are not able to really explain [it].”
Overall, he concluded that, on the basis of these results, he would agree with the notion that it is better to propose a rechallenge with anti-EGFR treatment as the fourth-line therapy in this patient population, before administering regorafenib.
Ducreux explained that, after a partial response, tumors acquire resistance to EGFR inhibitors through alterations and mutations that occur during treatment, via nongenetic mechanisms, and through treatment-induced selection for preexisting mutations.
Previous work has shown that mutations, such as in the RAS gene, are detectable early during EGFR inhibitor therapy, but that they then decay exponentially once the drugs are stopped, with the potential that tumors regain their sensitivity to them.
Germani said that this means that ctDNA-guided retreatment with anti-EGFR therapies is a “promising approach” in pretreated patients with RAS and BRAF wild-type mCRC, and that the sequencing of the drugs may be important. Indeed, the REVERCE trial showed that giving regorafenib followed by the anti-EGFR drug cetuzximab was associated with longer overall survival than the other way around in anti-EGFR medication-naive patients.
Methods and Results
For PARERE, the researchers enrolled patients aged at least 18 years with RAS and BRAF wild-type mCRC who were previously treated with a first-line anti-EGFR-containing regimen and had at least a partial response or stable disease for at least 6 months.
The patients were also required to have had at least one intervening anti-EGFR-free line of therapy, and to have previously received treatment with fluoropyrimidine, oxaliplatin, irinotecan, and anti-angiogenics. At least 4 months were required to have passed between the end of anti-EGFR administration and screening for the study.
In all, 428 patients were screened between December 2020 and December 2024, with 213 patients with RAS and BRAF wild-type mCRC, as detected on ctDNA, enrolled. They were randomized to panitumumab or regorafenib until first progression, followed by regorafenib, if they started on panitumumab, or panitumumab, if they started on regorafenib, until second progression.
The median age of the patients was 61 years among those who started on panitumumab and 64 years among those initially given regorafenib in the trial, and 63% and 57%, respectively, were male. The median number of prior lines of therapy was two in both groups, and 65% and 69%, respectively, had received pantitumumab as their first-line anti-EGFR.
Initial findings from the study presented at the 2025 ASCO Annual Meeting indicated that, after a median follow-up of 23.5 months, there was no significant difference in the median first PFS between the two treatment arms.
However, patients who started with panitumumab had a significant improvement in both the objective response and disease control rates (P < .001), as well as a signal for a potentially longer median second PFS, than those who started with regorafenib, particularly on the per-protocol analysis.
Presenting the overall survival results, Germani said that there was no significant difference between the groups on the intention-to-treat analysis, at a stratified hazard ratio of 1.13 (P = .440), or on the per-protocol analysis, at a hazard ratio of 1.07 (P = .730).
“We then ran a subgroup analysis,” he continued, “and we found out that an anti-EGFR-free interval before liquid biopsy shorter than 6 months was associated with less benefit from a panitumumab [first] sequence, which is biologically sound.”
It was also observed that women did significantly better when having panitumumab first, whereas men did not, for which “we do not have a clear biological explanation,” Germani added.
Confining the analysis to so-called “hyperselected” patients, who not only were RAS and BRAF wild type but also had no pathogenic mutations associated with anti-EGFR resistance, did not reveal any significant overall survival differences between the treatment groups.
However, Ducreux took issue with the way in which hyperselection, which is turning up more and more regularly in trials, is defined, as the choice of which mutations to include varies widely. He suggested that a consensus group be assembled to resolve this issue.
Looking more broadly, the researchers were able to show that, in this updated analysis, anti-EGFR re-treatment was superior to regorafenib regardless of the treatment sequence in terms of PFS, at 4.2 months vs 2.4 months (P = .103) when given first in the trial, and 3.9 months vs 2.7 months (P = .019) when given second in the trial, as well as in terms of objective response and disease control rates.
Adverse Events
In terms of safety, the results showed that, as expected, acneiform rash, fatigue, and hypomagnesemia were the most common adverse events associated with panitumumb, while those with regorafenib were fatigue, hand-foot skin reactions, and hypertension.
There were no notable differences in the number of patients receiving a post-study treatment nor in the post-study therapeutic choices, between the study arms.
The study was sponsored by GONO Foundation and partially supported by Amgen and Bayer. Germani declared having relationships with MSD and Amgen. Ducreux declared having relationships with Amgen, Bayer, BeiGene, Incyte, Jazz, Merck KGaA, Merck Serono, Merck Sharp & Dohme, Pierre Fabre, Roche, Servier, Keocyt, AbbVie, Abcely, Arcus, Bayer, BMS, Boehringer, GlaxoSmithKline, Sanofi, Scandion, and Zymeworks.
A version of this article first appeared on Medscape.com.
FROM ENDO 2025
Shifting Demographics: A Temporal Analysis of the Alarming Rise in Rectal Adenocarcinoma Among Young Adults
Background
Rectal adenocarcinoma has long been associated with older adults, with routine screening typically beginning at age 45 or older. However, recent data reveal a concerning rise in rectal cancer incidence among adults under 40. These early-onset cases often present at later stages and may have distinct biological features. While some research attributes this trend to genetic or environmental factors, the contribution of socioeconomic disparities and healthcare access has not been fully explored. Identifying these influences is essential to shaping targeted prevention and early detection strategies for younger populations.
Objective
To evaluate temporal trends in rectal adenocarcinoma among young adults and assess demographic and socioeconomic predictors of early-onset diagnosis.
Methods
Data were drawn from the National Cancer Database (NCDB) for patients diagnosed with rectal adenocarcinoma from 2004 to 2022. Among 440,316 cases, 17,842 (4.1%) occurred in individuals under 40. Linear regression assessed temporal trends, while logistic regression evaluated associations between early-onset diagnosis and variables including sex, race, insurance status, income level, Charlson-Deyo comorbidity score, and tumor stage. Statistical significance was defined as α = 0.05.
Results
The number of young adults diagnosed rose from 424 in 2004 to 937 in 2022—an increase of over 120%. Each year was associated with a 1.7% rise in odds of early diagnosis (OR = 1.017, p < 0.001). Male patients had 24.7% higher odds (OR = 1.247, p < 0.001), and Black patients had 59.3% higher odds compared to White patients (OR = 1.593, p < 0.001). Non-private insurance was linked to a 41.6% decrease in early diagnosis (OR = 0.584, p < 0.001). Income level was not significant (p = 0.426). Lower Charlson-Deyo scores and higher tumor stages were also associated with early-onset cases.
Conclusions
Rectal adenocarcinoma is increasingly affecting younger adults, with significant associations across demographic and insurance variables. These findings call for improved awareness, early diagnostic strategies, and further research into underlying causes to mitigate this growing public health concern.
Background
Rectal adenocarcinoma has long been associated with older adults, with routine screening typically beginning at age 45 or older. However, recent data reveal a concerning rise in rectal cancer incidence among adults under 40. These early-onset cases often present at later stages and may have distinct biological features. While some research attributes this trend to genetic or environmental factors, the contribution of socioeconomic disparities and healthcare access has not been fully explored. Identifying these influences is essential to shaping targeted prevention and early detection strategies for younger populations.
Objective
To evaluate temporal trends in rectal adenocarcinoma among young adults and assess demographic and socioeconomic predictors of early-onset diagnosis.
Methods
Data were drawn from the National Cancer Database (NCDB) for patients diagnosed with rectal adenocarcinoma from 2004 to 2022. Among 440,316 cases, 17,842 (4.1%) occurred in individuals under 40. Linear regression assessed temporal trends, while logistic regression evaluated associations between early-onset diagnosis and variables including sex, race, insurance status, income level, Charlson-Deyo comorbidity score, and tumor stage. Statistical significance was defined as α = 0.05.
Results
The number of young adults diagnosed rose from 424 in 2004 to 937 in 2022—an increase of over 120%. Each year was associated with a 1.7% rise in odds of early diagnosis (OR = 1.017, p < 0.001). Male patients had 24.7% higher odds (OR = 1.247, p < 0.001), and Black patients had 59.3% higher odds compared to White patients (OR = 1.593, p < 0.001). Non-private insurance was linked to a 41.6% decrease in early diagnosis (OR = 0.584, p < 0.001). Income level was not significant (p = 0.426). Lower Charlson-Deyo scores and higher tumor stages were also associated with early-onset cases.
Conclusions
Rectal adenocarcinoma is increasingly affecting younger adults, with significant associations across demographic and insurance variables. These findings call for improved awareness, early diagnostic strategies, and further research into underlying causes to mitigate this growing public health concern.
Background
Rectal adenocarcinoma has long been associated with older adults, with routine screening typically beginning at age 45 or older. However, recent data reveal a concerning rise in rectal cancer incidence among adults under 40. These early-onset cases often present at later stages and may have distinct biological features. While some research attributes this trend to genetic or environmental factors, the contribution of socioeconomic disparities and healthcare access has not been fully explored. Identifying these influences is essential to shaping targeted prevention and early detection strategies for younger populations.
Objective
To evaluate temporal trends in rectal adenocarcinoma among young adults and assess demographic and socioeconomic predictors of early-onset diagnosis.
Methods
Data were drawn from the National Cancer Database (NCDB) for patients diagnosed with rectal adenocarcinoma from 2004 to 2022. Among 440,316 cases, 17,842 (4.1%) occurred in individuals under 40. Linear regression assessed temporal trends, while logistic regression evaluated associations between early-onset diagnosis and variables including sex, race, insurance status, income level, Charlson-Deyo comorbidity score, and tumor stage. Statistical significance was defined as α = 0.05.
Results
The number of young adults diagnosed rose from 424 in 2004 to 937 in 2022—an increase of over 120%. Each year was associated with a 1.7% rise in odds of early diagnosis (OR = 1.017, p < 0.001). Male patients had 24.7% higher odds (OR = 1.247, p < 0.001), and Black patients had 59.3% higher odds compared to White patients (OR = 1.593, p < 0.001). Non-private insurance was linked to a 41.6% decrease in early diagnosis (OR = 0.584, p < 0.001). Income level was not significant (p = 0.426). Lower Charlson-Deyo scores and higher tumor stages were also associated with early-onset cases.
Conclusions
Rectal adenocarcinoma is increasingly affecting younger adults, with significant associations across demographic and insurance variables. These findings call for improved awareness, early diagnostic strategies, and further research into underlying causes to mitigate this growing public health concern.
An Unusual Metastasis of Anal Squamous Cell Carcinoma
Background
Anal squamous cell carcinoma is a rare cancer which usually has locoregional spread. We report a case of distant metastasis of primary anal squamous cell carcinoma to the posterior mediastinal lymph node without lung involvement.
Case Presentation
A 63-year-old female presented with a painful anal mass, bleeding, and fluid leakage for around six months. The patient was found to have a near-circumferential fungating anal mass with bilateral inguinal lymphadenopathy. MR imaging revealed an 8.7 x 5.9 cm anal mass extending beyond the mesorectal fascia, with lymphadenopathy involving inguinal, pelvic sidewall, and iliac regions. A biopsy of the mass confirmed anal squamous cell carcinoma (ASCC). Initial treatment included diverting colostomy followed by definitive chemoradiotherapy with Mitomycin and 5-Fluorouracil. Colonoscopy post-treatment revealed tubular adenomas and a hyperplastic polyp, with no malignancy detected. The patient demonstrated a strong therapeutic response, with resolution of the anal mass and improved symptoms. However, one year later, new FDG-avid mediastinal lymph node were detected on the CT/PET scan with no pulmonary involvement. Metastatic ASCC of the Mediastinal lymph node was confirmed by biopsy. Salvage chemotherapy with Carboplatin and Paclitaxel every three weeks for six cycles achieved complete resolution of metastases.
Conclusions
This case underscores the importance of a multidisciplinary approach in managing advanced ASCC and highlights the efficacy of salvage chemotherapy in addressing metastases. Close monitoring of disease progression following surgery and chemotherapy is crucial due to the risk of recurrence.
Background
Anal squamous cell carcinoma is a rare cancer which usually has locoregional spread. We report a case of distant metastasis of primary anal squamous cell carcinoma to the posterior mediastinal lymph node without lung involvement.
Case Presentation
A 63-year-old female presented with a painful anal mass, bleeding, and fluid leakage for around six months. The patient was found to have a near-circumferential fungating anal mass with bilateral inguinal lymphadenopathy. MR imaging revealed an 8.7 x 5.9 cm anal mass extending beyond the mesorectal fascia, with lymphadenopathy involving inguinal, pelvic sidewall, and iliac regions. A biopsy of the mass confirmed anal squamous cell carcinoma (ASCC). Initial treatment included diverting colostomy followed by definitive chemoradiotherapy with Mitomycin and 5-Fluorouracil. Colonoscopy post-treatment revealed tubular adenomas and a hyperplastic polyp, with no malignancy detected. The patient demonstrated a strong therapeutic response, with resolution of the anal mass and improved symptoms. However, one year later, new FDG-avid mediastinal lymph node were detected on the CT/PET scan with no pulmonary involvement. Metastatic ASCC of the Mediastinal lymph node was confirmed by biopsy. Salvage chemotherapy with Carboplatin and Paclitaxel every three weeks for six cycles achieved complete resolution of metastases.
Conclusions
This case underscores the importance of a multidisciplinary approach in managing advanced ASCC and highlights the efficacy of salvage chemotherapy in addressing metastases. Close monitoring of disease progression following surgery and chemotherapy is crucial due to the risk of recurrence.
Background
Anal squamous cell carcinoma is a rare cancer which usually has locoregional spread. We report a case of distant metastasis of primary anal squamous cell carcinoma to the posterior mediastinal lymph node without lung involvement.
Case Presentation
A 63-year-old female presented with a painful anal mass, bleeding, and fluid leakage for around six months. The patient was found to have a near-circumferential fungating anal mass with bilateral inguinal lymphadenopathy. MR imaging revealed an 8.7 x 5.9 cm anal mass extending beyond the mesorectal fascia, with lymphadenopathy involving inguinal, pelvic sidewall, and iliac regions. A biopsy of the mass confirmed anal squamous cell carcinoma (ASCC). Initial treatment included diverting colostomy followed by definitive chemoradiotherapy with Mitomycin and 5-Fluorouracil. Colonoscopy post-treatment revealed tubular adenomas and a hyperplastic polyp, with no malignancy detected. The patient demonstrated a strong therapeutic response, with resolution of the anal mass and improved symptoms. However, one year later, new FDG-avid mediastinal lymph node were detected on the CT/PET scan with no pulmonary involvement. Metastatic ASCC of the Mediastinal lymph node was confirmed by biopsy. Salvage chemotherapy with Carboplatin and Paclitaxel every three weeks for six cycles achieved complete resolution of metastases.
Conclusions
This case underscores the importance of a multidisciplinary approach in managing advanced ASCC and highlights the efficacy of salvage chemotherapy in addressing metastases. Close monitoring of disease progression following surgery and chemotherapy is crucial due to the risk of recurrence.
Eating More Cruciferous Vegetables May Cut Colon Cancer Risk
TOPLINE:
A higher consumption of cruciferous vegetables such as broccoli and cauliflower was associated with a notably reduced risk for colon cancer (CC), with an optimal intake of 40-60 g/d providing a risk reduction of 20% to 26%.
METHODOLOGY:
- Previous meta-analyses have studied the association between the intake of cruciferous vegetables and the risk for CC; however, the quantitative dose-response relationship remained uncharacterized, limiting insights for dietary guidance.
- Researchers performed a systematic review and meta-analysis of 17 studies (seven cohort and 10 case-control studies) to analyze the dose-response association between the consumption of cruciferous vegetables and CC risk.
- Studies were included if they enrolled adults without CC at baseline (cohort studies) or adults with diagnosed cases who were matched with control individuals (case-control studies), quantified the dietary intake of cruciferous vegetables through standardized questionnaires, and included comparator groups with lower or no intake of such vegetables.
- The studies included 639,539 participants, of whom 97,595 had CC. Incident cases of CC were confirmed via medical records, pathology, registries, or validated self-report.
TAKEAWAY:
- A pooled analysis revealed that people who consumed the largest amounts of cruciferous vegetables had a 20% lower risk for CC than those who consumed the lowest amounts.
- A dose-response analysis showed that risk reduction was near maximal at an intake of 40-60 g/d (odds ratio, 0.74-0.8), with benefits plateauing beyond this range.
- The peak protective effect per gram occurred at an intake of 20-40 g/d of cruciferous vegetables and fell after 60 g/d.
IN PRACTICE:
“The pathophysiology of CC has been linked to dietary factors, specifically inadequate intake of vegetables and dietary fiber, as well as excessive alcohol and caffeine use. These empirical findings lend credence to our results, suggesting a potential chemopreventive role of CV [cruciferous vegetables] against CC development,” the authors wrote.
SOURCE:
This study, led by Bo Lai, Department of Interventional Radiology, The Second Clinical Medical School of Inner Mongolia University for the Nationalities, Yakeshi, China, was published online in BMC Gastroenterology.
LIMITATIONS:
The inclusion of both case-control and cohort studies and variations in the assessment of cruciferous vegetable intake across studies may have introduced methodological heterogeneity and measurement error, respectively. This study did not measure factors such as pesticide exposure and genetic susceptibility. The predominance of studies from North America and Asia — regions with an elevated incidence of CC — may have limited generalizability to other populations.
DISCLOSURES:
This study received no financial support. The authors declared having no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A higher consumption of cruciferous vegetables such as broccoli and cauliflower was associated with a notably reduced risk for colon cancer (CC), with an optimal intake of 40-60 g/d providing a risk reduction of 20% to 26%.
METHODOLOGY:
- Previous meta-analyses have studied the association between the intake of cruciferous vegetables and the risk for CC; however, the quantitative dose-response relationship remained uncharacterized, limiting insights for dietary guidance.
- Researchers performed a systematic review and meta-analysis of 17 studies (seven cohort and 10 case-control studies) to analyze the dose-response association between the consumption of cruciferous vegetables and CC risk.
- Studies were included if they enrolled adults without CC at baseline (cohort studies) or adults with diagnosed cases who were matched with control individuals (case-control studies), quantified the dietary intake of cruciferous vegetables through standardized questionnaires, and included comparator groups with lower or no intake of such vegetables.
- The studies included 639,539 participants, of whom 97,595 had CC. Incident cases of CC were confirmed via medical records, pathology, registries, or validated self-report.
TAKEAWAY:
- A pooled analysis revealed that people who consumed the largest amounts of cruciferous vegetables had a 20% lower risk for CC than those who consumed the lowest amounts.
- A dose-response analysis showed that risk reduction was near maximal at an intake of 40-60 g/d (odds ratio, 0.74-0.8), with benefits plateauing beyond this range.
- The peak protective effect per gram occurred at an intake of 20-40 g/d of cruciferous vegetables and fell after 60 g/d.
IN PRACTICE:
“The pathophysiology of CC has been linked to dietary factors, specifically inadequate intake of vegetables and dietary fiber, as well as excessive alcohol and caffeine use. These empirical findings lend credence to our results, suggesting a potential chemopreventive role of CV [cruciferous vegetables] against CC development,” the authors wrote.
SOURCE:
This study, led by Bo Lai, Department of Interventional Radiology, The Second Clinical Medical School of Inner Mongolia University for the Nationalities, Yakeshi, China, was published online in BMC Gastroenterology.
LIMITATIONS:
The inclusion of both case-control and cohort studies and variations in the assessment of cruciferous vegetable intake across studies may have introduced methodological heterogeneity and measurement error, respectively. This study did not measure factors such as pesticide exposure and genetic susceptibility. The predominance of studies from North America and Asia — regions with an elevated incidence of CC — may have limited generalizability to other populations.
DISCLOSURES:
This study received no financial support. The authors declared having no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A higher consumption of cruciferous vegetables such as broccoli and cauliflower was associated with a notably reduced risk for colon cancer (CC), with an optimal intake of 40-60 g/d providing a risk reduction of 20% to 26%.
METHODOLOGY:
- Previous meta-analyses have studied the association between the intake of cruciferous vegetables and the risk for CC; however, the quantitative dose-response relationship remained uncharacterized, limiting insights for dietary guidance.
- Researchers performed a systematic review and meta-analysis of 17 studies (seven cohort and 10 case-control studies) to analyze the dose-response association between the consumption of cruciferous vegetables and CC risk.
- Studies were included if they enrolled adults without CC at baseline (cohort studies) or adults with diagnosed cases who were matched with control individuals (case-control studies), quantified the dietary intake of cruciferous vegetables through standardized questionnaires, and included comparator groups with lower or no intake of such vegetables.
- The studies included 639,539 participants, of whom 97,595 had CC. Incident cases of CC were confirmed via medical records, pathology, registries, or validated self-report.
TAKEAWAY:
- A pooled analysis revealed that people who consumed the largest amounts of cruciferous vegetables had a 20% lower risk for CC than those who consumed the lowest amounts.
- A dose-response analysis showed that risk reduction was near maximal at an intake of 40-60 g/d (odds ratio, 0.74-0.8), with benefits plateauing beyond this range.
- The peak protective effect per gram occurred at an intake of 20-40 g/d of cruciferous vegetables and fell after 60 g/d.
IN PRACTICE:
“The pathophysiology of CC has been linked to dietary factors, specifically inadequate intake of vegetables and dietary fiber, as well as excessive alcohol and caffeine use. These empirical findings lend credence to our results, suggesting a potential chemopreventive role of CV [cruciferous vegetables] against CC development,” the authors wrote.
SOURCE:
This study, led by Bo Lai, Department of Interventional Radiology, The Second Clinical Medical School of Inner Mongolia University for the Nationalities, Yakeshi, China, was published online in BMC Gastroenterology.
LIMITATIONS:
The inclusion of both case-control and cohort studies and variations in the assessment of cruciferous vegetable intake across studies may have introduced methodological heterogeneity and measurement error, respectively. This study did not measure factors such as pesticide exposure and genetic susceptibility. The predominance of studies from North America and Asia — regions with an elevated incidence of CC — may have limited generalizability to other populations.
DISCLOSURES:
This study received no financial support. The authors declared having no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Associations Between Prescreening Dietary Patterns and Longitudinal Colonoscopy Outcomes in Veterans
Associations Between Prescreening Dietary Patterns and Longitudinal Colonoscopy Outcomes in Veterans
Screening for colorectal cancer (CRC) with colonoscopy enables the identification and removal of CRC precursors (colonic adenomas) and has been associated with reduced risk of CRC incidence and mortality.1-3 Furthermore, there is consensus that diet and lifestyle may be associated with forestalling CRC pathogenesis at the intermediate adenoma stages.4-7 However, studies have shown that US veterans have poorer diet quality and a higher risk for neoplasia compared with nonveterans, reinforcing the need for tailored clinical approaches.8,9 Combining screening with conversations about modifiable environmental and lifestyle risk factors, such as poor diet, is a highly relevant and possibly easily leveraged prevention for those at high risk. However, there is limited evidence for any particular dietary patterns or dietary features that are most important over time.7
Several dietary components have been shown to be associated with CRC risk,10 either as potentially chemopreventive (fiber, fruits and vegetables,11 dairy,12 supplemental vitamin D,13 calcium,14 and multivitamins15) or carcinogenic (red meat16 and alcohol17). Previous studies of veterans have similarly shown that higher intake of fiber and vitamin D reduced risk, and red meat is associated with an increased risk for finding CRC precursors during colonoscopy.18 However, these dietary categories are often analyzed in isolation. Studying healthy dietary patterns in aggregate may be more clinically relevant and easier to implement for prevention of CRC and its precursors.19-21 Healthy dietary patterns, such as the US Dietary Guidelines for Americans represented by the Healthy Eating Index (HEI), the Mediterranean diet (MD), and the Dietary Approaches to Stop Hypertension (DASH) diet, have been associated with lower risk for chronic disease.22-24 Despite the extant literature, no known studies have compared these dietary patterns for associations with risk of CRC precursor or CRC development among US veterans undergoing long-term screening and follow-up after a baseline colonoscopy.
The objective of this study was to test for associations between baseline scores of healthy dietary patterns and the most severe colonoscopy findings (MSCFs) over ≥ 10 years following a baseline screening colonoscopy in veterans.
Methods
Participants in the Cooperative Studies Program (CSP) #380 cohort study included 3121 asymptomatic veterans aged 50 to 75 years at baseline who had consented to initial screening colonoscopy between 1994 and 1997, with subsequent follow-up and surveillance.25 Prior to their colonoscopy, all participants completed a baseline study survey that included questions about cancer risk factors including family history of CRC, diet, physical activity, and medication use.
Included in this cross-sectional analysis were data from a sample of veteran participants of the CSP #380 cohort with 1 baseline colonoscopy, follow-up surveillance through 2009, a cancer risk factor survey collected at baseline, and complete demographic and clinical indicator data. Excluded from the analysis were 67 participants with insufficient responses to the dietary food frequency questionnaire (FFQ) and 31 participants with missing body mass index (BMI), 3023 veterans.
Measures
MSCF. The outcome of interest in this study was the MSCF recorded across all participant colonoscopies during the study period. MSCF was categorized as either (1) no neoplasia; (2) < 2 nonadvanced adenomas, including small adenomas (diameter < 10 mm) with tubular histology; or (3) advanced neoplasia (AN), which is characterized by adenomas > 10 mm in diameter, with villous histology, with high-grade dysplasia, or CRC.
Dietary patterns. Dietary pattern scores representing dietary quality and calculated based on recommendations of the US Dietary Guidelines for Americans using the HEI, MD, and DASH diets were independent variables.26-28 These 3 dietary patterns were chosen for their hypothesized relationship with CRC risk, but each weighs food categories differently (Appendix 1).22-24,29 Dietary pattern scores were calculated using the CSP #380 self-reported responses to 129 baseline survey questions adapted from a well-established and previously validated semiquantitative FFQ.30 The form was administered by mail twice to a sample of 127 participants at baseline and at 1 year. During this interval, men completed 1-week diet records twice, spaced about 6 months apart. Mean values for intake of most nutrients assessed by the 2 methods were similar. Intraclass correlation coefficients for the baseline and 1-year FFQ-assessed nutrient intakes that ranged from 0.47 for vitamin E (without supplements) to 0.80 for vitamin C (with supplements). Correlation coefficients between the energy-adjusted nutrient intakes were measured by diet records and the 1-year FFQ, which asked about diet during the year encompassing the diet records. Higher raw and percent scores indicated better alignment with recommendations from each respective dietary pattern. Percent scores were calculated as a standardizing method and used in analyses for ease of comparing the dietary patterns. Scoring can be found in Appendix 2.
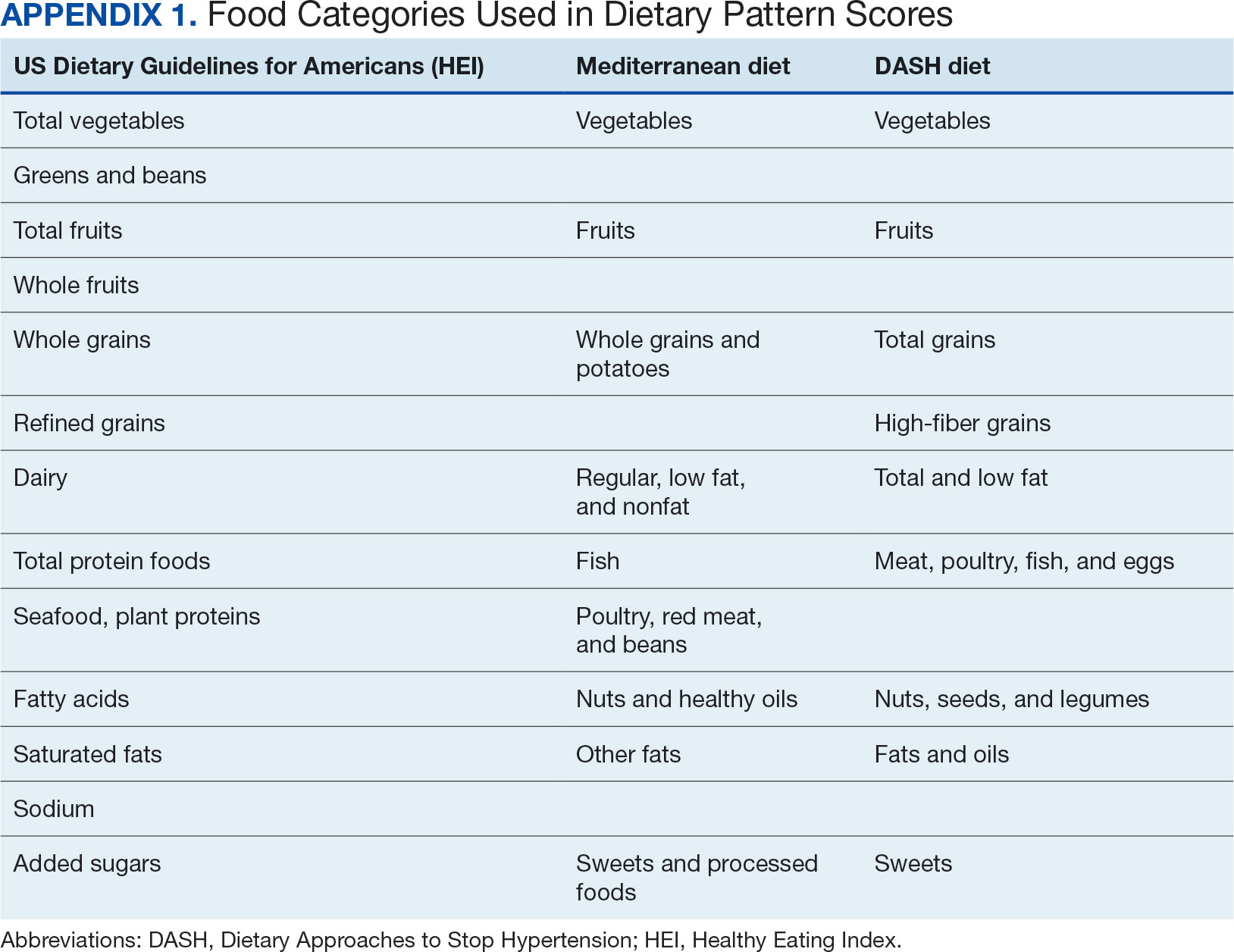
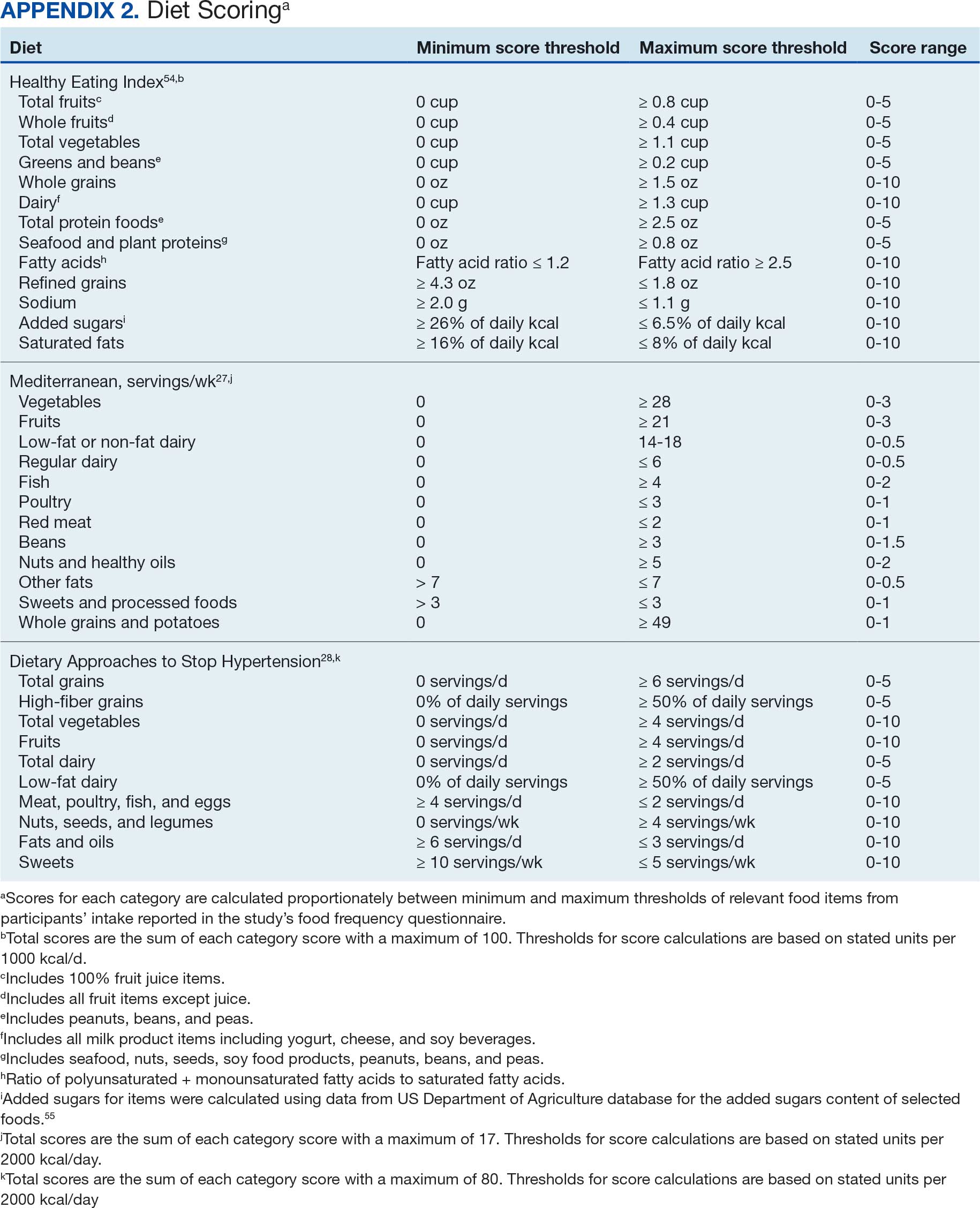
Demographic characteristics and clinical indicators. Demographic characteristics included age categories, sex, and race/ethnicity. Clinical indicators included BMI, the number of comorbid conditions used to calculate the Charlson Comorbidity Index, family history of CRC in first-degree relatives, number of follow-up colonoscopies across the study period, and food-based vitamin D intake.31 These variables were chosen for their applicability found in previous CSP #380 cohort studies.18,32,33 Self-reported race and ethnicity were collapsed due to small numbers in some groups. The authors acknowledge these are distinct concepts and the variable has limited utility other than for controlling for systemic racism in the model.
Statistical Analyses
Descriptive statistics were used to describe distributional assumptions for all variables, including demographics, clinical indicators, colonoscopy results, and dietary patterns. Pairwise correlations between the total dietary pattern scores and food category scores were calculated with Pearson correlation (r).
Multinomial logistic regression models were created using SAS procedure LOGISTIC with the outcome of the categorical MSCF (no neoplasia, nonadvanced adenoma, or AN).34 A model was created for each independent predictor variable of interest (ie, the HEI, MD, or DASH percentage-standardized dietary pattern score and each food category comprising each dietary pattern score). All models were adjusted for age, sex, race/ethnicity, BMI, number of comorbidities, family history of CRC, number of follow-up colonoscopies, and estimated daily food-derived vitamin D intake. The demographic and clinical indicators were included in the models as they are known to be associated with CRC risk.18 The number of colonoscopies was included to control for surveillance intensity presuming risk for AN is reduced as polyps are removed. Because colonoscopy findings from an initial screening have unique clinical implications compared with follow- up and surveillance, MSCF was observed in 2 ways in sensitivity analyses: (1) baseline and (2) aggregate follow-up and surveillance only, excluding baseline findings.
Adjusted odds ratios (aORs) and 95% CIs for each of the MSCF outcomes with a reference finding of no neoplasia for the models are presented. We chose not to adjust for multiple comparisons across the different dietary patterns given the correlation between dietary pattern total and category scores but did adjust for multiple comparisons for dietary categories within each dietary pattern. Tests for statistical significance used α= .05 for the dietary pattern total scores and P values for the dietary category scores for each dietary pattern controlled for false discovery rate using the MULTTEST SAS procedure.35 All data manipulations and analyses were performed using SAS version 9.4.
Results
The study included 3023 patients. All were aged 50 to 75 years, 2923 (96.7%) were male and 2532 (83.8%) were non-Hispanic White (Table 1). Most participants were overweight or obese (n = 2535 [83.8%]), 2024 (67.0%) had ≤ 2 comorbidities, and 2602 (86.1%) had no family history of CRC. The MSCF for 1628 patients (53.9%) was no neoplasia, 966 patients (32.0%) was nonadvanced adenoma, and 429 participants (14.2%) had AN.
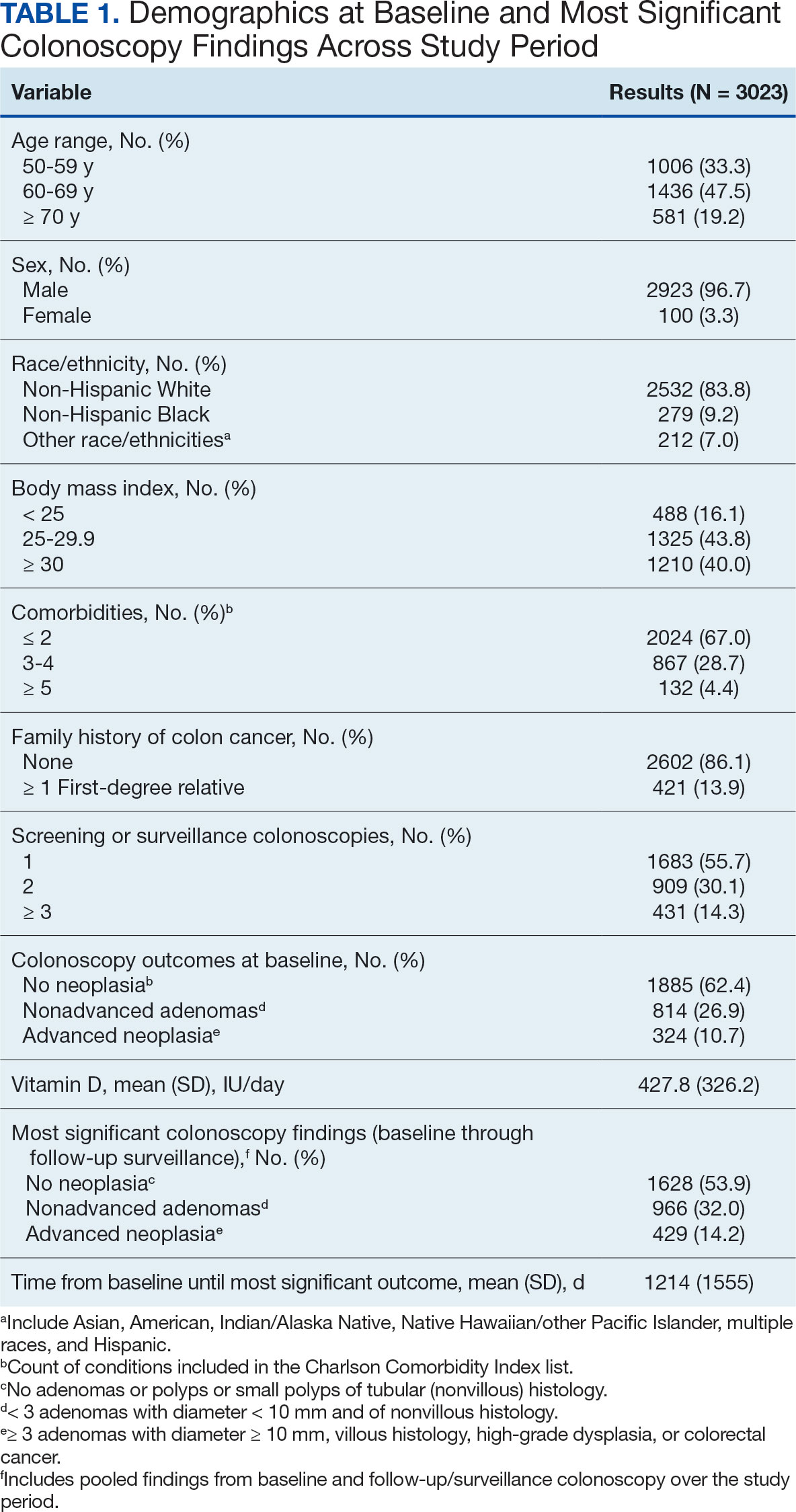
Mean percent scores were 58.5% for HEI, 38.2% for MD, and 63.1% for the DASH diet, with higher percentages indicating greater alignment with the recommendations for each diet (Table 2). All 3 dietary patterns scores standardized to percentages were strongly and significantly correlated in pairwise comparisons: HEI:MD, r = 0.62 (P < .001); HEI:DASH, r = 0.60 (P < .001); and MD:DASH, r = 0.72 (P < .001). Likewise, food category scores were significantly correlated across dietary patterns. For example, whole grain and fiber values from each dietary score were strongly correlated in pairwise comparisons: HEI Whole Grain:MD Grain, r = 0.64 (P < .001); HEI Whole Grain:DASH Fiber, r = 0.71 (P < .001); and MD Grain:DASH Fiber, r = 0.70 (P < .001).
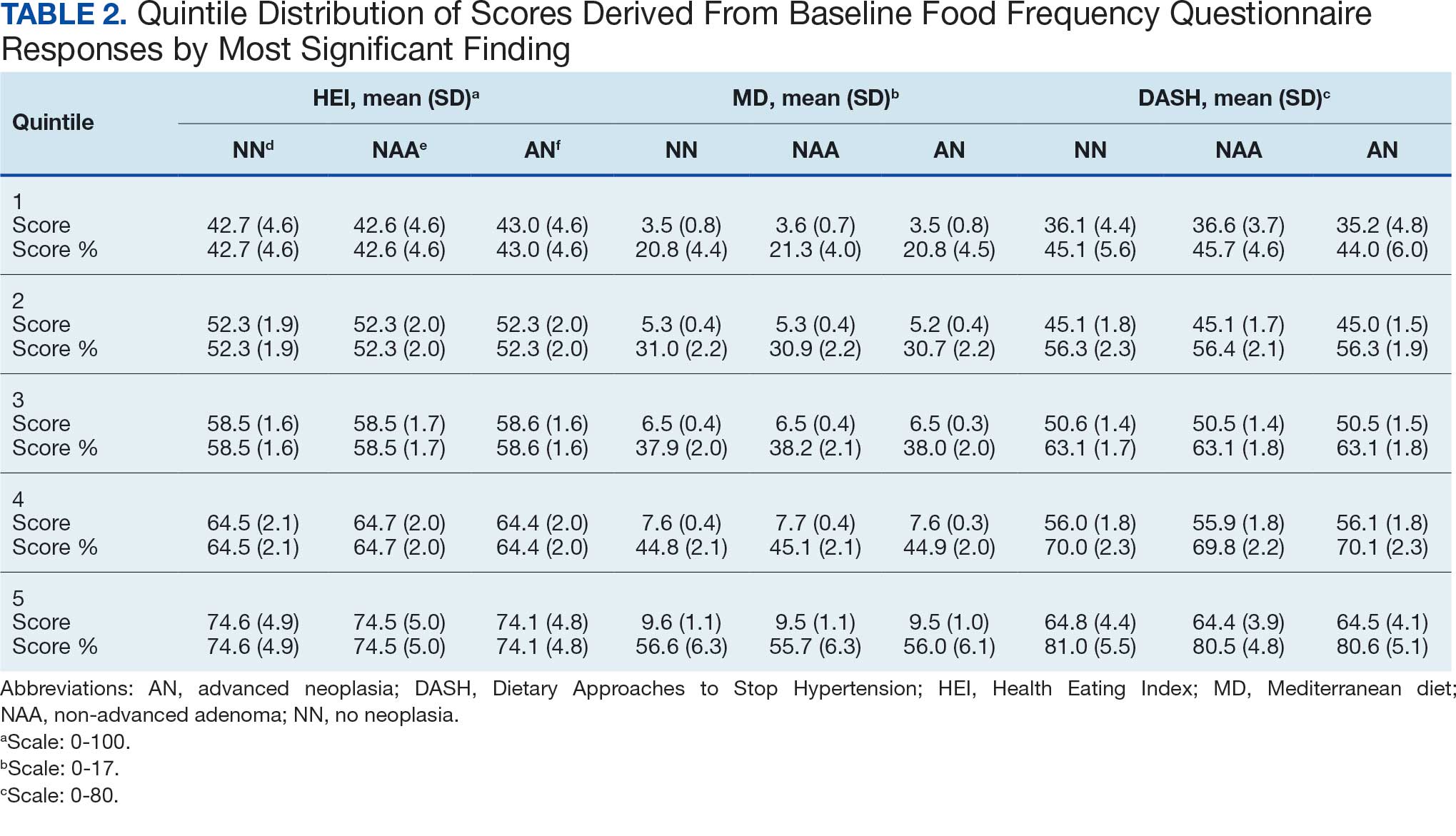
Associations between individual participants' dietary pattern scores and the outcome of their pooled MSCF from baseline screening and ≥ 10 years of surveillance are presented in Table 3. For each single-point increases in dietary pattern scores (reflecting better dietary quality), aORs for nonadvanced adenoma vs no neoplasia were slightly lower but not statistically significantly: HEI, aOR, 1.00 (95% CI, 0.99-1.01); MD, aOR, 0.98 (95% CI, 0.94-1.02); and DASH, aOR, 0.99 (95% CI, 0.99-1.00). aORs for AN vs no neoplasia were slightly lower for each dietary pattern assessed, and only the MD and DASH scores were significantly different from 1.00: HEI, aOR, 1.00 (95% CI, 0.99-1.01); MD, aOR, 0.95 (95% CI, 0.90-1.00); and DASH, aOR, 0.99 (95% CI, 0.98-1.00).
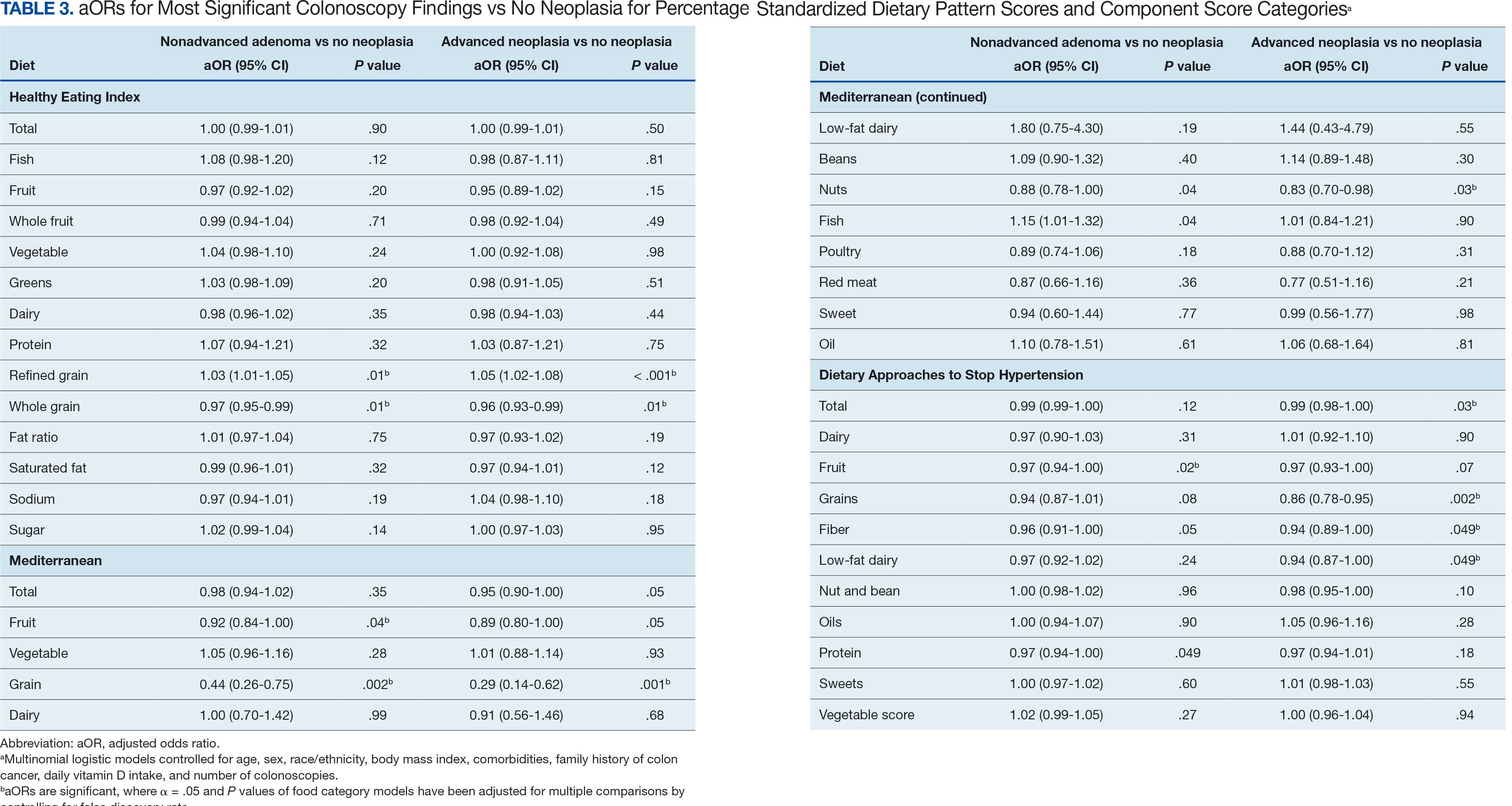
We observed lower odds for nonadvanced adenoma and AN among all these dietary patterns when there was greater alignment with the recommended intake of whole grains and fiber. In separate models conducted using food categories comprising the dietary patterns as independent variables and after correcting for multiple tests, higher scores for the HEI Refined Grain category were associated with higher odds for nonadvanced adenoma (aOR, 1.03 [95% CI, 1.01-1.05]; P = .01) and AN (aOR, 1.05 [95% CI, 1.02-1.08]; P < .001). Higher scores for the HEI Whole Grain category were associated with lower odds for nonadvanced adenoma (aOR, 0.97 [95% CI, 0.95-0.99]; P = .01) and AN (aOR, 0.96 [95% CI, 0.93-0.99]; P = .01). Higher scores for the MD Grain category were significantly associated with lower odds for nonadvanced adenoma (aOR, 0.44 [95% CI, 0.26-0.75]; P = .002) and AN (aOR, 0.29 [95% CI, 0.14-0.62]; P = .001). The DASH Grains category also was significantly associated with lower odds for AN (aOR, 0.86 [95% CI, 0.78-0.95]; P = .002).
Discussion
In this study of 3023 veterans undergoing first-time screening colonoscopy and ≥ 10 years of surveillance, we found that healthy dietary patterns, as assessed by the MD and DASH diet, were significantly associated with lower risk of AN. Additionally, we identified lower odds for AN and nonadvanced adenoma compared with no neoplasia for higher grain scores for all the dietary patterns studied. Other food categories that comprise the dietary pattern scores had mixed associations with the MSCF outcomes. Several other studies have examined associations between dietary patterns and risk for CRC but to our knowledge, no studies have explored these associations among US veterans.
These results also indicate study participants had better than average (based on a 50% threshold) dietary quality according to the HEI and DASH diet scoring methods we used, but poor dietary quality according to the MD scoring method. The mean HEI scores for the present study were higher than a US Department of Agriculture study by Dong et al that compared dietary quality between veterans and nonveterans using the HEI, for which veterans’ expected HEI score was 45.6 of 100.8 This could be explained by the fact that the participants needed to be healthy to be eligible and those with healthier behaviors overall may have self-selected into the study due to motivation for screening during a time when screening was not yet commonplace. 36 Similarly, participants of the present study had higher adherence to the DASH diet (63.1%) than adolescents with diabetes in a study by Günther et al. Conversely, firefighters who were coached to use a Mediterranean-style dietary pattern and dietary had higher adherence to MD than did participants in this study.27
A closer examination of specific food category component scores that comprise the 3 distinct dietary patterns revealed mixed results from the multinomial modeling, which may have to do with the guideline thresholds used to calculate the dietary scores. When analyzed separately in the logistic regression models for their associations with nonadvanced adenomas and AN compared with no neoplasia, higher MD and DASH fruit scores (but not HEI fruit scores) were found to be significant. Other studies have had mixed findings when attempting to test for associations of fruit intake with adenoma recurrence.10,37
This study had some unexpected findings. Vegetable intake was not associated with nonadvanced adenomas or AN risk. Studies of food categories have consistently found vegetable (specifically cruciferous ones) intake to be linked with lower odds for cancers.38 Likewise, the red meat category, which was only a unique food category in the MD score, was not associated with nonadvanced adenomas or AN. Despite consistent literature suggesting higher intake of red meat and processed meats increases CRC risk, in 2019 the Nutritional Recommendations Consortium indicated that the evidence was weak.39,40 This study showed higher DASH diet scores for low-fat dairy, which were maximized when participants reported at least 50% of their dairy servings per day as being low-fat, had lower odds for AN. Yet, the MD scores for low-fat dairy had no association with either outcome; their calculation was based on total number of servings per week. This difference in findings suggests the fat intake ratio may be more relevant to CRC risk than intake quantity.
The literature is mixed regarding fatty acid intake and CRC risk, which may be relevant to both dairy and meat intake. One systematic review and meta-analysis found dietary fat and types of fatty acid intake had no association with CRC risk.41 However, a more recent meta-analysis that assessed both dietary intake and plasma levels of fatty acids did find some statistically significant differences for various types of fatty acids and CRC risk.42
The findings in the present study that grain intake is associated with lower odds for more severe colonoscopy findings among veterans are notable.43 Lieberman et al, using the CSP #380 data, found that cereal fiber intake was associated with a lower odds for AN compared with hyperplastic polyps (OR, 0.98 [95% CI, 0.96- 1.00]).18 Similarly, Hullings et al determined that older adults in the highest quintile of cereal fiber intake had significantly lower odds of CRC than those in lower odds for CRC when compared with lowest quintile (OR, 0.89 [95% CI, 0.83- 0.96]; P < .001).44 These findings support existing guidance that prioritizes whole grains as a key source of dietary fiber for CRC prevention.
A recent literature review on fiber, fat, and CRC risk suggested a consensus regarding one protective mechanism: dietary fiber from grains modulates the gut microbiota by promoting butyrate synthesis.45 Butyrate is a short-chain fatty acid that supports energy production in colonocytes and has tumor-suppressing properties.46 Our findings suggest there could be more to learn about the relationship between butyrate production and reduction of CRC risk through metabolomic studies that use measurements of plasma butyrate. These studies may examine associations between not just a singular food or food category, but rather food patterns that include fruits, vegetables, nuts and seeds, and whole grains known to promote butyrate production and plasma butyrate.47
Improved understanding of mechanisms and risk-modifying lifestyle factors such as dietary patterns may enhance prevention strategies. Identifying the collective chemopreventive characteristics of a specific dietary pattern (eg, MD) will be helpful to clinicians and health care staff to promote healthy eating to reduce cancer risk. More studies are needed to understand whether such promotion is more clinically applicable and effective for patients, as compared with eating more or less of specific foods (eg, more whole grains, less red meat). Furthermore, considering important environmental factors collectively beyond dietary patterns may offer a way to better tailor screening and implement a variety of lifestyle interventions. In the literature, this is often referred to as a teachable moment when patients’ attentions are captured and may position them to be more receptive to guidance.48
Limitations
This study has several important limitations and leaves opportunities for future studies that explore the role of dietary patterns and AN or CRC risk. First, the FFQ data used to calculate dietary pattern scores used in analysis were only captured at baseline, and there are nearly 3 decades across the study period. However, it is widely assumed that the diets of older adults, like those included in this study, remain stable over time which is appropriate given our sample population was aged 50 to 75 years when the baseline FFQ data were collected.49-51 Additionally, while the HEI is a well-documented, standard scoring method for dietary quality, there are multitudes of dietary pattern scoring approaches for MD and DASH.23,52,53 Finally, findings from this study using the sample of veterans may not be generalizable to a broader population. Future longitudinal studies that test for a clinically significant change threshold are warranted.
Conclusion
Results of this study suggest future research should further explore the effects of dietary patterns, particularly intake of specific food groups in combination, as opposed to individual nutrients or food items, on AN and CRC risk. Possible studies might explore these dietary patterns for their mechanistic role in altering the microbiome metabolism, which may influence CRC outcomes or include diet in a more comprehensive, holistic risk score that could be used to predict colonic neoplasia risk or in intervention studies that assess the effects of dietary changes on long-term CRC prevention. We suggest there are differences in people’s dietary intake patterns that might be important to consider when implementing tailored approaches to CRC risk mitigation.
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectalcancer deaths. N Engl J Med. 2012;366(8):687-696. doi:10.1056/NEJMoa1100370
- Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-1105. doi:10.1056/NEJMoa1301969
- Bretthauer M, Løberg M, Wieszczy P, et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. 2022;387(17):1547-1556. doi:10.1056/NEJMoa2208375
- Cottet V, Bonithon-Kopp C, Kronborg O, et al. Dietary patterns and the risk of colorectal adenoma recurrence in a European intervention trial. Eur J Cancer Prev. 2005;14(1):21.
- Miller PE, Lesko SM, Muscat JE, Lazarus P, Hartman TJ. Dietary patterns and colorectal adenoma and cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2010;62(4):413-424. doi:10.1080/01635580903407114
- Godos J, Bella F, Torrisi A, Sciacca S, Galvano F, Grosso G. Dietary patterns and risk of colorectal adenoma: a systematic review and meta-analysis of observational studies. J Hum Nutr Diet Off J Br Diet Assoc. 2016;29(6):757-767. doi:10.1111/jhn.12395
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191-197. doi:10.1055/s-0029-1242458
- Dong D, Stewart H, Carlson AC. An Examination of Veterans’ Diet Quality. U.S. Department of Agriculture, Economic Research Service; 2019:32.
- El-Halabi MM, Rex DK, Saito A, Eckert GJ, Kahi CJ. Defining adenoma detection rate benchmarks in average-risk male veterans. Gastrointest Endosc. 2019;89(1):137-143. doi:10.1016/j.gie.2018.08.021
- Alberts DS, Hess LM, eds. Fundamentals of Cancer Prevention. Springer International Publishing; 2019. doi:10.1007/978-3-030-15935-1
- Dahm CC, Keogh RH, Spencer EA, et al. Dietary fiber and colorectal cancer risk: a nested case-control study using food diaries. J Natl Cancer Inst. 2010;102(9):614-626. doi:10.1093/jnci/djq092
- Aune D, Lau R, Chan DSM, et al. Dairy products and colorectal cancer risk: a systematic review and metaanalysis of cohort studies. Ann Oncol. 2012;23(1):37-45. doi:10.1093/annonc/mdr269
- Lee JE, Li H, Chan AT, et al. Circulating levels of vitamin D and colon and rectal cancer: the Physicians’ Health Study and a meta-analysis of prospective studies. Cancer Prev Res Phila Pa. 2011;4(5):735-743. doi:10.1158/1940-6207.CAPR-10-0289
- Carroll C, Cooper K, Papaioannou D, Hind D, Pilgrim H, Tappenden P. Supplemental calcium in the chemoprevention of colorectal cancer: a systematic review and meta-analysis. Clin Ther. 2010;32(5):789-803. doi:10.1016/j.clinthera.2010.04.024
- Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control CCC. 2010;21(11):1745- 1757. doi:10.1007/s10552-010-9549-y
- Alexander DD, Weed DL, Miller PE, Mohamed MA. Red meat and colorectal cancer: a quantitative update on the state of the epidemiologic science. J Am Coll Nutr. 2015;34(6):521-543. doi:10.1080/07315724.2014.992553
- Park SY, Wilkens LR, Setiawan VW, Monroe KR, Haiman CA, Le Marchand L. Alcohol intake and colorectal cancer risk in the multiethnic cohort study. Am J Epidemiol. 2019;188(1):67-76. doi:10.1093/aje/kwy208
- Lieberman DA. Risk Factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290(22):2959. doi:10.1001/jama.290.22.2959
- Archambault AN, Jeon J, Lin Y, et al. Risk stratification for early-onset colorectal cancer using a combination of genetic and environmental risk scores: an international multi-center study. J Natl Cancer Inst. 2022;114(4):528-539. doi:10.1093/jnci/djac003
- Carr PR, Weigl K, Edelmann D, et al. Estimation of absolute risk of colorectal cancer based on healthy lifestyle, genetic risk, and colonoscopy status in a populationbased study. Gastroenterology. 2020;159(1):129-138.e9. doi:10.1053/j.gastro.2020.03.016
- Sullivan BA, Qin X, Miller C, et al. Screening colonoscopy findings are associated with noncolorectal cancer mortality. Clin Transl Gastroenterol. 2022;13(4):e00479. doi:10.14309/ctg.0000000000000479
- Erben V, Carr PR, Holleczek B, Stegmaier C, Hoffmeister M, Brenner H. Dietary patterns and risk of advanced colorectal neoplasms: A large population based screening study in Germany. Prev Med. 2018;111:101-109. doi:10.1016/j.ypmed.2018.02.025
- Donovan MG, Selmin OI, Doetschman TC, Romagnolo DF. Mediterranean diet: prevention of colorectal cancer. Front Nutr. 2017;4:59. doi:10.3389/fnut.2017.00059
- Mohseni R, Mohseni F, Alizadeh S, Abbasi S. The Association of Dietary Approaches to Stop Hypertension (DASH) diet with the risk of colorectal cancer: a meta-analysis of observational studies.Nutr Cancer. 2020;72(5):778-790. doi:10.1080/01635581.2019.1651880
- Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
- Developing the Healthy Eating Index (HEI) | EGRP/ DCCPS/NCI/NIH. Accessed July 22, 2025. https://epi.grants.cancer.gov/hei/developing.html#2015c
- Reeve E, Piccici F, Feairheller DL. Validation of a Mediterranean diet scoring system for intervention based research. J Nutr Med Diet Care. 2021;7(1):053. doi:10.23937/2572-3278/1510053
- Günther AL, Liese AD, Bell RA, et al. ASSOCIATION BETWEEN THE DIETARY APPROACHES TO HYPERTENSION (DASH) DIET AND HYPERTENSION IN YOUTH WITH DIABETES. Hypertens Dallas Tex 1979. 2009;53(1):6-12. doi:10.1161/HYPERTENSIONAHA.108.116665
- Buckland G, Agudo A, Luján L, et al. Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Am J Clin Nutr. 2010;91(2):381- 390. doi:10.3945/ajcn.2009.28209
- Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114-1126. doi:10.1093/oxfordjournals.aje.a116211
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
- Lieberman DA, Weiss DG, Harford WV, et al. Fiveyear colon surveillance after screening colonoscopy. Gastroenterology. 2007;133(4):1077-1085. doi:10.1053/j.gastro.2007.07.006
- Lieberman D, Sullivan BA, Hauser ER, et al. Baseline colonoscopy findings associated with 10-year outcomes in a screening cohort undergoing colonoscopy surveillance. Gastroenterology. 2020;158(4):862-874.e8. doi:10.1053/j.gastro.2019.07.052
- PROC LOGISTIC: PROC LOGISTIC Statement : SAS/STAT(R) 9.22 User’s Guide. Accessed July 22, 2025. https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_logistic_sect004.htm
- PROC MULTTEST: PROC MULTTEST Statement : SAS/ STAT(R) 9.22 User’s Guide. Accessed July 22, 2025. https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_multtest_sect005.htm
- Elston DM. Participation bias, self-selection bias, and response bias. J Am Acad Dermatol. Published online June 18, 2021. doi:10.1016/j.jaad.2021.06.025
- Sansbury LB, Wanke K, Albert PS, et al. The effect of strict adherence to a high-fiber, high-fruit and -vegetable, and low-fat eating pattern on adenoma recurrence. Am J Epidemiol. 2009;170(5):576-584. doi:10.1093/aje/kwp169
- Borgas P, Gonzalez G, Veselkov K, Mirnezami R. Phytochemically rich dietary components and the risk of colorectal cancer: A systematic review and meta-analysis of observational studies. World J Clin Oncol. 2021;12(6):482- 499. doi:10.5306/wjco.v12.i6.482
- Papadimitriou N, Markozannes G, Kanellopoulou A, et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat Commun. 2021;12(1):4579. doi:10.1038/s41467-021-24861-8
- Johnston BC, Zeraatkar D, Han MA, et al. Unprocessed red meat and processed meat consumption: dietary guideline recommendations from the nutritional recommendations (NutriRECS) Consortium. Ann Intern Med. 2019;171(10):756-764. doi:10.7326/M19-1621
- Kim M, Park K. Dietary fat intake and risk of colorectal cancer: a systematic review and meta-analysis of prospective studies. Nutrients. 2018;10(12):1963. doi:10.3390/nu10121963
- Lu Y, Li D, Wang L, et al. Comprehensive investigation on associations between dietary intake and blood levels of fatty acids and colorectal cancer risk. Nutrients. 2023;15(3):730. doi:10.3390/nu15030730
- Gherasim A, Arhire LI, Ni.a O, Popa AD, Graur M, Mihalache L. The relationship between lifestyle components and dietary patterns. Proc Nutr Soc. 2020;79(3):311-323. doi:10.1017/S0029665120006898
- Hullings AG, Sinha R, Liao LM, Freedman ND, Graubard BI, Loftfield E. Whole grain and dietary fiber intake and risk of colorectal cancer in the NIH-AARP Diet and Health Study cohort. Am J Clin Nutr. 2020;112(3):603- 612. doi:10.1093/ajcn/nqaa161
- Ocvirk S, Wilson AS, Appolonia CN, Thomas TK, O’Keefe SJD. Fiber, fat, and colorectal cancer: new insight into modifiable dietary risk factors. Curr Gastroenterol Rep. 2019;21(11):62. doi:10.1007/s11894-019-0725-2
- O’Keefe SJD. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13(12):691-706. doi:10.1038/nrgastro.2016.165
- The health benefits and side effects of Butyrate Cleveland Clinic. July 11, 2022. Accessed July 22, 2025. https://health.clevelandclinic.org/butyrate-benefits/
- Knudsen MD, Wang L, Wang K, et al. Changes in lifestyle factors after endoscopic screening: a prospective study in the United States. Clin Gastroenterol Hepatol Off ClinPract J Am Gastroenterol Assoc. 2022;20(6):e1240-e1249. doi:10.1016/j.cgh.2021.07.014
- Thorpe MG, Milte CM, Crawford D, McNaughton SA. Education and lifestyle predict change in dietary patterns and diet quality of adults 55 years and over. Nutr J. 2019;18(1):67. doi:10.1186/s12937-019-0495-6
- Chapman K, Ogden J. How do people change their diet?: an exploration into mechanisms of dietary change. J Health Psychol. 2009;14(8):1229-1242. doi:10.1177/1359105309342289
- Djoussé L, Petrone AB, Weir NL, et al. Repeated versus single measurement of plasma omega-3 fatty acids and risk of heart failure. Eur J Nutr. 2014;53(6):1403-1408. doi:10.1007/s00394-013-0642-3
- Bach-Faig A, Berry EM, Lairon D, et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14(12A):2274-2284. doi:10.1017/S1368980011002515
- Miller PE, Cross AJ, Subar AF, et al. Comparison of 4 established DASH diet indexes: examining associations of index scores and colorectal cancer123. Am J Clin Nutr. 2013;98(3):794-803. doi:10.3945/ajcn.113.063602
- Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591-1602. doi:10.1016/j.jand.2018.05.021
- P.R. Pehrsson, Cutrufelli RL, Gebhardt SE, et al. USDA Database for the Added Sugars Content of Selected Foods. USDA; 2005. www.ars.usda.gov/nutrientdata
Screening for colorectal cancer (CRC) with colonoscopy enables the identification and removal of CRC precursors (colonic adenomas) and has been associated with reduced risk of CRC incidence and mortality.1-3 Furthermore, there is consensus that diet and lifestyle may be associated with forestalling CRC pathogenesis at the intermediate adenoma stages.4-7 However, studies have shown that US veterans have poorer diet quality and a higher risk for neoplasia compared with nonveterans, reinforcing the need for tailored clinical approaches.8,9 Combining screening with conversations about modifiable environmental and lifestyle risk factors, such as poor diet, is a highly relevant and possibly easily leveraged prevention for those at high risk. However, there is limited evidence for any particular dietary patterns or dietary features that are most important over time.7
Several dietary components have been shown to be associated with CRC risk,10 either as potentially chemopreventive (fiber, fruits and vegetables,11 dairy,12 supplemental vitamin D,13 calcium,14 and multivitamins15) or carcinogenic (red meat16 and alcohol17). Previous studies of veterans have similarly shown that higher intake of fiber and vitamin D reduced risk, and red meat is associated with an increased risk for finding CRC precursors during colonoscopy.18 However, these dietary categories are often analyzed in isolation. Studying healthy dietary patterns in aggregate may be more clinically relevant and easier to implement for prevention of CRC and its precursors.19-21 Healthy dietary patterns, such as the US Dietary Guidelines for Americans represented by the Healthy Eating Index (HEI), the Mediterranean diet (MD), and the Dietary Approaches to Stop Hypertension (DASH) diet, have been associated with lower risk for chronic disease.22-24 Despite the extant literature, no known studies have compared these dietary patterns for associations with risk of CRC precursor or CRC development among US veterans undergoing long-term screening and follow-up after a baseline colonoscopy.
The objective of this study was to test for associations between baseline scores of healthy dietary patterns and the most severe colonoscopy findings (MSCFs) over ≥ 10 years following a baseline screening colonoscopy in veterans.
Methods
Participants in the Cooperative Studies Program (CSP) #380 cohort study included 3121 asymptomatic veterans aged 50 to 75 years at baseline who had consented to initial screening colonoscopy between 1994 and 1997, with subsequent follow-up and surveillance.25 Prior to their colonoscopy, all participants completed a baseline study survey that included questions about cancer risk factors including family history of CRC, diet, physical activity, and medication use.
Included in this cross-sectional analysis were data from a sample of veteran participants of the CSP #380 cohort with 1 baseline colonoscopy, follow-up surveillance through 2009, a cancer risk factor survey collected at baseline, and complete demographic and clinical indicator data. Excluded from the analysis were 67 participants with insufficient responses to the dietary food frequency questionnaire (FFQ) and 31 participants with missing body mass index (BMI), 3023 veterans.
Measures
MSCF. The outcome of interest in this study was the MSCF recorded across all participant colonoscopies during the study period. MSCF was categorized as either (1) no neoplasia; (2) < 2 nonadvanced adenomas, including small adenomas (diameter < 10 mm) with tubular histology; or (3) advanced neoplasia (AN), which is characterized by adenomas > 10 mm in diameter, with villous histology, with high-grade dysplasia, or CRC.
Dietary patterns. Dietary pattern scores representing dietary quality and calculated based on recommendations of the US Dietary Guidelines for Americans using the HEI, MD, and DASH diets were independent variables.26-28 These 3 dietary patterns were chosen for their hypothesized relationship with CRC risk, but each weighs food categories differently (Appendix 1).22-24,29 Dietary pattern scores were calculated using the CSP #380 self-reported responses to 129 baseline survey questions adapted from a well-established and previously validated semiquantitative FFQ.30 The form was administered by mail twice to a sample of 127 participants at baseline and at 1 year. During this interval, men completed 1-week diet records twice, spaced about 6 months apart. Mean values for intake of most nutrients assessed by the 2 methods were similar. Intraclass correlation coefficients for the baseline and 1-year FFQ-assessed nutrient intakes that ranged from 0.47 for vitamin E (without supplements) to 0.80 for vitamin C (with supplements). Correlation coefficients between the energy-adjusted nutrient intakes were measured by diet records and the 1-year FFQ, which asked about diet during the year encompassing the diet records. Higher raw and percent scores indicated better alignment with recommendations from each respective dietary pattern. Percent scores were calculated as a standardizing method and used in analyses for ease of comparing the dietary patterns. Scoring can be found in Appendix 2.


Demographic characteristics and clinical indicators. Demographic characteristics included age categories, sex, and race/ethnicity. Clinical indicators included BMI, the number of comorbid conditions used to calculate the Charlson Comorbidity Index, family history of CRC in first-degree relatives, number of follow-up colonoscopies across the study period, and food-based vitamin D intake.31 These variables were chosen for their applicability found in previous CSP #380 cohort studies.18,32,33 Self-reported race and ethnicity were collapsed due to small numbers in some groups. The authors acknowledge these are distinct concepts and the variable has limited utility other than for controlling for systemic racism in the model.
Statistical Analyses
Descriptive statistics were used to describe distributional assumptions for all variables, including demographics, clinical indicators, colonoscopy results, and dietary patterns. Pairwise correlations between the total dietary pattern scores and food category scores were calculated with Pearson correlation (r).
Multinomial logistic regression models were created using SAS procedure LOGISTIC with the outcome of the categorical MSCF (no neoplasia, nonadvanced adenoma, or AN).34 A model was created for each independent predictor variable of interest (ie, the HEI, MD, or DASH percentage-standardized dietary pattern score and each food category comprising each dietary pattern score). All models were adjusted for age, sex, race/ethnicity, BMI, number of comorbidities, family history of CRC, number of follow-up colonoscopies, and estimated daily food-derived vitamin D intake. The demographic and clinical indicators were included in the models as they are known to be associated with CRC risk.18 The number of colonoscopies was included to control for surveillance intensity presuming risk for AN is reduced as polyps are removed. Because colonoscopy findings from an initial screening have unique clinical implications compared with follow- up and surveillance, MSCF was observed in 2 ways in sensitivity analyses: (1) baseline and (2) aggregate follow-up and surveillance only, excluding baseline findings.
Adjusted odds ratios (aORs) and 95% CIs for each of the MSCF outcomes with a reference finding of no neoplasia for the models are presented. We chose not to adjust for multiple comparisons across the different dietary patterns given the correlation between dietary pattern total and category scores but did adjust for multiple comparisons for dietary categories within each dietary pattern. Tests for statistical significance used α= .05 for the dietary pattern total scores and P values for the dietary category scores for each dietary pattern controlled for false discovery rate using the MULTTEST SAS procedure.35 All data manipulations and analyses were performed using SAS version 9.4.
Results
The study included 3023 patients. All were aged 50 to 75 years, 2923 (96.7%) were male and 2532 (83.8%) were non-Hispanic White (Table 1). Most participants were overweight or obese (n = 2535 [83.8%]), 2024 (67.0%) had ≤ 2 comorbidities, and 2602 (86.1%) had no family history of CRC. The MSCF for 1628 patients (53.9%) was no neoplasia, 966 patients (32.0%) was nonadvanced adenoma, and 429 participants (14.2%) had AN.

Mean percent scores were 58.5% for HEI, 38.2% for MD, and 63.1% for the DASH diet, with higher percentages indicating greater alignment with the recommendations for each diet (Table 2). All 3 dietary patterns scores standardized to percentages were strongly and significantly correlated in pairwise comparisons: HEI:MD, r = 0.62 (P < .001); HEI:DASH, r = 0.60 (P < .001); and MD:DASH, r = 0.72 (P < .001). Likewise, food category scores were significantly correlated across dietary patterns. For example, whole grain and fiber values from each dietary score were strongly correlated in pairwise comparisons: HEI Whole Grain:MD Grain, r = 0.64 (P < .001); HEI Whole Grain:DASH Fiber, r = 0.71 (P < .001); and MD Grain:DASH Fiber, r = 0.70 (P < .001).

Associations between individual participants' dietary pattern scores and the outcome of their pooled MSCF from baseline screening and ≥ 10 years of surveillance are presented in Table 3. For each single-point increases in dietary pattern scores (reflecting better dietary quality), aORs for nonadvanced adenoma vs no neoplasia were slightly lower but not statistically significantly: HEI, aOR, 1.00 (95% CI, 0.99-1.01); MD, aOR, 0.98 (95% CI, 0.94-1.02); and DASH, aOR, 0.99 (95% CI, 0.99-1.00). aORs for AN vs no neoplasia were slightly lower for each dietary pattern assessed, and only the MD and DASH scores were significantly different from 1.00: HEI, aOR, 1.00 (95% CI, 0.99-1.01); MD, aOR, 0.95 (95% CI, 0.90-1.00); and DASH, aOR, 0.99 (95% CI, 0.98-1.00).

We observed lower odds for nonadvanced adenoma and AN among all these dietary patterns when there was greater alignment with the recommended intake of whole grains and fiber. In separate models conducted using food categories comprising the dietary patterns as independent variables and after correcting for multiple tests, higher scores for the HEI Refined Grain category were associated with higher odds for nonadvanced adenoma (aOR, 1.03 [95% CI, 1.01-1.05]; P = .01) and AN (aOR, 1.05 [95% CI, 1.02-1.08]; P < .001). Higher scores for the HEI Whole Grain category were associated with lower odds for nonadvanced adenoma (aOR, 0.97 [95% CI, 0.95-0.99]; P = .01) and AN (aOR, 0.96 [95% CI, 0.93-0.99]; P = .01). Higher scores for the MD Grain category were significantly associated with lower odds for nonadvanced adenoma (aOR, 0.44 [95% CI, 0.26-0.75]; P = .002) and AN (aOR, 0.29 [95% CI, 0.14-0.62]; P = .001). The DASH Grains category also was significantly associated with lower odds for AN (aOR, 0.86 [95% CI, 0.78-0.95]; P = .002).
Discussion
In this study of 3023 veterans undergoing first-time screening colonoscopy and ≥ 10 years of surveillance, we found that healthy dietary patterns, as assessed by the MD and DASH diet, were significantly associated with lower risk of AN. Additionally, we identified lower odds for AN and nonadvanced adenoma compared with no neoplasia for higher grain scores for all the dietary patterns studied. Other food categories that comprise the dietary pattern scores had mixed associations with the MSCF outcomes. Several other studies have examined associations between dietary patterns and risk for CRC but to our knowledge, no studies have explored these associations among US veterans.
These results also indicate study participants had better than average (based on a 50% threshold) dietary quality according to the HEI and DASH diet scoring methods we used, but poor dietary quality according to the MD scoring method. The mean HEI scores for the present study were higher than a US Department of Agriculture study by Dong et al that compared dietary quality between veterans and nonveterans using the HEI, for which veterans’ expected HEI score was 45.6 of 100.8 This could be explained by the fact that the participants needed to be healthy to be eligible and those with healthier behaviors overall may have self-selected into the study due to motivation for screening during a time when screening was not yet commonplace. 36 Similarly, participants of the present study had higher adherence to the DASH diet (63.1%) than adolescents with diabetes in a study by Günther et al. Conversely, firefighters who were coached to use a Mediterranean-style dietary pattern and dietary had higher adherence to MD than did participants in this study.27
A closer examination of specific food category component scores that comprise the 3 distinct dietary patterns revealed mixed results from the multinomial modeling, which may have to do with the guideline thresholds used to calculate the dietary scores. When analyzed separately in the logistic regression models for their associations with nonadvanced adenomas and AN compared with no neoplasia, higher MD and DASH fruit scores (but not HEI fruit scores) were found to be significant. Other studies have had mixed findings when attempting to test for associations of fruit intake with adenoma recurrence.10,37
This study had some unexpected findings. Vegetable intake was not associated with nonadvanced adenomas or AN risk. Studies of food categories have consistently found vegetable (specifically cruciferous ones) intake to be linked with lower odds for cancers.38 Likewise, the red meat category, which was only a unique food category in the MD score, was not associated with nonadvanced adenomas or AN. Despite consistent literature suggesting higher intake of red meat and processed meats increases CRC risk, in 2019 the Nutritional Recommendations Consortium indicated that the evidence was weak.39,40 This study showed higher DASH diet scores for low-fat dairy, which were maximized when participants reported at least 50% of their dairy servings per day as being low-fat, had lower odds for AN. Yet, the MD scores for low-fat dairy had no association with either outcome; their calculation was based on total number of servings per week. This difference in findings suggests the fat intake ratio may be more relevant to CRC risk than intake quantity.
The literature is mixed regarding fatty acid intake and CRC risk, which may be relevant to both dairy and meat intake. One systematic review and meta-analysis found dietary fat and types of fatty acid intake had no association with CRC risk.41 However, a more recent meta-analysis that assessed both dietary intake and plasma levels of fatty acids did find some statistically significant differences for various types of fatty acids and CRC risk.42
The findings in the present study that grain intake is associated with lower odds for more severe colonoscopy findings among veterans are notable.43 Lieberman et al, using the CSP #380 data, found that cereal fiber intake was associated with a lower odds for AN compared with hyperplastic polyps (OR, 0.98 [95% CI, 0.96- 1.00]).18 Similarly, Hullings et al determined that older adults in the highest quintile of cereal fiber intake had significantly lower odds of CRC than those in lower odds for CRC when compared with lowest quintile (OR, 0.89 [95% CI, 0.83- 0.96]; P < .001).44 These findings support existing guidance that prioritizes whole grains as a key source of dietary fiber for CRC prevention.
A recent literature review on fiber, fat, and CRC risk suggested a consensus regarding one protective mechanism: dietary fiber from grains modulates the gut microbiota by promoting butyrate synthesis.45 Butyrate is a short-chain fatty acid that supports energy production in colonocytes and has tumor-suppressing properties.46 Our findings suggest there could be more to learn about the relationship between butyrate production and reduction of CRC risk through metabolomic studies that use measurements of plasma butyrate. These studies may examine associations between not just a singular food or food category, but rather food patterns that include fruits, vegetables, nuts and seeds, and whole grains known to promote butyrate production and plasma butyrate.47
Improved understanding of mechanisms and risk-modifying lifestyle factors such as dietary patterns may enhance prevention strategies. Identifying the collective chemopreventive characteristics of a specific dietary pattern (eg, MD) will be helpful to clinicians and health care staff to promote healthy eating to reduce cancer risk. More studies are needed to understand whether such promotion is more clinically applicable and effective for patients, as compared with eating more or less of specific foods (eg, more whole grains, less red meat). Furthermore, considering important environmental factors collectively beyond dietary patterns may offer a way to better tailor screening and implement a variety of lifestyle interventions. In the literature, this is often referred to as a teachable moment when patients’ attentions are captured and may position them to be more receptive to guidance.48
Limitations
This study has several important limitations and leaves opportunities for future studies that explore the role of dietary patterns and AN or CRC risk. First, the FFQ data used to calculate dietary pattern scores used in analysis were only captured at baseline, and there are nearly 3 decades across the study period. However, it is widely assumed that the diets of older adults, like those included in this study, remain stable over time which is appropriate given our sample population was aged 50 to 75 years when the baseline FFQ data were collected.49-51 Additionally, while the HEI is a well-documented, standard scoring method for dietary quality, there are multitudes of dietary pattern scoring approaches for MD and DASH.23,52,53 Finally, findings from this study using the sample of veterans may not be generalizable to a broader population. Future longitudinal studies that test for a clinically significant change threshold are warranted.
Conclusion
Results of this study suggest future research should further explore the effects of dietary patterns, particularly intake of specific food groups in combination, as opposed to individual nutrients or food items, on AN and CRC risk. Possible studies might explore these dietary patterns for their mechanistic role in altering the microbiome metabolism, which may influence CRC outcomes or include diet in a more comprehensive, holistic risk score that could be used to predict colonic neoplasia risk or in intervention studies that assess the effects of dietary changes on long-term CRC prevention. We suggest there are differences in people’s dietary intake patterns that might be important to consider when implementing tailored approaches to CRC risk mitigation.
Screening for colorectal cancer (CRC) with colonoscopy enables the identification and removal of CRC precursors (colonic adenomas) and has been associated with reduced risk of CRC incidence and mortality.1-3 Furthermore, there is consensus that diet and lifestyle may be associated with forestalling CRC pathogenesis at the intermediate adenoma stages.4-7 However, studies have shown that US veterans have poorer diet quality and a higher risk for neoplasia compared with nonveterans, reinforcing the need for tailored clinical approaches.8,9 Combining screening with conversations about modifiable environmental and lifestyle risk factors, such as poor diet, is a highly relevant and possibly easily leveraged prevention for those at high risk. However, there is limited evidence for any particular dietary patterns or dietary features that are most important over time.7
Several dietary components have been shown to be associated with CRC risk,10 either as potentially chemopreventive (fiber, fruits and vegetables,11 dairy,12 supplemental vitamin D,13 calcium,14 and multivitamins15) or carcinogenic (red meat16 and alcohol17). Previous studies of veterans have similarly shown that higher intake of fiber and vitamin D reduced risk, and red meat is associated with an increased risk for finding CRC precursors during colonoscopy.18 However, these dietary categories are often analyzed in isolation. Studying healthy dietary patterns in aggregate may be more clinically relevant and easier to implement for prevention of CRC and its precursors.19-21 Healthy dietary patterns, such as the US Dietary Guidelines for Americans represented by the Healthy Eating Index (HEI), the Mediterranean diet (MD), and the Dietary Approaches to Stop Hypertension (DASH) diet, have been associated with lower risk for chronic disease.22-24 Despite the extant literature, no known studies have compared these dietary patterns for associations with risk of CRC precursor or CRC development among US veterans undergoing long-term screening and follow-up after a baseline colonoscopy.
The objective of this study was to test for associations between baseline scores of healthy dietary patterns and the most severe colonoscopy findings (MSCFs) over ≥ 10 years following a baseline screening colonoscopy in veterans.
Methods
Participants in the Cooperative Studies Program (CSP) #380 cohort study included 3121 asymptomatic veterans aged 50 to 75 years at baseline who had consented to initial screening colonoscopy between 1994 and 1997, with subsequent follow-up and surveillance.25 Prior to their colonoscopy, all participants completed a baseline study survey that included questions about cancer risk factors including family history of CRC, diet, physical activity, and medication use.
Included in this cross-sectional analysis were data from a sample of veteran participants of the CSP #380 cohort with 1 baseline colonoscopy, follow-up surveillance through 2009, a cancer risk factor survey collected at baseline, and complete demographic and clinical indicator data. Excluded from the analysis were 67 participants with insufficient responses to the dietary food frequency questionnaire (FFQ) and 31 participants with missing body mass index (BMI), 3023 veterans.
Measures
MSCF. The outcome of interest in this study was the MSCF recorded across all participant colonoscopies during the study period. MSCF was categorized as either (1) no neoplasia; (2) < 2 nonadvanced adenomas, including small adenomas (diameter < 10 mm) with tubular histology; or (3) advanced neoplasia (AN), which is characterized by adenomas > 10 mm in diameter, with villous histology, with high-grade dysplasia, or CRC.
Dietary patterns. Dietary pattern scores representing dietary quality and calculated based on recommendations of the US Dietary Guidelines for Americans using the HEI, MD, and DASH diets were independent variables.26-28 These 3 dietary patterns were chosen for their hypothesized relationship with CRC risk, but each weighs food categories differently (Appendix 1).22-24,29 Dietary pattern scores were calculated using the CSP #380 self-reported responses to 129 baseline survey questions adapted from a well-established and previously validated semiquantitative FFQ.30 The form was administered by mail twice to a sample of 127 participants at baseline and at 1 year. During this interval, men completed 1-week diet records twice, spaced about 6 months apart. Mean values for intake of most nutrients assessed by the 2 methods were similar. Intraclass correlation coefficients for the baseline and 1-year FFQ-assessed nutrient intakes that ranged from 0.47 for vitamin E (without supplements) to 0.80 for vitamin C (with supplements). Correlation coefficients between the energy-adjusted nutrient intakes were measured by diet records and the 1-year FFQ, which asked about diet during the year encompassing the diet records. Higher raw and percent scores indicated better alignment with recommendations from each respective dietary pattern. Percent scores were calculated as a standardizing method and used in analyses for ease of comparing the dietary patterns. Scoring can be found in Appendix 2.


Demographic characteristics and clinical indicators. Demographic characteristics included age categories, sex, and race/ethnicity. Clinical indicators included BMI, the number of comorbid conditions used to calculate the Charlson Comorbidity Index, family history of CRC in first-degree relatives, number of follow-up colonoscopies across the study period, and food-based vitamin D intake.31 These variables were chosen for their applicability found in previous CSP #380 cohort studies.18,32,33 Self-reported race and ethnicity were collapsed due to small numbers in some groups. The authors acknowledge these are distinct concepts and the variable has limited utility other than for controlling for systemic racism in the model.
Statistical Analyses
Descriptive statistics were used to describe distributional assumptions for all variables, including demographics, clinical indicators, colonoscopy results, and dietary patterns. Pairwise correlations between the total dietary pattern scores and food category scores were calculated with Pearson correlation (r).
Multinomial logistic regression models were created using SAS procedure LOGISTIC with the outcome of the categorical MSCF (no neoplasia, nonadvanced adenoma, or AN).34 A model was created for each independent predictor variable of interest (ie, the HEI, MD, or DASH percentage-standardized dietary pattern score and each food category comprising each dietary pattern score). All models were adjusted for age, sex, race/ethnicity, BMI, number of comorbidities, family history of CRC, number of follow-up colonoscopies, and estimated daily food-derived vitamin D intake. The demographic and clinical indicators were included in the models as they are known to be associated with CRC risk.18 The number of colonoscopies was included to control for surveillance intensity presuming risk for AN is reduced as polyps are removed. Because colonoscopy findings from an initial screening have unique clinical implications compared with follow- up and surveillance, MSCF was observed in 2 ways in sensitivity analyses: (1) baseline and (2) aggregate follow-up and surveillance only, excluding baseline findings.
Adjusted odds ratios (aORs) and 95% CIs for each of the MSCF outcomes with a reference finding of no neoplasia for the models are presented. We chose not to adjust for multiple comparisons across the different dietary patterns given the correlation between dietary pattern total and category scores but did adjust for multiple comparisons for dietary categories within each dietary pattern. Tests for statistical significance used α= .05 for the dietary pattern total scores and P values for the dietary category scores for each dietary pattern controlled for false discovery rate using the MULTTEST SAS procedure.35 All data manipulations and analyses were performed using SAS version 9.4.
Results
The study included 3023 patients. All were aged 50 to 75 years, 2923 (96.7%) were male and 2532 (83.8%) were non-Hispanic White (Table 1). Most participants were overweight or obese (n = 2535 [83.8%]), 2024 (67.0%) had ≤ 2 comorbidities, and 2602 (86.1%) had no family history of CRC. The MSCF for 1628 patients (53.9%) was no neoplasia, 966 patients (32.0%) was nonadvanced adenoma, and 429 participants (14.2%) had AN.

Mean percent scores were 58.5% for HEI, 38.2% for MD, and 63.1% for the DASH diet, with higher percentages indicating greater alignment with the recommendations for each diet (Table 2). All 3 dietary patterns scores standardized to percentages were strongly and significantly correlated in pairwise comparisons: HEI:MD, r = 0.62 (P < .001); HEI:DASH, r = 0.60 (P < .001); and MD:DASH, r = 0.72 (P < .001). Likewise, food category scores were significantly correlated across dietary patterns. For example, whole grain and fiber values from each dietary score were strongly correlated in pairwise comparisons: HEI Whole Grain:MD Grain, r = 0.64 (P < .001); HEI Whole Grain:DASH Fiber, r = 0.71 (P < .001); and MD Grain:DASH Fiber, r = 0.70 (P < .001).

Associations between individual participants' dietary pattern scores and the outcome of their pooled MSCF from baseline screening and ≥ 10 years of surveillance are presented in Table 3. For each single-point increases in dietary pattern scores (reflecting better dietary quality), aORs for nonadvanced adenoma vs no neoplasia were slightly lower but not statistically significantly: HEI, aOR, 1.00 (95% CI, 0.99-1.01); MD, aOR, 0.98 (95% CI, 0.94-1.02); and DASH, aOR, 0.99 (95% CI, 0.99-1.00). aORs for AN vs no neoplasia were slightly lower for each dietary pattern assessed, and only the MD and DASH scores were significantly different from 1.00: HEI, aOR, 1.00 (95% CI, 0.99-1.01); MD, aOR, 0.95 (95% CI, 0.90-1.00); and DASH, aOR, 0.99 (95% CI, 0.98-1.00).

We observed lower odds for nonadvanced adenoma and AN among all these dietary patterns when there was greater alignment with the recommended intake of whole grains and fiber. In separate models conducted using food categories comprising the dietary patterns as independent variables and after correcting for multiple tests, higher scores for the HEI Refined Grain category were associated with higher odds for nonadvanced adenoma (aOR, 1.03 [95% CI, 1.01-1.05]; P = .01) and AN (aOR, 1.05 [95% CI, 1.02-1.08]; P < .001). Higher scores for the HEI Whole Grain category were associated with lower odds for nonadvanced adenoma (aOR, 0.97 [95% CI, 0.95-0.99]; P = .01) and AN (aOR, 0.96 [95% CI, 0.93-0.99]; P = .01). Higher scores for the MD Grain category were significantly associated with lower odds for nonadvanced adenoma (aOR, 0.44 [95% CI, 0.26-0.75]; P = .002) and AN (aOR, 0.29 [95% CI, 0.14-0.62]; P = .001). The DASH Grains category also was significantly associated with lower odds for AN (aOR, 0.86 [95% CI, 0.78-0.95]; P = .002).
Discussion
In this study of 3023 veterans undergoing first-time screening colonoscopy and ≥ 10 years of surveillance, we found that healthy dietary patterns, as assessed by the MD and DASH diet, were significantly associated with lower risk of AN. Additionally, we identified lower odds for AN and nonadvanced adenoma compared with no neoplasia for higher grain scores for all the dietary patterns studied. Other food categories that comprise the dietary pattern scores had mixed associations with the MSCF outcomes. Several other studies have examined associations between dietary patterns and risk for CRC but to our knowledge, no studies have explored these associations among US veterans.
These results also indicate study participants had better than average (based on a 50% threshold) dietary quality according to the HEI and DASH diet scoring methods we used, but poor dietary quality according to the MD scoring method. The mean HEI scores for the present study were higher than a US Department of Agriculture study by Dong et al that compared dietary quality between veterans and nonveterans using the HEI, for which veterans’ expected HEI score was 45.6 of 100.8 This could be explained by the fact that the participants needed to be healthy to be eligible and those with healthier behaviors overall may have self-selected into the study due to motivation for screening during a time when screening was not yet commonplace. 36 Similarly, participants of the present study had higher adherence to the DASH diet (63.1%) than adolescents with diabetes in a study by Günther et al. Conversely, firefighters who were coached to use a Mediterranean-style dietary pattern and dietary had higher adherence to MD than did participants in this study.27
A closer examination of specific food category component scores that comprise the 3 distinct dietary patterns revealed mixed results from the multinomial modeling, which may have to do with the guideline thresholds used to calculate the dietary scores. When analyzed separately in the logistic regression models for their associations with nonadvanced adenomas and AN compared with no neoplasia, higher MD and DASH fruit scores (but not HEI fruit scores) were found to be significant. Other studies have had mixed findings when attempting to test for associations of fruit intake with adenoma recurrence.10,37
This study had some unexpected findings. Vegetable intake was not associated with nonadvanced adenomas or AN risk. Studies of food categories have consistently found vegetable (specifically cruciferous ones) intake to be linked with lower odds for cancers.38 Likewise, the red meat category, which was only a unique food category in the MD score, was not associated with nonadvanced adenomas or AN. Despite consistent literature suggesting higher intake of red meat and processed meats increases CRC risk, in 2019 the Nutritional Recommendations Consortium indicated that the evidence was weak.39,40 This study showed higher DASH diet scores for low-fat dairy, which were maximized when participants reported at least 50% of their dairy servings per day as being low-fat, had lower odds for AN. Yet, the MD scores for low-fat dairy had no association with either outcome; their calculation was based on total number of servings per week. This difference in findings suggests the fat intake ratio may be more relevant to CRC risk than intake quantity.
The literature is mixed regarding fatty acid intake and CRC risk, which may be relevant to both dairy and meat intake. One systematic review and meta-analysis found dietary fat and types of fatty acid intake had no association with CRC risk.41 However, a more recent meta-analysis that assessed both dietary intake and plasma levels of fatty acids did find some statistically significant differences for various types of fatty acids and CRC risk.42
The findings in the present study that grain intake is associated with lower odds for more severe colonoscopy findings among veterans are notable.43 Lieberman et al, using the CSP #380 data, found that cereal fiber intake was associated with a lower odds for AN compared with hyperplastic polyps (OR, 0.98 [95% CI, 0.96- 1.00]).18 Similarly, Hullings et al determined that older adults in the highest quintile of cereal fiber intake had significantly lower odds of CRC than those in lower odds for CRC when compared with lowest quintile (OR, 0.89 [95% CI, 0.83- 0.96]; P < .001).44 These findings support existing guidance that prioritizes whole grains as a key source of dietary fiber for CRC prevention.
A recent literature review on fiber, fat, and CRC risk suggested a consensus regarding one protective mechanism: dietary fiber from grains modulates the gut microbiota by promoting butyrate synthesis.45 Butyrate is a short-chain fatty acid that supports energy production in colonocytes and has tumor-suppressing properties.46 Our findings suggest there could be more to learn about the relationship between butyrate production and reduction of CRC risk through metabolomic studies that use measurements of plasma butyrate. These studies may examine associations between not just a singular food or food category, but rather food patterns that include fruits, vegetables, nuts and seeds, and whole grains known to promote butyrate production and plasma butyrate.47
Improved understanding of mechanisms and risk-modifying lifestyle factors such as dietary patterns may enhance prevention strategies. Identifying the collective chemopreventive characteristics of a specific dietary pattern (eg, MD) will be helpful to clinicians and health care staff to promote healthy eating to reduce cancer risk. More studies are needed to understand whether such promotion is more clinically applicable and effective for patients, as compared with eating more or less of specific foods (eg, more whole grains, less red meat). Furthermore, considering important environmental factors collectively beyond dietary patterns may offer a way to better tailor screening and implement a variety of lifestyle interventions. In the literature, this is often referred to as a teachable moment when patients’ attentions are captured and may position them to be more receptive to guidance.48
Limitations
This study has several important limitations and leaves opportunities for future studies that explore the role of dietary patterns and AN or CRC risk. First, the FFQ data used to calculate dietary pattern scores used in analysis were only captured at baseline, and there are nearly 3 decades across the study period. However, it is widely assumed that the diets of older adults, like those included in this study, remain stable over time which is appropriate given our sample population was aged 50 to 75 years when the baseline FFQ data were collected.49-51 Additionally, while the HEI is a well-documented, standard scoring method for dietary quality, there are multitudes of dietary pattern scoring approaches for MD and DASH.23,52,53 Finally, findings from this study using the sample of veterans may not be generalizable to a broader population. Future longitudinal studies that test for a clinically significant change threshold are warranted.
Conclusion
Results of this study suggest future research should further explore the effects of dietary patterns, particularly intake of specific food groups in combination, as opposed to individual nutrients or food items, on AN and CRC risk. Possible studies might explore these dietary patterns for their mechanistic role in altering the microbiome metabolism, which may influence CRC outcomes or include diet in a more comprehensive, holistic risk score that could be used to predict colonic neoplasia risk or in intervention studies that assess the effects of dietary changes on long-term CRC prevention. We suggest there are differences in people’s dietary intake patterns that might be important to consider when implementing tailored approaches to CRC risk mitigation.
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectalcancer deaths. N Engl J Med. 2012;366(8):687-696. doi:10.1056/NEJMoa1100370
- Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-1105. doi:10.1056/NEJMoa1301969
- Bretthauer M, Løberg M, Wieszczy P, et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. 2022;387(17):1547-1556. doi:10.1056/NEJMoa2208375
- Cottet V, Bonithon-Kopp C, Kronborg O, et al. Dietary patterns and the risk of colorectal adenoma recurrence in a European intervention trial. Eur J Cancer Prev. 2005;14(1):21.
- Miller PE, Lesko SM, Muscat JE, Lazarus P, Hartman TJ. Dietary patterns and colorectal adenoma and cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2010;62(4):413-424. doi:10.1080/01635580903407114
- Godos J, Bella F, Torrisi A, Sciacca S, Galvano F, Grosso G. Dietary patterns and risk of colorectal adenoma: a systematic review and meta-analysis of observational studies. J Hum Nutr Diet Off J Br Diet Assoc. 2016;29(6):757-767. doi:10.1111/jhn.12395
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191-197. doi:10.1055/s-0029-1242458
- Dong D, Stewart H, Carlson AC. An Examination of Veterans’ Diet Quality. U.S. Department of Agriculture, Economic Research Service; 2019:32.
- El-Halabi MM, Rex DK, Saito A, Eckert GJ, Kahi CJ. Defining adenoma detection rate benchmarks in average-risk male veterans. Gastrointest Endosc. 2019;89(1):137-143. doi:10.1016/j.gie.2018.08.021
- Alberts DS, Hess LM, eds. Fundamentals of Cancer Prevention. Springer International Publishing; 2019. doi:10.1007/978-3-030-15935-1
- Dahm CC, Keogh RH, Spencer EA, et al. Dietary fiber and colorectal cancer risk: a nested case-control study using food diaries. J Natl Cancer Inst. 2010;102(9):614-626. doi:10.1093/jnci/djq092
- Aune D, Lau R, Chan DSM, et al. Dairy products and colorectal cancer risk: a systematic review and metaanalysis of cohort studies. Ann Oncol. 2012;23(1):37-45. doi:10.1093/annonc/mdr269
- Lee JE, Li H, Chan AT, et al. Circulating levels of vitamin D and colon and rectal cancer: the Physicians’ Health Study and a meta-analysis of prospective studies. Cancer Prev Res Phila Pa. 2011;4(5):735-743. doi:10.1158/1940-6207.CAPR-10-0289
- Carroll C, Cooper K, Papaioannou D, Hind D, Pilgrim H, Tappenden P. Supplemental calcium in the chemoprevention of colorectal cancer: a systematic review and meta-analysis. Clin Ther. 2010;32(5):789-803. doi:10.1016/j.clinthera.2010.04.024
- Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control CCC. 2010;21(11):1745- 1757. doi:10.1007/s10552-010-9549-y
- Alexander DD, Weed DL, Miller PE, Mohamed MA. Red meat and colorectal cancer: a quantitative update on the state of the epidemiologic science. J Am Coll Nutr. 2015;34(6):521-543. doi:10.1080/07315724.2014.992553
- Park SY, Wilkens LR, Setiawan VW, Monroe KR, Haiman CA, Le Marchand L. Alcohol intake and colorectal cancer risk in the multiethnic cohort study. Am J Epidemiol. 2019;188(1):67-76. doi:10.1093/aje/kwy208
- Lieberman DA. Risk Factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290(22):2959. doi:10.1001/jama.290.22.2959
- Archambault AN, Jeon J, Lin Y, et al. Risk stratification for early-onset colorectal cancer using a combination of genetic and environmental risk scores: an international multi-center study. J Natl Cancer Inst. 2022;114(4):528-539. doi:10.1093/jnci/djac003
- Carr PR, Weigl K, Edelmann D, et al. Estimation of absolute risk of colorectal cancer based on healthy lifestyle, genetic risk, and colonoscopy status in a populationbased study. Gastroenterology. 2020;159(1):129-138.e9. doi:10.1053/j.gastro.2020.03.016
- Sullivan BA, Qin X, Miller C, et al. Screening colonoscopy findings are associated with noncolorectal cancer mortality. Clin Transl Gastroenterol. 2022;13(4):e00479. doi:10.14309/ctg.0000000000000479
- Erben V, Carr PR, Holleczek B, Stegmaier C, Hoffmeister M, Brenner H. Dietary patterns and risk of advanced colorectal neoplasms: A large population based screening study in Germany. Prev Med. 2018;111:101-109. doi:10.1016/j.ypmed.2018.02.025
- Donovan MG, Selmin OI, Doetschman TC, Romagnolo DF. Mediterranean diet: prevention of colorectal cancer. Front Nutr. 2017;4:59. doi:10.3389/fnut.2017.00059
- Mohseni R, Mohseni F, Alizadeh S, Abbasi S. The Association of Dietary Approaches to Stop Hypertension (DASH) diet with the risk of colorectal cancer: a meta-analysis of observational studies.Nutr Cancer. 2020;72(5):778-790. doi:10.1080/01635581.2019.1651880
- Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
- Developing the Healthy Eating Index (HEI) | EGRP/ DCCPS/NCI/NIH. Accessed July 22, 2025. https://epi.grants.cancer.gov/hei/developing.html#2015c
- Reeve E, Piccici F, Feairheller DL. Validation of a Mediterranean diet scoring system for intervention based research. J Nutr Med Diet Care. 2021;7(1):053. doi:10.23937/2572-3278/1510053
- Günther AL, Liese AD, Bell RA, et al. ASSOCIATION BETWEEN THE DIETARY APPROACHES TO HYPERTENSION (DASH) DIET AND HYPERTENSION IN YOUTH WITH DIABETES. Hypertens Dallas Tex 1979. 2009;53(1):6-12. doi:10.1161/HYPERTENSIONAHA.108.116665
- Buckland G, Agudo A, Luján L, et al. Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Am J Clin Nutr. 2010;91(2):381- 390. doi:10.3945/ajcn.2009.28209
- Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114-1126. doi:10.1093/oxfordjournals.aje.a116211
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
- Lieberman DA, Weiss DG, Harford WV, et al. Fiveyear colon surveillance after screening colonoscopy. Gastroenterology. 2007;133(4):1077-1085. doi:10.1053/j.gastro.2007.07.006
- Lieberman D, Sullivan BA, Hauser ER, et al. Baseline colonoscopy findings associated with 10-year outcomes in a screening cohort undergoing colonoscopy surveillance. Gastroenterology. 2020;158(4):862-874.e8. doi:10.1053/j.gastro.2019.07.052
- PROC LOGISTIC: PROC LOGISTIC Statement : SAS/STAT(R) 9.22 User’s Guide. Accessed July 22, 2025. https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_logistic_sect004.htm
- PROC MULTTEST: PROC MULTTEST Statement : SAS/ STAT(R) 9.22 User’s Guide. Accessed July 22, 2025. https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_multtest_sect005.htm
- Elston DM. Participation bias, self-selection bias, and response bias. J Am Acad Dermatol. Published online June 18, 2021. doi:10.1016/j.jaad.2021.06.025
- Sansbury LB, Wanke K, Albert PS, et al. The effect of strict adherence to a high-fiber, high-fruit and -vegetable, and low-fat eating pattern on adenoma recurrence. Am J Epidemiol. 2009;170(5):576-584. doi:10.1093/aje/kwp169
- Borgas P, Gonzalez G, Veselkov K, Mirnezami R. Phytochemically rich dietary components and the risk of colorectal cancer: A systematic review and meta-analysis of observational studies. World J Clin Oncol. 2021;12(6):482- 499. doi:10.5306/wjco.v12.i6.482
- Papadimitriou N, Markozannes G, Kanellopoulou A, et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat Commun. 2021;12(1):4579. doi:10.1038/s41467-021-24861-8
- Johnston BC, Zeraatkar D, Han MA, et al. Unprocessed red meat and processed meat consumption: dietary guideline recommendations from the nutritional recommendations (NutriRECS) Consortium. Ann Intern Med. 2019;171(10):756-764. doi:10.7326/M19-1621
- Kim M, Park K. Dietary fat intake and risk of colorectal cancer: a systematic review and meta-analysis of prospective studies. Nutrients. 2018;10(12):1963. doi:10.3390/nu10121963
- Lu Y, Li D, Wang L, et al. Comprehensive investigation on associations between dietary intake and blood levels of fatty acids and colorectal cancer risk. Nutrients. 2023;15(3):730. doi:10.3390/nu15030730
- Gherasim A, Arhire LI, Ni.a O, Popa AD, Graur M, Mihalache L. The relationship between lifestyle components and dietary patterns. Proc Nutr Soc. 2020;79(3):311-323. doi:10.1017/S0029665120006898
- Hullings AG, Sinha R, Liao LM, Freedman ND, Graubard BI, Loftfield E. Whole grain and dietary fiber intake and risk of colorectal cancer in the NIH-AARP Diet and Health Study cohort. Am J Clin Nutr. 2020;112(3):603- 612. doi:10.1093/ajcn/nqaa161
- Ocvirk S, Wilson AS, Appolonia CN, Thomas TK, O’Keefe SJD. Fiber, fat, and colorectal cancer: new insight into modifiable dietary risk factors. Curr Gastroenterol Rep. 2019;21(11):62. doi:10.1007/s11894-019-0725-2
- O’Keefe SJD. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13(12):691-706. doi:10.1038/nrgastro.2016.165
- The health benefits and side effects of Butyrate Cleveland Clinic. July 11, 2022. Accessed July 22, 2025. https://health.clevelandclinic.org/butyrate-benefits/
- Knudsen MD, Wang L, Wang K, et al. Changes in lifestyle factors after endoscopic screening: a prospective study in the United States. Clin Gastroenterol Hepatol Off ClinPract J Am Gastroenterol Assoc. 2022;20(6):e1240-e1249. doi:10.1016/j.cgh.2021.07.014
- Thorpe MG, Milte CM, Crawford D, McNaughton SA. Education and lifestyle predict change in dietary patterns and diet quality of adults 55 years and over. Nutr J. 2019;18(1):67. doi:10.1186/s12937-019-0495-6
- Chapman K, Ogden J. How do people change their diet?: an exploration into mechanisms of dietary change. J Health Psychol. 2009;14(8):1229-1242. doi:10.1177/1359105309342289
- Djoussé L, Petrone AB, Weir NL, et al. Repeated versus single measurement of plasma omega-3 fatty acids and risk of heart failure. Eur J Nutr. 2014;53(6):1403-1408. doi:10.1007/s00394-013-0642-3
- Bach-Faig A, Berry EM, Lairon D, et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14(12A):2274-2284. doi:10.1017/S1368980011002515
- Miller PE, Cross AJ, Subar AF, et al. Comparison of 4 established DASH diet indexes: examining associations of index scores and colorectal cancer123. Am J Clin Nutr. 2013;98(3):794-803. doi:10.3945/ajcn.113.063602
- Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591-1602. doi:10.1016/j.jand.2018.05.021
- P.R. Pehrsson, Cutrufelli RL, Gebhardt SE, et al. USDA Database for the Added Sugars Content of Selected Foods. USDA; 2005. www.ars.usda.gov/nutrientdata
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectalcancer deaths. N Engl J Med. 2012;366(8):687-696. doi:10.1056/NEJMoa1100370
- Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-1105. doi:10.1056/NEJMoa1301969
- Bretthauer M, Løberg M, Wieszczy P, et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. 2022;387(17):1547-1556. doi:10.1056/NEJMoa2208375
- Cottet V, Bonithon-Kopp C, Kronborg O, et al. Dietary patterns and the risk of colorectal adenoma recurrence in a European intervention trial. Eur J Cancer Prev. 2005;14(1):21.
- Miller PE, Lesko SM, Muscat JE, Lazarus P, Hartman TJ. Dietary patterns and colorectal adenoma and cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2010;62(4):413-424. doi:10.1080/01635580903407114
- Godos J, Bella F, Torrisi A, Sciacca S, Galvano F, Grosso G. Dietary patterns and risk of colorectal adenoma: a systematic review and meta-analysis of observational studies. J Hum Nutr Diet Off J Br Diet Assoc. 2016;29(6):757-767. doi:10.1111/jhn.12395
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191-197. doi:10.1055/s-0029-1242458
- Dong D, Stewart H, Carlson AC. An Examination of Veterans’ Diet Quality. U.S. Department of Agriculture, Economic Research Service; 2019:32.
- El-Halabi MM, Rex DK, Saito A, Eckert GJ, Kahi CJ. Defining adenoma detection rate benchmarks in average-risk male veterans. Gastrointest Endosc. 2019;89(1):137-143. doi:10.1016/j.gie.2018.08.021
- Alberts DS, Hess LM, eds. Fundamentals of Cancer Prevention. Springer International Publishing; 2019. doi:10.1007/978-3-030-15935-1
- Dahm CC, Keogh RH, Spencer EA, et al. Dietary fiber and colorectal cancer risk: a nested case-control study using food diaries. J Natl Cancer Inst. 2010;102(9):614-626. doi:10.1093/jnci/djq092
- Aune D, Lau R, Chan DSM, et al. Dairy products and colorectal cancer risk: a systematic review and metaanalysis of cohort studies. Ann Oncol. 2012;23(1):37-45. doi:10.1093/annonc/mdr269
- Lee JE, Li H, Chan AT, et al. Circulating levels of vitamin D and colon and rectal cancer: the Physicians’ Health Study and a meta-analysis of prospective studies. Cancer Prev Res Phila Pa. 2011;4(5):735-743. doi:10.1158/1940-6207.CAPR-10-0289
- Carroll C, Cooper K, Papaioannou D, Hind D, Pilgrim H, Tappenden P. Supplemental calcium in the chemoprevention of colorectal cancer: a systematic review and meta-analysis. Clin Ther. 2010;32(5):789-803. doi:10.1016/j.clinthera.2010.04.024
- Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control CCC. 2010;21(11):1745- 1757. doi:10.1007/s10552-010-9549-y
- Alexander DD, Weed DL, Miller PE, Mohamed MA. Red meat and colorectal cancer: a quantitative update on the state of the epidemiologic science. J Am Coll Nutr. 2015;34(6):521-543. doi:10.1080/07315724.2014.992553
- Park SY, Wilkens LR, Setiawan VW, Monroe KR, Haiman CA, Le Marchand L. Alcohol intake and colorectal cancer risk in the multiethnic cohort study. Am J Epidemiol. 2019;188(1):67-76. doi:10.1093/aje/kwy208
- Lieberman DA. Risk Factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290(22):2959. doi:10.1001/jama.290.22.2959
- Archambault AN, Jeon J, Lin Y, et al. Risk stratification for early-onset colorectal cancer using a combination of genetic and environmental risk scores: an international multi-center study. J Natl Cancer Inst. 2022;114(4):528-539. doi:10.1093/jnci/djac003
- Carr PR, Weigl K, Edelmann D, et al. Estimation of absolute risk of colorectal cancer based on healthy lifestyle, genetic risk, and colonoscopy status in a populationbased study. Gastroenterology. 2020;159(1):129-138.e9. doi:10.1053/j.gastro.2020.03.016
- Sullivan BA, Qin X, Miller C, et al. Screening colonoscopy findings are associated with noncolorectal cancer mortality. Clin Transl Gastroenterol. 2022;13(4):e00479. doi:10.14309/ctg.0000000000000479
- Erben V, Carr PR, Holleczek B, Stegmaier C, Hoffmeister M, Brenner H. Dietary patterns and risk of advanced colorectal neoplasms: A large population based screening study in Germany. Prev Med. 2018;111:101-109. doi:10.1016/j.ypmed.2018.02.025
- Donovan MG, Selmin OI, Doetschman TC, Romagnolo DF. Mediterranean diet: prevention of colorectal cancer. Front Nutr. 2017;4:59. doi:10.3389/fnut.2017.00059
- Mohseni R, Mohseni F, Alizadeh S, Abbasi S. The Association of Dietary Approaches to Stop Hypertension (DASH) diet with the risk of colorectal cancer: a meta-analysis of observational studies.Nutr Cancer. 2020;72(5):778-790. doi:10.1080/01635581.2019.1651880
- Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
- Developing the Healthy Eating Index (HEI) | EGRP/ DCCPS/NCI/NIH. Accessed July 22, 2025. https://epi.grants.cancer.gov/hei/developing.html#2015c
- Reeve E, Piccici F, Feairheller DL. Validation of a Mediterranean diet scoring system for intervention based research. J Nutr Med Diet Care. 2021;7(1):053. doi:10.23937/2572-3278/1510053
- Günther AL, Liese AD, Bell RA, et al. ASSOCIATION BETWEEN THE DIETARY APPROACHES TO HYPERTENSION (DASH) DIET AND HYPERTENSION IN YOUTH WITH DIABETES. Hypertens Dallas Tex 1979. 2009;53(1):6-12. doi:10.1161/HYPERTENSIONAHA.108.116665
- Buckland G, Agudo A, Luján L, et al. Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Am J Clin Nutr. 2010;91(2):381- 390. doi:10.3945/ajcn.2009.28209
- Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114-1126. doi:10.1093/oxfordjournals.aje.a116211
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
- Lieberman DA, Weiss DG, Harford WV, et al. Fiveyear colon surveillance after screening colonoscopy. Gastroenterology. 2007;133(4):1077-1085. doi:10.1053/j.gastro.2007.07.006
- Lieberman D, Sullivan BA, Hauser ER, et al. Baseline colonoscopy findings associated with 10-year outcomes in a screening cohort undergoing colonoscopy surveillance. Gastroenterology. 2020;158(4):862-874.e8. doi:10.1053/j.gastro.2019.07.052
- PROC LOGISTIC: PROC LOGISTIC Statement : SAS/STAT(R) 9.22 User’s Guide. Accessed July 22, 2025. https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_logistic_sect004.htm
- PROC MULTTEST: PROC MULTTEST Statement : SAS/ STAT(R) 9.22 User’s Guide. Accessed July 22, 2025. https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_multtest_sect005.htm
- Elston DM. Participation bias, self-selection bias, and response bias. J Am Acad Dermatol. Published online June 18, 2021. doi:10.1016/j.jaad.2021.06.025
- Sansbury LB, Wanke K, Albert PS, et al. The effect of strict adherence to a high-fiber, high-fruit and -vegetable, and low-fat eating pattern on adenoma recurrence. Am J Epidemiol. 2009;170(5):576-584. doi:10.1093/aje/kwp169
- Borgas P, Gonzalez G, Veselkov K, Mirnezami R. Phytochemically rich dietary components and the risk of colorectal cancer: A systematic review and meta-analysis of observational studies. World J Clin Oncol. 2021;12(6):482- 499. doi:10.5306/wjco.v12.i6.482
- Papadimitriou N, Markozannes G, Kanellopoulou A, et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat Commun. 2021;12(1):4579. doi:10.1038/s41467-021-24861-8
- Johnston BC, Zeraatkar D, Han MA, et al. Unprocessed red meat and processed meat consumption: dietary guideline recommendations from the nutritional recommendations (NutriRECS) Consortium. Ann Intern Med. 2019;171(10):756-764. doi:10.7326/M19-1621
- Kim M, Park K. Dietary fat intake and risk of colorectal cancer: a systematic review and meta-analysis of prospective studies. Nutrients. 2018;10(12):1963. doi:10.3390/nu10121963
- Lu Y, Li D, Wang L, et al. Comprehensive investigation on associations between dietary intake and blood levels of fatty acids and colorectal cancer risk. Nutrients. 2023;15(3):730. doi:10.3390/nu15030730
- Gherasim A, Arhire LI, Ni.a O, Popa AD, Graur M, Mihalache L. The relationship between lifestyle components and dietary patterns. Proc Nutr Soc. 2020;79(3):311-323. doi:10.1017/S0029665120006898
- Hullings AG, Sinha R, Liao LM, Freedman ND, Graubard BI, Loftfield E. Whole grain and dietary fiber intake and risk of colorectal cancer in the NIH-AARP Diet and Health Study cohort. Am J Clin Nutr. 2020;112(3):603- 612. doi:10.1093/ajcn/nqaa161
- Ocvirk S, Wilson AS, Appolonia CN, Thomas TK, O’Keefe SJD. Fiber, fat, and colorectal cancer: new insight into modifiable dietary risk factors. Curr Gastroenterol Rep. 2019;21(11):62. doi:10.1007/s11894-019-0725-2
- O’Keefe SJD. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13(12):691-706. doi:10.1038/nrgastro.2016.165
- The health benefits and side effects of Butyrate Cleveland Clinic. July 11, 2022. Accessed July 22, 2025. https://health.clevelandclinic.org/butyrate-benefits/
- Knudsen MD, Wang L, Wang K, et al. Changes in lifestyle factors after endoscopic screening: a prospective study in the United States. Clin Gastroenterol Hepatol Off ClinPract J Am Gastroenterol Assoc. 2022;20(6):e1240-e1249. doi:10.1016/j.cgh.2021.07.014
- Thorpe MG, Milte CM, Crawford D, McNaughton SA. Education and lifestyle predict change in dietary patterns and diet quality of adults 55 years and over. Nutr J. 2019;18(1):67. doi:10.1186/s12937-019-0495-6
- Chapman K, Ogden J. How do people change their diet?: an exploration into mechanisms of dietary change. J Health Psychol. 2009;14(8):1229-1242. doi:10.1177/1359105309342289
- Djoussé L, Petrone AB, Weir NL, et al. Repeated versus single measurement of plasma omega-3 fatty acids and risk of heart failure. Eur J Nutr. 2014;53(6):1403-1408. doi:10.1007/s00394-013-0642-3
- Bach-Faig A, Berry EM, Lairon D, et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14(12A):2274-2284. doi:10.1017/S1368980011002515
- Miller PE, Cross AJ, Subar AF, et al. Comparison of 4 established DASH diet indexes: examining associations of index scores and colorectal cancer123. Am J Clin Nutr. 2013;98(3):794-803. doi:10.3945/ajcn.113.063602
- Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591-1602. doi:10.1016/j.jand.2018.05.021
- P.R. Pehrsson, Cutrufelli RL, Gebhardt SE, et al. USDA Database for the Added Sugars Content of Selected Foods. USDA; 2005. www.ars.usda.gov/nutrientdata
Associations Between Prescreening Dietary Patterns and Longitudinal Colonoscopy Outcomes in Veterans
Associations Between Prescreening Dietary Patterns and Longitudinal Colonoscopy Outcomes in Veterans