User login
Few antidepressant adverse effects backed by convincing evidence
Relatively few of the adverse health outcomes attributed to antidepressants are supported by convincing evidence, reported the authors of a systematic review of 45 meta-analyses.
The authors did find convincing evidence linking the use of antidepressants and suicide attempt or completion among people under age 19 years and use of the medication and autism risk among offspring. “However, the few [studies] with convincing evidence associations did not reflect causality, and none of them remained at the convincing evidence level after accounting for confounding by indication,” wrote Elena Dragioti, PhD, of the Pain and Rehabilitation Centre at Linköping (Sweden) University and coauthors. The study was published in JAMA Psychiatry.
Dr. Dragioti and coauthors undertook a systematic “umbrella review” grading the evidence from the 45 meta-analyses of 695 observational studies into the association between antidepressant use and the risk of adverse health outcomes. All the meta-analyses included a control group not exposed to antidepressants, with the exception of one that compared the risk of gastrointestinal bleeding between two classes of antidepressants.
They found 120 possible adverse health associations described in the meta-analyses, 61.7% of which related to maternal and pregnancy-related adverse health outcomes. Two-thirds of the adverse health outcome associations involved selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs).
However, among the 120 adverse health associations, only three (2.5%) were supported by “convincing” evidence. One was the association between SSRIs and increased risk of suicide attempts and completion in children and adolescents. Convincing evidence also was found between any antidepressant use before pregnancy and autism spectrum disorder and between SSRI use during pregnancy and autism spectrum disorder. The evidence for the association with suicide risk was deemed high quality, but the two associations with autism spectrum disorder were only of moderate quality.
The authors commented that these findings needed to be considered when prescribing antidepressants in adolescents and children, particularly as another networked meta-analysis had found fluoxetine was the only antidepressant that worked better than placebo in children and adolescents. “In addition, they wrote.
The review found that 11 adverse health outcomes (9.2%) had “highly suggestive” evidence linking them to antidepressant use. These were ADHD in children, cataract development, severe bleeding at any site, upper gastrointestinal tract bleeding, postpartum hemorrhage, preterm birth, lower Apgar score at 5 minutes, osteoporotic fracture, and hip fracture.
Seven of those – ADHD in children, lower Apgar score, severe bleeding at any site, cataract development, osteoporotic features, preterm birth, and upper GI bleeding – had moderate-quality evidence. However, the authors noted that the effect sizes were small and had low prevalence.
The study also found highly suggestive evidence linking antidepressant use to a decreased risk of suicide attempts or completion in adults.
The authors said several of those adverse events in adults, such as GI bleeding and osteoporotic fractures, could be prevented with medication, so the advantages of antidepressant use in adults could outweigh the disadvantage of those preventable safety issues.
Twenty-one adverse health outcomes showed either suggestive, weak, or no evidence for their association with antidepressant use.
They also conducted a sensitivity analysis that limited the analysis to cohort studies, prospective cohort studies, studies that controlled for confounding by the treatment indication, and studies from North America. This showed that none of the associations for which there was originally deemed to be convincing evidence retained that same rank.
“Overall, the results showed that the association between antidepressant use and adverse health outcomes was not supported by robust evidence and that the underlying disease likely inflated the findings in a relevant way,” the authors wrote.
However, when they looked solely at prospective cohort studies, the association between preterm birth and use of any antidepressant was upgraded to having convincing evidence.
When the analysis focused on SSRIs only, the association with lower Apgar scores at 5 minutes also was upgraded to having convincing evidence. Similarly, the evidence for an association with preterm birth also was found to be convincing when the analysis was limited to other or mixed antidepressants.
Dr. Dragioti and coauthors cited several limitations, including the inability of some randomized, controlled trials to address adverse outcomes.
“Antidepressant use appears to be safe for the treatment of psychiatric disorders, but more studies matching for underlying disease are needed to clarify the degree of confounding by indication and other biases,” the authors wrote.
The study was funded by several entities, including the National Institute for Health Research’s Biomedical Research Centre at South London and Maudsley NHS Foundation Trust. Dr. Dragioti reported no disclosures. Four authors declared funding, consultancies, personal fees, royalties, or shares in the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Dragioti E et al. JAMA Psychiatry. 2019 Oct 2. doi: 10.1001/jamapsychiatry.2019.2859.
Relatively few of the adverse health outcomes attributed to antidepressants are supported by convincing evidence, reported the authors of a systematic review of 45 meta-analyses.
The authors did find convincing evidence linking the use of antidepressants and suicide attempt or completion among people under age 19 years and use of the medication and autism risk among offspring. “However, the few [studies] with convincing evidence associations did not reflect causality, and none of them remained at the convincing evidence level after accounting for confounding by indication,” wrote Elena Dragioti, PhD, of the Pain and Rehabilitation Centre at Linköping (Sweden) University and coauthors. The study was published in JAMA Psychiatry.
Dr. Dragioti and coauthors undertook a systematic “umbrella review” grading the evidence from the 45 meta-analyses of 695 observational studies into the association between antidepressant use and the risk of adverse health outcomes. All the meta-analyses included a control group not exposed to antidepressants, with the exception of one that compared the risk of gastrointestinal bleeding between two classes of antidepressants.
They found 120 possible adverse health associations described in the meta-analyses, 61.7% of which related to maternal and pregnancy-related adverse health outcomes. Two-thirds of the adverse health outcome associations involved selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs).
However, among the 120 adverse health associations, only three (2.5%) were supported by “convincing” evidence. One was the association between SSRIs and increased risk of suicide attempts and completion in children and adolescents. Convincing evidence also was found between any antidepressant use before pregnancy and autism spectrum disorder and between SSRI use during pregnancy and autism spectrum disorder. The evidence for the association with suicide risk was deemed high quality, but the two associations with autism spectrum disorder were only of moderate quality.
The authors commented that these findings needed to be considered when prescribing antidepressants in adolescents and children, particularly as another networked meta-analysis had found fluoxetine was the only antidepressant that worked better than placebo in children and adolescents. “In addition, they wrote.
The review found that 11 adverse health outcomes (9.2%) had “highly suggestive” evidence linking them to antidepressant use. These were ADHD in children, cataract development, severe bleeding at any site, upper gastrointestinal tract bleeding, postpartum hemorrhage, preterm birth, lower Apgar score at 5 minutes, osteoporotic fracture, and hip fracture.
Seven of those – ADHD in children, lower Apgar score, severe bleeding at any site, cataract development, osteoporotic features, preterm birth, and upper GI bleeding – had moderate-quality evidence. However, the authors noted that the effect sizes were small and had low prevalence.
The study also found highly suggestive evidence linking antidepressant use to a decreased risk of suicide attempts or completion in adults.
The authors said several of those adverse events in adults, such as GI bleeding and osteoporotic fractures, could be prevented with medication, so the advantages of antidepressant use in adults could outweigh the disadvantage of those preventable safety issues.
Twenty-one adverse health outcomes showed either suggestive, weak, or no evidence for their association with antidepressant use.
They also conducted a sensitivity analysis that limited the analysis to cohort studies, prospective cohort studies, studies that controlled for confounding by the treatment indication, and studies from North America. This showed that none of the associations for which there was originally deemed to be convincing evidence retained that same rank.
“Overall, the results showed that the association between antidepressant use and adverse health outcomes was not supported by robust evidence and that the underlying disease likely inflated the findings in a relevant way,” the authors wrote.
However, when they looked solely at prospective cohort studies, the association between preterm birth and use of any antidepressant was upgraded to having convincing evidence.
When the analysis focused on SSRIs only, the association with lower Apgar scores at 5 minutes also was upgraded to having convincing evidence. Similarly, the evidence for an association with preterm birth also was found to be convincing when the analysis was limited to other or mixed antidepressants.
Dr. Dragioti and coauthors cited several limitations, including the inability of some randomized, controlled trials to address adverse outcomes.
“Antidepressant use appears to be safe for the treatment of psychiatric disorders, but more studies matching for underlying disease are needed to clarify the degree of confounding by indication and other biases,” the authors wrote.
The study was funded by several entities, including the National Institute for Health Research’s Biomedical Research Centre at South London and Maudsley NHS Foundation Trust. Dr. Dragioti reported no disclosures. Four authors declared funding, consultancies, personal fees, royalties, or shares in the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Dragioti E et al. JAMA Psychiatry. 2019 Oct 2. doi: 10.1001/jamapsychiatry.2019.2859.
Relatively few of the adverse health outcomes attributed to antidepressants are supported by convincing evidence, reported the authors of a systematic review of 45 meta-analyses.
The authors did find convincing evidence linking the use of antidepressants and suicide attempt or completion among people under age 19 years and use of the medication and autism risk among offspring. “However, the few [studies] with convincing evidence associations did not reflect causality, and none of them remained at the convincing evidence level after accounting for confounding by indication,” wrote Elena Dragioti, PhD, of the Pain and Rehabilitation Centre at Linköping (Sweden) University and coauthors. The study was published in JAMA Psychiatry.
Dr. Dragioti and coauthors undertook a systematic “umbrella review” grading the evidence from the 45 meta-analyses of 695 observational studies into the association between antidepressant use and the risk of adverse health outcomes. All the meta-analyses included a control group not exposed to antidepressants, with the exception of one that compared the risk of gastrointestinal bleeding between two classes of antidepressants.
They found 120 possible adverse health associations described in the meta-analyses, 61.7% of which related to maternal and pregnancy-related adverse health outcomes. Two-thirds of the adverse health outcome associations involved selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs).
However, among the 120 adverse health associations, only three (2.5%) were supported by “convincing” evidence. One was the association between SSRIs and increased risk of suicide attempts and completion in children and adolescents. Convincing evidence also was found between any antidepressant use before pregnancy and autism spectrum disorder and between SSRI use during pregnancy and autism spectrum disorder. The evidence for the association with suicide risk was deemed high quality, but the two associations with autism spectrum disorder were only of moderate quality.
The authors commented that these findings needed to be considered when prescribing antidepressants in adolescents and children, particularly as another networked meta-analysis had found fluoxetine was the only antidepressant that worked better than placebo in children and adolescents. “In addition, they wrote.
The review found that 11 adverse health outcomes (9.2%) had “highly suggestive” evidence linking them to antidepressant use. These were ADHD in children, cataract development, severe bleeding at any site, upper gastrointestinal tract bleeding, postpartum hemorrhage, preterm birth, lower Apgar score at 5 minutes, osteoporotic fracture, and hip fracture.
Seven of those – ADHD in children, lower Apgar score, severe bleeding at any site, cataract development, osteoporotic features, preterm birth, and upper GI bleeding – had moderate-quality evidence. However, the authors noted that the effect sizes were small and had low prevalence.
The study also found highly suggestive evidence linking antidepressant use to a decreased risk of suicide attempts or completion in adults.
The authors said several of those adverse events in adults, such as GI bleeding and osteoporotic fractures, could be prevented with medication, so the advantages of antidepressant use in adults could outweigh the disadvantage of those preventable safety issues.
Twenty-one adverse health outcomes showed either suggestive, weak, or no evidence for their association with antidepressant use.
They also conducted a sensitivity analysis that limited the analysis to cohort studies, prospective cohort studies, studies that controlled for confounding by the treatment indication, and studies from North America. This showed that none of the associations for which there was originally deemed to be convincing evidence retained that same rank.
“Overall, the results showed that the association between antidepressant use and adverse health outcomes was not supported by robust evidence and that the underlying disease likely inflated the findings in a relevant way,” the authors wrote.
However, when they looked solely at prospective cohort studies, the association between preterm birth and use of any antidepressant was upgraded to having convincing evidence.
When the analysis focused on SSRIs only, the association with lower Apgar scores at 5 minutes also was upgraded to having convincing evidence. Similarly, the evidence for an association with preterm birth also was found to be convincing when the analysis was limited to other or mixed antidepressants.
Dr. Dragioti and coauthors cited several limitations, including the inability of some randomized, controlled trials to address adverse outcomes.
“Antidepressant use appears to be safe for the treatment of psychiatric disorders, but more studies matching for underlying disease are needed to clarify the degree of confounding by indication and other biases,” the authors wrote.
The study was funded by several entities, including the National Institute for Health Research’s Biomedical Research Centre at South London and Maudsley NHS Foundation Trust. Dr. Dragioti reported no disclosures. Four authors declared funding, consultancies, personal fees, royalties, or shares in the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Dragioti E et al. JAMA Psychiatry. 2019 Oct 2. doi: 10.1001/jamapsychiatry.2019.2859.
FROM JAMA PSYCHIATRY
Key clinical point: “More studies [of antidepressants] matching for underlying disease are needed to clarify the degree of confounding by indication and other biases.”
Major finding: Increased suicide risk in children and adolescents is one of the few adverse health outcomes of antidepressants that is backed by evidence.
Study details: Systematic umbrella review of 45 meta-analyses of 695 observational studies.
Disclosures: The study was funded by several entities, including the National Institute for Health Research’s Biomedical Research Centre at South London and Maudsley NHS Foundation Trust. Dr. Dragioti reported no disclosures. Four authors declared funding, consultancies, personal fees, royalties, or shares in the pharmaceutical sector. No other conflicts of interest were declared.
Source: Dragioti E et al. JAMA Psychiatry. 2019 Oct 2. doi: 10.1001/jamapsychiatry.2019.2859.
Premature mortality across most psychiatric disorders
The evidence is robust and disheartening: As if the personal suffering and societal stigma of mental illness are not bad enough, psychiatric patients also have a shorter lifespan.1 In the past, most studies have focused on early mortality and loss of potential life-years in schizophrenia,2 but many subsequent reports indicate that premature death occurs in all major psychiatric disorders.
Here is a summary of the sobering facts:
- Schizophrenia. In a study of 30,210 patients with schizophrenia, compared with >5 million individuals in the general population in Denmark (where they have an excellent registry), mortality was 16-fold higher among patients with schizophrenia if they had a single somatic illness.3 The illnesses were mostly respiratory, gastrointestinal, or cardiovascular).3 The loss of potential years of life was staggeringly high: 18.7 years for men, 16.3 years for women.4 A study conducted in 8 US states reported a loss of 2 to 3 decades of life across each of these states.5 The causes of death in patients with schizophrenia were mainly heart disease, cancer, stroke, and pulmonary diseases. A national database in Sweden found that unmedicated patients with schizophrenia had a significantly higher death rate than those receiving antipsychotics.6,7 Similar findings were reported by researchers in Finland.8 The Swedish study by Tiihonen et al6 also found that mortality was highest in patients receiving benzodiazepines along with antipsychotics, but there was no increased mortality among patients with schizophrenia receiving antidepressants.
- Bipolar disorder. A shorter life expectancy has also been reported in bipolar disorder,9 with a loss of 13.6 years for men and 12.1 years for women. Early death was caused by physical illness (even when suicide deaths were excluded), especially cardiovascular disease.10
- Major depressive disorder (MDD). A reduction of life expectancy in persons with MDD (unipolar depression) has been reported, with a loss of 14 years in men and 10 years in women.11 Although suicide contributed to the shorter lifespan, death due to accidents was 500% higher among persons with unipolar depression; the largest causes of death were physical illnesses. Further, Zubenko et al12 reported alarming findings about excess mortality among first- and second-degree relatives of persons with early-onset depression (some of whom were bipolar). The relatives died an average of 8 years earlier than the local population, and 40% died before reaching age 65. Also, there was a 5-fold increase in infant mortality (in the first year of life) among the relatives. The most common causes of death in adult relatives were heart disease, cancer, and stroke. It is obvious that MDD has a significant negative impact on health and longevity in both patients and their relatives.
- Attention-deficit/hyperactivity disorder (ADHD). A 220% increase in mortality was reported in persons with ADHD at all ages.13 Accidents were the most common cause of death. The mortality rate ratio (MRR) was 1.86 for ADHD before age 6, 1.58 for ADHD between age 6 to 17, and 4.25 for those age ≥18. The rate of early mortality was higher in girls and women (MRR = 2.85) than boys and men (MRR = 1.27).
- Obsessive-compulsive disorder (OCD). A study from Denmark of 10,155 persons with OCD followed for 10 years reported a significantly higher risk of death from both natural (MRR = 1.68) and unnatural causes (MRR = 2.61), compared with the general population.14 Patients with OCD and comorbid depression, anxiety, or substance use had a further increase in mortality risk, but the mortality risk of individuals with OCD without psychiatric comorbidity was still 200% higher than that of the general population.
- Anxiety disorders. One study found no increase in mortality among patients who have generalized anxiety, unless it was associated with depression.15 Another study reported that the presence of anxiety reduced the risk of cardiovascular mortality in persons with depression.16 The absence of increased mortality in anxiety disorders was also confirmed in a meta-analysis of 36 studies.17 However, a study of postmenopausal women with panic attacks found a 3-fold increase in coronary artery disease and stroke in that cohort,18 which confirmed the findings of an older study19 that demonstrated a 2-fold increase of mortality among 155 men with panic disorder after a 12-year follow-up. Also, a 25-year follow-up study found that suicide accounted for 20% of deaths in the anxiety group compared with 16.2% in the depression group,20 showing a significant risk of suicide in panic disorder, even exceeding that of depression.
- Oppositional defiant disorder (ODD) and conduct disorder (CD). In a 12-year follow-up study of 9,495 individuals with “disruptive behavioral disorders,” which included ODD and CD, the mortality rate was >400% higher in these patients compared with 1.92 million individuals in the general population (9.66 vs 2.22 per 10,000 person-years).21 Comorbid substance use disorder and ADHD further increased the mortality rate in this cohort.
- Posttraumatic stress disorder (PTSD). Studies show that there is a significantly increased risk of early cardiovascular mortality in PTSD,22 and that the death rate may be associated with accelerated “DNA methylation age” that leads to a 13% increased risk for all-cause mortality.23
- Borderline personality disorder (BPD). A recent longitudinal study (24 years of follow-up with evaluation every 2 years) reported a significantly higher mortality in patients with BPD compared with those with other personality disorders. The age range when the study started was 18 to 35. The rate of suicide death was Palatino LT Std>400% higher in BPD (5.9% vs 1.4%). Also, non-suicidal death was 250% higher in BPD (14% vs 5.5%). The causes of non-suicidal death included cardiovascular disease, substance-related complications, cancer, and accidents.24
- Other personality disorders. Certain personality traits have been associated with shorter leukocyte telomeres, which signal early death. These traits include neuroticism, conscientiousness, harm avoidance, and reward dependence.25 Another study found shorter telomeres in persons with high neuroticism and low agreeableness26 regardless of age or sex. Short telomeres, which reflect accelerated cellular senescence and aging, have also been reported in several major psychiatric disorders (schizophrenia, bipolar disorder, MDD, and anxiety).27-29 The cumulative evidence is unassailable; psychiatric brain disorders are not only associated with premature death due to high suicide rates, but also with multiple medical diseases that lead to early mortality and a shorter lifespan. The shortened telomeres reflect high oxidative stress and inflammation, and both those toxic processes are known to be associated with major psychiatric disorders. Compounding the dismal facts about early mortality due to mental illness are the additional grave medical consequences of alcohol and substance use, which are highly comorbid with most psychiatric disorders, further exacerbating the premature death rates among psychiatric patients.
Continue to: There is an important take-home message...
There is an important take-home message in all of this: Our patients are at high risk for potentially fatal medical conditions that require early detection, and intensive ongoing treatment by a primary care clinician (not “provider”; I abhor the widespread use of that term for physicians or nurse practitioners) is an indispensable component of psychiatric care. Thus, collaborative care is vital to protect our psychiatric patients from early mortality and a shortened lifespan. Psychiatrists and psychiatric nurse practitioners must not only win the battle against mental illness, but also diligently avoid losing the war of life and death.
1. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
2. Laursen TM, Wahlbeck K, Hällgren J, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013;8(6):e67133. doi: 10.1371/journal.pone.0067133.
3. Kugathasan P, Stubbs B, Aagaard J, et al. Increased mortality from somatic multimorbidity in patients with schizophrenia: a Danish nationwide cohort study. Acta Psychiatr Scand. 2019. doi: 10.1111/acps.13076.
4. Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131(1-3):101-104.
5. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
6. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
7. Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41(3):656-663.
8. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
9. Wilson R, Gaughran F, Whitburn T, et al. Place of death and other factors associated with unnatural mortality in patients with serious mental disorders: population-based retrospective cohort study. BJPsych Open. 2019;5(2):e23. doi: 10.1192/bjo.2019.5.
10. Ösby U, Westman J, Hällgren J, et al. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987-2010. Eur J Public Health. 2016;26(5):867-871.
11. Laursen TM, Musliner KL, Benros ME, et al. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord. 2016;193:203-207.
12. Zubenko GS, Zubenko WN, Spiker DG, et al. Malignancy of recurrent, early-onset major depression: a family study. Am J Med Genet. 2001;105(8):690-699.
13. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
14. Meier SM, Mattheisen M, Mors O, et al. Mortality among persons with obsessive-compulsive disorder in Denmark. JAMA Psychiatry. 2016;73(3):268-274.
15. Holwerda TJ, Schoevers RA, Dekker J, et al. The relationship between generalized anxiety disorder, depression and mortality in old age. Int J Geriatr Psychiatry. 2007;22(3):241-249.
16. Ivanovs R, Kivite A, Ziedonis D, et al. Association of depression and anxiety with the 10-year risk of cardiovascular mortality in a primary care population of Latvia using the SCORE system. Front Psychiatry. 2018;9:276.
17. Miloyan B, Bulley A, Bandeen-Roche K, et al. Anxiety disorders and all-cause mortality: systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2016;51(11):1467-1475.
18. Smoller JW, Pollack MH, Wassertheil-Smoller S, et al. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women’s Health Initiative Observational Study. Arch Gen Psychiatry. 2007;64(10):1153-1160.
19. Coryell W, Noyes R Jr, House JD. Mortality among outpatients with anxiety disorders. Am J Psychiatry. 1986;143(4):508-510.
20. Coryell W, Noyes R, Clancy J. Excess mortality in panic disorder. A comparison with primary unipolar depression. Arch Gen Psychiatry. 1982;39(6):701-703.
21. Scott JG, Giørtz Pedersen M, Erskine HE, et al. Mortality in individuals with disruptive behavior disorders diagnosed by specialist services - a nationwide cohort study. Psychiatry Res. 2017;251:255-260.
22. Burg MM, Soufer R. Post-traumatic stress disorder and cardiovascular disease. Curr Cardiol Rep. 2016;18(10):94.
23. Wolf EJ, Logue MW, Stoop TB, et al. Accelerated DNA methylation age: associations with PTSD and mortality. Psychosom Med. 2017. doi: 10.1097/PSY.0000000000000506.
24. Temes CM, Frankenburg FR, Fitzmaurice MC, et al. Deaths by suicide and other causes among patients with borderline personality disorder and personality-disordered comparison subjects over 24 years of prospective follow-up. J Clin Psychiatry. 2019;80(1). doi: 10.4088/JCP.18m12436.
25. Sadahiro R, Suzuki A, Enokido M, et al. Relationship between leukocyte telomere length and personality traits in healthy subjects. Eur Psychiatry. 2015;30(2):291-295.
26. Schoormans D, Verhoeven JE, Denollet J, et al. Leukocyte telomere length and personality: associations with the Big Five and Type D personality traits. Psychol Med. 2018;48(6):1008-1019.
27. Muneer A, Minhas FA. Telomere biology in mood disorders: an updated, comprehensive review of the literature. Clin Psychopharmacol Neurosci. 2019;17(3):343-363.
28. Vakonaki E, Tsiminikaki K, Plaitis S, et al. Common mental disorders and association with telomere length. Biomed Rep. 2018;8(2):111-116.
29. Malouff
The evidence is robust and disheartening: As if the personal suffering and societal stigma of mental illness are not bad enough, psychiatric patients also have a shorter lifespan.1 In the past, most studies have focused on early mortality and loss of potential life-years in schizophrenia,2 but many subsequent reports indicate that premature death occurs in all major psychiatric disorders.
Here is a summary of the sobering facts:
- Schizophrenia. In a study of 30,210 patients with schizophrenia, compared with >5 million individuals in the general population in Denmark (where they have an excellent registry), mortality was 16-fold higher among patients with schizophrenia if they had a single somatic illness.3 The illnesses were mostly respiratory, gastrointestinal, or cardiovascular).3 The loss of potential years of life was staggeringly high: 18.7 years for men, 16.3 years for women.4 A study conducted in 8 US states reported a loss of 2 to 3 decades of life across each of these states.5 The causes of death in patients with schizophrenia were mainly heart disease, cancer, stroke, and pulmonary diseases. A national database in Sweden found that unmedicated patients with schizophrenia had a significantly higher death rate than those receiving antipsychotics.6,7 Similar findings were reported by researchers in Finland.8 The Swedish study by Tiihonen et al6 also found that mortality was highest in patients receiving benzodiazepines along with antipsychotics, but there was no increased mortality among patients with schizophrenia receiving antidepressants.
- Bipolar disorder. A shorter life expectancy has also been reported in bipolar disorder,9 with a loss of 13.6 years for men and 12.1 years for women. Early death was caused by physical illness (even when suicide deaths were excluded), especially cardiovascular disease.10
- Major depressive disorder (MDD). A reduction of life expectancy in persons with MDD (unipolar depression) has been reported, with a loss of 14 years in men and 10 years in women.11 Although suicide contributed to the shorter lifespan, death due to accidents was 500% higher among persons with unipolar depression; the largest causes of death were physical illnesses. Further, Zubenko et al12 reported alarming findings about excess mortality among first- and second-degree relatives of persons with early-onset depression (some of whom were bipolar). The relatives died an average of 8 years earlier than the local population, and 40% died before reaching age 65. Also, there was a 5-fold increase in infant mortality (in the first year of life) among the relatives. The most common causes of death in adult relatives were heart disease, cancer, and stroke. It is obvious that MDD has a significant negative impact on health and longevity in both patients and their relatives.
- Attention-deficit/hyperactivity disorder (ADHD). A 220% increase in mortality was reported in persons with ADHD at all ages.13 Accidents were the most common cause of death. The mortality rate ratio (MRR) was 1.86 for ADHD before age 6, 1.58 for ADHD between age 6 to 17, and 4.25 for those age ≥18. The rate of early mortality was higher in girls and women (MRR = 2.85) than boys and men (MRR = 1.27).
- Obsessive-compulsive disorder (OCD). A study from Denmark of 10,155 persons with OCD followed for 10 years reported a significantly higher risk of death from both natural (MRR = 1.68) and unnatural causes (MRR = 2.61), compared with the general population.14 Patients with OCD and comorbid depression, anxiety, or substance use had a further increase in mortality risk, but the mortality risk of individuals with OCD without psychiatric comorbidity was still 200% higher than that of the general population.
- Anxiety disorders. One study found no increase in mortality among patients who have generalized anxiety, unless it was associated with depression.15 Another study reported that the presence of anxiety reduced the risk of cardiovascular mortality in persons with depression.16 The absence of increased mortality in anxiety disorders was also confirmed in a meta-analysis of 36 studies.17 However, a study of postmenopausal women with panic attacks found a 3-fold increase in coronary artery disease and stroke in that cohort,18 which confirmed the findings of an older study19 that demonstrated a 2-fold increase of mortality among 155 men with panic disorder after a 12-year follow-up. Also, a 25-year follow-up study found that suicide accounted for 20% of deaths in the anxiety group compared with 16.2% in the depression group,20 showing a significant risk of suicide in panic disorder, even exceeding that of depression.
- Oppositional defiant disorder (ODD) and conduct disorder (CD). In a 12-year follow-up study of 9,495 individuals with “disruptive behavioral disorders,” which included ODD and CD, the mortality rate was >400% higher in these patients compared with 1.92 million individuals in the general population (9.66 vs 2.22 per 10,000 person-years).21 Comorbid substance use disorder and ADHD further increased the mortality rate in this cohort.
- Posttraumatic stress disorder (PTSD). Studies show that there is a significantly increased risk of early cardiovascular mortality in PTSD,22 and that the death rate may be associated with accelerated “DNA methylation age” that leads to a 13% increased risk for all-cause mortality.23
- Borderline personality disorder (BPD). A recent longitudinal study (24 years of follow-up with evaluation every 2 years) reported a significantly higher mortality in patients with BPD compared with those with other personality disorders. The age range when the study started was 18 to 35. The rate of suicide death was Palatino LT Std>400% higher in BPD (5.9% vs 1.4%). Also, non-suicidal death was 250% higher in BPD (14% vs 5.5%). The causes of non-suicidal death included cardiovascular disease, substance-related complications, cancer, and accidents.24
- Other personality disorders. Certain personality traits have been associated with shorter leukocyte telomeres, which signal early death. These traits include neuroticism, conscientiousness, harm avoidance, and reward dependence.25 Another study found shorter telomeres in persons with high neuroticism and low agreeableness26 regardless of age or sex. Short telomeres, which reflect accelerated cellular senescence and aging, have also been reported in several major psychiatric disorders (schizophrenia, bipolar disorder, MDD, and anxiety).27-29 The cumulative evidence is unassailable; psychiatric brain disorders are not only associated with premature death due to high suicide rates, but also with multiple medical diseases that lead to early mortality and a shorter lifespan. The shortened telomeres reflect high oxidative stress and inflammation, and both those toxic processes are known to be associated with major psychiatric disorders. Compounding the dismal facts about early mortality due to mental illness are the additional grave medical consequences of alcohol and substance use, which are highly comorbid with most psychiatric disorders, further exacerbating the premature death rates among psychiatric patients.
Continue to: There is an important take-home message...
There is an important take-home message in all of this: Our patients are at high risk for potentially fatal medical conditions that require early detection, and intensive ongoing treatment by a primary care clinician (not “provider”; I abhor the widespread use of that term for physicians or nurse practitioners) is an indispensable component of psychiatric care. Thus, collaborative care is vital to protect our psychiatric patients from early mortality and a shortened lifespan. Psychiatrists and psychiatric nurse practitioners must not only win the battle against mental illness, but also diligently avoid losing the war of life and death.
The evidence is robust and disheartening: As if the personal suffering and societal stigma of mental illness are not bad enough, psychiatric patients also have a shorter lifespan.1 In the past, most studies have focused on early mortality and loss of potential life-years in schizophrenia,2 but many subsequent reports indicate that premature death occurs in all major psychiatric disorders.
Here is a summary of the sobering facts:
- Schizophrenia. In a study of 30,210 patients with schizophrenia, compared with >5 million individuals in the general population in Denmark (where they have an excellent registry), mortality was 16-fold higher among patients with schizophrenia if they had a single somatic illness.3 The illnesses were mostly respiratory, gastrointestinal, or cardiovascular).3 The loss of potential years of life was staggeringly high: 18.7 years for men, 16.3 years for women.4 A study conducted in 8 US states reported a loss of 2 to 3 decades of life across each of these states.5 The causes of death in patients with schizophrenia were mainly heart disease, cancer, stroke, and pulmonary diseases. A national database in Sweden found that unmedicated patients with schizophrenia had a significantly higher death rate than those receiving antipsychotics.6,7 Similar findings were reported by researchers in Finland.8 The Swedish study by Tiihonen et al6 also found that mortality was highest in patients receiving benzodiazepines along with antipsychotics, but there was no increased mortality among patients with schizophrenia receiving antidepressants.
- Bipolar disorder. A shorter life expectancy has also been reported in bipolar disorder,9 with a loss of 13.6 years for men and 12.1 years for women. Early death was caused by physical illness (even when suicide deaths were excluded), especially cardiovascular disease.10
- Major depressive disorder (MDD). A reduction of life expectancy in persons with MDD (unipolar depression) has been reported, with a loss of 14 years in men and 10 years in women.11 Although suicide contributed to the shorter lifespan, death due to accidents was 500% higher among persons with unipolar depression; the largest causes of death were physical illnesses. Further, Zubenko et al12 reported alarming findings about excess mortality among first- and second-degree relatives of persons with early-onset depression (some of whom were bipolar). The relatives died an average of 8 years earlier than the local population, and 40% died before reaching age 65. Also, there was a 5-fold increase in infant mortality (in the first year of life) among the relatives. The most common causes of death in adult relatives were heart disease, cancer, and stroke. It is obvious that MDD has a significant negative impact on health and longevity in both patients and their relatives.
- Attention-deficit/hyperactivity disorder (ADHD). A 220% increase in mortality was reported in persons with ADHD at all ages.13 Accidents were the most common cause of death. The mortality rate ratio (MRR) was 1.86 for ADHD before age 6, 1.58 for ADHD between age 6 to 17, and 4.25 for those age ≥18. The rate of early mortality was higher in girls and women (MRR = 2.85) than boys and men (MRR = 1.27).
- Obsessive-compulsive disorder (OCD). A study from Denmark of 10,155 persons with OCD followed for 10 years reported a significantly higher risk of death from both natural (MRR = 1.68) and unnatural causes (MRR = 2.61), compared with the general population.14 Patients with OCD and comorbid depression, anxiety, or substance use had a further increase in mortality risk, but the mortality risk of individuals with OCD without psychiatric comorbidity was still 200% higher than that of the general population.
- Anxiety disorders. One study found no increase in mortality among patients who have generalized anxiety, unless it was associated with depression.15 Another study reported that the presence of anxiety reduced the risk of cardiovascular mortality in persons with depression.16 The absence of increased mortality in anxiety disorders was also confirmed in a meta-analysis of 36 studies.17 However, a study of postmenopausal women with panic attacks found a 3-fold increase in coronary artery disease and stroke in that cohort,18 which confirmed the findings of an older study19 that demonstrated a 2-fold increase of mortality among 155 men with panic disorder after a 12-year follow-up. Also, a 25-year follow-up study found that suicide accounted for 20% of deaths in the anxiety group compared with 16.2% in the depression group,20 showing a significant risk of suicide in panic disorder, even exceeding that of depression.
- Oppositional defiant disorder (ODD) and conduct disorder (CD). In a 12-year follow-up study of 9,495 individuals with “disruptive behavioral disorders,” which included ODD and CD, the mortality rate was >400% higher in these patients compared with 1.92 million individuals in the general population (9.66 vs 2.22 per 10,000 person-years).21 Comorbid substance use disorder and ADHD further increased the mortality rate in this cohort.
- Posttraumatic stress disorder (PTSD). Studies show that there is a significantly increased risk of early cardiovascular mortality in PTSD,22 and that the death rate may be associated with accelerated “DNA methylation age” that leads to a 13% increased risk for all-cause mortality.23
- Borderline personality disorder (BPD). A recent longitudinal study (24 years of follow-up with evaluation every 2 years) reported a significantly higher mortality in patients with BPD compared with those with other personality disorders. The age range when the study started was 18 to 35. The rate of suicide death was Palatino LT Std>400% higher in BPD (5.9% vs 1.4%). Also, non-suicidal death was 250% higher in BPD (14% vs 5.5%). The causes of non-suicidal death included cardiovascular disease, substance-related complications, cancer, and accidents.24
- Other personality disorders. Certain personality traits have been associated with shorter leukocyte telomeres, which signal early death. These traits include neuroticism, conscientiousness, harm avoidance, and reward dependence.25 Another study found shorter telomeres in persons with high neuroticism and low agreeableness26 regardless of age or sex. Short telomeres, which reflect accelerated cellular senescence and aging, have also been reported in several major psychiatric disorders (schizophrenia, bipolar disorder, MDD, and anxiety).27-29 The cumulative evidence is unassailable; psychiatric brain disorders are not only associated with premature death due to high suicide rates, but also with multiple medical diseases that lead to early mortality and a shorter lifespan. The shortened telomeres reflect high oxidative stress and inflammation, and both those toxic processes are known to be associated with major psychiatric disorders. Compounding the dismal facts about early mortality due to mental illness are the additional grave medical consequences of alcohol and substance use, which are highly comorbid with most psychiatric disorders, further exacerbating the premature death rates among psychiatric patients.
Continue to: There is an important take-home message...
There is an important take-home message in all of this: Our patients are at high risk for potentially fatal medical conditions that require early detection, and intensive ongoing treatment by a primary care clinician (not “provider”; I abhor the widespread use of that term for physicians or nurse practitioners) is an indispensable component of psychiatric care. Thus, collaborative care is vital to protect our psychiatric patients from early mortality and a shortened lifespan. Psychiatrists and psychiatric nurse practitioners must not only win the battle against mental illness, but also diligently avoid losing the war of life and death.
1. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
2. Laursen TM, Wahlbeck K, Hällgren J, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013;8(6):e67133. doi: 10.1371/journal.pone.0067133.
3. Kugathasan P, Stubbs B, Aagaard J, et al. Increased mortality from somatic multimorbidity in patients with schizophrenia: a Danish nationwide cohort study. Acta Psychiatr Scand. 2019. doi: 10.1111/acps.13076.
4. Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131(1-3):101-104.
5. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
6. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
7. Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41(3):656-663.
8. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
9. Wilson R, Gaughran F, Whitburn T, et al. Place of death and other factors associated with unnatural mortality in patients with serious mental disorders: population-based retrospective cohort study. BJPsych Open. 2019;5(2):e23. doi: 10.1192/bjo.2019.5.
10. Ösby U, Westman J, Hällgren J, et al. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987-2010. Eur J Public Health. 2016;26(5):867-871.
11. Laursen TM, Musliner KL, Benros ME, et al. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord. 2016;193:203-207.
12. Zubenko GS, Zubenko WN, Spiker DG, et al. Malignancy of recurrent, early-onset major depression: a family study. Am J Med Genet. 2001;105(8):690-699.
13. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
14. Meier SM, Mattheisen M, Mors O, et al. Mortality among persons with obsessive-compulsive disorder in Denmark. JAMA Psychiatry. 2016;73(3):268-274.
15. Holwerda TJ, Schoevers RA, Dekker J, et al. The relationship between generalized anxiety disorder, depression and mortality in old age. Int J Geriatr Psychiatry. 2007;22(3):241-249.
16. Ivanovs R, Kivite A, Ziedonis D, et al. Association of depression and anxiety with the 10-year risk of cardiovascular mortality in a primary care population of Latvia using the SCORE system. Front Psychiatry. 2018;9:276.
17. Miloyan B, Bulley A, Bandeen-Roche K, et al. Anxiety disorders and all-cause mortality: systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2016;51(11):1467-1475.
18. Smoller JW, Pollack MH, Wassertheil-Smoller S, et al. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women’s Health Initiative Observational Study. Arch Gen Psychiatry. 2007;64(10):1153-1160.
19. Coryell W, Noyes R Jr, House JD. Mortality among outpatients with anxiety disorders. Am J Psychiatry. 1986;143(4):508-510.
20. Coryell W, Noyes R, Clancy J. Excess mortality in panic disorder. A comparison with primary unipolar depression. Arch Gen Psychiatry. 1982;39(6):701-703.
21. Scott JG, Giørtz Pedersen M, Erskine HE, et al. Mortality in individuals with disruptive behavior disorders diagnosed by specialist services - a nationwide cohort study. Psychiatry Res. 2017;251:255-260.
22. Burg MM, Soufer R. Post-traumatic stress disorder and cardiovascular disease. Curr Cardiol Rep. 2016;18(10):94.
23. Wolf EJ, Logue MW, Stoop TB, et al. Accelerated DNA methylation age: associations with PTSD and mortality. Psychosom Med. 2017. doi: 10.1097/PSY.0000000000000506.
24. Temes CM, Frankenburg FR, Fitzmaurice MC, et al. Deaths by suicide and other causes among patients with borderline personality disorder and personality-disordered comparison subjects over 24 years of prospective follow-up. J Clin Psychiatry. 2019;80(1). doi: 10.4088/JCP.18m12436.
25. Sadahiro R, Suzuki A, Enokido M, et al. Relationship between leukocyte telomere length and personality traits in healthy subjects. Eur Psychiatry. 2015;30(2):291-295.
26. Schoormans D, Verhoeven JE, Denollet J, et al. Leukocyte telomere length and personality: associations with the Big Five and Type D personality traits. Psychol Med. 2018;48(6):1008-1019.
27. Muneer A, Minhas FA. Telomere biology in mood disorders: an updated, comprehensive review of the literature. Clin Psychopharmacol Neurosci. 2019;17(3):343-363.
28. Vakonaki E, Tsiminikaki K, Plaitis S, et al. Common mental disorders and association with telomere length. Biomed Rep. 2018;8(2):111-116.
29. Malouff
1. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
2. Laursen TM, Wahlbeck K, Hällgren J, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013;8(6):e67133. doi: 10.1371/journal.pone.0067133.
3. Kugathasan P, Stubbs B, Aagaard J, et al. Increased mortality from somatic multimorbidity in patients with schizophrenia: a Danish nationwide cohort study. Acta Psychiatr Scand. 2019. doi: 10.1111/acps.13076.
4. Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131(1-3):101-104.
5. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
6. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
7. Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41(3):656-663.
8. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
9. Wilson R, Gaughran F, Whitburn T, et al. Place of death and other factors associated with unnatural mortality in patients with serious mental disorders: population-based retrospective cohort study. BJPsych Open. 2019;5(2):e23. doi: 10.1192/bjo.2019.5.
10. Ösby U, Westman J, Hällgren J, et al. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987-2010. Eur J Public Health. 2016;26(5):867-871.
11. Laursen TM, Musliner KL, Benros ME, et al. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord. 2016;193:203-207.
12. Zubenko GS, Zubenko WN, Spiker DG, et al. Malignancy of recurrent, early-onset major depression: a family study. Am J Med Genet. 2001;105(8):690-699.
13. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
14. Meier SM, Mattheisen M, Mors O, et al. Mortality among persons with obsessive-compulsive disorder in Denmark. JAMA Psychiatry. 2016;73(3):268-274.
15. Holwerda TJ, Schoevers RA, Dekker J, et al. The relationship between generalized anxiety disorder, depression and mortality in old age. Int J Geriatr Psychiatry. 2007;22(3):241-249.
16. Ivanovs R, Kivite A, Ziedonis D, et al. Association of depression and anxiety with the 10-year risk of cardiovascular mortality in a primary care population of Latvia using the SCORE system. Front Psychiatry. 2018;9:276.
17. Miloyan B, Bulley A, Bandeen-Roche K, et al. Anxiety disorders and all-cause mortality: systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2016;51(11):1467-1475.
18. Smoller JW, Pollack MH, Wassertheil-Smoller S, et al. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women’s Health Initiative Observational Study. Arch Gen Psychiatry. 2007;64(10):1153-1160.
19. Coryell W, Noyes R Jr, House JD. Mortality among outpatients with anxiety disorders. Am J Psychiatry. 1986;143(4):508-510.
20. Coryell W, Noyes R, Clancy J. Excess mortality in panic disorder. A comparison with primary unipolar depression. Arch Gen Psychiatry. 1982;39(6):701-703.
21. Scott JG, Giørtz Pedersen M, Erskine HE, et al. Mortality in individuals with disruptive behavior disorders diagnosed by specialist services - a nationwide cohort study. Psychiatry Res. 2017;251:255-260.
22. Burg MM, Soufer R. Post-traumatic stress disorder and cardiovascular disease. Curr Cardiol Rep. 2016;18(10):94.
23. Wolf EJ, Logue MW, Stoop TB, et al. Accelerated DNA methylation age: associations with PTSD and mortality. Psychosom Med. 2017. doi: 10.1097/PSY.0000000000000506.
24. Temes CM, Frankenburg FR, Fitzmaurice MC, et al. Deaths by suicide and other causes among patients with borderline personality disorder and personality-disordered comparison subjects over 24 years of prospective follow-up. J Clin Psychiatry. 2019;80(1). doi: 10.4088/JCP.18m12436.
25. Sadahiro R, Suzuki A, Enokido M, et al. Relationship between leukocyte telomere length and personality traits in healthy subjects. Eur Psychiatry. 2015;30(2):291-295.
26. Schoormans D, Verhoeven JE, Denollet J, et al. Leukocyte telomere length and personality: associations with the Big Five and Type D personality traits. Psychol Med. 2018;48(6):1008-1019.
27. Muneer A, Minhas FA. Telomere biology in mood disorders: an updated, comprehensive review of the literature. Clin Psychopharmacol Neurosci. 2019;17(3):343-363.
28. Vakonaki E, Tsiminikaki K, Plaitis S, et al. Common mental disorders and association with telomere length. Biomed Rep. 2018;8(2):111-116.
29. Malouff
Physician burnout vs depression: Recognize the signs
Although all health care professionals are at risk for burnout, physicians have especially high rates of self-reported burnout—which is commonly understood as a work-related syndrome of emotional exhaustion, depersonalization, and a decreased sense of accomplishment that develops over time.1 In a 2019 report investigating burnout in approximately 15,000 physicians, 39% of psychiatrists and nearly 50% of physicians from multiple other specialities described themselves as “burned out.”2 In addition, 15% reported symptoms of clinical depression (4%) or subclinical depression (11%). In comparison, in 2017, 7.1% of US adults experienced at least 1 major depressive episode.3 Because physician burnout and depression can be associated with adverse outcomes in patient care and personal health, rapid identification and differentiation of the 2 conditions is paramount.
Differentiating burnout and depression
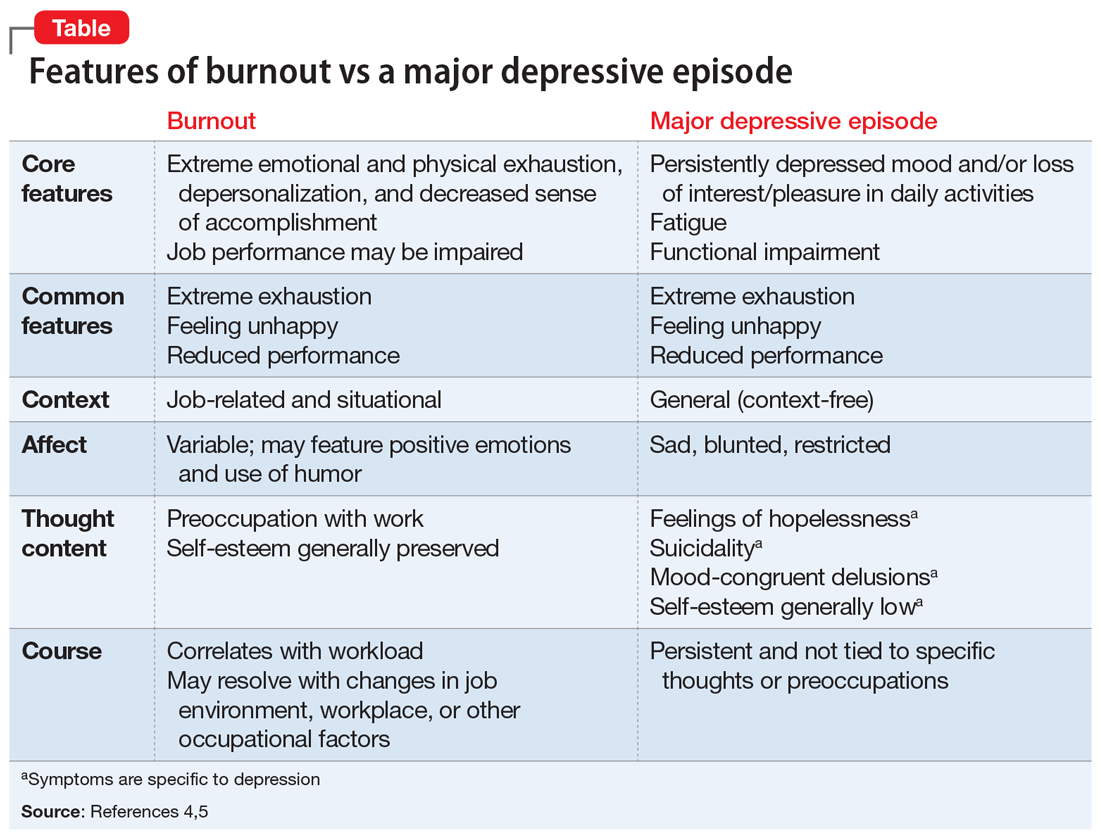
Burnout and depression are distinct but overlapping entities. Although burnout can be difficult to recognize and is not currently a DSM diagnosis, physicians can learn to identify the signs with reference to the more familiar features of depression (Table4,5). Many features of burnout are work-related, while the negative feelings and thoughts of depression pertain to all areas of life. Furthermore, a major depressive episode often includes hopelessness, suicidality, or mood-congruent delusions; burnout does not. Shared symptoms of burnout and depression include extreme exhaustion, feeling unhappy, and reduced performance.
Surprisingly, there is no universally accepted definition of burnout.4,5 Some researchers have proposed that physicians who are categorized as “burned out” may actually have underlying anxiety or depressive disorders that have been misdiagnosed and not appropriately treated.4,5 Others claim that burnout is best formulated as a depressive condition in need of formal diagnostic criteria.4,5 Because the definition of burnout is in question,4,5 strategies to prevent and detect burnout in individual clinicians remain elusive.
Key areas that contribute to vulnerability to burnout include one’s sense of community, fairness, and control in the workplace; personal and organization values; and work-life balance. We propose the mnemonic WORK to help clinicians quickly assess their vulnerability to burnout in these areas.
Workload. Outside of working hours, are you satisfied with the amount of time you devote to self-care, recreation, and other activities that are important to you? Do you honor your “down time”?
Oversight. Are you satisfied with the flexibility and autonomy in your professional life? Are you able to cope with the systemic demands of your practice while upholding your priorities within these restrictions?
Reward. Are the mechanisms for feedback, opportunities for advancement, and financial compensation in your workplace fair? Do you find positive meaning in the work that you do?
Continue to: Kinship
Kinship. Does your place of work support cooperation and collaboration, rather than competition and isolation? Do you approach and receive support from your colleagues when you need assistance?
Persistent dissatisfaction in any of these aspects should prompt clinicians to further develop strategies that promote workplace engagement, job satisfaction, and resilience. We hope this mnemonic helps clinicians to take responsibility for their own well-being and ultimately reap the rewards of a fulfilling professional life.
1. Brindley P. Psychological burnout and the intensive care practitioner: a practical and candid review for those who care. J Inten Care Soc. 2017;18(4):270-275.
2. Kane L. Medscape national physician b urnout & depression report 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056#1. Published January 16, 2019. Accessed September 17, 2019.
3. National Institute of Mental Health. Prevalence of major depressive episode among adults. https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Updated February 2019. Accessed September 17, 2019.
4. Messias E, Flynn V. The tired, retired, and recovered physician: professional burnout versus major depressive disorder. Am J Psychiatry. 2018;175(8):716-719.
5. Melnick ER, Powsner SM, Shanafelt TD. In reply—defining physician burnout, and differentiating between burnout and depression. Mayo Clinic Proc. 2017;92(9):1456-1458.
Although all health care professionals are at risk for burnout, physicians have especially high rates of self-reported burnout—which is commonly understood as a work-related syndrome of emotional exhaustion, depersonalization, and a decreased sense of accomplishment that develops over time.1 In a 2019 report investigating burnout in approximately 15,000 physicians, 39% of psychiatrists and nearly 50% of physicians from multiple other specialities described themselves as “burned out.”2 In addition, 15% reported symptoms of clinical depression (4%) or subclinical depression (11%). In comparison, in 2017, 7.1% of US adults experienced at least 1 major depressive episode.3 Because physician burnout and depression can be associated with adverse outcomes in patient care and personal health, rapid identification and differentiation of the 2 conditions is paramount.
Differentiating burnout and depression
Burnout and depression are distinct but overlapping entities. Although burnout can be difficult to recognize and is not currently a DSM diagnosis, physicians can learn to identify the signs with reference to the more familiar features of depression (Table4,5). Many features of burnout are work-related, while the negative feelings and thoughts of depression pertain to all areas of life. Furthermore, a major depressive episode often includes hopelessness, suicidality, or mood-congruent delusions; burnout does not. Shared symptoms of burnout and depression include extreme exhaustion, feeling unhappy, and reduced performance.
Surprisingly, there is no universally accepted definition of burnout.4,5 Some researchers have proposed that physicians who are categorized as “burned out” may actually have underlying anxiety or depressive disorders that have been misdiagnosed and not appropriately treated.4,5 Others claim that burnout is best formulated as a depressive condition in need of formal diagnostic criteria.4,5 Because the definition of burnout is in question,4,5 strategies to prevent and detect burnout in individual clinicians remain elusive.
Key areas that contribute to vulnerability to burnout include one’s sense of community, fairness, and control in the workplace; personal and organization values; and work-life balance. We propose the mnemonic WORK to help clinicians quickly assess their vulnerability to burnout in these areas.
Workload. Outside of working hours, are you satisfied with the amount of time you devote to self-care, recreation, and other activities that are important to you? Do you honor your “down time”?
Oversight. Are you satisfied with the flexibility and autonomy in your professional life? Are you able to cope with the systemic demands of your practice while upholding your priorities within these restrictions?
Reward. Are the mechanisms for feedback, opportunities for advancement, and financial compensation in your workplace fair? Do you find positive meaning in the work that you do?
Continue to: Kinship
Kinship. Does your place of work support cooperation and collaboration, rather than competition and isolation? Do you approach and receive support from your colleagues when you need assistance?
Persistent dissatisfaction in any of these aspects should prompt clinicians to further develop strategies that promote workplace engagement, job satisfaction, and resilience. We hope this mnemonic helps clinicians to take responsibility for their own well-being and ultimately reap the rewards of a fulfilling professional life.
Although all health care professionals are at risk for burnout, physicians have especially high rates of self-reported burnout—which is commonly understood as a work-related syndrome of emotional exhaustion, depersonalization, and a decreased sense of accomplishment that develops over time.1 In a 2019 report investigating burnout in approximately 15,000 physicians, 39% of psychiatrists and nearly 50% of physicians from multiple other specialities described themselves as “burned out.”2 In addition, 15% reported symptoms of clinical depression (4%) or subclinical depression (11%). In comparison, in 2017, 7.1% of US adults experienced at least 1 major depressive episode.3 Because physician burnout and depression can be associated with adverse outcomes in patient care and personal health, rapid identification and differentiation of the 2 conditions is paramount.
Differentiating burnout and depression
Burnout and depression are distinct but overlapping entities. Although burnout can be difficult to recognize and is not currently a DSM diagnosis, physicians can learn to identify the signs with reference to the more familiar features of depression (Table4,5). Many features of burnout are work-related, while the negative feelings and thoughts of depression pertain to all areas of life. Furthermore, a major depressive episode often includes hopelessness, suicidality, or mood-congruent delusions; burnout does not. Shared symptoms of burnout and depression include extreme exhaustion, feeling unhappy, and reduced performance.
Surprisingly, there is no universally accepted definition of burnout.4,5 Some researchers have proposed that physicians who are categorized as “burned out” may actually have underlying anxiety or depressive disorders that have been misdiagnosed and not appropriately treated.4,5 Others claim that burnout is best formulated as a depressive condition in need of formal diagnostic criteria.4,5 Because the definition of burnout is in question,4,5 strategies to prevent and detect burnout in individual clinicians remain elusive.
Key areas that contribute to vulnerability to burnout include one’s sense of community, fairness, and control in the workplace; personal and organization values; and work-life balance. We propose the mnemonic WORK to help clinicians quickly assess their vulnerability to burnout in these areas.
Workload. Outside of working hours, are you satisfied with the amount of time you devote to self-care, recreation, and other activities that are important to you? Do you honor your “down time”?
Oversight. Are you satisfied with the flexibility and autonomy in your professional life? Are you able to cope with the systemic demands of your practice while upholding your priorities within these restrictions?
Reward. Are the mechanisms for feedback, opportunities for advancement, and financial compensation in your workplace fair? Do you find positive meaning in the work that you do?
Continue to: Kinship
Kinship. Does your place of work support cooperation and collaboration, rather than competition and isolation? Do you approach and receive support from your colleagues when you need assistance?
Persistent dissatisfaction in any of these aspects should prompt clinicians to further develop strategies that promote workplace engagement, job satisfaction, and resilience. We hope this mnemonic helps clinicians to take responsibility for their own well-being and ultimately reap the rewards of a fulfilling professional life.
1. Brindley P. Psychological burnout and the intensive care practitioner: a practical and candid review for those who care. J Inten Care Soc. 2017;18(4):270-275.
2. Kane L. Medscape national physician b urnout & depression report 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056#1. Published January 16, 2019. Accessed September 17, 2019.
3. National Institute of Mental Health. Prevalence of major depressive episode among adults. https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Updated February 2019. Accessed September 17, 2019.
4. Messias E, Flynn V. The tired, retired, and recovered physician: professional burnout versus major depressive disorder. Am J Psychiatry. 2018;175(8):716-719.
5. Melnick ER, Powsner SM, Shanafelt TD. In reply—defining physician burnout, and differentiating between burnout and depression. Mayo Clinic Proc. 2017;92(9):1456-1458.
1. Brindley P. Psychological burnout and the intensive care practitioner: a practical and candid review for those who care. J Inten Care Soc. 2017;18(4):270-275.
2. Kane L. Medscape national physician b urnout & depression report 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056#1. Published January 16, 2019. Accessed September 17, 2019.
3. National Institute of Mental Health. Prevalence of major depressive episode among adults. https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Updated February 2019. Accessed September 17, 2019.
4. Messias E, Flynn V. The tired, retired, and recovered physician: professional burnout versus major depressive disorder. Am J Psychiatry. 2018;175(8):716-719.
5. Melnick ER, Powsner SM, Shanafelt TD. In reply—defining physician burnout, and differentiating between burnout and depression. Mayo Clinic Proc. 2017;92(9):1456-1458.
Four genetic variants link psychotic experiences to multiple mental disorders
Four genetic variations appear to link psychotic experiences with other psychiatric disorders, including schizophrenia, major depressive disorder, bipolar disorder, and neurodevelopmental disorders, a large genetic study has concluded.
, reported Sophie E. Legge, PhD, and colleagues. Their study was published in JAMA Psychiatry.
Although it is informative, the study is unlikely to expand the knowledge of schizophrenia-specific genetics.
“Consistent with other studies, the heritability estimate (1.71%) was low, and given that the variance explained in our polygenic risk analysis was also low, the finding suggests that understanding the genetics of psychotic experiences is unlikely to have an important effect on understanding the genetics of schizophrenia specifically,” wrote Dr. Legge, of the MRC Center for Neuropsychiatric Genetics and Genomics in the division of psychological medicine and clinical neurosciences at Cardiff (Wales) University, and colleagues.
The team conducted a genomewide association study (GWAS) using data from 127,966 individuals in the U.K. Biobank. Of these, 6,123 reported any psychotic experience, 2,143 reported distressing psychotic experiences, and 3,337 reported multiple experiences. The remainder served as controls. At the time of the biobank data collection, the subjects were a mean of 64 years of age; 56% were women.
First psychotic experience occurred at a mean of almost 32 years of age, but about a third reported that the first episode occurred before age 20, or that psychotic experiences had been happening ever since they could remember. Another third reported their first experience between ages 40 and 76 years.
The investigators conducted three GWAS studies: one for any psychotic experience, one for distressing experiences, and one for multiple experiences.
No significant genetic associations were found among those with multiple psychotic experiences, the authors said.
But they did find four variants significantly associated with the other experience categories.
Two variants were associated with any psychotic experience. Those with rs10994278, an intronic variant within Ankyrin-3 (ANK3), were 16% more likely to have a psychotic experience (odds ratio, 1.16). Those with intergenic variant rs549656827 were 39% less likely (OR, 0.61). “The ANK3 gene encodes ankyrin-G, a protein that has been shown to regulate the assembly of voltage-gated sodium channels and is essential for normal synaptic function,” the authors said. “ANK3 is one of strongest and most replicated genes for bipolar disorder, and variants within ANK3 have also been associated in the Psychiatric Genomics Consortium cross-disorder GWAS, and in a rare variant analysis of autism spectrum disorder.”
Two variants were linked to distressing psychotic experiences: rs75459873, intronic to cannabinoid receptor 2 (CNR2), decreased the risk by 34% (OR, 0.66). Intergenic variant rs3849810 increased the risk by 12% (OR, 1.12).
“CNR2 encodes for CB2, one of two well-characterized cannabinoid receptors. Several lines of evidence have implicated the endocannabinoid system in psychiatric disorders, including schizophrenia and depression. The main psychoactive agent of cannabis, tetrahydrocannabinol, can cause acute psychotic symptoms and cognitive impairment. Given that cannabis use is strongly associated with psychotic experiences, we tested, but found no evidence for, a mediating or moderating effect of cannabis use on the association of rs75459873 and distressing psychotic experiences. However, while no evidence was found in this study, a mediating effect of cannabis use cannot be ruled out given the relatively low power of such analyses and the potential measurement error.”
Also, significant genetic correlations were found between any psychotic experiences and major depressive disorder, autism spectrum disorder, ADHD, and schizophrenia. However, the polygenic risk scores for schizophrenia, major depressive disorder, bipolar disorder, ADHD, and autism spectrum disorder, were low.
“We also considered individual psychotic symptoms and found that polygenic risk scores for schizophrenia, bipolar disorder, depression, and ADHD were more strongly associated with delusions of persecution than with the other psychotic symptoms.”
Those with distressing psychotic experiences tended to have more copy number variations (CNVs) associated with schizophrenia (OR, 2.04) and neurodevelopmental disorders (OR, 1.75). The team also found significant associations between distressing experiences and major depressive disorder, ADHD, autism spectrum disorder, and schizophrenia.
“We found particular enrichment of these [polygenic risk scores] in distressing psychotic experiences and for delusions of persecution,” they noted. “ ... All schizophrenia-associated [copy number variations] are also associated with neurodevelopmental disorders such as intellectual disability and autism.”
The study’s strengths include its large sample size. Among its limitations, the researchers said, are the study’s retrospective measurement of psychotic experiences based on self-report from a questionnaire that was online. Gathering the data in that way raised the likelihood of possible error, they said.
Dr. Legge reported having no disclosures.
SOURCE: Legge SE et al. JAMA Psychiatry. 2019 Sep 25. doi: 10.1001/jamapsychiatry.2019.2508.
The genetic links uncovered in this study offer an intriguing, but incomplete look at the risks of psychotic experiences and their complicated intertwinings with other mental disorders, wrote Albert R. Powers III, MD, PhD.
“Penetrance of the genes in question likely depends at least in part on environmental influences, some of which have been studied extensively,” he wrote. “Recently, some have proposed risk stratification by exposome – a composite score of relevant exposures that may increase risk for psychosis and is analogous to the polygenic risk score used [here].
“The combination of environmental and genetic composite scores may lead to improved insight into individualized pathways toward psychotic experiences, highlighting genetic vulnerabilities to specific stressors likely to lead to phenotypic expression. Ultimately, this will require a more sophisticated mapping between phenomenology and biology than currently exists.”
One approach would be to combine deep phenotyping and behavioral analyses in a framework that could link all relevant levels from symptoms to neurophysiology.
“One such framework is predictive processing theory, which is linked closely with the free energy principle and the Bayesian brain hypothesis and attempts to explain perceptual and cognitive phenomena as manifestations of a drive to maintain as accurate an internal model of one’s surroundings as possible by minimizing prediction errors. This relatively simple scheme makes specific – and, importantly, falsifiable – assessments of the mathematical signatures of neurotypical processes and the ways they might break down to produce specific psychiatric symptoms.”
Dr. Powers is an assistant professor at the department of psychiatry at Yale University, New Haven, Conn., and serves as medical director of the PRIME Psychosis Research Clinic at Yale. His comments came in an accompanying editorial (JAMA Psychiatry. 2019 Sep 25. doi: 10.1001/jamapsychiatry.2019.2391 ).
The genetic links uncovered in this study offer an intriguing, but incomplete look at the risks of psychotic experiences and their complicated intertwinings with other mental disorders, wrote Albert R. Powers III, MD, PhD.
“Penetrance of the genes in question likely depends at least in part on environmental influences, some of which have been studied extensively,” he wrote. “Recently, some have proposed risk stratification by exposome – a composite score of relevant exposures that may increase risk for psychosis and is analogous to the polygenic risk score used [here].
“The combination of environmental and genetic composite scores may lead to improved insight into individualized pathways toward psychotic experiences, highlighting genetic vulnerabilities to specific stressors likely to lead to phenotypic expression. Ultimately, this will require a more sophisticated mapping between phenomenology and biology than currently exists.”
One approach would be to combine deep phenotyping and behavioral analyses in a framework that could link all relevant levels from symptoms to neurophysiology.
“One such framework is predictive processing theory, which is linked closely with the free energy principle and the Bayesian brain hypothesis and attempts to explain perceptual and cognitive phenomena as manifestations of a drive to maintain as accurate an internal model of one’s surroundings as possible by minimizing prediction errors. This relatively simple scheme makes specific – and, importantly, falsifiable – assessments of the mathematical signatures of neurotypical processes and the ways they might break down to produce specific psychiatric symptoms.”
Dr. Powers is an assistant professor at the department of psychiatry at Yale University, New Haven, Conn., and serves as medical director of the PRIME Psychosis Research Clinic at Yale. His comments came in an accompanying editorial (JAMA Psychiatry. 2019 Sep 25. doi: 10.1001/jamapsychiatry.2019.2391 ).
The genetic links uncovered in this study offer an intriguing, but incomplete look at the risks of psychotic experiences and their complicated intertwinings with other mental disorders, wrote Albert R. Powers III, MD, PhD.
“Penetrance of the genes in question likely depends at least in part on environmental influences, some of which have been studied extensively,” he wrote. “Recently, some have proposed risk stratification by exposome – a composite score of relevant exposures that may increase risk for psychosis and is analogous to the polygenic risk score used [here].
“The combination of environmental and genetic composite scores may lead to improved insight into individualized pathways toward psychotic experiences, highlighting genetic vulnerabilities to specific stressors likely to lead to phenotypic expression. Ultimately, this will require a more sophisticated mapping between phenomenology and biology than currently exists.”
One approach would be to combine deep phenotyping and behavioral analyses in a framework that could link all relevant levels from symptoms to neurophysiology.
“One such framework is predictive processing theory, which is linked closely with the free energy principle and the Bayesian brain hypothesis and attempts to explain perceptual and cognitive phenomena as manifestations of a drive to maintain as accurate an internal model of one’s surroundings as possible by minimizing prediction errors. This relatively simple scheme makes specific – and, importantly, falsifiable – assessments of the mathematical signatures of neurotypical processes and the ways they might break down to produce specific psychiatric symptoms.”
Dr. Powers is an assistant professor at the department of psychiatry at Yale University, New Haven, Conn., and serves as medical director of the PRIME Psychosis Research Clinic at Yale. His comments came in an accompanying editorial (JAMA Psychiatry. 2019 Sep 25. doi: 10.1001/jamapsychiatry.2019.2391 ).
Four genetic variations appear to link psychotic experiences with other psychiatric disorders, including schizophrenia, major depressive disorder, bipolar disorder, and neurodevelopmental disorders, a large genetic study has concluded.
, reported Sophie E. Legge, PhD, and colleagues. Their study was published in JAMA Psychiatry.
Although it is informative, the study is unlikely to expand the knowledge of schizophrenia-specific genetics.
“Consistent with other studies, the heritability estimate (1.71%) was low, and given that the variance explained in our polygenic risk analysis was also low, the finding suggests that understanding the genetics of psychotic experiences is unlikely to have an important effect on understanding the genetics of schizophrenia specifically,” wrote Dr. Legge, of the MRC Center for Neuropsychiatric Genetics and Genomics in the division of psychological medicine and clinical neurosciences at Cardiff (Wales) University, and colleagues.
The team conducted a genomewide association study (GWAS) using data from 127,966 individuals in the U.K. Biobank. Of these, 6,123 reported any psychotic experience, 2,143 reported distressing psychotic experiences, and 3,337 reported multiple experiences. The remainder served as controls. At the time of the biobank data collection, the subjects were a mean of 64 years of age; 56% were women.
First psychotic experience occurred at a mean of almost 32 years of age, but about a third reported that the first episode occurred before age 20, or that psychotic experiences had been happening ever since they could remember. Another third reported their first experience between ages 40 and 76 years.
The investigators conducted three GWAS studies: one for any psychotic experience, one for distressing experiences, and one for multiple experiences.
No significant genetic associations were found among those with multiple psychotic experiences, the authors said.
But they did find four variants significantly associated with the other experience categories.
Two variants were associated with any psychotic experience. Those with rs10994278, an intronic variant within Ankyrin-3 (ANK3), were 16% more likely to have a psychotic experience (odds ratio, 1.16). Those with intergenic variant rs549656827 were 39% less likely (OR, 0.61). “The ANK3 gene encodes ankyrin-G, a protein that has been shown to regulate the assembly of voltage-gated sodium channels and is essential for normal synaptic function,” the authors said. “ANK3 is one of strongest and most replicated genes for bipolar disorder, and variants within ANK3 have also been associated in the Psychiatric Genomics Consortium cross-disorder GWAS, and in a rare variant analysis of autism spectrum disorder.”
Two variants were linked to distressing psychotic experiences: rs75459873, intronic to cannabinoid receptor 2 (CNR2), decreased the risk by 34% (OR, 0.66). Intergenic variant rs3849810 increased the risk by 12% (OR, 1.12).
“CNR2 encodes for CB2, one of two well-characterized cannabinoid receptors. Several lines of evidence have implicated the endocannabinoid system in psychiatric disorders, including schizophrenia and depression. The main psychoactive agent of cannabis, tetrahydrocannabinol, can cause acute psychotic symptoms and cognitive impairment. Given that cannabis use is strongly associated with psychotic experiences, we tested, but found no evidence for, a mediating or moderating effect of cannabis use on the association of rs75459873 and distressing psychotic experiences. However, while no evidence was found in this study, a mediating effect of cannabis use cannot be ruled out given the relatively low power of such analyses and the potential measurement error.”
Also, significant genetic correlations were found between any psychotic experiences and major depressive disorder, autism spectrum disorder, ADHD, and schizophrenia. However, the polygenic risk scores for schizophrenia, major depressive disorder, bipolar disorder, ADHD, and autism spectrum disorder, were low.
“We also considered individual psychotic symptoms and found that polygenic risk scores for schizophrenia, bipolar disorder, depression, and ADHD were more strongly associated with delusions of persecution than with the other psychotic symptoms.”
Those with distressing psychotic experiences tended to have more copy number variations (CNVs) associated with schizophrenia (OR, 2.04) and neurodevelopmental disorders (OR, 1.75). The team also found significant associations between distressing experiences and major depressive disorder, ADHD, autism spectrum disorder, and schizophrenia.
“We found particular enrichment of these [polygenic risk scores] in distressing psychotic experiences and for delusions of persecution,” they noted. “ ... All schizophrenia-associated [copy number variations] are also associated with neurodevelopmental disorders such as intellectual disability and autism.”
The study’s strengths include its large sample size. Among its limitations, the researchers said, are the study’s retrospective measurement of psychotic experiences based on self-report from a questionnaire that was online. Gathering the data in that way raised the likelihood of possible error, they said.
Dr. Legge reported having no disclosures.
SOURCE: Legge SE et al. JAMA Psychiatry. 2019 Sep 25. doi: 10.1001/jamapsychiatry.2019.2508.
Four genetic variations appear to link psychotic experiences with other psychiatric disorders, including schizophrenia, major depressive disorder, bipolar disorder, and neurodevelopmental disorders, a large genetic study has concluded.
, reported Sophie E. Legge, PhD, and colleagues. Their study was published in JAMA Psychiatry.
Although it is informative, the study is unlikely to expand the knowledge of schizophrenia-specific genetics.
“Consistent with other studies, the heritability estimate (1.71%) was low, and given that the variance explained in our polygenic risk analysis was also low, the finding suggests that understanding the genetics of psychotic experiences is unlikely to have an important effect on understanding the genetics of schizophrenia specifically,” wrote Dr. Legge, of the MRC Center for Neuropsychiatric Genetics and Genomics in the division of psychological medicine and clinical neurosciences at Cardiff (Wales) University, and colleagues.
The team conducted a genomewide association study (GWAS) using data from 127,966 individuals in the U.K. Biobank. Of these, 6,123 reported any psychotic experience, 2,143 reported distressing psychotic experiences, and 3,337 reported multiple experiences. The remainder served as controls. At the time of the biobank data collection, the subjects were a mean of 64 years of age; 56% were women.
First psychotic experience occurred at a mean of almost 32 years of age, but about a third reported that the first episode occurred before age 20, or that psychotic experiences had been happening ever since they could remember. Another third reported their first experience between ages 40 and 76 years.
The investigators conducted three GWAS studies: one for any psychotic experience, one for distressing experiences, and one for multiple experiences.
No significant genetic associations were found among those with multiple psychotic experiences, the authors said.
But they did find four variants significantly associated with the other experience categories.
Two variants were associated with any psychotic experience. Those with rs10994278, an intronic variant within Ankyrin-3 (ANK3), were 16% more likely to have a psychotic experience (odds ratio, 1.16). Those with intergenic variant rs549656827 were 39% less likely (OR, 0.61). “The ANK3 gene encodes ankyrin-G, a protein that has been shown to regulate the assembly of voltage-gated sodium channels and is essential for normal synaptic function,” the authors said. “ANK3 is one of strongest and most replicated genes for bipolar disorder, and variants within ANK3 have also been associated in the Psychiatric Genomics Consortium cross-disorder GWAS, and in a rare variant analysis of autism spectrum disorder.”
Two variants were linked to distressing psychotic experiences: rs75459873, intronic to cannabinoid receptor 2 (CNR2), decreased the risk by 34% (OR, 0.66). Intergenic variant rs3849810 increased the risk by 12% (OR, 1.12).
“CNR2 encodes for CB2, one of two well-characterized cannabinoid receptors. Several lines of evidence have implicated the endocannabinoid system in psychiatric disorders, including schizophrenia and depression. The main psychoactive agent of cannabis, tetrahydrocannabinol, can cause acute psychotic symptoms and cognitive impairment. Given that cannabis use is strongly associated with psychotic experiences, we tested, but found no evidence for, a mediating or moderating effect of cannabis use on the association of rs75459873 and distressing psychotic experiences. However, while no evidence was found in this study, a mediating effect of cannabis use cannot be ruled out given the relatively low power of such analyses and the potential measurement error.”
Also, significant genetic correlations were found between any psychotic experiences and major depressive disorder, autism spectrum disorder, ADHD, and schizophrenia. However, the polygenic risk scores for schizophrenia, major depressive disorder, bipolar disorder, ADHD, and autism spectrum disorder, were low.
“We also considered individual psychotic symptoms and found that polygenic risk scores for schizophrenia, bipolar disorder, depression, and ADHD were more strongly associated with delusions of persecution than with the other psychotic symptoms.”
Those with distressing psychotic experiences tended to have more copy number variations (CNVs) associated with schizophrenia (OR, 2.04) and neurodevelopmental disorders (OR, 1.75). The team also found significant associations between distressing experiences and major depressive disorder, ADHD, autism spectrum disorder, and schizophrenia.
“We found particular enrichment of these [polygenic risk scores] in distressing psychotic experiences and for delusions of persecution,” they noted. “ ... All schizophrenia-associated [copy number variations] are also associated with neurodevelopmental disorders such as intellectual disability and autism.”
The study’s strengths include its large sample size. Among its limitations, the researchers said, are the study’s retrospective measurement of psychotic experiences based on self-report from a questionnaire that was online. Gathering the data in that way raised the likelihood of possible error, they said.
Dr. Legge reported having no disclosures.
SOURCE: Legge SE et al. JAMA Psychiatry. 2019 Sep 25. doi: 10.1001/jamapsychiatry.2019.2508.
FROM JAMA PSYCHIATRY
Esketamine nasal spray may get expanded indication
COPENHAGEN – Esketamine nasal spray achieved rapid reduction of major depressive disorder symptoms in patients at imminent risk for suicide in a pair of pivotal phase 3 clinical trials known as ASPIRE-1 and ASPIRE-2, Carla M. Canuso, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
These were groundbreaking studies, which addressed a major unmet need familiar to every mental health professional, given that more than 800,000 suicides per year occur worldwide. Standard antidepressants are of only limited value during the period of acute suicidal crisis because they take 4-6 weeks to work. Moreover, this population of seriously depressed and suicidal patients has been understudied.
“Patients with active suicidal ideation and intent are routinely excluded from antidepressant trials,” observed Dr. Canuso, a psychiatrist who serves as senior director of neuroscience clinical development at Janssen Research and Development in Titusville, N.J.
It’s very important for the field to know that we can actually study these patients safely and effectively in clinical trials,” she said.
ASPIRE-1 and -2 were identically designed, randomized, double-blind, placebo-controlled, multinational studies conducted in patients with moderate to severe major depressive disorder as evidenced by a baseline Montgomery-Åsberg Depression Rating Scale (MADRS) total score of about 40, along with moderate to extreme active suicidal ideation and intent as assessed using the Clinical Global Impression-Severity of Suicidality-Revised (CGI-SS-R).
“These were all patients in psychiatric crisis seeking clinical care,” according to Dr. Canuso.
All 456 participants in the two phase 3 studies underwent an initial 5-14 days of psychiatric hospitalization, during which they began treatment with esketamine nasal spray at 84 mg twice weekly or placebo coupled with comprehensive standard of care, which included a newly initiated and/or optimized oral antidepressant regimen.
The primary endpoint in the two clinical trials was the change in MADRS total score 24 hours after the first dose of study medication. The esketamine-treated patients demonstrated a mean reduction of 16.4 and 15.7 points, respectively, in the two trials, which was 3.82 points greater than in the pooled placebo group. This represents a clinically meaningful and statistically significant between-group difference.
The treatment effect size was even larger in some of the prespecified patient subgroups. Dr. Canuso drew attention to two key groups: In the roughly 60% of ASPIRE participants with a prior suicide attempt, esketamine resulted in a mean 4.81-point greater reduction in MADRS total score than placebo, and in the nearly 30% of participants with a suicide attempt during the past month, the difference was 5.22 points.
A word on the study design: Patients received intranasal esketamine at 84 mg per dose or placebo in double-blind fashion twice weekly for 4 weeks, thereby giving time for their oral antidepressant therapy to kick in, and were then followed on the conventional therapy out to 90 days.
A between-group difference in MADRS total score in favor of the esketamine group was evident as early as 4 hours after the first dose and continued through day 25, the end of the double-blind treatment period, at which point 54% and 47% of the esketamine-plus-conventional-antidepressant groups in the two trials had achieved remission as defined by a MADRS score of 12 or less, as had about one-third of the control group.
The prespecified key secondary efficacy endpoint in ASPIRE-1 and -2 was change in the CGI-SS-R 24 hours after the first dose. Both the esketamine and placebo-treated patients experienced significant improvement in this domain, with a disappointing absence of between-group statistical significance.
“We think that this may be due to the effect of acute hospitalization in diffusing the suicidal crisis,” Dr. Canuso said.
She noted, however, that other suicidality indices did show significant improvement in the esketamine-treated group during assessments at 4 hours, 24 hours, and 25 days after the first dose. For example, the double-blind esketamine-treated patients were 2.62-fold more likely than controls to show a significant improvement in MADRS Suicidal Thoughts at 4 hours after dose number one, and 6.15 times more likely to do so 4 hours after their day-25 dose. The CGI structured physician assessments of suicide risk and frequency of suicidal thinking, as well as patient-reported frequency of suicidal thinking, showed consistent favorable numeric trends for improvement with esketamine, with odds ratios of 1.46-2.11 from 4 hours through 25 days, although those results generally failed to achieve statistical significance.
In terms of safety, the rate of serious adverse events was just under 12% in both the esketamine and placebo arms. As in earlier studies, the most common adverse events associated with the novel antidepressant were dizziness, dissociation, nausea, and sleepiness, all several-fold more frequent than with placebo.
Esketamine is the S-enantiomer of racemic ketamine. It’s a noncompetitive N-methyl-D-aspartate receptor antagonist.
Janssen, which already markets intranasal esketamine as Spravato in the United States for treatment-resistant depression, plans to file for an expanded indication on the basis of the ASPIRE-1 and -2 results. The Food and Drug Administration already has granted Breakthrough Therapy designation for research on esketamine for reduction of major depression symptoms in patients with active suicidal ideation.
The ASPIRE studies were funded by Janssen.
COPENHAGEN – Esketamine nasal spray achieved rapid reduction of major depressive disorder symptoms in patients at imminent risk for suicide in a pair of pivotal phase 3 clinical trials known as ASPIRE-1 and ASPIRE-2, Carla M. Canuso, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
These were groundbreaking studies, which addressed a major unmet need familiar to every mental health professional, given that more than 800,000 suicides per year occur worldwide. Standard antidepressants are of only limited value during the period of acute suicidal crisis because they take 4-6 weeks to work. Moreover, this population of seriously depressed and suicidal patients has been understudied.
“Patients with active suicidal ideation and intent are routinely excluded from antidepressant trials,” observed Dr. Canuso, a psychiatrist who serves as senior director of neuroscience clinical development at Janssen Research and Development in Titusville, N.J.
It’s very important for the field to know that we can actually study these patients safely and effectively in clinical trials,” she said.
ASPIRE-1 and -2 were identically designed, randomized, double-blind, placebo-controlled, multinational studies conducted in patients with moderate to severe major depressive disorder as evidenced by a baseline Montgomery-Åsberg Depression Rating Scale (MADRS) total score of about 40, along with moderate to extreme active suicidal ideation and intent as assessed using the Clinical Global Impression-Severity of Suicidality-Revised (CGI-SS-R).
“These were all patients in psychiatric crisis seeking clinical care,” according to Dr. Canuso.
All 456 participants in the two phase 3 studies underwent an initial 5-14 days of psychiatric hospitalization, during which they began treatment with esketamine nasal spray at 84 mg twice weekly or placebo coupled with comprehensive standard of care, which included a newly initiated and/or optimized oral antidepressant regimen.
The primary endpoint in the two clinical trials was the change in MADRS total score 24 hours after the first dose of study medication. The esketamine-treated patients demonstrated a mean reduction of 16.4 and 15.7 points, respectively, in the two trials, which was 3.82 points greater than in the pooled placebo group. This represents a clinically meaningful and statistically significant between-group difference.
The treatment effect size was even larger in some of the prespecified patient subgroups. Dr. Canuso drew attention to two key groups: In the roughly 60% of ASPIRE participants with a prior suicide attempt, esketamine resulted in a mean 4.81-point greater reduction in MADRS total score than placebo, and in the nearly 30% of participants with a suicide attempt during the past month, the difference was 5.22 points.
A word on the study design: Patients received intranasal esketamine at 84 mg per dose or placebo in double-blind fashion twice weekly for 4 weeks, thereby giving time for their oral antidepressant therapy to kick in, and were then followed on the conventional therapy out to 90 days.
A between-group difference in MADRS total score in favor of the esketamine group was evident as early as 4 hours after the first dose and continued through day 25, the end of the double-blind treatment period, at which point 54% and 47% of the esketamine-plus-conventional-antidepressant groups in the two trials had achieved remission as defined by a MADRS score of 12 or less, as had about one-third of the control group.
The prespecified key secondary efficacy endpoint in ASPIRE-1 and -2 was change in the CGI-SS-R 24 hours after the first dose. Both the esketamine and placebo-treated patients experienced significant improvement in this domain, with a disappointing absence of between-group statistical significance.
“We think that this may be due to the effect of acute hospitalization in diffusing the suicidal crisis,” Dr. Canuso said.
She noted, however, that other suicidality indices did show significant improvement in the esketamine-treated group during assessments at 4 hours, 24 hours, and 25 days after the first dose. For example, the double-blind esketamine-treated patients were 2.62-fold more likely than controls to show a significant improvement in MADRS Suicidal Thoughts at 4 hours after dose number one, and 6.15 times more likely to do so 4 hours after their day-25 dose. The CGI structured physician assessments of suicide risk and frequency of suicidal thinking, as well as patient-reported frequency of suicidal thinking, showed consistent favorable numeric trends for improvement with esketamine, with odds ratios of 1.46-2.11 from 4 hours through 25 days, although those results generally failed to achieve statistical significance.
In terms of safety, the rate of serious adverse events was just under 12% in both the esketamine and placebo arms. As in earlier studies, the most common adverse events associated with the novel antidepressant were dizziness, dissociation, nausea, and sleepiness, all several-fold more frequent than with placebo.
Esketamine is the S-enantiomer of racemic ketamine. It’s a noncompetitive N-methyl-D-aspartate receptor antagonist.
Janssen, which already markets intranasal esketamine as Spravato in the United States for treatment-resistant depression, plans to file for an expanded indication on the basis of the ASPIRE-1 and -2 results. The Food and Drug Administration already has granted Breakthrough Therapy designation for research on esketamine for reduction of major depression symptoms in patients with active suicidal ideation.
The ASPIRE studies were funded by Janssen.
COPENHAGEN – Esketamine nasal spray achieved rapid reduction of major depressive disorder symptoms in patients at imminent risk for suicide in a pair of pivotal phase 3 clinical trials known as ASPIRE-1 and ASPIRE-2, Carla M. Canuso, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
These were groundbreaking studies, which addressed a major unmet need familiar to every mental health professional, given that more than 800,000 suicides per year occur worldwide. Standard antidepressants are of only limited value during the period of acute suicidal crisis because they take 4-6 weeks to work. Moreover, this population of seriously depressed and suicidal patients has been understudied.
“Patients with active suicidal ideation and intent are routinely excluded from antidepressant trials,” observed Dr. Canuso, a psychiatrist who serves as senior director of neuroscience clinical development at Janssen Research and Development in Titusville, N.J.
It’s very important for the field to know that we can actually study these patients safely and effectively in clinical trials,” she said.
ASPIRE-1 and -2 were identically designed, randomized, double-blind, placebo-controlled, multinational studies conducted in patients with moderate to severe major depressive disorder as evidenced by a baseline Montgomery-Åsberg Depression Rating Scale (MADRS) total score of about 40, along with moderate to extreme active suicidal ideation and intent as assessed using the Clinical Global Impression-Severity of Suicidality-Revised (CGI-SS-R).
“These were all patients in psychiatric crisis seeking clinical care,” according to Dr. Canuso.
All 456 participants in the two phase 3 studies underwent an initial 5-14 days of psychiatric hospitalization, during which they began treatment with esketamine nasal spray at 84 mg twice weekly or placebo coupled with comprehensive standard of care, which included a newly initiated and/or optimized oral antidepressant regimen.
The primary endpoint in the two clinical trials was the change in MADRS total score 24 hours after the first dose of study medication. The esketamine-treated patients demonstrated a mean reduction of 16.4 and 15.7 points, respectively, in the two trials, which was 3.82 points greater than in the pooled placebo group. This represents a clinically meaningful and statistically significant between-group difference.
The treatment effect size was even larger in some of the prespecified patient subgroups. Dr. Canuso drew attention to two key groups: In the roughly 60% of ASPIRE participants with a prior suicide attempt, esketamine resulted in a mean 4.81-point greater reduction in MADRS total score than placebo, and in the nearly 30% of participants with a suicide attempt during the past month, the difference was 5.22 points.
A word on the study design: Patients received intranasal esketamine at 84 mg per dose or placebo in double-blind fashion twice weekly for 4 weeks, thereby giving time for their oral antidepressant therapy to kick in, and were then followed on the conventional therapy out to 90 days.
A between-group difference in MADRS total score in favor of the esketamine group was evident as early as 4 hours after the first dose and continued through day 25, the end of the double-blind treatment period, at which point 54% and 47% of the esketamine-plus-conventional-antidepressant groups in the two trials had achieved remission as defined by a MADRS score of 12 or less, as had about one-third of the control group.
The prespecified key secondary efficacy endpoint in ASPIRE-1 and -2 was change in the CGI-SS-R 24 hours after the first dose. Both the esketamine and placebo-treated patients experienced significant improvement in this domain, with a disappointing absence of between-group statistical significance.
“We think that this may be due to the effect of acute hospitalization in diffusing the suicidal crisis,” Dr. Canuso said.
She noted, however, that other suicidality indices did show significant improvement in the esketamine-treated group during assessments at 4 hours, 24 hours, and 25 days after the first dose. For example, the double-blind esketamine-treated patients were 2.62-fold more likely than controls to show a significant improvement in MADRS Suicidal Thoughts at 4 hours after dose number one, and 6.15 times more likely to do so 4 hours after their day-25 dose. The CGI structured physician assessments of suicide risk and frequency of suicidal thinking, as well as patient-reported frequency of suicidal thinking, showed consistent favorable numeric trends for improvement with esketamine, with odds ratios of 1.46-2.11 from 4 hours through 25 days, although those results generally failed to achieve statistical significance.
In terms of safety, the rate of serious adverse events was just under 12% in both the esketamine and placebo arms. As in earlier studies, the most common adverse events associated with the novel antidepressant were dizziness, dissociation, nausea, and sleepiness, all several-fold more frequent than with placebo.
Esketamine is the S-enantiomer of racemic ketamine. It’s a noncompetitive N-methyl-D-aspartate receptor antagonist.
Janssen, which already markets intranasal esketamine as Spravato in the United States for treatment-resistant depression, plans to file for an expanded indication on the basis of the ASPIRE-1 and -2 results. The Food and Drug Administration already has granted Breakthrough Therapy designation for research on esketamine for reduction of major depression symptoms in patients with active suicidal ideation.
The ASPIRE studies were funded by Janssen.
REPORTING FROM ECNP 2019
Latest suicide prevention research highlights roles for clinicians, teachers, and parents
Joan Asarnow, PhD, said in a webinar presented on World Suicide Prevention Day, Sept. 10, 2019, to raise awareness of the latest research in suicide prevention and risk factors.
“Primary care doctors are the most trusted doctors for our teenagers,” Dr. Asarnow said during a question-and-answer session. Primary care can be the first-line screening to identify risk factors for suicide, and a close link with primary care “can make a very big difference in helping kids get through tough times.”
Other studies have shown that when doctors and nurses are able to recognize suicidality and link to behavioral health when needed, suicide attempts and ideation are reduced. Strategies including dialectical behavioral therapy and cognitive-behavior therapy have demonstrated success in reducing self -harm, she noted.
Schools have a role in suicide prevention as well, said Dr. Asarnow of the University of California, Los Angeles, and editor of a special issue of the Journal of Child Psychology and Psychiatry on suicide and self-harm.
She cited data from the Saving and Empowering Young Lives in Europe (SEYLE) study, a longitudinal study of school-based suicide prevention interventions, in which suicide attempts were significantly lower among teens who were exposed to a school-based program (Youth Aware of Mental Health) than they were among controls.
Additional findings from the SEYLE study recently were published in the Journal of Child Psychology and Psychiatry (2019. doi: 10.1111/jcpp.13119).
The authors investigated the interaction of three interventions with a certain model of suicide risk. The three interventions were Youth Aware of Mental Health (YAM); Question, Persuade and Refer (QPR); and ProfScreen. The latter two are established interventions for use by teachers. In the study, 11,110 high school students from 10 countries in the European Union completed questionnaires to assess baseline feelings of being a burden to others and feelings of loneliness and isolation from family and peers. The questionnaires also assessed health risk behaviors, self-injury, suicide ideation, and suicide attempts (SA), which were factors in the model being investigated. The participants were randomized to one of the interventions or to a control group that received educational posters with information about mental health resources.
In a reassessment of 8,972 adolescents 12 months later, the interventions all significantly reduced the association between repeated suicide attempts and the baseline interaction of self-injury and suicide ideation, compared with the control group.
“Among each of the three intervention groups, [suicide ideation] at baseline did not increase the risk of self-injury to be associated with repeated [suicide attempt]” at follow-up, Shira Barzilay, PhD, of Tel Aviv University, and coauthors said.
In addition, the researchers’ model found that “belongingness to parents” predicted lower odds of SI after controlling for depression, anxiety, and internalizing symptoms, and this prediction was similar across the intervention and control groups, although good relations with peers and lack of feeling like a burden on others were not significantly associated with lower odds of SI.
The study findings were limited by several factors including the limits of the model to fully capture the measures of belongingness or burdensomeness, and the use of a 12-month follow-up, which was too long to examine certain patterns of SA, the researchers noted. However, the results suggest that interventions can help reduce risk behaviors or self-harm that could lead to suicide. Areas for further study include examining spikes in risk variables that might have preceded suicide attempts, elevated stress, or interpersonal conflicts.
“The implications for suicide prevention, in both community and clinical settings, are to monitor youth who may be engaged in risky behaviors regardless of [suicide ideation] presentation and provide them with mental health education,” Dr. Barzilay and coauthors concluded.
The ongoing mission, Dr. Asarnow said, is “to send messages of hope, and that there is help out there.”
This is particularly important in the United States and the United Kingdom because, while suicide rates in adolescents have declined in some countries, they have increased in others, notably the two countries aforementioned, Dennis Ougrin, MD, said at the webinar.
Males are more likely to commit suicide than females by a ratio of 3 or 4 to 1 in most Western countries, said Dr. Ougrin, a child and adolescent psychiatrist at South London and Maudsley National Health Service Foundation Trust, leading the Child and Adolescent Mental Health Services Enhanced Treatment Service.
Although hanging is the most common method for suicides in most countries, followed by poisoning, more than 50% of suicides in the United States are caused by firearms, he noted.
Risk factors for completed suicide include male sex, low social status, restricted educational achievement, parental mental disorder, individual mental disorder, family history of suicidal behavior, problems with interpersonal relationships, drug and alcohol misuse, and feelings of hopelessness, said Dr. Ougrin, citing data from a 2012 study published in the Lancet (2012 Jun 23. doi: 10.1016/S0140-6736[12]60322-5).
The webinar was sponsored by Wiley partnership with the World Federation of Science Journalists and the Association of Health Care Journalists. Dr. Asarnow and Dr. Ougrin had no financial conflicts to disclose. The SEYLE project is supported by the European Union through the Seventh Framework Program. Dr. Barzilay and coauthors of the SEYLE study had no financial conflicts to disclose.
Joan Asarnow, PhD, said in a webinar presented on World Suicide Prevention Day, Sept. 10, 2019, to raise awareness of the latest research in suicide prevention and risk factors.
“Primary care doctors are the most trusted doctors for our teenagers,” Dr. Asarnow said during a question-and-answer session. Primary care can be the first-line screening to identify risk factors for suicide, and a close link with primary care “can make a very big difference in helping kids get through tough times.”
Other studies have shown that when doctors and nurses are able to recognize suicidality and link to behavioral health when needed, suicide attempts and ideation are reduced. Strategies including dialectical behavioral therapy and cognitive-behavior therapy have demonstrated success in reducing self -harm, she noted.
Schools have a role in suicide prevention as well, said Dr. Asarnow of the University of California, Los Angeles, and editor of a special issue of the Journal of Child Psychology and Psychiatry on suicide and self-harm.
She cited data from the Saving and Empowering Young Lives in Europe (SEYLE) study, a longitudinal study of school-based suicide prevention interventions, in which suicide attempts were significantly lower among teens who were exposed to a school-based program (Youth Aware of Mental Health) than they were among controls.
Additional findings from the SEYLE study recently were published in the Journal of Child Psychology and Psychiatry (2019. doi: 10.1111/jcpp.13119).
The authors investigated the interaction of three interventions with a certain model of suicide risk. The three interventions were Youth Aware of Mental Health (YAM); Question, Persuade and Refer (QPR); and ProfScreen. The latter two are established interventions for use by teachers. In the study, 11,110 high school students from 10 countries in the European Union completed questionnaires to assess baseline feelings of being a burden to others and feelings of loneliness and isolation from family and peers. The questionnaires also assessed health risk behaviors, self-injury, suicide ideation, and suicide attempts (SA), which were factors in the model being investigated. The participants were randomized to one of the interventions or to a control group that received educational posters with information about mental health resources.
In a reassessment of 8,972 adolescents 12 months later, the interventions all significantly reduced the association between repeated suicide attempts and the baseline interaction of self-injury and suicide ideation, compared with the control group.
“Among each of the three intervention groups, [suicide ideation] at baseline did not increase the risk of self-injury to be associated with repeated [suicide attempt]” at follow-up, Shira Barzilay, PhD, of Tel Aviv University, and coauthors said.
In addition, the researchers’ model found that “belongingness to parents” predicted lower odds of SI after controlling for depression, anxiety, and internalizing symptoms, and this prediction was similar across the intervention and control groups, although good relations with peers and lack of feeling like a burden on others were not significantly associated with lower odds of SI.
The study findings were limited by several factors including the limits of the model to fully capture the measures of belongingness or burdensomeness, and the use of a 12-month follow-up, which was too long to examine certain patterns of SA, the researchers noted. However, the results suggest that interventions can help reduce risk behaviors or self-harm that could lead to suicide. Areas for further study include examining spikes in risk variables that might have preceded suicide attempts, elevated stress, or interpersonal conflicts.
“The implications for suicide prevention, in both community and clinical settings, are to monitor youth who may be engaged in risky behaviors regardless of [suicide ideation] presentation and provide them with mental health education,” Dr. Barzilay and coauthors concluded.
The ongoing mission, Dr. Asarnow said, is “to send messages of hope, and that there is help out there.”
This is particularly important in the United States and the United Kingdom because, while suicide rates in adolescents have declined in some countries, they have increased in others, notably the two countries aforementioned, Dennis Ougrin, MD, said at the webinar.
Males are more likely to commit suicide than females by a ratio of 3 or 4 to 1 in most Western countries, said Dr. Ougrin, a child and adolescent psychiatrist at South London and Maudsley National Health Service Foundation Trust, leading the Child and Adolescent Mental Health Services Enhanced Treatment Service.
Although hanging is the most common method for suicides in most countries, followed by poisoning, more than 50% of suicides in the United States are caused by firearms, he noted.
Risk factors for completed suicide include male sex, low social status, restricted educational achievement, parental mental disorder, individual mental disorder, family history of suicidal behavior, problems with interpersonal relationships, drug and alcohol misuse, and feelings of hopelessness, said Dr. Ougrin, citing data from a 2012 study published in the Lancet (2012 Jun 23. doi: 10.1016/S0140-6736[12]60322-5).
The webinar was sponsored by Wiley partnership with the World Federation of Science Journalists and the Association of Health Care Journalists. Dr. Asarnow and Dr. Ougrin had no financial conflicts to disclose. The SEYLE project is supported by the European Union through the Seventh Framework Program. Dr. Barzilay and coauthors of the SEYLE study had no financial conflicts to disclose.
Joan Asarnow, PhD, said in a webinar presented on World Suicide Prevention Day, Sept. 10, 2019, to raise awareness of the latest research in suicide prevention and risk factors.
“Primary care doctors are the most trusted doctors for our teenagers,” Dr. Asarnow said during a question-and-answer session. Primary care can be the first-line screening to identify risk factors for suicide, and a close link with primary care “can make a very big difference in helping kids get through tough times.”
Other studies have shown that when doctors and nurses are able to recognize suicidality and link to behavioral health when needed, suicide attempts and ideation are reduced. Strategies including dialectical behavioral therapy and cognitive-behavior therapy have demonstrated success in reducing self -harm, she noted.
Schools have a role in suicide prevention as well, said Dr. Asarnow of the University of California, Los Angeles, and editor of a special issue of the Journal of Child Psychology and Psychiatry on suicide and self-harm.
She cited data from the Saving and Empowering Young Lives in Europe (SEYLE) study, a longitudinal study of school-based suicide prevention interventions, in which suicide attempts were significantly lower among teens who were exposed to a school-based program (Youth Aware of Mental Health) than they were among controls.
Additional findings from the SEYLE study recently were published in the Journal of Child Psychology and Psychiatry (2019. doi: 10.1111/jcpp.13119).
The authors investigated the interaction of three interventions with a certain model of suicide risk. The three interventions were Youth Aware of Mental Health (YAM); Question, Persuade and Refer (QPR); and ProfScreen. The latter two are established interventions for use by teachers. In the study, 11,110 high school students from 10 countries in the European Union completed questionnaires to assess baseline feelings of being a burden to others and feelings of loneliness and isolation from family and peers. The questionnaires also assessed health risk behaviors, self-injury, suicide ideation, and suicide attempts (SA), which were factors in the model being investigated. The participants were randomized to one of the interventions or to a control group that received educational posters with information about mental health resources.
In a reassessment of 8,972 adolescents 12 months later, the interventions all significantly reduced the association between repeated suicide attempts and the baseline interaction of self-injury and suicide ideation, compared with the control group.
“Among each of the three intervention groups, [suicide ideation] at baseline did not increase the risk of self-injury to be associated with repeated [suicide attempt]” at follow-up, Shira Barzilay, PhD, of Tel Aviv University, and coauthors said.
In addition, the researchers’ model found that “belongingness to parents” predicted lower odds of SI after controlling for depression, anxiety, and internalizing symptoms, and this prediction was similar across the intervention and control groups, although good relations with peers and lack of feeling like a burden on others were not significantly associated with lower odds of SI.
The study findings were limited by several factors including the limits of the model to fully capture the measures of belongingness or burdensomeness, and the use of a 12-month follow-up, which was too long to examine certain patterns of SA, the researchers noted. However, the results suggest that interventions can help reduce risk behaviors or self-harm that could lead to suicide. Areas for further study include examining spikes in risk variables that might have preceded suicide attempts, elevated stress, or interpersonal conflicts.
“The implications for suicide prevention, in both community and clinical settings, are to monitor youth who may be engaged in risky behaviors regardless of [suicide ideation] presentation and provide them with mental health education,” Dr. Barzilay and coauthors concluded.
The ongoing mission, Dr. Asarnow said, is “to send messages of hope, and that there is help out there.”
This is particularly important in the United States and the United Kingdom because, while suicide rates in adolescents have declined in some countries, they have increased in others, notably the two countries aforementioned, Dennis Ougrin, MD, said at the webinar.
Males are more likely to commit suicide than females by a ratio of 3 or 4 to 1 in most Western countries, said Dr. Ougrin, a child and adolescent psychiatrist at South London and Maudsley National Health Service Foundation Trust, leading the Child and Adolescent Mental Health Services Enhanced Treatment Service.
Although hanging is the most common method for suicides in most countries, followed by poisoning, more than 50% of suicides in the United States are caused by firearms, he noted.
Risk factors for completed suicide include male sex, low social status, restricted educational achievement, parental mental disorder, individual mental disorder, family history of suicidal behavior, problems with interpersonal relationships, drug and alcohol misuse, and feelings of hopelessness, said Dr. Ougrin, citing data from a 2012 study published in the Lancet (2012 Jun 23. doi: 10.1016/S0140-6736[12]60322-5).
The webinar was sponsored by Wiley partnership with the World Federation of Science Journalists and the Association of Health Care Journalists. Dr. Asarnow and Dr. Ougrin had no financial conflicts to disclose. The SEYLE project is supported by the European Union through the Seventh Framework Program. Dr. Barzilay and coauthors of the SEYLE study had no financial conflicts to disclose.
FROM A SCIENCE TALKS WEBINAR
Statins may do double duty as antidepressants
COPENHAGEN – The tantalizing prospect that statins could be repurposed as adjunctive antidepressant drugs in a defined subgroup of patients with major depression is finally about to undergo rigorous testing.
Several lines of preliminary evidence, including large observational cohort studies as well as three small, short-duration randomized trials, suggest that this might indeed be the case. It’s an extremely attractive possibility, since patients and physicians wish that antidepressant therapy were more effective, statins are among the most widely prescribed drugs worldwide, and their safety profile is thoroughly established. The expectation is that a definitive answer as to whether repurposing of statins as antidepressants is worthwhile will be provided by the SIMCODE trial, recently approved for funding by the German Federal Ministry of Education and Research, Christian Otte, MD, announced at the annual congress of the European College of Neuropsychopharmacology.
SIMCODE is a multicenter, double-blind, placebo-controlled randomized trial to be conducted at eight German academic medical centers. Participants, all of whom must have major depressive disorder and comorbid obesity, will be randomized to simvastatin or placebo on top of standard antidepressant therapy with escitalopram, an SSRI which, like simvastatin, is available as a relatively inexpensive generic, explained Dr. Otte, professor and vice director of the department of psychiatry and psychotherapy at Charite University in Berlin.
For Dr. Otte, SIMCODE will close a circle he helped open with his 2012 report from the Heart and Soul Study, a prospective longitudinal study of nearly 1,000 San Francisco Bay Area patients with coronary heart disease who were assessed annually for depressive symptoms for 6 years. The 65% of patients who were on statin therapy, albeit in nonrandomized fashion, had an adjusted 38% lower risk of developing depression (J Clin Psychiatry. 2012 May;73[5]:610-5).
His was one of seven observational studies involving more than 9,000 patients included in a subsequent meta-analysis showing that statin users were 37% less likely to develop depression than were nonusers (J Affect Disord. 2014 May;160:62-7).
All agreed that the verdict isn’t in yet as to statins’ effectiveness as adjunctive antidepressants, and that the subgroup of patients with major depression who are most likely to gain added antidepressive effect from a statin are those with what the speakers variously described as comorbid cardiometabolic disease, immunometabolic disease, or simply, as in SIMCODE, obesity. These are patients with a high degree of systemic inflammation, which often makes their depression less responsive to standard antidepressant therapies. The working hypothesis is that the pleiotropic anti-inflammatory effects of statins will result in a greater response to conventional antidepressants.
Animal studies point to multiple potential mechanisms by which statins might have antidepressant efficacy in clinical practice, according to Dr. Otte. Beyond their anti-inflammatory effects, these include the drugs’ documented effects on glutamatergic N-methyl-D-aspartate (NMDA) receptors, dopamine receptors, brain-derived neurotrophic factor, glucocorticoid receptors, and hippocampal serotonin 2A receptors.
Ole Kohler, MD, a psychiatrist at Aarhus (Denmark) University, presented highlights of his eye-popping population-based study of more than 872,000 Danes on an SSRI in 1997-2012, more than 113,000 of whom were on a concomitant statin. The key finding: During roughly 3 years of follow-up, the risk of contact with a psychiatric hospital for depression was 36% lower in the group on concomitant SSRI/statin therapy than in those not on a statin (Am J Psychiatry. 2016 Aug 1;173[8]:807-15).
He was quick to observe that a study such as this is vulnerable to various forms of confounding. This risk can be mitigated to a considerable extent by careful propensity score matching. Of note, however, none of the three studies that have been conducted with propensity score matching, including his own recent study of nearly 194,000 statin users and an equal number of matched nonusers, showed a difference in risk of depression between statin users and nonusers. All three studies were performed in general populations without known depression, leading Dr. Kohler to conclude that it’s unlikely that statins have a role in preventing depression in nondepressed individuals.
The focus should instead be on the possible role of statins in reducing the risk of depression in patients with cardiometabolic disease – that is, heart disease, metabolic syndrome, or type 2 diabetes – where more than a half-dozen cohort studies, including the Heart and Soul Study, have found that statins have a favorable impact, he added.
Estela Salagre, MD, a psychiatrist at the University of Barcelona, has carried out a meta-analysis of the three randomized, double-blind, placebo-controlled trials of add-on statin therapy in patients on standard therapies for moderate to severe depression published to date. She found that statin therapy was associated with a 27% greater reduction in scores on the Hamilton Depression Rating Scale, compared with placebo (J Affect Disord. 2016 Aug;200:235-42). Those findings recently were confirmed in a separate meta-analysis by other investigators using different methodologies (J Affect Disord. 2019 Oct 1;257:55-63).
Femke Lamers, PhD, presented evidence based on the nearly 3,000-subject longitudinal Netherlands Study of Depression and Anxiety that roughly one-quarter of individuals with major depressive disorder have a distinct subtype of nonmelancholic depression characterized by a clustering of obesity, inflammation, increased appetite, fatigue, hypersomnia, and increased levels of insulin and leptin. She calls it immunometabolic depression. She and her coinvestigators in the international Psychiatric Genomics Consortium have demonstrated that this phenotypic clustering is associated with a shared genetic vulnerability between major depression and obesity (JAMA Psychiatry. 2017 Dec 1;74[12]:1214-25).
“Major depressive disorder is not a one-size-fits-all disorder. There is an immunometabolic form of depression,” declared Dr. Lamers, an epidemiologist at the University of Amsterdam.
All speakers reported having no financial conflicts of interest.
COPENHAGEN – The tantalizing prospect that statins could be repurposed as adjunctive antidepressant drugs in a defined subgroup of patients with major depression is finally about to undergo rigorous testing.
Several lines of preliminary evidence, including large observational cohort studies as well as three small, short-duration randomized trials, suggest that this might indeed be the case. It’s an extremely attractive possibility, since patients and physicians wish that antidepressant therapy were more effective, statins are among the most widely prescribed drugs worldwide, and their safety profile is thoroughly established. The expectation is that a definitive answer as to whether repurposing of statins as antidepressants is worthwhile will be provided by the SIMCODE trial, recently approved for funding by the German Federal Ministry of Education and Research, Christian Otte, MD, announced at the annual congress of the European College of Neuropsychopharmacology.
SIMCODE is a multicenter, double-blind, placebo-controlled randomized trial to be conducted at eight German academic medical centers. Participants, all of whom must have major depressive disorder and comorbid obesity, will be randomized to simvastatin or placebo on top of standard antidepressant therapy with escitalopram, an SSRI which, like simvastatin, is available as a relatively inexpensive generic, explained Dr. Otte, professor and vice director of the department of psychiatry and psychotherapy at Charite University in Berlin.
For Dr. Otte, SIMCODE will close a circle he helped open with his 2012 report from the Heart and Soul Study, a prospective longitudinal study of nearly 1,000 San Francisco Bay Area patients with coronary heart disease who were assessed annually for depressive symptoms for 6 years. The 65% of patients who were on statin therapy, albeit in nonrandomized fashion, had an adjusted 38% lower risk of developing depression (J Clin Psychiatry. 2012 May;73[5]:610-5).
His was one of seven observational studies involving more than 9,000 patients included in a subsequent meta-analysis showing that statin users were 37% less likely to develop depression than were nonusers (J Affect Disord. 2014 May;160:62-7).
All agreed that the verdict isn’t in yet as to statins’ effectiveness as adjunctive antidepressants, and that the subgroup of patients with major depression who are most likely to gain added antidepressive effect from a statin are those with what the speakers variously described as comorbid cardiometabolic disease, immunometabolic disease, or simply, as in SIMCODE, obesity. These are patients with a high degree of systemic inflammation, which often makes their depression less responsive to standard antidepressant therapies. The working hypothesis is that the pleiotropic anti-inflammatory effects of statins will result in a greater response to conventional antidepressants.
Animal studies point to multiple potential mechanisms by which statins might have antidepressant efficacy in clinical practice, according to Dr. Otte. Beyond their anti-inflammatory effects, these include the drugs’ documented effects on glutamatergic N-methyl-D-aspartate (NMDA) receptors, dopamine receptors, brain-derived neurotrophic factor, glucocorticoid receptors, and hippocampal serotonin 2A receptors.
Ole Kohler, MD, a psychiatrist at Aarhus (Denmark) University, presented highlights of his eye-popping population-based study of more than 872,000 Danes on an SSRI in 1997-2012, more than 113,000 of whom were on a concomitant statin. The key finding: During roughly 3 years of follow-up, the risk of contact with a psychiatric hospital for depression was 36% lower in the group on concomitant SSRI/statin therapy than in those not on a statin (Am J Psychiatry. 2016 Aug 1;173[8]:807-15).
He was quick to observe that a study such as this is vulnerable to various forms of confounding. This risk can be mitigated to a considerable extent by careful propensity score matching. Of note, however, none of the three studies that have been conducted with propensity score matching, including his own recent study of nearly 194,000 statin users and an equal number of matched nonusers, showed a difference in risk of depression between statin users and nonusers. All three studies were performed in general populations without known depression, leading Dr. Kohler to conclude that it’s unlikely that statins have a role in preventing depression in nondepressed individuals.
The focus should instead be on the possible role of statins in reducing the risk of depression in patients with cardiometabolic disease – that is, heart disease, metabolic syndrome, or type 2 diabetes – where more than a half-dozen cohort studies, including the Heart and Soul Study, have found that statins have a favorable impact, he added.
Estela Salagre, MD, a psychiatrist at the University of Barcelona, has carried out a meta-analysis of the three randomized, double-blind, placebo-controlled trials of add-on statin therapy in patients on standard therapies for moderate to severe depression published to date. She found that statin therapy was associated with a 27% greater reduction in scores on the Hamilton Depression Rating Scale, compared with placebo (J Affect Disord. 2016 Aug;200:235-42). Those findings recently were confirmed in a separate meta-analysis by other investigators using different methodologies (J Affect Disord. 2019 Oct 1;257:55-63).
Femke Lamers, PhD, presented evidence based on the nearly 3,000-subject longitudinal Netherlands Study of Depression and Anxiety that roughly one-quarter of individuals with major depressive disorder have a distinct subtype of nonmelancholic depression characterized by a clustering of obesity, inflammation, increased appetite, fatigue, hypersomnia, and increased levels of insulin and leptin. She calls it immunometabolic depression. She and her coinvestigators in the international Psychiatric Genomics Consortium have demonstrated that this phenotypic clustering is associated with a shared genetic vulnerability between major depression and obesity (JAMA Psychiatry. 2017 Dec 1;74[12]:1214-25).
“Major depressive disorder is not a one-size-fits-all disorder. There is an immunometabolic form of depression,” declared Dr. Lamers, an epidemiologist at the University of Amsterdam.
All speakers reported having no financial conflicts of interest.
COPENHAGEN – The tantalizing prospect that statins could be repurposed as adjunctive antidepressant drugs in a defined subgroup of patients with major depression is finally about to undergo rigorous testing.
Several lines of preliminary evidence, including large observational cohort studies as well as three small, short-duration randomized trials, suggest that this might indeed be the case. It’s an extremely attractive possibility, since patients and physicians wish that antidepressant therapy were more effective, statins are among the most widely prescribed drugs worldwide, and their safety profile is thoroughly established. The expectation is that a definitive answer as to whether repurposing of statins as antidepressants is worthwhile will be provided by the SIMCODE trial, recently approved for funding by the German Federal Ministry of Education and Research, Christian Otte, MD, announced at the annual congress of the European College of Neuropsychopharmacology.
SIMCODE is a multicenter, double-blind, placebo-controlled randomized trial to be conducted at eight German academic medical centers. Participants, all of whom must have major depressive disorder and comorbid obesity, will be randomized to simvastatin or placebo on top of standard antidepressant therapy with escitalopram, an SSRI which, like simvastatin, is available as a relatively inexpensive generic, explained Dr. Otte, professor and vice director of the department of psychiatry and psychotherapy at Charite University in Berlin.
For Dr. Otte, SIMCODE will close a circle he helped open with his 2012 report from the Heart and Soul Study, a prospective longitudinal study of nearly 1,000 San Francisco Bay Area patients with coronary heart disease who were assessed annually for depressive symptoms for 6 years. The 65% of patients who were on statin therapy, albeit in nonrandomized fashion, had an adjusted 38% lower risk of developing depression (J Clin Psychiatry. 2012 May;73[5]:610-5).
His was one of seven observational studies involving more than 9,000 patients included in a subsequent meta-analysis showing that statin users were 37% less likely to develop depression than were nonusers (J Affect Disord. 2014 May;160:62-7).
All agreed that the verdict isn’t in yet as to statins’ effectiveness as adjunctive antidepressants, and that the subgroup of patients with major depression who are most likely to gain added antidepressive effect from a statin are those with what the speakers variously described as comorbid cardiometabolic disease, immunometabolic disease, or simply, as in SIMCODE, obesity. These are patients with a high degree of systemic inflammation, which often makes their depression less responsive to standard antidepressant therapies. The working hypothesis is that the pleiotropic anti-inflammatory effects of statins will result in a greater response to conventional antidepressants.
Animal studies point to multiple potential mechanisms by which statins might have antidepressant efficacy in clinical practice, according to Dr. Otte. Beyond their anti-inflammatory effects, these include the drugs’ documented effects on glutamatergic N-methyl-D-aspartate (NMDA) receptors, dopamine receptors, brain-derived neurotrophic factor, glucocorticoid receptors, and hippocampal serotonin 2A receptors.
Ole Kohler, MD, a psychiatrist at Aarhus (Denmark) University, presented highlights of his eye-popping population-based study of more than 872,000 Danes on an SSRI in 1997-2012, more than 113,000 of whom were on a concomitant statin. The key finding: During roughly 3 years of follow-up, the risk of contact with a psychiatric hospital for depression was 36% lower in the group on concomitant SSRI/statin therapy than in those not on a statin (Am J Psychiatry. 2016 Aug 1;173[8]:807-15).
He was quick to observe that a study such as this is vulnerable to various forms of confounding. This risk can be mitigated to a considerable extent by careful propensity score matching. Of note, however, none of the three studies that have been conducted with propensity score matching, including his own recent study of nearly 194,000 statin users and an equal number of matched nonusers, showed a difference in risk of depression between statin users and nonusers. All three studies were performed in general populations without known depression, leading Dr. Kohler to conclude that it’s unlikely that statins have a role in preventing depression in nondepressed individuals.
The focus should instead be on the possible role of statins in reducing the risk of depression in patients with cardiometabolic disease – that is, heart disease, metabolic syndrome, or type 2 diabetes – where more than a half-dozen cohort studies, including the Heart and Soul Study, have found that statins have a favorable impact, he added.
Estela Salagre, MD, a psychiatrist at the University of Barcelona, has carried out a meta-analysis of the three randomized, double-blind, placebo-controlled trials of add-on statin therapy in patients on standard therapies for moderate to severe depression published to date. She found that statin therapy was associated with a 27% greater reduction in scores on the Hamilton Depression Rating Scale, compared with placebo (J Affect Disord. 2016 Aug;200:235-42). Those findings recently were confirmed in a separate meta-analysis by other investigators using different methodologies (J Affect Disord. 2019 Oct 1;257:55-63).
Femke Lamers, PhD, presented evidence based on the nearly 3,000-subject longitudinal Netherlands Study of Depression and Anxiety that roughly one-quarter of individuals with major depressive disorder have a distinct subtype of nonmelancholic depression characterized by a clustering of obesity, inflammation, increased appetite, fatigue, hypersomnia, and increased levels of insulin and leptin. She calls it immunometabolic depression. She and her coinvestigators in the international Psychiatric Genomics Consortium have demonstrated that this phenotypic clustering is associated with a shared genetic vulnerability between major depression and obesity (JAMA Psychiatry. 2017 Dec 1;74[12]:1214-25).
“Major depressive disorder is not a one-size-fits-all disorder. There is an immunometabolic form of depression,” declared Dr. Lamers, an epidemiologist at the University of Amsterdam.
All speakers reported having no financial conflicts of interest.
REPORTING FROM ECNP 2019
Oral drug for postpartum depression aces phase 3 trial
COPENHAGEN – A first-in-class, once-daily, orally administered neuroactive steroid known for now as SAGE-217 aced all of its primary and secondary outcomes for the treatment of postpartum depression in the phase 3, randomized, double-blind, placebo-controlled ROBIN study, Eduard Vieta, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
“I think this changes the paradigm in the treatment of postpartum depression,” declared Dr. Vieta, professor of psychiatry and head of the bipolar disorders program at the University of Barcelona.
Like brexanolone (Zulresso), an intravenous formulation of allopregnanolone approved by the Food and Drug Administration in March 2019 as the first-ever drug specifically targeting postpartum depression, SAGE-217 is a positive allosteric modifier of synaptic and extrasynaptic GABA-A receptors. That differentiates the two drugs from benzodiazepines, which target only synaptic receptors. Both brexanolone and SAGE-217 are drugs developed by Sage Therapeutics. But SAGE-217, an investigational agent, is vastly more convenient to use than brexanolone since, as an oral drug, it doesn’t require hospitalization for intravenous administration.
Dr. Vieta ticked off five reasons why he considers SAGE-217 a game changer in the treatment of postpartum depression: “It’s an amazingly effective compound, with an effect size that’s bigger than we usually see with antidepressants. It has an early onset of action, similar to what we see with glutaminergic agents, although with an opposite mechanism: enhancing GABA rather than opposing glutamate. It has excellent tolerability, similar to placebo. It’s made to be used orally, a major advantage over other drugs that are available or close to becoming available, which have to be given IV. And last but not least, a patient will get it for only 2 weeks. The treatment can be stopped after 2 weeks, and there is long-term improvement.”
The ROBIN trial included 151 patients with postpartum depression as defined by a baseline Hamilton Rating Scale for Depression (HAM-D) score of at least 26 who were randomized double-blind to 14 days of SAGE-217 at 30 mg once daily or to placebo. The primary endpoint was the change in HAM-D scores between baseline and day 15. The key finding was that the SAGE-217 group averaged a 17.8-point reduction, significantly greater than the 13.6-point improvement with placebo. This advantage was maintained at assessment on day 45 – a full month after treatment stopped – with a 24.8-point improvement over baseline in the SAGE-217 recipients, compared with a 19-point reduction in controls. The advantage favoring SAGE-217 was significant as early as day 3, the first assessment, at which point the average improvement in HAM-D was 12.5 points, compared with 9.8 points in controls.
Other secondary endpoints included change from baseline to day 15 on the Montgomery-Åsberg Depression Rating Scale (MADRS): a 22.8-point improvement in the SAGE-217 group, significantly greater than the 17.6-point improvement in the placebo arm. The same pattern was evident at day 45, with reductions in MADRS of 24.8 and 19 points, respectively, in the SAGE-217 and placebo groups.
Another key prespecified secondary endpoint was change in scores on the Hamilton Rating Scale for Anxiety through day 15. There was a mean 16.6-point drop in the active treatment arm, compared with a 12.7-point improvement with placebo, again a statistically significant and clinically meaningful between-group difference. This is an important endpoint because comorbid anxiety is common in the setting of postpartum depression, the psychiatrist continued.
The SAGE-217 group also demonstrated significantly higher rates of HAM-D response as defined by a 50% or greater reduction in total score at day 15, as well as in HAM-D remission, which entails having a score of 7 or less.
Treatment-emergent adverse events in the SAGE-217 and placebo arms were similar in frequency and type. The most common adverse events associated with SAGE-217 – all occurring in single-digit frequencies – were sleepiness, headache, dizziness, upper respiratory infections, and diarrhea. There was no signal of increased suicidal thoughts or behavior as assessed using the Columbia Suicide Severity Rating Scale.
SAGE-217 also is the focus of an ongoing pivotal phase 3 trial in patients with major depression. In addition, the drug is under study for bipolar depression, major depressive disorder with comorbid insomnia, and generalized anxiety disorder.
Dr. Vieta reported serving on advisory boards for Sage Therapeutics, the study sponsor, as well as for two dozen other pharmaceutical companies. He receives research funding from the Spanish Ministry of Science and Education, the Stanley Medical Research Institute, and more than a dozen pharmaceutical companies.
COPENHAGEN – A first-in-class, once-daily, orally administered neuroactive steroid known for now as SAGE-217 aced all of its primary and secondary outcomes for the treatment of postpartum depression in the phase 3, randomized, double-blind, placebo-controlled ROBIN study, Eduard Vieta, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
“I think this changes the paradigm in the treatment of postpartum depression,” declared Dr. Vieta, professor of psychiatry and head of the bipolar disorders program at the University of Barcelona.
Like brexanolone (Zulresso), an intravenous formulation of allopregnanolone approved by the Food and Drug Administration in March 2019 as the first-ever drug specifically targeting postpartum depression, SAGE-217 is a positive allosteric modifier of synaptic and extrasynaptic GABA-A receptors. That differentiates the two drugs from benzodiazepines, which target only synaptic receptors. Both brexanolone and SAGE-217 are drugs developed by Sage Therapeutics. But SAGE-217, an investigational agent, is vastly more convenient to use than brexanolone since, as an oral drug, it doesn’t require hospitalization for intravenous administration.
Dr. Vieta ticked off five reasons why he considers SAGE-217 a game changer in the treatment of postpartum depression: “It’s an amazingly effective compound, with an effect size that’s bigger than we usually see with antidepressants. It has an early onset of action, similar to what we see with glutaminergic agents, although with an opposite mechanism: enhancing GABA rather than opposing glutamate. It has excellent tolerability, similar to placebo. It’s made to be used orally, a major advantage over other drugs that are available or close to becoming available, which have to be given IV. And last but not least, a patient will get it for only 2 weeks. The treatment can be stopped after 2 weeks, and there is long-term improvement.”
The ROBIN trial included 151 patients with postpartum depression as defined by a baseline Hamilton Rating Scale for Depression (HAM-D) score of at least 26 who were randomized double-blind to 14 days of SAGE-217 at 30 mg once daily or to placebo. The primary endpoint was the change in HAM-D scores between baseline and day 15. The key finding was that the SAGE-217 group averaged a 17.8-point reduction, significantly greater than the 13.6-point improvement with placebo. This advantage was maintained at assessment on day 45 – a full month after treatment stopped – with a 24.8-point improvement over baseline in the SAGE-217 recipients, compared with a 19-point reduction in controls. The advantage favoring SAGE-217 was significant as early as day 3, the first assessment, at which point the average improvement in HAM-D was 12.5 points, compared with 9.8 points in controls.
Other secondary endpoints included change from baseline to day 15 on the Montgomery-Åsberg Depression Rating Scale (MADRS): a 22.8-point improvement in the SAGE-217 group, significantly greater than the 17.6-point improvement in the placebo arm. The same pattern was evident at day 45, with reductions in MADRS of 24.8 and 19 points, respectively, in the SAGE-217 and placebo groups.
Another key prespecified secondary endpoint was change in scores on the Hamilton Rating Scale for Anxiety through day 15. There was a mean 16.6-point drop in the active treatment arm, compared with a 12.7-point improvement with placebo, again a statistically significant and clinically meaningful between-group difference. This is an important endpoint because comorbid anxiety is common in the setting of postpartum depression, the psychiatrist continued.
The SAGE-217 group also demonstrated significantly higher rates of HAM-D response as defined by a 50% or greater reduction in total score at day 15, as well as in HAM-D remission, which entails having a score of 7 or less.
Treatment-emergent adverse events in the SAGE-217 and placebo arms were similar in frequency and type. The most common adverse events associated with SAGE-217 – all occurring in single-digit frequencies – were sleepiness, headache, dizziness, upper respiratory infections, and diarrhea. There was no signal of increased suicidal thoughts or behavior as assessed using the Columbia Suicide Severity Rating Scale.
SAGE-217 also is the focus of an ongoing pivotal phase 3 trial in patients with major depression. In addition, the drug is under study for bipolar depression, major depressive disorder with comorbid insomnia, and generalized anxiety disorder.
Dr. Vieta reported serving on advisory boards for Sage Therapeutics, the study sponsor, as well as for two dozen other pharmaceutical companies. He receives research funding from the Spanish Ministry of Science and Education, the Stanley Medical Research Institute, and more than a dozen pharmaceutical companies.
COPENHAGEN – A first-in-class, once-daily, orally administered neuroactive steroid known for now as SAGE-217 aced all of its primary and secondary outcomes for the treatment of postpartum depression in the phase 3, randomized, double-blind, placebo-controlled ROBIN study, Eduard Vieta, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
“I think this changes the paradigm in the treatment of postpartum depression,” declared Dr. Vieta, professor of psychiatry and head of the bipolar disorders program at the University of Barcelona.
Like brexanolone (Zulresso), an intravenous formulation of allopregnanolone approved by the Food and Drug Administration in March 2019 as the first-ever drug specifically targeting postpartum depression, SAGE-217 is a positive allosteric modifier of synaptic and extrasynaptic GABA-A receptors. That differentiates the two drugs from benzodiazepines, which target only synaptic receptors. Both brexanolone and SAGE-217 are drugs developed by Sage Therapeutics. But SAGE-217, an investigational agent, is vastly more convenient to use than brexanolone since, as an oral drug, it doesn’t require hospitalization for intravenous administration.
Dr. Vieta ticked off five reasons why he considers SAGE-217 a game changer in the treatment of postpartum depression: “It’s an amazingly effective compound, with an effect size that’s bigger than we usually see with antidepressants. It has an early onset of action, similar to what we see with glutaminergic agents, although with an opposite mechanism: enhancing GABA rather than opposing glutamate. It has excellent tolerability, similar to placebo. It’s made to be used orally, a major advantage over other drugs that are available or close to becoming available, which have to be given IV. And last but not least, a patient will get it for only 2 weeks. The treatment can be stopped after 2 weeks, and there is long-term improvement.”
The ROBIN trial included 151 patients with postpartum depression as defined by a baseline Hamilton Rating Scale for Depression (HAM-D) score of at least 26 who were randomized double-blind to 14 days of SAGE-217 at 30 mg once daily or to placebo. The primary endpoint was the change in HAM-D scores between baseline and day 15. The key finding was that the SAGE-217 group averaged a 17.8-point reduction, significantly greater than the 13.6-point improvement with placebo. This advantage was maintained at assessment on day 45 – a full month after treatment stopped – with a 24.8-point improvement over baseline in the SAGE-217 recipients, compared with a 19-point reduction in controls. The advantage favoring SAGE-217 was significant as early as day 3, the first assessment, at which point the average improvement in HAM-D was 12.5 points, compared with 9.8 points in controls.
Other secondary endpoints included change from baseline to day 15 on the Montgomery-Åsberg Depression Rating Scale (MADRS): a 22.8-point improvement in the SAGE-217 group, significantly greater than the 17.6-point improvement in the placebo arm. The same pattern was evident at day 45, with reductions in MADRS of 24.8 and 19 points, respectively, in the SAGE-217 and placebo groups.
Another key prespecified secondary endpoint was change in scores on the Hamilton Rating Scale for Anxiety through day 15. There was a mean 16.6-point drop in the active treatment arm, compared with a 12.7-point improvement with placebo, again a statistically significant and clinically meaningful between-group difference. This is an important endpoint because comorbid anxiety is common in the setting of postpartum depression, the psychiatrist continued.
The SAGE-217 group also demonstrated significantly higher rates of HAM-D response as defined by a 50% or greater reduction in total score at day 15, as well as in HAM-D remission, which entails having a score of 7 or less.
Treatment-emergent adverse events in the SAGE-217 and placebo arms were similar in frequency and type. The most common adverse events associated with SAGE-217 – all occurring in single-digit frequencies – were sleepiness, headache, dizziness, upper respiratory infections, and diarrhea. There was no signal of increased suicidal thoughts or behavior as assessed using the Columbia Suicide Severity Rating Scale.
SAGE-217 also is the focus of an ongoing pivotal phase 3 trial in patients with major depression. In addition, the drug is under study for bipolar depression, major depressive disorder with comorbid insomnia, and generalized anxiety disorder.
Dr. Vieta reported serving on advisory boards for Sage Therapeutics, the study sponsor, as well as for two dozen other pharmaceutical companies. He receives research funding from the Spanish Ministry of Science and Education, the Stanley Medical Research Institute, and more than a dozen pharmaceutical companies.
REPORTING FROM THE ECNP 2019
SAGE-217 shows reduction in depression with no safety concerns
A new oral antidepressant that targets the gamma-aminobutyric acid type A (GABAA) receptors in the brain has been found to achieve a reduction in symptoms in adult patients with moderate to severe major depressive disorder, with no serious safety signals, results of a double-blind, phase 2 trial show.
The study involved 89 participants with major depression, excluding those with a history of treatment-resistant depression, who were randomized either to a once-daily dose of 30 mg of SAGE-217, a synthetic neurosteroid that acts as a positive allosteric modulator of GABAA receptors, or placebo for 14 days. Thirty-six of the 45 patients in the SAGE-217 group were black, as were 28 of the 44 patients in the placebo group, reported Handan Gunduz-Bruce, MD, of Sage Therapeutics and coauthors. Their study was published in the New England Journal of Medicine.
“One hypothesis for the mechanism of depression implicates deficits in gamma-aminobutyric acid and downstream alterations in monoaminergic neurotransmission,” wrote Dr. Gunduz-Bruce and coauthors. “Preclinical studies have shown that the naturally occurring neurosteroid allopregnanolone is a positive allosteric modulator of synaptic and extrasynaptic GABAA receptors that affects both phasic and tonic inhibition of neurons.”
At day 15 of the study, there was a significantly greater mean change from baseline in Hamilton Depression Rating Scale scores in the treatment group, compared with the placebo group (–17.4 vs. –10.3, P less than .001), and 79% of participants in the treatment arm showed a greater than 50% reduction in depression scores, compared with 41% of the placebo group.
At day 28, 62% of the treatment group and 46% of the placebo group had a reduction of more than 50% from baseline depression scores.
No serious or severe adverse events were seen in either group, and the most common adverse events in the SAGE-217 group included headache (18%), dizziness (11%), and nausea (11%). One patient in the treatment arm also reported euphoria.
The authors commented that somnolence and sedation were expected adverse events, based on the pharmacological properties of SAGE-217.
Six patients in the treatment arm also had dose reductions as a result of adverse events. Two patients in the SAGE-217 arm stopped treatment because they met prespecified criteria for discontinuation; the investigators reported nausea, dizziness, and headache in one patient, and increased levels of alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyltransferase in the other. However, the second patient had shown mildly elevated values of these at baseline, was asymptomatic throughout the trial, and the patient’s values returned to baseline or near-baseline after stopping treatment.
About one-quarter of both the SAGE-217 and placebo groups were receiving antidepressant treatment at baseline (27% and 23% respectively), with the duration of prior treatment ranging from 2 to 48 months. Investigators also gave three patients in the treatment arm and 11 in the placebo arm concomitant antidepressants during the follow-up period.
The small sample size and limited racial diversity among the participants were cited as limitations.
The study was supported by SAGE-217 manufacturer Sage Therapeutics. Ten authors were employees or directors of Sage Therapeutics, with stock options and patent interests. Three authors declared grants, personal fees, or advisory board positions with the pharmaceutical sector, including from Sage, and one also declared interest in a range of patents outside the study. One author had no disclosures.
SOURCE: Gunduz-Bruce H et al. N Engl J Med. 2019 Sep 5;381:903-11. doi: 10.1056/NEJMoa1815981.
Glutamate modulators, such as ketamine, recently have been found to achieve a rapid reduction in depressive symptoms – often within 24 hours. This is a significant development given that most existing antidepressants do not work quickly, and time is critical for patients with suicidal ideation.
This trial of SAGE-217 also shows a more rapid clinical response than is typical of existing antidepressants. However, the absence of a significant difference between the treatment and placebo arm in change of depression scores from baseline to day 28 suggests that the drug should be administered for longer than 14 days. It is also important to note that the trial excluded patients with treatment-resistant depression.
Emil F. Coccaro, MD, is affiliated with the department of psychiatry and behavioral neuroscience at the University of Chicago. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Sep 5;381:980-1. doi: 10.1056/NEJMe1907638). Dr. Coccaro declared grants from the National Institutes of Health and personal fees or stock options in the pharmaceutical sector.
Glutamate modulators, such as ketamine, recently have been found to achieve a rapid reduction in depressive symptoms – often within 24 hours. This is a significant development given that most existing antidepressants do not work quickly, and time is critical for patients with suicidal ideation.
This trial of SAGE-217 also shows a more rapid clinical response than is typical of existing antidepressants. However, the absence of a significant difference between the treatment and placebo arm in change of depression scores from baseline to day 28 suggests that the drug should be administered for longer than 14 days. It is also important to note that the trial excluded patients with treatment-resistant depression.
Emil F. Coccaro, MD, is affiliated with the department of psychiatry and behavioral neuroscience at the University of Chicago. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Sep 5;381:980-1. doi: 10.1056/NEJMe1907638). Dr. Coccaro declared grants from the National Institutes of Health and personal fees or stock options in the pharmaceutical sector.
Glutamate modulators, such as ketamine, recently have been found to achieve a rapid reduction in depressive symptoms – often within 24 hours. This is a significant development given that most existing antidepressants do not work quickly, and time is critical for patients with suicidal ideation.
This trial of SAGE-217 also shows a more rapid clinical response than is typical of existing antidepressants. However, the absence of a significant difference between the treatment and placebo arm in change of depression scores from baseline to day 28 suggests that the drug should be administered for longer than 14 days. It is also important to note that the trial excluded patients with treatment-resistant depression.
Emil F. Coccaro, MD, is affiliated with the department of psychiatry and behavioral neuroscience at the University of Chicago. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Sep 5;381:980-1. doi: 10.1056/NEJMe1907638). Dr. Coccaro declared grants from the National Institutes of Health and personal fees or stock options in the pharmaceutical sector.
A new oral antidepressant that targets the gamma-aminobutyric acid type A (GABAA) receptors in the brain has been found to achieve a reduction in symptoms in adult patients with moderate to severe major depressive disorder, with no serious safety signals, results of a double-blind, phase 2 trial show.
The study involved 89 participants with major depression, excluding those with a history of treatment-resistant depression, who were randomized either to a once-daily dose of 30 mg of SAGE-217, a synthetic neurosteroid that acts as a positive allosteric modulator of GABAA receptors, or placebo for 14 days. Thirty-six of the 45 patients in the SAGE-217 group were black, as were 28 of the 44 patients in the placebo group, reported Handan Gunduz-Bruce, MD, of Sage Therapeutics and coauthors. Their study was published in the New England Journal of Medicine.
“One hypothesis for the mechanism of depression implicates deficits in gamma-aminobutyric acid and downstream alterations in monoaminergic neurotransmission,” wrote Dr. Gunduz-Bruce and coauthors. “Preclinical studies have shown that the naturally occurring neurosteroid allopregnanolone is a positive allosteric modulator of synaptic and extrasynaptic GABAA receptors that affects both phasic and tonic inhibition of neurons.”
At day 15 of the study, there was a significantly greater mean change from baseline in Hamilton Depression Rating Scale scores in the treatment group, compared with the placebo group (–17.4 vs. –10.3, P less than .001), and 79% of participants in the treatment arm showed a greater than 50% reduction in depression scores, compared with 41% of the placebo group.
At day 28, 62% of the treatment group and 46% of the placebo group had a reduction of more than 50% from baseline depression scores.
No serious or severe adverse events were seen in either group, and the most common adverse events in the SAGE-217 group included headache (18%), dizziness (11%), and nausea (11%). One patient in the treatment arm also reported euphoria.
The authors commented that somnolence and sedation were expected adverse events, based on the pharmacological properties of SAGE-217.
Six patients in the treatment arm also had dose reductions as a result of adverse events. Two patients in the SAGE-217 arm stopped treatment because they met prespecified criteria for discontinuation; the investigators reported nausea, dizziness, and headache in one patient, and increased levels of alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyltransferase in the other. However, the second patient had shown mildly elevated values of these at baseline, was asymptomatic throughout the trial, and the patient’s values returned to baseline or near-baseline after stopping treatment.
About one-quarter of both the SAGE-217 and placebo groups were receiving antidepressant treatment at baseline (27% and 23% respectively), with the duration of prior treatment ranging from 2 to 48 months. Investigators also gave three patients in the treatment arm and 11 in the placebo arm concomitant antidepressants during the follow-up period.
The small sample size and limited racial diversity among the participants were cited as limitations.
The study was supported by SAGE-217 manufacturer Sage Therapeutics. Ten authors were employees or directors of Sage Therapeutics, with stock options and patent interests. Three authors declared grants, personal fees, or advisory board positions with the pharmaceutical sector, including from Sage, and one also declared interest in a range of patents outside the study. One author had no disclosures.
SOURCE: Gunduz-Bruce H et al. N Engl J Med. 2019 Sep 5;381:903-11. doi: 10.1056/NEJMoa1815981.
A new oral antidepressant that targets the gamma-aminobutyric acid type A (GABAA) receptors in the brain has been found to achieve a reduction in symptoms in adult patients with moderate to severe major depressive disorder, with no serious safety signals, results of a double-blind, phase 2 trial show.
The study involved 89 participants with major depression, excluding those with a history of treatment-resistant depression, who were randomized either to a once-daily dose of 30 mg of SAGE-217, a synthetic neurosteroid that acts as a positive allosteric modulator of GABAA receptors, or placebo for 14 days. Thirty-six of the 45 patients in the SAGE-217 group were black, as were 28 of the 44 patients in the placebo group, reported Handan Gunduz-Bruce, MD, of Sage Therapeutics and coauthors. Their study was published in the New England Journal of Medicine.
“One hypothesis for the mechanism of depression implicates deficits in gamma-aminobutyric acid and downstream alterations in monoaminergic neurotransmission,” wrote Dr. Gunduz-Bruce and coauthors. “Preclinical studies have shown that the naturally occurring neurosteroid allopregnanolone is a positive allosteric modulator of synaptic and extrasynaptic GABAA receptors that affects both phasic and tonic inhibition of neurons.”
At day 15 of the study, there was a significantly greater mean change from baseline in Hamilton Depression Rating Scale scores in the treatment group, compared with the placebo group (–17.4 vs. –10.3, P less than .001), and 79% of participants in the treatment arm showed a greater than 50% reduction in depression scores, compared with 41% of the placebo group.
At day 28, 62% of the treatment group and 46% of the placebo group had a reduction of more than 50% from baseline depression scores.
No serious or severe adverse events were seen in either group, and the most common adverse events in the SAGE-217 group included headache (18%), dizziness (11%), and nausea (11%). One patient in the treatment arm also reported euphoria.
The authors commented that somnolence and sedation were expected adverse events, based on the pharmacological properties of SAGE-217.
Six patients in the treatment arm also had dose reductions as a result of adverse events. Two patients in the SAGE-217 arm stopped treatment because they met prespecified criteria for discontinuation; the investigators reported nausea, dizziness, and headache in one patient, and increased levels of alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyltransferase in the other. However, the second patient had shown mildly elevated values of these at baseline, was asymptomatic throughout the trial, and the patient’s values returned to baseline or near-baseline after stopping treatment.
About one-quarter of both the SAGE-217 and placebo groups were receiving antidepressant treatment at baseline (27% and 23% respectively), with the duration of prior treatment ranging from 2 to 48 months. Investigators also gave three patients in the treatment arm and 11 in the placebo arm concomitant antidepressants during the follow-up period.
The small sample size and limited racial diversity among the participants were cited as limitations.
The study was supported by SAGE-217 manufacturer Sage Therapeutics. Ten authors were employees or directors of Sage Therapeutics, with stock options and patent interests. Three authors declared grants, personal fees, or advisory board positions with the pharmaceutical sector, including from Sage, and one also declared interest in a range of patents outside the study. One author had no disclosures.
SOURCE: Gunduz-Bruce H et al. N Engl J Med. 2019 Sep 5;381:903-11. doi: 10.1056/NEJMoa1815981.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Taking SAGE-217 – a new oral antidepressant – for 14 days leads to reductions in depressive symptoms at day 15.
Major finding: Treatment with SAGE-217 was associated with significantly greater improvements in depression scores, compared with placebo.
Study details: Phase 2, randomized, placebo-controlled trial in 89 patients with major depression.
Disclosures: The study was supported by SAGE-217 manufacturer Sage Therapeutics. Ten authors were employees or directors of Sage Therapeutics, with stock options and patent interests. Three authors declared grants, personal fees, or advisory board positions with the pharmaceutical sector, including from Sage, and one also declared interest in a range of patents outside the study. One author had no disclosures.
Source: Gunduz-Bruce H et al. N Engl J Med. 2019 Sep 5;381:903-11. doi: 10.1056/NEJMoa1815981.
Brexanolone injection for postpartum depression
Postpartum depression (PPD) is one of the most prevalent complications associated with pregnancy and childbirth in the United States, affecting more than 400,000 women annually.1 Postpartum depression is most commonly treated with psychotherapy and antidepressants approved for the treatment of major depressive disorder. Until recently, there was no pharmacologic therapy approved by the FDA specifically for the treatment of PPD. Considering the adverse outcomes associated with untreated or inadequately treated PPD, and the limitations of existing therapies, there is a significant unmet need for pharmacologic treatment options for PPD.2 To help address this need, the FDA recently approved brexanolone injection (brand name: ZULRESSO™) (Table 13) as a first-in-class therapy for the treatment of adults with PPD.3
Clinical implications
Postpartum depression can result in adverse outcomes for the patient, baby, and family when under- or untreated, and the need for rapid resolution of symptoms cannot be overstated.2 Suicide is strongly associated with depression and is a leading cause of pregnancy-related deaths.4 Additionally, PPD can impact the health, safety, and well-being of the child, with both short- and long-term consequences, including greater rates of psychological or behavioral difficulties among children of patients with PPD.5 Postpartum depression can also have negative effects on the patient’s partner, with 24% to 50% of partners experiencing depression.6 Current PPD management strategies include the use of psychotherapy and pharmacologic interventions for major depressive disorder that may take up to 4 to 6 weeks for some patients, and may not achieve remission for all patients.7-9
Brexanolone injection is a first-in-class medication with a novel mechanism of action. In clinical studies, it achieved rapid (by Hour 60) and sustained (through Day 30) reductions in depressive symptoms and could provide a meaningful new treatment option for adult women with PPD.10,11
How it works
Animal and human studies have established the re
Brexanolone is a neuroactive steroid that is chemically identical to endogenous allopregnanolone produced in the CNS. Brexanolone potentiates GABA-mediated currents from recombinant human GABAARs in mammalian cells expressing α1β2γ2 receptor subunits, α4β3δ receptor subunits, and α6β3δ receptor subunits.3 Positive allosteric modulation of both synaptic and extrasynaptic GABAARs differentiates brexanolone from other GABAAR modulators, such as benzodiazepines.10,11
Brexanolone’s mechanism of action in the treatment of PPD is not fully understood, but it is thought to be related to GABAAR PAM activity.3
Supporting evidence
The FDA approval of brexanolone injection was based on the efficacy demonstrated in 2 Phase III multicenter, randomized, double-blind, placebo-controlled studies in adult women (age 18 to 45) with PPD (defined by DSM-IV criteria for a major depressive episode, with onset of symptoms in the third trimester or within 4 weeks of delivery). Exclusion criteria included the presence of bipolar disorder or psychosis. In these studies, 60-hour continuous IV infusions of brexanolone or placebo were given, followed by 4 weeks of observation. Study 1 (202B) enrolled patients with severe PPD (Hamilton Rating Scale for Depression [HAM-D] total score ≥26), and Study 2 (202C) enrolled patients with moderate PPD (HAM-D score 20 to 25). A titration to the recommended target dosage of 90 μg/kg/hour was evaluated in both studies. BRX90 patients received 30 μg/kg/hour for 4 hours, 60 μg/kg/hour for 20 hours, 90 μg/kg/hour for 28 hours, followed by a taper to 60 μg/kg/hour for 4 hours and then 30 μg/kg/hour for 4 hours. The primary endpoint in both studies was the mean change from baseline in depressive symptoms as measured by HAM-D total score at the end of the 60-hour infusion. A pre-specified secondary efficacy endpoint was the mean change from baseline in HAM-D total score at Day 30.
Continue to: Efficacy
Efficacy. In both placebo-controlled studies, titration to a target dose of brexanolone 90 μg/kg/hour was superior to placebo in improvement of depressive symptoms (Table 33).
Pharmacological profile
Brexanolone exposure-response relationships and the time course of pharmacodynamic response are unknown.3
Adverse reactions. Safety was evaluated from all patients receiving brexanolone injection, regardless of dosing regimen (N = 140, including patients from a Phase IIb study, 202A).3,11
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were sedation/somnolence, dry mouth, loss of consciousness, and flushing/hot flush.3 The incidence of patients discontinuing due to any adverse reaction was 2% for brexanolone vs 1% for placebo.3
Sedation, somnolence, and loss of consciousness. In clinical studies, brexanolone caused sedation and somnolence that required dose interruption or reduction in some patients during the infusion (5% of brexanolone-treated patients compared with 0% of placebo-treated patients).3 Some patients were also reported to have loss of consciousness or altered state of consciousness during the brexanolone infusion (4% of patients treated with brexanolone compared with 0% of patients treated with placebo).3 All patients with loss of or altered state of consciousness recovered fully 15 to 60 minutes after dose interruption.3 There was no clear association between loss or alteration of consciousness and pattern or timing of dose, and not all patients who experienced a loss or alteration of consciousness reported sedation or somnolence before the episode.
Continue to: Suicidality
Suicidality. The risk of developing suicidal thoughts and behaviors with brexanolone is unknown, due to the relatively low number of exposures to brexanolone injection during clinical development and a mechanism of action distinct from that of existing antidepressant medications.3
Pharmacokinetics
In clinical trials, brexanolone exhibited dose-proportional pharmacokinetics, and the terminal half-life is approximately 9 hours (Table 43). Brexanolone is metabolized by non-cytochrome P450 (CYP)-based pathways, including keto-reduction, glucuronidation, and sulfation.3 No clinically significant differences in the pharmacokinetics of brexanolone were observed based on renal or hepatic impairment, and no studies were conducted to evaluate the effects of other drugs on brexanolone.3
Lactation. A population pharmacokinetics model constructed from studies in the clinical development program calculated the maximum relative infant dose for brexanolone during infusion as 1.3%.3 Given the low oral bioavailability of brexanolone (<5%) in adults, the potential for breastfed infant exposure is considered low.3
Clinical considerations
Risk Evaluation and Mitigation Strategies (REMS) requirements. Brexanolone injection is a Schedule IV controlled substance. It has a “black-box” warning regarding excessive sedation and sudden loss of consciousness, which has been taken into account within the REMS drug safety program. Health care facilities and pharmacies must enroll in the REMS program and ensure that brexanolone is administered only to patients who are enrolled in the REMS program. Staff must be trained on the processes and procedures to administer brexanolone, and the facility must have a fall precautions protocol in place and be equipped with a programmable peristaltic IV infusion pump and continuous pulse oximetry with alarms.3
Monitoring. A REMS-trained clinician must be available continuously on-site to oversee each patient for the duration of the continuous IV infusion, which lasts 60 hours (2.5 days) and should be initiated early enough in the day to allow for recognition of excessive sedation. Patients must be monitored for hypoxia using continuous pulse oximetry equipped with an alarm and should also be assessed for excessive sedation every 2 hours during planned, non-sleep periods. If excessive sedation occurs, the infusion should be stopped until symptoms resolve, after which the infusion may be resumed at the same or a lower dose as clinically appropriate. In case of overdosage, the infusion should be stopped immediately and supportive measures initiated as necessary. Patients must not be the primary caregiver of dependents, and must be accompanied during interactions with their child(ren).
Continue to: Contraindications
Contraindications. There are no contraindications for the use of brexanolone in adults with PPD.
End-stage renal disease (ESRD). Avoid using brexanolone in patients with ESRD because of the potential accumulation of the solubilizing agent, betadex sulfobutyl ether sodium.
Pregnancy. Brexanolone has not been studied in pregnant patients. Pregnant women and women of reproductive age should be informed of the potential risk to a fetus based on data from other drugs that enhance GABAergic inhibition.
Breastfeeding. There are no data on the effects of brexanolone on a breastfed infant. Breastfeeding should be a discussion of risk and benefit between the patient and her doctor. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for brexanolone and any potential adverse effects on the breastfed child from brexanolone or from the underlying maternal condition. However, based on the low relative infant dose (<2%) and the low oral bioavailability in adults, the risk to breastfed infants is thought to be low.16
Potential for abuse. Brexanolone injection is a Schedule IV controlled substance. Although it was not possible to assess physical dependency in the registrational trials due to dose tapering at the end of treatment, clinicians should advise patients about the theoretical possibility for brexanolone to be abused or lead to dependence based on other medications with similar primary pharmacology.
Continue to: Concomitant medications
Concomitant medications. Caution patients that taking opioids or other CNS depressants, such as benzodiazepines, in combination with brexanolone may increase the severity of sedative effects.
Suicidal thoughts and behaviors. Advise patients and caregivers to look for the emergence of suicidal thoughts and behavior and instruct them to report such symptoms to their clinician. Consider changing the therapeutic regimen, including discontinuing brexanolone, in patients whose depression becomes worse or who experience emergent suicidal thoughts and behaviors.
Why Rx?
Postpartum depression is a common and often devastating medical complication of childbirth that can result in adverse outcomes for the patient, baby, and family when left undertreated or untreated. There is a great need to identify and treat women who develop PPD. Rapid and sustained resolution of symptoms in women who experience PPD should be the goal of treatment, and consequently, brexanolone injection presents an important new tool in available treatment options for PPD.
Bottom Line
Brexanolone injection is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive allosteric modulator that’s been FDA-approved for the treatment of postpartum depression (PPD). It is administered as a continuous IV infusion over 60 hours. The rapid and sustained improvement of PPD observed in clinical trials with brexanolone injection may support a new treatment paradigm for women with PPD.
1. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms - 27 states, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66(6):153-158.
2. Frieder A, Fersh M, Hainline R, et al. Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs. 2019;33(3):265-282.
3. Brexanolone injection [package insert]. Cambridge, MA: Sage Therapeutics, Inc.; 2019.
4. Bodnar-Deren S, Klipstein K, Fersh M, et al. Suicidal ideation during the postpartum period. J Womens Health (Larchmt). 2016;25(12):1219-1224.
5. Netsi E, Pearson RM, Murray L, et al. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry. 2018;75(3):247-253.
6. Goodman JH. Paternal postpartum depression, its relationship to maternal postpartum depression, and implications for family health. J Adv Nurs. 2004;45(1):26-35.
7. Gelenberg AJ, Freeman MP, Markowitz JC, et al; American Psychiatric Association Work Group on Major Depressive Disorder. Practice guidelines for the treatment of patients with major depressive disorder. 3rd ed. Washington, DC: American Psychiatric Association; 2010.
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
9. Molyneaux E, Telesia LA, Henshaw C, et al. Antidepressants for preventing postnatal depression. Cochrane Database Syst Rev. 2018;4:CD004363.
10. Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480-489.
11. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
12. Melon LC, Hooper A, Yang X, et al. Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice. Psychoneuroendocrinology. 2018;90:182-193.
13. Deligiannidis KM, Fales CL, Kroll-Desrosiers AR, et al. Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2019;44(3):546-554.
14. Licheri V, Talani G, Gorule AA, et al. Plasticity of GABAA receptors during pregnancy and postpartum period: from gene to function. Neural Plast. 2015;2015:170435. doi: 10.1155/2015/170435.
15. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
16. Hoffmann E, Wald J, Dray D, et al. Brexanolone injection administration to lactating women: breast milk allopregnanolone levels [30J]. Obstetrics & Gynecology. 2019;133:115S.
Postpartum depression (PPD) is one of the most prevalent complications associated with pregnancy and childbirth in the United States, affecting more than 400,000 women annually.1 Postpartum depression is most commonly treated with psychotherapy and antidepressants approved for the treatment of major depressive disorder. Until recently, there was no pharmacologic therapy approved by the FDA specifically for the treatment of PPD. Considering the adverse outcomes associated with untreated or inadequately treated PPD, and the limitations of existing therapies, there is a significant unmet need for pharmacologic treatment options for PPD.2 To help address this need, the FDA recently approved brexanolone injection (brand name: ZULRESSO™) (Table 13) as a first-in-class therapy for the treatment of adults with PPD.3
Clinical implications
Postpartum depression can result in adverse outcomes for the patient, baby, and family when under- or untreated, and the need for rapid resolution of symptoms cannot be overstated.2 Suicide is strongly associated with depression and is a leading cause of pregnancy-related deaths.4 Additionally, PPD can impact the health, safety, and well-being of the child, with both short- and long-term consequences, including greater rates of psychological or behavioral difficulties among children of patients with PPD.5 Postpartum depression can also have negative effects on the patient’s partner, with 24% to 50% of partners experiencing depression.6 Current PPD management strategies include the use of psychotherapy and pharmacologic interventions for major depressive disorder that may take up to 4 to 6 weeks for some patients, and may not achieve remission for all patients.7-9
Brexanolone injection is a first-in-class medication with a novel mechanism of action. In clinical studies, it achieved rapid (by Hour 60) and sustained (through Day 30) reductions in depressive symptoms and could provide a meaningful new treatment option for adult women with PPD.10,11
How it works
Animal and human studies have established the re
Brexanolone is a neuroactive steroid that is chemically identical to endogenous allopregnanolone produced in the CNS. Brexanolone potentiates GABA-mediated currents from recombinant human GABAARs in mammalian cells expressing α1β2γ2 receptor subunits, α4β3δ receptor subunits, and α6β3δ receptor subunits.3 Positive allosteric modulation of both synaptic and extrasynaptic GABAARs differentiates brexanolone from other GABAAR modulators, such as benzodiazepines.10,11
Brexanolone’s mechanism of action in the treatment of PPD is not fully understood, but it is thought to be related to GABAAR PAM activity.3
Supporting evidence
The FDA approval of brexanolone injection was based on the efficacy demonstrated in 2 Phase III multicenter, randomized, double-blind, placebo-controlled studies in adult women (age 18 to 45) with PPD (defined by DSM-IV criteria for a major depressive episode, with onset of symptoms in the third trimester or within 4 weeks of delivery). Exclusion criteria included the presence of bipolar disorder or psychosis. In these studies, 60-hour continuous IV infusions of brexanolone or placebo were given, followed by 4 weeks of observation. Study 1 (202B) enrolled patients with severe PPD (Hamilton Rating Scale for Depression [HAM-D] total score ≥26), and Study 2 (202C) enrolled patients with moderate PPD (HAM-D score 20 to 25). A titration to the recommended target dosage of 90 μg/kg/hour was evaluated in both studies. BRX90 patients received 30 μg/kg/hour for 4 hours, 60 μg/kg/hour for 20 hours, 90 μg/kg/hour for 28 hours, followed by a taper to 60 μg/kg/hour for 4 hours and then 30 μg/kg/hour for 4 hours. The primary endpoint in both studies was the mean change from baseline in depressive symptoms as measured by HAM-D total score at the end of the 60-hour infusion. A pre-specified secondary efficacy endpoint was the mean change from baseline in HAM-D total score at Day 30.
Continue to: Efficacy
Efficacy. In both placebo-controlled studies, titration to a target dose of brexanolone 90 μg/kg/hour was superior to placebo in improvement of depressive symptoms (Table 33).
Pharmacological profile
Brexanolone exposure-response relationships and the time course of pharmacodynamic response are unknown.3
Adverse reactions. Safety was evaluated from all patients receiving brexanolone injection, regardless of dosing regimen (N = 140, including patients from a Phase IIb study, 202A).3,11
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were sedation/somnolence, dry mouth, loss of consciousness, and flushing/hot flush.3 The incidence of patients discontinuing due to any adverse reaction was 2% for brexanolone vs 1% for placebo.3
Sedation, somnolence, and loss of consciousness. In clinical studies, brexanolone caused sedation and somnolence that required dose interruption or reduction in some patients during the infusion (5% of brexanolone-treated patients compared with 0% of placebo-treated patients).3 Some patients were also reported to have loss of consciousness or altered state of consciousness during the brexanolone infusion (4% of patients treated with brexanolone compared with 0% of patients treated with placebo).3 All patients with loss of or altered state of consciousness recovered fully 15 to 60 minutes after dose interruption.3 There was no clear association between loss or alteration of consciousness and pattern or timing of dose, and not all patients who experienced a loss or alteration of consciousness reported sedation or somnolence before the episode.
Continue to: Suicidality
Suicidality. The risk of developing suicidal thoughts and behaviors with brexanolone is unknown, due to the relatively low number of exposures to brexanolone injection during clinical development and a mechanism of action distinct from that of existing antidepressant medications.3
Pharmacokinetics
In clinical trials, brexanolone exhibited dose-proportional pharmacokinetics, and the terminal half-life is approximately 9 hours (Table 43). Brexanolone is metabolized by non-cytochrome P450 (CYP)-based pathways, including keto-reduction, glucuronidation, and sulfation.3 No clinically significant differences in the pharmacokinetics of brexanolone were observed based on renal or hepatic impairment, and no studies were conducted to evaluate the effects of other drugs on brexanolone.3
Lactation. A population pharmacokinetics model constructed from studies in the clinical development program calculated the maximum relative infant dose for brexanolone during infusion as 1.3%.3 Given the low oral bioavailability of brexanolone (<5%) in adults, the potential for breastfed infant exposure is considered low.3
Clinical considerations
Risk Evaluation and Mitigation Strategies (REMS) requirements. Brexanolone injection is a Schedule IV controlled substance. It has a “black-box” warning regarding excessive sedation and sudden loss of consciousness, which has been taken into account within the REMS drug safety program. Health care facilities and pharmacies must enroll in the REMS program and ensure that brexanolone is administered only to patients who are enrolled in the REMS program. Staff must be trained on the processes and procedures to administer brexanolone, and the facility must have a fall precautions protocol in place and be equipped with a programmable peristaltic IV infusion pump and continuous pulse oximetry with alarms.3
Monitoring. A REMS-trained clinician must be available continuously on-site to oversee each patient for the duration of the continuous IV infusion, which lasts 60 hours (2.5 days) and should be initiated early enough in the day to allow for recognition of excessive sedation. Patients must be monitored for hypoxia using continuous pulse oximetry equipped with an alarm and should also be assessed for excessive sedation every 2 hours during planned, non-sleep periods. If excessive sedation occurs, the infusion should be stopped until symptoms resolve, after which the infusion may be resumed at the same or a lower dose as clinically appropriate. In case of overdosage, the infusion should be stopped immediately and supportive measures initiated as necessary. Patients must not be the primary caregiver of dependents, and must be accompanied during interactions with their child(ren).
Continue to: Contraindications
Contraindications. There are no contraindications for the use of brexanolone in adults with PPD.
End-stage renal disease (ESRD). Avoid using brexanolone in patients with ESRD because of the potential accumulation of the solubilizing agent, betadex sulfobutyl ether sodium.
Pregnancy. Brexanolone has not been studied in pregnant patients. Pregnant women and women of reproductive age should be informed of the potential risk to a fetus based on data from other drugs that enhance GABAergic inhibition.
Breastfeeding. There are no data on the effects of brexanolone on a breastfed infant. Breastfeeding should be a discussion of risk and benefit between the patient and her doctor. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for brexanolone and any potential adverse effects on the breastfed child from brexanolone or from the underlying maternal condition. However, based on the low relative infant dose (<2%) and the low oral bioavailability in adults, the risk to breastfed infants is thought to be low.16
Potential for abuse. Brexanolone injection is a Schedule IV controlled substance. Although it was not possible to assess physical dependency in the registrational trials due to dose tapering at the end of treatment, clinicians should advise patients about the theoretical possibility for brexanolone to be abused or lead to dependence based on other medications with similar primary pharmacology.
Continue to: Concomitant medications
Concomitant medications. Caution patients that taking opioids or other CNS depressants, such as benzodiazepines, in combination with brexanolone may increase the severity of sedative effects.
Suicidal thoughts and behaviors. Advise patients and caregivers to look for the emergence of suicidal thoughts and behavior and instruct them to report such symptoms to their clinician. Consider changing the therapeutic regimen, including discontinuing brexanolone, in patients whose depression becomes worse or who experience emergent suicidal thoughts and behaviors.
Why Rx?
Postpartum depression is a common and often devastating medical complication of childbirth that can result in adverse outcomes for the patient, baby, and family when left undertreated or untreated. There is a great need to identify and treat women who develop PPD. Rapid and sustained resolution of symptoms in women who experience PPD should be the goal of treatment, and consequently, brexanolone injection presents an important new tool in available treatment options for PPD.
Bottom Line
Brexanolone injection is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive allosteric modulator that’s been FDA-approved for the treatment of postpartum depression (PPD). It is administered as a continuous IV infusion over 60 hours. The rapid and sustained improvement of PPD observed in clinical trials with brexanolone injection may support a new treatment paradigm for women with PPD.
Postpartum depression (PPD) is one of the most prevalent complications associated with pregnancy and childbirth in the United States, affecting more than 400,000 women annually.1 Postpartum depression is most commonly treated with psychotherapy and antidepressants approved for the treatment of major depressive disorder. Until recently, there was no pharmacologic therapy approved by the FDA specifically for the treatment of PPD. Considering the adverse outcomes associated with untreated or inadequately treated PPD, and the limitations of existing therapies, there is a significant unmet need for pharmacologic treatment options for PPD.2 To help address this need, the FDA recently approved brexanolone injection (brand name: ZULRESSO™) (Table 13) as a first-in-class therapy for the treatment of adults with PPD.3
Clinical implications
Postpartum depression can result in adverse outcomes for the patient, baby, and family when under- or untreated, and the need for rapid resolution of symptoms cannot be overstated.2 Suicide is strongly associated with depression and is a leading cause of pregnancy-related deaths.4 Additionally, PPD can impact the health, safety, and well-being of the child, with both short- and long-term consequences, including greater rates of psychological or behavioral difficulties among children of patients with PPD.5 Postpartum depression can also have negative effects on the patient’s partner, with 24% to 50% of partners experiencing depression.6 Current PPD management strategies include the use of psychotherapy and pharmacologic interventions for major depressive disorder that may take up to 4 to 6 weeks for some patients, and may not achieve remission for all patients.7-9
Brexanolone injection is a first-in-class medication with a novel mechanism of action. In clinical studies, it achieved rapid (by Hour 60) and sustained (through Day 30) reductions in depressive symptoms and could provide a meaningful new treatment option for adult women with PPD.10,11
How it works
Animal and human studies have established the re
Brexanolone is a neuroactive steroid that is chemically identical to endogenous allopregnanolone produced in the CNS. Brexanolone potentiates GABA-mediated currents from recombinant human GABAARs in mammalian cells expressing α1β2γ2 receptor subunits, α4β3δ receptor subunits, and α6β3δ receptor subunits.3 Positive allosteric modulation of both synaptic and extrasynaptic GABAARs differentiates brexanolone from other GABAAR modulators, such as benzodiazepines.10,11
Brexanolone’s mechanism of action in the treatment of PPD is not fully understood, but it is thought to be related to GABAAR PAM activity.3
Supporting evidence
The FDA approval of brexanolone injection was based on the efficacy demonstrated in 2 Phase III multicenter, randomized, double-blind, placebo-controlled studies in adult women (age 18 to 45) with PPD (defined by DSM-IV criteria for a major depressive episode, with onset of symptoms in the third trimester or within 4 weeks of delivery). Exclusion criteria included the presence of bipolar disorder or psychosis. In these studies, 60-hour continuous IV infusions of brexanolone or placebo were given, followed by 4 weeks of observation. Study 1 (202B) enrolled patients with severe PPD (Hamilton Rating Scale for Depression [HAM-D] total score ≥26), and Study 2 (202C) enrolled patients with moderate PPD (HAM-D score 20 to 25). A titration to the recommended target dosage of 90 μg/kg/hour was evaluated in both studies. BRX90 patients received 30 μg/kg/hour for 4 hours, 60 μg/kg/hour for 20 hours, 90 μg/kg/hour for 28 hours, followed by a taper to 60 μg/kg/hour for 4 hours and then 30 μg/kg/hour for 4 hours. The primary endpoint in both studies was the mean change from baseline in depressive symptoms as measured by HAM-D total score at the end of the 60-hour infusion. A pre-specified secondary efficacy endpoint was the mean change from baseline in HAM-D total score at Day 30.
Continue to: Efficacy
Efficacy. In both placebo-controlled studies, titration to a target dose of brexanolone 90 μg/kg/hour was superior to placebo in improvement of depressive symptoms (Table 33).
Pharmacological profile
Brexanolone exposure-response relationships and the time course of pharmacodynamic response are unknown.3
Adverse reactions. Safety was evaluated from all patients receiving brexanolone injection, regardless of dosing regimen (N = 140, including patients from a Phase IIb study, 202A).3,11
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were sedation/somnolence, dry mouth, loss of consciousness, and flushing/hot flush.3 The incidence of patients discontinuing due to any adverse reaction was 2% for brexanolone vs 1% for placebo.3
Sedation, somnolence, and loss of consciousness. In clinical studies, brexanolone caused sedation and somnolence that required dose interruption or reduction in some patients during the infusion (5% of brexanolone-treated patients compared with 0% of placebo-treated patients).3 Some patients were also reported to have loss of consciousness or altered state of consciousness during the brexanolone infusion (4% of patients treated with brexanolone compared with 0% of patients treated with placebo).3 All patients with loss of or altered state of consciousness recovered fully 15 to 60 minutes after dose interruption.3 There was no clear association between loss or alteration of consciousness and pattern or timing of dose, and not all patients who experienced a loss or alteration of consciousness reported sedation or somnolence before the episode.
Continue to: Suicidality
Suicidality. The risk of developing suicidal thoughts and behaviors with brexanolone is unknown, due to the relatively low number of exposures to brexanolone injection during clinical development and a mechanism of action distinct from that of existing antidepressant medications.3
Pharmacokinetics
In clinical trials, brexanolone exhibited dose-proportional pharmacokinetics, and the terminal half-life is approximately 9 hours (Table 43). Brexanolone is metabolized by non-cytochrome P450 (CYP)-based pathways, including keto-reduction, glucuronidation, and sulfation.3 No clinically significant differences in the pharmacokinetics of brexanolone were observed based on renal or hepatic impairment, and no studies were conducted to evaluate the effects of other drugs on brexanolone.3
Lactation. A population pharmacokinetics model constructed from studies in the clinical development program calculated the maximum relative infant dose for brexanolone during infusion as 1.3%.3 Given the low oral bioavailability of brexanolone (<5%) in adults, the potential for breastfed infant exposure is considered low.3
Clinical considerations
Risk Evaluation and Mitigation Strategies (REMS) requirements. Brexanolone injection is a Schedule IV controlled substance. It has a “black-box” warning regarding excessive sedation and sudden loss of consciousness, which has been taken into account within the REMS drug safety program. Health care facilities and pharmacies must enroll in the REMS program and ensure that brexanolone is administered only to patients who are enrolled in the REMS program. Staff must be trained on the processes and procedures to administer brexanolone, and the facility must have a fall precautions protocol in place and be equipped with a programmable peristaltic IV infusion pump and continuous pulse oximetry with alarms.3
Monitoring. A REMS-trained clinician must be available continuously on-site to oversee each patient for the duration of the continuous IV infusion, which lasts 60 hours (2.5 days) and should be initiated early enough in the day to allow for recognition of excessive sedation. Patients must be monitored for hypoxia using continuous pulse oximetry equipped with an alarm and should also be assessed for excessive sedation every 2 hours during planned, non-sleep periods. If excessive sedation occurs, the infusion should be stopped until symptoms resolve, after which the infusion may be resumed at the same or a lower dose as clinically appropriate. In case of overdosage, the infusion should be stopped immediately and supportive measures initiated as necessary. Patients must not be the primary caregiver of dependents, and must be accompanied during interactions with their child(ren).
Continue to: Contraindications
Contraindications. There are no contraindications for the use of brexanolone in adults with PPD.
End-stage renal disease (ESRD). Avoid using brexanolone in patients with ESRD because of the potential accumulation of the solubilizing agent, betadex sulfobutyl ether sodium.
Pregnancy. Brexanolone has not been studied in pregnant patients. Pregnant women and women of reproductive age should be informed of the potential risk to a fetus based on data from other drugs that enhance GABAergic inhibition.
Breastfeeding. There are no data on the effects of brexanolone on a breastfed infant. Breastfeeding should be a discussion of risk and benefit between the patient and her doctor. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for brexanolone and any potential adverse effects on the breastfed child from brexanolone or from the underlying maternal condition. However, based on the low relative infant dose (<2%) and the low oral bioavailability in adults, the risk to breastfed infants is thought to be low.16
Potential for abuse. Brexanolone injection is a Schedule IV controlled substance. Although it was not possible to assess physical dependency in the registrational trials due to dose tapering at the end of treatment, clinicians should advise patients about the theoretical possibility for brexanolone to be abused or lead to dependence based on other medications with similar primary pharmacology.
Continue to: Concomitant medications
Concomitant medications. Caution patients that taking opioids or other CNS depressants, such as benzodiazepines, in combination with brexanolone may increase the severity of sedative effects.
Suicidal thoughts and behaviors. Advise patients and caregivers to look for the emergence of suicidal thoughts and behavior and instruct them to report such symptoms to their clinician. Consider changing the therapeutic regimen, including discontinuing brexanolone, in patients whose depression becomes worse or who experience emergent suicidal thoughts and behaviors.
Why Rx?
Postpartum depression is a common and often devastating medical complication of childbirth that can result in adverse outcomes for the patient, baby, and family when left undertreated or untreated. There is a great need to identify and treat women who develop PPD. Rapid and sustained resolution of symptoms in women who experience PPD should be the goal of treatment, and consequently, brexanolone injection presents an important new tool in available treatment options for PPD.
Bottom Line
Brexanolone injection is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive allosteric modulator that’s been FDA-approved for the treatment of postpartum depression (PPD). It is administered as a continuous IV infusion over 60 hours. The rapid and sustained improvement of PPD observed in clinical trials with brexanolone injection may support a new treatment paradigm for women with PPD.
1. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms - 27 states, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66(6):153-158.
2. Frieder A, Fersh M, Hainline R, et al. Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs. 2019;33(3):265-282.
3. Brexanolone injection [package insert]. Cambridge, MA: Sage Therapeutics, Inc.; 2019.
4. Bodnar-Deren S, Klipstein K, Fersh M, et al. Suicidal ideation during the postpartum period. J Womens Health (Larchmt). 2016;25(12):1219-1224.
5. Netsi E, Pearson RM, Murray L, et al. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry. 2018;75(3):247-253.
6. Goodman JH. Paternal postpartum depression, its relationship to maternal postpartum depression, and implications for family health. J Adv Nurs. 2004;45(1):26-35.
7. Gelenberg AJ, Freeman MP, Markowitz JC, et al; American Psychiatric Association Work Group on Major Depressive Disorder. Practice guidelines for the treatment of patients with major depressive disorder. 3rd ed. Washington, DC: American Psychiatric Association; 2010.
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
9. Molyneaux E, Telesia LA, Henshaw C, et al. Antidepressants for preventing postnatal depression. Cochrane Database Syst Rev. 2018;4:CD004363.
10. Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480-489.
11. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
12. Melon LC, Hooper A, Yang X, et al. Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice. Psychoneuroendocrinology. 2018;90:182-193.
13. Deligiannidis KM, Fales CL, Kroll-Desrosiers AR, et al. Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2019;44(3):546-554.
14. Licheri V, Talani G, Gorule AA, et al. Plasticity of GABAA receptors during pregnancy and postpartum period: from gene to function. Neural Plast. 2015;2015:170435. doi: 10.1155/2015/170435.
15. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
16. Hoffmann E, Wald J, Dray D, et al. Brexanolone injection administration to lactating women: breast milk allopregnanolone levels [30J]. Obstetrics & Gynecology. 2019;133:115S.
1. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms - 27 states, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66(6):153-158.
2. Frieder A, Fersh M, Hainline R, et al. Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs. 2019;33(3):265-282.
3. Brexanolone injection [package insert]. Cambridge, MA: Sage Therapeutics, Inc.; 2019.
4. Bodnar-Deren S, Klipstein K, Fersh M, et al. Suicidal ideation during the postpartum period. J Womens Health (Larchmt). 2016;25(12):1219-1224.
5. Netsi E, Pearson RM, Murray L, et al. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry. 2018;75(3):247-253.
6. Goodman JH. Paternal postpartum depression, its relationship to maternal postpartum depression, and implications for family health. J Adv Nurs. 2004;45(1):26-35.
7. Gelenberg AJ, Freeman MP, Markowitz JC, et al; American Psychiatric Association Work Group on Major Depressive Disorder. Practice guidelines for the treatment of patients with major depressive disorder. 3rd ed. Washington, DC: American Psychiatric Association; 2010.
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
9. Molyneaux E, Telesia LA, Henshaw C, et al. Antidepressants for preventing postnatal depression. Cochrane Database Syst Rev. 2018;4:CD004363.
10. Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480-489.
11. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
12. Melon LC, Hooper A, Yang X, et al. Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice. Psychoneuroendocrinology. 2018;90:182-193.
13. Deligiannidis KM, Fales CL, Kroll-Desrosiers AR, et al. Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2019;44(3):546-554.
14. Licheri V, Talani G, Gorule AA, et al. Plasticity of GABAA receptors during pregnancy and postpartum period: from gene to function. Neural Plast. 2015;2015:170435. doi: 10.1155/2015/170435.
15. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
16. Hoffmann E, Wald J, Dray D, et al. Brexanolone injection administration to lactating women: breast milk allopregnanolone levels [30J]. Obstetrics & Gynecology. 2019;133:115S.