User login
‘Miracle cures’ in psychiatry?
For a patient with a major mental illness, the road to wellness is long and uncertain. The medications commonly used to treat mood and thought disorders can take weeks to months to start providing benefits, and they carry significant risks for adverse effects, such as weight gain, sexual dysfunction, and movement disorders. Patients often have to take psychotropic medications for the rest of their lives. In addition to these downsides, there is no guarantee that these medications will provide complete or even partial relief.2,3
Recently, there has been growing excitement about new treatments that might be “miracle cures” for patients with mental illness, particularly for individuals with treatment-resistant depression (TRD). Two of these treatments—ketamine-related compounds, and hallucinogenic drugs—seem to promise therapeutic effects that are vastly different from those of other psychiatric medications: They appear to improve patients’ symptoms very quickly, and their effects may persist long after these drugs have been cleared from the body.
Intravenous ketamine is an older generic drug used in anesthesia; recently, it has been used off-label for TRD and other mental illnesses. On March 5, 2019, the FDA approved an intranasal formulation of esketamine—the S-enantiomer of ketamine—for TRD.4 Hallucinogens have also been tested in small studies and have seemingly significant effects in alleviating depression in patients with terminal illnesses5 and reducing smoking behavior in patients with tobacco use disorder.6,7
These miracle cures are becoming increasingly available to patients and continue to gain credibility among clinicians and researchers. How should we evaluate the usefulness of these new treatments? And how should we talk to our patients about them? To answer these questions, this article:
- explores our duty to our patients, ourselves, and our colleagues
- describes the dilemma
- discusses ways to evaluate claims made about these new miracle cures.
Duty: Protecting and helping our patients
The physician–patient relationship is a fiduciary relationship. According to both common law and medical ethics, a physician who enters into a treatment relationship with a patient creates a bond of special trust and confidence. Such a relationship requires a physician to act in good faith and in the patient’s best interests.8 As physicians, we have a duty to evaluate the safety and efficacy of new treatments that are available for our patients, whether or not they are FDA-approved.
We should also protect our patients from the adverse consequences of relatively untested drugs. For example, ketamine and hallucinogens both produce dissociative effects, and may carry high risks for patients who have a predisposition to psychosis.9 We should protect our patients from any false hopes that might lead them to abandon their current treatment regimens due to adverse effects and imperfect results. At the same time, we also have a duty to acknowledge our patients’ suffering and to recognize that they might be desperate for new treatment options. We should remain open-minded about new treatments, and acknowledge that they might work. Finally, we have a duty to be mindful of any financial benefits that we may derive from the development, marketing, and administration of these medications.
Dilemma: The need for new treatments
This is not the first time that novel treatments in mental health have seemed to hold incredible promise. In the late 1800s, Sigmund Freud began to regularly use a compound that led him to feel “the normal euphoria of a healthy person.” He wrote that this substance produced:
…exhilaration and lasting euphoria, which does not differ in any way from the normal euphoria of a healthy person. The feeling of excitement which accompanies stimulus by alcohol is completely lacking; the characteristic urge for immediate activity which alcohol produces is also absent. One senses an increase of self-control and feels more vigorous and more capable of work; on the other hand, if one works, one misses that heightening of the mental powers which alcohol, tea, or coffee induce. One is simply normal, and soon finds it difficult to believe that one is under the influence of any drug at all.1
Continue to: The compound Freud was describing...
The compound Freud was describing is cocaine, which we now know is an addictive and dangerous drug that can in fact worsen depression.10 Another treatment regarded as a miracle cure in its time involved placing patients with schizophrenia into an insulin-induced coma to treat their symptoms; this therapy was used from 1933 to 1960.11 We now recognize that this practice is unacceptably dangerous.
The past is filled with cautionary tales of the enthusiastic adoption of treatments for mental illness that later turned out to be ineffective, counterproductive, dangerous, or inhumane. Yet, the long, arduous journeys our patients go through continue to weigh heavily on us. We would love to offer our patients newer, more efficacious, and longer-lasting treatments with fewer adverse effects.
Discussion: How to best evaluate miracle cures
To help quickly assess a new treatment, the following 5 categories can help guide and organize our thought process.
1. Evidence
What type of evidence do we have that a new treatment is safe and effective? Psychiatric research may be even more susceptible to a placebo effect than other medical research, particularly for illnesses with subjective symptoms, such as depression.12 Double-blinded, placebo-controlled studies, such as the IV ketamine trial conducted by Singh et al,13 are the gold standard for separating a substance’s actual biologic effect from a placebo effect. Studies that do not include a control group should not be regarded as providing scientific evidence of efficacy.
2. Mechanism
If a new compound appears to have a beneficial effect on mental health, it is important to consider the potential mechanism underlying this effect to determine if it is biologically plausible. A compound that is claimed to be a panacea for every symptom of every mental illness should be heavily scrutinized. For example, based on available research, ketamine’s long-lasting effects seem to come from 2 mechanisms14,15:
- Activation of endogenous opioid receptors, which is also responsible for the euphoria induced by heroin and oxycodone.
- Blockade of N-methyl-
D -aspartate receptors. N-methyl-D -aspartate receptor activation is a key mechanism by which learning and memory function in the brain, and blocking these receptors may increase brain plasticity.
Continue to: Therefore, it seems plausible...
Therefore, it seems plausible that ketamine could produce both short- and long-term improvements in mood. Hallucinogenic drugs are thought to profoundly alter brain function through several mechanisms, including activating serotonin receptors, enhancing brain plasticity, and increasing brain connectivity.16
3. Reinforcement
Psychiatric medications that are acutely reinforcing have significant potential for abuse. Antidepressants and mood stabilizers are not acutely rewarding. They don’t make patients feel good right away. Medications such as stimulants and opioids do, and must be used with extreme care because of their abuse potential. The problem with acutely reinforcing medications is that in the long run, they can worsen depression by decreasing the brain’s ability to produce endogenous opioids.17
4.
A mental disorder is unlikely to have a single solution. Rather than regarding a new treatment as capable of rapidly alleviating every symptom of a patient’s illness, it should be viewed as a tool that can be helpful when used in combination with other treatments and lifestyle practices. In an interview with the web site STAT, Cristina Cusin, MD, co-director of the Intravenous Ketamine Clinic for Depression at Massachusetts General Hospital, said, “You don’t treat an advanced disease with just an infusion and a ‘see you next time.’ If [doctors] replace your knee but [you] don’t do physical therapy, you don’t walk again.”18 To sustain the benefits of a novel medication, patients with serious mental illnesses need to maintain strong social supports, see a mental health care provider regularly, and abstain from illicit drug and alcohol use.
5. Context matters
For a medication to obtain approval to treat a specific indication, the FDA usually require 2 trials that demonstrate efficacy. Off-label use of generic medications such as ketamine may have benefits, but it is unlikely that a generic drug would be put through a costly FDA-approval process.19
When learning about new medications, remember that patients might assume that these agents have undergone a thorough review process for safety and effectiveness. When our patients request such treatments—whether FDA-approved or off-label—it is our responsibility as physicians to educate them about the benefits, risks, effectiveness, and limitations of these treatments, as well as to evaluate the appropriateness of a treatment for a specific patient’s symptoms.
Continue to: Tempering excitement with caution
Tempering excitement with caution
Our patients are not the only ones desperate for a miracle cure. As psychiatrists, many of us are desperate, too. New compounds may ultimately change the way we treat mental illness. However, we have an obligation to temper our excitement with caution by remembering past mistakes, and systematically evaluating new miracle cures to determine if they are safe and effective.
1. Freud S. Cocaine papers. In: Freud S, Byck R. Sigmund Freud collection (Library of Congress). New York, NY: Stonehill; 1975;7.
2. Rush AJ. STAR*D: what have we learned? Am J Psychiatry. 2007;164(2):201-204.
3. Demjaha A, Lappin JM, Stahl D, et al. Antipsychotic treatment resistance in first-episode psychosis: prevalence, subtypes and predictors. Psychol Med. 2017;47(11):1981-1989.
4. Carey B. Fast-acting depression drug, newly approved, could help millions. The New York Times. https://www.nytimes.com/2019/03/05/health/depression-treatment-ketamine-fda.html. Published March 5, 2019. Accessed July 26, 2019.
5. Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30(12):1181-1197.
6. Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. 2017;43:55-60.
7. Garcia-Romeu A, Griffiths RR, Johnson MW. Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr Drug Abuse Rev 2014;7(3):157-164.
8. Simon RI. Clinical psychiatry and the law. 2nd ed. Washington, DC: American Psychiatric Press; 1992.
9. Lahti AC, Weiler MA, Tamara Michaelidis BA, et al. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25(4):455-467.
10. Perrine SA, Sheikh IS, Nwaneshiudu CA, et al. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology. 2008;54(2):355-364.
11. Doroshow DB. Performing a cure for schizophrenia: insulin coma therapy on the wards. J Hist Med Allied Sci. 2007;62(2):213-243.
12. Khan A, Kolts RL, Rapaport MH, et al. Magnitude of placebo response and drug-placebo differences across psychiatric disorders. Psychol Med. 2005;35(5):743-749.
13. Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173(8):816-826.
14. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
15. Duman RS, Aghajanian GK, Sanacora G, et al. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22(2):238-249.
16. Carhart-Harris RL. How do psychedelics work? Curr Opin Psychiatry. 2019;32(1):16-21.
17. Martins SS, Fenton MC, Keyes KM, et al. Mood and anxiety disorders and their association with non-medical prescription opioid use and prescription opioid-use disorder: longitudinal evidence from the National Epidemiologic Study on Alcohol and Related Conditions. Psychol Med. 2012;42(6):1261-1272.
18. Thielking M. Ketamine gives hope to patients with severe depression. But some clinics stray from the science and hype its benefits. STAT. https://www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment/. Published September 24, 2018. Accessed July 26, 2019.
19. Stafford RS. Regulating off-label drug use--rethinking the role of the FDA. N Engl J Med. 2008;358(14):1427-1429.
For a patient with a major mental illness, the road to wellness is long and uncertain. The medications commonly used to treat mood and thought disorders can take weeks to months to start providing benefits, and they carry significant risks for adverse effects, such as weight gain, sexual dysfunction, and movement disorders. Patients often have to take psychotropic medications for the rest of their lives. In addition to these downsides, there is no guarantee that these medications will provide complete or even partial relief.2,3
Recently, there has been growing excitement about new treatments that might be “miracle cures” for patients with mental illness, particularly for individuals with treatment-resistant depression (TRD). Two of these treatments—ketamine-related compounds, and hallucinogenic drugs—seem to promise therapeutic effects that are vastly different from those of other psychiatric medications: They appear to improve patients’ symptoms very quickly, and their effects may persist long after these drugs have been cleared from the body.
Intravenous ketamine is an older generic drug used in anesthesia; recently, it has been used off-label for TRD and other mental illnesses. On March 5, 2019, the FDA approved an intranasal formulation of esketamine—the S-enantiomer of ketamine—for TRD.4 Hallucinogens have also been tested in small studies and have seemingly significant effects in alleviating depression in patients with terminal illnesses5 and reducing smoking behavior in patients with tobacco use disorder.6,7
These miracle cures are becoming increasingly available to patients and continue to gain credibility among clinicians and researchers. How should we evaluate the usefulness of these new treatments? And how should we talk to our patients about them? To answer these questions, this article:
- explores our duty to our patients, ourselves, and our colleagues
- describes the dilemma
- discusses ways to evaluate claims made about these new miracle cures.
Duty: Protecting and helping our patients
The physician–patient relationship is a fiduciary relationship. According to both common law and medical ethics, a physician who enters into a treatment relationship with a patient creates a bond of special trust and confidence. Such a relationship requires a physician to act in good faith and in the patient’s best interests.8 As physicians, we have a duty to evaluate the safety and efficacy of new treatments that are available for our patients, whether or not they are FDA-approved.
We should also protect our patients from the adverse consequences of relatively untested drugs. For example, ketamine and hallucinogens both produce dissociative effects, and may carry high risks for patients who have a predisposition to psychosis.9 We should protect our patients from any false hopes that might lead them to abandon their current treatment regimens due to adverse effects and imperfect results. At the same time, we also have a duty to acknowledge our patients’ suffering and to recognize that they might be desperate for new treatment options. We should remain open-minded about new treatments, and acknowledge that they might work. Finally, we have a duty to be mindful of any financial benefits that we may derive from the development, marketing, and administration of these medications.
Dilemma: The need for new treatments
This is not the first time that novel treatments in mental health have seemed to hold incredible promise. In the late 1800s, Sigmund Freud began to regularly use a compound that led him to feel “the normal euphoria of a healthy person.” He wrote that this substance produced:
…exhilaration and lasting euphoria, which does not differ in any way from the normal euphoria of a healthy person. The feeling of excitement which accompanies stimulus by alcohol is completely lacking; the characteristic urge for immediate activity which alcohol produces is also absent. One senses an increase of self-control and feels more vigorous and more capable of work; on the other hand, if one works, one misses that heightening of the mental powers which alcohol, tea, or coffee induce. One is simply normal, and soon finds it difficult to believe that one is under the influence of any drug at all.1
Continue to: The compound Freud was describing...
The compound Freud was describing is cocaine, which we now know is an addictive and dangerous drug that can in fact worsen depression.10 Another treatment regarded as a miracle cure in its time involved placing patients with schizophrenia into an insulin-induced coma to treat their symptoms; this therapy was used from 1933 to 1960.11 We now recognize that this practice is unacceptably dangerous.
The past is filled with cautionary tales of the enthusiastic adoption of treatments for mental illness that later turned out to be ineffective, counterproductive, dangerous, or inhumane. Yet, the long, arduous journeys our patients go through continue to weigh heavily on us. We would love to offer our patients newer, more efficacious, and longer-lasting treatments with fewer adverse effects.
Discussion: How to best evaluate miracle cures
To help quickly assess a new treatment, the following 5 categories can help guide and organize our thought process.
1. Evidence
What type of evidence do we have that a new treatment is safe and effective? Psychiatric research may be even more susceptible to a placebo effect than other medical research, particularly for illnesses with subjective symptoms, such as depression.12 Double-blinded, placebo-controlled studies, such as the IV ketamine trial conducted by Singh et al,13 are the gold standard for separating a substance’s actual biologic effect from a placebo effect. Studies that do not include a control group should not be regarded as providing scientific evidence of efficacy.
2. Mechanism
If a new compound appears to have a beneficial effect on mental health, it is important to consider the potential mechanism underlying this effect to determine if it is biologically plausible. A compound that is claimed to be a panacea for every symptom of every mental illness should be heavily scrutinized. For example, based on available research, ketamine’s long-lasting effects seem to come from 2 mechanisms14,15:
- Activation of endogenous opioid receptors, which is also responsible for the euphoria induced by heroin and oxycodone.
- Blockade of N-methyl-
D -aspartate receptors. N-methyl-D -aspartate receptor activation is a key mechanism by which learning and memory function in the brain, and blocking these receptors may increase brain plasticity.
Continue to: Therefore, it seems plausible...
Therefore, it seems plausible that ketamine could produce both short- and long-term improvements in mood. Hallucinogenic drugs are thought to profoundly alter brain function through several mechanisms, including activating serotonin receptors, enhancing brain plasticity, and increasing brain connectivity.16
3. Reinforcement
Psychiatric medications that are acutely reinforcing have significant potential for abuse. Antidepressants and mood stabilizers are not acutely rewarding. They don’t make patients feel good right away. Medications such as stimulants and opioids do, and must be used with extreme care because of their abuse potential. The problem with acutely reinforcing medications is that in the long run, they can worsen depression by decreasing the brain’s ability to produce endogenous opioids.17
4.
A mental disorder is unlikely to have a single solution. Rather than regarding a new treatment as capable of rapidly alleviating every symptom of a patient’s illness, it should be viewed as a tool that can be helpful when used in combination with other treatments and lifestyle practices. In an interview with the web site STAT, Cristina Cusin, MD, co-director of the Intravenous Ketamine Clinic for Depression at Massachusetts General Hospital, said, “You don’t treat an advanced disease with just an infusion and a ‘see you next time.’ If [doctors] replace your knee but [you] don’t do physical therapy, you don’t walk again.”18 To sustain the benefits of a novel medication, patients with serious mental illnesses need to maintain strong social supports, see a mental health care provider regularly, and abstain from illicit drug and alcohol use.
5. Context matters
For a medication to obtain approval to treat a specific indication, the FDA usually require 2 trials that demonstrate efficacy. Off-label use of generic medications such as ketamine may have benefits, but it is unlikely that a generic drug would be put through a costly FDA-approval process.19
When learning about new medications, remember that patients might assume that these agents have undergone a thorough review process for safety and effectiveness. When our patients request such treatments—whether FDA-approved or off-label—it is our responsibility as physicians to educate them about the benefits, risks, effectiveness, and limitations of these treatments, as well as to evaluate the appropriateness of a treatment for a specific patient’s symptoms.
Continue to: Tempering excitement with caution
Tempering excitement with caution
Our patients are not the only ones desperate for a miracle cure. As psychiatrists, many of us are desperate, too. New compounds may ultimately change the way we treat mental illness. However, we have an obligation to temper our excitement with caution by remembering past mistakes, and systematically evaluating new miracle cures to determine if they are safe and effective.
For a patient with a major mental illness, the road to wellness is long and uncertain. The medications commonly used to treat mood and thought disorders can take weeks to months to start providing benefits, and they carry significant risks for adverse effects, such as weight gain, sexual dysfunction, and movement disorders. Patients often have to take psychotropic medications for the rest of their lives. In addition to these downsides, there is no guarantee that these medications will provide complete or even partial relief.2,3
Recently, there has been growing excitement about new treatments that might be “miracle cures” for patients with mental illness, particularly for individuals with treatment-resistant depression (TRD). Two of these treatments—ketamine-related compounds, and hallucinogenic drugs—seem to promise therapeutic effects that are vastly different from those of other psychiatric medications: They appear to improve patients’ symptoms very quickly, and their effects may persist long after these drugs have been cleared from the body.
Intravenous ketamine is an older generic drug used in anesthesia; recently, it has been used off-label for TRD and other mental illnesses. On March 5, 2019, the FDA approved an intranasal formulation of esketamine—the S-enantiomer of ketamine—for TRD.4 Hallucinogens have also been tested in small studies and have seemingly significant effects in alleviating depression in patients with terminal illnesses5 and reducing smoking behavior in patients with tobacco use disorder.6,7
These miracle cures are becoming increasingly available to patients and continue to gain credibility among clinicians and researchers. How should we evaluate the usefulness of these new treatments? And how should we talk to our patients about them? To answer these questions, this article:
- explores our duty to our patients, ourselves, and our colleagues
- describes the dilemma
- discusses ways to evaluate claims made about these new miracle cures.
Duty: Protecting and helping our patients
The physician–patient relationship is a fiduciary relationship. According to both common law and medical ethics, a physician who enters into a treatment relationship with a patient creates a bond of special trust and confidence. Such a relationship requires a physician to act in good faith and in the patient’s best interests.8 As physicians, we have a duty to evaluate the safety and efficacy of new treatments that are available for our patients, whether or not they are FDA-approved.
We should also protect our patients from the adverse consequences of relatively untested drugs. For example, ketamine and hallucinogens both produce dissociative effects, and may carry high risks for patients who have a predisposition to psychosis.9 We should protect our patients from any false hopes that might lead them to abandon their current treatment regimens due to adverse effects and imperfect results. At the same time, we also have a duty to acknowledge our patients’ suffering and to recognize that they might be desperate for new treatment options. We should remain open-minded about new treatments, and acknowledge that they might work. Finally, we have a duty to be mindful of any financial benefits that we may derive from the development, marketing, and administration of these medications.
Dilemma: The need for new treatments
This is not the first time that novel treatments in mental health have seemed to hold incredible promise. In the late 1800s, Sigmund Freud began to regularly use a compound that led him to feel “the normal euphoria of a healthy person.” He wrote that this substance produced:
…exhilaration and lasting euphoria, which does not differ in any way from the normal euphoria of a healthy person. The feeling of excitement which accompanies stimulus by alcohol is completely lacking; the characteristic urge for immediate activity which alcohol produces is also absent. One senses an increase of self-control and feels more vigorous and more capable of work; on the other hand, if one works, one misses that heightening of the mental powers which alcohol, tea, or coffee induce. One is simply normal, and soon finds it difficult to believe that one is under the influence of any drug at all.1
Continue to: The compound Freud was describing...
The compound Freud was describing is cocaine, which we now know is an addictive and dangerous drug that can in fact worsen depression.10 Another treatment regarded as a miracle cure in its time involved placing patients with schizophrenia into an insulin-induced coma to treat their symptoms; this therapy was used from 1933 to 1960.11 We now recognize that this practice is unacceptably dangerous.
The past is filled with cautionary tales of the enthusiastic adoption of treatments for mental illness that later turned out to be ineffective, counterproductive, dangerous, or inhumane. Yet, the long, arduous journeys our patients go through continue to weigh heavily on us. We would love to offer our patients newer, more efficacious, and longer-lasting treatments with fewer adverse effects.
Discussion: How to best evaluate miracle cures
To help quickly assess a new treatment, the following 5 categories can help guide and organize our thought process.
1. Evidence
What type of evidence do we have that a new treatment is safe and effective? Psychiatric research may be even more susceptible to a placebo effect than other medical research, particularly for illnesses with subjective symptoms, such as depression.12 Double-blinded, placebo-controlled studies, such as the IV ketamine trial conducted by Singh et al,13 are the gold standard for separating a substance’s actual biologic effect from a placebo effect. Studies that do not include a control group should not be regarded as providing scientific evidence of efficacy.
2. Mechanism
If a new compound appears to have a beneficial effect on mental health, it is important to consider the potential mechanism underlying this effect to determine if it is biologically plausible. A compound that is claimed to be a panacea for every symptom of every mental illness should be heavily scrutinized. For example, based on available research, ketamine’s long-lasting effects seem to come from 2 mechanisms14,15:
- Activation of endogenous opioid receptors, which is also responsible for the euphoria induced by heroin and oxycodone.
- Blockade of N-methyl-
D -aspartate receptors. N-methyl-D -aspartate receptor activation is a key mechanism by which learning and memory function in the brain, and blocking these receptors may increase brain plasticity.
Continue to: Therefore, it seems plausible...
Therefore, it seems plausible that ketamine could produce both short- and long-term improvements in mood. Hallucinogenic drugs are thought to profoundly alter brain function through several mechanisms, including activating serotonin receptors, enhancing brain plasticity, and increasing brain connectivity.16
3. Reinforcement
Psychiatric medications that are acutely reinforcing have significant potential for abuse. Antidepressants and mood stabilizers are not acutely rewarding. They don’t make patients feel good right away. Medications such as stimulants and opioids do, and must be used with extreme care because of their abuse potential. The problem with acutely reinforcing medications is that in the long run, they can worsen depression by decreasing the brain’s ability to produce endogenous opioids.17
4.
A mental disorder is unlikely to have a single solution. Rather than regarding a new treatment as capable of rapidly alleviating every symptom of a patient’s illness, it should be viewed as a tool that can be helpful when used in combination with other treatments and lifestyle practices. In an interview with the web site STAT, Cristina Cusin, MD, co-director of the Intravenous Ketamine Clinic for Depression at Massachusetts General Hospital, said, “You don’t treat an advanced disease with just an infusion and a ‘see you next time.’ If [doctors] replace your knee but [you] don’t do physical therapy, you don’t walk again.”18 To sustain the benefits of a novel medication, patients with serious mental illnesses need to maintain strong social supports, see a mental health care provider regularly, and abstain from illicit drug and alcohol use.
5. Context matters
For a medication to obtain approval to treat a specific indication, the FDA usually require 2 trials that demonstrate efficacy. Off-label use of generic medications such as ketamine may have benefits, but it is unlikely that a generic drug would be put through a costly FDA-approval process.19
When learning about new medications, remember that patients might assume that these agents have undergone a thorough review process for safety and effectiveness. When our patients request such treatments—whether FDA-approved or off-label—it is our responsibility as physicians to educate them about the benefits, risks, effectiveness, and limitations of these treatments, as well as to evaluate the appropriateness of a treatment for a specific patient’s symptoms.
Continue to: Tempering excitement with caution
Tempering excitement with caution
Our patients are not the only ones desperate for a miracle cure. As psychiatrists, many of us are desperate, too. New compounds may ultimately change the way we treat mental illness. However, we have an obligation to temper our excitement with caution by remembering past mistakes, and systematically evaluating new miracle cures to determine if they are safe and effective.
1. Freud S. Cocaine papers. In: Freud S, Byck R. Sigmund Freud collection (Library of Congress). New York, NY: Stonehill; 1975;7.
2. Rush AJ. STAR*D: what have we learned? Am J Psychiatry. 2007;164(2):201-204.
3. Demjaha A, Lappin JM, Stahl D, et al. Antipsychotic treatment resistance in first-episode psychosis: prevalence, subtypes and predictors. Psychol Med. 2017;47(11):1981-1989.
4. Carey B. Fast-acting depression drug, newly approved, could help millions. The New York Times. https://www.nytimes.com/2019/03/05/health/depression-treatment-ketamine-fda.html. Published March 5, 2019. Accessed July 26, 2019.
5. Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30(12):1181-1197.
6. Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. 2017;43:55-60.
7. Garcia-Romeu A, Griffiths RR, Johnson MW. Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr Drug Abuse Rev 2014;7(3):157-164.
8. Simon RI. Clinical psychiatry and the law. 2nd ed. Washington, DC: American Psychiatric Press; 1992.
9. Lahti AC, Weiler MA, Tamara Michaelidis BA, et al. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25(4):455-467.
10. Perrine SA, Sheikh IS, Nwaneshiudu CA, et al. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology. 2008;54(2):355-364.
11. Doroshow DB. Performing a cure for schizophrenia: insulin coma therapy on the wards. J Hist Med Allied Sci. 2007;62(2):213-243.
12. Khan A, Kolts RL, Rapaport MH, et al. Magnitude of placebo response and drug-placebo differences across psychiatric disorders. Psychol Med. 2005;35(5):743-749.
13. Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173(8):816-826.
14. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
15. Duman RS, Aghajanian GK, Sanacora G, et al. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22(2):238-249.
16. Carhart-Harris RL. How do psychedelics work? Curr Opin Psychiatry. 2019;32(1):16-21.
17. Martins SS, Fenton MC, Keyes KM, et al. Mood and anxiety disorders and their association with non-medical prescription opioid use and prescription opioid-use disorder: longitudinal evidence from the National Epidemiologic Study on Alcohol and Related Conditions. Psychol Med. 2012;42(6):1261-1272.
18. Thielking M. Ketamine gives hope to patients with severe depression. But some clinics stray from the science and hype its benefits. STAT. https://www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment/. Published September 24, 2018. Accessed July 26, 2019.
19. Stafford RS. Regulating off-label drug use--rethinking the role of the FDA. N Engl J Med. 2008;358(14):1427-1429.
1. Freud S. Cocaine papers. In: Freud S, Byck R. Sigmund Freud collection (Library of Congress). New York, NY: Stonehill; 1975;7.
2. Rush AJ. STAR*D: what have we learned? Am J Psychiatry. 2007;164(2):201-204.
3. Demjaha A, Lappin JM, Stahl D, et al. Antipsychotic treatment resistance in first-episode psychosis: prevalence, subtypes and predictors. Psychol Med. 2017;47(11):1981-1989.
4. Carey B. Fast-acting depression drug, newly approved, could help millions. The New York Times. https://www.nytimes.com/2019/03/05/health/depression-treatment-ketamine-fda.html. Published March 5, 2019. Accessed July 26, 2019.
5. Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30(12):1181-1197.
6. Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. 2017;43:55-60.
7. Garcia-Romeu A, Griffiths RR, Johnson MW. Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr Drug Abuse Rev 2014;7(3):157-164.
8. Simon RI. Clinical psychiatry and the law. 2nd ed. Washington, DC: American Psychiatric Press; 1992.
9. Lahti AC, Weiler MA, Tamara Michaelidis BA, et al. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25(4):455-467.
10. Perrine SA, Sheikh IS, Nwaneshiudu CA, et al. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology. 2008;54(2):355-364.
11. Doroshow DB. Performing a cure for schizophrenia: insulin coma therapy on the wards. J Hist Med Allied Sci. 2007;62(2):213-243.
12. Khan A, Kolts RL, Rapaport MH, et al. Magnitude of placebo response and drug-placebo differences across psychiatric disorders. Psychol Med. 2005;35(5):743-749.
13. Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173(8):816-826.
14. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
15. Duman RS, Aghajanian GK, Sanacora G, et al. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22(2):238-249.
16. Carhart-Harris RL. How do psychedelics work? Curr Opin Psychiatry. 2019;32(1):16-21.
17. Martins SS, Fenton MC, Keyes KM, et al. Mood and anxiety disorders and their association with non-medical prescription opioid use and prescription opioid-use disorder: longitudinal evidence from the National Epidemiologic Study on Alcohol and Related Conditions. Psychol Med. 2012;42(6):1261-1272.
18. Thielking M. Ketamine gives hope to patients with severe depression. But some clinics stray from the science and hype its benefits. STAT. https://www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment/. Published September 24, 2018. Accessed July 26, 2019.
19. Stafford RS. Regulating off-label drug use--rethinking the role of the FDA. N Engl J Med. 2008;358(14):1427-1429.
DBS vs TMS for treatment-resistant depression: A comparison
Approximately 20% to 30% of patients with major depressive disorder do not respond to pharmacotherapy.1 For patients with treatment-resistant depression (TRD)—typically defined as an inadequate response to at least 1 antidepressant trial of adequate dose and duration—neurostimulation may be an effective treatment option.
Two forms of neurostimulation used to treat TRD are deep brain stimulation (DBS) and transcranial magnetic stimulation (TMS). In DBS, electrodes are placed within the patient’s cranium and affixed to specific target locations. These electrodes are electrically stimulated at various frequencies. Transcranial magnetic stimulation is a noninvasive treatment in which a magnetic field is produced over a patient’s cranium, stimulating brain tissue via electromagnetic induction.
Media portraya
In this article, I compare DBS and TMS, and offer suggestions for educating patients about the potential adverse effects and therapeutic outcomes of each modality.
Deep brain stimulation
Deep brain stimulation is FDA-approved for treating Parkinson’s disease, essential tremor, dystonia, and obsessive-compulsive disorder (OCD).3 It has been used off-label for TRD, and some preliminary evidence suggests it is effective for this purpose. A review of 22 studies found that for patients with TRD, the rate of response to DBS (defined as >50% improvement on Hamilton Depression Rating Scale score) ranges from 40% to 70%.1 Additional research, including larger, randomized, sham-controlled trials, is needed.
A consensus on the optimal target location for DBS has not yet been reached. Studies have had varying degrees of symptom improvement targeting the subgenual cingulate gyrus, posterior gyrus rectus, nucleus accumbens, ventral capsule/ventral striatum, inferior thalamic peduncle, and lateral habenula.1
A worsening of depressive symptoms and increased risk of suicide have been reported in—but are not exclusive to—DBS. Patients treated with DBS may still meet the criteria for treatment resistance.
Continue to: The lack of insurance coverage...
The lack of insurance coverage for DBS for treating depression is a deterrent to its use. Because DBS is not FDA-approved for treating depression, the costs (approximately $65,000) that are not covered by a facility or study will fall on the patient.4 Patients may abandon hope for a positive therapeutic outcome if they must struggle with the financial responsibility for procedures and follow-up.4
Serious potential adverse events of DBS include infections, skin erosions, and postoperative seizure.4 Patients who are treated with DBS should be educated about these adverse effects, and how they may affect outcomes.
Transcranial magnetic stimulation
Transcranial magnetic stimulation is FDA-approved for treating depression, OCD, and migraine. Randomized, sham-controlled trials have found that TMS is effective for TRD.5 Studies have demonstrated varying degrees of efficacy, with response rates ranging from 47% to 58%.6
The most commonly used target area for TMS for patients with depression is the left dorsolateral prefrontal cortex.7 Potential adverse effects are relatively few and benign. The most serious adverse effect of TMS is a risk for seizure, which is reported to occur at a frequency of <0.1%.7
Although it varies by practice and location, the cost for an acute course of TMS (20 to 30 sessions) may range from $6,000 to $12,000.8 Most insurance companies cover TMS treatment for depression.
Continue to: TMS
TMS: A more accessible option
Compared with DBS, TMS is a more affordable and accessible therapy for patients with TRD. Further studies are needed to learn more about the therapeutic potential of DBS for TRD, and to develop methods that help decrease the risk of adverse effects. In addition, insurance coverage needs to be expanded to DBS to avoid having patients be responsible for the full costs of this treatment. Until then, TMS should be a recommended therapy for patients with TRD. If TRD persists in patients treated with TMS, consider electroconvulsive therapy.
1. Morishita T, Fayad SM, Higuchi MA, et al. Deep brain stimulation for treatment-resistant depression: systematic review of clinical outcomes. Neurotherapeutics. 2014;11(3):475-484.
2. Lawrence RE, Kaufmann CR, DeSilva RB, et al. Patients’ belief about deep brain stimulation for treatment resistant depression. AJOB Neuroscience, 2018;9(4):210-218.
3. Rossi PJ, Giordano J, Okun MS. The problem of funding off-label deep brain stimulation: bait-and-switch tactics and the need for policy reform. JAMA Neurol. 2017;74(1):9-10.
4. Holtzheimer PE, Husain MM, Lisanby SH, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4(11):839-849.
5. Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
6. Janicak PG, Sackett V, Kudrna K, et al. Advances in transcranial magnetic stimulation for managing major depressive disorders. Current Psychiatry. 2016;15(6):49-56.
7. Dobek CE, Blumberger DM, Downar J, et al. Risk of seizures in transcranial magnetic stimulation: a clinical review to inform consent process focused on bupropion. Neuropsychiatr Dis Treat. 2015;11:2975-2987.
8. McClintock SM, Reti IM, Carpenter LL, et al; National Network of Depression Centers rTMS Task Group; American Psychiatric Association Council on Research Task Force on Novel Biomarkers and Treatments. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. 2018;79(1). doi: 10.4088/JCP.16cs10905.
Approximately 20% to 30% of patients with major depressive disorder do not respond to pharmacotherapy.1 For patients with treatment-resistant depression (TRD)—typically defined as an inadequate response to at least 1 antidepressant trial of adequate dose and duration—neurostimulation may be an effective treatment option.
Two forms of neurostimulation used to treat TRD are deep brain stimulation (DBS) and transcranial magnetic stimulation (TMS). In DBS, electrodes are placed within the patient’s cranium and affixed to specific target locations. These electrodes are electrically stimulated at various frequencies. Transcranial magnetic stimulation is a noninvasive treatment in which a magnetic field is produced over a patient’s cranium, stimulating brain tissue via electromagnetic induction.
Media portraya
In this article, I compare DBS and TMS, and offer suggestions for educating patients about the potential adverse effects and therapeutic outcomes of each modality.
Deep brain stimulation
Deep brain stimulation is FDA-approved for treating Parkinson’s disease, essential tremor, dystonia, and obsessive-compulsive disorder (OCD).3 It has been used off-label for TRD, and some preliminary evidence suggests it is effective for this purpose. A review of 22 studies found that for patients with TRD, the rate of response to DBS (defined as >50% improvement on Hamilton Depression Rating Scale score) ranges from 40% to 70%.1 Additional research, including larger, randomized, sham-controlled trials, is needed.
A consensus on the optimal target location for DBS has not yet been reached. Studies have had varying degrees of symptom improvement targeting the subgenual cingulate gyrus, posterior gyrus rectus, nucleus accumbens, ventral capsule/ventral striatum, inferior thalamic peduncle, and lateral habenula.1
A worsening of depressive symptoms and increased risk of suicide have been reported in—but are not exclusive to—DBS. Patients treated with DBS may still meet the criteria for treatment resistance.
Continue to: The lack of insurance coverage...
The lack of insurance coverage for DBS for treating depression is a deterrent to its use. Because DBS is not FDA-approved for treating depression, the costs (approximately $65,000) that are not covered by a facility or study will fall on the patient.4 Patients may abandon hope for a positive therapeutic outcome if they must struggle with the financial responsibility for procedures and follow-up.4
Serious potential adverse events of DBS include infections, skin erosions, and postoperative seizure.4 Patients who are treated with DBS should be educated about these adverse effects, and how they may affect outcomes.
Transcranial magnetic stimulation
Transcranial magnetic stimulation is FDA-approved for treating depression, OCD, and migraine. Randomized, sham-controlled trials have found that TMS is effective for TRD.5 Studies have demonstrated varying degrees of efficacy, with response rates ranging from 47% to 58%.6
The most commonly used target area for TMS for patients with depression is the left dorsolateral prefrontal cortex.7 Potential adverse effects are relatively few and benign. The most serious adverse effect of TMS is a risk for seizure, which is reported to occur at a frequency of <0.1%.7
Although it varies by practice and location, the cost for an acute course of TMS (20 to 30 sessions) may range from $6,000 to $12,000.8 Most insurance companies cover TMS treatment for depression.
Continue to: TMS
TMS: A more accessible option
Compared with DBS, TMS is a more affordable and accessible therapy for patients with TRD. Further studies are needed to learn more about the therapeutic potential of DBS for TRD, and to develop methods that help decrease the risk of adverse effects. In addition, insurance coverage needs to be expanded to DBS to avoid having patients be responsible for the full costs of this treatment. Until then, TMS should be a recommended therapy for patients with TRD. If TRD persists in patients treated with TMS, consider electroconvulsive therapy.
Approximately 20% to 30% of patients with major depressive disorder do not respond to pharmacotherapy.1 For patients with treatment-resistant depression (TRD)—typically defined as an inadequate response to at least 1 antidepressant trial of adequate dose and duration—neurostimulation may be an effective treatment option.
Two forms of neurostimulation used to treat TRD are deep brain stimulation (DBS) and transcranial magnetic stimulation (TMS). In DBS, electrodes are placed within the patient’s cranium and affixed to specific target locations. These electrodes are electrically stimulated at various frequencies. Transcranial magnetic stimulation is a noninvasive treatment in which a magnetic field is produced over a patient’s cranium, stimulating brain tissue via electromagnetic induction.
Media portraya
In this article, I compare DBS and TMS, and offer suggestions for educating patients about the potential adverse effects and therapeutic outcomes of each modality.
Deep brain stimulation
Deep brain stimulation is FDA-approved for treating Parkinson’s disease, essential tremor, dystonia, and obsessive-compulsive disorder (OCD).3 It has been used off-label for TRD, and some preliminary evidence suggests it is effective for this purpose. A review of 22 studies found that for patients with TRD, the rate of response to DBS (defined as >50% improvement on Hamilton Depression Rating Scale score) ranges from 40% to 70%.1 Additional research, including larger, randomized, sham-controlled trials, is needed.
A consensus on the optimal target location for DBS has not yet been reached. Studies have had varying degrees of symptom improvement targeting the subgenual cingulate gyrus, posterior gyrus rectus, nucleus accumbens, ventral capsule/ventral striatum, inferior thalamic peduncle, and lateral habenula.1
A worsening of depressive symptoms and increased risk of suicide have been reported in—but are not exclusive to—DBS. Patients treated with DBS may still meet the criteria for treatment resistance.
Continue to: The lack of insurance coverage...
The lack of insurance coverage for DBS for treating depression is a deterrent to its use. Because DBS is not FDA-approved for treating depression, the costs (approximately $65,000) that are not covered by a facility or study will fall on the patient.4 Patients may abandon hope for a positive therapeutic outcome if they must struggle with the financial responsibility for procedures and follow-up.4
Serious potential adverse events of DBS include infections, skin erosions, and postoperative seizure.4 Patients who are treated with DBS should be educated about these adverse effects, and how they may affect outcomes.
Transcranial magnetic stimulation
Transcranial magnetic stimulation is FDA-approved for treating depression, OCD, and migraine. Randomized, sham-controlled trials have found that TMS is effective for TRD.5 Studies have demonstrated varying degrees of efficacy, with response rates ranging from 47% to 58%.6
The most commonly used target area for TMS for patients with depression is the left dorsolateral prefrontal cortex.7 Potential adverse effects are relatively few and benign. The most serious adverse effect of TMS is a risk for seizure, which is reported to occur at a frequency of <0.1%.7
Although it varies by practice and location, the cost for an acute course of TMS (20 to 30 sessions) may range from $6,000 to $12,000.8 Most insurance companies cover TMS treatment for depression.
Continue to: TMS
TMS: A more accessible option
Compared with DBS, TMS is a more affordable and accessible therapy for patients with TRD. Further studies are needed to learn more about the therapeutic potential of DBS for TRD, and to develop methods that help decrease the risk of adverse effects. In addition, insurance coverage needs to be expanded to DBS to avoid having patients be responsible for the full costs of this treatment. Until then, TMS should be a recommended therapy for patients with TRD. If TRD persists in patients treated with TMS, consider electroconvulsive therapy.
1. Morishita T, Fayad SM, Higuchi MA, et al. Deep brain stimulation for treatment-resistant depression: systematic review of clinical outcomes. Neurotherapeutics. 2014;11(3):475-484.
2. Lawrence RE, Kaufmann CR, DeSilva RB, et al. Patients’ belief about deep brain stimulation for treatment resistant depression. AJOB Neuroscience, 2018;9(4):210-218.
3. Rossi PJ, Giordano J, Okun MS. The problem of funding off-label deep brain stimulation: bait-and-switch tactics and the need for policy reform. JAMA Neurol. 2017;74(1):9-10.
4. Holtzheimer PE, Husain MM, Lisanby SH, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4(11):839-849.
5. Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
6. Janicak PG, Sackett V, Kudrna K, et al. Advances in transcranial magnetic stimulation for managing major depressive disorders. Current Psychiatry. 2016;15(6):49-56.
7. Dobek CE, Blumberger DM, Downar J, et al. Risk of seizures in transcranial magnetic stimulation: a clinical review to inform consent process focused on bupropion. Neuropsychiatr Dis Treat. 2015;11:2975-2987.
8. McClintock SM, Reti IM, Carpenter LL, et al; National Network of Depression Centers rTMS Task Group; American Psychiatric Association Council on Research Task Force on Novel Biomarkers and Treatments. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. 2018;79(1). doi: 10.4088/JCP.16cs10905.
1. Morishita T, Fayad SM, Higuchi MA, et al. Deep brain stimulation for treatment-resistant depression: systematic review of clinical outcomes. Neurotherapeutics. 2014;11(3):475-484.
2. Lawrence RE, Kaufmann CR, DeSilva RB, et al. Patients’ belief about deep brain stimulation for treatment resistant depression. AJOB Neuroscience, 2018;9(4):210-218.
3. Rossi PJ, Giordano J, Okun MS. The problem of funding off-label deep brain stimulation: bait-and-switch tactics and the need for policy reform. JAMA Neurol. 2017;74(1):9-10.
4. Holtzheimer PE, Husain MM, Lisanby SH, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4(11):839-849.
5. Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
6. Janicak PG, Sackett V, Kudrna K, et al. Advances in transcranial magnetic stimulation for managing major depressive disorders. Current Psychiatry. 2016;15(6):49-56.
7. Dobek CE, Blumberger DM, Downar J, et al. Risk of seizures in transcranial magnetic stimulation: a clinical review to inform consent process focused on bupropion. Neuropsychiatr Dis Treat. 2015;11:2975-2987.
8. McClintock SM, Reti IM, Carpenter LL, et al; National Network of Depression Centers rTMS Task Group; American Psychiatric Association Council on Research Task Force on Novel Biomarkers and Treatments. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. 2018;79(1). doi: 10.4088/JCP.16cs10905.
Antidepressants for pediatric patients
Major depressive disorder (MDD) is a significant pediatric health problem, with a lifetime prevalence as high as 20% by the end of adolescence.1-3 Major depressive disorder in adolescence is associated with significant morbidity, including poor social functioning, school difficulties, early pregnancy, and increased risk of physical illness and substance abuse.4-6 It is also linked with significant mortality, with increased risk for suicide, which is now the second leading cause of death in individuals age 10 to 24 years.1,7,8
As their name suggests, antidepressants comprise a group of medications that are used to treat MDD; they are also, however, first-line agents for generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD) in adults. Anxiety disorders (including GAD and other anxiety diagnoses) and PTSD are also common in childhood and adolescence with a combined lifetime prevalence ranging from 15% to 30%.9,10 These disorders are also associated with increased risk of suicide.11 For all of these disorders, depending on the severity of presentation and the preference of the patient, treatments are often a combination of psychotherapy and psychopharmacology.
Clinicians face several challenges when considering antidepressants for pediatric patients. Pediatricians and psychiatrists need to understand whether these medications work in children and adolescents, and whether there are unique developmental safety and tolerability issues. The evidence base in child psychiatry is considerably smaller compared with that of adult psychiatry. From this more limited evidence base also came the controversial “black-box” warning regarding a risk of emergent suicidality when starting antidepressants that accompanies all antidepressants for pediatric, but not adult, patients. This warning has had major effects on clinical encounters with children experiencing depression, including altering clinician prescribing behavior.12
Do antidepressants work in children?
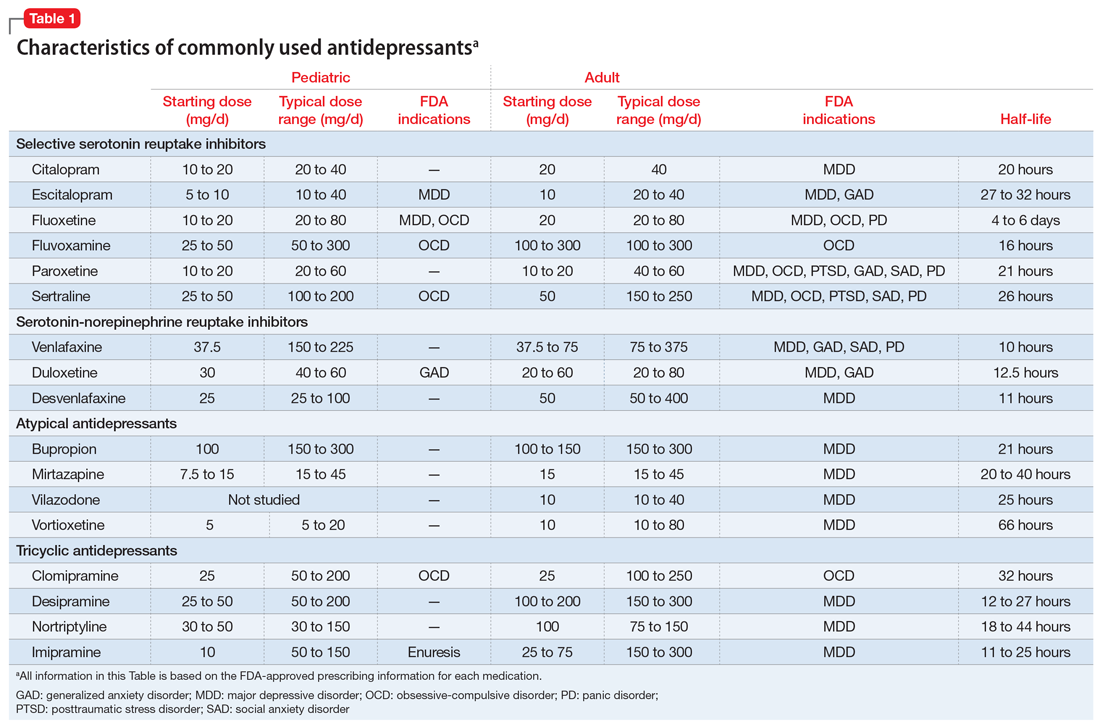
Selective serotonin reuptake inhibitors. Selective serotonin reuptake inhibitors (SSRIs) are the most commonly used class of antidepressants in both children and adults.13 While only a few SSRIs are FDA-approved for pediatric indications, the lack of FDA approval is typically related to a lack of sufficient testing in randomized controlled trials (RCTs) for specific pediatric indications, rather than to demonstrable differences in efficacy between antidepressant agents. Since there is currently no data to suggest inferiority of one agent compared to another in children or adults,14,15 efficacy data will be discussed here as applied to the class of SSRIs, generalizing from RCTs conducted on individual drugs. Table 1 lists FDA indications and dosing information for individual antidepressants.
There is strong evidence that SSRIs are effective for treating pediatric anxiety disorders (eg, social anxiety disorder and GAD)16 and OCD,17 with numbers needed to treat (NNT) between 3 and 5. For both of these disorders, SSRIs combined with cognitive-behavioral therapy (CBT) have the highest likelihood of improving symptoms or achieving remission.17,18
Selective serotonin reuptake inhibitors are also effective for treating pediatric MDD; however, the literature is more complex for this disorder compared to GAD and OCD as there are considerable differences in effect sizes between National Institute of Mental Health (NIMH)–funded studies and industry-sponsored trials.13 The major NIMH-sponsored adolescent depression trial, TADS (Treatment for Adolescents and Depression Study), showed that SSRIs (fluoxetine in this case) were quite effective, with an NNT of 4 over the acute phase (12 weeks).19 Ultimately, approximately 80% of adolescents improved over 9 months. Many industry-sponsored trials for MDD in pediatric patients had large placebo response rates (approximately 60%), which resulted in smaller between-group differences, and estimates of an NNT closer to 12,13 which has muddied the waters in meta-analyses that include all trials.20 Improvement in depressive symptoms also appears to be bolstered by concomitant CBT in MDD,19 but not as robustly as in GAD and OCD. While the full benefit of SSRIs for depression may take as long as 8 weeks, a meta-analysis of depression studies of pediatric patients suggests that significant benefits from placebo are observed as early as 2 weeks, and that further treatment gains are minimal after 4 weeks.15 Thus, we recommend at least a 4- to 6-week trial at therapeutic dosing before deeming a medication a treatment failure.
Continue to: Posttraumatic stress disorder...
Posttraumatic stress disorder is a fourth disorder in which SSRIs are a first-line treatment in adults. The data for using SSRIs to treat pediatric patients with PTSD is scant, with only a few RCTs, and no large NIMH-funded trials. Randomized controlled trials have not demonstrated significant differences between SSRIs and placebo21,22 and thus the current first-line recommendation in pediatric PTSD remains trauma-focused therapy, with good evidence for trauma-focused CBT.23 Practically speaking, there can be considerable overlap of PTSD, depression, and anxiety symptoms in children,23 and children with a history of trauma who also have comorbid MDD may benefit from medication if their symptoms persist despite an adequate trial of psychotherapy.
Taken together, the current evidence suggests that SSRIs are often effective in pediatric GAD, OCD, and MDD, with low NNTs (ranging from 3 to 5 based on NIMH-funded trials) for all of these disorders; there is not yet sufficient evidence of efficacy in pediatric patients with PTSD.
Fluoxetine has been studied more intensively than other SSRIs (for example, it was the antidepressant used in the TADS trial), and thus has the largest evidence base. For this reason, fluoxetine is often considered the first of the first-line options. Additionally, fluoxetine has a longer half-life than other antidepressants, which may make it more effective in situations where patients are likely to miss doses, and results in a lower risk of withdrawal symptoms when stopped due to “self-tapering.”
SNRIs and atypical antidepressants. Other antidepressants commonly used in pediatric patients but with far less evidence of efficacy include serotonin-norepinephrine reuptake inhibitors (SNRIs) and the atypical antidepressants bupropion and mirtazapine. The SNRI duloxetine is FDA-approved for treating GAD in children age 7 to 17, but there are no other pediatric indications for duloxetine, or for the other SNRIs.
In general, adverse effect profiles are worse for SNRIs compared to SSRIs, further limiting their utility. While there are no pediatric studies demonstrating SNRI efficacy for neuropathic pain, good data exists in adults.24 Thus, an SNRI could be a reasonable option if a pediatric patient has failed prior adequate SSRI trials and also has comorbid neuropathic pain.
Continue to: Neither bupropion nor mirtazapine...
Neither bupropion nor mirtazapine have undergone rigorous testing in pediatric patients, and therefore these agents should generally be considered only once other first-line treatments have failed. Bupropion has been evaluated for attention-deficit/hyperactivity disorder (ADHD)25 and for adolescent smoking cessation.26 However, the evidence is weak, and bupropion is not considered a first-line option for children and adolescents.
Tricyclic antidepressants. Randomized controlled trials have demonstrated that tricyclic antidepressants (TCAs) are efficacious for treating several pediatric conditions; however, their significant side effect profile, their monitoring requirements, as well as their lethality in overdose has left them replaced by SSRIs in most cases. That said, they can be appropriate in refractory ADHD (desipramine27,28) and refractory OCD (clomipramine is FDA-approved for this indication29); they are considered a third-line treatment for enuresis.30
Why did my patient stop the medication?
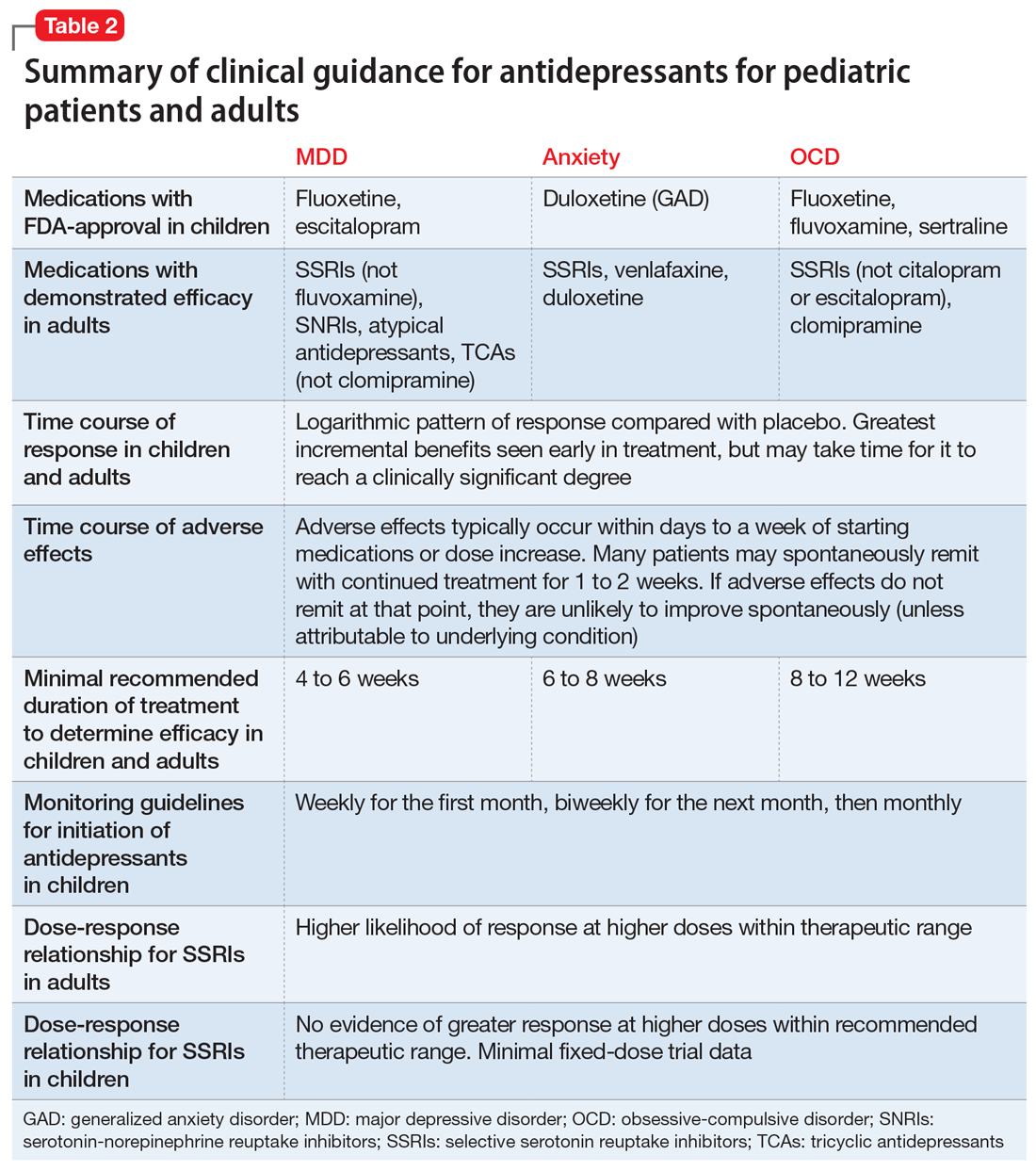
Common adverse effects. Although the greatest benefit of antidepressant medications compared with placebo is achieved relatively early on in treatment, it generally takes time for these benefits to accrue and become clinically apparent.15,31 By contrast, most adverse effects of antidepressants present and are at their most severe early in treatment. The combination of early adverse effects and delayed efficacy leads many patients, families, and clinicians to discontinue medications before they have an adequate chance to work. Thus, it is imperative to provide psychoeducation before starting a medication about the typical time-course of improvement and adverse effects (Table 2).
Adverse effects of SSRIs often appear or worsen transiently during initiation of a medication, during a dose increase,32 or, theoretically, with the addition of a medication that interferes with SSRI metabolism (eg, cimetidine inhibition of cytochrome P450 2D6).33 If families are prepared for this phenomenon and the therapeutic alliance is adequate, adverse effects can be tolerated to allow for a full medication trial. Common adverse effects of SSRIs include sleep problems (insomnia/sedation), gastrointestinal upset, sexual dysfunction, dry mouth, and hyperhidrosis. Although SSRIs differ somewhat in the frequency of these effects, as a class, they are more similar than different. Adequate psychoeducation is especially imperative in the treatment of OCD and anxiety disorders, where there is limited evidence of efficacy for any non-serotonergic antidepressants.
Serotonin-norepinephrine reuptake inhibitors are not considered first-line medications because of the reduced evidence base compared to SSRIs and their enhanced adverse effect profiles. Because SNRIs partially share a mechanism of action with SSRIs, they also share portions of the adverse effects profile. However, SNRIs have the additional adverse effect of hypertension, which is related to their noradrenergic activity. Thus, it is reasonable to obtain a baseline blood pressure before initiating an SNRI, as well as periodically after initiation and during dose increases, particularly if the patient has other risk factors for hypertension.34
Continue to: Although TCAs have efficacy...
Although TCAs have efficacy in some pediatric disorders,27-29,35 their adverse effect profile limits their use. Tricyclic antidepressants are highly anticholinergic (causing dizziness secondary to orthostatic hypotension, dry mouth, and urinary retention) and antihistaminergic (causing sedation and weight gain). Additionally, TCAs lower the seizure threshold and have adverse cardiac effects relating to their anti-alpha-1 adrenergic activity, resulting in dose-dependent increases in the QTc and cardiac toxicity in overdose that could lead to arrhythmia and death. These medications have their place, but their use requires careful informed consent, clear treatment goals, and baseline and periodic cardiac monitoring (via electrocardiogram).
Serious adverse effects. Clinicians may be hesitant to prescribe antidepressants for pediatric patients because of the potential for more serious adverse effects, including severe behavioral activation syndromes, serotonin syndrome, and emergent suicidality. However, current FDA-approved antidepressants arguably have one of the most positive risk/benefit profiles of any orally-administered medication approved for pediatric patients. Having a strong understanding of the evidence is critical to evaluating when it is appropriate to prescribe an antidepressant, how to properly monitor the patient, and how to obtain accurate informed consent.
Pediatric behavioral activation syndrome. Many clinicians report that children receiving antidepressants experience a pediatric behavioral activation syndrome, which exists along a spectrum from mild activation, increased energy, insomnia, or irritability up through more severe presentations of agitation, hyperactivity, or possibly mania. A recent meta-analysis suggested a positive association between antidepressant use and activation events on the milder end of this spectrum in pediatric patients with non-OCD anxiety disorders,16 and it is thought that compared with adolescents, younger children are more susceptible to activation adverse effects.36 The likelihood of activation events has been associated with higher antidepressant plasma levels,37 suggesting that dose or individual differences in metabolism may play a role. At the severe end of the spectrum, the risk of induction of mania in pediatric patients with depression or anxiety is relatively rare (<2%) and not statistically different from placebo in RCTs of pediatric participants.38 Meta-analyses of larger randomized, placebo-controlled trials of adults do not support the idea that SSRIs and other second-generation antidepressants carry an increased risk of mania compared with placebo.39,40 Children or adolescents with bona fide bipolar disorder (ie, patients who have had observed mania that meets all DSM-5 criteria) should be treated with a mood-stabilizing agent or antipsychotic if prescribed an antidepressant.41 These clear-cut cases are, however, relatively rare, and more often clinicians are confronted with ambiguous cases that include a family history of bipolar disorder along with “softer” symptoms of irritability, intrusiveness, or aggression. In these children, SSRIs may be appropriate for depressive, OCD, or anxiety symptoms, and should be strongly considered before prescribing antipsychotics or mood stabilizers, as long as initiated with proper monitoring.
Serotonin syndrome is a life-threatening condition caused by excess synaptic serotonin. It is characterized by confusion, sweating, diarrhea, hypertension, hyperthermia, and tachycardia. At its most severe, serotonin syndrome can result in seizures, arrhythmias, and death. The risk of serotonin syndrome is very low when using an SSRI as monotherapy. Risk increases with polypharmacy, particularly unexamined polypharmacy when multiple serotonergic agents are inadvertently on board. Commonly used serotonergic agents include other antidepressants, migraine medications (eg, triptans), some pain medications, and the cough suppressant dextromethorphan.
The easiest way to mitigate the risk of serotonin syndrome is to use an interaction index computer program, which can help ensure that the interacting agents are not prescribed without first discussing the risks and benefits. It is important to teach adolescents that certain recreational drugs are highly serotonergic and can cause serious interactions with antidepressants. For example, recreational use of dextromethorphan or 3,4-methylenedioxymethamphetamine (MDMA; commonly known as “ecstasy”) has been associated with serotonin syndrome in adolescents taking antidepressant medications.42,43
Continue to: Suicidality
Suicidality. The black-box warning regarding a risk of emergent suicidality when starting antidepressant treatment in children is controversial.44 The prospect that a medication intended to ameliorate depression might instead risk increasing suicidal thinking is alarming to parents and clinicians alike. To appropriately weigh and discuss the risks and benefits with families, it is important to understand the data upon which the warning is based.
In 2004, the FDA commissioned a review of 23 antidepressant trials, both published and unpublished, pooling studies across multiple indications (MDD, OCD, anxiety, and ADHD) and multiple antidepressant classes. This meta-analysis, which included nearly 4,400 pediatric patients, found a small but statistically significant increase in spontaneously-reported suicidal thoughts or actions, with a risk difference of 1% (95% confidence interval [CI], 1% to 2%).45 These data suggest that if one treats 100 pediatric patients, 1 to 2 of them may experience short-term increases in suicidal thinking or behavior.45 There were no differences in suicidal thinking when assessed systematically (ie, when all subjects reported symptoms of suicidal ideation on structured rating scales), and there were no completed suicides.45 A subsequent analysis that included 27 pediatric trials suggested an even lower, although still significant, risk difference (<1%), yielding a number needed to harm (NNH) of 143.46 Thus, with low NNT for efficacy (3 to 6) and relatively high NNH for emergent suicidal thoughts or behaviors (100 to 143), for many patients the benefits will outweigh the risks.
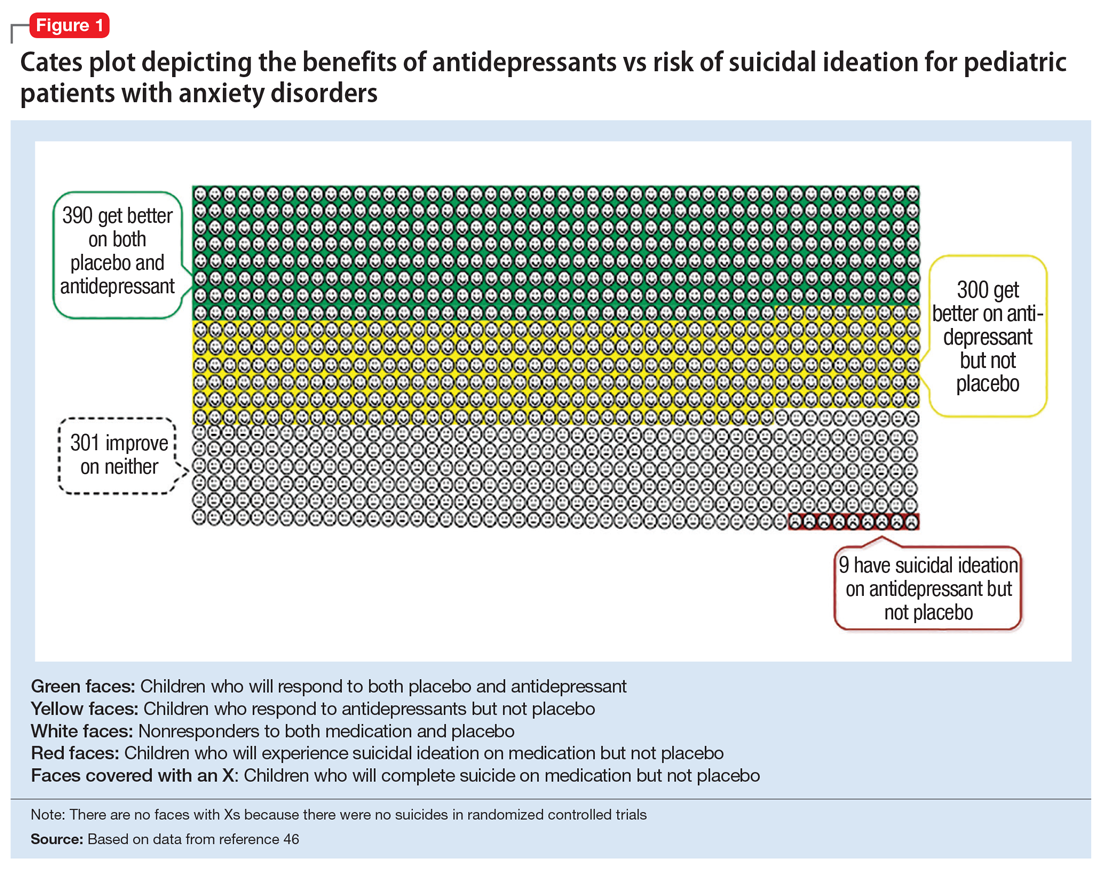
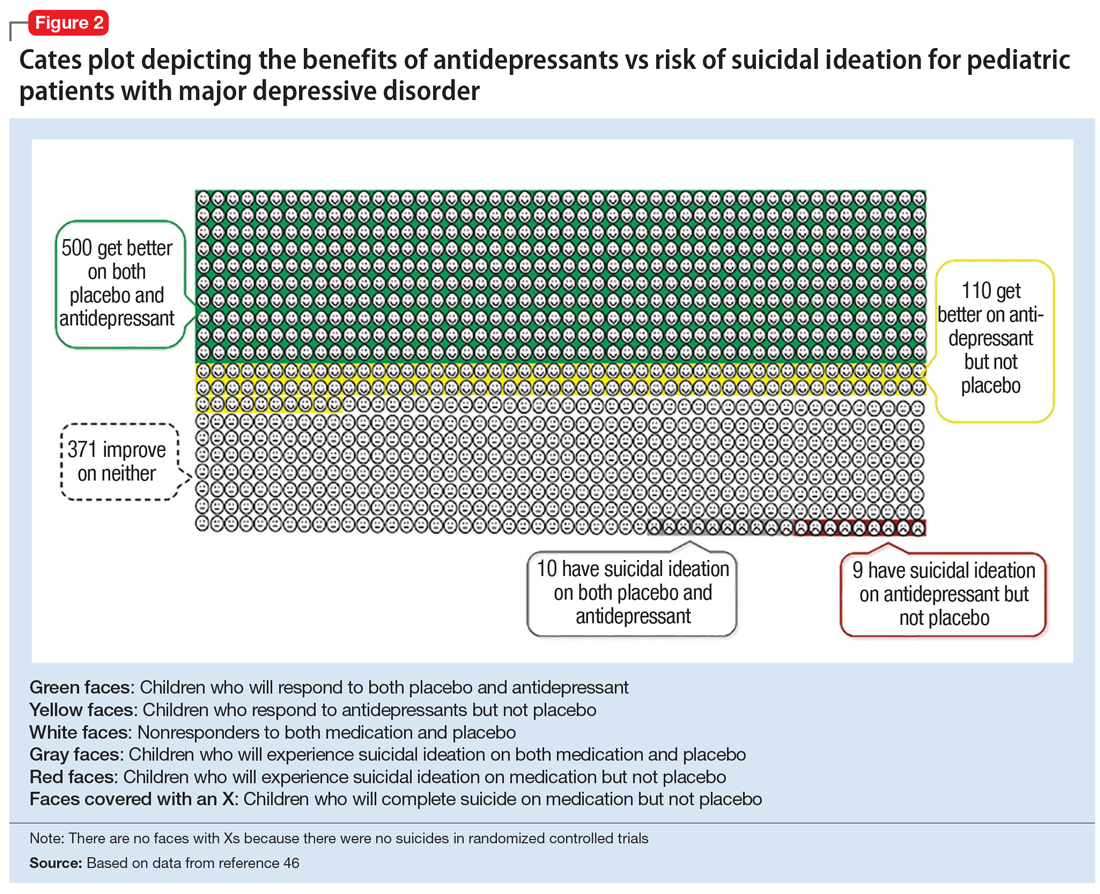
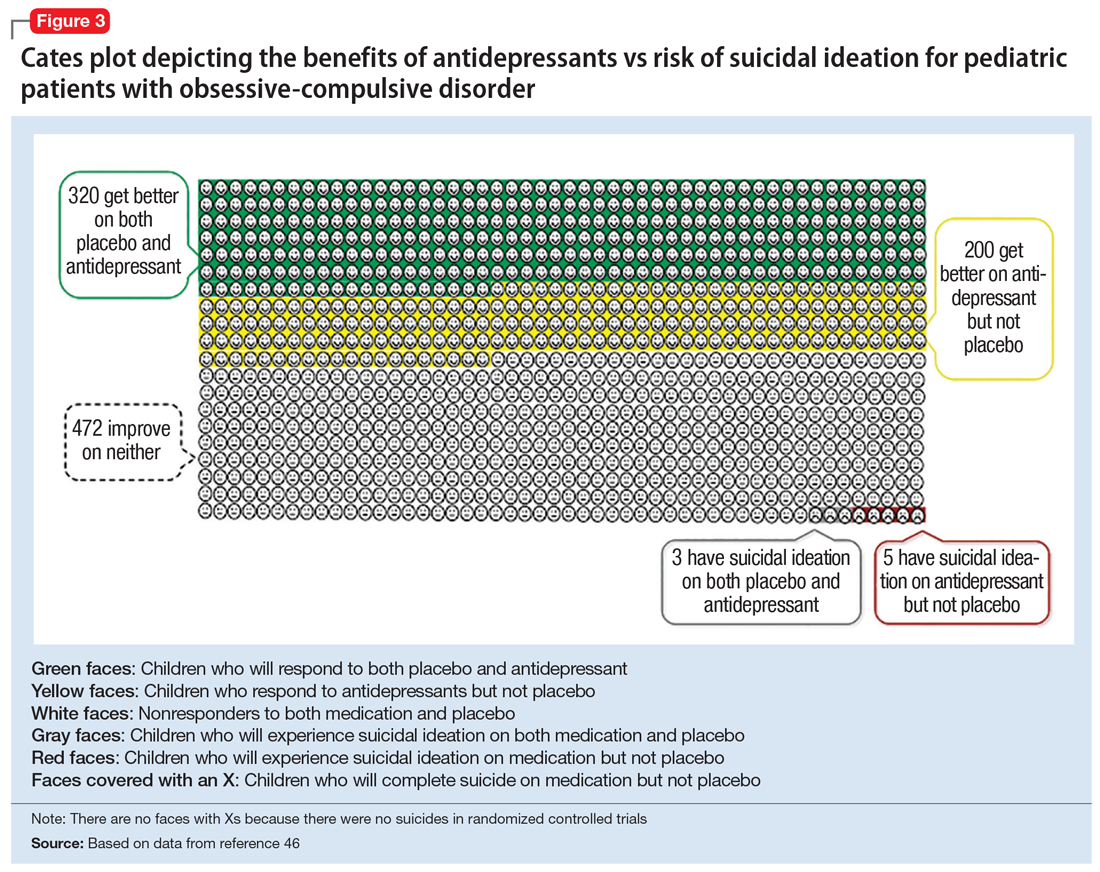
Figure 1, Figure 2, and Figure 3 are Cates plots that depict the absolute benefits of antidepressants compared with the risk of suicidality for pediatric patients with MDD, OCD, and anxiety disorders. Recent meta-analyses have suggested that the increased risk of suicidality in antidepressant trials is specific to studies that included children and adolescents, and is not observed in adult studies. A meta-analysis of 70 trials involving 18,526 participants suggested that the odds ratio of suicidality in trials of children and adolescents was 2.39 (95% CI, 1.31 to 4.33) compared with 0.81 (95% CI, 0.51 to 1.28) in adults.47 Additionally, a network meta-analysis exclusively focusing on pediatric antidepressant trials in MDD reported significantly higher suicidality-related adverse events in venlafaxine trials compared with placebo, duloxetine, and several SSRIs (fluoxetine, paroxetine, and escitalopram).20 These data should be interpreted with caution as differences in suicidality detected between agents is quite possibly related to differences in the method of assessment between trials, as opposed to actual differences in risk between agents.
Epidemiologic data further support the use of antidepressants in pediatric patients, showing that antidepressant use is associated with decreased teen suicide attempts and completions,48 and the decline in prescriptions that occurred following the black-box warning was accompanied by a 14% increase in teen suicides.49 Multiple hypotheses have been proposed to explain the pediatric clinical trial findings. One idea is that potential adverse effects of activation, or the intended effects of restoring the motivation, energy, and social engagement that is often impaired in depression, increases the likelihood of thinking about suicide or acting on thoughts. Another theory is that reporting of suicidality may be increased, rather than increased de novo suicidality itself. Antidepressants are effective for treating pediatric anxiety disorders, including social anxiety disorder,16 which could result in more willingness to report. Also, the manner in which adverse effects are generally ascertained in trials might have led to increased spontaneous reporting. In many trials, investigators ask whether participants have any adverse effects in general, and inquire about specific adverse effects only if the family answers affirmatively. Thus, the increased rate of other adverse effects associated with antidepressants (sleep problems, gastrointestinal upset, dry mouth, etc.) might trigger a specific question regarding suicidal ideation, which the child or family then may be more likely to report. Alternatively, any type of psychiatric treatment could increase an individual’s propensity to report; in adolescent psychotherapy trials, non-medic
Continue to: How long should the antidepressant be continued?
How long should the antidepressant be continued?
Many patients are concerned about how long they may be taking medication, and whether they will be taking an antidepressant “forever.” A treatment course can be broken into an acute phase, wherein remission is achieved and maintained for 6 to 8 weeks. This is followed by a continuation phase, with the goal of relapse prevention, lasting 16 to 20 weeks. The length of the last phase—the maintenance phase—depends both on the child’s history, the underlying therapeutic indication, the adverse effect burden experienced, and the family’s preferences/values. In general, for a first depressive episode, after treating for 1 year, a trial of discontinuation can be attempted with close monitoring. For a second depressive episode, we recommend 2 years of remission on antidepressant therapy before attempting discontinuation. In patients who have had 3 depressive episodes, or have had episodes of high severity, we recommend continuing antidepressant treatment indefinitely. Although much less well studied, the risk of relapse following SSRI discontinuation appears much more significant in OCD, whereas anxiety disorders and MDD have a relatively comparable risk.52
In general, stopping an antidepressant should be done carefully and slowly.
What to do when first-line treatments fail
Adequate psychotherapy? To determine whether children are receiving adequate CBT, ask:
- if the child receives homework from psychotherapy
- if the parents are included in treatment
- if therapy has involved identifying thought patterns that may be contributing to the child’s illness, and
- if the therapist has ever exposed the child to a challenge likely to produce anxiety or distress in a supervised environment and has developed an exposure hierarchy (for conditions with primarily exposure-based therapies, such as OCD or anxiety disorders).
If a family is not receiving most of these elements in psychotherapy, this is a good indicator that they may not be receiving evidence-based CBT.
Continue to: Adequate pharmacotherapy?
Adequate pharmacotherapy? Similarly, when determining the adequacy of previous pharmacotherapy, it is critical to determine whether the child received an adequate dose of medications (at least the FDA-recommended minimum dose) for an adequate duration of time at therapeutic dosing (at least 6 weeks for MDD, 8 weeks for anxiety disorders, and 8 to 12 weeks for pediatric patients with OCD), and that the child actually took the medication regularly during that period. Patient compliance can typically be tracked through checking refill requests or intervals through the patient’s pharmacy. Ensuring proper delivery of first-line treatments is imperative because (1) the adverse effects associated with second-line treatments are often more substantial; (2) the cost in terms of time and money is considerably higher with second-line treatments, and; (3) the evidence regarding the benefits of these treatments is much less certain.
Inadequate dosing is a common reason for non-response in pediatric patients. Therapeutic dose ranges for common antidepressants are displayed in Table 1. Many clinicians underdose antidepressants for pediatric patients initially (and often throughout treatment) because they fear that the typical dose titration used in clinical trials will increase the risk of adverse effects compared with more conservative dosing. There is limited evidence to suggest that this underdosing strategy is likely to be successful; adverse effects attributable to these medications are modest, and most that are experienced early in treatment (eg, headache, increased anxiety or irritability, sleep problems, gastrointestinal upset) are self-limiting and may be coincidental rather than medication-induced. Furthermore, there is no evidence for efficacy of subtherapeutic dosing in children in the acute phase of treatment or for preventing relapse.14 Thus, from an efficacy standpoint, a medication trial has not officially begun until the therapeutic dose range is reached.
Once dosing is within the therapeutic range, however, pediatric data differs from the adult literature. In most adult psychiatric conditions, higher doses of SSRIs within the therapeutic range are associated with an increased response rate.14,54 In pediatrics, there are few fixed dose trials, and once within the recommended therapeutic range, minimal data supports an association between higher dosing and higher efficacy.14 In general, pediatric guidelines are silent regarding optimal dosing of SSRIs within the recommended dose range, and higher antidepressant doses often result in a more significant adverse effect burden for children. One exception is pediatric OCD, where, similar to adults, the guidelines suggest that higher dosing of SSRIs often is required to induce a therapeutic response as compared to MDD and GAD.31,55
If a child does not respond to adequate first-line treatment (or has a treatment history that cannot be fully verified), repeating these first-line interventions carries little risk and can be quite effective. For example, 60% of adolescents with MDD who did not initially respond to an SSRI demonstrated a significant response when prescribed a second SSRI or venlafaxine (with or without CBT).56
When pediatric patients continue to experience significantly distressing and/or debilitating symptoms (particularly in MDD) despite multiple trials of antidepressants and psychotherapy, practitioners should consider a careful referral to interventional psychiatry services, which can include the more intensive treatments of electroconvulsive therapy, repetitive transcranial magnetic stimulation, or ketamine (see Box 1). Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and under-utilized clinically in pediatric populations.
Continue to: Antidepressants in general...
Antidepressants in general, and SSRIs in particular, are the first-line pharmacotherapy for pediatric anxiety, OCD, and MDD. For PTSD, although they are a first-line treatment in adults, their efficacy has not been demonstrated in children and adolescents. Antidepressants are generally safe, well-tolerated, and effective, with low NNTs (3 to 5 for anxiety and OCD; 4 to 12 in MDD, depending on whether industry trials are included). It is important that clinicians and families be educated about possible adverse effects and their time course in order to anticipate difficulties, ensure adequate informed consent, and monitor appropriately. The black-box warning regarding treatment-emergent suicidal thoughts or behaviors must be discussed (for suggested talking points, see Box 2). The NNH is large (100 to 143), and for many patients, the benefits will outweigh the risks. For pediatric patients who fail to respond to multiple adequate trials of antidepressants and psychotherapy, referrals for interventional psychiatry consultation should be considered.
Bottom Line
Although the evidence base for prescribing antidepressants for children and adolescents is smaller compared to the adult literature, properly understanding and prescribing these agents, and explaining their risks and benefits to families, can make a major difference in patient compliance, satisfaction, and outcomes. Antidepressants (particularly selective serotonin reuptake inhibitors) are the firstline pharmacologic intervention for pediatric patients with anxiety disorders, obsessive-compulsive disorder, or major depressive disorder.
Related Resource
- Bonin L, Moreland CS. Overview of prevention and treatment for pediatric depression. https://www.uptodate.com/contents/overview-of-prevention-and-treatment-for-pediatric-depression. Last reviewed July 2019.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cimetidine • Tagamet
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Box 1
Continuing severe depression is associated with reduced educational attainment and quality of life, as well as increased risk of substance abuse and suicide,1,2 which is the second leading cause of death in individuals age 10 to 24 years.3 Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and underutilized in pediatric patients. Interventional antidepressants in adults include electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), and, most recently, ketamine.
Electroconvulsive therapy is the most effective therapy available for depression in adults, alleviating depressive symptoms in treatment-refractory patients and outperforming both pharmacotherapy4 and rTMS.5 Despite its track record of effectiveness and safety in adults, ECT continues to suffer considerable stigma.4 Cognitive adverse effects and memory problems in adults are generally self-limited, and some aspects of cognition actually improve after ECT as depression lifts.6 The combination of stigma and the concern about possible cognitive adverse effects during periods of brain development have likely impeded the rigorous testing of ECT in treatment-refractory pediatric patients. Several case series and other retrospective analyses suggest, however, that ECT has strong efficacy and limited adverse effects in adolescents who have severe depression or psychotic symptoms.7-9 Despite these positive preliminary data in pediatric patients, and a large body of literature in adults, no controlled trials of ECT have been conducted in the pediatric population, and it remains a rarely used treatment in severe pediatric mental illness.
Repetitive transcranial magnetic stimulation is a technique in which magnetic stimulation is used to activate the left dorsolateral prefrontal cortex (DLPFC), a target thought to be important in the pathophysiology of MDD. Repetitive transcranial magnetic stimulation is FDAapproved to treat medication-refractory major depressive disorder (MDD) in adults, and has been shown to be effective as both a monotherapy10 and an adjunctive treatment.11 The estimated number needed to treat (NNT) for rTMS ranges from 6 to 8, which is quite effective, although less so than ECT (and probably initial pharmacotherapy).5 Similar to ECT, however, there are no large randomized controlled trials (RCTs) in children or adolescents. Pilot RCTs12 and open trials13 suggest that DLPFC rTMS may be effective as an adjunctive treatment, speeding or augmenting response to a selective serotonin reuptake inhibitor in adolescents with MDD. Larger trials studying rTMS in pediatric patients are needed. While rTMS is generally well tolerated, disadvantages include the time-consuming schedule (the initial treatment is typically 5 days/week for several weeks) and local adverse effects of headache and scalp pain.
Ketamine, which traditionally is used as a dissociative anesthetic, is a rapidly emerging novel treatment in adult treatment-refractory MDD. It acts quickly (within hours to days) and cause significant improvement in difficult symptoms such as anhedonia14 and suicidal ideation.15 In adult studies, ketamine has a robust average effect size of >1.2, and an NNT ranging from 3 to 5 in medication-refractory patients.16,17 Ketamine is a glutamatergic modulator, acting outside of the monoamine neurochemical systems traditionally targeted by standard antidepressants.16 The efficacy of ketamine in treatment-refractory adults is impressive, but the effects of a single treatment are ephemeral, dissipating within 1 to 2 weeks, which has led to significant discussion surrounding optimal dosing strategies.16 Although small RCTs in pediatric patients are currently underway, at this time, the only evidence for ketamine for pediatric MDD is based on case series/report data18,19 which was positive.
For all of these interventional modalities, it is critical to refer children with treatmentrefractory disorders to interventionists who have appropriate experience and monitoring capabilities.
References
1. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
2. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
3. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
4. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and metaanalysis. Lancet. 2003;361(9360):799-808.
5. Berlim MT, Van den Eynde F, Daskalakis ZJ. Efficacy and acceptability of high frequency repetitive transcranial magnetic stimulation (rTMS) versus electroconvulsive therapy (ECT) for major depression: a systematic review and meta-analysis of randomized trials. Depress Anxiety. 2013;30(7):614-623.
6. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
7. Jacob P, Gogi PK, Srinath S, et al. Review of electroconvulsive therapy practice from a tertiary child and adolescent psychiatry centre. Asian J Psychiatr. 2014;12(1):95-99.
8. Zhand N, Courtney DB, Flament MF. Use of electroconvulsive therapy in adolescents with treatment-resistant depressive disorders: a case series. J ECT. 2015;31(4):238-245.
9. Puffer CC, Wall CA, Huxsahl JE, et al. A 20 year practice review of electroconvulsive therapy for adolescents. J Child Adolesc Psychopharmacol. 2016;26(7):632-636.
10. Berlim MT, van den Eynde F, Tovar-Perdomo S, et al. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44(2):225-239.
11. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342.
12. Huang ML, Luo BY, Hu JB, et al. Repetitive transcranial magnetic stimulation in combination with citalopram in young patients with first-episode major depressive disorder: a double-blind, randomized, sham-controlled trial. Aust N Z J Psychiatry. 2012;46(3):257-264.
13. Wall CA, Croarkin PE, Sim LA, et al. Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. J Clin Psychiatry. 2011;72(9):1263-1269.
14. Lally N, Nugent AC, Luckenbaugh DA, et al. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105.
15. Ballard ED, Ionescu DF, Vande Voort JL, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161-166.
16. Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
17. McGirr A, Berlim MT, Bond DJ, et al. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45(4):693-704.
18. Dwyer JB, Beyer C, Wilkinson ST, et al. Ketamine as a treatment for adolescent depression: a case report. J Am Acad Child Adolesc Psychiatry. 2017;56(4):352-354.
19. Cullen KR, Amatya P, Roback MG, et al. Intravenous ketamine for adolescents with treatment-resistant depression: an open-label study. J Child Adolesc Psychopharmacol. 2018;28(7):437-444.
Box 2
Efficacy
- Selective serotonin reuptake inhibitors are the most effective pharmacologic treatment we have for pediatric depression, OCD, and anxiety
- More than one-half of children who are prescribed SSRIs have a significant improvement, regardless of condition
- Based on current estimates, we need to treat 4 to 6 children with an SSRI to find one that will improve who would not improve with placebo
- The clinical benefits of SSRIs generally take a while to accrue; therefore, it is advisable to take the medication for at least 2 to 3 months before concluding that it is ineffective
- In addition to medication, evidence-based psychotherapies provide significant benefit for pediatric depression, OCD, and anxiety
Tolerability
- Most commonly prescribed pediatric antidepressants have been used safely in children for 2 to 3 decades. The safety profiles of SSRIs are among the best of any medications used for children and adolescents
- While many children get better when taking these medications, it’s important that we also talk about potential adverse effects. Some children will experience sleep problems (either sleepier than usual or difficulty sleeping), changes in energy levels, headache, gastrointestinal upset, and dry mouth. These are most likely at the beginning of treatment, or when we increase the dose; they usually are time-limited and go away on their own
- Often adverse effects occur first and the benefits come later. Because it may take at least a few weeks to start to see the mood/anxiety benefits, it’s important for us to talk about any adverse effects your child experiences and remember that they usually are short-lived
Suicidality
- The FDA placed a “black-box” warning on antidepressants after pediatric studies found a small but statistically significant increased risk of reporting suicidal thoughts or behaviors over the short-term compared with placebo
- The increased risk of spontaneously reporting suicidal ideation was quite small. Studies suggested that one would need to treat 100 to 140 children to see 1 child report suicidal ideation compared to placebo. Suicidal ideation is a common symptom in children with depression and anxiety
- Studies found no increased risk when suicidal ideation was systematically assessed using structured rating scales
- In the studies evaluated, there were no completed suicides by patients taking medication or placebo
- Population studies show that higher rates of antidepressant prescriptions are associated with lower rates of attempted and completed teen suicide, which underscores that in general, these medicines treat the underlying causes of suicidality
- No scientific consensus exists on whether these medications are truly associated with an increased risk of new-onset suicidal ideation, or if this association is due to other factors (eg, improvement in anxiety and depressive symptoms that make patients more comfortable to report suicidal ideation spontaneously)
- Regardless, the FDA recommends frequent monitoring of children for suicidal thoughts when these medications are started. This should be done anyway in children experiencing depression and anxiety, and it’s why we will plan to have more frequent appointments as the medication is initiated
OCD: obsessive-compulsive disorder; SSRIs: selective serotonin reuptake inhibitors
1. Williams SB, O’Connor EA, Eder M, et al. Screening for child and adolescent depression in primary care settings: a systematic evidence review for the US Preventive Services Task Force. Pediatrics. 2009;123(4):e716-e735. doi: 10.1542/peds.2008-2415.
2. Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49(12):1002-1014.
3. Lewinsohn PM, Clarke GN, Seeley JR, et al. Major depression in community adolescents: age at onset, episode duration, and time to recurrence. J Am Acad Child Adolesc Psychiatry. 1994;33(6):809-818.
4. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
5. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
6. Keenan-Miller D, Hammen CL, Brennan PA. Health outcomes related to early adolescent depression. J Adolesc Health. 2007; 41(3): 256-62.
7. Shaffer D, Gould MS, Fisher P, et al. Psychiatric diagnosis in child and adolescent suicide. Arch Gen Psychiatry. 1996;53(4):339-348.
8. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
9. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
10. Wittchen HU, Nelson CB, Lachner G. Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychol Med. 1998;28(1):109-126.
11. Foley DL, Goldston DB, Costello EJ, et al. Proximal psychiatric risk factors for suicidality in youth: the Great Smoky Mountains Study. Arch Gen Psychiatry. 2006;63(9):1017-1024.
12. Cheung A, Sacks D, Dewa CS, et al. Pediatric prescribing practices and the FDA black-box warning on antidepressants. J Dev Behav Pediatr. 2008 29(3):213-215.
13. Walkup JT. Antidepressant efficacy for depression in children and adolescents: industry- and NIMH-funded studies. Am J Psychiatry. 2017;174(5):430-437.
14. Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173(2):174-183.
15. Varigonda AL, Jakubovski E, Taylor MJ, et al. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors in pediatric major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(7):557-564.
16. Strawn JR, Welge JA, Wehry AM, et al. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32(3):149-157.
17. March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA. 1998;280(20):1752-1756.
18. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
19. Kennard BD, Silva SG, Tonev S, et al. Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48(2):186-195.
20. Cipriani A, Zhou X, Del Giovane C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388(10047):881-890.
21. Cohen JA, Mannarino AP, Perel JM, et al. A pilot randomized controlled trial of combined trauma-focused CBT and sertraline for childhood PTSD symptoms. J Am Acad Child Adolesc Psychiatry. 2007;46(7):811-819.
22. Robb AS, Cueva JE, Sporn J, et al. Sertraline treatment of children and adolescents with posttraumatic stress disorder: a double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2010;20(6):463-471.
23. Diehle J, Opmeer BC, Boer F, et al. Trauma-focused cognitive behavioral therapy or eye movement desensitization and reprocessing: what works in children with posttraumatic stress symptoms? A randomized controlled trial. Eur Child Adolesc Psychiatry. 2015;24(2):227-236.
24. Aiyer R, Barkin RL, Bhatia A. Treatment of neuropathic pain with venlafaxine: a systematic review. Pain Med. 2017;18(10):1999-2012.
25. Barrickman LL, Perry PJ, Allen AJ, et al. Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34(5):649-657.
26. Monuteaux MC, Spencer TJ, Faraone SV, et al. A randomized, placebo-controlled clinical trial of bupropion for the prevention of smoking in children and adolescents with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2007;68(7):1094-1101.
27. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
28. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656.
29. DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder--a multicenter trial. J Am Acad Child Adolesc Psychiatry. 1992;31(1):45-49.
30. Caldwell PH, Sureshkumar P, Wong WC. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev. 2016;(1):CD002117.
31. Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(10):851-859.e2. doi: 10.1016/j.jaac.2016.07.768.
32. Walkup J, Labellarte M. Complications of SSRI treatment. J Child Adolesc Psychopharmacol. 2001;11(1):1-4.
33. Leo RJ, Lichter DG, Hershey LA. Parkinsonism associated with fluoxetine and cimetidine: a case report. J Geriatr Psychiatry Neurol. 1995;8(4):231-233.
34. Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(4):283-293.
35. Bernstein GA, Borchardt CM, Perwien AR, et al. Imipramine plus cognitive-behavioral therapy in the treatment of school refusal. J Am Acad Child Adolesc Psychiatry. 2000;39(3): 276-283.
36. Safer DJ, Zito JM. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: children versus adolescents. J Child Adolesc Psychopharmacol. 2006;16(1-2):159-169.
37. Reinblatt SP, DosReis S, Walkup JT, et al. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119-126.
38. Goldsmith M, Singh M, Chang K. Antidepressants and psychostimulants in pediatric populations: is there an association with mania? Paediatr Drugs. 2011;13(4): 225-243.
39. Sidor MM, Macqueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
40. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
41. McClellan J, Kowatch R, Findling RL. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):107-125.
42. Dobry Y, Rice T, Sher L. Ecstasy use and serotonin syndrome: a neglected danger to adolescents and young adults prescribed selective serotonin reuptake inhibitors. Int J Adolesc Med Health. 2013; 25(3):193-199.
43. Schwartz AR, Pizon AF, Brooks DE. Dextromethorphan-induced serotonin syndrome. Clin Toxicol (Phila). 2008;46(8):771-773.
44. Gibbons RD, Brown CH, Hur K, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry. 2007;164(9):1356-1363.
45. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):332-339.
46. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683-1696.
47. Sharma T, Guski LS, Freund N, et al. Suicidality and aggression during antidepressant treatment: systematic review and meta-analyses based on clinical study reports. BMJ. 2016;352: i65. doi: https://doi.org/10.1136/bmj.i65.
48. Olfson M, Shaffer D, Marcus SC, et al. Relationship between antidepressant medication treatment and suicide in adolescents. Arch Gen Psychiatry. 2003;60(10):978-982.
49. Garland JE, Kutcher S, Virani A, et al. Update on the Use of SSRIs and SNRIs with children and adolescents in clinical practice. J Can Acad Child Adolesc Psychiatry. 2016;25(1):4-10.
50. Bridge JA, Barbe RP, Birmaher B, et al. Emergent suicidality in a clinical psychotherapy trial for adolescent depression. Am J Psychiatry. 2005;162(11):2173-2175.
51. Birmaher B, Brent D, Bernet W, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1503-1526.
52. Ravizza L, Maina G, Bogetto F, et al. Long term treatment of obsessive-compulsive disorder. CNS Drugs. 1998;10(4):247-255.
53. Hosenbocus S, Chahal R. SSRIs and SNRIs: a review of the discontinuation syndrome in children and adolescents. J Can Acad Child Adolesc Psychiatry. 2011;20(1):60-67.
54. Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850-855.
55. Issari Y, Jakubovski E, Bartley CA, et al. Early onset of response with selective serotonin reuptake inhibitors in obsessive-compulsive disorder: a meta-analysis. J Clin Psychiatry. 2016; 77(5):e605-e611. doi: 10.4088/JCP.14r09758.
56. Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299(8):901-913.
Major depressive disorder (MDD) is a significant pediatric health problem, with a lifetime prevalence as high as 20% by the end of adolescence.1-3 Major depressive disorder in adolescence is associated with significant morbidity, including poor social functioning, school difficulties, early pregnancy, and increased risk of physical illness and substance abuse.4-6 It is also linked with significant mortality, with increased risk for suicide, which is now the second leading cause of death in individuals age 10 to 24 years.1,7,8
As their name suggests, antidepressants comprise a group of medications that are used to treat MDD; they are also, however, first-line agents for generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD) in adults. Anxiety disorders (including GAD and other anxiety diagnoses) and PTSD are also common in childhood and adolescence with a combined lifetime prevalence ranging from 15% to 30%.9,10 These disorders are also associated with increased risk of suicide.11 For all of these disorders, depending on the severity of presentation and the preference of the patient, treatments are often a combination of psychotherapy and psychopharmacology.
Clinicians face several challenges when considering antidepressants for pediatric patients. Pediatricians and psychiatrists need to understand whether these medications work in children and adolescents, and whether there are unique developmental safety and tolerability issues. The evidence base in child psychiatry is considerably smaller compared with that of adult psychiatry. From this more limited evidence base also came the controversial “black-box” warning regarding a risk of emergent suicidality when starting antidepressants that accompanies all antidepressants for pediatric, but not adult, patients. This warning has had major effects on clinical encounters with children experiencing depression, including altering clinician prescribing behavior.12
Do antidepressants work in children?
Selective serotonin reuptake inhibitors. Selective serotonin reuptake inhibitors (SSRIs) are the most commonly used class of antidepressants in both children and adults.13 While only a few SSRIs are FDA-approved for pediatric indications, the lack of FDA approval is typically related to a lack of sufficient testing in randomized controlled trials (RCTs) for specific pediatric indications, rather than to demonstrable differences in efficacy between antidepressant agents. Since there is currently no data to suggest inferiority of one agent compared to another in children or adults,14,15 efficacy data will be discussed here as applied to the class of SSRIs, generalizing from RCTs conducted on individual drugs. Table 1 lists FDA indications and dosing information for individual antidepressants.
There is strong evidence that SSRIs are effective for treating pediatric anxiety disorders (eg, social anxiety disorder and GAD)16 and OCD,17 with numbers needed to treat (NNT) between 3 and 5. For both of these disorders, SSRIs combined with cognitive-behavioral therapy (CBT) have the highest likelihood of improving symptoms or achieving remission.17,18
Selective serotonin reuptake inhibitors are also effective for treating pediatric MDD; however, the literature is more complex for this disorder compared to GAD and OCD as there are considerable differences in effect sizes between National Institute of Mental Health (NIMH)–funded studies and industry-sponsored trials.13 The major NIMH-sponsored adolescent depression trial, TADS (Treatment for Adolescents and Depression Study), showed that SSRIs (fluoxetine in this case) were quite effective, with an NNT of 4 over the acute phase (12 weeks).19 Ultimately, approximately 80% of adolescents improved over 9 months. Many industry-sponsored trials for MDD in pediatric patients had large placebo response rates (approximately 60%), which resulted in smaller between-group differences, and estimates of an NNT closer to 12,13 which has muddied the waters in meta-analyses that include all trials.20 Improvement in depressive symptoms also appears to be bolstered by concomitant CBT in MDD,19 but not as robustly as in GAD and OCD. While the full benefit of SSRIs for depression may take as long as 8 weeks, a meta-analysis of depression studies of pediatric patients suggests that significant benefits from placebo are observed as early as 2 weeks, and that further treatment gains are minimal after 4 weeks.15 Thus, we recommend at least a 4- to 6-week trial at therapeutic dosing before deeming a medication a treatment failure.
Continue to: Posttraumatic stress disorder...
Posttraumatic stress disorder is a fourth disorder in which SSRIs are a first-line treatment in adults. The data for using SSRIs to treat pediatric patients with PTSD is scant, with only a few RCTs, and no large NIMH-funded trials. Randomized controlled trials have not demonstrated significant differences between SSRIs and placebo21,22 and thus the current first-line recommendation in pediatric PTSD remains trauma-focused therapy, with good evidence for trauma-focused CBT.23 Practically speaking, there can be considerable overlap of PTSD, depression, and anxiety symptoms in children,23 and children with a history of trauma who also have comorbid MDD may benefit from medication if their symptoms persist despite an adequate trial of psychotherapy.
Taken together, the current evidence suggests that SSRIs are often effective in pediatric GAD, OCD, and MDD, with low NNTs (ranging from 3 to 5 based on NIMH-funded trials) for all of these disorders; there is not yet sufficient evidence of efficacy in pediatric patients with PTSD.
Fluoxetine has been studied more intensively than other SSRIs (for example, it was the antidepressant used in the TADS trial), and thus has the largest evidence base. For this reason, fluoxetine is often considered the first of the first-line options. Additionally, fluoxetine has a longer half-life than other antidepressants, which may make it more effective in situations where patients are likely to miss doses, and results in a lower risk of withdrawal symptoms when stopped due to “self-tapering.”
SNRIs and atypical antidepressants. Other antidepressants commonly used in pediatric patients but with far less evidence of efficacy include serotonin-norepinephrine reuptake inhibitors (SNRIs) and the atypical antidepressants bupropion and mirtazapine. The SNRI duloxetine is FDA-approved for treating GAD in children age 7 to 17, but there are no other pediatric indications for duloxetine, or for the other SNRIs.
In general, adverse effect profiles are worse for SNRIs compared to SSRIs, further limiting their utility. While there are no pediatric studies demonstrating SNRI efficacy for neuropathic pain, good data exists in adults.24 Thus, an SNRI could be a reasonable option if a pediatric patient has failed prior adequate SSRI trials and also has comorbid neuropathic pain.
Continue to: Neither bupropion nor mirtazapine...
Neither bupropion nor mirtazapine have undergone rigorous testing in pediatric patients, and therefore these agents should generally be considered only once other first-line treatments have failed. Bupropion has been evaluated for attention-deficit/hyperactivity disorder (ADHD)25 and for adolescent smoking cessation.26 However, the evidence is weak, and bupropion is not considered a first-line option for children and adolescents.
Tricyclic antidepressants. Randomized controlled trials have demonstrated that tricyclic antidepressants (TCAs) are efficacious for treating several pediatric conditions; however, their significant side effect profile, their monitoring requirements, as well as their lethality in overdose has left them replaced by SSRIs in most cases. That said, they can be appropriate in refractory ADHD (desipramine27,28) and refractory OCD (clomipramine is FDA-approved for this indication29); they are considered a third-line treatment for enuresis.30
Why did my patient stop the medication?
Common adverse effects. Although the greatest benefit of antidepressant medications compared with placebo is achieved relatively early on in treatment, it generally takes time for these benefits to accrue and become clinically apparent.15,31 By contrast, most adverse effects of antidepressants present and are at their most severe early in treatment. The combination of early adverse effects and delayed efficacy leads many patients, families, and clinicians to discontinue medications before they have an adequate chance to work. Thus, it is imperative to provide psychoeducation before starting a medication about the typical time-course of improvement and adverse effects (Table 2).
Adverse effects of SSRIs often appear or worsen transiently during initiation of a medication, during a dose increase,32 or, theoretically, with the addition of a medication that interferes with SSRI metabolism (eg, cimetidine inhibition of cytochrome P450 2D6).33 If families are prepared for this phenomenon and the therapeutic alliance is adequate, adverse effects can be tolerated to allow for a full medication trial. Common adverse effects of SSRIs include sleep problems (insomnia/sedation), gastrointestinal upset, sexual dysfunction, dry mouth, and hyperhidrosis. Although SSRIs differ somewhat in the frequency of these effects, as a class, they are more similar than different. Adequate psychoeducation is especially imperative in the treatment of OCD and anxiety disorders, where there is limited evidence of efficacy for any non-serotonergic antidepressants.
Serotonin-norepinephrine reuptake inhibitors are not considered first-line medications because of the reduced evidence base compared to SSRIs and their enhanced adverse effect profiles. Because SNRIs partially share a mechanism of action with SSRIs, they also share portions of the adverse effects profile. However, SNRIs have the additional adverse effect of hypertension, which is related to their noradrenergic activity. Thus, it is reasonable to obtain a baseline blood pressure before initiating an SNRI, as well as periodically after initiation and during dose increases, particularly if the patient has other risk factors for hypertension.34
Continue to: Although TCAs have efficacy...
Although TCAs have efficacy in some pediatric disorders,27-29,35 their adverse effect profile limits their use. Tricyclic antidepressants are highly anticholinergic (causing dizziness secondary to orthostatic hypotension, dry mouth, and urinary retention) and antihistaminergic (causing sedation and weight gain). Additionally, TCAs lower the seizure threshold and have adverse cardiac effects relating to their anti-alpha-1 adrenergic activity, resulting in dose-dependent increases in the QTc and cardiac toxicity in overdose that could lead to arrhythmia and death. These medications have their place, but their use requires careful informed consent, clear treatment goals, and baseline and periodic cardiac monitoring (via electrocardiogram).
Serious adverse effects. Clinicians may be hesitant to prescribe antidepressants for pediatric patients because of the potential for more serious adverse effects, including severe behavioral activation syndromes, serotonin syndrome, and emergent suicidality. However, current FDA-approved antidepressants arguably have one of the most positive risk/benefit profiles of any orally-administered medication approved for pediatric patients. Having a strong understanding of the evidence is critical to evaluating when it is appropriate to prescribe an antidepressant, how to properly monitor the patient, and how to obtain accurate informed consent.
Pediatric behavioral activation syndrome. Many clinicians report that children receiving antidepressants experience a pediatric behavioral activation syndrome, which exists along a spectrum from mild activation, increased energy, insomnia, or irritability up through more severe presentations of agitation, hyperactivity, or possibly mania. A recent meta-analysis suggested a positive association between antidepressant use and activation events on the milder end of this spectrum in pediatric patients with non-OCD anxiety disorders,16 and it is thought that compared with adolescents, younger children are more susceptible to activation adverse effects.36 The likelihood of activation events has been associated with higher antidepressant plasma levels,37 suggesting that dose or individual differences in metabolism may play a role. At the severe end of the spectrum, the risk of induction of mania in pediatric patients with depression or anxiety is relatively rare (<2%) and not statistically different from placebo in RCTs of pediatric participants.38 Meta-analyses of larger randomized, placebo-controlled trials of adults do not support the idea that SSRIs and other second-generation antidepressants carry an increased risk of mania compared with placebo.39,40 Children or adolescents with bona fide bipolar disorder (ie, patients who have had observed mania that meets all DSM-5 criteria) should be treated with a mood-stabilizing agent or antipsychotic if prescribed an antidepressant.41 These clear-cut cases are, however, relatively rare, and more often clinicians are confronted with ambiguous cases that include a family history of bipolar disorder along with “softer” symptoms of irritability, intrusiveness, or aggression. In these children, SSRIs may be appropriate for depressive, OCD, or anxiety symptoms, and should be strongly considered before prescribing antipsychotics or mood stabilizers, as long as initiated with proper monitoring.
Serotonin syndrome is a life-threatening condition caused by excess synaptic serotonin. It is characterized by confusion, sweating, diarrhea, hypertension, hyperthermia, and tachycardia. At its most severe, serotonin syndrome can result in seizures, arrhythmias, and death. The risk of serotonin syndrome is very low when using an SSRI as monotherapy. Risk increases with polypharmacy, particularly unexamined polypharmacy when multiple serotonergic agents are inadvertently on board. Commonly used serotonergic agents include other antidepressants, migraine medications (eg, triptans), some pain medications, and the cough suppressant dextromethorphan.
The easiest way to mitigate the risk of serotonin syndrome is to use an interaction index computer program, which can help ensure that the interacting agents are not prescribed without first discussing the risks and benefits. It is important to teach adolescents that certain recreational drugs are highly serotonergic and can cause serious interactions with antidepressants. For example, recreational use of dextromethorphan or 3,4-methylenedioxymethamphetamine (MDMA; commonly known as “ecstasy”) has been associated with serotonin syndrome in adolescents taking antidepressant medications.42,43
Continue to: Suicidality
Suicidality. The black-box warning regarding a risk of emergent suicidality when starting antidepressant treatment in children is controversial.44 The prospect that a medication intended to ameliorate depression might instead risk increasing suicidal thinking is alarming to parents and clinicians alike. To appropriately weigh and discuss the risks and benefits with families, it is important to understand the data upon which the warning is based.
In 2004, the FDA commissioned a review of 23 antidepressant trials, both published and unpublished, pooling studies across multiple indications (MDD, OCD, anxiety, and ADHD) and multiple antidepressant classes. This meta-analysis, which included nearly 4,400 pediatric patients, found a small but statistically significant increase in spontaneously-reported suicidal thoughts or actions, with a risk difference of 1% (95% confidence interval [CI], 1% to 2%).45 These data suggest that if one treats 100 pediatric patients, 1 to 2 of them may experience short-term increases in suicidal thinking or behavior.45 There were no differences in suicidal thinking when assessed systematically (ie, when all subjects reported symptoms of suicidal ideation on structured rating scales), and there were no completed suicides.45 A subsequent analysis that included 27 pediatric trials suggested an even lower, although still significant, risk difference (<1%), yielding a number needed to harm (NNH) of 143.46 Thus, with low NNT for efficacy (3 to 6) and relatively high NNH for emergent suicidal thoughts or behaviors (100 to 143), for many patients the benefits will outweigh the risks.
Figure 1, Figure 2, and Figure 3 are Cates plots that depict the absolute benefits of antidepressants compared with the risk of suicidality for pediatric patients with MDD, OCD, and anxiety disorders. Recent meta-analyses have suggested that the increased risk of suicidality in antidepressant trials is specific to studies that included children and adolescents, and is not observed in adult studies. A meta-analysis of 70 trials involving 18,526 participants suggested that the odds ratio of suicidality in trials of children and adolescents was 2.39 (95% CI, 1.31 to 4.33) compared with 0.81 (95% CI, 0.51 to 1.28) in adults.47 Additionally, a network meta-analysis exclusively focusing on pediatric antidepressant trials in MDD reported significantly higher suicidality-related adverse events in venlafaxine trials compared with placebo, duloxetine, and several SSRIs (fluoxetine, paroxetine, and escitalopram).20 These data should be interpreted with caution as differences in suicidality detected between agents is quite possibly related to differences in the method of assessment between trials, as opposed to actual differences in risk between agents.
Epidemiologic data further support the use of antidepressants in pediatric patients, showing that antidepressant use is associated with decreased teen suicide attempts and completions,48 and the decline in prescriptions that occurred following the black-box warning was accompanied by a 14% increase in teen suicides.49 Multiple hypotheses have been proposed to explain the pediatric clinical trial findings. One idea is that potential adverse effects of activation, or the intended effects of restoring the motivation, energy, and social engagement that is often impaired in depression, increases the likelihood of thinking about suicide or acting on thoughts. Another theory is that reporting of suicidality may be increased, rather than increased de novo suicidality itself. Antidepressants are effective for treating pediatric anxiety disorders, including social anxiety disorder,16 which could result in more willingness to report. Also, the manner in which adverse effects are generally ascertained in trials might have led to increased spontaneous reporting. In many trials, investigators ask whether participants have any adverse effects in general, and inquire about specific adverse effects only if the family answers affirmatively. Thus, the increased rate of other adverse effects associated with antidepressants (sleep problems, gastrointestinal upset, dry mouth, etc.) might trigger a specific question regarding suicidal ideation, which the child or family then may be more likely to report. Alternatively, any type of psychiatric treatment could increase an individual’s propensity to report; in adolescent psychotherapy trials, non-medic
Continue to: How long should the antidepressant be continued?
How long should the antidepressant be continued?
Many patients are concerned about how long they may be taking medication, and whether they will be taking an antidepressant “forever.” A treatment course can be broken into an acute phase, wherein remission is achieved and maintained for 6 to 8 weeks. This is followed by a continuation phase, with the goal of relapse prevention, lasting 16 to 20 weeks. The length of the last phase—the maintenance phase—depends both on the child’s history, the underlying therapeutic indication, the adverse effect burden experienced, and the family’s preferences/values. In general, for a first depressive episode, after treating for 1 year, a trial of discontinuation can be attempted with close monitoring. For a second depressive episode, we recommend 2 years of remission on antidepressant therapy before attempting discontinuation. In patients who have had 3 depressive episodes, or have had episodes of high severity, we recommend continuing antidepressant treatment indefinitely. Although much less well studied, the risk of relapse following SSRI discontinuation appears much more significant in OCD, whereas anxiety disorders and MDD have a relatively comparable risk.52
In general, stopping an antidepressant should be done carefully and slowly.
What to do when first-line treatments fail
Adequate psychotherapy? To determine whether children are receiving adequate CBT, ask:
- if the child receives homework from psychotherapy
- if the parents are included in treatment
- if therapy has involved identifying thought patterns that may be contributing to the child’s illness, and
- if the therapist has ever exposed the child to a challenge likely to produce anxiety or distress in a supervised environment and has developed an exposure hierarchy (for conditions with primarily exposure-based therapies, such as OCD or anxiety disorders).
If a family is not receiving most of these elements in psychotherapy, this is a good indicator that they may not be receiving evidence-based CBT.
Continue to: Adequate pharmacotherapy?
Adequate pharmacotherapy? Similarly, when determining the adequacy of previous pharmacotherapy, it is critical to determine whether the child received an adequate dose of medications (at least the FDA-recommended minimum dose) for an adequate duration of time at therapeutic dosing (at least 6 weeks for MDD, 8 weeks for anxiety disorders, and 8 to 12 weeks for pediatric patients with OCD), and that the child actually took the medication regularly during that period. Patient compliance can typically be tracked through checking refill requests or intervals through the patient’s pharmacy. Ensuring proper delivery of first-line treatments is imperative because (1) the adverse effects associated with second-line treatments are often more substantial; (2) the cost in terms of time and money is considerably higher with second-line treatments, and; (3) the evidence regarding the benefits of these treatments is much less certain.
Inadequate dosing is a common reason for non-response in pediatric patients. Therapeutic dose ranges for common antidepressants are displayed in Table 1. Many clinicians underdose antidepressants for pediatric patients initially (and often throughout treatment) because they fear that the typical dose titration used in clinical trials will increase the risk of adverse effects compared with more conservative dosing. There is limited evidence to suggest that this underdosing strategy is likely to be successful; adverse effects attributable to these medications are modest, and most that are experienced early in treatment (eg, headache, increased anxiety or irritability, sleep problems, gastrointestinal upset) are self-limiting and may be coincidental rather than medication-induced. Furthermore, there is no evidence for efficacy of subtherapeutic dosing in children in the acute phase of treatment or for preventing relapse.14 Thus, from an efficacy standpoint, a medication trial has not officially begun until the therapeutic dose range is reached.
Once dosing is within the therapeutic range, however, pediatric data differs from the adult literature. In most adult psychiatric conditions, higher doses of SSRIs within the therapeutic range are associated with an increased response rate.14,54 In pediatrics, there are few fixed dose trials, and once within the recommended therapeutic range, minimal data supports an association between higher dosing and higher efficacy.14 In general, pediatric guidelines are silent regarding optimal dosing of SSRIs within the recommended dose range, and higher antidepressant doses often result in a more significant adverse effect burden for children. One exception is pediatric OCD, where, similar to adults, the guidelines suggest that higher dosing of SSRIs often is required to induce a therapeutic response as compared to MDD and GAD.31,55
If a child does not respond to adequate first-line treatment (or has a treatment history that cannot be fully verified), repeating these first-line interventions carries little risk and can be quite effective. For example, 60% of adolescents with MDD who did not initially respond to an SSRI demonstrated a significant response when prescribed a second SSRI or venlafaxine (with or without CBT).56
When pediatric patients continue to experience significantly distressing and/or debilitating symptoms (particularly in MDD) despite multiple trials of antidepressants and psychotherapy, practitioners should consider a careful referral to interventional psychiatry services, which can include the more intensive treatments of electroconvulsive therapy, repetitive transcranial magnetic stimulation, or ketamine (see Box 1). Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and under-utilized clinically in pediatric populations.
Continue to: Antidepressants in general...
Antidepressants in general, and SSRIs in particular, are the first-line pharmacotherapy for pediatric anxiety, OCD, and MDD. For PTSD, although they are a first-line treatment in adults, their efficacy has not been demonstrated in children and adolescents. Antidepressants are generally safe, well-tolerated, and effective, with low NNTs (3 to 5 for anxiety and OCD; 4 to 12 in MDD, depending on whether industry trials are included). It is important that clinicians and families be educated about possible adverse effects and their time course in order to anticipate difficulties, ensure adequate informed consent, and monitor appropriately. The black-box warning regarding treatment-emergent suicidal thoughts or behaviors must be discussed (for suggested talking points, see Box 2). The NNH is large (100 to 143), and for many patients, the benefits will outweigh the risks. For pediatric patients who fail to respond to multiple adequate trials of antidepressants and psychotherapy, referrals for interventional psychiatry consultation should be considered.
Bottom Line
Although the evidence base for prescribing antidepressants for children and adolescents is smaller compared to the adult literature, properly understanding and prescribing these agents, and explaining their risks and benefits to families, can make a major difference in patient compliance, satisfaction, and outcomes. Antidepressants (particularly selective serotonin reuptake inhibitors) are the firstline pharmacologic intervention for pediatric patients with anxiety disorders, obsessive-compulsive disorder, or major depressive disorder.
Related Resource
- Bonin L, Moreland CS. Overview of prevention and treatment for pediatric depression. https://www.uptodate.com/contents/overview-of-prevention-and-treatment-for-pediatric-depression. Last reviewed July 2019.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cimetidine • Tagamet
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Box 1
Continuing severe depression is associated with reduced educational attainment and quality of life, as well as increased risk of substance abuse and suicide,1,2 which is the second leading cause of death in individuals age 10 to 24 years.3 Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and underutilized in pediatric patients. Interventional antidepressants in adults include electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), and, most recently, ketamine.
Electroconvulsive therapy is the most effective therapy available for depression in adults, alleviating depressive symptoms in treatment-refractory patients and outperforming both pharmacotherapy4 and rTMS.5 Despite its track record of effectiveness and safety in adults, ECT continues to suffer considerable stigma.4 Cognitive adverse effects and memory problems in adults are generally self-limited, and some aspects of cognition actually improve after ECT as depression lifts.6 The combination of stigma and the concern about possible cognitive adverse effects during periods of brain development have likely impeded the rigorous testing of ECT in treatment-refractory pediatric patients. Several case series and other retrospective analyses suggest, however, that ECT has strong efficacy and limited adverse effects in adolescents who have severe depression or psychotic symptoms.7-9 Despite these positive preliminary data in pediatric patients, and a large body of literature in adults, no controlled trials of ECT have been conducted in the pediatric population, and it remains a rarely used treatment in severe pediatric mental illness.
Repetitive transcranial magnetic stimulation is a technique in which magnetic stimulation is used to activate the left dorsolateral prefrontal cortex (DLPFC), a target thought to be important in the pathophysiology of MDD. Repetitive transcranial magnetic stimulation is FDAapproved to treat medication-refractory major depressive disorder (MDD) in adults, and has been shown to be effective as both a monotherapy10 and an adjunctive treatment.11 The estimated number needed to treat (NNT) for rTMS ranges from 6 to 8, which is quite effective, although less so than ECT (and probably initial pharmacotherapy).5 Similar to ECT, however, there are no large randomized controlled trials (RCTs) in children or adolescents. Pilot RCTs12 and open trials13 suggest that DLPFC rTMS may be effective as an adjunctive treatment, speeding or augmenting response to a selective serotonin reuptake inhibitor in adolescents with MDD. Larger trials studying rTMS in pediatric patients are needed. While rTMS is generally well tolerated, disadvantages include the time-consuming schedule (the initial treatment is typically 5 days/week for several weeks) and local adverse effects of headache and scalp pain.
Ketamine, which traditionally is used as a dissociative anesthetic, is a rapidly emerging novel treatment in adult treatment-refractory MDD. It acts quickly (within hours to days) and cause significant improvement in difficult symptoms such as anhedonia14 and suicidal ideation.15 In adult studies, ketamine has a robust average effect size of >1.2, and an NNT ranging from 3 to 5 in medication-refractory patients.16,17 Ketamine is a glutamatergic modulator, acting outside of the monoamine neurochemical systems traditionally targeted by standard antidepressants.16 The efficacy of ketamine in treatment-refractory adults is impressive, but the effects of a single treatment are ephemeral, dissipating within 1 to 2 weeks, which has led to significant discussion surrounding optimal dosing strategies.16 Although small RCTs in pediatric patients are currently underway, at this time, the only evidence for ketamine for pediatric MDD is based on case series/report data18,19 which was positive.
For all of these interventional modalities, it is critical to refer children with treatmentrefractory disorders to interventionists who have appropriate experience and monitoring capabilities.
References
1. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
2. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
3. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
4. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and metaanalysis. Lancet. 2003;361(9360):799-808.
5. Berlim MT, Van den Eynde F, Daskalakis ZJ. Efficacy and acceptability of high frequency repetitive transcranial magnetic stimulation (rTMS) versus electroconvulsive therapy (ECT) for major depression: a systematic review and meta-analysis of randomized trials. Depress Anxiety. 2013;30(7):614-623.
6. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
7. Jacob P, Gogi PK, Srinath S, et al. Review of electroconvulsive therapy practice from a tertiary child and adolescent psychiatry centre. Asian J Psychiatr. 2014;12(1):95-99.
8. Zhand N, Courtney DB, Flament MF. Use of electroconvulsive therapy in adolescents with treatment-resistant depressive disorders: a case series. J ECT. 2015;31(4):238-245.
9. Puffer CC, Wall CA, Huxsahl JE, et al. A 20 year practice review of electroconvulsive therapy for adolescents. J Child Adolesc Psychopharmacol. 2016;26(7):632-636.
10. Berlim MT, van den Eynde F, Tovar-Perdomo S, et al. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44(2):225-239.
11. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342.
12. Huang ML, Luo BY, Hu JB, et al. Repetitive transcranial magnetic stimulation in combination with citalopram in young patients with first-episode major depressive disorder: a double-blind, randomized, sham-controlled trial. Aust N Z J Psychiatry. 2012;46(3):257-264.
13. Wall CA, Croarkin PE, Sim LA, et al. Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. J Clin Psychiatry. 2011;72(9):1263-1269.
14. Lally N, Nugent AC, Luckenbaugh DA, et al. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105.
15. Ballard ED, Ionescu DF, Vande Voort JL, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161-166.
16. Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
17. McGirr A, Berlim MT, Bond DJ, et al. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45(4):693-704.
18. Dwyer JB, Beyer C, Wilkinson ST, et al. Ketamine as a treatment for adolescent depression: a case report. J Am Acad Child Adolesc Psychiatry. 2017;56(4):352-354.
19. Cullen KR, Amatya P, Roback MG, et al. Intravenous ketamine for adolescents with treatment-resistant depression: an open-label study. J Child Adolesc Psychopharmacol. 2018;28(7):437-444.
Box 2
Efficacy
- Selective serotonin reuptake inhibitors are the most effective pharmacologic treatment we have for pediatric depression, OCD, and anxiety
- More than one-half of children who are prescribed SSRIs have a significant improvement, regardless of condition
- Based on current estimates, we need to treat 4 to 6 children with an SSRI to find one that will improve who would not improve with placebo
- The clinical benefits of SSRIs generally take a while to accrue; therefore, it is advisable to take the medication for at least 2 to 3 months before concluding that it is ineffective
- In addition to medication, evidence-based psychotherapies provide significant benefit for pediatric depression, OCD, and anxiety
Tolerability
- Most commonly prescribed pediatric antidepressants have been used safely in children for 2 to 3 decades. The safety profiles of SSRIs are among the best of any medications used for children and adolescents
- While many children get better when taking these medications, it’s important that we also talk about potential adverse effects. Some children will experience sleep problems (either sleepier than usual or difficulty sleeping), changes in energy levels, headache, gastrointestinal upset, and dry mouth. These are most likely at the beginning of treatment, or when we increase the dose; they usually are time-limited and go away on their own
- Often adverse effects occur first and the benefits come later. Because it may take at least a few weeks to start to see the mood/anxiety benefits, it’s important for us to talk about any adverse effects your child experiences and remember that they usually are short-lived
Suicidality
- The FDA placed a “black-box” warning on antidepressants after pediatric studies found a small but statistically significant increased risk of reporting suicidal thoughts or behaviors over the short-term compared with placebo
- The increased risk of spontaneously reporting suicidal ideation was quite small. Studies suggested that one would need to treat 100 to 140 children to see 1 child report suicidal ideation compared to placebo. Suicidal ideation is a common symptom in children with depression and anxiety
- Studies found no increased risk when suicidal ideation was systematically assessed using structured rating scales
- In the studies evaluated, there were no completed suicides by patients taking medication or placebo
- Population studies show that higher rates of antidepressant prescriptions are associated with lower rates of attempted and completed teen suicide, which underscores that in general, these medicines treat the underlying causes of suicidality
- No scientific consensus exists on whether these medications are truly associated with an increased risk of new-onset suicidal ideation, or if this association is due to other factors (eg, improvement in anxiety and depressive symptoms that make patients more comfortable to report suicidal ideation spontaneously)
- Regardless, the FDA recommends frequent monitoring of children for suicidal thoughts when these medications are started. This should be done anyway in children experiencing depression and anxiety, and it’s why we will plan to have more frequent appointments as the medication is initiated
OCD: obsessive-compulsive disorder; SSRIs: selective serotonin reuptake inhibitors
Major depressive disorder (MDD) is a significant pediatric health problem, with a lifetime prevalence as high as 20% by the end of adolescence.1-3 Major depressive disorder in adolescence is associated with significant morbidity, including poor social functioning, school difficulties, early pregnancy, and increased risk of physical illness and substance abuse.4-6 It is also linked with significant mortality, with increased risk for suicide, which is now the second leading cause of death in individuals age 10 to 24 years.1,7,8
As their name suggests, antidepressants comprise a group of medications that are used to treat MDD; they are also, however, first-line agents for generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD) in adults. Anxiety disorders (including GAD and other anxiety diagnoses) and PTSD are also common in childhood and adolescence with a combined lifetime prevalence ranging from 15% to 30%.9,10 These disorders are also associated with increased risk of suicide.11 For all of these disorders, depending on the severity of presentation and the preference of the patient, treatments are often a combination of psychotherapy and psychopharmacology.
Clinicians face several challenges when considering antidepressants for pediatric patients. Pediatricians and psychiatrists need to understand whether these medications work in children and adolescents, and whether there are unique developmental safety and tolerability issues. The evidence base in child psychiatry is considerably smaller compared with that of adult psychiatry. From this more limited evidence base also came the controversial “black-box” warning regarding a risk of emergent suicidality when starting antidepressants that accompanies all antidepressants for pediatric, but not adult, patients. This warning has had major effects on clinical encounters with children experiencing depression, including altering clinician prescribing behavior.12
Do antidepressants work in children?
Selective serotonin reuptake inhibitors. Selective serotonin reuptake inhibitors (SSRIs) are the most commonly used class of antidepressants in both children and adults.13 While only a few SSRIs are FDA-approved for pediatric indications, the lack of FDA approval is typically related to a lack of sufficient testing in randomized controlled trials (RCTs) for specific pediatric indications, rather than to demonstrable differences in efficacy between antidepressant agents. Since there is currently no data to suggest inferiority of one agent compared to another in children or adults,14,15 efficacy data will be discussed here as applied to the class of SSRIs, generalizing from RCTs conducted on individual drugs. Table 1 lists FDA indications and dosing information for individual antidepressants.
There is strong evidence that SSRIs are effective for treating pediatric anxiety disorders (eg, social anxiety disorder and GAD)16 and OCD,17 with numbers needed to treat (NNT) between 3 and 5. For both of these disorders, SSRIs combined with cognitive-behavioral therapy (CBT) have the highest likelihood of improving symptoms or achieving remission.17,18
Selective serotonin reuptake inhibitors are also effective for treating pediatric MDD; however, the literature is more complex for this disorder compared to GAD and OCD as there are considerable differences in effect sizes between National Institute of Mental Health (NIMH)–funded studies and industry-sponsored trials.13 The major NIMH-sponsored adolescent depression trial, TADS (Treatment for Adolescents and Depression Study), showed that SSRIs (fluoxetine in this case) were quite effective, with an NNT of 4 over the acute phase (12 weeks).19 Ultimately, approximately 80% of adolescents improved over 9 months. Many industry-sponsored trials for MDD in pediatric patients had large placebo response rates (approximately 60%), which resulted in smaller between-group differences, and estimates of an NNT closer to 12,13 which has muddied the waters in meta-analyses that include all trials.20 Improvement in depressive symptoms also appears to be bolstered by concomitant CBT in MDD,19 but not as robustly as in GAD and OCD. While the full benefit of SSRIs for depression may take as long as 8 weeks, a meta-analysis of depression studies of pediatric patients suggests that significant benefits from placebo are observed as early as 2 weeks, and that further treatment gains are minimal after 4 weeks.15 Thus, we recommend at least a 4- to 6-week trial at therapeutic dosing before deeming a medication a treatment failure.
Continue to: Posttraumatic stress disorder...
Posttraumatic stress disorder is a fourth disorder in which SSRIs are a first-line treatment in adults. The data for using SSRIs to treat pediatric patients with PTSD is scant, with only a few RCTs, and no large NIMH-funded trials. Randomized controlled trials have not demonstrated significant differences between SSRIs and placebo21,22 and thus the current first-line recommendation in pediatric PTSD remains trauma-focused therapy, with good evidence for trauma-focused CBT.23 Practically speaking, there can be considerable overlap of PTSD, depression, and anxiety symptoms in children,23 and children with a history of trauma who also have comorbid MDD may benefit from medication if their symptoms persist despite an adequate trial of psychotherapy.
Taken together, the current evidence suggests that SSRIs are often effective in pediatric GAD, OCD, and MDD, with low NNTs (ranging from 3 to 5 based on NIMH-funded trials) for all of these disorders; there is not yet sufficient evidence of efficacy in pediatric patients with PTSD.
Fluoxetine has been studied more intensively than other SSRIs (for example, it was the antidepressant used in the TADS trial), and thus has the largest evidence base. For this reason, fluoxetine is often considered the first of the first-line options. Additionally, fluoxetine has a longer half-life than other antidepressants, which may make it more effective in situations where patients are likely to miss doses, and results in a lower risk of withdrawal symptoms when stopped due to “self-tapering.”
SNRIs and atypical antidepressants. Other antidepressants commonly used in pediatric patients but with far less evidence of efficacy include serotonin-norepinephrine reuptake inhibitors (SNRIs) and the atypical antidepressants bupropion and mirtazapine. The SNRI duloxetine is FDA-approved for treating GAD in children age 7 to 17, but there are no other pediatric indications for duloxetine, or for the other SNRIs.
In general, adverse effect profiles are worse for SNRIs compared to SSRIs, further limiting their utility. While there are no pediatric studies demonstrating SNRI efficacy for neuropathic pain, good data exists in adults.24 Thus, an SNRI could be a reasonable option if a pediatric patient has failed prior adequate SSRI trials and also has comorbid neuropathic pain.
Continue to: Neither bupropion nor mirtazapine...
Neither bupropion nor mirtazapine have undergone rigorous testing in pediatric patients, and therefore these agents should generally be considered only once other first-line treatments have failed. Bupropion has been evaluated for attention-deficit/hyperactivity disorder (ADHD)25 and for adolescent smoking cessation.26 However, the evidence is weak, and bupropion is not considered a first-line option for children and adolescents.
Tricyclic antidepressants. Randomized controlled trials have demonstrated that tricyclic antidepressants (TCAs) are efficacious for treating several pediatric conditions; however, their significant side effect profile, their monitoring requirements, as well as their lethality in overdose has left them replaced by SSRIs in most cases. That said, they can be appropriate in refractory ADHD (desipramine27,28) and refractory OCD (clomipramine is FDA-approved for this indication29); they are considered a third-line treatment for enuresis.30
Why did my patient stop the medication?
Common adverse effects. Although the greatest benefit of antidepressant medications compared with placebo is achieved relatively early on in treatment, it generally takes time for these benefits to accrue and become clinically apparent.15,31 By contrast, most adverse effects of antidepressants present and are at their most severe early in treatment. The combination of early adverse effects and delayed efficacy leads many patients, families, and clinicians to discontinue medications before they have an adequate chance to work. Thus, it is imperative to provide psychoeducation before starting a medication about the typical time-course of improvement and adverse effects (Table 2).
Adverse effects of SSRIs often appear or worsen transiently during initiation of a medication, during a dose increase,32 or, theoretically, with the addition of a medication that interferes with SSRI metabolism (eg, cimetidine inhibition of cytochrome P450 2D6).33 If families are prepared for this phenomenon and the therapeutic alliance is adequate, adverse effects can be tolerated to allow for a full medication trial. Common adverse effects of SSRIs include sleep problems (insomnia/sedation), gastrointestinal upset, sexual dysfunction, dry mouth, and hyperhidrosis. Although SSRIs differ somewhat in the frequency of these effects, as a class, they are more similar than different. Adequate psychoeducation is especially imperative in the treatment of OCD and anxiety disorders, where there is limited evidence of efficacy for any non-serotonergic antidepressants.
Serotonin-norepinephrine reuptake inhibitors are not considered first-line medications because of the reduced evidence base compared to SSRIs and their enhanced adverse effect profiles. Because SNRIs partially share a mechanism of action with SSRIs, they also share portions of the adverse effects profile. However, SNRIs have the additional adverse effect of hypertension, which is related to their noradrenergic activity. Thus, it is reasonable to obtain a baseline blood pressure before initiating an SNRI, as well as periodically after initiation and during dose increases, particularly if the patient has other risk factors for hypertension.34
Continue to: Although TCAs have efficacy...
Although TCAs have efficacy in some pediatric disorders,27-29,35 their adverse effect profile limits their use. Tricyclic antidepressants are highly anticholinergic (causing dizziness secondary to orthostatic hypotension, dry mouth, and urinary retention) and antihistaminergic (causing sedation and weight gain). Additionally, TCAs lower the seizure threshold and have adverse cardiac effects relating to their anti-alpha-1 adrenergic activity, resulting in dose-dependent increases in the QTc and cardiac toxicity in overdose that could lead to arrhythmia and death. These medications have their place, but their use requires careful informed consent, clear treatment goals, and baseline and periodic cardiac monitoring (via electrocardiogram).
Serious adverse effects. Clinicians may be hesitant to prescribe antidepressants for pediatric patients because of the potential for more serious adverse effects, including severe behavioral activation syndromes, serotonin syndrome, and emergent suicidality. However, current FDA-approved antidepressants arguably have one of the most positive risk/benefit profiles of any orally-administered medication approved for pediatric patients. Having a strong understanding of the evidence is critical to evaluating when it is appropriate to prescribe an antidepressant, how to properly monitor the patient, and how to obtain accurate informed consent.
Pediatric behavioral activation syndrome. Many clinicians report that children receiving antidepressants experience a pediatric behavioral activation syndrome, which exists along a spectrum from mild activation, increased energy, insomnia, or irritability up through more severe presentations of agitation, hyperactivity, or possibly mania. A recent meta-analysis suggested a positive association between antidepressant use and activation events on the milder end of this spectrum in pediatric patients with non-OCD anxiety disorders,16 and it is thought that compared with adolescents, younger children are more susceptible to activation adverse effects.36 The likelihood of activation events has been associated with higher antidepressant plasma levels,37 suggesting that dose or individual differences in metabolism may play a role. At the severe end of the spectrum, the risk of induction of mania in pediatric patients with depression or anxiety is relatively rare (<2%) and not statistically different from placebo in RCTs of pediatric participants.38 Meta-analyses of larger randomized, placebo-controlled trials of adults do not support the idea that SSRIs and other second-generation antidepressants carry an increased risk of mania compared with placebo.39,40 Children or adolescents with bona fide bipolar disorder (ie, patients who have had observed mania that meets all DSM-5 criteria) should be treated with a mood-stabilizing agent or antipsychotic if prescribed an antidepressant.41 These clear-cut cases are, however, relatively rare, and more often clinicians are confronted with ambiguous cases that include a family history of bipolar disorder along with “softer” symptoms of irritability, intrusiveness, or aggression. In these children, SSRIs may be appropriate for depressive, OCD, or anxiety symptoms, and should be strongly considered before prescribing antipsychotics or mood stabilizers, as long as initiated with proper monitoring.
Serotonin syndrome is a life-threatening condition caused by excess synaptic serotonin. It is characterized by confusion, sweating, diarrhea, hypertension, hyperthermia, and tachycardia. At its most severe, serotonin syndrome can result in seizures, arrhythmias, and death. The risk of serotonin syndrome is very low when using an SSRI as monotherapy. Risk increases with polypharmacy, particularly unexamined polypharmacy when multiple serotonergic agents are inadvertently on board. Commonly used serotonergic agents include other antidepressants, migraine medications (eg, triptans), some pain medications, and the cough suppressant dextromethorphan.
The easiest way to mitigate the risk of serotonin syndrome is to use an interaction index computer program, which can help ensure that the interacting agents are not prescribed without first discussing the risks and benefits. It is important to teach adolescents that certain recreational drugs are highly serotonergic and can cause serious interactions with antidepressants. For example, recreational use of dextromethorphan or 3,4-methylenedioxymethamphetamine (MDMA; commonly known as “ecstasy”) has been associated with serotonin syndrome in adolescents taking antidepressant medications.42,43
Continue to: Suicidality
Suicidality. The black-box warning regarding a risk of emergent suicidality when starting antidepressant treatment in children is controversial.44 The prospect that a medication intended to ameliorate depression might instead risk increasing suicidal thinking is alarming to parents and clinicians alike. To appropriately weigh and discuss the risks and benefits with families, it is important to understand the data upon which the warning is based.
In 2004, the FDA commissioned a review of 23 antidepressant trials, both published and unpublished, pooling studies across multiple indications (MDD, OCD, anxiety, and ADHD) and multiple antidepressant classes. This meta-analysis, which included nearly 4,400 pediatric patients, found a small but statistically significant increase in spontaneously-reported suicidal thoughts or actions, with a risk difference of 1% (95% confidence interval [CI], 1% to 2%).45 These data suggest that if one treats 100 pediatric patients, 1 to 2 of them may experience short-term increases in suicidal thinking or behavior.45 There were no differences in suicidal thinking when assessed systematically (ie, when all subjects reported symptoms of suicidal ideation on structured rating scales), and there were no completed suicides.45 A subsequent analysis that included 27 pediatric trials suggested an even lower, although still significant, risk difference (<1%), yielding a number needed to harm (NNH) of 143.46 Thus, with low NNT for efficacy (3 to 6) and relatively high NNH for emergent suicidal thoughts or behaviors (100 to 143), for many patients the benefits will outweigh the risks.
Figure 1, Figure 2, and Figure 3 are Cates plots that depict the absolute benefits of antidepressants compared with the risk of suicidality for pediatric patients with MDD, OCD, and anxiety disorders. Recent meta-analyses have suggested that the increased risk of suicidality in antidepressant trials is specific to studies that included children and adolescents, and is not observed in adult studies. A meta-analysis of 70 trials involving 18,526 participants suggested that the odds ratio of suicidality in trials of children and adolescents was 2.39 (95% CI, 1.31 to 4.33) compared with 0.81 (95% CI, 0.51 to 1.28) in adults.47 Additionally, a network meta-analysis exclusively focusing on pediatric antidepressant trials in MDD reported significantly higher suicidality-related adverse events in venlafaxine trials compared with placebo, duloxetine, and several SSRIs (fluoxetine, paroxetine, and escitalopram).20 These data should be interpreted with caution as differences in suicidality detected between agents is quite possibly related to differences in the method of assessment between trials, as opposed to actual differences in risk between agents.
Epidemiologic data further support the use of antidepressants in pediatric patients, showing that antidepressant use is associated with decreased teen suicide attempts and completions,48 and the decline in prescriptions that occurred following the black-box warning was accompanied by a 14% increase in teen suicides.49 Multiple hypotheses have been proposed to explain the pediatric clinical trial findings. One idea is that potential adverse effects of activation, or the intended effects of restoring the motivation, energy, and social engagement that is often impaired in depression, increases the likelihood of thinking about suicide or acting on thoughts. Another theory is that reporting of suicidality may be increased, rather than increased de novo suicidality itself. Antidepressants are effective for treating pediatric anxiety disorders, including social anxiety disorder,16 which could result in more willingness to report. Also, the manner in which adverse effects are generally ascertained in trials might have led to increased spontaneous reporting. In many trials, investigators ask whether participants have any adverse effects in general, and inquire about specific adverse effects only if the family answers affirmatively. Thus, the increased rate of other adverse effects associated with antidepressants (sleep problems, gastrointestinal upset, dry mouth, etc.) might trigger a specific question regarding suicidal ideation, which the child or family then may be more likely to report. Alternatively, any type of psychiatric treatment could increase an individual’s propensity to report; in adolescent psychotherapy trials, non-medic
Continue to: How long should the antidepressant be continued?
How long should the antidepressant be continued?
Many patients are concerned about how long they may be taking medication, and whether they will be taking an antidepressant “forever.” A treatment course can be broken into an acute phase, wherein remission is achieved and maintained for 6 to 8 weeks. This is followed by a continuation phase, with the goal of relapse prevention, lasting 16 to 20 weeks. The length of the last phase—the maintenance phase—depends both on the child’s history, the underlying therapeutic indication, the adverse effect burden experienced, and the family’s preferences/values. In general, for a first depressive episode, after treating for 1 year, a trial of discontinuation can be attempted with close monitoring. For a second depressive episode, we recommend 2 years of remission on antidepressant therapy before attempting discontinuation. In patients who have had 3 depressive episodes, or have had episodes of high severity, we recommend continuing antidepressant treatment indefinitely. Although much less well studied, the risk of relapse following SSRI discontinuation appears much more significant in OCD, whereas anxiety disorders and MDD have a relatively comparable risk.52
In general, stopping an antidepressant should be done carefully and slowly.
What to do when first-line treatments fail
Adequate psychotherapy? To determine whether children are receiving adequate CBT, ask:
- if the child receives homework from psychotherapy
- if the parents are included in treatment
- if therapy has involved identifying thought patterns that may be contributing to the child’s illness, and
- if the therapist has ever exposed the child to a challenge likely to produce anxiety or distress in a supervised environment and has developed an exposure hierarchy (for conditions with primarily exposure-based therapies, such as OCD or anxiety disorders).
If a family is not receiving most of these elements in psychotherapy, this is a good indicator that they may not be receiving evidence-based CBT.
Continue to: Adequate pharmacotherapy?
Adequate pharmacotherapy? Similarly, when determining the adequacy of previous pharmacotherapy, it is critical to determine whether the child received an adequate dose of medications (at least the FDA-recommended minimum dose) for an adequate duration of time at therapeutic dosing (at least 6 weeks for MDD, 8 weeks for anxiety disorders, and 8 to 12 weeks for pediatric patients with OCD), and that the child actually took the medication regularly during that period. Patient compliance can typically be tracked through checking refill requests or intervals through the patient’s pharmacy. Ensuring proper delivery of first-line treatments is imperative because (1) the adverse effects associated with second-line treatments are often more substantial; (2) the cost in terms of time and money is considerably higher with second-line treatments, and; (3) the evidence regarding the benefits of these treatments is much less certain.
Inadequate dosing is a common reason for non-response in pediatric patients. Therapeutic dose ranges for common antidepressants are displayed in Table 1. Many clinicians underdose antidepressants for pediatric patients initially (and often throughout treatment) because they fear that the typical dose titration used in clinical trials will increase the risk of adverse effects compared with more conservative dosing. There is limited evidence to suggest that this underdosing strategy is likely to be successful; adverse effects attributable to these medications are modest, and most that are experienced early in treatment (eg, headache, increased anxiety or irritability, sleep problems, gastrointestinal upset) are self-limiting and may be coincidental rather than medication-induced. Furthermore, there is no evidence for efficacy of subtherapeutic dosing in children in the acute phase of treatment or for preventing relapse.14 Thus, from an efficacy standpoint, a medication trial has not officially begun until the therapeutic dose range is reached.
Once dosing is within the therapeutic range, however, pediatric data differs from the adult literature. In most adult psychiatric conditions, higher doses of SSRIs within the therapeutic range are associated with an increased response rate.14,54 In pediatrics, there are few fixed dose trials, and once within the recommended therapeutic range, minimal data supports an association between higher dosing and higher efficacy.14 In general, pediatric guidelines are silent regarding optimal dosing of SSRIs within the recommended dose range, and higher antidepressant doses often result in a more significant adverse effect burden for children. One exception is pediatric OCD, where, similar to adults, the guidelines suggest that higher dosing of SSRIs often is required to induce a therapeutic response as compared to MDD and GAD.31,55
If a child does not respond to adequate first-line treatment (or has a treatment history that cannot be fully verified), repeating these first-line interventions carries little risk and can be quite effective. For example, 60% of adolescents with MDD who did not initially respond to an SSRI demonstrated a significant response when prescribed a second SSRI or venlafaxine (with or without CBT).56
When pediatric patients continue to experience significantly distressing and/or debilitating symptoms (particularly in MDD) despite multiple trials of antidepressants and psychotherapy, practitioners should consider a careful referral to interventional psychiatry services, which can include the more intensive treatments of electroconvulsive therapy, repetitive transcranial magnetic stimulation, or ketamine (see Box 1). Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and under-utilized clinically in pediatric populations.
Continue to: Antidepressants in general...
Antidepressants in general, and SSRIs in particular, are the first-line pharmacotherapy for pediatric anxiety, OCD, and MDD. For PTSD, although they are a first-line treatment in adults, their efficacy has not been demonstrated in children and adolescents. Antidepressants are generally safe, well-tolerated, and effective, with low NNTs (3 to 5 for anxiety and OCD; 4 to 12 in MDD, depending on whether industry trials are included). It is important that clinicians and families be educated about possible adverse effects and their time course in order to anticipate difficulties, ensure adequate informed consent, and monitor appropriately. The black-box warning regarding treatment-emergent suicidal thoughts or behaviors must be discussed (for suggested talking points, see Box 2). The NNH is large (100 to 143), and for many patients, the benefits will outweigh the risks. For pediatric patients who fail to respond to multiple adequate trials of antidepressants and psychotherapy, referrals for interventional psychiatry consultation should be considered.
Bottom Line
Although the evidence base for prescribing antidepressants for children and adolescents is smaller compared to the adult literature, properly understanding and prescribing these agents, and explaining their risks and benefits to families, can make a major difference in patient compliance, satisfaction, and outcomes. Antidepressants (particularly selective serotonin reuptake inhibitors) are the firstline pharmacologic intervention for pediatric patients with anxiety disorders, obsessive-compulsive disorder, or major depressive disorder.
Related Resource
- Bonin L, Moreland CS. Overview of prevention and treatment for pediatric depression. https://www.uptodate.com/contents/overview-of-prevention-and-treatment-for-pediatric-depression. Last reviewed July 2019.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cimetidine • Tagamet
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Box 1
Continuing severe depression is associated with reduced educational attainment and quality of life, as well as increased risk of substance abuse and suicide,1,2 which is the second leading cause of death in individuals age 10 to 24 years.3 Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and underutilized in pediatric patients. Interventional antidepressants in adults include electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), and, most recently, ketamine.
Electroconvulsive therapy is the most effective therapy available for depression in adults, alleviating depressive symptoms in treatment-refractory patients and outperforming both pharmacotherapy4 and rTMS.5 Despite its track record of effectiveness and safety in adults, ECT continues to suffer considerable stigma.4 Cognitive adverse effects and memory problems in adults are generally self-limited, and some aspects of cognition actually improve after ECT as depression lifts.6 The combination of stigma and the concern about possible cognitive adverse effects during periods of brain development have likely impeded the rigorous testing of ECT in treatment-refractory pediatric patients. Several case series and other retrospective analyses suggest, however, that ECT has strong efficacy and limited adverse effects in adolescents who have severe depression or psychotic symptoms.7-9 Despite these positive preliminary data in pediatric patients, and a large body of literature in adults, no controlled trials of ECT have been conducted in the pediatric population, and it remains a rarely used treatment in severe pediatric mental illness.
Repetitive transcranial magnetic stimulation is a technique in which magnetic stimulation is used to activate the left dorsolateral prefrontal cortex (DLPFC), a target thought to be important in the pathophysiology of MDD. Repetitive transcranial magnetic stimulation is FDAapproved to treat medication-refractory major depressive disorder (MDD) in adults, and has been shown to be effective as both a monotherapy10 and an adjunctive treatment.11 The estimated number needed to treat (NNT) for rTMS ranges from 6 to 8, which is quite effective, although less so than ECT (and probably initial pharmacotherapy).5 Similar to ECT, however, there are no large randomized controlled trials (RCTs) in children or adolescents. Pilot RCTs12 and open trials13 suggest that DLPFC rTMS may be effective as an adjunctive treatment, speeding or augmenting response to a selective serotonin reuptake inhibitor in adolescents with MDD. Larger trials studying rTMS in pediatric patients are needed. While rTMS is generally well tolerated, disadvantages include the time-consuming schedule (the initial treatment is typically 5 days/week for several weeks) and local adverse effects of headache and scalp pain.
Ketamine, which traditionally is used as a dissociative anesthetic, is a rapidly emerging novel treatment in adult treatment-refractory MDD. It acts quickly (within hours to days) and cause significant improvement in difficult symptoms such as anhedonia14 and suicidal ideation.15 In adult studies, ketamine has a robust average effect size of >1.2, and an NNT ranging from 3 to 5 in medication-refractory patients.16,17 Ketamine is a glutamatergic modulator, acting outside of the monoamine neurochemical systems traditionally targeted by standard antidepressants.16 The efficacy of ketamine in treatment-refractory adults is impressive, but the effects of a single treatment are ephemeral, dissipating within 1 to 2 weeks, which has led to significant discussion surrounding optimal dosing strategies.16 Although small RCTs in pediatric patients are currently underway, at this time, the only evidence for ketamine for pediatric MDD is based on case series/report data18,19 which was positive.
For all of these interventional modalities, it is critical to refer children with treatmentrefractory disorders to interventionists who have appropriate experience and monitoring capabilities.
References
1. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
2. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
3. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
4. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and metaanalysis. Lancet. 2003;361(9360):799-808.
5. Berlim MT, Van den Eynde F, Daskalakis ZJ. Efficacy and acceptability of high frequency repetitive transcranial magnetic stimulation (rTMS) versus electroconvulsive therapy (ECT) for major depression: a systematic review and meta-analysis of randomized trials. Depress Anxiety. 2013;30(7):614-623.
6. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
7. Jacob P, Gogi PK, Srinath S, et al. Review of electroconvulsive therapy practice from a tertiary child and adolescent psychiatry centre. Asian J Psychiatr. 2014;12(1):95-99.
8. Zhand N, Courtney DB, Flament MF. Use of electroconvulsive therapy in adolescents with treatment-resistant depressive disorders: a case series. J ECT. 2015;31(4):238-245.
9. Puffer CC, Wall CA, Huxsahl JE, et al. A 20 year practice review of electroconvulsive therapy for adolescents. J Child Adolesc Psychopharmacol. 2016;26(7):632-636.
10. Berlim MT, van den Eynde F, Tovar-Perdomo S, et al. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44(2):225-239.
11. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342.
12. Huang ML, Luo BY, Hu JB, et al. Repetitive transcranial magnetic stimulation in combination with citalopram in young patients with first-episode major depressive disorder: a double-blind, randomized, sham-controlled trial. Aust N Z J Psychiatry. 2012;46(3):257-264.
13. Wall CA, Croarkin PE, Sim LA, et al. Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. J Clin Psychiatry. 2011;72(9):1263-1269.
14. Lally N, Nugent AC, Luckenbaugh DA, et al. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105.
15. Ballard ED, Ionescu DF, Vande Voort JL, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161-166.
16. Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
17. McGirr A, Berlim MT, Bond DJ, et al. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45(4):693-704.
18. Dwyer JB, Beyer C, Wilkinson ST, et al. Ketamine as a treatment for adolescent depression: a case report. J Am Acad Child Adolesc Psychiatry. 2017;56(4):352-354.
19. Cullen KR, Amatya P, Roback MG, et al. Intravenous ketamine for adolescents with treatment-resistant depression: an open-label study. J Child Adolesc Psychopharmacol. 2018;28(7):437-444.
Box 2
Efficacy
- Selective serotonin reuptake inhibitors are the most effective pharmacologic treatment we have for pediatric depression, OCD, and anxiety
- More than one-half of children who are prescribed SSRIs have a significant improvement, regardless of condition
- Based on current estimates, we need to treat 4 to 6 children with an SSRI to find one that will improve who would not improve with placebo
- The clinical benefits of SSRIs generally take a while to accrue; therefore, it is advisable to take the medication for at least 2 to 3 months before concluding that it is ineffective
- In addition to medication, evidence-based psychotherapies provide significant benefit for pediatric depression, OCD, and anxiety
Tolerability
- Most commonly prescribed pediatric antidepressants have been used safely in children for 2 to 3 decades. The safety profiles of SSRIs are among the best of any medications used for children and adolescents
- While many children get better when taking these medications, it’s important that we also talk about potential adverse effects. Some children will experience sleep problems (either sleepier than usual or difficulty sleeping), changes in energy levels, headache, gastrointestinal upset, and dry mouth. These are most likely at the beginning of treatment, or when we increase the dose; they usually are time-limited and go away on their own
- Often adverse effects occur first and the benefits come later. Because it may take at least a few weeks to start to see the mood/anxiety benefits, it’s important for us to talk about any adverse effects your child experiences and remember that they usually are short-lived
Suicidality
- The FDA placed a “black-box” warning on antidepressants after pediatric studies found a small but statistically significant increased risk of reporting suicidal thoughts or behaviors over the short-term compared with placebo
- The increased risk of spontaneously reporting suicidal ideation was quite small. Studies suggested that one would need to treat 100 to 140 children to see 1 child report suicidal ideation compared to placebo. Suicidal ideation is a common symptom in children with depression and anxiety
- Studies found no increased risk when suicidal ideation was systematically assessed using structured rating scales
- In the studies evaluated, there were no completed suicides by patients taking medication or placebo
- Population studies show that higher rates of antidepressant prescriptions are associated with lower rates of attempted and completed teen suicide, which underscores that in general, these medicines treat the underlying causes of suicidality
- No scientific consensus exists on whether these medications are truly associated with an increased risk of new-onset suicidal ideation, or if this association is due to other factors (eg, improvement in anxiety and depressive symptoms that make patients more comfortable to report suicidal ideation spontaneously)
- Regardless, the FDA recommends frequent monitoring of children for suicidal thoughts when these medications are started. This should be done anyway in children experiencing depression and anxiety, and it’s why we will plan to have more frequent appointments as the medication is initiated
OCD: obsessive-compulsive disorder; SSRIs: selective serotonin reuptake inhibitors
1. Williams SB, O’Connor EA, Eder M, et al. Screening for child and adolescent depression in primary care settings: a systematic evidence review for the US Preventive Services Task Force. Pediatrics. 2009;123(4):e716-e735. doi: 10.1542/peds.2008-2415.
2. Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49(12):1002-1014.
3. Lewinsohn PM, Clarke GN, Seeley JR, et al. Major depression in community adolescents: age at onset, episode duration, and time to recurrence. J Am Acad Child Adolesc Psychiatry. 1994;33(6):809-818.
4. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
5. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
6. Keenan-Miller D, Hammen CL, Brennan PA. Health outcomes related to early adolescent depression. J Adolesc Health. 2007; 41(3): 256-62.
7. Shaffer D, Gould MS, Fisher P, et al. Psychiatric diagnosis in child and adolescent suicide. Arch Gen Psychiatry. 1996;53(4):339-348.
8. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
9. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
10. Wittchen HU, Nelson CB, Lachner G. Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychol Med. 1998;28(1):109-126.
11. Foley DL, Goldston DB, Costello EJ, et al. Proximal psychiatric risk factors for suicidality in youth: the Great Smoky Mountains Study. Arch Gen Psychiatry. 2006;63(9):1017-1024.
12. Cheung A, Sacks D, Dewa CS, et al. Pediatric prescribing practices and the FDA black-box warning on antidepressants. J Dev Behav Pediatr. 2008 29(3):213-215.
13. Walkup JT. Antidepressant efficacy for depression in children and adolescents: industry- and NIMH-funded studies. Am J Psychiatry. 2017;174(5):430-437.
14. Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173(2):174-183.
15. Varigonda AL, Jakubovski E, Taylor MJ, et al. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors in pediatric major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(7):557-564.
16. Strawn JR, Welge JA, Wehry AM, et al. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32(3):149-157.
17. March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA. 1998;280(20):1752-1756.
18. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
19. Kennard BD, Silva SG, Tonev S, et al. Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48(2):186-195.
20. Cipriani A, Zhou X, Del Giovane C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388(10047):881-890.
21. Cohen JA, Mannarino AP, Perel JM, et al. A pilot randomized controlled trial of combined trauma-focused CBT and sertraline for childhood PTSD symptoms. J Am Acad Child Adolesc Psychiatry. 2007;46(7):811-819.
22. Robb AS, Cueva JE, Sporn J, et al. Sertraline treatment of children and adolescents with posttraumatic stress disorder: a double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2010;20(6):463-471.
23. Diehle J, Opmeer BC, Boer F, et al. Trauma-focused cognitive behavioral therapy or eye movement desensitization and reprocessing: what works in children with posttraumatic stress symptoms? A randomized controlled trial. Eur Child Adolesc Psychiatry. 2015;24(2):227-236.
24. Aiyer R, Barkin RL, Bhatia A. Treatment of neuropathic pain with venlafaxine: a systematic review. Pain Med. 2017;18(10):1999-2012.
25. Barrickman LL, Perry PJ, Allen AJ, et al. Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34(5):649-657.
26. Monuteaux MC, Spencer TJ, Faraone SV, et al. A randomized, placebo-controlled clinical trial of bupropion for the prevention of smoking in children and adolescents with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2007;68(7):1094-1101.
27. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
28. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656.
29. DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder--a multicenter trial. J Am Acad Child Adolesc Psychiatry. 1992;31(1):45-49.
30. Caldwell PH, Sureshkumar P, Wong WC. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev. 2016;(1):CD002117.
31. Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(10):851-859.e2. doi: 10.1016/j.jaac.2016.07.768.
32. Walkup J, Labellarte M. Complications of SSRI treatment. J Child Adolesc Psychopharmacol. 2001;11(1):1-4.
33. Leo RJ, Lichter DG, Hershey LA. Parkinsonism associated with fluoxetine and cimetidine: a case report. J Geriatr Psychiatry Neurol. 1995;8(4):231-233.
34. Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(4):283-293.
35. Bernstein GA, Borchardt CM, Perwien AR, et al. Imipramine plus cognitive-behavioral therapy in the treatment of school refusal. J Am Acad Child Adolesc Psychiatry. 2000;39(3): 276-283.
36. Safer DJ, Zito JM. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: children versus adolescents. J Child Adolesc Psychopharmacol. 2006;16(1-2):159-169.
37. Reinblatt SP, DosReis S, Walkup JT, et al. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119-126.
38. Goldsmith M, Singh M, Chang K. Antidepressants and psychostimulants in pediatric populations: is there an association with mania? Paediatr Drugs. 2011;13(4): 225-243.
39. Sidor MM, Macqueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
40. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
41. McClellan J, Kowatch R, Findling RL. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):107-125.
42. Dobry Y, Rice T, Sher L. Ecstasy use and serotonin syndrome: a neglected danger to adolescents and young adults prescribed selective serotonin reuptake inhibitors. Int J Adolesc Med Health. 2013; 25(3):193-199.
43. Schwartz AR, Pizon AF, Brooks DE. Dextromethorphan-induced serotonin syndrome. Clin Toxicol (Phila). 2008;46(8):771-773.
44. Gibbons RD, Brown CH, Hur K, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry. 2007;164(9):1356-1363.
45. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):332-339.
46. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683-1696.
47. Sharma T, Guski LS, Freund N, et al. Suicidality and aggression during antidepressant treatment: systematic review and meta-analyses based on clinical study reports. BMJ. 2016;352: i65. doi: https://doi.org/10.1136/bmj.i65.
48. Olfson M, Shaffer D, Marcus SC, et al. Relationship between antidepressant medication treatment and suicide in adolescents. Arch Gen Psychiatry. 2003;60(10):978-982.
49. Garland JE, Kutcher S, Virani A, et al. Update on the Use of SSRIs and SNRIs with children and adolescents in clinical practice. J Can Acad Child Adolesc Psychiatry. 2016;25(1):4-10.
50. Bridge JA, Barbe RP, Birmaher B, et al. Emergent suicidality in a clinical psychotherapy trial for adolescent depression. Am J Psychiatry. 2005;162(11):2173-2175.
51. Birmaher B, Brent D, Bernet W, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1503-1526.
52. Ravizza L, Maina G, Bogetto F, et al. Long term treatment of obsessive-compulsive disorder. CNS Drugs. 1998;10(4):247-255.
53. Hosenbocus S, Chahal R. SSRIs and SNRIs: a review of the discontinuation syndrome in children and adolescents. J Can Acad Child Adolesc Psychiatry. 2011;20(1):60-67.
54. Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850-855.
55. Issari Y, Jakubovski E, Bartley CA, et al. Early onset of response with selective serotonin reuptake inhibitors in obsessive-compulsive disorder: a meta-analysis. J Clin Psychiatry. 2016; 77(5):e605-e611. doi: 10.4088/JCP.14r09758.
56. Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299(8):901-913.
1. Williams SB, O’Connor EA, Eder M, et al. Screening for child and adolescent depression in primary care settings: a systematic evidence review for the US Preventive Services Task Force. Pediatrics. 2009;123(4):e716-e735. doi: 10.1542/peds.2008-2415.
2. Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49(12):1002-1014.
3. Lewinsohn PM, Clarke GN, Seeley JR, et al. Major depression in community adolescents: age at onset, episode duration, and time to recurrence. J Am Acad Child Adolesc Psychiatry. 1994;33(6):809-818.
4. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
5. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
6. Keenan-Miller D, Hammen CL, Brennan PA. Health outcomes related to early adolescent depression. J Adolesc Health. 2007; 41(3): 256-62.
7. Shaffer D, Gould MS, Fisher P, et al. Psychiatric diagnosis in child and adolescent suicide. Arch Gen Psychiatry. 1996;53(4):339-348.
8. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
9. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
10. Wittchen HU, Nelson CB, Lachner G. Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychol Med. 1998;28(1):109-126.
11. Foley DL, Goldston DB, Costello EJ, et al. Proximal psychiatric risk factors for suicidality in youth: the Great Smoky Mountains Study. Arch Gen Psychiatry. 2006;63(9):1017-1024.
12. Cheung A, Sacks D, Dewa CS, et al. Pediatric prescribing practices and the FDA black-box warning on antidepressants. J Dev Behav Pediatr. 2008 29(3):213-215.
13. Walkup JT. Antidepressant efficacy for depression in children and adolescents: industry- and NIMH-funded studies. Am J Psychiatry. 2017;174(5):430-437.
14. Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173(2):174-183.
15. Varigonda AL, Jakubovski E, Taylor MJ, et al. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors in pediatric major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(7):557-564.
16. Strawn JR, Welge JA, Wehry AM, et al. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32(3):149-157.
17. March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA. 1998;280(20):1752-1756.
18. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
19. Kennard BD, Silva SG, Tonev S, et al. Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48(2):186-195.
20. Cipriani A, Zhou X, Del Giovane C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388(10047):881-890.
21. Cohen JA, Mannarino AP, Perel JM, et al. A pilot randomized controlled trial of combined trauma-focused CBT and sertraline for childhood PTSD symptoms. J Am Acad Child Adolesc Psychiatry. 2007;46(7):811-819.
22. Robb AS, Cueva JE, Sporn J, et al. Sertraline treatment of children and adolescents with posttraumatic stress disorder: a double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2010;20(6):463-471.
23. Diehle J, Opmeer BC, Boer F, et al. Trauma-focused cognitive behavioral therapy or eye movement desensitization and reprocessing: what works in children with posttraumatic stress symptoms? A randomized controlled trial. Eur Child Adolesc Psychiatry. 2015;24(2):227-236.
24. Aiyer R, Barkin RL, Bhatia A. Treatment of neuropathic pain with venlafaxine: a systematic review. Pain Med. 2017;18(10):1999-2012.
25. Barrickman LL, Perry PJ, Allen AJ, et al. Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34(5):649-657.
26. Monuteaux MC, Spencer TJ, Faraone SV, et al. A randomized, placebo-controlled clinical trial of bupropion for the prevention of smoking in children and adolescents with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2007;68(7):1094-1101.
27. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
28. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656.
29. DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder--a multicenter trial. J Am Acad Child Adolesc Psychiatry. 1992;31(1):45-49.
30. Caldwell PH, Sureshkumar P, Wong WC. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev. 2016;(1):CD002117.
31. Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(10):851-859.e2. doi: 10.1016/j.jaac.2016.07.768.
32. Walkup J, Labellarte M. Complications of SSRI treatment. J Child Adolesc Psychopharmacol. 2001;11(1):1-4.
33. Leo RJ, Lichter DG, Hershey LA. Parkinsonism associated with fluoxetine and cimetidine: a case report. J Geriatr Psychiatry Neurol. 1995;8(4):231-233.
34. Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(4):283-293.
35. Bernstein GA, Borchardt CM, Perwien AR, et al. Imipramine plus cognitive-behavioral therapy in the treatment of school refusal. J Am Acad Child Adolesc Psychiatry. 2000;39(3): 276-283.
36. Safer DJ, Zito JM. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: children versus adolescents. J Child Adolesc Psychopharmacol. 2006;16(1-2):159-169.
37. Reinblatt SP, DosReis S, Walkup JT, et al. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119-126.
38. Goldsmith M, Singh M, Chang K. Antidepressants and psychostimulants in pediatric populations: is there an association with mania? Paediatr Drugs. 2011;13(4): 225-243.
39. Sidor MM, Macqueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
40. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
41. McClellan J, Kowatch R, Findling RL. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):107-125.
42. Dobry Y, Rice T, Sher L. Ecstasy use and serotonin syndrome: a neglected danger to adolescents and young adults prescribed selective serotonin reuptake inhibitors. Int J Adolesc Med Health. 2013; 25(3):193-199.
43. Schwartz AR, Pizon AF, Brooks DE. Dextromethorphan-induced serotonin syndrome. Clin Toxicol (Phila). 2008;46(8):771-773.
44. Gibbons RD, Brown CH, Hur K, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry. 2007;164(9):1356-1363.
45. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):332-339.
46. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683-1696.
47. Sharma T, Guski LS, Freund N, et al. Suicidality and aggression during antidepressant treatment: systematic review and meta-analyses based on clinical study reports. BMJ. 2016;352: i65. doi: https://doi.org/10.1136/bmj.i65.
48. Olfson M, Shaffer D, Marcus SC, et al. Relationship between antidepressant medication treatment and suicide in adolescents. Arch Gen Psychiatry. 2003;60(10):978-982.
49. Garland JE, Kutcher S, Virani A, et al. Update on the Use of SSRIs and SNRIs with children and adolescents in clinical practice. J Can Acad Child Adolesc Psychiatry. 2016;25(1):4-10.
50. Bridge JA, Barbe RP, Birmaher B, et al. Emergent suicidality in a clinical psychotherapy trial for adolescent depression. Am J Psychiatry. 2005;162(11):2173-2175.
51. Birmaher B, Brent D, Bernet W, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1503-1526.
52. Ravizza L, Maina G, Bogetto F, et al. Long term treatment of obsessive-compulsive disorder. CNS Drugs. 1998;10(4):247-255.
53. Hosenbocus S, Chahal R. SSRIs and SNRIs: a review of the discontinuation syndrome in children and adolescents. J Can Acad Child Adolesc Psychiatry. 2011;20(1):60-67.
54. Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850-855.
55. Issari Y, Jakubovski E, Bartley CA, et al. Early onset of response with selective serotonin reuptake inhibitors in obsessive-compulsive disorder: a meta-analysis. J Clin Psychiatry. 2016; 77(5):e605-e611. doi: 10.4088/JCP.14r09758.
56. Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299(8):901-913.
FCC backs designating 988 as suicide prevention hotline
A Federal Communications Commission report recommends that 988 be designated as a nationwide suicide and mental health crisis support hotline, because a three-digit number “could be more effective” than the current 10-digit number, according to an FCC release.
The report was prepared by the FCC’s Wireline Competition Bureau and Office of Economics and Analytics at the direction of the National Suicide Hotline Improvement Act of 2018. The current service, known as the National Suicide Prevention Lifeline, uses the 10-digit number of 800-273-8255 (TALK). The report noted that the hotline has proved effective: The service answered 2.2 million calls in 2018 and various assessments have shown significant reductions in hopelessness and suicidal ideation among callers.
However, based on data and conclusions from some of those assessments, the report determined “that the Lifeline could be more effective in preventing suicides and providing crisis intervention if it were accessible via a simple, easy-to-remember, 3-digit dialing code.” The report examined the feasibility of three-digit options, including N11 options such as 211 and 511, but based on an analysis by the North American Numbering Council, it found that “technical and operational concerns related to the 988 code could be more easily and quickly addressed and resolved than any re-education efforts related to repurposing a N11 code.”
Suicide and mental health crises have been on the rise, according to numbers from the Centers for Disease Control and Prevention cited by the FCC report. Among the 50 states, 49 saw increases during 1999-2016, and more than half saw increases greater than 20%. The report describes how some groups, such as veterans and LGBTQ youth, are at especially high risk:
A Federal Communications Commission report recommends that 988 be designated as a nationwide suicide and mental health crisis support hotline, because a three-digit number “could be more effective” than the current 10-digit number, according to an FCC release.
The report was prepared by the FCC’s Wireline Competition Bureau and Office of Economics and Analytics at the direction of the National Suicide Hotline Improvement Act of 2018. The current service, known as the National Suicide Prevention Lifeline, uses the 10-digit number of 800-273-8255 (TALK). The report noted that the hotline has proved effective: The service answered 2.2 million calls in 2018 and various assessments have shown significant reductions in hopelessness and suicidal ideation among callers.
However, based on data and conclusions from some of those assessments, the report determined “that the Lifeline could be more effective in preventing suicides and providing crisis intervention if it were accessible via a simple, easy-to-remember, 3-digit dialing code.” The report examined the feasibility of three-digit options, including N11 options such as 211 and 511, but based on an analysis by the North American Numbering Council, it found that “technical and operational concerns related to the 988 code could be more easily and quickly addressed and resolved than any re-education efforts related to repurposing a N11 code.”
Suicide and mental health crises have been on the rise, according to numbers from the Centers for Disease Control and Prevention cited by the FCC report. Among the 50 states, 49 saw increases during 1999-2016, and more than half saw increases greater than 20%. The report describes how some groups, such as veterans and LGBTQ youth, are at especially high risk:
A Federal Communications Commission report recommends that 988 be designated as a nationwide suicide and mental health crisis support hotline, because a three-digit number “could be more effective” than the current 10-digit number, according to an FCC release.
The report was prepared by the FCC’s Wireline Competition Bureau and Office of Economics and Analytics at the direction of the National Suicide Hotline Improvement Act of 2018. The current service, known as the National Suicide Prevention Lifeline, uses the 10-digit number of 800-273-8255 (TALK). The report noted that the hotline has proved effective: The service answered 2.2 million calls in 2018 and various assessments have shown significant reductions in hopelessness and suicidal ideation among callers.
However, based on data and conclusions from some of those assessments, the report determined “that the Lifeline could be more effective in preventing suicides and providing crisis intervention if it were accessible via a simple, easy-to-remember, 3-digit dialing code.” The report examined the feasibility of three-digit options, including N11 options such as 211 and 511, but based on an analysis by the North American Numbering Council, it found that “technical and operational concerns related to the 988 code could be more easily and quickly addressed and resolved than any re-education efforts related to repurposing a N11 code.”
Suicide and mental health crises have been on the rise, according to numbers from the Centers for Disease Control and Prevention cited by the FCC report. Among the 50 states, 49 saw increases during 1999-2016, and more than half saw increases greater than 20%. The report describes how some groups, such as veterans and LGBTQ youth, are at especially high risk:
Addressing suicidality among Indigenous women, girls
Historical trauma and current social factors contribute to depression, PTSD, anxiety disorders
The history of abuse and genocide has its precursors in antiquity. A brief sketch of this history will provide some insights into the impact of intergenerational trauma and a rationale for the crisis of missing and murdered Indigenous women and girls in the United States and Canada, or Turtle Island, as the Indigenous People call it.
Such a review also will provide a partial explanation of why the suicide rate among non-Hispanic Native American or Alaska Native women increased by 139%1 during 1999-2017 – a time when more Indigenous women were gaining access to law and medical school, as well as positions of authority in their tribes.
Church-, state-sanctioned transgressions
The psychological impact of our past history haunts us today. Papal bulletins – decrees from the pope – gave permission to Christian explorers to take land, wealth, and slaves from any nonbeliever. This permission was labeled the Doctrine of Discovery. It was incorporated into U.S. law in 1823, and by the Supreme Court case, Johnson v. M’intosh. It also provided rationale for the Indian Removal Act, which was passed on May 28, 1830, and signed into law by U.S. President Andrew Jackson. As a result of that law, Indigenous People were forced onto reservations, often removed from their traditional and sacred homelands. Many died during forced relocation.2
From the time of “discovery” by settlers until well into the 19th century, the U.S. governmental intent was genocide. It was manifest by the outright murder of Indigenous People, displacement from land, and the disruption of families when children were taken, put into boarding schools, and were forbidden to speak their language. Indigenous medicine people were killed or jailed for practicing their traditional ceremonies. Indigenous nations had their laws, languages, and agricultural practices denied them. Even today, they must practice U.S. law, adapt colonizing forms of land ownership, and engage in the economic practices of the dominant culture. The economic system currently in place rewards rape of the land and creates a trickle-up economy that keeps rewarding the rich at the expense of the poor. The economic system even gives corporations legal status as individuals, and, in some cases, is allowed to supersede the rights of Indigenous nations.
Today, the federal government still can appropriate land for minerals, pipelines,3 and even put indigenous land and water sovereignty at risk of contamination and pollution by mines established upstream.4 Most of those practices are repugnant to Indigenous nations. The Doctrine of Discovery established prior to 1492 is still alive and well on Turtle Island.
It is this background that denies the rights of Mother Earth, and this backdrop that, in turn, generalizes the denial of the rights of Indigenous women. There are women today, who, against their will and knowledge, have been sterilized.5 There are cases in which women have been raped and beaten, and their perpetrators were never been brought to justice.6 There are jurisdictional issues in the federal law that keep non-native perpetrators from being punished for their actions on tribal sovereign land.
This history and those current practices affect Indigenous families. Historical trauma produces epigenetic changes7 that create more anxiety and depression. Families in which one or both parents were taken away have a harder time providing a loving, safe, addiction-free environment for their children. Children often have high scores on measurements of adverse childhood experiences and suffer PTSD. As psychiatrists, we have treated PTSD from residential and boarding school survivors, families with family members who were victims of being missing or murdered, and survivors of sexual abuse – both in the United States and Canada. According to the final Canadian report of the inquiry into missing and murdered Indigenous women and girls, the murder rate for Indigenous women was 12 times that of non-Indigenous women.8
We assert that this combination of historical trauma and current social factors contributes to depression, PTSD, and anxiety disorders that currently feed the rise in attempted and completed suicide. Less-than-optimal educational opportunities and unemployment, often above 10% on reservations,9 along with food insecurity, accentuate the settings in which women and girls live.
Women achieving despite challenges
Yet, Indigenous women are making great strides within their cultures and communities. For example, Indigenous women are leading language revitalization, and within their culture, are healers and carriers of knowledge. Many Indigenous women are doctors, lawyers, dentists, teachers, poets, authors, and artists.10 Voters in last year’s midterms elected two Native American women to the U.S. Congress. Often, however, those achievements within the Western culture come at a cost, and some might have difficulty balancing those roles with their traditional cultures.
Current societal pressures feed the rise of suicide. Santa Fe, N.M., is known for its affluence and reputation as a tricultural city of Anglos, Hispanics, and Native Americans, and yet, a recent health impact assessment survey of urban Indigenous families stated that food insecurity was the leading concern for those families. Unemployment on the Navajo Nation is above 50%.11 The Indian Health Service (IHS) in the United States, which provides the majority of mental services to the Indigenous population, has identified mental health issues as the No. 1 health problem. However, only 7% of the IHS budget is allocated for mental health and substance abuse services. This represents an underfudging of services to American Indian and Alaska Native communities. In fact, there were only two psychiatrists per 100,000 people served by the IHS, which is one-seventh the number of psychiatrists available to the general population in the United States.12
Best practices for psychiatrists working with Indigenous women demands that we know the history, know how that history is still being manifest in subtle ways, and understand how such antiquated papal bulletins as the Doctrine of Discovery still operate to justify the taking and misuse of indigenous land. We must realize that the dominant economic systems, laws, and policing strategies are imposed on cultures that are sophisticated in their own right. This will then allow compassionate care with a level of understanding.
13
We can advocate at all levels, considering that the role of the federal government, the state, corporations, tribes, families, and provision of quality care to individuals can continue the positive collective advancement of women, and reduce the morbidity and mortality associated with suicide attempts.
We need to be sensitive to our patients and their risks of suicide. Treat suicidal ideation as the serious threat that it is. Address the depression, anxiety, PTSD, historical trauma, substance abuse, emotional dysregulation, and loss of relationship in persons with attachment disorders as serious and valid life events than can lead to serious consequences – including completed suicide.
Indigenous women are resilient, and the approach should be to also balance knowledge of those potential barriers with validating the feminine, and supporting the traditional roles of women and men that value women and children, and revere the matriarchs. Encouraging and supporting Indigenous resurgence of cultural practices and values is significant for positive outcomes for healing and wellness. Doing so can carry a greater meaning within Indigenous and First Nations society.
References
1. Curtin SC and H Hedegaard. Suicide rates for females and males by race and ethnicity: United States, 1999 and 2017. NCHS Health E-Stat. 2019.
2. Anderson GC. Ethnic cleansing and the Indian: The crime that should haunt America. Norman, Okla.: University of Oklahoma Press, 2014.
3. Rausch N. “Standing Rock, Morton County work to mend relationships post-DAPL protests.” Billingsgazette.com. Aug 10, 2019.
4. Roy A. “5 ways the government keeps Native Americans in poverty.” Forbes.com. Mar 13, 2014.
5. Blakemore E. “The little-known history of forced sterilization of Native American women.” JSTOR.org. Aug 25, 2016.
6. Bleir G and A Zoledziowski. “Murdered and missing Native American women challenge police and courts.” Publicintegrity.org. Aug 27, 2018.
7. Brockie TN et al. A framework to examine the role of epigenetics in health disparities among Native Americans. Nurs Res Prac. 2013;2013:410395.
8. “Reclaiming power and place: The final report of the national inquiry into missing and murdered Indigenous women and girls.” Vancouver: Privy Office. Jun 3, 2019.
9. Hagan S. “Where U.S. unemployment is still sky-high: Indian reservations.” Bloomberg.com. Apr 5, 2018.
10. Morin B. “Meet 10 Indigenous women who are making the world a better place.” Indian Country Today. Jul 1, 2019.
11. Fact sheet. Discovernavajo.com.
12. Sarche M and P Spicer. Poverty and health disparities for American Indian and Alaska Native children: Current knowledge and future prospects. Ann NY Acad Sci. 2008 Jul 25;1136:126-36.
13. Lewis-Fernández R et al. Culture and psychiatric evaluation: Operationalizing cultural formulation for DSM-5. Psychiatry. 2014 Summer;77(2):130-54.
Dr. Roessel is a Navajo board-certified psychiatrist practicing in Santa Fe, N.M., working with the local Indigenous population. She has special expertise in cultural psychiatry; her childhood was spent growing up in the Navajo nation with her grandfather, who was a Navajo medicine man. Her psychiatric practice focuses on integrating Indigenous knowledge and principles. Dr. Roessel is a distinguished fellow of the American Psychiatric Association.
Dr. Neidhardt is a board-certified psychiatrist who lives in Santa Fe and has an integrative, holistic psychiatric practice that also specializes in trauma-focused therapy. He has provided care for Indigenous People in the Southwest United States and in Canada, and has worked with Navajo medicine people to develop training for mental health professionals with his wife, Dr. Mary Hasbah Roessel. Dr. Reinhardt is a life fellow of the APA.
Historical trauma and current social factors contribute to depression, PTSD, anxiety disorders
Historical trauma and current social factors contribute to depression, PTSD, anxiety disorders
The history of abuse and genocide has its precursors in antiquity. A brief sketch of this history will provide some insights into the impact of intergenerational trauma and a rationale for the crisis of missing and murdered Indigenous women and girls in the United States and Canada, or Turtle Island, as the Indigenous People call it.
Such a review also will provide a partial explanation of why the suicide rate among non-Hispanic Native American or Alaska Native women increased by 139%1 during 1999-2017 – a time when more Indigenous women were gaining access to law and medical school, as well as positions of authority in their tribes.
Church-, state-sanctioned transgressions
The psychological impact of our past history haunts us today. Papal bulletins – decrees from the pope – gave permission to Christian explorers to take land, wealth, and slaves from any nonbeliever. This permission was labeled the Doctrine of Discovery. It was incorporated into U.S. law in 1823, and by the Supreme Court case, Johnson v. M’intosh. It also provided rationale for the Indian Removal Act, which was passed on May 28, 1830, and signed into law by U.S. President Andrew Jackson. As a result of that law, Indigenous People were forced onto reservations, often removed from their traditional and sacred homelands. Many died during forced relocation.2
From the time of “discovery” by settlers until well into the 19th century, the U.S. governmental intent was genocide. It was manifest by the outright murder of Indigenous People, displacement from land, and the disruption of families when children were taken, put into boarding schools, and were forbidden to speak their language. Indigenous medicine people were killed or jailed for practicing their traditional ceremonies. Indigenous nations had their laws, languages, and agricultural practices denied them. Even today, they must practice U.S. law, adapt colonizing forms of land ownership, and engage in the economic practices of the dominant culture. The economic system currently in place rewards rape of the land and creates a trickle-up economy that keeps rewarding the rich at the expense of the poor. The economic system even gives corporations legal status as individuals, and, in some cases, is allowed to supersede the rights of Indigenous nations.
Today, the federal government still can appropriate land for minerals, pipelines,3 and even put indigenous land and water sovereignty at risk of contamination and pollution by mines established upstream.4 Most of those practices are repugnant to Indigenous nations. The Doctrine of Discovery established prior to 1492 is still alive and well on Turtle Island.
It is this background that denies the rights of Mother Earth, and this backdrop that, in turn, generalizes the denial of the rights of Indigenous women. There are women today, who, against their will and knowledge, have been sterilized.5 There are cases in which women have been raped and beaten, and their perpetrators were never been brought to justice.6 There are jurisdictional issues in the federal law that keep non-native perpetrators from being punished for their actions on tribal sovereign land.
This history and those current practices affect Indigenous families. Historical trauma produces epigenetic changes7 that create more anxiety and depression. Families in which one or both parents were taken away have a harder time providing a loving, safe, addiction-free environment for their children. Children often have high scores on measurements of adverse childhood experiences and suffer PTSD. As psychiatrists, we have treated PTSD from residential and boarding school survivors, families with family members who were victims of being missing or murdered, and survivors of sexual abuse – both in the United States and Canada. According to the final Canadian report of the inquiry into missing and murdered Indigenous women and girls, the murder rate for Indigenous women was 12 times that of non-Indigenous women.8
We assert that this combination of historical trauma and current social factors contributes to depression, PTSD, and anxiety disorders that currently feed the rise in attempted and completed suicide. Less-than-optimal educational opportunities and unemployment, often above 10% on reservations,9 along with food insecurity, accentuate the settings in which women and girls live.
Women achieving despite challenges
Yet, Indigenous women are making great strides within their cultures and communities. For example, Indigenous women are leading language revitalization, and within their culture, are healers and carriers of knowledge. Many Indigenous women are doctors, lawyers, dentists, teachers, poets, authors, and artists.10 Voters in last year’s midterms elected two Native American women to the U.S. Congress. Often, however, those achievements within the Western culture come at a cost, and some might have difficulty balancing those roles with their traditional cultures.
Current societal pressures feed the rise of suicide. Santa Fe, N.M., is known for its affluence and reputation as a tricultural city of Anglos, Hispanics, and Native Americans, and yet, a recent health impact assessment survey of urban Indigenous families stated that food insecurity was the leading concern for those families. Unemployment on the Navajo Nation is above 50%.11 The Indian Health Service (IHS) in the United States, which provides the majority of mental services to the Indigenous population, has identified mental health issues as the No. 1 health problem. However, only 7% of the IHS budget is allocated for mental health and substance abuse services. This represents an underfudging of services to American Indian and Alaska Native communities. In fact, there were only two psychiatrists per 100,000 people served by the IHS, which is one-seventh the number of psychiatrists available to the general population in the United States.12
Best practices for psychiatrists working with Indigenous women demands that we know the history, know how that history is still being manifest in subtle ways, and understand how such antiquated papal bulletins as the Doctrine of Discovery still operate to justify the taking and misuse of indigenous land. We must realize that the dominant economic systems, laws, and policing strategies are imposed on cultures that are sophisticated in their own right. This will then allow compassionate care with a level of understanding.
13
We can advocate at all levels, considering that the role of the federal government, the state, corporations, tribes, families, and provision of quality care to individuals can continue the positive collective advancement of women, and reduce the morbidity and mortality associated with suicide attempts.
We need to be sensitive to our patients and their risks of suicide. Treat suicidal ideation as the serious threat that it is. Address the depression, anxiety, PTSD, historical trauma, substance abuse, emotional dysregulation, and loss of relationship in persons with attachment disorders as serious and valid life events than can lead to serious consequences – including completed suicide.
Indigenous women are resilient, and the approach should be to also balance knowledge of those potential barriers with validating the feminine, and supporting the traditional roles of women and men that value women and children, and revere the matriarchs. Encouraging and supporting Indigenous resurgence of cultural practices and values is significant for positive outcomes for healing and wellness. Doing so can carry a greater meaning within Indigenous and First Nations society.
References
1. Curtin SC and H Hedegaard. Suicide rates for females and males by race and ethnicity: United States, 1999 and 2017. NCHS Health E-Stat. 2019.
2. Anderson GC. Ethnic cleansing and the Indian: The crime that should haunt America. Norman, Okla.: University of Oklahoma Press, 2014.
3. Rausch N. “Standing Rock, Morton County work to mend relationships post-DAPL protests.” Billingsgazette.com. Aug 10, 2019.
4. Roy A. “5 ways the government keeps Native Americans in poverty.” Forbes.com. Mar 13, 2014.
5. Blakemore E. “The little-known history of forced sterilization of Native American women.” JSTOR.org. Aug 25, 2016.
6. Bleir G and A Zoledziowski. “Murdered and missing Native American women challenge police and courts.” Publicintegrity.org. Aug 27, 2018.
7. Brockie TN et al. A framework to examine the role of epigenetics in health disparities among Native Americans. Nurs Res Prac. 2013;2013:410395.
8. “Reclaiming power and place: The final report of the national inquiry into missing and murdered Indigenous women and girls.” Vancouver: Privy Office. Jun 3, 2019.
9. Hagan S. “Where U.S. unemployment is still sky-high: Indian reservations.” Bloomberg.com. Apr 5, 2018.
10. Morin B. “Meet 10 Indigenous women who are making the world a better place.” Indian Country Today. Jul 1, 2019.
11. Fact sheet. Discovernavajo.com.
12. Sarche M and P Spicer. Poverty and health disparities for American Indian and Alaska Native children: Current knowledge and future prospects. Ann NY Acad Sci. 2008 Jul 25;1136:126-36.
13. Lewis-Fernández R et al. Culture and psychiatric evaluation: Operationalizing cultural formulation for DSM-5. Psychiatry. 2014 Summer;77(2):130-54.
Dr. Roessel is a Navajo board-certified psychiatrist practicing in Santa Fe, N.M., working with the local Indigenous population. She has special expertise in cultural psychiatry; her childhood was spent growing up in the Navajo nation with her grandfather, who was a Navajo medicine man. Her psychiatric practice focuses on integrating Indigenous knowledge and principles. Dr. Roessel is a distinguished fellow of the American Psychiatric Association.
Dr. Neidhardt is a board-certified psychiatrist who lives in Santa Fe and has an integrative, holistic psychiatric practice that also specializes in trauma-focused therapy. He has provided care for Indigenous People in the Southwest United States and in Canada, and has worked with Navajo medicine people to develop training for mental health professionals with his wife, Dr. Mary Hasbah Roessel. Dr. Reinhardt is a life fellow of the APA.
The history of abuse and genocide has its precursors in antiquity. A brief sketch of this history will provide some insights into the impact of intergenerational trauma and a rationale for the crisis of missing and murdered Indigenous women and girls in the United States and Canada, or Turtle Island, as the Indigenous People call it.
Such a review also will provide a partial explanation of why the suicide rate among non-Hispanic Native American or Alaska Native women increased by 139%1 during 1999-2017 – a time when more Indigenous women were gaining access to law and medical school, as well as positions of authority in their tribes.
Church-, state-sanctioned transgressions
The psychological impact of our past history haunts us today. Papal bulletins – decrees from the pope – gave permission to Christian explorers to take land, wealth, and slaves from any nonbeliever. This permission was labeled the Doctrine of Discovery. It was incorporated into U.S. law in 1823, and by the Supreme Court case, Johnson v. M’intosh. It also provided rationale for the Indian Removal Act, which was passed on May 28, 1830, and signed into law by U.S. President Andrew Jackson. As a result of that law, Indigenous People were forced onto reservations, often removed from their traditional and sacred homelands. Many died during forced relocation.2
From the time of “discovery” by settlers until well into the 19th century, the U.S. governmental intent was genocide. It was manifest by the outright murder of Indigenous People, displacement from land, and the disruption of families when children were taken, put into boarding schools, and were forbidden to speak their language. Indigenous medicine people were killed or jailed for practicing their traditional ceremonies. Indigenous nations had their laws, languages, and agricultural practices denied them. Even today, they must practice U.S. law, adapt colonizing forms of land ownership, and engage in the economic practices of the dominant culture. The economic system currently in place rewards rape of the land and creates a trickle-up economy that keeps rewarding the rich at the expense of the poor. The economic system even gives corporations legal status as individuals, and, in some cases, is allowed to supersede the rights of Indigenous nations.
Today, the federal government still can appropriate land for minerals, pipelines,3 and even put indigenous land and water sovereignty at risk of contamination and pollution by mines established upstream.4 Most of those practices are repugnant to Indigenous nations. The Doctrine of Discovery established prior to 1492 is still alive and well on Turtle Island.
It is this background that denies the rights of Mother Earth, and this backdrop that, in turn, generalizes the denial of the rights of Indigenous women. There are women today, who, against their will and knowledge, have been sterilized.5 There are cases in which women have been raped and beaten, and their perpetrators were never been brought to justice.6 There are jurisdictional issues in the federal law that keep non-native perpetrators from being punished for their actions on tribal sovereign land.
This history and those current practices affect Indigenous families. Historical trauma produces epigenetic changes7 that create more anxiety and depression. Families in which one or both parents were taken away have a harder time providing a loving, safe, addiction-free environment for their children. Children often have high scores on measurements of adverse childhood experiences and suffer PTSD. As psychiatrists, we have treated PTSD from residential and boarding school survivors, families with family members who were victims of being missing or murdered, and survivors of sexual abuse – both in the United States and Canada. According to the final Canadian report of the inquiry into missing and murdered Indigenous women and girls, the murder rate for Indigenous women was 12 times that of non-Indigenous women.8
We assert that this combination of historical trauma and current social factors contributes to depression, PTSD, and anxiety disorders that currently feed the rise in attempted and completed suicide. Less-than-optimal educational opportunities and unemployment, often above 10% on reservations,9 along with food insecurity, accentuate the settings in which women and girls live.
Women achieving despite challenges
Yet, Indigenous women are making great strides within their cultures and communities. For example, Indigenous women are leading language revitalization, and within their culture, are healers and carriers of knowledge. Many Indigenous women are doctors, lawyers, dentists, teachers, poets, authors, and artists.10 Voters in last year’s midterms elected two Native American women to the U.S. Congress. Often, however, those achievements within the Western culture come at a cost, and some might have difficulty balancing those roles with their traditional cultures.
Current societal pressures feed the rise of suicide. Santa Fe, N.M., is known for its affluence and reputation as a tricultural city of Anglos, Hispanics, and Native Americans, and yet, a recent health impact assessment survey of urban Indigenous families stated that food insecurity was the leading concern for those families. Unemployment on the Navajo Nation is above 50%.11 The Indian Health Service (IHS) in the United States, which provides the majority of mental services to the Indigenous population, has identified mental health issues as the No. 1 health problem. However, only 7% of the IHS budget is allocated for mental health and substance abuse services. This represents an underfudging of services to American Indian and Alaska Native communities. In fact, there were only two psychiatrists per 100,000 people served by the IHS, which is one-seventh the number of psychiatrists available to the general population in the United States.12
Best practices for psychiatrists working with Indigenous women demands that we know the history, know how that history is still being manifest in subtle ways, and understand how such antiquated papal bulletins as the Doctrine of Discovery still operate to justify the taking and misuse of indigenous land. We must realize that the dominant economic systems, laws, and policing strategies are imposed on cultures that are sophisticated in their own right. This will then allow compassionate care with a level of understanding.
13
We can advocate at all levels, considering that the role of the federal government, the state, corporations, tribes, families, and provision of quality care to individuals can continue the positive collective advancement of women, and reduce the morbidity and mortality associated with suicide attempts.
We need to be sensitive to our patients and their risks of suicide. Treat suicidal ideation as the serious threat that it is. Address the depression, anxiety, PTSD, historical trauma, substance abuse, emotional dysregulation, and loss of relationship in persons with attachment disorders as serious and valid life events than can lead to serious consequences – including completed suicide.
Indigenous women are resilient, and the approach should be to also balance knowledge of those potential barriers with validating the feminine, and supporting the traditional roles of women and men that value women and children, and revere the matriarchs. Encouraging and supporting Indigenous resurgence of cultural practices and values is significant for positive outcomes for healing and wellness. Doing so can carry a greater meaning within Indigenous and First Nations society.
References
1. Curtin SC and H Hedegaard. Suicide rates for females and males by race and ethnicity: United States, 1999 and 2017. NCHS Health E-Stat. 2019.
2. Anderson GC. Ethnic cleansing and the Indian: The crime that should haunt America. Norman, Okla.: University of Oklahoma Press, 2014.
3. Rausch N. “Standing Rock, Morton County work to mend relationships post-DAPL protests.” Billingsgazette.com. Aug 10, 2019.
4. Roy A. “5 ways the government keeps Native Americans in poverty.” Forbes.com. Mar 13, 2014.
5. Blakemore E. “The little-known history of forced sterilization of Native American women.” JSTOR.org. Aug 25, 2016.
6. Bleir G and A Zoledziowski. “Murdered and missing Native American women challenge police and courts.” Publicintegrity.org. Aug 27, 2018.
7. Brockie TN et al. A framework to examine the role of epigenetics in health disparities among Native Americans. Nurs Res Prac. 2013;2013:410395.
8. “Reclaiming power and place: The final report of the national inquiry into missing and murdered Indigenous women and girls.” Vancouver: Privy Office. Jun 3, 2019.
9. Hagan S. “Where U.S. unemployment is still sky-high: Indian reservations.” Bloomberg.com. Apr 5, 2018.
10. Morin B. “Meet 10 Indigenous women who are making the world a better place.” Indian Country Today. Jul 1, 2019.
11. Fact sheet. Discovernavajo.com.
12. Sarche M and P Spicer. Poverty and health disparities for American Indian and Alaska Native children: Current knowledge and future prospects. Ann NY Acad Sci. 2008 Jul 25;1136:126-36.
13. Lewis-Fernández R et al. Culture and psychiatric evaluation: Operationalizing cultural formulation for DSM-5. Psychiatry. 2014 Summer;77(2):130-54.
Dr. Roessel is a Navajo board-certified psychiatrist practicing in Santa Fe, N.M., working with the local Indigenous population. She has special expertise in cultural psychiatry; her childhood was spent growing up in the Navajo nation with her grandfather, who was a Navajo medicine man. Her psychiatric practice focuses on integrating Indigenous knowledge and principles. Dr. Roessel is a distinguished fellow of the American Psychiatric Association.
Dr. Neidhardt is a board-certified psychiatrist who lives in Santa Fe and has an integrative, holistic psychiatric practice that also specializes in trauma-focused therapy. He has provided care for Indigenous People in the Southwest United States and in Canada, and has worked with Navajo medicine people to develop training for mental health professionals with his wife, Dr. Mary Hasbah Roessel. Dr. Reinhardt is a life fellow of the APA.
Commentary: Medical educators must do more to prevent physician suicides
while describing two exemplars at providing physicians with mental health support, in a recently published commentary.
“I want to call attention to the gap between what educators think and say is available, in terms of mental health support, and what trainees experience,” Dr. Poorman said in a statement on her piece in the Journal of Patient Safety and Risk Management. She shared the results of an anonymous depression survey of interns, which showed that 41.8% of participants screened positive for depression. Dr. Poorman also provided statistics on physician suicide, including that at least 66 residents killed themselves between 2000 and 2014, according to ACGME, and that another source estimated that 300-400 physician suicide deaths occur annually.
“When it comes to mental illness and suicide, we are all at risk, but we too often lacked compassion in the way we approach our colleagues. We have lacked the courage to fight the stigma that is killing us. We have not asked whether our unwillingness to reform medical training has eroded the empathy of generations of doctors. We have not done enough to fight medical boards that ask doctors about mental health diagnoses in the same way they ask if we have domestic violence charges,” wrote Dr. Poorman, who practices at a University of Washington neighborhood clinic in Kent.
“A focus on the occupational risks we face would shift us away from ‘wellness’ and ‘resilience,’ and place the onus on schools, training programs, and hospitals to do better for providers and patients,” continued Dr. Poorman, who serves on the editorial advisory board of Internal Medicine News.
She commended Oregon Health and Science University, Portland, and Stanford (Calif.) University’s divisions of general surgery for providing “rigorously confidential mental health support” through a wellness and suicide prevention program for residents and faculty, and a wellness program for residents “that emphasizes relationships, structural support, and psychological safety,” respectively. Dr. Poorman also applauded both for speaking openly about physician suicides.
SOURCE: Poorman E. J Patient Saf Risk Manag. 2019 Aug 5. doi: 10.1177/2516043519866993.
This article was updated 8/5/19.
while describing two exemplars at providing physicians with mental health support, in a recently published commentary.
“I want to call attention to the gap between what educators think and say is available, in terms of mental health support, and what trainees experience,” Dr. Poorman said in a statement on her piece in the Journal of Patient Safety and Risk Management. She shared the results of an anonymous depression survey of interns, which showed that 41.8% of participants screened positive for depression. Dr. Poorman also provided statistics on physician suicide, including that at least 66 residents killed themselves between 2000 and 2014, according to ACGME, and that another source estimated that 300-400 physician suicide deaths occur annually.
“When it comes to mental illness and suicide, we are all at risk, but we too often lacked compassion in the way we approach our colleagues. We have lacked the courage to fight the stigma that is killing us. We have not asked whether our unwillingness to reform medical training has eroded the empathy of generations of doctors. We have not done enough to fight medical boards that ask doctors about mental health diagnoses in the same way they ask if we have domestic violence charges,” wrote Dr. Poorman, who practices at a University of Washington neighborhood clinic in Kent.
“A focus on the occupational risks we face would shift us away from ‘wellness’ and ‘resilience,’ and place the onus on schools, training programs, and hospitals to do better for providers and patients,” continued Dr. Poorman, who serves on the editorial advisory board of Internal Medicine News.
She commended Oregon Health and Science University, Portland, and Stanford (Calif.) University’s divisions of general surgery for providing “rigorously confidential mental health support” through a wellness and suicide prevention program for residents and faculty, and a wellness program for residents “that emphasizes relationships, structural support, and psychological safety,” respectively. Dr. Poorman also applauded both for speaking openly about physician suicides.
SOURCE: Poorman E. J Patient Saf Risk Manag. 2019 Aug 5. doi: 10.1177/2516043519866993.
This article was updated 8/5/19.
while describing two exemplars at providing physicians with mental health support, in a recently published commentary.
“I want to call attention to the gap between what educators think and say is available, in terms of mental health support, and what trainees experience,” Dr. Poorman said in a statement on her piece in the Journal of Patient Safety and Risk Management. She shared the results of an anonymous depression survey of interns, which showed that 41.8% of participants screened positive for depression. Dr. Poorman also provided statistics on physician suicide, including that at least 66 residents killed themselves between 2000 and 2014, according to ACGME, and that another source estimated that 300-400 physician suicide deaths occur annually.
“When it comes to mental illness and suicide, we are all at risk, but we too often lacked compassion in the way we approach our colleagues. We have lacked the courage to fight the stigma that is killing us. We have not asked whether our unwillingness to reform medical training has eroded the empathy of generations of doctors. We have not done enough to fight medical boards that ask doctors about mental health diagnoses in the same way they ask if we have domestic violence charges,” wrote Dr. Poorman, who practices at a University of Washington neighborhood clinic in Kent.
“A focus on the occupational risks we face would shift us away from ‘wellness’ and ‘resilience,’ and place the onus on schools, training programs, and hospitals to do better for providers and patients,” continued Dr. Poorman, who serves on the editorial advisory board of Internal Medicine News.
She commended Oregon Health and Science University, Portland, and Stanford (Calif.) University’s divisions of general surgery for providing “rigorously confidential mental health support” through a wellness and suicide prevention program for residents and faculty, and a wellness program for residents “that emphasizes relationships, structural support, and psychological safety,” respectively. Dr. Poorman also applauded both for speaking openly about physician suicides.
SOURCE: Poorman E. J Patient Saf Risk Manag. 2019 Aug 5. doi: 10.1177/2516043519866993.
This article was updated 8/5/19.
FROM THE JOURNAL OF PATIENT SAFETY AND RISK MANAGEMENT
Technology, counseling, and CBT apps for primary care
There is probably no area where human contact is more important than in the area of counseling and psychotherapy. Or so most of us have thought. It turns out that, even in behavioral medicine, technology has made fantastic inroads in helping patients achieve real improvement in troublesome behavioral symptoms. We will not go over that evidence in this column, other than to say that the evidence is there, but rather we will review some of the best apps that those of us in primary care can utilize in the care of our patients. It is our opinion that these apps are best used in conjunction with our care to supplement the counseling we are giving our patients in the office. Many of the apps listed may be used for both anxiety and depression, as well as in areas related to problem solving, self-esteem, anger management, creating lifestyle changes, and coping with uncertainty.
MoodKit
MoodKit is a CBT app with four main tools: a collection of activities focused on coping self-efficacy (a person’s belief in success in specific situations) that includes individual productivity, social relationships, physical activity, and healthy habits; a thought checker; mood tracker; and journal. MoodKit is accessed in an unstructured way and can be used as an unguided self-help app. It is useful in patient interactions to access interventions in areas such as social engagement and options for choosing a healthy lifestyle. It is available in Apple’s App Store, and it costs $4.99.
Moodnotes
Based on CBT and positive psychology, Moodnotes assists in recognizing and learning about “traps” in thinking, as well as emphasizing healthier thinking habits. Traps in thinking include “catastrophic thinking” where patients with depression may think that a small error or behavioral indiscretion may lead to a consequence that far exceeds what is likely, or “mind-reading” where a person assumes that others are critical of them without actually having evidence that this is the case. Moodnotes tracks mood over a period of time while identifying factors that influence it. It is helpful in between visits to aid clinicians in gaining perspective on mood patterns. It is available in the App Store; it costs $4.99.
MoodMission
This app recommends strategies based in CBT after input of low moods or feelings of anxiety. MoodMission provides five “missions” to engage in that promote confidence in handling stressors and promotes coping self-efficacy. The app learns what style works best and tailors techniques according to when a patient uses it most frequently. Rewards in the app are used to promote motivation and to increase pleasure and self-confidence. It is useful for patients who could use a lift in mood or decrease in symptoms of anxiety and depression. It available in the App Store and Google Play, and it’s free.
What’s Up
In line with its development based on principles from CBT and Acceptance and Commitment Therapy (ACT), What’s Up identifies common negative thinking patterns and methods to overcome them with useful metaphors, a catastrophe scale, grounding techniques, and breathing exercises. What’s Up syncs data across multiple devices and uses a unique passcode to protect this information. One of the abilities that separates it from other apps is that it can become active in forums where people discuss similar feelings and strategies that have been useful for them. It is available in the App Store and Google Play, and it’s free.
Moodpath
Moodpath uses daily screenings to create better understanding of thoughts, feelings, and emotions. If needed, it provides a discussion guide to talking with a medical professional based on answers to its daily screenings. Included in the app are over 150 psychological exercises and videos to promote and strengthen overall mental health. It is useful in introducing how to discuss mental health with a professional. It is available in the App Store and Google Play free of cost.
MindShift CBT
Designed to assist youth and young adults in coping with anxiety, MindShift constructs an individualized toolbox to help individuals deal with test anxiety, perfectionism, social anxiety, worry, panic, and conflict. The app includes directions on how to construct “belief experiments” to test common beliefs that fuel anxiety, guided relaxation, as well as tools and tips to help set and accomplish goals. It is useful in helping teens and young adults learn about helpful and unhelpful anxiety, as well as to overcome fears by gradually facing them in manageable steps. It is available in the App Store and Google Play for free.
CBT-i Coach
CBT-i Coach, based on principles of cognitive behavioral therapy for insomnia (CBT-i), is a structured program to learn about sleep, develop positive sleep routines, and improve sleep environment. The CBT methods used attempt to change behaviors, which in turn provides confidence that patients will sleep better on a regular basis. It useful as a first-line intervention in treating symptoms of insomnia. It is available in the App Store and Google Play for no cost.
Getselfhelp.co.uk
This website provides free self-help and therapy resources grounded in methods that teach the change agents in CBT that can influence negative and destructive thought patterns. Negative thought patterns include thinking in terms of all or nothing: “Nothing ever works out for me,” fortune telling: “I shouldn’t even try,” and overgeneralization: “This didn’t work so this will not either.” Getselfhelp.co.uk provides handouts on a wide array of symptoms related to anxiety, depression, low self-esteem, panic attacks, social disorder, and more. The solution section of the website supplies interventions that can be printed and saved for future use. It is helpful for clinicians and patients in identifying an area of need and creating an action plan. It is also useful for clinicians to have as an augmented supplement for counseling and is free of cost.
The bottom line
When used correctly the resources that we have reviewed can essentially be deployed in a manner similar to how we use finger-stick blood sugar monitoring in the treatment of diabetes. Each of these technologies works best when combined with clinician input and periodic review. When used to supplement clinician counseling, the apps may help sustain motivation and provide insights and exercises that improve patient engagement and supplement the effect of counseling and/or medications that are prescribed in the office.
Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Aaron Sutton is a behavioral health consultant and faculty member in the family medicine residency program at Abington Jefferson Health.
There is probably no area where human contact is more important than in the area of counseling and psychotherapy. Or so most of us have thought. It turns out that, even in behavioral medicine, technology has made fantastic inroads in helping patients achieve real improvement in troublesome behavioral symptoms. We will not go over that evidence in this column, other than to say that the evidence is there, but rather we will review some of the best apps that those of us in primary care can utilize in the care of our patients. It is our opinion that these apps are best used in conjunction with our care to supplement the counseling we are giving our patients in the office. Many of the apps listed may be used for both anxiety and depression, as well as in areas related to problem solving, self-esteem, anger management, creating lifestyle changes, and coping with uncertainty.
MoodKit
MoodKit is a CBT app with four main tools: a collection of activities focused on coping self-efficacy (a person’s belief in success in specific situations) that includes individual productivity, social relationships, physical activity, and healthy habits; a thought checker; mood tracker; and journal. MoodKit is accessed in an unstructured way and can be used as an unguided self-help app. It is useful in patient interactions to access interventions in areas such as social engagement and options for choosing a healthy lifestyle. It is available in Apple’s App Store, and it costs $4.99.
Moodnotes
Based on CBT and positive psychology, Moodnotes assists in recognizing and learning about “traps” in thinking, as well as emphasizing healthier thinking habits. Traps in thinking include “catastrophic thinking” where patients with depression may think that a small error or behavioral indiscretion may lead to a consequence that far exceeds what is likely, or “mind-reading” where a person assumes that others are critical of them without actually having evidence that this is the case. Moodnotes tracks mood over a period of time while identifying factors that influence it. It is helpful in between visits to aid clinicians in gaining perspective on mood patterns. It is available in the App Store; it costs $4.99.
MoodMission
This app recommends strategies based in CBT after input of low moods or feelings of anxiety. MoodMission provides five “missions” to engage in that promote confidence in handling stressors and promotes coping self-efficacy. The app learns what style works best and tailors techniques according to when a patient uses it most frequently. Rewards in the app are used to promote motivation and to increase pleasure and self-confidence. It is useful for patients who could use a lift in mood or decrease in symptoms of anxiety and depression. It available in the App Store and Google Play, and it’s free.
What’s Up
In line with its development based on principles from CBT and Acceptance and Commitment Therapy (ACT), What’s Up identifies common negative thinking patterns and methods to overcome them with useful metaphors, a catastrophe scale, grounding techniques, and breathing exercises. What’s Up syncs data across multiple devices and uses a unique passcode to protect this information. One of the abilities that separates it from other apps is that it can become active in forums where people discuss similar feelings and strategies that have been useful for them. It is available in the App Store and Google Play, and it’s free.
Moodpath
Moodpath uses daily screenings to create better understanding of thoughts, feelings, and emotions. If needed, it provides a discussion guide to talking with a medical professional based on answers to its daily screenings. Included in the app are over 150 psychological exercises and videos to promote and strengthen overall mental health. It is useful in introducing how to discuss mental health with a professional. It is available in the App Store and Google Play free of cost.
MindShift CBT
Designed to assist youth and young adults in coping with anxiety, MindShift constructs an individualized toolbox to help individuals deal with test anxiety, perfectionism, social anxiety, worry, panic, and conflict. The app includes directions on how to construct “belief experiments” to test common beliefs that fuel anxiety, guided relaxation, as well as tools and tips to help set and accomplish goals. It is useful in helping teens and young adults learn about helpful and unhelpful anxiety, as well as to overcome fears by gradually facing them in manageable steps. It is available in the App Store and Google Play for free.
CBT-i Coach
CBT-i Coach, based on principles of cognitive behavioral therapy for insomnia (CBT-i), is a structured program to learn about sleep, develop positive sleep routines, and improve sleep environment. The CBT methods used attempt to change behaviors, which in turn provides confidence that patients will sleep better on a regular basis. It useful as a first-line intervention in treating symptoms of insomnia. It is available in the App Store and Google Play for no cost.
Getselfhelp.co.uk
This website provides free self-help and therapy resources grounded in methods that teach the change agents in CBT that can influence negative and destructive thought patterns. Negative thought patterns include thinking in terms of all or nothing: “Nothing ever works out for me,” fortune telling: “I shouldn’t even try,” and overgeneralization: “This didn’t work so this will not either.” Getselfhelp.co.uk provides handouts on a wide array of symptoms related to anxiety, depression, low self-esteem, panic attacks, social disorder, and more. The solution section of the website supplies interventions that can be printed and saved for future use. It is helpful for clinicians and patients in identifying an area of need and creating an action plan. It is also useful for clinicians to have as an augmented supplement for counseling and is free of cost.
The bottom line
When used correctly the resources that we have reviewed can essentially be deployed in a manner similar to how we use finger-stick blood sugar monitoring in the treatment of diabetes. Each of these technologies works best when combined with clinician input and periodic review. When used to supplement clinician counseling, the apps may help sustain motivation and provide insights and exercises that improve patient engagement and supplement the effect of counseling and/or medications that are prescribed in the office.
Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Aaron Sutton is a behavioral health consultant and faculty member in the family medicine residency program at Abington Jefferson Health.
There is probably no area where human contact is more important than in the area of counseling and psychotherapy. Or so most of us have thought. It turns out that, even in behavioral medicine, technology has made fantastic inroads in helping patients achieve real improvement in troublesome behavioral symptoms. We will not go over that evidence in this column, other than to say that the evidence is there, but rather we will review some of the best apps that those of us in primary care can utilize in the care of our patients. It is our opinion that these apps are best used in conjunction with our care to supplement the counseling we are giving our patients in the office. Many of the apps listed may be used for both anxiety and depression, as well as in areas related to problem solving, self-esteem, anger management, creating lifestyle changes, and coping with uncertainty.
MoodKit
MoodKit is a CBT app with four main tools: a collection of activities focused on coping self-efficacy (a person’s belief in success in specific situations) that includes individual productivity, social relationships, physical activity, and healthy habits; a thought checker; mood tracker; and journal. MoodKit is accessed in an unstructured way and can be used as an unguided self-help app. It is useful in patient interactions to access interventions in areas such as social engagement and options for choosing a healthy lifestyle. It is available in Apple’s App Store, and it costs $4.99.
Moodnotes
Based on CBT and positive psychology, Moodnotes assists in recognizing and learning about “traps” in thinking, as well as emphasizing healthier thinking habits. Traps in thinking include “catastrophic thinking” where patients with depression may think that a small error or behavioral indiscretion may lead to a consequence that far exceeds what is likely, or “mind-reading” where a person assumes that others are critical of them without actually having evidence that this is the case. Moodnotes tracks mood over a period of time while identifying factors that influence it. It is helpful in between visits to aid clinicians in gaining perspective on mood patterns. It is available in the App Store; it costs $4.99.
MoodMission
This app recommends strategies based in CBT after input of low moods or feelings of anxiety. MoodMission provides five “missions” to engage in that promote confidence in handling stressors and promotes coping self-efficacy. The app learns what style works best and tailors techniques according to when a patient uses it most frequently. Rewards in the app are used to promote motivation and to increase pleasure and self-confidence. It is useful for patients who could use a lift in mood or decrease in symptoms of anxiety and depression. It available in the App Store and Google Play, and it’s free.
What’s Up
In line with its development based on principles from CBT and Acceptance and Commitment Therapy (ACT), What’s Up identifies common negative thinking patterns and methods to overcome them with useful metaphors, a catastrophe scale, grounding techniques, and breathing exercises. What’s Up syncs data across multiple devices and uses a unique passcode to protect this information. One of the abilities that separates it from other apps is that it can become active in forums where people discuss similar feelings and strategies that have been useful for them. It is available in the App Store and Google Play, and it’s free.
Moodpath
Moodpath uses daily screenings to create better understanding of thoughts, feelings, and emotions. If needed, it provides a discussion guide to talking with a medical professional based on answers to its daily screenings. Included in the app are over 150 psychological exercises and videos to promote and strengthen overall mental health. It is useful in introducing how to discuss mental health with a professional. It is available in the App Store and Google Play free of cost.
MindShift CBT
Designed to assist youth and young adults in coping with anxiety, MindShift constructs an individualized toolbox to help individuals deal with test anxiety, perfectionism, social anxiety, worry, panic, and conflict. The app includes directions on how to construct “belief experiments” to test common beliefs that fuel anxiety, guided relaxation, as well as tools and tips to help set and accomplish goals. It is useful in helping teens and young adults learn about helpful and unhelpful anxiety, as well as to overcome fears by gradually facing them in manageable steps. It is available in the App Store and Google Play for free.
CBT-i Coach
CBT-i Coach, based on principles of cognitive behavioral therapy for insomnia (CBT-i), is a structured program to learn about sleep, develop positive sleep routines, and improve sleep environment. The CBT methods used attempt to change behaviors, which in turn provides confidence that patients will sleep better on a regular basis. It useful as a first-line intervention in treating symptoms of insomnia. It is available in the App Store and Google Play for no cost.
Getselfhelp.co.uk
This website provides free self-help and therapy resources grounded in methods that teach the change agents in CBT that can influence negative and destructive thought patterns. Negative thought patterns include thinking in terms of all or nothing: “Nothing ever works out for me,” fortune telling: “I shouldn’t even try,” and overgeneralization: “This didn’t work so this will not either.” Getselfhelp.co.uk provides handouts on a wide array of symptoms related to anxiety, depression, low self-esteem, panic attacks, social disorder, and more. The solution section of the website supplies interventions that can be printed and saved for future use. It is helpful for clinicians and patients in identifying an area of need and creating an action plan. It is also useful for clinicians to have as an augmented supplement for counseling and is free of cost.
The bottom line
When used correctly the resources that we have reviewed can essentially be deployed in a manner similar to how we use finger-stick blood sugar monitoring in the treatment of diabetes. Each of these technologies works best when combined with clinician input and periodic review. When used to supplement clinician counseling, the apps may help sustain motivation and provide insights and exercises that improve patient engagement and supplement the effect of counseling and/or medications that are prescribed in the office.
Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Aaron Sutton is a behavioral health consultant and faculty member in the family medicine residency program at Abington Jefferson Health.
Brain imaging could predict response to CBT in depression
Researchers have used functional MRI to identify key differences between individuals with depression who respond to cognitive-behavioral therapy and those who don’t.
In a paper published in Science Advances, Filippo Queirazza, MD, PhD, and coauthors reported the outcomes of a study of 37 individuals with untreated depression who took part in an online, self-guided cognitive-behavioral therapy (CBT) program.
The participants – 18 of whom were women – attended an appointment before and 2 months after completing the therapy program, at which they were clinically evaluated by a psychiatrist, and underwent fMRI scanning. Only 26 subjects completed the program and attended the posttreatment appointment, wrote Dr. Queirazza of the Institute of Neuroscience and Psychology at the University of Glasgow, Scotland.
To look for indication of treatment response in fMRI scanning, researchers got participants to do a series of reverse-learning tasks while they were being scanned. That activity involved learning which of two stimuli gave the highest payoff rate.
“The choice of a relevant generative model in MDD is dictated by a wealth of behavioral and neural findings, suggesting that learning from positive (reward) and negative (punishment) feedback [also known as reinforcement learning] is substantially impaired in depressed subjects,” the authors wrote.
The imaging suggested that individuals who responded showed more pretreatment neural activity in areas of the brain that deal with the acquisition and processing of feedback information, and that the level of activity was in proportion to the magnitude of their later treatment response.
They also found that activity in right striatum was best at discriminating responders from nonresponders. Based on their findings, they were able to predict responders as well as did pretreatment BDI-II scores.
“Our finding that neural activity in the right striatum is positively correlated with CBT response is consistent with a previous report that, in a group of adolescents with depression, greater pretreatment striatal responses to both anticipation and presentation of positive feedback during a monetary reward task were linked to posttreatment reduction in depression severity, particularly of anxiety symptoms,” they wrote.
The study was supported by several entities, including the Chief Scientist Office and the Dr Mortimer and Theresa Sackler Foundation. No competing interests were declared.
SOURCE: Queirazza F et al. Sci Adv. 2019 Jul 31. doi: 10.1126/sciadv.aav4962.
Researchers have used functional MRI to identify key differences between individuals with depression who respond to cognitive-behavioral therapy and those who don’t.
In a paper published in Science Advances, Filippo Queirazza, MD, PhD, and coauthors reported the outcomes of a study of 37 individuals with untreated depression who took part in an online, self-guided cognitive-behavioral therapy (CBT) program.
The participants – 18 of whom were women – attended an appointment before and 2 months after completing the therapy program, at which they were clinically evaluated by a psychiatrist, and underwent fMRI scanning. Only 26 subjects completed the program and attended the posttreatment appointment, wrote Dr. Queirazza of the Institute of Neuroscience and Psychology at the University of Glasgow, Scotland.
To look for indication of treatment response in fMRI scanning, researchers got participants to do a series of reverse-learning tasks while they were being scanned. That activity involved learning which of two stimuli gave the highest payoff rate.
“The choice of a relevant generative model in MDD is dictated by a wealth of behavioral and neural findings, suggesting that learning from positive (reward) and negative (punishment) feedback [also known as reinforcement learning] is substantially impaired in depressed subjects,” the authors wrote.
The imaging suggested that individuals who responded showed more pretreatment neural activity in areas of the brain that deal with the acquisition and processing of feedback information, and that the level of activity was in proportion to the magnitude of their later treatment response.
They also found that activity in right striatum was best at discriminating responders from nonresponders. Based on their findings, they were able to predict responders as well as did pretreatment BDI-II scores.
“Our finding that neural activity in the right striatum is positively correlated with CBT response is consistent with a previous report that, in a group of adolescents with depression, greater pretreatment striatal responses to both anticipation and presentation of positive feedback during a monetary reward task were linked to posttreatment reduction in depression severity, particularly of anxiety symptoms,” they wrote.
The study was supported by several entities, including the Chief Scientist Office and the Dr Mortimer and Theresa Sackler Foundation. No competing interests were declared.
SOURCE: Queirazza F et al. Sci Adv. 2019 Jul 31. doi: 10.1126/sciadv.aav4962.
Researchers have used functional MRI to identify key differences between individuals with depression who respond to cognitive-behavioral therapy and those who don’t.
In a paper published in Science Advances, Filippo Queirazza, MD, PhD, and coauthors reported the outcomes of a study of 37 individuals with untreated depression who took part in an online, self-guided cognitive-behavioral therapy (CBT) program.
The participants – 18 of whom were women – attended an appointment before and 2 months after completing the therapy program, at which they were clinically evaluated by a psychiatrist, and underwent fMRI scanning. Only 26 subjects completed the program and attended the posttreatment appointment, wrote Dr. Queirazza of the Institute of Neuroscience and Psychology at the University of Glasgow, Scotland.
To look for indication of treatment response in fMRI scanning, researchers got participants to do a series of reverse-learning tasks while they were being scanned. That activity involved learning which of two stimuli gave the highest payoff rate.
“The choice of a relevant generative model in MDD is dictated by a wealth of behavioral and neural findings, suggesting that learning from positive (reward) and negative (punishment) feedback [also known as reinforcement learning] is substantially impaired in depressed subjects,” the authors wrote.
The imaging suggested that individuals who responded showed more pretreatment neural activity in areas of the brain that deal with the acquisition and processing of feedback information, and that the level of activity was in proportion to the magnitude of their later treatment response.
They also found that activity in right striatum was best at discriminating responders from nonresponders. Based on their findings, they were able to predict responders as well as did pretreatment BDI-II scores.
“Our finding that neural activity in the right striatum is positively correlated with CBT response is consistent with a previous report that, in a group of adolescents with depression, greater pretreatment striatal responses to both anticipation and presentation of positive feedback during a monetary reward task were linked to posttreatment reduction in depression severity, particularly of anxiety symptoms,” they wrote.
The study was supported by several entities, including the Chief Scientist Office and the Dr Mortimer and Theresa Sackler Foundation. No competing interests were declared.
SOURCE: Queirazza F et al. Sci Adv. 2019 Jul 31. doi: 10.1126/sciadv.aav4962.
FROM SCIENCE ADVANCES
Psychiatrists face challenges in creating Spravato practices
At Actify Neurotherapies, a longtime provider of ketamine infusion therapy, Steven P. Levine, MD, and staff are busy making preparations for a new offering: intranasal esketamine.
The nasal spray, called Spravato and developed by Janssen Pharmaceuticals, was approved by the Food and Drug Administration in March 2019 for treatment-resistant depression in adults who have failed at least two oral antidepressants of different classes.
“We’ve been anticipating this for years,” said Dr. Levine, founder and chief medical officer for Actify Neurotherapies, formerly Ketamine Treatment Centers.“Being used to giving IV ketamine every day, this really fits right into our daily practice. For us, [it means] adding a few staff members here and there to make sure we have adequate monitoring, [and] setting up recovery spaces so that patients have a nice place to spend time.”
Dr. Levine is far from alone. After the recent intranasal esketamine approval, many psychiatrists are considering how they might integrate the medication into their practice. The FDA is managing the drug under a Risk Evaluation and Mitigation Strategy (REMS) program, which requires prescriber training on esketamine risks and patient monitoring. Facilities must be licensed to dispense esketamine and are required to monitor patients in person for at least 2 hours after administration.
The rigid requirements are important for safety, but they mean that a limited number of psychiatrists will likely have the capacity to deliver the medication, said Gerard Sanacora, MD, PhD, director of the Yale Depression Research Program in New Haven, Conn., and a principal investigator for several clinical trials associated with the esketamine nasal spray. Psychiatrists interested in providing the medication have much to consider in the way of logistics, structure, and staffing.
“The requirements do [present] hurdles,” Dr. Sanacora said. “This is a medication that requires a fair amount of infrastructure. This is not going to be a treatment that is going to be given by every corner psychiatrist.”
In addition to delivery obstacles, questions remain about the ideal Spravato regimen, particularly the optimal dose frequency during maintenance and the best way to track long-term outcomes. Some have also raised concerns about the potential for esketamine/ketamine abuse following the FDA approval.
Despite the questions, one thing most psychiatrists agree on is the need for intranasal esketamine and its promising impact.
“There’s no secret that at this point: We really haven’t put a dent in the rates of depression and the rates of suicide over the past several decades,” Dr. Levine said. “We’ve needed something that’s truly new, something that’s innovative. It makes the FDA approval of intranasal esketamine a really big deal. It’s a new mechanism, a new approach to depression, and provides hope to patients and the field of psychiatry.”
Addressing delivery challenges
After the FDA approval, Jacqueline Posada, MD, was tasked with helping a small psychiatry practice develop the framework to incorporate intranasal esketamine. Dr. Posada, a consultation-liaison psychiatry fellow at the Inova Fairfax Hospital-George Washington University program in Falls Church, Va., started by creating a comprehensive screening form for esketamine candidates, developing an informed-consent protocol, and researching storage guidelines for an outpatient practice.
Before the practice could move forward, however, Dr. Posada learned that building regulations for the office prohibited “medical procedures” from occurring in the space. While the 10-doctor practice is associated with George Washington University, Washington, it is located several blocks away in an office setting.
“It was pretty disappointing,” Dr. Posada said. “Our organization is looking for another spot that allows psychiatry to administer a medical procedure. There’s a lot of people who are in private practice who are in a type of office building that may restrict medical procedures. Depending on the type of space they are renting, they need to know what is allowed in their office building.”
Patient privacy is also a primary consideration when delivering the medication. Dr. Posada said she and her staff had contemplated conducting the 2-hour patient monitoring in a group setting, but that idea was quickly thwarted.
“Our plan was in 15- to 20-minute appointments, administer the esketamine, and have maybe 5-10 people monitored in a room with barriers between each chair,” she said. “We were advised by our privacy officer that that could hinder patient confidentiality and was not a viable option for administering the medication in our practice.”
Practices should also determine a long-term treatment plan for each patient receiving esketamine, Dr. Sanacora said, particularly in light of data suggesting that a substantial number of patients will relapse if treatment is stopped. A long-term treatment plan should be considered prior to, or at the same time that esketamine treatment is initiated.
Comanagement of patients by clinicians is another essential part of esketamine treatment, Dr. Sanacora added.
“It is going to be important for the community clinicians not directly offering the treatment to develop close and ongoing relationships with the clinicians and sites that are offering this treatment to optimize the care of their patients,” he said. “Conversely, it is going to be imperative that the esketamine providers develop effective and efficient methods of maintaining close communications with the referring clinicians.”
Reimbursement comes with upfront cost
As far as payment, Dr. Levine expects that the nasal spray will be widely reimbursed. Clinics must first purchase the medication and insurers will reimburse them later for the drug, a method commonly referred to as “buy and bill.” The upfront cost could be too expensive for some physicians.
“That’s not something psychiatrists are used to, storing a controlled substance on site, having the financial outlay of keeping inventory of an expensive drug, and waiting for reimbursement,” Dr. Levine said.
Practices can also bill an evaluation and management code for the treatment as well as for observation time. At this point, it appears insurers will not cover psychotherapy delivered at the same time, which is disappointing, he said.
Sanjay J. Mathew, MD, a psychiatry professor and vice chair for research at Baylor College of Medicine, Houston, said because many psychiatrists who specialize in psychopharmacology and who see treatment-resistant depression patients do not accept private insurance or Medicare/Medicaid, patient access to the drug may be limited.
“Given the REMS and the need for 2-hour monitoring following the treatment, I believe that primarily, specialized clinics and centers for ‘interventional psychiatry’ will be the early adopters,” he said. “The typical office-based psychiatrist who does not accept insurance, and does not have means to store and dispense a controlled substance or monitor patients, may not readily adopt this new approach.”
Experts say abuse potential low
Meanwhile, the potential for abuse with intranasal esketamine is low, considering the REMS requirements, said Mark S. Gold, MD, an addiction medicine specialist. If practices follow the guidelines, which restrict patients from taking the medication home, misuse is not likely.
However, the drug’s counterpart, ketamine, which has been abused globally for decades, may see an uptick in illicit use after the recent FDA approval of esketamine, Dr. Gold said. Ketamine is currently produced in clandestine labs for use on the club scene and for date rape purposes, Dr. Gold said. Most recently, ketamine has been mixed with cocaine and referred to as “Calvin Klein” for street use.
“You do have the unintended consequence when a medication is approved, [that] the general public may not be sophisticated enough to understand dose, duration, and supervised administration, and think that street ketamine is equally a medication,” Dr. Gold said. “You have the capacity for black market ketamine to undercut the price and be distributed in a form that can be extremely dangerous and life threatening. Just calling it ketamine provides confusion and the opportunity for black market sales distribution and increases in use.”
Dr. Mathew hopes the REMS designation will not only aid in risk mitigation for esketamine, but also result in valuable postapproval information with respect to longer term safety and efficacy. Questions that still need to be addressed include the best antidepressant regimens in conjunction with intranasal esketamine, the ideal long-term monitoring method, and the potential impact of benzodiazepines on response to Spravato.
“These answers will come with more research – phase 4 postmarketing studies – and also clinical experience in the community, as clinicians begin to prescribe esketamine,” Dr. Mathew said. “Input from organizations, such as the American Society of Ketamine Physicians, will be important as well.”
Dr. Levine, Dr. Posada, and Dr. Gold reported no disclosures. Dr. Sanacora served as principal investigator for several clinical trials associated with intranasal esketamine. Dr. Mathew served as a consultant to several pharmaceutical companies, including Janssen.
At Actify Neurotherapies, a longtime provider of ketamine infusion therapy, Steven P. Levine, MD, and staff are busy making preparations for a new offering: intranasal esketamine.
The nasal spray, called Spravato and developed by Janssen Pharmaceuticals, was approved by the Food and Drug Administration in March 2019 for treatment-resistant depression in adults who have failed at least two oral antidepressants of different classes.
“We’ve been anticipating this for years,” said Dr. Levine, founder and chief medical officer for Actify Neurotherapies, formerly Ketamine Treatment Centers.“Being used to giving IV ketamine every day, this really fits right into our daily practice. For us, [it means] adding a few staff members here and there to make sure we have adequate monitoring, [and] setting up recovery spaces so that patients have a nice place to spend time.”
Dr. Levine is far from alone. After the recent intranasal esketamine approval, many psychiatrists are considering how they might integrate the medication into their practice. The FDA is managing the drug under a Risk Evaluation and Mitigation Strategy (REMS) program, which requires prescriber training on esketamine risks and patient monitoring. Facilities must be licensed to dispense esketamine and are required to monitor patients in person for at least 2 hours after administration.
The rigid requirements are important for safety, but they mean that a limited number of psychiatrists will likely have the capacity to deliver the medication, said Gerard Sanacora, MD, PhD, director of the Yale Depression Research Program in New Haven, Conn., and a principal investigator for several clinical trials associated with the esketamine nasal spray. Psychiatrists interested in providing the medication have much to consider in the way of logistics, structure, and staffing.
“The requirements do [present] hurdles,” Dr. Sanacora said. “This is a medication that requires a fair amount of infrastructure. This is not going to be a treatment that is going to be given by every corner psychiatrist.”
In addition to delivery obstacles, questions remain about the ideal Spravato regimen, particularly the optimal dose frequency during maintenance and the best way to track long-term outcomes. Some have also raised concerns about the potential for esketamine/ketamine abuse following the FDA approval.
Despite the questions, one thing most psychiatrists agree on is the need for intranasal esketamine and its promising impact.
“There’s no secret that at this point: We really haven’t put a dent in the rates of depression and the rates of suicide over the past several decades,” Dr. Levine said. “We’ve needed something that’s truly new, something that’s innovative. It makes the FDA approval of intranasal esketamine a really big deal. It’s a new mechanism, a new approach to depression, and provides hope to patients and the field of psychiatry.”
Addressing delivery challenges
After the FDA approval, Jacqueline Posada, MD, was tasked with helping a small psychiatry practice develop the framework to incorporate intranasal esketamine. Dr. Posada, a consultation-liaison psychiatry fellow at the Inova Fairfax Hospital-George Washington University program in Falls Church, Va., started by creating a comprehensive screening form for esketamine candidates, developing an informed-consent protocol, and researching storage guidelines for an outpatient practice.
Before the practice could move forward, however, Dr. Posada learned that building regulations for the office prohibited “medical procedures” from occurring in the space. While the 10-doctor practice is associated with George Washington University, Washington, it is located several blocks away in an office setting.
“It was pretty disappointing,” Dr. Posada said. “Our organization is looking for another spot that allows psychiatry to administer a medical procedure. There’s a lot of people who are in private practice who are in a type of office building that may restrict medical procedures. Depending on the type of space they are renting, they need to know what is allowed in their office building.”
Patient privacy is also a primary consideration when delivering the medication. Dr. Posada said she and her staff had contemplated conducting the 2-hour patient monitoring in a group setting, but that idea was quickly thwarted.
“Our plan was in 15- to 20-minute appointments, administer the esketamine, and have maybe 5-10 people monitored in a room with barriers between each chair,” she said. “We were advised by our privacy officer that that could hinder patient confidentiality and was not a viable option for administering the medication in our practice.”
Practices should also determine a long-term treatment plan for each patient receiving esketamine, Dr. Sanacora said, particularly in light of data suggesting that a substantial number of patients will relapse if treatment is stopped. A long-term treatment plan should be considered prior to, or at the same time that esketamine treatment is initiated.
Comanagement of patients by clinicians is another essential part of esketamine treatment, Dr. Sanacora added.
“It is going to be important for the community clinicians not directly offering the treatment to develop close and ongoing relationships with the clinicians and sites that are offering this treatment to optimize the care of their patients,” he said. “Conversely, it is going to be imperative that the esketamine providers develop effective and efficient methods of maintaining close communications with the referring clinicians.”
Reimbursement comes with upfront cost
As far as payment, Dr. Levine expects that the nasal spray will be widely reimbursed. Clinics must first purchase the medication and insurers will reimburse them later for the drug, a method commonly referred to as “buy and bill.” The upfront cost could be too expensive for some physicians.
“That’s not something psychiatrists are used to, storing a controlled substance on site, having the financial outlay of keeping inventory of an expensive drug, and waiting for reimbursement,” Dr. Levine said.
Practices can also bill an evaluation and management code for the treatment as well as for observation time. At this point, it appears insurers will not cover psychotherapy delivered at the same time, which is disappointing, he said.
Sanjay J. Mathew, MD, a psychiatry professor and vice chair for research at Baylor College of Medicine, Houston, said because many psychiatrists who specialize in psychopharmacology and who see treatment-resistant depression patients do not accept private insurance or Medicare/Medicaid, patient access to the drug may be limited.
“Given the REMS and the need for 2-hour monitoring following the treatment, I believe that primarily, specialized clinics and centers for ‘interventional psychiatry’ will be the early adopters,” he said. “The typical office-based psychiatrist who does not accept insurance, and does not have means to store and dispense a controlled substance or monitor patients, may not readily adopt this new approach.”
Experts say abuse potential low
Meanwhile, the potential for abuse with intranasal esketamine is low, considering the REMS requirements, said Mark S. Gold, MD, an addiction medicine specialist. If practices follow the guidelines, which restrict patients from taking the medication home, misuse is not likely.
However, the drug’s counterpart, ketamine, which has been abused globally for decades, may see an uptick in illicit use after the recent FDA approval of esketamine, Dr. Gold said. Ketamine is currently produced in clandestine labs for use on the club scene and for date rape purposes, Dr. Gold said. Most recently, ketamine has been mixed with cocaine and referred to as “Calvin Klein” for street use.
“You do have the unintended consequence when a medication is approved, [that] the general public may not be sophisticated enough to understand dose, duration, and supervised administration, and think that street ketamine is equally a medication,” Dr. Gold said. “You have the capacity for black market ketamine to undercut the price and be distributed in a form that can be extremely dangerous and life threatening. Just calling it ketamine provides confusion and the opportunity for black market sales distribution and increases in use.”
Dr. Mathew hopes the REMS designation will not only aid in risk mitigation for esketamine, but also result in valuable postapproval information with respect to longer term safety and efficacy. Questions that still need to be addressed include the best antidepressant regimens in conjunction with intranasal esketamine, the ideal long-term monitoring method, and the potential impact of benzodiazepines on response to Spravato.
“These answers will come with more research – phase 4 postmarketing studies – and also clinical experience in the community, as clinicians begin to prescribe esketamine,” Dr. Mathew said. “Input from organizations, such as the American Society of Ketamine Physicians, will be important as well.”
Dr. Levine, Dr. Posada, and Dr. Gold reported no disclosures. Dr. Sanacora served as principal investigator for several clinical trials associated with intranasal esketamine. Dr. Mathew served as a consultant to several pharmaceutical companies, including Janssen.
At Actify Neurotherapies, a longtime provider of ketamine infusion therapy, Steven P. Levine, MD, and staff are busy making preparations for a new offering: intranasal esketamine.
The nasal spray, called Spravato and developed by Janssen Pharmaceuticals, was approved by the Food and Drug Administration in March 2019 for treatment-resistant depression in adults who have failed at least two oral antidepressants of different classes.
“We’ve been anticipating this for years,” said Dr. Levine, founder and chief medical officer for Actify Neurotherapies, formerly Ketamine Treatment Centers.“Being used to giving IV ketamine every day, this really fits right into our daily practice. For us, [it means] adding a few staff members here and there to make sure we have adequate monitoring, [and] setting up recovery spaces so that patients have a nice place to spend time.”
Dr. Levine is far from alone. After the recent intranasal esketamine approval, many psychiatrists are considering how they might integrate the medication into their practice. The FDA is managing the drug under a Risk Evaluation and Mitigation Strategy (REMS) program, which requires prescriber training on esketamine risks and patient monitoring. Facilities must be licensed to dispense esketamine and are required to monitor patients in person for at least 2 hours after administration.
The rigid requirements are important for safety, but they mean that a limited number of psychiatrists will likely have the capacity to deliver the medication, said Gerard Sanacora, MD, PhD, director of the Yale Depression Research Program in New Haven, Conn., and a principal investigator for several clinical trials associated with the esketamine nasal spray. Psychiatrists interested in providing the medication have much to consider in the way of logistics, structure, and staffing.
“The requirements do [present] hurdles,” Dr. Sanacora said. “This is a medication that requires a fair amount of infrastructure. This is not going to be a treatment that is going to be given by every corner psychiatrist.”
In addition to delivery obstacles, questions remain about the ideal Spravato regimen, particularly the optimal dose frequency during maintenance and the best way to track long-term outcomes. Some have also raised concerns about the potential for esketamine/ketamine abuse following the FDA approval.
Despite the questions, one thing most psychiatrists agree on is the need for intranasal esketamine and its promising impact.
“There’s no secret that at this point: We really haven’t put a dent in the rates of depression and the rates of suicide over the past several decades,” Dr. Levine said. “We’ve needed something that’s truly new, something that’s innovative. It makes the FDA approval of intranasal esketamine a really big deal. It’s a new mechanism, a new approach to depression, and provides hope to patients and the field of psychiatry.”
Addressing delivery challenges
After the FDA approval, Jacqueline Posada, MD, was tasked with helping a small psychiatry practice develop the framework to incorporate intranasal esketamine. Dr. Posada, a consultation-liaison psychiatry fellow at the Inova Fairfax Hospital-George Washington University program in Falls Church, Va., started by creating a comprehensive screening form for esketamine candidates, developing an informed-consent protocol, and researching storage guidelines for an outpatient practice.
Before the practice could move forward, however, Dr. Posada learned that building regulations for the office prohibited “medical procedures” from occurring in the space. While the 10-doctor practice is associated with George Washington University, Washington, it is located several blocks away in an office setting.
“It was pretty disappointing,” Dr. Posada said. “Our organization is looking for another spot that allows psychiatry to administer a medical procedure. There’s a lot of people who are in private practice who are in a type of office building that may restrict medical procedures. Depending on the type of space they are renting, they need to know what is allowed in their office building.”
Patient privacy is also a primary consideration when delivering the medication. Dr. Posada said she and her staff had contemplated conducting the 2-hour patient monitoring in a group setting, but that idea was quickly thwarted.
“Our plan was in 15- to 20-minute appointments, administer the esketamine, and have maybe 5-10 people monitored in a room with barriers between each chair,” she said. “We were advised by our privacy officer that that could hinder patient confidentiality and was not a viable option for administering the medication in our practice.”
Practices should also determine a long-term treatment plan for each patient receiving esketamine, Dr. Sanacora said, particularly in light of data suggesting that a substantial number of patients will relapse if treatment is stopped. A long-term treatment plan should be considered prior to, or at the same time that esketamine treatment is initiated.
Comanagement of patients by clinicians is another essential part of esketamine treatment, Dr. Sanacora added.
“It is going to be important for the community clinicians not directly offering the treatment to develop close and ongoing relationships with the clinicians and sites that are offering this treatment to optimize the care of their patients,” he said. “Conversely, it is going to be imperative that the esketamine providers develop effective and efficient methods of maintaining close communications with the referring clinicians.”
Reimbursement comes with upfront cost
As far as payment, Dr. Levine expects that the nasal spray will be widely reimbursed. Clinics must first purchase the medication and insurers will reimburse them later for the drug, a method commonly referred to as “buy and bill.” The upfront cost could be too expensive for some physicians.
“That’s not something psychiatrists are used to, storing a controlled substance on site, having the financial outlay of keeping inventory of an expensive drug, and waiting for reimbursement,” Dr. Levine said.
Practices can also bill an evaluation and management code for the treatment as well as for observation time. At this point, it appears insurers will not cover psychotherapy delivered at the same time, which is disappointing, he said.
Sanjay J. Mathew, MD, a psychiatry professor and vice chair for research at Baylor College of Medicine, Houston, said because many psychiatrists who specialize in psychopharmacology and who see treatment-resistant depression patients do not accept private insurance or Medicare/Medicaid, patient access to the drug may be limited.
“Given the REMS and the need for 2-hour monitoring following the treatment, I believe that primarily, specialized clinics and centers for ‘interventional psychiatry’ will be the early adopters,” he said. “The typical office-based psychiatrist who does not accept insurance, and does not have means to store and dispense a controlled substance or monitor patients, may not readily adopt this new approach.”
Experts say abuse potential low
Meanwhile, the potential for abuse with intranasal esketamine is low, considering the REMS requirements, said Mark S. Gold, MD, an addiction medicine specialist. If practices follow the guidelines, which restrict patients from taking the medication home, misuse is not likely.
However, the drug’s counterpart, ketamine, which has been abused globally for decades, may see an uptick in illicit use after the recent FDA approval of esketamine, Dr. Gold said. Ketamine is currently produced in clandestine labs for use on the club scene and for date rape purposes, Dr. Gold said. Most recently, ketamine has been mixed with cocaine and referred to as “Calvin Klein” for street use.
“You do have the unintended consequence when a medication is approved, [that] the general public may not be sophisticated enough to understand dose, duration, and supervised administration, and think that street ketamine is equally a medication,” Dr. Gold said. “You have the capacity for black market ketamine to undercut the price and be distributed in a form that can be extremely dangerous and life threatening. Just calling it ketamine provides confusion and the opportunity for black market sales distribution and increases in use.”
Dr. Mathew hopes the REMS designation will not only aid in risk mitigation for esketamine, but also result in valuable postapproval information with respect to longer term safety and efficacy. Questions that still need to be addressed include the best antidepressant regimens in conjunction with intranasal esketamine, the ideal long-term monitoring method, and the potential impact of benzodiazepines on response to Spravato.
“These answers will come with more research – phase 4 postmarketing studies – and also clinical experience in the community, as clinicians begin to prescribe esketamine,” Dr. Mathew said. “Input from organizations, such as the American Society of Ketamine Physicians, will be important as well.”
Dr. Levine, Dr. Posada, and Dr. Gold reported no disclosures. Dr. Sanacora served as principal investigator for several clinical trials associated with intranasal esketamine. Dr. Mathew served as a consultant to several pharmaceutical companies, including Janssen.
First-time fathers at risk of postnatal depressive symptoms
First-time fathers may be at risk of experiencing depressive symptoms as they transition to parenthood – especially if risk factors such as poor sleep are present, results of a prospective study of more than 600 new fathers show.
“Strategies to promote better sleep, mobilize social support, and strengthen the couple relationship may be important to address in innovative interventions tailored to new fathers at risk for depression during the perinatal period,” wrote Deborah Da Costa, PhD, of McGill University, Montreal, and colleagues. The study was published in the Journal of Affective Disorders.
To determine the prevalence of depressive symptoms in first-time fathers and identify notable risk factors, the researchers surveyed 622 Canadian men during their partner’s third trimester. The same group was surveyed again at 2 and 6 months postpartum. Depression was assessed via the Edinburgh Postnatal Depression Scale (EPDS), and additional variables such as sleep quality, social support, and stress were gathered as well.
Of the initial 622 men surveyed, 487 (78.3%) and 375 (60.3%) completed the questionnaires at 2 and 6 months postpartum, respectively. The prevalence of paternal depressive symptoms was 13.76% (95% confidence interval, 10.70-16.82) at 2 months and 13.6% (95% CI, 10.13-17.07) at 6 months. Of the men who reported depressive symptoms at 2 months postpartum, 40.3% also experienced symptoms during the third trimester. Of the men who reported depressive symptoms at 6 months postpartum, 24% experienced symptoms during the third trimester and after 2 months.
At 2 months, the risk of depressive symptoms increased for men with worse sleep quality (odds ratio, 1.25; 95% CI, 1.10-1.42), poorer couple relationship adjustment (OR, 0.97; 95% CI, 0.94-0.99), and higher parenting stress (OR, 1.07; 95% CI, 1.02-1.11). At 6 months, there was a significant association between paternal depressive symptoms and unemployment (OR, 3.75; 95% CI, 1.00-13.72), poorer sleep quality (OR, 1.37; 95% CI, 1.16-1.65), lower social support (OR, 0.92; 95% CI, 0.84-1.00), poorer couple relationship adjustment (OR, 0.95; 95% CI, 0.92-0.98), and higher financial stress (OR, 1.21; 95% CI, 1.04-1.42).
The authors acknowledged their study’s limitations, including a middling response rate that could affect the accuracy of prevalence estimates and a well-educated, largely middle-class sample that could limit generalizability. In addition, they assessed depressive symptoms by self-report and not diagnostic clinical interviews. However, they also noted that “the EPDS is the most widely used tool to assess depressive symptoms in parents during the perinatal period and was validated in expectant and new fathers.”
The study was funded by the Canadian Institutes of Health Research. No conflicts of interest were reported.
SOURCE: Da Costa D et al. J Affect Disord. 2019 Apr 15;249:371-7.
First-time fathers may be at risk of experiencing depressive symptoms as they transition to parenthood – especially if risk factors such as poor sleep are present, results of a prospective study of more than 600 new fathers show.
“Strategies to promote better sleep, mobilize social support, and strengthen the couple relationship may be important to address in innovative interventions tailored to new fathers at risk for depression during the perinatal period,” wrote Deborah Da Costa, PhD, of McGill University, Montreal, and colleagues. The study was published in the Journal of Affective Disorders.
To determine the prevalence of depressive symptoms in first-time fathers and identify notable risk factors, the researchers surveyed 622 Canadian men during their partner’s third trimester. The same group was surveyed again at 2 and 6 months postpartum. Depression was assessed via the Edinburgh Postnatal Depression Scale (EPDS), and additional variables such as sleep quality, social support, and stress were gathered as well.
Of the initial 622 men surveyed, 487 (78.3%) and 375 (60.3%) completed the questionnaires at 2 and 6 months postpartum, respectively. The prevalence of paternal depressive symptoms was 13.76% (95% confidence interval, 10.70-16.82) at 2 months and 13.6% (95% CI, 10.13-17.07) at 6 months. Of the men who reported depressive symptoms at 2 months postpartum, 40.3% also experienced symptoms during the third trimester. Of the men who reported depressive symptoms at 6 months postpartum, 24% experienced symptoms during the third trimester and after 2 months.
At 2 months, the risk of depressive symptoms increased for men with worse sleep quality (odds ratio, 1.25; 95% CI, 1.10-1.42), poorer couple relationship adjustment (OR, 0.97; 95% CI, 0.94-0.99), and higher parenting stress (OR, 1.07; 95% CI, 1.02-1.11). At 6 months, there was a significant association between paternal depressive symptoms and unemployment (OR, 3.75; 95% CI, 1.00-13.72), poorer sleep quality (OR, 1.37; 95% CI, 1.16-1.65), lower social support (OR, 0.92; 95% CI, 0.84-1.00), poorer couple relationship adjustment (OR, 0.95; 95% CI, 0.92-0.98), and higher financial stress (OR, 1.21; 95% CI, 1.04-1.42).
The authors acknowledged their study’s limitations, including a middling response rate that could affect the accuracy of prevalence estimates and a well-educated, largely middle-class sample that could limit generalizability. In addition, they assessed depressive symptoms by self-report and not diagnostic clinical interviews. However, they also noted that “the EPDS is the most widely used tool to assess depressive symptoms in parents during the perinatal period and was validated in expectant and new fathers.”
The study was funded by the Canadian Institutes of Health Research. No conflicts of interest were reported.
SOURCE: Da Costa D et al. J Affect Disord. 2019 Apr 15;249:371-7.
First-time fathers may be at risk of experiencing depressive symptoms as they transition to parenthood – especially if risk factors such as poor sleep are present, results of a prospective study of more than 600 new fathers show.
“Strategies to promote better sleep, mobilize social support, and strengthen the couple relationship may be important to address in innovative interventions tailored to new fathers at risk for depression during the perinatal period,” wrote Deborah Da Costa, PhD, of McGill University, Montreal, and colleagues. The study was published in the Journal of Affective Disorders.
To determine the prevalence of depressive symptoms in first-time fathers and identify notable risk factors, the researchers surveyed 622 Canadian men during their partner’s third trimester. The same group was surveyed again at 2 and 6 months postpartum. Depression was assessed via the Edinburgh Postnatal Depression Scale (EPDS), and additional variables such as sleep quality, social support, and stress were gathered as well.
Of the initial 622 men surveyed, 487 (78.3%) and 375 (60.3%) completed the questionnaires at 2 and 6 months postpartum, respectively. The prevalence of paternal depressive symptoms was 13.76% (95% confidence interval, 10.70-16.82) at 2 months and 13.6% (95% CI, 10.13-17.07) at 6 months. Of the men who reported depressive symptoms at 2 months postpartum, 40.3% also experienced symptoms during the third trimester. Of the men who reported depressive symptoms at 6 months postpartum, 24% experienced symptoms during the third trimester and after 2 months.
At 2 months, the risk of depressive symptoms increased for men with worse sleep quality (odds ratio, 1.25; 95% CI, 1.10-1.42), poorer couple relationship adjustment (OR, 0.97; 95% CI, 0.94-0.99), and higher parenting stress (OR, 1.07; 95% CI, 1.02-1.11). At 6 months, there was a significant association between paternal depressive symptoms and unemployment (OR, 3.75; 95% CI, 1.00-13.72), poorer sleep quality (OR, 1.37; 95% CI, 1.16-1.65), lower social support (OR, 0.92; 95% CI, 0.84-1.00), poorer couple relationship adjustment (OR, 0.95; 95% CI, 0.92-0.98), and higher financial stress (OR, 1.21; 95% CI, 1.04-1.42).
The authors acknowledged their study’s limitations, including a middling response rate that could affect the accuracy of prevalence estimates and a well-educated, largely middle-class sample that could limit generalizability. In addition, they assessed depressive symptoms by self-report and not diagnostic clinical interviews. However, they also noted that “the EPDS is the most widely used tool to assess depressive symptoms in parents during the perinatal period and was validated in expectant and new fathers.”
The study was funded by the Canadian Institutes of Health Research. No conflicts of interest were reported.
SOURCE: Da Costa D et al. J Affect Disord. 2019 Apr 15;249:371-7.
FROM THE JOURNAL OF AFFECTIVE DISORDERS