User login
Postpartum depression often tricky to diagnose
LAS VEGAS – Diagnosing postpartum depression can be tricky because of the wide range of body changes that occur during the postpartum period, but vigilance is warranted with mothers who express a lack of sleep and a lack of social support.
at an annual psychopharmacology update held by the Nevada Psychiatric Association. “This gives you information about depression and insomnia. Make sure to ask about anxiety symptoms. Also ask about any thoughts of suicide or harming the infant, and support from family and friends when she’s under stress and taking care of the baby.”
According to Dr. Friedman, a perinatal and forensic psychiatrist at Case Western Reserve University, Cleveland, social risk factors for postpartum depression (PPD) include being a victim of intimate partner violence and/or abuse, negative life events, decreased social support, relationship issues, and socioeconomic status. Psychological risk factors include anxiety/depression in pregnancy, personal or family history of PPD, and substance misuse. Biological risk factors include medical illness, multiple births, and having an infant with low birth weight/prematurity.
PPD affects 10%-20% of new mothers and peaks at 12 weeks. Postpartum psychosis, meanwhile, occurs in about 1-2 of every 1,000 deliveries. Anxiety comorbidity is common.
In the neonatal intensive care unit (NICU), PPD rates might increase from 28% to 70% depending on the study. Risk factors include personal or family history, disturbed relationships, unfavorable socioeconomic factors, and stressful life events. Obstetrical risk factors might include conception by assisted reproductive technologies and having a stillbirth in the year before conception. NICU-specific risk factors include less-effective coping strategies, greater perception of maternal role disruption, and decreased perception of nursing support. “A lot of mothers [in the NICU] talk to me about being on a roller roaster every day about what’s going to happen with their baby,” Dr. Friedman said.
The most widely used measure to screen for PPD is the 10-item self-rating Edinburgh Postnatal Depression Scale . A total score of 10 or more is considered a flag for the need to follow up for possible depressive symptoms. She advises clinicians to pay particular attention to how patients respond to item No. 10 on the scale, which reads, “The thought of harming myself has occurred to me.” (Optional answers range from “Yes, quite often” to “Never.”) She also recommends administering the screen at both pediatric and obstetrical office visits, “because mothers are more likely to attend a pediatrics appointment than her own [postpartum] follow-up.”
The differential diagnosis of PPD includes the baby blues, postpartum psychosis, postpartum anxiety/PTSD, medical causes, substance use disorder, and PPD in bipolar disorder. Baby blues is not synonymous with PPD. It affects the majority (50%-80%) of new mothers and is characterized by emotional sensitivity, mood lability, and irritability. It usually occurs within 5 days and resolves by the second week post partum.
Postpartum psychosis (PPP) occurs in about 1-2 of every 1,000 deliveries, typically in the first 2 weeks after delivery. The onset occurs rapidly, and PPP is most frequently correlated with bipolar disorder over time. PPP itself is characterized by grandiose bizarre delusions, mood lability, hallucinations, confusion, and disorganized behavior. “This can occur as a new onset of mental illness as well, so getting collateral information about her behaviors is important,” she said.
Dr. Friedman explained that those events occur post partum largely because of sleep deprivation and increasing stress as the woman adjusts to a mothering role. Hormonal shifts also occur, with a drop in estrogen levels. Obstetrical complications also might factor in.
Postpartum obsessive-compulsive disorder (OCD) is commonly comorbid with PPD and is distinguished by ego-dystonic intrusive thoughts. The mother might have intense distress that she is going to harm the infant and might start to avoid holding the baby out of concern. “Common things I’ve heard from women with postpartum OCD are: ‘I’m afraid I’m going to put the baby in the microwave or in the oven instead of dinner’ or ‘I’m afraid I’m going to leave the baby in the car overnight and she’ll freeze to death,’ ” she said.
Postpartum PTSD can be triggered by a traumatic event experience in the birthing process, such as an emergency C-section. Affected mothers avoid the infant and hospital, “reexperience” the trauma, are easily startled, irritable, and disconnected. Dr. Friedman also noted that early parental PTSD symptoms predict sleep and eating problems in childhood and less sensitive/more controlling maternal behaviors.
Medical conditions that mimic PPD include anemia, thyroid disease, hypoactive delirium, infections, and alcohol/substance use disorder.
The best available data show that mothers with PPD are more withdrawn, disengaged, display more hostility, and are more likely to have disrupted attachment with their babies, Dr. Friedman said. They also are less likely to employ healthy child development practices and to breastfeed. Untreated depression might lead to psychotic symptoms, suicide, or homicide. Paternal PPD also occurs in an estimated 10% of fathers and is moderately correlated with maternal PPD.
Potential risks of PPD include impaired bonding, attachment disturbance, language development, cognitive skills, and behavior problems.
Potential risks of untreated PPD include child neglect or abuse because of active symptoms, suicide, and psychotic or maltreatment-related infanticide. “If the mother is taking about harming herself, I often ask: ‘Have you thought of what would happen to your baby if you were to take your own life?’ ” Dr. Friedman offered. Peripartum suicide risk is lower than in the general female population, but it represents about 20% of peripartum deaths. Overdose is the most common method. “However, uncommon and dramatic methods are more common in this population,” she said. “Teens and stigmatized single mothers are at greater risk.”
Dr. Friedman noted that clinicians face risk of a malpractice lawsuit if they fail to treat, abandon the patient, fail to provide informed consent, and if there are bad outcomes. The best approach is to proactively communicate with the patient, partner, pediatrics, and obstetrics. “Conduct an individual risk-benefit assessment with the individual patient’s history,” she advised. “Don’t do anything knee jerk. Consult when needed, document, and consider lactation and future pregnancy possibility in women of reproductive age.”
Nonpharmacologic therapy might be the first line of treatment for mild to moderate symptoms. Options include cognitive-behavioral therapy, interpersonal psychotherapy, family therapy, psychodynamic psychotherapy, and supportive psychotherapy. She recommends close follow-up and conducting a careful medication history. Electroconvulsive therapy remains a possibility.
If medication use is warranted, “weigh the benefits of breastfeeding with the usually low drug exposure of the infant,” Dr. Friedman advised. “We want to use the least number of medications at an effective dose to optimize treatment. Newer medications have less perinatal data. Sertraline and paroxetine are usually the preferred selective serotonin reuptake inhibitors in lactation. However, fluoxetine or citalopram might be used depending on the patient’s response history/use in pregnancy.”
Dr. Friedman reported no disclosures.
LAS VEGAS – Diagnosing postpartum depression can be tricky because of the wide range of body changes that occur during the postpartum period, but vigilance is warranted with mothers who express a lack of sleep and a lack of social support.
at an annual psychopharmacology update held by the Nevada Psychiatric Association. “This gives you information about depression and insomnia. Make sure to ask about anxiety symptoms. Also ask about any thoughts of suicide or harming the infant, and support from family and friends when she’s under stress and taking care of the baby.”
According to Dr. Friedman, a perinatal and forensic psychiatrist at Case Western Reserve University, Cleveland, social risk factors for postpartum depression (PPD) include being a victim of intimate partner violence and/or abuse, negative life events, decreased social support, relationship issues, and socioeconomic status. Psychological risk factors include anxiety/depression in pregnancy, personal or family history of PPD, and substance misuse. Biological risk factors include medical illness, multiple births, and having an infant with low birth weight/prematurity.
PPD affects 10%-20% of new mothers and peaks at 12 weeks. Postpartum psychosis, meanwhile, occurs in about 1-2 of every 1,000 deliveries. Anxiety comorbidity is common.
In the neonatal intensive care unit (NICU), PPD rates might increase from 28% to 70% depending on the study. Risk factors include personal or family history, disturbed relationships, unfavorable socioeconomic factors, and stressful life events. Obstetrical risk factors might include conception by assisted reproductive technologies and having a stillbirth in the year before conception. NICU-specific risk factors include less-effective coping strategies, greater perception of maternal role disruption, and decreased perception of nursing support. “A lot of mothers [in the NICU] talk to me about being on a roller roaster every day about what’s going to happen with their baby,” Dr. Friedman said.
The most widely used measure to screen for PPD is the 10-item self-rating Edinburgh Postnatal Depression Scale . A total score of 10 or more is considered a flag for the need to follow up for possible depressive symptoms. She advises clinicians to pay particular attention to how patients respond to item No. 10 on the scale, which reads, “The thought of harming myself has occurred to me.” (Optional answers range from “Yes, quite often” to “Never.”) She also recommends administering the screen at both pediatric and obstetrical office visits, “because mothers are more likely to attend a pediatrics appointment than her own [postpartum] follow-up.”
The differential diagnosis of PPD includes the baby blues, postpartum psychosis, postpartum anxiety/PTSD, medical causes, substance use disorder, and PPD in bipolar disorder. Baby blues is not synonymous with PPD. It affects the majority (50%-80%) of new mothers and is characterized by emotional sensitivity, mood lability, and irritability. It usually occurs within 5 days and resolves by the second week post partum.
Postpartum psychosis (PPP) occurs in about 1-2 of every 1,000 deliveries, typically in the first 2 weeks after delivery. The onset occurs rapidly, and PPP is most frequently correlated with bipolar disorder over time. PPP itself is characterized by grandiose bizarre delusions, mood lability, hallucinations, confusion, and disorganized behavior. “This can occur as a new onset of mental illness as well, so getting collateral information about her behaviors is important,” she said.
Dr. Friedman explained that those events occur post partum largely because of sleep deprivation and increasing stress as the woman adjusts to a mothering role. Hormonal shifts also occur, with a drop in estrogen levels. Obstetrical complications also might factor in.
Postpartum obsessive-compulsive disorder (OCD) is commonly comorbid with PPD and is distinguished by ego-dystonic intrusive thoughts. The mother might have intense distress that she is going to harm the infant and might start to avoid holding the baby out of concern. “Common things I’ve heard from women with postpartum OCD are: ‘I’m afraid I’m going to put the baby in the microwave or in the oven instead of dinner’ or ‘I’m afraid I’m going to leave the baby in the car overnight and she’ll freeze to death,’ ” she said.
Postpartum PTSD can be triggered by a traumatic event experience in the birthing process, such as an emergency C-section. Affected mothers avoid the infant and hospital, “reexperience” the trauma, are easily startled, irritable, and disconnected. Dr. Friedman also noted that early parental PTSD symptoms predict sleep and eating problems in childhood and less sensitive/more controlling maternal behaviors.
Medical conditions that mimic PPD include anemia, thyroid disease, hypoactive delirium, infections, and alcohol/substance use disorder.
The best available data show that mothers with PPD are more withdrawn, disengaged, display more hostility, and are more likely to have disrupted attachment with their babies, Dr. Friedman said. They also are less likely to employ healthy child development practices and to breastfeed. Untreated depression might lead to psychotic symptoms, suicide, or homicide. Paternal PPD also occurs in an estimated 10% of fathers and is moderately correlated with maternal PPD.
Potential risks of PPD include impaired bonding, attachment disturbance, language development, cognitive skills, and behavior problems.
Potential risks of untreated PPD include child neglect or abuse because of active symptoms, suicide, and psychotic or maltreatment-related infanticide. “If the mother is taking about harming herself, I often ask: ‘Have you thought of what would happen to your baby if you were to take your own life?’ ” Dr. Friedman offered. Peripartum suicide risk is lower than in the general female population, but it represents about 20% of peripartum deaths. Overdose is the most common method. “However, uncommon and dramatic methods are more common in this population,” she said. “Teens and stigmatized single mothers are at greater risk.”
Dr. Friedman noted that clinicians face risk of a malpractice lawsuit if they fail to treat, abandon the patient, fail to provide informed consent, and if there are bad outcomes. The best approach is to proactively communicate with the patient, partner, pediatrics, and obstetrics. “Conduct an individual risk-benefit assessment with the individual patient’s history,” she advised. “Don’t do anything knee jerk. Consult when needed, document, and consider lactation and future pregnancy possibility in women of reproductive age.”
Nonpharmacologic therapy might be the first line of treatment for mild to moderate symptoms. Options include cognitive-behavioral therapy, interpersonal psychotherapy, family therapy, psychodynamic psychotherapy, and supportive psychotherapy. She recommends close follow-up and conducting a careful medication history. Electroconvulsive therapy remains a possibility.
If medication use is warranted, “weigh the benefits of breastfeeding with the usually low drug exposure of the infant,” Dr. Friedman advised. “We want to use the least number of medications at an effective dose to optimize treatment. Newer medications have less perinatal data. Sertraline and paroxetine are usually the preferred selective serotonin reuptake inhibitors in lactation. However, fluoxetine or citalopram might be used depending on the patient’s response history/use in pregnancy.”
Dr. Friedman reported no disclosures.
LAS VEGAS – Diagnosing postpartum depression can be tricky because of the wide range of body changes that occur during the postpartum period, but vigilance is warranted with mothers who express a lack of sleep and a lack of social support.
at an annual psychopharmacology update held by the Nevada Psychiatric Association. “This gives you information about depression and insomnia. Make sure to ask about anxiety symptoms. Also ask about any thoughts of suicide or harming the infant, and support from family and friends when she’s under stress and taking care of the baby.”
According to Dr. Friedman, a perinatal and forensic psychiatrist at Case Western Reserve University, Cleveland, social risk factors for postpartum depression (PPD) include being a victim of intimate partner violence and/or abuse, negative life events, decreased social support, relationship issues, and socioeconomic status. Psychological risk factors include anxiety/depression in pregnancy, personal or family history of PPD, and substance misuse. Biological risk factors include medical illness, multiple births, and having an infant with low birth weight/prematurity.
PPD affects 10%-20% of new mothers and peaks at 12 weeks. Postpartum psychosis, meanwhile, occurs in about 1-2 of every 1,000 deliveries. Anxiety comorbidity is common.
In the neonatal intensive care unit (NICU), PPD rates might increase from 28% to 70% depending on the study. Risk factors include personal or family history, disturbed relationships, unfavorable socioeconomic factors, and stressful life events. Obstetrical risk factors might include conception by assisted reproductive technologies and having a stillbirth in the year before conception. NICU-specific risk factors include less-effective coping strategies, greater perception of maternal role disruption, and decreased perception of nursing support. “A lot of mothers [in the NICU] talk to me about being on a roller roaster every day about what’s going to happen with their baby,” Dr. Friedman said.
The most widely used measure to screen for PPD is the 10-item self-rating Edinburgh Postnatal Depression Scale . A total score of 10 or more is considered a flag for the need to follow up for possible depressive symptoms. She advises clinicians to pay particular attention to how patients respond to item No. 10 on the scale, which reads, “The thought of harming myself has occurred to me.” (Optional answers range from “Yes, quite often” to “Never.”) She also recommends administering the screen at both pediatric and obstetrical office visits, “because mothers are more likely to attend a pediatrics appointment than her own [postpartum] follow-up.”
The differential diagnosis of PPD includes the baby blues, postpartum psychosis, postpartum anxiety/PTSD, medical causes, substance use disorder, and PPD in bipolar disorder. Baby blues is not synonymous with PPD. It affects the majority (50%-80%) of new mothers and is characterized by emotional sensitivity, mood lability, and irritability. It usually occurs within 5 days and resolves by the second week post partum.
Postpartum psychosis (PPP) occurs in about 1-2 of every 1,000 deliveries, typically in the first 2 weeks after delivery. The onset occurs rapidly, and PPP is most frequently correlated with bipolar disorder over time. PPP itself is characterized by grandiose bizarre delusions, mood lability, hallucinations, confusion, and disorganized behavior. “This can occur as a new onset of mental illness as well, so getting collateral information about her behaviors is important,” she said.
Dr. Friedman explained that those events occur post partum largely because of sleep deprivation and increasing stress as the woman adjusts to a mothering role. Hormonal shifts also occur, with a drop in estrogen levels. Obstetrical complications also might factor in.
Postpartum obsessive-compulsive disorder (OCD) is commonly comorbid with PPD and is distinguished by ego-dystonic intrusive thoughts. The mother might have intense distress that she is going to harm the infant and might start to avoid holding the baby out of concern. “Common things I’ve heard from women with postpartum OCD are: ‘I’m afraid I’m going to put the baby in the microwave or in the oven instead of dinner’ or ‘I’m afraid I’m going to leave the baby in the car overnight and she’ll freeze to death,’ ” she said.
Postpartum PTSD can be triggered by a traumatic event experience in the birthing process, such as an emergency C-section. Affected mothers avoid the infant and hospital, “reexperience” the trauma, are easily startled, irritable, and disconnected. Dr. Friedman also noted that early parental PTSD symptoms predict sleep and eating problems in childhood and less sensitive/more controlling maternal behaviors.
Medical conditions that mimic PPD include anemia, thyroid disease, hypoactive delirium, infections, and alcohol/substance use disorder.
The best available data show that mothers with PPD are more withdrawn, disengaged, display more hostility, and are more likely to have disrupted attachment with their babies, Dr. Friedman said. They also are less likely to employ healthy child development practices and to breastfeed. Untreated depression might lead to psychotic symptoms, suicide, or homicide. Paternal PPD also occurs in an estimated 10% of fathers and is moderately correlated with maternal PPD.
Potential risks of PPD include impaired bonding, attachment disturbance, language development, cognitive skills, and behavior problems.
Potential risks of untreated PPD include child neglect or abuse because of active symptoms, suicide, and psychotic or maltreatment-related infanticide. “If the mother is taking about harming herself, I often ask: ‘Have you thought of what would happen to your baby if you were to take your own life?’ ” Dr. Friedman offered. Peripartum suicide risk is lower than in the general female population, but it represents about 20% of peripartum deaths. Overdose is the most common method. “However, uncommon and dramatic methods are more common in this population,” she said. “Teens and stigmatized single mothers are at greater risk.”
Dr. Friedman noted that clinicians face risk of a malpractice lawsuit if they fail to treat, abandon the patient, fail to provide informed consent, and if there are bad outcomes. The best approach is to proactively communicate with the patient, partner, pediatrics, and obstetrics. “Conduct an individual risk-benefit assessment with the individual patient’s history,” she advised. “Don’t do anything knee jerk. Consult when needed, document, and consider lactation and future pregnancy possibility in women of reproductive age.”
Nonpharmacologic therapy might be the first line of treatment for mild to moderate symptoms. Options include cognitive-behavioral therapy, interpersonal psychotherapy, family therapy, psychodynamic psychotherapy, and supportive psychotherapy. She recommends close follow-up and conducting a careful medication history. Electroconvulsive therapy remains a possibility.
If medication use is warranted, “weigh the benefits of breastfeeding with the usually low drug exposure of the infant,” Dr. Friedman advised. “We want to use the least number of medications at an effective dose to optimize treatment. Newer medications have less perinatal data. Sertraline and paroxetine are usually the preferred selective serotonin reuptake inhibitors in lactation. However, fluoxetine or citalopram might be used depending on the patient’s response history/use in pregnancy.”
Dr. Friedman reported no disclosures.
EXPERT ANALYSIS FROM NPA 2019
Suicide: Igor Galynker
One major topic of conversation centers around suicide-specific diagnosis. And later, Renee Kohanski, MD, talks about the importance of communication. You can listen to Dr. Galynker’s first appearance on the Psychcast here.
One major topic of conversation centers around suicide-specific diagnosis. And later, Renee Kohanski, MD, talks about the importance of communication. You can listen to Dr. Galynker’s first appearance on the Psychcast here.
One major topic of conversation centers around suicide-specific diagnosis. And later, Renee Kohanski, MD, talks about the importance of communication. You can listen to Dr. Galynker’s first appearance on the Psychcast here.
PTSD, cardiovascular disease link likely caused by higher comorbidity burden
Although PTSD is strongly linked to cardiovascular disease, it is not an independent risk factor, results of a recent analysis suggest.
Instead, the association between PTSD and cardiovascular disease (CVD) is likely explained by factors such as smoking and physical and psychiatric disorders, according to authors of the analysis based on EHR data for more than 4,000 U.S. veterans.
Individuals with PTSD were 41% more likely than those without it to develop cardiovascular disease, according to the researchers, led by Jeffrey F. Scherrer, PhD, of Saint Louis University and the Harry S. Truman Veterans Administration Medical Center in Columbia, Mo.
However, PTSD was not associated with CVD in the study after adjustment for physical, psychiatric, and behavioral conditions, Dr. Scherrer and his colleagues reported in the Journal of the American Heart Association.
“Recognizing that PTSD does not preordain CVD may empower patients to seek care to prevent and/or manage CVD risk factors,” they wrote.
Health behavior change and management of chronic disease can mitigate risk of CVD in patients with or without PTSD, they added.
This result contrasts with earlier work associating PTSD with CVD, the authors wrote. In particular, a few well-designed studies did indicate that the link between PTSD and CVD was weakened, but still significant, when controlling for traditional CVD risk factors such as smoking, diabetes, and hypertension.
In the current study, investigators controlled for a variety of physical and psychiatric conditions, as well as smoking, in data for Veterans Affairs patients, of whom 2,519 had a PTSD diagnosis and 1,659 did not. These patients were 87% male, 60% white, and had an average age of 50 years.
The investigators found that PTSD was significantly associated with incident CVD after adjusting for age, with a hazard ratio of 1.41 (95% confidence interval, 1.21-1.63; P less than .0001).
That association remained significant after adjusting for diabetes, obesity, hypertension, and hyperlipidemia, but the magnitude of the association dropped considerably (HR, 1.23; 95% CI, 1.06-1.44; P less than .007) and dropped out altogether after controlling for smoking, substance abuse, sleep disorders, anxiety, and depression (HR, 0.96; 95% CI, 0.81-1.15; P = .691).
Taken together, these findings suggest the association with incident CVD may be explained by combinations of comorbidities that are more prevalent in patients who have PTSD than in those who do not, Dr. Scherrer and his coauthors wrote.
“Because these conditions are more common in patients with PTSD, closer monitoring for comorbidities may be warranted,” they concluded. “Early detection and effective management may reduce the burden of CVD associated with PTSD.”
One study coinvestigator reported consulting for Noblis Therapeutics and grant-related disclosures with the Department of Veterans Affairs, Department of Defense, and National Institute of Mental Health.
SOURCE: Scherrer JF et al. J Am Heart Assoc. 2019 Feb 13. doi: 10.1161/JAHA.118.011133.
Although PTSD is strongly linked to cardiovascular disease, it is not an independent risk factor, results of a recent analysis suggest.
Instead, the association between PTSD and cardiovascular disease (CVD) is likely explained by factors such as smoking and physical and psychiatric disorders, according to authors of the analysis based on EHR data for more than 4,000 U.S. veterans.
Individuals with PTSD were 41% more likely than those without it to develop cardiovascular disease, according to the researchers, led by Jeffrey F. Scherrer, PhD, of Saint Louis University and the Harry S. Truman Veterans Administration Medical Center in Columbia, Mo.
However, PTSD was not associated with CVD in the study after adjustment for physical, psychiatric, and behavioral conditions, Dr. Scherrer and his colleagues reported in the Journal of the American Heart Association.
“Recognizing that PTSD does not preordain CVD may empower patients to seek care to prevent and/or manage CVD risk factors,” they wrote.
Health behavior change and management of chronic disease can mitigate risk of CVD in patients with or without PTSD, they added.
This result contrasts with earlier work associating PTSD with CVD, the authors wrote. In particular, a few well-designed studies did indicate that the link between PTSD and CVD was weakened, but still significant, when controlling for traditional CVD risk factors such as smoking, diabetes, and hypertension.
In the current study, investigators controlled for a variety of physical and psychiatric conditions, as well as smoking, in data for Veterans Affairs patients, of whom 2,519 had a PTSD diagnosis and 1,659 did not. These patients were 87% male, 60% white, and had an average age of 50 years.
The investigators found that PTSD was significantly associated with incident CVD after adjusting for age, with a hazard ratio of 1.41 (95% confidence interval, 1.21-1.63; P less than .0001).
That association remained significant after adjusting for diabetes, obesity, hypertension, and hyperlipidemia, but the magnitude of the association dropped considerably (HR, 1.23; 95% CI, 1.06-1.44; P less than .007) and dropped out altogether after controlling for smoking, substance abuse, sleep disorders, anxiety, and depression (HR, 0.96; 95% CI, 0.81-1.15; P = .691).
Taken together, these findings suggest the association with incident CVD may be explained by combinations of comorbidities that are more prevalent in patients who have PTSD than in those who do not, Dr. Scherrer and his coauthors wrote.
“Because these conditions are more common in patients with PTSD, closer monitoring for comorbidities may be warranted,” they concluded. “Early detection and effective management may reduce the burden of CVD associated with PTSD.”
One study coinvestigator reported consulting for Noblis Therapeutics and grant-related disclosures with the Department of Veterans Affairs, Department of Defense, and National Institute of Mental Health.
SOURCE: Scherrer JF et al. J Am Heart Assoc. 2019 Feb 13. doi: 10.1161/JAHA.118.011133.
Although PTSD is strongly linked to cardiovascular disease, it is not an independent risk factor, results of a recent analysis suggest.
Instead, the association between PTSD and cardiovascular disease (CVD) is likely explained by factors such as smoking and physical and psychiatric disorders, according to authors of the analysis based on EHR data for more than 4,000 U.S. veterans.
Individuals with PTSD were 41% more likely than those without it to develop cardiovascular disease, according to the researchers, led by Jeffrey F. Scherrer, PhD, of Saint Louis University and the Harry S. Truman Veterans Administration Medical Center in Columbia, Mo.
However, PTSD was not associated with CVD in the study after adjustment for physical, psychiatric, and behavioral conditions, Dr. Scherrer and his colleagues reported in the Journal of the American Heart Association.
“Recognizing that PTSD does not preordain CVD may empower patients to seek care to prevent and/or manage CVD risk factors,” they wrote.
Health behavior change and management of chronic disease can mitigate risk of CVD in patients with or without PTSD, they added.
This result contrasts with earlier work associating PTSD with CVD, the authors wrote. In particular, a few well-designed studies did indicate that the link between PTSD and CVD was weakened, but still significant, when controlling for traditional CVD risk factors such as smoking, diabetes, and hypertension.
In the current study, investigators controlled for a variety of physical and psychiatric conditions, as well as smoking, in data for Veterans Affairs patients, of whom 2,519 had a PTSD diagnosis and 1,659 did not. These patients were 87% male, 60% white, and had an average age of 50 years.
The investigators found that PTSD was significantly associated with incident CVD after adjusting for age, with a hazard ratio of 1.41 (95% confidence interval, 1.21-1.63; P less than .0001).
That association remained significant after adjusting for diabetes, obesity, hypertension, and hyperlipidemia, but the magnitude of the association dropped considerably (HR, 1.23; 95% CI, 1.06-1.44; P less than .007) and dropped out altogether after controlling for smoking, substance abuse, sleep disorders, anxiety, and depression (HR, 0.96; 95% CI, 0.81-1.15; P = .691).
Taken together, these findings suggest the association with incident CVD may be explained by combinations of comorbidities that are more prevalent in patients who have PTSD than in those who do not, Dr. Scherrer and his coauthors wrote.
“Because these conditions are more common in patients with PTSD, closer monitoring for comorbidities may be warranted,” they concluded. “Early detection and effective management may reduce the burden of CVD associated with PTSD.”
One study coinvestigator reported consulting for Noblis Therapeutics and grant-related disclosures with the Department of Veterans Affairs, Department of Defense, and National Institute of Mental Health.
SOURCE: Scherrer JF et al. J Am Heart Assoc. 2019 Feb 13. doi: 10.1161/JAHA.118.011133.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Key clinical point: The link between PTSD and cardiovascular disease may be explained by higher prevalence of comorbidities in individuals with the disorder, rather than the disorder itself.
Major finding: PTSD was significantly associated with incident cardiovascular disease after adjusting for age, but was no longer associated with cardiovascular disease after further adjustment for physical and psychological comorbidities and smoking (hazard ratio, 0.96; 95% confidence interval, 0.81-1.15; P = 0.691).
Study details: A retrospective study of EHR data for Veterans Affairs patients, of whom 2,519 had a PTSD diagnosis and 1,659 did not.
Disclosures: One study author reported financial disclosures related to Noblis Therapeutics, the Department of Veterans Affairs, Department of Defense, the National Institute of Mental Health.
Source: Scherrer JF et al. J Am Heart Assoc. 2019 Feb 13. doi: 10.1161/JAHA.118.011133.
FDA panels back intranasal esketamine for refractory depression
ROCKVILLE, MD – If approved for treatment-resistant depression, intranasal esketamine will be strictly regulated in the clinic, with federal monitoring requirements designed to prevent misuse, abuse, or diversion of the drug.
Managed under a Food and Drug Administration Risk Evaluation and Mitigation Strategy (REMS), such a program would establish a stringent post-administration protocol of observation and blood pressure monitoring and require every provider – whether a large health care center or a single clinician – to obtain federal certification to dispense the medication.
At a joint meeting of FDA’s Psychopharmacologic Drugs Advisory and Drug Safety and Risk Management Advisory committees, some members offered a more tempered view while still supporting the approval pathway of the N-methyl-D-aspartate receptor antagonist. By a vote of 14-2, with one abstention, they agreed Feb. 12 that the benefits outweigh the risks of esketamine for treatment-resistant depression.
“I think it has the potential to be a game changer in treatment-resistant depression,” said Walter Dunn, MD, PhD, of the University of California, Los Angeles. “We may someday talk about 2019 in the same way we now talk about the late ’80s, when the first [selective serotonin reuptake inhibitors] were approved.”
Janssen Pharmaceuticals, which is developing the drug, incorporated concerns about misuse from the beginning. Even the delivery device is designed to prevent such issues, a company spokesman said.
Each disposable intranasal delivery device contains 28 mg esketamine; it will come in prepackaged units of one, two, or three devices to deliver the prescribed doses of 28 mg, 56 mg, or 84 mg, respectively. The device does not require priming and, after use, contains only about 30 microliters of residual medication. Its interlocking design, with a glass vial inside the plastic outer assembly, would make it very difficult to pull apart, should anyone want to obtain the residue.
The proposed REMS – the key requirement for approval at this point – would include the following measures:
- Prescriber training on the risks of esketamine and importance of monitoring patients after their dose is administered and the need to register patients
- Administration of esketamine only in certain health care settings that ensure patient monitoring by a health care clinician for 2 hours after administration
- Pharmacies, clinicians, or health care settings that dispense the drug are specially certified to ensure that esketamine is not dispensed directly to patients and that patients are monitored
- Enrollment of patients who are treated with esketamine in a registry to better characterize the risks associated with esketamine administration and inform risk mitigation strategies
After administration, patients would be monitored for at least 2 hours for the common side effects, sedation and dissociation that typically clear within that time. Transient blood pressure fluctuations also can occur shortly after administration and would be monitored until stable. Patients should also be counseled not to drive the day of treatment, and to bring a companion along to drive them home.
Dr. Dunn, however, suggested that some facets of the proposed REMS might create unnecessary barriers for some patients and that stringent monitoring after every single dose – potentially for years – might not be necessary for everyone.
“The REM is certainly important to address the potential for diversion and misuse and adverse effects, but there needs to be a pathway to reduce monitoring requirements” on an individual basis. “If a patient is doing well for a year or so, in remission with no side effects, we should have a way to reduce the need for monitoring. If we make it too much of a burden to go in, get the medication, stay for a couple of hours for monitoring, it’s easy to skip a dose. And we know the number one predictor of relapse is medication nonadherence.”
The facility certification requirement also could curtail access to esketamine, said Steven B. Meisel, PharmD, of Minneapolis.
“How do we define a medically supervised center? Is it somewhere with a nurse onsite? A physician onsite? Does it have to have access to emergency services? This issue of access vs. control and safety is a very important one.”
He posed a clinical conundrum: A patient doing well on regular esketamine who wants to go on an extended trip. Under the proposed REMS, that patient would not be able to access his regular dose, which could only be handled, sorted, and administered by a certified health care clinician. “How are we going to deal with this? There will be great pressure to loosen this up in some manner. But if we allow a patient who’s been doing well on regular treatment with no relapse to have this at home, do we open the way for a teenager to take a bottle or two to a party? Those are real-world issues and must be considered when we establish a REM in a real world that demands access to needed therapy.”
Erring on the side of caution is the responsibility of policymakers, argued Kim Witczak, executive director of Woodymatters, a consumer-driven, nonprofit drug safety organization dedicated to FDA reform. Ms. Witczak was one of two dissenting voices on the vote.
“This has so much potential for so many people who just want a quick fix [for their mood disorders], and the marketing side will see this,” she predicted. “I would want to be very cautious. Once it gets out there into the real world, there will be a lot of people trying to get it. We don’t want to have ‘Esketamines “R” Us’ clinics popping up everywhere.”
The FDA usually follows its panels’ recommendations, which are not binding.
“The REMS program that was proposed by the company and seemingly endorsed by the FDA provides adequate protection,” Sanjay J. Mathew, MD, said in an interview. “I think that was one of the reasons it sailed through the panels.”
An important aspect of intranasal ketamine is that, as an N-methyl-D-aspartate receptor antagonist, it is “an entirely new class” for treating depression, said Dr. Mathew. “This is the first approval that does not work on serotonin or norepinephrine or dopamine. This is a big, big development. We can’t overstate that.”
Also, the nasal spray had to beat a placebo and a newly administered antidepressant. “There was a relatively high bar for showing convincing efficacy,” he said. “So if approved, this drug would be prescribed with an oral antidepressant. Intranasal esketamine represents 20 years’ worth of effort. Today was an important day for psychiatry,” he said. “It was an important day for patients with depression.”
Dr. Mathew is the Marjorie Bintliff Johnson and Raleigh White Johnson Jr. Vice Chair for Research and professor in the Menninger department of psychiatry & behavioral sciences at the Baylor College of Medicine in Houston. He has served as a consultant for and has had research funded by Janssen.
“The REMS program that was proposed by the company and seemingly endorsed by the FDA provides adequate protection,” Sanjay J. Mathew, MD, said in an interview. “I think that was one of the reasons it sailed through the panels.”
An important aspect of intranasal ketamine is that, as an N-methyl-D-aspartate receptor antagonist, it is “an entirely new class” for treating depression, said Dr. Mathew. “This is the first approval that does not work on serotonin or norepinephrine or dopamine. This is a big, big development. We can’t overstate that.”
Also, the nasal spray had to beat a placebo and a newly administered antidepressant. “There was a relatively high bar for showing convincing efficacy,” he said. “So if approved, this drug would be prescribed with an oral antidepressant. Intranasal esketamine represents 20 years’ worth of effort. Today was an important day for psychiatry,” he said. “It was an important day for patients with depression.”
Dr. Mathew is the Marjorie Bintliff Johnson and Raleigh White Johnson Jr. Vice Chair for Research and professor in the Menninger department of psychiatry & behavioral sciences at the Baylor College of Medicine in Houston. He has served as a consultant for and has had research funded by Janssen.
“The REMS program that was proposed by the company and seemingly endorsed by the FDA provides adequate protection,” Sanjay J. Mathew, MD, said in an interview. “I think that was one of the reasons it sailed through the panels.”
An important aspect of intranasal ketamine is that, as an N-methyl-D-aspartate receptor antagonist, it is “an entirely new class” for treating depression, said Dr. Mathew. “This is the first approval that does not work on serotonin or norepinephrine or dopamine. This is a big, big development. We can’t overstate that.”
Also, the nasal spray had to beat a placebo and a newly administered antidepressant. “There was a relatively high bar for showing convincing efficacy,” he said. “So if approved, this drug would be prescribed with an oral antidepressant. Intranasal esketamine represents 20 years’ worth of effort. Today was an important day for psychiatry,” he said. “It was an important day for patients with depression.”
Dr. Mathew is the Marjorie Bintliff Johnson and Raleigh White Johnson Jr. Vice Chair for Research and professor in the Menninger department of psychiatry & behavioral sciences at the Baylor College of Medicine in Houston. He has served as a consultant for and has had research funded by Janssen.
ROCKVILLE, MD – If approved for treatment-resistant depression, intranasal esketamine will be strictly regulated in the clinic, with federal monitoring requirements designed to prevent misuse, abuse, or diversion of the drug.
Managed under a Food and Drug Administration Risk Evaluation and Mitigation Strategy (REMS), such a program would establish a stringent post-administration protocol of observation and blood pressure monitoring and require every provider – whether a large health care center or a single clinician – to obtain federal certification to dispense the medication.
At a joint meeting of FDA’s Psychopharmacologic Drugs Advisory and Drug Safety and Risk Management Advisory committees, some members offered a more tempered view while still supporting the approval pathway of the N-methyl-D-aspartate receptor antagonist. By a vote of 14-2, with one abstention, they agreed Feb. 12 that the benefits outweigh the risks of esketamine for treatment-resistant depression.
“I think it has the potential to be a game changer in treatment-resistant depression,” said Walter Dunn, MD, PhD, of the University of California, Los Angeles. “We may someday talk about 2019 in the same way we now talk about the late ’80s, when the first [selective serotonin reuptake inhibitors] were approved.”
Janssen Pharmaceuticals, which is developing the drug, incorporated concerns about misuse from the beginning. Even the delivery device is designed to prevent such issues, a company spokesman said.
Each disposable intranasal delivery device contains 28 mg esketamine; it will come in prepackaged units of one, two, or three devices to deliver the prescribed doses of 28 mg, 56 mg, or 84 mg, respectively. The device does not require priming and, after use, contains only about 30 microliters of residual medication. Its interlocking design, with a glass vial inside the plastic outer assembly, would make it very difficult to pull apart, should anyone want to obtain the residue.
The proposed REMS – the key requirement for approval at this point – would include the following measures:
- Prescriber training on the risks of esketamine and importance of monitoring patients after their dose is administered and the need to register patients
- Administration of esketamine only in certain health care settings that ensure patient monitoring by a health care clinician for 2 hours after administration
- Pharmacies, clinicians, or health care settings that dispense the drug are specially certified to ensure that esketamine is not dispensed directly to patients and that patients are monitored
- Enrollment of patients who are treated with esketamine in a registry to better characterize the risks associated with esketamine administration and inform risk mitigation strategies
After administration, patients would be monitored for at least 2 hours for the common side effects, sedation and dissociation that typically clear within that time. Transient blood pressure fluctuations also can occur shortly after administration and would be monitored until stable. Patients should also be counseled not to drive the day of treatment, and to bring a companion along to drive them home.
Dr. Dunn, however, suggested that some facets of the proposed REMS might create unnecessary barriers for some patients and that stringent monitoring after every single dose – potentially for years – might not be necessary for everyone.
“The REM is certainly important to address the potential for diversion and misuse and adverse effects, but there needs to be a pathway to reduce monitoring requirements” on an individual basis. “If a patient is doing well for a year or so, in remission with no side effects, we should have a way to reduce the need for monitoring. If we make it too much of a burden to go in, get the medication, stay for a couple of hours for monitoring, it’s easy to skip a dose. And we know the number one predictor of relapse is medication nonadherence.”
The facility certification requirement also could curtail access to esketamine, said Steven B. Meisel, PharmD, of Minneapolis.
“How do we define a medically supervised center? Is it somewhere with a nurse onsite? A physician onsite? Does it have to have access to emergency services? This issue of access vs. control and safety is a very important one.”
He posed a clinical conundrum: A patient doing well on regular esketamine who wants to go on an extended trip. Under the proposed REMS, that patient would not be able to access his regular dose, which could only be handled, sorted, and administered by a certified health care clinician. “How are we going to deal with this? There will be great pressure to loosen this up in some manner. But if we allow a patient who’s been doing well on regular treatment with no relapse to have this at home, do we open the way for a teenager to take a bottle or two to a party? Those are real-world issues and must be considered when we establish a REM in a real world that demands access to needed therapy.”
Erring on the side of caution is the responsibility of policymakers, argued Kim Witczak, executive director of Woodymatters, a consumer-driven, nonprofit drug safety organization dedicated to FDA reform. Ms. Witczak was one of two dissenting voices on the vote.
“This has so much potential for so many people who just want a quick fix [for their mood disorders], and the marketing side will see this,” she predicted. “I would want to be very cautious. Once it gets out there into the real world, there will be a lot of people trying to get it. We don’t want to have ‘Esketamines “R” Us’ clinics popping up everywhere.”
The FDA usually follows its panels’ recommendations, which are not binding.
ROCKVILLE, MD – If approved for treatment-resistant depression, intranasal esketamine will be strictly regulated in the clinic, with federal monitoring requirements designed to prevent misuse, abuse, or diversion of the drug.
Managed under a Food and Drug Administration Risk Evaluation and Mitigation Strategy (REMS), such a program would establish a stringent post-administration protocol of observation and blood pressure monitoring and require every provider – whether a large health care center or a single clinician – to obtain federal certification to dispense the medication.
At a joint meeting of FDA’s Psychopharmacologic Drugs Advisory and Drug Safety and Risk Management Advisory committees, some members offered a more tempered view while still supporting the approval pathway of the N-methyl-D-aspartate receptor antagonist. By a vote of 14-2, with one abstention, they agreed Feb. 12 that the benefits outweigh the risks of esketamine for treatment-resistant depression.
“I think it has the potential to be a game changer in treatment-resistant depression,” said Walter Dunn, MD, PhD, of the University of California, Los Angeles. “We may someday talk about 2019 in the same way we now talk about the late ’80s, when the first [selective serotonin reuptake inhibitors] were approved.”
Janssen Pharmaceuticals, which is developing the drug, incorporated concerns about misuse from the beginning. Even the delivery device is designed to prevent such issues, a company spokesman said.
Each disposable intranasal delivery device contains 28 mg esketamine; it will come in prepackaged units of one, two, or three devices to deliver the prescribed doses of 28 mg, 56 mg, or 84 mg, respectively. The device does not require priming and, after use, contains only about 30 microliters of residual medication. Its interlocking design, with a glass vial inside the plastic outer assembly, would make it very difficult to pull apart, should anyone want to obtain the residue.
The proposed REMS – the key requirement for approval at this point – would include the following measures:
- Prescriber training on the risks of esketamine and importance of monitoring patients after their dose is administered and the need to register patients
- Administration of esketamine only in certain health care settings that ensure patient monitoring by a health care clinician for 2 hours after administration
- Pharmacies, clinicians, or health care settings that dispense the drug are specially certified to ensure that esketamine is not dispensed directly to patients and that patients are monitored
- Enrollment of patients who are treated with esketamine in a registry to better characterize the risks associated with esketamine administration and inform risk mitigation strategies
After administration, patients would be monitored for at least 2 hours for the common side effects, sedation and dissociation that typically clear within that time. Transient blood pressure fluctuations also can occur shortly after administration and would be monitored until stable. Patients should also be counseled not to drive the day of treatment, and to bring a companion along to drive them home.
Dr. Dunn, however, suggested that some facets of the proposed REMS might create unnecessary barriers for some patients and that stringent monitoring after every single dose – potentially for years – might not be necessary for everyone.
“The REM is certainly important to address the potential for diversion and misuse and adverse effects, but there needs to be a pathway to reduce monitoring requirements” on an individual basis. “If a patient is doing well for a year or so, in remission with no side effects, we should have a way to reduce the need for monitoring. If we make it too much of a burden to go in, get the medication, stay for a couple of hours for monitoring, it’s easy to skip a dose. And we know the number one predictor of relapse is medication nonadherence.”
The facility certification requirement also could curtail access to esketamine, said Steven B. Meisel, PharmD, of Minneapolis.
“How do we define a medically supervised center? Is it somewhere with a nurse onsite? A physician onsite? Does it have to have access to emergency services? This issue of access vs. control and safety is a very important one.”
He posed a clinical conundrum: A patient doing well on regular esketamine who wants to go on an extended trip. Under the proposed REMS, that patient would not be able to access his regular dose, which could only be handled, sorted, and administered by a certified health care clinician. “How are we going to deal with this? There will be great pressure to loosen this up in some manner. But if we allow a patient who’s been doing well on regular treatment with no relapse to have this at home, do we open the way for a teenager to take a bottle or two to a party? Those are real-world issues and must be considered when we establish a REM in a real world that demands access to needed therapy.”
Erring on the side of caution is the responsibility of policymakers, argued Kim Witczak, executive director of Woodymatters, a consumer-driven, nonprofit drug safety organization dedicated to FDA reform. Ms. Witczak was one of two dissenting voices on the vote.
“This has so much potential for so many people who just want a quick fix [for their mood disorders], and the marketing side will see this,” she predicted. “I would want to be very cautious. Once it gets out there into the real world, there will be a lot of people trying to get it. We don’t want to have ‘Esketamines “R” Us’ clinics popping up everywhere.”
The FDA usually follows its panels’ recommendations, which are not binding.
Medical students and psychiatry
I have the unfortunate task of trying to teach medical students about psychiatry. I say “unfortunate,” as most of them find psychiatry a difficult art to understand, and they seem reluctant to classify psychiatry as a branch of medicine.
In my efforts to keep things simple, I tell that them psychiatry is one of the most difficult branches of medicine as there are very few objective measures we can rely on to make sense of people’s behavior. Regrettably, the American Psychiatric Association’s Diagnostic and Statistical Manual only seems to confuse them more. So, I remind them that, in medicine, 90%-95% of diagnoses can be obtained from doing a good history, and, if we are lucky a drug level will show drugs in the system, a CT scan without contrast will show cerebral atrophy, or there will be a lab result that will be abnormal and point to a diagnosis. But mostly what they will be seeing is unusual behavior they are unable to classify.
So I identifiable brain damage, psychosis, affective disorders, anxiety disorders, and personality disorders. Under the brain damage category, I include the short- and long-term effects of drugs, major neurocognitive disorders (called dementia before DSM-5), cerebrovascular infarcts, traumatic brain injury, and neurodevelopmental disorders. For their exams and, if they are interested in psychiatry, I tell them to study the DSM. I explain to them that when I was in medical school my dermatology professor told us that if we could recognize the 10 most common dermatologic disorders, we would be able to recognize 90% of the skin disorders we would see. It is similar in psychiatry – thus, my five categories.
However, because I do not want them thinking that only schizophrenia causes psychosis, I let them know that at least 40 different factors cause people to be psychotic indicated by auditory hallucinations. Those 40 factors are: 1) acute alcohol intoxication, 2) alcohol withdrawal, 3) alcoholism, 4) Alzheimer’s disease, 5) benzodiazepine withdrawal, 6) cocaine abuse and addiction, 7) chemical poisoning, 8) dehydration, 9) delirium, 10) dissociative disorders, 11) electrolyte imbalances, 12) encephalopathy of various forms, 13) ecstasy, 14) extreme fatigue, 15) falling asleep, 16) fetal alcohol exposure, 17) grief, 18) hallucinogen use, 19) heroin abuse and dependence, 20) high fever, 21) hyperglycemia, 22) hypoglycemia, 23) intellectual disability, 24) lupus, 25) major depression, 26) mania, 27) methamphetamine use, 28) Parkinson’s disease, 29) phencyclidine, 30) postictal states, 31) posttraumatic stress disorder, 32) schizoid or schizotypal personality disorder, 33) schizophrenia, 34) sleep deprivation, 35) sleep paralysis, 36) solvent abuse, 37) traumatic brain injury, 38) temporal lobe epilepsy, 39) uremia. Lastly, I ask them about No. 40 – “normal” (For example, have you ever been walking down the street and thought you heard someone calling your name, but when you turned around no one was there?). Of course, there are many more causes of psychosis, but keeping it simple makes the principle easier to remember.
Regarding affective disorders, I point out to them, as I did in a previous column, that there is a huge difference between major depressive disorders, unhappiness, or sadness, grief, and demoralization. Regarding anxiety disorders, I let the medical students know that, like personality disorders, there is a lot of comorbidity. Yet, if they can distinguish brain damage, psychosis, and affective disorders from anxiety and personality disorders, that will be good enough.
In keeping with trying to help medical students not make assumptions, I always ask them what’s wrong with people who wash their hands 30 times a day. Invariably, the answer is obsessive-compulsive disorder. So, next I ask: Isn’t it possible that the person who washes his hands 30 times a day is a surgeon – or perhaps a patient with schizophrenia who thinks that Martians are beaming germs to his hands?
I guess I raise this issue because I am concerned with the future of psychiatry, and I think that my approach to medical school education provides a framework that can help students learn how to think about and provide care for psychiatric patients.
Dr. Bell is a staff psychiatrist at Jackson Park Hospital’s Medical/Surgical-Psychiatry Inpatient Unit in Chicago, clinical psychiatrist emeritus in the department of psychiatry at the University of Illinois at Chicago, former president/CEO of the Community Mental Health Council, and former director of the Institute for Juvenile Research (birthplace of child psychiatry), also in Chicago. If you have tricks of the medical school teaching trade that you would like to share, email Dr. Bell at cpnews@mededge.com.
I have the unfortunate task of trying to teach medical students about psychiatry. I say “unfortunate,” as most of them find psychiatry a difficult art to understand, and they seem reluctant to classify psychiatry as a branch of medicine.
In my efforts to keep things simple, I tell that them psychiatry is one of the most difficult branches of medicine as there are very few objective measures we can rely on to make sense of people’s behavior. Regrettably, the American Psychiatric Association’s Diagnostic and Statistical Manual only seems to confuse them more. So, I remind them that, in medicine, 90%-95% of diagnoses can be obtained from doing a good history, and, if we are lucky a drug level will show drugs in the system, a CT scan without contrast will show cerebral atrophy, or there will be a lab result that will be abnormal and point to a diagnosis. But mostly what they will be seeing is unusual behavior they are unable to classify.
So I identifiable brain damage, psychosis, affective disorders, anxiety disorders, and personality disorders. Under the brain damage category, I include the short- and long-term effects of drugs, major neurocognitive disorders (called dementia before DSM-5), cerebrovascular infarcts, traumatic brain injury, and neurodevelopmental disorders. For their exams and, if they are interested in psychiatry, I tell them to study the DSM. I explain to them that when I was in medical school my dermatology professor told us that if we could recognize the 10 most common dermatologic disorders, we would be able to recognize 90% of the skin disorders we would see. It is similar in psychiatry – thus, my five categories.
However, because I do not want them thinking that only schizophrenia causes psychosis, I let them know that at least 40 different factors cause people to be psychotic indicated by auditory hallucinations. Those 40 factors are: 1) acute alcohol intoxication, 2) alcohol withdrawal, 3) alcoholism, 4) Alzheimer’s disease, 5) benzodiazepine withdrawal, 6) cocaine abuse and addiction, 7) chemical poisoning, 8) dehydration, 9) delirium, 10) dissociative disorders, 11) electrolyte imbalances, 12) encephalopathy of various forms, 13) ecstasy, 14) extreme fatigue, 15) falling asleep, 16) fetal alcohol exposure, 17) grief, 18) hallucinogen use, 19) heroin abuse and dependence, 20) high fever, 21) hyperglycemia, 22) hypoglycemia, 23) intellectual disability, 24) lupus, 25) major depression, 26) mania, 27) methamphetamine use, 28) Parkinson’s disease, 29) phencyclidine, 30) postictal states, 31) posttraumatic stress disorder, 32) schizoid or schizotypal personality disorder, 33) schizophrenia, 34) sleep deprivation, 35) sleep paralysis, 36) solvent abuse, 37) traumatic brain injury, 38) temporal lobe epilepsy, 39) uremia. Lastly, I ask them about No. 40 – “normal” (For example, have you ever been walking down the street and thought you heard someone calling your name, but when you turned around no one was there?). Of course, there are many more causes of psychosis, but keeping it simple makes the principle easier to remember.
Regarding affective disorders, I point out to them, as I did in a previous column, that there is a huge difference between major depressive disorders, unhappiness, or sadness, grief, and demoralization. Regarding anxiety disorders, I let the medical students know that, like personality disorders, there is a lot of comorbidity. Yet, if they can distinguish brain damage, psychosis, and affective disorders from anxiety and personality disorders, that will be good enough.
In keeping with trying to help medical students not make assumptions, I always ask them what’s wrong with people who wash their hands 30 times a day. Invariably, the answer is obsessive-compulsive disorder. So, next I ask: Isn’t it possible that the person who washes his hands 30 times a day is a surgeon – or perhaps a patient with schizophrenia who thinks that Martians are beaming germs to his hands?
I guess I raise this issue because I am concerned with the future of psychiatry, and I think that my approach to medical school education provides a framework that can help students learn how to think about and provide care for psychiatric patients.
Dr. Bell is a staff psychiatrist at Jackson Park Hospital’s Medical/Surgical-Psychiatry Inpatient Unit in Chicago, clinical psychiatrist emeritus in the department of psychiatry at the University of Illinois at Chicago, former president/CEO of the Community Mental Health Council, and former director of the Institute for Juvenile Research (birthplace of child psychiatry), also in Chicago. If you have tricks of the medical school teaching trade that you would like to share, email Dr. Bell at cpnews@mededge.com.
I have the unfortunate task of trying to teach medical students about psychiatry. I say “unfortunate,” as most of them find psychiatry a difficult art to understand, and they seem reluctant to classify psychiatry as a branch of medicine.
In my efforts to keep things simple, I tell that them psychiatry is one of the most difficult branches of medicine as there are very few objective measures we can rely on to make sense of people’s behavior. Regrettably, the American Psychiatric Association’s Diagnostic and Statistical Manual only seems to confuse them more. So, I remind them that, in medicine, 90%-95% of diagnoses can be obtained from doing a good history, and, if we are lucky a drug level will show drugs in the system, a CT scan without contrast will show cerebral atrophy, or there will be a lab result that will be abnormal and point to a diagnosis. But mostly what they will be seeing is unusual behavior they are unable to classify.
So I identifiable brain damage, psychosis, affective disorders, anxiety disorders, and personality disorders. Under the brain damage category, I include the short- and long-term effects of drugs, major neurocognitive disorders (called dementia before DSM-5), cerebrovascular infarcts, traumatic brain injury, and neurodevelopmental disorders. For their exams and, if they are interested in psychiatry, I tell them to study the DSM. I explain to them that when I was in medical school my dermatology professor told us that if we could recognize the 10 most common dermatologic disorders, we would be able to recognize 90% of the skin disorders we would see. It is similar in psychiatry – thus, my five categories.
However, because I do not want them thinking that only schizophrenia causes psychosis, I let them know that at least 40 different factors cause people to be psychotic indicated by auditory hallucinations. Those 40 factors are: 1) acute alcohol intoxication, 2) alcohol withdrawal, 3) alcoholism, 4) Alzheimer’s disease, 5) benzodiazepine withdrawal, 6) cocaine abuse and addiction, 7) chemical poisoning, 8) dehydration, 9) delirium, 10) dissociative disorders, 11) electrolyte imbalances, 12) encephalopathy of various forms, 13) ecstasy, 14) extreme fatigue, 15) falling asleep, 16) fetal alcohol exposure, 17) grief, 18) hallucinogen use, 19) heroin abuse and dependence, 20) high fever, 21) hyperglycemia, 22) hypoglycemia, 23) intellectual disability, 24) lupus, 25) major depression, 26) mania, 27) methamphetamine use, 28) Parkinson’s disease, 29) phencyclidine, 30) postictal states, 31) posttraumatic stress disorder, 32) schizoid or schizotypal personality disorder, 33) schizophrenia, 34) sleep deprivation, 35) sleep paralysis, 36) solvent abuse, 37) traumatic brain injury, 38) temporal lobe epilepsy, 39) uremia. Lastly, I ask them about No. 40 – “normal” (For example, have you ever been walking down the street and thought you heard someone calling your name, but when you turned around no one was there?). Of course, there are many more causes of psychosis, but keeping it simple makes the principle easier to remember.
Regarding affective disorders, I point out to them, as I did in a previous column, that there is a huge difference between major depressive disorders, unhappiness, or sadness, grief, and demoralization. Regarding anxiety disorders, I let the medical students know that, like personality disorders, there is a lot of comorbidity. Yet, if they can distinguish brain damage, psychosis, and affective disorders from anxiety and personality disorders, that will be good enough.
In keeping with trying to help medical students not make assumptions, I always ask them what’s wrong with people who wash their hands 30 times a day. Invariably, the answer is obsessive-compulsive disorder. So, next I ask: Isn’t it possible that the person who washes his hands 30 times a day is a surgeon – or perhaps a patient with schizophrenia who thinks that Martians are beaming germs to his hands?
I guess I raise this issue because I am concerned with the future of psychiatry, and I think that my approach to medical school education provides a framework that can help students learn how to think about and provide care for psychiatric patients.
Dr. Bell is a staff psychiatrist at Jackson Park Hospital’s Medical/Surgical-Psychiatry Inpatient Unit in Chicago, clinical psychiatrist emeritus in the department of psychiatry at the University of Illinois at Chicago, former president/CEO of the Community Mental Health Council, and former director of the Institute for Juvenile Research (birthplace of child psychiatry), also in Chicago. If you have tricks of the medical school teaching trade that you would like to share, email Dr. Bell at cpnews@mededge.com.
USPSTF recommends counseling for perinatal depression prevention
according to a B recommendation from the U.S. Preventive Services Task Force.
The Task Force determined that counseling interventions are effective in preventing perinatal depression, defined as a major or minor depressive episode during pregnancy or within the first year after delivery. The condition affects an estimated 12% of new mothers in the United States each year, according to lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and her colleagues.
The recommendation, published in JAMA, applies to “pregnant persons and persons who are less than 1 year postpartum who do not have a current diagnosis of depression but are at increased risk of developing depression,” according to the authors (JAMA. 2019 Feb 12;321(6):580-7).
Risk factors for development of perinatal depression include:
- Past history of depression.
- Current depressive symptoms (that do not reach a diagnostic threshold).
- History of physical or sexual abuse.
- Unplanned or unwanted pregnancy.
- Stressful life events.
- Lack of social and financial support.
- Intimate partner violence.
- Pregestational or gestational diabetes.
- Complications during pregnancy.
- Adolescent parenthood.
- Low socioeconomic status.
- Lack of social support.
After reviewing the evidence, the USPSTF found a moderate net benefit for counseling interventions, particularly cognitive behavioral therapy and interpersonal therapy, for preventing perinatal depression in women at risk. Counseling sessions reviewed for this recommendation ranged from 4 to 20 meetings (median, 8 meetings).
The USPSTF found inadequate evidence to assess the harms and benefits of other noncounseling interventions, including pharmacologic therapy.
In the evidence review accompanying the recommendations, Elizabeth A. O’Connor, PhD, of Kaiser Permanente, Portland, Ore., and her colleagues analyzed data from 50 studies including 22,385 individuals; 20 of these studies were randomized, controlled trials of counseling interventions (JAMA. 2019 Feb 12;321(6):588-601).
Overall, the likelihood of perinatal depression was significantly lower among women who received counseling, compared with controls, among more than 3,000 women in those studies (pooled risk ratio 0.61). Absolute risk differences for perinatal depression ranged from a 1% increased reduction in controls to a 32% increased reduction among women who received counseling. The effects were strongest for cognitive behavioral therapy and interpersonal therapy as interventions. No adverse events were reported in the counseling intervention studies.
In three studies of health system interventions, the researchers found a benefit for interventions vs. controls, but the difference was not statistically significant.
Trials of most other alternative interventions including infant sleep advice, birth-experience postpartum debriefing, omega-3 fatty acid supplementation, expressive writing, antidepressants, and yoga did not show statistical significance in benefit for reducing perinatal depression.
Only one of three randomized controlled trials of physical activity found a statistically significant group difference.
A trial of nortriptyline to prevent perinatal depression showed no benefit, compared with placebo. A sertraline study of found “a smaller percentage of participants taking sertraline had a depression recurrence, compared with those taking placebo,” the investigators wrote. In these two studies, women who took nortriptyline showed no adverse effects, and those in a trial involving sertraline reported significantly more dizziness and drowsiness compared with placebo patients.
The evidence review was limited by the small number of quality studies, especially studies of alternative interventions. More research is needed; however, the findings support data from similar reviews and support the potential for counseling to prevent perinatal depression, particularly in women at increased risk for perinatal depression, Dr. O’Connor and her associates said.
The USPSTF is supported by the Agency for Healthcare Research and Quality. Coauthor Dr. Michelle L. Henninger reported receiving grants from Pfizer IGLC (Independent Grants for Learning & Change) outside the submitted work. Coauthor Dr. Bradley N. Gaynes reported receiving personal fees from LivaNova and Johnson & Johnson outside the submitted work. The remaining researchers had no financial conflicts to disclose.
SOURCE: Curry SJ et al. JAMA. 2019;321(6):580-7; O’Connor E et al. JAMA. 2019;321(6):588-601.
A proactive approach to prevention and management of perinatal depression as recommended by the USPSTF can potentially improve outcomes for new mothers and their babies, Marlene P. Freeman, MD, wrote in an accompanying editorial. She identified three key challenges to implementing the USPSTF recommendations: identifying women at risk, connecting them to evidence-based treatment, and assessing outcomes after treatment.
The development of screening tools would help clinicians identify women at risk for perinatal depression, Dr. Freeman said. No such tool currently exists, but in the meantime, “women at risk may be identified by targeting those with histories of depression, subthreshold depressive symptoms, and certain sociodemographic factors (i.e., economically disadvantaged, single/young, unplanned pregnancy).”
The counseling interventions shown to be effective in preventing perinatal depression – cognitive behavioral therapy and interpersonal psychotherapy – require education and training outside the time limitations and expertise of many clinicians providing obstetric care, she noted.
“The delivery of effective care on a large scale will require creative solutions,” such as the use of telehealth and smartphone platforms, and the involvement of multidisciplinary teams to care for women with severe illness, Dr. Freeman said. “In addition, a substantial number of reproductive-aged women have serious psychiatric disorders and will be identified as at risk for perinatal depression, although their needs may be more comprehensive,” she wrote. “Women who are identified as at risk for perinatal depression may have psychotic disorders, bipolar spectrum disorders, anxiety disorders, and substance use disorders, and there is comorbidity among psychiatric disorders. Therefore, systematic provisions for referral and treatment for other psychiatric disorders should be considered.” Further research is needed to explore treatment options including pharmacotherapy for women with severe psychiatric disorders.
However, she expressed optimism that the recommendations for screening and counseling for perinatal depression are valuable, and they “may return great dividends in the form of enhanced well-being of mothers and their offspring.”
Dr. Freeman is affiliated with the department of psychiatry at Massachusetts General Hospital, Boston. She commented in an editorial accompanying the article by Curry et al. (JAMA. 2019 Feb 12;321[6]:550-2). Dr. Freeman disclosed relationships with companies including Takeda, JayMac, Sage, Otsuka, Alkermes, Janssen, and Sunovion; she also disclosed serving on an independent data safety and monitoring committee for Janssen (Johnson & Johnson); and editing the GOED (Global Organization for EPA & DHA Omega-3) newsletter.
A proactive approach to prevention and management of perinatal depression as recommended by the USPSTF can potentially improve outcomes for new mothers and their babies, Marlene P. Freeman, MD, wrote in an accompanying editorial. She identified three key challenges to implementing the USPSTF recommendations: identifying women at risk, connecting them to evidence-based treatment, and assessing outcomes after treatment.
The development of screening tools would help clinicians identify women at risk for perinatal depression, Dr. Freeman said. No such tool currently exists, but in the meantime, “women at risk may be identified by targeting those with histories of depression, subthreshold depressive symptoms, and certain sociodemographic factors (i.e., economically disadvantaged, single/young, unplanned pregnancy).”
The counseling interventions shown to be effective in preventing perinatal depression – cognitive behavioral therapy and interpersonal psychotherapy – require education and training outside the time limitations and expertise of many clinicians providing obstetric care, she noted.
“The delivery of effective care on a large scale will require creative solutions,” such as the use of telehealth and smartphone platforms, and the involvement of multidisciplinary teams to care for women with severe illness, Dr. Freeman said. “In addition, a substantial number of reproductive-aged women have serious psychiatric disorders and will be identified as at risk for perinatal depression, although their needs may be more comprehensive,” she wrote. “Women who are identified as at risk for perinatal depression may have psychotic disorders, bipolar spectrum disorders, anxiety disorders, and substance use disorders, and there is comorbidity among psychiatric disorders. Therefore, systematic provisions for referral and treatment for other psychiatric disorders should be considered.” Further research is needed to explore treatment options including pharmacotherapy for women with severe psychiatric disorders.
However, she expressed optimism that the recommendations for screening and counseling for perinatal depression are valuable, and they “may return great dividends in the form of enhanced well-being of mothers and their offspring.”
Dr. Freeman is affiliated with the department of psychiatry at Massachusetts General Hospital, Boston. She commented in an editorial accompanying the article by Curry et al. (JAMA. 2019 Feb 12;321[6]:550-2). Dr. Freeman disclosed relationships with companies including Takeda, JayMac, Sage, Otsuka, Alkermes, Janssen, and Sunovion; she also disclosed serving on an independent data safety and monitoring committee for Janssen (Johnson & Johnson); and editing the GOED (Global Organization for EPA & DHA Omega-3) newsletter.
A proactive approach to prevention and management of perinatal depression as recommended by the USPSTF can potentially improve outcomes for new mothers and their babies, Marlene P. Freeman, MD, wrote in an accompanying editorial. She identified three key challenges to implementing the USPSTF recommendations: identifying women at risk, connecting them to evidence-based treatment, and assessing outcomes after treatment.
The development of screening tools would help clinicians identify women at risk for perinatal depression, Dr. Freeman said. No such tool currently exists, but in the meantime, “women at risk may be identified by targeting those with histories of depression, subthreshold depressive symptoms, and certain sociodemographic factors (i.e., economically disadvantaged, single/young, unplanned pregnancy).”
The counseling interventions shown to be effective in preventing perinatal depression – cognitive behavioral therapy and interpersonal psychotherapy – require education and training outside the time limitations and expertise of many clinicians providing obstetric care, she noted.
“The delivery of effective care on a large scale will require creative solutions,” such as the use of telehealth and smartphone platforms, and the involvement of multidisciplinary teams to care for women with severe illness, Dr. Freeman said. “In addition, a substantial number of reproductive-aged women have serious psychiatric disorders and will be identified as at risk for perinatal depression, although their needs may be more comprehensive,” she wrote. “Women who are identified as at risk for perinatal depression may have psychotic disorders, bipolar spectrum disorders, anxiety disorders, and substance use disorders, and there is comorbidity among psychiatric disorders. Therefore, systematic provisions for referral and treatment for other psychiatric disorders should be considered.” Further research is needed to explore treatment options including pharmacotherapy for women with severe psychiatric disorders.
However, she expressed optimism that the recommendations for screening and counseling for perinatal depression are valuable, and they “may return great dividends in the form of enhanced well-being of mothers and their offspring.”
Dr. Freeman is affiliated with the department of psychiatry at Massachusetts General Hospital, Boston. She commented in an editorial accompanying the article by Curry et al. (JAMA. 2019 Feb 12;321[6]:550-2). Dr. Freeman disclosed relationships with companies including Takeda, JayMac, Sage, Otsuka, Alkermes, Janssen, and Sunovion; she also disclosed serving on an independent data safety and monitoring committee for Janssen (Johnson & Johnson); and editing the GOED (Global Organization for EPA & DHA Omega-3) newsletter.
according to a B recommendation from the U.S. Preventive Services Task Force.
The Task Force determined that counseling interventions are effective in preventing perinatal depression, defined as a major or minor depressive episode during pregnancy or within the first year after delivery. The condition affects an estimated 12% of new mothers in the United States each year, according to lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and her colleagues.
The recommendation, published in JAMA, applies to “pregnant persons and persons who are less than 1 year postpartum who do not have a current diagnosis of depression but are at increased risk of developing depression,” according to the authors (JAMA. 2019 Feb 12;321(6):580-7).
Risk factors for development of perinatal depression include:
- Past history of depression.
- Current depressive symptoms (that do not reach a diagnostic threshold).
- History of physical or sexual abuse.
- Unplanned or unwanted pregnancy.
- Stressful life events.
- Lack of social and financial support.
- Intimate partner violence.
- Pregestational or gestational diabetes.
- Complications during pregnancy.
- Adolescent parenthood.
- Low socioeconomic status.
- Lack of social support.
After reviewing the evidence, the USPSTF found a moderate net benefit for counseling interventions, particularly cognitive behavioral therapy and interpersonal therapy, for preventing perinatal depression in women at risk. Counseling sessions reviewed for this recommendation ranged from 4 to 20 meetings (median, 8 meetings).
The USPSTF found inadequate evidence to assess the harms and benefits of other noncounseling interventions, including pharmacologic therapy.
In the evidence review accompanying the recommendations, Elizabeth A. O’Connor, PhD, of Kaiser Permanente, Portland, Ore., and her colleagues analyzed data from 50 studies including 22,385 individuals; 20 of these studies were randomized, controlled trials of counseling interventions (JAMA. 2019 Feb 12;321(6):588-601).
Overall, the likelihood of perinatal depression was significantly lower among women who received counseling, compared with controls, among more than 3,000 women in those studies (pooled risk ratio 0.61). Absolute risk differences for perinatal depression ranged from a 1% increased reduction in controls to a 32% increased reduction among women who received counseling. The effects were strongest for cognitive behavioral therapy and interpersonal therapy as interventions. No adverse events were reported in the counseling intervention studies.
In three studies of health system interventions, the researchers found a benefit for interventions vs. controls, but the difference was not statistically significant.
Trials of most other alternative interventions including infant sleep advice, birth-experience postpartum debriefing, omega-3 fatty acid supplementation, expressive writing, antidepressants, and yoga did not show statistical significance in benefit for reducing perinatal depression.
Only one of three randomized controlled trials of physical activity found a statistically significant group difference.
A trial of nortriptyline to prevent perinatal depression showed no benefit, compared with placebo. A sertraline study of found “a smaller percentage of participants taking sertraline had a depression recurrence, compared with those taking placebo,” the investigators wrote. In these two studies, women who took nortriptyline showed no adverse effects, and those in a trial involving sertraline reported significantly more dizziness and drowsiness compared with placebo patients.
The evidence review was limited by the small number of quality studies, especially studies of alternative interventions. More research is needed; however, the findings support data from similar reviews and support the potential for counseling to prevent perinatal depression, particularly in women at increased risk for perinatal depression, Dr. O’Connor and her associates said.
The USPSTF is supported by the Agency for Healthcare Research and Quality. Coauthor Dr. Michelle L. Henninger reported receiving grants from Pfizer IGLC (Independent Grants for Learning & Change) outside the submitted work. Coauthor Dr. Bradley N. Gaynes reported receiving personal fees from LivaNova and Johnson & Johnson outside the submitted work. The remaining researchers had no financial conflicts to disclose.
SOURCE: Curry SJ et al. JAMA. 2019;321(6):580-7; O’Connor E et al. JAMA. 2019;321(6):588-601.
according to a B recommendation from the U.S. Preventive Services Task Force.
The Task Force determined that counseling interventions are effective in preventing perinatal depression, defined as a major or minor depressive episode during pregnancy or within the first year after delivery. The condition affects an estimated 12% of new mothers in the United States each year, according to lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and her colleagues.
The recommendation, published in JAMA, applies to “pregnant persons and persons who are less than 1 year postpartum who do not have a current diagnosis of depression but are at increased risk of developing depression,” according to the authors (JAMA. 2019 Feb 12;321(6):580-7).
Risk factors for development of perinatal depression include:
- Past history of depression.
- Current depressive symptoms (that do not reach a diagnostic threshold).
- History of physical or sexual abuse.
- Unplanned or unwanted pregnancy.
- Stressful life events.
- Lack of social and financial support.
- Intimate partner violence.
- Pregestational or gestational diabetes.
- Complications during pregnancy.
- Adolescent parenthood.
- Low socioeconomic status.
- Lack of social support.
After reviewing the evidence, the USPSTF found a moderate net benefit for counseling interventions, particularly cognitive behavioral therapy and interpersonal therapy, for preventing perinatal depression in women at risk. Counseling sessions reviewed for this recommendation ranged from 4 to 20 meetings (median, 8 meetings).
The USPSTF found inadequate evidence to assess the harms and benefits of other noncounseling interventions, including pharmacologic therapy.
In the evidence review accompanying the recommendations, Elizabeth A. O’Connor, PhD, of Kaiser Permanente, Portland, Ore., and her colleagues analyzed data from 50 studies including 22,385 individuals; 20 of these studies were randomized, controlled trials of counseling interventions (JAMA. 2019 Feb 12;321(6):588-601).
Overall, the likelihood of perinatal depression was significantly lower among women who received counseling, compared with controls, among more than 3,000 women in those studies (pooled risk ratio 0.61). Absolute risk differences for perinatal depression ranged from a 1% increased reduction in controls to a 32% increased reduction among women who received counseling. The effects were strongest for cognitive behavioral therapy and interpersonal therapy as interventions. No adverse events were reported in the counseling intervention studies.
In three studies of health system interventions, the researchers found a benefit for interventions vs. controls, but the difference was not statistically significant.
Trials of most other alternative interventions including infant sleep advice, birth-experience postpartum debriefing, omega-3 fatty acid supplementation, expressive writing, antidepressants, and yoga did not show statistical significance in benefit for reducing perinatal depression.
Only one of three randomized controlled trials of physical activity found a statistically significant group difference.
A trial of nortriptyline to prevent perinatal depression showed no benefit, compared with placebo. A sertraline study of found “a smaller percentage of participants taking sertraline had a depression recurrence, compared with those taking placebo,” the investigators wrote. In these two studies, women who took nortriptyline showed no adverse effects, and those in a trial involving sertraline reported significantly more dizziness and drowsiness compared with placebo patients.
The evidence review was limited by the small number of quality studies, especially studies of alternative interventions. More research is needed; however, the findings support data from similar reviews and support the potential for counseling to prevent perinatal depression, particularly in women at increased risk for perinatal depression, Dr. O’Connor and her associates said.
The USPSTF is supported by the Agency for Healthcare Research and Quality. Coauthor Dr. Michelle L. Henninger reported receiving grants from Pfizer IGLC (Independent Grants for Learning & Change) outside the submitted work. Coauthor Dr. Bradley N. Gaynes reported receiving personal fees from LivaNova and Johnson & Johnson outside the submitted work. The remaining researchers had no financial conflicts to disclose.
SOURCE: Curry SJ et al. JAMA. 2019;321(6):580-7; O’Connor E et al. JAMA. 2019;321(6):588-601.
FROM JAMA
Mood and behavior are different targets for irritability in children
BROOKLYN, N.Y. – As a target of therapy in children with a psychiatric disorder, irritability expressed as grumpy mood or anger should be uncoupled from irritability expressed as threatening behavior, according to an exploration of this common clinical issue at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
“Irritability is like fever,” reported Gabrielle A. Carlson, MD, professor of psychiatry and pediatrics, State University of New York at Stony Brook. “It is a nonspecific symptom that only tells you that something is wrong.”
Irritability might be nothing more than a negative mood, but it also can be the source of explosive aggression, leading to tantrums and destructive behaviors, according to Dr. Carlson. She placed them into two different categories when considering treatment. Irritability leading to annoyance, grumpiness, withdrawal, or persistent anger is characterized as the “internalizing” or “tonic” form of the symptom. As opposed to the aggressive subtype, the tonic form is more closely associated with depression or anxiety. Irritability leading to extreme verbal outbursts or physical violence is characterized as the “externalizing” or “phasic” form, Dr. Carlson said. This type of irritability, defined by behavior more than mood, might signal disruptive mood dysregulation disorder (DMDD). But it is important to recognize that DMDD can overlap with other conditions, such as attention-deficit/hyperactivity disorder (ADHD), bipolar disorder, oppositional defiant disorder (ODD), and autism spectrum disorders.
In defining the impact of treatments on tonic versus phasic symptoms of irritability within the context of the underlying diagnoses, studies have not done a good job in separating relative effects on the two key forms of irritability, Dr. Carlson said.
“Irritability needs to be measured not only by how one feels but what one does,” said Dr. Carlson, explaining that the impact of therapy has not always been adequately described in therapy studies.
For the tonic form, irritability is likely to improve or resolve with control of the underlying psychiatric condition. Although this might also be true of the phasic form, this type of irritability often accompanies conditions that are less readily controlled even through the threat of self-harm, harm to others, or other destructive behaviors invites intervention specifically targeted at this symptom.
Unfortunately, the best approach to irritability is unclear for many underling pathologies.
“Clinicians should recognize that empirical evidence is still lacking as to aggression-targeted treatments with favorable benefit-risk profiles for children and adolescents with ADHD and severe aggression,” said Dr. Carlson, providing ADHD as one of several examples.
Psychological interventions, such as dialectical behavior therapy in children (DBT-C), have been associated with control of both tonic and phasic forms of irritability, but Dr. Carlson cautioned that few studies have adequately differentiated improvement in irritability as measured by behavior relative to mood. In addition, the baseline severity and the degree to which improvement meant adequate control have been unclear.
“Many psychological treatments are school based or group delivered, making it likely that patients are less impaired than explosive kids in psychiatry clinics and hospitals,” Dr. Carlson said.
Providing some practical tips for addressing the phasic form of irritability, She advised clinicians to “maximize the treatment of the base condition” but to add pharmacologic therapies to psychological interventions if symptoms persist.
“Our pendulum has swung from dishing out atypicals to eschewing them completely,” Dr. Carlson noted. Although she agreed these are no longer appropriate as first-line therapies, she suggested they might be employed judiciously if weight gain is monitored carefully.
“If they don’t work, stop them. If they do work, try to limit the duration of use,” Dr. Carlson said.
She reported having no relevant financial relationships to disclose.
BROOKLYN, N.Y. – As a target of therapy in children with a psychiatric disorder, irritability expressed as grumpy mood or anger should be uncoupled from irritability expressed as threatening behavior, according to an exploration of this common clinical issue at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
“Irritability is like fever,” reported Gabrielle A. Carlson, MD, professor of psychiatry and pediatrics, State University of New York at Stony Brook. “It is a nonspecific symptom that only tells you that something is wrong.”
Irritability might be nothing more than a negative mood, but it also can be the source of explosive aggression, leading to tantrums and destructive behaviors, according to Dr. Carlson. She placed them into two different categories when considering treatment. Irritability leading to annoyance, grumpiness, withdrawal, or persistent anger is characterized as the “internalizing” or “tonic” form of the symptom. As opposed to the aggressive subtype, the tonic form is more closely associated with depression or anxiety. Irritability leading to extreme verbal outbursts or physical violence is characterized as the “externalizing” or “phasic” form, Dr. Carlson said. This type of irritability, defined by behavior more than mood, might signal disruptive mood dysregulation disorder (DMDD). But it is important to recognize that DMDD can overlap with other conditions, such as attention-deficit/hyperactivity disorder (ADHD), bipolar disorder, oppositional defiant disorder (ODD), and autism spectrum disorders.
In defining the impact of treatments on tonic versus phasic symptoms of irritability within the context of the underlying diagnoses, studies have not done a good job in separating relative effects on the two key forms of irritability, Dr. Carlson said.
“Irritability needs to be measured not only by how one feels but what one does,” said Dr. Carlson, explaining that the impact of therapy has not always been adequately described in therapy studies.
For the tonic form, irritability is likely to improve or resolve with control of the underlying psychiatric condition. Although this might also be true of the phasic form, this type of irritability often accompanies conditions that are less readily controlled even through the threat of self-harm, harm to others, or other destructive behaviors invites intervention specifically targeted at this symptom.
Unfortunately, the best approach to irritability is unclear for many underling pathologies.
“Clinicians should recognize that empirical evidence is still lacking as to aggression-targeted treatments with favorable benefit-risk profiles for children and adolescents with ADHD and severe aggression,” said Dr. Carlson, providing ADHD as one of several examples.
Psychological interventions, such as dialectical behavior therapy in children (DBT-C), have been associated with control of both tonic and phasic forms of irritability, but Dr. Carlson cautioned that few studies have adequately differentiated improvement in irritability as measured by behavior relative to mood. In addition, the baseline severity and the degree to which improvement meant adequate control have been unclear.
“Many psychological treatments are school based or group delivered, making it likely that patients are less impaired than explosive kids in psychiatry clinics and hospitals,” Dr. Carlson said.
Providing some practical tips for addressing the phasic form of irritability, She advised clinicians to “maximize the treatment of the base condition” but to add pharmacologic therapies to psychological interventions if symptoms persist.
“Our pendulum has swung from dishing out atypicals to eschewing them completely,” Dr. Carlson noted. Although she agreed these are no longer appropriate as first-line therapies, she suggested they might be employed judiciously if weight gain is monitored carefully.
“If they don’t work, stop them. If they do work, try to limit the duration of use,” Dr. Carlson said.
She reported having no relevant financial relationships to disclose.
BROOKLYN, N.Y. – As a target of therapy in children with a psychiatric disorder, irritability expressed as grumpy mood or anger should be uncoupled from irritability expressed as threatening behavior, according to an exploration of this common clinical issue at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
“Irritability is like fever,” reported Gabrielle A. Carlson, MD, professor of psychiatry and pediatrics, State University of New York at Stony Brook. “It is a nonspecific symptom that only tells you that something is wrong.”
Irritability might be nothing more than a negative mood, but it also can be the source of explosive aggression, leading to tantrums and destructive behaviors, according to Dr. Carlson. She placed them into two different categories when considering treatment. Irritability leading to annoyance, grumpiness, withdrawal, or persistent anger is characterized as the “internalizing” or “tonic” form of the symptom. As opposed to the aggressive subtype, the tonic form is more closely associated with depression or anxiety. Irritability leading to extreme verbal outbursts or physical violence is characterized as the “externalizing” or “phasic” form, Dr. Carlson said. This type of irritability, defined by behavior more than mood, might signal disruptive mood dysregulation disorder (DMDD). But it is important to recognize that DMDD can overlap with other conditions, such as attention-deficit/hyperactivity disorder (ADHD), bipolar disorder, oppositional defiant disorder (ODD), and autism spectrum disorders.
In defining the impact of treatments on tonic versus phasic symptoms of irritability within the context of the underlying diagnoses, studies have not done a good job in separating relative effects on the two key forms of irritability, Dr. Carlson said.
“Irritability needs to be measured not only by how one feels but what one does,” said Dr. Carlson, explaining that the impact of therapy has not always been adequately described in therapy studies.
For the tonic form, irritability is likely to improve or resolve with control of the underlying psychiatric condition. Although this might also be true of the phasic form, this type of irritability often accompanies conditions that are less readily controlled even through the threat of self-harm, harm to others, or other destructive behaviors invites intervention specifically targeted at this symptom.
Unfortunately, the best approach to irritability is unclear for many underling pathologies.
“Clinicians should recognize that empirical evidence is still lacking as to aggression-targeted treatments with favorable benefit-risk profiles for children and adolescents with ADHD and severe aggression,” said Dr. Carlson, providing ADHD as one of several examples.
Psychological interventions, such as dialectical behavior therapy in children (DBT-C), have been associated with control of both tonic and phasic forms of irritability, but Dr. Carlson cautioned that few studies have adequately differentiated improvement in irritability as measured by behavior relative to mood. In addition, the baseline severity and the degree to which improvement meant adequate control have been unclear.
“Many psychological treatments are school based or group delivered, making it likely that patients are less impaired than explosive kids in psychiatry clinics and hospitals,” Dr. Carlson said.
Providing some practical tips for addressing the phasic form of irritability, She advised clinicians to “maximize the treatment of the base condition” but to add pharmacologic therapies to psychological interventions if symptoms persist.
“Our pendulum has swung from dishing out atypicals to eschewing them completely,” Dr. Carlson noted. Although she agreed these are no longer appropriate as first-line therapies, she suggested they might be employed judiciously if weight gain is monitored carefully.
“If they don’t work, stop them. If they do work, try to limit the duration of use,” Dr. Carlson said.
She reported having no relevant financial relationships to disclose.
REPORTING FROM The PSYCHOPHARMACOLOGY UPDATE INSTITUTE
The blinding lies of depression
Numb and empty, I continued to drive home in a daze. My mind focused only on the light ahead changing from yellow to red. “Remember to step on the brake,” commanded the internal boss to my stunned mind. No tears, I continued to drive as green blinked its eye.
Earlier that afternoon as I stepped out of my second outpatient appointment of the day, the office administrator’s assistant gingerly informed me, “The guy who answered the phone for your no-show said she passed.”
“Passed? Like … died?” I asked in shock.
She nodded. “I looked her up in the system. She passed away 2 Saturdays ago.”
That was only 2 days after the last time I met with her when we celebrated her progress.
“Too soon, too soon in your career,” my attending bemoaned as I shared the news.
Gathering my scattered wit, I smoothed my furrowed brow and forced a smile back into my eyes. I had other patients to see.
Continue to: Soothed by the hum of my car...
Soothed by the hum of my car, my mind replayed our last meeting where hope and active plans had replaced broken hopelessness. For the past 2 weeks, I had erroneously dismissed her no-shows as her volatile borderline personality’s decision to fire me. I was wrong.
Holding things together until a silly domestic dispute unleashed the brewing tornado inside, I stormed upstairs to contain the pain. Behind locked doors, my body shuddered from uncontrollable tears that blinded my eyes. She was the first patient I helped through psychotherapy and the first I lost through suicide.
The news of her death triggered anguish from past suicides of dear friends. Chopper, who blew off his face during our sophomore y
A few years later, another classmate, Aaron, sank into depression. He, too, shot himself. Just months before I’d received the call requesting my presence at his funeral, he had asked me if I would be his Valentine. Jokingly, I agreed, knowing our paths would never cross after our graduation. At his funeral, his parents insisted that I sat as a member of his immediate family.
Oh … the blinding lies of depression. Those who have fallen prey to suicide never knew the truth: Their lives and their deaths matter.
Even strangers weep.
Numb and empty, I continued to drive home in a daze. My mind focused only on the light ahead changing from yellow to red. “Remember to step on the brake,” commanded the internal boss to my stunned mind. No tears, I continued to drive as green blinked its eye.
Earlier that afternoon as I stepped out of my second outpatient appointment of the day, the office administrator’s assistant gingerly informed me, “The guy who answered the phone for your no-show said she passed.”
“Passed? Like … died?” I asked in shock.
She nodded. “I looked her up in the system. She passed away 2 Saturdays ago.”
That was only 2 days after the last time I met with her when we celebrated her progress.
“Too soon, too soon in your career,” my attending bemoaned as I shared the news.
Gathering my scattered wit, I smoothed my furrowed brow and forced a smile back into my eyes. I had other patients to see.
Continue to: Soothed by the hum of my car...
Soothed by the hum of my car, my mind replayed our last meeting where hope and active plans had replaced broken hopelessness. For the past 2 weeks, I had erroneously dismissed her no-shows as her volatile borderline personality’s decision to fire me. I was wrong.
Holding things together until a silly domestic dispute unleashed the brewing tornado inside, I stormed upstairs to contain the pain. Behind locked doors, my body shuddered from uncontrollable tears that blinded my eyes. She was the first patient I helped through psychotherapy and the first I lost through suicide.
The news of her death triggered anguish from past suicides of dear friends. Chopper, who blew off his face during our sophomore y
A few years later, another classmate, Aaron, sank into depression. He, too, shot himself. Just months before I’d received the call requesting my presence at his funeral, he had asked me if I would be his Valentine. Jokingly, I agreed, knowing our paths would never cross after our graduation. At his funeral, his parents insisted that I sat as a member of his immediate family.
Oh … the blinding lies of depression. Those who have fallen prey to suicide never knew the truth: Their lives and their deaths matter.
Even strangers weep.
Numb and empty, I continued to drive home in a daze. My mind focused only on the light ahead changing from yellow to red. “Remember to step on the brake,” commanded the internal boss to my stunned mind. No tears, I continued to drive as green blinked its eye.
Earlier that afternoon as I stepped out of my second outpatient appointment of the day, the office administrator’s assistant gingerly informed me, “The guy who answered the phone for your no-show said she passed.”
“Passed? Like … died?” I asked in shock.
She nodded. “I looked her up in the system. She passed away 2 Saturdays ago.”
That was only 2 days after the last time I met with her when we celebrated her progress.
“Too soon, too soon in your career,” my attending bemoaned as I shared the news.
Gathering my scattered wit, I smoothed my furrowed brow and forced a smile back into my eyes. I had other patients to see.
Continue to: Soothed by the hum of my car...
Soothed by the hum of my car, my mind replayed our last meeting where hope and active plans had replaced broken hopelessness. For the past 2 weeks, I had erroneously dismissed her no-shows as her volatile borderline personality’s decision to fire me. I was wrong.
Holding things together until a silly domestic dispute unleashed the brewing tornado inside, I stormed upstairs to contain the pain. Behind locked doors, my body shuddered from uncontrollable tears that blinded my eyes. She was the first patient I helped through psychotherapy and the first I lost through suicide.
The news of her death triggered anguish from past suicides of dear friends. Chopper, who blew off his face during our sophomore y
A few years later, another classmate, Aaron, sank into depression. He, too, shot himself. Just months before I’d received the call requesting my presence at his funeral, he had asked me if I would be his Valentine. Jokingly, I agreed, knowing our paths would never cross after our graduation. At his funeral, his parents insisted that I sat as a member of his immediate family.
Oh … the blinding lies of depression. Those who have fallen prey to suicide never knew the truth: Their lives and their deaths matter.
Even strangers weep.
Differentiating serotonin syndrome and neuroleptic malignant syndrome
Serotonin syndrome (SS) and neuroleptic malignant syndrome (NMS) are each rare psychiatric emergencies that can lead to fatal outcomes. Their clinical presentations can overlap, which can make it difficult to differentiate between the 2 syndromes; however, their treatments are distinct, and it is imperative to know how to identify symptoms and accurately diagnose each of them to provide appropriate intervention. This article summarizes the 2 syndromes and their treatments, with a focus on how clinicians can distinguish them, provide prompt intervention, and prevent occurrence.
Serotonin syndrome
Mechanism. The decarboxylation and hydroxylation of tryptophan forms serotonin, also known as 5-hydroxytryptamine (5-HT), which can then be metabolized by monoamine oxidase-A (MAO-A) into 5-hydroxyindoleacetic acid (5-HIAA).1Medications can disrupt this pathway of serotonin production or its metabolism, and result in excessive levels of serotonin, which subsequently leads to an overactivation of central and peripheral serotonin receptors.1 Increased receptor activation leads to further upregulation, and ultimately more serotonin transmission. This can be caused by monotherapy or use of multiple serotonergic agents, polypharmacy with a combination of medication classes, drug interactions, or overdose. The wide variety of medications often prescribed by different clinicians can make identification of excessive serotonergic activity difficult, especially because mood stabilizers such as lithium,2 and non-psychiatric medications such as
- inhibition of serotonin uptake (seen with selective serotonin reuptake inhibitors [SSRIs], serotonin-norepinephrine reuptake inhibitors [SNRIs], and tricyclic antidepressants [TCAs])
- inhibition of serotonin metabolism (seen with monoamine oxidase inhibitors [MAOIs])
- increased serotonin synthesis (seen with stimulants)
- increased serotonin release (seen with stimulants and opiates)
- activation of serotonin receptors (seen with lithium)
- inhibition of certain cytochrome P450 (CYP450) enzymes (seen with ciprofloxacin, fluconazole, etc.).
It is important to recognize that various serotonergic agents are involved in the CYP450 system. Inhibition of the CYP450 pathway by common antibiotics such as ciprofloxacin, or antifungals such as fluconazole, may result in an accumulation of serotonergic agents and place patients at increased risk for developing SS.
Clinical presentation. The clinical presentation of SS can range from mild to fatal. There is no specific laboratory test for diagnosis, although an elevation of the total creatine kinase (CK) and leukocyte count, as well as increased transaminase levels or lower bicarbonate levels, have been reported in the literature.4
Symptoms of SS generally present within 24 hours of starting/changing therapy and include a triad of mental status changes (altered mental status [AMS]), autonomic instability, and abnormalities of neuromuscular tone. Examples of AMS include agitation, anxiety, disorientation, and restlessness. Symptoms of autonomic instability include hypertension, tachycardia, tachypnea, hyperthermia, diaphoresis, flushed skin, vomiting, diarrhea, and arrhythmias. Symptoms stemming from changes in neuromuscular tone include tremors, clonus, hyperreflexia, and muscle rigidity.1 The multiple possible clinical presentations, as well as symptoms that overlap with those of other syndromes, can make SS difficult to recognize quickly in a clinical setting.
Diagnostic criteria. Sternbach’s diagnostic criteria for SS are defined as the presence of 3 or more of the 10 most common clinical features (Table 25). Due to concerns that Sternbach’s diagnostic criteria overemphasized an abnormal mental state (leading to possible confusion of SS with other AMS syndromes), the Hunter serotonin toxicity criteria6 (Figure6) were developed in 2003, and were found to be more sensitive and specific than Sternbach’s criteria. Both tools are often used in clinical practice.
Treatment. Treatment of SS begins with prompt discontinuation of all serotonergic agents. The intensity of treatment depends on the severity of the symptoms. Mild symptoms can be managed with supportive care,3 and in such cases, the syndrome generally resolves within 24 hours.7 Clinicians may use supportive care to normalize vital signs (oxygenation to maintain SpO2 >94%, IV fluids for volume depletion, cooling agents, antihypertensives, benzodiazepines for sedation or control of agitation, etc.). Patients who are more ill may require more aggressive treatment, such as the use of a serotonergic antagonist (ie, cyproheptadine) and those who are severely hyperthermic (temperature >41.1ºC) may require neuromuscular sedation, paralysis, and possibly endotracheal intubation.3
Continue to: Management pitfalls include...
Management pitfalls include misdiagnosis of SS, failure to recognize its rapid rate of progression, and adverse effects of pharmacologic therapy.3 The most effective treatment for SS is prevention. SS can be prevented by astute pharmacologic understanding, avoidance of polypharmacy, and physician education.3
Neuroleptic malignant syndrome
Possible mechanisms. Neuromuscular malignant syndrome is thought to result from dopamine receptor antagonism leading to a hypodopaminergic state in the striatum and hypothalamus.8 The pathophysiology behind NMS has not fully been elucidated; however, several hypotheses attempt to explain this life-threatening reaction. The first focuses on dopamine D2 receptor antagonism, because many of the neuroleptic (antipsychotic) medications that can precipitate NMS are involved in dopamine blockade. In this theory, blocking dopamine D2 receptors in the anterior hypothalamus explains the hyperthermia seen in NMS, while blockade in the corpus striatum is believed to lead to muscle rigidity.9
The second hypothesis suggests that neuroleptics may have a direct toxic effect to muscle cells. Neuroleptics influence calcium transport across the sarcoplasmic reticulum and can lead to increased calcium release, which may contribute to the muscle rigidity and hyperthermia seen in NMS.9
The third hypothesis involves hyperactivity of the sympathetic nervous system; it is thought that psychologic stressors alter frontal lobe function, with neuroleptics disrupting the inhibitory pathways of the sympathetic nervous system. The autonomic nervous system innervates multiple organ systems, so this excessively dysregulated sympathetic nervous system may be responsible for multiple NMS symptoms (hyperthermia, muscle rigidity, hypertension, diaphoresis, tachycardia, elevated CK.10
NMS can be caused by neuroleptic agents (both first- and second-generation antipsychotics) as well as antiemetics (Table 31). The time between use of these medications and onset of symptoms is highly variable. NMS can occur after a single dose, after a dose adjustment, or possibly after years of treatment with the same medication. It is not dose-dependent.11 In certain individuals, NMS may occur at therapeutic doses.
Continue to: Clinical presentation
Clinical presentation. Patients with NMS typically present with a tetrad of symptoms: mental status changes, muscular rigidity, hyperthermia, and autonomic instability.12 Mental status changes can include confusion and agitation, as well as catatonic signs and mutism. The muscular rigidity of NMS is characterized by “lead pipe rigidity” and may be accompanied by tremor, dystonia, or dyskinesias. Laboratory findings include elevated serum CK (from severe rigidity), often >1,000 U/L, although normal levels can be observed if rigidity has not yet developed.13
Treatment. The first step for treatment is to discontinue the causative medication.14 Initiate supportive therapy immediately to restrict the progression of symptoms. Interventions include cooling blankets, fluid resuscitation, and antihypertensives to maintain autonomic stability15 or benzodiazepines to control agitation. In severe cases, muscular rigidity may extend to the airways and intubation may be required. The severity of these symptoms may warrant admission to the ICU for close monitoring. Pharmacologic treatment with
Differentiating between SS and NMS
Differentiating between these 2 syndromes (Table 417) is critical to direct appropriate intervention. Table 517 outlines the treatment overview for SS and NMS.
Detailed history. A detailed history is imperative in making accurate diagnoses. Useful components of the history include a patient’s duration of symptoms and medication history (prescription medications as well as over-the-counter medications, supplements, and illicit drugs). Also assess for medical comorbidities, because certain medical diagnoses may alert the clinician that it is likely the patient had been prescribed serotonergic agents or neuroleptics, and renal or liver impairment may alert the clinician of decreased metabolism rates. Medication history is arguably the most useful piece of the interview, because serotonergic agents can cause SS, whereas dopamine blockers cause NMS. It should be noted that excess serotonin acts as a true toxidrome and is concentration-dependent in causing SS, whereas NMS is an idiosyncratic reaction to a drug.
Physical exam. Although there are many overlapping clinical manifestations, SS produces neuromuscular hyperactivity (ie, clonus, hyperreflexia), whereas NMS is characterized by more sluggish responses (ie, rigidity, bradyreflexia).18
Continue to: Laboratory findings
Laboratory findings. Overlap between NMS and SS also occurs with lab findings; both syndromes can result in leukocytosis, elevated CK from muscle damage, and low serum iron levels. However, these findings are more commonly associated with NMS and are seen in 75% of cases.17,19
Course of illness. Duration of symptoms can also help differentiate the 2 syndromes. SS typically develops within 24 hours of starting/changing therapy, whereas NMS symptoms can be present for days to weeks. Resolution of symptoms may also be helpful in differentiation because SS typically resolves within a few days of initiating treatment, whereas NMS resolves within 9 to 14 days of starting treatment.19
Bottom Line
The clinical presentations of serotonin syndrome (SS) and neuroleptic malignant syndrome (NMS) overlap, which can make them difficult to differentiate; however, they each have distinct approaches to treatment. Features in SS that are distinct from NMS include a history of serotonergic agents, rapid onset of symptoms, hyperreflexia, and clonus. NMS is slower in onset and can be found in patients who are prescribed dopamine antagonists, with distinct symptoms of rigidity and hyporeflexia.
Related Resources
- Kimmel R. Serotonin syndrome or NMS? Clues to diagnosis. Current Psychiatry. 2010;9(2):92.
- Strawn JR, Keck Jr PE, Caroff SN. Neuroleptic malignant syndrome: Answers to 6 tough questions. Current Psychiatry. 2008;7(1):95-101.
Drug Brand Names
Amantadine • Symmetrel
Amitriptyline • Elavil, Endep
Aripiprazole • Abilify
Bromocriptine • Cycloset, Parlodel
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Ciprofloxacin • Cipro
Citalopram • Celexa
Clomipramine • Anafranil
Clozapine • Clozaril
Cyclobenzaprine • Amrix, Flexeril
Cyproheptadine • Periactin
Dantrolene • Dantrium
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextromethorphan • Benylin, Dexalone
Dolasetron • Anzemet
Doxepin • Silenor
Droperidol • Inapsine
Duloxetine • Cymbalt
Escitalopram • Lexapro
Fentanyl • Actiq, Duragesic
Fluconazole • Diflucan
Fluoxetine • Prozac
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Granisetron • Kytril
Haloperidol • Haldol
Isocarboxazid • Marplan
Levomilnacipran • Fetzima
Linezolid • Zyvox
Lithium • Eskalith, Lithobid
Meperidone • Demerol
Metoclopramide • Reglan
Milnacipran • Savella
Nefazodone • Serzone
Olanzapine • Zyprexa
Ondansetron • Zofran
Paliperidone • Invega
Palonosetron • Aloxi
Paroxetine • Paxil
Pentazocine • Talwin, Talacen
Perphenazine • Trilafon
Phenelzine • Nardil
Procarbazine • Matulane
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Safinamide • Xadago
Selegiline • Eldepryl, Zelapar
Sertraline • Zoloft
Sibutramine • Meridia
Tedizolid • Sivextro
Thioridazine • Mellaril
Tranylcypromine • Parnate
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Valproate • Depacon
Ziprasidone • Geodon
1. Volpi-Abadie J, Kaye AM, Kaye AD. Serotonin syndrome. Ochsner J. 2013;13(4):533-540.
2. Werneke U, Jamshidi F, Taylor D, et al. Conundrums in neurology: diagnosing serotonin syndrome – a meta-analysis of cases. BMC Neurol. 2016;16:97.
3. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
4. Birmes P, Coppin D, Schmitt L, et al. Serotonin syndrome: a brief review. CMAJ. 2003;168(11):1439-1442.
5. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148:705-713.
6. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter serotonin toxicity criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003; 96(9):635-642.
7. Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331(15):1021-1022.
8. Nisijima K. Serotonin syndrome overlapping with neuroleptic malignant syndrome: A case report and approaches for differentially diagnosing the two syndromes. Asian J Psychiatr. 2015;18:100-101.
9. Adnet P, Lestavel P, Krivosic-Horber R. Neuroleptic malignant syndrome. Br J Anaesth. 2000;85(1):129-135.
10. Gurrera R. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry. 1999;156:169-180.
11. Pope HG Jr, Aizley HG, Keck PE Jr, et al. Neuroleptic malignant syndrome: long-term follow-up of 20 cases. J Clin Psychiatry. 1991;52(5):208-212.
12. Velamoor VR, Norman RM, Caroff SN, et al. Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis. 1994;182(3):168-173.
13. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am. 1993;77(1):185-202.
14. Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973-981.
15. San Gabriel MC, Eddula-Changala B, Tan Y, et al. Electroconvulsive in a schizophrenic patient with neuroleptic malignant syndrome and rhabdomyolysis. J ECT. 2015;31(3):197-200.
16. Buggenhout S, Vandenberghe J, Sienaert P. Electroconvulsion therapy for neuroleptic malignant syndrome. Tijdschr Psychiatr. 2014;56(9):612-615.
17. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
18. Mills KC. Serotonin syndrome. A clinical update. Crit Care Clin. 1997;13(4):763-783.
19. Dosi R, Ambaliya A, Joshi H, et al. Serotonin syndrome versus neuroleptic malignant syndrome: a challenge clinical quandary. BMJ Case Rep. 2014;2014:bcr201404154. doi:10.1136/bcr-2014-204154.
Serotonin syndrome (SS) and neuroleptic malignant syndrome (NMS) are each rare psychiatric emergencies that can lead to fatal outcomes. Their clinical presentations can overlap, which can make it difficult to differentiate between the 2 syndromes; however, their treatments are distinct, and it is imperative to know how to identify symptoms and accurately diagnose each of them to provide appropriate intervention. This article summarizes the 2 syndromes and their treatments, with a focus on how clinicians can distinguish them, provide prompt intervention, and prevent occurrence.
Serotonin syndrome
Mechanism. The decarboxylation and hydroxylation of tryptophan forms serotonin, also known as 5-hydroxytryptamine (5-HT), which can then be metabolized by monoamine oxidase-A (MAO-A) into 5-hydroxyindoleacetic acid (5-HIAA).1Medications can disrupt this pathway of serotonin production or its metabolism, and result in excessive levels of serotonin, which subsequently leads to an overactivation of central and peripheral serotonin receptors.1 Increased receptor activation leads to further upregulation, and ultimately more serotonin transmission. This can be caused by monotherapy or use of multiple serotonergic agents, polypharmacy with a combination of medication classes, drug interactions, or overdose. The wide variety of medications often prescribed by different clinicians can make identification of excessive serotonergic activity difficult, especially because mood stabilizers such as lithium,2 and non-psychiatric medications such as
- inhibition of serotonin uptake (seen with selective serotonin reuptake inhibitors [SSRIs], serotonin-norepinephrine reuptake inhibitors [SNRIs], and tricyclic antidepressants [TCAs])
- inhibition of serotonin metabolism (seen with monoamine oxidase inhibitors [MAOIs])
- increased serotonin synthesis (seen with stimulants)
- increased serotonin release (seen with stimulants and opiates)
- activation of serotonin receptors (seen with lithium)
- inhibition of certain cytochrome P450 (CYP450) enzymes (seen with ciprofloxacin, fluconazole, etc.).
It is important to recognize that various serotonergic agents are involved in the CYP450 system. Inhibition of the CYP450 pathway by common antibiotics such as ciprofloxacin, or antifungals such as fluconazole, may result in an accumulation of serotonergic agents and place patients at increased risk for developing SS.
Clinical presentation. The clinical presentation of SS can range from mild to fatal. There is no specific laboratory test for diagnosis, although an elevation of the total creatine kinase (CK) and leukocyte count, as well as increased transaminase levels or lower bicarbonate levels, have been reported in the literature.4
Symptoms of SS generally present within 24 hours of starting/changing therapy and include a triad of mental status changes (altered mental status [AMS]), autonomic instability, and abnormalities of neuromuscular tone. Examples of AMS include agitation, anxiety, disorientation, and restlessness. Symptoms of autonomic instability include hypertension, tachycardia, tachypnea, hyperthermia, diaphoresis, flushed skin, vomiting, diarrhea, and arrhythmias. Symptoms stemming from changes in neuromuscular tone include tremors, clonus, hyperreflexia, and muscle rigidity.1 The multiple possible clinical presentations, as well as symptoms that overlap with those of other syndromes, can make SS difficult to recognize quickly in a clinical setting.
Diagnostic criteria. Sternbach’s diagnostic criteria for SS are defined as the presence of 3 or more of the 10 most common clinical features (Table 25). Due to concerns that Sternbach’s diagnostic criteria overemphasized an abnormal mental state (leading to possible confusion of SS with other AMS syndromes), the Hunter serotonin toxicity criteria6 (Figure6) were developed in 2003, and were found to be more sensitive and specific than Sternbach’s criteria. Both tools are often used in clinical practice.
Treatment. Treatment of SS begins with prompt discontinuation of all serotonergic agents. The intensity of treatment depends on the severity of the symptoms. Mild symptoms can be managed with supportive care,3 and in such cases, the syndrome generally resolves within 24 hours.7 Clinicians may use supportive care to normalize vital signs (oxygenation to maintain SpO2 >94%, IV fluids for volume depletion, cooling agents, antihypertensives, benzodiazepines for sedation or control of agitation, etc.). Patients who are more ill may require more aggressive treatment, such as the use of a serotonergic antagonist (ie, cyproheptadine) and those who are severely hyperthermic (temperature >41.1ºC) may require neuromuscular sedation, paralysis, and possibly endotracheal intubation.3
Continue to: Management pitfalls include...
Management pitfalls include misdiagnosis of SS, failure to recognize its rapid rate of progression, and adverse effects of pharmacologic therapy.3 The most effective treatment for SS is prevention. SS can be prevented by astute pharmacologic understanding, avoidance of polypharmacy, and physician education.3
Neuroleptic malignant syndrome
Possible mechanisms. Neuromuscular malignant syndrome is thought to result from dopamine receptor antagonism leading to a hypodopaminergic state in the striatum and hypothalamus.8 The pathophysiology behind NMS has not fully been elucidated; however, several hypotheses attempt to explain this life-threatening reaction. The first focuses on dopamine D2 receptor antagonism, because many of the neuroleptic (antipsychotic) medications that can precipitate NMS are involved in dopamine blockade. In this theory, blocking dopamine D2 receptors in the anterior hypothalamus explains the hyperthermia seen in NMS, while blockade in the corpus striatum is believed to lead to muscle rigidity.9
The second hypothesis suggests that neuroleptics may have a direct toxic effect to muscle cells. Neuroleptics influence calcium transport across the sarcoplasmic reticulum and can lead to increased calcium release, which may contribute to the muscle rigidity and hyperthermia seen in NMS.9
The third hypothesis involves hyperactivity of the sympathetic nervous system; it is thought that psychologic stressors alter frontal lobe function, with neuroleptics disrupting the inhibitory pathways of the sympathetic nervous system. The autonomic nervous system innervates multiple organ systems, so this excessively dysregulated sympathetic nervous system may be responsible for multiple NMS symptoms (hyperthermia, muscle rigidity, hypertension, diaphoresis, tachycardia, elevated CK.10
NMS can be caused by neuroleptic agents (both first- and second-generation antipsychotics) as well as antiemetics (Table 31). The time between use of these medications and onset of symptoms is highly variable. NMS can occur after a single dose, after a dose adjustment, or possibly after years of treatment with the same medication. It is not dose-dependent.11 In certain individuals, NMS may occur at therapeutic doses.
Continue to: Clinical presentation
Clinical presentation. Patients with NMS typically present with a tetrad of symptoms: mental status changes, muscular rigidity, hyperthermia, and autonomic instability.12 Mental status changes can include confusion and agitation, as well as catatonic signs and mutism. The muscular rigidity of NMS is characterized by “lead pipe rigidity” and may be accompanied by tremor, dystonia, or dyskinesias. Laboratory findings include elevated serum CK (from severe rigidity), often >1,000 U/L, although normal levels can be observed if rigidity has not yet developed.13
Treatment. The first step for treatment is to discontinue the causative medication.14 Initiate supportive therapy immediately to restrict the progression of symptoms. Interventions include cooling blankets, fluid resuscitation, and antihypertensives to maintain autonomic stability15 or benzodiazepines to control agitation. In severe cases, muscular rigidity may extend to the airways and intubation may be required. The severity of these symptoms may warrant admission to the ICU for close monitoring. Pharmacologic treatment with
Differentiating between SS and NMS
Differentiating between these 2 syndromes (Table 417) is critical to direct appropriate intervention. Table 517 outlines the treatment overview for SS and NMS.
Detailed history. A detailed history is imperative in making accurate diagnoses. Useful components of the history include a patient’s duration of symptoms and medication history (prescription medications as well as over-the-counter medications, supplements, and illicit drugs). Also assess for medical comorbidities, because certain medical diagnoses may alert the clinician that it is likely the patient had been prescribed serotonergic agents or neuroleptics, and renal or liver impairment may alert the clinician of decreased metabolism rates. Medication history is arguably the most useful piece of the interview, because serotonergic agents can cause SS, whereas dopamine blockers cause NMS. It should be noted that excess serotonin acts as a true toxidrome and is concentration-dependent in causing SS, whereas NMS is an idiosyncratic reaction to a drug.
Physical exam. Although there are many overlapping clinical manifestations, SS produces neuromuscular hyperactivity (ie, clonus, hyperreflexia), whereas NMS is characterized by more sluggish responses (ie, rigidity, bradyreflexia).18
Continue to: Laboratory findings
Laboratory findings. Overlap between NMS and SS also occurs with lab findings; both syndromes can result in leukocytosis, elevated CK from muscle damage, and low serum iron levels. However, these findings are more commonly associated with NMS and are seen in 75% of cases.17,19
Course of illness. Duration of symptoms can also help differentiate the 2 syndromes. SS typically develops within 24 hours of starting/changing therapy, whereas NMS symptoms can be present for days to weeks. Resolution of symptoms may also be helpful in differentiation because SS typically resolves within a few days of initiating treatment, whereas NMS resolves within 9 to 14 days of starting treatment.19
Bottom Line
The clinical presentations of serotonin syndrome (SS) and neuroleptic malignant syndrome (NMS) overlap, which can make them difficult to differentiate; however, they each have distinct approaches to treatment. Features in SS that are distinct from NMS include a history of serotonergic agents, rapid onset of symptoms, hyperreflexia, and clonus. NMS is slower in onset and can be found in patients who are prescribed dopamine antagonists, with distinct symptoms of rigidity and hyporeflexia.
Related Resources
- Kimmel R. Serotonin syndrome or NMS? Clues to diagnosis. Current Psychiatry. 2010;9(2):92.
- Strawn JR, Keck Jr PE, Caroff SN. Neuroleptic malignant syndrome: Answers to 6 tough questions. Current Psychiatry. 2008;7(1):95-101.
Drug Brand Names
Amantadine • Symmetrel
Amitriptyline • Elavil, Endep
Aripiprazole • Abilify
Bromocriptine • Cycloset, Parlodel
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Ciprofloxacin • Cipro
Citalopram • Celexa
Clomipramine • Anafranil
Clozapine • Clozaril
Cyclobenzaprine • Amrix, Flexeril
Cyproheptadine • Periactin
Dantrolene • Dantrium
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextromethorphan • Benylin, Dexalone
Dolasetron • Anzemet
Doxepin • Silenor
Droperidol • Inapsine
Duloxetine • Cymbalt
Escitalopram • Lexapro
Fentanyl • Actiq, Duragesic
Fluconazole • Diflucan
Fluoxetine • Prozac
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Granisetron • Kytril
Haloperidol • Haldol
Isocarboxazid • Marplan
Levomilnacipran • Fetzima
Linezolid • Zyvox
Lithium • Eskalith, Lithobid
Meperidone • Demerol
Metoclopramide • Reglan
Milnacipran • Savella
Nefazodone • Serzone
Olanzapine • Zyprexa
Ondansetron • Zofran
Paliperidone • Invega
Palonosetron • Aloxi
Paroxetine • Paxil
Pentazocine • Talwin, Talacen
Perphenazine • Trilafon
Phenelzine • Nardil
Procarbazine • Matulane
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Safinamide • Xadago
Selegiline • Eldepryl, Zelapar
Sertraline • Zoloft
Sibutramine • Meridia
Tedizolid • Sivextro
Thioridazine • Mellaril
Tranylcypromine • Parnate
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Valproate • Depacon
Ziprasidone • Geodon
Serotonin syndrome (SS) and neuroleptic malignant syndrome (NMS) are each rare psychiatric emergencies that can lead to fatal outcomes. Their clinical presentations can overlap, which can make it difficult to differentiate between the 2 syndromes; however, their treatments are distinct, and it is imperative to know how to identify symptoms and accurately diagnose each of them to provide appropriate intervention. This article summarizes the 2 syndromes and their treatments, with a focus on how clinicians can distinguish them, provide prompt intervention, and prevent occurrence.
Serotonin syndrome
Mechanism. The decarboxylation and hydroxylation of tryptophan forms serotonin, also known as 5-hydroxytryptamine (5-HT), which can then be metabolized by monoamine oxidase-A (MAO-A) into 5-hydroxyindoleacetic acid (5-HIAA).1Medications can disrupt this pathway of serotonin production or its metabolism, and result in excessive levels of serotonin, which subsequently leads to an overactivation of central and peripheral serotonin receptors.1 Increased receptor activation leads to further upregulation, and ultimately more serotonin transmission. This can be caused by monotherapy or use of multiple serotonergic agents, polypharmacy with a combination of medication classes, drug interactions, or overdose. The wide variety of medications often prescribed by different clinicians can make identification of excessive serotonergic activity difficult, especially because mood stabilizers such as lithium,2 and non-psychiatric medications such as
- inhibition of serotonin uptake (seen with selective serotonin reuptake inhibitors [SSRIs], serotonin-norepinephrine reuptake inhibitors [SNRIs], and tricyclic antidepressants [TCAs])
- inhibition of serotonin metabolism (seen with monoamine oxidase inhibitors [MAOIs])
- increased serotonin synthesis (seen with stimulants)
- increased serotonin release (seen with stimulants and opiates)
- activation of serotonin receptors (seen with lithium)
- inhibition of certain cytochrome P450 (CYP450) enzymes (seen with ciprofloxacin, fluconazole, etc.).
It is important to recognize that various serotonergic agents are involved in the CYP450 system. Inhibition of the CYP450 pathway by common antibiotics such as ciprofloxacin, or antifungals such as fluconazole, may result in an accumulation of serotonergic agents and place patients at increased risk for developing SS.
Clinical presentation. The clinical presentation of SS can range from mild to fatal. There is no specific laboratory test for diagnosis, although an elevation of the total creatine kinase (CK) and leukocyte count, as well as increased transaminase levels or lower bicarbonate levels, have been reported in the literature.4
Symptoms of SS generally present within 24 hours of starting/changing therapy and include a triad of mental status changes (altered mental status [AMS]), autonomic instability, and abnormalities of neuromuscular tone. Examples of AMS include agitation, anxiety, disorientation, and restlessness. Symptoms of autonomic instability include hypertension, tachycardia, tachypnea, hyperthermia, diaphoresis, flushed skin, vomiting, diarrhea, and arrhythmias. Symptoms stemming from changes in neuromuscular tone include tremors, clonus, hyperreflexia, and muscle rigidity.1 The multiple possible clinical presentations, as well as symptoms that overlap with those of other syndromes, can make SS difficult to recognize quickly in a clinical setting.
Diagnostic criteria. Sternbach’s diagnostic criteria for SS are defined as the presence of 3 or more of the 10 most common clinical features (Table 25). Due to concerns that Sternbach’s diagnostic criteria overemphasized an abnormal mental state (leading to possible confusion of SS with other AMS syndromes), the Hunter serotonin toxicity criteria6 (Figure6) were developed in 2003, and were found to be more sensitive and specific than Sternbach’s criteria. Both tools are often used in clinical practice.
Treatment. Treatment of SS begins with prompt discontinuation of all serotonergic agents. The intensity of treatment depends on the severity of the symptoms. Mild symptoms can be managed with supportive care,3 and in such cases, the syndrome generally resolves within 24 hours.7 Clinicians may use supportive care to normalize vital signs (oxygenation to maintain SpO2 >94%, IV fluids for volume depletion, cooling agents, antihypertensives, benzodiazepines for sedation or control of agitation, etc.). Patients who are more ill may require more aggressive treatment, such as the use of a serotonergic antagonist (ie, cyproheptadine) and those who are severely hyperthermic (temperature >41.1ºC) may require neuromuscular sedation, paralysis, and possibly endotracheal intubation.3
Continue to: Management pitfalls include...
Management pitfalls include misdiagnosis of SS, failure to recognize its rapid rate of progression, and adverse effects of pharmacologic therapy.3 The most effective treatment for SS is prevention. SS can be prevented by astute pharmacologic understanding, avoidance of polypharmacy, and physician education.3
Neuroleptic malignant syndrome
Possible mechanisms. Neuromuscular malignant syndrome is thought to result from dopamine receptor antagonism leading to a hypodopaminergic state in the striatum and hypothalamus.8 The pathophysiology behind NMS has not fully been elucidated; however, several hypotheses attempt to explain this life-threatening reaction. The first focuses on dopamine D2 receptor antagonism, because many of the neuroleptic (antipsychotic) medications that can precipitate NMS are involved in dopamine blockade. In this theory, blocking dopamine D2 receptors in the anterior hypothalamus explains the hyperthermia seen in NMS, while blockade in the corpus striatum is believed to lead to muscle rigidity.9
The second hypothesis suggests that neuroleptics may have a direct toxic effect to muscle cells. Neuroleptics influence calcium transport across the sarcoplasmic reticulum and can lead to increased calcium release, which may contribute to the muscle rigidity and hyperthermia seen in NMS.9
The third hypothesis involves hyperactivity of the sympathetic nervous system; it is thought that psychologic stressors alter frontal lobe function, with neuroleptics disrupting the inhibitory pathways of the sympathetic nervous system. The autonomic nervous system innervates multiple organ systems, so this excessively dysregulated sympathetic nervous system may be responsible for multiple NMS symptoms (hyperthermia, muscle rigidity, hypertension, diaphoresis, tachycardia, elevated CK.10
NMS can be caused by neuroleptic agents (both first- and second-generation antipsychotics) as well as antiemetics (Table 31). The time between use of these medications and onset of symptoms is highly variable. NMS can occur after a single dose, after a dose adjustment, or possibly after years of treatment with the same medication. It is not dose-dependent.11 In certain individuals, NMS may occur at therapeutic doses.
Continue to: Clinical presentation
Clinical presentation. Patients with NMS typically present with a tetrad of symptoms: mental status changes, muscular rigidity, hyperthermia, and autonomic instability.12 Mental status changes can include confusion and agitation, as well as catatonic signs and mutism. The muscular rigidity of NMS is characterized by “lead pipe rigidity” and may be accompanied by tremor, dystonia, or dyskinesias. Laboratory findings include elevated serum CK (from severe rigidity), often >1,000 U/L, although normal levels can be observed if rigidity has not yet developed.13
Treatment. The first step for treatment is to discontinue the causative medication.14 Initiate supportive therapy immediately to restrict the progression of symptoms. Interventions include cooling blankets, fluid resuscitation, and antihypertensives to maintain autonomic stability15 or benzodiazepines to control agitation. In severe cases, muscular rigidity may extend to the airways and intubation may be required. The severity of these symptoms may warrant admission to the ICU for close monitoring. Pharmacologic treatment with
Differentiating between SS and NMS
Differentiating between these 2 syndromes (Table 417) is critical to direct appropriate intervention. Table 517 outlines the treatment overview for SS and NMS.
Detailed history. A detailed history is imperative in making accurate diagnoses. Useful components of the history include a patient’s duration of symptoms and medication history (prescription medications as well as over-the-counter medications, supplements, and illicit drugs). Also assess for medical comorbidities, because certain medical diagnoses may alert the clinician that it is likely the patient had been prescribed serotonergic agents or neuroleptics, and renal or liver impairment may alert the clinician of decreased metabolism rates. Medication history is arguably the most useful piece of the interview, because serotonergic agents can cause SS, whereas dopamine blockers cause NMS. It should be noted that excess serotonin acts as a true toxidrome and is concentration-dependent in causing SS, whereas NMS is an idiosyncratic reaction to a drug.
Physical exam. Although there are many overlapping clinical manifestations, SS produces neuromuscular hyperactivity (ie, clonus, hyperreflexia), whereas NMS is characterized by more sluggish responses (ie, rigidity, bradyreflexia).18
Continue to: Laboratory findings
Laboratory findings. Overlap between NMS and SS also occurs with lab findings; both syndromes can result in leukocytosis, elevated CK from muscle damage, and low serum iron levels. However, these findings are more commonly associated with NMS and are seen in 75% of cases.17,19
Course of illness. Duration of symptoms can also help differentiate the 2 syndromes. SS typically develops within 24 hours of starting/changing therapy, whereas NMS symptoms can be present for days to weeks. Resolution of symptoms may also be helpful in differentiation because SS typically resolves within a few days of initiating treatment, whereas NMS resolves within 9 to 14 days of starting treatment.19
Bottom Line
The clinical presentations of serotonin syndrome (SS) and neuroleptic malignant syndrome (NMS) overlap, which can make them difficult to differentiate; however, they each have distinct approaches to treatment. Features in SS that are distinct from NMS include a history of serotonergic agents, rapid onset of symptoms, hyperreflexia, and clonus. NMS is slower in onset and can be found in patients who are prescribed dopamine antagonists, with distinct symptoms of rigidity and hyporeflexia.
Related Resources
- Kimmel R. Serotonin syndrome or NMS? Clues to diagnosis. Current Psychiatry. 2010;9(2):92.
- Strawn JR, Keck Jr PE, Caroff SN. Neuroleptic malignant syndrome: Answers to 6 tough questions. Current Psychiatry. 2008;7(1):95-101.
Drug Brand Names
Amantadine • Symmetrel
Amitriptyline • Elavil, Endep
Aripiprazole • Abilify
Bromocriptine • Cycloset, Parlodel
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Ciprofloxacin • Cipro
Citalopram • Celexa
Clomipramine • Anafranil
Clozapine • Clozaril
Cyclobenzaprine • Amrix, Flexeril
Cyproheptadine • Periactin
Dantrolene • Dantrium
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextromethorphan • Benylin, Dexalone
Dolasetron • Anzemet
Doxepin • Silenor
Droperidol • Inapsine
Duloxetine • Cymbalt
Escitalopram • Lexapro
Fentanyl • Actiq, Duragesic
Fluconazole • Diflucan
Fluoxetine • Prozac
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Granisetron • Kytril
Haloperidol • Haldol
Isocarboxazid • Marplan
Levomilnacipran • Fetzima
Linezolid • Zyvox
Lithium • Eskalith, Lithobid
Meperidone • Demerol
Metoclopramide • Reglan
Milnacipran • Savella
Nefazodone • Serzone
Olanzapine • Zyprexa
Ondansetron • Zofran
Paliperidone • Invega
Palonosetron • Aloxi
Paroxetine • Paxil
Pentazocine • Talwin, Talacen
Perphenazine • Trilafon
Phenelzine • Nardil
Procarbazine • Matulane
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Safinamide • Xadago
Selegiline • Eldepryl, Zelapar
Sertraline • Zoloft
Sibutramine • Meridia
Tedizolid • Sivextro
Thioridazine • Mellaril
Tranylcypromine • Parnate
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Valproate • Depacon
Ziprasidone • Geodon
1. Volpi-Abadie J, Kaye AM, Kaye AD. Serotonin syndrome. Ochsner J. 2013;13(4):533-540.
2. Werneke U, Jamshidi F, Taylor D, et al. Conundrums in neurology: diagnosing serotonin syndrome – a meta-analysis of cases. BMC Neurol. 2016;16:97.
3. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
4. Birmes P, Coppin D, Schmitt L, et al. Serotonin syndrome: a brief review. CMAJ. 2003;168(11):1439-1442.
5. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148:705-713.
6. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter serotonin toxicity criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003; 96(9):635-642.
7. Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331(15):1021-1022.
8. Nisijima K. Serotonin syndrome overlapping with neuroleptic malignant syndrome: A case report and approaches for differentially diagnosing the two syndromes. Asian J Psychiatr. 2015;18:100-101.
9. Adnet P, Lestavel P, Krivosic-Horber R. Neuroleptic malignant syndrome. Br J Anaesth. 2000;85(1):129-135.
10. Gurrera R. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry. 1999;156:169-180.
11. Pope HG Jr, Aizley HG, Keck PE Jr, et al. Neuroleptic malignant syndrome: long-term follow-up of 20 cases. J Clin Psychiatry. 1991;52(5):208-212.
12. Velamoor VR, Norman RM, Caroff SN, et al. Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis. 1994;182(3):168-173.
13. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am. 1993;77(1):185-202.
14. Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973-981.
15. San Gabriel MC, Eddula-Changala B, Tan Y, et al. Electroconvulsive in a schizophrenic patient with neuroleptic malignant syndrome and rhabdomyolysis. J ECT. 2015;31(3):197-200.
16. Buggenhout S, Vandenberghe J, Sienaert P. Electroconvulsion therapy for neuroleptic malignant syndrome. Tijdschr Psychiatr. 2014;56(9):612-615.
17. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
18. Mills KC. Serotonin syndrome. A clinical update. Crit Care Clin. 1997;13(4):763-783.
19. Dosi R, Ambaliya A, Joshi H, et al. Serotonin syndrome versus neuroleptic malignant syndrome: a challenge clinical quandary. BMJ Case Rep. 2014;2014:bcr201404154. doi:10.1136/bcr-2014-204154.
1. Volpi-Abadie J, Kaye AM, Kaye AD. Serotonin syndrome. Ochsner J. 2013;13(4):533-540.
2. Werneke U, Jamshidi F, Taylor D, et al. Conundrums in neurology: diagnosing serotonin syndrome – a meta-analysis of cases. BMC Neurol. 2016;16:97.
3. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
4. Birmes P, Coppin D, Schmitt L, et al. Serotonin syndrome: a brief review. CMAJ. 2003;168(11):1439-1442.
5. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148:705-713.
6. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter serotonin toxicity criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003; 96(9):635-642.
7. Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331(15):1021-1022.
8. Nisijima K. Serotonin syndrome overlapping with neuroleptic malignant syndrome: A case report and approaches for differentially diagnosing the two syndromes. Asian J Psychiatr. 2015;18:100-101.
9. Adnet P, Lestavel P, Krivosic-Horber R. Neuroleptic malignant syndrome. Br J Anaesth. 2000;85(1):129-135.
10. Gurrera R. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry. 1999;156:169-180.
11. Pope HG Jr, Aizley HG, Keck PE Jr, et al. Neuroleptic malignant syndrome: long-term follow-up of 20 cases. J Clin Psychiatry. 1991;52(5):208-212.
12. Velamoor VR, Norman RM, Caroff SN, et al. Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis. 1994;182(3):168-173.
13. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am. 1993;77(1):185-202.
14. Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973-981.
15. San Gabriel MC, Eddula-Changala B, Tan Y, et al. Electroconvulsive in a schizophrenic patient with neuroleptic malignant syndrome and rhabdomyolysis. J ECT. 2015;31(3):197-200.
16. Buggenhout S, Vandenberghe J, Sienaert P. Electroconvulsion therapy for neuroleptic malignant syndrome. Tijdschr Psychiatr. 2014;56(9):612-615.
17. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
18. Mills KC. Serotonin syndrome. A clinical update. Crit Care Clin. 1997;13(4):763-783.
19. Dosi R, Ambaliya A, Joshi H, et al. Serotonin syndrome versus neuroleptic malignant syndrome: a challenge clinical quandary. BMJ Case Rep. 2014;2014:bcr201404154. doi:10.1136/bcr-2014-204154.
Antidepressants for chronic pain
Approximately 55 years ago, tricyclic antidepressants (TCAs) began to be used to treat neuropathic pain.1 Eventually, clinical trials emerged suggesting the utility of TCAs for other chronic pain conditions, such as fibromyalgia (FM) and migraine prophylaxis. However, despite TCAs’ effectiveness in mitigating painful conditions, their adverse effects limited their use.
Pharmacologic advancements have led to the development of other antidepressant classes, including selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), and the use of these agents has come to eclipse that of TCAs. In the realm of pain management, such developments have raised the hope of possible alternative co-analgesic agents that could avoid the adverse effects associated with TCAs. Some of these agents have demonstrated efficacy for managing chronic pain states, while others have demonstrated only limited utility.
This article provides a synopsis of systematic reviews and meta-analyses examining the role of antidepressant therapy for managing several chronic pain conditions, including pain associated with neuropathy, FM, headache, and irritable bowel syndrome (IBS). Because the literature base is rapidly evolving, it is necessary to revisit the information gleaned from clinical data with respect to treatment effectiveness, and to clarify how antidepressants might be positioned in the management of chronic pain.
The effectiveness of antidepressants for pain
The pathophysiologic processes that precipitate and maintain chronic pain conditions are complex (Box 12-10). The pain-mitigating effects of antidepressants can be thought of in terms of direct analgesic effects and indirect effects (Box 22,3,8,10,11-33).
Box 1
The pathophysiologic processes precipitating and maintaining chronic pain conditions are complex. Persistent and chronic pain results from changes in sensitivity within both ascending pathways (relaying pain information from the periphery to the spinal cord and brain) and descending pain pathways (functioning to modulate ascending pain information).2,3 Tissue damage or peripheral nerve injury can lead to a cascade of neuroplastic changes within the CNS, resulting in hyperexcitability within the ascending pain pathways.
The descending pain pathways consist of the midbrain periaqueductal gray area (PGA), the rostroventral medulla (RVM), and the dorsolateral pontomesencephalic tegmentum (DLPT). The axons of the RVM (the outflow of which is serotonergic) and DLPT (the outflow of which is noradrenergic) terminate in the dorsal horn of the spinal cord,4 and thereby dampen pain signals arising from the periphery. Diminished output from descending pain pathways can heighten the pain experience. Input from the cortex, hypothalamus, and amygdala (among other structures) converges upon the PGA, RVM and DLPT, and can influence the degree of pain modulation emerging from descending pathways. In this way, thoughts, appraisals, and mood are believed to comprise cognitive and affective modifiers of pain experiences.
Devising effective chronic pain treatment becomes challenging; multimodal treatment approaches often are advocated, including pharmacologic treatment with analgesics in combination with co-analgesic medications such as antidepressants. Although a description of multimodal treatment is beyond the scope of this article, such treatment also would encompass physical therapy, occupational therapy, and psychotherapeutic interventions to augment rehabilitative efforts and the functional capabilities of patients who struggle with persisting pain.
Although the direct pain-mitigating effects of antidepressants are not fully understood, it is believed that augmentation of monoamine neurotransmission from supraspinal nuclei (ie, the RVM and DLPT) modulate pain transmission from the periphery.5,6 In addition, there is evidence that some effects of tricyclic antidepressants can modulate several other functions that impact peripheral and central sensitization.7-10
During the last several decades, antidepressants have been used to address—and have demonstrated clinical utility for—a variety of chronic pain states. However, antidepressants are not a panacea; some chronic pain conditions are more responsive to antidepressants than are others. The chronic painful states most amenable to antidepressants are those that result primarily from a process of neural sensitization, as opposed to acute somatic or visceral nociception. Hence, several meta-analyses and evidence-based reviews have long suggested the usefulness of antidepressants for mitigating pain associated with neuropathy,34,35 FM,36,37 headache,38 and IBS.39,40
Box 2
The pain-mitigating effects of antidepressants can be thought of in terms of direct analgesic effects (impacting neurotransmission of descending pathways independent of influences on mood) and indirect effects (presumably impacting cortical and limbic output to the periaqueductal gray area, the rostroventral medulla, and the dorsolateral pontomesencephalic tegmentum brought about by improvement in mood and/or cognitive appraisals) (Figure2,3,8,10,11,15,20,22,28,29). Support for the direct analgesic effects has been garnered from initial empirical work that demonstrated pain relief among patients with pain who are not depressed. Additionally, among patients who have depression and experience pain, analgesia reportedly often occurs within 2 weeks, which is before antidepressant effects are appreciated,11-15 and, at least for some antidepressants, occurs at doses far lower than those required to produce mood-elevating effects.11,12,16
On the other hand, it is well established that significant comorbidities exist between chronic pain states and psychiatric disorders (eg, depression and somatic symptom and related disorders).17-21 There may be common physiological substrates underlying chronic pain and depression.20,22 There are bidirectional influences of limbic (affective) systems and those CNS structures involved in pain processing and integration. The effects of pain and depression are reciprocal; the presence of one makes the management of the other more challenging.23-27 Mood disturbances can, therefore, impact pain processing by acting as affective and cognitive amplifiers of pain by leading to catastrophizing, pain severity augmentation, poor coping with pain-related stress, etc.28,29 It is plausible that the mood-elevating effects of antidepressants can improve pain by indirect effects, by modulating limbic activity, which in turn, impacts coping, cognitive appraisals of pain, etc.
Patients with somatoform disorders (using pre-DSM-5 terminology) frequently present with chronic pain, often in multiple sites.19 Such patients are characterized by hypervigilance for, and a predisposition to focus on, physical sensations and to appraise these sensations as reflecting a pathological state.30 Neuroimaging studies have begun to identify those neural circuits involved in somatoform disorders, many of which act as cognitive and affective amplifiers of visceral-somatic sensory processing. Many of these neural circuits overlap, and interact with, those involved in pain processing.31 Antidepressants can mitigate the severity of unexplained physical complaints, including pain, among patients who somatize32,33; however, due to the heterogeneity of studies upon which this claim is based, the quality of the evidence is reportedly low.33 There is uncertainty whether, or to what extent, antidepressant benefits among patients who somatize are due to a direct impact on pain modulation, or indirect effects on mood or cognitive appraisals/perceptions.
Despite the uncertainties about the exact mechanisms through which antidepressants exert analgesic effects, antidepressants can be appropriately used to treat patients with selected chronic pain syndromes, regardless of whether or not the patient has a psychiatric comorbidity. For those patients with pain and psychiatric comorbidities, the benefits may be brought about via direct mechanisms, indirect mechanisms, or a combination of both.
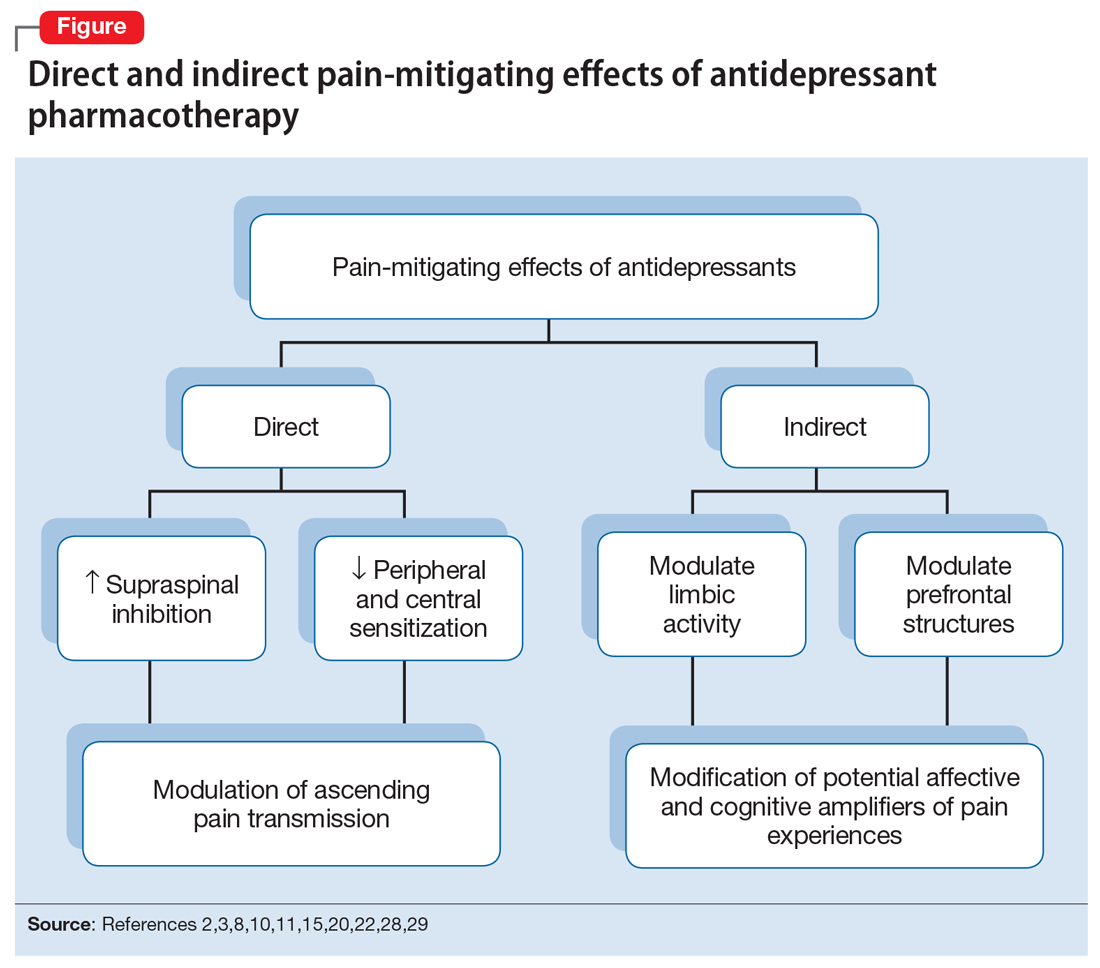
Continue to: Neuropathic pain
Neuropathic pain
Several treatment guidelines advocate for the use of antidepressants for neuropathic pain.41-44 For decades, TCAs have been employed off-label to successfully treat many patients with neuropathic pain states. Early investigations suggested that TCAs were robustly efficacious in managing patients with neuropathy.45-48 Calculated number-needed-to-treat (NNT) values for TCAs were quite low (ie, reflecting that few patients would need to be treated to yield a positive response in one patient compared with placebo), and were comparable to, if not slightly better than, the NNTs generated for anticonvulsants and α2-δ ligands, such as gabapentin or pregabalin.45-48
Unfortunately, early studies involving TCAs conducted many years ago do not meet contemporary standards of methodological rigor; they featured relatively small samples of patients assessed for brief post-treatment intervals with variable outcome measures. Thus, the NNT values obtained in meta-analyses based on these studies may overestimate treatment benefits.49 Further, NNT values derived from meta-analyses tended to combine all drugs within a particular antidepressant class (eg, amitriptyline, nortriptyline, desipramine, and imipramine among the TCAs) employed at diverse doses. Taken together, these limitations raise questions about the results of those meta-analyses.
Subsequent meta-analyses, which employed strict criteria to eliminate data from studies with potential sources of bias and used a primary outcome of frequencies of patients reporting at least 30% pain reduction compared with a placebo-controlled sample, suggest that the effectiveness of TCAs as a class for treating neuropathic pain is not as compelling as once was thought. Meta-analyses of studies employing specific TCAs revealed that there was little evidence to support the use of desipramine,50 imipramine,51 or nortriptyline52 in managing diabetic neuropathy or postherpetic neuralgia. Studies evaluating amitriptyline (dose range 12.5 to 150 mg/d), found low-level evidence of effectiveness; the benefit was expected to be present for a small subset (approximately 25%) of patients with neuropathic pain.53
There is moderate-quality evidence that duloxetine (60 to 120 mg/d) can produce a ≥50% improvement in pain severity ratings among patients with diabetic peripheral neuropathy.54 Although head-to-head studies with other antidepressants are limited, it appears that duloxetine and amitriptyline have comparable efficacy, even though the NNTs for amitriptyline were derived from lower-quality studies than those for duloxetine. Duloxetine is the only antidepressant to receive FDA approval for managing diabetic neuropathy. By contrast, studies assessing the utility of venlafaxine in neuropathic pain comprised small samples for brief durations, which limits the ability to draw clear (unbiased) support for its usefulness.55
Given the diversity of pathophysiologic processes underlying the disturbances that cause neuropathic pain disorders, it is unsurprising that the effectiveness of amitriptyline and duloxetine were not generalizable to all neuropathic pain states. Although amitriptyline produced pain-mitigating effects in patients with diabetic neuropathy and post-herpetic neuralgia, and duloxetine mitigated pain among patients with diabetic neuropathy, there was no evidence to suggest their effectiveness in phantom limb pain or human immunodeficiency virus-related and spinal cord-related neuropathies.35,53,54,56-58
Continue to: Fibromyalgia
Fibromyalgia
As with the issues encountered in interpreting the effectiveness of antidepressants in neuropathic pain, interpreting results gleaned from clinical trials of antidepressants for treating FM are fraught with similar difficulties. Although amitriptyline has been a first-line treatment for FM for many years, the evidence upon which such recommendations were based consisted of low-level studies that had a significant potential for bias.59 Large randomized trials would offer more compelling data regarding the efficacy of amitriptyline, but the prohibitive costs of such studies makes it unlikely they will be conducted. Amitriptyline (25 to 50 mg/d) was effective in mitigating FM-related pain in a small percentage of patients studied, with an estimated NNT of 4.59 Adverse effects, often contributing to treatment discontinuation, were encountered more frequently among patients who received amitriptyline compared with placebo.
Selective serotonin reuptake inhibitors failed to demonstrate significant pain relief (estimated NNT of 10), or improvement in fatigue or sleep problems, even though the studies upon which such conclusions were based were low-level studies with a high potential for bias.60 Although SSRIs have limited utility for mitigating pain, they are still quite useful for reducing depression among patients with FM.60
By contrast, the SNRIs duloxetine and milnacipran provided clinically relevant benefit over placebo in the frequency of patients reporting pain relief of ≥30%, as well as patients’ global impression of change.61 These agents, however, failed to provide clinically relevant benefit over placebo in improving health-related quality of life, reducing sleep problems, or improving fatigue. Nonetheless, duloxetine and milnacipran are FDA-approved for managing pain in FM. Studies assessing the efficacy of venlafaxine in the treatment of FM to date have been limited by small sample sizes, inconsistent dosing, lack of a placebo control, and lack of blinding, which limits the ability to clearly delineate the role of venlafaxine in managing FM.62
Mirtazapine (15 to 45 mg/d) showed a clinically relevant benefit compared with placebo for participant-reported pain relief of ≥30% and sleep disturbances. There was no benefit in terms of participant-reported improvement of quality of life, fatigue, or negative mood.63 The evidence was considered to be of low quality overall.
Headache
Amitriptyline has been employed off-label to address headache prophylaxis since 1964.64 Compared with placebo, it is efficacious in ameliorating migraine frequency and intensity as well as the frequency of tension headache.65,66 However, SSRIs and SNRIs (venlafaxine) failed to produce significant reductions in migraine frequency or severity or the frequencies of tension headache when compared with placebo.67,68
Continue to: Irritable bowel syndrome
Irritable bowel syndrome
Early studies addressing antidepressant efficacy in IBS reveal inconsistencies. For example, whereas some suggest that TCAs are effective in mitigating chronic, severe abdominal pain,39,40 others concluded that TCAs failed to demonstrate a significant analgesic benefit.69 A recent meta-analysis that restricted analysis of efficacy to randomized controlled trials (RCTs) with more rigorous methodological adherence found that pain relief in IBS is possible with both TCAs as well as SSRIs. However, adverse effects were more commonly encountered with TCAs than with SSRIs. Some of the inconsistencies in treatment efficacy reported in early studies may be due to variations in responsiveness of subsets of IBS patients. Specifically, the utility of TCAs appears to be best among patients with diarrheal-type (as opposed to constipation-type) IBS, presumably due to TCAs’ anticholinergic effects, whereas SSRIs may provide more of a benefit for patients with predominantly constipation-type IBS.40,70
Other chronic pain conditions
Antidepressants have been used to assist in the management of several other pain conditions, including oral-facial pain, interstitial cystitis, non-cardiac chest pain, and others. The role of antidepressants for such conditions remains unclear due to limitations in the prevailing empirical work, such as few trials, small sample sizes, variations in outcome measures, and insufficient randomization and blinding.71-76 The interpretation of results from systematic reviews and meta-analyses is limited because of these shortcomings.77 Hence, it has not always been possible to determine whether, and to what extent, patients with such conditions may benefit from antidepressants.
Neuromodulatory effects and efficacy for pain
The interplay of norepinephrine (NE) and serotonin (5-HT) neurotransmitter systems and cellular mechanisms involved in the descending modulation of pain pathways is complex. Experimental animal models of pain modulation suggest that 5-HT can both inhibit as well as promote pain perception by different physiological mechanisms, in contrast to NE, which is predominately inhibitory. While 5-HT in the descending modulating system can inhibit pain transmission ascending to the brain from the periphery, it appears that an intact noradrenergic system is necessary for the inhibitory influences of the serotonergic system to be appreciated.16,78,79 Deficiencies in one or both of these neurotransmitter systems may contribute to hyperactive pain processing, and thereby precipitate or maintain chronic pain.
Pain mitigation may be achieved best by enhancing both neurotransmitters simultaneously, less so by enhancing NE alone, and least by enhancing 5-HT alone.6 The ability to impact pain modulation would, therefore, depend on the degree to which an antidepressant capitalizes on both noradrenergic and serotonergic neurotransmission. Antidepressants commonly employed to manage pain are presented in Table 147,60,68,80-88 according to their primary neurotransmitter effects. Thus, the literature summarized above suggests that antidepressants that influence both NE and 5-HT transmission have greater analgesic effects than antidepressants with more specific effects, such as influencing 5-HT reuptake alone.80-85 It is unsurprising, therefore, that the SSRIs have not been demonstrated to be as consistently analgesic.47,60,68,80,86-88
Similarly, pharmacodynamic and pharmacokinetic differences within antidepressant classes may influence analgesic effectiveness. Simultaneous effects on NE and 5-HT are achieved at low doses with duloxetine and milnacipran. By contrast, 5-HT effects predominate at low doses for venlafaxine. To achieve pain-mitigating effects, higher doses of venlafaxine generally are required.89 Therefore, inconsistencies across studies regarding the analgesic benefits of venlafaxine may be attributable to variability in dosing; patients treated with lower doses may not have experienced sufficient NE effects to garner positive results.
Continue to: The differences in analgesic efficacy...
The differences in analgesic efficacy among specific TCAs may be understood in a similar fashion. Specifically, tertiary TCAs (imipramine and amitriptyline) inhibit both 5-HT and NE reuptake.6,90 Secondary amines (desipramine and nortriptyline) predominantly impact NE reuptake, possibly accounting for the lesser pain-mitigating benefit achieved with these agents, such as for treating neuropathic pain. Further, in vivo imipramine and amitriptyline are rapidly metabolized to secondary amines that are potent and selective NE reuptake inhibitors. In this way, the secondary amines may substantially lose the ability to modulate pain transmission because of the loss of concurrent 5-HT influences.90
Clinical pearls
The following practical points can help guide clinicians regarding the usefulness of antidepressants for pain management:
- Antidepressants can alleviate symptoms of depression and pain. The pain-mitigating effects of antidepressants are possible even among chronic pain patients who are not depressed. Antidepressants may confer benefits for chronic pain patients with depression and other comorbid conditions, such as somatic symptom and related disorders.
- Antidepressants are useful for select chronic pain states. Although the noradrenergic and serotonergic antidepressants (SNRIs and, to some extent, amitriptyline) appear to have efficacy for neuropathic pain and FM, the benefits of SSRIs appear to be less robust. On the other hand, SSRIs and TCAs may have potential benefit for patients with IBS. However, the results of meta-analyses are limited in the ability to provide information about which patients will best respond to which specific antidepressant or how well. Future research directed at identifying characteristics that can predict which patients are likely to benefit from one antidepressant vs another would help inform how best to tailor treatment to individual needs.
- The pain-mitigating effects of antidepressants often emerge early in the course of treatment (often before mood-elevating effects are observed). For example, in the case of amitriptyline, pain relief may be possible for some patients at doses generally lower than those required for mood-elevating effects. To date, there is limited information in the literature to determine what constitutes a sufficient duration of treatment, or when treatment should be modified.
- Failure to reduce pain should raise questions about whether the dose should be increased, an alternative agent should be tried, or combinations with other analgesic agents should be considered. Failure to achieve pain-mitigating effects with one antidepressant does not mean failure with others. Hence, failure to achieve desired effects with one agent might warrant an empirical trial with another agent. Presently, too few double-blind RCTs have been conducted to assess the pain-mitigating effects of other antidepressants (eg, bupropion and newer SNRIs such as desvenlafaxine and levomilnacipran). Meta-analysis of the analgesic effectiveness of these agents or comparisons to the efficacy of other antidepressant classes is, therefore, impossible at this time.
Because many chronic pain states are complex, patients will seldom experience clinically relevant benefit from any one intervention.53 The bigger implication for clinical research is to determine whether there is a sequence or combination of medication use that will provide overall better clinical effectiveness.53 Only limited data are available exploring the utility of combining pharmacologic approaches to address pain.91 For example, preliminary evidence suggests that combinations of complementary strategies, such as duloxetine combined with pregabalin, may result in significantly greater numbers of FM patients achieving ≥30% pain reduction compared with monotherapy with either agent alone or placebo.92
- Antidepressant selection may need to be based on medication-related adverse effect profiles and the potential for drug interactions. These factors are useful to consider in delineating multimodal treatment regimens for chronic pain in light of patients’ comorbidities and co-medication regimen. For example, the adverse effects of TCAs (anticholinergic and alpha-adrenergic influences) limit their utility for treating pain. Some of these effects can be more problematic in select populations, such as older adults or those with orthostatic difficulties, among others. TCAs are contraindicated in patients with closed-angle glaucoma, recent myocardial infarction, cardiac arrhythmias, poorly controlled seizures, or severe benign prostatic hypertrophy. Although the pain-mitigating effects of SNRIs have not been demonstrated to significantly exceed those of TCAs,68,93,94 SNRIs would offer an advantage of greater tolerability of adverse effects and relative safety in patients with comorbid medical conditions that would otherwise preclude TCA use. The adverse effects and common drug interactions associated with antidepressants are summarized in Table 295.
Conclusion
Chronic, nonmalignant pain conditions afflict many patients and significantly impair their ability to function. Because of heightened concerns related to the appropriateness of, and restricting inordinate access to, long-term opioid analgesics, clinicians need to explore the usefulness of co-analgesic agents, such as antidepressants. Significant comorbidities exist between psychiatric disorders and chronic pain, and psychiatrists are uniquely positioned to diagnose and treat psychiatric comorbidities, as well as pain, among their patients, especially since they understand the kinetics and dynamics of antidepressants.
Bottom Line
Antidepressants can alleviate symptoms of depression and pain. Noradrenergic and serotonergic antidepressants appear to have efficacy for pain associated with neuropathy and fibromyalgia, while selective serotonin reuptake inhibitors and tricyclic antidepressants may have benefit for patients with irritable bowel syndrome. However, evidence regarding which patients will best respond to which specific antidepressant is limited.
Continue to: Related Resources
Related Resources
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
- Maletic V, Demuri B. Chronic pain and depression: treatment of 2 culprits in common. Current Psychiatry. 2016;15(3):41,47-50,52.
Drug Brand Names
Amitriptyline • Elavil, Endep
Bupropion • Wellbutrin, Zyban
Carisoprodol • Rela, Soma
Cyclobenzaprine • Amrix, Flexeril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Horizant, Neurontin
Imipramine • Tofranil
Levomilnacipran • Fetzima
Methadone • Dolophine, Methadose
Milnacipran • Savella
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Pregabalin • Lyrica, Lyrica CR
Tapentadol • Nucynta
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Warfarin • Coumadin, Jantoven
1. Paoli F, Darcourt G, Cossa P. Preliminary note on the action of imipramine in painful states [in French]. Rev Neurol (Paris). 1960;102:503-504.
2. Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219-245.
3. Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2(2):83-91.
4. Lamont LA, Tranquilli WJ, Grimm KA. Physiology of pain. Vet Clin North Am Small Anim Pract. 2000;30(4):703-728, v.
5. Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, eds. Wall and Melzack’s Textbook of Pain. 5th ed. Burlington, MA: Elsevier Health Sciences; 2005:125-142.
6. Marks DM, Shah MJ, Patkar AA, et al. Serotonin-norepinephrine reuptake inhibitors for pain control: premise and promise. Curr Neuropharmacol. 2009;7(4):331-336.
7. Baba H, Shimoji K, Yoshimura M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology. 2000;92(2):473-484.
8. Carter GT, Sullivan MD. Antidepressants in pain management. Curr Opin Investig Drugs. 2002;3(3):454-458.
9. Kawasaki Y, Kumamoto E, Furue H, et al. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology. 2003;98(3):682-689.
10. McCleane G. Antidepressants as analgesics. CNS Drugs. 2008;22(2):139-156.
11. Ansari A. The efficacy of newer antidepressants in the treatment of chronic pain: a review of current literature. Harv Rev Psychiatry. 2000;7(5):257-277.
12. Egbunike IG, Chaffee BJ. Antidepressants in the management of chronic pain syndromes. Pharmacotherapy. 1990;10(4):262-270.
13. Fishbain DA. Evidence-based data on pain relief with antidepressants. Ann Med. 2000;32(5):305-316.
14. Fishbain DA, Detke MJ, Wernicke J, et al. The relationship between antidepressant and analgesic responses: findings from six placebo-controlled trials assessing the efficacy of duloxetine in patients with major depressive disorder. Curr Med Res Opin. 2008;24(11):3105-3115.
15. Harada E, Tokuoka H, Fujikoshi S, et al. Is duloxetine’s effect on painful physical symptoms in depression an indirect result of improvement of depressive symptoms? Pooled analyses of three randomized controlled trials. Pain. 2016;157(3):577-584.
16. Kehoe WA. Antidepressants for chronic pain: selection and dosing considerations. Am J Pain Med. 1993;3(4):161-165.
17. Damush TM, Kroenke K, Bair MJ, et al. Pain self-management training increases self-efficacy, self-management behaviours and pain and depression outcomes. Eur J Pain. 2016;20(2):1070-1078.
18. DeVeaugh-Geiss AM, West SL, Miller WC, et al. The adverse effects of comorbid pain on depression outcomes in primary care patients: results from the ARTIST trial. Pain Medicine. 2010;11(5):732-741.
19. Egloff N, Cámara RJ, von Känel R, et al. Hypersensitivity and hyperalgesia in somatoform pain disorders. Gen Hosp Psychiatry. 2014;36(3):284-290.
20. Goesling J, Clauw DW, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psych Reports. 2013;15(12):421.
21. Kroenke K, Wu J, Bair MJ, et al. Reciprocal relationship between pain and depression: a 12-Month longitudinal analysis in primary care. J Pain. 2011;12(9):964-973.
22. Leo RJ. Chronic pain and comorbid depression. Curr Treat Options Neurol. 2005;7(5):403-412.
23. Bair MJ, Robinson RL, Eckert GJ, et al. Impact of pain on depression treatment response in primary care. Psychosom Med. 2004;66(1):17-22.
24. Karp JF, Scott J, Houck P, et al. Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry. 2005;66(5):591-597.
25. Kroenke K, Shen J, Oxman TE, et al. Impact of pain on the outcomes of depression treatment: results from the RESPECT trial. Pain. 2008;134(1-2):209-215.
26. Mavandadi S, Ten Have TR, Katz IR, et al. Effect of depression treatment on depressive symptoms in older adulthood: the moderating role of pain. J Am Geriatr Soc. 2007;55(2):202-211.
27. Thielke SM, Fan MY, Sullivan M, et al. Pain limits the effectiveness of collaborative care for depression. Am J Geriatr Psychiatry. 2007;15(8):699-707.
28. Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68(2):262-268.
29. Demyttenaere K, Bonnewyn A, Bruffaerts R, et al. Comorbid painful physical symptoms and depression: Prevalence, work loss, and help seeking. J Affect Disord. 2006;92(2-3):185-193.
30. Nakao M, Barsky AJ. Clinical application of somatosensory amplification in psychosomatic medicine. Biopsychosoc Med. 2007;1:17.
31. Perez DL, Barsky AJ, Vago DR, et al. A neural circuit framework for somatosensory amplification in somatoform disorders. J Neuropsychiatry Clin Neurosci. 2015;27(1):e40-e50.
32. Fishbain DA, Cutler RB, Rosomoff HL, et al. Do antidepressants have an analgesic effect in psychogenic pain and somatoform pain disorder? A meta-analysis. Psychosom Med. 1998;60(4):503-509.
33. Kleinstäuber M, Witthöft M, Steffanowski A, et al. Pharmacological interventions for somatoform disorders in adults. Cochrane Database Syst Rev. 2014;(11):CD010628.
34. Collins SL, Moore RA, McQuay HJ, et al. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: a quantitative systematic review. J Pain Symptom Manage. 2000;20(6):449-458.
35. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain: a Cochrane review. J Neurol Neurosurg Psychiatry. 2010;81(12):1372-1373.
36. Arnold LM, Keck PE, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41(2):104-113.
37. O’Malley PG, Balden E, Tomkins G, et al. Treatment of fibromyalgia with antidepressants: a meta-analysis. J Gen Intern Med. 2000;15(9):659-666.
38. Tomkins GE, Jackson JL, O’Malley PG, et al. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med. 2001;111(1):54-63.
39. Jackson JL, O’Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108(1):65-72.
40. Lesbros-Pantoflickova D, Michetti P, Fried M et al. Meta-analysis: the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(11-12):1253-1269.
41. Centre for Clinical Practice at NICE (UK). Neuropathic pain: the pharmacological management of neuropathic pain in adults in non-specialist settings. London, UK: National Institute for Health and Care Excellence, (UK); 2013.
42. O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(suppl 10):S22-S32.
43. Moulin D, Boulanger A, Clark AJ, et al; Canadian Pain Society. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328-35.
44. Mu A, Weinberg E, Moulin DE, et al. Pharmacologic management of chronic neuropathic pain: Review of the Canadian Pain Society consensus statement. Can Fam Physician. 2017;63(11):844-852.
45. Finnerup NB, Otto M, McQuay HJ, et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289-305.
46. Hempenstall K, Nurmikko TJ, Johnson RW, et al. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2(7):e164.
47. Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83(3):389-400.
48. Wu CL, Raja SN. An update on the treatment of postherpetic neuralgia. J Pain. 2008;9(suppl 1):S19-S30.
49. Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206-219.
50. Hearn L, Moore RA, Derry S, et al. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(9):CD011003.
51. Hearn L, Derry S, Phillips T, et al. Imipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(5):CD010769.
52. Derry S, Wiffen PJ, Aldington D, et al. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;1:CD011209.
53. Moore R, Derry S, Aldington D, et al. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(7):CD008242.
54. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115.
55. Gallagher HC, Gallagher RM, Butler M, et al. Venlafaxine for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(8):CD011091.
56. Alviar MJ, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev. 2016;10:CD006380.
57. Dinat N, Marinda E, Moch S, et al. Randomized, Double-Blind, Crossover Trial of Amitriptyline for Analgesia in Painful HIV-Associated Sensory Neuropathy. PLoS One. 2015;10(5):e0126297. doi: 10.1371/journal.pone.0126297.eCollection 2015.
58. Mehta S, McIntyre A, Janzen S, et al; Spinal Cord Injury Rehabilitation Evidence Team. Systematic review of pharmacologic treatments of pain after spinal cord injury: an update. Arch Phys Med Rehabil. 2016;97(8):1381-1391.e1.
59. Moore RA, Derry S, Aldington D, et al. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;(12):CD008242..
60. Walitt B, Urrútia G, Nishishinya MB, et al. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015;(6):CD011735.
61. Welsch P, Üçeyler N, Klose P, et al. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia. Cochrane Database Syst Rev. 2018;(2):CD010292.
62. VanderWeide LA, Smith SM, Trinkley KE. A systematic review of the efficacy of venlafaxine for the treatment of fibromyalgia. J Clin Pharm Ther. 2015;40(1):1-6.
63. Welsch P, Bernardy K, Derry S, et al. Mirtazapine for fibromyalgia in adults. Cochrane Database Syst Rev. 2018;(8):CD012708.
64. Lance JW, Curran DA. Treatment of chronic tension headache. Lancet. 1964;283(7345):1236-1239.
65. Jackson JL, William S, Laura S, et al. Tricyclic antidepressants and headaches: systematic review and meta-analysis. BMJ. 2010;341:c5222. doi: https://doi.org/10.1136/bmj.c5222
66. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine. 2017;96(22):e6989. doi: 10.1097/MD.0000000000006989.
67. Banzi R, Cusi C, Randazzo C, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of migraine in adults. Cochrane Database Syst Rev. 2015;(4):CD002919.
68. Banzi R, Cusi C, Randazzo C, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of tension-type headache in adults. Cochrane Database Syst Rev. 2015;(5):CD011681.
69. Quartero AO, Meineche-Schmidt V, Muris J, et al. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2005;(2):CD003460.
70. Ford AC, Talley NJ, Schoenfeld PS, et al. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58(3):367-378.
71. Coss-Adame E, Erdogan A, Rao SS. Treatment of esophageal (noncardiac) chest pain: an expert review. Clin Gastroenterol Hepatol. 2014;12(8):1224-1245.
72. Kelada E, Jones A. Interstitial cystitis. Arch Gynecol Obstet. 2007;275(4):223-229.
73. Leo RJ, Dewani S. A systematic review of the utility of antidepressant pharmacotherapy in the treatment of vulvodynia pain. J Sex Med. 2013;10(10):2497-2505.
74. McMillan R, Forssell H, Buchanan JA, et al. Interventions for treating burning mouth syndrome. Cochrane Database Syst Rev. 2016;11:CD002779.
75. Patel DN. Inconclusive results of a systematic review of efficacy of antidepressants on orofacial pain disorders. Evid Based Dent. 2013;14(2):55-56.
76. Wang W, Sun YH, Wang YY, et al. Treatment of functional chest pain with antidepressants: a meta-analysis. Pain Physician. 2012;15(2):E131-E142.
77. Lavis JN. How can we support the use of systematic reviews in policymaking? PLoS Med. 2009;6(11):e1000141. doi: 10.1371/journal.pmed.1000141.
78. Sorkin L. Nociceptive transmission within the spinal cord. Mt Sinai J Med. 1991;58(3):208-216.
79. Yokogawa F, Kiuchi Y, Ishikawa Y, et al. An investigation of monoamine receptors involved in antinociceptive effects of antidepressants. Anesth Analg. 2002;95(1):163-168, table of contents.
80. Lynch ME. Antidepressants as analgesics: a review of randomized controlled trials. J Psychiatry Neurosci. 2001;26(1):30-36.
81. Max MB. Treatment of post-herpetic neuralgia: antidepressants. Ann Neurol. 1994;35(suppl):S50-S53.
82. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
83. McQuay HJ, Tramèr M, Nye BA, et al. A systematic review of antidepressants in neuropathic pain. Pain. 1996;68(2-3):217-227.
84. Mochizucki D. Serotonin and noradrenaline reuptake inhibitors in animal models of pain. Hum Psychopharmacol Clin Exp. 2004;19(suppl 1):15-19.
85. Sussman N. SNRIs versus SSRIs: mechanisms of action in treating depression and painful physical symptoms. Primary Care Companion J Clin Psychiatry. 2003;5(suppl 7):19-26.
86. Bundeff AW, Woodis CB. Selective serotonin reuptake inhibitors for the treatment of irritable bowel syndrome. Ann Pharmacother. 2014;48(6):777-784.
87. Jung AC, Staiger T, Sullivan M. The efficacy of selective serotonin reuptake inhibitors for the management of chronic pain. J Gen Intern Med. 1997;12(6):384-389.
88. Xie C, Tang Y, Wang Y, et al. Efficacy and safety of antidepressants for the treatment of irritable bowel syndrome: a meta-analysis. PLoS One. 2015;10(8):e0127815. doi: 10.1371/journal.pone.0127815. eCollection 2015.
89. Zijlstra TR , Barendregt PJ , van de Laar MA. Venlafaxine in fibromyalgia: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 2002;46(suppl 9):S105.
90. Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology. 2001;25(6):871-880.
91. Thorpe J, Shum B, Moore RA, et al. Combination pharmacotherapy for the treatment of fibromyalgia in adults. Cochrane Database Syst Rev. 2018;(2):CD010585.
92. Gilron I, Chaparro LE, Tu D, et al. Combination of pregabalin with duloxetine for fibromyalgia: a randomized controlled trial. Pain. 2016;157(7):1532-1540.
93. Häuser W, Petzke F, Üçeyler N, et al. Comparative efficacy and acceptability of amitriptyline, duloxetine and milnacipran in fibromyalgia syndrome: a systematic review with meta-analysis. Rheumatology (Oxford). 2011;50(3):532-543.
94. Hossain SM, Hussain SM, Ekram AR. Duloxetine in painful diabetic neuropathy: a systematic review. Clin J Pain. 2016;32(11):1005-1010.
95. Riediger C, Schuster T, Barlinn K, et al. Adverse effects of antidepressants for chronic pain: a systematic review and meta-analysis. Front Neurol. 2017;8:307.
Approximately 55 years ago, tricyclic antidepressants (TCAs) began to be used to treat neuropathic pain.1 Eventually, clinical trials emerged suggesting the utility of TCAs for other chronic pain conditions, such as fibromyalgia (FM) and migraine prophylaxis. However, despite TCAs’ effectiveness in mitigating painful conditions, their adverse effects limited their use.
Pharmacologic advancements have led to the development of other antidepressant classes, including selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), and the use of these agents has come to eclipse that of TCAs. In the realm of pain management, such developments have raised the hope of possible alternative co-analgesic agents that could avoid the adverse effects associated with TCAs. Some of these agents have demonstrated efficacy for managing chronic pain states, while others have demonstrated only limited utility.
This article provides a synopsis of systematic reviews and meta-analyses examining the role of antidepressant therapy for managing several chronic pain conditions, including pain associated with neuropathy, FM, headache, and irritable bowel syndrome (IBS). Because the literature base is rapidly evolving, it is necessary to revisit the information gleaned from clinical data with respect to treatment effectiveness, and to clarify how antidepressants might be positioned in the management of chronic pain.
The effectiveness of antidepressants for pain
The pathophysiologic processes that precipitate and maintain chronic pain conditions are complex (Box 12-10). The pain-mitigating effects of antidepressants can be thought of in terms of direct analgesic effects and indirect effects (Box 22,3,8,10,11-33).
Box 1
The pathophysiologic processes precipitating and maintaining chronic pain conditions are complex. Persistent and chronic pain results from changes in sensitivity within both ascending pathways (relaying pain information from the periphery to the spinal cord and brain) and descending pain pathways (functioning to modulate ascending pain information).2,3 Tissue damage or peripheral nerve injury can lead to a cascade of neuroplastic changes within the CNS, resulting in hyperexcitability within the ascending pain pathways.
The descending pain pathways consist of the midbrain periaqueductal gray area (PGA), the rostroventral medulla (RVM), and the dorsolateral pontomesencephalic tegmentum (DLPT). The axons of the RVM (the outflow of which is serotonergic) and DLPT (the outflow of which is noradrenergic) terminate in the dorsal horn of the spinal cord,4 and thereby dampen pain signals arising from the periphery. Diminished output from descending pain pathways can heighten the pain experience. Input from the cortex, hypothalamus, and amygdala (among other structures) converges upon the PGA, RVM and DLPT, and can influence the degree of pain modulation emerging from descending pathways. In this way, thoughts, appraisals, and mood are believed to comprise cognitive and affective modifiers of pain experiences.
Devising effective chronic pain treatment becomes challenging; multimodal treatment approaches often are advocated, including pharmacologic treatment with analgesics in combination with co-analgesic medications such as antidepressants. Although a description of multimodal treatment is beyond the scope of this article, such treatment also would encompass physical therapy, occupational therapy, and psychotherapeutic interventions to augment rehabilitative efforts and the functional capabilities of patients who struggle with persisting pain.
Although the direct pain-mitigating effects of antidepressants are not fully understood, it is believed that augmentation of monoamine neurotransmission from supraspinal nuclei (ie, the RVM and DLPT) modulate pain transmission from the periphery.5,6 In addition, there is evidence that some effects of tricyclic antidepressants can modulate several other functions that impact peripheral and central sensitization.7-10
During the last several decades, antidepressants have been used to address—and have demonstrated clinical utility for—a variety of chronic pain states. However, antidepressants are not a panacea; some chronic pain conditions are more responsive to antidepressants than are others. The chronic painful states most amenable to antidepressants are those that result primarily from a process of neural sensitization, as opposed to acute somatic or visceral nociception. Hence, several meta-analyses and evidence-based reviews have long suggested the usefulness of antidepressants for mitigating pain associated with neuropathy,34,35 FM,36,37 headache,38 and IBS.39,40
Box 2
The pain-mitigating effects of antidepressants can be thought of in terms of direct analgesic effects (impacting neurotransmission of descending pathways independent of influences on mood) and indirect effects (presumably impacting cortical and limbic output to the periaqueductal gray area, the rostroventral medulla, and the dorsolateral pontomesencephalic tegmentum brought about by improvement in mood and/or cognitive appraisals) (Figure2,3,8,10,11,15,20,22,28,29). Support for the direct analgesic effects has been garnered from initial empirical work that demonstrated pain relief among patients with pain who are not depressed. Additionally, among patients who have depression and experience pain, analgesia reportedly often occurs within 2 weeks, which is before antidepressant effects are appreciated,11-15 and, at least for some antidepressants, occurs at doses far lower than those required to produce mood-elevating effects.11,12,16
On the other hand, it is well established that significant comorbidities exist between chronic pain states and psychiatric disorders (eg, depression and somatic symptom and related disorders).17-21 There may be common physiological substrates underlying chronic pain and depression.20,22 There are bidirectional influences of limbic (affective) systems and those CNS structures involved in pain processing and integration. The effects of pain and depression are reciprocal; the presence of one makes the management of the other more challenging.23-27 Mood disturbances can, therefore, impact pain processing by acting as affective and cognitive amplifiers of pain by leading to catastrophizing, pain severity augmentation, poor coping with pain-related stress, etc.28,29 It is plausible that the mood-elevating effects of antidepressants can improve pain by indirect effects, by modulating limbic activity, which in turn, impacts coping, cognitive appraisals of pain, etc.
Patients with somatoform disorders (using pre-DSM-5 terminology) frequently present with chronic pain, often in multiple sites.19 Such patients are characterized by hypervigilance for, and a predisposition to focus on, physical sensations and to appraise these sensations as reflecting a pathological state.30 Neuroimaging studies have begun to identify those neural circuits involved in somatoform disorders, many of which act as cognitive and affective amplifiers of visceral-somatic sensory processing. Many of these neural circuits overlap, and interact with, those involved in pain processing.31 Antidepressants can mitigate the severity of unexplained physical complaints, including pain, among patients who somatize32,33; however, due to the heterogeneity of studies upon which this claim is based, the quality of the evidence is reportedly low.33 There is uncertainty whether, or to what extent, antidepressant benefits among patients who somatize are due to a direct impact on pain modulation, or indirect effects on mood or cognitive appraisals/perceptions.
Despite the uncertainties about the exact mechanisms through which antidepressants exert analgesic effects, antidepressants can be appropriately used to treat patients with selected chronic pain syndromes, regardless of whether or not the patient has a psychiatric comorbidity. For those patients with pain and psychiatric comorbidities, the benefits may be brought about via direct mechanisms, indirect mechanisms, or a combination of both.

Continue to: Neuropathic pain
Neuropathic pain
Several treatment guidelines advocate for the use of antidepressants for neuropathic pain.41-44 For decades, TCAs have been employed off-label to successfully treat many patients with neuropathic pain states. Early investigations suggested that TCAs were robustly efficacious in managing patients with neuropathy.45-48 Calculated number-needed-to-treat (NNT) values for TCAs were quite low (ie, reflecting that few patients would need to be treated to yield a positive response in one patient compared with placebo), and were comparable to, if not slightly better than, the NNTs generated for anticonvulsants and α2-δ ligands, such as gabapentin or pregabalin.45-48
Unfortunately, early studies involving TCAs conducted many years ago do not meet contemporary standards of methodological rigor; they featured relatively small samples of patients assessed for brief post-treatment intervals with variable outcome measures. Thus, the NNT values obtained in meta-analyses based on these studies may overestimate treatment benefits.49 Further, NNT values derived from meta-analyses tended to combine all drugs within a particular antidepressant class (eg, amitriptyline, nortriptyline, desipramine, and imipramine among the TCAs) employed at diverse doses. Taken together, these limitations raise questions about the results of those meta-analyses.
Subsequent meta-analyses, which employed strict criteria to eliminate data from studies with potential sources of bias and used a primary outcome of frequencies of patients reporting at least 30% pain reduction compared with a placebo-controlled sample, suggest that the effectiveness of TCAs as a class for treating neuropathic pain is not as compelling as once was thought. Meta-analyses of studies employing specific TCAs revealed that there was little evidence to support the use of desipramine,50 imipramine,51 or nortriptyline52 in managing diabetic neuropathy or postherpetic neuralgia. Studies evaluating amitriptyline (dose range 12.5 to 150 mg/d), found low-level evidence of effectiveness; the benefit was expected to be present for a small subset (approximately 25%) of patients with neuropathic pain.53
There is moderate-quality evidence that duloxetine (60 to 120 mg/d) can produce a ≥50% improvement in pain severity ratings among patients with diabetic peripheral neuropathy.54 Although head-to-head studies with other antidepressants are limited, it appears that duloxetine and amitriptyline have comparable efficacy, even though the NNTs for amitriptyline were derived from lower-quality studies than those for duloxetine. Duloxetine is the only antidepressant to receive FDA approval for managing diabetic neuropathy. By contrast, studies assessing the utility of venlafaxine in neuropathic pain comprised small samples for brief durations, which limits the ability to draw clear (unbiased) support for its usefulness.55
Given the diversity of pathophysiologic processes underlying the disturbances that cause neuropathic pain disorders, it is unsurprising that the effectiveness of amitriptyline and duloxetine were not generalizable to all neuropathic pain states. Although amitriptyline produced pain-mitigating effects in patients with diabetic neuropathy and post-herpetic neuralgia, and duloxetine mitigated pain among patients with diabetic neuropathy, there was no evidence to suggest their effectiveness in phantom limb pain or human immunodeficiency virus-related and spinal cord-related neuropathies.35,53,54,56-58
Continue to: Fibromyalgia
Fibromyalgia
As with the issues encountered in interpreting the effectiveness of antidepressants in neuropathic pain, interpreting results gleaned from clinical trials of antidepressants for treating FM are fraught with similar difficulties. Although amitriptyline has been a first-line treatment for FM for many years, the evidence upon which such recommendations were based consisted of low-level studies that had a significant potential for bias.59 Large randomized trials would offer more compelling data regarding the efficacy of amitriptyline, but the prohibitive costs of such studies makes it unlikely they will be conducted. Amitriptyline (25 to 50 mg/d) was effective in mitigating FM-related pain in a small percentage of patients studied, with an estimated NNT of 4.59 Adverse effects, often contributing to treatment discontinuation, were encountered more frequently among patients who received amitriptyline compared with placebo.
Selective serotonin reuptake inhibitors failed to demonstrate significant pain relief (estimated NNT of 10), or improvement in fatigue or sleep problems, even though the studies upon which such conclusions were based were low-level studies with a high potential for bias.60 Although SSRIs have limited utility for mitigating pain, they are still quite useful for reducing depression among patients with FM.60
By contrast, the SNRIs duloxetine and milnacipran provided clinically relevant benefit over placebo in the frequency of patients reporting pain relief of ≥30%, as well as patients’ global impression of change.61 These agents, however, failed to provide clinically relevant benefit over placebo in improving health-related quality of life, reducing sleep problems, or improving fatigue. Nonetheless, duloxetine and milnacipran are FDA-approved for managing pain in FM. Studies assessing the efficacy of venlafaxine in the treatment of FM to date have been limited by small sample sizes, inconsistent dosing, lack of a placebo control, and lack of blinding, which limits the ability to clearly delineate the role of venlafaxine in managing FM.62
Mirtazapine (15 to 45 mg/d) showed a clinically relevant benefit compared with placebo for participant-reported pain relief of ≥30% and sleep disturbances. There was no benefit in terms of participant-reported improvement of quality of life, fatigue, or negative mood.63 The evidence was considered to be of low quality overall.
Headache
Amitriptyline has been employed off-label to address headache prophylaxis since 1964.64 Compared with placebo, it is efficacious in ameliorating migraine frequency and intensity as well as the frequency of tension headache.65,66 However, SSRIs and SNRIs (venlafaxine) failed to produce significant reductions in migraine frequency or severity or the frequencies of tension headache when compared with placebo.67,68
Continue to: Irritable bowel syndrome
Irritable bowel syndrome
Early studies addressing antidepressant efficacy in IBS reveal inconsistencies. For example, whereas some suggest that TCAs are effective in mitigating chronic, severe abdominal pain,39,40 others concluded that TCAs failed to demonstrate a significant analgesic benefit.69 A recent meta-analysis that restricted analysis of efficacy to randomized controlled trials (RCTs) with more rigorous methodological adherence found that pain relief in IBS is possible with both TCAs as well as SSRIs. However, adverse effects were more commonly encountered with TCAs than with SSRIs. Some of the inconsistencies in treatment efficacy reported in early studies may be due to variations in responsiveness of subsets of IBS patients. Specifically, the utility of TCAs appears to be best among patients with diarrheal-type (as opposed to constipation-type) IBS, presumably due to TCAs’ anticholinergic effects, whereas SSRIs may provide more of a benefit for patients with predominantly constipation-type IBS.40,70
Other chronic pain conditions
Antidepressants have been used to assist in the management of several other pain conditions, including oral-facial pain, interstitial cystitis, non-cardiac chest pain, and others. The role of antidepressants for such conditions remains unclear due to limitations in the prevailing empirical work, such as few trials, small sample sizes, variations in outcome measures, and insufficient randomization and blinding.71-76 The interpretation of results from systematic reviews and meta-analyses is limited because of these shortcomings.77 Hence, it has not always been possible to determine whether, and to what extent, patients with such conditions may benefit from antidepressants.
Neuromodulatory effects and efficacy for pain
The interplay of norepinephrine (NE) and serotonin (5-HT) neurotransmitter systems and cellular mechanisms involved in the descending modulation of pain pathways is complex. Experimental animal models of pain modulation suggest that 5-HT can both inhibit as well as promote pain perception by different physiological mechanisms, in contrast to NE, which is predominately inhibitory. While 5-HT in the descending modulating system can inhibit pain transmission ascending to the brain from the periphery, it appears that an intact noradrenergic system is necessary for the inhibitory influences of the serotonergic system to be appreciated.16,78,79 Deficiencies in one or both of these neurotransmitter systems may contribute to hyperactive pain processing, and thereby precipitate or maintain chronic pain.
Pain mitigation may be achieved best by enhancing both neurotransmitters simultaneously, less so by enhancing NE alone, and least by enhancing 5-HT alone.6 The ability to impact pain modulation would, therefore, depend on the degree to which an antidepressant capitalizes on both noradrenergic and serotonergic neurotransmission. Antidepressants commonly employed to manage pain are presented in Table 147,60,68,80-88 according to their primary neurotransmitter effects. Thus, the literature summarized above suggests that antidepressants that influence both NE and 5-HT transmission have greater analgesic effects than antidepressants with more specific effects, such as influencing 5-HT reuptake alone.80-85 It is unsurprising, therefore, that the SSRIs have not been demonstrated to be as consistently analgesic.47,60,68,80,86-88
Similarly, pharmacodynamic and pharmacokinetic differences within antidepressant classes may influence analgesic effectiveness. Simultaneous effects on NE and 5-HT are achieved at low doses with duloxetine and milnacipran. By contrast, 5-HT effects predominate at low doses for venlafaxine. To achieve pain-mitigating effects, higher doses of venlafaxine generally are required.89 Therefore, inconsistencies across studies regarding the analgesic benefits of venlafaxine may be attributable to variability in dosing; patients treated with lower doses may not have experienced sufficient NE effects to garner positive results.
Continue to: The differences in analgesic efficacy...
The differences in analgesic efficacy among specific TCAs may be understood in a similar fashion. Specifically, tertiary TCAs (imipramine and amitriptyline) inhibit both 5-HT and NE reuptake.6,90 Secondary amines (desipramine and nortriptyline) predominantly impact NE reuptake, possibly accounting for the lesser pain-mitigating benefit achieved with these agents, such as for treating neuropathic pain. Further, in vivo imipramine and amitriptyline are rapidly metabolized to secondary amines that are potent and selective NE reuptake inhibitors. In this way, the secondary amines may substantially lose the ability to modulate pain transmission because of the loss of concurrent 5-HT influences.90
Clinical pearls
The following practical points can help guide clinicians regarding the usefulness of antidepressants for pain management:
- Antidepressants can alleviate symptoms of depression and pain. The pain-mitigating effects of antidepressants are possible even among chronic pain patients who are not depressed. Antidepressants may confer benefits for chronic pain patients with depression and other comorbid conditions, such as somatic symptom and related disorders.
- Antidepressants are useful for select chronic pain states. Although the noradrenergic and serotonergic antidepressants (SNRIs and, to some extent, amitriptyline) appear to have efficacy for neuropathic pain and FM, the benefits of SSRIs appear to be less robust. On the other hand, SSRIs and TCAs may have potential benefit for patients with IBS. However, the results of meta-analyses are limited in the ability to provide information about which patients will best respond to which specific antidepressant or how well. Future research directed at identifying characteristics that can predict which patients are likely to benefit from one antidepressant vs another would help inform how best to tailor treatment to individual needs.
- The pain-mitigating effects of antidepressants often emerge early in the course of treatment (often before mood-elevating effects are observed). For example, in the case of amitriptyline, pain relief may be possible for some patients at doses generally lower than those required for mood-elevating effects. To date, there is limited information in the literature to determine what constitutes a sufficient duration of treatment, or when treatment should be modified.
- Failure to reduce pain should raise questions about whether the dose should be increased, an alternative agent should be tried, or combinations with other analgesic agents should be considered. Failure to achieve pain-mitigating effects with one antidepressant does not mean failure with others. Hence, failure to achieve desired effects with one agent might warrant an empirical trial with another agent. Presently, too few double-blind RCTs have been conducted to assess the pain-mitigating effects of other antidepressants (eg, bupropion and newer SNRIs such as desvenlafaxine and levomilnacipran). Meta-analysis of the analgesic effectiveness of these agents or comparisons to the efficacy of other antidepressant classes is, therefore, impossible at this time.
Because many chronic pain states are complex, patients will seldom experience clinically relevant benefit from any one intervention.53 The bigger implication for clinical research is to determine whether there is a sequence or combination of medication use that will provide overall better clinical effectiveness.53 Only limited data are available exploring the utility of combining pharmacologic approaches to address pain.91 For example, preliminary evidence suggests that combinations of complementary strategies, such as duloxetine combined with pregabalin, may result in significantly greater numbers of FM patients achieving ≥30% pain reduction compared with monotherapy with either agent alone or placebo.92
- Antidepressant selection may need to be based on medication-related adverse effect profiles and the potential for drug interactions. These factors are useful to consider in delineating multimodal treatment regimens for chronic pain in light of patients’ comorbidities and co-medication regimen. For example, the adverse effects of TCAs (anticholinergic and alpha-adrenergic influences) limit their utility for treating pain. Some of these effects can be more problematic in select populations, such as older adults or those with orthostatic difficulties, among others. TCAs are contraindicated in patients with closed-angle glaucoma, recent myocardial infarction, cardiac arrhythmias, poorly controlled seizures, or severe benign prostatic hypertrophy. Although the pain-mitigating effects of SNRIs have not been demonstrated to significantly exceed those of TCAs,68,93,94 SNRIs would offer an advantage of greater tolerability of adverse effects and relative safety in patients with comorbid medical conditions that would otherwise preclude TCA use. The adverse effects and common drug interactions associated with antidepressants are summarized in Table 295.
Conclusion
Chronic, nonmalignant pain conditions afflict many patients and significantly impair their ability to function. Because of heightened concerns related to the appropriateness of, and restricting inordinate access to, long-term opioid analgesics, clinicians need to explore the usefulness of co-analgesic agents, such as antidepressants. Significant comorbidities exist between psychiatric disorders and chronic pain, and psychiatrists are uniquely positioned to diagnose and treat psychiatric comorbidities, as well as pain, among their patients, especially since they understand the kinetics and dynamics of antidepressants.
Bottom Line
Antidepressants can alleviate symptoms of depression and pain. Noradrenergic and serotonergic antidepressants appear to have efficacy for pain associated with neuropathy and fibromyalgia, while selective serotonin reuptake inhibitors and tricyclic antidepressants may have benefit for patients with irritable bowel syndrome. However, evidence regarding which patients will best respond to which specific antidepressant is limited.
Continue to: Related Resources
Related Resources
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
- Maletic V, Demuri B. Chronic pain and depression: treatment of 2 culprits in common. Current Psychiatry. 2016;15(3):41,47-50,52.
Drug Brand Names
Amitriptyline • Elavil, Endep
Bupropion • Wellbutrin, Zyban
Carisoprodol • Rela, Soma
Cyclobenzaprine • Amrix, Flexeril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Horizant, Neurontin
Imipramine • Tofranil
Levomilnacipran • Fetzima
Methadone • Dolophine, Methadose
Milnacipran • Savella
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Pregabalin • Lyrica, Lyrica CR
Tapentadol • Nucynta
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Warfarin • Coumadin, Jantoven
Approximately 55 years ago, tricyclic antidepressants (TCAs) began to be used to treat neuropathic pain.1 Eventually, clinical trials emerged suggesting the utility of TCAs for other chronic pain conditions, such as fibromyalgia (FM) and migraine prophylaxis. However, despite TCAs’ effectiveness in mitigating painful conditions, their adverse effects limited their use.
Pharmacologic advancements have led to the development of other antidepressant classes, including selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), and the use of these agents has come to eclipse that of TCAs. In the realm of pain management, such developments have raised the hope of possible alternative co-analgesic agents that could avoid the adverse effects associated with TCAs. Some of these agents have demonstrated efficacy for managing chronic pain states, while others have demonstrated only limited utility.
This article provides a synopsis of systematic reviews and meta-analyses examining the role of antidepressant therapy for managing several chronic pain conditions, including pain associated with neuropathy, FM, headache, and irritable bowel syndrome (IBS). Because the literature base is rapidly evolving, it is necessary to revisit the information gleaned from clinical data with respect to treatment effectiveness, and to clarify how antidepressants might be positioned in the management of chronic pain.
The effectiveness of antidepressants for pain
The pathophysiologic processes that precipitate and maintain chronic pain conditions are complex (Box 12-10). The pain-mitigating effects of antidepressants can be thought of in terms of direct analgesic effects and indirect effects (Box 22,3,8,10,11-33).
Box 1
The pathophysiologic processes precipitating and maintaining chronic pain conditions are complex. Persistent and chronic pain results from changes in sensitivity within both ascending pathways (relaying pain information from the periphery to the spinal cord and brain) and descending pain pathways (functioning to modulate ascending pain information).2,3 Tissue damage or peripheral nerve injury can lead to a cascade of neuroplastic changes within the CNS, resulting in hyperexcitability within the ascending pain pathways.
The descending pain pathways consist of the midbrain periaqueductal gray area (PGA), the rostroventral medulla (RVM), and the dorsolateral pontomesencephalic tegmentum (DLPT). The axons of the RVM (the outflow of which is serotonergic) and DLPT (the outflow of which is noradrenergic) terminate in the dorsal horn of the spinal cord,4 and thereby dampen pain signals arising from the periphery. Diminished output from descending pain pathways can heighten the pain experience. Input from the cortex, hypothalamus, and amygdala (among other structures) converges upon the PGA, RVM and DLPT, and can influence the degree of pain modulation emerging from descending pathways. In this way, thoughts, appraisals, and mood are believed to comprise cognitive and affective modifiers of pain experiences.
Devising effective chronic pain treatment becomes challenging; multimodal treatment approaches often are advocated, including pharmacologic treatment with analgesics in combination with co-analgesic medications such as antidepressants. Although a description of multimodal treatment is beyond the scope of this article, such treatment also would encompass physical therapy, occupational therapy, and psychotherapeutic interventions to augment rehabilitative efforts and the functional capabilities of patients who struggle with persisting pain.
Although the direct pain-mitigating effects of antidepressants are not fully understood, it is believed that augmentation of monoamine neurotransmission from supraspinal nuclei (ie, the RVM and DLPT) modulate pain transmission from the periphery.5,6 In addition, there is evidence that some effects of tricyclic antidepressants can modulate several other functions that impact peripheral and central sensitization.7-10
During the last several decades, antidepressants have been used to address—and have demonstrated clinical utility for—a variety of chronic pain states. However, antidepressants are not a panacea; some chronic pain conditions are more responsive to antidepressants than are others. The chronic painful states most amenable to antidepressants are those that result primarily from a process of neural sensitization, as opposed to acute somatic or visceral nociception. Hence, several meta-analyses and evidence-based reviews have long suggested the usefulness of antidepressants for mitigating pain associated with neuropathy,34,35 FM,36,37 headache,38 and IBS.39,40
Box 2
The pain-mitigating effects of antidepressants can be thought of in terms of direct analgesic effects (impacting neurotransmission of descending pathways independent of influences on mood) and indirect effects (presumably impacting cortical and limbic output to the periaqueductal gray area, the rostroventral medulla, and the dorsolateral pontomesencephalic tegmentum brought about by improvement in mood and/or cognitive appraisals) (Figure2,3,8,10,11,15,20,22,28,29). Support for the direct analgesic effects has been garnered from initial empirical work that demonstrated pain relief among patients with pain who are not depressed. Additionally, among patients who have depression and experience pain, analgesia reportedly often occurs within 2 weeks, which is before antidepressant effects are appreciated,11-15 and, at least for some antidepressants, occurs at doses far lower than those required to produce mood-elevating effects.11,12,16
On the other hand, it is well established that significant comorbidities exist between chronic pain states and psychiatric disorders (eg, depression and somatic symptom and related disorders).17-21 There may be common physiological substrates underlying chronic pain and depression.20,22 There are bidirectional influences of limbic (affective) systems and those CNS structures involved in pain processing and integration. The effects of pain and depression are reciprocal; the presence of one makes the management of the other more challenging.23-27 Mood disturbances can, therefore, impact pain processing by acting as affective and cognitive amplifiers of pain by leading to catastrophizing, pain severity augmentation, poor coping with pain-related stress, etc.28,29 It is plausible that the mood-elevating effects of antidepressants can improve pain by indirect effects, by modulating limbic activity, which in turn, impacts coping, cognitive appraisals of pain, etc.
Patients with somatoform disorders (using pre-DSM-5 terminology) frequently present with chronic pain, often in multiple sites.19 Such patients are characterized by hypervigilance for, and a predisposition to focus on, physical sensations and to appraise these sensations as reflecting a pathological state.30 Neuroimaging studies have begun to identify those neural circuits involved in somatoform disorders, many of which act as cognitive and affective amplifiers of visceral-somatic sensory processing. Many of these neural circuits overlap, and interact with, those involved in pain processing.31 Antidepressants can mitigate the severity of unexplained physical complaints, including pain, among patients who somatize32,33; however, due to the heterogeneity of studies upon which this claim is based, the quality of the evidence is reportedly low.33 There is uncertainty whether, or to what extent, antidepressant benefits among patients who somatize are due to a direct impact on pain modulation, or indirect effects on mood or cognitive appraisals/perceptions.
Despite the uncertainties about the exact mechanisms through which antidepressants exert analgesic effects, antidepressants can be appropriately used to treat patients with selected chronic pain syndromes, regardless of whether or not the patient has a psychiatric comorbidity. For those patients with pain and psychiatric comorbidities, the benefits may be brought about via direct mechanisms, indirect mechanisms, or a combination of both.

Continue to: Neuropathic pain
Neuropathic pain
Several treatment guidelines advocate for the use of antidepressants for neuropathic pain.41-44 For decades, TCAs have been employed off-label to successfully treat many patients with neuropathic pain states. Early investigations suggested that TCAs were robustly efficacious in managing patients with neuropathy.45-48 Calculated number-needed-to-treat (NNT) values for TCAs were quite low (ie, reflecting that few patients would need to be treated to yield a positive response in one patient compared with placebo), and were comparable to, if not slightly better than, the NNTs generated for anticonvulsants and α2-δ ligands, such as gabapentin or pregabalin.45-48
Unfortunately, early studies involving TCAs conducted many years ago do not meet contemporary standards of methodological rigor; they featured relatively small samples of patients assessed for brief post-treatment intervals with variable outcome measures. Thus, the NNT values obtained in meta-analyses based on these studies may overestimate treatment benefits.49 Further, NNT values derived from meta-analyses tended to combine all drugs within a particular antidepressant class (eg, amitriptyline, nortriptyline, desipramine, and imipramine among the TCAs) employed at diverse doses. Taken together, these limitations raise questions about the results of those meta-analyses.
Subsequent meta-analyses, which employed strict criteria to eliminate data from studies with potential sources of bias and used a primary outcome of frequencies of patients reporting at least 30% pain reduction compared with a placebo-controlled sample, suggest that the effectiveness of TCAs as a class for treating neuropathic pain is not as compelling as once was thought. Meta-analyses of studies employing specific TCAs revealed that there was little evidence to support the use of desipramine,50 imipramine,51 or nortriptyline52 in managing diabetic neuropathy or postherpetic neuralgia. Studies evaluating amitriptyline (dose range 12.5 to 150 mg/d), found low-level evidence of effectiveness; the benefit was expected to be present for a small subset (approximately 25%) of patients with neuropathic pain.53
There is moderate-quality evidence that duloxetine (60 to 120 mg/d) can produce a ≥50% improvement in pain severity ratings among patients with diabetic peripheral neuropathy.54 Although head-to-head studies with other antidepressants are limited, it appears that duloxetine and amitriptyline have comparable efficacy, even though the NNTs for amitriptyline were derived from lower-quality studies than those for duloxetine. Duloxetine is the only antidepressant to receive FDA approval for managing diabetic neuropathy. By contrast, studies assessing the utility of venlafaxine in neuropathic pain comprised small samples for brief durations, which limits the ability to draw clear (unbiased) support for its usefulness.55
Given the diversity of pathophysiologic processes underlying the disturbances that cause neuropathic pain disorders, it is unsurprising that the effectiveness of amitriptyline and duloxetine were not generalizable to all neuropathic pain states. Although amitriptyline produced pain-mitigating effects in patients with diabetic neuropathy and post-herpetic neuralgia, and duloxetine mitigated pain among patients with diabetic neuropathy, there was no evidence to suggest their effectiveness in phantom limb pain or human immunodeficiency virus-related and spinal cord-related neuropathies.35,53,54,56-58
Continue to: Fibromyalgia
Fibromyalgia
As with the issues encountered in interpreting the effectiveness of antidepressants in neuropathic pain, interpreting results gleaned from clinical trials of antidepressants for treating FM are fraught with similar difficulties. Although amitriptyline has been a first-line treatment for FM for many years, the evidence upon which such recommendations were based consisted of low-level studies that had a significant potential for bias.59 Large randomized trials would offer more compelling data regarding the efficacy of amitriptyline, but the prohibitive costs of such studies makes it unlikely they will be conducted. Amitriptyline (25 to 50 mg/d) was effective in mitigating FM-related pain in a small percentage of patients studied, with an estimated NNT of 4.59 Adverse effects, often contributing to treatment discontinuation, were encountered more frequently among patients who received amitriptyline compared with placebo.
Selective serotonin reuptake inhibitors failed to demonstrate significant pain relief (estimated NNT of 10), or improvement in fatigue or sleep problems, even though the studies upon which such conclusions were based were low-level studies with a high potential for bias.60 Although SSRIs have limited utility for mitigating pain, they are still quite useful for reducing depression among patients with FM.60
By contrast, the SNRIs duloxetine and milnacipran provided clinically relevant benefit over placebo in the frequency of patients reporting pain relief of ≥30%, as well as patients’ global impression of change.61 These agents, however, failed to provide clinically relevant benefit over placebo in improving health-related quality of life, reducing sleep problems, or improving fatigue. Nonetheless, duloxetine and milnacipran are FDA-approved for managing pain in FM. Studies assessing the efficacy of venlafaxine in the treatment of FM to date have been limited by small sample sizes, inconsistent dosing, lack of a placebo control, and lack of blinding, which limits the ability to clearly delineate the role of venlafaxine in managing FM.62
Mirtazapine (15 to 45 mg/d) showed a clinically relevant benefit compared with placebo for participant-reported pain relief of ≥30% and sleep disturbances. There was no benefit in terms of participant-reported improvement of quality of life, fatigue, or negative mood.63 The evidence was considered to be of low quality overall.
Headache
Amitriptyline has been employed off-label to address headache prophylaxis since 1964.64 Compared with placebo, it is efficacious in ameliorating migraine frequency and intensity as well as the frequency of tension headache.65,66 However, SSRIs and SNRIs (venlafaxine) failed to produce significant reductions in migraine frequency or severity or the frequencies of tension headache when compared with placebo.67,68
Continue to: Irritable bowel syndrome
Irritable bowel syndrome
Early studies addressing antidepressant efficacy in IBS reveal inconsistencies. For example, whereas some suggest that TCAs are effective in mitigating chronic, severe abdominal pain,39,40 others concluded that TCAs failed to demonstrate a significant analgesic benefit.69 A recent meta-analysis that restricted analysis of efficacy to randomized controlled trials (RCTs) with more rigorous methodological adherence found that pain relief in IBS is possible with both TCAs as well as SSRIs. However, adverse effects were more commonly encountered with TCAs than with SSRIs. Some of the inconsistencies in treatment efficacy reported in early studies may be due to variations in responsiveness of subsets of IBS patients. Specifically, the utility of TCAs appears to be best among patients with diarrheal-type (as opposed to constipation-type) IBS, presumably due to TCAs’ anticholinergic effects, whereas SSRIs may provide more of a benefit for patients with predominantly constipation-type IBS.40,70
Other chronic pain conditions
Antidepressants have been used to assist in the management of several other pain conditions, including oral-facial pain, interstitial cystitis, non-cardiac chest pain, and others. The role of antidepressants for such conditions remains unclear due to limitations in the prevailing empirical work, such as few trials, small sample sizes, variations in outcome measures, and insufficient randomization and blinding.71-76 The interpretation of results from systematic reviews and meta-analyses is limited because of these shortcomings.77 Hence, it has not always been possible to determine whether, and to what extent, patients with such conditions may benefit from antidepressants.
Neuromodulatory effects and efficacy for pain
The interplay of norepinephrine (NE) and serotonin (5-HT) neurotransmitter systems and cellular mechanisms involved in the descending modulation of pain pathways is complex. Experimental animal models of pain modulation suggest that 5-HT can both inhibit as well as promote pain perception by different physiological mechanisms, in contrast to NE, which is predominately inhibitory. While 5-HT in the descending modulating system can inhibit pain transmission ascending to the brain from the periphery, it appears that an intact noradrenergic system is necessary for the inhibitory influences of the serotonergic system to be appreciated.16,78,79 Deficiencies in one or both of these neurotransmitter systems may contribute to hyperactive pain processing, and thereby precipitate or maintain chronic pain.
Pain mitigation may be achieved best by enhancing both neurotransmitters simultaneously, less so by enhancing NE alone, and least by enhancing 5-HT alone.6 The ability to impact pain modulation would, therefore, depend on the degree to which an antidepressant capitalizes on both noradrenergic and serotonergic neurotransmission. Antidepressants commonly employed to manage pain are presented in Table 147,60,68,80-88 according to their primary neurotransmitter effects. Thus, the literature summarized above suggests that antidepressants that influence both NE and 5-HT transmission have greater analgesic effects than antidepressants with more specific effects, such as influencing 5-HT reuptake alone.80-85 It is unsurprising, therefore, that the SSRIs have not been demonstrated to be as consistently analgesic.47,60,68,80,86-88
Similarly, pharmacodynamic and pharmacokinetic differences within antidepressant classes may influence analgesic effectiveness. Simultaneous effects on NE and 5-HT are achieved at low doses with duloxetine and milnacipran. By contrast, 5-HT effects predominate at low doses for venlafaxine. To achieve pain-mitigating effects, higher doses of venlafaxine generally are required.89 Therefore, inconsistencies across studies regarding the analgesic benefits of venlafaxine may be attributable to variability in dosing; patients treated with lower doses may not have experienced sufficient NE effects to garner positive results.
Continue to: The differences in analgesic efficacy...
The differences in analgesic efficacy among specific TCAs may be understood in a similar fashion. Specifically, tertiary TCAs (imipramine and amitriptyline) inhibit both 5-HT and NE reuptake.6,90 Secondary amines (desipramine and nortriptyline) predominantly impact NE reuptake, possibly accounting for the lesser pain-mitigating benefit achieved with these agents, such as for treating neuropathic pain. Further, in vivo imipramine and amitriptyline are rapidly metabolized to secondary amines that are potent and selective NE reuptake inhibitors. In this way, the secondary amines may substantially lose the ability to modulate pain transmission because of the loss of concurrent 5-HT influences.90
Clinical pearls
The following practical points can help guide clinicians regarding the usefulness of antidepressants for pain management:
- Antidepressants can alleviate symptoms of depression and pain. The pain-mitigating effects of antidepressants are possible even among chronic pain patients who are not depressed. Antidepressants may confer benefits for chronic pain patients with depression and other comorbid conditions, such as somatic symptom and related disorders.
- Antidepressants are useful for select chronic pain states. Although the noradrenergic and serotonergic antidepressants (SNRIs and, to some extent, amitriptyline) appear to have efficacy for neuropathic pain and FM, the benefits of SSRIs appear to be less robust. On the other hand, SSRIs and TCAs may have potential benefit for patients with IBS. However, the results of meta-analyses are limited in the ability to provide information about which patients will best respond to which specific antidepressant or how well. Future research directed at identifying characteristics that can predict which patients are likely to benefit from one antidepressant vs another would help inform how best to tailor treatment to individual needs.
- The pain-mitigating effects of antidepressants often emerge early in the course of treatment (often before mood-elevating effects are observed). For example, in the case of amitriptyline, pain relief may be possible for some patients at doses generally lower than those required for mood-elevating effects. To date, there is limited information in the literature to determine what constitutes a sufficient duration of treatment, or when treatment should be modified.
- Failure to reduce pain should raise questions about whether the dose should be increased, an alternative agent should be tried, or combinations with other analgesic agents should be considered. Failure to achieve pain-mitigating effects with one antidepressant does not mean failure with others. Hence, failure to achieve desired effects with one agent might warrant an empirical trial with another agent. Presently, too few double-blind RCTs have been conducted to assess the pain-mitigating effects of other antidepressants (eg, bupropion and newer SNRIs such as desvenlafaxine and levomilnacipran). Meta-analysis of the analgesic effectiveness of these agents or comparisons to the efficacy of other antidepressant classes is, therefore, impossible at this time.
Because many chronic pain states are complex, patients will seldom experience clinically relevant benefit from any one intervention.53 The bigger implication for clinical research is to determine whether there is a sequence or combination of medication use that will provide overall better clinical effectiveness.53 Only limited data are available exploring the utility of combining pharmacologic approaches to address pain.91 For example, preliminary evidence suggests that combinations of complementary strategies, such as duloxetine combined with pregabalin, may result in significantly greater numbers of FM patients achieving ≥30% pain reduction compared with monotherapy with either agent alone or placebo.92
- Antidepressant selection may need to be based on medication-related adverse effect profiles and the potential for drug interactions. These factors are useful to consider in delineating multimodal treatment regimens for chronic pain in light of patients’ comorbidities and co-medication regimen. For example, the adverse effects of TCAs (anticholinergic and alpha-adrenergic influences) limit their utility for treating pain. Some of these effects can be more problematic in select populations, such as older adults or those with orthostatic difficulties, among others. TCAs are contraindicated in patients with closed-angle glaucoma, recent myocardial infarction, cardiac arrhythmias, poorly controlled seizures, or severe benign prostatic hypertrophy. Although the pain-mitigating effects of SNRIs have not been demonstrated to significantly exceed those of TCAs,68,93,94 SNRIs would offer an advantage of greater tolerability of adverse effects and relative safety in patients with comorbid medical conditions that would otherwise preclude TCA use. The adverse effects and common drug interactions associated with antidepressants are summarized in Table 295.
Conclusion
Chronic, nonmalignant pain conditions afflict many patients and significantly impair their ability to function. Because of heightened concerns related to the appropriateness of, and restricting inordinate access to, long-term opioid analgesics, clinicians need to explore the usefulness of co-analgesic agents, such as antidepressants. Significant comorbidities exist between psychiatric disorders and chronic pain, and psychiatrists are uniquely positioned to diagnose and treat psychiatric comorbidities, as well as pain, among their patients, especially since they understand the kinetics and dynamics of antidepressants.
Bottom Line
Antidepressants can alleviate symptoms of depression and pain. Noradrenergic and serotonergic antidepressants appear to have efficacy for pain associated with neuropathy and fibromyalgia, while selective serotonin reuptake inhibitors and tricyclic antidepressants may have benefit for patients with irritable bowel syndrome. However, evidence regarding which patients will best respond to which specific antidepressant is limited.
Continue to: Related Resources
Related Resources
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
- Maletic V, Demuri B. Chronic pain and depression: treatment of 2 culprits in common. Current Psychiatry. 2016;15(3):41,47-50,52.
Drug Brand Names
Amitriptyline • Elavil, Endep
Bupropion • Wellbutrin, Zyban
Carisoprodol • Rela, Soma
Cyclobenzaprine • Amrix, Flexeril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Horizant, Neurontin
Imipramine • Tofranil
Levomilnacipran • Fetzima
Methadone • Dolophine, Methadose
Milnacipran • Savella
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Pregabalin • Lyrica, Lyrica CR
Tapentadol • Nucynta
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Warfarin • Coumadin, Jantoven
1. Paoli F, Darcourt G, Cossa P. Preliminary note on the action of imipramine in painful states [in French]. Rev Neurol (Paris). 1960;102:503-504.
2. Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219-245.
3. Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2(2):83-91.
4. Lamont LA, Tranquilli WJ, Grimm KA. Physiology of pain. Vet Clin North Am Small Anim Pract. 2000;30(4):703-728, v.
5. Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, eds. Wall and Melzack’s Textbook of Pain. 5th ed. Burlington, MA: Elsevier Health Sciences; 2005:125-142.
6. Marks DM, Shah MJ, Patkar AA, et al. Serotonin-norepinephrine reuptake inhibitors for pain control: premise and promise. Curr Neuropharmacol. 2009;7(4):331-336.
7. Baba H, Shimoji K, Yoshimura M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology. 2000;92(2):473-484.
8. Carter GT, Sullivan MD. Antidepressants in pain management. Curr Opin Investig Drugs. 2002;3(3):454-458.
9. Kawasaki Y, Kumamoto E, Furue H, et al. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology. 2003;98(3):682-689.
10. McCleane G. Antidepressants as analgesics. CNS Drugs. 2008;22(2):139-156.
11. Ansari A. The efficacy of newer antidepressants in the treatment of chronic pain: a review of current literature. Harv Rev Psychiatry. 2000;7(5):257-277.
12. Egbunike IG, Chaffee BJ. Antidepressants in the management of chronic pain syndromes. Pharmacotherapy. 1990;10(4):262-270.
13. Fishbain DA. Evidence-based data on pain relief with antidepressants. Ann Med. 2000;32(5):305-316.
14. Fishbain DA, Detke MJ, Wernicke J, et al. The relationship between antidepressant and analgesic responses: findings from six placebo-controlled trials assessing the efficacy of duloxetine in patients with major depressive disorder. Curr Med Res Opin. 2008;24(11):3105-3115.
15. Harada E, Tokuoka H, Fujikoshi S, et al. Is duloxetine’s effect on painful physical symptoms in depression an indirect result of improvement of depressive symptoms? Pooled analyses of three randomized controlled trials. Pain. 2016;157(3):577-584.
16. Kehoe WA. Antidepressants for chronic pain: selection and dosing considerations. Am J Pain Med. 1993;3(4):161-165.
17. Damush TM, Kroenke K, Bair MJ, et al. Pain self-management training increases self-efficacy, self-management behaviours and pain and depression outcomes. Eur J Pain. 2016;20(2):1070-1078.
18. DeVeaugh-Geiss AM, West SL, Miller WC, et al. The adverse effects of comorbid pain on depression outcomes in primary care patients: results from the ARTIST trial. Pain Medicine. 2010;11(5):732-741.
19. Egloff N, Cámara RJ, von Känel R, et al. Hypersensitivity and hyperalgesia in somatoform pain disorders. Gen Hosp Psychiatry. 2014;36(3):284-290.
20. Goesling J, Clauw DW, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psych Reports. 2013;15(12):421.
21. Kroenke K, Wu J, Bair MJ, et al. Reciprocal relationship between pain and depression: a 12-Month longitudinal analysis in primary care. J Pain. 2011;12(9):964-973.
22. Leo RJ. Chronic pain and comorbid depression. Curr Treat Options Neurol. 2005;7(5):403-412.
23. Bair MJ, Robinson RL, Eckert GJ, et al. Impact of pain on depression treatment response in primary care. Psychosom Med. 2004;66(1):17-22.
24. Karp JF, Scott J, Houck P, et al. Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry. 2005;66(5):591-597.
25. Kroenke K, Shen J, Oxman TE, et al. Impact of pain on the outcomes of depression treatment: results from the RESPECT trial. Pain. 2008;134(1-2):209-215.
26. Mavandadi S, Ten Have TR, Katz IR, et al. Effect of depression treatment on depressive symptoms in older adulthood: the moderating role of pain. J Am Geriatr Soc. 2007;55(2):202-211.
27. Thielke SM, Fan MY, Sullivan M, et al. Pain limits the effectiveness of collaborative care for depression. Am J Geriatr Psychiatry. 2007;15(8):699-707.
28. Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68(2):262-268.
29. Demyttenaere K, Bonnewyn A, Bruffaerts R, et al. Comorbid painful physical symptoms and depression: Prevalence, work loss, and help seeking. J Affect Disord. 2006;92(2-3):185-193.
30. Nakao M, Barsky AJ. Clinical application of somatosensory amplification in psychosomatic medicine. Biopsychosoc Med. 2007;1:17.
31. Perez DL, Barsky AJ, Vago DR, et al. A neural circuit framework for somatosensory amplification in somatoform disorders. J Neuropsychiatry Clin Neurosci. 2015;27(1):e40-e50.
32. Fishbain DA, Cutler RB, Rosomoff HL, et al. Do antidepressants have an analgesic effect in psychogenic pain and somatoform pain disorder? A meta-analysis. Psychosom Med. 1998;60(4):503-509.
33. Kleinstäuber M, Witthöft M, Steffanowski A, et al. Pharmacological interventions for somatoform disorders in adults. Cochrane Database Syst Rev. 2014;(11):CD010628.
34. Collins SL, Moore RA, McQuay HJ, et al. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: a quantitative systematic review. J Pain Symptom Manage. 2000;20(6):449-458.
35. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain: a Cochrane review. J Neurol Neurosurg Psychiatry. 2010;81(12):1372-1373.
36. Arnold LM, Keck PE, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41(2):104-113.
37. O’Malley PG, Balden E, Tomkins G, et al. Treatment of fibromyalgia with antidepressants: a meta-analysis. J Gen Intern Med. 2000;15(9):659-666.
38. Tomkins GE, Jackson JL, O’Malley PG, et al. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med. 2001;111(1):54-63.
39. Jackson JL, O’Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108(1):65-72.
40. Lesbros-Pantoflickova D, Michetti P, Fried M et al. Meta-analysis: the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(11-12):1253-1269.
41. Centre for Clinical Practice at NICE (UK). Neuropathic pain: the pharmacological management of neuropathic pain in adults in non-specialist settings. London, UK: National Institute for Health and Care Excellence, (UK); 2013.
42. O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(suppl 10):S22-S32.
43. Moulin D, Boulanger A, Clark AJ, et al; Canadian Pain Society. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328-35.
44. Mu A, Weinberg E, Moulin DE, et al. Pharmacologic management of chronic neuropathic pain: Review of the Canadian Pain Society consensus statement. Can Fam Physician. 2017;63(11):844-852.
45. Finnerup NB, Otto M, McQuay HJ, et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289-305.
46. Hempenstall K, Nurmikko TJ, Johnson RW, et al. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2(7):e164.
47. Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83(3):389-400.
48. Wu CL, Raja SN. An update on the treatment of postherpetic neuralgia. J Pain. 2008;9(suppl 1):S19-S30.
49. Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206-219.
50. Hearn L, Moore RA, Derry S, et al. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(9):CD011003.
51. Hearn L, Derry S, Phillips T, et al. Imipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(5):CD010769.
52. Derry S, Wiffen PJ, Aldington D, et al. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;1:CD011209.
53. Moore R, Derry S, Aldington D, et al. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(7):CD008242.
54. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115.
55. Gallagher HC, Gallagher RM, Butler M, et al. Venlafaxine for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(8):CD011091.
56. Alviar MJ, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev. 2016;10:CD006380.
57. Dinat N, Marinda E, Moch S, et al. Randomized, Double-Blind, Crossover Trial of Amitriptyline for Analgesia in Painful HIV-Associated Sensory Neuropathy. PLoS One. 2015;10(5):e0126297. doi: 10.1371/journal.pone.0126297.eCollection 2015.
58. Mehta S, McIntyre A, Janzen S, et al; Spinal Cord Injury Rehabilitation Evidence Team. Systematic review of pharmacologic treatments of pain after spinal cord injury: an update. Arch Phys Med Rehabil. 2016;97(8):1381-1391.e1.
59. Moore RA, Derry S, Aldington D, et al. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;(12):CD008242..
60. Walitt B, Urrútia G, Nishishinya MB, et al. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015;(6):CD011735.
61. Welsch P, Üçeyler N, Klose P, et al. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia. Cochrane Database Syst Rev. 2018;(2):CD010292.
62. VanderWeide LA, Smith SM, Trinkley KE. A systematic review of the efficacy of venlafaxine for the treatment of fibromyalgia. J Clin Pharm Ther. 2015;40(1):1-6.
63. Welsch P, Bernardy K, Derry S, et al. Mirtazapine for fibromyalgia in adults. Cochrane Database Syst Rev. 2018;(8):CD012708.
64. Lance JW, Curran DA. Treatment of chronic tension headache. Lancet. 1964;283(7345):1236-1239.
65. Jackson JL, William S, Laura S, et al. Tricyclic antidepressants and headaches: systematic review and meta-analysis. BMJ. 2010;341:c5222. doi: https://doi.org/10.1136/bmj.c5222
66. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine. 2017;96(22):e6989. doi: 10.1097/MD.0000000000006989.
67. Banzi R, Cusi C, Randazzo C, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of migraine in adults. Cochrane Database Syst Rev. 2015;(4):CD002919.
68. Banzi R, Cusi C, Randazzo C, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of tension-type headache in adults. Cochrane Database Syst Rev. 2015;(5):CD011681.
69. Quartero AO, Meineche-Schmidt V, Muris J, et al. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2005;(2):CD003460.
70. Ford AC, Talley NJ, Schoenfeld PS, et al. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58(3):367-378.
71. Coss-Adame E, Erdogan A, Rao SS. Treatment of esophageal (noncardiac) chest pain: an expert review. Clin Gastroenterol Hepatol. 2014;12(8):1224-1245.
72. Kelada E, Jones A. Interstitial cystitis. Arch Gynecol Obstet. 2007;275(4):223-229.
73. Leo RJ, Dewani S. A systematic review of the utility of antidepressant pharmacotherapy in the treatment of vulvodynia pain. J Sex Med. 2013;10(10):2497-2505.
74. McMillan R, Forssell H, Buchanan JA, et al. Interventions for treating burning mouth syndrome. Cochrane Database Syst Rev. 2016;11:CD002779.
75. Patel DN. Inconclusive results of a systematic review of efficacy of antidepressants on orofacial pain disorders. Evid Based Dent. 2013;14(2):55-56.
76. Wang W, Sun YH, Wang YY, et al. Treatment of functional chest pain with antidepressants: a meta-analysis. Pain Physician. 2012;15(2):E131-E142.
77. Lavis JN. How can we support the use of systematic reviews in policymaking? PLoS Med. 2009;6(11):e1000141. doi: 10.1371/journal.pmed.1000141.
78. Sorkin L. Nociceptive transmission within the spinal cord. Mt Sinai J Med. 1991;58(3):208-216.
79. Yokogawa F, Kiuchi Y, Ishikawa Y, et al. An investigation of monoamine receptors involved in antinociceptive effects of antidepressants. Anesth Analg. 2002;95(1):163-168, table of contents.
80. Lynch ME. Antidepressants as analgesics: a review of randomized controlled trials. J Psychiatry Neurosci. 2001;26(1):30-36.
81. Max MB. Treatment of post-herpetic neuralgia: antidepressants. Ann Neurol. 1994;35(suppl):S50-S53.
82. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
83. McQuay HJ, Tramèr M, Nye BA, et al. A systematic review of antidepressants in neuropathic pain. Pain. 1996;68(2-3):217-227.
84. Mochizucki D. Serotonin and noradrenaline reuptake inhibitors in animal models of pain. Hum Psychopharmacol Clin Exp. 2004;19(suppl 1):15-19.
85. Sussman N. SNRIs versus SSRIs: mechanisms of action in treating depression and painful physical symptoms. Primary Care Companion J Clin Psychiatry. 2003;5(suppl 7):19-26.
86. Bundeff AW, Woodis CB. Selective serotonin reuptake inhibitors for the treatment of irritable bowel syndrome. Ann Pharmacother. 2014;48(6):777-784.
87. Jung AC, Staiger T, Sullivan M. The efficacy of selective serotonin reuptake inhibitors for the management of chronic pain. J Gen Intern Med. 1997;12(6):384-389.
88. Xie C, Tang Y, Wang Y, et al. Efficacy and safety of antidepressants for the treatment of irritable bowel syndrome: a meta-analysis. PLoS One. 2015;10(8):e0127815. doi: 10.1371/journal.pone.0127815. eCollection 2015.
89. Zijlstra TR , Barendregt PJ , van de Laar MA. Venlafaxine in fibromyalgia: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 2002;46(suppl 9):S105.
90. Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology. 2001;25(6):871-880.
91. Thorpe J, Shum B, Moore RA, et al. Combination pharmacotherapy for the treatment of fibromyalgia in adults. Cochrane Database Syst Rev. 2018;(2):CD010585.
92. Gilron I, Chaparro LE, Tu D, et al. Combination of pregabalin with duloxetine for fibromyalgia: a randomized controlled trial. Pain. 2016;157(7):1532-1540.
93. Häuser W, Petzke F, Üçeyler N, et al. Comparative efficacy and acceptability of amitriptyline, duloxetine and milnacipran in fibromyalgia syndrome: a systematic review with meta-analysis. Rheumatology (Oxford). 2011;50(3):532-543.
94. Hossain SM, Hussain SM, Ekram AR. Duloxetine in painful diabetic neuropathy: a systematic review. Clin J Pain. 2016;32(11):1005-1010.
95. Riediger C, Schuster T, Barlinn K, et al. Adverse effects of antidepressants for chronic pain: a systematic review and meta-analysis. Front Neurol. 2017;8:307.
1. Paoli F, Darcourt G, Cossa P. Preliminary note on the action of imipramine in painful states [in French]. Rev Neurol (Paris). 1960;102:503-504.
2. Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219-245.
3. Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2(2):83-91.
4. Lamont LA, Tranquilli WJ, Grimm KA. Physiology of pain. Vet Clin North Am Small Anim Pract. 2000;30(4):703-728, v.
5. Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, eds. Wall and Melzack’s Textbook of Pain. 5th ed. Burlington, MA: Elsevier Health Sciences; 2005:125-142.
6. Marks DM, Shah MJ, Patkar AA, et al. Serotonin-norepinephrine reuptake inhibitors for pain control: premise and promise. Curr Neuropharmacol. 2009;7(4):331-336.
7. Baba H, Shimoji K, Yoshimura M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology. 2000;92(2):473-484.
8. Carter GT, Sullivan MD. Antidepressants in pain management. Curr Opin Investig Drugs. 2002;3(3):454-458.
9. Kawasaki Y, Kumamoto E, Furue H, et al. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology. 2003;98(3):682-689.
10. McCleane G. Antidepressants as analgesics. CNS Drugs. 2008;22(2):139-156.
11. Ansari A. The efficacy of newer antidepressants in the treatment of chronic pain: a review of current literature. Harv Rev Psychiatry. 2000;7(5):257-277.
12. Egbunike IG, Chaffee BJ. Antidepressants in the management of chronic pain syndromes. Pharmacotherapy. 1990;10(4):262-270.
13. Fishbain DA. Evidence-based data on pain relief with antidepressants. Ann Med. 2000;32(5):305-316.
14. Fishbain DA, Detke MJ, Wernicke J, et al. The relationship between antidepressant and analgesic responses: findings from six placebo-controlled trials assessing the efficacy of duloxetine in patients with major depressive disorder. Curr Med Res Opin. 2008;24(11):3105-3115.
15. Harada E, Tokuoka H, Fujikoshi S, et al. Is duloxetine’s effect on painful physical symptoms in depression an indirect result of improvement of depressive symptoms? Pooled analyses of three randomized controlled trials. Pain. 2016;157(3):577-584.
16. Kehoe WA. Antidepressants for chronic pain: selection and dosing considerations. Am J Pain Med. 1993;3(4):161-165.
17. Damush TM, Kroenke K, Bair MJ, et al. Pain self-management training increases self-efficacy, self-management behaviours and pain and depression outcomes. Eur J Pain. 2016;20(2):1070-1078.
18. DeVeaugh-Geiss AM, West SL, Miller WC, et al. The adverse effects of comorbid pain on depression outcomes in primary care patients: results from the ARTIST trial. Pain Medicine. 2010;11(5):732-741.
19. Egloff N, Cámara RJ, von Känel R, et al. Hypersensitivity and hyperalgesia in somatoform pain disorders. Gen Hosp Psychiatry. 2014;36(3):284-290.
20. Goesling J, Clauw DW, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psych Reports. 2013;15(12):421.
21. Kroenke K, Wu J, Bair MJ, et al. Reciprocal relationship between pain and depression: a 12-Month longitudinal analysis in primary care. J Pain. 2011;12(9):964-973.
22. Leo RJ. Chronic pain and comorbid depression. Curr Treat Options Neurol. 2005;7(5):403-412.
23. Bair MJ, Robinson RL, Eckert GJ, et al. Impact of pain on depression treatment response in primary care. Psychosom Med. 2004;66(1):17-22.
24. Karp JF, Scott J, Houck P, et al. Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry. 2005;66(5):591-597.
25. Kroenke K, Shen J, Oxman TE, et al. Impact of pain on the outcomes of depression treatment: results from the RESPECT trial. Pain. 2008;134(1-2):209-215.
26. Mavandadi S, Ten Have TR, Katz IR, et al. Effect of depression treatment on depressive symptoms in older adulthood: the moderating role of pain. J Am Geriatr Soc. 2007;55(2):202-211.
27. Thielke SM, Fan MY, Sullivan M, et al. Pain limits the effectiveness of collaborative care for depression. Am J Geriatr Psychiatry. 2007;15(8):699-707.
28. Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68(2):262-268.
29. Demyttenaere K, Bonnewyn A, Bruffaerts R, et al. Comorbid painful physical symptoms and depression: Prevalence, work loss, and help seeking. J Affect Disord. 2006;92(2-3):185-193.
30. Nakao M, Barsky AJ. Clinical application of somatosensory amplification in psychosomatic medicine. Biopsychosoc Med. 2007;1:17.
31. Perez DL, Barsky AJ, Vago DR, et al. A neural circuit framework for somatosensory amplification in somatoform disorders. J Neuropsychiatry Clin Neurosci. 2015;27(1):e40-e50.
32. Fishbain DA, Cutler RB, Rosomoff HL, et al. Do antidepressants have an analgesic effect in psychogenic pain and somatoform pain disorder? A meta-analysis. Psychosom Med. 1998;60(4):503-509.
33. Kleinstäuber M, Witthöft M, Steffanowski A, et al. Pharmacological interventions for somatoform disorders in adults. Cochrane Database Syst Rev. 2014;(11):CD010628.
34. Collins SL, Moore RA, McQuay HJ, et al. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: a quantitative systematic review. J Pain Symptom Manage. 2000;20(6):449-458.
35. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain: a Cochrane review. J Neurol Neurosurg Psychiatry. 2010;81(12):1372-1373.
36. Arnold LM, Keck PE, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41(2):104-113.
37. O’Malley PG, Balden E, Tomkins G, et al. Treatment of fibromyalgia with antidepressants: a meta-analysis. J Gen Intern Med. 2000;15(9):659-666.
38. Tomkins GE, Jackson JL, O’Malley PG, et al. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med. 2001;111(1):54-63.
39. Jackson JL, O’Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108(1):65-72.
40. Lesbros-Pantoflickova D, Michetti P, Fried M et al. Meta-analysis: the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(11-12):1253-1269.
41. Centre for Clinical Practice at NICE (UK). Neuropathic pain: the pharmacological management of neuropathic pain in adults in non-specialist settings. London, UK: National Institute for Health and Care Excellence, (UK); 2013.
42. O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(suppl 10):S22-S32.
43. Moulin D, Boulanger A, Clark AJ, et al; Canadian Pain Society. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328-35.
44. Mu A, Weinberg E, Moulin DE, et al. Pharmacologic management of chronic neuropathic pain: Review of the Canadian Pain Society consensus statement. Can Fam Physician. 2017;63(11):844-852.
45. Finnerup NB, Otto M, McQuay HJ, et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289-305.
46. Hempenstall K, Nurmikko TJ, Johnson RW, et al. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2(7):e164.
47. Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83(3):389-400.
48. Wu CL, Raja SN. An update on the treatment of postherpetic neuralgia. J Pain. 2008;9(suppl 1):S19-S30.
49. Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206-219.
50. Hearn L, Moore RA, Derry S, et al. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(9):CD011003.
51. Hearn L, Derry S, Phillips T, et al. Imipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(5):CD010769.
52. Derry S, Wiffen PJ, Aldington D, et al. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;1:CD011209.
53. Moore R, Derry S, Aldington D, et al. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(7):CD008242.
54. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115.
55. Gallagher HC, Gallagher RM, Butler M, et al. Venlafaxine for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(8):CD011091.
56. Alviar MJ, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev. 2016;10:CD006380.
57. Dinat N, Marinda E, Moch S, et al. Randomized, Double-Blind, Crossover Trial of Amitriptyline for Analgesia in Painful HIV-Associated Sensory Neuropathy. PLoS One. 2015;10(5):e0126297. doi: 10.1371/journal.pone.0126297.eCollection 2015.
58. Mehta S, McIntyre A, Janzen S, et al; Spinal Cord Injury Rehabilitation Evidence Team. Systematic review of pharmacologic treatments of pain after spinal cord injury: an update. Arch Phys Med Rehabil. 2016;97(8):1381-1391.e1.
59. Moore RA, Derry S, Aldington D, et al. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;(12):CD008242..
60. Walitt B, Urrútia G, Nishishinya MB, et al. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015;(6):CD011735.
61. Welsch P, Üçeyler N, Klose P, et al. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia. Cochrane Database Syst Rev. 2018;(2):CD010292.
62. VanderWeide LA, Smith SM, Trinkley KE. A systematic review of the efficacy of venlafaxine for the treatment of fibromyalgia. J Clin Pharm Ther. 2015;40(1):1-6.
63. Welsch P, Bernardy K, Derry S, et al. Mirtazapine for fibromyalgia in adults. Cochrane Database Syst Rev. 2018;(8):CD012708.
64. Lance JW, Curran DA. Treatment of chronic tension headache. Lancet. 1964;283(7345):1236-1239.
65. Jackson JL, William S, Laura S, et al. Tricyclic antidepressants and headaches: systematic review and meta-analysis. BMJ. 2010;341:c5222. doi: https://doi.org/10.1136/bmj.c5222
66. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine. 2017;96(22):e6989. doi: 10.1097/MD.0000000000006989.
67. Banzi R, Cusi C, Randazzo C, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of migraine in adults. Cochrane Database Syst Rev. 2015;(4):CD002919.
68. Banzi R, Cusi C, Randazzo C, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of tension-type headache in adults. Cochrane Database Syst Rev. 2015;(5):CD011681.
69. Quartero AO, Meineche-Schmidt V, Muris J, et al. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2005;(2):CD003460.
70. Ford AC, Talley NJ, Schoenfeld PS, et al. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58(3):367-378.
71. Coss-Adame E, Erdogan A, Rao SS. Treatment of esophageal (noncardiac) chest pain: an expert review. Clin Gastroenterol Hepatol. 2014;12(8):1224-1245.
72. Kelada E, Jones A. Interstitial cystitis. Arch Gynecol Obstet. 2007;275(4):223-229.
73. Leo RJ, Dewani S. A systematic review of the utility of antidepressant pharmacotherapy in the treatment of vulvodynia pain. J Sex Med. 2013;10(10):2497-2505.
74. McMillan R, Forssell H, Buchanan JA, et al. Interventions for treating burning mouth syndrome. Cochrane Database Syst Rev. 2016;11:CD002779.
75. Patel DN. Inconclusive results of a systematic review of efficacy of antidepressants on orofacial pain disorders. Evid Based Dent. 2013;14(2):55-56.
76. Wang W, Sun YH, Wang YY, et al. Treatment of functional chest pain with antidepressants: a meta-analysis. Pain Physician. 2012;15(2):E131-E142.
77. Lavis JN. How can we support the use of systematic reviews in policymaking? PLoS Med. 2009;6(11):e1000141. doi: 10.1371/journal.pmed.1000141.
78. Sorkin L. Nociceptive transmission within the spinal cord. Mt Sinai J Med. 1991;58(3):208-216.
79. Yokogawa F, Kiuchi Y, Ishikawa Y, et al. An investigation of monoamine receptors involved in antinociceptive effects of antidepressants. Anesth Analg. 2002;95(1):163-168, table of contents.
80. Lynch ME. Antidepressants as analgesics: a review of randomized controlled trials. J Psychiatry Neurosci. 2001;26(1):30-36.
81. Max MB. Treatment of post-herpetic neuralgia: antidepressants. Ann Neurol. 1994;35(suppl):S50-S53.
82. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
83. McQuay HJ, Tramèr M, Nye BA, et al. A systematic review of antidepressants in neuropathic pain. Pain. 1996;68(2-3):217-227.
84. Mochizucki D. Serotonin and noradrenaline reuptake inhibitors in animal models of pain. Hum Psychopharmacol Clin Exp. 2004;19(suppl 1):15-19.
85. Sussman N. SNRIs versus SSRIs: mechanisms of action in treating depression and painful physical symptoms. Primary Care Companion J Clin Psychiatry. 2003;5(suppl 7):19-26.
86. Bundeff AW, Woodis CB. Selective serotonin reuptake inhibitors for the treatment of irritable bowel syndrome. Ann Pharmacother. 2014;48(6):777-784.
87. Jung AC, Staiger T, Sullivan M. The efficacy of selective serotonin reuptake inhibitors for the management of chronic pain. J Gen Intern Med. 1997;12(6):384-389.
88. Xie C, Tang Y, Wang Y, et al. Efficacy and safety of antidepressants for the treatment of irritable bowel syndrome: a meta-analysis. PLoS One. 2015;10(8):e0127815. doi: 10.1371/journal.pone.0127815. eCollection 2015.
89. Zijlstra TR , Barendregt PJ , van de Laar MA. Venlafaxine in fibromyalgia: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 2002;46(suppl 9):S105.
90. Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology. 2001;25(6):871-880.
91. Thorpe J, Shum B, Moore RA, et al. Combination pharmacotherapy for the treatment of fibromyalgia in adults. Cochrane Database Syst Rev. 2018;(2):CD010585.
92. Gilron I, Chaparro LE, Tu D, et al. Combination of pregabalin with duloxetine for fibromyalgia: a randomized controlled trial. Pain. 2016;157(7):1532-1540.
93. Häuser W, Petzke F, Üçeyler N, et al. Comparative efficacy and acceptability of amitriptyline, duloxetine and milnacipran in fibromyalgia syndrome: a systematic review with meta-analysis. Rheumatology (Oxford). 2011;50(3):532-543.
94. Hossain SM, Hussain SM, Ekram AR. Duloxetine in painful diabetic neuropathy: a systematic review. Clin J Pain. 2016;32(11):1005-1010.
95. Riediger C, Schuster T, Barlinn K, et al. Adverse effects of antidepressants for chronic pain: a systematic review and meta-analysis. Front Neurol. 2017;8:307.