User login
FDA approves intranasal esketamine for refractory major depressive disorder
The spray (Spravato; Janssen Pharmaceuticals) will come in tamper-resistant prepackaged units of one, two, or three devices to deliver the prescribed doses of 28 mg, 56 mg, or 84 mg, respectively. To reduce the risk of diversion, misuse, or abuse, the drug will managed under an FDA Risk Evaluation and Management Strategy (REMS). It will only be available to prescribing clinicians who have undergone training on the risks of esketamine and the importance of monitoring patients after their dose is administered. Facilities licensed to dispense esketamine must have the ability to medically monitor patients for at least 2 hours after administration. Patients will self-administer the spray and will not be able to take any of it home.
The REMS will require both prescriber and the patient to both sign a Patient Enrollment Form clearly stating that patients understand the necessity of assisted transport to leave the health care facility and that there should be no driving or use of heavy machinery for the rest of the day on which they are treated.
A boxed warning on the label will note that patients are at risk for sedation and difficulty with attention, judgment, and thinking (dissociation); abuse and misuse; and suicidal thoughts and behaviors after administration of the drug.
Despite the FDA’s caveats, the approval of intranasal esketamine is seen as a substantial win for the psychiatric community, Tiffany Farchione, MD, acting director of the FDA division of psychiatry products, said in a statement. “There has been a long-standing need for additional effective treatments for treatment-resistant depression, a serious and life-threatening condition.”
“Spravato has the potential to change the treatment paradigm and offer new hope to the estimated one-third of people with major depressive disorder who have not responded to existing therapies,” said Mathai Mammen, MD, PhD, global head of Janssen Research and Development.
The company “is working quickly to educate and certify treatment centers in accordance with the REMS so that health care providers can offer Spravato to appropriate patients,” according to a statement from Janssen. “Later this month, patients can visit www.SPRAVATO.com for a locater tool and to sign up to receive alerts when new treatment centers are available.”
Intranasal esketamine was evaluated in three short-term clinical trials and one longer-term maintenance-of-effect trial. One of the studies demonstrated a clinically significant effect in depression severity, in as little as 2 days for some patients. The two other short-term trials did not show significant benefit. However, in the maintenance study, patients in stable remission or with stable response who continued treatment with esketamine plus an oral antidepressant experienced a significantly longer time to relapse of depressive symptoms than patients on placebo spray plus an oral antidepressant. The most common side effects were disassociation, dizziness, nausea, sedation, vertigo, hypoesthesia, anxiety, lethargy, increased blood pressure, vomiting, and feeling drunk.
Patients with unstable or poorly controlled hypertension or pre-existing aneurysmal vascular disorders might be at increased risk for adverse cardiovascular or cerebrovascular effects. Esketamine might impair attention, judgment, thinking, reaction speed, and motor skills. It may cause fetal harm; women of childbearing age should be on reliable contraception. Breastfeeding women should not use it.
.
I’m not surprised by the FDA decision, given the strong endorsement from the advisory committees in mid-February based on the drug’s benefit-to-risk evaluation. This is an important advance for our field, and the FDA approval will allow more patients who suffer from treatment-resistant depression to gain access to this medication.
To date, ketamine (not the intranasal esketamine spray) has been offered primarily on a fee-for-service basis or in the context of a clinical trial. I anticipate this treatment to receive broad insurance coverage, but this remains to be determined.
Dr. Sanjay J. Mathew is the Marjorie Bintliff Johnson and Raleigh White Johnson Jr. Vice Chair for Research and professor in the Menninger department of psychiatry & behavioral sciences at the Baylor College of Medicine. He also is affiliated with the Michael E. Debakey VA Medical Center in Houston. Dr. Mathew has served as a consultant for and has had research funded by Janssen Pharmaceuticals.
I’m not surprised by the FDA decision, given the strong endorsement from the advisory committees in mid-February based on the drug’s benefit-to-risk evaluation. This is an important advance for our field, and the FDA approval will allow more patients who suffer from treatment-resistant depression to gain access to this medication.
To date, ketamine (not the intranasal esketamine spray) has been offered primarily on a fee-for-service basis or in the context of a clinical trial. I anticipate this treatment to receive broad insurance coverage, but this remains to be determined.
Dr. Sanjay J. Mathew is the Marjorie Bintliff Johnson and Raleigh White Johnson Jr. Vice Chair for Research and professor in the Menninger department of psychiatry & behavioral sciences at the Baylor College of Medicine. He also is affiliated with the Michael E. Debakey VA Medical Center in Houston. Dr. Mathew has served as a consultant for and has had research funded by Janssen Pharmaceuticals.
I’m not surprised by the FDA decision, given the strong endorsement from the advisory committees in mid-February based on the drug’s benefit-to-risk evaluation. This is an important advance for our field, and the FDA approval will allow more patients who suffer from treatment-resistant depression to gain access to this medication.
To date, ketamine (not the intranasal esketamine spray) has been offered primarily on a fee-for-service basis or in the context of a clinical trial. I anticipate this treatment to receive broad insurance coverage, but this remains to be determined.
Dr. Sanjay J. Mathew is the Marjorie Bintliff Johnson and Raleigh White Johnson Jr. Vice Chair for Research and professor in the Menninger department of psychiatry & behavioral sciences at the Baylor College of Medicine. He also is affiliated with the Michael E. Debakey VA Medical Center in Houston. Dr. Mathew has served as a consultant for and has had research funded by Janssen Pharmaceuticals.
The spray (Spravato; Janssen Pharmaceuticals) will come in tamper-resistant prepackaged units of one, two, or three devices to deliver the prescribed doses of 28 mg, 56 mg, or 84 mg, respectively. To reduce the risk of diversion, misuse, or abuse, the drug will managed under an FDA Risk Evaluation and Management Strategy (REMS). It will only be available to prescribing clinicians who have undergone training on the risks of esketamine and the importance of monitoring patients after their dose is administered. Facilities licensed to dispense esketamine must have the ability to medically monitor patients for at least 2 hours after administration. Patients will self-administer the spray and will not be able to take any of it home.
The REMS will require both prescriber and the patient to both sign a Patient Enrollment Form clearly stating that patients understand the necessity of assisted transport to leave the health care facility and that there should be no driving or use of heavy machinery for the rest of the day on which they are treated.
A boxed warning on the label will note that patients are at risk for sedation and difficulty with attention, judgment, and thinking (dissociation); abuse and misuse; and suicidal thoughts and behaviors after administration of the drug.
Despite the FDA’s caveats, the approval of intranasal esketamine is seen as a substantial win for the psychiatric community, Tiffany Farchione, MD, acting director of the FDA division of psychiatry products, said in a statement. “There has been a long-standing need for additional effective treatments for treatment-resistant depression, a serious and life-threatening condition.”
“Spravato has the potential to change the treatment paradigm and offer new hope to the estimated one-third of people with major depressive disorder who have not responded to existing therapies,” said Mathai Mammen, MD, PhD, global head of Janssen Research and Development.
The company “is working quickly to educate and certify treatment centers in accordance with the REMS so that health care providers can offer Spravato to appropriate patients,” according to a statement from Janssen. “Later this month, patients can visit www.SPRAVATO.com for a locater tool and to sign up to receive alerts when new treatment centers are available.”
Intranasal esketamine was evaluated in three short-term clinical trials and one longer-term maintenance-of-effect trial. One of the studies demonstrated a clinically significant effect in depression severity, in as little as 2 days for some patients. The two other short-term trials did not show significant benefit. However, in the maintenance study, patients in stable remission or with stable response who continued treatment with esketamine plus an oral antidepressant experienced a significantly longer time to relapse of depressive symptoms than patients on placebo spray plus an oral antidepressant. The most common side effects were disassociation, dizziness, nausea, sedation, vertigo, hypoesthesia, anxiety, lethargy, increased blood pressure, vomiting, and feeling drunk.
Patients with unstable or poorly controlled hypertension or pre-existing aneurysmal vascular disorders might be at increased risk for adverse cardiovascular or cerebrovascular effects. Esketamine might impair attention, judgment, thinking, reaction speed, and motor skills. It may cause fetal harm; women of childbearing age should be on reliable contraception. Breastfeeding women should not use it.
.
The spray (Spravato; Janssen Pharmaceuticals) will come in tamper-resistant prepackaged units of one, two, or three devices to deliver the prescribed doses of 28 mg, 56 mg, or 84 mg, respectively. To reduce the risk of diversion, misuse, or abuse, the drug will managed under an FDA Risk Evaluation and Management Strategy (REMS). It will only be available to prescribing clinicians who have undergone training on the risks of esketamine and the importance of monitoring patients after their dose is administered. Facilities licensed to dispense esketamine must have the ability to medically monitor patients for at least 2 hours after administration. Patients will self-administer the spray and will not be able to take any of it home.
The REMS will require both prescriber and the patient to both sign a Patient Enrollment Form clearly stating that patients understand the necessity of assisted transport to leave the health care facility and that there should be no driving or use of heavy machinery for the rest of the day on which they are treated.
A boxed warning on the label will note that patients are at risk for sedation and difficulty with attention, judgment, and thinking (dissociation); abuse and misuse; and suicidal thoughts and behaviors after administration of the drug.
Despite the FDA’s caveats, the approval of intranasal esketamine is seen as a substantial win for the psychiatric community, Tiffany Farchione, MD, acting director of the FDA division of psychiatry products, said in a statement. “There has been a long-standing need for additional effective treatments for treatment-resistant depression, a serious and life-threatening condition.”
“Spravato has the potential to change the treatment paradigm and offer new hope to the estimated one-third of people with major depressive disorder who have not responded to existing therapies,” said Mathai Mammen, MD, PhD, global head of Janssen Research and Development.
The company “is working quickly to educate and certify treatment centers in accordance with the REMS so that health care providers can offer Spravato to appropriate patients,” according to a statement from Janssen. “Later this month, patients can visit www.SPRAVATO.com for a locater tool and to sign up to receive alerts when new treatment centers are available.”
Intranasal esketamine was evaluated in three short-term clinical trials and one longer-term maintenance-of-effect trial. One of the studies demonstrated a clinically significant effect in depression severity, in as little as 2 days for some patients. The two other short-term trials did not show significant benefit. However, in the maintenance study, patients in stable remission or with stable response who continued treatment with esketamine plus an oral antidepressant experienced a significantly longer time to relapse of depressive symptoms than patients on placebo spray plus an oral antidepressant. The most common side effects were disassociation, dizziness, nausea, sedation, vertigo, hypoesthesia, anxiety, lethargy, increased blood pressure, vomiting, and feeling drunk.
Patients with unstable or poorly controlled hypertension or pre-existing aneurysmal vascular disorders might be at increased risk for adverse cardiovascular or cerebrovascular effects. Esketamine might impair attention, judgment, thinking, reaction speed, and motor skills. It may cause fetal harm; women of childbearing age should be on reliable contraception. Breastfeeding women should not use it.
.
Behavioral intervention improves physical activity in patients with diabetes
A behavioral intervention that involves regular counseling sessions could help patients with type 2 diabetes increase their levels of physical activity and decrease their amount of sedentary time, according to findings from a prospective, randomized trial of 300 physically inactive patients with type 2 diabetes.
“The primary strength of this study is the application of an intervention targeting both physical activity and sedentary time across all settings (e.g., leisure, transportation, household, and occupation), based on theoretical grounds and using several behavior-change techniques,” wrote Stefano Balducci, MD, of Sapienza University in Rome and his colleagues. The findings were published in JAMA.
Half the participants were randomized to an intervention that involved one individual theoretical counseling session with a diabetologist and eight biweekly theoretical and practical counseling sessions with an exercise specialist each year for 3 years. The other half received standard care in the form of recommendations from their general physician about increasing physical activity and decreasing sedentary time. Both groups also received the same general treatment regimen according to guidelines.
The findings showed significant increases in volume of physical activity, light-intensity physical activity, and moderate to vigorous physical activity in the intervention group during the first 4 months of the trial. Those increases also were greater than the increases seen in the usual care group. Patients in the intervention group also showed greater decreases in sedentary time, compared with those in the control group during the same time.
After 4 months, the increases in physical activity in the intervention group plateaued but remained stable until 2 years. After that, the levels of activity declined but still remained significantly higher than at baseline. The level of sedentary time also increased after 2 years but was still lower than at baseline.
By the end of the study, the intervention group accumulated 13.8 metabolic equivalent hours/week of physical activity volume, compared with 10.5 hours in the control group; 18.9 minutes/day of moderate to vigorous intensity physical activity, compared with 12.5 minutes in the control group; and 4.6 hours/day of light-intensity physical activity, compared with 3.8 hours in the control group. In regard to sedentary time, the intervention group accumulated 10.9 hours/day, compared with 11.7 hours in the control group. All differences were statistically significant.
“The present findings support the need for interventions targeting all domains of behavior to obtain substantial lifestyle changes, not limited to moderate- to vigorous-intensity physical activity, which has little effect on sedentary time,” Dr. Balducci and his coauthors wrote. “This concept is consistent with a 2018 report showing that physical activity, sedentary time, and cardiorespiratory fitness are all important for cardiometabolic health.”
For the secondary outcomes of cardiorespiratory fitness and lower-body strength, the authors saw significant improvements in the intervention group, whereas the control group showed a worsening in those outcomes. The intervention group also showed significant improvements in fasting plasma glucose level, systolic blood pressure, total coronary heart disease 10-year risk score, and fatal coronary heart disease 10-year risk score. They also had significantly greater improvements than did the control group in total stroke risk score, hemoglobin A1c, fasting plasma glucose levels, and coronary heart disease risk.
In all, there were 41 adverse events in the intervention group, compared with 59 in the control group, outside of the sessions. During the sessions, participants in the intervention group experienced mild hypoglycemia (8 episodes), tachycardia/arrhythmia (3), and musculoskeletal injury or discomfort (19).
One of the limitations highlighted by the authors was that the benefits of their strategy could vary in other cohorts because of differences in climatic, socioeconomic, or cultural settings.
The study was supported by the Metabolic Fitness Association. Three authors declared grants and personal fees from pharmaceutical companies, and one author was an employee of Technogym. No other conflicts of interest were declared.
SOURCE: Balducci S et al. JAMA. 2019;321:880-90.
A behavioral intervention that involves regular counseling sessions could help patients with type 2 diabetes increase their levels of physical activity and decrease their amount of sedentary time, according to findings from a prospective, randomized trial of 300 physically inactive patients with type 2 diabetes.
“The primary strength of this study is the application of an intervention targeting both physical activity and sedentary time across all settings (e.g., leisure, transportation, household, and occupation), based on theoretical grounds and using several behavior-change techniques,” wrote Stefano Balducci, MD, of Sapienza University in Rome and his colleagues. The findings were published in JAMA.
Half the participants were randomized to an intervention that involved one individual theoretical counseling session with a diabetologist and eight biweekly theoretical and practical counseling sessions with an exercise specialist each year for 3 years. The other half received standard care in the form of recommendations from their general physician about increasing physical activity and decreasing sedentary time. Both groups also received the same general treatment regimen according to guidelines.
The findings showed significant increases in volume of physical activity, light-intensity physical activity, and moderate to vigorous physical activity in the intervention group during the first 4 months of the trial. Those increases also were greater than the increases seen in the usual care group. Patients in the intervention group also showed greater decreases in sedentary time, compared with those in the control group during the same time.
After 4 months, the increases in physical activity in the intervention group plateaued but remained stable until 2 years. After that, the levels of activity declined but still remained significantly higher than at baseline. The level of sedentary time also increased after 2 years but was still lower than at baseline.
By the end of the study, the intervention group accumulated 13.8 metabolic equivalent hours/week of physical activity volume, compared with 10.5 hours in the control group; 18.9 minutes/day of moderate to vigorous intensity physical activity, compared with 12.5 minutes in the control group; and 4.6 hours/day of light-intensity physical activity, compared with 3.8 hours in the control group. In regard to sedentary time, the intervention group accumulated 10.9 hours/day, compared with 11.7 hours in the control group. All differences were statistically significant.
“The present findings support the need for interventions targeting all domains of behavior to obtain substantial lifestyle changes, not limited to moderate- to vigorous-intensity physical activity, which has little effect on sedentary time,” Dr. Balducci and his coauthors wrote. “This concept is consistent with a 2018 report showing that physical activity, sedentary time, and cardiorespiratory fitness are all important for cardiometabolic health.”
For the secondary outcomes of cardiorespiratory fitness and lower-body strength, the authors saw significant improvements in the intervention group, whereas the control group showed a worsening in those outcomes. The intervention group also showed significant improvements in fasting plasma glucose level, systolic blood pressure, total coronary heart disease 10-year risk score, and fatal coronary heart disease 10-year risk score. They also had significantly greater improvements than did the control group in total stroke risk score, hemoglobin A1c, fasting plasma glucose levels, and coronary heart disease risk.
In all, there were 41 adverse events in the intervention group, compared with 59 in the control group, outside of the sessions. During the sessions, participants in the intervention group experienced mild hypoglycemia (8 episodes), tachycardia/arrhythmia (3), and musculoskeletal injury or discomfort (19).
One of the limitations highlighted by the authors was that the benefits of their strategy could vary in other cohorts because of differences in climatic, socioeconomic, or cultural settings.
The study was supported by the Metabolic Fitness Association. Three authors declared grants and personal fees from pharmaceutical companies, and one author was an employee of Technogym. No other conflicts of interest were declared.
SOURCE: Balducci S et al. JAMA. 2019;321:880-90.
A behavioral intervention that involves regular counseling sessions could help patients with type 2 diabetes increase their levels of physical activity and decrease their amount of sedentary time, according to findings from a prospective, randomized trial of 300 physically inactive patients with type 2 diabetes.
“The primary strength of this study is the application of an intervention targeting both physical activity and sedentary time across all settings (e.g., leisure, transportation, household, and occupation), based on theoretical grounds and using several behavior-change techniques,” wrote Stefano Balducci, MD, of Sapienza University in Rome and his colleagues. The findings were published in JAMA.
Half the participants were randomized to an intervention that involved one individual theoretical counseling session with a diabetologist and eight biweekly theoretical and practical counseling sessions with an exercise specialist each year for 3 years. The other half received standard care in the form of recommendations from their general physician about increasing physical activity and decreasing sedentary time. Both groups also received the same general treatment regimen according to guidelines.
The findings showed significant increases in volume of physical activity, light-intensity physical activity, and moderate to vigorous physical activity in the intervention group during the first 4 months of the trial. Those increases also were greater than the increases seen in the usual care group. Patients in the intervention group also showed greater decreases in sedentary time, compared with those in the control group during the same time.
After 4 months, the increases in physical activity in the intervention group plateaued but remained stable until 2 years. After that, the levels of activity declined but still remained significantly higher than at baseline. The level of sedentary time also increased after 2 years but was still lower than at baseline.
By the end of the study, the intervention group accumulated 13.8 metabolic equivalent hours/week of physical activity volume, compared with 10.5 hours in the control group; 18.9 minutes/day of moderate to vigorous intensity physical activity, compared with 12.5 minutes in the control group; and 4.6 hours/day of light-intensity physical activity, compared with 3.8 hours in the control group. In regard to sedentary time, the intervention group accumulated 10.9 hours/day, compared with 11.7 hours in the control group. All differences were statistically significant.
“The present findings support the need for interventions targeting all domains of behavior to obtain substantial lifestyle changes, not limited to moderate- to vigorous-intensity physical activity, which has little effect on sedentary time,” Dr. Balducci and his coauthors wrote. “This concept is consistent with a 2018 report showing that physical activity, sedentary time, and cardiorespiratory fitness are all important for cardiometabolic health.”
For the secondary outcomes of cardiorespiratory fitness and lower-body strength, the authors saw significant improvements in the intervention group, whereas the control group showed a worsening in those outcomes. The intervention group also showed significant improvements in fasting plasma glucose level, systolic blood pressure, total coronary heart disease 10-year risk score, and fatal coronary heart disease 10-year risk score. They also had significantly greater improvements than did the control group in total stroke risk score, hemoglobin A1c, fasting plasma glucose levels, and coronary heart disease risk.
In all, there were 41 adverse events in the intervention group, compared with 59 in the control group, outside of the sessions. During the sessions, participants in the intervention group experienced mild hypoglycemia (8 episodes), tachycardia/arrhythmia (3), and musculoskeletal injury or discomfort (19).
One of the limitations highlighted by the authors was that the benefits of their strategy could vary in other cohorts because of differences in climatic, socioeconomic, or cultural settings.
The study was supported by the Metabolic Fitness Association. Three authors declared grants and personal fees from pharmaceutical companies, and one author was an employee of Technogym. No other conflicts of interest were declared.
SOURCE: Balducci S et al. JAMA. 2019;321:880-90.
FROM JAMA
Supplements and food-related therapy do not prevent depression in overweight adults
Multinutrient supplements and food-related therapy, together or separately, do not reduce major depressive disorder (MDD) episodes, according to a clinical trial of overweight adults with subsyndromal depressive symptoms.
“These findings do not support the use of these interventions for prevention of major depressive disorder in this population,” wrote lead author Mariska Bot, PhD, of Amsterdam University Medical Center, and her coauthors. The study was published in JAMA.
For this randomized clinical trial, Dr. Bot and her colleagues recruited 1,025 overweight adults from four European countries. All had at least mild depressive symptoms – determined through Patient Health Questionnaire–9 scores of 5 or higher – but no MDD episode in the last 6 months. The patients were allocated into four groups: placebo without therapy (n = 257), placebo with therapy (n = 256), supplements without therapy (n = 256), and supplements with therapy (n = 256). The supplements included 1,412 mg of omega-3 fatty acids, 30 mcg of selenium, 400 mcg of folic acid, and 20 mcg of vitamin D3 plus 100 mg of calcium. The therapy sessions were focused on food-related behavioral activation and emphasized a Mediterranean-style diet.
Only 779 (76%) of the patients completed the trial. Of the 105 participants who developed an MDD episode during 12-month follow-up, 25 (9.7%) were receiving placebo alone, 26 (10.2%) were receiving placebo with therapy, 32 (12.5%) were receiving supplements alone, and 22 (8.6%) were receiving supplements with therapy. Three of the four groups had 24 patients hospitalized, and the supplements-only group saw 26 patients hospitalized.
“This study showed that multinutrient supplements containing omega-3 [polyunsaturated fatty acids], vitamin D, folic acid, and selenium neither reduced depressive symptoms, anxiety symptoms nor improved health utility measures,” Dr. Bot and her coauthors wrote. “In fact, they appeared to result in slightly poorer depressive and anxiety symptoms scores compared with placebo.”
, roughly a quarter of patients lost to follow-up, and the likelihood that patients in the placebo group might have realized that they were not taking a multivitamin. In addition, participants were not selected based on deficiencies in the nutrients provided, making it possible that “deficient individuals will be more likely to benefit from supplementation.”
The study was funded by the European Union FP7 MooDFOOD Project Multi-country Collaborative Project on the Role of Diet, Food-related Behavior, and Obesity in the Prevention of Depression. Dr. Bot reported no disclosures. Her coauthors reported receiving funding from numerous pharmaceutical companies, the European Union, and Guilford Press.
SOURCE: Bot M et al. JAMA. 2019 Mar 5. doi: 10.1001/jama.2019.0556.
Though links between mental and physical health disorders have been established, this study from Bot et al. reinforces the murkiness of a nascent field like nutritional psychiatry, according to Michael Berk, MD, PhD, and Felice N. Jacka, PhD, of Deakin University in Victoria, Australia.
“Prevention of major depressive disorder is difficult to study,” they wrote, which makes a trial like this so important. However, its findings also emphasize the ambiguous nature of oft-touted remedies, specifically the “liberal and mostly non–evidence-based use of nutrient supplement combinations for psychiatric disorders.”
This study raises as many questions as it answers. Only 71% of those in the food-related behavioral activation therapy group attended more than 8 of the 21 offered sessions, and there is no way to prove how many followed the dietary restrictions. Those who did attend at least of 8 the sessions “showed a significant reduction in risk of depression,” however, which supports other trials that have associated dietary adherence and symptom improvement.
Diet is not a sole treatment for depression. However, the authors acknowledged that it is likely a piece of the complex puzzle that nutritional psychiatry wishes to solve. “These recent findings,” they wrote, “highlight that an integrated care package incorporating first-line psychological and pharmacological treatments, along with evidence-based lifestyle interventions addressing ... diet quality, may have a more robust effect on this burdensome disorder.”
These comments are adapted from an accompanying editorial (JAMA. 2019 Mar 5. doi: 10.1001/jama.2019.0273 ). Both coauthors reported conflicts of interest, including receiving grants, consulting fees, and research support from numerous boards, pharmaceutical companies, and foundations.
Though links between mental and physical health disorders have been established, this study from Bot et al. reinforces the murkiness of a nascent field like nutritional psychiatry, according to Michael Berk, MD, PhD, and Felice N. Jacka, PhD, of Deakin University in Victoria, Australia.
“Prevention of major depressive disorder is difficult to study,” they wrote, which makes a trial like this so important. However, its findings also emphasize the ambiguous nature of oft-touted remedies, specifically the “liberal and mostly non–evidence-based use of nutrient supplement combinations for psychiatric disorders.”
This study raises as many questions as it answers. Only 71% of those in the food-related behavioral activation therapy group attended more than 8 of the 21 offered sessions, and there is no way to prove how many followed the dietary restrictions. Those who did attend at least of 8 the sessions “showed a significant reduction in risk of depression,” however, which supports other trials that have associated dietary adherence and symptom improvement.
Diet is not a sole treatment for depression. However, the authors acknowledged that it is likely a piece of the complex puzzle that nutritional psychiatry wishes to solve. “These recent findings,” they wrote, “highlight that an integrated care package incorporating first-line psychological and pharmacological treatments, along with evidence-based lifestyle interventions addressing ... diet quality, may have a more robust effect on this burdensome disorder.”
These comments are adapted from an accompanying editorial (JAMA. 2019 Mar 5. doi: 10.1001/jama.2019.0273 ). Both coauthors reported conflicts of interest, including receiving grants, consulting fees, and research support from numerous boards, pharmaceutical companies, and foundations.
Though links between mental and physical health disorders have been established, this study from Bot et al. reinforces the murkiness of a nascent field like nutritional psychiatry, according to Michael Berk, MD, PhD, and Felice N. Jacka, PhD, of Deakin University in Victoria, Australia.
“Prevention of major depressive disorder is difficult to study,” they wrote, which makes a trial like this so important. However, its findings also emphasize the ambiguous nature of oft-touted remedies, specifically the “liberal and mostly non–evidence-based use of nutrient supplement combinations for psychiatric disorders.”
This study raises as many questions as it answers. Only 71% of those in the food-related behavioral activation therapy group attended more than 8 of the 21 offered sessions, and there is no way to prove how many followed the dietary restrictions. Those who did attend at least of 8 the sessions “showed a significant reduction in risk of depression,” however, which supports other trials that have associated dietary adherence and symptom improvement.
Diet is not a sole treatment for depression. However, the authors acknowledged that it is likely a piece of the complex puzzle that nutritional psychiatry wishes to solve. “These recent findings,” they wrote, “highlight that an integrated care package incorporating first-line psychological and pharmacological treatments, along with evidence-based lifestyle interventions addressing ... diet quality, may have a more robust effect on this burdensome disorder.”
These comments are adapted from an accompanying editorial (JAMA. 2019 Mar 5. doi: 10.1001/jama.2019.0273 ). Both coauthors reported conflicts of interest, including receiving grants, consulting fees, and research support from numerous boards, pharmaceutical companies, and foundations.
Multinutrient supplements and food-related therapy, together or separately, do not reduce major depressive disorder (MDD) episodes, according to a clinical trial of overweight adults with subsyndromal depressive symptoms.
“These findings do not support the use of these interventions for prevention of major depressive disorder in this population,” wrote lead author Mariska Bot, PhD, of Amsterdam University Medical Center, and her coauthors. The study was published in JAMA.
For this randomized clinical trial, Dr. Bot and her colleagues recruited 1,025 overweight adults from four European countries. All had at least mild depressive symptoms – determined through Patient Health Questionnaire–9 scores of 5 or higher – but no MDD episode in the last 6 months. The patients were allocated into four groups: placebo without therapy (n = 257), placebo with therapy (n = 256), supplements without therapy (n = 256), and supplements with therapy (n = 256). The supplements included 1,412 mg of omega-3 fatty acids, 30 mcg of selenium, 400 mcg of folic acid, and 20 mcg of vitamin D3 plus 100 mg of calcium. The therapy sessions were focused on food-related behavioral activation and emphasized a Mediterranean-style diet.
Only 779 (76%) of the patients completed the trial. Of the 105 participants who developed an MDD episode during 12-month follow-up, 25 (9.7%) were receiving placebo alone, 26 (10.2%) were receiving placebo with therapy, 32 (12.5%) were receiving supplements alone, and 22 (8.6%) were receiving supplements with therapy. Three of the four groups had 24 patients hospitalized, and the supplements-only group saw 26 patients hospitalized.
“This study showed that multinutrient supplements containing omega-3 [polyunsaturated fatty acids], vitamin D, folic acid, and selenium neither reduced depressive symptoms, anxiety symptoms nor improved health utility measures,” Dr. Bot and her coauthors wrote. “In fact, they appeared to result in slightly poorer depressive and anxiety symptoms scores compared with placebo.”
, roughly a quarter of patients lost to follow-up, and the likelihood that patients in the placebo group might have realized that they were not taking a multivitamin. In addition, participants were not selected based on deficiencies in the nutrients provided, making it possible that “deficient individuals will be more likely to benefit from supplementation.”
The study was funded by the European Union FP7 MooDFOOD Project Multi-country Collaborative Project on the Role of Diet, Food-related Behavior, and Obesity in the Prevention of Depression. Dr. Bot reported no disclosures. Her coauthors reported receiving funding from numerous pharmaceutical companies, the European Union, and Guilford Press.
SOURCE: Bot M et al. JAMA. 2019 Mar 5. doi: 10.1001/jama.2019.0556.
Multinutrient supplements and food-related therapy, together or separately, do not reduce major depressive disorder (MDD) episodes, according to a clinical trial of overweight adults with subsyndromal depressive symptoms.
“These findings do not support the use of these interventions for prevention of major depressive disorder in this population,” wrote lead author Mariska Bot, PhD, of Amsterdam University Medical Center, and her coauthors. The study was published in JAMA.
For this randomized clinical trial, Dr. Bot and her colleagues recruited 1,025 overweight adults from four European countries. All had at least mild depressive symptoms – determined through Patient Health Questionnaire–9 scores of 5 or higher – but no MDD episode in the last 6 months. The patients were allocated into four groups: placebo without therapy (n = 257), placebo with therapy (n = 256), supplements without therapy (n = 256), and supplements with therapy (n = 256). The supplements included 1,412 mg of omega-3 fatty acids, 30 mcg of selenium, 400 mcg of folic acid, and 20 mcg of vitamin D3 plus 100 mg of calcium. The therapy sessions were focused on food-related behavioral activation and emphasized a Mediterranean-style diet.
Only 779 (76%) of the patients completed the trial. Of the 105 participants who developed an MDD episode during 12-month follow-up, 25 (9.7%) were receiving placebo alone, 26 (10.2%) were receiving placebo with therapy, 32 (12.5%) were receiving supplements alone, and 22 (8.6%) were receiving supplements with therapy. Three of the four groups had 24 patients hospitalized, and the supplements-only group saw 26 patients hospitalized.
“This study showed that multinutrient supplements containing omega-3 [polyunsaturated fatty acids], vitamin D, folic acid, and selenium neither reduced depressive symptoms, anxiety symptoms nor improved health utility measures,” Dr. Bot and her coauthors wrote. “In fact, they appeared to result in slightly poorer depressive and anxiety symptoms scores compared with placebo.”
, roughly a quarter of patients lost to follow-up, and the likelihood that patients in the placebo group might have realized that they were not taking a multivitamin. In addition, participants were not selected based on deficiencies in the nutrients provided, making it possible that “deficient individuals will be more likely to benefit from supplementation.”
The study was funded by the European Union FP7 MooDFOOD Project Multi-country Collaborative Project on the Role of Diet, Food-related Behavior, and Obesity in the Prevention of Depression. Dr. Bot reported no disclosures. Her coauthors reported receiving funding from numerous pharmaceutical companies, the European Union, and Guilford Press.
SOURCE: Bot M et al. JAMA. 2019 Mar 5. doi: 10.1001/jama.2019.0556.
FROM JAMA
Key clinical point: A combination of multinutrient supplements and food-related behavioral activation therapy did not reduce episodes of major depressive disorder in overweight adults.
Major finding: Of the 105 participants who developed an MDD episode, 25 (9.7%) were receiving placebo alone, 26 (10.2%) were receiving placebo with therapy, 32 (12.5%) were receiving supplements alone, and 22 (8.6%) were receiving supplements with therapy.
Study details: A 2 x 2 factorial randomized clinical trial of 1,025 overweight adults from four European countries with elevated depressive symptoms and no major depressive disorder episode in the past 6 months.
Disclosures: The study was funded by the European Union FP7 MooDFOOD Project Multi-country Collaborative Project on the Role of Diet, Food-related Behavior, and Obesity in the Prevention of Depression. Dr. Bot reported no disclosures. Her coauthors reported receiving funding from numerous pharmaceutical companies, the European Union, and Guilford Press.
Source: Bot M et al. JAMA. 2019 Mar 5. doi: 10.1001/jama.2019.0556.
Watch for depression symptom trajectory in high-risk young adults
Severity, variability of symptoms may be only predictor of suicide attempts.
Among the trajectories of clinical predictors of suicide attempt, depression symptoms were the only ones linked with an increased risk of suicide attempt in young adults whose parents have mood disorders, according to a longitudinal study.
Psychiatric diagnoses are well established as predictors of suicidal behavior; however, symptoms and risk can vary over the course of illness, and it is important to identify symptoms that can change over time, wrote Nadine M. Melhem, PhD, associate professor of psychiatry at the University of Pittsburgh, and her associates. The report is in JAMA Psychiatry.
Between July 15, 1997, and Sept. 6, 2005, 663 adolescents and young adults (mean age, 23.8 years) whose parents have mood disorders were recruited and followed until Jan. 21, 2014. All participants were assessed at baseline and every year for up to 12 years (median follow-up, 8.1 years) for lifetime and current psychiatric disorders as well as suicidal ideation. In addition, participants were assessed at baseline and at each follow-up for the trajectory of depression symptoms, hopelessness, impulsivity, aggression, impulsive aggression, and irritability.
After the study period, participants were analyzed for all trajectories and separated into classes based on mean scores and variability. All trajectories except for depression had two classes, in which participants in class 2 had higher mean scores and variability; for depression, patients were separated into three classes, in which class 3 had the highest mean score and variability.
Over the study period, 71 of the 663 patients attempted suicide (10.7%), with 51 patients attempting suicide for the first time. The mean number of attempts was 1.2, and the median time from the last assessment to the attempt was 45 weeks.
(22.9% with vs. 27 without), class 2 impulsivity (38.8% vs. 21.7%), class 2 aggression (29.0% vs. 15.6%), class 2 impulsive aggression (76.5% vs. 52.2%), and class 2 irritability (39.4% vs. 22.7%). However, after adjustment for demographics, parental suicide attempts, and additional clinical characteristics, only class 3 depression remained associated with suicide attempts (odds ratio, 4.72; 95% confidence interval, 1.47-15.21; P = .01).
Other significant predictors of suicide attempts were younger age (OR, 0.82; 95% CI, 0.74-0.90; P less than .001), lifetime history of unipolar disorder (OR, 4.71; 95% CI, 1.63-13.58; P = .004), lifetime history of bipolar disorder (OR, 3.4; 95% CI, 0.96-12.04; P = .06), history of childhood abuse (OR, 2.98; 95% CI, 1.40-6.38; P = .01), and parental suicide attempt (OR, 2.24; 95% CI, 1.06-4.75; P = .04).
The investigators concluded that clinicians should “pay particular attention to the severity of both current and past depression and the variability in these symptoms, and monitor and treat depression symptoms over time to reduce symptom severity and fluctuation, and thus the likelihood for suicide attempt, in high-risk young adults.”
Dr. Melhem reported receiving research support from the National Institute of Mental Health, the Brain and Behavior Research Foundation, and the American Foundation for Suicide Prevention. Several other coauthors also reported conflicts of interest.
SOURCE: Melhem NM et al. JAMA Psychiatry. 2019 Feb 27. doi: 10.1001/jamapsychiatry.2018.4513.
Severity, variability of symptoms may be only predictor of suicide attempts.
Severity, variability of symptoms may be only predictor of suicide attempts.
Among the trajectories of clinical predictors of suicide attempt, depression symptoms were the only ones linked with an increased risk of suicide attempt in young adults whose parents have mood disorders, according to a longitudinal study.
Psychiatric diagnoses are well established as predictors of suicidal behavior; however, symptoms and risk can vary over the course of illness, and it is important to identify symptoms that can change over time, wrote Nadine M. Melhem, PhD, associate professor of psychiatry at the University of Pittsburgh, and her associates. The report is in JAMA Psychiatry.
Between July 15, 1997, and Sept. 6, 2005, 663 adolescents and young adults (mean age, 23.8 years) whose parents have mood disorders were recruited and followed until Jan. 21, 2014. All participants were assessed at baseline and every year for up to 12 years (median follow-up, 8.1 years) for lifetime and current psychiatric disorders as well as suicidal ideation. In addition, participants were assessed at baseline and at each follow-up for the trajectory of depression symptoms, hopelessness, impulsivity, aggression, impulsive aggression, and irritability.
After the study period, participants were analyzed for all trajectories and separated into classes based on mean scores and variability. All trajectories except for depression had two classes, in which participants in class 2 had higher mean scores and variability; for depression, patients were separated into three classes, in which class 3 had the highest mean score and variability.
Over the study period, 71 of the 663 patients attempted suicide (10.7%), with 51 patients attempting suicide for the first time. The mean number of attempts was 1.2, and the median time from the last assessment to the attempt was 45 weeks.
(22.9% with vs. 27 without), class 2 impulsivity (38.8% vs. 21.7%), class 2 aggression (29.0% vs. 15.6%), class 2 impulsive aggression (76.5% vs. 52.2%), and class 2 irritability (39.4% vs. 22.7%). However, after adjustment for demographics, parental suicide attempts, and additional clinical characteristics, only class 3 depression remained associated with suicide attempts (odds ratio, 4.72; 95% confidence interval, 1.47-15.21; P = .01).
Other significant predictors of suicide attempts were younger age (OR, 0.82; 95% CI, 0.74-0.90; P less than .001), lifetime history of unipolar disorder (OR, 4.71; 95% CI, 1.63-13.58; P = .004), lifetime history of bipolar disorder (OR, 3.4; 95% CI, 0.96-12.04; P = .06), history of childhood abuse (OR, 2.98; 95% CI, 1.40-6.38; P = .01), and parental suicide attempt (OR, 2.24; 95% CI, 1.06-4.75; P = .04).
The investigators concluded that clinicians should “pay particular attention to the severity of both current and past depression and the variability in these symptoms, and monitor and treat depression symptoms over time to reduce symptom severity and fluctuation, and thus the likelihood for suicide attempt, in high-risk young adults.”
Dr. Melhem reported receiving research support from the National Institute of Mental Health, the Brain and Behavior Research Foundation, and the American Foundation for Suicide Prevention. Several other coauthors also reported conflicts of interest.
SOURCE: Melhem NM et al. JAMA Psychiatry. 2019 Feb 27. doi: 10.1001/jamapsychiatry.2018.4513.
Among the trajectories of clinical predictors of suicide attempt, depression symptoms were the only ones linked with an increased risk of suicide attempt in young adults whose parents have mood disorders, according to a longitudinal study.
Psychiatric diagnoses are well established as predictors of suicidal behavior; however, symptoms and risk can vary over the course of illness, and it is important to identify symptoms that can change over time, wrote Nadine M. Melhem, PhD, associate professor of psychiatry at the University of Pittsburgh, and her associates. The report is in JAMA Psychiatry.
Between July 15, 1997, and Sept. 6, 2005, 663 adolescents and young adults (mean age, 23.8 years) whose parents have mood disorders were recruited and followed until Jan. 21, 2014. All participants were assessed at baseline and every year for up to 12 years (median follow-up, 8.1 years) for lifetime and current psychiatric disorders as well as suicidal ideation. In addition, participants were assessed at baseline and at each follow-up for the trajectory of depression symptoms, hopelessness, impulsivity, aggression, impulsive aggression, and irritability.
After the study period, participants were analyzed for all trajectories and separated into classes based on mean scores and variability. All trajectories except for depression had two classes, in which participants in class 2 had higher mean scores and variability; for depression, patients were separated into three classes, in which class 3 had the highest mean score and variability.
Over the study period, 71 of the 663 patients attempted suicide (10.7%), with 51 patients attempting suicide for the first time. The mean number of attempts was 1.2, and the median time from the last assessment to the attempt was 45 weeks.
(22.9% with vs. 27 without), class 2 impulsivity (38.8% vs. 21.7%), class 2 aggression (29.0% vs. 15.6%), class 2 impulsive aggression (76.5% vs. 52.2%), and class 2 irritability (39.4% vs. 22.7%). However, after adjustment for demographics, parental suicide attempts, and additional clinical characteristics, only class 3 depression remained associated with suicide attempts (odds ratio, 4.72; 95% confidence interval, 1.47-15.21; P = .01).
Other significant predictors of suicide attempts were younger age (OR, 0.82; 95% CI, 0.74-0.90; P less than .001), lifetime history of unipolar disorder (OR, 4.71; 95% CI, 1.63-13.58; P = .004), lifetime history of bipolar disorder (OR, 3.4; 95% CI, 0.96-12.04; P = .06), history of childhood abuse (OR, 2.98; 95% CI, 1.40-6.38; P = .01), and parental suicide attempt (OR, 2.24; 95% CI, 1.06-4.75; P = .04).
The investigators concluded that clinicians should “pay particular attention to the severity of both current and past depression and the variability in these symptoms, and monitor and treat depression symptoms over time to reduce symptom severity and fluctuation, and thus the likelihood for suicide attempt, in high-risk young adults.”
Dr. Melhem reported receiving research support from the National Institute of Mental Health, the Brain and Behavior Research Foundation, and the American Foundation for Suicide Prevention. Several other coauthors also reported conflicts of interest.
SOURCE: Melhem NM et al. JAMA Psychiatry. 2019 Feb 27. doi: 10.1001/jamapsychiatry.2018.4513.
FROM JAMA PSYCHIATRY
Key clinical point: Only depression symptoms were associated with a higher suicide attempt risk in young adults whose parents have mood disorders.
Major finding: The depression symptom trajectory with the highest mean scores and variability over time was the only measured trajectory that predicted suicide attempts (odds ratio, 4.72; 95% confidence interval, 1.47-15.21; P = .01).
Study details: A longitudinal study of 663 adolescents and younger adults whose parents have mood disorders.
Disclosures: Dr. Melhem reported receiving research support from the National Institute of Mental Health, the Brain and Behavior Research Foundation, and the American Foundation for Suicide Prevention. Several other coauthors also reported conflicts of interest.
Source: Melhem NM et al. JAMA Psychiatry. 2019 Feb 27. doi: 10.1001/jamapsychiatry.2018.4513.
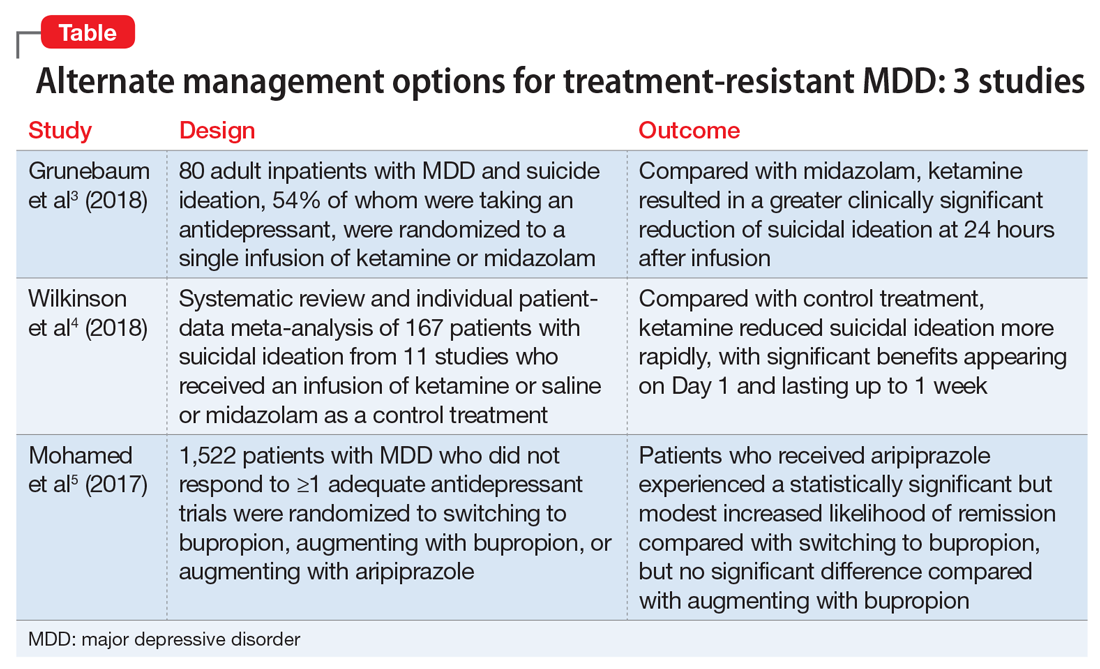
Management of treatment-resistant depression: A review of 3 studies
An estimated 7.1% of the adults in United States had a major depressive episode in 2017, and this prevalence has been trending upward over the past few years.1 The prevalence is even higher in adults between age 18 and 25 (13.1%).1 Like other psychiatric diagnoses, major depressive disorder (MDD) has a significant impact on productivity as well as daily functioning. Only one-third of patients with MDD achieve remission on the first antidepressant medication.2 This leaves an estimated 11.47 million people in the United States in need of an alternate regimen for management of their depressive episode.
The data on evidence-based biologic treatments for treatment-resistant depression are limited (other than for electroconvulsive therapy). Pharmacologic options include switching to a different medication, combining medications, and augmentation strategies or novel approaches such as ketamine and related agents. Here we summarize the findings from 3 recent studies that investigate alternate management options for MDD.
Ketamine: Randomized controlled trial
Traditional antidepressants may reduce suicidal ideation by improving depressive symptoms, but this effect may take weeks. Ketamine, an N-methyl-
_
1. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
Grunebaum et al3 evaluated the acute effect of adjunctive subanesthetic IV ketamine on clinically significant suicidal ideation in patients with MDD, with a comparison arm that received an infusion of midazolam.
Study design
- 80 inpatients (age 18 to 65 years) with MDD who had a score ≥16 on the Hamilton Depression Rating Scale (HAM-D) and a score ≥4 on the Scale for Suicidal Ideation (SSI). Approximately one-half (54%) were taking an antidepressant
- Patients were randomly assigned to IV racemic ketamine hydrochloride, .5 mg/kg, or IV midazolam, .02 mg/kg, both administered in 100 mL normal saline over 40 minutes.
Outcomes
- Scale for Suicidal Ideation scores were assessed at screening, before infusion, 230 minutes after infusion, 24 hours after infusion, and after 1 to 6 weeks of follow-up. The average SSI score on Day 1 was 4.96 points lower in the ketamine group compared with the midazolam group. The proportion of responders (defined as patients who experienced a 50% reduction in SSI score) on Day 1 was 55% for patients in the ketamine group compared with 30% in the midazolam group.
Conclusion
- Compared with midazolam, ketamine produced a greater clinically meaningful reduction in suicidal ideation 24 hours after infusion.
Apart from the primary outcome of reduction in suicidal ideation, greater reductions were also found in overall mood disturbance, depression subscale, and fatigue subscale scores as assessed on the Profile of Mood States (POMS). Although the study noted improvement in depression scores, the proportion of responders on Day 1 in depression scales, including HAM-D and the self-rated Beck Depression Inventory, fell short of statistical significance. Overall, compared with the midazolam infusion, a single adjunctive subanesthetic ketamine infusion was associated with a greater clinically significant reduction in suicidal ideation on Day 1.
Continue to: Ketamine
Ketamine: Review and meta-analysis
Wilkinson et al4 conducted a systematic review and individual participant data meta-analysis of 11 similar comparison intervention studies examining the effects of ketamine in reducing suicidal thoughts.
2. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
Study design
- Review of 11 studies of a single dose of IV ketamine for treatment of any psychiatric disorder. Only comparison intervention trials using saline placebo or midazolam were included:
- Individual patient-level data of 298 patients were obtained from 10 of the 11 trials. Analysis was performed on 167 patients who had suicidal ideation at baseline.
- Results were assessed by clinician-administered rating scales.
Outcomes
- Ketamine reduced suicidal ideation more rapidly compared with control infusions as assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS) and HAM-D, with significant benefits appearing on Day 1 and extending up to Day 7. The mean MADRS score in the ketamine group decreased to 19.5 from 33.8 within 1 day of infusion, compared with a reduction to 29.2 from 32.9 in the control groups.
- The number needed to treat to be free of suicidal ideation for ketamine (compared with control) was 3.1 to 4.0 for all time points in the first week after infusion.
Conclusion
- This meta-analysis provided evidence from the largest sample to date (N = 298) that ketamine reduces suicidal ideation partially independently of mood symptoms.
While the anti-suicidal effects of ketamine appear to be robust in the above studies, the possibility of rebound suicidal ideation remains in the weeks or months following exposure. Also, these studies only prove a reduction in suicidal ideation; reduction in suicidal behavior was not studied. Nevertheless, ketamine holds considerable promise as a potential rapid-acting agent in patients at risk of suicide.
Continue to: Strategies for augmentation or switching
Strategies for augmentation or switching
Only one-third of the patients with depression achieve remission on the first antidepressant medication. The American Psychiatric Association’s current management guidelines2 for patients who do not respond to the first-choice antidepressant include multiple options. Switching strategies recommended in these guidelines include changing to an antidepressant of the same class, or to one from a different class (eg, from a selective serotonin reuptake inhibitor [SSRI] to a serotonin-norepinephrine reuptake inhibitor, or from an SSRI to a tricyclic antidepressant). Augmentation strategies include augmenting with a non-monoamine oxidase inhibitor antidepressant from a different class, lithium, thyroid hormone, or an atypical antipsychotic.
The VAST-D trial5 evaluated the relative effectiveness and safety of 3 common treatments for treatment-resistant MDD:
- switching to bupropion
- augmenting the current treatment with bupropion
- augmenting the current treatment with the second-generation antipsychotic aripiprazole.
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
Study design
- A multi-site, randomized, single-blind, parallel-assignment trial of 1,522 patients at 35 US Veteran Health Administration medical centers with nonpsychotic MDD with a suboptimal response to at least one antidepressant (defined as a score of ≥16 on the Quick Inventory Depressive Symptomatology-Clinician Rated questionnaire [QIDS-C16]).
- Participants were randomly assigned to 1 of 3 groups: switching to bupropion (n = 511), augmenting with bupropion (n = 506), or augmenting with aripiprazole (n = 505).
- The primary outcome was remission (defined as a QIDS-C16 score ≤5 at 2 consecutively scheduled follow-up visits). Secondary outcome was a reduction in QIDS-C16 score by ≥50%, or a Clinical Global Impression (CGI) Improvement scale score of 1 (very much improved) or 2 (much improved).
Outcomes
- The aripiprazole group showed a modest, statistically significant remission rate (28.9%) compared with the bupropion switch group (22.3%), but did not show any statistically significant difference compared with the bupropion augmentation group.
- For the secondary outcome, there was a significantly higher response rate in the aripiprazole group (74.3%) compared with the bupropion switch group (62.4%) and bupropion augmentation group (65.6%). Response measured by the CGI– Improvement scale score also favored the aripiprazole group (79%) compared with the bupropion switch group (70%) and bupropion augmentation group (74%).
Continue to: Conclusion
Conclusion
- Overall, the study found a statistically significant but modest increased likelihood of remission during 12 weeks of augmentation treatment with aripiprazole, compared with switching to bupropion monotherapy.
The studies discussed here, which are summarized in the Table,3-5 provide some potential avenues for research into interventions for patients who are acutely suicidal and those with treatment-resistant depression. Further research into long-term outcomes and adverse effects of ketamine use for suicidality in patients with depression is needed. The VAST-D trial suggests a need for further exploration into the efficacy of augmentation with second-generation antipsychotics for treatment-resistant depression.
1. Substance Abuse and Mental Health Services Administration. Reports and detailed tables from the 2017 National Survey on Drug Use and Health (NSDUH). https://www.samhsa.gov/data/nsduh/reports-detailed-tables-2017-NSDUH. Accessed November 12, 2018.
2. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published 2010. Accessed November 12, 2018.
3. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
4. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
5. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
An estimated 7.1% of the adults in United States had a major depressive episode in 2017, and this prevalence has been trending upward over the past few years.1 The prevalence is even higher in adults between age 18 and 25 (13.1%).1 Like other psychiatric diagnoses, major depressive disorder (MDD) has a significant impact on productivity as well as daily functioning. Only one-third of patients with MDD achieve remission on the first antidepressant medication.2 This leaves an estimated 11.47 million people in the United States in need of an alternate regimen for management of their depressive episode.
The data on evidence-based biologic treatments for treatment-resistant depression are limited (other than for electroconvulsive therapy). Pharmacologic options include switching to a different medication, combining medications, and augmentation strategies or novel approaches such as ketamine and related agents. Here we summarize the findings from 3 recent studies that investigate alternate management options for MDD.
Ketamine: Randomized controlled trial
Traditional antidepressants may reduce suicidal ideation by improving depressive symptoms, but this effect may take weeks. Ketamine, an N-methyl-
_
1. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
Grunebaum et al3 evaluated the acute effect of adjunctive subanesthetic IV ketamine on clinically significant suicidal ideation in patients with MDD, with a comparison arm that received an infusion of midazolam.
Study design
- 80 inpatients (age 18 to 65 years) with MDD who had a score ≥16 on the Hamilton Depression Rating Scale (HAM-D) and a score ≥4 on the Scale for Suicidal Ideation (SSI). Approximately one-half (54%) were taking an antidepressant
- Patients were randomly assigned to IV racemic ketamine hydrochloride, .5 mg/kg, or IV midazolam, .02 mg/kg, both administered in 100 mL normal saline over 40 minutes.
Outcomes
- Scale for Suicidal Ideation scores were assessed at screening, before infusion, 230 minutes after infusion, 24 hours after infusion, and after 1 to 6 weeks of follow-up. The average SSI score on Day 1 was 4.96 points lower in the ketamine group compared with the midazolam group. The proportion of responders (defined as patients who experienced a 50% reduction in SSI score) on Day 1 was 55% for patients in the ketamine group compared with 30% in the midazolam group.
Conclusion
- Compared with midazolam, ketamine produced a greater clinically meaningful reduction in suicidal ideation 24 hours after infusion.
Apart from the primary outcome of reduction in suicidal ideation, greater reductions were also found in overall mood disturbance, depression subscale, and fatigue subscale scores as assessed on the Profile of Mood States (POMS). Although the study noted improvement in depression scores, the proportion of responders on Day 1 in depression scales, including HAM-D and the self-rated Beck Depression Inventory, fell short of statistical significance. Overall, compared with the midazolam infusion, a single adjunctive subanesthetic ketamine infusion was associated with a greater clinically significant reduction in suicidal ideation on Day 1.
Continue to: Ketamine
Ketamine: Review and meta-analysis
Wilkinson et al4 conducted a systematic review and individual participant data meta-analysis of 11 similar comparison intervention studies examining the effects of ketamine in reducing suicidal thoughts.
2. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
Study design
- Review of 11 studies of a single dose of IV ketamine for treatment of any psychiatric disorder. Only comparison intervention trials using saline placebo or midazolam were included:
- Individual patient-level data of 298 patients were obtained from 10 of the 11 trials. Analysis was performed on 167 patients who had suicidal ideation at baseline.
- Results were assessed by clinician-administered rating scales.
Outcomes
- Ketamine reduced suicidal ideation more rapidly compared with control infusions as assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS) and HAM-D, with significant benefits appearing on Day 1 and extending up to Day 7. The mean MADRS score in the ketamine group decreased to 19.5 from 33.8 within 1 day of infusion, compared with a reduction to 29.2 from 32.9 in the control groups.
- The number needed to treat to be free of suicidal ideation for ketamine (compared with control) was 3.1 to 4.0 for all time points in the first week after infusion.
Conclusion
- This meta-analysis provided evidence from the largest sample to date (N = 298) that ketamine reduces suicidal ideation partially independently of mood symptoms.
While the anti-suicidal effects of ketamine appear to be robust in the above studies, the possibility of rebound suicidal ideation remains in the weeks or months following exposure. Also, these studies only prove a reduction in suicidal ideation; reduction in suicidal behavior was not studied. Nevertheless, ketamine holds considerable promise as a potential rapid-acting agent in patients at risk of suicide.
Continue to: Strategies for augmentation or switching
Strategies for augmentation or switching
Only one-third of the patients with depression achieve remission on the first antidepressant medication. The American Psychiatric Association’s current management guidelines2 for patients who do not respond to the first-choice antidepressant include multiple options. Switching strategies recommended in these guidelines include changing to an antidepressant of the same class, or to one from a different class (eg, from a selective serotonin reuptake inhibitor [SSRI] to a serotonin-norepinephrine reuptake inhibitor, or from an SSRI to a tricyclic antidepressant). Augmentation strategies include augmenting with a non-monoamine oxidase inhibitor antidepressant from a different class, lithium, thyroid hormone, or an atypical antipsychotic.
The VAST-D trial5 evaluated the relative effectiveness and safety of 3 common treatments for treatment-resistant MDD:
- switching to bupropion
- augmenting the current treatment with bupropion
- augmenting the current treatment with the second-generation antipsychotic aripiprazole.
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
Study design
- A multi-site, randomized, single-blind, parallel-assignment trial of 1,522 patients at 35 US Veteran Health Administration medical centers with nonpsychotic MDD with a suboptimal response to at least one antidepressant (defined as a score of ≥16 on the Quick Inventory Depressive Symptomatology-Clinician Rated questionnaire [QIDS-C16]).
- Participants were randomly assigned to 1 of 3 groups: switching to bupropion (n = 511), augmenting with bupropion (n = 506), or augmenting with aripiprazole (n = 505).
- The primary outcome was remission (defined as a QIDS-C16 score ≤5 at 2 consecutively scheduled follow-up visits). Secondary outcome was a reduction in QIDS-C16 score by ≥50%, or a Clinical Global Impression (CGI) Improvement scale score of 1 (very much improved) or 2 (much improved).
Outcomes
- The aripiprazole group showed a modest, statistically significant remission rate (28.9%) compared with the bupropion switch group (22.3%), but did not show any statistically significant difference compared with the bupropion augmentation group.
- For the secondary outcome, there was a significantly higher response rate in the aripiprazole group (74.3%) compared with the bupropion switch group (62.4%) and bupropion augmentation group (65.6%). Response measured by the CGI– Improvement scale score also favored the aripiprazole group (79%) compared with the bupropion switch group (70%) and bupropion augmentation group (74%).
Continue to: Conclusion
Conclusion
- Overall, the study found a statistically significant but modest increased likelihood of remission during 12 weeks of augmentation treatment with aripiprazole, compared with switching to bupropion monotherapy.
The studies discussed here, which are summarized in the Table,3-5 provide some potential avenues for research into interventions for patients who are acutely suicidal and those with treatment-resistant depression. Further research into long-term outcomes and adverse effects of ketamine use for suicidality in patients with depression is needed. The VAST-D trial suggests a need for further exploration into the efficacy of augmentation with second-generation antipsychotics for treatment-resistant depression.
An estimated 7.1% of the adults in United States had a major depressive episode in 2017, and this prevalence has been trending upward over the past few years.1 The prevalence is even higher in adults between age 18 and 25 (13.1%).1 Like other psychiatric diagnoses, major depressive disorder (MDD) has a significant impact on productivity as well as daily functioning. Only one-third of patients with MDD achieve remission on the first antidepressant medication.2 This leaves an estimated 11.47 million people in the United States in need of an alternate regimen for management of their depressive episode.
The data on evidence-based biologic treatments for treatment-resistant depression are limited (other than for electroconvulsive therapy). Pharmacologic options include switching to a different medication, combining medications, and augmentation strategies or novel approaches such as ketamine and related agents. Here we summarize the findings from 3 recent studies that investigate alternate management options for MDD.
Ketamine: Randomized controlled trial
Traditional antidepressants may reduce suicidal ideation by improving depressive symptoms, but this effect may take weeks. Ketamine, an N-methyl-
_
1. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
Grunebaum et al3 evaluated the acute effect of adjunctive subanesthetic IV ketamine on clinically significant suicidal ideation in patients with MDD, with a comparison arm that received an infusion of midazolam.
Study design
- 80 inpatients (age 18 to 65 years) with MDD who had a score ≥16 on the Hamilton Depression Rating Scale (HAM-D) and a score ≥4 on the Scale for Suicidal Ideation (SSI). Approximately one-half (54%) were taking an antidepressant
- Patients were randomly assigned to IV racemic ketamine hydrochloride, .5 mg/kg, or IV midazolam, .02 mg/kg, both administered in 100 mL normal saline over 40 minutes.
Outcomes
- Scale for Suicidal Ideation scores were assessed at screening, before infusion, 230 minutes after infusion, 24 hours after infusion, and after 1 to 6 weeks of follow-up. The average SSI score on Day 1 was 4.96 points lower in the ketamine group compared with the midazolam group. The proportion of responders (defined as patients who experienced a 50% reduction in SSI score) on Day 1 was 55% for patients in the ketamine group compared with 30% in the midazolam group.
Conclusion
- Compared with midazolam, ketamine produced a greater clinically meaningful reduction in suicidal ideation 24 hours after infusion.
Apart from the primary outcome of reduction in suicidal ideation, greater reductions were also found in overall mood disturbance, depression subscale, and fatigue subscale scores as assessed on the Profile of Mood States (POMS). Although the study noted improvement in depression scores, the proportion of responders on Day 1 in depression scales, including HAM-D and the self-rated Beck Depression Inventory, fell short of statistical significance. Overall, compared with the midazolam infusion, a single adjunctive subanesthetic ketamine infusion was associated with a greater clinically significant reduction in suicidal ideation on Day 1.
Continue to: Ketamine
Ketamine: Review and meta-analysis
Wilkinson et al4 conducted a systematic review and individual participant data meta-analysis of 11 similar comparison intervention studies examining the effects of ketamine in reducing suicidal thoughts.
2. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
Study design
- Review of 11 studies of a single dose of IV ketamine for treatment of any psychiatric disorder. Only comparison intervention trials using saline placebo or midazolam were included:
- Individual patient-level data of 298 patients were obtained from 10 of the 11 trials. Analysis was performed on 167 patients who had suicidal ideation at baseline.
- Results were assessed by clinician-administered rating scales.
Outcomes
- Ketamine reduced suicidal ideation more rapidly compared with control infusions as assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS) and HAM-D, with significant benefits appearing on Day 1 and extending up to Day 7. The mean MADRS score in the ketamine group decreased to 19.5 from 33.8 within 1 day of infusion, compared with a reduction to 29.2 from 32.9 in the control groups.
- The number needed to treat to be free of suicidal ideation for ketamine (compared with control) was 3.1 to 4.0 for all time points in the first week after infusion.
Conclusion
- This meta-analysis provided evidence from the largest sample to date (N = 298) that ketamine reduces suicidal ideation partially independently of mood symptoms.
While the anti-suicidal effects of ketamine appear to be robust in the above studies, the possibility of rebound suicidal ideation remains in the weeks or months following exposure. Also, these studies only prove a reduction in suicidal ideation; reduction in suicidal behavior was not studied. Nevertheless, ketamine holds considerable promise as a potential rapid-acting agent in patients at risk of suicide.
Continue to: Strategies for augmentation or switching
Strategies for augmentation or switching
Only one-third of the patients with depression achieve remission on the first antidepressant medication. The American Psychiatric Association’s current management guidelines2 for patients who do not respond to the first-choice antidepressant include multiple options. Switching strategies recommended in these guidelines include changing to an antidepressant of the same class, or to one from a different class (eg, from a selective serotonin reuptake inhibitor [SSRI] to a serotonin-norepinephrine reuptake inhibitor, or from an SSRI to a tricyclic antidepressant). Augmentation strategies include augmenting with a non-monoamine oxidase inhibitor antidepressant from a different class, lithium, thyroid hormone, or an atypical antipsychotic.
The VAST-D trial5 evaluated the relative effectiveness and safety of 3 common treatments for treatment-resistant MDD:
- switching to bupropion
- augmenting the current treatment with bupropion
- augmenting the current treatment with the second-generation antipsychotic aripiprazole.
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
Study design
- A multi-site, randomized, single-blind, parallel-assignment trial of 1,522 patients at 35 US Veteran Health Administration medical centers with nonpsychotic MDD with a suboptimal response to at least one antidepressant (defined as a score of ≥16 on the Quick Inventory Depressive Symptomatology-Clinician Rated questionnaire [QIDS-C16]).
- Participants were randomly assigned to 1 of 3 groups: switching to bupropion (n = 511), augmenting with bupropion (n = 506), or augmenting with aripiprazole (n = 505).
- The primary outcome was remission (defined as a QIDS-C16 score ≤5 at 2 consecutively scheduled follow-up visits). Secondary outcome was a reduction in QIDS-C16 score by ≥50%, or a Clinical Global Impression (CGI) Improvement scale score of 1 (very much improved) or 2 (much improved).
Outcomes
- The aripiprazole group showed a modest, statistically significant remission rate (28.9%) compared with the bupropion switch group (22.3%), but did not show any statistically significant difference compared with the bupropion augmentation group.
- For the secondary outcome, there was a significantly higher response rate in the aripiprazole group (74.3%) compared with the bupropion switch group (62.4%) and bupropion augmentation group (65.6%). Response measured by the CGI– Improvement scale score also favored the aripiprazole group (79%) compared with the bupropion switch group (70%) and bupropion augmentation group (74%).
Continue to: Conclusion
Conclusion
- Overall, the study found a statistically significant but modest increased likelihood of remission during 12 weeks of augmentation treatment with aripiprazole, compared with switching to bupropion monotherapy.
The studies discussed here, which are summarized in the Table,3-5 provide some potential avenues for research into interventions for patients who are acutely suicidal and those with treatment-resistant depression. Further research into long-term outcomes and adverse effects of ketamine use for suicidality in patients with depression is needed. The VAST-D trial suggests a need for further exploration into the efficacy of augmentation with second-generation antipsychotics for treatment-resistant depression.
1. Substance Abuse and Mental Health Services Administration. Reports and detailed tables from the 2017 National Survey on Drug Use and Health (NSDUH). https://www.samhsa.gov/data/nsduh/reports-detailed-tables-2017-NSDUH. Accessed November 12, 2018.
2. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published 2010. Accessed November 12, 2018.
3. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
4. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
5. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
1. Substance Abuse and Mental Health Services Administration. Reports and detailed tables from the 2017 National Survey on Drug Use and Health (NSDUH). https://www.samhsa.gov/data/nsduh/reports-detailed-tables-2017-NSDUH. Accessed November 12, 2018.
2. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published 2010. Accessed November 12, 2018.
3. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
4. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
5. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
What’s new in transcranial magnetic stimulation
Therapeutic neuromodulation takes advantage of the brain’s electrochemical makeup. This allows for treatment devices that modulate neurocircuits relevant to behaviors disrupted in disorders such as major depressive disorder (MDD) (eg, sleep quality, appetite, cognitive, and executive functions). The default mode network (comprised of structures such as the medial prefrontal cortex [MPFC], the posterior cingulate cortex, the hippocampus, and their functional connectivity) serves as a prime example of circuitry that can be targeted by this approach.1
For 80 years, electroconvulsive therapy (ECT) has been an important neuromodulation option for patients with more severe illness. Recently, additional neuromodulatory approaches have been FDA-cleared, including transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), and deep brain stimulation (DBS). Another approach, transcranial direct current stimulation (tDCS), has been extensively studied for its potential clinical utility but is not FDA-cleared. The Table provides descriptions of these therapies.
Since being cleared by the FDA in 2008, TMS has arguably made the greatest strides in providing an alternate neuromodulation treatment option for patients with MDD, with >1,000 centers nationally and 7 TMS devices FDA-cleared for treatment of depression. In this article, we review recent developments in TMS.
An evolving therapeutic option
While primarily studied as a monotherapy for MDD, in clinical practice TMS (Box) is typically used as an adjunct to medication and psychotherapy.2,3 In this context, it has demonstrated efficacy for more difficult-to-treat mood disorders with an excellent safety and tolerability profile whether used with or without medication.4-6
To further improve the efficiency and efficacy of TMS while maintaining its safety and tolerability, researchers and clinicians have been exploring a few initiatives.
Box
- Transcranial magnetic stimulation (TMS) utilizes intense, localized magnetic fields to alter activity in neural circuits implicated in the pathophysiology of depression
- Randomized, sham-controlled acute trials have demonstrated the efficacy of TMS for treatment-resistant depression
- Clinical availability of TMS has grown steadily over the past 10 years as >1,000 centers have been opened and additional devices have been FDA-cleared
- TMS has the potential to avoid safety and tolerability concerns associated with antidepressant pharmacotherapy (eg, weight gain, sexual dysfunction) and electroconvulsive therapy (eg, cognitive deficits)
- Greater sophistication in the choice of stimulation parameters, as well as other ongoing efforts to optimize the benefits of TMS, are yielding better clinical outcomes
Altered treatment parameters
One initiative is assessing the feasibility of altering various treatment parameters, such as the total number of treatment sessions (30 to 60 sessions); the frequency of sessions (eg, more than once daily); the total number of magnetic pulses per session (eg, >3,000); the stimulation coil localization (eg, left vs right dorsal lateral prefrontal cortex [DLPFC]; MPFC; and various methods to determine optimal coil placement (eg, EEG F3 coordinate or MRI-guided neuro-navigational methods). Such refinements offer the potential for enhanced efficacy, shorter treatment sessions, and/or improved tolerability. For example, lower frequency right DLPFC stimulations (eg, 1 Hz) can decrease the risk of seizures and improve overall tolerability. While this has not been studied as extensively as higher frequency left DLPFC stimulations (eg, 5 to 20 Hz), existing evidence supports similar efficacy between these 2 approaches.7
Theta burst stimulation. Some TMS devices can be adapted to deliver theta burst stimulation (TBS). This produces trains of triple, 50 Hz, pulsed bursts (usually with 200 ms inter-burst intervals occurring at a rate of 5 Hz; at 80% MT) to model naturally occurring theta rhythms. These bursts can be administered in stimulation protocols using intermittent TBS (iTBS) (eg, 10 bursts of triplets over 2 seconds every 10 seconds; 30 pulses per burst; for approximately 3 minutes; totaling 600 pulses) or continuous TBS (cTBS) bursts given in an uninterrupted train (eg, 40 seconds, 600 pulses). Evidence indicates these protocols facilitate long-term potentiation (ie, iTBS) and long-term depression (ie, cTBS), which in turn can modulate synaptic plasticity.
Continue to: While some clinicians are using...
While some clinicians are using TBS off-label, a recent non-inferiority trial (N = 395) reported similar efficacy and safety comparing standard 10 Hz TMS to an iTBS protocol at 120% of resting motor threshold (both over the left DLPFC).8 This has led to FDA clearance of the TMS device adapted to provide iTBS in this trial.8
From a more practical perspective, TBS has the potential to reduce the number of pulses (eg, 600 vs 3,000) and the total number of sessions required, as well as the duration of treatment sessions (eg, 37.5 minutes to <5 minutes). This can accelerate the time to response and decrease patient and staff commitment, with resulting cost savings.9 Despite this recent progress, ongoing research still needs to clarify issues such as the risk/benefit profile, particularly in younger and older populations, as well as assessment of duration of initial benefit and appropriate maintenance strategies.
New devices
Another initiative is the development of alternative TMS equipment. For example, newer coil designs with enhanced cooling ability allow for a substantial decrease in the required inter-train interval duration between stimulation trains, thus shortening the total session duration by approximately 50% (eg, from 37.5 to 19 minutes). The use of different coil arrays (eg, the H-coil capable of deeper vs surface stimulation) may allow for more direct stimulation of relevant neurocircuitry (eg, cingulate cortex), possibly improving efficacy and shortening time to onset of benefit. However, in head-to-head comparisons with single-coil devices, enhanced efficacy for depression has not been clearly demonstrated. One caveat is that the increase in depth of magnetic field penetration results in a loss of focality, resulting in the stimulation of larger brain areas. This might increase the risk of adverse effects such as seizures.
Increasing durability of effect
Because high relapse and recurrence rates compromise the initial benefit of any antidepressant therapy, appropriate maintenance strategies are essential. Several studies have evaluated strategies to maintain the acute benefit of TMS for treatment-resistant depression.
One was a 6-month, open-label TMS durability of effect trial for acute responders (n = 99) in the pivotal registration study.5 During this study, all participants were given antidepressant medication monotherapy. In addition, with early indication of relapse, patients received a reintroduction of TMS sessions (32/99 patients; mean number of sessions = 14.3). With this protocol, approximately 84% re-achieved their response status. The overall relapse rate was approximately 13%.5
Continue to: In a 1-year naturalistic study...
In a 1-year naturalistic study, 63% of patients (75/120) who met response or remission criteria after an acute course of TMS still met response criteria after 12 months. These patients received clinician-determined maintenance treatment that included reintroduction of TMS when indicated.3
In a prospective, 12-month, multisite, randomized pilot study, 67 patients with treatment-resistant MDD underwent an antidepressant medication washout and then received 30 sessions of TMS monotherapy.10 Those who met criteria for improvement (n = 49) were then randomized to once-monthly TMS or observation only. All patients remained medication-free but could receive TMS re-introduction if they deteriorated. At the end of the study, both groups demonstrated comparable outcomes, with a trend to a longer time before relapse among participants who received once-monthly TMS. Although these results are preliminary, they suggest that some patients could be treated both acutely and then maintained with TMS alone.
Re-introducing TMS in patients who show early signs of relapse after having an initial response achieves rates of sustained improvement that compare favorably with those of other strategies used to manage patients with treatment-resistant depression.
TMS vs ECT
The question often arises as to whether TMS is a viable alternate treatment to ECT. I believe the answer is unequivocally yes and no. By this, I mean some patients who in the past only had ECT as their next option when medications and psychotherapy were insufficient may now consider TMS. In support, there is evidence of comparable efficacy between TMS and ECT in a subgroup of patients who were considered clinically appropriate for ECT.11-13
How to best identify this group remains unclear, but investigators are exploring predictive biomarkers. For example, a large study (N = 1,188), with functional magnetic resonance imaging (fMRI) reported that depressed patients could be divided into 4 neurophysiological “biotypes” based on different patterns of aberrant connectivity in limbic and fronto-striatal networks.14 The authors further noted that such distinctions were helpful in predicting response in a subgroup of patients (n = 154) who received TMS.
Continue to: For now...
For now, experience indicates certain clinical factors may provide some guidance. Patients are usually better served by ECT if they:
- have depressive episodes of longer duration (eg, >3 years)
- have a high risk of suicide
- have psychotic or catatonic features associated with their depression
- have difficulty maintaining their physical well-being
- have bipolar depression.
Although existing evidence supports a possible benefit with TMS for bipolar depression (used in combination with a mood stabilizer), the lack of a definitive trial (precluding FDA clearance for this indication) and the lack of insurance coverage both limit the routine use of TMS for this indication.15
One potential advantage of TMS over ECT is a lower cost.13 Transcranial magnetic stimulation also may make it possible to achieve similar efficacy as ECT with fewer cognitive adverse effects when used in combination with ECT to reduce the number of acute ECT treatments required or as part of a maintenance strategy after a patient experiences an acute response to ECT.13
Magnetic seizure therapy (MST) vs ECT. An experimental treatment, MST uses a TMS device capable of producing more intense magnetic fields sufficient to induce a seizure.16 The advantage of MST over ECT-induced seizures is better control of intra-cerebral current path and density, thus avoiding deeper cortical areas associated with memory (eg, hippocampus) and minimizing cognitive adverse effects. As with ECT, however, anesthesia and muscle relaxation are required. Presently, MST remains investigational.
Other potential indications
In addition to MDD, TMS is also being studied as a potential treatment for other neuropsychiatric disorders.
Continue to: Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD). A recent double-blind study that evaluated a deep TMS (DTMS) device reported a significantly better outcome based on the Yale-Brown Obsessive-Compulsive Scale score with active high-frequency (20 Hz) DTMS (n = 18) vs a sham control (n = 15).17 The initial benefit persisted up to 1 month after the end of treatment. The authors speculated that this benefit may be due to direct modulation of the anterior cingulate cortex. These results led to the first FDA clearance of a deep TMS device for treating OCD.
Cognition. Because TMS does not require a seizure to produce its antidepressant effect and does not require anesthesia, the risk of neurocognitive disruption is low. In fact, evidence suggests TMS may have beneficial cognitive effects.18
In an effort to take advantage of this benefit, researchers have explored providing psychoeducation and psychotherapy sessions (eg, behavioral activation) during TMS treatments (“online”).19,20 The rationale is that neurocircuitry subserving various cognitive functions may be in a heightened state of receptivity during a TMS treatment, which would allow patients to assimilate and better utilize the therapeutic information provided.19,20
Researchers are also looking at the use of TMS to treat patients with mild cognitive impairment or early dementia. These patients often experience comorbid depression, and TMS could potentially improve memory via both its pro-cognitive and antidepressant effects.1 The lack of effective treatments for dementia supports pursuing TMS as a therapeutic option for these patients.
Other neuropsychiatric disorders. In addition to early-onset cognitive problems, other neurologic indications with promising data for TMS include chronic pain syndromes, Parkinson’s disease, tinnitus, and migraine headaches (a hand-held FDA-cleared device is now available for treating migraines). In addition to OCD and bipolar depression, other psychiatric indications with promising data include schizophrenia (eg, refractory auditory hallucinations, negative symptoms), posttraumatic stress disorder, and various addictive disorders.21 Because results have been mixed for most of these disorders, definitive trials are needed to clearly characterize the potential role of TMS.
Continue to: An ongoing evolution
An ongoing evolution
Neuromodulation is undergoing a renaissance spurred on by the need for more effective treatments to manage some of our most challenging illnesses. Transcranial magnetic stimulation and other forms of therapeutic neuromodulation are welcome additions for managing treatment-resistant depression, OCD, and possibly other disorders. But perhaps their greatest value is as a bellwether for what’s to come. In addition to the ongoing refinements to existing neuromodulation devices, newer modulation approaches (eg, temporal interference stimulation) and the search for reliable biomarkers may dramatically expand and enhance our clinical options.14,22
Bottom Line
Transcranial magnetic stimulation (TMS) continues to evolve as a nonpharmacologic treatment for mood disorders, obsessive-compulsive disorder, and potentially for other indications. Recent developments, including altered treatment parameters, new devices, and strategies for increasing the durability of antidepressant effects, have enhanced the benefits of TMS.
Related Resources
- Ziemann U. Thirty years of transcranial magnetic stimulation: where do we stand? Exp Brain Res. 2017;235(4):973-984.
- Janicak PG, Sackett V, Kudrna K, et al. Transcranial magnetic stimulation for the treatment of major depression: an update on recent advances. Current Psychiatry. 2016:15(6):49-56.
1. Koch G, Bonnì S, Pellicciari MC, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage. 2018;169: 302-310.
2. O’Reardon JP, Solvason B, Janicak PG, et al. Efficacy and safety of repetitive transcranial magnetic stimulation (rTMS) in the acute treatment of major depression: results of a multicenter randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
3. Dunner DL, Aaronson ST, Sackheim HA, et al. A multisite, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a one-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-1401.
4. Janicak PG, O’Reardon JP, Sampson SM, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. 2008;69:222-232.
5. Janicak PG, Nahas Z, Lisanby SH, et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010;3(4):187-199.
6. Janicak PG. Risk management issues in transcranial magnetic stimulation for treatment of major depression. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
7. Chen J, Zhou C, Wu B, et al. Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Res. 2013;210(3):1260-1264.
8. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683-1692.
9. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. 2015;32(3):182-192.
10. Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul. 2016;9(2):251-257.
11. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prop Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
12. Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depressive: preliminary results of a randomized trial. Biol Psychiatry. 2002;51(8):659-667.
13. Lanocha K, Janicak PG. TMS for depression: relationship to ECT and other therapeutic neuromodulation approaches. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
14. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38.
15. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
16. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi: 10.1155/2015/521398.
17. Carmi L, Alyagon U, Barnea-Ygael N, et al. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018;11(1):158-165.
18. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114:1125-1132.
19. Donse L, Padberg F, Sack AT, et al. Simultaneous rTMS and psychotherapy in major depressive disorder: Clinical outcomes and predictors from a large naturalistic study. Brain Stimul. 2018;11(2):337-345.
20. Russo GB, Tirrell E, Busch A, et al. Behavioral activation therapy during transcranial magnetic stimulation for major depressive disorder. J Affect Disord. 2018;236:101-104.
21. Pannu J, DE Souza DD, Samara Z, et al. Transcranial magnetic stimulation for disorders other than depression. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
22. Grossman N. Modulation without surgical intervention. Science. 2018;361:461-462.
Therapeutic neuromodulation takes advantage of the brain’s electrochemical makeup. This allows for treatment devices that modulate neurocircuits relevant to behaviors disrupted in disorders such as major depressive disorder (MDD) (eg, sleep quality, appetite, cognitive, and executive functions). The default mode network (comprised of structures such as the medial prefrontal cortex [MPFC], the posterior cingulate cortex, the hippocampus, and their functional connectivity) serves as a prime example of circuitry that can be targeted by this approach.1
For 80 years, electroconvulsive therapy (ECT) has been an important neuromodulation option for patients with more severe illness. Recently, additional neuromodulatory approaches have been FDA-cleared, including transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), and deep brain stimulation (DBS). Another approach, transcranial direct current stimulation (tDCS), has been extensively studied for its potential clinical utility but is not FDA-cleared. The Table provides descriptions of these therapies.
Since being cleared by the FDA in 2008, TMS has arguably made the greatest strides in providing an alternate neuromodulation treatment option for patients with MDD, with >1,000 centers nationally and 7 TMS devices FDA-cleared for treatment of depression. In this article, we review recent developments in TMS.
An evolving therapeutic option
While primarily studied as a monotherapy for MDD, in clinical practice TMS (Box) is typically used as an adjunct to medication and psychotherapy.2,3 In this context, it has demonstrated efficacy for more difficult-to-treat mood disorders with an excellent safety and tolerability profile whether used with or without medication.4-6
To further improve the efficiency and efficacy of TMS while maintaining its safety and tolerability, researchers and clinicians have been exploring a few initiatives.
Box
- Transcranial magnetic stimulation (TMS) utilizes intense, localized magnetic fields to alter activity in neural circuits implicated in the pathophysiology of depression
- Randomized, sham-controlled acute trials have demonstrated the efficacy of TMS for treatment-resistant depression
- Clinical availability of TMS has grown steadily over the past 10 years as >1,000 centers have been opened and additional devices have been FDA-cleared
- TMS has the potential to avoid safety and tolerability concerns associated with antidepressant pharmacotherapy (eg, weight gain, sexual dysfunction) and electroconvulsive therapy (eg, cognitive deficits)
- Greater sophistication in the choice of stimulation parameters, as well as other ongoing efforts to optimize the benefits of TMS, are yielding better clinical outcomes
Altered treatment parameters
One initiative is assessing the feasibility of altering various treatment parameters, such as the total number of treatment sessions (30 to 60 sessions); the frequency of sessions (eg, more than once daily); the total number of magnetic pulses per session (eg, >3,000); the stimulation coil localization (eg, left vs right dorsal lateral prefrontal cortex [DLPFC]; MPFC; and various methods to determine optimal coil placement (eg, EEG F3 coordinate or MRI-guided neuro-navigational methods). Such refinements offer the potential for enhanced efficacy, shorter treatment sessions, and/or improved tolerability. For example, lower frequency right DLPFC stimulations (eg, 1 Hz) can decrease the risk of seizures and improve overall tolerability. While this has not been studied as extensively as higher frequency left DLPFC stimulations (eg, 5 to 20 Hz), existing evidence supports similar efficacy between these 2 approaches.7
Theta burst stimulation. Some TMS devices can be adapted to deliver theta burst stimulation (TBS). This produces trains of triple, 50 Hz, pulsed bursts (usually with 200 ms inter-burst intervals occurring at a rate of 5 Hz; at 80% MT) to model naturally occurring theta rhythms. These bursts can be administered in stimulation protocols using intermittent TBS (iTBS) (eg, 10 bursts of triplets over 2 seconds every 10 seconds; 30 pulses per burst; for approximately 3 minutes; totaling 600 pulses) or continuous TBS (cTBS) bursts given in an uninterrupted train (eg, 40 seconds, 600 pulses). Evidence indicates these protocols facilitate long-term potentiation (ie, iTBS) and long-term depression (ie, cTBS), which in turn can modulate synaptic plasticity.
Continue to: While some clinicians are using...
While some clinicians are using TBS off-label, a recent non-inferiority trial (N = 395) reported similar efficacy and safety comparing standard 10 Hz TMS to an iTBS protocol at 120% of resting motor threshold (both over the left DLPFC).8 This has led to FDA clearance of the TMS device adapted to provide iTBS in this trial.8
From a more practical perspective, TBS has the potential to reduce the number of pulses (eg, 600 vs 3,000) and the total number of sessions required, as well as the duration of treatment sessions (eg, 37.5 minutes to <5 minutes). This can accelerate the time to response and decrease patient and staff commitment, with resulting cost savings.9 Despite this recent progress, ongoing research still needs to clarify issues such as the risk/benefit profile, particularly in younger and older populations, as well as assessment of duration of initial benefit and appropriate maintenance strategies.
New devices
Another initiative is the development of alternative TMS equipment. For example, newer coil designs with enhanced cooling ability allow for a substantial decrease in the required inter-train interval duration between stimulation trains, thus shortening the total session duration by approximately 50% (eg, from 37.5 to 19 minutes). The use of different coil arrays (eg, the H-coil capable of deeper vs surface stimulation) may allow for more direct stimulation of relevant neurocircuitry (eg, cingulate cortex), possibly improving efficacy and shortening time to onset of benefit. However, in head-to-head comparisons with single-coil devices, enhanced efficacy for depression has not been clearly demonstrated. One caveat is that the increase in depth of magnetic field penetration results in a loss of focality, resulting in the stimulation of larger brain areas. This might increase the risk of adverse effects such as seizures.
Increasing durability of effect
Because high relapse and recurrence rates compromise the initial benefit of any antidepressant therapy, appropriate maintenance strategies are essential. Several studies have evaluated strategies to maintain the acute benefit of TMS for treatment-resistant depression.
One was a 6-month, open-label TMS durability of effect trial for acute responders (n = 99) in the pivotal registration study.5 During this study, all participants were given antidepressant medication monotherapy. In addition, with early indication of relapse, patients received a reintroduction of TMS sessions (32/99 patients; mean number of sessions = 14.3). With this protocol, approximately 84% re-achieved their response status. The overall relapse rate was approximately 13%.5
Continue to: In a 1-year naturalistic study...
In a 1-year naturalistic study, 63% of patients (75/120) who met response or remission criteria after an acute course of TMS still met response criteria after 12 months. These patients received clinician-determined maintenance treatment that included reintroduction of TMS when indicated.3
In a prospective, 12-month, multisite, randomized pilot study, 67 patients with treatment-resistant MDD underwent an antidepressant medication washout and then received 30 sessions of TMS monotherapy.10 Those who met criteria for improvement (n = 49) were then randomized to once-monthly TMS or observation only. All patients remained medication-free but could receive TMS re-introduction if they deteriorated. At the end of the study, both groups demonstrated comparable outcomes, with a trend to a longer time before relapse among participants who received once-monthly TMS. Although these results are preliminary, they suggest that some patients could be treated both acutely and then maintained with TMS alone.
Re-introducing TMS in patients who show early signs of relapse after having an initial response achieves rates of sustained improvement that compare favorably with those of other strategies used to manage patients with treatment-resistant depression.
TMS vs ECT
The question often arises as to whether TMS is a viable alternate treatment to ECT. I believe the answer is unequivocally yes and no. By this, I mean some patients who in the past only had ECT as their next option when medications and psychotherapy were insufficient may now consider TMS. In support, there is evidence of comparable efficacy between TMS and ECT in a subgroup of patients who were considered clinically appropriate for ECT.11-13
How to best identify this group remains unclear, but investigators are exploring predictive biomarkers. For example, a large study (N = 1,188), with functional magnetic resonance imaging (fMRI) reported that depressed patients could be divided into 4 neurophysiological “biotypes” based on different patterns of aberrant connectivity in limbic and fronto-striatal networks.14 The authors further noted that such distinctions were helpful in predicting response in a subgroup of patients (n = 154) who received TMS.
Continue to: For now...
For now, experience indicates certain clinical factors may provide some guidance. Patients are usually better served by ECT if they:
- have depressive episodes of longer duration (eg, >3 years)
- have a high risk of suicide
- have psychotic or catatonic features associated with their depression
- have difficulty maintaining their physical well-being
- have bipolar depression.
Although existing evidence supports a possible benefit with TMS for bipolar depression (used in combination with a mood stabilizer), the lack of a definitive trial (precluding FDA clearance for this indication) and the lack of insurance coverage both limit the routine use of TMS for this indication.15
One potential advantage of TMS over ECT is a lower cost.13 Transcranial magnetic stimulation also may make it possible to achieve similar efficacy as ECT with fewer cognitive adverse effects when used in combination with ECT to reduce the number of acute ECT treatments required or as part of a maintenance strategy after a patient experiences an acute response to ECT.13
Magnetic seizure therapy (MST) vs ECT. An experimental treatment, MST uses a TMS device capable of producing more intense magnetic fields sufficient to induce a seizure.16 The advantage of MST over ECT-induced seizures is better control of intra-cerebral current path and density, thus avoiding deeper cortical areas associated with memory (eg, hippocampus) and minimizing cognitive adverse effects. As with ECT, however, anesthesia and muscle relaxation are required. Presently, MST remains investigational.
Other potential indications
In addition to MDD, TMS is also being studied as a potential treatment for other neuropsychiatric disorders.
Continue to: Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD). A recent double-blind study that evaluated a deep TMS (DTMS) device reported a significantly better outcome based on the Yale-Brown Obsessive-Compulsive Scale score with active high-frequency (20 Hz) DTMS (n = 18) vs a sham control (n = 15).17 The initial benefit persisted up to 1 month after the end of treatment. The authors speculated that this benefit may be due to direct modulation of the anterior cingulate cortex. These results led to the first FDA clearance of a deep TMS device for treating OCD.
Cognition. Because TMS does not require a seizure to produce its antidepressant effect and does not require anesthesia, the risk of neurocognitive disruption is low. In fact, evidence suggests TMS may have beneficial cognitive effects.18
In an effort to take advantage of this benefit, researchers have explored providing psychoeducation and psychotherapy sessions (eg, behavioral activation) during TMS treatments (“online”).19,20 The rationale is that neurocircuitry subserving various cognitive functions may be in a heightened state of receptivity during a TMS treatment, which would allow patients to assimilate and better utilize the therapeutic information provided.19,20
Researchers are also looking at the use of TMS to treat patients with mild cognitive impairment or early dementia. These patients often experience comorbid depression, and TMS could potentially improve memory via both its pro-cognitive and antidepressant effects.1 The lack of effective treatments for dementia supports pursuing TMS as a therapeutic option for these patients.
Other neuropsychiatric disorders. In addition to early-onset cognitive problems, other neurologic indications with promising data for TMS include chronic pain syndromes, Parkinson’s disease, tinnitus, and migraine headaches (a hand-held FDA-cleared device is now available for treating migraines). In addition to OCD and bipolar depression, other psychiatric indications with promising data include schizophrenia (eg, refractory auditory hallucinations, negative symptoms), posttraumatic stress disorder, and various addictive disorders.21 Because results have been mixed for most of these disorders, definitive trials are needed to clearly characterize the potential role of TMS.
Continue to: An ongoing evolution
An ongoing evolution
Neuromodulation is undergoing a renaissance spurred on by the need for more effective treatments to manage some of our most challenging illnesses. Transcranial magnetic stimulation and other forms of therapeutic neuromodulation are welcome additions for managing treatment-resistant depression, OCD, and possibly other disorders. But perhaps their greatest value is as a bellwether for what’s to come. In addition to the ongoing refinements to existing neuromodulation devices, newer modulation approaches (eg, temporal interference stimulation) and the search for reliable biomarkers may dramatically expand and enhance our clinical options.14,22
Bottom Line
Transcranial magnetic stimulation (TMS) continues to evolve as a nonpharmacologic treatment for mood disorders, obsessive-compulsive disorder, and potentially for other indications. Recent developments, including altered treatment parameters, new devices, and strategies for increasing the durability of antidepressant effects, have enhanced the benefits of TMS.
Related Resources
- Ziemann U. Thirty years of transcranial magnetic stimulation: where do we stand? Exp Brain Res. 2017;235(4):973-984.
- Janicak PG, Sackett V, Kudrna K, et al. Transcranial magnetic stimulation for the treatment of major depression: an update on recent advances. Current Psychiatry. 2016:15(6):49-56.
Therapeutic neuromodulation takes advantage of the brain’s electrochemical makeup. This allows for treatment devices that modulate neurocircuits relevant to behaviors disrupted in disorders such as major depressive disorder (MDD) (eg, sleep quality, appetite, cognitive, and executive functions). The default mode network (comprised of structures such as the medial prefrontal cortex [MPFC], the posterior cingulate cortex, the hippocampus, and their functional connectivity) serves as a prime example of circuitry that can be targeted by this approach.1
For 80 years, electroconvulsive therapy (ECT) has been an important neuromodulation option for patients with more severe illness. Recently, additional neuromodulatory approaches have been FDA-cleared, including transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), and deep brain stimulation (DBS). Another approach, transcranial direct current stimulation (tDCS), has been extensively studied for its potential clinical utility but is not FDA-cleared. The Table provides descriptions of these therapies.
Since being cleared by the FDA in 2008, TMS has arguably made the greatest strides in providing an alternate neuromodulation treatment option for patients with MDD, with >1,000 centers nationally and 7 TMS devices FDA-cleared for treatment of depression. In this article, we review recent developments in TMS.
An evolving therapeutic option
While primarily studied as a monotherapy for MDD, in clinical practice TMS (Box) is typically used as an adjunct to medication and psychotherapy.2,3 In this context, it has demonstrated efficacy for more difficult-to-treat mood disorders with an excellent safety and tolerability profile whether used with or without medication.4-6
To further improve the efficiency and efficacy of TMS while maintaining its safety and tolerability, researchers and clinicians have been exploring a few initiatives.
Box
- Transcranial magnetic stimulation (TMS) utilizes intense, localized magnetic fields to alter activity in neural circuits implicated in the pathophysiology of depression
- Randomized, sham-controlled acute trials have demonstrated the efficacy of TMS for treatment-resistant depression
- Clinical availability of TMS has grown steadily over the past 10 years as >1,000 centers have been opened and additional devices have been FDA-cleared
- TMS has the potential to avoid safety and tolerability concerns associated with antidepressant pharmacotherapy (eg, weight gain, sexual dysfunction) and electroconvulsive therapy (eg, cognitive deficits)
- Greater sophistication in the choice of stimulation parameters, as well as other ongoing efforts to optimize the benefits of TMS, are yielding better clinical outcomes
Altered treatment parameters
One initiative is assessing the feasibility of altering various treatment parameters, such as the total number of treatment sessions (30 to 60 sessions); the frequency of sessions (eg, more than once daily); the total number of magnetic pulses per session (eg, >3,000); the stimulation coil localization (eg, left vs right dorsal lateral prefrontal cortex [DLPFC]; MPFC; and various methods to determine optimal coil placement (eg, EEG F3 coordinate or MRI-guided neuro-navigational methods). Such refinements offer the potential for enhanced efficacy, shorter treatment sessions, and/or improved tolerability. For example, lower frequency right DLPFC stimulations (eg, 1 Hz) can decrease the risk of seizures and improve overall tolerability. While this has not been studied as extensively as higher frequency left DLPFC stimulations (eg, 5 to 20 Hz), existing evidence supports similar efficacy between these 2 approaches.7
Theta burst stimulation. Some TMS devices can be adapted to deliver theta burst stimulation (TBS). This produces trains of triple, 50 Hz, pulsed bursts (usually with 200 ms inter-burst intervals occurring at a rate of 5 Hz; at 80% MT) to model naturally occurring theta rhythms. These bursts can be administered in stimulation protocols using intermittent TBS (iTBS) (eg, 10 bursts of triplets over 2 seconds every 10 seconds; 30 pulses per burst; for approximately 3 minutes; totaling 600 pulses) or continuous TBS (cTBS) bursts given in an uninterrupted train (eg, 40 seconds, 600 pulses). Evidence indicates these protocols facilitate long-term potentiation (ie, iTBS) and long-term depression (ie, cTBS), which in turn can modulate synaptic plasticity.
Continue to: While some clinicians are using...
While some clinicians are using TBS off-label, a recent non-inferiority trial (N = 395) reported similar efficacy and safety comparing standard 10 Hz TMS to an iTBS protocol at 120% of resting motor threshold (both over the left DLPFC).8 This has led to FDA clearance of the TMS device adapted to provide iTBS in this trial.8
From a more practical perspective, TBS has the potential to reduce the number of pulses (eg, 600 vs 3,000) and the total number of sessions required, as well as the duration of treatment sessions (eg, 37.5 minutes to <5 minutes). This can accelerate the time to response and decrease patient and staff commitment, with resulting cost savings.9 Despite this recent progress, ongoing research still needs to clarify issues such as the risk/benefit profile, particularly in younger and older populations, as well as assessment of duration of initial benefit and appropriate maintenance strategies.
New devices
Another initiative is the development of alternative TMS equipment. For example, newer coil designs with enhanced cooling ability allow for a substantial decrease in the required inter-train interval duration between stimulation trains, thus shortening the total session duration by approximately 50% (eg, from 37.5 to 19 minutes). The use of different coil arrays (eg, the H-coil capable of deeper vs surface stimulation) may allow for more direct stimulation of relevant neurocircuitry (eg, cingulate cortex), possibly improving efficacy and shortening time to onset of benefit. However, in head-to-head comparisons with single-coil devices, enhanced efficacy for depression has not been clearly demonstrated. One caveat is that the increase in depth of magnetic field penetration results in a loss of focality, resulting in the stimulation of larger brain areas. This might increase the risk of adverse effects such as seizures.
Increasing durability of effect
Because high relapse and recurrence rates compromise the initial benefit of any antidepressant therapy, appropriate maintenance strategies are essential. Several studies have evaluated strategies to maintain the acute benefit of TMS for treatment-resistant depression.
One was a 6-month, open-label TMS durability of effect trial for acute responders (n = 99) in the pivotal registration study.5 During this study, all participants were given antidepressant medication monotherapy. In addition, with early indication of relapse, patients received a reintroduction of TMS sessions (32/99 patients; mean number of sessions = 14.3). With this protocol, approximately 84% re-achieved their response status. The overall relapse rate was approximately 13%.5
Continue to: In a 1-year naturalistic study...
In a 1-year naturalistic study, 63% of patients (75/120) who met response or remission criteria after an acute course of TMS still met response criteria after 12 months. These patients received clinician-determined maintenance treatment that included reintroduction of TMS when indicated.3
In a prospective, 12-month, multisite, randomized pilot study, 67 patients with treatment-resistant MDD underwent an antidepressant medication washout and then received 30 sessions of TMS monotherapy.10 Those who met criteria for improvement (n = 49) were then randomized to once-monthly TMS or observation only. All patients remained medication-free but could receive TMS re-introduction if they deteriorated. At the end of the study, both groups demonstrated comparable outcomes, with a trend to a longer time before relapse among participants who received once-monthly TMS. Although these results are preliminary, they suggest that some patients could be treated both acutely and then maintained with TMS alone.
Re-introducing TMS in patients who show early signs of relapse after having an initial response achieves rates of sustained improvement that compare favorably with those of other strategies used to manage patients with treatment-resistant depression.
TMS vs ECT
The question often arises as to whether TMS is a viable alternate treatment to ECT. I believe the answer is unequivocally yes and no. By this, I mean some patients who in the past only had ECT as their next option when medications and psychotherapy were insufficient may now consider TMS. In support, there is evidence of comparable efficacy between TMS and ECT in a subgroup of patients who were considered clinically appropriate for ECT.11-13
How to best identify this group remains unclear, but investigators are exploring predictive biomarkers. For example, a large study (N = 1,188), with functional magnetic resonance imaging (fMRI) reported that depressed patients could be divided into 4 neurophysiological “biotypes” based on different patterns of aberrant connectivity in limbic and fronto-striatal networks.14 The authors further noted that such distinctions were helpful in predicting response in a subgroup of patients (n = 154) who received TMS.
Continue to: For now...
For now, experience indicates certain clinical factors may provide some guidance. Patients are usually better served by ECT if they:
- have depressive episodes of longer duration (eg, >3 years)
- have a high risk of suicide
- have psychotic or catatonic features associated with their depression
- have difficulty maintaining their physical well-being
- have bipolar depression.
Although existing evidence supports a possible benefit with TMS for bipolar depression (used in combination with a mood stabilizer), the lack of a definitive trial (precluding FDA clearance for this indication) and the lack of insurance coverage both limit the routine use of TMS for this indication.15
One potential advantage of TMS over ECT is a lower cost.13 Transcranial magnetic stimulation also may make it possible to achieve similar efficacy as ECT with fewer cognitive adverse effects when used in combination with ECT to reduce the number of acute ECT treatments required or as part of a maintenance strategy after a patient experiences an acute response to ECT.13
Magnetic seizure therapy (MST) vs ECT. An experimental treatment, MST uses a TMS device capable of producing more intense magnetic fields sufficient to induce a seizure.16 The advantage of MST over ECT-induced seizures is better control of intra-cerebral current path and density, thus avoiding deeper cortical areas associated with memory (eg, hippocampus) and minimizing cognitive adverse effects. As with ECT, however, anesthesia and muscle relaxation are required. Presently, MST remains investigational.
Other potential indications
In addition to MDD, TMS is also being studied as a potential treatment for other neuropsychiatric disorders.
Continue to: Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD). A recent double-blind study that evaluated a deep TMS (DTMS) device reported a significantly better outcome based on the Yale-Brown Obsessive-Compulsive Scale score with active high-frequency (20 Hz) DTMS (n = 18) vs a sham control (n = 15).17 The initial benefit persisted up to 1 month after the end of treatment. The authors speculated that this benefit may be due to direct modulation of the anterior cingulate cortex. These results led to the first FDA clearance of a deep TMS device for treating OCD.
Cognition. Because TMS does not require a seizure to produce its antidepressant effect and does not require anesthesia, the risk of neurocognitive disruption is low. In fact, evidence suggests TMS may have beneficial cognitive effects.18
In an effort to take advantage of this benefit, researchers have explored providing psychoeducation and psychotherapy sessions (eg, behavioral activation) during TMS treatments (“online”).19,20 The rationale is that neurocircuitry subserving various cognitive functions may be in a heightened state of receptivity during a TMS treatment, which would allow patients to assimilate and better utilize the therapeutic information provided.19,20
Researchers are also looking at the use of TMS to treat patients with mild cognitive impairment or early dementia. These patients often experience comorbid depression, and TMS could potentially improve memory via both its pro-cognitive and antidepressant effects.1 The lack of effective treatments for dementia supports pursuing TMS as a therapeutic option for these patients.
Other neuropsychiatric disorders. In addition to early-onset cognitive problems, other neurologic indications with promising data for TMS include chronic pain syndromes, Parkinson’s disease, tinnitus, and migraine headaches (a hand-held FDA-cleared device is now available for treating migraines). In addition to OCD and bipolar depression, other psychiatric indications with promising data include schizophrenia (eg, refractory auditory hallucinations, negative symptoms), posttraumatic stress disorder, and various addictive disorders.21 Because results have been mixed for most of these disorders, definitive trials are needed to clearly characterize the potential role of TMS.
Continue to: An ongoing evolution
An ongoing evolution
Neuromodulation is undergoing a renaissance spurred on by the need for more effective treatments to manage some of our most challenging illnesses. Transcranial magnetic stimulation and other forms of therapeutic neuromodulation are welcome additions for managing treatment-resistant depression, OCD, and possibly other disorders. But perhaps their greatest value is as a bellwether for what’s to come. In addition to the ongoing refinements to existing neuromodulation devices, newer modulation approaches (eg, temporal interference stimulation) and the search for reliable biomarkers may dramatically expand and enhance our clinical options.14,22
Bottom Line
Transcranial magnetic stimulation (TMS) continues to evolve as a nonpharmacologic treatment for mood disorders, obsessive-compulsive disorder, and potentially for other indications. Recent developments, including altered treatment parameters, new devices, and strategies for increasing the durability of antidepressant effects, have enhanced the benefits of TMS.
Related Resources
- Ziemann U. Thirty years of transcranial magnetic stimulation: where do we stand? Exp Brain Res. 2017;235(4):973-984.
- Janicak PG, Sackett V, Kudrna K, et al. Transcranial magnetic stimulation for the treatment of major depression: an update on recent advances. Current Psychiatry. 2016:15(6):49-56.
1. Koch G, Bonnì S, Pellicciari MC, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage. 2018;169: 302-310.
2. O’Reardon JP, Solvason B, Janicak PG, et al. Efficacy and safety of repetitive transcranial magnetic stimulation (rTMS) in the acute treatment of major depression: results of a multicenter randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
3. Dunner DL, Aaronson ST, Sackheim HA, et al. A multisite, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a one-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-1401.
4. Janicak PG, O’Reardon JP, Sampson SM, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. 2008;69:222-232.
5. Janicak PG, Nahas Z, Lisanby SH, et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010;3(4):187-199.
6. Janicak PG. Risk management issues in transcranial magnetic stimulation for treatment of major depression. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
7. Chen J, Zhou C, Wu B, et al. Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Res. 2013;210(3):1260-1264.
8. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683-1692.
9. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. 2015;32(3):182-192.
10. Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul. 2016;9(2):251-257.
11. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prop Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
12. Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depressive: preliminary results of a randomized trial. Biol Psychiatry. 2002;51(8):659-667.
13. Lanocha K, Janicak PG. TMS for depression: relationship to ECT and other therapeutic neuromodulation approaches. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
14. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38.
15. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
16. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi: 10.1155/2015/521398.
17. Carmi L, Alyagon U, Barnea-Ygael N, et al. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018;11(1):158-165.
18. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114:1125-1132.
19. Donse L, Padberg F, Sack AT, et al. Simultaneous rTMS and psychotherapy in major depressive disorder: Clinical outcomes and predictors from a large naturalistic study. Brain Stimul. 2018;11(2):337-345.
20. Russo GB, Tirrell E, Busch A, et al. Behavioral activation therapy during transcranial magnetic stimulation for major depressive disorder. J Affect Disord. 2018;236:101-104.
21. Pannu J, DE Souza DD, Samara Z, et al. Transcranial magnetic stimulation for disorders other than depression. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
22. Grossman N. Modulation without surgical intervention. Science. 2018;361:461-462.
1. Koch G, Bonnì S, Pellicciari MC, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage. 2018;169: 302-310.
2. O’Reardon JP, Solvason B, Janicak PG, et al. Efficacy and safety of repetitive transcranial magnetic stimulation (rTMS) in the acute treatment of major depression: results of a multicenter randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
3. Dunner DL, Aaronson ST, Sackheim HA, et al. A multisite, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a one-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-1401.
4. Janicak PG, O’Reardon JP, Sampson SM, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. 2008;69:222-232.
5. Janicak PG, Nahas Z, Lisanby SH, et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010;3(4):187-199.
6. Janicak PG. Risk management issues in transcranial magnetic stimulation for treatment of major depression. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
7. Chen J, Zhou C, Wu B, et al. Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Res. 2013;210(3):1260-1264.
8. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683-1692.
9. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. 2015;32(3):182-192.
10. Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul. 2016;9(2):251-257.
11. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prop Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
12. Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depressive: preliminary results of a randomized trial. Biol Psychiatry. 2002;51(8):659-667.
13. Lanocha K, Janicak PG. TMS for depression: relationship to ECT and other therapeutic neuromodulation approaches. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
14. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38.
15. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
16. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi: 10.1155/2015/521398.
17. Carmi L, Alyagon U, Barnea-Ygael N, et al. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018;11(1):158-165.
18. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114:1125-1132.
19. Donse L, Padberg F, Sack AT, et al. Simultaneous rTMS and psychotherapy in major depressive disorder: Clinical outcomes and predictors from a large naturalistic study. Brain Stimul. 2018;11(2):337-345.
20. Russo GB, Tirrell E, Busch A, et al. Behavioral activation therapy during transcranial magnetic stimulation for major depressive disorder. J Affect Disord. 2018;236:101-104.
21. Pannu J, DE Souza DD, Samara Z, et al. Transcranial magnetic stimulation for disorders other than depression. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
22. Grossman N. Modulation without surgical intervention. Science. 2018;361:461-462.
App, role-playing used as interventions
Help for people with mental health and substance use issues has become more accessible now that the Georgia Crisis & Access Line launched a mobile app. The aim of the My GCAL app is to guide people to free and confidential access, and the targets are those who would rather send a text message than speak to someone over the phone. In Georgia and elsewhere, this tends to be younger people. “We are trying to be proactive,” said Georgia Gov. Brian Kemp. Atlanta Journal-Constitution.
Ninth-grade students at the Uplift Hampton Preparatory school in Dallas have been taking part in classroom sessions where role-playing activities are helping them spot the signs of depression in themselves and others. “It’s kind of like ‘Mental Health 101,’ ” said Tony Walker, senior director of student support services at Uplift Education, in an Associated Press article published in the National Post. “So they talk about depression and anxiety and just common mental health issues, and then I think the most important thing is they talk about what to do if you feel that way.” The Youth Aware of Mental Health (YAM) program, administered by Madhukar H. Trivedi, MD, of the University of Texas Southwestern Medical Center is offered to all 9th-graders in the 20-school Uplift Education network in the Dallas area. The program, consisting of five 45-minute sessions, originally was developed at the Karolinska Institute in Stockholm and Columbia University in New York. A similar initiative offered by the National Alliance on Mental Illness (NAMI) teaches students the warning signs of mental health problems. Since the NAMI program launched in 2014, it has reached almost 450,000 youth in 41 states. National Post.
Identifying the source of students’ frustration and anger can prevent them from lashing out, according to a National Public Radio report. But responses rooted in compassion can help diffuse potentially tragic outcomes. The report describes the story of a young man whose struggles started in middle school. An encounter with bullies left him with severe damage in his right eye, and he spent his high school years getting into fights. After school officials stepped in, acknowledged that he had reasons to be angry, and connected him with a mentor who was able to talk and reason with him, the young man graduated on time. He’s now 25 and works full time for a security firm. “Moving kids from despair to hope. That’s the bumper sticker for what we do,” said school psychologist John Van Dreal. The approach “really works,” he added. NPR.
An exhibition now running at the Science Center of Iowa in Des Moines is helping patrons explore the reality of mental illness. The Mental Health: Mind Matters exhibition is intended to show the real lives of people with mental illness, with the hope of inspiring better appreciation and empathy. It features audio renderings of what the world can sound like to someone with psychosis, walk-through rooms that take patrons inside the homes of people with depression, and exercises that inspire worry or fear, as well as photography. The aim is to take the patron inside the heads of those with mental illnesses. “A possibility now exists to utilize a constellation of exhibits like Mind Matters to revolutionize understanding, prevention, and wellness nationally, all while unlocking economic benefits and advancing human dignity,” wrote Paul Piwko, the author of an article describing the exhibit. Des Moines Register.
Students are creating a dialogue about suicide awareness and prevention at Gardner (Kansas) Edgerton High School. At a recent basketball game with Shawnee Mission West High School, Gardner Edgerton team members, students, cheerleaders, and fans donned T-shirts emblazoned with “#ZeroReasonsWhy.” The student-led campaign is aimed at encouraging students to seek help rather than consider suicide. At the game, T-shirts and bracelets also were handed out to Shawnee Mission West players in an effort to spread the message. The Kansas City Star.
Help for people with mental health and substance use issues has become more accessible now that the Georgia Crisis & Access Line launched a mobile app. The aim of the My GCAL app is to guide people to free and confidential access, and the targets are those who would rather send a text message than speak to someone over the phone. In Georgia and elsewhere, this tends to be younger people. “We are trying to be proactive,” said Georgia Gov. Brian Kemp. Atlanta Journal-Constitution.
Ninth-grade students at the Uplift Hampton Preparatory school in Dallas have been taking part in classroom sessions where role-playing activities are helping them spot the signs of depression in themselves and others. “It’s kind of like ‘Mental Health 101,’ ” said Tony Walker, senior director of student support services at Uplift Education, in an Associated Press article published in the National Post. “So they talk about depression and anxiety and just common mental health issues, and then I think the most important thing is they talk about what to do if you feel that way.” The Youth Aware of Mental Health (YAM) program, administered by Madhukar H. Trivedi, MD, of the University of Texas Southwestern Medical Center is offered to all 9th-graders in the 20-school Uplift Education network in the Dallas area. The program, consisting of five 45-minute sessions, originally was developed at the Karolinska Institute in Stockholm and Columbia University in New York. A similar initiative offered by the National Alliance on Mental Illness (NAMI) teaches students the warning signs of mental health problems. Since the NAMI program launched in 2014, it has reached almost 450,000 youth in 41 states. National Post.
Identifying the source of students’ frustration and anger can prevent them from lashing out, according to a National Public Radio report. But responses rooted in compassion can help diffuse potentially tragic outcomes. The report describes the story of a young man whose struggles started in middle school. An encounter with bullies left him with severe damage in his right eye, and he spent his high school years getting into fights. After school officials stepped in, acknowledged that he had reasons to be angry, and connected him with a mentor who was able to talk and reason with him, the young man graduated on time. He’s now 25 and works full time for a security firm. “Moving kids from despair to hope. That’s the bumper sticker for what we do,” said school psychologist John Van Dreal. The approach “really works,” he added. NPR.
An exhibition now running at the Science Center of Iowa in Des Moines is helping patrons explore the reality of mental illness. The Mental Health: Mind Matters exhibition is intended to show the real lives of people with mental illness, with the hope of inspiring better appreciation and empathy. It features audio renderings of what the world can sound like to someone with psychosis, walk-through rooms that take patrons inside the homes of people with depression, and exercises that inspire worry or fear, as well as photography. The aim is to take the patron inside the heads of those with mental illnesses. “A possibility now exists to utilize a constellation of exhibits like Mind Matters to revolutionize understanding, prevention, and wellness nationally, all while unlocking economic benefits and advancing human dignity,” wrote Paul Piwko, the author of an article describing the exhibit. Des Moines Register.
Students are creating a dialogue about suicide awareness and prevention at Gardner (Kansas) Edgerton High School. At a recent basketball game with Shawnee Mission West High School, Gardner Edgerton team members, students, cheerleaders, and fans donned T-shirts emblazoned with “#ZeroReasonsWhy.” The student-led campaign is aimed at encouraging students to seek help rather than consider suicide. At the game, T-shirts and bracelets also were handed out to Shawnee Mission West players in an effort to spread the message. The Kansas City Star.
Help for people with mental health and substance use issues has become more accessible now that the Georgia Crisis & Access Line launched a mobile app. The aim of the My GCAL app is to guide people to free and confidential access, and the targets are those who would rather send a text message than speak to someone over the phone. In Georgia and elsewhere, this tends to be younger people. “We are trying to be proactive,” said Georgia Gov. Brian Kemp. Atlanta Journal-Constitution.
Ninth-grade students at the Uplift Hampton Preparatory school in Dallas have been taking part in classroom sessions where role-playing activities are helping them spot the signs of depression in themselves and others. “It’s kind of like ‘Mental Health 101,’ ” said Tony Walker, senior director of student support services at Uplift Education, in an Associated Press article published in the National Post. “So they talk about depression and anxiety and just common mental health issues, and then I think the most important thing is they talk about what to do if you feel that way.” The Youth Aware of Mental Health (YAM) program, administered by Madhukar H. Trivedi, MD, of the University of Texas Southwestern Medical Center is offered to all 9th-graders in the 20-school Uplift Education network in the Dallas area. The program, consisting of five 45-minute sessions, originally was developed at the Karolinska Institute in Stockholm and Columbia University in New York. A similar initiative offered by the National Alliance on Mental Illness (NAMI) teaches students the warning signs of mental health problems. Since the NAMI program launched in 2014, it has reached almost 450,000 youth in 41 states. National Post.
Identifying the source of students’ frustration and anger can prevent them from lashing out, according to a National Public Radio report. But responses rooted in compassion can help diffuse potentially tragic outcomes. The report describes the story of a young man whose struggles started in middle school. An encounter with bullies left him with severe damage in his right eye, and he spent his high school years getting into fights. After school officials stepped in, acknowledged that he had reasons to be angry, and connected him with a mentor who was able to talk and reason with him, the young man graduated on time. He’s now 25 and works full time for a security firm. “Moving kids from despair to hope. That’s the bumper sticker for what we do,” said school psychologist John Van Dreal. The approach “really works,” he added. NPR.
An exhibition now running at the Science Center of Iowa in Des Moines is helping patrons explore the reality of mental illness. The Mental Health: Mind Matters exhibition is intended to show the real lives of people with mental illness, with the hope of inspiring better appreciation and empathy. It features audio renderings of what the world can sound like to someone with psychosis, walk-through rooms that take patrons inside the homes of people with depression, and exercises that inspire worry or fear, as well as photography. The aim is to take the patron inside the heads of those with mental illnesses. “A possibility now exists to utilize a constellation of exhibits like Mind Matters to revolutionize understanding, prevention, and wellness nationally, all while unlocking economic benefits and advancing human dignity,” wrote Paul Piwko, the author of an article describing the exhibit. Des Moines Register.
Students are creating a dialogue about suicide awareness and prevention at Gardner (Kansas) Edgerton High School. At a recent basketball game with Shawnee Mission West High School, Gardner Edgerton team members, students, cheerleaders, and fans donned T-shirts emblazoned with “#ZeroReasonsWhy.” The student-led campaign is aimed at encouraging students to seek help rather than consider suicide. At the game, T-shirts and bracelets also were handed out to Shawnee Mission West players in an effort to spread the message. The Kansas City Star.
ICU admissions raise chronic condition risk
SAN DIEGO – The research showed rising likelihood of conditions such as depression, diabetes, and heart disease.
By merging two existing databases, the researchers were able to capture a more comprehensive picture of post-ICU patients. “We were able to include almost the entire country,” Ilse van Beusekom, a PhD candidate in health sciences at the University of Amsterdam and data manager at the National Intensive Care Evaluation (NICE) foundation, said in an interview.
Ms. van Beusekom presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. The study was simultaneously published in Critical Care Medicine.
The work compared 56,760 ICU survivors from 81 facilities across the Netherlands to 75,232 age-, sex-, and socioeconomic status–matched controls. The mean age was 65 years and 60% of the population was male. “The types of chronic conditions are the same, only the prevalences are different,” said Ms. van Beusekom.
The researchers compared chronic conditions in the year before ICU admission and the year after, based on data pulled from the NICE national quality database, which includes data describing the first 24 hours of ICU admission, and the Vektis insurance claims database, which includes information on medical treatment. Before ICU admission, 45% of the ICU population was free of chronic conditions, as were 62% of controls. One chronic condition was present in 36% of ICU patients, versus 29% of controls, and two or more conditions were present in 19% versus 9% of controls.
The ICU population was more likely to have high cholesterol (16% vs. 14%), heart disease (14% vs. 6%), chronic obstructive pulmonary disease (8% vs. 3%), type II diabetes (8% vs. 6%), type I diabetes (6% vs. 3%), and depression (6% vs. 4%).
The ICU population also was at greater risk of developing one or more new chronic conditions during the year following their stay. The risk was three- to fourfold higher throughout age ranges.
The study suggests the need for greater follow-up after an ICU admission in order to help patients cope with lingering problems. Ms. van Beusekom noted that there are follow-up programs in the Netherlands for several patient groups, but none for ICU survivors. One possibility would be to have the patient return to the ICU 3 months or so after release to discuss their diagnosis, treatment, and any lingering concerns. “A lot of people don’t know that their complaints are linked with the ICU visit,” said Ms. van Beusekom.
Timothy G. Buchman, MD, professor of surgery at Emory University, Atlanta, who moderated the session, wondered why the ICU seems to be an inflection point for developing new chronic conditions. Could it simply be because patients are sicker to begin with and have reached an inflection point of their illness, or could the treatments in ICU be contributing to or exposing those conditions? Ms. van Beusekom believed it was likely a combination of factors, and she referred to data she had not presented showing that even control patients who had been to the hospital (though not the ICU) during the study period were at lower risk of new chronic conditions than ICU patients.
Ms. van Beusekom’s group plans to investigate ICU-related variables that might be associated with risk of chronic conditions.
The study was not funded. Ms. van Beusekom had no relevant disclosures.
SOURCE: van Beusekom I et al. CCC48, Abstract Crit Care Med. 2019;47:324-30.
SAN DIEGO – The research showed rising likelihood of conditions such as depression, diabetes, and heart disease.
By merging two existing databases, the researchers were able to capture a more comprehensive picture of post-ICU patients. “We were able to include almost the entire country,” Ilse van Beusekom, a PhD candidate in health sciences at the University of Amsterdam and data manager at the National Intensive Care Evaluation (NICE) foundation, said in an interview.
Ms. van Beusekom presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. The study was simultaneously published in Critical Care Medicine.
The work compared 56,760 ICU survivors from 81 facilities across the Netherlands to 75,232 age-, sex-, and socioeconomic status–matched controls. The mean age was 65 years and 60% of the population was male. “The types of chronic conditions are the same, only the prevalences are different,” said Ms. van Beusekom.
The researchers compared chronic conditions in the year before ICU admission and the year after, based on data pulled from the NICE national quality database, which includes data describing the first 24 hours of ICU admission, and the Vektis insurance claims database, which includes information on medical treatment. Before ICU admission, 45% of the ICU population was free of chronic conditions, as were 62% of controls. One chronic condition was present in 36% of ICU patients, versus 29% of controls, and two or more conditions were present in 19% versus 9% of controls.
The ICU population was more likely to have high cholesterol (16% vs. 14%), heart disease (14% vs. 6%), chronic obstructive pulmonary disease (8% vs. 3%), type II diabetes (8% vs. 6%), type I diabetes (6% vs. 3%), and depression (6% vs. 4%).
The ICU population also was at greater risk of developing one or more new chronic conditions during the year following their stay. The risk was three- to fourfold higher throughout age ranges.
The study suggests the need for greater follow-up after an ICU admission in order to help patients cope with lingering problems. Ms. van Beusekom noted that there are follow-up programs in the Netherlands for several patient groups, but none for ICU survivors. One possibility would be to have the patient return to the ICU 3 months or so after release to discuss their diagnosis, treatment, and any lingering concerns. “A lot of people don’t know that their complaints are linked with the ICU visit,” said Ms. van Beusekom.
Timothy G. Buchman, MD, professor of surgery at Emory University, Atlanta, who moderated the session, wondered why the ICU seems to be an inflection point for developing new chronic conditions. Could it simply be because patients are sicker to begin with and have reached an inflection point of their illness, or could the treatments in ICU be contributing to or exposing those conditions? Ms. van Beusekom believed it was likely a combination of factors, and she referred to data she had not presented showing that even control patients who had been to the hospital (though not the ICU) during the study period were at lower risk of new chronic conditions than ICU patients.
Ms. van Beusekom’s group plans to investigate ICU-related variables that might be associated with risk of chronic conditions.
The study was not funded. Ms. van Beusekom had no relevant disclosures.
SOURCE: van Beusekom I et al. CCC48, Abstract Crit Care Med. 2019;47:324-30.
SAN DIEGO – The research showed rising likelihood of conditions such as depression, diabetes, and heart disease.
By merging two existing databases, the researchers were able to capture a more comprehensive picture of post-ICU patients. “We were able to include almost the entire country,” Ilse van Beusekom, a PhD candidate in health sciences at the University of Amsterdam and data manager at the National Intensive Care Evaluation (NICE) foundation, said in an interview.
Ms. van Beusekom presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. The study was simultaneously published in Critical Care Medicine.
The work compared 56,760 ICU survivors from 81 facilities across the Netherlands to 75,232 age-, sex-, and socioeconomic status–matched controls. The mean age was 65 years and 60% of the population was male. “The types of chronic conditions are the same, only the prevalences are different,” said Ms. van Beusekom.
The researchers compared chronic conditions in the year before ICU admission and the year after, based on data pulled from the NICE national quality database, which includes data describing the first 24 hours of ICU admission, and the Vektis insurance claims database, which includes information on medical treatment. Before ICU admission, 45% of the ICU population was free of chronic conditions, as were 62% of controls. One chronic condition was present in 36% of ICU patients, versus 29% of controls, and two or more conditions were present in 19% versus 9% of controls.
The ICU population was more likely to have high cholesterol (16% vs. 14%), heart disease (14% vs. 6%), chronic obstructive pulmonary disease (8% vs. 3%), type II diabetes (8% vs. 6%), type I diabetes (6% vs. 3%), and depression (6% vs. 4%).
The ICU population also was at greater risk of developing one or more new chronic conditions during the year following their stay. The risk was three- to fourfold higher throughout age ranges.
The study suggests the need for greater follow-up after an ICU admission in order to help patients cope with lingering problems. Ms. van Beusekom noted that there are follow-up programs in the Netherlands for several patient groups, but none for ICU survivors. One possibility would be to have the patient return to the ICU 3 months or so after release to discuss their diagnosis, treatment, and any lingering concerns. “A lot of people don’t know that their complaints are linked with the ICU visit,” said Ms. van Beusekom.
Timothy G. Buchman, MD, professor of surgery at Emory University, Atlanta, who moderated the session, wondered why the ICU seems to be an inflection point for developing new chronic conditions. Could it simply be because patients are sicker to begin with and have reached an inflection point of their illness, or could the treatments in ICU be contributing to or exposing those conditions? Ms. van Beusekom believed it was likely a combination of factors, and she referred to data she had not presented showing that even control patients who had been to the hospital (though not the ICU) during the study period were at lower risk of new chronic conditions than ICU patients.
Ms. van Beusekom’s group plans to investigate ICU-related variables that might be associated with risk of chronic conditions.
The study was not funded. Ms. van Beusekom had no relevant disclosures.
SOURCE: van Beusekom I et al. CCC48, Abstract Crit Care Med. 2019;47:324-30.
REPORTING FROM CCC48
Checklists, colleagues key when psychiatric patient overdoses
BONITA SPRINGS, FLA. — Specialized checklists and colleague support prove crucial to psychiatrists when one of their patients in treatment for substance use disorder dies from an overdose, an expert said at the Annual Meeting of the American Academy of Addiction Psychiatry.
Much of the knowledge about how psychiatrists are affected by overdose deaths, and what can help them handle them better, is drawn from the literature on patient suicide – both types of death are sudden and unexpected, and both involve stigma and can isolate the patients’ families and providers, said Amy Yule, MD, medical director of the Addiction Recovery Management Service at Massachusetts General Hospital in Boston.
“To our knowledge, the provider’s experience after an overdose has not been studied, and [there are] no practice guidelines to guide providers after an overdose death,” she said.
The overdose death of a patient is a particularly difficult matter because psychiatrists struggle with the emotional toll at the same time that they are dealing with fairly urgent details, including some with important legal implications, Dr. Yule said.
“Literature on the provider experience after suicide death indicates that providers are highly impacted by a patient’s suicide,” she said.
A key question is whether to contact the patient’s family. And generally, the answer should be yes.
“It’s really important to offer the option to meet with family members since these families may feel very isolated stigma as they grieve,” Dr. Yule said. What’s more, when families are not contacted by the physician, they might turn to litigation to try to seek information to help them understand their loss, she said.
In a survey of therapists whose patients died by suicide, 73% said they made contact with patient families and, in most instances, the family was not critical and expressed gratitude.
She emphasized the importance of knowing whether a patient’s family knew of the treatment. Because privacy laws extend after a patient’s death, providers cannot disclose treatment to families who did not already know, she said.
Also, she said, “communication with families should be focused on addressing the family members’ feelings and not the clinical details of the case.”
Most states have “apology statutes” that prevent expressions of sympathy – such as, “I’m sorry for your loss” – to be used as admission of liability, but providers should check the laws in their own states, she said.
If you have a colleague whose patient has overdosed or lost their lives to suicide, certain approaches are better than others, Dr. Yule said.
“It’s helpful when colleagues share their own experience with the suicide of a patient or patient who has overdosed and died,” she said. “What’s not helpful is the premature reassurance that the clinician has done nothing wrong. We may feel in these instances that we want to provide that premature reassurance, but it’s important not to do that because it doesn’t help providers resolve their grief.”
For solo providers, it’s especially important to be part of a physician network because they might otherwise not have the same support that those in larger organizations have, she said.
Beyond the grieving process, logistical details also need tending to, she said. The malpractice insurance carrier should be notified, even when there was no sign of a contentious interaction with the family. And, in her organization, the staff run down a checklist that includes not only calling the family and sending a condolence card, notifying staff promptly, and documenting the death, but also easily overlooked details like canceling future appointments in the scheduling system.
“You really don’t want a phone call going to the patient’s family with an appointment reminder after the patient is deceased,” Dr. Yule said. “These are the little details that you may not remember when you’re acutely grieving a patient’s death. And that’s why we feel it’s important to have a list.”
Dr. Yule reported no relevant disclosures.
BONITA SPRINGS, FLA. — Specialized checklists and colleague support prove crucial to psychiatrists when one of their patients in treatment for substance use disorder dies from an overdose, an expert said at the Annual Meeting of the American Academy of Addiction Psychiatry.
Much of the knowledge about how psychiatrists are affected by overdose deaths, and what can help them handle them better, is drawn from the literature on patient suicide – both types of death are sudden and unexpected, and both involve stigma and can isolate the patients’ families and providers, said Amy Yule, MD, medical director of the Addiction Recovery Management Service at Massachusetts General Hospital in Boston.
“To our knowledge, the provider’s experience after an overdose has not been studied, and [there are] no practice guidelines to guide providers after an overdose death,” she said.
The overdose death of a patient is a particularly difficult matter because psychiatrists struggle with the emotional toll at the same time that they are dealing with fairly urgent details, including some with important legal implications, Dr. Yule said.
“Literature on the provider experience after suicide death indicates that providers are highly impacted by a patient’s suicide,” she said.
A key question is whether to contact the patient’s family. And generally, the answer should be yes.
“It’s really important to offer the option to meet with family members since these families may feel very isolated stigma as they grieve,” Dr. Yule said. What’s more, when families are not contacted by the physician, they might turn to litigation to try to seek information to help them understand their loss, she said.
In a survey of therapists whose patients died by suicide, 73% said they made contact with patient families and, in most instances, the family was not critical and expressed gratitude.
She emphasized the importance of knowing whether a patient’s family knew of the treatment. Because privacy laws extend after a patient’s death, providers cannot disclose treatment to families who did not already know, she said.
Also, she said, “communication with families should be focused on addressing the family members’ feelings and not the clinical details of the case.”
Most states have “apology statutes” that prevent expressions of sympathy – such as, “I’m sorry for your loss” – to be used as admission of liability, but providers should check the laws in their own states, she said.
If you have a colleague whose patient has overdosed or lost their lives to suicide, certain approaches are better than others, Dr. Yule said.
“It’s helpful when colleagues share their own experience with the suicide of a patient or patient who has overdosed and died,” she said. “What’s not helpful is the premature reassurance that the clinician has done nothing wrong. We may feel in these instances that we want to provide that premature reassurance, but it’s important not to do that because it doesn’t help providers resolve their grief.”
For solo providers, it’s especially important to be part of a physician network because they might otherwise not have the same support that those in larger organizations have, she said.
Beyond the grieving process, logistical details also need tending to, she said. The malpractice insurance carrier should be notified, even when there was no sign of a contentious interaction with the family. And, in her organization, the staff run down a checklist that includes not only calling the family and sending a condolence card, notifying staff promptly, and documenting the death, but also easily overlooked details like canceling future appointments in the scheduling system.
“You really don’t want a phone call going to the patient’s family with an appointment reminder after the patient is deceased,” Dr. Yule said. “These are the little details that you may not remember when you’re acutely grieving a patient’s death. And that’s why we feel it’s important to have a list.”
Dr. Yule reported no relevant disclosures.
BONITA SPRINGS, FLA. — Specialized checklists and colleague support prove crucial to psychiatrists when one of their patients in treatment for substance use disorder dies from an overdose, an expert said at the Annual Meeting of the American Academy of Addiction Psychiatry.
Much of the knowledge about how psychiatrists are affected by overdose deaths, and what can help them handle them better, is drawn from the literature on patient suicide – both types of death are sudden and unexpected, and both involve stigma and can isolate the patients’ families and providers, said Amy Yule, MD, medical director of the Addiction Recovery Management Service at Massachusetts General Hospital in Boston.
“To our knowledge, the provider’s experience after an overdose has not been studied, and [there are] no practice guidelines to guide providers after an overdose death,” she said.
The overdose death of a patient is a particularly difficult matter because psychiatrists struggle with the emotional toll at the same time that they are dealing with fairly urgent details, including some with important legal implications, Dr. Yule said.
“Literature on the provider experience after suicide death indicates that providers are highly impacted by a patient’s suicide,” she said.
A key question is whether to contact the patient’s family. And generally, the answer should be yes.
“It’s really important to offer the option to meet with family members since these families may feel very isolated stigma as they grieve,” Dr. Yule said. What’s more, when families are not contacted by the physician, they might turn to litigation to try to seek information to help them understand their loss, she said.
In a survey of therapists whose patients died by suicide, 73% said they made contact with patient families and, in most instances, the family was not critical and expressed gratitude.
She emphasized the importance of knowing whether a patient’s family knew of the treatment. Because privacy laws extend after a patient’s death, providers cannot disclose treatment to families who did not already know, she said.
Also, she said, “communication with families should be focused on addressing the family members’ feelings and not the clinical details of the case.”
Most states have “apology statutes” that prevent expressions of sympathy – such as, “I’m sorry for your loss” – to be used as admission of liability, but providers should check the laws in their own states, she said.
If you have a colleague whose patient has overdosed or lost their lives to suicide, certain approaches are better than others, Dr. Yule said.
“It’s helpful when colleagues share their own experience with the suicide of a patient or patient who has overdosed and died,” she said. “What’s not helpful is the premature reassurance that the clinician has done nothing wrong. We may feel in these instances that we want to provide that premature reassurance, but it’s important not to do that because it doesn’t help providers resolve their grief.”
For solo providers, it’s especially important to be part of a physician network because they might otherwise not have the same support that those in larger organizations have, she said.
Beyond the grieving process, logistical details also need tending to, she said. The malpractice insurance carrier should be notified, even when there was no sign of a contentious interaction with the family. And, in her organization, the staff run down a checklist that includes not only calling the family and sending a condolence card, notifying staff promptly, and documenting the death, but also easily overlooked details like canceling future appointments in the scheduling system.
“You really don’t want a phone call going to the patient’s family with an appointment reminder after the patient is deceased,” Dr. Yule said. “These are the little details that you may not remember when you’re acutely grieving a patient’s death. And that’s why we feel it’s important to have a list.”
Dr. Yule reported no relevant disclosures.
EXPERT ANALYSIS FROM AAAP 2018
Child suicides rock Kentucky county; lack of access to care burdens rural Arizona
The people of Fayette County, Ky., reportedly have experienced five suicides by children in the last year. The latest suicide occurred several weeks ago and involved a 12-year-old girl. This followed the suicides of children aged 10, 11, 13, and 14 years.
The deaths are not related, and there seems to be no connection to race or gender. All involved hanging, and one child might have been bullied.
The cases reflect a disturbing trend in Kentucky and across the country. In 2015, 25% of suicides of children under 17 years in Kentucky involved those aged 10-14, a 14% increase from 4 years earlier. The percentage of 6th-grade students who have thought about or planned their suicide also has climbed in recent years.
Fayette County Coroner Gary W. Ginn said the deaths in Kentucky provide another example of how events in life that might be less traumatic to adults can cause mental anguish for children, anguish which can lead some to take their own lives.
“We should be very worried to have this many cases, but we should not be hopeless,“ said Susan H. Pollack, MD, a pediatrician at the University of Kentucky Children’s Hospital, Lexington, in an interview with the Lexington Herald-Leader. She added that resources and programs are available but that stronger support systems focusing on youth are needed.
“Our families have limited options when their child needs a higher level of care. ... Local agencies often have a wait list,” Fayette County Public Schools spokeswoman Lisa Deffendall said in the article. “We have made referrals and seen it take weeks for children to get the help they need. Where do families turn when their child is in crisis? Who provides care when school is not in session?”
Rural Arizona facing crisis
Living in rural areas can prove isolating, and gaining access to health care, including mental health care, can be challenging. A segment presented on KOLD News 13 in Tucson provided yet another examples of the mental health crisis in rural America.
Cochise County is an area of about 6,200 square miles in the southeast corner of Arizona. The area, which is about eight times bigger than New York City, is home to about 125,000 people. For those with mental health issues, it’s a bleak place to live, with only two psychiatrists available and no mental health facility.
“It’s as if we got a fire going that we can’t put out,” said James P. Reed, DO, in an interview. He is one of the two psychiatrists practicing in the county. In the last 2 months alone, 64 new people have sought his help, and he has had to turn many away.
Dr. Reed has been practicing in the country for 35 years, which gives him a longer-term perspective. “It’s so much worse now. I don’t know what it is, if it’s a consequence of our society and the direction it’s going. I just can’t put my finger on it.”
The main reason behind the paucity of mental health professionals comes down to economics. Burdened with student loan debts after graduation from medical school, the low salaries of rural positions cripple the recruitment of psychiatrists and other medical professionals.
In Cochise County, as elsewhere, the main refuge for people with mental illness is jail. “We have people in there [who] really shouldn’t be in there,” said Cochise County Sheriff Mark J. Dannels. “These people need special help that I can’t provide to them. It’s almost a misjustice to have them in our jail. Unfortunately, there’s no other place to put them.”
Perils of involuntary mental health holds
South Dakota is one of five states where jailing people with mental illness is part of a deliberate strategy, and the state’s new governor wants to change the practice.
“They’re not criminals,” Gov. Kristi Noem said in an article published in the Sioux Falls Argus Leader. “They’re having a crisis at a point in time when they need to be observed, but unfortunately, in a lot of communities, that’s the only option that folks have.”
The article cited the case of Nick Johnson, a 14-year-old whose mental health struggles include the loss of control that can include aggressiveness. Although not charged with any offense, the response had been stints in the Minnehaha County Juvenile Detention Center. “It feels like I’m in prison,” Nick said. “Why would a kid have to go through that?”
National advocacy groups have criticized the practice of imprisoning people with mental illness, and local jail officials have complained that their facilities have not been designed to deliver mental health treatment. “If you look at it from a strictly medical perspective, being in a jail setting is almost guaranteed to make somebody’s mental health crisis worse, not better,” said Lisa Dailey, legislative and policy counsel for the Treatment Advocacy Center, a national nonprofit that surveys and ranks states for their mental health policies. “It’s the worst possible thing you could do.” The report said the other states that take this approach toward people with mental illness are Texas, Wyoming, New Mexico, and North Dakota.
Cutbacks may hit recovery centers
Idaho’s nine crisis centers are in peril in the first year of the administration of Gov. Brad Little. The centers, located in nine of the state’s cities, collectively had requested about $890,000 from the Idaho Millennium Fund that would enable them to stabilize precarious financing and improve outreach efforts in surrounding rural communities.
However, as the Idaho Statesman reported, the funding request did not make it to the governor’s budget recommendation for 2020. Instead, the governor intends to use the Millennium Fund funds to expand Medicaid coverage. The consequence of the lack of state financial support could be the shuttering of all nine centers. If that happens, it would be much harder for those in the throes of or recovering from addiction or mental health issues to find the support they need.
“[The centers’] continued survival has been something of a miracle already,” said Norma Jaeger, executive director of Recovery Idaho. The organization had submitted the funding request on behalf of the nine centers. “Hopefully, a better funding solution is on the horizon,” said Ms. Jaeger.
Some in government disagree with that view, contending that the ongoing use of the Millennium Fund was never in the plans and that the centers were expected to seek other nongovernmental sources of funding. This tact is contrary to the traditional state funding of mental health and substance abuse programs. The legality of the move is being considered by the state Supreme Court.
Mental health and religious faith
A recent article in the Philadelphia Inquirer related the downsides and upsides of religious belief in the struggle against depression. The article discussed Yashi Brown, who at age 20, dealt with a bout of depression that led to manic episodes and contemplation of suicide. To her dismay, Ms. Brown found that her Jehovah’s Witness faith community was of little help.
Twenty years later, Ms. Brown, who now works as a mental health advocate in Los Angeles, better understands why relying on the faith’s teachings did not work. “I was in the throes of a manic episode,” she said. “I didn’t even have the tools to say a prayer.”
Ms. Brown’s experience highlights the burden faced by some religious traditions in helping church members with mental health issues. Instead of receiving tangible care from mental health professionals, those who are suffering can be told to rely on faith alone. The fallout is especially profound for African Americans like Ms. Brown, research shows. African Americans are 20% more likely to experience serious psychological distress than their white counterparts but are less likely to seek help – even if they can financially afford it.
The racial/ethnic disparities in health care that affect African Americans partly explains this reticence to seek treatment. Another factor is the multigenerational acceptance among some African Americans of mental trauma as a normal part of life, according to Meagan McLeod, a pastor and spiritual care director for Friends Hospital, a psychiatric hospital in northeast Philadelphia. “The idea is, if prayer worked for our ancestors when we were in slavery, then prayer has to be able to work now,” said Ms. McLeod.
Meanwhile, the article said, mental health professionals and clergy think that faith and mental health treatment “can – and should – work together. Research suggests higher levels of religiosity or spirituality are associated with lower rates of depression, anxiety, substance use disorder, and suicidal thoughts. Prayer can have the same calming effects as meditation,” such as both lowering blood pressure and respiratory rates. Incorporating religious approaches into the mental health treatment of African American patients might be particularly helpful, the article said.
The people of Fayette County, Ky., reportedly have experienced five suicides by children in the last year. The latest suicide occurred several weeks ago and involved a 12-year-old girl. This followed the suicides of children aged 10, 11, 13, and 14 years.
The deaths are not related, and there seems to be no connection to race or gender. All involved hanging, and one child might have been bullied.
The cases reflect a disturbing trend in Kentucky and across the country. In 2015, 25% of suicides of children under 17 years in Kentucky involved those aged 10-14, a 14% increase from 4 years earlier. The percentage of 6th-grade students who have thought about or planned their suicide also has climbed in recent years.
Fayette County Coroner Gary W. Ginn said the deaths in Kentucky provide another example of how events in life that might be less traumatic to adults can cause mental anguish for children, anguish which can lead some to take their own lives.
“We should be very worried to have this many cases, but we should not be hopeless,“ said Susan H. Pollack, MD, a pediatrician at the University of Kentucky Children’s Hospital, Lexington, in an interview with the Lexington Herald-Leader. She added that resources and programs are available but that stronger support systems focusing on youth are needed.
“Our families have limited options when their child needs a higher level of care. ... Local agencies often have a wait list,” Fayette County Public Schools spokeswoman Lisa Deffendall said in the article. “We have made referrals and seen it take weeks for children to get the help they need. Where do families turn when their child is in crisis? Who provides care when school is not in session?”
Rural Arizona facing crisis
Living in rural areas can prove isolating, and gaining access to health care, including mental health care, can be challenging. A segment presented on KOLD News 13 in Tucson provided yet another examples of the mental health crisis in rural America.
Cochise County is an area of about 6,200 square miles in the southeast corner of Arizona. The area, which is about eight times bigger than New York City, is home to about 125,000 people. For those with mental health issues, it’s a bleak place to live, with only two psychiatrists available and no mental health facility.
“It’s as if we got a fire going that we can’t put out,” said James P. Reed, DO, in an interview. He is one of the two psychiatrists practicing in the county. In the last 2 months alone, 64 new people have sought his help, and he has had to turn many away.
Dr. Reed has been practicing in the country for 35 years, which gives him a longer-term perspective. “It’s so much worse now. I don’t know what it is, if it’s a consequence of our society and the direction it’s going. I just can’t put my finger on it.”
The main reason behind the paucity of mental health professionals comes down to economics. Burdened with student loan debts after graduation from medical school, the low salaries of rural positions cripple the recruitment of psychiatrists and other medical professionals.
In Cochise County, as elsewhere, the main refuge for people with mental illness is jail. “We have people in there [who] really shouldn’t be in there,” said Cochise County Sheriff Mark J. Dannels. “These people need special help that I can’t provide to them. It’s almost a misjustice to have them in our jail. Unfortunately, there’s no other place to put them.”
Perils of involuntary mental health holds
South Dakota is one of five states where jailing people with mental illness is part of a deliberate strategy, and the state’s new governor wants to change the practice.
“They’re not criminals,” Gov. Kristi Noem said in an article published in the Sioux Falls Argus Leader. “They’re having a crisis at a point in time when they need to be observed, but unfortunately, in a lot of communities, that’s the only option that folks have.”
The article cited the case of Nick Johnson, a 14-year-old whose mental health struggles include the loss of control that can include aggressiveness. Although not charged with any offense, the response had been stints in the Minnehaha County Juvenile Detention Center. “It feels like I’m in prison,” Nick said. “Why would a kid have to go through that?”
National advocacy groups have criticized the practice of imprisoning people with mental illness, and local jail officials have complained that their facilities have not been designed to deliver mental health treatment. “If you look at it from a strictly medical perspective, being in a jail setting is almost guaranteed to make somebody’s mental health crisis worse, not better,” said Lisa Dailey, legislative and policy counsel for the Treatment Advocacy Center, a national nonprofit that surveys and ranks states for their mental health policies. “It’s the worst possible thing you could do.” The report said the other states that take this approach toward people with mental illness are Texas, Wyoming, New Mexico, and North Dakota.
Cutbacks may hit recovery centers
Idaho’s nine crisis centers are in peril in the first year of the administration of Gov. Brad Little. The centers, located in nine of the state’s cities, collectively had requested about $890,000 from the Idaho Millennium Fund that would enable them to stabilize precarious financing and improve outreach efforts in surrounding rural communities.
However, as the Idaho Statesman reported, the funding request did not make it to the governor’s budget recommendation for 2020. Instead, the governor intends to use the Millennium Fund funds to expand Medicaid coverage. The consequence of the lack of state financial support could be the shuttering of all nine centers. If that happens, it would be much harder for those in the throes of or recovering from addiction or mental health issues to find the support they need.
“[The centers’] continued survival has been something of a miracle already,” said Norma Jaeger, executive director of Recovery Idaho. The organization had submitted the funding request on behalf of the nine centers. “Hopefully, a better funding solution is on the horizon,” said Ms. Jaeger.
Some in government disagree with that view, contending that the ongoing use of the Millennium Fund was never in the plans and that the centers were expected to seek other nongovernmental sources of funding. This tact is contrary to the traditional state funding of mental health and substance abuse programs. The legality of the move is being considered by the state Supreme Court.
Mental health and religious faith
A recent article in the Philadelphia Inquirer related the downsides and upsides of religious belief in the struggle against depression. The article discussed Yashi Brown, who at age 20, dealt with a bout of depression that led to manic episodes and contemplation of suicide. To her dismay, Ms. Brown found that her Jehovah’s Witness faith community was of little help.
Twenty years later, Ms. Brown, who now works as a mental health advocate in Los Angeles, better understands why relying on the faith’s teachings did not work. “I was in the throes of a manic episode,” she said. “I didn’t even have the tools to say a prayer.”
Ms. Brown’s experience highlights the burden faced by some religious traditions in helping church members with mental health issues. Instead of receiving tangible care from mental health professionals, those who are suffering can be told to rely on faith alone. The fallout is especially profound for African Americans like Ms. Brown, research shows. African Americans are 20% more likely to experience serious psychological distress than their white counterparts but are less likely to seek help – even if they can financially afford it.
The racial/ethnic disparities in health care that affect African Americans partly explains this reticence to seek treatment. Another factor is the multigenerational acceptance among some African Americans of mental trauma as a normal part of life, according to Meagan McLeod, a pastor and spiritual care director for Friends Hospital, a psychiatric hospital in northeast Philadelphia. “The idea is, if prayer worked for our ancestors when we were in slavery, then prayer has to be able to work now,” said Ms. McLeod.
Meanwhile, the article said, mental health professionals and clergy think that faith and mental health treatment “can – and should – work together. Research suggests higher levels of religiosity or spirituality are associated with lower rates of depression, anxiety, substance use disorder, and suicidal thoughts. Prayer can have the same calming effects as meditation,” such as both lowering blood pressure and respiratory rates. Incorporating religious approaches into the mental health treatment of African American patients might be particularly helpful, the article said.
The people of Fayette County, Ky., reportedly have experienced five suicides by children in the last year. The latest suicide occurred several weeks ago and involved a 12-year-old girl. This followed the suicides of children aged 10, 11, 13, and 14 years.
The deaths are not related, and there seems to be no connection to race or gender. All involved hanging, and one child might have been bullied.
The cases reflect a disturbing trend in Kentucky and across the country. In 2015, 25% of suicides of children under 17 years in Kentucky involved those aged 10-14, a 14% increase from 4 years earlier. The percentage of 6th-grade students who have thought about or planned their suicide also has climbed in recent years.
Fayette County Coroner Gary W. Ginn said the deaths in Kentucky provide another example of how events in life that might be less traumatic to adults can cause mental anguish for children, anguish which can lead some to take their own lives.
“We should be very worried to have this many cases, but we should not be hopeless,“ said Susan H. Pollack, MD, a pediatrician at the University of Kentucky Children’s Hospital, Lexington, in an interview with the Lexington Herald-Leader. She added that resources and programs are available but that stronger support systems focusing on youth are needed.
“Our families have limited options when their child needs a higher level of care. ... Local agencies often have a wait list,” Fayette County Public Schools spokeswoman Lisa Deffendall said in the article. “We have made referrals and seen it take weeks for children to get the help they need. Where do families turn when their child is in crisis? Who provides care when school is not in session?”
Rural Arizona facing crisis
Living in rural areas can prove isolating, and gaining access to health care, including mental health care, can be challenging. A segment presented on KOLD News 13 in Tucson provided yet another examples of the mental health crisis in rural America.
Cochise County is an area of about 6,200 square miles in the southeast corner of Arizona. The area, which is about eight times bigger than New York City, is home to about 125,000 people. For those with mental health issues, it’s a bleak place to live, with only two psychiatrists available and no mental health facility.
“It’s as if we got a fire going that we can’t put out,” said James P. Reed, DO, in an interview. He is one of the two psychiatrists practicing in the county. In the last 2 months alone, 64 new people have sought his help, and he has had to turn many away.
Dr. Reed has been practicing in the country for 35 years, which gives him a longer-term perspective. “It’s so much worse now. I don’t know what it is, if it’s a consequence of our society and the direction it’s going. I just can’t put my finger on it.”
The main reason behind the paucity of mental health professionals comes down to economics. Burdened with student loan debts after graduation from medical school, the low salaries of rural positions cripple the recruitment of psychiatrists and other medical professionals.
In Cochise County, as elsewhere, the main refuge for people with mental illness is jail. “We have people in there [who] really shouldn’t be in there,” said Cochise County Sheriff Mark J. Dannels. “These people need special help that I can’t provide to them. It’s almost a misjustice to have them in our jail. Unfortunately, there’s no other place to put them.”
Perils of involuntary mental health holds
South Dakota is one of five states where jailing people with mental illness is part of a deliberate strategy, and the state’s new governor wants to change the practice.
“They’re not criminals,” Gov. Kristi Noem said in an article published in the Sioux Falls Argus Leader. “They’re having a crisis at a point in time when they need to be observed, but unfortunately, in a lot of communities, that’s the only option that folks have.”
The article cited the case of Nick Johnson, a 14-year-old whose mental health struggles include the loss of control that can include aggressiveness. Although not charged with any offense, the response had been stints in the Minnehaha County Juvenile Detention Center. “It feels like I’m in prison,” Nick said. “Why would a kid have to go through that?”
National advocacy groups have criticized the practice of imprisoning people with mental illness, and local jail officials have complained that their facilities have not been designed to deliver mental health treatment. “If you look at it from a strictly medical perspective, being in a jail setting is almost guaranteed to make somebody’s mental health crisis worse, not better,” said Lisa Dailey, legislative and policy counsel for the Treatment Advocacy Center, a national nonprofit that surveys and ranks states for their mental health policies. “It’s the worst possible thing you could do.” The report said the other states that take this approach toward people with mental illness are Texas, Wyoming, New Mexico, and North Dakota.
Cutbacks may hit recovery centers
Idaho’s nine crisis centers are in peril in the first year of the administration of Gov. Brad Little. The centers, located in nine of the state’s cities, collectively had requested about $890,000 from the Idaho Millennium Fund that would enable them to stabilize precarious financing and improve outreach efforts in surrounding rural communities.
However, as the Idaho Statesman reported, the funding request did not make it to the governor’s budget recommendation for 2020. Instead, the governor intends to use the Millennium Fund funds to expand Medicaid coverage. The consequence of the lack of state financial support could be the shuttering of all nine centers. If that happens, it would be much harder for those in the throes of or recovering from addiction or mental health issues to find the support they need.
“[The centers’] continued survival has been something of a miracle already,” said Norma Jaeger, executive director of Recovery Idaho. The organization had submitted the funding request on behalf of the nine centers. “Hopefully, a better funding solution is on the horizon,” said Ms. Jaeger.
Some in government disagree with that view, contending that the ongoing use of the Millennium Fund was never in the plans and that the centers were expected to seek other nongovernmental sources of funding. This tact is contrary to the traditional state funding of mental health and substance abuse programs. The legality of the move is being considered by the state Supreme Court.
Mental health and religious faith
A recent article in the Philadelphia Inquirer related the downsides and upsides of religious belief in the struggle against depression. The article discussed Yashi Brown, who at age 20, dealt with a bout of depression that led to manic episodes and contemplation of suicide. To her dismay, Ms. Brown found that her Jehovah’s Witness faith community was of little help.
Twenty years later, Ms. Brown, who now works as a mental health advocate in Los Angeles, better understands why relying on the faith’s teachings did not work. “I was in the throes of a manic episode,” she said. “I didn’t even have the tools to say a prayer.”
Ms. Brown’s experience highlights the burden faced by some religious traditions in helping church members with mental health issues. Instead of receiving tangible care from mental health professionals, those who are suffering can be told to rely on faith alone. The fallout is especially profound for African Americans like Ms. Brown, research shows. African Americans are 20% more likely to experience serious psychological distress than their white counterparts but are less likely to seek help – even if they can financially afford it.
The racial/ethnic disparities in health care that affect African Americans partly explains this reticence to seek treatment. Another factor is the multigenerational acceptance among some African Americans of mental trauma as a normal part of life, according to Meagan McLeod, a pastor and spiritual care director for Friends Hospital, a psychiatric hospital in northeast Philadelphia. “The idea is, if prayer worked for our ancestors when we were in slavery, then prayer has to be able to work now,” said Ms. McLeod.
Meanwhile, the article said, mental health professionals and clergy think that faith and mental health treatment “can – and should – work together. Research suggests higher levels of religiosity or spirituality are associated with lower rates of depression, anxiety, substance use disorder, and suicidal thoughts. Prayer can have the same calming effects as meditation,” such as both lowering blood pressure and respiratory rates. Incorporating religious approaches into the mental health treatment of African American patients might be particularly helpful, the article said.