User login
Mood disorders worsen multiple sclerosis disability
BERLIN – Depression and bipolar disorder are major risk factors for worsening disability in people with multiple sclerosis, according to the results of a large Swedish registry-based study.
The presence of depression increased the risk of having a sustained Expanded Disability Status Scale (EDSS) score of 3.0 by 54% and 4.0 by 87%, and it doubled the risk of an EDSS of 6.0.
Selective serotonin reuptake inhibitor treatment also upped the risk of greater disability, with patients exposed to SSRIs having a 40% increased risk of a sustained EDSS of 3.0, a 97% chance of having a sustained EDSS of 4.0, and 2.2-fold increased risk of a sustained EDSS of 6.0.
“We know that mood disorders are highly prevalent in people with multiple sclerosis,” Stefanie Binzer, MD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis. She gave her presentation at the meeting on Oct. 10, which was World Mental Health Day.
The presence of mood disorders is associated with reduced quality of life, said Dr. Binzer of the department of clinical neuroscience at the Karolinska Institute in Stockholm. Furthermore, depression is the major risk factor for suicidality in patients with MS. However, before this study the effect of having a comorbid mood disorder on MS patients’ disability levels had not been established.
The investigators analyzed data from 5,875 patients in the Swedish MS registry between 2001 and 2014. By matching these patients to records in the Swedish National Patient Registry and the Swedish National Prescribed Drug Registry, they found that 8.5% (n = 502) had an International Classification of Diseases, 10th revision (ICD-10), code for depression. Of these, 261 had received a diagnosis of depression before their diagnosis of MS.
Of 3,817 patients with MS onset between 2005 and 2014, 27.4% (n = 1,048) had collected at least one prescription for an SSRI.
“What we found was that MS patients with either an ICD code for depression or having been exposed to SSRIs had a significantly increased risk of reaching EDSS 3.0,” Dr. Binzer reported. The age at which patients reached these milestones were younger in both groups when compared with MS patients without depression, she observed.
“The difference between the groups [MS with and MS without depression] seemed to increased with EDSS,” Dr. Binzer said.
Although not statistically significant, there was a trend for patients with depression to be more likely to convert to secondary progressive MS, with a hazard ratio of 1.38 (95% confidence interval, 0.91-2.1).
“For a sensitivity analysis, we found that those who had depression prior to their first MS symptom, the median age when they reached EDSS 3.0 and 4.0 was reduced by 3 and 7 years, respectively,” Dr. Binzer said, adding that, unfortunately, there wasn’t enough power to look at the other endpoints.
In regard to bipolar disorder, 1.5% (n = 200) of 13,125 MS patients diagnosed between 1973 and 2014 were identified with this mood disorder. Its presence significantly increased the risk of MS patients reaching an EDSS score of 4.0 by 58% (95% CI, 1.1-2.28), but not EDSS 3.0 (HR = 1.34; 95% CI, 0.94-1.92) or 6.0 (HR = 1.16; 95% CI, 0.79-1.69). The latter could be due to smaller sample size, Dr. Binzer suggested.
The investigators’ analysis of the results stratified by sex, conducted because men tend to fare worse than women with MS and progress faster, showed that for both depression and bipolar disorder, men were at significantly higher risk of reaching sustained disability milestones. Indeed, compared with women, men with depression had a 61% increased risk and those with bipolar disorder a 31% increased risk of reaching an EDSS score of 6.0. They also had 51% and 32% increased risks of conversion to secondary progressive MS.
“We don’t know the mechanisms that underlie these associations,” Dr. Binzer noted. “Irrespective of the underlying mechanisms, [the study] clearly shows that it’s imperative that we recognize, early, mood disorders in MS patients, and manage them effectively in order to provide better care and hopefully reduce MS disability worsening.”
The research was funded by the Swedish Research Council and the Swedish Brain Foundation. Dr. Binzer has received speaker fees and travel grants from Biogen.
SOURCE: Binzer S et al. Mult Scler. 2018;24(Suppl 2):41. Abstract 99.
BERLIN – Depression and bipolar disorder are major risk factors for worsening disability in people with multiple sclerosis, according to the results of a large Swedish registry-based study.
The presence of depression increased the risk of having a sustained Expanded Disability Status Scale (EDSS) score of 3.0 by 54% and 4.0 by 87%, and it doubled the risk of an EDSS of 6.0.
Selective serotonin reuptake inhibitor treatment also upped the risk of greater disability, with patients exposed to SSRIs having a 40% increased risk of a sustained EDSS of 3.0, a 97% chance of having a sustained EDSS of 4.0, and 2.2-fold increased risk of a sustained EDSS of 6.0.
“We know that mood disorders are highly prevalent in people with multiple sclerosis,” Stefanie Binzer, MD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis. She gave her presentation at the meeting on Oct. 10, which was World Mental Health Day.
The presence of mood disorders is associated with reduced quality of life, said Dr. Binzer of the department of clinical neuroscience at the Karolinska Institute in Stockholm. Furthermore, depression is the major risk factor for suicidality in patients with MS. However, before this study the effect of having a comorbid mood disorder on MS patients’ disability levels had not been established.
The investigators analyzed data from 5,875 patients in the Swedish MS registry between 2001 and 2014. By matching these patients to records in the Swedish National Patient Registry and the Swedish National Prescribed Drug Registry, they found that 8.5% (n = 502) had an International Classification of Diseases, 10th revision (ICD-10), code for depression. Of these, 261 had received a diagnosis of depression before their diagnosis of MS.
Of 3,817 patients with MS onset between 2005 and 2014, 27.4% (n = 1,048) had collected at least one prescription for an SSRI.
“What we found was that MS patients with either an ICD code for depression or having been exposed to SSRIs had a significantly increased risk of reaching EDSS 3.0,” Dr. Binzer reported. The age at which patients reached these milestones were younger in both groups when compared with MS patients without depression, she observed.
“The difference between the groups [MS with and MS without depression] seemed to increased with EDSS,” Dr. Binzer said.
Although not statistically significant, there was a trend for patients with depression to be more likely to convert to secondary progressive MS, with a hazard ratio of 1.38 (95% confidence interval, 0.91-2.1).
“For a sensitivity analysis, we found that those who had depression prior to their first MS symptom, the median age when they reached EDSS 3.0 and 4.0 was reduced by 3 and 7 years, respectively,” Dr. Binzer said, adding that, unfortunately, there wasn’t enough power to look at the other endpoints.
In regard to bipolar disorder, 1.5% (n = 200) of 13,125 MS patients diagnosed between 1973 and 2014 were identified with this mood disorder. Its presence significantly increased the risk of MS patients reaching an EDSS score of 4.0 by 58% (95% CI, 1.1-2.28), but not EDSS 3.0 (HR = 1.34; 95% CI, 0.94-1.92) or 6.0 (HR = 1.16; 95% CI, 0.79-1.69). The latter could be due to smaller sample size, Dr. Binzer suggested.
The investigators’ analysis of the results stratified by sex, conducted because men tend to fare worse than women with MS and progress faster, showed that for both depression and bipolar disorder, men were at significantly higher risk of reaching sustained disability milestones. Indeed, compared with women, men with depression had a 61% increased risk and those with bipolar disorder a 31% increased risk of reaching an EDSS score of 6.0. They also had 51% and 32% increased risks of conversion to secondary progressive MS.
“We don’t know the mechanisms that underlie these associations,” Dr. Binzer noted. “Irrespective of the underlying mechanisms, [the study] clearly shows that it’s imperative that we recognize, early, mood disorders in MS patients, and manage them effectively in order to provide better care and hopefully reduce MS disability worsening.”
The research was funded by the Swedish Research Council and the Swedish Brain Foundation. Dr. Binzer has received speaker fees and travel grants from Biogen.
SOURCE: Binzer S et al. Mult Scler. 2018;24(Suppl 2):41. Abstract 99.
BERLIN – Depression and bipolar disorder are major risk factors for worsening disability in people with multiple sclerosis, according to the results of a large Swedish registry-based study.
The presence of depression increased the risk of having a sustained Expanded Disability Status Scale (EDSS) score of 3.0 by 54% and 4.0 by 87%, and it doubled the risk of an EDSS of 6.0.
Selective serotonin reuptake inhibitor treatment also upped the risk of greater disability, with patients exposed to SSRIs having a 40% increased risk of a sustained EDSS of 3.0, a 97% chance of having a sustained EDSS of 4.0, and 2.2-fold increased risk of a sustained EDSS of 6.0.
“We know that mood disorders are highly prevalent in people with multiple sclerosis,” Stefanie Binzer, MD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis. She gave her presentation at the meeting on Oct. 10, which was World Mental Health Day.
The presence of mood disorders is associated with reduced quality of life, said Dr. Binzer of the department of clinical neuroscience at the Karolinska Institute in Stockholm. Furthermore, depression is the major risk factor for suicidality in patients with MS. However, before this study the effect of having a comorbid mood disorder on MS patients’ disability levels had not been established.
The investigators analyzed data from 5,875 patients in the Swedish MS registry between 2001 and 2014. By matching these patients to records in the Swedish National Patient Registry and the Swedish National Prescribed Drug Registry, they found that 8.5% (n = 502) had an International Classification of Diseases, 10th revision (ICD-10), code for depression. Of these, 261 had received a diagnosis of depression before their diagnosis of MS.
Of 3,817 patients with MS onset between 2005 and 2014, 27.4% (n = 1,048) had collected at least one prescription for an SSRI.
“What we found was that MS patients with either an ICD code for depression or having been exposed to SSRIs had a significantly increased risk of reaching EDSS 3.0,” Dr. Binzer reported. The age at which patients reached these milestones were younger in both groups when compared with MS patients without depression, she observed.
“The difference between the groups [MS with and MS without depression] seemed to increased with EDSS,” Dr. Binzer said.
Although not statistically significant, there was a trend for patients with depression to be more likely to convert to secondary progressive MS, with a hazard ratio of 1.38 (95% confidence interval, 0.91-2.1).
“For a sensitivity analysis, we found that those who had depression prior to their first MS symptom, the median age when they reached EDSS 3.0 and 4.0 was reduced by 3 and 7 years, respectively,” Dr. Binzer said, adding that, unfortunately, there wasn’t enough power to look at the other endpoints.
In regard to bipolar disorder, 1.5% (n = 200) of 13,125 MS patients diagnosed between 1973 and 2014 were identified with this mood disorder. Its presence significantly increased the risk of MS patients reaching an EDSS score of 4.0 by 58% (95% CI, 1.1-2.28), but not EDSS 3.0 (HR = 1.34; 95% CI, 0.94-1.92) or 6.0 (HR = 1.16; 95% CI, 0.79-1.69). The latter could be due to smaller sample size, Dr. Binzer suggested.
The investigators’ analysis of the results stratified by sex, conducted because men tend to fare worse than women with MS and progress faster, showed that for both depression and bipolar disorder, men were at significantly higher risk of reaching sustained disability milestones. Indeed, compared with women, men with depression had a 61% increased risk and those with bipolar disorder a 31% increased risk of reaching an EDSS score of 6.0. They also had 51% and 32% increased risks of conversion to secondary progressive MS.
“We don’t know the mechanisms that underlie these associations,” Dr. Binzer noted. “Irrespective of the underlying mechanisms, [the study] clearly shows that it’s imperative that we recognize, early, mood disorders in MS patients, and manage them effectively in order to provide better care and hopefully reduce MS disability worsening.”
The research was funded by the Swedish Research Council and the Swedish Brain Foundation. Dr. Binzer has received speaker fees and travel grants from Biogen.
SOURCE: Binzer S et al. Mult Scler. 2018;24(Suppl 2):41. Abstract 99.
REPORTING FROM ECTRIMS 2018
Key clinical point:
Major finding: Depression and bipolar disorder increased the risk of reaching Expanded Disability Status Scale scores of 3.0, 4.0, and 6.0, particularly in men with MS.
Study details: Swedish registry study of nearly 6,000 individuals with confirmed MS, 8.5% of whom had depression and 1.5% of whom had bipolar disorder.
Disclosures: The research was funded by the Swedish Research Council and the Swedish Brain Foundation. Dr. Binzer has received speaker fees and travel grants from Biogen.
Source: Binzer S et al. Mult Scler. 2018;24(Suppl 2):41. Abstract 99.
Anxiety and depression widespread among arthritis patients
Adults with arthritis are almost twice as likely to have symptoms of anxiety than depression, but the depressed patients are more likely to receive treatment, according to the Centers for Disease Control and Prevention.
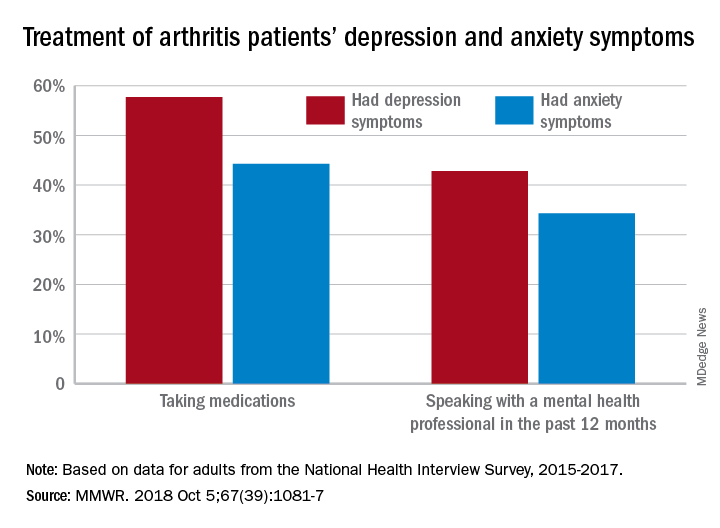
During 2015-2017, the prevalence of anxiety symptoms was 22.5% in adults with arthritis, compared with 12.1% for depression symptoms. Treatment of those symptoms, however, was another story: 57.7% of arthritis patients with depression symptoms were taking medications, versus 44.3% of those with anxiety symptoms, and 42.8% of those with symptoms of depression reported seeing a mental health professional the past 12 months, compared with 34.3% of adults with anxiety, Dana Guglielmo, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and her associates reported in the Morbidity and Mortality Weekly Report.
Prevalences of anxiety and depression symptoms varied considerably by sociodemographic characteristic during 2015-2017. Anxiety and depression were both more common in those aged 18-44 years (28.3% and 13.7%, respectively) than in those aged over 65 (9.7% and 6.2%), and women with arthritis were more likely than were men to experience symptoms of anxiety (26.9% vs. 16%) and depression (14% vs. 9.2%), the investigators said, based on data from the National Health Interview Survey.
Among racial/ethnic groups, the prevalence of anxiety was highest for whites (23.9%) and lowest for Asians (10.6%), who also had lowest depression symptom prevalence at 3.3%, with American Indians/Alaska Natives highest at 15.4%. Adults categorized as other/multiple race, however, were highest in both cases at 32.3% for anxiety and 17.4% for depression, Ms. Guglielmo and her associates said.
The overall prevalence of anxiety and depression symptoms in patients with arthritis was much higher than in those without arthritis – 10.7% for anxiety and 4.7% for depression – which “suggests that all adults with arthritis would benefit from mental health screening,” they noted.
, and encouraging physical activity, which is an effective nonpharmacologic strategy that can help reduce the symptoms of anxiety and depression, improve arthritis symptoms, and promote better quality of life,” the investigators wrote.
SOURCE: Guglielmo D et al. MMWR. 2018 Oct 5;67(39):1081-7.
Adults with arthritis are almost twice as likely to have symptoms of anxiety than depression, but the depressed patients are more likely to receive treatment, according to the Centers for Disease Control and Prevention.

During 2015-2017, the prevalence of anxiety symptoms was 22.5% in adults with arthritis, compared with 12.1% for depression symptoms. Treatment of those symptoms, however, was another story: 57.7% of arthritis patients with depression symptoms were taking medications, versus 44.3% of those with anxiety symptoms, and 42.8% of those with symptoms of depression reported seeing a mental health professional the past 12 months, compared with 34.3% of adults with anxiety, Dana Guglielmo, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and her associates reported in the Morbidity and Mortality Weekly Report.
Prevalences of anxiety and depression symptoms varied considerably by sociodemographic characteristic during 2015-2017. Anxiety and depression were both more common in those aged 18-44 years (28.3% and 13.7%, respectively) than in those aged over 65 (9.7% and 6.2%), and women with arthritis were more likely than were men to experience symptoms of anxiety (26.9% vs. 16%) and depression (14% vs. 9.2%), the investigators said, based on data from the National Health Interview Survey.
Among racial/ethnic groups, the prevalence of anxiety was highest for whites (23.9%) and lowest for Asians (10.6%), who also had lowest depression symptom prevalence at 3.3%, with American Indians/Alaska Natives highest at 15.4%. Adults categorized as other/multiple race, however, were highest in both cases at 32.3% for anxiety and 17.4% for depression, Ms. Guglielmo and her associates said.
The overall prevalence of anxiety and depression symptoms in patients with arthritis was much higher than in those without arthritis – 10.7% for anxiety and 4.7% for depression – which “suggests that all adults with arthritis would benefit from mental health screening,” they noted.
, and encouraging physical activity, which is an effective nonpharmacologic strategy that can help reduce the symptoms of anxiety and depression, improve arthritis symptoms, and promote better quality of life,” the investigators wrote.
SOURCE: Guglielmo D et al. MMWR. 2018 Oct 5;67(39):1081-7.
Adults with arthritis are almost twice as likely to have symptoms of anxiety than depression, but the depressed patients are more likely to receive treatment, according to the Centers for Disease Control and Prevention.

During 2015-2017, the prevalence of anxiety symptoms was 22.5% in adults with arthritis, compared with 12.1% for depression symptoms. Treatment of those symptoms, however, was another story: 57.7% of arthritis patients with depression symptoms were taking medications, versus 44.3% of those with anxiety symptoms, and 42.8% of those with symptoms of depression reported seeing a mental health professional the past 12 months, compared with 34.3% of adults with anxiety, Dana Guglielmo, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and her associates reported in the Morbidity and Mortality Weekly Report.
Prevalences of anxiety and depression symptoms varied considerably by sociodemographic characteristic during 2015-2017. Anxiety and depression were both more common in those aged 18-44 years (28.3% and 13.7%, respectively) than in those aged over 65 (9.7% and 6.2%), and women with arthritis were more likely than were men to experience symptoms of anxiety (26.9% vs. 16%) and depression (14% vs. 9.2%), the investigators said, based on data from the National Health Interview Survey.
Among racial/ethnic groups, the prevalence of anxiety was highest for whites (23.9%) and lowest for Asians (10.6%), who also had lowest depression symptom prevalence at 3.3%, with American Indians/Alaska Natives highest at 15.4%. Adults categorized as other/multiple race, however, were highest in both cases at 32.3% for anxiety and 17.4% for depression, Ms. Guglielmo and her associates said.
The overall prevalence of anxiety and depression symptoms in patients with arthritis was much higher than in those without arthritis – 10.7% for anxiety and 4.7% for depression – which “suggests that all adults with arthritis would benefit from mental health screening,” they noted.
, and encouraging physical activity, which is an effective nonpharmacologic strategy that can help reduce the symptoms of anxiety and depression, improve arthritis symptoms, and promote better quality of life,” the investigators wrote.
SOURCE: Guglielmo D et al. MMWR. 2018 Oct 5;67(39):1081-7.
FROM MMWR
Promising novel antidepressant cruising in pipeline
BARCELONA – An investigational antidepressant known for now simply as MIN-117 shows the potential – at least, in phase 2 development – of offering significant advantages over currently available antidepressants in patients with major depressive disorder, Michael Davidson, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“This is a compound with a very, very rich pharmacology – so rich that we don’t know exactly which of the pharmacologic effects are really making the difference. This rich pharmacology is related to pathways that may confer to MIN-117 a unique positioning in the field of antidepressants and address unmet medical needs not well covered by existing therapies. For example, faster onset of action, complete restoration of euthymia, and beneficial effects on cognition and sexual functioning,” according to Michael Davidson, MD, chief medical officer at Minerva Neurosciences, which is developing the drug..
In the completed phase 2 study, the drug also displayed a strong anxiolytic effect and no prolongation of REM sleep latency in polysomnographic testing.
“So it may be that this is the first antidepressant which does not affect sleep architecture,” observed Dr. Davidson, professor of psychiatry at the Sackler School of Medicine in Tel Aviv.
Preclinical studies established that MIN-117 has high affinity for the 5HT serotonin transporter, serotonin 1A and 2A receptors, the dopamine transporter, and the alpha-1A and -1B adrenergic receptors. In animal models of depression, the drug results in sustained release of dopamine and serotonin. In human studies, the drug has a long half-life of roughly 60 hours.
“One possibility is that MIN-117 will be administered not once a day, but once or twice a week. It may be that here we have an antidepressant that doesn’t have to be administered every day,” the psychiatrist said.
In the completed phase 2 study, however, the drug was given once daily in what he described as a “classic design” for an antidepressant clinical trial: a 4-week washout period, then 6 weeks of double-blind treatment with MIN-117 at 2.5 or 0.5 mg/day, paroxetine at 20 mg/day, or placebo, then a 2-week posttreatment follow-up phase.
In describing the results of the study of 84 patients with major depressive disorder, Dr. Davidson painted a picture of MIN-117’s safety and efficacy with broad strokes because the trial wasn’t powered to demonstrate statistically significant differences. But the treatment effect sizes for the novel drug were impressive. A much more complete picture of the safety and efficacy of MIN-117 will be provided by an ongoing 324-patient phase 2b multicenter U.S. and European trial of the drug given at 2.5 or 5 mg/day or placebo.
The primary endpoint in the completed trial was change from baseline to 6 weeks in mean Montgomery-Åsberg Rating Depression Scale (MADRS) score. From a baseline score of about 33, MADRS scores improved by 9 points with placebo, 11 with MIN-117 at 0.5 mg, and by 12 points with 2.5 mg of the drug. MIN-117 was superior to placebo in this regard from the time of the earliest assessment, at 2 weeks.
One-quarter of patients on MIN-117 at 2.5 mg/day achieved remission, prospectively defined as a MADRS score below 12. Remission was 2.1-fold more likely in this group than with placebo at week 4 and 3.1-fold more likely at 6 weeks. The remission rate with MIN-117 also was better than with paroxetine.
“So , and that we’ll be able to produce full remission of the depressive symptoms,” Dr. Davidson said.
MIN-117 also showed a solid anxiolytic effect, with mean scores on the Hamilton Anxiety Rating Scale – a secondary endpoint – improving by 10 points in both the 0.5- and 2.5-mg groups from a baseline of 26 points. As a result of this impressive showing, the large ongoing phase 2b study is recruiting patients with an ongoing episode of major depressive disorder and prominent secondary anxiety.
Both doses of MIN-117 were well tolerated. Scores on the Arizona Sexual Experiences Scale showed that the drug did not result in any impairment of sexual function. Nor was MIN-117 associated with evidence of cognitive impairment; in fact, scores on the Digit-Symbol Substitution Test and Digit Span Backwards tool were better than in the placebo arm.
The study was sponsored by Minerva Neurosciences and presented by the company’s chief medical officer.
BARCELONA – An investigational antidepressant known for now simply as MIN-117 shows the potential – at least, in phase 2 development – of offering significant advantages over currently available antidepressants in patients with major depressive disorder, Michael Davidson, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“This is a compound with a very, very rich pharmacology – so rich that we don’t know exactly which of the pharmacologic effects are really making the difference. This rich pharmacology is related to pathways that may confer to MIN-117 a unique positioning in the field of antidepressants and address unmet medical needs not well covered by existing therapies. For example, faster onset of action, complete restoration of euthymia, and beneficial effects on cognition and sexual functioning,” according to Michael Davidson, MD, chief medical officer at Minerva Neurosciences, which is developing the drug..
In the completed phase 2 study, the drug also displayed a strong anxiolytic effect and no prolongation of REM sleep latency in polysomnographic testing.
“So it may be that this is the first antidepressant which does not affect sleep architecture,” observed Dr. Davidson, professor of psychiatry at the Sackler School of Medicine in Tel Aviv.
Preclinical studies established that MIN-117 has high affinity for the 5HT serotonin transporter, serotonin 1A and 2A receptors, the dopamine transporter, and the alpha-1A and -1B adrenergic receptors. In animal models of depression, the drug results in sustained release of dopamine and serotonin. In human studies, the drug has a long half-life of roughly 60 hours.
“One possibility is that MIN-117 will be administered not once a day, but once or twice a week. It may be that here we have an antidepressant that doesn’t have to be administered every day,” the psychiatrist said.
In the completed phase 2 study, however, the drug was given once daily in what he described as a “classic design” for an antidepressant clinical trial: a 4-week washout period, then 6 weeks of double-blind treatment with MIN-117 at 2.5 or 0.5 mg/day, paroxetine at 20 mg/day, or placebo, then a 2-week posttreatment follow-up phase.
In describing the results of the study of 84 patients with major depressive disorder, Dr. Davidson painted a picture of MIN-117’s safety and efficacy with broad strokes because the trial wasn’t powered to demonstrate statistically significant differences. But the treatment effect sizes for the novel drug were impressive. A much more complete picture of the safety and efficacy of MIN-117 will be provided by an ongoing 324-patient phase 2b multicenter U.S. and European trial of the drug given at 2.5 or 5 mg/day or placebo.
The primary endpoint in the completed trial was change from baseline to 6 weeks in mean Montgomery-Åsberg Rating Depression Scale (MADRS) score. From a baseline score of about 33, MADRS scores improved by 9 points with placebo, 11 with MIN-117 at 0.5 mg, and by 12 points with 2.5 mg of the drug. MIN-117 was superior to placebo in this regard from the time of the earliest assessment, at 2 weeks.
One-quarter of patients on MIN-117 at 2.5 mg/day achieved remission, prospectively defined as a MADRS score below 12. Remission was 2.1-fold more likely in this group than with placebo at week 4 and 3.1-fold more likely at 6 weeks. The remission rate with MIN-117 also was better than with paroxetine.
“So , and that we’ll be able to produce full remission of the depressive symptoms,” Dr. Davidson said.
MIN-117 also showed a solid anxiolytic effect, with mean scores on the Hamilton Anxiety Rating Scale – a secondary endpoint – improving by 10 points in both the 0.5- and 2.5-mg groups from a baseline of 26 points. As a result of this impressive showing, the large ongoing phase 2b study is recruiting patients with an ongoing episode of major depressive disorder and prominent secondary anxiety.
Both doses of MIN-117 were well tolerated. Scores on the Arizona Sexual Experiences Scale showed that the drug did not result in any impairment of sexual function. Nor was MIN-117 associated with evidence of cognitive impairment; in fact, scores on the Digit-Symbol Substitution Test and Digit Span Backwards tool were better than in the placebo arm.
The study was sponsored by Minerva Neurosciences and presented by the company’s chief medical officer.
BARCELONA – An investigational antidepressant known for now simply as MIN-117 shows the potential – at least, in phase 2 development – of offering significant advantages over currently available antidepressants in patients with major depressive disorder, Michael Davidson, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“This is a compound with a very, very rich pharmacology – so rich that we don’t know exactly which of the pharmacologic effects are really making the difference. This rich pharmacology is related to pathways that may confer to MIN-117 a unique positioning in the field of antidepressants and address unmet medical needs not well covered by existing therapies. For example, faster onset of action, complete restoration of euthymia, and beneficial effects on cognition and sexual functioning,” according to Michael Davidson, MD, chief medical officer at Minerva Neurosciences, which is developing the drug..
In the completed phase 2 study, the drug also displayed a strong anxiolytic effect and no prolongation of REM sleep latency in polysomnographic testing.
“So it may be that this is the first antidepressant which does not affect sleep architecture,” observed Dr. Davidson, professor of psychiatry at the Sackler School of Medicine in Tel Aviv.
Preclinical studies established that MIN-117 has high affinity for the 5HT serotonin transporter, serotonin 1A and 2A receptors, the dopamine transporter, and the alpha-1A and -1B adrenergic receptors. In animal models of depression, the drug results in sustained release of dopamine and serotonin. In human studies, the drug has a long half-life of roughly 60 hours.
“One possibility is that MIN-117 will be administered not once a day, but once or twice a week. It may be that here we have an antidepressant that doesn’t have to be administered every day,” the psychiatrist said.
In the completed phase 2 study, however, the drug was given once daily in what he described as a “classic design” for an antidepressant clinical trial: a 4-week washout period, then 6 weeks of double-blind treatment with MIN-117 at 2.5 or 0.5 mg/day, paroxetine at 20 mg/day, or placebo, then a 2-week posttreatment follow-up phase.
In describing the results of the study of 84 patients with major depressive disorder, Dr. Davidson painted a picture of MIN-117’s safety and efficacy with broad strokes because the trial wasn’t powered to demonstrate statistically significant differences. But the treatment effect sizes for the novel drug were impressive. A much more complete picture of the safety and efficacy of MIN-117 will be provided by an ongoing 324-patient phase 2b multicenter U.S. and European trial of the drug given at 2.5 or 5 mg/day or placebo.
The primary endpoint in the completed trial was change from baseline to 6 weeks in mean Montgomery-Åsberg Rating Depression Scale (MADRS) score. From a baseline score of about 33, MADRS scores improved by 9 points with placebo, 11 with MIN-117 at 0.5 mg, and by 12 points with 2.5 mg of the drug. MIN-117 was superior to placebo in this regard from the time of the earliest assessment, at 2 weeks.
One-quarter of patients on MIN-117 at 2.5 mg/day achieved remission, prospectively defined as a MADRS score below 12. Remission was 2.1-fold more likely in this group than with placebo at week 4 and 3.1-fold more likely at 6 weeks. The remission rate with MIN-117 also was better than with paroxetine.
“So , and that we’ll be able to produce full remission of the depressive symptoms,” Dr. Davidson said.
MIN-117 also showed a solid anxiolytic effect, with mean scores on the Hamilton Anxiety Rating Scale – a secondary endpoint – improving by 10 points in both the 0.5- and 2.5-mg groups from a baseline of 26 points. As a result of this impressive showing, the large ongoing phase 2b study is recruiting patients with an ongoing episode of major depressive disorder and prominent secondary anxiety.
Both doses of MIN-117 were well tolerated. Scores on the Arizona Sexual Experiences Scale showed that the drug did not result in any impairment of sexual function. Nor was MIN-117 associated with evidence of cognitive impairment; in fact, scores on the Digit-Symbol Substitution Test and Digit Span Backwards tool were better than in the placebo arm.
The study was sponsored by Minerva Neurosciences and presented by the company’s chief medical officer.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: An investigational antidepressant might address important unmet needs in the treatment of major depression.
Major finding: Patients with major depressive disorder were 3.1-fold more likely to be in remission after 6 weeks on MIN-117 at 2.5 mg/day than with placebo.
Study details: This prospective, double-blind, randomized, double-blind, placebo- and active-controlled study included 84 patients with major depressive disorder.
Disclosures: The study was sponsored by Minerva Neurosciences and presented by the company’s chief medical officer.
Sexual assault and harassment linked to hypertension, depression, and anxiety
Sexual harassment and assault may have significant health impacts on women in midlife, including greater risk of hypertension, poor sleep, depression, and anxiety, research suggests.
In the Oct. 3 online edition of JAMA Internal Medicine, a study of 304 women aged 40-60 years showed that 19% reported a history of workplace sexual harassment, 22% reported a history of sexual assault, and 10% reported both. The report was presented simultaneously at the North American Menopause Society annual meeting in San Diego.
The researchers found that those with a history of sexual assault had an almost threefold higher odds of clinically elevated depressive symptoms (OR, 2.86, P = .003), and more than twofold greater odds of anxiety and poor sleep (OR, 2.26, P = .006 and OR, 2.15, P = .007 respectively).
Women who reported experiencing sexual harassment in the workplace – and who were not taking antihypertensive medication – were more than twice as likely to have stage 1 or 2 hypertension, compared with women who had not experienced sexual harassment (OR, 2.36, P = .03). They also had 89% higher odds of poor sleep consistent with clinical insomnia (P = .03).
These associations all persisted even after adjustment for demographic and biomedical factors such as age, ethnicity, body mass index, snoring, and the use of antihypertensive, antidepressant, and anti-anxiety medications.
“Given the high prevalence of sexual harassment and assault, addressing these prevalent and potent social exposures may be critical to promoting health and preventing disease in women,” wrote Rebecca C. Thurston, PhD, of the department of psychiatry at the University of Pittsburgh, and her coauthors.
They noted that the 1-in-5 rate of sexual harassment or assault seen in the study was actually lower than that seen in national samples, which may be have been because of the exclusion of women who smoked, had undergone hysterectomies, or were using common antidepressants or cardiovascular medications.
“Few characteristics distinguished between women who had been sexually harassed and those who had been sexually assaulted, with the exception that women who were sexually harassed were more highly educated yet more financially strained,” they wrote. “Notably, women who are younger or are in more precarious employment situations are more likely to be harassed, and financially stressed women can lack the financial security to leave abusive work situations.”
The study was supported by the National Institutes of Health, National Heart Lung and Blood Institute, and the University of Pittsburgh Clinical and Translational Science Institute. Dr. Thurston declared consultancies for MAS Innovations, Procter & Gamble, and Pfizer, but no other conflicts of interest were declared.
SOURCE: Thurston R et al. JAMA Intern Med. 2018, Oct 3. doi: 10.1001/jamainternmed.2018.4886.
Sexual harassment and assault may have significant health impacts on women in midlife, including greater risk of hypertension, poor sleep, depression, and anxiety, research suggests.
In the Oct. 3 online edition of JAMA Internal Medicine, a study of 304 women aged 40-60 years showed that 19% reported a history of workplace sexual harassment, 22% reported a history of sexual assault, and 10% reported both. The report was presented simultaneously at the North American Menopause Society annual meeting in San Diego.
The researchers found that those with a history of sexual assault had an almost threefold higher odds of clinically elevated depressive symptoms (OR, 2.86, P = .003), and more than twofold greater odds of anxiety and poor sleep (OR, 2.26, P = .006 and OR, 2.15, P = .007 respectively).
Women who reported experiencing sexual harassment in the workplace – and who were not taking antihypertensive medication – were more than twice as likely to have stage 1 or 2 hypertension, compared with women who had not experienced sexual harassment (OR, 2.36, P = .03). They also had 89% higher odds of poor sleep consistent with clinical insomnia (P = .03).
These associations all persisted even after adjustment for demographic and biomedical factors such as age, ethnicity, body mass index, snoring, and the use of antihypertensive, antidepressant, and anti-anxiety medications.
“Given the high prevalence of sexual harassment and assault, addressing these prevalent and potent social exposures may be critical to promoting health and preventing disease in women,” wrote Rebecca C. Thurston, PhD, of the department of psychiatry at the University of Pittsburgh, and her coauthors.
They noted that the 1-in-5 rate of sexual harassment or assault seen in the study was actually lower than that seen in national samples, which may be have been because of the exclusion of women who smoked, had undergone hysterectomies, or were using common antidepressants or cardiovascular medications.
“Few characteristics distinguished between women who had been sexually harassed and those who had been sexually assaulted, with the exception that women who were sexually harassed were more highly educated yet more financially strained,” they wrote. “Notably, women who are younger or are in more precarious employment situations are more likely to be harassed, and financially stressed women can lack the financial security to leave abusive work situations.”
The study was supported by the National Institutes of Health, National Heart Lung and Blood Institute, and the University of Pittsburgh Clinical and Translational Science Institute. Dr. Thurston declared consultancies for MAS Innovations, Procter & Gamble, and Pfizer, but no other conflicts of interest were declared.
SOURCE: Thurston R et al. JAMA Intern Med. 2018, Oct 3. doi: 10.1001/jamainternmed.2018.4886.
Sexual harassment and assault may have significant health impacts on women in midlife, including greater risk of hypertension, poor sleep, depression, and anxiety, research suggests.
In the Oct. 3 online edition of JAMA Internal Medicine, a study of 304 women aged 40-60 years showed that 19% reported a history of workplace sexual harassment, 22% reported a history of sexual assault, and 10% reported both. The report was presented simultaneously at the North American Menopause Society annual meeting in San Diego.
The researchers found that those with a history of sexual assault had an almost threefold higher odds of clinically elevated depressive symptoms (OR, 2.86, P = .003), and more than twofold greater odds of anxiety and poor sleep (OR, 2.26, P = .006 and OR, 2.15, P = .007 respectively).
Women who reported experiencing sexual harassment in the workplace – and who were not taking antihypertensive medication – were more than twice as likely to have stage 1 or 2 hypertension, compared with women who had not experienced sexual harassment (OR, 2.36, P = .03). They also had 89% higher odds of poor sleep consistent with clinical insomnia (P = .03).
These associations all persisted even after adjustment for demographic and biomedical factors such as age, ethnicity, body mass index, snoring, and the use of antihypertensive, antidepressant, and anti-anxiety medications.
“Given the high prevalence of sexual harassment and assault, addressing these prevalent and potent social exposures may be critical to promoting health and preventing disease in women,” wrote Rebecca C. Thurston, PhD, of the department of psychiatry at the University of Pittsburgh, and her coauthors.
They noted that the 1-in-5 rate of sexual harassment or assault seen in the study was actually lower than that seen in national samples, which may be have been because of the exclusion of women who smoked, had undergone hysterectomies, or were using common antidepressants or cardiovascular medications.
“Few characteristics distinguished between women who had been sexually harassed and those who had been sexually assaulted, with the exception that women who were sexually harassed were more highly educated yet more financially strained,” they wrote. “Notably, women who are younger or are in more precarious employment situations are more likely to be harassed, and financially stressed women can lack the financial security to leave abusive work situations.”
The study was supported by the National Institutes of Health, National Heart Lung and Blood Institute, and the University of Pittsburgh Clinical and Translational Science Institute. Dr. Thurston declared consultancies for MAS Innovations, Procter & Gamble, and Pfizer, but no other conflicts of interest were declared.
SOURCE: Thurston R et al. JAMA Intern Med. 2018, Oct 3. doi: 10.1001/jamainternmed.2018.4886.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: Women who have experienced sexual assault showed nearly threefold higher odds of depressive symptoms.
Study details: Study of 304 women aged 40-60 years.
Disclosures: The study was supported by the National Institutes of Health, National Heart Lung and Blood Institute, and the University of Pittsburgh Clinical and Translational Science Institute. Dr. Thurston declared consultancies for MAS Innovations, Procter & Gamble, and Pfizer, but no other conflicts of interest were declared.
Source: Thurston R et al. JAMA Intern Med. 2018 Oct 3. doi: 10.1001/jamainternmed.2018.4886.
Could group CBT help survivors of Florence?
Rising waters forced hundreds of people, mainly in the Carolinas, to call for emergency rescues, and some people were forced to abandon their cars because of flooding. One man reportedly died by electrocution while trying to hook up a generator. Another man died after going out to check the status of hunting dogs, according to media reports. And in one of the most heart-wrenching tragedies, a mother and her infant were killed when a tree fell on their home.
Watching the TV reports and listening to the news of Hurricane Florence’s devastating impact on so many millions of people has been shocking. The death toll from this catastrophic weather event as of this writing stands at 39. Besides the current and future physical problems and illnesses left in Florence’s wake, the extent of property damage and loss must be overwhelming for the survivors.
I worry about the extent of the emotional toll left behind by Florence, just as Hurricane Maria did last year in Puerto Rico. The storm and its subsequent damage to the individual psyche – including the loss of identity and the fracturing of social structures and networks – almost certainly will lead to posttraumatic stress disorder, depression, and utter despair for many survivors.
While monitoring Florence’s impact, I thought about Hurricane Sandy, which upended me personally when it hit New York in 2012. As I’ve written previously, Sandy’s impact left me without power, running water, or toilet facilities. Almost 3 days of this uncertainty shook me from my comfort zone and truly affected my emotions. Before day 3, I left my home and drove (yes, I could still use my car; the roads were clear and my garage was not flooded) to my older son’s home – where I had a great support system and was able to continue to live a relatively normal life while watching the storm’s developments on TV. To this day, many areas of New York, New Jersey, and Connecticut that were hit by Sandy have not fully recovered.
Back to the human tragedy still unfolding for the survivors of Florence: I believe – and the data suggest – that early intervention and treatment of PTSD leads to better outcomes and should be addressed sooner than later. There is no specific medicinal “magic bullet” for PTSD, although some medications may help as well as treat a depressive component of the disorder and other medications may assist in improving sleep and disruptive sleep patterns. It’s been shown, time and again, that cognitive-behavioral therapy, various types of prolonged exposure therapy, and eye movement desensitization therapies work best. The most updated federal guidelines from the Department of Veterans Affairs and the Department of Defense, coauthored by Lori L. Davis, MD, of the University of Alabama at Birmingham, reinforce those treatments.
I also believe that, in situations in which masses of people are affected or potentially affected by PTSD, another first line of care that should be added is supportive, educational, interactive group therapy. In other words, it is possible that a cognitive-behavioral group therapy (CBGT) approach would reach many more people, make psychiatric intervention acceptable, and help the survivors of Florence. A recent study by researchers at the University of Massachusetts Boston that examined the role of “decentering” as part of CBGT for patients with specific anxiety disorders, for example, social anxiety disorder, might provide some hints. Decentering involves learning to observe thoughts and feelings as objective events in the mind rather than identifying with them personally. Aaron T. Beck, MD, and others hypothesized decentering as a mechanism of change in CBT.
In the UMass study, researchers recruited 81 people with a principal diagnosis of social anxiety disorder based on the Anxiety Disorders Interview Scheduled for DSM-IV. Other inclusion criteria for the study included stability on medications for 3 months or 1 month on benzodiazepines (Behav Ther. 2018 Sep;49[5]:809-12). Sixty-three of participants had 12 sessions of CBGT. The researchers found that people who received the CBGT experienced an increase in decentering. An increase in decentering, in turn, predicted improvement on most outcome measures.
Just as primary care physicians and surgeons know how to address serious physical health issues related natural and man-made disasters, psychiatrists must quickly know how to address the mental health aspects of care. Group therapy has the greatest potential to help more people and perhaps treat – and even prevent not only PTSD but many anxiety disorders as well.
Dr. London, a psychiatrist who practices in New York, developed and ran a short-term psychotherapy program for 20 years at NYU Langone Medical Center and has been writing columns for 35 years. His new book about helping people feel better fast is expected to be published in fall 2018. He has no disclosures.
Rising waters forced hundreds of people, mainly in the Carolinas, to call for emergency rescues, and some people were forced to abandon their cars because of flooding. One man reportedly died by electrocution while trying to hook up a generator. Another man died after going out to check the status of hunting dogs, according to media reports. And in one of the most heart-wrenching tragedies, a mother and her infant were killed when a tree fell on their home.
Watching the TV reports and listening to the news of Hurricane Florence’s devastating impact on so many millions of people has been shocking. The death toll from this catastrophic weather event as of this writing stands at 39. Besides the current and future physical problems and illnesses left in Florence’s wake, the extent of property damage and loss must be overwhelming for the survivors.
I worry about the extent of the emotional toll left behind by Florence, just as Hurricane Maria did last year in Puerto Rico. The storm and its subsequent damage to the individual psyche – including the loss of identity and the fracturing of social structures and networks – almost certainly will lead to posttraumatic stress disorder, depression, and utter despair for many survivors.
While monitoring Florence’s impact, I thought about Hurricane Sandy, which upended me personally when it hit New York in 2012. As I’ve written previously, Sandy’s impact left me without power, running water, or toilet facilities. Almost 3 days of this uncertainty shook me from my comfort zone and truly affected my emotions. Before day 3, I left my home and drove (yes, I could still use my car; the roads were clear and my garage was not flooded) to my older son’s home – where I had a great support system and was able to continue to live a relatively normal life while watching the storm’s developments on TV. To this day, many areas of New York, New Jersey, and Connecticut that were hit by Sandy have not fully recovered.
Back to the human tragedy still unfolding for the survivors of Florence: I believe – and the data suggest – that early intervention and treatment of PTSD leads to better outcomes and should be addressed sooner than later. There is no specific medicinal “magic bullet” for PTSD, although some medications may help as well as treat a depressive component of the disorder and other medications may assist in improving sleep and disruptive sleep patterns. It’s been shown, time and again, that cognitive-behavioral therapy, various types of prolonged exposure therapy, and eye movement desensitization therapies work best. The most updated federal guidelines from the Department of Veterans Affairs and the Department of Defense, coauthored by Lori L. Davis, MD, of the University of Alabama at Birmingham, reinforce those treatments.
I also believe that, in situations in which masses of people are affected or potentially affected by PTSD, another first line of care that should be added is supportive, educational, interactive group therapy. In other words, it is possible that a cognitive-behavioral group therapy (CBGT) approach would reach many more people, make psychiatric intervention acceptable, and help the survivors of Florence. A recent study by researchers at the University of Massachusetts Boston that examined the role of “decentering” as part of CBGT for patients with specific anxiety disorders, for example, social anxiety disorder, might provide some hints. Decentering involves learning to observe thoughts and feelings as objective events in the mind rather than identifying with them personally. Aaron T. Beck, MD, and others hypothesized decentering as a mechanism of change in CBT.
In the UMass study, researchers recruited 81 people with a principal diagnosis of social anxiety disorder based on the Anxiety Disorders Interview Scheduled for DSM-IV. Other inclusion criteria for the study included stability on medications for 3 months or 1 month on benzodiazepines (Behav Ther. 2018 Sep;49[5]:809-12). Sixty-three of participants had 12 sessions of CBGT. The researchers found that people who received the CBGT experienced an increase in decentering. An increase in decentering, in turn, predicted improvement on most outcome measures.
Just as primary care physicians and surgeons know how to address serious physical health issues related natural and man-made disasters, psychiatrists must quickly know how to address the mental health aspects of care. Group therapy has the greatest potential to help more people and perhaps treat – and even prevent not only PTSD but many anxiety disorders as well.
Dr. London, a psychiatrist who practices in New York, developed and ran a short-term psychotherapy program for 20 years at NYU Langone Medical Center and has been writing columns for 35 years. His new book about helping people feel better fast is expected to be published in fall 2018. He has no disclosures.
Rising waters forced hundreds of people, mainly in the Carolinas, to call for emergency rescues, and some people were forced to abandon their cars because of flooding. One man reportedly died by electrocution while trying to hook up a generator. Another man died after going out to check the status of hunting dogs, according to media reports. And in one of the most heart-wrenching tragedies, a mother and her infant were killed when a tree fell on their home.
Watching the TV reports and listening to the news of Hurricane Florence’s devastating impact on so many millions of people has been shocking. The death toll from this catastrophic weather event as of this writing stands at 39. Besides the current and future physical problems and illnesses left in Florence’s wake, the extent of property damage and loss must be overwhelming for the survivors.
I worry about the extent of the emotional toll left behind by Florence, just as Hurricane Maria did last year in Puerto Rico. The storm and its subsequent damage to the individual psyche – including the loss of identity and the fracturing of social structures and networks – almost certainly will lead to posttraumatic stress disorder, depression, and utter despair for many survivors.
While monitoring Florence’s impact, I thought about Hurricane Sandy, which upended me personally when it hit New York in 2012. As I’ve written previously, Sandy’s impact left me without power, running water, or toilet facilities. Almost 3 days of this uncertainty shook me from my comfort zone and truly affected my emotions. Before day 3, I left my home and drove (yes, I could still use my car; the roads were clear and my garage was not flooded) to my older son’s home – where I had a great support system and was able to continue to live a relatively normal life while watching the storm’s developments on TV. To this day, many areas of New York, New Jersey, and Connecticut that were hit by Sandy have not fully recovered.
Back to the human tragedy still unfolding for the survivors of Florence: I believe – and the data suggest – that early intervention and treatment of PTSD leads to better outcomes and should be addressed sooner than later. There is no specific medicinal “magic bullet” for PTSD, although some medications may help as well as treat a depressive component of the disorder and other medications may assist in improving sleep and disruptive sleep patterns. It’s been shown, time and again, that cognitive-behavioral therapy, various types of prolonged exposure therapy, and eye movement desensitization therapies work best. The most updated federal guidelines from the Department of Veterans Affairs and the Department of Defense, coauthored by Lori L. Davis, MD, of the University of Alabama at Birmingham, reinforce those treatments.
I also believe that, in situations in which masses of people are affected or potentially affected by PTSD, another first line of care that should be added is supportive, educational, interactive group therapy. In other words, it is possible that a cognitive-behavioral group therapy (CBGT) approach would reach many more people, make psychiatric intervention acceptable, and help the survivors of Florence. A recent study by researchers at the University of Massachusetts Boston that examined the role of “decentering” as part of CBGT for patients with specific anxiety disorders, for example, social anxiety disorder, might provide some hints. Decentering involves learning to observe thoughts and feelings as objective events in the mind rather than identifying with them personally. Aaron T. Beck, MD, and others hypothesized decentering as a mechanism of change in CBT.
In the UMass study, researchers recruited 81 people with a principal diagnosis of social anxiety disorder based on the Anxiety Disorders Interview Scheduled for DSM-IV. Other inclusion criteria for the study included stability on medications for 3 months or 1 month on benzodiazepines (Behav Ther. 2018 Sep;49[5]:809-12). Sixty-three of participants had 12 sessions of CBGT. The researchers found that people who received the CBGT experienced an increase in decentering. An increase in decentering, in turn, predicted improvement on most outcome measures.
Just as primary care physicians and surgeons know how to address serious physical health issues related natural and man-made disasters, psychiatrists must quickly know how to address the mental health aspects of care. Group therapy has the greatest potential to help more people and perhaps treat – and even prevent not only PTSD but many anxiety disorders as well.
Dr. London, a psychiatrist who practices in New York, developed and ran a short-term psychotherapy program for 20 years at NYU Langone Medical Center and has been writing columns for 35 years. His new book about helping people feel better fast is expected to be published in fall 2018. He has no disclosures.
Previous psychiatric admissions predict suicide attempts
SAN DIEGO – Among patients presenting to the emergency department with suicidal ideation, the number of previous psychiatric admissions is the most important predictor of a subsequent suicide attempt, results from a 2-year-long study showed.
Momentary measures – significant current difficulties with sleep, energy, or appetite, and anhedonia – “are important considerations during suicide risk assessment, regardless of whether the patient meets the threshold for major depressive disorder, lead study author Anne C. Knorr said in an interview in advance of the annual meeting of the American College of Emergency Physicians.
“Many traditionally studied psychiatric risk factors do not significantly differ between individuals who think about suicide and those who act on their suicidal thoughts, an important distinction as only one-third of those who think about suicide carry out a suicide attempt. While there is support for some psychiatric factors – for example, anxiety, substance use disorders, sleep disturbance – being useful in differentiating these groups, the current study is unique in its use of a longitudinal design to identify influential risk factors that predict future suicide attempt among those with suicidal ideation,” she said.
Ms. Knorr, a research project manager at Geisinger Medical Center, Danville, Pa., and her colleagues collected electronic medical record data from 908 patients who had received a psychiatric evaluation at an index emergency department visit for suicidal ideation over a 2-year period. The mean age of patients was 39 years. The target sample was 30 patients who had returned to the ED following a suicide attempt within 6 months of their index ED visit.
The researchers analyzed 32 predictor variables from patient charts, including demographics, psychiatric history, and current psychiatric presentation. The evaluation was done with “a machine learning statistical approach which is more capable than traditional statistical approaches in handling a large number of predictor variables,” Ms. Knorr said.
The number of previous psychiatric admissions was the most important predictor of a subsequent suicide attempt. The next nine most important variables were sleep disturbance, history of family suicide, low energy, patient age, psychiatrist determination of severe suicide risk, psychiatrist determination of moderate suicide risk, appetite issues, presence of a support system, and loss of interest/pleasure.
These symptoms may not typically be weighed heavily in risk assessments, Ms. Knorr said. “Additionally, given that research suggests that the clinical determination of suicide risk is historically poor, it was interesting that psychiatrist determination of moderate or high risk was influential in predicting a return visit for suicide attempt. Finally, we were surprised that a past history of suicide attempt, often viewed as the strongest predictor of a future suicide attempt, did not emerge as one of the top 10 influential predictors.
“Limitations of this study include the use of a return visit to a Geisinger emergency department as the only measure of a future suicide attempt and the utilization of data from only one health system,” she noted.
The study was funded by the Geisinger Clinic Research Fund. One of the coauthors, Andrei Nemoianu, MD, reported receipt of a research grant from Takeda Pharmaceuticals for a separate study.
SOURCE: Knorr A et al. Ann Emerg Med. 2018 Oct;72;4:S23. doi. 10.1016/j.annemergmed.2018.08.055.
SAN DIEGO – Among patients presenting to the emergency department with suicidal ideation, the number of previous psychiatric admissions is the most important predictor of a subsequent suicide attempt, results from a 2-year-long study showed.
Momentary measures – significant current difficulties with sleep, energy, or appetite, and anhedonia – “are important considerations during suicide risk assessment, regardless of whether the patient meets the threshold for major depressive disorder, lead study author Anne C. Knorr said in an interview in advance of the annual meeting of the American College of Emergency Physicians.
“Many traditionally studied psychiatric risk factors do not significantly differ between individuals who think about suicide and those who act on their suicidal thoughts, an important distinction as only one-third of those who think about suicide carry out a suicide attempt. While there is support for some psychiatric factors – for example, anxiety, substance use disorders, sleep disturbance – being useful in differentiating these groups, the current study is unique in its use of a longitudinal design to identify influential risk factors that predict future suicide attempt among those with suicidal ideation,” she said.
Ms. Knorr, a research project manager at Geisinger Medical Center, Danville, Pa., and her colleagues collected electronic medical record data from 908 patients who had received a psychiatric evaluation at an index emergency department visit for suicidal ideation over a 2-year period. The mean age of patients was 39 years. The target sample was 30 patients who had returned to the ED following a suicide attempt within 6 months of their index ED visit.
The researchers analyzed 32 predictor variables from patient charts, including demographics, psychiatric history, and current psychiatric presentation. The evaluation was done with “a machine learning statistical approach which is more capable than traditional statistical approaches in handling a large number of predictor variables,” Ms. Knorr said.
The number of previous psychiatric admissions was the most important predictor of a subsequent suicide attempt. The next nine most important variables were sleep disturbance, history of family suicide, low energy, patient age, psychiatrist determination of severe suicide risk, psychiatrist determination of moderate suicide risk, appetite issues, presence of a support system, and loss of interest/pleasure.
These symptoms may not typically be weighed heavily in risk assessments, Ms. Knorr said. “Additionally, given that research suggests that the clinical determination of suicide risk is historically poor, it was interesting that psychiatrist determination of moderate or high risk was influential in predicting a return visit for suicide attempt. Finally, we were surprised that a past history of suicide attempt, often viewed as the strongest predictor of a future suicide attempt, did not emerge as one of the top 10 influential predictors.
“Limitations of this study include the use of a return visit to a Geisinger emergency department as the only measure of a future suicide attempt and the utilization of data from only one health system,” she noted.
The study was funded by the Geisinger Clinic Research Fund. One of the coauthors, Andrei Nemoianu, MD, reported receipt of a research grant from Takeda Pharmaceuticals for a separate study.
SOURCE: Knorr A et al. Ann Emerg Med. 2018 Oct;72;4:S23. doi. 10.1016/j.annemergmed.2018.08.055.
SAN DIEGO – Among patients presenting to the emergency department with suicidal ideation, the number of previous psychiatric admissions is the most important predictor of a subsequent suicide attempt, results from a 2-year-long study showed.
Momentary measures – significant current difficulties with sleep, energy, or appetite, and anhedonia – “are important considerations during suicide risk assessment, regardless of whether the patient meets the threshold for major depressive disorder, lead study author Anne C. Knorr said in an interview in advance of the annual meeting of the American College of Emergency Physicians.
“Many traditionally studied psychiatric risk factors do not significantly differ between individuals who think about suicide and those who act on their suicidal thoughts, an important distinction as only one-third of those who think about suicide carry out a suicide attempt. While there is support for some psychiatric factors – for example, anxiety, substance use disorders, sleep disturbance – being useful in differentiating these groups, the current study is unique in its use of a longitudinal design to identify influential risk factors that predict future suicide attempt among those with suicidal ideation,” she said.
Ms. Knorr, a research project manager at Geisinger Medical Center, Danville, Pa., and her colleagues collected electronic medical record data from 908 patients who had received a psychiatric evaluation at an index emergency department visit for suicidal ideation over a 2-year period. The mean age of patients was 39 years. The target sample was 30 patients who had returned to the ED following a suicide attempt within 6 months of their index ED visit.
The researchers analyzed 32 predictor variables from patient charts, including demographics, psychiatric history, and current psychiatric presentation. The evaluation was done with “a machine learning statistical approach which is more capable than traditional statistical approaches in handling a large number of predictor variables,” Ms. Knorr said.
The number of previous psychiatric admissions was the most important predictor of a subsequent suicide attempt. The next nine most important variables were sleep disturbance, history of family suicide, low energy, patient age, psychiatrist determination of severe suicide risk, psychiatrist determination of moderate suicide risk, appetite issues, presence of a support system, and loss of interest/pleasure.
These symptoms may not typically be weighed heavily in risk assessments, Ms. Knorr said. “Additionally, given that research suggests that the clinical determination of suicide risk is historically poor, it was interesting that psychiatrist determination of moderate or high risk was influential in predicting a return visit for suicide attempt. Finally, we were surprised that a past history of suicide attempt, often viewed as the strongest predictor of a future suicide attempt, did not emerge as one of the top 10 influential predictors.
“Limitations of this study include the use of a return visit to a Geisinger emergency department as the only measure of a future suicide attempt and the utilization of data from only one health system,” she noted.
The study was funded by the Geisinger Clinic Research Fund. One of the coauthors, Andrei Nemoianu, MD, reported receipt of a research grant from Takeda Pharmaceuticals for a separate study.
SOURCE: Knorr A et al. Ann Emerg Med. 2018 Oct;72;4:S23. doi. 10.1016/j.annemergmed.2018.08.055.
AT ACEP18
Key clinical point: A number of factors representing momentary experiences such as low energy emerged as predictors of suicide attempts among suicidal ideators following an ED visit.
Major finding: The number of past psychiatric admissions was the most influential predictor of a subsequent suicide attempt.
Study details: A study of 30 patients who had returned to the ED following a suicide attempt within 6 months of their index ED visit.
Disclosures: The Geisinger Clinic Research Fund supported the study. A coauthor, Andrei Nemoianu, MD, reported receipt of a research grant from Takeda Pharmaceuticals for a separate study.
Source: Knorr A et al. Ann Emerg Med. 2018 Oct;72;4:S23.
Family therapy and cultural conflicts
I recently had the privilege of treating a family who spoke my first language, Hindi. My patient, Ms. M, was 16 years old and struggling to adjust to her new life in the United States, having recently come from India. America’s schooling, culture, and “open society” was a contrast to her life in a semi-rural town, especially her close-knit family structure in which her parents and siblings are everything. Due to their cultural beliefs and religious faith in Islam, both Ms. M and her father were initially resistant to begin treatment for her depression and anxiety. “Let’s give it a trial” was the attitude I finally got from the father. But to me, there was a clear discordance in the communication among the family members in addition to the primary mental illness that led them to come for treatment. I was attracted to work with this family because I had a reasonable understanding of their faith, their culture, and their family system, and I have an inclination toward spirituality. Even though I recognized this family’s social isolation, I wondered why they were still in a state of unrest, given their deep commitment to their faith.
Ms. M was isolating herself at home, in an environment that wasn’t supportive of talking about her concerns. These included being bullied for being “different,” for how she dressed, and for having home-cooked traditional meals for lunch, and being unable to socialize with most of her male peers, except for those from her same community. This led her to dream of returning to India.
The family did not have a social life. Ms. M told me, “I wanted to socialize, but I cannot because of my faith and religion.” So she chose to wear attire to identify with her mother and her culture of origin. She also did this to hide her emotional pain from enduring trauma related to bullying at her school. It was a challenge to understand how faith, resilience, and trauma were intermingled in Ms. M and her family.
I saw Ms. M and her family for 12 one-hour family psychotherapy sessions. The initial session unfolded uneasily. It was a challenge to build rapport and help them understand how family therapy works. Circular inquiries to each family member, specifically to get the mother’s point of view, brought mourning, shame, and guilt to this family. The importance of marriage, education, and immigration were processed in reference to their culture and their incomplete acculturation to life in the United States.
I wondered if there were other families with different cultural backgrounds who struggled with similar conflicts. I also wondered if those families understood the value of family therapy or had ever experienced this therapeutic process.
The 3 key signs that made me believe that this family was making progress through our work together included:
- They complied with treatment; the family never missed a session.
- The parents acknowledged that their daughter was doing better.
- The mother brought me a dinner as a gesture of gratitude in our last session. This is a particularly meaningful gesture on the part of people with their cultural background.
I clearly remember our first meeting, when Ms. M asked
I recently had the privilege of treating a family who spoke my first language, Hindi. My patient, Ms. M, was 16 years old and struggling to adjust to her new life in the United States, having recently come from India. America’s schooling, culture, and “open society” was a contrast to her life in a semi-rural town, especially her close-knit family structure in which her parents and siblings are everything. Due to their cultural beliefs and religious faith in Islam, both Ms. M and her father were initially resistant to begin treatment for her depression and anxiety. “Let’s give it a trial” was the attitude I finally got from the father. But to me, there was a clear discordance in the communication among the family members in addition to the primary mental illness that led them to come for treatment. I was attracted to work with this family because I had a reasonable understanding of their faith, their culture, and their family system, and I have an inclination toward spirituality. Even though I recognized this family’s social isolation, I wondered why they were still in a state of unrest, given their deep commitment to their faith.
Ms. M was isolating herself at home, in an environment that wasn’t supportive of talking about her concerns. These included being bullied for being “different,” for how she dressed, and for having home-cooked traditional meals for lunch, and being unable to socialize with most of her male peers, except for those from her same community. This led her to dream of returning to India.
The family did not have a social life. Ms. M told me, “I wanted to socialize, but I cannot because of my faith and religion.” So she chose to wear attire to identify with her mother and her culture of origin. She also did this to hide her emotional pain from enduring trauma related to bullying at her school. It was a challenge to understand how faith, resilience, and trauma were intermingled in Ms. M and her family.
I saw Ms. M and her family for 12 one-hour family psychotherapy sessions. The initial session unfolded uneasily. It was a challenge to build rapport and help them understand how family therapy works. Circular inquiries to each family member, specifically to get the mother’s point of view, brought mourning, shame, and guilt to this family. The importance of marriage, education, and immigration were processed in reference to their culture and their incomplete acculturation to life in the United States.
I wondered if there were other families with different cultural backgrounds who struggled with similar conflicts. I also wondered if those families understood the value of family therapy or had ever experienced this therapeutic process.
The 3 key signs that made me believe that this family was making progress through our work together included:
- They complied with treatment; the family never missed a session.
- The parents acknowledged that their daughter was doing better.
- The mother brought me a dinner as a gesture of gratitude in our last session. This is a particularly meaningful gesture on the part of people with their cultural background.
I clearly remember our first meeting, when Ms. M asked
I recently had the privilege of treating a family who spoke my first language, Hindi. My patient, Ms. M, was 16 years old and struggling to adjust to her new life in the United States, having recently come from India. America’s schooling, culture, and “open society” was a contrast to her life in a semi-rural town, especially her close-knit family structure in which her parents and siblings are everything. Due to their cultural beliefs and religious faith in Islam, both Ms. M and her father were initially resistant to begin treatment for her depression and anxiety. “Let’s give it a trial” was the attitude I finally got from the father. But to me, there was a clear discordance in the communication among the family members in addition to the primary mental illness that led them to come for treatment. I was attracted to work with this family because I had a reasonable understanding of their faith, their culture, and their family system, and I have an inclination toward spirituality. Even though I recognized this family’s social isolation, I wondered why they were still in a state of unrest, given their deep commitment to their faith.
Ms. M was isolating herself at home, in an environment that wasn’t supportive of talking about her concerns. These included being bullied for being “different,” for how she dressed, and for having home-cooked traditional meals for lunch, and being unable to socialize with most of her male peers, except for those from her same community. This led her to dream of returning to India.
The family did not have a social life. Ms. M told me, “I wanted to socialize, but I cannot because of my faith and religion.” So she chose to wear attire to identify with her mother and her culture of origin. She also did this to hide her emotional pain from enduring trauma related to bullying at her school. It was a challenge to understand how faith, resilience, and trauma were intermingled in Ms. M and her family.
I saw Ms. M and her family for 12 one-hour family psychotherapy sessions. The initial session unfolded uneasily. It was a challenge to build rapport and help them understand how family therapy works. Circular inquiries to each family member, specifically to get the mother’s point of view, brought mourning, shame, and guilt to this family. The importance of marriage, education, and immigration were processed in reference to their culture and their incomplete acculturation to life in the United States.
I wondered if there were other families with different cultural backgrounds who struggled with similar conflicts. I also wondered if those families understood the value of family therapy or had ever experienced this therapeutic process.
The 3 key signs that made me believe that this family was making progress through our work together included:
- They complied with treatment; the family never missed a session.
- The parents acknowledged that their daughter was doing better.
- The mother brought me a dinner as a gesture of gratitude in our last session. This is a particularly meaningful gesture on the part of people with their cultural background.
I clearly remember our first meeting, when Ms. M asked
Psychiatric considerations in menopause
Mrs. J, age 49, presents to your psychiatric clinic. For the last few years, she has been experiencing night sweats and hot flashes, which she has attributed to being perimenopausal. Over the last year, she has noticed that her mood has declined; however, she has suffered several life events that she feels have contributed. Her mother was diagnosed with Alzheimer’s disease and had to move into a nursing home, which Mrs. J found very stressful. At the same time, her daughter left home for college, and her son is exploring his college options. Recently, Mrs. J has not been able to work due to her mood, and she is afraid she may lose her job as a consequence. She has struggled to talk to her husband about how she is feeling, and feels increasingly isolated. Over the last month, she has had increased problems sleeping and less energy; some days she struggles to get out of bed. She is finding it difficult to concentrate and is more forgetful. She has lost interest in her hobbies and is no longer meeting with her friends. She has no history of depression or anxiety, although she recalls feeling very low in mood for months after the birth of each of her children.
Are Mrs. J’s symptoms related to menopause or depression? What further investigations are necessary? Would you modify your treatment plan because of her menopausal status?
Women are at elevated risk of developing psychiatric symptoms and disorders throughout their reproductive lives, including during menopause. Menopause is a time of life transition, when women may experience multiple physical symptoms, including vasomotor symptoms (night sweats and hot flashes), sexual symptoms, and sleep difficulties. Depressive symptoms occur more frequently during menopause, and symptoms of schizophrenia may worsen.
Estrogen plays a role in mental illness throughout a woman’s life. In menopause, decreasing estrogen levels may correlate with increased mood symptoms, physical symptoms, and psychotic symptoms. As such, psychiatrists should consider whether collaboration regarding adjunctive hormone replacement therapy would be beneficial, and whether the benefits outweigh the potential risks. Otherwise, treatment of depression in menopause is similar to treatment outside of the menopausal transition, though serotonergic antidepressants may help target vasomotor symptoms while therapy may focus on role transition and loss. In this article, we review why women are at increased risk for mental illness during menopause, the role of estrogen, and treatment of mood and psychotic disorders during this phase of a woman’s life.
Increased vulnerability across the lifespan
Continued to: Why menopause?
Why menopause?
Perimenopausal mood disorders
However, one should keep in mind that new-onset mania in menopause is rare and should trigger a medical work-up and a dementia evaluation.13 Table 414 provides recommendations for evaluation of women undergoing menopause.
Menopause and serious mental illnesses
A study of 91 perimenopausal and postmenopausal women (age 45 to 55) who were diagnosed with schizophrenia/schizoaffective disorder, bipolar disorder, or major depressive disorder (MDD) found that women with severe mental illness experienced significant vasomotor, physical, sexual, and psychosocial symptoms related to menopause.15 Furthermore, on 7 of 29 items on the Menopause Specific Quality of Life Scale, including hot flashes, women diagnosed with MDD reported problems significantly more often than women with other serious mental illnesses.15
Women with serious mental illness often have deficits in their knowledge about menopause.3 More than half of the 91 women in the study diagnosed with schizophrenia/schizoaffective disorder, bipolar disorder, or MDD felt more stressed related to menopause, and reported that menopause had a negative effect on their mental health.3 These women rated their top 5 symptoms potentially related to menopause as feeling depressed, anxious, or tired; lacking energy; and experiencing poor memory.3
Continued to: Role of estrogen on mood and psychosis
Role of estrogen on mood and psychosis
Women are at higher risk throughout their reproductive life than are men for MDD, anxiety disorders, and trauma-related disorders.12 Factors associated with depression during the menopause transition are reproductive hormonal changes (rise of follicle-stimulating hormone [FSH] and luteinizing hormone levels, and variability in estrogen [E2] and FSH levels); menopausal symptoms, particularly vasomotor symptoms; prior depression; psychosocial factors (adverse life events, financial strain, poor social supports); high body mass index, smoking, and poor physical health.6,7 Decreasing estrogen in the menopause transition may increase susceptibility to depression in some women.16 The Box17,18 provides more information on the relationship between estrogen and brain function.
Box
Estrogen and brain function
Numerous molecular and clinical studies have established the role of 17-beta estradiol in modulating brain functions via alterations in neurotransmission.17 Estrogen increases serotonin availability in the synapse by various pathways. It increases the rate of degradation of monoamine oxidase; monoamine oxidase enzymes are responsible for catabolizing serotonin, dopamine, and norepinephrine. Estrogen also increases tryptophan hydroxylase expression (rate-limiting enzyme in serotonin synthesis) and promotes intraneuronal serotonin transport in brain regions associated with affect regulation by increasing gene expression of the serotonin reuptake transporter. Studies have linked brain-derived neurotropic factor (BDNF) to increased serotonin turnover and proposed that estrogen may influence depression by increasing BDNF levels within the brain.18
Depressive disorders, including premenstrual dysphoric disorder, postpartum depression, and perimenopausal depression, have been linked to changes in hormonal status in women. Symptomatic menopause transition occurs in at least 20% of women, and a retrospective cohort study suggests that symptomatic menopause transition might increase the risk of new-onset depressive disorders, bipolar disorders, anxiety disorders, and sleep disorders.19 Symptomatic menopause transition also is a vulnerable time for relapse of MDD. Among women experiencing menopausal symptoms, including hot flashes, one-third also report depression—which correlates with a poorer quality of life, less work productivity, and greater use of health care services.9
Women who undergo surgical menopause are at greater risk for depression.8,10,11 This may be due to abrupt deprivation of estrogen—or related to a psychological reaction to the loss of fertility.
The observation that hormonal fluctuations related to women’s reproductive cycle have a significant impact on psychotic symptomatology has resulted in the “hypo-estrogenism hypothesis,” which proposes that gonadal dysfunction may increase vulnerability to schizophrenia, or that schizophrenia may lead to gonadal dysfunction.20 The “estrogen protection hypothesis” proposes that estrogen may protect women from schizophrenia, and may be a factor in the delayed onset of schizophrenia compared with men, less severe psychopathology, better outcomes, and premenstrual and postmenopausal deterioration in women. Many women of reproductive age with schizophrenia experience improvement in symptoms during the high estrogen phase of their menstrual cycle.
Pope et al21 have suggested that a hormone sensitivity syndrome may underlie why some women experience physical, psychological, and emotional symptoms at times of hormonal shifts such as menopause. This may represent a critical window of vulnerability, and also an opportunity to consider E2 as a therapeutic intervention.
Continued to: Treating mental illness in menopause
Treating mental illness in menopause
Changes to drug pharmacokinetics occur because some metabolising enzymes are estrogen-dependent and their levels decline after menopause, which leads to greater variability in drug response, particularly for oral medications. Other factors that can contribute to variability in medication response are polypharmacy, alcohol, illicit drugs, liver mass, smoking, caffeine, and nutritional intake.
While antidepressants are the first-line treatment for MDD and anxiety disorders, some patients remain unresponsive or inadequately responsive to currently available medications. In perimenopausal women with MDD, there may be an indication for adjunctive therapy with transdermal E2 in refractory cases; estrogen may augment the effects of selective serotonin reuptake inhibitor (SSRI) antidepressants as well as hasten the onset of antidepressant action.22 Estrogen also may be worth considering in women with mild depressive symptoms. For MDD, SSRIs plus estrogen may be more beneficial in improving mood than either agent alone. The effectiveness of E2 is less certain in postmenopausal depression.
Hormonal therapy for mental health disorders has equivocal evidence. The individual’s history and risk factors (eg, cardiovascular and osteoporosis risks) must be considered. A recent trial found that treatment with either venlafaxine or low-dose estrogen improved quality of life in menopausal women with vasomotor symptoms.23 Venlafaxine improved the psychosocial domain, while estrogen improved quality of life in other domains. Escitalopram, duloxetine, and citalopram have also been identified as having a possible positive impact on menopausal symptoms.22 SSRIs and serotonin-norepinephrine reuptake inhibitors may help reduce hot flashes and improve sleep.11
Regarding schizophrenia and estrogen, there may be improved symptoms during the high estrogen phase of the menstrual cycle, followed by a premenstrual aggravation of symptoms. Recall that women have a second peak of onset of schizophrenia after age 45, around the age of the onset of menopause.24 In a study of geropsychiatric hospital admissions, women were overrepresented among those with schizophrenia and schizoaffective disorder, compared with other psychiatric disorders.25 Postmenopausally, some women experience a decreased responsiveness to antipsychotics and worsening symptoms. In menopausal women with schizophrenia, check prolactin levels to help determine whether they are experiencing a natural menopause or medication-induced amenorrhea. Gender differences in pharmacotherapy responses and the decreasing response to antipsychotics in women older than age 50 have been observed26 and have led to exploration of the role of estrogen for treating schizophrenia in menopausal women. There have been contradictory results regarding use of estrogen as an adjunct to antipsychotics, with some reports finding this approach is effective and results in lower average doses of antipsychotics. Kulkarni et al27,28 have reported improvements in positive symptoms of treatment-resistant schizophrenia with transdermal use of E2, 200 mcg, as an adjunct to antipsychotics in women of childbearing age. However, they expressed caution regarding the health risks associated with prolonged use of E2. Long-term risks of high-dose estrogen therapies include thromboembolism, endometrial hyperplasia, and breast cancer, and individual factors should be considered before starting any form of hormone therapy. Selective estrogen receptor modulators (SERMs), such as raloxifene, which can cause activation of E2 receptors in a tissue-specific fashion and have less estrogen-related adverse effects, offer hope for future development in this field.27,28 While the use of adjunctive hormone therapy to manage psychotic symptoms in menopause is not routinely advised, the dosages of previously effective antipsychotics may need to be reviewed, or long-acting depot routes considered.29 Increased risk of prolonged QTc interval and tardive dyskinesia in geriatric women also should be considered in decisions regarding changes to antipsychotics or dosages.30
There are no guidelines regarding change in dosage of either individual antidepressants or antipsychotics in women at the time of menopause for managing pre-existing conditions. This may be due to the high variability in the effect of menopause on mental health and recognition that menopause is also a time for deterioration in physical health, as well as psychosocial changes for women, and thus other forms of intervention need to be considered.
Continued to: The biopsychosocial approach to treatment...
The biopsychosocial approach to treatment is particularly important in menopause.11 Common transitions in midlife include changes in relationships, employment, and financial status, and illness or death of family and friends.31 Therapy may focus on accepting a role transition and coping with loss of fertility. Cognitive-behavioral therapy may be helpful for menopausal symptoms, including hot flashes,4 as well as depressive symptoms.11
Although there are overlapping symptoms with both MDD and the perimenopause, these are typically restricted to impaired energy, sleep, and concentration, or changes in libido and weight.32 Therefore, it is vital to obtain a clear history and explore these symptoms in greater depth, as well as collect further information related to additional criteria such as appetite, agitation, feelings of worthlessness or guilt, and suicidal ideation.
Starting an antidepressant
On evaluation, Mrs. J discloses that she had experienced thoughts of wanting to end her life by overdose, although she had not acted on these thoughts. She appears subdued with poor eye contact, latency of response, and a slowed thought process. Mrs. J has blood tests to rule out thyroid abnormality or anemia. FSH and LH levels also are measured; these could provide a useful reference for later.
After a discussion with Mrs. J, she agrees to start an antidepressant. She also plans to speak to her gynecologist about the possibility of hormone replacement therapy. She is referred for psychotherapy to help support her with current life stressors. Mrs. J is started on escitalopram, 10 mg/d, and, after a month, she notices some improvement in her mood, psychomotor symptoms, sleep, and energy levels.
Bottom Line
Menopause is an important transition in our patients’ lives—both biologically and psychosocially. Women’s symptom patterns and medication needs may change during menopause.
Related Resource
- The North American Menopause Society. Depression & menopause. https://www.menopause.org/for-women/menopauseflashes/mental-health-at-menopause/depressionmenopause.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Raloxifene • Evista
Venlafaxine • Effexor
1. Bromberger JT, Kravitz HM. Mood and menopause: findings from the study of women’s health across the nation (SWAN) over 10 years. Obstet Gynecol Clin North Am. 2011;38(3):609-625.
2. Almeida OP, Marsh K, Flicker L, et al. Depressive symptoms in midlife: the role of reproductive stage. Menopause. 2016;23(6):669-765.
3. Sajatovic M, Friedman SH, Schuermeyer IN, et al. Menopause knowledge and subjective experience among peri- and postmenopausal women with bipolar disorder, schizophrenia and major depression. J Nerv Ment Dis. 2006;194(3):173-178.
4. Ayers BN, Forshaw MJ, Hunter MS. The menopause. The Psychologist. 2011;24:348-353.
5. Bromberger JT, Kravitz HM, Chang YF, et al. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychol Med. 2011;41(9):1879-1888.
6. Cohen LS, Soares CN, Vitonis AF, et al. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385-390.
7. Freeman EW, Sammel MD, Lin H, et al. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375-382.
8. Georgakis MK, Thomopoulos TP, Diamantaras AA, et al. Association of age at menopause and duration of reproductive period with depression after menopause: a systematic review and meta-analysis. JAMA Psychiatry 2016;73(2):139-149.
9. DiBonaventura MC, Wagner JS, Alvir J, et al. Depression, quality of life, work productivity, resource use, and costs among women experiencing menopause and hot flashes: a cross-sectional study [published online November 1, 2012]. Prim Care Companion CNS Disord. 2012;14(6): pii: PCC.12m01410. doi: 10.4088/PCC.12m01410.
10. Llaneza P, Garcia-Portilla MP, Llaneza-Suárez D, et al. Depressive disorders and the menopause transition. Maturitas. 2012;71(2):120-130.
11. Vivian-Taylor J, Hickey M. Menopause and depression: is there a link? Maturitas. 2014;79(2):142-146.
12. Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. 1: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29(2-3):85-96.
13. Friedman SH, Stankowski JE, Sajatovic M. Bipolar disorder in women. The Female Patient. 2007;32:15-24.
14. Soares C, Cohen L. The perimenopause, depressive disorders, and hormonal variability. Sao Paulo Med J. 2001;119(2):78-83.
15. Friedman SH, Sajatovic M, Schuermeyer IN, et al. Menopause-related quality of life in chronically mentally ill women. Int J Psychiatry Med. 2005;35(3):259-271.
16. Schmidt PJ, Ben Dor R, Martinez PE, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):714-726.
17. Carretti N, Florio P, Bertolin A et al. Serum fluctuations of total and free tryptophan levels during the menstrual cycle are related to gonadotrophins and reflect brain serotonin utilization. Hum Reprod. 2005;20(6):1548-1553.
18. Borrow AP, Cameron NM. Estrogenic mediation of serotonergic and neurotrophic systems: implications for female mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:13-25.
19. Hu LY, Shen CC, Hung JH et al. Risk of psychiatric disorders following symptomatic menopausal transition: a nationwide population-based retrospective cohort study. Medicine (Baltimore). 2016;95(6):e2800. doi: 10.1097/MD.0000000000002800.
20. Riecher-Rossler AW. Estrogens and schizophrenia. In: Bergemann N, Riecher-Rossler A, eds. Estrogen effects in psychiatric disorders. Wien, Austria: Springer-Verlag Wien; 2005:31-52.
21. Pope CJ, Oinonen K, Mazmanian D, et al. The hormonal sensitivity hypothesis: a review and new findings. Med Hypotheses. 2017;102:69-77.
22. Dennerstein L, Soares CN. The unique challenges of managing depression in mid-life women. World Psychiatry. 2008;7(3):137-142.
23. Caan B, LaCroix AZ, Joffe H, et al. Effects of estrogen and venlafaxine on menopause-related quality of life in healthy postmenopausal women with hot flashes: a placebo-controlled randomized trial. Menopause. 2015;22(6):607-615.
24. Seeman MV. Psychosis in women: Consider midlife medical and psychological triggers. Current Psychiatry. 2010;9(2):64-68,75-76.
25. Sajatovic M, Friedman SH, Sabharwal J, et al. Clinical characteristics and length of hospital stay among older adults with bipolar disorder, schizophrenia or schizoaffective disorder, depression, and dementia. J Geriatr Psychiatry Neurol. 2004;17(1):3-8.
26. Grover S, Talwar P, Baghel R, et al. Genetic variability in estrogen disposition: potential clinical implications for neuropsychiatric disorders. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(8):1391-1410.
27. Kulkarni J, Gavrilidis E, Wang W, et al. Estradiol for treatment-resistant schizophrenia: a large-scale randomized-controlled trial in women of child-bearing age. Mol Psychiatry. 2015;20(6):695-702.
28. Kulkarni J, Gavrilidis E, Gwini SM, et al. Effect of adjunctive raloxifene therapy on severity of refractory schizophrenia in women: a randomized clinical trial. JAMA Psychiatry. 2016;73(9):947-954.
29. Brzezinski A, Brzezinski-Sinai NA, Seeman MV. Treating schizophrenia during menopause. Menopause. 2017;24(5):582-588.
30. Lange B, Mueller JK, Leweke FM, et al. How gender affects the pharmacotherapeutic approach to treating psychosis - a systematic review. Expert Opin Pharmacother. 2017;18(4):351-362.
31. Ballard KD, Kuh DJ, Wadsworth MEJ. The role of the menopause in women’s experiences of the ‘change of life.’ Sociology of Health & Illness. 2001;23(4):397-424.
32. Clayton AH, Ninan PT. Depression or menopause? Presentation and management of major depressive disorder in perimenopausal and postmenopausal women. Prim Care Companion J Clin Psychiatry. 2010;12(1):PCC.08r00747. doi: 10.4088/PCC.08r00747blu.
Mrs. J, age 49, presents to your psychiatric clinic. For the last few years, she has been experiencing night sweats and hot flashes, which she has attributed to being perimenopausal. Over the last year, she has noticed that her mood has declined; however, she has suffered several life events that she feels have contributed. Her mother was diagnosed with Alzheimer’s disease and had to move into a nursing home, which Mrs. J found very stressful. At the same time, her daughter left home for college, and her son is exploring his college options. Recently, Mrs. J has not been able to work due to her mood, and she is afraid she may lose her job as a consequence. She has struggled to talk to her husband about how she is feeling, and feels increasingly isolated. Over the last month, she has had increased problems sleeping and less energy; some days she struggles to get out of bed. She is finding it difficult to concentrate and is more forgetful. She has lost interest in her hobbies and is no longer meeting with her friends. She has no history of depression or anxiety, although she recalls feeling very low in mood for months after the birth of each of her children.
Are Mrs. J’s symptoms related to menopause or depression? What further investigations are necessary? Would you modify your treatment plan because of her menopausal status?
Women are at elevated risk of developing psychiatric symptoms and disorders throughout their reproductive lives, including during menopause. Menopause is a time of life transition, when women may experience multiple physical symptoms, including vasomotor symptoms (night sweats and hot flashes), sexual symptoms, and sleep difficulties. Depressive symptoms occur more frequently during menopause, and symptoms of schizophrenia may worsen.
Estrogen plays a role in mental illness throughout a woman’s life. In menopause, decreasing estrogen levels may correlate with increased mood symptoms, physical symptoms, and psychotic symptoms. As such, psychiatrists should consider whether collaboration regarding adjunctive hormone replacement therapy would be beneficial, and whether the benefits outweigh the potential risks. Otherwise, treatment of depression in menopause is similar to treatment outside of the menopausal transition, though serotonergic antidepressants may help target vasomotor symptoms while therapy may focus on role transition and loss. In this article, we review why women are at increased risk for mental illness during menopause, the role of estrogen, and treatment of mood and psychotic disorders during this phase of a woman’s life.
Increased vulnerability across the lifespan
Continued to: Why menopause?
Why menopause?
Perimenopausal mood disorders
However, one should keep in mind that new-onset mania in menopause is rare and should trigger a medical work-up and a dementia evaluation.13 Table 414 provides recommendations for evaluation of women undergoing menopause.
Menopause and serious mental illnesses
A study of 91 perimenopausal and postmenopausal women (age 45 to 55) who were diagnosed with schizophrenia/schizoaffective disorder, bipolar disorder, or major depressive disorder (MDD) found that women with severe mental illness experienced significant vasomotor, physical, sexual, and psychosocial symptoms related to menopause.15 Furthermore, on 7 of 29 items on the Menopause Specific Quality of Life Scale, including hot flashes, women diagnosed with MDD reported problems significantly more often than women with other serious mental illnesses.15
Women with serious mental illness often have deficits in their knowledge about menopause.3 More than half of the 91 women in the study diagnosed with schizophrenia/schizoaffective disorder, bipolar disorder, or MDD felt more stressed related to menopause, and reported that menopause had a negative effect on their mental health.3 These women rated their top 5 symptoms potentially related to menopause as feeling depressed, anxious, or tired; lacking energy; and experiencing poor memory.3
Continued to: Role of estrogen on mood and psychosis
Role of estrogen on mood and psychosis
Women are at higher risk throughout their reproductive life than are men for MDD, anxiety disorders, and trauma-related disorders.12 Factors associated with depression during the menopause transition are reproductive hormonal changes (rise of follicle-stimulating hormone [FSH] and luteinizing hormone levels, and variability in estrogen [E2] and FSH levels); menopausal symptoms, particularly vasomotor symptoms; prior depression; psychosocial factors (adverse life events, financial strain, poor social supports); high body mass index, smoking, and poor physical health.6,7 Decreasing estrogen in the menopause transition may increase susceptibility to depression in some women.16 The Box17,18 provides more information on the relationship between estrogen and brain function.
Box
Estrogen and brain function
Numerous molecular and clinical studies have established the role of 17-beta estradiol in modulating brain functions via alterations in neurotransmission.17 Estrogen increases serotonin availability in the synapse by various pathways. It increases the rate of degradation of monoamine oxidase; monoamine oxidase enzymes are responsible for catabolizing serotonin, dopamine, and norepinephrine. Estrogen also increases tryptophan hydroxylase expression (rate-limiting enzyme in serotonin synthesis) and promotes intraneuronal serotonin transport in brain regions associated with affect regulation by increasing gene expression of the serotonin reuptake transporter. Studies have linked brain-derived neurotropic factor (BDNF) to increased serotonin turnover and proposed that estrogen may influence depression by increasing BDNF levels within the brain.18
Depressive disorders, including premenstrual dysphoric disorder, postpartum depression, and perimenopausal depression, have been linked to changes in hormonal status in women. Symptomatic menopause transition occurs in at least 20% of women, and a retrospective cohort study suggests that symptomatic menopause transition might increase the risk of new-onset depressive disorders, bipolar disorders, anxiety disorders, and sleep disorders.19 Symptomatic menopause transition also is a vulnerable time for relapse of MDD. Among women experiencing menopausal symptoms, including hot flashes, one-third also report depression—which correlates with a poorer quality of life, less work productivity, and greater use of health care services.9
Women who undergo surgical menopause are at greater risk for depression.8,10,11 This may be due to abrupt deprivation of estrogen—or related to a psychological reaction to the loss of fertility.
The observation that hormonal fluctuations related to women’s reproductive cycle have a significant impact on psychotic symptomatology has resulted in the “hypo-estrogenism hypothesis,” which proposes that gonadal dysfunction may increase vulnerability to schizophrenia, or that schizophrenia may lead to gonadal dysfunction.20 The “estrogen protection hypothesis” proposes that estrogen may protect women from schizophrenia, and may be a factor in the delayed onset of schizophrenia compared with men, less severe psychopathology, better outcomes, and premenstrual and postmenopausal deterioration in women. Many women of reproductive age with schizophrenia experience improvement in symptoms during the high estrogen phase of their menstrual cycle.
Pope et al21 have suggested that a hormone sensitivity syndrome may underlie why some women experience physical, psychological, and emotional symptoms at times of hormonal shifts such as menopause. This may represent a critical window of vulnerability, and also an opportunity to consider E2 as a therapeutic intervention.
Continued to: Treating mental illness in menopause
Treating mental illness in menopause
Changes to drug pharmacokinetics occur because some metabolising enzymes are estrogen-dependent and their levels decline after menopause, which leads to greater variability in drug response, particularly for oral medications. Other factors that can contribute to variability in medication response are polypharmacy, alcohol, illicit drugs, liver mass, smoking, caffeine, and nutritional intake.
While antidepressants are the first-line treatment for MDD and anxiety disorders, some patients remain unresponsive or inadequately responsive to currently available medications. In perimenopausal women with MDD, there may be an indication for adjunctive therapy with transdermal E2 in refractory cases; estrogen may augment the effects of selective serotonin reuptake inhibitor (SSRI) antidepressants as well as hasten the onset of antidepressant action.22 Estrogen also may be worth considering in women with mild depressive symptoms. For MDD, SSRIs plus estrogen may be more beneficial in improving mood than either agent alone. The effectiveness of E2 is less certain in postmenopausal depression.
Hormonal therapy for mental health disorders has equivocal evidence. The individual’s history and risk factors (eg, cardiovascular and osteoporosis risks) must be considered. A recent trial found that treatment with either venlafaxine or low-dose estrogen improved quality of life in menopausal women with vasomotor symptoms.23 Venlafaxine improved the psychosocial domain, while estrogen improved quality of life in other domains. Escitalopram, duloxetine, and citalopram have also been identified as having a possible positive impact on menopausal symptoms.22 SSRIs and serotonin-norepinephrine reuptake inhibitors may help reduce hot flashes and improve sleep.11
Regarding schizophrenia and estrogen, there may be improved symptoms during the high estrogen phase of the menstrual cycle, followed by a premenstrual aggravation of symptoms. Recall that women have a second peak of onset of schizophrenia after age 45, around the age of the onset of menopause.24 In a study of geropsychiatric hospital admissions, women were overrepresented among those with schizophrenia and schizoaffective disorder, compared with other psychiatric disorders.25 Postmenopausally, some women experience a decreased responsiveness to antipsychotics and worsening symptoms. In menopausal women with schizophrenia, check prolactin levels to help determine whether they are experiencing a natural menopause or medication-induced amenorrhea. Gender differences in pharmacotherapy responses and the decreasing response to antipsychotics in women older than age 50 have been observed26 and have led to exploration of the role of estrogen for treating schizophrenia in menopausal women. There have been contradictory results regarding use of estrogen as an adjunct to antipsychotics, with some reports finding this approach is effective and results in lower average doses of antipsychotics. Kulkarni et al27,28 have reported improvements in positive symptoms of treatment-resistant schizophrenia with transdermal use of E2, 200 mcg, as an adjunct to antipsychotics in women of childbearing age. However, they expressed caution regarding the health risks associated with prolonged use of E2. Long-term risks of high-dose estrogen therapies include thromboembolism, endometrial hyperplasia, and breast cancer, and individual factors should be considered before starting any form of hormone therapy. Selective estrogen receptor modulators (SERMs), such as raloxifene, which can cause activation of E2 receptors in a tissue-specific fashion and have less estrogen-related adverse effects, offer hope for future development in this field.27,28 While the use of adjunctive hormone therapy to manage psychotic symptoms in menopause is not routinely advised, the dosages of previously effective antipsychotics may need to be reviewed, or long-acting depot routes considered.29 Increased risk of prolonged QTc interval and tardive dyskinesia in geriatric women also should be considered in decisions regarding changes to antipsychotics or dosages.30
There are no guidelines regarding change in dosage of either individual antidepressants or antipsychotics in women at the time of menopause for managing pre-existing conditions. This may be due to the high variability in the effect of menopause on mental health and recognition that menopause is also a time for deterioration in physical health, as well as psychosocial changes for women, and thus other forms of intervention need to be considered.
Continued to: The biopsychosocial approach to treatment...
The biopsychosocial approach to treatment is particularly important in menopause.11 Common transitions in midlife include changes in relationships, employment, and financial status, and illness or death of family and friends.31 Therapy may focus on accepting a role transition and coping with loss of fertility. Cognitive-behavioral therapy may be helpful for menopausal symptoms, including hot flashes,4 as well as depressive symptoms.11
Although there are overlapping symptoms with both MDD and the perimenopause, these are typically restricted to impaired energy, sleep, and concentration, or changes in libido and weight.32 Therefore, it is vital to obtain a clear history and explore these symptoms in greater depth, as well as collect further information related to additional criteria such as appetite, agitation, feelings of worthlessness or guilt, and suicidal ideation.
Starting an antidepressant
On evaluation, Mrs. J discloses that she had experienced thoughts of wanting to end her life by overdose, although she had not acted on these thoughts. She appears subdued with poor eye contact, latency of response, and a slowed thought process. Mrs. J has blood tests to rule out thyroid abnormality or anemia. FSH and LH levels also are measured; these could provide a useful reference for later.
After a discussion with Mrs. J, she agrees to start an antidepressant. She also plans to speak to her gynecologist about the possibility of hormone replacement therapy. She is referred for psychotherapy to help support her with current life stressors. Mrs. J is started on escitalopram, 10 mg/d, and, after a month, she notices some improvement in her mood, psychomotor symptoms, sleep, and energy levels.
Bottom Line
Menopause is an important transition in our patients’ lives—both biologically and psychosocially. Women’s symptom patterns and medication needs may change during menopause.
Related Resource
- The North American Menopause Society. Depression & menopause. https://www.menopause.org/for-women/menopauseflashes/mental-health-at-menopause/depressionmenopause.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Raloxifene • Evista
Venlafaxine • Effexor
Mrs. J, age 49, presents to your psychiatric clinic. For the last few years, she has been experiencing night sweats and hot flashes, which she has attributed to being perimenopausal. Over the last year, she has noticed that her mood has declined; however, she has suffered several life events that she feels have contributed. Her mother was diagnosed with Alzheimer’s disease and had to move into a nursing home, which Mrs. J found very stressful. At the same time, her daughter left home for college, and her son is exploring his college options. Recently, Mrs. J has not been able to work due to her mood, and she is afraid she may lose her job as a consequence. She has struggled to talk to her husband about how she is feeling, and feels increasingly isolated. Over the last month, she has had increased problems sleeping and less energy; some days she struggles to get out of bed. She is finding it difficult to concentrate and is more forgetful. She has lost interest in her hobbies and is no longer meeting with her friends. She has no history of depression or anxiety, although she recalls feeling very low in mood for months after the birth of each of her children.
Are Mrs. J’s symptoms related to menopause or depression? What further investigations are necessary? Would you modify your treatment plan because of her menopausal status?
Women are at elevated risk of developing psychiatric symptoms and disorders throughout their reproductive lives, including during menopause. Menopause is a time of life transition, when women may experience multiple physical symptoms, including vasomotor symptoms (night sweats and hot flashes), sexual symptoms, and sleep difficulties. Depressive symptoms occur more frequently during menopause, and symptoms of schizophrenia may worsen.
Estrogen plays a role in mental illness throughout a woman’s life. In menopause, decreasing estrogen levels may correlate with increased mood symptoms, physical symptoms, and psychotic symptoms. As such, psychiatrists should consider whether collaboration regarding adjunctive hormone replacement therapy would be beneficial, and whether the benefits outweigh the potential risks. Otherwise, treatment of depression in menopause is similar to treatment outside of the menopausal transition, though serotonergic antidepressants may help target vasomotor symptoms while therapy may focus on role transition and loss. In this article, we review why women are at increased risk for mental illness during menopause, the role of estrogen, and treatment of mood and psychotic disorders during this phase of a woman’s life.
Increased vulnerability across the lifespan
Continued to: Why menopause?
Why menopause?
Perimenopausal mood disorders
However, one should keep in mind that new-onset mania in menopause is rare and should trigger a medical work-up and a dementia evaluation.13 Table 414 provides recommendations for evaluation of women undergoing menopause.
Menopause and serious mental illnesses
A study of 91 perimenopausal and postmenopausal women (age 45 to 55) who were diagnosed with schizophrenia/schizoaffective disorder, bipolar disorder, or major depressive disorder (MDD) found that women with severe mental illness experienced significant vasomotor, physical, sexual, and psychosocial symptoms related to menopause.15 Furthermore, on 7 of 29 items on the Menopause Specific Quality of Life Scale, including hot flashes, women diagnosed with MDD reported problems significantly more often than women with other serious mental illnesses.15
Women with serious mental illness often have deficits in their knowledge about menopause.3 More than half of the 91 women in the study diagnosed with schizophrenia/schizoaffective disorder, bipolar disorder, or MDD felt more stressed related to menopause, and reported that menopause had a negative effect on their mental health.3 These women rated their top 5 symptoms potentially related to menopause as feeling depressed, anxious, or tired; lacking energy; and experiencing poor memory.3
Continued to: Role of estrogen on mood and psychosis
Role of estrogen on mood and psychosis
Women are at higher risk throughout their reproductive life than are men for MDD, anxiety disorders, and trauma-related disorders.12 Factors associated with depression during the menopause transition are reproductive hormonal changes (rise of follicle-stimulating hormone [FSH] and luteinizing hormone levels, and variability in estrogen [E2] and FSH levels); menopausal symptoms, particularly vasomotor symptoms; prior depression; psychosocial factors (adverse life events, financial strain, poor social supports); high body mass index, smoking, and poor physical health.6,7 Decreasing estrogen in the menopause transition may increase susceptibility to depression in some women.16 The Box17,18 provides more information on the relationship between estrogen and brain function.
Box
Estrogen and brain function
Numerous molecular and clinical studies have established the role of 17-beta estradiol in modulating brain functions via alterations in neurotransmission.17 Estrogen increases serotonin availability in the synapse by various pathways. It increases the rate of degradation of monoamine oxidase; monoamine oxidase enzymes are responsible for catabolizing serotonin, dopamine, and norepinephrine. Estrogen also increases tryptophan hydroxylase expression (rate-limiting enzyme in serotonin synthesis) and promotes intraneuronal serotonin transport in brain regions associated with affect regulation by increasing gene expression of the serotonin reuptake transporter. Studies have linked brain-derived neurotropic factor (BDNF) to increased serotonin turnover and proposed that estrogen may influence depression by increasing BDNF levels within the brain.18
Depressive disorders, including premenstrual dysphoric disorder, postpartum depression, and perimenopausal depression, have been linked to changes in hormonal status in women. Symptomatic menopause transition occurs in at least 20% of women, and a retrospective cohort study suggests that symptomatic menopause transition might increase the risk of new-onset depressive disorders, bipolar disorders, anxiety disorders, and sleep disorders.19 Symptomatic menopause transition also is a vulnerable time for relapse of MDD. Among women experiencing menopausal symptoms, including hot flashes, one-third also report depression—which correlates with a poorer quality of life, less work productivity, and greater use of health care services.9
Women who undergo surgical menopause are at greater risk for depression.8,10,11 This may be due to abrupt deprivation of estrogen—or related to a psychological reaction to the loss of fertility.
The observation that hormonal fluctuations related to women’s reproductive cycle have a significant impact on psychotic symptomatology has resulted in the “hypo-estrogenism hypothesis,” which proposes that gonadal dysfunction may increase vulnerability to schizophrenia, or that schizophrenia may lead to gonadal dysfunction.20 The “estrogen protection hypothesis” proposes that estrogen may protect women from schizophrenia, and may be a factor in the delayed onset of schizophrenia compared with men, less severe psychopathology, better outcomes, and premenstrual and postmenopausal deterioration in women. Many women of reproductive age with schizophrenia experience improvement in symptoms during the high estrogen phase of their menstrual cycle.
Pope et al21 have suggested that a hormone sensitivity syndrome may underlie why some women experience physical, psychological, and emotional symptoms at times of hormonal shifts such as menopause. This may represent a critical window of vulnerability, and also an opportunity to consider E2 as a therapeutic intervention.
Continued to: Treating mental illness in menopause
Treating mental illness in menopause
Changes to drug pharmacokinetics occur because some metabolising enzymes are estrogen-dependent and their levels decline after menopause, which leads to greater variability in drug response, particularly for oral medications. Other factors that can contribute to variability in medication response are polypharmacy, alcohol, illicit drugs, liver mass, smoking, caffeine, and nutritional intake.
While antidepressants are the first-line treatment for MDD and anxiety disorders, some patients remain unresponsive or inadequately responsive to currently available medications. In perimenopausal women with MDD, there may be an indication for adjunctive therapy with transdermal E2 in refractory cases; estrogen may augment the effects of selective serotonin reuptake inhibitor (SSRI) antidepressants as well as hasten the onset of antidepressant action.22 Estrogen also may be worth considering in women with mild depressive symptoms. For MDD, SSRIs plus estrogen may be more beneficial in improving mood than either agent alone. The effectiveness of E2 is less certain in postmenopausal depression.
Hormonal therapy for mental health disorders has equivocal evidence. The individual’s history and risk factors (eg, cardiovascular and osteoporosis risks) must be considered. A recent trial found that treatment with either venlafaxine or low-dose estrogen improved quality of life in menopausal women with vasomotor symptoms.23 Venlafaxine improved the psychosocial domain, while estrogen improved quality of life in other domains. Escitalopram, duloxetine, and citalopram have also been identified as having a possible positive impact on menopausal symptoms.22 SSRIs and serotonin-norepinephrine reuptake inhibitors may help reduce hot flashes and improve sleep.11
Regarding schizophrenia and estrogen, there may be improved symptoms during the high estrogen phase of the menstrual cycle, followed by a premenstrual aggravation of symptoms. Recall that women have a second peak of onset of schizophrenia after age 45, around the age of the onset of menopause.24 In a study of geropsychiatric hospital admissions, women were overrepresented among those with schizophrenia and schizoaffective disorder, compared with other psychiatric disorders.25 Postmenopausally, some women experience a decreased responsiveness to antipsychotics and worsening symptoms. In menopausal women with schizophrenia, check prolactin levels to help determine whether they are experiencing a natural menopause or medication-induced amenorrhea. Gender differences in pharmacotherapy responses and the decreasing response to antipsychotics in women older than age 50 have been observed26 and have led to exploration of the role of estrogen for treating schizophrenia in menopausal women. There have been contradictory results regarding use of estrogen as an adjunct to antipsychotics, with some reports finding this approach is effective and results in lower average doses of antipsychotics. Kulkarni et al27,28 have reported improvements in positive symptoms of treatment-resistant schizophrenia with transdermal use of E2, 200 mcg, as an adjunct to antipsychotics in women of childbearing age. However, they expressed caution regarding the health risks associated with prolonged use of E2. Long-term risks of high-dose estrogen therapies include thromboembolism, endometrial hyperplasia, and breast cancer, and individual factors should be considered before starting any form of hormone therapy. Selective estrogen receptor modulators (SERMs), such as raloxifene, which can cause activation of E2 receptors in a tissue-specific fashion and have less estrogen-related adverse effects, offer hope for future development in this field.27,28 While the use of adjunctive hormone therapy to manage psychotic symptoms in menopause is not routinely advised, the dosages of previously effective antipsychotics may need to be reviewed, or long-acting depot routes considered.29 Increased risk of prolonged QTc interval and tardive dyskinesia in geriatric women also should be considered in decisions regarding changes to antipsychotics or dosages.30
There are no guidelines regarding change in dosage of either individual antidepressants or antipsychotics in women at the time of menopause for managing pre-existing conditions. This may be due to the high variability in the effect of menopause on mental health and recognition that menopause is also a time for deterioration in physical health, as well as psychosocial changes for women, and thus other forms of intervention need to be considered.
Continued to: The biopsychosocial approach to treatment...
The biopsychosocial approach to treatment is particularly important in menopause.11 Common transitions in midlife include changes in relationships, employment, and financial status, and illness or death of family and friends.31 Therapy may focus on accepting a role transition and coping with loss of fertility. Cognitive-behavioral therapy may be helpful for menopausal symptoms, including hot flashes,4 as well as depressive symptoms.11
Although there are overlapping symptoms with both MDD and the perimenopause, these are typically restricted to impaired energy, sleep, and concentration, or changes in libido and weight.32 Therefore, it is vital to obtain a clear history and explore these symptoms in greater depth, as well as collect further information related to additional criteria such as appetite, agitation, feelings of worthlessness or guilt, and suicidal ideation.
Starting an antidepressant
On evaluation, Mrs. J discloses that she had experienced thoughts of wanting to end her life by overdose, although she had not acted on these thoughts. She appears subdued with poor eye contact, latency of response, and a slowed thought process. Mrs. J has blood tests to rule out thyroid abnormality or anemia. FSH and LH levels also are measured; these could provide a useful reference for later.
After a discussion with Mrs. J, she agrees to start an antidepressant. She also plans to speak to her gynecologist about the possibility of hormone replacement therapy. She is referred for psychotherapy to help support her with current life stressors. Mrs. J is started on escitalopram, 10 mg/d, and, after a month, she notices some improvement in her mood, psychomotor symptoms, sleep, and energy levels.
Bottom Line
Menopause is an important transition in our patients’ lives—both biologically and psychosocially. Women’s symptom patterns and medication needs may change during menopause.
Related Resource
- The North American Menopause Society. Depression & menopause. https://www.menopause.org/for-women/menopauseflashes/mental-health-at-menopause/depressionmenopause.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Raloxifene • Evista
Venlafaxine • Effexor
1. Bromberger JT, Kravitz HM. Mood and menopause: findings from the study of women’s health across the nation (SWAN) over 10 years. Obstet Gynecol Clin North Am. 2011;38(3):609-625.
2. Almeida OP, Marsh K, Flicker L, et al. Depressive symptoms in midlife: the role of reproductive stage. Menopause. 2016;23(6):669-765.
3. Sajatovic M, Friedman SH, Schuermeyer IN, et al. Menopause knowledge and subjective experience among peri- and postmenopausal women with bipolar disorder, schizophrenia and major depression. J Nerv Ment Dis. 2006;194(3):173-178.
4. Ayers BN, Forshaw MJ, Hunter MS. The menopause. The Psychologist. 2011;24:348-353.
5. Bromberger JT, Kravitz HM, Chang YF, et al. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychol Med. 2011;41(9):1879-1888.
6. Cohen LS, Soares CN, Vitonis AF, et al. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385-390.
7. Freeman EW, Sammel MD, Lin H, et al. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375-382.
8. Georgakis MK, Thomopoulos TP, Diamantaras AA, et al. Association of age at menopause and duration of reproductive period with depression after menopause: a systematic review and meta-analysis. JAMA Psychiatry 2016;73(2):139-149.
9. DiBonaventura MC, Wagner JS, Alvir J, et al. Depression, quality of life, work productivity, resource use, and costs among women experiencing menopause and hot flashes: a cross-sectional study [published online November 1, 2012]. Prim Care Companion CNS Disord. 2012;14(6): pii: PCC.12m01410. doi: 10.4088/PCC.12m01410.
10. Llaneza P, Garcia-Portilla MP, Llaneza-Suárez D, et al. Depressive disorders and the menopause transition. Maturitas. 2012;71(2):120-130.
11. Vivian-Taylor J, Hickey M. Menopause and depression: is there a link? Maturitas. 2014;79(2):142-146.
12. Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. 1: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29(2-3):85-96.
13. Friedman SH, Stankowski JE, Sajatovic M. Bipolar disorder in women. The Female Patient. 2007;32:15-24.
14. Soares C, Cohen L. The perimenopause, depressive disorders, and hormonal variability. Sao Paulo Med J. 2001;119(2):78-83.
15. Friedman SH, Sajatovic M, Schuermeyer IN, et al. Menopause-related quality of life in chronically mentally ill women. Int J Psychiatry Med. 2005;35(3):259-271.
16. Schmidt PJ, Ben Dor R, Martinez PE, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):714-726.
17. Carretti N, Florio P, Bertolin A et al. Serum fluctuations of total and free tryptophan levels during the menstrual cycle are related to gonadotrophins and reflect brain serotonin utilization. Hum Reprod. 2005;20(6):1548-1553.
18. Borrow AP, Cameron NM. Estrogenic mediation of serotonergic and neurotrophic systems: implications for female mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:13-25.
19. Hu LY, Shen CC, Hung JH et al. Risk of psychiatric disorders following symptomatic menopausal transition: a nationwide population-based retrospective cohort study. Medicine (Baltimore). 2016;95(6):e2800. doi: 10.1097/MD.0000000000002800.
20. Riecher-Rossler AW. Estrogens and schizophrenia. In: Bergemann N, Riecher-Rossler A, eds. Estrogen effects in psychiatric disorders. Wien, Austria: Springer-Verlag Wien; 2005:31-52.
21. Pope CJ, Oinonen K, Mazmanian D, et al. The hormonal sensitivity hypothesis: a review and new findings. Med Hypotheses. 2017;102:69-77.
22. Dennerstein L, Soares CN. The unique challenges of managing depression in mid-life women. World Psychiatry. 2008;7(3):137-142.
23. Caan B, LaCroix AZ, Joffe H, et al. Effects of estrogen and venlafaxine on menopause-related quality of life in healthy postmenopausal women with hot flashes: a placebo-controlled randomized trial. Menopause. 2015;22(6):607-615.
24. Seeman MV. Psychosis in women: Consider midlife medical and psychological triggers. Current Psychiatry. 2010;9(2):64-68,75-76.
25. Sajatovic M, Friedman SH, Sabharwal J, et al. Clinical characteristics and length of hospital stay among older adults with bipolar disorder, schizophrenia or schizoaffective disorder, depression, and dementia. J Geriatr Psychiatry Neurol. 2004;17(1):3-8.
26. Grover S, Talwar P, Baghel R, et al. Genetic variability in estrogen disposition: potential clinical implications for neuropsychiatric disorders. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(8):1391-1410.
27. Kulkarni J, Gavrilidis E, Wang W, et al. Estradiol for treatment-resistant schizophrenia: a large-scale randomized-controlled trial in women of child-bearing age. Mol Psychiatry. 2015;20(6):695-702.
28. Kulkarni J, Gavrilidis E, Gwini SM, et al. Effect of adjunctive raloxifene therapy on severity of refractory schizophrenia in women: a randomized clinical trial. JAMA Psychiatry. 2016;73(9):947-954.
29. Brzezinski A, Brzezinski-Sinai NA, Seeman MV. Treating schizophrenia during menopause. Menopause. 2017;24(5):582-588.
30. Lange B, Mueller JK, Leweke FM, et al. How gender affects the pharmacotherapeutic approach to treating psychosis - a systematic review. Expert Opin Pharmacother. 2017;18(4):351-362.
31. Ballard KD, Kuh DJ, Wadsworth MEJ. The role of the menopause in women’s experiences of the ‘change of life.’ Sociology of Health & Illness. 2001;23(4):397-424.
32. Clayton AH, Ninan PT. Depression or menopause? Presentation and management of major depressive disorder in perimenopausal and postmenopausal women. Prim Care Companion J Clin Psychiatry. 2010;12(1):PCC.08r00747. doi: 10.4088/PCC.08r00747blu.
1. Bromberger JT, Kravitz HM. Mood and menopause: findings from the study of women’s health across the nation (SWAN) over 10 years. Obstet Gynecol Clin North Am. 2011;38(3):609-625.
2. Almeida OP, Marsh K, Flicker L, et al. Depressive symptoms in midlife: the role of reproductive stage. Menopause. 2016;23(6):669-765.
3. Sajatovic M, Friedman SH, Schuermeyer IN, et al. Menopause knowledge and subjective experience among peri- and postmenopausal women with bipolar disorder, schizophrenia and major depression. J Nerv Ment Dis. 2006;194(3):173-178.
4. Ayers BN, Forshaw MJ, Hunter MS. The menopause. The Psychologist. 2011;24:348-353.
5. Bromberger JT, Kravitz HM, Chang YF, et al. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychol Med. 2011;41(9):1879-1888.
6. Cohen LS, Soares CN, Vitonis AF, et al. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385-390.
7. Freeman EW, Sammel MD, Lin H, et al. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375-382.
8. Georgakis MK, Thomopoulos TP, Diamantaras AA, et al. Association of age at menopause and duration of reproductive period with depression after menopause: a systematic review and meta-analysis. JAMA Psychiatry 2016;73(2):139-149.
9. DiBonaventura MC, Wagner JS, Alvir J, et al. Depression, quality of life, work productivity, resource use, and costs among women experiencing menopause and hot flashes: a cross-sectional study [published online November 1, 2012]. Prim Care Companion CNS Disord. 2012;14(6): pii: PCC.12m01410. doi: 10.4088/PCC.12m01410.
10. Llaneza P, Garcia-Portilla MP, Llaneza-Suárez D, et al. Depressive disorders and the menopause transition. Maturitas. 2012;71(2):120-130.
11. Vivian-Taylor J, Hickey M. Menopause and depression: is there a link? Maturitas. 2014;79(2):142-146.
12. Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. 1: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29(2-3):85-96.
13. Friedman SH, Stankowski JE, Sajatovic M. Bipolar disorder in women. The Female Patient. 2007;32:15-24.
14. Soares C, Cohen L. The perimenopause, depressive disorders, and hormonal variability. Sao Paulo Med J. 2001;119(2):78-83.
15. Friedman SH, Sajatovic M, Schuermeyer IN, et al. Menopause-related quality of life in chronically mentally ill women. Int J Psychiatry Med. 2005;35(3):259-271.
16. Schmidt PJ, Ben Dor R, Martinez PE, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):714-726.
17. Carretti N, Florio P, Bertolin A et al. Serum fluctuations of total and free tryptophan levels during the menstrual cycle are related to gonadotrophins and reflect brain serotonin utilization. Hum Reprod. 2005;20(6):1548-1553.
18. Borrow AP, Cameron NM. Estrogenic mediation of serotonergic and neurotrophic systems: implications for female mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:13-25.
19. Hu LY, Shen CC, Hung JH et al. Risk of psychiatric disorders following symptomatic menopausal transition: a nationwide population-based retrospective cohort study. Medicine (Baltimore). 2016;95(6):e2800. doi: 10.1097/MD.0000000000002800.
20. Riecher-Rossler AW. Estrogens and schizophrenia. In: Bergemann N, Riecher-Rossler A, eds. Estrogen effects in psychiatric disorders. Wien, Austria: Springer-Verlag Wien; 2005:31-52.
21. Pope CJ, Oinonen K, Mazmanian D, et al. The hormonal sensitivity hypothesis: a review and new findings. Med Hypotheses. 2017;102:69-77.
22. Dennerstein L, Soares CN. The unique challenges of managing depression in mid-life women. World Psychiatry. 2008;7(3):137-142.
23. Caan B, LaCroix AZ, Joffe H, et al. Effects of estrogen and venlafaxine on menopause-related quality of life in healthy postmenopausal women with hot flashes: a placebo-controlled randomized trial. Menopause. 2015;22(6):607-615.
24. Seeman MV. Psychosis in women: Consider midlife medical and psychological triggers. Current Psychiatry. 2010;9(2):64-68,75-76.
25. Sajatovic M, Friedman SH, Sabharwal J, et al. Clinical characteristics and length of hospital stay among older adults with bipolar disorder, schizophrenia or schizoaffective disorder, depression, and dementia. J Geriatr Psychiatry Neurol. 2004;17(1):3-8.
26. Grover S, Talwar P, Baghel R, et al. Genetic variability in estrogen disposition: potential clinical implications for neuropsychiatric disorders. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(8):1391-1410.
27. Kulkarni J, Gavrilidis E, Wang W, et al. Estradiol for treatment-resistant schizophrenia: a large-scale randomized-controlled trial in women of child-bearing age. Mol Psychiatry. 2015;20(6):695-702.
28. Kulkarni J, Gavrilidis E, Gwini SM, et al. Effect of adjunctive raloxifene therapy on severity of refractory schizophrenia in women: a randomized clinical trial. JAMA Psychiatry. 2016;73(9):947-954.
29. Brzezinski A, Brzezinski-Sinai NA, Seeman MV. Treating schizophrenia during menopause. Menopause. 2017;24(5):582-588.
30. Lange B, Mueller JK, Leweke FM, et al. How gender affects the pharmacotherapeutic approach to treating psychosis - a systematic review. Expert Opin Pharmacother. 2017;18(4):351-362.
31. Ballard KD, Kuh DJ, Wadsworth MEJ. The role of the menopause in women’s experiences of the ‘change of life.’ Sociology of Health & Illness. 2001;23(4):397-424.
32. Clayton AH, Ninan PT. Depression or menopause? Presentation and management of major depressive disorder in perimenopausal and postmenopausal women. Prim Care Companion J Clin Psychiatry. 2010;12(1):PCC.08r00747. doi: 10.4088/PCC.08r00747blu.
Unrelenting depression: ‘I would rather be dead than feel this way’
CASE Suicidal ideation, flare-up of ulcerative colitis
Mr. J, age 56, who has a history of major depressive disorder (MDD), generalized anxiety disorder (GAD), and ulcerative colitis (UC), presents to the emergency department (ED) with suicidal ideation and a plan to overdose on his medications. He reports no current emotional or financial stressors in his personal life. Home medications documented at the time of his arrival to the ED include sertraline, 100 mg/d, bupropion, 150 mg/d, buspirone, 10 mg 3 times daily, diazepam 10 mg 3 times daily, as needed, adalimumab, 40 mg IM every 2 weeks, and diphenhydramine, 50 mg every night.
A recent flare-up of UC resulted in Mr. J being placed on a 15-week prednisone taper, beginning at 80 mg/d and decreasing by 5 mg weekly, which was completed 2 weeks before he presented to the ED. After completing the prednisone taper, Mr. J went to his primary care physician (PCP) on 3 separate occasions due to episodes of severe depression. Although the PCP prescribed multiple medications to target Mr. J’s depressive symptoms, he continued to decline.
Subsequently, Mr. J came to the ED and is admitted to the psychiatric unit for safety and stabilization. Upon admission, Mr. J becomes bedridden, and reports that his current depressive episode is the most severe that he has ever experienced in his more than 30 years of having MDD. He says that neither bupropion nor buspirone are helping with his depression, anxiety, or any related symptom.
[polldaddy:10120537]
The authors’ observations
At admission, all of Mr. J’s home medications, except sertraline and adalimumab, which had been prescribed to treat UC (Box1,2), were discontinued. His diazepam was discontinued because the clinician felt it may have been contributing to Mr. J’s inability to walk or get out of bed. Diazepam was not tapered because it was initiated 7 days prior to admission and was thought to be exacerbating his depression and suicidal ideation. Bupropion and buspirone, which were initiated 2 weeks prior, were discontinued because Mr. J reported that neither medication was helping with his depression, anxiety, or any related symptom.
Box
Ulcerative colitis and depressive episodes
Ulcerative colitis (UC) is a chronic condition associated with inflammation in the colon causing extreme abdominal discomfort during acute flare-ups. Moderate to severe UC flare-ups are commonly treated with corticosteroids due to these medications’ anti-inflammatory properties. Although rare, corticosteroid withdrawal has been documented to induce episodes of depression. The pathophysiology of corticosteroid withdrawal inducing neuropsychiatric sequelae remains unclear; however, it is thought to be due to hypothalamic-pituitary-adrenocortical suppression.1 Fardet et al2 concluded that incident rates per 100 person-years at risk during the withdrawal period were 11.1 (95% confidence interval, 10.0, 12.3) for depression.
EVALUATION Poor appetite, anxiety, and continued suicidality
During evaluation, vital signs, laboratory findings, and diagnostic testing are found to be unremarkable. Mr. J’s presentation and complaints are entirely subjective, and include poor appetite, fatigue, difficulty sleeping, sorrow, anxiety, and continued suicidality. Mr. J reports that he feels miserable, which is reflected by his poor eye contact, soft speech, and body language.
Continued to: The authors' observations
The authors’ observations
MDD is a mood disorder characterized by depressed mood and/or loss of interest or pleasure for more than 2 weeks.3 First-line pharmacotherapy for MDD includes monotherapy with a selective serotonin reuptake inhibitor (SSRI), serotonin-norepinephrine reuptake inhibitor (SNRI), mirtazapine, or bupropion.4 Medication selection is typically based on patient-specific factors, adverse effect profile, drug–drug interactions, and cost. Other treatments include electroconvulsive therapy (ECT) or cognitive-behavioral therapy (CBT).4,5 Augmentation agents, such as second-generation antipsychotics, lithium, thyroid hormone supplementation, buspirone, anticonvulsants, and combinations of antidepressants, may also be considered.4
TREATMENT Condition worsens
On Day 2 of hospitalization, Mr. J is started on aripiprazole, 5 mg/d, clonazepam, 1 mg twice daily, and melatonin, 5 mg, each night for sleep. Aripiprazole, 5 mg/d, is initiated as an adjunct to sertraline for MDD because Mr. J reports feeling much worse and continues to report that he would “rather die than feel this way.” Mr. J begins to believe that his current state is his new baseline, and that feeling better is no longer possible.
On Day 3 of hospitalization, records are obtained from a clinician at an outside facility who previously treated Mr
By Day 8 of hospitalization, there is no notable change in Mr. J’s depressive symptoms. On Day 9, sertraline is increased to 200 mg/d, with little improvement from Mr. J’s perspective. The multidisciplinary team evaluates him, and when directly asked, Mr. J cites his 4 greatest complaints to be poor sleep, fatigue, no appetite, and depressed mood. Once again, he states, “I would rather be dead than go on feeling this way.”
[polldaddy:10120587]
The authors’ observations
Due to Mr. J’s severe, unrelenting depressive episode, the treatment team obtained his informed consent to undergo ECT. On Day 9, before initiating ECT, the pharmacist recommended mirtazapine, even though the patient weighed almost 89 kg (196.21 lb) and had a body mass index of 27.8 kg/m2. The treatment team thought that mirtazapine augmentation could potentially help the sertraline work more quickly while targeting Mr. J’s 4 greatest complaints.
Mirtazapine is a central alpha-2 antagonist or noradrenergic and specific serotonergic antidepressant (NaSSA) that works through antagonism of the presynaptic alpha-2 adrenergic receptors to indirectly regulate release of monoamines and increase the release of serotonin and norepinephrine.6 Additionally, mirtazapine has antagonist actions at 5HT2A, 5HT2C, 5HT3, and histamine-1 receptors.6 Potential adverse effects include drowsiness and increased appetite leading to weight gain.7 Mirtazapine’s therapeutic efficacy is similar to SSRIs for treating depression.4 Mirtazapine in combination with an SNRI has been referred to as “California rocket fuel” due to the theoretical pharmacologic synergy and resulting strong antidepressant action.6 It was hypothesized that similar effects could be seen by augmenting the SSRI sertraline with mirtazapine.
Continued to: The time to efficacy with mirtazapine...
The time to efficacy with mirtazapine is approximately 2 to 4 weeks, but anxiety symptoms and poor sleep or insomnia may improve in the first week.8 Studies have suggested the possibility of a more rapid onset of efficacy with mirtazapine than with SSRIs, as well as potential response acceleration in MDD and other psychiatric illnesses such as anxiety disorders or obsessive-compulsive disorder (OCD).9,10 A review that included several double-blind studies and compared mirtazapine with SSRIs found the amount of responders with persistent improvement with onset in Week 1 was more pronounced with mirtazapine.9
Augmenting an SSRI with mirtazapine is a potential therapeutic option because it can help boost the efficacy of the prescribed SSRI while enhancing appetite and blunting the activating or anxiety-like effects of some SSRIs, which may help with relaxation and sleep.4 The combination of an SSRI plus mirtazapine has been studied in patients with MDD, posttraumatic stress disorder, and OCD; it was found to improve symptoms of those conditions due to the medications’ complementary mechanisms of action.4,11-13 Also, mirtazapine has been shown to decrease the rates of relapse after an acute phase of depression.4,14
OUTCOME Rapid improvement
On Day 9, Mr. J receives the first dose of mirtazapine, 7.5 mg at bedtime. On Day 10, when Mr. J wakes, his mood is notably improved. He is more interactive (sitting up in bed reading and making eye contact with the staff during an interview), and he reports improved sleep and eats most of his breakfast.
After receiving 3 doses of mirtazapine, Mr. J reports that he feels back to his normal self; he is interactive, alert, and eating well. Due to the rapid improvement in mood, ECT is discontinued, and he does not receive any ECT treatment during the remainder of his hospitalization.
On Day 11, divalproex is discontinued. Because Mr. J receives only 5 days of therapy with this agent, his divalproex level is not checked. At this point, the treatment team feels confident in ruling out bipolar disorder.
On Day 15, Mr. J is discharged with sertraline, 200 mg/d, mirtazapine, 7.5 mg/d at 7
Ten months after his depressive episode, Mr. J has had no further admissions at the hospital where he received the treatment described here.
Bottom Line
Evidence for the treatment of major depressive disorder induced by corticosteroid withdrawal is limited. Despite trials of agents from multiple medication classes, the depressive episode may not improve. Adding mirtazapine to a selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor may prove successful.
Related Resources
- Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528.
- Kenna HA, Poon AW, de los Angeles CP, et al. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65(6):549-560.
Drug Brand Names
Adalimumab • Humira
Aripiprazole • Abilify
Bupropion • Wellbutrin, Zyban
Buspirone • Buspar
Clonazepam • Klonopin
Diazepam • Valium
Diphenhydramine • Benadryl
Divalproex • Depakote, Depakote ER
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Prednisone • Deltasone
Sertraline • Zoloft
1. Dixon R, Christy N. On the various forms of corticosteroid withdrawal syndrome. Am J Med. 1980;68(2):224-30.
2. Fardet L, Petersen I, Nazareth I. Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry. 2012;169(5):491-497.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Arlington Virginia: American Psychiatric Association. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published October 2010. Accessed March 15, 2017.
5. National Institute for Health and Clinical Excellence (NICE) Clinical Guideline 90. Depression in adults: recognition and management. https://www.nice.org.uk/guidance/cg90. Accessed March 15, 2017.
6. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013;317-322; 363-364.
7. Remeron [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2018.
8. Gorman JM. Mirtazapine: clinical overview. J Clin Psychiatry. 1999;60(suppl 17):9-13; discussion 46-48.
9. Quitkin FM, Taylor BP, Kremer C. Does mirtazapine have a more rapid onset than SSRIs? J Clin Psychiatry. 2001;62(5):358-361.
10. Pallanti S, Quercioli L, Bruscoli M. Response acceleration with mirtazapine augmentation of citalopram in obsessive-compulsive disorder patients without comorbid depression: a pilot study. J Clin Psychiatry. 2004;65(10):1394-1399.
11. Blier P, Gobbi G, Turcotte JE, et al. Mirtazapine and paroxetine in major depression: a comparison of monotherapy versus their combination from treatment initiation. Eur Neuropsychopharmacol. 2009;19(7):457-465.
12. Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288.
13. Carpenter LL, Yasmin S, Price LH. A double-blind, placebo-controlled study of antidepressant augmentation with mirtazapine. Biol Psychiatry. 2002;51(2):183-188.
14. Schneier FR, Campeas R, Carcamo J, et al. Combined mirtazapine and SSRI treatment of PTSD: a placebo-controlled trial. Depress Anxiety. 2015;32(8):570-579.
CASE Suicidal ideation, flare-up of ulcerative colitis
Mr. J, age 56, who has a history of major depressive disorder (MDD), generalized anxiety disorder (GAD), and ulcerative colitis (UC), presents to the emergency department (ED) with suicidal ideation and a plan to overdose on his medications. He reports no current emotional or financial stressors in his personal life. Home medications documented at the time of his arrival to the ED include sertraline, 100 mg/d, bupropion, 150 mg/d, buspirone, 10 mg 3 times daily, diazepam 10 mg 3 times daily, as needed, adalimumab, 40 mg IM every 2 weeks, and diphenhydramine, 50 mg every night.
A recent flare-up of UC resulted in Mr. J being placed on a 15-week prednisone taper, beginning at 80 mg/d and decreasing by 5 mg weekly, which was completed 2 weeks before he presented to the ED. After completing the prednisone taper, Mr. J went to his primary care physician (PCP) on 3 separate occasions due to episodes of severe depression. Although the PCP prescribed multiple medications to target Mr. J’s depressive symptoms, he continued to decline.
Subsequently, Mr. J came to the ED and is admitted to the psychiatric unit for safety and stabilization. Upon admission, Mr. J becomes bedridden, and reports that his current depressive episode is the most severe that he has ever experienced in his more than 30 years of having MDD. He says that neither bupropion nor buspirone are helping with his depression, anxiety, or any related symptom.
[polldaddy:10120537]
The authors’ observations
At admission, all of Mr. J’s home medications, except sertraline and adalimumab, which had been prescribed to treat UC (Box1,2), were discontinued. His diazepam was discontinued because the clinician felt it may have been contributing to Mr. J’s inability to walk or get out of bed. Diazepam was not tapered because it was initiated 7 days prior to admission and was thought to be exacerbating his depression and suicidal ideation. Bupropion and buspirone, which were initiated 2 weeks prior, were discontinued because Mr. J reported that neither medication was helping with his depression, anxiety, or any related symptom.
Box
Ulcerative colitis and depressive episodes
Ulcerative colitis (UC) is a chronic condition associated with inflammation in the colon causing extreme abdominal discomfort during acute flare-ups. Moderate to severe UC flare-ups are commonly treated with corticosteroids due to these medications’ anti-inflammatory properties. Although rare, corticosteroid withdrawal has been documented to induce episodes of depression. The pathophysiology of corticosteroid withdrawal inducing neuropsychiatric sequelae remains unclear; however, it is thought to be due to hypothalamic-pituitary-adrenocortical suppression.1 Fardet et al2 concluded that incident rates per 100 person-years at risk during the withdrawal period were 11.1 (95% confidence interval, 10.0, 12.3) for depression.
EVALUATION Poor appetite, anxiety, and continued suicidality
During evaluation, vital signs, laboratory findings, and diagnostic testing are found to be unremarkable. Mr. J’s presentation and complaints are entirely subjective, and include poor appetite, fatigue, difficulty sleeping, sorrow, anxiety, and continued suicidality. Mr. J reports that he feels miserable, which is reflected by his poor eye contact, soft speech, and body language.
Continued to: The authors' observations
The authors’ observations
MDD is a mood disorder characterized by depressed mood and/or loss of interest or pleasure for more than 2 weeks.3 First-line pharmacotherapy for MDD includes monotherapy with a selective serotonin reuptake inhibitor (SSRI), serotonin-norepinephrine reuptake inhibitor (SNRI), mirtazapine, or bupropion.4 Medication selection is typically based on patient-specific factors, adverse effect profile, drug–drug interactions, and cost. Other treatments include electroconvulsive therapy (ECT) or cognitive-behavioral therapy (CBT).4,5 Augmentation agents, such as second-generation antipsychotics, lithium, thyroid hormone supplementation, buspirone, anticonvulsants, and combinations of antidepressants, may also be considered.4
TREATMENT Condition worsens
On Day 2 of hospitalization, Mr. J is started on aripiprazole, 5 mg/d, clonazepam, 1 mg twice daily, and melatonin, 5 mg, each night for sleep. Aripiprazole, 5 mg/d, is initiated as an adjunct to sertraline for MDD because Mr. J reports feeling much worse and continues to report that he would “rather die than feel this way.” Mr. J begins to believe that his current state is his new baseline, and that feeling better is no longer possible.
On Day 3 of hospitalization, records are obtained from a clinician at an outside facility who previously treated Mr
By Day 8 of hospitalization, there is no notable change in Mr. J’s depressive symptoms. On Day 9, sertraline is increased to 200 mg/d, with little improvement from Mr. J’s perspective. The multidisciplinary team evaluates him, and when directly asked, Mr. J cites his 4 greatest complaints to be poor sleep, fatigue, no appetite, and depressed mood. Once again, he states, “I would rather be dead than go on feeling this way.”
[polldaddy:10120587]
The authors’ observations
Due to Mr. J’s severe, unrelenting depressive episode, the treatment team obtained his informed consent to undergo ECT. On Day 9, before initiating ECT, the pharmacist recommended mirtazapine, even though the patient weighed almost 89 kg (196.21 lb) and had a body mass index of 27.8 kg/m2. The treatment team thought that mirtazapine augmentation could potentially help the sertraline work more quickly while targeting Mr. J’s 4 greatest complaints.
Mirtazapine is a central alpha-2 antagonist or noradrenergic and specific serotonergic antidepressant (NaSSA) that works through antagonism of the presynaptic alpha-2 adrenergic receptors to indirectly regulate release of monoamines and increase the release of serotonin and norepinephrine.6 Additionally, mirtazapine has antagonist actions at 5HT2A, 5HT2C, 5HT3, and histamine-1 receptors.6 Potential adverse effects include drowsiness and increased appetite leading to weight gain.7 Mirtazapine’s therapeutic efficacy is similar to SSRIs for treating depression.4 Mirtazapine in combination with an SNRI has been referred to as “California rocket fuel” due to the theoretical pharmacologic synergy and resulting strong antidepressant action.6 It was hypothesized that similar effects could be seen by augmenting the SSRI sertraline with mirtazapine.
Continued to: The time to efficacy with mirtazapine...
The time to efficacy with mirtazapine is approximately 2 to 4 weeks, but anxiety symptoms and poor sleep or insomnia may improve in the first week.8 Studies have suggested the possibility of a more rapid onset of efficacy with mirtazapine than with SSRIs, as well as potential response acceleration in MDD and other psychiatric illnesses such as anxiety disorders or obsessive-compulsive disorder (OCD).9,10 A review that included several double-blind studies and compared mirtazapine with SSRIs found the amount of responders with persistent improvement with onset in Week 1 was more pronounced with mirtazapine.9
Augmenting an SSRI with mirtazapine is a potential therapeutic option because it can help boost the efficacy of the prescribed SSRI while enhancing appetite and blunting the activating or anxiety-like effects of some SSRIs, which may help with relaxation and sleep.4 The combination of an SSRI plus mirtazapine has been studied in patients with MDD, posttraumatic stress disorder, and OCD; it was found to improve symptoms of those conditions due to the medications’ complementary mechanisms of action.4,11-13 Also, mirtazapine has been shown to decrease the rates of relapse after an acute phase of depression.4,14
OUTCOME Rapid improvement
On Day 9, Mr. J receives the first dose of mirtazapine, 7.5 mg at bedtime. On Day 10, when Mr. J wakes, his mood is notably improved. He is more interactive (sitting up in bed reading and making eye contact with the staff during an interview), and he reports improved sleep and eats most of his breakfast.
After receiving 3 doses of mirtazapine, Mr. J reports that he feels back to his normal self; he is interactive, alert, and eating well. Due to the rapid improvement in mood, ECT is discontinued, and he does not receive any ECT treatment during the remainder of his hospitalization.
On Day 11, divalproex is discontinued. Because Mr. J receives only 5 days of therapy with this agent, his divalproex level is not checked. At this point, the treatment team feels confident in ruling out bipolar disorder.
On Day 15, Mr. J is discharged with sertraline, 200 mg/d, mirtazapine, 7.5 mg/d at 7
Ten months after his depressive episode, Mr. J has had no further admissions at the hospital where he received the treatment described here.
Bottom Line
Evidence for the treatment of major depressive disorder induced by corticosteroid withdrawal is limited. Despite trials of agents from multiple medication classes, the depressive episode may not improve. Adding mirtazapine to a selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor may prove successful.
Related Resources
- Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528.
- Kenna HA, Poon AW, de los Angeles CP, et al. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65(6):549-560.
Drug Brand Names
Adalimumab • Humira
Aripiprazole • Abilify
Bupropion • Wellbutrin, Zyban
Buspirone • Buspar
Clonazepam • Klonopin
Diazepam • Valium
Diphenhydramine • Benadryl
Divalproex • Depakote, Depakote ER
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Prednisone • Deltasone
Sertraline • Zoloft
CASE Suicidal ideation, flare-up of ulcerative colitis
Mr. J, age 56, who has a history of major depressive disorder (MDD), generalized anxiety disorder (GAD), and ulcerative colitis (UC), presents to the emergency department (ED) with suicidal ideation and a plan to overdose on his medications. He reports no current emotional or financial stressors in his personal life. Home medications documented at the time of his arrival to the ED include sertraline, 100 mg/d, bupropion, 150 mg/d, buspirone, 10 mg 3 times daily, diazepam 10 mg 3 times daily, as needed, adalimumab, 40 mg IM every 2 weeks, and diphenhydramine, 50 mg every night.
A recent flare-up of UC resulted in Mr. J being placed on a 15-week prednisone taper, beginning at 80 mg/d and decreasing by 5 mg weekly, which was completed 2 weeks before he presented to the ED. After completing the prednisone taper, Mr. J went to his primary care physician (PCP) on 3 separate occasions due to episodes of severe depression. Although the PCP prescribed multiple medications to target Mr. J’s depressive symptoms, he continued to decline.
Subsequently, Mr. J came to the ED and is admitted to the psychiatric unit for safety and stabilization. Upon admission, Mr. J becomes bedridden, and reports that his current depressive episode is the most severe that he has ever experienced in his more than 30 years of having MDD. He says that neither bupropion nor buspirone are helping with his depression, anxiety, or any related symptom.
[polldaddy:10120537]
The authors’ observations
At admission, all of Mr. J’s home medications, except sertraline and adalimumab, which had been prescribed to treat UC (Box1,2), were discontinued. His diazepam was discontinued because the clinician felt it may have been contributing to Mr. J’s inability to walk or get out of bed. Diazepam was not tapered because it was initiated 7 days prior to admission and was thought to be exacerbating his depression and suicidal ideation. Bupropion and buspirone, which were initiated 2 weeks prior, were discontinued because Mr. J reported that neither medication was helping with his depression, anxiety, or any related symptom.
Box
Ulcerative colitis and depressive episodes
Ulcerative colitis (UC) is a chronic condition associated with inflammation in the colon causing extreme abdominal discomfort during acute flare-ups. Moderate to severe UC flare-ups are commonly treated with corticosteroids due to these medications’ anti-inflammatory properties. Although rare, corticosteroid withdrawal has been documented to induce episodes of depression. The pathophysiology of corticosteroid withdrawal inducing neuropsychiatric sequelae remains unclear; however, it is thought to be due to hypothalamic-pituitary-adrenocortical suppression.1 Fardet et al2 concluded that incident rates per 100 person-years at risk during the withdrawal period were 11.1 (95% confidence interval, 10.0, 12.3) for depression.
EVALUATION Poor appetite, anxiety, and continued suicidality
During evaluation, vital signs, laboratory findings, and diagnostic testing are found to be unremarkable. Mr. J’s presentation and complaints are entirely subjective, and include poor appetite, fatigue, difficulty sleeping, sorrow, anxiety, and continued suicidality. Mr. J reports that he feels miserable, which is reflected by his poor eye contact, soft speech, and body language.
Continued to: The authors' observations
The authors’ observations
MDD is a mood disorder characterized by depressed mood and/or loss of interest or pleasure for more than 2 weeks.3 First-line pharmacotherapy for MDD includes monotherapy with a selective serotonin reuptake inhibitor (SSRI), serotonin-norepinephrine reuptake inhibitor (SNRI), mirtazapine, or bupropion.4 Medication selection is typically based on patient-specific factors, adverse effect profile, drug–drug interactions, and cost. Other treatments include electroconvulsive therapy (ECT) or cognitive-behavioral therapy (CBT).4,5 Augmentation agents, such as second-generation antipsychotics, lithium, thyroid hormone supplementation, buspirone, anticonvulsants, and combinations of antidepressants, may also be considered.4
TREATMENT Condition worsens
On Day 2 of hospitalization, Mr. J is started on aripiprazole, 5 mg/d, clonazepam, 1 mg twice daily, and melatonin, 5 mg, each night for sleep. Aripiprazole, 5 mg/d, is initiated as an adjunct to sertraline for MDD because Mr. J reports feeling much worse and continues to report that he would “rather die than feel this way.” Mr. J begins to believe that his current state is his new baseline, and that feeling better is no longer possible.
On Day 3 of hospitalization, records are obtained from a clinician at an outside facility who previously treated Mr
By Day 8 of hospitalization, there is no notable change in Mr. J’s depressive symptoms. On Day 9, sertraline is increased to 200 mg/d, with little improvement from Mr. J’s perspective. The multidisciplinary team evaluates him, and when directly asked, Mr. J cites his 4 greatest complaints to be poor sleep, fatigue, no appetite, and depressed mood. Once again, he states, “I would rather be dead than go on feeling this way.”
[polldaddy:10120587]
The authors’ observations
Due to Mr. J’s severe, unrelenting depressive episode, the treatment team obtained his informed consent to undergo ECT. On Day 9, before initiating ECT, the pharmacist recommended mirtazapine, even though the patient weighed almost 89 kg (196.21 lb) and had a body mass index of 27.8 kg/m2. The treatment team thought that mirtazapine augmentation could potentially help the sertraline work more quickly while targeting Mr. J’s 4 greatest complaints.
Mirtazapine is a central alpha-2 antagonist or noradrenergic and specific serotonergic antidepressant (NaSSA) that works through antagonism of the presynaptic alpha-2 adrenergic receptors to indirectly regulate release of monoamines and increase the release of serotonin and norepinephrine.6 Additionally, mirtazapine has antagonist actions at 5HT2A, 5HT2C, 5HT3, and histamine-1 receptors.6 Potential adverse effects include drowsiness and increased appetite leading to weight gain.7 Mirtazapine’s therapeutic efficacy is similar to SSRIs for treating depression.4 Mirtazapine in combination with an SNRI has been referred to as “California rocket fuel” due to the theoretical pharmacologic synergy and resulting strong antidepressant action.6 It was hypothesized that similar effects could be seen by augmenting the SSRI sertraline with mirtazapine.
Continued to: The time to efficacy with mirtazapine...
The time to efficacy with mirtazapine is approximately 2 to 4 weeks, but anxiety symptoms and poor sleep or insomnia may improve in the first week.8 Studies have suggested the possibility of a more rapid onset of efficacy with mirtazapine than with SSRIs, as well as potential response acceleration in MDD and other psychiatric illnesses such as anxiety disorders or obsessive-compulsive disorder (OCD).9,10 A review that included several double-blind studies and compared mirtazapine with SSRIs found the amount of responders with persistent improvement with onset in Week 1 was more pronounced with mirtazapine.9
Augmenting an SSRI with mirtazapine is a potential therapeutic option because it can help boost the efficacy of the prescribed SSRI while enhancing appetite and blunting the activating or anxiety-like effects of some SSRIs, which may help with relaxation and sleep.4 The combination of an SSRI plus mirtazapine has been studied in patients with MDD, posttraumatic stress disorder, and OCD; it was found to improve symptoms of those conditions due to the medications’ complementary mechanisms of action.4,11-13 Also, mirtazapine has been shown to decrease the rates of relapse after an acute phase of depression.4,14
OUTCOME Rapid improvement
On Day 9, Mr. J receives the first dose of mirtazapine, 7.5 mg at bedtime. On Day 10, when Mr. J wakes, his mood is notably improved. He is more interactive (sitting up in bed reading and making eye contact with the staff during an interview), and he reports improved sleep and eats most of his breakfast.
After receiving 3 doses of mirtazapine, Mr. J reports that he feels back to his normal self; he is interactive, alert, and eating well. Due to the rapid improvement in mood, ECT is discontinued, and he does not receive any ECT treatment during the remainder of his hospitalization.
On Day 11, divalproex is discontinued. Because Mr. J receives only 5 days of therapy with this agent, his divalproex level is not checked. At this point, the treatment team feels confident in ruling out bipolar disorder.
On Day 15, Mr. J is discharged with sertraline, 200 mg/d, mirtazapine, 7.5 mg/d at 7
Ten months after his depressive episode, Mr. J has had no further admissions at the hospital where he received the treatment described here.
Bottom Line
Evidence for the treatment of major depressive disorder induced by corticosteroid withdrawal is limited. Despite trials of agents from multiple medication classes, the depressive episode may not improve. Adding mirtazapine to a selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor may prove successful.
Related Resources
- Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528.
- Kenna HA, Poon AW, de los Angeles CP, et al. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65(6):549-560.
Drug Brand Names
Adalimumab • Humira
Aripiprazole • Abilify
Bupropion • Wellbutrin, Zyban
Buspirone • Buspar
Clonazepam • Klonopin
Diazepam • Valium
Diphenhydramine • Benadryl
Divalproex • Depakote, Depakote ER
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Prednisone • Deltasone
Sertraline • Zoloft
1. Dixon R, Christy N. On the various forms of corticosteroid withdrawal syndrome. Am J Med. 1980;68(2):224-30.
2. Fardet L, Petersen I, Nazareth I. Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry. 2012;169(5):491-497.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Arlington Virginia: American Psychiatric Association. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published October 2010. Accessed March 15, 2017.
5. National Institute for Health and Clinical Excellence (NICE) Clinical Guideline 90. Depression in adults: recognition and management. https://www.nice.org.uk/guidance/cg90. Accessed March 15, 2017.
6. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013;317-322; 363-364.
7. Remeron [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2018.
8. Gorman JM. Mirtazapine: clinical overview. J Clin Psychiatry. 1999;60(suppl 17):9-13; discussion 46-48.
9. Quitkin FM, Taylor BP, Kremer C. Does mirtazapine have a more rapid onset than SSRIs? J Clin Psychiatry. 2001;62(5):358-361.
10. Pallanti S, Quercioli L, Bruscoli M. Response acceleration with mirtazapine augmentation of citalopram in obsessive-compulsive disorder patients without comorbid depression: a pilot study. J Clin Psychiatry. 2004;65(10):1394-1399.
11. Blier P, Gobbi G, Turcotte JE, et al. Mirtazapine and paroxetine in major depression: a comparison of monotherapy versus their combination from treatment initiation. Eur Neuropsychopharmacol. 2009;19(7):457-465.
12. Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288.
13. Carpenter LL, Yasmin S, Price LH. A double-blind, placebo-controlled study of antidepressant augmentation with mirtazapine. Biol Psychiatry. 2002;51(2):183-188.
14. Schneier FR, Campeas R, Carcamo J, et al. Combined mirtazapine and SSRI treatment of PTSD: a placebo-controlled trial. Depress Anxiety. 2015;32(8):570-579.
1. Dixon R, Christy N. On the various forms of corticosteroid withdrawal syndrome. Am J Med. 1980;68(2):224-30.
2. Fardet L, Petersen I, Nazareth I. Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry. 2012;169(5):491-497.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Arlington Virginia: American Psychiatric Association. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published October 2010. Accessed March 15, 2017.
5. National Institute for Health and Clinical Excellence (NICE) Clinical Guideline 90. Depression in adults: recognition and management. https://www.nice.org.uk/guidance/cg90. Accessed March 15, 2017.
6. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013;317-322; 363-364.
7. Remeron [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2018.
8. Gorman JM. Mirtazapine: clinical overview. J Clin Psychiatry. 1999;60(suppl 17):9-13; discussion 46-48.
9. Quitkin FM, Taylor BP, Kremer C. Does mirtazapine have a more rapid onset than SSRIs? J Clin Psychiatry. 2001;62(5):358-361.
10. Pallanti S, Quercioli L, Bruscoli M. Response acceleration with mirtazapine augmentation of citalopram in obsessive-compulsive disorder patients without comorbid depression: a pilot study. J Clin Psychiatry. 2004;65(10):1394-1399.
11. Blier P, Gobbi G, Turcotte JE, et al. Mirtazapine and paroxetine in major depression: a comparison of monotherapy versus their combination from treatment initiation. Eur Neuropsychopharmacol. 2009;19(7):457-465.
12. Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288.
13. Carpenter LL, Yasmin S, Price LH. A double-blind, placebo-controlled study of antidepressant augmentation with mirtazapine. Biol Psychiatry. 2002;51(2):183-188.
14. Schneier FR, Campeas R, Carcamo J, et al. Combined mirtazapine and SSRI treatment of PTSD: a placebo-controlled trial. Depress Anxiety. 2015;32(8):570-579.
Behavioral checklist IDs children at risk of depressive, anxiety disorders
disorders in later adolescent or adult life, according to research published in the Journal of Pediatrics.
“If confirmed, this observation could have very significant clinical and public health implications in the identification of children at the highest risk for the development of major depressive disorder,” Mai Uchida, MD, of the department of psychiatry at Massachusetts General Hospital in Boston, and colleagues wrote. “Early identification is the first step toward effectively allocating limited societal resources to help prevent illness progression and its associated impairments in children most in need.”
Dr. Uchida and her colleagues analyzed a sample of 537 children aged 6-17 years from two longitudinal studies of children and their parents with and without ADHD, excluding the ADHD probands in their analysis. Parents answered the CBCL, which consisted of behavioral questions translated into scores for subscales involving being anxious and/or depressed, withdrawn and/or depressed, social problems, thought problems, aggressive behavior, rule-breaking behavior, attention problems, and somatic complaints.
The sample was then divided into four groups; there were 172 children with parents who had a mood disorder but the children did not have CBCL anxiety/depression subsyndromal elevations (high risk group); 22 children without a parental history of mood disorder but had CBCL anxiety/depression subsyndromal elevations (subsyndromal major depressive disorder); 22 children with a parental history of mood disorder and CBCL anxiety/depression subsyndromal elevations (high-risk and subsyndromal major depressive disorder); and 186 children in a control group with no parental history of mood disorder or CBCL anxiety/depression subsyndromal elevations.
Compared with the control group, children with a history of parental mood disorders and children with baseline CBCL anxiety/depression subsyndromal elevations had intermediate risk of developing major depression and anxiety disorders. However, the highest risk was among children with both a parental history of mood disorder and baseline CBCL anxiety/depression subsyndromal elevations.
Using data from two previously collected longitudinal studies was a potential limitation of the study, Dr. Uchida and her associates said, but they noted the CBCL scale has predictive utility for identifying anxiety and depressive disorders in children later in life.
“Considering its simplicity, strong psychometric properties, and limited cost, the CBCL scale lends itself to be used by health professionals and educators in the community,” they wrote.
This study was partly supported by National Institutes of Health and the Massachusetts General Hospital Pediatric Psychopharmacology Council Fund. Joseph Biederman, MD, received research support from Lundbeck AS, Neurocentria, Pfizer, Shire, Sunovion, and others, and has relationships with multiple other associations and pharmaceutical companies. The other authors have no relevant financial disclosures.
SOURCE: Uchida M et al. J Pediatr. 2018 Oct;201:252-8.
disorders in later adolescent or adult life, according to research published in the Journal of Pediatrics.
“If confirmed, this observation could have very significant clinical and public health implications in the identification of children at the highest risk for the development of major depressive disorder,” Mai Uchida, MD, of the department of psychiatry at Massachusetts General Hospital in Boston, and colleagues wrote. “Early identification is the first step toward effectively allocating limited societal resources to help prevent illness progression and its associated impairments in children most in need.”
Dr. Uchida and her colleagues analyzed a sample of 537 children aged 6-17 years from two longitudinal studies of children and their parents with and without ADHD, excluding the ADHD probands in their analysis. Parents answered the CBCL, which consisted of behavioral questions translated into scores for subscales involving being anxious and/or depressed, withdrawn and/or depressed, social problems, thought problems, aggressive behavior, rule-breaking behavior, attention problems, and somatic complaints.
The sample was then divided into four groups; there were 172 children with parents who had a mood disorder but the children did not have CBCL anxiety/depression subsyndromal elevations (high risk group); 22 children without a parental history of mood disorder but had CBCL anxiety/depression subsyndromal elevations (subsyndromal major depressive disorder); 22 children with a parental history of mood disorder and CBCL anxiety/depression subsyndromal elevations (high-risk and subsyndromal major depressive disorder); and 186 children in a control group with no parental history of mood disorder or CBCL anxiety/depression subsyndromal elevations.
Compared with the control group, children with a history of parental mood disorders and children with baseline CBCL anxiety/depression subsyndromal elevations had intermediate risk of developing major depression and anxiety disorders. However, the highest risk was among children with both a parental history of mood disorder and baseline CBCL anxiety/depression subsyndromal elevations.
Using data from two previously collected longitudinal studies was a potential limitation of the study, Dr. Uchida and her associates said, but they noted the CBCL scale has predictive utility for identifying anxiety and depressive disorders in children later in life.
“Considering its simplicity, strong psychometric properties, and limited cost, the CBCL scale lends itself to be used by health professionals and educators in the community,” they wrote.
This study was partly supported by National Institutes of Health and the Massachusetts General Hospital Pediatric Psychopharmacology Council Fund. Joseph Biederman, MD, received research support from Lundbeck AS, Neurocentria, Pfizer, Shire, Sunovion, and others, and has relationships with multiple other associations and pharmaceutical companies. The other authors have no relevant financial disclosures.
SOURCE: Uchida M et al. J Pediatr. 2018 Oct;201:252-8.
disorders in later adolescent or adult life, according to research published in the Journal of Pediatrics.
“If confirmed, this observation could have very significant clinical and public health implications in the identification of children at the highest risk for the development of major depressive disorder,” Mai Uchida, MD, of the department of psychiatry at Massachusetts General Hospital in Boston, and colleagues wrote. “Early identification is the first step toward effectively allocating limited societal resources to help prevent illness progression and its associated impairments in children most in need.”
Dr. Uchida and her colleagues analyzed a sample of 537 children aged 6-17 years from two longitudinal studies of children and their parents with and without ADHD, excluding the ADHD probands in their analysis. Parents answered the CBCL, which consisted of behavioral questions translated into scores for subscales involving being anxious and/or depressed, withdrawn and/or depressed, social problems, thought problems, aggressive behavior, rule-breaking behavior, attention problems, and somatic complaints.
The sample was then divided into four groups; there were 172 children with parents who had a mood disorder but the children did not have CBCL anxiety/depression subsyndromal elevations (high risk group); 22 children without a parental history of mood disorder but had CBCL anxiety/depression subsyndromal elevations (subsyndromal major depressive disorder); 22 children with a parental history of mood disorder and CBCL anxiety/depression subsyndromal elevations (high-risk and subsyndromal major depressive disorder); and 186 children in a control group with no parental history of mood disorder or CBCL anxiety/depression subsyndromal elevations.
Compared with the control group, children with a history of parental mood disorders and children with baseline CBCL anxiety/depression subsyndromal elevations had intermediate risk of developing major depression and anxiety disorders. However, the highest risk was among children with both a parental history of mood disorder and baseline CBCL anxiety/depression subsyndromal elevations.
Using data from two previously collected longitudinal studies was a potential limitation of the study, Dr. Uchida and her associates said, but they noted the CBCL scale has predictive utility for identifying anxiety and depressive disorders in children later in life.
“Considering its simplicity, strong psychometric properties, and limited cost, the CBCL scale lends itself to be used by health professionals and educators in the community,” they wrote.
This study was partly supported by National Institutes of Health and the Massachusetts General Hospital Pediatric Psychopharmacology Council Fund. Joseph Biederman, MD, received research support from Lundbeck AS, Neurocentria, Pfizer, Shire, Sunovion, and others, and has relationships with multiple other associations and pharmaceutical companies. The other authors have no relevant financial disclosures.
SOURCE: Uchida M et al. J Pediatr. 2018 Oct;201:252-8.
FROM THE JOURNAL OF PEDIATRICS
Key clinical point: Children with elevated CBCL anxiety/depression scale scores at baseline as well as a parent with a major depressive disorder were at risk of developing a major depressive disorder or anxiety disorder at 10-year follow-up.
Major finding:
Study details: An analysis of 537 children in a sample from two longitudinal case studies of families with and without child and parental history of ADHD.
Disclosures: This study was partly supported by National Institutes of Health and the Massachusetts General Hospital Pediatric Psychopharmacology Council Fund. Joseph Biederman, MD, received research support from Neurocentria, Pfizer, Shire, Sunovion, and others, and has relationships with multiple other associations and pharmaceutical companies. The other authors have no relevant financial disclosures.
Source: Uchida M et al. J Pediatr. 2018 Oct;201:252-8.