User login
Symptoms of psychosis and OCD in a patient with postpartum depression
CASE Thoughts of harming baby
Ms. A, age 37, is G4P2, 4 months postpartum, and breastfeeding. She has major depressive disorder (MDD) with peripartum onset, posttraumatic stress disorder, and mild intellectual disability. For years she has been stable on fluoxetine 40 mg/d and prazosin 2 mg/d. Despite recent titration of her medications, at her most recent outpatient appointment Ms. A reports having a depressed mood with frequent crying, insomnia, a lack of desire to bond with her baby, and feelings of shame. She also says she has had auditory hallucinations and thoughts of harming her baby. Ms. A’s outpatient physician makes an urgent request for her to be evaluated at the psychiatric emergency department (ED).
HISTORY Depression and possible auditory hallucinations
Ms. A developed MDD following the birth of her first child, for which her care team initiated fluoxetine at 20 mg/d and titrated it to 40 mg/d,which was effective. At that time, her outpatient physician documented potential psychotic features, including vague descriptions of derogatory auditory hallucinations. However, it was unclear if these auditory hallucinations were more representative of a distressing inner monologue without the quality of an external voice. The team determined that Ms. A was not at acute risk for harm to herself or her baby and was appropriate for outpatient care. Because the nature of these possible auditory hallucinations was mild, nondistressing, and nonthreatening, the treatment team did not initiate an antipsychotic and Ms. A was not hospitalized. She has no history of hypomanic/manic episodes and has never met criteria for a psychotic disorder.
EVALUATION Distressing thoughts and discontinued medications
During the evaluation by psychiatric emergency services, Ms. A reports that 2 weeks after giving birth she experienced a worsening of her depressive symptoms. She says she began hearing voices telling her to harm herself and her baby and describes frequent distressing thoughts, such as stabbing her baby with a knife and running over her baby with a car. Ms. A says she repeatedly wakes up at night to check on her baby’s breathing, overfeeds her baby due to a fear of inadequate nutrition, and notes intermittent feelings of confusion. Afraid of being alone with her infant, Ms. A asks her partner and mother to move in with her. Additionally, she says 2 weeks ago she discontinued all her medications at the suggestion of her partner, who recommended herbal supplements. Ms. A’s initial routine laboratory results are unremarkable and her urine drug screen is negative for all substances.
[polldaddy:13041928]
The authors’ observations
Approximately 85% of birthing parents experience some form of postpartum mood disturbance; 10% to 15% develop more significant symptoms of anxiety or depression.3 The etiology of postpartum illness is multifactorial, and includes psychiatric personal/family history, insomnia, acute and chronic psychosocial stressors, and rapid hormone fluctuations.1 As a result, the postpartum period represents a vulnerable time for birthing parents, particularly those with previously established psychiatric illness.
Ms. A’s initial presentation was concerning for a possible diagnosis of postpartum psychosis vs obsessive-compulsive disorder (OCD) with postpartum onset; other differential diagnoses included MDD with peripartum onset and psychotic features (Table1-6). Ms. A’s subjective clinical history was significant for critical pertinent findings of both OCD with postpartum onset (ie, egodystonic intrusive thoughts, checking behaviors, feelings of shame, and seeking reassurance) and postpartum psychosis (ie, command auditory hallucinations and waxing/waning confusion), which added to diagnostic complexity.
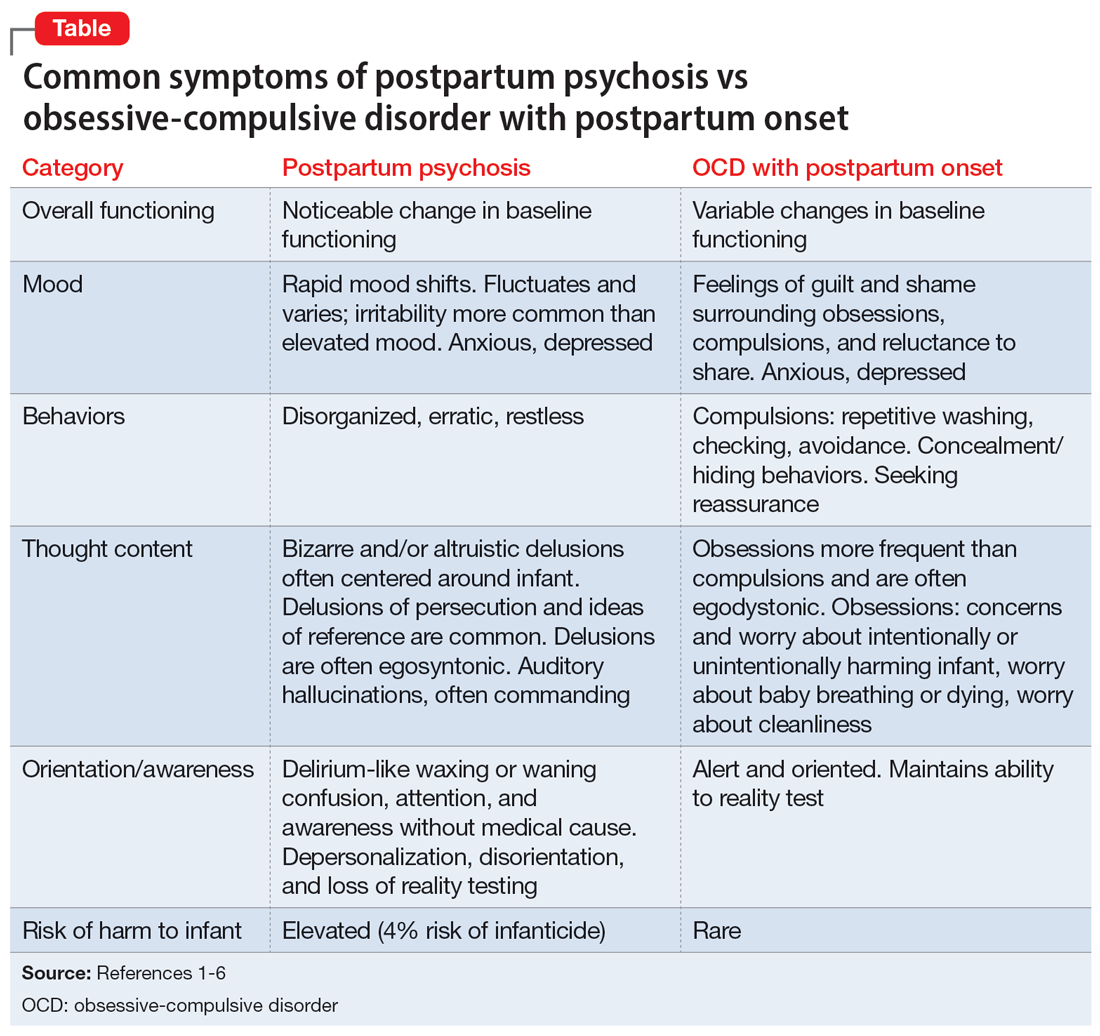
Although postpartum psychosis is rare (1 to 2 cases per 1,000 women),5 it is considered a psychiatric emergency because it has significant potential for infanticide, morbidity, and mortality. Most symptoms develop within the first 2 weeks of the postpartum period.2 There are many risk factors for the development of postpartum psychosis; however, in first-time pregnancies, a previous diagnosis of BD I is the single most important risk factor.1 Approximately 20% to 30% of women with BD experience postpartum psychosis.4
For many patients (approximately 56.7%, according to 1 meta-analysis7), postpartum psychosis denotes an episode of BD, representing a more severe form of illness with increased risk of recurrence. Most manic or mixed mood episodes reoccur within the first year removed from the perinatal period. In contrast, for some patients (approximately 43.5% according to the same meta-analysis), the episode denotes “isolated postpartum psychosis.”7 Isolated postpartum psychosis is a psychotic episode that occurs only in the postpartum period with no recurrence of psychosis or recurrence of psychosis exclusive to postpartum periods. If treated, this type of postpartum psychosis has a more favorable prognosis than postpartum psychosis in a patient with BD.7 As such, a BD diagnosis should not be established at the onset of a patient’s first postpartum psychosis presentation. Regardless of type, all presentations of postpartum psychosis are considered a psychiatry emergency.
Continue to: The prevalence of OCD...
The prevalence of OCD with postpartum onset varies. One study estimated it occurs in 2.43% of cases.4 However, the true prevalence is likely underreported due to feelings of guilt or shame associated with intrusive thoughts, and fear of stigmatization and separation from the baby. Approximately 70.6% of women experiencing OCD with postpartum onset have a comorbid depressive disorder.4
Ms. A’s presentation to the psychiatric ED carried with it diagnostic complexity and uncertainty. Her initial presentation was concerning for elements of both postpartum psychosis and OCD with postpartum onset. After her evaluation in the psychiatric ED, there remained a lack of clear and convincing evidence for a diagnosis of OCD with postpartum onset, which eliminated the possibility of discharging Ms. A with robust safety planning and reinitiation of a selective serotonin reuptake inhibitor.
Additionally, because auditory hallucinations are atypical in OCD, the treatment team remained concerned for a diagnosis of postpartum psychosis, which would warrant hospitalization. With assistance from the institution’s reproductive psychiatrists, the treatment team discussed the importance of inpatient hospitalization for risk mitigation, close observation, and thorough evaluation for greater diagnostic clarity and certainty.
TREATMENT Involuntary hospitalization
The treatment team counsels Ms. A and her partner on her differential diagnoses, including the elevated acute risk of harm to herself and her baby if she has postpartum psychosis, as well as the need for continued observation and evaluation. When alone with a clinician, Ms. A says she understands and agrees to voluntary hospitalization. However, following a subsequent risk-benefit discussion with her partner, they both grew increasingly concerned about her separation from the baby and reinitiating her medications. Amid these concerns, the treatment team notices that Ms. A attempts to minimize her symptoms. Ms. A changes her mind and no longer consents to hospitalization. She is placed on a psychiatric hold for involuntary hospitalization on the psychiatric inpatient unit.
On the inpatient unit, the inpatient clinicians and a reproductive psychiatrist continue to evaluate Ms. A. Though her diagnosis remains unclear, Ms. A agrees to start a trial of quetiapine 100 mg/d titrated to 150 mg/d to manage her potential postpartum psychosis, depressed mood, insomnia (off-label), anxiety (off-label), and OCD (off-label). Lithium is deferred because Ms. A is breastfeeding.
[polldaddy:13041932]
Continue to: The authors' observations
The authors’ observations
Due to an elevated acute risk of suicide and infanticide, postpartum psychosis represents a psychiatric emergency and often requires hospitalization. The Figure outlines steps in evaluating a patient with concerns for postpartum psychosis in a psychiatric emergency service setting. Due to the waxing and waning nature of symptoms, patients may appear psychiatrically stable at any time but remain at an overall elevated acute risk of harm to self and/or their baby.
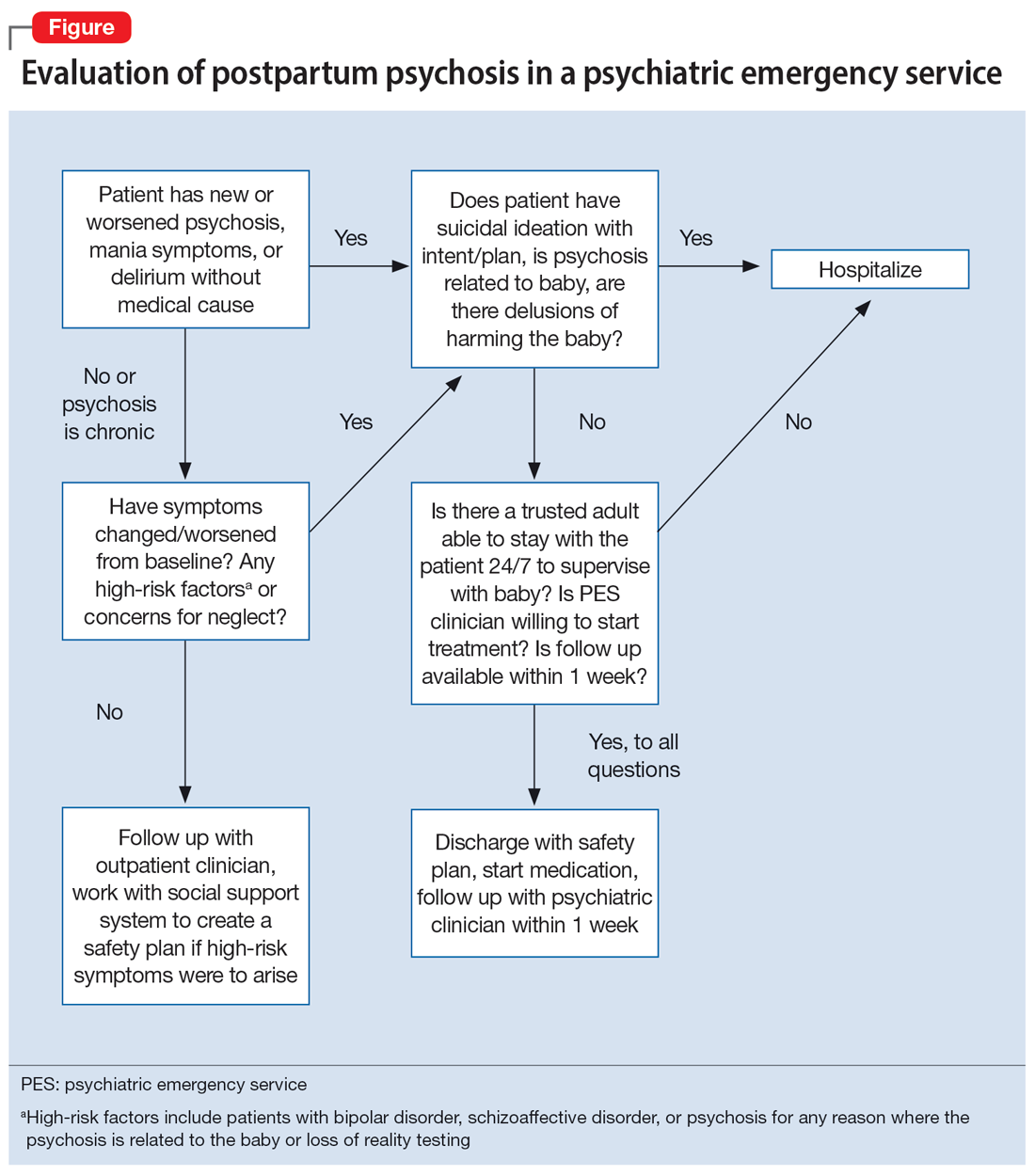
If a patient is being considered for discharge based on yes answers to all questions in Step 2 of the Figure, the emergency psychiatric clinician must initiate appropriate psychotropic medications and complete robust safety planning with the patient and a trusted adult who will provide direct supervision. Safety planning may include (but is not limited to) strict return precautions, education on concerning symptoms and behaviors, psychotropic education and agreement of compliance, and detailed instructions on outpatient follow-up within 1 week. Ideally—and as was the case for Ms. A—a reproductive psychiatrist should be consulted in the emergency setting for shared decision-making on admission vs discharge, medication management, and outpatient follow-up considerations.
Because postpartum psychosis carries significant risks and hospitalization generally results in separating the patient from their baby, initiating psychotropics should not be delayed. Clinicians must consider the patient’s psychiatric history, allergies, and breastfeeding status.
Based on current evidence, first-line treatment for postpartum psychosis includes a mood stabilizer, an antipsychotic, and possibly a benzodiazepine.6 Thus, an appropriate initial treatment regimen would be a benzodiazepine (particularly lorazepam due to its relatively shorter half-life) and an antipsychotic (eg, haloperidol, olanzapine, or quetiapine) for acute psychosis, plus lithium for mood stabilization.1,5
If the postpartum psychosis represents an episode of BD, use of a long-term mood stabilizer may be required. In contrast, for isolated postpartum psychosis, clinicians may consider initiating psychotropics only in the immediate postpartum period, with an eventual slow taper. In future pregnancies, psychotropics may be reintroduced postpartum, which will avoid peripartum fetal exposure.8 If the patient is breastfeeding, lithium may be deferred in an acute care setting. For patients with evidence of catatonia, severe suicidality, refusal of oral intake with compromised nutrition, severe agitation, or treatment resistance, electroconvulsive therapy remains a safe and effective treatment option.6 Additionally, the safety of continued breastfeeding in acute psychosis must be considered, with the potential for recommending discontinuation, which would decrease sleep disruptions at night and increase the ability of others to feed the baby. Comprehensive care requires nonpharmacologic interventions, including psychoeducation for the patient and their family, individual psychotherapy, and expansion of psychosocial supports.
Continue to: Patients who have experienced...
Patients who have experienced an episode of postpartum psychosis are predisposed to another episode in future pregnancies.1 Current research recommends prophylaxis of recurrence with lithium monotherapy.1,2,5,6 Similar to other psychotropics in reproductive psychiatry, maintenance therapy on lithium requires a thorough “risk vs risk” discussion with the patient. The risk of lithium use while pregnant and/or breastfeeding must be weighed against the risks associated with postpartum psychosis (ie, infanticide, suicide, poor peripartum care, or poor infant bonding).
OUTCOME Improved mood
After 7 days of inpatient treatment with quetiapine, Ms. A demonstrates improvement in the targeted depressive symptoms (including improved motivation/energy and insomnia, decreased feelings of guilt, and denial of ongoing suicidal ideation). Additionally, the thoughts of harming her baby are less frequent, and command auditory hallucinations resolve. Upon discharge, Ms. A and her partner meet with inpatient clinicians for continued counseling, safety planning, and plans for outpatient follow-up with the institution’s reproductive psychiatrist.
The authors’ observations
Many aspects of Ms. A’s initial presentation in the psychiatric ED were challenging. Given the presence of symptoms of both psychosis and OCD, a diagnosis was difficult to ascertain in the emergency setting. Since command auditory hallucinations are atypical in patients with postpartum OCD, the treatment team maintained high suspicion for postpartum psychosis, which represented an emergency requiring inpatient care.
Hospitalization separated Ms. A from her baby, for whom she was the primary caregiver. Additional considerations for inpatient admission and psychotropic initiation were necessary, because Ms. A was breastfeeding. Although Ms. A’s partner was able to provide full-time childcare, the patient ultimately did not agree to hospitalization and required an emergency hold for involuntary admission, which was an additional barrier to care. Furthermore, her partner held unfavorable beliefs regarding psychotropic medications and Ms. A’s need for hospital admission, which required ongoing patient and partner education in the emergency, inpatient, and outpatient settings. Moreover, if Ms. A’s symptoms were ultimately attributable to postpartum OCD, the patient’s involuntary hospitalization might have increased the risk of stigmatization of mental illness and treatment with psychotropics.
Bottom Line
The peripartum period is a vulnerable time for patients, particularly those with previously diagnosed psychiatric illnesses. Postpartum psychosis is the most severe form of postpartum psychiatric illness and often represents an episode of bipolar disorder. Due to an elevated acute risk of suicide and infanticide, postpartum psychosis is a psychiatric emergency and warrants inpatient hospitalization for immediate intervention.
Related Resources
- Sharma V. Does your patient have postpartum OCD? Current Psychiatry. 2019;18(5):9-10.
- Hatters Friedman S, Prakash C, Nagel-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
Drug Brand Names
Fluoxetine • Prozac
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Valproic acid • Depakene
1. Raza SK, Raza S. Postpartum Psychosis. StatPearls Publishing; 2023. Updated June 26, 2023. https://www.ncbi.nlm.nih.gov/books/NBK544304/
2. MGH Center for Women’s Mental Health. What Is Postpartum Psychosis: This Is What You Need to Know. MGH Center for Women’s Mental Health. Published November 15, 2019. Accessed June 22, 2023. https://womensmentalhealth.org/posts/postpartum-psychosis-ten-things-need-know-2/
3. MGH Center for Women’s Mental Health. Postpartum Psychiatric Disorders. MGH Center for Women’s Mental Health. Accessed October 7, 2023. https://womensmentalhealth.org/specialty-clinics-2/postpartum-psychiatric-disorders-2/
4. Sharma V, Sommerdyk C. Obsessive-compulsive disorder in the postpartum period: diagnosis, differential diagnosis and management. Womens Health (Lond). 2015;11(4):543-552. doi:10.2217/whe.15.20
5. Osborne LM. Recognizing and managing postpartum psychosis: a clinical guide for obstetric providers. Obstet Gynecol Clin North Am. 2018;45(3):455-468. doi:10.1016/j.ogc.2018.04.005
6. Hutner LA, Catapano LA, Nagle-Yang SM, et al, eds. Textbook of Women’s Reproductive Mental Health. American Psychiatric Association; 2022.
7. Gilden J, Kamperman AM, Munk-Olsen T, et al. Long-term outcomes of postpartum psychosis: a systematic review and meta-analysis. J Clin Psychiatry. 2020;81(2):19r12906. doi:10.4088/JCP.19r12906
8. Bergink V, Boyce P, Munk-Olsen T. Postpartum psychosis: a valuable misnomer. Aust N Z J Psychiatry. 2015;49(2):102-103. doi:10.1177/0004867414564698
CASE Thoughts of harming baby
Ms. A, age 37, is G4P2, 4 months postpartum, and breastfeeding. She has major depressive disorder (MDD) with peripartum onset, posttraumatic stress disorder, and mild intellectual disability. For years she has been stable on fluoxetine 40 mg/d and prazosin 2 mg/d. Despite recent titration of her medications, at her most recent outpatient appointment Ms. A reports having a depressed mood with frequent crying, insomnia, a lack of desire to bond with her baby, and feelings of shame. She also says she has had auditory hallucinations and thoughts of harming her baby. Ms. A’s outpatient physician makes an urgent request for her to be evaluated at the psychiatric emergency department (ED).
HISTORY Depression and possible auditory hallucinations
Ms. A developed MDD following the birth of her first child, for which her care team initiated fluoxetine at 20 mg/d and titrated it to 40 mg/d,which was effective. At that time, her outpatient physician documented potential psychotic features, including vague descriptions of derogatory auditory hallucinations. However, it was unclear if these auditory hallucinations were more representative of a distressing inner monologue without the quality of an external voice. The team determined that Ms. A was not at acute risk for harm to herself or her baby and was appropriate for outpatient care. Because the nature of these possible auditory hallucinations was mild, nondistressing, and nonthreatening, the treatment team did not initiate an antipsychotic and Ms. A was not hospitalized. She has no history of hypomanic/manic episodes and has never met criteria for a psychotic disorder.
EVALUATION Distressing thoughts and discontinued medications
During the evaluation by psychiatric emergency services, Ms. A reports that 2 weeks after giving birth she experienced a worsening of her depressive symptoms. She says she began hearing voices telling her to harm herself and her baby and describes frequent distressing thoughts, such as stabbing her baby with a knife and running over her baby with a car. Ms. A says she repeatedly wakes up at night to check on her baby’s breathing, overfeeds her baby due to a fear of inadequate nutrition, and notes intermittent feelings of confusion. Afraid of being alone with her infant, Ms. A asks her partner and mother to move in with her. Additionally, she says 2 weeks ago she discontinued all her medications at the suggestion of her partner, who recommended herbal supplements. Ms. A’s initial routine laboratory results are unremarkable and her urine drug screen is negative for all substances.
[polldaddy:13041928]
The authors’ observations
Approximately 85% of birthing parents experience some form of postpartum mood disturbance; 10% to 15% develop more significant symptoms of anxiety or depression.3 The etiology of postpartum illness is multifactorial, and includes psychiatric personal/family history, insomnia, acute and chronic psychosocial stressors, and rapid hormone fluctuations.1 As a result, the postpartum period represents a vulnerable time for birthing parents, particularly those with previously established psychiatric illness.
Ms. A’s initial presentation was concerning for a possible diagnosis of postpartum psychosis vs obsessive-compulsive disorder (OCD) with postpartum onset; other differential diagnoses included MDD with peripartum onset and psychotic features (Table1-6). Ms. A’s subjective clinical history was significant for critical pertinent findings of both OCD with postpartum onset (ie, egodystonic intrusive thoughts, checking behaviors, feelings of shame, and seeking reassurance) and postpartum psychosis (ie, command auditory hallucinations and waxing/waning confusion), which added to diagnostic complexity.

Although postpartum psychosis is rare (1 to 2 cases per 1,000 women),5 it is considered a psychiatric emergency because it has significant potential for infanticide, morbidity, and mortality. Most symptoms develop within the first 2 weeks of the postpartum period.2 There are many risk factors for the development of postpartum psychosis; however, in first-time pregnancies, a previous diagnosis of BD I is the single most important risk factor.1 Approximately 20% to 30% of women with BD experience postpartum psychosis.4
For many patients (approximately 56.7%, according to 1 meta-analysis7), postpartum psychosis denotes an episode of BD, representing a more severe form of illness with increased risk of recurrence. Most manic or mixed mood episodes reoccur within the first year removed from the perinatal period. In contrast, for some patients (approximately 43.5% according to the same meta-analysis), the episode denotes “isolated postpartum psychosis.”7 Isolated postpartum psychosis is a psychotic episode that occurs only in the postpartum period with no recurrence of psychosis or recurrence of psychosis exclusive to postpartum periods. If treated, this type of postpartum psychosis has a more favorable prognosis than postpartum psychosis in a patient with BD.7 As such, a BD diagnosis should not be established at the onset of a patient’s first postpartum psychosis presentation. Regardless of type, all presentations of postpartum psychosis are considered a psychiatry emergency.
Continue to: The prevalence of OCD...
The prevalence of OCD with postpartum onset varies. One study estimated it occurs in 2.43% of cases.4 However, the true prevalence is likely underreported due to feelings of guilt or shame associated with intrusive thoughts, and fear of stigmatization and separation from the baby. Approximately 70.6% of women experiencing OCD with postpartum onset have a comorbid depressive disorder.4
Ms. A’s presentation to the psychiatric ED carried with it diagnostic complexity and uncertainty. Her initial presentation was concerning for elements of both postpartum psychosis and OCD with postpartum onset. After her evaluation in the psychiatric ED, there remained a lack of clear and convincing evidence for a diagnosis of OCD with postpartum onset, which eliminated the possibility of discharging Ms. A with robust safety planning and reinitiation of a selective serotonin reuptake inhibitor.
Additionally, because auditory hallucinations are atypical in OCD, the treatment team remained concerned for a diagnosis of postpartum psychosis, which would warrant hospitalization. With assistance from the institution’s reproductive psychiatrists, the treatment team discussed the importance of inpatient hospitalization for risk mitigation, close observation, and thorough evaluation for greater diagnostic clarity and certainty.
TREATMENT Involuntary hospitalization
The treatment team counsels Ms. A and her partner on her differential diagnoses, including the elevated acute risk of harm to herself and her baby if she has postpartum psychosis, as well as the need for continued observation and evaluation. When alone with a clinician, Ms. A says she understands and agrees to voluntary hospitalization. However, following a subsequent risk-benefit discussion with her partner, they both grew increasingly concerned about her separation from the baby and reinitiating her medications. Amid these concerns, the treatment team notices that Ms. A attempts to minimize her symptoms. Ms. A changes her mind and no longer consents to hospitalization. She is placed on a psychiatric hold for involuntary hospitalization on the psychiatric inpatient unit.
On the inpatient unit, the inpatient clinicians and a reproductive psychiatrist continue to evaluate Ms. A. Though her diagnosis remains unclear, Ms. A agrees to start a trial of quetiapine 100 mg/d titrated to 150 mg/d to manage her potential postpartum psychosis, depressed mood, insomnia (off-label), anxiety (off-label), and OCD (off-label). Lithium is deferred because Ms. A is breastfeeding.
[polldaddy:13041932]
Continue to: The authors' observations
The authors’ observations
Due to an elevated acute risk of suicide and infanticide, postpartum psychosis represents a psychiatric emergency and often requires hospitalization. The Figure outlines steps in evaluating a patient with concerns for postpartum psychosis in a psychiatric emergency service setting. Due to the waxing and waning nature of symptoms, patients may appear psychiatrically stable at any time but remain at an overall elevated acute risk of harm to self and/or their baby.

If a patient is being considered for discharge based on yes answers to all questions in Step 2 of the Figure, the emergency psychiatric clinician must initiate appropriate psychotropic medications and complete robust safety planning with the patient and a trusted adult who will provide direct supervision. Safety planning may include (but is not limited to) strict return precautions, education on concerning symptoms and behaviors, psychotropic education and agreement of compliance, and detailed instructions on outpatient follow-up within 1 week. Ideally—and as was the case for Ms. A—a reproductive psychiatrist should be consulted in the emergency setting for shared decision-making on admission vs discharge, medication management, and outpatient follow-up considerations.
Because postpartum psychosis carries significant risks and hospitalization generally results in separating the patient from their baby, initiating psychotropics should not be delayed. Clinicians must consider the patient’s psychiatric history, allergies, and breastfeeding status.
Based on current evidence, first-line treatment for postpartum psychosis includes a mood stabilizer, an antipsychotic, and possibly a benzodiazepine.6 Thus, an appropriate initial treatment regimen would be a benzodiazepine (particularly lorazepam due to its relatively shorter half-life) and an antipsychotic (eg, haloperidol, olanzapine, or quetiapine) for acute psychosis, plus lithium for mood stabilization.1,5
If the postpartum psychosis represents an episode of BD, use of a long-term mood stabilizer may be required. In contrast, for isolated postpartum psychosis, clinicians may consider initiating psychotropics only in the immediate postpartum period, with an eventual slow taper. In future pregnancies, psychotropics may be reintroduced postpartum, which will avoid peripartum fetal exposure.8 If the patient is breastfeeding, lithium may be deferred in an acute care setting. For patients with evidence of catatonia, severe suicidality, refusal of oral intake with compromised nutrition, severe agitation, or treatment resistance, electroconvulsive therapy remains a safe and effective treatment option.6 Additionally, the safety of continued breastfeeding in acute psychosis must be considered, with the potential for recommending discontinuation, which would decrease sleep disruptions at night and increase the ability of others to feed the baby. Comprehensive care requires nonpharmacologic interventions, including psychoeducation for the patient and their family, individual psychotherapy, and expansion of psychosocial supports.
Continue to: Patients who have experienced...
Patients who have experienced an episode of postpartum psychosis are predisposed to another episode in future pregnancies.1 Current research recommends prophylaxis of recurrence with lithium monotherapy.1,2,5,6 Similar to other psychotropics in reproductive psychiatry, maintenance therapy on lithium requires a thorough “risk vs risk” discussion with the patient. The risk of lithium use while pregnant and/or breastfeeding must be weighed against the risks associated with postpartum psychosis (ie, infanticide, suicide, poor peripartum care, or poor infant bonding).
OUTCOME Improved mood
After 7 days of inpatient treatment with quetiapine, Ms. A demonstrates improvement in the targeted depressive symptoms (including improved motivation/energy and insomnia, decreased feelings of guilt, and denial of ongoing suicidal ideation). Additionally, the thoughts of harming her baby are less frequent, and command auditory hallucinations resolve. Upon discharge, Ms. A and her partner meet with inpatient clinicians for continued counseling, safety planning, and plans for outpatient follow-up with the institution’s reproductive psychiatrist.
The authors’ observations
Many aspects of Ms. A’s initial presentation in the psychiatric ED were challenging. Given the presence of symptoms of both psychosis and OCD, a diagnosis was difficult to ascertain in the emergency setting. Since command auditory hallucinations are atypical in patients with postpartum OCD, the treatment team maintained high suspicion for postpartum psychosis, which represented an emergency requiring inpatient care.
Hospitalization separated Ms. A from her baby, for whom she was the primary caregiver. Additional considerations for inpatient admission and psychotropic initiation were necessary, because Ms. A was breastfeeding. Although Ms. A’s partner was able to provide full-time childcare, the patient ultimately did not agree to hospitalization and required an emergency hold for involuntary admission, which was an additional barrier to care. Furthermore, her partner held unfavorable beliefs regarding psychotropic medications and Ms. A’s need for hospital admission, which required ongoing patient and partner education in the emergency, inpatient, and outpatient settings. Moreover, if Ms. A’s symptoms were ultimately attributable to postpartum OCD, the patient’s involuntary hospitalization might have increased the risk of stigmatization of mental illness and treatment with psychotropics.
Bottom Line
The peripartum period is a vulnerable time for patients, particularly those with previously diagnosed psychiatric illnesses. Postpartum psychosis is the most severe form of postpartum psychiatric illness and often represents an episode of bipolar disorder. Due to an elevated acute risk of suicide and infanticide, postpartum psychosis is a psychiatric emergency and warrants inpatient hospitalization for immediate intervention.
Related Resources
- Sharma V. Does your patient have postpartum OCD? Current Psychiatry. 2019;18(5):9-10.
- Hatters Friedman S, Prakash C, Nagel-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
Drug Brand Names
Fluoxetine • Prozac
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Valproic acid • Depakene
CASE Thoughts of harming baby
Ms. A, age 37, is G4P2, 4 months postpartum, and breastfeeding. She has major depressive disorder (MDD) with peripartum onset, posttraumatic stress disorder, and mild intellectual disability. For years she has been stable on fluoxetine 40 mg/d and prazosin 2 mg/d. Despite recent titration of her medications, at her most recent outpatient appointment Ms. A reports having a depressed mood with frequent crying, insomnia, a lack of desire to bond with her baby, and feelings of shame. She also says she has had auditory hallucinations and thoughts of harming her baby. Ms. A’s outpatient physician makes an urgent request for her to be evaluated at the psychiatric emergency department (ED).
HISTORY Depression and possible auditory hallucinations
Ms. A developed MDD following the birth of her first child, for which her care team initiated fluoxetine at 20 mg/d and titrated it to 40 mg/d,which was effective. At that time, her outpatient physician documented potential psychotic features, including vague descriptions of derogatory auditory hallucinations. However, it was unclear if these auditory hallucinations were more representative of a distressing inner monologue without the quality of an external voice. The team determined that Ms. A was not at acute risk for harm to herself or her baby and was appropriate for outpatient care. Because the nature of these possible auditory hallucinations was mild, nondistressing, and nonthreatening, the treatment team did not initiate an antipsychotic and Ms. A was not hospitalized. She has no history of hypomanic/manic episodes and has never met criteria for a psychotic disorder.
EVALUATION Distressing thoughts and discontinued medications
During the evaluation by psychiatric emergency services, Ms. A reports that 2 weeks after giving birth she experienced a worsening of her depressive symptoms. She says she began hearing voices telling her to harm herself and her baby and describes frequent distressing thoughts, such as stabbing her baby with a knife and running over her baby with a car. Ms. A says she repeatedly wakes up at night to check on her baby’s breathing, overfeeds her baby due to a fear of inadequate nutrition, and notes intermittent feelings of confusion. Afraid of being alone with her infant, Ms. A asks her partner and mother to move in with her. Additionally, she says 2 weeks ago she discontinued all her medications at the suggestion of her partner, who recommended herbal supplements. Ms. A’s initial routine laboratory results are unremarkable and her urine drug screen is negative for all substances.
[polldaddy:13041928]
The authors’ observations
Approximately 85% of birthing parents experience some form of postpartum mood disturbance; 10% to 15% develop more significant symptoms of anxiety or depression.3 The etiology of postpartum illness is multifactorial, and includes psychiatric personal/family history, insomnia, acute and chronic psychosocial stressors, and rapid hormone fluctuations.1 As a result, the postpartum period represents a vulnerable time for birthing parents, particularly those with previously established psychiatric illness.
Ms. A’s initial presentation was concerning for a possible diagnosis of postpartum psychosis vs obsessive-compulsive disorder (OCD) with postpartum onset; other differential diagnoses included MDD with peripartum onset and psychotic features (Table1-6). Ms. A’s subjective clinical history was significant for critical pertinent findings of both OCD with postpartum onset (ie, egodystonic intrusive thoughts, checking behaviors, feelings of shame, and seeking reassurance) and postpartum psychosis (ie, command auditory hallucinations and waxing/waning confusion), which added to diagnostic complexity.

Although postpartum psychosis is rare (1 to 2 cases per 1,000 women),5 it is considered a psychiatric emergency because it has significant potential for infanticide, morbidity, and mortality. Most symptoms develop within the first 2 weeks of the postpartum period.2 There are many risk factors for the development of postpartum psychosis; however, in first-time pregnancies, a previous diagnosis of BD I is the single most important risk factor.1 Approximately 20% to 30% of women with BD experience postpartum psychosis.4
For many patients (approximately 56.7%, according to 1 meta-analysis7), postpartum psychosis denotes an episode of BD, representing a more severe form of illness with increased risk of recurrence. Most manic or mixed mood episodes reoccur within the first year removed from the perinatal period. In contrast, for some patients (approximately 43.5% according to the same meta-analysis), the episode denotes “isolated postpartum psychosis.”7 Isolated postpartum psychosis is a psychotic episode that occurs only in the postpartum period with no recurrence of psychosis or recurrence of psychosis exclusive to postpartum periods. If treated, this type of postpartum psychosis has a more favorable prognosis than postpartum psychosis in a patient with BD.7 As such, a BD diagnosis should not be established at the onset of a patient’s first postpartum psychosis presentation. Regardless of type, all presentations of postpartum psychosis are considered a psychiatry emergency.
Continue to: The prevalence of OCD...
The prevalence of OCD with postpartum onset varies. One study estimated it occurs in 2.43% of cases.4 However, the true prevalence is likely underreported due to feelings of guilt or shame associated with intrusive thoughts, and fear of stigmatization and separation from the baby. Approximately 70.6% of women experiencing OCD with postpartum onset have a comorbid depressive disorder.4
Ms. A’s presentation to the psychiatric ED carried with it diagnostic complexity and uncertainty. Her initial presentation was concerning for elements of both postpartum psychosis and OCD with postpartum onset. After her evaluation in the psychiatric ED, there remained a lack of clear and convincing evidence for a diagnosis of OCD with postpartum onset, which eliminated the possibility of discharging Ms. A with robust safety planning and reinitiation of a selective serotonin reuptake inhibitor.
Additionally, because auditory hallucinations are atypical in OCD, the treatment team remained concerned for a diagnosis of postpartum psychosis, which would warrant hospitalization. With assistance from the institution’s reproductive psychiatrists, the treatment team discussed the importance of inpatient hospitalization for risk mitigation, close observation, and thorough evaluation for greater diagnostic clarity and certainty.
TREATMENT Involuntary hospitalization
The treatment team counsels Ms. A and her partner on her differential diagnoses, including the elevated acute risk of harm to herself and her baby if she has postpartum psychosis, as well as the need for continued observation and evaluation. When alone with a clinician, Ms. A says she understands and agrees to voluntary hospitalization. However, following a subsequent risk-benefit discussion with her partner, they both grew increasingly concerned about her separation from the baby and reinitiating her medications. Amid these concerns, the treatment team notices that Ms. A attempts to minimize her symptoms. Ms. A changes her mind and no longer consents to hospitalization. She is placed on a psychiatric hold for involuntary hospitalization on the psychiatric inpatient unit.
On the inpatient unit, the inpatient clinicians and a reproductive psychiatrist continue to evaluate Ms. A. Though her diagnosis remains unclear, Ms. A agrees to start a trial of quetiapine 100 mg/d titrated to 150 mg/d to manage her potential postpartum psychosis, depressed mood, insomnia (off-label), anxiety (off-label), and OCD (off-label). Lithium is deferred because Ms. A is breastfeeding.
[polldaddy:13041932]
Continue to: The authors' observations
The authors’ observations
Due to an elevated acute risk of suicide and infanticide, postpartum psychosis represents a psychiatric emergency and often requires hospitalization. The Figure outlines steps in evaluating a patient with concerns for postpartum psychosis in a psychiatric emergency service setting. Due to the waxing and waning nature of symptoms, patients may appear psychiatrically stable at any time but remain at an overall elevated acute risk of harm to self and/or their baby.

If a patient is being considered for discharge based on yes answers to all questions in Step 2 of the Figure, the emergency psychiatric clinician must initiate appropriate psychotropic medications and complete robust safety planning with the patient and a trusted adult who will provide direct supervision. Safety planning may include (but is not limited to) strict return precautions, education on concerning symptoms and behaviors, psychotropic education and agreement of compliance, and detailed instructions on outpatient follow-up within 1 week. Ideally—and as was the case for Ms. A—a reproductive psychiatrist should be consulted in the emergency setting for shared decision-making on admission vs discharge, medication management, and outpatient follow-up considerations.
Because postpartum psychosis carries significant risks and hospitalization generally results in separating the patient from their baby, initiating psychotropics should not be delayed. Clinicians must consider the patient’s psychiatric history, allergies, and breastfeeding status.
Based on current evidence, first-line treatment for postpartum psychosis includes a mood stabilizer, an antipsychotic, and possibly a benzodiazepine.6 Thus, an appropriate initial treatment regimen would be a benzodiazepine (particularly lorazepam due to its relatively shorter half-life) and an antipsychotic (eg, haloperidol, olanzapine, or quetiapine) for acute psychosis, plus lithium for mood stabilization.1,5
If the postpartum psychosis represents an episode of BD, use of a long-term mood stabilizer may be required. In contrast, for isolated postpartum psychosis, clinicians may consider initiating psychotropics only in the immediate postpartum period, with an eventual slow taper. In future pregnancies, psychotropics may be reintroduced postpartum, which will avoid peripartum fetal exposure.8 If the patient is breastfeeding, lithium may be deferred in an acute care setting. For patients with evidence of catatonia, severe suicidality, refusal of oral intake with compromised nutrition, severe agitation, or treatment resistance, electroconvulsive therapy remains a safe and effective treatment option.6 Additionally, the safety of continued breastfeeding in acute psychosis must be considered, with the potential for recommending discontinuation, which would decrease sleep disruptions at night and increase the ability of others to feed the baby. Comprehensive care requires nonpharmacologic interventions, including psychoeducation for the patient and their family, individual psychotherapy, and expansion of psychosocial supports.
Continue to: Patients who have experienced...
Patients who have experienced an episode of postpartum psychosis are predisposed to another episode in future pregnancies.1 Current research recommends prophylaxis of recurrence with lithium monotherapy.1,2,5,6 Similar to other psychotropics in reproductive psychiatry, maintenance therapy on lithium requires a thorough “risk vs risk” discussion with the patient. The risk of lithium use while pregnant and/or breastfeeding must be weighed against the risks associated with postpartum psychosis (ie, infanticide, suicide, poor peripartum care, or poor infant bonding).
OUTCOME Improved mood
After 7 days of inpatient treatment with quetiapine, Ms. A demonstrates improvement in the targeted depressive symptoms (including improved motivation/energy and insomnia, decreased feelings of guilt, and denial of ongoing suicidal ideation). Additionally, the thoughts of harming her baby are less frequent, and command auditory hallucinations resolve. Upon discharge, Ms. A and her partner meet with inpatient clinicians for continued counseling, safety planning, and plans for outpatient follow-up with the institution’s reproductive psychiatrist.
The authors’ observations
Many aspects of Ms. A’s initial presentation in the psychiatric ED were challenging. Given the presence of symptoms of both psychosis and OCD, a diagnosis was difficult to ascertain in the emergency setting. Since command auditory hallucinations are atypical in patients with postpartum OCD, the treatment team maintained high suspicion for postpartum psychosis, which represented an emergency requiring inpatient care.
Hospitalization separated Ms. A from her baby, for whom she was the primary caregiver. Additional considerations for inpatient admission and psychotropic initiation were necessary, because Ms. A was breastfeeding. Although Ms. A’s partner was able to provide full-time childcare, the patient ultimately did not agree to hospitalization and required an emergency hold for involuntary admission, which was an additional barrier to care. Furthermore, her partner held unfavorable beliefs regarding psychotropic medications and Ms. A’s need for hospital admission, which required ongoing patient and partner education in the emergency, inpatient, and outpatient settings. Moreover, if Ms. A’s symptoms were ultimately attributable to postpartum OCD, the patient’s involuntary hospitalization might have increased the risk of stigmatization of mental illness and treatment with psychotropics.
Bottom Line
The peripartum period is a vulnerable time for patients, particularly those with previously diagnosed psychiatric illnesses. Postpartum psychosis is the most severe form of postpartum psychiatric illness and often represents an episode of bipolar disorder. Due to an elevated acute risk of suicide and infanticide, postpartum psychosis is a psychiatric emergency and warrants inpatient hospitalization for immediate intervention.
Related Resources
- Sharma V. Does your patient have postpartum OCD? Current Psychiatry. 2019;18(5):9-10.
- Hatters Friedman S, Prakash C, Nagel-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
Drug Brand Names
Fluoxetine • Prozac
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Valproic acid • Depakene
1. Raza SK, Raza S. Postpartum Psychosis. StatPearls Publishing; 2023. Updated June 26, 2023. https://www.ncbi.nlm.nih.gov/books/NBK544304/
2. MGH Center for Women’s Mental Health. What Is Postpartum Psychosis: This Is What You Need to Know. MGH Center for Women’s Mental Health. Published November 15, 2019. Accessed June 22, 2023. https://womensmentalhealth.org/posts/postpartum-psychosis-ten-things-need-know-2/
3. MGH Center for Women’s Mental Health. Postpartum Psychiatric Disorders. MGH Center for Women’s Mental Health. Accessed October 7, 2023. https://womensmentalhealth.org/specialty-clinics-2/postpartum-psychiatric-disorders-2/
4. Sharma V, Sommerdyk C. Obsessive-compulsive disorder in the postpartum period: diagnosis, differential diagnosis and management. Womens Health (Lond). 2015;11(4):543-552. doi:10.2217/whe.15.20
5. Osborne LM. Recognizing and managing postpartum psychosis: a clinical guide for obstetric providers. Obstet Gynecol Clin North Am. 2018;45(3):455-468. doi:10.1016/j.ogc.2018.04.005
6. Hutner LA, Catapano LA, Nagle-Yang SM, et al, eds. Textbook of Women’s Reproductive Mental Health. American Psychiatric Association; 2022.
7. Gilden J, Kamperman AM, Munk-Olsen T, et al. Long-term outcomes of postpartum psychosis: a systematic review and meta-analysis. J Clin Psychiatry. 2020;81(2):19r12906. doi:10.4088/JCP.19r12906
8. Bergink V, Boyce P, Munk-Olsen T. Postpartum psychosis: a valuable misnomer. Aust N Z J Psychiatry. 2015;49(2):102-103. doi:10.1177/0004867414564698
1. Raza SK, Raza S. Postpartum Psychosis. StatPearls Publishing; 2023. Updated June 26, 2023. https://www.ncbi.nlm.nih.gov/books/NBK544304/
2. MGH Center for Women’s Mental Health. What Is Postpartum Psychosis: This Is What You Need to Know. MGH Center for Women’s Mental Health. Published November 15, 2019. Accessed June 22, 2023. https://womensmentalhealth.org/posts/postpartum-psychosis-ten-things-need-know-2/
3. MGH Center for Women’s Mental Health. Postpartum Psychiatric Disorders. MGH Center for Women’s Mental Health. Accessed October 7, 2023. https://womensmentalhealth.org/specialty-clinics-2/postpartum-psychiatric-disorders-2/
4. Sharma V, Sommerdyk C. Obsessive-compulsive disorder in the postpartum period: diagnosis, differential diagnosis and management. Womens Health (Lond). 2015;11(4):543-552. doi:10.2217/whe.15.20
5. Osborne LM. Recognizing and managing postpartum psychosis: a clinical guide for obstetric providers. Obstet Gynecol Clin North Am. 2018;45(3):455-468. doi:10.1016/j.ogc.2018.04.005
6. Hutner LA, Catapano LA, Nagle-Yang SM, et al, eds. Textbook of Women’s Reproductive Mental Health. American Psychiatric Association; 2022.
7. Gilden J, Kamperman AM, Munk-Olsen T, et al. Long-term outcomes of postpartum psychosis: a systematic review and meta-analysis. J Clin Psychiatry. 2020;81(2):19r12906. doi:10.4088/JCP.19r12906
8. Bergink V, Boyce P, Munk-Olsen T. Postpartum psychosis: a valuable misnomer. Aust N Z J Psychiatry. 2015;49(2):102-103. doi:10.1177/0004867414564698
Perinatal psychiatric screening: What to ask
Perinatal psychiatry focuses on the evaluation, diagnosis, and treatment of mental health disorders during the preconception, pregnancy, and postpartum periods. Mood disorders, anxiety disorders, and posttraumatic stress disorder are the most common mental health conditions that arise during the perinatal period.1 Mediating factors include hormone fluctuations, sleep deprivation, trauma exposure, financial stress, having a history of psychiatric illness, and factors related to newborn care.
During the perinatal period, a comprehensive psychiatric interview is crucial. Effective screening and identification of maternal mental health conditions necessitate more than merely checking off boxes on a questionnaire. It requires compassionate, informed, and individualized conversations between physicians and their patients.
In addition to asking about pertinent positive and negative psychiatric symptoms, the following screening questions could be asked during a structured interview to identify perinatal issues during pregnancy and the postpartum period.
During pregnancy
- How do you feel about your pregnancy?
- Was this pregnancy planned or unplanned, desired or not?
- Was fertility treatment needed or used?
- Did you think about stopping the pregnancy? If so, was your decision influenced by laws that restrict abortion in your state?
- Do you feel connected to the fetus?
- Do you have a room or crib at home for the baby? A car seat? Clothing? Baby supplies?
- Are you planning on breastfeeding?
- Do you have thoughts on future desired fertility and/or contraception?
- Who is your support system at home?
- How is your relationship with the baby’s father?
- How is the baby’s father’s mental well-being?
- Have you been subject to any abuse, intimate partner violence, or neglect?
- Are your other children being taken care of properly? What is the plan for them during delivery days at the hospital?
During the postpartum period
- Was your baby born prematurely?
- Did you have a vaginal or cesarean delivery?
- Did you encounter any delivery complications?
- Did you see the baby after the delivery?
- Do you feel connected to or able to bond with the baby?
- Do you have access to maternity leave from work?
- Have you had scary or upsetting thoughts about hurting your baby?
- Do you have any concerns about your treatment plan, such as medication use?
- In case of an emergency, are you aware of perinatal psychiatry resources in your area or the national maternal mental health hotline (833-852-6262)?
The American College of Obstetricians and Gynecologists clinical practice guidelines recommend that clinicians conduct depression and anxiety screening at least once during the perinatal period by using a standardized, validated tool.2 Psychiatry residents should receive adequate guidance and education about perinatal psychiatric evaluation, risk assessment, and treatment counseling. Early detection of mental health symptoms allows for early referral, close surveillance during episodes of vulnerability, and better access to mental health care during the perinatal period.
1. Howard LM, Khalifeh H. Perinatal mental health: a review of progress and challenges. World Psychiatry. 2020;19(3):313-327. doi:10.1002/wps.20769
2. American College of Obstetricians and Gynecologists. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline Number 4. June 2023. Accessed November 3, 2023. https://www.acog.org/clinical/clinical-guidance/clinical-practice-guideline/articles/2023/06/screening-and-diagnosis-of-mental-health-conditions-during-pregnancy-and-postpartum
Perinatal psychiatry focuses on the evaluation, diagnosis, and treatment of mental health disorders during the preconception, pregnancy, and postpartum periods. Mood disorders, anxiety disorders, and posttraumatic stress disorder are the most common mental health conditions that arise during the perinatal period.1 Mediating factors include hormone fluctuations, sleep deprivation, trauma exposure, financial stress, having a history of psychiatric illness, and factors related to newborn care.
During the perinatal period, a comprehensive psychiatric interview is crucial. Effective screening and identification of maternal mental health conditions necessitate more than merely checking off boxes on a questionnaire. It requires compassionate, informed, and individualized conversations between physicians and their patients.
In addition to asking about pertinent positive and negative psychiatric symptoms, the following screening questions could be asked during a structured interview to identify perinatal issues during pregnancy and the postpartum period.
During pregnancy
- How do you feel about your pregnancy?
- Was this pregnancy planned or unplanned, desired or not?
- Was fertility treatment needed or used?
- Did you think about stopping the pregnancy? If so, was your decision influenced by laws that restrict abortion in your state?
- Do you feel connected to the fetus?
- Do you have a room or crib at home for the baby? A car seat? Clothing? Baby supplies?
- Are you planning on breastfeeding?
- Do you have thoughts on future desired fertility and/or contraception?
- Who is your support system at home?
- How is your relationship with the baby’s father?
- How is the baby’s father’s mental well-being?
- Have you been subject to any abuse, intimate partner violence, or neglect?
- Are your other children being taken care of properly? What is the plan for them during delivery days at the hospital?
During the postpartum period
- Was your baby born prematurely?
- Did you have a vaginal or cesarean delivery?
- Did you encounter any delivery complications?
- Did you see the baby after the delivery?
- Do you feel connected to or able to bond with the baby?
- Do you have access to maternity leave from work?
- Have you had scary or upsetting thoughts about hurting your baby?
- Do you have any concerns about your treatment plan, such as medication use?
- In case of an emergency, are you aware of perinatal psychiatry resources in your area or the national maternal mental health hotline (833-852-6262)?
The American College of Obstetricians and Gynecologists clinical practice guidelines recommend that clinicians conduct depression and anxiety screening at least once during the perinatal period by using a standardized, validated tool.2 Psychiatry residents should receive adequate guidance and education about perinatal psychiatric evaluation, risk assessment, and treatment counseling. Early detection of mental health symptoms allows for early referral, close surveillance during episodes of vulnerability, and better access to mental health care during the perinatal period.
Perinatal psychiatry focuses on the evaluation, diagnosis, and treatment of mental health disorders during the preconception, pregnancy, and postpartum periods. Mood disorders, anxiety disorders, and posttraumatic stress disorder are the most common mental health conditions that arise during the perinatal period.1 Mediating factors include hormone fluctuations, sleep deprivation, trauma exposure, financial stress, having a history of psychiatric illness, and factors related to newborn care.
During the perinatal period, a comprehensive psychiatric interview is crucial. Effective screening and identification of maternal mental health conditions necessitate more than merely checking off boxes on a questionnaire. It requires compassionate, informed, and individualized conversations between physicians and their patients.
In addition to asking about pertinent positive and negative psychiatric symptoms, the following screening questions could be asked during a structured interview to identify perinatal issues during pregnancy and the postpartum period.
During pregnancy
- How do you feel about your pregnancy?
- Was this pregnancy planned or unplanned, desired or not?
- Was fertility treatment needed or used?
- Did you think about stopping the pregnancy? If so, was your decision influenced by laws that restrict abortion in your state?
- Do you feel connected to the fetus?
- Do you have a room or crib at home for the baby? A car seat? Clothing? Baby supplies?
- Are you planning on breastfeeding?
- Do you have thoughts on future desired fertility and/or contraception?
- Who is your support system at home?
- How is your relationship with the baby’s father?
- How is the baby’s father’s mental well-being?
- Have you been subject to any abuse, intimate partner violence, or neglect?
- Are your other children being taken care of properly? What is the plan for them during delivery days at the hospital?
During the postpartum period
- Was your baby born prematurely?
- Did you have a vaginal or cesarean delivery?
- Did you encounter any delivery complications?
- Did you see the baby after the delivery?
- Do you feel connected to or able to bond with the baby?
- Do you have access to maternity leave from work?
- Have you had scary or upsetting thoughts about hurting your baby?
- Do you have any concerns about your treatment plan, such as medication use?
- In case of an emergency, are you aware of perinatal psychiatry resources in your area or the national maternal mental health hotline (833-852-6262)?
The American College of Obstetricians and Gynecologists clinical practice guidelines recommend that clinicians conduct depression and anxiety screening at least once during the perinatal period by using a standardized, validated tool.2 Psychiatry residents should receive adequate guidance and education about perinatal psychiatric evaluation, risk assessment, and treatment counseling. Early detection of mental health symptoms allows for early referral, close surveillance during episodes of vulnerability, and better access to mental health care during the perinatal period.
1. Howard LM, Khalifeh H. Perinatal mental health: a review of progress and challenges. World Psychiatry. 2020;19(3):313-327. doi:10.1002/wps.20769
2. American College of Obstetricians and Gynecologists. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline Number 4. June 2023. Accessed November 3, 2023. https://www.acog.org/clinical/clinical-guidance/clinical-practice-guideline/articles/2023/06/screening-and-diagnosis-of-mental-health-conditions-during-pregnancy-and-postpartum
1. Howard LM, Khalifeh H. Perinatal mental health: a review of progress and challenges. World Psychiatry. 2020;19(3):313-327. doi:10.1002/wps.20769
2. American College of Obstetricians and Gynecologists. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline Number 4. June 2023. Accessed November 3, 2023. https://www.acog.org/clinical/clinical-guidance/clinical-practice-guideline/articles/2023/06/screening-and-diagnosis-of-mental-health-conditions-during-pregnancy-and-postpartum
Worsening mania while receiving low-dose quetiapine: A case report
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
The second-generation antipsychotic quetiapine is commonly used to treat several psychiatric disorders, including bipolar disorder (BD) and insomnia. In this case report, we discuss a patient with a history of unipolar depression and initial signs of mania who experienced an exacerbation of manic symptoms following administration of low-dose quetiapine. This case underscores the need for careful monitoring of patients receiving quetiapine, especially at lower doses, and the potential limitations of its efficacy in controlling manic symptoms.
Depressed with racing thoughts
Mr. X, age 58, is an Army veteran who lives with his wife of 29 years and works as a contractor. He has a history of depression and a suicide attempt 10 years ago by self-inflicted gunshot wound to the head, which left him with a bullet lodged in his sinus cavity and residual dysarthria after tongue surgery. After the suicide attempt, Mr. X was medically hospitalized, but not psychiatrically hospitalized. Shortly after, he self-discontinued all psychotropic medications and follow-up.
Mr. X has no other medical history and takes no other medications or supplements. His family history includes a mother with schizoaffective disorder, 1 brother with BD, and another brother with developmental delay.
Mr. X remained euthymic until his brother died. Soon after, he began to experience low mood, heightened anxiety, racing thoughts, tearfulness, and mild insomnia. He was prescribed quetiapine 25 mg/d at bedtime and instructed to titrate up to 50 mg/d.
Ten days later, Mr. X was brought to the hospital by his wife, who reported that after starting quetiapine, her husband began to act erratically. He had disorganized and racing thoughts, loose associations, labile affect, hyperactivity/restlessness, and was not sleeping. In the morning before presenting to the hospital, Mr. X had gone to work, laid down on the floor, began mumbling to himself, and would not respond to coworkers. Upon evaluation, Mr. X was noted to have pressured speech, disorganized speech, delusions, anxiety, and hallucinations. A CT scan of his head was normal, and a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, B12, folate, and hemoglobin A1c were within normal limits. Mr. X’s vitamin D level was low at 22 ng/mL, and a syphilis screen was negative.
Mr. X was admitted to the hospital for his safety. The treatment team discontinued quetiapine and started risperidone 3 mg twice a day for psychotic symptoms and mood stabilization. At the time of discharge 7 days later, Mr. X was no longer experiencing any hallucinations or delusions, his thought process was linear and goal-directed, his mood was stable, and his insomnia had improved. Based on the temporal relationship between the initiation of quetiapine and the onset of Mr. X’s manic symptoms, along with an absence of organic causes, the treatment team suspected Mr. X had experienced a worsening of manic symptoms induced by quetiapine. Before starting quetiapine, he had presented with an initial manic symptom of racing thoughts.
At his next outpatient appointment, Mr. X exhibited significant akathisia. The treatment team initiated propranolol 20 mg twice a day but Mr. X did not experience much improvement. Risperidone was reduced to 1 mg twice a day and Mr. X was started on clonazepam 0.5 mg twice a day. The akathisia resolved. The treatment team decided to discontinue all medications and observe Mr. X for any recurrence of symptoms. One year after his manic episode. Mr. X remained euthymic. He was able to resume full-time work and began psychotherapy to process the grief over the loss of his brother.
Quetiapine’s unique profile
This case sheds light on the potential limitations of quetiapine, especially at lower doses, for managing manic symptoms. Quetiapine exhibits antidepressant effects, even at doses as low as 50 mg/d.1 At higher doses, quetiapine acts as an antagonist at serotonin (5-HT1A and 5-HT2A), dopamine (D1 and D2), histamine H1, and adrenergic receptors.2 At doses <300 mg/d, there is an absence of dopamine receptor blockade and a higher affinity for 5-HT2A receptors, which could explain why higher doses are generally necessary for treating mania and psychotic symptoms.3-5 High 5-HT2A antagonism may disinhibit the dopaminergic system and paradoxically increase dopaminergic activity, which could be the mechanism responsible for lack of control of manic symptoms with low doses of quetiapine.2 Another possible explanation is that the metabolite of quetiapine, N-desalkylquetiapine, acts as a norepinephrine reuptake blocker and partial 5-HT1Aantagonist, which acts as an antidepressant, and antidepressants are known to induce mania in vulnerable patients.4
The antimanic property of most antipsychotics (except possibly clozapine) is attributed to their D2 antagonistic potency. Because quetiapine is among the weaker D2 antagonists, its inability to prevent the progression of mania, especially at 50 mg/d, is not unexpected. Mr. X’s subsequent need for a stronger D2 antagonist—risperidone—at a significant dose further supports this observation. A common misconception is that quetiapine’s sedating effects make it effective for treating mania, but that is not the case. Clinicians should be cautious when prescribing quetiapine, especially at lower doses, to patients who exhibit signs of mania. Given the potential risk, clinicians should consider alternative treatments before resorting to low-dose quetiapine for insomnia. Regular monitoring for manic symptoms is crucial for all patients receiving quetiapine. If patients present with signs of mania or hypomania, a therapeutic dose range of 600 to 800 mg/d is recommended.6
- Weisler R, Joyce M, McGill L, et al. Extended release quetiapine fumarate monotherapy for major depressive disorder: results of a double-blind, randomized, placebo-controlled study. CNS Spectr. 2009;14(6):299-313. doi:10.1017/s1092852900020307
- Khalil RB, Baddoura C. Quetiapine induced hypomania: a case report and a review of the literature. Curr Drug Saf. 2012;7(3):250-253. doi:10.2174/157488612803251333
- Benyamina A, Samalin L. Atypical antipsychotic-induced mania/hypomania: a review of recent case reports and clinical studies. Int J Psychiatry Clin Pract. 2012;16(1):2-7. doi:10.3109/13651501.2011.605957
- Gnanavel S. Quetiapine-induced manic episode: a paradox for contemplation. BMJ Case Rep. 2013;2013:bcr2013201761. doi:10.1136/bcr-2013-201761
- Pacchiarotti I, Manfredi G, Kotzalidis GD, et al. Quetiapine-induced mania. Aust N Z J Psychiatry. 2003;37(5):626.
- Millard HY, Wilson BA, Noordsy DL. Low-dose quetiapine induced or worsened mania in the context of possible undertreatment. J Am Board Fam Med. 2015;28(1):154-158. doi:10.3122/jabfm.2015.01.140105
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
The second-generation antipsychotic quetiapine is commonly used to treat several psychiatric disorders, including bipolar disorder (BD) and insomnia. In this case report, we discuss a patient with a history of unipolar depression and initial signs of mania who experienced an exacerbation of manic symptoms following administration of low-dose quetiapine. This case underscores the need for careful monitoring of patients receiving quetiapine, especially at lower doses, and the potential limitations of its efficacy in controlling manic symptoms.
Depressed with racing thoughts
Mr. X, age 58, is an Army veteran who lives with his wife of 29 years and works as a contractor. He has a history of depression and a suicide attempt 10 years ago by self-inflicted gunshot wound to the head, which left him with a bullet lodged in his sinus cavity and residual dysarthria after tongue surgery. After the suicide attempt, Mr. X was medically hospitalized, but not psychiatrically hospitalized. Shortly after, he self-discontinued all psychotropic medications and follow-up.
Mr. X has no other medical history and takes no other medications or supplements. His family history includes a mother with schizoaffective disorder, 1 brother with BD, and another brother with developmental delay.
Mr. X remained euthymic until his brother died. Soon after, he began to experience low mood, heightened anxiety, racing thoughts, tearfulness, and mild insomnia. He was prescribed quetiapine 25 mg/d at bedtime and instructed to titrate up to 50 mg/d.
Ten days later, Mr. X was brought to the hospital by his wife, who reported that after starting quetiapine, her husband began to act erratically. He had disorganized and racing thoughts, loose associations, labile affect, hyperactivity/restlessness, and was not sleeping. In the morning before presenting to the hospital, Mr. X had gone to work, laid down on the floor, began mumbling to himself, and would not respond to coworkers. Upon evaluation, Mr. X was noted to have pressured speech, disorganized speech, delusions, anxiety, and hallucinations. A CT scan of his head was normal, and a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, B12, folate, and hemoglobin A1c were within normal limits. Mr. X’s vitamin D level was low at 22 ng/mL, and a syphilis screen was negative.
Mr. X was admitted to the hospital for his safety. The treatment team discontinued quetiapine and started risperidone 3 mg twice a day for psychotic symptoms and mood stabilization. At the time of discharge 7 days later, Mr. X was no longer experiencing any hallucinations or delusions, his thought process was linear and goal-directed, his mood was stable, and his insomnia had improved. Based on the temporal relationship between the initiation of quetiapine and the onset of Mr. X’s manic symptoms, along with an absence of organic causes, the treatment team suspected Mr. X had experienced a worsening of manic symptoms induced by quetiapine. Before starting quetiapine, he had presented with an initial manic symptom of racing thoughts.
At his next outpatient appointment, Mr. X exhibited significant akathisia. The treatment team initiated propranolol 20 mg twice a day but Mr. X did not experience much improvement. Risperidone was reduced to 1 mg twice a day and Mr. X was started on clonazepam 0.5 mg twice a day. The akathisia resolved. The treatment team decided to discontinue all medications and observe Mr. X for any recurrence of symptoms. One year after his manic episode. Mr. X remained euthymic. He was able to resume full-time work and began psychotherapy to process the grief over the loss of his brother.
Quetiapine’s unique profile
This case sheds light on the potential limitations of quetiapine, especially at lower doses, for managing manic symptoms. Quetiapine exhibits antidepressant effects, even at doses as low as 50 mg/d.1 At higher doses, quetiapine acts as an antagonist at serotonin (5-HT1A and 5-HT2A), dopamine (D1 and D2), histamine H1, and adrenergic receptors.2 At doses <300 mg/d, there is an absence of dopamine receptor blockade and a higher affinity for 5-HT2A receptors, which could explain why higher doses are generally necessary for treating mania and psychotic symptoms.3-5 High 5-HT2A antagonism may disinhibit the dopaminergic system and paradoxically increase dopaminergic activity, which could be the mechanism responsible for lack of control of manic symptoms with low doses of quetiapine.2 Another possible explanation is that the metabolite of quetiapine, N-desalkylquetiapine, acts as a norepinephrine reuptake blocker and partial 5-HT1Aantagonist, which acts as an antidepressant, and antidepressants are known to induce mania in vulnerable patients.4
The antimanic property of most antipsychotics (except possibly clozapine) is attributed to their D2 antagonistic potency. Because quetiapine is among the weaker D2 antagonists, its inability to prevent the progression of mania, especially at 50 mg/d, is not unexpected. Mr. X’s subsequent need for a stronger D2 antagonist—risperidone—at a significant dose further supports this observation. A common misconception is that quetiapine’s sedating effects make it effective for treating mania, but that is not the case. Clinicians should be cautious when prescribing quetiapine, especially at lower doses, to patients who exhibit signs of mania. Given the potential risk, clinicians should consider alternative treatments before resorting to low-dose quetiapine for insomnia. Regular monitoring for manic symptoms is crucial for all patients receiving quetiapine. If patients present with signs of mania or hypomania, a therapeutic dose range of 600 to 800 mg/d is recommended.6
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
The second-generation antipsychotic quetiapine is commonly used to treat several psychiatric disorders, including bipolar disorder (BD) and insomnia. In this case report, we discuss a patient with a history of unipolar depression and initial signs of mania who experienced an exacerbation of manic symptoms following administration of low-dose quetiapine. This case underscores the need for careful monitoring of patients receiving quetiapine, especially at lower doses, and the potential limitations of its efficacy in controlling manic symptoms.
Depressed with racing thoughts
Mr. X, age 58, is an Army veteran who lives with his wife of 29 years and works as a contractor. He has a history of depression and a suicide attempt 10 years ago by self-inflicted gunshot wound to the head, which left him with a bullet lodged in his sinus cavity and residual dysarthria after tongue surgery. After the suicide attempt, Mr. X was medically hospitalized, but not psychiatrically hospitalized. Shortly after, he self-discontinued all psychotropic medications and follow-up.
Mr. X has no other medical history and takes no other medications or supplements. His family history includes a mother with schizoaffective disorder, 1 brother with BD, and another brother with developmental delay.
Mr. X remained euthymic until his brother died. Soon after, he began to experience low mood, heightened anxiety, racing thoughts, tearfulness, and mild insomnia. He was prescribed quetiapine 25 mg/d at bedtime and instructed to titrate up to 50 mg/d.
Ten days later, Mr. X was brought to the hospital by his wife, who reported that after starting quetiapine, her husband began to act erratically. He had disorganized and racing thoughts, loose associations, labile affect, hyperactivity/restlessness, and was not sleeping. In the morning before presenting to the hospital, Mr. X had gone to work, laid down on the floor, began mumbling to himself, and would not respond to coworkers. Upon evaluation, Mr. X was noted to have pressured speech, disorganized speech, delusions, anxiety, and hallucinations. A CT scan of his head was normal, and a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, B12, folate, and hemoglobin A1c were within normal limits. Mr. X’s vitamin D level was low at 22 ng/mL, and a syphilis screen was negative.
Mr. X was admitted to the hospital for his safety. The treatment team discontinued quetiapine and started risperidone 3 mg twice a day for psychotic symptoms and mood stabilization. At the time of discharge 7 days later, Mr. X was no longer experiencing any hallucinations or delusions, his thought process was linear and goal-directed, his mood was stable, and his insomnia had improved. Based on the temporal relationship between the initiation of quetiapine and the onset of Mr. X’s manic symptoms, along with an absence of organic causes, the treatment team suspected Mr. X had experienced a worsening of manic symptoms induced by quetiapine. Before starting quetiapine, he had presented with an initial manic symptom of racing thoughts.
At his next outpatient appointment, Mr. X exhibited significant akathisia. The treatment team initiated propranolol 20 mg twice a day but Mr. X did not experience much improvement. Risperidone was reduced to 1 mg twice a day and Mr. X was started on clonazepam 0.5 mg twice a day. The akathisia resolved. The treatment team decided to discontinue all medications and observe Mr. X for any recurrence of symptoms. One year after his manic episode. Mr. X remained euthymic. He was able to resume full-time work and began psychotherapy to process the grief over the loss of his brother.
Quetiapine’s unique profile
This case sheds light on the potential limitations of quetiapine, especially at lower doses, for managing manic symptoms. Quetiapine exhibits antidepressant effects, even at doses as low as 50 mg/d.1 At higher doses, quetiapine acts as an antagonist at serotonin (5-HT1A and 5-HT2A), dopamine (D1 and D2), histamine H1, and adrenergic receptors.2 At doses <300 mg/d, there is an absence of dopamine receptor blockade and a higher affinity for 5-HT2A receptors, which could explain why higher doses are generally necessary for treating mania and psychotic symptoms.3-5 High 5-HT2A antagonism may disinhibit the dopaminergic system and paradoxically increase dopaminergic activity, which could be the mechanism responsible for lack of control of manic symptoms with low doses of quetiapine.2 Another possible explanation is that the metabolite of quetiapine, N-desalkylquetiapine, acts as a norepinephrine reuptake blocker and partial 5-HT1Aantagonist, which acts as an antidepressant, and antidepressants are known to induce mania in vulnerable patients.4
The antimanic property of most antipsychotics (except possibly clozapine) is attributed to their D2 antagonistic potency. Because quetiapine is among the weaker D2 antagonists, its inability to prevent the progression of mania, especially at 50 mg/d, is not unexpected. Mr. X’s subsequent need for a stronger D2 antagonist—risperidone—at a significant dose further supports this observation. A common misconception is that quetiapine’s sedating effects make it effective for treating mania, but that is not the case. Clinicians should be cautious when prescribing quetiapine, especially at lower doses, to patients who exhibit signs of mania. Given the potential risk, clinicians should consider alternative treatments before resorting to low-dose quetiapine for insomnia. Regular monitoring for manic symptoms is crucial for all patients receiving quetiapine. If patients present with signs of mania or hypomania, a therapeutic dose range of 600 to 800 mg/d is recommended.6
- Weisler R, Joyce M, McGill L, et al. Extended release quetiapine fumarate monotherapy for major depressive disorder: results of a double-blind, randomized, placebo-controlled study. CNS Spectr. 2009;14(6):299-313. doi:10.1017/s1092852900020307
- Khalil RB, Baddoura C. Quetiapine induced hypomania: a case report and a review of the literature. Curr Drug Saf. 2012;7(3):250-253. doi:10.2174/157488612803251333
- Benyamina A, Samalin L. Atypical antipsychotic-induced mania/hypomania: a review of recent case reports and clinical studies. Int J Psychiatry Clin Pract. 2012;16(1):2-7. doi:10.3109/13651501.2011.605957
- Gnanavel S. Quetiapine-induced manic episode: a paradox for contemplation. BMJ Case Rep. 2013;2013:bcr2013201761. doi:10.1136/bcr-2013-201761
- Pacchiarotti I, Manfredi G, Kotzalidis GD, et al. Quetiapine-induced mania. Aust N Z J Psychiatry. 2003;37(5):626.
- Millard HY, Wilson BA, Noordsy DL. Low-dose quetiapine induced or worsened mania in the context of possible undertreatment. J Am Board Fam Med. 2015;28(1):154-158. doi:10.3122/jabfm.2015.01.140105
- Weisler R, Joyce M, McGill L, et al. Extended release quetiapine fumarate monotherapy for major depressive disorder: results of a double-blind, randomized, placebo-controlled study. CNS Spectr. 2009;14(6):299-313. doi:10.1017/s1092852900020307
- Khalil RB, Baddoura C. Quetiapine induced hypomania: a case report and a review of the literature. Curr Drug Saf. 2012;7(3):250-253. doi:10.2174/157488612803251333
- Benyamina A, Samalin L. Atypical antipsychotic-induced mania/hypomania: a review of recent case reports and clinical studies. Int J Psychiatry Clin Pract. 2012;16(1):2-7. doi:10.3109/13651501.2011.605957
- Gnanavel S. Quetiapine-induced manic episode: a paradox for contemplation. BMJ Case Rep. 2013;2013:bcr2013201761. doi:10.1136/bcr-2013-201761
- Pacchiarotti I, Manfredi G, Kotzalidis GD, et al. Quetiapine-induced mania. Aust N Z J Psychiatry. 2003;37(5):626.
- Millard HY, Wilson BA, Noordsy DL. Low-dose quetiapine induced or worsened mania in the context of possible undertreatment. J Am Board Fam Med. 2015;28(1):154-158. doi:10.3122/jabfm.2015.01.140105
Rx for resilience: Five prescriptions for physician burnout
Physician burnout persists even as the height of the COVID-19 crisis fades farther into the rear-view mirror. The causes for the sadness, stress, and frustration among doctors vary, but the effects are universal and often debilitating: exhaustion, emotional detachment, lethargy, feeling useless, and lacking purpose.
When surveyed, physicians pointed to many systemic solutions for burnout in Medscape’s Physician Burnout & Depression Report 2023, such as a need for greater compensation, more manageable workloads and schedules, and more support staff. But for many doctors, these fixes may be years if not decades away. Equally important are strategies for relieving burnout symptoms now, especially as we head into a busy holiday season.
Because not every stress-relief practice works for everyone, it’s crucial to try various methods until you find something that makes a difference for you, said Christine Gibson, MD, a family physician and trauma therapist in Calgary, Alta., and author of The Modern Trauma Toolkit.
“Every person should have a toolkit of the things that bring them out of the psychological and physical distress that dysregulates their nervous system,” said Dr. Gibson.
Once you learn the personal ways to alleviate your specific brand of burnout, you can start working on systemic changes that might help the culture of medicine overall.
Symptoms speak louder than words
It seems obvious, but if you aren’t aware that what you’re feeling is burnout, you probably aren’t going to find effective steps to relieve it. Jessi Gold, MD, assistant professor and director of wellness, engagement, and outreach in the department of psychiatry, Washington University in St. Louis, is a psychiatrist who treats health care professionals, including frontline workers during the height of the pandemic. But even as a burnout expert, she admits that she misses the signs in herself.
“I was fighting constant fatigue, falling asleep the minute I got home from work every day, but I thought a B12 shot would solve all my problems. I didn’t realize I was having symptoms of burnout until my own therapist told me,” said Dr. Gold. “As doctors, we spend so much time focusing on other people that we don’t necessarily notice very much in ourselves – usually once it starts to impact our job.”
Practices like meditation and mindfulness can help you delve into your feelings and emotions and notice how you’re doing. But you may also need to ask spouses, partners, and friends and family – or better yet, a mental health professional – if they notice that you seem burnt out.
Practice ‘in the moment’ relief
Sometimes, walking away at the moment of stress helps like when stepping away from a heated argument. “Step out of a frustrating staff meeting to go to the bathroom and splash your face,” said Eran Magan, PhD, a psychologist at the University of Pennsylvania, Philadelphia, and founder and CEO of the suicide prevention system EarlyAlert.me. “Tell a patient you need to check something in the next room, so you have time to take a breath.”
Dr. Magan recommended finding techniques that help lower acute stress while it’s actually happening. First, find a way to escape or excuse yourself from the event, and when possible, stop situations that are actively upsetting or triggering in their tracks.
Next, recharge by doing something that helps you feel better, like looking at a cute video of your child or grandchild or closing your eyes and taking a deep breath. You can also try to “catch” good feelings from someone else, said Dr. Magan. Ask someone about a trip, vacation, holiday, or pleasant event. “Ask a colleague about something that makes [them] happy,” he said. “Happiness can be infectious too.”
Burnout is also in the body
“Body psychotherapy” or somatic therapy is a treatment that focuses on how emotions appear within your body. Dr. Gibson said it’s a valuable tool for addressing trauma and a mainstay in many a medical career; it’s useful to help physicians learn to “befriend” their nervous system.
Somatic therapy exercises involve things like body scanning, scanning for physical sensations; conscious breathing, connecting to each inhale and exhale; grounding your weight by releasing tension through your feet, doing a total body stretch; or releasing shoulder and neck tension by consciously relaxing each of these muscle groups.
“We spend our whole day in sympathetic tone; our amygdala’s are firing, telling us that we’re in danger,” said Dr. Gibson. “We actually have to practice getting into and spending time in our parasympathetic nervous system to restore the balance in our autonomic nervous system.”
Somatic therapy includes a wide array of exercises that help reconnect you to your body through calming or activation. The movements release tension, ground you, and restore balance.
Bite-sized tools for well-being
Because of the prevalence of physician burnout, there’s been a groundswell of researchers and organizations who have turned their focus toward improving the well-being in the health care workforce.
One such effort comes from the Duke Center for the Advancement of Well-being Science, which “camouflages” well-being tools as continuing education credits to make them accessible for busy, stressed, and overworked physicians.
“They’re called bite-sized tools for well-being, and they have actual evidence behind them,” said Dr. Gold. For example, she said, one tools is a text program called Three Good Things that encourages physicians to send a text listing three positive things that happened during the day. The exercise lasts 15 days, and texters have access to others’ answers as well. After 3 months, participants’ baseline depression, gratitude, and life satisfaction had all “significantly improved.”
“It feels almost ridiculous that that could work, but it does,” said Dr. Gold. “I’ve had patients push back and say: ‘Well, isn’t that toxic positivity?’ But really what it is is dialectics. It’s not saying there’s only positive; it’s just making you realize there is more than just the negative.”
These and other short interventions focus on concepts such as joy, humor, awe, engagement, and self-kindness to build resilience and help physicians recover from burnout symptoms.
Cognitive restructuring could work
Cognitive restructuring is a therapeutic process of learning new ways of interpreting and responding to people and situations. It helps you change the “filter” through which you interact with your environment. Dr. Gibson said it’s a tool to use with care after other modes of therapy that help you understand your patterns and how they developed because of how you view and understand the world.
“The message of [cognitive-behavioral therapy] or cognitive restructuring is there’s something wrong with the way you’re thinking, and we need to change it or fix it, but in a traumatic system [like health care], you’re thinking has been an adaptive process related to the harm in the environment you’re in,” said Dr. Gibson.
“So, if you [jump straight to cognitive restructuring before other types of therapy], then we just gaslight ourselves into believing that there’s something wrong with us, that we haven’t adapted sufficiently to an environment that’s actually harmful.”
Strive for a few systemic changes
Systemic changes can be small ones within your own sphere. For example, Dr. Magan said, work toward making little tweaks to the flow of your day that will increase calm and reduce frustration.
“Make a ‘bug list,’ little, regular demands that drain your energy, and discuss them with your colleagues and supervisors to see if they can be improved,” he said. Examples include everyday frustrations like having unsolicited visitors popping into your office, scheduling complex patients too late in the day, or having a computer freeze whenever you access patient charts.
Though not always financially feasible, affecting real change and finding relief from all these insidious bugs can improve your mental health and burnout symptoms.
“Physicians tend to work extremely hard in order to keep holding together a system that is often not inherently sustainable, like the fascia of a body under tremendous strain,” said Dr. Magan. “Sometimes the brave thing to do is to refuse to continue being the lynchpin and let things break, so the system will have to start improving itself, rather than demanding more and more of the people in it.”
A version of this article first appeared on Medscape.com.
Physician burnout persists even as the height of the COVID-19 crisis fades farther into the rear-view mirror. The causes for the sadness, stress, and frustration among doctors vary, but the effects are universal and often debilitating: exhaustion, emotional detachment, lethargy, feeling useless, and lacking purpose.
When surveyed, physicians pointed to many systemic solutions for burnout in Medscape’s Physician Burnout & Depression Report 2023, such as a need for greater compensation, more manageable workloads and schedules, and more support staff. But for many doctors, these fixes may be years if not decades away. Equally important are strategies for relieving burnout symptoms now, especially as we head into a busy holiday season.
Because not every stress-relief practice works for everyone, it’s crucial to try various methods until you find something that makes a difference for you, said Christine Gibson, MD, a family physician and trauma therapist in Calgary, Alta., and author of The Modern Trauma Toolkit.
“Every person should have a toolkit of the things that bring them out of the psychological and physical distress that dysregulates their nervous system,” said Dr. Gibson.
Once you learn the personal ways to alleviate your specific brand of burnout, you can start working on systemic changes that might help the culture of medicine overall.
Symptoms speak louder than words
It seems obvious, but if you aren’t aware that what you’re feeling is burnout, you probably aren’t going to find effective steps to relieve it. Jessi Gold, MD, assistant professor and director of wellness, engagement, and outreach in the department of psychiatry, Washington University in St. Louis, is a psychiatrist who treats health care professionals, including frontline workers during the height of the pandemic. But even as a burnout expert, she admits that she misses the signs in herself.
“I was fighting constant fatigue, falling asleep the minute I got home from work every day, but I thought a B12 shot would solve all my problems. I didn’t realize I was having symptoms of burnout until my own therapist told me,” said Dr. Gold. “As doctors, we spend so much time focusing on other people that we don’t necessarily notice very much in ourselves – usually once it starts to impact our job.”
Practices like meditation and mindfulness can help you delve into your feelings and emotions and notice how you’re doing. But you may also need to ask spouses, partners, and friends and family – or better yet, a mental health professional – if they notice that you seem burnt out.
Practice ‘in the moment’ relief
Sometimes, walking away at the moment of stress helps like when stepping away from a heated argument. “Step out of a frustrating staff meeting to go to the bathroom and splash your face,” said Eran Magan, PhD, a psychologist at the University of Pennsylvania, Philadelphia, and founder and CEO of the suicide prevention system EarlyAlert.me. “Tell a patient you need to check something in the next room, so you have time to take a breath.”
Dr. Magan recommended finding techniques that help lower acute stress while it’s actually happening. First, find a way to escape or excuse yourself from the event, and when possible, stop situations that are actively upsetting or triggering in their tracks.
Next, recharge by doing something that helps you feel better, like looking at a cute video of your child or grandchild or closing your eyes and taking a deep breath. You can also try to “catch” good feelings from someone else, said Dr. Magan. Ask someone about a trip, vacation, holiday, or pleasant event. “Ask a colleague about something that makes [them] happy,” he said. “Happiness can be infectious too.”
Burnout is also in the body
“Body psychotherapy” or somatic therapy is a treatment that focuses on how emotions appear within your body. Dr. Gibson said it’s a valuable tool for addressing trauma and a mainstay in many a medical career; it’s useful to help physicians learn to “befriend” their nervous system.
Somatic therapy exercises involve things like body scanning, scanning for physical sensations; conscious breathing, connecting to each inhale and exhale; grounding your weight by releasing tension through your feet, doing a total body stretch; or releasing shoulder and neck tension by consciously relaxing each of these muscle groups.
“We spend our whole day in sympathetic tone; our amygdala’s are firing, telling us that we’re in danger,” said Dr. Gibson. “We actually have to practice getting into and spending time in our parasympathetic nervous system to restore the balance in our autonomic nervous system.”
Somatic therapy includes a wide array of exercises that help reconnect you to your body through calming or activation. The movements release tension, ground you, and restore balance.
Bite-sized tools for well-being
Because of the prevalence of physician burnout, there’s been a groundswell of researchers and organizations who have turned their focus toward improving the well-being in the health care workforce.
One such effort comes from the Duke Center for the Advancement of Well-being Science, which “camouflages” well-being tools as continuing education credits to make them accessible for busy, stressed, and overworked physicians.
“They’re called bite-sized tools for well-being, and they have actual evidence behind them,” said Dr. Gold. For example, she said, one tools is a text program called Three Good Things that encourages physicians to send a text listing three positive things that happened during the day. The exercise lasts 15 days, and texters have access to others’ answers as well. After 3 months, participants’ baseline depression, gratitude, and life satisfaction had all “significantly improved.”
“It feels almost ridiculous that that could work, but it does,” said Dr. Gold. “I’ve had patients push back and say: ‘Well, isn’t that toxic positivity?’ But really what it is is dialectics. It’s not saying there’s only positive; it’s just making you realize there is more than just the negative.”
These and other short interventions focus on concepts such as joy, humor, awe, engagement, and self-kindness to build resilience and help physicians recover from burnout symptoms.
Cognitive restructuring could work
Cognitive restructuring is a therapeutic process of learning new ways of interpreting and responding to people and situations. It helps you change the “filter” through which you interact with your environment. Dr. Gibson said it’s a tool to use with care after other modes of therapy that help you understand your patterns and how they developed because of how you view and understand the world.
“The message of [cognitive-behavioral therapy] or cognitive restructuring is there’s something wrong with the way you’re thinking, and we need to change it or fix it, but in a traumatic system [like health care], you’re thinking has been an adaptive process related to the harm in the environment you’re in,” said Dr. Gibson.
“So, if you [jump straight to cognitive restructuring before other types of therapy], then we just gaslight ourselves into believing that there’s something wrong with us, that we haven’t adapted sufficiently to an environment that’s actually harmful.”
Strive for a few systemic changes
Systemic changes can be small ones within your own sphere. For example, Dr. Magan said, work toward making little tweaks to the flow of your day that will increase calm and reduce frustration.
“Make a ‘bug list,’ little, regular demands that drain your energy, and discuss them with your colleagues and supervisors to see if they can be improved,” he said. Examples include everyday frustrations like having unsolicited visitors popping into your office, scheduling complex patients too late in the day, or having a computer freeze whenever you access patient charts.
Though not always financially feasible, affecting real change and finding relief from all these insidious bugs can improve your mental health and burnout symptoms.
“Physicians tend to work extremely hard in order to keep holding together a system that is often not inherently sustainable, like the fascia of a body under tremendous strain,” said Dr. Magan. “Sometimes the brave thing to do is to refuse to continue being the lynchpin and let things break, so the system will have to start improving itself, rather than demanding more and more of the people in it.”
A version of this article first appeared on Medscape.com.
Physician burnout persists even as the height of the COVID-19 crisis fades farther into the rear-view mirror. The causes for the sadness, stress, and frustration among doctors vary, but the effects are universal and often debilitating: exhaustion, emotional detachment, lethargy, feeling useless, and lacking purpose.
When surveyed, physicians pointed to many systemic solutions for burnout in Medscape’s Physician Burnout & Depression Report 2023, such as a need for greater compensation, more manageable workloads and schedules, and more support staff. But for many doctors, these fixes may be years if not decades away. Equally important are strategies for relieving burnout symptoms now, especially as we head into a busy holiday season.
Because not every stress-relief practice works for everyone, it’s crucial to try various methods until you find something that makes a difference for you, said Christine Gibson, MD, a family physician and trauma therapist in Calgary, Alta., and author of The Modern Trauma Toolkit.
“Every person should have a toolkit of the things that bring them out of the psychological and physical distress that dysregulates their nervous system,” said Dr. Gibson.
Once you learn the personal ways to alleviate your specific brand of burnout, you can start working on systemic changes that might help the culture of medicine overall.
Symptoms speak louder than words
It seems obvious, but if you aren’t aware that what you’re feeling is burnout, you probably aren’t going to find effective steps to relieve it. Jessi Gold, MD, assistant professor and director of wellness, engagement, and outreach in the department of psychiatry, Washington University in St. Louis, is a psychiatrist who treats health care professionals, including frontline workers during the height of the pandemic. But even as a burnout expert, she admits that she misses the signs in herself.
“I was fighting constant fatigue, falling asleep the minute I got home from work every day, but I thought a B12 shot would solve all my problems. I didn’t realize I was having symptoms of burnout until my own therapist told me,” said Dr. Gold. “As doctors, we spend so much time focusing on other people that we don’t necessarily notice very much in ourselves – usually once it starts to impact our job.”
Practices like meditation and mindfulness can help you delve into your feelings and emotions and notice how you’re doing. But you may also need to ask spouses, partners, and friends and family – or better yet, a mental health professional – if they notice that you seem burnt out.
Practice ‘in the moment’ relief
Sometimes, walking away at the moment of stress helps like when stepping away from a heated argument. “Step out of a frustrating staff meeting to go to the bathroom and splash your face,” said Eran Magan, PhD, a psychologist at the University of Pennsylvania, Philadelphia, and founder and CEO of the suicide prevention system EarlyAlert.me. “Tell a patient you need to check something in the next room, so you have time to take a breath.”
Dr. Magan recommended finding techniques that help lower acute stress while it’s actually happening. First, find a way to escape or excuse yourself from the event, and when possible, stop situations that are actively upsetting or triggering in their tracks.
Next, recharge by doing something that helps you feel better, like looking at a cute video of your child or grandchild or closing your eyes and taking a deep breath. You can also try to “catch” good feelings from someone else, said Dr. Magan. Ask someone about a trip, vacation, holiday, or pleasant event. “Ask a colleague about something that makes [them] happy,” he said. “Happiness can be infectious too.”
Burnout is also in the body
“Body psychotherapy” or somatic therapy is a treatment that focuses on how emotions appear within your body. Dr. Gibson said it’s a valuable tool for addressing trauma and a mainstay in many a medical career; it’s useful to help physicians learn to “befriend” their nervous system.
Somatic therapy exercises involve things like body scanning, scanning for physical sensations; conscious breathing, connecting to each inhale and exhale; grounding your weight by releasing tension through your feet, doing a total body stretch; or releasing shoulder and neck tension by consciously relaxing each of these muscle groups.
“We spend our whole day in sympathetic tone; our amygdala’s are firing, telling us that we’re in danger,” said Dr. Gibson. “We actually have to practice getting into and spending time in our parasympathetic nervous system to restore the balance in our autonomic nervous system.”
Somatic therapy includes a wide array of exercises that help reconnect you to your body through calming or activation. The movements release tension, ground you, and restore balance.
Bite-sized tools for well-being
Because of the prevalence of physician burnout, there’s been a groundswell of researchers and organizations who have turned their focus toward improving the well-being in the health care workforce.
One such effort comes from the Duke Center for the Advancement of Well-being Science, which “camouflages” well-being tools as continuing education credits to make them accessible for busy, stressed, and overworked physicians.
“They’re called bite-sized tools for well-being, and they have actual evidence behind them,” said Dr. Gold. For example, she said, one tools is a text program called Three Good Things that encourages physicians to send a text listing three positive things that happened during the day. The exercise lasts 15 days, and texters have access to others’ answers as well. After 3 months, participants’ baseline depression, gratitude, and life satisfaction had all “significantly improved.”
“It feels almost ridiculous that that could work, but it does,” said Dr. Gold. “I’ve had patients push back and say: ‘Well, isn’t that toxic positivity?’ But really what it is is dialectics. It’s not saying there’s only positive; it’s just making you realize there is more than just the negative.”
These and other short interventions focus on concepts such as joy, humor, awe, engagement, and self-kindness to build resilience and help physicians recover from burnout symptoms.
Cognitive restructuring could work
Cognitive restructuring is a therapeutic process of learning new ways of interpreting and responding to people and situations. It helps you change the “filter” through which you interact with your environment. Dr. Gibson said it’s a tool to use with care after other modes of therapy that help you understand your patterns and how they developed because of how you view and understand the world.
“The message of [cognitive-behavioral therapy] or cognitive restructuring is there’s something wrong with the way you’re thinking, and we need to change it or fix it, but in a traumatic system [like health care], you’re thinking has been an adaptive process related to the harm in the environment you’re in,” said Dr. Gibson.
“So, if you [jump straight to cognitive restructuring before other types of therapy], then we just gaslight ourselves into believing that there’s something wrong with us, that we haven’t adapted sufficiently to an environment that’s actually harmful.”
Strive for a few systemic changes
Systemic changes can be small ones within your own sphere. For example, Dr. Magan said, work toward making little tweaks to the flow of your day that will increase calm and reduce frustration.
“Make a ‘bug list,’ little, regular demands that drain your energy, and discuss them with your colleagues and supervisors to see if they can be improved,” he said. Examples include everyday frustrations like having unsolicited visitors popping into your office, scheduling complex patients too late in the day, or having a computer freeze whenever you access patient charts.
Though not always financially feasible, affecting real change and finding relief from all these insidious bugs can improve your mental health and burnout symptoms.
“Physicians tend to work extremely hard in order to keep holding together a system that is often not inherently sustainable, like the fascia of a body under tremendous strain,” said Dr. Magan. “Sometimes the brave thing to do is to refuse to continue being the lynchpin and let things break, so the system will have to start improving itself, rather than demanding more and more of the people in it.”
A version of this article first appeared on Medscape.com.
Novel approach curbs the impact of racism on mental health in Black youth
TOPLINE:
, results of a post hoc analysis of a randomized controlled trial show.
METHODOLOGY:
- SAAF is a 7-week family skills training program delivered at local community centers that targets effective parenting behavior, adolescent self-regulation, and Black pride.
- In the original trial, 472 Black children aged 11-12 years were randomly allocated to SAAF or no treatment control.
- The post hoc analysis investigated changes in adolescent-reported depressive symptoms from age 13 to 14 years using the 20-item Center for Epidemiologic Studies Depression Scale for Children.
TAKEAWAY:
- Exposure to racial discrimination at age 13 years correlated with increased depressive symptoms at age 14 years (P < .001).
- Participation in the SAAF program significantly attenuated the association of racial discrimination at age 13 with increases in depressive symptoms at age 14 (P = .01).
- Racial discrimination was significantly associated with increases in depressive symptoms in the control group (P < .001) but not in the SAAF group.
- This moderating effect was observed using intent-to-treat design; the investigators accounted for family socioeconomic disadvantage and youth gender.
IN PRACTICE:
The findings add to other evidence suggesting that “prevention programs targeting aspects of racial identity, racial socialization processes, and parenting behavior may, to some extent, mitigate the mental health effects associated with racial discrimination. These processes appear to increase positive coping in the aftermath of discrimination, and prevent adolescents internalization of toxic messages regarding racial inferiority,” the authors wrote.
SOURCE:
The study, with first author Steven M. Kogan, PhD, University of Georgia in Athens, was published online in JAMA Network Open with a commentary by Kevin M. Simon, MD, MPH, with Boston Children’s Hospital.
LIMITATIONS:
This was a post hoc analysis of trial data. The sample consisted of Black adolescents from rural areas of Georgia, and the results may not be generalizable to Black adolescents from urban areas or adolescents from other racial groups. Because of the study’s focus on individual-level racial discrimination, the potential for SAAF to buffer the effects of structural and institutional forms of racism is unknown.
DISCLOSURES:
The study had no specific funding. The authors have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, results of a post hoc analysis of a randomized controlled trial show.
METHODOLOGY:
- SAAF is a 7-week family skills training program delivered at local community centers that targets effective parenting behavior, adolescent self-regulation, and Black pride.
- In the original trial, 472 Black children aged 11-12 years were randomly allocated to SAAF or no treatment control.
- The post hoc analysis investigated changes in adolescent-reported depressive symptoms from age 13 to 14 years using the 20-item Center for Epidemiologic Studies Depression Scale for Children.
TAKEAWAY:
- Exposure to racial discrimination at age 13 years correlated with increased depressive symptoms at age 14 years (P < .001).
- Participation in the SAAF program significantly attenuated the association of racial discrimination at age 13 with increases in depressive symptoms at age 14 (P = .01).
- Racial discrimination was significantly associated with increases in depressive symptoms in the control group (P < .001) but not in the SAAF group.
- This moderating effect was observed using intent-to-treat design; the investigators accounted for family socioeconomic disadvantage and youth gender.
IN PRACTICE:
The findings add to other evidence suggesting that “prevention programs targeting aspects of racial identity, racial socialization processes, and parenting behavior may, to some extent, mitigate the mental health effects associated with racial discrimination. These processes appear to increase positive coping in the aftermath of discrimination, and prevent adolescents internalization of toxic messages regarding racial inferiority,” the authors wrote.
SOURCE:
The study, with first author Steven M. Kogan, PhD, University of Georgia in Athens, was published online in JAMA Network Open with a commentary by Kevin M. Simon, MD, MPH, with Boston Children’s Hospital.
LIMITATIONS:
This was a post hoc analysis of trial data. The sample consisted of Black adolescents from rural areas of Georgia, and the results may not be generalizable to Black adolescents from urban areas or adolescents from other racial groups. Because of the study’s focus on individual-level racial discrimination, the potential for SAAF to buffer the effects of structural and institutional forms of racism is unknown.
DISCLOSURES:
The study had no specific funding. The authors have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, results of a post hoc analysis of a randomized controlled trial show.
METHODOLOGY:
- SAAF is a 7-week family skills training program delivered at local community centers that targets effective parenting behavior, adolescent self-regulation, and Black pride.
- In the original trial, 472 Black children aged 11-12 years were randomly allocated to SAAF or no treatment control.
- The post hoc analysis investigated changes in adolescent-reported depressive symptoms from age 13 to 14 years using the 20-item Center for Epidemiologic Studies Depression Scale for Children.
TAKEAWAY:
- Exposure to racial discrimination at age 13 years correlated with increased depressive symptoms at age 14 years (P < .001).
- Participation in the SAAF program significantly attenuated the association of racial discrimination at age 13 with increases in depressive symptoms at age 14 (P = .01).
- Racial discrimination was significantly associated with increases in depressive symptoms in the control group (P < .001) but not in the SAAF group.
- This moderating effect was observed using intent-to-treat design; the investigators accounted for family socioeconomic disadvantage and youth gender.
IN PRACTICE:
The findings add to other evidence suggesting that “prevention programs targeting aspects of racial identity, racial socialization processes, and parenting behavior may, to some extent, mitigate the mental health effects associated with racial discrimination. These processes appear to increase positive coping in the aftermath of discrimination, and prevent adolescents internalization of toxic messages regarding racial inferiority,” the authors wrote.
SOURCE:
The study, with first author Steven M. Kogan, PhD, University of Georgia in Athens, was published online in JAMA Network Open with a commentary by Kevin M. Simon, MD, MPH, with Boston Children’s Hospital.
LIMITATIONS:
This was a post hoc analysis of trial data. The sample consisted of Black adolescents from rural areas of Georgia, and the results may not be generalizable to Black adolescents from urban areas or adolescents from other racial groups. Because of the study’s focus on individual-level racial discrimination, the potential for SAAF to buffer the effects of structural and institutional forms of racism is unknown.
DISCLOSURES:
The study had no specific funding. The authors have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Patients with Parkinson’s at elevated risk for suicidal thoughts, behavior
Adults with Parkinson’s disease are twice as likely to engage in suicidal behavior as the general population, results of a large meta-analysis show.
Given that up to half of patients with PD suffer from depression and anxiety, physicians should maintain a “high index of suspicion” for early recognition and management of suicidality, write the investigators, led by Eng-King Tan, MD, of Duke-NUS Medical School, Singapore.
“Management of both medical, such as sleep disorders, and psychosocial risk factors, such as feelings of loneliness, hopelessness, and depressed mood, could be useful in lowering suicide risk in patients with PD,” they add.
The study was published online in JAMA Neurology.
Suicide risk neglected in PD?
The analysis included 505,950 patients with PD across 28 cross-sectional, case-control, and cohort studies.
Across 14 studies, the prevalence of suicidal ideation in patients with PD was 22.2% (95% confidence interval, 14.6-32.3). In a sensitivity analysis excluding three outliers, the prevalence of suicidal ideation was higher at 24% (95% CI, 19.1-29.7).
Across 21 studies, the prevalence of suicidal behavior was “substantial” at 1.25% (95% CI, 0.64-2.41), the authors report. The prevalence of suicidal behavior was significantly higher in prospective studies (1.75%; 95% CI, 1.03-2.95) than retrospective studies (0.50%; 95% CI, 0.24 to 1.01).
Across 10 studies, the likelihood of suicidal behavior was about twofold higher among patients with PD than general population controls (odds ratio, 2.15; 95% CI, 1.22-3.78; P = .01). Across nine studies, the hazard ratio for suicidal behavior was 1.73 (95% CI, 1.40-2.14; P < .001).
There was no evidence of sex-related differences in suicidal behavior, although the analysis was limited by the paucity of data, the researchers note.
They note the quality of included studies was generally high, although eight of them did not explicitly identify and adjust for confounders.
Higher rate of mood, anxiety disorders
Paul Nestadt, MD, with Johns Hopkins Bloomberg School of Public Health, Baltimore, said this analysis reiterates what several reviews have found over the past few years, including his own.
“In general, rates of mood and anxiety disorders are much higher in PD than in other dementias, such as Alzheimer’s disease. This is reason enough to allocate resources to the mental health care of those diagnosed with PD and to pay special attention to at risk periods, such as early in the diagnosis, when suicide rates seem to be higher in dementias in general,” said Dr. Nestadt, who wasn’t involved in the study.
He noted that research has shown that suicides among people with PD are more likely to involve a firearm – likely because people with PD are more likely to be over age 65 and to be male – “both huge risk factors for firearm suicide.”
“Therefore, it is essential that caregivers be aware of the risks posed by firearms in the homes of people suffering from Parkinson’s or other dementias. It is the clinician’s responsibility to inform families of this risk, but it is all too often neglected,” Dr. Nestadt said.
Support for the study was provided in part by the National Medical Research Council. Dr. Tan reported honoraria from Eisai and Elsevier outside the submitted work. Dr. Nestadt reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adults with Parkinson’s disease are twice as likely to engage in suicidal behavior as the general population, results of a large meta-analysis show.
Given that up to half of patients with PD suffer from depression and anxiety, physicians should maintain a “high index of suspicion” for early recognition and management of suicidality, write the investigators, led by Eng-King Tan, MD, of Duke-NUS Medical School, Singapore.
“Management of both medical, such as sleep disorders, and psychosocial risk factors, such as feelings of loneliness, hopelessness, and depressed mood, could be useful in lowering suicide risk in patients with PD,” they add.
The study was published online in JAMA Neurology.
Suicide risk neglected in PD?
The analysis included 505,950 patients with PD across 28 cross-sectional, case-control, and cohort studies.
Across 14 studies, the prevalence of suicidal ideation in patients with PD was 22.2% (95% confidence interval, 14.6-32.3). In a sensitivity analysis excluding three outliers, the prevalence of suicidal ideation was higher at 24% (95% CI, 19.1-29.7).
Across 21 studies, the prevalence of suicidal behavior was “substantial” at 1.25% (95% CI, 0.64-2.41), the authors report. The prevalence of suicidal behavior was significantly higher in prospective studies (1.75%; 95% CI, 1.03-2.95) than retrospective studies (0.50%; 95% CI, 0.24 to 1.01).
Across 10 studies, the likelihood of suicidal behavior was about twofold higher among patients with PD than general population controls (odds ratio, 2.15; 95% CI, 1.22-3.78; P = .01). Across nine studies, the hazard ratio for suicidal behavior was 1.73 (95% CI, 1.40-2.14; P < .001).
There was no evidence of sex-related differences in suicidal behavior, although the analysis was limited by the paucity of data, the researchers note.
They note the quality of included studies was generally high, although eight of them did not explicitly identify and adjust for confounders.
Higher rate of mood, anxiety disorders
Paul Nestadt, MD, with Johns Hopkins Bloomberg School of Public Health, Baltimore, said this analysis reiterates what several reviews have found over the past few years, including his own.
“In general, rates of mood and anxiety disorders are much higher in PD than in other dementias, such as Alzheimer’s disease. This is reason enough to allocate resources to the mental health care of those diagnosed with PD and to pay special attention to at risk periods, such as early in the diagnosis, when suicide rates seem to be higher in dementias in general,” said Dr. Nestadt, who wasn’t involved in the study.
He noted that research has shown that suicides among people with PD are more likely to involve a firearm – likely because people with PD are more likely to be over age 65 and to be male – “both huge risk factors for firearm suicide.”
“Therefore, it is essential that caregivers be aware of the risks posed by firearms in the homes of people suffering from Parkinson’s or other dementias. It is the clinician’s responsibility to inform families of this risk, but it is all too often neglected,” Dr. Nestadt said.
Support for the study was provided in part by the National Medical Research Council. Dr. Tan reported honoraria from Eisai and Elsevier outside the submitted work. Dr. Nestadt reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adults with Parkinson’s disease are twice as likely to engage in suicidal behavior as the general population, results of a large meta-analysis show.
Given that up to half of patients with PD suffer from depression and anxiety, physicians should maintain a “high index of suspicion” for early recognition and management of suicidality, write the investigators, led by Eng-King Tan, MD, of Duke-NUS Medical School, Singapore.
“Management of both medical, such as sleep disorders, and psychosocial risk factors, such as feelings of loneliness, hopelessness, and depressed mood, could be useful in lowering suicide risk in patients with PD,” they add.
The study was published online in JAMA Neurology.
Suicide risk neglected in PD?
The analysis included 505,950 patients with PD across 28 cross-sectional, case-control, and cohort studies.
Across 14 studies, the prevalence of suicidal ideation in patients with PD was 22.2% (95% confidence interval, 14.6-32.3). In a sensitivity analysis excluding three outliers, the prevalence of suicidal ideation was higher at 24% (95% CI, 19.1-29.7).
Across 21 studies, the prevalence of suicidal behavior was “substantial” at 1.25% (95% CI, 0.64-2.41), the authors report. The prevalence of suicidal behavior was significantly higher in prospective studies (1.75%; 95% CI, 1.03-2.95) than retrospective studies (0.50%; 95% CI, 0.24 to 1.01).
Across 10 studies, the likelihood of suicidal behavior was about twofold higher among patients with PD than general population controls (odds ratio, 2.15; 95% CI, 1.22-3.78; P = .01). Across nine studies, the hazard ratio for suicidal behavior was 1.73 (95% CI, 1.40-2.14; P < .001).
There was no evidence of sex-related differences in suicidal behavior, although the analysis was limited by the paucity of data, the researchers note.
They note the quality of included studies was generally high, although eight of them did not explicitly identify and adjust for confounders.
Higher rate of mood, anxiety disorders
Paul Nestadt, MD, with Johns Hopkins Bloomberg School of Public Health, Baltimore, said this analysis reiterates what several reviews have found over the past few years, including his own.
“In general, rates of mood and anxiety disorders are much higher in PD than in other dementias, such as Alzheimer’s disease. This is reason enough to allocate resources to the mental health care of those diagnosed with PD and to pay special attention to at risk periods, such as early in the diagnosis, when suicide rates seem to be higher in dementias in general,” said Dr. Nestadt, who wasn’t involved in the study.
He noted that research has shown that suicides among people with PD are more likely to involve a firearm – likely because people with PD are more likely to be over age 65 and to be male – “both huge risk factors for firearm suicide.”
“Therefore, it is essential that caregivers be aware of the risks posed by firearms in the homes of people suffering from Parkinson’s or other dementias. It is the clinician’s responsibility to inform families of this risk, but it is all too often neglected,” Dr. Nestadt said.
Support for the study was provided in part by the National Medical Research Council. Dr. Tan reported honoraria from Eisai and Elsevier outside the submitted work. Dr. Nestadt reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cold-water swimming for your health? These docs say jump in
Adam Boggon, MBChB, was working at the Royal Free Hospital in North London during the city’s second wave of COVID-19. “I was effectively living in the hospital,” he recalled. “It felt like I was going 10,000 miles per hour, trying to corral hundreds of medical students and doctors.”
During a national lockdown, there were few places Dr. Boggon could escape to, but the Hampstead Heath swimming ponds mostly remained open. He swam there regularly to exercise and recharge even in winter.
“Swimming in cold water takes you out of yourself,” Dr. Boggon said. “It was such a release for someone who grew up in a rural place and had access to green space, even though the water is murky.” It also hovers around 50 °F (10 °C).
Jumping into cold water, well, kind of stinks. So why do it? It’s not only for bragging rights. , specifically to improve depression symptoms and even ease inflammatory conditions.
And a lot of that research is driven by medical pros who love to do it themselves.
For Dr. Boggon, swimming in frigid water is uncomfortable, but he feels that a sensation of calmness follows that makes the plunge more than worth it. Now a Fulbright Scholar at Harvard, where he studies public health and health management, Dr. Boggon is able to frequent the fabled Walden Pond just outside of Boston.
As Thoreau himself said, “You can never have enough of nature.”
Yes, even if it’s really, really cold.
Taking a deeper dive
Heather Massey, PhD, a senior lecturer in Sport, Health, and Exercise Science at University of Portsmouth, blames her father, a dinghy sailor, for her affinity for cold-water swimming.
And she’s done more than most, including an epic 16-hour crossing of the English Channel. The water temperature was in the upper-50s °F, and she swam without a wetsuit. “Time just seemed to collapse,” she has shared about the experience.
While working on her PhD and studying the effects of environmental physiology, in particular what happens to the body when it gets hot or cold, Dr. Massey’s hobby and studies seemed to coalesce.
Her research initially focused on the hazards around being in cold open water. But she also noticed a growing trend of people claiming health benefits from the practice. “People started to talk about experiencing improved symptoms of depression or improved mental health from their activities in the water,” she said.
She partnered with another outdoor swimming enthusiast, Hannah Denton, a counseling psychologist working for the National Health Service in the United Kingdom. Ms. Denton was publishing papers on the potential impact that outdoor swimming may have on people with depression and how it could improve mental health in general. She also regularly engages in cold-water swims to boost feelings of mindfulness and peace.
“Having the experience of being so close to nature, as well as the strong sensory experience of being in cold water, does really encourage you to be in the moment,” Ms. Denton wrote in an article for the Sussex Mindfulness Centre. “My experiences of sea swimming and mindfulness support each other. Both have made me feel more comfortable with my body, to have more of a present moment focus, to pay attention to my breathing, and to gain distance from difficult thoughts.”
Over the past few years, Dr. Massey and Ms. Denton have moved from fairly small-scale studies with no real controls to today, completing a randomized controlled trial and looking at the impact that outdoor swimming may have on people living with mild to moderate depression.
“At first, people sort of thought our idea was a bit wacky,” said Dr. Massey. “Now, the popularity of open-water swimming has really blossomed, and so has this area of research. We’re starting to build more rigor into the work.”
Like all the researchers and physicians interviewed for this article, Dr. Massey hesitates to claim that cold-water swimming is a “cure” that should be medicalized.
“It’s not about prescribing it or forcing people to do it,” said Dr. Massey. “This is not something that a doctor should write on a prescription and say you should go and have eight 1-hour sessions of swimming.”
(Not yet) a common cure
Enter into the conversation Mark Harper, MD, PhD, consultant anesthetist at Sussex University Hospitals in the United Kingdom and Kristiansand, Norway. Dr. Harper is the author of the 2022 book, Chill: The Cold Water Swim Cure – A Transformative Guide to Renew Your Body and Mind.
Dr. Harper grew up swimming in pools, and it wasn’t until his pool closed for 2 weeks that he ventured into the sea. He recalled walking up the beach afterward, thinking, God, this feels good, and from that moment on, he became hooked on outdoor swimming and curious about its therapeutic potential.
The “cure” in the book’s title, Dr. Harper explained, is being used in the historical sense of “treatment,” as in the first medical book about sea-bathing written over 250 years ago. Dr. Harper acknowledged that the connection to health is still speculative. “However, the circumstantial evidence, the feedback from participants and early study data for its benefits are now very strong,” he said.
In a small study published in 2022, Dr. Harper and colleagues took 59 people with anxiety and depression and put them through a sea-swimming course. Afterward, 80% showed a clinically significant improvement in their mental health.
More recently, Dr. Harper and his team of researchers released a survey to determine how many people were using cold-water swimming as a treatment for a mental or physical ailment. “We thought 30 or 40 people would respond, but we ended up with over 700,” he said. “The majority were using it for mental health but also included inflammation-related conditions.”
Over 2 decades, Dr. Harper has seen dramatic success stories. In his book, he recalled a good friend who, in his early 20s, suffered from Crohn’s disease so badly he couldn’t walk up the steps to his parents’ house. The friend turned to outdoor cold swimming as a low-impact workout and began noticing the symptoms of his disease were improving. Within months, he was able to go off his medications. In 2022, he completed 52 triathlons: one per week for the entire year.
How cold exposure may play with your brain
Vaibhav Diwadkar, PhD, professor of psychiatry and behavioral neurosciences at Wayne State University, in Detroit, is studying how human brain networks respond to cold exposure. Dr. Diwadkar and his colleague, Otto Muzik, PhD, began by putting volunteers in a rubber suit with thin tubing and infusing the tubing with temperature-controlled water. Meanwhile, they collected functional brain imaging data to analyze which parts of the brain were responding as body temperature changed.
The data showed that the cold exposure made certain areas of the brain very active, including some that have been associated with the regulation of mood.
Dr. Diwadkar posits that controlled exposure to cold serves as a low-level stressor that knocks different systems within the brain and body out of homeostasis. Once the stress is removed, the brain responds by releasing neurotransmitters that enhance mood, frequently leading to feelings of euphoria in participants.
“We don’t have direct evidence of such a mechanism, but it’s a reasonable speculation,” said Dr. Diwadkar.
However, he pointed out that science writers in the media often portray topics such as this one in black and white, which is “oversimplifying the scientific complexity of biology.”
Clearly, more research needs to be done on the potential therapeutic benefits of cold-water swimming. But for those suffering from anxiety, depression, or chronic illness, if taking a cold dip makes you feel better, the why and how might be beside the point.
Plus, as Dr. Harper pointed out, it’s an easy and accessible therapy.
“All you need is some water – enough to submerge your entire body in – that’s less than 68 °F (20 °C),” he said. “If you stay long enough to get over that initial shock, which is just 2 or 3 minutes, then you’ve got the effect. If you get out and want to go back in again, then you’ve done it right.”
A version of this article first appeared on Medscape.com.
Adam Boggon, MBChB, was working at the Royal Free Hospital in North London during the city’s second wave of COVID-19. “I was effectively living in the hospital,” he recalled. “It felt like I was going 10,000 miles per hour, trying to corral hundreds of medical students and doctors.”
During a national lockdown, there were few places Dr. Boggon could escape to, but the Hampstead Heath swimming ponds mostly remained open. He swam there regularly to exercise and recharge even in winter.
“Swimming in cold water takes you out of yourself,” Dr. Boggon said. “It was such a release for someone who grew up in a rural place and had access to green space, even though the water is murky.” It also hovers around 50 °F (10 °C).
Jumping into cold water, well, kind of stinks. So why do it? It’s not only for bragging rights. , specifically to improve depression symptoms and even ease inflammatory conditions.
And a lot of that research is driven by medical pros who love to do it themselves.
For Dr. Boggon, swimming in frigid water is uncomfortable, but he feels that a sensation of calmness follows that makes the plunge more than worth it. Now a Fulbright Scholar at Harvard, where he studies public health and health management, Dr. Boggon is able to frequent the fabled Walden Pond just outside of Boston.
As Thoreau himself said, “You can never have enough of nature.”
Yes, even if it’s really, really cold.
Taking a deeper dive
Heather Massey, PhD, a senior lecturer in Sport, Health, and Exercise Science at University of Portsmouth, blames her father, a dinghy sailor, for her affinity for cold-water swimming.
And she’s done more than most, including an epic 16-hour crossing of the English Channel. The water temperature was in the upper-50s °F, and she swam without a wetsuit. “Time just seemed to collapse,” she has shared about the experience.
While working on her PhD and studying the effects of environmental physiology, in particular what happens to the body when it gets hot or cold, Dr. Massey’s hobby and studies seemed to coalesce.
Her research initially focused on the hazards around being in cold open water. But she also noticed a growing trend of people claiming health benefits from the practice. “People started to talk about experiencing improved symptoms of depression or improved mental health from their activities in the water,” she said.
She partnered with another outdoor swimming enthusiast, Hannah Denton, a counseling psychologist working for the National Health Service in the United Kingdom. Ms. Denton was publishing papers on the potential impact that outdoor swimming may have on people with depression and how it could improve mental health in general. She also regularly engages in cold-water swims to boost feelings of mindfulness and peace.
“Having the experience of being so close to nature, as well as the strong sensory experience of being in cold water, does really encourage you to be in the moment,” Ms. Denton wrote in an article for the Sussex Mindfulness Centre. “My experiences of sea swimming and mindfulness support each other. Both have made me feel more comfortable with my body, to have more of a present moment focus, to pay attention to my breathing, and to gain distance from difficult thoughts.”
Over the past few years, Dr. Massey and Ms. Denton have moved from fairly small-scale studies with no real controls to today, completing a randomized controlled trial and looking at the impact that outdoor swimming may have on people living with mild to moderate depression.
“At first, people sort of thought our idea was a bit wacky,” said Dr. Massey. “Now, the popularity of open-water swimming has really blossomed, and so has this area of research. We’re starting to build more rigor into the work.”
Like all the researchers and physicians interviewed for this article, Dr. Massey hesitates to claim that cold-water swimming is a “cure” that should be medicalized.
“It’s not about prescribing it or forcing people to do it,” said Dr. Massey. “This is not something that a doctor should write on a prescription and say you should go and have eight 1-hour sessions of swimming.”
(Not yet) a common cure
Enter into the conversation Mark Harper, MD, PhD, consultant anesthetist at Sussex University Hospitals in the United Kingdom and Kristiansand, Norway. Dr. Harper is the author of the 2022 book, Chill: The Cold Water Swim Cure – A Transformative Guide to Renew Your Body and Mind.
Dr. Harper grew up swimming in pools, and it wasn’t until his pool closed for 2 weeks that he ventured into the sea. He recalled walking up the beach afterward, thinking, God, this feels good, and from that moment on, he became hooked on outdoor swimming and curious about its therapeutic potential.
The “cure” in the book’s title, Dr. Harper explained, is being used in the historical sense of “treatment,” as in the first medical book about sea-bathing written over 250 years ago. Dr. Harper acknowledged that the connection to health is still speculative. “However, the circumstantial evidence, the feedback from participants and early study data for its benefits are now very strong,” he said.
In a small study published in 2022, Dr. Harper and colleagues took 59 people with anxiety and depression and put them through a sea-swimming course. Afterward, 80% showed a clinically significant improvement in their mental health.
More recently, Dr. Harper and his team of researchers released a survey to determine how many people were using cold-water swimming as a treatment for a mental or physical ailment. “We thought 30 or 40 people would respond, but we ended up with over 700,” he said. “The majority were using it for mental health but also included inflammation-related conditions.”
Over 2 decades, Dr. Harper has seen dramatic success stories. In his book, he recalled a good friend who, in his early 20s, suffered from Crohn’s disease so badly he couldn’t walk up the steps to his parents’ house. The friend turned to outdoor cold swimming as a low-impact workout and began noticing the symptoms of his disease were improving. Within months, he was able to go off his medications. In 2022, he completed 52 triathlons: one per week for the entire year.
How cold exposure may play with your brain
Vaibhav Diwadkar, PhD, professor of psychiatry and behavioral neurosciences at Wayne State University, in Detroit, is studying how human brain networks respond to cold exposure. Dr. Diwadkar and his colleague, Otto Muzik, PhD, began by putting volunteers in a rubber suit with thin tubing and infusing the tubing with temperature-controlled water. Meanwhile, they collected functional brain imaging data to analyze which parts of the brain were responding as body temperature changed.
The data showed that the cold exposure made certain areas of the brain very active, including some that have been associated with the regulation of mood.
Dr. Diwadkar posits that controlled exposure to cold serves as a low-level stressor that knocks different systems within the brain and body out of homeostasis. Once the stress is removed, the brain responds by releasing neurotransmitters that enhance mood, frequently leading to feelings of euphoria in participants.
“We don’t have direct evidence of such a mechanism, but it’s a reasonable speculation,” said Dr. Diwadkar.
However, he pointed out that science writers in the media often portray topics such as this one in black and white, which is “oversimplifying the scientific complexity of biology.”
Clearly, more research needs to be done on the potential therapeutic benefits of cold-water swimming. But for those suffering from anxiety, depression, or chronic illness, if taking a cold dip makes you feel better, the why and how might be beside the point.
Plus, as Dr. Harper pointed out, it’s an easy and accessible therapy.
“All you need is some water – enough to submerge your entire body in – that’s less than 68 °F (20 °C),” he said. “If you stay long enough to get over that initial shock, which is just 2 or 3 minutes, then you’ve got the effect. If you get out and want to go back in again, then you’ve done it right.”
A version of this article first appeared on Medscape.com.
Adam Boggon, MBChB, was working at the Royal Free Hospital in North London during the city’s second wave of COVID-19. “I was effectively living in the hospital,” he recalled. “It felt like I was going 10,000 miles per hour, trying to corral hundreds of medical students and doctors.”
During a national lockdown, there were few places Dr. Boggon could escape to, but the Hampstead Heath swimming ponds mostly remained open. He swam there regularly to exercise and recharge even in winter.
“Swimming in cold water takes you out of yourself,” Dr. Boggon said. “It was such a release for someone who grew up in a rural place and had access to green space, even though the water is murky.” It also hovers around 50 °F (10 °C).
Jumping into cold water, well, kind of stinks. So why do it? It’s not only for bragging rights. , specifically to improve depression symptoms and even ease inflammatory conditions.
And a lot of that research is driven by medical pros who love to do it themselves.
For Dr. Boggon, swimming in frigid water is uncomfortable, but he feels that a sensation of calmness follows that makes the plunge more than worth it. Now a Fulbright Scholar at Harvard, where he studies public health and health management, Dr. Boggon is able to frequent the fabled Walden Pond just outside of Boston.
As Thoreau himself said, “You can never have enough of nature.”
Yes, even if it’s really, really cold.
Taking a deeper dive
Heather Massey, PhD, a senior lecturer in Sport, Health, and Exercise Science at University of Portsmouth, blames her father, a dinghy sailor, for her affinity for cold-water swimming.
And she’s done more than most, including an epic 16-hour crossing of the English Channel. The water temperature was in the upper-50s °F, and she swam without a wetsuit. “Time just seemed to collapse,” she has shared about the experience.
While working on her PhD and studying the effects of environmental physiology, in particular what happens to the body when it gets hot or cold, Dr. Massey’s hobby and studies seemed to coalesce.
Her research initially focused on the hazards around being in cold open water. But she also noticed a growing trend of people claiming health benefits from the practice. “People started to talk about experiencing improved symptoms of depression or improved mental health from their activities in the water,” she said.
She partnered with another outdoor swimming enthusiast, Hannah Denton, a counseling psychologist working for the National Health Service in the United Kingdom. Ms. Denton was publishing papers on the potential impact that outdoor swimming may have on people with depression and how it could improve mental health in general. She also regularly engages in cold-water swims to boost feelings of mindfulness and peace.
“Having the experience of being so close to nature, as well as the strong sensory experience of being in cold water, does really encourage you to be in the moment,” Ms. Denton wrote in an article for the Sussex Mindfulness Centre. “My experiences of sea swimming and mindfulness support each other. Both have made me feel more comfortable with my body, to have more of a present moment focus, to pay attention to my breathing, and to gain distance from difficult thoughts.”
Over the past few years, Dr. Massey and Ms. Denton have moved from fairly small-scale studies with no real controls to today, completing a randomized controlled trial and looking at the impact that outdoor swimming may have on people living with mild to moderate depression.
“At first, people sort of thought our idea was a bit wacky,” said Dr. Massey. “Now, the popularity of open-water swimming has really blossomed, and so has this area of research. We’re starting to build more rigor into the work.”
Like all the researchers and physicians interviewed for this article, Dr. Massey hesitates to claim that cold-water swimming is a “cure” that should be medicalized.
“It’s not about prescribing it or forcing people to do it,” said Dr. Massey. “This is not something that a doctor should write on a prescription and say you should go and have eight 1-hour sessions of swimming.”
(Not yet) a common cure
Enter into the conversation Mark Harper, MD, PhD, consultant anesthetist at Sussex University Hospitals in the United Kingdom and Kristiansand, Norway. Dr. Harper is the author of the 2022 book, Chill: The Cold Water Swim Cure – A Transformative Guide to Renew Your Body and Mind.
Dr. Harper grew up swimming in pools, and it wasn’t until his pool closed for 2 weeks that he ventured into the sea. He recalled walking up the beach afterward, thinking, God, this feels good, and from that moment on, he became hooked on outdoor swimming and curious about its therapeutic potential.
The “cure” in the book’s title, Dr. Harper explained, is being used in the historical sense of “treatment,” as in the first medical book about sea-bathing written over 250 years ago. Dr. Harper acknowledged that the connection to health is still speculative. “However, the circumstantial evidence, the feedback from participants and early study data for its benefits are now very strong,” he said.
In a small study published in 2022, Dr. Harper and colleagues took 59 people with anxiety and depression and put them through a sea-swimming course. Afterward, 80% showed a clinically significant improvement in their mental health.
More recently, Dr. Harper and his team of researchers released a survey to determine how many people were using cold-water swimming as a treatment for a mental or physical ailment. “We thought 30 or 40 people would respond, but we ended up with over 700,” he said. “The majority were using it for mental health but also included inflammation-related conditions.”
Over 2 decades, Dr. Harper has seen dramatic success stories. In his book, he recalled a good friend who, in his early 20s, suffered from Crohn’s disease so badly he couldn’t walk up the steps to his parents’ house. The friend turned to outdoor cold swimming as a low-impact workout and began noticing the symptoms of his disease were improving. Within months, he was able to go off his medications. In 2022, he completed 52 triathlons: one per week for the entire year.
How cold exposure may play with your brain
Vaibhav Diwadkar, PhD, professor of psychiatry and behavioral neurosciences at Wayne State University, in Detroit, is studying how human brain networks respond to cold exposure. Dr. Diwadkar and his colleague, Otto Muzik, PhD, began by putting volunteers in a rubber suit with thin tubing and infusing the tubing with temperature-controlled water. Meanwhile, they collected functional brain imaging data to analyze which parts of the brain were responding as body temperature changed.
The data showed that the cold exposure made certain areas of the brain very active, including some that have been associated with the regulation of mood.
Dr. Diwadkar posits that controlled exposure to cold serves as a low-level stressor that knocks different systems within the brain and body out of homeostasis. Once the stress is removed, the brain responds by releasing neurotransmitters that enhance mood, frequently leading to feelings of euphoria in participants.
“We don’t have direct evidence of such a mechanism, but it’s a reasonable speculation,” said Dr. Diwadkar.
However, he pointed out that science writers in the media often portray topics such as this one in black and white, which is “oversimplifying the scientific complexity of biology.”
Clearly, more research needs to be done on the potential therapeutic benefits of cold-water swimming. But for those suffering from anxiety, depression, or chronic illness, if taking a cold dip makes you feel better, the why and how might be beside the point.
Plus, as Dr. Harper pointed out, it’s an easy and accessible therapy.
“All you need is some water – enough to submerge your entire body in – that’s less than 68 °F (20 °C),” he said. “If you stay long enough to get over that initial shock, which is just 2 or 3 minutes, then you’ve got the effect. If you get out and want to go back in again, then you’ve done it right.”
A version of this article first appeared on Medscape.com.
Pharmacogenomic testing for antidepressants could save time, money
Scientists developed a microsimulation model to evaluate the effectiveness of pharmacogenomic testing for adult patients in British Columbia, with newly diagnosed moderate to severe major depressive disorder (MDD). The model predicted that testing could result in 37% fewer patients developing refractory depression, 15% more time of patients feeling well, and a health system cost-savings of $956 million CAD over 20 years.
“Our study shows that, if pharmacogenomic testing guides the prescription of an effective antidepressant, it can reduce the lengthy trial-and-error process many patients experience and dramatically reduce the financial burden on the health care system,” Shahzad Ghanbarian, PhD, the lead author and a research analyst at the University of British Columbia’s Centre for Clinical Epidemiology and Evaluation, Vancouver, said in an interview.
“The next step should be developing implementation strategies and identifying the most suitable health care professionals to provide pharmacogenomic-guided care,” she said.
The study was published online on in the Canadian Medical Association Journal.
Developing a model
The World Health Organization has predicted that depression will be the leading cause of disability worldwide by 2030. However, about half of patients don’t respond to the antidepressant that they are initially prescribed, and more than one-quarter report adverse effects. Previous studies have found that up to 42% of the lack in response stems from genetic factors that affect medication metabolism.
Pharmacogenomic testing, which uses a blood, saliva, or buccal swab sample, could help identify genetic variants involved in drug metabolism and response as well as guide prescribing and reduce adverse effects, the authors wrote.
Dr. Ghanbarian and colleagues developed a microsimulation model in collaboration with patient partners, clinicians, and the health system to evaluate the effectiveness and cost-effectiveness of pharmacogenomic testing for adult patients with MDD in British Columbia. The model included unique patient characteristics, such as metabolizer phenotypes, and followed the experience of patients through diagnosis, treatment, and recurrence.
According to British Columbia administrative data from 2015 to 2020, the model simulated a population of 194,149 adults and incorporated 40 different antidepressants and other treatments, including electroconvulsive therapy (ECT) and psychotherapy. The research team compared treatment pathways for patients with and without pharmacogenomic testing over 20 years.
Overall, the model showed that pharmacogenomic-guided treatment resulted in higher remission rates and lower discontinuation rates, with 23,216 (37%) fewer patients developing refractory depression and decreased use of resource-intensive treatment options such as ECT and psychotherapy (by 28% and 22%, respectively). According to the model, these reductions would save the British Columbia health system $4,926 CAD per patient, or about $56 million CAD over 20 years.
These findings provide a solid economic justification for clinical implementation of pharmacogenomic-guided depression treatment in Canada.
In addition, the model found that patients who underwent pharmacogenomic testing spent 15% more time in the “well” state without depression symptoms and 18% less time in the MDD state with recurrent episodes or refractory depression. In turn, this would mean 1,869 fewer deaths and 21,346 fewer all-cause hospital admissions over 20 years.
Pharmacogenomic testing also led to gains of 0.064 life-years and 0.381 quality-adjusted life-years per patient, or 12,436 life-years and 74,023 QALYs for all of British Columbia over 20 years.
From a cost perspective, the $121 million CAD cost of pharmacogenomic testing and $524 million CAD increase in episodic care were offset by a decrease in the cost of refractory MDD care. In sensitivity analyses, up-front investment in pharmacogenomic testing was typically offset after 2 years through lower direct medical costs, and it was also considered a cost-saving measure from that point forward.
“By incorporating the perspectives of patients with lived and living experience into this model, alongside robust data sets, we were able to carefully simulate the treatment journey of people with major depression,” Dr. Ghanbarian said. “The simulation model is designed to be flexible and could be applied to other jurisdictions beyond British Columbia, where we might expect to see similar benefits, particularly within a comparable Canadian context.”
Implementing the model
Now, Dr. Ghanbarian and colleagues are interested in potential implementation strategies at a system-wide level. For now, pharmacogenomic tests aren’t offered through the public health systems across Canada, but patients can pay for them through private companies.
“These findings provide a solid economic justification for clinical implementation of pharmacogenomic-guided depression treatment in Canada,” Chad Bousman, PhD, associate professor of physiology and pharmacology at the University of Calgary (Alta.), said in an interview.
Dr. Bousman, who was not involved with this study, coauthored the Clinical Pharmacogenetics Implementation Consortium guideline for several genotypes and serotonin reuptake inhibitor antidepressants. He and colleagues have also developed and evaluated web-based tools that translate pharmacogenetic data into evidence-based prescribing recommendations.
“The hope is that this work will facilitate investment in the establishment of the necessary infrastructure to ensure Canadians have equitable access to pharmacogenomic testing and ultimately improve mental health outcomes,” he said.
The study was funded by Genome BC, Genome Canada, and Michael Smith Health Research British Columbia. Dr. Ghanbarian and Dr. Bousman reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Scientists developed a microsimulation model to evaluate the effectiveness of pharmacogenomic testing for adult patients in British Columbia, with newly diagnosed moderate to severe major depressive disorder (MDD). The model predicted that testing could result in 37% fewer patients developing refractory depression, 15% more time of patients feeling well, and a health system cost-savings of $956 million CAD over 20 years.
“Our study shows that, if pharmacogenomic testing guides the prescription of an effective antidepressant, it can reduce the lengthy trial-and-error process many patients experience and dramatically reduce the financial burden on the health care system,” Shahzad Ghanbarian, PhD, the lead author and a research analyst at the University of British Columbia’s Centre for Clinical Epidemiology and Evaluation, Vancouver, said in an interview.
“The next step should be developing implementation strategies and identifying the most suitable health care professionals to provide pharmacogenomic-guided care,” she said.
The study was published online on in the Canadian Medical Association Journal.
Developing a model
The World Health Organization has predicted that depression will be the leading cause of disability worldwide by 2030. However, about half of patients don’t respond to the antidepressant that they are initially prescribed, and more than one-quarter report adverse effects. Previous studies have found that up to 42% of the lack in response stems from genetic factors that affect medication metabolism.
Pharmacogenomic testing, which uses a blood, saliva, or buccal swab sample, could help identify genetic variants involved in drug metabolism and response as well as guide prescribing and reduce adverse effects, the authors wrote.
Dr. Ghanbarian and colleagues developed a microsimulation model in collaboration with patient partners, clinicians, and the health system to evaluate the effectiveness and cost-effectiveness of pharmacogenomic testing for adult patients with MDD in British Columbia. The model included unique patient characteristics, such as metabolizer phenotypes, and followed the experience of patients through diagnosis, treatment, and recurrence.
According to British Columbia administrative data from 2015 to 2020, the model simulated a population of 194,149 adults and incorporated 40 different antidepressants and other treatments, including electroconvulsive therapy (ECT) and psychotherapy. The research team compared treatment pathways for patients with and without pharmacogenomic testing over 20 years.
Overall, the model showed that pharmacogenomic-guided treatment resulted in higher remission rates and lower discontinuation rates, with 23,216 (37%) fewer patients developing refractory depression and decreased use of resource-intensive treatment options such as ECT and psychotherapy (by 28% and 22%, respectively). According to the model, these reductions would save the British Columbia health system $4,926 CAD per patient, or about $56 million CAD over 20 years.
These findings provide a solid economic justification for clinical implementation of pharmacogenomic-guided depression treatment in Canada.
In addition, the model found that patients who underwent pharmacogenomic testing spent 15% more time in the “well” state without depression symptoms and 18% less time in the MDD state with recurrent episodes or refractory depression. In turn, this would mean 1,869 fewer deaths and 21,346 fewer all-cause hospital admissions over 20 years.
Pharmacogenomic testing also led to gains of 0.064 life-years and 0.381 quality-adjusted life-years per patient, or 12,436 life-years and 74,023 QALYs for all of British Columbia over 20 years.
From a cost perspective, the $121 million CAD cost of pharmacogenomic testing and $524 million CAD increase in episodic care were offset by a decrease in the cost of refractory MDD care. In sensitivity analyses, up-front investment in pharmacogenomic testing was typically offset after 2 years through lower direct medical costs, and it was also considered a cost-saving measure from that point forward.
“By incorporating the perspectives of patients with lived and living experience into this model, alongside robust data sets, we were able to carefully simulate the treatment journey of people with major depression,” Dr. Ghanbarian said. “The simulation model is designed to be flexible and could be applied to other jurisdictions beyond British Columbia, where we might expect to see similar benefits, particularly within a comparable Canadian context.”
Implementing the model
Now, Dr. Ghanbarian and colleagues are interested in potential implementation strategies at a system-wide level. For now, pharmacogenomic tests aren’t offered through the public health systems across Canada, but patients can pay for them through private companies.
“These findings provide a solid economic justification for clinical implementation of pharmacogenomic-guided depression treatment in Canada,” Chad Bousman, PhD, associate professor of physiology and pharmacology at the University of Calgary (Alta.), said in an interview.
Dr. Bousman, who was not involved with this study, coauthored the Clinical Pharmacogenetics Implementation Consortium guideline for several genotypes and serotonin reuptake inhibitor antidepressants. He and colleagues have also developed and evaluated web-based tools that translate pharmacogenetic data into evidence-based prescribing recommendations.
“The hope is that this work will facilitate investment in the establishment of the necessary infrastructure to ensure Canadians have equitable access to pharmacogenomic testing and ultimately improve mental health outcomes,” he said.
The study was funded by Genome BC, Genome Canada, and Michael Smith Health Research British Columbia. Dr. Ghanbarian and Dr. Bousman reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Scientists developed a microsimulation model to evaluate the effectiveness of pharmacogenomic testing for adult patients in British Columbia, with newly diagnosed moderate to severe major depressive disorder (MDD). The model predicted that testing could result in 37% fewer patients developing refractory depression, 15% more time of patients feeling well, and a health system cost-savings of $956 million CAD over 20 years.
“Our study shows that, if pharmacogenomic testing guides the prescription of an effective antidepressant, it can reduce the lengthy trial-and-error process many patients experience and dramatically reduce the financial burden on the health care system,” Shahzad Ghanbarian, PhD, the lead author and a research analyst at the University of British Columbia’s Centre for Clinical Epidemiology and Evaluation, Vancouver, said in an interview.
“The next step should be developing implementation strategies and identifying the most suitable health care professionals to provide pharmacogenomic-guided care,” she said.
The study was published online on in the Canadian Medical Association Journal.
Developing a model
The World Health Organization has predicted that depression will be the leading cause of disability worldwide by 2030. However, about half of patients don’t respond to the antidepressant that they are initially prescribed, and more than one-quarter report adverse effects. Previous studies have found that up to 42% of the lack in response stems from genetic factors that affect medication metabolism.
Pharmacogenomic testing, which uses a blood, saliva, or buccal swab sample, could help identify genetic variants involved in drug metabolism and response as well as guide prescribing and reduce adverse effects, the authors wrote.
Dr. Ghanbarian and colleagues developed a microsimulation model in collaboration with patient partners, clinicians, and the health system to evaluate the effectiveness and cost-effectiveness of pharmacogenomic testing for adult patients with MDD in British Columbia. The model included unique patient characteristics, such as metabolizer phenotypes, and followed the experience of patients through diagnosis, treatment, and recurrence.
According to British Columbia administrative data from 2015 to 2020, the model simulated a population of 194,149 adults and incorporated 40 different antidepressants and other treatments, including electroconvulsive therapy (ECT) and psychotherapy. The research team compared treatment pathways for patients with and without pharmacogenomic testing over 20 years.
Overall, the model showed that pharmacogenomic-guided treatment resulted in higher remission rates and lower discontinuation rates, with 23,216 (37%) fewer patients developing refractory depression and decreased use of resource-intensive treatment options such as ECT and psychotherapy (by 28% and 22%, respectively). According to the model, these reductions would save the British Columbia health system $4,926 CAD per patient, or about $56 million CAD over 20 years.
These findings provide a solid economic justification for clinical implementation of pharmacogenomic-guided depression treatment in Canada.
In addition, the model found that patients who underwent pharmacogenomic testing spent 15% more time in the “well” state without depression symptoms and 18% less time in the MDD state with recurrent episodes or refractory depression. In turn, this would mean 1,869 fewer deaths and 21,346 fewer all-cause hospital admissions over 20 years.
Pharmacogenomic testing also led to gains of 0.064 life-years and 0.381 quality-adjusted life-years per patient, or 12,436 life-years and 74,023 QALYs for all of British Columbia over 20 years.
From a cost perspective, the $121 million CAD cost of pharmacogenomic testing and $524 million CAD increase in episodic care were offset by a decrease in the cost of refractory MDD care. In sensitivity analyses, up-front investment in pharmacogenomic testing was typically offset after 2 years through lower direct medical costs, and it was also considered a cost-saving measure from that point forward.
“By incorporating the perspectives of patients with lived and living experience into this model, alongside robust data sets, we were able to carefully simulate the treatment journey of people with major depression,” Dr. Ghanbarian said. “The simulation model is designed to be flexible and could be applied to other jurisdictions beyond British Columbia, where we might expect to see similar benefits, particularly within a comparable Canadian context.”
Implementing the model
Now, Dr. Ghanbarian and colleagues are interested in potential implementation strategies at a system-wide level. For now, pharmacogenomic tests aren’t offered through the public health systems across Canada, but patients can pay for them through private companies.
“These findings provide a solid economic justification for clinical implementation of pharmacogenomic-guided depression treatment in Canada,” Chad Bousman, PhD, associate professor of physiology and pharmacology at the University of Calgary (Alta.), said in an interview.
Dr. Bousman, who was not involved with this study, coauthored the Clinical Pharmacogenetics Implementation Consortium guideline for several genotypes and serotonin reuptake inhibitor antidepressants. He and colleagues have also developed and evaluated web-based tools that translate pharmacogenetic data into evidence-based prescribing recommendations.
“The hope is that this work will facilitate investment in the establishment of the necessary infrastructure to ensure Canadians have equitable access to pharmacogenomic testing and ultimately improve mental health outcomes,” he said.
The study was funded by Genome BC, Genome Canada, and Michael Smith Health Research British Columbia. Dr. Ghanbarian and Dr. Bousman reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM CMAJ
Maternal depressive symptoms may start at pregnancy
, new research suggests.
The analysis of more than 11,000 pregnant women with depressive symptoms from seven prospective cohorts in Canada, the United Kingdom, and Singapore suggests that depressive symptoms (low, mild, or high levels) start sooner and last longer than is commonly thought.
The term “postnatal depression” is “at odds with existing scientific literature and the experience of clinicians who treat mental disorders in the context of obstetric practice,” said Michael J. Meaney, PhD, professor at McGill University, Montreal, and director of the Translational Neuroscience Program at the Agency for Science, Technology and Research (A*STAR), Singapore.
“Although we anticipated that the prenatal period would be the primary time of onset and that symptom levels would be largely stable, I was nevertheless surprised at how this pattern was so universal across so many studies,” he said in an interview. “In truth, we saw very little evidence for a postnatal onset.”
This suggests that depressive symptoms start earlier than previously thought, and “that the relevant clinical settings for prevention are those treating women in routine health care, including family medicine,” he added.
Start screening sooner
The investigators examined the course and stability of self-reported depressive symptoms at multiple time points across the perinatal period among 11,563 pregnant women in seven cohorts from the United Kingdom, Canada, and Singapore. Participants’ mean age was 29 years; 87.6% were White, 4.9% were East Asian, and 2.6% were Southeast Asian.
The analysis tracked depressive symptoms from preconception through pregnancy to 2 years after childbirth. Three groups of mothers were identified in each cohort on the basis of their level of depressive symptoms (low, mild, or high) as assessed by the Edinburgh Postnatal Depression Scale (EPDS) or the Center for Epidemiological Studies Depression (CES-D).
The team found that all mothers within and across all cohorts had stable trajectories of maternal depressive symptoms from pregnancy onward. Trajectories for mothers who passed clinically validated cutoffs for “probable” depression also showed stable trajectories from pregnancy into the postnatal period.
“Taken together, these findings suggest that maternal depressive symptom levels in community-based cohort studies are apparent during pregnancy and remain stable into the postnatal period,” the authors write. “The results point to the early antenatal period as a timepoint for the identification of stable trajectories of maternal depressive symptoms. Public health policies should emphasize the early antenatal period as the optimal timing for interventions targeting maternal depressive symptoms.”
The findings, they note, “underscore the American Psychiatric Association’s recent approach in renaming postpartum depression as peripartum depression.”
Furthermore, a recent paper of the group’s findings details that depressive symptoms may often predate conception.
“Our findings should serve to universally align practice to prenatal screening,” even though depression screening often takes place in a mid-gestational visit during the second trimester, Dr. Meaney said. “Our findings and those on the effects on child development strongly suggest the timing of the screening must be advanced into the first confirmation of pregnancy.”
Depression is likely worse in the United States
Catherine Monk, PhD, chief of the Division of Women’s Mental Health and professor of medical psychology at Columbia University, Vagelos College of Physicians and Surgeons, New York, said in an interview that the results of the study “amplify similar research findings and the experience of most perinatal clinicians: Depression is stable from pregnancy onwards.”
Dr. Monk, who was not involved in the research, said that “as the authors note, the common focus on postpartum depression misses the months of prior suffering and an opportunity for earlier intervention.” Dr. Monk said she would have liked the results to have been examined further by race and ethnicity and socioeconomic factors. “Also, the combined sample does not include a U.S. cohort. This is significant as the U.S. has the highest maternal morbidity and mortality rate of developed nations, and some reports identify mental health factors as the number-one cause of maternal mortality.”
“Given the tremendous economic, racial, and ethnic inequities in health care – the lack of any kind of health justice – it is quite possible that in the U.S., depression that starts in pregnancy worsens over time, at least for some demographic groups,” Dr. Monk said. “Rates of depression, levels of depression, and the course of it during the peripartum period may be even more dire [in the U.S.] than what is represented in this article.” “What should be practice-changing about this article, and so many others demonstrating the persistent and often high levels of life-threatening depression during pregnancy, is the need for mental health providers to advocate for changes to the low rates of insurance reimbursement that push providers away from accepting insurance and into private practice, making access to affordable mental care nearly impossible for most,” she concluded.
This study was supported by the Singapore Institute for Clinical Sciences, Agency for Science, Technology, and Research; the Toxic Stress Network of the JPB Foundation; the Hope for Depression Research Foundation; and the Jacob’s Foundation. Dr. Meaney and Dr. Monk report no conflicts of interest.
A version of this article appeared on Medscape.com.
, new research suggests.
The analysis of more than 11,000 pregnant women with depressive symptoms from seven prospective cohorts in Canada, the United Kingdom, and Singapore suggests that depressive symptoms (low, mild, or high levels) start sooner and last longer than is commonly thought.
The term “postnatal depression” is “at odds with existing scientific literature and the experience of clinicians who treat mental disorders in the context of obstetric practice,” said Michael J. Meaney, PhD, professor at McGill University, Montreal, and director of the Translational Neuroscience Program at the Agency for Science, Technology and Research (A*STAR), Singapore.
“Although we anticipated that the prenatal period would be the primary time of onset and that symptom levels would be largely stable, I was nevertheless surprised at how this pattern was so universal across so many studies,” he said in an interview. “In truth, we saw very little evidence for a postnatal onset.”
This suggests that depressive symptoms start earlier than previously thought, and “that the relevant clinical settings for prevention are those treating women in routine health care, including family medicine,” he added.
Start screening sooner
The investigators examined the course and stability of self-reported depressive symptoms at multiple time points across the perinatal period among 11,563 pregnant women in seven cohorts from the United Kingdom, Canada, and Singapore. Participants’ mean age was 29 years; 87.6% were White, 4.9% were East Asian, and 2.6% were Southeast Asian.
The analysis tracked depressive symptoms from preconception through pregnancy to 2 years after childbirth. Three groups of mothers were identified in each cohort on the basis of their level of depressive symptoms (low, mild, or high) as assessed by the Edinburgh Postnatal Depression Scale (EPDS) or the Center for Epidemiological Studies Depression (CES-D).
The team found that all mothers within and across all cohorts had stable trajectories of maternal depressive symptoms from pregnancy onward. Trajectories for mothers who passed clinically validated cutoffs for “probable” depression also showed stable trajectories from pregnancy into the postnatal period.
“Taken together, these findings suggest that maternal depressive symptom levels in community-based cohort studies are apparent during pregnancy and remain stable into the postnatal period,” the authors write. “The results point to the early antenatal period as a timepoint for the identification of stable trajectories of maternal depressive symptoms. Public health policies should emphasize the early antenatal period as the optimal timing for interventions targeting maternal depressive symptoms.”
The findings, they note, “underscore the American Psychiatric Association’s recent approach in renaming postpartum depression as peripartum depression.”
Furthermore, a recent paper of the group’s findings details that depressive symptoms may often predate conception.
“Our findings should serve to universally align practice to prenatal screening,” even though depression screening often takes place in a mid-gestational visit during the second trimester, Dr. Meaney said. “Our findings and those on the effects on child development strongly suggest the timing of the screening must be advanced into the first confirmation of pregnancy.”
Depression is likely worse in the United States
Catherine Monk, PhD, chief of the Division of Women’s Mental Health and professor of medical psychology at Columbia University, Vagelos College of Physicians and Surgeons, New York, said in an interview that the results of the study “amplify similar research findings and the experience of most perinatal clinicians: Depression is stable from pregnancy onwards.”
Dr. Monk, who was not involved in the research, said that “as the authors note, the common focus on postpartum depression misses the months of prior suffering and an opportunity for earlier intervention.” Dr. Monk said she would have liked the results to have been examined further by race and ethnicity and socioeconomic factors. “Also, the combined sample does not include a U.S. cohort. This is significant as the U.S. has the highest maternal morbidity and mortality rate of developed nations, and some reports identify mental health factors as the number-one cause of maternal mortality.”
“Given the tremendous economic, racial, and ethnic inequities in health care – the lack of any kind of health justice – it is quite possible that in the U.S., depression that starts in pregnancy worsens over time, at least for some demographic groups,” Dr. Monk said. “Rates of depression, levels of depression, and the course of it during the peripartum period may be even more dire [in the U.S.] than what is represented in this article.” “What should be practice-changing about this article, and so many others demonstrating the persistent and often high levels of life-threatening depression during pregnancy, is the need for mental health providers to advocate for changes to the low rates of insurance reimbursement that push providers away from accepting insurance and into private practice, making access to affordable mental care nearly impossible for most,” she concluded.
This study was supported by the Singapore Institute for Clinical Sciences, Agency for Science, Technology, and Research; the Toxic Stress Network of the JPB Foundation; the Hope for Depression Research Foundation; and the Jacob’s Foundation. Dr. Meaney and Dr. Monk report no conflicts of interest.
A version of this article appeared on Medscape.com.
, new research suggests.
The analysis of more than 11,000 pregnant women with depressive symptoms from seven prospective cohorts in Canada, the United Kingdom, and Singapore suggests that depressive symptoms (low, mild, or high levels) start sooner and last longer than is commonly thought.
The term “postnatal depression” is “at odds with existing scientific literature and the experience of clinicians who treat mental disorders in the context of obstetric practice,” said Michael J. Meaney, PhD, professor at McGill University, Montreal, and director of the Translational Neuroscience Program at the Agency for Science, Technology and Research (A*STAR), Singapore.
“Although we anticipated that the prenatal period would be the primary time of onset and that symptom levels would be largely stable, I was nevertheless surprised at how this pattern was so universal across so many studies,” he said in an interview. “In truth, we saw very little evidence for a postnatal onset.”
This suggests that depressive symptoms start earlier than previously thought, and “that the relevant clinical settings for prevention are those treating women in routine health care, including family medicine,” he added.
Start screening sooner
The investigators examined the course and stability of self-reported depressive symptoms at multiple time points across the perinatal period among 11,563 pregnant women in seven cohorts from the United Kingdom, Canada, and Singapore. Participants’ mean age was 29 years; 87.6% were White, 4.9% were East Asian, and 2.6% were Southeast Asian.
The analysis tracked depressive symptoms from preconception through pregnancy to 2 years after childbirth. Three groups of mothers were identified in each cohort on the basis of their level of depressive symptoms (low, mild, or high) as assessed by the Edinburgh Postnatal Depression Scale (EPDS) or the Center for Epidemiological Studies Depression (CES-D).
The team found that all mothers within and across all cohorts had stable trajectories of maternal depressive symptoms from pregnancy onward. Trajectories for mothers who passed clinically validated cutoffs for “probable” depression also showed stable trajectories from pregnancy into the postnatal period.
“Taken together, these findings suggest that maternal depressive symptom levels in community-based cohort studies are apparent during pregnancy and remain stable into the postnatal period,” the authors write. “The results point to the early antenatal period as a timepoint for the identification of stable trajectories of maternal depressive symptoms. Public health policies should emphasize the early antenatal period as the optimal timing for interventions targeting maternal depressive symptoms.”
The findings, they note, “underscore the American Psychiatric Association’s recent approach in renaming postpartum depression as peripartum depression.”
Furthermore, a recent paper of the group’s findings details that depressive symptoms may often predate conception.
“Our findings should serve to universally align practice to prenatal screening,” even though depression screening often takes place in a mid-gestational visit during the second trimester, Dr. Meaney said. “Our findings and those on the effects on child development strongly suggest the timing of the screening must be advanced into the first confirmation of pregnancy.”
Depression is likely worse in the United States
Catherine Monk, PhD, chief of the Division of Women’s Mental Health and professor of medical psychology at Columbia University, Vagelos College of Physicians and Surgeons, New York, said in an interview that the results of the study “amplify similar research findings and the experience of most perinatal clinicians: Depression is stable from pregnancy onwards.”
Dr. Monk, who was not involved in the research, said that “as the authors note, the common focus on postpartum depression misses the months of prior suffering and an opportunity for earlier intervention.” Dr. Monk said she would have liked the results to have been examined further by race and ethnicity and socioeconomic factors. “Also, the combined sample does not include a U.S. cohort. This is significant as the U.S. has the highest maternal morbidity and mortality rate of developed nations, and some reports identify mental health factors as the number-one cause of maternal mortality.”
“Given the tremendous economic, racial, and ethnic inequities in health care – the lack of any kind of health justice – it is quite possible that in the U.S., depression that starts in pregnancy worsens over time, at least for some demographic groups,” Dr. Monk said. “Rates of depression, levels of depression, and the course of it during the peripartum period may be even more dire [in the U.S.] than what is represented in this article.” “What should be practice-changing about this article, and so many others demonstrating the persistent and often high levels of life-threatening depression during pregnancy, is the need for mental health providers to advocate for changes to the low rates of insurance reimbursement that push providers away from accepting insurance and into private practice, making access to affordable mental care nearly impossible for most,” she concluded.
This study was supported by the Singapore Institute for Clinical Sciences, Agency for Science, Technology, and Research; the Toxic Stress Network of the JPB Foundation; the Hope for Depression Research Foundation; and the Jacob’s Foundation. Dr. Meaney and Dr. Monk report no conflicts of interest.
A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN
‘Love more’: Why doctors should promote social connection
Those who embrace lifestyle medicine are familiar with the slogan Dean Ornish, MD, likes to use: Eat well, move more, stress less, love more.
That last one, love, was the renowned physician and author’s focus at the recent American College of Lifestyle Medicine Conference in Denver. That’s because love – essentially the support, connectedness, and caring that patients feel when they join a lifestyle-change program – is “where healing occurs at the deepest level.”
Indeed, social connectedness is emerging as a vital pillar in the burgeoning field of lifestyle medicine, a specialty that uses lifestyle interventions to treat chronic conditions. About 300 lifestyle medicine programs are now integrated into residencies in medical schools across the country, up from a handful just 5 years ago, said Meagan Grega, MD, the conference chair.
“The energy and growth in American lifestyle medicine is unparalleled by anything else I see in the health care world right now,” said Dr. Grega, a family physician for 25 years in eastern Pennsylvania.
The field applies volumes of research, from the 1990s to today, demonstrating the healing effects of lifestyle changes. Dr. Ornish’s Preventive Medicine Research Institute has published research on small changes (like pomegranate juice helping blood flow in the heart) and huge ones: Coronary heart patients reversed the narrowing of arteries without lipid-lowering drugs after 1 year of lifestyle changes, including a vegetarian diet, aerobic exercise, stress management, and group support.
Ranking alongside bedrocks such as healthy diet, sleep, exercise, and stress management is positive social connection. That part, the “love more” part, often draws skepticism but is vital, said Dr. Ornish, who is sometimes referred to as the father of lifestyle medicine.
It’s “invariably the part that’s the most meaningful – that sense of connection to community that can come when you bring total strangers together,” Dr. Ornish said. “The ‘love more’ part, in many ways, is not only as important, but in some ways even more because everything really flows from that.”
Patients in a support group, who can “let down their emotional defenses and talk openly and authentically,” are much more likely to make and maintain healthy changes, Dr. Ornish said.
Love as medicine
Mounting evidence links loneliness and isolation with a range of health issues, from mood disorders such as depression to chronic conditions such as cardiovascular disease. What’s more, data suggest that loneliness and social isolation in the United States are on the rise, and the COVID pandemic made that more clear. In May 2023, Surgeon General Vivek Murthy, MD, called loneliness, isolation, and lack of connection in the United States a “public health crisis.”
“Good relationships keep us happier and healthier,” said Robert Waldinger, MD, a psychiatrist at Massachusetts General Hospital, Boston.
Dr. Waldinger, who was not affiliated with the conference, is head of the Harvard Study of Adult Development, one of the longest studies of adult life. Beginning in 1938, the study has tracked 724 people plus more than 1,300 of their descendants and found that embracing community and close relationships helps us live longer and be happier.
In the study, the people who were most satisfied with their relationships at age 50 years were the healthiest at age 80 years. Knowing you have someone to rely on protects the brain: “Those people’s memories stay sharper longer,” Dr. Waldinger said.
He draws a distinction between connection and love. “Love is, I think, more of a feeling,” Dr. Waldinger noted. “Connection is a feeling, but it’s also an activity.”
One in five Americans say they’re lonely, he said, “and loneliness is a stressor.” People who are isolated don’t sleep as well, he added. Their health declines earlier in midlife, brain function slips sooner, and their lives are shorter.
“You don’t have anyone to complain to,” he said. If you do, “you can feel your body start to calm down.” Those without social connections may stay in a low-level “fight-or-flight mode.”
“What we think happens is that you have low levels of inflammation chronically, and those can gradually break down body systems.” Moreover, higher rates of cardiac reactivity, for instance, a racing heartbeat when upset, can lead to high blood pressure and lower immune function.
In his talk, Dr. Ornish said, “Anger is that one emotion that has consistently been shown to make heart disease worse.”
Helping people in those straits is gratifying, Dr. Ornish said. “If we can work with people as lifestyle medicine practitioners when they’re suffering, there’s an opportunity for transformation.”
Future
Of course, that can be easier said than done. Dr. Ornish relayed a patient’s typical reaction to a lifestyle program: “This is kind of weird stuff. Like, I get diet. But a plant-based diet, really? Meditation? Loving more? Really?”
He told the conference, “Part of our job as lifestyle medicine practitioners is to spend a little extra time with them. It doesn’t even take that much time. And to really help them understand what brings them a sense of hope and meaning and purpose.”
The results can be motivating. “Most people feel so much better so quickly,” Dr. Ornish said. “It reframes the reason for change from fear of dying to joy of living.”
Dr. Grega, for one, is optimistic for the future, citing survey results showing that 95% of medical students think that they›d be better counselors with lifestyle training. ‘They passionately want this type of thing,” she said.
A version of this article first appeared on Medscape.com.
Those who embrace lifestyle medicine are familiar with the slogan Dean Ornish, MD, likes to use: Eat well, move more, stress less, love more.
That last one, love, was the renowned physician and author’s focus at the recent American College of Lifestyle Medicine Conference in Denver. That’s because love – essentially the support, connectedness, and caring that patients feel when they join a lifestyle-change program – is “where healing occurs at the deepest level.”
Indeed, social connectedness is emerging as a vital pillar in the burgeoning field of lifestyle medicine, a specialty that uses lifestyle interventions to treat chronic conditions. About 300 lifestyle medicine programs are now integrated into residencies in medical schools across the country, up from a handful just 5 years ago, said Meagan Grega, MD, the conference chair.
“The energy and growth in American lifestyle medicine is unparalleled by anything else I see in the health care world right now,” said Dr. Grega, a family physician for 25 years in eastern Pennsylvania.
The field applies volumes of research, from the 1990s to today, demonstrating the healing effects of lifestyle changes. Dr. Ornish’s Preventive Medicine Research Institute has published research on small changes (like pomegranate juice helping blood flow in the heart) and huge ones: Coronary heart patients reversed the narrowing of arteries without lipid-lowering drugs after 1 year of lifestyle changes, including a vegetarian diet, aerobic exercise, stress management, and group support.
Ranking alongside bedrocks such as healthy diet, sleep, exercise, and stress management is positive social connection. That part, the “love more” part, often draws skepticism but is vital, said Dr. Ornish, who is sometimes referred to as the father of lifestyle medicine.
It’s “invariably the part that’s the most meaningful – that sense of connection to community that can come when you bring total strangers together,” Dr. Ornish said. “The ‘love more’ part, in many ways, is not only as important, but in some ways even more because everything really flows from that.”
Patients in a support group, who can “let down their emotional defenses and talk openly and authentically,” are much more likely to make and maintain healthy changes, Dr. Ornish said.
Love as medicine
Mounting evidence links loneliness and isolation with a range of health issues, from mood disorders such as depression to chronic conditions such as cardiovascular disease. What’s more, data suggest that loneliness and social isolation in the United States are on the rise, and the COVID pandemic made that more clear. In May 2023, Surgeon General Vivek Murthy, MD, called loneliness, isolation, and lack of connection in the United States a “public health crisis.”
“Good relationships keep us happier and healthier,” said Robert Waldinger, MD, a psychiatrist at Massachusetts General Hospital, Boston.
Dr. Waldinger, who was not affiliated with the conference, is head of the Harvard Study of Adult Development, one of the longest studies of adult life. Beginning in 1938, the study has tracked 724 people plus more than 1,300 of their descendants and found that embracing community and close relationships helps us live longer and be happier.
In the study, the people who were most satisfied with their relationships at age 50 years were the healthiest at age 80 years. Knowing you have someone to rely on protects the brain: “Those people’s memories stay sharper longer,” Dr. Waldinger said.
He draws a distinction between connection and love. “Love is, I think, more of a feeling,” Dr. Waldinger noted. “Connection is a feeling, but it’s also an activity.”
One in five Americans say they’re lonely, he said, “and loneliness is a stressor.” People who are isolated don’t sleep as well, he added. Their health declines earlier in midlife, brain function slips sooner, and their lives are shorter.
“You don’t have anyone to complain to,” he said. If you do, “you can feel your body start to calm down.” Those without social connections may stay in a low-level “fight-or-flight mode.”
“What we think happens is that you have low levels of inflammation chronically, and those can gradually break down body systems.” Moreover, higher rates of cardiac reactivity, for instance, a racing heartbeat when upset, can lead to high blood pressure and lower immune function.
In his talk, Dr. Ornish said, “Anger is that one emotion that has consistently been shown to make heart disease worse.”
Helping people in those straits is gratifying, Dr. Ornish said. “If we can work with people as lifestyle medicine practitioners when they’re suffering, there’s an opportunity for transformation.”
Future
Of course, that can be easier said than done. Dr. Ornish relayed a patient’s typical reaction to a lifestyle program: “This is kind of weird stuff. Like, I get diet. But a plant-based diet, really? Meditation? Loving more? Really?”
He told the conference, “Part of our job as lifestyle medicine practitioners is to spend a little extra time with them. It doesn’t even take that much time. And to really help them understand what brings them a sense of hope and meaning and purpose.”
The results can be motivating. “Most people feel so much better so quickly,” Dr. Ornish said. “It reframes the reason for change from fear of dying to joy of living.”
Dr. Grega, for one, is optimistic for the future, citing survey results showing that 95% of medical students think that they›d be better counselors with lifestyle training. ‘They passionately want this type of thing,” she said.
A version of this article first appeared on Medscape.com.
Those who embrace lifestyle medicine are familiar with the slogan Dean Ornish, MD, likes to use: Eat well, move more, stress less, love more.
That last one, love, was the renowned physician and author’s focus at the recent American College of Lifestyle Medicine Conference in Denver. That’s because love – essentially the support, connectedness, and caring that patients feel when they join a lifestyle-change program – is “where healing occurs at the deepest level.”
Indeed, social connectedness is emerging as a vital pillar in the burgeoning field of lifestyle medicine, a specialty that uses lifestyle interventions to treat chronic conditions. About 300 lifestyle medicine programs are now integrated into residencies in medical schools across the country, up from a handful just 5 years ago, said Meagan Grega, MD, the conference chair.
“The energy and growth in American lifestyle medicine is unparalleled by anything else I see in the health care world right now,” said Dr. Grega, a family physician for 25 years in eastern Pennsylvania.
The field applies volumes of research, from the 1990s to today, demonstrating the healing effects of lifestyle changes. Dr. Ornish’s Preventive Medicine Research Institute has published research on small changes (like pomegranate juice helping blood flow in the heart) and huge ones: Coronary heart patients reversed the narrowing of arteries without lipid-lowering drugs after 1 year of lifestyle changes, including a vegetarian diet, aerobic exercise, stress management, and group support.
Ranking alongside bedrocks such as healthy diet, sleep, exercise, and stress management is positive social connection. That part, the “love more” part, often draws skepticism but is vital, said Dr. Ornish, who is sometimes referred to as the father of lifestyle medicine.
It’s “invariably the part that’s the most meaningful – that sense of connection to community that can come when you bring total strangers together,” Dr. Ornish said. “The ‘love more’ part, in many ways, is not only as important, but in some ways even more because everything really flows from that.”
Patients in a support group, who can “let down their emotional defenses and talk openly and authentically,” are much more likely to make and maintain healthy changes, Dr. Ornish said.
Love as medicine
Mounting evidence links loneliness and isolation with a range of health issues, from mood disorders such as depression to chronic conditions such as cardiovascular disease. What’s more, data suggest that loneliness and social isolation in the United States are on the rise, and the COVID pandemic made that more clear. In May 2023, Surgeon General Vivek Murthy, MD, called loneliness, isolation, and lack of connection in the United States a “public health crisis.”
“Good relationships keep us happier and healthier,” said Robert Waldinger, MD, a psychiatrist at Massachusetts General Hospital, Boston.
Dr. Waldinger, who was not affiliated with the conference, is head of the Harvard Study of Adult Development, one of the longest studies of adult life. Beginning in 1938, the study has tracked 724 people plus more than 1,300 of their descendants and found that embracing community and close relationships helps us live longer and be happier.
In the study, the people who were most satisfied with their relationships at age 50 years were the healthiest at age 80 years. Knowing you have someone to rely on protects the brain: “Those people’s memories stay sharper longer,” Dr. Waldinger said.
He draws a distinction between connection and love. “Love is, I think, more of a feeling,” Dr. Waldinger noted. “Connection is a feeling, but it’s also an activity.”
One in five Americans say they’re lonely, he said, “and loneliness is a stressor.” People who are isolated don’t sleep as well, he added. Their health declines earlier in midlife, brain function slips sooner, and their lives are shorter.
“You don’t have anyone to complain to,” he said. If you do, “you can feel your body start to calm down.” Those without social connections may stay in a low-level “fight-or-flight mode.”
“What we think happens is that you have low levels of inflammation chronically, and those can gradually break down body systems.” Moreover, higher rates of cardiac reactivity, for instance, a racing heartbeat when upset, can lead to high blood pressure and lower immune function.
In his talk, Dr. Ornish said, “Anger is that one emotion that has consistently been shown to make heart disease worse.”
Helping people in those straits is gratifying, Dr. Ornish said. “If we can work with people as lifestyle medicine practitioners when they’re suffering, there’s an opportunity for transformation.”
Future
Of course, that can be easier said than done. Dr. Ornish relayed a patient’s typical reaction to a lifestyle program: “This is kind of weird stuff. Like, I get diet. But a plant-based diet, really? Meditation? Loving more? Really?”
He told the conference, “Part of our job as lifestyle medicine practitioners is to spend a little extra time with them. It doesn’t even take that much time. And to really help them understand what brings them a sense of hope and meaning and purpose.”
The results can be motivating. “Most people feel so much better so quickly,” Dr. Ornish said. “It reframes the reason for change from fear of dying to joy of living.”
Dr. Grega, for one, is optimistic for the future, citing survey results showing that 95% of medical students think that they›d be better counselors with lifestyle training. ‘They passionately want this type of thing,” she said.
A version of this article first appeared on Medscape.com.




