User login
The cult of the suicide risk assessment
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
Psilocybin reduces symptoms, disability in major depression
The randomized, phase 2 trial was conducted at 11 sites across the United States and is the latest to demonstrate the psychedelic drug’s potential as a treatment for depression.
The project was funded by Usona Institute, a nonprofit medical research organization based in Madison, Wisc. The institute issued a press statement, but researchers did not comment further on the findings.
“As the largest and most rigorous study conducted across a wide spectrum of individuals with major depressive disorder, the results show promise for all people struggling with this condition,” lead author Charles Raison, MD, director of clinical and translational research at Usona, said in the statement.
The 34 coauthors on the study are affiliated with public universities, research centers, and private companies. Eight of the investigators are identified as employees of Usona Institute.
Declining further comment, an institute spokesperson told this news organization that, “Usona has chosen the approach of no interviews, and this applies for all coauthors.”
The findings were published online in JAMA.
Largest study to date
Usona’s investigational psilocybin drug has been granted a breakthrough designation by the Food and Drug Administration, a process designed to speed drug development and review.
Previous smaller studies have suggested a rapid antidepressant response with psilocybin, but they have been small, unblinded, and have had short duration of follow-up, they write. This randomized, double-blind, phase 2 clinical trial is the largest study of psilocybin for depression to date, the researchers note.
It included 104 adults aged 21-65 years with MDD who had a current depressive episode of at least 60 days and a Montgomery-Åsberg Depression Rating Scale (MADRS) total score of 28 or more at baseline.
Participants had to be free of psychedelic drugs for at least 5 years, have had no active suicidal ideation or suicidal behavior in the prior 12 months, no personal or first-degree family history of psychosis or mania, and no history of moderate/severe alcohol or drug use disorder.
Before the study, participants had a 7- to 35-day screening period for psychiatric medication tapering, underwent baseline assessments, and received 6-8 hours of preparation with two facilitators who would be with them during dosing.
Dosing occurred within 7 days of baseline assessments. During the 6- to 8-hour session, participants received either a single 25-mg oral dose of psilocybin or 100-mg dose of niacin. One participant randomly assigned to receive psilocybin received the incorrect treatment, resulting in 50 participants receiving psilocybin and 54 receiving niacin.
Participants returned the next day, the next week, and then every 2 weeks for assessments, for a follow-up of 6 weeks.
Psychosocial support
Participants who received psilocybin reported significantly greater improvements in MDD symptoms, compared with those who received niacin. MADRS scores – a scale from 0 to 60 where higher scores indicate more severe depression – showed greater reductions with treatment vs. placebo at 8 days (mean difference, −12.0; 95% confidence interval, −16.6 to −7.4; P < .001), and at day 43 (mean difference, −12.3; 95% CI, −17.5 to −7.2; P < .001).
More participants receiving psilocybin had sustained depressive symptom response (42% vs. 11%; P = .002) and more improvement in the Sheehan Disability Scale score, which measures functional disability, 43 days after treatment (P < .001).
The effects persisted through the end of the study, although the differences between groups were no longer significant by week 6.
“This is another exciting piece of evidence that adds to the current literature regarding the potential efficacy of psilocybin for the treatment of mental health conditions, particularly depression,” said Greg Fonzo, MD, codirector of the Center for Psychedelic Research and Therapy at the University of Texas at Austin, who commented on the findings.
Significantly more people in the psilocybin group reported at least one treatment-related adverse event (AE, 82% vs. 44%), although most were mild to moderate. Headache and nausea were the most common side effects and most resolved within 1 day of dosing.
While those numbers are high, Dr. Fonzo said they’re not out of line with AEs reported in other studies.
“Particularly with the types of adverse events reported here, like headache and nausea, those are things you would typically expect to see in this treatment,” said Dr. Fonzo, who was not part of the research.
“But it is high, and it underscores that this is not a treatment without certain risks, even though it was good that they were primarily mild in severity,” he added.
A ‘stepping stone’ to FDA approval?
The use of tools to measure disability in addition to symptoms of depression severity is a strength of the study, Dr. Fonzo added. The use of an active comparator and the 6-week follow-up also offer something new over previous studies.
Despite the longer follow up, questions remain about the durability of response, something only a longer study could answer, Dr. Fonzo said. The small and homogeneous sample-size are also a concern. Nearly 90% of participants were White, and more than half had an income of $75,000 a year or higher.
“It’s another stepping stone in the process to FDA approval, but the next step in that process would be much larger phase 3 trials that would have much larger samples, a longer follow-up, and hopefully have a more inclusive swath of the population,” Dr. Fonzo said.
But perhaps one of the most significant limitations is the use of niacin as an active comparator, said Caleb Alexander, MD, codirector of the Center for Drug Safety and Effectiveness at Johns Hopkins University in Baltimore.
The use of an agent that doesn’t produce effects similar to those expected from a psychedelic introduced the potential for functional unblinding, Dr. Alexander said. Investigators did not ask participants to guess whether they received psilocybin or niacin, so the quality of the blinding was not assessed in the study.
“We’d like to see the use of [an] active comparator that might have a chance of obscuring to people as to whether they’ve been randomized to the treatment arm or control arm,” said Dr. Alexander, who wasn’t involved in the study. “Why not use a benzodiazepine or another drug that produces a transient euphoria that would better obscure whether or not people were receiving the psilocybin?”
The authors of an accompanying editorial shared these concerns, also noting that the study included “a significant number of patients who did not respond to therapy.”
“It is important to analyze and understand adverse outcomes in psychedelic trials and conduct longitudinal studies to determine how sustained the effects will be and what may initiate a recrudescence of symptoms,” write Rachel Yehuda, PhD, and Amy Lehrner, PhD, both of the Peters VA Medical Center and Icahn School of Medicine at Mount Sinai, New York.
“Future studies will help identify who is most likely to benefit from psychedelics, whether booster or repeated treatment is safe and beneficial, and what the optimal dose and therapeutic frameworks are.”
A long-term follow-up of the current trial was terminated last year because of low enrollment. The spokesperson with Usona Institute did not respond to questions about that study, and the institute’s statement only added that preparations are underway to launch another study that “will provide additional safety and efficacy data to support submission of a new drug application to the FDA.”
Usona published its manufacturing process that it used to synthesize psilocybin in an open-access journal and signed a statement on “open science and open praxis” with psilocybin and similar substances, which appears on their website. That statement was signed by 31 research and service organizations around the world and nearly 150 scientists, scholars, and practitioners.
The study was funded by Usona Institute. Dr. Raison reported receiving personal fees from Usona Institute and grants to Usona Institute from Dr. Bronner’s All-One, Fournier Family Foundation, Good Ventures, Steven and Alexandra Cohen Foundation, Tiny Blue Dot Foundation, Turnbull Family Foundation, and William A. Linton during the conduct of the study; and personal fees from Novartis, Sage/Biogen, Emory Healthcare, and Vail Health outside the submitted work. Dr. Fonzo and Dr. Alexander report no relevant financial relationships. Dr. Yehuda reports receiving nonfinancial support from the Multidisciplinary Association for Psychedelic Studies Public Benefit (MAPS PBC) and grants from COMPASS Pathways. Dr. Lehrner is an investigator on trials sponsored by MAPS PBC and COMPASS Pathways.
A version of this article first appeared on Medscape.com.
The randomized, phase 2 trial was conducted at 11 sites across the United States and is the latest to demonstrate the psychedelic drug’s potential as a treatment for depression.
The project was funded by Usona Institute, a nonprofit medical research organization based in Madison, Wisc. The institute issued a press statement, but researchers did not comment further on the findings.
“As the largest and most rigorous study conducted across a wide spectrum of individuals with major depressive disorder, the results show promise for all people struggling with this condition,” lead author Charles Raison, MD, director of clinical and translational research at Usona, said in the statement.
The 34 coauthors on the study are affiliated with public universities, research centers, and private companies. Eight of the investigators are identified as employees of Usona Institute.
Declining further comment, an institute spokesperson told this news organization that, “Usona has chosen the approach of no interviews, and this applies for all coauthors.”
The findings were published online in JAMA.
Largest study to date
Usona’s investigational psilocybin drug has been granted a breakthrough designation by the Food and Drug Administration, a process designed to speed drug development and review.
Previous smaller studies have suggested a rapid antidepressant response with psilocybin, but they have been small, unblinded, and have had short duration of follow-up, they write. This randomized, double-blind, phase 2 clinical trial is the largest study of psilocybin for depression to date, the researchers note.
It included 104 adults aged 21-65 years with MDD who had a current depressive episode of at least 60 days and a Montgomery-Åsberg Depression Rating Scale (MADRS) total score of 28 or more at baseline.
Participants had to be free of psychedelic drugs for at least 5 years, have had no active suicidal ideation or suicidal behavior in the prior 12 months, no personal or first-degree family history of psychosis or mania, and no history of moderate/severe alcohol or drug use disorder.
Before the study, participants had a 7- to 35-day screening period for psychiatric medication tapering, underwent baseline assessments, and received 6-8 hours of preparation with two facilitators who would be with them during dosing.
Dosing occurred within 7 days of baseline assessments. During the 6- to 8-hour session, participants received either a single 25-mg oral dose of psilocybin or 100-mg dose of niacin. One participant randomly assigned to receive psilocybin received the incorrect treatment, resulting in 50 participants receiving psilocybin and 54 receiving niacin.
Participants returned the next day, the next week, and then every 2 weeks for assessments, for a follow-up of 6 weeks.
Psychosocial support
Participants who received psilocybin reported significantly greater improvements in MDD symptoms, compared with those who received niacin. MADRS scores – a scale from 0 to 60 where higher scores indicate more severe depression – showed greater reductions with treatment vs. placebo at 8 days (mean difference, −12.0; 95% confidence interval, −16.6 to −7.4; P < .001), and at day 43 (mean difference, −12.3; 95% CI, −17.5 to −7.2; P < .001).
More participants receiving psilocybin had sustained depressive symptom response (42% vs. 11%; P = .002) and more improvement in the Sheehan Disability Scale score, which measures functional disability, 43 days after treatment (P < .001).
The effects persisted through the end of the study, although the differences between groups were no longer significant by week 6.
“This is another exciting piece of evidence that adds to the current literature regarding the potential efficacy of psilocybin for the treatment of mental health conditions, particularly depression,” said Greg Fonzo, MD, codirector of the Center for Psychedelic Research and Therapy at the University of Texas at Austin, who commented on the findings.
Significantly more people in the psilocybin group reported at least one treatment-related adverse event (AE, 82% vs. 44%), although most were mild to moderate. Headache and nausea were the most common side effects and most resolved within 1 day of dosing.
While those numbers are high, Dr. Fonzo said they’re not out of line with AEs reported in other studies.
“Particularly with the types of adverse events reported here, like headache and nausea, those are things you would typically expect to see in this treatment,” said Dr. Fonzo, who was not part of the research.
“But it is high, and it underscores that this is not a treatment without certain risks, even though it was good that they were primarily mild in severity,” he added.
A ‘stepping stone’ to FDA approval?
The use of tools to measure disability in addition to symptoms of depression severity is a strength of the study, Dr. Fonzo added. The use of an active comparator and the 6-week follow-up also offer something new over previous studies.
Despite the longer follow up, questions remain about the durability of response, something only a longer study could answer, Dr. Fonzo said. The small and homogeneous sample-size are also a concern. Nearly 90% of participants were White, and more than half had an income of $75,000 a year or higher.
“It’s another stepping stone in the process to FDA approval, but the next step in that process would be much larger phase 3 trials that would have much larger samples, a longer follow-up, and hopefully have a more inclusive swath of the population,” Dr. Fonzo said.
But perhaps one of the most significant limitations is the use of niacin as an active comparator, said Caleb Alexander, MD, codirector of the Center for Drug Safety and Effectiveness at Johns Hopkins University in Baltimore.
The use of an agent that doesn’t produce effects similar to those expected from a psychedelic introduced the potential for functional unblinding, Dr. Alexander said. Investigators did not ask participants to guess whether they received psilocybin or niacin, so the quality of the blinding was not assessed in the study.
“We’d like to see the use of [an] active comparator that might have a chance of obscuring to people as to whether they’ve been randomized to the treatment arm or control arm,” said Dr. Alexander, who wasn’t involved in the study. “Why not use a benzodiazepine or another drug that produces a transient euphoria that would better obscure whether or not people were receiving the psilocybin?”
The authors of an accompanying editorial shared these concerns, also noting that the study included “a significant number of patients who did not respond to therapy.”
“It is important to analyze and understand adverse outcomes in psychedelic trials and conduct longitudinal studies to determine how sustained the effects will be and what may initiate a recrudescence of symptoms,” write Rachel Yehuda, PhD, and Amy Lehrner, PhD, both of the Peters VA Medical Center and Icahn School of Medicine at Mount Sinai, New York.
“Future studies will help identify who is most likely to benefit from psychedelics, whether booster or repeated treatment is safe and beneficial, and what the optimal dose and therapeutic frameworks are.”
A long-term follow-up of the current trial was terminated last year because of low enrollment. The spokesperson with Usona Institute did not respond to questions about that study, and the institute’s statement only added that preparations are underway to launch another study that “will provide additional safety and efficacy data to support submission of a new drug application to the FDA.”
Usona published its manufacturing process that it used to synthesize psilocybin in an open-access journal and signed a statement on “open science and open praxis” with psilocybin and similar substances, which appears on their website. That statement was signed by 31 research and service organizations around the world and nearly 150 scientists, scholars, and practitioners.
The study was funded by Usona Institute. Dr. Raison reported receiving personal fees from Usona Institute and grants to Usona Institute from Dr. Bronner’s All-One, Fournier Family Foundation, Good Ventures, Steven and Alexandra Cohen Foundation, Tiny Blue Dot Foundation, Turnbull Family Foundation, and William A. Linton during the conduct of the study; and personal fees from Novartis, Sage/Biogen, Emory Healthcare, and Vail Health outside the submitted work. Dr. Fonzo and Dr. Alexander report no relevant financial relationships. Dr. Yehuda reports receiving nonfinancial support from the Multidisciplinary Association for Psychedelic Studies Public Benefit (MAPS PBC) and grants from COMPASS Pathways. Dr. Lehrner is an investigator on trials sponsored by MAPS PBC and COMPASS Pathways.
A version of this article first appeared on Medscape.com.
The randomized, phase 2 trial was conducted at 11 sites across the United States and is the latest to demonstrate the psychedelic drug’s potential as a treatment for depression.
The project was funded by Usona Institute, a nonprofit medical research organization based in Madison, Wisc. The institute issued a press statement, but researchers did not comment further on the findings.
“As the largest and most rigorous study conducted across a wide spectrum of individuals with major depressive disorder, the results show promise for all people struggling with this condition,” lead author Charles Raison, MD, director of clinical and translational research at Usona, said in the statement.
The 34 coauthors on the study are affiliated with public universities, research centers, and private companies. Eight of the investigators are identified as employees of Usona Institute.
Declining further comment, an institute spokesperson told this news organization that, “Usona has chosen the approach of no interviews, and this applies for all coauthors.”
The findings were published online in JAMA.
Largest study to date
Usona’s investigational psilocybin drug has been granted a breakthrough designation by the Food and Drug Administration, a process designed to speed drug development and review.
Previous smaller studies have suggested a rapid antidepressant response with psilocybin, but they have been small, unblinded, and have had short duration of follow-up, they write. This randomized, double-blind, phase 2 clinical trial is the largest study of psilocybin for depression to date, the researchers note.
It included 104 adults aged 21-65 years with MDD who had a current depressive episode of at least 60 days and a Montgomery-Åsberg Depression Rating Scale (MADRS) total score of 28 or more at baseline.
Participants had to be free of psychedelic drugs for at least 5 years, have had no active suicidal ideation or suicidal behavior in the prior 12 months, no personal or first-degree family history of psychosis or mania, and no history of moderate/severe alcohol or drug use disorder.
Before the study, participants had a 7- to 35-day screening period for psychiatric medication tapering, underwent baseline assessments, and received 6-8 hours of preparation with two facilitators who would be with them during dosing.
Dosing occurred within 7 days of baseline assessments. During the 6- to 8-hour session, participants received either a single 25-mg oral dose of psilocybin or 100-mg dose of niacin. One participant randomly assigned to receive psilocybin received the incorrect treatment, resulting in 50 participants receiving psilocybin and 54 receiving niacin.
Participants returned the next day, the next week, and then every 2 weeks for assessments, for a follow-up of 6 weeks.
Psychosocial support
Participants who received psilocybin reported significantly greater improvements in MDD symptoms, compared with those who received niacin. MADRS scores – a scale from 0 to 60 where higher scores indicate more severe depression – showed greater reductions with treatment vs. placebo at 8 days (mean difference, −12.0; 95% confidence interval, −16.6 to −7.4; P < .001), and at day 43 (mean difference, −12.3; 95% CI, −17.5 to −7.2; P < .001).
More participants receiving psilocybin had sustained depressive symptom response (42% vs. 11%; P = .002) and more improvement in the Sheehan Disability Scale score, which measures functional disability, 43 days after treatment (P < .001).
The effects persisted through the end of the study, although the differences between groups were no longer significant by week 6.
“This is another exciting piece of evidence that adds to the current literature regarding the potential efficacy of psilocybin for the treatment of mental health conditions, particularly depression,” said Greg Fonzo, MD, codirector of the Center for Psychedelic Research and Therapy at the University of Texas at Austin, who commented on the findings.
Significantly more people in the psilocybin group reported at least one treatment-related adverse event (AE, 82% vs. 44%), although most were mild to moderate. Headache and nausea were the most common side effects and most resolved within 1 day of dosing.
While those numbers are high, Dr. Fonzo said they’re not out of line with AEs reported in other studies.
“Particularly with the types of adverse events reported here, like headache and nausea, those are things you would typically expect to see in this treatment,” said Dr. Fonzo, who was not part of the research.
“But it is high, and it underscores that this is not a treatment without certain risks, even though it was good that they were primarily mild in severity,” he added.
A ‘stepping stone’ to FDA approval?
The use of tools to measure disability in addition to symptoms of depression severity is a strength of the study, Dr. Fonzo added. The use of an active comparator and the 6-week follow-up also offer something new over previous studies.
Despite the longer follow up, questions remain about the durability of response, something only a longer study could answer, Dr. Fonzo said. The small and homogeneous sample-size are also a concern. Nearly 90% of participants were White, and more than half had an income of $75,000 a year or higher.
“It’s another stepping stone in the process to FDA approval, but the next step in that process would be much larger phase 3 trials that would have much larger samples, a longer follow-up, and hopefully have a more inclusive swath of the population,” Dr. Fonzo said.
But perhaps one of the most significant limitations is the use of niacin as an active comparator, said Caleb Alexander, MD, codirector of the Center for Drug Safety and Effectiveness at Johns Hopkins University in Baltimore.
The use of an agent that doesn’t produce effects similar to those expected from a psychedelic introduced the potential for functional unblinding, Dr. Alexander said. Investigators did not ask participants to guess whether they received psilocybin or niacin, so the quality of the blinding was not assessed in the study.
“We’d like to see the use of [an] active comparator that might have a chance of obscuring to people as to whether they’ve been randomized to the treatment arm or control arm,” said Dr. Alexander, who wasn’t involved in the study. “Why not use a benzodiazepine or another drug that produces a transient euphoria that would better obscure whether or not people were receiving the psilocybin?”
The authors of an accompanying editorial shared these concerns, also noting that the study included “a significant number of patients who did not respond to therapy.”
“It is important to analyze and understand adverse outcomes in psychedelic trials and conduct longitudinal studies to determine how sustained the effects will be and what may initiate a recrudescence of symptoms,” write Rachel Yehuda, PhD, and Amy Lehrner, PhD, both of the Peters VA Medical Center and Icahn School of Medicine at Mount Sinai, New York.
“Future studies will help identify who is most likely to benefit from psychedelics, whether booster or repeated treatment is safe and beneficial, and what the optimal dose and therapeutic frameworks are.”
A long-term follow-up of the current trial was terminated last year because of low enrollment. The spokesperson with Usona Institute did not respond to questions about that study, and the institute’s statement only added that preparations are underway to launch another study that “will provide additional safety and efficacy data to support submission of a new drug application to the FDA.”
Usona published its manufacturing process that it used to synthesize psilocybin in an open-access journal and signed a statement on “open science and open praxis” with psilocybin and similar substances, which appears on their website. That statement was signed by 31 research and service organizations around the world and nearly 150 scientists, scholars, and practitioners.
The study was funded by Usona Institute. Dr. Raison reported receiving personal fees from Usona Institute and grants to Usona Institute from Dr. Bronner’s All-One, Fournier Family Foundation, Good Ventures, Steven and Alexandra Cohen Foundation, Tiny Blue Dot Foundation, Turnbull Family Foundation, and William A. Linton during the conduct of the study; and personal fees from Novartis, Sage/Biogen, Emory Healthcare, and Vail Health outside the submitted work. Dr. Fonzo and Dr. Alexander report no relevant financial relationships. Dr. Yehuda reports receiving nonfinancial support from the Multidisciplinary Association for Psychedelic Studies Public Benefit (MAPS PBC) and grants from COMPASS Pathways. Dr. Lehrner is an investigator on trials sponsored by MAPS PBC and COMPASS Pathways.
A version of this article first appeared on Medscape.com.
FROM JAMA
Suicidal behavior tied to increased all-cause mortality in MDD
Investigators studied close to 143,000 patients, encompassing more than 150,000 MDD episodes. Episodes of depression with suicidal behavior (MDD-SB) were compared to MDD episodes without suicidal behavior (MDD-non-SB).
Suicidal behavior was associated with a 2.6-fold higher rate of all-cause mortality, as well as considerably higher health care resource utilization (HCRU) and work loss, compared with matched controls.
Patients with depression who had attempted suicide were younger and more commonly suffering from other psychiatric comorbidities, such as anxiety and addiction. Important risk factors for suicidal acts within a year after the onset of a depressive episode were previous suicide attempts, substance use disorder, anxiety, and sleeping disorders.
“The findings tell us that the care provided for this particular group needs to be developed,” lead author Johan Lundberg, MD, PhD, adjunct professor in psychiatry and senior physician in psychiatry, Karolinska Institute, Stockholm, told this news organization.
“The take-home message is that, when treating patients with increased risk of suicidal behavior, one should offer treatments with this in mind,” said Dr. Lundberg, also the head of the section of mood disorders, Northern Stockholm Psychiatry Clinic. “One possible option is lithium augmentation.”
The study was published online in JAMA Psychiatry.
Identifying subgroups
Depression is associated with increased all-cause mortality, the authors write. Suicidal behavior and previous suicide attempts are known to increase the risk of suicide-associated mortality, with up to 13% of patients with nonfatal suicide attempts dying of suicide at a later time.
Previous studies investigating the association between suicidal behavior and mortality have been limited by nonrandom sampling due to “nonuniversal access to health care and/or exclusion of primary care data,” they state.
For this reason, it’s not established to what extent these estimates actually represent patients with MDD as a whole, or to what extent suicidal behavior is a risk factor for all-cause mortality.
“We think there is a need to identify subgroups within the very large group of individuals with MDD in order to improve treatment outcomes,” Dr. Lundberg said.
To do so, the researchers turned to data from the Stockholm MDD Cohort (SMC), which comprises all patients diagnosed with MDD in any health care setting in the regions of Stockholm from 2010 to 2018. They identified 5 years of recorded MDD episodes (n = 158,169) in patients aged 18 years and older (n = 145,577). A single patient could contribute more than one episode.
At index, MDD-SB patients (n = 2,219; mean age, 41 years) were matched with MDD-non-SB patients (9,574; mean age, 41 years) based on age, sex, year of MDD diagnosis, and socioeconomic status. In total, 2,219 episodes (63.2% in women, 36.8% in men) were compared to 11,109 episodes (63.4% in women, 36.6% in men), respectively.
Enhanced monitoring, optimized treatment
The median time from the start of the episode until the first suicidal behavior was 165 days.
The all-cause mortality rate in the MDD-SB and MDD-non-SB groups was 2.5 per 100 person-years vs. 1 per 100 person-years, respectively (based on 466 deaths), corresponding to a hazard ratio of 2.62 (95% confidence interval, 2.15-3.20).
Patients in the MDD-SB group were younger, were more frequently diagnosed while in specialized care, and had sustained more work loss than their counterparts in the MDD-non-SB group. They also showed a gradual increase in the prevalence of comorbid conditions from about 12 months before index, with this increase being “most pronounced” for anxiety, stress, substance use, and personality disorders.
MDD-SB episodes were associated with higher HCRU and more work loss, compared with MDD-non-SB episodes.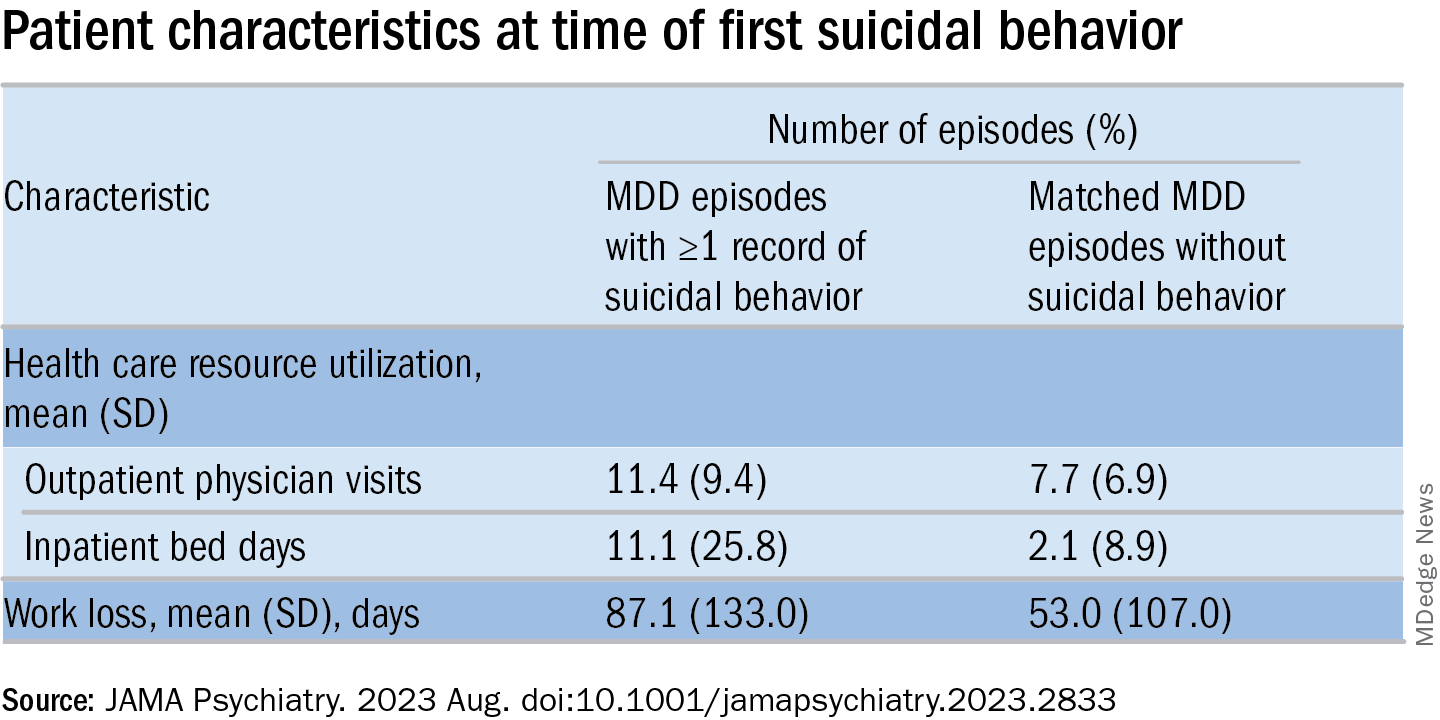
The researchers calculated a risk score for factors associated with suicidal behavior within 1 year after the start of an MDD episode (outcome). The two most important risk factors for suicidal behavior were a history of suicidal behavior together with age, which had a “U-shaped association” with the outcome, they write, with individuals younger than age 20 and older than age 70 having the highest risks.
The final risk score included additional factors that increased the risk of the outcome (in descending order): history of substance use, history of sleep disorders, health care level in which MDD was diagnosed, history of antidepressant use, and history of anxiety disorders.
These results “indicate that patients at risk for suicidal behavior can be identified at an early stage to allow for enhanced monitoring and optimized treatment with the goal of preventing suicidal behavior and reducing mortality,” the authors state.
The specific causes of death weren’t analyzed in this particular paper, Dr. Lundberg noted. A previous study conducted by the same group found the risk of death was doubled in MDD patients, compared with controls.
“We don’t speculate about which causes other than suicide might explain the difference” and account for the increased mortality risk, he said. “This should be studied in future projects.”
Complicated family of destructive behaviors
In a comment, Russell Copelan, MD, a former emergency department psychiatrist at the University of Colorado Affiliated Hospital and currently an expert consultant to the American Association of Suicidology, said a take-home message of the study is that suicide is “a complex and complicated family of destructive behaviors.”
The findings “should not suggest a wait-and-see clinical approach,” warned Dr. Copelan, who wasn’t involved with the study.
Underrecognized or misdiagnosed anxiety, agitation, and insomnia may be “barriers to remission and treatment response,” he noted.
Dr. Copelan, who is also the founder and CEO of eMed Logic, which offers assessment tools for suicide and violence, encouraged clinicians “not to minimize the proportion of patients who experience anxiety, agitation, and insomnia in response to what some may consider a personal misfortune, such as interpersonal, employment, or financial crisis.”
A version of this article first appeared on Medscape.com.
Investigators studied close to 143,000 patients, encompassing more than 150,000 MDD episodes. Episodes of depression with suicidal behavior (MDD-SB) were compared to MDD episodes without suicidal behavior (MDD-non-SB).
Suicidal behavior was associated with a 2.6-fold higher rate of all-cause mortality, as well as considerably higher health care resource utilization (HCRU) and work loss, compared with matched controls.
Patients with depression who had attempted suicide were younger and more commonly suffering from other psychiatric comorbidities, such as anxiety and addiction. Important risk factors for suicidal acts within a year after the onset of a depressive episode were previous suicide attempts, substance use disorder, anxiety, and sleeping disorders.
“The findings tell us that the care provided for this particular group needs to be developed,” lead author Johan Lundberg, MD, PhD, adjunct professor in psychiatry and senior physician in psychiatry, Karolinska Institute, Stockholm, told this news organization.
“The take-home message is that, when treating patients with increased risk of suicidal behavior, one should offer treatments with this in mind,” said Dr. Lundberg, also the head of the section of mood disorders, Northern Stockholm Psychiatry Clinic. “One possible option is lithium augmentation.”
The study was published online in JAMA Psychiatry.
Identifying subgroups
Depression is associated with increased all-cause mortality, the authors write. Suicidal behavior and previous suicide attempts are known to increase the risk of suicide-associated mortality, with up to 13% of patients with nonfatal suicide attempts dying of suicide at a later time.
Previous studies investigating the association between suicidal behavior and mortality have been limited by nonrandom sampling due to “nonuniversal access to health care and/or exclusion of primary care data,” they state.
For this reason, it’s not established to what extent these estimates actually represent patients with MDD as a whole, or to what extent suicidal behavior is a risk factor for all-cause mortality.
“We think there is a need to identify subgroups within the very large group of individuals with MDD in order to improve treatment outcomes,” Dr. Lundberg said.
To do so, the researchers turned to data from the Stockholm MDD Cohort (SMC), which comprises all patients diagnosed with MDD in any health care setting in the regions of Stockholm from 2010 to 2018. They identified 5 years of recorded MDD episodes (n = 158,169) in patients aged 18 years and older (n = 145,577). A single patient could contribute more than one episode.
At index, MDD-SB patients (n = 2,219; mean age, 41 years) were matched with MDD-non-SB patients (9,574; mean age, 41 years) based on age, sex, year of MDD diagnosis, and socioeconomic status. In total, 2,219 episodes (63.2% in women, 36.8% in men) were compared to 11,109 episodes (63.4% in women, 36.6% in men), respectively.
Enhanced monitoring, optimized treatment
The median time from the start of the episode until the first suicidal behavior was 165 days.
The all-cause mortality rate in the MDD-SB and MDD-non-SB groups was 2.5 per 100 person-years vs. 1 per 100 person-years, respectively (based on 466 deaths), corresponding to a hazard ratio of 2.62 (95% confidence interval, 2.15-3.20).
Patients in the MDD-SB group were younger, were more frequently diagnosed while in specialized care, and had sustained more work loss than their counterparts in the MDD-non-SB group. They also showed a gradual increase in the prevalence of comorbid conditions from about 12 months before index, with this increase being “most pronounced” for anxiety, stress, substance use, and personality disorders.
MDD-SB episodes were associated with higher HCRU and more work loss, compared with MDD-non-SB episodes.
The researchers calculated a risk score for factors associated with suicidal behavior within 1 year after the start of an MDD episode (outcome). The two most important risk factors for suicidal behavior were a history of suicidal behavior together with age, which had a “U-shaped association” with the outcome, they write, with individuals younger than age 20 and older than age 70 having the highest risks.
The final risk score included additional factors that increased the risk of the outcome (in descending order): history of substance use, history of sleep disorders, health care level in which MDD was diagnosed, history of antidepressant use, and history of anxiety disorders.
These results “indicate that patients at risk for suicidal behavior can be identified at an early stage to allow for enhanced monitoring and optimized treatment with the goal of preventing suicidal behavior and reducing mortality,” the authors state.
The specific causes of death weren’t analyzed in this particular paper, Dr. Lundberg noted. A previous study conducted by the same group found the risk of death was doubled in MDD patients, compared with controls.
“We don’t speculate about which causes other than suicide might explain the difference” and account for the increased mortality risk, he said. “This should be studied in future projects.”
Complicated family of destructive behaviors
In a comment, Russell Copelan, MD, a former emergency department psychiatrist at the University of Colorado Affiliated Hospital and currently an expert consultant to the American Association of Suicidology, said a take-home message of the study is that suicide is “a complex and complicated family of destructive behaviors.”
The findings “should not suggest a wait-and-see clinical approach,” warned Dr. Copelan, who wasn’t involved with the study.
Underrecognized or misdiagnosed anxiety, agitation, and insomnia may be “barriers to remission and treatment response,” he noted.
Dr. Copelan, who is also the founder and CEO of eMed Logic, which offers assessment tools for suicide and violence, encouraged clinicians “not to minimize the proportion of patients who experience anxiety, agitation, and insomnia in response to what some may consider a personal misfortune, such as interpersonal, employment, or financial crisis.”
A version of this article first appeared on Medscape.com.
Investigators studied close to 143,000 patients, encompassing more than 150,000 MDD episodes. Episodes of depression with suicidal behavior (MDD-SB) were compared to MDD episodes without suicidal behavior (MDD-non-SB).
Suicidal behavior was associated with a 2.6-fold higher rate of all-cause mortality, as well as considerably higher health care resource utilization (HCRU) and work loss, compared with matched controls.
Patients with depression who had attempted suicide were younger and more commonly suffering from other psychiatric comorbidities, such as anxiety and addiction. Important risk factors for suicidal acts within a year after the onset of a depressive episode were previous suicide attempts, substance use disorder, anxiety, and sleeping disorders.
“The findings tell us that the care provided for this particular group needs to be developed,” lead author Johan Lundberg, MD, PhD, adjunct professor in psychiatry and senior physician in psychiatry, Karolinska Institute, Stockholm, told this news organization.
“The take-home message is that, when treating patients with increased risk of suicidal behavior, one should offer treatments with this in mind,” said Dr. Lundberg, also the head of the section of mood disorders, Northern Stockholm Psychiatry Clinic. “One possible option is lithium augmentation.”
The study was published online in JAMA Psychiatry.
Identifying subgroups
Depression is associated with increased all-cause mortality, the authors write. Suicidal behavior and previous suicide attempts are known to increase the risk of suicide-associated mortality, with up to 13% of patients with nonfatal suicide attempts dying of suicide at a later time.
Previous studies investigating the association between suicidal behavior and mortality have been limited by nonrandom sampling due to “nonuniversal access to health care and/or exclusion of primary care data,” they state.
For this reason, it’s not established to what extent these estimates actually represent patients with MDD as a whole, or to what extent suicidal behavior is a risk factor for all-cause mortality.
“We think there is a need to identify subgroups within the very large group of individuals with MDD in order to improve treatment outcomes,” Dr. Lundberg said.
To do so, the researchers turned to data from the Stockholm MDD Cohort (SMC), which comprises all patients diagnosed with MDD in any health care setting in the regions of Stockholm from 2010 to 2018. They identified 5 years of recorded MDD episodes (n = 158,169) in patients aged 18 years and older (n = 145,577). A single patient could contribute more than one episode.
At index, MDD-SB patients (n = 2,219; mean age, 41 years) were matched with MDD-non-SB patients (9,574; mean age, 41 years) based on age, sex, year of MDD diagnosis, and socioeconomic status. In total, 2,219 episodes (63.2% in women, 36.8% in men) were compared to 11,109 episodes (63.4% in women, 36.6% in men), respectively.
Enhanced monitoring, optimized treatment
The median time from the start of the episode until the first suicidal behavior was 165 days.
The all-cause mortality rate in the MDD-SB and MDD-non-SB groups was 2.5 per 100 person-years vs. 1 per 100 person-years, respectively (based on 466 deaths), corresponding to a hazard ratio of 2.62 (95% confidence interval, 2.15-3.20).
Patients in the MDD-SB group were younger, were more frequently diagnosed while in specialized care, and had sustained more work loss than their counterparts in the MDD-non-SB group. They also showed a gradual increase in the prevalence of comorbid conditions from about 12 months before index, with this increase being “most pronounced” for anxiety, stress, substance use, and personality disorders.
MDD-SB episodes were associated with higher HCRU and more work loss, compared with MDD-non-SB episodes.
The researchers calculated a risk score for factors associated with suicidal behavior within 1 year after the start of an MDD episode (outcome). The two most important risk factors for suicidal behavior were a history of suicidal behavior together with age, which had a “U-shaped association” with the outcome, they write, with individuals younger than age 20 and older than age 70 having the highest risks.
The final risk score included additional factors that increased the risk of the outcome (in descending order): history of substance use, history of sleep disorders, health care level in which MDD was diagnosed, history of antidepressant use, and history of anxiety disorders.
These results “indicate that patients at risk for suicidal behavior can be identified at an early stage to allow for enhanced monitoring and optimized treatment with the goal of preventing suicidal behavior and reducing mortality,” the authors state.
The specific causes of death weren’t analyzed in this particular paper, Dr. Lundberg noted. A previous study conducted by the same group found the risk of death was doubled in MDD patients, compared with controls.
“We don’t speculate about which causes other than suicide might explain the difference” and account for the increased mortality risk, he said. “This should be studied in future projects.”
Complicated family of destructive behaviors
In a comment, Russell Copelan, MD, a former emergency department psychiatrist at the University of Colorado Affiliated Hospital and currently an expert consultant to the American Association of Suicidology, said a take-home message of the study is that suicide is “a complex and complicated family of destructive behaviors.”
The findings “should not suggest a wait-and-see clinical approach,” warned Dr. Copelan, who wasn’t involved with the study.
Underrecognized or misdiagnosed anxiety, agitation, and insomnia may be “barriers to remission and treatment response,” he noted.
Dr. Copelan, who is also the founder and CEO of eMed Logic, which offers assessment tools for suicide and violence, encouraged clinicians “not to minimize the proportion of patients who experience anxiety, agitation, and insomnia in response to what some may consider a personal misfortune, such as interpersonal, employment, or financial crisis.”
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Climate change and mental illness: What psychiatrists can do
“ Hope is engagement with the act of mapping our destinies.” 1
—Valerie Braithwaite
Why should psychiatrists care about climate change and try to mitigate its effects? First, we are tasked by society with managing the psychological and neuropsychiatric sequelae from disasters, which include climate change. The American Psychiatric Association’s position statement on climate change includes it as a legitimate focus for our specialty.2 Second, as physicians, we are morally obligated to do no harm. Since the health care sector contributes significantly to climate change (8.5% of national carbon emissions stem from health care) and causes demonstrable health impacts,3 managing these impacts and decarbonizing the health care industry is morally imperative.4 And third, psychiatric clinicians have transferrable skills that can address fears of climate change, challenge climate change denialism,5 motivate people to adopt more pro-environmental behaviors, and help communities not only endure the emotional impact of climate change but become more psychologically resilient.6
Most psychiatrists, however, did not receive formal training on climate change and the related field of disaster preparedness. For example, Harvard Medical School did not include a course on climate change in their medical student curriculum until 2023.7 In this article, we provide a basic framework of climate change and its impact on mental health, with particular focus on patients with serious mental illness (SMI). We offer concrete steps clinicians can take to prevent or mitigate harm from climate change for their patients, prepare for disasters at the level of individual patient encounters, and strengthen their clinics and communities. We also encourage clinicians to take active leadership roles in their professional organizations to be part of climate solutions, building on the trust patients continue to have in their physicians.8 Even if clinicians do not view climate change concerns under their conceived clinical care mandate, having a working knowledge about it is important because patients, paraprofessional staff, or medical trainees are likely to bring it up.9
Climate change and mental health
Climate change is harmful to human health, including mental health.10 It can impact mental health directly via its impact on brain function and neuropsychiatric sequelae, and indirectly via climate-related disasters leading to acute or chronic stress, losses, and displacement with psychiatric and psychological sequelae (Table 111-29).
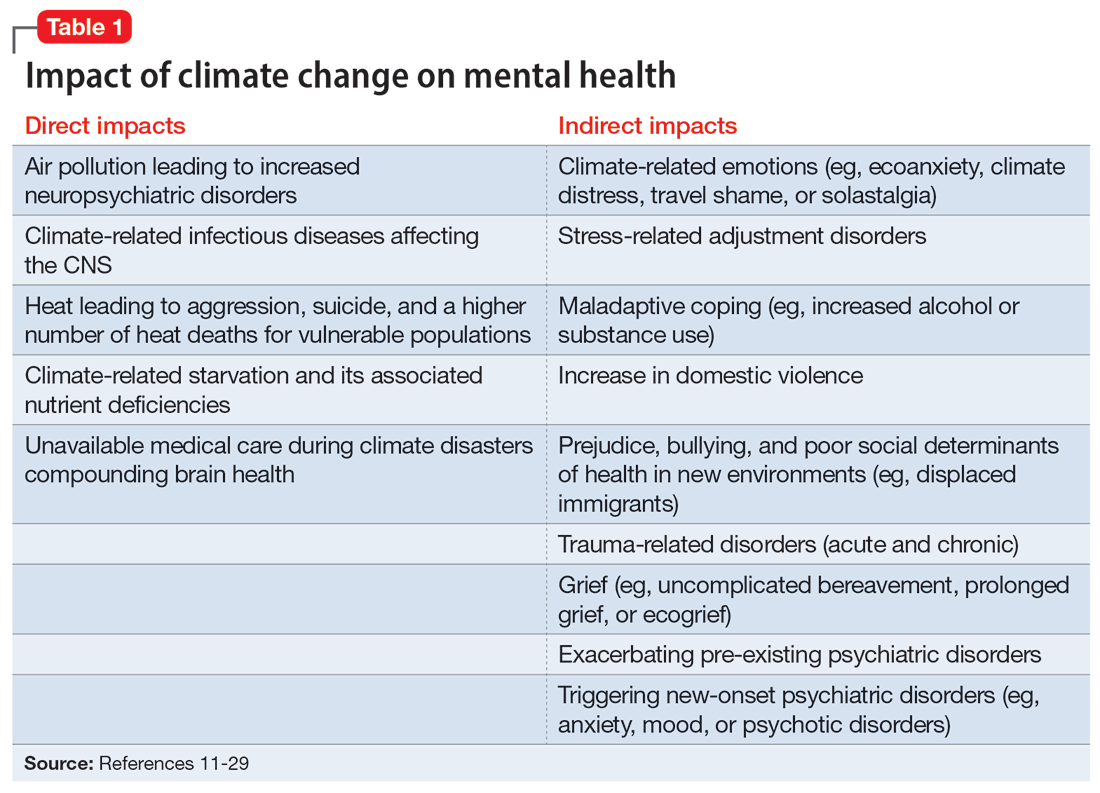
Direct impact
The effects of air pollution, heat, infections, and starvation are examples of how climate change directly impacts mental health. Air pollution and brain health are a concern for psychiatry, given the well-described effects of air deterioration on the developing brain.11 In animal models, airborne pollutants lead to widespread neuroinflammation and cell loss via a multitude of mechanisms.12 This is consistent with worse cognitive and behavioral functions across a wide range of cognitive domains seen in children exposed to pollution compared to those who grew up in environments with healthy air.13 Even low-level exposure to air pollution increases the risk for later onset of depression, suicide, and anxiety.14 Hippocampal atrophy observed in patients with first-episode psychosis may also be partially attributable to air pollution.15 An association between heat and suicide (and to a lesser extent, aggression) has also been reported.16
Worse physical health (eg, strokes) due to excessive heat can further compound mental health via elevated rates of depression. Data from the United States and Mexico show that for each degree Celsius increase in ambient temperature, suicide rates may increase by approximately 1%.17 A meta-analysis by Frangione et al18 similarly concluded that each degree Celsius increase results in an overall risk ratio of 1.016 (95% CI, 1.012 to 1.019) for deaths by suicide and suicide attempts. Additionally, global warming is shifting the endemic areas for many infectious agents, particularly vector-borne diseases,19 to regions in which they had hitherto been unknown, increasing the risk for future outbreaks and even pandemics.20 These infectious illnesses often carry neuropsychiatric morbidity, with seizures, encephalopathy with incomplete recovery, and psychiatric syndromes occurring in many cases. Crop failure can lead to starvation during pregnancy and childhood, which has wide-ranging consequences for brain development and later physical and psychological health in adults.21,22 Mothers affected by starvation also experience negative impacts on childbearing and childrearing.23
Indirect impact
Climate change’s indirect impact on mental health can stem from the stress of living through a disaster such as an extreme weather event; from losses, including the death of friends and family members; and from becoming temporarily displaced.24 Some climate change–driven disasters can be viewed as slow-moving, such as drought and the rising of sea levels, where displacement becomes permanent. Managing mass migration from internally or externally displaced people who must abandon their communities because of climate change will have significant repercussions for all societies.25 The term “climate refugee” is not (yet) included in the United Nations’ official definition of refugees; it defines refugees as individuals who have fled their countries because of war, violence, or persecution.26 These and other bureaucratic issues can come up when clinicians are trying to help migrants with immigration-related paperwork.
Continue to: As the inevitability of climate change...
As the inevitability of climate change sinks in, its long-term ramifications have introduced a new lexicon of psychological suffering related to the crisis.27 Common terms for such distress include ecoanxiety (fear of what is happening and will happen with climate change), ecogrief (sadness about the destruction of species and natural habitats), solastalgia28 (the nostalgia an individual feels for emotionally treasured landscapes that have changed), and terrafuria or ecorage (the reaction to betrayal and inaction by governments and leaders).29 Climate-related emotions can lead to pessimism about the future and a nihilistic outlook on an individual’s ability to effect change and have agency over their life’s outcomes.
The categories of direct and indirect impacts are not mutually exclusive. A child may be starving due to weather-related crop failure as the family is forced to move to another country, then have to contend with prejudice and bullying as an immigrant, and later become anxiously preoccupied with climate change and its ability to cause further distress.
Effect on individuals with serious mental illness
Patients with SMI are particularly vulnerable to the impact of climate change. They are less resilient to climate change–related events, such as heat waves or temporary displacement from flooding, both at the personal level due to illness factors (eg, negative symptoms or cognitive impairment) and at the community level due to social factors (eg, weaker social support or poverty).
Recognizing the increased vulnerability to heat waves and preparing for them is particularly important for patients with SMI because they are at an increased risk for heat-related illnesses.30 For example, patients may not appreciate the danger from heat and live in conditions that put them at risk (ie, not having air conditioning in their home or living alone). Their illness alone impairs heat regulation31; patients with depression and anxiety also dissipate heat less effectively.32,33 Additionally, many psychiatric medications, particularly antipsychotics, impair key mechanisms of heat dissipation.34,35 Antipsychotics render organisms more poikilothermic (susceptible to environmental temperature, like cold-blooded animals) and can be anticholinergic, which impedes sweating. A recent analysis of heat-related deaths during a period of extreme and prolonged heat in British Columbia in 2021 affirmed these concerns, reporting that patients with schizophrenia had the highest odds of death during this heat-related event.36
COVID-19 has shown that flexible models of care are needed to prevent disengagement from medical and psychiatric care37 and assure continued treatment with essential medications such as clozapine38 and long-acting injectable antipsychotics39 during periods of social change, as with climate change. While telehealth was critical during the COVID-19 pandemic40 and is here to stay, it alone may be insufficient given the digital divide (patients with SMI may be less likely to have access to or be proficient in the use of digital technologies). The pandemic has shown the importance of public health efforts, including benefits from targeted outreach, with regards to vaccinations for this patient group.41,42Table 2 summarizes things clinicians should consider when preparing patients with SMI for the effects of climate change.

Continue to: The psychiatrist's role
The psychiatrist’s role
There are many ways a psychiatrist can professionally get involved in addressing climate change. Table 343-53 outlines the 3 Ps of climate action (taking actions to mitigate the effects of climate change): personal, patient (and clinic), and political (advocacy).
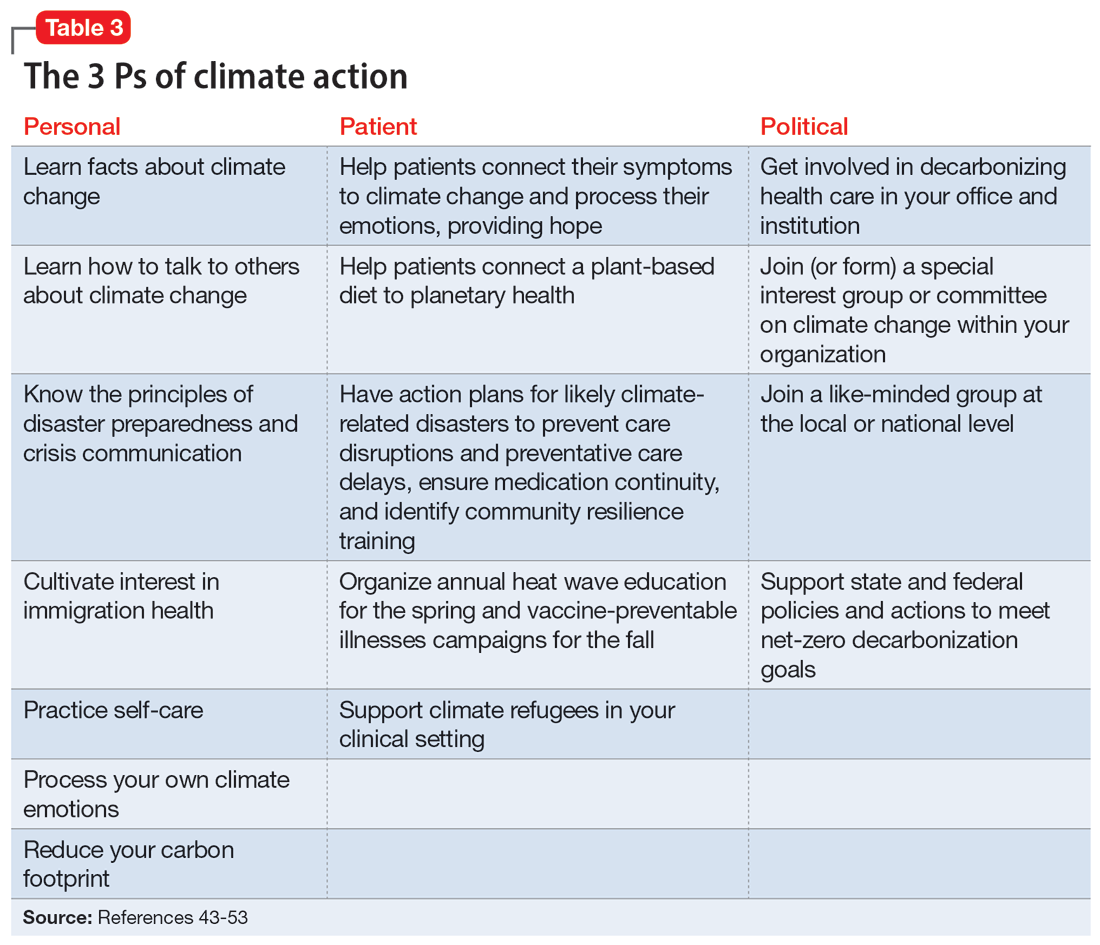
Personal
Even if clinicians believe climate change is important for their clinical work, they may still feel overwhelmed and unsure what to do in the context of competing responsibilities. A necessary first step is overcoming paralysis from the enormity of the problem, including the need to shift away from an expanding consumption model to environmental sustainability in a short period of time.
A good starting point is to get educated on the facts of climate change and how to discuss it in an office setting as well as in your personal life. A basic principle of climate change communication is that constructive hope (progress achieved despite everything) coupled with constructive doubt (the reality of the threat) can mobilize people towards action, whereas false hope or fatalistic doubt impedes action.43 The importance of optimal public health messaging cannot be overstated; well-meaning campaigns to change behavior can fail if they emphasize the wrong message. For example, in a study examining COVID-19 messaging in >80 countries, Dorison et al44 found that negatively framed messages mostly increased anxiety but had no benefit with regard to shifting people toward desired behaviors.
In addition, clinicians can learn how to confront climate disavowal and difficult emotions in themselves and even plan to shift to carbon neutrality, such as purchasing carbon offsets or green sources of energy and transportation. They may not be familiar with principles of disaster preparedness or crisis communication.46 Acquiring those professional skills may suggest next steps for action. Being familiar with the challenges and resources for immigrants, including individuals displaced due to climate change, may be necessary.47 Finally, to reduce the risk of burnout, it is important to practice self-care, including strategies to reduce feelings of being overwhelmed.
Patient
In clinical encounters, clinicians can be proactive in helping patients understand their climate-related anxieties around an uncertain future, including identifying barriers to climate action.48
Continue to: Clinics must prepare for disasters...
Clinics must prepare for disasters in their communities to prevent disruption of psychiatric care by having an action plan, including the provision of medications. Such action plans should be prioritized for the most likely scenarios in an individual’s setting (eg, heat waves, wildfires, hurricanes, or flooding).
It is important to educate clinic staff and include them in planning for emergencies, because an all-hands approach and buy-in from all team members is critical. Clinicians should review how patients would continue to receive services, particularly medications, in the event of a disaster. In some cases, providing a 90-day medication supply will suffice, while in others (eg, patients receiving long-acting antipsychotics or clozapine) more preparation is necessary. Some events are predictable and can be organized annually, such as clinicians becoming vaccine ambassadors and organizing vaccine campaigns every fall50; winter-related disaster preparation every fall; and heat wave education every spring (leaflets for patients, staff, and family members; review of safety of medications during heat waves). Plan for, monitor, and coordinate medical care and services for climate refugees and other populations that may otherwise delay medical care and impede illness prevention. Finally, support climate refugees, including connecting them to services or providing trauma-informed care.
Political
Some clinicians may feel compelled to become politically active to advocate for changes within the health care system. Two initiatives related to decarbonizing the health care sector are My Green Doctor51 and Health Care Without Harm,52 which offer help in shifting your office, clinic, or hospital towards carbon neutrality.
Climate change unevenly affects people and will continue to exacerbate inequalities in society, including individuals with mental illness.53 To work toward climate justice on behalf of their patients, clinicians could join (or form) climate committees of special interest groups in their professional organizations or setting. Joining like-minded groups working on climate change at the local or national level prevents an omission of a psychiatric voice and counteracts burnout. It is important to stay focused on the root causes of the problem during activism: doing something to reduce fossil fuel use is ultimately most important.54 The concrete goal of reaching the Paris 1.5-degree Celsius climate goal is a critical benchmark against which any other action can be measured.54
Planning for the future
Over the course of history, societies have always faced difficult periods in which they needed to rebuild after natural disasters or self-inflicted catastrophes such as terrorist attacks or wars. Since the advent of the nuclear age, people have lived under the existential threat of nuclear war. The Anthropocene is a proposed geological term that reflects the enormous and possibly disastrous impact human activity has had on our planet.55 While not yet formally adopted, this term has heuristic value, directing attention and reflection to our role and its now undisputed consequences. In the future, historians will debate if the scale of our current climate crisis has been different. It is, however, not controversial that humanity will be faced with the effects of climate change for the foreseeable future.10 Already, even “normal” weather events are fueled by energy in overcharged and altered weather systems due to global warming, leading to weather events ranging from droughts to floods and storms that are more severe, more frequent, and have longer-lasting effects on communities.56
Continue to: As physicians, we are tasked...
As physicians, we are tasked by society to create and maintain a health care system that addresses the needs of our patients and the communities in which they live. Increasingly, we are forced to contend with an addition to the traditional 5 phases of acute disaster management (prevention, mitigation, preparedness, response, and recovery) to manage prolonged or even parallel disasters, where a series of disasters occurs before the community has recovered and healed. We must grapple with a sense of an “extended period of insecurity and instability” (permacrisis) and must better prepare for and prevent the polycrisis (many simultaneous crises) or the metacrisis of our “age of turmoil”57 in which we must limit global warming, mitigate its damage, and increase community resilience to adapt.
Leading by personal example and providing hope may be what some patients need, as the reality of climate change contributes to the general uneasiness about the future and doomsday scenarios to which many fall victim. At the level of professional societies, many are calling for leadership, including from mental health organizations, to bolster the “social climate,” to help us strengthen our emotional resilience and social bonds to better withstand climate change together.58 It is becoming harder to justify standing on the sidelines,59 and it may be better for both our world and a clinician’s own sanity to be engaged in professional and private hopeful action1 to address climate change. Without ecological or planetary health, there can be no mental health.
Bottom Line
Clinicians can prepare their patients for climate-related disruptions and manage the impact climate change has on their mental health. Addressing climate change at clinical and political levels is consistent with the leadership roles and professional ethics clinicians face in daily practice.
Related Resources
- Lim C, MacLaurin S, Freudenreich O. Preparing patients with serious mental illness for extreme HEAT. Current Psychiatry. 2022;21(9):27-28. doi:10.12788/cp.0287
- My Green Doctor. https://mygreendoctor.org/
- The Climate Resilience for Frontline Clinics Toolkit from Americares. https://www.americares.org/what-we-do/community-health/climate-resilient-health-clinics
- Climate Psychiatry Alliance. https://www.climatepsychiatry.org/
Drug Brand Names
Clozapine • Clozaril
1. Kretz L. Hope in environmental philosophy. J Agricult Environ Ethics. 2013;26:925-944. doi:10.1007/s10806-012-9425-8
2. Ursano RJ, Morganstein JC, Cooper R. Position statement on mental health and climate change. American Psychiatric Association. March 2023. Accessed August 6, 2023. https://www.psychiatry.org/getattachment/0ce71f37-61a6-44d0-8fcd-c752b7e935fd/Position-Mental-Health-Climate-Change.pdf
3. Eckelman MJ, Huang K, Lagasse R, et al. Health care pollution and public health damage in the United States: an update. Health Aff (Millwood). 2020;39:2071-2079.
4. Dzau VJ, Levine R, Barrett G, et al. Decarbonizing the U.S. health sector - a call to action. N Engl J Med. 2021;385(23):2117-2119. doi:10.1056/NEJMp2115675
5. Haase E, Augustinavicius JH, K. Climate change and psychiatry. In: Tasman A, Riba MB, Alarcón RD, et al, eds. Tasman’s Psychiatry. 5th ed. Springer; 2023.
6. Belkin G. Mental health and the global race to resilience. Psychiatr Times. 2023;40(3):26.
7. Hu SR, Yang JQ. Harvard Medical School will integrate climate change into M.D. curriculum. The Harvard Crimson. February 3, 2023. Accessed August 6, 2023. https://www.thecrimson.com/article/2023/2/3/hms-climate-curriculum/#:~:text=The%20new%20climate%20change%20curriculum,in%20arriving%20at%20climate%20solutions
8. Funk C, Gramlich J. Amid coronavirus threat, Americans generally have a high level of trust in medical doctors. Pew Research Center. March 13, 2020. Accessed August 6, 2023. https://www.pewresearch.org/fact-tank/2020/03/13/amid-coronavirus-threat-americans-generally-have-a-high-level-of-trust-in-medical-doctors/
9. Coverdale J, Balon R, Beresin EV, et al. Climate change: a call to action for the psychiatric profession. Acad Psychiatry. 2018;42(3):317-323. doi:10.1007/s40596-018-0885-7
10. Intergovernmental Panel on Climate Change. AR6 synthesis report: climate change 2023. Accessed August 6, 2023. https://www.ipcc.ch/report/sixth-assessment-report-cycle/
11. Perera FP. Multiple threats to child health from fossil fuel combustion: impacts of air pollution and climate change. Environ Health Perspect. 2017;125(2):141-148. doi:10.1289/EHP299
12. Hahad O, Lelieveldz J, Birklein F, et al. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int J Mol Sci. 2020;21(12):4306. doi:10.3390/ijms21124306
13. Brockmeyer S, D’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Translational Neurosci. 2016;7(1):24-30. doi:10.1515/tnsci-2016-0005
14. Yang T, Wang J, Huang J, et al. Long-term exposure to multiple ambient air pollutants and association with incident depression and anxiety. JAMA Psychiatry. 2023;80:305-313. doi:10.1001/jamapsychiatry.2022.4812
15. Worthington MA, Petkova E, Freudenreich O, et al. Air pollution and hippocampal atrophy in first episode schizophrenia. Schizophr Res. 2020;218:63-69. doi:10.1016/j.schres.2020.03.001
16. Dumont C, Haase E, Dolber T, et al. Climate change and risk of completed suicide. J Nerv Ment Dis. 2020;208(7):559-565. doi:10.1097/NMD.0000000000001162
17. Burke M, Gonzales F, Bayis P, et al. Higher temperatures increase suicide rates in the United States and Mexico. Nat Climate Change. 2018;8:723-729. doi:10.1038/s41558-018-0222-x
18. Frangione B, Villamizar LAR, Lang JJ, et al. Short-term changes in meteorological conditions and suicide: a systematic review and meta-analysis. Environ Res. 2022;207:112230. doi:10.1016/j.envres.2021.112230
19. Rocklov J, Dubrow R. Climate change: an enduring challenge for vector-borne disease prevention and control. Nat Immunol. 2020;21(5):479-483. doi:10.1038/s41590-020-0648-y
20. Carlson CJ, Albery GF, Merow C, et al. Climate change increases cross-species viral transmission risk. Nature. 2022;607(7919):555-562. doi:10.1038/s41586-022-04788-w
21. Roseboom TJ, Painter RC, van Abeelen AFM, et al. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70(2):141-145. doi:10.1016/j.maturitas.2011.06.017
22. Liu Y, Diao L, Xu L. The impact of childhood experience of starvations on the health of older adults: evidence from China. Int J Health Plann Manage. 2021;36(2):515-531. doi:10.1002/hpm.3099
23. Rothschild J, Haase E. The mental health of women and climate change: direct neuropsychiatric impacts and associated psychological concerns. Int J Gynaecol Obstet. 2023;160(2):405-413. doi:10.1002/ijgo.14479
24. Cianconi P, Betro S, Janiri L. The impact of climate change on mental health: a systematic descriptive review. Frontiers Psychiatry. 2020;11:74. doi:10.3389/fpsyt.2020.00074
25. World Economic Forum. Climate refugees – the world’s forgotten victims. June 18, 2021. Accessed August 6, 2023. https://www.weforum.org/agenda/2021/06/climate-refugees-the-world-s-forgotten-victims
26. Climate Refugees. Accessed August 6, 2023. https://www.climate-refugees.org/why
27. Pihkala P. Anxiety and the ecological crisis: an analysis of eco-anxiety and climate anxiety. Sustainability. 2020;12(19):7836. doi:10.3390/su12197836
28. Galway LP, Beery T, Jones-Casey K, et al. Mapping the solastalgia literature: a scoping review study. Int J Environ Res Public Health. 2019;16(15):2662. doi:10.3390/ijerph16152662
29. Albrecht GA. Earth Emotions. New Words for a New World. Cornell University Press; 2019.
30. Sorensen C, Hess J. Treatment and prevention of heat-related illness. N Engl J Med. 2022;387(15):1404-1413. doi:10.1056/NEJMcp2210623
31. Chong TWH, Castle DJ. Layer upon layer: thermoregulation in schizophrenia. Schizophr Res. 2004;69(2-3):149-157. doi:10.1016/s0920-9964(03)00222-6
32. von Salis S, Ehlert U, Fischer S. Altered experienced thermoregulation in depression--no evidence for an effect of early life stress. Front Psychiatry. 2021;12:620656. doi:10.3389/fpsyt.2021.620656
33. Sarchiapone M, Gramaglia C, Iosue M, et al. The association between electrodermal activity (EDA), depression and suicidal behaviour: a systematic review and narrative synthesis. BMC Psychiatry. 2018;18(1):22. doi:10.1186/s12888-017-1551-4
34. Martin-Latry K, Goumy MP, Latry P, et al. Psychotropic drugs use and risk of heat-related hospitalisation. Eur Psychiatry. 2007;22(6):335-338. doi:10.1016/j.eurpsy.2007.03.007
35. Ebi KL, Capon A, Berry P, et al. Hot weather and heat extremes: health risks. Lancet. 2021;398(10301):698-708. doi:10.1016/S0140-6736(21)01208-3
36. Lee MJ, McLean KE, Kuo M, et al. Chronic diseases associated with mortality in British Columbia, Canada during the 2021 Western North America extreme heat event. Geohealth. 2023;7(3):e2022GH000729. doi:10.1029/2022GH000729
37. Busch AB, Huskamp HA, Raja P, et al. Disruptions in care for Medicare beneficiaries with severe mental illness during the COVID-19 pandemic. JAMA Netw Open. 2022;5(1):e2145677. doi:10.1001/jamanetworkopen.2021.45677
38. Siskind D, Honer WG, Clark S, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci. 2020;45(3):222-223. doi:10.1503/jpn.200061
39. MacLaurin SA, Mulligan C, Van Alphen MU, et al. Optimal long-acting injectable antipsychotic management during COVID-19. J Clin Psychiatry. 2021;82(1): 20l13730. doi:10.4088/JCP.20l13730
40. Bartels SJ, Baggett TP, Freudenreich O, et al. COVID-19 emergency reforms in Massachusetts to support behavioral health care and reduce mortality of people with serious mental illness. Psychiatr Serv. 2020;71(10):1078-1081. doi:10.1176/appi.ps.202000244
41. Van Alphen MU, Lim C, Freudenreich O. Mobile vaccine clinics for patients with serious mental illness and health care workers in outpatient mental health clinics. Psychiatr Serv. February 8, 2023. doi:10.1176/appi.ps.20220460
42. Lim C, Van Alphen MU, Maclaurin S, et al. Increasing COVID-19 vaccination rates among patients with serious mental illness: a pilot intervention study. Psychiatr Serv. 2022;73(11):1274-1277. doi:10.1176/appi.ps.202100702
43. Marlon JR, Bloodhart B, Ballew MT, et al. How hope and doubt affect climate change mobilization. Front Commun. May 21, 2019. doi:10.3389/fcomm.2019.00020
44. Dorison CA, Lerner JS, Heller BH, et al. In COVID-19 health messaging, loss framing increases anxiety with little-to-no concomitant benefits: experimental evidence from 84 countries. Affective Sci. 2022;3(3):577-602. doi:10.1007/s42761-022-00128-3
45. Maibach E. Increasing public awareness and facilitating behavior change: two guiding heuristics. George Mason University, Center for Climate Change Communication. September 2015. Accessed August 6, 2023. https://www.climatechangecommunication.org/wp-content/uploads/2018/06/Maibach-Two-hueristics-September-2015-revised.pdf
46. Koh KA, Raviola G, Stoddard FJ Jr. Psychiatry and crisis communication during COVID-19: a view from the trenches. Psychiatr Serv. 2021;72(5):615. doi:10.1176/appi.ps.202000912
47. Velez G, Adam B, Shadid O, et al. The clock is ticking: are we prepared for mass climate migration? Psychiatr News. March 24, 2023. Accessed August 6, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2023.04.4.3
48. Ingle HE, Mikulewicz M. Mental health and climate change: tackling invisible injustice. Lancet Planet Health. 2020;4:e128-e130. doi:10.1016/S2542-5196(20)30081-4
49. Shah UA, Merlo G. Personal and planetary health--the connection with dietary choices. JAMA. 2023;329(21):1823-1824. doi:10.1001/jama.2023.6118
50. Lim C, Van Alphen MU, Freudenreich O. Becoming vaccine ambassadors: a new role for psychiatrists. Current Psychiatry. 2021;20(8):10-11,17-21,26-28,38. doi:10.12788/cp.0155
51. My Green Doctor. Accessed August 6, 2023. https://mygreendoctor.org/
52. Healthcare Without Harm. Accessed August 6, 2023. https://noharm.org/
53. Levy BS, Patz JA. Climate change, human rights, and social justice. Ann Glob Health. 2015;81:310-322.
54. Intergovernmental Panel on Climate Change. Global warming of 1.5° C 2018. Accessed August 6, 2023. https://www.ipcc.ch/sr15/
55. Steffen W, Crutzen J, McNeill JR. The Anthropocene: are humans now overwhelming the great forces of nature? Ambio. 2007;36(8):614-621. doi:10.1579/0044-7447(2007)36[614:taahno]2.0.co;2
56. American Meteorological Society. Explaining extreme events from a climate perspective. Accessed August 6, 2023. https://www.ametsoc.org/ams/index.cfm/publications/bulletin-of-the-american-meteorological-society-bams/explaining-extreme-events-from-a-climate-perspective/
57. Nierenberg AA. Coping in the age of turmoil. Psychiatr Ann. 2022;52(7):263. July 1, 2022. doi:10.3928/23258160-20220701-01
58. Belkin G. Leadership for the social climate. N Engl J Med. 2020;382(21):1975-1977. doi:10.1056/NEJMp2001507
59. Skinner JR. Doctors and climate change: first do no harm. J Paediatr Child Health. 2021;57(11):1754-1758. doi:10.1111/jpc.15658
“ Hope is engagement with the act of mapping our destinies.” 1
—Valerie Braithwaite
Why should psychiatrists care about climate change and try to mitigate its effects? First, we are tasked by society with managing the psychological and neuropsychiatric sequelae from disasters, which include climate change. The American Psychiatric Association’s position statement on climate change includes it as a legitimate focus for our specialty.2 Second, as physicians, we are morally obligated to do no harm. Since the health care sector contributes significantly to climate change (8.5% of national carbon emissions stem from health care) and causes demonstrable health impacts,3 managing these impacts and decarbonizing the health care industry is morally imperative.4 And third, psychiatric clinicians have transferrable skills that can address fears of climate change, challenge climate change denialism,5 motivate people to adopt more pro-environmental behaviors, and help communities not only endure the emotional impact of climate change but become more psychologically resilient.6
Most psychiatrists, however, did not receive formal training on climate change and the related field of disaster preparedness. For example, Harvard Medical School did not include a course on climate change in their medical student curriculum until 2023.7 In this article, we provide a basic framework of climate change and its impact on mental health, with particular focus on patients with serious mental illness (SMI). We offer concrete steps clinicians can take to prevent or mitigate harm from climate change for their patients, prepare for disasters at the level of individual patient encounters, and strengthen their clinics and communities. We also encourage clinicians to take active leadership roles in their professional organizations to be part of climate solutions, building on the trust patients continue to have in their physicians.8 Even if clinicians do not view climate change concerns under their conceived clinical care mandate, having a working knowledge about it is important because patients, paraprofessional staff, or medical trainees are likely to bring it up.9
Climate change and mental health
Climate change is harmful to human health, including mental health.10 It can impact mental health directly via its impact on brain function and neuropsychiatric sequelae, and indirectly via climate-related disasters leading to acute or chronic stress, losses, and displacement with psychiatric and psychological sequelae (Table 111-29).

Direct impact
The effects of air pollution, heat, infections, and starvation are examples of how climate change directly impacts mental health. Air pollution and brain health are a concern for psychiatry, given the well-described effects of air deterioration on the developing brain.11 In animal models, airborne pollutants lead to widespread neuroinflammation and cell loss via a multitude of mechanisms.12 This is consistent with worse cognitive and behavioral functions across a wide range of cognitive domains seen in children exposed to pollution compared to those who grew up in environments with healthy air.13 Even low-level exposure to air pollution increases the risk for later onset of depression, suicide, and anxiety.14 Hippocampal atrophy observed in patients with first-episode psychosis may also be partially attributable to air pollution.15 An association between heat and suicide (and to a lesser extent, aggression) has also been reported.16
Worse physical health (eg, strokes) due to excessive heat can further compound mental health via elevated rates of depression. Data from the United States and Mexico show that for each degree Celsius increase in ambient temperature, suicide rates may increase by approximately 1%.17 A meta-analysis by Frangione et al18 similarly concluded that each degree Celsius increase results in an overall risk ratio of 1.016 (95% CI, 1.012 to 1.019) for deaths by suicide and suicide attempts. Additionally, global warming is shifting the endemic areas for many infectious agents, particularly vector-borne diseases,19 to regions in which they had hitherto been unknown, increasing the risk for future outbreaks and even pandemics.20 These infectious illnesses often carry neuropsychiatric morbidity, with seizures, encephalopathy with incomplete recovery, and psychiatric syndromes occurring in many cases. Crop failure can lead to starvation during pregnancy and childhood, which has wide-ranging consequences for brain development and later physical and psychological health in adults.21,22 Mothers affected by starvation also experience negative impacts on childbearing and childrearing.23
Indirect impact
Climate change’s indirect impact on mental health can stem from the stress of living through a disaster such as an extreme weather event; from losses, including the death of friends and family members; and from becoming temporarily displaced.24 Some climate change–driven disasters can be viewed as slow-moving, such as drought and the rising of sea levels, where displacement becomes permanent. Managing mass migration from internally or externally displaced people who must abandon their communities because of climate change will have significant repercussions for all societies.25 The term “climate refugee” is not (yet) included in the United Nations’ official definition of refugees; it defines refugees as individuals who have fled their countries because of war, violence, or persecution.26 These and other bureaucratic issues can come up when clinicians are trying to help migrants with immigration-related paperwork.
Continue to: As the inevitability of climate change...
As the inevitability of climate change sinks in, its long-term ramifications have introduced a new lexicon of psychological suffering related to the crisis.27 Common terms for such distress include ecoanxiety (fear of what is happening and will happen with climate change), ecogrief (sadness about the destruction of species and natural habitats), solastalgia28 (the nostalgia an individual feels for emotionally treasured landscapes that have changed), and terrafuria or ecorage (the reaction to betrayal and inaction by governments and leaders).29 Climate-related emotions can lead to pessimism about the future and a nihilistic outlook on an individual’s ability to effect change and have agency over their life’s outcomes.
The categories of direct and indirect impacts are not mutually exclusive. A child may be starving due to weather-related crop failure as the family is forced to move to another country, then have to contend with prejudice and bullying as an immigrant, and later become anxiously preoccupied with climate change and its ability to cause further distress.
Effect on individuals with serious mental illness
Patients with SMI are particularly vulnerable to the impact of climate change. They are less resilient to climate change–related events, such as heat waves or temporary displacement from flooding, both at the personal level due to illness factors (eg, negative symptoms or cognitive impairment) and at the community level due to social factors (eg, weaker social support or poverty).
Recognizing the increased vulnerability to heat waves and preparing for them is particularly important for patients with SMI because they are at an increased risk for heat-related illnesses.30 For example, patients may not appreciate the danger from heat and live in conditions that put them at risk (ie, not having air conditioning in their home or living alone). Their illness alone impairs heat regulation31; patients with depression and anxiety also dissipate heat less effectively.32,33 Additionally, many psychiatric medications, particularly antipsychotics, impair key mechanisms of heat dissipation.34,35 Antipsychotics render organisms more poikilothermic (susceptible to environmental temperature, like cold-blooded animals) and can be anticholinergic, which impedes sweating. A recent analysis of heat-related deaths during a period of extreme and prolonged heat in British Columbia in 2021 affirmed these concerns, reporting that patients with schizophrenia had the highest odds of death during this heat-related event.36
COVID-19 has shown that flexible models of care are needed to prevent disengagement from medical and psychiatric care37 and assure continued treatment with essential medications such as clozapine38 and long-acting injectable antipsychotics39 during periods of social change, as with climate change. While telehealth was critical during the COVID-19 pandemic40 and is here to stay, it alone may be insufficient given the digital divide (patients with SMI may be less likely to have access to or be proficient in the use of digital technologies). The pandemic has shown the importance of public health efforts, including benefits from targeted outreach, with regards to vaccinations for this patient group.41,42Table 2 summarizes things clinicians should consider when preparing patients with SMI for the effects of climate change.

Continue to: The psychiatrist's role
The psychiatrist’s role
There are many ways a psychiatrist can professionally get involved in addressing climate change. Table 343-53 outlines the 3 Ps of climate action (taking actions to mitigate the effects of climate change): personal, patient (and clinic), and political (advocacy).

Personal
Even if clinicians believe climate change is important for their clinical work, they may still feel overwhelmed and unsure what to do in the context of competing responsibilities. A necessary first step is overcoming paralysis from the enormity of the problem, including the need to shift away from an expanding consumption model to environmental sustainability in a short period of time.
A good starting point is to get educated on the facts of climate change and how to discuss it in an office setting as well as in your personal life. A basic principle of climate change communication is that constructive hope (progress achieved despite everything) coupled with constructive doubt (the reality of the threat) can mobilize people towards action, whereas false hope or fatalistic doubt impedes action.43 The importance of optimal public health messaging cannot be overstated; well-meaning campaigns to change behavior can fail if they emphasize the wrong message. For example, in a study examining COVID-19 messaging in >80 countries, Dorison et al44 found that negatively framed messages mostly increased anxiety but had no benefit with regard to shifting people toward desired behaviors.
In addition, clinicians can learn how to confront climate disavowal and difficult emotions in themselves and even plan to shift to carbon neutrality, such as purchasing carbon offsets or green sources of energy and transportation. They may not be familiar with principles of disaster preparedness or crisis communication.46 Acquiring those professional skills may suggest next steps for action. Being familiar with the challenges and resources for immigrants, including individuals displaced due to climate change, may be necessary.47 Finally, to reduce the risk of burnout, it is important to practice self-care, including strategies to reduce feelings of being overwhelmed.
Patient
In clinical encounters, clinicians can be proactive in helping patients understand their climate-related anxieties around an uncertain future, including identifying barriers to climate action.48
Continue to: Clinics must prepare for disasters...
Clinics must prepare for disasters in their communities to prevent disruption of psychiatric care by having an action plan, including the provision of medications. Such action plans should be prioritized for the most likely scenarios in an individual’s setting (eg, heat waves, wildfires, hurricanes, or flooding).
It is important to educate clinic staff and include them in planning for emergencies, because an all-hands approach and buy-in from all team members is critical. Clinicians should review how patients would continue to receive services, particularly medications, in the event of a disaster. In some cases, providing a 90-day medication supply will suffice, while in others (eg, patients receiving long-acting antipsychotics or clozapine) more preparation is necessary. Some events are predictable and can be organized annually, such as clinicians becoming vaccine ambassadors and organizing vaccine campaigns every fall50; winter-related disaster preparation every fall; and heat wave education every spring (leaflets for patients, staff, and family members; review of safety of medications during heat waves). Plan for, monitor, and coordinate medical care and services for climate refugees and other populations that may otherwise delay medical care and impede illness prevention. Finally, support climate refugees, including connecting them to services or providing trauma-informed care.
Political
Some clinicians may feel compelled to become politically active to advocate for changes within the health care system. Two initiatives related to decarbonizing the health care sector are My Green Doctor51 and Health Care Without Harm,52 which offer help in shifting your office, clinic, or hospital towards carbon neutrality.
Climate change unevenly affects people and will continue to exacerbate inequalities in society, including individuals with mental illness.53 To work toward climate justice on behalf of their patients, clinicians could join (or form) climate committees of special interest groups in their professional organizations or setting. Joining like-minded groups working on climate change at the local or national level prevents an omission of a psychiatric voice and counteracts burnout. It is important to stay focused on the root causes of the problem during activism: doing something to reduce fossil fuel use is ultimately most important.54 The concrete goal of reaching the Paris 1.5-degree Celsius climate goal is a critical benchmark against which any other action can be measured.54
Planning for the future
Over the course of history, societies have always faced difficult periods in which they needed to rebuild after natural disasters or self-inflicted catastrophes such as terrorist attacks or wars. Since the advent of the nuclear age, people have lived under the existential threat of nuclear war. The Anthropocene is a proposed geological term that reflects the enormous and possibly disastrous impact human activity has had on our planet.55 While not yet formally adopted, this term has heuristic value, directing attention and reflection to our role and its now undisputed consequences. In the future, historians will debate if the scale of our current climate crisis has been different. It is, however, not controversial that humanity will be faced with the effects of climate change for the foreseeable future.10 Already, even “normal” weather events are fueled by energy in overcharged and altered weather systems due to global warming, leading to weather events ranging from droughts to floods and storms that are more severe, more frequent, and have longer-lasting effects on communities.56
Continue to: As physicians, we are tasked...
As physicians, we are tasked by society to create and maintain a health care system that addresses the needs of our patients and the communities in which they live. Increasingly, we are forced to contend with an addition to the traditional 5 phases of acute disaster management (prevention, mitigation, preparedness, response, and recovery) to manage prolonged or even parallel disasters, where a series of disasters occurs before the community has recovered and healed. We must grapple with a sense of an “extended period of insecurity and instability” (permacrisis) and must better prepare for and prevent the polycrisis (many simultaneous crises) or the metacrisis of our “age of turmoil”57 in which we must limit global warming, mitigate its damage, and increase community resilience to adapt.
Leading by personal example and providing hope may be what some patients need, as the reality of climate change contributes to the general uneasiness about the future and doomsday scenarios to which many fall victim. At the level of professional societies, many are calling for leadership, including from mental health organizations, to bolster the “social climate,” to help us strengthen our emotional resilience and social bonds to better withstand climate change together.58 It is becoming harder to justify standing on the sidelines,59 and it may be better for both our world and a clinician’s own sanity to be engaged in professional and private hopeful action1 to address climate change. Without ecological or planetary health, there can be no mental health.
Bottom Line
Clinicians can prepare their patients for climate-related disruptions and manage the impact climate change has on their mental health. Addressing climate change at clinical and political levels is consistent with the leadership roles and professional ethics clinicians face in daily practice.
Related Resources
- Lim C, MacLaurin S, Freudenreich O. Preparing patients with serious mental illness for extreme HEAT. Current Psychiatry. 2022;21(9):27-28. doi:10.12788/cp.0287
- My Green Doctor. https://mygreendoctor.org/
- The Climate Resilience for Frontline Clinics Toolkit from Americares. https://www.americares.org/what-we-do/community-health/climate-resilient-health-clinics
- Climate Psychiatry Alliance. https://www.climatepsychiatry.org/
Drug Brand Names
Clozapine • Clozaril
“ Hope is engagement with the act of mapping our destinies.” 1
—Valerie Braithwaite
Why should psychiatrists care about climate change and try to mitigate its effects? First, we are tasked by society with managing the psychological and neuropsychiatric sequelae from disasters, which include climate change. The American Psychiatric Association’s position statement on climate change includes it as a legitimate focus for our specialty.2 Second, as physicians, we are morally obligated to do no harm. Since the health care sector contributes significantly to climate change (8.5% of national carbon emissions stem from health care) and causes demonstrable health impacts,3 managing these impacts and decarbonizing the health care industry is morally imperative.4 And third, psychiatric clinicians have transferrable skills that can address fears of climate change, challenge climate change denialism,5 motivate people to adopt more pro-environmental behaviors, and help communities not only endure the emotional impact of climate change but become more psychologically resilient.6
Most psychiatrists, however, did not receive formal training on climate change and the related field of disaster preparedness. For example, Harvard Medical School did not include a course on climate change in their medical student curriculum until 2023.7 In this article, we provide a basic framework of climate change and its impact on mental health, with particular focus on patients with serious mental illness (SMI). We offer concrete steps clinicians can take to prevent or mitigate harm from climate change for their patients, prepare for disasters at the level of individual patient encounters, and strengthen their clinics and communities. We also encourage clinicians to take active leadership roles in their professional organizations to be part of climate solutions, building on the trust patients continue to have in their physicians.8 Even if clinicians do not view climate change concerns under their conceived clinical care mandate, having a working knowledge about it is important because patients, paraprofessional staff, or medical trainees are likely to bring it up.9
Climate change and mental health
Climate change is harmful to human health, including mental health.10 It can impact mental health directly via its impact on brain function and neuropsychiatric sequelae, and indirectly via climate-related disasters leading to acute or chronic stress, losses, and displacement with psychiatric and psychological sequelae (Table 111-29).

Direct impact
The effects of air pollution, heat, infections, and starvation are examples of how climate change directly impacts mental health. Air pollution and brain health are a concern for psychiatry, given the well-described effects of air deterioration on the developing brain.11 In animal models, airborne pollutants lead to widespread neuroinflammation and cell loss via a multitude of mechanisms.12 This is consistent with worse cognitive and behavioral functions across a wide range of cognitive domains seen in children exposed to pollution compared to those who grew up in environments with healthy air.13 Even low-level exposure to air pollution increases the risk for later onset of depression, suicide, and anxiety.14 Hippocampal atrophy observed in patients with first-episode psychosis may also be partially attributable to air pollution.15 An association between heat and suicide (and to a lesser extent, aggression) has also been reported.16
Worse physical health (eg, strokes) due to excessive heat can further compound mental health via elevated rates of depression. Data from the United States and Mexico show that for each degree Celsius increase in ambient temperature, suicide rates may increase by approximately 1%.17 A meta-analysis by Frangione et al18 similarly concluded that each degree Celsius increase results in an overall risk ratio of 1.016 (95% CI, 1.012 to 1.019) for deaths by suicide and suicide attempts. Additionally, global warming is shifting the endemic areas for many infectious agents, particularly vector-borne diseases,19 to regions in which they had hitherto been unknown, increasing the risk for future outbreaks and even pandemics.20 These infectious illnesses often carry neuropsychiatric morbidity, with seizures, encephalopathy with incomplete recovery, and psychiatric syndromes occurring in many cases. Crop failure can lead to starvation during pregnancy and childhood, which has wide-ranging consequences for brain development and later physical and psychological health in adults.21,22 Mothers affected by starvation also experience negative impacts on childbearing and childrearing.23
Indirect impact
Climate change’s indirect impact on mental health can stem from the stress of living through a disaster such as an extreme weather event; from losses, including the death of friends and family members; and from becoming temporarily displaced.24 Some climate change–driven disasters can be viewed as slow-moving, such as drought and the rising of sea levels, where displacement becomes permanent. Managing mass migration from internally or externally displaced people who must abandon their communities because of climate change will have significant repercussions for all societies.25 The term “climate refugee” is not (yet) included in the United Nations’ official definition of refugees; it defines refugees as individuals who have fled their countries because of war, violence, or persecution.26 These and other bureaucratic issues can come up when clinicians are trying to help migrants with immigration-related paperwork.
Continue to: As the inevitability of climate change...
As the inevitability of climate change sinks in, its long-term ramifications have introduced a new lexicon of psychological suffering related to the crisis.27 Common terms for such distress include ecoanxiety (fear of what is happening and will happen with climate change), ecogrief (sadness about the destruction of species and natural habitats), solastalgia28 (the nostalgia an individual feels for emotionally treasured landscapes that have changed), and terrafuria or ecorage (the reaction to betrayal and inaction by governments and leaders).29 Climate-related emotions can lead to pessimism about the future and a nihilistic outlook on an individual’s ability to effect change and have agency over their life’s outcomes.
The categories of direct and indirect impacts are not mutually exclusive. A child may be starving due to weather-related crop failure as the family is forced to move to another country, then have to contend with prejudice and bullying as an immigrant, and later become anxiously preoccupied with climate change and its ability to cause further distress.
Effect on individuals with serious mental illness
Patients with SMI are particularly vulnerable to the impact of climate change. They are less resilient to climate change–related events, such as heat waves or temporary displacement from flooding, both at the personal level due to illness factors (eg, negative symptoms or cognitive impairment) and at the community level due to social factors (eg, weaker social support or poverty).
Recognizing the increased vulnerability to heat waves and preparing for them is particularly important for patients with SMI because they are at an increased risk for heat-related illnesses.30 For example, patients may not appreciate the danger from heat and live in conditions that put them at risk (ie, not having air conditioning in their home or living alone). Their illness alone impairs heat regulation31; patients with depression and anxiety also dissipate heat less effectively.32,33 Additionally, many psychiatric medications, particularly antipsychotics, impair key mechanisms of heat dissipation.34,35 Antipsychotics render organisms more poikilothermic (susceptible to environmental temperature, like cold-blooded animals) and can be anticholinergic, which impedes sweating. A recent analysis of heat-related deaths during a period of extreme and prolonged heat in British Columbia in 2021 affirmed these concerns, reporting that patients with schizophrenia had the highest odds of death during this heat-related event.36
COVID-19 has shown that flexible models of care are needed to prevent disengagement from medical and psychiatric care37 and assure continued treatment with essential medications such as clozapine38 and long-acting injectable antipsychotics39 during periods of social change, as with climate change. While telehealth was critical during the COVID-19 pandemic40 and is here to stay, it alone may be insufficient given the digital divide (patients with SMI may be less likely to have access to or be proficient in the use of digital technologies). The pandemic has shown the importance of public health efforts, including benefits from targeted outreach, with regards to vaccinations for this patient group.41,42Table 2 summarizes things clinicians should consider when preparing patients with SMI for the effects of climate change.

Continue to: The psychiatrist's role
The psychiatrist’s role
There are many ways a psychiatrist can professionally get involved in addressing climate change. Table 343-53 outlines the 3 Ps of climate action (taking actions to mitigate the effects of climate change): personal, patient (and clinic), and political (advocacy).

Personal
Even if clinicians believe climate change is important for their clinical work, they may still feel overwhelmed and unsure what to do in the context of competing responsibilities. A necessary first step is overcoming paralysis from the enormity of the problem, including the need to shift away from an expanding consumption model to environmental sustainability in a short period of time.
A good starting point is to get educated on the facts of climate change and how to discuss it in an office setting as well as in your personal life. A basic principle of climate change communication is that constructive hope (progress achieved despite everything) coupled with constructive doubt (the reality of the threat) can mobilize people towards action, whereas false hope or fatalistic doubt impedes action.43 The importance of optimal public health messaging cannot be overstated; well-meaning campaigns to change behavior can fail if they emphasize the wrong message. For example, in a study examining COVID-19 messaging in >80 countries, Dorison et al44 found that negatively framed messages mostly increased anxiety but had no benefit with regard to shifting people toward desired behaviors.
In addition, clinicians can learn how to confront climate disavowal and difficult emotions in themselves and even plan to shift to carbon neutrality, such as purchasing carbon offsets or green sources of energy and transportation. They may not be familiar with principles of disaster preparedness or crisis communication.46 Acquiring those professional skills may suggest next steps for action. Being familiar with the challenges and resources for immigrants, including individuals displaced due to climate change, may be necessary.47 Finally, to reduce the risk of burnout, it is important to practice self-care, including strategies to reduce feelings of being overwhelmed.
Patient
In clinical encounters, clinicians can be proactive in helping patients understand their climate-related anxieties around an uncertain future, including identifying barriers to climate action.48
Continue to: Clinics must prepare for disasters...
Clinics must prepare for disasters in their communities to prevent disruption of psychiatric care by having an action plan, including the provision of medications. Such action plans should be prioritized for the most likely scenarios in an individual’s setting (eg, heat waves, wildfires, hurricanes, or flooding).
It is important to educate clinic staff and include them in planning for emergencies, because an all-hands approach and buy-in from all team members is critical. Clinicians should review how patients would continue to receive services, particularly medications, in the event of a disaster. In some cases, providing a 90-day medication supply will suffice, while in others (eg, patients receiving long-acting antipsychotics or clozapine) more preparation is necessary. Some events are predictable and can be organized annually, such as clinicians becoming vaccine ambassadors and organizing vaccine campaigns every fall50; winter-related disaster preparation every fall; and heat wave education every spring (leaflets for patients, staff, and family members; review of safety of medications during heat waves). Plan for, monitor, and coordinate medical care and services for climate refugees and other populations that may otherwise delay medical care and impede illness prevention. Finally, support climate refugees, including connecting them to services or providing trauma-informed care.
Political
Some clinicians may feel compelled to become politically active to advocate for changes within the health care system. Two initiatives related to decarbonizing the health care sector are My Green Doctor51 and Health Care Without Harm,52 which offer help in shifting your office, clinic, or hospital towards carbon neutrality.
Climate change unevenly affects people and will continue to exacerbate inequalities in society, including individuals with mental illness.53 To work toward climate justice on behalf of their patients, clinicians could join (or form) climate committees of special interest groups in their professional organizations or setting. Joining like-minded groups working on climate change at the local or national level prevents an omission of a psychiatric voice and counteracts burnout. It is important to stay focused on the root causes of the problem during activism: doing something to reduce fossil fuel use is ultimately most important.54 The concrete goal of reaching the Paris 1.5-degree Celsius climate goal is a critical benchmark against which any other action can be measured.54
Planning for the future
Over the course of history, societies have always faced difficult periods in which they needed to rebuild after natural disasters or self-inflicted catastrophes such as terrorist attacks or wars. Since the advent of the nuclear age, people have lived under the existential threat of nuclear war. The Anthropocene is a proposed geological term that reflects the enormous and possibly disastrous impact human activity has had on our planet.55 While not yet formally adopted, this term has heuristic value, directing attention and reflection to our role and its now undisputed consequences. In the future, historians will debate if the scale of our current climate crisis has been different. It is, however, not controversial that humanity will be faced with the effects of climate change for the foreseeable future.10 Already, even “normal” weather events are fueled by energy in overcharged and altered weather systems due to global warming, leading to weather events ranging from droughts to floods and storms that are more severe, more frequent, and have longer-lasting effects on communities.56
Continue to: As physicians, we are tasked...
As physicians, we are tasked by society to create and maintain a health care system that addresses the needs of our patients and the communities in which they live. Increasingly, we are forced to contend with an addition to the traditional 5 phases of acute disaster management (prevention, mitigation, preparedness, response, and recovery) to manage prolonged or even parallel disasters, where a series of disasters occurs before the community has recovered and healed. We must grapple with a sense of an “extended period of insecurity and instability” (permacrisis) and must better prepare for and prevent the polycrisis (many simultaneous crises) or the metacrisis of our “age of turmoil”57 in which we must limit global warming, mitigate its damage, and increase community resilience to adapt.
Leading by personal example and providing hope may be what some patients need, as the reality of climate change contributes to the general uneasiness about the future and doomsday scenarios to which many fall victim. At the level of professional societies, many are calling for leadership, including from mental health organizations, to bolster the “social climate,” to help us strengthen our emotional resilience and social bonds to better withstand climate change together.58 It is becoming harder to justify standing on the sidelines,59 and it may be better for both our world and a clinician’s own sanity to be engaged in professional and private hopeful action1 to address climate change. Without ecological or planetary health, there can be no mental health.
Bottom Line
Clinicians can prepare their patients for climate-related disruptions and manage the impact climate change has on their mental health. Addressing climate change at clinical and political levels is consistent with the leadership roles and professional ethics clinicians face in daily practice.
Related Resources
- Lim C, MacLaurin S, Freudenreich O. Preparing patients with serious mental illness for extreme HEAT. Current Psychiatry. 2022;21(9):27-28. doi:10.12788/cp.0287
- My Green Doctor. https://mygreendoctor.org/
- The Climate Resilience for Frontline Clinics Toolkit from Americares. https://www.americares.org/what-we-do/community-health/climate-resilient-health-clinics
- Climate Psychiatry Alliance. https://www.climatepsychiatry.org/
Drug Brand Names
Clozapine • Clozaril
1. Kretz L. Hope in environmental philosophy. J Agricult Environ Ethics. 2013;26:925-944. doi:10.1007/s10806-012-9425-8
2. Ursano RJ, Morganstein JC, Cooper R. Position statement on mental health and climate change. American Psychiatric Association. March 2023. Accessed August 6, 2023. https://www.psychiatry.org/getattachment/0ce71f37-61a6-44d0-8fcd-c752b7e935fd/Position-Mental-Health-Climate-Change.pdf
3. Eckelman MJ, Huang K, Lagasse R, et al. Health care pollution and public health damage in the United States: an update. Health Aff (Millwood). 2020;39:2071-2079.
4. Dzau VJ, Levine R, Barrett G, et al. Decarbonizing the U.S. health sector - a call to action. N Engl J Med. 2021;385(23):2117-2119. doi:10.1056/NEJMp2115675
5. Haase E, Augustinavicius JH, K. Climate change and psychiatry. In: Tasman A, Riba MB, Alarcón RD, et al, eds. Tasman’s Psychiatry. 5th ed. Springer; 2023.
6. Belkin G. Mental health and the global race to resilience. Psychiatr Times. 2023;40(3):26.
7. Hu SR, Yang JQ. Harvard Medical School will integrate climate change into M.D. curriculum. The Harvard Crimson. February 3, 2023. Accessed August 6, 2023. https://www.thecrimson.com/article/2023/2/3/hms-climate-curriculum/#:~:text=The%20new%20climate%20change%20curriculum,in%20arriving%20at%20climate%20solutions
8. Funk C, Gramlich J. Amid coronavirus threat, Americans generally have a high level of trust in medical doctors. Pew Research Center. March 13, 2020. Accessed August 6, 2023. https://www.pewresearch.org/fact-tank/2020/03/13/amid-coronavirus-threat-americans-generally-have-a-high-level-of-trust-in-medical-doctors/
9. Coverdale J, Balon R, Beresin EV, et al. Climate change: a call to action for the psychiatric profession. Acad Psychiatry. 2018;42(3):317-323. doi:10.1007/s40596-018-0885-7
10. Intergovernmental Panel on Climate Change. AR6 synthesis report: climate change 2023. Accessed August 6, 2023. https://www.ipcc.ch/report/sixth-assessment-report-cycle/
11. Perera FP. Multiple threats to child health from fossil fuel combustion: impacts of air pollution and climate change. Environ Health Perspect. 2017;125(2):141-148. doi:10.1289/EHP299
12. Hahad O, Lelieveldz J, Birklein F, et al. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int J Mol Sci. 2020;21(12):4306. doi:10.3390/ijms21124306
13. Brockmeyer S, D’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Translational Neurosci. 2016;7(1):24-30. doi:10.1515/tnsci-2016-0005
14. Yang T, Wang J, Huang J, et al. Long-term exposure to multiple ambient air pollutants and association with incident depression and anxiety. JAMA Psychiatry. 2023;80:305-313. doi:10.1001/jamapsychiatry.2022.4812
15. Worthington MA, Petkova E, Freudenreich O, et al. Air pollution and hippocampal atrophy in first episode schizophrenia. Schizophr Res. 2020;218:63-69. doi:10.1016/j.schres.2020.03.001
16. Dumont C, Haase E, Dolber T, et al. Climate change and risk of completed suicide. J Nerv Ment Dis. 2020;208(7):559-565. doi:10.1097/NMD.0000000000001162
17. Burke M, Gonzales F, Bayis P, et al. Higher temperatures increase suicide rates in the United States and Mexico. Nat Climate Change. 2018;8:723-729. doi:10.1038/s41558-018-0222-x
18. Frangione B, Villamizar LAR, Lang JJ, et al. Short-term changes in meteorological conditions and suicide: a systematic review and meta-analysis. Environ Res. 2022;207:112230. doi:10.1016/j.envres.2021.112230
19. Rocklov J, Dubrow R. Climate change: an enduring challenge for vector-borne disease prevention and control. Nat Immunol. 2020;21(5):479-483. doi:10.1038/s41590-020-0648-y
20. Carlson CJ, Albery GF, Merow C, et al. Climate change increases cross-species viral transmission risk. Nature. 2022;607(7919):555-562. doi:10.1038/s41586-022-04788-w
21. Roseboom TJ, Painter RC, van Abeelen AFM, et al. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70(2):141-145. doi:10.1016/j.maturitas.2011.06.017
22. Liu Y, Diao L, Xu L. The impact of childhood experience of starvations on the health of older adults: evidence from China. Int J Health Plann Manage. 2021;36(2):515-531. doi:10.1002/hpm.3099
23. Rothschild J, Haase E. The mental health of women and climate change: direct neuropsychiatric impacts and associated psychological concerns. Int J Gynaecol Obstet. 2023;160(2):405-413. doi:10.1002/ijgo.14479
24. Cianconi P, Betro S, Janiri L. The impact of climate change on mental health: a systematic descriptive review. Frontiers Psychiatry. 2020;11:74. doi:10.3389/fpsyt.2020.00074
25. World Economic Forum. Climate refugees – the world’s forgotten victims. June 18, 2021. Accessed August 6, 2023. https://www.weforum.org/agenda/2021/06/climate-refugees-the-world-s-forgotten-victims
26. Climate Refugees. Accessed August 6, 2023. https://www.climate-refugees.org/why
27. Pihkala P. Anxiety and the ecological crisis: an analysis of eco-anxiety and climate anxiety. Sustainability. 2020;12(19):7836. doi:10.3390/su12197836
28. Galway LP, Beery T, Jones-Casey K, et al. Mapping the solastalgia literature: a scoping review study. Int J Environ Res Public Health. 2019;16(15):2662. doi:10.3390/ijerph16152662
29. Albrecht GA. Earth Emotions. New Words for a New World. Cornell University Press; 2019.
30. Sorensen C, Hess J. Treatment and prevention of heat-related illness. N Engl J Med. 2022;387(15):1404-1413. doi:10.1056/NEJMcp2210623
31. Chong TWH, Castle DJ. Layer upon layer: thermoregulation in schizophrenia. Schizophr Res. 2004;69(2-3):149-157. doi:10.1016/s0920-9964(03)00222-6
32. von Salis S, Ehlert U, Fischer S. Altered experienced thermoregulation in depression--no evidence for an effect of early life stress. Front Psychiatry. 2021;12:620656. doi:10.3389/fpsyt.2021.620656
33. Sarchiapone M, Gramaglia C, Iosue M, et al. The association between electrodermal activity (EDA), depression and suicidal behaviour: a systematic review and narrative synthesis. BMC Psychiatry. 2018;18(1):22. doi:10.1186/s12888-017-1551-4
34. Martin-Latry K, Goumy MP, Latry P, et al. Psychotropic drugs use and risk of heat-related hospitalisation. Eur Psychiatry. 2007;22(6):335-338. doi:10.1016/j.eurpsy.2007.03.007
35. Ebi KL, Capon A, Berry P, et al. Hot weather and heat extremes: health risks. Lancet. 2021;398(10301):698-708. doi:10.1016/S0140-6736(21)01208-3
36. Lee MJ, McLean KE, Kuo M, et al. Chronic diseases associated with mortality in British Columbia, Canada during the 2021 Western North America extreme heat event. Geohealth. 2023;7(3):e2022GH000729. doi:10.1029/2022GH000729
37. Busch AB, Huskamp HA, Raja P, et al. Disruptions in care for Medicare beneficiaries with severe mental illness during the COVID-19 pandemic. JAMA Netw Open. 2022;5(1):e2145677. doi:10.1001/jamanetworkopen.2021.45677
38. Siskind D, Honer WG, Clark S, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci. 2020;45(3):222-223. doi:10.1503/jpn.200061
39. MacLaurin SA, Mulligan C, Van Alphen MU, et al. Optimal long-acting injectable antipsychotic management during COVID-19. J Clin Psychiatry. 2021;82(1): 20l13730. doi:10.4088/JCP.20l13730
40. Bartels SJ, Baggett TP, Freudenreich O, et al. COVID-19 emergency reforms in Massachusetts to support behavioral health care and reduce mortality of people with serious mental illness. Psychiatr Serv. 2020;71(10):1078-1081. doi:10.1176/appi.ps.202000244
41. Van Alphen MU, Lim C, Freudenreich O. Mobile vaccine clinics for patients with serious mental illness and health care workers in outpatient mental health clinics. Psychiatr Serv. February 8, 2023. doi:10.1176/appi.ps.20220460
42. Lim C, Van Alphen MU, Maclaurin S, et al. Increasing COVID-19 vaccination rates among patients with serious mental illness: a pilot intervention study. Psychiatr Serv. 2022;73(11):1274-1277. doi:10.1176/appi.ps.202100702
43. Marlon JR, Bloodhart B, Ballew MT, et al. How hope and doubt affect climate change mobilization. Front Commun. May 21, 2019. doi:10.3389/fcomm.2019.00020
44. Dorison CA, Lerner JS, Heller BH, et al. In COVID-19 health messaging, loss framing increases anxiety with little-to-no concomitant benefits: experimental evidence from 84 countries. Affective Sci. 2022;3(3):577-602. doi:10.1007/s42761-022-00128-3
45. Maibach E. Increasing public awareness and facilitating behavior change: two guiding heuristics. George Mason University, Center for Climate Change Communication. September 2015. Accessed August 6, 2023. https://www.climatechangecommunication.org/wp-content/uploads/2018/06/Maibach-Two-hueristics-September-2015-revised.pdf
46. Koh KA, Raviola G, Stoddard FJ Jr. Psychiatry and crisis communication during COVID-19: a view from the trenches. Psychiatr Serv. 2021;72(5):615. doi:10.1176/appi.ps.202000912
47. Velez G, Adam B, Shadid O, et al. The clock is ticking: are we prepared for mass climate migration? Psychiatr News. March 24, 2023. Accessed August 6, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2023.04.4.3
48. Ingle HE, Mikulewicz M. Mental health and climate change: tackling invisible injustice. Lancet Planet Health. 2020;4:e128-e130. doi:10.1016/S2542-5196(20)30081-4
49. Shah UA, Merlo G. Personal and planetary health--the connection with dietary choices. JAMA. 2023;329(21):1823-1824. doi:10.1001/jama.2023.6118
50. Lim C, Van Alphen MU, Freudenreich O. Becoming vaccine ambassadors: a new role for psychiatrists. Current Psychiatry. 2021;20(8):10-11,17-21,26-28,38. doi:10.12788/cp.0155
51. My Green Doctor. Accessed August 6, 2023. https://mygreendoctor.org/
52. Healthcare Without Harm. Accessed August 6, 2023. https://noharm.org/
53. Levy BS, Patz JA. Climate change, human rights, and social justice. Ann Glob Health. 2015;81:310-322.
54. Intergovernmental Panel on Climate Change. Global warming of 1.5° C 2018. Accessed August 6, 2023. https://www.ipcc.ch/sr15/
55. Steffen W, Crutzen J, McNeill JR. The Anthropocene: are humans now overwhelming the great forces of nature? Ambio. 2007;36(8):614-621. doi:10.1579/0044-7447(2007)36[614:taahno]2.0.co;2
56. American Meteorological Society. Explaining extreme events from a climate perspective. Accessed August 6, 2023. https://www.ametsoc.org/ams/index.cfm/publications/bulletin-of-the-american-meteorological-society-bams/explaining-extreme-events-from-a-climate-perspective/
57. Nierenberg AA. Coping in the age of turmoil. Psychiatr Ann. 2022;52(7):263. July 1, 2022. doi:10.3928/23258160-20220701-01
58. Belkin G. Leadership for the social climate. N Engl J Med. 2020;382(21):1975-1977. doi:10.1056/NEJMp2001507
59. Skinner JR. Doctors and climate change: first do no harm. J Paediatr Child Health. 2021;57(11):1754-1758. doi:10.1111/jpc.15658
1. Kretz L. Hope in environmental philosophy. J Agricult Environ Ethics. 2013;26:925-944. doi:10.1007/s10806-012-9425-8
2. Ursano RJ, Morganstein JC, Cooper R. Position statement on mental health and climate change. American Psychiatric Association. March 2023. Accessed August 6, 2023. https://www.psychiatry.org/getattachment/0ce71f37-61a6-44d0-8fcd-c752b7e935fd/Position-Mental-Health-Climate-Change.pdf
3. Eckelman MJ, Huang K, Lagasse R, et al. Health care pollution and public health damage in the United States: an update. Health Aff (Millwood). 2020;39:2071-2079.
4. Dzau VJ, Levine R, Barrett G, et al. Decarbonizing the U.S. health sector - a call to action. N Engl J Med. 2021;385(23):2117-2119. doi:10.1056/NEJMp2115675
5. Haase E, Augustinavicius JH, K. Climate change and psychiatry. In: Tasman A, Riba MB, Alarcón RD, et al, eds. Tasman’s Psychiatry. 5th ed. Springer; 2023.
6. Belkin G. Mental health and the global race to resilience. Psychiatr Times. 2023;40(3):26.
7. Hu SR, Yang JQ. Harvard Medical School will integrate climate change into M.D. curriculum. The Harvard Crimson. February 3, 2023. Accessed August 6, 2023. https://www.thecrimson.com/article/2023/2/3/hms-climate-curriculum/#:~:text=The%20new%20climate%20change%20curriculum,in%20arriving%20at%20climate%20solutions
8. Funk C, Gramlich J. Amid coronavirus threat, Americans generally have a high level of trust in medical doctors. Pew Research Center. March 13, 2020. Accessed August 6, 2023. https://www.pewresearch.org/fact-tank/2020/03/13/amid-coronavirus-threat-americans-generally-have-a-high-level-of-trust-in-medical-doctors/
9. Coverdale J, Balon R, Beresin EV, et al. Climate change: a call to action for the psychiatric profession. Acad Psychiatry. 2018;42(3):317-323. doi:10.1007/s40596-018-0885-7
10. Intergovernmental Panel on Climate Change. AR6 synthesis report: climate change 2023. Accessed August 6, 2023. https://www.ipcc.ch/report/sixth-assessment-report-cycle/
11. Perera FP. Multiple threats to child health from fossil fuel combustion: impacts of air pollution and climate change. Environ Health Perspect. 2017;125(2):141-148. doi:10.1289/EHP299
12. Hahad O, Lelieveldz J, Birklein F, et al. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int J Mol Sci. 2020;21(12):4306. doi:10.3390/ijms21124306
13. Brockmeyer S, D’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Translational Neurosci. 2016;7(1):24-30. doi:10.1515/tnsci-2016-0005
14. Yang T, Wang J, Huang J, et al. Long-term exposure to multiple ambient air pollutants and association with incident depression and anxiety. JAMA Psychiatry. 2023;80:305-313. doi:10.1001/jamapsychiatry.2022.4812
15. Worthington MA, Petkova E, Freudenreich O, et al. Air pollution and hippocampal atrophy in first episode schizophrenia. Schizophr Res. 2020;218:63-69. doi:10.1016/j.schres.2020.03.001
16. Dumont C, Haase E, Dolber T, et al. Climate change and risk of completed suicide. J Nerv Ment Dis. 2020;208(7):559-565. doi:10.1097/NMD.0000000000001162
17. Burke M, Gonzales F, Bayis P, et al. Higher temperatures increase suicide rates in the United States and Mexico. Nat Climate Change. 2018;8:723-729. doi:10.1038/s41558-018-0222-x
18. Frangione B, Villamizar LAR, Lang JJ, et al. Short-term changes in meteorological conditions and suicide: a systematic review and meta-analysis. Environ Res. 2022;207:112230. doi:10.1016/j.envres.2021.112230
19. Rocklov J, Dubrow R. Climate change: an enduring challenge for vector-borne disease prevention and control. Nat Immunol. 2020;21(5):479-483. doi:10.1038/s41590-020-0648-y
20. Carlson CJ, Albery GF, Merow C, et al. Climate change increases cross-species viral transmission risk. Nature. 2022;607(7919):555-562. doi:10.1038/s41586-022-04788-w
21. Roseboom TJ, Painter RC, van Abeelen AFM, et al. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70(2):141-145. doi:10.1016/j.maturitas.2011.06.017
22. Liu Y, Diao L, Xu L. The impact of childhood experience of starvations on the health of older adults: evidence from China. Int J Health Plann Manage. 2021;36(2):515-531. doi:10.1002/hpm.3099
23. Rothschild J, Haase E. The mental health of women and climate change: direct neuropsychiatric impacts and associated psychological concerns. Int J Gynaecol Obstet. 2023;160(2):405-413. doi:10.1002/ijgo.14479
24. Cianconi P, Betro S, Janiri L. The impact of climate change on mental health: a systematic descriptive review. Frontiers Psychiatry. 2020;11:74. doi:10.3389/fpsyt.2020.00074
25. World Economic Forum. Climate refugees – the world’s forgotten victims. June 18, 2021. Accessed August 6, 2023. https://www.weforum.org/agenda/2021/06/climate-refugees-the-world-s-forgotten-victims
26. Climate Refugees. Accessed August 6, 2023. https://www.climate-refugees.org/why
27. Pihkala P. Anxiety and the ecological crisis: an analysis of eco-anxiety and climate anxiety. Sustainability. 2020;12(19):7836. doi:10.3390/su12197836
28. Galway LP, Beery T, Jones-Casey K, et al. Mapping the solastalgia literature: a scoping review study. Int J Environ Res Public Health. 2019;16(15):2662. doi:10.3390/ijerph16152662
29. Albrecht GA. Earth Emotions. New Words for a New World. Cornell University Press; 2019.
30. Sorensen C, Hess J. Treatment and prevention of heat-related illness. N Engl J Med. 2022;387(15):1404-1413. doi:10.1056/NEJMcp2210623
31. Chong TWH, Castle DJ. Layer upon layer: thermoregulation in schizophrenia. Schizophr Res. 2004;69(2-3):149-157. doi:10.1016/s0920-9964(03)00222-6
32. von Salis S, Ehlert U, Fischer S. Altered experienced thermoregulation in depression--no evidence for an effect of early life stress. Front Psychiatry. 2021;12:620656. doi:10.3389/fpsyt.2021.620656
33. Sarchiapone M, Gramaglia C, Iosue M, et al. The association between electrodermal activity (EDA), depression and suicidal behaviour: a systematic review and narrative synthesis. BMC Psychiatry. 2018;18(1):22. doi:10.1186/s12888-017-1551-4
34. Martin-Latry K, Goumy MP, Latry P, et al. Psychotropic drugs use and risk of heat-related hospitalisation. Eur Psychiatry. 2007;22(6):335-338. doi:10.1016/j.eurpsy.2007.03.007
35. Ebi KL, Capon A, Berry P, et al. Hot weather and heat extremes: health risks. Lancet. 2021;398(10301):698-708. doi:10.1016/S0140-6736(21)01208-3
36. Lee MJ, McLean KE, Kuo M, et al. Chronic diseases associated with mortality in British Columbia, Canada during the 2021 Western North America extreme heat event. Geohealth. 2023;7(3):e2022GH000729. doi:10.1029/2022GH000729
37. Busch AB, Huskamp HA, Raja P, et al. Disruptions in care for Medicare beneficiaries with severe mental illness during the COVID-19 pandemic. JAMA Netw Open. 2022;5(1):e2145677. doi:10.1001/jamanetworkopen.2021.45677
38. Siskind D, Honer WG, Clark S, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci. 2020;45(3):222-223. doi:10.1503/jpn.200061
39. MacLaurin SA, Mulligan C, Van Alphen MU, et al. Optimal long-acting injectable antipsychotic management during COVID-19. J Clin Psychiatry. 2021;82(1): 20l13730. doi:10.4088/JCP.20l13730
40. Bartels SJ, Baggett TP, Freudenreich O, et al. COVID-19 emergency reforms in Massachusetts to support behavioral health care and reduce mortality of people with serious mental illness. Psychiatr Serv. 2020;71(10):1078-1081. doi:10.1176/appi.ps.202000244
41. Van Alphen MU, Lim C, Freudenreich O. Mobile vaccine clinics for patients with serious mental illness and health care workers in outpatient mental health clinics. Psychiatr Serv. February 8, 2023. doi:10.1176/appi.ps.20220460
42. Lim C, Van Alphen MU, Maclaurin S, et al. Increasing COVID-19 vaccination rates among patients with serious mental illness: a pilot intervention study. Psychiatr Serv. 2022;73(11):1274-1277. doi:10.1176/appi.ps.202100702
43. Marlon JR, Bloodhart B, Ballew MT, et al. How hope and doubt affect climate change mobilization. Front Commun. May 21, 2019. doi:10.3389/fcomm.2019.00020
44. Dorison CA, Lerner JS, Heller BH, et al. In COVID-19 health messaging, loss framing increases anxiety with little-to-no concomitant benefits: experimental evidence from 84 countries. Affective Sci. 2022;3(3):577-602. doi:10.1007/s42761-022-00128-3
45. Maibach E. Increasing public awareness and facilitating behavior change: two guiding heuristics. George Mason University, Center for Climate Change Communication. September 2015. Accessed August 6, 2023. https://www.climatechangecommunication.org/wp-content/uploads/2018/06/Maibach-Two-hueristics-September-2015-revised.pdf
46. Koh KA, Raviola G, Stoddard FJ Jr. Psychiatry and crisis communication during COVID-19: a view from the trenches. Psychiatr Serv. 2021;72(5):615. doi:10.1176/appi.ps.202000912
47. Velez G, Adam B, Shadid O, et al. The clock is ticking: are we prepared for mass climate migration? Psychiatr News. March 24, 2023. Accessed August 6, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2023.04.4.3
48. Ingle HE, Mikulewicz M. Mental health and climate change: tackling invisible injustice. Lancet Planet Health. 2020;4:e128-e130. doi:10.1016/S2542-5196(20)30081-4
49. Shah UA, Merlo G. Personal and planetary health--the connection with dietary choices. JAMA. 2023;329(21):1823-1824. doi:10.1001/jama.2023.6118
50. Lim C, Van Alphen MU, Freudenreich O. Becoming vaccine ambassadors: a new role for psychiatrists. Current Psychiatry. 2021;20(8):10-11,17-21,26-28,38. doi:10.12788/cp.0155
51. My Green Doctor. Accessed August 6, 2023. https://mygreendoctor.org/
52. Healthcare Without Harm. Accessed August 6, 2023. https://noharm.org/
53. Levy BS, Patz JA. Climate change, human rights, and social justice. Ann Glob Health. 2015;81:310-322.
54. Intergovernmental Panel on Climate Change. Global warming of 1.5° C 2018. Accessed August 6, 2023. https://www.ipcc.ch/sr15/
55. Steffen W, Crutzen J, McNeill JR. The Anthropocene: are humans now overwhelming the great forces of nature? Ambio. 2007;36(8):614-621. doi:10.1579/0044-7447(2007)36[614:taahno]2.0.co;2
56. American Meteorological Society. Explaining extreme events from a climate perspective. Accessed August 6, 2023. https://www.ametsoc.org/ams/index.cfm/publications/bulletin-of-the-american-meteorological-society-bams/explaining-extreme-events-from-a-climate-perspective/
57. Nierenberg AA. Coping in the age of turmoil. Psychiatr Ann. 2022;52(7):263. July 1, 2022. doi:10.3928/23258160-20220701-01
58. Belkin G. Leadership for the social climate. N Engl J Med. 2020;382(21):1975-1977. doi:10.1056/NEJMp2001507
59. Skinner JR. Doctors and climate change: first do no harm. J Paediatr Child Health. 2021;57(11):1754-1758. doi:10.1111/jpc.15658
Agitated and depressed with a traumatic brain injury
CASE TBI as a result of self-harm
Mr. N, age 46, presents to the emergency department (ED) after his neighbors report hearing “loud banging sounds” coming from his apartment for approximately 3 days. Emergency medical services found him repeatedly beating his head into a table. Upon admission to the ED, his injuries include a right temporal lobe contusion, right temporal subdural hematoma, facial fractures, bilateral foot fractures, and prevertebral swelling at the C4 vertebrate.
Mr. N is admitted to the surgical intensive care unit for hourly neurology checks. Neurosurgery recommends nonoperative management and for Mr. N to wear a cervical collar for 1 month. He is sedated after he experiences auditory hallucinations and becomes agitated toward the staff, which is later determined to be delirium. The Psychiatry team recommends inpatient psychiatric hospitalization because Mr. N’s self-harming behavior resulted in severe and dangerous injuries.
HISTORY Alcohol use disorder, insomnia, anxiety, and depression
As Mr. N becomes alert and oriented, he reports a history of alcohol use disorder (AUD), insomnia, anxiety, and major depressive disorder (MDD), but no personal or family history of bipolar disorder (BD). He says he has had insomnia and anxiety since age 18, for which he received diazepam and zolpidem for 16 years. He stopped diazepam soon after a recent change in psychiatrists and subsequently had difficulty sleeping. Mr. N started taking mirtazapine, but found minimal relief and stopped it several months ago.
[polldaddy:12704471]
The authors’ observations
The term “agitated depression” refers to a mixed state that includes symptoms of depression plus marked anxiety, restlessness, and delusions. Agitated depression is not a distinct diagnosis in DSM-5, but is classified as depression with mixed features.1 To meet the criteria for the mixed features specifier, a patient who meets the criteria for a major depressive episode needs to have ≥3 of the following manic/hypomanic symptoms1:
- Elevated, expansive mood
- Inflated self-esteem or grandiosity
- More talkative than usual
- Flight of ideas or racing thoughts
- Increase in energy or goal-directed activity
- Increased involvement in activities that have a high potential for painful consequences
- Decreased need for sleep.
The diagnosis for individuals who meet the full criteria for mania or hypomania would be BD I or BD II.1 Additionally, mixed features associated with a major depressive episode are a significant risk factor for BD.1
EVALUATION Agitation and hallucinations
Mr. N recalls multiple falls at home in the weeks prior to hospitalization, but says he does not remember repeatedly hitting his head against a table. He reports sleeping for approximately 2 hours per night since his father’s death 2 months ago, an acute stressor that likely precipitated this depressive episode. Mr. N says he had been experiencing visual hallucinations of his father and a younger version of himself for weeks before presenting to the ED. It is not clear if Mr. N does not recall beating his head on the table due to his traumatic brain injury (TBI) or because it occurred during an acute manic or psychotic episode with hallucinations.
The treatment team assigns Mr. N a working diagnosis of agitated depression with a risk for BD, mixed episode. He meets the criteria for agitated depression (major depressive episode, motor agitation, and psychic agitation), but also has many features of BD; a manic episode may have led to hospitalization. The treating clinicians continue to monitor the progression of Mr. N’s symptoms to clarify his diagnoses. During the course of his hospitalization, Mr. N’s psychiatric diagnoses include delirium (resolved), alcohol withdrawal, catatonia, substance-induced mood disorder, and agitated depression. Mixed episode BD is ruled out.
Continue to: The authors' observations
The authors’ observations
There is significant symptomatic overlap between agitated depression and BD. It can be difficult to differentiate the diagnoses, as psychomotor agitation can be seen in MDD and agitated depression can be seen in BD. Serra et al2 investigated the prevalence of agitated depression in patients with BD and found that agitation accompanied bipolar depression in at least one-third of cases and was associated with concurrent somatic depressive symptoms, which are common features of mixed manic states. Psychomotor agitation was also associated with lifetime experience of mixed mania, comorbid panic disorder, and increased suicidal behavior.2
Though antidepressants are considered a first-line treatment for depression, they should not be used to treat agitated depression because they may increase insomnia, agitation, and suicide risk, and may trigger the onset of psychotic symptoms. In a similar vein, antidepressant monotherapy is contraindicated in BD because it may induce mania or hypomania states.2
TREATMENT Neuroprotective psychotropics
Due to Mr. N’s medical complexity (particularly cervical collar and physical therapy needs), he is not transferred to a psychiatric facility. Instead, the consultation-liaison psychiatry team follows him and provides psychiatric care in the hospital.
Due to concerns for continued self-harm, Mr. N is observed by continuous video monitoring. After initial stabilization, the care team starts valproic acid 250 mg twice daily and titrates it to 500 mg/d in the morning and 1,000 mg/d in the evening for mood stabilization, gabapentin 300 mg 3 times daily, melatonin 3 mg/d at bedtime for insomnia, and lorazepam 1 mg/d at bedtime to rule out catatonia and 1 mg/d as needed for agitation. After starting valproic acid, the care team routinely checks Mr. N’s ammonia levels throughout his hospitalization.
[polldaddy:12704473]
The authors’ observations
Treatment of agitated depression both in isolation and in the context of BD presents a clinical challenge because antidepressants are contraindicated for both agitated depression and BD. In the context of TBI, treatment of agitated depression becomes more complicated because neuroprotection is the priority. Neuroprotection refers to a medication’s ability to prevent neuronal cell death or further injury or damage through neurochemical modulation.
Continue to: To treat agitation associated with MDD...
To treat agitation associated with MDD, second-generation antipsychotics and valproic acid have shown significant neuroprotective effects. The proposed mechanisms for neuroprotection include not only antioxidant effects but 5HT1A agonist properties, with the latter thought to protect against excitotoxic injury that may exacerbate agitation due to brain trauma.3
There is no consensus on which antipsychotics are most efficacious for treating agitation in the setting of an acute TBI. Williamson et al4 reviewed various medications that may treat agitation in the setting of acute TBI with fewer adverse effects.
Though haloperidol is often prescribed to treat agitation in patients with TBI, animal studies have shown it is inferior to second-generation antipsychotics in protecting against excitotoxic/oxidative injury, and haloperidol has been associated with neuronal loss. Haloperidol has been linked to adverse clinical outcomes for patients with aggression after TBI, including prolonged amnesia, which is thought to be linked to haloperidol’s strong and selective dopamine-2 receptor antagonism and the mesocortical and nigrostriatal pathways involved.4
Carbamazepine, phenytoin, and methylphenidate cause oxidative stress and/or apoptosis, and therefore offer no neuroprotection. Data on gabapentin are mixed; a few studies suggest it may block synapse formation or decrease quantities of antioxidant enzymes in the brain, though it’s known to protect against glutamate-induced neuronal injury.3
Additional research is needed to assess which second-generation antipsychotics offer the most neuroprotection. However, based on existing literature, olanzapine and aripiprazole may offer the most benefit because they have the greatest antioxidant—and thus, neuroprotective—activity. Cognitive enhancers such as memantine and donepezil exhibit neuroprotection, particularly in Alzheimer disease. Anticonvulsants such as levetiracetam, lacosamide, and lamotrigine offer neuroprotection and may be considered for seizure prevention.3 The Table3-6 lists psychotropic medications used to treat TBI.
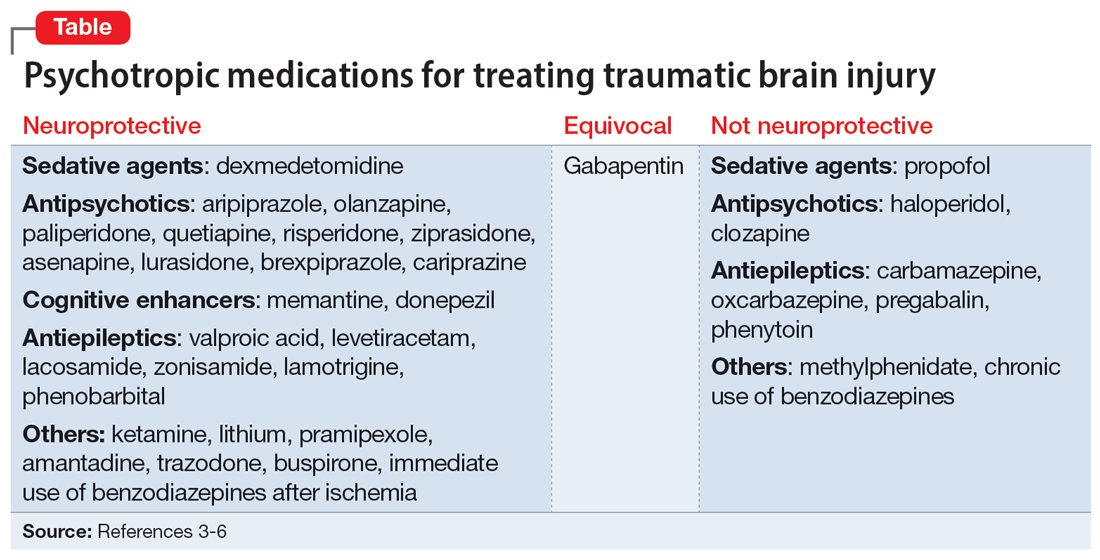
Continue to: Valproic acid stands out among...
Valproic acid stands out among anticonvulsants because its superior antioxidant effects, in combination with its antiepileptic effect in patients with TBI, offer more neuroprotection than other medications.5 It is important to regularly monitor ammonia levels in patients receiving valproic acid because elevated levels can cause hyperammonemic encephalopathy.
A 2005 study by DeBattista et al5 investigated the impact of valproic acid on agitation in 12 adults with MDD who were being treated with antidepressants. Participants were given a low dose of valproic acid for 4 weeks and their agitation, anxiety, and depressed mood were independently assessed by separate rating scales. There was a modest decrease in scores for mood symptoms but a particularly sharp decrease in agitation scores.5
Valproic acid has been shown to be a potentially safe and efficacious treatment for alcohol withdrawal. A clinical trial examining patients with moderate alcohol withdrawal found a faster and more consistent resolution of symptoms in patients given valproic acid detoxification compared to a control group that received the standard benzodiazepine detoxification.6 Additionally, patients who continued maintenance valproic acid following detoxification were completely abstinent at 6-week follow-up compared to patients who did not receive this maintenance therapy.6
Valproic acid was a particularly optimal medication choice for Mr. N due to its neuroprotective properties in the context of TBI, its ability to treat delirium,7 its lack of abuse potential compared with benzodiazepines, and its potential efficacy for managing alcohol withdrawal and AUD.
OUTCOME Improvement and discharge
Mr. N is medically cleared for discharge. Although the psychiatry team initially was concerned about his willingness to attend follow-up appointments and adhere to proper cervical collar use, Mr. N becomes more cooperative with psychiatric care as his stay continues, and he is psychiatrically cleared for discharge 1 month after admission. Discharge plans include attending an intensive outpatient program, continuing the inpatient psychiatric medication regimen, participating in regular outpatient psychiatric follow-up, as well as following up with orthopedic surgery, neurosurgery, podiatry, and ear, nose, and throat for medical conditions.
Bottom Line
Agitated depression is a mixed state that includes features of depression and manic/hypomanic symptoms. Diagnosis and treatment can be challenging because symptoms of agitated depression overlap with bipolar disorder and antidepressants are contraindicated. In a patient with a traumatic brain injury, pharmacotherapy that provides neuroprotection is a priority.
Related Resources
- Ramaswamy S, Driscoll D, Rodriguez A, et al. Nutraceuticals for traumatic brain injury: should you recommend their use? Current Psychiatry. 2017;16(7):34-38,40,41-45.
- Sampogna G, Del Vecchio V, Giallonardo V, et al. Diagnosis, clinical features, and therapeutic implications of agitated depression. Psychiatr Clin North Am. 2020;43(1):47-57. doi: 10.1016/j.psc.2019.10.011
Drug Brand Names
Amantadine • Gocovri
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Buspirone • BuSpar
Carbamazepine • Tegretol
Cariprazine • Vraylar
Clozapine • Clozaril
Dexmedetomidine • Igalmi
Diazepam • Valium
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Ketamine • Ketalar
Lacosamide • Vimpat
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lithium • Lithobid
Lorazepam • Ativan
Lurasidone • Latuda
Memantine • Namenda
Methylphenidate • Concerta
Mirtazapine • Remeron
Olanzapine • Zyprexa
Oxcarbazepine • Trileptal
Paliperidone • Invega
Phenytoin • Dilantin
Pramipexole • Mirapex
Pregabalin • Lyrica
Quetiapine • Seroquel
Risperidone • Risperdal
Trazodone • Oleptro
Valproic acid • Depakene
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Serra F, Gordon‐Smith K, Perry A, et al. Agitated depression in bipolar disorder. Bipolar Disord. 2019;21(6):547-555. doi:10.1111/bdi.12778
3. Meresh E, Daniels D, Owens JH, et al. Psychotropics and neuroprotection: literature review and case series report. OBM Neurobiol. 2020;4(1). doi:10.21926/obm.neurobiol.2001048
4. Williamson DR, Frenette AJ, Burry L, et al. Pharmacological interventions for agitation in patients with traumatic brain injury: protocol for a systematic review and meta-analysis. Syst Rev. 2016;5(1):193. doi:10.1186/s13643-016-0374-6
5. DeBattista C, Solomon A, Arnow B, et al. The efficacy of divalproex sodium in the treatment of agitation associated with major depression. J Clin Psychopharmacol. 2005;25(5):476-479. doi:10.1097/01.jcp.0000177552.21338.b0
6. Longo LP, Campbell T, Hubatch, S. Divalproex sodium (Depakote) for alcohol withdrawal and relapse prevention. J Addict Dis. 2002;21(2):55-64. doi:10.1300/J069v21n02_05
7. Sher Y, Cramer ACM, Ament A, et al. Valproic acid for treatment of hyperactive or mixed delirium: rationale and literature review. Psychosomatics. 2015;56(6):615-625. doi:10.1016/j.psym.2015.09.008
CASE TBI as a result of self-harm
Mr. N, age 46, presents to the emergency department (ED) after his neighbors report hearing “loud banging sounds” coming from his apartment for approximately 3 days. Emergency medical services found him repeatedly beating his head into a table. Upon admission to the ED, his injuries include a right temporal lobe contusion, right temporal subdural hematoma, facial fractures, bilateral foot fractures, and prevertebral swelling at the C4 vertebrate.
Mr. N is admitted to the surgical intensive care unit for hourly neurology checks. Neurosurgery recommends nonoperative management and for Mr. N to wear a cervical collar for 1 month. He is sedated after he experiences auditory hallucinations and becomes agitated toward the staff, which is later determined to be delirium. The Psychiatry team recommends inpatient psychiatric hospitalization because Mr. N’s self-harming behavior resulted in severe and dangerous injuries.
HISTORY Alcohol use disorder, insomnia, anxiety, and depression
As Mr. N becomes alert and oriented, he reports a history of alcohol use disorder (AUD), insomnia, anxiety, and major depressive disorder (MDD), but no personal or family history of bipolar disorder (BD). He says he has had insomnia and anxiety since age 18, for which he received diazepam and zolpidem for 16 years. He stopped diazepam soon after a recent change in psychiatrists and subsequently had difficulty sleeping. Mr. N started taking mirtazapine, but found minimal relief and stopped it several months ago.
[polldaddy:12704471]
The authors’ observations
The term “agitated depression” refers to a mixed state that includes symptoms of depression plus marked anxiety, restlessness, and delusions. Agitated depression is not a distinct diagnosis in DSM-5, but is classified as depression with mixed features.1 To meet the criteria for the mixed features specifier, a patient who meets the criteria for a major depressive episode needs to have ≥3 of the following manic/hypomanic symptoms1:
- Elevated, expansive mood
- Inflated self-esteem or grandiosity
- More talkative than usual
- Flight of ideas or racing thoughts
- Increase in energy or goal-directed activity
- Increased involvement in activities that have a high potential for painful consequences
- Decreased need for sleep.
The diagnosis for individuals who meet the full criteria for mania or hypomania would be BD I or BD II.1 Additionally, mixed features associated with a major depressive episode are a significant risk factor for BD.1
EVALUATION Agitation and hallucinations
Mr. N recalls multiple falls at home in the weeks prior to hospitalization, but says he does not remember repeatedly hitting his head against a table. He reports sleeping for approximately 2 hours per night since his father’s death 2 months ago, an acute stressor that likely precipitated this depressive episode. Mr. N says he had been experiencing visual hallucinations of his father and a younger version of himself for weeks before presenting to the ED. It is not clear if Mr. N does not recall beating his head on the table due to his traumatic brain injury (TBI) or because it occurred during an acute manic or psychotic episode with hallucinations.
The treatment team assigns Mr. N a working diagnosis of agitated depression with a risk for BD, mixed episode. He meets the criteria for agitated depression (major depressive episode, motor agitation, and psychic agitation), but also has many features of BD; a manic episode may have led to hospitalization. The treating clinicians continue to monitor the progression of Mr. N’s symptoms to clarify his diagnoses. During the course of his hospitalization, Mr. N’s psychiatric diagnoses include delirium (resolved), alcohol withdrawal, catatonia, substance-induced mood disorder, and agitated depression. Mixed episode BD is ruled out.
Continue to: The authors' observations
The authors’ observations
There is significant symptomatic overlap between agitated depression and BD. It can be difficult to differentiate the diagnoses, as psychomotor agitation can be seen in MDD and agitated depression can be seen in BD. Serra et al2 investigated the prevalence of agitated depression in patients with BD and found that agitation accompanied bipolar depression in at least one-third of cases and was associated with concurrent somatic depressive symptoms, which are common features of mixed manic states. Psychomotor agitation was also associated with lifetime experience of mixed mania, comorbid panic disorder, and increased suicidal behavior.2
Though antidepressants are considered a first-line treatment for depression, they should not be used to treat agitated depression because they may increase insomnia, agitation, and suicide risk, and may trigger the onset of psychotic symptoms. In a similar vein, antidepressant monotherapy is contraindicated in BD because it may induce mania or hypomania states.2
TREATMENT Neuroprotective psychotropics
Due to Mr. N’s medical complexity (particularly cervical collar and physical therapy needs), he is not transferred to a psychiatric facility. Instead, the consultation-liaison psychiatry team follows him and provides psychiatric care in the hospital.
Due to concerns for continued self-harm, Mr. N is observed by continuous video monitoring. After initial stabilization, the care team starts valproic acid 250 mg twice daily and titrates it to 500 mg/d in the morning and 1,000 mg/d in the evening for mood stabilization, gabapentin 300 mg 3 times daily, melatonin 3 mg/d at bedtime for insomnia, and lorazepam 1 mg/d at bedtime to rule out catatonia and 1 mg/d as needed for agitation. After starting valproic acid, the care team routinely checks Mr. N’s ammonia levels throughout his hospitalization.
[polldaddy:12704473]
The authors’ observations
Treatment of agitated depression both in isolation and in the context of BD presents a clinical challenge because antidepressants are contraindicated for both agitated depression and BD. In the context of TBI, treatment of agitated depression becomes more complicated because neuroprotection is the priority. Neuroprotection refers to a medication’s ability to prevent neuronal cell death or further injury or damage through neurochemical modulation.
Continue to: To treat agitation associated with MDD...
To treat agitation associated with MDD, second-generation antipsychotics and valproic acid have shown significant neuroprotective effects. The proposed mechanisms for neuroprotection include not only antioxidant effects but 5HT1A agonist properties, with the latter thought to protect against excitotoxic injury that may exacerbate agitation due to brain trauma.3
There is no consensus on which antipsychotics are most efficacious for treating agitation in the setting of an acute TBI. Williamson et al4 reviewed various medications that may treat agitation in the setting of acute TBI with fewer adverse effects.
Though haloperidol is often prescribed to treat agitation in patients with TBI, animal studies have shown it is inferior to second-generation antipsychotics in protecting against excitotoxic/oxidative injury, and haloperidol has been associated with neuronal loss. Haloperidol has been linked to adverse clinical outcomes for patients with aggression after TBI, including prolonged amnesia, which is thought to be linked to haloperidol’s strong and selective dopamine-2 receptor antagonism and the mesocortical and nigrostriatal pathways involved.4
Carbamazepine, phenytoin, and methylphenidate cause oxidative stress and/or apoptosis, and therefore offer no neuroprotection. Data on gabapentin are mixed; a few studies suggest it may block synapse formation or decrease quantities of antioxidant enzymes in the brain, though it’s known to protect against glutamate-induced neuronal injury.3
Additional research is needed to assess which second-generation antipsychotics offer the most neuroprotection. However, based on existing literature, olanzapine and aripiprazole may offer the most benefit because they have the greatest antioxidant—and thus, neuroprotective—activity. Cognitive enhancers such as memantine and donepezil exhibit neuroprotection, particularly in Alzheimer disease. Anticonvulsants such as levetiracetam, lacosamide, and lamotrigine offer neuroprotection and may be considered for seizure prevention.3 The Table3-6 lists psychotropic medications used to treat TBI.

Continue to: Valproic acid stands out among...
Valproic acid stands out among anticonvulsants because its superior antioxidant effects, in combination with its antiepileptic effect in patients with TBI, offer more neuroprotection than other medications.5 It is important to regularly monitor ammonia levels in patients receiving valproic acid because elevated levels can cause hyperammonemic encephalopathy.
A 2005 study by DeBattista et al5 investigated the impact of valproic acid on agitation in 12 adults with MDD who were being treated with antidepressants. Participants were given a low dose of valproic acid for 4 weeks and their agitation, anxiety, and depressed mood were independently assessed by separate rating scales. There was a modest decrease in scores for mood symptoms but a particularly sharp decrease in agitation scores.5
Valproic acid has been shown to be a potentially safe and efficacious treatment for alcohol withdrawal. A clinical trial examining patients with moderate alcohol withdrawal found a faster and more consistent resolution of symptoms in patients given valproic acid detoxification compared to a control group that received the standard benzodiazepine detoxification.6 Additionally, patients who continued maintenance valproic acid following detoxification were completely abstinent at 6-week follow-up compared to patients who did not receive this maintenance therapy.6
Valproic acid was a particularly optimal medication choice for Mr. N due to its neuroprotective properties in the context of TBI, its ability to treat delirium,7 its lack of abuse potential compared with benzodiazepines, and its potential efficacy for managing alcohol withdrawal and AUD.
OUTCOME Improvement and discharge
Mr. N is medically cleared for discharge. Although the psychiatry team initially was concerned about his willingness to attend follow-up appointments and adhere to proper cervical collar use, Mr. N becomes more cooperative with psychiatric care as his stay continues, and he is psychiatrically cleared for discharge 1 month after admission. Discharge plans include attending an intensive outpatient program, continuing the inpatient psychiatric medication regimen, participating in regular outpatient psychiatric follow-up, as well as following up with orthopedic surgery, neurosurgery, podiatry, and ear, nose, and throat for medical conditions.
Bottom Line
Agitated depression is a mixed state that includes features of depression and manic/hypomanic symptoms. Diagnosis and treatment can be challenging because symptoms of agitated depression overlap with bipolar disorder and antidepressants are contraindicated. In a patient with a traumatic brain injury, pharmacotherapy that provides neuroprotection is a priority.
Related Resources
- Ramaswamy S, Driscoll D, Rodriguez A, et al. Nutraceuticals for traumatic brain injury: should you recommend their use? Current Psychiatry. 2017;16(7):34-38,40,41-45.
- Sampogna G, Del Vecchio V, Giallonardo V, et al. Diagnosis, clinical features, and therapeutic implications of agitated depression. Psychiatr Clin North Am. 2020;43(1):47-57. doi: 10.1016/j.psc.2019.10.011
Drug Brand Names
Amantadine • Gocovri
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Buspirone • BuSpar
Carbamazepine • Tegretol
Cariprazine • Vraylar
Clozapine • Clozaril
Dexmedetomidine • Igalmi
Diazepam • Valium
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Ketamine • Ketalar
Lacosamide • Vimpat
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lithium • Lithobid
Lorazepam • Ativan
Lurasidone • Latuda
Memantine • Namenda
Methylphenidate • Concerta
Mirtazapine • Remeron
Olanzapine • Zyprexa
Oxcarbazepine • Trileptal
Paliperidone • Invega
Phenytoin • Dilantin
Pramipexole • Mirapex
Pregabalin • Lyrica
Quetiapine • Seroquel
Risperidone • Risperdal
Trazodone • Oleptro
Valproic acid • Depakene
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
CASE TBI as a result of self-harm
Mr. N, age 46, presents to the emergency department (ED) after his neighbors report hearing “loud banging sounds” coming from his apartment for approximately 3 days. Emergency medical services found him repeatedly beating his head into a table. Upon admission to the ED, his injuries include a right temporal lobe contusion, right temporal subdural hematoma, facial fractures, bilateral foot fractures, and prevertebral swelling at the C4 vertebrate.
Mr. N is admitted to the surgical intensive care unit for hourly neurology checks. Neurosurgery recommends nonoperative management and for Mr. N to wear a cervical collar for 1 month. He is sedated after he experiences auditory hallucinations and becomes agitated toward the staff, which is later determined to be delirium. The Psychiatry team recommends inpatient psychiatric hospitalization because Mr. N’s self-harming behavior resulted in severe and dangerous injuries.
HISTORY Alcohol use disorder, insomnia, anxiety, and depression
As Mr. N becomes alert and oriented, he reports a history of alcohol use disorder (AUD), insomnia, anxiety, and major depressive disorder (MDD), but no personal or family history of bipolar disorder (BD). He says he has had insomnia and anxiety since age 18, for which he received diazepam and zolpidem for 16 years. He stopped diazepam soon after a recent change in psychiatrists and subsequently had difficulty sleeping. Mr. N started taking mirtazapine, but found minimal relief and stopped it several months ago.
[polldaddy:12704471]
The authors’ observations
The term “agitated depression” refers to a mixed state that includes symptoms of depression plus marked anxiety, restlessness, and delusions. Agitated depression is not a distinct diagnosis in DSM-5, but is classified as depression with mixed features.1 To meet the criteria for the mixed features specifier, a patient who meets the criteria for a major depressive episode needs to have ≥3 of the following manic/hypomanic symptoms1:
- Elevated, expansive mood
- Inflated self-esteem or grandiosity
- More talkative than usual
- Flight of ideas or racing thoughts
- Increase in energy or goal-directed activity
- Increased involvement in activities that have a high potential for painful consequences
- Decreased need for sleep.
The diagnosis for individuals who meet the full criteria for mania or hypomania would be BD I or BD II.1 Additionally, mixed features associated with a major depressive episode are a significant risk factor for BD.1
EVALUATION Agitation and hallucinations
Mr. N recalls multiple falls at home in the weeks prior to hospitalization, but says he does not remember repeatedly hitting his head against a table. He reports sleeping for approximately 2 hours per night since his father’s death 2 months ago, an acute stressor that likely precipitated this depressive episode. Mr. N says he had been experiencing visual hallucinations of his father and a younger version of himself for weeks before presenting to the ED. It is not clear if Mr. N does not recall beating his head on the table due to his traumatic brain injury (TBI) or because it occurred during an acute manic or psychotic episode with hallucinations.
The treatment team assigns Mr. N a working diagnosis of agitated depression with a risk for BD, mixed episode. He meets the criteria for agitated depression (major depressive episode, motor agitation, and psychic agitation), but also has many features of BD; a manic episode may have led to hospitalization. The treating clinicians continue to monitor the progression of Mr. N’s symptoms to clarify his diagnoses. During the course of his hospitalization, Mr. N’s psychiatric diagnoses include delirium (resolved), alcohol withdrawal, catatonia, substance-induced mood disorder, and agitated depression. Mixed episode BD is ruled out.
Continue to: The authors' observations
The authors’ observations
There is significant symptomatic overlap between agitated depression and BD. It can be difficult to differentiate the diagnoses, as psychomotor agitation can be seen in MDD and agitated depression can be seen in BD. Serra et al2 investigated the prevalence of agitated depression in patients with BD and found that agitation accompanied bipolar depression in at least one-third of cases and was associated with concurrent somatic depressive symptoms, which are common features of mixed manic states. Psychomotor agitation was also associated with lifetime experience of mixed mania, comorbid panic disorder, and increased suicidal behavior.2
Though antidepressants are considered a first-line treatment for depression, they should not be used to treat agitated depression because they may increase insomnia, agitation, and suicide risk, and may trigger the onset of psychotic symptoms. In a similar vein, antidepressant monotherapy is contraindicated in BD because it may induce mania or hypomania states.2
TREATMENT Neuroprotective psychotropics
Due to Mr. N’s medical complexity (particularly cervical collar and physical therapy needs), he is not transferred to a psychiatric facility. Instead, the consultation-liaison psychiatry team follows him and provides psychiatric care in the hospital.
Due to concerns for continued self-harm, Mr. N is observed by continuous video monitoring. After initial stabilization, the care team starts valproic acid 250 mg twice daily and titrates it to 500 mg/d in the morning and 1,000 mg/d in the evening for mood stabilization, gabapentin 300 mg 3 times daily, melatonin 3 mg/d at bedtime for insomnia, and lorazepam 1 mg/d at bedtime to rule out catatonia and 1 mg/d as needed for agitation. After starting valproic acid, the care team routinely checks Mr. N’s ammonia levels throughout his hospitalization.
[polldaddy:12704473]
The authors’ observations
Treatment of agitated depression both in isolation and in the context of BD presents a clinical challenge because antidepressants are contraindicated for both agitated depression and BD. In the context of TBI, treatment of agitated depression becomes more complicated because neuroprotection is the priority. Neuroprotection refers to a medication’s ability to prevent neuronal cell death or further injury or damage through neurochemical modulation.
Continue to: To treat agitation associated with MDD...
To treat agitation associated with MDD, second-generation antipsychotics and valproic acid have shown significant neuroprotective effects. The proposed mechanisms for neuroprotection include not only antioxidant effects but 5HT1A agonist properties, with the latter thought to protect against excitotoxic injury that may exacerbate agitation due to brain trauma.3
There is no consensus on which antipsychotics are most efficacious for treating agitation in the setting of an acute TBI. Williamson et al4 reviewed various medications that may treat agitation in the setting of acute TBI with fewer adverse effects.
Though haloperidol is often prescribed to treat agitation in patients with TBI, animal studies have shown it is inferior to second-generation antipsychotics in protecting against excitotoxic/oxidative injury, and haloperidol has been associated with neuronal loss. Haloperidol has been linked to adverse clinical outcomes for patients with aggression after TBI, including prolonged amnesia, which is thought to be linked to haloperidol’s strong and selective dopamine-2 receptor antagonism and the mesocortical and nigrostriatal pathways involved.4
Carbamazepine, phenytoin, and methylphenidate cause oxidative stress and/or apoptosis, and therefore offer no neuroprotection. Data on gabapentin are mixed; a few studies suggest it may block synapse formation or decrease quantities of antioxidant enzymes in the brain, though it’s known to protect against glutamate-induced neuronal injury.3
Additional research is needed to assess which second-generation antipsychotics offer the most neuroprotection. However, based on existing literature, olanzapine and aripiprazole may offer the most benefit because they have the greatest antioxidant—and thus, neuroprotective—activity. Cognitive enhancers such as memantine and donepezil exhibit neuroprotection, particularly in Alzheimer disease. Anticonvulsants such as levetiracetam, lacosamide, and lamotrigine offer neuroprotection and may be considered for seizure prevention.3 The Table3-6 lists psychotropic medications used to treat TBI.

Continue to: Valproic acid stands out among...
Valproic acid stands out among anticonvulsants because its superior antioxidant effects, in combination with its antiepileptic effect in patients with TBI, offer more neuroprotection than other medications.5 It is important to regularly monitor ammonia levels in patients receiving valproic acid because elevated levels can cause hyperammonemic encephalopathy.
A 2005 study by DeBattista et al5 investigated the impact of valproic acid on agitation in 12 adults with MDD who were being treated with antidepressants. Participants were given a low dose of valproic acid for 4 weeks and their agitation, anxiety, and depressed mood were independently assessed by separate rating scales. There was a modest decrease in scores for mood symptoms but a particularly sharp decrease in agitation scores.5
Valproic acid has been shown to be a potentially safe and efficacious treatment for alcohol withdrawal. A clinical trial examining patients with moderate alcohol withdrawal found a faster and more consistent resolution of symptoms in patients given valproic acid detoxification compared to a control group that received the standard benzodiazepine detoxification.6 Additionally, patients who continued maintenance valproic acid following detoxification were completely abstinent at 6-week follow-up compared to patients who did not receive this maintenance therapy.6
Valproic acid was a particularly optimal medication choice for Mr. N due to its neuroprotective properties in the context of TBI, its ability to treat delirium,7 its lack of abuse potential compared with benzodiazepines, and its potential efficacy for managing alcohol withdrawal and AUD.
OUTCOME Improvement and discharge
Mr. N is medically cleared for discharge. Although the psychiatry team initially was concerned about his willingness to attend follow-up appointments and adhere to proper cervical collar use, Mr. N becomes more cooperative with psychiatric care as his stay continues, and he is psychiatrically cleared for discharge 1 month after admission. Discharge plans include attending an intensive outpatient program, continuing the inpatient psychiatric medication regimen, participating in regular outpatient psychiatric follow-up, as well as following up with orthopedic surgery, neurosurgery, podiatry, and ear, nose, and throat for medical conditions.
Bottom Line
Agitated depression is a mixed state that includes features of depression and manic/hypomanic symptoms. Diagnosis and treatment can be challenging because symptoms of agitated depression overlap with bipolar disorder and antidepressants are contraindicated. In a patient with a traumatic brain injury, pharmacotherapy that provides neuroprotection is a priority.
Related Resources
- Ramaswamy S, Driscoll D, Rodriguez A, et al. Nutraceuticals for traumatic brain injury: should you recommend their use? Current Psychiatry. 2017;16(7):34-38,40,41-45.
- Sampogna G, Del Vecchio V, Giallonardo V, et al. Diagnosis, clinical features, and therapeutic implications of agitated depression. Psychiatr Clin North Am. 2020;43(1):47-57. doi: 10.1016/j.psc.2019.10.011
Drug Brand Names
Amantadine • Gocovri
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Buspirone • BuSpar
Carbamazepine • Tegretol
Cariprazine • Vraylar
Clozapine • Clozaril
Dexmedetomidine • Igalmi
Diazepam • Valium
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Ketamine • Ketalar
Lacosamide • Vimpat
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lithium • Lithobid
Lorazepam • Ativan
Lurasidone • Latuda
Memantine • Namenda
Methylphenidate • Concerta
Mirtazapine • Remeron
Olanzapine • Zyprexa
Oxcarbazepine • Trileptal
Paliperidone • Invega
Phenytoin • Dilantin
Pramipexole • Mirapex
Pregabalin • Lyrica
Quetiapine • Seroquel
Risperidone • Risperdal
Trazodone • Oleptro
Valproic acid • Depakene
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Serra F, Gordon‐Smith K, Perry A, et al. Agitated depression in bipolar disorder. Bipolar Disord. 2019;21(6):547-555. doi:10.1111/bdi.12778
3. Meresh E, Daniels D, Owens JH, et al. Psychotropics and neuroprotection: literature review and case series report. OBM Neurobiol. 2020;4(1). doi:10.21926/obm.neurobiol.2001048
4. Williamson DR, Frenette AJ, Burry L, et al. Pharmacological interventions for agitation in patients with traumatic brain injury: protocol for a systematic review and meta-analysis. Syst Rev. 2016;5(1):193. doi:10.1186/s13643-016-0374-6
5. DeBattista C, Solomon A, Arnow B, et al. The efficacy of divalproex sodium in the treatment of agitation associated with major depression. J Clin Psychopharmacol. 2005;25(5):476-479. doi:10.1097/01.jcp.0000177552.21338.b0
6. Longo LP, Campbell T, Hubatch, S. Divalproex sodium (Depakote) for alcohol withdrawal and relapse prevention. J Addict Dis. 2002;21(2):55-64. doi:10.1300/J069v21n02_05
7. Sher Y, Cramer ACM, Ament A, et al. Valproic acid for treatment of hyperactive or mixed delirium: rationale and literature review. Psychosomatics. 2015;56(6):615-625. doi:10.1016/j.psym.2015.09.008
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Serra F, Gordon‐Smith K, Perry A, et al. Agitated depression in bipolar disorder. Bipolar Disord. 2019;21(6):547-555. doi:10.1111/bdi.12778
3. Meresh E, Daniels D, Owens JH, et al. Psychotropics and neuroprotection: literature review and case series report. OBM Neurobiol. 2020;4(1). doi:10.21926/obm.neurobiol.2001048
4. Williamson DR, Frenette AJ, Burry L, et al. Pharmacological interventions for agitation in patients with traumatic brain injury: protocol for a systematic review and meta-analysis. Syst Rev. 2016;5(1):193. doi:10.1186/s13643-016-0374-6
5. DeBattista C, Solomon A, Arnow B, et al. The efficacy of divalproex sodium in the treatment of agitation associated with major depression. J Clin Psychopharmacol. 2005;25(5):476-479. doi:10.1097/01.jcp.0000177552.21338.b0
6. Longo LP, Campbell T, Hubatch, S. Divalproex sodium (Depakote) for alcohol withdrawal and relapse prevention. J Addict Dis. 2002;21(2):55-64. doi:10.1300/J069v21n02_05
7. Sher Y, Cramer ACM, Ament A, et al. Valproic acid for treatment of hyperactive or mixed delirium: rationale and literature review. Psychosomatics. 2015;56(6):615-625. doi:10.1016/j.psym.2015.09.008
No link between most cancers and depression/anxiety: Study
from a large, individual participant data meta-analysis.
An exception was for lung and smoking-related cancers, but key covariates appeared to explain the relationship between depression, anxiety, and these cancer types, the investigators reported.
The findings challenge a common theory that depression and anxiety increase cancer risk and should “change current thinking,” they argue.
“Our results may come as a relief to many patients with cancer who believe their diagnosis is attributed to previous anxiety or depression,” first author Lonneke A. van Tuijl, PhD, of the University of Groningen and Utrecht University, the Netherlands, noted in a press release.
Analyses included data from up to nearly 320,000 individuals from the 18 prospective cohorts included in the international Psychosocial Factors and Cancer Incidence (PSY-CA) consortium. The cohorts are from studies conducted in the Netherlands, United Kingdom, Norway, and Canada, and included 25,803 patients with cancer. During follow-up of up to 26 years and more than 3.2 million person-years, depression and anxiety symptoms and diagnoses showed no association with overall breast, prostate, colorectal, and alcohol-related cancers (hazard ratios, 0.98-1.05).
For the specific cancer types, the investigators “found no evidence for an association between depression or anxiety and the incidence of colorectal cancer (HRs, 0.88-1.13), prostate cancer (HRs, 0.97-1.17), or alcohol-related cancers (HRs, 0.97-1.06).”
“For breast cancer, all pooled HRs were consistently negative but mean pooled HRs were close to 1 (HRs, 0.92-0.98) and the upper limit of the 95% confidence intervals all exceeded 1 (with the exception of anxiety symptoms),” they noted.
An increase in risk observed between depression and anxiety symptoms and diagnoses and lung cancer (HRs, 1.12-1.60) and smoking-related cancers (HRs, 1.06-1.60), in minimally adjusted models, was substantially attenuated after adjusting for known risk factors such as smoking, alcohol use, and body mass index (HRs, 1.04-1.08), the investigators reported.
The findings were published online in Cancer.
“Depression and anxiety have long been hypothesized to increase the risk for cancer. It is thought that the increased cancer risk can occur via several pathways, including health behaviors, or by influencing mutation, viral oncogenes, cell proliferation, or DNA repair,” the authors explained, noting that “[c]onclusions drawn in meta-analyses vary greatly, with some supporting an association between depression, anxiety, and cancer incidence and others finding no or a negligible association.”
The current findings “may help health professionals to alleviate feelings of guilt and self-blame in patients with cancer who attribute their diagnosis to previous depression or anxiety,” they said, noting that the findings “also underscore the importance of addressing tobacco smoking and other unhealthy behaviors – including those that may develop as a result of anxiety or depression.”
“However, further research is needed to understand exactly how depression, anxiety, health behaviors, and lung cancer are related,” said Dr. Tuijl.
Dr. Tuijl has received grants and travel support from the Dutch Cancer Society (KWF).
from a large, individual participant data meta-analysis.
An exception was for lung and smoking-related cancers, but key covariates appeared to explain the relationship between depression, anxiety, and these cancer types, the investigators reported.
The findings challenge a common theory that depression and anxiety increase cancer risk and should “change current thinking,” they argue.
“Our results may come as a relief to many patients with cancer who believe their diagnosis is attributed to previous anxiety or depression,” first author Lonneke A. van Tuijl, PhD, of the University of Groningen and Utrecht University, the Netherlands, noted in a press release.
Analyses included data from up to nearly 320,000 individuals from the 18 prospective cohorts included in the international Psychosocial Factors and Cancer Incidence (PSY-CA) consortium. The cohorts are from studies conducted in the Netherlands, United Kingdom, Norway, and Canada, and included 25,803 patients with cancer. During follow-up of up to 26 years and more than 3.2 million person-years, depression and anxiety symptoms and diagnoses showed no association with overall breast, prostate, colorectal, and alcohol-related cancers (hazard ratios, 0.98-1.05).
For the specific cancer types, the investigators “found no evidence for an association between depression or anxiety and the incidence of colorectal cancer (HRs, 0.88-1.13), prostate cancer (HRs, 0.97-1.17), or alcohol-related cancers (HRs, 0.97-1.06).”
“For breast cancer, all pooled HRs were consistently negative but mean pooled HRs were close to 1 (HRs, 0.92-0.98) and the upper limit of the 95% confidence intervals all exceeded 1 (with the exception of anxiety symptoms),” they noted.
An increase in risk observed between depression and anxiety symptoms and diagnoses and lung cancer (HRs, 1.12-1.60) and smoking-related cancers (HRs, 1.06-1.60), in minimally adjusted models, was substantially attenuated after adjusting for known risk factors such as smoking, alcohol use, and body mass index (HRs, 1.04-1.08), the investigators reported.
The findings were published online in Cancer.
“Depression and anxiety have long been hypothesized to increase the risk for cancer. It is thought that the increased cancer risk can occur via several pathways, including health behaviors, or by influencing mutation, viral oncogenes, cell proliferation, or DNA repair,” the authors explained, noting that “[c]onclusions drawn in meta-analyses vary greatly, with some supporting an association between depression, anxiety, and cancer incidence and others finding no or a negligible association.”
The current findings “may help health professionals to alleviate feelings of guilt and self-blame in patients with cancer who attribute their diagnosis to previous depression or anxiety,” they said, noting that the findings “also underscore the importance of addressing tobacco smoking and other unhealthy behaviors – including those that may develop as a result of anxiety or depression.”
“However, further research is needed to understand exactly how depression, anxiety, health behaviors, and lung cancer are related,” said Dr. Tuijl.
Dr. Tuijl has received grants and travel support from the Dutch Cancer Society (KWF).
from a large, individual participant data meta-analysis.
An exception was for lung and smoking-related cancers, but key covariates appeared to explain the relationship between depression, anxiety, and these cancer types, the investigators reported.
The findings challenge a common theory that depression and anxiety increase cancer risk and should “change current thinking,” they argue.
“Our results may come as a relief to many patients with cancer who believe their diagnosis is attributed to previous anxiety or depression,” first author Lonneke A. van Tuijl, PhD, of the University of Groningen and Utrecht University, the Netherlands, noted in a press release.
Analyses included data from up to nearly 320,000 individuals from the 18 prospective cohorts included in the international Psychosocial Factors and Cancer Incidence (PSY-CA) consortium. The cohorts are from studies conducted in the Netherlands, United Kingdom, Norway, and Canada, and included 25,803 patients with cancer. During follow-up of up to 26 years and more than 3.2 million person-years, depression and anxiety symptoms and diagnoses showed no association with overall breast, prostate, colorectal, and alcohol-related cancers (hazard ratios, 0.98-1.05).
For the specific cancer types, the investigators “found no evidence for an association between depression or anxiety and the incidence of colorectal cancer (HRs, 0.88-1.13), prostate cancer (HRs, 0.97-1.17), or alcohol-related cancers (HRs, 0.97-1.06).”
“For breast cancer, all pooled HRs were consistently negative but mean pooled HRs were close to 1 (HRs, 0.92-0.98) and the upper limit of the 95% confidence intervals all exceeded 1 (with the exception of anxiety symptoms),” they noted.
An increase in risk observed between depression and anxiety symptoms and diagnoses and lung cancer (HRs, 1.12-1.60) and smoking-related cancers (HRs, 1.06-1.60), in minimally adjusted models, was substantially attenuated after adjusting for known risk factors such as smoking, alcohol use, and body mass index (HRs, 1.04-1.08), the investigators reported.
The findings were published online in Cancer.
“Depression and anxiety have long been hypothesized to increase the risk for cancer. It is thought that the increased cancer risk can occur via several pathways, including health behaviors, or by influencing mutation, viral oncogenes, cell proliferation, or DNA repair,” the authors explained, noting that “[c]onclusions drawn in meta-analyses vary greatly, with some supporting an association between depression, anxiety, and cancer incidence and others finding no or a negligible association.”
The current findings “may help health professionals to alleviate feelings of guilt and self-blame in patients with cancer who attribute their diagnosis to previous depression or anxiety,” they said, noting that the findings “also underscore the importance of addressing tobacco smoking and other unhealthy behaviors – including those that may develop as a result of anxiety or depression.”
“However, further research is needed to understand exactly how depression, anxiety, health behaviors, and lung cancer are related,” said Dr. Tuijl.
Dr. Tuijl has received grants and travel support from the Dutch Cancer Society (KWF).
FROM CANCER
PTSD: Written exposure therapy matches prolonged exposure therapy
Investigators also found that participants randomly assigned to receive WET were significantly less likely to drop out of treatment than those receiving PE.
Written exposure therapy involves writing about thoughts and feelings during a specific traumatic event during five supervised, 30-minute sessions and discussing the writing process with the therapist supervising the sessions.
In the latter sessions, the participant talks about how the event affected them.
“Clinicians should consider using WET in their practices as some clients would prefer a shorter treatment approach, and it may be the only option for some clients – for instance, those who have limited time for therapy and may not be able to do a longer treatment,” study investigator Denise Sloan, PhD, said in an interview.
She also noted that WET is covered by insurance and that “most providers I know indicate that they list it as CBT [cognitive-behavioral therapy] code to insurance companies.”
Sloan is senior clinician investigator of the National Center for PTSD at VA Boston Healthcare System and professor of psychiatry at Boston University.
The findings were published online in JAMA Psychiatry.
High attrition rates
The disadvantage to the three major types of therapy used most often to treat PTSD in veterans – eye movement desensitization and reprocessing, cognitive processing therapy (CPT), and PE – are the dropout rates, that range from 18% to as high as 50%.
Prior studies have shown that WET is briefer and just as effective as CPT, but investigators noted that it had never been tested against PE in a randomized clinical trial.
To find out how the two types of therapy compare, Dr. Sloan and associates randomized 178 veterans with PTSD from three VA centers – Boston; Charleston, S.C.; and Madison, Wisc. – to receive either WET or PE.
PE consisted of 8-15 90-minute therapy sessions during which participants imagine the most distressing aspect of their traumatic memory, and between sessions, they confront the people, places, or situations they have been avoiding because of the trauma.
The investigators used the Structured Clinical Interview for DSM-5 at baseline to screen participants at high risk for suicide, comorbid substance use disorder, and unstable bipolar disorder, who were excluded from the study.
At baseline, 10, 20, and 30 weeks after the first treatment session, the investigators measured the severity of each patient’s PTSD symptoms with the Clinician-Administered PTSD Scale for DSM-5, which has a range of 0 (no PTSD symptoms) to 80 (most severe PTSD symptoms).
Of the 178 veterans, 134 were men, and their mean age was 45 years. The majority (63%) was White, while 21% were Black.
The researchers found that study participants were not significantly more likely to meet PTSD diagnostic criteria in the WET or PE conditions at any assessment.
WET briefer, better retention
Investigators noted the largest difference in PTSD scores in favor of WET at the 10-month assessment: The mean score for those receiving WET was 27.7, and the mean score for those receiving PE was 30.1 (odds ratio, 0.72; 95% CI, 0.35-1.46).
Among those who finished treatment, the mean number of treatment sessions was 12.5 for PE and 6 for WET.
Participants assigned to receive PE were significantly more likely to drop out of the study prematurely; 32 (35.6%) dropped out, compared with 11 (12.5%) participants assigned to WET.
Notably, of the 32 participants who dropped out of PE, 30 did so by session 7, so the increased dropout in PE was not related to the greater number of sessions, the investigators noted.
They added that findings could have been limited by stressors related to the global COVID-19 pandemic, which was taking place during the treatment, and the fact that all of the participants were veterans, which could limit the generalizability of the findings.
In an editorial, Charles Taylor, PhD, and Murray Stein, MD, MPH, both from the department of psychiatry at the University of California, San Diego, wrote that “WET achieved comparable reductions in PTSD symptoms through fewer sessions, shorter duration sessions, less therapist involvement, and no explicit prescription of homework.
“These findings should galvanize the psychotherapy field to design parsimonious treatments from the start, systematically testing the effects of different dose parameters,” they concluded.
The study was supported by the VA. Dr. Sloan reported receiving royalty payments for the published Written Exposure Therapy manual from the American Psychological Association outside the submitted work.
A version of this article appeared on Medscape.com.
Investigators also found that participants randomly assigned to receive WET were significantly less likely to drop out of treatment than those receiving PE.
Written exposure therapy involves writing about thoughts and feelings during a specific traumatic event during five supervised, 30-minute sessions and discussing the writing process with the therapist supervising the sessions.
In the latter sessions, the participant talks about how the event affected them.
“Clinicians should consider using WET in their practices as some clients would prefer a shorter treatment approach, and it may be the only option for some clients – for instance, those who have limited time for therapy and may not be able to do a longer treatment,” study investigator Denise Sloan, PhD, said in an interview.
She also noted that WET is covered by insurance and that “most providers I know indicate that they list it as CBT [cognitive-behavioral therapy] code to insurance companies.”
Sloan is senior clinician investigator of the National Center for PTSD at VA Boston Healthcare System and professor of psychiatry at Boston University.
The findings were published online in JAMA Psychiatry.
High attrition rates
The disadvantage to the three major types of therapy used most often to treat PTSD in veterans – eye movement desensitization and reprocessing, cognitive processing therapy (CPT), and PE – are the dropout rates, that range from 18% to as high as 50%.
Prior studies have shown that WET is briefer and just as effective as CPT, but investigators noted that it had never been tested against PE in a randomized clinical trial.
To find out how the two types of therapy compare, Dr. Sloan and associates randomized 178 veterans with PTSD from three VA centers – Boston; Charleston, S.C.; and Madison, Wisc. – to receive either WET or PE.
PE consisted of 8-15 90-minute therapy sessions during which participants imagine the most distressing aspect of their traumatic memory, and between sessions, they confront the people, places, or situations they have been avoiding because of the trauma.
The investigators used the Structured Clinical Interview for DSM-5 at baseline to screen participants at high risk for suicide, comorbid substance use disorder, and unstable bipolar disorder, who were excluded from the study.
At baseline, 10, 20, and 30 weeks after the first treatment session, the investigators measured the severity of each patient’s PTSD symptoms with the Clinician-Administered PTSD Scale for DSM-5, which has a range of 0 (no PTSD symptoms) to 80 (most severe PTSD symptoms).
Of the 178 veterans, 134 were men, and their mean age was 45 years. The majority (63%) was White, while 21% were Black.
The researchers found that study participants were not significantly more likely to meet PTSD diagnostic criteria in the WET or PE conditions at any assessment.
WET briefer, better retention
Investigators noted the largest difference in PTSD scores in favor of WET at the 10-month assessment: The mean score for those receiving WET was 27.7, and the mean score for those receiving PE was 30.1 (odds ratio, 0.72; 95% CI, 0.35-1.46).
Among those who finished treatment, the mean number of treatment sessions was 12.5 for PE and 6 for WET.
Participants assigned to receive PE were significantly more likely to drop out of the study prematurely; 32 (35.6%) dropped out, compared with 11 (12.5%) participants assigned to WET.
Notably, of the 32 participants who dropped out of PE, 30 did so by session 7, so the increased dropout in PE was not related to the greater number of sessions, the investigators noted.
They added that findings could have been limited by stressors related to the global COVID-19 pandemic, which was taking place during the treatment, and the fact that all of the participants were veterans, which could limit the generalizability of the findings.
In an editorial, Charles Taylor, PhD, and Murray Stein, MD, MPH, both from the department of psychiatry at the University of California, San Diego, wrote that “WET achieved comparable reductions in PTSD symptoms through fewer sessions, shorter duration sessions, less therapist involvement, and no explicit prescription of homework.
“These findings should galvanize the psychotherapy field to design parsimonious treatments from the start, systematically testing the effects of different dose parameters,” they concluded.
The study was supported by the VA. Dr. Sloan reported receiving royalty payments for the published Written Exposure Therapy manual from the American Psychological Association outside the submitted work.
A version of this article appeared on Medscape.com.
Investigators also found that participants randomly assigned to receive WET were significantly less likely to drop out of treatment than those receiving PE.
Written exposure therapy involves writing about thoughts and feelings during a specific traumatic event during five supervised, 30-minute sessions and discussing the writing process with the therapist supervising the sessions.
In the latter sessions, the participant talks about how the event affected them.
“Clinicians should consider using WET in their practices as some clients would prefer a shorter treatment approach, and it may be the only option for some clients – for instance, those who have limited time for therapy and may not be able to do a longer treatment,” study investigator Denise Sloan, PhD, said in an interview.
She also noted that WET is covered by insurance and that “most providers I know indicate that they list it as CBT [cognitive-behavioral therapy] code to insurance companies.”
Sloan is senior clinician investigator of the National Center for PTSD at VA Boston Healthcare System and professor of psychiatry at Boston University.
The findings were published online in JAMA Psychiatry.
High attrition rates
The disadvantage to the three major types of therapy used most often to treat PTSD in veterans – eye movement desensitization and reprocessing, cognitive processing therapy (CPT), and PE – are the dropout rates, that range from 18% to as high as 50%.
Prior studies have shown that WET is briefer and just as effective as CPT, but investigators noted that it had never been tested against PE in a randomized clinical trial.
To find out how the two types of therapy compare, Dr. Sloan and associates randomized 178 veterans with PTSD from three VA centers – Boston; Charleston, S.C.; and Madison, Wisc. – to receive either WET or PE.
PE consisted of 8-15 90-minute therapy sessions during which participants imagine the most distressing aspect of their traumatic memory, and between sessions, they confront the people, places, or situations they have been avoiding because of the trauma.
The investigators used the Structured Clinical Interview for DSM-5 at baseline to screen participants at high risk for suicide, comorbid substance use disorder, and unstable bipolar disorder, who were excluded from the study.
At baseline, 10, 20, and 30 weeks after the first treatment session, the investigators measured the severity of each patient’s PTSD symptoms with the Clinician-Administered PTSD Scale for DSM-5, which has a range of 0 (no PTSD symptoms) to 80 (most severe PTSD symptoms).
Of the 178 veterans, 134 were men, and their mean age was 45 years. The majority (63%) was White, while 21% were Black.
The researchers found that study participants were not significantly more likely to meet PTSD diagnostic criteria in the WET or PE conditions at any assessment.
WET briefer, better retention
Investigators noted the largest difference in PTSD scores in favor of WET at the 10-month assessment: The mean score for those receiving WET was 27.7, and the mean score for those receiving PE was 30.1 (odds ratio, 0.72; 95% CI, 0.35-1.46).
Among those who finished treatment, the mean number of treatment sessions was 12.5 for PE and 6 for WET.
Participants assigned to receive PE were significantly more likely to drop out of the study prematurely; 32 (35.6%) dropped out, compared with 11 (12.5%) participants assigned to WET.
Notably, of the 32 participants who dropped out of PE, 30 did so by session 7, so the increased dropout in PE was not related to the greater number of sessions, the investigators noted.
They added that findings could have been limited by stressors related to the global COVID-19 pandemic, which was taking place during the treatment, and the fact that all of the participants were veterans, which could limit the generalizability of the findings.
In an editorial, Charles Taylor, PhD, and Murray Stein, MD, MPH, both from the department of psychiatry at the University of California, San Diego, wrote that “WET achieved comparable reductions in PTSD symptoms through fewer sessions, shorter duration sessions, less therapist involvement, and no explicit prescription of homework.
“These findings should galvanize the psychotherapy field to design parsimonious treatments from the start, systematically testing the effects of different dose parameters,” they concluded.
The study was supported by the VA. Dr. Sloan reported receiving royalty payments for the published Written Exposure Therapy manual from the American Psychological Association outside the submitted work.
A version of this article appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Use of mental health services soared during pandemic
By the end of August 2022, overall use of mental health services was almost 40% higher than before the COVID-19 pandemic, while spending increased by 54%, according to a new study by researchers at the RAND Corporation.
During the early phase of the pandemic, from mid-March to mid-December 2020, before the vaccine was available, in-person visits decreased by 40%, while telehealth visits increased by 1,000%, reported Jonathan H. Cantor, PhD, and colleagues at RAND, and at Castlight Health, a benefit coordination provider, in a paper published online in JAMA Health Forum.
Between December 2020 and August 2022, telehealth visits stayed stable, but in-person visits creeped back up, eventually reaching 80% of prepandemic levels. However, “total utilization was higher than before the pandemic,” Dr. Cantor, a policy researcher at RAND, told this news organization.
“It could be that it’s easier for individuals to receive care via telehealth, but it could also just be that there’s a greater demand or need since the pandemic,” said Dr. Cantor. “We’ll just need more research to actually unpack what’s going on,” he said.
Initial per capita spending increased by about a third and was up overall by more than half. But it’s not clear how much of that is due to utilization or to price of services, said Dr. Cantor. Spending for telehealth services remained stable in the post-vaccine period, while spending on in-person visits returned to prepandemic levels.
Dr. Cantor and his colleagues were not able to determine whether utilization was by new or existing patients, but he said that would be good data to have. “It would be really important to know whether or not folks are initiating care because telehealth is making it easier,” he said.
The authors analyzed about 1.5 million claims for anxiety disorders, major depressive disorder, bipolar disorder, schizophrenia, and posttraumatic stress disorder, out of claims submitted by 7 million commercially insured adults whose self-insured employers used the Castlight benefit.
Dr. Cantor noted that this is just a small subset of the U.S. population. He said he’d like to have data from Medicare and Medicaid to fully assess the impact of the COVID-19 pandemic on mental health and of telehealth visits.
“This is a still-burgeoning field,” he said about telehealth. “We’re still trying to get a handle on how things are operating, given that there’s been so much change so rapidly.”
Meanwhile, 152 major employers responding to a large national survey this summer said that they’ve been grappling with how COVID-19 has affected workers. The employers include 72 Fortune 100 companies and provide health coverage for more than 60 million workers, retirees, and their families.
Seventy-seven percent said they are currently seeing an increase in depression, anxiety, and substance use disorders as a result of the pandemic, according to the Business Group on Health’s survey. That’s up from 44% in 2022.
Going forward, employers will focus on increasing access to mental health services, the survey reported.
“Our survey found that in 2024 and for the near future, employers will be acutely focused on addressing employees’ mental health needs while ensuring access and lowering cost barriers,” Ellen Kelsay, president and CEO of Business Group on Health, said in a statement.
The study was supported by grants from the National Institute of Mental Health and the National Institute on Aging. Coauthor Dena Bravata, MD, a Castlight employee, reported receiving personal fees from Castlight Health during the conduct of the study. Coauthor Christopher M. Whaley, a RAND employee, reported receiving personal fees from Castlight Health outside the submitted work.
A version of this article appeared on Medscape.com.
By the end of August 2022, overall use of mental health services was almost 40% higher than before the COVID-19 pandemic, while spending increased by 54%, according to a new study by researchers at the RAND Corporation.
During the early phase of the pandemic, from mid-March to mid-December 2020, before the vaccine was available, in-person visits decreased by 40%, while telehealth visits increased by 1,000%, reported Jonathan H. Cantor, PhD, and colleagues at RAND, and at Castlight Health, a benefit coordination provider, in a paper published online in JAMA Health Forum.
Between December 2020 and August 2022, telehealth visits stayed stable, but in-person visits creeped back up, eventually reaching 80% of prepandemic levels. However, “total utilization was higher than before the pandemic,” Dr. Cantor, a policy researcher at RAND, told this news organization.
“It could be that it’s easier for individuals to receive care via telehealth, but it could also just be that there’s a greater demand or need since the pandemic,” said Dr. Cantor. “We’ll just need more research to actually unpack what’s going on,” he said.
Initial per capita spending increased by about a third and was up overall by more than half. But it’s not clear how much of that is due to utilization or to price of services, said Dr. Cantor. Spending for telehealth services remained stable in the post-vaccine period, while spending on in-person visits returned to prepandemic levels.
Dr. Cantor and his colleagues were not able to determine whether utilization was by new or existing patients, but he said that would be good data to have. “It would be really important to know whether or not folks are initiating care because telehealth is making it easier,” he said.
The authors analyzed about 1.5 million claims for anxiety disorders, major depressive disorder, bipolar disorder, schizophrenia, and posttraumatic stress disorder, out of claims submitted by 7 million commercially insured adults whose self-insured employers used the Castlight benefit.
Dr. Cantor noted that this is just a small subset of the U.S. population. He said he’d like to have data from Medicare and Medicaid to fully assess the impact of the COVID-19 pandemic on mental health and of telehealth visits.
“This is a still-burgeoning field,” he said about telehealth. “We’re still trying to get a handle on how things are operating, given that there’s been so much change so rapidly.”
Meanwhile, 152 major employers responding to a large national survey this summer said that they’ve been grappling with how COVID-19 has affected workers. The employers include 72 Fortune 100 companies and provide health coverage for more than 60 million workers, retirees, and their families.
Seventy-seven percent said they are currently seeing an increase in depression, anxiety, and substance use disorders as a result of the pandemic, according to the Business Group on Health’s survey. That’s up from 44% in 2022.
Going forward, employers will focus on increasing access to mental health services, the survey reported.
“Our survey found that in 2024 and for the near future, employers will be acutely focused on addressing employees’ mental health needs while ensuring access and lowering cost barriers,” Ellen Kelsay, president and CEO of Business Group on Health, said in a statement.
The study was supported by grants from the National Institute of Mental Health and the National Institute on Aging. Coauthor Dena Bravata, MD, a Castlight employee, reported receiving personal fees from Castlight Health during the conduct of the study. Coauthor Christopher M. Whaley, a RAND employee, reported receiving personal fees from Castlight Health outside the submitted work.
A version of this article appeared on Medscape.com.
By the end of August 2022, overall use of mental health services was almost 40% higher than before the COVID-19 pandemic, while spending increased by 54%, according to a new study by researchers at the RAND Corporation.
During the early phase of the pandemic, from mid-March to mid-December 2020, before the vaccine was available, in-person visits decreased by 40%, while telehealth visits increased by 1,000%, reported Jonathan H. Cantor, PhD, and colleagues at RAND, and at Castlight Health, a benefit coordination provider, in a paper published online in JAMA Health Forum.
Between December 2020 and August 2022, telehealth visits stayed stable, but in-person visits creeped back up, eventually reaching 80% of prepandemic levels. However, “total utilization was higher than before the pandemic,” Dr. Cantor, a policy researcher at RAND, told this news organization.
“It could be that it’s easier for individuals to receive care via telehealth, but it could also just be that there’s a greater demand or need since the pandemic,” said Dr. Cantor. “We’ll just need more research to actually unpack what’s going on,” he said.
Initial per capita spending increased by about a third and was up overall by more than half. But it’s not clear how much of that is due to utilization or to price of services, said Dr. Cantor. Spending for telehealth services remained stable in the post-vaccine period, while spending on in-person visits returned to prepandemic levels.
Dr. Cantor and his colleagues were not able to determine whether utilization was by new or existing patients, but he said that would be good data to have. “It would be really important to know whether or not folks are initiating care because telehealth is making it easier,” he said.
The authors analyzed about 1.5 million claims for anxiety disorders, major depressive disorder, bipolar disorder, schizophrenia, and posttraumatic stress disorder, out of claims submitted by 7 million commercially insured adults whose self-insured employers used the Castlight benefit.
Dr. Cantor noted that this is just a small subset of the U.S. population. He said he’d like to have data from Medicare and Medicaid to fully assess the impact of the COVID-19 pandemic on mental health and of telehealth visits.
“This is a still-burgeoning field,” he said about telehealth. “We’re still trying to get a handle on how things are operating, given that there’s been so much change so rapidly.”
Meanwhile, 152 major employers responding to a large national survey this summer said that they’ve been grappling with how COVID-19 has affected workers. The employers include 72 Fortune 100 companies and provide health coverage for more than 60 million workers, retirees, and their families.
Seventy-seven percent said they are currently seeing an increase in depression, anxiety, and substance use disorders as a result of the pandemic, according to the Business Group on Health’s survey. That’s up from 44% in 2022.
Going forward, employers will focus on increasing access to mental health services, the survey reported.
“Our survey found that in 2024 and for the near future, employers will be acutely focused on addressing employees’ mental health needs while ensuring access and lowering cost barriers,” Ellen Kelsay, president and CEO of Business Group on Health, said in a statement.
The study was supported by grants from the National Institute of Mental Health and the National Institute on Aging. Coauthor Dena Bravata, MD, a Castlight employee, reported receiving personal fees from Castlight Health during the conduct of the study. Coauthor Christopher M. Whaley, a RAND employee, reported receiving personal fees from Castlight Health outside the submitted work.
A version of this article appeared on Medscape.com.
Sleep disturbance may predict increased risk of suicidal thoughts
Suicide remains the second leading cause of death in young adults, but factors that may predict increased suicide risk have not been characterized, wrote Rebecca C. Cox, PhD, of the University of Colorado Boulder, and colleagues.
“Sleep disturbance is a promising modifiable risk factor for acute changes in suicide risk,” they noted. “Previous research has found multiple aspects of sleep disturbance are linked to elevated SI, including insomnia symptoms, both short and long sleep duration, nocturnal wakefulness, and nightmares.”
However, data on the impact of nightly sleep disturbance on suicide risk are limited, the researchers said. They hypothesized that use of ecological momentary assessment (EMA) to assess daily variability in sleep might offer more insight into the relationship between various components of sleep disturbance and changes in suicide risk.
In a study published in Psychiatry Research , the investigators recruited 102 young adults aged 18-35 years who had a history of suicidal behavior; 74.5% were female, 64.7% were White. Participants completed seven semi-random surveys per day for between wake and sleep schedules over 21 days. Each survey asked participants to report on whether they had experienced suicidal ideation (SI) since the last survey. The researchers examined within-person and between-person sleep variables including bedtime, sleep onset latency, sleep onset, number of awakenings, wake after sleep onset, sleep duration, sleep timing, sleep quality, and nightmares.
Overall, nightmares had a significant, positive effect on passive SI at both within- and between-person levels, but no significant effect on active SI. Sleep latency showed a significant, positive effect on passive and active SI at the between-person level, meaning that “participants who took longer to fall asleep on average were more likely to experience passive and active SI during the sampling period,” the researchers noted.
In addition, days following nights of more time awake between sleep onset and offset were days with increased likelihood of passive and active SI. Similarly, days following nights of worse sleep quality than normally reported for an individual were days with increased likelihood of passive and active SI. Sleep timing and duration had no significant effects on SI at the within- or between-person level.
“Notably, tests of reverse models found no relation between daily passive or active SI and any component of the subsequent night’s sleep, suggesting a unidirectional relation between sleep disturbance and subsequent SI,” the researchers wrote in their discussion. If future research replicates the study findings, the results could support the inclusion of sleep difficulties on standard risk assessments as a way to identify risk for SI and initiate prevention approaches, they said.
The findings were limited by several factors including the potential for unmeasured variables impacting the associations between sleep and SI, the researchers noted. Other limitations included the lack of data on more severe levels of SI such as planning and intent, and on suicidal behaviors such as preparatory behaviors, aborted attempts, and actual attempts. The findings also may not generalize to other age groups such as children, adolescents, or older adults, they said.
More research is needed to determine which sleep disturbance components are acute risk factors for which suicide-related outcomes, the researchers said. However, the study is the first to provide evidence for daily sleep disturbances as a near-term predictor of SI in young adults, they concluded.
The study was supported in part by the National Institutes of Health. The researchers had no financial conflicts to disclose.
Suicide remains the second leading cause of death in young adults, but factors that may predict increased suicide risk have not been characterized, wrote Rebecca C. Cox, PhD, of the University of Colorado Boulder, and colleagues.
“Sleep disturbance is a promising modifiable risk factor for acute changes in suicide risk,” they noted. “Previous research has found multiple aspects of sleep disturbance are linked to elevated SI, including insomnia symptoms, both short and long sleep duration, nocturnal wakefulness, and nightmares.”
However, data on the impact of nightly sleep disturbance on suicide risk are limited, the researchers said. They hypothesized that use of ecological momentary assessment (EMA) to assess daily variability in sleep might offer more insight into the relationship between various components of sleep disturbance and changes in suicide risk.
In a study published in Psychiatry Research , the investigators recruited 102 young adults aged 18-35 years who had a history of suicidal behavior; 74.5% were female, 64.7% were White. Participants completed seven semi-random surveys per day for between wake and sleep schedules over 21 days. Each survey asked participants to report on whether they had experienced suicidal ideation (SI) since the last survey. The researchers examined within-person and between-person sleep variables including bedtime, sleep onset latency, sleep onset, number of awakenings, wake after sleep onset, sleep duration, sleep timing, sleep quality, and nightmares.
Overall, nightmares had a significant, positive effect on passive SI at both within- and between-person levels, but no significant effect on active SI. Sleep latency showed a significant, positive effect on passive and active SI at the between-person level, meaning that “participants who took longer to fall asleep on average were more likely to experience passive and active SI during the sampling period,” the researchers noted.
In addition, days following nights of more time awake between sleep onset and offset were days with increased likelihood of passive and active SI. Similarly, days following nights of worse sleep quality than normally reported for an individual were days with increased likelihood of passive and active SI. Sleep timing and duration had no significant effects on SI at the within- or between-person level.
“Notably, tests of reverse models found no relation between daily passive or active SI and any component of the subsequent night’s sleep, suggesting a unidirectional relation between sleep disturbance and subsequent SI,” the researchers wrote in their discussion. If future research replicates the study findings, the results could support the inclusion of sleep difficulties on standard risk assessments as a way to identify risk for SI and initiate prevention approaches, they said.
The findings were limited by several factors including the potential for unmeasured variables impacting the associations between sleep and SI, the researchers noted. Other limitations included the lack of data on more severe levels of SI such as planning and intent, and on suicidal behaviors such as preparatory behaviors, aborted attempts, and actual attempts. The findings also may not generalize to other age groups such as children, adolescents, or older adults, they said.
More research is needed to determine which sleep disturbance components are acute risk factors for which suicide-related outcomes, the researchers said. However, the study is the first to provide evidence for daily sleep disturbances as a near-term predictor of SI in young adults, they concluded.
The study was supported in part by the National Institutes of Health. The researchers had no financial conflicts to disclose.
Suicide remains the second leading cause of death in young adults, but factors that may predict increased suicide risk have not been characterized, wrote Rebecca C. Cox, PhD, of the University of Colorado Boulder, and colleagues.
“Sleep disturbance is a promising modifiable risk factor for acute changes in suicide risk,” they noted. “Previous research has found multiple aspects of sleep disturbance are linked to elevated SI, including insomnia symptoms, both short and long sleep duration, nocturnal wakefulness, and nightmares.”
However, data on the impact of nightly sleep disturbance on suicide risk are limited, the researchers said. They hypothesized that use of ecological momentary assessment (EMA) to assess daily variability in sleep might offer more insight into the relationship between various components of sleep disturbance and changes in suicide risk.
In a study published in Psychiatry Research , the investigators recruited 102 young adults aged 18-35 years who had a history of suicidal behavior; 74.5% were female, 64.7% were White. Participants completed seven semi-random surveys per day for between wake and sleep schedules over 21 days. Each survey asked participants to report on whether they had experienced suicidal ideation (SI) since the last survey. The researchers examined within-person and between-person sleep variables including bedtime, sleep onset latency, sleep onset, number of awakenings, wake after sleep onset, sleep duration, sleep timing, sleep quality, and nightmares.
Overall, nightmares had a significant, positive effect on passive SI at both within- and between-person levels, but no significant effect on active SI. Sleep latency showed a significant, positive effect on passive and active SI at the between-person level, meaning that “participants who took longer to fall asleep on average were more likely to experience passive and active SI during the sampling period,” the researchers noted.
In addition, days following nights of more time awake between sleep onset and offset were days with increased likelihood of passive and active SI. Similarly, days following nights of worse sleep quality than normally reported for an individual were days with increased likelihood of passive and active SI. Sleep timing and duration had no significant effects on SI at the within- or between-person level.
“Notably, tests of reverse models found no relation between daily passive or active SI and any component of the subsequent night’s sleep, suggesting a unidirectional relation between sleep disturbance and subsequent SI,” the researchers wrote in their discussion. If future research replicates the study findings, the results could support the inclusion of sleep difficulties on standard risk assessments as a way to identify risk for SI and initiate prevention approaches, they said.
The findings were limited by several factors including the potential for unmeasured variables impacting the associations between sleep and SI, the researchers noted. Other limitations included the lack of data on more severe levels of SI such as planning and intent, and on suicidal behaviors such as preparatory behaviors, aborted attempts, and actual attempts. The findings also may not generalize to other age groups such as children, adolescents, or older adults, they said.
More research is needed to determine which sleep disturbance components are acute risk factors for which suicide-related outcomes, the researchers said. However, the study is the first to provide evidence for daily sleep disturbances as a near-term predictor of SI in young adults, they concluded.
The study was supported in part by the National Institutes of Health. The researchers had no financial conflicts to disclose.
FROM PSYCHIATRY RESEARCH
The three pillars of perinatal care: Babies, parents, dyadic relationships
Perinatal depression (PND) is the most common obstetric complication in the United States. Even when screening results are positive, mothers often do not receive further evaluation, and even when PND is diagnosed, mothers do not receive evidence-based treatments.
Meta-analytic estimates show that pregnant women suffer from PND at rates from 6.5% to 12.9% across pregnancy to 3-months post partum.1 Women from low-income families and adolescent mothers are at highest risk, where rates are double and triple respectively.
Fathers also suffer from PND, with a prevalence rate from 2% to 25%, increasing to 50% when the mother experiences PND.
The American Academy of Pediatrics issued a Policy Statement (January 2019) about the need to recognize and manage PND. They recommended that pediatric medical homes establish a system to implement the screening of mothers at the 1-, 2-, 4-, and 6-month well-child visits, to use community resources for the treatment and referral of the mother with depression, and to provide support for the maternal-child relationship.2
The American Academy of Pediatrics also recommends advocacy for workforce development for mental health professionals who care for young children and mother-infant dyads, and for promotion of evidence-based interventions focused on healthy attachment and parent-child relationships.
Family research
There is a bidirectional association between family relational stress and PND. Lack of family support is both a predictor and a consequence of perinatal depression. Frequent arguments, conflict because one or both partners did not want the pregnancy, division of labor, poor support following stressful life events, lack of partner availability, and low intimacy are associated with increased perinatal depressive symptoms.
Gender role stress is also included as a risk factor. For example, men may fear performance failure related to work and sex, and women may fear disruption in the couple relationship due to the introduction of a child.
When depressed and nondepressed women at 2 months post delivery were compared, the women with depressive symptoms perceived that their partners did not share similar interests, provided little companionship, expressed disinterest in infant care, did not provide a feeling of connection, did not encourage them to get assistance to cope with difficulties, and expressed disagreement in infant care.3
A high-quality intimate relationship is protective for many illnesses and PND is no exception.4
Assessment
Despite the availability of effective treatments, perinatal mental health utilization rates are strikingly low. There are limited providers and a general lack of awareness of the need for this care. The stigma for assessing and treating PND is high because the perception is that pregnancy is supposed to be a joyous time and with time, PND will pass.
The first step is a timely and accurate assessment of the mother, which should, if possible, include the father and other family support people. The preferred standard for women is the Edinburgh Postnatal Depression Scale (EPDS), a checklist of 10 items (listed below) with a maximum score of 30, and any score over 10 warrants further assessment.5 This scale is used worldwide in obstetric clinics and has been used to identify PND in fathers.
- I have been able to laugh and see the funny side of things.
- I have looked forward with enjoyment to things.
- I have blamed myself unnecessarily when things went wrong.
- I have been anxious or worried for no good reason.
- I have felt scared or panicky for no good reason.
- Things have been getting to me.
- I have been so unhappy that I have had difficulty sleeping.
- I have felt sad or miserable.
- I have been so unhappy that I have been crying.
- The thought of harming myself has occurred to me.
A new ultrabrief tool with only four questions is the Brief Multidimensional Assessment Scale (BMAS), which measures the ability to get things done, emotional support in important relationships, quality of life, and sense of purpose in life. It demonstrates concurrent validity with other measures and discriminates between nonclinical participants and participants from most clinical contexts.6
For those interested in assessing family health, an easy-to-use assessment tool is the 12-item Family Assessment Device (FAD).7
Family therapy interventions
A systematic review and meta-analysis of the current evidence on the usefulness of family therapy interventions in the prevention and treatment of PND identified seven studies.
In these studies, there were statistically significant reductions in depressive symptoms at postintervention in intervention group mothers. Intervention intensity and level of family involvement moderated the impacts of intervention on maternal depression, and there was a trend in improved family functioning in intervention group couples.8
Evidence-based interventions are usually psychoeducational or cognitive-behavioral family therapy models where focused interventions target the following three areas:
- Communication skills related to expectations (including those that pertain to gender roles and the transition to parenthood) and emotional support.
- Conflict management.
- Problem-solving skills related to shared responsibility in infant care and household activities.
Intensive day program for mothers and babies
There is a growing awareness of the effectiveness of specialized mother-baby day hospital programs for women with psychiatric distress during the peripartum period.9
The Women & Infants’ Hospital (WIH) in Providence, R.I., established a mother-baby postpartum depression day program in 2000, adjacent to the obstetrical hospital, the ninth largest obstetrical service in the United States. The day program is integrated with the hospital’s obstetric medicine team and referrals are also accepted from the perinatal practices in the surrounding community. The treatment day includes group, individual, and milieu treatment, as well as consultation with psychiatrists, nutritionists, social workers, lactation specialists and others.
The primary theoretical model utilized by the program is interpersonal psychotherapy (IPT), with essential elements of the program incorporating cognitive behavioral therapy (CBT), and experiential strategies (for instance, mindfulness, breathing, progressive muscle relaxation) to improve self-care and relaxation skills. Patient satisfaction surveys collected from 800 women, (54% identified as White) treated at the program between 2007 and 2012 found that women were highly satisfied with the treatment received, noting that the inclusion of the baby in their treatment is a highly valued aspect of care.
A similar program in Minnesota reported that 328 women who consented to participation in research had significant improvements (P < .001) in self-report scales assessing depression, anxiety, and maternal functioning, improving mental health and parenting functioning.10
Lastly, a recent study out of Brussels, on the benefit of a mother-baby day program analyzed patient data from 2015 and 2020. This clinical population of 92 patients (43% identifying as North African) was comparable to the population of the inpatient mother-baby units in terms of psychosocial fragility except that the parents entering the day program had less severe illnesses, more anxiety disorder, and less postpartum psychosis. In the day program, all the babies improved in terms of symptoms and relationships, except for those with significant developmental difficulties.
The dyadic relationship was measured using “levels of adaptation of the parent–child relationship” scale which has four general levels of adjustment, from well-adjusted to troubled or dangerous relationship. Unlike programs in the United States, this program takes children up to 2.5 years old and the assessment period is up to 8 weeks.11
Prevention of mental illness is best achieved by reducing the known determinants of illness. For PND, the research is clear, so why not start at the earliest possible stage, when we know that change is possible? Pushing health care systems to change is not easy, but as the research accumulates and the positive results grow, our arguments become stronger.
Dr. Heru is a psychiatrist in Aurora, Colo. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at alisonheru@gmail.com.
References
1. Gavin NI et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005 Nov;106(5 Pt 1):1071-83. doi: 10.1097/01.AOG.0000183597.31630.db.
2. Rafferty J et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019 Jan;143(1):e20183260. doi: 10.1542/peds.2018-3260.
3. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
4. Kumar SA et al. Promoting resilience to depression among couples during pregnancy: The protective functions of intimate relationship satisfaction and self-compassion. Family Process. 2022 May;62(1):387-405. doi: 10.1111/famp.12788.
5. Cox JL et al. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987 Jun;150:782-6. doi: 10.1192/bjp.150.6.782.
6. Keitner GI et al. The Brief Multidimensional Assessment Scale (BMAS): A broad measure of patient well-being. Am J Psychother. 2023 Feb 1;76(2):75-81. doi: 10.1176/appi.psychotherapy.20220032.
7. Boterhoven de Haan KL et al. Reliability and validity of a short version of the general functioning subscale of the McMaster Family Assessment Device. Fam Process. 2015 Mar;54(1):116-23. doi: 10.1111/famp.12113.
8. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
9. Battle CL, Howard MM. A mother-baby psychiatric day hospital: History, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014 Jun;7(2):66-70. doi: 10.1177/1753495X13514402.
10. Kim HG et al. Keeping Parent, Child, and Relationship in Mind: Clinical Effectiveness of a Trauma-informed, Multigenerational, Attachment-Based, Mother-Baby Partial Hospital Program in an Urban Safety Net Hospital. Matern Child Health J. 2021 Nov;25(11):1776-86. doi: 10.1007/s10995-021-03221-4.
11. Moureau A et al. A 5 years’ experience of a parent-baby day unit: impact on baby’s development. Front Psychiatry. 2023 June 15;14. doi: 10.3389/fpsyt.2023.1121894.
Perinatal depression (PND) is the most common obstetric complication in the United States. Even when screening results are positive, mothers often do not receive further evaluation, and even when PND is diagnosed, mothers do not receive evidence-based treatments.
Meta-analytic estimates show that pregnant women suffer from PND at rates from 6.5% to 12.9% across pregnancy to 3-months post partum.1 Women from low-income families and adolescent mothers are at highest risk, where rates are double and triple respectively.
Fathers also suffer from PND, with a prevalence rate from 2% to 25%, increasing to 50% when the mother experiences PND.
The American Academy of Pediatrics issued a Policy Statement (January 2019) about the need to recognize and manage PND. They recommended that pediatric medical homes establish a system to implement the screening of mothers at the 1-, 2-, 4-, and 6-month well-child visits, to use community resources for the treatment and referral of the mother with depression, and to provide support for the maternal-child relationship.2
The American Academy of Pediatrics also recommends advocacy for workforce development for mental health professionals who care for young children and mother-infant dyads, and for promotion of evidence-based interventions focused on healthy attachment and parent-child relationships.
Family research
There is a bidirectional association between family relational stress and PND. Lack of family support is both a predictor and a consequence of perinatal depression. Frequent arguments, conflict because one or both partners did not want the pregnancy, division of labor, poor support following stressful life events, lack of partner availability, and low intimacy are associated with increased perinatal depressive symptoms.
Gender role stress is also included as a risk factor. For example, men may fear performance failure related to work and sex, and women may fear disruption in the couple relationship due to the introduction of a child.
When depressed and nondepressed women at 2 months post delivery were compared, the women with depressive symptoms perceived that their partners did not share similar interests, provided little companionship, expressed disinterest in infant care, did not provide a feeling of connection, did not encourage them to get assistance to cope with difficulties, and expressed disagreement in infant care.3
A high-quality intimate relationship is protective for many illnesses and PND is no exception.4
Assessment
Despite the availability of effective treatments, perinatal mental health utilization rates are strikingly low. There are limited providers and a general lack of awareness of the need for this care. The stigma for assessing and treating PND is high because the perception is that pregnancy is supposed to be a joyous time and with time, PND will pass.
The first step is a timely and accurate assessment of the mother, which should, if possible, include the father and other family support people. The preferred standard for women is the Edinburgh Postnatal Depression Scale (EPDS), a checklist of 10 items (listed below) with a maximum score of 30, and any score over 10 warrants further assessment.5 This scale is used worldwide in obstetric clinics and has been used to identify PND in fathers.
- I have been able to laugh and see the funny side of things.
- I have looked forward with enjoyment to things.
- I have blamed myself unnecessarily when things went wrong.
- I have been anxious or worried for no good reason.
- I have felt scared or panicky for no good reason.
- Things have been getting to me.
- I have been so unhappy that I have had difficulty sleeping.
- I have felt sad or miserable.
- I have been so unhappy that I have been crying.
- The thought of harming myself has occurred to me.
A new ultrabrief tool with only four questions is the Brief Multidimensional Assessment Scale (BMAS), which measures the ability to get things done, emotional support in important relationships, quality of life, and sense of purpose in life. It demonstrates concurrent validity with other measures and discriminates between nonclinical participants and participants from most clinical contexts.6
For those interested in assessing family health, an easy-to-use assessment tool is the 12-item Family Assessment Device (FAD).7
Family therapy interventions
A systematic review and meta-analysis of the current evidence on the usefulness of family therapy interventions in the prevention and treatment of PND identified seven studies.
In these studies, there were statistically significant reductions in depressive symptoms at postintervention in intervention group mothers. Intervention intensity and level of family involvement moderated the impacts of intervention on maternal depression, and there was a trend in improved family functioning in intervention group couples.8
Evidence-based interventions are usually psychoeducational or cognitive-behavioral family therapy models where focused interventions target the following three areas:
- Communication skills related to expectations (including those that pertain to gender roles and the transition to parenthood) and emotional support.
- Conflict management.
- Problem-solving skills related to shared responsibility in infant care and household activities.
Intensive day program for mothers and babies
There is a growing awareness of the effectiveness of specialized mother-baby day hospital programs for women with psychiatric distress during the peripartum period.9
The Women & Infants’ Hospital (WIH) in Providence, R.I., established a mother-baby postpartum depression day program in 2000, adjacent to the obstetrical hospital, the ninth largest obstetrical service in the United States. The day program is integrated with the hospital’s obstetric medicine team and referrals are also accepted from the perinatal practices in the surrounding community. The treatment day includes group, individual, and milieu treatment, as well as consultation with psychiatrists, nutritionists, social workers, lactation specialists and others.
The primary theoretical model utilized by the program is interpersonal psychotherapy (IPT), with essential elements of the program incorporating cognitive behavioral therapy (CBT), and experiential strategies (for instance, mindfulness, breathing, progressive muscle relaxation) to improve self-care and relaxation skills. Patient satisfaction surveys collected from 800 women, (54% identified as White) treated at the program between 2007 and 2012 found that women were highly satisfied with the treatment received, noting that the inclusion of the baby in their treatment is a highly valued aspect of care.
A similar program in Minnesota reported that 328 women who consented to participation in research had significant improvements (P < .001) in self-report scales assessing depression, anxiety, and maternal functioning, improving mental health and parenting functioning.10
Lastly, a recent study out of Brussels, on the benefit of a mother-baby day program analyzed patient data from 2015 and 2020. This clinical population of 92 patients (43% identifying as North African) was comparable to the population of the inpatient mother-baby units in terms of psychosocial fragility except that the parents entering the day program had less severe illnesses, more anxiety disorder, and less postpartum psychosis. In the day program, all the babies improved in terms of symptoms and relationships, except for those with significant developmental difficulties.
The dyadic relationship was measured using “levels of adaptation of the parent–child relationship” scale which has four general levels of adjustment, from well-adjusted to troubled or dangerous relationship. Unlike programs in the United States, this program takes children up to 2.5 years old and the assessment period is up to 8 weeks.11
Prevention of mental illness is best achieved by reducing the known determinants of illness. For PND, the research is clear, so why not start at the earliest possible stage, when we know that change is possible? Pushing health care systems to change is not easy, but as the research accumulates and the positive results grow, our arguments become stronger.
Dr. Heru is a psychiatrist in Aurora, Colo. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at alisonheru@gmail.com.
References
1. Gavin NI et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005 Nov;106(5 Pt 1):1071-83. doi: 10.1097/01.AOG.0000183597.31630.db.
2. Rafferty J et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019 Jan;143(1):e20183260. doi: 10.1542/peds.2018-3260.
3. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
4. Kumar SA et al. Promoting resilience to depression among couples during pregnancy: The protective functions of intimate relationship satisfaction and self-compassion. Family Process. 2022 May;62(1):387-405. doi: 10.1111/famp.12788.
5. Cox JL et al. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987 Jun;150:782-6. doi: 10.1192/bjp.150.6.782.
6. Keitner GI et al. The Brief Multidimensional Assessment Scale (BMAS): A broad measure of patient well-being. Am J Psychother. 2023 Feb 1;76(2):75-81. doi: 10.1176/appi.psychotherapy.20220032.
7. Boterhoven de Haan KL et al. Reliability and validity of a short version of the general functioning subscale of the McMaster Family Assessment Device. Fam Process. 2015 Mar;54(1):116-23. doi: 10.1111/famp.12113.
8. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
9. Battle CL, Howard MM. A mother-baby psychiatric day hospital: History, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014 Jun;7(2):66-70. doi: 10.1177/1753495X13514402.
10. Kim HG et al. Keeping Parent, Child, and Relationship in Mind: Clinical Effectiveness of a Trauma-informed, Multigenerational, Attachment-Based, Mother-Baby Partial Hospital Program in an Urban Safety Net Hospital. Matern Child Health J. 2021 Nov;25(11):1776-86. doi: 10.1007/s10995-021-03221-4.
11. Moureau A et al. A 5 years’ experience of a parent-baby day unit: impact on baby’s development. Front Psychiatry. 2023 June 15;14. doi: 10.3389/fpsyt.2023.1121894.
Perinatal depression (PND) is the most common obstetric complication in the United States. Even when screening results are positive, mothers often do not receive further evaluation, and even when PND is diagnosed, mothers do not receive evidence-based treatments.
Meta-analytic estimates show that pregnant women suffer from PND at rates from 6.5% to 12.9% across pregnancy to 3-months post partum.1 Women from low-income families and adolescent mothers are at highest risk, where rates are double and triple respectively.
Fathers also suffer from PND, with a prevalence rate from 2% to 25%, increasing to 50% when the mother experiences PND.
The American Academy of Pediatrics issued a Policy Statement (January 2019) about the need to recognize and manage PND. They recommended that pediatric medical homes establish a system to implement the screening of mothers at the 1-, 2-, 4-, and 6-month well-child visits, to use community resources for the treatment and referral of the mother with depression, and to provide support for the maternal-child relationship.2
The American Academy of Pediatrics also recommends advocacy for workforce development for mental health professionals who care for young children and mother-infant dyads, and for promotion of evidence-based interventions focused on healthy attachment and parent-child relationships.
Family research
There is a bidirectional association between family relational stress and PND. Lack of family support is both a predictor and a consequence of perinatal depression. Frequent arguments, conflict because one or both partners did not want the pregnancy, division of labor, poor support following stressful life events, lack of partner availability, and low intimacy are associated with increased perinatal depressive symptoms.
Gender role stress is also included as a risk factor. For example, men may fear performance failure related to work and sex, and women may fear disruption in the couple relationship due to the introduction of a child.
When depressed and nondepressed women at 2 months post delivery were compared, the women with depressive symptoms perceived that their partners did not share similar interests, provided little companionship, expressed disinterest in infant care, did not provide a feeling of connection, did not encourage them to get assistance to cope with difficulties, and expressed disagreement in infant care.3
A high-quality intimate relationship is protective for many illnesses and PND is no exception.4
Assessment
Despite the availability of effective treatments, perinatal mental health utilization rates are strikingly low. There are limited providers and a general lack of awareness of the need for this care. The stigma for assessing and treating PND is high because the perception is that pregnancy is supposed to be a joyous time and with time, PND will pass.
The first step is a timely and accurate assessment of the mother, which should, if possible, include the father and other family support people. The preferred standard for women is the Edinburgh Postnatal Depression Scale (EPDS), a checklist of 10 items (listed below) with a maximum score of 30, and any score over 10 warrants further assessment.5 This scale is used worldwide in obstetric clinics and has been used to identify PND in fathers.
- I have been able to laugh and see the funny side of things.
- I have looked forward with enjoyment to things.
- I have blamed myself unnecessarily when things went wrong.
- I have been anxious or worried for no good reason.
- I have felt scared or panicky for no good reason.
- Things have been getting to me.
- I have been so unhappy that I have had difficulty sleeping.
- I have felt sad or miserable.
- I have been so unhappy that I have been crying.
- The thought of harming myself has occurred to me.
A new ultrabrief tool with only four questions is the Brief Multidimensional Assessment Scale (BMAS), which measures the ability to get things done, emotional support in important relationships, quality of life, and sense of purpose in life. It demonstrates concurrent validity with other measures and discriminates between nonclinical participants and participants from most clinical contexts.6
For those interested in assessing family health, an easy-to-use assessment tool is the 12-item Family Assessment Device (FAD).7
Family therapy interventions
A systematic review and meta-analysis of the current evidence on the usefulness of family therapy interventions in the prevention and treatment of PND identified seven studies.
In these studies, there were statistically significant reductions in depressive symptoms at postintervention in intervention group mothers. Intervention intensity and level of family involvement moderated the impacts of intervention on maternal depression, and there was a trend in improved family functioning in intervention group couples.8
Evidence-based interventions are usually psychoeducational or cognitive-behavioral family therapy models where focused interventions target the following three areas:
- Communication skills related to expectations (including those that pertain to gender roles and the transition to parenthood) and emotional support.
- Conflict management.
- Problem-solving skills related to shared responsibility in infant care and household activities.
Intensive day program for mothers and babies
There is a growing awareness of the effectiveness of specialized mother-baby day hospital programs for women with psychiatric distress during the peripartum period.9
The Women & Infants’ Hospital (WIH) in Providence, R.I., established a mother-baby postpartum depression day program in 2000, adjacent to the obstetrical hospital, the ninth largest obstetrical service in the United States. The day program is integrated with the hospital’s obstetric medicine team and referrals are also accepted from the perinatal practices in the surrounding community. The treatment day includes group, individual, and milieu treatment, as well as consultation with psychiatrists, nutritionists, social workers, lactation specialists and others.
The primary theoretical model utilized by the program is interpersonal psychotherapy (IPT), with essential elements of the program incorporating cognitive behavioral therapy (CBT), and experiential strategies (for instance, mindfulness, breathing, progressive muscle relaxation) to improve self-care and relaxation skills. Patient satisfaction surveys collected from 800 women, (54% identified as White) treated at the program between 2007 and 2012 found that women were highly satisfied with the treatment received, noting that the inclusion of the baby in their treatment is a highly valued aspect of care.
A similar program in Minnesota reported that 328 women who consented to participation in research had significant improvements (P < .001) in self-report scales assessing depression, anxiety, and maternal functioning, improving mental health and parenting functioning.10
Lastly, a recent study out of Brussels, on the benefit of a mother-baby day program analyzed patient data from 2015 and 2020. This clinical population of 92 patients (43% identifying as North African) was comparable to the population of the inpatient mother-baby units in terms of psychosocial fragility except that the parents entering the day program had less severe illnesses, more anxiety disorder, and less postpartum psychosis. In the day program, all the babies improved in terms of symptoms and relationships, except for those with significant developmental difficulties.
The dyadic relationship was measured using “levels of adaptation of the parent–child relationship” scale which has four general levels of adjustment, from well-adjusted to troubled or dangerous relationship. Unlike programs in the United States, this program takes children up to 2.5 years old and the assessment period is up to 8 weeks.11
Prevention of mental illness is best achieved by reducing the known determinants of illness. For PND, the research is clear, so why not start at the earliest possible stage, when we know that change is possible? Pushing health care systems to change is not easy, but as the research accumulates and the positive results grow, our arguments become stronger.
Dr. Heru is a psychiatrist in Aurora, Colo. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at alisonheru@gmail.com.
References
1. Gavin NI et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005 Nov;106(5 Pt 1):1071-83. doi: 10.1097/01.AOG.0000183597.31630.db.
2. Rafferty J et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019 Jan;143(1):e20183260. doi: 10.1542/peds.2018-3260.
3. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
4. Kumar SA et al. Promoting resilience to depression among couples during pregnancy: The protective functions of intimate relationship satisfaction and self-compassion. Family Process. 2022 May;62(1):387-405. doi: 10.1111/famp.12788.
5. Cox JL et al. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987 Jun;150:782-6. doi: 10.1192/bjp.150.6.782.
6. Keitner GI et al. The Brief Multidimensional Assessment Scale (BMAS): A broad measure of patient well-being. Am J Psychother. 2023 Feb 1;76(2):75-81. doi: 10.1176/appi.psychotherapy.20220032.
7. Boterhoven de Haan KL et al. Reliability and validity of a short version of the general functioning subscale of the McMaster Family Assessment Device. Fam Process. 2015 Mar;54(1):116-23. doi: 10.1111/famp.12113.
8. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
9. Battle CL, Howard MM. A mother-baby psychiatric day hospital: History, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014 Jun;7(2):66-70. doi: 10.1177/1753495X13514402.
10. Kim HG et al. Keeping Parent, Child, and Relationship in Mind: Clinical Effectiveness of a Trauma-informed, Multigenerational, Attachment-Based, Mother-Baby Partial Hospital Program in an Urban Safety Net Hospital. Matern Child Health J. 2021 Nov;25(11):1776-86. doi: 10.1007/s10995-021-03221-4.
11. Moureau A et al. A 5 years’ experience of a parent-baby day unit: impact on baby’s development. Front Psychiatry. 2023 June 15;14. doi: 10.3389/fpsyt.2023.1121894.