User login
Evaluation after a suicide attempt: What to ask
In 2021, suicide was the 11th leading cause of death in the United States.1 Suicide resulted in 49,000 US deaths during 2021; it was the second most common cause of death in individuals age 10 to 34, and the fifth leading cause among children.1,2 Women are 3 to 4 times more likely than men to attempt suicide, but men are 4 times more likely to die by suicide.2
The evaluation of patients with suicidal ideation who have not made an attempt generally involves assessing 4 factors: the specific plan, access to lethal means, any recent social stressors, and the presence of a psychiatric disorder.3 The clinician should also assess which potential deterrents, such as religious beliefs or dependent children, might be present.
Mental health clinicians are often called upon to evaluate a patient after a suicide attempt to assess intent for continued self-harm and to determine appropriate disposition. Such an evaluation must consider multiple factors, including the method used, premeditation, consequences of the attempt, the presence of severe depression and/or psychosis, and the role of substance use. Assessment after a suicide attempt differs from the examination of individuals who harbor suicidal thoughts but have not made an attempt; the latter group may be more likely to respond to interventions such as intensive outpatient care, mobilization of family support, and religious proscriptions against suicide. However, for patients who make an attempt to end their life, whatever potential safeguards or deterrents to suicide that were in place obviously did not prevent the self-harm act. The consequences of the attempt, such as disabling injuries or medical complications, and possible involuntary commitment, need to be considered. Assessment of the patient’s feelings about having survived the attempt is important because the psychological impact of the attempt on family members may serve to intensify the patient’s depression and make a subsequent attempt more likely.
Many individuals who think of suicide have communicated self-harm thoughts or intentions, but such comments are often minimized or ignored. There is a common but erroneous belief that if patients are encouraged to discuss thoughts of self-harm, they will be more likely to act upon them. Because the opposite is true,4 clinicians should ask vulnerable patients about suicidal ideation or intent. Importantly, noncompliance with life-saving medical care, risk-taking behaviors, and substance use may also signal a desire for self-harm. Passive thoughts of death, typified by comments such as “I don’t care whether I wake up or not,” should also be elicited. Many patients who think of suicide speak of being in a “bad place” where reason and logic give way to an intense desire to end their misery.

The evaluation of a patient who has attempted suicide is an important component of providing psychiatric care. This article reflects our 45 years of evaluating such patients. As such, it reflects our clinical experience and is not evidence-based. We offer a checklist of 14 questions that we have found helpful when determining if it would be best for a patient to receive inpatient psychiatric hospitalization or a discharge referral for outpatient care (Table). Questions 1 through 6 are specific for patients who have made a suicide attempt, while questions 7 through 14 are helpful for assessing global risk factors for suicide.
1. Was the attempt premeditated?
Determining premeditation vs impulsivity is an essential element of the assessment following a suicide attempt. Many such acts may occur without forethought in response to an unexpected stressor, such as an altercation between partners or family conflicts. Impulsive attempts can occur when an individual is involved in a distressing event and/or while intoxicated. Conversely, premeditation involves forethought and planning, which may increase the risk of suicide in the near future.
Examples of premeditated behavior include:
- Contemplating the attempt days or weeks beforehand
- Researching the effects of a medication or combination of medications in terms of potential lethality
- Engaging in behavior that would decrease the likelihood of their body being discovered after the attempt
- Obtaining weapons and/or stockpiling pills
- Canvassing potential sites such as bridges or tall buildings
- Engaging in a suicide attempt “practice run”
- Leaving a suicide note or message on social media
- Making funeral arrangements, such as choosing burial clothing
- Writing a will and arranging for the custody of dependent children
- Purchasing life insurance that does not deny payment of benefits in cases of death by suicide.
Continue to: Patients with a premeditated...
Patients with a premeditated suicide attempt generally do not expect to survive and are often surprised or upset that the act was not fatal. The presence of indicators that the attempt was premeditated should direct the disposition more toward hospitalization than discharge. In assessing the impact of premeditation, it is important to gauge not just the examples listed above, but also the patient’s perception of these issues (such as potential loss of child custody). Consider how much the patient is emotionally affected by such thinking.
2. What were the consequences of the attempt?
Assessing the reason for the attempt (if any) and determining whether the inciting circumstance has changed due to the suicide attempt are an important part of the evaluation. A suicide attempt may result in reconciliation with and/or renewed support from family members or partners, who might not have been aware of the patient’s emotional distress. Such unexpected support often results in the patient exhibiting improved mood and affect, and possibly temporary resolution of suicidal thoughts. This “flight into health” may be short-lived, but it also may be enough to engage the patient in a therapeutic alliance. That may permit a discharge with safe disposition to the outpatient clinic while in the custody of a family member, partner, or close friend.
Alternatively, some people experience a troubling worsening of precipitants following a suicide attempt. Preexisting medical conditions and financial, occupational, and/or social woes may be exacerbated. Child custody determinations may be affected, assuming the patient understands the possibility of this adverse consequence. Violent methods may result in disfigurement and body image issues. Individuals from small, close-knit communities may experience stigmatization and unwanted notoriety because of their suicide attempt. Such negative consequences may render some patients more likely to make another attempt to die by suicide. It is crucial to consider how a suicide attempt may have changed the original stress that led to the attempt.
3. Which method was used?
Most fatal suicides in the US are by firearms, and many individuals who survive such attempts do so because of unfamiliarity with the weapon, gun malfunction, faulty aim, or alcohol use.5-7 Some survivors report intending to shoot themselves in the heart, but instead suffered shoulder injuries. Unfortunately, for a patient who survives self-inflicted gunshot wounds, the sequelae of chronic pain, multiple surgical procedures, disability, and disfigurement may serve as constant negative reminders of the event. Some individuals with suicidal intent eschew the idea of using firearms because they hope to avoid having a family member be the first to discover them. Witnessing the aftermath of a fatal suicide by gunshot can induce symptoms of posttraumatic stress disorder in family members and/or partners.8
For a patient with self-inflicted gunshot wounds, always determine whether the weapon has been secured or if the patient still has access to it. Asking about weapon availability is essential during the evaluation of any patient with depression, major life crises, or other factors that may yield a desire to die; this is especially true for individuals with substance use disorders (SUDs). Whenever readily available to such individuals, weapons need to be safely removed.
Continue to: Other self-harm methods...
Other self-harm methods with a high degree of lethality include jumping from bridges or buildings, poisonings, self-immolation, cutting, and hangings. Individuals who choose these approaches generally do not intend to survive. Many of these methods also entail premeditation, as in the case of individuals who canvass bridges and note time when traffic is light so they are less likely to be interrupted. Between 1937 and 2012, there were >1,600 deaths by suicide from San Francisco’s Golden Gate Bridge.9 Patients who choose highly lethal methods are often irritated during the postattempt evaluation because their plans were not fatal. Usually, patients who choose such potentially lethal methods are hospitalized initially on medical and surgical floors, and receive most of their psychiatric care from consultation psychiatrists. Following discharge, these patients may be at high risk for subsequent suicide attempts.
In the US, the most common method of attempting suicide is by overdose.4 Lethality is determined by the agent or combination of substances ingested, the amount taken, the person’s health status, and the length of time before they are discovered. Many patients mistakenly assume that readily available agents such as acetaminophen and aspirin are less likely to be fatal than prescription medications. Evaluators may want to assess for suicidality in individuals with erratic, risk-taking behaviors, who are at especially high risk for death. Learning about the method the patient used can help the clinician determine the imminent risk of another suicide attempt. The more potentially fatal the patient’s method, the more serious their suicide intent, and the higher the risk they will make another suicide attempt, possibly using an even more lethal method.
4. What was the intent?
“What did you want to happen when you made this attempt?” Many patients will respond that they wanted to die, sleep, not wake up, or did not care what happened. Others say it was a gesture to evoke a certain response from another person. If this is the case, it is important to know whether the desired outcome was achieved. These so-called gestures often involve making sure the intended person is aware of the attempt, often by writing a letter, sending a text, or posting on social media. Such behaviors may be exhibited by patients with personality disorders. While such attempts often are impulsive, if the attempt fails to generate the anticipated effect, the patient may try to gain more attention by escalating their suicide actions.
Conversely, if a spouse or partner reconciles with the patient solely because of a suicide attempt, this may set a pattern for future self-harm events in which the patient hopes to achieve the same outcome. Nevertheless, it is better to err for safety because some of these patients will make another attempt, just to prove that they should have been taken more seriously. An exploration of such intent can help the evaluation because even supposed “gestures” can have dangerous consequences. Acts that do not result in the desired outcome should precipitate hospitalization rather than discharge.
5. What facilitated the patient’s rescue?
“Why is this patient still alive?” Determine if the patient did anything to save themself, such as calling an ambulance, inducing emesis, telling someone what they did, or coming to the hospital on their own. If yes, asking them what changed their mind may provide information about what exists in their lives to potentially prevent future attempts, or about wishes to stay alive. These issues can be used to guide outpatient therapy.
Continue to: How does the patient feel about having survived?
6. How does the patient feel about having survived?
When a patient is asked how they feel about having survived a suicide attempt, some will label their act “stupid” and profess embarrassment. Others exhibit future-oriented thought, which is a very good prognostic sign. More ominous is subsequent dysphoria or lamenting that “I could not even do this right.” Patients often express anger toward anyone who rescued them, especially those whose attempts were carefully planned or were discovered by accident. Some patients might also express ambivalence about having survived.
The patient’s response to this question may be shaped by their desire to avoid hospitalization, so beyond their verbal answers, be attentive to clinical cues that may suggest the patient is not being fully transparent. Anger or ambivalence about having survived, a lack of future-oriented thought, and a restricted affect despite verbalizing joy about still being alive are features that suggest psychiatric hospitalization may be warranted.
7. Has the patient made previous suicide attempts?
Compared to individuals with no previous suicide attempts, patients with a history of suicide attempts are 30 to 40 times more likely to die by suicide.2 Many patients who present after a suicide attempt have tried to kill themselves multiple times. Exploring the number of past attempts, how recent the attempts were, and what dispositions were made can be of benefit. Reviewing the potential lethality of past attempts (eg, was hospitalization required, was the patient placed in an intensive care unit, and/or was intubation needed) is recommended. If outpatient care was suggested or medication prescribed, was the patient adherent? Consider asking about passive suicidal behavior, such as not seeking care for medical issues, discontinuing life-saving medication, or engaging in reckless behavior. While such behaviors may not have been classified as a suicide attempt, it might indicate a feeling of indifference toward staying alive. A patient with a past attempt, especially if recent, merits consideration for inpatient care. Once again, referring previously nonadherent patients to outpatient treatment is less likely to be effective.
8. Does the patient have a support network?
Before discharging a patient who has made a suicide attempt, consider the quality of their support network. Gauging the response of the family and friends to the patient’s attempt can be beneficial. Indifference or resentment on the part of loved ones is a bad sign. Some patients have access to support networks they either did not know were available or chose not to utilize. In other instances, after realizing how depressed the patient has been, the family might provide a new safety net. Strong religious affiliations can also be valuable because devout spirituality can be a deterrent to suicide behaviors.10 For an individual whose attempt was motivated by loneliness or feeling unloved or underappreciated, a newly realized support network can be an additional protective deterrent.
9. Does the patient have a family history of suicide?
There may be a familial component to suicide. Knowing about any suicide history in the family contributes to future therapeutic planning. The clinician may want to explore the patient’s family suicide history in detail because such information can have substantial impact on the patient’s motivation for attempting suicide. The evaluator may want to determine if the anniversary of a family suicide is coming. Triggers for a suicide attempt could include the anniversary of a death, birthdays, family-oriented holidays, and similar events. It is productive to understand how the patient feels about family members who have died by suicide. Some will empathize with the deceased, commenting that they did the “right thing.” Others, upon realizing how their own attempt affected others, will be remorseful and determined not to inflict more pain on their family. Such patients may need to be reminded of the misery associated with their family being left without them. These understandings are helpful at setting a safe disposition. However, a history of death by suicide in the family should always be thoroughly evaluated, regardless of the patient’s attitude about that death.
Continue to: Was the attempt the result of depression?
10. Was the attempt the result of depression?
For a patient experiencing depressive symptoms, the prognosis is less positive; they are more likely to harbor serious intent, premeditation, hopelessness, and social isolation, and less likely to express future-oriented thought. They often exhibit a temporary “flight into health.” Such progress is often transitory and may not represent recovery. Because mood disorders may still be present despite a temporary improvement, inpatient and pharmacologic treatment may be needed. If a patient’s suicide attempt occurred as a result of severe depression, it is possible they will make another suicide attempt unless their depression is addressed in a safe and secure setting, such as inpatient hospitalization, or through close family observation while the patient is receiving intensive outpatient treatment.
11. Does the patient have a psychotic disorder?
Many patients with a psychotic illness die following their first attempt without ever having contact with a mental health professional.11 Features of psychosis might include malevolent auditory hallucinations that suggest self-destruction.11 Such “voices” can be intense and self-deprecating; many patients with this type of hallucination report having made a suicide attempt “just to make the voices stop.”
Symptoms of paranoia can make it less likely for individuals with psychosis to confide in family members, friends, or medical personnel. Religious elements are often of a delusional nature and can be dangerous. Psychosis is more difficult to hide than depression and the presence of psychoses concurrent with major depressive disorder (MDD) increases the probability of suicidality.11 Psychosis secondary to substance use may diminish inhibitions and heighten impulsivity, thereby exacerbating the likelihood of self-harm. Usually, the presence of psychotic features precipitating or following a suicide attempt leads to psychiatric hospitalization.
12. Is the patient in a high-risk demographic group?
When evaluating a patient who has attempted suicide, it helps to consider not just what they did, but who they are. Specifically, does the individual belong to a demographic group that traditionally has a high rate of suicide? For example, patients who are Native American or Alaska Natives warrant extra caution.2 Older White males, especially those who are divorced, widowed, retired, and/or have chronic health problems, are also at greater risk. Compared to the general population, individuals age >80 have a massively elevated chance for self-induced death.12 Some of the reasons include:
- medical comorbidities make surviving an attempt less likely
- access to large amounts of medications
- more irreversible issues, such as chronic pain, disability, or widowhood
- living alone, which may delay discovery.
Patients who are members of any of these demographic groups may deserve serious consideration for inpatient psychiatric admission, regardless of other factors.
Continue to: Were drugs or alcohol involved?
13. Were drugs or alcohol involved?
This factor is unique in that it is both a chronic risk factor (SUDs) and a warning sign for imminent suicide, as in the case of an individual who gets intoxicated to disinhibit their fear of death so they can attempt suicide. Alcohol use disorders are associated with depression and suicide. Overdoses by fentanyl and other opiates have become more frequent.13 In many cases, fatalities are unintentional because users overestimate their tolerance or ingest contaminated substances.14 Disinhibition by alcohol and/or other drugs is a risk factor for attempting suicide and can intensify the depth of MDD. Some patients will ingest substances before an attempt just to give them the courage to act; many think of suicide only when intoxicated. Toxicology screens are indicated as part of the evaluation after a suicide attempt.
Depressive and suicidal thoughts often occur in people “coming down” from cocaine or other stimulants. These circumstances require determining whether to refer the patient for treatment for an SUD or psychiatric hospitalization.
In summary, getting intoxicated solely to diminish anxiety about suicide is a dangerous feature, whereas attempting suicide due to intoxication is less concerning. The latter patient may not consider suicide unless they become intoxicated again. When available, dual diagnosis treatment facilities can be an appropriate referral for such patients. Emergency department holding beds can allow these individuals to detoxify prior to the evaluation.
14. Does the patient have future-oriented thoughts?
When evaluating a patient who has attempted suicide, the presence of future planning and anticipation can be reassuring, but these features should be carefully assessed.14-16
After-the-fact comments may be more reliable when a patient offers them spontaneously, as opposed to in response to direct questioning.
- the specificity of the future plans
- corroboration from the family and others about the patient’s previous investment in the upcoming event
- whether the patient mentions such plans spontaneously or only in response to direct questioning
- the patient’s emotional expression or affect when discussing their future
- whether such plans are reasonable, grandiose, and/or unrealistic.
Bottom Line
When assessing a patient after a suicide attempt, both the patient’s presentation and history and the clinician’s instincts are important. Careful consideration of the method, stated intent, premeditation vs impulsivity, feelings about having survived, presence of psychiatric illness, high-risk demographic, postattempt demeanor and affect, quality of support, presence of self-rescue behaviors, future-oriented thoughts, and other factors can help in making the appropriate disposition.
Related Resources
- Kim H, Kim Y, Shin MH, et al. Early psychiatric referral after attempted suicide helps prevent suicide reattempts: a longitudinal national cohort study in South Korea. Front Psychiatry. 2022;13:607892. doi:10.3389/fpsyt.2022.607892
- Michaud L, Berva S, Ostertag L, et al. When to discharge and when to voluntary or compulsory hospitalize? Factors associated with treatment decision after self-harm. Psychiatry Res. 2022;317:114810. doi:10.1016/j.psychres.2022.114810
1. Ten Leading Causes of Death, United States 2020. Centers for Disease Control and Prevention WISQARS. Accessed March 4, 2022. https://wisqars.cdc.gov/data/lcd/home
2. Norris D, Clark MS. Evaluation and treatment of suicidal patients. Am Fam Physician. 2012;15;85(6):602-605.
3. Gliatto MF, Rai AK. Evaluation and treatment patients with suicidal ideation. Am Fam Phys. 1999;59(6):1500-1506.
4. Dazzi T, Gribble R, Wessely S, et al. Does asking about suicide and related behaviors induce suicidal ideation? What is the evidence? Psychol Med. 2014;44(16):3361-3363.
5. Lewiecki EM, Miller SA. Suicide, guns and public policy. Am J Public Health. 2013;103(1):27-31.
6. Frierson RL. Women who shoot themselves. Hosp Community Psychiatry. 1989;40(8):841-843.
7. Frierson RL, Lippmann SB. Psychiatric consultation for patients with self-inflicted gunshot wounds. Psychosomatics. 1990;31(1):67-74.
8. Mitchell AM, Terhorst L. PTSD symptoms in survivors bereaved by the suicide of a significant other. J Am Psychiatr Nurses Assoc. 2017;23(1):61-65.
9. Bateson J. The Golden Gate Bridge’s fatal flaw. Los Angeles Times. May 25, 2012. Accessed March 2, 2022. https://www.latimes.com/opinion/la-xpm-2012-may-25-la-oe-adv-bateson-golden-gate-20120525-story.html
10. Dervic K, Oquendoma MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004;161(12):2303-2308.
11. Nordentoft H, Madsen T, Fedyszyn IF. Suicidal behavior and mortality in first episode psychosis. J Nerv Ment Dis. 2015;203(5):387-392.
12. Frierson R, Lippmann S. Suicide attempts by the old and the very old. Arch Intern Med. 1991;151(1):141-144.
13. Braden JB, Edlund MJ, Sullivan MD. Suicide deaths with opiate poisonings in the United States: 1999-2014. Am J Public Health. 2017;107(3):421-426.
14. Morin KA, Acharya S, Eibl JK, et al: Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treated patients in Ontario, Canada. Int J Drug Policy. 2021;90:103088.
15. Shobassy A, Abu-Mohammad AS. Assessing imminent suicide risk: what about future planning? Current Psychiatry. 2022;21(2):12-17.
16. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
17. Macleod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2010;44(Pt 4):495-504.
In 2021, suicide was the 11th leading cause of death in the United States.1 Suicide resulted in 49,000 US deaths during 2021; it was the second most common cause of death in individuals age 10 to 34, and the fifth leading cause among children.1,2 Women are 3 to 4 times more likely than men to attempt suicide, but men are 4 times more likely to die by suicide.2
The evaluation of patients with suicidal ideation who have not made an attempt generally involves assessing 4 factors: the specific plan, access to lethal means, any recent social stressors, and the presence of a psychiatric disorder.3 The clinician should also assess which potential deterrents, such as religious beliefs or dependent children, might be present.
Mental health clinicians are often called upon to evaluate a patient after a suicide attempt to assess intent for continued self-harm and to determine appropriate disposition. Such an evaluation must consider multiple factors, including the method used, premeditation, consequences of the attempt, the presence of severe depression and/or psychosis, and the role of substance use. Assessment after a suicide attempt differs from the examination of individuals who harbor suicidal thoughts but have not made an attempt; the latter group may be more likely to respond to interventions such as intensive outpatient care, mobilization of family support, and religious proscriptions against suicide. However, for patients who make an attempt to end their life, whatever potential safeguards or deterrents to suicide that were in place obviously did not prevent the self-harm act. The consequences of the attempt, such as disabling injuries or medical complications, and possible involuntary commitment, need to be considered. Assessment of the patient’s feelings about having survived the attempt is important because the psychological impact of the attempt on family members may serve to intensify the patient’s depression and make a subsequent attempt more likely.
Many individuals who think of suicide have communicated self-harm thoughts or intentions, but such comments are often minimized or ignored. There is a common but erroneous belief that if patients are encouraged to discuss thoughts of self-harm, they will be more likely to act upon them. Because the opposite is true,4 clinicians should ask vulnerable patients about suicidal ideation or intent. Importantly, noncompliance with life-saving medical care, risk-taking behaviors, and substance use may also signal a desire for self-harm. Passive thoughts of death, typified by comments such as “I don’t care whether I wake up or not,” should also be elicited. Many patients who think of suicide speak of being in a “bad place” where reason and logic give way to an intense desire to end their misery.

The evaluation of a patient who has attempted suicide is an important component of providing psychiatric care. This article reflects our 45 years of evaluating such patients. As such, it reflects our clinical experience and is not evidence-based. We offer a checklist of 14 questions that we have found helpful when determining if it would be best for a patient to receive inpatient psychiatric hospitalization or a discharge referral for outpatient care (Table). Questions 1 through 6 are specific for patients who have made a suicide attempt, while questions 7 through 14 are helpful for assessing global risk factors for suicide.
1. Was the attempt premeditated?
Determining premeditation vs impulsivity is an essential element of the assessment following a suicide attempt. Many such acts may occur without forethought in response to an unexpected stressor, such as an altercation between partners or family conflicts. Impulsive attempts can occur when an individual is involved in a distressing event and/or while intoxicated. Conversely, premeditation involves forethought and planning, which may increase the risk of suicide in the near future.
Examples of premeditated behavior include:
- Contemplating the attempt days or weeks beforehand
- Researching the effects of a medication or combination of medications in terms of potential lethality
- Engaging in behavior that would decrease the likelihood of their body being discovered after the attempt
- Obtaining weapons and/or stockpiling pills
- Canvassing potential sites such as bridges or tall buildings
- Engaging in a suicide attempt “practice run”
- Leaving a suicide note or message on social media
- Making funeral arrangements, such as choosing burial clothing
- Writing a will and arranging for the custody of dependent children
- Purchasing life insurance that does not deny payment of benefits in cases of death by suicide.
Continue to: Patients with a premeditated...
Patients with a premeditated suicide attempt generally do not expect to survive and are often surprised or upset that the act was not fatal. The presence of indicators that the attempt was premeditated should direct the disposition more toward hospitalization than discharge. In assessing the impact of premeditation, it is important to gauge not just the examples listed above, but also the patient’s perception of these issues (such as potential loss of child custody). Consider how much the patient is emotionally affected by such thinking.
2. What were the consequences of the attempt?
Assessing the reason for the attempt (if any) and determining whether the inciting circumstance has changed due to the suicide attempt are an important part of the evaluation. A suicide attempt may result in reconciliation with and/or renewed support from family members or partners, who might not have been aware of the patient’s emotional distress. Such unexpected support often results in the patient exhibiting improved mood and affect, and possibly temporary resolution of suicidal thoughts. This “flight into health” may be short-lived, but it also may be enough to engage the patient in a therapeutic alliance. That may permit a discharge with safe disposition to the outpatient clinic while in the custody of a family member, partner, or close friend.
Alternatively, some people experience a troubling worsening of precipitants following a suicide attempt. Preexisting medical conditions and financial, occupational, and/or social woes may be exacerbated. Child custody determinations may be affected, assuming the patient understands the possibility of this adverse consequence. Violent methods may result in disfigurement and body image issues. Individuals from small, close-knit communities may experience stigmatization and unwanted notoriety because of their suicide attempt. Such negative consequences may render some patients more likely to make another attempt to die by suicide. It is crucial to consider how a suicide attempt may have changed the original stress that led to the attempt.
3. Which method was used?
Most fatal suicides in the US are by firearms, and many individuals who survive such attempts do so because of unfamiliarity with the weapon, gun malfunction, faulty aim, or alcohol use.5-7 Some survivors report intending to shoot themselves in the heart, but instead suffered shoulder injuries. Unfortunately, for a patient who survives self-inflicted gunshot wounds, the sequelae of chronic pain, multiple surgical procedures, disability, and disfigurement may serve as constant negative reminders of the event. Some individuals with suicidal intent eschew the idea of using firearms because they hope to avoid having a family member be the first to discover them. Witnessing the aftermath of a fatal suicide by gunshot can induce symptoms of posttraumatic stress disorder in family members and/or partners.8
For a patient with self-inflicted gunshot wounds, always determine whether the weapon has been secured or if the patient still has access to it. Asking about weapon availability is essential during the evaluation of any patient with depression, major life crises, or other factors that may yield a desire to die; this is especially true for individuals with substance use disorders (SUDs). Whenever readily available to such individuals, weapons need to be safely removed.
Continue to: Other self-harm methods...
Other self-harm methods with a high degree of lethality include jumping from bridges or buildings, poisonings, self-immolation, cutting, and hangings. Individuals who choose these approaches generally do not intend to survive. Many of these methods also entail premeditation, as in the case of individuals who canvass bridges and note time when traffic is light so they are less likely to be interrupted. Between 1937 and 2012, there were >1,600 deaths by suicide from San Francisco’s Golden Gate Bridge.9 Patients who choose highly lethal methods are often irritated during the postattempt evaluation because their plans were not fatal. Usually, patients who choose such potentially lethal methods are hospitalized initially on medical and surgical floors, and receive most of their psychiatric care from consultation psychiatrists. Following discharge, these patients may be at high risk for subsequent suicide attempts.
In the US, the most common method of attempting suicide is by overdose.4 Lethality is determined by the agent or combination of substances ingested, the amount taken, the person’s health status, and the length of time before they are discovered. Many patients mistakenly assume that readily available agents such as acetaminophen and aspirin are less likely to be fatal than prescription medications. Evaluators may want to assess for suicidality in individuals with erratic, risk-taking behaviors, who are at especially high risk for death. Learning about the method the patient used can help the clinician determine the imminent risk of another suicide attempt. The more potentially fatal the patient’s method, the more serious their suicide intent, and the higher the risk they will make another suicide attempt, possibly using an even more lethal method.
4. What was the intent?
“What did you want to happen when you made this attempt?” Many patients will respond that they wanted to die, sleep, not wake up, or did not care what happened. Others say it was a gesture to evoke a certain response from another person. If this is the case, it is important to know whether the desired outcome was achieved. These so-called gestures often involve making sure the intended person is aware of the attempt, often by writing a letter, sending a text, or posting on social media. Such behaviors may be exhibited by patients with personality disorders. While such attempts often are impulsive, if the attempt fails to generate the anticipated effect, the patient may try to gain more attention by escalating their suicide actions.
Conversely, if a spouse or partner reconciles with the patient solely because of a suicide attempt, this may set a pattern for future self-harm events in which the patient hopes to achieve the same outcome. Nevertheless, it is better to err for safety because some of these patients will make another attempt, just to prove that they should have been taken more seriously. An exploration of such intent can help the evaluation because even supposed “gestures” can have dangerous consequences. Acts that do not result in the desired outcome should precipitate hospitalization rather than discharge.
5. What facilitated the patient’s rescue?
“Why is this patient still alive?” Determine if the patient did anything to save themself, such as calling an ambulance, inducing emesis, telling someone what they did, or coming to the hospital on their own. If yes, asking them what changed their mind may provide information about what exists in their lives to potentially prevent future attempts, or about wishes to stay alive. These issues can be used to guide outpatient therapy.
Continue to: How does the patient feel about having survived?
6. How does the patient feel about having survived?
When a patient is asked how they feel about having survived a suicide attempt, some will label their act “stupid” and profess embarrassment. Others exhibit future-oriented thought, which is a very good prognostic sign. More ominous is subsequent dysphoria or lamenting that “I could not even do this right.” Patients often express anger toward anyone who rescued them, especially those whose attempts were carefully planned or were discovered by accident. Some patients might also express ambivalence about having survived.
The patient’s response to this question may be shaped by their desire to avoid hospitalization, so beyond their verbal answers, be attentive to clinical cues that may suggest the patient is not being fully transparent. Anger or ambivalence about having survived, a lack of future-oriented thought, and a restricted affect despite verbalizing joy about still being alive are features that suggest psychiatric hospitalization may be warranted.
7. Has the patient made previous suicide attempts?
Compared to individuals with no previous suicide attempts, patients with a history of suicide attempts are 30 to 40 times more likely to die by suicide.2 Many patients who present after a suicide attempt have tried to kill themselves multiple times. Exploring the number of past attempts, how recent the attempts were, and what dispositions were made can be of benefit. Reviewing the potential lethality of past attempts (eg, was hospitalization required, was the patient placed in an intensive care unit, and/or was intubation needed) is recommended. If outpatient care was suggested or medication prescribed, was the patient adherent? Consider asking about passive suicidal behavior, such as not seeking care for medical issues, discontinuing life-saving medication, or engaging in reckless behavior. While such behaviors may not have been classified as a suicide attempt, it might indicate a feeling of indifference toward staying alive. A patient with a past attempt, especially if recent, merits consideration for inpatient care. Once again, referring previously nonadherent patients to outpatient treatment is less likely to be effective.
8. Does the patient have a support network?
Before discharging a patient who has made a suicide attempt, consider the quality of their support network. Gauging the response of the family and friends to the patient’s attempt can be beneficial. Indifference or resentment on the part of loved ones is a bad sign. Some patients have access to support networks they either did not know were available or chose not to utilize. In other instances, after realizing how depressed the patient has been, the family might provide a new safety net. Strong religious affiliations can also be valuable because devout spirituality can be a deterrent to suicide behaviors.10 For an individual whose attempt was motivated by loneliness or feeling unloved or underappreciated, a newly realized support network can be an additional protective deterrent.
9. Does the patient have a family history of suicide?
There may be a familial component to suicide. Knowing about any suicide history in the family contributes to future therapeutic planning. The clinician may want to explore the patient’s family suicide history in detail because such information can have substantial impact on the patient’s motivation for attempting suicide. The evaluator may want to determine if the anniversary of a family suicide is coming. Triggers for a suicide attempt could include the anniversary of a death, birthdays, family-oriented holidays, and similar events. It is productive to understand how the patient feels about family members who have died by suicide. Some will empathize with the deceased, commenting that they did the “right thing.” Others, upon realizing how their own attempt affected others, will be remorseful and determined not to inflict more pain on their family. Such patients may need to be reminded of the misery associated with their family being left without them. These understandings are helpful at setting a safe disposition. However, a history of death by suicide in the family should always be thoroughly evaluated, regardless of the patient’s attitude about that death.
Continue to: Was the attempt the result of depression?
10. Was the attempt the result of depression?
For a patient experiencing depressive symptoms, the prognosis is less positive; they are more likely to harbor serious intent, premeditation, hopelessness, and social isolation, and less likely to express future-oriented thought. They often exhibit a temporary “flight into health.” Such progress is often transitory and may not represent recovery. Because mood disorders may still be present despite a temporary improvement, inpatient and pharmacologic treatment may be needed. If a patient’s suicide attempt occurred as a result of severe depression, it is possible they will make another suicide attempt unless their depression is addressed in a safe and secure setting, such as inpatient hospitalization, or through close family observation while the patient is receiving intensive outpatient treatment.
11. Does the patient have a psychotic disorder?
Many patients with a psychotic illness die following their first attempt without ever having contact with a mental health professional.11 Features of psychosis might include malevolent auditory hallucinations that suggest self-destruction.11 Such “voices” can be intense and self-deprecating; many patients with this type of hallucination report having made a suicide attempt “just to make the voices stop.”
Symptoms of paranoia can make it less likely for individuals with psychosis to confide in family members, friends, or medical personnel. Religious elements are often of a delusional nature and can be dangerous. Psychosis is more difficult to hide than depression and the presence of psychoses concurrent with major depressive disorder (MDD) increases the probability of suicidality.11 Psychosis secondary to substance use may diminish inhibitions and heighten impulsivity, thereby exacerbating the likelihood of self-harm. Usually, the presence of psychotic features precipitating or following a suicide attempt leads to psychiatric hospitalization.
12. Is the patient in a high-risk demographic group?
When evaluating a patient who has attempted suicide, it helps to consider not just what they did, but who they are. Specifically, does the individual belong to a demographic group that traditionally has a high rate of suicide? For example, patients who are Native American or Alaska Natives warrant extra caution.2 Older White males, especially those who are divorced, widowed, retired, and/or have chronic health problems, are also at greater risk. Compared to the general population, individuals age >80 have a massively elevated chance for self-induced death.12 Some of the reasons include:
- medical comorbidities make surviving an attempt less likely
- access to large amounts of medications
- more irreversible issues, such as chronic pain, disability, or widowhood
- living alone, which may delay discovery.
Patients who are members of any of these demographic groups may deserve serious consideration for inpatient psychiatric admission, regardless of other factors.
Continue to: Were drugs or alcohol involved?
13. Were drugs or alcohol involved?
This factor is unique in that it is both a chronic risk factor (SUDs) and a warning sign for imminent suicide, as in the case of an individual who gets intoxicated to disinhibit their fear of death so they can attempt suicide. Alcohol use disorders are associated with depression and suicide. Overdoses by fentanyl and other opiates have become more frequent.13 In many cases, fatalities are unintentional because users overestimate their tolerance or ingest contaminated substances.14 Disinhibition by alcohol and/or other drugs is a risk factor for attempting suicide and can intensify the depth of MDD. Some patients will ingest substances before an attempt just to give them the courage to act; many think of suicide only when intoxicated. Toxicology screens are indicated as part of the evaluation after a suicide attempt.
Depressive and suicidal thoughts often occur in people “coming down” from cocaine or other stimulants. These circumstances require determining whether to refer the patient for treatment for an SUD or psychiatric hospitalization.
In summary, getting intoxicated solely to diminish anxiety about suicide is a dangerous feature, whereas attempting suicide due to intoxication is less concerning. The latter patient may not consider suicide unless they become intoxicated again. When available, dual diagnosis treatment facilities can be an appropriate referral for such patients. Emergency department holding beds can allow these individuals to detoxify prior to the evaluation.
14. Does the patient have future-oriented thoughts?
When evaluating a patient who has attempted suicide, the presence of future planning and anticipation can be reassuring, but these features should be carefully assessed.14-16
After-the-fact comments may be more reliable when a patient offers them spontaneously, as opposed to in response to direct questioning.
- the specificity of the future plans
- corroboration from the family and others about the patient’s previous investment in the upcoming event
- whether the patient mentions such plans spontaneously or only in response to direct questioning
- the patient’s emotional expression or affect when discussing their future
- whether such plans are reasonable, grandiose, and/or unrealistic.
Bottom Line
When assessing a patient after a suicide attempt, both the patient’s presentation and history and the clinician’s instincts are important. Careful consideration of the method, stated intent, premeditation vs impulsivity, feelings about having survived, presence of psychiatric illness, high-risk demographic, postattempt demeanor and affect, quality of support, presence of self-rescue behaviors, future-oriented thoughts, and other factors can help in making the appropriate disposition.
Related Resources
- Kim H, Kim Y, Shin MH, et al. Early psychiatric referral after attempted suicide helps prevent suicide reattempts: a longitudinal national cohort study in South Korea. Front Psychiatry. 2022;13:607892. doi:10.3389/fpsyt.2022.607892
- Michaud L, Berva S, Ostertag L, et al. When to discharge and when to voluntary or compulsory hospitalize? Factors associated with treatment decision after self-harm. Psychiatry Res. 2022;317:114810. doi:10.1016/j.psychres.2022.114810
In 2021, suicide was the 11th leading cause of death in the United States.1 Suicide resulted in 49,000 US deaths during 2021; it was the second most common cause of death in individuals age 10 to 34, and the fifth leading cause among children.1,2 Women are 3 to 4 times more likely than men to attempt suicide, but men are 4 times more likely to die by suicide.2
The evaluation of patients with suicidal ideation who have not made an attempt generally involves assessing 4 factors: the specific plan, access to lethal means, any recent social stressors, and the presence of a psychiatric disorder.3 The clinician should also assess which potential deterrents, such as religious beliefs or dependent children, might be present.
Mental health clinicians are often called upon to evaluate a patient after a suicide attempt to assess intent for continued self-harm and to determine appropriate disposition. Such an evaluation must consider multiple factors, including the method used, premeditation, consequences of the attempt, the presence of severe depression and/or psychosis, and the role of substance use. Assessment after a suicide attempt differs from the examination of individuals who harbor suicidal thoughts but have not made an attempt; the latter group may be more likely to respond to interventions such as intensive outpatient care, mobilization of family support, and religious proscriptions against suicide. However, for patients who make an attempt to end their life, whatever potential safeguards or deterrents to suicide that were in place obviously did not prevent the self-harm act. The consequences of the attempt, such as disabling injuries or medical complications, and possible involuntary commitment, need to be considered. Assessment of the patient’s feelings about having survived the attempt is important because the psychological impact of the attempt on family members may serve to intensify the patient’s depression and make a subsequent attempt more likely.
Many individuals who think of suicide have communicated self-harm thoughts or intentions, but such comments are often minimized or ignored. There is a common but erroneous belief that if patients are encouraged to discuss thoughts of self-harm, they will be more likely to act upon them. Because the opposite is true,4 clinicians should ask vulnerable patients about suicidal ideation or intent. Importantly, noncompliance with life-saving medical care, risk-taking behaviors, and substance use may also signal a desire for self-harm. Passive thoughts of death, typified by comments such as “I don’t care whether I wake up or not,” should also be elicited. Many patients who think of suicide speak of being in a “bad place” where reason and logic give way to an intense desire to end their misery.

The evaluation of a patient who has attempted suicide is an important component of providing psychiatric care. This article reflects our 45 years of evaluating such patients. As such, it reflects our clinical experience and is not evidence-based. We offer a checklist of 14 questions that we have found helpful when determining if it would be best for a patient to receive inpatient psychiatric hospitalization or a discharge referral for outpatient care (Table). Questions 1 through 6 are specific for patients who have made a suicide attempt, while questions 7 through 14 are helpful for assessing global risk factors for suicide.
1. Was the attempt premeditated?
Determining premeditation vs impulsivity is an essential element of the assessment following a suicide attempt. Many such acts may occur without forethought in response to an unexpected stressor, such as an altercation between partners or family conflicts. Impulsive attempts can occur when an individual is involved in a distressing event and/or while intoxicated. Conversely, premeditation involves forethought and planning, which may increase the risk of suicide in the near future.
Examples of premeditated behavior include:
- Contemplating the attempt days or weeks beforehand
- Researching the effects of a medication or combination of medications in terms of potential lethality
- Engaging in behavior that would decrease the likelihood of their body being discovered after the attempt
- Obtaining weapons and/or stockpiling pills
- Canvassing potential sites such as bridges or tall buildings
- Engaging in a suicide attempt “practice run”
- Leaving a suicide note or message on social media
- Making funeral arrangements, such as choosing burial clothing
- Writing a will and arranging for the custody of dependent children
- Purchasing life insurance that does not deny payment of benefits in cases of death by suicide.
Continue to: Patients with a premeditated...
Patients with a premeditated suicide attempt generally do not expect to survive and are often surprised or upset that the act was not fatal. The presence of indicators that the attempt was premeditated should direct the disposition more toward hospitalization than discharge. In assessing the impact of premeditation, it is important to gauge not just the examples listed above, but also the patient’s perception of these issues (such as potential loss of child custody). Consider how much the patient is emotionally affected by such thinking.
2. What were the consequences of the attempt?
Assessing the reason for the attempt (if any) and determining whether the inciting circumstance has changed due to the suicide attempt are an important part of the evaluation. A suicide attempt may result in reconciliation with and/or renewed support from family members or partners, who might not have been aware of the patient’s emotional distress. Such unexpected support often results in the patient exhibiting improved mood and affect, and possibly temporary resolution of suicidal thoughts. This “flight into health” may be short-lived, but it also may be enough to engage the patient in a therapeutic alliance. That may permit a discharge with safe disposition to the outpatient clinic while in the custody of a family member, partner, or close friend.
Alternatively, some people experience a troubling worsening of precipitants following a suicide attempt. Preexisting medical conditions and financial, occupational, and/or social woes may be exacerbated. Child custody determinations may be affected, assuming the patient understands the possibility of this adverse consequence. Violent methods may result in disfigurement and body image issues. Individuals from small, close-knit communities may experience stigmatization and unwanted notoriety because of their suicide attempt. Such negative consequences may render some patients more likely to make another attempt to die by suicide. It is crucial to consider how a suicide attempt may have changed the original stress that led to the attempt.
3. Which method was used?
Most fatal suicides in the US are by firearms, and many individuals who survive such attempts do so because of unfamiliarity with the weapon, gun malfunction, faulty aim, or alcohol use.5-7 Some survivors report intending to shoot themselves in the heart, but instead suffered shoulder injuries. Unfortunately, for a patient who survives self-inflicted gunshot wounds, the sequelae of chronic pain, multiple surgical procedures, disability, and disfigurement may serve as constant negative reminders of the event. Some individuals with suicidal intent eschew the idea of using firearms because they hope to avoid having a family member be the first to discover them. Witnessing the aftermath of a fatal suicide by gunshot can induce symptoms of posttraumatic stress disorder in family members and/or partners.8
For a patient with self-inflicted gunshot wounds, always determine whether the weapon has been secured or if the patient still has access to it. Asking about weapon availability is essential during the evaluation of any patient with depression, major life crises, or other factors that may yield a desire to die; this is especially true for individuals with substance use disorders (SUDs). Whenever readily available to such individuals, weapons need to be safely removed.
Continue to: Other self-harm methods...
Other self-harm methods with a high degree of lethality include jumping from bridges or buildings, poisonings, self-immolation, cutting, and hangings. Individuals who choose these approaches generally do not intend to survive. Many of these methods also entail premeditation, as in the case of individuals who canvass bridges and note time when traffic is light so they are less likely to be interrupted. Between 1937 and 2012, there were >1,600 deaths by suicide from San Francisco’s Golden Gate Bridge.9 Patients who choose highly lethal methods are often irritated during the postattempt evaluation because their plans were not fatal. Usually, patients who choose such potentially lethal methods are hospitalized initially on medical and surgical floors, and receive most of their psychiatric care from consultation psychiatrists. Following discharge, these patients may be at high risk for subsequent suicide attempts.
In the US, the most common method of attempting suicide is by overdose.4 Lethality is determined by the agent or combination of substances ingested, the amount taken, the person’s health status, and the length of time before they are discovered. Many patients mistakenly assume that readily available agents such as acetaminophen and aspirin are less likely to be fatal than prescription medications. Evaluators may want to assess for suicidality in individuals with erratic, risk-taking behaviors, who are at especially high risk for death. Learning about the method the patient used can help the clinician determine the imminent risk of another suicide attempt. The more potentially fatal the patient’s method, the more serious their suicide intent, and the higher the risk they will make another suicide attempt, possibly using an even more lethal method.
4. What was the intent?
“What did you want to happen when you made this attempt?” Many patients will respond that they wanted to die, sleep, not wake up, or did not care what happened. Others say it was a gesture to evoke a certain response from another person. If this is the case, it is important to know whether the desired outcome was achieved. These so-called gestures often involve making sure the intended person is aware of the attempt, often by writing a letter, sending a text, or posting on social media. Such behaviors may be exhibited by patients with personality disorders. While such attempts often are impulsive, if the attempt fails to generate the anticipated effect, the patient may try to gain more attention by escalating their suicide actions.
Conversely, if a spouse or partner reconciles with the patient solely because of a suicide attempt, this may set a pattern for future self-harm events in which the patient hopes to achieve the same outcome. Nevertheless, it is better to err for safety because some of these patients will make another attempt, just to prove that they should have been taken more seriously. An exploration of such intent can help the evaluation because even supposed “gestures” can have dangerous consequences. Acts that do not result in the desired outcome should precipitate hospitalization rather than discharge.
5. What facilitated the patient’s rescue?
“Why is this patient still alive?” Determine if the patient did anything to save themself, such as calling an ambulance, inducing emesis, telling someone what they did, or coming to the hospital on their own. If yes, asking them what changed their mind may provide information about what exists in their lives to potentially prevent future attempts, or about wishes to stay alive. These issues can be used to guide outpatient therapy.
Continue to: How does the patient feel about having survived?
6. How does the patient feel about having survived?
When a patient is asked how they feel about having survived a suicide attempt, some will label their act “stupid” and profess embarrassment. Others exhibit future-oriented thought, which is a very good prognostic sign. More ominous is subsequent dysphoria or lamenting that “I could not even do this right.” Patients often express anger toward anyone who rescued them, especially those whose attempts were carefully planned or were discovered by accident. Some patients might also express ambivalence about having survived.
The patient’s response to this question may be shaped by their desire to avoid hospitalization, so beyond their verbal answers, be attentive to clinical cues that may suggest the patient is not being fully transparent. Anger or ambivalence about having survived, a lack of future-oriented thought, and a restricted affect despite verbalizing joy about still being alive are features that suggest psychiatric hospitalization may be warranted.
7. Has the patient made previous suicide attempts?
Compared to individuals with no previous suicide attempts, patients with a history of suicide attempts are 30 to 40 times more likely to die by suicide.2 Many patients who present after a suicide attempt have tried to kill themselves multiple times. Exploring the number of past attempts, how recent the attempts were, and what dispositions were made can be of benefit. Reviewing the potential lethality of past attempts (eg, was hospitalization required, was the patient placed in an intensive care unit, and/or was intubation needed) is recommended. If outpatient care was suggested or medication prescribed, was the patient adherent? Consider asking about passive suicidal behavior, such as not seeking care for medical issues, discontinuing life-saving medication, or engaging in reckless behavior. While such behaviors may not have been classified as a suicide attempt, it might indicate a feeling of indifference toward staying alive. A patient with a past attempt, especially if recent, merits consideration for inpatient care. Once again, referring previously nonadherent patients to outpatient treatment is less likely to be effective.
8. Does the patient have a support network?
Before discharging a patient who has made a suicide attempt, consider the quality of their support network. Gauging the response of the family and friends to the patient’s attempt can be beneficial. Indifference or resentment on the part of loved ones is a bad sign. Some patients have access to support networks they either did not know were available or chose not to utilize. In other instances, after realizing how depressed the patient has been, the family might provide a new safety net. Strong religious affiliations can also be valuable because devout spirituality can be a deterrent to suicide behaviors.10 For an individual whose attempt was motivated by loneliness or feeling unloved or underappreciated, a newly realized support network can be an additional protective deterrent.
9. Does the patient have a family history of suicide?
There may be a familial component to suicide. Knowing about any suicide history in the family contributes to future therapeutic planning. The clinician may want to explore the patient’s family suicide history in detail because such information can have substantial impact on the patient’s motivation for attempting suicide. The evaluator may want to determine if the anniversary of a family suicide is coming. Triggers for a suicide attempt could include the anniversary of a death, birthdays, family-oriented holidays, and similar events. It is productive to understand how the patient feels about family members who have died by suicide. Some will empathize with the deceased, commenting that they did the “right thing.” Others, upon realizing how their own attempt affected others, will be remorseful and determined not to inflict more pain on their family. Such patients may need to be reminded of the misery associated with their family being left without them. These understandings are helpful at setting a safe disposition. However, a history of death by suicide in the family should always be thoroughly evaluated, regardless of the patient’s attitude about that death.
Continue to: Was the attempt the result of depression?
10. Was the attempt the result of depression?
For a patient experiencing depressive symptoms, the prognosis is less positive; they are more likely to harbor serious intent, premeditation, hopelessness, and social isolation, and less likely to express future-oriented thought. They often exhibit a temporary “flight into health.” Such progress is often transitory and may not represent recovery. Because mood disorders may still be present despite a temporary improvement, inpatient and pharmacologic treatment may be needed. If a patient’s suicide attempt occurred as a result of severe depression, it is possible they will make another suicide attempt unless their depression is addressed in a safe and secure setting, such as inpatient hospitalization, or through close family observation while the patient is receiving intensive outpatient treatment.
11. Does the patient have a psychotic disorder?
Many patients with a psychotic illness die following their first attempt without ever having contact with a mental health professional.11 Features of psychosis might include malevolent auditory hallucinations that suggest self-destruction.11 Such “voices” can be intense and self-deprecating; many patients with this type of hallucination report having made a suicide attempt “just to make the voices stop.”
Symptoms of paranoia can make it less likely for individuals with psychosis to confide in family members, friends, or medical personnel. Religious elements are often of a delusional nature and can be dangerous. Psychosis is more difficult to hide than depression and the presence of psychoses concurrent with major depressive disorder (MDD) increases the probability of suicidality.11 Psychosis secondary to substance use may diminish inhibitions and heighten impulsivity, thereby exacerbating the likelihood of self-harm. Usually, the presence of psychotic features precipitating or following a suicide attempt leads to psychiatric hospitalization.
12. Is the patient in a high-risk demographic group?
When evaluating a patient who has attempted suicide, it helps to consider not just what they did, but who they are. Specifically, does the individual belong to a demographic group that traditionally has a high rate of suicide? For example, patients who are Native American or Alaska Natives warrant extra caution.2 Older White males, especially those who are divorced, widowed, retired, and/or have chronic health problems, are also at greater risk. Compared to the general population, individuals age >80 have a massively elevated chance for self-induced death.12 Some of the reasons include:
- medical comorbidities make surviving an attempt less likely
- access to large amounts of medications
- more irreversible issues, such as chronic pain, disability, or widowhood
- living alone, which may delay discovery.
Patients who are members of any of these demographic groups may deserve serious consideration for inpatient psychiatric admission, regardless of other factors.
Continue to: Were drugs or alcohol involved?
13. Were drugs or alcohol involved?
This factor is unique in that it is both a chronic risk factor (SUDs) and a warning sign for imminent suicide, as in the case of an individual who gets intoxicated to disinhibit their fear of death so they can attempt suicide. Alcohol use disorders are associated with depression and suicide. Overdoses by fentanyl and other opiates have become more frequent.13 In many cases, fatalities are unintentional because users overestimate their tolerance or ingest contaminated substances.14 Disinhibition by alcohol and/or other drugs is a risk factor for attempting suicide and can intensify the depth of MDD. Some patients will ingest substances before an attempt just to give them the courage to act; many think of suicide only when intoxicated. Toxicology screens are indicated as part of the evaluation after a suicide attempt.
Depressive and suicidal thoughts often occur in people “coming down” from cocaine or other stimulants. These circumstances require determining whether to refer the patient for treatment for an SUD or psychiatric hospitalization.
In summary, getting intoxicated solely to diminish anxiety about suicide is a dangerous feature, whereas attempting suicide due to intoxication is less concerning. The latter patient may not consider suicide unless they become intoxicated again. When available, dual diagnosis treatment facilities can be an appropriate referral for such patients. Emergency department holding beds can allow these individuals to detoxify prior to the evaluation.
14. Does the patient have future-oriented thoughts?
When evaluating a patient who has attempted suicide, the presence of future planning and anticipation can be reassuring, but these features should be carefully assessed.14-16
After-the-fact comments may be more reliable when a patient offers them spontaneously, as opposed to in response to direct questioning.
- the specificity of the future plans
- corroboration from the family and others about the patient’s previous investment in the upcoming event
- whether the patient mentions such plans spontaneously or only in response to direct questioning
- the patient’s emotional expression or affect when discussing their future
- whether such plans are reasonable, grandiose, and/or unrealistic.
Bottom Line
When assessing a patient after a suicide attempt, both the patient’s presentation and history and the clinician’s instincts are important. Careful consideration of the method, stated intent, premeditation vs impulsivity, feelings about having survived, presence of psychiatric illness, high-risk demographic, postattempt demeanor and affect, quality of support, presence of self-rescue behaviors, future-oriented thoughts, and other factors can help in making the appropriate disposition.
Related Resources
- Kim H, Kim Y, Shin MH, et al. Early psychiatric referral after attempted suicide helps prevent suicide reattempts: a longitudinal national cohort study in South Korea. Front Psychiatry. 2022;13:607892. doi:10.3389/fpsyt.2022.607892
- Michaud L, Berva S, Ostertag L, et al. When to discharge and when to voluntary or compulsory hospitalize? Factors associated with treatment decision after self-harm. Psychiatry Res. 2022;317:114810. doi:10.1016/j.psychres.2022.114810
1. Ten Leading Causes of Death, United States 2020. Centers for Disease Control and Prevention WISQARS. Accessed March 4, 2022. https://wisqars.cdc.gov/data/lcd/home
2. Norris D, Clark MS. Evaluation and treatment of suicidal patients. Am Fam Physician. 2012;15;85(6):602-605.
3. Gliatto MF, Rai AK. Evaluation and treatment patients with suicidal ideation. Am Fam Phys. 1999;59(6):1500-1506.
4. Dazzi T, Gribble R, Wessely S, et al. Does asking about suicide and related behaviors induce suicidal ideation? What is the evidence? Psychol Med. 2014;44(16):3361-3363.
5. Lewiecki EM, Miller SA. Suicide, guns and public policy. Am J Public Health. 2013;103(1):27-31.
6. Frierson RL. Women who shoot themselves. Hosp Community Psychiatry. 1989;40(8):841-843.
7. Frierson RL, Lippmann SB. Psychiatric consultation for patients with self-inflicted gunshot wounds. Psychosomatics. 1990;31(1):67-74.
8. Mitchell AM, Terhorst L. PTSD symptoms in survivors bereaved by the suicide of a significant other. J Am Psychiatr Nurses Assoc. 2017;23(1):61-65.
9. Bateson J. The Golden Gate Bridge’s fatal flaw. Los Angeles Times. May 25, 2012. Accessed March 2, 2022. https://www.latimes.com/opinion/la-xpm-2012-may-25-la-oe-adv-bateson-golden-gate-20120525-story.html
10. Dervic K, Oquendoma MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004;161(12):2303-2308.
11. Nordentoft H, Madsen T, Fedyszyn IF. Suicidal behavior and mortality in first episode psychosis. J Nerv Ment Dis. 2015;203(5):387-392.
12. Frierson R, Lippmann S. Suicide attempts by the old and the very old. Arch Intern Med. 1991;151(1):141-144.
13. Braden JB, Edlund MJ, Sullivan MD. Suicide deaths with opiate poisonings in the United States: 1999-2014. Am J Public Health. 2017;107(3):421-426.
14. Morin KA, Acharya S, Eibl JK, et al: Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treated patients in Ontario, Canada. Int J Drug Policy. 2021;90:103088.
15. Shobassy A, Abu-Mohammad AS. Assessing imminent suicide risk: what about future planning? Current Psychiatry. 2022;21(2):12-17.
16. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
17. Macleod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2010;44(Pt 4):495-504.
1. Ten Leading Causes of Death, United States 2020. Centers for Disease Control and Prevention WISQARS. Accessed March 4, 2022. https://wisqars.cdc.gov/data/lcd/home
2. Norris D, Clark MS. Evaluation and treatment of suicidal patients. Am Fam Physician. 2012;15;85(6):602-605.
3. Gliatto MF, Rai AK. Evaluation and treatment patients with suicidal ideation. Am Fam Phys. 1999;59(6):1500-1506.
4. Dazzi T, Gribble R, Wessely S, et al. Does asking about suicide and related behaviors induce suicidal ideation? What is the evidence? Psychol Med. 2014;44(16):3361-3363.
5. Lewiecki EM, Miller SA. Suicide, guns and public policy. Am J Public Health. 2013;103(1):27-31.
6. Frierson RL. Women who shoot themselves. Hosp Community Psychiatry. 1989;40(8):841-843.
7. Frierson RL, Lippmann SB. Psychiatric consultation for patients with self-inflicted gunshot wounds. Psychosomatics. 1990;31(1):67-74.
8. Mitchell AM, Terhorst L. PTSD symptoms in survivors bereaved by the suicide of a significant other. J Am Psychiatr Nurses Assoc. 2017;23(1):61-65.
9. Bateson J. The Golden Gate Bridge’s fatal flaw. Los Angeles Times. May 25, 2012. Accessed March 2, 2022. https://www.latimes.com/opinion/la-xpm-2012-may-25-la-oe-adv-bateson-golden-gate-20120525-story.html
10. Dervic K, Oquendoma MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004;161(12):2303-2308.
11. Nordentoft H, Madsen T, Fedyszyn IF. Suicidal behavior and mortality in first episode psychosis. J Nerv Ment Dis. 2015;203(5):387-392.
12. Frierson R, Lippmann S. Suicide attempts by the old and the very old. Arch Intern Med. 1991;151(1):141-144.
13. Braden JB, Edlund MJ, Sullivan MD. Suicide deaths with opiate poisonings in the United States: 1999-2014. Am J Public Health. 2017;107(3):421-426.
14. Morin KA, Acharya S, Eibl JK, et al: Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treated patients in Ontario, Canada. Int J Drug Policy. 2021;90:103088.
15. Shobassy A, Abu-Mohammad AS. Assessing imminent suicide risk: what about future planning? Current Psychiatry. 2022;21(2):12-17.
16. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
17. Macleod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2010;44(Pt 4):495-504.
Depression guidelines fall short in characterizing withdrawal
Previous research suggests that approximately half of patients who discontinue or decrease dosage of antidepressants experience withdrawal symptoms, wrote Anders Sørensen, MD, of Copenhagen University Hospital, and colleagues. These symptoms are diverse and may include flulike symptoms, fatigue, anxiety, and sensations of electric shock, they noted. Most withdrawal effects last for a few weeks, but some persist for months or years, sometimes described as persistent postwithdrawal disorder, they added.
“Symptoms of withdrawal and depression overlap considerably but constitute two fundamentally different clinical conditions, which makes it important to distinguish between the two,” the researchers emphasized.
In a study published in the Journal of Affective Disorders, the researchers identified 21 clinical practice guidelines (CPGs) for depression published between 1998 and 2022. The guidelines were published in the United Kingdom, the United States, Canada, Australia, Singapore, Ireland, and New Zealand. They compared descriptions of withdrawal from antidepressants and calculated the proportion of CPGs with different information.
Overall, 15 of the 21 studies in the review (71%) noted that antidepressants are associated with withdrawal symptoms, but less than half (43%) used the term “withdrawal symptoms,” or similar. Of the nine guidelines that mentioned withdrawal symptoms, five used the term interchangeably with “discontinuation symptoms” and six used the term “discontinuation symptoms” only when discussing antidepressant withdrawal. In addition, six CPGs specifically stated that patients who stop antidepressants can experience withdrawal symptoms, and five stated that these symptoms also can occur in patients who are reducing or tapering their doses.
The type of withdrawal symptoms was mentioned in 10 CPGs, and the other 11 had no information on potential withdrawal symptoms, the researchers noted. Of the CPGs that mentioned symptoms specifically associated with withdrawal, the number of potential symptoms ranged from 4 to 39.
“None of the CPGs provided an exhaustive list of the potential withdrawal symptoms identified in the research literature,” the researchers wrote in their discussion.
Only four of the guidelines (19%) mentioned the overlap in symptoms between withdrawal from antidepressants and depression relapse, and only one provided guidance on distinguishing between the two conditions. Most of the symptoms of withdrawal, when described, were characterized as mild, brief, or self-limiting, the researchers noted.
“Being in withdrawal is a fundamentally different clinical situation than experiencing relapse, requiring two distinctly different treatment approaches,” the researchers emphasized. “Withdrawal reactions that are more severe and longer lasting than currently defined in the CPGs could risk getting misinterpreted as relapse, potentially leading to resumed unnecessary long-term antidepressant treatment in some patients,” they added.
The findings were limited by several factors including the inclusion only of guidelines from English-speaking countries, which may limit generalizability, the researchers noted. Other potential limitations include the subjective judgments involved in creating different guidelines, they said.
However, the results support the need for improved CPGs that help clinicians distinguish potential withdrawal reactions from depression relapse, and the need for more research on optimal dose reduction strategies for antidepressants, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Previous research suggests that approximately half of patients who discontinue or decrease dosage of antidepressants experience withdrawal symptoms, wrote Anders Sørensen, MD, of Copenhagen University Hospital, and colleagues. These symptoms are diverse and may include flulike symptoms, fatigue, anxiety, and sensations of electric shock, they noted. Most withdrawal effects last for a few weeks, but some persist for months or years, sometimes described as persistent postwithdrawal disorder, they added.
“Symptoms of withdrawal and depression overlap considerably but constitute two fundamentally different clinical conditions, which makes it important to distinguish between the two,” the researchers emphasized.
In a study published in the Journal of Affective Disorders, the researchers identified 21 clinical practice guidelines (CPGs) for depression published between 1998 and 2022. The guidelines were published in the United Kingdom, the United States, Canada, Australia, Singapore, Ireland, and New Zealand. They compared descriptions of withdrawal from antidepressants and calculated the proportion of CPGs with different information.
Overall, 15 of the 21 studies in the review (71%) noted that antidepressants are associated with withdrawal symptoms, but less than half (43%) used the term “withdrawal symptoms,” or similar. Of the nine guidelines that mentioned withdrawal symptoms, five used the term interchangeably with “discontinuation symptoms” and six used the term “discontinuation symptoms” only when discussing antidepressant withdrawal. In addition, six CPGs specifically stated that patients who stop antidepressants can experience withdrawal symptoms, and five stated that these symptoms also can occur in patients who are reducing or tapering their doses.
The type of withdrawal symptoms was mentioned in 10 CPGs, and the other 11 had no information on potential withdrawal symptoms, the researchers noted. Of the CPGs that mentioned symptoms specifically associated with withdrawal, the number of potential symptoms ranged from 4 to 39.
“None of the CPGs provided an exhaustive list of the potential withdrawal symptoms identified in the research literature,” the researchers wrote in their discussion.
Only four of the guidelines (19%) mentioned the overlap in symptoms between withdrawal from antidepressants and depression relapse, and only one provided guidance on distinguishing between the two conditions. Most of the symptoms of withdrawal, when described, were characterized as mild, brief, or self-limiting, the researchers noted.
“Being in withdrawal is a fundamentally different clinical situation than experiencing relapse, requiring two distinctly different treatment approaches,” the researchers emphasized. “Withdrawal reactions that are more severe and longer lasting than currently defined in the CPGs could risk getting misinterpreted as relapse, potentially leading to resumed unnecessary long-term antidepressant treatment in some patients,” they added.
The findings were limited by several factors including the inclusion only of guidelines from English-speaking countries, which may limit generalizability, the researchers noted. Other potential limitations include the subjective judgments involved in creating different guidelines, they said.
However, the results support the need for improved CPGs that help clinicians distinguish potential withdrawal reactions from depression relapse, and the need for more research on optimal dose reduction strategies for antidepressants, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Previous research suggests that approximately half of patients who discontinue or decrease dosage of antidepressants experience withdrawal symptoms, wrote Anders Sørensen, MD, of Copenhagen University Hospital, and colleagues. These symptoms are diverse and may include flulike symptoms, fatigue, anxiety, and sensations of electric shock, they noted. Most withdrawal effects last for a few weeks, but some persist for months or years, sometimes described as persistent postwithdrawal disorder, they added.
“Symptoms of withdrawal and depression overlap considerably but constitute two fundamentally different clinical conditions, which makes it important to distinguish between the two,” the researchers emphasized.
In a study published in the Journal of Affective Disorders, the researchers identified 21 clinical practice guidelines (CPGs) for depression published between 1998 and 2022. The guidelines were published in the United Kingdom, the United States, Canada, Australia, Singapore, Ireland, and New Zealand. They compared descriptions of withdrawal from antidepressants and calculated the proportion of CPGs with different information.
Overall, 15 of the 21 studies in the review (71%) noted that antidepressants are associated with withdrawal symptoms, but less than half (43%) used the term “withdrawal symptoms,” or similar. Of the nine guidelines that mentioned withdrawal symptoms, five used the term interchangeably with “discontinuation symptoms” and six used the term “discontinuation symptoms” only when discussing antidepressant withdrawal. In addition, six CPGs specifically stated that patients who stop antidepressants can experience withdrawal symptoms, and five stated that these symptoms also can occur in patients who are reducing or tapering their doses.
The type of withdrawal symptoms was mentioned in 10 CPGs, and the other 11 had no information on potential withdrawal symptoms, the researchers noted. Of the CPGs that mentioned symptoms specifically associated with withdrawal, the number of potential symptoms ranged from 4 to 39.
“None of the CPGs provided an exhaustive list of the potential withdrawal symptoms identified in the research literature,” the researchers wrote in their discussion.
Only four of the guidelines (19%) mentioned the overlap in symptoms between withdrawal from antidepressants and depression relapse, and only one provided guidance on distinguishing between the two conditions. Most of the symptoms of withdrawal, when described, were characterized as mild, brief, or self-limiting, the researchers noted.
“Being in withdrawal is a fundamentally different clinical situation than experiencing relapse, requiring two distinctly different treatment approaches,” the researchers emphasized. “Withdrawal reactions that are more severe and longer lasting than currently defined in the CPGs could risk getting misinterpreted as relapse, potentially leading to resumed unnecessary long-term antidepressant treatment in some patients,” they added.
The findings were limited by several factors including the inclusion only of guidelines from English-speaking countries, which may limit generalizability, the researchers noted. Other potential limitations include the subjective judgments involved in creating different guidelines, they said.
However, the results support the need for improved CPGs that help clinicians distinguish potential withdrawal reactions from depression relapse, and the need for more research on optimal dose reduction strategies for antidepressants, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Depression and schizophrenia: Many biological and clinical similarities
Clinicians generally regard major depressive disorder (MDD) and schizophrenia as 2 separate and distinct psychiatric brain disorders. However, despite some differences, those 2 psychiatric syndromes have numerous similarities across clinical features and neurobiologic parameters.
Biological similarities
Both disorders share the following variables:
- Highly genetic in etiology but with environmental influences and epigenetics
- Associated with childhood maltreatment, abuse, or neglect
- Disrupted neuroplasticity, especially shrinkage in hippocampal volume
- Significant drop in brain-derived neurotrophic factor resulting in decreased neurogenesis
- Extensive white matter pathology across interhemispheric and intrahemispheric bundles
- Increased levels of serum cortisol, a stress hormone and inflammatory biomarker
- Hypofrontal cerebral blood flow during acute episodes of both MDD and schizophrenia
- Reduced dendritic spines (in number and size) and impaired experiential neuroplasticity
- Neuroinflammation (eg, cytokines, tumor necrosis factor-alpha, C-reactive protein) during acute episodes
- Elevated oxidative stress biomarkers, indicating an increase in free radicals
- Overactive default mode network associated with ruminations in MDD and “daydreaming” in schizophrenia
- Decrease in gamma-aminobutyric acid (GABA) and its inhibitory activity, translating into dysregulation of glutamatergic pathways and other neurotransmitters
- Immune dysregulation and comorbid autoimmune disorders
Clinical similarities
- Psychotic symptoms, especially delusional thinking such as paranoia in schizophrenia and severe self-deprecation in MDD
- Significantly elevated lifetime suicide risk
- Cognitive impairment (more severe in schizophrenia across several cognitive functions)
- Similarity of depressive and negative symptoms (especially anhedonia, apathy, restricted facial expression, social withdrawal)
- Antidepressant medications im-prove depressive and negative symptoms (though not completely in the case of negative symptoms of schizophrenia)
- Both have treatment-resistant subtypes that fail to respond to standard therapies
- Both are associated with comorbid generalized anxiety disorder
- Both are associated with comorbid obsessive-compulsive disorder
- Both are associated with serious alcohol and drug use
- Early mortality from general medical conditions, especially cardiovascular risks due to obesity, diabetes, hypertension, dyslipidemia
- Elevated risk of dementia with aging compared to the unaffected general population
- Opioids improve MDD and psychosis (buprenorphine in MDD and morphine in schizophrenia)
- Several second-generation antipsychotic medications are approved for both MDD and schizophrenia
- Electroconvulsive therapy is effective when pharmacotherapy fails in both MDD and schizophrenia
Biological differences
- Glutamate N-methyl-D-aspartate receptor antagonists (eg, ketamine) improve MDD but worsen schizophrenia
- Muscarinic agonists improve psychosis but worsen depression
- High pain threshold in schizophrenia (pain insensitivity) and low threshold in MDD (in which pain is a common comorbidity)
- Cortical thinning more severe in schizophrenia
- Hippocampal atrophy is reversible with successful treatment in MDD but not in schizophrenia
- Hypofrontality is reversible with remission in MDD but not in schizophrenia
Clinical differences
- Auditory and visual hallucinations are more common in schizophrenia than in MDD
- Anosognosia is common in schizophrenia but not in MDD
- Implausible delusions are more common in schizophrenia than in MDD
- Mood-congruent delusions are more common in MDD than in schizophrenia
- Sadness, crying, pessimism, and self-deprecation are common in MDD but not in schizophrenia
- Achieving full remission is more common in MDD than in schizophrenia
- Long-acting injectable medications are available for schizophrenia but not for MDD
- Evidence-based psychotherapy, without pharmacotherapy, is more likely to be effective in MDD than in schizophrenia
A transdiagnostic model of psychopathology
The significant overlap between MDD and schizophrenia should not be surprising. They are both generated by the same organ, the human brain, with disrupted neurochemical and physiological circuits in the brain.
The overlap is also consistent with the emerging transdiagnostic model of psychopathology.1-9 This model proposes that there is a “core” genetic risk for psychopathology with different iterations. The transdiagnostic model is in stark contrast to the prevailing DSM-5, which categorizes psychiatric disorders in “silos,” as if they are completely independent from each other despite many shared features. This is highly debatable according to the substantial evidence that multiple psychiatric disorders share many genes that influence brain development in utero and predispose individuals to a variety of clinical symptoms in adolescence and young adulthood.
The origin of mental illness is being disentangled by emerging research, which is identifying the common links among the various disorders currently listed in DSM-5.10 However, the evolution of psychiatric diagnosis has come full circle from a single entity before DSM, to multiple entities with DSM, and now back to a unified transdiagnostic model that is rapidly emerging.11 This has implications for the FDA’s persistent dogma that clinical trials for new drugs must be targeted for 1 of the DSM-5 categories, a flawed and narrow assumption. Given the accelerating body of evidence for a unified, transdiagnostic model, it makes much more sense for the FDA to approve medications that target a psychiatric symptom that is shared by multiple psychiatric conditions within a transdiagnostic clinical system. When medications are approved for a symptom regardless of a DSM diagnosis, the term “off-label” and its “stigma” will then fade into history, along with the malignant preauthorization racket that was invented by greedy insurance companies that exploit the off-label use of medications (even when an FDA-approved medication for the patient’s condition does not yet exist) simply to deny coverage, lower their expenses, and fatten their profits.
1. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305-315.
2. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
3. Krueger RF, Easton NR. Transdiagnostic factors in mental disorders. World Psychiatry. 2015;14(1):27-29.
4. Hyman SE. New evidence for shared risk architecture for mental disorders. JAMA Psychiatry. 2019;76(3):235-236.
5. Selzam S, Coleman JRI, Caspi A, et al. A polygenic p factor for major psychiatric disorders. Translational Psychiatry. 2018;8(1):205.
6. Barch DM. What it means to be transdiagnostic and how do we know? Am J Psychiatry. 2020;177(5):370-372.
7. Nasrallah HA. Is there only 1 neurobiologic psychiatric disorder with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
8. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
9. Nasrallah HA. Beyond DSM-5: clinical and biological features shared by major psychiatric syndromes. Current Psychiatry. 2017;16(10):4-7.
10. Marshall M. Roots of mental illness: researchers are beginning to untangle the common biology that links supposedly distinct psychiatric conditions. Nature. 2020;581:19-21.
11. Kendler KS. From many to one to many--the search for causes of psychiatric illness. JAMA Psychiatry. 2019;76(10):1085-1091.
Clinicians generally regard major depressive disorder (MDD) and schizophrenia as 2 separate and distinct psychiatric brain disorders. However, despite some differences, those 2 psychiatric syndromes have numerous similarities across clinical features and neurobiologic parameters.
Biological similarities
Both disorders share the following variables:
- Highly genetic in etiology but with environmental influences and epigenetics
- Associated with childhood maltreatment, abuse, or neglect
- Disrupted neuroplasticity, especially shrinkage in hippocampal volume
- Significant drop in brain-derived neurotrophic factor resulting in decreased neurogenesis
- Extensive white matter pathology across interhemispheric and intrahemispheric bundles
- Increased levels of serum cortisol, a stress hormone and inflammatory biomarker
- Hypofrontal cerebral blood flow during acute episodes of both MDD and schizophrenia
- Reduced dendritic spines (in number and size) and impaired experiential neuroplasticity
- Neuroinflammation (eg, cytokines, tumor necrosis factor-alpha, C-reactive protein) during acute episodes
- Elevated oxidative stress biomarkers, indicating an increase in free radicals
- Overactive default mode network associated with ruminations in MDD and “daydreaming” in schizophrenia
- Decrease in gamma-aminobutyric acid (GABA) and its inhibitory activity, translating into dysregulation of glutamatergic pathways and other neurotransmitters
- Immune dysregulation and comorbid autoimmune disorders
Clinical similarities
- Psychotic symptoms, especially delusional thinking such as paranoia in schizophrenia and severe self-deprecation in MDD
- Significantly elevated lifetime suicide risk
- Cognitive impairment (more severe in schizophrenia across several cognitive functions)
- Similarity of depressive and negative symptoms (especially anhedonia, apathy, restricted facial expression, social withdrawal)
- Antidepressant medications im-prove depressive and negative symptoms (though not completely in the case of negative symptoms of schizophrenia)
- Both have treatment-resistant subtypes that fail to respond to standard therapies
- Both are associated with comorbid generalized anxiety disorder
- Both are associated with comorbid obsessive-compulsive disorder
- Both are associated with serious alcohol and drug use
- Early mortality from general medical conditions, especially cardiovascular risks due to obesity, diabetes, hypertension, dyslipidemia
- Elevated risk of dementia with aging compared to the unaffected general population
- Opioids improve MDD and psychosis (buprenorphine in MDD and morphine in schizophrenia)
- Several second-generation antipsychotic medications are approved for both MDD and schizophrenia
- Electroconvulsive therapy is effective when pharmacotherapy fails in both MDD and schizophrenia
Biological differences
- Glutamate N-methyl-D-aspartate receptor antagonists (eg, ketamine) improve MDD but worsen schizophrenia
- Muscarinic agonists improve psychosis but worsen depression
- High pain threshold in schizophrenia (pain insensitivity) and low threshold in MDD (in which pain is a common comorbidity)
- Cortical thinning more severe in schizophrenia
- Hippocampal atrophy is reversible with successful treatment in MDD but not in schizophrenia
- Hypofrontality is reversible with remission in MDD but not in schizophrenia
Clinical differences
- Auditory and visual hallucinations are more common in schizophrenia than in MDD
- Anosognosia is common in schizophrenia but not in MDD
- Implausible delusions are more common in schizophrenia than in MDD
- Mood-congruent delusions are more common in MDD than in schizophrenia
- Sadness, crying, pessimism, and self-deprecation are common in MDD but not in schizophrenia
- Achieving full remission is more common in MDD than in schizophrenia
- Long-acting injectable medications are available for schizophrenia but not for MDD
- Evidence-based psychotherapy, without pharmacotherapy, is more likely to be effective in MDD than in schizophrenia
A transdiagnostic model of psychopathology
The significant overlap between MDD and schizophrenia should not be surprising. They are both generated by the same organ, the human brain, with disrupted neurochemical and physiological circuits in the brain.
The overlap is also consistent with the emerging transdiagnostic model of psychopathology.1-9 This model proposes that there is a “core” genetic risk for psychopathology with different iterations. The transdiagnostic model is in stark contrast to the prevailing DSM-5, which categorizes psychiatric disorders in “silos,” as if they are completely independent from each other despite many shared features. This is highly debatable according to the substantial evidence that multiple psychiatric disorders share many genes that influence brain development in utero and predispose individuals to a variety of clinical symptoms in adolescence and young adulthood.
The origin of mental illness is being disentangled by emerging research, which is identifying the common links among the various disorders currently listed in DSM-5.10 However, the evolution of psychiatric diagnosis has come full circle from a single entity before DSM, to multiple entities with DSM, and now back to a unified transdiagnostic model that is rapidly emerging.11 This has implications for the FDA’s persistent dogma that clinical trials for new drugs must be targeted for 1 of the DSM-5 categories, a flawed and narrow assumption. Given the accelerating body of evidence for a unified, transdiagnostic model, it makes much more sense for the FDA to approve medications that target a psychiatric symptom that is shared by multiple psychiatric conditions within a transdiagnostic clinical system. When medications are approved for a symptom regardless of a DSM diagnosis, the term “off-label” and its “stigma” will then fade into history, along with the malignant preauthorization racket that was invented by greedy insurance companies that exploit the off-label use of medications (even when an FDA-approved medication for the patient’s condition does not yet exist) simply to deny coverage, lower their expenses, and fatten their profits.
Clinicians generally regard major depressive disorder (MDD) and schizophrenia as 2 separate and distinct psychiatric brain disorders. However, despite some differences, those 2 psychiatric syndromes have numerous similarities across clinical features and neurobiologic parameters.
Biological similarities
Both disorders share the following variables:
- Highly genetic in etiology but with environmental influences and epigenetics
- Associated with childhood maltreatment, abuse, or neglect
- Disrupted neuroplasticity, especially shrinkage in hippocampal volume
- Significant drop in brain-derived neurotrophic factor resulting in decreased neurogenesis
- Extensive white matter pathology across interhemispheric and intrahemispheric bundles
- Increased levels of serum cortisol, a stress hormone and inflammatory biomarker
- Hypofrontal cerebral blood flow during acute episodes of both MDD and schizophrenia
- Reduced dendritic spines (in number and size) and impaired experiential neuroplasticity
- Neuroinflammation (eg, cytokines, tumor necrosis factor-alpha, C-reactive protein) during acute episodes
- Elevated oxidative stress biomarkers, indicating an increase in free radicals
- Overactive default mode network associated with ruminations in MDD and “daydreaming” in schizophrenia
- Decrease in gamma-aminobutyric acid (GABA) and its inhibitory activity, translating into dysregulation of glutamatergic pathways and other neurotransmitters
- Immune dysregulation and comorbid autoimmune disorders
Clinical similarities
- Psychotic symptoms, especially delusional thinking such as paranoia in schizophrenia and severe self-deprecation in MDD
- Significantly elevated lifetime suicide risk
- Cognitive impairment (more severe in schizophrenia across several cognitive functions)
- Similarity of depressive and negative symptoms (especially anhedonia, apathy, restricted facial expression, social withdrawal)
- Antidepressant medications im-prove depressive and negative symptoms (though not completely in the case of negative symptoms of schizophrenia)
- Both have treatment-resistant subtypes that fail to respond to standard therapies
- Both are associated with comorbid generalized anxiety disorder
- Both are associated with comorbid obsessive-compulsive disorder
- Both are associated with serious alcohol and drug use
- Early mortality from general medical conditions, especially cardiovascular risks due to obesity, diabetes, hypertension, dyslipidemia
- Elevated risk of dementia with aging compared to the unaffected general population
- Opioids improve MDD and psychosis (buprenorphine in MDD and morphine in schizophrenia)
- Several second-generation antipsychotic medications are approved for both MDD and schizophrenia
- Electroconvulsive therapy is effective when pharmacotherapy fails in both MDD and schizophrenia
Biological differences
- Glutamate N-methyl-D-aspartate receptor antagonists (eg, ketamine) improve MDD but worsen schizophrenia
- Muscarinic agonists improve psychosis but worsen depression
- High pain threshold in schizophrenia (pain insensitivity) and low threshold in MDD (in which pain is a common comorbidity)
- Cortical thinning more severe in schizophrenia
- Hippocampal atrophy is reversible with successful treatment in MDD but not in schizophrenia
- Hypofrontality is reversible with remission in MDD but not in schizophrenia
Clinical differences
- Auditory and visual hallucinations are more common in schizophrenia than in MDD
- Anosognosia is common in schizophrenia but not in MDD
- Implausible delusions are more common in schizophrenia than in MDD
- Mood-congruent delusions are more common in MDD than in schizophrenia
- Sadness, crying, pessimism, and self-deprecation are common in MDD but not in schizophrenia
- Achieving full remission is more common in MDD than in schizophrenia
- Long-acting injectable medications are available for schizophrenia but not for MDD
- Evidence-based psychotherapy, without pharmacotherapy, is more likely to be effective in MDD than in schizophrenia
A transdiagnostic model of psychopathology
The significant overlap between MDD and schizophrenia should not be surprising. They are both generated by the same organ, the human brain, with disrupted neurochemical and physiological circuits in the brain.
The overlap is also consistent with the emerging transdiagnostic model of psychopathology.1-9 This model proposes that there is a “core” genetic risk for psychopathology with different iterations. The transdiagnostic model is in stark contrast to the prevailing DSM-5, which categorizes psychiatric disorders in “silos,” as if they are completely independent from each other despite many shared features. This is highly debatable according to the substantial evidence that multiple psychiatric disorders share many genes that influence brain development in utero and predispose individuals to a variety of clinical symptoms in adolescence and young adulthood.
The origin of mental illness is being disentangled by emerging research, which is identifying the common links among the various disorders currently listed in DSM-5.10 However, the evolution of psychiatric diagnosis has come full circle from a single entity before DSM, to multiple entities with DSM, and now back to a unified transdiagnostic model that is rapidly emerging.11 This has implications for the FDA’s persistent dogma that clinical trials for new drugs must be targeted for 1 of the DSM-5 categories, a flawed and narrow assumption. Given the accelerating body of evidence for a unified, transdiagnostic model, it makes much more sense for the FDA to approve medications that target a psychiatric symptom that is shared by multiple psychiatric conditions within a transdiagnostic clinical system. When medications are approved for a symptom regardless of a DSM diagnosis, the term “off-label” and its “stigma” will then fade into history, along with the malignant preauthorization racket that was invented by greedy insurance companies that exploit the off-label use of medications (even when an FDA-approved medication for the patient’s condition does not yet exist) simply to deny coverage, lower their expenses, and fatten their profits.
1. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305-315.
2. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
3. Krueger RF, Easton NR. Transdiagnostic factors in mental disorders. World Psychiatry. 2015;14(1):27-29.
4. Hyman SE. New evidence for shared risk architecture for mental disorders. JAMA Psychiatry. 2019;76(3):235-236.
5. Selzam S, Coleman JRI, Caspi A, et al. A polygenic p factor for major psychiatric disorders. Translational Psychiatry. 2018;8(1):205.
6. Barch DM. What it means to be transdiagnostic and how do we know? Am J Psychiatry. 2020;177(5):370-372.
7. Nasrallah HA. Is there only 1 neurobiologic psychiatric disorder with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
8. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
9. Nasrallah HA. Beyond DSM-5: clinical and biological features shared by major psychiatric syndromes. Current Psychiatry. 2017;16(10):4-7.
10. Marshall M. Roots of mental illness: researchers are beginning to untangle the common biology that links supposedly distinct psychiatric conditions. Nature. 2020;581:19-21.
11. Kendler KS. From many to one to many--the search for causes of psychiatric illness. JAMA Psychiatry. 2019;76(10):1085-1091.
1. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305-315.
2. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
3. Krueger RF, Easton NR. Transdiagnostic factors in mental disorders. World Psychiatry. 2015;14(1):27-29.
4. Hyman SE. New evidence for shared risk architecture for mental disorders. JAMA Psychiatry. 2019;76(3):235-236.
5. Selzam S, Coleman JRI, Caspi A, et al. A polygenic p factor for major psychiatric disorders. Translational Psychiatry. 2018;8(1):205.
6. Barch DM. What it means to be transdiagnostic and how do we know? Am J Psychiatry. 2020;177(5):370-372.
7. Nasrallah HA. Is there only 1 neurobiologic psychiatric disorder with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
8. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
9. Nasrallah HA. Beyond DSM-5: clinical and biological features shared by major psychiatric syndromes. Current Psychiatry. 2017;16(10):4-7.
10. Marshall M. Roots of mental illness: researchers are beginning to untangle the common biology that links supposedly distinct psychiatric conditions. Nature. 2020;581:19-21.
11. Kendler KS. From many to one to many--the search for causes of psychiatric illness. JAMA Psychiatry. 2019;76(10):1085-1091.
Subtle cognitive decline in a patient with depression and anxiety
CASE Anxious and confused
Mr. M, age 53, a surgeon, presents to the emergency department (ED) following a panic attack and concerns from his staff that he appears confused. Specifically, staff members report that in the past 4 months, Mr. M was observed having problems completing some postoperative tasks related to chart documentation. Mr. M has a history of major depressive disorder (MDD), hypertension, hyperlipidemia, and type 2 diabetes.
HISTORY A long-standing diagnosis of depression
Mr. M reports that 30 years ago, he received care from a psychiatrist to address symptoms of MDD. He says that around the time he arrived at the ED, he had noticed subtle but gradual changes in his cognition, which led him to skip words and often struggle to find the correct words. These episodes left him confused. Mr. M started getting anxious about these cognitive issues because they disrupted his work and forced him to reduce his duties. He does not have any known family history of mental illness, is single, and lives alone.
EVALUATION After stroke is ruled out, a psychiatric workup
In the ED, a comprehensive exam rules out an acute cerebrovascular event. A neurologic evaluation notes some delay in processing information and observes Mr. M having difficulty following simple commands. Laboratory investigations, including a comprehensive metabolic panel, are unremarkable. An MRI of Mr. M’s brain, with and without contrast, notes no acute findings. He is discharged from the ED with a diagnosis of MDD.
Before he presented to the ED, Mr. M’s medication regimen included duloxetine 60 mg/d, buspirone 10 mg 3 times a day, and aripiprazole 5 mg/d for MDD and anxiety. After the ED visit, Mr. M’s physician refers him to an outpatient psychiatrist for management of worsening depression and panic attacks. During the psychiatrist’s evaluation, Mr. M reports a decreased interest in activities, decreased motivation, being easily fatigued, and having poor sleep. He denies having a depressed mood, difficulty concentrating, or having problems with his appetite. He also denies suicidal thoughts, both past and present.
Mr. M describes his mood as anxious, primarily surrounding his recent cognitive changes. He does not have a substance use disorder, psychotic illness, mania or hypomania, posttraumatic stress disorder, or obsessive-compulsive disorder. He reports adherence to his psychiatric medications. A mental status exam reveals Mr. M to be anxious. His attention is not well sustained, and he has difficulty describing details of his cognitive struggles, providing vague descriptions such as “skipping thought” and “skipping words.” Mr. M’s affect is congruent to his mood with some restriction and the psychiatrist notes that he is experiencing thought latency, poverty of content of thoughts, word-finding difficulties, and circumlocution. Mr. M denies any perceptual abnormalities, and there is no evidence of delusions.
[polldaddy:11320112]
The authors’ observations
Mr. M’s symptoms are significant for subacute cognitive decline that is subtle but gradual and can be easily missed, especially in the beginning. Though his ED evaluation—including brain imaging—ruled out acute or focal neurologic findings and his primary psychiatric presentation was anxiety, Mr. M’s medical history and mental status exam were suggestive of cognitive deficits.
Collateral information was obtained from his work colleagues, which confirmed both cognitive problems and comorbid anxiety. Additionally, given Mr. M’s high cognitive baseline as a surgeon, the new-onset cognitive changes over 4 months warranted further cognitive and neurologic evaluation. There are many causes of cognitive impairment (vascular, cancer, infection, autoimmune, medications, substances or toxins, neurodegenerative, psychiatric, vitamin deficiencies), all of which need to be considered in a patient with a nonspecific presentation such as Mr. M’s. The psychiatrist confirmed Mr. M’s current medication regimen, and discussed tapering aripiprazole while continuing duloxetine and buspirone.
Continue to: EVALUATION A closer look at cognitive deficits
EVALUATION A closer look at cognitive deficits
Mr. M scores 12/30 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment (Table 1). The psychiatrist refers Mr. M to Neurology. During his neurologic evaluation, Mr. M continues to report feeling anxious that “something is wrong” and skips his words. The neurologist confirms Mr. M’s symptoms may have started 2 to 3 months before he presented to the ED. Mr. M reports unusual eating habits, including yogurt and cookies for breakfast, Mexican food for lunch, and more cookies for dinner. He denies having a fever, gaining or losing weight, rashes, headaches, neck stiffness, tingling or weakness or stiffness of limbs, vertigo, visual changes, photophobia, unsteady gait, bowel or bladder incontinence, or tremors.
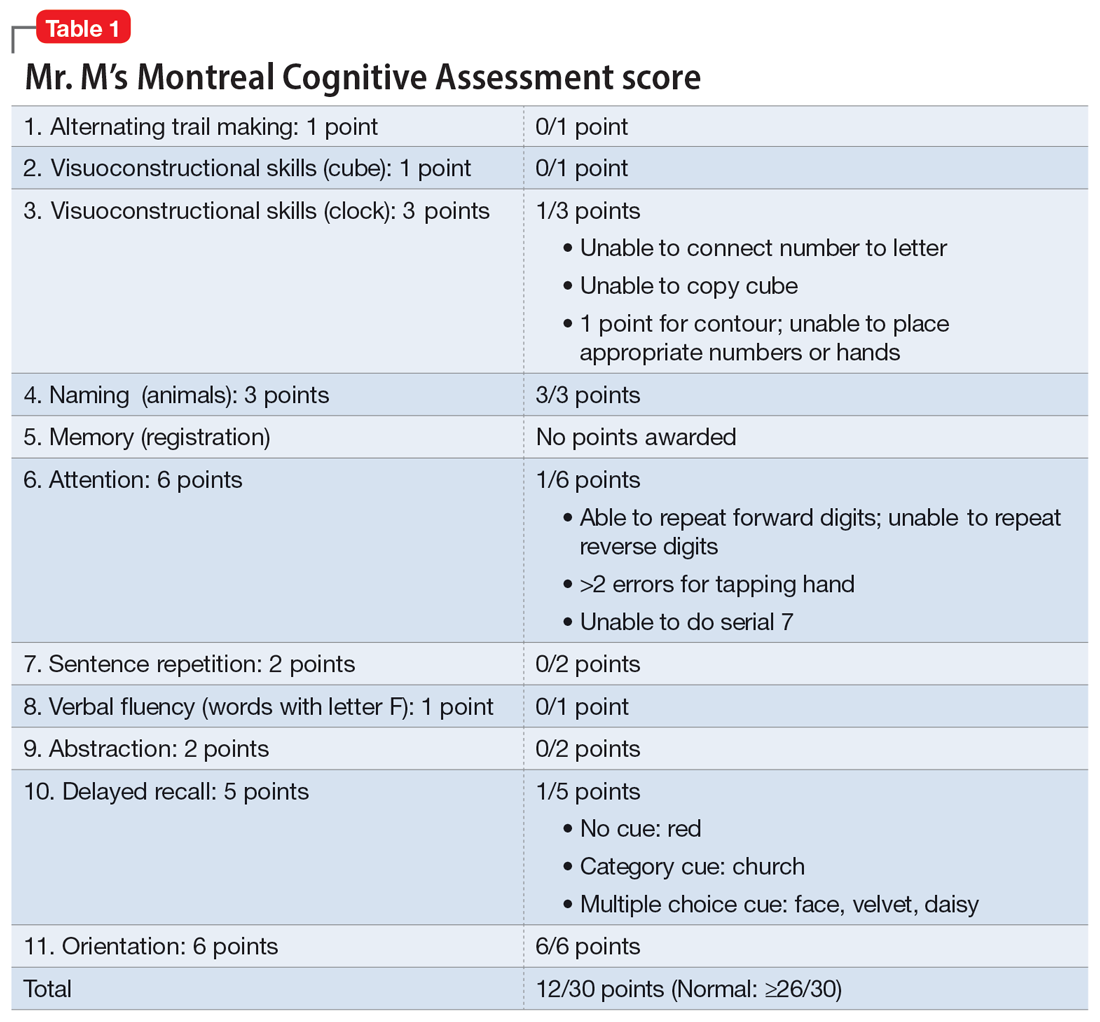
When the neurologist repeats the MoCA, Mr. M again scores 12. The neurologist notes that Mr. M answers questions a little slowly and pauses for thoughts when unable to find an answer. Mr. M has difficulty following some simple commands, such as “touch a finger to your nose.” Other in-office neurologic physical exams (cranial nerves, involuntary movements or tremors, sensation, muscle strength, reflexes, cerebellar signs) are unremarkable except for mildly decreased vibration sense of his toes. The neurologist concludes that Mr. M’s presentation is suggestive of subacute to chronic bradyphrenia and orders additional evaluation, including neuropsychological testing.
[polldaddy:11320114]
The authors’ observations
Physical and neurologic exams were not suggestive of any obvious causes of cognitive decline. Both the mental status exam and 2 serial MoCAs suggested deficits in executive function, language, and memory. Each of the differential diagnoses considered was ruled out with workup or exams (Table 2), which led to a most likely diagnosis of neurodegenerative disorder with PPA. Neuropsychological testing confirmed the diagnosis of nonfluent PPA.
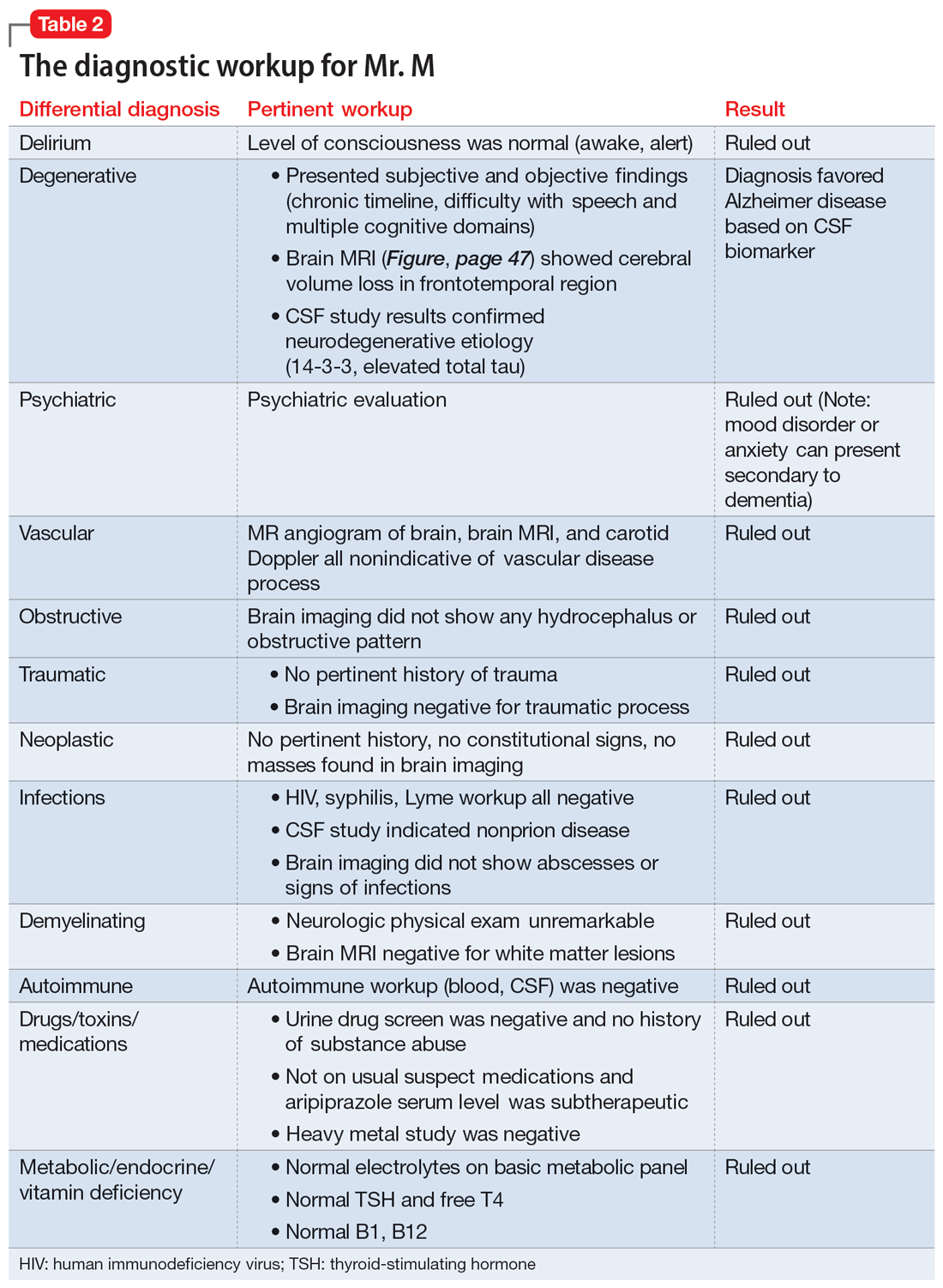
Primary progressive aphasia
PPA is an uncommon, heterogeneous group of disorders stemming from focal degeneration of language-governing centers of the brain.1,2 The estimated prevalence of PPA is 3 in 100,000 cases.2,3 There are 4 major variants of PPA (Table 34), and each presents with distinct language, cognitive, neuroanatomical, and neuropathological characteristics.4 PPA is usually diagnosed in late middle life; however, diagnosis is often delayed due to the relative obscurity of the disorder.4 In Mr. M’s case, it took approximately 4 months of evaluations by various specialists before a diagnosis was confirmed.
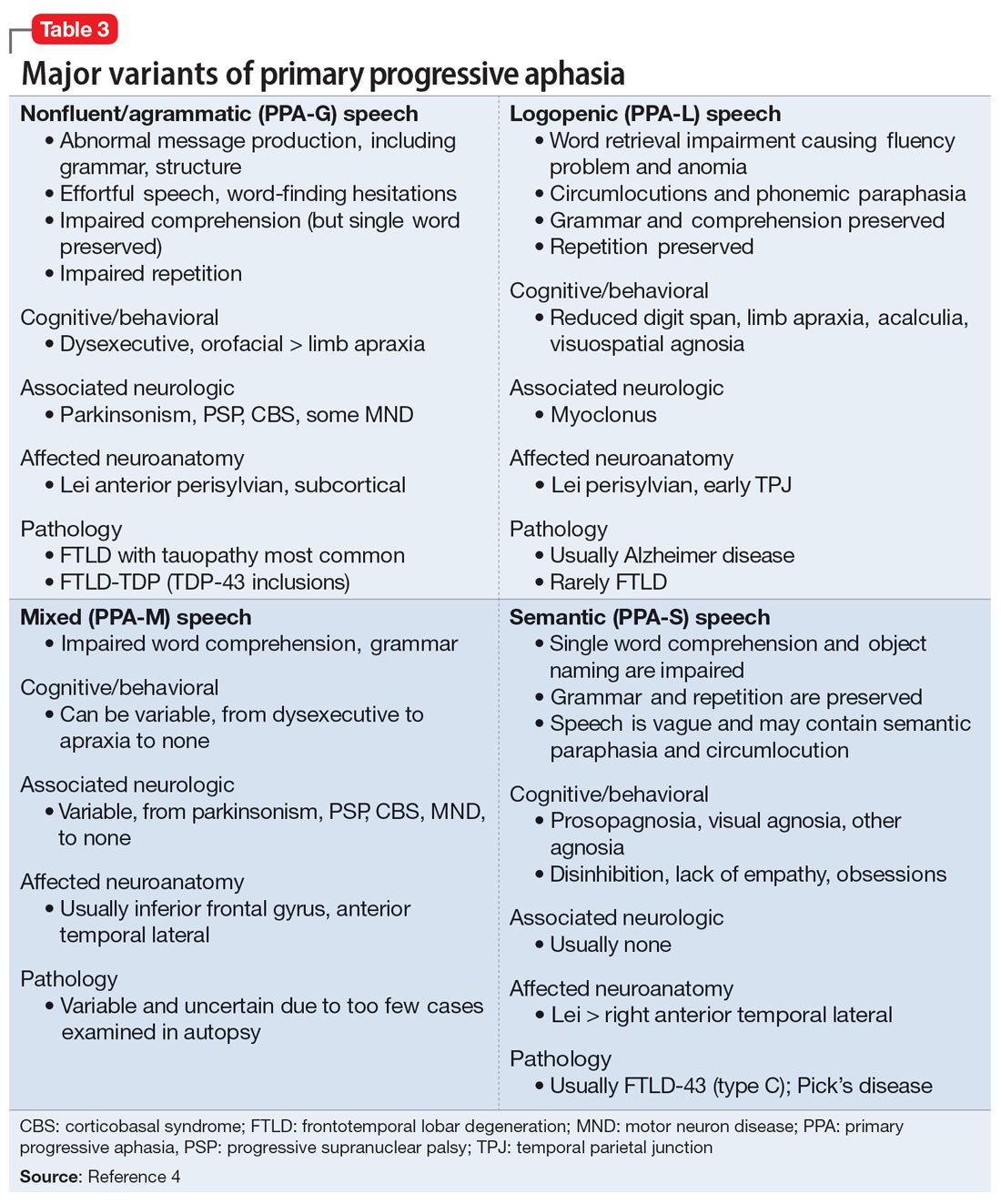
The initial phase of PPA can present as a diagnostic challenge because patients can have difficulty articulating their cognitive and language deficits. PPA can be commonly mistaken for a primary psychiatric disorder such as MDD or anxiety, which can further delay an accurate diagnosis and treatment. Special attention to the mental status exam, close observation of the patient’s language, and assessment of cognitive abilities using standardized screenings such as the MoCA or Mini-Mental State Examination can be helpful in clarifying the diagnosis. It is also important to rule out developmental problems (eg, dyslexia) and hearing difficulties, particularly in older patients.
Continue to: TREATMENT Adjusting the medication regimen
TREATMENT Adjusting the medication regimen
The neurologist completes additional examinations to rule out causes of rare neurodegenerative disorders, including CSF autoimmune disorders, Creutzfeldt-Jakob disease, and Alzheimer disease (AD) (Table 4). Mr. M continues to follow up with his outpatient psychiatrist and his medication regimen is adjusted. Aripiprazole and buspirone are discontinued, and duloxetine is titrated to 60 mg twice a day. During follow-up visits, Mr. M discusses his understanding of his neurologic condition. His concerns shift to his illness and prognosis. During these visits, he continues to deny suicidality.
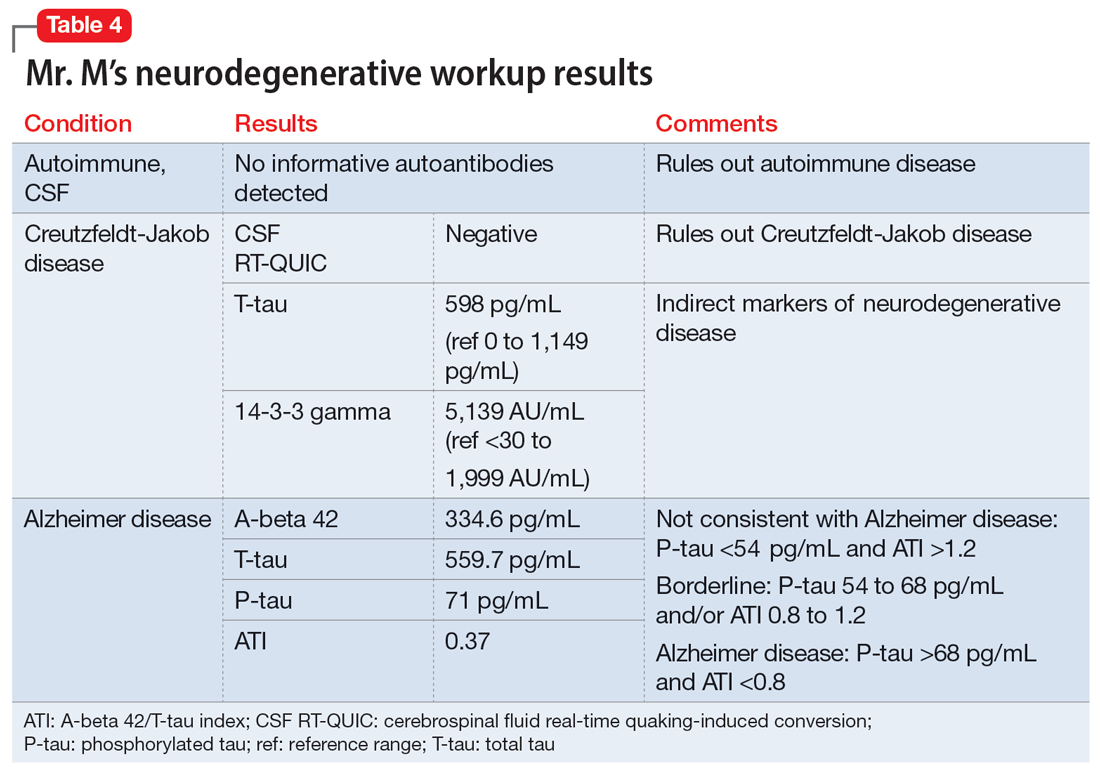
[polldaddy:11320115]
The authors’ observations
Mr. M’s neurodegenerative workup identified an intriguing diagnostic challenge. A repeat brain MRI (Figure) showed atrophy patterns suggestive of frontotemporal lobar degeneration (FTLD). On the other hand, his CSF ATI (A-beta 42/T-tau index, a value used to aid in the diagnosis of AD) was <1, suggesting early-onset AD.5,6 Although significant advances have been made to distinguish AD and FTLD following an autopsy, there are still no reliable or definitive biomarkers to distinguish AD from FTLD (particularly in the early stages of FTLD). This can often leave the confirmatory diagnosis as a question.7
A PPA diagnosis (and other dementias) can have a significant impact on the patient and their family due to the uncertain nature of the progression of the disease and quality-of-life issues related to language and other cognitive deficits. Early identification and accurate diagnosis of PPA and its etiology (ie, AD vs FTLD) is important to avoid unnecessary exposure to medications or the use of polypharmacy to treat an inaccurate diagnosis of a primary psychiatric illness. For example, Mr. M was being treated with 3 psychiatric medications (aripiprazole, buspirone, and duloxetine) for depression and anxiety prior to the diagnosis of PPA.
Nonpharmacologic interventions can play an important role in the management of patients with PPA. These include educating the patient and their family about the diagnosis and discussions about future planning, including appropriate social support, employment, and finances.4 Pharmacologic interventions may be limited, as there are currently no disease-modifying treatments for PPA or FTLD. For patients with nonfluent PPA or AD, cholinesterase inhibitors such as donepezil or N-methyl
Psychiatrists should continue to treat patients with PPA for comorbid anxiety or depression, with appropriate medications and/or supportive therapy to guide the patient through the process of grief. Assessing for suicide risk is also important in patients diagnosed with dementia. A retrospective cohort study of patients age ≥60 with a diagnosis of dementia suggested that the majority of suicides occurred in those with a new dementia diagnosis.9 End-of-life decisions such as advanced directives should be made when the patient still has legal capacity, ideally as soon as possible after diagnosis.10
OUTCOME Remaining engaged in treatment
Mr. M continues to follow-up with the Neurology team. He has also been regularly seeing his psychiatric team for medication management and supportive therapy, and his psychiatric medications have been optimized to reduce polypharmacy. During his sessions, Mr. M discusses his grief and plans for the future. Despite his anxiety about the uncertainty of his prognosis, Mr. M continues to report that he is doing reasonably well and remains engaged in treatment.
Bottom Line
Patients with primary progressive aphasia and rare neurodegenerative disorders may present to an outpatient or emergency setting with symptoms of anxiety and confusion. They are frequently misdiagnosed with a primary psychiatric disorder due to the nature of cognitive and language deficits, particularly in the early stages of the disease. Paying close attention to language and conducting cognitive screening are critical in identifying the true cause of a patient’s symptoms.
Related Resources
- Primary progressive aphasia. National Center for Advancing Translational Sciences. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/8541/primary-progressive-aphasia
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: A useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
Drug Brand Names
Aripiprazole • Abilify
Donepezil • Aricept
Duloxetine • Cymbalta
Memantine • Namenda
1. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88-97. doi:10.1038/nrneurol.2009.216
2. Mesulam M-M, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10(10):554-569. doi:10.1038/nrneurol.2014.159
3. Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi:10.1212/WNL.0000000000002638
4. Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: a clinical approach. J Neurol. 2018;265(6):1474-1490. doi:10.1007/s00415-018-8762-6
5. Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1(2):213-225. doi:10.1602/neurorx.1.2.213
6. Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555-1562. doi:10.1212/wnl.52.8.1555
7. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. doi:10.1038/s41591-020-0762-2
8. Newhart M, Davis C, Kannan V, et al. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7-8):823-834. doi:10.1080/02687030802661762
9. Seyfried LS, Kales HC, Ignacio RV, et al. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567-573. doi:10.1016/j.jalz.2011.01.006
10. Porteri C. Advance directives as a tool to respect patients’ values and preferences: discussion on the case of Alzheimer’s disease. BMC Med Ethics. 2018;19(1):9. doi:10.1186/s12910-018-0249-6
CASE Anxious and confused
Mr. M, age 53, a surgeon, presents to the emergency department (ED) following a panic attack and concerns from his staff that he appears confused. Specifically, staff members report that in the past 4 months, Mr. M was observed having problems completing some postoperative tasks related to chart documentation. Mr. M has a history of major depressive disorder (MDD), hypertension, hyperlipidemia, and type 2 diabetes.
HISTORY A long-standing diagnosis of depression
Mr. M reports that 30 years ago, he received care from a psychiatrist to address symptoms of MDD. He says that around the time he arrived at the ED, he had noticed subtle but gradual changes in his cognition, which led him to skip words and often struggle to find the correct words. These episodes left him confused. Mr. M started getting anxious about these cognitive issues because they disrupted his work and forced him to reduce his duties. He does not have any known family history of mental illness, is single, and lives alone.
EVALUATION After stroke is ruled out, a psychiatric workup
In the ED, a comprehensive exam rules out an acute cerebrovascular event. A neurologic evaluation notes some delay in processing information and observes Mr. M having difficulty following simple commands. Laboratory investigations, including a comprehensive metabolic panel, are unremarkable. An MRI of Mr. M’s brain, with and without contrast, notes no acute findings. He is discharged from the ED with a diagnosis of MDD.
Before he presented to the ED, Mr. M’s medication regimen included duloxetine 60 mg/d, buspirone 10 mg 3 times a day, and aripiprazole 5 mg/d for MDD and anxiety. After the ED visit, Mr. M’s physician refers him to an outpatient psychiatrist for management of worsening depression and panic attacks. During the psychiatrist’s evaluation, Mr. M reports a decreased interest in activities, decreased motivation, being easily fatigued, and having poor sleep. He denies having a depressed mood, difficulty concentrating, or having problems with his appetite. He also denies suicidal thoughts, both past and present.
Mr. M describes his mood as anxious, primarily surrounding his recent cognitive changes. He does not have a substance use disorder, psychotic illness, mania or hypomania, posttraumatic stress disorder, or obsessive-compulsive disorder. He reports adherence to his psychiatric medications. A mental status exam reveals Mr. M to be anxious. His attention is not well sustained, and he has difficulty describing details of his cognitive struggles, providing vague descriptions such as “skipping thought” and “skipping words.” Mr. M’s affect is congruent to his mood with some restriction and the psychiatrist notes that he is experiencing thought latency, poverty of content of thoughts, word-finding difficulties, and circumlocution. Mr. M denies any perceptual abnormalities, and there is no evidence of delusions.
[polldaddy:11320112]
The authors’ observations
Mr. M’s symptoms are significant for subacute cognitive decline that is subtle but gradual and can be easily missed, especially in the beginning. Though his ED evaluation—including brain imaging—ruled out acute or focal neurologic findings and his primary psychiatric presentation was anxiety, Mr. M’s medical history and mental status exam were suggestive of cognitive deficits.
Collateral information was obtained from his work colleagues, which confirmed both cognitive problems and comorbid anxiety. Additionally, given Mr. M’s high cognitive baseline as a surgeon, the new-onset cognitive changes over 4 months warranted further cognitive and neurologic evaluation. There are many causes of cognitive impairment (vascular, cancer, infection, autoimmune, medications, substances or toxins, neurodegenerative, psychiatric, vitamin deficiencies), all of which need to be considered in a patient with a nonspecific presentation such as Mr. M’s. The psychiatrist confirmed Mr. M’s current medication regimen, and discussed tapering aripiprazole while continuing duloxetine and buspirone.
Continue to: EVALUATION A closer look at cognitive deficits
EVALUATION A closer look at cognitive deficits
Mr. M scores 12/30 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment (Table 1). The psychiatrist refers Mr. M to Neurology. During his neurologic evaluation, Mr. M continues to report feeling anxious that “something is wrong” and skips his words. The neurologist confirms Mr. M’s symptoms may have started 2 to 3 months before he presented to the ED. Mr. M reports unusual eating habits, including yogurt and cookies for breakfast, Mexican food for lunch, and more cookies for dinner. He denies having a fever, gaining or losing weight, rashes, headaches, neck stiffness, tingling or weakness or stiffness of limbs, vertigo, visual changes, photophobia, unsteady gait, bowel or bladder incontinence, or tremors.

When the neurologist repeats the MoCA, Mr. M again scores 12. The neurologist notes that Mr. M answers questions a little slowly and pauses for thoughts when unable to find an answer. Mr. M has difficulty following some simple commands, such as “touch a finger to your nose.” Other in-office neurologic physical exams (cranial nerves, involuntary movements or tremors, sensation, muscle strength, reflexes, cerebellar signs) are unremarkable except for mildly decreased vibration sense of his toes. The neurologist concludes that Mr. M’s presentation is suggestive of subacute to chronic bradyphrenia and orders additional evaluation, including neuropsychological testing.
[polldaddy:11320114]
The authors’ observations
Physical and neurologic exams were not suggestive of any obvious causes of cognitive decline. Both the mental status exam and 2 serial MoCAs suggested deficits in executive function, language, and memory. Each of the differential diagnoses considered was ruled out with workup or exams (Table 2), which led to a most likely diagnosis of neurodegenerative disorder with PPA. Neuropsychological testing confirmed the diagnosis of nonfluent PPA.

Primary progressive aphasia
PPA is an uncommon, heterogeneous group of disorders stemming from focal degeneration of language-governing centers of the brain.1,2 The estimated prevalence of PPA is 3 in 100,000 cases.2,3 There are 4 major variants of PPA (Table 34), and each presents with distinct language, cognitive, neuroanatomical, and neuropathological characteristics.4 PPA is usually diagnosed in late middle life; however, diagnosis is often delayed due to the relative obscurity of the disorder.4 In Mr. M’s case, it took approximately 4 months of evaluations by various specialists before a diagnosis was confirmed.

The initial phase of PPA can present as a diagnostic challenge because patients can have difficulty articulating their cognitive and language deficits. PPA can be commonly mistaken for a primary psychiatric disorder such as MDD or anxiety, which can further delay an accurate diagnosis and treatment. Special attention to the mental status exam, close observation of the patient’s language, and assessment of cognitive abilities using standardized screenings such as the MoCA or Mini-Mental State Examination can be helpful in clarifying the diagnosis. It is also important to rule out developmental problems (eg, dyslexia) and hearing difficulties, particularly in older patients.
Continue to: TREATMENT Adjusting the medication regimen
TREATMENT Adjusting the medication regimen
The neurologist completes additional examinations to rule out causes of rare neurodegenerative disorders, including CSF autoimmune disorders, Creutzfeldt-Jakob disease, and Alzheimer disease (AD) (Table 4). Mr. M continues to follow up with his outpatient psychiatrist and his medication regimen is adjusted. Aripiprazole and buspirone are discontinued, and duloxetine is titrated to 60 mg twice a day. During follow-up visits, Mr. M discusses his understanding of his neurologic condition. His concerns shift to his illness and prognosis. During these visits, he continues to deny suicidality.

[polldaddy:11320115]
The authors’ observations
Mr. M’s neurodegenerative workup identified an intriguing diagnostic challenge. A repeat brain MRI (Figure) showed atrophy patterns suggestive of frontotemporal lobar degeneration (FTLD). On the other hand, his CSF ATI (A-beta 42/T-tau index, a value used to aid in the diagnosis of AD) was <1, suggesting early-onset AD.5,6 Although significant advances have been made to distinguish AD and FTLD following an autopsy, there are still no reliable or definitive biomarkers to distinguish AD from FTLD (particularly in the early stages of FTLD). This can often leave the confirmatory diagnosis as a question.7

A PPA diagnosis (and other dementias) can have a significant impact on the patient and their family due to the uncertain nature of the progression of the disease and quality-of-life issues related to language and other cognitive deficits. Early identification and accurate diagnosis of PPA and its etiology (ie, AD vs FTLD) is important to avoid unnecessary exposure to medications or the use of polypharmacy to treat an inaccurate diagnosis of a primary psychiatric illness. For example, Mr. M was being treated with 3 psychiatric medications (aripiprazole, buspirone, and duloxetine) for depression and anxiety prior to the diagnosis of PPA.
Nonpharmacologic interventions can play an important role in the management of patients with PPA. These include educating the patient and their family about the diagnosis and discussions about future planning, including appropriate social support, employment, and finances.4 Pharmacologic interventions may be limited, as there are currently no disease-modifying treatments for PPA or FTLD. For patients with nonfluent PPA or AD, cholinesterase inhibitors such as donepezil or N-methyl
Psychiatrists should continue to treat patients with PPA for comorbid anxiety or depression, with appropriate medications and/or supportive therapy to guide the patient through the process of grief. Assessing for suicide risk is also important in patients diagnosed with dementia. A retrospective cohort study of patients age ≥60 with a diagnosis of dementia suggested that the majority of suicides occurred in those with a new dementia diagnosis.9 End-of-life decisions such as advanced directives should be made when the patient still has legal capacity, ideally as soon as possible after diagnosis.10
OUTCOME Remaining engaged in treatment
Mr. M continues to follow-up with the Neurology team. He has also been regularly seeing his psychiatric team for medication management and supportive therapy, and his psychiatric medications have been optimized to reduce polypharmacy. During his sessions, Mr. M discusses his grief and plans for the future. Despite his anxiety about the uncertainty of his prognosis, Mr. M continues to report that he is doing reasonably well and remains engaged in treatment.
Bottom Line
Patients with primary progressive aphasia and rare neurodegenerative disorders may present to an outpatient or emergency setting with symptoms of anxiety and confusion. They are frequently misdiagnosed with a primary psychiatric disorder due to the nature of cognitive and language deficits, particularly in the early stages of the disease. Paying close attention to language and conducting cognitive screening are critical in identifying the true cause of a patient’s symptoms.
Related Resources
- Primary progressive aphasia. National Center for Advancing Translational Sciences. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/8541/primary-progressive-aphasia
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: A useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
Drug Brand Names
Aripiprazole • Abilify
Donepezil • Aricept
Duloxetine • Cymbalta
Memantine • Namenda
CASE Anxious and confused
Mr. M, age 53, a surgeon, presents to the emergency department (ED) following a panic attack and concerns from his staff that he appears confused. Specifically, staff members report that in the past 4 months, Mr. M was observed having problems completing some postoperative tasks related to chart documentation. Mr. M has a history of major depressive disorder (MDD), hypertension, hyperlipidemia, and type 2 diabetes.
HISTORY A long-standing diagnosis of depression
Mr. M reports that 30 years ago, he received care from a psychiatrist to address symptoms of MDD. He says that around the time he arrived at the ED, he had noticed subtle but gradual changes in his cognition, which led him to skip words and often struggle to find the correct words. These episodes left him confused. Mr. M started getting anxious about these cognitive issues because they disrupted his work and forced him to reduce his duties. He does not have any known family history of mental illness, is single, and lives alone.
EVALUATION After stroke is ruled out, a psychiatric workup
In the ED, a comprehensive exam rules out an acute cerebrovascular event. A neurologic evaluation notes some delay in processing information and observes Mr. M having difficulty following simple commands. Laboratory investigations, including a comprehensive metabolic panel, are unremarkable. An MRI of Mr. M’s brain, with and without contrast, notes no acute findings. He is discharged from the ED with a diagnosis of MDD.
Before he presented to the ED, Mr. M’s medication regimen included duloxetine 60 mg/d, buspirone 10 mg 3 times a day, and aripiprazole 5 mg/d for MDD and anxiety. After the ED visit, Mr. M’s physician refers him to an outpatient psychiatrist for management of worsening depression and panic attacks. During the psychiatrist’s evaluation, Mr. M reports a decreased interest in activities, decreased motivation, being easily fatigued, and having poor sleep. He denies having a depressed mood, difficulty concentrating, or having problems with his appetite. He also denies suicidal thoughts, both past and present.
Mr. M describes his mood as anxious, primarily surrounding his recent cognitive changes. He does not have a substance use disorder, psychotic illness, mania or hypomania, posttraumatic stress disorder, or obsessive-compulsive disorder. He reports adherence to his psychiatric medications. A mental status exam reveals Mr. M to be anxious. His attention is not well sustained, and he has difficulty describing details of his cognitive struggles, providing vague descriptions such as “skipping thought” and “skipping words.” Mr. M’s affect is congruent to his mood with some restriction and the psychiatrist notes that he is experiencing thought latency, poverty of content of thoughts, word-finding difficulties, and circumlocution. Mr. M denies any perceptual abnormalities, and there is no evidence of delusions.
[polldaddy:11320112]
The authors’ observations
Mr. M’s symptoms are significant for subacute cognitive decline that is subtle but gradual and can be easily missed, especially in the beginning. Though his ED evaluation—including brain imaging—ruled out acute or focal neurologic findings and his primary psychiatric presentation was anxiety, Mr. M’s medical history and mental status exam were suggestive of cognitive deficits.
Collateral information was obtained from his work colleagues, which confirmed both cognitive problems and comorbid anxiety. Additionally, given Mr. M’s high cognitive baseline as a surgeon, the new-onset cognitive changes over 4 months warranted further cognitive and neurologic evaluation. There are many causes of cognitive impairment (vascular, cancer, infection, autoimmune, medications, substances or toxins, neurodegenerative, psychiatric, vitamin deficiencies), all of which need to be considered in a patient with a nonspecific presentation such as Mr. M’s. The psychiatrist confirmed Mr. M’s current medication regimen, and discussed tapering aripiprazole while continuing duloxetine and buspirone.
Continue to: EVALUATION A closer look at cognitive deficits
EVALUATION A closer look at cognitive deficits
Mr. M scores 12/30 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment (Table 1). The psychiatrist refers Mr. M to Neurology. During his neurologic evaluation, Mr. M continues to report feeling anxious that “something is wrong” and skips his words. The neurologist confirms Mr. M’s symptoms may have started 2 to 3 months before he presented to the ED. Mr. M reports unusual eating habits, including yogurt and cookies for breakfast, Mexican food for lunch, and more cookies for dinner. He denies having a fever, gaining or losing weight, rashes, headaches, neck stiffness, tingling or weakness or stiffness of limbs, vertigo, visual changes, photophobia, unsteady gait, bowel or bladder incontinence, or tremors.

When the neurologist repeats the MoCA, Mr. M again scores 12. The neurologist notes that Mr. M answers questions a little slowly and pauses for thoughts when unable to find an answer. Mr. M has difficulty following some simple commands, such as “touch a finger to your nose.” Other in-office neurologic physical exams (cranial nerves, involuntary movements or tremors, sensation, muscle strength, reflexes, cerebellar signs) are unremarkable except for mildly decreased vibration sense of his toes. The neurologist concludes that Mr. M’s presentation is suggestive of subacute to chronic bradyphrenia and orders additional evaluation, including neuropsychological testing.
[polldaddy:11320114]
The authors’ observations
Physical and neurologic exams were not suggestive of any obvious causes of cognitive decline. Both the mental status exam and 2 serial MoCAs suggested deficits in executive function, language, and memory. Each of the differential diagnoses considered was ruled out with workup or exams (Table 2), which led to a most likely diagnosis of neurodegenerative disorder with PPA. Neuropsychological testing confirmed the diagnosis of nonfluent PPA.

Primary progressive aphasia
PPA is an uncommon, heterogeneous group of disorders stemming from focal degeneration of language-governing centers of the brain.1,2 The estimated prevalence of PPA is 3 in 100,000 cases.2,3 There are 4 major variants of PPA (Table 34), and each presents with distinct language, cognitive, neuroanatomical, and neuropathological characteristics.4 PPA is usually diagnosed in late middle life; however, diagnosis is often delayed due to the relative obscurity of the disorder.4 In Mr. M’s case, it took approximately 4 months of evaluations by various specialists before a diagnosis was confirmed.

The initial phase of PPA can present as a diagnostic challenge because patients can have difficulty articulating their cognitive and language deficits. PPA can be commonly mistaken for a primary psychiatric disorder such as MDD or anxiety, which can further delay an accurate diagnosis and treatment. Special attention to the mental status exam, close observation of the patient’s language, and assessment of cognitive abilities using standardized screenings such as the MoCA or Mini-Mental State Examination can be helpful in clarifying the diagnosis. It is also important to rule out developmental problems (eg, dyslexia) and hearing difficulties, particularly in older patients.
Continue to: TREATMENT Adjusting the medication regimen
TREATMENT Adjusting the medication regimen
The neurologist completes additional examinations to rule out causes of rare neurodegenerative disorders, including CSF autoimmune disorders, Creutzfeldt-Jakob disease, and Alzheimer disease (AD) (Table 4). Mr. M continues to follow up with his outpatient psychiatrist and his medication regimen is adjusted. Aripiprazole and buspirone are discontinued, and duloxetine is titrated to 60 mg twice a day. During follow-up visits, Mr. M discusses his understanding of his neurologic condition. His concerns shift to his illness and prognosis. During these visits, he continues to deny suicidality.

[polldaddy:11320115]
The authors’ observations
Mr. M’s neurodegenerative workup identified an intriguing diagnostic challenge. A repeat brain MRI (Figure) showed atrophy patterns suggestive of frontotemporal lobar degeneration (FTLD). On the other hand, his CSF ATI (A-beta 42/T-tau index, a value used to aid in the diagnosis of AD) was <1, suggesting early-onset AD.5,6 Although significant advances have been made to distinguish AD and FTLD following an autopsy, there are still no reliable or definitive biomarkers to distinguish AD from FTLD (particularly in the early stages of FTLD). This can often leave the confirmatory diagnosis as a question.7

A PPA diagnosis (and other dementias) can have a significant impact on the patient and their family due to the uncertain nature of the progression of the disease and quality-of-life issues related to language and other cognitive deficits. Early identification and accurate diagnosis of PPA and its etiology (ie, AD vs FTLD) is important to avoid unnecessary exposure to medications or the use of polypharmacy to treat an inaccurate diagnosis of a primary psychiatric illness. For example, Mr. M was being treated with 3 psychiatric medications (aripiprazole, buspirone, and duloxetine) for depression and anxiety prior to the diagnosis of PPA.
Nonpharmacologic interventions can play an important role in the management of patients with PPA. These include educating the patient and their family about the diagnosis and discussions about future planning, including appropriate social support, employment, and finances.4 Pharmacologic interventions may be limited, as there are currently no disease-modifying treatments for PPA or FTLD. For patients with nonfluent PPA or AD, cholinesterase inhibitors such as donepezil or N-methyl
Psychiatrists should continue to treat patients with PPA for comorbid anxiety or depression, with appropriate medications and/or supportive therapy to guide the patient through the process of grief. Assessing for suicide risk is also important in patients diagnosed with dementia. A retrospective cohort study of patients age ≥60 with a diagnosis of dementia suggested that the majority of suicides occurred in those with a new dementia diagnosis.9 End-of-life decisions such as advanced directives should be made when the patient still has legal capacity, ideally as soon as possible after diagnosis.10
OUTCOME Remaining engaged in treatment
Mr. M continues to follow-up with the Neurology team. He has also been regularly seeing his psychiatric team for medication management and supportive therapy, and his psychiatric medications have been optimized to reduce polypharmacy. During his sessions, Mr. M discusses his grief and plans for the future. Despite his anxiety about the uncertainty of his prognosis, Mr. M continues to report that he is doing reasonably well and remains engaged in treatment.
Bottom Line
Patients with primary progressive aphasia and rare neurodegenerative disorders may present to an outpatient or emergency setting with symptoms of anxiety and confusion. They are frequently misdiagnosed with a primary psychiatric disorder due to the nature of cognitive and language deficits, particularly in the early stages of the disease. Paying close attention to language and conducting cognitive screening are critical in identifying the true cause of a patient’s symptoms.
Related Resources
- Primary progressive aphasia. National Center for Advancing Translational Sciences. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/8541/primary-progressive-aphasia
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: A useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
Drug Brand Names
Aripiprazole • Abilify
Donepezil • Aricept
Duloxetine • Cymbalta
Memantine • Namenda
1. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88-97. doi:10.1038/nrneurol.2009.216
2. Mesulam M-M, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10(10):554-569. doi:10.1038/nrneurol.2014.159
3. Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi:10.1212/WNL.0000000000002638
4. Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: a clinical approach. J Neurol. 2018;265(6):1474-1490. doi:10.1007/s00415-018-8762-6
5. Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1(2):213-225. doi:10.1602/neurorx.1.2.213
6. Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555-1562. doi:10.1212/wnl.52.8.1555
7. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. doi:10.1038/s41591-020-0762-2
8. Newhart M, Davis C, Kannan V, et al. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7-8):823-834. doi:10.1080/02687030802661762
9. Seyfried LS, Kales HC, Ignacio RV, et al. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567-573. doi:10.1016/j.jalz.2011.01.006
10. Porteri C. Advance directives as a tool to respect patients’ values and preferences: discussion on the case of Alzheimer’s disease. BMC Med Ethics. 2018;19(1):9. doi:10.1186/s12910-018-0249-6
1. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88-97. doi:10.1038/nrneurol.2009.216
2. Mesulam M-M, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10(10):554-569. doi:10.1038/nrneurol.2014.159
3. Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi:10.1212/WNL.0000000000002638
4. Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: a clinical approach. J Neurol. 2018;265(6):1474-1490. doi:10.1007/s00415-018-8762-6
5. Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1(2):213-225. doi:10.1602/neurorx.1.2.213
6. Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555-1562. doi:10.1212/wnl.52.8.1555
7. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. doi:10.1038/s41591-020-0762-2
8. Newhart M, Davis C, Kannan V, et al. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7-8):823-834. doi:10.1080/02687030802661762
9. Seyfried LS, Kales HC, Ignacio RV, et al. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567-573. doi:10.1016/j.jalz.2011.01.006
10. Porteri C. Advance directives as a tool to respect patients’ values and preferences: discussion on the case of Alzheimer’s disease. BMC Med Ethics. 2018;19(1):9. doi:10.1186/s12910-018-0249-6
More on psilocybin
I would like to remark on “Psychedelics for treating psychiatric disorders: Are they safe?” (
The Oregon Psilocybin Services that will begin in 2023 are not specific to therapeutic use; this is a common misconception. These are specifically referred to as “psilocybin services” in the Oregon Administrative Rules (OAR), and psilocybin facilitators are required to limit their scope such that they are not practicing psychotherapy or other interventions, even if they do have a medical or psychotherapy background. The intention of the Oregon Psilocybin Services rollout was that these services would not be of the medical model. In the spirit of this, services do not require a medical diagnosis or referral, and services are not a medical or clinical treatment (OAR 333-333-5040). Additionally, services cannot be provided in a health care facility (OAR 441). Facilitators receive robust training as defined by Oregon law, and licensed facilitators provide this information during preparation for services. When discussing this model on a large public scale, I have noticed substantial misconceptions; it is imperative that we refer to these services as they are defined so that individuals with mental health conditions who seek them are aware that such services are different from psilocybin-assisted psychotherapy. Instead, Oregon Psilocybin Services might be better categorized as supported psilocybin use.
I would like to remark on “Psychedelics for treating psychiatric disorders: Are they safe?” (
The Oregon Psilocybin Services that will begin in 2023 are not specific to therapeutic use; this is a common misconception. These are specifically referred to as “psilocybin services” in the Oregon Administrative Rules (OAR), and psilocybin facilitators are required to limit their scope such that they are not practicing psychotherapy or other interventions, even if they do have a medical or psychotherapy background. The intention of the Oregon Psilocybin Services rollout was that these services would not be of the medical model. In the spirit of this, services do not require a medical diagnosis or referral, and services are not a medical or clinical treatment (OAR 333-333-5040). Additionally, services cannot be provided in a health care facility (OAR 441). Facilitators receive robust training as defined by Oregon law, and licensed facilitators provide this information during preparation for services. When discussing this model on a large public scale, I have noticed substantial misconceptions; it is imperative that we refer to these services as they are defined so that individuals with mental health conditions who seek them are aware that such services are different from psilocybin-assisted psychotherapy. Instead, Oregon Psilocybin Services might be better categorized as supported psilocybin use.
I would like to remark on “Psychedelics for treating psychiatric disorders: Are they safe?” (
The Oregon Psilocybin Services that will begin in 2023 are not specific to therapeutic use; this is a common misconception. These are specifically referred to as “psilocybin services” in the Oregon Administrative Rules (OAR), and psilocybin facilitators are required to limit their scope such that they are not practicing psychotherapy or other interventions, even if they do have a medical or psychotherapy background. The intention of the Oregon Psilocybin Services rollout was that these services would not be of the medical model. In the spirit of this, services do not require a medical diagnosis or referral, and services are not a medical or clinical treatment (OAR 333-333-5040). Additionally, services cannot be provided in a health care facility (OAR 441). Facilitators receive robust training as defined by Oregon law, and licensed facilitators provide this information during preparation for services. When discussing this model on a large public scale, I have noticed substantial misconceptions; it is imperative that we refer to these services as they are defined so that individuals with mental health conditions who seek them are aware that such services are different from psilocybin-assisted psychotherapy. Instead, Oregon Psilocybin Services might be better categorized as supported psilocybin use.
Guidelines recommend CBT alone for mild acute depression, more options for more severe cases
The guidelines also state that patients with mild depression should start with CBT alone, and if a patient with moderate to severe depression prefers, they can use a combination of both CBT and an SGA.
These nuanced recommendations contrast sharply with the 2016 ACP guidelines for depression, which lumped all stages and severity levels together, and came with just one recommendation: Clinicians should choose between CBT and an SGA.
More data have come to light over the years, requiring the present update, reported lead author Amir Qaseem, MD, PhD, vice president of Clinical Policy and the Center for Evidence Reviews at the ACP, and adjunct faculty at Thomas Jefferson University, Philadelphia, and colleagues.
In addition to the focus on acute depression, Dr. Qaseem and colleagues highlighted the new guidelines' “consideration of patient values and preferences, and costs,” as well as responses to therapy.
Recommendations were derived from a network meta-analysis that included studies evaluating nonpharmacologic and pharmacologic therapies, the authors wrote in Annals of Internal Medicine. They compared effectiveness across a range of SGAs, “including selective serotonin reuptake inhibitors; serotonin-norepinephrine reuptake inhibitors; and others such as bupropion, mirtazapine, nefazodone, trazodone, vilazodone, and vortioxetine.”
This analysis yielded three pieces of clinical advice.
First, patients in the acute phase of mild depression should receive CBT alone as their initial treatment.
Dr. Qaseem and colleagues noted that many depression studies for pharmacologic therapies excluded these patients in favor of those with moderate to severe depression, leaving an evidence gap.
“Furthermore, the Clinical Guidelines Committee had concerns about adverse effects of SGAs in these patients and suggests that the use of SGAs as initial treatment of these patients should be based on additional considerations, such as limited access to or cost of CBT, history of moderate or severe major depressive disorder, or patient preferences,” they added.
The committee’s next recommendation, based on moderate-certainty evidence, suggested that CBT alone or an SGA alone should be considered for patients in the acute phase of moderate to severe depression. This call for monotherapy is balanced by a conditional recommendation based on low-certainty evidence that the same group may benefit from initial combination therapy with both CBT and an SGA.
“The informed decision on the options of monotherapy with CBT versus SGAs, or combination therapy, should be personalized and based on discussion of potential treatment benefits, harms, adverse effect profiles, cost, feasibility, patients’ specific symptoms (such as insomnia, hypersomnia, or fluctuation in appetite), comorbidities, concomitant medication use, and patient preferences,” the guidelines state.
The third and final recommendation offers an algorithm for patients who do not respond to initial therapy with an SGA. Multiple pathways are provided: Switch to CBT or augment with CBT; or switch to a different SGA or augment with a second pharmacologic therapy, such as mirtazapine, bupropion, or buspirone.
“These second-line treatment strategies show similar efficacy when compared with each other,” the guidelines committee noted.
Again, the guidelines suggest that second-line choices should be personalized based on the various factors previously discussed.
A timely update
“The new guideline is very different from the last guideline,” said Ryan Mire, MD, president of the ACP and practicing internal medicine physician in Nashville, Tenn. in a written comment. “ACP decided to update the depression guidelines with a focus on acute depression because approximately 70% of patients with major depressive disorder do not achieve remission and remain in the acute phase after the initial pharmacologic treatment attempt. In addition, there is new evidence on second-line treatments since the 2016 ACP guideline was published.”
Neil S. Skolnik, MD, of Thomas Jefferson University, Philadelphia, agreed that the guidelines offer a necessary and fresh perspective on caring for patients with depression.
“These guidelines are a helpful update, assuring us that we are using the latest, evidence-based therapies, and [they] are written in a practical, easy-to-implement manner,” Dr. Skolnik said in a written comment.
“First, the guidelines reaffirm that CBT is an effective first-line option, with or without the concurrent use of an SGA,” Dr. Skolnik said, noting that CBT alone may reduce likelihood of recurrence, compared with an SGA alone. “Many patients do not like the idea of medication, or the potential side effects of medications, and CBT is an evidenced-based approach that can be very helpful for patients.”
Dr. Skolnik also applauded the guidelines authors for offering a clear path forward for patients who do not have full remission after treatment – a common clinical scenario.
He went on to offer some more detailed steps forward.
“If someone chooses to be treated with an SGA alone and has not had much response at all to an initial SGA, usually a selective serotonin reuptake inhibitor, I’ll usually switch to a different SSRI or serotonin and norepinephrine reuptake inhibitor (SNRI) and/or add CBT,” Dr. Skolnik said. “If they have had a partial response, I’ll often encourage CBT and consider the addition of augmentation with an additional medication as discussed in the guidelines.”
Valuable despite the gaps
Other experts expressed mixed impressions of the update, noting both highs and lows.
“Although [this guideline] has some gaps, it is more valuable in several ways than other widely consulted practice guidelines for depression,” wrote Miriam Shuchman, MD and Elia Abi-Jaoude, MSc, MD, PhD, of the University of Toronto, in an accompanying editorial.
Specifically, they praised the publication’s focus on shared decision-making in the treatment planning process.
“This effort to respond to patient preferences is crucial and may even increase the chance that patients will improve with treatment,” they wrote.
They also applauded the ACP’s efforts to recuse any committee members who may have had conflicts of interest “that could affect their judgment about treatments for depression.”
After highlighting these attributes, Dr. Shuchman and Dr. Abi-Jaoude noted that the guidelines still contain “significant gaps.”
Foremost, they pointed out the guidelines' emphasis on CBT to the exclusion of other nonpharmacologic options.
“The guideline does patients a disservice by leaving out several nonmedication treatment options that clinicians can offer as first- or second-line therapies,” they wrote.
This oversight may increase risk that patients simply hop from one SGA to another, which is a common, and often ineffective, strategy, according to Dr. Shuchman and Dr. Abi-Jaoude.
“Patients often go from one drug to the next in the hopes of landing on one that ‘works,’ ” the editorialists wrote. “This narrow clinical approach of pursuing medication-based treatments ignores the ways difficulties in a person’s work or relationships may contribute to their struggles with depression. At a time when the COVID-19 pandemic has underscored the importance of the social context of mental health, clinicians may need to consider other forms of support and tailor prescribing to what is most relevant and accessible for a particular patient.”
Dr. Shuchman and Dr. Abi-Jaoude went on to suggest several nonpharmacologic options beyond CBT, including interpersonal therapy, psychodynamic therapy, problem solving, behavioral activation, and guided self-help.
The other key gap they pointed out relates to withdrawal.
Although the guideline does advise physicians to taper antidepressants to reduce risk of withdrawal, the editorialists suggested that this recommendation lacked sufficient emphasis, as it can be a particularly difficult period in the treatment process.
“Tapering of an antidepressant may need to be done over months or years, not weeks, and a patient may need to visit a compounding pharmacy to obtain doses of a second-generation antidepressant not marketed by drug manufacturers so that prescriptions can be tapered even more slowly,” they suggested.
Financial costs remain unclear
Beyond the above medical considerations, one other piece of the depression puzzle remains unsolved: cost.
In a simultaneously published rapid review, Andreea Dobrescu, MD, PhD, of Cochrane Austria, and colleagues evaluated the relative cost-effectiveness of first- and second-step treatment strategies.
For most comparisons, evidence was insufficient to reach a conclusion, although they suggested that CBT may be more cost effective at the 5-year mark.
“For most pharmacologic and nonpharmacologic interventions for major depressive disorder, evidence was missing or was insufficient to draw conclusions about the cost-effectiveness of first- or second-step treatments for MDD,” Dr. Dobrescu and colleagues wrote. “The strongest evidence (albeit still low certainty of evidence) was for the cost-effectiveness of CBT compared with SGA as a first-step treatment over a 5-year time horizon from the societal and health care sector perspectives. However, this evidence should also be interpreted cautiously considering it is based on a single study.”
When asked about the financial findings, Dr. Mire agreed that more data are needed, especially because CBT and SGA costs range widely. He suggested that cost, for each patient, should be considered in the personalized approach now highlighted by the new guidelines.
The guidelines and the Cochrane cost-effectiveness study were supported by the ACP. The guidelines' authors and other individuals quoted in this article reported no conflicts of interest.
The guidelines also state that patients with mild depression should start with CBT alone, and if a patient with moderate to severe depression prefers, they can use a combination of both CBT and an SGA.
These nuanced recommendations contrast sharply with the 2016 ACP guidelines for depression, which lumped all stages and severity levels together, and came with just one recommendation: Clinicians should choose between CBT and an SGA.
More data have come to light over the years, requiring the present update, reported lead author Amir Qaseem, MD, PhD, vice president of Clinical Policy and the Center for Evidence Reviews at the ACP, and adjunct faculty at Thomas Jefferson University, Philadelphia, and colleagues.
In addition to the focus on acute depression, Dr. Qaseem and colleagues highlighted the new guidelines' “consideration of patient values and preferences, and costs,” as well as responses to therapy.
Recommendations were derived from a network meta-analysis that included studies evaluating nonpharmacologic and pharmacologic therapies, the authors wrote in Annals of Internal Medicine. They compared effectiveness across a range of SGAs, “including selective serotonin reuptake inhibitors; serotonin-norepinephrine reuptake inhibitors; and others such as bupropion, mirtazapine, nefazodone, trazodone, vilazodone, and vortioxetine.”
This analysis yielded three pieces of clinical advice.
First, patients in the acute phase of mild depression should receive CBT alone as their initial treatment.
Dr. Qaseem and colleagues noted that many depression studies for pharmacologic therapies excluded these patients in favor of those with moderate to severe depression, leaving an evidence gap.
“Furthermore, the Clinical Guidelines Committee had concerns about adverse effects of SGAs in these patients and suggests that the use of SGAs as initial treatment of these patients should be based on additional considerations, such as limited access to or cost of CBT, history of moderate or severe major depressive disorder, or patient preferences,” they added.
The committee’s next recommendation, based on moderate-certainty evidence, suggested that CBT alone or an SGA alone should be considered for patients in the acute phase of moderate to severe depression. This call for monotherapy is balanced by a conditional recommendation based on low-certainty evidence that the same group may benefit from initial combination therapy with both CBT and an SGA.
“The informed decision on the options of monotherapy with CBT versus SGAs, or combination therapy, should be personalized and based on discussion of potential treatment benefits, harms, adverse effect profiles, cost, feasibility, patients’ specific symptoms (such as insomnia, hypersomnia, or fluctuation in appetite), comorbidities, concomitant medication use, and patient preferences,” the guidelines state.
The third and final recommendation offers an algorithm for patients who do not respond to initial therapy with an SGA. Multiple pathways are provided: Switch to CBT or augment with CBT; or switch to a different SGA or augment with a second pharmacologic therapy, such as mirtazapine, bupropion, or buspirone.
“These second-line treatment strategies show similar efficacy when compared with each other,” the guidelines committee noted.
Again, the guidelines suggest that second-line choices should be personalized based on the various factors previously discussed.
A timely update
“The new guideline is very different from the last guideline,” said Ryan Mire, MD, president of the ACP and practicing internal medicine physician in Nashville, Tenn. in a written comment. “ACP decided to update the depression guidelines with a focus on acute depression because approximately 70% of patients with major depressive disorder do not achieve remission and remain in the acute phase after the initial pharmacologic treatment attempt. In addition, there is new evidence on second-line treatments since the 2016 ACP guideline was published.”
Neil S. Skolnik, MD, of Thomas Jefferson University, Philadelphia, agreed that the guidelines offer a necessary and fresh perspective on caring for patients with depression.
“These guidelines are a helpful update, assuring us that we are using the latest, evidence-based therapies, and [they] are written in a practical, easy-to-implement manner,” Dr. Skolnik said in a written comment.
“First, the guidelines reaffirm that CBT is an effective first-line option, with or without the concurrent use of an SGA,” Dr. Skolnik said, noting that CBT alone may reduce likelihood of recurrence, compared with an SGA alone. “Many patients do not like the idea of medication, or the potential side effects of medications, and CBT is an evidenced-based approach that can be very helpful for patients.”
Dr. Skolnik also applauded the guidelines authors for offering a clear path forward for patients who do not have full remission after treatment – a common clinical scenario.
He went on to offer some more detailed steps forward.
“If someone chooses to be treated with an SGA alone and has not had much response at all to an initial SGA, usually a selective serotonin reuptake inhibitor, I’ll usually switch to a different SSRI or serotonin and norepinephrine reuptake inhibitor (SNRI) and/or add CBT,” Dr. Skolnik said. “If they have had a partial response, I’ll often encourage CBT and consider the addition of augmentation with an additional medication as discussed in the guidelines.”
Valuable despite the gaps
Other experts expressed mixed impressions of the update, noting both highs and lows.
“Although [this guideline] has some gaps, it is more valuable in several ways than other widely consulted practice guidelines for depression,” wrote Miriam Shuchman, MD and Elia Abi-Jaoude, MSc, MD, PhD, of the University of Toronto, in an accompanying editorial.
Specifically, they praised the publication’s focus on shared decision-making in the treatment planning process.
“This effort to respond to patient preferences is crucial and may even increase the chance that patients will improve with treatment,” they wrote.
They also applauded the ACP’s efforts to recuse any committee members who may have had conflicts of interest “that could affect their judgment about treatments for depression.”
After highlighting these attributes, Dr. Shuchman and Dr. Abi-Jaoude noted that the guidelines still contain “significant gaps.”
Foremost, they pointed out the guidelines' emphasis on CBT to the exclusion of other nonpharmacologic options.
“The guideline does patients a disservice by leaving out several nonmedication treatment options that clinicians can offer as first- or second-line therapies,” they wrote.
This oversight may increase risk that patients simply hop from one SGA to another, which is a common, and often ineffective, strategy, according to Dr. Shuchman and Dr. Abi-Jaoude.
“Patients often go from one drug to the next in the hopes of landing on one that ‘works,’ ” the editorialists wrote. “This narrow clinical approach of pursuing medication-based treatments ignores the ways difficulties in a person’s work or relationships may contribute to their struggles with depression. At a time when the COVID-19 pandemic has underscored the importance of the social context of mental health, clinicians may need to consider other forms of support and tailor prescribing to what is most relevant and accessible for a particular patient.”
Dr. Shuchman and Dr. Abi-Jaoude went on to suggest several nonpharmacologic options beyond CBT, including interpersonal therapy, psychodynamic therapy, problem solving, behavioral activation, and guided self-help.
The other key gap they pointed out relates to withdrawal.
Although the guideline does advise physicians to taper antidepressants to reduce risk of withdrawal, the editorialists suggested that this recommendation lacked sufficient emphasis, as it can be a particularly difficult period in the treatment process.
“Tapering of an antidepressant may need to be done over months or years, not weeks, and a patient may need to visit a compounding pharmacy to obtain doses of a second-generation antidepressant not marketed by drug manufacturers so that prescriptions can be tapered even more slowly,” they suggested.
Financial costs remain unclear
Beyond the above medical considerations, one other piece of the depression puzzle remains unsolved: cost.
In a simultaneously published rapid review, Andreea Dobrescu, MD, PhD, of Cochrane Austria, and colleagues evaluated the relative cost-effectiveness of first- and second-step treatment strategies.
For most comparisons, evidence was insufficient to reach a conclusion, although they suggested that CBT may be more cost effective at the 5-year mark.
“For most pharmacologic and nonpharmacologic interventions for major depressive disorder, evidence was missing or was insufficient to draw conclusions about the cost-effectiveness of first- or second-step treatments for MDD,” Dr. Dobrescu and colleagues wrote. “The strongest evidence (albeit still low certainty of evidence) was for the cost-effectiveness of CBT compared with SGA as a first-step treatment over a 5-year time horizon from the societal and health care sector perspectives. However, this evidence should also be interpreted cautiously considering it is based on a single study.”
When asked about the financial findings, Dr. Mire agreed that more data are needed, especially because CBT and SGA costs range widely. He suggested that cost, for each patient, should be considered in the personalized approach now highlighted by the new guidelines.
The guidelines and the Cochrane cost-effectiveness study were supported by the ACP. The guidelines' authors and other individuals quoted in this article reported no conflicts of interest.
The guidelines also state that patients with mild depression should start with CBT alone, and if a patient with moderate to severe depression prefers, they can use a combination of both CBT and an SGA.
These nuanced recommendations contrast sharply with the 2016 ACP guidelines for depression, which lumped all stages and severity levels together, and came with just one recommendation: Clinicians should choose between CBT and an SGA.
More data have come to light over the years, requiring the present update, reported lead author Amir Qaseem, MD, PhD, vice president of Clinical Policy and the Center for Evidence Reviews at the ACP, and adjunct faculty at Thomas Jefferson University, Philadelphia, and colleagues.
In addition to the focus on acute depression, Dr. Qaseem and colleagues highlighted the new guidelines' “consideration of patient values and preferences, and costs,” as well as responses to therapy.
Recommendations were derived from a network meta-analysis that included studies evaluating nonpharmacologic and pharmacologic therapies, the authors wrote in Annals of Internal Medicine. They compared effectiveness across a range of SGAs, “including selective serotonin reuptake inhibitors; serotonin-norepinephrine reuptake inhibitors; and others such as bupropion, mirtazapine, nefazodone, trazodone, vilazodone, and vortioxetine.”
This analysis yielded three pieces of clinical advice.
First, patients in the acute phase of mild depression should receive CBT alone as their initial treatment.
Dr. Qaseem and colleagues noted that many depression studies for pharmacologic therapies excluded these patients in favor of those with moderate to severe depression, leaving an evidence gap.
“Furthermore, the Clinical Guidelines Committee had concerns about adverse effects of SGAs in these patients and suggests that the use of SGAs as initial treatment of these patients should be based on additional considerations, such as limited access to or cost of CBT, history of moderate or severe major depressive disorder, or patient preferences,” they added.
The committee’s next recommendation, based on moderate-certainty evidence, suggested that CBT alone or an SGA alone should be considered for patients in the acute phase of moderate to severe depression. This call for monotherapy is balanced by a conditional recommendation based on low-certainty evidence that the same group may benefit from initial combination therapy with both CBT and an SGA.
“The informed decision on the options of monotherapy with CBT versus SGAs, or combination therapy, should be personalized and based on discussion of potential treatment benefits, harms, adverse effect profiles, cost, feasibility, patients’ specific symptoms (such as insomnia, hypersomnia, or fluctuation in appetite), comorbidities, concomitant medication use, and patient preferences,” the guidelines state.
The third and final recommendation offers an algorithm for patients who do not respond to initial therapy with an SGA. Multiple pathways are provided: Switch to CBT or augment with CBT; or switch to a different SGA or augment with a second pharmacologic therapy, such as mirtazapine, bupropion, or buspirone.
“These second-line treatment strategies show similar efficacy when compared with each other,” the guidelines committee noted.
Again, the guidelines suggest that second-line choices should be personalized based on the various factors previously discussed.
A timely update
“The new guideline is very different from the last guideline,” said Ryan Mire, MD, president of the ACP and practicing internal medicine physician in Nashville, Tenn. in a written comment. “ACP decided to update the depression guidelines with a focus on acute depression because approximately 70% of patients with major depressive disorder do not achieve remission and remain in the acute phase after the initial pharmacologic treatment attempt. In addition, there is new evidence on second-line treatments since the 2016 ACP guideline was published.”
Neil S. Skolnik, MD, of Thomas Jefferson University, Philadelphia, agreed that the guidelines offer a necessary and fresh perspective on caring for patients with depression.
“These guidelines are a helpful update, assuring us that we are using the latest, evidence-based therapies, and [they] are written in a practical, easy-to-implement manner,” Dr. Skolnik said in a written comment.
“First, the guidelines reaffirm that CBT is an effective first-line option, with or without the concurrent use of an SGA,” Dr. Skolnik said, noting that CBT alone may reduce likelihood of recurrence, compared with an SGA alone. “Many patients do not like the idea of medication, or the potential side effects of medications, and CBT is an evidenced-based approach that can be very helpful for patients.”
Dr. Skolnik also applauded the guidelines authors for offering a clear path forward for patients who do not have full remission after treatment – a common clinical scenario.
He went on to offer some more detailed steps forward.
“If someone chooses to be treated with an SGA alone and has not had much response at all to an initial SGA, usually a selective serotonin reuptake inhibitor, I’ll usually switch to a different SSRI or serotonin and norepinephrine reuptake inhibitor (SNRI) and/or add CBT,” Dr. Skolnik said. “If they have had a partial response, I’ll often encourage CBT and consider the addition of augmentation with an additional medication as discussed in the guidelines.”
Valuable despite the gaps
Other experts expressed mixed impressions of the update, noting both highs and lows.
“Although [this guideline] has some gaps, it is more valuable in several ways than other widely consulted practice guidelines for depression,” wrote Miriam Shuchman, MD and Elia Abi-Jaoude, MSc, MD, PhD, of the University of Toronto, in an accompanying editorial.
Specifically, they praised the publication’s focus on shared decision-making in the treatment planning process.
“This effort to respond to patient preferences is crucial and may even increase the chance that patients will improve with treatment,” they wrote.
They also applauded the ACP’s efforts to recuse any committee members who may have had conflicts of interest “that could affect their judgment about treatments for depression.”
After highlighting these attributes, Dr. Shuchman and Dr. Abi-Jaoude noted that the guidelines still contain “significant gaps.”
Foremost, they pointed out the guidelines' emphasis on CBT to the exclusion of other nonpharmacologic options.
“The guideline does patients a disservice by leaving out several nonmedication treatment options that clinicians can offer as first- or second-line therapies,” they wrote.
This oversight may increase risk that patients simply hop from one SGA to another, which is a common, and often ineffective, strategy, according to Dr. Shuchman and Dr. Abi-Jaoude.
“Patients often go from one drug to the next in the hopes of landing on one that ‘works,’ ” the editorialists wrote. “This narrow clinical approach of pursuing medication-based treatments ignores the ways difficulties in a person’s work or relationships may contribute to their struggles with depression. At a time when the COVID-19 pandemic has underscored the importance of the social context of mental health, clinicians may need to consider other forms of support and tailor prescribing to what is most relevant and accessible for a particular patient.”
Dr. Shuchman and Dr. Abi-Jaoude went on to suggest several nonpharmacologic options beyond CBT, including interpersonal therapy, psychodynamic therapy, problem solving, behavioral activation, and guided self-help.
The other key gap they pointed out relates to withdrawal.
Although the guideline does advise physicians to taper antidepressants to reduce risk of withdrawal, the editorialists suggested that this recommendation lacked sufficient emphasis, as it can be a particularly difficult period in the treatment process.
“Tapering of an antidepressant may need to be done over months or years, not weeks, and a patient may need to visit a compounding pharmacy to obtain doses of a second-generation antidepressant not marketed by drug manufacturers so that prescriptions can be tapered even more slowly,” they suggested.
Financial costs remain unclear
Beyond the above medical considerations, one other piece of the depression puzzle remains unsolved: cost.
In a simultaneously published rapid review, Andreea Dobrescu, MD, PhD, of Cochrane Austria, and colleagues evaluated the relative cost-effectiveness of first- and second-step treatment strategies.
For most comparisons, evidence was insufficient to reach a conclusion, although they suggested that CBT may be more cost effective at the 5-year mark.
“For most pharmacologic and nonpharmacologic interventions for major depressive disorder, evidence was missing or was insufficient to draw conclusions about the cost-effectiveness of first- or second-step treatments for MDD,” Dr. Dobrescu and colleagues wrote. “The strongest evidence (albeit still low certainty of evidence) was for the cost-effectiveness of CBT compared with SGA as a first-step treatment over a 5-year time horizon from the societal and health care sector perspectives. However, this evidence should also be interpreted cautiously considering it is based on a single study.”
When asked about the financial findings, Dr. Mire agreed that more data are needed, especially because CBT and SGA costs range widely. He suggested that cost, for each patient, should be considered in the personalized approach now highlighted by the new guidelines.
The guidelines and the Cochrane cost-effectiveness study were supported by the ACP. The guidelines' authors and other individuals quoted in this article reported no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Geriatrician advises on use of vitamin D supplementation, lecanemab, and texting for her patients
Vitamin D supplementation and incident fractures
Vitamin D supplementation is a commonly recommended intervention for bone health, but data to support its impact on reducing fracture risk has been variable.
A study in the New England Journal of Medicine by LeBoff and colleagues has garnered much attention since its publication in July 2022.1 In the ancillary study of the Vitamin D and Omega-3-Trial (VITAL), the authors examined the impact of vitamin D supplementation versus placebo on incident fractures. The study found that vitamin D supplementation, as compared with placebo, led to no significant difference in the incidence of total, nonvertebral, and hip fractures in midlife and older adults over the 5-year period of follow-up.
The generalizability of these findings has been raised as a concern as the study does not describe adults at higher risk for fracture. The authors of the study specified in their conclusion that vitamin D supplementation does not reduce fracture risk in “generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass or osteoporosis.”
With a mean participant age of 67 and exclusion of participants with a history of cardiovascular disease, stroke, cirrhosis and other serious illnesses, the study does not reflect the multimorbid older adult population that geriatricians typically care for. Furthermore, efficacy of vitamin D supplementation on fracture risk may be the most impactful in those with osteoporosis and with severe vitamin D deficiency (defined by vitamin D 25[OH]D level less than 12 ng/mL).
In post hoc analyses, there was no significant difference in fracture risk in these subgroups, however the authors acknowledged that the findings may be limited by the small percentage of participants with severe vitamin D deficiency (2.4%) and osteoporosis included in the study (5%).
Lecanemab for mild cognitive impairment and early Alzheimer’s dementia
On Jan. 6, 2023, the Food and Drug Administration approved lecanemab, the second-ever disease-modifying treatment for Alzheimer’s dementia following the approval of aducanumab in 2021. Lecanemab is a monoclonal antibody targeting larger amyloid-beta oligomers, which has been shown in vitro to have higher affinity for amyloid-beta, compared with aducanumab. FDA approval followed shortly after the publication of the CLARITY-AD trial, which investigated the effect of lecanemab versus placebo on cognitive decline and burden of amyloid in adults with mild cognitive impairment and mild Alzheimer’s dementia. Over an 18-month period, the study found that participants who received lecanemab, compared with placebo, had a significantly smaller decline in cognition and function, and reduction in amyloid burden on PET CT.2
The clinical significance of these findings, however, is unclear. As noted by an editorial published in the Lancet in 2022, the difference in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scale between the treatment and placebo groups was 0.45. On an 18-point scale, prior research has noted that a minimal clinically significance difference of 0.98 is necessary in those with mild cognitive impairment and 1.63 in mild Alzheimer dementia.3
Additionally, the CLARITY-AD trial reported that lecanemab resulted in infusion reactions in 26.4% of participants and brain edema (an amyloid-related imaging abnormality referred to as ARIA-E) in 12.6% of participants. This finding highlights concerns for safety and the need for close monitoring, as well as ongoing implications of economic feasibility and equitable access for all those who qualify for treatment.2
Social isolation and dementia risk
There is growing awareness of the impact of social isolation on health outcomes, particularly among older adults. Prior research has reported that one in four older adults are considered socially isolated and that social isolation increases risk of premature death, dementia, depression, and cardiovascular disease.4
A study by Huang and colleagues is the first nationally representative cohort study examining the association between social isolation and incident dementia for older adults in community dwelling settings. A cohort of 5,022 older adults participating in the National Health and Aging Trends Study was followed from 2011 to 2020. When adjusting for demographic and health factors, including race, level of education, and number of chronic health conditions, socially isolated adults had a greater risk of developing dementia, compared with adults who were not socially isolated (hazard ratio, 1.27; 95% confidence interval, 1.08-1.49). Potential mechanisms to explain this association include the increased risk of cardiovascular disease and depression in older adults who are socially isolated, thereby increasing dementia risk.
Decreased cognitive activity/engagement and access to resources such as caregiving and health care may also be linked to the increased risk of dementia in socially isolated older adults.5
Another observational cohort study from the National Health and Aging Trends Study investigated whether access and use of technology can lower the risk of social isolation. The study found that older adults who used email or text messaging had a lower risk of social isolation than older adults who did not use technology (incidence rate ratio, 0.64; 95% CI, 0.51-0.80).6 These findings highlight the importance of addressing social isolation as an important modifiable health risk factor, and the need for providing equitable access to technology in vulnerable populations as health intervention.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. LeBoff MS et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299-30.
2. van Dyck CH et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
3. The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022; 400:1899.
4. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: 2020, The National Academies Press.
5. Huang, AR et al. Social isolation and 9-year dementia risk in community dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023 Jan 11. doi: 10.1111/jgs18140.
6. Umoh ME etal. Impact of technology on social isolation: Longitudinal analysis from the National Health Aging Trends Study. J Am Geriatr Soc. 2022 Dec 15. doi 10.1111/jgs.18179.
Vitamin D supplementation and incident fractures
Vitamin D supplementation is a commonly recommended intervention for bone health, but data to support its impact on reducing fracture risk has been variable.
A study in the New England Journal of Medicine by LeBoff and colleagues has garnered much attention since its publication in July 2022.1 In the ancillary study of the Vitamin D and Omega-3-Trial (VITAL), the authors examined the impact of vitamin D supplementation versus placebo on incident fractures. The study found that vitamin D supplementation, as compared with placebo, led to no significant difference in the incidence of total, nonvertebral, and hip fractures in midlife and older adults over the 5-year period of follow-up.
The generalizability of these findings has been raised as a concern as the study does not describe adults at higher risk for fracture. The authors of the study specified in their conclusion that vitamin D supplementation does not reduce fracture risk in “generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass or osteoporosis.”
With a mean participant age of 67 and exclusion of participants with a history of cardiovascular disease, stroke, cirrhosis and other serious illnesses, the study does not reflect the multimorbid older adult population that geriatricians typically care for. Furthermore, efficacy of vitamin D supplementation on fracture risk may be the most impactful in those with osteoporosis and with severe vitamin D deficiency (defined by vitamin D 25[OH]D level less than 12 ng/mL).
In post hoc analyses, there was no significant difference in fracture risk in these subgroups, however the authors acknowledged that the findings may be limited by the small percentage of participants with severe vitamin D deficiency (2.4%) and osteoporosis included in the study (5%).
Lecanemab for mild cognitive impairment and early Alzheimer’s dementia
On Jan. 6, 2023, the Food and Drug Administration approved lecanemab, the second-ever disease-modifying treatment for Alzheimer’s dementia following the approval of aducanumab in 2021. Lecanemab is a monoclonal antibody targeting larger amyloid-beta oligomers, which has been shown in vitro to have higher affinity for amyloid-beta, compared with aducanumab. FDA approval followed shortly after the publication of the CLARITY-AD trial, which investigated the effect of lecanemab versus placebo on cognitive decline and burden of amyloid in adults with mild cognitive impairment and mild Alzheimer’s dementia. Over an 18-month period, the study found that participants who received lecanemab, compared with placebo, had a significantly smaller decline in cognition and function, and reduction in amyloid burden on PET CT.2
The clinical significance of these findings, however, is unclear. As noted by an editorial published in the Lancet in 2022, the difference in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scale between the treatment and placebo groups was 0.45. On an 18-point scale, prior research has noted that a minimal clinically significance difference of 0.98 is necessary in those with mild cognitive impairment and 1.63 in mild Alzheimer dementia.3
Additionally, the CLARITY-AD trial reported that lecanemab resulted in infusion reactions in 26.4% of participants and brain edema (an amyloid-related imaging abnormality referred to as ARIA-E) in 12.6% of participants. This finding highlights concerns for safety and the need for close monitoring, as well as ongoing implications of economic feasibility and equitable access for all those who qualify for treatment.2
Social isolation and dementia risk
There is growing awareness of the impact of social isolation on health outcomes, particularly among older adults. Prior research has reported that one in four older adults are considered socially isolated and that social isolation increases risk of premature death, dementia, depression, and cardiovascular disease.4
A study by Huang and colleagues is the first nationally representative cohort study examining the association between social isolation and incident dementia for older adults in community dwelling settings. A cohort of 5,022 older adults participating in the National Health and Aging Trends Study was followed from 2011 to 2020. When adjusting for demographic and health factors, including race, level of education, and number of chronic health conditions, socially isolated adults had a greater risk of developing dementia, compared with adults who were not socially isolated (hazard ratio, 1.27; 95% confidence interval, 1.08-1.49). Potential mechanisms to explain this association include the increased risk of cardiovascular disease and depression in older adults who are socially isolated, thereby increasing dementia risk.
Decreased cognitive activity/engagement and access to resources such as caregiving and health care may also be linked to the increased risk of dementia in socially isolated older adults.5
Another observational cohort study from the National Health and Aging Trends Study investigated whether access and use of technology can lower the risk of social isolation. The study found that older adults who used email or text messaging had a lower risk of social isolation than older adults who did not use technology (incidence rate ratio, 0.64; 95% CI, 0.51-0.80).6 These findings highlight the importance of addressing social isolation as an important modifiable health risk factor, and the need for providing equitable access to technology in vulnerable populations as health intervention.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. LeBoff MS et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299-30.
2. van Dyck CH et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
3. The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022; 400:1899.
4. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: 2020, The National Academies Press.
5. Huang, AR et al. Social isolation and 9-year dementia risk in community dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023 Jan 11. doi: 10.1111/jgs18140.
6. Umoh ME etal. Impact of technology on social isolation: Longitudinal analysis from the National Health Aging Trends Study. J Am Geriatr Soc. 2022 Dec 15. doi 10.1111/jgs.18179.
Vitamin D supplementation and incident fractures
Vitamin D supplementation is a commonly recommended intervention for bone health, but data to support its impact on reducing fracture risk has been variable.
A study in the New England Journal of Medicine by LeBoff and colleagues has garnered much attention since its publication in July 2022.1 In the ancillary study of the Vitamin D and Omega-3-Trial (VITAL), the authors examined the impact of vitamin D supplementation versus placebo on incident fractures. The study found that vitamin D supplementation, as compared with placebo, led to no significant difference in the incidence of total, nonvertebral, and hip fractures in midlife and older adults over the 5-year period of follow-up.
The generalizability of these findings has been raised as a concern as the study does not describe adults at higher risk for fracture. The authors of the study specified in their conclusion that vitamin D supplementation does not reduce fracture risk in “generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass or osteoporosis.”
With a mean participant age of 67 and exclusion of participants with a history of cardiovascular disease, stroke, cirrhosis and other serious illnesses, the study does not reflect the multimorbid older adult population that geriatricians typically care for. Furthermore, efficacy of vitamin D supplementation on fracture risk may be the most impactful in those with osteoporosis and with severe vitamin D deficiency (defined by vitamin D 25[OH]D level less than 12 ng/mL).
In post hoc analyses, there was no significant difference in fracture risk in these subgroups, however the authors acknowledged that the findings may be limited by the small percentage of participants with severe vitamin D deficiency (2.4%) and osteoporosis included in the study (5%).
Lecanemab for mild cognitive impairment and early Alzheimer’s dementia
On Jan. 6, 2023, the Food and Drug Administration approved lecanemab, the second-ever disease-modifying treatment for Alzheimer’s dementia following the approval of aducanumab in 2021. Lecanemab is a monoclonal antibody targeting larger amyloid-beta oligomers, which has been shown in vitro to have higher affinity for amyloid-beta, compared with aducanumab. FDA approval followed shortly after the publication of the CLARITY-AD trial, which investigated the effect of lecanemab versus placebo on cognitive decline and burden of amyloid in adults with mild cognitive impairment and mild Alzheimer’s dementia. Over an 18-month period, the study found that participants who received lecanemab, compared with placebo, had a significantly smaller decline in cognition and function, and reduction in amyloid burden on PET CT.2
The clinical significance of these findings, however, is unclear. As noted by an editorial published in the Lancet in 2022, the difference in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scale between the treatment and placebo groups was 0.45. On an 18-point scale, prior research has noted that a minimal clinically significance difference of 0.98 is necessary in those with mild cognitive impairment and 1.63 in mild Alzheimer dementia.3
Additionally, the CLARITY-AD trial reported that lecanemab resulted in infusion reactions in 26.4% of participants and brain edema (an amyloid-related imaging abnormality referred to as ARIA-E) in 12.6% of participants. This finding highlights concerns for safety and the need for close monitoring, as well as ongoing implications of economic feasibility and equitable access for all those who qualify for treatment.2
Social isolation and dementia risk
There is growing awareness of the impact of social isolation on health outcomes, particularly among older adults. Prior research has reported that one in four older adults are considered socially isolated and that social isolation increases risk of premature death, dementia, depression, and cardiovascular disease.4
A study by Huang and colleagues is the first nationally representative cohort study examining the association between social isolation and incident dementia for older adults in community dwelling settings. A cohort of 5,022 older adults participating in the National Health and Aging Trends Study was followed from 2011 to 2020. When adjusting for demographic and health factors, including race, level of education, and number of chronic health conditions, socially isolated adults had a greater risk of developing dementia, compared with adults who were not socially isolated (hazard ratio, 1.27; 95% confidence interval, 1.08-1.49). Potential mechanisms to explain this association include the increased risk of cardiovascular disease and depression in older adults who are socially isolated, thereby increasing dementia risk.
Decreased cognitive activity/engagement and access to resources such as caregiving and health care may also be linked to the increased risk of dementia in socially isolated older adults.5
Another observational cohort study from the National Health and Aging Trends Study investigated whether access and use of technology can lower the risk of social isolation. The study found that older adults who used email or text messaging had a lower risk of social isolation than older adults who did not use technology (incidence rate ratio, 0.64; 95% CI, 0.51-0.80).6 These findings highlight the importance of addressing social isolation as an important modifiable health risk factor, and the need for providing equitable access to technology in vulnerable populations as health intervention.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. LeBoff MS et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299-30.
2. van Dyck CH et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
3. The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022; 400:1899.
4. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: 2020, The National Academies Press.
5. Huang, AR et al. Social isolation and 9-year dementia risk in community dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023 Jan 11. doi: 10.1111/jgs18140.
6. Umoh ME etal. Impact of technology on social isolation: Longitudinal analysis from the National Health Aging Trends Study. J Am Geriatr Soc. 2022 Dec 15. doi 10.1111/jgs.18179.
Canadian Task Force recommendation on screening for postpartum depression misses the mark
Postpartum/perinatal depression (PPD) remains the most common complication in modern obstetrics, with a prevalence of 10%-15% based on multiple studies over the last 2 decades. Over those same 2 decades, there has been growing interest and motivation across the country – from small community hospitals to major academic centers – to promote screening. Such screening is integrated into obstetrical practices, typically using the Edinburgh Postnatal Depression Scale (EPDS), the most widely used validated screen for PPD globally.
As mentioned in previous columns, the U.S. Preventive Services Task Force recommended screening for PPD in 2016, which includes screening women at highest risk, and both acutely treating and preventing PPD.
Since then, screening women for a common clinical problem like PPD has been widely adopted by clinicians representing a broad spectrum of interdisciplinary care. Providers who are engaged in the treatment of postpartum women – obstetricians, psychiatrists, doulas, lactation consultants, facilitators of postpartum support groups, and advocacy groups among others – are included.
An open question and one of great concern recently to our group and others has been what happens after screening. It is clear that identification of PPD per se is not necessarily a challenge, and we have multiple effective treatments from antidepressants to mindfulness-based cognitive therapy to cognitive-behavioral interventions. There is also a growing number of digital applications aimed at mitigation of depressive symptoms in women with postpartum major depressive disorder. One unanswered question is how to engage women after identification of PPD and how to facilitate access to care in a way that maximizes the likelihood that women who actually are suffering from PPD get adequate treatment.
The “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression. This is perhaps the greatest challenge to the field and to clinicians – how do we, on the other side of screening, see that these women get access to care and get well?
With that backdrop, it is surprising that the Canadian Task Force on Preventive Health Care has recently recommended against screening with systematic questionnaires, noting that benefits were unclear and not a particular advantage relative to standard practice. The recommendation carries an assumption that standard practice involves queries about mental health. While the task force continues to recommend screening for PPD, their recommendation against screening with a standardized questionnaire represents a bold, sweeping, if not myopic view.
While the Canadian Task Force on Preventive Health Care made their recommendation based on a single randomized controlled trial with the assumption that women were getting mental health counseling, and that women liked getting mental health engagement around their depression, that is not a uniform part of practice. Thus, it is puzzling why the task force would make the recommendation based on such sparse data.
The way to optimize access to care and referral systems for women who are suffering from PPD is not to remove a part of the system that’s already working. Well-validated questionnaires such as the EPDS are easy to administer and are routinely integrated into the electronic health systems records of both small and large centers. These questionnaires are an inexpensive way to increase the likelihood that women get identified and referred for a spectrum of potentially helpful interventions.
PPD is also easy to treat with medications and a wide spectrum of nonpharmacologic interventions. Novel interventions are also being explored to maximize access for women with postpartum mood and anxiety disorders such as peer-delivered behavioral activation and cognitive-behavioral therapy, which could be community based and implemented from urban to rural settings across the United States.
What may need the greatest study is the path to accessing effective treatments and resources for these women and this problem has prompted our group to explore these issues in our more recent investigations. Better understanding of those factors that limit access to mental health providers with expertise in perinatal mental health to the logistical issues of navigating the health care system for sleep-deprived new moms and their families demands greater attention and clearer answers.
The whole field has an obligation to postpartum women to figure out the amalgam of practitioners, resources, and platforms that need to be used to engage women so that they get effective treatment – because we have effective treatments. But the solution to improving perinatal mental health outcomes, unlike the approach of our colleagues in Canada, is not to be found in abandoning questionnaire-based screening, but in identifying the best ways to prevent PPD and to maximize access to care.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
Postpartum/perinatal depression (PPD) remains the most common complication in modern obstetrics, with a prevalence of 10%-15% based on multiple studies over the last 2 decades. Over those same 2 decades, there has been growing interest and motivation across the country – from small community hospitals to major academic centers – to promote screening. Such screening is integrated into obstetrical practices, typically using the Edinburgh Postnatal Depression Scale (EPDS), the most widely used validated screen for PPD globally.
As mentioned in previous columns, the U.S. Preventive Services Task Force recommended screening for PPD in 2016, which includes screening women at highest risk, and both acutely treating and preventing PPD.
Since then, screening women for a common clinical problem like PPD has been widely adopted by clinicians representing a broad spectrum of interdisciplinary care. Providers who are engaged in the treatment of postpartum women – obstetricians, psychiatrists, doulas, lactation consultants, facilitators of postpartum support groups, and advocacy groups among others – are included.
An open question and one of great concern recently to our group and others has been what happens after screening. It is clear that identification of PPD per se is not necessarily a challenge, and we have multiple effective treatments from antidepressants to mindfulness-based cognitive therapy to cognitive-behavioral interventions. There is also a growing number of digital applications aimed at mitigation of depressive symptoms in women with postpartum major depressive disorder. One unanswered question is how to engage women after identification of PPD and how to facilitate access to care in a way that maximizes the likelihood that women who actually are suffering from PPD get adequate treatment.
The “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression. This is perhaps the greatest challenge to the field and to clinicians – how do we, on the other side of screening, see that these women get access to care and get well?
With that backdrop, it is surprising that the Canadian Task Force on Preventive Health Care has recently recommended against screening with systematic questionnaires, noting that benefits were unclear and not a particular advantage relative to standard practice. The recommendation carries an assumption that standard practice involves queries about mental health. While the task force continues to recommend screening for PPD, their recommendation against screening with a standardized questionnaire represents a bold, sweeping, if not myopic view.
While the Canadian Task Force on Preventive Health Care made their recommendation based on a single randomized controlled trial with the assumption that women were getting mental health counseling, and that women liked getting mental health engagement around their depression, that is not a uniform part of practice. Thus, it is puzzling why the task force would make the recommendation based on such sparse data.
The way to optimize access to care and referral systems for women who are suffering from PPD is not to remove a part of the system that’s already working. Well-validated questionnaires such as the EPDS are easy to administer and are routinely integrated into the electronic health systems records of both small and large centers. These questionnaires are an inexpensive way to increase the likelihood that women get identified and referred for a spectrum of potentially helpful interventions.
PPD is also easy to treat with medications and a wide spectrum of nonpharmacologic interventions. Novel interventions are also being explored to maximize access for women with postpartum mood and anxiety disorders such as peer-delivered behavioral activation and cognitive-behavioral therapy, which could be community based and implemented from urban to rural settings across the United States.
What may need the greatest study is the path to accessing effective treatments and resources for these women and this problem has prompted our group to explore these issues in our more recent investigations. Better understanding of those factors that limit access to mental health providers with expertise in perinatal mental health to the logistical issues of navigating the health care system for sleep-deprived new moms and their families demands greater attention and clearer answers.
The whole field has an obligation to postpartum women to figure out the amalgam of practitioners, resources, and platforms that need to be used to engage women so that they get effective treatment – because we have effective treatments. But the solution to improving perinatal mental health outcomes, unlike the approach of our colleagues in Canada, is not to be found in abandoning questionnaire-based screening, but in identifying the best ways to prevent PPD and to maximize access to care.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
Postpartum/perinatal depression (PPD) remains the most common complication in modern obstetrics, with a prevalence of 10%-15% based on multiple studies over the last 2 decades. Over those same 2 decades, there has been growing interest and motivation across the country – from small community hospitals to major academic centers – to promote screening. Such screening is integrated into obstetrical practices, typically using the Edinburgh Postnatal Depression Scale (EPDS), the most widely used validated screen for PPD globally.
As mentioned in previous columns, the U.S. Preventive Services Task Force recommended screening for PPD in 2016, which includes screening women at highest risk, and both acutely treating and preventing PPD.
Since then, screening women for a common clinical problem like PPD has been widely adopted by clinicians representing a broad spectrum of interdisciplinary care. Providers who are engaged in the treatment of postpartum women – obstetricians, psychiatrists, doulas, lactation consultants, facilitators of postpartum support groups, and advocacy groups among others – are included.
An open question and one of great concern recently to our group and others has been what happens after screening. It is clear that identification of PPD per se is not necessarily a challenge, and we have multiple effective treatments from antidepressants to mindfulness-based cognitive therapy to cognitive-behavioral interventions. There is also a growing number of digital applications aimed at mitigation of depressive symptoms in women with postpartum major depressive disorder. One unanswered question is how to engage women after identification of PPD and how to facilitate access to care in a way that maximizes the likelihood that women who actually are suffering from PPD get adequate treatment.
The “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression. This is perhaps the greatest challenge to the field and to clinicians – how do we, on the other side of screening, see that these women get access to care and get well?
With that backdrop, it is surprising that the Canadian Task Force on Preventive Health Care has recently recommended against screening with systematic questionnaires, noting that benefits were unclear and not a particular advantage relative to standard practice. The recommendation carries an assumption that standard practice involves queries about mental health. While the task force continues to recommend screening for PPD, their recommendation against screening with a standardized questionnaire represents a bold, sweeping, if not myopic view.
While the Canadian Task Force on Preventive Health Care made their recommendation based on a single randomized controlled trial with the assumption that women were getting mental health counseling, and that women liked getting mental health engagement around their depression, that is not a uniform part of practice. Thus, it is puzzling why the task force would make the recommendation based on such sparse data.
The way to optimize access to care and referral systems for women who are suffering from PPD is not to remove a part of the system that’s already working. Well-validated questionnaires such as the EPDS are easy to administer and are routinely integrated into the electronic health systems records of both small and large centers. These questionnaires are an inexpensive way to increase the likelihood that women get identified and referred for a spectrum of potentially helpful interventions.
PPD is also easy to treat with medications and a wide spectrum of nonpharmacologic interventions. Novel interventions are also being explored to maximize access for women with postpartum mood and anxiety disorders such as peer-delivered behavioral activation and cognitive-behavioral therapy, which could be community based and implemented from urban to rural settings across the United States.
What may need the greatest study is the path to accessing effective treatments and resources for these women and this problem has prompted our group to explore these issues in our more recent investigations. Better understanding of those factors that limit access to mental health providers with expertise in perinatal mental health to the logistical issues of navigating the health care system for sleep-deprived new moms and their families demands greater attention and clearer answers.
The whole field has an obligation to postpartum women to figure out the amalgam of practitioners, resources, and platforms that need to be used to engage women so that they get effective treatment – because we have effective treatments. But the solution to improving perinatal mental health outcomes, unlike the approach of our colleagues in Canada, is not to be found in abandoning questionnaire-based screening, but in identifying the best ways to prevent PPD and to maximize access to care.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
Anxiety sensitivity fuels depression in dissociative identity disorder
Anxiety sensitivity refers to fear of the signs and symptoms of anxiety based on the individual’s belief that the signs of anxiety will have harmful consequences, wrote Xi Pan, LICSW, MPA, of McLean Hospital, Belmont, Mass., and colleagues.
Anxiety sensitivity can include cognitive, physical, and social elements: for example, fear that the inability to focus signals mental illness, fear that a racing heart might cause a heart attack, or fear that exhibiting anxiety signs in public (e.g., sweaty palms) will cause embarrassment, the researchers said.
Previous studies have found associations between anxiety sensitivity and panic attacks, and anxiety sensitivity has been shown to contribute to worsening symptoms in patients with anxiety disorders, depressive disorders, and trauma-related disorders such as posttraumatic stress disorder. However, “anxiety sensitivity has not been studied in individuals with complex dissociative disorders such as dissociative identity disorder (DID)” – who often have co-occurring PTSD and depression, the researchers said.
In a study published in the Journal of Psychiatric Research, the authors analyzed data from 21 treatment-seeking adult women with histories of childhood trauma, current PTSD, and dissociative identity disorder. Participants completed the Anxiety Sensitivity Index (ASI), Beck Depression Inventory-II, Childhood Trauma Questionnaire, Multidimensional Inventory of Dissociation, and PTSD Checklist for DSM-5.
Anxiety sensitivity in cognitive, physical, and social domains was assessed using ASI subscales.
Pearson correlations showed that symptoms of depression were significantly associated with anxiety sensitivity total scores and across all anxiety subscales. However, no direct associations appeared between anxiety sensitivity and PTSD or severe dissociative symptoms.
In a multiple regression analysis, the ASI cognitive subscale was a positive predictor of depressive symptoms, although physical and social subscale scores were not.
The researchers also tested for an indirect relationship between anxiety sensitivity and dissociative symptoms through depression. “Specifically, more severe ASI cognitive concerns were associated with more depressive symptoms, and more depressive symptoms predicted more severe pathological dissociation symptoms,” they wrote.
The findings were limited by the inability to show a direct causal relationship between anxiety sensitivity and depression, the researchers noted. Other limitations included the small sample size, use of self-reports, and the population of mainly White women, which may not generalize to other populations, they said.
However, the results represent the first empirical investigation of the relationship between anxiety sensitivity and DID symptoms, and support the value of assessment for anxiety sensitivity in DID patients in clinical practice, they said.
“If high levels of anxiety sensitivity are identified, the individual may benefit from targeted interventions, which in turn may alleviate some symptoms of depression and dissociation in DID,” the researchers concluded.
The study was supported by the National Institute of Mental Health and the Julia Kasparian Fund for Neuroscience Research. The researchers had no financial conflicts to disclose.
Anxiety sensitivity refers to fear of the signs and symptoms of anxiety based on the individual’s belief that the signs of anxiety will have harmful consequences, wrote Xi Pan, LICSW, MPA, of McLean Hospital, Belmont, Mass., and colleagues.
Anxiety sensitivity can include cognitive, physical, and social elements: for example, fear that the inability to focus signals mental illness, fear that a racing heart might cause a heart attack, or fear that exhibiting anxiety signs in public (e.g., sweaty palms) will cause embarrassment, the researchers said.
Previous studies have found associations between anxiety sensitivity and panic attacks, and anxiety sensitivity has been shown to contribute to worsening symptoms in patients with anxiety disorders, depressive disorders, and trauma-related disorders such as posttraumatic stress disorder. However, “anxiety sensitivity has not been studied in individuals with complex dissociative disorders such as dissociative identity disorder (DID)” – who often have co-occurring PTSD and depression, the researchers said.
In a study published in the Journal of Psychiatric Research, the authors analyzed data from 21 treatment-seeking adult women with histories of childhood trauma, current PTSD, and dissociative identity disorder. Participants completed the Anxiety Sensitivity Index (ASI), Beck Depression Inventory-II, Childhood Trauma Questionnaire, Multidimensional Inventory of Dissociation, and PTSD Checklist for DSM-5.
Anxiety sensitivity in cognitive, physical, and social domains was assessed using ASI subscales.
Pearson correlations showed that symptoms of depression were significantly associated with anxiety sensitivity total scores and across all anxiety subscales. However, no direct associations appeared between anxiety sensitivity and PTSD or severe dissociative symptoms.
In a multiple regression analysis, the ASI cognitive subscale was a positive predictor of depressive symptoms, although physical and social subscale scores were not.
The researchers also tested for an indirect relationship between anxiety sensitivity and dissociative symptoms through depression. “Specifically, more severe ASI cognitive concerns were associated with more depressive symptoms, and more depressive symptoms predicted more severe pathological dissociation symptoms,” they wrote.
The findings were limited by the inability to show a direct causal relationship between anxiety sensitivity and depression, the researchers noted. Other limitations included the small sample size, use of self-reports, and the population of mainly White women, which may not generalize to other populations, they said.
However, the results represent the first empirical investigation of the relationship between anxiety sensitivity and DID symptoms, and support the value of assessment for anxiety sensitivity in DID patients in clinical practice, they said.
“If high levels of anxiety sensitivity are identified, the individual may benefit from targeted interventions, which in turn may alleviate some symptoms of depression and dissociation in DID,” the researchers concluded.
The study was supported by the National Institute of Mental Health and the Julia Kasparian Fund for Neuroscience Research. The researchers had no financial conflicts to disclose.
Anxiety sensitivity refers to fear of the signs and symptoms of anxiety based on the individual’s belief that the signs of anxiety will have harmful consequences, wrote Xi Pan, LICSW, MPA, of McLean Hospital, Belmont, Mass., and colleagues.
Anxiety sensitivity can include cognitive, physical, and social elements: for example, fear that the inability to focus signals mental illness, fear that a racing heart might cause a heart attack, or fear that exhibiting anxiety signs in public (e.g., sweaty palms) will cause embarrassment, the researchers said.
Previous studies have found associations between anxiety sensitivity and panic attacks, and anxiety sensitivity has been shown to contribute to worsening symptoms in patients with anxiety disorders, depressive disorders, and trauma-related disorders such as posttraumatic stress disorder. However, “anxiety sensitivity has not been studied in individuals with complex dissociative disorders such as dissociative identity disorder (DID)” – who often have co-occurring PTSD and depression, the researchers said.
In a study published in the Journal of Psychiatric Research, the authors analyzed data from 21 treatment-seeking adult women with histories of childhood trauma, current PTSD, and dissociative identity disorder. Participants completed the Anxiety Sensitivity Index (ASI), Beck Depression Inventory-II, Childhood Trauma Questionnaire, Multidimensional Inventory of Dissociation, and PTSD Checklist for DSM-5.
Anxiety sensitivity in cognitive, physical, and social domains was assessed using ASI subscales.
Pearson correlations showed that symptoms of depression were significantly associated with anxiety sensitivity total scores and across all anxiety subscales. However, no direct associations appeared between anxiety sensitivity and PTSD or severe dissociative symptoms.
In a multiple regression analysis, the ASI cognitive subscale was a positive predictor of depressive symptoms, although physical and social subscale scores were not.
The researchers also tested for an indirect relationship between anxiety sensitivity and dissociative symptoms through depression. “Specifically, more severe ASI cognitive concerns were associated with more depressive symptoms, and more depressive symptoms predicted more severe pathological dissociation symptoms,” they wrote.
The findings were limited by the inability to show a direct causal relationship between anxiety sensitivity and depression, the researchers noted. Other limitations included the small sample size, use of self-reports, and the population of mainly White women, which may not generalize to other populations, they said.
However, the results represent the first empirical investigation of the relationship between anxiety sensitivity and DID symptoms, and support the value of assessment for anxiety sensitivity in DID patients in clinical practice, they said.
“If high levels of anxiety sensitivity are identified, the individual may benefit from targeted interventions, which in turn may alleviate some symptoms of depression and dissociation in DID,” the researchers concluded.
The study was supported by the National Institute of Mental Health and the Julia Kasparian Fund for Neuroscience Research. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
Borderline patients have longer time to depression remission
Major depressive episodes (MDEs) occur in major depressive disorder (MDD) and bipolar disorder (BD), John J. Söderholm, MD, of the University of Helsinki and colleagues wrote. Borderline personality disorder (BPD) includes an increased risk for depression, but data on the relationship between BPD symptoms and depressive illness are limited. In particular, they noted “a lack of studies prospectively comparing the presence of (hypo)manic symptoms over time during the recovery process from MDE between MDD, MDE/BD, and MDE/BPD patients.”
In a cohort study published in the Journal of Affective Disorders, the researchers collected data from 39 adult MDE patients with MDD, 33 with BD, and 23 with BPD. The patients were diagnosed with MDE using the SCID-I/P and SCID-II interviews, mixed symptoms were identified using the Mix-MDE scale, and borderline symptoms were identified using the Borderline Personality Disorder Severity Index.
Over a 6-month follow-up period, the participants completed biweekly online assessments. The primary outcomes were time to first full remission of symptoms and duration and nature of mood episodes.
Overall, the mean number of distinct mood states was 5.75, and the median duration was 60.9 days. When identified by subcohorts, the median number of mood state periods for MDD, BD, and BPD was 4.49, 8.05, and 4.67, respectively. The median durations were 69.2 days, 40.30 days, and 75.6 days, respectively.
The rates of remission for depressive symptoms were similar for MDD, MDE/BD, and MDE/BPD patients. However, MDE/BD patients had a significantly shorter time to first remission (hazard ratio, 2.44). Patients in the BPD group had a significantly longer time to first remission (HR, 0.95).
“When the cohort was divided into quintiles according to BPD feature severity, there was an approximately 1-month difference in time to first period of remission between the first and third and between the third and fifth quintiles, with longer times seen in patients with more severe BPD symptoms,” the researchers wrote.
The study findings were limited by several factors including the small sample size and short follow-up period that prevented investigation of depressive recurrence, the researchers noted. Other limitations included the lack of diagnostic blinding and variation in patients’ treatment schedules.
However, the results were strengthened by the representative samples of subjects with various disorders, the prospective and multimodal assessment of affective states, and the comparison of three patient groups in a single study.
As BPD was associated with a longer time to remission from depressive symptoms, the results suggest that BPD severity may be an indicator of more severe disease in patients with MDD in the context of depression, the researchers concluded.
The study was supported by the Finska Lakaresallskapet, the City of Helsinki, the Hospital District of Helsinki and Uusimaa, and the Finnish Psychiatric Association. The researchers had no financial conflicts to disclose.
Major depressive episodes (MDEs) occur in major depressive disorder (MDD) and bipolar disorder (BD), John J. Söderholm, MD, of the University of Helsinki and colleagues wrote. Borderline personality disorder (BPD) includes an increased risk for depression, but data on the relationship between BPD symptoms and depressive illness are limited. In particular, they noted “a lack of studies prospectively comparing the presence of (hypo)manic symptoms over time during the recovery process from MDE between MDD, MDE/BD, and MDE/BPD patients.”
In a cohort study published in the Journal of Affective Disorders, the researchers collected data from 39 adult MDE patients with MDD, 33 with BD, and 23 with BPD. The patients were diagnosed with MDE using the SCID-I/P and SCID-II interviews, mixed symptoms were identified using the Mix-MDE scale, and borderline symptoms were identified using the Borderline Personality Disorder Severity Index.
Over a 6-month follow-up period, the participants completed biweekly online assessments. The primary outcomes were time to first full remission of symptoms and duration and nature of mood episodes.
Overall, the mean number of distinct mood states was 5.75, and the median duration was 60.9 days. When identified by subcohorts, the median number of mood state periods for MDD, BD, and BPD was 4.49, 8.05, and 4.67, respectively. The median durations were 69.2 days, 40.30 days, and 75.6 days, respectively.
The rates of remission for depressive symptoms were similar for MDD, MDE/BD, and MDE/BPD patients. However, MDE/BD patients had a significantly shorter time to first remission (hazard ratio, 2.44). Patients in the BPD group had a significantly longer time to first remission (HR, 0.95).
“When the cohort was divided into quintiles according to BPD feature severity, there was an approximately 1-month difference in time to first period of remission between the first and third and between the third and fifth quintiles, with longer times seen in patients with more severe BPD symptoms,” the researchers wrote.
The study findings were limited by several factors including the small sample size and short follow-up period that prevented investigation of depressive recurrence, the researchers noted. Other limitations included the lack of diagnostic blinding and variation in patients’ treatment schedules.
However, the results were strengthened by the representative samples of subjects with various disorders, the prospective and multimodal assessment of affective states, and the comparison of three patient groups in a single study.
As BPD was associated with a longer time to remission from depressive symptoms, the results suggest that BPD severity may be an indicator of more severe disease in patients with MDD in the context of depression, the researchers concluded.
The study was supported by the Finska Lakaresallskapet, the City of Helsinki, the Hospital District of Helsinki and Uusimaa, and the Finnish Psychiatric Association. The researchers had no financial conflicts to disclose.
Major depressive episodes (MDEs) occur in major depressive disorder (MDD) and bipolar disorder (BD), John J. Söderholm, MD, of the University of Helsinki and colleagues wrote. Borderline personality disorder (BPD) includes an increased risk for depression, but data on the relationship between BPD symptoms and depressive illness are limited. In particular, they noted “a lack of studies prospectively comparing the presence of (hypo)manic symptoms over time during the recovery process from MDE between MDD, MDE/BD, and MDE/BPD patients.”
In a cohort study published in the Journal of Affective Disorders, the researchers collected data from 39 adult MDE patients with MDD, 33 with BD, and 23 with BPD. The patients were diagnosed with MDE using the SCID-I/P and SCID-II interviews, mixed symptoms were identified using the Mix-MDE scale, and borderline symptoms were identified using the Borderline Personality Disorder Severity Index.
Over a 6-month follow-up period, the participants completed biweekly online assessments. The primary outcomes were time to first full remission of symptoms and duration and nature of mood episodes.
Overall, the mean number of distinct mood states was 5.75, and the median duration was 60.9 days. When identified by subcohorts, the median number of mood state periods for MDD, BD, and BPD was 4.49, 8.05, and 4.67, respectively. The median durations were 69.2 days, 40.30 days, and 75.6 days, respectively.
The rates of remission for depressive symptoms were similar for MDD, MDE/BD, and MDE/BPD patients. However, MDE/BD patients had a significantly shorter time to first remission (hazard ratio, 2.44). Patients in the BPD group had a significantly longer time to first remission (HR, 0.95).
“When the cohort was divided into quintiles according to BPD feature severity, there was an approximately 1-month difference in time to first period of remission between the first and third and between the third and fifth quintiles, with longer times seen in patients with more severe BPD symptoms,” the researchers wrote.
The study findings were limited by several factors including the small sample size and short follow-up period that prevented investigation of depressive recurrence, the researchers noted. Other limitations included the lack of diagnostic blinding and variation in patients’ treatment schedules.
However, the results were strengthened by the representative samples of subjects with various disorders, the prospective and multimodal assessment of affective states, and the comparison of three patient groups in a single study.
As BPD was associated with a longer time to remission from depressive symptoms, the results suggest that BPD severity may be an indicator of more severe disease in patients with MDD in the context of depression, the researchers concluded.
The study was supported by the Finska Lakaresallskapet, the City of Helsinki, the Hospital District of Helsinki and Uusimaa, and the Finnish Psychiatric Association. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS














