User login
Breast cancer risk in type 2 diabetes related to adiposity
ORLANDO – findings from meta-analyses suggest.
In one meta-analysis of data from 21 prospective studies with a total of nearly 15.2 million women, 325,117 breast cancer cases, and a mean follow-up time of 8 years (nearly 33 million person-years), the risk of breast cancer was significantly greater among patients with diabetes than it was among patients without diabetes (summary relative risk, 1.11), Maria Bota reported at the annual scientific sessions of the American Diabetes Association.
However, there was substantial unexplained heterogeneity of results across the individual studies (I2 = 82%), said Ms. Bota, a faculty member at the International Prevention Research Institute, Lyon, France.
“When the analysis was restricted to the 12 studies that adjusted for [body mass index], the summary relative risk decreased to 1.09 and the heterogeneity also decreased to a moderate value of 32%; when the analysis was restricted to the 9 studies that did not adjust for BMI, the summary relative risk increased to 1.14 again, and the heterogeneity increased even more to 91%,” she said.
In an analysis that combined the results of the nine studies that did not adjust for BMI along with crude relative risks from studies that reported both crude and BMI-adjusted relative risks (17 studies in all), the SRR was 1.12, and heterogeneity among the studies was high at 84%.
Additionally, an analysis by menopausal status based on four studies that reported breast cancer in both pre- and postmenopausal women showed SRRs for breast cancer of 0.97 (a 3% decrease in risk) and 1.14 among diabetic vs. nondiabetic premenopausal women and postmenopausal women, respectively, she said, noting that heterogeneity was low (I2 = 0%) among the premenopausal breast cancer study groups and high (I2 = 70%) among the postmenopausal study groups.
The findings provide evidence for a moderately increased risk of breast cancer in women with T2DM, Ms. Bota said.
“However, the effect of the adjustment or lack of adjustment on the heterogeneity suggests that the higher risk of breast cancer in women with diabetes may not be due to diabetes itself, but to adiposity,” she said, adding that “this hypothesis is equally supported by our subgroup analysis according to menopausal status because we saw that the risk of breast cancer was only associated with diabetes in postmenopausal women and this pattern resembles the pattern of the risk of breast cancer associated with adiposity, which is also only increased in postmenopausal women.”
This study was limited by insufficient data for investigating the sources of heterogeneity, she said.
“Therefore we propose ... future pooled analyses based on individual data from good quality prospective studies in order to increase the study power and to do some detailed analysis of the links between adiposity, diabetes, and breast cancer,” she concluded, adding that new studies to examine those relationships only in premenopausal women are also needed
In a separate meta-analysis, she and her colleagues, including Peter Boyle, PhD, president of the International Prevention Research Institute, assessed the association between insulin treatment and breast cancer risk in patients with diabetes.
“The long-acting insulin analogues glargine and detemir have been shown in some studies to be associated with increased risk of breast cancer, and other studies have shown no association between the use of these two compounds and the risk of breast cancer,” Dr. Boyle said in a separate presentation at the ADA meeting. “It was important to sort out a little bit what was going on in the literature.”
Overall, the meta-analysis of data from 12 longitudinal cohort studies – including more than 6,000 cases of breast cancer – showed a slight increase in breast cancer risk in patients taking long-acting insulin (SRR, 1.13) with “a relatively reasonable” level of heterogeneity (I2 = 23%).
“But we see that the story is not that simple,” he said.
For example, some studies included only patients who were prescribed insulin for the first time after the study began (new users), some included only patients who were prescribed insulin before the study began (prevalent users), and some included both (ever users), which may have introduced bias in the results, he explained.
Studies of glargine included 4,168 breast cancer cases over a total of 1,418,743 person-years of observation, and studies of detemir included fewer than 2,047 breast cancer cases (not all studies reported case numbers). Among both new users of glargine and detemir, the SRR was 1.12, suggesting no real association between either glargine or detemir use and breast cancer, he said.
“One important take-home message is that you have to be careful that these pharmaco-epidemiological studies, even when working with the same database, may have conflicting results ... so we still need more robust standards in methods for [such] studies,” he concluded.
Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
SOURCE: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.
ORLANDO – findings from meta-analyses suggest.
In one meta-analysis of data from 21 prospective studies with a total of nearly 15.2 million women, 325,117 breast cancer cases, and a mean follow-up time of 8 years (nearly 33 million person-years), the risk of breast cancer was significantly greater among patients with diabetes than it was among patients without diabetes (summary relative risk, 1.11), Maria Bota reported at the annual scientific sessions of the American Diabetes Association.
However, there was substantial unexplained heterogeneity of results across the individual studies (I2 = 82%), said Ms. Bota, a faculty member at the International Prevention Research Institute, Lyon, France.
“When the analysis was restricted to the 12 studies that adjusted for [body mass index], the summary relative risk decreased to 1.09 and the heterogeneity also decreased to a moderate value of 32%; when the analysis was restricted to the 9 studies that did not adjust for BMI, the summary relative risk increased to 1.14 again, and the heterogeneity increased even more to 91%,” she said.
In an analysis that combined the results of the nine studies that did not adjust for BMI along with crude relative risks from studies that reported both crude and BMI-adjusted relative risks (17 studies in all), the SRR was 1.12, and heterogeneity among the studies was high at 84%.
Additionally, an analysis by menopausal status based on four studies that reported breast cancer in both pre- and postmenopausal women showed SRRs for breast cancer of 0.97 (a 3% decrease in risk) and 1.14 among diabetic vs. nondiabetic premenopausal women and postmenopausal women, respectively, she said, noting that heterogeneity was low (I2 = 0%) among the premenopausal breast cancer study groups and high (I2 = 70%) among the postmenopausal study groups.
The findings provide evidence for a moderately increased risk of breast cancer in women with T2DM, Ms. Bota said.
“However, the effect of the adjustment or lack of adjustment on the heterogeneity suggests that the higher risk of breast cancer in women with diabetes may not be due to diabetes itself, but to adiposity,” she said, adding that “this hypothesis is equally supported by our subgroup analysis according to menopausal status because we saw that the risk of breast cancer was only associated with diabetes in postmenopausal women and this pattern resembles the pattern of the risk of breast cancer associated with adiposity, which is also only increased in postmenopausal women.”
This study was limited by insufficient data for investigating the sources of heterogeneity, she said.
“Therefore we propose ... future pooled analyses based on individual data from good quality prospective studies in order to increase the study power and to do some detailed analysis of the links between adiposity, diabetes, and breast cancer,” she concluded, adding that new studies to examine those relationships only in premenopausal women are also needed
In a separate meta-analysis, she and her colleagues, including Peter Boyle, PhD, president of the International Prevention Research Institute, assessed the association between insulin treatment and breast cancer risk in patients with diabetes.
“The long-acting insulin analogues glargine and detemir have been shown in some studies to be associated with increased risk of breast cancer, and other studies have shown no association between the use of these two compounds and the risk of breast cancer,” Dr. Boyle said in a separate presentation at the ADA meeting. “It was important to sort out a little bit what was going on in the literature.”
Overall, the meta-analysis of data from 12 longitudinal cohort studies – including more than 6,000 cases of breast cancer – showed a slight increase in breast cancer risk in patients taking long-acting insulin (SRR, 1.13) with “a relatively reasonable” level of heterogeneity (I2 = 23%).
“But we see that the story is not that simple,” he said.
For example, some studies included only patients who were prescribed insulin for the first time after the study began (new users), some included only patients who were prescribed insulin before the study began (prevalent users), and some included both (ever users), which may have introduced bias in the results, he explained.
Studies of glargine included 4,168 breast cancer cases over a total of 1,418,743 person-years of observation, and studies of detemir included fewer than 2,047 breast cancer cases (not all studies reported case numbers). Among both new users of glargine and detemir, the SRR was 1.12, suggesting no real association between either glargine or detemir use and breast cancer, he said.
“One important take-home message is that you have to be careful that these pharmaco-epidemiological studies, even when working with the same database, may have conflicting results ... so we still need more robust standards in methods for [such] studies,” he concluded.
Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
SOURCE: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.
ORLANDO – findings from meta-analyses suggest.
In one meta-analysis of data from 21 prospective studies with a total of nearly 15.2 million women, 325,117 breast cancer cases, and a mean follow-up time of 8 years (nearly 33 million person-years), the risk of breast cancer was significantly greater among patients with diabetes than it was among patients without diabetes (summary relative risk, 1.11), Maria Bota reported at the annual scientific sessions of the American Diabetes Association.
However, there was substantial unexplained heterogeneity of results across the individual studies (I2 = 82%), said Ms. Bota, a faculty member at the International Prevention Research Institute, Lyon, France.
“When the analysis was restricted to the 12 studies that adjusted for [body mass index], the summary relative risk decreased to 1.09 and the heterogeneity also decreased to a moderate value of 32%; when the analysis was restricted to the 9 studies that did not adjust for BMI, the summary relative risk increased to 1.14 again, and the heterogeneity increased even more to 91%,” she said.
In an analysis that combined the results of the nine studies that did not adjust for BMI along with crude relative risks from studies that reported both crude and BMI-adjusted relative risks (17 studies in all), the SRR was 1.12, and heterogeneity among the studies was high at 84%.
Additionally, an analysis by menopausal status based on four studies that reported breast cancer in both pre- and postmenopausal women showed SRRs for breast cancer of 0.97 (a 3% decrease in risk) and 1.14 among diabetic vs. nondiabetic premenopausal women and postmenopausal women, respectively, she said, noting that heterogeneity was low (I2 = 0%) among the premenopausal breast cancer study groups and high (I2 = 70%) among the postmenopausal study groups.
The findings provide evidence for a moderately increased risk of breast cancer in women with T2DM, Ms. Bota said.
“However, the effect of the adjustment or lack of adjustment on the heterogeneity suggests that the higher risk of breast cancer in women with diabetes may not be due to diabetes itself, but to adiposity,” she said, adding that “this hypothesis is equally supported by our subgroup analysis according to menopausal status because we saw that the risk of breast cancer was only associated with diabetes in postmenopausal women and this pattern resembles the pattern of the risk of breast cancer associated with adiposity, which is also only increased in postmenopausal women.”
This study was limited by insufficient data for investigating the sources of heterogeneity, she said.
“Therefore we propose ... future pooled analyses based on individual data from good quality prospective studies in order to increase the study power and to do some detailed analysis of the links between adiposity, diabetes, and breast cancer,” she concluded, adding that new studies to examine those relationships only in premenopausal women are also needed
In a separate meta-analysis, she and her colleagues, including Peter Boyle, PhD, president of the International Prevention Research Institute, assessed the association between insulin treatment and breast cancer risk in patients with diabetes.
“The long-acting insulin analogues glargine and detemir have been shown in some studies to be associated with increased risk of breast cancer, and other studies have shown no association between the use of these two compounds and the risk of breast cancer,” Dr. Boyle said in a separate presentation at the ADA meeting. “It was important to sort out a little bit what was going on in the literature.”
Overall, the meta-analysis of data from 12 longitudinal cohort studies – including more than 6,000 cases of breast cancer – showed a slight increase in breast cancer risk in patients taking long-acting insulin (SRR, 1.13) with “a relatively reasonable” level of heterogeneity (I2 = 23%).
“But we see that the story is not that simple,” he said.
For example, some studies included only patients who were prescribed insulin for the first time after the study began (new users), some included only patients who were prescribed insulin before the study began (prevalent users), and some included both (ever users), which may have introduced bias in the results, he explained.
Studies of glargine included 4,168 breast cancer cases over a total of 1,418,743 person-years of observation, and studies of detemir included fewer than 2,047 breast cancer cases (not all studies reported case numbers). Among both new users of glargine and detemir, the SRR was 1.12, suggesting no real association between either glargine or detemir use and breast cancer, he said.
“One important take-home message is that you have to be careful that these pharmaco-epidemiological studies, even when working with the same database, may have conflicting results ... so we still need more robust standards in methods for [such] studies,” he concluded.
Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
SOURCE: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.
REPORTING FROM ADA 2018
Key clinical point: Adiposity accounts for the increased risk of breast cancer among women with diabetes.
Major finding: An analysis of 12 studies that adjusted for BMI showed a summary relative risk for breast cancer of 1.09 in diabetic versus nondiabetic women, with moderate study heterogeneity.
Study details: Meta-analyses including 21 and 12 studies, respectively.
Disclosures: Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
Source: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.
Obesity: When to consider surgery
Patients with overweight and obesity are at increased risk of multiple morbidities, including cardiovascular disease, stroke, type 2 diabetes (T2D), osteoarthritis, obstructive sleep apnea (OSA), and all-cause mortality.1 Even modest weight loss—5% to 10%—can lead to a clinically relevant reduction in this risk of disease.2,3 The American Academy of Family Physicians recognizes obesity as a disease, and recommends screening of all adults for obesity and referral for those with body mass index (BMI)* ≥30 to intensive, multicomponent behavioral interventions.4,5
For some patients, diet, exercise, and behavioral modifications are sufficient; for the great majority, however, weight loss achieved by lifestyle modification is counteracted by metabolic adaptations that promote weight regain.6 For patients with obesity who are unable to achieve or maintain sufficient weight loss to improve health outcomes with lifestyle modification alone, options include pharmacotherapy, devices, endoscopic bariatric therapies, and bariatric surgery.
Bariatric surgery is the most effective of these treatments, due to its association with significant and sustained weight loss, reduction in obesity-related comorbidities, and improved quality of life.1,7 Furthermore, compared with usual care, bariatric surgery is associated with a reduced number of cardiovascular deaths, a lower incidence of cardiovascular events in adults with obesity, and a long-term reduction in overall mortality.8-10
What are the options? Who is a candidate?
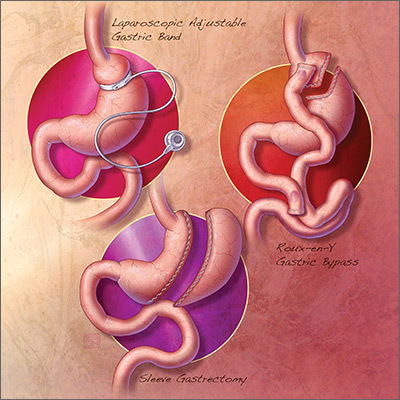
The 3 most common bariatric procedures in the United States are sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), and laparoscopic adjustable gastric band (LAGB).11 SG and RYGB are performed more often than the LAGB, consequent to greater efficacy and fewer complications.12 Weight loss is maximal at 1 to 2 years, and is estimated to be 15% of total body weight for LAGB; 25% for SG; and 35% for RYGB.13,14
Not all patients are candidates for bariatric surgery. Contraindications include chronic obstructive pulmonary disease or respiratory dysfunction, poor cardiac reserve, nonadherence to medical treatment, and severe psychological disorders.15 Because some patients have difficulty maintaining weight loss following bariatric surgery and, on average, patients regain at least some weight, patients must understand that long-term lifestyle changes and follow-up are critical to the success of bariatric surgery.16
When should bariatric surgery be considered?
American Heart Association/American College of Cardiology/The Obesity Society guidelines16 conceptualize 2 indications for bariatric surgery:
- adults with BMI ≥40
- adults with BMI ≥35 who have obesity-related comorbid conditions and are motivated to lose weight but have not responded to behavioral treatment, with or without pharmacotherapy, to achieve sufficient weight loss for target health goals.
American Association of Clinical Endocrinologists guidelines17 conceptualize 3 indications for bariatric surgery:
- adults with BMI ≥40
- adults with BMI ≥35 with 1 or more severe obesity-related complications
- adults with BMI 30-34.9 with diabetes or metabolic syndrome (evidence for this recommendation is limited).
Continue to: The 3 illustrative vignettes presented...
The 3 illustrative vignettes presented in this article offer examples of patients with obesity who could benefit from bariatric surgery. Each has been unable to achieve or maintain sufficient weight loss to improve health outcomes with nonsurgical interventions alone.
CASE 1
Sleep apnea persists despite weight loss
Robin W, a 50-year-old woman with class-II obesity (5’8”; 250 lb; BMI, 38 ), OSA requiring continuous positive airway pressure (CPAP), hyperlipidemia, hypertension, and iron-deficiency anemia secondary to menorrhagia, and taking an iron supplement, presents for weight management. She has lost 50 lb, reducing her BMI from 45.6 with behavioral modifications and pharmacotherapy, but she has been unsuccessful at achieving further weight loss despite a reduced-calorie diet and at least 30 minutes of physical activity most days.
Ms. W is frustrated that she has reached a weight plateau; she is motivated to lose more weight. Her goal is to improve her weight-related comorbid conditions and reduce her medication requirement. Despite the initial weight loss, she continues to require CPAP therapy for OSA and remains on 3 medications for hypertension. She does not have cardiac or respiratory disease, psychiatric diagnoses, or a history of gastroesophageal reflux disease (GERD).
Is bariatric surgery a reasonable option for Ms. W? If so, which procedure would you recommend?
Good option for Ms. W: Sleeve gastrectomy
It is reasonable to consider bariatric surgery—in particular, SG—for this patient with class-II obesity and multiple weight-related comorbid conditions because she has been unable to achieve further weight loss with more conservative measures.
Continue to: How does the procedure work?
How does the procedure work? SG removes a large portion of the stomach along the greater curvature, reducing the organ to approximately 15% to 25% of its original size.18 The procedure leaves the pyloric valve intact and does not involve removal or bypass of the intestines.
How appealing and successful is it? The majority of patients who undergo SG experience significant weight loss; studies report approximately 25% total body weight loss after 1 to 2 years.14 Furthermore, most patients with T2D experience resolution of, or improvement in, disease markers.19 Because SG leaves the pylorus intact, there are fewer restrictions on what a patient can eat after surgery, compared with RYGB. With further weight loss, Ms. W may experience improvement in, or resolution of, hypertension, hyperlipidemia, and OSA.
The SG procedure itself is simpler than some other bariatric procedures and presents less risk of malabsorption because the intestines are left intact. Patients who undergo SG report feeling less hungry because the fundus of the stomach, which secretes ghrelin (the so-called hunger hormone), is removed.18,20
What are special considerations, including candidacy? Patients with GERD are not ideal candidates for this procedure because exacerbation of the disease is a potential associated adverse event. SG is a reasonable surgical option for Ms. W because the procedure is less likely to exacerbate her nutritional deficiency (iron-deficiency anemia), compared to RYGB, and she does not have a history of GERD.
What are the complications? Complications of SG occur at a lower rate than they do with RYGB, which is associated with a greater risk of nutritional deficiency.18 Common early complications of SG include leaking, bleeding, stenosis, GERD, and vomiting due to excessive eating. Late complications include stomach expansion by 12 months, leading to decreased restriction.15 Unlike RYGB and LAGB, SG is not reversible.
Continue to: CASE 2
CASE 2
Severe obesity, polypharmacy for type 2 diabetes
Anne P, a 42-year-old woman with class-III obesity (5’6”; 290 lb; BMI, 46.8 kg/m2), presents to discuss bariatric surgery. Comorbidities include T2D, for which she takes metformin, a glucagon-like peptide-1 (GLP-1) receptor agonist, and a sodium–glucose cotransporter-2 (SGLT-2) inhibitor; GERD; hypertension, for which she takes an angiotensin-converting enzyme inhibitor and a calcium-channel blocker; hyperlipidemia, for which she takes a statin; and osteoarthritis.
Ms. P lost 30 pounds—reducing her BMI from 51.6—when the sulfonylurea and thiazolidinedione she was taking were switched to the GLP-1 receptor agonist and the SGLT2 inhibitor. She also made behavioral modifications, including 30 minutes a day of physical activity and a reduced-calorie meal plan under the guidance of a dietitian.
However, Ms. P has been unable to lose more weight or reduce her hemoglobin A1c (HbA1c) level below 8%. Her goal is to avoid the need to take insulin (which several members of her family take), lower her HbA1c level, and decrease her medication requirement.
Ms. P does not have cardiac or respiratory disease or psychiatric diagnoses. Which surgical intervention would you recommend for her?
Good option for Ms. P: Roux-en-Y gastric bypass
RYGB is a reasonable option for a patient with class-III obesity and multiple comorbidities, including poorly controlled T2D and GERD, who has failed conservative measures but wants to lose more weight, reduce her HbA1c, reduce her medication requirement, and avoid the need for insulin.
Continue to: How does the procedure work?
How does the procedure work? RYGB constructs a small pouch from the proximal portion of the stomach and attaches it directly to the jejunum, thus bypassing part of the stomach and duodenum. The procedure is effective for weight loss because it is both restrictive and malabsorptive: patients not only eat smaller portions, but cannot absorb all they eat. Other mechanisms attributed to RYGB that are hypothesized to promote weight loss include21:
- alteration of endogenous gut hormones, which promotes postprandial satiety
- increased levels of bile acids, which promotes alteration of the gut microbiome
- intestinal hypertrophy.
How successful is it? RYGB is associated with significant total body weight loss of approximately 35% at 2 years.9 The procedure has been shown to produce superior outcomes in reducing comorbid disease compared to other bariatric procedures or medical therapy alone. Of the procedures discussed in this article, RYGB is associated with the greatest reduction in triglycerides, HbA1c, and use of diabetes medications, including insulin.22
What are special considerations, including candidacy? For patients with mild or moderate T2D (calculated using the Individualized Metabolic Surgery Score [http://riskcalc.org/Metabolic_Surgery_Score/], which categorizes patients by number of diabetes medications, insulin use, duration of diabetes before surgery, and HbA1c), RYGB is recommended over SG because it leads to greater long-term remission of T2D.
RYGB is associated with a lower rate of GERD than SG and can even alleviate GERD in patients who have the disease. Furthermore, for patients with limited pancreatic beta cell reserve, RYBG and SG have similarly low efficacy for T2D remission; SG is therefore recommended over RYGB in this specific circumstance, given its slightly lower risk profile.23
What are the complications? Patients who undergo any bariatric surgical procedure require long-term follow-up and vitamin supplementation, but those who undergo RYGB require stricter dietary adherence after the procedure; lifelong vitamin (D, B12, folic acid, and thiamine), iron, and calcium supplementation; and long-term follow-up to reduce the risk and severity of complications and to monitor for nutritional deficiencies.7 As such, patients who have shown poor adherence to medical treatment are not good candidates for the procedure.
Continue to: Early complications include...
Early complications include leak, stricture, obstruction, and failure of the staple partition of the upper stomach. Late complications include nutritional deficiencies, as noted, and ulceration of the anastomosis. Dumping syndrome (overly rapid transit of food from the stomach into the small intestine) can develop early or late; early dumping leads to osmotic diarrhea and abdominal cramping, and late dumping leads to reactive hypoglycemia.15
Technically, RYGB is a reversible procedure, although generally it is reversed only in extreme circumstances.
CASE 3
Fatty liver disease, hesitation to undergo surgery
Walt Z, a 35 year-old-man with class-II obesity (5’10”; 265 lb; BMI, 38 kg/m2), T2D, and hepatic steatosis, presents for weight management. He has been able to lose modest weight over the years with behavioral modifications, but has been unsuccessful in maintaining that loss. He requests referral to a bariatric surgeon but is concerned about the permanence and invasiveness of most bariatric procedures.
Which surgical intervention would you recommend for this patient?
Good option for Mr. Z: Laparoscopic adjustable gastric band
Given that Mr. Z is a candidate for a surgical intervention but does not want a permanent or invasive procedure, LAGB is a reasonable option.
Continue to: How does the procedure work?
How does the procedure work? LAGB is a reversible procedure in which an inflatable band is placed around the fundus of the stomach to create a small pouch. The band can be adjusted to regulate food intake by adding or removing saline through a subcutaneous access port.
How appealing and successful is it? LAGB results in approximately 15% total body weight loss at 2 years.13 Because the procedure is purely restrictive, it carries a reduced risk of nutritional deficiency associated more commonly with malabsorptive procedures.
What are special considerations, including candidacy? As noted, Mr. Z expressed concern about the permanence and invasiveness of most bariatric procedures, and therefore wants to undergo a reversible procedure; LAGB can be a reasonable option for such a patient. Patients who want a reversible or minimally invasive procedure should also be made aware that endoscopic bariatric therapies and other devices are being developed to fill the treatment gap in the management of obesity.
What are the complications? Although LAGB is the least invasive procedure discussed here, it is associated with the highest rate of complications—most commonly, complications associated with the band itself (eg, nausea, vomiting, obstruction, band erosion or migration, esophageal dysmotility leading to acid reflux) and failure to lose weight.7 LAGB also requires more postoperative visits than other procedures, to optimize band tightness. A high number of bands are removed eventually because of complications or inadequate weight loss, or both.13,24
Shared decision-making and dialogue are essential to overcome obstacles
Despite the known benefits of bariatric surgery, including greater reduction in the risk and severity of obesity-related comorbid conditions than seen with other interventions and a long-term reduction in overall mortality when compared with usual care, fewer than 1% of eligible patients undergo a weight-loss procedure.25 Likely, this is due to:
- limited patient knowledge of the health benefits of surgery
- limited provider comfort recommending surgery
- inadequate insurance coverage, which might, in part, be due to a lack of prospective studies comparing various bariatric procedures.18
Continue to: Ultimately, the decision whether to undergo a bariatric procedure...
Ultimately, the decision whether to undergo a bariatric procedure, and which one(s) to consider, should be the product of a thorough conversation between patient and provider.
CORRESPONDENCE
Sarah R. Barenbaum, MD, Department of Internal Medicine, New York–Presbyterian Hospital/Weill Cornell Medical College, 530 East 70th Street, M-507, New York, NY 10021; srb9023@nyp.org
1. Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523-1529.
2. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
3. Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
4. American Academy of Family Physicians. Clinical preventive service recommendation: Obesity. www.aafp.org/patient-care/clinical-recommendations/all/obesity.html. Accessed August 22, 2018.
5. American Academy of Family Physicians: USPSTF draft recommendation: Intensive behavioral interventions recommended for obesity. www.aafp.org/news/health-of-the-public/20180221uspstfobesity.html. Published February 21, 2018. Accessed August 22, 2018.
6. Saunders KH, Shukla AP, Igel LI, Aronne LJ. Obesity: When to consider medication. J Fam Pract. 2017;66:608-616.
7. Roux CW, Heneghan HM. Bariatric surgery for obesity. Med Clin North Am. 2018;102:165-182.
8. Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56-65.
9. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234.
10. Reges O, Greenland P, Dicker D, et al. Association of bariatric surgery using laparoscopic banding, Roux-en-Y, gastric bypass, or laparoscopic sleeve gastrectomy vs usual care obesity management with all-cause mortality. JAMA. 2018;319:279-290.
11. Lee JH, Nguyen QN, Le QA. Comparative effectiveness of 3 bariatric surgery procedures: Roux-en-Y gastric bypass, laparoscopic adjustable gastric band, and sleeve gastrectomy. Surg Obes Relat Dis. 2016;12:997-1002.
12. American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Published June 2018. Accessed August 22, 2018.
13. Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018;153:427-434.
14. Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254-266.
15. Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;(8):CD003641.
16. Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138.
17. Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for comprehensive medical care of patients with obesity. Endocr Pract. 2016;22 Suppl 3:1-203.
18. Carlin Am, Zeni Tm, English WJ, et al; Michigan Bariatric Surgery Collaborative. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg. 2013;257:791-797.
19. Gill RS, Birch DW, Shi X, et al. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review. Surg Obes Relat Dis. 2010;6:707-713.
20. Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy. Ann Surg. 2008;247:401-407.
21. Abdeen G, le Roux CW. Mechanism underlying the weight loss and complications of Roux-en-Y gastric bypass. Obes Surg. 2016;26:410-421.
22. Schauer PR, Bhatt DL, Kirwan JP et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641-651.
23. Aminian A, Brethauer SA, Andalib A, et al. Individualized metabolic surgery score: procedure selection based on diabetes severity. Ann Surg. 2017;266:4:650-657.
24. Smetana GW, Jones DB, Wee CC. Beyond the guidelines: Should this patient have weight loss surgery? Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med. 2017;166:808-817.
25. Wolfe BM, Morton JM. Weighing in on bariatric surgery: procedure use, readmission rates, and mortality [editorial]. JAMA. 2005;294:1960-1963.
Patients with overweight and obesity are at increased risk of multiple morbidities, including cardiovascular disease, stroke, type 2 diabetes (T2D), osteoarthritis, obstructive sleep apnea (OSA), and all-cause mortality.1 Even modest weight loss—5% to 10%—can lead to a clinically relevant reduction in this risk of disease.2,3 The American Academy of Family Physicians recognizes obesity as a disease, and recommends screening of all adults for obesity and referral for those with body mass index (BMI)* ≥30 to intensive, multicomponent behavioral interventions.4,5
For some patients, diet, exercise, and behavioral modifications are sufficient; for the great majority, however, weight loss achieved by lifestyle modification is counteracted by metabolic adaptations that promote weight regain.6 For patients with obesity who are unable to achieve or maintain sufficient weight loss to improve health outcomes with lifestyle modification alone, options include pharmacotherapy, devices, endoscopic bariatric therapies, and bariatric surgery.
Bariatric surgery is the most effective of these treatments, due to its association with significant and sustained weight loss, reduction in obesity-related comorbidities, and improved quality of life.1,7 Furthermore, compared with usual care, bariatric surgery is associated with a reduced number of cardiovascular deaths, a lower incidence of cardiovascular events in adults with obesity, and a long-term reduction in overall mortality.8-10
What are the options? Who is a candidate?
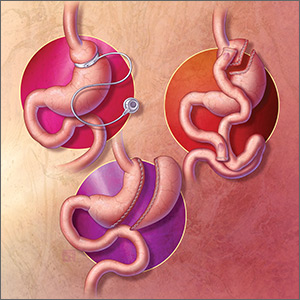
The 3 most common bariatric procedures in the United States are sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), and laparoscopic adjustable gastric band (LAGB).11 SG and RYGB are performed more often than the LAGB, consequent to greater efficacy and fewer complications.12 Weight loss is maximal at 1 to 2 years, and is estimated to be 15% of total body weight for LAGB; 25% for SG; and 35% for RYGB.13,14
Not all patients are candidates for bariatric surgery. Contraindications include chronic obstructive pulmonary disease or respiratory dysfunction, poor cardiac reserve, nonadherence to medical treatment, and severe psychological disorders.15 Because some patients have difficulty maintaining weight loss following bariatric surgery and, on average, patients regain at least some weight, patients must understand that long-term lifestyle changes and follow-up are critical to the success of bariatric surgery.16
When should bariatric surgery be considered?
American Heart Association/American College of Cardiology/The Obesity Society guidelines16 conceptualize 2 indications for bariatric surgery:
- adults with BMI ≥40
- adults with BMI ≥35 who have obesity-related comorbid conditions and are motivated to lose weight but have not responded to behavioral treatment, with or without pharmacotherapy, to achieve sufficient weight loss for target health goals.
American Association of Clinical Endocrinologists guidelines17 conceptualize 3 indications for bariatric surgery:
- adults with BMI ≥40
- adults with BMI ≥35 with 1 or more severe obesity-related complications
- adults with BMI 30-34.9 with diabetes or metabolic syndrome (evidence for this recommendation is limited).
Continue to: The 3 illustrative vignettes presented...
The 3 illustrative vignettes presented in this article offer examples of patients with obesity who could benefit from bariatric surgery. Each has been unable to achieve or maintain sufficient weight loss to improve health outcomes with nonsurgical interventions alone.
CASE 1
Sleep apnea persists despite weight loss
Robin W, a 50-year-old woman with class-II obesity (5’8”; 250 lb; BMI, 38 ), OSA requiring continuous positive airway pressure (CPAP), hyperlipidemia, hypertension, and iron-deficiency anemia secondary to menorrhagia, and taking an iron supplement, presents for weight management. She has lost 50 lb, reducing her BMI from 45.6 with behavioral modifications and pharmacotherapy, but she has been unsuccessful at achieving further weight loss despite a reduced-calorie diet and at least 30 minutes of physical activity most days.
Ms. W is frustrated that she has reached a weight plateau; she is motivated to lose more weight. Her goal is to improve her weight-related comorbid conditions and reduce her medication requirement. Despite the initial weight loss, she continues to require CPAP therapy for OSA and remains on 3 medications for hypertension. She does not have cardiac or respiratory disease, psychiatric diagnoses, or a history of gastroesophageal reflux disease (GERD).
Is bariatric surgery a reasonable option for Ms. W? If so, which procedure would you recommend?
Good option for Ms. W: Sleeve gastrectomy
It is reasonable to consider bariatric surgery—in particular, SG—for this patient with class-II obesity and multiple weight-related comorbid conditions because she has been unable to achieve further weight loss with more conservative measures.
Continue to: How does the procedure work?
How does the procedure work? SG removes a large portion of the stomach along the greater curvature, reducing the organ to approximately 15% to 25% of its original size.18 The procedure leaves the pyloric valve intact and does not involve removal or bypass of the intestines.
How appealing and successful is it? The majority of patients who undergo SG experience significant weight loss; studies report approximately 25% total body weight loss after 1 to 2 years.14 Furthermore, most patients with T2D experience resolution of, or improvement in, disease markers.19 Because SG leaves the pylorus intact, there are fewer restrictions on what a patient can eat after surgery, compared with RYGB. With further weight loss, Ms. W may experience improvement in, or resolution of, hypertension, hyperlipidemia, and OSA.
The SG procedure itself is simpler than some other bariatric procedures and presents less risk of malabsorption because the intestines are left intact. Patients who undergo SG report feeling less hungry because the fundus of the stomach, which secretes ghrelin (the so-called hunger hormone), is removed.18,20
What are special considerations, including candidacy? Patients with GERD are not ideal candidates for this procedure because exacerbation of the disease is a potential associated adverse event. SG is a reasonable surgical option for Ms. W because the procedure is less likely to exacerbate her nutritional deficiency (iron-deficiency anemia), compared to RYGB, and she does not have a history of GERD.
What are the complications? Complications of SG occur at a lower rate than they do with RYGB, which is associated with a greater risk of nutritional deficiency.18 Common early complications of SG include leaking, bleeding, stenosis, GERD, and vomiting due to excessive eating. Late complications include stomach expansion by 12 months, leading to decreased restriction.15 Unlike RYGB and LAGB, SG is not reversible.
Continue to: CASE 2
CASE 2
Severe obesity, polypharmacy for type 2 diabetes
Anne P, a 42-year-old woman with class-III obesity (5’6”; 290 lb; BMI, 46.8 kg/m2), presents to discuss bariatric surgery. Comorbidities include T2D, for which she takes metformin, a glucagon-like peptide-1 (GLP-1) receptor agonist, and a sodium–glucose cotransporter-2 (SGLT-2) inhibitor; GERD; hypertension, for which she takes an angiotensin-converting enzyme inhibitor and a calcium-channel blocker; hyperlipidemia, for which she takes a statin; and osteoarthritis.
Ms. P lost 30 pounds—reducing her BMI from 51.6—when the sulfonylurea and thiazolidinedione she was taking were switched to the GLP-1 receptor agonist and the SGLT2 inhibitor. She also made behavioral modifications, including 30 minutes a day of physical activity and a reduced-calorie meal plan under the guidance of a dietitian.
However, Ms. P has been unable to lose more weight or reduce her hemoglobin A1c (HbA1c) level below 8%. Her goal is to avoid the need to take insulin (which several members of her family take), lower her HbA1c level, and decrease her medication requirement.
Ms. P does not have cardiac or respiratory disease or psychiatric diagnoses. Which surgical intervention would you recommend for her?
Good option for Ms. P: Roux-en-Y gastric bypass
RYGB is a reasonable option for a patient with class-III obesity and multiple comorbidities, including poorly controlled T2D and GERD, who has failed conservative measures but wants to lose more weight, reduce her HbA1c, reduce her medication requirement, and avoid the need for insulin.
Continue to: How does the procedure work?
How does the procedure work? RYGB constructs a small pouch from the proximal portion of the stomach and attaches it directly to the jejunum, thus bypassing part of the stomach and duodenum. The procedure is effective for weight loss because it is both restrictive and malabsorptive: patients not only eat smaller portions, but cannot absorb all they eat. Other mechanisms attributed to RYGB that are hypothesized to promote weight loss include21:
- alteration of endogenous gut hormones, which promotes postprandial satiety
- increased levels of bile acids, which promotes alteration of the gut microbiome
- intestinal hypertrophy.
How successful is it? RYGB is associated with significant total body weight loss of approximately 35% at 2 years.9 The procedure has been shown to produce superior outcomes in reducing comorbid disease compared to other bariatric procedures or medical therapy alone. Of the procedures discussed in this article, RYGB is associated with the greatest reduction in triglycerides, HbA1c, and use of diabetes medications, including insulin.22
What are special considerations, including candidacy? For patients with mild or moderate T2D (calculated using the Individualized Metabolic Surgery Score [http://riskcalc.org/Metabolic_Surgery_Score/], which categorizes patients by number of diabetes medications, insulin use, duration of diabetes before surgery, and HbA1c), RYGB is recommended over SG because it leads to greater long-term remission of T2D.
RYGB is associated with a lower rate of GERD than SG and can even alleviate GERD in patients who have the disease. Furthermore, for patients with limited pancreatic beta cell reserve, RYBG and SG have similarly low efficacy for T2D remission; SG is therefore recommended over RYGB in this specific circumstance, given its slightly lower risk profile.23
What are the complications? Patients who undergo any bariatric surgical procedure require long-term follow-up and vitamin supplementation, but those who undergo RYGB require stricter dietary adherence after the procedure; lifelong vitamin (D, B12, folic acid, and thiamine), iron, and calcium supplementation; and long-term follow-up to reduce the risk and severity of complications and to monitor for nutritional deficiencies.7 As such, patients who have shown poor adherence to medical treatment are not good candidates for the procedure.
Continue to: Early complications include...
Early complications include leak, stricture, obstruction, and failure of the staple partition of the upper stomach. Late complications include nutritional deficiencies, as noted, and ulceration of the anastomosis. Dumping syndrome (overly rapid transit of food from the stomach into the small intestine) can develop early or late; early dumping leads to osmotic diarrhea and abdominal cramping, and late dumping leads to reactive hypoglycemia.15
Technically, RYGB is a reversible procedure, although generally it is reversed only in extreme circumstances.
CASE 3
Fatty liver disease, hesitation to undergo surgery
Walt Z, a 35 year-old-man with class-II obesity (5’10”; 265 lb; BMI, 38 kg/m2), T2D, and hepatic steatosis, presents for weight management. He has been able to lose modest weight over the years with behavioral modifications, but has been unsuccessful in maintaining that loss. He requests referral to a bariatric surgeon but is concerned about the permanence and invasiveness of most bariatric procedures.
Which surgical intervention would you recommend for this patient?
Good option for Mr. Z: Laparoscopic adjustable gastric band
Given that Mr. Z is a candidate for a surgical intervention but does not want a permanent or invasive procedure, LAGB is a reasonable option.
Continue to: How does the procedure work?
How does the procedure work? LAGB is a reversible procedure in which an inflatable band is placed around the fundus of the stomach to create a small pouch. The band can be adjusted to regulate food intake by adding or removing saline through a subcutaneous access port.
How appealing and successful is it? LAGB results in approximately 15% total body weight loss at 2 years.13 Because the procedure is purely restrictive, it carries a reduced risk of nutritional deficiency associated more commonly with malabsorptive procedures.
What are special considerations, including candidacy? As noted, Mr. Z expressed concern about the permanence and invasiveness of most bariatric procedures, and therefore wants to undergo a reversible procedure; LAGB can be a reasonable option for such a patient. Patients who want a reversible or minimally invasive procedure should also be made aware that endoscopic bariatric therapies and other devices are being developed to fill the treatment gap in the management of obesity.
What are the complications? Although LAGB is the least invasive procedure discussed here, it is associated with the highest rate of complications—most commonly, complications associated with the band itself (eg, nausea, vomiting, obstruction, band erosion or migration, esophageal dysmotility leading to acid reflux) and failure to lose weight.7 LAGB also requires more postoperative visits than other procedures, to optimize band tightness. A high number of bands are removed eventually because of complications or inadequate weight loss, or both.13,24
Shared decision-making and dialogue are essential to overcome obstacles
Despite the known benefits of bariatric surgery, including greater reduction in the risk and severity of obesity-related comorbid conditions than seen with other interventions and a long-term reduction in overall mortality when compared with usual care, fewer than 1% of eligible patients undergo a weight-loss procedure.25 Likely, this is due to:
- limited patient knowledge of the health benefits of surgery
- limited provider comfort recommending surgery
- inadequate insurance coverage, which might, in part, be due to a lack of prospective studies comparing various bariatric procedures.18
Continue to: Ultimately, the decision whether to undergo a bariatric procedure...
Ultimately, the decision whether to undergo a bariatric procedure, and which one(s) to consider, should be the product of a thorough conversation between patient and provider.
CORRESPONDENCE
Sarah R. Barenbaum, MD, Department of Internal Medicine, New York–Presbyterian Hospital/Weill Cornell Medical College, 530 East 70th Street, M-507, New York, NY 10021; srb9023@nyp.org
Patients with overweight and obesity are at increased risk of multiple morbidities, including cardiovascular disease, stroke, type 2 diabetes (T2D), osteoarthritis, obstructive sleep apnea (OSA), and all-cause mortality.1 Even modest weight loss—5% to 10%—can lead to a clinically relevant reduction in this risk of disease.2,3 The American Academy of Family Physicians recognizes obesity as a disease, and recommends screening of all adults for obesity and referral for those with body mass index (BMI)* ≥30 to intensive, multicomponent behavioral interventions.4,5
For some patients, diet, exercise, and behavioral modifications are sufficient; for the great majority, however, weight loss achieved by lifestyle modification is counteracted by metabolic adaptations that promote weight regain.6 For patients with obesity who are unable to achieve or maintain sufficient weight loss to improve health outcomes with lifestyle modification alone, options include pharmacotherapy, devices, endoscopic bariatric therapies, and bariatric surgery.
Bariatric surgery is the most effective of these treatments, due to its association with significant and sustained weight loss, reduction in obesity-related comorbidities, and improved quality of life.1,7 Furthermore, compared with usual care, bariatric surgery is associated with a reduced number of cardiovascular deaths, a lower incidence of cardiovascular events in adults with obesity, and a long-term reduction in overall mortality.8-10
What are the options? Who is a candidate?
The 3 most common bariatric procedures in the United States are sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), and laparoscopic adjustable gastric band (LAGB).11 SG and RYGB are performed more often than the LAGB, consequent to greater efficacy and fewer complications.12 Weight loss is maximal at 1 to 2 years, and is estimated to be 15% of total body weight for LAGB; 25% for SG; and 35% for RYGB.13,14
Not all patients are candidates for bariatric surgery. Contraindications include chronic obstructive pulmonary disease or respiratory dysfunction, poor cardiac reserve, nonadherence to medical treatment, and severe psychological disorders.15 Because some patients have difficulty maintaining weight loss following bariatric surgery and, on average, patients regain at least some weight, patients must understand that long-term lifestyle changes and follow-up are critical to the success of bariatric surgery.16
When should bariatric surgery be considered?
American Heart Association/American College of Cardiology/The Obesity Society guidelines16 conceptualize 2 indications for bariatric surgery:
- adults with BMI ≥40
- adults with BMI ≥35 who have obesity-related comorbid conditions and are motivated to lose weight but have not responded to behavioral treatment, with or without pharmacotherapy, to achieve sufficient weight loss for target health goals.
American Association of Clinical Endocrinologists guidelines17 conceptualize 3 indications for bariatric surgery:
- adults with BMI ≥40
- adults with BMI ≥35 with 1 or more severe obesity-related complications
- adults with BMI 30-34.9 with diabetes or metabolic syndrome (evidence for this recommendation is limited).
Continue to: The 3 illustrative vignettes presented...
The 3 illustrative vignettes presented in this article offer examples of patients with obesity who could benefit from bariatric surgery. Each has been unable to achieve or maintain sufficient weight loss to improve health outcomes with nonsurgical interventions alone.
CASE 1
Sleep apnea persists despite weight loss
Robin W, a 50-year-old woman with class-II obesity (5’8”; 250 lb; BMI, 38 ), OSA requiring continuous positive airway pressure (CPAP), hyperlipidemia, hypertension, and iron-deficiency anemia secondary to menorrhagia, and taking an iron supplement, presents for weight management. She has lost 50 lb, reducing her BMI from 45.6 with behavioral modifications and pharmacotherapy, but she has been unsuccessful at achieving further weight loss despite a reduced-calorie diet and at least 30 minutes of physical activity most days.
Ms. W is frustrated that she has reached a weight plateau; she is motivated to lose more weight. Her goal is to improve her weight-related comorbid conditions and reduce her medication requirement. Despite the initial weight loss, she continues to require CPAP therapy for OSA and remains on 3 medications for hypertension. She does not have cardiac or respiratory disease, psychiatric diagnoses, or a history of gastroesophageal reflux disease (GERD).
Is bariatric surgery a reasonable option for Ms. W? If so, which procedure would you recommend?
Good option for Ms. W: Sleeve gastrectomy
It is reasonable to consider bariatric surgery—in particular, SG—for this patient with class-II obesity and multiple weight-related comorbid conditions because she has been unable to achieve further weight loss with more conservative measures.
Continue to: How does the procedure work?
How does the procedure work? SG removes a large portion of the stomach along the greater curvature, reducing the organ to approximately 15% to 25% of its original size.18 The procedure leaves the pyloric valve intact and does not involve removal or bypass of the intestines.
How appealing and successful is it? The majority of patients who undergo SG experience significant weight loss; studies report approximately 25% total body weight loss after 1 to 2 years.14 Furthermore, most patients with T2D experience resolution of, or improvement in, disease markers.19 Because SG leaves the pylorus intact, there are fewer restrictions on what a patient can eat after surgery, compared with RYGB. With further weight loss, Ms. W may experience improvement in, or resolution of, hypertension, hyperlipidemia, and OSA.
The SG procedure itself is simpler than some other bariatric procedures and presents less risk of malabsorption because the intestines are left intact. Patients who undergo SG report feeling less hungry because the fundus of the stomach, which secretes ghrelin (the so-called hunger hormone), is removed.18,20
What are special considerations, including candidacy? Patients with GERD are not ideal candidates for this procedure because exacerbation of the disease is a potential associated adverse event. SG is a reasonable surgical option for Ms. W because the procedure is less likely to exacerbate her nutritional deficiency (iron-deficiency anemia), compared to RYGB, and she does not have a history of GERD.
What are the complications? Complications of SG occur at a lower rate than they do with RYGB, which is associated with a greater risk of nutritional deficiency.18 Common early complications of SG include leaking, bleeding, stenosis, GERD, and vomiting due to excessive eating. Late complications include stomach expansion by 12 months, leading to decreased restriction.15 Unlike RYGB and LAGB, SG is not reversible.
Continue to: CASE 2
CASE 2
Severe obesity, polypharmacy for type 2 diabetes
Anne P, a 42-year-old woman with class-III obesity (5’6”; 290 lb; BMI, 46.8 kg/m2), presents to discuss bariatric surgery. Comorbidities include T2D, for which she takes metformin, a glucagon-like peptide-1 (GLP-1) receptor agonist, and a sodium–glucose cotransporter-2 (SGLT-2) inhibitor; GERD; hypertension, for which she takes an angiotensin-converting enzyme inhibitor and a calcium-channel blocker; hyperlipidemia, for which she takes a statin; and osteoarthritis.
Ms. P lost 30 pounds—reducing her BMI from 51.6—when the sulfonylurea and thiazolidinedione she was taking were switched to the GLP-1 receptor agonist and the SGLT2 inhibitor. She also made behavioral modifications, including 30 minutes a day of physical activity and a reduced-calorie meal plan under the guidance of a dietitian.
However, Ms. P has been unable to lose more weight or reduce her hemoglobin A1c (HbA1c) level below 8%. Her goal is to avoid the need to take insulin (which several members of her family take), lower her HbA1c level, and decrease her medication requirement.
Ms. P does not have cardiac or respiratory disease or psychiatric diagnoses. Which surgical intervention would you recommend for her?
Good option for Ms. P: Roux-en-Y gastric bypass
RYGB is a reasonable option for a patient with class-III obesity and multiple comorbidities, including poorly controlled T2D and GERD, who has failed conservative measures but wants to lose more weight, reduce her HbA1c, reduce her medication requirement, and avoid the need for insulin.
Continue to: How does the procedure work?
How does the procedure work? RYGB constructs a small pouch from the proximal portion of the stomach and attaches it directly to the jejunum, thus bypassing part of the stomach and duodenum. The procedure is effective for weight loss because it is both restrictive and malabsorptive: patients not only eat smaller portions, but cannot absorb all they eat. Other mechanisms attributed to RYGB that are hypothesized to promote weight loss include21:
- alteration of endogenous gut hormones, which promotes postprandial satiety
- increased levels of bile acids, which promotes alteration of the gut microbiome
- intestinal hypertrophy.
How successful is it? RYGB is associated with significant total body weight loss of approximately 35% at 2 years.9 The procedure has been shown to produce superior outcomes in reducing comorbid disease compared to other bariatric procedures or medical therapy alone. Of the procedures discussed in this article, RYGB is associated with the greatest reduction in triglycerides, HbA1c, and use of diabetes medications, including insulin.22
What are special considerations, including candidacy? For patients with mild or moderate T2D (calculated using the Individualized Metabolic Surgery Score [http://riskcalc.org/Metabolic_Surgery_Score/], which categorizes patients by number of diabetes medications, insulin use, duration of diabetes before surgery, and HbA1c), RYGB is recommended over SG because it leads to greater long-term remission of T2D.
RYGB is associated with a lower rate of GERD than SG and can even alleviate GERD in patients who have the disease. Furthermore, for patients with limited pancreatic beta cell reserve, RYBG and SG have similarly low efficacy for T2D remission; SG is therefore recommended over RYGB in this specific circumstance, given its slightly lower risk profile.23
What are the complications? Patients who undergo any bariatric surgical procedure require long-term follow-up and vitamin supplementation, but those who undergo RYGB require stricter dietary adherence after the procedure; lifelong vitamin (D, B12, folic acid, and thiamine), iron, and calcium supplementation; and long-term follow-up to reduce the risk and severity of complications and to monitor for nutritional deficiencies.7 As such, patients who have shown poor adherence to medical treatment are not good candidates for the procedure.
Continue to: Early complications include...
Early complications include leak, stricture, obstruction, and failure of the staple partition of the upper stomach. Late complications include nutritional deficiencies, as noted, and ulceration of the anastomosis. Dumping syndrome (overly rapid transit of food from the stomach into the small intestine) can develop early or late; early dumping leads to osmotic diarrhea and abdominal cramping, and late dumping leads to reactive hypoglycemia.15
Technically, RYGB is a reversible procedure, although generally it is reversed only in extreme circumstances.
CASE 3
Fatty liver disease, hesitation to undergo surgery
Walt Z, a 35 year-old-man with class-II obesity (5’10”; 265 lb; BMI, 38 kg/m2), T2D, and hepatic steatosis, presents for weight management. He has been able to lose modest weight over the years with behavioral modifications, but has been unsuccessful in maintaining that loss. He requests referral to a bariatric surgeon but is concerned about the permanence and invasiveness of most bariatric procedures.
Which surgical intervention would you recommend for this patient?
Good option for Mr. Z: Laparoscopic adjustable gastric band
Given that Mr. Z is a candidate for a surgical intervention but does not want a permanent or invasive procedure, LAGB is a reasonable option.
Continue to: How does the procedure work?
How does the procedure work? LAGB is a reversible procedure in which an inflatable band is placed around the fundus of the stomach to create a small pouch. The band can be adjusted to regulate food intake by adding or removing saline through a subcutaneous access port.
How appealing and successful is it? LAGB results in approximately 15% total body weight loss at 2 years.13 Because the procedure is purely restrictive, it carries a reduced risk of nutritional deficiency associated more commonly with malabsorptive procedures.
What are special considerations, including candidacy? As noted, Mr. Z expressed concern about the permanence and invasiveness of most bariatric procedures, and therefore wants to undergo a reversible procedure; LAGB can be a reasonable option for such a patient. Patients who want a reversible or minimally invasive procedure should also be made aware that endoscopic bariatric therapies and other devices are being developed to fill the treatment gap in the management of obesity.
What are the complications? Although LAGB is the least invasive procedure discussed here, it is associated with the highest rate of complications—most commonly, complications associated with the band itself (eg, nausea, vomiting, obstruction, band erosion or migration, esophageal dysmotility leading to acid reflux) and failure to lose weight.7 LAGB also requires more postoperative visits than other procedures, to optimize band tightness. A high number of bands are removed eventually because of complications or inadequate weight loss, or both.13,24
Shared decision-making and dialogue are essential to overcome obstacles
Despite the known benefits of bariatric surgery, including greater reduction in the risk and severity of obesity-related comorbid conditions than seen with other interventions and a long-term reduction in overall mortality when compared with usual care, fewer than 1% of eligible patients undergo a weight-loss procedure.25 Likely, this is due to:
- limited patient knowledge of the health benefits of surgery
- limited provider comfort recommending surgery
- inadequate insurance coverage, which might, in part, be due to a lack of prospective studies comparing various bariatric procedures.18
Continue to: Ultimately, the decision whether to undergo a bariatric procedure...
Ultimately, the decision whether to undergo a bariatric procedure, and which one(s) to consider, should be the product of a thorough conversation between patient and provider.
CORRESPONDENCE
Sarah R. Barenbaum, MD, Department of Internal Medicine, New York–Presbyterian Hospital/Weill Cornell Medical College, 530 East 70th Street, M-507, New York, NY 10021; srb9023@nyp.org
1. Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523-1529.
2. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
3. Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
4. American Academy of Family Physicians. Clinical preventive service recommendation: Obesity. www.aafp.org/patient-care/clinical-recommendations/all/obesity.html. Accessed August 22, 2018.
5. American Academy of Family Physicians: USPSTF draft recommendation: Intensive behavioral interventions recommended for obesity. www.aafp.org/news/health-of-the-public/20180221uspstfobesity.html. Published February 21, 2018. Accessed August 22, 2018.
6. Saunders KH, Shukla AP, Igel LI, Aronne LJ. Obesity: When to consider medication. J Fam Pract. 2017;66:608-616.
7. Roux CW, Heneghan HM. Bariatric surgery for obesity. Med Clin North Am. 2018;102:165-182.
8. Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56-65.
9. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234.
10. Reges O, Greenland P, Dicker D, et al. Association of bariatric surgery using laparoscopic banding, Roux-en-Y, gastric bypass, or laparoscopic sleeve gastrectomy vs usual care obesity management with all-cause mortality. JAMA. 2018;319:279-290.
11. Lee JH, Nguyen QN, Le QA. Comparative effectiveness of 3 bariatric surgery procedures: Roux-en-Y gastric bypass, laparoscopic adjustable gastric band, and sleeve gastrectomy. Surg Obes Relat Dis. 2016;12:997-1002.
12. American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Published June 2018. Accessed August 22, 2018.
13. Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018;153:427-434.
14. Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254-266.
15. Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;(8):CD003641.
16. Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138.
17. Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for comprehensive medical care of patients with obesity. Endocr Pract. 2016;22 Suppl 3:1-203.
18. Carlin Am, Zeni Tm, English WJ, et al; Michigan Bariatric Surgery Collaborative. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg. 2013;257:791-797.
19. Gill RS, Birch DW, Shi X, et al. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review. Surg Obes Relat Dis. 2010;6:707-713.
20. Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy. Ann Surg. 2008;247:401-407.
21. Abdeen G, le Roux CW. Mechanism underlying the weight loss and complications of Roux-en-Y gastric bypass. Obes Surg. 2016;26:410-421.
22. Schauer PR, Bhatt DL, Kirwan JP et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641-651.
23. Aminian A, Brethauer SA, Andalib A, et al. Individualized metabolic surgery score: procedure selection based on diabetes severity. Ann Surg. 2017;266:4:650-657.
24. Smetana GW, Jones DB, Wee CC. Beyond the guidelines: Should this patient have weight loss surgery? Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med. 2017;166:808-817.
25. Wolfe BM, Morton JM. Weighing in on bariatric surgery: procedure use, readmission rates, and mortality [editorial]. JAMA. 2005;294:1960-1963.
1. Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523-1529.
2. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
3. Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
4. American Academy of Family Physicians. Clinical preventive service recommendation: Obesity. www.aafp.org/patient-care/clinical-recommendations/all/obesity.html. Accessed August 22, 2018.
5. American Academy of Family Physicians: USPSTF draft recommendation: Intensive behavioral interventions recommended for obesity. www.aafp.org/news/health-of-the-public/20180221uspstfobesity.html. Published February 21, 2018. Accessed August 22, 2018.
6. Saunders KH, Shukla AP, Igel LI, Aronne LJ. Obesity: When to consider medication. J Fam Pract. 2017;66:608-616.
7. Roux CW, Heneghan HM. Bariatric surgery for obesity. Med Clin North Am. 2018;102:165-182.
8. Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56-65.
9. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234.
10. Reges O, Greenland P, Dicker D, et al. Association of bariatric surgery using laparoscopic banding, Roux-en-Y, gastric bypass, or laparoscopic sleeve gastrectomy vs usual care obesity management with all-cause mortality. JAMA. 2018;319:279-290.
11. Lee JH, Nguyen QN, Le QA. Comparative effectiveness of 3 bariatric surgery procedures: Roux-en-Y gastric bypass, laparoscopic adjustable gastric band, and sleeve gastrectomy. Surg Obes Relat Dis. 2016;12:997-1002.
12. American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Published June 2018. Accessed August 22, 2018.
13. Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018;153:427-434.
14. Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254-266.
15. Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;(8):CD003641.
16. Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138.
17. Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for comprehensive medical care of patients with obesity. Endocr Pract. 2016;22 Suppl 3:1-203.
18. Carlin Am, Zeni Tm, English WJ, et al; Michigan Bariatric Surgery Collaborative. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg. 2013;257:791-797.
19. Gill RS, Birch DW, Shi X, et al. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review. Surg Obes Relat Dis. 2010;6:707-713.
20. Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy. Ann Surg. 2008;247:401-407.
21. Abdeen G, le Roux CW. Mechanism underlying the weight loss and complications of Roux-en-Y gastric bypass. Obes Surg. 2016;26:410-421.
22. Schauer PR, Bhatt DL, Kirwan JP et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641-651.
23. Aminian A, Brethauer SA, Andalib A, et al. Individualized metabolic surgery score: procedure selection based on diabetes severity. Ann Surg. 2017;266:4:650-657.
24. Smetana GW, Jones DB, Wee CC. Beyond the guidelines: Should this patient have weight loss surgery? Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med. 2017;166:808-817.
25. Wolfe BM, Morton JM. Weighing in on bariatric surgery: procedure use, readmission rates, and mortality [editorial]. JAMA. 2005;294:1960-1963.
PRACTICE RECOMMENDATIONS
Among adult patients with body mass index* ≥40, or ≥35 with obesity-related comorbid conditions:
› Consider bariatric surgery in those who are motivated to lose weight but who have not responded to lifestyle modification with or without pharmacotherapy in order to achieve sufficient and sustained weight loss. A
› Consider bariatric surgery to help patients achieve target health goals and reduce/improve obesity-related comorbidities. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
*Calculated as weight in kilograms divided by height in meters squared.
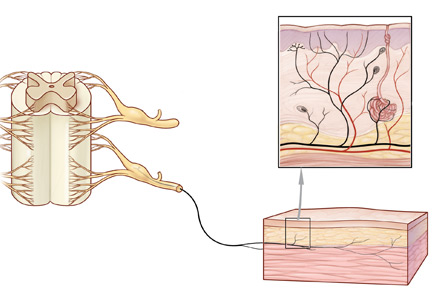
Office approach to small fiber neuropathy
Peripheral neuropathy is the most common reason for an outpatient neurology visit in the United States and accounts for over $10 billion in healthcare spending each year.1,2 When the disorder affects only small, thinly myelinated or unmyelinated nerve fibers, it is referred to as small fiber neuropathy, which commonly presents as numbness and burning pain in the feet.
This article details the manifestations and evaluation of small fiber neuropathy, with an eye toward diagnosing an underlying cause amenable to treatment.
OLDER PATIENTS MOST AFFECTED
The epidemiology of small fiber neuropathy is not well established. It occurs more commonly in older patients, but data are mixed on prevalence by sex.3–6 In a Dutch study,3 the overall prevalence was at least 53 cases per 100,000, with the highest rate in men over age 65.
CHARACTERISTIC SENSORY DISTURBANCES
Sensations vary in quality and time
Patients with small fiber neuropathy typically present with a symmetric length-dependent (“stocking-glove”) distribution of sensory changes, starting in the feet and gradually ascending up the legs and then to the hands.
Commonly reported neuropathic symptoms include various combinations of burning, numbness, tingling, itching, sunburn-like, and frostbite-like sensations. Nonneuropathic symptoms may include tightness, a vise-like squeezing of the feet, and the sensation of a sock rolled up at the end of the shoe. Cramps or spasms may also be reported but rarely occur in isolation.7
Symptoms are typically worse at the end of the day and while sitting or lying down at night. They can arise spontaneously but may also be triggered by something as minor as the touch of clothing or cool air against the skin. Bedsheet sensitivity of the feet is reported so often that it is used as an outcome measure in clinical trials. Symptoms can also be exacerbated by extremes in ambient temperature and are especially worse in cold weather.
Random patterns suggest an immune cause
Symptoms may also have a non–length-dependent distribution that is asymmetric, patchy, intermittent, and migratory, and can involve the face, proximal limbs, and trunk. Symptoms may vary throughout the day, eg, starting with electric-shock sensations on one side of the face, followed by perineal numbness and then tingling in the arms lasting for a few minutes to several hours. While such patterns may be seen with diabetes and other common etiologies, they often suggest an underlying immune-mediated disorder such as Sjögren syndrome or sarcoidosis.8–10 Although large fiber polyneuropathy may also be non–length-dependent, the deficits are usually fixed, with no migratory component.
Autonomic features may be prominent
Autonomic symptoms occur in nearly half of patients and can be as troublesome as neuropathic pain.3 Small nerve fibers mediate somatic and autonomic functions, an evolutionary link that may reflect visceral defense mechanisms responding to pain as a signal of danger.11 This may help explain the multisystemic nature of symptoms, which can include sweating abnormalities, bowel and bladder disturbances, dry eyes, dry mouth, gastrointestinal dysmotility, skin changes (eg, discoloration, loss of hair, shiny skin), sexual dysfunction, orthostatic hypotension, and palpitations. In some cases, isolated dysautonomia may be seen.
TARGETED EXAMINATION
History: Medications, alcohol, infections
When a patient presents with neuropathic pain in the feet, a detailed history should be obtained, including alcohol use, family history of neuropathy, and use of neurotoxic medications such as metronidazole, colchicine, and chemotherapeutic agents.
Human immunodeficiency virus (HIV) and hepatitis C infection are well known to be associated with small fiber neuropathy, so relevant risk factors (eg, blood transfusions, sexual history, intravenous drug use) should be asked about. Recent illnesses and vaccinations are another important line of questioning, as a small-fiber variant of Guillain-Barré syndrome has been described.12
Assess reflexes, strength, sensation
On physical examination, particular attention should be focused on searching for abnormalities indicating large nerve fiber involvement (eg, absent deep tendon reflexes, weakness of the toes). However, absent ankle deep tendon reflexes and reduced vibratory sense may also occur in healthy elderly people.
Similarly, proprioception, motor strength, balance, and vibratory sensation are functions of large myelinated nerve fibers, and thus remain unaffected in patients with only small fiber neuropathy.
Evidence of a systemic disorder should also be sought, as it may indicate an underlying etiology.
DIAGNOSTIC TESTING
Although patients with either large or small fiber neuropathy may have subjective hyperesthesia or numbness of the distal lower extremities, the absence of significant abnormalities on neurologic examination should prompt consideration of small fiber neuropathy.
Electromyography worthwhile
Nerve conduction studies and needle electrode examination evaluate only large nerve fiber conditions. While electromyographic results are normal in patients with isolated small fiber neuropathy, the test can help evaluate subclinical large nerve fiber involvement and alternative diagnoses such as bilateral S1 radiculopathy. Nerve conduction studies may be less useful in patients over age 75, as they may lack sural sensory responses because of aging changes.13
Skin biopsy easy to do
Skin biopsy for evaluating intraepidermal nerve fiber density is one of the most widely used tests for small fiber neuropathy. This minimally invasive procedure can now be performed in a primary care office using readily available tools or prepackaged kits and analyzed by several commercial laboratories.
Reduced intraepidermal nerve fiber density on skin biopsy has been described in various other conditions such as fibromyalgia and chronic pain syndromes.16,17 The clinical significance of these findings remains uncertain.
Quantitative sudomotor axon reflex testing
Quantitative sudomotor axon reflex testing (QSART) is a noninvasive autonomic study that assesses the volume of sweat produced by the limbs in response to acetylcholine. A measure of postganglionic sympathetic sudomotor nerve function, QSART has a sensitivity of up to 80% and can be used to diagnose small fiber neuropathy.18 In a series of 115 patients with sarcoidosis small fiber neuropathy,9 the QSART and skin biopsy findings were concordant in 17 cases and complementary in 29, allowing for confirmation of small fiber neuropathy in patients whose condition would have remained undiagnosed had only one test been performed. QSART can also be considered in cases where skin biopsy may be contraindicated (eg, patient use of anticoagulation). Of note, the study may be affected by a number of external factors, including caffeine, tobacco, antihistamines, and tricyclic antidepressants; these should be held before testing.
Other diagnostic studies
Other tests may be helpful, as follows:
Tilt-table and cardiovagal testing may be useful for patients with orthostasis and palpitations.
Thermoregulatory sweat testing can be used to evaluate patients with abnormal patterns of sweating, eg, hyperhidrosis of the face and head.
INITIAL TESTING FOR AN UNDERLYING CAUSE
Glucose tolerance test for diabetes
Diabetes is the most common identifiable cause of small fiber neuropathy and accounts for about a third of all cases.5 Impaired glucose tolerance is also thought to be a risk factor and has been found in up to 50% of idiopathic cases, but the association is still being debated.21
While testing for hemoglobin A1c is more convenient for the patient, especially because it does not require fasting, a 2-hour oral glucose tolerance test is more sensitive for detecting glucose dysmetabolism.22
Lipid panel for metabolic syndrome
Small fiber neuropathy is associated with individual components of the metabolic syndrome, which include obesity, hyperglycemia, and dyslipidemia. Of these, dyslipidemia has emerged as the primary factor involved in the development of small fiber neuropathy, via an inflammatory pathway or oxidative stress mechanism.23,24
Vitamin B12 deficiency testing
Vitamin B12 deficiency, a potentially correctable cause of small fiber neuropathy, may be underdiagnosed, especially as values obtained by blood testing may not reflect tissue uptake. Causes of vitamin B12 deficiency include reduced intake, pernicious anemia, and medications that can affect absorption of vitamin B12 (eg, proton pump inhibitors, histamine 2 receptor antagonists, metformin).
Testing should include:
- Complete blood cell count to evaluate for vitamin B12-related macrocytic anemia and other hematologic abnormalities
- Serum vitamin B12 level
- Methylmalonic acid or homocysteine level in patients with subclinical or mild vitamin B12 deficiency, manifested as low to normal vitamin B12 levels (< 400 pg/mL); methylmalonic acid and homocysteine require vitamin B12 as a cofactor for enzymatic conversion, and either or both may be elevated in early vitamin B12 deficiency.
Celiac antibody panel
Celiac disease, a T-cell mediated enteropathy characterized by gluten intolerance and a herpetiform-like rash, can be associated with small fiber neuropathy.25 In some cases, neuropathy symptoms are preceded by the onset of gastrointestinal symptoms, or they may occur in isolation.25
Inflammatory disease testing
Sjögren syndrome accounts for nearly 10% of cases of small fiber neuropathy. Associated neuropathic symptoms are often non–length-dependent, can precede sicca symptoms for up to 6 years, and in some cases are the sole manifestation of the disease.10 Small fiber neuropathy may also be associated with vasculitis, systemic lupus erythematosus, and other connective tissue disorders.
Testing should include:
- Erythrocyte sedimentation rate, C-reactive protein, and antinuclear antibodies: though these are nonspecific markers of inflammation, they may support an immune-mediated etiology if positive
- Extractable nuclear antigen panel: Sjögren syndrome A and B autoantibodies are the most important components in this setting5,11
- The Schirmer test or salivary gland biopsy should be considered for seronegative patients with sicca or a suspected immune-mediated etiology, as the sensitivity of antibody testing ranges from only 10% to 55%.10
Thyroid function testing
Hypothyroidism, and less commonly hyperthyroidism, are associated with small fiber neuropathy.
Metabolic tests for liver and kidney disease
Renal insufficiency and liver impairment are well-known causes of small nerve fiber dysfunction. Testing should include:
- Comprehensive metabolic panel
- Gamma-glutamyltransferase if alcohol abuse is suspected, since heavy alcohol use is one of the most common causes of both large and small fiber neuropathy.
HIV and hepatitis C testing
For patients with relevant risk factors, HIV and hepatitis C testing should be part of the initial workup (and as second-tier testing for others). Patients who test positive for hepatitis C should undergo further testing for cryoglobulinemia, which can present with painful small fiber neuropathy.26
Serum and urine immunoelectrophoresis
Paraproteinemia, with causes ranging from monoclonal gammopathy of uncertain significance to multiple myeloma, has been associated with small fiber neuropathy. An abnormal serum or urine immunoelectrophoresis test warrants further investigation and possibly referral to a hematology-oncology specialist.
SECOND-TIER TESTING
Less common treatable causes of small fiber neuropathy may also be evaluated.
Copper, vitamin B1 (thiamine), or vitamin B6 (pyridoxine) deficiency testing. Although vitamin B6 toxicity may also result in neuropathy due to its toxic effect on the dorsal root ganglia, the mildly elevated vitamin B6 levels often found in patients being evaluated for neuropathy are unlikely to be the primary cause of symptoms. Many laboratories require fasting samples for accurate vitamin B6 levels.
Angiotensin-converting enzyme levels for sarcoidosis. Small fiber neuropathy is common in sarcoidosis, occurring in more than 30% of patients with systemic disease.27 However, screening for sarcoidosis by measuring serum levels is often falsely positive and is not cost-effective. In a study of 195 patients with idiopathic small fiber neuropathy,11 44% had an elevated serum level, but no evidence of sarcoidosis was seen on further testing, which included computed tomography of the chest in 29 patients.12 Thus, this test is best used for patients with evidence of systemic disease.
Amyloid testing for amyloidosis. Fat pad or bone marrow biopsy should be considered in the appropriate clinical setting.
Paraneoplastic autoantibody panel for occult cancer. Such testing may also be considered if clinically warranted. However, if a patient is found to have low positive titers of paraneoplastic antibodies and suspicion is low for an occult cancer (eg, no weight loss or early satiety), repeat confirmatory testing at another laboratory should be done before embarking on an extensive search for malignancy.
Ganglionic acetylcholine receptor antibody testing for autoimmune autonomic ganglionopathy. This should be ordered for patients with prominent autonomic dysfunction. The antibody test can be ordered separately or as part of an autoantibody panel. The antibody may indicate a primary immune-mediated process or a paraneoplastic disease.28
Genetic mutation testing. Recent discoveries of gene mutations leading to peripheral nerve hyperexcitability of voltage-gated sodium channels have elucidated a hereditary cause of small fiber neuropathy in nearly 30% of cases that were once thought to be idiopathic.29,30 Genetic testing for mutations in SCN9A and SCN10 (which code for the Nav1.7 and Nav1.8 sodium channels, respectively) is commercially available and may be considered for those with a family history of neuropathic pain in the feet or for young, otherwise healthy patients.
Fabry disease is an X-linked lysosomal disorder characterized by angiokeratomas, cardiac and renal impairment, and small fiber neuropathy. Treatment is now available, but screening is not cost-efficient and should only be pursued in patients with other symptoms of the disease.31,32
OTHER POSSIBLE CAUSES
Guillain-Barré syndrome
A Guillain-Barré syndrome variant has been reported that is characterized by ascending limb paresthesias and cerebrospinal fluid albuminocytologic dissociation in the setting of preserved deep tendon reflexes and normal findings on EMG.12 The clinical course is similar to that of typical Guillain-Barré syndrome, in that symptoms follow an upper respiratory or gastrointestinal tract infection, reach their nadir at 4 weeks, and then gradually improve. Some patients respond to intravenous immune globulin.
Vaccine-associated
Postvaccination small fiber neuropathy has also been reported. The nature of the association is unclear.33
Parkinson disease
Small fiber neuropathy is associated with Parkinson disease. It is attributed to a number of proposed factors, including neurodegeneration that occurs parallel to central nervous system decline, as well as intestinal malabsorption with resultant vitamin deficiency.34,35
Rapid glycemic lowering
Aggressive treatment of diabetes, defined as at least a 2-point reduction of serum hemoglobin A1c level over 3 months, may result in acute small fiber neuropathy. It manifests as severe distal extremity pain and dysautonomia.
In a retrospective study,36 104 (10.9%) of 954 patients presenting to a tertiary diabetic clinic developed treatment-induced diabetic neuropathy with symptoms occurring within 8 weeks of rapid glycemic control. The severity of neuropathy correlated with the degree and rate of glycemic lowering. The condition was reversible in some cases.
TREATING SPECIFIC DISORDERS
For patients with an identified cause of neuropathy, targeted treatment offers the best chance of halting progression and possibly improving symptoms. Below are recommendations for addressing neuropathy associated with the common diagnoses.
Diabetes, impaired glucose tolerance, and metabolic syndrome. In addition to glycemic- and lipid-lowering therapies, lifestyle modifications with a specific focus on exercise and nutrition are integral to treating diabetes and related disorders.
In the Look AHEAD (Action for Health in Diabetes) study,37 which evaluated the effects of intensive lifestyle intervention on neuropathy in 5,145 overweight patients with type 2 diabetes, patients in the intervention group had lower pain scores and better touch sensation in the toes compared with controls at 1 year. Differences correlated with the degree of weight loss and reduction of hemoglobin A1c and lipid levels.
As running and walking may not be feasible for many patients owing to pain, stationary cycling, aqua therapy, and swimming are other options. A stationary recumbent bike may be useful for older patients with balance issues.
Vitamin B12 deficiency. As reduced absorption rather than low dietary intake is the primary cause of vitamin B12 deficiency for many patients, parenteral rather than oral supplementation may be best. A suggested regimen is subcutaneous or intramuscular methylcobalamin injection of 1,000 µg given daily for 1 week, then once weekly for 1 month, followed by a maintenance dose once a month for at least 6 to 12 months. Alternatively, a daily dose of vitamin B12 1,000 µg can be taken sublingually.
Sjögren syndrome. According to anecdotal case reports, intravenous immune globulin, corticosteroids, and other immunosuppressants help painful small fiber neuropathy and dysautonomia associated with Sjögren syndrome.10
Sarcoidosis. Sarcoidosis-associated small fiber neuropathy may also respond to intravenous immune globulin, as well as infliximab and combination therapy.9 Culver et al38 found that cibinetide, an experimental erythropoetin agonist, resulted in improved corneal nerve fiber measures in patients with small fiber neuropathy associated with sarcoidosis.
Celiac disease. A gluten-free diet is the treatment for celiac disease and can help some patients.
GENERAL MANAGEMENT
For all patients, regardless of whether the cause of small fiber neuropathy has been identified, managing symptoms remains key, as pain and autonomic dysfunction can markedly impair quality of life. A multidisciplinary approach that incorporates pain medications, physical therapy, and lifestyle modifications is ideal. Integrative holistic treatments such as natural supplements, yoga, and other mind-body therapies may also help.
Pain control
Mexiletine, a voltage-gated sodium channel blocker used as an antiarrhythmic, may help refractory pain or hereditary small fiber neuropathy related to sodium channel dysfunction. However, it is not recommended for diabetic neuropathy.39
Combination regimens that use drugs with different mechanisms of action can be effective. In one study, combined gabapentin and nortriptyline were more effective than either drug alone for neuropathic pain.40
Inhaled cannabis reduced pain in patients with HIV and diabetic neuropathy in a number of studies. Side effects included euphoria, somnolence, and cognitive impairment.41,42 The use of medical marijuana is not yet legal nationwide and may affect employability even in states in which it has been legalized.
Owing to the opioid epidemic and high addiction potential, opioids are no longer a preferred recommendation for chronic treatment of noncancer-related neuropathy. A population-based study of 2,892 patients with neuropathy found that those on chronic opioid therapy (≥ 90 days) had worse functional outcomes and higher rates of addiction and overdose than those on short-term therapy.43 However, the opioid agonist tramadol was found to be effective in reducing neuropathic pain and may be a safer option for patients with chronic small fiber neuropathy.44
Integrative, holistic therapies
PROGNOSIS
For many patients, small fiber neuropathy is a slowly progressive disorder that reaches a clinical plateau lasting for years, with progression to large fiber involvement reported in 13% to 36% of cases; over half of patients in one series either improved or remained stable over a period of 2 years.5,57 Long-term studies are needed to fully understand the natural disease course. In the meantime, treating underlying disease and managing symptoms are imperative to patient care.
- Burke JF, Skolarus LE, Callaghan BC, Kerber KA. Choosing Wisely: highest-cost tests in outpatient neurology. Ann Neurol 2013; 73(5):679–683. doi:10.1002/ana.23865
- Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care 2003; 26(6):1790–1795. pmid:12766111
- Peters MJ, Bakkers M, Merkies IS, Hoeijmakers JG, van Raak EP, Faber CG. Incidence and prevalence of small-fiber neuropathy: a survey in the Netherlands. Neurology 2013; 81(15):1356–1360. doi:10.1212/WNL.0b013e3182a8236e
- Periquet MI, Novak V, Collins MP, et al. Painful sensory neuropathy: prospective evaluation using skin biopsy. Neurology 1999; 53(8):1641–1647. pmid:10563606
- Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008; 131(pt 7):1912–1925. doi:10.1093/brain/awn093
- Lacomis D. Small-fiber neuropathy. Muscle Nerve 2002; 26(2):173–188. doi:10.1002/mus.10181
- Lopate G, Streif E, Harms M, Weihl C, Pestronk A. Cramps and small-fiber neuropathy. Muscle Nerve 2013; 48(2):252–255. doi:10.1002/mus.23757
- Khan S, Zhou L. Characterization of non-length-dependent small-fiber sensory neuropathy. Muscle Nerve 2012; 45(1):86–91. doi:10.1002/mus.22255
- Tavee JO, Karwa K, Ahmed Z, Thompson N, Parambil J, Culver DA. Sarcoidosis-associated small fiber neuropathy in a large cohort: clinical aspects and response to IVIG and anti-TNF alpha treatment. Respir Med 2017; 126:135–138. doi:10.1016/j.rmed.2017.03.011
- Berkowitz AL, Samuels MA. The neurology of Sjogren’s syndrome and the rheumatology of peripheral neuropathy and myelitis. Pract Neurol 2014; 14(1):14–22. doi:10.1136/practneurol-2013-000651
- Lang M, Treister R, Oaklander AL. Diagnostic value of blood tests for occult causes of initially idiopathic small-fiber polyneuropathy. J Neurol 2016; 263(12):2515–2527. doi:10.1007/s00415-016-8270-5
- Seneviratne U, Gunasekera S. Acute small fibre sensory neuropathy: another variant of Guillain-Barré syndrome? J Neurol Neurosurg Psychiatry 2002; 72(4):540–542. pmid:11909922
- Tavee JO, Polston D, Zhou L, Shields RW, Butler RS, Levin KH. Sural sensory nerve action potential, epidermal nerve fiber density, and quantitative sudomotor axon reflex in the healthy elderly. Muscle Nerve 2014; 49(4):564–569. doi:10.1002/mus.23971
- Tavee J, Zhou L. Small fiber neuropathy: a burning problem. Cleve Clin J Med 2009; 76(5):297–305. doi:10.3949/ccjm.76a.08070
- Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology 1999; 53(8):1634–1640. pmid:10563605
- Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 2013; 154(11):2310–2316. doi:10.1016/j.pain.2013.06.001
- Üçeyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013; 136(pt 6):1857–1867. doi:10.1093/brain/awt053
- Stewart JD, Low PA, Fealey RD. Distal small fiber neuropathy: results of tests of sweating and autonomic cardiovascular reflexes. Muscle Nerve 1992; 15(6):661–665. doi:10.1002/mus.880150605
- Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia 2003; 46(5):683–688. doi:10.1007/s00125-003-1086-8
- de Greef BTA, Hoeijmakers JGJ, Gorissen-Brouwers CML, Geerts M, Faber CG, Merkies ISJ. Associated conditions in small fiber neuropathy—a large cohort study and review of the literature. Eur J Neurol 2018; 25(2):348–355. doi:10.1111/ene.13508
- Smith AG. Impaired glucose tolerance and metabolic syndrome in idiopathic neuropathy. J Peripher Nerv Syst 2012; 17(suppl 2):15–21. doi:10.1111/j.1529-8027.2012.00390.x
- Hoffman-Snyder C, Smith BE, Ross MA, Hernandez J, Bosch EP. Value of the oral glucose tolerance test in the evaluation of chronic idiopathic axonal polyneuropathy. Arch Neurol 2006; 63(8):1075–1079. doi:10.1001/archneur.63.8.noc50336
- Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst 2009; 14(4):257–267. doi:10.1111/j.1529-8027.2009.00237.x
- Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes 2009; 58(7):1634–1640. doi:10.2337/db08-1771
- Chin RL, Sander HW, Brannagan TH, et al. Celiac neuropathy. Neurology 2003; 60(10):1581–1585. pmid:12771245
- Gemignani F, Brindani F, Alfieri S, et al. Clinical spectrum of cryoglobulinaemic neuropathy. J Neurol Neurosurg Psychiatry 2005; 76(10):1410–1414. doi:10.1136/jnnp.2004.057620
- Bakkers M, Merkies IS, Lauria G, et al. Intraepidermal nerve fiber density and its application in sarcoidosis. Neurology 2009; 73(14):1142–1148. doi:10.1212/WNL.0b013e3181bacf05
- Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med 2000; 343(12):847–855. doi:10.1056/NEJM200009213431204
- Faber CG, Hoeijmakers JG, Ahn HS, et al. Gain of function Nav1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol 2012; 71(1):26–39. doi:10.1002/ana.22485
- Brouwer BA, Merkies IS, Gerrits MM, Waxman SG, Hoeijmakers JG, Faber CG. Painful neuropathies: the emerging role of sodium channelopathies. J Peripher Nerv Syst 2014; 19(2):53–65. doi:10.1111/jns5.12071
- Samuelsson K, Kostulas K, Vrethem M, Rolfs A, Press R. Idiopathic small fiber neuropathy: phenotype, etiologies, and the search for Fabry disease. J Clin Neurol 2014; 10(2):108–118. doi:10.3988/jcn.2014.10.2.108
- de Greef BT, Hoeijmakers JG, Wolters EE, et al. No Fabry disease in patients presenting with isolated small fiber neuropathy. PLoS One 2016; 11(2):e0148316. doi:10.1371/journal.pone.0148316
- Souayah N, Ajroud-Driss S, Sander HW, Brannagan TH, Hays AP, Chin RL. Small fiber neuropathy following vaccination for rabies, varicella or Lyme disease. Vaccine 2009; 27(52):7322–7325. doi:10.1016/j.vaccine.2009.09.077
- Nolano M, Provitera V, Manganelli F, et al. Loss of cutaneous large and small fibers in naive and l-dopa–treated PD patients. Neurology 2017; 89(8):776–784. doi:10.1212/WNL.0000000000004274
- Zis P, Grünewald RA, Chaudhuri RK, Hadjivassiliou M. Peripheral neuropathy in idiopathic Parkinson’s disease: a systematic review. J Neurol Sci 2017; 378:204–209. doi:10.1016/j.jns.2017.05.023
- Gibbons CH, Freeman R. Treatment-induced neuropathy of diabetes: an acute, iatrogenic complication of diabetes. Brain 2015; 138(pt 1):43–52. doi:10.1093/brain/awu307
- Look AHEAD Research Group. Effects of a long-term lifestyle modification programme on peripheral neuropathy in overweight or obese adults with type 2 diabetes: the Look AHEAD study. Diabetologia 2017; 60(6):980–988. doi:10.1007/s00125-017-4253-z
- Culver DA, Dahan A, Bajorunas D, et al. Cibinetide improves corneal nerve fiber abundance in patients with sarcoidosis-associated small nerve fiber loss and neuropathic pain. Invest Ophthalmol Vis Sci 2017; 58(6):BIO52–BIO60. doi:10.1167/iovs.16-21291
- Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R 2011; 3(4):345–352.e21. doi:10.1016/j.pmrj.2011.03.008
- Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet 2009; 374(9697):1252–1261. doi:10.1016/S0140-6736(09)61081-3
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009; 34(3):672–680. doi:10.1038/npp.2008.120
- Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015; 16(7):616–627. doi:10.1016/j.jpain.2015.03.008
- Hoffman EM, Watson JC, St Sauver J, Staff NP, Klein CJ. Association of long-term opioid therapy with functional status, adverse outcomes, and mortality among patients with polyneuropathy. JAMA Neurol 2017; 74(7):773–779. doi:10.1001/jamaneurol.2017.0486
- Harati Y, Gooch C, Swenson M, et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology 1998; 50(6):1842–1846. pmid:9633738
- Sima AA, Calvani M, Mehra M, Amato A; Acetyl-L-Carnitine Study Group. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care 2005; 28(1):89–94. pmid:15616239
- Ziegler D, Hanefeld M, Ruhnau KJ, et al. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multicentre randomized controlled trial (ALADIN Study). Diabetologia 1995; 38(12):1425–1433. pmid:8786016
- Scarpini E, Sacilotto G, Baron P, Cusini M, Scarlato G. Effect of acetyl-L-carnitine in the treatment of painful peripheral neuropathies in HIV+ patients. J Peripher Nerv Syst 1997; 2(3):250-252. pmid: 10975731
- Hershman DL, Unger JM, Crew KD, et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J Clin Oncol 2013; 31(20):2627-2633. doi:10.1200/JCO.2012.44.8738
- Amara S. Oral glutamine for the prevention of chemotherapy-induced peripheral neuropathy. Ann Pharmacother 2008; 42(10):1481-1485. doi:10.1345/aph.1L179
- Huang JS, Wu CL, Fan CW, Chen WH, Yeh KY, Chang PH. Intravenous glutamine appears to reduce the severity of symptomatic platinum-induced neuropathy: a prospective randomized study. J Chemother 2015; 27(4):235-240. doi:10.1179/1973947815Y.0000000011
- Banafshe HR, Hamidi GA, Noureddini M, Mirhashemi SM, Mokhtari R, Shoferpour M. Effect of curcumin on diabetic peripheral neuropathic pain: possible involvement of opioid system. Eur J Pharmacol 2014; 723:202-206. doi:10.1016/j.ejphar.2013.11.033
- Mendonça LM, da Silva Machado C, Teixeira CC, de Freitas LA, Bianchi MD, Antunes LM. Curcumin reduces cisplatin-induced neurotoxicity in NGF-differentiated PC12 cells. Neurotoxicology 2013; 34:205-211. doi:10.1016/j.neuro.2012.09.011
- Wagner K, Lee KS, Yang J, Hammock BD. Epoxy fatty acids mediate analgesia in murine diabetic neuropathy. Eur J Pain 2017; 21(3):456-465. doi:10.1002/ejp.939
- Lewis EJ, Perkins BA, Lovblom LE, Bazinet RP, Wolever TMS, Bril V. Effect of omega-3 supplementation on neuropathy in type 1 diabetes: a 12-month pilot trial. Neurology 2017; 88(24):2294–2301. doi:10.1212/WNL.0000000000004033
- Hu D, Wang C, Li F, et al. A combined water extract of frankincense and myrrh alleviates neuropathic pain in mice via modulation of TRPV1. Neural Plast 2017; 2017:3710821. doi:10.1155/2017/3710821
- Tavee J, Rensel M, Planchon SM, Butler RS, Stone L. Effects of meditation on pain and quality of life in multiple sclerosis and peripheral neuropathy: a pilot study. Int J MS Care 2011; 13(4):163–168. doi:10.7224/1537-2073-13.4.163
- Khoshnoodi MA, Truelove S, Burakgazi A, Hoke A, Mammen AL, Polydefkis M. Longitudinal assessment of small fiber neuropathy: evidence of a non-length-dependent distal axonopathy. JAMA Neurol 2016; 73(6):684–690. doi:10.1001/jamaneurol.2016.0057
Peripheral neuropathy is the most common reason for an outpatient neurology visit in the United States and accounts for over $10 billion in healthcare spending each year.1,2 When the disorder affects only small, thinly myelinated or unmyelinated nerve fibers, it is referred to as small fiber neuropathy, which commonly presents as numbness and burning pain in the feet.
This article details the manifestations and evaluation of small fiber neuropathy, with an eye toward diagnosing an underlying cause amenable to treatment.
OLDER PATIENTS MOST AFFECTED
The epidemiology of small fiber neuropathy is not well established. It occurs more commonly in older patients, but data are mixed on prevalence by sex.3–6 In a Dutch study,3 the overall prevalence was at least 53 cases per 100,000, with the highest rate in men over age 65.
CHARACTERISTIC SENSORY DISTURBANCES
Sensations vary in quality and time
Patients with small fiber neuropathy typically present with a symmetric length-dependent (“stocking-glove”) distribution of sensory changes, starting in the feet and gradually ascending up the legs and then to the hands.
Commonly reported neuropathic symptoms include various combinations of burning, numbness, tingling, itching, sunburn-like, and frostbite-like sensations. Nonneuropathic symptoms may include tightness, a vise-like squeezing of the feet, and the sensation of a sock rolled up at the end of the shoe. Cramps or spasms may also be reported but rarely occur in isolation.7
Symptoms are typically worse at the end of the day and while sitting or lying down at night. They can arise spontaneously but may also be triggered by something as minor as the touch of clothing or cool air against the skin. Bedsheet sensitivity of the feet is reported so often that it is used as an outcome measure in clinical trials. Symptoms can also be exacerbated by extremes in ambient temperature and are especially worse in cold weather.
Random patterns suggest an immune cause
Symptoms may also have a non–length-dependent distribution that is asymmetric, patchy, intermittent, and migratory, and can involve the face, proximal limbs, and trunk. Symptoms may vary throughout the day, eg, starting with electric-shock sensations on one side of the face, followed by perineal numbness and then tingling in the arms lasting for a few minutes to several hours. While such patterns may be seen with diabetes and other common etiologies, they often suggest an underlying immune-mediated disorder such as Sjögren syndrome or sarcoidosis.8–10 Although large fiber polyneuropathy may also be non–length-dependent, the deficits are usually fixed, with no migratory component.
Autonomic features may be prominent
Autonomic symptoms occur in nearly half of patients and can be as troublesome as neuropathic pain.3 Small nerve fibers mediate somatic and autonomic functions, an evolutionary link that may reflect visceral defense mechanisms responding to pain as a signal of danger.11 This may help explain the multisystemic nature of symptoms, which can include sweating abnormalities, bowel and bladder disturbances, dry eyes, dry mouth, gastrointestinal dysmotility, skin changes (eg, discoloration, loss of hair, shiny skin), sexual dysfunction, orthostatic hypotension, and palpitations. In some cases, isolated dysautonomia may be seen.
TARGETED EXAMINATION
History: Medications, alcohol, infections
When a patient presents with neuropathic pain in the feet, a detailed history should be obtained, including alcohol use, family history of neuropathy, and use of neurotoxic medications such as metronidazole, colchicine, and chemotherapeutic agents.
Human immunodeficiency virus (HIV) and hepatitis C infection are well known to be associated with small fiber neuropathy, so relevant risk factors (eg, blood transfusions, sexual history, intravenous drug use) should be asked about. Recent illnesses and vaccinations are another important line of questioning, as a small-fiber variant of Guillain-Barré syndrome has been described.12
Assess reflexes, strength, sensation
On physical examination, particular attention should be focused on searching for abnormalities indicating large nerve fiber involvement (eg, absent deep tendon reflexes, weakness of the toes). However, absent ankle deep tendon reflexes and reduced vibratory sense may also occur in healthy elderly people.
Similarly, proprioception, motor strength, balance, and vibratory sensation are functions of large myelinated nerve fibers, and thus remain unaffected in patients with only small fiber neuropathy.
Evidence of a systemic disorder should also be sought, as it may indicate an underlying etiology.
DIAGNOSTIC TESTING
Although patients with either large or small fiber neuropathy may have subjective hyperesthesia or numbness of the distal lower extremities, the absence of significant abnormalities on neurologic examination should prompt consideration of small fiber neuropathy.
Electromyography worthwhile
Nerve conduction studies and needle electrode examination evaluate only large nerve fiber conditions. While electromyographic results are normal in patients with isolated small fiber neuropathy, the test can help evaluate subclinical large nerve fiber involvement and alternative diagnoses such as bilateral S1 radiculopathy. Nerve conduction studies may be less useful in patients over age 75, as they may lack sural sensory responses because of aging changes.13
Skin biopsy easy to do
Skin biopsy for evaluating intraepidermal nerve fiber density is one of the most widely used tests for small fiber neuropathy. This minimally invasive procedure can now be performed in a primary care office using readily available tools or prepackaged kits and analyzed by several commercial laboratories.
Reduced intraepidermal nerve fiber density on skin biopsy has been described in various other conditions such as fibromyalgia and chronic pain syndromes.16,17 The clinical significance of these findings remains uncertain.
Quantitative sudomotor axon reflex testing
Quantitative sudomotor axon reflex testing (QSART) is a noninvasive autonomic study that assesses the volume of sweat produced by the limbs in response to acetylcholine. A measure of postganglionic sympathetic sudomotor nerve function, QSART has a sensitivity of up to 80% and can be used to diagnose small fiber neuropathy.18 In a series of 115 patients with sarcoidosis small fiber neuropathy,9 the QSART and skin biopsy findings were concordant in 17 cases and complementary in 29, allowing for confirmation of small fiber neuropathy in patients whose condition would have remained undiagnosed had only one test been performed. QSART can also be considered in cases where skin biopsy may be contraindicated (eg, patient use of anticoagulation). Of note, the study may be affected by a number of external factors, including caffeine, tobacco, antihistamines, and tricyclic antidepressants; these should be held before testing.
Other diagnostic studies
Other tests may be helpful, as follows:
Tilt-table and cardiovagal testing may be useful for patients with orthostasis and palpitations.
Thermoregulatory sweat testing can be used to evaluate patients with abnormal patterns of sweating, eg, hyperhidrosis of the face and head.
INITIAL TESTING FOR AN UNDERLYING CAUSE
Glucose tolerance test for diabetes
Diabetes is the most common identifiable cause of small fiber neuropathy and accounts for about a third of all cases.5 Impaired glucose tolerance is also thought to be a risk factor and has been found in up to 50% of idiopathic cases, but the association is still being debated.21
While testing for hemoglobin A1c is more convenient for the patient, especially because it does not require fasting, a 2-hour oral glucose tolerance test is more sensitive for detecting glucose dysmetabolism.22
Lipid panel for metabolic syndrome
Small fiber neuropathy is associated with individual components of the metabolic syndrome, which include obesity, hyperglycemia, and dyslipidemia. Of these, dyslipidemia has emerged as the primary factor involved in the development of small fiber neuropathy, via an inflammatory pathway or oxidative stress mechanism.23,24
Vitamin B12 deficiency testing
Vitamin B12 deficiency, a potentially correctable cause of small fiber neuropathy, may be underdiagnosed, especially as values obtained by blood testing may not reflect tissue uptake. Causes of vitamin B12 deficiency include reduced intake, pernicious anemia, and medications that can affect absorption of vitamin B12 (eg, proton pump inhibitors, histamine 2 receptor antagonists, metformin).
Testing should include:
- Complete blood cell count to evaluate for vitamin B12-related macrocytic anemia and other hematologic abnormalities
- Serum vitamin B12 level
- Methylmalonic acid or homocysteine level in patients with subclinical or mild vitamin B12 deficiency, manifested as low to normal vitamin B12 levels (< 400 pg/mL); methylmalonic acid and homocysteine require vitamin B12 as a cofactor for enzymatic conversion, and either or both may be elevated in early vitamin B12 deficiency.
Celiac antibody panel
Celiac disease, a T-cell mediated enteropathy characterized by gluten intolerance and a herpetiform-like rash, can be associated with small fiber neuropathy.25 In some cases, neuropathy symptoms are preceded by the onset of gastrointestinal symptoms, or they may occur in isolation.25
Inflammatory disease testing
Sjögren syndrome accounts for nearly 10% of cases of small fiber neuropathy. Associated neuropathic symptoms are often non–length-dependent, can precede sicca symptoms for up to 6 years, and in some cases are the sole manifestation of the disease.10 Small fiber neuropathy may also be associated with vasculitis, systemic lupus erythematosus, and other connective tissue disorders.
Testing should include:
- Erythrocyte sedimentation rate, C-reactive protein, and antinuclear antibodies: though these are nonspecific markers of inflammation, they may support an immune-mediated etiology if positive
- Extractable nuclear antigen panel: Sjögren syndrome A and B autoantibodies are the most important components in this setting5,11
- The Schirmer test or salivary gland biopsy should be considered for seronegative patients with sicca or a suspected immune-mediated etiology, as the sensitivity of antibody testing ranges from only 10% to 55%.10
Thyroid function testing
Hypothyroidism, and less commonly hyperthyroidism, are associated with small fiber neuropathy.
Metabolic tests for liver and kidney disease
Renal insufficiency and liver impairment are well-known causes of small nerve fiber dysfunction. Testing should include:
- Comprehensive metabolic panel
- Gamma-glutamyltransferase if alcohol abuse is suspected, since heavy alcohol use is one of the most common causes of both large and small fiber neuropathy.
HIV and hepatitis C testing
For patients with relevant risk factors, HIV and hepatitis C testing should be part of the initial workup (and as second-tier testing for others). Patients who test positive for hepatitis C should undergo further testing for cryoglobulinemia, which can present with painful small fiber neuropathy.26
Serum and urine immunoelectrophoresis
Paraproteinemia, with causes ranging from monoclonal gammopathy of uncertain significance to multiple myeloma, has been associated with small fiber neuropathy. An abnormal serum or urine immunoelectrophoresis test warrants further investigation and possibly referral to a hematology-oncology specialist.
SECOND-TIER TESTING
Less common treatable causes of small fiber neuropathy may also be evaluated.
Copper, vitamin B1 (thiamine), or vitamin B6 (pyridoxine) deficiency testing. Although vitamin B6 toxicity may also result in neuropathy due to its toxic effect on the dorsal root ganglia, the mildly elevated vitamin B6 levels often found in patients being evaluated for neuropathy are unlikely to be the primary cause of symptoms. Many laboratories require fasting samples for accurate vitamin B6 levels.
Angiotensin-converting enzyme levels for sarcoidosis. Small fiber neuropathy is common in sarcoidosis, occurring in more than 30% of patients with systemic disease.27 However, screening for sarcoidosis by measuring serum levels is often falsely positive and is not cost-effective. In a study of 195 patients with idiopathic small fiber neuropathy,11 44% had an elevated serum level, but no evidence of sarcoidosis was seen on further testing, which included computed tomography of the chest in 29 patients.12 Thus, this test is best used for patients with evidence of systemic disease.
Amyloid testing for amyloidosis. Fat pad or bone marrow biopsy should be considered in the appropriate clinical setting.
Paraneoplastic autoantibody panel for occult cancer. Such testing may also be considered if clinically warranted. However, if a patient is found to have low positive titers of paraneoplastic antibodies and suspicion is low for an occult cancer (eg, no weight loss or early satiety), repeat confirmatory testing at another laboratory should be done before embarking on an extensive search for malignancy.
Ganglionic acetylcholine receptor antibody testing for autoimmune autonomic ganglionopathy. This should be ordered for patients with prominent autonomic dysfunction. The antibody test can be ordered separately or as part of an autoantibody panel. The antibody may indicate a primary immune-mediated process or a paraneoplastic disease.28
Genetic mutation testing. Recent discoveries of gene mutations leading to peripheral nerve hyperexcitability of voltage-gated sodium channels have elucidated a hereditary cause of small fiber neuropathy in nearly 30% of cases that were once thought to be idiopathic.29,30 Genetic testing for mutations in SCN9A and SCN10 (which code for the Nav1.7 and Nav1.8 sodium channels, respectively) is commercially available and may be considered for those with a family history of neuropathic pain in the feet or for young, otherwise healthy patients.
Fabry disease is an X-linked lysosomal disorder characterized by angiokeratomas, cardiac and renal impairment, and small fiber neuropathy. Treatment is now available, but screening is not cost-efficient and should only be pursued in patients with other symptoms of the disease.31,32
OTHER POSSIBLE CAUSES
Guillain-Barré syndrome
A Guillain-Barré syndrome variant has been reported that is characterized by ascending limb paresthesias and cerebrospinal fluid albuminocytologic dissociation in the setting of preserved deep tendon reflexes and normal findings on EMG.12 The clinical course is similar to that of typical Guillain-Barré syndrome, in that symptoms follow an upper respiratory or gastrointestinal tract infection, reach their nadir at 4 weeks, and then gradually improve. Some patients respond to intravenous immune globulin.
Vaccine-associated
Postvaccination small fiber neuropathy has also been reported. The nature of the association is unclear.33
Parkinson disease
Small fiber neuropathy is associated with Parkinson disease. It is attributed to a number of proposed factors, including neurodegeneration that occurs parallel to central nervous system decline, as well as intestinal malabsorption with resultant vitamin deficiency.34,35
Rapid glycemic lowering
Aggressive treatment of diabetes, defined as at least a 2-point reduction of serum hemoglobin A1c level over 3 months, may result in acute small fiber neuropathy. It manifests as severe distal extremity pain and dysautonomia.
In a retrospective study,36 104 (10.9%) of 954 patients presenting to a tertiary diabetic clinic developed treatment-induced diabetic neuropathy with symptoms occurring within 8 weeks of rapid glycemic control. The severity of neuropathy correlated with the degree and rate of glycemic lowering. The condition was reversible in some cases.
TREATING SPECIFIC DISORDERS
For patients with an identified cause of neuropathy, targeted treatment offers the best chance of halting progression and possibly improving symptoms. Below are recommendations for addressing neuropathy associated with the common diagnoses.
Diabetes, impaired glucose tolerance, and metabolic syndrome. In addition to glycemic- and lipid-lowering therapies, lifestyle modifications with a specific focus on exercise and nutrition are integral to treating diabetes and related disorders.
In the Look AHEAD (Action for Health in Diabetes) study,37 which evaluated the effects of intensive lifestyle intervention on neuropathy in 5,145 overweight patients with type 2 diabetes, patients in the intervention group had lower pain scores and better touch sensation in the toes compared with controls at 1 year. Differences correlated with the degree of weight loss and reduction of hemoglobin A1c and lipid levels.
As running and walking may not be feasible for many patients owing to pain, stationary cycling, aqua therapy, and swimming are other options. A stationary recumbent bike may be useful for older patients with balance issues.
Vitamin B12 deficiency. As reduced absorption rather than low dietary intake is the primary cause of vitamin B12 deficiency for many patients, parenteral rather than oral supplementation may be best. A suggested regimen is subcutaneous or intramuscular methylcobalamin injection of 1,000 µg given daily for 1 week, then once weekly for 1 month, followed by a maintenance dose once a month for at least 6 to 12 months. Alternatively, a daily dose of vitamin B12 1,000 µg can be taken sublingually.
Sjögren syndrome. According to anecdotal case reports, intravenous immune globulin, corticosteroids, and other immunosuppressants help painful small fiber neuropathy and dysautonomia associated with Sjögren syndrome.10
Sarcoidosis. Sarcoidosis-associated small fiber neuropathy may also respond to intravenous immune globulin, as well as infliximab and combination therapy.9 Culver et al38 found that cibinetide, an experimental erythropoetin agonist, resulted in improved corneal nerve fiber measures in patients with small fiber neuropathy associated with sarcoidosis.
Celiac disease. A gluten-free diet is the treatment for celiac disease and can help some patients.
GENERAL MANAGEMENT
For all patients, regardless of whether the cause of small fiber neuropathy has been identified, managing symptoms remains key, as pain and autonomic dysfunction can markedly impair quality of life. A multidisciplinary approach that incorporates pain medications, physical therapy, and lifestyle modifications is ideal. Integrative holistic treatments such as natural supplements, yoga, and other mind-body therapies may also help.
Pain control
Mexiletine, a voltage-gated sodium channel blocker used as an antiarrhythmic, may help refractory pain or hereditary small fiber neuropathy related to sodium channel dysfunction. However, it is not recommended for diabetic neuropathy.39
Combination regimens that use drugs with different mechanisms of action can be effective. In one study, combined gabapentin and nortriptyline were more effective than either drug alone for neuropathic pain.40
Inhaled cannabis reduced pain in patients with HIV and diabetic neuropathy in a number of studies. Side effects included euphoria, somnolence, and cognitive impairment.41,42 The use of medical marijuana is not yet legal nationwide and may affect employability even in states in which it has been legalized.
Owing to the opioid epidemic and high addiction potential, opioids are no longer a preferred recommendation for chronic treatment of noncancer-related neuropathy. A population-based study of 2,892 patients with neuropathy found that those on chronic opioid therapy (≥ 90 days) had worse functional outcomes and higher rates of addiction and overdose than those on short-term therapy.43 However, the opioid agonist tramadol was found to be effective in reducing neuropathic pain and may be a safer option for patients with chronic small fiber neuropathy.44
Integrative, holistic therapies
PROGNOSIS
For many patients, small fiber neuropathy is a slowly progressive disorder that reaches a clinical plateau lasting for years, with progression to large fiber involvement reported in 13% to 36% of cases; over half of patients in one series either improved or remained stable over a period of 2 years.5,57 Long-term studies are needed to fully understand the natural disease course. In the meantime, treating underlying disease and managing symptoms are imperative to patient care.
Peripheral neuropathy is the most common reason for an outpatient neurology visit in the United States and accounts for over $10 billion in healthcare spending each year.1,2 When the disorder affects only small, thinly myelinated or unmyelinated nerve fibers, it is referred to as small fiber neuropathy, which commonly presents as numbness and burning pain in the feet.
This article details the manifestations and evaluation of small fiber neuropathy, with an eye toward diagnosing an underlying cause amenable to treatment.
OLDER PATIENTS MOST AFFECTED
The epidemiology of small fiber neuropathy is not well established. It occurs more commonly in older patients, but data are mixed on prevalence by sex.3–6 In a Dutch study,3 the overall prevalence was at least 53 cases per 100,000, with the highest rate in men over age 65.
CHARACTERISTIC SENSORY DISTURBANCES
Sensations vary in quality and time
Patients with small fiber neuropathy typically present with a symmetric length-dependent (“stocking-glove”) distribution of sensory changes, starting in the feet and gradually ascending up the legs and then to the hands.
Commonly reported neuropathic symptoms include various combinations of burning, numbness, tingling, itching, sunburn-like, and frostbite-like sensations. Nonneuropathic symptoms may include tightness, a vise-like squeezing of the feet, and the sensation of a sock rolled up at the end of the shoe. Cramps or spasms may also be reported but rarely occur in isolation.7
Symptoms are typically worse at the end of the day and while sitting or lying down at night. They can arise spontaneously but may also be triggered by something as minor as the touch of clothing or cool air against the skin. Bedsheet sensitivity of the feet is reported so often that it is used as an outcome measure in clinical trials. Symptoms can also be exacerbated by extremes in ambient temperature and are especially worse in cold weather.
Random patterns suggest an immune cause
Symptoms may also have a non–length-dependent distribution that is asymmetric, patchy, intermittent, and migratory, and can involve the face, proximal limbs, and trunk. Symptoms may vary throughout the day, eg, starting with electric-shock sensations on one side of the face, followed by perineal numbness and then tingling in the arms lasting for a few minutes to several hours. While such patterns may be seen with diabetes and other common etiologies, they often suggest an underlying immune-mediated disorder such as Sjögren syndrome or sarcoidosis.8–10 Although large fiber polyneuropathy may also be non–length-dependent, the deficits are usually fixed, with no migratory component.
Autonomic features may be prominent
Autonomic symptoms occur in nearly half of patients and can be as troublesome as neuropathic pain.3 Small nerve fibers mediate somatic and autonomic functions, an evolutionary link that may reflect visceral defense mechanisms responding to pain as a signal of danger.11 This may help explain the multisystemic nature of symptoms, which can include sweating abnormalities, bowel and bladder disturbances, dry eyes, dry mouth, gastrointestinal dysmotility, skin changes (eg, discoloration, loss of hair, shiny skin), sexual dysfunction, orthostatic hypotension, and palpitations. In some cases, isolated dysautonomia may be seen.
TARGETED EXAMINATION
History: Medications, alcohol, infections
When a patient presents with neuropathic pain in the feet, a detailed history should be obtained, including alcohol use, family history of neuropathy, and use of neurotoxic medications such as metronidazole, colchicine, and chemotherapeutic agents.
Human immunodeficiency virus (HIV) and hepatitis C infection are well known to be associated with small fiber neuropathy, so relevant risk factors (eg, blood transfusions, sexual history, intravenous drug use) should be asked about. Recent illnesses and vaccinations are another important line of questioning, as a small-fiber variant of Guillain-Barré syndrome has been described.12
Assess reflexes, strength, sensation
On physical examination, particular attention should be focused on searching for abnormalities indicating large nerve fiber involvement (eg, absent deep tendon reflexes, weakness of the toes). However, absent ankle deep tendon reflexes and reduced vibratory sense may also occur in healthy elderly people.
Similarly, proprioception, motor strength, balance, and vibratory sensation are functions of large myelinated nerve fibers, and thus remain unaffected in patients with only small fiber neuropathy.
Evidence of a systemic disorder should also be sought, as it may indicate an underlying etiology.
DIAGNOSTIC TESTING
Although patients with either large or small fiber neuropathy may have subjective hyperesthesia or numbness of the distal lower extremities, the absence of significant abnormalities on neurologic examination should prompt consideration of small fiber neuropathy.
Electromyography worthwhile
Nerve conduction studies and needle electrode examination evaluate only large nerve fiber conditions. While electromyographic results are normal in patients with isolated small fiber neuropathy, the test can help evaluate subclinical large nerve fiber involvement and alternative diagnoses such as bilateral S1 radiculopathy. Nerve conduction studies may be less useful in patients over age 75, as they may lack sural sensory responses because of aging changes.13
Skin biopsy easy to do
Skin biopsy for evaluating intraepidermal nerve fiber density is one of the most widely used tests for small fiber neuropathy. This minimally invasive procedure can now be performed in a primary care office using readily available tools or prepackaged kits and analyzed by several commercial laboratories.
Reduced intraepidermal nerve fiber density on skin biopsy has been described in various other conditions such as fibromyalgia and chronic pain syndromes.16,17 The clinical significance of these findings remains uncertain.
Quantitative sudomotor axon reflex testing
Quantitative sudomotor axon reflex testing (QSART) is a noninvasive autonomic study that assesses the volume of sweat produced by the limbs in response to acetylcholine. A measure of postganglionic sympathetic sudomotor nerve function, QSART has a sensitivity of up to 80% and can be used to diagnose small fiber neuropathy.18 In a series of 115 patients with sarcoidosis small fiber neuropathy,9 the QSART and skin biopsy findings were concordant in 17 cases and complementary in 29, allowing for confirmation of small fiber neuropathy in patients whose condition would have remained undiagnosed had only one test been performed. QSART can also be considered in cases where skin biopsy may be contraindicated (eg, patient use of anticoagulation). Of note, the study may be affected by a number of external factors, including caffeine, tobacco, antihistamines, and tricyclic antidepressants; these should be held before testing.
Other diagnostic studies
Other tests may be helpful, as follows:
Tilt-table and cardiovagal testing may be useful for patients with orthostasis and palpitations.
Thermoregulatory sweat testing can be used to evaluate patients with abnormal patterns of sweating, eg, hyperhidrosis of the face and head.
INITIAL TESTING FOR AN UNDERLYING CAUSE
Glucose tolerance test for diabetes
Diabetes is the most common identifiable cause of small fiber neuropathy and accounts for about a third of all cases.5 Impaired glucose tolerance is also thought to be a risk factor and has been found in up to 50% of idiopathic cases, but the association is still being debated.21
While testing for hemoglobin A1c is more convenient for the patient, especially because it does not require fasting, a 2-hour oral glucose tolerance test is more sensitive for detecting glucose dysmetabolism.22
Lipid panel for metabolic syndrome
Small fiber neuropathy is associated with individual components of the metabolic syndrome, which include obesity, hyperglycemia, and dyslipidemia. Of these, dyslipidemia has emerged as the primary factor involved in the development of small fiber neuropathy, via an inflammatory pathway or oxidative stress mechanism.23,24
Vitamin B12 deficiency testing
Vitamin B12 deficiency, a potentially correctable cause of small fiber neuropathy, may be underdiagnosed, especially as values obtained by blood testing may not reflect tissue uptake. Causes of vitamin B12 deficiency include reduced intake, pernicious anemia, and medications that can affect absorption of vitamin B12 (eg, proton pump inhibitors, histamine 2 receptor antagonists, metformin).
Testing should include:
- Complete blood cell count to evaluate for vitamin B12-related macrocytic anemia and other hematologic abnormalities
- Serum vitamin B12 level
- Methylmalonic acid or homocysteine level in patients with subclinical or mild vitamin B12 deficiency, manifested as low to normal vitamin B12 levels (< 400 pg/mL); methylmalonic acid and homocysteine require vitamin B12 as a cofactor for enzymatic conversion, and either or both may be elevated in early vitamin B12 deficiency.
Celiac antibody panel
Celiac disease, a T-cell mediated enteropathy characterized by gluten intolerance and a herpetiform-like rash, can be associated with small fiber neuropathy.25 In some cases, neuropathy symptoms are preceded by the onset of gastrointestinal symptoms, or they may occur in isolation.25
Inflammatory disease testing
Sjögren syndrome accounts for nearly 10% of cases of small fiber neuropathy. Associated neuropathic symptoms are often non–length-dependent, can precede sicca symptoms for up to 6 years, and in some cases are the sole manifestation of the disease.10 Small fiber neuropathy may also be associated with vasculitis, systemic lupus erythematosus, and other connective tissue disorders.
Testing should include:
- Erythrocyte sedimentation rate, C-reactive protein, and antinuclear antibodies: though these are nonspecific markers of inflammation, they may support an immune-mediated etiology if positive
- Extractable nuclear antigen panel: Sjögren syndrome A and B autoantibodies are the most important components in this setting5,11
- The Schirmer test or salivary gland biopsy should be considered for seronegative patients with sicca or a suspected immune-mediated etiology, as the sensitivity of antibody testing ranges from only 10% to 55%.10
Thyroid function testing
Hypothyroidism, and less commonly hyperthyroidism, are associated with small fiber neuropathy.
Metabolic tests for liver and kidney disease
Renal insufficiency and liver impairment are well-known causes of small nerve fiber dysfunction. Testing should include:
- Comprehensive metabolic panel
- Gamma-glutamyltransferase if alcohol abuse is suspected, since heavy alcohol use is one of the most common causes of both large and small fiber neuropathy.
HIV and hepatitis C testing
For patients with relevant risk factors, HIV and hepatitis C testing should be part of the initial workup (and as second-tier testing for others). Patients who test positive for hepatitis C should undergo further testing for cryoglobulinemia, which can present with painful small fiber neuropathy.26
Serum and urine immunoelectrophoresis
Paraproteinemia, with causes ranging from monoclonal gammopathy of uncertain significance to multiple myeloma, has been associated with small fiber neuropathy. An abnormal serum or urine immunoelectrophoresis test warrants further investigation and possibly referral to a hematology-oncology specialist.
SECOND-TIER TESTING
Less common treatable causes of small fiber neuropathy may also be evaluated.
Copper, vitamin B1 (thiamine), or vitamin B6 (pyridoxine) deficiency testing. Although vitamin B6 toxicity may also result in neuropathy due to its toxic effect on the dorsal root ganglia, the mildly elevated vitamin B6 levels often found in patients being evaluated for neuropathy are unlikely to be the primary cause of symptoms. Many laboratories require fasting samples for accurate vitamin B6 levels.
Angiotensin-converting enzyme levels for sarcoidosis. Small fiber neuropathy is common in sarcoidosis, occurring in more than 30% of patients with systemic disease.27 However, screening for sarcoidosis by measuring serum levels is often falsely positive and is not cost-effective. In a study of 195 patients with idiopathic small fiber neuropathy,11 44% had an elevated serum level, but no evidence of sarcoidosis was seen on further testing, which included computed tomography of the chest in 29 patients.12 Thus, this test is best used for patients with evidence of systemic disease.
Amyloid testing for amyloidosis. Fat pad or bone marrow biopsy should be considered in the appropriate clinical setting.
Paraneoplastic autoantibody panel for occult cancer. Such testing may also be considered if clinically warranted. However, if a patient is found to have low positive titers of paraneoplastic antibodies and suspicion is low for an occult cancer (eg, no weight loss or early satiety), repeat confirmatory testing at another laboratory should be done before embarking on an extensive search for malignancy.
Ganglionic acetylcholine receptor antibody testing for autoimmune autonomic ganglionopathy. This should be ordered for patients with prominent autonomic dysfunction. The antibody test can be ordered separately or as part of an autoantibody panel. The antibody may indicate a primary immune-mediated process or a paraneoplastic disease.28
Genetic mutation testing. Recent discoveries of gene mutations leading to peripheral nerve hyperexcitability of voltage-gated sodium channels have elucidated a hereditary cause of small fiber neuropathy in nearly 30% of cases that were once thought to be idiopathic.29,30 Genetic testing for mutations in SCN9A and SCN10 (which code for the Nav1.7 and Nav1.8 sodium channels, respectively) is commercially available and may be considered for those with a family history of neuropathic pain in the feet or for young, otherwise healthy patients.
Fabry disease is an X-linked lysosomal disorder characterized by angiokeratomas, cardiac and renal impairment, and small fiber neuropathy. Treatment is now available, but screening is not cost-efficient and should only be pursued in patients with other symptoms of the disease.31,32
OTHER POSSIBLE CAUSES
Guillain-Barré syndrome
A Guillain-Barré syndrome variant has been reported that is characterized by ascending limb paresthesias and cerebrospinal fluid albuminocytologic dissociation in the setting of preserved deep tendon reflexes and normal findings on EMG.12 The clinical course is similar to that of typical Guillain-Barré syndrome, in that symptoms follow an upper respiratory or gastrointestinal tract infection, reach their nadir at 4 weeks, and then gradually improve. Some patients respond to intravenous immune globulin.
Vaccine-associated
Postvaccination small fiber neuropathy has also been reported. The nature of the association is unclear.33
Parkinson disease
Small fiber neuropathy is associated with Parkinson disease. It is attributed to a number of proposed factors, including neurodegeneration that occurs parallel to central nervous system decline, as well as intestinal malabsorption with resultant vitamin deficiency.34,35
Rapid glycemic lowering
Aggressive treatment of diabetes, defined as at least a 2-point reduction of serum hemoglobin A1c level over 3 months, may result in acute small fiber neuropathy. It manifests as severe distal extremity pain and dysautonomia.
In a retrospective study,36 104 (10.9%) of 954 patients presenting to a tertiary diabetic clinic developed treatment-induced diabetic neuropathy with symptoms occurring within 8 weeks of rapid glycemic control. The severity of neuropathy correlated with the degree and rate of glycemic lowering. The condition was reversible in some cases.
TREATING SPECIFIC DISORDERS
For patients with an identified cause of neuropathy, targeted treatment offers the best chance of halting progression and possibly improving symptoms. Below are recommendations for addressing neuropathy associated with the common diagnoses.
Diabetes, impaired glucose tolerance, and metabolic syndrome. In addition to glycemic- and lipid-lowering therapies, lifestyle modifications with a specific focus on exercise and nutrition are integral to treating diabetes and related disorders.
In the Look AHEAD (Action for Health in Diabetes) study,37 which evaluated the effects of intensive lifestyle intervention on neuropathy in 5,145 overweight patients with type 2 diabetes, patients in the intervention group had lower pain scores and better touch sensation in the toes compared with controls at 1 year. Differences correlated with the degree of weight loss and reduction of hemoglobin A1c and lipid levels.
As running and walking may not be feasible for many patients owing to pain, stationary cycling, aqua therapy, and swimming are other options. A stationary recumbent bike may be useful for older patients with balance issues.
Vitamin B12 deficiency. As reduced absorption rather than low dietary intake is the primary cause of vitamin B12 deficiency for many patients, parenteral rather than oral supplementation may be best. A suggested regimen is subcutaneous or intramuscular methylcobalamin injection of 1,000 µg given daily for 1 week, then once weekly for 1 month, followed by a maintenance dose once a month for at least 6 to 12 months. Alternatively, a daily dose of vitamin B12 1,000 µg can be taken sublingually.
Sjögren syndrome. According to anecdotal case reports, intravenous immune globulin, corticosteroids, and other immunosuppressants help painful small fiber neuropathy and dysautonomia associated with Sjögren syndrome.10
Sarcoidosis. Sarcoidosis-associated small fiber neuropathy may also respond to intravenous immune globulin, as well as infliximab and combination therapy.9 Culver et al38 found that cibinetide, an experimental erythropoetin agonist, resulted in improved corneal nerve fiber measures in patients with small fiber neuropathy associated with sarcoidosis.
Celiac disease. A gluten-free diet is the treatment for celiac disease and can help some patients.
GENERAL MANAGEMENT
For all patients, regardless of whether the cause of small fiber neuropathy has been identified, managing symptoms remains key, as pain and autonomic dysfunction can markedly impair quality of life. A multidisciplinary approach that incorporates pain medications, physical therapy, and lifestyle modifications is ideal. Integrative holistic treatments such as natural supplements, yoga, and other mind-body therapies may also help.
Pain control
Mexiletine, a voltage-gated sodium channel blocker used as an antiarrhythmic, may help refractory pain or hereditary small fiber neuropathy related to sodium channel dysfunction. However, it is not recommended for diabetic neuropathy.39
Combination regimens that use drugs with different mechanisms of action can be effective. In one study, combined gabapentin and nortriptyline were more effective than either drug alone for neuropathic pain.40
Inhaled cannabis reduced pain in patients with HIV and diabetic neuropathy in a number of studies. Side effects included euphoria, somnolence, and cognitive impairment.41,42 The use of medical marijuana is not yet legal nationwide and may affect employability even in states in which it has been legalized.
Owing to the opioid epidemic and high addiction potential, opioids are no longer a preferred recommendation for chronic treatment of noncancer-related neuropathy. A population-based study of 2,892 patients with neuropathy found that those on chronic opioid therapy (≥ 90 days) had worse functional outcomes and higher rates of addiction and overdose than those on short-term therapy.43 However, the opioid agonist tramadol was found to be effective in reducing neuropathic pain and may be a safer option for patients with chronic small fiber neuropathy.44
Integrative, holistic therapies
PROGNOSIS
For many patients, small fiber neuropathy is a slowly progressive disorder that reaches a clinical plateau lasting for years, with progression to large fiber involvement reported in 13% to 36% of cases; over half of patients in one series either improved or remained stable over a period of 2 years.5,57 Long-term studies are needed to fully understand the natural disease course. In the meantime, treating underlying disease and managing symptoms are imperative to patient care.
- Burke JF, Skolarus LE, Callaghan BC, Kerber KA. Choosing Wisely: highest-cost tests in outpatient neurology. Ann Neurol 2013; 73(5):679–683. doi:10.1002/ana.23865
- Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care 2003; 26(6):1790–1795. pmid:12766111
- Peters MJ, Bakkers M, Merkies IS, Hoeijmakers JG, van Raak EP, Faber CG. Incidence and prevalence of small-fiber neuropathy: a survey in the Netherlands. Neurology 2013; 81(15):1356–1360. doi:10.1212/WNL.0b013e3182a8236e
- Periquet MI, Novak V, Collins MP, et al. Painful sensory neuropathy: prospective evaluation using skin biopsy. Neurology 1999; 53(8):1641–1647. pmid:10563606
- Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008; 131(pt 7):1912–1925. doi:10.1093/brain/awn093
- Lacomis D. Small-fiber neuropathy. Muscle Nerve 2002; 26(2):173–188. doi:10.1002/mus.10181
- Lopate G, Streif E, Harms M, Weihl C, Pestronk A. Cramps and small-fiber neuropathy. Muscle Nerve 2013; 48(2):252–255. doi:10.1002/mus.23757
- Khan S, Zhou L. Characterization of non-length-dependent small-fiber sensory neuropathy. Muscle Nerve 2012; 45(1):86–91. doi:10.1002/mus.22255
- Tavee JO, Karwa K, Ahmed Z, Thompson N, Parambil J, Culver DA. Sarcoidosis-associated small fiber neuropathy in a large cohort: clinical aspects and response to IVIG and anti-TNF alpha treatment. Respir Med 2017; 126:135–138. doi:10.1016/j.rmed.2017.03.011
- Berkowitz AL, Samuels MA. The neurology of Sjogren’s syndrome and the rheumatology of peripheral neuropathy and myelitis. Pract Neurol 2014; 14(1):14–22. doi:10.1136/practneurol-2013-000651
- Lang M, Treister R, Oaklander AL. Diagnostic value of blood tests for occult causes of initially idiopathic small-fiber polyneuropathy. J Neurol 2016; 263(12):2515–2527. doi:10.1007/s00415-016-8270-5
- Seneviratne U, Gunasekera S. Acute small fibre sensory neuropathy: another variant of Guillain-Barré syndrome? J Neurol Neurosurg Psychiatry 2002; 72(4):540–542. pmid:11909922
- Tavee JO, Polston D, Zhou L, Shields RW, Butler RS, Levin KH. Sural sensory nerve action potential, epidermal nerve fiber density, and quantitative sudomotor axon reflex in the healthy elderly. Muscle Nerve 2014; 49(4):564–569. doi:10.1002/mus.23971
- Tavee J, Zhou L. Small fiber neuropathy: a burning problem. Cleve Clin J Med 2009; 76(5):297–305. doi:10.3949/ccjm.76a.08070
- Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology 1999; 53(8):1634–1640. pmid:10563605
- Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 2013; 154(11):2310–2316. doi:10.1016/j.pain.2013.06.001
- Üçeyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013; 136(pt 6):1857–1867. doi:10.1093/brain/awt053
- Stewart JD, Low PA, Fealey RD. Distal small fiber neuropathy: results of tests of sweating and autonomic cardiovascular reflexes. Muscle Nerve 1992; 15(6):661–665. doi:10.1002/mus.880150605
- Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia 2003; 46(5):683–688. doi:10.1007/s00125-003-1086-8
- de Greef BTA, Hoeijmakers JGJ, Gorissen-Brouwers CML, Geerts M, Faber CG, Merkies ISJ. Associated conditions in small fiber neuropathy—a large cohort study and review of the literature. Eur J Neurol 2018; 25(2):348–355. doi:10.1111/ene.13508
- Smith AG. Impaired glucose tolerance and metabolic syndrome in idiopathic neuropathy. J Peripher Nerv Syst 2012; 17(suppl 2):15–21. doi:10.1111/j.1529-8027.2012.00390.x
- Hoffman-Snyder C, Smith BE, Ross MA, Hernandez J, Bosch EP. Value of the oral glucose tolerance test in the evaluation of chronic idiopathic axonal polyneuropathy. Arch Neurol 2006; 63(8):1075–1079. doi:10.1001/archneur.63.8.noc50336
- Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst 2009; 14(4):257–267. doi:10.1111/j.1529-8027.2009.00237.x
- Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes 2009; 58(7):1634–1640. doi:10.2337/db08-1771
- Chin RL, Sander HW, Brannagan TH, et al. Celiac neuropathy. Neurology 2003; 60(10):1581–1585. pmid:12771245
- Gemignani F, Brindani F, Alfieri S, et al. Clinical spectrum of cryoglobulinaemic neuropathy. J Neurol Neurosurg Psychiatry 2005; 76(10):1410–1414. doi:10.1136/jnnp.2004.057620
- Bakkers M, Merkies IS, Lauria G, et al. Intraepidermal nerve fiber density and its application in sarcoidosis. Neurology 2009; 73(14):1142–1148. doi:10.1212/WNL.0b013e3181bacf05
- Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med 2000; 343(12):847–855. doi:10.1056/NEJM200009213431204
- Faber CG, Hoeijmakers JG, Ahn HS, et al. Gain of function Nav1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol 2012; 71(1):26–39. doi:10.1002/ana.22485
- Brouwer BA, Merkies IS, Gerrits MM, Waxman SG, Hoeijmakers JG, Faber CG. Painful neuropathies: the emerging role of sodium channelopathies. J Peripher Nerv Syst 2014; 19(2):53–65. doi:10.1111/jns5.12071
- Samuelsson K, Kostulas K, Vrethem M, Rolfs A, Press R. Idiopathic small fiber neuropathy: phenotype, etiologies, and the search for Fabry disease. J Clin Neurol 2014; 10(2):108–118. doi:10.3988/jcn.2014.10.2.108
- de Greef BT, Hoeijmakers JG, Wolters EE, et al. No Fabry disease in patients presenting with isolated small fiber neuropathy. PLoS One 2016; 11(2):e0148316. doi:10.1371/journal.pone.0148316
- Souayah N, Ajroud-Driss S, Sander HW, Brannagan TH, Hays AP, Chin RL. Small fiber neuropathy following vaccination for rabies, varicella or Lyme disease. Vaccine 2009; 27(52):7322–7325. doi:10.1016/j.vaccine.2009.09.077
- Nolano M, Provitera V, Manganelli F, et al. Loss of cutaneous large and small fibers in naive and l-dopa–treated PD patients. Neurology 2017; 89(8):776–784. doi:10.1212/WNL.0000000000004274
- Zis P, Grünewald RA, Chaudhuri RK, Hadjivassiliou M. Peripheral neuropathy in idiopathic Parkinson’s disease: a systematic review. J Neurol Sci 2017; 378:204–209. doi:10.1016/j.jns.2017.05.023
- Gibbons CH, Freeman R. Treatment-induced neuropathy of diabetes: an acute, iatrogenic complication of diabetes. Brain 2015; 138(pt 1):43–52. doi:10.1093/brain/awu307
- Look AHEAD Research Group. Effects of a long-term lifestyle modification programme on peripheral neuropathy in overweight or obese adults with type 2 diabetes: the Look AHEAD study. Diabetologia 2017; 60(6):980–988. doi:10.1007/s00125-017-4253-z
- Culver DA, Dahan A, Bajorunas D, et al. Cibinetide improves corneal nerve fiber abundance in patients with sarcoidosis-associated small nerve fiber loss and neuropathic pain. Invest Ophthalmol Vis Sci 2017; 58(6):BIO52–BIO60. doi:10.1167/iovs.16-21291
- Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R 2011; 3(4):345–352.e21. doi:10.1016/j.pmrj.2011.03.008
- Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet 2009; 374(9697):1252–1261. doi:10.1016/S0140-6736(09)61081-3
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009; 34(3):672–680. doi:10.1038/npp.2008.120
- Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015; 16(7):616–627. doi:10.1016/j.jpain.2015.03.008
- Hoffman EM, Watson JC, St Sauver J, Staff NP, Klein CJ. Association of long-term opioid therapy with functional status, adverse outcomes, and mortality among patients with polyneuropathy. JAMA Neurol 2017; 74(7):773–779. doi:10.1001/jamaneurol.2017.0486
- Harati Y, Gooch C, Swenson M, et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology 1998; 50(6):1842–1846. pmid:9633738
- Sima AA, Calvani M, Mehra M, Amato A; Acetyl-L-Carnitine Study Group. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care 2005; 28(1):89–94. pmid:15616239
- Ziegler D, Hanefeld M, Ruhnau KJ, et al. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multicentre randomized controlled trial (ALADIN Study). Diabetologia 1995; 38(12):1425–1433. pmid:8786016
- Scarpini E, Sacilotto G, Baron P, Cusini M, Scarlato G. Effect of acetyl-L-carnitine in the treatment of painful peripheral neuropathies in HIV+ patients. J Peripher Nerv Syst 1997; 2(3):250-252. pmid: 10975731
- Hershman DL, Unger JM, Crew KD, et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J Clin Oncol 2013; 31(20):2627-2633. doi:10.1200/JCO.2012.44.8738
- Amara S. Oral glutamine for the prevention of chemotherapy-induced peripheral neuropathy. Ann Pharmacother 2008; 42(10):1481-1485. doi:10.1345/aph.1L179
- Huang JS, Wu CL, Fan CW, Chen WH, Yeh KY, Chang PH. Intravenous glutamine appears to reduce the severity of symptomatic platinum-induced neuropathy: a prospective randomized study. J Chemother 2015; 27(4):235-240. doi:10.1179/1973947815Y.0000000011
- Banafshe HR, Hamidi GA, Noureddini M, Mirhashemi SM, Mokhtari R, Shoferpour M. Effect of curcumin on diabetic peripheral neuropathic pain: possible involvement of opioid system. Eur J Pharmacol 2014; 723:202-206. doi:10.1016/j.ejphar.2013.11.033
- Mendonça LM, da Silva Machado C, Teixeira CC, de Freitas LA, Bianchi MD, Antunes LM. Curcumin reduces cisplatin-induced neurotoxicity in NGF-differentiated PC12 cells. Neurotoxicology 2013; 34:205-211. doi:10.1016/j.neuro.2012.09.011
- Wagner K, Lee KS, Yang J, Hammock BD. Epoxy fatty acids mediate analgesia in murine diabetic neuropathy. Eur J Pain 2017; 21(3):456-465. doi:10.1002/ejp.939
- Lewis EJ, Perkins BA, Lovblom LE, Bazinet RP, Wolever TMS, Bril V. Effect of omega-3 supplementation on neuropathy in type 1 diabetes: a 12-month pilot trial. Neurology 2017; 88(24):2294–2301. doi:10.1212/WNL.0000000000004033
- Hu D, Wang C, Li F, et al. A combined water extract of frankincense and myrrh alleviates neuropathic pain in mice via modulation of TRPV1. Neural Plast 2017; 2017:3710821. doi:10.1155/2017/3710821
- Tavee J, Rensel M, Planchon SM, Butler RS, Stone L. Effects of meditation on pain and quality of life in multiple sclerosis and peripheral neuropathy: a pilot study. Int J MS Care 2011; 13(4):163–168. doi:10.7224/1537-2073-13.4.163
- Khoshnoodi MA, Truelove S, Burakgazi A, Hoke A, Mammen AL, Polydefkis M. Longitudinal assessment of small fiber neuropathy: evidence of a non-length-dependent distal axonopathy. JAMA Neurol 2016; 73(6):684–690. doi:10.1001/jamaneurol.2016.0057
- Burke JF, Skolarus LE, Callaghan BC, Kerber KA. Choosing Wisely: highest-cost tests in outpatient neurology. Ann Neurol 2013; 73(5):679–683. doi:10.1002/ana.23865
- Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care 2003; 26(6):1790–1795. pmid:12766111
- Peters MJ, Bakkers M, Merkies IS, Hoeijmakers JG, van Raak EP, Faber CG. Incidence and prevalence of small-fiber neuropathy: a survey in the Netherlands. Neurology 2013; 81(15):1356–1360. doi:10.1212/WNL.0b013e3182a8236e
- Periquet MI, Novak V, Collins MP, et al. Painful sensory neuropathy: prospective evaluation using skin biopsy. Neurology 1999; 53(8):1641–1647. pmid:10563606
- Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008; 131(pt 7):1912–1925. doi:10.1093/brain/awn093
- Lacomis D. Small-fiber neuropathy. Muscle Nerve 2002; 26(2):173–188. doi:10.1002/mus.10181
- Lopate G, Streif E, Harms M, Weihl C, Pestronk A. Cramps and small-fiber neuropathy. Muscle Nerve 2013; 48(2):252–255. doi:10.1002/mus.23757
- Khan S, Zhou L. Characterization of non-length-dependent small-fiber sensory neuropathy. Muscle Nerve 2012; 45(1):86–91. doi:10.1002/mus.22255
- Tavee JO, Karwa K, Ahmed Z, Thompson N, Parambil J, Culver DA. Sarcoidosis-associated small fiber neuropathy in a large cohort: clinical aspects and response to IVIG and anti-TNF alpha treatment. Respir Med 2017; 126:135–138. doi:10.1016/j.rmed.2017.03.011
- Berkowitz AL, Samuels MA. The neurology of Sjogren’s syndrome and the rheumatology of peripheral neuropathy and myelitis. Pract Neurol 2014; 14(1):14–22. doi:10.1136/practneurol-2013-000651
- Lang M, Treister R, Oaklander AL. Diagnostic value of blood tests for occult causes of initially idiopathic small-fiber polyneuropathy. J Neurol 2016; 263(12):2515–2527. doi:10.1007/s00415-016-8270-5
- Seneviratne U, Gunasekera S. Acute small fibre sensory neuropathy: another variant of Guillain-Barré syndrome? J Neurol Neurosurg Psychiatry 2002; 72(4):540–542. pmid:11909922
- Tavee JO, Polston D, Zhou L, Shields RW, Butler RS, Levin KH. Sural sensory nerve action potential, epidermal nerve fiber density, and quantitative sudomotor axon reflex in the healthy elderly. Muscle Nerve 2014; 49(4):564–569. doi:10.1002/mus.23971
- Tavee J, Zhou L. Small fiber neuropathy: a burning problem. Cleve Clin J Med 2009; 76(5):297–305. doi:10.3949/ccjm.76a.08070
- Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology 1999; 53(8):1634–1640. pmid:10563605
- Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 2013; 154(11):2310–2316. doi:10.1016/j.pain.2013.06.001
- Üçeyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013; 136(pt 6):1857–1867. doi:10.1093/brain/awt053
- Stewart JD, Low PA, Fealey RD. Distal small fiber neuropathy: results of tests of sweating and autonomic cardiovascular reflexes. Muscle Nerve 1992; 15(6):661–665. doi:10.1002/mus.880150605
- Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia 2003; 46(5):683–688. doi:10.1007/s00125-003-1086-8
- de Greef BTA, Hoeijmakers JGJ, Gorissen-Brouwers CML, Geerts M, Faber CG, Merkies ISJ. Associated conditions in small fiber neuropathy—a large cohort study and review of the literature. Eur J Neurol 2018; 25(2):348–355. doi:10.1111/ene.13508
- Smith AG. Impaired glucose tolerance and metabolic syndrome in idiopathic neuropathy. J Peripher Nerv Syst 2012; 17(suppl 2):15–21. doi:10.1111/j.1529-8027.2012.00390.x
- Hoffman-Snyder C, Smith BE, Ross MA, Hernandez J, Bosch EP. Value of the oral glucose tolerance test in the evaluation of chronic idiopathic axonal polyneuropathy. Arch Neurol 2006; 63(8):1075–1079. doi:10.1001/archneur.63.8.noc50336
- Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst 2009; 14(4):257–267. doi:10.1111/j.1529-8027.2009.00237.x
- Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes 2009; 58(7):1634–1640. doi:10.2337/db08-1771
- Chin RL, Sander HW, Brannagan TH, et al. Celiac neuropathy. Neurology 2003; 60(10):1581–1585. pmid:12771245
- Gemignani F, Brindani F, Alfieri S, et al. Clinical spectrum of cryoglobulinaemic neuropathy. J Neurol Neurosurg Psychiatry 2005; 76(10):1410–1414. doi:10.1136/jnnp.2004.057620
- Bakkers M, Merkies IS, Lauria G, et al. Intraepidermal nerve fiber density and its application in sarcoidosis. Neurology 2009; 73(14):1142–1148. doi:10.1212/WNL.0b013e3181bacf05
- Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med 2000; 343(12):847–855. doi:10.1056/NEJM200009213431204
- Faber CG, Hoeijmakers JG, Ahn HS, et al. Gain of function Nav1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol 2012; 71(1):26–39. doi:10.1002/ana.22485
- Brouwer BA, Merkies IS, Gerrits MM, Waxman SG, Hoeijmakers JG, Faber CG. Painful neuropathies: the emerging role of sodium channelopathies. J Peripher Nerv Syst 2014; 19(2):53–65. doi:10.1111/jns5.12071
- Samuelsson K, Kostulas K, Vrethem M, Rolfs A, Press R. Idiopathic small fiber neuropathy: phenotype, etiologies, and the search for Fabry disease. J Clin Neurol 2014; 10(2):108–118. doi:10.3988/jcn.2014.10.2.108
- de Greef BT, Hoeijmakers JG, Wolters EE, et al. No Fabry disease in patients presenting with isolated small fiber neuropathy. PLoS One 2016; 11(2):e0148316. doi:10.1371/journal.pone.0148316
- Souayah N, Ajroud-Driss S, Sander HW, Brannagan TH, Hays AP, Chin RL. Small fiber neuropathy following vaccination for rabies, varicella or Lyme disease. Vaccine 2009; 27(52):7322–7325. doi:10.1016/j.vaccine.2009.09.077
- Nolano M, Provitera V, Manganelli F, et al. Loss of cutaneous large and small fibers in naive and l-dopa–treated PD patients. Neurology 2017; 89(8):776–784. doi:10.1212/WNL.0000000000004274
- Zis P, Grünewald RA, Chaudhuri RK, Hadjivassiliou M. Peripheral neuropathy in idiopathic Parkinson’s disease: a systematic review. J Neurol Sci 2017; 378:204–209. doi:10.1016/j.jns.2017.05.023
- Gibbons CH, Freeman R. Treatment-induced neuropathy of diabetes: an acute, iatrogenic complication of diabetes. Brain 2015; 138(pt 1):43–52. doi:10.1093/brain/awu307
- Look AHEAD Research Group. Effects of a long-term lifestyle modification programme on peripheral neuropathy in overweight or obese adults with type 2 diabetes: the Look AHEAD study. Diabetologia 2017; 60(6):980–988. doi:10.1007/s00125-017-4253-z
- Culver DA, Dahan A, Bajorunas D, et al. Cibinetide improves corneal nerve fiber abundance in patients with sarcoidosis-associated small nerve fiber loss and neuropathic pain. Invest Ophthalmol Vis Sci 2017; 58(6):BIO52–BIO60. doi:10.1167/iovs.16-21291
- Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R 2011; 3(4):345–352.e21. doi:10.1016/j.pmrj.2011.03.008
- Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet 2009; 374(9697):1252–1261. doi:10.1016/S0140-6736(09)61081-3
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009; 34(3):672–680. doi:10.1038/npp.2008.120
- Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015; 16(7):616–627. doi:10.1016/j.jpain.2015.03.008
- Hoffman EM, Watson JC, St Sauver J, Staff NP, Klein CJ. Association of long-term opioid therapy with functional status, adverse outcomes, and mortality among patients with polyneuropathy. JAMA Neurol 2017; 74(7):773–779. doi:10.1001/jamaneurol.2017.0486
- Harati Y, Gooch C, Swenson M, et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology 1998; 50(6):1842–1846. pmid:9633738
- Sima AA, Calvani M, Mehra M, Amato A; Acetyl-L-Carnitine Study Group. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care 2005; 28(1):89–94. pmid:15616239
- Ziegler D, Hanefeld M, Ruhnau KJ, et al. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multicentre randomized controlled trial (ALADIN Study). Diabetologia 1995; 38(12):1425–1433. pmid:8786016
- Scarpini E, Sacilotto G, Baron P, Cusini M, Scarlato G. Effect of acetyl-L-carnitine in the treatment of painful peripheral neuropathies in HIV+ patients. J Peripher Nerv Syst 1997; 2(3):250-252. pmid: 10975731
- Hershman DL, Unger JM, Crew KD, et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J Clin Oncol 2013; 31(20):2627-2633. doi:10.1200/JCO.2012.44.8738
- Amara S. Oral glutamine for the prevention of chemotherapy-induced peripheral neuropathy. Ann Pharmacother 2008; 42(10):1481-1485. doi:10.1345/aph.1L179
- Huang JS, Wu CL, Fan CW, Chen WH, Yeh KY, Chang PH. Intravenous glutamine appears to reduce the severity of symptomatic platinum-induced neuropathy: a prospective randomized study. J Chemother 2015; 27(4):235-240. doi:10.1179/1973947815Y.0000000011
- Banafshe HR, Hamidi GA, Noureddini M, Mirhashemi SM, Mokhtari R, Shoferpour M. Effect of curcumin on diabetic peripheral neuropathic pain: possible involvement of opioid system. Eur J Pharmacol 2014; 723:202-206. doi:10.1016/j.ejphar.2013.11.033
- Mendonça LM, da Silva Machado C, Teixeira CC, de Freitas LA, Bianchi MD, Antunes LM. Curcumin reduces cisplatin-induced neurotoxicity in NGF-differentiated PC12 cells. Neurotoxicology 2013; 34:205-211. doi:10.1016/j.neuro.2012.09.011
- Wagner K, Lee KS, Yang J, Hammock BD. Epoxy fatty acids mediate analgesia in murine diabetic neuropathy. Eur J Pain 2017; 21(3):456-465. doi:10.1002/ejp.939
- Lewis EJ, Perkins BA, Lovblom LE, Bazinet RP, Wolever TMS, Bril V. Effect of omega-3 supplementation on neuropathy in type 1 diabetes: a 12-month pilot trial. Neurology 2017; 88(24):2294–2301. doi:10.1212/WNL.0000000000004033
- Hu D, Wang C, Li F, et al. A combined water extract of frankincense and myrrh alleviates neuropathic pain in mice via modulation of TRPV1. Neural Plast 2017; 2017:3710821. doi:10.1155/2017/3710821
- Tavee J, Rensel M, Planchon SM, Butler RS, Stone L. Effects of meditation on pain and quality of life in multiple sclerosis and peripheral neuropathy: a pilot study. Int J MS Care 2011; 13(4):163–168. doi:10.7224/1537-2073-13.4.163
- Khoshnoodi MA, Truelove S, Burakgazi A, Hoke A, Mammen AL, Polydefkis M. Longitudinal assessment of small fiber neuropathy: evidence of a non-length-dependent distal axonopathy. JAMA Neurol 2016; 73(6):684–690. doi:10.1001/jamaneurol.2016.0057
KEY POINTS
- Patients typically develop a symmetric “stocking-glove” pattern of sensory loss in the feet and hands.
- The diagnosis may be confirmed with skin biopsy for nerve fiber density, which can easily be done in a clinic setting with commercially available kits.
- Diabetes is the most common identifiable cause of small fiber neuropathy.
- Serologic testing can help uncover a vitamin deficiency or other potentially treatable condition.
- Antiepileptics, antidepressants, and topical agents are first-line drugs for managing pain.
Small fibers, large impact
The details about an individual’s search for information tell us a lot about healthcare concerns and uncertainty across the medical universe. For nearly a decade, one of the most “clicked on” papers we have published in the Journal has been a review of small fiber neuropathy—a clinical entity with a prevalence of perhaps 1 in 1,000 to 2,000 people and, to my knowledge, no associated walkathons or arm bracelets. Yet it certainly piques the interest of clinicians from many specialties far broader than neurology. In this issue of the Journal, Dr. Jinny Tavee updates her 2009 review and provides us with a clinical overview of the disorder and the opportunity to assess how much further we need to more fully understand its management and associated comorbid conditions.
The wide interest in this disorder plugs into our current seeming epidemic of patients with chronic pain. It seems that almost half of my new patients have issues related to chronic pain that are not directly explained by active inflammation or anatomic damage. Many of these patients have diffuse body pains with associated fatigue and sleep disorders and are diagnosed with fibromyalgia. But others describe pain with a burning and tingling quality that seems of neurologic origin, yet their neurologic examination is normal. A few describe a predominantly distal symmetric stocking-and-glove distribution, but most do not. In some patients these pains are spatially random and evanescent, which to me are usually the hardest to fathom. Nerve conduction studies, when performed, are unrevealing.
A number of systemic autoimmune disorders, as discussed by Dr. Tavee in her article, are suggested to have an association with these symptoms. Given the chronicity and the frustrating nature of the symptoms, it is no surprise that a panoply of immune serologies are frequently ordered. Invariably, since serologies (eg, ANA, SSA, SSB, rheumatoid factor) are not specific for any single entity, some will return as positive. The strength of many of these associations is weak; even when the clinical diagnosis of lupus, for example, is definite, treatment of the underlying disease does not necessarily improve the dysesthetic pain. In an alternative scenario, the small fiber neuropathy is ascribed to a systemic autoimmune disorder that has been diagnosed because an autoantibody has been detected, but this rarely helps the patient and may in fact worsen symptoms by increasing anxiety and concern over having a systemic disease such as Sjögren syndrome or lupus (both of which sound terrible when reviewed on the Internet).
Some patients describe autonomic symptoms. Given the biologic basis that has been defined for this entity, it is no surprise that some patients have marked symptoms of decreased exocrine gland function, gastrointestinal dysmotility, and orthostasis. These symptoms may not be recognized unless specifically sought out when interviewing the patient.
Given the chronicity and sometimes the vagaries of symptoms, it is often comforting for patients to get an actual diagnosis. Dr. Tavee notes the relative simplicity of diagnostic procedures. But determining the clinical implications of the results may not be straightforward, and devising a fully and uniformly effective therapeutic approach eludes us still. As she points out, a multidisciplinary approach to therapy and diagnosis can be quite helpful.
The details about an individual’s search for information tell us a lot about healthcare concerns and uncertainty across the medical universe. For nearly a decade, one of the most “clicked on” papers we have published in the Journal has been a review of small fiber neuropathy—a clinical entity with a prevalence of perhaps 1 in 1,000 to 2,000 people and, to my knowledge, no associated walkathons or arm bracelets. Yet it certainly piques the interest of clinicians from many specialties far broader than neurology. In this issue of the Journal, Dr. Jinny Tavee updates her 2009 review and provides us with a clinical overview of the disorder and the opportunity to assess how much further we need to more fully understand its management and associated comorbid conditions.
The wide interest in this disorder plugs into our current seeming epidemic of patients with chronic pain. It seems that almost half of my new patients have issues related to chronic pain that are not directly explained by active inflammation or anatomic damage. Many of these patients have diffuse body pains with associated fatigue and sleep disorders and are diagnosed with fibromyalgia. But others describe pain with a burning and tingling quality that seems of neurologic origin, yet their neurologic examination is normal. A few describe a predominantly distal symmetric stocking-and-glove distribution, but most do not. In some patients these pains are spatially random and evanescent, which to me are usually the hardest to fathom. Nerve conduction studies, when performed, are unrevealing.
A number of systemic autoimmune disorders, as discussed by Dr. Tavee in her article, are suggested to have an association with these symptoms. Given the chronicity and the frustrating nature of the symptoms, it is no surprise that a panoply of immune serologies are frequently ordered. Invariably, since serologies (eg, ANA, SSA, SSB, rheumatoid factor) are not specific for any single entity, some will return as positive. The strength of many of these associations is weak; even when the clinical diagnosis of lupus, for example, is definite, treatment of the underlying disease does not necessarily improve the dysesthetic pain. In an alternative scenario, the small fiber neuropathy is ascribed to a systemic autoimmune disorder that has been diagnosed because an autoantibody has been detected, but this rarely helps the patient and may in fact worsen symptoms by increasing anxiety and concern over having a systemic disease such as Sjögren syndrome or lupus (both of which sound terrible when reviewed on the Internet).
Some patients describe autonomic symptoms. Given the biologic basis that has been defined for this entity, it is no surprise that some patients have marked symptoms of decreased exocrine gland function, gastrointestinal dysmotility, and orthostasis. These symptoms may not be recognized unless specifically sought out when interviewing the patient.
Given the chronicity and sometimes the vagaries of symptoms, it is often comforting for patients to get an actual diagnosis. Dr. Tavee notes the relative simplicity of diagnostic procedures. But determining the clinical implications of the results may not be straightforward, and devising a fully and uniformly effective therapeutic approach eludes us still. As she points out, a multidisciplinary approach to therapy and diagnosis can be quite helpful.
The details about an individual’s search for information tell us a lot about healthcare concerns and uncertainty across the medical universe. For nearly a decade, one of the most “clicked on” papers we have published in the Journal has been a review of small fiber neuropathy—a clinical entity with a prevalence of perhaps 1 in 1,000 to 2,000 people and, to my knowledge, no associated walkathons or arm bracelets. Yet it certainly piques the interest of clinicians from many specialties far broader than neurology. In this issue of the Journal, Dr. Jinny Tavee updates her 2009 review and provides us with a clinical overview of the disorder and the opportunity to assess how much further we need to more fully understand its management and associated comorbid conditions.
The wide interest in this disorder plugs into our current seeming epidemic of patients with chronic pain. It seems that almost half of my new patients have issues related to chronic pain that are not directly explained by active inflammation or anatomic damage. Many of these patients have diffuse body pains with associated fatigue and sleep disorders and are diagnosed with fibromyalgia. But others describe pain with a burning and tingling quality that seems of neurologic origin, yet their neurologic examination is normal. A few describe a predominantly distal symmetric stocking-and-glove distribution, but most do not. In some patients these pains are spatially random and evanescent, which to me are usually the hardest to fathom. Nerve conduction studies, when performed, are unrevealing.
A number of systemic autoimmune disorders, as discussed by Dr. Tavee in her article, are suggested to have an association with these symptoms. Given the chronicity and the frustrating nature of the symptoms, it is no surprise that a panoply of immune serologies are frequently ordered. Invariably, since serologies (eg, ANA, SSA, SSB, rheumatoid factor) are not specific for any single entity, some will return as positive. The strength of many of these associations is weak; even when the clinical diagnosis of lupus, for example, is definite, treatment of the underlying disease does not necessarily improve the dysesthetic pain. In an alternative scenario, the small fiber neuropathy is ascribed to a systemic autoimmune disorder that has been diagnosed because an autoantibody has been detected, but this rarely helps the patient and may in fact worsen symptoms by increasing anxiety and concern over having a systemic disease such as Sjögren syndrome or lupus (both of which sound terrible when reviewed on the Internet).
Some patients describe autonomic symptoms. Given the biologic basis that has been defined for this entity, it is no surprise that some patients have marked symptoms of decreased exocrine gland function, gastrointestinal dysmotility, and orthostasis. These symptoms may not be recognized unless specifically sought out when interviewing the patient.
Given the chronicity and sometimes the vagaries of symptoms, it is often comforting for patients to get an actual diagnosis. Dr. Tavee notes the relative simplicity of diagnostic procedures. But determining the clinical implications of the results may not be straightforward, and devising a fully and uniformly effective therapeutic approach eludes us still. As she points out, a multidisciplinary approach to therapy and diagnosis can be quite helpful.
Postsurgical hypoparathyroidism is not primary hypoparathyroidism
To the Editor: I read with interest the case of a 67-year-old woman with bilateral hand numbness, published in the March 2018 issue of the Journal, and I would like to suggest 2 important corrections to this article.1
The authors present a case of hypocalcemia secondary to postsurgical hypoparathyroidism but describe it as due to primary hypoparathyroidism. The patient had undergone thyroidectomy 10 years earlier and since then had hypocalcemia, secondary to postsurgical hypoparathyroidism, that was treated with calcium and vitamin D, until she stopped taking these agents. Postsurgical hypothyroidism is the most common cause of acquired or secondary hypoparathyroidism and is not primary hypoparathyroidism. I strongly feel that this requires an update or correction to the article. This patient may have associated malabsorption, as the authors alluded to, as the cause of her “normal” serum parathyroid hormone level.
The patient also had hypomagnesemia, which the authors state could have been due to furosemide use and “uncontrolled” diabetes mellitus. Diabetes doesn’t need to be uncontrolled to cause hypomagnesemia. Hypomagnesemia is common in patients with type 2 diabetes mellitus, with a prevalence of 14% to 48% in patients with diabetes compared with 2.5% to 15% in the general population.2 It is often multifactorial and may be secondary to one or more of the following factors: poor dietary intake, autonomic dysfunction, altered insulin resistance, glomerular hyperfiltration, osmotic diuresis (uncontrolled diabetes), recurrent metabolic acidosis, hypophosphatemia, hypokalemia, and therapy with drugs such as metformin and sulfonylureas.
Patients with type 2 diabetes and hypomagnesemia often enter a vicious cycle in which hypomagnesemia worsens insulin resistance and insulin resistance, by reducing the activity of renal magnesium channel transient receptor potential melastatin (TRPM) type 6, perpetuates hypomagnesemia.3
- Radwan SS, Hamo KN, Zayed AA. A 67-year-old woman with bilateral hand numbness. Cleve Clin J Med 2018; 85(3):200–208. doi:10.3949/ccjm.85a.17026
- Pham PC, Pham PM, Pham SV, Miller JM, Pham PT. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol 2007; 2(2):366–373. doi:10.2215/CJN.02960906
- Gommers LM, Hoenderop JG, Bindels RJ, de Baaij JH. Hypomagnesemia in type 2 diabetes: a vicious circle? Diabetes 2016; 65(1):3–13. doi:10.2337/db15-1028
To the Editor: I read with interest the case of a 67-year-old woman with bilateral hand numbness, published in the March 2018 issue of the Journal, and I would like to suggest 2 important corrections to this article.1
The authors present a case of hypocalcemia secondary to postsurgical hypoparathyroidism but describe it as due to primary hypoparathyroidism. The patient had undergone thyroidectomy 10 years earlier and since then had hypocalcemia, secondary to postsurgical hypoparathyroidism, that was treated with calcium and vitamin D, until she stopped taking these agents. Postsurgical hypothyroidism is the most common cause of acquired or secondary hypoparathyroidism and is not primary hypoparathyroidism. I strongly feel that this requires an update or correction to the article. This patient may have associated malabsorption, as the authors alluded to, as the cause of her “normal” serum parathyroid hormone level.
The patient also had hypomagnesemia, which the authors state could have been due to furosemide use and “uncontrolled” diabetes mellitus. Diabetes doesn’t need to be uncontrolled to cause hypomagnesemia. Hypomagnesemia is common in patients with type 2 diabetes mellitus, with a prevalence of 14% to 48% in patients with diabetes compared with 2.5% to 15% in the general population.2 It is often multifactorial and may be secondary to one or more of the following factors: poor dietary intake, autonomic dysfunction, altered insulin resistance, glomerular hyperfiltration, osmotic diuresis (uncontrolled diabetes), recurrent metabolic acidosis, hypophosphatemia, hypokalemia, and therapy with drugs such as metformin and sulfonylureas.
Patients with type 2 diabetes and hypomagnesemia often enter a vicious cycle in which hypomagnesemia worsens insulin resistance and insulin resistance, by reducing the activity of renal magnesium channel transient receptor potential melastatin (TRPM) type 6, perpetuates hypomagnesemia.3
To the Editor: I read with interest the case of a 67-year-old woman with bilateral hand numbness, published in the March 2018 issue of the Journal, and I would like to suggest 2 important corrections to this article.1
The authors present a case of hypocalcemia secondary to postsurgical hypoparathyroidism but describe it as due to primary hypoparathyroidism. The patient had undergone thyroidectomy 10 years earlier and since then had hypocalcemia, secondary to postsurgical hypoparathyroidism, that was treated with calcium and vitamin D, until she stopped taking these agents. Postsurgical hypothyroidism is the most common cause of acquired or secondary hypoparathyroidism and is not primary hypoparathyroidism. I strongly feel that this requires an update or correction to the article. This patient may have associated malabsorption, as the authors alluded to, as the cause of her “normal” serum parathyroid hormone level.
The patient also had hypomagnesemia, which the authors state could have been due to furosemide use and “uncontrolled” diabetes mellitus. Diabetes doesn’t need to be uncontrolled to cause hypomagnesemia. Hypomagnesemia is common in patients with type 2 diabetes mellitus, with a prevalence of 14% to 48% in patients with diabetes compared with 2.5% to 15% in the general population.2 It is often multifactorial and may be secondary to one or more of the following factors: poor dietary intake, autonomic dysfunction, altered insulin resistance, glomerular hyperfiltration, osmotic diuresis (uncontrolled diabetes), recurrent metabolic acidosis, hypophosphatemia, hypokalemia, and therapy with drugs such as metformin and sulfonylureas.
Patients with type 2 diabetes and hypomagnesemia often enter a vicious cycle in which hypomagnesemia worsens insulin resistance and insulin resistance, by reducing the activity of renal magnesium channel transient receptor potential melastatin (TRPM) type 6, perpetuates hypomagnesemia.3
- Radwan SS, Hamo KN, Zayed AA. A 67-year-old woman with bilateral hand numbness. Cleve Clin J Med 2018; 85(3):200–208. doi:10.3949/ccjm.85a.17026
- Pham PC, Pham PM, Pham SV, Miller JM, Pham PT. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol 2007; 2(2):366–373. doi:10.2215/CJN.02960906
- Gommers LM, Hoenderop JG, Bindels RJ, de Baaij JH. Hypomagnesemia in type 2 diabetes: a vicious circle? Diabetes 2016; 65(1):3–13. doi:10.2337/db15-1028
- Radwan SS, Hamo KN, Zayed AA. A 67-year-old woman with bilateral hand numbness. Cleve Clin J Med 2018; 85(3):200–208. doi:10.3949/ccjm.85a.17026
- Pham PC, Pham PM, Pham SV, Miller JM, Pham PT. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol 2007; 2(2):366–373. doi:10.2215/CJN.02960906
- Gommers LM, Hoenderop JG, Bindels RJ, de Baaij JH. Hypomagnesemia in type 2 diabetes: a vicious circle? Diabetes 2016; 65(1):3–13. doi:10.2337/db15-1028
In reply: Postsurgical hypoparathyroidism is not primary hypoparathyroidism
In Reply: We thank Dr. Parmar and appreciate his important comments.
Regarding the difference between primary and secondary hypoparathyroidism, the definition varies among investigators. Some define primary hypoparathyroidism as a condition characterized by primary absence or deficiency of parathyroid hormone (PTH), which results in hypocalcemia and which can be congenital or acquired, including postsurgical hypoparathyroidism.1–4 In principle, this is similar to the classification of disorders affecting other endocrine glands as primary and secondary. For example, primary hypothyroidism refers to a state of low thyroid hormones resulting from impairment or loss of function of the thyroid gland itself, such as in Hashimoto thyroiditis, radioactive iodine therapy, or thyroidectomy, among others.5 We adopted this definition in our article. In contrast, secondary hypoparathyroidism is characterized by low PTH secretion in response to certain conditions that cause hypercalcemia. Non-PTH-mediated hypercalcemia is a more common term used to describe this state of secondary hypoparathyroidism.
Other investigators restrict the term “primary hypoparathyroidism” to nonacquired (congenital or hereditary) etiologies, while applying the term “secondary hypoparathyroidism” to acquired etiologies.6
Concerning the association between diabetes mellitus and hypomagnesemia, we agree that diabetes does not need to be uncontrolled to cause hypomagnesemia. However, the patient described in our article presented with severe hypomagnesemia (serum level 0.6 mg/dL), which is not commonly associated with diabetes. Most cases of hypomagnesemia in patients with type 2 diabetes mellitus are mild and asymptomatic, whereas severe manifestations including seizures, cardiac arrhythmias, and acute tetany are rarely encountered in clinical practice.7 Furthermore, numerous studies have shown a negative correlation between serum magnesium level and glycemic control.7–11 A recent study reported that plasma triglyceride and glucose levels are the main determinants of the plasma magnesium concentration in patients with type 2 diabetes.12
Our patient’s diabetes was uncontrolled, as evidenced by her hemoglobin A1c level of 9.7% and her random serum glucose level of 224 mg/dL. Therefore, it is more likely that “uncontrolled diabetes mellitus” (in addition to diuretic use) was the cause of her symptomatic severe hypomagnesemia rather than controlled diabetes mellitus.
- Mendes EM, Meireles-Brandão L, Meira C, Morais N, Ribeiro C, Guerra D. Primary hypoparathyroidism presenting as basal ganglia calcification secondary to extreme hypocalcemia. Clin Pract 2018; 8(1):1007. doi:10.4081/cp.2018.1007
- Vadiveloo T, Donnan PT, Leese GP. A population-based study of the epidemiology of chronic hypoparathyroidism. J Bone Miner Res 2018; 33(3):478-485. doi:10.1002/jbmr.3329
- Hendy GN, Cole DEC, Bastepe M. Hypoparathyroidism and pseudohypoparathyroidism. In: De Groot LJ, Chrousos G, Dungan K, et al, eds. Endotext [Internet], South Dartmouth (MA): MDText.com, Inc.; 2017. www.ncbi.nlm.nih.gov/books/NBK279165. Accessed August 20, 2018.
- Rosa RG, Barros AJ, de Lima AR, et al. Mood disorder as a manifestation of primary hypoparathyroidism: a case report. J Med Case Rep 2014; 8:326. doi:10.1186/1752-1947-8-326
- Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am 2012; 96(2):203–221. doi:10.1016/j.mcna.2012.01.005
- Fouda UM, Fouda RM, Ammar HM, Salem M, Darouti ME. Impetigo herpetiformis during the puerperium triggered by secondary hypoparathyroidism: a case report. Cases J 2009; 2:9338. doi:10.1186/1757-1626-2-9338
- Tosiello L. Hypomagnesemia and diabetes mellitus. A review of clinical implications. Arch Intern Med 1996; 156(11):1143–1148. pmid: 8639008
- Pham PC, Pham PM, Pham PA, et al. Lower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2. Clin Nephrol 2005; 63(6):429–436. pmid:15960144
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20(1):3–17. doi:10.1177/0885066604271539
- Resnick LM, Altura BT, Gupta RK, Laragh JH, Alderman MH, Altura BM. Intracellular and extracellular magnesium depletion in type 2 (non-insulin-independent) diabetes mellitus. Diabetologia 1993; 36(8):767–770. pmid:8405745
- Pun KK, Ho PW. Subclinical hyponatremia, hyperkalemia and hypomagnesemia in patients with poorly controlled diabetes mellitus. Diabetes Res Clin Pract 1989; 7(3)163–167. pmid: 2605984
- Kurstjens S, de Baaij JH, Bouras H, Bindels RJ, Tack CJ, Hoenderop JG. Determinants of hypomagnesemia in patients with type 2 diabetes mellitus. Eur J Endocrinol 2017; 176(1):11–19. doi:10.1530/EJE-16-0517
In Reply: We thank Dr. Parmar and appreciate his important comments.
Regarding the difference between primary and secondary hypoparathyroidism, the definition varies among investigators. Some define primary hypoparathyroidism as a condition characterized by primary absence or deficiency of parathyroid hormone (PTH), which results in hypocalcemia and which can be congenital or acquired, including postsurgical hypoparathyroidism.1–4 In principle, this is similar to the classification of disorders affecting other endocrine glands as primary and secondary. For example, primary hypothyroidism refers to a state of low thyroid hormones resulting from impairment or loss of function of the thyroid gland itself, such as in Hashimoto thyroiditis, radioactive iodine therapy, or thyroidectomy, among others.5 We adopted this definition in our article. In contrast, secondary hypoparathyroidism is characterized by low PTH secretion in response to certain conditions that cause hypercalcemia. Non-PTH-mediated hypercalcemia is a more common term used to describe this state of secondary hypoparathyroidism.
Other investigators restrict the term “primary hypoparathyroidism” to nonacquired (congenital or hereditary) etiologies, while applying the term “secondary hypoparathyroidism” to acquired etiologies.6
Concerning the association between diabetes mellitus and hypomagnesemia, we agree that diabetes does not need to be uncontrolled to cause hypomagnesemia. However, the patient described in our article presented with severe hypomagnesemia (serum level 0.6 mg/dL), which is not commonly associated with diabetes. Most cases of hypomagnesemia in patients with type 2 diabetes mellitus are mild and asymptomatic, whereas severe manifestations including seizures, cardiac arrhythmias, and acute tetany are rarely encountered in clinical practice.7 Furthermore, numerous studies have shown a negative correlation between serum magnesium level and glycemic control.7–11 A recent study reported that plasma triglyceride and glucose levels are the main determinants of the plasma magnesium concentration in patients with type 2 diabetes.12
Our patient’s diabetes was uncontrolled, as evidenced by her hemoglobin A1c level of 9.7% and her random serum glucose level of 224 mg/dL. Therefore, it is more likely that “uncontrolled diabetes mellitus” (in addition to diuretic use) was the cause of her symptomatic severe hypomagnesemia rather than controlled diabetes mellitus.
In Reply: We thank Dr. Parmar and appreciate his important comments.
Regarding the difference between primary and secondary hypoparathyroidism, the definition varies among investigators. Some define primary hypoparathyroidism as a condition characterized by primary absence or deficiency of parathyroid hormone (PTH), which results in hypocalcemia and which can be congenital or acquired, including postsurgical hypoparathyroidism.1–4 In principle, this is similar to the classification of disorders affecting other endocrine glands as primary and secondary. For example, primary hypothyroidism refers to a state of low thyroid hormones resulting from impairment or loss of function of the thyroid gland itself, such as in Hashimoto thyroiditis, radioactive iodine therapy, or thyroidectomy, among others.5 We adopted this definition in our article. In contrast, secondary hypoparathyroidism is characterized by low PTH secretion in response to certain conditions that cause hypercalcemia. Non-PTH-mediated hypercalcemia is a more common term used to describe this state of secondary hypoparathyroidism.
Other investigators restrict the term “primary hypoparathyroidism” to nonacquired (congenital or hereditary) etiologies, while applying the term “secondary hypoparathyroidism” to acquired etiologies.6
Concerning the association between diabetes mellitus and hypomagnesemia, we agree that diabetes does not need to be uncontrolled to cause hypomagnesemia. However, the patient described in our article presented with severe hypomagnesemia (serum level 0.6 mg/dL), which is not commonly associated with diabetes. Most cases of hypomagnesemia in patients with type 2 diabetes mellitus are mild and asymptomatic, whereas severe manifestations including seizures, cardiac arrhythmias, and acute tetany are rarely encountered in clinical practice.7 Furthermore, numerous studies have shown a negative correlation between serum magnesium level and glycemic control.7–11 A recent study reported that plasma triglyceride and glucose levels are the main determinants of the plasma magnesium concentration in patients with type 2 diabetes.12
Our patient’s diabetes was uncontrolled, as evidenced by her hemoglobin A1c level of 9.7% and her random serum glucose level of 224 mg/dL. Therefore, it is more likely that “uncontrolled diabetes mellitus” (in addition to diuretic use) was the cause of her symptomatic severe hypomagnesemia rather than controlled diabetes mellitus.
- Mendes EM, Meireles-Brandão L, Meira C, Morais N, Ribeiro C, Guerra D. Primary hypoparathyroidism presenting as basal ganglia calcification secondary to extreme hypocalcemia. Clin Pract 2018; 8(1):1007. doi:10.4081/cp.2018.1007
- Vadiveloo T, Donnan PT, Leese GP. A population-based study of the epidemiology of chronic hypoparathyroidism. J Bone Miner Res 2018; 33(3):478-485. doi:10.1002/jbmr.3329
- Hendy GN, Cole DEC, Bastepe M. Hypoparathyroidism and pseudohypoparathyroidism. In: De Groot LJ, Chrousos G, Dungan K, et al, eds. Endotext [Internet], South Dartmouth (MA): MDText.com, Inc.; 2017. www.ncbi.nlm.nih.gov/books/NBK279165. Accessed August 20, 2018.
- Rosa RG, Barros AJ, de Lima AR, et al. Mood disorder as a manifestation of primary hypoparathyroidism: a case report. J Med Case Rep 2014; 8:326. doi:10.1186/1752-1947-8-326
- Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am 2012; 96(2):203–221. doi:10.1016/j.mcna.2012.01.005
- Fouda UM, Fouda RM, Ammar HM, Salem M, Darouti ME. Impetigo herpetiformis during the puerperium triggered by secondary hypoparathyroidism: a case report. Cases J 2009; 2:9338. doi:10.1186/1757-1626-2-9338
- Tosiello L. Hypomagnesemia and diabetes mellitus. A review of clinical implications. Arch Intern Med 1996; 156(11):1143–1148. pmid: 8639008
- Pham PC, Pham PM, Pham PA, et al. Lower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2. Clin Nephrol 2005; 63(6):429–436. pmid:15960144
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20(1):3–17. doi:10.1177/0885066604271539
- Resnick LM, Altura BT, Gupta RK, Laragh JH, Alderman MH, Altura BM. Intracellular and extracellular magnesium depletion in type 2 (non-insulin-independent) diabetes mellitus. Diabetologia 1993; 36(8):767–770. pmid:8405745
- Pun KK, Ho PW. Subclinical hyponatremia, hyperkalemia and hypomagnesemia in patients with poorly controlled diabetes mellitus. Diabetes Res Clin Pract 1989; 7(3)163–167. pmid: 2605984
- Kurstjens S, de Baaij JH, Bouras H, Bindels RJ, Tack CJ, Hoenderop JG. Determinants of hypomagnesemia in patients with type 2 diabetes mellitus. Eur J Endocrinol 2017; 176(1):11–19. doi:10.1530/EJE-16-0517
- Mendes EM, Meireles-Brandão L, Meira C, Morais N, Ribeiro C, Guerra D. Primary hypoparathyroidism presenting as basal ganglia calcification secondary to extreme hypocalcemia. Clin Pract 2018; 8(1):1007. doi:10.4081/cp.2018.1007
- Vadiveloo T, Donnan PT, Leese GP. A population-based study of the epidemiology of chronic hypoparathyroidism. J Bone Miner Res 2018; 33(3):478-485. doi:10.1002/jbmr.3329
- Hendy GN, Cole DEC, Bastepe M. Hypoparathyroidism and pseudohypoparathyroidism. In: De Groot LJ, Chrousos G, Dungan K, et al, eds. Endotext [Internet], South Dartmouth (MA): MDText.com, Inc.; 2017. www.ncbi.nlm.nih.gov/books/NBK279165. Accessed August 20, 2018.
- Rosa RG, Barros AJ, de Lima AR, et al. Mood disorder as a manifestation of primary hypoparathyroidism: a case report. J Med Case Rep 2014; 8:326. doi:10.1186/1752-1947-8-326
- Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am 2012; 96(2):203–221. doi:10.1016/j.mcna.2012.01.005
- Fouda UM, Fouda RM, Ammar HM, Salem M, Darouti ME. Impetigo herpetiformis during the puerperium triggered by secondary hypoparathyroidism: a case report. Cases J 2009; 2:9338. doi:10.1186/1757-1626-2-9338
- Tosiello L. Hypomagnesemia and diabetes mellitus. A review of clinical implications. Arch Intern Med 1996; 156(11):1143–1148. pmid: 8639008
- Pham PC, Pham PM, Pham PA, et al. Lower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2. Clin Nephrol 2005; 63(6):429–436. pmid:15960144
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20(1):3–17. doi:10.1177/0885066604271539
- Resnick LM, Altura BT, Gupta RK, Laragh JH, Alderman MH, Altura BM. Intracellular and extracellular magnesium depletion in type 2 (non-insulin-independent) diabetes mellitus. Diabetologia 1993; 36(8):767–770. pmid:8405745
- Pun KK, Ho PW. Subclinical hyponatremia, hyperkalemia and hypomagnesemia in patients with poorly controlled diabetes mellitus. Diabetes Res Clin Pract 1989; 7(3)163–167. pmid: 2605984
- Kurstjens S, de Baaij JH, Bouras H, Bindels RJ, Tack CJ, Hoenderop JG. Determinants of hypomagnesemia in patients with type 2 diabetes mellitus. Eur J Endocrinol 2017; 176(1):11–19. doi:10.1530/EJE-16-0517
Swings in four metabolic measures predicted death in healthy people
, based on data from a 5.5-year population-based study in Korea.
In a model adjusted for age, sex, smoking, alcohol consumption, regular exercise, and income status the group with high variability for all four parameters had a significantly higher risk for all-cause mortality (hazard ratio, 2.27; 95% confidence interval, 2.13-2.42), for MI (HR, 1.43; 95% CI, 1.25-1.64), and for stroke (HR, 1.41; 95% CI, 1.25-1.60), compared with the group with low variability for all four parameters. The association with risk was graded and persisted after multivariable adjustment.
“Variability in metabolic parameters may be prognostic surrogate markers for predicting mortality and cardiovascular outcomes,” wrote senior author Seung-Hwan Lee, MD, PhD, and professor of endocrinology at the College of Medicine of the Catholic University of Korea in Seoul, South Korea, and colleagues. “High variability in metabolic parameters (may be) associated with adverse health outcomes not only in a diseased population, but also in the relatively healthy population although the mechanism could be somewhat different.”
Korea has a single-payer system, the Korean National Health Insurance system, that includes health information on its entire population. The researchers selected data from 6,748,773 people who were free of diabetes mellitus, hypertension, and dyslipidemia, and who underwent three or more health examinations during 2005-2012 that documented body mass index (BMI), fasting blood glucose, systolic blood pressure, and total cholesterol. Participants were followed to the end of 2015, for a median follow-up of 5.5 years. There were 54,785 deaths (0.8%), 22,498 cases of stroke (0.3%), and 21,452 MIs (0.3%).
The research team defined high variability as the highest quartile, classifying participants according to the number of high-variability parameters. A score of 4 indicated high variability in all four metabolic parameters – body weight, systolic blood pressure, total cholesterol, and fasting blood glucose.
In the highest quartile in fasting blood glucose variability, compared with the lowest quartile, the risk of all-cause mortality increased by 20% (HR, 1.20; 95% CI, 1.18-1.23), MI by 16% (HR, 1.16; 95% CI, 1.12-1.21), and stroke by 13% (HR, 1.13; 95% CI, 1.09-1.17).
For the highest quartile in total cholesterol variability, compared with the lowest quartile, the risk of all-cause mortality increased by 31% (HR, 1.31; 95% CI, 1.28-1.34), MI by 10% (HR, 1.10; 95% CI, 1.06-1.14), and stroke by 6% (HR, 1.06; 95% CI, 1.03-1.10).
For the highest quartile in systolic BP variability, compared with the lowest quartile, the risk of all-cause mortality increased by 19% (HR, 1.19; 95% CI, 1.16-1.22), MI by 7% (HR, 1.07; 95% CI, 1.03-1.11), and stroke by 14% (HR, 1.14; 95% CI, 1.10-1.18).
For the highest quartile in BMI variability, compared with the lowest quartile, the risk of all-cause mortality increased by 53% (HR, 1.53; 95% CI, 1.50-1.57), MI by 14% (HR, 1.14; 95% CI, 1.09-1.18), and stroke by 14% (HR, 1.14; 95% CI, 1.10-1.18).
“It is not certain whether these results from Korea would apply to the United States. However, several previous studies on variability were performed in other populations, suggesting that it is likely to be a common phenomenon,” the authors wrote.
The study was supported in part by the National Research Foundation of Korea Grant funded by the Korean Government. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
SOURCE: Lee S-H et al. Circulation. 2018 Oct.
, based on data from a 5.5-year population-based study in Korea.
In a model adjusted for age, sex, smoking, alcohol consumption, regular exercise, and income status the group with high variability for all four parameters had a significantly higher risk for all-cause mortality (hazard ratio, 2.27; 95% confidence interval, 2.13-2.42), for MI (HR, 1.43; 95% CI, 1.25-1.64), and for stroke (HR, 1.41; 95% CI, 1.25-1.60), compared with the group with low variability for all four parameters. The association with risk was graded and persisted after multivariable adjustment.
“Variability in metabolic parameters may be prognostic surrogate markers for predicting mortality and cardiovascular outcomes,” wrote senior author Seung-Hwan Lee, MD, PhD, and professor of endocrinology at the College of Medicine of the Catholic University of Korea in Seoul, South Korea, and colleagues. “High variability in metabolic parameters (may be) associated with adverse health outcomes not only in a diseased population, but also in the relatively healthy population although the mechanism could be somewhat different.”
Korea has a single-payer system, the Korean National Health Insurance system, that includes health information on its entire population. The researchers selected data from 6,748,773 people who were free of diabetes mellitus, hypertension, and dyslipidemia, and who underwent three or more health examinations during 2005-2012 that documented body mass index (BMI), fasting blood glucose, systolic blood pressure, and total cholesterol. Participants were followed to the end of 2015, for a median follow-up of 5.5 years. There were 54,785 deaths (0.8%), 22,498 cases of stroke (0.3%), and 21,452 MIs (0.3%).
The research team defined high variability as the highest quartile, classifying participants according to the number of high-variability parameters. A score of 4 indicated high variability in all four metabolic parameters – body weight, systolic blood pressure, total cholesterol, and fasting blood glucose.
In the highest quartile in fasting blood glucose variability, compared with the lowest quartile, the risk of all-cause mortality increased by 20% (HR, 1.20; 95% CI, 1.18-1.23), MI by 16% (HR, 1.16; 95% CI, 1.12-1.21), and stroke by 13% (HR, 1.13; 95% CI, 1.09-1.17).
For the highest quartile in total cholesterol variability, compared with the lowest quartile, the risk of all-cause mortality increased by 31% (HR, 1.31; 95% CI, 1.28-1.34), MI by 10% (HR, 1.10; 95% CI, 1.06-1.14), and stroke by 6% (HR, 1.06; 95% CI, 1.03-1.10).
For the highest quartile in systolic BP variability, compared with the lowest quartile, the risk of all-cause mortality increased by 19% (HR, 1.19; 95% CI, 1.16-1.22), MI by 7% (HR, 1.07; 95% CI, 1.03-1.11), and stroke by 14% (HR, 1.14; 95% CI, 1.10-1.18).
For the highest quartile in BMI variability, compared with the lowest quartile, the risk of all-cause mortality increased by 53% (HR, 1.53; 95% CI, 1.50-1.57), MI by 14% (HR, 1.14; 95% CI, 1.09-1.18), and stroke by 14% (HR, 1.14; 95% CI, 1.10-1.18).
“It is not certain whether these results from Korea would apply to the United States. However, several previous studies on variability were performed in other populations, suggesting that it is likely to be a common phenomenon,” the authors wrote.
The study was supported in part by the National Research Foundation of Korea Grant funded by the Korean Government. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
SOURCE: Lee S-H et al. Circulation. 2018 Oct.
, based on data from a 5.5-year population-based study in Korea.
In a model adjusted for age, sex, smoking, alcohol consumption, regular exercise, and income status the group with high variability for all four parameters had a significantly higher risk for all-cause mortality (hazard ratio, 2.27; 95% confidence interval, 2.13-2.42), for MI (HR, 1.43; 95% CI, 1.25-1.64), and for stroke (HR, 1.41; 95% CI, 1.25-1.60), compared with the group with low variability for all four parameters. The association with risk was graded and persisted after multivariable adjustment.
“Variability in metabolic parameters may be prognostic surrogate markers for predicting mortality and cardiovascular outcomes,” wrote senior author Seung-Hwan Lee, MD, PhD, and professor of endocrinology at the College of Medicine of the Catholic University of Korea in Seoul, South Korea, and colleagues. “High variability in metabolic parameters (may be) associated with adverse health outcomes not only in a diseased population, but also in the relatively healthy population although the mechanism could be somewhat different.”
Korea has a single-payer system, the Korean National Health Insurance system, that includes health information on its entire population. The researchers selected data from 6,748,773 people who were free of diabetes mellitus, hypertension, and dyslipidemia, and who underwent three or more health examinations during 2005-2012 that documented body mass index (BMI), fasting blood glucose, systolic blood pressure, and total cholesterol. Participants were followed to the end of 2015, for a median follow-up of 5.5 years. There were 54,785 deaths (0.8%), 22,498 cases of stroke (0.3%), and 21,452 MIs (0.3%).
The research team defined high variability as the highest quartile, classifying participants according to the number of high-variability parameters. A score of 4 indicated high variability in all four metabolic parameters – body weight, systolic blood pressure, total cholesterol, and fasting blood glucose.
In the highest quartile in fasting blood glucose variability, compared with the lowest quartile, the risk of all-cause mortality increased by 20% (HR, 1.20; 95% CI, 1.18-1.23), MI by 16% (HR, 1.16; 95% CI, 1.12-1.21), and stroke by 13% (HR, 1.13; 95% CI, 1.09-1.17).
For the highest quartile in total cholesterol variability, compared with the lowest quartile, the risk of all-cause mortality increased by 31% (HR, 1.31; 95% CI, 1.28-1.34), MI by 10% (HR, 1.10; 95% CI, 1.06-1.14), and stroke by 6% (HR, 1.06; 95% CI, 1.03-1.10).
For the highest quartile in systolic BP variability, compared with the lowest quartile, the risk of all-cause mortality increased by 19% (HR, 1.19; 95% CI, 1.16-1.22), MI by 7% (HR, 1.07; 95% CI, 1.03-1.11), and stroke by 14% (HR, 1.14; 95% CI, 1.10-1.18).
For the highest quartile in BMI variability, compared with the lowest quartile, the risk of all-cause mortality increased by 53% (HR, 1.53; 95% CI, 1.50-1.57), MI by 14% (HR, 1.14; 95% CI, 1.09-1.18), and stroke by 14% (HR, 1.14; 95% CI, 1.10-1.18).
“It is not certain whether these results from Korea would apply to the United States. However, several previous studies on variability were performed in other populations, suggesting that it is likely to be a common phenomenon,” the authors wrote.
The study was supported in part by the National Research Foundation of Korea Grant funded by the Korean Government. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
SOURCE: Lee S-H et al. Circulation. 2018 Oct.
FROM CIRCULATION
Key clinical point: Fluctuations in fasting glucose and cholesterol levels, systolic blood pressure, and body mass index are associated with a higher risk for all-cause mortality, myocardial infarction, and stroke in otherwise healthy people.
Major finding: The hazard ratios were 2.27 (95% CI, 2.13-2.42) for all-cause mortality, 1.43 (95% CI, 1.25-1.64) for MI, and 1.41 (95% CI, 1.25-1.60) for stroke.
Study details: An observational population-based study involving more than 6.7 million Koreans age 20 and older.
Disclosures: The study was funded by the National Research Foundation of Korea. The authors had no relevant conflicts of interest to declare.
Source: Lee S-H et al. Circulation. 2018 Oct.
Online diabetes prevention programs as good as face-to-face programs
, researchers report.
Writing in background information to their paper, Tannaz Moin, MD, an endocrinologist at the VA Greater Los Angeles Healthcare System and the Veterans Affairs’ Health Services Research and Development Center for the Study of Healthcare Innovation, Implementation, and Policy, and her associates, said intensive lifestyle interventions such as diabetes prevention programs (DPP) could lower the risk of incident diabetes by 58%, but a lack of reach significantly attenuated their population impact in real-world settings.
“Building evidence for online DPP is important because of its potential for increasing reach because most U.S. adults (87%) use the Internet,” they wrote in their paper, published in the American Journal of Preventive Medicine.
They therefore set out to compare weight loss results from 114 veterans taking part in the Veterans Administration’s face-to-face standard-of-care weight management program MOVE! with an online program involving 268 obese or overweight veterans with prediabetes and 273 people taking part in an in-person program.
MOVE! included 8-12 face-to-face healthy-lifestyle sessions and monthly maintenance sessions but with no specified goals. The online program involved virtual groups of participants: live e-coaches who monitored group interactions and provided the participants with feedback via phone and private online messages; weekly educational modules on healthy eating and exercise; and wireless scales to record participant weights.
The in-person program consisted of 8-22 group-based face-to-face sessions focused on 7% weight loss and at least 150 minutes per session of moderate physical activity.
Weight loss, considered by the authors to be a significant predictor of diabetes risk reduction, was recorded at 6 months and then again at 12 months in all three interventions.
An analysis of 242 participants enrolled in the intensive, multifaceted online DPP intervention (26 were excluded because they did not have more than two available weights) revealed a significant weight change of –4.7 kg at 6 months and –4 kg at 12 months’ follow-up. On average, these participants lost 3.7% of their baseline weight at 12 months.
At both times weight change (kg and percentage) was not significantly different between the online intervention and those taking part in the in-person DPP (–4.8 and –4.1 kg for online vs –4 kg and –3.9 kg in-person for those completing more than one module/session). Both groups also had higher weight loss (percentage and kg) at 6 and 12 months compared with MOVE! participants (–1.1kg and 0.10 kg).
The research team noted that the online program had better participation than did the in-person program, with 87% of online participants completing eight or more sessions, compared with 59% for the in-person program and 55% for MOVE!
They suggested this was because the online program had several user-friendly features that increased the frequency of potential “touches” participants received over time.
“Future studies examining how inline DPP intervention components can work together to impact participation and engagement are key,” they said.
“This is one of the first studies to report weight outcomes irrespective of the level of engagement with an online DPP intervention and to examine outcomes compared with in person DPP. Overall, these findings may have important implications for national efforts to disseminate DPP,” they concluded.
The authors conceded that the generalizability of their study was limited as it included veterans receiving care in the VHA.
SOURCE: Am J Prev Med. 2018 Sep 24. doi: 10.1016/j.amepre.2018.06.028
, researchers report.
Writing in background information to their paper, Tannaz Moin, MD, an endocrinologist at the VA Greater Los Angeles Healthcare System and the Veterans Affairs’ Health Services Research and Development Center for the Study of Healthcare Innovation, Implementation, and Policy, and her associates, said intensive lifestyle interventions such as diabetes prevention programs (DPP) could lower the risk of incident diabetes by 58%, but a lack of reach significantly attenuated their population impact in real-world settings.
“Building evidence for online DPP is important because of its potential for increasing reach because most U.S. adults (87%) use the Internet,” they wrote in their paper, published in the American Journal of Preventive Medicine.
They therefore set out to compare weight loss results from 114 veterans taking part in the Veterans Administration’s face-to-face standard-of-care weight management program MOVE! with an online program involving 268 obese or overweight veterans with prediabetes and 273 people taking part in an in-person program.
MOVE! included 8-12 face-to-face healthy-lifestyle sessions and monthly maintenance sessions but with no specified goals. The online program involved virtual groups of participants: live e-coaches who monitored group interactions and provided the participants with feedback via phone and private online messages; weekly educational modules on healthy eating and exercise; and wireless scales to record participant weights.
The in-person program consisted of 8-22 group-based face-to-face sessions focused on 7% weight loss and at least 150 minutes per session of moderate physical activity.
Weight loss, considered by the authors to be a significant predictor of diabetes risk reduction, was recorded at 6 months and then again at 12 months in all three interventions.
An analysis of 242 participants enrolled in the intensive, multifaceted online DPP intervention (26 were excluded because they did not have more than two available weights) revealed a significant weight change of –4.7 kg at 6 months and –4 kg at 12 months’ follow-up. On average, these participants lost 3.7% of their baseline weight at 12 months.
At both times weight change (kg and percentage) was not significantly different between the online intervention and those taking part in the in-person DPP (–4.8 and –4.1 kg for online vs –4 kg and –3.9 kg in-person for those completing more than one module/session). Both groups also had higher weight loss (percentage and kg) at 6 and 12 months compared with MOVE! participants (–1.1kg and 0.10 kg).
The research team noted that the online program had better participation than did the in-person program, with 87% of online participants completing eight or more sessions, compared with 59% for the in-person program and 55% for MOVE!
They suggested this was because the online program had several user-friendly features that increased the frequency of potential “touches” participants received over time.
“Future studies examining how inline DPP intervention components can work together to impact participation and engagement are key,” they said.
“This is one of the first studies to report weight outcomes irrespective of the level of engagement with an online DPP intervention and to examine outcomes compared with in person DPP. Overall, these findings may have important implications for national efforts to disseminate DPP,” they concluded.
The authors conceded that the generalizability of their study was limited as it included veterans receiving care in the VHA.
SOURCE: Am J Prev Med. 2018 Sep 24. doi: 10.1016/j.amepre.2018.06.028
, researchers report.
Writing in background information to their paper, Tannaz Moin, MD, an endocrinologist at the VA Greater Los Angeles Healthcare System and the Veterans Affairs’ Health Services Research and Development Center for the Study of Healthcare Innovation, Implementation, and Policy, and her associates, said intensive lifestyle interventions such as diabetes prevention programs (DPP) could lower the risk of incident diabetes by 58%, but a lack of reach significantly attenuated their population impact in real-world settings.
“Building evidence for online DPP is important because of its potential for increasing reach because most U.S. adults (87%) use the Internet,” they wrote in their paper, published in the American Journal of Preventive Medicine.
They therefore set out to compare weight loss results from 114 veterans taking part in the Veterans Administration’s face-to-face standard-of-care weight management program MOVE! with an online program involving 268 obese or overweight veterans with prediabetes and 273 people taking part in an in-person program.
MOVE! included 8-12 face-to-face healthy-lifestyle sessions and monthly maintenance sessions but with no specified goals. The online program involved virtual groups of participants: live e-coaches who monitored group interactions and provided the participants with feedback via phone and private online messages; weekly educational modules on healthy eating and exercise; and wireless scales to record participant weights.
The in-person program consisted of 8-22 group-based face-to-face sessions focused on 7% weight loss and at least 150 minutes per session of moderate physical activity.
Weight loss, considered by the authors to be a significant predictor of diabetes risk reduction, was recorded at 6 months and then again at 12 months in all three interventions.
An analysis of 242 participants enrolled in the intensive, multifaceted online DPP intervention (26 were excluded because they did not have more than two available weights) revealed a significant weight change of –4.7 kg at 6 months and –4 kg at 12 months’ follow-up. On average, these participants lost 3.7% of their baseline weight at 12 months.
At both times weight change (kg and percentage) was not significantly different between the online intervention and those taking part in the in-person DPP (–4.8 and –4.1 kg for online vs –4 kg and –3.9 kg in-person for those completing more than one module/session). Both groups also had higher weight loss (percentage and kg) at 6 and 12 months compared with MOVE! participants (–1.1kg and 0.10 kg).
The research team noted that the online program had better participation than did the in-person program, with 87% of online participants completing eight or more sessions, compared with 59% for the in-person program and 55% for MOVE!
They suggested this was because the online program had several user-friendly features that increased the frequency of potential “touches” participants received over time.
“Future studies examining how inline DPP intervention components can work together to impact participation and engagement are key,” they said.
“This is one of the first studies to report weight outcomes irrespective of the level of engagement with an online DPP intervention and to examine outcomes compared with in person DPP. Overall, these findings may have important implications for national efforts to disseminate DPP,” they concluded.
The authors conceded that the generalizability of their study was limited as it included veterans receiving care in the VHA.
SOURCE: Am J Prev Med. 2018 Sep 24. doi: 10.1016/j.amepre.2018.06.028
FROM AMERICAN JOURNAL OF PREVENTIVE MEDICINE
Key clinical point: Online diabetes prevention programs (DPP) are as effective as in-person programs in terms of weight loss, and they have a wider reach.
Major finding: Participants enrolled in an intensive, multifaceted online DPP intervention had significant weight change of −4.7 kg at 6 months and −4.0 kg at 12-month follow-up, similar to that of participants enrolled in a face-to face program.
Study details: A large nonrandomized trial and a comparative analysis of individuals from a concurrent trial of two parallel in-person programs.
Disclosures: The Department of Veteran Affairs funded the study. One author reported co-owning shares in Amgen, and another reported receiving personal fees from two pharmaceutical companies.
Source: Am J Prev Med. 2018 Sep 24. doi: 10.1016/j.amepre.2018.06.028.
Fish oil phoenix
A 4 g/day dose of the triglyceride-reducing drug Vascepa (Amarin), compared to placebo, was associated with a 25% lower risk of a heart attack, a stroke, an intervention for arterial thrombosis, or chest pain requiring a hospitalization in a study of 8,179 people who had high triglycerides and previous cardiovascular disorders or diabetes and another risk factor for heart disease.
In the ASCEND study, presented at the annual congress of the European Society of Cardiology, 1 g/day of omega-3 fatty acids showed no net cardiovascular benefits in people with diabetes and no known cardiovascular disease.
The results of the Amarin study, announced in a press release, are slated for presentation at the American Heart Association scientific sessions in November.
A 4 g/day dose of the triglyceride-reducing drug Vascepa (Amarin), compared to placebo, was associated with a 25% lower risk of a heart attack, a stroke, an intervention for arterial thrombosis, or chest pain requiring a hospitalization in a study of 8,179 people who had high triglycerides and previous cardiovascular disorders or diabetes and another risk factor for heart disease.
In the ASCEND study, presented at the annual congress of the European Society of Cardiology, 1 g/day of omega-3 fatty acids showed no net cardiovascular benefits in people with diabetes and no known cardiovascular disease.
The results of the Amarin study, announced in a press release, are slated for presentation at the American Heart Association scientific sessions in November.
A 4 g/day dose of the triglyceride-reducing drug Vascepa (Amarin), compared to placebo, was associated with a 25% lower risk of a heart attack, a stroke, an intervention for arterial thrombosis, or chest pain requiring a hospitalization in a study of 8,179 people who had high triglycerides and previous cardiovascular disorders or diabetes and another risk factor for heart disease.
In the ASCEND study, presented at the annual congress of the European Society of Cardiology, 1 g/day of omega-3 fatty acids showed no net cardiovascular benefits in people with diabetes and no known cardiovascular disease.
The results of the Amarin study, announced in a press release, are slated for presentation at the American Heart Association scientific sessions in November.
Dapagliflozin meets endpoint in top-line DECLARE results, AstraZeneca says
AstraZeneca announced top-line results of its phase III DECLARE-TIMI 58 cardiovascular outcomes trial for dapagliflozin (Farxiga). The 5-year international trial evaluated the cardiovascular outcomes of dapagliflozin compared with placebo in more than 17,000 adults with type 2 diabetes at high cardiovascular risk or established disease.
DECLARE met its primary safety endpoint of noninferiority for major adverse cardiovascular events for dabigatran the company said. Specifically, it achieved a statistically significant reduction in the composite endpoint of hospitalization for heart failure or cardiovascular death.
Dapagliflozin, approved in 2014, is a sodium-glucose cotransporter 2 (SGLT2) inhibitor indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.
Full results of DECLARE will be presented on November 10 at the American Heart Association annual meeting, according to the AstraZeneca release.
AstraZeneca announced top-line results of its phase III DECLARE-TIMI 58 cardiovascular outcomes trial for dapagliflozin (Farxiga). The 5-year international trial evaluated the cardiovascular outcomes of dapagliflozin compared with placebo in more than 17,000 adults with type 2 diabetes at high cardiovascular risk or established disease.
DECLARE met its primary safety endpoint of noninferiority for major adverse cardiovascular events for dabigatran the company said. Specifically, it achieved a statistically significant reduction in the composite endpoint of hospitalization for heart failure or cardiovascular death.
Dapagliflozin, approved in 2014, is a sodium-glucose cotransporter 2 (SGLT2) inhibitor indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.
Full results of DECLARE will be presented on November 10 at the American Heart Association annual meeting, according to the AstraZeneca release.
AstraZeneca announced top-line results of its phase III DECLARE-TIMI 58 cardiovascular outcomes trial for dapagliflozin (Farxiga). The 5-year international trial evaluated the cardiovascular outcomes of dapagliflozin compared with placebo in more than 17,000 adults with type 2 diabetes at high cardiovascular risk or established disease.
DECLARE met its primary safety endpoint of noninferiority for major adverse cardiovascular events for dabigatran the company said. Specifically, it achieved a statistically significant reduction in the composite endpoint of hospitalization for heart failure or cardiovascular death.
Dapagliflozin, approved in 2014, is a sodium-glucose cotransporter 2 (SGLT2) inhibitor indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.
Full results of DECLARE will be presented on November 10 at the American Heart Association annual meeting, according to the AstraZeneca release.