User login
Nocturnal Dexmedetomidine for Prevention of Delirium in the ICU
Study Overview
Objective. To determine if nocturnal dexmedetomidine prevents delirium and improves sleep in critically ill patients.
Design. Two-center, double-blind, placebo-controlled, randomized, trial.
Setting and participants. This study was conducted in the intensive care units (ICU) at 2 centers in North America between 2013 and 2016. Adults admitted to the ICU and receiving intermittent or continuous sedatives and expected to require at least 48 hours of ICU care were included in the study. Exclusion criteria were presence of delirium, severe dementia, acute neurologic injury, severe bradycardia, hepatic encephalopathy, end-stage liver disease, and expected death within 24 hours.
Intervention. Patients were randomized 1:1 to receive nocturnal dexmedetomidine (0.2–0.7 mcg/kg/hr) or dextrose 5% in water. Patients, clinicians, bedside nurses, and all study personnel were blinded to study drug assignment throughout the study. All sedatives were halved before the study drug was administered each evening. As-needed intravenous midazolam was used while titrating up the study drug. Study drug was administered nightly until either ICU discharge or an adverse event occurred. Decisions regarding use of other analgesic and sedative therapy, including opioids, oral benzodiazepines, acetaminophen, and nonsteroidal anti-inflammatory drugs, were left to the discretion of the clinician. Sleep-promoting agents such as melatonin or trazodone were not allowed.
Main outcome measures. The primary outcome was the proportion of patients who remained free of delirium during their critical illness. Secondary outcomes included ICU days spent without delirium; duration of delirium; sleep quality; proportion of patients who ever developed coma; proportion of nocturnal hours spent at each Richmond Agitation and Sedation Scale (RASS) score; maximal nocturnal pain levels; antipsychotic, corticosteroid, and oral analgesic use; days of mechanical ventilation; ICU and hospital stay duration; and ICU and hospital mortality.
Main results. 100 patients were randomized, with 50 patients in each group. 89% of patients were mechanically ventilated, and the Prediction of Delirium in ICU (PRE-DELIRIC) score [1] was 54 in the dexmedetomidine group and 51 in the placebo group. Continuous propofol and fentanyl infusion at randomization was used in 49% and 80%, respectively. Duration of median ICU stay was 10 days in the dexmedetomidine group and 9 days in the placebo group. More patients in the dexmedetomidine group (40 of 50 patients [80%]) than in the placebo group (27 of 50 patients [54%]) remained free of delirium (relative risk [RR], 0.44, 95% confidence interval {CI} 0.23 to 0.82; P = 0.006). The median (interquartile range [IQR]) duration of the first episode of delirium was similar between the dexmedetomidine (IQR 2.0 [0.6–2.7] days) and placebo (2.2 [0.7–3.2] days) groups (P = 0.73). The average Leeds Sleep Evaluation Questionnaire score also was similar (mean difference, 0.02, 95% CI 0.42 to 1.92) between the 2 groups. Incidence of hypotension or bradycardia did not differ significantly between the groups.
Conclusion. Nocturnal administration of low-dose dexmedetomidine in critically ill adults reduces the incidence of delirium during the ICU stay, and patient-reported sleep quality appears unchanged.
Commentary
Delirium is a sudden state of confusion and/or disturbance of consciousness and cognition that is believed to result from acute brain dysfunction, including neurochemical disequilibrium. It often occurs in association with a general medical condition, such as various types of shock, sepsis, surgery, anesthesia, or electrolyte imbalance. Studies have shown that delirium is associated with increased mortality in critically ill patients [2]. Most ICUs use a systematic assessment tool for early detection of delirium, such as the Confusion Assessment Method for the ICU (CAM-ICU), the Intensive Care Delirium Screening Checklist (ICDSC), or the DSM-IV TR score system. The CAM-ICU is the most frequently used tool to evaluate for the presence of delirium in critically ill patients; it is scored as positive if the patient manifests both an acute change in mental status and inattention, and has either a RASS greater than 0 or disorganized thinking [3].
The level of evidence regarding delirium prevention is low. Ear plugs, eye masks, educational staff, supportive reorientation, and music have been studied as nonpharmacologic methods for preventing delirium [4]. From a pharmacologic standpoint, the dopamine D2 antagonist haloperidol has been explored as a therapy for both treating and preventing delirium, since the condition is thought to be associated with anticholinergic and excessive dopaminergic mechanisms. A randomized controlled study in 142 patients who received haloperidol 2.5 mg intravenously every 8 hours found that the duration of delirium did not differ between the haloperidol and the placebo groups [5]. The most feared adverse effects of haloperidol, such as akathisia, muscle stiffness, arrhythmia, or QT prolongation, did not occur more frequently in the haloperidol group. Similar results have been reported by Al-Qadheeb et al [6]. Pharmacologic prophylaxis of delirium using atypical antipsychotics such as quetiapine has also been explored, but the level of evidence for this intervention remains very low. Current American College of Critical Care Medicine guidelines recommend nonpharmacologic management and do not firmly recommend any pharmacologic prevention for ICU delirium [7].
Dexmedetomidine is a selective alpha-2 adrenergic receptor agonist that acts at the locus ceruleus, providing sedation and analgesia. Studies assessing the choice of sedation in the ICU found that the use of dexmedetomidine or propofol, compared to benzodiazepines, is associated with a lower rate of delirium occurrence, especially in mechanically ventilated patients [8,9]. Dexmedetomidine offers several potential advantages over other sedative drugs: it has little effect on cognition, has minimal anticholinergic effect, and may restore a natural sleep pattern. While propofol causes hypotension, respiratory depression, and deeper sedation, dexmedetomidine is associated with lighter sedation, a minimal effect on respiratory drive, and a milder hemodynamic effect. In a randomized controlled trial involving post-surgery ICU patients, dexmedetomidine partially restored a normal sleep pattern (eg, increased percentage of stage 2 non-rapid eye movement sleep), prolonged total sleep time, improved sleep efficiency, and increased sleep quality [10]; by improving overall sleep quality, dexmedetomidine potentially may prevent delirium. Another study that randomly assigned 700 ICU patients who underwent noncardiac surgery to dexmedetomidine infusion (0.1 mcg/kg/hr from ICU admission on the day of surgery until the following morning) or placebo reported a significantly reduced incidence of delirium in the dexmedetomidine group [11]. On the other hand, a 2015 Cochrane meta-analysis that included 7 randomized controlled studies did not find a significant risk reduction of delirium with dexmedetomidine [12].
The current study by Skrobik et al was a randomized, placebo-controlled trial that examined the role of nocturnal dexmedetomidine in ICU delirium prevention in 100 ICU patients. Nocturnal administration of low-dose dexmedetomidine led to a statistically significant reduction in delirium incidence compared to placebo (RR of delirium, 0.44, 95% CI 0.23 to 0.82, which is similar to that suggested by previous studies). This study adds additional evidence regarding the use of dexmedetomidine for pharmacologic delirium prevention. It included many mechanically ventilated patients (89% of study population), strengthening the applicability of the result. Mechanical ventilation is a known risk factor for ICU delirium, and therefore this is an important population to study; previous trials largely included patients who were not mechanically ventilated. This study also supports the safety of dexmedetomidine infusion, especially in lower doses in critically ill patients, without significantly increasing the incidence of adverse events (mainly hypotension and bradycardia). The study protocol closely approximated real practice by allowing other analgesics, including opioids, and therefore suggests safety and real world applicability.
There are several confounding issues in this study. The study was blinded, and there was concern that the bedside nurses may have been able to identify the study drug based on the effects on heart rate. In addition, 50% of patients received antipsychotics. While baseline RASS score was significantly different between the 2 groups, patients in the dexmedetomidine group reached a deeper level of sedation during the study. Also, the protocol mandated halving the pre-existing sedative on the night of study drug initiation, which could have led to inadequate sedation in the placebo group. Placebo patients received propofol for a similar duration but at a higher dose compared to dexmedetomidine patients, and midazolam and fentanyl infusion was used in a similar pattern between the groups. The high exclusion rate (71%) limits the ability to generalize the results to all ICU patients.
Applications for Clinical Practice
ICU delirium is an important complication of critical illness and is potentially preventable. Benzodiazepines are associated with an increased risk of delirium, while there has been increasing interest in dexmedetomidine, a selective alpha-2 adrenergic receptor agonist, because of its potential for delirium prevention. Evidence to date does not strongly support routine use of pharmacologic prevention of delirium; however, dexmedetomidine may be an option for sedation, as opposed to benzodiazepines or propofol, in selected patients and may potentially prevent delirium.
—Minkyung Kwon, MD, Neal Patel, MD, and Vichaya Arunthari, MD, Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL
1. van den Boogaard M, Pickkers P, Slooter AJ, et al. Development and validation of PRE-DELIRIC (PREdiction of DELIRium in ICu patients) delirium prediction model for intensive care patients: observational multicentre study. BMJ 2012;344:e420.
2. Slooter AJ, Van De Leur RR, Zaal IJ. Delirium in critically ill patients. Handb Clin Neurol 2017;141:449–66.
3. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001;286:2703–10.
4. Abraha I, Trotta F, Rimland JM, et al. Efficacy of non-pharmacological interventions to prevent and treat delirium in older patients: a systematic overview. The SENATOR project ONTOP Series. PLoS One 2015;10:e0123090.
5. Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2013;1:515–23.
6. Al-Qadheeb NS, Skrobik Y, Schumaker G, et al. Preventing ICU subsyndromal delirium conversion to delirium with low-dose IV haloperidol: a double-blind, placebo-controlled pilot study. Crit Care Med 2016;44:583–91.
7. Barr J, Fraser GL, Puntillo K, et al; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263–306.
8. Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009;301:489–99.
9. Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007;298:2644–53.
10. Wu XH, Cui F, Zhang C, et al. Low-dose dexmedetomidine improves sleep quality pattern in elderly patients after noncardiac surgery in the intensive care unit: a pilot randomized controlled trial. Anesthesiology 2016;125:979–91.
11. Su X, Meng Z-T, Wu X-H, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 2016;388:1893–1902.
12. Chen K, Lu Z, Xin YC, et al. Alpha-2 agonists for long-term sedation during mechanical ventilation in critically ill patients. Cochrane Database Syst Rev 2015;1:CD010269.
Study Overview
Objective. To determine if nocturnal dexmedetomidine prevents delirium and improves sleep in critically ill patients.
Design. Two-center, double-blind, placebo-controlled, randomized, trial.
Setting and participants. This study was conducted in the intensive care units (ICU) at 2 centers in North America between 2013 and 2016. Adults admitted to the ICU and receiving intermittent or continuous sedatives and expected to require at least 48 hours of ICU care were included in the study. Exclusion criteria were presence of delirium, severe dementia, acute neurologic injury, severe bradycardia, hepatic encephalopathy, end-stage liver disease, and expected death within 24 hours.
Intervention. Patients were randomized 1:1 to receive nocturnal dexmedetomidine (0.2–0.7 mcg/kg/hr) or dextrose 5% in water. Patients, clinicians, bedside nurses, and all study personnel were blinded to study drug assignment throughout the study. All sedatives were halved before the study drug was administered each evening. As-needed intravenous midazolam was used while titrating up the study drug. Study drug was administered nightly until either ICU discharge or an adverse event occurred. Decisions regarding use of other analgesic and sedative therapy, including opioids, oral benzodiazepines, acetaminophen, and nonsteroidal anti-inflammatory drugs, were left to the discretion of the clinician. Sleep-promoting agents such as melatonin or trazodone were not allowed.
Main outcome measures. The primary outcome was the proportion of patients who remained free of delirium during their critical illness. Secondary outcomes included ICU days spent without delirium; duration of delirium; sleep quality; proportion of patients who ever developed coma; proportion of nocturnal hours spent at each Richmond Agitation and Sedation Scale (RASS) score; maximal nocturnal pain levels; antipsychotic, corticosteroid, and oral analgesic use; days of mechanical ventilation; ICU and hospital stay duration; and ICU and hospital mortality.
Main results. 100 patients were randomized, with 50 patients in each group. 89% of patients were mechanically ventilated, and the Prediction of Delirium in ICU (PRE-DELIRIC) score [1] was 54 in the dexmedetomidine group and 51 in the placebo group. Continuous propofol and fentanyl infusion at randomization was used in 49% and 80%, respectively. Duration of median ICU stay was 10 days in the dexmedetomidine group and 9 days in the placebo group. More patients in the dexmedetomidine group (40 of 50 patients [80%]) than in the placebo group (27 of 50 patients [54%]) remained free of delirium (relative risk [RR], 0.44, 95% confidence interval {CI} 0.23 to 0.82; P = 0.006). The median (interquartile range [IQR]) duration of the first episode of delirium was similar between the dexmedetomidine (IQR 2.0 [0.6–2.7] days) and placebo (2.2 [0.7–3.2] days) groups (P = 0.73). The average Leeds Sleep Evaluation Questionnaire score also was similar (mean difference, 0.02, 95% CI 0.42 to 1.92) between the 2 groups. Incidence of hypotension or bradycardia did not differ significantly between the groups.
Conclusion. Nocturnal administration of low-dose dexmedetomidine in critically ill adults reduces the incidence of delirium during the ICU stay, and patient-reported sleep quality appears unchanged.
Commentary
Delirium is a sudden state of confusion and/or disturbance of consciousness and cognition that is believed to result from acute brain dysfunction, including neurochemical disequilibrium. It often occurs in association with a general medical condition, such as various types of shock, sepsis, surgery, anesthesia, or electrolyte imbalance. Studies have shown that delirium is associated with increased mortality in critically ill patients [2]. Most ICUs use a systematic assessment tool for early detection of delirium, such as the Confusion Assessment Method for the ICU (CAM-ICU), the Intensive Care Delirium Screening Checklist (ICDSC), or the DSM-IV TR score system. The CAM-ICU is the most frequently used tool to evaluate for the presence of delirium in critically ill patients; it is scored as positive if the patient manifests both an acute change in mental status and inattention, and has either a RASS greater than 0 or disorganized thinking [3].
The level of evidence regarding delirium prevention is low. Ear plugs, eye masks, educational staff, supportive reorientation, and music have been studied as nonpharmacologic methods for preventing delirium [4]. From a pharmacologic standpoint, the dopamine D2 antagonist haloperidol has been explored as a therapy for both treating and preventing delirium, since the condition is thought to be associated with anticholinergic and excessive dopaminergic mechanisms. A randomized controlled study in 142 patients who received haloperidol 2.5 mg intravenously every 8 hours found that the duration of delirium did not differ between the haloperidol and the placebo groups [5]. The most feared adverse effects of haloperidol, such as akathisia, muscle stiffness, arrhythmia, or QT prolongation, did not occur more frequently in the haloperidol group. Similar results have been reported by Al-Qadheeb et al [6]. Pharmacologic prophylaxis of delirium using atypical antipsychotics such as quetiapine has also been explored, but the level of evidence for this intervention remains very low. Current American College of Critical Care Medicine guidelines recommend nonpharmacologic management and do not firmly recommend any pharmacologic prevention for ICU delirium [7].
Dexmedetomidine is a selective alpha-2 adrenergic receptor agonist that acts at the locus ceruleus, providing sedation and analgesia. Studies assessing the choice of sedation in the ICU found that the use of dexmedetomidine or propofol, compared to benzodiazepines, is associated with a lower rate of delirium occurrence, especially in mechanically ventilated patients [8,9]. Dexmedetomidine offers several potential advantages over other sedative drugs: it has little effect on cognition, has minimal anticholinergic effect, and may restore a natural sleep pattern. While propofol causes hypotension, respiratory depression, and deeper sedation, dexmedetomidine is associated with lighter sedation, a minimal effect on respiratory drive, and a milder hemodynamic effect. In a randomized controlled trial involving post-surgery ICU patients, dexmedetomidine partially restored a normal sleep pattern (eg, increased percentage of stage 2 non-rapid eye movement sleep), prolonged total sleep time, improved sleep efficiency, and increased sleep quality [10]; by improving overall sleep quality, dexmedetomidine potentially may prevent delirium. Another study that randomly assigned 700 ICU patients who underwent noncardiac surgery to dexmedetomidine infusion (0.1 mcg/kg/hr from ICU admission on the day of surgery until the following morning) or placebo reported a significantly reduced incidence of delirium in the dexmedetomidine group [11]. On the other hand, a 2015 Cochrane meta-analysis that included 7 randomized controlled studies did not find a significant risk reduction of delirium with dexmedetomidine [12].
The current study by Skrobik et al was a randomized, placebo-controlled trial that examined the role of nocturnal dexmedetomidine in ICU delirium prevention in 100 ICU patients. Nocturnal administration of low-dose dexmedetomidine led to a statistically significant reduction in delirium incidence compared to placebo (RR of delirium, 0.44, 95% CI 0.23 to 0.82, which is similar to that suggested by previous studies). This study adds additional evidence regarding the use of dexmedetomidine for pharmacologic delirium prevention. It included many mechanically ventilated patients (89% of study population), strengthening the applicability of the result. Mechanical ventilation is a known risk factor for ICU delirium, and therefore this is an important population to study; previous trials largely included patients who were not mechanically ventilated. This study also supports the safety of dexmedetomidine infusion, especially in lower doses in critically ill patients, without significantly increasing the incidence of adverse events (mainly hypotension and bradycardia). The study protocol closely approximated real practice by allowing other analgesics, including opioids, and therefore suggests safety and real world applicability.
There are several confounding issues in this study. The study was blinded, and there was concern that the bedside nurses may have been able to identify the study drug based on the effects on heart rate. In addition, 50% of patients received antipsychotics. While baseline RASS score was significantly different between the 2 groups, patients in the dexmedetomidine group reached a deeper level of sedation during the study. Also, the protocol mandated halving the pre-existing sedative on the night of study drug initiation, which could have led to inadequate sedation in the placebo group. Placebo patients received propofol for a similar duration but at a higher dose compared to dexmedetomidine patients, and midazolam and fentanyl infusion was used in a similar pattern between the groups. The high exclusion rate (71%) limits the ability to generalize the results to all ICU patients.
Applications for Clinical Practice
ICU delirium is an important complication of critical illness and is potentially preventable. Benzodiazepines are associated with an increased risk of delirium, while there has been increasing interest in dexmedetomidine, a selective alpha-2 adrenergic receptor agonist, because of its potential for delirium prevention. Evidence to date does not strongly support routine use of pharmacologic prevention of delirium; however, dexmedetomidine may be an option for sedation, as opposed to benzodiazepines or propofol, in selected patients and may potentially prevent delirium.
—Minkyung Kwon, MD, Neal Patel, MD, and Vichaya Arunthari, MD, Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL
Study Overview
Objective. To determine if nocturnal dexmedetomidine prevents delirium and improves sleep in critically ill patients.
Design. Two-center, double-blind, placebo-controlled, randomized, trial.
Setting and participants. This study was conducted in the intensive care units (ICU) at 2 centers in North America between 2013 and 2016. Adults admitted to the ICU and receiving intermittent or continuous sedatives and expected to require at least 48 hours of ICU care were included in the study. Exclusion criteria were presence of delirium, severe dementia, acute neurologic injury, severe bradycardia, hepatic encephalopathy, end-stage liver disease, and expected death within 24 hours.
Intervention. Patients were randomized 1:1 to receive nocturnal dexmedetomidine (0.2–0.7 mcg/kg/hr) or dextrose 5% in water. Patients, clinicians, bedside nurses, and all study personnel were blinded to study drug assignment throughout the study. All sedatives were halved before the study drug was administered each evening. As-needed intravenous midazolam was used while titrating up the study drug. Study drug was administered nightly until either ICU discharge or an adverse event occurred. Decisions regarding use of other analgesic and sedative therapy, including opioids, oral benzodiazepines, acetaminophen, and nonsteroidal anti-inflammatory drugs, were left to the discretion of the clinician. Sleep-promoting agents such as melatonin or trazodone were not allowed.
Main outcome measures. The primary outcome was the proportion of patients who remained free of delirium during their critical illness. Secondary outcomes included ICU days spent without delirium; duration of delirium; sleep quality; proportion of patients who ever developed coma; proportion of nocturnal hours spent at each Richmond Agitation and Sedation Scale (RASS) score; maximal nocturnal pain levels; antipsychotic, corticosteroid, and oral analgesic use; days of mechanical ventilation; ICU and hospital stay duration; and ICU and hospital mortality.
Main results. 100 patients were randomized, with 50 patients in each group. 89% of patients were mechanically ventilated, and the Prediction of Delirium in ICU (PRE-DELIRIC) score [1] was 54 in the dexmedetomidine group and 51 in the placebo group. Continuous propofol and fentanyl infusion at randomization was used in 49% and 80%, respectively. Duration of median ICU stay was 10 days in the dexmedetomidine group and 9 days in the placebo group. More patients in the dexmedetomidine group (40 of 50 patients [80%]) than in the placebo group (27 of 50 patients [54%]) remained free of delirium (relative risk [RR], 0.44, 95% confidence interval {CI} 0.23 to 0.82; P = 0.006). The median (interquartile range [IQR]) duration of the first episode of delirium was similar between the dexmedetomidine (IQR 2.0 [0.6–2.7] days) and placebo (2.2 [0.7–3.2] days) groups (P = 0.73). The average Leeds Sleep Evaluation Questionnaire score also was similar (mean difference, 0.02, 95% CI 0.42 to 1.92) between the 2 groups. Incidence of hypotension or bradycardia did not differ significantly between the groups.
Conclusion. Nocturnal administration of low-dose dexmedetomidine in critically ill adults reduces the incidence of delirium during the ICU stay, and patient-reported sleep quality appears unchanged.
Commentary
Delirium is a sudden state of confusion and/or disturbance of consciousness and cognition that is believed to result from acute brain dysfunction, including neurochemical disequilibrium. It often occurs in association with a general medical condition, such as various types of shock, sepsis, surgery, anesthesia, or electrolyte imbalance. Studies have shown that delirium is associated with increased mortality in critically ill patients [2]. Most ICUs use a systematic assessment tool for early detection of delirium, such as the Confusion Assessment Method for the ICU (CAM-ICU), the Intensive Care Delirium Screening Checklist (ICDSC), or the DSM-IV TR score system. The CAM-ICU is the most frequently used tool to evaluate for the presence of delirium in critically ill patients; it is scored as positive if the patient manifests both an acute change in mental status and inattention, and has either a RASS greater than 0 or disorganized thinking [3].
The level of evidence regarding delirium prevention is low. Ear plugs, eye masks, educational staff, supportive reorientation, and music have been studied as nonpharmacologic methods for preventing delirium [4]. From a pharmacologic standpoint, the dopamine D2 antagonist haloperidol has been explored as a therapy for both treating and preventing delirium, since the condition is thought to be associated with anticholinergic and excessive dopaminergic mechanisms. A randomized controlled study in 142 patients who received haloperidol 2.5 mg intravenously every 8 hours found that the duration of delirium did not differ between the haloperidol and the placebo groups [5]. The most feared adverse effects of haloperidol, such as akathisia, muscle stiffness, arrhythmia, or QT prolongation, did not occur more frequently in the haloperidol group. Similar results have been reported by Al-Qadheeb et al [6]. Pharmacologic prophylaxis of delirium using atypical antipsychotics such as quetiapine has also been explored, but the level of evidence for this intervention remains very low. Current American College of Critical Care Medicine guidelines recommend nonpharmacologic management and do not firmly recommend any pharmacologic prevention for ICU delirium [7].
Dexmedetomidine is a selective alpha-2 adrenergic receptor agonist that acts at the locus ceruleus, providing sedation and analgesia. Studies assessing the choice of sedation in the ICU found that the use of dexmedetomidine or propofol, compared to benzodiazepines, is associated with a lower rate of delirium occurrence, especially in mechanically ventilated patients [8,9]. Dexmedetomidine offers several potential advantages over other sedative drugs: it has little effect on cognition, has minimal anticholinergic effect, and may restore a natural sleep pattern. While propofol causes hypotension, respiratory depression, and deeper sedation, dexmedetomidine is associated with lighter sedation, a minimal effect on respiratory drive, and a milder hemodynamic effect. In a randomized controlled trial involving post-surgery ICU patients, dexmedetomidine partially restored a normal sleep pattern (eg, increased percentage of stage 2 non-rapid eye movement sleep), prolonged total sleep time, improved sleep efficiency, and increased sleep quality [10]; by improving overall sleep quality, dexmedetomidine potentially may prevent delirium. Another study that randomly assigned 700 ICU patients who underwent noncardiac surgery to dexmedetomidine infusion (0.1 mcg/kg/hr from ICU admission on the day of surgery until the following morning) or placebo reported a significantly reduced incidence of delirium in the dexmedetomidine group [11]. On the other hand, a 2015 Cochrane meta-analysis that included 7 randomized controlled studies did not find a significant risk reduction of delirium with dexmedetomidine [12].
The current study by Skrobik et al was a randomized, placebo-controlled trial that examined the role of nocturnal dexmedetomidine in ICU delirium prevention in 100 ICU patients. Nocturnal administration of low-dose dexmedetomidine led to a statistically significant reduction in delirium incidence compared to placebo (RR of delirium, 0.44, 95% CI 0.23 to 0.82, which is similar to that suggested by previous studies). This study adds additional evidence regarding the use of dexmedetomidine for pharmacologic delirium prevention. It included many mechanically ventilated patients (89% of study population), strengthening the applicability of the result. Mechanical ventilation is a known risk factor for ICU delirium, and therefore this is an important population to study; previous trials largely included patients who were not mechanically ventilated. This study also supports the safety of dexmedetomidine infusion, especially in lower doses in critically ill patients, without significantly increasing the incidence of adverse events (mainly hypotension and bradycardia). The study protocol closely approximated real practice by allowing other analgesics, including opioids, and therefore suggests safety and real world applicability.
There are several confounding issues in this study. The study was blinded, and there was concern that the bedside nurses may have been able to identify the study drug based on the effects on heart rate. In addition, 50% of patients received antipsychotics. While baseline RASS score was significantly different between the 2 groups, patients in the dexmedetomidine group reached a deeper level of sedation during the study. Also, the protocol mandated halving the pre-existing sedative on the night of study drug initiation, which could have led to inadequate sedation in the placebo group. Placebo patients received propofol for a similar duration but at a higher dose compared to dexmedetomidine patients, and midazolam and fentanyl infusion was used in a similar pattern between the groups. The high exclusion rate (71%) limits the ability to generalize the results to all ICU patients.
Applications for Clinical Practice
ICU delirium is an important complication of critical illness and is potentially preventable. Benzodiazepines are associated with an increased risk of delirium, while there has been increasing interest in dexmedetomidine, a selective alpha-2 adrenergic receptor agonist, because of its potential for delirium prevention. Evidence to date does not strongly support routine use of pharmacologic prevention of delirium; however, dexmedetomidine may be an option for sedation, as opposed to benzodiazepines or propofol, in selected patients and may potentially prevent delirium.
—Minkyung Kwon, MD, Neal Patel, MD, and Vichaya Arunthari, MD, Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL
1. van den Boogaard M, Pickkers P, Slooter AJ, et al. Development and validation of PRE-DELIRIC (PREdiction of DELIRium in ICu patients) delirium prediction model for intensive care patients: observational multicentre study. BMJ 2012;344:e420.
2. Slooter AJ, Van De Leur RR, Zaal IJ. Delirium in critically ill patients. Handb Clin Neurol 2017;141:449–66.
3. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001;286:2703–10.
4. Abraha I, Trotta F, Rimland JM, et al. Efficacy of non-pharmacological interventions to prevent and treat delirium in older patients: a systematic overview. The SENATOR project ONTOP Series. PLoS One 2015;10:e0123090.
5. Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2013;1:515–23.
6. Al-Qadheeb NS, Skrobik Y, Schumaker G, et al. Preventing ICU subsyndromal delirium conversion to delirium with low-dose IV haloperidol: a double-blind, placebo-controlled pilot study. Crit Care Med 2016;44:583–91.
7. Barr J, Fraser GL, Puntillo K, et al; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263–306.
8. Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009;301:489–99.
9. Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007;298:2644–53.
10. Wu XH, Cui F, Zhang C, et al. Low-dose dexmedetomidine improves sleep quality pattern in elderly patients after noncardiac surgery in the intensive care unit: a pilot randomized controlled trial. Anesthesiology 2016;125:979–91.
11. Su X, Meng Z-T, Wu X-H, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 2016;388:1893–1902.
12. Chen K, Lu Z, Xin YC, et al. Alpha-2 agonists for long-term sedation during mechanical ventilation in critically ill patients. Cochrane Database Syst Rev 2015;1:CD010269.
1. van den Boogaard M, Pickkers P, Slooter AJ, et al. Development and validation of PRE-DELIRIC (PREdiction of DELIRium in ICu patients) delirium prediction model for intensive care patients: observational multicentre study. BMJ 2012;344:e420.
2. Slooter AJ, Van De Leur RR, Zaal IJ. Delirium in critically ill patients. Handb Clin Neurol 2017;141:449–66.
3. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001;286:2703–10.
4. Abraha I, Trotta F, Rimland JM, et al. Efficacy of non-pharmacological interventions to prevent and treat delirium in older patients: a systematic overview. The SENATOR project ONTOP Series. PLoS One 2015;10:e0123090.
5. Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2013;1:515–23.
6. Al-Qadheeb NS, Skrobik Y, Schumaker G, et al. Preventing ICU subsyndromal delirium conversion to delirium with low-dose IV haloperidol: a double-blind, placebo-controlled pilot study. Crit Care Med 2016;44:583–91.
7. Barr J, Fraser GL, Puntillo K, et al; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263–306.
8. Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009;301:489–99.
9. Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007;298:2644–53.
10. Wu XH, Cui F, Zhang C, et al. Low-dose dexmedetomidine improves sleep quality pattern in elderly patients after noncardiac surgery in the intensive care unit: a pilot randomized controlled trial. Anesthesiology 2016;125:979–91.
11. Su X, Meng Z-T, Wu X-H, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 2016;388:1893–1902.
12. Chen K, Lu Z, Xin YC, et al. Alpha-2 agonists for long-term sedation during mechanical ventilation in critically ill patients. Cochrane Database Syst Rev 2015;1:CD010269.
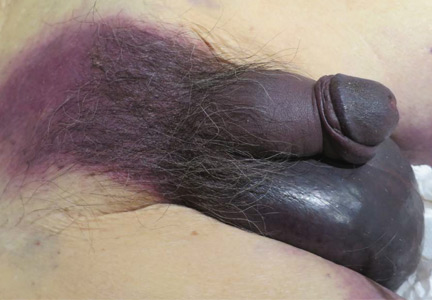
Fournier gangrene
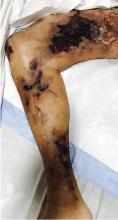
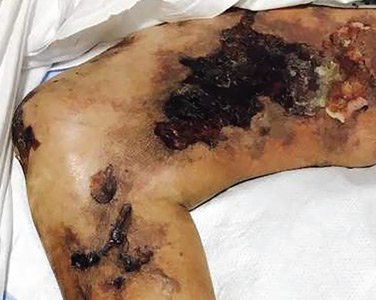
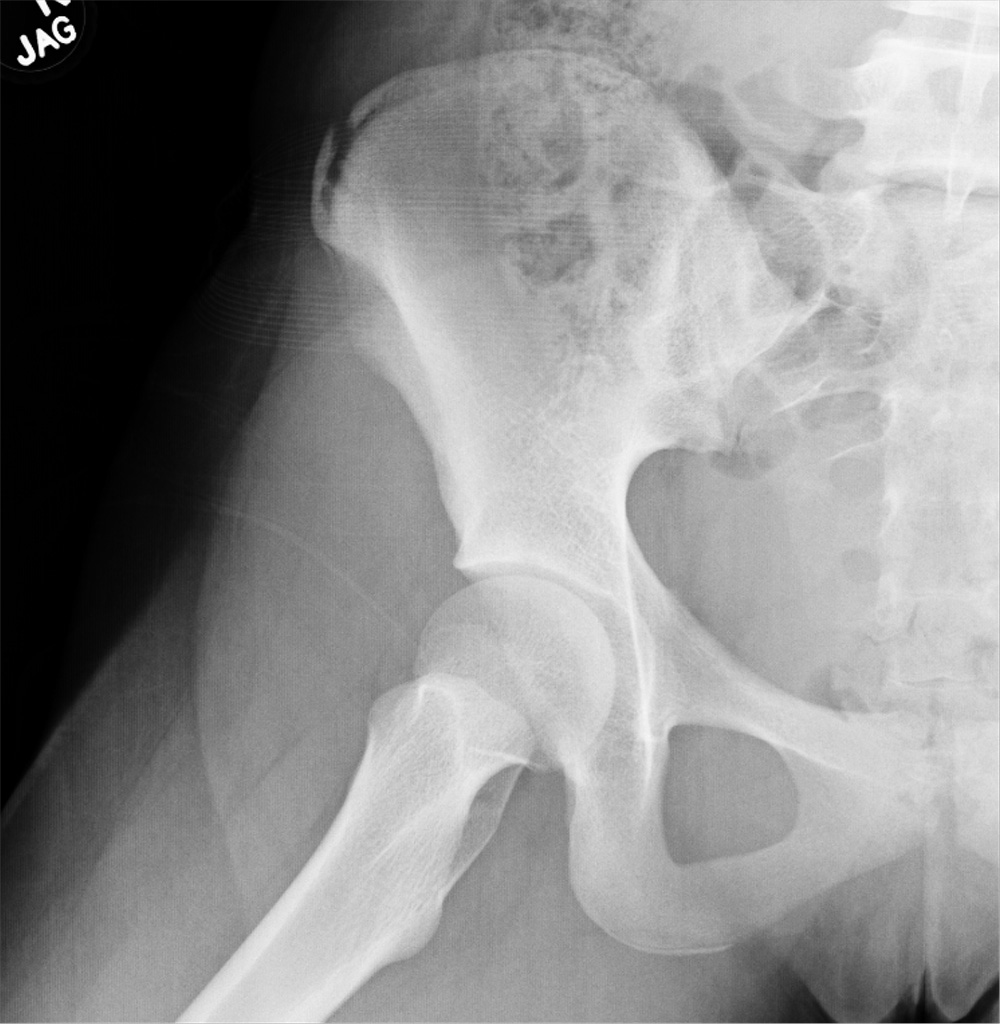
An 88-year-old man with a 1-day history of fever and altered mental status was transferred to the emergency department. He had been receiving conservative management for low-risk localized prostate cancer but had no previous cardiovascular or gastrointestinal problems.
Based on these findings, the diagnosis was Fournier gangrene. Despite aggressive treatment, the patient’s condition deteriorated rapidly, and he died 2 hours after admission.
FOURNIER GANGRENE: NECROTIZING FASCIITIS OF THE PERINEUM
Fournier gangrene is a rare but rapidly progressive necrotizing fasciitis of the perineum with a high death rate.
Predisposing factors for Fournier gangrene include older age, diabetes mellitus, morbid obesity, cardiovascular disorders, chronic alcoholism, long-term corticosteroid treatment, malignancy, and human immunodeficiency virus infection.1,2 Urethral obstruction, instrumentation, urinary extravasation, and trauma have also been associated with this condition.3
In general, organisms from the urinary tract spread along the fascial planes to involve the penis and scrotum.
The differential diagnosis of Fournier gangrene includes scrotal and perineal disorders, as well as intra-abdominal disorders such as cellulitis, abscess, strangulated hernia, pyoderma gangrenosum, allergic vasculitis, vascular occlusion syndromes, and warfarin necrosis.
Delay in the diagnosis of Fournier gangrene leads to an extremely high death rate due to rapid progression of the disease, leading to sepsis, multiple organ failure, and disseminated intravascular coagulation. Immediate diagnosis and appropriate treatment such as broad-spectrum antibiotics and extensive surgical debridement reduce morbidity and control the infection. Antibiotics for methicillin-resistant Staphylococcus aureus should be considered if there is a history of or risk factors for this organism.4
Necrotizing fasciitis, including Fournier gangrene, is a common indication for intravenous immunoglobulin, and this treatment has been reported to be effective in a few cases. However, a double-blind, placebo-controlled trial that evaluated the benefit of this treatment was terminated early due to slow patient recruitment.5
A delay of even a few hours from suspicion of Fournier gangrene to surgical debridement significantly increases the risk of death.6 Thus, when it is suspected, immediate surgical intervention may be necessary to confirm the diagnosis and to treat it. The usual combination of antibiotic therapy for Fournier gangrene includes penicillin for the streptococcal species, a third-generation cephalosporin with or without an aminoglycoside for the gram-negative organisms, and metronidazole for anaerobic bacteria.
- Wang YK, Li YH, Wu ST, Meng E. Fournier’s gangrene. QJM 2017; 110(10):671–672. doi:10.1093/qjmed/hcx124
- Yanar H, Taviloglu K, Ertekin C, et al. Fournier’s gangrene: risk factors and strategies for management. World J Surg 2006; 30(9):1750–1754. doi:10.1007/s00268-005-0777-3
- Paonam SS, Bag S. Fournier gangrene with extensive necrosis of urethra and bladder mucosa: a rare occurrence in a patient with advanced prostate cancer. Urol Ann 2015; 7(4):507–509. doi:10.4103/0974-7796.157975
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg 2008; 6(4):328–338. doi:10.1016/j.ijsu.2007.07.001
- Koch C, Hecker A, Grau V, Padberg W, Wolff M, Henrich M. Intravenous immunoglobulin in necrotizing fasciitis—a case report and review of recent literature. Ann Med Surg (Lond) 2015; 4(3):260–263. doi:10.1016/j.amsu.2015.07.017
- Singh A, Ahmed K, Aydin A, Khan MS, Dasgupta P. Fournier's gangrene. A clinical review. Arch Ital Urol Androl 2016; 88(3):157–164. doi:10.4081/aiua.2016.3.157
An 88-year-old man with a 1-day history of fever and altered mental status was transferred to the emergency department. He had been receiving conservative management for low-risk localized prostate cancer but had no previous cardiovascular or gastrointestinal problems.
Based on these findings, the diagnosis was Fournier gangrene. Despite aggressive treatment, the patient’s condition deteriorated rapidly, and he died 2 hours after admission.
FOURNIER GANGRENE: NECROTIZING FASCIITIS OF THE PERINEUM
Fournier gangrene is a rare but rapidly progressive necrotizing fasciitis of the perineum with a high death rate.
Predisposing factors for Fournier gangrene include older age, diabetes mellitus, morbid obesity, cardiovascular disorders, chronic alcoholism, long-term corticosteroid treatment, malignancy, and human immunodeficiency virus infection.1,2 Urethral obstruction, instrumentation, urinary extravasation, and trauma have also been associated with this condition.3
In general, organisms from the urinary tract spread along the fascial planes to involve the penis and scrotum.
The differential diagnosis of Fournier gangrene includes scrotal and perineal disorders, as well as intra-abdominal disorders such as cellulitis, abscess, strangulated hernia, pyoderma gangrenosum, allergic vasculitis, vascular occlusion syndromes, and warfarin necrosis.
Delay in the diagnosis of Fournier gangrene leads to an extremely high death rate due to rapid progression of the disease, leading to sepsis, multiple organ failure, and disseminated intravascular coagulation. Immediate diagnosis and appropriate treatment such as broad-spectrum antibiotics and extensive surgical debridement reduce morbidity and control the infection. Antibiotics for methicillin-resistant Staphylococcus aureus should be considered if there is a history of or risk factors for this organism.4
Necrotizing fasciitis, including Fournier gangrene, is a common indication for intravenous immunoglobulin, and this treatment has been reported to be effective in a few cases. However, a double-blind, placebo-controlled trial that evaluated the benefit of this treatment was terminated early due to slow patient recruitment.5
A delay of even a few hours from suspicion of Fournier gangrene to surgical debridement significantly increases the risk of death.6 Thus, when it is suspected, immediate surgical intervention may be necessary to confirm the diagnosis and to treat it. The usual combination of antibiotic therapy for Fournier gangrene includes penicillin for the streptococcal species, a third-generation cephalosporin with or without an aminoglycoside for the gram-negative organisms, and metronidazole for anaerobic bacteria.
An 88-year-old man with a 1-day history of fever and altered mental status was transferred to the emergency department. He had been receiving conservative management for low-risk localized prostate cancer but had no previous cardiovascular or gastrointestinal problems.
Based on these findings, the diagnosis was Fournier gangrene. Despite aggressive treatment, the patient’s condition deteriorated rapidly, and he died 2 hours after admission.
FOURNIER GANGRENE: NECROTIZING FASCIITIS OF THE PERINEUM
Fournier gangrene is a rare but rapidly progressive necrotizing fasciitis of the perineum with a high death rate.
Predisposing factors for Fournier gangrene include older age, diabetes mellitus, morbid obesity, cardiovascular disorders, chronic alcoholism, long-term corticosteroid treatment, malignancy, and human immunodeficiency virus infection.1,2 Urethral obstruction, instrumentation, urinary extravasation, and trauma have also been associated with this condition.3
In general, organisms from the urinary tract spread along the fascial planes to involve the penis and scrotum.
The differential diagnosis of Fournier gangrene includes scrotal and perineal disorders, as well as intra-abdominal disorders such as cellulitis, abscess, strangulated hernia, pyoderma gangrenosum, allergic vasculitis, vascular occlusion syndromes, and warfarin necrosis.
Delay in the diagnosis of Fournier gangrene leads to an extremely high death rate due to rapid progression of the disease, leading to sepsis, multiple organ failure, and disseminated intravascular coagulation. Immediate diagnosis and appropriate treatment such as broad-spectrum antibiotics and extensive surgical debridement reduce morbidity and control the infection. Antibiotics for methicillin-resistant Staphylococcus aureus should be considered if there is a history of or risk factors for this organism.4
Necrotizing fasciitis, including Fournier gangrene, is a common indication for intravenous immunoglobulin, and this treatment has been reported to be effective in a few cases. However, a double-blind, placebo-controlled trial that evaluated the benefit of this treatment was terminated early due to slow patient recruitment.5
A delay of even a few hours from suspicion of Fournier gangrene to surgical debridement significantly increases the risk of death.6 Thus, when it is suspected, immediate surgical intervention may be necessary to confirm the diagnosis and to treat it. The usual combination of antibiotic therapy for Fournier gangrene includes penicillin for the streptococcal species, a third-generation cephalosporin with or without an aminoglycoside for the gram-negative organisms, and metronidazole for anaerobic bacteria.
- Wang YK, Li YH, Wu ST, Meng E. Fournier’s gangrene. QJM 2017; 110(10):671–672. doi:10.1093/qjmed/hcx124
- Yanar H, Taviloglu K, Ertekin C, et al. Fournier’s gangrene: risk factors and strategies for management. World J Surg 2006; 30(9):1750–1754. doi:10.1007/s00268-005-0777-3
- Paonam SS, Bag S. Fournier gangrene with extensive necrosis of urethra and bladder mucosa: a rare occurrence in a patient with advanced prostate cancer. Urol Ann 2015; 7(4):507–509. doi:10.4103/0974-7796.157975
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg 2008; 6(4):328–338. doi:10.1016/j.ijsu.2007.07.001
- Koch C, Hecker A, Grau V, Padberg W, Wolff M, Henrich M. Intravenous immunoglobulin in necrotizing fasciitis—a case report and review of recent literature. Ann Med Surg (Lond) 2015; 4(3):260–263. doi:10.1016/j.amsu.2015.07.017
- Singh A, Ahmed K, Aydin A, Khan MS, Dasgupta P. Fournier's gangrene. A clinical review. Arch Ital Urol Androl 2016; 88(3):157–164. doi:10.4081/aiua.2016.3.157
- Wang YK, Li YH, Wu ST, Meng E. Fournier’s gangrene. QJM 2017; 110(10):671–672. doi:10.1093/qjmed/hcx124
- Yanar H, Taviloglu K, Ertekin C, et al. Fournier’s gangrene: risk factors and strategies for management. World J Surg 2006; 30(9):1750–1754. doi:10.1007/s00268-005-0777-3
- Paonam SS, Bag S. Fournier gangrene with extensive necrosis of urethra and bladder mucosa: a rare occurrence in a patient with advanced prostate cancer. Urol Ann 2015; 7(4):507–509. doi:10.4103/0974-7796.157975
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg 2008; 6(4):328–338. doi:10.1016/j.ijsu.2007.07.001
- Koch C, Hecker A, Grau V, Padberg W, Wolff M, Henrich M. Intravenous immunoglobulin in necrotizing fasciitis—a case report and review of recent literature. Ann Med Surg (Lond) 2015; 4(3):260–263. doi:10.1016/j.amsu.2015.07.017
- Singh A, Ahmed K, Aydin A, Khan MS, Dasgupta P. Fournier's gangrene. A clinical review. Arch Ital Urol Androl 2016; 88(3):157–164. doi:10.4081/aiua.2016.3.157
Diabetic Ketoacidosis and Hyperosmolar Hyperglycemic Syndrome Management
In this review, the authors discuss the similarities and differences between diabetic ketoacidosis and the hyperosmolar hyperglycemic state, providing clinical pearls and common pitfalls to help guide the clinician in the diagnosis and management.
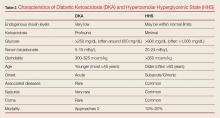
Diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic state (HHS) are similar but distinct diabetic emergencies that are frequently encountered in the ED. Patients with DKA or HHS present with hyperglycemia and dehydration and frequently appear quite ill physically. In both syndromes, there is insufficient insulin levels to transport glucose into cells.
As previously noted, although DKA and HHS share similar characteristic signs and symptoms, they are two distinct conditions that must be differentiated in the clinical work-up. One characteristic that helps the emergency physician (EP) to distinguish between the two conditions is the patient age at symptom onset. Although both conditions can occur at any age, diabetic ketoacidosis typically develops in younger patients, less than 45 years, who have little or no endogenous insulin production, whereas HHS usually occurs in much older non-insulin-dependent patients (who are often greater than 60 years old). 1-3 This review discusses the similarities and differences in the etiology, diagnosis, and treatment of DKA and HHS to guide evaluation and simplify management, highlighting practical tips and clinical pearls. When applicable, information has been organized into groups of five to facilitate retention and recall.
The Etiology of DKA Vs HHS
The fundamental underlying issue in both DKA and HHS is an absolute or relative lack of insulin that results in an increase in counter-regulatory hormones, including glucagon, cortisol, and catecholamines.
Insulin has five main actions: (1) to drive glucose into cells; (2) to drive potassium into cells; (3) to create an anabolic environment; (4) to inhibit breakdown of fat; and (5) to block the breakdown of proteins. (Table 1).
Diabetic ketoacidosis typically develops in patients who lack significant endogenous insulin; this insufficiency of circulating insulin causes hyperglycemia and hyperkalemia, the creation of a catabolic state with high levels of both ketone bodies and free-fatty acids due to the breakdown of proteins and fats.
In contrast, HHS occurs in patients who produce a sufficient amount of insulin to drive potassium into cells and to inhibit the breakdown of proteins and fats; as such these patients are not ketoacidotic. However, patients with HHS do not produce enough insulin to drive glucose intracellularly. As the glucose levels increase, patients with HHS become increasingly hyperosmolar and dehydrated, resulting in further elevation of glucose levels, causing a perpetual cycle of increasing glucose and resultant hyperosmolarity and dehydration.1-3 It is important to appreciate that both hyperglycemic crises result in an osmotic diuresis leading to severe dehydration and urinary wasting of electrolytes.
Diagnosis and Workup
Laboratory Evaluation
In addition to hyperglycemia, another key finding in DKA is elevated anion gap metabolic acidosis resulting from ketoacid production. Laboratory evaluation will demonstrate a pH less than 7.3 and serum bicarbonate level less than 18 mEq/L; urinalysis will be positive for ketones.
Though HHS patients also present with hyperglycemia, laboratory evaluation will demonstrate no, or only mild, acidosis, normal pH (typically >7.3), bicarbonate level greater than 18 mEq/L, and a high-serum osmolality (>350 mOsm/kg). While urinalysis may show low or no ketones, patients with HHS do not develop the marked ketoacidosis seen in DKA. Moreover, glucose values are more elevated in patients with HHS, frequently exceeding 1,000 mg/dL.
Signs and Symptoms
Patients with either of these hyperglycemic crises often present with fatigue, polyuria, and polydipsia. They appear dehydrated on examination with dry mucous membranes, tachycardia, and in severe cases, hypotension. Patients with HHS often present with altered mental status, seizures, and even coma, while patients with DKA typically only experience changes in mental status in the most severe cases (Table 2). As many as one-third of patients with a hyperglycemic crisis will have an overlapping DKA/HHS syndrome.1-3 With respect to patient age, HHS tends to develop in older patients who have type 2 diabetes and several underlying comorbidities.2
Precipitating Causes of DKA and HHS
The five causes of DKA/HHS can be remembered as the “Five I’s”: (1) infection; (2) infarction; (3) infant (pregnancy), (4) indiscretion, and (5) [lack of] insulin (Table 3).
Infection. Infection is one of the most common precipitating factors in both DKA and HHS.1 During the history intake, the EP should ask the patient if he or she has had any recent urinary and respiratory symptoms, as positive responses prompt further investigation with urinalysis and chest radiography. A thorough dermatological examination should be performed to assess for visible signs of infection, particularly in the extremities and intertriginous areas.
When assessing patients with DKA or HHS for signs of infection, body temperature to assess for the presence of fever is not always a reliable indicator. This is because many patients with DKA/HHS develop tachypnea, which can affect the efficacy of an oral thermometer. In such cases, the rectal temperature is more sensitive for detecting fever. It should be noted, however, that some patients with severe infection or immunocompromised state—regardless of the presence or absence of tachypnea—may be normothermic or even hypothermic due to peripheral vasodilation—a poor prognostic sign.3 Blood and urine cultures should be obtained for all patients in whom infection is suspected.
Laboratory evaluation of patients with DKA may demonstrate a mild leukocytosis, a very common finding in DKA even in the absence of infection; leukocytosis is believed to result from elevated stress hormones such as cortisol and epinephrine.3
Infarction. Infarction is another important underlying cause of DKA/HHS and one that must not be overlooked. Screening for acute coronary syndrome through patient history, electrocardiography, and cardiac biomarkers should be performed in all patients older than age 40 years and in patients in whom there is any suggestion of myocardial ischemia.
A thorough neurological examination should be performed to assess for deficits indicative of stroke. Although neurological deficits can be due to severe hyperglycemia, most patients with focal neurological deficits from hyperglycemia also have altered mental status. Focal neurological deficits without a change in mental status are more likely to represent an actual stroke.4
Infant (Pregnancy). Pregnant patients are at an increased risk of developing DKA for several reasons, most notably due to the increased production of insulin-antagonistic hormones, which can lead to higher insulin resistance and thus increased insulin requirements during pregnancy.5 A pregnancy test is therefore indicated for all female patients of child-bearing age.
Indiscretion. Non-compliance with diet, such as taking in too many calories without appropriate insulin correction, and the ingestion of significant amounts of alcohol can lead to DKA. Eating disorders, particularly in young patients, may also contribute to recurrent cases.3
[Lack of] Insulin. In insulin-dependent diabetes, skipped insulin doses or insulin pump failure can trigger DKA/HHS. In fact, missed insulin doses are becoming a more frequent cause of DKA than infections.1
Both DKA and HHS are usually triggered by an underlying illness or event. Therefore, clinicians should always focus on identifying precipitating causes such as acute infection, stroke, or myocardial infarction, as some require immediate treatment. In fact, DKA or HHS is rarely the primary cause of death; patients are much more likely to die from the precipitating event that caused DKA or HHS.1,3
Assessing Disease Severity
Mental status and pH and serum bicarbonate levels help clinicians determine the extent of disease severity, classifying patients as having mild, moderate, or severe DKA (Table 4).2 Patients at the highest risk for poor outcomes include those at the extremes of age, who have severe comorbidities, who have underlying infection, and who are hypotensive and/or in a comatose state.3
Patients who have HHS are much more likely to present with altered mental status, including coma. There is a linear relationship between osmolality and degree of altered mental status. Thus, diabetic patients with major changes in mental status but without high serum osmolality warrant immediate workup for alternative causes of their altered mental status.3 In addition, seizures, especially focal seizures, are relatively common in severe cases of HHS. Finally, highly abnormal blood pressure, pulse, and respiratory rate can also provide additional clues regarding the severity of hyperglycemic crisis.
Arterial Blood Gas Assessment: To Stick or Not to Stick?
In the past, measuring arterial blood gases (ABG) has been considered a mainstay in the evaluation of patients with DKA. But does an arterial stick, which is associated with some risk, really add essential information? One study by Ma et al6 evaluated whether ABG results significantly alter how physicians manage patients with DKA. In the study, the authors evaluated 200 ED patients and found that ABG analysis only changed diagnosis in 1% of patients, altered treatment in 3.5% of patients, and changed disposition in only 1% of patients. Arterial stick partial pressure of oxygen and partial pressure of carbon dioxide altered treatment and disposition in only 1% of patients. Furthermore, the study results showed venous pH correlated very strongly with arterial pH (r = 0.95).6 These findings demonstrate that ABG measurements rarely affect or alter DKA management, and support the use of venous pH as an adequate substitute for ABG testing.
Euglycemia
Euglycemic DKA has been reported in patients with type 1 diabetes who had been fasting or vomiting or who had received exogenous insulin prior to presentation.1Euglycemia has also been reported in pregnant patients with type I diabetes.1 More recently, sodium glucose cotransporter 2 inhibitors (SGLT2) have been shown to cause euglycemic DKA. While the therapeutic mechanism of this drug class is to inhibit proximal tubular resorption of glucose, they can cause DKA by decreasing renal clearance of ketone bodies and increasing glucagon levels and promoting hepatic ketogenesis. Patients with DKA who are on SGLT2 inhibitors may present with only modestly elevated glucose levels (typically in the 200- to 300-mg/dL range), but have profound wide gap metabolic acidosis due to β-hydroxybutyrate acid and acetoacetate accumulation.7 When evaluating patients on SGLT2 inhibitors for DKA, EPs should not solely rely on glucose values but rather assess the patient’s overall clinical picture, including the physical examination, vital signs, and pH. Additionally, once resuscitation with intravenous (IV) fluids and insulin is initiated, it may take longer for patients who use SGLT2 inhibitors to clear ketoacids than patients with DKA who do not use these medications.8
Treatment
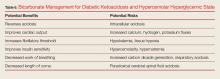
The goal of treatment for DKA and HHS is to correct volume deficits, hyperglycemia, and electrolyte abnormalities (Table 5). Three of the five therapies to manage DKA and HHS are mandatory: IV fluid resuscitation, IV insulin, and IV potassium. The other therapies, IV bicarbonate, and IV phosphate, should be considered, but are rarely required for DKA or HHS.
In general, management of HHS is less aggressive than that of DKA because HHS develops over a period of weeks—unlike DKA, which develops over only 1 to 2 days. Treatment of any underlying causes of HHS should occur simultaneously.
Intravenous Fluids
The goal of IV fluid therapy for patients with DKA is rehydration—not to “wash-out” ketones. Ketone elimination occurs through insulin-stimulated metabolism. When determining volume-replacement goals, it is helpful to keep in mind that patients with moderate-to-mild DKA typically have fluid deficiencies of 3 to 5 L, and patients with severe DKA have fluid deficiencies between 5 and 6 L. Patients with HHS present with significantly higher fluid deficiencies of around 9 to 12 L.
The initial goal of fluid management is to correct hypoperfusion with bolus fluids, followed by a more gradual repletion of remaining deficits. After bolus fluids are administered, the rate and type of subsequent IV fluid infusion varies depending on hemodynamics, hydration state, and serum sodium levels.2,3
Nonaggressive Vs Aggressive Fluid Management. One study by Adrogué et al9 compared the effects of managing DKA with aggressive vs nonaggressive fluid repletion. In the study, one group of patients received normal saline at 1,000 mL per hour for 4 hours, followed by normal saline at 500 mL per hour for 4 hours. The other group of patients received normal saline at a more modest rate of 500 mL per hour for 4 hours, followed by normal saline at 250 mL per hour for 4 hours. The authors found that patients in the less aggressive volume therapy group achieved a prompt and adequate recovery and maintained higher serum bicarbonate levels.9
Current recommendations for patients with DKA are to first treat patients with an initial bolus of 1,000 mL or 20 cc/kg of normal saline. Patients without profound dehydration should then receive 500 cc of normal saline per hour for the first 4 hours of treatment, after which the flow rate may be reduced to 250 cc per hour. For patients with mild DKA, therapy can start at 250 cc per hour with a smaller bolus dose or no bolus dose. Patients with profound dehydration and poor perfusion, should receive crystalloid fluids wide open until perfusion has improved. Overall, volume resuscitation in HHS is similar to DKA. However, the EP should be cautious with respect to total fluid volume and infusion rates to avoid fluid overload, since many patients with HHS are elderly and may have congestive heart failure.
Crystalloid Fluid Type for Initial Resuscitation. Normal saline has been the traditional crystalloid fluid of choice for managing DKA and HHS. Recent studies, however, have shown some benefit to using balanced solutions (Ringer’s lactate or PlasmaLyte) instead of normal saline. A recently published large study by Semler et al10 compared balanced crystalloid, in most cases lactated Ringers solution, to normal saline in critically ill adult patients, some of whom were diagnosed with DKA. The study demonstrated decreased mortality (from any cause) in the group who received balanced crystalloid fluid therapy and reduced need for renal-replacement therapy and reduced incidence of persistent renal dysfunction. The findings by Semler et al10 and findings from other smaller studies, calls into question whether normal saline is the best crystalloid to manage DKA and HHS.11,12 It remains to be seen what modification the American Diabetes Association (ADA) will make to its current recommendations for fluid therapy, which were last updated in 2009.
It appears that though the use of Ringers lactate or PlasmaLyte to treat DKA usually raises a patient’s serum bicarbonate level to 18 mEq/L at a more rapid rate than normal saline, the use of balanced solutions may result in longer time to lower blood glucose to 250 mg/dL.13 However, by using a balanced solution, the hyperchloremic metabolic acidosis often seen with normal saline treatment will be avoided.12
Half-Normal Saline. An initially normal or increased serum sodium level, despite significant hyperglycemia, suggests a substantial free-water deficit. Calculating a corrected serum sodium can help quantify the degree of free-water deficit.3 While isotonic fluids remain the standard for initial volume load, clinicians should consider switching patients to half-normal saline following initial resuscitation if the corrected serum sodium is elevated above normal. The simplest estimation to correct sodium levels in DKA is to expect a decrease in sodium levels at a rate of at least 2 mEq/L per 100-mg/dL increase in glucose levels above 100 mg/dL. For a more accurate calculation, providers can expect a drop in sodium of 1.6 mEq/L per 100 mg/dL increase of glucose up to a level of 400 mg/dL and then a fall of 2.4 mEq/L in sodium per every 100 mg/dL rise in glucose thereafter.14
Glucose. Patients with DKA require insulin therapy until ketoacidosis resolves. However, the average time to correct ketoacidosis from initiating treatment is about 12 hours compared to only 6 hours for correction of hyperglycemia. Since insulin therapy must be continued despite lower glucose levels, patients are at risk for developing hypoglycemia if glucose is not added to IV fluids. To prevent hypoglycemia and provide an energy source for ketone metabolism, patients should be switched to fluids containing dextrose when their serum glucose approaches 200 to 250 mg/dL.1,3 Typically, 5% dextrose in half-normal saline at 150 to 250 cc per hour is usually adequate to achieve this goal.
Insulin
As previously noted, insulin therapy is required to treat hyperglycemic crises from DKA and HHS. In DKA there is an absolute insulin deficiency, whereas in HHS, there is a relative insulin deficiency. In HHS, there is not enough endogenous insulin to move glucose into the cells, but there is enough insulin to block a catabolic state. That is why the breakdown of fats and proteins does not occur, and why ketoacidosis and hyperkalemia are not seen in HHS. On the other hand, glucose elevations do occur, and are usually more extreme in HHS than DKA. There are five major therapeutic actions of insulin in DKA (Table 5), and it is imperative to determine serum potassium before starting an insulin infusion as insulin will drive potassium into the cell, worsening hypokalemia and promoting the development of life-threatening arrhythmias, including ventricular fibrillation, ventricular tachycardia, and torsades de pointes. The electrocardiogram does not accurately predict severity of hypokalemia and should not be used as a substitute for direct potassium measurement.
Loading Dose and Drip Rate. When treating adult patients with DKA or HHS, the ADA recommends an IV push loading dose of 0.1 U/kg insulin, followed by an hourly maintenance dose of 0.1 U/kg. Alternatively, a continuous infusion of 0.16 U/kg/hr can be used without a bolus. The rationale behind a bolus is the rapid saturation of insulin receptors, followed by a drip to maintain saturation of receptors. However, a recent prospective observational cohort study by Goyal et al15 questions the utility of the initial insulin bolus. The study compared DKA patients who received an initial insulin bolus to those who did not. Both groups were similar at baseline and received equivalent IV fluids and insulin drips. They found no statistically significant differences in the incidence of hypoglycemia, rate of serum glucose change, anion gap change, or length of stay in the ED or hospital. The authors concluded that administration of an insulin bolus has no significant benefit to patients and does not change clinically relevant end-points.15 At this time, there is no proven benefit to giving DKA patients an IV insulin bolus; moreover, doing so may further increase hypoglycemia. The use of an insulin bolus is particularly not recommended for use in pediatric patients with DKA due to a higher incidence of hypoglycemia in this patient population.16
As with DKA, the ADA3 recommends giving HHS patients an insulin bolus of 0.1 U/ kg followed by a continuous infusion at 0.1 U/kg per hour. It is crucial to monitor patients closely to ensure glucose levels do not fall too rapidly. Glucose levels should be kept between 150 to 200 mg/dL for patients with DKA and 200 to 300 mg/dL for patients with HHS until the conditions resolve; this may necessitate lowering the infusion rate to 0.02 to 0.05 U/kg per hour. In addition to frequent glucose monitoring, a basic metabolic panel and venous pH should be obtained every 2 to 4 hours while a patient is on an insulin drip.3
Subcutaneous Vs Intravenous Insulin for DKA. Several small studies evaluating patients with mild-moderate DKA demonstrated similar outcomes when subcutaneous (SQ) insulin was used instead of IV insulin. However, SQ injections require more frequent dosing (every 1 to 2 hours) and still require close monitoring of blood glucose. This monitoring frequency is usually not feasible on a hospital floor, but may be feasible on step-down units, thus avoiding admission to the intensive care unit (ICU) for patients who do not otherwise require ICU-level of care.17 Subcutaneous insulin should not be given to patients with severe acidosis, hypotension, or altered mental status. The ADA consensus statement continues to recommend IV infusion of regular insulin as the preferred route due to its short half-life and easy titration.3
Determining When to Switch to Subcutaneous Insulin. Ideally, patients are not in the ED long enough to have their metabolic abnormalities corrected, as this usually requires several hours. In DKA, the insulin drip should continue until the blood glucose is less than 200 and at least two of the following conditions are met: the anion gap is less than 12, venous pH greater than 7.3, and serum bicarbonate >15. In HHS, osmolality and mental status should both return to normal prior to stopping the infusion. In both cases, subcutaneous insulin should be administered at least 1 to 2 hours before stopping the drip to prevent recurrent crisis.1,3
Refractory Acidosis. First and foremost, refractory acidosis should prompt a diligent source for dead gut, abscess, and underlying sepsis. While vomiting and diffuse abdominal pain are common in DKA and are related to ketoacidosis, these symptoms are atypical of HHS and should raise suspicion for underlying pathology.3 Additionally, a lower than expected bicarbonate level can also occur from resuscitation with large volumes of normal saline, resulting in a hyperchloremic non-gap metabolic acidosis.
Potassium
Both DKA and HHS patients have total body potassium deficits due to osmotic diuresis that require careful repletion. Deficits can be substantial: The average total whole body potassium deficit in DKA is 3 to 5 mEq/kg.2 Clinicians should exercise caution, however, since DKA patients may be hyperkalemic initially despite a total body potassium deficit. Early hyperkalemia is due to the transmembrane shift of potassium secondary to acidosis and insulin deficiency as well as hypertonicity. If initial potassium is greater than 5.2 mEq/L, potassium should not be administered but instead rechecked in 1 to 2 hours. If the potassium level is 4.0 to 5.2 mEq/L, then 10 mEq per hour is usually adequate. For levels between 3.3 and 4.0 mEq/L, administer potassium chloride at 20 mEq per hour. For levels less than 3.3 mEq/L, insulin should be held and potassium chloride should be aggressively repleted at 20 to 30 mEq per hour with continuous cardiac monitoring.1-3 Failure to recognize and act on critical potassium levels is a known cause of unexpected death in DKA. During the first hour of DKA onset, patients are more likely to die from hyperkalemia. Later, while the patient is “stabilizing” on an insulin infusion, potassium levels will fall as insulin drives potassium back into cells.
Bicarbonate
Bicarbonate has many theoretical benefits but also has potential risks (Table 6).
Phosphate
Since there is no proven benefit to giving phosphate to adult patients with DKA, it is rarely used, except in specific situations other than pediatric DKA. Similar to potassium, initial serum phosphate levels do not reflect total body phosphate levels due to transmembrane shifts.2,3 Phosphate repletionis most beneficial for patients who have cachexia, respiratory depression, anemia, cardiac dysfunction, or phosphate values lower than 1.0 to 1.5. If given, 20 to 30 mEq/L potassium phosphate (K2PO4) added to fluids is usually sufficient.3 Overly aggressive phosphate administration (>4.5 mmol/h or 1.5 mL/h potassium phosphate) can cause severe hypocalcemia and should be avoided.1,3 In pediatric patients, up to one-half of potassium requirements are often given as potassium phosphate, but this may vary by institution.
Conclusion
Both DKA and HHS are diabetic emergencies that must be approached and managed systematically to correct underlying dehydration and metabolic abnormalities. Patient care begins by determining the etiology of these conditions, especially HHS. Once the cause has been identified, patients should be treated with bolus fluids to obtain adequate perfusion, followed by IV fluid infusion. Clinicians should carefully monitor the serum sodium level of patients with DKA or HHS to determine the ideal amount and type of fluid required, and also should measure potassium levels prior to starting patients on insulin. (Tables 7 and 8 summarize important clinical pearls when treating patients with DKA or HHS.)
1. Nyenwe EA, Kitabchi AE. The evolution of diabetic ketoacidosis: an update of its etiology, pathogenesis and management. Metabolism. 2016;65(4):507-521. doi:10.1016/j.metabol.2015.12.007.
2. Fayfman M, Pasquel FJ, Umpierrez GE. Management of hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Med Clin North Am. 2017;101(3):587-606. doi:10.1016/j.mcna.2016.12.011.
3. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343. doi:10.2337/dc09-9032.
4. Fugate JE, Rabinstein AA. Absolute and relative contraindications to IV rt-PA for acute ischemic stroke. Neurohospitalist. 2015;5(3):110-121. doi:10.1177/1941874415578532.
5. Kamalakannan D, Baskar V, Barton DM, Abdu TA. Diabetic ketoacidosis in pregnancy. Postgrad Med J. 2003;79(9):454-457.
6. Ma OJ, Rush MD, Godfrey MM, Gaddis G. Arterial blood gas results rarely influence emergency physician management of patients with suspected diabetic ketoacidosis. Acad Emerg Med. 2003;10(8):836-841.
7. Taylor S, Blau J, Rother K. SGLT2 Inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100(8):2849-2852. doi:10.1210/jc.2015-1884.
8. Kum-Nji JS, Gosmanov AR, Steinberg H, Dagogo-Jack S. Hyperglycemic, high anion-gap metabolic acidosis in patients receiving SGLT-2 inhibitors for diabetes management. J Diabetes Complications. 2017;31(3):611-614. doi:10.1016/j.jdiacomp.2016.11.004.
9. Adrogué HJ, Barrero J, Eknoyan G. Salutary effects of modest fluid replacement in the treatment of adults with diabetic ketoacidosis. Use in patients without extreme volume deficit. JAMA. 1989;262(15):2108-2013.
10. Semler MW, Self WH, Wanderer JP, et al; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloid versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. doi:10.1056/NEJMoa1711584.
11. Chua HR, Venkatesh B, Stachowski E, et al. Plasma-Lyte 148 vs 0.9% saline for fluid resuscitation in diabetic ketoacidosis. J Crit Care. 2012;27(2):138-145. doi:10.1016/j.jcrc.2012.01.007.
12. Mahler S, Conrad S, Wang H, Arnold T. Resuscitation with balanced electrolyte solution prevents hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis. Am J Emerg Med. 2011;29(9):1194-1197. doi:10.1016/j.ajem.2010.07.015.
11. Van Zyl DG, Rheeder P, Delport E. Fluid management in diabetic-acidosis--Ringer’s lactate versus normal saline: a randomized controlled trial. QJM. 2012;105(4):337-343. doi:10.1093/qjmed/hcr226.
14. Penne EL, Thijssen S, Raimann JG, Levin NW, Kotanko P. Correction of serum sodium for glucose concentration in hemodialysis patients with poor glucose control. Diabetes Care. 2010;33(7):e91. doi:10.2337/dc10-0557.
15. Goyal N, Miller JB, Sankey SS, Mossallam U. Utility of initial bolus insulin in the treatment of diabetic ketoacidosis. J Emerg Med. 2010;38(4):422-427. doi:10.1016/j.jemermed.2007.11.033.
16. Wolfsdorf JI, Allgrove J, Craig ME, et al; International Society for Pediatric and Adolescent Diabetes. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2014;15(Suppl 20):154-179. doi:10.1111/pedi.12165.
17. Cohn BG, Keim SM, Watkins JW, Camargo CA. Does management of diabetic ketoacidosis with subcutaneous rapid-acting insulin reduce the need for intensive care unit admission? J Emerg Med. 2015;49(4):530-538. doi:10.1016/j.jemermed.2015.05.016.
18. Glaser N, Barnett P, McCaslin I, et al; Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N Engl J Med. 2001;344(4):264-269. doi:10.1056/NEJM200101253440404.
In this review, the authors discuss the similarities and differences between diabetic ketoacidosis and the hyperosmolar hyperglycemic state, providing clinical pearls and common pitfalls to help guide the clinician in the diagnosis and management.
In this review, the authors discuss the similarities and differences between diabetic ketoacidosis and the hyperosmolar hyperglycemic state, providing clinical pearls and common pitfalls to help guide the clinician in the diagnosis and management.
Diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic state (HHS) are similar but distinct diabetic emergencies that are frequently encountered in the ED. Patients with DKA or HHS present with hyperglycemia and dehydration and frequently appear quite ill physically. In both syndromes, there is insufficient insulin levels to transport glucose into cells.
As previously noted, although DKA and HHS share similar characteristic signs and symptoms, they are two distinct conditions that must be differentiated in the clinical work-up. One characteristic that helps the emergency physician (EP) to distinguish between the two conditions is the patient age at symptom onset. Although both conditions can occur at any age, diabetic ketoacidosis typically develops in younger patients, less than 45 years, who have little or no endogenous insulin production, whereas HHS usually occurs in much older non-insulin-dependent patients (who are often greater than 60 years old). 1-3 This review discusses the similarities and differences in the etiology, diagnosis, and treatment of DKA and HHS to guide evaluation and simplify management, highlighting practical tips and clinical pearls. When applicable, information has been organized into groups of five to facilitate retention and recall.
The Etiology of DKA Vs HHS
The fundamental underlying issue in both DKA and HHS is an absolute or relative lack of insulin that results in an increase in counter-regulatory hormones, including glucagon, cortisol, and catecholamines.
Insulin has five main actions: (1) to drive glucose into cells; (2) to drive potassium into cells; (3) to create an anabolic environment; (4) to inhibit breakdown of fat; and (5) to block the breakdown of proteins. (Table 1).
Diabetic ketoacidosis typically develops in patients who lack significant endogenous insulin; this insufficiency of circulating insulin causes hyperglycemia and hyperkalemia, the creation of a catabolic state with high levels of both ketone bodies and free-fatty acids due to the breakdown of proteins and fats.
In contrast, HHS occurs in patients who produce a sufficient amount of insulin to drive potassium into cells and to inhibit the breakdown of proteins and fats; as such these patients are not ketoacidotic. However, patients with HHS do not produce enough insulin to drive glucose intracellularly. As the glucose levels increase, patients with HHS become increasingly hyperosmolar and dehydrated, resulting in further elevation of glucose levels, causing a perpetual cycle of increasing glucose and resultant hyperosmolarity and dehydration.1-3 It is important to appreciate that both hyperglycemic crises result in an osmotic diuresis leading to severe dehydration and urinary wasting of electrolytes.
Diagnosis and Workup
Laboratory Evaluation
In addition to hyperglycemia, another key finding in DKA is elevated anion gap metabolic acidosis resulting from ketoacid production. Laboratory evaluation will demonstrate a pH less than 7.3 and serum bicarbonate level less than 18 mEq/L; urinalysis will be positive for ketones.
Though HHS patients also present with hyperglycemia, laboratory evaluation will demonstrate no, or only mild, acidosis, normal pH (typically >7.3), bicarbonate level greater than 18 mEq/L, and a high-serum osmolality (>350 mOsm/kg). While urinalysis may show low or no ketones, patients with HHS do not develop the marked ketoacidosis seen in DKA. Moreover, glucose values are more elevated in patients with HHS, frequently exceeding 1,000 mg/dL.
Signs and Symptoms
Patients with either of these hyperglycemic crises often present with fatigue, polyuria, and polydipsia. They appear dehydrated on examination with dry mucous membranes, tachycardia, and in severe cases, hypotension. Patients with HHS often present with altered mental status, seizures, and even coma, while patients with DKA typically only experience changes in mental status in the most severe cases (Table 2). As many as one-third of patients with a hyperglycemic crisis will have an overlapping DKA/HHS syndrome.1-3 With respect to patient age, HHS tends to develop in older patients who have type 2 diabetes and several underlying comorbidities.2
Precipitating Causes of DKA and HHS
The five causes of DKA/HHS can be remembered as the “Five I’s”: (1) infection; (2) infarction; (3) infant (pregnancy), (4) indiscretion, and (5) [lack of] insulin (Table 3).
Infection. Infection is one of the most common precipitating factors in both DKA and HHS.1 During the history intake, the EP should ask the patient if he or she has had any recent urinary and respiratory symptoms, as positive responses prompt further investigation with urinalysis and chest radiography. A thorough dermatological examination should be performed to assess for visible signs of infection, particularly in the extremities and intertriginous areas.
When assessing patients with DKA or HHS for signs of infection, body temperature to assess for the presence of fever is not always a reliable indicator. This is because many patients with DKA/HHS develop tachypnea, which can affect the efficacy of an oral thermometer. In such cases, the rectal temperature is more sensitive for detecting fever. It should be noted, however, that some patients with severe infection or immunocompromised state—regardless of the presence or absence of tachypnea—may be normothermic or even hypothermic due to peripheral vasodilation—a poor prognostic sign.3 Blood and urine cultures should be obtained for all patients in whom infection is suspected.
Laboratory evaluation of patients with DKA may demonstrate a mild leukocytosis, a very common finding in DKA even in the absence of infection; leukocytosis is believed to result from elevated stress hormones such as cortisol and epinephrine.3
Infarction. Infarction is another important underlying cause of DKA/HHS and one that must not be overlooked. Screening for acute coronary syndrome through patient history, electrocardiography, and cardiac biomarkers should be performed in all patients older than age 40 years and in patients in whom there is any suggestion of myocardial ischemia.
A thorough neurological examination should be performed to assess for deficits indicative of stroke. Although neurological deficits can be due to severe hyperglycemia, most patients with focal neurological deficits from hyperglycemia also have altered mental status. Focal neurological deficits without a change in mental status are more likely to represent an actual stroke.4
Infant (Pregnancy). Pregnant patients are at an increased risk of developing DKA for several reasons, most notably due to the increased production of insulin-antagonistic hormones, which can lead to higher insulin resistance and thus increased insulin requirements during pregnancy.5 A pregnancy test is therefore indicated for all female patients of child-bearing age.
Indiscretion. Non-compliance with diet, such as taking in too many calories without appropriate insulin correction, and the ingestion of significant amounts of alcohol can lead to DKA. Eating disorders, particularly in young patients, may also contribute to recurrent cases.3
[Lack of] Insulin. In insulin-dependent diabetes, skipped insulin doses or insulin pump failure can trigger DKA/HHS. In fact, missed insulin doses are becoming a more frequent cause of DKA than infections.1
Both DKA and HHS are usually triggered by an underlying illness or event. Therefore, clinicians should always focus on identifying precipitating causes such as acute infection, stroke, or myocardial infarction, as some require immediate treatment. In fact, DKA or HHS is rarely the primary cause of death; patients are much more likely to die from the precipitating event that caused DKA or HHS.1,3
Assessing Disease Severity
Mental status and pH and serum bicarbonate levels help clinicians determine the extent of disease severity, classifying patients as having mild, moderate, or severe DKA (Table 4).2 Patients at the highest risk for poor outcomes include those at the extremes of age, who have severe comorbidities, who have underlying infection, and who are hypotensive and/or in a comatose state.3
Patients who have HHS are much more likely to present with altered mental status, including coma. There is a linear relationship between osmolality and degree of altered mental status. Thus, diabetic patients with major changes in mental status but without high serum osmolality warrant immediate workup for alternative causes of their altered mental status.3 In addition, seizures, especially focal seizures, are relatively common in severe cases of HHS. Finally, highly abnormal blood pressure, pulse, and respiratory rate can also provide additional clues regarding the severity of hyperglycemic crisis.
Arterial Blood Gas Assessment: To Stick or Not to Stick?
In the past, measuring arterial blood gases (ABG) has been considered a mainstay in the evaluation of patients with DKA. But does an arterial stick, which is associated with some risk, really add essential information? One study by Ma et al6 evaluated whether ABG results significantly alter how physicians manage patients with DKA. In the study, the authors evaluated 200 ED patients and found that ABG analysis only changed diagnosis in 1% of patients, altered treatment in 3.5% of patients, and changed disposition in only 1% of patients. Arterial stick partial pressure of oxygen and partial pressure of carbon dioxide altered treatment and disposition in only 1% of patients. Furthermore, the study results showed venous pH correlated very strongly with arterial pH (r = 0.95).6 These findings demonstrate that ABG measurements rarely affect or alter DKA management, and support the use of venous pH as an adequate substitute for ABG testing.
Euglycemia
Euglycemic DKA has been reported in patients with type 1 diabetes who had been fasting or vomiting or who had received exogenous insulin prior to presentation.1Euglycemia has also been reported in pregnant patients with type I diabetes.1 More recently, sodium glucose cotransporter 2 inhibitors (SGLT2) have been shown to cause euglycemic DKA. While the therapeutic mechanism of this drug class is to inhibit proximal tubular resorption of glucose, they can cause DKA by decreasing renal clearance of ketone bodies and increasing glucagon levels and promoting hepatic ketogenesis. Patients with DKA who are on SGLT2 inhibitors may present with only modestly elevated glucose levels (typically in the 200- to 300-mg/dL range), but have profound wide gap metabolic acidosis due to β-hydroxybutyrate acid and acetoacetate accumulation.7 When evaluating patients on SGLT2 inhibitors for DKA, EPs should not solely rely on glucose values but rather assess the patient’s overall clinical picture, including the physical examination, vital signs, and pH. Additionally, once resuscitation with intravenous (IV) fluids and insulin is initiated, it may take longer for patients who use SGLT2 inhibitors to clear ketoacids than patients with DKA who do not use these medications.8
Treatment
The goal of treatment for DKA and HHS is to correct volume deficits, hyperglycemia, and electrolyte abnormalities (Table 5). Three of the five therapies to manage DKA and HHS are mandatory: IV fluid resuscitation, IV insulin, and IV potassium. The other therapies, IV bicarbonate, and IV phosphate, should be considered, but are rarely required for DKA or HHS.
In general, management of HHS is less aggressive than that of DKA because HHS develops over a period of weeks—unlike DKA, which develops over only 1 to 2 days. Treatment of any underlying causes of HHS should occur simultaneously.
Intravenous Fluids
The goal of IV fluid therapy for patients with DKA is rehydration—not to “wash-out” ketones. Ketone elimination occurs through insulin-stimulated metabolism. When determining volume-replacement goals, it is helpful to keep in mind that patients with moderate-to-mild DKA typically have fluid deficiencies of 3 to 5 L, and patients with severe DKA have fluid deficiencies between 5 and 6 L. Patients with HHS present with significantly higher fluid deficiencies of around 9 to 12 L.
The initial goal of fluid management is to correct hypoperfusion with bolus fluids, followed by a more gradual repletion of remaining deficits. After bolus fluids are administered, the rate and type of subsequent IV fluid infusion varies depending on hemodynamics, hydration state, and serum sodium levels.2,3
Nonaggressive Vs Aggressive Fluid Management. One study by Adrogué et al9 compared the effects of managing DKA with aggressive vs nonaggressive fluid repletion. In the study, one group of patients received normal saline at 1,000 mL per hour for 4 hours, followed by normal saline at 500 mL per hour for 4 hours. The other group of patients received normal saline at a more modest rate of 500 mL per hour for 4 hours, followed by normal saline at 250 mL per hour for 4 hours. The authors found that patients in the less aggressive volume therapy group achieved a prompt and adequate recovery and maintained higher serum bicarbonate levels.9
Current recommendations for patients with DKA are to first treat patients with an initial bolus of 1,000 mL or 20 cc/kg of normal saline. Patients without profound dehydration should then receive 500 cc of normal saline per hour for the first 4 hours of treatment, after which the flow rate may be reduced to 250 cc per hour. For patients with mild DKA, therapy can start at 250 cc per hour with a smaller bolus dose or no bolus dose. Patients with profound dehydration and poor perfusion, should receive crystalloid fluids wide open until perfusion has improved. Overall, volume resuscitation in HHS is similar to DKA. However, the EP should be cautious with respect to total fluid volume and infusion rates to avoid fluid overload, since many patients with HHS are elderly and may have congestive heart failure.
Crystalloid Fluid Type for Initial Resuscitation. Normal saline has been the traditional crystalloid fluid of choice for managing DKA and HHS. Recent studies, however, have shown some benefit to using balanced solutions (Ringer’s lactate or PlasmaLyte) instead of normal saline. A recently published large study by Semler et al10 compared balanced crystalloid, in most cases lactated Ringers solution, to normal saline in critically ill adult patients, some of whom were diagnosed with DKA. The study demonstrated decreased mortality (from any cause) in the group who received balanced crystalloid fluid therapy and reduced need for renal-replacement therapy and reduced incidence of persistent renal dysfunction. The findings by Semler et al10 and findings from other smaller studies, calls into question whether normal saline is the best crystalloid to manage DKA and HHS.11,12 It remains to be seen what modification the American Diabetes Association (ADA) will make to its current recommendations for fluid therapy, which were last updated in 2009.
It appears that though the use of Ringers lactate or PlasmaLyte to treat DKA usually raises a patient’s serum bicarbonate level to 18 mEq/L at a more rapid rate than normal saline, the use of balanced solutions may result in longer time to lower blood glucose to 250 mg/dL.13 However, by using a balanced solution, the hyperchloremic metabolic acidosis often seen with normal saline treatment will be avoided.12
Half-Normal Saline. An initially normal or increased serum sodium level, despite significant hyperglycemia, suggests a substantial free-water deficit. Calculating a corrected serum sodium can help quantify the degree of free-water deficit.3 While isotonic fluids remain the standard for initial volume load, clinicians should consider switching patients to half-normal saline following initial resuscitation if the corrected serum sodium is elevated above normal. The simplest estimation to correct sodium levels in DKA is to expect a decrease in sodium levels at a rate of at least 2 mEq/L per 100-mg/dL increase in glucose levels above 100 mg/dL. For a more accurate calculation, providers can expect a drop in sodium of 1.6 mEq/L per 100 mg/dL increase of glucose up to a level of 400 mg/dL and then a fall of 2.4 mEq/L in sodium per every 100 mg/dL rise in glucose thereafter.14
Glucose. Patients with DKA require insulin therapy until ketoacidosis resolves. However, the average time to correct ketoacidosis from initiating treatment is about 12 hours compared to only 6 hours for correction of hyperglycemia. Since insulin therapy must be continued despite lower glucose levels, patients are at risk for developing hypoglycemia if glucose is not added to IV fluids. To prevent hypoglycemia and provide an energy source for ketone metabolism, patients should be switched to fluids containing dextrose when their serum glucose approaches 200 to 250 mg/dL.1,3 Typically, 5% dextrose in half-normal saline at 150 to 250 cc per hour is usually adequate to achieve this goal.
Insulin
As previously noted, insulin therapy is required to treat hyperglycemic crises from DKA and HHS. In DKA there is an absolute insulin deficiency, whereas in HHS, there is a relative insulin deficiency. In HHS, there is not enough endogenous insulin to move glucose into the cells, but there is enough insulin to block a catabolic state. That is why the breakdown of fats and proteins does not occur, and why ketoacidosis and hyperkalemia are not seen in HHS. On the other hand, glucose elevations do occur, and are usually more extreme in HHS than DKA. There are five major therapeutic actions of insulin in DKA (Table 5), and it is imperative to determine serum potassium before starting an insulin infusion as insulin will drive potassium into the cell, worsening hypokalemia and promoting the development of life-threatening arrhythmias, including ventricular fibrillation, ventricular tachycardia, and torsades de pointes. The electrocardiogram does not accurately predict severity of hypokalemia and should not be used as a substitute for direct potassium measurement.
Loading Dose and Drip Rate. When treating adult patients with DKA or HHS, the ADA recommends an IV push loading dose of 0.1 U/kg insulin, followed by an hourly maintenance dose of 0.1 U/kg. Alternatively, a continuous infusion of 0.16 U/kg/hr can be used without a bolus. The rationale behind a bolus is the rapid saturation of insulin receptors, followed by a drip to maintain saturation of receptors. However, a recent prospective observational cohort study by Goyal et al15 questions the utility of the initial insulin bolus. The study compared DKA patients who received an initial insulin bolus to those who did not. Both groups were similar at baseline and received equivalent IV fluids and insulin drips. They found no statistically significant differences in the incidence of hypoglycemia, rate of serum glucose change, anion gap change, or length of stay in the ED or hospital. The authors concluded that administration of an insulin bolus has no significant benefit to patients and does not change clinically relevant end-points.15 At this time, there is no proven benefit to giving DKA patients an IV insulin bolus; moreover, doing so may further increase hypoglycemia. The use of an insulin bolus is particularly not recommended for use in pediatric patients with DKA due to a higher incidence of hypoglycemia in this patient population.16
As with DKA, the ADA3 recommends giving HHS patients an insulin bolus of 0.1 U/ kg followed by a continuous infusion at 0.1 U/kg per hour. It is crucial to monitor patients closely to ensure glucose levels do not fall too rapidly. Glucose levels should be kept between 150 to 200 mg/dL for patients with DKA and 200 to 300 mg/dL for patients with HHS until the conditions resolve; this may necessitate lowering the infusion rate to 0.02 to 0.05 U/kg per hour. In addition to frequent glucose monitoring, a basic metabolic panel and venous pH should be obtained every 2 to 4 hours while a patient is on an insulin drip.3
Subcutaneous Vs Intravenous Insulin for DKA. Several small studies evaluating patients with mild-moderate DKA demonstrated similar outcomes when subcutaneous (SQ) insulin was used instead of IV insulin. However, SQ injections require more frequent dosing (every 1 to 2 hours) and still require close monitoring of blood glucose. This monitoring frequency is usually not feasible on a hospital floor, but may be feasible on step-down units, thus avoiding admission to the intensive care unit (ICU) for patients who do not otherwise require ICU-level of care.17 Subcutaneous insulin should not be given to patients with severe acidosis, hypotension, or altered mental status. The ADA consensus statement continues to recommend IV infusion of regular insulin as the preferred route due to its short half-life and easy titration.3
Determining When to Switch to Subcutaneous Insulin. Ideally, patients are not in the ED long enough to have their metabolic abnormalities corrected, as this usually requires several hours. In DKA, the insulin drip should continue until the blood glucose is less than 200 and at least two of the following conditions are met: the anion gap is less than 12, venous pH greater than 7.3, and serum bicarbonate >15. In HHS, osmolality and mental status should both return to normal prior to stopping the infusion. In both cases, subcutaneous insulin should be administered at least 1 to 2 hours before stopping the drip to prevent recurrent crisis.1,3
Refractory Acidosis. First and foremost, refractory acidosis should prompt a diligent source for dead gut, abscess, and underlying sepsis. While vomiting and diffuse abdominal pain are common in DKA and are related to ketoacidosis, these symptoms are atypical of HHS and should raise suspicion for underlying pathology.3 Additionally, a lower than expected bicarbonate level can also occur from resuscitation with large volumes of normal saline, resulting in a hyperchloremic non-gap metabolic acidosis.
Potassium
Both DKA and HHS patients have total body potassium deficits due to osmotic diuresis that require careful repletion. Deficits can be substantial: The average total whole body potassium deficit in DKA is 3 to 5 mEq/kg.2 Clinicians should exercise caution, however, since DKA patients may be hyperkalemic initially despite a total body potassium deficit. Early hyperkalemia is due to the transmembrane shift of potassium secondary to acidosis and insulin deficiency as well as hypertonicity. If initial potassium is greater than 5.2 mEq/L, potassium should not be administered but instead rechecked in 1 to 2 hours. If the potassium level is 4.0 to 5.2 mEq/L, then 10 mEq per hour is usually adequate. For levels between 3.3 and 4.0 mEq/L, administer potassium chloride at 20 mEq per hour. For levels less than 3.3 mEq/L, insulin should be held and potassium chloride should be aggressively repleted at 20 to 30 mEq per hour with continuous cardiac monitoring.1-3 Failure to recognize and act on critical potassium levels is a known cause of unexpected death in DKA. During the first hour of DKA onset, patients are more likely to die from hyperkalemia. Later, while the patient is “stabilizing” on an insulin infusion, potassium levels will fall as insulin drives potassium back into cells.
Bicarbonate
Bicarbonate has many theoretical benefits but also has potential risks (Table 6).
Phosphate
Since there is no proven benefit to giving phosphate to adult patients with DKA, it is rarely used, except in specific situations other than pediatric DKA. Similar to potassium, initial serum phosphate levels do not reflect total body phosphate levels due to transmembrane shifts.2,3 Phosphate repletionis most beneficial for patients who have cachexia, respiratory depression, anemia, cardiac dysfunction, or phosphate values lower than 1.0 to 1.5. If given, 20 to 30 mEq/L potassium phosphate (K2PO4) added to fluids is usually sufficient.3 Overly aggressive phosphate administration (>4.5 mmol/h or 1.5 mL/h potassium phosphate) can cause severe hypocalcemia and should be avoided.1,3 In pediatric patients, up to one-half of potassium requirements are often given as potassium phosphate, but this may vary by institution.
Conclusion
Both DKA and HHS are diabetic emergencies that must be approached and managed systematically to correct underlying dehydration and metabolic abnormalities. Patient care begins by determining the etiology of these conditions, especially HHS. Once the cause has been identified, patients should be treated with bolus fluids to obtain adequate perfusion, followed by IV fluid infusion. Clinicians should carefully monitor the serum sodium level of patients with DKA or HHS to determine the ideal amount and type of fluid required, and also should measure potassium levels prior to starting patients on insulin. (Tables 7 and 8 summarize important clinical pearls when treating patients with DKA or HHS.)
Diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic state (HHS) are similar but distinct diabetic emergencies that are frequently encountered in the ED. Patients with DKA or HHS present with hyperglycemia and dehydration and frequently appear quite ill physically. In both syndromes, there is insufficient insulin levels to transport glucose into cells.
As previously noted, although DKA and HHS share similar characteristic signs and symptoms, they are two distinct conditions that must be differentiated in the clinical work-up. One characteristic that helps the emergency physician (EP) to distinguish between the two conditions is the patient age at symptom onset. Although both conditions can occur at any age, diabetic ketoacidosis typically develops in younger patients, less than 45 years, who have little or no endogenous insulin production, whereas HHS usually occurs in much older non-insulin-dependent patients (who are often greater than 60 years old). 1-3 This review discusses the similarities and differences in the etiology, diagnosis, and treatment of DKA and HHS to guide evaluation and simplify management, highlighting practical tips and clinical pearls. When applicable, information has been organized into groups of five to facilitate retention and recall.
The Etiology of DKA Vs HHS
The fundamental underlying issue in both DKA and HHS is an absolute or relative lack of insulin that results in an increase in counter-regulatory hormones, including glucagon, cortisol, and catecholamines.
Insulin has five main actions: (1) to drive glucose into cells; (2) to drive potassium into cells; (3) to create an anabolic environment; (4) to inhibit breakdown of fat; and (5) to block the breakdown of proteins. (Table 1).
Diabetic ketoacidosis typically develops in patients who lack significant endogenous insulin; this insufficiency of circulating insulin causes hyperglycemia and hyperkalemia, the creation of a catabolic state with high levels of both ketone bodies and free-fatty acids due to the breakdown of proteins and fats.
In contrast, HHS occurs in patients who produce a sufficient amount of insulin to drive potassium into cells and to inhibit the breakdown of proteins and fats; as such these patients are not ketoacidotic. However, patients with HHS do not produce enough insulin to drive glucose intracellularly. As the glucose levels increase, patients with HHS become increasingly hyperosmolar and dehydrated, resulting in further elevation of glucose levels, causing a perpetual cycle of increasing glucose and resultant hyperosmolarity and dehydration.1-3 It is important to appreciate that both hyperglycemic crises result in an osmotic diuresis leading to severe dehydration and urinary wasting of electrolytes.
Diagnosis and Workup
Laboratory Evaluation
In addition to hyperglycemia, another key finding in DKA is elevated anion gap metabolic acidosis resulting from ketoacid production. Laboratory evaluation will demonstrate a pH less than 7.3 and serum bicarbonate level less than 18 mEq/L; urinalysis will be positive for ketones.
Though HHS patients also present with hyperglycemia, laboratory evaluation will demonstrate no, or only mild, acidosis, normal pH (typically >7.3), bicarbonate level greater than 18 mEq/L, and a high-serum osmolality (>350 mOsm/kg). While urinalysis may show low or no ketones, patients with HHS do not develop the marked ketoacidosis seen in DKA. Moreover, glucose values are more elevated in patients with HHS, frequently exceeding 1,000 mg/dL.
Signs and Symptoms
Patients with either of these hyperglycemic crises often present with fatigue, polyuria, and polydipsia. They appear dehydrated on examination with dry mucous membranes, tachycardia, and in severe cases, hypotension. Patients with HHS often present with altered mental status, seizures, and even coma, while patients with DKA typically only experience changes in mental status in the most severe cases (Table 2). As many as one-third of patients with a hyperglycemic crisis will have an overlapping DKA/HHS syndrome.1-3 With respect to patient age, HHS tends to develop in older patients who have type 2 diabetes and several underlying comorbidities.2
Precipitating Causes of DKA and HHS
The five causes of DKA/HHS can be remembered as the “Five I’s”: (1) infection; (2) infarction; (3) infant (pregnancy), (4) indiscretion, and (5) [lack of] insulin (Table 3).
Infection. Infection is one of the most common precipitating factors in both DKA and HHS.1 During the history intake, the EP should ask the patient if he or she has had any recent urinary and respiratory symptoms, as positive responses prompt further investigation with urinalysis and chest radiography. A thorough dermatological examination should be performed to assess for visible signs of infection, particularly in the extremities and intertriginous areas.
When assessing patients with DKA or HHS for signs of infection, body temperature to assess for the presence of fever is not always a reliable indicator. This is because many patients with DKA/HHS develop tachypnea, which can affect the efficacy of an oral thermometer. In such cases, the rectal temperature is more sensitive for detecting fever. It should be noted, however, that some patients with severe infection or immunocompromised state—regardless of the presence or absence of tachypnea—may be normothermic or even hypothermic due to peripheral vasodilation—a poor prognostic sign.3 Blood and urine cultures should be obtained for all patients in whom infection is suspected.
Laboratory evaluation of patients with DKA may demonstrate a mild leukocytosis, a very common finding in DKA even in the absence of infection; leukocytosis is believed to result from elevated stress hormones such as cortisol and epinephrine.3
Infarction. Infarction is another important underlying cause of DKA/HHS and one that must not be overlooked. Screening for acute coronary syndrome through patient history, electrocardiography, and cardiac biomarkers should be performed in all patients older than age 40 years and in patients in whom there is any suggestion of myocardial ischemia.
A thorough neurological examination should be performed to assess for deficits indicative of stroke. Although neurological deficits can be due to severe hyperglycemia, most patients with focal neurological deficits from hyperglycemia also have altered mental status. Focal neurological deficits without a change in mental status are more likely to represent an actual stroke.4
Infant (Pregnancy). Pregnant patients are at an increased risk of developing DKA for several reasons, most notably due to the increased production of insulin-antagonistic hormones, which can lead to higher insulin resistance and thus increased insulin requirements during pregnancy.5 A pregnancy test is therefore indicated for all female patients of child-bearing age.
Indiscretion. Non-compliance with diet, such as taking in too many calories without appropriate insulin correction, and the ingestion of significant amounts of alcohol can lead to DKA. Eating disorders, particularly in young patients, may also contribute to recurrent cases.3
[Lack of] Insulin. In insulin-dependent diabetes, skipped insulin doses or insulin pump failure can trigger DKA/HHS. In fact, missed insulin doses are becoming a more frequent cause of DKA than infections.1
Both DKA and HHS are usually triggered by an underlying illness or event. Therefore, clinicians should always focus on identifying precipitating causes such as acute infection, stroke, or myocardial infarction, as some require immediate treatment. In fact, DKA or HHS is rarely the primary cause of death; patients are much more likely to die from the precipitating event that caused DKA or HHS.1,3
Assessing Disease Severity
Mental status and pH and serum bicarbonate levels help clinicians determine the extent of disease severity, classifying patients as having mild, moderate, or severe DKA (Table 4).2 Patients at the highest risk for poor outcomes include those at the extremes of age, who have severe comorbidities, who have underlying infection, and who are hypotensive and/or in a comatose state.3
Patients who have HHS are much more likely to present with altered mental status, including coma. There is a linear relationship between osmolality and degree of altered mental status. Thus, diabetic patients with major changes in mental status but without high serum osmolality warrant immediate workup for alternative causes of their altered mental status.3 In addition, seizures, especially focal seizures, are relatively common in severe cases of HHS. Finally, highly abnormal blood pressure, pulse, and respiratory rate can also provide additional clues regarding the severity of hyperglycemic crisis.
Arterial Blood Gas Assessment: To Stick or Not to Stick?
In the past, measuring arterial blood gases (ABG) has been considered a mainstay in the evaluation of patients with DKA. But does an arterial stick, which is associated with some risk, really add essential information? One study by Ma et al6 evaluated whether ABG results significantly alter how physicians manage patients with DKA. In the study, the authors evaluated 200 ED patients and found that ABG analysis only changed diagnosis in 1% of patients, altered treatment in 3.5% of patients, and changed disposition in only 1% of patients. Arterial stick partial pressure of oxygen and partial pressure of carbon dioxide altered treatment and disposition in only 1% of patients. Furthermore, the study results showed venous pH correlated very strongly with arterial pH (r = 0.95).6 These findings demonstrate that ABG measurements rarely affect or alter DKA management, and support the use of venous pH as an adequate substitute for ABG testing.
Euglycemia
Euglycemic DKA has been reported in patients with type 1 diabetes who had been fasting or vomiting or who had received exogenous insulin prior to presentation.1Euglycemia has also been reported in pregnant patients with type I diabetes.1 More recently, sodium glucose cotransporter 2 inhibitors (SGLT2) have been shown to cause euglycemic DKA. While the therapeutic mechanism of this drug class is to inhibit proximal tubular resorption of glucose, they can cause DKA by decreasing renal clearance of ketone bodies and increasing glucagon levels and promoting hepatic ketogenesis. Patients with DKA who are on SGLT2 inhibitors may present with only modestly elevated glucose levels (typically in the 200- to 300-mg/dL range), but have profound wide gap metabolic acidosis due to β-hydroxybutyrate acid and acetoacetate accumulation.7 When evaluating patients on SGLT2 inhibitors for DKA, EPs should not solely rely on glucose values but rather assess the patient’s overall clinical picture, including the physical examination, vital signs, and pH. Additionally, once resuscitation with intravenous (IV) fluids and insulin is initiated, it may take longer for patients who use SGLT2 inhibitors to clear ketoacids than patients with DKA who do not use these medications.8
Treatment
The goal of treatment for DKA and HHS is to correct volume deficits, hyperglycemia, and electrolyte abnormalities (Table 5). Three of the five therapies to manage DKA and HHS are mandatory: IV fluid resuscitation, IV insulin, and IV potassium. The other therapies, IV bicarbonate, and IV phosphate, should be considered, but are rarely required for DKA or HHS.
In general, management of HHS is less aggressive than that of DKA because HHS develops over a period of weeks—unlike DKA, which develops over only 1 to 2 days. Treatment of any underlying causes of HHS should occur simultaneously.
Intravenous Fluids
The goal of IV fluid therapy for patients with DKA is rehydration—not to “wash-out” ketones. Ketone elimination occurs through insulin-stimulated metabolism. When determining volume-replacement goals, it is helpful to keep in mind that patients with moderate-to-mild DKA typically have fluid deficiencies of 3 to 5 L, and patients with severe DKA have fluid deficiencies between 5 and 6 L. Patients with HHS present with significantly higher fluid deficiencies of around 9 to 12 L.
The initial goal of fluid management is to correct hypoperfusion with bolus fluids, followed by a more gradual repletion of remaining deficits. After bolus fluids are administered, the rate and type of subsequent IV fluid infusion varies depending on hemodynamics, hydration state, and serum sodium levels.2,3
Nonaggressive Vs Aggressive Fluid Management. One study by Adrogué et al9 compared the effects of managing DKA with aggressive vs nonaggressive fluid repletion. In the study, one group of patients received normal saline at 1,000 mL per hour for 4 hours, followed by normal saline at 500 mL per hour for 4 hours. The other group of patients received normal saline at a more modest rate of 500 mL per hour for 4 hours, followed by normal saline at 250 mL per hour for 4 hours. The authors found that patients in the less aggressive volume therapy group achieved a prompt and adequate recovery and maintained higher serum bicarbonate levels.9
Current recommendations for patients with DKA are to first treat patients with an initial bolus of 1,000 mL or 20 cc/kg of normal saline. Patients without profound dehydration should then receive 500 cc of normal saline per hour for the first 4 hours of treatment, after which the flow rate may be reduced to 250 cc per hour. For patients with mild DKA, therapy can start at 250 cc per hour with a smaller bolus dose or no bolus dose. Patients with profound dehydration and poor perfusion, should receive crystalloid fluids wide open until perfusion has improved. Overall, volume resuscitation in HHS is similar to DKA. However, the EP should be cautious with respect to total fluid volume and infusion rates to avoid fluid overload, since many patients with HHS are elderly and may have congestive heart failure.
Crystalloid Fluid Type for Initial Resuscitation. Normal saline has been the traditional crystalloid fluid of choice for managing DKA and HHS. Recent studies, however, have shown some benefit to using balanced solutions (Ringer’s lactate or PlasmaLyte) instead of normal saline. A recently published large study by Semler et al10 compared balanced crystalloid, in most cases lactated Ringers solution, to normal saline in critically ill adult patients, some of whom were diagnosed with DKA. The study demonstrated decreased mortality (from any cause) in the group who received balanced crystalloid fluid therapy and reduced need for renal-replacement therapy and reduced incidence of persistent renal dysfunction. The findings by Semler et al10 and findings from other smaller studies, calls into question whether normal saline is the best crystalloid to manage DKA and HHS.11,12 It remains to be seen what modification the American Diabetes Association (ADA) will make to its current recommendations for fluid therapy, which were last updated in 2009.
It appears that though the use of Ringers lactate or PlasmaLyte to treat DKA usually raises a patient’s serum bicarbonate level to 18 mEq/L at a more rapid rate than normal saline, the use of balanced solutions may result in longer time to lower blood glucose to 250 mg/dL.13 However, by using a balanced solution, the hyperchloremic metabolic acidosis often seen with normal saline treatment will be avoided.12
Half-Normal Saline. An initially normal or increased serum sodium level, despite significant hyperglycemia, suggests a substantial free-water deficit. Calculating a corrected serum sodium can help quantify the degree of free-water deficit.3 While isotonic fluids remain the standard for initial volume load, clinicians should consider switching patients to half-normal saline following initial resuscitation if the corrected serum sodium is elevated above normal. The simplest estimation to correct sodium levels in DKA is to expect a decrease in sodium levels at a rate of at least 2 mEq/L per 100-mg/dL increase in glucose levels above 100 mg/dL. For a more accurate calculation, providers can expect a drop in sodium of 1.6 mEq/L per 100 mg/dL increase of glucose up to a level of 400 mg/dL and then a fall of 2.4 mEq/L in sodium per every 100 mg/dL rise in glucose thereafter.14
Glucose. Patients with DKA require insulin therapy until ketoacidosis resolves. However, the average time to correct ketoacidosis from initiating treatment is about 12 hours compared to only 6 hours for correction of hyperglycemia. Since insulin therapy must be continued despite lower glucose levels, patients are at risk for developing hypoglycemia if glucose is not added to IV fluids. To prevent hypoglycemia and provide an energy source for ketone metabolism, patients should be switched to fluids containing dextrose when their serum glucose approaches 200 to 250 mg/dL.1,3 Typically, 5% dextrose in half-normal saline at 150 to 250 cc per hour is usually adequate to achieve this goal.
Insulin
As previously noted, insulin therapy is required to treat hyperglycemic crises from DKA and HHS. In DKA there is an absolute insulin deficiency, whereas in HHS, there is a relative insulin deficiency. In HHS, there is not enough endogenous insulin to move glucose into the cells, but there is enough insulin to block a catabolic state. That is why the breakdown of fats and proteins does not occur, and why ketoacidosis and hyperkalemia are not seen in HHS. On the other hand, glucose elevations do occur, and are usually more extreme in HHS than DKA. There are five major therapeutic actions of insulin in DKA (Table 5), and it is imperative to determine serum potassium before starting an insulin infusion as insulin will drive potassium into the cell, worsening hypokalemia and promoting the development of life-threatening arrhythmias, including ventricular fibrillation, ventricular tachycardia, and torsades de pointes. The electrocardiogram does not accurately predict severity of hypokalemia and should not be used as a substitute for direct potassium measurement.
Loading Dose and Drip Rate. When treating adult patients with DKA or HHS, the ADA recommends an IV push loading dose of 0.1 U/kg insulin, followed by an hourly maintenance dose of 0.1 U/kg. Alternatively, a continuous infusion of 0.16 U/kg/hr can be used without a bolus. The rationale behind a bolus is the rapid saturation of insulin receptors, followed by a drip to maintain saturation of receptors. However, a recent prospective observational cohort study by Goyal et al15 questions the utility of the initial insulin bolus. The study compared DKA patients who received an initial insulin bolus to those who did not. Both groups were similar at baseline and received equivalent IV fluids and insulin drips. They found no statistically significant differences in the incidence of hypoglycemia, rate of serum glucose change, anion gap change, or length of stay in the ED or hospital. The authors concluded that administration of an insulin bolus has no significant benefit to patients and does not change clinically relevant end-points.15 At this time, there is no proven benefit to giving DKA patients an IV insulin bolus; moreover, doing so may further increase hypoglycemia. The use of an insulin bolus is particularly not recommended for use in pediatric patients with DKA due to a higher incidence of hypoglycemia in this patient population.16
As with DKA, the ADA3 recommends giving HHS patients an insulin bolus of 0.1 U/ kg followed by a continuous infusion at 0.1 U/kg per hour. It is crucial to monitor patients closely to ensure glucose levels do not fall too rapidly. Glucose levels should be kept between 150 to 200 mg/dL for patients with DKA and 200 to 300 mg/dL for patients with HHS until the conditions resolve; this may necessitate lowering the infusion rate to 0.02 to 0.05 U/kg per hour. In addition to frequent glucose monitoring, a basic metabolic panel and venous pH should be obtained every 2 to 4 hours while a patient is on an insulin drip.3
Subcutaneous Vs Intravenous Insulin for DKA. Several small studies evaluating patients with mild-moderate DKA demonstrated similar outcomes when subcutaneous (SQ) insulin was used instead of IV insulin. However, SQ injections require more frequent dosing (every 1 to 2 hours) and still require close monitoring of blood glucose. This monitoring frequency is usually not feasible on a hospital floor, but may be feasible on step-down units, thus avoiding admission to the intensive care unit (ICU) for patients who do not otherwise require ICU-level of care.17 Subcutaneous insulin should not be given to patients with severe acidosis, hypotension, or altered mental status. The ADA consensus statement continues to recommend IV infusion of regular insulin as the preferred route due to its short half-life and easy titration.3
Determining When to Switch to Subcutaneous Insulin. Ideally, patients are not in the ED long enough to have their metabolic abnormalities corrected, as this usually requires several hours. In DKA, the insulin drip should continue until the blood glucose is less than 200 and at least two of the following conditions are met: the anion gap is less than 12, venous pH greater than 7.3, and serum bicarbonate >15. In HHS, osmolality and mental status should both return to normal prior to stopping the infusion. In both cases, subcutaneous insulin should be administered at least 1 to 2 hours before stopping the drip to prevent recurrent crisis.1,3
Refractory Acidosis. First and foremost, refractory acidosis should prompt a diligent source for dead gut, abscess, and underlying sepsis. While vomiting and diffuse abdominal pain are common in DKA and are related to ketoacidosis, these symptoms are atypical of HHS and should raise suspicion for underlying pathology.3 Additionally, a lower than expected bicarbonate level can also occur from resuscitation with large volumes of normal saline, resulting in a hyperchloremic non-gap metabolic acidosis.
Potassium
Both DKA and HHS patients have total body potassium deficits due to osmotic diuresis that require careful repletion. Deficits can be substantial: The average total whole body potassium deficit in DKA is 3 to 5 mEq/kg.2 Clinicians should exercise caution, however, since DKA patients may be hyperkalemic initially despite a total body potassium deficit. Early hyperkalemia is due to the transmembrane shift of potassium secondary to acidosis and insulin deficiency as well as hypertonicity. If initial potassium is greater than 5.2 mEq/L, potassium should not be administered but instead rechecked in 1 to 2 hours. If the potassium level is 4.0 to 5.2 mEq/L, then 10 mEq per hour is usually adequate. For levels between 3.3 and 4.0 mEq/L, administer potassium chloride at 20 mEq per hour. For levels less than 3.3 mEq/L, insulin should be held and potassium chloride should be aggressively repleted at 20 to 30 mEq per hour with continuous cardiac monitoring.1-3 Failure to recognize and act on critical potassium levels is a known cause of unexpected death in DKA. During the first hour of DKA onset, patients are more likely to die from hyperkalemia. Later, while the patient is “stabilizing” on an insulin infusion, potassium levels will fall as insulin drives potassium back into cells.
Bicarbonate
Bicarbonate has many theoretical benefits but also has potential risks (Table 6).
Phosphate
Since there is no proven benefit to giving phosphate to adult patients with DKA, it is rarely used, except in specific situations other than pediatric DKA. Similar to potassium, initial serum phosphate levels do not reflect total body phosphate levels due to transmembrane shifts.2,3 Phosphate repletionis most beneficial for patients who have cachexia, respiratory depression, anemia, cardiac dysfunction, or phosphate values lower than 1.0 to 1.5. If given, 20 to 30 mEq/L potassium phosphate (K2PO4) added to fluids is usually sufficient.3 Overly aggressive phosphate administration (>4.5 mmol/h or 1.5 mL/h potassium phosphate) can cause severe hypocalcemia and should be avoided.1,3 In pediatric patients, up to one-half of potassium requirements are often given as potassium phosphate, but this may vary by institution.
Conclusion
Both DKA and HHS are diabetic emergencies that must be approached and managed systematically to correct underlying dehydration and metabolic abnormalities. Patient care begins by determining the etiology of these conditions, especially HHS. Once the cause has been identified, patients should be treated with bolus fluids to obtain adequate perfusion, followed by IV fluid infusion. Clinicians should carefully monitor the serum sodium level of patients with DKA or HHS to determine the ideal amount and type of fluid required, and also should measure potassium levels prior to starting patients on insulin. (Tables 7 and 8 summarize important clinical pearls when treating patients with DKA or HHS.)
1. Nyenwe EA, Kitabchi AE. The evolution of diabetic ketoacidosis: an update of its etiology, pathogenesis and management. Metabolism. 2016;65(4):507-521. doi:10.1016/j.metabol.2015.12.007.
2. Fayfman M, Pasquel FJ, Umpierrez GE. Management of hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Med Clin North Am. 2017;101(3):587-606. doi:10.1016/j.mcna.2016.12.011.
3. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343. doi:10.2337/dc09-9032.
4. Fugate JE, Rabinstein AA. Absolute and relative contraindications to IV rt-PA for acute ischemic stroke. Neurohospitalist. 2015;5(3):110-121. doi:10.1177/1941874415578532.
5. Kamalakannan D, Baskar V, Barton DM, Abdu TA. Diabetic ketoacidosis in pregnancy. Postgrad Med J. 2003;79(9):454-457.
6. Ma OJ, Rush MD, Godfrey MM, Gaddis G. Arterial blood gas results rarely influence emergency physician management of patients with suspected diabetic ketoacidosis. Acad Emerg Med. 2003;10(8):836-841.
7. Taylor S, Blau J, Rother K. SGLT2 Inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100(8):2849-2852. doi:10.1210/jc.2015-1884.
8. Kum-Nji JS, Gosmanov AR, Steinberg H, Dagogo-Jack S. Hyperglycemic, high anion-gap metabolic acidosis in patients receiving SGLT-2 inhibitors for diabetes management. J Diabetes Complications. 2017;31(3):611-614. doi:10.1016/j.jdiacomp.2016.11.004.
9. Adrogué HJ, Barrero J, Eknoyan G. Salutary effects of modest fluid replacement in the treatment of adults with diabetic ketoacidosis. Use in patients without extreme volume deficit. JAMA. 1989;262(15):2108-2013.
10. Semler MW, Self WH, Wanderer JP, et al; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloid versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. doi:10.1056/NEJMoa1711584.
11. Chua HR, Venkatesh B, Stachowski E, et al. Plasma-Lyte 148 vs 0.9% saline for fluid resuscitation in diabetic ketoacidosis. J Crit Care. 2012;27(2):138-145. doi:10.1016/j.jcrc.2012.01.007.
12. Mahler S, Conrad S, Wang H, Arnold T. Resuscitation with balanced electrolyte solution prevents hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis. Am J Emerg Med. 2011;29(9):1194-1197. doi:10.1016/j.ajem.2010.07.015.
11. Van Zyl DG, Rheeder P, Delport E. Fluid management in diabetic-acidosis--Ringer’s lactate versus normal saline: a randomized controlled trial. QJM. 2012;105(4):337-343. doi:10.1093/qjmed/hcr226.
14. Penne EL, Thijssen S, Raimann JG, Levin NW, Kotanko P. Correction of serum sodium for glucose concentration in hemodialysis patients with poor glucose control. Diabetes Care. 2010;33(7):e91. doi:10.2337/dc10-0557.
15. Goyal N, Miller JB, Sankey SS, Mossallam U. Utility of initial bolus insulin in the treatment of diabetic ketoacidosis. J Emerg Med. 2010;38(4):422-427. doi:10.1016/j.jemermed.2007.11.033.
16. Wolfsdorf JI, Allgrove J, Craig ME, et al; International Society for Pediatric and Adolescent Diabetes. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2014;15(Suppl 20):154-179. doi:10.1111/pedi.12165.
17. Cohn BG, Keim SM, Watkins JW, Camargo CA. Does management of diabetic ketoacidosis with subcutaneous rapid-acting insulin reduce the need for intensive care unit admission? J Emerg Med. 2015;49(4):530-538. doi:10.1016/j.jemermed.2015.05.016.
18. Glaser N, Barnett P, McCaslin I, et al; Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N Engl J Med. 2001;344(4):264-269. doi:10.1056/NEJM200101253440404.
1. Nyenwe EA, Kitabchi AE. The evolution of diabetic ketoacidosis: an update of its etiology, pathogenesis and management. Metabolism. 2016;65(4):507-521. doi:10.1016/j.metabol.2015.12.007.
2. Fayfman M, Pasquel FJ, Umpierrez GE. Management of hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Med Clin North Am. 2017;101(3):587-606. doi:10.1016/j.mcna.2016.12.011.
3. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343. doi:10.2337/dc09-9032.
4. Fugate JE, Rabinstein AA. Absolute and relative contraindications to IV rt-PA for acute ischemic stroke. Neurohospitalist. 2015;5(3):110-121. doi:10.1177/1941874415578532.
5. Kamalakannan D, Baskar V, Barton DM, Abdu TA. Diabetic ketoacidosis in pregnancy. Postgrad Med J. 2003;79(9):454-457.
6. Ma OJ, Rush MD, Godfrey MM, Gaddis G. Arterial blood gas results rarely influence emergency physician management of patients with suspected diabetic ketoacidosis. Acad Emerg Med. 2003;10(8):836-841.
7. Taylor S, Blau J, Rother K. SGLT2 Inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100(8):2849-2852. doi:10.1210/jc.2015-1884.
8. Kum-Nji JS, Gosmanov AR, Steinberg H, Dagogo-Jack S. Hyperglycemic, high anion-gap metabolic acidosis in patients receiving SGLT-2 inhibitors for diabetes management. J Diabetes Complications. 2017;31(3):611-614. doi:10.1016/j.jdiacomp.2016.11.004.
9. Adrogué HJ, Barrero J, Eknoyan G. Salutary effects of modest fluid replacement in the treatment of adults with diabetic ketoacidosis. Use in patients without extreme volume deficit. JAMA. 1989;262(15):2108-2013.
10. Semler MW, Self WH, Wanderer JP, et al; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloid versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. doi:10.1056/NEJMoa1711584.
11. Chua HR, Venkatesh B, Stachowski E, et al. Plasma-Lyte 148 vs 0.9% saline for fluid resuscitation in diabetic ketoacidosis. J Crit Care. 2012;27(2):138-145. doi:10.1016/j.jcrc.2012.01.007.
12. Mahler S, Conrad S, Wang H, Arnold T. Resuscitation with balanced electrolyte solution prevents hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis. Am J Emerg Med. 2011;29(9):1194-1197. doi:10.1016/j.ajem.2010.07.015.
11. Van Zyl DG, Rheeder P, Delport E. Fluid management in diabetic-acidosis--Ringer’s lactate versus normal saline: a randomized controlled trial. QJM. 2012;105(4):337-343. doi:10.1093/qjmed/hcr226.
14. Penne EL, Thijssen S, Raimann JG, Levin NW, Kotanko P. Correction of serum sodium for glucose concentration in hemodialysis patients with poor glucose control. Diabetes Care. 2010;33(7):e91. doi:10.2337/dc10-0557.
15. Goyal N, Miller JB, Sankey SS, Mossallam U. Utility of initial bolus insulin in the treatment of diabetic ketoacidosis. J Emerg Med. 2010;38(4):422-427. doi:10.1016/j.jemermed.2007.11.033.
16. Wolfsdorf JI, Allgrove J, Craig ME, et al; International Society for Pediatric and Adolescent Diabetes. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2014;15(Suppl 20):154-179. doi:10.1111/pedi.12165.
17. Cohn BG, Keim SM, Watkins JW, Camargo CA. Does management of diabetic ketoacidosis with subcutaneous rapid-acting insulin reduce the need for intensive care unit admission? J Emerg Med. 2015;49(4):530-538. doi:10.1016/j.jemermed.2015.05.016.
18. Glaser N, Barnett P, McCaslin I, et al; Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N Engl J Med. 2001;344(4):264-269. doi:10.1056/NEJM200101253440404.
Everything’s Fine … Except His Spine
ANSWER
The chest radiograph shows an approximately 3-cm cavitary lesion in the right upper lobe. Such a lesion can indicate lung abscess, neoplasm, or tuberculosis.
Subsequent workup determined that he did, in fact, have tuberculosis, with involvement in his spine (known as Pott disease).
ANSWER
The chest radiograph shows an approximately 3-cm cavitary lesion in the right upper lobe. Such a lesion can indicate lung abscess, neoplasm, or tuberculosis.
Subsequent workup determined that he did, in fact, have tuberculosis, with involvement in his spine (known as Pott disease).
ANSWER
The chest radiograph shows an approximately 3-cm cavitary lesion in the right upper lobe. Such a lesion can indicate lung abscess, neoplasm, or tuberculosis.
Subsequent workup determined that he did, in fact, have tuberculosis, with involvement in his spine (known as Pott disease).
A 25-year-old man is admitted to your facility for a possible infection in his spine. He reports a two-week history of severe back pain with no history of injury or trauma. Imaging performed at an outside facility suggested compression and erosion of his vertebral bodies at the thoracolumbar junction, and the radiologist raised concern for possible osteomyelitis and diskitis.
The patient is otherwise healthy and denies any medical problems. He denies drug use of any form. Review of systems is significant for a three-month history of anorexia and night sweats but no fever.
Physical exam reveals a healthy-appearing male with normal vital signs. His heart and lung sounds are normal.
A chest radiograph is obtained (shown). What is your impression?
ED visits up for acute pancreatitis linked to younger age, alcohol, chronic disease
, an analysis of a nationally representative database has suggested.
Meanwhile, hospital admissions and length of stay dropped, but ED and inpatient charges increased, according to the analysis by Sushil K. Garg, MD, of the division of gastroenterology and hepatology at the Mayo Clinic, Rochester, Minn., and his coauthors.
“This study identifies important patient populations, specifically young patients with alcohol abuse, to target in order to develop programs to assist in reduction of ED utilization for acute pancreatitis,” Dr. Garg and his colleagues reported in the Journal of Clinical Gastroenterology.
The retrospective analysis was focused on nearly 2.2 million ED visits during 2006-2012 in the National Emergency Department Sample (NEDS) database. The cohort was limited to adults at least 18 years of age with a primary diagnosis of acute pancreatitis.
Overall, there was a nonsignificant 5.5% increase in visits per 10,000 U.S. population during 2006-2012, the researchers found. However, the total number of ED visits in this sample increased significantly – from 292,902 in 2006 to a peak of 326,376, an average rate of increase of 7,213 visits per year (P = .0086), according to the report.
Younger patients had a significant increase in the number of pancreatitis-related ED visits over the study period, while older patients had a significant decrease, according to investigators. Visits were up 9.2% for patients aged 18-44 years and 8.6% for those aged 45-64 but down 13.4% for patients aged 65-84 years and 20.1% for those aged 85 years or older.
The incidence of visits secondary to biliary disease was virtually flat over time, Dr. Garg and his coinvestigators found when looking at visits grouped by the most common presenting etiologies. By contrast, there were significant increases in visits for acute pancreatitis associated with alcohol abuse or chronic pancreatitis.
Specifically, acute pancreatitis associated with biliary disease averaged 20.7% of yearly pancreatitis-related ED visits and did not significantly change over time, the researchers reported.
By contrast, acute pancreatitis associated with alcohol abuse, which accounted for 24.1% of visits on average, increased by 15.9% over the study period, an increase driven by an increase among age groups younger than 65 years.
Acute pancreatitis associated with chronic pancreatitis, which made up 11.5% of visits on average, increased “substantially” in all age groups, according to study authors, with the largest increase in the group aged 45-64 years. Overall, the percentage increase over 7 years was 59.5%.
Rates of hospitalization decreased significantly over time, from 76.2% in 2006 to 72.7% in 2012 (P = .0026), and likewise, the length of stay dropped from 5.36 to 4.64 days (P = .0001), according to the analysis.
Inpatient charges, adjusted for inflation and expressed in 2012 dollars, increased from $32,130.63 to $34,652.00 (P = .0011), an average rate of increase of $489/year.
Predictors of hospitalization included age older than 84 years, alcohol use, smoking, and a Charlson comorbidity score of 1 or greater, according to the results of a multivariate regression analysis.
“Factors which may place patients at higher risk for severe or complicated acute pancreatitis requiring admission, such as obesity, alcohol use, and increasing age, are identified and should be explored in further studies and potentially targeted to improve ED and inpatient care,” Dr. Garg and his coauthors said.
Dr. Garg and his coauthors had no disclosures related to the study.
Help your patients better understand pancreatitis and available tests and treatments by using AGA patient education materials, https://www.gastro.org/practice-guidance/gi-patient-center/topic/pancreatitis.
SOURCE: Garg SK et al. J Clin Gastroenterol. 2018 Apr 6. doi: 10.1097/MCG.0000000000001030.
, an analysis of a nationally representative database has suggested.
Meanwhile, hospital admissions and length of stay dropped, but ED and inpatient charges increased, according to the analysis by Sushil K. Garg, MD, of the division of gastroenterology and hepatology at the Mayo Clinic, Rochester, Minn., and his coauthors.
“This study identifies important patient populations, specifically young patients with alcohol abuse, to target in order to develop programs to assist in reduction of ED utilization for acute pancreatitis,” Dr. Garg and his colleagues reported in the Journal of Clinical Gastroenterology.
The retrospective analysis was focused on nearly 2.2 million ED visits during 2006-2012 in the National Emergency Department Sample (NEDS) database. The cohort was limited to adults at least 18 years of age with a primary diagnosis of acute pancreatitis.
Overall, there was a nonsignificant 5.5% increase in visits per 10,000 U.S. population during 2006-2012, the researchers found. However, the total number of ED visits in this sample increased significantly – from 292,902 in 2006 to a peak of 326,376, an average rate of increase of 7,213 visits per year (P = .0086), according to the report.
Younger patients had a significant increase in the number of pancreatitis-related ED visits over the study period, while older patients had a significant decrease, according to investigators. Visits were up 9.2% for patients aged 18-44 years and 8.6% for those aged 45-64 but down 13.4% for patients aged 65-84 years and 20.1% for those aged 85 years or older.
The incidence of visits secondary to biliary disease was virtually flat over time, Dr. Garg and his coinvestigators found when looking at visits grouped by the most common presenting etiologies. By contrast, there were significant increases in visits for acute pancreatitis associated with alcohol abuse or chronic pancreatitis.
Specifically, acute pancreatitis associated with biliary disease averaged 20.7% of yearly pancreatitis-related ED visits and did not significantly change over time, the researchers reported.
By contrast, acute pancreatitis associated with alcohol abuse, which accounted for 24.1% of visits on average, increased by 15.9% over the study period, an increase driven by an increase among age groups younger than 65 years.
Acute pancreatitis associated with chronic pancreatitis, which made up 11.5% of visits on average, increased “substantially” in all age groups, according to study authors, with the largest increase in the group aged 45-64 years. Overall, the percentage increase over 7 years was 59.5%.
Rates of hospitalization decreased significantly over time, from 76.2% in 2006 to 72.7% in 2012 (P = .0026), and likewise, the length of stay dropped from 5.36 to 4.64 days (P = .0001), according to the analysis.
Inpatient charges, adjusted for inflation and expressed in 2012 dollars, increased from $32,130.63 to $34,652.00 (P = .0011), an average rate of increase of $489/year.
Predictors of hospitalization included age older than 84 years, alcohol use, smoking, and a Charlson comorbidity score of 1 or greater, according to the results of a multivariate regression analysis.
“Factors which may place patients at higher risk for severe or complicated acute pancreatitis requiring admission, such as obesity, alcohol use, and increasing age, are identified and should be explored in further studies and potentially targeted to improve ED and inpatient care,” Dr. Garg and his coauthors said.
Dr. Garg and his coauthors had no disclosures related to the study.
Help your patients better understand pancreatitis and available tests and treatments by using AGA patient education materials, https://www.gastro.org/practice-guidance/gi-patient-center/topic/pancreatitis.
SOURCE: Garg SK et al. J Clin Gastroenterol. 2018 Apr 6. doi: 10.1097/MCG.0000000000001030.
, an analysis of a nationally representative database has suggested.
Meanwhile, hospital admissions and length of stay dropped, but ED and inpatient charges increased, according to the analysis by Sushil K. Garg, MD, of the division of gastroenterology and hepatology at the Mayo Clinic, Rochester, Minn., and his coauthors.
“This study identifies important patient populations, specifically young patients with alcohol abuse, to target in order to develop programs to assist in reduction of ED utilization for acute pancreatitis,” Dr. Garg and his colleagues reported in the Journal of Clinical Gastroenterology.
The retrospective analysis was focused on nearly 2.2 million ED visits during 2006-2012 in the National Emergency Department Sample (NEDS) database. The cohort was limited to adults at least 18 years of age with a primary diagnosis of acute pancreatitis.
Overall, there was a nonsignificant 5.5% increase in visits per 10,000 U.S. population during 2006-2012, the researchers found. However, the total number of ED visits in this sample increased significantly – from 292,902 in 2006 to a peak of 326,376, an average rate of increase of 7,213 visits per year (P = .0086), according to the report.
Younger patients had a significant increase in the number of pancreatitis-related ED visits over the study period, while older patients had a significant decrease, according to investigators. Visits were up 9.2% for patients aged 18-44 years and 8.6% for those aged 45-64 but down 13.4% for patients aged 65-84 years and 20.1% for those aged 85 years or older.
The incidence of visits secondary to biliary disease was virtually flat over time, Dr. Garg and his coinvestigators found when looking at visits grouped by the most common presenting etiologies. By contrast, there were significant increases in visits for acute pancreatitis associated with alcohol abuse or chronic pancreatitis.
Specifically, acute pancreatitis associated with biliary disease averaged 20.7% of yearly pancreatitis-related ED visits and did not significantly change over time, the researchers reported.
By contrast, acute pancreatitis associated with alcohol abuse, which accounted for 24.1% of visits on average, increased by 15.9% over the study period, an increase driven by an increase among age groups younger than 65 years.
Acute pancreatitis associated with chronic pancreatitis, which made up 11.5% of visits on average, increased “substantially” in all age groups, according to study authors, with the largest increase in the group aged 45-64 years. Overall, the percentage increase over 7 years was 59.5%.
Rates of hospitalization decreased significantly over time, from 76.2% in 2006 to 72.7% in 2012 (P = .0026), and likewise, the length of stay dropped from 5.36 to 4.64 days (P = .0001), according to the analysis.
Inpatient charges, adjusted for inflation and expressed in 2012 dollars, increased from $32,130.63 to $34,652.00 (P = .0011), an average rate of increase of $489/year.
Predictors of hospitalization included age older than 84 years, alcohol use, smoking, and a Charlson comorbidity score of 1 or greater, according to the results of a multivariate regression analysis.
“Factors which may place patients at higher risk for severe or complicated acute pancreatitis requiring admission, such as obesity, alcohol use, and increasing age, are identified and should be explored in further studies and potentially targeted to improve ED and inpatient care,” Dr. Garg and his coauthors said.
Dr. Garg and his coauthors had no disclosures related to the study.
Help your patients better understand pancreatitis and available tests and treatments by using AGA patient education materials, https://www.gastro.org/practice-guidance/gi-patient-center/topic/pancreatitis.
SOURCE: Garg SK et al. J Clin Gastroenterol. 2018 Apr 6. doi: 10.1097/MCG.0000000000001030.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Key clinical point: The number of U.S. emergency visits for acute pancreatitis associated with alcohol abuse, chronic pancreatitis, and younger age has risen in recent years.
Major finding: From 2006 to 2012, visits were up about 9% for patients under 65 years of age, 15.9% for acute pancreatitis associated with alcohol abuse, and 59.5% for acute on chronic pancreatitis.
Study details: Retrospective analysis of ED visits during 2006-2012 for nearly 2.2 million adults.
Disclosures: The authors had no disclosures.
Source: Garg SK et al. J Clin Gastroenterol. 2018 Apr 6. doi: 10.1097/MCG.0000000000001030.
Calcific uremic arteriolopathy
A 51-year-old man with end-stage renal disease, on peritoneal dialysis for the past 4 years, presented to the emergency department with severe pain in both legs. The pain had started 2 months previously and had progressively worsened. After multiple admissions in the past for hyperkalemia and volume overload due to noncompliance, he had been advised to switch to hemodialysis.
See related article and editorial
Laboratory analysis revealed the following values:
- Serum creatinine 12.62 mg/dL (reference range 0.73–1.22)
- Blood urea nitrogen 159 mg/dL (9–24)
- Serum calcium corrected for serum albumin 8.1 mg/dL (8.4–10.0)
- Serum phosphorus 10.6 mg/dL (2.7–4.8).
His history of end-stage renal disease, failure of peritoneal dialysis, high calcium-phosphorus product (8.1 mg/dL × 10.6 mg/dL = 85.9 mg2/dL2, reference range ≤ 55), and characteristic physical findings led to the diagnosis of calcific uremic arteriolopathy.
CALCIFIC UREMIC ARTERIOLOPATHY
Calcific uremic arteriolopathy or “calciphylaxis,” seen most often in patients with end-stage renal disease, is caused by calcium deposition in the media of the dermo-hypodermic arterioles, leading to infarction of adjacent tissue.1–3 A high calcium-phosphorus product (> 55) has been implicated in its development; however, the calcium-phosphorus product can be normal despite hyperphosphatemia, which itself may promote ectopic calcification.
Early ischemic manifestations include livedo reticularis and painful retiform purpura on the thighs and other areas of high adiposity. Lesions evolve into violaceous plaquelike subcutaneous nodules that can infarct, become necrotic, ulcerate, and become infected. Punch biopsy demonstrating arteriolar calcification, subintimal fibrosis, and thrombosis confirms the diagnosis.
Differential diagnosis
Warfarin necrosis can cause large, irregular, bloody bullae that ulcerate and turn into eschar that may resemble lesions of calcific uremic arteriolopathy. Our patient, however, had no exposure to warfarin.
Pemphigus foliaceus, an immunoglobulin G4-mediated autoimmune disorder targeted against desmoglein-1, leads to the formation of fragile blisters that easily rupture when rubbed (Nikolsky sign). Lesions evolve into scaling, crusty erosions on an erythematous base. With tender blisters and lack of mucous membrane involvement, pemphigus foliaceus shares similarities with calcific uremic arteriolopathy, but the presence of necrotic eschar surrounded by violaceous plaques in our patient made it an unlikely diagnosis.
Cryofibrinogenemia. In the right clinical scenario, ie, in a patient with vasculitis, malignancy, infection, cryoglobulinemia, or collagen diseases, cryofibrinogen-mediated cold-induced occlusive lesions may mimic calcific uremic arteriolopathy, with painful or pruritic erythema, purpura, livedo reticularis, necrosis, and ulceration.4 Our patient had no color changes with exposure to cold, nor any history of Raynaud phenomenon or joint pain, making the diagnosis of cryofibrinogenemia less likely.
Nephrogenic systemic fibrosis. Gadolinium contrast medium in magnetic resonance imaging can cause nephrogenic systemic fibrosis, characterized by erythematous papules that coalesce into brawny plaques with surrounding woody induration, which may resemble lesions of calcific uremic arteriolopathy.5 However, our patient had not been exposed to gadolinium.
Management
Management is multidisciplinary and includes the following1:
- Hemodialysis, modified to optimize calcium balance2
- Intravenous sodium thiosulfate: the exact mechanism of action remains unclear, but it is thought to play a role in chelating calcium from tissue deposits, thus decreasing pain and promoting regression of skin lesions3
- Wound care, including chemical debridement agents, negative-pressure wound therapy, and surgical debridement for infected wounds6
- Pain management with opioid analgesics.
The patient was treated with all these measures. However, he died of sudden cardiac arrest during the same admission.
- Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol 2007; 56(4):569–579. doi:10.1016/j.jaad.2006.08.065
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis 2015; 66(1):133–146. doi:10.1053/j.ajkd.2015.01.034
- Janigan DT, Hirsch DJ, Klassen GA, MacDonald AS. Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“calciphylaxis”) in chronic renal failure. Am J Kidney Dis 2000; 35(4):588–597. pmid:10739777
- Michaud M, Pourrat J. Cryofibrinogenemia. J Clin Rheumatol 2013; 19(3):142–148. doi:10.1097/RHU.0b013e318289e06e
- Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol 2006; 18(6):614–617. doi:10.1097/01.bor.0000245725.94887.8d
- Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, eds. Fitzpatrick’s Dermatology in General Medicine. 6th ed. New York, NY: McGraw-Hill Professional; 2003:558–562.
A 51-year-old man with end-stage renal disease, on peritoneal dialysis for the past 4 years, presented to the emergency department with severe pain in both legs. The pain had started 2 months previously and had progressively worsened. After multiple admissions in the past for hyperkalemia and volume overload due to noncompliance, he had been advised to switch to hemodialysis.
See related article and editorial
Laboratory analysis revealed the following values:
- Serum creatinine 12.62 mg/dL (reference range 0.73–1.22)
- Blood urea nitrogen 159 mg/dL (9–24)
- Serum calcium corrected for serum albumin 8.1 mg/dL (8.4–10.0)
- Serum phosphorus 10.6 mg/dL (2.7–4.8).
His history of end-stage renal disease, failure of peritoneal dialysis, high calcium-phosphorus product (8.1 mg/dL × 10.6 mg/dL = 85.9 mg2/dL2, reference range ≤ 55), and characteristic physical findings led to the diagnosis of calcific uremic arteriolopathy.
CALCIFIC UREMIC ARTERIOLOPATHY
Calcific uremic arteriolopathy or “calciphylaxis,” seen most often in patients with end-stage renal disease, is caused by calcium deposition in the media of the dermo-hypodermic arterioles, leading to infarction of adjacent tissue.1–3 A high calcium-phosphorus product (> 55) has been implicated in its development; however, the calcium-phosphorus product can be normal despite hyperphosphatemia, which itself may promote ectopic calcification.
Early ischemic manifestations include livedo reticularis and painful retiform purpura on the thighs and other areas of high adiposity. Lesions evolve into violaceous plaquelike subcutaneous nodules that can infarct, become necrotic, ulcerate, and become infected. Punch biopsy demonstrating arteriolar calcification, subintimal fibrosis, and thrombosis confirms the diagnosis.
Differential diagnosis
Warfarin necrosis can cause large, irregular, bloody bullae that ulcerate and turn into eschar that may resemble lesions of calcific uremic arteriolopathy. Our patient, however, had no exposure to warfarin.
Pemphigus foliaceus, an immunoglobulin G4-mediated autoimmune disorder targeted against desmoglein-1, leads to the formation of fragile blisters that easily rupture when rubbed (Nikolsky sign). Lesions evolve into scaling, crusty erosions on an erythematous base. With tender blisters and lack of mucous membrane involvement, pemphigus foliaceus shares similarities with calcific uremic arteriolopathy, but the presence of necrotic eschar surrounded by violaceous plaques in our patient made it an unlikely diagnosis.
Cryofibrinogenemia. In the right clinical scenario, ie, in a patient with vasculitis, malignancy, infection, cryoglobulinemia, or collagen diseases, cryofibrinogen-mediated cold-induced occlusive lesions may mimic calcific uremic arteriolopathy, with painful or pruritic erythema, purpura, livedo reticularis, necrosis, and ulceration.4 Our patient had no color changes with exposure to cold, nor any history of Raynaud phenomenon or joint pain, making the diagnosis of cryofibrinogenemia less likely.
Nephrogenic systemic fibrosis. Gadolinium contrast medium in magnetic resonance imaging can cause nephrogenic systemic fibrosis, characterized by erythematous papules that coalesce into brawny plaques with surrounding woody induration, which may resemble lesions of calcific uremic arteriolopathy.5 However, our patient had not been exposed to gadolinium.
Management
Management is multidisciplinary and includes the following1:
- Hemodialysis, modified to optimize calcium balance2
- Intravenous sodium thiosulfate: the exact mechanism of action remains unclear, but it is thought to play a role in chelating calcium from tissue deposits, thus decreasing pain and promoting regression of skin lesions3
- Wound care, including chemical debridement agents, negative-pressure wound therapy, and surgical debridement for infected wounds6
- Pain management with opioid analgesics.
The patient was treated with all these measures. However, he died of sudden cardiac arrest during the same admission.
A 51-year-old man with end-stage renal disease, on peritoneal dialysis for the past 4 years, presented to the emergency department with severe pain in both legs. The pain had started 2 months previously and had progressively worsened. After multiple admissions in the past for hyperkalemia and volume overload due to noncompliance, he had been advised to switch to hemodialysis.
See related article and editorial
Laboratory analysis revealed the following values:
- Serum creatinine 12.62 mg/dL (reference range 0.73–1.22)
- Blood urea nitrogen 159 mg/dL (9–24)
- Serum calcium corrected for serum albumin 8.1 mg/dL (8.4–10.0)
- Serum phosphorus 10.6 mg/dL (2.7–4.8).
His history of end-stage renal disease, failure of peritoneal dialysis, high calcium-phosphorus product (8.1 mg/dL × 10.6 mg/dL = 85.9 mg2/dL2, reference range ≤ 55), and characteristic physical findings led to the diagnosis of calcific uremic arteriolopathy.
CALCIFIC UREMIC ARTERIOLOPATHY
Calcific uremic arteriolopathy or “calciphylaxis,” seen most often in patients with end-stage renal disease, is caused by calcium deposition in the media of the dermo-hypodermic arterioles, leading to infarction of adjacent tissue.1–3 A high calcium-phosphorus product (> 55) has been implicated in its development; however, the calcium-phosphorus product can be normal despite hyperphosphatemia, which itself may promote ectopic calcification.
Early ischemic manifestations include livedo reticularis and painful retiform purpura on the thighs and other areas of high adiposity. Lesions evolve into violaceous plaquelike subcutaneous nodules that can infarct, become necrotic, ulcerate, and become infected. Punch biopsy demonstrating arteriolar calcification, subintimal fibrosis, and thrombosis confirms the diagnosis.
Differential diagnosis
Warfarin necrosis can cause large, irregular, bloody bullae that ulcerate and turn into eschar that may resemble lesions of calcific uremic arteriolopathy. Our patient, however, had no exposure to warfarin.
Pemphigus foliaceus, an immunoglobulin G4-mediated autoimmune disorder targeted against desmoglein-1, leads to the formation of fragile blisters that easily rupture when rubbed (Nikolsky sign). Lesions evolve into scaling, crusty erosions on an erythematous base. With tender blisters and lack of mucous membrane involvement, pemphigus foliaceus shares similarities with calcific uremic arteriolopathy, but the presence of necrotic eschar surrounded by violaceous plaques in our patient made it an unlikely diagnosis.
Cryofibrinogenemia. In the right clinical scenario, ie, in a patient with vasculitis, malignancy, infection, cryoglobulinemia, or collagen diseases, cryofibrinogen-mediated cold-induced occlusive lesions may mimic calcific uremic arteriolopathy, with painful or pruritic erythema, purpura, livedo reticularis, necrosis, and ulceration.4 Our patient had no color changes with exposure to cold, nor any history of Raynaud phenomenon or joint pain, making the diagnosis of cryofibrinogenemia less likely.
Nephrogenic systemic fibrosis. Gadolinium contrast medium in magnetic resonance imaging can cause nephrogenic systemic fibrosis, characterized by erythematous papules that coalesce into brawny plaques with surrounding woody induration, which may resemble lesions of calcific uremic arteriolopathy.5 However, our patient had not been exposed to gadolinium.
Management
Management is multidisciplinary and includes the following1:
- Hemodialysis, modified to optimize calcium balance2
- Intravenous sodium thiosulfate: the exact mechanism of action remains unclear, but it is thought to play a role in chelating calcium from tissue deposits, thus decreasing pain and promoting regression of skin lesions3
- Wound care, including chemical debridement agents, negative-pressure wound therapy, and surgical debridement for infected wounds6
- Pain management with opioid analgesics.
The patient was treated with all these measures. However, he died of sudden cardiac arrest during the same admission.
- Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol 2007; 56(4):569–579. doi:10.1016/j.jaad.2006.08.065
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis 2015; 66(1):133–146. doi:10.1053/j.ajkd.2015.01.034
- Janigan DT, Hirsch DJ, Klassen GA, MacDonald AS. Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“calciphylaxis”) in chronic renal failure. Am J Kidney Dis 2000; 35(4):588–597. pmid:10739777
- Michaud M, Pourrat J. Cryofibrinogenemia. J Clin Rheumatol 2013; 19(3):142–148. doi:10.1097/RHU.0b013e318289e06e
- Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol 2006; 18(6):614–617. doi:10.1097/01.bor.0000245725.94887.8d
- Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, eds. Fitzpatrick’s Dermatology in General Medicine. 6th ed. New York, NY: McGraw-Hill Professional; 2003:558–562.
- Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol 2007; 56(4):569–579. doi:10.1016/j.jaad.2006.08.065
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis 2015; 66(1):133–146. doi:10.1053/j.ajkd.2015.01.034
- Janigan DT, Hirsch DJ, Klassen GA, MacDonald AS. Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“calciphylaxis”) in chronic renal failure. Am J Kidney Dis 2000; 35(4):588–597. pmid:10739777
- Michaud M, Pourrat J. Cryofibrinogenemia. J Clin Rheumatol 2013; 19(3):142–148. doi:10.1097/RHU.0b013e318289e06e
- Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol 2006; 18(6):614–617. doi:10.1097/01.bor.0000245725.94887.8d
- Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, eds. Fitzpatrick’s Dermatology in General Medicine. 6th ed. New York, NY: McGraw-Hill Professional; 2003:558–562.
Neuroimaging may often be unneeded in ED seizure treatment
Neuroimaging may be appropriate only for specific types of patients with recurrent seizures who present at emergency departments because the scans are otherwise unlikely to prompt acute changes in treatment, a new multicenter study suggests.
“Going forward, our results should help ED providers determine which patients are more likely to derive benefit from neuroimaging and which patients are not likely to benefit,” lead author Martin Salinsky, MD, of Oregon Health & Science University, Portland, said in an interview. “They can be more selective in ordering scans and reduce the total number obtained.”
As the study authors noted in their report, published July 18 in Epilepsia, “head CT is generally considered a benign procedure. However, overuse is problematic.”
The scans are costly and expose patients to radiation equivalent to 10 chest x-rays, the authors wrote. Scans can complicate care by clogging ED work flow and producing false positives, and patients often seize while undergoing scans, creating even more complications, they added.
“There is very little information in the medical literature that would help guide ED providers in their decision of whether to obtain neuroimaging on a patient who presents with a recurrent seizure,” Dr. Salinsky said. “Without this information, the tendency is to be cautious and obtain scans in more patients.”
For the study, the researchers tracked 822 consecutive ED visits for nonindex – recurrent – epileptic seizures at Oregon Health & Science University and VA Portland Health Care medical centers. (Nonindex seizures accounted for 78% of the total seizures that prompted ED care.)
The study subjects were adults treated for seizure as the main complaint. Patients who had a history of seizures but hadn’t had one for at least 5 years were excluded.
Of the total nonindex seizures, 46% of those resulted in neuroimaging.
“The overall yield of neuroimaging in this patient group was 2%-3%,” Dr. Salinsky said, referring to the percentage of patients whose scans resulted in an acute change in management.
False positives due to imaging artifacts were subsequently discovered in 3 of the 11 patients whose neuroimaging prompted an acute change in management. When the false positives were removed, the yield of acute management changes prompted by neuroimaging decreased to 2.1% overall.
“Three clinical factors – acute head trauma, prolonged alteration of consciousness, and focal neurological examination [at presentation] – were associated with an increased yield of imaging,” he said. “Absent all three factors, the yield in our patients was zero.”
At the two medical centers, the percentages of patients with acute head trauma were 10% and 15%. Prolonged alteration of consciousness occurred in 6% at both centers, and focal neurological examination at presentation was observed in 12% and 14%.
A fourth factor, presentation with status epilepticus/acute repetitive seizures, “bordered on statistical significance and might have reached significance in a larger series,” the authors wrote.
As they put it, “these results support a more conservative use of ED neuroimaging for nonindex seizures, based on clinical factors at the time of presentation. ... without specific indications, ED neuroimaging for nonindex seizures is unlikely to result in an acute change in care.”
The study authors estimated that hundreds of millions of dollars could be saved annually in the United States if neuroimaging in these ED patients could be cut in half.
No study funding was reported, and the authors reported no relevant disclosures.
SOURCE: Salinsky M et al. Epilepsia. 2018 July 18. doi: 10.1111/epi.14518
Neuroimaging may be appropriate only for specific types of patients with recurrent seizures who present at emergency departments because the scans are otherwise unlikely to prompt acute changes in treatment, a new multicenter study suggests.
“Going forward, our results should help ED providers determine which patients are more likely to derive benefit from neuroimaging and which patients are not likely to benefit,” lead author Martin Salinsky, MD, of Oregon Health & Science University, Portland, said in an interview. “They can be more selective in ordering scans and reduce the total number obtained.”
As the study authors noted in their report, published July 18 in Epilepsia, “head CT is generally considered a benign procedure. However, overuse is problematic.”
The scans are costly and expose patients to radiation equivalent to 10 chest x-rays, the authors wrote. Scans can complicate care by clogging ED work flow and producing false positives, and patients often seize while undergoing scans, creating even more complications, they added.
“There is very little information in the medical literature that would help guide ED providers in their decision of whether to obtain neuroimaging on a patient who presents with a recurrent seizure,” Dr. Salinsky said. “Without this information, the tendency is to be cautious and obtain scans in more patients.”
For the study, the researchers tracked 822 consecutive ED visits for nonindex – recurrent – epileptic seizures at Oregon Health & Science University and VA Portland Health Care medical centers. (Nonindex seizures accounted for 78% of the total seizures that prompted ED care.)
The study subjects were adults treated for seizure as the main complaint. Patients who had a history of seizures but hadn’t had one for at least 5 years were excluded.
Of the total nonindex seizures, 46% of those resulted in neuroimaging.
“The overall yield of neuroimaging in this patient group was 2%-3%,” Dr. Salinsky said, referring to the percentage of patients whose scans resulted in an acute change in management.
False positives due to imaging artifacts were subsequently discovered in 3 of the 11 patients whose neuroimaging prompted an acute change in management. When the false positives were removed, the yield of acute management changes prompted by neuroimaging decreased to 2.1% overall.
“Three clinical factors – acute head trauma, prolonged alteration of consciousness, and focal neurological examination [at presentation] – were associated with an increased yield of imaging,” he said. “Absent all three factors, the yield in our patients was zero.”
At the two medical centers, the percentages of patients with acute head trauma were 10% and 15%. Prolonged alteration of consciousness occurred in 6% at both centers, and focal neurological examination at presentation was observed in 12% and 14%.
A fourth factor, presentation with status epilepticus/acute repetitive seizures, “bordered on statistical significance and might have reached significance in a larger series,” the authors wrote.
As they put it, “these results support a more conservative use of ED neuroimaging for nonindex seizures, based on clinical factors at the time of presentation. ... without specific indications, ED neuroimaging for nonindex seizures is unlikely to result in an acute change in care.”
The study authors estimated that hundreds of millions of dollars could be saved annually in the United States if neuroimaging in these ED patients could be cut in half.
No study funding was reported, and the authors reported no relevant disclosures.
SOURCE: Salinsky M et al. Epilepsia. 2018 July 18. doi: 10.1111/epi.14518
Neuroimaging may be appropriate only for specific types of patients with recurrent seizures who present at emergency departments because the scans are otherwise unlikely to prompt acute changes in treatment, a new multicenter study suggests.
“Going forward, our results should help ED providers determine which patients are more likely to derive benefit from neuroimaging and which patients are not likely to benefit,” lead author Martin Salinsky, MD, of Oregon Health & Science University, Portland, said in an interview. “They can be more selective in ordering scans and reduce the total number obtained.”
As the study authors noted in their report, published July 18 in Epilepsia, “head CT is generally considered a benign procedure. However, overuse is problematic.”
The scans are costly and expose patients to radiation equivalent to 10 chest x-rays, the authors wrote. Scans can complicate care by clogging ED work flow and producing false positives, and patients often seize while undergoing scans, creating even more complications, they added.
“There is very little information in the medical literature that would help guide ED providers in their decision of whether to obtain neuroimaging on a patient who presents with a recurrent seizure,” Dr. Salinsky said. “Without this information, the tendency is to be cautious and obtain scans in more patients.”
For the study, the researchers tracked 822 consecutive ED visits for nonindex – recurrent – epileptic seizures at Oregon Health & Science University and VA Portland Health Care medical centers. (Nonindex seizures accounted for 78% of the total seizures that prompted ED care.)
The study subjects were adults treated for seizure as the main complaint. Patients who had a history of seizures but hadn’t had one for at least 5 years were excluded.
Of the total nonindex seizures, 46% of those resulted in neuroimaging.
“The overall yield of neuroimaging in this patient group was 2%-3%,” Dr. Salinsky said, referring to the percentage of patients whose scans resulted in an acute change in management.
False positives due to imaging artifacts were subsequently discovered in 3 of the 11 patients whose neuroimaging prompted an acute change in management. When the false positives were removed, the yield of acute management changes prompted by neuroimaging decreased to 2.1% overall.
“Three clinical factors – acute head trauma, prolonged alteration of consciousness, and focal neurological examination [at presentation] – were associated with an increased yield of imaging,” he said. “Absent all three factors, the yield in our patients was zero.”
At the two medical centers, the percentages of patients with acute head trauma were 10% and 15%. Prolonged alteration of consciousness occurred in 6% at both centers, and focal neurological examination at presentation was observed in 12% and 14%.
A fourth factor, presentation with status epilepticus/acute repetitive seizures, “bordered on statistical significance and might have reached significance in a larger series,” the authors wrote.
As they put it, “these results support a more conservative use of ED neuroimaging for nonindex seizures, based on clinical factors at the time of presentation. ... without specific indications, ED neuroimaging for nonindex seizures is unlikely to result in an acute change in care.”
The study authors estimated that hundreds of millions of dollars could be saved annually in the United States if neuroimaging in these ED patients could be cut in half.
No study funding was reported, and the authors reported no relevant disclosures.
SOURCE: Salinsky M et al. Epilepsia. 2018 July 18. doi: 10.1111/epi.14518
FROM EPILEPSIA
Key clinical point: In emergency departments, patients with seizure disorders and nonindex seizures may need neuroimaging only if they have acute head trauma, prolonged alteration of consciousness, or focal neurological examination at presentation.
Major finding: Absent the three factors above, neuroimaging did not prompt any acute changes in management.
Study details: Retrospective examination of 822 consecutive ED visits for nonindex seizures in patients with seizure disorders at two medical centers.
Disclosures: No study funding was reported, and the study authors reported no relevant disclosures.
Source: Salinsky M et al. Epilepsia. 2018 Jul 18. doi: 10.1111/epi.14518.
Supporting Suicidal Patients After Discharge from the Emergency Department
From the Department of Psychiatry, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA.
Abstract
- Objective: To provide a review of emergency department (ED)-based psychosocial interventions that support adult patients with an identified suicide risk towards a goal of reducing subsequent suicidal behavior through the period after discharge, which is known to be a time of high risk for suicidal behavior.
- Methods: Non-systematic review of the literature.
- Results: Multiple methods of engaging patients after discharge from the ED have been shown to reduce subsequent suicidal behaviors. These methods include sending caring letters in the mail, facilitating supportive phone conversations, case management, and protocols that combine different services. Overall, the existing literature is insufficient to recommend widespread adoption of any individual strategy or protocol. However, providing psychosocial and emotional support to patients with an identified suicide risk after they are discharged from the ED is feasible and may reduce subsequent suicidal behaviors. Templates for providing supportive outreach using different modalities now exist, and these may help guide the ongoing development and widespread adoption of more effective and cost-effective solutions.
- Conclusion: Many ED–based interventions that provide enhanced support to patients with suicide risk after they are discharged have demonstrated a potential to reduce the risk of future suicidal behavior.
Key words: suicide; emergency department.
Despite the fact that emergency department (ED) providers often feel unprepared to manage suicide risk, patients with significant suicide risk frequently receive care in EDs, whether or not they have sustained physical injuries resulting from suicidal behavior [1,2]. Patients make greater than 400,000 visits to EDs in the United States each year for suicidal and self-injurious behaviors (suicide attempts and self-injurious behaviors are typically coded in ways that make them indistinguishable from each other in retrospective analyses) [3], and it is estimated that 6% to 10% of all patients in EDs endorse suicidal ideation when asked, regardless of their original chief complaints [4]. Meanwhile, suicide has become the 10th leading cause of death in the United States [5], and the Joint Commission has charged all accredited health care organizations with providing comprehensive treatment to suicidal patients, which may range from immediately containing an acute risk to ensuring continuity of care in follow-up [5].
When an acute suicide risk is identified in the ED, the provider’s immediate next steps should be to place the patient in a safe area under constant observation and to provide an emergency assessment [5,6]. Although psychiatric consultation and/or psychiatric admission may follow this assessment, suicide risk does not require admission in all cases; and some patients with suicide risk may be discharged to an outpatient setting even without receiving a psychiatric consultation [1]. Regardless of whether an outpatient disposition from the ED is appropriate, however, the period that immediately follows discharge is a time of high risk for repeated suicidal behavior and suicide death [7–9], and only 30% to 50% of patients who are discharged from EDs after a self-harm incident actually keep a follow-up mental health appointment [9,10]. Therefore, any support given to patients through this transition out of the emergency care setting could be especially high-yield.
The Joint Commission recommends that all patients with suicidal ideation receive, at minimum, a referral to treatment, telephone numbers for local and national crisis support resources (including the National Suicide Prevention Lifeline 1-800-273-TALK), collaborative safety planning, and counseling to restrict access to lethal means upon discharge [5]. However, some programs have demonstrated the capacity to provide enhanced support to patients beyond discharge from the ED, with some success in reducing the rates of subsequent suicidal behaviors. This non-systematic review describes interventions that can be initiated in the context of an ED encounter with the purpose of reducing future suicidal behavior among adult patients. They are primarily psychosocial rather than clinical. Clinical interventions that apply psychotherapy [11–13] psychopharmacology [14], and specialized inpatient treatments [15] have been studied as well but are beyond the scope of this review.
[polldaddy:10107269]
Interventions to Support Patients At Risk of Suicide After Discharge from the ED
Brief Contact Interventions
The idea that maintaining written correspondence with patients who have a known suicide risk after discharge can reduce subsequent suicide rates originated with a study of psychiatric inpatients conducted by Motto and Bostrom, in which patients who had been admitted for depression but had declined outpatient treatment were randomly assigned to periodically receive letters containing supportive messages from staff members over a period of 5 years [16]. This study remarkably found that these so-called brief contact interventions (BCIs), which were personalized to each recipient but did not contain psychotherapy per se, were associated with a reduced rate of suicide throughout the duration of the program compared with no written contacts [16].
BCIs have since been adapted to other communication formats and have been studied in patients who were discharged directly from the ED after an evaluation of suicide risk or suicidal behavior. Typically, BCIs consist of short, supportive messages that are delivered at regular intervals (often once every 1–2 months) over a period of 1 to 5 years [17,18]. They notably do not contain psychotherapy content, although they may reinforce coping strategies or remind recipients of how to access help if needed [17,19]. They may arrive as postcards [20,21], letters [22], telephone outreach [23–25], or a combination of modalities [26].
Protocols that rely on BCIs alone vary in their structure and have yielded mixed results [18]. A meta-analysis of 12 BCI protocols conducted by Milner et al found that, overall, BCIs administered after a presentation to the ED for self-harm have been associated with a significant reduction in repeat suicide attempts per recipient but not in total suicide deaths [27]. Milner’s group did not recommend large-scale promotion of BCIs based on the inadequacy of data so far, but suggested that this strategy may yet show promise upon further study [27]. A key advantage of BCIs is that they are inexpensive to implement, particularly if they do not include a telephone outreach component [28]. Thus, even if the potential benefit to patients is small, administering BCIs can be cost-effective [28].
It should not come as a surprise, therefore, that the potential for incorporation of BCIs into mobile smartphone technology is currently under investigation. Individuals who own mobile phones typically keep them on their persons and turned on continuously, and thus this is a reliable platform for maintaining contact with a wide range of patients in real-time [17,29]. Developers of at least 2 BCI smartphone programs that rely on mobile text messaging have published their protocols [17,30]. However, whether these programs will succeed in meaningfully reducing suicide rates remains to be determined by future research.
Green Cards
Morgan et al conducted a study in the United Kingdom in which individuals who presented to EDs after a self-harm event received a “green card,” which contained encouraging messages about seeking help and provided contact information for emergency services with 24-hour availability [31]. The green card also facilitated access to a crisis admission if necessary. The green card was distributed first in the ED and a second time by mail 3 weeks later. No suicides occurred in either the intervention or control group, which received usual care, and no statistically significant differences in suicide reattempt rate were found between groups after 1 year [31].
Evans et al studied an updated version of the green card intervention in which the green card facilitated access to an on-call psychiatrist with 24-hour availability by telephone [32]. The updated card included encouraging messages about seeking help similar to the original green card described by Morgan; however, the psychiatry consultation via telephone replaced the offer of hospital admission [32]. This second trial of green cards also failed to show a reduction in the rate of suicide reattempts among green card recipients at 6 months and 1 year [32,33].
Brief Intervention and Contact
The World Health Organization’s Brief Intervention and Contact (BIC) protocol is a standardized, multi-step suicide prevention program that has been studied primarily in patients who present to EDs after a suicide attempt in middle-income countries [34]. BIC includes a 1-hour information session that is administered shortly prior to discharge, and subsequently provides 9 follow-up contact interventions at specified intervals over an 18-month period. Unlike in a typical BCI, the contacts in BIC are conducted by a clinician either face-to-face or over the phone and include standardized assessments of the patient’s condition, although they still do not include psychotherapy. BIC has been shown to reduce suicide attempts, suicide deaths, or both in India [34–36], Iran [34,36,37], China [34,36], Brazil [34,36], and Sri Lanka [34,36] but was not found to directly improve clinical outcomes in a study conducted in French Polynesia [38]. A meta-analysis conducted by Riblet et al concluded that BIC is effective in reducing suicide risk overall [39].
ED-SAFE
The Emergency Department Safety Assessment and Follow-up Evaluation (ED-SAFE) protocol was validated in 8 EDs in 7 states in the US that did not already provide psychiatric services internally [40]. Under this model, all patients in the ED receive a screening for suicide risk, and those with an initial positive screen receive a secondary screen administered by the ED physician, a self-administered safety plan, and a series of up to 11 phone contacts over the following year that are administered by trained mental health clinicians in a central location. The ED-SAFE phone contacts follow the Coping Long Term with Active Suicide Program (CLASP) protocol [41] and provide support around safety planning and treatment engagement. They have the capacity to engage the patients’ significant others directly if a significant other is available and the patient chooses to involve that person.
In a single multicenter study, ED-SAFE reduced the absolute risk of suicide attempt by 5%, and the relative risk by 20% compared to usual treatment [40]. An intermediate phase of the study compared the universal suicide screening alone (ie, without the safety plan or follow-up contacts) with usual care and did not find this to improve outcomes [40].
Case Management
Kawanishi et al conducted a randomized controlled trial of assertive case management, the ACTION-J study, for patients with psychiatric diagnoses who presented with self-harm to 17 participating EDs in Japan [42]. In the ACTION-J study, case managers were mental health clinicians who provided clinical evaluations, treatment planning, encouragement, and care coordination over the course of 7 scheduled face-to-face or phone contacts in the first 18 months, and additional contacts at 6-month intervals until the completion of the trial (up to a total of 5 years) [43]. The comparison intervention, enhanced usual care, consisted of psychoeducation provided at the time of the encounter in the ED without case management services. The assertive case management intervention was associated with a decrease in suicidal behavior in the first 6 months but not for the duration of the study, except in women, for whom the benefit lasted the full 18 months [42]. A subsequent analysis also found a decrease in the total number of self-harm episodes per person-year compared to enhanced usual care, although there was not a difference in the number of participants who experienced a repeat self-harm episode [43]. The benefit was most strongly pronounced among patients who had presented with an index suicide attempt [43].
Morthorst et al applied an alternative case management model for the assertive intervention for deliberate self harm (AID) trial, which took place in Denmark [44]. Participants were aged 12 and older and could have been recruited from medical or pediatric inpatient units as well as the ED after a self-harm event. AID employed psychiatric nurses to provide crisis intervention, crisis planning, problem solving, motivational support, family mediation, and assistance with keeping appointments over a period of 6 months following discharge. Outreach took place over the phone, by text message, in participants’ homes, in cafes, and at health and social services appointments. The intervention required at least 4 contacts, although additional contacts could be made if appropriate. In comparison with a control group, in which participants received only usual care (which included ready access to short-term psychotherapy), the AID intervention was not associated with statistically significant differences in recurrent suicidal behaviors [44]. Subgroup analyses examining adult participants aged 20–39 and 40 and older also did not find differences in recurrent suicidal behavior between groups [44].
The Baerum Model and OPAC
A municipal suicide prevention team that provides comprehensive social services to suicide attempters has operated in Baerum, Norway, since 1983 [45]. Under the Baerum model, patients who attempt suicide, can be discharged from the general hospital without psychiatric admission, and are determined to have a high level of need for support are connected by a hospital-based suicide prevention team to a community-based team consisting of nurses and a consulting psychologist, who subsequently engage patients in own their homes and through follow-up phone calls. The services they provide include care coordination, encouragement, activation of social networks, psychological first-aid, and counseling focused on problem-solving. The ostensible goal of the suicide prevention team is to provide a bridge between inpatient medical care and outpatient mental health treatment; however, the intervention lasts approximately 1 year regardless of whether the patient connects with a treatment program [45].
A retrospective comparison of outcomes between recipients of the original Baerum program and non-recipients failed to find a difference in suicide attempts or suicide deaths between groups [45]. However, this was not a controlled study, and suicide attempters were preferentially referred to the program based on whether they had a higher level of need at baseline. Hvid and Wang adapted this model to patients who presented to EDs and general hospitals in Amager, Denmark [46] and have since conducted a series of randomized controlled trials comparing their adaptation to usual care. The Danish version of the Baerum model, renamed OPAC (for “outreach, problem solving, adherence, continuity”), provides similar case management and counseling services but for a maximum of 6 months. In their studies, OPAC significantly reduced the number of patients with a repeat suicide attempt and the total number of repeat suicide attempts at a 1-year interval, and this effect on total number of suicide attempts was sustained at 5 years [47,48]. Although the OPAC protocol begins with a patient’s presentation to the ED, the intervention is initiated after admission to the general hospital. Therefore, while this may inspire a model that provides similar services directly from the ED to patients who do not require general hospital admission, the existing model is not entirely based in the ED.
Discussion
The needs of suicidal patients are often multidimensional, and in some cases their risks are driven by psychosocial problems in addition to, or instead of, medically modifiable psychiatric conditions [49]. However, developing an ED-based program to support patients who are at risk of suicide after they are discharged from the ED is possible. Many such programs that provide or facilitate caring contacts, family support, case management, and/or treatment engagement with discharged patients have demonstrated that similar strategies may have the potential to impact future suicidal behavior. Nonetheless, it would be a stretch to say that all hospital systems should immediately begin doing so.
A new post-discharge support program is an investment of financial resources, personnel, and sometimes technology. Successful delivery of support or messages in any format requires that the intended recipient be able to receive it via reliable access to a working address, telephone number, or electronic device. Nonetheless, programs that rely on BCIs alone (excluding those conducted via telephone) cost relatively little to implement and thus would require a smaller investment than programs that require synchronous telephone or face-to-face contacts with staff in addition to or instead of BCIs. Costs for synchronous programs will also vary depending on the frequency and duration of contacts and the licensure and training required of the staff who provide them.
A trend toward better outcomes associating with more resource-intensive programs is easy to imagine but has not been definitively demonstrated. The wide variation between protocols in all types of programs makes comparisons between those that do and do not include synchronous contacts, and between types of synchronous contacts, difficult. Meanwhile, the low cost of BCIs alone could increase their attractiveness as an investment regardless of the magnitude of outcome improvement.
Denchev et al constructed a cost/benefit comparison model that included the postcard BCI study conducted by Carter et al [20], the telephone outreach study conducted by Vaiva et al [23], and a study of cognitive behavioral therapy (CBT) [11], all of which showed a clinical benefit. This model relied upon some numeric estimations and did not account for variation in outcomes between individual studies of each intervention strategy. However, it concluded that both telephone outreach and CBT were likely to be cost-prohibitive compared to asynchronous BCIs, which were associated with a reduction in costs overall [28].
Conclusion
There remains much to learn regarding how best to reduce suicide risk among adult patients in the period after discharge from the ED, during which patients with an identified suicide risk are known to be vulnerable. However, providing psychosocial and emotional support to patients with an identified suicide risk after they are discharged from the ED is feasible and may reduce subsequent suicidal behaviors. Templates for providing supportive outreach using different modalities now exist, and these may help guide the ongoing development and widespread adoption of more effective and cost-effective solutions.
Corresponding author: David S. Kroll, MD, dskroll@bwh.harvard.edu.
Financial disclosure: Dr. Kroll has received research funding from Brigham and Women’s Hospital to study and develop technological solutions for supporting suicidal patients after discharge from the emergency department. He has additionally received research funding and a speaking honorarium from Avasure.
1. Betz ME, Boudreaux ED. Managing suicidal patients in the emergency department. Ann Emerg Med 2016;67:276–82.
2. McManus MC, Cramer RJ, Boshier M, et al. Mental health and drivers of need in emergent and non-emergent emergency department (ED) use: do living location and non-emergent care sources matter? Int J Environ Res Public Health 2018;15:129.
3. Ting SA, Sullivan AF, Boudreaux ED, et al. Trends in US emergency department visits for attempted suicide and self-inflicted injury, 1993-2008. Gen Hosp Psychiatry 2012;34:557–65.
4. Betz ME, Wintersteen M, Boudreaux ED, Brown G, Capoccia L, Currier G, et al. reducing suicide risk: challenges and opportunities in the emergency department. Ann Emerg Med 2016;68:758–65.
5. The Joint Commission. Sentinel event alert 56: detecting and treating suicide ideation in all settings. www.jointcommission.org/sea_issue_56/. Published February 24, 2016. Accessed June 4, 2018.
6. Mills PD, Watts BV, Hemphill RR. Suicide attempts and completions on medical-surgical and intensive care units. J Hosp Med 2014;9:182–5.
7. Crane EH. Patients with drug-related emergency department visits involving suicide attempts who left against medical advice. The CBHSQ Report. http://www.ncbi.nlm.nih.gov/books/NBK396153/ . Published September 13, 2016. Accessed June 4, 2018.
8. Fedyszyn IE, Erlangsen A, Hjorthøj C, et al. Repeated suicide attempts and suicide among individuals with a first emergency department contact for attempted suicide: a prospective, nationwide, Danish register-based study. J Clin Psychiatry 2016;77:832–40.
9. Hunter J, Maunder R, Kurdyak P, et al. Mental health follow-up after deliberate self-harm and risk for repeat self-harm and death. Psychiatry Res 2018;259:333–9.
10. Costemale-Lacoste JF, Balaguer E, Boniface B, et al. Outpatient treatment engagement after suicidal attempt: a multisite prospective study. Psychiatry Res 2017;258:21–3.
11. Brown GK, Ten Have T, Henriques GR, et al. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA 2005;294:563–70.
12. Gysin-Maillart A, Schwab S, Soravia L, Megert M, Michel K. A novel brief therapy for patients who attempt suicide: a 24-months follow-up randomized controlled study of the attempted suicide short intervention program (ASSIP). PLoS Med 2016;13:e1001968.
13. Hawton K, Witt KG, Salisbury TLT, et al. Psychosocial interventions following self-harm in adults: a systematic review and meta-analysis. Lancet Psychiatry. 2016;3:740–50.
14. Battaglia J, Wolff TK, Wagner-Johnson DS, et al. Structured diagnostic assessment and depot fluphenazine treatment of multiple suicide attempters in the emergency department. Int Clin Psychopharmacol 1999;14:361–72.
15. van der Sande R, van Rooijen L, Buskens E, et al. Intensive in-patient and community intervention versus routine care after attempted suicide. A randomised controlled intervention study. Br J Psychiatry 1997;171:35–41.
16. Motto JA, Bostrom AG. A randomized controlled trial of postcrisis suicide prevention. Psychiatr Serv 2001;52:828–33.
17. Berrouiguet S, Larsen ME, Mesmeur C, Gravey M, Billot R, Walter M, et al. Toward mHealth brief contact interventions in suicide prevention: case series from the suicide intervention assisted by messages (SIAM) randomized controlled trial. JMIR MHealth UHealth 2018;6:e8.
18. Falcone G, Nardella A, Lamis DA, et al. Taking care of suicidal patients with new technologies and reaching-out means in the post-discharge period. World J Psychiatry 2017;7:163–76.
19. Milner A, Spittal MJ, Kapur N, et al. Mechanisms of brief contact interventions in clinical populations: a systematic review. BMC Psychiatry 2016;16:194.
20. Carter GL, Clover K, Whyte IM, et al. Postcards from the EDge: 5-year outcomes of a randomised controlled trial for hospital-treated self-poisoning. Br J Psychiatry 2013;202:372–80.
21. Hassanian-Moghaddam H, Sarjami S, Kolahi AA, Carter GL. Postcards in Persia: randomised controlled trial to reduce suicidal behaviours 12 months after hospital-treated self-poisoning. Br J Psychiatry 2011;198:309–16.
22. Luxton DD, Thomas EK, Chipps J, et al. Caring letters for suicide prevention: implementation of a multi-site randomized clinical trial in the U.S. military and Veteran Affairs healthcare systems. Contemp Clin Trials 2014;37(2):252–60.
23. Vaiva G, Vaiva G, Ducrocq F, et al. Effect of telephone contact on further suicide attempts in patients discharged from an emergency department: randomised controlled study. BMJ 2006;332:1241–5.
24. Cebrià AI, Parra I, Pàmias M, et al. Effectiveness of a telephone management programme for patients discharged from an emergency department after a suicide attempt: controlled study in a Spanish population. J Affect Disord 2013;147:269–76.
25. Cedereke M, Monti K, Ojehagen A. Telephone contact with patients in the year after a suicide attempt: does it affect treatment attendance and outcome? A randomised controlled study. Eur Psychiatry. 2002;17:82–91.
26. Vaiva G, Walter M, Al Arab AS, et al. ALGOS: the development of a randomized controlled trial testing a case management algorithm designed to reduce suicide risk among suicide attempters. BMC Psychiatry 2011;11:1.
27. Milner AJ, Carter G, Pirkis J, et al. Letters, green cards, telephone calls and postcards: systematic and meta-analytic review of brief contact interventions for reducing self-harm, suicide attempts and suicide. Br J Psychiatry. 2015;206:184–90.
28. Denchev P, Pearson JL, Allen MH, Claassen CA, Currier GW, Zatzick DF, et al. Modeling the cost-effectiveness of interventions to reduce suicide risk among hospital emergency department patients. Psychiatr Serv 2018;69:23–31.
29. Berrouiguet S, Courtet P, Larsen ME, et al. Suicide prevention: towards integrative, innovative and individualized brief contact interventions. Eur Psychiatry 2018;47:25–6.
30. Larsen ME, Shand F, Morley K, Batterham PJ, Petrie K, Reda B, et al. A mobile text message intervention to reduce repeat suicidal episodes: design and development of reconnecting after a suicide attempt (RAFT). JMIR Ment Health 2017;4:e56.
31. Morgan HG, Jones EM, Owen JH. Secondary prevention of non-fatal deliberate self-harm. The green card study. Br J Psychiatry 1993;163:111–2.
32. Evans MO, Morgan HG, Hayward A, Gunnell DJ. Crisis telephone consultation for deliberate self-harm patients: effects on repetition. Br J Psychiatry 1999;175:23–7.
33. Evans J, Evans M, Morgan HG, et al. Crisis card following self-harm: 12-month follow-up of a randomised controlled trial. Br J Psychiatry J 2005;187:186–7.
34. Fleischmann A, Bertolote JM, Wasserman D, et al. Effectiveness of brief intervention and contact for suicide attempters: a randomized controlled trial in five countries. Bull World Health Organ 2008;86:703–9.
35. Vijayakumar L, Umamaheswari C, Shujaath Ali ZS, et al. Intervention for suicide attempters: a randomized controlled study. Indian J Psychiatry 2011;53:244–8.
36. Bertolote JM, Fleischmann A, De Leo D, et al. Repetition of suicide attempts: data from emergency care settings in five culturally different low- and middle-income countries participating in the WHO SUPRE-MISS Study. Crisis 2010;31:194–201.
37. Mousavi SG, Zohreh R, Maracy MR, et al. The efficacy of telephonic follow up in prevention of suicidal reattempt in patients with suicide attempt history. Adv Biomed Res 2014;3:198.
38. Amadéo S, Rereao M, Malogne A, et al. Testing brief intervention and phone contact among subjects with suicidal behavior: a randomized controlled trial in French Polynesia in the frames of the World Health Organization/suicide trends in at-risk territories study. Ment Illn 2015;7:5818.
39. Riblet NBV, Shiner B, Young-Xu Y, Watts BV. Strategies to prevent death by suicide: meta-analysis of randomised controlled trials. Br J Psychiatry 2017;210:396–402.
40. Miller IW, Camargo CA Jr, Arias SA, et al. Suicide prevention in an emergency department population: the ED-SAFE study. JAMA Psychiatry 2017;74:563–70.
41. Miller IW, Gaudiano BA, Weinstock LM. The coping long term with active suicide program: description and pilot data. Suicide Life Threat Behav 2016;46:752–61.
42. Kawanishi C, Aruga T, Ishizuka N, et al. Assertive case management versus enhanced usual care for people with mental health problems who had attempted suicide and were admitted to hospital emergency departments in Japan (ACTION-J): a multicentre, randomised controlled trial. Lancet Psychiatry 2014;1:193–201.
43. Furuno T, Nakagawa M, Hino K, et al. Effectiveness of assertive case management on repeat self-harm in patients admitted for suicide attempt: findings from ACTION-J study. J Affect Disord 2018;225:460–5.
44. Morthorst B, Krogh J, Erlangsen A, et al. Effect of assertive outreach after suicide attempt in the AID (assertive intervention for deliberate self harm) trial: randomised controlled trial. BMJ 2012;345:e4972.
45. Johannessen HA, Dieserud G, De Leo D, Claussen B, et al. Chain of care for patients who have attempted suicide: a follow-up study from Bærum, Norway. BMC Public Health 2011;11:81.
46. Hvid M, Wang AG. Preventing repetition of attempted suicide—I. Feasibility (acceptability, adherence, and effectiveness) of a Baerum-model like aftercare. Nord J Psychiatry 2009;63:148–53.
47. Hvid M, Vangborg K, Sørensen HJ, et al. Preventing repetition of attempted suicide-II. The Amager project, a randomized controlled trial. Nord J Psychiatry 2011;65:292–8.
48. Lahoz T, Hvid M, Wang AG. Preventing repetition of attempted suicide-III. The Amager project, 5-year follow-up of a randomized controlled trial. Nord J Psychiatry 2016;70:547–53.
49. Kroll DS, Karno J, Mullen B, et al. Clinical severity alone does not determine disposition decisions for patients in the emergency department with suicide risk. Psychosomatics 2017; pii: S0033-3182(17)30247–5.
From the Department of Psychiatry, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA.
Abstract
- Objective: To provide a review of emergency department (ED)-based psychosocial interventions that support adult patients with an identified suicide risk towards a goal of reducing subsequent suicidal behavior through the period after discharge, which is known to be a time of high risk for suicidal behavior.
- Methods: Non-systematic review of the literature.
- Results: Multiple methods of engaging patients after discharge from the ED have been shown to reduce subsequent suicidal behaviors. These methods include sending caring letters in the mail, facilitating supportive phone conversations, case management, and protocols that combine different services. Overall, the existing literature is insufficient to recommend widespread adoption of any individual strategy or protocol. However, providing psychosocial and emotional support to patients with an identified suicide risk after they are discharged from the ED is feasible and may reduce subsequent suicidal behaviors. Templates for providing supportive outreach using different modalities now exist, and these may help guide the ongoing development and widespread adoption of more effective and cost-effective solutions.
- Conclusion: Many ED–based interventions that provide enhanced support to patients with suicide risk after they are discharged have demonstrated a potential to reduce the risk of future suicidal behavior.
Key words: suicide; emergency department.
Despite the fact that emergency department (ED) providers often feel unprepared to manage suicide risk, patients with significant suicide risk frequently receive care in EDs, whether or not they have sustained physical injuries resulting from suicidal behavior [1,2]. Patients make greater than 400,000 visits to EDs in the United States each year for suicidal and self-injurious behaviors (suicide attempts and self-injurious behaviors are typically coded in ways that make them indistinguishable from each other in retrospective analyses) [3], and it is estimated that 6% to 10% of all patients in EDs endorse suicidal ideation when asked, regardless of their original chief complaints [4]. Meanwhile, suicide has become the 10th leading cause of death in the United States [5], and the Joint Commission has charged all accredited health care organizations with providing comprehensive treatment to suicidal patients, which may range from immediately containing an acute risk to ensuring continuity of care in follow-up [5].
When an acute suicide risk is identified in the ED, the provider’s immediate next steps should be to place the patient in a safe area under constant observation and to provide an emergency assessment [5,6]. Although psychiatric consultation and/or psychiatric admission may follow this assessment, suicide risk does not require admission in all cases; and some patients with suicide risk may be discharged to an outpatient setting even without receiving a psychiatric consultation [1]. Regardless of whether an outpatient disposition from the ED is appropriate, however, the period that immediately follows discharge is a time of high risk for repeated suicidal behavior and suicide death [7–9], and only 30% to 50% of patients who are discharged from EDs after a self-harm incident actually keep a follow-up mental health appointment [9,10]. Therefore, any support given to patients through this transition out of the emergency care setting could be especially high-yield.
The Joint Commission recommends that all patients with suicidal ideation receive, at minimum, a referral to treatment, telephone numbers for local and national crisis support resources (including the National Suicide Prevention Lifeline 1-800-273-TALK), collaborative safety planning, and counseling to restrict access to lethal means upon discharge [5]. However, some programs have demonstrated the capacity to provide enhanced support to patients beyond discharge from the ED, with some success in reducing the rates of subsequent suicidal behaviors. This non-systematic review describes interventions that can be initiated in the context of an ED encounter with the purpose of reducing future suicidal behavior among adult patients. They are primarily psychosocial rather than clinical. Clinical interventions that apply psychotherapy [11–13] psychopharmacology [14], and specialized inpatient treatments [15] have been studied as well but are beyond the scope of this review.
[polldaddy:10107269]
Interventions to Support Patients At Risk of Suicide After Discharge from the ED
Brief Contact Interventions
The idea that maintaining written correspondence with patients who have a known suicide risk after discharge can reduce subsequent suicide rates originated with a study of psychiatric inpatients conducted by Motto and Bostrom, in which patients who had been admitted for depression but had declined outpatient treatment were randomly assigned to periodically receive letters containing supportive messages from staff members over a period of 5 years [16]. This study remarkably found that these so-called brief contact interventions (BCIs), which were personalized to each recipient but did not contain psychotherapy per se, were associated with a reduced rate of suicide throughout the duration of the program compared with no written contacts [16].
BCIs have since been adapted to other communication formats and have been studied in patients who were discharged directly from the ED after an evaluation of suicide risk or suicidal behavior. Typically, BCIs consist of short, supportive messages that are delivered at regular intervals (often once every 1–2 months) over a period of 1 to 5 years [17,18]. They notably do not contain psychotherapy content, although they may reinforce coping strategies or remind recipients of how to access help if needed [17,19]. They may arrive as postcards [20,21], letters [22], telephone outreach [23–25], or a combination of modalities [26].
Protocols that rely on BCIs alone vary in their structure and have yielded mixed results [18]. A meta-analysis of 12 BCI protocols conducted by Milner et al found that, overall, BCIs administered after a presentation to the ED for self-harm have been associated with a significant reduction in repeat suicide attempts per recipient but not in total suicide deaths [27]. Milner’s group did not recommend large-scale promotion of BCIs based on the inadequacy of data so far, but suggested that this strategy may yet show promise upon further study [27]. A key advantage of BCIs is that they are inexpensive to implement, particularly if they do not include a telephone outreach component [28]. Thus, even if the potential benefit to patients is small, administering BCIs can be cost-effective [28].
It should not come as a surprise, therefore, that the potential for incorporation of BCIs into mobile smartphone technology is currently under investigation. Individuals who own mobile phones typically keep them on their persons and turned on continuously, and thus this is a reliable platform for maintaining contact with a wide range of patients in real-time [17,29]. Developers of at least 2 BCI smartphone programs that rely on mobile text messaging have published their protocols [17,30]. However, whether these programs will succeed in meaningfully reducing suicide rates remains to be determined by future research.
Green Cards
Morgan et al conducted a study in the United Kingdom in which individuals who presented to EDs after a self-harm event received a “green card,” which contained encouraging messages about seeking help and provided contact information for emergency services with 24-hour availability [31]. The green card also facilitated access to a crisis admission if necessary. The green card was distributed first in the ED and a second time by mail 3 weeks later. No suicides occurred in either the intervention or control group, which received usual care, and no statistically significant differences in suicide reattempt rate were found between groups after 1 year [31].
Evans et al studied an updated version of the green card intervention in which the green card facilitated access to an on-call psychiatrist with 24-hour availability by telephone [32]. The updated card included encouraging messages about seeking help similar to the original green card described by Morgan; however, the psychiatry consultation via telephone replaced the offer of hospital admission [32]. This second trial of green cards also failed to show a reduction in the rate of suicide reattempts among green card recipients at 6 months and 1 year [32,33].
Brief Intervention and Contact
The World Health Organization’s Brief Intervention and Contact (BIC) protocol is a standardized, multi-step suicide prevention program that has been studied primarily in patients who present to EDs after a suicide attempt in middle-income countries [34]. BIC includes a 1-hour information session that is administered shortly prior to discharge, and subsequently provides 9 follow-up contact interventions at specified intervals over an 18-month period. Unlike in a typical BCI, the contacts in BIC are conducted by a clinician either face-to-face or over the phone and include standardized assessments of the patient’s condition, although they still do not include psychotherapy. BIC has been shown to reduce suicide attempts, suicide deaths, or both in India [34–36], Iran [34,36,37], China [34,36], Brazil [34,36], and Sri Lanka [34,36] but was not found to directly improve clinical outcomes in a study conducted in French Polynesia [38]. A meta-analysis conducted by Riblet et al concluded that BIC is effective in reducing suicide risk overall [39].
ED-SAFE
The Emergency Department Safety Assessment and Follow-up Evaluation (ED-SAFE) protocol was validated in 8 EDs in 7 states in the US that did not already provide psychiatric services internally [40]. Under this model, all patients in the ED receive a screening for suicide risk, and those with an initial positive screen receive a secondary screen administered by the ED physician, a self-administered safety plan, and a series of up to 11 phone contacts over the following year that are administered by trained mental health clinicians in a central location. The ED-SAFE phone contacts follow the Coping Long Term with Active Suicide Program (CLASP) protocol [41] and provide support around safety planning and treatment engagement. They have the capacity to engage the patients’ significant others directly if a significant other is available and the patient chooses to involve that person.
In a single multicenter study, ED-SAFE reduced the absolute risk of suicide attempt by 5%, and the relative risk by 20% compared to usual treatment [40]. An intermediate phase of the study compared the universal suicide screening alone (ie, without the safety plan or follow-up contacts) with usual care and did not find this to improve outcomes [40].
Case Management
Kawanishi et al conducted a randomized controlled trial of assertive case management, the ACTION-J study, for patients with psychiatric diagnoses who presented with self-harm to 17 participating EDs in Japan [42]. In the ACTION-J study, case managers were mental health clinicians who provided clinical evaluations, treatment planning, encouragement, and care coordination over the course of 7 scheduled face-to-face or phone contacts in the first 18 months, and additional contacts at 6-month intervals until the completion of the trial (up to a total of 5 years) [43]. The comparison intervention, enhanced usual care, consisted of psychoeducation provided at the time of the encounter in the ED without case management services. The assertive case management intervention was associated with a decrease in suicidal behavior in the first 6 months but not for the duration of the study, except in women, for whom the benefit lasted the full 18 months [42]. A subsequent analysis also found a decrease in the total number of self-harm episodes per person-year compared to enhanced usual care, although there was not a difference in the number of participants who experienced a repeat self-harm episode [43]. The benefit was most strongly pronounced among patients who had presented with an index suicide attempt [43].
Morthorst et al applied an alternative case management model for the assertive intervention for deliberate self harm (AID) trial, which took place in Denmark [44]. Participants were aged 12 and older and could have been recruited from medical or pediatric inpatient units as well as the ED after a self-harm event. AID employed psychiatric nurses to provide crisis intervention, crisis planning, problem solving, motivational support, family mediation, and assistance with keeping appointments over a period of 6 months following discharge. Outreach took place over the phone, by text message, in participants’ homes, in cafes, and at health and social services appointments. The intervention required at least 4 contacts, although additional contacts could be made if appropriate. In comparison with a control group, in which participants received only usual care (which included ready access to short-term psychotherapy), the AID intervention was not associated with statistically significant differences in recurrent suicidal behaviors [44]. Subgroup analyses examining adult participants aged 20–39 and 40 and older also did not find differences in recurrent suicidal behavior between groups [44].
The Baerum Model and OPAC
A municipal suicide prevention team that provides comprehensive social services to suicide attempters has operated in Baerum, Norway, since 1983 [45]. Under the Baerum model, patients who attempt suicide, can be discharged from the general hospital without psychiatric admission, and are determined to have a high level of need for support are connected by a hospital-based suicide prevention team to a community-based team consisting of nurses and a consulting psychologist, who subsequently engage patients in own their homes and through follow-up phone calls. The services they provide include care coordination, encouragement, activation of social networks, psychological first-aid, and counseling focused on problem-solving. The ostensible goal of the suicide prevention team is to provide a bridge between inpatient medical care and outpatient mental health treatment; however, the intervention lasts approximately 1 year regardless of whether the patient connects with a treatment program [45].
A retrospective comparison of outcomes between recipients of the original Baerum program and non-recipients failed to find a difference in suicide attempts or suicide deaths between groups [45]. However, this was not a controlled study, and suicide attempters were preferentially referred to the program based on whether they had a higher level of need at baseline. Hvid and Wang adapted this model to patients who presented to EDs and general hospitals in Amager, Denmark [46] and have since conducted a series of randomized controlled trials comparing their adaptation to usual care. The Danish version of the Baerum model, renamed OPAC (for “outreach, problem solving, adherence, continuity”), provides similar case management and counseling services but for a maximum of 6 months. In their studies, OPAC significantly reduced the number of patients with a repeat suicide attempt and the total number of repeat suicide attempts at a 1-year interval, and this effect on total number of suicide attempts was sustained at 5 years [47,48]. Although the OPAC protocol begins with a patient’s presentation to the ED, the intervention is initiated after admission to the general hospital. Therefore, while this may inspire a model that provides similar services directly from the ED to patients who do not require general hospital admission, the existing model is not entirely based in the ED.
Discussion
The needs of suicidal patients are often multidimensional, and in some cases their risks are driven by psychosocial problems in addition to, or instead of, medically modifiable psychiatric conditions [49]. However, developing an ED-based program to support patients who are at risk of suicide after they are discharged from the ED is possible. Many such programs that provide or facilitate caring contacts, family support, case management, and/or treatment engagement with discharged patients have demonstrated that similar strategies may have the potential to impact future suicidal behavior. Nonetheless, it would be a stretch to say that all hospital systems should immediately begin doing so.
A new post-discharge support program is an investment of financial resources, personnel, and sometimes technology. Successful delivery of support or messages in any format requires that the intended recipient be able to receive it via reliable access to a working address, telephone number, or electronic device. Nonetheless, programs that rely on BCIs alone (excluding those conducted via telephone) cost relatively little to implement and thus would require a smaller investment than programs that require synchronous telephone or face-to-face contacts with staff in addition to or instead of BCIs. Costs for synchronous programs will also vary depending on the frequency and duration of contacts and the licensure and training required of the staff who provide them.
A trend toward better outcomes associating with more resource-intensive programs is easy to imagine but has not been definitively demonstrated. The wide variation between protocols in all types of programs makes comparisons between those that do and do not include synchronous contacts, and between types of synchronous contacts, difficult. Meanwhile, the low cost of BCIs alone could increase their attractiveness as an investment regardless of the magnitude of outcome improvement.
Denchev et al constructed a cost/benefit comparison model that included the postcard BCI study conducted by Carter et al [20], the telephone outreach study conducted by Vaiva et al [23], and a study of cognitive behavioral therapy (CBT) [11], all of which showed a clinical benefit. This model relied upon some numeric estimations and did not account for variation in outcomes between individual studies of each intervention strategy. However, it concluded that both telephone outreach and CBT were likely to be cost-prohibitive compared to asynchronous BCIs, which were associated with a reduction in costs overall [28].
Conclusion
There remains much to learn regarding how best to reduce suicide risk among adult patients in the period after discharge from the ED, during which patients with an identified suicide risk are known to be vulnerable. However, providing psychosocial and emotional support to patients with an identified suicide risk after they are discharged from the ED is feasible and may reduce subsequent suicidal behaviors. Templates for providing supportive outreach using different modalities now exist, and these may help guide the ongoing development and widespread adoption of more effective and cost-effective solutions.
Corresponding author: David S. Kroll, MD, dskroll@bwh.harvard.edu.
Financial disclosure: Dr. Kroll has received research funding from Brigham and Women’s Hospital to study and develop technological solutions for supporting suicidal patients after discharge from the emergency department. He has additionally received research funding and a speaking honorarium from Avasure.
From the Department of Psychiatry, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA.
Abstract
- Objective: To provide a review of emergency department (ED)-based psychosocial interventions that support adult patients with an identified suicide risk towards a goal of reducing subsequent suicidal behavior through the period after discharge, which is known to be a time of high risk for suicidal behavior.
- Methods: Non-systematic review of the literature.
- Results: Multiple methods of engaging patients after discharge from the ED have been shown to reduce subsequent suicidal behaviors. These methods include sending caring letters in the mail, facilitating supportive phone conversations, case management, and protocols that combine different services. Overall, the existing literature is insufficient to recommend widespread adoption of any individual strategy or protocol. However, providing psychosocial and emotional support to patients with an identified suicide risk after they are discharged from the ED is feasible and may reduce subsequent suicidal behaviors. Templates for providing supportive outreach using different modalities now exist, and these may help guide the ongoing development and widespread adoption of more effective and cost-effective solutions.
- Conclusion: Many ED–based interventions that provide enhanced support to patients with suicide risk after they are discharged have demonstrated a potential to reduce the risk of future suicidal behavior.
Key words: suicide; emergency department.
Despite the fact that emergency department (ED) providers often feel unprepared to manage suicide risk, patients with significant suicide risk frequently receive care in EDs, whether or not they have sustained physical injuries resulting from suicidal behavior [1,2]. Patients make greater than 400,000 visits to EDs in the United States each year for suicidal and self-injurious behaviors (suicide attempts and self-injurious behaviors are typically coded in ways that make them indistinguishable from each other in retrospective analyses) [3], and it is estimated that 6% to 10% of all patients in EDs endorse suicidal ideation when asked, regardless of their original chief complaints [4]. Meanwhile, suicide has become the 10th leading cause of death in the United States [5], and the Joint Commission has charged all accredited health care organizations with providing comprehensive treatment to suicidal patients, which may range from immediately containing an acute risk to ensuring continuity of care in follow-up [5].
When an acute suicide risk is identified in the ED, the provider’s immediate next steps should be to place the patient in a safe area under constant observation and to provide an emergency assessment [5,6]. Although psychiatric consultation and/or psychiatric admission may follow this assessment, suicide risk does not require admission in all cases; and some patients with suicide risk may be discharged to an outpatient setting even without receiving a psychiatric consultation [1]. Regardless of whether an outpatient disposition from the ED is appropriate, however, the period that immediately follows discharge is a time of high risk for repeated suicidal behavior and suicide death [7–9], and only 30% to 50% of patients who are discharged from EDs after a self-harm incident actually keep a follow-up mental health appointment [9,10]. Therefore, any support given to patients through this transition out of the emergency care setting could be especially high-yield.
The Joint Commission recommends that all patients with suicidal ideation receive, at minimum, a referral to treatment, telephone numbers for local and national crisis support resources (including the National Suicide Prevention Lifeline 1-800-273-TALK), collaborative safety planning, and counseling to restrict access to lethal means upon discharge [5]. However, some programs have demonstrated the capacity to provide enhanced support to patients beyond discharge from the ED, with some success in reducing the rates of subsequent suicidal behaviors. This non-systematic review describes interventions that can be initiated in the context of an ED encounter with the purpose of reducing future suicidal behavior among adult patients. They are primarily psychosocial rather than clinical. Clinical interventions that apply psychotherapy [11–13] psychopharmacology [14], and specialized inpatient treatments [15] have been studied as well but are beyond the scope of this review.
[polldaddy:10107269]
Interventions to Support Patients At Risk of Suicide After Discharge from the ED
Brief Contact Interventions
The idea that maintaining written correspondence with patients who have a known suicide risk after discharge can reduce subsequent suicide rates originated with a study of psychiatric inpatients conducted by Motto and Bostrom, in which patients who had been admitted for depression but had declined outpatient treatment were randomly assigned to periodically receive letters containing supportive messages from staff members over a period of 5 years [16]. This study remarkably found that these so-called brief contact interventions (BCIs), which were personalized to each recipient but did not contain psychotherapy per se, were associated with a reduced rate of suicide throughout the duration of the program compared with no written contacts [16].
BCIs have since been adapted to other communication formats and have been studied in patients who were discharged directly from the ED after an evaluation of suicide risk or suicidal behavior. Typically, BCIs consist of short, supportive messages that are delivered at regular intervals (often once every 1–2 months) over a period of 1 to 5 years [17,18]. They notably do not contain psychotherapy content, although they may reinforce coping strategies or remind recipients of how to access help if needed [17,19]. They may arrive as postcards [20,21], letters [22], telephone outreach [23–25], or a combination of modalities [26].
Protocols that rely on BCIs alone vary in their structure and have yielded mixed results [18]. A meta-analysis of 12 BCI protocols conducted by Milner et al found that, overall, BCIs administered after a presentation to the ED for self-harm have been associated with a significant reduction in repeat suicide attempts per recipient but not in total suicide deaths [27]. Milner’s group did not recommend large-scale promotion of BCIs based on the inadequacy of data so far, but suggested that this strategy may yet show promise upon further study [27]. A key advantage of BCIs is that they are inexpensive to implement, particularly if they do not include a telephone outreach component [28]. Thus, even if the potential benefit to patients is small, administering BCIs can be cost-effective [28].
It should not come as a surprise, therefore, that the potential for incorporation of BCIs into mobile smartphone technology is currently under investigation. Individuals who own mobile phones typically keep them on their persons and turned on continuously, and thus this is a reliable platform for maintaining contact with a wide range of patients in real-time [17,29]. Developers of at least 2 BCI smartphone programs that rely on mobile text messaging have published their protocols [17,30]. However, whether these programs will succeed in meaningfully reducing suicide rates remains to be determined by future research.
Green Cards
Morgan et al conducted a study in the United Kingdom in which individuals who presented to EDs after a self-harm event received a “green card,” which contained encouraging messages about seeking help and provided contact information for emergency services with 24-hour availability [31]. The green card also facilitated access to a crisis admission if necessary. The green card was distributed first in the ED and a second time by mail 3 weeks later. No suicides occurred in either the intervention or control group, which received usual care, and no statistically significant differences in suicide reattempt rate were found between groups after 1 year [31].
Evans et al studied an updated version of the green card intervention in which the green card facilitated access to an on-call psychiatrist with 24-hour availability by telephone [32]. The updated card included encouraging messages about seeking help similar to the original green card described by Morgan; however, the psychiatry consultation via telephone replaced the offer of hospital admission [32]. This second trial of green cards also failed to show a reduction in the rate of suicide reattempts among green card recipients at 6 months and 1 year [32,33].
Brief Intervention and Contact
The World Health Organization’s Brief Intervention and Contact (BIC) protocol is a standardized, multi-step suicide prevention program that has been studied primarily in patients who present to EDs after a suicide attempt in middle-income countries [34]. BIC includes a 1-hour information session that is administered shortly prior to discharge, and subsequently provides 9 follow-up contact interventions at specified intervals over an 18-month period. Unlike in a typical BCI, the contacts in BIC are conducted by a clinician either face-to-face or over the phone and include standardized assessments of the patient’s condition, although they still do not include psychotherapy. BIC has been shown to reduce suicide attempts, suicide deaths, or both in India [34–36], Iran [34,36,37], China [34,36], Brazil [34,36], and Sri Lanka [34,36] but was not found to directly improve clinical outcomes in a study conducted in French Polynesia [38]. A meta-analysis conducted by Riblet et al concluded that BIC is effective in reducing suicide risk overall [39].
ED-SAFE
The Emergency Department Safety Assessment and Follow-up Evaluation (ED-SAFE) protocol was validated in 8 EDs in 7 states in the US that did not already provide psychiatric services internally [40]. Under this model, all patients in the ED receive a screening for suicide risk, and those with an initial positive screen receive a secondary screen administered by the ED physician, a self-administered safety plan, and a series of up to 11 phone contacts over the following year that are administered by trained mental health clinicians in a central location. The ED-SAFE phone contacts follow the Coping Long Term with Active Suicide Program (CLASP) protocol [41] and provide support around safety planning and treatment engagement. They have the capacity to engage the patients’ significant others directly if a significant other is available and the patient chooses to involve that person.
In a single multicenter study, ED-SAFE reduced the absolute risk of suicide attempt by 5%, and the relative risk by 20% compared to usual treatment [40]. An intermediate phase of the study compared the universal suicide screening alone (ie, without the safety plan or follow-up contacts) with usual care and did not find this to improve outcomes [40].
Case Management
Kawanishi et al conducted a randomized controlled trial of assertive case management, the ACTION-J study, for patients with psychiatric diagnoses who presented with self-harm to 17 participating EDs in Japan [42]. In the ACTION-J study, case managers were mental health clinicians who provided clinical evaluations, treatment planning, encouragement, and care coordination over the course of 7 scheduled face-to-face or phone contacts in the first 18 months, and additional contacts at 6-month intervals until the completion of the trial (up to a total of 5 years) [43]. The comparison intervention, enhanced usual care, consisted of psychoeducation provided at the time of the encounter in the ED without case management services. The assertive case management intervention was associated with a decrease in suicidal behavior in the first 6 months but not for the duration of the study, except in women, for whom the benefit lasted the full 18 months [42]. A subsequent analysis also found a decrease in the total number of self-harm episodes per person-year compared to enhanced usual care, although there was not a difference in the number of participants who experienced a repeat self-harm episode [43]. The benefit was most strongly pronounced among patients who had presented with an index suicide attempt [43].
Morthorst et al applied an alternative case management model for the assertive intervention for deliberate self harm (AID) trial, which took place in Denmark [44]. Participants were aged 12 and older and could have been recruited from medical or pediatric inpatient units as well as the ED after a self-harm event. AID employed psychiatric nurses to provide crisis intervention, crisis planning, problem solving, motivational support, family mediation, and assistance with keeping appointments over a period of 6 months following discharge. Outreach took place over the phone, by text message, in participants’ homes, in cafes, and at health and social services appointments. The intervention required at least 4 contacts, although additional contacts could be made if appropriate. In comparison with a control group, in which participants received only usual care (which included ready access to short-term psychotherapy), the AID intervention was not associated with statistically significant differences in recurrent suicidal behaviors [44]. Subgroup analyses examining adult participants aged 20–39 and 40 and older also did not find differences in recurrent suicidal behavior between groups [44].
The Baerum Model and OPAC
A municipal suicide prevention team that provides comprehensive social services to suicide attempters has operated in Baerum, Norway, since 1983 [45]. Under the Baerum model, patients who attempt suicide, can be discharged from the general hospital without psychiatric admission, and are determined to have a high level of need for support are connected by a hospital-based suicide prevention team to a community-based team consisting of nurses and a consulting psychologist, who subsequently engage patients in own their homes and through follow-up phone calls. The services they provide include care coordination, encouragement, activation of social networks, psychological first-aid, and counseling focused on problem-solving. The ostensible goal of the suicide prevention team is to provide a bridge between inpatient medical care and outpatient mental health treatment; however, the intervention lasts approximately 1 year regardless of whether the patient connects with a treatment program [45].
A retrospective comparison of outcomes between recipients of the original Baerum program and non-recipients failed to find a difference in suicide attempts or suicide deaths between groups [45]. However, this was not a controlled study, and suicide attempters were preferentially referred to the program based on whether they had a higher level of need at baseline. Hvid and Wang adapted this model to patients who presented to EDs and general hospitals in Amager, Denmark [46] and have since conducted a series of randomized controlled trials comparing their adaptation to usual care. The Danish version of the Baerum model, renamed OPAC (for “outreach, problem solving, adherence, continuity”), provides similar case management and counseling services but for a maximum of 6 months. In their studies, OPAC significantly reduced the number of patients with a repeat suicide attempt and the total number of repeat suicide attempts at a 1-year interval, and this effect on total number of suicide attempts was sustained at 5 years [47,48]. Although the OPAC protocol begins with a patient’s presentation to the ED, the intervention is initiated after admission to the general hospital. Therefore, while this may inspire a model that provides similar services directly from the ED to patients who do not require general hospital admission, the existing model is not entirely based in the ED.
Discussion
The needs of suicidal patients are often multidimensional, and in some cases their risks are driven by psychosocial problems in addition to, or instead of, medically modifiable psychiatric conditions [49]. However, developing an ED-based program to support patients who are at risk of suicide after they are discharged from the ED is possible. Many such programs that provide or facilitate caring contacts, family support, case management, and/or treatment engagement with discharged patients have demonstrated that similar strategies may have the potential to impact future suicidal behavior. Nonetheless, it would be a stretch to say that all hospital systems should immediately begin doing so.
A new post-discharge support program is an investment of financial resources, personnel, and sometimes technology. Successful delivery of support or messages in any format requires that the intended recipient be able to receive it via reliable access to a working address, telephone number, or electronic device. Nonetheless, programs that rely on BCIs alone (excluding those conducted via telephone) cost relatively little to implement and thus would require a smaller investment than programs that require synchronous telephone or face-to-face contacts with staff in addition to or instead of BCIs. Costs for synchronous programs will also vary depending on the frequency and duration of contacts and the licensure and training required of the staff who provide them.
A trend toward better outcomes associating with more resource-intensive programs is easy to imagine but has not been definitively demonstrated. The wide variation between protocols in all types of programs makes comparisons between those that do and do not include synchronous contacts, and between types of synchronous contacts, difficult. Meanwhile, the low cost of BCIs alone could increase their attractiveness as an investment regardless of the magnitude of outcome improvement.
Denchev et al constructed a cost/benefit comparison model that included the postcard BCI study conducted by Carter et al [20], the telephone outreach study conducted by Vaiva et al [23], and a study of cognitive behavioral therapy (CBT) [11], all of which showed a clinical benefit. This model relied upon some numeric estimations and did not account for variation in outcomes between individual studies of each intervention strategy. However, it concluded that both telephone outreach and CBT were likely to be cost-prohibitive compared to asynchronous BCIs, which were associated with a reduction in costs overall [28].
Conclusion
There remains much to learn regarding how best to reduce suicide risk among adult patients in the period after discharge from the ED, during which patients with an identified suicide risk are known to be vulnerable. However, providing psychosocial and emotional support to patients with an identified suicide risk after they are discharged from the ED is feasible and may reduce subsequent suicidal behaviors. Templates for providing supportive outreach using different modalities now exist, and these may help guide the ongoing development and widespread adoption of more effective and cost-effective solutions.
Corresponding author: David S. Kroll, MD, dskroll@bwh.harvard.edu.
Financial disclosure: Dr. Kroll has received research funding from Brigham and Women’s Hospital to study and develop technological solutions for supporting suicidal patients after discharge from the emergency department. He has additionally received research funding and a speaking honorarium from Avasure.
1. Betz ME, Boudreaux ED. Managing suicidal patients in the emergency department. Ann Emerg Med 2016;67:276–82.
2. McManus MC, Cramer RJ, Boshier M, et al. Mental health and drivers of need in emergent and non-emergent emergency department (ED) use: do living location and non-emergent care sources matter? Int J Environ Res Public Health 2018;15:129.
3. Ting SA, Sullivan AF, Boudreaux ED, et al. Trends in US emergency department visits for attempted suicide and self-inflicted injury, 1993-2008. Gen Hosp Psychiatry 2012;34:557–65.
4. Betz ME, Wintersteen M, Boudreaux ED, Brown G, Capoccia L, Currier G, et al. reducing suicide risk: challenges and opportunities in the emergency department. Ann Emerg Med 2016;68:758–65.
5. The Joint Commission. Sentinel event alert 56: detecting and treating suicide ideation in all settings. www.jointcommission.org/sea_issue_56/. Published February 24, 2016. Accessed June 4, 2018.
6. Mills PD, Watts BV, Hemphill RR. Suicide attempts and completions on medical-surgical and intensive care units. J Hosp Med 2014;9:182–5.
7. Crane EH. Patients with drug-related emergency department visits involving suicide attempts who left against medical advice. The CBHSQ Report. http://www.ncbi.nlm.nih.gov/books/NBK396153/ . Published September 13, 2016. Accessed June 4, 2018.
8. Fedyszyn IE, Erlangsen A, Hjorthøj C, et al. Repeated suicide attempts and suicide among individuals with a first emergency department contact for attempted suicide: a prospective, nationwide, Danish register-based study. J Clin Psychiatry 2016;77:832–40.
9. Hunter J, Maunder R, Kurdyak P, et al. Mental health follow-up after deliberate self-harm and risk for repeat self-harm and death. Psychiatry Res 2018;259:333–9.
10. Costemale-Lacoste JF, Balaguer E, Boniface B, et al. Outpatient treatment engagement after suicidal attempt: a multisite prospective study. Psychiatry Res 2017;258:21–3.
11. Brown GK, Ten Have T, Henriques GR, et al. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA 2005;294:563–70.
12. Gysin-Maillart A, Schwab S, Soravia L, Megert M, Michel K. A novel brief therapy for patients who attempt suicide: a 24-months follow-up randomized controlled study of the attempted suicide short intervention program (ASSIP). PLoS Med 2016;13:e1001968.
13. Hawton K, Witt KG, Salisbury TLT, et al. Psychosocial interventions following self-harm in adults: a systematic review and meta-analysis. Lancet Psychiatry. 2016;3:740–50.
14. Battaglia J, Wolff TK, Wagner-Johnson DS, et al. Structured diagnostic assessment and depot fluphenazine treatment of multiple suicide attempters in the emergency department. Int Clin Psychopharmacol 1999;14:361–72.
15. van der Sande R, van Rooijen L, Buskens E, et al. Intensive in-patient and community intervention versus routine care after attempted suicide. A randomised controlled intervention study. Br J Psychiatry 1997;171:35–41.
16. Motto JA, Bostrom AG. A randomized controlled trial of postcrisis suicide prevention. Psychiatr Serv 2001;52:828–33.
17. Berrouiguet S, Larsen ME, Mesmeur C, Gravey M, Billot R, Walter M, et al. Toward mHealth brief contact interventions in suicide prevention: case series from the suicide intervention assisted by messages (SIAM) randomized controlled trial. JMIR MHealth UHealth 2018;6:e8.
18. Falcone G, Nardella A, Lamis DA, et al. Taking care of suicidal patients with new technologies and reaching-out means in the post-discharge period. World J Psychiatry 2017;7:163–76.
19. Milner A, Spittal MJ, Kapur N, et al. Mechanisms of brief contact interventions in clinical populations: a systematic review. BMC Psychiatry 2016;16:194.
20. Carter GL, Clover K, Whyte IM, et al. Postcards from the EDge: 5-year outcomes of a randomised controlled trial for hospital-treated self-poisoning. Br J Psychiatry 2013;202:372–80.
21. Hassanian-Moghaddam H, Sarjami S, Kolahi AA, Carter GL. Postcards in Persia: randomised controlled trial to reduce suicidal behaviours 12 months after hospital-treated self-poisoning. Br J Psychiatry 2011;198:309–16.
22. Luxton DD, Thomas EK, Chipps J, et al. Caring letters for suicide prevention: implementation of a multi-site randomized clinical trial in the U.S. military and Veteran Affairs healthcare systems. Contemp Clin Trials 2014;37(2):252–60.
23. Vaiva G, Vaiva G, Ducrocq F, et al. Effect of telephone contact on further suicide attempts in patients discharged from an emergency department: randomised controlled study. BMJ 2006;332:1241–5.
24. Cebrià AI, Parra I, Pàmias M, et al. Effectiveness of a telephone management programme for patients discharged from an emergency department after a suicide attempt: controlled study in a Spanish population. J Affect Disord 2013;147:269–76.
25. Cedereke M, Monti K, Ojehagen A. Telephone contact with patients in the year after a suicide attempt: does it affect treatment attendance and outcome? A randomised controlled study. Eur Psychiatry. 2002;17:82–91.
26. Vaiva G, Walter M, Al Arab AS, et al. ALGOS: the development of a randomized controlled trial testing a case management algorithm designed to reduce suicide risk among suicide attempters. BMC Psychiatry 2011;11:1.
27. Milner AJ, Carter G, Pirkis J, et al. Letters, green cards, telephone calls and postcards: systematic and meta-analytic review of brief contact interventions for reducing self-harm, suicide attempts and suicide. Br J Psychiatry. 2015;206:184–90.
28. Denchev P, Pearson JL, Allen MH, Claassen CA, Currier GW, Zatzick DF, et al. Modeling the cost-effectiveness of interventions to reduce suicide risk among hospital emergency department patients. Psychiatr Serv 2018;69:23–31.
29. Berrouiguet S, Courtet P, Larsen ME, et al. Suicide prevention: towards integrative, innovative and individualized brief contact interventions. Eur Psychiatry 2018;47:25–6.
30. Larsen ME, Shand F, Morley K, Batterham PJ, Petrie K, Reda B, et al. A mobile text message intervention to reduce repeat suicidal episodes: design and development of reconnecting after a suicide attempt (RAFT). JMIR Ment Health 2017;4:e56.
31. Morgan HG, Jones EM, Owen JH. Secondary prevention of non-fatal deliberate self-harm. The green card study. Br J Psychiatry 1993;163:111–2.
32. Evans MO, Morgan HG, Hayward A, Gunnell DJ. Crisis telephone consultation for deliberate self-harm patients: effects on repetition. Br J Psychiatry 1999;175:23–7.
33. Evans J, Evans M, Morgan HG, et al. Crisis card following self-harm: 12-month follow-up of a randomised controlled trial. Br J Psychiatry J 2005;187:186–7.
34. Fleischmann A, Bertolote JM, Wasserman D, et al. Effectiveness of brief intervention and contact for suicide attempters: a randomized controlled trial in five countries. Bull World Health Organ 2008;86:703–9.
35. Vijayakumar L, Umamaheswari C, Shujaath Ali ZS, et al. Intervention for suicide attempters: a randomized controlled study. Indian J Psychiatry 2011;53:244–8.
36. Bertolote JM, Fleischmann A, De Leo D, et al. Repetition of suicide attempts: data from emergency care settings in five culturally different low- and middle-income countries participating in the WHO SUPRE-MISS Study. Crisis 2010;31:194–201.
37. Mousavi SG, Zohreh R, Maracy MR, et al. The efficacy of telephonic follow up in prevention of suicidal reattempt in patients with suicide attempt history. Adv Biomed Res 2014;3:198.
38. Amadéo S, Rereao M, Malogne A, et al. Testing brief intervention and phone contact among subjects with suicidal behavior: a randomized controlled trial in French Polynesia in the frames of the World Health Organization/suicide trends in at-risk territories study. Ment Illn 2015;7:5818.
39. Riblet NBV, Shiner B, Young-Xu Y, Watts BV. Strategies to prevent death by suicide: meta-analysis of randomised controlled trials. Br J Psychiatry 2017;210:396–402.
40. Miller IW, Camargo CA Jr, Arias SA, et al. Suicide prevention in an emergency department population: the ED-SAFE study. JAMA Psychiatry 2017;74:563–70.
41. Miller IW, Gaudiano BA, Weinstock LM. The coping long term with active suicide program: description and pilot data. Suicide Life Threat Behav 2016;46:752–61.
42. Kawanishi C, Aruga T, Ishizuka N, et al. Assertive case management versus enhanced usual care for people with mental health problems who had attempted suicide and were admitted to hospital emergency departments in Japan (ACTION-J): a multicentre, randomised controlled trial. Lancet Psychiatry 2014;1:193–201.
43. Furuno T, Nakagawa M, Hino K, et al. Effectiveness of assertive case management on repeat self-harm in patients admitted for suicide attempt: findings from ACTION-J study. J Affect Disord 2018;225:460–5.
44. Morthorst B, Krogh J, Erlangsen A, et al. Effect of assertive outreach after suicide attempt in the AID (assertive intervention for deliberate self harm) trial: randomised controlled trial. BMJ 2012;345:e4972.
45. Johannessen HA, Dieserud G, De Leo D, Claussen B, et al. Chain of care for patients who have attempted suicide: a follow-up study from Bærum, Norway. BMC Public Health 2011;11:81.
46. Hvid M, Wang AG. Preventing repetition of attempted suicide—I. Feasibility (acceptability, adherence, and effectiveness) of a Baerum-model like aftercare. Nord J Psychiatry 2009;63:148–53.
47. Hvid M, Vangborg K, Sørensen HJ, et al. Preventing repetition of attempted suicide-II. The Amager project, a randomized controlled trial. Nord J Psychiatry 2011;65:292–8.
48. Lahoz T, Hvid M, Wang AG. Preventing repetition of attempted suicide-III. The Amager project, 5-year follow-up of a randomized controlled trial. Nord J Psychiatry 2016;70:547–53.
49. Kroll DS, Karno J, Mullen B, et al. Clinical severity alone does not determine disposition decisions for patients in the emergency department with suicide risk. Psychosomatics 2017; pii: S0033-3182(17)30247–5.
1. Betz ME, Boudreaux ED. Managing suicidal patients in the emergency department. Ann Emerg Med 2016;67:276–82.
2. McManus MC, Cramer RJ, Boshier M, et al. Mental health and drivers of need in emergent and non-emergent emergency department (ED) use: do living location and non-emergent care sources matter? Int J Environ Res Public Health 2018;15:129.
3. Ting SA, Sullivan AF, Boudreaux ED, et al. Trends in US emergency department visits for attempted suicide and self-inflicted injury, 1993-2008. Gen Hosp Psychiatry 2012;34:557–65.
4. Betz ME, Wintersteen M, Boudreaux ED, Brown G, Capoccia L, Currier G, et al. reducing suicide risk: challenges and opportunities in the emergency department. Ann Emerg Med 2016;68:758–65.
5. The Joint Commission. Sentinel event alert 56: detecting and treating suicide ideation in all settings. www.jointcommission.org/sea_issue_56/. Published February 24, 2016. Accessed June 4, 2018.
6. Mills PD, Watts BV, Hemphill RR. Suicide attempts and completions on medical-surgical and intensive care units. J Hosp Med 2014;9:182–5.
7. Crane EH. Patients with drug-related emergency department visits involving suicide attempts who left against medical advice. The CBHSQ Report. http://www.ncbi.nlm.nih.gov/books/NBK396153/ . Published September 13, 2016. Accessed June 4, 2018.
8. Fedyszyn IE, Erlangsen A, Hjorthøj C, et al. Repeated suicide attempts and suicide among individuals with a first emergency department contact for attempted suicide: a prospective, nationwide, Danish register-based study. J Clin Psychiatry 2016;77:832–40.
9. Hunter J, Maunder R, Kurdyak P, et al. Mental health follow-up after deliberate self-harm and risk for repeat self-harm and death. Psychiatry Res 2018;259:333–9.
10. Costemale-Lacoste JF, Balaguer E, Boniface B, et al. Outpatient treatment engagement after suicidal attempt: a multisite prospective study. Psychiatry Res 2017;258:21–3.
11. Brown GK, Ten Have T, Henriques GR, et al. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA 2005;294:563–70.
12. Gysin-Maillart A, Schwab S, Soravia L, Megert M, Michel K. A novel brief therapy for patients who attempt suicide: a 24-months follow-up randomized controlled study of the attempted suicide short intervention program (ASSIP). PLoS Med 2016;13:e1001968.
13. Hawton K, Witt KG, Salisbury TLT, et al. Psychosocial interventions following self-harm in adults: a systematic review and meta-analysis. Lancet Psychiatry. 2016;3:740–50.
14. Battaglia J, Wolff TK, Wagner-Johnson DS, et al. Structured diagnostic assessment and depot fluphenazine treatment of multiple suicide attempters in the emergency department. Int Clin Psychopharmacol 1999;14:361–72.
15. van der Sande R, van Rooijen L, Buskens E, et al. Intensive in-patient and community intervention versus routine care after attempted suicide. A randomised controlled intervention study. Br J Psychiatry 1997;171:35–41.
16. Motto JA, Bostrom AG. A randomized controlled trial of postcrisis suicide prevention. Psychiatr Serv 2001;52:828–33.
17. Berrouiguet S, Larsen ME, Mesmeur C, Gravey M, Billot R, Walter M, et al. Toward mHealth brief contact interventions in suicide prevention: case series from the suicide intervention assisted by messages (SIAM) randomized controlled trial. JMIR MHealth UHealth 2018;6:e8.
18. Falcone G, Nardella A, Lamis DA, et al. Taking care of suicidal patients with new technologies and reaching-out means in the post-discharge period. World J Psychiatry 2017;7:163–76.
19. Milner A, Spittal MJ, Kapur N, et al. Mechanisms of brief contact interventions in clinical populations: a systematic review. BMC Psychiatry 2016;16:194.
20. Carter GL, Clover K, Whyte IM, et al. Postcards from the EDge: 5-year outcomes of a randomised controlled trial for hospital-treated self-poisoning. Br J Psychiatry 2013;202:372–80.
21. Hassanian-Moghaddam H, Sarjami S, Kolahi AA, Carter GL. Postcards in Persia: randomised controlled trial to reduce suicidal behaviours 12 months after hospital-treated self-poisoning. Br J Psychiatry 2011;198:309–16.
22. Luxton DD, Thomas EK, Chipps J, et al. Caring letters for suicide prevention: implementation of a multi-site randomized clinical trial in the U.S. military and Veteran Affairs healthcare systems. Contemp Clin Trials 2014;37(2):252–60.
23. Vaiva G, Vaiva G, Ducrocq F, et al. Effect of telephone contact on further suicide attempts in patients discharged from an emergency department: randomised controlled study. BMJ 2006;332:1241–5.
24. Cebrià AI, Parra I, Pàmias M, et al. Effectiveness of a telephone management programme for patients discharged from an emergency department after a suicide attempt: controlled study in a Spanish population. J Affect Disord 2013;147:269–76.
25. Cedereke M, Monti K, Ojehagen A. Telephone contact with patients in the year after a suicide attempt: does it affect treatment attendance and outcome? A randomised controlled study. Eur Psychiatry. 2002;17:82–91.
26. Vaiva G, Walter M, Al Arab AS, et al. ALGOS: the development of a randomized controlled trial testing a case management algorithm designed to reduce suicide risk among suicide attempters. BMC Psychiatry 2011;11:1.
27. Milner AJ, Carter G, Pirkis J, et al. Letters, green cards, telephone calls and postcards: systematic and meta-analytic review of brief contact interventions for reducing self-harm, suicide attempts and suicide. Br J Psychiatry. 2015;206:184–90.
28. Denchev P, Pearson JL, Allen MH, Claassen CA, Currier GW, Zatzick DF, et al. Modeling the cost-effectiveness of interventions to reduce suicide risk among hospital emergency department patients. Psychiatr Serv 2018;69:23–31.
29. Berrouiguet S, Courtet P, Larsen ME, et al. Suicide prevention: towards integrative, innovative and individualized brief contact interventions. Eur Psychiatry 2018;47:25–6.
30. Larsen ME, Shand F, Morley K, Batterham PJ, Petrie K, Reda B, et al. A mobile text message intervention to reduce repeat suicidal episodes: design and development of reconnecting after a suicide attempt (RAFT). JMIR Ment Health 2017;4:e56.
31. Morgan HG, Jones EM, Owen JH. Secondary prevention of non-fatal deliberate self-harm. The green card study. Br J Psychiatry 1993;163:111–2.
32. Evans MO, Morgan HG, Hayward A, Gunnell DJ. Crisis telephone consultation for deliberate self-harm patients: effects on repetition. Br J Psychiatry 1999;175:23–7.
33. Evans J, Evans M, Morgan HG, et al. Crisis card following self-harm: 12-month follow-up of a randomised controlled trial. Br J Psychiatry J 2005;187:186–7.
34. Fleischmann A, Bertolote JM, Wasserman D, et al. Effectiveness of brief intervention and contact for suicide attempters: a randomized controlled trial in five countries. Bull World Health Organ 2008;86:703–9.
35. Vijayakumar L, Umamaheswari C, Shujaath Ali ZS, et al. Intervention for suicide attempters: a randomized controlled study. Indian J Psychiatry 2011;53:244–8.
36. Bertolote JM, Fleischmann A, De Leo D, et al. Repetition of suicide attempts: data from emergency care settings in five culturally different low- and middle-income countries participating in the WHO SUPRE-MISS Study. Crisis 2010;31:194–201.
37. Mousavi SG, Zohreh R, Maracy MR, et al. The efficacy of telephonic follow up in prevention of suicidal reattempt in patients with suicide attempt history. Adv Biomed Res 2014;3:198.
38. Amadéo S, Rereao M, Malogne A, et al. Testing brief intervention and phone contact among subjects with suicidal behavior: a randomized controlled trial in French Polynesia in the frames of the World Health Organization/suicide trends in at-risk territories study. Ment Illn 2015;7:5818.
39. Riblet NBV, Shiner B, Young-Xu Y, Watts BV. Strategies to prevent death by suicide: meta-analysis of randomised controlled trials. Br J Psychiatry 2017;210:396–402.
40. Miller IW, Camargo CA Jr, Arias SA, et al. Suicide prevention in an emergency department population: the ED-SAFE study. JAMA Psychiatry 2017;74:563–70.
41. Miller IW, Gaudiano BA, Weinstock LM. The coping long term with active suicide program: description and pilot data. Suicide Life Threat Behav 2016;46:752–61.
42. Kawanishi C, Aruga T, Ishizuka N, et al. Assertive case management versus enhanced usual care for people with mental health problems who had attempted suicide and were admitted to hospital emergency departments in Japan (ACTION-J): a multicentre, randomised controlled trial. Lancet Psychiatry 2014;1:193–201.
43. Furuno T, Nakagawa M, Hino K, et al. Effectiveness of assertive case management on repeat self-harm in patients admitted for suicide attempt: findings from ACTION-J study. J Affect Disord 2018;225:460–5.
44. Morthorst B, Krogh J, Erlangsen A, et al. Effect of assertive outreach after suicide attempt in the AID (assertive intervention for deliberate self harm) trial: randomised controlled trial. BMJ 2012;345:e4972.
45. Johannessen HA, Dieserud G, De Leo D, Claussen B, et al. Chain of care for patients who have attempted suicide: a follow-up study from Bærum, Norway. BMC Public Health 2011;11:81.
46. Hvid M, Wang AG. Preventing repetition of attempted suicide—I. Feasibility (acceptability, adherence, and effectiveness) of a Baerum-model like aftercare. Nord J Psychiatry 2009;63:148–53.
47. Hvid M, Vangborg K, Sørensen HJ, et al. Preventing repetition of attempted suicide-II. The Amager project, a randomized controlled trial. Nord J Psychiatry 2011;65:292–8.
48. Lahoz T, Hvid M, Wang AG. Preventing repetition of attempted suicide-III. The Amager project, 5-year follow-up of a randomized controlled trial. Nord J Psychiatry 2016;70:547–53.
49. Kroll DS, Karno J, Mullen B, et al. Clinical severity alone does not determine disposition decisions for patients in the emergency department with suicide risk. Psychosomatics 2017; pii: S0033-3182(17)30247–5.
The Pop That Stopped the Soccer Game
ANSWER
The radiograph shows an avulsion fracture of the right iliac crest. While the patient does have a growth plate in this location, there is asymmetry between the right and left sides.
Pelvic avulsion fractures can be easy to overlook and are often misdiagnosed as strains. Providers must remember that the pelvis serves as an insertion site for multiple muscles; in both adolescent and adult patients, certain activities (eg, sprinting, jumping, kicking) can increase tension and result in a bone avulsion. Affected patients typically report a popping sensation, pain with range of motion, and point tenderness over the fracture.
Avulsion fractures can usually be identified on x-ray; CT and MRI are used only when definitive diagnosis is unclear. Treatment consists of conservative management—rest, protected weight bearing, and physical therapy. Surgery is typically reserved for those with > 2 cm displacement of the fracture fragment.
In athletes, a gradual return to sports is advised, with full participation at four to 12 weeks postinjury. Possible complications include recurrent symptoms, prolonged healing time, nonunion, malunion, or hip weakness.
This patient was placed on crutches with non-weight-bearing status for one week. She used OTC pain medication as needed. The patient completed a four-week course of physical therapy and returned to full weight-bearing status. After six weeks, the patient had returned to full activity with pain-free range of motion and full strength.
ANSWER
The radiograph shows an avulsion fracture of the right iliac crest. While the patient does have a growth plate in this location, there is asymmetry between the right and left sides.
Pelvic avulsion fractures can be easy to overlook and are often misdiagnosed as strains. Providers must remember that the pelvis serves as an insertion site for multiple muscles; in both adolescent and adult patients, certain activities (eg, sprinting, jumping, kicking) can increase tension and result in a bone avulsion. Affected patients typically report a popping sensation, pain with range of motion, and point tenderness over the fracture.
Avulsion fractures can usually be identified on x-ray; CT and MRI are used only when definitive diagnosis is unclear. Treatment consists of conservative management—rest, protected weight bearing, and physical therapy. Surgery is typically reserved for those with > 2 cm displacement of the fracture fragment.
In athletes, a gradual return to sports is advised, with full participation at four to 12 weeks postinjury. Possible complications include recurrent symptoms, prolonged healing time, nonunion, malunion, or hip weakness.
This patient was placed on crutches with non-weight-bearing status for one week. She used OTC pain medication as needed. The patient completed a four-week course of physical therapy and returned to full weight-bearing status. After six weeks, the patient had returned to full activity with pain-free range of motion and full strength.
ANSWER
The radiograph shows an avulsion fracture of the right iliac crest. While the patient does have a growth plate in this location, there is asymmetry between the right and left sides.
Pelvic avulsion fractures can be easy to overlook and are often misdiagnosed as strains. Providers must remember that the pelvis serves as an insertion site for multiple muscles; in both adolescent and adult patients, certain activities (eg, sprinting, jumping, kicking) can increase tension and result in a bone avulsion. Affected patients typically report a popping sensation, pain with range of motion, and point tenderness over the fracture.
Avulsion fractures can usually be identified on x-ray; CT and MRI are used only when definitive diagnosis is unclear. Treatment consists of conservative management—rest, protected weight bearing, and physical therapy. Surgery is typically reserved for those with > 2 cm displacement of the fracture fragment.
In athletes, a gradual return to sports is advised, with full participation at four to 12 weeks postinjury. Possible complications include recurrent symptoms, prolonged healing time, nonunion, malunion, or hip weakness.
This patient was placed on crutches with non-weight-bearing status for one week. She used OTC pain medication as needed. The patient completed a four-week course of physical therapy and returned to full weight-bearing status. After six weeks, the patient had returned to full activity with pain-free range of motion and full strength.
A 13-year-old girl presents with her mother for evaluation of right hip pain following a soccer game two days ago. The patient says she felt a “pop” in her right hip while running and kicking the ball. She was escorted off the field, unable to finish the game.
Since then, she has had pain over the right superior pelvic region. She rates the pain as a 1/10 at rest but 7/10 with ambulation. She is unwilling to bear weight secondary to discomfort and has been using crutches provided by her trainer. She has been using ice and ibuprofen without relief. Her medical history is unremarkable.
On physical exam, you note a well-developed, well-nourished female in no acute distress. No ecchymosis, erythema, or abrasions can be seen on skin exam. The patient has point tenderness over the right iliac crest. She has mild pain and weakness with hip flexion and significant pain with abduction. The extremity is neurovascularly intact.
A pelvic radiograph is obtained. What is your impression?
Thrown Off Track
ANSWER
The radiograph shows rib fractures on the left side (arrows); on the same side, there is a moderate-sized pleural effusion—presumably a hemothorax from the trauma.
A closer look at the mid-thoracic spine reveals some irregularity and possible deformity—note the slight offset. This finding is strongly suspicious for a fracture.
A subsequent CT revealed a thoracic burst fracture with retropulsion into the spinal canal.
ANSWER
The radiograph shows rib fractures on the left side (arrows); on the same side, there is a moderate-sized pleural effusion—presumably a hemothorax from the trauma.
A closer look at the mid-thoracic spine reveals some irregularity and possible deformity—note the slight offset. This finding is strongly suspicious for a fracture.
A subsequent CT revealed a thoracic burst fracture with retropulsion into the spinal canal.
ANSWER
The radiograph shows rib fractures on the left side (arrows); on the same side, there is a moderate-sized pleural effusion—presumably a hemothorax from the trauma.
A closer look at the mid-thoracic spine reveals some irregularity and possible deformity—note the slight offset. This finding is strongly suspicious for a fracture.
A subsequent CT revealed a thoracic burst fracture with retropulsion into the spinal canal.
A 20-year-old man is riding a four-wheel all-terrain vehicle at a high rate of speed when he loses control and is thrown off. He is not wearin
As you begin your primary survey, you note a young male who is anxious but awake and able to converse. He is receiving 100% oxygen via a non-rebreather mask. His heart rate is 130 beats/min and his blood pressure, 80/40 mm Hg. Breath sounds are somewhat decreased on the left side. The patient can move both arms, and his strength is normal. However, he is insensate from his mid-chest down and is unable to move his legs at all.
Portable radiographs are obtained, including a chest radiograph (shown). What is your impression?