User login
Preventing a first episode of esophageal variceal hemorrhage
Variceal hemorrhage is a medical emergency in which up to 20% of patients die.1 Even if the patient survives an initial episode of variceal bleeding, the probability of another episode is high: the rebleeding rate without treatment is 70% within 1 year. The mortality rate with rebleeding is 33%.
With such overwhelming consequences, the best strategy in any patient with cirrhosis and known varices is to try to prevent the first episode of bleeding.
WHO IS AT RISK?
Esophageal varices are present in 30% of patients with compensated cirrhosis and in up to 60% of those with decompensated cirrhosis (ie, with evidence of ascites or encephalopathy).2
The risk of variceal hemorrhage is related to three factors:
- The size of the varices. Varices 5 mm in diameter or smaller have a 7% risk of bleeding in 2 years, while those larger than 5 mm have a 30% risk of bleeding within 2 years.3
- The appearance of the varices. Morphologic features of varices, including red wale signs (red streaks of the mucosa overlying the varix), have been correlated with an increased risk of hemorrhage.
- The severity of liver dysfunction, as assessed by the Child-Pugh classification—an index of liver dysfunction based on serum albumin concentration, bilirubin level, prothrombin time, and the presence of ascites and encephalopathy. A high Child-Pugh score (ie, class B or C), representing decompensated cirrhosis, is associated with an increased risk of bleeding.
HOW VARICES DEVELOP: PORTAL HYPERTENSION
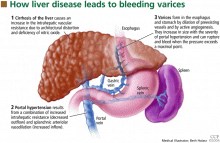
Esophageal varices form as a result of increased portal pressure, the product of increased portal venous inflow and resistance to outflow from the portal venous system. Portal hypertension is a major complication of chronic liver disease. In cirrhosis, architectural distortion of the liver causes an increase in the intrahepatic vascular resistance.
Portal venous inflow depends on mesenteric arteriolar tone, increasing when tone decreases. In cirrhotic patients, the increase in portal pressure results from a combination of increased portal blood flow secondary to splanchnic arteriolar vasodilation and elevated resistance to outflow through distorted hepatic sinusoids.
The potent vasodilator nitric oxide (NO) plays an important role in portal hypertension. In patients with cirrhosis, NO bioavailability is decreased in the intrahepatic circulation due to defects in the posttranslational regulation of endothelial NO synthase.4 This deficiency of NO, along with mechanical factors in the sinusoids, contributes to the increase in intrahepatic resistance. In the systemic and splanchnic circulation, NO bioavailability is increased due to upregulation and posttranslational regulation of endothelial NO synthase, thereby increasing splanchnic vasodilatation and leading to increased portal venous inflow.5 This results in a marked increase in cardiac output and so-called hyperdynamic circulation.
Portal hypertension results in the development of collateral circulation, including venous channels in the esophagus and stomach, by the dilation of preexisting vessels and active angiogenesis. Esophagogastric varices increase in size with the severity of portal hypertension and can rupture when the tension in their walls exceeds a maximal point.
HEPATIC VEIN PRESSURE GRADIENT: A PROXY FOR PORTAL PRESSURE
Ideally, the portal venous pressure should be directly measured. However, since direct measurement is invasive and impractical, the hepatic vein pressure gradient (HVPG) can be measured instead and correlates well with the portal pressure.6
The normal HVPG is 5 mm Hg or less; anything above this value denotes portal hypertension. However, studies have shown that varices may develop but do not bleed if the HVPG is less than 12 mm Hg.8
TWO WAYS TO PREVENT BLEEDING
Bleeding can be prevented either by reducing the portal venous pressure or by obliterating the varices. Portal pressure can be reduced by placing a portosystemic shunt either surgically or percutaneously with radiographic guidance or by giving drugs such as nonselective beta-blockers, nitrates, or a combination of these drugs. Variceal obliteration is typically done by endoscopic methods with either injection of a sclerosant or band ligation.
NONSELECTIVE BETA-BLOCKERS: THE MAINSTAY OF TREATMENT
Nonselective beta-blockers, the most commonly used drugs for preventing first esophageal variceal bleeding, decrease portal pressure by blocking both beta-1 and beta-2 adrenergic receptors.9 Beta-1 blockade decreases portal flow by decreasing the heart rate and cardiac output, while blockade of beta-2 receptors results in unopposed alpha-adrenergic-mediated vasoconstriction.
Selective beta-blockers do not appear to be as useful for primary prophylaxis. More than 2 decades ago, metoprolol (Toprol, Lopressor), a beta-1 selective antagonist, was compared with propranolol (Inderal), a nons-elective agent, in patients with cirrhosis and portal hypertension.10 Although both drugs significantly reduced the heart rate and cardiac output, only those taking propranolol showed a marked fall in portal pressure (mean decrease of 6.8 mm Hg vs 3.8 mm Hg with metoprolol) and a significant reduction in hepatic blood flow. The differences were thought to be related to beta-2 blockade of vasodilator receptors in the splanchnic circulation, which occurs only with nonselective beta-blockers such as propranolol.
The two nonselective beta-blockers most often used to prevent variceal bleeding are nadolol (Corgard) and propranolol. Both have been extensively studied in preventing a first variceal hemorrhage.
Effectiveness of beta-blockers
D’Amico et al11 performed a meta-analysis in 1995, examining nine trials (996 patients total) of the effectiveness of beta-blockers in preventing a first variceal hemorrhage. Seven trials found that bleeding risk was reduced with beta-blockers (significantly in four), one trial found that risk was unchanged, and one trial found that risk was increased—an outlier due to a small sample size. The meta-analysis showed a significant bleeding reduction with the use of a beta-blocker, either including the outlier trial (pooled odds ratio 0.54; 95% confidence interval 0.39–0.74) or excluding it (pooled odds ratio 0.48; 95% confidence interval 0.35–0.66).
Mortality rates were also reduced in seven trials, but the reduction was statistically significant in only one. However, in the pooled estimate, the mortality risk reduction approached statistical significance (pooled odds ratio 0.75; 95% confidence interval 0.57–1.06).
Ideo et al12 gave either nadolol or placebo to 79 patients with cirrhosis and large esophageal varices that had never bled. Nadolol was found to protect against a first variceal hemorrhage: at 2-year follow-up, only 1 of the 30 patients allocated to nadolol had had bleeding, vs 11 of the 49 patients in the placebo group.
Merkel et al13 found that the risk of variceal bleeding was lower in patients who started treatment with beta-blockers when their varices were small (12% at 5 years) than in those who started treatment after a diagnosis of large esophageal varices (22% at 5 years). They concluded that nadolol helps prevent small varices from growing into larger ones.
Response to beta-blockers is not uniform
Although beta-blockers decrease the portal pressure in many cirrhotic patients, the response is not uniform. In a study of 60 cirrhotic patients,14 40% showed no reduction or even a slight increase in HVPG with propranolol. Most patients showed a significant reduction in heart rate (17.5% ± 10%) after receiving 40 mg of propranolol. In the patients whose HVPG did not decrease by at least 10% with 40 mg of propranolol, increasing the dose caused a decrease in HVPG without a further decrease in heart rate. This suggests that 40 mg of propranolol successfully produced beta-1 blockade but that a higher dose was required for effective beta-2 blockade.
Failure to respond in certain patients may be due to a concurrent rise in collateral or hepatic sinusoidal resistance, or both. This was confirmed in a study in portal-hypertensive rats treated with propranolol.15 The reduction in portal blood flow expected was accompanied by a disproportionately small reduction in portal pressure, which was thought to be due to a rise in portal and collateral vascular resistance.
How to tell if beta-blocker treatment is ‘working’
An HVPG ≤ 12 mm Hg? Studies have shown that the most important predictor of efficacy of prophylaxis for variceal bleeding is a decrease in the HVPG to 12 mm Hg or less or a decrease in the initial HVPG of more than 20%.9 Although measuring the HVPG is invasive, expensive, and not routinely done in clinical practice, several studies have investigated the role of measuring hemodynamic response to medication.
Merkel et al16 measured the HVPG in 49 cirrhotic patients with previously nonbleeding varices before starting therapy with beta-blockers with or without nitrates and after 1 to 3 months of treatment. They followed the patients for up to 5 years. The mean HVPG value at baseline was 18.8 mm Hg. At 3 years of follow-up, 7% of those who had responded well to therapy (defined as achieving an HVPG less than 13 mm Hg or a decrease of more than 20%) had experienced a bleeding episode, which was significantly less than the rate (41%) in those who did not meet those hemodynamic end points. No patient reaching an HVPG of 12 mm Hg or less during treatment had variceal bleeding during follow-up.
Groszmann et al17 also prospectively measured the HVPG in patients with cirrhosis and varices, but their patients received either propranolol or placebo. Variceal hemorrhage occurred in 13 patients (11 of 51 in the placebo group and 2 of 51 in the propranolol group), all of whom had an HVPG greater than 12 mm Hg. Again, none of the patients whose HVPG was decreased to 12 mm Hg or less bled from esophageal varices.
Unfortunately, routine HVPG measurement to guide primary prophylaxis is an expensive strategy. Data suggest that measuring the HVPG is cost-effective only when the cost of measuring the HVPG is very low, the risk of variceal bleeding is very high, or the patient is expected to survive at least 3 to 5 years.18
A heart rate of 55 to 60? An alternative to HVPG measurement to monitor the effectiveness of beta-blocker therapy is to follow the heart rate. A 25% reduction from baseline or a heart rate of 55 to 60 beats per minute is the standard goal19,20; yet, at least 40% of patients treated with enough propranolol to decrease the heart rate by 25% do not respond with significant HVPG reductions.14,21
So, although beta-blockade is effective peripherally, it may not reduce HVPG to less than 12 mm Hg or 20% from baseline, and direct HVPG measurement is still the gold standard.
Treatment should be lifelong
Once a patient is started on a beta-blocker to prevent variceal hemorrhage, the treatment should be lifelong.
In 2001, a group of patients (most of them in Child-Pugh class A or B) completing a prospective randomized controlled trial of propranolol for primary prevention of variceal hemorrhage were tapered off propranolol or placebo.22 Of the 49 patients, 9 experienced variceal hemorrhage (6 of 25 former propranolol recipients and 3 of 24 former placebo recipients), and 17 patients died (12 former propranolol and 5 former placebo recipients), suggesting that treatment should be maintained for life.
Therefore, when beta-blocker therapy is discontinued, the risk of variceal hemorrhage returns to what would be expected in an untreated population.
Beta-blockers may not prevent varices
Although many trials have shown that beta-blockers are effective as prophylaxis against a first variceal hemorrhage, there is no evidence that these drugs prevent varices from forming in cirrhotic patients.
Groszmann et al23 treated more than 200 patients who had biopsy-proven cirrhosis and portal hypertension (HVPG > 6 mm Hg) but no varices with timolol (Blocadren), a nonselective beta-blocker, or placebo. At a median follow-up of about 55 months, the groups did not differ significantly in the incidence of primary events (development of varices or variceal hemorrhage) or treatment failures (transplantation or death). Varices developed less frequently among patients with a baseline HVPG of less than 10 mm Hg and among those whose HVPG had decreased by more than 10% at 1 year. In patients whose HVPG increased by more than 10%, varices developed more frequently.
Contraindications, side effects
The major drawbacks to therapy with beta-blockers are their contraindications and side effects.
Contraindications include chronic obstructive lung disease, psychosis, atrioventricular heart blocks, and aortic-valve disease.
Side effects are reported in 15% of patients but severe events are rare.24 Still, an estimated 10% to 20% of patients discontinue treatment because they cannot tolerate it.25 The more common complaints include fatigue, shortness of breath, sexual dysfunction, and sleep disorders.
Dosage
No specific starting dose of beta-blockers is agreed upon, but nadolol 20 to 40 mg once daily or long-acting propranolol 60 mg once daily can be used as initial therapy.25 Once-daily dosing increases the likelihood of compliance.
Since portal pressure progressively declines from 12 noon to 7 PM and then increases throughout the night and back to baseline by 9 AM,26 we recommend that the medication be taken in the evening to counteract increases in portal pressure that occur in the middle of the night.
ENDOSCOPIC VARICEAL LIGATION
Endoscopic variceal ligation has been investigated extensively for use as prophylaxis against first variceal hemorrhage. The procedure involves placing a rubber band around a varix aspirated into a cylinder on the tip of an endoscope.
Effectiveness of ligation
Lay et al,27 in a prospective, randomized trial in 126 cirrhotic patients endoscopically judged to be at high risk of hemorrhage, found that ligation significantly reduced the 2-year cumulative bleeding rate (19% with ligation vs 60% in an untreated control group) and the overall mortality rate (28% vs 58%). The lower risk of bleeding in the ligation group was attributed to a rapid reduction of variceal size; 60% of those in the ligation group had complete eradication of varices and 38% had varices reduced in size.
Imperiale and Chalasani28 performed a meta-analysis in 2001 that included 601 patients in five trials comparing prophylactic ligation with untreated controls and 283 patients in four trials comparing ligation with beta-blocker therapy. Compared with no treatment, ligation reduced the risk of first variceal hemorrhage, bleeding-related death, and death from any cause. Compared with propranolol, ligation reduced the risk of first-time bleeding but had no effect on the death rate.
Schepke et al,29 in a randomized controlled multicenter trial in 152 cirrhotic patients with two or more esophageal varices, found that neither bleeding incidence nor death rate differed significantly between ligation and propranolol.
Lui et al30 followed 172 cirrhotic patients with grade II or III esophageal varices for 6 years and found that ligation was equivalent to propranolol. However, many patients reported side effects with propranolol, and 30% of patients withdrew from propranolol treatment, making ligation a more attractive option.
Khuroo et al31 performed a meta-analysis of eight randomized controlled trials including 596 patients and found that ligation significantly reduced the rates of first gastrointestinal hemorrhage by 31% and of first variceal hemorrhage by 43%. In subgroup analysis, ligation had a significant advantage compared with beta-blockers in trials with patients with a high bleeding risk, ie, trials in which more than 30% of patients were in Child-Pugh class C and more than 50% of the patients had large varices.
Jutabha et al32 performed a multicenter, prospective trial (published in 2005) in 62 patients with high-risk esophageal varices randomized to propranolol or banding. The trial was ended early after an interim analysis showed that the failure rate of propranolol was significantly higher than that of banding (6/31 vs 0/31, P = .0098). Esophageal variceal hemorrhage occurred in 4 (12.9%) of the patients in the propranolol group compared with 0 in the ligation group. Similarly, 4 patients in the propranolol group died, compared with 0 in the ligation group. All the patients in this trial were liver transplant candidates and therefore all had severe liver disease.
In another trial favoring variceal banding over beta-blockers, Psilopoulos et al33 in 2005 followed 60 patients with cirrhosis and esophageal varices with no history of bleeding. Thirty percent of the patients in the propranolol group developed variceal bleeding compared with 6.7% in the ligation group (P = .043).
Lay et al34 followed 100 cirrhotic patients for 2 years and found comparable cumulative bleeding rates with ligation vs propranolol (18% vs 16%, respectively) and also comparable rates of death (28% vs 24%, respectively).
Sarin et al35 investigated the role of propranolol in addition to ligation in the prevention of first hemorrhage in 144 patients. Adding propranolol did not further decrease the incidence of initial bleeding (7% in the combination group vs 11% in the ligation-only group). Survival rates were similar at 20 months: 92% in the combination group vs 85% in the ligation-only group. However, the rate of variceal recurrence was lower with combination therapy: 6% in the combination group vs 15% with ligation alone.
Does esophageal variceal ligation increase gastric varices?
A less researched topic is whether variceal ligation results in gastric hemodynamic changes that increase the size of fundal varices and worsen portal hypertensive gastropathy.
Yuksel et al36 found that 37 of 85 patients had fundal varices before they underwent ligation of esophageal varices, increasing to 46 after the procedure, a statistically significant increment. The severity of portal hypertensive gastropathy also increased.
Further research is required regarding the long-term consequences of these findings.
ANGIOTENSIN II RECEPTOR ANTAGONISTS: ROLE UNKNOWN
Angiotensin II increases portal pressure, and angiotensin II levels are elevated in patients with cirrhosis, suggesting that this hormone plays a role in the pathogenesis of portal hypertension.
Losartan (Cozaar), an angiotensin II receptor antagonist, was found to decrease the HVPG significantly in patients with severe and moderate portal hypertension in a pilot study37 in 1999. However, in two subsequent studies,38,39 losartan only moderately reduced the HVPG and caused hypotension and a reduction in the glomerular filtration rate. The role of angiotensin II receptor blockers in primary prevention of variceal bleeding is still unknown.
SURGICAL PORTAL DECOMPRESSION HAS BEEN ABANDONED
The first method investigated to prevent variceal bleeding was surgical portal decompression.
A meta-analysis of four randomized controlled trials in 302 patients with varices of all sizes compared portocaval shunt surgery and medical therapy.11 Although shunt surgery was very effective in preventing variceal bleeding, the risk of chronic or recurrent encephalopathy was significantly increased (odds ratio 2.0), as was the risk of death (odds ratio 1.6).
These poor results, combined with advances in endoscopic procedures, led to the abandonment of surgical shunting for primary prophylaxis.
TIPS PROCEDURE: NO ROLE AT PRESENT
The transjugular intrahepatic portosystemic shunt (TIPS) procedure is used to treat the main consequences of portal hypertension, including ascites and variceal hemorrhage. The procedure entails accessing the hepatic vein via the right jugular vein and placing a stent to the portal vein, forming a low-resistance channel and allowing blood to return to the systemic circulation.
TIPS placement increases the risk of encephalopathy; liver failure is a rare complication, and procedural complications (ie, shunt dysfunction) also occur. Trials comparing the TIPS procedure with other forms of therapy to prevent first variceal hemorrhages have not been performed.40 Research to improve the outcome of the TIPS procedure is ongoing, but currently this procedure has no role in primary prevention of variceal bleeding.
ENDOSCOPIC SCLEROTHERAPY MAY INCREASE THE RISK OF DEATH
Numerous clinical trials evaluated sclerotherapy as prophylaxis against a first esophageal variceal hemorrhage. The procedure involves injecting a sclerosant in and around varices.
In a large Veterans Administration study,41 sclerotherapy was compared with sham treatment in 281 men with alcoholic liver disease who had documented varices but no history of bleeding. The trial was terminated after 22.5 months because the rate of all-cause mortality was significantly higher in the sclerotherapy group (32.5%) than in the sham therapy group (17.4%). The higher death rate did not persist after the treatment was discontinued, and it was speculated that, although sclerotherapy had reduced new episodes of variceal hemorrhage, the procedure might have caused bleeding from esophageal ulcers, leading to an increased mortality rate in that group.
The PROVA Study Group from Norway and Denmark found similar results when 286 cirrhotic patients were randomized to receive sclerotherapy, propranolol, combination sclerotherapy and propranolol, or no treatment to prevent a first variceal hemorrhage.42 The incidence of variceal bleeding was almost identical in the four groups, but the mortality rate with variceal bleeding was 2.75 times higher in the sclerotherapy groups than in the other groups (P = .002). It was speculated that repeated sclerotherapy sessions might be poorly tolerated by patients in Child-Pugh classes B and C and might have contributed to the precipitation of liver failure and other common complications of cirrhosis.
A meta-analysis by D’Amico et al11 evaluated 19 trials (1,630 patients) comparing sclerotherapy with nonactive treatment. Sclerotherapy tended to be favorable in trials with a high bleeding rate in the control patients and unfavorable in trials with a low bleeding rate. The benefit seen in patients at high risk is consistent with the efficacy of sclerotherapy for preventing rebleeding, whereas the harmful effect in the low-risk patients points towards side effects and complications exceeding the potential benefits.
In general, currently available evidence suggests that the benefits of prophylactic sclerotherapy are marginal, and therefore sclerotherapy is not recommended as primary prophylaxis for variceal hemorrhage.
NITRATES: NO LONGER USED AS MONOTHERAPY
Unlike vasoconstrictors, which decrease portal pressure by decreasing blood flow, vasodilators reduce hepatic pressure by decreasing intrahepatic and portocollateral vascular resistance.43 In addition, larger doses directly affect the arterial circulation, lowering systemic and therefore splanchnic perfusion pressure.44 Unfortunately, the systemic vasodilatory effects of nitrates exacerbate the hyperdynamic state that is characteristic of cirrhosis, thereby limiting their use and tolerability in many patients.
A trial comparing propranolol vs isosorbide mononitrate initially found that the groups did not differ significantly with regard to bleeding rates and 2-year survival rates,45 but a 6-year follow-up found the likelihood of death greater in patients older than 50 years in the nitrate group.46 In an additional study comparing isosorbide mononitrate vs placebo in patients with contraindications to or intolerance of beta-blockers, no difference in the relative risk of first variceal hemorrhage was found between the two groups.47 Therefore, nitrates are no longer used as monotherapy to prevent variceal bleeding.
Combination therapy with beta-blockers plus nitrates is controversial. In a trial in 1996, Merkel et al48 found the cumulative risk of variceal bleeding was 18% at 40 months with nadolol alone vs 7.5% with nadolol plus isosorbide mononitrate. However, in a later trial, Garcia-Pagán et al49 found no significant advantage to combination therapy. The incidence of variceal bleeding at 1 year was 8.3% in the group receiving propranolol plus placebo and 5% in the group receiving propranolol plus isosorbide mononitrate; at 2 years, the rates were 10.6% vs 12.5%.
RECOMMENDATIONS FOR SCREENING AND PROPHYLAXIS
- All patients with cirrhosis should be screened for varices at the time of diagnosis.
- The size of the varices, including small (≤ 5 mm) and large (> 5 mm), and the presence of red wale marks on the varices should be recorded.
- Patients who have no varices on screening endoscopy should be rescreened every 3 years if their liver function is stable or every year if their liver function deteriorates. (Varices grow at a rate proportional to the severity of the liver disease.)
- Patients with portal hypertension but without varices do not need treatment with nonselective beta-blockers. Endoscopy should be performed at the intervals suggested above.
- Those who are found to have small varices on screening endoscopy but who have well-compensated liver disease (Child-Pugh class A) and no red wale marks should be rescreened every other year because the development of large varices is greater in patients with small varices on initial endoscopy than in patients with no varices. Emerging data support the use of beta-blockers to prevent varices from increasing in size.
- Patients who have small varices with red wale signs or who are in Child-Pugh class B or C have an increased risk of bleeding and should be treated with beta-blockers. If beta-blockers are not used, endoscopy should be done every year to look for an increase in variceal size.
- Patients who have large varices without red wale signs or who are in Child-Pugh class B or C should be treated with nonselective beta-blockers. The dose should be adjusted to achieve maximal tolerable decrease in heart rate to a minimum of 55 beats per minute, and treatment should be continued indefinitely.
- Endoscopic variceal ligation is an acceptable alternative to beta-blocker treatment as first-line therapy in those who cannot tolerate beta-blockers or who have contraindications to their use, or in those who have red wale marking or who are in Child-Pugh class B or C.
- D’Amico G, De Franchis R Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003; 38:599–612.
- D’Amico G, Luca A. Natural history. Clinicalhaemodynamic correlations. Prediction of the risk of bleeding. Baillieres Clin Gastroenterol 1997; 11:243–256.
- Kamath PS. Esophageal variceal bleeding: primary prophylaxis. Clin Gastroenterol Hepatol 2005; 3:90–93.
- Shah V, Toruner M, Haddad F, et al. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology 1999; 117:1222–1228.
- Morales-Ruiz M, Jimenez W, Perez-Sala D, et al. Increased nitric oxide synthase expression in arterial vessels of cirrhotic rats with ascites. Hepatology 1996; 24:1481–1486.
- De Franchis R, Dell’Era A, Iannuzzi F. Diagnosis and treatment of portal hypertension. Dig Liver Dis 2004; 36:787–798.
- Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology 2004; 39:280–282.
- Garcia-Tsao G, Groszmann RJ, Fisher RL, Conn HO, Atterbury CE, Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology 1985; 5:419–424.
- Bosch J, Masti R, Kravetz D, et al. Effects of propranolol on azygos venous blood flow and hepatic and systemic hemodynamics in cirrhosis. Hepatology 1984; 4:1200–1205.
- Westaby D, Bihari DJ, Gimson AE, Crossley IR, Williams R. Selective and non-selective beta receptor blockade in the reduction of portal pressure in patients with cirrhosis and portal hypertension. Gut 1984; 25:121–124.
- D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology 1995; 22:332–354.
- Ideo G, Bellati G, Fesce E, Grimoldi D. Nadolol can prevent the first gastrointestinal bleeding in cirrhotics: a prospective, randomized study. Hepatology 1988; 8:6–9.
- Merkel C, Marin R, Angeli P, et al. Gruppo Triveneto per l’Ipertensione Portale. A placebo-controlled clinical trial of nadolol in the prophylaxis of growth of small esophageal varices in cirrhosis. Gastroenterology 2004; 127:476–484.
- Garcia-Tsao G, Grace ND, Groszmann RJ, et al. Short-term effects of propranolol on portal venous pressure. Hepatology 1986; 6:101–106.
- Kroeger RJ, Groszmann RJ. Increased portal venous resistance hinders portal pressure reduction during the administration of beta-adrenergic blocking agents in a portal hypertensive model. Hepatology 1985; 5:97–101.
- Merkel C, Bolognesi M, Sacerdoti D, et al. The hemodynamic response to medical treatment of portal hypertension as a predictor of clinical effectiveness in the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology 2000; 32:930–934.
- Groszmann RJ, Bosch J, Grace ND, et al. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology 1990; 99:1401–1407.
- Hicken BL, Sharara AI, Abrams GA, Eloubeidi M, Fallon MB, Arguedas MR. Hepatic venous pressure gradient measurements to assess response to primary prophylaxis in patients with cirrhosis: a decision analytical study. Aliment Pharmacol Ther 2003; 17:145–153.
- Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol 1997; 92:1081–1091.
- Grace ND, Groszmann RJ, Garcia-Tsao G, et al. Portal hypertension and variceal bleeding: an AASLD single topic symposium. Hepatology 1998; 28:868–880.
- Feu F, Garcia-Pagán JC, Bosch J, et al. Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal haemorrhage in patients with cirrhosis. Lancet 1995; 346:1056–1059.
- Abraczinskas DR, Ookubo R, Grace ND, et al. Propranolol for the prevention of first esophageal variceal hemorrhage: a lifetime commitment? Hepatology 2001; 34:1096–1102.
- Groszmann RJ, Garcia-Tsao G, Bosch J, et al Portal Hypertension Collaborative Group. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med 2005; 353:2254–2261.
- Garcia-Pagán JC, Bosch J. Pharmacological prevention of variceal bleeding. New developments. Baillier Clin Gastroenterol 1997; 11:271–287.
- Talwalkar JA, Kamath PS. An evidence-based medicine approach to beta-blocker therapy in patients with cirrhosis. Am J Med 2004; 116:759–766.
- Garcia-Pagan JC, Feu F, Castells A, et al. Circadian variations of portal pressure and variceal hemorrhage in patients with cirrhosis. Hepatology 1994; 19:595–601.
- Lay CS, Tsai YT, Teg CY, et al. Endoscopic variceal ligation in prophylaxis of first variceal bleeding in cirrhotic patients with high-risk esophageal varices. Hepatology 1997; 25:1346–1350.
- Imperiale TF, Chalasani N. A meta-analysis of endoscopic variceal ligation for primary prophylaxis of esophageal variceal bleeding. Hepatology 2001; 33:802–807.
- Schepke M, Kleber G, Nurnberg D, et al. Ligation versus propranolol for the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology 2004; 40:65–72.
- Lui HF, Stanley AJ, Forrest EH, et al. Primary prophylaxis of variceal hemorrhage: a randomized controlled trial comparing band ligation, propranolol, and isosorbide mononitrate. Gastroenterology 2002; 123:735–744.
- Khuroo MS, Khuroo NS, Farahat KL, Khuroo YS, Sofi AA, Dahab ST. Meta-analysis: endoscopic variceal ligation for primary prophylaxis of oesophageal variceal bleeding. Aliment Pharmacol Ther 2005; 21:347–361.
- Jutabha R, Jensen DM, Martin P, Savides T, Han SH, Gornbein J. Randomized study comparing banding and propranolol to prevent initial variceal hemorrhage in cirrhotics with high-risk esophageal varices. Gastroenterology 2005; 128:870–881.
- Psilopoulos D, Galanis P, Goulas S, et al. Endoscopic variceal ligation vs. propranolol for prevention of first variceal bleeding: a randomized controlled trial. Eur J Gastroenterol Hepatol 2005; 17:1111–1117.
- Lay CS, Tsai YT, Lee FY, et al. Endoscopic variceal ligation versus propranolol in prophylaxis of first variceal bleeding in patients with cirrhosis. J Gastroenterol Hepatol 2006; 21:413–419.
- Sarin SK, Wadhawan M, Agarwal SR, Tyagi P, Sharma BC. Endoscopic variceal ligation plus propranolol versus endoscopic variceal ligation alone in primary prophylaxis of variceal bleeding. Am J Gastroenterol 2005; 100:797–804.
- Yuksel O, Koklu S, Arhan M, Yolcu OF, et al. Effects of esophageal variceal eradication on portal hypertensive gastropathy and fundal varices: a retrospective and comparative study. Dig Dis Sci 2006; 51:27–30.
- Schneider AW, Kalk JF, Klein CP. Effect of losartan, an angiotensin II receptor antagonist, on portal pressure in cirrhosis. Hepatology 1999; 29:334–339.
- Schepke M, Werner E, Biecker E, et al. Hemodynamic effects of the angiotensin II receptor antagonist irbesartan in patients with cirrhosis and portal hypertension. Gastroenterology 2001; 121:389–395.
- Gonzalez-Abraldes J, Albillos A, Banares R, et al. Randomized comparison of long-term losartan versus propranolol in lowering portal pressure in cirrhosis. Gastroenterology 2001; 121:382–388.
- Boyer TD, Haskal ZJ American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology 2005; 41:386–400.
- Prophylactic sclerotherapy for esophageal varices in men with alcoholic liver disease. A randomized, single-blind, multicenter clinical trial. The Veterans Affairs Cooperative Variceal Sclerotherapy Group. N Engl J Med 1991; 324:1779–1784.
- The PROVA Study Group. Prophylaxis of first hemorrhage from esophageal varices by sclerotherapy, propranolol or both in cirrhotic patients: a randomized multicenter trial. Hepatology 1991; 14:1016–1024.
- Escorsell A, Feu F, Bordas JM, et al. Effects of isosorbide-5-mononitrate on variceal pressure and systemic and splanchnic haemodynamics in patients with cirrhosis. J Hepatol 1996; 24:423–429.
- Hayes PC, Westaby D, Williams R. Effect and mechanism of action of isosorbide-5-mononitrate. Gut 1988; 29:752–755.
- Angelico M, Carli L, Piat C, et al. Isosorbide-5-mononitrate versus propranolol in the prevention of first bleeding in cirrhosis. Gastroenterology 1993; 104:1460–1465.
- Angelico M, Carli L, Piat C, Gentile S, Capocaccia L. Effects of isosorbide-5-mononitrate compared with propranolol on first bleeding and long-term survival in cirrhosis. Gastroenterology 1997; 113:1632–1639.
- Garcia-Pagan JC, Villanueva C, Vila MC, et al. MOVE Group. Mononitrato Varices Esofagicas. Isosorbide mononitrate in the prevention of first variceal bleed in patients who cannot receive beta-blockers. Gastroenterology 2001; 121:908–914.
- Merkel C, Marin R, Enzo E, et al. Randomised trial of nadolol alone or with isosorbide mononitrate for primary prophylaxis of variceal bleeding in cirrhosis. Gruppo-Triveneto per L’ipertensione portale (GTIP). Lancet 1996; 348:1677–1681.
- Garcia-Pagán JC, Morillas R, Banares R, et al Spanish Variceal Bleeding Study Group. Propranolol plus placebo versus propranolol plus isosorbide-5-mononitrate in the prevention of a first variceal bleed: a double-blind RCT. Hepatology 2003; 37:1260–1266.
- Garcia-Tsao G, Sanyal A, Grace N, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007; 46:922–938.
Variceal hemorrhage is a medical emergency in which up to 20% of patients die.1 Even if the patient survives an initial episode of variceal bleeding, the probability of another episode is high: the rebleeding rate without treatment is 70% within 1 year. The mortality rate with rebleeding is 33%.
With such overwhelming consequences, the best strategy in any patient with cirrhosis and known varices is to try to prevent the first episode of bleeding.
WHO IS AT RISK?
Esophageal varices are present in 30% of patients with compensated cirrhosis and in up to 60% of those with decompensated cirrhosis (ie, with evidence of ascites or encephalopathy).2
The risk of variceal hemorrhage is related to three factors:
- The size of the varices. Varices 5 mm in diameter or smaller have a 7% risk of bleeding in 2 years, while those larger than 5 mm have a 30% risk of bleeding within 2 years.3
- The appearance of the varices. Morphologic features of varices, including red wale signs (red streaks of the mucosa overlying the varix), have been correlated with an increased risk of hemorrhage.
- The severity of liver dysfunction, as assessed by the Child-Pugh classification—an index of liver dysfunction based on serum albumin concentration, bilirubin level, prothrombin time, and the presence of ascites and encephalopathy. A high Child-Pugh score (ie, class B or C), representing decompensated cirrhosis, is associated with an increased risk of bleeding.
HOW VARICES DEVELOP: PORTAL HYPERTENSION
Esophageal varices form as a result of increased portal pressure, the product of increased portal venous inflow and resistance to outflow from the portal venous system. Portal hypertension is a major complication of chronic liver disease. In cirrhosis, architectural distortion of the liver causes an increase in the intrahepatic vascular resistance.
Portal venous inflow depends on mesenteric arteriolar tone, increasing when tone decreases. In cirrhotic patients, the increase in portal pressure results from a combination of increased portal blood flow secondary to splanchnic arteriolar vasodilation and elevated resistance to outflow through distorted hepatic sinusoids.
The potent vasodilator nitric oxide (NO) plays an important role in portal hypertension. In patients with cirrhosis, NO bioavailability is decreased in the intrahepatic circulation due to defects in the posttranslational regulation of endothelial NO synthase.4 This deficiency of NO, along with mechanical factors in the sinusoids, contributes to the increase in intrahepatic resistance. In the systemic and splanchnic circulation, NO bioavailability is increased due to upregulation and posttranslational regulation of endothelial NO synthase, thereby increasing splanchnic vasodilatation and leading to increased portal venous inflow.5 This results in a marked increase in cardiac output and so-called hyperdynamic circulation.
Portal hypertension results in the development of collateral circulation, including venous channels in the esophagus and stomach, by the dilation of preexisting vessels and active angiogenesis. Esophagogastric varices increase in size with the severity of portal hypertension and can rupture when the tension in their walls exceeds a maximal point.
HEPATIC VEIN PRESSURE GRADIENT: A PROXY FOR PORTAL PRESSURE
Ideally, the portal venous pressure should be directly measured. However, since direct measurement is invasive and impractical, the hepatic vein pressure gradient (HVPG) can be measured instead and correlates well with the portal pressure.6
The normal HVPG is 5 mm Hg or less; anything above this value denotes portal hypertension. However, studies have shown that varices may develop but do not bleed if the HVPG is less than 12 mm Hg.8
TWO WAYS TO PREVENT BLEEDING
Bleeding can be prevented either by reducing the portal venous pressure or by obliterating the varices. Portal pressure can be reduced by placing a portosystemic shunt either surgically or percutaneously with radiographic guidance or by giving drugs such as nonselective beta-blockers, nitrates, or a combination of these drugs. Variceal obliteration is typically done by endoscopic methods with either injection of a sclerosant or band ligation.
NONSELECTIVE BETA-BLOCKERS: THE MAINSTAY OF TREATMENT
Nonselective beta-blockers, the most commonly used drugs for preventing first esophageal variceal bleeding, decrease portal pressure by blocking both beta-1 and beta-2 adrenergic receptors.9 Beta-1 blockade decreases portal flow by decreasing the heart rate and cardiac output, while blockade of beta-2 receptors results in unopposed alpha-adrenergic-mediated vasoconstriction.
Selective beta-blockers do not appear to be as useful for primary prophylaxis. More than 2 decades ago, metoprolol (Toprol, Lopressor), a beta-1 selective antagonist, was compared with propranolol (Inderal), a nons-elective agent, in patients with cirrhosis and portal hypertension.10 Although both drugs significantly reduced the heart rate and cardiac output, only those taking propranolol showed a marked fall in portal pressure (mean decrease of 6.8 mm Hg vs 3.8 mm Hg with metoprolol) and a significant reduction in hepatic blood flow. The differences were thought to be related to beta-2 blockade of vasodilator receptors in the splanchnic circulation, which occurs only with nonselective beta-blockers such as propranolol.
The two nonselective beta-blockers most often used to prevent variceal bleeding are nadolol (Corgard) and propranolol. Both have been extensively studied in preventing a first variceal hemorrhage.
Effectiveness of beta-blockers
D’Amico et al11 performed a meta-analysis in 1995, examining nine trials (996 patients total) of the effectiveness of beta-blockers in preventing a first variceal hemorrhage. Seven trials found that bleeding risk was reduced with beta-blockers (significantly in four), one trial found that risk was unchanged, and one trial found that risk was increased—an outlier due to a small sample size. The meta-analysis showed a significant bleeding reduction with the use of a beta-blocker, either including the outlier trial (pooled odds ratio 0.54; 95% confidence interval 0.39–0.74) or excluding it (pooled odds ratio 0.48; 95% confidence interval 0.35–0.66).
Mortality rates were also reduced in seven trials, but the reduction was statistically significant in only one. However, in the pooled estimate, the mortality risk reduction approached statistical significance (pooled odds ratio 0.75; 95% confidence interval 0.57–1.06).
Ideo et al12 gave either nadolol or placebo to 79 patients with cirrhosis and large esophageal varices that had never bled. Nadolol was found to protect against a first variceal hemorrhage: at 2-year follow-up, only 1 of the 30 patients allocated to nadolol had had bleeding, vs 11 of the 49 patients in the placebo group.
Merkel et al13 found that the risk of variceal bleeding was lower in patients who started treatment with beta-blockers when their varices were small (12% at 5 years) than in those who started treatment after a diagnosis of large esophageal varices (22% at 5 years). They concluded that nadolol helps prevent small varices from growing into larger ones.
Response to beta-blockers is not uniform
Although beta-blockers decrease the portal pressure in many cirrhotic patients, the response is not uniform. In a study of 60 cirrhotic patients,14 40% showed no reduction or even a slight increase in HVPG with propranolol. Most patients showed a significant reduction in heart rate (17.5% ± 10%) after receiving 40 mg of propranolol. In the patients whose HVPG did not decrease by at least 10% with 40 mg of propranolol, increasing the dose caused a decrease in HVPG without a further decrease in heart rate. This suggests that 40 mg of propranolol successfully produced beta-1 blockade but that a higher dose was required for effective beta-2 blockade.
Failure to respond in certain patients may be due to a concurrent rise in collateral or hepatic sinusoidal resistance, or both. This was confirmed in a study in portal-hypertensive rats treated with propranolol.15 The reduction in portal blood flow expected was accompanied by a disproportionately small reduction in portal pressure, which was thought to be due to a rise in portal and collateral vascular resistance.
How to tell if beta-blocker treatment is ‘working’
An HVPG ≤ 12 mm Hg? Studies have shown that the most important predictor of efficacy of prophylaxis for variceal bleeding is a decrease in the HVPG to 12 mm Hg or less or a decrease in the initial HVPG of more than 20%.9 Although measuring the HVPG is invasive, expensive, and not routinely done in clinical practice, several studies have investigated the role of measuring hemodynamic response to medication.
Merkel et al16 measured the HVPG in 49 cirrhotic patients with previously nonbleeding varices before starting therapy with beta-blockers with or without nitrates and after 1 to 3 months of treatment. They followed the patients for up to 5 years. The mean HVPG value at baseline was 18.8 mm Hg. At 3 years of follow-up, 7% of those who had responded well to therapy (defined as achieving an HVPG less than 13 mm Hg or a decrease of more than 20%) had experienced a bleeding episode, which was significantly less than the rate (41%) in those who did not meet those hemodynamic end points. No patient reaching an HVPG of 12 mm Hg or less during treatment had variceal bleeding during follow-up.
Groszmann et al17 also prospectively measured the HVPG in patients with cirrhosis and varices, but their patients received either propranolol or placebo. Variceal hemorrhage occurred in 13 patients (11 of 51 in the placebo group and 2 of 51 in the propranolol group), all of whom had an HVPG greater than 12 mm Hg. Again, none of the patients whose HVPG was decreased to 12 mm Hg or less bled from esophageal varices.
Unfortunately, routine HVPG measurement to guide primary prophylaxis is an expensive strategy. Data suggest that measuring the HVPG is cost-effective only when the cost of measuring the HVPG is very low, the risk of variceal bleeding is very high, or the patient is expected to survive at least 3 to 5 years.18
A heart rate of 55 to 60? An alternative to HVPG measurement to monitor the effectiveness of beta-blocker therapy is to follow the heart rate. A 25% reduction from baseline or a heart rate of 55 to 60 beats per minute is the standard goal19,20; yet, at least 40% of patients treated with enough propranolol to decrease the heart rate by 25% do not respond with significant HVPG reductions.14,21
So, although beta-blockade is effective peripherally, it may not reduce HVPG to less than 12 mm Hg or 20% from baseline, and direct HVPG measurement is still the gold standard.
Treatment should be lifelong
Once a patient is started on a beta-blocker to prevent variceal hemorrhage, the treatment should be lifelong.
In 2001, a group of patients (most of them in Child-Pugh class A or B) completing a prospective randomized controlled trial of propranolol for primary prevention of variceal hemorrhage were tapered off propranolol or placebo.22 Of the 49 patients, 9 experienced variceal hemorrhage (6 of 25 former propranolol recipients and 3 of 24 former placebo recipients), and 17 patients died (12 former propranolol and 5 former placebo recipients), suggesting that treatment should be maintained for life.
Therefore, when beta-blocker therapy is discontinued, the risk of variceal hemorrhage returns to what would be expected in an untreated population.
Beta-blockers may not prevent varices
Although many trials have shown that beta-blockers are effective as prophylaxis against a first variceal hemorrhage, there is no evidence that these drugs prevent varices from forming in cirrhotic patients.
Groszmann et al23 treated more than 200 patients who had biopsy-proven cirrhosis and portal hypertension (HVPG > 6 mm Hg) but no varices with timolol (Blocadren), a nonselective beta-blocker, or placebo. At a median follow-up of about 55 months, the groups did not differ significantly in the incidence of primary events (development of varices or variceal hemorrhage) or treatment failures (transplantation or death). Varices developed less frequently among patients with a baseline HVPG of less than 10 mm Hg and among those whose HVPG had decreased by more than 10% at 1 year. In patients whose HVPG increased by more than 10%, varices developed more frequently.
Contraindications, side effects
The major drawbacks to therapy with beta-blockers are their contraindications and side effects.
Contraindications include chronic obstructive lung disease, psychosis, atrioventricular heart blocks, and aortic-valve disease.
Side effects are reported in 15% of patients but severe events are rare.24 Still, an estimated 10% to 20% of patients discontinue treatment because they cannot tolerate it.25 The more common complaints include fatigue, shortness of breath, sexual dysfunction, and sleep disorders.
Dosage
No specific starting dose of beta-blockers is agreed upon, but nadolol 20 to 40 mg once daily or long-acting propranolol 60 mg once daily can be used as initial therapy.25 Once-daily dosing increases the likelihood of compliance.
Since portal pressure progressively declines from 12 noon to 7 PM and then increases throughout the night and back to baseline by 9 AM,26 we recommend that the medication be taken in the evening to counteract increases in portal pressure that occur in the middle of the night.
ENDOSCOPIC VARICEAL LIGATION
Endoscopic variceal ligation has been investigated extensively for use as prophylaxis against first variceal hemorrhage. The procedure involves placing a rubber band around a varix aspirated into a cylinder on the tip of an endoscope.
Effectiveness of ligation
Lay et al,27 in a prospective, randomized trial in 126 cirrhotic patients endoscopically judged to be at high risk of hemorrhage, found that ligation significantly reduced the 2-year cumulative bleeding rate (19% with ligation vs 60% in an untreated control group) and the overall mortality rate (28% vs 58%). The lower risk of bleeding in the ligation group was attributed to a rapid reduction of variceal size; 60% of those in the ligation group had complete eradication of varices and 38% had varices reduced in size.
Imperiale and Chalasani28 performed a meta-analysis in 2001 that included 601 patients in five trials comparing prophylactic ligation with untreated controls and 283 patients in four trials comparing ligation with beta-blocker therapy. Compared with no treatment, ligation reduced the risk of first variceal hemorrhage, bleeding-related death, and death from any cause. Compared with propranolol, ligation reduced the risk of first-time bleeding but had no effect on the death rate.
Schepke et al,29 in a randomized controlled multicenter trial in 152 cirrhotic patients with two or more esophageal varices, found that neither bleeding incidence nor death rate differed significantly between ligation and propranolol.
Lui et al30 followed 172 cirrhotic patients with grade II or III esophageal varices for 6 years and found that ligation was equivalent to propranolol. However, many patients reported side effects with propranolol, and 30% of patients withdrew from propranolol treatment, making ligation a more attractive option.
Khuroo et al31 performed a meta-analysis of eight randomized controlled trials including 596 patients and found that ligation significantly reduced the rates of first gastrointestinal hemorrhage by 31% and of first variceal hemorrhage by 43%. In subgroup analysis, ligation had a significant advantage compared with beta-blockers in trials with patients with a high bleeding risk, ie, trials in which more than 30% of patients were in Child-Pugh class C and more than 50% of the patients had large varices.
Jutabha et al32 performed a multicenter, prospective trial (published in 2005) in 62 patients with high-risk esophageal varices randomized to propranolol or banding. The trial was ended early after an interim analysis showed that the failure rate of propranolol was significantly higher than that of banding (6/31 vs 0/31, P = .0098). Esophageal variceal hemorrhage occurred in 4 (12.9%) of the patients in the propranolol group compared with 0 in the ligation group. Similarly, 4 patients in the propranolol group died, compared with 0 in the ligation group. All the patients in this trial were liver transplant candidates and therefore all had severe liver disease.
In another trial favoring variceal banding over beta-blockers, Psilopoulos et al33 in 2005 followed 60 patients with cirrhosis and esophageal varices with no history of bleeding. Thirty percent of the patients in the propranolol group developed variceal bleeding compared with 6.7% in the ligation group (P = .043).
Lay et al34 followed 100 cirrhotic patients for 2 years and found comparable cumulative bleeding rates with ligation vs propranolol (18% vs 16%, respectively) and also comparable rates of death (28% vs 24%, respectively).
Sarin et al35 investigated the role of propranolol in addition to ligation in the prevention of first hemorrhage in 144 patients. Adding propranolol did not further decrease the incidence of initial bleeding (7% in the combination group vs 11% in the ligation-only group). Survival rates were similar at 20 months: 92% in the combination group vs 85% in the ligation-only group. However, the rate of variceal recurrence was lower with combination therapy: 6% in the combination group vs 15% with ligation alone.
Does esophageal variceal ligation increase gastric varices?
A less researched topic is whether variceal ligation results in gastric hemodynamic changes that increase the size of fundal varices and worsen portal hypertensive gastropathy.
Yuksel et al36 found that 37 of 85 patients had fundal varices before they underwent ligation of esophageal varices, increasing to 46 after the procedure, a statistically significant increment. The severity of portal hypertensive gastropathy also increased.
Further research is required regarding the long-term consequences of these findings.
ANGIOTENSIN II RECEPTOR ANTAGONISTS: ROLE UNKNOWN
Angiotensin II increases portal pressure, and angiotensin II levels are elevated in patients with cirrhosis, suggesting that this hormone plays a role in the pathogenesis of portal hypertension.
Losartan (Cozaar), an angiotensin II receptor antagonist, was found to decrease the HVPG significantly in patients with severe and moderate portal hypertension in a pilot study37 in 1999. However, in two subsequent studies,38,39 losartan only moderately reduced the HVPG and caused hypotension and a reduction in the glomerular filtration rate. The role of angiotensin II receptor blockers in primary prevention of variceal bleeding is still unknown.
SURGICAL PORTAL DECOMPRESSION HAS BEEN ABANDONED
The first method investigated to prevent variceal bleeding was surgical portal decompression.
A meta-analysis of four randomized controlled trials in 302 patients with varices of all sizes compared portocaval shunt surgery and medical therapy.11 Although shunt surgery was very effective in preventing variceal bleeding, the risk of chronic or recurrent encephalopathy was significantly increased (odds ratio 2.0), as was the risk of death (odds ratio 1.6).
These poor results, combined with advances in endoscopic procedures, led to the abandonment of surgical shunting for primary prophylaxis.
TIPS PROCEDURE: NO ROLE AT PRESENT
The transjugular intrahepatic portosystemic shunt (TIPS) procedure is used to treat the main consequences of portal hypertension, including ascites and variceal hemorrhage. The procedure entails accessing the hepatic vein via the right jugular vein and placing a stent to the portal vein, forming a low-resistance channel and allowing blood to return to the systemic circulation.
TIPS placement increases the risk of encephalopathy; liver failure is a rare complication, and procedural complications (ie, shunt dysfunction) also occur. Trials comparing the TIPS procedure with other forms of therapy to prevent first variceal hemorrhages have not been performed.40 Research to improve the outcome of the TIPS procedure is ongoing, but currently this procedure has no role in primary prevention of variceal bleeding.
ENDOSCOPIC SCLEROTHERAPY MAY INCREASE THE RISK OF DEATH
Numerous clinical trials evaluated sclerotherapy as prophylaxis against a first esophageal variceal hemorrhage. The procedure involves injecting a sclerosant in and around varices.
In a large Veterans Administration study,41 sclerotherapy was compared with sham treatment in 281 men with alcoholic liver disease who had documented varices but no history of bleeding. The trial was terminated after 22.5 months because the rate of all-cause mortality was significantly higher in the sclerotherapy group (32.5%) than in the sham therapy group (17.4%). The higher death rate did not persist after the treatment was discontinued, and it was speculated that, although sclerotherapy had reduced new episodes of variceal hemorrhage, the procedure might have caused bleeding from esophageal ulcers, leading to an increased mortality rate in that group.
The PROVA Study Group from Norway and Denmark found similar results when 286 cirrhotic patients were randomized to receive sclerotherapy, propranolol, combination sclerotherapy and propranolol, or no treatment to prevent a first variceal hemorrhage.42 The incidence of variceal bleeding was almost identical in the four groups, but the mortality rate with variceal bleeding was 2.75 times higher in the sclerotherapy groups than in the other groups (P = .002). It was speculated that repeated sclerotherapy sessions might be poorly tolerated by patients in Child-Pugh classes B and C and might have contributed to the precipitation of liver failure and other common complications of cirrhosis.
A meta-analysis by D’Amico et al11 evaluated 19 trials (1,630 patients) comparing sclerotherapy with nonactive treatment. Sclerotherapy tended to be favorable in trials with a high bleeding rate in the control patients and unfavorable in trials with a low bleeding rate. The benefit seen in patients at high risk is consistent with the efficacy of sclerotherapy for preventing rebleeding, whereas the harmful effect in the low-risk patients points towards side effects and complications exceeding the potential benefits.
In general, currently available evidence suggests that the benefits of prophylactic sclerotherapy are marginal, and therefore sclerotherapy is not recommended as primary prophylaxis for variceal hemorrhage.
NITRATES: NO LONGER USED AS MONOTHERAPY
Unlike vasoconstrictors, which decrease portal pressure by decreasing blood flow, vasodilators reduce hepatic pressure by decreasing intrahepatic and portocollateral vascular resistance.43 In addition, larger doses directly affect the arterial circulation, lowering systemic and therefore splanchnic perfusion pressure.44 Unfortunately, the systemic vasodilatory effects of nitrates exacerbate the hyperdynamic state that is characteristic of cirrhosis, thereby limiting their use and tolerability in many patients.
A trial comparing propranolol vs isosorbide mononitrate initially found that the groups did not differ significantly with regard to bleeding rates and 2-year survival rates,45 but a 6-year follow-up found the likelihood of death greater in patients older than 50 years in the nitrate group.46 In an additional study comparing isosorbide mononitrate vs placebo in patients with contraindications to or intolerance of beta-blockers, no difference in the relative risk of first variceal hemorrhage was found between the two groups.47 Therefore, nitrates are no longer used as monotherapy to prevent variceal bleeding.
Combination therapy with beta-blockers plus nitrates is controversial. In a trial in 1996, Merkel et al48 found the cumulative risk of variceal bleeding was 18% at 40 months with nadolol alone vs 7.5% with nadolol plus isosorbide mononitrate. However, in a later trial, Garcia-Pagán et al49 found no significant advantage to combination therapy. The incidence of variceal bleeding at 1 year was 8.3% in the group receiving propranolol plus placebo and 5% in the group receiving propranolol plus isosorbide mononitrate; at 2 years, the rates were 10.6% vs 12.5%.
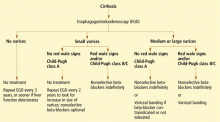
RECOMMENDATIONS FOR SCREENING AND PROPHYLAXIS
- All patients with cirrhosis should be screened for varices at the time of diagnosis.
- The size of the varices, including small (≤ 5 mm) and large (> 5 mm), and the presence of red wale marks on the varices should be recorded.
- Patients who have no varices on screening endoscopy should be rescreened every 3 years if their liver function is stable or every year if their liver function deteriorates. (Varices grow at a rate proportional to the severity of the liver disease.)
- Patients with portal hypertension but without varices do not need treatment with nonselective beta-blockers. Endoscopy should be performed at the intervals suggested above.
- Those who are found to have small varices on screening endoscopy but who have well-compensated liver disease (Child-Pugh class A) and no red wale marks should be rescreened every other year because the development of large varices is greater in patients with small varices on initial endoscopy than in patients with no varices. Emerging data support the use of beta-blockers to prevent varices from increasing in size.
- Patients who have small varices with red wale signs or who are in Child-Pugh class B or C have an increased risk of bleeding and should be treated with beta-blockers. If beta-blockers are not used, endoscopy should be done every year to look for an increase in variceal size.
- Patients who have large varices without red wale signs or who are in Child-Pugh class B or C should be treated with nonselective beta-blockers. The dose should be adjusted to achieve maximal tolerable decrease in heart rate to a minimum of 55 beats per minute, and treatment should be continued indefinitely.
- Endoscopic variceal ligation is an acceptable alternative to beta-blocker treatment as first-line therapy in those who cannot tolerate beta-blockers or who have contraindications to their use, or in those who have red wale marking or who are in Child-Pugh class B or C.
Variceal hemorrhage is a medical emergency in which up to 20% of patients die.1 Even if the patient survives an initial episode of variceal bleeding, the probability of another episode is high: the rebleeding rate without treatment is 70% within 1 year. The mortality rate with rebleeding is 33%.
With such overwhelming consequences, the best strategy in any patient with cirrhosis and known varices is to try to prevent the first episode of bleeding.
WHO IS AT RISK?
Esophageal varices are present in 30% of patients with compensated cirrhosis and in up to 60% of those with decompensated cirrhosis (ie, with evidence of ascites or encephalopathy).2
The risk of variceal hemorrhage is related to three factors:
- The size of the varices. Varices 5 mm in diameter or smaller have a 7% risk of bleeding in 2 years, while those larger than 5 mm have a 30% risk of bleeding within 2 years.3
- The appearance of the varices. Morphologic features of varices, including red wale signs (red streaks of the mucosa overlying the varix), have been correlated with an increased risk of hemorrhage.
- The severity of liver dysfunction, as assessed by the Child-Pugh classification—an index of liver dysfunction based on serum albumin concentration, bilirubin level, prothrombin time, and the presence of ascites and encephalopathy. A high Child-Pugh score (ie, class B or C), representing decompensated cirrhosis, is associated with an increased risk of bleeding.
HOW VARICES DEVELOP: PORTAL HYPERTENSION
Esophageal varices form as a result of increased portal pressure, the product of increased portal venous inflow and resistance to outflow from the portal venous system. Portal hypertension is a major complication of chronic liver disease. In cirrhosis, architectural distortion of the liver causes an increase in the intrahepatic vascular resistance.
Portal venous inflow depends on mesenteric arteriolar tone, increasing when tone decreases. In cirrhotic patients, the increase in portal pressure results from a combination of increased portal blood flow secondary to splanchnic arteriolar vasodilation and elevated resistance to outflow through distorted hepatic sinusoids.
The potent vasodilator nitric oxide (NO) plays an important role in portal hypertension. In patients with cirrhosis, NO bioavailability is decreased in the intrahepatic circulation due to defects in the posttranslational regulation of endothelial NO synthase.4 This deficiency of NO, along with mechanical factors in the sinusoids, contributes to the increase in intrahepatic resistance. In the systemic and splanchnic circulation, NO bioavailability is increased due to upregulation and posttranslational regulation of endothelial NO synthase, thereby increasing splanchnic vasodilatation and leading to increased portal venous inflow.5 This results in a marked increase in cardiac output and so-called hyperdynamic circulation.
Portal hypertension results in the development of collateral circulation, including venous channels in the esophagus and stomach, by the dilation of preexisting vessels and active angiogenesis. Esophagogastric varices increase in size with the severity of portal hypertension and can rupture when the tension in their walls exceeds a maximal point.
HEPATIC VEIN PRESSURE GRADIENT: A PROXY FOR PORTAL PRESSURE
Ideally, the portal venous pressure should be directly measured. However, since direct measurement is invasive and impractical, the hepatic vein pressure gradient (HVPG) can be measured instead and correlates well with the portal pressure.6
The normal HVPG is 5 mm Hg or less; anything above this value denotes portal hypertension. However, studies have shown that varices may develop but do not bleed if the HVPG is less than 12 mm Hg.8
TWO WAYS TO PREVENT BLEEDING
Bleeding can be prevented either by reducing the portal venous pressure or by obliterating the varices. Portal pressure can be reduced by placing a portosystemic shunt either surgically or percutaneously with radiographic guidance or by giving drugs such as nonselective beta-blockers, nitrates, or a combination of these drugs. Variceal obliteration is typically done by endoscopic methods with either injection of a sclerosant or band ligation.
NONSELECTIVE BETA-BLOCKERS: THE MAINSTAY OF TREATMENT
Nonselective beta-blockers, the most commonly used drugs for preventing first esophageal variceal bleeding, decrease portal pressure by blocking both beta-1 and beta-2 adrenergic receptors.9 Beta-1 blockade decreases portal flow by decreasing the heart rate and cardiac output, while blockade of beta-2 receptors results in unopposed alpha-adrenergic-mediated vasoconstriction.
Selective beta-blockers do not appear to be as useful for primary prophylaxis. More than 2 decades ago, metoprolol (Toprol, Lopressor), a beta-1 selective antagonist, was compared with propranolol (Inderal), a nons-elective agent, in patients with cirrhosis and portal hypertension.10 Although both drugs significantly reduced the heart rate and cardiac output, only those taking propranolol showed a marked fall in portal pressure (mean decrease of 6.8 mm Hg vs 3.8 mm Hg with metoprolol) and a significant reduction in hepatic blood flow. The differences were thought to be related to beta-2 blockade of vasodilator receptors in the splanchnic circulation, which occurs only with nonselective beta-blockers such as propranolol.
The two nonselective beta-blockers most often used to prevent variceal bleeding are nadolol (Corgard) and propranolol. Both have been extensively studied in preventing a first variceal hemorrhage.
Effectiveness of beta-blockers
D’Amico et al11 performed a meta-analysis in 1995, examining nine trials (996 patients total) of the effectiveness of beta-blockers in preventing a first variceal hemorrhage. Seven trials found that bleeding risk was reduced with beta-blockers (significantly in four), one trial found that risk was unchanged, and one trial found that risk was increased—an outlier due to a small sample size. The meta-analysis showed a significant bleeding reduction with the use of a beta-blocker, either including the outlier trial (pooled odds ratio 0.54; 95% confidence interval 0.39–0.74) or excluding it (pooled odds ratio 0.48; 95% confidence interval 0.35–0.66).
Mortality rates were also reduced in seven trials, but the reduction was statistically significant in only one. However, in the pooled estimate, the mortality risk reduction approached statistical significance (pooled odds ratio 0.75; 95% confidence interval 0.57–1.06).
Ideo et al12 gave either nadolol or placebo to 79 patients with cirrhosis and large esophageal varices that had never bled. Nadolol was found to protect against a first variceal hemorrhage: at 2-year follow-up, only 1 of the 30 patients allocated to nadolol had had bleeding, vs 11 of the 49 patients in the placebo group.
Merkel et al13 found that the risk of variceal bleeding was lower in patients who started treatment with beta-blockers when their varices were small (12% at 5 years) than in those who started treatment after a diagnosis of large esophageal varices (22% at 5 years). They concluded that nadolol helps prevent small varices from growing into larger ones.
Response to beta-blockers is not uniform
Although beta-blockers decrease the portal pressure in many cirrhotic patients, the response is not uniform. In a study of 60 cirrhotic patients,14 40% showed no reduction or even a slight increase in HVPG with propranolol. Most patients showed a significant reduction in heart rate (17.5% ± 10%) after receiving 40 mg of propranolol. In the patients whose HVPG did not decrease by at least 10% with 40 mg of propranolol, increasing the dose caused a decrease in HVPG without a further decrease in heart rate. This suggests that 40 mg of propranolol successfully produced beta-1 blockade but that a higher dose was required for effective beta-2 blockade.
Failure to respond in certain patients may be due to a concurrent rise in collateral or hepatic sinusoidal resistance, or both. This was confirmed in a study in portal-hypertensive rats treated with propranolol.15 The reduction in portal blood flow expected was accompanied by a disproportionately small reduction in portal pressure, which was thought to be due to a rise in portal and collateral vascular resistance.
How to tell if beta-blocker treatment is ‘working’
An HVPG ≤ 12 mm Hg? Studies have shown that the most important predictor of efficacy of prophylaxis for variceal bleeding is a decrease in the HVPG to 12 mm Hg or less or a decrease in the initial HVPG of more than 20%.9 Although measuring the HVPG is invasive, expensive, and not routinely done in clinical practice, several studies have investigated the role of measuring hemodynamic response to medication.
Merkel et al16 measured the HVPG in 49 cirrhotic patients with previously nonbleeding varices before starting therapy with beta-blockers with or without nitrates and after 1 to 3 months of treatment. They followed the patients for up to 5 years. The mean HVPG value at baseline was 18.8 mm Hg. At 3 years of follow-up, 7% of those who had responded well to therapy (defined as achieving an HVPG less than 13 mm Hg or a decrease of more than 20%) had experienced a bleeding episode, which was significantly less than the rate (41%) in those who did not meet those hemodynamic end points. No patient reaching an HVPG of 12 mm Hg or less during treatment had variceal bleeding during follow-up.
Groszmann et al17 also prospectively measured the HVPG in patients with cirrhosis and varices, but their patients received either propranolol or placebo. Variceal hemorrhage occurred in 13 patients (11 of 51 in the placebo group and 2 of 51 in the propranolol group), all of whom had an HVPG greater than 12 mm Hg. Again, none of the patients whose HVPG was decreased to 12 mm Hg or less bled from esophageal varices.
Unfortunately, routine HVPG measurement to guide primary prophylaxis is an expensive strategy. Data suggest that measuring the HVPG is cost-effective only when the cost of measuring the HVPG is very low, the risk of variceal bleeding is very high, or the patient is expected to survive at least 3 to 5 years.18
A heart rate of 55 to 60? An alternative to HVPG measurement to monitor the effectiveness of beta-blocker therapy is to follow the heart rate. A 25% reduction from baseline or a heart rate of 55 to 60 beats per minute is the standard goal19,20; yet, at least 40% of patients treated with enough propranolol to decrease the heart rate by 25% do not respond with significant HVPG reductions.14,21
So, although beta-blockade is effective peripherally, it may not reduce HVPG to less than 12 mm Hg or 20% from baseline, and direct HVPG measurement is still the gold standard.
Treatment should be lifelong
Once a patient is started on a beta-blocker to prevent variceal hemorrhage, the treatment should be lifelong.
In 2001, a group of patients (most of them in Child-Pugh class A or B) completing a prospective randomized controlled trial of propranolol for primary prevention of variceal hemorrhage were tapered off propranolol or placebo.22 Of the 49 patients, 9 experienced variceal hemorrhage (6 of 25 former propranolol recipients and 3 of 24 former placebo recipients), and 17 patients died (12 former propranolol and 5 former placebo recipients), suggesting that treatment should be maintained for life.
Therefore, when beta-blocker therapy is discontinued, the risk of variceal hemorrhage returns to what would be expected in an untreated population.
Beta-blockers may not prevent varices
Although many trials have shown that beta-blockers are effective as prophylaxis against a first variceal hemorrhage, there is no evidence that these drugs prevent varices from forming in cirrhotic patients.
Groszmann et al23 treated more than 200 patients who had biopsy-proven cirrhosis and portal hypertension (HVPG > 6 mm Hg) but no varices with timolol (Blocadren), a nonselective beta-blocker, or placebo. At a median follow-up of about 55 months, the groups did not differ significantly in the incidence of primary events (development of varices or variceal hemorrhage) or treatment failures (transplantation or death). Varices developed less frequently among patients with a baseline HVPG of less than 10 mm Hg and among those whose HVPG had decreased by more than 10% at 1 year. In patients whose HVPG increased by more than 10%, varices developed more frequently.
Contraindications, side effects
The major drawbacks to therapy with beta-blockers are their contraindications and side effects.
Contraindications include chronic obstructive lung disease, psychosis, atrioventricular heart blocks, and aortic-valve disease.
Side effects are reported in 15% of patients but severe events are rare.24 Still, an estimated 10% to 20% of patients discontinue treatment because they cannot tolerate it.25 The more common complaints include fatigue, shortness of breath, sexual dysfunction, and sleep disorders.
Dosage
No specific starting dose of beta-blockers is agreed upon, but nadolol 20 to 40 mg once daily or long-acting propranolol 60 mg once daily can be used as initial therapy.25 Once-daily dosing increases the likelihood of compliance.
Since portal pressure progressively declines from 12 noon to 7 PM and then increases throughout the night and back to baseline by 9 AM,26 we recommend that the medication be taken in the evening to counteract increases in portal pressure that occur in the middle of the night.
ENDOSCOPIC VARICEAL LIGATION
Endoscopic variceal ligation has been investigated extensively for use as prophylaxis against first variceal hemorrhage. The procedure involves placing a rubber band around a varix aspirated into a cylinder on the tip of an endoscope.
Effectiveness of ligation
Lay et al,27 in a prospective, randomized trial in 126 cirrhotic patients endoscopically judged to be at high risk of hemorrhage, found that ligation significantly reduced the 2-year cumulative bleeding rate (19% with ligation vs 60% in an untreated control group) and the overall mortality rate (28% vs 58%). The lower risk of bleeding in the ligation group was attributed to a rapid reduction of variceal size; 60% of those in the ligation group had complete eradication of varices and 38% had varices reduced in size.
Imperiale and Chalasani28 performed a meta-analysis in 2001 that included 601 patients in five trials comparing prophylactic ligation with untreated controls and 283 patients in four trials comparing ligation with beta-blocker therapy. Compared with no treatment, ligation reduced the risk of first variceal hemorrhage, bleeding-related death, and death from any cause. Compared with propranolol, ligation reduced the risk of first-time bleeding but had no effect on the death rate.
Schepke et al,29 in a randomized controlled multicenter trial in 152 cirrhotic patients with two or more esophageal varices, found that neither bleeding incidence nor death rate differed significantly between ligation and propranolol.
Lui et al30 followed 172 cirrhotic patients with grade II or III esophageal varices for 6 years and found that ligation was equivalent to propranolol. However, many patients reported side effects with propranolol, and 30% of patients withdrew from propranolol treatment, making ligation a more attractive option.
Khuroo et al31 performed a meta-analysis of eight randomized controlled trials including 596 patients and found that ligation significantly reduced the rates of first gastrointestinal hemorrhage by 31% and of first variceal hemorrhage by 43%. In subgroup analysis, ligation had a significant advantage compared with beta-blockers in trials with patients with a high bleeding risk, ie, trials in which more than 30% of patients were in Child-Pugh class C and more than 50% of the patients had large varices.
Jutabha et al32 performed a multicenter, prospective trial (published in 2005) in 62 patients with high-risk esophageal varices randomized to propranolol or banding. The trial was ended early after an interim analysis showed that the failure rate of propranolol was significantly higher than that of banding (6/31 vs 0/31, P = .0098). Esophageal variceal hemorrhage occurred in 4 (12.9%) of the patients in the propranolol group compared with 0 in the ligation group. Similarly, 4 patients in the propranolol group died, compared with 0 in the ligation group. All the patients in this trial were liver transplant candidates and therefore all had severe liver disease.
In another trial favoring variceal banding over beta-blockers, Psilopoulos et al33 in 2005 followed 60 patients with cirrhosis and esophageal varices with no history of bleeding. Thirty percent of the patients in the propranolol group developed variceal bleeding compared with 6.7% in the ligation group (P = .043).
Lay et al34 followed 100 cirrhotic patients for 2 years and found comparable cumulative bleeding rates with ligation vs propranolol (18% vs 16%, respectively) and also comparable rates of death (28% vs 24%, respectively).
Sarin et al35 investigated the role of propranolol in addition to ligation in the prevention of first hemorrhage in 144 patients. Adding propranolol did not further decrease the incidence of initial bleeding (7% in the combination group vs 11% in the ligation-only group). Survival rates were similar at 20 months: 92% in the combination group vs 85% in the ligation-only group. However, the rate of variceal recurrence was lower with combination therapy: 6% in the combination group vs 15% with ligation alone.
Does esophageal variceal ligation increase gastric varices?
A less researched topic is whether variceal ligation results in gastric hemodynamic changes that increase the size of fundal varices and worsen portal hypertensive gastropathy.
Yuksel et al36 found that 37 of 85 patients had fundal varices before they underwent ligation of esophageal varices, increasing to 46 after the procedure, a statistically significant increment. The severity of portal hypertensive gastropathy also increased.
Further research is required regarding the long-term consequences of these findings.
ANGIOTENSIN II RECEPTOR ANTAGONISTS: ROLE UNKNOWN
Angiotensin II increases portal pressure, and angiotensin II levels are elevated in patients with cirrhosis, suggesting that this hormone plays a role in the pathogenesis of portal hypertension.
Losartan (Cozaar), an angiotensin II receptor antagonist, was found to decrease the HVPG significantly in patients with severe and moderate portal hypertension in a pilot study37 in 1999. However, in two subsequent studies,38,39 losartan only moderately reduced the HVPG and caused hypotension and a reduction in the glomerular filtration rate. The role of angiotensin II receptor blockers in primary prevention of variceal bleeding is still unknown.
SURGICAL PORTAL DECOMPRESSION HAS BEEN ABANDONED
The first method investigated to prevent variceal bleeding was surgical portal decompression.
A meta-analysis of four randomized controlled trials in 302 patients with varices of all sizes compared portocaval shunt surgery and medical therapy.11 Although shunt surgery was very effective in preventing variceal bleeding, the risk of chronic or recurrent encephalopathy was significantly increased (odds ratio 2.0), as was the risk of death (odds ratio 1.6).
These poor results, combined with advances in endoscopic procedures, led to the abandonment of surgical shunting for primary prophylaxis.
TIPS PROCEDURE: NO ROLE AT PRESENT
The transjugular intrahepatic portosystemic shunt (TIPS) procedure is used to treat the main consequences of portal hypertension, including ascites and variceal hemorrhage. The procedure entails accessing the hepatic vein via the right jugular vein and placing a stent to the portal vein, forming a low-resistance channel and allowing blood to return to the systemic circulation.
TIPS placement increases the risk of encephalopathy; liver failure is a rare complication, and procedural complications (ie, shunt dysfunction) also occur. Trials comparing the TIPS procedure with other forms of therapy to prevent first variceal hemorrhages have not been performed.40 Research to improve the outcome of the TIPS procedure is ongoing, but currently this procedure has no role in primary prevention of variceal bleeding.
ENDOSCOPIC SCLEROTHERAPY MAY INCREASE THE RISK OF DEATH
Numerous clinical trials evaluated sclerotherapy as prophylaxis against a first esophageal variceal hemorrhage. The procedure involves injecting a sclerosant in and around varices.
In a large Veterans Administration study,41 sclerotherapy was compared with sham treatment in 281 men with alcoholic liver disease who had documented varices but no history of bleeding. The trial was terminated after 22.5 months because the rate of all-cause mortality was significantly higher in the sclerotherapy group (32.5%) than in the sham therapy group (17.4%). The higher death rate did not persist after the treatment was discontinued, and it was speculated that, although sclerotherapy had reduced new episodes of variceal hemorrhage, the procedure might have caused bleeding from esophageal ulcers, leading to an increased mortality rate in that group.
The PROVA Study Group from Norway and Denmark found similar results when 286 cirrhotic patients were randomized to receive sclerotherapy, propranolol, combination sclerotherapy and propranolol, or no treatment to prevent a first variceal hemorrhage.42 The incidence of variceal bleeding was almost identical in the four groups, but the mortality rate with variceal bleeding was 2.75 times higher in the sclerotherapy groups than in the other groups (P = .002). It was speculated that repeated sclerotherapy sessions might be poorly tolerated by patients in Child-Pugh classes B and C and might have contributed to the precipitation of liver failure and other common complications of cirrhosis.
A meta-analysis by D’Amico et al11 evaluated 19 trials (1,630 patients) comparing sclerotherapy with nonactive treatment. Sclerotherapy tended to be favorable in trials with a high bleeding rate in the control patients and unfavorable in trials with a low bleeding rate. The benefit seen in patients at high risk is consistent with the efficacy of sclerotherapy for preventing rebleeding, whereas the harmful effect in the low-risk patients points towards side effects and complications exceeding the potential benefits.
In general, currently available evidence suggests that the benefits of prophylactic sclerotherapy are marginal, and therefore sclerotherapy is not recommended as primary prophylaxis for variceal hemorrhage.
NITRATES: NO LONGER USED AS MONOTHERAPY
Unlike vasoconstrictors, which decrease portal pressure by decreasing blood flow, vasodilators reduce hepatic pressure by decreasing intrahepatic and portocollateral vascular resistance.43 In addition, larger doses directly affect the arterial circulation, lowering systemic and therefore splanchnic perfusion pressure.44 Unfortunately, the systemic vasodilatory effects of nitrates exacerbate the hyperdynamic state that is characteristic of cirrhosis, thereby limiting their use and tolerability in many patients.
A trial comparing propranolol vs isosorbide mononitrate initially found that the groups did not differ significantly with regard to bleeding rates and 2-year survival rates,45 but a 6-year follow-up found the likelihood of death greater in patients older than 50 years in the nitrate group.46 In an additional study comparing isosorbide mononitrate vs placebo in patients with contraindications to or intolerance of beta-blockers, no difference in the relative risk of first variceal hemorrhage was found between the two groups.47 Therefore, nitrates are no longer used as monotherapy to prevent variceal bleeding.
Combination therapy with beta-blockers plus nitrates is controversial. In a trial in 1996, Merkel et al48 found the cumulative risk of variceal bleeding was 18% at 40 months with nadolol alone vs 7.5% with nadolol plus isosorbide mononitrate. However, in a later trial, Garcia-Pagán et al49 found no significant advantage to combination therapy. The incidence of variceal bleeding at 1 year was 8.3% in the group receiving propranolol plus placebo and 5% in the group receiving propranolol plus isosorbide mononitrate; at 2 years, the rates were 10.6% vs 12.5%.
RECOMMENDATIONS FOR SCREENING AND PROPHYLAXIS
- All patients with cirrhosis should be screened for varices at the time of diagnosis.
- The size of the varices, including small (≤ 5 mm) and large (> 5 mm), and the presence of red wale marks on the varices should be recorded.
- Patients who have no varices on screening endoscopy should be rescreened every 3 years if their liver function is stable or every year if their liver function deteriorates. (Varices grow at a rate proportional to the severity of the liver disease.)
- Patients with portal hypertension but without varices do not need treatment with nonselective beta-blockers. Endoscopy should be performed at the intervals suggested above.
- Those who are found to have small varices on screening endoscopy but who have well-compensated liver disease (Child-Pugh class A) and no red wale marks should be rescreened every other year because the development of large varices is greater in patients with small varices on initial endoscopy than in patients with no varices. Emerging data support the use of beta-blockers to prevent varices from increasing in size.
- Patients who have small varices with red wale signs or who are in Child-Pugh class B or C have an increased risk of bleeding and should be treated with beta-blockers. If beta-blockers are not used, endoscopy should be done every year to look for an increase in variceal size.
- Patients who have large varices without red wale signs or who are in Child-Pugh class B or C should be treated with nonselective beta-blockers. The dose should be adjusted to achieve maximal tolerable decrease in heart rate to a minimum of 55 beats per minute, and treatment should be continued indefinitely.
- Endoscopic variceal ligation is an acceptable alternative to beta-blocker treatment as first-line therapy in those who cannot tolerate beta-blockers or who have contraindications to their use, or in those who have red wale marking or who are in Child-Pugh class B or C.
- D’Amico G, De Franchis R Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003; 38:599–612.
- D’Amico G, Luca A. Natural history. Clinicalhaemodynamic correlations. Prediction of the risk of bleeding. Baillieres Clin Gastroenterol 1997; 11:243–256.
- Kamath PS. Esophageal variceal bleeding: primary prophylaxis. Clin Gastroenterol Hepatol 2005; 3:90–93.
- Shah V, Toruner M, Haddad F, et al. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology 1999; 117:1222–1228.
- Morales-Ruiz M, Jimenez W, Perez-Sala D, et al. Increased nitric oxide synthase expression in arterial vessels of cirrhotic rats with ascites. Hepatology 1996; 24:1481–1486.
- De Franchis R, Dell’Era A, Iannuzzi F. Diagnosis and treatment of portal hypertension. Dig Liver Dis 2004; 36:787–798.
- Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology 2004; 39:280–282.
- Garcia-Tsao G, Groszmann RJ, Fisher RL, Conn HO, Atterbury CE, Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology 1985; 5:419–424.
- Bosch J, Masti R, Kravetz D, et al. Effects of propranolol on azygos venous blood flow and hepatic and systemic hemodynamics in cirrhosis. Hepatology 1984; 4:1200–1205.
- Westaby D, Bihari DJ, Gimson AE, Crossley IR, Williams R. Selective and non-selective beta receptor blockade in the reduction of portal pressure in patients with cirrhosis and portal hypertension. Gut 1984; 25:121–124.
- D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology 1995; 22:332–354.
- Ideo G, Bellati G, Fesce E, Grimoldi D. Nadolol can prevent the first gastrointestinal bleeding in cirrhotics: a prospective, randomized study. Hepatology 1988; 8:6–9.
- Merkel C, Marin R, Angeli P, et al. Gruppo Triveneto per l’Ipertensione Portale. A placebo-controlled clinical trial of nadolol in the prophylaxis of growth of small esophageal varices in cirrhosis. Gastroenterology 2004; 127:476–484.
- Garcia-Tsao G, Grace ND, Groszmann RJ, et al. Short-term effects of propranolol on portal venous pressure. Hepatology 1986; 6:101–106.
- Kroeger RJ, Groszmann RJ. Increased portal venous resistance hinders portal pressure reduction during the administration of beta-adrenergic blocking agents in a portal hypertensive model. Hepatology 1985; 5:97–101.
- Merkel C, Bolognesi M, Sacerdoti D, et al. The hemodynamic response to medical treatment of portal hypertension as a predictor of clinical effectiveness in the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology 2000; 32:930–934.
- Groszmann RJ, Bosch J, Grace ND, et al. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology 1990; 99:1401–1407.
- Hicken BL, Sharara AI, Abrams GA, Eloubeidi M, Fallon MB, Arguedas MR. Hepatic venous pressure gradient measurements to assess response to primary prophylaxis in patients with cirrhosis: a decision analytical study. Aliment Pharmacol Ther 2003; 17:145–153.
- Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol 1997; 92:1081–1091.
- Grace ND, Groszmann RJ, Garcia-Tsao G, et al. Portal hypertension and variceal bleeding: an AASLD single topic symposium. Hepatology 1998; 28:868–880.
- Feu F, Garcia-Pagán JC, Bosch J, et al. Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal haemorrhage in patients with cirrhosis. Lancet 1995; 346:1056–1059.
- Abraczinskas DR, Ookubo R, Grace ND, et al. Propranolol for the prevention of first esophageal variceal hemorrhage: a lifetime commitment? Hepatology 2001; 34:1096–1102.
- Groszmann RJ, Garcia-Tsao G, Bosch J, et al Portal Hypertension Collaborative Group. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med 2005; 353:2254–2261.
- Garcia-Pagán JC, Bosch J. Pharmacological prevention of variceal bleeding. New developments. Baillier Clin Gastroenterol 1997; 11:271–287.
- Talwalkar JA, Kamath PS. An evidence-based medicine approach to beta-blocker therapy in patients with cirrhosis. Am J Med 2004; 116:759–766.
- Garcia-Pagan JC, Feu F, Castells A, et al. Circadian variations of portal pressure and variceal hemorrhage in patients with cirrhosis. Hepatology 1994; 19:595–601.
- Lay CS, Tsai YT, Teg CY, et al. Endoscopic variceal ligation in prophylaxis of first variceal bleeding in cirrhotic patients with high-risk esophageal varices. Hepatology 1997; 25:1346–1350.
- Imperiale TF, Chalasani N. A meta-analysis of endoscopic variceal ligation for primary prophylaxis of esophageal variceal bleeding. Hepatology 2001; 33:802–807.
- Schepke M, Kleber G, Nurnberg D, et al. Ligation versus propranolol for the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology 2004; 40:65–72.
- Lui HF, Stanley AJ, Forrest EH, et al. Primary prophylaxis of variceal hemorrhage: a randomized controlled trial comparing band ligation, propranolol, and isosorbide mononitrate. Gastroenterology 2002; 123:735–744.
- Khuroo MS, Khuroo NS, Farahat KL, Khuroo YS, Sofi AA, Dahab ST. Meta-analysis: endoscopic variceal ligation for primary prophylaxis of oesophageal variceal bleeding. Aliment Pharmacol Ther 2005; 21:347–361.
- Jutabha R, Jensen DM, Martin P, Savides T, Han SH, Gornbein J. Randomized study comparing banding and propranolol to prevent initial variceal hemorrhage in cirrhotics with high-risk esophageal varices. Gastroenterology 2005; 128:870–881.
- Psilopoulos D, Galanis P, Goulas S, et al. Endoscopic variceal ligation vs. propranolol for prevention of first variceal bleeding: a randomized controlled trial. Eur J Gastroenterol Hepatol 2005; 17:1111–1117.
- Lay CS, Tsai YT, Lee FY, et al. Endoscopic variceal ligation versus propranolol in prophylaxis of first variceal bleeding in patients with cirrhosis. J Gastroenterol Hepatol 2006; 21:413–419.
- Sarin SK, Wadhawan M, Agarwal SR, Tyagi P, Sharma BC. Endoscopic variceal ligation plus propranolol versus endoscopic variceal ligation alone in primary prophylaxis of variceal bleeding. Am J Gastroenterol 2005; 100:797–804.
- Yuksel O, Koklu S, Arhan M, Yolcu OF, et al. Effects of esophageal variceal eradication on portal hypertensive gastropathy and fundal varices: a retrospective and comparative study. Dig Dis Sci 2006; 51:27–30.
- Schneider AW, Kalk JF, Klein CP. Effect of losartan, an angiotensin II receptor antagonist, on portal pressure in cirrhosis. Hepatology 1999; 29:334–339.
- Schepke M, Werner E, Biecker E, et al. Hemodynamic effects of the angiotensin II receptor antagonist irbesartan in patients with cirrhosis and portal hypertension. Gastroenterology 2001; 121:389–395.
- Gonzalez-Abraldes J, Albillos A, Banares R, et al. Randomized comparison of long-term losartan versus propranolol in lowering portal pressure in cirrhosis. Gastroenterology 2001; 121:382–388.
- Boyer TD, Haskal ZJ American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology 2005; 41:386–400.
- Prophylactic sclerotherapy for esophageal varices in men with alcoholic liver disease. A randomized, single-blind, multicenter clinical trial. The Veterans Affairs Cooperative Variceal Sclerotherapy Group. N Engl J Med 1991; 324:1779–1784.
- The PROVA Study Group. Prophylaxis of first hemorrhage from esophageal varices by sclerotherapy, propranolol or both in cirrhotic patients: a randomized multicenter trial. Hepatology 1991; 14:1016–1024.
- Escorsell A, Feu F, Bordas JM, et al. Effects of isosorbide-5-mononitrate on variceal pressure and systemic and splanchnic haemodynamics in patients with cirrhosis. J Hepatol 1996; 24:423–429.
- Hayes PC, Westaby D, Williams R. Effect and mechanism of action of isosorbide-5-mononitrate. Gut 1988; 29:752–755.
- Angelico M, Carli L, Piat C, et al. Isosorbide-5-mononitrate versus propranolol in the prevention of first bleeding in cirrhosis. Gastroenterology 1993; 104:1460–1465.
- Angelico M, Carli L, Piat C, Gentile S, Capocaccia L. Effects of isosorbide-5-mononitrate compared with propranolol on first bleeding and long-term survival in cirrhosis. Gastroenterology 1997; 113:1632–1639.
- Garcia-Pagan JC, Villanueva C, Vila MC, et al. MOVE Group. Mononitrato Varices Esofagicas. Isosorbide mononitrate in the prevention of first variceal bleed in patients who cannot receive beta-blockers. Gastroenterology 2001; 121:908–914.
- Merkel C, Marin R, Enzo E, et al. Randomised trial of nadolol alone or with isosorbide mononitrate for primary prophylaxis of variceal bleeding in cirrhosis. Gruppo-Triveneto per L’ipertensione portale (GTIP). Lancet 1996; 348:1677–1681.
- Garcia-Pagán JC, Morillas R, Banares R, et al Spanish Variceal Bleeding Study Group. Propranolol plus placebo versus propranolol plus isosorbide-5-mononitrate in the prevention of a first variceal bleed: a double-blind RCT. Hepatology 2003; 37:1260–1266.
- Garcia-Tsao G, Sanyal A, Grace N, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007; 46:922–938.
- D’Amico G, De Franchis R Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003; 38:599–612.
- D’Amico G, Luca A. Natural history. Clinicalhaemodynamic correlations. Prediction of the risk of bleeding. Baillieres Clin Gastroenterol 1997; 11:243–256.
- Kamath PS. Esophageal variceal bleeding: primary prophylaxis. Clin Gastroenterol Hepatol 2005; 3:90–93.
- Shah V, Toruner M, Haddad F, et al. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology 1999; 117:1222–1228.
- Morales-Ruiz M, Jimenez W, Perez-Sala D, et al. Increased nitric oxide synthase expression in arterial vessels of cirrhotic rats with ascites. Hepatology 1996; 24:1481–1486.
- De Franchis R, Dell’Era A, Iannuzzi F. Diagnosis and treatment of portal hypertension. Dig Liver Dis 2004; 36:787–798.
- Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology 2004; 39:280–282.
- Garcia-Tsao G, Groszmann RJ, Fisher RL, Conn HO, Atterbury CE, Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology 1985; 5:419–424.
- Bosch J, Masti R, Kravetz D, et al. Effects of propranolol on azygos venous blood flow and hepatic and systemic hemodynamics in cirrhosis. Hepatology 1984; 4:1200–1205.
- Westaby D, Bihari DJ, Gimson AE, Crossley IR, Williams R. Selective and non-selective beta receptor blockade in the reduction of portal pressure in patients with cirrhosis and portal hypertension. Gut 1984; 25:121–124.
- D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology 1995; 22:332–354.
- Ideo G, Bellati G, Fesce E, Grimoldi D. Nadolol can prevent the first gastrointestinal bleeding in cirrhotics: a prospective, randomized study. Hepatology 1988; 8:6–9.
- Merkel C, Marin R, Angeli P, et al. Gruppo Triveneto per l’Ipertensione Portale. A placebo-controlled clinical trial of nadolol in the prophylaxis of growth of small esophageal varices in cirrhosis. Gastroenterology 2004; 127:476–484.
- Garcia-Tsao G, Grace ND, Groszmann RJ, et al. Short-term effects of propranolol on portal venous pressure. Hepatology 1986; 6:101–106.
- Kroeger RJ, Groszmann RJ. Increased portal venous resistance hinders portal pressure reduction during the administration of beta-adrenergic blocking agents in a portal hypertensive model. Hepatology 1985; 5:97–101.
- Merkel C, Bolognesi M, Sacerdoti D, et al. The hemodynamic response to medical treatment of portal hypertension as a predictor of clinical effectiveness in the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology 2000; 32:930–934.
- Groszmann RJ, Bosch J, Grace ND, et al. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology 1990; 99:1401–1407.
- Hicken BL, Sharara AI, Abrams GA, Eloubeidi M, Fallon MB, Arguedas MR. Hepatic venous pressure gradient measurements to assess response to primary prophylaxis in patients with cirrhosis: a decision analytical study. Aliment Pharmacol Ther 2003; 17:145–153.
- Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol 1997; 92:1081–1091.
- Grace ND, Groszmann RJ, Garcia-Tsao G, et al. Portal hypertension and variceal bleeding: an AASLD single topic symposium. Hepatology 1998; 28:868–880.
- Feu F, Garcia-Pagán JC, Bosch J, et al. Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal haemorrhage in patients with cirrhosis. Lancet 1995; 346:1056–1059.
- Abraczinskas DR, Ookubo R, Grace ND, et al. Propranolol for the prevention of first esophageal variceal hemorrhage: a lifetime commitment? Hepatology 2001; 34:1096–1102.
- Groszmann RJ, Garcia-Tsao G, Bosch J, et al Portal Hypertension Collaborative Group. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med 2005; 353:2254–2261.
- Garcia-Pagán JC, Bosch J. Pharmacological prevention of variceal bleeding. New developments. Baillier Clin Gastroenterol 1997; 11:271–287.
- Talwalkar JA, Kamath PS. An evidence-based medicine approach to beta-blocker therapy in patients with cirrhosis. Am J Med 2004; 116:759–766.
- Garcia-Pagan JC, Feu F, Castells A, et al. Circadian variations of portal pressure and variceal hemorrhage in patients with cirrhosis. Hepatology 1994; 19:595–601.
- Lay CS, Tsai YT, Teg CY, et al. Endoscopic variceal ligation in prophylaxis of first variceal bleeding in cirrhotic patients with high-risk esophageal varices. Hepatology 1997; 25:1346–1350.
- Imperiale TF, Chalasani N. A meta-analysis of endoscopic variceal ligation for primary prophylaxis of esophageal variceal bleeding. Hepatology 2001; 33:802–807.
- Schepke M, Kleber G, Nurnberg D, et al. Ligation versus propranolol for the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology 2004; 40:65–72.
- Lui HF, Stanley AJ, Forrest EH, et al. Primary prophylaxis of variceal hemorrhage: a randomized controlled trial comparing band ligation, propranolol, and isosorbide mononitrate. Gastroenterology 2002; 123:735–744.
- Khuroo MS, Khuroo NS, Farahat KL, Khuroo YS, Sofi AA, Dahab ST. Meta-analysis: endoscopic variceal ligation for primary prophylaxis of oesophageal variceal bleeding. Aliment Pharmacol Ther 2005; 21:347–361.
- Jutabha R, Jensen DM, Martin P, Savides T, Han SH, Gornbein J. Randomized study comparing banding and propranolol to prevent initial variceal hemorrhage in cirrhotics with high-risk esophageal varices. Gastroenterology 2005; 128:870–881.
- Psilopoulos D, Galanis P, Goulas S, et al. Endoscopic variceal ligation vs. propranolol for prevention of first variceal bleeding: a randomized controlled trial. Eur J Gastroenterol Hepatol 2005; 17:1111–1117.
- Lay CS, Tsai YT, Lee FY, et al. Endoscopic variceal ligation versus propranolol in prophylaxis of first variceal bleeding in patients with cirrhosis. J Gastroenterol Hepatol 2006; 21:413–419.
- Sarin SK, Wadhawan M, Agarwal SR, Tyagi P, Sharma BC. Endoscopic variceal ligation plus propranolol versus endoscopic variceal ligation alone in primary prophylaxis of variceal bleeding. Am J Gastroenterol 2005; 100:797–804.
- Yuksel O, Koklu S, Arhan M, Yolcu OF, et al. Effects of esophageal variceal eradication on portal hypertensive gastropathy and fundal varices: a retrospective and comparative study. Dig Dis Sci 2006; 51:27–30.
- Schneider AW, Kalk JF, Klein CP. Effect of losartan, an angiotensin II receptor antagonist, on portal pressure in cirrhosis. Hepatology 1999; 29:334–339.
- Schepke M, Werner E, Biecker E, et al. Hemodynamic effects of the angiotensin II receptor antagonist irbesartan in patients with cirrhosis and portal hypertension. Gastroenterology 2001; 121:389–395.
- Gonzalez-Abraldes J, Albillos A, Banares R, et al. Randomized comparison of long-term losartan versus propranolol in lowering portal pressure in cirrhosis. Gastroenterology 2001; 121:382–388.
- Boyer TD, Haskal ZJ American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology 2005; 41:386–400.
- Prophylactic sclerotherapy for esophageal varices in men with alcoholic liver disease. A randomized, single-blind, multicenter clinical trial. The Veterans Affairs Cooperative Variceal Sclerotherapy Group. N Engl J Med 1991; 324:1779–1784.
- The PROVA Study Group. Prophylaxis of first hemorrhage from esophageal varices by sclerotherapy, propranolol or both in cirrhotic patients: a randomized multicenter trial. Hepatology 1991; 14:1016–1024.
- Escorsell A, Feu F, Bordas JM, et al. Effects of isosorbide-5-mononitrate on variceal pressure and systemic and splanchnic haemodynamics in patients with cirrhosis. J Hepatol 1996; 24:423–429.
- Hayes PC, Westaby D, Williams R. Effect and mechanism of action of isosorbide-5-mononitrate. Gut 1988; 29:752–755.
- Angelico M, Carli L, Piat C, et al. Isosorbide-5-mononitrate versus propranolol in the prevention of first bleeding in cirrhosis. Gastroenterology 1993; 104:1460–1465.
- Angelico M, Carli L, Piat C, Gentile S, Capocaccia L. Effects of isosorbide-5-mononitrate compared with propranolol on first bleeding and long-term survival in cirrhosis. Gastroenterology 1997; 113:1632–1639.
- Garcia-Pagan JC, Villanueva C, Vila MC, et al. MOVE Group. Mononitrato Varices Esofagicas. Isosorbide mononitrate in the prevention of first variceal bleed in patients who cannot receive beta-blockers. Gastroenterology 2001; 121:908–914.
- Merkel C, Marin R, Enzo E, et al. Randomised trial of nadolol alone or with isosorbide mononitrate for primary prophylaxis of variceal bleeding in cirrhosis. Gruppo-Triveneto per L’ipertensione portale (GTIP). Lancet 1996; 348:1677–1681.
- Garcia-Pagán JC, Morillas R, Banares R, et al Spanish Variceal Bleeding Study Group. Propranolol plus placebo versus propranolol plus isosorbide-5-mononitrate in the prevention of a first variceal bleed: a double-blind RCT. Hepatology 2003; 37:1260–1266.
- Garcia-Tsao G, Sanyal A, Grace N, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007; 46:922–938.
KEY POINTS
- The hepatic vein pressure gradient (HVPG) correlates well with the portal pressure and is easier to measure. However, whether it is cost-effective to measure the HVPG in clinical practice is controversial.
- Nonselective beta-blockers are the mainstay of treatment; selective beta-blockers do not reduce portal pressure to the same degree and are not recommended for preventing variceal bleeding.
- Endoscopic variceal ligation is an acceptable alternative to beta-blocker therapy for patients who cannot tolerate these drugs and for patients with varices at high risk of bleeding.
- Nitrates are no longer used as monotherapy for preventing variceal hemorrhage, and their use in combination with beta-blockers is controversial. Surgical portal decompression, transjugular intrahepatic portosystemic shunting, and endoscopic sclerotherapy are not recommended.
Acute aortic syndromes: Time to talk of many things
“The time has come,” the Walrus said,
“To talk of many things:
Of shoes—and ships—and sealing-wax—
Of cabbages—and kings—
And why the sea is boiling hot
And whether pigs have wings.”
—Lewis Carroll, The Walrus and the Carpenter (from Through the Looking-Glass and What Alice Found There, 1872).
Lewis Carroll's poem of 1872 is a useful starting point for identifying issues resulting from confusion over the variously described acute aortic syndromes—and, for oysters, the dangers of listening to walruses.
TALK OF MANY THINGS
In cases of aortic dissection (splitting or separation of the layers of the aortic wall), it is important to establish the type (ie, the location and extent) and class (ie, the structure) of the dissection, because these distinctions determine the treatment.1 Similarly, in cases of painful or leaking degenerative aneurysms, we need to know the location of the aneurysm and whether the presenting pain is from compression of surrounding tissue, particularly of the vertebral bodies, or from leakage.
The location and extent of an aortic dissection can be classified in three ways (see Figure 3 in Smith and Schoenhagen’s excellent review of the use of computed tomography [CT] in acute aortic syndromes in this issue of the Cleveland Clinic Journal of Medicine2):
- The DeBakey system (type I, II, or III)
- The Stanford system (type A or B)
- Distal or proximal to the left subclavian artery.
Of note, the DeBakey system does not include tears in the arch that extend distally without ascending involvement. The original Stanford system included arch tears with distal extension in type B; hence, type B excluded all patients without ascending involvement.
The importance of the extent of dissection is that most patients with Stanford type A or DeBakey type I or II dissections should undergo immediate surgery, as most of them would die without it. Surgery is also indicated for arch tears (non-DeBakey, original Stanford type B).
Because these classifications are somewhat confusing, the simplest approach is to note whether the dissection extends proximal or distal to the left subclavian artery, because proximal dissections need surgery and distal ones are first managed medically.
The classes of dissection also have bearing on treatment.1 These are:
- Class I—classic aortic dissection in the media with two lumens separated by a “flap” or septum
- Class II—intramural hematoma in the aortic wall from dissection in which the intimal tear cannot be imaged (these are nearly always found duringsurgery or autopsy)
- Class III—localized confined intimal tears without extensive undermining of the intima or flap formation. These are often seen with Marfan syndrome and can rupture or cause tamponade, as can any type of proximal dissection. The typical appearance is of a bulging bubble in the aortic wall.
- Class IV—penetrating atherosclerotic ulcers with localized dissections or wall hematomas, often with calcium at the base of a mushroom-shaped area of extraluminal contrast. Of note, the plane of dissection is often between the media and adventitia.
- Class V—iatrogenic or posttraumatic dissection.
All class I to class IV tears of the proximal aorta require surgery, whereas distal class IV and V tears may require either open or endovascular surgical intervention. Surgery is also indicated for patients with distal dissections who have severe narrowing of the true lumen, distal ischemia, uncontrolled pain, severe hypertension, or evidence of leaking, particularly with class IV tears.
In distal dissections that are subacute (2–6 week sold), the Investigation of Stent grafts in Patients With Type B Aortic Dissection (INSTEAD) trial found that inserting a stent prophylactically provided no benefit. Further-more, there is no proof that stenting is beneficial if the aortic dissection is chronic, ie, more than 6 weeks old.1,3–5
WHICH SHOE FITS?
There is no ideal procedure to detect dissection, although the trend is towards CT angiography, as Smith and Schoenhagen report.2 Although some investigators have optimistically estimated CT’s sensitivity and specificity as 100%, cardiovascular surgeons are well aware of both false-positive and false-negative CT studies. Thus, for emergency repairs of proximal dissections, transesophageal echocardiography should be done after intubation and before opening a patient’s chest if time allows. Magnetic resonance imaging, CT, and transesophageal echocardiography may all miss class III tears, but these are frequently evidenced by eccentric “bubbles”or “ballooning.”1
SHIPS
Patients with either acute aortic dissection or severe pain associated with degenerative aneurysms need to be “shipped” promptly to a tertiary medical center after diagnosis, since larger volumes of procedures appear to be associated with better outcomes.
SEALING WAX
Using current surgical methods, the aortic valve can be preserved during aortic dissection repair unless the valve is bicuspid or the patient has Marfan syndrome.1,3,4,6–8
Sealing wax in the form of biological glues, rather than for letters, is a new innovation. A caveat remains, however: we have seen patients who have required reoperation for false aneurysms or infection. Hence, glues should be used with caution.
CABBAGES
A dilemma is whether patients should undergo coronary catheterization (or CT angiography—a separate question) and subsequent coronary artery bypass grafting (CABG), if needed, at the time of aortic dissection repair. The problem is that approximately one-third of patients have coronary artery disease that may require CABG, but the delay for catheterization increases the risk of rupture or tamponade before surgery.
Indeed, 40% of patients with proximal dissections die immediately, and 1% to 3% die in the hour before surgery. The short-term (in-hospital and 30-day) mortality rates range from 3.4% (Cleveland Clinic 2006 data) to 25%, and of the survivors only about 50% area live 5 years after surgery.
Though dismal, the prognosis is improving. In 162 patients with aortic dissection and Marfan syndrome or connective tissue disorders who underwent surgery at Cleveland Clinic in the years 1978–2003, the 5-year survival rate in those with aortic dissection was 75% and the 10-year rate was 55%.7 In those without dissection, the 10-year survival rate was approximately 90% (P < .001).
KINGS
Noted personalities who have had aortic dissection include King George II of England (who died in 1760), Lucille Ball, Conway Twitty, Jan Larson, and most recently John Ritter. None of these famous people survived their aortic dissections. Indeed, dissection and diseases of the aorta or its branches cause between 43,000 and 47,000 deaths annually,9 more than from breast cancer, murders, or motor vehicle accidents. The main reason for these dismal statistics is that the disease is often misdiagnosed at the time of presentation.
BOILING SEA
Careful studies from Olmsted County, Minnesota,10 have shown a tripling of the incidence of aortic disease, particularly in women, even though the rate of deaths from coronary artery disease has been decreasing. Furthermore, Olsson et al11 report that the incidence of aortic dissection in men in Sweden increased to approximately16 per 100,000 per year from 1987 to 2002, a 52% increase. The aging of the population must play a large role, but other factors may exist that are not well understood or defined and require further research.
PIGS HAVE WINGS
Will it be possible to overcome this rising problem? The answer is a definite yes. The results of aortic surgery have never been better. Many new innovations are available, such as aortic root preservation and endovascular stenting procedures. It may be possible to slow the growth of or prevent some aneurysms and aortic dissections, particularly with beta-blockers and, potentially, with losartan (Cozaar) for Marfan syndrome patients.
One of the keys to preventing aortic catastrophes and aortic dissection is to repair aortic aneurysms. The threshold for surgery, however, depends on a surgeon’s experience and results, the underlying pathology, and the aortic size.
We observed that 12.5% of dissections in patients with bicuspid valves and 15% of those in patients with Marfan syndrome were in aortas smaller than 5.0 cm in diameter, that aortic dissection occurred at smaller diameters in shorter patients, and that the risk of dissection increased exponentially with the size of the aorta. Subsequently, we found that a better measure of risk is the maximal aortic cross-sectional area in cm2 divided by the patient’s height in meters; if this ratio exceeds 10, then surgery is recommended.12
Results of surgery are good in experienced hands. In patients who undergo surgical repair of bicuspid aortic valves with or without concurrent repair of the ascending aorta (mostly in patients with an aortic cross-section-to-height ratio > 10), the perioperative mortality rate is about 1.0% for both groups, and at 10 years about 98% of patients are free from re-operation on the aorta and more than 90% are free from re-operation on the aortic valve.8 This is important because these are typically young patients who would do better without biological replacement valves (which are not very durable) or mechanical valves (which necessitate lifelong anticoagulation). Results are also good in surgery of the aortic arch and even better in patients with tricuspid aortic valves.4,6,8
Increasingly, in patients at high risk, we are inserting thoracic, abdominal, and thoracoabdominal stent grafts, with excellent early results. An even newer innovation is to replace the aortic valve in high-risk patients via a transcatheter balloon-expandable valve stent inserted through the groin or left ventricular apex.
These treatment innovations have been big strides, but aortic disease continues to increase. Indeed, our volume of thoracic aortic surgery at Cleveland Clinic increased from 190 procedures in 1999 to 717 in 2006. Early detection—before acute emergency surgery is required, with its concomitant high risk of death—is the key to successful surgical outcome and long-term survival.
- Svensson LG, Labib SB, Eisenhauer AC, Butterly JR. Intimal tear without hematoma: an important variant of aortic dissection that can elude current imaging techniques. Circulation 1999; 99:1331–1336.
- Smith AD, Schoenhagen P. CT imaging for acute aortic syndrome. Cleve Clin J Med 2008; 75:7–24.
- Svensson LG, Nadolny EM, Kimmel WA. Multimodal protocol influence on stroke and neurocognitive deficit prevention after ascending/arch aortic operations. Ann Thorac Surg 2002; 74:2040–2046.
- Svensson LG, Kim KH, Blackstone EH, et al. Elephant trunk procedure: newer indications and uses. Ann Thorac Surg 2004; 78:109–116.
- Greenberg RK, Haddad F, Svensson L, et al. Hybrid approaches to thoracic aortic aneurysms: the role of endovascular elephant trunk completion. Circulation 2005; 112:2619–2626.
- Svensson LG. Sizing for modified David’s reimplantation procedure. Ann Thorac Surg 2003; 76:1751–1753.
- Svensson LG, Blackstone EH, Feng J, et al. Are Marfan syndrome and marfanoid patients distinguishable on long-term follow-up? Ann Thorac Surg 2007; 83:1067–1074.
- Svensson LG, Blackstone EH, Cosgrove DM 3rd. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Svensson LG, Rodriguez ER. Aortic organ disease epidemic, and why do balloons pop? Circulation 2005; 112:1082–1084.
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998; 280:1926–1929.
- Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 114:2611–2618.
- Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection inpatients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003;126:892–893.
“The time has come,” the Walrus said,
“To talk of many things:
Of shoes—and ships—and sealing-wax—
Of cabbages—and kings—
And why the sea is boiling hot
And whether pigs have wings.”
—Lewis Carroll, The Walrus and the Carpenter (from Through the Looking-Glass and What Alice Found There, 1872).
Lewis Carroll's poem of 1872 is a useful starting point for identifying issues resulting from confusion over the variously described acute aortic syndromes—and, for oysters, the dangers of listening to walruses.
TALK OF MANY THINGS
In cases of aortic dissection (splitting or separation of the layers of the aortic wall), it is important to establish the type (ie, the location and extent) and class (ie, the structure) of the dissection, because these distinctions determine the treatment.1 Similarly, in cases of painful or leaking degenerative aneurysms, we need to know the location of the aneurysm and whether the presenting pain is from compression of surrounding tissue, particularly of the vertebral bodies, or from leakage.
The location and extent of an aortic dissection can be classified in three ways (see Figure 3 in Smith and Schoenhagen’s excellent review of the use of computed tomography [CT] in acute aortic syndromes in this issue of the Cleveland Clinic Journal of Medicine2):
- The DeBakey system (type I, II, or III)
- The Stanford system (type A or B)
- Distal or proximal to the left subclavian artery.
Of note, the DeBakey system does not include tears in the arch that extend distally without ascending involvement. The original Stanford system included arch tears with distal extension in type B; hence, type B excluded all patients without ascending involvement.
The importance of the extent of dissection is that most patients with Stanford type A or DeBakey type I or II dissections should undergo immediate surgery, as most of them would die without it. Surgery is also indicated for arch tears (non-DeBakey, original Stanford type B).
Because these classifications are somewhat confusing, the simplest approach is to note whether the dissection extends proximal or distal to the left subclavian artery, because proximal dissections need surgery and distal ones are first managed medically.
The classes of dissection also have bearing on treatment.1 These are:
- Class I—classic aortic dissection in the media with two lumens separated by a “flap” or septum
- Class II—intramural hematoma in the aortic wall from dissection in which the intimal tear cannot be imaged (these are nearly always found duringsurgery or autopsy)
- Class III—localized confined intimal tears without extensive undermining of the intima or flap formation. These are often seen with Marfan syndrome and can rupture or cause tamponade, as can any type of proximal dissection. The typical appearance is of a bulging bubble in the aortic wall.
- Class IV—penetrating atherosclerotic ulcers with localized dissections or wall hematomas, often with calcium at the base of a mushroom-shaped area of extraluminal contrast. Of note, the plane of dissection is often between the media and adventitia.
- Class V—iatrogenic or posttraumatic dissection.
All class I to class IV tears of the proximal aorta require surgery, whereas distal class IV and V tears may require either open or endovascular surgical intervention. Surgery is also indicated for patients with distal dissections who have severe narrowing of the true lumen, distal ischemia, uncontrolled pain, severe hypertension, or evidence of leaking, particularly with class IV tears.
In distal dissections that are subacute (2–6 week sold), the Investigation of Stent grafts in Patients With Type B Aortic Dissection (INSTEAD) trial found that inserting a stent prophylactically provided no benefit. Further-more, there is no proof that stenting is beneficial if the aortic dissection is chronic, ie, more than 6 weeks old.1,3–5
WHICH SHOE FITS?
There is no ideal procedure to detect dissection, although the trend is towards CT angiography, as Smith and Schoenhagen report.2 Although some investigators have optimistically estimated CT’s sensitivity and specificity as 100%, cardiovascular surgeons are well aware of both false-positive and false-negative CT studies. Thus, for emergency repairs of proximal dissections, transesophageal echocardiography should be done after intubation and before opening a patient’s chest if time allows. Magnetic resonance imaging, CT, and transesophageal echocardiography may all miss class III tears, but these are frequently evidenced by eccentric “bubbles”or “ballooning.”1
SHIPS
Patients with either acute aortic dissection or severe pain associated with degenerative aneurysms need to be “shipped” promptly to a tertiary medical center after diagnosis, since larger volumes of procedures appear to be associated with better outcomes.
SEALING WAX
Using current surgical methods, the aortic valve can be preserved during aortic dissection repair unless the valve is bicuspid or the patient has Marfan syndrome.1,3,4,6–8
Sealing wax in the form of biological glues, rather than for letters, is a new innovation. A caveat remains, however: we have seen patients who have required reoperation for false aneurysms or infection. Hence, glues should be used with caution.
CABBAGES
A dilemma is whether patients should undergo coronary catheterization (or CT angiography—a separate question) and subsequent coronary artery bypass grafting (CABG), if needed, at the time of aortic dissection repair. The problem is that approximately one-third of patients have coronary artery disease that may require CABG, but the delay for catheterization increases the risk of rupture or tamponade before surgery.
Indeed, 40% of patients with proximal dissections die immediately, and 1% to 3% die in the hour before surgery. The short-term (in-hospital and 30-day) mortality rates range from 3.4% (Cleveland Clinic 2006 data) to 25%, and of the survivors only about 50% area live 5 years after surgery.
Though dismal, the prognosis is improving. In 162 patients with aortic dissection and Marfan syndrome or connective tissue disorders who underwent surgery at Cleveland Clinic in the years 1978–2003, the 5-year survival rate in those with aortic dissection was 75% and the 10-year rate was 55%.7 In those without dissection, the 10-year survival rate was approximately 90% (P < .001).
KINGS
Noted personalities who have had aortic dissection include King George II of England (who died in 1760), Lucille Ball, Conway Twitty, Jan Larson, and most recently John Ritter. None of these famous people survived their aortic dissections. Indeed, dissection and diseases of the aorta or its branches cause between 43,000 and 47,000 deaths annually,9 more than from breast cancer, murders, or motor vehicle accidents. The main reason for these dismal statistics is that the disease is often misdiagnosed at the time of presentation.
BOILING SEA
Careful studies from Olmsted County, Minnesota,10 have shown a tripling of the incidence of aortic disease, particularly in women, even though the rate of deaths from coronary artery disease has been decreasing. Furthermore, Olsson et al11 report that the incidence of aortic dissection in men in Sweden increased to approximately16 per 100,000 per year from 1987 to 2002, a 52% increase. The aging of the population must play a large role, but other factors may exist that are not well understood or defined and require further research.
PIGS HAVE WINGS
Will it be possible to overcome this rising problem? The answer is a definite yes. The results of aortic surgery have never been better. Many new innovations are available, such as aortic root preservation and endovascular stenting procedures. It may be possible to slow the growth of or prevent some aneurysms and aortic dissections, particularly with beta-blockers and, potentially, with losartan (Cozaar) for Marfan syndrome patients.
One of the keys to preventing aortic catastrophes and aortic dissection is to repair aortic aneurysms. The threshold for surgery, however, depends on a surgeon’s experience and results, the underlying pathology, and the aortic size.
We observed that 12.5% of dissections in patients with bicuspid valves and 15% of those in patients with Marfan syndrome were in aortas smaller than 5.0 cm in diameter, that aortic dissection occurred at smaller diameters in shorter patients, and that the risk of dissection increased exponentially with the size of the aorta. Subsequently, we found that a better measure of risk is the maximal aortic cross-sectional area in cm2 divided by the patient’s height in meters; if this ratio exceeds 10, then surgery is recommended.12
Results of surgery are good in experienced hands. In patients who undergo surgical repair of bicuspid aortic valves with or without concurrent repair of the ascending aorta (mostly in patients with an aortic cross-section-to-height ratio > 10), the perioperative mortality rate is about 1.0% for both groups, and at 10 years about 98% of patients are free from re-operation on the aorta and more than 90% are free from re-operation on the aortic valve.8 This is important because these are typically young patients who would do better without biological replacement valves (which are not very durable) or mechanical valves (which necessitate lifelong anticoagulation). Results are also good in surgery of the aortic arch and even better in patients with tricuspid aortic valves.4,6,8
Increasingly, in patients at high risk, we are inserting thoracic, abdominal, and thoracoabdominal stent grafts, with excellent early results. An even newer innovation is to replace the aortic valve in high-risk patients via a transcatheter balloon-expandable valve stent inserted through the groin or left ventricular apex.
These treatment innovations have been big strides, but aortic disease continues to increase. Indeed, our volume of thoracic aortic surgery at Cleveland Clinic increased from 190 procedures in 1999 to 717 in 2006. Early detection—before acute emergency surgery is required, with its concomitant high risk of death—is the key to successful surgical outcome and long-term survival.
“The time has come,” the Walrus said,
“To talk of many things:
Of shoes—and ships—and sealing-wax—
Of cabbages—and kings—
And why the sea is boiling hot
And whether pigs have wings.”
—Lewis Carroll, The Walrus and the Carpenter (from Through the Looking-Glass and What Alice Found There, 1872).
Lewis Carroll's poem of 1872 is a useful starting point for identifying issues resulting from confusion over the variously described acute aortic syndromes—and, for oysters, the dangers of listening to walruses.
TALK OF MANY THINGS
In cases of aortic dissection (splitting or separation of the layers of the aortic wall), it is important to establish the type (ie, the location and extent) and class (ie, the structure) of the dissection, because these distinctions determine the treatment.1 Similarly, in cases of painful or leaking degenerative aneurysms, we need to know the location of the aneurysm and whether the presenting pain is from compression of surrounding tissue, particularly of the vertebral bodies, or from leakage.
The location and extent of an aortic dissection can be classified in three ways (see Figure 3 in Smith and Schoenhagen’s excellent review of the use of computed tomography [CT] in acute aortic syndromes in this issue of the Cleveland Clinic Journal of Medicine2):
- The DeBakey system (type I, II, or III)
- The Stanford system (type A or B)
- Distal or proximal to the left subclavian artery.
Of note, the DeBakey system does not include tears in the arch that extend distally without ascending involvement. The original Stanford system included arch tears with distal extension in type B; hence, type B excluded all patients without ascending involvement.
The importance of the extent of dissection is that most patients with Stanford type A or DeBakey type I or II dissections should undergo immediate surgery, as most of them would die without it. Surgery is also indicated for arch tears (non-DeBakey, original Stanford type B).
Because these classifications are somewhat confusing, the simplest approach is to note whether the dissection extends proximal or distal to the left subclavian artery, because proximal dissections need surgery and distal ones are first managed medically.
The classes of dissection also have bearing on treatment.1 These are:
- Class I—classic aortic dissection in the media with two lumens separated by a “flap” or septum
- Class II—intramural hematoma in the aortic wall from dissection in which the intimal tear cannot be imaged (these are nearly always found duringsurgery or autopsy)
- Class III—localized confined intimal tears without extensive undermining of the intima or flap formation. These are often seen with Marfan syndrome and can rupture or cause tamponade, as can any type of proximal dissection. The typical appearance is of a bulging bubble in the aortic wall.
- Class IV—penetrating atherosclerotic ulcers with localized dissections or wall hematomas, often with calcium at the base of a mushroom-shaped area of extraluminal contrast. Of note, the plane of dissection is often between the media and adventitia.
- Class V—iatrogenic or posttraumatic dissection.
All class I to class IV tears of the proximal aorta require surgery, whereas distal class IV and V tears may require either open or endovascular surgical intervention. Surgery is also indicated for patients with distal dissections who have severe narrowing of the true lumen, distal ischemia, uncontrolled pain, severe hypertension, or evidence of leaking, particularly with class IV tears.
In distal dissections that are subacute (2–6 week sold), the Investigation of Stent grafts in Patients With Type B Aortic Dissection (INSTEAD) trial found that inserting a stent prophylactically provided no benefit. Further-more, there is no proof that stenting is beneficial if the aortic dissection is chronic, ie, more than 6 weeks old.1,3–5
WHICH SHOE FITS?
There is no ideal procedure to detect dissection, although the trend is towards CT angiography, as Smith and Schoenhagen report.2 Although some investigators have optimistically estimated CT’s sensitivity and specificity as 100%, cardiovascular surgeons are well aware of both false-positive and false-negative CT studies. Thus, for emergency repairs of proximal dissections, transesophageal echocardiography should be done after intubation and before opening a patient’s chest if time allows. Magnetic resonance imaging, CT, and transesophageal echocardiography may all miss class III tears, but these are frequently evidenced by eccentric “bubbles”or “ballooning.”1
SHIPS
Patients with either acute aortic dissection or severe pain associated with degenerative aneurysms need to be “shipped” promptly to a tertiary medical center after diagnosis, since larger volumes of procedures appear to be associated with better outcomes.
SEALING WAX
Using current surgical methods, the aortic valve can be preserved during aortic dissection repair unless the valve is bicuspid or the patient has Marfan syndrome.1,3,4,6–8
Sealing wax in the form of biological glues, rather than for letters, is a new innovation. A caveat remains, however: we have seen patients who have required reoperation for false aneurysms or infection. Hence, glues should be used with caution.
CABBAGES
A dilemma is whether patients should undergo coronary catheterization (or CT angiography—a separate question) and subsequent coronary artery bypass grafting (CABG), if needed, at the time of aortic dissection repair. The problem is that approximately one-third of patients have coronary artery disease that may require CABG, but the delay for catheterization increases the risk of rupture or tamponade before surgery.
Indeed, 40% of patients with proximal dissections die immediately, and 1% to 3% die in the hour before surgery. The short-term (in-hospital and 30-day) mortality rates range from 3.4% (Cleveland Clinic 2006 data) to 25%, and of the survivors only about 50% area live 5 years after surgery.
Though dismal, the prognosis is improving. In 162 patients with aortic dissection and Marfan syndrome or connective tissue disorders who underwent surgery at Cleveland Clinic in the years 1978–2003, the 5-year survival rate in those with aortic dissection was 75% and the 10-year rate was 55%.7 In those without dissection, the 10-year survival rate was approximately 90% (P < .001).
KINGS
Noted personalities who have had aortic dissection include King George II of England (who died in 1760), Lucille Ball, Conway Twitty, Jan Larson, and most recently John Ritter. None of these famous people survived their aortic dissections. Indeed, dissection and diseases of the aorta or its branches cause between 43,000 and 47,000 deaths annually,9 more than from breast cancer, murders, or motor vehicle accidents. The main reason for these dismal statistics is that the disease is often misdiagnosed at the time of presentation.
BOILING SEA
Careful studies from Olmsted County, Minnesota,10 have shown a tripling of the incidence of aortic disease, particularly in women, even though the rate of deaths from coronary artery disease has been decreasing. Furthermore, Olsson et al11 report that the incidence of aortic dissection in men in Sweden increased to approximately16 per 100,000 per year from 1987 to 2002, a 52% increase. The aging of the population must play a large role, but other factors may exist that are not well understood or defined and require further research.
PIGS HAVE WINGS
Will it be possible to overcome this rising problem? The answer is a definite yes. The results of aortic surgery have never been better. Many new innovations are available, such as aortic root preservation and endovascular stenting procedures. It may be possible to slow the growth of or prevent some aneurysms and aortic dissections, particularly with beta-blockers and, potentially, with losartan (Cozaar) for Marfan syndrome patients.
One of the keys to preventing aortic catastrophes and aortic dissection is to repair aortic aneurysms. The threshold for surgery, however, depends on a surgeon’s experience and results, the underlying pathology, and the aortic size.
We observed that 12.5% of dissections in patients with bicuspid valves and 15% of those in patients with Marfan syndrome were in aortas smaller than 5.0 cm in diameter, that aortic dissection occurred at smaller diameters in shorter patients, and that the risk of dissection increased exponentially with the size of the aorta. Subsequently, we found that a better measure of risk is the maximal aortic cross-sectional area in cm2 divided by the patient’s height in meters; if this ratio exceeds 10, then surgery is recommended.12
Results of surgery are good in experienced hands. In patients who undergo surgical repair of bicuspid aortic valves with or without concurrent repair of the ascending aorta (mostly in patients with an aortic cross-section-to-height ratio > 10), the perioperative mortality rate is about 1.0% for both groups, and at 10 years about 98% of patients are free from re-operation on the aorta and more than 90% are free from re-operation on the aortic valve.8 This is important because these are typically young patients who would do better without biological replacement valves (which are not very durable) or mechanical valves (which necessitate lifelong anticoagulation). Results are also good in surgery of the aortic arch and even better in patients with tricuspid aortic valves.4,6,8
Increasingly, in patients at high risk, we are inserting thoracic, abdominal, and thoracoabdominal stent grafts, with excellent early results. An even newer innovation is to replace the aortic valve in high-risk patients via a transcatheter balloon-expandable valve stent inserted through the groin or left ventricular apex.
These treatment innovations have been big strides, but aortic disease continues to increase. Indeed, our volume of thoracic aortic surgery at Cleveland Clinic increased from 190 procedures in 1999 to 717 in 2006. Early detection—before acute emergency surgery is required, with its concomitant high risk of death—is the key to successful surgical outcome and long-term survival.
- Svensson LG, Labib SB, Eisenhauer AC, Butterly JR. Intimal tear without hematoma: an important variant of aortic dissection that can elude current imaging techniques. Circulation 1999; 99:1331–1336.
- Smith AD, Schoenhagen P. CT imaging for acute aortic syndrome. Cleve Clin J Med 2008; 75:7–24.
- Svensson LG, Nadolny EM, Kimmel WA. Multimodal protocol influence on stroke and neurocognitive deficit prevention after ascending/arch aortic operations. Ann Thorac Surg 2002; 74:2040–2046.
- Svensson LG, Kim KH, Blackstone EH, et al. Elephant trunk procedure: newer indications and uses. Ann Thorac Surg 2004; 78:109–116.
- Greenberg RK, Haddad F, Svensson L, et al. Hybrid approaches to thoracic aortic aneurysms: the role of endovascular elephant trunk completion. Circulation 2005; 112:2619–2626.
- Svensson LG. Sizing for modified David’s reimplantation procedure. Ann Thorac Surg 2003; 76:1751–1753.
- Svensson LG, Blackstone EH, Feng J, et al. Are Marfan syndrome and marfanoid patients distinguishable on long-term follow-up? Ann Thorac Surg 2007; 83:1067–1074.
- Svensson LG, Blackstone EH, Cosgrove DM 3rd. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Svensson LG, Rodriguez ER. Aortic organ disease epidemic, and why do balloons pop? Circulation 2005; 112:1082–1084.
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998; 280:1926–1929.
- Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 114:2611–2618.
- Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection inpatients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003;126:892–893.
- Svensson LG, Labib SB, Eisenhauer AC, Butterly JR. Intimal tear without hematoma: an important variant of aortic dissection that can elude current imaging techniques. Circulation 1999; 99:1331–1336.
- Smith AD, Schoenhagen P. CT imaging for acute aortic syndrome. Cleve Clin J Med 2008; 75:7–24.
- Svensson LG, Nadolny EM, Kimmel WA. Multimodal protocol influence on stroke and neurocognitive deficit prevention after ascending/arch aortic operations. Ann Thorac Surg 2002; 74:2040–2046.
- Svensson LG, Kim KH, Blackstone EH, et al. Elephant trunk procedure: newer indications and uses. Ann Thorac Surg 2004; 78:109–116.
- Greenberg RK, Haddad F, Svensson L, et al. Hybrid approaches to thoracic aortic aneurysms: the role of endovascular elephant trunk completion. Circulation 2005; 112:2619–2626.
- Svensson LG. Sizing for modified David’s reimplantation procedure. Ann Thorac Surg 2003; 76:1751–1753.
- Svensson LG, Blackstone EH, Feng J, et al. Are Marfan syndrome and marfanoid patients distinguishable on long-term follow-up? Ann Thorac Surg 2007; 83:1067–1074.
- Svensson LG, Blackstone EH, Cosgrove DM 3rd. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Svensson LG, Rodriguez ER. Aortic organ disease epidemic, and why do balloons pop? Circulation 2005; 112:1082–1084.
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998; 280:1926–1929.
- Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 114:2611–2618.
- Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection inpatients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003;126:892–893.