User login
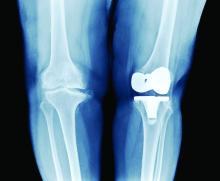
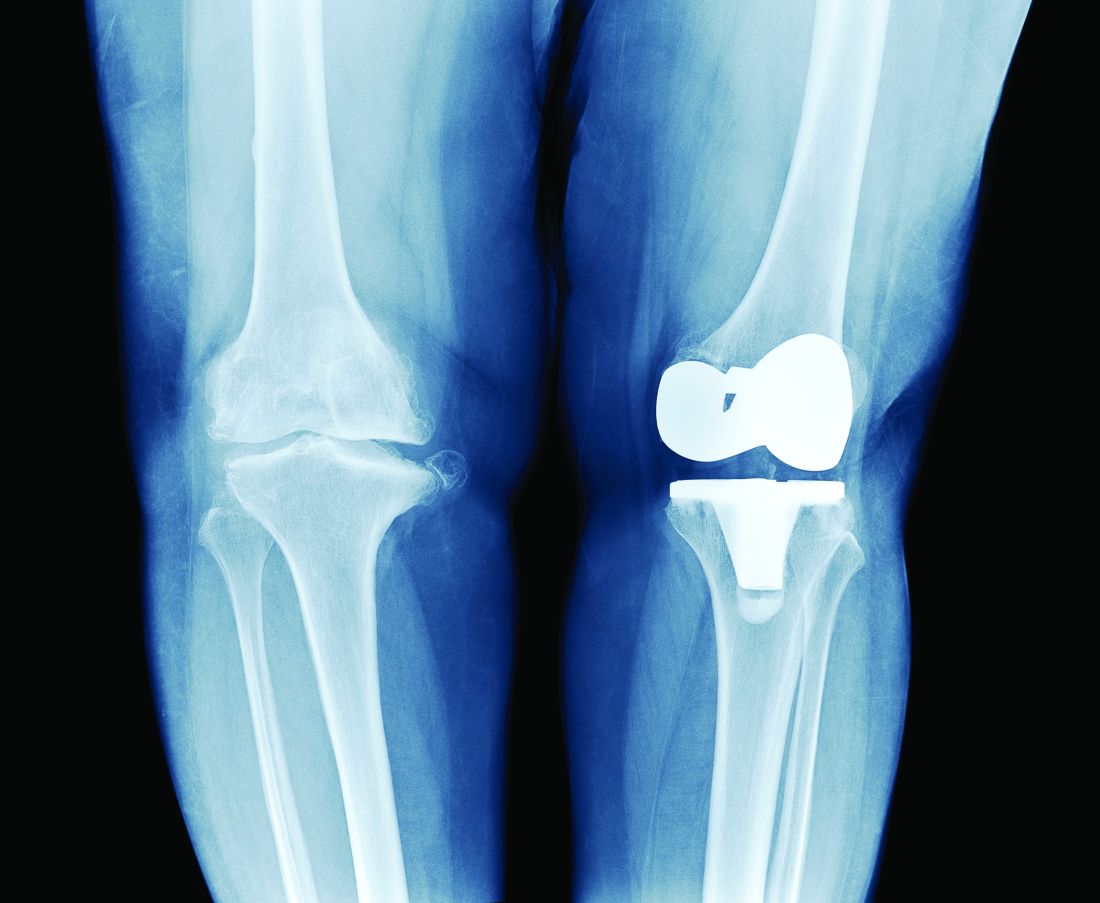
PT may lower risk of long-term opioid use after knee replacement
A new study has found that physical therapy may lead to a reduced risk of long-term opioid use in patients who have undergone total knee replacement (TKR).
“Greater number of PT intervention sessions and earlier initiation of outpatient PT care after TKR were associated with lower odds of long-term opioid use,” authors from Boston University wrote in their report on the study, which was published online Oct. 27 in JAMA Network Open.
“In previous large studies, we’ve seen that physical therapy can reduce pain in people with knee osteoarthritis, which is usually the primary indication for TKR,” study coauthor Deepak Kumar, PT, PhD, said in an interview. “But the association of physical therapy with opioid use in people with knee replacement has not yet been explored.
“The reason we focused on opioid use in these patients is because the number of knee replacement surgeries is going up exponentially,” Dr. Kumar said. “And, depending on which data you look at, from one-third to up to half of people who undergo knee replacement and have used opioids before end up becoming long-term users. Even in people who have not used them before, 5%-8% become long-term users after the surgery.
“Given how many surgeries are happening – and that number is expected to keep going up – the number of people who are becoming long-term opioid users is not trivial,” he said.
Study details
To assess the value of PT in reducing opioid use in this subset of patients, the authors reviewed records from the OptumLabs Data Warehouse insurance claims database to identify 67,322 eligible participants aged 40 or older who underwent TKR from Jan. 1, 2001, to Dec. 31, 2016. Of those patients, 38,408 were opioid naive and 28,914 had taken opioids before. The authors evaluated long-term opioid use – defined as 90 days or more of filled prescriptions – during a 12-month outcome assessment period that varied depending on differences in post-TKR PT start date and duration.
The researchers found a significantly lower likelihood of long-term opioid use associated with receipt of any PT before TKR among patients who had not taken opioids before (adjusted odds ratio [aOR], 0.75; 95% confidence interval, 0.60-0.95) and those who had taken opioids in the past (aOR, 0.75; 95% CI, 0.70-0.80).
Investigators found that 2.2% of participants in the opioid-naive group and 32.5% of those in the opioid-experienced group used opioids long-term after TKR. Approximately 76% of participants overall received outpatient PT within the 90 days after surgery, and the receipt of post-TKR PT at any point was associated with lower odds of long-term opioid use in the opioid-experienced group (aOR, 0.75; 95% CI, 0.70-0.79).
Among the opioid-experienced group, receiving between 6 and 12 PT sessions (aOR, 0.82; 95% CI, 0.75-0.90) or ≥ 13 sessions (aOR, 0.71; 95% CI, 0.65-0.77) were both associated with lower odds of long-term opioid use, compared with those who received 1-5 sessions. Beginning PT 31-60 days or 61-90 days after surgery was associated with greater odds of long-term opioid use across both cohorts, compared with those who initiated therapy within 30 days of TKR.
Physical therapy: Underexplored option for pain in knee replacement
One finding caught the researchers slightly off guard: There was no association between active physical therapy and reduced odds of long-term opioid use. “From prior studies, at least in people with knee osteoarthritis, we know that active interventions were more useful than passive interventions,” Dr. Kumar said.
That said, he added that there is still some professional uncertainty regarding “the right type or the right components of physical therapy for managing pain in this population.” Regardless, he believes their study emphasizes the benefits of PT as a pain alleviator in these patients, especially those who have previously used opioids.
“Pharmaceuticals have side effects. Injections are not super effective,” he said. “The idea behind focusing on physical therapy interventions is that it’s widely available, it does you no harm, and it could potentially be lower cost to both the payers and the providers.”
The authors acknowledged their study’s limitations, including not adjusting for opioid use within the 90 days after surgery as well as the different outcome assessment periods for pre-TKR and post-TKR PT exposures. In addition, they admitted that some of the patients who received PT could have been among those less likely to be treated with opioids, and vice versa. “A randomized clinical trial,” they wrote, “would be required to disentangle these issues.”
The study was supported by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kumar reported receiving grants from the National Institutes of Health during the conduct of the study and grants from Pfizer for unrelated projects outside the submitted work. The full list of author disclosures can be found with the original article.
A version of this article first appeared on Medscape.com.
A new study has found that physical therapy may lead to a reduced risk of long-term opioid use in patients who have undergone total knee replacement (TKR).
“Greater number of PT intervention sessions and earlier initiation of outpatient PT care after TKR were associated with lower odds of long-term opioid use,” authors from Boston University wrote in their report on the study, which was published online Oct. 27 in JAMA Network Open.
“In previous large studies, we’ve seen that physical therapy can reduce pain in people with knee osteoarthritis, which is usually the primary indication for TKR,” study coauthor Deepak Kumar, PT, PhD, said in an interview. “But the association of physical therapy with opioid use in people with knee replacement has not yet been explored.
“The reason we focused on opioid use in these patients is because the number of knee replacement surgeries is going up exponentially,” Dr. Kumar said. “And, depending on which data you look at, from one-third to up to half of people who undergo knee replacement and have used opioids before end up becoming long-term users. Even in people who have not used them before, 5%-8% become long-term users after the surgery.
“Given how many surgeries are happening – and that number is expected to keep going up – the number of people who are becoming long-term opioid users is not trivial,” he said.
Study details
To assess the value of PT in reducing opioid use in this subset of patients, the authors reviewed records from the OptumLabs Data Warehouse insurance claims database to identify 67,322 eligible participants aged 40 or older who underwent TKR from Jan. 1, 2001, to Dec. 31, 2016. Of those patients, 38,408 were opioid naive and 28,914 had taken opioids before. The authors evaluated long-term opioid use – defined as 90 days or more of filled prescriptions – during a 12-month outcome assessment period that varied depending on differences in post-TKR PT start date and duration.
The researchers found a significantly lower likelihood of long-term opioid use associated with receipt of any PT before TKR among patients who had not taken opioids before (adjusted odds ratio [aOR], 0.75; 95% confidence interval, 0.60-0.95) and those who had taken opioids in the past (aOR, 0.75; 95% CI, 0.70-0.80).
Investigators found that 2.2% of participants in the opioid-naive group and 32.5% of those in the opioid-experienced group used opioids long-term after TKR. Approximately 76% of participants overall received outpatient PT within the 90 days after surgery, and the receipt of post-TKR PT at any point was associated with lower odds of long-term opioid use in the opioid-experienced group (aOR, 0.75; 95% CI, 0.70-0.79).
Among the opioid-experienced group, receiving between 6 and 12 PT sessions (aOR, 0.82; 95% CI, 0.75-0.90) or ≥ 13 sessions (aOR, 0.71; 95% CI, 0.65-0.77) were both associated with lower odds of long-term opioid use, compared with those who received 1-5 sessions. Beginning PT 31-60 days or 61-90 days after surgery was associated with greater odds of long-term opioid use across both cohorts, compared with those who initiated therapy within 30 days of TKR.
Physical therapy: Underexplored option for pain in knee replacement
One finding caught the researchers slightly off guard: There was no association between active physical therapy and reduced odds of long-term opioid use. “From prior studies, at least in people with knee osteoarthritis, we know that active interventions were more useful than passive interventions,” Dr. Kumar said.
That said, he added that there is still some professional uncertainty regarding “the right type or the right components of physical therapy for managing pain in this population.” Regardless, he believes their study emphasizes the benefits of PT as a pain alleviator in these patients, especially those who have previously used opioids.
“Pharmaceuticals have side effects. Injections are not super effective,” he said. “The idea behind focusing on physical therapy interventions is that it’s widely available, it does you no harm, and it could potentially be lower cost to both the payers and the providers.”
The authors acknowledged their study’s limitations, including not adjusting for opioid use within the 90 days after surgery as well as the different outcome assessment periods for pre-TKR and post-TKR PT exposures. In addition, they admitted that some of the patients who received PT could have been among those less likely to be treated with opioids, and vice versa. “A randomized clinical trial,” they wrote, “would be required to disentangle these issues.”
The study was supported by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kumar reported receiving grants from the National Institutes of Health during the conduct of the study and grants from Pfizer for unrelated projects outside the submitted work. The full list of author disclosures can be found with the original article.
A version of this article first appeared on Medscape.com.
A new study has found that physical therapy may lead to a reduced risk of long-term opioid use in patients who have undergone total knee replacement (TKR).
“Greater number of PT intervention sessions and earlier initiation of outpatient PT care after TKR were associated with lower odds of long-term opioid use,” authors from Boston University wrote in their report on the study, which was published online Oct. 27 in JAMA Network Open.
“In previous large studies, we’ve seen that physical therapy can reduce pain in people with knee osteoarthritis, which is usually the primary indication for TKR,” study coauthor Deepak Kumar, PT, PhD, said in an interview. “But the association of physical therapy with opioid use in people with knee replacement has not yet been explored.
“The reason we focused on opioid use in these patients is because the number of knee replacement surgeries is going up exponentially,” Dr. Kumar said. “And, depending on which data you look at, from one-third to up to half of people who undergo knee replacement and have used opioids before end up becoming long-term users. Even in people who have not used them before, 5%-8% become long-term users after the surgery.
“Given how many surgeries are happening – and that number is expected to keep going up – the number of people who are becoming long-term opioid users is not trivial,” he said.
Study details
To assess the value of PT in reducing opioid use in this subset of patients, the authors reviewed records from the OptumLabs Data Warehouse insurance claims database to identify 67,322 eligible participants aged 40 or older who underwent TKR from Jan. 1, 2001, to Dec. 31, 2016. Of those patients, 38,408 were opioid naive and 28,914 had taken opioids before. The authors evaluated long-term opioid use – defined as 90 days or more of filled prescriptions – during a 12-month outcome assessment period that varied depending on differences in post-TKR PT start date and duration.
The researchers found a significantly lower likelihood of long-term opioid use associated with receipt of any PT before TKR among patients who had not taken opioids before (adjusted odds ratio [aOR], 0.75; 95% confidence interval, 0.60-0.95) and those who had taken opioids in the past (aOR, 0.75; 95% CI, 0.70-0.80).
Investigators found that 2.2% of participants in the opioid-naive group and 32.5% of those in the opioid-experienced group used opioids long-term after TKR. Approximately 76% of participants overall received outpatient PT within the 90 days after surgery, and the receipt of post-TKR PT at any point was associated with lower odds of long-term opioid use in the opioid-experienced group (aOR, 0.75; 95% CI, 0.70-0.79).
Among the opioid-experienced group, receiving between 6 and 12 PT sessions (aOR, 0.82; 95% CI, 0.75-0.90) or ≥ 13 sessions (aOR, 0.71; 95% CI, 0.65-0.77) were both associated with lower odds of long-term opioid use, compared with those who received 1-5 sessions. Beginning PT 31-60 days or 61-90 days after surgery was associated with greater odds of long-term opioid use across both cohorts, compared with those who initiated therapy within 30 days of TKR.
Physical therapy: Underexplored option for pain in knee replacement
One finding caught the researchers slightly off guard: There was no association between active physical therapy and reduced odds of long-term opioid use. “From prior studies, at least in people with knee osteoarthritis, we know that active interventions were more useful than passive interventions,” Dr. Kumar said.
That said, he added that there is still some professional uncertainty regarding “the right type or the right components of physical therapy for managing pain in this population.” Regardless, he believes their study emphasizes the benefits of PT as a pain alleviator in these patients, especially those who have previously used opioids.
“Pharmaceuticals have side effects. Injections are not super effective,” he said. “The idea behind focusing on physical therapy interventions is that it’s widely available, it does you no harm, and it could potentially be lower cost to both the payers and the providers.”
The authors acknowledged their study’s limitations, including not adjusting for opioid use within the 90 days after surgery as well as the different outcome assessment periods for pre-TKR and post-TKR PT exposures. In addition, they admitted that some of the patients who received PT could have been among those less likely to be treated with opioids, and vice versa. “A randomized clinical trial,” they wrote, “would be required to disentangle these issues.”
The study was supported by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kumar reported receiving grants from the National Institutes of Health during the conduct of the study and grants from Pfizer for unrelated projects outside the submitted work. The full list of author disclosures can be found with the original article.
A version of this article first appeared on Medscape.com.
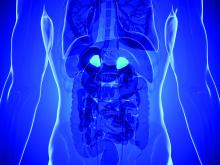
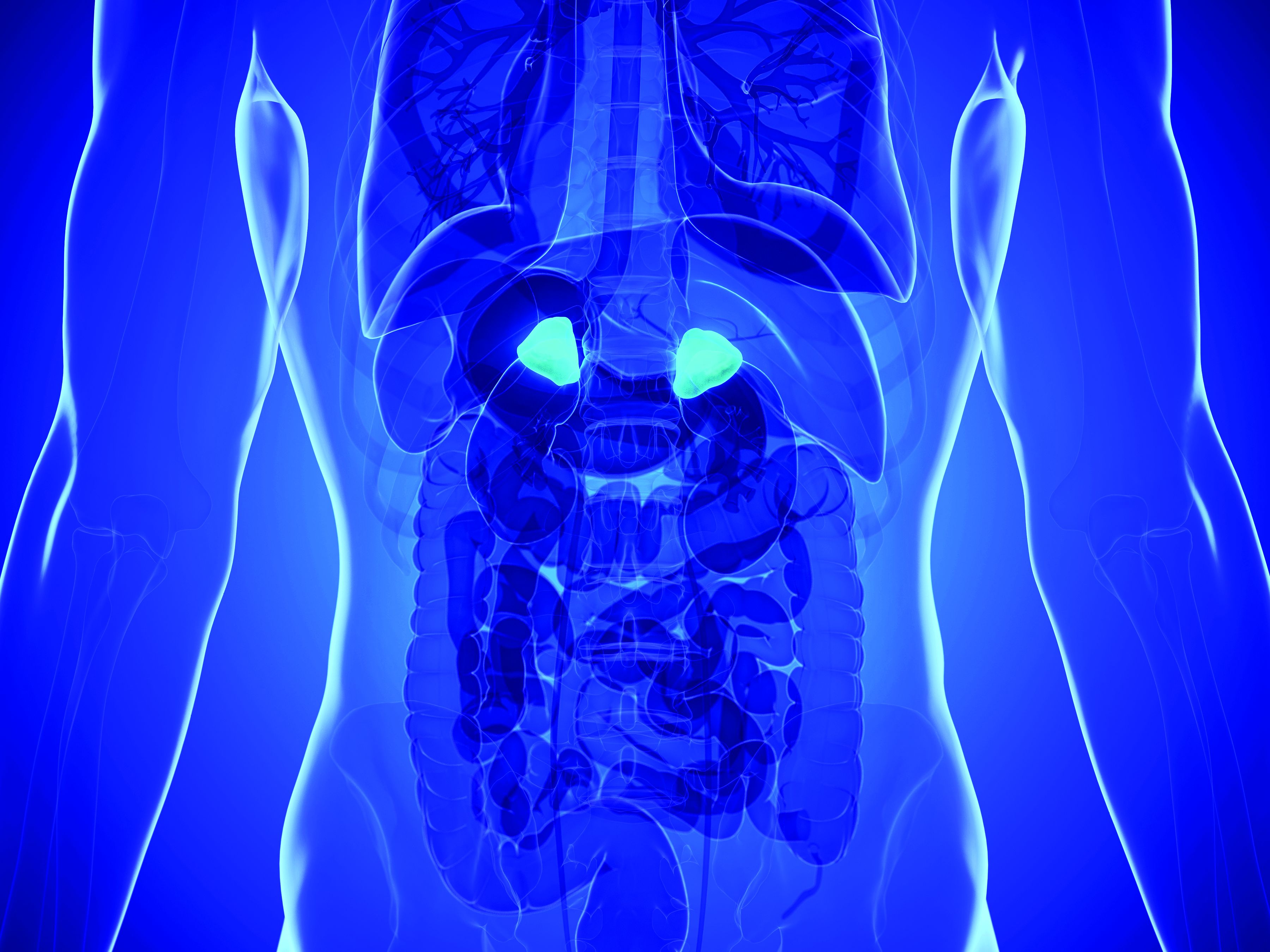
Adrenal vein sampling looms as choke point for aldosteronism assessment of hypertensives
At a time when new evidence strongly suggests that roughly a fifth of patents with hypertension have primary aldosteronism as the cause, other recent findings suggest that many of these possibly tens of millions of patients with aldosterone-driven high blood pressure may as a consequence need an expensive and not-widely-available diagnostic test – adrenal vein sampling – to determine whether they are candidates for a definitive surgical cure to their aldosteronism.
Some endocrinologists worry the worldwide infrastructure for running adrenal vein sampling (AVS) isn’t close to being in place to deliver on this looming need for patients with primary aldosteronism (PA), especially given the burgeoning numbers now being cited for PA prevalence.
“The system could be overwhelmed,” warned Robert M. Carey, MD, a cardiovascular endocrinologist and professor of medicine at the University of Virginia in Charlottesville. “Right now, adrenal vein sampling [AVS] is the gold standard,” for distinguishing unilateral and bilateral excess aldosterone secretion, “but not every radiologist can do AVS. Until we find a surrogate biomarker that can distinguish unilateral and bilateral PA” many patients will need AVS, Dr. Carey said in an interview.
“AVS is important for accurate lateralization of aldosterone excess in patients, but it may not be feasible for all patients with PA to undergo AVS. If the prevalence of PA truly is on the order of 15% [of all patients with hypertension] then health systems would be stretched to offer all of them AVS, which is technically challenging and requires dedicated training and is therefore limited to expert centers,” commented Jun Yang, MBBS, a cardiovascular endocrinologist at the Hudson Institute of Medical Research and a hypertension researcher at Monash University, both in Melbourne. “At Monash, our interventional radiologists have increased their [AVS] success rate from 40% to more than 90% during the past 10 years, and our waiting list for patients scheduled for AVS is now 3-4 months long,” Dr. Yang said in an interview.
Finding a unilateral adrenal nodule as the cause of PA means that surgical removal is an option, a step that often fully resolves the PA and normalizes blood pressure. Patients with a bilateral source of the aldosterone are not candidates for surgical cure and must be managed with medical treatment, usually a mineralocorticoid receptor antagonist such as spironolactone that can neutralize or at least reduce the impact of hyperaldosteronism.
AVS finds unilateral adenomas when imaging can’t
The evidence that raised concerns about the reliability of imaging as an easier and noninvasive means to identify hypertensive patients with PA and a unilateral adrenal nodule that makes them candidates for surgical removal to resolve their PA and hypertension came out in May 2020 in a review of 174 PA patients who underwent AVS at a single center in Calgary, Alta., during 2006-2018.
The review included 366 patients with PA referred to the University of Calgary for assessment, of whom 179 had no adrenal nodule visible with either CT or MRI imaging, with 174 of these patients also undergoing successful AVS. The procedure revealed 70 patients (40%) had unilateral aldosterone secretion (Can J Cardiol. 2020 May 16. doi: 10.1016/j.cjca.2020.05.013).
In an editorial about this report that appeared a few weeks later, Ross D. Feldman, MD, a hypertension-management researcher and professor of medicine at the University of Manitoba in Winnipeg, Man., said the finding was “amazing,” and “confirms that lateralization of aldosterone secretion in a patient with PA but without an identifiable mass on that side is not a zebra,” but instead a presentation that “occurs in almost half of patients with PA and no discernible adenoma on the side that lateralizes.” (Can J. Cardiol. 2020 Jul 3. doi: 10.1016/j.cjca.2020.06.022).
Although this was just one center’s experience, the authors are not alone in making this finding, although prior reports seem to have been largely forgotten or ignored until now.
“The discordance between AVS and adrenal imaging has been documented by numerous groups, and in our own experience [in Melbourne] around 40% of patients with unilateral aldosterone excess do not have a distinct unilateral adenoma on CT,” said Dr. Yang.
“Here’s the problem,” summed up Dr. Feldman in an interview. “Nearly half of patients with hyperaldosteronism don’t localize based on a CT or MRI, so you have to do AVS, but AVS is not generally available; it’s only at tertiary centers; and you have to do a lot of them,” to do them well. “It’s a half-day procedure, and you have to hit the correct adrenal vein.”
AVS for millions?
Compounding the challenge is the other bit of bombshell news recently dropped on the endocrinology and hypertension communities: PA may be much more prevalent that previously suspected, occurring in roughly 20% of patients with hypertension, according to study results that also came out in 2020 (Ann Int Med. 2020 Jul 7;173[1]:10-20).
The upshot, according to Dr. Feldman and others, is that researchers will need to find reliable criteria besides imaging for identifying PA patients with an increased likelihood of having a lateralized source for their excess aldosterone production. That’s “the only hope,” said Dr. Feldman, “so we won’t have to do AVS on 20 million Americans.”
Unfortunately, the path toward a successful screen to winnow down candidates for AVS has been long and not especially fruitful, with efforts dating back at least 50 years, and with one of the most recent efforts at stratifying PA patients by certain laboratory measures getting dismissed as producing a benefit that “might not be substantial,” wrote Michael Stowasser, MBBS, in a published commentary (J Hypertension. 2020 Jul;38[7]:1259-61).
In contrast to Dr. Feldman, Dr. Stowasser was more optimistic about the prospects for avoiding an immediate crisis in AVS assessment of PA patients, mostly because so few patients with PA are now identified by clinicians. Given the poor record clinicians have historically rung up diagnosing PA, “it would seem unlikely that we are going to be flooded with AVS requests any time soon,” he wrote. There is also reason to hope that increased demand for AVS will help broaden availability, and innovative testing methods promise to speed up the procedure, said Dr. Stowasser, a professor of medicine at the University of Queensland in Brisbane, Australia and director of the Endocrine Hypertension Research Centre at Greenslopes and Princess Alexandra Hospitals in Brisbane, in an interview.
But regardless of whether AVS testing becomes more available or streamlined, recent events suggest there will be little way to avoid eventually having to run millions of these diagnostic procedures.
Patients with PA “who decide they will not want surgery do not need AVS. For all other patients with PA, you need AVS. The medical system will just have to respond,” Dr. Carey concluded.
Dr. Carey, Dr. Yang, Dr. Feldman, and Dr. Stowasser had no relevant disclosures.
At a time when new evidence strongly suggests that roughly a fifth of patents with hypertension have primary aldosteronism as the cause, other recent findings suggest that many of these possibly tens of millions of patients with aldosterone-driven high blood pressure may as a consequence need an expensive and not-widely-available diagnostic test – adrenal vein sampling – to determine whether they are candidates for a definitive surgical cure to their aldosteronism.
Some endocrinologists worry the worldwide infrastructure for running adrenal vein sampling (AVS) isn’t close to being in place to deliver on this looming need for patients with primary aldosteronism (PA), especially given the burgeoning numbers now being cited for PA prevalence.
“The system could be overwhelmed,” warned Robert M. Carey, MD, a cardiovascular endocrinologist and professor of medicine at the University of Virginia in Charlottesville. “Right now, adrenal vein sampling [AVS] is the gold standard,” for distinguishing unilateral and bilateral excess aldosterone secretion, “but not every radiologist can do AVS. Until we find a surrogate biomarker that can distinguish unilateral and bilateral PA” many patients will need AVS, Dr. Carey said in an interview.
“AVS is important for accurate lateralization of aldosterone excess in patients, but it may not be feasible for all patients with PA to undergo AVS. If the prevalence of PA truly is on the order of 15% [of all patients with hypertension] then health systems would be stretched to offer all of them AVS, which is technically challenging and requires dedicated training and is therefore limited to expert centers,” commented Jun Yang, MBBS, a cardiovascular endocrinologist at the Hudson Institute of Medical Research and a hypertension researcher at Monash University, both in Melbourne. “At Monash, our interventional radiologists have increased their [AVS] success rate from 40% to more than 90% during the past 10 years, and our waiting list for patients scheduled for AVS is now 3-4 months long,” Dr. Yang said in an interview.
Finding a unilateral adrenal nodule as the cause of PA means that surgical removal is an option, a step that often fully resolves the PA and normalizes blood pressure. Patients with a bilateral source of the aldosterone are not candidates for surgical cure and must be managed with medical treatment, usually a mineralocorticoid receptor antagonist such as spironolactone that can neutralize or at least reduce the impact of hyperaldosteronism.
AVS finds unilateral adenomas when imaging can’t
The evidence that raised concerns about the reliability of imaging as an easier and noninvasive means to identify hypertensive patients with PA and a unilateral adrenal nodule that makes them candidates for surgical removal to resolve their PA and hypertension came out in May 2020 in a review of 174 PA patients who underwent AVS at a single center in Calgary, Alta., during 2006-2018.
The review included 366 patients with PA referred to the University of Calgary for assessment, of whom 179 had no adrenal nodule visible with either CT or MRI imaging, with 174 of these patients also undergoing successful AVS. The procedure revealed 70 patients (40%) had unilateral aldosterone secretion (Can J Cardiol. 2020 May 16. doi: 10.1016/j.cjca.2020.05.013).
In an editorial about this report that appeared a few weeks later, Ross D. Feldman, MD, a hypertension-management researcher and professor of medicine at the University of Manitoba in Winnipeg, Man., said the finding was “amazing,” and “confirms that lateralization of aldosterone secretion in a patient with PA but without an identifiable mass on that side is not a zebra,” but instead a presentation that “occurs in almost half of patients with PA and no discernible adenoma on the side that lateralizes.” (Can J. Cardiol. 2020 Jul 3. doi: 10.1016/j.cjca.2020.06.022).
Although this was just one center’s experience, the authors are not alone in making this finding, although prior reports seem to have been largely forgotten or ignored until now.
“The discordance between AVS and adrenal imaging has been documented by numerous groups, and in our own experience [in Melbourne] around 40% of patients with unilateral aldosterone excess do not have a distinct unilateral adenoma on CT,” said Dr. Yang.
“Here’s the problem,” summed up Dr. Feldman in an interview. “Nearly half of patients with hyperaldosteronism don’t localize based on a CT or MRI, so you have to do AVS, but AVS is not generally available; it’s only at tertiary centers; and you have to do a lot of them,” to do them well. “It’s a half-day procedure, and you have to hit the correct adrenal vein.”
AVS for millions?
Compounding the challenge is the other bit of bombshell news recently dropped on the endocrinology and hypertension communities: PA may be much more prevalent that previously suspected, occurring in roughly 20% of patients with hypertension, according to study results that also came out in 2020 (Ann Int Med. 2020 Jul 7;173[1]:10-20).
The upshot, according to Dr. Feldman and others, is that researchers will need to find reliable criteria besides imaging for identifying PA patients with an increased likelihood of having a lateralized source for their excess aldosterone production. That’s “the only hope,” said Dr. Feldman, “so we won’t have to do AVS on 20 million Americans.”
Unfortunately, the path toward a successful screen to winnow down candidates for AVS has been long and not especially fruitful, with efforts dating back at least 50 years, and with one of the most recent efforts at stratifying PA patients by certain laboratory measures getting dismissed as producing a benefit that “might not be substantial,” wrote Michael Stowasser, MBBS, in a published commentary (J Hypertension. 2020 Jul;38[7]:1259-61).
In contrast to Dr. Feldman, Dr. Stowasser was more optimistic about the prospects for avoiding an immediate crisis in AVS assessment of PA patients, mostly because so few patients with PA are now identified by clinicians. Given the poor record clinicians have historically rung up diagnosing PA, “it would seem unlikely that we are going to be flooded with AVS requests any time soon,” he wrote. There is also reason to hope that increased demand for AVS will help broaden availability, and innovative testing methods promise to speed up the procedure, said Dr. Stowasser, a professor of medicine at the University of Queensland in Brisbane, Australia and director of the Endocrine Hypertension Research Centre at Greenslopes and Princess Alexandra Hospitals in Brisbane, in an interview.
But regardless of whether AVS testing becomes more available or streamlined, recent events suggest there will be little way to avoid eventually having to run millions of these diagnostic procedures.
Patients with PA “who decide they will not want surgery do not need AVS. For all other patients with PA, you need AVS. The medical system will just have to respond,” Dr. Carey concluded.
Dr. Carey, Dr. Yang, Dr. Feldman, and Dr. Stowasser had no relevant disclosures.
At a time when new evidence strongly suggests that roughly a fifth of patents with hypertension have primary aldosteronism as the cause, other recent findings suggest that many of these possibly tens of millions of patients with aldosterone-driven high blood pressure may as a consequence need an expensive and not-widely-available diagnostic test – adrenal vein sampling – to determine whether they are candidates for a definitive surgical cure to their aldosteronism.
Some endocrinologists worry the worldwide infrastructure for running adrenal vein sampling (AVS) isn’t close to being in place to deliver on this looming need for patients with primary aldosteronism (PA), especially given the burgeoning numbers now being cited for PA prevalence.
“The system could be overwhelmed,” warned Robert M. Carey, MD, a cardiovascular endocrinologist and professor of medicine at the University of Virginia in Charlottesville. “Right now, adrenal vein sampling [AVS] is the gold standard,” for distinguishing unilateral and bilateral excess aldosterone secretion, “but not every radiologist can do AVS. Until we find a surrogate biomarker that can distinguish unilateral and bilateral PA” many patients will need AVS, Dr. Carey said in an interview.
“AVS is important for accurate lateralization of aldosterone excess in patients, but it may not be feasible for all patients with PA to undergo AVS. If the prevalence of PA truly is on the order of 15% [of all patients with hypertension] then health systems would be stretched to offer all of them AVS, which is technically challenging and requires dedicated training and is therefore limited to expert centers,” commented Jun Yang, MBBS, a cardiovascular endocrinologist at the Hudson Institute of Medical Research and a hypertension researcher at Monash University, both in Melbourne. “At Monash, our interventional radiologists have increased their [AVS] success rate from 40% to more than 90% during the past 10 years, and our waiting list for patients scheduled for AVS is now 3-4 months long,” Dr. Yang said in an interview.
Finding a unilateral adrenal nodule as the cause of PA means that surgical removal is an option, a step that often fully resolves the PA and normalizes blood pressure. Patients with a bilateral source of the aldosterone are not candidates for surgical cure and must be managed with medical treatment, usually a mineralocorticoid receptor antagonist such as spironolactone that can neutralize or at least reduce the impact of hyperaldosteronism.
AVS finds unilateral adenomas when imaging can’t
The evidence that raised concerns about the reliability of imaging as an easier and noninvasive means to identify hypertensive patients with PA and a unilateral adrenal nodule that makes them candidates for surgical removal to resolve their PA and hypertension came out in May 2020 in a review of 174 PA patients who underwent AVS at a single center in Calgary, Alta., during 2006-2018.
The review included 366 patients with PA referred to the University of Calgary for assessment, of whom 179 had no adrenal nodule visible with either CT or MRI imaging, with 174 of these patients also undergoing successful AVS. The procedure revealed 70 patients (40%) had unilateral aldosterone secretion (Can J Cardiol. 2020 May 16. doi: 10.1016/j.cjca.2020.05.013).
In an editorial about this report that appeared a few weeks later, Ross D. Feldman, MD, a hypertension-management researcher and professor of medicine at the University of Manitoba in Winnipeg, Man., said the finding was “amazing,” and “confirms that lateralization of aldosterone secretion in a patient with PA but without an identifiable mass on that side is not a zebra,” but instead a presentation that “occurs in almost half of patients with PA and no discernible adenoma on the side that lateralizes.” (Can J. Cardiol. 2020 Jul 3. doi: 10.1016/j.cjca.2020.06.022).
Although this was just one center’s experience, the authors are not alone in making this finding, although prior reports seem to have been largely forgotten or ignored until now.
“The discordance between AVS and adrenal imaging has been documented by numerous groups, and in our own experience [in Melbourne] around 40% of patients with unilateral aldosterone excess do not have a distinct unilateral adenoma on CT,” said Dr. Yang.
“Here’s the problem,” summed up Dr. Feldman in an interview. “Nearly half of patients with hyperaldosteronism don’t localize based on a CT or MRI, so you have to do AVS, but AVS is not generally available; it’s only at tertiary centers; and you have to do a lot of them,” to do them well. “It’s a half-day procedure, and you have to hit the correct adrenal vein.”
AVS for millions?
Compounding the challenge is the other bit of bombshell news recently dropped on the endocrinology and hypertension communities: PA may be much more prevalent that previously suspected, occurring in roughly 20% of patients with hypertension, according to study results that also came out in 2020 (Ann Int Med. 2020 Jul 7;173[1]:10-20).
The upshot, according to Dr. Feldman and others, is that researchers will need to find reliable criteria besides imaging for identifying PA patients with an increased likelihood of having a lateralized source for their excess aldosterone production. That’s “the only hope,” said Dr. Feldman, “so we won’t have to do AVS on 20 million Americans.”
Unfortunately, the path toward a successful screen to winnow down candidates for AVS has been long and not especially fruitful, with efforts dating back at least 50 years, and with one of the most recent efforts at stratifying PA patients by certain laboratory measures getting dismissed as producing a benefit that “might not be substantial,” wrote Michael Stowasser, MBBS, in a published commentary (J Hypertension. 2020 Jul;38[7]:1259-61).
In contrast to Dr. Feldman, Dr. Stowasser was more optimistic about the prospects for avoiding an immediate crisis in AVS assessment of PA patients, mostly because so few patients with PA are now identified by clinicians. Given the poor record clinicians have historically rung up diagnosing PA, “it would seem unlikely that we are going to be flooded with AVS requests any time soon,” he wrote. There is also reason to hope that increased demand for AVS will help broaden availability, and innovative testing methods promise to speed up the procedure, said Dr. Stowasser, a professor of medicine at the University of Queensland in Brisbane, Australia and director of the Endocrine Hypertension Research Centre at Greenslopes and Princess Alexandra Hospitals in Brisbane, in an interview.
But regardless of whether AVS testing becomes more available or streamlined, recent events suggest there will be little way to avoid eventually having to run millions of these diagnostic procedures.
Patients with PA “who decide they will not want surgery do not need AVS. For all other patients with PA, you need AVS. The medical system will just have to respond,” Dr. Carey concluded.
Dr. Carey, Dr. Yang, Dr. Feldman, and Dr. Stowasser had no relevant disclosures.
Antibiotics or appendectomy? Both good options
Patients given antibiotics for appendicitis fared no worse in quality of life, at least in the short term, than did patients whose appendix was removed, according to a large, randomized, nonblinded, noninferiority study published online Oct. 5 in The New England Journal of Medicine.
One expert says the body of data, including this trial, indicates that the best appendicitis treatment now comes down to individual patients and choice.
David Flum, MD, director of the Surgical Outcomes Research Center at the University of Washington in Seattle, and colleagues conducted the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial, which compared a 10-day course of antibiotics with appendectomy for patients with appendicitis at 25 US centers.
Although some may interpret the study as praising the potential role of antibiotics, the author of an accompanying editorial warns against rushing to antibiotics, even during a pandemic when hospital resources may be strained.
In the study of 1552 adults (414 with an appendicolith), 776 were randomly assigned to the antibiotics group and 776 to appendectomy (96% of whom underwent a laparoscopic procedure).
After 30 days, antibiotics were found to be noninferior to appendectomy, the standard of treatment for 120 years, as determined on the basis of 30-day scores for the European Quality of Life–5 Dimensions (EQ-5D) questionnaire (mean difference, 0.01 points; 95% CI, −0.001 to 0.03).
EQ-5D at 30 days was chosen as the primary endpoint because it has been validated as an overall measure of health after appendicitis treatment and the 30-day time frame mimics the typical recovery period for appendectomy, Flum and colleagues explain.
Some results favored appendectomy
However, editorialist Danny Jacobs, MD, MPH, president of Oregon Health and Science University in Portland, points out that about a third (29%) of the patients in the antibiotics group had undergone appendectomy by 90 days.
Appendicolith, a well-established potential complication, he acknowledges, was the main driver of the need for surgery (41% with that complication needed appendectomy), but it was not the sole reason.
Complications were more common in the antibiotics group than in the appendectomy group (8.1 vs 3.5 per 100 participants; rate ratio, 2.28; 95% CI, 1.30 – 3.98). The rate of serious adverse events was 4.0 per 100 participants in the antibiotics group and 3.0 per 100 participants in the appendectomy group (rate ratio, 1.29; 95% CI, 0.67 – 2.50). Additionally, the number of emergency department visits was nearly three times higher in the antibiotics group, and more time was spent in the hospital by that group, Jacobs points out.
He notes that the article mentions circumstances such as the COVID-19 pandemic may figure into consideration when weighing antibiotics against appendectomy. But he warns that there also may be a danger of treatment bias in vulnerable populations and that COVID-19 has highlighted disparities in care overall.
“It will be important to ensure that some people, in particular vulnerable populations, are not offered antibiotic therapy preferentially or without adequate education regarding the longer-term implications,” Jacobs writes.
Flum told Medscape Medical News he agrees with Jacobs that the potential for bias is important.
“We should all be worried that new healthcare options won’t be equally applied,” he said.
But he and his coauthors offer an alternative view of the results of the study.
“In the antibiotics group,” they write, “more than 7 in 10 participants avoided surgery, many were treated on an outpatient basis, and participants and caregivers missed less time at work than with appendectomy.”
Flum said, “[T]hat’s going to be attractive to some patients. Not all, but some.”
Douglas Smink, MD, MPH, chief of surgery at Brigham and Women’s Faulkner Hospital in Boston, told Medscape Medical News that he sees this study as an argument for surgery remaining the go-to option for appendicitis, unless there is a safety reason for not performing the surgery.
Patients come in and want their appendix out immediately, he said, and surgery offers a quick option with short length of stay and few complications.
Additionally, he said, if patients are told that, with antibiotics, “there’s a 1 in 3 chance you’re going to need [an appendectomy] in the next 3 months, I think most people would say, ‘Just take it out then,’ ” he said.
Can research decide which is best?
The controversy has been well studied. But with no clear answer in any of the studies about whether appendectomy or use of antibiotics is better, should the current study put the research to rest?
Flum told Medscape Medical News that this study, which is three times the size of the next-largest study, makes clear “there are choices.”
Previous trials in Europe “did not move the needle” on the issue, he said, “in part because they didn’t include the patients who typically get appendectomies.”
He said their team tried to build on those studies and include “typical patients in typical hospitals with typical appendicitis” and found that both surgery and antibiotics are safe and have advantages and disadvantages, depending on the patient.
Smink says one thing that has been definitively answered with this trial is that patients with appendicolith are “more likely to fail with antibiotics.”
Previous trials have excluded patients with appendicolith, and this one did not.
“That’s something we’ve not really known for sure but we’ve assumed,” he said.
But now, Smink says, he thinks the research on the topic has gone about as far as it can go.
He notes that none of the trials has shown antibiotics to be better than appendectomy. “I have a hard time believing we are going to find anything different if we did another study like this. This is a really well-done one,” he said.
“If the best you can do is show noninferiority, which is where we are with these studies on appendicitis, you’re always going to have both options, which is great for patients and doctors,” he said.
The study was funded by the Patient-Centered Outcomes Research Institute. The original article lists the authors’ relevant financial relationships. Jacobs and Smink reported no such relationships.
This article first appeared on Medscape.com.
Patients given antibiotics for appendicitis fared no worse in quality of life, at least in the short term, than did patients whose appendix was removed, according to a large, randomized, nonblinded, noninferiority study published online Oct. 5 in The New England Journal of Medicine.
One expert says the body of data, including this trial, indicates that the best appendicitis treatment now comes down to individual patients and choice.
David Flum, MD, director of the Surgical Outcomes Research Center at the University of Washington in Seattle, and colleagues conducted the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial, which compared a 10-day course of antibiotics with appendectomy for patients with appendicitis at 25 US centers.
Although some may interpret the study as praising the potential role of antibiotics, the author of an accompanying editorial warns against rushing to antibiotics, even during a pandemic when hospital resources may be strained.
In the study of 1552 adults (414 with an appendicolith), 776 were randomly assigned to the antibiotics group and 776 to appendectomy (96% of whom underwent a laparoscopic procedure).
After 30 days, antibiotics were found to be noninferior to appendectomy, the standard of treatment for 120 years, as determined on the basis of 30-day scores for the European Quality of Life–5 Dimensions (EQ-5D) questionnaire (mean difference, 0.01 points; 95% CI, −0.001 to 0.03).
EQ-5D at 30 days was chosen as the primary endpoint because it has been validated as an overall measure of health after appendicitis treatment and the 30-day time frame mimics the typical recovery period for appendectomy, Flum and colleagues explain.
Some results favored appendectomy
However, editorialist Danny Jacobs, MD, MPH, president of Oregon Health and Science University in Portland, points out that about a third (29%) of the patients in the antibiotics group had undergone appendectomy by 90 days.
Appendicolith, a well-established potential complication, he acknowledges, was the main driver of the need for surgery (41% with that complication needed appendectomy), but it was not the sole reason.
Complications were more common in the antibiotics group than in the appendectomy group (8.1 vs 3.5 per 100 participants; rate ratio, 2.28; 95% CI, 1.30 – 3.98). The rate of serious adverse events was 4.0 per 100 participants in the antibiotics group and 3.0 per 100 participants in the appendectomy group (rate ratio, 1.29; 95% CI, 0.67 – 2.50). Additionally, the number of emergency department visits was nearly three times higher in the antibiotics group, and more time was spent in the hospital by that group, Jacobs points out.
He notes that the article mentions circumstances such as the COVID-19 pandemic may figure into consideration when weighing antibiotics against appendectomy. But he warns that there also may be a danger of treatment bias in vulnerable populations and that COVID-19 has highlighted disparities in care overall.
“It will be important to ensure that some people, in particular vulnerable populations, are not offered antibiotic therapy preferentially or without adequate education regarding the longer-term implications,” Jacobs writes.
Flum told Medscape Medical News he agrees with Jacobs that the potential for bias is important.
“We should all be worried that new healthcare options won’t be equally applied,” he said.
But he and his coauthors offer an alternative view of the results of the study.
“In the antibiotics group,” they write, “more than 7 in 10 participants avoided surgery, many were treated on an outpatient basis, and participants and caregivers missed less time at work than with appendectomy.”
Flum said, “[T]hat’s going to be attractive to some patients. Not all, but some.”
Douglas Smink, MD, MPH, chief of surgery at Brigham and Women’s Faulkner Hospital in Boston, told Medscape Medical News that he sees this study as an argument for surgery remaining the go-to option for appendicitis, unless there is a safety reason for not performing the surgery.
Patients come in and want their appendix out immediately, he said, and surgery offers a quick option with short length of stay and few complications.
Additionally, he said, if patients are told that, with antibiotics, “there’s a 1 in 3 chance you’re going to need [an appendectomy] in the next 3 months, I think most people would say, ‘Just take it out then,’ ” he said.
Can research decide which is best?
The controversy has been well studied. But with no clear answer in any of the studies about whether appendectomy or use of antibiotics is better, should the current study put the research to rest?
Flum told Medscape Medical News that this study, which is three times the size of the next-largest study, makes clear “there are choices.”
Previous trials in Europe “did not move the needle” on the issue, he said, “in part because they didn’t include the patients who typically get appendectomies.”
He said their team tried to build on those studies and include “typical patients in typical hospitals with typical appendicitis” and found that both surgery and antibiotics are safe and have advantages and disadvantages, depending on the patient.
Smink says one thing that has been definitively answered with this trial is that patients with appendicolith are “more likely to fail with antibiotics.”
Previous trials have excluded patients with appendicolith, and this one did not.
“That’s something we’ve not really known for sure but we’ve assumed,” he said.
But now, Smink says, he thinks the research on the topic has gone about as far as it can go.
He notes that none of the trials has shown antibiotics to be better than appendectomy. “I have a hard time believing we are going to find anything different if we did another study like this. This is a really well-done one,” he said.
“If the best you can do is show noninferiority, which is where we are with these studies on appendicitis, you’re always going to have both options, which is great for patients and doctors,” he said.
The study was funded by the Patient-Centered Outcomes Research Institute. The original article lists the authors’ relevant financial relationships. Jacobs and Smink reported no such relationships.
This article first appeared on Medscape.com.
Patients given antibiotics for appendicitis fared no worse in quality of life, at least in the short term, than did patients whose appendix was removed, according to a large, randomized, nonblinded, noninferiority study published online Oct. 5 in The New England Journal of Medicine.
One expert says the body of data, including this trial, indicates that the best appendicitis treatment now comes down to individual patients and choice.
David Flum, MD, director of the Surgical Outcomes Research Center at the University of Washington in Seattle, and colleagues conducted the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial, which compared a 10-day course of antibiotics with appendectomy for patients with appendicitis at 25 US centers.
Although some may interpret the study as praising the potential role of antibiotics, the author of an accompanying editorial warns against rushing to antibiotics, even during a pandemic when hospital resources may be strained.
In the study of 1552 adults (414 with an appendicolith), 776 were randomly assigned to the antibiotics group and 776 to appendectomy (96% of whom underwent a laparoscopic procedure).
After 30 days, antibiotics were found to be noninferior to appendectomy, the standard of treatment for 120 years, as determined on the basis of 30-day scores for the European Quality of Life–5 Dimensions (EQ-5D) questionnaire (mean difference, 0.01 points; 95% CI, −0.001 to 0.03).
EQ-5D at 30 days was chosen as the primary endpoint because it has been validated as an overall measure of health after appendicitis treatment and the 30-day time frame mimics the typical recovery period for appendectomy, Flum and colleagues explain.
Some results favored appendectomy
However, editorialist Danny Jacobs, MD, MPH, president of Oregon Health and Science University in Portland, points out that about a third (29%) of the patients in the antibiotics group had undergone appendectomy by 90 days.
Appendicolith, a well-established potential complication, he acknowledges, was the main driver of the need for surgery (41% with that complication needed appendectomy), but it was not the sole reason.
Complications were more common in the antibiotics group than in the appendectomy group (8.1 vs 3.5 per 100 participants; rate ratio, 2.28; 95% CI, 1.30 – 3.98). The rate of serious adverse events was 4.0 per 100 participants in the antibiotics group and 3.0 per 100 participants in the appendectomy group (rate ratio, 1.29; 95% CI, 0.67 – 2.50). Additionally, the number of emergency department visits was nearly three times higher in the antibiotics group, and more time was spent in the hospital by that group, Jacobs points out.
He notes that the article mentions circumstances such as the COVID-19 pandemic may figure into consideration when weighing antibiotics against appendectomy. But he warns that there also may be a danger of treatment bias in vulnerable populations and that COVID-19 has highlighted disparities in care overall.
“It will be important to ensure that some people, in particular vulnerable populations, are not offered antibiotic therapy preferentially or without adequate education regarding the longer-term implications,” Jacobs writes.
Flum told Medscape Medical News he agrees with Jacobs that the potential for bias is important.
“We should all be worried that new healthcare options won’t be equally applied,” he said.
But he and his coauthors offer an alternative view of the results of the study.
“In the antibiotics group,” they write, “more than 7 in 10 participants avoided surgery, many were treated on an outpatient basis, and participants and caregivers missed less time at work than with appendectomy.”
Flum said, “[T]hat’s going to be attractive to some patients. Not all, but some.”
Douglas Smink, MD, MPH, chief of surgery at Brigham and Women’s Faulkner Hospital in Boston, told Medscape Medical News that he sees this study as an argument for surgery remaining the go-to option for appendicitis, unless there is a safety reason for not performing the surgery.
Patients come in and want their appendix out immediately, he said, and surgery offers a quick option with short length of stay and few complications.
Additionally, he said, if patients are told that, with antibiotics, “there’s a 1 in 3 chance you’re going to need [an appendectomy] in the next 3 months, I think most people would say, ‘Just take it out then,’ ” he said.
Can research decide which is best?
The controversy has been well studied. But with no clear answer in any of the studies about whether appendectomy or use of antibiotics is better, should the current study put the research to rest?
Flum told Medscape Medical News that this study, which is three times the size of the next-largest study, makes clear “there are choices.”
Previous trials in Europe “did not move the needle” on the issue, he said, “in part because they didn’t include the patients who typically get appendectomies.”
He said their team tried to build on those studies and include “typical patients in typical hospitals with typical appendicitis” and found that both surgery and antibiotics are safe and have advantages and disadvantages, depending on the patient.
Smink says one thing that has been definitively answered with this trial is that patients with appendicolith are “more likely to fail with antibiotics.”
Previous trials have excluded patients with appendicolith, and this one did not.
“That’s something we’ve not really known for sure but we’ve assumed,” he said.
But now, Smink says, he thinks the research on the topic has gone about as far as it can go.
He notes that none of the trials has shown antibiotics to be better than appendectomy. “I have a hard time believing we are going to find anything different if we did another study like this. This is a really well-done one,” he said.
“If the best you can do is show noninferiority, which is where we are with these studies on appendicitis, you’re always going to have both options, which is great for patients and doctors,” he said.
The study was funded by the Patient-Centered Outcomes Research Institute. The original article lists the authors’ relevant financial relationships. Jacobs and Smink reported no such relationships.
This article first appeared on Medscape.com.
Diabetes-related amputations on the rise in older adults
The recent resurgence in diabetes-related lower-extremity amputations in the United States is not limited to younger adults, according to the author of a recent study that documents similar increases among an older population of Medicare beneficiaries.
While the rate of amputations fell among these older adults from 2000 to 2009, it increased significantly from 2009 to 2017, albeit at a “less severe rate” than recently reported in younger populations, said study investigator Jessica Harding, PhD.
The rate of nontraumatic lower extremity amputation (NLEA) was ticking upward by more than 1% per year over the 2009-2017 period, according to Dr. Harding, assistant professor in the department of surgery at Emory University, Atlanta.
This latest report follows one from last year, published in Diabetes Care, that documented an annual percentage increase approaching 6% between 2009 and 2015, driven by larger increases among adults 18-64 years of age, as well as an increase among men.
It’s not clear why rates of NLEA would be on the rise among younger and older adults in the United States, Dr. Harding said, though factors she said could be implicated include changes in amputation practice, increased comorbidities, higher insulin costs, or shortcomings in early prevention programs.
“We need large-scale studies with granular data to tease out key risk factors that could help identify the drivers of these increases in amputations,” Dr. Harding said in a presentation at the virtual annual scientific sessions of the American Diabetes Association.
“In the interim, increased attention to preventive foot care across the age spectrum could benefit adults with diabetes,” she added.
Devastating complication in older adults
The latest findings from Dr. Harding and coauthors emphasize the importance of a “team approach” to early prevention in older adults with diabetes, said Derek LeRoith, MD, PhD, director of research in the division of endocrinology, diabetes, and bone diseases with Icahn School of Medicine at Mount Sinai, New York.
“If you take a 75-year-old or even an 80-year-old, their life expectancy can still be a good 10 years or more,” Dr. LeRoith said in an interview. “We shouldn’t give up on them – we should be treating them to prevent complications.”
Lower-extremity amputation is a “particularly devastating” complication that can compromise mobility, ability to exercise, and motivation, according to Dr. LeRoith, lead author of a recent Endocrine Society clinical practice guideline that urges referral of older adults with diabetes to a podiatrist, orthopedist, or vascular specialist for preventive care.
“Quite often, treating their glucose or high blood pressure will be much more difficult because of these changes,” he said.
Lower extremity amputation trends upward
Rates of NLEA declined for years, only to rebound by 50%, according to authors of a recent analysis of Nationwide Inpatient Sample (NIS) data reported last year. In their report, the age-standardized diabetes-related NLEA rate per 1,000 adults with diabetes went from 5.30 in 2000, down to 3.07 in 2009/2010, and back up to 4.62 by 2015 (Diabetes Care. 2019 Jan;42:50-4).
The resurgence was fueled mainly by an increased rate of amputations in younger and middle-aged adults and men, and through increases in minor amputations, notably the toe, according to the investigators. “These changes in trend are concerning because of the disabling and costly consequences of NLEAs as well as what they may mean for the direction of efforts to reduce diabetes-related complications,” authors of that report said at the time.
In the current study, Dr. Harding and colleagues included Medicare Parts A and B claims data for beneficiaries enrolled from 2000 to 2017. There were 4.6 million Medicare fee-for-service beneficiaries with diabetes in 2000, increasing to 6.9 million in 2017, she reported at the virtual ADA meeting.
Rates of NLEA followed a trajectory similar to what was seen in the earlier NIS report, falling from 8.5 per 1,000 persons in 2000 to 4.4 in 2009, for an annual percentage change of –7.9 (P < .001), Dr. Harding said. Then rates ticked upward again, to 4.8 in 2017, for an annual percentage change of 1.2 over that later period (P < .001).
While the trend was similar for most subgroups analyzed, the absolute rates were highest among men and black individuals in this older patient population, reported Dr. Harding and coauthors.
Dr. Harding said she and coauthors had no disclosures related to the research, which was performed as a collaboration between Emory University and the Centers for Disease Control and Prevention Division of Diabetes Translation.
SOURCE: Harding J. ADA 2020, Abstract 106-OR.
The recent resurgence in diabetes-related lower-extremity amputations in the United States is not limited to younger adults, according to the author of a recent study that documents similar increases among an older population of Medicare beneficiaries.
While the rate of amputations fell among these older adults from 2000 to 2009, it increased significantly from 2009 to 2017, albeit at a “less severe rate” than recently reported in younger populations, said study investigator Jessica Harding, PhD.
The rate of nontraumatic lower extremity amputation (NLEA) was ticking upward by more than 1% per year over the 2009-2017 period, according to Dr. Harding, assistant professor in the department of surgery at Emory University, Atlanta.
This latest report follows one from last year, published in Diabetes Care, that documented an annual percentage increase approaching 6% between 2009 and 2015, driven by larger increases among adults 18-64 years of age, as well as an increase among men.
It’s not clear why rates of NLEA would be on the rise among younger and older adults in the United States, Dr. Harding said, though factors she said could be implicated include changes in amputation practice, increased comorbidities, higher insulin costs, or shortcomings in early prevention programs.
“We need large-scale studies with granular data to tease out key risk factors that could help identify the drivers of these increases in amputations,” Dr. Harding said in a presentation at the virtual annual scientific sessions of the American Diabetes Association.
“In the interim, increased attention to preventive foot care across the age spectrum could benefit adults with diabetes,” she added.
Devastating complication in older adults
The latest findings from Dr. Harding and coauthors emphasize the importance of a “team approach” to early prevention in older adults with diabetes, said Derek LeRoith, MD, PhD, director of research in the division of endocrinology, diabetes, and bone diseases with Icahn School of Medicine at Mount Sinai, New York.
“If you take a 75-year-old or even an 80-year-old, their life expectancy can still be a good 10 years or more,” Dr. LeRoith said in an interview. “We shouldn’t give up on them – we should be treating them to prevent complications.”
Lower-extremity amputation is a “particularly devastating” complication that can compromise mobility, ability to exercise, and motivation, according to Dr. LeRoith, lead author of a recent Endocrine Society clinical practice guideline that urges referral of older adults with diabetes to a podiatrist, orthopedist, or vascular specialist for preventive care.
“Quite often, treating their glucose or high blood pressure will be much more difficult because of these changes,” he said.
Lower extremity amputation trends upward
Rates of NLEA declined for years, only to rebound by 50%, according to authors of a recent analysis of Nationwide Inpatient Sample (NIS) data reported last year. In their report, the age-standardized diabetes-related NLEA rate per 1,000 adults with diabetes went from 5.30 in 2000, down to 3.07 in 2009/2010, and back up to 4.62 by 2015 (Diabetes Care. 2019 Jan;42:50-4).
The resurgence was fueled mainly by an increased rate of amputations in younger and middle-aged adults and men, and through increases in minor amputations, notably the toe, according to the investigators. “These changes in trend are concerning because of the disabling and costly consequences of NLEAs as well as what they may mean for the direction of efforts to reduce diabetes-related complications,” authors of that report said at the time.
In the current study, Dr. Harding and colleagues included Medicare Parts A and B claims data for beneficiaries enrolled from 2000 to 2017. There were 4.6 million Medicare fee-for-service beneficiaries with diabetes in 2000, increasing to 6.9 million in 2017, she reported at the virtual ADA meeting.
Rates of NLEA followed a trajectory similar to what was seen in the earlier NIS report, falling from 8.5 per 1,000 persons in 2000 to 4.4 in 2009, for an annual percentage change of –7.9 (P < .001), Dr. Harding said. Then rates ticked upward again, to 4.8 in 2017, for an annual percentage change of 1.2 over that later period (P < .001).
While the trend was similar for most subgroups analyzed, the absolute rates were highest among men and black individuals in this older patient population, reported Dr. Harding and coauthors.
Dr. Harding said she and coauthors had no disclosures related to the research, which was performed as a collaboration between Emory University and the Centers for Disease Control and Prevention Division of Diabetes Translation.
SOURCE: Harding J. ADA 2020, Abstract 106-OR.
The recent resurgence in diabetes-related lower-extremity amputations in the United States is not limited to younger adults, according to the author of a recent study that documents similar increases among an older population of Medicare beneficiaries.
While the rate of amputations fell among these older adults from 2000 to 2009, it increased significantly from 2009 to 2017, albeit at a “less severe rate” than recently reported in younger populations, said study investigator Jessica Harding, PhD.
The rate of nontraumatic lower extremity amputation (NLEA) was ticking upward by more than 1% per year over the 2009-2017 period, according to Dr. Harding, assistant professor in the department of surgery at Emory University, Atlanta.
This latest report follows one from last year, published in Diabetes Care, that documented an annual percentage increase approaching 6% between 2009 and 2015, driven by larger increases among adults 18-64 years of age, as well as an increase among men.
It’s not clear why rates of NLEA would be on the rise among younger and older adults in the United States, Dr. Harding said, though factors she said could be implicated include changes in amputation practice, increased comorbidities, higher insulin costs, or shortcomings in early prevention programs.
“We need large-scale studies with granular data to tease out key risk factors that could help identify the drivers of these increases in amputations,” Dr. Harding said in a presentation at the virtual annual scientific sessions of the American Diabetes Association.
“In the interim, increased attention to preventive foot care across the age spectrum could benefit adults with diabetes,” she added.
Devastating complication in older adults
The latest findings from Dr. Harding and coauthors emphasize the importance of a “team approach” to early prevention in older adults with diabetes, said Derek LeRoith, MD, PhD, director of research in the division of endocrinology, diabetes, and bone diseases with Icahn School of Medicine at Mount Sinai, New York.
“If you take a 75-year-old or even an 80-year-old, their life expectancy can still be a good 10 years or more,” Dr. LeRoith said in an interview. “We shouldn’t give up on them – we should be treating them to prevent complications.”
Lower-extremity amputation is a “particularly devastating” complication that can compromise mobility, ability to exercise, and motivation, according to Dr. LeRoith, lead author of a recent Endocrine Society clinical practice guideline that urges referral of older adults with diabetes to a podiatrist, orthopedist, or vascular specialist for preventive care.
“Quite often, treating their glucose or high blood pressure will be much more difficult because of these changes,” he said.
Lower extremity amputation trends upward
Rates of NLEA declined for years, only to rebound by 50%, according to authors of a recent analysis of Nationwide Inpatient Sample (NIS) data reported last year. In their report, the age-standardized diabetes-related NLEA rate per 1,000 adults with diabetes went from 5.30 in 2000, down to 3.07 in 2009/2010, and back up to 4.62 by 2015 (Diabetes Care. 2019 Jan;42:50-4).
The resurgence was fueled mainly by an increased rate of amputations in younger and middle-aged adults and men, and through increases in minor amputations, notably the toe, according to the investigators. “These changes in trend are concerning because of the disabling and costly consequences of NLEAs as well as what they may mean for the direction of efforts to reduce diabetes-related complications,” authors of that report said at the time.
In the current study, Dr. Harding and colleagues included Medicare Parts A and B claims data for beneficiaries enrolled from 2000 to 2017. There were 4.6 million Medicare fee-for-service beneficiaries with diabetes in 2000, increasing to 6.9 million in 2017, she reported at the virtual ADA meeting.
Rates of NLEA followed a trajectory similar to what was seen in the earlier NIS report, falling from 8.5 per 1,000 persons in 2000 to 4.4 in 2009, for an annual percentage change of –7.9 (P < .001), Dr. Harding said. Then rates ticked upward again, to 4.8 in 2017, for an annual percentage change of 1.2 over that later period (P < .001).
While the trend was similar for most subgroups analyzed, the absolute rates were highest among men and black individuals in this older patient population, reported Dr. Harding and coauthors.
Dr. Harding said she and coauthors had no disclosures related to the research, which was performed as a collaboration between Emory University and the Centers for Disease Control and Prevention Division of Diabetes Translation.
SOURCE: Harding J. ADA 2020, Abstract 106-OR.
FROM ADA 2020
Metformin use linked to improved surgery outcomes
Patients with type 2 diabetes who take metformin may have lower risk-adjusted mortality and readmission rates after surgery than do those who don’t take metformin, findings from a large retrospective cohort study suggest.
Of 10,088 individuals with diabetes who underwent a major surgery requiring hospital admission between January 1, 2010, and January 1, 2016, a total of 5,962 (59%) had received a prescription for metformin in the 180 days before surgery, and 5,460 of those patients were propensity score–matched to controls who did not receive a metformin prescription.
The study participants had a mean age of 67.7 years and underwent surgery requiring general anesthesia and postoperative admission at any of 15 hospitals in a single Pennsylvania health system. In addition to being prescribed metformin within 180 days before surgery, they also had metformin on their list of active medications at their most recent preoperative encounter before the surgery. The were followed until December 18, 2018.
In all, the 90-day and 5-year mortality hazards were reduced by 28% and 26%, respectively, in the metformin prescription recipients, compared with the propensity score–matched controls (hazard ratios, 0.72 and 0.74, respectively), Katherine M. Reitz, MD, and colleagues at the University of Pittsburgh reported in JAMA Surgery.
The readmission hazard – with mortality as a competing risk – was reduced by 16% at 30 days and 14% at 90 days (sub-HRs, 0.84 and 0.86, respectively), the researchers found.
“Hospital readmissions among those with preoperative metformin prescriptions were observed by postdischarge days 30 and 90 (304 [11%] and 538 [20.1%], respectively), whereas among those without prescriptions, 361 readmissions (13%) occurred by day 30 and 614 (23%) by day 90,” they wrote.
The investigators also noted that inflammation was reduced in patients who received a metformin prescription, compared with those who did not (mean preoperative neutrophil to leukocyte ratio, 4.5 vs. 5.0, respectively).
“In the full cohort, multivariable regression analysis similarly demonstrated that metformin was associated with a reduced hazard for both 90-day and 5-year mortality (adjusted HRs, 0.77 and 0.80, respectively) and for 30-day and 90-day readmission (aHR, 0.83 and 0.86), with mortality as a competing risk,” they added.
The findings support those from previous studies showing a decrease in all-cause mortality among diabetes patients taking metformin, said the researchers, noting that those patients had fewer age-related chronic diseases.
“These associations may reflect the anti-aging properties of metformin against the onset of disease or diabetes-associated complications. This study extends these finding by demonstrating that preoperative metformin prescriptions were associated with a reduction in postoperative mortality and readmission, a surrogate for postoperative complications, and with long-term mortality,” they wrote.
The study was limited by a number of factors, such as the potential for residual confounding inherent in retrospective analyses and a lack of adequate power to evaluate the association between metformin use and outcomes for individual surgical procedures. But the authors added that the findings are of note, because adults with comorbidities, such as diabetes, have less physiological reserve and an increased postoperative mortality and readmission rate. The results, therefore, warrant investigation with a prospective randomized clinical trial, they concluded.
In an accompanying editorial, Elizabeth L. George, MD, and Sherry M. Wren, MD, of Stanford (Calif.) University wrote that the study “demonstrates how variables, besides coexisting medical diseases, can affect surgical outcomes.”
“Metformin now joins beta-blockers, statins, and immunonutrition as preoperative agents associated with improved surgical outcomes,” they wrote, adding that future studies should factor in statin use and whether those and other medications should be continued postoperatively because metformin is often held after surgery owing to concerns about contrast agent interactions, whereas statin continuation is recommended.
Future studies of metformin in this setting should exclude patients who are taking statins, or look at possible interactions between the two agents, they said, adding that they would be “interested in seeing a subanalysis of this data set that excludes patients who were prescribed statins.”
“Those data would further solidify the role of metformin as a possible modifiable perioperative factor,” they wrote.
The study was funded by the University of Pittsburgh Medical Center and by grants from the National Heart, Lung, and Blood Institute and the National Institutes of Health. Dr. Reitz reported having no disclosures. Dr. George and Dr. Wren also reported having no disclosures.
SOURCE: Reitz K et al. JAMA Surg. 2020 Apr 8. doi: 10.1001/jamasurg.2020.0416.
Patients with type 2 diabetes who take metformin may have lower risk-adjusted mortality and readmission rates after surgery than do those who don’t take metformin, findings from a large retrospective cohort study suggest.
Of 10,088 individuals with diabetes who underwent a major surgery requiring hospital admission between January 1, 2010, and January 1, 2016, a total of 5,962 (59%) had received a prescription for metformin in the 180 days before surgery, and 5,460 of those patients were propensity score–matched to controls who did not receive a metformin prescription.
The study participants had a mean age of 67.7 years and underwent surgery requiring general anesthesia and postoperative admission at any of 15 hospitals in a single Pennsylvania health system. In addition to being prescribed metformin within 180 days before surgery, they also had metformin on their list of active medications at their most recent preoperative encounter before the surgery. The were followed until December 18, 2018.
In all, the 90-day and 5-year mortality hazards were reduced by 28% and 26%, respectively, in the metformin prescription recipients, compared with the propensity score–matched controls (hazard ratios, 0.72 and 0.74, respectively), Katherine M. Reitz, MD, and colleagues at the University of Pittsburgh reported in JAMA Surgery.
The readmission hazard – with mortality as a competing risk – was reduced by 16% at 30 days and 14% at 90 days (sub-HRs, 0.84 and 0.86, respectively), the researchers found.
“Hospital readmissions among those with preoperative metformin prescriptions were observed by postdischarge days 30 and 90 (304 [11%] and 538 [20.1%], respectively), whereas among those without prescriptions, 361 readmissions (13%) occurred by day 30 and 614 (23%) by day 90,” they wrote.
The investigators also noted that inflammation was reduced in patients who received a metformin prescription, compared with those who did not (mean preoperative neutrophil to leukocyte ratio, 4.5 vs. 5.0, respectively).
“In the full cohort, multivariable regression analysis similarly demonstrated that metformin was associated with a reduced hazard for both 90-day and 5-year mortality (adjusted HRs, 0.77 and 0.80, respectively) and for 30-day and 90-day readmission (aHR, 0.83 and 0.86), with mortality as a competing risk,” they added.
The findings support those from previous studies showing a decrease in all-cause mortality among diabetes patients taking metformin, said the researchers, noting that those patients had fewer age-related chronic diseases.
“These associations may reflect the anti-aging properties of metformin against the onset of disease or diabetes-associated complications. This study extends these finding by demonstrating that preoperative metformin prescriptions were associated with a reduction in postoperative mortality and readmission, a surrogate for postoperative complications, and with long-term mortality,” they wrote.
The study was limited by a number of factors, such as the potential for residual confounding inherent in retrospective analyses and a lack of adequate power to evaluate the association between metformin use and outcomes for individual surgical procedures. But the authors added that the findings are of note, because adults with comorbidities, such as diabetes, have less physiological reserve and an increased postoperative mortality and readmission rate. The results, therefore, warrant investigation with a prospective randomized clinical trial, they concluded.
In an accompanying editorial, Elizabeth L. George, MD, and Sherry M. Wren, MD, of Stanford (Calif.) University wrote that the study “demonstrates how variables, besides coexisting medical diseases, can affect surgical outcomes.”
“Metformin now joins beta-blockers, statins, and immunonutrition as preoperative agents associated with improved surgical outcomes,” they wrote, adding that future studies should factor in statin use and whether those and other medications should be continued postoperatively because metformin is often held after surgery owing to concerns about contrast agent interactions, whereas statin continuation is recommended.
Future studies of metformin in this setting should exclude patients who are taking statins, or look at possible interactions between the two agents, they said, adding that they would be “interested in seeing a subanalysis of this data set that excludes patients who were prescribed statins.”
“Those data would further solidify the role of metformin as a possible modifiable perioperative factor,” they wrote.
The study was funded by the University of Pittsburgh Medical Center and by grants from the National Heart, Lung, and Blood Institute and the National Institutes of Health. Dr. Reitz reported having no disclosures. Dr. George and Dr. Wren also reported having no disclosures.
SOURCE: Reitz K et al. JAMA Surg. 2020 Apr 8. doi: 10.1001/jamasurg.2020.0416.
Patients with type 2 diabetes who take metformin may have lower risk-adjusted mortality and readmission rates after surgery than do those who don’t take metformin, findings from a large retrospective cohort study suggest.
Of 10,088 individuals with diabetes who underwent a major surgery requiring hospital admission between January 1, 2010, and January 1, 2016, a total of 5,962 (59%) had received a prescription for metformin in the 180 days before surgery, and 5,460 of those patients were propensity score–matched to controls who did not receive a metformin prescription.
The study participants had a mean age of 67.7 years and underwent surgery requiring general anesthesia and postoperative admission at any of 15 hospitals in a single Pennsylvania health system. In addition to being prescribed metformin within 180 days before surgery, they also had metformin on their list of active medications at their most recent preoperative encounter before the surgery. The were followed until December 18, 2018.
In all, the 90-day and 5-year mortality hazards were reduced by 28% and 26%, respectively, in the metformin prescription recipients, compared with the propensity score–matched controls (hazard ratios, 0.72 and 0.74, respectively), Katherine M. Reitz, MD, and colleagues at the University of Pittsburgh reported in JAMA Surgery.
The readmission hazard – with mortality as a competing risk – was reduced by 16% at 30 days and 14% at 90 days (sub-HRs, 0.84 and 0.86, respectively), the researchers found.
“Hospital readmissions among those with preoperative metformin prescriptions were observed by postdischarge days 30 and 90 (304 [11%] and 538 [20.1%], respectively), whereas among those without prescriptions, 361 readmissions (13%) occurred by day 30 and 614 (23%) by day 90,” they wrote.
The investigators also noted that inflammation was reduced in patients who received a metformin prescription, compared with those who did not (mean preoperative neutrophil to leukocyte ratio, 4.5 vs. 5.0, respectively).
“In the full cohort, multivariable regression analysis similarly demonstrated that metformin was associated with a reduced hazard for both 90-day and 5-year mortality (adjusted HRs, 0.77 and 0.80, respectively) and for 30-day and 90-day readmission (aHR, 0.83 and 0.86), with mortality as a competing risk,” they added.
The findings support those from previous studies showing a decrease in all-cause mortality among diabetes patients taking metformin, said the researchers, noting that those patients had fewer age-related chronic diseases.
“These associations may reflect the anti-aging properties of metformin against the onset of disease or diabetes-associated complications. This study extends these finding by demonstrating that preoperative metformin prescriptions were associated with a reduction in postoperative mortality and readmission, a surrogate for postoperative complications, and with long-term mortality,” they wrote.
The study was limited by a number of factors, such as the potential for residual confounding inherent in retrospective analyses and a lack of adequate power to evaluate the association between metformin use and outcomes for individual surgical procedures. But the authors added that the findings are of note, because adults with comorbidities, such as diabetes, have less physiological reserve and an increased postoperative mortality and readmission rate. The results, therefore, warrant investigation with a prospective randomized clinical trial, they concluded.
In an accompanying editorial, Elizabeth L. George, MD, and Sherry M. Wren, MD, of Stanford (Calif.) University wrote that the study “demonstrates how variables, besides coexisting medical diseases, can affect surgical outcomes.”
“Metformin now joins beta-blockers, statins, and immunonutrition as preoperative agents associated with improved surgical outcomes,” they wrote, adding that future studies should factor in statin use and whether those and other medications should be continued postoperatively because metformin is often held after surgery owing to concerns about contrast agent interactions, whereas statin continuation is recommended.
Future studies of metformin in this setting should exclude patients who are taking statins, or look at possible interactions between the two agents, they said, adding that they would be “interested in seeing a subanalysis of this data set that excludes patients who were prescribed statins.”
“Those data would further solidify the role of metformin as a possible modifiable perioperative factor,” they wrote.
The study was funded by the University of Pittsburgh Medical Center and by grants from the National Heart, Lung, and Blood Institute and the National Institutes of Health. Dr. Reitz reported having no disclosures. Dr. George and Dr. Wren also reported having no disclosures.
SOURCE: Reitz K et al. JAMA Surg. 2020 Apr 8. doi: 10.1001/jamasurg.2020.0416.
FROM JAMA SURGERY
After PCI, stopping antiplatelet therapy for surgery appears safe
NATIONAL HARBOR, MD. – Following a percutaneous intervention with a second-generation drug-eluting stent, a judicious interruption of antiplatelet therapy for noncardiac surgery does not increase risk of net adverse clinical events, according to a large dataset presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
Drawn from a multicenter registry in South Korea, it is likely that those in whom antiplatelet therapy was stopped during the perioperative period were at a lower relative risk, but the data remain reassuring, according to Jung-Sun Kim, MD, PhD, professor of medicine at Yonsei University, Seoul, South Korea.
In the registry of patients with a second-generation drug-eluting stent (DES) undergoing noncardiac surgery, “antiplatelet therapy was discontinued in almost half of the patients,” Dr. Kim reported. When these patients were compared with those who did not discontinue antiplatelet therapy, the data, called an “exploratory analysis,” suggested “no increased risk” of a composite of major adverse cardiac events (MACE) or major bleeding.
The retrospective analysis involved 3,582 percutaneous intervention (PCI) patients who had received a second-generation DES and subsequently underwent noncardiac surgery. In 1,750 of these patients, antiplatelet therapy was temporarily discontinued. The remaining 1,832 remained on some form of antiplatelet treatment, whether aspirin, a P2Y12 inhibitor, or dual-antiplatelet therapy.
There were no significant differences in crude rates between groups in rates at 30 days of a composite endpoint of MACE, major bleeding as defined by the International Society on Thrombosis and Haemostasis, or net adverse clinical events (NACE), a composite of adverse events that included MACE and major bleeding.
Relative risks for antiplatelet discontinuation remained generally low even after multiple stratifications performed to explore different variables, including the types of antiplatelet therapy being taken at the time of discontinuation, the types of noncardiac surgery performed, and the duration of discontinuation.
Of these variables, the interval of discontinuation appeared to be most relevant. Antiplatelet discontinuation of 3 days or less appeared to be associated with a higher risk of bleeding, although the difference did not reach significance. Discontinuations of 9 days or more were associated with increased risk of MACE, and this difference did reach statistical significance (hazard ratio, 3.38; 95% confidence interval, 1.36-8.38).
“Discontinuation of antiplatelet therapy for a period of 4-8 days appears to be optimal,” Dr. Kim said.
In general, risk of MACE, major bleeding, or NACE could not be linked to type of surgery, with the exception of intra-abdominal surgery. For this procedure, there appeared to be a lower risk of MACE in those who discontinued relative to those who remained on antiplatelet therapy, Dr. Kim reported.
Importantly, because of the fact that the decision to stop antiplatelet treatment was made by treating physicians, the characteristics of those who discontinued or remained on antiplatelet therapy differed meaningfully. Specifically, those in the discontinuation group were younger and were less likely to have additional risks for thrombotic events such as diabetes or chronic kidney disease. In those who discontinued antiplatelets, the average time since PCI was 23 months versus 16 months in the continuation group.
In addition, “more of the patients underwent higher-risk surgeries in the discontinuation group,” Dr. Kim added.
Relative rates of MACE and NACE remained similar even after risk adjustment, but Dr. Kim advised that the data should be “interpreted cautiously” because of the retrospective nature of the analysis.
A panel of experts invited to comment on the presentation agreed. These data were considered reassuring for clinicians considering an interruption of antiplatelet therapy following PCI with a second-generation DES, but there was uncertainty about their value for defining which patients are the best candidates.
The decision to discontinue antiplatelet drugs for noncardiac surgery is an important and common dilemma, but these data might be best characterized as “a testament to Korean cardiologists making good decisions,” said David J. Moliterno, MD, chairman of the department of medicine at University of Kentucky Health Care, Lexington.
Dr. Kim reported no potential financial conflicts of interest.
NATIONAL HARBOR, MD. – Following a percutaneous intervention with a second-generation drug-eluting stent, a judicious interruption of antiplatelet therapy for noncardiac surgery does not increase risk of net adverse clinical events, according to a large dataset presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
Drawn from a multicenter registry in South Korea, it is likely that those in whom antiplatelet therapy was stopped during the perioperative period were at a lower relative risk, but the data remain reassuring, according to Jung-Sun Kim, MD, PhD, professor of medicine at Yonsei University, Seoul, South Korea.
In the registry of patients with a second-generation drug-eluting stent (DES) undergoing noncardiac surgery, “antiplatelet therapy was discontinued in almost half of the patients,” Dr. Kim reported. When these patients were compared with those who did not discontinue antiplatelet therapy, the data, called an “exploratory analysis,” suggested “no increased risk” of a composite of major adverse cardiac events (MACE) or major bleeding.
The retrospective analysis involved 3,582 percutaneous intervention (PCI) patients who had received a second-generation DES and subsequently underwent noncardiac surgery. In 1,750 of these patients, antiplatelet therapy was temporarily discontinued. The remaining 1,832 remained on some form of antiplatelet treatment, whether aspirin, a P2Y12 inhibitor, or dual-antiplatelet therapy.
There were no significant differences in crude rates between groups in rates at 30 days of a composite endpoint of MACE, major bleeding as defined by the International Society on Thrombosis and Haemostasis, or net adverse clinical events (NACE), a composite of adverse events that included MACE and major bleeding.
Relative risks for antiplatelet discontinuation remained generally low even after multiple stratifications performed to explore different variables, including the types of antiplatelet therapy being taken at the time of discontinuation, the types of noncardiac surgery performed, and the duration of discontinuation.
Of these variables, the interval of discontinuation appeared to be most relevant. Antiplatelet discontinuation of 3 days or less appeared to be associated with a higher risk of bleeding, although the difference did not reach significance. Discontinuations of 9 days or more were associated with increased risk of MACE, and this difference did reach statistical significance (hazard ratio, 3.38; 95% confidence interval, 1.36-8.38).
“Discontinuation of antiplatelet therapy for a period of 4-8 days appears to be optimal,” Dr. Kim said.
In general, risk of MACE, major bleeding, or NACE could not be linked to type of surgery, with the exception of intra-abdominal surgery. For this procedure, there appeared to be a lower risk of MACE in those who discontinued relative to those who remained on antiplatelet therapy, Dr. Kim reported.
Importantly, because of the fact that the decision to stop antiplatelet treatment was made by treating physicians, the characteristics of those who discontinued or remained on antiplatelet therapy differed meaningfully. Specifically, those in the discontinuation group were younger and were less likely to have additional risks for thrombotic events such as diabetes or chronic kidney disease. In those who discontinued antiplatelets, the average time since PCI was 23 months versus 16 months in the continuation group.
In addition, “more of the patients underwent higher-risk surgeries in the discontinuation group,” Dr. Kim added.
Relative rates of MACE and NACE remained similar even after risk adjustment, but Dr. Kim advised that the data should be “interpreted cautiously” because of the retrospective nature of the analysis.
A panel of experts invited to comment on the presentation agreed. These data were considered reassuring for clinicians considering an interruption of antiplatelet therapy following PCI with a second-generation DES, but there was uncertainty about their value for defining which patients are the best candidates.
The decision to discontinue antiplatelet drugs for noncardiac surgery is an important and common dilemma, but these data might be best characterized as “a testament to Korean cardiologists making good decisions,” said David J. Moliterno, MD, chairman of the department of medicine at University of Kentucky Health Care, Lexington.
Dr. Kim reported no potential financial conflicts of interest.
NATIONAL HARBOR, MD. – Following a percutaneous intervention with a second-generation drug-eluting stent, a judicious interruption of antiplatelet therapy for noncardiac surgery does not increase risk of net adverse clinical events, according to a large dataset presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
Drawn from a multicenter registry in South Korea, it is likely that those in whom antiplatelet therapy was stopped during the perioperative period were at a lower relative risk, but the data remain reassuring, according to Jung-Sun Kim, MD, PhD, professor of medicine at Yonsei University, Seoul, South Korea.
In the registry of patients with a second-generation drug-eluting stent (DES) undergoing noncardiac surgery, “antiplatelet therapy was discontinued in almost half of the patients,” Dr. Kim reported. When these patients were compared with those who did not discontinue antiplatelet therapy, the data, called an “exploratory analysis,” suggested “no increased risk” of a composite of major adverse cardiac events (MACE) or major bleeding.
The retrospective analysis involved 3,582 percutaneous intervention (PCI) patients who had received a second-generation DES and subsequently underwent noncardiac surgery. In 1,750 of these patients, antiplatelet therapy was temporarily discontinued. The remaining 1,832 remained on some form of antiplatelet treatment, whether aspirin, a P2Y12 inhibitor, or dual-antiplatelet therapy.
There were no significant differences in crude rates between groups in rates at 30 days of a composite endpoint of MACE, major bleeding as defined by the International Society on Thrombosis and Haemostasis, or net adverse clinical events (NACE), a composite of adverse events that included MACE and major bleeding.
Relative risks for antiplatelet discontinuation remained generally low even after multiple stratifications performed to explore different variables, including the types of antiplatelet therapy being taken at the time of discontinuation, the types of noncardiac surgery performed, and the duration of discontinuation.
Of these variables, the interval of discontinuation appeared to be most relevant. Antiplatelet discontinuation of 3 days or less appeared to be associated with a higher risk of bleeding, although the difference did not reach significance. Discontinuations of 9 days or more were associated with increased risk of MACE, and this difference did reach statistical significance (hazard ratio, 3.38; 95% confidence interval, 1.36-8.38).
“Discontinuation of antiplatelet therapy for a period of 4-8 days appears to be optimal,” Dr. Kim said.
In general, risk of MACE, major bleeding, or NACE could not be linked to type of surgery, with the exception of intra-abdominal surgery. For this procedure, there appeared to be a lower risk of MACE in those who discontinued relative to those who remained on antiplatelet therapy, Dr. Kim reported.
Importantly, because of the fact that the decision to stop antiplatelet treatment was made by treating physicians, the characteristics of those who discontinued or remained on antiplatelet therapy differed meaningfully. Specifically, those in the discontinuation group were younger and were less likely to have additional risks for thrombotic events such as diabetes or chronic kidney disease. In those who discontinued antiplatelets, the average time since PCI was 23 months versus 16 months in the continuation group.
In addition, “more of the patients underwent higher-risk surgeries in the discontinuation group,” Dr. Kim added.
Relative rates of MACE and NACE remained similar even after risk adjustment, but Dr. Kim advised that the data should be “interpreted cautiously” because of the retrospective nature of the analysis.
A panel of experts invited to comment on the presentation agreed. These data were considered reassuring for clinicians considering an interruption of antiplatelet therapy following PCI with a second-generation DES, but there was uncertainty about their value for defining which patients are the best candidates.
The decision to discontinue antiplatelet drugs for noncardiac surgery is an important and common dilemma, but these data might be best characterized as “a testament to Korean cardiologists making good decisions,” said David J. Moliterno, MD, chairman of the department of medicine at University of Kentucky Health Care, Lexington.
Dr. Kim reported no potential financial conflicts of interest.
REPORTING FROM CRT 2020
ERAS takes its place in IBD surgery
AUSTIN, TEX. – Enhanced recovery after surgery (ERAS) protocols have been around for decades, but typically excluded patients having surgery for inflammatory bowel disease (IBD). However, recent studies have shown strategies to optimize these patients, including presurgery carbohydrate loading and early postsurgery feeding, can improve outcomes, according to a review of evidence presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“It’s really important that we implement strategies to help mitigate the impact that malnutrition is going to have on our perioperative patients, and one of the ways we do that is by using an ERAS or enhanced recovery after surgery protocol,” said Kelly Issokson, MS, RD, of Cedars-Sinai Medical Center, Los Angeles. She noted that patients with IBD are five times more likely to be malnourished than non-IBD patients, and those with fistulizing Crohn’s disease and bowel resections are at greatest risk (Inflamm Bowel Dis. 2008;14:1139-46).
“I constantly see patients who are kept NPO [nothing by mouth] 12 or 24 hours before surgery, maybe even longer sometimes, unfortunately,” she said. “We should really be minimizing that NPO to help mitigate the catabolic effect that surgery has on our patients and help them recover more quickly.”
To screen surgery patients for nutrition risk, Ms. Issokson said that gastroenterologists can ask two questions from the malnutrition screening tool: Did the patient have recent unintentional weight loss, and is the patient eating less because of poor appetite? A yes to either question merits referral to a registered dietician. Malnutrition, weight loss of 5%-10% of total body weight, and sarcopenia are predictors of surgical complications for IBD patients, the latter an independent predictor in patients aged 40 years and older.
The ERAS protocol involves optimizing preoperative and postoperative nutrition, she said. It has been linked with improved outcomes in elective colorectal surgery (World J Surg. 2014;38:1531-41), although the evidence in IBD isn’t as robust. She cited a retrospective study reported at the 2019 annual Digestive Disease Week of patients with Crohn’s disease that found no difference in readmissions, complications, or reoperations between ERAS and standard-care patients.
Preoperative nutrition optimization in ERAS involves anemia and fluid management, oral nutrition supplementation, and – based on European Society for Clinical Nutrition and Metabolism (ESPEN) 2017 guidelines – delaying the operation where possible if the patient is malnourished. “Patients who receive preoperative nutrition support have been shown to have better outcomes postoperatively,” Ms. Issokson said, citing a meta-analysis of 1,111 Crohn’s disease patients that reported the complication rate was 20% in patients on nutrition support versus 60% for those on standard care; in those on enteral nutrition, the disparity was more pronounced: 21% versus 73% (Eur J Gastro Hep. 2018;30:997-1002).
Gastroenterologists should not be afraid of implementing total parenteral nutrition (TPN) perioperatively in these patients, Ms. Issokson said. “This can really help to improve outcomes and quality of life in our patients, and it’s something that we really should not shy away from,” she added in an interview. “If our patients are malnourished and meet the criteria for TPN, then we should really not be withholding it.” Patients with severe IBD who are not absorbing from their gut and can’t meet 60% of their needs by mouth are prime candidates for TPN, she said, referencing a 2019 study that reported that preoperative TPN in malnourished IBD patients resulted in a rate of overall noninfectious complications half that of no-TPN patients: 8.3% versus 16.8% (Gastroenterol Rep. 2019 Apr;7:107-14).
Carbohydrate loading before surgery is a big part of ERAS in these patients. “Surgery has a huge impact on the catabolic state of a patient,” Ms. Issokson said. “It’s similar to running a marathon; you wouldn’t go out and run a marathon without fueling up the night before with a whole bunch of carbohydrates. So we use this same strategy in our surgical patients.”
ERAS society guidelines call for 100 g of carbohydrates the night before and 50 g 2 hours before surgery in the form of a clear liquid beverage, along with permitting a light meal up to 6 hours before, with exceptions in gastroparesis, motility disorders, and emergency surgery.
Another key component of ERAS in IBD is early postoperative feeding. “Postoperatively we want to feed our patients as soon as possible,” Ms. Issokson said. ESPEN guidelines call for feeding patients with new nondiverted colorectal anastomosis within 4 hours. “Studies show that patients aren’t able to eat enough calories to help them recover postoperatively, so implementing an oral nutrition supplement might be helpful there,” she added.
Ms. Issokson is a Crohn’s & Colitis Foundation board member, and disclosed financial relationships with Orgain, RMEI, and Medscape.
SOURCE: Issokson K et al. Crohn’s & Colitis Congress 2020, Session Sp83.
AUSTIN, TEX. – Enhanced recovery after surgery (ERAS) protocols have been around for decades, but typically excluded patients having surgery for inflammatory bowel disease (IBD). However, recent studies have shown strategies to optimize these patients, including presurgery carbohydrate loading and early postsurgery feeding, can improve outcomes, according to a review of evidence presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“It’s really important that we implement strategies to help mitigate the impact that malnutrition is going to have on our perioperative patients, and one of the ways we do that is by using an ERAS or enhanced recovery after surgery protocol,” said Kelly Issokson, MS, RD, of Cedars-Sinai Medical Center, Los Angeles. She noted that patients with IBD are five times more likely to be malnourished than non-IBD patients, and those with fistulizing Crohn’s disease and bowel resections are at greatest risk (Inflamm Bowel Dis. 2008;14:1139-46).
“I constantly see patients who are kept NPO [nothing by mouth] 12 or 24 hours before surgery, maybe even longer sometimes, unfortunately,” she said. “We should really be minimizing that NPO to help mitigate the catabolic effect that surgery has on our patients and help them recover more quickly.”
To screen surgery patients for nutrition risk, Ms. Issokson said that gastroenterologists can ask two questions from the malnutrition screening tool: Did the patient have recent unintentional weight loss, and is the patient eating less because of poor appetite? A yes to either question merits referral to a registered dietician. Malnutrition, weight loss of 5%-10% of total body weight, and sarcopenia are predictors of surgical complications for IBD patients, the latter an independent predictor in patients aged 40 years and older.
The ERAS protocol involves optimizing preoperative and postoperative nutrition, she said. It has been linked with improved outcomes in elective colorectal surgery (World J Surg. 2014;38:1531-41), although the evidence in IBD isn’t as robust. She cited a retrospective study reported at the 2019 annual Digestive Disease Week of patients with Crohn’s disease that found no difference in readmissions, complications, or reoperations between ERAS and standard-care patients.
Preoperative nutrition optimization in ERAS involves anemia and fluid management, oral nutrition supplementation, and – based on European Society for Clinical Nutrition and Metabolism (ESPEN) 2017 guidelines – delaying the operation where possible if the patient is malnourished. “Patients who receive preoperative nutrition support have been shown to have better outcomes postoperatively,” Ms. Issokson said, citing a meta-analysis of 1,111 Crohn’s disease patients that reported the complication rate was 20% in patients on nutrition support versus 60% for those on standard care; in those on enteral nutrition, the disparity was more pronounced: 21% versus 73% (Eur J Gastro Hep. 2018;30:997-1002).
Gastroenterologists should not be afraid of implementing total parenteral nutrition (TPN) perioperatively in these patients, Ms. Issokson said. “This can really help to improve outcomes and quality of life in our patients, and it’s something that we really should not shy away from,” she added in an interview. “If our patients are malnourished and meet the criteria for TPN, then we should really not be withholding it.” Patients with severe IBD who are not absorbing from their gut and can’t meet 60% of their needs by mouth are prime candidates for TPN, she said, referencing a 2019 study that reported that preoperative TPN in malnourished IBD patients resulted in a rate of overall noninfectious complications half that of no-TPN patients: 8.3% versus 16.8% (Gastroenterol Rep. 2019 Apr;7:107-14).
Carbohydrate loading before surgery is a big part of ERAS in these patients. “Surgery has a huge impact on the catabolic state of a patient,” Ms. Issokson said. “It’s similar to running a marathon; you wouldn’t go out and run a marathon without fueling up the night before with a whole bunch of carbohydrates. So we use this same strategy in our surgical patients.”
ERAS society guidelines call for 100 g of carbohydrates the night before and 50 g 2 hours before surgery in the form of a clear liquid beverage, along with permitting a light meal up to 6 hours before, with exceptions in gastroparesis, motility disorders, and emergency surgery.
Another key component of ERAS in IBD is early postoperative feeding. “Postoperatively we want to feed our patients as soon as possible,” Ms. Issokson said. ESPEN guidelines call for feeding patients with new nondiverted colorectal anastomosis within 4 hours. “Studies show that patients aren’t able to eat enough calories to help them recover postoperatively, so implementing an oral nutrition supplement might be helpful there,” she added.
Ms. Issokson is a Crohn’s & Colitis Foundation board member, and disclosed financial relationships with Orgain, RMEI, and Medscape.
SOURCE: Issokson K et al. Crohn’s & Colitis Congress 2020, Session Sp83.
AUSTIN, TEX. – Enhanced recovery after surgery (ERAS) protocols have been around for decades, but typically excluded patients having surgery for inflammatory bowel disease (IBD). However, recent studies have shown strategies to optimize these patients, including presurgery carbohydrate loading and early postsurgery feeding, can improve outcomes, according to a review of evidence presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“It’s really important that we implement strategies to help mitigate the impact that malnutrition is going to have on our perioperative patients, and one of the ways we do that is by using an ERAS or enhanced recovery after surgery protocol,” said Kelly Issokson, MS, RD, of Cedars-Sinai Medical Center, Los Angeles. She noted that patients with IBD are five times more likely to be malnourished than non-IBD patients, and those with fistulizing Crohn’s disease and bowel resections are at greatest risk (Inflamm Bowel Dis. 2008;14:1139-46).
“I constantly see patients who are kept NPO [nothing by mouth] 12 or 24 hours before surgery, maybe even longer sometimes, unfortunately,” she said. “We should really be minimizing that NPO to help mitigate the catabolic effect that surgery has on our patients and help them recover more quickly.”
To screen surgery patients for nutrition risk, Ms. Issokson said that gastroenterologists can ask two questions from the malnutrition screening tool: Did the patient have recent unintentional weight loss, and is the patient eating less because of poor appetite? A yes to either question merits referral to a registered dietician. Malnutrition, weight loss of 5%-10% of total body weight, and sarcopenia are predictors of surgical complications for IBD patients, the latter an independent predictor in patients aged 40 years and older.
The ERAS protocol involves optimizing preoperative and postoperative nutrition, she said. It has been linked with improved outcomes in elective colorectal surgery (World J Surg. 2014;38:1531-41), although the evidence in IBD isn’t as robust. She cited a retrospective study reported at the 2019 annual Digestive Disease Week of patients with Crohn’s disease that found no difference in readmissions, complications, or reoperations between ERAS and standard-care patients.
Preoperative nutrition optimization in ERAS involves anemia and fluid management, oral nutrition supplementation, and – based on European Society for Clinical Nutrition and Metabolism (ESPEN) 2017 guidelines – delaying the operation where possible if the patient is malnourished. “Patients who receive preoperative nutrition support have been shown to have better outcomes postoperatively,” Ms. Issokson said, citing a meta-analysis of 1,111 Crohn’s disease patients that reported the complication rate was 20% in patients on nutrition support versus 60% for those on standard care; in those on enteral nutrition, the disparity was more pronounced: 21% versus 73% (Eur J Gastro Hep. 2018;30:997-1002).
Gastroenterologists should not be afraid of implementing total parenteral nutrition (TPN) perioperatively in these patients, Ms. Issokson said. “This can really help to improve outcomes and quality of life in our patients, and it’s something that we really should not shy away from,” she added in an interview. “If our patients are malnourished and meet the criteria for TPN, then we should really not be withholding it.” Patients with severe IBD who are not absorbing from their gut and can’t meet 60% of their needs by mouth are prime candidates for TPN, she said, referencing a 2019 study that reported that preoperative TPN in malnourished IBD patients resulted in a rate of overall noninfectious complications half that of no-TPN patients: 8.3% versus 16.8% (Gastroenterol Rep. 2019 Apr;7:107-14).
Carbohydrate loading before surgery is a big part of ERAS in these patients. “Surgery has a huge impact on the catabolic state of a patient,” Ms. Issokson said. “It’s similar to running a marathon; you wouldn’t go out and run a marathon without fueling up the night before with a whole bunch of carbohydrates. So we use this same strategy in our surgical patients.”
ERAS society guidelines call for 100 g of carbohydrates the night before and 50 g 2 hours before surgery in the form of a clear liquid beverage, along with permitting a light meal up to 6 hours before, with exceptions in gastroparesis, motility disorders, and emergency surgery.
Another key component of ERAS in IBD is early postoperative feeding. “Postoperatively we want to feed our patients as soon as possible,” Ms. Issokson said. ESPEN guidelines call for feeding patients with new nondiverted colorectal anastomosis within 4 hours. “Studies show that patients aren’t able to eat enough calories to help them recover postoperatively, so implementing an oral nutrition supplement might be helpful there,” she added.
Ms. Issokson is a Crohn’s & Colitis Foundation board member, and disclosed financial relationships with Orgain, RMEI, and Medscape.
SOURCE: Issokson K et al. Crohn’s & Colitis Congress 2020, Session Sp83.
REPORTING FROM THE CROHN’S & COLITIS CONGRESS
Initial ultrasound assessment of appendicitis curbs costs
Assessing appendicitis in children with initial ultrasound followed by computed tomography in the absence of appendix visualization and presence of secondary signs was the most cost-effective approach, according to data from a modeling study of 10 strategies.
Ultrasound is safer and less expensive than computed tomography and avoids radiation exposure; however, cost-effectiveness models of various approaches to imaging have not been well studied, wrote Rebecca Jennings, MD, of Seattle Children’s Hospital, Washington, and colleagues.
In a study published in Pediatrics, the researchers simulated a hypothetical patient population using a Markov cohort model and compared 10 different strategies including CT only, MRI only, and ultrasound followed by CT or MRI after ultrasounds that are negative or fail to visualize the appendix.
Overall, the most cost-effective strategy for moderate-risk patients was the use of ultrasound followed by CT or MRI if the ultrasound failed to visualize the appendix and secondary signs of inflammation were present in the right lower quadrant. The cost of this strategy was $4,815, with effectiveness of 0.99694 quality-adjusted life-years. “The most cost-effective strategy is highly dependent on a patient’s risk stratification,” the researchers noted. Based on their model, imaging was not cost effective for patients with a prevalence less than 16% or greater than 95%. However, those with appendicitis risk between 16% and 95% and no secondary signs of inflammation can forgo further imaging, even without visualization of the appendix for maximum cost-effectiveness, the researchers said.
The study was limited by several factors, including the inability to account for all potential costs related to imaging and outcomes, lack of accounting for the use of sedation when assessing costs, and inability to separate imaging costs from total hospital costs, the researchers noted. However, the results suggest that tailored imaging approaches based on patient risk are the most cost-effective strategies to assess appendicitis, they said.
“The diagnosis and exclusion of appendicitis continues to be one of the primary concerns of providers who care for children with abdominal pain,” wrote Rebecca M. Rentea, MD, and Charles L. Snyder, MD, of Children’s Mercy Hospital Kansas City, Mo., in an accompanying editorial (Pediatrics. 2020 Feb;145:e20193349).
“The best diagnostic and imaging approach to appendicitis has been a topic of interest for some time, and improvements such as appendicitis scoring systems, decreased use of ionized radiation, and adoption of clinical algorithms have been incremental but steady,” they said. Despite the potential of missed appendicitis, the use of an algorithm based on an initial ultrasound and previous possibility of appendicitis described in the study was the most cost effective, they said. In addition, “the ability to visualize the appendix did not alter the most cost-effective approach in those with a moderate risk of appendicitis (most patients),” they concluded.
The study was supported by the University of Washington and Seattle Children’s Hospital Quality Improvement Scholars Program. The researchers had no financial conflicts to disclose.
Dr. Rentea and Dr. Snyder had no financial conflicts to disclose.
SOURCE: Jennings R et al. Pediatrics. 2020. doi: 10.1542/peds.2019-1352.
Assessing appendicitis in children with initial ultrasound followed by computed tomography in the absence of appendix visualization and presence of secondary signs was the most cost-effective approach, according to data from a modeling study of 10 strategies.
Ultrasound is safer and less expensive than computed tomography and avoids radiation exposure; however, cost-effectiveness models of various approaches to imaging have not been well studied, wrote Rebecca Jennings, MD, of Seattle Children’s Hospital, Washington, and colleagues.
In a study published in Pediatrics, the researchers simulated a hypothetical patient population using a Markov cohort model and compared 10 different strategies including CT only, MRI only, and ultrasound followed by CT or MRI after ultrasounds that are negative or fail to visualize the appendix.
Overall, the most cost-effective strategy for moderate-risk patients was the use of ultrasound followed by CT or MRI if the ultrasound failed to visualize the appendix and secondary signs of inflammation were present in the right lower quadrant. The cost of this strategy was $4,815, with effectiveness of 0.99694 quality-adjusted life-years. “The most cost-effective strategy is highly dependent on a patient’s risk stratification,” the researchers noted. Based on their model, imaging was not cost effective for patients with a prevalence less than 16% or greater than 95%. However, those with appendicitis risk between 16% and 95% and no secondary signs of inflammation can forgo further imaging, even without visualization of the appendix for maximum cost-effectiveness, the researchers said.
The study was limited by several factors, including the inability to account for all potential costs related to imaging and outcomes, lack of accounting for the use of sedation when assessing costs, and inability to separate imaging costs from total hospital costs, the researchers noted. However, the results suggest that tailored imaging approaches based on patient risk are the most cost-effective strategies to assess appendicitis, they said.
“The diagnosis and exclusion of appendicitis continues to be one of the primary concerns of providers who care for children with abdominal pain,” wrote Rebecca M. Rentea, MD, and Charles L. Snyder, MD, of Children’s Mercy Hospital Kansas City, Mo., in an accompanying editorial (Pediatrics. 2020 Feb;145:e20193349).
“The best diagnostic and imaging approach to appendicitis has been a topic of interest for some time, and improvements such as appendicitis scoring systems, decreased use of ionized radiation, and adoption of clinical algorithms have been incremental but steady,” they said. Despite the potential of missed appendicitis, the use of an algorithm based on an initial ultrasound and previous possibility of appendicitis described in the study was the most cost effective, they said. In addition, “the ability to visualize the appendix did not alter the most cost-effective approach in those with a moderate risk of appendicitis (most patients),” they concluded.
The study was supported by the University of Washington and Seattle Children’s Hospital Quality Improvement Scholars Program. The researchers had no financial conflicts to disclose.
Dr. Rentea and Dr. Snyder had no financial conflicts to disclose.
SOURCE: Jennings R et al. Pediatrics. 2020. doi: 10.1542/peds.2019-1352.
Assessing appendicitis in children with initial ultrasound followed by computed tomography in the absence of appendix visualization and presence of secondary signs was the most cost-effective approach, according to data from a modeling study of 10 strategies.
Ultrasound is safer and less expensive than computed tomography and avoids radiation exposure; however, cost-effectiveness models of various approaches to imaging have not been well studied, wrote Rebecca Jennings, MD, of Seattle Children’s Hospital, Washington, and colleagues.
In a study published in Pediatrics, the researchers simulated a hypothetical patient population using a Markov cohort model and compared 10 different strategies including CT only, MRI only, and ultrasound followed by CT or MRI after ultrasounds that are negative or fail to visualize the appendix.
Overall, the most cost-effective strategy for moderate-risk patients was the use of ultrasound followed by CT or MRI if the ultrasound failed to visualize the appendix and secondary signs of inflammation were present in the right lower quadrant. The cost of this strategy was $4,815, with effectiveness of 0.99694 quality-adjusted life-years. “The most cost-effective strategy is highly dependent on a patient’s risk stratification,” the researchers noted. Based on their model, imaging was not cost effective for patients with a prevalence less than 16% or greater than 95%. However, those with appendicitis risk between 16% and 95% and no secondary signs of inflammation can forgo further imaging, even without visualization of the appendix for maximum cost-effectiveness, the researchers said.
The study was limited by several factors, including the inability to account for all potential costs related to imaging and outcomes, lack of accounting for the use of sedation when assessing costs, and inability to separate imaging costs from total hospital costs, the researchers noted. However, the results suggest that tailored imaging approaches based on patient risk are the most cost-effective strategies to assess appendicitis, they said.
“The diagnosis and exclusion of appendicitis continues to be one of the primary concerns of providers who care for children with abdominal pain,” wrote Rebecca M. Rentea, MD, and Charles L. Snyder, MD, of Children’s Mercy Hospital Kansas City, Mo., in an accompanying editorial (Pediatrics. 2020 Feb;145:e20193349).
“The best diagnostic and imaging approach to appendicitis has been a topic of interest for some time, and improvements such as appendicitis scoring systems, decreased use of ionized radiation, and adoption of clinical algorithms have been incremental but steady,” they said. Despite the potential of missed appendicitis, the use of an algorithm based on an initial ultrasound and previous possibility of appendicitis described in the study was the most cost effective, they said. In addition, “the ability to visualize the appendix did not alter the most cost-effective approach in those with a moderate risk of appendicitis (most patients),” they concluded.
The study was supported by the University of Washington and Seattle Children’s Hospital Quality Improvement Scholars Program. The researchers had no financial conflicts to disclose.
Dr. Rentea and Dr. Snyder had no financial conflicts to disclose.
SOURCE: Jennings R et al. Pediatrics. 2020. doi: 10.1542/peds.2019-1352.
FROM PEDIATRICS
Over half of NASH patients have improvement in liver fibrosis after bariatric surgery
BOSTON – For almost half of patients with nonalcoholic steatohepatitis (NASH), bariatric surgery does not resolve severe fibrosis, even after significant weight loss and resolution of multiple metabolic comorbidities, according to investigators.
Still, bariatric surgery was highly effective at improving liver histology in patients without severe fibrosis, reported lead author Raluca Pais, MD, of Sorbonne University, Paris, and colleagues.
“There are many targeted agents for NASH at this time, but their response rate is limited to less than 30%,” Dr. Pais said in a presentation at the annual meeting of the American Association for the Study of Liver Diseases. “In very selective patients, bariatric surgery is a very attractive therapeutic option, as it promotes massive weight loss and sustained improvement in metabolic comorbidities, which are concomitant with very high rates of hepatic histological improvement in most but not all patients.” Despite the known benefits of bariatric surgery, histologic outcomes have been minimally studied, Dr. Pais said, which prompted the present trial.
The investigators began by analyzing data from 868 patients with NASH who underwent bariatric surgery with perioperative liver biopsy between 2004 and 2014. Of these patients, 181 had advanced NASH, a diagnosis that was subclassified by severe fibrosis (F3 or F4) or high activity (steatosis, activity, and fibrosis score of 3-4 with F0-2). Out of the 181 patients with advanced NASH, 65 consented to follow-up liver biopsy, which was conducted a mean of 6 years after surgery. Among these patients, 53 had undergone gastric bypass surgery, while 12 had undergone sleeve gastrectomy.
Almost one-third (29%) of the 65 patients who underwent bariatric surgery had normal livers at follow-up biopsy. Among the 35 patients who had severe fibrosis at baseline, slightly more than half (54%) had resolution of severe fibrosis. In contrast, resolution of high activity occurred in almost all affected patients (97%).
While the findings highlighted some of the benefits associated with bariatric surgery, Dr. Pais emphasized the other side of the coin; many patients did not have resolution of severe fibrosis, even years after surgery. Specifically, 45% of patients had persistent severe fibrosis at follow-up biopsy, despite improvements in comorbidities. On average, these patients lost 23% of their baseline body weight, and two-thirds of the group achieved normal ALT and resolution of NASH. Many also had improvements in insulin resistance and nonalcoholic fatty liver disease activity scores. These findings suggest that changes in severe fibrosis occur independently of many other improvements, Dr. Pais said.
Although multiple comorbidities were not correlated with changes in severe fibrosis, several other predictors were identified. Compared with patients who had resolution of severe fibrosis, nonresponders were typically older (56 vs. 49 years), more often had persistent diabetes (79% vs. 50%), and generally had shorter time between surgery and follow-up biopsy (4.2 vs. 7.5 years). Compared with nonresponders, responders were more likely to have undergone gastric bypass surgery (100% vs. 69%), suggesting that this procedure was more effective at resolving severe fibrosis than sleeve gastrectomy.
During the question-and-answer session following the presentation, multiple conference attendees suggested that the title of the study, “Persistence of severe liver fibrosis despite substantial weight loss with bariatric surgery,” was unnecessarily negative, when in fact the study offered strong support for bariatric surgery.
“This is wonderful data that shows surgery is very effective,” one attendee said.
“This is really positive data,” said another. “I mean, you’ve got reversal of advanced fibrosis in half of the population by the time you follow up for several years, so I would say this is really, very positive data.”
The investigators disclosed relationships with Allergan, Boehringer Ingelheim, Novo Nordisk, and others.
*This story was updated on November 15, 2019.
SOURCE: Pais R et al. The Liver Meeting 2019, Abstract 66.
BOSTON – For almost half of patients with nonalcoholic steatohepatitis (NASH), bariatric surgery does not resolve severe fibrosis, even after significant weight loss and resolution of multiple metabolic comorbidities, according to investigators.
Still, bariatric surgery was highly effective at improving liver histology in patients without severe fibrosis, reported lead author Raluca Pais, MD, of Sorbonne University, Paris, and colleagues.
“There are many targeted agents for NASH at this time, but their response rate is limited to less than 30%,” Dr. Pais said in a presentation at the annual meeting of the American Association for the Study of Liver Diseases. “In very selective patients, bariatric surgery is a very attractive therapeutic option, as it promotes massive weight loss and sustained improvement in metabolic comorbidities, which are concomitant with very high rates of hepatic histological improvement in most but not all patients.” Despite the known benefits of bariatric surgery, histologic outcomes have been minimally studied, Dr. Pais said, which prompted the present trial.
The investigators began by analyzing data from 868 patients with NASH who underwent bariatric surgery with perioperative liver biopsy between 2004 and 2014. Of these patients, 181 had advanced NASH, a diagnosis that was subclassified by severe fibrosis (F3 or F4) or high activity (steatosis, activity, and fibrosis score of 3-4 with F0-2). Out of the 181 patients with advanced NASH, 65 consented to follow-up liver biopsy, which was conducted a mean of 6 years after surgery. Among these patients, 53 had undergone gastric bypass surgery, while 12 had undergone sleeve gastrectomy.
Almost one-third (29%) of the 65 patients who underwent bariatric surgery had normal livers at follow-up biopsy. Among the 35 patients who had severe fibrosis at baseline, slightly more than half (54%) had resolution of severe fibrosis. In contrast, resolution of high activity occurred in almost all affected patients (97%).
While the findings highlighted some of the benefits associated with bariatric surgery, Dr. Pais emphasized the other side of the coin; many patients did not have resolution of severe fibrosis, even years after surgery. Specifically, 45% of patients had persistent severe fibrosis at follow-up biopsy, despite improvements in comorbidities. On average, these patients lost 23% of their baseline body weight, and two-thirds of the group achieved normal ALT and resolution of NASH. Many also had improvements in insulin resistance and nonalcoholic fatty liver disease activity scores. These findings suggest that changes in severe fibrosis occur independently of many other improvements, Dr. Pais said.
Although multiple comorbidities were not correlated with changes in severe fibrosis, several other predictors were identified. Compared with patients who had resolution of severe fibrosis, nonresponders were typically older (56 vs. 49 years), more often had persistent diabetes (79% vs. 50%), and generally had shorter time between surgery and follow-up biopsy (4.2 vs. 7.5 years). Compared with nonresponders, responders were more likely to have undergone gastric bypass surgery (100% vs. 69%), suggesting that this procedure was more effective at resolving severe fibrosis than sleeve gastrectomy.
During the question-and-answer session following the presentation, multiple conference attendees suggested that the title of the study, “Persistence of severe liver fibrosis despite substantial weight loss with bariatric surgery,” was unnecessarily negative, when in fact the study offered strong support for bariatric surgery.
“This is wonderful data that shows surgery is very effective,” one attendee said.
“This is really positive data,” said another. “I mean, you’ve got reversal of advanced fibrosis in half of the population by the time you follow up for several years, so I would say this is really, very positive data.”
The investigators disclosed relationships with Allergan, Boehringer Ingelheim, Novo Nordisk, and others.
*This story was updated on November 15, 2019.
SOURCE: Pais R et al. The Liver Meeting 2019, Abstract 66.
BOSTON – For almost half of patients with nonalcoholic steatohepatitis (NASH), bariatric surgery does not resolve severe fibrosis, even after significant weight loss and resolution of multiple metabolic comorbidities, according to investigators.
Still, bariatric surgery was highly effective at improving liver histology in patients without severe fibrosis, reported lead author Raluca Pais, MD, of Sorbonne University, Paris, and colleagues.
“There are many targeted agents for NASH at this time, but their response rate is limited to less than 30%,” Dr. Pais said in a presentation at the annual meeting of the American Association for the Study of Liver Diseases. “In very selective patients, bariatric surgery is a very attractive therapeutic option, as it promotes massive weight loss and sustained improvement in metabolic comorbidities, which are concomitant with very high rates of hepatic histological improvement in most but not all patients.” Despite the known benefits of bariatric surgery, histologic outcomes have been minimally studied, Dr. Pais said, which prompted the present trial.
The investigators began by analyzing data from 868 patients with NASH who underwent bariatric surgery with perioperative liver biopsy between 2004 and 2014. Of these patients, 181 had advanced NASH, a diagnosis that was subclassified by severe fibrosis (F3 or F4) or high activity (steatosis, activity, and fibrosis score of 3-4 with F0-2). Out of the 181 patients with advanced NASH, 65 consented to follow-up liver biopsy, which was conducted a mean of 6 years after surgery. Among these patients, 53 had undergone gastric bypass surgery, while 12 had undergone sleeve gastrectomy.
Almost one-third (29%) of the 65 patients who underwent bariatric surgery had normal livers at follow-up biopsy. Among the 35 patients who had severe fibrosis at baseline, slightly more than half (54%) had resolution of severe fibrosis. In contrast, resolution of high activity occurred in almost all affected patients (97%).
While the findings highlighted some of the benefits associated with bariatric surgery, Dr. Pais emphasized the other side of the coin; many patients did not have resolution of severe fibrosis, even years after surgery. Specifically, 45% of patients had persistent severe fibrosis at follow-up biopsy, despite improvements in comorbidities. On average, these patients lost 23% of their baseline body weight, and two-thirds of the group achieved normal ALT and resolution of NASH. Many also had improvements in insulin resistance and nonalcoholic fatty liver disease activity scores. These findings suggest that changes in severe fibrosis occur independently of many other improvements, Dr. Pais said.
Although multiple comorbidities were not correlated with changes in severe fibrosis, several other predictors were identified. Compared with patients who had resolution of severe fibrosis, nonresponders were typically older (56 vs. 49 years), more often had persistent diabetes (79% vs. 50%), and generally had shorter time between surgery and follow-up biopsy (4.2 vs. 7.5 years). Compared with nonresponders, responders were more likely to have undergone gastric bypass surgery (100% vs. 69%), suggesting that this procedure was more effective at resolving severe fibrosis than sleeve gastrectomy.
During the question-and-answer session following the presentation, multiple conference attendees suggested that the title of the study, “Persistence of severe liver fibrosis despite substantial weight loss with bariatric surgery,” was unnecessarily negative, when in fact the study offered strong support for bariatric surgery.
“This is wonderful data that shows surgery is very effective,” one attendee said.
“This is really positive data,” said another. “I mean, you’ve got reversal of advanced fibrosis in half of the population by the time you follow up for several years, so I would say this is really, very positive data.”
The investigators disclosed relationships with Allergan, Boehringer Ingelheim, Novo Nordisk, and others.
*This story was updated on November 15, 2019.
SOURCE: Pais R et al. The Liver Meeting 2019, Abstract 66.
REPORTING FROM THE LIVER MEETING 2019
Machine-learning model predicts NASH based on common clinical and lab values
BOSTON – A machine-learning model based on standard clinical and laboratory values is able to predict nonalcoholic steatohepatitis (NASH) with a sensitivity of 72%-81%, an investigator reported at the annual meeting of the American Association for the Study of Liver Diseases.
The tool, dubbed NASHmap, could serve as an initial screening tool to reveal more potential undiagnosed patients with NASH, according to Jörn M. Schattenberg, MD, with the metabolic liver research program in the department of medicine at University Medical Centre Mainz (Germany).
While not intended to replace current scoring systems, NASHmap potentially could be applied as a clinical decision support tool within electronic medical record systems, allowing greater numbers of patients with suspected NASH to be evaluated and referred to specialists for further testing, according to Dr. Schattenberg.
“To me, this is an at-risk population,” Dr. Schattenberg said in an interview. “There’s a lot of talk of increasing numbers of end-stage liver disease, and these are the cases that are waiting to happen. I think if we identify them, we can manage them better.”
“I’m not saying they should all get drugs – I’m saying they have to be informed about their condition because they may not have a clue they have liver disease,” he continued.
The machine-learning approach described here by Dr. Schattenberg included an exploratory analysis based on 704 patients with NASH or non-NASH nonalcoholic fatty liver disease (NAFLD) in the NAFLD Adult Database from the National Institute of Diabetes, Digestive, and Kidney Diseases.
The best-performing model they identified included 14 variables. Ranked by contribution to predictive power, those variables included hemoglobin A1c, aspartate aminotransferase, alanine aminotransferase, total protein, AST/ALT ratio, body mass index, triglycerides, height, platelets, white blood cells, hematocrit, albumin, hypertension, and sex.
That 14-variable model had good performance when tested on the Optum Humedica electronic medical record database, according to Dr. Schattenberg and colleagues.
In the analysis for final evaluation, including 1,016 patients with histologically confirmed NASH, the area under the curve was 0.76, they reported.
A simplified, five-variable model including just hemoglobin A1c, AST, ALT, total protein, and AST/ALT ratio also had good performance, with an area under the curve of 0.74, they said in their report.
Dr. Schattenberg provided disclosures related to AbbVie, Novartis, MSD, Pfizer, Boehringer Ingelheim, BMS, Intercept Pharmaceuticals, Genfit, Gilead, and Echosens. Several study coauthors reported employment with Novartis and stock ownership.
BOSTON – A machine-learning model based on standard clinical and laboratory values is able to predict nonalcoholic steatohepatitis (NASH) with a sensitivity of 72%-81%, an investigator reported at the annual meeting of the American Association for the Study of Liver Diseases.
The tool, dubbed NASHmap, could serve as an initial screening tool to reveal more potential undiagnosed patients with NASH, according to Jörn M. Schattenberg, MD, with the metabolic liver research program in the department of medicine at University Medical Centre Mainz (Germany).
While not intended to replace current scoring systems, NASHmap potentially could be applied as a clinical decision support tool within electronic medical record systems, allowing greater numbers of patients with suspected NASH to be evaluated and referred to specialists for further testing, according to Dr. Schattenberg.
“To me, this is an at-risk population,” Dr. Schattenberg said in an interview. “There’s a lot of talk of increasing numbers of end-stage liver disease, and these are the cases that are waiting to happen. I think if we identify them, we can manage them better.”
“I’m not saying they should all get drugs – I’m saying they have to be informed about their condition because they may not have a clue they have liver disease,” he continued.
The machine-learning approach described here by Dr. Schattenberg included an exploratory analysis based on 704 patients with NASH or non-NASH nonalcoholic fatty liver disease (NAFLD) in the NAFLD Adult Database from the National Institute of Diabetes, Digestive, and Kidney Diseases.
The best-performing model they identified included 14 variables. Ranked by contribution to predictive power, those variables included hemoglobin A1c, aspartate aminotransferase, alanine aminotransferase, total protein, AST/ALT ratio, body mass index, triglycerides, height, platelets, white blood cells, hematocrit, albumin, hypertension, and sex.
That 14-variable model had good performance when tested on the Optum Humedica electronic medical record database, according to Dr. Schattenberg and colleagues.
In the analysis for final evaluation, including 1,016 patients with histologically confirmed NASH, the area under the curve was 0.76, they reported.
A simplified, five-variable model including just hemoglobin A1c, AST, ALT, total protein, and AST/ALT ratio also had good performance, with an area under the curve of 0.74, they said in their report.
Dr. Schattenberg provided disclosures related to AbbVie, Novartis, MSD, Pfizer, Boehringer Ingelheim, BMS, Intercept Pharmaceuticals, Genfit, Gilead, and Echosens. Several study coauthors reported employment with Novartis and stock ownership.
BOSTON – A machine-learning model based on standard clinical and laboratory values is able to predict nonalcoholic steatohepatitis (NASH) with a sensitivity of 72%-81%, an investigator reported at the annual meeting of the American Association for the Study of Liver Diseases.
The tool, dubbed NASHmap, could serve as an initial screening tool to reveal more potential undiagnosed patients with NASH, according to Jörn M. Schattenberg, MD, with the metabolic liver research program in the department of medicine at University Medical Centre Mainz (Germany).
While not intended to replace current scoring systems, NASHmap potentially could be applied as a clinical decision support tool within electronic medical record systems, allowing greater numbers of patients with suspected NASH to be evaluated and referred to specialists for further testing, according to Dr. Schattenberg.
“To me, this is an at-risk population,” Dr. Schattenberg said in an interview. “There’s a lot of talk of increasing numbers of end-stage liver disease, and these are the cases that are waiting to happen. I think if we identify them, we can manage them better.”
“I’m not saying they should all get drugs – I’m saying they have to be informed about their condition because they may not have a clue they have liver disease,” he continued.
The machine-learning approach described here by Dr. Schattenberg included an exploratory analysis based on 704 patients with NASH or non-NASH nonalcoholic fatty liver disease (NAFLD) in the NAFLD Adult Database from the National Institute of Diabetes, Digestive, and Kidney Diseases.
The best-performing model they identified included 14 variables. Ranked by contribution to predictive power, those variables included hemoglobin A1c, aspartate aminotransferase, alanine aminotransferase, total protein, AST/ALT ratio, body mass index, triglycerides, height, platelets, white blood cells, hematocrit, albumin, hypertension, and sex.
That 14-variable model had good performance when tested on the Optum Humedica electronic medical record database, according to Dr. Schattenberg and colleagues.
In the analysis for final evaluation, including 1,016 patients with histologically confirmed NASH, the area under the curve was 0.76, they reported.
A simplified, five-variable model including just hemoglobin A1c, AST, ALT, total protein, and AST/ALT ratio also had good performance, with an area under the curve of 0.74, they said in their report.
Dr. Schattenberg provided disclosures related to AbbVie, Novartis, MSD, Pfizer, Boehringer Ingelheim, BMS, Intercept Pharmaceuticals, Genfit, Gilead, and Echosens. Several study coauthors reported employment with Novartis and stock ownership.
REPORTING FROM THE LIVER MEETING 2019