User login
Immunotherapy may hold the key to defeating virally associated cancers
Infection with certain viruses has been causally linked to the development of cancer. In recent years, an improved understanding of the unique pathology and molecular underpinnings of these virally associated cancers has prompted the development of more personalized treatment strategies, with a particular focus on immunotherapy. Here, we describe some of the latest developments.
The link between viruses and cancer
Suspicions about a possible role of viral infections in the development of cancer were first aroused in the early 1900s. The seminal discovery is traced back to Peyton Rous, who showed that a malignant tumor growing in a chicken could be transferred to a healthy bird by injecting it with tumor extracts that contained no actual tumor cells.1
The infectious etiology of human cancer, however, remained controversial until many years later when the first cancer-causing virus, Epstein-Barr virus (EBV), was identified in cell cultures from patients with Burkitt lymphoma. Shortly afterward, the Rous sarcoma virus was unveiled as the oncogenic agent behind Rous’ observations.2Seven viruses have now been linked to the development of cancers and are thought to be responsible for around 12% of all cancer cases worldwide. The burden is likely to increase as technological advancements make it easier to establish a causal link between viruses and cancer development.3
In addition to making these links, researchers have also made significant headway in understanding how viruses cause cancer. Cancerous transformation of host cells occurs in only a minority of those who are infected with oncogenic viruses and often occurs in the setting of chronic infection.
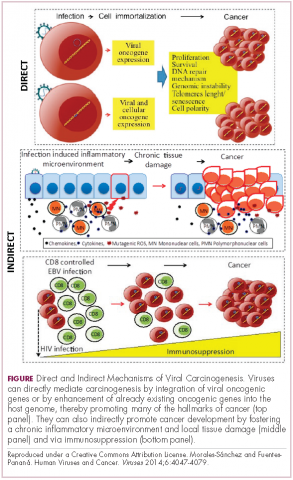
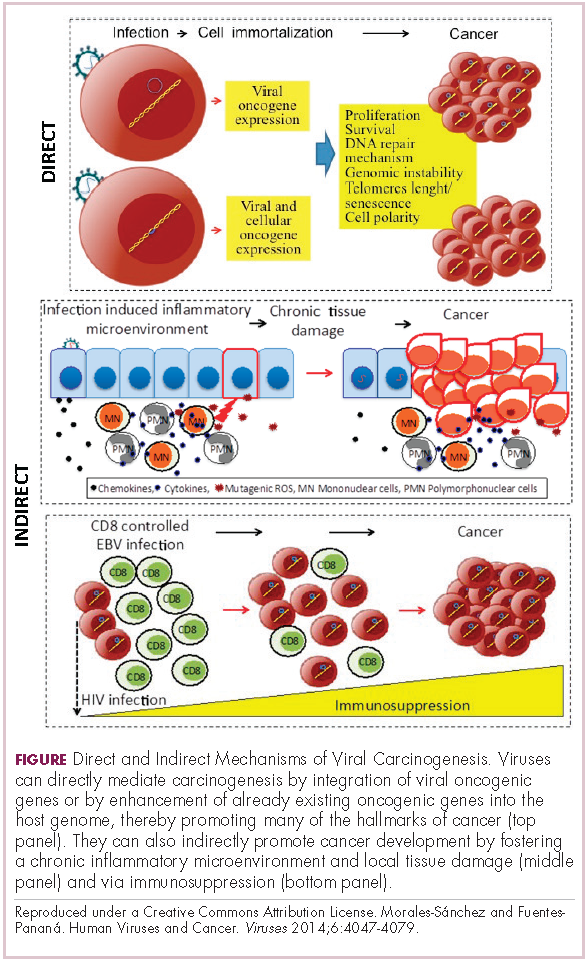
Viruses can mediate carcinogenesis by direct and/or indirect mechanisms (Figure 1). Many of the hallmarks of cancer, the key attributes that drive the transformation from a normal cell to a malignant one, are compatible with the virus’s needs, such as needing to avoid cell death, increasing cell proliferation, and avoiding detection by the immune system.
Viruses hijack the cellular machinery to meet those needs and they can do this either by producing viral proteins that have an oncogenic effect or by integrating their genetic material into the host cell genome. When the latter occurs, the process of integration can also cause damage to the DNA, which further increases the risk of cancer-promoting changes occurring in the host genome.
Viruses can indirectly contribute to carcinogenesis by fostering a microenvironment of chronic inflammation, causing oxidative stress and local tissue damage, and by suppressing the antitumor immune response.4,5
Screening and prevention efforts have helped to reduce the burden of several different virally associated cancers. However, for the substantial proportion of patients who are still affected by these cancers, there is a pressing need for new therapeutic options, particularly since genome sequencing studies have revealed that these cancers can often have distinct underlying molecular mechanisms.
Vaccines lead the charge in HPV-driven cancers
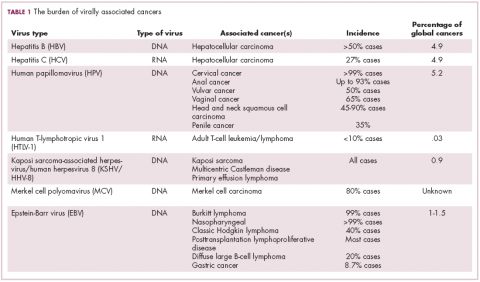
German virologist Harald zur Hausen received the Nobel Prize in 2008 for his discovery of the oncogenic role of human papillomaviruses (HPVs), a large family of more than 100 DNA viruses that infect the epithelial cells of the skin and mucous membranes. They are responsible for the largest number of virally associated cancer cases globally – around 5% (Table 1).
A number of different cancer types are linked to HPV infection, but it is best known as the cause of cervical cancer. The development of diagnostic blood tests and prophylactic vaccines for prevention and early intervention in HPV infection has helped to reduce the incidence of cervical cancer. Conversely, another type of HPV-associated cancer, head and neck squamous cell carcinoma (HNSCC), has seen increased incidence in recent years.
HPVs are categorized according to their oncogenic potential as high, intermediate, or low risk. The high-risk HPV16 and HPV18 strains are most commonly associated with cancer. They are thought to cause cancer predominantly through integration into the host genome. The HPV genome is composed of 8 genes encoding proteins that regulate viral replication and assembly. The E6 and E7 genes are the most highly oncogenic; as the HPV DNA is inserted into the host genome, the transcriptional regulator of E6/E7 is lost, leading to their increased expression. These genes have significant oncogenic potential because of their interaction with 2 tumor suppressor proteins, p53 and pRb.6,7
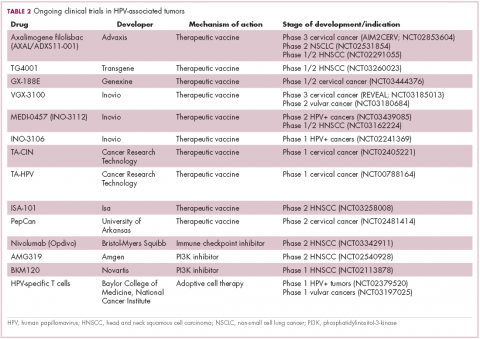
The largest investment in therapeutic development for HPV-positive cancers has been in the realm of immunotherapy in an effort to boost the anti-tumor immune response. In particular, there has been a focus on the development of therapeutic vaccines, designed to prime the anti-tumor immune response to recognize viral antigens. A variety of different types of vaccines are being developed, including live, attenuated and inactivated vaccines that are protein, DNA, or peptide based. Most developed to date target the E6/E7 proteins from the HPV16/18 strains (Table 2).8,9
Other immunotherapies are also being evaluated, including immune checkpoint inhibitors, antibodies designed to target one of the principal mechanisms of immune evasion exploited by cancer cells. The combination of immune checkpoint inhibitors with vaccines is a particularly promising strategy in HPV-associated cancers. At the European Society for Medical Oncology Congress in 2017, the results of a phase 2 trial of nivolumab in combination with ISA-101 were presented.
Among 24 patients with HPV-positive tumors, the majority oropharyngeal cancers, the combination elicited an overall response rate (ORR) of 33%, including 2 complete responses (CRs). Most adverse events (AEs) were mild to moderate in severity and included fever, injection site reactions, fatigue and nausea.14
Hepatocellular carcinoma: a tale of two viruses
The hepatitis viruses are a group of 5 unrelated viruses that causes inflammation of the liver. Hepatitis B (HBV), a DNA virus, and hepatitis C (HCV), an RNA virus, are also oncoviruses; HBV in particular is one of the main causes of hepatocellular carcinoma (HCC), the most common type of liver cancer.
The highly inflammatory environment fostered by HBV and HCV infection causes liver damage that often leads to cirrhosis. Continued infection can drive permanent damage to the hepatocytes, leading to genetic and epigenetic damage and driving oncogenesis. As an RNA virus, HCV doesn’t integrate into the genome and no confirmed viral oncoproteins have been identified to date, therefore it mostly drives cancer through these indirect mechanisms, which is also reflected in the fact that HCV-associated HCC predominantly occurs against a backdrop of liver cirrhosis.
HBV does integrate into the host genome. Genome sequencing studies revealed hundreds of integration sites, but most commonly they disrupted host genes involved in telomere stability and cell cycle regulation, providing some insight into the mechanisms by which HBV-associated HCC develops. In addition, HBV produces several oncoproteins, including HBx, which disrupts gene transcription, cell signaling pathways, cell cycle progress, apoptosis and other cellular processes.15,16
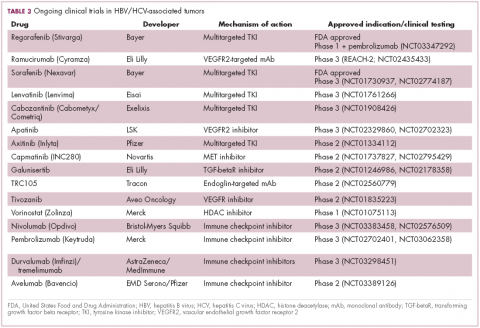
Multitargeted tyrosine kinase inhibitors (TKIs) have been the focal point of therapeutic development in HCC. However, following the approval of sorafenib in 2008, there was a dearth of effective new treatment options despite substantial efforts and numerous phase 3 trials. More recently, immunotherapy has also come to the forefront, especially immune checkpoint inhibitors.
Last year marked the first new drug approvals in nearly a decade – the TKI regorafenib (Stivarga) and immune checkpoint inhibitor nivolumab (Opdivo), both in the second-line setting after failure of sorafenib. Treatment options in this setting may continue to expand, with the TKIs cabozantinib and lenvatinib and the immune checkpoint inhibitor pembrolizumab and the combination of durvalumab and tremelimumab hot on their heels.17-20 Many of these drugs are also being evaluated in the front-line setting in comparison with sorafenib (Table 3).
At the current time, the treatment strategy for patients with HCC is independent of etiology, however, there are significant ongoing efforts to try to tease out the implications of infection for treatment efficacy. A recent meta-analysis of patients treated with sorafenib in 3 randomized phase 3 trials (n = 3,526) suggested that it improved overall survival (OS) among patients who were HCV-positive, but HBV-negative.21
Studies of the vascular endothelial growth factor receptor 2-targeting monoclonal antibody ramucirumab, on the other hand, suggested that it may have a greater OS benefit in patients with HBV, while regorafenib seemed to have a comparable OS benefit in both subgroups.22-25 The immune checkpoint inhibitors studied thus far seem to elicit responses irrespective of infection status.
A phase 2 trial of the immune checkpoint inhibitor tremelimumab was conducted specifically in patients with advanced HCC and chronic HCV infection. The disease control rate (DCR) was 76.4%, with 17.6% partial response (PR) rate. There was also a significant drop in viral load, suggesting that tremelimumab may have antiviral effects.26,27,28
Adoptive cell therapy promising in EBV-positive cancers
More than 90% of the global population is infected with EBV, making it one of the most common human viruses. It is a member of the herpesvirus family that is probably best known as the cause of infectious mononucleosis. On rare occasions, however, EBV can cause tumor development, though our understanding of its exact pathogenic role in cancer is still incomplete.
EBV is a DNA virus that doesn’t tend to integrate into the host genome, but instead remains in the nucleus in the form of episomes and produces several oncoproteins, including latent membrane protein-1. It is associated with a range of different cancer types, including Burkitt lymphoma and other B-cell malignancies. It also infects epithelial cells and can cause nasopharyngeal carcinoma and gastric cancer, however, much less is known about the molecular underpinnings of these EBV-positive cancer types.26,27Gastric cancers actually comprise the largest group of EBV-associated tumors because of the global incidence of this cancer type. The Cancer Genome Atlas Research Network recently characterized gastric cancer on a molecular level and identified an EBV-positive subgroup as a distinct clinical entity with unique molecular characteristics.29
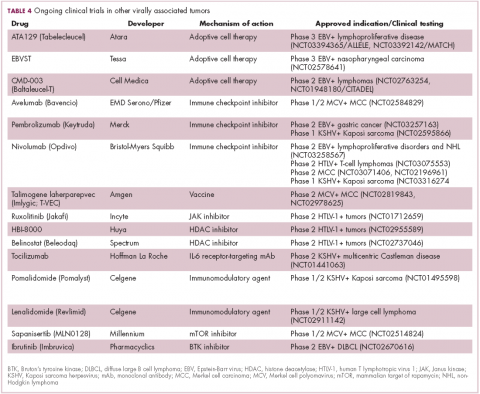
The focus of therapeutic development has again been on immunotherapy, however in this case the idea of collecting the patients T cells, engineering them to recognize EBV, and then reinfusing them into the patient – adoptive cell therapy – has gained the most traction (Table 4).
Two presentations at the American Society of Hematology annual meeting in 2017 detailed ongoing clinical trials of Atara Biotherapeutics’ ATA129 and Cell Medica’s CMD-003. ATA129 was associated with a high response rate and a low rate of serious AEs in patients with posttransplant lymphoproliferative disorder; ORR was 80% in 6 patients treated after hematopoietic stem cell transplantation, and 83% in 6 patients after solid organ transplant.30
CMD-003, meanwhile, demonstrated preliminary signs of activity and safety in patients with relapsed extranodal NK/T-cell lymphoma, according to early results from the phase 2 CITADEL trial. Among 6 evaluable patients, the ORR was 50% and the DCR was 67%.31
Newest oncovirus on the block
The most recently discovered cancer-associated virus is Merkel cell polyomavirus (MCV), a DNA virus that was identified in 2008. Like EBV, virtually the whole global adult population is infected with MCV. It is linked to the development of a highly aggressive and lethal, though rare, form of skin cancer – Merkel cell carcinoma.
MCV is found in around 80% of MCC cases and in fewer than 10% of melanomas and other skin cancers. Thus far, several direct mechanisms of oncogenesis have been described, including integration of MCV into the host genome and the production of viral oncogenes, though their precise function is as yet unclear.32-34
The American Cancer Society estimates that only 1500 cases of MCC are diagnosed each year in the United States.35 Its rarity makes it difficult to conduct clinical trials with sufficient power, yet some headway has still been made.
Around half of MCCs express the programmed cell death ligand 1 (PD-L1) on their surface, making them a logical candidate for immune checkpoint inhibition. In 2017, avelumab became the first FDA-approved drug for the treatment of MCC. Approval was based on the JAVELIN Merkel 200 study in which 88 patients received avelumab. After 1 year of follow-up the ORR was 31.8%, with a CR rate of 9%.36
Genome sequencing studies suggest that the mutational profile of MCV-positive tumors is quite different to those that are MCV-negative, which could have therapeutic implications. To date, these implications have not been delineated, given the challenge of small patient numbers, however an ongoing phase 1/2 trial is evaluating the combination of avelumab and radiation therapy or recombinant interferon beta, with or without MCV-specific cytotoxic T cells in patients with MCC and MCV infection.
The 2 other known cancer-causing viruses are human T-lymphotropic virus 1 (HTLV-1), a retrovirus associated with adult T-cell leukemia/lymphoma (ATL) and Kaposi sarcoma herpesvirus (KSHV). The latter is the causative agent of Kaposi sarcoma, often in combination with human immunodeficiency virus (HIV), a rare skin tumor that became renowned in the 1980s as an AIDS-defining illness.
The incidence of HTLV-1- and KSHV-positive tumors is substantially lower than the other virally associated cancers and, like MCC, this makes studying them and conducting clinical trials of novel therapeutic options a challenge. Nonetheless, several trials of targeted therapies and immunotherapies are underway.
1. Rous PA. Transmissible avain neoplasm. (Sarcoma of the common fowl). J Exp Med. 1910;12(5):696-705.
2. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1(7335):702-703.
3. Mesri Enrique A, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe. 2014;15(3):266-282.
4. Santana-Davila R, Bhatia S, Chow LQ. Harnessing the immune system as a therapeutic tool in virus-associated cancers. JAMA Oncol. 2017;3(1):106-112.
5. Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27(1):59-73.
6. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80-85.
7. Tulay P, Serakinci N. The route to HPV-associated neoplastic transformation: a review of the literature. Crit Rev Eukaryot Gene Expr. 2016;26(1):27-39.
8. Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9(9).
9. Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World Journal of Clinical Oncology. 2014;5(5):1002-1019.
10. Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract. 2017;4:10.
11. Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract. 2017;4:9.
12. Huh W, Dizon D, Powell M, Landrum L, Leath C. A prospective phase II trial of the listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second and third-line metastatic cervical cancer: A NRG oncology group trial. Paper presented at: Annual Meeting on Women's Cancer; March 12-15, 2017, 2017; National Harbor, MD.
13. Petit RG, Mehta A, Jain M, et al. ADXS11-001 immunotherapy targeting HPV-E7: final results from a Phase II study in Indian women with recurrent cervical cancer. Journal for Immunotherapy of Cancer. 2014;2(Suppl 3):P92-P92.
14. Glisson B, Massarelli E, William W, et al. Nivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer. Ann Oncol. 2017;28(suppl_5):v403-v427.
15. Ding X-X, Zhu Q-G, Zhang S-M, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715-55730.
16. Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1732):20160274.
17. Abou-Alfa G, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2017;36(Suppl 4S):abstr 207.
18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018.
19. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36(Suppl 4S):Abstr 209.
20. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. Journal of Clinical Oncology. 2017;35(15_suppl):4073-4073.
21. Jackson R, Psarelli E-E, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. Journal of Clinical Oncology. 2017;35(6):622-628.
22. Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017;6(2):101-112.
23. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650-656.
24. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
25. Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412-3419.
26. Neparidze N, Lacy J. Malignancies associated with epstein-barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12(6):358-371.
27. Ozoya OO, Sokol L, Dalia S. EBV-Related Malignancies, Outcomes and Novel Prevention Strategies. Infect Disord Drug Targets. 2016;16(1):4-21.
28. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81-88.
29. The Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202.
30. Prockop S, Li A, Baiocchi R, et al. Efficacy and safety of ATA129, partially matched allogeneic third-party Epstein-Barr virus-targeted cytotoxic T lymphocytes in a multicenter study for post-transplant lymphoproliferative disorder. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
31. Kim W, Ardeshna K, Lin Y, et al. Autologous EBV-specific T cells (CMD-003): Early results from a multicenter, multinational Phase 2 trial for treatment of EBV-associated NK/T-cell lymphoma. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
32. Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. European Journal of Cancer. 2017;71:53-69.
33. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118-130.
34. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: An update and review: Current and future therapy. J Am Acad Dermatol. 2018;78(3):445-454.
35. American Cancer Society. Key Statistics for Merkel Cell Carcinoma. 2015; https://www.cancer.org/cancer/merkel-cell-skin-cancer/about/key-statistics.html#written_by. Accessed March 7th, 2017.
36. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology.17(10):1374-1385.
Infection with certain viruses has been causally linked to the development of cancer. In recent years, an improved understanding of the unique pathology and molecular underpinnings of these virally associated cancers has prompted the development of more personalized treatment strategies, with a particular focus on immunotherapy. Here, we describe some of the latest developments.
The link between viruses and cancer
Suspicions about a possible role of viral infections in the development of cancer were first aroused in the early 1900s. The seminal discovery is traced back to Peyton Rous, who showed that a malignant tumor growing in a chicken could be transferred to a healthy bird by injecting it with tumor extracts that contained no actual tumor cells.1
The infectious etiology of human cancer, however, remained controversial until many years later when the first cancer-causing virus, Epstein-Barr virus (EBV), was identified in cell cultures from patients with Burkitt lymphoma. Shortly afterward, the Rous sarcoma virus was unveiled as the oncogenic agent behind Rous’ observations.2Seven viruses have now been linked to the development of cancers and are thought to be responsible for around 12% of all cancer cases worldwide. The burden is likely to increase as technological advancements make it easier to establish a causal link between viruses and cancer development.3
In addition to making these links, researchers have also made significant headway in understanding how viruses cause cancer. Cancerous transformation of host cells occurs in only a minority of those who are infected with oncogenic viruses and often occurs in the setting of chronic infection.
Viruses can mediate carcinogenesis by direct and/or indirect mechanisms (Figure 1). Many of the hallmarks of cancer, the key attributes that drive the transformation from a normal cell to a malignant one, are compatible with the virus’s needs, such as needing to avoid cell death, increasing cell proliferation, and avoiding detection by the immune system.
Viruses hijack the cellular machinery to meet those needs and they can do this either by producing viral proteins that have an oncogenic effect or by integrating their genetic material into the host cell genome. When the latter occurs, the process of integration can also cause damage to the DNA, which further increases the risk of cancer-promoting changes occurring in the host genome.
Viruses can indirectly contribute to carcinogenesis by fostering a microenvironment of chronic inflammation, causing oxidative stress and local tissue damage, and by suppressing the antitumor immune response.4,5
Screening and prevention efforts have helped to reduce the burden of several different virally associated cancers. However, for the substantial proportion of patients who are still affected by these cancers, there is a pressing need for new therapeutic options, particularly since genome sequencing studies have revealed that these cancers can often have distinct underlying molecular mechanisms.
Vaccines lead the charge in HPV-driven cancers
German virologist Harald zur Hausen received the Nobel Prize in 2008 for his discovery of the oncogenic role of human papillomaviruses (HPVs), a large family of more than 100 DNA viruses that infect the epithelial cells of the skin and mucous membranes. They are responsible for the largest number of virally associated cancer cases globally – around 5% (Table 1).
A number of different cancer types are linked to HPV infection, but it is best known as the cause of cervical cancer. The development of diagnostic blood tests and prophylactic vaccines for prevention and early intervention in HPV infection has helped to reduce the incidence of cervical cancer. Conversely, another type of HPV-associated cancer, head and neck squamous cell carcinoma (HNSCC), has seen increased incidence in recent years.
HPVs are categorized according to their oncogenic potential as high, intermediate, or low risk. The high-risk HPV16 and HPV18 strains are most commonly associated with cancer. They are thought to cause cancer predominantly through integration into the host genome. The HPV genome is composed of 8 genes encoding proteins that regulate viral replication and assembly. The E6 and E7 genes are the most highly oncogenic; as the HPV DNA is inserted into the host genome, the transcriptional regulator of E6/E7 is lost, leading to their increased expression. These genes have significant oncogenic potential because of their interaction with 2 tumor suppressor proteins, p53 and pRb.6,7
The largest investment in therapeutic development for HPV-positive cancers has been in the realm of immunotherapy in an effort to boost the anti-tumor immune response. In particular, there has been a focus on the development of therapeutic vaccines, designed to prime the anti-tumor immune response to recognize viral antigens. A variety of different types of vaccines are being developed, including live, attenuated and inactivated vaccines that are protein, DNA, or peptide based. Most developed to date target the E6/E7 proteins from the HPV16/18 strains (Table 2).8,9
Other immunotherapies are also being evaluated, including immune checkpoint inhibitors, antibodies designed to target one of the principal mechanisms of immune evasion exploited by cancer cells. The combination of immune checkpoint inhibitors with vaccines is a particularly promising strategy in HPV-associated cancers. At the European Society for Medical Oncology Congress in 2017, the results of a phase 2 trial of nivolumab in combination with ISA-101 were presented.
Among 24 patients with HPV-positive tumors, the majority oropharyngeal cancers, the combination elicited an overall response rate (ORR) of 33%, including 2 complete responses (CRs). Most adverse events (AEs) were mild to moderate in severity and included fever, injection site reactions, fatigue and nausea.14
Hepatocellular carcinoma: a tale of two viruses
The hepatitis viruses are a group of 5 unrelated viruses that causes inflammation of the liver. Hepatitis B (HBV), a DNA virus, and hepatitis C (HCV), an RNA virus, are also oncoviruses; HBV in particular is one of the main causes of hepatocellular carcinoma (HCC), the most common type of liver cancer.
The highly inflammatory environment fostered by HBV and HCV infection causes liver damage that often leads to cirrhosis. Continued infection can drive permanent damage to the hepatocytes, leading to genetic and epigenetic damage and driving oncogenesis. As an RNA virus, HCV doesn’t integrate into the genome and no confirmed viral oncoproteins have been identified to date, therefore it mostly drives cancer through these indirect mechanisms, which is also reflected in the fact that HCV-associated HCC predominantly occurs against a backdrop of liver cirrhosis.
HBV does integrate into the host genome. Genome sequencing studies revealed hundreds of integration sites, but most commonly they disrupted host genes involved in telomere stability and cell cycle regulation, providing some insight into the mechanisms by which HBV-associated HCC develops. In addition, HBV produces several oncoproteins, including HBx, which disrupts gene transcription, cell signaling pathways, cell cycle progress, apoptosis and other cellular processes.15,16
Multitargeted tyrosine kinase inhibitors (TKIs) have been the focal point of therapeutic development in HCC. However, following the approval of sorafenib in 2008, there was a dearth of effective new treatment options despite substantial efforts and numerous phase 3 trials. More recently, immunotherapy has also come to the forefront, especially immune checkpoint inhibitors.
Last year marked the first new drug approvals in nearly a decade – the TKI regorafenib (Stivarga) and immune checkpoint inhibitor nivolumab (Opdivo), both in the second-line setting after failure of sorafenib. Treatment options in this setting may continue to expand, with the TKIs cabozantinib and lenvatinib and the immune checkpoint inhibitor pembrolizumab and the combination of durvalumab and tremelimumab hot on their heels.17-20 Many of these drugs are also being evaluated in the front-line setting in comparison with sorafenib (Table 3).
At the current time, the treatment strategy for patients with HCC is independent of etiology, however, there are significant ongoing efforts to try to tease out the implications of infection for treatment efficacy. A recent meta-analysis of patients treated with sorafenib in 3 randomized phase 3 trials (n = 3,526) suggested that it improved overall survival (OS) among patients who were HCV-positive, but HBV-negative.21
Studies of the vascular endothelial growth factor receptor 2-targeting monoclonal antibody ramucirumab, on the other hand, suggested that it may have a greater OS benefit in patients with HBV, while regorafenib seemed to have a comparable OS benefit in both subgroups.22-25 The immune checkpoint inhibitors studied thus far seem to elicit responses irrespective of infection status.
A phase 2 trial of the immune checkpoint inhibitor tremelimumab was conducted specifically in patients with advanced HCC and chronic HCV infection. The disease control rate (DCR) was 76.4%, with 17.6% partial response (PR) rate. There was also a significant drop in viral load, suggesting that tremelimumab may have antiviral effects.26,27,28
Adoptive cell therapy promising in EBV-positive cancers
More than 90% of the global population is infected with EBV, making it one of the most common human viruses. It is a member of the herpesvirus family that is probably best known as the cause of infectious mononucleosis. On rare occasions, however, EBV can cause tumor development, though our understanding of its exact pathogenic role in cancer is still incomplete.
EBV is a DNA virus that doesn’t tend to integrate into the host genome, but instead remains in the nucleus in the form of episomes and produces several oncoproteins, including latent membrane protein-1. It is associated with a range of different cancer types, including Burkitt lymphoma and other B-cell malignancies. It also infects epithelial cells and can cause nasopharyngeal carcinoma and gastric cancer, however, much less is known about the molecular underpinnings of these EBV-positive cancer types.26,27Gastric cancers actually comprise the largest group of EBV-associated tumors because of the global incidence of this cancer type. The Cancer Genome Atlas Research Network recently characterized gastric cancer on a molecular level and identified an EBV-positive subgroup as a distinct clinical entity with unique molecular characteristics.29
The focus of therapeutic development has again been on immunotherapy, however in this case the idea of collecting the patients T cells, engineering them to recognize EBV, and then reinfusing them into the patient – adoptive cell therapy – has gained the most traction (Table 4).
Two presentations at the American Society of Hematology annual meeting in 2017 detailed ongoing clinical trials of Atara Biotherapeutics’ ATA129 and Cell Medica’s CMD-003. ATA129 was associated with a high response rate and a low rate of serious AEs in patients with posttransplant lymphoproliferative disorder; ORR was 80% in 6 patients treated after hematopoietic stem cell transplantation, and 83% in 6 patients after solid organ transplant.30
CMD-003, meanwhile, demonstrated preliminary signs of activity and safety in patients with relapsed extranodal NK/T-cell lymphoma, according to early results from the phase 2 CITADEL trial. Among 6 evaluable patients, the ORR was 50% and the DCR was 67%.31
Newest oncovirus on the block
The most recently discovered cancer-associated virus is Merkel cell polyomavirus (MCV), a DNA virus that was identified in 2008. Like EBV, virtually the whole global adult population is infected with MCV. It is linked to the development of a highly aggressive and lethal, though rare, form of skin cancer – Merkel cell carcinoma.
MCV is found in around 80% of MCC cases and in fewer than 10% of melanomas and other skin cancers. Thus far, several direct mechanisms of oncogenesis have been described, including integration of MCV into the host genome and the production of viral oncogenes, though their precise function is as yet unclear.32-34
The American Cancer Society estimates that only 1500 cases of MCC are diagnosed each year in the United States.35 Its rarity makes it difficult to conduct clinical trials with sufficient power, yet some headway has still been made.
Around half of MCCs express the programmed cell death ligand 1 (PD-L1) on their surface, making them a logical candidate for immune checkpoint inhibition. In 2017, avelumab became the first FDA-approved drug for the treatment of MCC. Approval was based on the JAVELIN Merkel 200 study in which 88 patients received avelumab. After 1 year of follow-up the ORR was 31.8%, with a CR rate of 9%.36
Genome sequencing studies suggest that the mutational profile of MCV-positive tumors is quite different to those that are MCV-negative, which could have therapeutic implications. To date, these implications have not been delineated, given the challenge of small patient numbers, however an ongoing phase 1/2 trial is evaluating the combination of avelumab and radiation therapy or recombinant interferon beta, with or without MCV-specific cytotoxic T cells in patients with MCC and MCV infection.
The 2 other known cancer-causing viruses are human T-lymphotropic virus 1 (HTLV-1), a retrovirus associated with adult T-cell leukemia/lymphoma (ATL) and Kaposi sarcoma herpesvirus (KSHV). The latter is the causative agent of Kaposi sarcoma, often in combination with human immunodeficiency virus (HIV), a rare skin tumor that became renowned in the 1980s as an AIDS-defining illness.
The incidence of HTLV-1- and KSHV-positive tumors is substantially lower than the other virally associated cancers and, like MCC, this makes studying them and conducting clinical trials of novel therapeutic options a challenge. Nonetheless, several trials of targeted therapies and immunotherapies are underway.
Infection with certain viruses has been causally linked to the development of cancer. In recent years, an improved understanding of the unique pathology and molecular underpinnings of these virally associated cancers has prompted the development of more personalized treatment strategies, with a particular focus on immunotherapy. Here, we describe some of the latest developments.
The link between viruses and cancer
Suspicions about a possible role of viral infections in the development of cancer were first aroused in the early 1900s. The seminal discovery is traced back to Peyton Rous, who showed that a malignant tumor growing in a chicken could be transferred to a healthy bird by injecting it with tumor extracts that contained no actual tumor cells.1
The infectious etiology of human cancer, however, remained controversial until many years later when the first cancer-causing virus, Epstein-Barr virus (EBV), was identified in cell cultures from patients with Burkitt lymphoma. Shortly afterward, the Rous sarcoma virus was unveiled as the oncogenic agent behind Rous’ observations.2Seven viruses have now been linked to the development of cancers and are thought to be responsible for around 12% of all cancer cases worldwide. The burden is likely to increase as technological advancements make it easier to establish a causal link between viruses and cancer development.3
In addition to making these links, researchers have also made significant headway in understanding how viruses cause cancer. Cancerous transformation of host cells occurs in only a minority of those who are infected with oncogenic viruses and often occurs in the setting of chronic infection.
Viruses can mediate carcinogenesis by direct and/or indirect mechanisms (Figure 1). Many of the hallmarks of cancer, the key attributes that drive the transformation from a normal cell to a malignant one, are compatible with the virus’s needs, such as needing to avoid cell death, increasing cell proliferation, and avoiding detection by the immune system.
Viruses hijack the cellular machinery to meet those needs and they can do this either by producing viral proteins that have an oncogenic effect or by integrating their genetic material into the host cell genome. When the latter occurs, the process of integration can also cause damage to the DNA, which further increases the risk of cancer-promoting changes occurring in the host genome.
Viruses can indirectly contribute to carcinogenesis by fostering a microenvironment of chronic inflammation, causing oxidative stress and local tissue damage, and by suppressing the antitumor immune response.4,5
Screening and prevention efforts have helped to reduce the burden of several different virally associated cancers. However, for the substantial proportion of patients who are still affected by these cancers, there is a pressing need for new therapeutic options, particularly since genome sequencing studies have revealed that these cancers can often have distinct underlying molecular mechanisms.
Vaccines lead the charge in HPV-driven cancers
German virologist Harald zur Hausen received the Nobel Prize in 2008 for his discovery of the oncogenic role of human papillomaviruses (HPVs), a large family of more than 100 DNA viruses that infect the epithelial cells of the skin and mucous membranes. They are responsible for the largest number of virally associated cancer cases globally – around 5% (Table 1).
A number of different cancer types are linked to HPV infection, but it is best known as the cause of cervical cancer. The development of diagnostic blood tests and prophylactic vaccines for prevention and early intervention in HPV infection has helped to reduce the incidence of cervical cancer. Conversely, another type of HPV-associated cancer, head and neck squamous cell carcinoma (HNSCC), has seen increased incidence in recent years.
HPVs are categorized according to their oncogenic potential as high, intermediate, or low risk. The high-risk HPV16 and HPV18 strains are most commonly associated with cancer. They are thought to cause cancer predominantly through integration into the host genome. The HPV genome is composed of 8 genes encoding proteins that regulate viral replication and assembly. The E6 and E7 genes are the most highly oncogenic; as the HPV DNA is inserted into the host genome, the transcriptional regulator of E6/E7 is lost, leading to their increased expression. These genes have significant oncogenic potential because of their interaction with 2 tumor suppressor proteins, p53 and pRb.6,7
The largest investment in therapeutic development for HPV-positive cancers has been in the realm of immunotherapy in an effort to boost the anti-tumor immune response. In particular, there has been a focus on the development of therapeutic vaccines, designed to prime the anti-tumor immune response to recognize viral antigens. A variety of different types of vaccines are being developed, including live, attenuated and inactivated vaccines that are protein, DNA, or peptide based. Most developed to date target the E6/E7 proteins from the HPV16/18 strains (Table 2).8,9
Other immunotherapies are also being evaluated, including immune checkpoint inhibitors, antibodies designed to target one of the principal mechanisms of immune evasion exploited by cancer cells. The combination of immune checkpoint inhibitors with vaccines is a particularly promising strategy in HPV-associated cancers. At the European Society for Medical Oncology Congress in 2017, the results of a phase 2 trial of nivolumab in combination with ISA-101 were presented.
Among 24 patients with HPV-positive tumors, the majority oropharyngeal cancers, the combination elicited an overall response rate (ORR) of 33%, including 2 complete responses (CRs). Most adverse events (AEs) were mild to moderate in severity and included fever, injection site reactions, fatigue and nausea.14
Hepatocellular carcinoma: a tale of two viruses
The hepatitis viruses are a group of 5 unrelated viruses that causes inflammation of the liver. Hepatitis B (HBV), a DNA virus, and hepatitis C (HCV), an RNA virus, are also oncoviruses; HBV in particular is one of the main causes of hepatocellular carcinoma (HCC), the most common type of liver cancer.
The highly inflammatory environment fostered by HBV and HCV infection causes liver damage that often leads to cirrhosis. Continued infection can drive permanent damage to the hepatocytes, leading to genetic and epigenetic damage and driving oncogenesis. As an RNA virus, HCV doesn’t integrate into the genome and no confirmed viral oncoproteins have been identified to date, therefore it mostly drives cancer through these indirect mechanisms, which is also reflected in the fact that HCV-associated HCC predominantly occurs against a backdrop of liver cirrhosis.
HBV does integrate into the host genome. Genome sequencing studies revealed hundreds of integration sites, but most commonly they disrupted host genes involved in telomere stability and cell cycle regulation, providing some insight into the mechanisms by which HBV-associated HCC develops. In addition, HBV produces several oncoproteins, including HBx, which disrupts gene transcription, cell signaling pathways, cell cycle progress, apoptosis and other cellular processes.15,16
Multitargeted tyrosine kinase inhibitors (TKIs) have been the focal point of therapeutic development in HCC. However, following the approval of sorafenib in 2008, there was a dearth of effective new treatment options despite substantial efforts and numerous phase 3 trials. More recently, immunotherapy has also come to the forefront, especially immune checkpoint inhibitors.
Last year marked the first new drug approvals in nearly a decade – the TKI regorafenib (Stivarga) and immune checkpoint inhibitor nivolumab (Opdivo), both in the second-line setting after failure of sorafenib. Treatment options in this setting may continue to expand, with the TKIs cabozantinib and lenvatinib and the immune checkpoint inhibitor pembrolizumab and the combination of durvalumab and tremelimumab hot on their heels.17-20 Many of these drugs are also being evaluated in the front-line setting in comparison with sorafenib (Table 3).
At the current time, the treatment strategy for patients with HCC is independent of etiology, however, there are significant ongoing efforts to try to tease out the implications of infection for treatment efficacy. A recent meta-analysis of patients treated with sorafenib in 3 randomized phase 3 trials (n = 3,526) suggested that it improved overall survival (OS) among patients who were HCV-positive, but HBV-negative.21
Studies of the vascular endothelial growth factor receptor 2-targeting monoclonal antibody ramucirumab, on the other hand, suggested that it may have a greater OS benefit in patients with HBV, while regorafenib seemed to have a comparable OS benefit in both subgroups.22-25 The immune checkpoint inhibitors studied thus far seem to elicit responses irrespective of infection status.
A phase 2 trial of the immune checkpoint inhibitor tremelimumab was conducted specifically in patients with advanced HCC and chronic HCV infection. The disease control rate (DCR) was 76.4%, with 17.6% partial response (PR) rate. There was also a significant drop in viral load, suggesting that tremelimumab may have antiviral effects.26,27,28
Adoptive cell therapy promising in EBV-positive cancers
More than 90% of the global population is infected with EBV, making it one of the most common human viruses. It is a member of the herpesvirus family that is probably best known as the cause of infectious mononucleosis. On rare occasions, however, EBV can cause tumor development, though our understanding of its exact pathogenic role in cancer is still incomplete.
EBV is a DNA virus that doesn’t tend to integrate into the host genome, but instead remains in the nucleus in the form of episomes and produces several oncoproteins, including latent membrane protein-1. It is associated with a range of different cancer types, including Burkitt lymphoma and other B-cell malignancies. It also infects epithelial cells and can cause nasopharyngeal carcinoma and gastric cancer, however, much less is known about the molecular underpinnings of these EBV-positive cancer types.26,27Gastric cancers actually comprise the largest group of EBV-associated tumors because of the global incidence of this cancer type. The Cancer Genome Atlas Research Network recently characterized gastric cancer on a molecular level and identified an EBV-positive subgroup as a distinct clinical entity with unique molecular characteristics.29
The focus of therapeutic development has again been on immunotherapy, however in this case the idea of collecting the patients T cells, engineering them to recognize EBV, and then reinfusing them into the patient – adoptive cell therapy – has gained the most traction (Table 4).
Two presentations at the American Society of Hematology annual meeting in 2017 detailed ongoing clinical trials of Atara Biotherapeutics’ ATA129 and Cell Medica’s CMD-003. ATA129 was associated with a high response rate and a low rate of serious AEs in patients with posttransplant lymphoproliferative disorder; ORR was 80% in 6 patients treated after hematopoietic stem cell transplantation, and 83% in 6 patients after solid organ transplant.30
CMD-003, meanwhile, demonstrated preliminary signs of activity and safety in patients with relapsed extranodal NK/T-cell lymphoma, according to early results from the phase 2 CITADEL trial. Among 6 evaluable patients, the ORR was 50% and the DCR was 67%.31
Newest oncovirus on the block
The most recently discovered cancer-associated virus is Merkel cell polyomavirus (MCV), a DNA virus that was identified in 2008. Like EBV, virtually the whole global adult population is infected with MCV. It is linked to the development of a highly aggressive and lethal, though rare, form of skin cancer – Merkel cell carcinoma.
MCV is found in around 80% of MCC cases and in fewer than 10% of melanomas and other skin cancers. Thus far, several direct mechanisms of oncogenesis have been described, including integration of MCV into the host genome and the production of viral oncogenes, though their precise function is as yet unclear.32-34
The American Cancer Society estimates that only 1500 cases of MCC are diagnosed each year in the United States.35 Its rarity makes it difficult to conduct clinical trials with sufficient power, yet some headway has still been made.
Around half of MCCs express the programmed cell death ligand 1 (PD-L1) on their surface, making them a logical candidate for immune checkpoint inhibition. In 2017, avelumab became the first FDA-approved drug for the treatment of MCC. Approval was based on the JAVELIN Merkel 200 study in which 88 patients received avelumab. After 1 year of follow-up the ORR was 31.8%, with a CR rate of 9%.36
Genome sequencing studies suggest that the mutational profile of MCV-positive tumors is quite different to those that are MCV-negative, which could have therapeutic implications. To date, these implications have not been delineated, given the challenge of small patient numbers, however an ongoing phase 1/2 trial is evaluating the combination of avelumab and radiation therapy or recombinant interferon beta, with or without MCV-specific cytotoxic T cells in patients with MCC and MCV infection.
The 2 other known cancer-causing viruses are human T-lymphotropic virus 1 (HTLV-1), a retrovirus associated with adult T-cell leukemia/lymphoma (ATL) and Kaposi sarcoma herpesvirus (KSHV). The latter is the causative agent of Kaposi sarcoma, often in combination with human immunodeficiency virus (HIV), a rare skin tumor that became renowned in the 1980s as an AIDS-defining illness.
The incidence of HTLV-1- and KSHV-positive tumors is substantially lower than the other virally associated cancers and, like MCC, this makes studying them and conducting clinical trials of novel therapeutic options a challenge. Nonetheless, several trials of targeted therapies and immunotherapies are underway.
1. Rous PA. Transmissible avain neoplasm. (Sarcoma of the common fowl). J Exp Med. 1910;12(5):696-705.
2. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1(7335):702-703.
3. Mesri Enrique A, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe. 2014;15(3):266-282.
4. Santana-Davila R, Bhatia S, Chow LQ. Harnessing the immune system as a therapeutic tool in virus-associated cancers. JAMA Oncol. 2017;3(1):106-112.
5. Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27(1):59-73.
6. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80-85.
7. Tulay P, Serakinci N. The route to HPV-associated neoplastic transformation: a review of the literature. Crit Rev Eukaryot Gene Expr. 2016;26(1):27-39.
8. Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9(9).
9. Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World Journal of Clinical Oncology. 2014;5(5):1002-1019.
10. Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract. 2017;4:10.
11. Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract. 2017;4:9.
12. Huh W, Dizon D, Powell M, Landrum L, Leath C. A prospective phase II trial of the listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second and third-line metastatic cervical cancer: A NRG oncology group trial. Paper presented at: Annual Meeting on Women's Cancer; March 12-15, 2017, 2017; National Harbor, MD.
13. Petit RG, Mehta A, Jain M, et al. ADXS11-001 immunotherapy targeting HPV-E7: final results from a Phase II study in Indian women with recurrent cervical cancer. Journal for Immunotherapy of Cancer. 2014;2(Suppl 3):P92-P92.
14. Glisson B, Massarelli E, William W, et al. Nivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer. Ann Oncol. 2017;28(suppl_5):v403-v427.
15. Ding X-X, Zhu Q-G, Zhang S-M, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715-55730.
16. Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1732):20160274.
17. Abou-Alfa G, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2017;36(Suppl 4S):abstr 207.
18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018.
19. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36(Suppl 4S):Abstr 209.
20. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. Journal of Clinical Oncology. 2017;35(15_suppl):4073-4073.
21. Jackson R, Psarelli E-E, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. Journal of Clinical Oncology. 2017;35(6):622-628.
22. Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017;6(2):101-112.
23. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650-656.
24. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
25. Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412-3419.
26. Neparidze N, Lacy J. Malignancies associated with epstein-barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12(6):358-371.
27. Ozoya OO, Sokol L, Dalia S. EBV-Related Malignancies, Outcomes and Novel Prevention Strategies. Infect Disord Drug Targets. 2016;16(1):4-21.
28. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81-88.
29. The Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202.
30. Prockop S, Li A, Baiocchi R, et al. Efficacy and safety of ATA129, partially matched allogeneic third-party Epstein-Barr virus-targeted cytotoxic T lymphocytes in a multicenter study for post-transplant lymphoproliferative disorder. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
31. Kim W, Ardeshna K, Lin Y, et al. Autologous EBV-specific T cells (CMD-003): Early results from a multicenter, multinational Phase 2 trial for treatment of EBV-associated NK/T-cell lymphoma. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
32. Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. European Journal of Cancer. 2017;71:53-69.
33. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118-130.
34. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: An update and review: Current and future therapy. J Am Acad Dermatol. 2018;78(3):445-454.
35. American Cancer Society. Key Statistics for Merkel Cell Carcinoma. 2015; https://www.cancer.org/cancer/merkel-cell-skin-cancer/about/key-statistics.html#written_by. Accessed March 7th, 2017.
36. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology.17(10):1374-1385.
1. Rous PA. Transmissible avain neoplasm. (Sarcoma of the common fowl). J Exp Med. 1910;12(5):696-705.
2. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1(7335):702-703.
3. Mesri Enrique A, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe. 2014;15(3):266-282.
4. Santana-Davila R, Bhatia S, Chow LQ. Harnessing the immune system as a therapeutic tool in virus-associated cancers. JAMA Oncol. 2017;3(1):106-112.
5. Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27(1):59-73.
6. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80-85.
7. Tulay P, Serakinci N. The route to HPV-associated neoplastic transformation: a review of the literature. Crit Rev Eukaryot Gene Expr. 2016;26(1):27-39.
8. Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9(9).
9. Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World Journal of Clinical Oncology. 2014;5(5):1002-1019.
10. Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract. 2017;4:10.
11. Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract. 2017;4:9.
12. Huh W, Dizon D, Powell M, Landrum L, Leath C. A prospective phase II trial of the listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second and third-line metastatic cervical cancer: A NRG oncology group trial. Paper presented at: Annual Meeting on Women's Cancer; March 12-15, 2017, 2017; National Harbor, MD.
13. Petit RG, Mehta A, Jain M, et al. ADXS11-001 immunotherapy targeting HPV-E7: final results from a Phase II study in Indian women with recurrent cervical cancer. Journal for Immunotherapy of Cancer. 2014;2(Suppl 3):P92-P92.
14. Glisson B, Massarelli E, William W, et al. Nivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer. Ann Oncol. 2017;28(suppl_5):v403-v427.
15. Ding X-X, Zhu Q-G, Zhang S-M, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715-55730.
16. Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1732):20160274.
17. Abou-Alfa G, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2017;36(Suppl 4S):abstr 207.
18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018.
19. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36(Suppl 4S):Abstr 209.
20. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. Journal of Clinical Oncology. 2017;35(15_suppl):4073-4073.
21. Jackson R, Psarelli E-E, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. Journal of Clinical Oncology. 2017;35(6):622-628.
22. Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017;6(2):101-112.
23. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650-656.
24. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
25. Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412-3419.
26. Neparidze N, Lacy J. Malignancies associated with epstein-barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12(6):358-371.
27. Ozoya OO, Sokol L, Dalia S. EBV-Related Malignancies, Outcomes and Novel Prevention Strategies. Infect Disord Drug Targets. 2016;16(1):4-21.
28. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81-88.
29. The Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202.
30. Prockop S, Li A, Baiocchi R, et al. Efficacy and safety of ATA129, partially matched allogeneic third-party Epstein-Barr virus-targeted cytotoxic T lymphocytes in a multicenter study for post-transplant lymphoproliferative disorder. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
31. Kim W, Ardeshna K, Lin Y, et al. Autologous EBV-specific T cells (CMD-003): Early results from a multicenter, multinational Phase 2 trial for treatment of EBV-associated NK/T-cell lymphoma. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
32. Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. European Journal of Cancer. 2017;71:53-69.
33. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118-130.
34. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: An update and review: Current and future therapy. J Am Acad Dermatol. 2018;78(3):445-454.
35. American Cancer Society. Key Statistics for Merkel Cell Carcinoma. 2015; https://www.cancer.org/cancer/merkel-cell-skin-cancer/about/key-statistics.html#written_by. Accessed March 7th, 2017.
36. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology.17(10):1374-1385.
BAP1 expression augurs prognosis of metastatic RCC
Expression in metastatic tissues of BAP1, a gene encoding for a tumor suppressor protein, is a significant marker for progression-free and overall survival in patients with metastatic clear cell renal cell carcinoma (ccRCC), investigators contended.
An analysis of tissue samples from 124 patients with metastatic ccRCC showed that patients with metastatic tissues expressing BAP1 had a 5-year overall survival (OS) rate of 53.2%, compared with 35.1% for patients whose tissues did not express the gene (P = .004), reported Walter Henriques da Costa, MD, PhD, and colleagues from the A.C. Camargo Cancer Center in Sao Paulo.
BAP1 expression was also associated with significantly better progression-free survival (PFS), but expression of a different gene thought to be associated with prognosis in metastatic ccRCC, PBRM1, was not a significant predictor of either overall survival (OS) or PFS.
“The pattern of immunohistochemical expression of both PBRM1 and BAP1 was shown to be significantly discordant when comparing the expression of primary tumor and metastatic tumor tissue. The use of prognostic biomarkers identified in the primary tumor tissue might be not reliable in the metastatic disease scenario. Patients with metastatic ccRCC that present loss of BAP1 expression in metastatic tissue demonstrated poor survival rates and represent a relevant risk group for tumor recurrence and death,” the investigators wrote in Urologic Oncology.
Both BAP1 and PBRM1 have been shown to be frequently mutated in ccRCC. These and other mutated genes recently identified through next-generation sequencing encode proteins that are involved in chromatin regulation and act as gene suppressors.
To see whether expression of the genes had prognostic value, they performed immunohistochemical studies of tissues from 124 consecutive patients in their center who underwent metastasectomy or biopsy of metastases and from 38 paired cases with tissues from the primary tumors of patients who underwent partial or radical nephrectomies.
They found that 98 of the metastatic samples (79%) stained negative for PBRM1 and 26 (21%) stained positive for expression of the gene; 62.1% of samples were negative for BAP1 expression and 37.9% were positive.
There were discordant expression patterns between primary tumors and metastases for BAP1 in 17 of the 38 (44.7%) samples from patients with both primary and metastatic tissues and for PBRM1 in 19 of 38 (50%) samples.
As noted before, the 5-year OS rate was 53.2% for patients with BAP1-positive metastases, compared with 35.1% for patients with BAP1-negative tissues (P = .004). The respective 5-year PFS rates for BAP1 expression were 14.9% and 3.9% (P = .003).
There were no significant differences associated with PBRM1 expression for either PFS or OS, however.
Negative expression of BAP1 in metastases was associated in multivariate analysis with both higher risk of death (hazard ratio, 2.017; P = .045) and with disease progression (HR, 1.586; P = .012).
The finding of discordance in expression between the primary tumor and metastases shows that the “strategy of using tissue markers of the primary tumor in the prediction of response of metastatic disease is not reliable. Such fact reinforces the imminent need for identification and validation of tumor markers in metastatic tissue,” the authors wrote.
No funding source or author disclosures were reported.
SOURCE: da Costa WH et al. Urol Oncol. 2018 Nov 13. doi: 10.1016/j.urolonc.2018.10.017.
Expression in metastatic tissues of BAP1, a gene encoding for a tumor suppressor protein, is a significant marker for progression-free and overall survival in patients with metastatic clear cell renal cell carcinoma (ccRCC), investigators contended.
An analysis of tissue samples from 124 patients with metastatic ccRCC showed that patients with metastatic tissues expressing BAP1 had a 5-year overall survival (OS) rate of 53.2%, compared with 35.1% for patients whose tissues did not express the gene (P = .004), reported Walter Henriques da Costa, MD, PhD, and colleagues from the A.C. Camargo Cancer Center in Sao Paulo.
BAP1 expression was also associated with significantly better progression-free survival (PFS), but expression of a different gene thought to be associated with prognosis in metastatic ccRCC, PBRM1, was not a significant predictor of either overall survival (OS) or PFS.
“The pattern of immunohistochemical expression of both PBRM1 and BAP1 was shown to be significantly discordant when comparing the expression of primary tumor and metastatic tumor tissue. The use of prognostic biomarkers identified in the primary tumor tissue might be not reliable in the metastatic disease scenario. Patients with metastatic ccRCC that present loss of BAP1 expression in metastatic tissue demonstrated poor survival rates and represent a relevant risk group for tumor recurrence and death,” the investigators wrote in Urologic Oncology.
Both BAP1 and PBRM1 have been shown to be frequently mutated in ccRCC. These and other mutated genes recently identified through next-generation sequencing encode proteins that are involved in chromatin regulation and act as gene suppressors.
To see whether expression of the genes had prognostic value, they performed immunohistochemical studies of tissues from 124 consecutive patients in their center who underwent metastasectomy or biopsy of metastases and from 38 paired cases with tissues from the primary tumors of patients who underwent partial or radical nephrectomies.
They found that 98 of the metastatic samples (79%) stained negative for PBRM1 and 26 (21%) stained positive for expression of the gene; 62.1% of samples were negative for BAP1 expression and 37.9% were positive.
There were discordant expression patterns between primary tumors and metastases for BAP1 in 17 of the 38 (44.7%) samples from patients with both primary and metastatic tissues and for PBRM1 in 19 of 38 (50%) samples.
As noted before, the 5-year OS rate was 53.2% for patients with BAP1-positive metastases, compared with 35.1% for patients with BAP1-negative tissues (P = .004). The respective 5-year PFS rates for BAP1 expression were 14.9% and 3.9% (P = .003).
There were no significant differences associated with PBRM1 expression for either PFS or OS, however.
Negative expression of BAP1 in metastases was associated in multivariate analysis with both higher risk of death (hazard ratio, 2.017; P = .045) and with disease progression (HR, 1.586; P = .012).
The finding of discordance in expression between the primary tumor and metastases shows that the “strategy of using tissue markers of the primary tumor in the prediction of response of metastatic disease is not reliable. Such fact reinforces the imminent need for identification and validation of tumor markers in metastatic tissue,” the authors wrote.
No funding source or author disclosures were reported.
SOURCE: da Costa WH et al. Urol Oncol. 2018 Nov 13. doi: 10.1016/j.urolonc.2018.10.017.
Expression in metastatic tissues of BAP1, a gene encoding for a tumor suppressor protein, is a significant marker for progression-free and overall survival in patients with metastatic clear cell renal cell carcinoma (ccRCC), investigators contended.
An analysis of tissue samples from 124 patients with metastatic ccRCC showed that patients with metastatic tissues expressing BAP1 had a 5-year overall survival (OS) rate of 53.2%, compared with 35.1% for patients whose tissues did not express the gene (P = .004), reported Walter Henriques da Costa, MD, PhD, and colleagues from the A.C. Camargo Cancer Center in Sao Paulo.
BAP1 expression was also associated with significantly better progression-free survival (PFS), but expression of a different gene thought to be associated with prognosis in metastatic ccRCC, PBRM1, was not a significant predictor of either overall survival (OS) or PFS.
“The pattern of immunohistochemical expression of both PBRM1 and BAP1 was shown to be significantly discordant when comparing the expression of primary tumor and metastatic tumor tissue. The use of prognostic biomarkers identified in the primary tumor tissue might be not reliable in the metastatic disease scenario. Patients with metastatic ccRCC that present loss of BAP1 expression in metastatic tissue demonstrated poor survival rates and represent a relevant risk group for tumor recurrence and death,” the investigators wrote in Urologic Oncology.
Both BAP1 and PBRM1 have been shown to be frequently mutated in ccRCC. These and other mutated genes recently identified through next-generation sequencing encode proteins that are involved in chromatin regulation and act as gene suppressors.
To see whether expression of the genes had prognostic value, they performed immunohistochemical studies of tissues from 124 consecutive patients in their center who underwent metastasectomy or biopsy of metastases and from 38 paired cases with tissues from the primary tumors of patients who underwent partial or radical nephrectomies.
They found that 98 of the metastatic samples (79%) stained negative for PBRM1 and 26 (21%) stained positive for expression of the gene; 62.1% of samples were negative for BAP1 expression and 37.9% were positive.
There were discordant expression patterns between primary tumors and metastases for BAP1 in 17 of the 38 (44.7%) samples from patients with both primary and metastatic tissues and for PBRM1 in 19 of 38 (50%) samples.
As noted before, the 5-year OS rate was 53.2% for patients with BAP1-positive metastases, compared with 35.1% for patients with BAP1-negative tissues (P = .004). The respective 5-year PFS rates for BAP1 expression were 14.9% and 3.9% (P = .003).
There were no significant differences associated with PBRM1 expression for either PFS or OS, however.
Negative expression of BAP1 in metastases was associated in multivariate analysis with both higher risk of death (hazard ratio, 2.017; P = .045) and with disease progression (HR, 1.586; P = .012).
The finding of discordance in expression between the primary tumor and metastases shows that the “strategy of using tissue markers of the primary tumor in the prediction of response of metastatic disease is not reliable. Such fact reinforces the imminent need for identification and validation of tumor markers in metastatic tissue,” the authors wrote.
No funding source or author disclosures were reported.
SOURCE: da Costa WH et al. Urol Oncol. 2018 Nov 13. doi: 10.1016/j.urolonc.2018.10.017.
FROM UROLOGIC ONCOLOGY
Key clinical point: Metastatic clear cell renal cell carcinoma tissue expression of BAP1 is a marker for better overall and progression-free survival.
Major finding: The 5-year overall OS rate was 53.2% for patients with BAP1-positive metastases versus 35.1% for those with BAP1-negative tissues (P = .004).
Study details: A retrospective analysis of tumor samples from 124 patients with metastatic clear cell renal cell carcinoma and 38 patients with paired primary tumor and metastases samples.
Disclosures: No funding source or author disclosures were reported.
Source: da Costa WH et al. Urol Oncol. 2018 Nov 13. doi: 10.1016/j.urolonc.2018.10.017.
PD-L1 correlates with worse sporadic, hereditary ccRCC outcomes
In patients with either sporadic or hereditary clear cell renal cell carcinoma, tumor expression of programmed death–ligand 1 (PD-L1) is associated with aggressive disease, investigators have found.
Analysis of tumor samples from patients with sporadic clear cell renal cell carcinoma (ccRCC) and others with Von Hippel-Lindau (VHL)–associated hereditary ccRCC showed that positive PD-L1 correlated with aggressive clinicopathologic features, reported Baoan Hong, MD, from Peking University First Hospital in Beijing, and colleagues.
“PD-L1 is a promising predictive biomarker for the utilization of PD-1/PD-L1 checkpoint inhibitors in ccRCC patients,” they wrote in Genitourinary Cancer.
The investigators conducted a retrospective analysis of PD-L1 expression and its potential correlation with disease features using samples from 129 patients with sporadic ccRCC and 26 patients with VHL disease who underwent partial or radical nephrectomy at their center from 2010 to 2017.
Sporadic ccRCC
The median age of patients with sporadic ccRCC was 61 years and the median tumor size was 4.3 cm. Of the 129 patients, 56 had pathological stage T1a at diagnosis, 44 had stage T1b, 8 had stage T2, and 21 had stage T3. In all, 25 patients had Fuhrman nuclear grade 3 tumors and 104 had grade 1 or 2 tumors. A total of 7 patients had metastases to lymph nodes, 41 had microvascular invasion, and 16 had tumor necrosis.
In all, 61 of these patients had PD-L1-positive tumors and 68 were PD-L1 negative. Positive PD-L1 was significantly associated with male gender (P = .025) and worse disease features, including higher T stage (P = .0011) and higher Fuhrman nuclear grade (P = .022).
After a median follow-up of 68 months, 9 patients in this group died and 17 others developed distant metastases or recurrent disease. Patients whose tumors were PD-L1 negative had significantly longer disease-free survival than patients with PD-L1-positive tumors, at a median 36 versus 28 months (P = .037).
VHL-associated ccRCC
Of the 26 patients with VHL-associated hereditary ccRCC (13 men and 13 women; median age, 42 years), 13 had pathological stage T1a disease, 7 had T1b, and 2 each has stage T2a, T3a, and T3b tumors. A total of 18 patients had Fuhrman nuclear grade 1 tumors and 8 had grade 2 tumors.
In this cohort, 17 patients had PD-L1-negative tumors, and 9 had PD-L1-positive tumors. PD-L1 expression was more common in patients with Fuhrman nuclear grade 2 tumors (six of eight cases). Patients with Fuhrman nuclear grade 1 tumors were more likely to be PD-L1 negative (15 of 18, P = .008). PD-L1 expression was not significantly correlated with either gender or tumor stage in this cohort.
There were no associations in this population between PD-L1 status and either age, tumor size, microvascular invasion, tumor necrosis, or lymph node metastases.
The investigators also compared the age of onset of all VHL-associated tumors in this cohort in PD-L1-positive versus PD-L1-negative patients, but found no statistically significant differences.
The authors acknowledged that the cohort sizes were small and that follow-up was relatively short, which could have a bearing on the analysis of associations between PD-L1 expression and disease features.
“Whether PD-L1 expression level in ccRCC is related to the effectiveness of PD-1/PD-L1 checkpoint inhibitor immunotherapy needs to be further investigated,” they wrote.
The study was supported by the National Natural Science Foundation of China and Special Health Development Research Project of Capital. The authors reported having no relevant disclosures.
SOURCE: Hong B et al. Clin Genitourin Cancer. 2018 Nov 13. doi: 10.1016/j.clgc.2018.11.001.
In patients with either sporadic or hereditary clear cell renal cell carcinoma, tumor expression of programmed death–ligand 1 (PD-L1) is associated with aggressive disease, investigators have found.
Analysis of tumor samples from patients with sporadic clear cell renal cell carcinoma (ccRCC) and others with Von Hippel-Lindau (VHL)–associated hereditary ccRCC showed that positive PD-L1 correlated with aggressive clinicopathologic features, reported Baoan Hong, MD, from Peking University First Hospital in Beijing, and colleagues.
“PD-L1 is a promising predictive biomarker for the utilization of PD-1/PD-L1 checkpoint inhibitors in ccRCC patients,” they wrote in Genitourinary Cancer.
The investigators conducted a retrospective analysis of PD-L1 expression and its potential correlation with disease features using samples from 129 patients with sporadic ccRCC and 26 patients with VHL disease who underwent partial or radical nephrectomy at their center from 2010 to 2017.
Sporadic ccRCC
The median age of patients with sporadic ccRCC was 61 years and the median tumor size was 4.3 cm. Of the 129 patients, 56 had pathological stage T1a at diagnosis, 44 had stage T1b, 8 had stage T2, and 21 had stage T3. In all, 25 patients had Fuhrman nuclear grade 3 tumors and 104 had grade 1 or 2 tumors. A total of 7 patients had metastases to lymph nodes, 41 had microvascular invasion, and 16 had tumor necrosis.
In all, 61 of these patients had PD-L1-positive tumors and 68 were PD-L1 negative. Positive PD-L1 was significantly associated with male gender (P = .025) and worse disease features, including higher T stage (P = .0011) and higher Fuhrman nuclear grade (P = .022).
After a median follow-up of 68 months, 9 patients in this group died and 17 others developed distant metastases or recurrent disease. Patients whose tumors were PD-L1 negative had significantly longer disease-free survival than patients with PD-L1-positive tumors, at a median 36 versus 28 months (P = .037).
VHL-associated ccRCC
Of the 26 patients with VHL-associated hereditary ccRCC (13 men and 13 women; median age, 42 years), 13 had pathological stage T1a disease, 7 had T1b, and 2 each has stage T2a, T3a, and T3b tumors. A total of 18 patients had Fuhrman nuclear grade 1 tumors and 8 had grade 2 tumors.
In this cohort, 17 patients had PD-L1-negative tumors, and 9 had PD-L1-positive tumors. PD-L1 expression was more common in patients with Fuhrman nuclear grade 2 tumors (six of eight cases). Patients with Fuhrman nuclear grade 1 tumors were more likely to be PD-L1 negative (15 of 18, P = .008). PD-L1 expression was not significantly correlated with either gender or tumor stage in this cohort.
There were no associations in this population between PD-L1 status and either age, tumor size, microvascular invasion, tumor necrosis, or lymph node metastases.
The investigators also compared the age of onset of all VHL-associated tumors in this cohort in PD-L1-positive versus PD-L1-negative patients, but found no statistically significant differences.
The authors acknowledged that the cohort sizes were small and that follow-up was relatively short, which could have a bearing on the analysis of associations between PD-L1 expression and disease features.
“Whether PD-L1 expression level in ccRCC is related to the effectiveness of PD-1/PD-L1 checkpoint inhibitor immunotherapy needs to be further investigated,” they wrote.
The study was supported by the National Natural Science Foundation of China and Special Health Development Research Project of Capital. The authors reported having no relevant disclosures.
SOURCE: Hong B et al. Clin Genitourin Cancer. 2018 Nov 13. doi: 10.1016/j.clgc.2018.11.001.
In patients with either sporadic or hereditary clear cell renal cell carcinoma, tumor expression of programmed death–ligand 1 (PD-L1) is associated with aggressive disease, investigators have found.
Analysis of tumor samples from patients with sporadic clear cell renal cell carcinoma (ccRCC) and others with Von Hippel-Lindau (VHL)–associated hereditary ccRCC showed that positive PD-L1 correlated with aggressive clinicopathologic features, reported Baoan Hong, MD, from Peking University First Hospital in Beijing, and colleagues.
“PD-L1 is a promising predictive biomarker for the utilization of PD-1/PD-L1 checkpoint inhibitors in ccRCC patients,” they wrote in Genitourinary Cancer.
The investigators conducted a retrospective analysis of PD-L1 expression and its potential correlation with disease features using samples from 129 patients with sporadic ccRCC and 26 patients with VHL disease who underwent partial or radical nephrectomy at their center from 2010 to 2017.
Sporadic ccRCC
The median age of patients with sporadic ccRCC was 61 years and the median tumor size was 4.3 cm. Of the 129 patients, 56 had pathological stage T1a at diagnosis, 44 had stage T1b, 8 had stage T2, and 21 had stage T3. In all, 25 patients had Fuhrman nuclear grade 3 tumors and 104 had grade 1 or 2 tumors. A total of 7 patients had metastases to lymph nodes, 41 had microvascular invasion, and 16 had tumor necrosis.
In all, 61 of these patients had PD-L1-positive tumors and 68 were PD-L1 negative. Positive PD-L1 was significantly associated with male gender (P = .025) and worse disease features, including higher T stage (P = .0011) and higher Fuhrman nuclear grade (P = .022).
After a median follow-up of 68 months, 9 patients in this group died and 17 others developed distant metastases or recurrent disease. Patients whose tumors were PD-L1 negative had significantly longer disease-free survival than patients with PD-L1-positive tumors, at a median 36 versus 28 months (P = .037).
VHL-associated ccRCC
Of the 26 patients with VHL-associated hereditary ccRCC (13 men and 13 women; median age, 42 years), 13 had pathological stage T1a disease, 7 had T1b, and 2 each has stage T2a, T3a, and T3b tumors. A total of 18 patients had Fuhrman nuclear grade 1 tumors and 8 had grade 2 tumors.
In this cohort, 17 patients had PD-L1-negative tumors, and 9 had PD-L1-positive tumors. PD-L1 expression was more common in patients with Fuhrman nuclear grade 2 tumors (six of eight cases). Patients with Fuhrman nuclear grade 1 tumors were more likely to be PD-L1 negative (15 of 18, P = .008). PD-L1 expression was not significantly correlated with either gender or tumor stage in this cohort.
There were no associations in this population between PD-L1 status and either age, tumor size, microvascular invasion, tumor necrosis, or lymph node metastases.
The investigators also compared the age of onset of all VHL-associated tumors in this cohort in PD-L1-positive versus PD-L1-negative patients, but found no statistically significant differences.
The authors acknowledged that the cohort sizes were small and that follow-up was relatively short, which could have a bearing on the analysis of associations between PD-L1 expression and disease features.
“Whether PD-L1 expression level in ccRCC is related to the effectiveness of PD-1/PD-L1 checkpoint inhibitor immunotherapy needs to be further investigated,” they wrote.
The study was supported by the National Natural Science Foundation of China and Special Health Development Research Project of Capital. The authors reported having no relevant disclosures.
SOURCE: Hong B et al. Clin Genitourin Cancer. 2018 Nov 13. doi: 10.1016/j.clgc.2018.11.001.
FROM CLINICAL GENITOURINARY CANCER
Key clinical point: Expression of programmed death–ligand 1 (PD-L1) in both sporadic and hereditary clear cell renal cell carcinoma (ccRCC) was associated with worse prognosis.
Major finding: Median disease-free survival of patients with sporadic ccRCC tumors negative for PD-L1 was 36 months, compared with 28 months for patients with PD-L1-positive tumors.
Study details: A retrospective analysis of tissues from 129 patients with sporadic ccRCC and 26 with Von Hippel-Lindau–associated hereditary ccRCC.
Disclosures: The study was supported by the National Natural Science Foundation of China and Special Health Development Research Project of Capital. The authors reported having no relevant disclosures.
Source: Hong B et al. Clin Genitourin Cancer. 2018 Nov 13. doi: 10.1016/j.clgc.2018.11.001.
Abiraterone also benefits low-risk metastatic prostate cancer patients
MUNICH – Men with metastatic hormone-naive prostate cancer may benefit from treatment with the combination of abiraterone (Zytiga), prednisolone, and androgen deprivation regardless of risk group or disease volume, STAMPEDE trialists contend.
Results of the STAMPEDE and LATITUDE trials, published in 2017 in the New England Journal of Medicine, showed significant improvements in overall survival with abiraterone, androgen deprivation therapy (ADT) and either prednisone (in LATITUDE) or prednisolone (in STAMPEDE) compared with ADT alone.
Data from the LATITUDE trial were used to support approval by both the Food and Drug Administration and European Medicines Agency of abiraterone in combination with ADT and a glucocorticoid for the new indication of treatment of men with metastatic high-risk castration-sensitive prostate cancer.
“So where we stand, at the minute, in terms of guidance: the EAU [European Urology Association] and the NCCN [National Comprehensive Cancer Network] have suggested that we consider treatment for men with hormone-naive metastatic prostatic cancer, but in 2018 the FDA and EMA licensed the drug for high-risk disease, so there’s therefore an evolving uncertainty about what we should be doing in low-risk disease,” Alex Hoyle, MBChB, of Christie NHS Foundation Trust, Manchester, England, said on behalf of colleagues in the STAMPEDE trial group.
The problem is that there is no international consensus on what constitutes low-risk disease, Dr. Hoyle said at the European Society for Medical Oncology Congress.
For example, in the CHAARTED trial, risk was defined by volume, with high-risk patients defined as those with visceral metastases and/or four or more bone metastases with one or more outside the vertebral column or pelvis. In contrast, the LATITUDE investigators defined high-risk patients as those with two or more high-risk features, including three or more bone metastases, visceral metastases, and/or a Gleason score of 8 or more.
To determine whether men with low-risk disease could also benefit from the combination, Dr. Hoyle and colleagues performed a retrospective analysis of the STAMPEDE trial, using staging scans to stratify patients with M1 disease into either high- or low-risk categories according to the LATITUDE risk criteria. The reviewers were blinded to the treatment arm for each patient. They also performed a secondary differential analysis by tumor volume according to the CHAARTED criteria.
The investigators then retrospectively reviewed outcomes for 901 evaluable patients, median age 67 years, with a median PSA of 96 ng/mL, followed for a median of 42 months. The sample included 428 patients determined to have low-risk disease, and 473 determined to have high-risk disease.
Overall survival (OS), the primary endpoint, was significantly better for patients treated with the combination vs. ADT alone in both high- and low-risk groups. The 3-year OS in high-risk patients treated with the abiraterone/prednisolone/ADT was 64.7% compared with 45% for patients treated with AD alone, an absolute difference of 19.7% that translated into a hazard ratio (HR) for death of 0.54 (P less than .001).
For patients in the low-risk group, 3-year OS was 82.4% with the combination vs. 78% with ADT alone, an absolute difference of 4.4%, translating into an HR of 0.66 (P = .041).
Three-year prostate cancer-specific survival, a secondary endpoint, was better with abiraterone in the high-risk (67% vs. 47.9%, HR 0.57, P less than .001) and low-risk (88.7% vs. 81.6%, HR 0.51, P = .008) populations.
The results were even more pronounced in favor of the abiraterone combination for the secondary endpoint of failure-free survival (FFS) in both groups, with 45.1% of high-risk patients on abiraterone having no biochemical failure at 3 years vs. 12.2% for those treated with ADT alone (HR 0.48, P less than .001). The respective FFS rates in the low-risk group were 80.8% vs. 56.4% (HR 0.66, P = .041).
ADT was superior in analyses of skeletal related event-free survival (HR 0.48 for high risk and 0.31 for low risk, P less than .001 for both comparisons), and metastasis progression-free survival (HR 0.54, P less than .001 for high risk, HR 0.66, P = .041 for low risk).
An exploratory analysis using the CHAARTED risk criteria showed similar results, with the combination significantly better in every category except prostate cancer–specific survival in patients with low-volume disease, although here, too, there was a clear trend favoring abiraterone.
“Abiraterone plus prednisolone in addition to ADT improves survival endpoints in metastatic hormone-naive prostate cancer,” Dr. Hoyle said.
Invited discussant Karim Fizazi, MD, PhD, of Gustave Roussy Cancer Institute at the University of Paris-Sud, France, said that the study, despite some limitations, was very important.
“For patients with high-risk de novo disease, until today we’ve had two standards of care: castration plus abiraterone or castration plus docetaxel. For patients with low risk, that was strongly debated – either castration alone or castration plus docetaxel. After this publication, I think it’s fair to say that for patients with high-risk disease the role of abiraterone is being strengthened, while for patients with low-risk disease, ADT plus abiraterone probably is going to become the new standard,” he said.
The STAMPEDE trial is supported by the Medical Research Council of the United Kingdom, the Salford Royal and the Christie NHS Foundation trusts, and Manchester Cancer Research Centre. Dr. Hoyle reported having no conflicts of interest. Dr. Fizazi reported advisory board participation and/or honoraria from Amgen, Astellas, AstraZeneca, Bayer, Clovis, CureVac, Essa, Genentech, Janssen, MSD, Orion, and Sanofi.
SOURCE: Hoyle AP et al. ESMO 2018. Abstract LBA4.
MUNICH – Men with metastatic hormone-naive prostate cancer may benefit from treatment with the combination of abiraterone (Zytiga), prednisolone, and androgen deprivation regardless of risk group or disease volume, STAMPEDE trialists contend.
Results of the STAMPEDE and LATITUDE trials, published in 2017 in the New England Journal of Medicine, showed significant improvements in overall survival with abiraterone, androgen deprivation therapy (ADT) and either prednisone (in LATITUDE) or prednisolone (in STAMPEDE) compared with ADT alone.
Data from the LATITUDE trial were used to support approval by both the Food and Drug Administration and European Medicines Agency of abiraterone in combination with ADT and a glucocorticoid for the new indication of treatment of men with metastatic high-risk castration-sensitive prostate cancer.
“So where we stand, at the minute, in terms of guidance: the EAU [European Urology Association] and the NCCN [National Comprehensive Cancer Network] have suggested that we consider treatment for men with hormone-naive metastatic prostatic cancer, but in 2018 the FDA and EMA licensed the drug for high-risk disease, so there’s therefore an evolving uncertainty about what we should be doing in low-risk disease,” Alex Hoyle, MBChB, of Christie NHS Foundation Trust, Manchester, England, said on behalf of colleagues in the STAMPEDE trial group.
The problem is that there is no international consensus on what constitutes low-risk disease, Dr. Hoyle said at the European Society for Medical Oncology Congress.
For example, in the CHAARTED trial, risk was defined by volume, with high-risk patients defined as those with visceral metastases and/or four or more bone metastases with one or more outside the vertebral column or pelvis. In contrast, the LATITUDE investigators defined high-risk patients as those with two or more high-risk features, including three or more bone metastases, visceral metastases, and/or a Gleason score of 8 or more.
To determine whether men with low-risk disease could also benefit from the combination, Dr. Hoyle and colleagues performed a retrospective analysis of the STAMPEDE trial, using staging scans to stratify patients with M1 disease into either high- or low-risk categories according to the LATITUDE risk criteria. The reviewers were blinded to the treatment arm for each patient. They also performed a secondary differential analysis by tumor volume according to the CHAARTED criteria.
The investigators then retrospectively reviewed outcomes for 901 evaluable patients, median age 67 years, with a median PSA of 96 ng/mL, followed for a median of 42 months. The sample included 428 patients determined to have low-risk disease, and 473 determined to have high-risk disease.
Overall survival (OS), the primary endpoint, was significantly better for patients treated with the combination vs. ADT alone in both high- and low-risk groups. The 3-year OS in high-risk patients treated with the abiraterone/prednisolone/ADT was 64.7% compared with 45% for patients treated with AD alone, an absolute difference of 19.7% that translated into a hazard ratio (HR) for death of 0.54 (P less than .001).
For patients in the low-risk group, 3-year OS was 82.4% with the combination vs. 78% with ADT alone, an absolute difference of 4.4%, translating into an HR of 0.66 (P = .041).
Three-year prostate cancer-specific survival, a secondary endpoint, was better with abiraterone in the high-risk (67% vs. 47.9%, HR 0.57, P less than .001) and low-risk (88.7% vs. 81.6%, HR 0.51, P = .008) populations.
The results were even more pronounced in favor of the abiraterone combination for the secondary endpoint of failure-free survival (FFS) in both groups, with 45.1% of high-risk patients on abiraterone having no biochemical failure at 3 years vs. 12.2% for those treated with ADT alone (HR 0.48, P less than .001). The respective FFS rates in the low-risk group were 80.8% vs. 56.4% (HR 0.66, P = .041).
ADT was superior in analyses of skeletal related event-free survival (HR 0.48 for high risk and 0.31 for low risk, P less than .001 for both comparisons), and metastasis progression-free survival (HR 0.54, P less than .001 for high risk, HR 0.66, P = .041 for low risk).
An exploratory analysis using the CHAARTED risk criteria showed similar results, with the combination significantly better in every category except prostate cancer–specific survival in patients with low-volume disease, although here, too, there was a clear trend favoring abiraterone.
“Abiraterone plus prednisolone in addition to ADT improves survival endpoints in metastatic hormone-naive prostate cancer,” Dr. Hoyle said.
Invited discussant Karim Fizazi, MD, PhD, of Gustave Roussy Cancer Institute at the University of Paris-Sud, France, said that the study, despite some limitations, was very important.
“For patients with high-risk de novo disease, until today we’ve had two standards of care: castration plus abiraterone or castration plus docetaxel. For patients with low risk, that was strongly debated – either castration alone or castration plus docetaxel. After this publication, I think it’s fair to say that for patients with high-risk disease the role of abiraterone is being strengthened, while for patients with low-risk disease, ADT plus abiraterone probably is going to become the new standard,” he said.
The STAMPEDE trial is supported by the Medical Research Council of the United Kingdom, the Salford Royal and the Christie NHS Foundation trusts, and Manchester Cancer Research Centre. Dr. Hoyle reported having no conflicts of interest. Dr. Fizazi reported advisory board participation and/or honoraria from Amgen, Astellas, AstraZeneca, Bayer, Clovis, CureVac, Essa, Genentech, Janssen, MSD, Orion, and Sanofi.
SOURCE: Hoyle AP et al. ESMO 2018. Abstract LBA4.
MUNICH – Men with metastatic hormone-naive prostate cancer may benefit from treatment with the combination of abiraterone (Zytiga), prednisolone, and androgen deprivation regardless of risk group or disease volume, STAMPEDE trialists contend.
Results of the STAMPEDE and LATITUDE trials, published in 2017 in the New England Journal of Medicine, showed significant improvements in overall survival with abiraterone, androgen deprivation therapy (ADT) and either prednisone (in LATITUDE) or prednisolone (in STAMPEDE) compared with ADT alone.
Data from the LATITUDE trial were used to support approval by both the Food and Drug Administration and European Medicines Agency of abiraterone in combination with ADT and a glucocorticoid for the new indication of treatment of men with metastatic high-risk castration-sensitive prostate cancer.
“So where we stand, at the minute, in terms of guidance: the EAU [European Urology Association] and the NCCN [National Comprehensive Cancer Network] have suggested that we consider treatment for men with hormone-naive metastatic prostatic cancer, but in 2018 the FDA and EMA licensed the drug for high-risk disease, so there’s therefore an evolving uncertainty about what we should be doing in low-risk disease,” Alex Hoyle, MBChB, of Christie NHS Foundation Trust, Manchester, England, said on behalf of colleagues in the STAMPEDE trial group.
The problem is that there is no international consensus on what constitutes low-risk disease, Dr. Hoyle said at the European Society for Medical Oncology Congress.
For example, in the CHAARTED trial, risk was defined by volume, with high-risk patients defined as those with visceral metastases and/or four or more bone metastases with one or more outside the vertebral column or pelvis. In contrast, the LATITUDE investigators defined high-risk patients as those with two or more high-risk features, including three or more bone metastases, visceral metastases, and/or a Gleason score of 8 or more.
To determine whether men with low-risk disease could also benefit from the combination, Dr. Hoyle and colleagues performed a retrospective analysis of the STAMPEDE trial, using staging scans to stratify patients with M1 disease into either high- or low-risk categories according to the LATITUDE risk criteria. The reviewers were blinded to the treatment arm for each patient. They also performed a secondary differential analysis by tumor volume according to the CHAARTED criteria.
The investigators then retrospectively reviewed outcomes for 901 evaluable patients, median age 67 years, with a median PSA of 96 ng/mL, followed for a median of 42 months. The sample included 428 patients determined to have low-risk disease, and 473 determined to have high-risk disease.
Overall survival (OS), the primary endpoint, was significantly better for patients treated with the combination vs. ADT alone in both high- and low-risk groups. The 3-year OS in high-risk patients treated with the abiraterone/prednisolone/ADT was 64.7% compared with 45% for patients treated with AD alone, an absolute difference of 19.7% that translated into a hazard ratio (HR) for death of 0.54 (P less than .001).
For patients in the low-risk group, 3-year OS was 82.4% with the combination vs. 78% with ADT alone, an absolute difference of 4.4%, translating into an HR of 0.66 (P = .041).
Three-year prostate cancer-specific survival, a secondary endpoint, was better with abiraterone in the high-risk (67% vs. 47.9%, HR 0.57, P less than .001) and low-risk (88.7% vs. 81.6%, HR 0.51, P = .008) populations.
The results were even more pronounced in favor of the abiraterone combination for the secondary endpoint of failure-free survival (FFS) in both groups, with 45.1% of high-risk patients on abiraterone having no biochemical failure at 3 years vs. 12.2% for those treated with ADT alone (HR 0.48, P less than .001). The respective FFS rates in the low-risk group were 80.8% vs. 56.4% (HR 0.66, P = .041).
ADT was superior in analyses of skeletal related event-free survival (HR 0.48 for high risk and 0.31 for low risk, P less than .001 for both comparisons), and metastasis progression-free survival (HR 0.54, P less than .001 for high risk, HR 0.66, P = .041 for low risk).
An exploratory analysis using the CHAARTED risk criteria showed similar results, with the combination significantly better in every category except prostate cancer–specific survival in patients with low-volume disease, although here, too, there was a clear trend favoring abiraterone.
“Abiraterone plus prednisolone in addition to ADT improves survival endpoints in metastatic hormone-naive prostate cancer,” Dr. Hoyle said.
Invited discussant Karim Fizazi, MD, PhD, of Gustave Roussy Cancer Institute at the University of Paris-Sud, France, said that the study, despite some limitations, was very important.
“For patients with high-risk de novo disease, until today we’ve had two standards of care: castration plus abiraterone or castration plus docetaxel. For patients with low risk, that was strongly debated – either castration alone or castration plus docetaxel. After this publication, I think it’s fair to say that for patients with high-risk disease the role of abiraterone is being strengthened, while for patients with low-risk disease, ADT plus abiraterone probably is going to become the new standard,” he said.
The STAMPEDE trial is supported by the Medical Research Council of the United Kingdom, the Salford Royal and the Christie NHS Foundation trusts, and Manchester Cancer Research Centre. Dr. Hoyle reported having no conflicts of interest. Dr. Fizazi reported advisory board participation and/or honoraria from Amgen, Astellas, AstraZeneca, Bayer, Clovis, CureVac, Essa, Genentech, Janssen, MSD, Orion, and Sanofi.
SOURCE: Hoyle AP et al. ESMO 2018. Abstract LBA4.
AT ESMO 2018
Key clinical point: Men with metastatic hormone-naive prostate cancer at both low and high risk have better outcomes with abiraterone plus androgen deprivation and prednisolone or prednisone.
Major finding: Patients with low-risk disease treated with the abiraterone combination had 3-year OS of 82.4% vs. 78% with ADT alone (HR 0.66, P = .041).
Study details: Retrospective analysis of data from the STAMPEDE trial using risk criteria from the LATITUDE and CHAARTED trials.
Disclosures: The STAMPEDE trial is supported by the Medical Research Council of the United Kingdom, the Salford Royal and the Christie NHS Foundation trusts, and Manchester Cancer Research Centre. Dr. Hoyle reported having no conflicts of interest. Dr. Fizazi reported advisory board participation and/or honoraria from Amgen, Astellas, AstraZeneca, Bayer, Clovis, CureVac, Essa, Genentech, Janssen, MSD, Orion, and Sanofi.
Source: Hoyle AP et al. ESMO 2018. Abstract LBA4.
Refractory immune-mediated colitis: Fecal transplant may be the answer
WASHINGTON – , according to Yinghong Wang, MD.
In two patients who developed severe, refractory, immune-mediated colitis (IMC), FMT led to recovery, Dr. Wang of M.D. Anderson Cancer Center, Houston, reported at the annual meeting of the Society for Immunotherapy of Cancer.
Patient 1 was a woman with renal cell cancer who developed grade 2+ IMC within 1 month of initiation of treatment with combined ipilimumab and nivolumab. Infectious etiology was ruled out, and her symptoms and ulcers persisted despite 3 months of treatment with corticosteroids, two doses of infliximab, and one dose of vedolizumab.
A single FMT delivered via colonoscopy led to complete symptom resolution within 10 days, and a repeat colonoscopy showed “very nice healing of inflammation and ulcers,” Dr. Wang said.
Patient 2 was a man with prostate cancer who developed grade 2+ IMC 3 months after receiving two doses of ipilimumab. Infectious etiologies were ruled out, and like patient 1, his symptoms and mucosal ulcerations persisted despite 5 months of immunosuppression with corticosteroids, two doses of infliximab, and three doses of vedolizumab. He underwent two FMTs via colonoscopy.
“The first fecal transplant achieved partial response, and the second fecal transplant achieved complete clinical response, and this remission was sustained for a total of 8 months,” Dr. Wang said.
Immune checkpoint inhibitor–related IMC is typically treated with immunosuppressive therapy that is associated with significant morbidity, including a possible adverse impact on the antitumor effects of checkpoint inhibitors, Dr. Wang said.
However, studies have suggested that “the microbiome in healthy people potentially plays a very important and synergistic role for tumor regression in combination with immunotherapy,” and animal models also suggest that patients who develop IMC have differential bacterial signatures in their gut microbiome, she said.
“Based on that preliminary information, we performed fecal transplant as a compassionate treatment for cases refractory to all immunosuppression in June 2017 at M.D. Anderson,” she said.
Stool microbiome analyses showed successful engraftment of donor microbiome in recipient stool samples, and microbiome taxonomy showed increases in specific Escherichia species that “we think potentially play a role in this colitis recovery,” she said.
“Fecal transplant is safe and effective based on our preliminary study,” she said, adding that restoration of a healthy microbiome seems to reverse IMC. “Future large-scale studies are needed to evaluate this finding.”
Dr. Wang reported having no disclosures.
SOURCE: Wang Y et al. SITC 2018, Abstract P194.
WASHINGTON – , according to Yinghong Wang, MD.
In two patients who developed severe, refractory, immune-mediated colitis (IMC), FMT led to recovery, Dr. Wang of M.D. Anderson Cancer Center, Houston, reported at the annual meeting of the Society for Immunotherapy of Cancer.
Patient 1 was a woman with renal cell cancer who developed grade 2+ IMC within 1 month of initiation of treatment with combined ipilimumab and nivolumab. Infectious etiology was ruled out, and her symptoms and ulcers persisted despite 3 months of treatment with corticosteroids, two doses of infliximab, and one dose of vedolizumab.
A single FMT delivered via colonoscopy led to complete symptom resolution within 10 days, and a repeat colonoscopy showed “very nice healing of inflammation and ulcers,” Dr. Wang said.
Patient 2 was a man with prostate cancer who developed grade 2+ IMC 3 months after receiving two doses of ipilimumab. Infectious etiologies were ruled out, and like patient 1, his symptoms and mucosal ulcerations persisted despite 5 months of immunosuppression with corticosteroids, two doses of infliximab, and three doses of vedolizumab. He underwent two FMTs via colonoscopy.
“The first fecal transplant achieved partial response, and the second fecal transplant achieved complete clinical response, and this remission was sustained for a total of 8 months,” Dr. Wang said.
Immune checkpoint inhibitor–related IMC is typically treated with immunosuppressive therapy that is associated with significant morbidity, including a possible adverse impact on the antitumor effects of checkpoint inhibitors, Dr. Wang said.
However, studies have suggested that “the microbiome in healthy people potentially plays a very important and synergistic role for tumor regression in combination with immunotherapy,” and animal models also suggest that patients who develop IMC have differential bacterial signatures in their gut microbiome, she said.
“Based on that preliminary information, we performed fecal transplant as a compassionate treatment for cases refractory to all immunosuppression in June 2017 at M.D. Anderson,” she said.
Stool microbiome analyses showed successful engraftment of donor microbiome in recipient stool samples, and microbiome taxonomy showed increases in specific Escherichia species that “we think potentially play a role in this colitis recovery,” she said.
“Fecal transplant is safe and effective based on our preliminary study,” she said, adding that restoration of a healthy microbiome seems to reverse IMC. “Future large-scale studies are needed to evaluate this finding.”
Dr. Wang reported having no disclosures.
SOURCE: Wang Y et al. SITC 2018, Abstract P194.
WASHINGTON – , according to Yinghong Wang, MD.
In two patients who developed severe, refractory, immune-mediated colitis (IMC), FMT led to recovery, Dr. Wang of M.D. Anderson Cancer Center, Houston, reported at the annual meeting of the Society for Immunotherapy of Cancer.
Patient 1 was a woman with renal cell cancer who developed grade 2+ IMC within 1 month of initiation of treatment with combined ipilimumab and nivolumab. Infectious etiology was ruled out, and her symptoms and ulcers persisted despite 3 months of treatment with corticosteroids, two doses of infliximab, and one dose of vedolizumab.
A single FMT delivered via colonoscopy led to complete symptom resolution within 10 days, and a repeat colonoscopy showed “very nice healing of inflammation and ulcers,” Dr. Wang said.
Patient 2 was a man with prostate cancer who developed grade 2+ IMC 3 months after receiving two doses of ipilimumab. Infectious etiologies were ruled out, and like patient 1, his symptoms and mucosal ulcerations persisted despite 5 months of immunosuppression with corticosteroids, two doses of infliximab, and three doses of vedolizumab. He underwent two FMTs via colonoscopy.
“The first fecal transplant achieved partial response, and the second fecal transplant achieved complete clinical response, and this remission was sustained for a total of 8 months,” Dr. Wang said.
Immune checkpoint inhibitor–related IMC is typically treated with immunosuppressive therapy that is associated with significant morbidity, including a possible adverse impact on the antitumor effects of checkpoint inhibitors, Dr. Wang said.
However, studies have suggested that “the microbiome in healthy people potentially plays a very important and synergistic role for tumor regression in combination with immunotherapy,” and animal models also suggest that patients who develop IMC have differential bacterial signatures in their gut microbiome, she said.
“Based on that preliminary information, we performed fecal transplant as a compassionate treatment for cases refractory to all immunosuppression in June 2017 at M.D. Anderson,” she said.
Stool microbiome analyses showed successful engraftment of donor microbiome in recipient stool samples, and microbiome taxonomy showed increases in specific Escherichia species that “we think potentially play a role in this colitis recovery,” she said.
“Fecal transplant is safe and effective based on our preliminary study,” she said, adding that restoration of a healthy microbiome seems to reverse IMC. “Future large-scale studies are needed to evaluate this finding.”
Dr. Wang reported having no disclosures.
SOURCE: Wang Y et al. SITC 2018, Abstract P194.
REPORTING FROM SITC 2018
Key clinical point: FMT lead to recovery in two patients with refractory IMC.
Major finding: FMT was effective for the treatment of IMC in two patients.
Study details: Two case reports.
Disclosures: Dr. Wang reported having no disclosures.
Source: Wang Y et al. SITC 2018, Abstract P194.
SRS beats surgery in early control of brain mets, advantage fades with time
Stereotactic radiosurgery (SRS) provides better early local control of brain metastases than complete surgical resection, but this advantage fades with time, according to investigators.
By 6 months, lower risks associated with SRS shifted in favor of those who had surgical resection, reported lead author Thomas Churilla, MD, of Fox Chase Cancer Center in Philadelphia and his colleagues.
“Outside recognized indications for surgery such as establishing diagnosis or relieving mass effect, little evidence is available to guide the therapeutic choice of SRS vs. surgical resection in the treatment of patients with limited brain metastases,” the investigators wrote in JAMA Oncology.
The investigators performed an exploratory analysis of data from the European Organization for the Research and Treatment of Cancer (EORTC) 22952-26001 phase 3 trial, which was designed to evaluate whole-brain radiotherapy for patients with one to three brain metastases who had undergone SRS or complete surgical resection. The present analysis involved 268 patients, of whom 154 had SRS and 114 had complete surgical resection.
Primary tumors included lung, breast, colorectum, kidney, and melanoma. Initial analysis showed that patients undergoing surgical resection, compared with those who had SRS, typically had larger brain metastases (median, 28 mm vs. 20 mm) and more often had 1 brain metastasis (98.2% vs. 74.0%). Mass locality also differed between groups; compared with patients receiving SRS, surgical patients more often had metastases in the posterior fossa (26.3% vs. 7.8%) and less often in the parietal lobe (18.4% vs. 39.6%).
After median follow-up of 39.9 months, risks of local recurrence were similar between surgical and SRS groups (hazard ratio, 1.15). Stratifying by interval, however, showed that surgical patients were at much higher risk of local recurrence in the first 3 months following treatment (HR for 0-3 months, 5.94). Of note, this risk faded with time (HR for 3-6 months, 1.37; HR for 6-9 months, 0.75; HR for 9 months or longer, 0.36). From the 6-9 months interval onward, surgical patients had lower risk of recurrence, compared with SRS patients, and the risk even decreased after the 6-9 month interval.
“Prospective controlled trials are warranted to direct the optimal local approach for patients with brain metastases and to define whether any population may benefit from escalation in local therapy,” the investigators concluded.
The study was funded by the National Cancer Institute, National Institutes of Health, and Fonds Cancer in Belgium. One author reported receiving financial compensation from Pfizer via her institution.
SOURCE: Churilla T et al. JAMA Onc. 2018. doi: 10.1001/jamaoncol.2018.4610.
Stereotactic radiosurgery (SRS) provides better early local control of brain metastases than complete surgical resection, but this advantage fades with time, according to investigators.
By 6 months, lower risks associated with SRS shifted in favor of those who had surgical resection, reported lead author Thomas Churilla, MD, of Fox Chase Cancer Center in Philadelphia and his colleagues.
“Outside recognized indications for surgery such as establishing diagnosis or relieving mass effect, little evidence is available to guide the therapeutic choice of SRS vs. surgical resection in the treatment of patients with limited brain metastases,” the investigators wrote in JAMA Oncology.
The investigators performed an exploratory analysis of data from the European Organization for the Research and Treatment of Cancer (EORTC) 22952-26001 phase 3 trial, which was designed to evaluate whole-brain radiotherapy for patients with one to three brain metastases who had undergone SRS or complete surgical resection. The present analysis involved 268 patients, of whom 154 had SRS and 114 had complete surgical resection.
Primary tumors included lung, breast, colorectum, kidney, and melanoma. Initial analysis showed that patients undergoing surgical resection, compared with those who had SRS, typically had larger brain metastases (median, 28 mm vs. 20 mm) and more often had 1 brain metastasis (98.2% vs. 74.0%). Mass locality also differed between groups; compared with patients receiving SRS, surgical patients more often had metastases in the posterior fossa (26.3% vs. 7.8%) and less often in the parietal lobe (18.4% vs. 39.6%).
After median follow-up of 39.9 months, risks of local recurrence were similar between surgical and SRS groups (hazard ratio, 1.15). Stratifying by interval, however, showed that surgical patients were at much higher risk of local recurrence in the first 3 months following treatment (HR for 0-3 months, 5.94). Of note, this risk faded with time (HR for 3-6 months, 1.37; HR for 6-9 months, 0.75; HR for 9 months or longer, 0.36). From the 6-9 months interval onward, surgical patients had lower risk of recurrence, compared with SRS patients, and the risk even decreased after the 6-9 month interval.
“Prospective controlled trials are warranted to direct the optimal local approach for patients with brain metastases and to define whether any population may benefit from escalation in local therapy,” the investigators concluded.
The study was funded by the National Cancer Institute, National Institutes of Health, and Fonds Cancer in Belgium. One author reported receiving financial compensation from Pfizer via her institution.
SOURCE: Churilla T et al. JAMA Onc. 2018. doi: 10.1001/jamaoncol.2018.4610.
Stereotactic radiosurgery (SRS) provides better early local control of brain metastases than complete surgical resection, but this advantage fades with time, according to investigators.
By 6 months, lower risks associated with SRS shifted in favor of those who had surgical resection, reported lead author Thomas Churilla, MD, of Fox Chase Cancer Center in Philadelphia and his colleagues.
“Outside recognized indications for surgery such as establishing diagnosis or relieving mass effect, little evidence is available to guide the therapeutic choice of SRS vs. surgical resection in the treatment of patients with limited brain metastases,” the investigators wrote in JAMA Oncology.
The investigators performed an exploratory analysis of data from the European Organization for the Research and Treatment of Cancer (EORTC) 22952-26001 phase 3 trial, which was designed to evaluate whole-brain radiotherapy for patients with one to three brain metastases who had undergone SRS or complete surgical resection. The present analysis involved 268 patients, of whom 154 had SRS and 114 had complete surgical resection.
Primary tumors included lung, breast, colorectum, kidney, and melanoma. Initial analysis showed that patients undergoing surgical resection, compared with those who had SRS, typically had larger brain metastases (median, 28 mm vs. 20 mm) and more often had 1 brain metastasis (98.2% vs. 74.0%). Mass locality also differed between groups; compared with patients receiving SRS, surgical patients more often had metastases in the posterior fossa (26.3% vs. 7.8%) and less often in the parietal lobe (18.4% vs. 39.6%).
After median follow-up of 39.9 months, risks of local recurrence were similar between surgical and SRS groups (hazard ratio, 1.15). Stratifying by interval, however, showed that surgical patients were at much higher risk of local recurrence in the first 3 months following treatment (HR for 0-3 months, 5.94). Of note, this risk faded with time (HR for 3-6 months, 1.37; HR for 6-9 months, 0.75; HR for 9 months or longer, 0.36). From the 6-9 months interval onward, surgical patients had lower risk of recurrence, compared with SRS patients, and the risk even decreased after the 6-9 month interval.
“Prospective controlled trials are warranted to direct the optimal local approach for patients with brain metastases and to define whether any population may benefit from escalation in local therapy,” the investigators concluded.
The study was funded by the National Cancer Institute, National Institutes of Health, and Fonds Cancer in Belgium. One author reported receiving financial compensation from Pfizer via her institution.
SOURCE: Churilla T et al. JAMA Onc. 2018. doi: 10.1001/jamaoncol.2018.4610.
FROM JAMA ONCOLOGY
Key clinical point: Stereotactic radiosurgery (SRS) provides better early local control of brain metastases than surgical resection, but this advantage fades with time.
Major finding: Patients treated with surgery were more likely to have local recurrence in the first 3 months following treatment, compared with patients treated with SRS (hazard ratio, 5.94).
Study details: An exploratory analysis of data from the European Organization for the Research and Treatment of Cancer (EORTC) 22952-26001 phase 3 trial. Analysis involved 268 patients with one to three brain metastases who underwent whole-brain radiotherapy or observation after SRS (n = 154) or complete surgical resection (n = 114).
Disclosures: The study was funded by the National Cancer Institute, National Institutes of Health, and Fonds Cancer in Belgium. Dr. Handorf reported financial compensation from Pfizer, via her institution.
Source: Churilla T et al. JAMA Onc. 2018. doi: 10.1001/jamaoncol.2018.4610.
Circulating tumor DNA identified by fragment size
Circulating tumor DNA could be effectively isolated from plasma by focusing on a particular range of fragment sizes, which paves the way for noninvasive genomic analysis of tumor DNA, new research suggests.
In a study of 344 plasma samples from 200 patients with 18 cancer types and 65 samples from healthy controls, DNA fragment length could be used to distinguish circulating tumor DNA (ctDNA) from other cell-free DNA (cfDNA), investigators reported in Science Translational Medicine.
“We hypothesized that we could improve the sensitivity for noninvasive cancer genomics by selective sequencing of ctDNA fragments and by leveraging differences in the biology that determine DNA fragmentation,” wrote Florent Mouliere, PhD, from the Cancer Research UK Cambridge Institute, and coauthors.
Cell-free plasma fragments are often cleaved at around 167 base pairs in length and differences in length between circulating fetal and maternal DNA are already used for noninvasive prenatal diagnosis. However, the authors said that only a few studies, with conflicting results, have looked at the size distribution of tumor-derived cfDNA.
The study used two approaches to determining the size profile of mutant ctDNA. The first looked at tumor and nontumor cfDNA in mice with human ovarian cancer xenografts and the second approach used deep sequencing in 19 cancer patients. This revealed that tumor-derived cfDNA was most commonly found in fragments between 90-150 base pairs or 250-320 base pairs in size.
The researchers also noted that mutant circulating tumor DNA was generally more fragmented than nonmutant cfDNA and that patients with untreated advanced cancer showed consistently shorter lengths of mutant DNA.
The next question was whether size selection and other biological properties – such as somatic alterations – of the cfDNA could be used to enhance detection of ctDNA via machine learning technology.
Two models, designed to distinguish between healthy and cancerous samples, were developed using 153 samples, then validated on two datasets of 94 and 83 samples.
One of these models correctly classified cancerous samples in 94% of samples from patients with cancers known to have high levels of ctDNA – colorectal, cholangiocarcinoma, ovarian, breast, and melanoma – and in 65% of samples from low-ctDNA cancers – pancreatic, renal, and glioma.
Another model focused just on fragmentation patterns and was still able to distinguish cancer samples from those of healthy controls, although with slightly reduced area under the curve.
“Our results indicate that exploiting fundamental properties of cfDNA with fragment-specific analyses can allow more sensitive evaluation of ctDNA,” the authors wrote. “We identified features that could determine the presence and amount of ctDNA in plasma samples, without a prior knowledge of somatic aberrations.”
The authors pointed out that size selection of DNA fragments was relatively simple and cheap, and was also compatible with other genome-wide and targeted genomic analyses, “greatly increasing the potential value and utility of liquid biopsies as well as the cost-effectiveness of cfDNA sequencing.”
However, they cautioned that their catalogue had focused solely on double-stranded DNA and was subject to potential biases from the DNA extraction and sequencing methods they used in the study. They also commented that other biological effects could help refine the analysis of ctDNA.
“Other bodily fluids [urine, cerebrospinal fluid, and saliva], different nucleic acids and structures, altered mechanisms of release into circulation, or sample processing methods could exhibit varying fragment size signatures and could offer additional exploitable biological patterns for selective sequencing,” they wrote.
The study was supported by the University of Cambridge, Cancer Research UK, and the Engineering and Physical Sciences Research Council. Research supporting the study was also funded by the European Research Council, the National Institute for Health Research Cambridge, National Cancer Research Network, Cambridge Experimental Cancer Medicine Centre, Hutchison Whampoa, Target Ovarian Cancer, the Medical Research Council, and AstraZeneca. Three authors are cofounders, shareholders, and officers/consultants in a company specializing in ctDNA analysis. One author declared research funding and advisory board fees from private industry. Seven authors are listed on related patents.
SOURCE: Mouliere F et al. Sci Transl Med. 2018 Nov 7. doi: 10.1126/scitranslmed.aat4921.
Cell-free DNA analysis has tremendous diagnostic potential and so is a very active area of research. In this study, researchers were able to identify five variables and develop models for the detection of cancer following analysis of circulating tumor DNA. One of these models based on DNA fragmentation pattern performed very well, and so fragment length analyses could develop into a general test for the presence of cancer.
However confirmation of these findings in large, multicenter clinical trials is still needed. There is also the problem that size selection can result in a loss of circulating tumor DNA for analysis or may introduce biases. We also need to understand the mechanisms underpinning the different fragment size patterns seen in the study. But this study still substantially extends the potential of cell-free, DNA-based diagnostic tests.
Ellen Heitzer, PhD, and Michael R. Speicher, MD, are from the Medical University of Graz (Austria). These comments are taken from an accompanying editorial (Sci Transl Med. 2018 Nov 7. doi: 10.1126/scitranslmed.aav3873). Both authors declared research funding from Servier and Dr. Heitzer declared laboratory research funding from Freenome and PreAnalytiX.
Cell-free DNA analysis has tremendous diagnostic potential and so is a very active area of research. In this study, researchers were able to identify five variables and develop models for the detection of cancer following analysis of circulating tumor DNA. One of these models based on DNA fragmentation pattern performed very well, and so fragment length analyses could develop into a general test for the presence of cancer.
However confirmation of these findings in large, multicenter clinical trials is still needed. There is also the problem that size selection can result in a loss of circulating tumor DNA for analysis or may introduce biases. We also need to understand the mechanisms underpinning the different fragment size patterns seen in the study. But this study still substantially extends the potential of cell-free, DNA-based diagnostic tests.
Ellen Heitzer, PhD, and Michael R. Speicher, MD, are from the Medical University of Graz (Austria). These comments are taken from an accompanying editorial (Sci Transl Med. 2018 Nov 7. doi: 10.1126/scitranslmed.aav3873). Both authors declared research funding from Servier and Dr. Heitzer declared laboratory research funding from Freenome and PreAnalytiX.
Cell-free DNA analysis has tremendous diagnostic potential and so is a very active area of research. In this study, researchers were able to identify five variables and develop models for the detection of cancer following analysis of circulating tumor DNA. One of these models based on DNA fragmentation pattern performed very well, and so fragment length analyses could develop into a general test for the presence of cancer.
However confirmation of these findings in large, multicenter clinical trials is still needed. There is also the problem that size selection can result in a loss of circulating tumor DNA for analysis or may introduce biases. We also need to understand the mechanisms underpinning the different fragment size patterns seen in the study. But this study still substantially extends the potential of cell-free, DNA-based diagnostic tests.
Ellen Heitzer, PhD, and Michael R. Speicher, MD, are from the Medical University of Graz (Austria). These comments are taken from an accompanying editorial (Sci Transl Med. 2018 Nov 7. doi: 10.1126/scitranslmed.aav3873). Both authors declared research funding from Servier and Dr. Heitzer declared laboratory research funding from Freenome and PreAnalytiX.
Circulating tumor DNA could be effectively isolated from plasma by focusing on a particular range of fragment sizes, which paves the way for noninvasive genomic analysis of tumor DNA, new research suggests.
In a study of 344 plasma samples from 200 patients with 18 cancer types and 65 samples from healthy controls, DNA fragment length could be used to distinguish circulating tumor DNA (ctDNA) from other cell-free DNA (cfDNA), investigators reported in Science Translational Medicine.
“We hypothesized that we could improve the sensitivity for noninvasive cancer genomics by selective sequencing of ctDNA fragments and by leveraging differences in the biology that determine DNA fragmentation,” wrote Florent Mouliere, PhD, from the Cancer Research UK Cambridge Institute, and coauthors.
Cell-free plasma fragments are often cleaved at around 167 base pairs in length and differences in length between circulating fetal and maternal DNA are already used for noninvasive prenatal diagnosis. However, the authors said that only a few studies, with conflicting results, have looked at the size distribution of tumor-derived cfDNA.
The study used two approaches to determining the size profile of mutant ctDNA. The first looked at tumor and nontumor cfDNA in mice with human ovarian cancer xenografts and the second approach used deep sequencing in 19 cancer patients. This revealed that tumor-derived cfDNA was most commonly found in fragments between 90-150 base pairs or 250-320 base pairs in size.
The researchers also noted that mutant circulating tumor DNA was generally more fragmented than nonmutant cfDNA and that patients with untreated advanced cancer showed consistently shorter lengths of mutant DNA.
The next question was whether size selection and other biological properties – such as somatic alterations – of the cfDNA could be used to enhance detection of ctDNA via machine learning technology.
Two models, designed to distinguish between healthy and cancerous samples, were developed using 153 samples, then validated on two datasets of 94 and 83 samples.
One of these models correctly classified cancerous samples in 94% of samples from patients with cancers known to have high levels of ctDNA – colorectal, cholangiocarcinoma, ovarian, breast, and melanoma – and in 65% of samples from low-ctDNA cancers – pancreatic, renal, and glioma.
Another model focused just on fragmentation patterns and was still able to distinguish cancer samples from those of healthy controls, although with slightly reduced area under the curve.
“Our results indicate that exploiting fundamental properties of cfDNA with fragment-specific analyses can allow more sensitive evaluation of ctDNA,” the authors wrote. “We identified features that could determine the presence and amount of ctDNA in plasma samples, without a prior knowledge of somatic aberrations.”
The authors pointed out that size selection of DNA fragments was relatively simple and cheap, and was also compatible with other genome-wide and targeted genomic analyses, “greatly increasing the potential value and utility of liquid biopsies as well as the cost-effectiveness of cfDNA sequencing.”
However, they cautioned that their catalogue had focused solely on double-stranded DNA and was subject to potential biases from the DNA extraction and sequencing methods they used in the study. They also commented that other biological effects could help refine the analysis of ctDNA.
“Other bodily fluids [urine, cerebrospinal fluid, and saliva], different nucleic acids and structures, altered mechanisms of release into circulation, or sample processing methods could exhibit varying fragment size signatures and could offer additional exploitable biological patterns for selective sequencing,” they wrote.
The study was supported by the University of Cambridge, Cancer Research UK, and the Engineering and Physical Sciences Research Council. Research supporting the study was also funded by the European Research Council, the National Institute for Health Research Cambridge, National Cancer Research Network, Cambridge Experimental Cancer Medicine Centre, Hutchison Whampoa, Target Ovarian Cancer, the Medical Research Council, and AstraZeneca. Three authors are cofounders, shareholders, and officers/consultants in a company specializing in ctDNA analysis. One author declared research funding and advisory board fees from private industry. Seven authors are listed on related patents.
SOURCE: Mouliere F et al. Sci Transl Med. 2018 Nov 7. doi: 10.1126/scitranslmed.aat4921.
Circulating tumor DNA could be effectively isolated from plasma by focusing on a particular range of fragment sizes, which paves the way for noninvasive genomic analysis of tumor DNA, new research suggests.
In a study of 344 plasma samples from 200 patients with 18 cancer types and 65 samples from healthy controls, DNA fragment length could be used to distinguish circulating tumor DNA (ctDNA) from other cell-free DNA (cfDNA), investigators reported in Science Translational Medicine.
“We hypothesized that we could improve the sensitivity for noninvasive cancer genomics by selective sequencing of ctDNA fragments and by leveraging differences in the biology that determine DNA fragmentation,” wrote Florent Mouliere, PhD, from the Cancer Research UK Cambridge Institute, and coauthors.
Cell-free plasma fragments are often cleaved at around 167 base pairs in length and differences in length between circulating fetal and maternal DNA are already used for noninvasive prenatal diagnosis. However, the authors said that only a few studies, with conflicting results, have looked at the size distribution of tumor-derived cfDNA.
The study used two approaches to determining the size profile of mutant ctDNA. The first looked at tumor and nontumor cfDNA in mice with human ovarian cancer xenografts and the second approach used deep sequencing in 19 cancer patients. This revealed that tumor-derived cfDNA was most commonly found in fragments between 90-150 base pairs or 250-320 base pairs in size.
The researchers also noted that mutant circulating tumor DNA was generally more fragmented than nonmutant cfDNA and that patients with untreated advanced cancer showed consistently shorter lengths of mutant DNA.
The next question was whether size selection and other biological properties – such as somatic alterations – of the cfDNA could be used to enhance detection of ctDNA via machine learning technology.
Two models, designed to distinguish between healthy and cancerous samples, were developed using 153 samples, then validated on two datasets of 94 and 83 samples.
One of these models correctly classified cancerous samples in 94% of samples from patients with cancers known to have high levels of ctDNA – colorectal, cholangiocarcinoma, ovarian, breast, and melanoma – and in 65% of samples from low-ctDNA cancers – pancreatic, renal, and glioma.
Another model focused just on fragmentation patterns and was still able to distinguish cancer samples from those of healthy controls, although with slightly reduced area under the curve.
“Our results indicate that exploiting fundamental properties of cfDNA with fragment-specific analyses can allow more sensitive evaluation of ctDNA,” the authors wrote. “We identified features that could determine the presence and amount of ctDNA in plasma samples, without a prior knowledge of somatic aberrations.”
The authors pointed out that size selection of DNA fragments was relatively simple and cheap, and was also compatible with other genome-wide and targeted genomic analyses, “greatly increasing the potential value and utility of liquid biopsies as well as the cost-effectiveness of cfDNA sequencing.”
However, they cautioned that their catalogue had focused solely on double-stranded DNA and was subject to potential biases from the DNA extraction and sequencing methods they used in the study. They also commented that other biological effects could help refine the analysis of ctDNA.
“Other bodily fluids [urine, cerebrospinal fluid, and saliva], different nucleic acids and structures, altered mechanisms of release into circulation, or sample processing methods could exhibit varying fragment size signatures and could offer additional exploitable biological patterns for selective sequencing,” they wrote.
The study was supported by the University of Cambridge, Cancer Research UK, and the Engineering and Physical Sciences Research Council. Research supporting the study was also funded by the European Research Council, the National Institute for Health Research Cambridge, National Cancer Research Network, Cambridge Experimental Cancer Medicine Centre, Hutchison Whampoa, Target Ovarian Cancer, the Medical Research Council, and AstraZeneca. Three authors are cofounders, shareholders, and officers/consultants in a company specializing in ctDNA analysis. One author declared research funding and advisory board fees from private industry. Seven authors are listed on related patents.
SOURCE: Mouliere F et al. Sci Transl Med. 2018 Nov 7. doi: 10.1126/scitranslmed.aat4921.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: The size of cell-free DNA could be used to single out circulating tumor DNA.
Major finding: Circulating tumor DNA fragments are more commonly found in the 90-150 base pair range.
Study details: A study of 344 plasma samples from 200 patients with 18 cancer types and 65 samples from healthy controls.
Disclosures: The study was supported by the University of Cambridge, Cancer Research UK, and the Engineering and Physical Sciences Research Council. Research supporting the study was also funded by the European Research Council, the National Institute for Health Research Cambridge, National Cancer Research Network, Cambridge Experimental Cancer Medicine Centre, Hutchison Whampoa, Target Ovarian Cancer, the Medical Research Council, and AstraZeneca. Three authors are cofounders, shareholders, and officers/consultants in a company specializing in circulating tumor DNA analysis. One author declared research funding and advisory board fees from private industry. Seven authors are listed on related patents.
Source: Mouliere F et al. Sci Transl Med. 2018 Nov 7. doi: 10.1126/scitranslmed.aat4921.
Tumor burden in active surveillance for mRCC may inform treatment decisions
suggests an Italian cohort study.
Investigators led by Davide Bimbatti, MD, of Azienda Ospedaliera Universitaria Integrata in Verona, Italy, retrospectively studied 52 patients with mRCC who started active surveillance as their initial strategy for disease management. They assessed three predictors of outcomes: International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk class, number of metastatic sites, and tumor burden.
Patients remained on active surveillance for a median of 18.3 months and had a median total overall survival of 80.1 months, according to study results published in Urologic Oncology. Fully 69.2% started first-line systemic therapy during a median follow-up of 38.5 months.
The only baseline factor predicting time on active surveillance was IMDC class (hazard ratio, 2.15; P = .011).
An increasing number of metastatic sites during active surveillance was associated with poorer total overall survival (HR, 2.86; P = .010) and a trend toward poorer postsurveillance overall survival (HR, 2.37; P = .060).
Increasing tumor burden, measured as the sum in millimeters of the longest tumor diameter of each measurable lesion, during active surveillance was associated with both poorer total overall survival (HR, 1.16; P = .024) and poorer postsurveillance overall survival (HR, 1.21; P = .004).
Finally, an IMDC class of good or intermediate versus poor at the start of systemic therapy was a favorable predictor (HR, 0.07; P = .010; and HR, 0.12; P = .044, respectively) and an increase in tumor burden was an unfavorable predictor (HR, 1.26; P = .005) of postsurveillance overall survival.
“Our study confirms that active surveillance is a safe option for certain patients, with a median time on surveillance of 1.5 years delaying the beginning of systemic therapies and avoiding drug-related toxicities, with a median overall survival greater than 6.5 years,” wrote Dr. Bimbatti and coinvestigators.
“During active surveillance, patients rarely show any deterioration of the IMDC prognostic class. Meanwhile, the tumor burden changes, more than the increase of metastatic sites, account for the heterogeneity of the disease and may help physicians to make decisions about the early termination of active surveillance and the start of systemic therapy,” they concluded.
SOURCE: Bimbatti D et al. Urol Oncol. 2018 Oct 6. doi: 10.1016/j.urolonc.2018.08.018.
suggests an Italian cohort study.
Investigators led by Davide Bimbatti, MD, of Azienda Ospedaliera Universitaria Integrata in Verona, Italy, retrospectively studied 52 patients with mRCC who started active surveillance as their initial strategy for disease management. They assessed three predictors of outcomes: International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk class, number of metastatic sites, and tumor burden.
Patients remained on active surveillance for a median of 18.3 months and had a median total overall survival of 80.1 months, according to study results published in Urologic Oncology. Fully 69.2% started first-line systemic therapy during a median follow-up of 38.5 months.
The only baseline factor predicting time on active surveillance was IMDC class (hazard ratio, 2.15; P = .011).
An increasing number of metastatic sites during active surveillance was associated with poorer total overall survival (HR, 2.86; P = .010) and a trend toward poorer postsurveillance overall survival (HR, 2.37; P = .060).
Increasing tumor burden, measured as the sum in millimeters of the longest tumor diameter of each measurable lesion, during active surveillance was associated with both poorer total overall survival (HR, 1.16; P = .024) and poorer postsurveillance overall survival (HR, 1.21; P = .004).
Finally, an IMDC class of good or intermediate versus poor at the start of systemic therapy was a favorable predictor (HR, 0.07; P = .010; and HR, 0.12; P = .044, respectively) and an increase in tumor burden was an unfavorable predictor (HR, 1.26; P = .005) of postsurveillance overall survival.
“Our study confirms that active surveillance is a safe option for certain patients, with a median time on surveillance of 1.5 years delaying the beginning of systemic therapies and avoiding drug-related toxicities, with a median overall survival greater than 6.5 years,” wrote Dr. Bimbatti and coinvestigators.
“During active surveillance, patients rarely show any deterioration of the IMDC prognostic class. Meanwhile, the tumor burden changes, more than the increase of metastatic sites, account for the heterogeneity of the disease and may help physicians to make decisions about the early termination of active surveillance and the start of systemic therapy,” they concluded.
SOURCE: Bimbatti D et al. Urol Oncol. 2018 Oct 6. doi: 10.1016/j.urolonc.2018.08.018.
suggests an Italian cohort study.
Investigators led by Davide Bimbatti, MD, of Azienda Ospedaliera Universitaria Integrata in Verona, Italy, retrospectively studied 52 patients with mRCC who started active surveillance as their initial strategy for disease management. They assessed three predictors of outcomes: International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk class, number of metastatic sites, and tumor burden.
Patients remained on active surveillance for a median of 18.3 months and had a median total overall survival of 80.1 months, according to study results published in Urologic Oncology. Fully 69.2% started first-line systemic therapy during a median follow-up of 38.5 months.
The only baseline factor predicting time on active surveillance was IMDC class (hazard ratio, 2.15; P = .011).
An increasing number of metastatic sites during active surveillance was associated with poorer total overall survival (HR, 2.86; P = .010) and a trend toward poorer postsurveillance overall survival (HR, 2.37; P = .060).
Increasing tumor burden, measured as the sum in millimeters of the longest tumor diameter of each measurable lesion, during active surveillance was associated with both poorer total overall survival (HR, 1.16; P = .024) and poorer postsurveillance overall survival (HR, 1.21; P = .004).
Finally, an IMDC class of good or intermediate versus poor at the start of systemic therapy was a favorable predictor (HR, 0.07; P = .010; and HR, 0.12; P = .044, respectively) and an increase in tumor burden was an unfavorable predictor (HR, 1.26; P = .005) of postsurveillance overall survival.
“Our study confirms that active surveillance is a safe option for certain patients, with a median time on surveillance of 1.5 years delaying the beginning of systemic therapies and avoiding drug-related toxicities, with a median overall survival greater than 6.5 years,” wrote Dr. Bimbatti and coinvestigators.
“During active surveillance, patients rarely show any deterioration of the IMDC prognostic class. Meanwhile, the tumor burden changes, more than the increase of metastatic sites, account for the heterogeneity of the disease and may help physicians to make decisions about the early termination of active surveillance and the start of systemic therapy,” they concluded.
SOURCE: Bimbatti D et al. Urol Oncol. 2018 Oct 6. doi: 10.1016/j.urolonc.2018.08.018.
FROM UROLOGIC ONCOLOGY
Key clinical point: Change in tumor burden during active surveillance for metastatic RCC is prognostic.
Major finding: Each millimeter increase in total tumor burden during surveillance was associated with a 16% higher risk of death overall and a 21% increase in risk of death after stopping surveillance and starting first-line systemic therapy.
Study details: Retrospective cohort study of 52 patients with metastatic RCC who started with active surveillance.
Disclosures: The authors declared having no conflicts of interest.
Source: Bimbatti D et al. Urol Oncol. 2018 Oct 6. doi: 10.1016/j.urolonc.2018.08.018.
Striking racial/ethnic differences seen in RCC features
In a southwestern U.S. population having renal cell carcinoma (RCC), patient and disease characteristics differ by race/ethnicity in ways that may have implications for prevention, diagnosis, prognosis, and treatment, finds a single-center cohort study.
Investigators led by Ken Batai, PhD, of University of Arizona, Tucson, retrospectively reviewed the medical records of 294 patients with RCC as their first cancer who underwent partial or radical nephrectomy: 151 European Americans, 95 Hispanic Americans, 22 Native Americans, 9 African Americans, and 17 other race/ethnicity. About 12% overall had metastases at presentation.
On average, compared with European Americans, Hispanic Americans were about 5 years younger at diagnosis (55.8 vs. 60.5) and had higher odds of diagnosis before the age of 50 (odds ratio, 2.77), according to results published in Clinical Genitourinary Cancer.
Native Americans were even younger (49.7) and had dramatically elevated odds of diagnosis before that age (odds ratio, 6.23).
Relative to their European American counterparts, Hispanic Americans less commonly smoked (30.5% vs 48.6%) and African Americans more commonly had chronic kidney disease (37.5% vs. 5.8%). Both groups had higher prevalence of diabetes (45.6% and 54.5% vs. 21.7%). In addition, Native Americans had higher body mass index (35.2 vs. 30.7).
Clear cell histology was seen in 78.8% of European Americans, but in 92.6% of Hispanic Americans (odds ratio, 2.79) and 86.4% of Native Americans. African Americans more commonly had stage III or IV disease at diagnosis (77.8% vs. 35.3%; odds ratio, 6.51), but the racial/ethnic groups did not differ significantly on grade, tumor size, or presence of necrosis.
Among the Hispanic American patients undergoing radical nephrectomy, disease was more commonly of stage III or IV at diagnosis in those who were aged 65 or older (odds ratio, 10.48) and those who spoke Spanish as their primary language (odds ratios, 4.61).
The reasons for the observed racial/ethnic disparities remain unclear, according to Dr. Batai and his coinvestigators. Nonetheless, “it is necessary to better understand the clinical characteristics of these underserved Hispanic American and Native American populations with high kidney cancer burden,” they wrote.
“Our findings can direct future research toward elucidating the difference in tumor behavior among the different ethnic groups and health care issues causing poor outcomes,” they concluded. The findings also “bring ... awareness to practitioners treating patients from these racial/ethnic minority groups regarding the clinical characteristics and underlying issues in these patient populations.”
The study was supported by the American Cancer Society and the Partnership for Native American Cancer Prevention.
SOURCE: Batai K et al. Clin Genitourin Cancer. 2018 Oct 26. doi: 10.1016/j.clgc.2018.10.012.
In a southwestern U.S. population having renal cell carcinoma (RCC), patient and disease characteristics differ by race/ethnicity in ways that may have implications for prevention, diagnosis, prognosis, and treatment, finds a single-center cohort study.
Investigators led by Ken Batai, PhD, of University of Arizona, Tucson, retrospectively reviewed the medical records of 294 patients with RCC as their first cancer who underwent partial or radical nephrectomy: 151 European Americans, 95 Hispanic Americans, 22 Native Americans, 9 African Americans, and 17 other race/ethnicity. About 12% overall had metastases at presentation.
On average, compared with European Americans, Hispanic Americans were about 5 years younger at diagnosis (55.8 vs. 60.5) and had higher odds of diagnosis before the age of 50 (odds ratio, 2.77), according to results published in Clinical Genitourinary Cancer.
Native Americans were even younger (49.7) and had dramatically elevated odds of diagnosis before that age (odds ratio, 6.23).
Relative to their European American counterparts, Hispanic Americans less commonly smoked (30.5% vs 48.6%) and African Americans more commonly had chronic kidney disease (37.5% vs. 5.8%). Both groups had higher prevalence of diabetes (45.6% and 54.5% vs. 21.7%). In addition, Native Americans had higher body mass index (35.2 vs. 30.7).
Clear cell histology was seen in 78.8% of European Americans, but in 92.6% of Hispanic Americans (odds ratio, 2.79) and 86.4% of Native Americans. African Americans more commonly had stage III or IV disease at diagnosis (77.8% vs. 35.3%; odds ratio, 6.51), but the racial/ethnic groups did not differ significantly on grade, tumor size, or presence of necrosis.
Among the Hispanic American patients undergoing radical nephrectomy, disease was more commonly of stage III or IV at diagnosis in those who were aged 65 or older (odds ratio, 10.48) and those who spoke Spanish as their primary language (odds ratios, 4.61).
The reasons for the observed racial/ethnic disparities remain unclear, according to Dr. Batai and his coinvestigators. Nonetheless, “it is necessary to better understand the clinical characteristics of these underserved Hispanic American and Native American populations with high kidney cancer burden,” they wrote.
“Our findings can direct future research toward elucidating the difference in tumor behavior among the different ethnic groups and health care issues causing poor outcomes,” they concluded. The findings also “bring ... awareness to practitioners treating patients from these racial/ethnic minority groups regarding the clinical characteristics and underlying issues in these patient populations.”
The study was supported by the American Cancer Society and the Partnership for Native American Cancer Prevention.
SOURCE: Batai K et al. Clin Genitourin Cancer. 2018 Oct 26. doi: 10.1016/j.clgc.2018.10.012.
In a southwestern U.S. population having renal cell carcinoma (RCC), patient and disease characteristics differ by race/ethnicity in ways that may have implications for prevention, diagnosis, prognosis, and treatment, finds a single-center cohort study.
Investigators led by Ken Batai, PhD, of University of Arizona, Tucson, retrospectively reviewed the medical records of 294 patients with RCC as their first cancer who underwent partial or radical nephrectomy: 151 European Americans, 95 Hispanic Americans, 22 Native Americans, 9 African Americans, and 17 other race/ethnicity. About 12% overall had metastases at presentation.
On average, compared with European Americans, Hispanic Americans were about 5 years younger at diagnosis (55.8 vs. 60.5) and had higher odds of diagnosis before the age of 50 (odds ratio, 2.77), according to results published in Clinical Genitourinary Cancer.
Native Americans were even younger (49.7) and had dramatically elevated odds of diagnosis before that age (odds ratio, 6.23).
Relative to their European American counterparts, Hispanic Americans less commonly smoked (30.5% vs 48.6%) and African Americans more commonly had chronic kidney disease (37.5% vs. 5.8%). Both groups had higher prevalence of diabetes (45.6% and 54.5% vs. 21.7%). In addition, Native Americans had higher body mass index (35.2 vs. 30.7).
Clear cell histology was seen in 78.8% of European Americans, but in 92.6% of Hispanic Americans (odds ratio, 2.79) and 86.4% of Native Americans. African Americans more commonly had stage III or IV disease at diagnosis (77.8% vs. 35.3%; odds ratio, 6.51), but the racial/ethnic groups did not differ significantly on grade, tumor size, or presence of necrosis.
Among the Hispanic American patients undergoing radical nephrectomy, disease was more commonly of stage III or IV at diagnosis in those who were aged 65 or older (odds ratio, 10.48) and those who spoke Spanish as their primary language (odds ratios, 4.61).
The reasons for the observed racial/ethnic disparities remain unclear, according to Dr. Batai and his coinvestigators. Nonetheless, “it is necessary to better understand the clinical characteristics of these underserved Hispanic American and Native American populations with high kidney cancer burden,” they wrote.
“Our findings can direct future research toward elucidating the difference in tumor behavior among the different ethnic groups and health care issues causing poor outcomes,” they concluded. The findings also “bring ... awareness to practitioners treating patients from these racial/ethnic minority groups regarding the clinical characteristics and underlying issues in these patient populations.”
The study was supported by the American Cancer Society and the Partnership for Native American Cancer Prevention.
SOURCE: Batai K et al. Clin Genitourin Cancer. 2018 Oct 26. doi: 10.1016/j.clgc.2018.10.012.
FROM CLINICAL GENITOURINARY CANCER
Key clinical point: .
Major finding: Compared with European Americans, Hispanic Americans and Native Americans were younger at diagnosis (55.8 and 49.7 vs. 60.5 years) and more often had clear cell histology (92.6% and 86.4% vs. 78.8%), and African Americans more often had stage III/IV disease (77.8% vs. 35.3%).
Study details: U.S. single-center retrospective cohort study of 294 patients with RCC who underwent partial or radical nephrectomy.
Disclosures: The authors declared that they did not have any conflicts of interest. The study was supported by the American Cancer Society and the Partnership for Native American Cancer Prevention.
Source: Batai K et al. Clin Genitourin Cancer. 2018 Oct 26. doi: 10.1016/j.clgc.2018.10.012.
KPNA2 is “novel prognostic factor” in RCC
High expression of karyopherin alpha 2 (KPNA2), a carrier protein that helps shuttle cancer-associated proteins from the nucleus to the cytoplasm, is an adverse prognostic factor in patients with clear cell or papillary renal cell carcinoma (RCC), according to a retrospective cohort study.
Senior author Glen Kristiansen, MD, director of the Institute of Pathology at the University Hospital Bonn (Germany), and his colleagues assessed tumor levels of KPNA2 protein by immunohistochemistry in 240 clinic patients with RCC (217 with clear cell histology, 23 with papillary histology). They also assessed tumor levels of KPNA2 mRNA in 771 patients with RCC (481 with clear cell histology, 290 with papillary cell histology) using publicly available gene expression data from the Cancer Genome Atlas (CGA).
Overall, 19% of the clinic patients’ tumors showed high expression of KPNA2 protein, according to results reported in Clinical Genitourinary Cancer. In addition, 26% of the CGA patients’ tumors showed high expression of KPNA2 mRNA.
Among patients with clear cell RCC, those with high tumor levels of KPNA2 protein survived roughly half as long as counterparts with low levels or none (74 months vs. 171 months); the difference was significant in univariate analysis (P = .012) but not in multivariate analysis that included well-known prognostic factors (HR, 1.491; P = .237). On the other hand, those with high tumor levels of KPNA2 mRNA had an elevated risk of death in both univariate analysis (HR, 2.31; P less than .001) and multivariate analysis (HR, 1.45; P = .035).
Among patients with papillary RCC, tumor levels of KPNA2 protein were not significantly associated with survival. However, those with high tumor levels of KPNA2 mRNA had a sharply elevated risk of death in both univariate analysis (HR, 9.7; P less than .001) and multivariate analysis (HR, 6.2; P = .004).
KPNA2 expression “represents a novel prognostic factor in these subtypes of RCC,” concluded Dr. Kristiansen and his coinvestigators. Therefore, this biomarker “could be used to stratify risk groups within RCC.” Collectively, the study’s findings suggest that KPNA2 is involved in both the pathogenesis and the progression of RCC. Given evidence that it helps transport p53 and fibroblast growth factor 2 at least in tumors with clear cell histology, “investigation of the nucleocytoplasmic transport through KPNA2 in [clear cell] RCC is an important task for future studies to better understand the link between elevated KPNA2 expression and altered biological processes like proliferation, cell growth, migration, invasion and tumor formation in RCC. In addition, examination of other members of [the] karyopherin-alpha family could elucidate the role of the nucleocytoplasmic transport system in pathogenesis of RCC.”
The authors reported that they had no conflict of interests.
SOURCE: Kristiansen G et al. Clin Genitourin Cancer. 2018 Oct 22. doi: 10.1016/j.clgc.2018.10.008.
High expression of karyopherin alpha 2 (KPNA2), a carrier protein that helps shuttle cancer-associated proteins from the nucleus to the cytoplasm, is an adverse prognostic factor in patients with clear cell or papillary renal cell carcinoma (RCC), according to a retrospective cohort study.
Senior author Glen Kristiansen, MD, director of the Institute of Pathology at the University Hospital Bonn (Germany), and his colleagues assessed tumor levels of KPNA2 protein by immunohistochemistry in 240 clinic patients with RCC (217 with clear cell histology, 23 with papillary histology). They also assessed tumor levels of KPNA2 mRNA in 771 patients with RCC (481 with clear cell histology, 290 with papillary cell histology) using publicly available gene expression data from the Cancer Genome Atlas (CGA).
Overall, 19% of the clinic patients’ tumors showed high expression of KPNA2 protein, according to results reported in Clinical Genitourinary Cancer. In addition, 26% of the CGA patients’ tumors showed high expression of KPNA2 mRNA.
Among patients with clear cell RCC, those with high tumor levels of KPNA2 protein survived roughly half as long as counterparts with low levels or none (74 months vs. 171 months); the difference was significant in univariate analysis (P = .012) but not in multivariate analysis that included well-known prognostic factors (HR, 1.491; P = .237). On the other hand, those with high tumor levels of KPNA2 mRNA had an elevated risk of death in both univariate analysis (HR, 2.31; P less than .001) and multivariate analysis (HR, 1.45; P = .035).
Among patients with papillary RCC, tumor levels of KPNA2 protein were not significantly associated with survival. However, those with high tumor levels of KPNA2 mRNA had a sharply elevated risk of death in both univariate analysis (HR, 9.7; P less than .001) and multivariate analysis (HR, 6.2; P = .004).
KPNA2 expression “represents a novel prognostic factor in these subtypes of RCC,” concluded Dr. Kristiansen and his coinvestigators. Therefore, this biomarker “could be used to stratify risk groups within RCC.” Collectively, the study’s findings suggest that KPNA2 is involved in both the pathogenesis and the progression of RCC. Given evidence that it helps transport p53 and fibroblast growth factor 2 at least in tumors with clear cell histology, “investigation of the nucleocytoplasmic transport through KPNA2 in [clear cell] RCC is an important task for future studies to better understand the link between elevated KPNA2 expression and altered biological processes like proliferation, cell growth, migration, invasion and tumor formation in RCC. In addition, examination of other members of [the] karyopherin-alpha family could elucidate the role of the nucleocytoplasmic transport system in pathogenesis of RCC.”
The authors reported that they had no conflict of interests.
SOURCE: Kristiansen G et al. Clin Genitourin Cancer. 2018 Oct 22. doi: 10.1016/j.clgc.2018.10.008.
High expression of karyopherin alpha 2 (KPNA2), a carrier protein that helps shuttle cancer-associated proteins from the nucleus to the cytoplasm, is an adverse prognostic factor in patients with clear cell or papillary renal cell carcinoma (RCC), according to a retrospective cohort study.
Senior author Glen Kristiansen, MD, director of the Institute of Pathology at the University Hospital Bonn (Germany), and his colleagues assessed tumor levels of KPNA2 protein by immunohistochemistry in 240 clinic patients with RCC (217 with clear cell histology, 23 with papillary histology). They also assessed tumor levels of KPNA2 mRNA in 771 patients with RCC (481 with clear cell histology, 290 with papillary cell histology) using publicly available gene expression data from the Cancer Genome Atlas (CGA).
Overall, 19% of the clinic patients’ tumors showed high expression of KPNA2 protein, according to results reported in Clinical Genitourinary Cancer. In addition, 26% of the CGA patients’ tumors showed high expression of KPNA2 mRNA.
Among patients with clear cell RCC, those with high tumor levels of KPNA2 protein survived roughly half as long as counterparts with low levels or none (74 months vs. 171 months); the difference was significant in univariate analysis (P = .012) but not in multivariate analysis that included well-known prognostic factors (HR, 1.491; P = .237). On the other hand, those with high tumor levels of KPNA2 mRNA had an elevated risk of death in both univariate analysis (HR, 2.31; P less than .001) and multivariate analysis (HR, 1.45; P = .035).
Among patients with papillary RCC, tumor levels of KPNA2 protein were not significantly associated with survival. However, those with high tumor levels of KPNA2 mRNA had a sharply elevated risk of death in both univariate analysis (HR, 9.7; P less than .001) and multivariate analysis (HR, 6.2; P = .004).
KPNA2 expression “represents a novel prognostic factor in these subtypes of RCC,” concluded Dr. Kristiansen and his coinvestigators. Therefore, this biomarker “could be used to stratify risk groups within RCC.” Collectively, the study’s findings suggest that KPNA2 is involved in both the pathogenesis and the progression of RCC. Given evidence that it helps transport p53 and fibroblast growth factor 2 at least in tumors with clear cell histology, “investigation of the nucleocytoplasmic transport through KPNA2 in [clear cell] RCC is an important task for future studies to better understand the link between elevated KPNA2 expression and altered biological processes like proliferation, cell growth, migration, invasion and tumor formation in RCC. In addition, examination of other members of [the] karyopherin-alpha family could elucidate the role of the nucleocytoplasmic transport system in pathogenesis of RCC.”
The authors reported that they had no conflict of interests.
SOURCE: Kristiansen G et al. Clin Genitourin Cancer. 2018 Oct 22. doi: 10.1016/j.clgc.2018.10.008.
FROM CLINICAL GENITOURINARY CANCER
Key clinical point:
Major finding: Survival was poorer for patients with clear cell RCC having high KPNA2 protein levels (74 vs. 171 months) or mRNA levels (hazard ratio for death, 1.45) and for patients with papillary RCC having high KPNA2 mRNA levels (HR, 6.2).
Study details: A retrospective dual cohort study of 240 clinic patients and 771 Cancer Genome Atlas patients.
Disclosures: The authors reported that they had no conflict of interests.
Source: Kristiansen G et al. Clin Genitourin Cancer. 2018 Oct 22. doi: 10.1016/j.clgc.2018.10.008.