User login
Female cancer researchers receive less funding than male counterparts
Female cancer researchers receive significantly less funding than their male counterparts in terms of total investment, number of awards, and mean and median funding, according to an analysis of data on public and philanthropic cancer research funding awarded to U.K. institutions between 2000 and 2013.
In an analysis of 4,186 awards totaling 2.33 billion pounds, 2,890 grants (69%) with a total value of 1.82 billion pounds (78%) were awarded to male primary investigators (PIs), compared with just 1,296 grants (31%) with a total value of 512 million pounds(22%) for female PIs, investigators reported in BMJ Open.
Investigators studied openly accessible information on funding awards from public and philanthropic sources including the Medical Research Council, Department of Health, Biotechnology and Biological Sciences Research Council, Engineering and Physical Science Research Council, Wellcome Trust, European Commission, and nine members of the Association of Medical Research Charities. Awards were excluded if they were not relevant to oncology, led by a non-U.K. institution, and/or not considered a research and development activity, wrote Charlie D. Zhou, MD, of the Royal Free NHS Foundation Trust Department of Nuclear Medicine in London, and coauthors.
Median grant value was greater for men (252,647 pounds; interquartile range, 127,343-553,560 pounds) than for women (198,485 pounds; IQR, 99,317-382,650 pounds) (P less than .001). Mean grant value was also greater for men (630,324 pounds; standard deviation, 1,662,559 pounds) than for women (394,730 pounds; SD, 666,574 pounds), Dr. Zhou and colleagues reported.
Large funding discrepancies were seen for sex-specific cancer research. For instance, males received 13.8, 3.5, and 2.0 times the investment of their female counterparts in total, mean, and median prostate cancer funding, respectively. Likewise, men received 9.9, 6.6, and 2.9 times the funding of women PIs in total, mean, and median funding, respectively, for cervical cancer research. This pattern was true for ovarian cancer and breast cancer research, as well.
Men also received significantly greater median funding at all points of the research and development pipeline. For preclinical, phase 1, 2, or 3 clinical trials; and public health, men received 20%, 90%, and 50% more, respectively (P less than .001); for product development and cross-disciplinary research, the difference was 50% and 20%, respectively (P less than .01).
The results of the analysis demonstrate that “female PIs clearly and consistently receive less funding than their male counterparts,” the authors wrote. Although the study results are descriptive in nature and do not identify the underlying mechanisms for these discrepancies, they “demonstrate substantial gender imbalances in cancer research investment.
“We would strongly urge policy makers, funders and the academic and scientific community to investigate the factors leading to our observed differences and seek to ensure that women are appropriately supported in scientific endeavor,” they concluded.
No disclosures or conflicts of interest were reported.
SOURCE: Zhou CD et al. BMJ Open. 2018 Apr 30. doi: 10.1136/bmjopen-2017-018625.
Female cancer researchers receive significantly less funding than their male counterparts in terms of total investment, number of awards, and mean and median funding, according to an analysis of data on public and philanthropic cancer research funding awarded to U.K. institutions between 2000 and 2013.
In an analysis of 4,186 awards totaling 2.33 billion pounds, 2,890 grants (69%) with a total value of 1.82 billion pounds (78%) were awarded to male primary investigators (PIs), compared with just 1,296 grants (31%) with a total value of 512 million pounds(22%) for female PIs, investigators reported in BMJ Open.
Investigators studied openly accessible information on funding awards from public and philanthropic sources including the Medical Research Council, Department of Health, Biotechnology and Biological Sciences Research Council, Engineering and Physical Science Research Council, Wellcome Trust, European Commission, and nine members of the Association of Medical Research Charities. Awards were excluded if they were not relevant to oncology, led by a non-U.K. institution, and/or not considered a research and development activity, wrote Charlie D. Zhou, MD, of the Royal Free NHS Foundation Trust Department of Nuclear Medicine in London, and coauthors.
Median grant value was greater for men (252,647 pounds; interquartile range, 127,343-553,560 pounds) than for women (198,485 pounds; IQR, 99,317-382,650 pounds) (P less than .001). Mean grant value was also greater for men (630,324 pounds; standard deviation, 1,662,559 pounds) than for women (394,730 pounds; SD, 666,574 pounds), Dr. Zhou and colleagues reported.
Large funding discrepancies were seen for sex-specific cancer research. For instance, males received 13.8, 3.5, and 2.0 times the investment of their female counterparts in total, mean, and median prostate cancer funding, respectively. Likewise, men received 9.9, 6.6, and 2.9 times the funding of women PIs in total, mean, and median funding, respectively, for cervical cancer research. This pattern was true for ovarian cancer and breast cancer research, as well.
Men also received significantly greater median funding at all points of the research and development pipeline. For preclinical, phase 1, 2, or 3 clinical trials; and public health, men received 20%, 90%, and 50% more, respectively (P less than .001); for product development and cross-disciplinary research, the difference was 50% and 20%, respectively (P less than .01).
The results of the analysis demonstrate that “female PIs clearly and consistently receive less funding than their male counterparts,” the authors wrote. Although the study results are descriptive in nature and do not identify the underlying mechanisms for these discrepancies, they “demonstrate substantial gender imbalances in cancer research investment.
“We would strongly urge policy makers, funders and the academic and scientific community to investigate the factors leading to our observed differences and seek to ensure that women are appropriately supported in scientific endeavor,” they concluded.
No disclosures or conflicts of interest were reported.
SOURCE: Zhou CD et al. BMJ Open. 2018 Apr 30. doi: 10.1136/bmjopen-2017-018625.
Female cancer researchers receive significantly less funding than their male counterparts in terms of total investment, number of awards, and mean and median funding, according to an analysis of data on public and philanthropic cancer research funding awarded to U.K. institutions between 2000 and 2013.
In an analysis of 4,186 awards totaling 2.33 billion pounds, 2,890 grants (69%) with a total value of 1.82 billion pounds (78%) were awarded to male primary investigators (PIs), compared with just 1,296 grants (31%) with a total value of 512 million pounds(22%) for female PIs, investigators reported in BMJ Open.
Investigators studied openly accessible information on funding awards from public and philanthropic sources including the Medical Research Council, Department of Health, Biotechnology and Biological Sciences Research Council, Engineering and Physical Science Research Council, Wellcome Trust, European Commission, and nine members of the Association of Medical Research Charities. Awards were excluded if they were not relevant to oncology, led by a non-U.K. institution, and/or not considered a research and development activity, wrote Charlie D. Zhou, MD, of the Royal Free NHS Foundation Trust Department of Nuclear Medicine in London, and coauthors.
Median grant value was greater for men (252,647 pounds; interquartile range, 127,343-553,560 pounds) than for women (198,485 pounds; IQR, 99,317-382,650 pounds) (P less than .001). Mean grant value was also greater for men (630,324 pounds; standard deviation, 1,662,559 pounds) than for women (394,730 pounds; SD, 666,574 pounds), Dr. Zhou and colleagues reported.
Large funding discrepancies were seen for sex-specific cancer research. For instance, males received 13.8, 3.5, and 2.0 times the investment of their female counterparts in total, mean, and median prostate cancer funding, respectively. Likewise, men received 9.9, 6.6, and 2.9 times the funding of women PIs in total, mean, and median funding, respectively, for cervical cancer research. This pattern was true for ovarian cancer and breast cancer research, as well.
Men also received significantly greater median funding at all points of the research and development pipeline. For preclinical, phase 1, 2, or 3 clinical trials; and public health, men received 20%, 90%, and 50% more, respectively (P less than .001); for product development and cross-disciplinary research, the difference was 50% and 20%, respectively (P less than .01).
The results of the analysis demonstrate that “female PIs clearly and consistently receive less funding than their male counterparts,” the authors wrote. Although the study results are descriptive in nature and do not identify the underlying mechanisms for these discrepancies, they “demonstrate substantial gender imbalances in cancer research investment.
“We would strongly urge policy makers, funders and the academic and scientific community to investigate the factors leading to our observed differences and seek to ensure that women are appropriately supported in scientific endeavor,” they concluded.
No disclosures or conflicts of interest were reported.
SOURCE: Zhou CD et al. BMJ Open. 2018 Apr 30. doi: 10.1136/bmjopen-2017-018625.
FROM BMJ OPEN
Key clinical point: Female cancer researchers receive significantly less funding than their male counterparts.
Major finding: Of 4,186 awards, 2,890 grants (69%) were awarded to male primary investigators (PIs), compared with 1,296 grants (31%) for female PIs.
Study details: An analysis of data on public and philanthropic cancer research funding awarded to U.K. institutions between 2000 and 2013.
Disclosures: No disclosures or conflicts of interest were reported.
Source: Zhou CD et al. BMJ Open. 2018 Apr 30. doi: 10.1136/bmjopen-2017-018625.
Immediate postresection gemcitabine tops saline in low-grade non–muscle-invasive bladder cancer
For patients with suspected low-grade non–muscle-invasive bladder cancer, immediate postresection treatment with intravesicular gemcitabine significantly cut recurrence rates in a double-blind, multicenter, randomized, placebo-controlled trial.
In the intention-to-treat analysis, estimated rates of 4-year recurrence were 35% with gemcitabine and 47% with placebo saline (hazard ratio, 0.66; 95% confidence interval, 0.48-0.90; P less than .001), reported Edward D. Messing of the University of Rochester, New York, and his associates. Gemcitabine also significantly outperformed placebo in the preplanned analysis of patients with confirmed low-grade non–muscle-invasive urothelial cancer (estimated 4-year recurrence rates , 4% and 54% respectively; HR, 0.53; 95% CI, 0.35-0.81; P = .001).
Intravesicular gemcitabine did not significantly reduce all-cause mortality or tumor progression to muscle invasion. “In an underpowered post hoc subgroup analysis, there [also] was no evidence of a benefit of immediate post-TURBT [transurethral resection of bladder tumor] gemcitabine in patients with high-grade non–muscle-invasive urothelial cancer,” the researchers wrote. The report was published May 8 in JAMA.
Robust data already support single-dose intravesicular chemotherapy with mitomycin C or epirubicin immediately after patients undergo TURBT. But in reality, this practice is uncommon in the United States. Meanwhile, systemic gemcitabine already is used to treat bladder cancer, and its intravesicular use appears safe and at least as effective as other chemotherapies, the investigators noted. Therefore, the SWOG S0337 trial enrolled 416 symptomatic patients with suspected low-grade papillary urothelial cancer who received a single intravesicular instillation of either gemcitabine (2 g in 100 mL saline) or saline (100 mL) within 3 hours after transurethral resection of TURBT.
Ten percent of patients did not receive study drug instillation, usually for medical reasons. There were no grade 4-5 adverse events. Grade 3 or lower adverse events did not significantly differ between groups. The study did not capture reliable data on tumor size or treatment at or after recurrence, the researchers said. Taken together, the findings “support using this therapy, but further research is needed to compare gemcitabine with other intravesical agents.”
The National Cancer Institute provided funding. Eli Lilly provided the gemcitabine used in the study. Dr. Messing reported having no relevant conflicts of interest. Three coinvestigators disclosed ties to BioCancell, Incyte, and various other biopharmaceutical companies.
SOURCE: Messing EM et al. JAMA. 2018 May 8;319(18):1880-8.
The well designed and executed study “provides important results for patients and physicians alike,” Samuel D. Kaffenberger, MD, David C. Miller, MD, MPH, and Matthew E. Nielsen, MD, MS, wrote in an editorial accompanying the study report in JAMA.
Recurrent bladder cancer exacts major emotional, medical, and monetary costs, the experts stressed. “The natural history of frequent recurrences drives a uniquely intensive and costly program of invasive surveillance and treatment.”
Thus, while the trial results are promising, their “ultimate benefit” will depend on “more consistent and proficient use of intravesical gemcitabine than has been observed for mitomycin,” they said. Disseminating this “simple, safe, effective, and affordable” treatment will require education and mobilization of patients and physicians, advocacy organizations, and health care system leaders.
Dr. Kaffenberger and Dr. Miller are at the University of Michigan, Ann Arbor, and Dr. Nielsen is at the University of North Carolina at Chapel Hill. Dr. Nielsen disclosed stock options via the Grand Rounds Medical Advisory Board. Dr. Miller disclosed ties to Blue Cross Blue Shield of Michigan. Dr. Kaffenberger reported having no conflicts of interest. These comments summarize their editorial (JAMA. 2018 319;18:1864-65).
The well designed and executed study “provides important results for patients and physicians alike,” Samuel D. Kaffenberger, MD, David C. Miller, MD, MPH, and Matthew E. Nielsen, MD, MS, wrote in an editorial accompanying the study report in JAMA.
Recurrent bladder cancer exacts major emotional, medical, and monetary costs, the experts stressed. “The natural history of frequent recurrences drives a uniquely intensive and costly program of invasive surveillance and treatment.”
Thus, while the trial results are promising, their “ultimate benefit” will depend on “more consistent and proficient use of intravesical gemcitabine than has been observed for mitomycin,” they said. Disseminating this “simple, safe, effective, and affordable” treatment will require education and mobilization of patients and physicians, advocacy organizations, and health care system leaders.
Dr. Kaffenberger and Dr. Miller are at the University of Michigan, Ann Arbor, and Dr. Nielsen is at the University of North Carolina at Chapel Hill. Dr. Nielsen disclosed stock options via the Grand Rounds Medical Advisory Board. Dr. Miller disclosed ties to Blue Cross Blue Shield of Michigan. Dr. Kaffenberger reported having no conflicts of interest. These comments summarize their editorial (JAMA. 2018 319;18:1864-65).
The well designed and executed study “provides important results for patients and physicians alike,” Samuel D. Kaffenberger, MD, David C. Miller, MD, MPH, and Matthew E. Nielsen, MD, MS, wrote in an editorial accompanying the study report in JAMA.
Recurrent bladder cancer exacts major emotional, medical, and monetary costs, the experts stressed. “The natural history of frequent recurrences drives a uniquely intensive and costly program of invasive surveillance and treatment.”
Thus, while the trial results are promising, their “ultimate benefit” will depend on “more consistent and proficient use of intravesical gemcitabine than has been observed for mitomycin,” they said. Disseminating this “simple, safe, effective, and affordable” treatment will require education and mobilization of patients and physicians, advocacy organizations, and health care system leaders.
Dr. Kaffenberger and Dr. Miller are at the University of Michigan, Ann Arbor, and Dr. Nielsen is at the University of North Carolina at Chapel Hill. Dr. Nielsen disclosed stock options via the Grand Rounds Medical Advisory Board. Dr. Miller disclosed ties to Blue Cross Blue Shield of Michigan. Dr. Kaffenberger reported having no conflicts of interest. These comments summarize their editorial (JAMA. 2018 319;18:1864-65).
For patients with suspected low-grade non–muscle-invasive bladder cancer, immediate postresection treatment with intravesicular gemcitabine significantly cut recurrence rates in a double-blind, multicenter, randomized, placebo-controlled trial.
In the intention-to-treat analysis, estimated rates of 4-year recurrence were 35% with gemcitabine and 47% with placebo saline (hazard ratio, 0.66; 95% confidence interval, 0.48-0.90; P less than .001), reported Edward D. Messing of the University of Rochester, New York, and his associates. Gemcitabine also significantly outperformed placebo in the preplanned analysis of patients with confirmed low-grade non–muscle-invasive urothelial cancer (estimated 4-year recurrence rates , 4% and 54% respectively; HR, 0.53; 95% CI, 0.35-0.81; P = .001).
Intravesicular gemcitabine did not significantly reduce all-cause mortality or tumor progression to muscle invasion. “In an underpowered post hoc subgroup analysis, there [also] was no evidence of a benefit of immediate post-TURBT [transurethral resection of bladder tumor] gemcitabine in patients with high-grade non–muscle-invasive urothelial cancer,” the researchers wrote. The report was published May 8 in JAMA.
Robust data already support single-dose intravesicular chemotherapy with mitomycin C or epirubicin immediately after patients undergo TURBT. But in reality, this practice is uncommon in the United States. Meanwhile, systemic gemcitabine already is used to treat bladder cancer, and its intravesicular use appears safe and at least as effective as other chemotherapies, the investigators noted. Therefore, the SWOG S0337 trial enrolled 416 symptomatic patients with suspected low-grade papillary urothelial cancer who received a single intravesicular instillation of either gemcitabine (2 g in 100 mL saline) or saline (100 mL) within 3 hours after transurethral resection of TURBT.
Ten percent of patients did not receive study drug instillation, usually for medical reasons. There were no grade 4-5 adverse events. Grade 3 or lower adverse events did not significantly differ between groups. The study did not capture reliable data on tumor size or treatment at or after recurrence, the researchers said. Taken together, the findings “support using this therapy, but further research is needed to compare gemcitabine with other intravesical agents.”
The National Cancer Institute provided funding. Eli Lilly provided the gemcitabine used in the study. Dr. Messing reported having no relevant conflicts of interest. Three coinvestigators disclosed ties to BioCancell, Incyte, and various other biopharmaceutical companies.
SOURCE: Messing EM et al. JAMA. 2018 May 8;319(18):1880-8.
For patients with suspected low-grade non–muscle-invasive bladder cancer, immediate postresection treatment with intravesicular gemcitabine significantly cut recurrence rates in a double-blind, multicenter, randomized, placebo-controlled trial.
In the intention-to-treat analysis, estimated rates of 4-year recurrence were 35% with gemcitabine and 47% with placebo saline (hazard ratio, 0.66; 95% confidence interval, 0.48-0.90; P less than .001), reported Edward D. Messing of the University of Rochester, New York, and his associates. Gemcitabine also significantly outperformed placebo in the preplanned analysis of patients with confirmed low-grade non–muscle-invasive urothelial cancer (estimated 4-year recurrence rates , 4% and 54% respectively; HR, 0.53; 95% CI, 0.35-0.81; P = .001).
Intravesicular gemcitabine did not significantly reduce all-cause mortality or tumor progression to muscle invasion. “In an underpowered post hoc subgroup analysis, there [also] was no evidence of a benefit of immediate post-TURBT [transurethral resection of bladder tumor] gemcitabine in patients with high-grade non–muscle-invasive urothelial cancer,” the researchers wrote. The report was published May 8 in JAMA.
Robust data already support single-dose intravesicular chemotherapy with mitomycin C or epirubicin immediately after patients undergo TURBT. But in reality, this practice is uncommon in the United States. Meanwhile, systemic gemcitabine already is used to treat bladder cancer, and its intravesicular use appears safe and at least as effective as other chemotherapies, the investigators noted. Therefore, the SWOG S0337 trial enrolled 416 symptomatic patients with suspected low-grade papillary urothelial cancer who received a single intravesicular instillation of either gemcitabine (2 g in 100 mL saline) or saline (100 mL) within 3 hours after transurethral resection of TURBT.
Ten percent of patients did not receive study drug instillation, usually for medical reasons. There were no grade 4-5 adverse events. Grade 3 or lower adverse events did not significantly differ between groups. The study did not capture reliable data on tumor size or treatment at or after recurrence, the researchers said. Taken together, the findings “support using this therapy, but further research is needed to compare gemcitabine with other intravesical agents.”
The National Cancer Institute provided funding. Eli Lilly provided the gemcitabine used in the study. Dr. Messing reported having no relevant conflicts of interest. Three coinvestigators disclosed ties to BioCancell, Incyte, and various other biopharmaceutical companies.
SOURCE: Messing EM et al. JAMA. 2018 May 8;319(18):1880-8.
FROM JAMA
Key clinical point: Immediate postresection intravesicular gemcitabine significantly reduced the risk of recurrence in patients with suspected low-grade non–muscle-invasive bladder cancer.
Major finding: Estimated rates of 4-year recurrence were 35% with gemcitabine and 47% with placebo (hazard ratio, 0.66; P less than .001).
Study details: Phase 3 multicenter trial of 416 patients randomly assigned to receive gemcitabine (2 g in 100 mL saline) or placebo saline (100 mL) (SWOG S0337).
Disclosures: The National Cancer Institute provided funding. Eli Lilly provided the gemcitabine used in the study. Dr. Messing reported having no relevant conflicts of interest. Three coinvestigators disclosed ties to BioCancell, Incyte, and various other biopharmaceutical companies.
Source: Messing EM et al. JAMA. 2018 May 8;319(18):1880-8.
Rare paraneoplastic dermatomyositis secondary to high-grade bladder cancer
The clinical presentation of bladder cancer typically presents with hematuria; changes in voiding habits such as urgency, frequency, and pain; or less commonly, obstructive symptoms. Rarely does bladder cancer first present as part of a paraneoplastic syndrome with an inflammatory myopathy. Inflammatory myopathies such as dermatomyositis have been known to be associated with malignancy, however, in a meta-analysis by Yang and colleagues of 449 patients with dermatomyositis and malignancy there were only 8 cases reported of bladder cancer.1 Herein, we report a paraneoplastic dermatomyositis in the setting of a bladder cancer.
Case presentation and summary
A 65-year-old man with a medical history of hypertension and alcohol use presented to the emergency department with worsening pain, stiffness in the neck, shoulders, and inability to lift his arms above his shoulders. During the physical exam, an erythematous purple rash was noted over his chest, neck, and arms. Upon further evaluation, his creatine phosphokinase was 3,500 U/L (reference range 52-336 U/L) suggesting muscle breakdown and possible inflammatory myopathy. A biopsy of the left deltoid and quadriceps muscles was performed and yielded a diagnosis of dermatomyositis. He was treated with prednisone 60 mg daily for his inflammatory myopathy. The patient also reported an unintentional weight loss of 20 lbs. and increasing weakness and inability to swallow, which caused aspiration events without developing pneumonia.
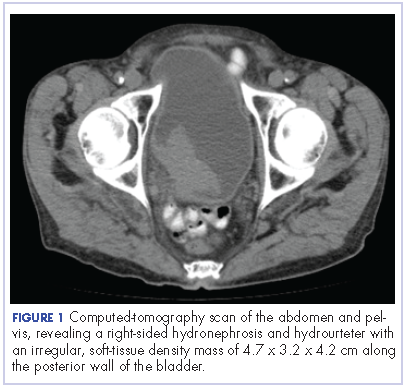
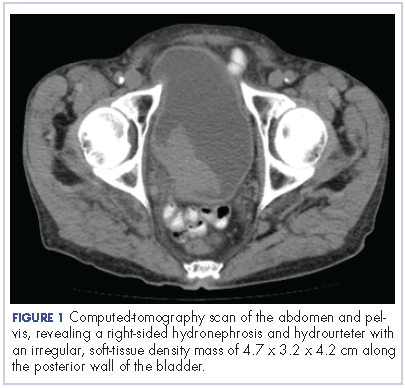
The patient’s symptoms worsened while he was on steroids, and we became concerned about the possibility of a primary malignancy, which led to further work-up. The results of a computed-tomography (CT) scan of the abdomen and pelvis showed right-sided hydronephrosis and hydrourteter with an irregular, soft-tissue density mass of 4.7 x 3.2 x 4.2 cm along the posterior wall of the bladder (Figure 1).
A cystoscopy was performed with transurethral resection of a bladder tumor that was more than 8 cm in diameter. Because the mass was not fully resectable, only 25% of the tumor burden was removed. The pathology report revealed an invasive, high-grade urothelial cell carcinoma (Figure 2, see PDF). Further imaging ruled out metastatic spread. The patient was continued on steroids. He was not a candidate for neoadjuvant chemotherapy because of his comorbidities and cisplatin ineligibility owing to his significant bilateral hearing deficiencies. Members of a multidisciplinary tumor board decided to move forward with definitive surgery. The patient underwent a robotic-assisted laparoscoptic cystoprostatectomy with bilateral pelvic lymph node dissection and open ileal conduit urinary diversion. Staging of tumor was determined as pT3b N1 (1/30) M0, LVI+. After the surgery, the patient had resolution of his rash and significant improvement in his muscle weakness with the ability to raise his arms over his head and climb stairs. Adjuvant chemotherapy was not given since he was cisplatin ineligible as a result of his hearing loss. Active surveillance was preferred.
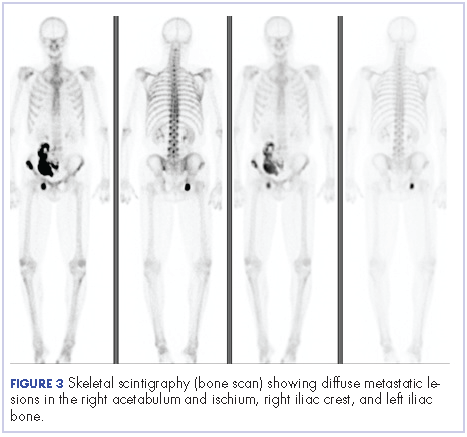
Four months after his cystoprostatectomy, he experienced new-onset hip pain and further imaging, including a bone scan, was performed. It showed metastatic disease in the ischium and iliac crest (Figure 3).
The patient decided to forgo any palliative chemotherapy and to have palliative radiation for pain and enroll in hospice. He died nine months after the initial diagnosis of urothelial cell carcinoma.
Discussion
Dermatomyositis is one of the inflammatory myopathies with a clinical presentation of proximal muscle weakness and characteristic skin findings of Gottron papules and heliotrope eruption. The most common subgroups of inflammatory myopathies are dermatomyositis, polymyositis, necrotizing autoimmune myopathy, and inclusion body myopathy. The pathogenesis of inflammatory myopathies is not well understood; however, some theories have been described, including: type 1 interferon signaling causing myofiber injury and antibody-complement mediated processes causing ischemia resulting in myofiber injury. 2,3 The diagnoses of inflammatory myopathies may be suggested based on history, physical examination findings, laboratory values showing muscle injury (creatine kinase, aldolase, ALT, AST, LDH), myositis-specific antibodies (antisynthetase autoantibodies), electromyogram, and magnetic-resonance imaging. However, muscle biopsy remains the gold standard.4
The initial treatment of inflammatory myopathies begins with glucocorticoid therapy at 0.5-1.0 mg/kg. This regimen may be titrated down over 6 weeks to a level adequate to control symptoms. Even while on glucocorticoid therapy, this patient’s symptoms continued, along with the development of dysphagia. Dysphagia is another notable symptom of dermatomyositis that may result in aspiration pneumonia with fatal outcomes.5,6,7 Not only did this patient initially respond poorly to corticosteroids, but the unintentional weight loss was another alarming feature prompting further evaluation. That led to the diagnosis of urothelial cell carcinoma, which was causing the paraneoplastic syndrome.
A paraneoplastic syndrome is a collection of symptoms that are observed in organ systems separate from the primary disease. This process is mostly caused by an autoimmune response to the tumor and nervous system.8 Inflammatory myopathies, such as dermatomyositis, have been shown to be associated with a variety of malignancies as part of a paraneoplastic syndrome. The most common cancers associated with dermatomyositis are ovarian, lung, pancreatic, stomach, colorectal, and non-Hodgkin lymphoma.9 Although an association between dermatomyositis and bladder cancer has been established, very few cases have been reported in the literature. In the Yang meta-analysis, the relative risk of malignancy for patients with dermatomyositis was 5.5%, and of the 449 patients with dermatomyositis who had malignancy, only 8 cases of bladder cancer were reported.1
After a patient has been diagnosed with an inflammatory myopathy, there should be further evaluation for an underling malignancy causing a paraneoplastic process. The risk of these patients having a malignancy overall is 4.5 times higher than patients without dermatomyositis.1 Definite screening recommendations have not been established, but screening should be based on patient’s age, gender, and clinical scenario. The European Federation of Neurological Societies formed a task force to focus on malignancy screening of paraneoplastic neurological syndromes and included dermatomyositis as one of the signs.10 Patients should have a CT scan of the chest, abdomen, and pelvis. Women should have a mammogram and a pelvis ultrasound. Men younger than 50 years should consider testes ultrasound, and patients older than 50 years should undergo usual colonoscopy screening.
The risk of malignancy is highest in the first year after diagnosis, but may extend to 5 years after the diagnosis, so repeat screening should be performed 3-6 months after diagnosis, followed with biannual testing for 4 years. If a malignancy is present, then treatment should be tailored to the neoplasm to improve symptoms of myositis; however, response is generally worse than it would be with dermatomyositis in the absence of malignancy. In the present case with bladder cancer, therapies may include platinum-based-chemotherapy, resection, and radiation. Dermatomyositis as a result of a bladder cancer paraneoplastic syndrome is associated with a poor prognosis as demonstrated in the case of this patient and others reported in the literature.11
Even though dermatomyositis is usually a chronic disease process, 87% of patients respond initially to corticosteroid treatment.12 Therefore, treatment should be escalated with an agent such as azathioprine or methotrexate, or, like in this case, an underlying malignancy should be suspected. This case emphasizes the importance of screening patients appropriately for malignancy in patients with an inflammatory myopathy and reveals the poor prognosis associated with this disease.
1. Yang Z, Lin F, Qin B, Liang Y, Zhong R. Polymyositis/dermatomyositis and malignancy risk: a metaanalysis study. J Rheumatol. 2015;42(2):282-291.
2. Greenberg, SA. Dermatomyositis and type 1 interferons. Curr Rheumatol Rep. 2010;12(3):198-203.
3. Dalakas, MC, Hohlfeld, R. Polymyositis and dermatomyositis. Lancet. 2003;362(9388):971-982.
4. Malik A, Hayat G, Kalia JS, Guzman MA. Idiopathic inflammatory myopathies: clinical approach and management. Front Neurol. 2016;7:64.
5. Sabio JM, Vargas-Hitos JA, Jiménez-Alonso J. Paraneoplastic dermatomyositis associated with bladder cancer. Lupus. 2006;15(9):619-620.
6. Mallon E, Osborne G, Dinneen M, Lane RJ, Glaser M, Bunker CB. Dermatomyositis in association with transitional cell carcinoma of the bladder. Clin Exp Dermatol. 1999;24(2):94-96.
7. Hafejee A, Coulson IH. Dysphagia in dermatomyositis secondary to bladder cancer: rapid response to combined immunoglobulin and methylprednisolone. Clin Exp Dermatol. 2005;30(1):93-94.
8. Dalmau J, Gultekin HS, Posner JB. Paraneoplastic neurologic syndromes: pathogenesis and physiopathology. Brain Pathol. 1999;9(2):275-284.
9. Hill CL, Zhang Y, Sigureirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357(9250):96-100.
10. Titulaer, MJ, Soffietti R, Dalmau J, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS Task Force. Eur J Neurol. 2011;18(1):19-e3.
11. Xu R, Zhong Z, Jiang H, Zhang L, Zhao X. A rare paraneoplastic dermatomyositis in bladder cancer with fatal outcome. Urol J. 2013;10(1):815-817.
12. Troyanov Y, Targoff IN, Tremblay JL, Goulet JR, Raymond Y, Senecal JL. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients. Medicine (Baltimore), 2005;84(4):231-249.
The clinical presentation of bladder cancer typically presents with hematuria; changes in voiding habits such as urgency, frequency, and pain; or less commonly, obstructive symptoms. Rarely does bladder cancer first present as part of a paraneoplastic syndrome with an inflammatory myopathy. Inflammatory myopathies such as dermatomyositis have been known to be associated with malignancy, however, in a meta-analysis by Yang and colleagues of 449 patients with dermatomyositis and malignancy there were only 8 cases reported of bladder cancer.1 Herein, we report a paraneoplastic dermatomyositis in the setting of a bladder cancer.
Case presentation and summary
A 65-year-old man with a medical history of hypertension and alcohol use presented to the emergency department with worsening pain, stiffness in the neck, shoulders, and inability to lift his arms above his shoulders. During the physical exam, an erythematous purple rash was noted over his chest, neck, and arms. Upon further evaluation, his creatine phosphokinase was 3,500 U/L (reference range 52-336 U/L) suggesting muscle breakdown and possible inflammatory myopathy. A biopsy of the left deltoid and quadriceps muscles was performed and yielded a diagnosis of dermatomyositis. He was treated with prednisone 60 mg daily for his inflammatory myopathy. The patient also reported an unintentional weight loss of 20 lbs. and increasing weakness and inability to swallow, which caused aspiration events without developing pneumonia.
The patient’s symptoms worsened while he was on steroids, and we became concerned about the possibility of a primary malignancy, which led to further work-up. The results of a computed-tomography (CT) scan of the abdomen and pelvis showed right-sided hydronephrosis and hydrourteter with an irregular, soft-tissue density mass of 4.7 x 3.2 x 4.2 cm along the posterior wall of the bladder (Figure 1).
A cystoscopy was performed with transurethral resection of a bladder tumor that was more than 8 cm in diameter. Because the mass was not fully resectable, only 25% of the tumor burden was removed. The pathology report revealed an invasive, high-grade urothelial cell carcinoma (Figure 2, see PDF). Further imaging ruled out metastatic spread. The patient was continued on steroids. He was not a candidate for neoadjuvant chemotherapy because of his comorbidities and cisplatin ineligibility owing to his significant bilateral hearing deficiencies. Members of a multidisciplinary tumor board decided to move forward with definitive surgery. The patient underwent a robotic-assisted laparoscoptic cystoprostatectomy with bilateral pelvic lymph node dissection and open ileal conduit urinary diversion. Staging of tumor was determined as pT3b N1 (1/30) M0, LVI+. After the surgery, the patient had resolution of his rash and significant improvement in his muscle weakness with the ability to raise his arms over his head and climb stairs. Adjuvant chemotherapy was not given since he was cisplatin ineligible as a result of his hearing loss. Active surveillance was preferred.
Four months after his cystoprostatectomy, he experienced new-onset hip pain and further imaging, including a bone scan, was performed. It showed metastatic disease in the ischium and iliac crest (Figure 3).
The patient decided to forgo any palliative chemotherapy and to have palliative radiation for pain and enroll in hospice. He died nine months after the initial diagnosis of urothelial cell carcinoma.
Discussion
Dermatomyositis is one of the inflammatory myopathies with a clinical presentation of proximal muscle weakness and characteristic skin findings of Gottron papules and heliotrope eruption. The most common subgroups of inflammatory myopathies are dermatomyositis, polymyositis, necrotizing autoimmune myopathy, and inclusion body myopathy. The pathogenesis of inflammatory myopathies is not well understood; however, some theories have been described, including: type 1 interferon signaling causing myofiber injury and antibody-complement mediated processes causing ischemia resulting in myofiber injury. 2,3 The diagnoses of inflammatory myopathies may be suggested based on history, physical examination findings, laboratory values showing muscle injury (creatine kinase, aldolase, ALT, AST, LDH), myositis-specific antibodies (antisynthetase autoantibodies), electromyogram, and magnetic-resonance imaging. However, muscle biopsy remains the gold standard.4
The initial treatment of inflammatory myopathies begins with glucocorticoid therapy at 0.5-1.0 mg/kg. This regimen may be titrated down over 6 weeks to a level adequate to control symptoms. Even while on glucocorticoid therapy, this patient’s symptoms continued, along with the development of dysphagia. Dysphagia is another notable symptom of dermatomyositis that may result in aspiration pneumonia with fatal outcomes.5,6,7 Not only did this patient initially respond poorly to corticosteroids, but the unintentional weight loss was another alarming feature prompting further evaluation. That led to the diagnosis of urothelial cell carcinoma, which was causing the paraneoplastic syndrome.
A paraneoplastic syndrome is a collection of symptoms that are observed in organ systems separate from the primary disease. This process is mostly caused by an autoimmune response to the tumor and nervous system.8 Inflammatory myopathies, such as dermatomyositis, have been shown to be associated with a variety of malignancies as part of a paraneoplastic syndrome. The most common cancers associated with dermatomyositis are ovarian, lung, pancreatic, stomach, colorectal, and non-Hodgkin lymphoma.9 Although an association between dermatomyositis and bladder cancer has been established, very few cases have been reported in the literature. In the Yang meta-analysis, the relative risk of malignancy for patients with dermatomyositis was 5.5%, and of the 449 patients with dermatomyositis who had malignancy, only 8 cases of bladder cancer were reported.1
After a patient has been diagnosed with an inflammatory myopathy, there should be further evaluation for an underling malignancy causing a paraneoplastic process. The risk of these patients having a malignancy overall is 4.5 times higher than patients without dermatomyositis.1 Definite screening recommendations have not been established, but screening should be based on patient’s age, gender, and clinical scenario. The European Federation of Neurological Societies formed a task force to focus on malignancy screening of paraneoplastic neurological syndromes and included dermatomyositis as one of the signs.10 Patients should have a CT scan of the chest, abdomen, and pelvis. Women should have a mammogram and a pelvis ultrasound. Men younger than 50 years should consider testes ultrasound, and patients older than 50 years should undergo usual colonoscopy screening.
The risk of malignancy is highest in the first year after diagnosis, but may extend to 5 years after the diagnosis, so repeat screening should be performed 3-6 months after diagnosis, followed with biannual testing for 4 years. If a malignancy is present, then treatment should be tailored to the neoplasm to improve symptoms of myositis; however, response is generally worse than it would be with dermatomyositis in the absence of malignancy. In the present case with bladder cancer, therapies may include platinum-based-chemotherapy, resection, and radiation. Dermatomyositis as a result of a bladder cancer paraneoplastic syndrome is associated with a poor prognosis as demonstrated in the case of this patient and others reported in the literature.11
Even though dermatomyositis is usually a chronic disease process, 87% of patients respond initially to corticosteroid treatment.12 Therefore, treatment should be escalated with an agent such as azathioprine or methotrexate, or, like in this case, an underlying malignancy should be suspected. This case emphasizes the importance of screening patients appropriately for malignancy in patients with an inflammatory myopathy and reveals the poor prognosis associated with this disease.
The clinical presentation of bladder cancer typically presents with hematuria; changes in voiding habits such as urgency, frequency, and pain; or less commonly, obstructive symptoms. Rarely does bladder cancer first present as part of a paraneoplastic syndrome with an inflammatory myopathy. Inflammatory myopathies such as dermatomyositis have been known to be associated with malignancy, however, in a meta-analysis by Yang and colleagues of 449 patients with dermatomyositis and malignancy there were only 8 cases reported of bladder cancer.1 Herein, we report a paraneoplastic dermatomyositis in the setting of a bladder cancer.
Case presentation and summary
A 65-year-old man with a medical history of hypertension and alcohol use presented to the emergency department with worsening pain, stiffness in the neck, shoulders, and inability to lift his arms above his shoulders. During the physical exam, an erythematous purple rash was noted over his chest, neck, and arms. Upon further evaluation, his creatine phosphokinase was 3,500 U/L (reference range 52-336 U/L) suggesting muscle breakdown and possible inflammatory myopathy. A biopsy of the left deltoid and quadriceps muscles was performed and yielded a diagnosis of dermatomyositis. He was treated with prednisone 60 mg daily for his inflammatory myopathy. The patient also reported an unintentional weight loss of 20 lbs. and increasing weakness and inability to swallow, which caused aspiration events without developing pneumonia.
The patient’s symptoms worsened while he was on steroids, and we became concerned about the possibility of a primary malignancy, which led to further work-up. The results of a computed-tomography (CT) scan of the abdomen and pelvis showed right-sided hydronephrosis and hydrourteter with an irregular, soft-tissue density mass of 4.7 x 3.2 x 4.2 cm along the posterior wall of the bladder (Figure 1).
A cystoscopy was performed with transurethral resection of a bladder tumor that was more than 8 cm in diameter. Because the mass was not fully resectable, only 25% of the tumor burden was removed. The pathology report revealed an invasive, high-grade urothelial cell carcinoma (Figure 2, see PDF). Further imaging ruled out metastatic spread. The patient was continued on steroids. He was not a candidate for neoadjuvant chemotherapy because of his comorbidities and cisplatin ineligibility owing to his significant bilateral hearing deficiencies. Members of a multidisciplinary tumor board decided to move forward with definitive surgery. The patient underwent a robotic-assisted laparoscoptic cystoprostatectomy with bilateral pelvic lymph node dissection and open ileal conduit urinary diversion. Staging of tumor was determined as pT3b N1 (1/30) M0, LVI+. After the surgery, the patient had resolution of his rash and significant improvement in his muscle weakness with the ability to raise his arms over his head and climb stairs. Adjuvant chemotherapy was not given since he was cisplatin ineligible as a result of his hearing loss. Active surveillance was preferred.
Four months after his cystoprostatectomy, he experienced new-onset hip pain and further imaging, including a bone scan, was performed. It showed metastatic disease in the ischium and iliac crest (Figure 3).
The patient decided to forgo any palliative chemotherapy and to have palliative radiation for pain and enroll in hospice. He died nine months after the initial diagnosis of urothelial cell carcinoma.
Discussion
Dermatomyositis is one of the inflammatory myopathies with a clinical presentation of proximal muscle weakness and characteristic skin findings of Gottron papules and heliotrope eruption. The most common subgroups of inflammatory myopathies are dermatomyositis, polymyositis, necrotizing autoimmune myopathy, and inclusion body myopathy. The pathogenesis of inflammatory myopathies is not well understood; however, some theories have been described, including: type 1 interferon signaling causing myofiber injury and antibody-complement mediated processes causing ischemia resulting in myofiber injury. 2,3 The diagnoses of inflammatory myopathies may be suggested based on history, physical examination findings, laboratory values showing muscle injury (creatine kinase, aldolase, ALT, AST, LDH), myositis-specific antibodies (antisynthetase autoantibodies), electromyogram, and magnetic-resonance imaging. However, muscle biopsy remains the gold standard.4
The initial treatment of inflammatory myopathies begins with glucocorticoid therapy at 0.5-1.0 mg/kg. This regimen may be titrated down over 6 weeks to a level adequate to control symptoms. Even while on glucocorticoid therapy, this patient’s symptoms continued, along with the development of dysphagia. Dysphagia is another notable symptom of dermatomyositis that may result in aspiration pneumonia with fatal outcomes.5,6,7 Not only did this patient initially respond poorly to corticosteroids, but the unintentional weight loss was another alarming feature prompting further evaluation. That led to the diagnosis of urothelial cell carcinoma, which was causing the paraneoplastic syndrome.
A paraneoplastic syndrome is a collection of symptoms that are observed in organ systems separate from the primary disease. This process is mostly caused by an autoimmune response to the tumor and nervous system.8 Inflammatory myopathies, such as dermatomyositis, have been shown to be associated with a variety of malignancies as part of a paraneoplastic syndrome. The most common cancers associated with dermatomyositis are ovarian, lung, pancreatic, stomach, colorectal, and non-Hodgkin lymphoma.9 Although an association between dermatomyositis and bladder cancer has been established, very few cases have been reported in the literature. In the Yang meta-analysis, the relative risk of malignancy for patients with dermatomyositis was 5.5%, and of the 449 patients with dermatomyositis who had malignancy, only 8 cases of bladder cancer were reported.1
After a patient has been diagnosed with an inflammatory myopathy, there should be further evaluation for an underling malignancy causing a paraneoplastic process. The risk of these patients having a malignancy overall is 4.5 times higher than patients without dermatomyositis.1 Definite screening recommendations have not been established, but screening should be based on patient’s age, gender, and clinical scenario. The European Federation of Neurological Societies formed a task force to focus on malignancy screening of paraneoplastic neurological syndromes and included dermatomyositis as one of the signs.10 Patients should have a CT scan of the chest, abdomen, and pelvis. Women should have a mammogram and a pelvis ultrasound. Men younger than 50 years should consider testes ultrasound, and patients older than 50 years should undergo usual colonoscopy screening.
The risk of malignancy is highest in the first year after diagnosis, but may extend to 5 years after the diagnosis, so repeat screening should be performed 3-6 months after diagnosis, followed with biannual testing for 4 years. If a malignancy is present, then treatment should be tailored to the neoplasm to improve symptoms of myositis; however, response is generally worse than it would be with dermatomyositis in the absence of malignancy. In the present case with bladder cancer, therapies may include platinum-based-chemotherapy, resection, and radiation. Dermatomyositis as a result of a bladder cancer paraneoplastic syndrome is associated with a poor prognosis as demonstrated in the case of this patient and others reported in the literature.11
Even though dermatomyositis is usually a chronic disease process, 87% of patients respond initially to corticosteroid treatment.12 Therefore, treatment should be escalated with an agent such as azathioprine or methotrexate, or, like in this case, an underlying malignancy should be suspected. This case emphasizes the importance of screening patients appropriately for malignancy in patients with an inflammatory myopathy and reveals the poor prognosis associated with this disease.
1. Yang Z, Lin F, Qin B, Liang Y, Zhong R. Polymyositis/dermatomyositis and malignancy risk: a metaanalysis study. J Rheumatol. 2015;42(2):282-291.
2. Greenberg, SA. Dermatomyositis and type 1 interferons. Curr Rheumatol Rep. 2010;12(3):198-203.
3. Dalakas, MC, Hohlfeld, R. Polymyositis and dermatomyositis. Lancet. 2003;362(9388):971-982.
4. Malik A, Hayat G, Kalia JS, Guzman MA. Idiopathic inflammatory myopathies: clinical approach and management. Front Neurol. 2016;7:64.
5. Sabio JM, Vargas-Hitos JA, Jiménez-Alonso J. Paraneoplastic dermatomyositis associated with bladder cancer. Lupus. 2006;15(9):619-620.
6. Mallon E, Osborne G, Dinneen M, Lane RJ, Glaser M, Bunker CB. Dermatomyositis in association with transitional cell carcinoma of the bladder. Clin Exp Dermatol. 1999;24(2):94-96.
7. Hafejee A, Coulson IH. Dysphagia in dermatomyositis secondary to bladder cancer: rapid response to combined immunoglobulin and methylprednisolone. Clin Exp Dermatol. 2005;30(1):93-94.
8. Dalmau J, Gultekin HS, Posner JB. Paraneoplastic neurologic syndromes: pathogenesis and physiopathology. Brain Pathol. 1999;9(2):275-284.
9. Hill CL, Zhang Y, Sigureirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357(9250):96-100.
10. Titulaer, MJ, Soffietti R, Dalmau J, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS Task Force. Eur J Neurol. 2011;18(1):19-e3.
11. Xu R, Zhong Z, Jiang H, Zhang L, Zhao X. A rare paraneoplastic dermatomyositis in bladder cancer with fatal outcome. Urol J. 2013;10(1):815-817.
12. Troyanov Y, Targoff IN, Tremblay JL, Goulet JR, Raymond Y, Senecal JL. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients. Medicine (Baltimore), 2005;84(4):231-249.
1. Yang Z, Lin F, Qin B, Liang Y, Zhong R. Polymyositis/dermatomyositis and malignancy risk: a metaanalysis study. J Rheumatol. 2015;42(2):282-291.
2. Greenberg, SA. Dermatomyositis and type 1 interferons. Curr Rheumatol Rep. 2010;12(3):198-203.
3. Dalakas, MC, Hohlfeld, R. Polymyositis and dermatomyositis. Lancet. 2003;362(9388):971-982.
4. Malik A, Hayat G, Kalia JS, Guzman MA. Idiopathic inflammatory myopathies: clinical approach and management. Front Neurol. 2016;7:64.
5. Sabio JM, Vargas-Hitos JA, Jiménez-Alonso J. Paraneoplastic dermatomyositis associated with bladder cancer. Lupus. 2006;15(9):619-620.
6. Mallon E, Osborne G, Dinneen M, Lane RJ, Glaser M, Bunker CB. Dermatomyositis in association with transitional cell carcinoma of the bladder. Clin Exp Dermatol. 1999;24(2):94-96.
7. Hafejee A, Coulson IH. Dysphagia in dermatomyositis secondary to bladder cancer: rapid response to combined immunoglobulin and methylprednisolone. Clin Exp Dermatol. 2005;30(1):93-94.
8. Dalmau J, Gultekin HS, Posner JB. Paraneoplastic neurologic syndromes: pathogenesis and physiopathology. Brain Pathol. 1999;9(2):275-284.
9. Hill CL, Zhang Y, Sigureirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357(9250):96-100.
10. Titulaer, MJ, Soffietti R, Dalmau J, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS Task Force. Eur J Neurol. 2011;18(1):19-e3.
11. Xu R, Zhong Z, Jiang H, Zhang L, Zhao X. A rare paraneoplastic dermatomyositis in bladder cancer with fatal outcome. Urol J. 2013;10(1):815-817.
12. Troyanov Y, Targoff IN, Tremblay JL, Goulet JR, Raymond Y, Senecal JL. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients. Medicine (Baltimore), 2005;84(4):231-249.
World Trade Center responders face greater cancer burden, including greater risk of multiple myeloma
Rescue and recovery workers who were involved in the aftermath of the World Trade Center disaster may face a greater cancer burden than the general population, according to two studies published in JAMA Oncology.
In particular, they may be at risk of developing multiple myeloma at an earlier age.
The first study was a closed-cohort study of 14,474 employees of the Fire Department of the City of New York (FDNY) who were exposed to the World Trade Center disaster but were cancer-free as of Jan. 1, 2012. The aim was to project cancer incidence from 2012 through 2031, based on data from the FDNY World Trade Center Health Program, and compare those rates with age-, race-, and sex-specific New York cancer rates from the general population.
The modeling projected a “modestly” higher number of cancer cases in the white male subgroup of rescue and recovery workers exposed to the World Trade Center (2,714 vs. 2,596 for the general population of New York; P less than .001). Specifically, the investigators projected significantly higher case counts of prostate cancer (1,437 vs. 863), thyroid cancer (73 vs. 57), and melanoma (201 vs. 131), compared with the general population in New York, but fewer lung (237 vs. 373), colorectal (172 vs. 267), and kidney cancers (66 vs. 132) (P less than .001 for all).
“Our findings suggest that the FDNY WTC-exposed cohort may experience a greater burden of cancer than would be expected from a population with similar demographic characteristics,” wrote Rachel Zeig-Owens, DrPH, from the Montefiore Medical Center and Albert Einstein College of Medicine, both in New York, and coauthors, highlighting prostate cancer as a particular concern.
However, they also acknowledged that the elevated rates observed in people exposed to the World Trade Center disaster could be a result of increased surveillance, even though they did attempt to correct for that, and that firefighters in general might face higher risks.
“It is possible that firefighters have a higher risk of cancer than the general population owing to exposures associated with the occupation,” they wrote. However occupation could also have the opposite effect, as rescue and recovery workers tend to have lower smoking rates, which may explain the relatively low rates of certain cancers such as lung cancer, they said.
A second study examined the effect of the World Trade Center disaster on the risk of multiple myeloma and monoclonal gammopathies in exposed firefighters.
The seroprevalence study of monoclonal gammopathies of undetermined significance (MGUS) in 781 exposed firefighters revealed that the age-standardized prevalence of these was 76% higher in this population than it was in a white male reference population living in Minnesota.
In particular, the age-standardized prevalence of light-chain MGUS was more than threefold higher in exposed firefighters, compared with the reference population.
Researchers also analyzed a case series of 16 exposed white male firefighters who received a diagnosis of multiple myeloma after Sept. 11, 2001. Of the 14 patients for whom data on the monoclonal protein isotype was available, half had light-chain multiple myeloma.
“These findings are of interest due to previously observed associations between light-chain multiple myeloma and light-chain MGUS and exposure to toxins, and chronic immune stimulation,” wrote Ola Landgren, MD, PhD, from the Memorial Sloan Kettering Cancer Center and his coauthors.
Seven patients were also assessed for CD20 expression – a marker of poorer prognosis – and 71% were found to be CD20-positive, a prevalence around 3.5-fold higher than that seen in the general population.
The cohort with multiple myeloma was diagnosed on average 12 years younger than those in the general population. The authors commented that this was unlikely to be caused by lead-time bias because the time from first symptoms to clinical manifestation of the disease is usually around 1 year.
“Taken together, our results show that environmental exposure due to the WTC attacks is associated with myeloma precursor disease (MGUS and light-chain MGUS) and may be a risk factor for the development of multiple myeloma at an earlier age, particularly the light-chain subtype,” the authors wrote.
The first study was supported by the National Institute of Occupational Safety and Health; no conflicts of interest were declared.
The second study was supported by the V Foundation for Cancer Research, the Byrne Fund for the benefit of Memorial Sloan-Kettering Cancer Center, the National Cancer Institute, the Albert Einstein Cancer Center, and the National Institute for Occupational Safety and Health; no conflicts of interest were declared.
SOURCE: Zeig-Owens R et al. JAMA Oncology. 2018 April 26. doi: 10.1001/jamaoncol.2018.0504. Landgren O et al. JAMA Oncology. 2018 April 16. doi: 10.1001/jamaoncol.2018.0509.
When the heroes of the World Trade Center are diagnosed with even a common cancer, there is a natural tendency to assume that the diagnosis is the result of their service during the disaster. However, it is important to appreciate that the firefighting profession is known to be associated with higher risks of monoclonal gammopathy of undetermined significance and multiple myelomas, compared with the general population.
Given that, it would have been preferable to compare the World Trade Center–exposed populations with an equally intensively screened, age-matched cohort of firefighters from another major city.
If we apply Sir Richard Doll’s rule that a single epidemiologic study cannot be persuasive until the lower bound of the 95% confidence interval is greater than three, the relative risks in the study by Landgren and colleagues are too small to be persuasive.
The predicted increases in cancers of the prostate, thyroid, and myeloma are interesting, but these have also been previously reported in firefighters from other cities.
Despite this, we owe it to these men and women to find the truth and determine the illnesses that are associated with their service.
Otis W. Brawley, MD, is chief medical and scientific officer and executive vice president of the American Cancer Society and a professor at Emory University, Atlanta. These comments are taken from an accompanying editorial (JAMA Oncology. 2018 April 26. doi: 10.1001/jamaoncol.2018.0498.) No conflicts of interest were declared.
When the heroes of the World Trade Center are diagnosed with even a common cancer, there is a natural tendency to assume that the diagnosis is the result of their service during the disaster. However, it is important to appreciate that the firefighting profession is known to be associated with higher risks of monoclonal gammopathy of undetermined significance and multiple myelomas, compared with the general population.
Given that, it would have been preferable to compare the World Trade Center–exposed populations with an equally intensively screened, age-matched cohort of firefighters from another major city.
If we apply Sir Richard Doll’s rule that a single epidemiologic study cannot be persuasive until the lower bound of the 95% confidence interval is greater than three, the relative risks in the study by Landgren and colleagues are too small to be persuasive.
The predicted increases in cancers of the prostate, thyroid, and myeloma are interesting, but these have also been previously reported in firefighters from other cities.
Despite this, we owe it to these men and women to find the truth and determine the illnesses that are associated with their service.
Otis W. Brawley, MD, is chief medical and scientific officer and executive vice president of the American Cancer Society and a professor at Emory University, Atlanta. These comments are taken from an accompanying editorial (JAMA Oncology. 2018 April 26. doi: 10.1001/jamaoncol.2018.0498.) No conflicts of interest were declared.
When the heroes of the World Trade Center are diagnosed with even a common cancer, there is a natural tendency to assume that the diagnosis is the result of their service during the disaster. However, it is important to appreciate that the firefighting profession is known to be associated with higher risks of monoclonal gammopathy of undetermined significance and multiple myelomas, compared with the general population.
Given that, it would have been preferable to compare the World Trade Center–exposed populations with an equally intensively screened, age-matched cohort of firefighters from another major city.
If we apply Sir Richard Doll’s rule that a single epidemiologic study cannot be persuasive until the lower bound of the 95% confidence interval is greater than three, the relative risks in the study by Landgren and colleagues are too small to be persuasive.
The predicted increases in cancers of the prostate, thyroid, and myeloma are interesting, but these have also been previously reported in firefighters from other cities.
Despite this, we owe it to these men and women to find the truth and determine the illnesses that are associated with their service.
Otis W. Brawley, MD, is chief medical and scientific officer and executive vice president of the American Cancer Society and a professor at Emory University, Atlanta. These comments are taken from an accompanying editorial (JAMA Oncology. 2018 April 26. doi: 10.1001/jamaoncol.2018.0498.) No conflicts of interest were declared.
Rescue and recovery workers who were involved in the aftermath of the World Trade Center disaster may face a greater cancer burden than the general population, according to two studies published in JAMA Oncology.
In particular, they may be at risk of developing multiple myeloma at an earlier age.
The first study was a closed-cohort study of 14,474 employees of the Fire Department of the City of New York (FDNY) who were exposed to the World Trade Center disaster but were cancer-free as of Jan. 1, 2012. The aim was to project cancer incidence from 2012 through 2031, based on data from the FDNY World Trade Center Health Program, and compare those rates with age-, race-, and sex-specific New York cancer rates from the general population.
The modeling projected a “modestly” higher number of cancer cases in the white male subgroup of rescue and recovery workers exposed to the World Trade Center (2,714 vs. 2,596 for the general population of New York; P less than .001). Specifically, the investigators projected significantly higher case counts of prostate cancer (1,437 vs. 863), thyroid cancer (73 vs. 57), and melanoma (201 vs. 131), compared with the general population in New York, but fewer lung (237 vs. 373), colorectal (172 vs. 267), and kidney cancers (66 vs. 132) (P less than .001 for all).
“Our findings suggest that the FDNY WTC-exposed cohort may experience a greater burden of cancer than would be expected from a population with similar demographic characteristics,” wrote Rachel Zeig-Owens, DrPH, from the Montefiore Medical Center and Albert Einstein College of Medicine, both in New York, and coauthors, highlighting prostate cancer as a particular concern.
However, they also acknowledged that the elevated rates observed in people exposed to the World Trade Center disaster could be a result of increased surveillance, even though they did attempt to correct for that, and that firefighters in general might face higher risks.
“It is possible that firefighters have a higher risk of cancer than the general population owing to exposures associated with the occupation,” they wrote. However occupation could also have the opposite effect, as rescue and recovery workers tend to have lower smoking rates, which may explain the relatively low rates of certain cancers such as lung cancer, they said.
A second study examined the effect of the World Trade Center disaster on the risk of multiple myeloma and monoclonal gammopathies in exposed firefighters.
The seroprevalence study of monoclonal gammopathies of undetermined significance (MGUS) in 781 exposed firefighters revealed that the age-standardized prevalence of these was 76% higher in this population than it was in a white male reference population living in Minnesota.
In particular, the age-standardized prevalence of light-chain MGUS was more than threefold higher in exposed firefighters, compared with the reference population.
Researchers also analyzed a case series of 16 exposed white male firefighters who received a diagnosis of multiple myeloma after Sept. 11, 2001. Of the 14 patients for whom data on the monoclonal protein isotype was available, half had light-chain multiple myeloma.
“These findings are of interest due to previously observed associations between light-chain multiple myeloma and light-chain MGUS and exposure to toxins, and chronic immune stimulation,” wrote Ola Landgren, MD, PhD, from the Memorial Sloan Kettering Cancer Center and his coauthors.
Seven patients were also assessed for CD20 expression – a marker of poorer prognosis – and 71% were found to be CD20-positive, a prevalence around 3.5-fold higher than that seen in the general population.
The cohort with multiple myeloma was diagnosed on average 12 years younger than those in the general population. The authors commented that this was unlikely to be caused by lead-time bias because the time from first symptoms to clinical manifestation of the disease is usually around 1 year.
“Taken together, our results show that environmental exposure due to the WTC attacks is associated with myeloma precursor disease (MGUS and light-chain MGUS) and may be a risk factor for the development of multiple myeloma at an earlier age, particularly the light-chain subtype,” the authors wrote.
The first study was supported by the National Institute of Occupational Safety and Health; no conflicts of interest were declared.
The second study was supported by the V Foundation for Cancer Research, the Byrne Fund for the benefit of Memorial Sloan-Kettering Cancer Center, the National Cancer Institute, the Albert Einstein Cancer Center, and the National Institute for Occupational Safety and Health; no conflicts of interest were declared.
SOURCE: Zeig-Owens R et al. JAMA Oncology. 2018 April 26. doi: 10.1001/jamaoncol.2018.0504. Landgren O et al. JAMA Oncology. 2018 April 16. doi: 10.1001/jamaoncol.2018.0509.
Rescue and recovery workers who were involved in the aftermath of the World Trade Center disaster may face a greater cancer burden than the general population, according to two studies published in JAMA Oncology.
In particular, they may be at risk of developing multiple myeloma at an earlier age.
The first study was a closed-cohort study of 14,474 employees of the Fire Department of the City of New York (FDNY) who were exposed to the World Trade Center disaster but were cancer-free as of Jan. 1, 2012. The aim was to project cancer incidence from 2012 through 2031, based on data from the FDNY World Trade Center Health Program, and compare those rates with age-, race-, and sex-specific New York cancer rates from the general population.
The modeling projected a “modestly” higher number of cancer cases in the white male subgroup of rescue and recovery workers exposed to the World Trade Center (2,714 vs. 2,596 for the general population of New York; P less than .001). Specifically, the investigators projected significantly higher case counts of prostate cancer (1,437 vs. 863), thyroid cancer (73 vs. 57), and melanoma (201 vs. 131), compared with the general population in New York, but fewer lung (237 vs. 373), colorectal (172 vs. 267), and kidney cancers (66 vs. 132) (P less than .001 for all).
“Our findings suggest that the FDNY WTC-exposed cohort may experience a greater burden of cancer than would be expected from a population with similar demographic characteristics,” wrote Rachel Zeig-Owens, DrPH, from the Montefiore Medical Center and Albert Einstein College of Medicine, both in New York, and coauthors, highlighting prostate cancer as a particular concern.
However, they also acknowledged that the elevated rates observed in people exposed to the World Trade Center disaster could be a result of increased surveillance, even though they did attempt to correct for that, and that firefighters in general might face higher risks.
“It is possible that firefighters have a higher risk of cancer than the general population owing to exposures associated with the occupation,” they wrote. However occupation could also have the opposite effect, as rescue and recovery workers tend to have lower smoking rates, which may explain the relatively low rates of certain cancers such as lung cancer, they said.
A second study examined the effect of the World Trade Center disaster on the risk of multiple myeloma and monoclonal gammopathies in exposed firefighters.
The seroprevalence study of monoclonal gammopathies of undetermined significance (MGUS) in 781 exposed firefighters revealed that the age-standardized prevalence of these was 76% higher in this population than it was in a white male reference population living in Minnesota.
In particular, the age-standardized prevalence of light-chain MGUS was more than threefold higher in exposed firefighters, compared with the reference population.
Researchers also analyzed a case series of 16 exposed white male firefighters who received a diagnosis of multiple myeloma after Sept. 11, 2001. Of the 14 patients for whom data on the monoclonal protein isotype was available, half had light-chain multiple myeloma.
“These findings are of interest due to previously observed associations between light-chain multiple myeloma and light-chain MGUS and exposure to toxins, and chronic immune stimulation,” wrote Ola Landgren, MD, PhD, from the Memorial Sloan Kettering Cancer Center and his coauthors.
Seven patients were also assessed for CD20 expression – a marker of poorer prognosis – and 71% were found to be CD20-positive, a prevalence around 3.5-fold higher than that seen in the general population.
The cohort with multiple myeloma was diagnosed on average 12 years younger than those in the general population. The authors commented that this was unlikely to be caused by lead-time bias because the time from first symptoms to clinical manifestation of the disease is usually around 1 year.
“Taken together, our results show that environmental exposure due to the WTC attacks is associated with myeloma precursor disease (MGUS and light-chain MGUS) and may be a risk factor for the development of multiple myeloma at an earlier age, particularly the light-chain subtype,” the authors wrote.
The first study was supported by the National Institute of Occupational Safety and Health; no conflicts of interest were declared.
The second study was supported by the V Foundation for Cancer Research, the Byrne Fund for the benefit of Memorial Sloan-Kettering Cancer Center, the National Cancer Institute, the Albert Einstein Cancer Center, and the National Institute for Occupational Safety and Health; no conflicts of interest were declared.
SOURCE: Zeig-Owens R et al. JAMA Oncology. 2018 April 26. doi: 10.1001/jamaoncol.2018.0504. Landgren O et al. JAMA Oncology. 2018 April 16. doi: 10.1001/jamaoncol.2018.0509.
FROM JAMA ONCOLOGY
Key clinical point: Monoclonal gammopathies and multiple myeloma may occur more often and earlier in World Trade Center rescue workers.
Major finding: Prevalence of light-chain monoclonal gammopathies is threefold higher in exposed firefighters than in a reference population of white males.
Study details: A cohort study in 14,474 employees of the Fire Department of the City of New York exposed to the Sept. 11, 2001, World Trade Center disaster, a case series of 16 exposed white male firefighters diagnosed with multiple myeloma, and a seroprevalence study of monoclonal gammopathies of undetermined significance in 781 exposed firefighters.
Disclosures: The first study was supported by the National Institute of Occupational Safety and Health; no conflicts of interest were declared. The second study was supported by the V Foundation for Cancer Research, the Byrne Fund for the benefit of Memorial Sloan-Kettering Cancer Center, the National Cancer Institute, the Albert Einstein Cancer Center, and the National Institute for Occupational Safety and Health; no conflicts of interest were declared.
Source: Zeig-Owens R et al. JAMA Oncology 2018, Apr 26. doi: 10.1001/jamaoncol.2018.0504. Landgren O et al. JAMA Oncology 2018, Apr 26. doi: 10.1001/jamaoncol.2018.0509.
Company discontinues phase 3 ADAPT for mRCC
A second interim analysis of
In ADAPT, 462 patients with previously untreated advanced or metastatic renal cell carcinoma (mRCC) were randomized 2:1 between combination treatment with Rocapuldencel-T and sunitinib versus sunitinib monotherapy, after undergoing cytoreductive nephrectomy.
In February 2017, the trial’s Independent Data Monitoring Committee had reviewed the data and concluded that the trial was unlikely to demonstrate a statistically significant improvement in median overall survival in the combination arm and recommended halting the trial. However, the principal investigators and the company, Argos Therapeutics, considered the data too immature to observe the delayed effects associated with immunotherapy and decided to continue the trial. They submitted a protocol amendment to the Food and Drug Administration adding additional co-primary endpoints, and in April of last year, met with the Food and Drug Administration, which accepted the amendment and agreed to continuation of the trial, according to a company press release issued in November.
In the latest interim analysis, which was conducted following an additional 51 deaths, median overall survival for the intent-to-treat patient population was 28.2 months for the combination arm (95% confidence interval, 23.4, 35.2) compared with 31.2 months (95% CI, 23.0, 44.5) for the control arm; this was one of four new co-primary endpoints. The hazard ratio was 1.10 (95% CI, 0.85, 1.42).
Other co-primary endpoints that were evaluated, including overall survival for the patients who remained alive at the time of the February 2017 interim analysis and overall survival for all patients for whom at least 12 months of follow-up was available, did not demonstrate a favorable result, Argos Therapeutics said in a recent press release.
Rocapuldencel-T “consists of autologous dendritic cells programmed with amplified RNA from a patient’s primary tumor” and is “designed to overcome immunosuppression and induce broadly reactive, long-lasting anti-tumor memory T cells” according to the early interim analysis presented at the European Society for Medical Oncology (ESMO) 2017. The drug is also being evaluated in non–small cell lung cancer and bladder cancer.
A second interim analysis of
In ADAPT, 462 patients with previously untreated advanced or metastatic renal cell carcinoma (mRCC) were randomized 2:1 between combination treatment with Rocapuldencel-T and sunitinib versus sunitinib monotherapy, after undergoing cytoreductive nephrectomy.
In February 2017, the trial’s Independent Data Monitoring Committee had reviewed the data and concluded that the trial was unlikely to demonstrate a statistically significant improvement in median overall survival in the combination arm and recommended halting the trial. However, the principal investigators and the company, Argos Therapeutics, considered the data too immature to observe the delayed effects associated with immunotherapy and decided to continue the trial. They submitted a protocol amendment to the Food and Drug Administration adding additional co-primary endpoints, and in April of last year, met with the Food and Drug Administration, which accepted the amendment and agreed to continuation of the trial, according to a company press release issued in November.
In the latest interim analysis, which was conducted following an additional 51 deaths, median overall survival for the intent-to-treat patient population was 28.2 months for the combination arm (95% confidence interval, 23.4, 35.2) compared with 31.2 months (95% CI, 23.0, 44.5) for the control arm; this was one of four new co-primary endpoints. The hazard ratio was 1.10 (95% CI, 0.85, 1.42).
Other co-primary endpoints that were evaluated, including overall survival for the patients who remained alive at the time of the February 2017 interim analysis and overall survival for all patients for whom at least 12 months of follow-up was available, did not demonstrate a favorable result, Argos Therapeutics said in a recent press release.
Rocapuldencel-T “consists of autologous dendritic cells programmed with amplified RNA from a patient’s primary tumor” and is “designed to overcome immunosuppression and induce broadly reactive, long-lasting anti-tumor memory T cells” according to the early interim analysis presented at the European Society for Medical Oncology (ESMO) 2017. The drug is also being evaluated in non–small cell lung cancer and bladder cancer.
A second interim analysis of
In ADAPT, 462 patients with previously untreated advanced or metastatic renal cell carcinoma (mRCC) were randomized 2:1 between combination treatment with Rocapuldencel-T and sunitinib versus sunitinib monotherapy, after undergoing cytoreductive nephrectomy.
In February 2017, the trial’s Independent Data Monitoring Committee had reviewed the data and concluded that the trial was unlikely to demonstrate a statistically significant improvement in median overall survival in the combination arm and recommended halting the trial. However, the principal investigators and the company, Argos Therapeutics, considered the data too immature to observe the delayed effects associated with immunotherapy and decided to continue the trial. They submitted a protocol amendment to the Food and Drug Administration adding additional co-primary endpoints, and in April of last year, met with the Food and Drug Administration, which accepted the amendment and agreed to continuation of the trial, according to a company press release issued in November.
In the latest interim analysis, which was conducted following an additional 51 deaths, median overall survival for the intent-to-treat patient population was 28.2 months for the combination arm (95% confidence interval, 23.4, 35.2) compared with 31.2 months (95% CI, 23.0, 44.5) for the control arm; this was one of four new co-primary endpoints. The hazard ratio was 1.10 (95% CI, 0.85, 1.42).
Other co-primary endpoints that were evaluated, including overall survival for the patients who remained alive at the time of the February 2017 interim analysis and overall survival for all patients for whom at least 12 months of follow-up was available, did not demonstrate a favorable result, Argos Therapeutics said in a recent press release.
Rocapuldencel-T “consists of autologous dendritic cells programmed with amplified RNA from a patient’s primary tumor” and is “designed to overcome immunosuppression and induce broadly reactive, long-lasting anti-tumor memory T cells” according to the early interim analysis presented at the European Society for Medical Oncology (ESMO) 2017. The drug is also being evaluated in non–small cell lung cancer and bladder cancer.
New regimen looks good in stage IV favorable histology Wilms
Tailoring chemoradiation therapy while minimizing anthracycline exposure produced strong survival results in stage IV favorable histology Wilms tumor (FHWT), according to new results from the Children’s Oncology Group AREN0533 study.
In the new regimen, patients whose isolated lung nodules completely respond to 6 weeks of vincristine/dactinomycin/doxorubicin (DD4A) therapy continue DD4A and forgo lung radiation therapy (RT), explained David B. Dix, MBChB, of British Columbia Children’s Hospital, Vancouver, B.C., and his associates. Incomplete responders and patients with loss of heterozygosity at chromosomes 1p/16q receive lung RT plus boosted chemotherapy consisting of DD4 plus four cycles of cyclophosphamide/etoposide (Regimen M).
Among 133 assessable complete responders who received the DD4A regimen and were followed for a median of 4.7 years, 4-year event-free survival (EFS) was 79.5% (95% confidence interval, 71%-88%) and 4-year overall survival (OS) was 96% (95% CI, 92%-100%), Dr. Dix and his associates wrote. The report was published in the Journal of Clinical Oncology.
Among 159 incomplete responders receiving Regimen M, 4-year EFS was 88.5% (95% CI, 82%-95%) and 4-year OS was 95% (95% CI, 91%-100%). Regimen M produced superior EFS and OS (P less than .001 for both comparisons) than the protocol used in the National Wilms Tumor Study (NWTS) 5 study, in which all patients with lung metastases received DD4A plus RT, regardless of lung nodule response. “These results provide a benchmark for future studies,” Dr. Dix and his associates concluded.
Most patients with FHWT have pulmonary metastases and historically have fared worse than peers with localized disease. Until now, patients have had two main treatment options. The Society of Pediatric Oncology (SIOP) protocol focuses on pre-nephrectomy DD4A and forgoes lung RT if chemotherapy or surgical resection achieves lung nodule CR. Patients in the most recently reported SIOP trial (93-01) received a high cumulative anthracycline dose of 350 mg/m2 and had 5-year EFS of 77% and 5-year OS of 87%. The second option – the NWTS protocol – more than halves the cumulative doxorubicin dose (150 mg/m2), but all patients undergo lung RT.
In contrast, the AREN0533 protocol involved cumulative doxorubicin doses of 150 mg/m2 for DD4A and 195 mg/m2 for Regimen M. Among complete responders, the expected event rate was 15% and the actual rate was 20% (P = .05). Among incomplete responders, observed and expected event rates were 25% and. 12%, respectively (P less than .001). The higher-than-expected event rates might stem from lower chemotherapy doses, but the SIOP study also did not include central image review and may have defined CR less stringently, Dr. Dix and his coinvestigators said.
They concluded that AREN0533 showed “excellent” survival results for patients with CR and that certain late risks of Regimen M – including an increased risk of leukemia from exposure to cyclophosphamide and etoposide – should be balanced against its superior 4-year EFS.
Funders included the National Cancer Institute, National Institutes of Health, and St. Baldrick’s Foundation. Senior author Jeffrey S. Dome, MD, PhD, disclosed intellectual property with Rockland Immunochemicals. Three coinvestigators disclosed ties to healthcare and pharmaceutical companies.
SOURCE: Dix DB et al. J Clin Oncol. 2018 Apr 11. doi: 10.1200/JCO. 2017.77.1931.
Tailoring chemoradiation therapy while minimizing anthracycline exposure produced strong survival results in stage IV favorable histology Wilms tumor (FHWT), according to new results from the Children’s Oncology Group AREN0533 study.
In the new regimen, patients whose isolated lung nodules completely respond to 6 weeks of vincristine/dactinomycin/doxorubicin (DD4A) therapy continue DD4A and forgo lung radiation therapy (RT), explained David B. Dix, MBChB, of British Columbia Children’s Hospital, Vancouver, B.C., and his associates. Incomplete responders and patients with loss of heterozygosity at chromosomes 1p/16q receive lung RT plus boosted chemotherapy consisting of DD4 plus four cycles of cyclophosphamide/etoposide (Regimen M).
Among 133 assessable complete responders who received the DD4A regimen and were followed for a median of 4.7 years, 4-year event-free survival (EFS) was 79.5% (95% confidence interval, 71%-88%) and 4-year overall survival (OS) was 96% (95% CI, 92%-100%), Dr. Dix and his associates wrote. The report was published in the Journal of Clinical Oncology.
Among 159 incomplete responders receiving Regimen M, 4-year EFS was 88.5% (95% CI, 82%-95%) and 4-year OS was 95% (95% CI, 91%-100%). Regimen M produced superior EFS and OS (P less than .001 for both comparisons) than the protocol used in the National Wilms Tumor Study (NWTS) 5 study, in which all patients with lung metastases received DD4A plus RT, regardless of lung nodule response. “These results provide a benchmark for future studies,” Dr. Dix and his associates concluded.
Most patients with FHWT have pulmonary metastases and historically have fared worse than peers with localized disease. Until now, patients have had two main treatment options. The Society of Pediatric Oncology (SIOP) protocol focuses on pre-nephrectomy DD4A and forgoes lung RT if chemotherapy or surgical resection achieves lung nodule CR. Patients in the most recently reported SIOP trial (93-01) received a high cumulative anthracycline dose of 350 mg/m2 and had 5-year EFS of 77% and 5-year OS of 87%. The second option – the NWTS protocol – more than halves the cumulative doxorubicin dose (150 mg/m2), but all patients undergo lung RT.
In contrast, the AREN0533 protocol involved cumulative doxorubicin doses of 150 mg/m2 for DD4A and 195 mg/m2 for Regimen M. Among complete responders, the expected event rate was 15% and the actual rate was 20% (P = .05). Among incomplete responders, observed and expected event rates were 25% and. 12%, respectively (P less than .001). The higher-than-expected event rates might stem from lower chemotherapy doses, but the SIOP study also did not include central image review and may have defined CR less stringently, Dr. Dix and his coinvestigators said.
They concluded that AREN0533 showed “excellent” survival results for patients with CR and that certain late risks of Regimen M – including an increased risk of leukemia from exposure to cyclophosphamide and etoposide – should be balanced against its superior 4-year EFS.
Funders included the National Cancer Institute, National Institutes of Health, and St. Baldrick’s Foundation. Senior author Jeffrey S. Dome, MD, PhD, disclosed intellectual property with Rockland Immunochemicals. Three coinvestigators disclosed ties to healthcare and pharmaceutical companies.
SOURCE: Dix DB et al. J Clin Oncol. 2018 Apr 11. doi: 10.1200/JCO. 2017.77.1931.
Tailoring chemoradiation therapy while minimizing anthracycline exposure produced strong survival results in stage IV favorable histology Wilms tumor (FHWT), according to new results from the Children’s Oncology Group AREN0533 study.
In the new regimen, patients whose isolated lung nodules completely respond to 6 weeks of vincristine/dactinomycin/doxorubicin (DD4A) therapy continue DD4A and forgo lung radiation therapy (RT), explained David B. Dix, MBChB, of British Columbia Children’s Hospital, Vancouver, B.C., and his associates. Incomplete responders and patients with loss of heterozygosity at chromosomes 1p/16q receive lung RT plus boosted chemotherapy consisting of DD4 plus four cycles of cyclophosphamide/etoposide (Regimen M).
Among 133 assessable complete responders who received the DD4A regimen and were followed for a median of 4.7 years, 4-year event-free survival (EFS) was 79.5% (95% confidence interval, 71%-88%) and 4-year overall survival (OS) was 96% (95% CI, 92%-100%), Dr. Dix and his associates wrote. The report was published in the Journal of Clinical Oncology.
Among 159 incomplete responders receiving Regimen M, 4-year EFS was 88.5% (95% CI, 82%-95%) and 4-year OS was 95% (95% CI, 91%-100%). Regimen M produced superior EFS and OS (P less than .001 for both comparisons) than the protocol used in the National Wilms Tumor Study (NWTS) 5 study, in which all patients with lung metastases received DD4A plus RT, regardless of lung nodule response. “These results provide a benchmark for future studies,” Dr. Dix and his associates concluded.
Most patients with FHWT have pulmonary metastases and historically have fared worse than peers with localized disease. Until now, patients have had two main treatment options. The Society of Pediatric Oncology (SIOP) protocol focuses on pre-nephrectomy DD4A and forgoes lung RT if chemotherapy or surgical resection achieves lung nodule CR. Patients in the most recently reported SIOP trial (93-01) received a high cumulative anthracycline dose of 350 mg/m2 and had 5-year EFS of 77% and 5-year OS of 87%. The second option – the NWTS protocol – more than halves the cumulative doxorubicin dose (150 mg/m2), but all patients undergo lung RT.
In contrast, the AREN0533 protocol involved cumulative doxorubicin doses of 150 mg/m2 for DD4A and 195 mg/m2 for Regimen M. Among complete responders, the expected event rate was 15% and the actual rate was 20% (P = .05). Among incomplete responders, observed and expected event rates were 25% and. 12%, respectively (P less than .001). The higher-than-expected event rates might stem from lower chemotherapy doses, but the SIOP study also did not include central image review and may have defined CR less stringently, Dr. Dix and his coinvestigators said.
They concluded that AREN0533 showed “excellent” survival results for patients with CR and that certain late risks of Regimen M – including an increased risk of leukemia from exposure to cyclophosphamide and etoposide – should be balanced against its superior 4-year EFS.
Funders included the National Cancer Institute, National Institutes of Health, and St. Baldrick’s Foundation. Senior author Jeffrey S. Dome, MD, PhD, disclosed intellectual property with Rockland Immunochemicals. Three coinvestigators disclosed ties to healthcare and pharmaceutical companies.
SOURCE: Dix DB et al. J Clin Oncol. 2018 Apr 11. doi: 10.1200/JCO. 2017.77.1931.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Tailored chemoradiation therapy that reduces anthracycline exposure produced good outcomes in stage IV favorable histology Wilms tumor.
Major finding: Four-year event-free survival rates were 79.5% among complete responders and 89% among incomplete responders.
Study details: Study of 292 patients from AREN0533 (Treatment of Newly Diagnosed Higher-Risk Favorable Histology Wilms Tumors).
Disclosures: Funders included the National Cancer Institute, National Institutes of Health, and St. Baldrick’s Foundation. Senior author Jeffrey S. Dome, MD, PhD, disclosed intellectual property with Rockland Immunochemicals. Three coinvestigators disclosed ties to health care and pharmaceutical companies.
Source: Dix DB et al. J Clin Oncol. 2018 Apr 16. doi: 10.1200/JCO.2017.77.1931.
FDA approves immunotherapy combo for advanced RCC
The Food and Drug Administration has granted approvals to
The approvals were based on statistically significant improvements in overall survival (OS) and objective response rate (ORR) for patients receiving the combination of nivolumab and ipilimumab (n = 425), compared with those receiving sunitinib (n = 422) in CheckMate 214, the FDA said in a press statement.
Median OS was not yet reached in the combination arm at follow-up of 32 months, compared with 25.9 months in the sunitinib arm (hazard ratio, 0.63; 95% confidence interval, 0.44-0.89; P less than .0001). The ORR was 41.6% (95% CI, 36.9-46.5) for the combination versus 26.5% (95% CI, 22.4-31) in the sunitinib arm (P less than .0001).
Efficacy of the combination was not established for patients with favorable-risk disease.
The most common adverse reactions were fatigue, rash, diarrhea, musculoskeletal pain, pruritus, nausea, cough, pyrexia, arthralgia, and decreased appetite.
The recommended schedule and dose is 3 mg/kg nivolumab, followed by 1 mg/kg ipilimumab, on the same day every 3 weeks for four doses, then 240 mg nivolumab every 2 weeks or 480 mg every 4 weeks, the FDA said.
Nivolumab is marketed as Opdivo and ipilimumab as Yervoy by Bristol-Myers Squibb.
The Food and Drug Administration has granted approvals to
The approvals were based on statistically significant improvements in overall survival (OS) and objective response rate (ORR) for patients receiving the combination of nivolumab and ipilimumab (n = 425), compared with those receiving sunitinib (n = 422) in CheckMate 214, the FDA said in a press statement.
Median OS was not yet reached in the combination arm at follow-up of 32 months, compared with 25.9 months in the sunitinib arm (hazard ratio, 0.63; 95% confidence interval, 0.44-0.89; P less than .0001). The ORR was 41.6% (95% CI, 36.9-46.5) for the combination versus 26.5% (95% CI, 22.4-31) in the sunitinib arm (P less than .0001).
Efficacy of the combination was not established for patients with favorable-risk disease.
The most common adverse reactions were fatigue, rash, diarrhea, musculoskeletal pain, pruritus, nausea, cough, pyrexia, arthralgia, and decreased appetite.
The recommended schedule and dose is 3 mg/kg nivolumab, followed by 1 mg/kg ipilimumab, on the same day every 3 weeks for four doses, then 240 mg nivolumab every 2 weeks or 480 mg every 4 weeks, the FDA said.
Nivolumab is marketed as Opdivo and ipilimumab as Yervoy by Bristol-Myers Squibb.
The Food and Drug Administration has granted approvals to
The approvals were based on statistically significant improvements in overall survival (OS) and objective response rate (ORR) for patients receiving the combination of nivolumab and ipilimumab (n = 425), compared with those receiving sunitinib (n = 422) in CheckMate 214, the FDA said in a press statement.
Median OS was not yet reached in the combination arm at follow-up of 32 months, compared with 25.9 months in the sunitinib arm (hazard ratio, 0.63; 95% confidence interval, 0.44-0.89; P less than .0001). The ORR was 41.6% (95% CI, 36.9-46.5) for the combination versus 26.5% (95% CI, 22.4-31) in the sunitinib arm (P less than .0001).
Efficacy of the combination was not established for patients with favorable-risk disease.
The most common adverse reactions were fatigue, rash, diarrhea, musculoskeletal pain, pruritus, nausea, cough, pyrexia, arthralgia, and decreased appetite.
The recommended schedule and dose is 3 mg/kg nivolumab, followed by 1 mg/kg ipilimumab, on the same day every 3 weeks for four doses, then 240 mg nivolumab every 2 weeks or 480 mg every 4 weeks, the FDA said.
Nivolumab is marketed as Opdivo and ipilimumab as Yervoy by Bristol-Myers Squibb.
Diabetes from checkpoint inhibitors probably means lifelong insulin
CHICAGO – , according to Priyanka Iyer, MD, an endocrinology fellow at MD Anderson Cancer Center, Houston.
“As long as we get glycemic control, they can continue,” she said at the annual meeting of the Endocrine Society.
Diabetes is a known side effect of immune checkpoint inhibitors (ICIs) but it’s rare, occurring in maybe 0.17% of patients, and its natural history and risk factors are unknown.
ICIs are fairly new agents, and as their use expands beyond clinical trials, “we anticipate seeing larger numbers of cases. Patients should be educated about the symptoms of uncontrolled blood sugars while on ICIs,” and endocrinologists “have to get involved and recognize this entity sooner,” Dr. Iyer said.
In short, her team found that ICI-mediated diabetes can occur in patients with or without preexisting diabetes, and that most patients have evidence of beta-cell failure, likely T-cell mediated destruction due to immune activation. In all but one case, patients remained on insulin at a median follow-up of 44 weeks, even after stopping ICIs. For most, ICI-mediated diabetes likely means lifelong insulin.
They were all on the programmed cell death protein (PD-1) inhibitors nivolumab (Opdivo) or pembrolizumab (Keytruda), or the PD-1 ligand (PD-L) inhibitor durvalumab (Imfinzi). The agents are used for a range of cancers, including renal cell, melanoma, and Hodgkin lymphoma. There were no diabetes cases in patients on single-agent ipilimumab (Yervoy) or tremelimumab, which target cytotoxic T-lymphocyte associated antigen-4 and are used for melanoma and mesothelioma.
Median time to diabetes presentation after the start of ICI treatment was 12.3 weeks but ranged from 1 to 67.2 weeks. Half of the cases presented in diabetic ketoacidosis (DKA). Patients had upward trending hyperglycemia and most had diabetes symptoms for a while before diagnosis. They presented with a blood glucose above 250 mg/dL, and more than half above 500 mg/dL. Median hemoglobin A1c at presentation was 8%, but ranged up to 12.5%.
Every patient required insulin, including the six that discontinued ICIs after developing diabetes. Diabetes resolved in just one patient at 10.2 months; she presented with DKA.
There were no obvious predisposing factors. None of the patients had histories of type 1 diabetes or other autoimmune disease. Five patients had well-controlled type 2 diabetes prior to ICI initiation; four had prediabetes. Some had family members with type 2 diabetes, but not type 1. Four had prior ICI exposure. Just three patients were on concomitant steroids.
A few patients also developed thyroid or pituitary dysfunction, which are more common side effects of ICIs.
The median age at diabetes presentation was 61 years and ranged from 32 to 82 years. The majority of patients were men, which reflects MD Anderson demographics, not a predisposing risk factor, Dr. Iyer said.
Melanoma was the most common cancer, followed by renal cell and prostate; patients had stage 2-4 disease. About half the subjects were on single agent anti-PD-1 treatment, about a third on anti-PD-1 combination treatment, and the rest on anti-PD-L1 combination therapy. C-peptide levels were below 0.9 ng/mL at diabetes diagnosis in most of the patients. Eleven of the 20 tested (55%) were positive for the pancreatic islet cell antibody GAD65.
The investigators had no disclosures. A funding source was not reported.
SOURCE: Iyer PC et al. Abstract OR05-5.
CHICAGO – , according to Priyanka Iyer, MD, an endocrinology fellow at MD Anderson Cancer Center, Houston.
“As long as we get glycemic control, they can continue,” she said at the annual meeting of the Endocrine Society.
Diabetes is a known side effect of immune checkpoint inhibitors (ICIs) but it’s rare, occurring in maybe 0.17% of patients, and its natural history and risk factors are unknown.
ICIs are fairly new agents, and as their use expands beyond clinical trials, “we anticipate seeing larger numbers of cases. Patients should be educated about the symptoms of uncontrolled blood sugars while on ICIs,” and endocrinologists “have to get involved and recognize this entity sooner,” Dr. Iyer said.
In short, her team found that ICI-mediated diabetes can occur in patients with or without preexisting diabetes, and that most patients have evidence of beta-cell failure, likely T-cell mediated destruction due to immune activation. In all but one case, patients remained on insulin at a median follow-up of 44 weeks, even after stopping ICIs. For most, ICI-mediated diabetes likely means lifelong insulin.
They were all on the programmed cell death protein (PD-1) inhibitors nivolumab (Opdivo) or pembrolizumab (Keytruda), or the PD-1 ligand (PD-L) inhibitor durvalumab (Imfinzi). The agents are used for a range of cancers, including renal cell, melanoma, and Hodgkin lymphoma. There were no diabetes cases in patients on single-agent ipilimumab (Yervoy) or tremelimumab, which target cytotoxic T-lymphocyte associated antigen-4 and are used for melanoma and mesothelioma.
Median time to diabetes presentation after the start of ICI treatment was 12.3 weeks but ranged from 1 to 67.2 weeks. Half of the cases presented in diabetic ketoacidosis (DKA). Patients had upward trending hyperglycemia and most had diabetes symptoms for a while before diagnosis. They presented with a blood glucose above 250 mg/dL, and more than half above 500 mg/dL. Median hemoglobin A1c at presentation was 8%, but ranged up to 12.5%.
Every patient required insulin, including the six that discontinued ICIs after developing diabetes. Diabetes resolved in just one patient at 10.2 months; she presented with DKA.
There were no obvious predisposing factors. None of the patients had histories of type 1 diabetes or other autoimmune disease. Five patients had well-controlled type 2 diabetes prior to ICI initiation; four had prediabetes. Some had family members with type 2 diabetes, but not type 1. Four had prior ICI exposure. Just three patients were on concomitant steroids.
A few patients also developed thyroid or pituitary dysfunction, which are more common side effects of ICIs.
The median age at diabetes presentation was 61 years and ranged from 32 to 82 years. The majority of patients were men, which reflects MD Anderson demographics, not a predisposing risk factor, Dr. Iyer said.
Melanoma was the most common cancer, followed by renal cell and prostate; patients had stage 2-4 disease. About half the subjects were on single agent anti-PD-1 treatment, about a third on anti-PD-1 combination treatment, and the rest on anti-PD-L1 combination therapy. C-peptide levels were below 0.9 ng/mL at diabetes diagnosis in most of the patients. Eleven of the 20 tested (55%) were positive for the pancreatic islet cell antibody GAD65.
The investigators had no disclosures. A funding source was not reported.
SOURCE: Iyer PC et al. Abstract OR05-5.
CHICAGO – , according to Priyanka Iyer, MD, an endocrinology fellow at MD Anderson Cancer Center, Houston.
“As long as we get glycemic control, they can continue,” she said at the annual meeting of the Endocrine Society.
Diabetes is a known side effect of immune checkpoint inhibitors (ICIs) but it’s rare, occurring in maybe 0.17% of patients, and its natural history and risk factors are unknown.
ICIs are fairly new agents, and as their use expands beyond clinical trials, “we anticipate seeing larger numbers of cases. Patients should be educated about the symptoms of uncontrolled blood sugars while on ICIs,” and endocrinologists “have to get involved and recognize this entity sooner,” Dr. Iyer said.
In short, her team found that ICI-mediated diabetes can occur in patients with or without preexisting diabetes, and that most patients have evidence of beta-cell failure, likely T-cell mediated destruction due to immune activation. In all but one case, patients remained on insulin at a median follow-up of 44 weeks, even after stopping ICIs. For most, ICI-mediated diabetes likely means lifelong insulin.
They were all on the programmed cell death protein (PD-1) inhibitors nivolumab (Opdivo) or pembrolizumab (Keytruda), or the PD-1 ligand (PD-L) inhibitor durvalumab (Imfinzi). The agents are used for a range of cancers, including renal cell, melanoma, and Hodgkin lymphoma. There were no diabetes cases in patients on single-agent ipilimumab (Yervoy) or tremelimumab, which target cytotoxic T-lymphocyte associated antigen-4 and are used for melanoma and mesothelioma.
Median time to diabetes presentation after the start of ICI treatment was 12.3 weeks but ranged from 1 to 67.2 weeks. Half of the cases presented in diabetic ketoacidosis (DKA). Patients had upward trending hyperglycemia and most had diabetes symptoms for a while before diagnosis. They presented with a blood glucose above 250 mg/dL, and more than half above 500 mg/dL. Median hemoglobin A1c at presentation was 8%, but ranged up to 12.5%.
Every patient required insulin, including the six that discontinued ICIs after developing diabetes. Diabetes resolved in just one patient at 10.2 months; she presented with DKA.
There were no obvious predisposing factors. None of the patients had histories of type 1 diabetes or other autoimmune disease. Five patients had well-controlled type 2 diabetes prior to ICI initiation; four had prediabetes. Some had family members with type 2 diabetes, but not type 1. Four had prior ICI exposure. Just three patients were on concomitant steroids.
A few patients also developed thyroid or pituitary dysfunction, which are more common side effects of ICIs.
The median age at diabetes presentation was 61 years and ranged from 32 to 82 years. The majority of patients were men, which reflects MD Anderson demographics, not a predisposing risk factor, Dr. Iyer said.
Melanoma was the most common cancer, followed by renal cell and prostate; patients had stage 2-4 disease. About half the subjects were on single agent anti-PD-1 treatment, about a third on anti-PD-1 combination treatment, and the rest on anti-PD-L1 combination therapy. C-peptide levels were below 0.9 ng/mL at diabetes diagnosis in most of the patients. Eleven of the 20 tested (55%) were positive for the pancreatic islet cell antibody GAD65.
The investigators had no disclosures. A funding source was not reported.
SOURCE: Iyer PC et al. Abstract OR05-5.
REPORTING FROM ENDO 2018
Key clinical point: Be on the lookout for new-onset diabetes when patients start immune checkpoint inhibitors.
Major finding: In all but one case, patients remained on insulin at a median follow-up of 44 weeks, even after stopping ICIs.
Study details: Review of 24 cases.
Disclosures: The investigators had no disclosures. A funding source was not reported.
Source: Iyer PC et al. Abstract OR05-5.
Analgesic management in radiation oncology for painful bone metastases
Bone metastases are a common cause of pain in patients with advanced cancer, with about three-quarters of patients with bone metastases experiencing pain as the dominant symptom.1 Inadequately treated cancer pain impairs patient quality of life, and is associated with higher rates of depression, anxiety, and fatigue. Palliative radiotherapy (RT) is effective in alleviating pain from bone metastases.4 Local field external beam radiotherapy can provide some pain relief at the site of treated metastasis in 80%-90% of cases, with complete pain relief in 50%-60% of cases.5,6 However, maximal pain relief from RT is delayed, in some cases taking days to up to multiple weeks to attain.7,8 Therefore, optimal management of bone metastases pain may require the use of analgesics until RT takes adequate effect.
National Comprehensive Cancer Network (NCCN) Guidelines for Adult Cancer Pain (v. 2.2015) recommend that pain intensity rating (PIR; range, 0-10, where 0 denotes no pain and 10, worst pain imaginable) be used to quantify pain for all symptomatic patients. These guidelines also recommend the pain medication regimen be assessed for all symptomatic patients. For patients with moderate or severe pain (PIR of ≥4), NCCN guidelines recommend that analgesic regimen be intervened upon by alteration of the analgesic regimen (initiating, rotating, or titrating analgesic) or consideration of referral to pain/symptom management specialty.
Previous findings have demonstrated inadequate analgesic management for cancer pain,2,9 including within the radiation oncology (RO) clinic, suggesting that patients seen in consultation for palliative RT may experience uncontrolled pain for days to weeks before the onset of relief from RT. Possible reasons for inadequate acute pain intervention in the RO clinic may be provider discomfort with analgesic management and infrequent formal integration of palliative care within RO.10
Limited single-institution data from the few institutions with dedicated palliative RO services have suggested that these services improve the quality of palliative care delivery, as demonstrated by providers perceptions’ of the clinical impact of a dedicated service11 and the implementation of expedited palliative RT delivery for acute cancer pain.12,13 To our knowledge, the impact of a dedicated palliative RO service on analgesic management for cancer pain has not been assessed.
Here, we report how often patients with symptomatic bone metastases had assessments of existing analgesic regimens and interventions at RO consultation at 2 cancer centers. Center 1 had implemented a dedicated palliative RO service in 2011, consisting of rotating attending physicians and residents as well as dedicated palliative care trained nurse practitioners and a fellow, with the service structured around daily rounds,11 whereas Center 2 had not yet implemented a dedicated service. Using data from both centers, we assessed the impact of a palliative RO service on analgesic assessment and management in patients with bone metastases.
Methods
We searched our institutional databases for patients seen in RO consultation for bone metastases using ICD-9 code 198.5, and retrospectively reviewed consultation notes for those patients during June-July 2008, January-February 2010, January-February 2013, and June-July 2014. Those time periods were chosen as evenly spaced representative samples before and after implementation of a dedicated palliative RO service in 2011 at Center 1. Center 2 did not implement a dedicated palliative RO service in these time periods.
Within consultation notes, we recorded the following data from the History of the Present Illness section: symptoms from bone metastases (symptomatic was defined as any pain present); PIR (range, 0-10); and whether or not the preconsultation analgesic regimen was reported for symptomatic patients (including analgesic type, dosing, effectiveness, and adherence).
Documentation of the analgesic regimen in the history section of the notes was considered the proxy for analgesic regimen assessment at time of RO consultation. Analgesics within the Medications list, which were autopopulated in the consultation note by the electronic medical record, were recorded.
Whether or not pain was addressed with initiation or titration of analgesics for patients with a PIR of ≥4 was recorded from the Assessment and Plan portion of the notes, and that metric was considered the proxy for pain intervention. In addition, the case was coded as having had pain intervention if there was documentation of the patient declining recommended analgesic intervention, or the patient had been referred to a symptom management service for intervention (eg, referral to a specialty palliative care clinic), or there was recommendation for the patient to discuss uncontrolled pain with the original prescriber. A PIR of 4 was chosen as the threshold for analgesic intervention because at that level, NCCN guidelines for cancer pain state that the analgesic regimen should be titrated, whereas for a PIR of 3 or less, the guidelines recommend only consideration of titrating the analgesic. Only patients with a documented PIR were included in the pain intervention analysis.
Frequencies of analgesic assessment and analgesic intervention were compared using t tests (Wizard Pro, v1.8.5; Evan Miller, Chicago IL).
Results
A total of 271 patients with RO consultation notes were identified at the 2 centers within the 4 time periods (Table 1).
Among symptomatic patients, any component of the preconsultation analgesic regimen (including analgesic type, dosing, pain response, and adherence) was documented for 37.9% of the entire cohort at RO consultation (Table 3). At Centers 1 and 2, the frequencies of analgesic regimen assessment were documented for 41.3% and 28.1%, respectively (P = .06). Among symptomatic patients, 81.5% had an opioid or nonopioid analgesic listed in the Medications section in the electronic medical record at time of consultation.
Patients seen on the dedicated palliative RO service at Center 1 had an analgesic assessment documentation rate of 59.5%, whereas the patients not seen on a palliative RO service (ie, patients seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had an assessment documentation rate of 33.5% (P = .002; Figure 1). There was no significant difference between rates of analgesic regimen assessment between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (28.1% vs 35.9%, respectively; P = .27).
In patients seen at Center 1 only, those seen on the palliative RO service had a higher documentation rate of analgesic assessment compared with those seen by other services after implementation of the dedicated service (59.5% vs 38%, respectively; P = .018). Time period (after versus before 2011) was not significantly associated with the rate of documentation of analgesic assessment at either Center 1 (after vs before 2011: 44.4% vs 31%, P = .23) or Center 2 (31.4% vs 24.1%, P = .60).
Among patients with a PIR of ≥4, analgesic intervention was reported for 17.2% of patients within the entire cohort (20.8% at Center 1 and 0% at Center 2, P = .05). Among those with a PIR of ≥4, documentation of analgesic assessment noted in the History of the Present Illness section was associated with increased documentation of an analgesic intervention in the Assessment and Plan section (25% vs 7.3%; odds ratio [OR], 4.22; 95% confidence interval [CI], 1.1-16.0; P = .03).
Patients seen on the dedicated palliative RO service at Center 1 had a documented analgesic intervention rate of 31.6%, whereas the patients not seen on a palliative RO service (ie, those seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had a documented analgesic intervention rate of 9.2% (P = .01; Figure 2). There was no statistically significant difference between rates of documentation of an analgesic regimen intervention between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (0% vs 17.2%, respectively; P = .07).
Looking at only patients seen at Center 1, patients with a PIR of ≥4 seen on the dedicated palliative RO service had a nearly significant higher rate of documented analgesic interventions in the time period after implementation of the dedicate service (31.6% if seen on the dedicated service vs 12% if seen on a nondedicated service, P = .06).
Discussion
Multiple studies demonstrate the undertreatment of cancer pain in the outpatient setting.2,9,14,15 At 2 cancer centers, we found that about half of patients who present for consideration of palliative RT for bone metastases had a PIR of ≥4, yet only 17% of them had documentation of analgesic intervention as recommended by NCCN guidelines for cancer pain. Underlying this low rate of appropriate intervention may be the assumption of rapid pain relief by RT. However, RT often does not begin at time of consultation,16 and maximal pain relief may take days to weeks after commencement of RT.17 It is estimated that a quarter of all patients with cancer develop bone metastases during the course of their disease,12 and most of those patients suffer from pain. Thus, inherent delay in pain relief before, during, and after RT results in significant morbidity for the cancer patient population if adequate analgesic management is not provided.
The low rate of appropriate analgesic intervention at the time of RO consultation may also be related to the low incidence of proper analgesic assessment. In our cohort, 80% of symptomatic patients had an opioid or nonopioid analgesic listed in their medications within the electronic medical record at time of consultation, but only 38% had the analgesic regimen and/or its effectiveness described in the History of the Present Illness section of the record. Inattentiveness to analgesic type, dosing, and effectiveness during consultation may result in any inadequacies of the analgesic regimen going unnoticed. Consistent with this notion, we found that the rate of appropriate intervention for patients with a PIR of ≥4 was higher among patients who had analgesic regimen reported in the consultation note. Thus, interventions to implement routine review and documentation of the analgesic regimen, for example within the electronic medical record, may be one way to improve pain management.
Another possible reason for low rates of acute pain management within the RO clinic is low provider confidence in regard to analgesic management. In a recent national survey, 96% of radiation oncologists stated they were at least moderately confident with assessment of pain, yet only 77% were at least moderately confident with titrating opioids, and just 56% were at least moderately confident with rotating opioids.10 Educational interventions that improve providers’ facility with analgesic management may increase the frequency of pain management in the RO clinic.
Patients seen on the dedicated palliative RO service had significantly higher rates of documented analgesic regimen assessment and appropriate intervention during RO consultation, compared with patients seen at Center 2 and those not seen on the dedicated palliative RO service at Center 1. The improvements we observed in analgesic assessment and intervention at Center 1 for patients seen on the palliative RO service are likely owing to involvement of palliative RO and not to secular trends, because there were not similar improvements for patients at Center 1 who were not seen by the palliative RO service and those at Center 2, where there was no service.
At Center 1, the dedicated palliative RO service was created to provide specialized care to patients with metastatic disease undergoing palliative radiation. Within its structure, topics within palliative RO, such as technical aspects of palliative RT, symptom management, and communication are taught and reinforced in a case-based approach. Such palliative care awareness, integration, and education within RO achieved by the palliative RO service likely contribute to the improved rates of analgesic management we found in our study. We do note that rate of analgesic intervention in the palliative RO cohort, though higher than in the nonpalliative RO group, was still low, with only a third of patients receiving proper analgesic management. These findings highlight the importance of continued effort in increasing providers’ awareness of the need to assess pain and raise comfort with analgesic initiation and titration and of having dedicated palliative care clinicians embedded within the RO setting.
Since the data for this study was acquired, Center 2 has implemented a short palliative RO didactic course for residents, which improved their comfort levels in assessing analgesic effectiveness and intervening for uncontrolled pain.18 The impact of this intervention on clinical care will need to be evaluated, but the improved provider comfort levels may translate into better-quality care.
Limitations
An important limitation of this retrospective study is the reliance on the documentation provided in the consultation note for determining frequencies of analgesic regimen assessment and intervention. The actual rates of analgesic management that occurred in clinic may have been higher than reported in the documentation. However, such discrepancy in documentation of analgesic management would also be an area for quality improvement. Inadequate documentation limits the ability for proper follow-up of cancer pain as recommended by a joint guidance statement from the American Society of Clinical Oncology and the American Academy of Hospice and Palliative Medicine.19,20 The results of our study may also partly reflect a positive impact in documentation of analgesic management by a dedicated palliative RO service.
Given the multi-institutional nature of this study, it may be that general practice differences confound the impact of the dedicated palliative RO service at Center 1. However, with excluding Center 2, the dedicated service was still strongly associated with a higher rate of analgesic assessment within Center 1 and was almost significantly associated with appropriate analgesic intervention within Center 1.
We used a PIR of ≥4 as a threshold for appropriate analgesic regimen intervention because it is what is recommended by the NCCN guidelines. However, close attention should be paid to the impact that any amount of pain has on an individual patient. The functional, spiritual, and existential impact of pain is unique to each patient’s experience, and optimal symptom management should take those elements into account.
Conclusion
In conclusion, this study indicates that advanced cancer patient pain assessment and intervention according to NCCN cancer pain management guidelines is not common in the RO setting, and it is an area that should be targeted for quality improvement because of the positive implications for patient well-being. Pain assessment and intervention were greater in the setting of a dedicated structure for palliative care within RO, suggesting that the integration of palliative care within RO is a promising means of improving quality of pain management.
This work was presented at the 2016 ASCO Palliative Care in Oncology Symposium (September 9-10, 2016), where this work received a Conquer Cancer Foundation Merit Award.
1. Amichetti M, Orrù P, Madeddu A, et al. Comparative evaluation of two hypofractionated radiotherapy regimens for painful bone metastases. Tumori. 2004;90(1):91-95.
2. Vuong S, Pulenzas N, DeAngelis C, et al. Inadequate pain management in cancer patients attending an outpatient palliative radiotherapy clinic. Support Care Cancer. 2016;24(2):887-892.
3. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1-2):129-134.
4. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Sze WM, ed. Cochrane Database Syst Rev. 2004;(2):CD004721-CD004721.
5. Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44(1):1-18.
6. Kirou-Mauro A, Hird A, Wong J, et al. Is response to radiotherapy in patients related to the severity of pretreatment pain? Int J Radiat Oncol Biol Phys. 2008;71(4):1208-1212.
7. Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S158-S164.
8. McDonald R, Ding K, Brundage M, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol. 2017 Jul 1;3(7):953-959.
9. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149-4154.
10. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the united states: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
11. Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol. 2014;4(4):247-253.
12. Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163-170.
13. de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer. 2009;17(7):757-762.
14. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-1991.
15. Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39(2):259-267.
16. Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14(1):38-43.
17. Feyer PC, Steingraeber M. Radiotherapy of bone metastasis in breast cancer patients – current approaches. Breast Care (Basel). 2012;7(2):108-112.
18. Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys. 2017;97(5):884-885.
19. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
20. Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: an American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J Oncol Pract. 2016;12(9):e828-e838.
Bone metastases are a common cause of pain in patients with advanced cancer, with about three-quarters of patients with bone metastases experiencing pain as the dominant symptom.1 Inadequately treated cancer pain impairs patient quality of life, and is associated with higher rates of depression, anxiety, and fatigue. Palliative radiotherapy (RT) is effective in alleviating pain from bone metastases.4 Local field external beam radiotherapy can provide some pain relief at the site of treated metastasis in 80%-90% of cases, with complete pain relief in 50%-60% of cases.5,6 However, maximal pain relief from RT is delayed, in some cases taking days to up to multiple weeks to attain.7,8 Therefore, optimal management of bone metastases pain may require the use of analgesics until RT takes adequate effect.
National Comprehensive Cancer Network (NCCN) Guidelines for Adult Cancer Pain (v. 2.2015) recommend that pain intensity rating (PIR; range, 0-10, where 0 denotes no pain and 10, worst pain imaginable) be used to quantify pain for all symptomatic patients. These guidelines also recommend the pain medication regimen be assessed for all symptomatic patients. For patients with moderate or severe pain (PIR of ≥4), NCCN guidelines recommend that analgesic regimen be intervened upon by alteration of the analgesic regimen (initiating, rotating, or titrating analgesic) or consideration of referral to pain/symptom management specialty.
Previous findings have demonstrated inadequate analgesic management for cancer pain,2,9 including within the radiation oncology (RO) clinic, suggesting that patients seen in consultation for palliative RT may experience uncontrolled pain for days to weeks before the onset of relief from RT. Possible reasons for inadequate acute pain intervention in the RO clinic may be provider discomfort with analgesic management and infrequent formal integration of palliative care within RO.10
Limited single-institution data from the few institutions with dedicated palliative RO services have suggested that these services improve the quality of palliative care delivery, as demonstrated by providers perceptions’ of the clinical impact of a dedicated service11 and the implementation of expedited palliative RT delivery for acute cancer pain.12,13 To our knowledge, the impact of a dedicated palliative RO service on analgesic management for cancer pain has not been assessed.
Here, we report how often patients with symptomatic bone metastases had assessments of existing analgesic regimens and interventions at RO consultation at 2 cancer centers. Center 1 had implemented a dedicated palliative RO service in 2011, consisting of rotating attending physicians and residents as well as dedicated palliative care trained nurse practitioners and a fellow, with the service structured around daily rounds,11 whereas Center 2 had not yet implemented a dedicated service. Using data from both centers, we assessed the impact of a palliative RO service on analgesic assessment and management in patients with bone metastases.
Methods
We searched our institutional databases for patients seen in RO consultation for bone metastases using ICD-9 code 198.5, and retrospectively reviewed consultation notes for those patients during June-July 2008, January-February 2010, January-February 2013, and June-July 2014. Those time periods were chosen as evenly spaced representative samples before and after implementation of a dedicated palliative RO service in 2011 at Center 1. Center 2 did not implement a dedicated palliative RO service in these time periods.
Within consultation notes, we recorded the following data from the History of the Present Illness section: symptoms from bone metastases (symptomatic was defined as any pain present); PIR (range, 0-10); and whether or not the preconsultation analgesic regimen was reported for symptomatic patients (including analgesic type, dosing, effectiveness, and adherence).
Documentation of the analgesic regimen in the history section of the notes was considered the proxy for analgesic regimen assessment at time of RO consultation. Analgesics within the Medications list, which were autopopulated in the consultation note by the electronic medical record, were recorded.
Whether or not pain was addressed with initiation or titration of analgesics for patients with a PIR of ≥4 was recorded from the Assessment and Plan portion of the notes, and that metric was considered the proxy for pain intervention. In addition, the case was coded as having had pain intervention if there was documentation of the patient declining recommended analgesic intervention, or the patient had been referred to a symptom management service for intervention (eg, referral to a specialty palliative care clinic), or there was recommendation for the patient to discuss uncontrolled pain with the original prescriber. A PIR of 4 was chosen as the threshold for analgesic intervention because at that level, NCCN guidelines for cancer pain state that the analgesic regimen should be titrated, whereas for a PIR of 3 or less, the guidelines recommend only consideration of titrating the analgesic. Only patients with a documented PIR were included in the pain intervention analysis.
Frequencies of analgesic assessment and analgesic intervention were compared using t tests (Wizard Pro, v1.8.5; Evan Miller, Chicago IL).
Results
A total of 271 patients with RO consultation notes were identified at the 2 centers within the 4 time periods (Table 1).
Among symptomatic patients, any component of the preconsultation analgesic regimen (including analgesic type, dosing, pain response, and adherence) was documented for 37.9% of the entire cohort at RO consultation (Table 3). At Centers 1 and 2, the frequencies of analgesic regimen assessment were documented for 41.3% and 28.1%, respectively (P = .06). Among symptomatic patients, 81.5% had an opioid or nonopioid analgesic listed in the Medications section in the electronic medical record at time of consultation.
Patients seen on the dedicated palliative RO service at Center 1 had an analgesic assessment documentation rate of 59.5%, whereas the patients not seen on a palliative RO service (ie, patients seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had an assessment documentation rate of 33.5% (P = .002; Figure 1). There was no significant difference between rates of analgesic regimen assessment between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (28.1% vs 35.9%, respectively; P = .27).
In patients seen at Center 1 only, those seen on the palliative RO service had a higher documentation rate of analgesic assessment compared with those seen by other services after implementation of the dedicated service (59.5% vs 38%, respectively; P = .018). Time period (after versus before 2011) was not significantly associated with the rate of documentation of analgesic assessment at either Center 1 (after vs before 2011: 44.4% vs 31%, P = .23) or Center 2 (31.4% vs 24.1%, P = .60).
Among patients with a PIR of ≥4, analgesic intervention was reported for 17.2% of patients within the entire cohort (20.8% at Center 1 and 0% at Center 2, P = .05). Among those with a PIR of ≥4, documentation of analgesic assessment noted in the History of the Present Illness section was associated with increased documentation of an analgesic intervention in the Assessment and Plan section (25% vs 7.3%; odds ratio [OR], 4.22; 95% confidence interval [CI], 1.1-16.0; P = .03).
Patients seen on the dedicated palliative RO service at Center 1 had a documented analgesic intervention rate of 31.6%, whereas the patients not seen on a palliative RO service (ie, those seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had a documented analgesic intervention rate of 9.2% (P = .01; Figure 2). There was no statistically significant difference between rates of documentation of an analgesic regimen intervention between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (0% vs 17.2%, respectively; P = .07).
Looking at only patients seen at Center 1, patients with a PIR of ≥4 seen on the dedicated palliative RO service had a nearly significant higher rate of documented analgesic interventions in the time period after implementation of the dedicate service (31.6% if seen on the dedicated service vs 12% if seen on a nondedicated service, P = .06).
Discussion
Multiple studies demonstrate the undertreatment of cancer pain in the outpatient setting.2,9,14,15 At 2 cancer centers, we found that about half of patients who present for consideration of palliative RT for bone metastases had a PIR of ≥4, yet only 17% of them had documentation of analgesic intervention as recommended by NCCN guidelines for cancer pain. Underlying this low rate of appropriate intervention may be the assumption of rapid pain relief by RT. However, RT often does not begin at time of consultation,16 and maximal pain relief may take days to weeks after commencement of RT.17 It is estimated that a quarter of all patients with cancer develop bone metastases during the course of their disease,12 and most of those patients suffer from pain. Thus, inherent delay in pain relief before, during, and after RT results in significant morbidity for the cancer patient population if adequate analgesic management is not provided.
The low rate of appropriate analgesic intervention at the time of RO consultation may also be related to the low incidence of proper analgesic assessment. In our cohort, 80% of symptomatic patients had an opioid or nonopioid analgesic listed in their medications within the electronic medical record at time of consultation, but only 38% had the analgesic regimen and/or its effectiveness described in the History of the Present Illness section of the record. Inattentiveness to analgesic type, dosing, and effectiveness during consultation may result in any inadequacies of the analgesic regimen going unnoticed. Consistent with this notion, we found that the rate of appropriate intervention for patients with a PIR of ≥4 was higher among patients who had analgesic regimen reported in the consultation note. Thus, interventions to implement routine review and documentation of the analgesic regimen, for example within the electronic medical record, may be one way to improve pain management.
Another possible reason for low rates of acute pain management within the RO clinic is low provider confidence in regard to analgesic management. In a recent national survey, 96% of radiation oncologists stated they were at least moderately confident with assessment of pain, yet only 77% were at least moderately confident with titrating opioids, and just 56% were at least moderately confident with rotating opioids.10 Educational interventions that improve providers’ facility with analgesic management may increase the frequency of pain management in the RO clinic.
Patients seen on the dedicated palliative RO service had significantly higher rates of documented analgesic regimen assessment and appropriate intervention during RO consultation, compared with patients seen at Center 2 and those not seen on the dedicated palliative RO service at Center 1. The improvements we observed in analgesic assessment and intervention at Center 1 for patients seen on the palliative RO service are likely owing to involvement of palliative RO and not to secular trends, because there were not similar improvements for patients at Center 1 who were not seen by the palliative RO service and those at Center 2, where there was no service.
At Center 1, the dedicated palliative RO service was created to provide specialized care to patients with metastatic disease undergoing palliative radiation. Within its structure, topics within palliative RO, such as technical aspects of palliative RT, symptom management, and communication are taught and reinforced in a case-based approach. Such palliative care awareness, integration, and education within RO achieved by the palliative RO service likely contribute to the improved rates of analgesic management we found in our study. We do note that rate of analgesic intervention in the palliative RO cohort, though higher than in the nonpalliative RO group, was still low, with only a third of patients receiving proper analgesic management. These findings highlight the importance of continued effort in increasing providers’ awareness of the need to assess pain and raise comfort with analgesic initiation and titration and of having dedicated palliative care clinicians embedded within the RO setting.
Since the data for this study was acquired, Center 2 has implemented a short palliative RO didactic course for residents, which improved their comfort levels in assessing analgesic effectiveness and intervening for uncontrolled pain.18 The impact of this intervention on clinical care will need to be evaluated, but the improved provider comfort levels may translate into better-quality care.
Limitations
An important limitation of this retrospective study is the reliance on the documentation provided in the consultation note for determining frequencies of analgesic regimen assessment and intervention. The actual rates of analgesic management that occurred in clinic may have been higher than reported in the documentation. However, such discrepancy in documentation of analgesic management would also be an area for quality improvement. Inadequate documentation limits the ability for proper follow-up of cancer pain as recommended by a joint guidance statement from the American Society of Clinical Oncology and the American Academy of Hospice and Palliative Medicine.19,20 The results of our study may also partly reflect a positive impact in documentation of analgesic management by a dedicated palliative RO service.
Given the multi-institutional nature of this study, it may be that general practice differences confound the impact of the dedicated palliative RO service at Center 1. However, with excluding Center 2, the dedicated service was still strongly associated with a higher rate of analgesic assessment within Center 1 and was almost significantly associated with appropriate analgesic intervention within Center 1.
We used a PIR of ≥4 as a threshold for appropriate analgesic regimen intervention because it is what is recommended by the NCCN guidelines. However, close attention should be paid to the impact that any amount of pain has on an individual patient. The functional, spiritual, and existential impact of pain is unique to each patient’s experience, and optimal symptom management should take those elements into account.
Conclusion
In conclusion, this study indicates that advanced cancer patient pain assessment and intervention according to NCCN cancer pain management guidelines is not common in the RO setting, and it is an area that should be targeted for quality improvement because of the positive implications for patient well-being. Pain assessment and intervention were greater in the setting of a dedicated structure for palliative care within RO, suggesting that the integration of palliative care within RO is a promising means of improving quality of pain management.
This work was presented at the 2016 ASCO Palliative Care in Oncology Symposium (September 9-10, 2016), where this work received a Conquer Cancer Foundation Merit Award.
Bone metastases are a common cause of pain in patients with advanced cancer, with about three-quarters of patients with bone metastases experiencing pain as the dominant symptom.1 Inadequately treated cancer pain impairs patient quality of life, and is associated with higher rates of depression, anxiety, and fatigue. Palliative radiotherapy (RT) is effective in alleviating pain from bone metastases.4 Local field external beam radiotherapy can provide some pain relief at the site of treated metastasis in 80%-90% of cases, with complete pain relief in 50%-60% of cases.5,6 However, maximal pain relief from RT is delayed, in some cases taking days to up to multiple weeks to attain.7,8 Therefore, optimal management of bone metastases pain may require the use of analgesics until RT takes adequate effect.
National Comprehensive Cancer Network (NCCN) Guidelines for Adult Cancer Pain (v. 2.2015) recommend that pain intensity rating (PIR; range, 0-10, where 0 denotes no pain and 10, worst pain imaginable) be used to quantify pain for all symptomatic patients. These guidelines also recommend the pain medication regimen be assessed for all symptomatic patients. For patients with moderate or severe pain (PIR of ≥4), NCCN guidelines recommend that analgesic regimen be intervened upon by alteration of the analgesic regimen (initiating, rotating, or titrating analgesic) or consideration of referral to pain/symptom management specialty.
Previous findings have demonstrated inadequate analgesic management for cancer pain,2,9 including within the radiation oncology (RO) clinic, suggesting that patients seen in consultation for palliative RT may experience uncontrolled pain for days to weeks before the onset of relief from RT. Possible reasons for inadequate acute pain intervention in the RO clinic may be provider discomfort with analgesic management and infrequent formal integration of palliative care within RO.10
Limited single-institution data from the few institutions with dedicated palliative RO services have suggested that these services improve the quality of palliative care delivery, as demonstrated by providers perceptions’ of the clinical impact of a dedicated service11 and the implementation of expedited palliative RT delivery for acute cancer pain.12,13 To our knowledge, the impact of a dedicated palliative RO service on analgesic management for cancer pain has not been assessed.
Here, we report how often patients with symptomatic bone metastases had assessments of existing analgesic regimens and interventions at RO consultation at 2 cancer centers. Center 1 had implemented a dedicated palliative RO service in 2011, consisting of rotating attending physicians and residents as well as dedicated palliative care trained nurse practitioners and a fellow, with the service structured around daily rounds,11 whereas Center 2 had not yet implemented a dedicated service. Using data from both centers, we assessed the impact of a palliative RO service on analgesic assessment and management in patients with bone metastases.
Methods
We searched our institutional databases for patients seen in RO consultation for bone metastases using ICD-9 code 198.5, and retrospectively reviewed consultation notes for those patients during June-July 2008, January-February 2010, January-February 2013, and June-July 2014. Those time periods were chosen as evenly spaced representative samples before and after implementation of a dedicated palliative RO service in 2011 at Center 1. Center 2 did not implement a dedicated palliative RO service in these time periods.
Within consultation notes, we recorded the following data from the History of the Present Illness section: symptoms from bone metastases (symptomatic was defined as any pain present); PIR (range, 0-10); and whether or not the preconsultation analgesic regimen was reported for symptomatic patients (including analgesic type, dosing, effectiveness, and adherence).
Documentation of the analgesic regimen in the history section of the notes was considered the proxy for analgesic regimen assessment at time of RO consultation. Analgesics within the Medications list, which were autopopulated in the consultation note by the electronic medical record, were recorded.
Whether or not pain was addressed with initiation or titration of analgesics for patients with a PIR of ≥4 was recorded from the Assessment and Plan portion of the notes, and that metric was considered the proxy for pain intervention. In addition, the case was coded as having had pain intervention if there was documentation of the patient declining recommended analgesic intervention, or the patient had been referred to a symptom management service for intervention (eg, referral to a specialty palliative care clinic), or there was recommendation for the patient to discuss uncontrolled pain with the original prescriber. A PIR of 4 was chosen as the threshold for analgesic intervention because at that level, NCCN guidelines for cancer pain state that the analgesic regimen should be titrated, whereas for a PIR of 3 or less, the guidelines recommend only consideration of titrating the analgesic. Only patients with a documented PIR were included in the pain intervention analysis.
Frequencies of analgesic assessment and analgesic intervention were compared using t tests (Wizard Pro, v1.8.5; Evan Miller, Chicago IL).
Results
A total of 271 patients with RO consultation notes were identified at the 2 centers within the 4 time periods (Table 1).
Among symptomatic patients, any component of the preconsultation analgesic regimen (including analgesic type, dosing, pain response, and adherence) was documented for 37.9% of the entire cohort at RO consultation (Table 3). At Centers 1 and 2, the frequencies of analgesic regimen assessment were documented for 41.3% and 28.1%, respectively (P = .06). Among symptomatic patients, 81.5% had an opioid or nonopioid analgesic listed in the Medications section in the electronic medical record at time of consultation.
Patients seen on the dedicated palliative RO service at Center 1 had an analgesic assessment documentation rate of 59.5%, whereas the patients not seen on a palliative RO service (ie, patients seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had an assessment documentation rate of 33.5% (P = .002; Figure 1). There was no significant difference between rates of analgesic regimen assessment between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (28.1% vs 35.9%, respectively; P = .27).
In patients seen at Center 1 only, those seen on the palliative RO service had a higher documentation rate of analgesic assessment compared with those seen by other services after implementation of the dedicated service (59.5% vs 38%, respectively; P = .018). Time period (after versus before 2011) was not significantly associated with the rate of documentation of analgesic assessment at either Center 1 (after vs before 2011: 44.4% vs 31%, P = .23) or Center 2 (31.4% vs 24.1%, P = .60).
Among patients with a PIR of ≥4, analgesic intervention was reported for 17.2% of patients within the entire cohort (20.8% at Center 1 and 0% at Center 2, P = .05). Among those with a PIR of ≥4, documentation of analgesic assessment noted in the History of the Present Illness section was associated with increased documentation of an analgesic intervention in the Assessment and Plan section (25% vs 7.3%; odds ratio [OR], 4.22; 95% confidence interval [CI], 1.1-16.0; P = .03).
Patients seen on the dedicated palliative RO service at Center 1 had a documented analgesic intervention rate of 31.6%, whereas the patients not seen on a palliative RO service (ie, those seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had a documented analgesic intervention rate of 9.2% (P = .01; Figure 2). There was no statistically significant difference between rates of documentation of an analgesic regimen intervention between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (0% vs 17.2%, respectively; P = .07).
Looking at only patients seen at Center 1, patients with a PIR of ≥4 seen on the dedicated palliative RO service had a nearly significant higher rate of documented analgesic interventions in the time period after implementation of the dedicate service (31.6% if seen on the dedicated service vs 12% if seen on a nondedicated service, P = .06).
Discussion
Multiple studies demonstrate the undertreatment of cancer pain in the outpatient setting.2,9,14,15 At 2 cancer centers, we found that about half of patients who present for consideration of palliative RT for bone metastases had a PIR of ≥4, yet only 17% of them had documentation of analgesic intervention as recommended by NCCN guidelines for cancer pain. Underlying this low rate of appropriate intervention may be the assumption of rapid pain relief by RT. However, RT often does not begin at time of consultation,16 and maximal pain relief may take days to weeks after commencement of RT.17 It is estimated that a quarter of all patients with cancer develop bone metastases during the course of their disease,12 and most of those patients suffer from pain. Thus, inherent delay in pain relief before, during, and after RT results in significant morbidity for the cancer patient population if adequate analgesic management is not provided.
The low rate of appropriate analgesic intervention at the time of RO consultation may also be related to the low incidence of proper analgesic assessment. In our cohort, 80% of symptomatic patients had an opioid or nonopioid analgesic listed in their medications within the electronic medical record at time of consultation, but only 38% had the analgesic regimen and/or its effectiveness described in the History of the Present Illness section of the record. Inattentiveness to analgesic type, dosing, and effectiveness during consultation may result in any inadequacies of the analgesic regimen going unnoticed. Consistent with this notion, we found that the rate of appropriate intervention for patients with a PIR of ≥4 was higher among patients who had analgesic regimen reported in the consultation note. Thus, interventions to implement routine review and documentation of the analgesic regimen, for example within the electronic medical record, may be one way to improve pain management.
Another possible reason for low rates of acute pain management within the RO clinic is low provider confidence in regard to analgesic management. In a recent national survey, 96% of radiation oncologists stated they were at least moderately confident with assessment of pain, yet only 77% were at least moderately confident with titrating opioids, and just 56% were at least moderately confident with rotating opioids.10 Educational interventions that improve providers’ facility with analgesic management may increase the frequency of pain management in the RO clinic.
Patients seen on the dedicated palliative RO service had significantly higher rates of documented analgesic regimen assessment and appropriate intervention during RO consultation, compared with patients seen at Center 2 and those not seen on the dedicated palliative RO service at Center 1. The improvements we observed in analgesic assessment and intervention at Center 1 for patients seen on the palliative RO service are likely owing to involvement of palliative RO and not to secular trends, because there were not similar improvements for patients at Center 1 who were not seen by the palliative RO service and those at Center 2, where there was no service.
At Center 1, the dedicated palliative RO service was created to provide specialized care to patients with metastatic disease undergoing palliative radiation. Within its structure, topics within palliative RO, such as technical aspects of palliative RT, symptom management, and communication are taught and reinforced in a case-based approach. Such palliative care awareness, integration, and education within RO achieved by the palliative RO service likely contribute to the improved rates of analgesic management we found in our study. We do note that rate of analgesic intervention in the palliative RO cohort, though higher than in the nonpalliative RO group, was still low, with only a third of patients receiving proper analgesic management. These findings highlight the importance of continued effort in increasing providers’ awareness of the need to assess pain and raise comfort with analgesic initiation and titration and of having dedicated palliative care clinicians embedded within the RO setting.
Since the data for this study was acquired, Center 2 has implemented a short palliative RO didactic course for residents, which improved their comfort levels in assessing analgesic effectiveness and intervening for uncontrolled pain.18 The impact of this intervention on clinical care will need to be evaluated, but the improved provider comfort levels may translate into better-quality care.
Limitations
An important limitation of this retrospective study is the reliance on the documentation provided in the consultation note for determining frequencies of analgesic regimen assessment and intervention. The actual rates of analgesic management that occurred in clinic may have been higher than reported in the documentation. However, such discrepancy in documentation of analgesic management would also be an area for quality improvement. Inadequate documentation limits the ability for proper follow-up of cancer pain as recommended by a joint guidance statement from the American Society of Clinical Oncology and the American Academy of Hospice and Palliative Medicine.19,20 The results of our study may also partly reflect a positive impact in documentation of analgesic management by a dedicated palliative RO service.
Given the multi-institutional nature of this study, it may be that general practice differences confound the impact of the dedicated palliative RO service at Center 1. However, with excluding Center 2, the dedicated service was still strongly associated with a higher rate of analgesic assessment within Center 1 and was almost significantly associated with appropriate analgesic intervention within Center 1.
We used a PIR of ≥4 as a threshold for appropriate analgesic regimen intervention because it is what is recommended by the NCCN guidelines. However, close attention should be paid to the impact that any amount of pain has on an individual patient. The functional, spiritual, and existential impact of pain is unique to each patient’s experience, and optimal symptom management should take those elements into account.
Conclusion
In conclusion, this study indicates that advanced cancer patient pain assessment and intervention according to NCCN cancer pain management guidelines is not common in the RO setting, and it is an area that should be targeted for quality improvement because of the positive implications for patient well-being. Pain assessment and intervention were greater in the setting of a dedicated structure for palliative care within RO, suggesting that the integration of palliative care within RO is a promising means of improving quality of pain management.
This work was presented at the 2016 ASCO Palliative Care in Oncology Symposium (September 9-10, 2016), where this work received a Conquer Cancer Foundation Merit Award.
1. Amichetti M, Orrù P, Madeddu A, et al. Comparative evaluation of two hypofractionated radiotherapy regimens for painful bone metastases. Tumori. 2004;90(1):91-95.
2. Vuong S, Pulenzas N, DeAngelis C, et al. Inadequate pain management in cancer patients attending an outpatient palliative radiotherapy clinic. Support Care Cancer. 2016;24(2):887-892.
3. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1-2):129-134.
4. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Sze WM, ed. Cochrane Database Syst Rev. 2004;(2):CD004721-CD004721.
5. Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44(1):1-18.
6. Kirou-Mauro A, Hird A, Wong J, et al. Is response to radiotherapy in patients related to the severity of pretreatment pain? Int J Radiat Oncol Biol Phys. 2008;71(4):1208-1212.
7. Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S158-S164.
8. McDonald R, Ding K, Brundage M, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol. 2017 Jul 1;3(7):953-959.
9. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149-4154.
10. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the united states: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
11. Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol. 2014;4(4):247-253.
12. Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163-170.
13. de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer. 2009;17(7):757-762.
14. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-1991.
15. Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39(2):259-267.
16. Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14(1):38-43.
17. Feyer PC, Steingraeber M. Radiotherapy of bone metastasis in breast cancer patients – current approaches. Breast Care (Basel). 2012;7(2):108-112.
18. Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys. 2017;97(5):884-885.
19. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
20. Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: an American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J Oncol Pract. 2016;12(9):e828-e838.
1. Amichetti M, Orrù P, Madeddu A, et al. Comparative evaluation of two hypofractionated radiotherapy regimens for painful bone metastases. Tumori. 2004;90(1):91-95.
2. Vuong S, Pulenzas N, DeAngelis C, et al. Inadequate pain management in cancer patients attending an outpatient palliative radiotherapy clinic. Support Care Cancer. 2016;24(2):887-892.
3. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1-2):129-134.
4. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Sze WM, ed. Cochrane Database Syst Rev. 2004;(2):CD004721-CD004721.
5. Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44(1):1-18.
6. Kirou-Mauro A, Hird A, Wong J, et al. Is response to radiotherapy in patients related to the severity of pretreatment pain? Int J Radiat Oncol Biol Phys. 2008;71(4):1208-1212.
7. Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S158-S164.
8. McDonald R, Ding K, Brundage M, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol. 2017 Jul 1;3(7):953-959.
9. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149-4154.
10. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the united states: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
11. Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol. 2014;4(4):247-253.
12. Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163-170.
13. de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer. 2009;17(7):757-762.
14. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-1991.
15. Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39(2):259-267.
16. Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14(1):38-43.
17. Feyer PC, Steingraeber M. Radiotherapy of bone metastasis in breast cancer patients – current approaches. Breast Care (Basel). 2012;7(2):108-112.
18. Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys. 2017;97(5):884-885.
19. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
20. Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: an American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J Oncol Pract. 2016;12(9):e828-e838.
Tivozanib after sorafenib promising in patients with advanced RCC
For patients with advanced renal cell carcinoma (RCC) progressing after sorafenib treatment, tivozanib was well tolerated and provided promising survival outcomes, investigators in a phase 2 study have reported.
Incidence of adverse events on tivozanib was low, and the safety profile was favorable in comparison with other agents in its class, the investigators said in the European Journal of Cancer.
The findings also help clarify results of a previous randomized phase 3 trial of tivozanib versus sorafenib where the investigators say crossover may have confounded overall survival results to the detriment of the tivozanib arm.
“Collectively, these data provide evidence of the anti-tumor activity of tivozanib and may be used to help frame future studies in recurrent disease,” wrote Ana M. Molina, MD, of Weill Cornell Medicine, New York, and her coauthors.
Tivozanib, recently approved in Europe for untreated RCC, is characterized by highly potent and selective inhibition of the three known vascular endothelial growth factor (VEGF) receptors.
Dr. Molina and her colleagues reported a single-arm crossover study of patients who were previously enrolled in the randomized phase 3 TIVO-1 trial of tivozanib versus sorafenib.
They enrolled a total of 161 patients who were randomized to the sorafenib arm of TIVO-1 and went on to receive tivozanib after disease progression.
Median progression-free survival was 11.0 months and median overall survival was 21.6 months for these crossover patients, Dr. Molina and co-investigators reported.
No patients in the study had a complete response, while 29 (18%) had a partial response and 83 (52%) had stable disease.
“These data compare favorably with other second-line therapies for RCC,” Dr. Molina and co-authors said.
Grade 3 or greater adverse events occurred in 48% of patients, including 24% that were treatment related. The most common grade 3 treatment-related adverse event was hypertension in 11%.
Approximately 4% of patients discontinued tivozanib due to adverse events.
“This study also provided clarity of the TIVO-1 trial, in which patient crossover was thought to have confounded the overall survival results,” Dr. Molina and colleagues said.
In TIVO-1, the primary end point of progression-free survival was improved for tivozanib versus sorafenib (median of 11.9 vs 9.1 months; P = .042), they noted.
However, median overall survival was not statistically different between arms, possibly because 74% of patients randomized to sorafenib were treated with next-line therapy, mainly tivozanib, investigators said.
AVEO Oncology and Astellas Pharma US, Inc. funded the study. Dr. Molina reported receiving honoraria from AVEO, Novartis, and Eisai.
SOURCE: Molina AM, et al. Eur J Cancer. 2018 Mar 13. doi: 10.1016/j.ejca.2018.02.009.
For patients with advanced renal cell carcinoma (RCC) progressing after sorafenib treatment, tivozanib was well tolerated and provided promising survival outcomes, investigators in a phase 2 study have reported.
Incidence of adverse events on tivozanib was low, and the safety profile was favorable in comparison with other agents in its class, the investigators said in the European Journal of Cancer.
The findings also help clarify results of a previous randomized phase 3 trial of tivozanib versus sorafenib where the investigators say crossover may have confounded overall survival results to the detriment of the tivozanib arm.
“Collectively, these data provide evidence of the anti-tumor activity of tivozanib and may be used to help frame future studies in recurrent disease,” wrote Ana M. Molina, MD, of Weill Cornell Medicine, New York, and her coauthors.
Tivozanib, recently approved in Europe for untreated RCC, is characterized by highly potent and selective inhibition of the three known vascular endothelial growth factor (VEGF) receptors.
Dr. Molina and her colleagues reported a single-arm crossover study of patients who were previously enrolled in the randomized phase 3 TIVO-1 trial of tivozanib versus sorafenib.
They enrolled a total of 161 patients who were randomized to the sorafenib arm of TIVO-1 and went on to receive tivozanib after disease progression.
Median progression-free survival was 11.0 months and median overall survival was 21.6 months for these crossover patients, Dr. Molina and co-investigators reported.
No patients in the study had a complete response, while 29 (18%) had a partial response and 83 (52%) had stable disease.
“These data compare favorably with other second-line therapies for RCC,” Dr. Molina and co-authors said.
Grade 3 or greater adverse events occurred in 48% of patients, including 24% that were treatment related. The most common grade 3 treatment-related adverse event was hypertension in 11%.
Approximately 4% of patients discontinued tivozanib due to adverse events.
“This study also provided clarity of the TIVO-1 trial, in which patient crossover was thought to have confounded the overall survival results,” Dr. Molina and colleagues said.
In TIVO-1, the primary end point of progression-free survival was improved for tivozanib versus sorafenib (median of 11.9 vs 9.1 months; P = .042), they noted.
However, median overall survival was not statistically different between arms, possibly because 74% of patients randomized to sorafenib were treated with next-line therapy, mainly tivozanib, investigators said.
AVEO Oncology and Astellas Pharma US, Inc. funded the study. Dr. Molina reported receiving honoraria from AVEO, Novartis, and Eisai.
SOURCE: Molina AM, et al. Eur J Cancer. 2018 Mar 13. doi: 10.1016/j.ejca.2018.02.009.
For patients with advanced renal cell carcinoma (RCC) progressing after sorafenib treatment, tivozanib was well tolerated and provided promising survival outcomes, investigators in a phase 2 study have reported.
Incidence of adverse events on tivozanib was low, and the safety profile was favorable in comparison with other agents in its class, the investigators said in the European Journal of Cancer.
The findings also help clarify results of a previous randomized phase 3 trial of tivozanib versus sorafenib where the investigators say crossover may have confounded overall survival results to the detriment of the tivozanib arm.
“Collectively, these data provide evidence of the anti-tumor activity of tivozanib and may be used to help frame future studies in recurrent disease,” wrote Ana M. Molina, MD, of Weill Cornell Medicine, New York, and her coauthors.
Tivozanib, recently approved in Europe for untreated RCC, is characterized by highly potent and selective inhibition of the three known vascular endothelial growth factor (VEGF) receptors.
Dr. Molina and her colleagues reported a single-arm crossover study of patients who were previously enrolled in the randomized phase 3 TIVO-1 trial of tivozanib versus sorafenib.
They enrolled a total of 161 patients who were randomized to the sorafenib arm of TIVO-1 and went on to receive tivozanib after disease progression.
Median progression-free survival was 11.0 months and median overall survival was 21.6 months for these crossover patients, Dr. Molina and co-investigators reported.
No patients in the study had a complete response, while 29 (18%) had a partial response and 83 (52%) had stable disease.
“These data compare favorably with other second-line therapies for RCC,” Dr. Molina and co-authors said.
Grade 3 or greater adverse events occurred in 48% of patients, including 24% that were treatment related. The most common grade 3 treatment-related adverse event was hypertension in 11%.
Approximately 4% of patients discontinued tivozanib due to adverse events.
“This study also provided clarity of the TIVO-1 trial, in which patient crossover was thought to have confounded the overall survival results,” Dr. Molina and colleagues said.
In TIVO-1, the primary end point of progression-free survival was improved for tivozanib versus sorafenib (median of 11.9 vs 9.1 months; P = .042), they noted.
However, median overall survival was not statistically different between arms, possibly because 74% of patients randomized to sorafenib were treated with next-line therapy, mainly tivozanib, investigators said.
AVEO Oncology and Astellas Pharma US, Inc. funded the study. Dr. Molina reported receiving honoraria from AVEO, Novartis, and Eisai.
SOURCE: Molina AM, et al. Eur J Cancer. 2018 Mar 13. doi: 10.1016/j.ejca.2018.02.009.
FROM THE EUROPEAN JOURNAL OF CANCER
Key clinical point: Tivozanib has potent antitumor activity in patients with advanced renal cell carcinoma (RCC) who previously progressed on sorafenib.
Major finding: Median progression-free survival was 11.0 months, and median overall survival was 21.6 months for patients receiving tivozanib.
Study details: A single-arm, phase 2 crossover study of patients previously randomized to the sorafenib arm of the phase 3 TIVO-1 study.
Disclosures: AVEO Oncology and Astellas Pharma US, Inc. funded the study. Investigators reported potential conflict of interests related to AVEO, Novartis, Eisai, Pfizer, and others.
Source: Molina AM, et al. Eur J Cancer. 2018 Mar 13. doi: 10.1016/j.ejca.2018.02.009.