User login
Exercise type matters for fall prevention among elderly
according to a Cochrane Review meta-analysis of 108 randomized controlled trials.
Exercise has been shown to prevent falls in older people, but given the potential consequences, the investigators thought an up-to-date synthesis of the evidence was in order. The analysis focused on people living independently who had not recently been discharged from a hospital. The trials involved 23,407 subjects from 25 countries. The review was exhaustive; the final report is almost 600 pages long (Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2).
The type of exercise matters. The researchers cited “high-certainty evidence” that exercise involving balance and functional training reduces falls. “Tai chi may also prevent falls,” they noted, adding that they were uncertain of the effect of dance, walking, and resistance training by itself. There was no evidence to determine the effects of flexibility or endurance exercises, added the researchers, led by Cathie Sherrington, PhD, of the University of Sydney Institute for Musculoskeletal Health.
Functional exercise mimics everyday movement, with the goal of improving performance. Multidirectional lunges are an example, helping the body prepare for vacuuming, yard work, and other common activities.
“Exercise [programs] carried out in group classes or done at home prescribed by a health professional ... or a trained exercise leader were effective. Exercises were mostly done while standing as this better enhances balance and the ability to do daily activities such as standing up from a low chair or climbing stairs,” according to a Cochrane press release regarding the study.
Overall, exercise reduced the number of falls by 23%, and the number of fallers by 15%, with high-certainty evidence.
Exercise also brought down the number of people facing fall fractures by over 27%, the number of people requiring medical attention for a fall by 39%, and the number ending up in the hospital for a fall by 22%.
Balance and functional exercises reduced the rate of falls by 24%, and the number of fallers by 13%. The effects were even greater when resistance exercises were added to the mix; drops in fall rates and the number of people experiencing falls were 34% and 22%, respectively. There was low-certainty evidence that tai chi reduces the rate of falls by 19% and the number of people experiencing falls by 20%.
Despite fall prevention, “exercise may make little important difference to health-related quality of life;” when results were converted to EQ-5D and 36-Item Short Form Survey scores, “the respective 95% [confidence intervals] were much smaller than minimally important differences,” the investigators said.
Serious adverse events occurred in participants in one of the 27 trials that reported adverse events. These two serious adverse events were a pelvic stress fracture and an inguinal hernia surgery. Most of the other adverse events reported, all non-serious, were musculoskeletal.
On average, participants were 76 years old, and 77% were women. Disease specific trials – such as exercise for stroke rehabilitation – were excluded.
The work was supported primarily by the Cochrane Bone, Joint and Muscle Trauma Group, based at the University of Manchester, England, and Cochrane’s Acute and Emergency Care Network. There were no industry disclosures.
SOURCE: Sherrington C et al. Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2.
according to a Cochrane Review meta-analysis of 108 randomized controlled trials.
Exercise has been shown to prevent falls in older people, but given the potential consequences, the investigators thought an up-to-date synthesis of the evidence was in order. The analysis focused on people living independently who had not recently been discharged from a hospital. The trials involved 23,407 subjects from 25 countries. The review was exhaustive; the final report is almost 600 pages long (Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2).
The type of exercise matters. The researchers cited “high-certainty evidence” that exercise involving balance and functional training reduces falls. “Tai chi may also prevent falls,” they noted, adding that they were uncertain of the effect of dance, walking, and resistance training by itself. There was no evidence to determine the effects of flexibility or endurance exercises, added the researchers, led by Cathie Sherrington, PhD, of the University of Sydney Institute for Musculoskeletal Health.
Functional exercise mimics everyday movement, with the goal of improving performance. Multidirectional lunges are an example, helping the body prepare for vacuuming, yard work, and other common activities.
“Exercise [programs] carried out in group classes or done at home prescribed by a health professional ... or a trained exercise leader were effective. Exercises were mostly done while standing as this better enhances balance and the ability to do daily activities such as standing up from a low chair or climbing stairs,” according to a Cochrane press release regarding the study.
Overall, exercise reduced the number of falls by 23%, and the number of fallers by 15%, with high-certainty evidence.
Exercise also brought down the number of people facing fall fractures by over 27%, the number of people requiring medical attention for a fall by 39%, and the number ending up in the hospital for a fall by 22%.
Balance and functional exercises reduced the rate of falls by 24%, and the number of fallers by 13%. The effects were even greater when resistance exercises were added to the mix; drops in fall rates and the number of people experiencing falls were 34% and 22%, respectively. There was low-certainty evidence that tai chi reduces the rate of falls by 19% and the number of people experiencing falls by 20%.
Despite fall prevention, “exercise may make little important difference to health-related quality of life;” when results were converted to EQ-5D and 36-Item Short Form Survey scores, “the respective 95% [confidence intervals] were much smaller than minimally important differences,” the investigators said.
Serious adverse events occurred in participants in one of the 27 trials that reported adverse events. These two serious adverse events were a pelvic stress fracture and an inguinal hernia surgery. Most of the other adverse events reported, all non-serious, were musculoskeletal.
On average, participants were 76 years old, and 77% were women. Disease specific trials – such as exercise for stroke rehabilitation – were excluded.
The work was supported primarily by the Cochrane Bone, Joint and Muscle Trauma Group, based at the University of Manchester, England, and Cochrane’s Acute and Emergency Care Network. There were no industry disclosures.
SOURCE: Sherrington C et al. Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2.
according to a Cochrane Review meta-analysis of 108 randomized controlled trials.
Exercise has been shown to prevent falls in older people, but given the potential consequences, the investigators thought an up-to-date synthesis of the evidence was in order. The analysis focused on people living independently who had not recently been discharged from a hospital. The trials involved 23,407 subjects from 25 countries. The review was exhaustive; the final report is almost 600 pages long (Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2).
The type of exercise matters. The researchers cited “high-certainty evidence” that exercise involving balance and functional training reduces falls. “Tai chi may also prevent falls,” they noted, adding that they were uncertain of the effect of dance, walking, and resistance training by itself. There was no evidence to determine the effects of flexibility or endurance exercises, added the researchers, led by Cathie Sherrington, PhD, of the University of Sydney Institute for Musculoskeletal Health.
Functional exercise mimics everyday movement, with the goal of improving performance. Multidirectional lunges are an example, helping the body prepare for vacuuming, yard work, and other common activities.
“Exercise [programs] carried out in group classes or done at home prescribed by a health professional ... or a trained exercise leader were effective. Exercises were mostly done while standing as this better enhances balance and the ability to do daily activities such as standing up from a low chair or climbing stairs,” according to a Cochrane press release regarding the study.
Overall, exercise reduced the number of falls by 23%, and the number of fallers by 15%, with high-certainty evidence.
Exercise also brought down the number of people facing fall fractures by over 27%, the number of people requiring medical attention for a fall by 39%, and the number ending up in the hospital for a fall by 22%.
Balance and functional exercises reduced the rate of falls by 24%, and the number of fallers by 13%. The effects were even greater when resistance exercises were added to the mix; drops in fall rates and the number of people experiencing falls were 34% and 22%, respectively. There was low-certainty evidence that tai chi reduces the rate of falls by 19% and the number of people experiencing falls by 20%.
Despite fall prevention, “exercise may make little important difference to health-related quality of life;” when results were converted to EQ-5D and 36-Item Short Form Survey scores, “the respective 95% [confidence intervals] were much smaller than minimally important differences,” the investigators said.
Serious adverse events occurred in participants in one of the 27 trials that reported adverse events. These two serious adverse events were a pelvic stress fracture and an inguinal hernia surgery. Most of the other adverse events reported, all non-serious, were musculoskeletal.
On average, participants were 76 years old, and 77% were women. Disease specific trials – such as exercise for stroke rehabilitation – were excluded.
The work was supported primarily by the Cochrane Bone, Joint and Muscle Trauma Group, based at the University of Manchester, England, and Cochrane’s Acute and Emergency Care Network. There were no industry disclosures.
SOURCE: Sherrington C et al. Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2.
FROM COCHRANE DATABASE OF SYSTEMATIC REVIEWS
Key clinical point: Exercise helps elderly people avoid falls, especially if it focuses on balance and mimics daily activities.
Major finding: Balance and functional exercises reduce the rate of falls by 24%, and the number of fallers by 13%. The effects were even greater when resistance exercises were added to the mix; drops in fall rates and the number of people experiencing falls were 34% and 22%, respectively.
Study details: A meta-analysis of 108 randomized, controlled trials.
Disclosures: The work was supported by Cochrane. There were no industry disclosures.
Source: Sherrington C et al. Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2.
Novel plasma biomarkers may predict preclinical Alzheimer’s disease
, researchers reported in Science Advances.
“To our knowledge, this is the first time that a multianalyte plasma biomarker panel for an Alzheimer’s disease–related phenotype has been found and independently replicated by a nontargeted mass spectrometry approach,” said Nicholas J. Ashton, PhD, of King’s College London and the University of Gothenburg in Sweden, and his research colleagues.
Blood-based measures that predict amyloid-beta burden in preclinical Alzheimer’s disease have the potential to help investigators conduct clinical trials and aid in diagnostic management. However, this novel approach needs to be validated and translated “to a simpler automated platform suitable for wider utility,” the investigators noted. In addition, it is unclear whether their classifier can track changes in amyloid-beta or differentiate between other diseases with amyloid-beta pathology.
Advances in mass spectrometry technology have renewed interest in the analysis of plasma proteins in patients with various diseases. To assess whether proteomic discovery in plasma can help predict amyloid-beta burden in preclinical Alzheimer’s disease, Dr. Ashton and his colleagues studied 238 cognitively unimpaired individuals from the Australian Imaging, Biomarker and Lifestyle Flagship Study of Ageing (AIBL) and the Kerr Anglican Retirement Village Initiative in Ageing Health (KARVIAH). The participants had undergone PET to determine their amyloid-beta status. In the AIBL cohort (n = 144), 100 participants were amyloid-beta negative, and 44 were amyloid-beta positive. In the KARVIAH cohort (n = 94), 59 participants were amyloid-beta negative, and 35 were amyloid-beta positive. There were significantly more APOE4 carriers in the amyloid-beta–positive groups than in the amyloid-beta–negative groups. In addition, the amyloid-beta–positive groups tended to be older.
A support vector machine analysis created classifiers predicting amyloid-beta positivity in the AIBL cohort using demographics, proteins, or both. The researchers then tested each classifier in the KARVIAH dataset to identify which model best predicted amyloid-beta positivity. The optimal model included 10 protein features (prothrombin, adhesion G protein–coupled receptor, amyloid-beta A4 protein, NGN2, DNAH10, REST, NfL, RPS6KA3, GPSM2, FHAD1) and two demographic features (APOE4 count and age).
The classifier achieved a testing area under the receiver operator characteristic curve of 0.891 in the KARVIAH cohort to predict amyloid-beta positivity in cognitively unimpaired individuals with a sensitivity of 0.78 and specificity of 0.77.
The 10 protein features “represent a diverse array of pathways,” and the highest ranked feature was the serine protease prothrombin, which is a precursor to thrombin, the authors noted. “Multiple lines of evidence support that cerebrovascular disease may play a role in AD and that amyloid-beta may be involved in thrombosis, fibrinolysis, and inflammation via its interaction with the coagulation cascade,” the researchers wrote.
Two of the biomarkers – amyloid-beta A4 protein and NfL – have been examined in prior research and had a greater effect size in a secondary analysis that included participants with mild cognitive impairment and Alzheimer’s disease. This finding confirms “their connection with the more established disease state,” Dr. Ashton and colleagues said. In the secondary analysis, the optimal classifier included one demographic factor (APOE4 count) and nine protein features, eight of which also were used in the cognitively unimpaired classifier.
The study was funded in part by the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, and many authors reported additional research support from various institutions. One author is an employee of Johnson & Johnson and a named inventor on unrelated biomarker intellectual property owned by Proteome Science and King’s College London.
SOURCE: Ashton NJ et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aau7220.
, researchers reported in Science Advances.
“To our knowledge, this is the first time that a multianalyte plasma biomarker panel for an Alzheimer’s disease–related phenotype has been found and independently replicated by a nontargeted mass spectrometry approach,” said Nicholas J. Ashton, PhD, of King’s College London and the University of Gothenburg in Sweden, and his research colleagues.
Blood-based measures that predict amyloid-beta burden in preclinical Alzheimer’s disease have the potential to help investigators conduct clinical trials and aid in diagnostic management. However, this novel approach needs to be validated and translated “to a simpler automated platform suitable for wider utility,” the investigators noted. In addition, it is unclear whether their classifier can track changes in amyloid-beta or differentiate between other diseases with amyloid-beta pathology.
Advances in mass spectrometry technology have renewed interest in the analysis of plasma proteins in patients with various diseases. To assess whether proteomic discovery in plasma can help predict amyloid-beta burden in preclinical Alzheimer’s disease, Dr. Ashton and his colleagues studied 238 cognitively unimpaired individuals from the Australian Imaging, Biomarker and Lifestyle Flagship Study of Ageing (AIBL) and the Kerr Anglican Retirement Village Initiative in Ageing Health (KARVIAH). The participants had undergone PET to determine their amyloid-beta status. In the AIBL cohort (n = 144), 100 participants were amyloid-beta negative, and 44 were amyloid-beta positive. In the KARVIAH cohort (n = 94), 59 participants were amyloid-beta negative, and 35 were amyloid-beta positive. There were significantly more APOE4 carriers in the amyloid-beta–positive groups than in the amyloid-beta–negative groups. In addition, the amyloid-beta–positive groups tended to be older.
A support vector machine analysis created classifiers predicting amyloid-beta positivity in the AIBL cohort using demographics, proteins, or both. The researchers then tested each classifier in the KARVIAH dataset to identify which model best predicted amyloid-beta positivity. The optimal model included 10 protein features (prothrombin, adhesion G protein–coupled receptor, amyloid-beta A4 protein, NGN2, DNAH10, REST, NfL, RPS6KA3, GPSM2, FHAD1) and two demographic features (APOE4 count and age).
The classifier achieved a testing area under the receiver operator characteristic curve of 0.891 in the KARVIAH cohort to predict amyloid-beta positivity in cognitively unimpaired individuals with a sensitivity of 0.78 and specificity of 0.77.
The 10 protein features “represent a diverse array of pathways,” and the highest ranked feature was the serine protease prothrombin, which is a precursor to thrombin, the authors noted. “Multiple lines of evidence support that cerebrovascular disease may play a role in AD and that amyloid-beta may be involved in thrombosis, fibrinolysis, and inflammation via its interaction with the coagulation cascade,” the researchers wrote.
Two of the biomarkers – amyloid-beta A4 protein and NfL – have been examined in prior research and had a greater effect size in a secondary analysis that included participants with mild cognitive impairment and Alzheimer’s disease. This finding confirms “their connection with the more established disease state,” Dr. Ashton and colleagues said. In the secondary analysis, the optimal classifier included one demographic factor (APOE4 count) and nine protein features, eight of which also were used in the cognitively unimpaired classifier.
The study was funded in part by the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, and many authors reported additional research support from various institutions. One author is an employee of Johnson & Johnson and a named inventor on unrelated biomarker intellectual property owned by Proteome Science and King’s College London.
SOURCE: Ashton NJ et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aau7220.
, researchers reported in Science Advances.
“To our knowledge, this is the first time that a multianalyte plasma biomarker panel for an Alzheimer’s disease–related phenotype has been found and independently replicated by a nontargeted mass spectrometry approach,” said Nicholas J. Ashton, PhD, of King’s College London and the University of Gothenburg in Sweden, and his research colleagues.
Blood-based measures that predict amyloid-beta burden in preclinical Alzheimer’s disease have the potential to help investigators conduct clinical trials and aid in diagnostic management. However, this novel approach needs to be validated and translated “to a simpler automated platform suitable for wider utility,” the investigators noted. In addition, it is unclear whether their classifier can track changes in amyloid-beta or differentiate between other diseases with amyloid-beta pathology.
Advances in mass spectrometry technology have renewed interest in the analysis of plasma proteins in patients with various diseases. To assess whether proteomic discovery in plasma can help predict amyloid-beta burden in preclinical Alzheimer’s disease, Dr. Ashton and his colleagues studied 238 cognitively unimpaired individuals from the Australian Imaging, Biomarker and Lifestyle Flagship Study of Ageing (AIBL) and the Kerr Anglican Retirement Village Initiative in Ageing Health (KARVIAH). The participants had undergone PET to determine their amyloid-beta status. In the AIBL cohort (n = 144), 100 participants were amyloid-beta negative, and 44 were amyloid-beta positive. In the KARVIAH cohort (n = 94), 59 participants were amyloid-beta negative, and 35 were amyloid-beta positive. There were significantly more APOE4 carriers in the amyloid-beta–positive groups than in the amyloid-beta–negative groups. In addition, the amyloid-beta–positive groups tended to be older.
A support vector machine analysis created classifiers predicting amyloid-beta positivity in the AIBL cohort using demographics, proteins, or both. The researchers then tested each classifier in the KARVIAH dataset to identify which model best predicted amyloid-beta positivity. The optimal model included 10 protein features (prothrombin, adhesion G protein–coupled receptor, amyloid-beta A4 protein, NGN2, DNAH10, REST, NfL, RPS6KA3, GPSM2, FHAD1) and two demographic features (APOE4 count and age).
The classifier achieved a testing area under the receiver operator characteristic curve of 0.891 in the KARVIAH cohort to predict amyloid-beta positivity in cognitively unimpaired individuals with a sensitivity of 0.78 and specificity of 0.77.
The 10 protein features “represent a diverse array of pathways,” and the highest ranked feature was the serine protease prothrombin, which is a precursor to thrombin, the authors noted. “Multiple lines of evidence support that cerebrovascular disease may play a role in AD and that amyloid-beta may be involved in thrombosis, fibrinolysis, and inflammation via its interaction with the coagulation cascade,” the researchers wrote.
Two of the biomarkers – amyloid-beta A4 protein and NfL – have been examined in prior research and had a greater effect size in a secondary analysis that included participants with mild cognitive impairment and Alzheimer’s disease. This finding confirms “their connection with the more established disease state,” Dr. Ashton and colleagues said. In the secondary analysis, the optimal classifier included one demographic factor (APOE4 count) and nine protein features, eight of which also were used in the cognitively unimpaired classifier.
The study was funded in part by the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, and many authors reported additional research support from various institutions. One author is an employee of Johnson & Johnson and a named inventor on unrelated biomarker intellectual property owned by Proteome Science and King’s College London.
SOURCE: Ashton NJ et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aau7220.
FROM SCIENCE ADVANCES
Key clinical point: Blood-based measures that predict amyloid-beta burden in preclinical Alzheimer’s disease have the potential to help investigators conduct clinical trials and aid in diagnostic management.
Major finding: A classifier developed using plasma proteomic analysis achieved an area under the receiver operator characteristic curve of 0.891.
Study details: An analysis of data from 238 cognitively unimpaired individuals from the Australian Imaging, Biomarker and Lifestyle Flagship Study of Ageing (AIBL) and the Kerr Anglican Retirement Village Initiative in Ageing Health (KARVIAH).
Disclosures: The study was funded in part by the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, and many authors reported additional research support from various institutions. One author is an employee of Johnson & Johnson and a named inventor on unrelated biomarker intellectual property owned by Proteome Science and King’s College London.
Source: Ashton NJ et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aau7220.
Statins cut vascular events in elderly patients
Statin therapy appears to reduce the risk of major vascular events for patients of all age groups, but there is less evidence that older patients with evidence of occlusive vascular disease benefit from the treatment, according to a recent meta-analysis of 28 trials from the Cholesterol Treatment Trialists’ Collaboration published in The Lancet.
Statins are “useful and affordable drug[s] that reduce heart attacks and strokes in older patients. Until now there has been an evidence gap and we wanted to look at their efficacy and safety in older people,” Jordan Fulcher, BSc (Med), MBBS, from the Cholesterol Treatment Trialists’ (CTT) Collaboration and the University of Sydney stated in a press release. “Our analysis indicates that major cardiovascular events were reduced by about a fifth, per mmol/L lower LDL cholesterol, by statin therapy across all age groups. Despite previous concerns, we found no adverse effect on cancer or nonvascular mortality in any age group.”
The researchers examined 186,854 participants from 28 CTT trials undergoing statin therapy, of whom 14,483 (8%) were older than 75 years. Patients were divided into six groups based on age and examined the risk of major cardiovascular events such as stroke, coronary revascularization and major coronary events, as well as the incidence of cancer and vascular mortality.
Among all age groups, there was a significant reduction in major vascular events, with a 21% proportional per 1.0-mmol/L reduction in LDL cholesterol (risk ratio, 0.79; 95% confidence interval, 0.77-0.81) among patients receiving statin therapy or a more intensive statin regimen, and there was a 24% proportional reduction (RR, 0.76; 95% CI, 0.73-0.79) of major coronary events per 1.0-mmol/L reduction in LDL cholesterol, with older age resulting in a lower proportional reduction of major coronary events (P = .009). The researchers also found a proportional reduction of coronary revascularization procedures by 25% (RR, 0.75; 95% CI, 0.73-0.78) and stroke by 16% (RR, 0.84; 95% CI, 0.80-0.89) among patients of any age group receiving statin therapy or more intensive statin regimen, with no significant differences between age groups.
There was a 12% proportional reduction in vascular mortality per 1.0-mmol/L reduction in LDL cholesterol (RR, 0.88; 95% CI, 0.85-0.91), but this statistic did not remain significant after the researchers excluded four trials that included patients with heart failure or who were receiving renal dialysis. After excluding these trials from the overall analysis, the researchers found the smaller proportional reductions persisted for older patients for major coronary events (P = .01) but was no longer significant for major vascular events.
The researchers noted their study was limited by the highly selected patient population, low percentage of patients older than 75 years, including trials with efficacy endpoints where some nonserious adverse events may not have been recorded, and not including some trials in the meta-analysis if they were not part of the CTT.
This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
SOURCE: Fulcher J et al. Lancet. 2019;393:407-15.
Statin therapy is often discontinued for older patients who have concomitant disease or other considerations, but it should still be considered in older patients when the benefits outweigh the risks, Bernard M.Y. Cheung, PhD, and Karen S.L. Lam, MD, wrote in a related editorial.
“Even if the relative risk reduction in people older than 75 years is less than expected, statin therapy might still be justified by a high baseline cardiovascular risk, which is usually present in older people,” they said.
One explanation for the decreased relative risk reduction among older patients from the results by Fulcher et al. in the Cholesterol Treatment Trialists’ (CTT) Collaboration trial could have been the inclusion of older patients with cardiac and renal failure, and treating patients with lower cardiac risk or lowering LDL cholesterol in patients at risk of cardiovascular events can help prevent major vascular events later.
Ultimately, no drug is harmless and the risk and benefits must be weighed before making a decision to use statins with older patients just as they would in any other patient population. “The challenge for the health-care profession and the media is to convey risks and benefits in ways that patients can understand, enabling them to make an informed choice,” the authors wrote.
Dr. Cheung and Dr. Lam are from the department of medicine at Queen Mary Hospital, University of Hong Kong in Hong Kong Special Administrative Region, China. They had no relevant disclosures.
Statin therapy is often discontinued for older patients who have concomitant disease or other considerations, but it should still be considered in older patients when the benefits outweigh the risks, Bernard M.Y. Cheung, PhD, and Karen S.L. Lam, MD, wrote in a related editorial.
“Even if the relative risk reduction in people older than 75 years is less than expected, statin therapy might still be justified by a high baseline cardiovascular risk, which is usually present in older people,” they said.
One explanation for the decreased relative risk reduction among older patients from the results by Fulcher et al. in the Cholesterol Treatment Trialists’ (CTT) Collaboration trial could have been the inclusion of older patients with cardiac and renal failure, and treating patients with lower cardiac risk or lowering LDL cholesterol in patients at risk of cardiovascular events can help prevent major vascular events later.
Ultimately, no drug is harmless and the risk and benefits must be weighed before making a decision to use statins with older patients just as they would in any other patient population. “The challenge for the health-care profession and the media is to convey risks and benefits in ways that patients can understand, enabling them to make an informed choice,” the authors wrote.
Dr. Cheung and Dr. Lam are from the department of medicine at Queen Mary Hospital, University of Hong Kong in Hong Kong Special Administrative Region, China. They had no relevant disclosures.
Statin therapy is often discontinued for older patients who have concomitant disease or other considerations, but it should still be considered in older patients when the benefits outweigh the risks, Bernard M.Y. Cheung, PhD, and Karen S.L. Lam, MD, wrote in a related editorial.
“Even if the relative risk reduction in people older than 75 years is less than expected, statin therapy might still be justified by a high baseline cardiovascular risk, which is usually present in older people,” they said.
One explanation for the decreased relative risk reduction among older patients from the results by Fulcher et al. in the Cholesterol Treatment Trialists’ (CTT) Collaboration trial could have been the inclusion of older patients with cardiac and renal failure, and treating patients with lower cardiac risk or lowering LDL cholesterol in patients at risk of cardiovascular events can help prevent major vascular events later.
Ultimately, no drug is harmless and the risk and benefits must be weighed before making a decision to use statins with older patients just as they would in any other patient population. “The challenge for the health-care profession and the media is to convey risks and benefits in ways that patients can understand, enabling them to make an informed choice,” the authors wrote.
Dr. Cheung and Dr. Lam are from the department of medicine at Queen Mary Hospital, University of Hong Kong in Hong Kong Special Administrative Region, China. They had no relevant disclosures.
Statin therapy appears to reduce the risk of major vascular events for patients of all age groups, but there is less evidence that older patients with evidence of occlusive vascular disease benefit from the treatment, according to a recent meta-analysis of 28 trials from the Cholesterol Treatment Trialists’ Collaboration published in The Lancet.
Statins are “useful and affordable drug[s] that reduce heart attacks and strokes in older patients. Until now there has been an evidence gap and we wanted to look at their efficacy and safety in older people,” Jordan Fulcher, BSc (Med), MBBS, from the Cholesterol Treatment Trialists’ (CTT) Collaboration and the University of Sydney stated in a press release. “Our analysis indicates that major cardiovascular events were reduced by about a fifth, per mmol/L lower LDL cholesterol, by statin therapy across all age groups. Despite previous concerns, we found no adverse effect on cancer or nonvascular mortality in any age group.”
The researchers examined 186,854 participants from 28 CTT trials undergoing statin therapy, of whom 14,483 (8%) were older than 75 years. Patients were divided into six groups based on age and examined the risk of major cardiovascular events such as stroke, coronary revascularization and major coronary events, as well as the incidence of cancer and vascular mortality.
Among all age groups, there was a significant reduction in major vascular events, with a 21% proportional per 1.0-mmol/L reduction in LDL cholesterol (risk ratio, 0.79; 95% confidence interval, 0.77-0.81) among patients receiving statin therapy or a more intensive statin regimen, and there was a 24% proportional reduction (RR, 0.76; 95% CI, 0.73-0.79) of major coronary events per 1.0-mmol/L reduction in LDL cholesterol, with older age resulting in a lower proportional reduction of major coronary events (P = .009). The researchers also found a proportional reduction of coronary revascularization procedures by 25% (RR, 0.75; 95% CI, 0.73-0.78) and stroke by 16% (RR, 0.84; 95% CI, 0.80-0.89) among patients of any age group receiving statin therapy or more intensive statin regimen, with no significant differences between age groups.
There was a 12% proportional reduction in vascular mortality per 1.0-mmol/L reduction in LDL cholesterol (RR, 0.88; 95% CI, 0.85-0.91), but this statistic did not remain significant after the researchers excluded four trials that included patients with heart failure or who were receiving renal dialysis. After excluding these trials from the overall analysis, the researchers found the smaller proportional reductions persisted for older patients for major coronary events (P = .01) but was no longer significant for major vascular events.
The researchers noted their study was limited by the highly selected patient population, low percentage of patients older than 75 years, including trials with efficacy endpoints where some nonserious adverse events may not have been recorded, and not including some trials in the meta-analysis if they were not part of the CTT.
This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
SOURCE: Fulcher J et al. Lancet. 2019;393:407-15.
Statin therapy appears to reduce the risk of major vascular events for patients of all age groups, but there is less evidence that older patients with evidence of occlusive vascular disease benefit from the treatment, according to a recent meta-analysis of 28 trials from the Cholesterol Treatment Trialists’ Collaboration published in The Lancet.
Statins are “useful and affordable drug[s] that reduce heart attacks and strokes in older patients. Until now there has been an evidence gap and we wanted to look at their efficacy and safety in older people,” Jordan Fulcher, BSc (Med), MBBS, from the Cholesterol Treatment Trialists’ (CTT) Collaboration and the University of Sydney stated in a press release. “Our analysis indicates that major cardiovascular events were reduced by about a fifth, per mmol/L lower LDL cholesterol, by statin therapy across all age groups. Despite previous concerns, we found no adverse effect on cancer or nonvascular mortality in any age group.”
The researchers examined 186,854 participants from 28 CTT trials undergoing statin therapy, of whom 14,483 (8%) were older than 75 years. Patients were divided into six groups based on age and examined the risk of major cardiovascular events such as stroke, coronary revascularization and major coronary events, as well as the incidence of cancer and vascular mortality.
Among all age groups, there was a significant reduction in major vascular events, with a 21% proportional per 1.0-mmol/L reduction in LDL cholesterol (risk ratio, 0.79; 95% confidence interval, 0.77-0.81) among patients receiving statin therapy or a more intensive statin regimen, and there was a 24% proportional reduction (RR, 0.76; 95% CI, 0.73-0.79) of major coronary events per 1.0-mmol/L reduction in LDL cholesterol, with older age resulting in a lower proportional reduction of major coronary events (P = .009). The researchers also found a proportional reduction of coronary revascularization procedures by 25% (RR, 0.75; 95% CI, 0.73-0.78) and stroke by 16% (RR, 0.84; 95% CI, 0.80-0.89) among patients of any age group receiving statin therapy or more intensive statin regimen, with no significant differences between age groups.
There was a 12% proportional reduction in vascular mortality per 1.0-mmol/L reduction in LDL cholesterol (RR, 0.88; 95% CI, 0.85-0.91), but this statistic did not remain significant after the researchers excluded four trials that included patients with heart failure or who were receiving renal dialysis. After excluding these trials from the overall analysis, the researchers found the smaller proportional reductions persisted for older patients for major coronary events (P = .01) but was no longer significant for major vascular events.
The researchers noted their study was limited by the highly selected patient population, low percentage of patients older than 75 years, including trials with efficacy endpoints where some nonserious adverse events may not have been recorded, and not including some trials in the meta-analysis if they were not part of the CTT.
This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
SOURCE: Fulcher J et al. Lancet. 2019;393:407-15.
FROM THE LANCET
Key clinical point: but patients older than 75 years with occlusive vascular disease have a smaller reduction in major coronary events.
Major finding: Major vascular coronary events were reduced by 24% (risk ratio, 0.76; 95% confidence interval, 0.73-0.79) with a decrease in the reduction of coronary events among patients older than 75 years. Study details: A meta-analysis of 28 trials with 186,854 individuals undergoing statin therapy from the Cholesterol Treatment Trialists’ Collaboration.
Disclosures: This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
Source: Fulcher J et al. Lancet. 2019;393:407-15.
Click for Credit: Missed HIV screening opps; aspirin & preeclampsia; more
Here are 5 articles from the February issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term lung function better predicts mortality risk in SSc
To take the posttest, go to: https://bit.ly/2RrRuIY
Expires November 26, 2019
2. Healthier lifestyle in midlife women reduces subclinical carotid atherosclerosis
To take the posttest, go to: https://bit.ly/2TvDH5G
Expires November 28, 2019
3. Three commonly used quick cognitive assessments often yield flawed results
To take the posttest, go to: https://bit.ly/2G1qkHn
Expires November 28, 2019
4. Missed HIV screening opportunities found among subsequently infected youth
To take the posttest, go to: https://bit.ly/2HGa8Nm
Expires November 29, 2019
5. Aspirin appears underused to prevent preeclampsia in SLE patients
To take the posttest, go to: https://bit.ly/2G0dU2v
Expires January 2, 2019
Here are 5 articles from the February issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term lung function better predicts mortality risk in SSc
To take the posttest, go to: https://bit.ly/2RrRuIY
Expires November 26, 2019
2. Healthier lifestyle in midlife women reduces subclinical carotid atherosclerosis
To take the posttest, go to: https://bit.ly/2TvDH5G
Expires November 28, 2019
3. Three commonly used quick cognitive assessments often yield flawed results
To take the posttest, go to: https://bit.ly/2G1qkHn
Expires November 28, 2019
4. Missed HIV screening opportunities found among subsequently infected youth
To take the posttest, go to: https://bit.ly/2HGa8Nm
Expires November 29, 2019
5. Aspirin appears underused to prevent preeclampsia in SLE patients
To take the posttest, go to: https://bit.ly/2G0dU2v
Expires January 2, 2019
Here are 5 articles from the February issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term lung function better predicts mortality risk in SSc
To take the posttest, go to: https://bit.ly/2RrRuIY
Expires November 26, 2019
2. Healthier lifestyle in midlife women reduces subclinical carotid atherosclerosis
To take the posttest, go to: https://bit.ly/2TvDH5G
Expires November 28, 2019
3. Three commonly used quick cognitive assessments often yield flawed results
To take the posttest, go to: https://bit.ly/2G1qkHn
Expires November 28, 2019
4. Missed HIV screening opportunities found among subsequently infected youth
To take the posttest, go to: https://bit.ly/2HGa8Nm
Expires November 29, 2019
5. Aspirin appears underused to prevent preeclampsia in SLE patients
To take the posttest, go to: https://bit.ly/2G0dU2v
Expires January 2, 2019
Subclinical hypothyroidism: When to treat
Whether subclinical hypothyroidism is clinically important and should be treated remains controversial. Studies have differed in their findings, and although most have found this condition to be associated with a variety of adverse outcomes, large randomized controlled trials are needed to clearly demonstrate its clinical impact in various age groups and the benefit of levothyroxine therapy.
Currently, the best practical approach is to base treatment decisions on the magnitude of elevation of thyroid-stimulating hormone (TSH) and whether the patient has thyroid autoantibodies and associated comorbid conditions.
HIGH TSH, NORMAL FREE T4 LEVELS
Subclinical hypothyroidism is defined by elevated TSH along with a normal free thyroxine (T4).1
The hypothalamic-pituitary-thyroid axis is a balanced homeostatic system, and TSH and thyroid hormone levels have an inverse log-linear relation: if free T4 and triiodothyronine (T3) levels go down even a little, TSH levels go up a lot.2
TSH secretion is pulsatile and has a circadian rhythm: serum TSH levels are 50% higher at night and early in the morning than during the rest of the day. Thus, repeated measurements in the same patient can vary by as much as half of the reference range.3
WHAT IS THE UPPER LIMIT OF NORMAL FOR TSH?
The upper limit of normal for TSH, defined as the 97.5th percentile, is approximately 4 or 5 mIU/L depending on the laboratory and the population, but some experts believe it should be lower.3
In favor of a lower upper limit: the distribution of serum TSH levels in the healthy general population does not seem to be a typical bell-shaped Gaussian curve, but rather has a tail at the high end. Some argue that some of the individuals with values in the upper end of the normal range may actually have undiagnosed hypothyroidism and that the upper 97.5th percentile cutoff would be 2.5 mIU/L if these people were excluded.4 Also, TSH levels higher than 2.5 mIU/L have been associated with a higher prevalence of antithyroid antibodies and a higher risk of clinical hypothyroidism.5
On the other hand, lowering the upper limit of normal to 2.5 mIU/L would result in 4 times as many people receiving a diagnosis of subclinical hypothyroidism, or 22 to 28 million people in the United States.4,6 Thus, lowering the cutoff may lead to unnecessary therapy and could even harm from overtreatment.
Another argument against lowering the upper limit of normal for TSH is that, with age, serum TSH levels shift higher.7 The third National Health and Nutrition Education Survey (NHANES III) found that the 97.5th percentile for serum TSH was 3.56 mIU/L for age group 20 to 29 but 7.49 mIU/L for octogenarians.7,8
It has been suggested that the upper limit of normal for TSH be adjusted for age, race, sex, and iodine intake.3 Currently available TSH reference ranges are not adjusted for these variables, and there is not enough evidence to suggest age-appropriate ranges,9 although higher TSH cutoffs for treatment are advised in elderly patients.10 Interestingly, higher TSH in older people has been linked to lower mortality rates in some studies.11
Authors of the NHANES III8 and Hanford Thyroid Disease study12 have proposed a cutoff of 4.1 mIU/L for the upper limit of normal for serum TSH in patients with negative antithyroid antibodies and normal findings on thyroid ultrasonography.
SUBCLINICAL HYPOTHYROIDISM IS COMMON
In different studies, the prevalence of subclinical hypothyroidism has been as low as 4% and as high as 20%.1,8,13 The prevalence is higher in women and increases with age.8 It is higher in iodine-sufficient areas, and it increases in iodine-deficient areas with iodine supplementation.14 Genetics also plays a role, as subclinical hypothyroidism is more common in white people than in African Americans.8
A difficulty in estimating the prevalence is the disagreement about the cutoff for TSH, which may differ from that in the general population in certain subgroups such as adolescents, the elderly, and pregnant women.10,15
A VARIETY OF CAUSES
The most common cause of subclinical hypothyroidism, accounting for 60% to 80% of cases, is Hashimoto (autoimmune) thyroiditis,2 in which thyroid peroxidase antibodies are usually present.2,16
Also important to rule out are false-positive elevations due to substances that interfere with TSH assays (eg, heterophile antibodies, rheumatoid factor, biotin, macro-TSH); reversible causes such as the recovery phase of euthyroid sick syndrome; subacute, painless, or postpartum thyroiditis; central hypo- or hyperthyroidism; and thyroid hormone resistance.
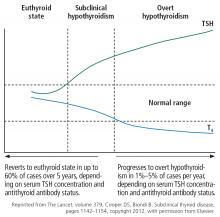
SUBCLINICAL HYPOTHYROIDISM CAN RESOLVE OR PROGRESS
“Subclinical” suggests that the disease is in its early stage, with changes in TSH already apparent but decreases in thyroid hormone levels yet to come.17 And indeed, subclinical hypothyroidism can progress to overt hypothyroidism,18 although it has been reported to resolve spontaneously in half of cases within 2 years,19 typically in patients with TSH values of 4 to 6 mIU/L.20 The rate of progression to overt hypothyroidism is estimated to be 33% to 55% over 10 to 20 years of follow-up.18
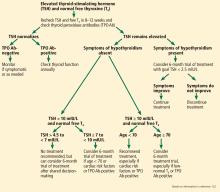
Figure 1 shows the natural history of subclinical hypothyroidism.1
GUIDELINES FOR SCREENING DIFFER
Guidelines differ on screening for thyroid disease in the general population, owing to lack of large-scale randomized controlled trials showing treatment benefit in otherwise-healthy people with mildly elevated TSH values.
Various professional societies have adopted different criteria for aggressive case-finding in patients at risk of thyroid disease. Risk factors include family history of thyroid disease, neck irradiation, partial thyroidectomy, dyslipidemia, atrial fibrillation, unexplained weight loss, hyperprolactinemia, autoimmune disorders, and use of medications affecting thyroid function.23
The US Preventive Services Task Force in 2014 found insufficient evidence on the benefits and harms of screening.24
The American Thyroid Association (ATA) recommends screening adults starting at age 35, with repeat testing every 5 years in patients who have no signs or symptoms of hypothyroidism, and more frequently in those who do.25
The American Association of Clinical Endocrinologists recommends screening in women and older patients. Their guidelines and those of the ATA also suggest screening people at high risk of thyroid disease due to risk factors such as history of autoimmune diseases, neck irradiation, or medications affecting thyroid function.26
The American Academy of Family Physicians recommends screening after age 60.18
The American College of Physicians recommends screening patients over age 50 who have symptoms.18
Our approach. Although evidence is lacking to recommend routine screening in adults, aggressive case-finding and treatment in patients at risk of thyroid disease can, we believe, offset the risks associated with subclinical hypothyroidism.24
CLINICAL PRESENTATION
About 70% of patients with subclinical hypothyroidism have no symptoms.13
Tiredness was more common in subclinical hypothyroid patients with TSH levels lower than 10 mIU/L compared with euthyroid controls in 1 study, but other studies have been unable to replicate this finding.27,28
Other frequently reported symptoms include dry skin, cognitive slowing, poor memory, muscle weakness, cold intolerance, constipation, puffy eyes, and hoarseness.13
The evidence in favor of levothyroxine therapy to improve symptoms in subclinical hypothyroidism has varied, with some studies showing an improvement in symptom scores compared with placebo, while others have not shown any benefit.29–31
In one study, the average TSH value for patients whose symptoms did not improve with therapy was 4.6 mIU/L.31 An explanation for the lack of effect in this group may be that the TSH values for these patients were in the high-normal range. Also, because most subclinical hypothyroid patients have no symptoms, it is difficult to ascertain symptomatic improvement. Though it is possible to conclude that levothyroxine therapy has a limited role in this group, it is important to also consider the suggestive evidence that untreated subclinical hypothyroidism may lead to increased morbidity and mortality.
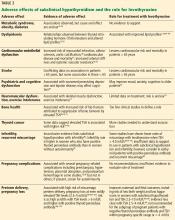
ADVERSE EFFECTS OF SUBCLINICAL HYPOTHYROIDISM, EFFECTS OF THERAPY
INDIVIDUALIZED MANAGEMENT AND SHARED DECISION-MAKING
The management of subclinical hypothyroidism should be individualized on the basis of extent of thyroid dysfunction, comorbid conditions, risk factors, and patient preference.118 Shared decision-making is key, weighing the risks and benefits of levothyroxine treatment and the patient’s goals.
The risks of treatment should be kept in mind and explained to the patient. Levothyroxine has a narrow therapeutic range, causing a possibility of overreplacement, and a half-life of 7 days that can cause dosing errors to have longer effect.118,119
Adherence can be a challenge. The drug needs to be taken on an empty stomach because foods and supplements interfere with its absorption.118,120 In addition, the cost of medication, frequent biochemical monitoring, and possible need for titration can add to financial burden.
When choosing the dose, one should consider the degree of hypothyroidism or TSH elevation and the patient’s weight, and adjust the dose gently.
If the TSH is high-normal
It is proposed that a TSH range of 3 to 5 mIU/L overlaps with normal thyroid function in a great segment of the population, and at this level it is probably not associated with clinically significant consequences. For these reasons, levothyroxine therapy is not thought to be beneficial for those with TSH in this range.
Pollock et al121 found that, in patients with symptoms suggesting hypothyroidism and TSH values in the upper end of the normal range, there was no improvement in cognitive function or psychological well-being after 12 weeks of levothyroxine therapy.
However, due to the concern for possible adverse maternal and fetal outcomes and low IQ in children of pregnant patients with subclinical hypothyroidism, levothyroxine therapy is advised in those who are pregnant or planning pregnancy who have TSH levels higher than 2.5 mIU/L, especially if they have thyroid peroxidase antibody. Levothyroxine therapy is not recommended for pregnant patients with negative thyroid peroxidase antibody and TSH within the pregnancy-specific range or less than 4 mIU/L if the reference ranges are unavailable.
Keep in mind that, even at these TSH values, there is risk of progression to overt hypothyroidism, especially in the presence of thyroid peroxidase antibody, so patients in this group should be monitored closely.
If TSH is mildly elevated
The evidence to support levothyroxine therapy in patients with subclinical hypothyroidism with TSH levels less than 10 mIU/L remains inconclusive, and the decision to treat should be based on clinical judgment.2 The studies that have looked at the benefit of treating subclinical hypothyroidism in terms of cardiac, neuromuscular, cognitive, and neuropsychiatric outcomes have included patients with a wide range of TSH levels, and some of these studies were not stratified on the basis of degree of TSH elevation.
The risk that subclinical hypothyroidism will progress to overt hypothyroidism in patients with TSH higher than 8 mIU/L is high, and in 70% of these patients, the TSH level rises to more than 10 mIU/L within 4 years. Early treatment should be considered if the TSH is higher than 7 or 8 mIU/L.
If TSH is higher than 10 mIU/L
Figure 2 outlines an algorithmic approach to subclinical hypothyroidism in nonpregnant patients as suggested by Peeters.122
- Cooper DS, Biondi B. Subclinical thyroid disease. Lancet 2012; 379(9821):1142–1154. doi:10.1016/S0140-6736(11)60276-6
- Fatourechi V. Subclinical hypothyroidism: an update for primary care physicians. Mayo Clin Proc 2009; 84(1):65–71. doi:10.4065/84.1.65
- Laurberg P, Andersen S, Carle A, Karmisholt J, Knudsen N, Pedersen IB. The TSH upper reference limit: where are we at? Nat Rev Endocrinol 2011; 7(4):232–239. doi:10.1038/nrendo.2011.13
- Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab 2005; 90(9):5483–5488. doi:10.1210/jc.2005-0455
- Spencer CA, Hollowell JG, Kazarosyan M, Braverman LE. National Health and Nutrition Examination Survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab 2007; 92(11):4236–4240. doi:10.1210/jc.2007-0287
- Fatourechi V, Klee GG, Grebe SK, et al. Effects of reducing the upper limit of normal TSH values. JAMA 2003; 290(24):3195–3196. doi:10.1001/jama.290.24.3195-a
- Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 2007; 92(12):4575–4582. doi:10.1210/jc.2007-1499
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002; 87(2):489–499. doi:10.1210/jcem.87.2.8182
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014; 24(12):1670–1751. doi:10.1089/thy.2014.0028
- Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc 2015; 63(8):1663–1673. doi:10.1111/jgs.13532
- Razvi S, Shakoor A, Vanderpump M, Weaver JU, Pearce SH. The influence of age on the relationship between subclinical hypothyroidism and ischemic heart disease: a metaanalysis. J Clin Endocrinol Metab 2008; 93(8):2998–3007. doi:10.1210/jc.2008-0167
- Hamilton TE, Davis S, Onstad L, Kopecky KJ. Thyrotropin levels in a population with no clinical, autoantibody, or ultrasonographic evidence of thyroid disease: implications for the diagnosis of subclinical hypothyroidism. J Clin Endocrinol Metab 2008; 93(4):1224–1230. doi:10.1210/jc.2006-2300
- Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med 2000; 160(4):526–534. pmid:10695693
- Teng W, Shan Z, Teng X, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med 2006; 354(26):2783–2793. doi:10.1056/NEJMoa054022
- Negro R, Stagnaro-Green A. Diagnosis and management of subclinical hypothyroidism in pregnancy. BMJ 2014; 349:g4929. doi:10.1136/bmj.g4929
- Baumgartner C, Blum MR, Rodondi N. Subclinical hypothyroidism: summary of evidence in 2014. Swiss Med Wkly 2014; 144:w14058. doi:10.4414/smw.2014.14058
- Stedman TL. Stedman’s Medical Dictionary. 28th ed. Baltimore, MD: Lippincott Williams and Wilkins; 2006.
- Raza SA, Mahmood N. Subclinical hypothyroidism: controversies to consensus. Indian J Endocrinol Metab 2013; 17(suppl 3):S636–S642. doi:10.4103/2230-8210.123555
- Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab 2002; 87(7):3221–3226. doi:10.1210/jcem.87.7.8678
- Diez JJ, Iglesias P, Burman KD. Spontaneous normalization of thyrotropin concentrations in patients with subclinical hypothyroidism. J Clin Endocrinol Metab 2005; 90(7):4124–4127. doi:10.1210/jc.2005-0375
- Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol (Oxf) 1995; 43(1):55–68. pmid:7641412
- Li Y, Teng D, Shan Z, et al. Antithyroperoxidase and antithyroglobulin antibodies in a five-year follow-up survey of populations with different iodine intakes. J Clin Endocrinol Metab 2008; 93(5):1751–1757. doi:10.1210/jc.2007-2368
- Hennessey JV, Klein I, Woeber KA, Cobin R, Garber JR. Aggressive case finding: a clinical strategy for the documentation of thyroid dysfunction. Ann Intern Med 2015; 163(4):311–312. doi:10.7326/M15-0762
- Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: an evidence review for the US.Preventive Services Task Force. Ann Intern Med 2015; 162(1):35–45. doi:10.7326/M14-1456
- Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med 2000; 160(11):1573–1575. pmid:10847249
- Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 2012; 18(6):988–1028. doi:10.4158/EP12280.GL
- Jorde R, Waterloo K, Storhaug H, Nyrnes A, Sundsfjord J, Jenssen TG. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab 2006; 91(1):145–153. doi:10.1210/jc.2005-1775
- Joffe RT, Pearce EN, Hennessey JV, Ryan JJ, Stern RA. Subclinical hypothyroidism, mood, and cognition in older adults: a review. Int J Geriatr Psychiatry 2013; 28(2):111–118. doi:10.1002/gps.3796
- Cooper DS, Halpern R, Wood LC, Levin AA, Ridgway EC. L-thyroxine therapy in subclinical hypothyroidism. A double-blind, placebo-controlled trial. Ann Intern Med 1984; 101(1):18–24. pmid:6428290
- Nystrom E, Caidahl K, Fager G, Wikkelso C, Lundberg PA, Lindstedt G. A double-blind cross-over 12-month study of L-thyroxine treatment of women with ‘subclinical’ hypothyroidism. Clin Endocrinol (Oxf) 1988; 29(1):63–75. pmid:3073880
- Monzani F, Del Guerra P, Caraccio N, et al. Subclinical hypothyroidism: neurobehavioral features and beneficial effect of L-thyroxine treatment. Clin Investig 1993; 71(5):367–371. pmid:8508006
- Biondi B. Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab 2010; 95(8):3614–3617. doi:10.1210/jc.2010-1245
- Erdogan M, Canataroglu A, Ganidagli S, Kulaksizoglu M. Metabolic syndrome prevalence in subclinic and overt hypothyroid patients and the relation among metabolic syndrome parameters. J Endocrinol Invest 2011; 34(7):488–492. doi:10.3275/7202
- Javed Z, Sathyapalan T. Levothyroxine treatment of mild subclinical hypothyroidism: a review of potential risks and benefits. Ther Adv Endocrinol Metab 2016; 7(1):12–23. doi:10.1177/2042018815616543
- Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J 2013; 2(4):215–228. doi:10.1159/000356507
- Wang C. The relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res 2013; 2013:390534. doi:10.1155/2013/390534
- Razvi S, Weaver JU, Vanderpump MP, Pearce SH. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: reanalysis of the Whickham survey cohort. J Clin Endocrinol Metab 2010; 95(4):1734–1740. doi:10.1210/jc.2009-1749
- Bindels AJ, Westendorp RG, Frolich M, Seidell JC, Blokstra A, Smelt AH. The prevalence of subclinical hypothyroidism at different total plasma cholesterol levels in middle aged men and women: a need for case-finding? Clin Endocrinol (Oxf) 1999; 50(2):217–220. pmid:10396365
- Pearce EN. Hypothyroidism and dyslipidemia: modern concepts and approaches. Curr Cardiol Rep 2004; 6(6):451–456. pmid:15485607
- Pearce EN. Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab 2012; 97(2):326–333. doi:10.1210/jc.2011-2532
- Rizos CV, Elisaf MS, Liberopoulos EN. Effects of thyroid dysfunction on lipid profile. Open Cardiovasc Med J 2011; 5:76–84. doi:10.2174/1874192401105010076
- Peppa M, Betsi G, Dimitriadis G. Lipid abnormalities and cardiometabolic risk in patients with overt and subclinical thyroid disease. J Lipids 2011; 2011:575840. doi:10.1155/2011/575840
- Asvold BO, Vatten LJ, Nilsen TI, Bjoro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. the HUNT study. Eur J Endocrinol 2007;1 56(2):181–186. doi:10.1530/eje.1.02333
- Danese MD, Ladenson PW, Meinert CL, Powe NR. Clinical review 115: effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: a quantitative review of the literature. J Clin Endocrinol Metab 2000; 85(9):2993–3001. doi:10.1210/jcem.85.9.6841
- Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of L-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab 2007; 92(5):1715–1723. doi:10.1210/jc.2006-1869
- Abreu IM, Lau E, de Sousa Pinto B, Carvalho D. Subclinical hypothyroidism: to treat or not to treat, that is the question! A systematic review with meta-analysis on lipid profile. Endocr Connect 2017; 6(3):188–199. doi:10.1530/EC-17-0028
- Robison CD, Bair TL, Horne BD, et al. Hypothyroidism as a risk factor for statin intolerance. J Clin Lipidol 2014; 8(4):401–407. doi:10.1016/j.jacl.2014.05.005
- Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam study. Ann Intern Med 2000; 132(4):270–278. pmid:10681281
- Boekholdt SM, Titan SM, Wiersinga WM, et al. Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol (Oxf) 2010; 72(3):404–410. doi:10.1111/j.1365-2265.2009.03640.x
- Andersen MN, Olsen AM, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One 2015; 10(6):e0129793. doi:10.1371/journal.pone.0129793
- Biondi B. Cardiovascular effects of mild hypothyroidism. Thyroid 2007; 17(7):625–630. doi:10.1089/thy.2007.0158
- Brenta G, Mutti LA, Schnitman M, Fretes O, Perrone A, Matute ML. Assessment of left ventricular diastolic function by radionuclide ventriculography at rest and exercise in subclinical hypothyroidism, and its response to L-thyroxine therapy. Am J Cardiol 2003; 91(11):1327–1330. pmid:12767425
- Taddei S, Caraccio N, Virdis A, et al. Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: beneficial effect of levothyroxine therapy. J Clin Endocrinol Metab 2003; 88(8):3731–3737. doi:10.1210/jc.2003-030039
- Gao N, Zhang W, Zhang YZ, Yang Q, Chen SH. Carotid intima-media thickness in patients with subclinical hypothyroidism: a meta-analysis. Atherosclerosis 2013; 227(1):18–25. doi:10.1016/j.atherosclerosis.2012.10.070
- Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 2008; 29(1):76–131. doi:10.1210/er.2006-0043
- Chaker L, Baumgartner C, den Elzen WP, et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of stroke events and fatal stroke: an individual participant data analysis. J Clin Endocrinol Metab 2015; 100(6):2181–2191. doi:10.1210/jc.2015-1438
- Monzani F, Di Bello V, Caraccio N, et al. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. J Clin Endocrinol Metab 2001; 86(3):1110–1115. doi:10.1210/jcem.86.3.7291
- Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet 2001; 358(9285):861-865. doi:10.1016/S0140-6736(01)06067-6
- Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med 2012; 172(10):811–817. doi:10.1001/archinternmed.2012.1159
- Pasqualetti G, Tognini S, Polini A, Caraccio N, Monzani F. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab 2013; 98(6):2256–2266. doi:10.1210/jc.2012-3818
- Mooijaart SP, IEMO 80-plus Thyroid Trial Collaboration. Subclinical thyroid disorders. Lancet 2012; 380(9839):335. doi:10.1016/S0140-6736(12)61241-0
- Rodondi N, Bauer DC. Subclinical hypothyroidism and cardiovascular risk: how to end the controversy. J Clin Endocrinol Metab 2013; 98(6):2267–2269. doi:10.1210/jc.2013-1875
- Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med 2005; 165(21):2460–2466. doi:10.1001/archinte.165.21.2460
- Rodondi N, Bauer DC, Cappola AR, et al. Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure. The Cardiovascular Health study. J Am Coll Cardiol 2008; 52(14):1152–1159. doi:10.1016/j.jacc.2008.07.009
- Haggerty JJ Jr, Garbutt JC, Evans DL, et al. Subclinical hypothyroidism: a review of neuropsychiatric aspects. Int J Psychiatry Med 1990; 20(2):193–208. doi:10.2190/ADLY-1UU0-1A8L-HPXY
- Baldini IM, Vita A, Mauri MC, et al. Psychopathological and cognitive features in subclinical hypothyroidism. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21(6):925–935. pmid:9380789
- del Ser Quijano T, Delgado C, Martinez Espinosa S, Vazquez C. Cognitive deficiency in mild hypothyroidism. Neurologia 2000; 15(5):193–198. Spanish. pmid:10850118
- Correia N, Mullally S, Cooke G, et al. Evidence for a specific defect in hippocampal memory in overt and subclinical hypothyroidism. J Clin Endocrinol Metab 2009; 94(10):3789–3797. doi:10.1210/jc.2008-2702
- Aghili R, Khamseh ME, Malek M, et al. Changes of subtests of Wechsler memory scale and cognitive function in subjects with subclinical hypothyroidism following treatment with levothyroxine. Arch Med Sci 2012; 8(6):1096–1101. doi:10.5114/aoms.2012.32423
- Pasqualetti G, Pagano G, Rengo G, Ferrara N, Monzani F. Subclinical hypothyroidism and cognitive impairment: systematic review and meta-analysis. J Clin Endocrinol Metab 2015; 100(11):4240–4248. doi:10.1210/jc.2015-2046
- Christ-Crain M, Meier C, Huber PR, Staub J, Muller B. Effect of L-thyroxine replacement therapy on surrogate markers of skeletal and cardiac function in subclinical hypothyroidism. Endocrinologist 2004; 14(3):161–166. doi:10.1097/01.ten.0000127932.31710.4f
- Brennan MD, Powell C, Kaufman KR, Sun PC, Bahn RS, Nair KS. The impact of overt and subclinical hyperthyroidism on skeletal muscle. Thyroid 2006; 16(4):375–380. doi:10.1089/thy.2006.16.375
- Reuters VS, Teixeira Pde F, Vigario PS, et al. Functional capacity and muscular abnormalities in subclinical hypothyroidism. Am J Med Sci 2009; 338(4):259–263. doi:10.1097/MAJ.0b013e3181af7c7c
- Mainenti MR, Vigario PS, Teixeira PF, Maia MD, Oliveira FP, Vaisman M. Effect of levothyroxine replacement on exercise performance in subclinical hypothyroidism. J Endocrinol Invest 2009; 32(5):470–473. doi:10.3275/6106
- Lankhaar JA, de Vries WR, Jansen JA, Zelissen PM, Backx FJ. Impact of overt and subclinical hypothyroidism on exercise tolerance: a systematic review. Res Q Exerc Sport 2014; 85(3):365–389. doi:10.1080/02701367.2014.930405
- Lee JS, Buzkova P, Fink HA, et al. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch Intern Med 2010; 170(21):1876–1883. doi:10.1001/archinternmed.2010.424
- Svare A, Nilsen TI, Asvold BO, et al. Does thyroid function influence fracture risk? Prospective data from the HUNT2 study, Norway. Eur J Endocrinol 2013; 169(6):845–852. doi:10.1530/EJE-13-0546
- Di Mase R, Cerbone M, Improda N, et al. Bone health in children with long-term idiopathic subclinical hypothyroidism. Ital J Pediatr 2012; 38:56. doi:10.1186/1824-7288-38-56
- Boelaert K. The association between serum TSH concentration and thyroid cancer. Endocr Relat Cancer 2009; 16(4):1065–1072. doi:10.1677/ERC-09-0150
- Haymart MR, Glinberg SL, Liu J, Sippel RS, Jaume JC, Chen H. Higher serum TSH in thyroid cancer patients occurs independent of age and correlates with extrathyroidal extension. Clin Endocrinol (Oxf) 2009; 71(3):434–439. doi:10.1111/j.1365-2265.2008.03489.x
- Fiore E, Vitti P. Serum TSH and risk of papillary thyroid cancer in nodular thyroid disease. J Clin Endocrinol Metab 2012; 97(4):1134–1145. doi:10.1210/jc.2011-2735
- Fiore E, Rago T, Provenzale MA, et al. L-thyroxine-treated patients with nodular goiter have lower serum TSH and lower frequency of papillary thyroid cancer: results of a cross-sectional study on 27,914 patients. Endocr Relat Cancer 2010; 17(1):231–239. doi:10.1677/ERC-09-0251
- Hercbergs AH, Ashur-Fabian O, Garfield D. Thyroid hormones and cancer: clinical studies of hypothyroidism in oncology. Curr Opin Endocrinol Diabetes Obes 2010; 17(5):432–436. doi:10.1097/MED.0b013e32833d9710
- Thvilum M, Brandt F, Brix TH, Hegedus L. A review of the evidence for and against increased mortality in hypothyroidism. Nat Rev Endocrinol 2012; 8(7):417–424. doi:10.1038/nrendo.2012.29
- Stott DJ, Rodondi N, Kearney PM, et al; TRUST Study Group. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med 2017; 376(26):2534–2544. doi:10.1056/NEJMoa1603825
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril 2015; 104(3):545–753. doi:10.1016/j.fertnstert.2015.05.028
- Stagnaro-Green A, Abalovich M, Alexander E, et al; American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011; 21(10):1081–1125. doi:10.1089/thy.2011.0087
- Goldsmith RE, Sturgis SH, Lerman J, Stanbury JB. The menstrual pattern in thyroid disease. J Clin Endocrinol Metab. 1952; 12(7):846-855. doi:10.1210/jcem-12-7-846
- Plowden TC, Schisterman EF, Sjaarda LA, et al. Subclinical hypothyroidism and thyroid autoimmunity are not associated with fecundity, pregnancy loss, or live birth. J Clin Endocrinol Metab 2016; 101(6):2358–2365. doi:10.1210/jc.2016-1049
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017; 27(3):315–389. doi:10.1089/thy.2016.0457
- Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab 2006; 91(7):2587–2591. doi:10.1210/jc.2005-1603
- Panesar NS, Li CY, Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem 2001; 38(pt 4):329–332. doi:10.1258/0004563011900830
- Lepoutre T, Debieve F, Gruson D, Daumerie C. Reduction of miscarriages through universal screening and treatment of thyroid autoimmune diseases. Gynecol Obstet Invest 2012; 74(4):265–273. doi:10.1159/000343759
- De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97(8):2543–2565. doi:10.1210/jc.2011-2803
- Crawford NM, Steiner AZ. Thyroid autoimmunity and reproductive function. Semin Reprod Med 2016; 34(6):343–350. doi:10.1055/s-0036-1593485
- Maraka S, Ospina NM, O’Keeffe DT, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid 2016; 26(4):580–590. doi:10.1089/thy.2015.0418
- Wiles KS, Jarvis S, Nelson-Piercy C. Are we overtreating subclinical hypothyroidism in pregnancy? BMJ 2015; 351:h4726. doi:10.1136/bmj.h4726
- Tudela CM, Casey BM, McIntire DD, Cunningham FG. Relationship of subclinical thyroid disease to the incidence of gestational diabetes. Obstet Gynecol 2012; 119(5):983–988. doi:10.1097/AOG.0b013e318250aeeb
- Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European Thyroid Association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J 2014; 3(2):76–94. doi:10.1159/000362597
- Karakosta P, Alegakis D, Georgiou V, et al. Thyroid dysfunction and autoantibodies in early pregnancy are associated with increased risk of gestational diabetes and adverse birth outcomes. J Clin Endocrinol Metab 2012; 97(12):4464–4472. doi:10.1210/jc.2012-2540
- Toulis KA, Stagnaro-Green A, Negro R. Maternal subclinical hypothyroidsm and gestational diabetes mellitus: a meta-analysis. Endocr Pract 2014; 20(7):703–714. doi:10.4158/EP13440.RA
- van den Boogaard E, Vissenberg R, Land JA, et al. Significance of subclinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update 2011; 17(5):605–619. doi:10.1093/humupd/dmr024
- Wilson KL, Casey BM, McIntire DD, Halvorson LM, Cunningham FG. Subclinical thyroid disease and the incidence of hypertension in pregnancy. Obstet Gynecol 2012; 119(2 Pt 1):315–320. doi:10.1097/AOG.0b013e318240de6a
- Ashoor G, Maiz N, Rotas M, Jawdat F, Nicolaides KH. Maternal thyroid function at 11 to 13 weeks of gestation and subsequent fetal death. Thyroid 2010; 20(9):989–993. doi:10.1089/thy.2010.0058
- Casey BM, Dashe JS, Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol 2005; 105(2):239–245. doi:10.1097/01.AOG.0000152345.99421.22
- Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab 2010; 95(9):E44–E48. doi:10.1210/jc.2010-0340
- Su PY, Huang K, Hao JH, et al. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab 2011; 96(10):3234–3241. doi:10.1210/jc.2011-0274
- Allan WC, Haddow JE, Palomaki GE, et al. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen 2000; 7(3):127–130. doi:10.1136/jms.7.3.127
- Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol 2009; 160(6):985–991. doi:10.1530/EJE-08-0953
- Korevaar TI, Medici M, de Rijke YB, et al. Ethnic differences in maternal thyroid parameters during pregnancy: the generation R study. J Clin Endocrinol Metab 2013; 98(9):3678–3686. doi:10.1210/jc.2013-2005
- Cleary-Goldman J, Malone FD, Lambert-Messerlian G, et al. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol 2008; 112(1):85–92. doi:10.1097/AOG.0b013e3181788dd7
- Li Y, Shan Z, Teng W, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin Endocrinol (Oxf) 2010; 72(6):825–829. doi:10.1111/j.1365-2265.2009.03743.x
- Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 1999; 341(8):549–555. doi:10.1056/NEJM199908193410801
- Henrichs J, Bongers-Schokking JJ, Schenk JJ, et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab 2010; 95(9):4227–4234. doi:10.1210/jc.2010-0415
- Behrooz HG, Tohidi M, Mehrabi Y, Behrooz EG, Tehranidoost M, Azizi F. Subclinical hypothyroidism in pregnancy: intellectual development of offspring. Thyroid 2011; 21(10):1143–1147. doi:10.1089/thy.2011.0053
- Julvez J, Alvarez-Pedrerol M, Rebagliato M, et al. Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiology 2013; 24(1):150–157. doi:10.1097/EDE.0b013e318276ccd3
- Casey BM, Thom EA, Peaceman AM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med 2017; 376(9):815–825. doi:10.1056/NEJMoa1606205
- Burns RB, Bates CK, Hartzband P, Smetana GW. Should we treat for subclinical hypothyroidism?: Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med 2016; 164(11):764–770. doi:10.7326/M16-0857
- Kucukler FK, Akbaba G, Arduc A, Simsek Y, Guler S. Evaluation of the common mistakes made by patients in the use of levothyroxine. Eur J Intern Med 2014; 25(9):e107–e108. doi:10.1016/j.ejim.2014.09.002
- McMillan M, Rotenberg KS, Vora K, et al. Comorbidities, concomitant medications, and diet as factors affecting levothyroxine therapy: results of the CONTROL surveillance project. Drugs R D 2016; 16(1):53–68. doi:10.1007/s40268-015-0116-6
- Pollock MA, Sturrock A, Marshall K, et al. Thyroxine treatment in patients with symptoms of hypothyroidism but thyroid function tests within the reference range: Randomised double blind placebo controlled crossover trial. BMJ 2001; 323(7318):891–895. pmid:11668132
- Peeters RP. Subclinical hypothyroidism. N Engl J Med 2017; 376(26):2556–2565. doi:10.1056/NEJMcp1611144
Whether subclinical hypothyroidism is clinically important and should be treated remains controversial. Studies have differed in their findings, and although most have found this condition to be associated with a variety of adverse outcomes, large randomized controlled trials are needed to clearly demonstrate its clinical impact in various age groups and the benefit of levothyroxine therapy.
Currently, the best practical approach is to base treatment decisions on the magnitude of elevation of thyroid-stimulating hormone (TSH) and whether the patient has thyroid autoantibodies and associated comorbid conditions.
HIGH TSH, NORMAL FREE T4 LEVELS
Subclinical hypothyroidism is defined by elevated TSH along with a normal free thyroxine (T4).1
The hypothalamic-pituitary-thyroid axis is a balanced homeostatic system, and TSH and thyroid hormone levels have an inverse log-linear relation: if free T4 and triiodothyronine (T3) levels go down even a little, TSH levels go up a lot.2
TSH secretion is pulsatile and has a circadian rhythm: serum TSH levels are 50% higher at night and early in the morning than during the rest of the day. Thus, repeated measurements in the same patient can vary by as much as half of the reference range.3
WHAT IS THE UPPER LIMIT OF NORMAL FOR TSH?
The upper limit of normal for TSH, defined as the 97.5th percentile, is approximately 4 or 5 mIU/L depending on the laboratory and the population, but some experts believe it should be lower.3
In favor of a lower upper limit: the distribution of serum TSH levels in the healthy general population does not seem to be a typical bell-shaped Gaussian curve, but rather has a tail at the high end. Some argue that some of the individuals with values in the upper end of the normal range may actually have undiagnosed hypothyroidism and that the upper 97.5th percentile cutoff would be 2.5 mIU/L if these people were excluded.4 Also, TSH levels higher than 2.5 mIU/L have been associated with a higher prevalence of antithyroid antibodies and a higher risk of clinical hypothyroidism.5
On the other hand, lowering the upper limit of normal to 2.5 mIU/L would result in 4 times as many people receiving a diagnosis of subclinical hypothyroidism, or 22 to 28 million people in the United States.4,6 Thus, lowering the cutoff may lead to unnecessary therapy and could even harm from overtreatment.
Another argument against lowering the upper limit of normal for TSH is that, with age, serum TSH levels shift higher.7 The third National Health and Nutrition Education Survey (NHANES III) found that the 97.5th percentile for serum TSH was 3.56 mIU/L for age group 20 to 29 but 7.49 mIU/L for octogenarians.7,8
It has been suggested that the upper limit of normal for TSH be adjusted for age, race, sex, and iodine intake.3 Currently available TSH reference ranges are not adjusted for these variables, and there is not enough evidence to suggest age-appropriate ranges,9 although higher TSH cutoffs for treatment are advised in elderly patients.10 Interestingly, higher TSH in older people has been linked to lower mortality rates in some studies.11
Authors of the NHANES III8 and Hanford Thyroid Disease study12 have proposed a cutoff of 4.1 mIU/L for the upper limit of normal for serum TSH in patients with negative antithyroid antibodies and normal findings on thyroid ultrasonography.
SUBCLINICAL HYPOTHYROIDISM IS COMMON
In different studies, the prevalence of subclinical hypothyroidism has been as low as 4% and as high as 20%.1,8,13 The prevalence is higher in women and increases with age.8 It is higher in iodine-sufficient areas, and it increases in iodine-deficient areas with iodine supplementation.14 Genetics also plays a role, as subclinical hypothyroidism is more common in white people than in African Americans.8
A difficulty in estimating the prevalence is the disagreement about the cutoff for TSH, which may differ from that in the general population in certain subgroups such as adolescents, the elderly, and pregnant women.10,15
A VARIETY OF CAUSES
The most common cause of subclinical hypothyroidism, accounting for 60% to 80% of cases, is Hashimoto (autoimmune) thyroiditis,2 in which thyroid peroxidase antibodies are usually present.2,16
Also important to rule out are false-positive elevations due to substances that interfere with TSH assays (eg, heterophile antibodies, rheumatoid factor, biotin, macro-TSH); reversible causes such as the recovery phase of euthyroid sick syndrome; subacute, painless, or postpartum thyroiditis; central hypo- or hyperthyroidism; and thyroid hormone resistance.
SUBCLINICAL HYPOTHYROIDISM CAN RESOLVE OR PROGRESS
“Subclinical” suggests that the disease is in its early stage, with changes in TSH already apparent but decreases in thyroid hormone levels yet to come.17 And indeed, subclinical hypothyroidism can progress to overt hypothyroidism,18 although it has been reported to resolve spontaneously in half of cases within 2 years,19 typically in patients with TSH values of 4 to 6 mIU/L.20 The rate of progression to overt hypothyroidism is estimated to be 33% to 55% over 10 to 20 years of follow-up.18
Figure 1 shows the natural history of subclinical hypothyroidism.1
GUIDELINES FOR SCREENING DIFFER
Guidelines differ on screening for thyroid disease in the general population, owing to lack of large-scale randomized controlled trials showing treatment benefit in otherwise-healthy people with mildly elevated TSH values.
Various professional societies have adopted different criteria for aggressive case-finding in patients at risk of thyroid disease. Risk factors include family history of thyroid disease, neck irradiation, partial thyroidectomy, dyslipidemia, atrial fibrillation, unexplained weight loss, hyperprolactinemia, autoimmune disorders, and use of medications affecting thyroid function.23
The US Preventive Services Task Force in 2014 found insufficient evidence on the benefits and harms of screening.24
The American Thyroid Association (ATA) recommends screening adults starting at age 35, with repeat testing every 5 years in patients who have no signs or symptoms of hypothyroidism, and more frequently in those who do.25
The American Association of Clinical Endocrinologists recommends screening in women and older patients. Their guidelines and those of the ATA also suggest screening people at high risk of thyroid disease due to risk factors such as history of autoimmune diseases, neck irradiation, or medications affecting thyroid function.26
The American Academy of Family Physicians recommends screening after age 60.18
The American College of Physicians recommends screening patients over age 50 who have symptoms.18
Our approach. Although evidence is lacking to recommend routine screening in adults, aggressive case-finding and treatment in patients at risk of thyroid disease can, we believe, offset the risks associated with subclinical hypothyroidism.24
CLINICAL PRESENTATION
About 70% of patients with subclinical hypothyroidism have no symptoms.13
Tiredness was more common in subclinical hypothyroid patients with TSH levels lower than 10 mIU/L compared with euthyroid controls in 1 study, but other studies have been unable to replicate this finding.27,28
Other frequently reported symptoms include dry skin, cognitive slowing, poor memory, muscle weakness, cold intolerance, constipation, puffy eyes, and hoarseness.13
The evidence in favor of levothyroxine therapy to improve symptoms in subclinical hypothyroidism has varied, with some studies showing an improvement in symptom scores compared with placebo, while others have not shown any benefit.29–31
In one study, the average TSH value for patients whose symptoms did not improve with therapy was 4.6 mIU/L.31 An explanation for the lack of effect in this group may be that the TSH values for these patients were in the high-normal range. Also, because most subclinical hypothyroid patients have no symptoms, it is difficult to ascertain symptomatic improvement. Though it is possible to conclude that levothyroxine therapy has a limited role in this group, it is important to also consider the suggestive evidence that untreated subclinical hypothyroidism may lead to increased morbidity and mortality.
ADVERSE EFFECTS OF SUBCLINICAL HYPOTHYROIDISM, EFFECTS OF THERAPY
INDIVIDUALIZED MANAGEMENT AND SHARED DECISION-MAKING
The management of subclinical hypothyroidism should be individualized on the basis of extent of thyroid dysfunction, comorbid conditions, risk factors, and patient preference.118 Shared decision-making is key, weighing the risks and benefits of levothyroxine treatment and the patient’s goals.
The risks of treatment should be kept in mind and explained to the patient. Levothyroxine has a narrow therapeutic range, causing a possibility of overreplacement, and a half-life of 7 days that can cause dosing errors to have longer effect.118,119
Adherence can be a challenge. The drug needs to be taken on an empty stomach because foods and supplements interfere with its absorption.118,120 In addition, the cost of medication, frequent biochemical monitoring, and possible need for titration can add to financial burden.
When choosing the dose, one should consider the degree of hypothyroidism or TSH elevation and the patient’s weight, and adjust the dose gently.
If the TSH is high-normal
It is proposed that a TSH range of 3 to 5 mIU/L overlaps with normal thyroid function in a great segment of the population, and at this level it is probably not associated with clinically significant consequences. For these reasons, levothyroxine therapy is not thought to be beneficial for those with TSH in this range.
Pollock et al121 found that, in patients with symptoms suggesting hypothyroidism and TSH values in the upper end of the normal range, there was no improvement in cognitive function or psychological well-being after 12 weeks of levothyroxine therapy.
However, due to the concern for possible adverse maternal and fetal outcomes and low IQ in children of pregnant patients with subclinical hypothyroidism, levothyroxine therapy is advised in those who are pregnant or planning pregnancy who have TSH levels higher than 2.5 mIU/L, especially if they have thyroid peroxidase antibody. Levothyroxine therapy is not recommended for pregnant patients with negative thyroid peroxidase antibody and TSH within the pregnancy-specific range or less than 4 mIU/L if the reference ranges are unavailable.
Keep in mind that, even at these TSH values, there is risk of progression to overt hypothyroidism, especially in the presence of thyroid peroxidase antibody, so patients in this group should be monitored closely.
If TSH is mildly elevated
The evidence to support levothyroxine therapy in patients with subclinical hypothyroidism with TSH levels less than 10 mIU/L remains inconclusive, and the decision to treat should be based on clinical judgment.2 The studies that have looked at the benefit of treating subclinical hypothyroidism in terms of cardiac, neuromuscular, cognitive, and neuropsychiatric outcomes have included patients with a wide range of TSH levels, and some of these studies were not stratified on the basis of degree of TSH elevation.
The risk that subclinical hypothyroidism will progress to overt hypothyroidism in patients with TSH higher than 8 mIU/L is high, and in 70% of these patients, the TSH level rises to more than 10 mIU/L within 4 years. Early treatment should be considered if the TSH is higher than 7 or 8 mIU/L.
If TSH is higher than 10 mIU/L
Figure 2 outlines an algorithmic approach to subclinical hypothyroidism in nonpregnant patients as suggested by Peeters.122
Whether subclinical hypothyroidism is clinically important and should be treated remains controversial. Studies have differed in their findings, and although most have found this condition to be associated with a variety of adverse outcomes, large randomized controlled trials are needed to clearly demonstrate its clinical impact in various age groups and the benefit of levothyroxine therapy.
Currently, the best practical approach is to base treatment decisions on the magnitude of elevation of thyroid-stimulating hormone (TSH) and whether the patient has thyroid autoantibodies and associated comorbid conditions.
HIGH TSH, NORMAL FREE T4 LEVELS
Subclinical hypothyroidism is defined by elevated TSH along with a normal free thyroxine (T4).1
The hypothalamic-pituitary-thyroid axis is a balanced homeostatic system, and TSH and thyroid hormone levels have an inverse log-linear relation: if free T4 and triiodothyronine (T3) levels go down even a little, TSH levels go up a lot.2
TSH secretion is pulsatile and has a circadian rhythm: serum TSH levels are 50% higher at night and early in the morning than during the rest of the day. Thus, repeated measurements in the same patient can vary by as much as half of the reference range.3
WHAT IS THE UPPER LIMIT OF NORMAL FOR TSH?
The upper limit of normal for TSH, defined as the 97.5th percentile, is approximately 4 or 5 mIU/L depending on the laboratory and the population, but some experts believe it should be lower.3
In favor of a lower upper limit: the distribution of serum TSH levels in the healthy general population does not seem to be a typical bell-shaped Gaussian curve, but rather has a tail at the high end. Some argue that some of the individuals with values in the upper end of the normal range may actually have undiagnosed hypothyroidism and that the upper 97.5th percentile cutoff would be 2.5 mIU/L if these people were excluded.4 Also, TSH levels higher than 2.5 mIU/L have been associated with a higher prevalence of antithyroid antibodies and a higher risk of clinical hypothyroidism.5
On the other hand, lowering the upper limit of normal to 2.5 mIU/L would result in 4 times as many people receiving a diagnosis of subclinical hypothyroidism, or 22 to 28 million people in the United States.4,6 Thus, lowering the cutoff may lead to unnecessary therapy and could even harm from overtreatment.
Another argument against lowering the upper limit of normal for TSH is that, with age, serum TSH levels shift higher.7 The third National Health and Nutrition Education Survey (NHANES III) found that the 97.5th percentile for serum TSH was 3.56 mIU/L for age group 20 to 29 but 7.49 mIU/L for octogenarians.7,8
It has been suggested that the upper limit of normal for TSH be adjusted for age, race, sex, and iodine intake.3 Currently available TSH reference ranges are not adjusted for these variables, and there is not enough evidence to suggest age-appropriate ranges,9 although higher TSH cutoffs for treatment are advised in elderly patients.10 Interestingly, higher TSH in older people has been linked to lower mortality rates in some studies.11
Authors of the NHANES III8 and Hanford Thyroid Disease study12 have proposed a cutoff of 4.1 mIU/L for the upper limit of normal for serum TSH in patients with negative antithyroid antibodies and normal findings on thyroid ultrasonography.
SUBCLINICAL HYPOTHYROIDISM IS COMMON
In different studies, the prevalence of subclinical hypothyroidism has been as low as 4% and as high as 20%.1,8,13 The prevalence is higher in women and increases with age.8 It is higher in iodine-sufficient areas, and it increases in iodine-deficient areas with iodine supplementation.14 Genetics also plays a role, as subclinical hypothyroidism is more common in white people than in African Americans.8
A difficulty in estimating the prevalence is the disagreement about the cutoff for TSH, which may differ from that in the general population in certain subgroups such as adolescents, the elderly, and pregnant women.10,15
A VARIETY OF CAUSES
The most common cause of subclinical hypothyroidism, accounting for 60% to 80% of cases, is Hashimoto (autoimmune) thyroiditis,2 in which thyroid peroxidase antibodies are usually present.2,16
Also important to rule out are false-positive elevations due to substances that interfere with TSH assays (eg, heterophile antibodies, rheumatoid factor, biotin, macro-TSH); reversible causes such as the recovery phase of euthyroid sick syndrome; subacute, painless, or postpartum thyroiditis; central hypo- or hyperthyroidism; and thyroid hormone resistance.
SUBCLINICAL HYPOTHYROIDISM CAN RESOLVE OR PROGRESS
“Subclinical” suggests that the disease is in its early stage, with changes in TSH already apparent but decreases in thyroid hormone levels yet to come.17 And indeed, subclinical hypothyroidism can progress to overt hypothyroidism,18 although it has been reported to resolve spontaneously in half of cases within 2 years,19 typically in patients with TSH values of 4 to 6 mIU/L.20 The rate of progression to overt hypothyroidism is estimated to be 33% to 55% over 10 to 20 years of follow-up.18
Figure 1 shows the natural history of subclinical hypothyroidism.1
GUIDELINES FOR SCREENING DIFFER
Guidelines differ on screening for thyroid disease in the general population, owing to lack of large-scale randomized controlled trials showing treatment benefit in otherwise-healthy people with mildly elevated TSH values.
Various professional societies have adopted different criteria for aggressive case-finding in patients at risk of thyroid disease. Risk factors include family history of thyroid disease, neck irradiation, partial thyroidectomy, dyslipidemia, atrial fibrillation, unexplained weight loss, hyperprolactinemia, autoimmune disorders, and use of medications affecting thyroid function.23
The US Preventive Services Task Force in 2014 found insufficient evidence on the benefits and harms of screening.24
The American Thyroid Association (ATA) recommends screening adults starting at age 35, with repeat testing every 5 years in patients who have no signs or symptoms of hypothyroidism, and more frequently in those who do.25
The American Association of Clinical Endocrinologists recommends screening in women and older patients. Their guidelines and those of the ATA also suggest screening people at high risk of thyroid disease due to risk factors such as history of autoimmune diseases, neck irradiation, or medications affecting thyroid function.26
The American Academy of Family Physicians recommends screening after age 60.18
The American College of Physicians recommends screening patients over age 50 who have symptoms.18
Our approach. Although evidence is lacking to recommend routine screening in adults, aggressive case-finding and treatment in patients at risk of thyroid disease can, we believe, offset the risks associated with subclinical hypothyroidism.24
CLINICAL PRESENTATION
About 70% of patients with subclinical hypothyroidism have no symptoms.13
Tiredness was more common in subclinical hypothyroid patients with TSH levels lower than 10 mIU/L compared with euthyroid controls in 1 study, but other studies have been unable to replicate this finding.27,28
Other frequently reported symptoms include dry skin, cognitive slowing, poor memory, muscle weakness, cold intolerance, constipation, puffy eyes, and hoarseness.13
The evidence in favor of levothyroxine therapy to improve symptoms in subclinical hypothyroidism has varied, with some studies showing an improvement in symptom scores compared with placebo, while others have not shown any benefit.29–31
In one study, the average TSH value for patients whose symptoms did not improve with therapy was 4.6 mIU/L.31 An explanation for the lack of effect in this group may be that the TSH values for these patients were in the high-normal range. Also, because most subclinical hypothyroid patients have no symptoms, it is difficult to ascertain symptomatic improvement. Though it is possible to conclude that levothyroxine therapy has a limited role in this group, it is important to also consider the suggestive evidence that untreated subclinical hypothyroidism may lead to increased morbidity and mortality.
ADVERSE EFFECTS OF SUBCLINICAL HYPOTHYROIDISM, EFFECTS OF THERAPY
INDIVIDUALIZED MANAGEMENT AND SHARED DECISION-MAKING
The management of subclinical hypothyroidism should be individualized on the basis of extent of thyroid dysfunction, comorbid conditions, risk factors, and patient preference.118 Shared decision-making is key, weighing the risks and benefits of levothyroxine treatment and the patient’s goals.
The risks of treatment should be kept in mind and explained to the patient. Levothyroxine has a narrow therapeutic range, causing a possibility of overreplacement, and a half-life of 7 days that can cause dosing errors to have longer effect.118,119
Adherence can be a challenge. The drug needs to be taken on an empty stomach because foods and supplements interfere with its absorption.118,120 In addition, the cost of medication, frequent biochemical monitoring, and possible need for titration can add to financial burden.
When choosing the dose, one should consider the degree of hypothyroidism or TSH elevation and the patient’s weight, and adjust the dose gently.
If the TSH is high-normal
It is proposed that a TSH range of 3 to 5 mIU/L overlaps with normal thyroid function in a great segment of the population, and at this level it is probably not associated with clinically significant consequences. For these reasons, levothyroxine therapy is not thought to be beneficial for those with TSH in this range.
Pollock et al121 found that, in patients with symptoms suggesting hypothyroidism and TSH values in the upper end of the normal range, there was no improvement in cognitive function or psychological well-being after 12 weeks of levothyroxine therapy.
However, due to the concern for possible adverse maternal and fetal outcomes and low IQ in children of pregnant patients with subclinical hypothyroidism, levothyroxine therapy is advised in those who are pregnant or planning pregnancy who have TSH levels higher than 2.5 mIU/L, especially if they have thyroid peroxidase antibody. Levothyroxine therapy is not recommended for pregnant patients with negative thyroid peroxidase antibody and TSH within the pregnancy-specific range or less than 4 mIU/L if the reference ranges are unavailable.
Keep in mind that, even at these TSH values, there is risk of progression to overt hypothyroidism, especially in the presence of thyroid peroxidase antibody, so patients in this group should be monitored closely.
If TSH is mildly elevated
The evidence to support levothyroxine therapy in patients with subclinical hypothyroidism with TSH levels less than 10 mIU/L remains inconclusive, and the decision to treat should be based on clinical judgment.2 The studies that have looked at the benefit of treating subclinical hypothyroidism in terms of cardiac, neuromuscular, cognitive, and neuropsychiatric outcomes have included patients with a wide range of TSH levels, and some of these studies were not stratified on the basis of degree of TSH elevation.
The risk that subclinical hypothyroidism will progress to overt hypothyroidism in patients with TSH higher than 8 mIU/L is high, and in 70% of these patients, the TSH level rises to more than 10 mIU/L within 4 years. Early treatment should be considered if the TSH is higher than 7 or 8 mIU/L.
If TSH is higher than 10 mIU/L
Figure 2 outlines an algorithmic approach to subclinical hypothyroidism in nonpregnant patients as suggested by Peeters.122
- Cooper DS, Biondi B. Subclinical thyroid disease. Lancet 2012; 379(9821):1142–1154. doi:10.1016/S0140-6736(11)60276-6
- Fatourechi V. Subclinical hypothyroidism: an update for primary care physicians. Mayo Clin Proc 2009; 84(1):65–71. doi:10.4065/84.1.65
- Laurberg P, Andersen S, Carle A, Karmisholt J, Knudsen N, Pedersen IB. The TSH upper reference limit: where are we at? Nat Rev Endocrinol 2011; 7(4):232–239. doi:10.1038/nrendo.2011.13
- Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab 2005; 90(9):5483–5488. doi:10.1210/jc.2005-0455
- Spencer CA, Hollowell JG, Kazarosyan M, Braverman LE. National Health and Nutrition Examination Survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab 2007; 92(11):4236–4240. doi:10.1210/jc.2007-0287
- Fatourechi V, Klee GG, Grebe SK, et al. Effects of reducing the upper limit of normal TSH values. JAMA 2003; 290(24):3195–3196. doi:10.1001/jama.290.24.3195-a
- Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 2007; 92(12):4575–4582. doi:10.1210/jc.2007-1499
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002; 87(2):489–499. doi:10.1210/jcem.87.2.8182
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014; 24(12):1670–1751. doi:10.1089/thy.2014.0028
- Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc 2015; 63(8):1663–1673. doi:10.1111/jgs.13532
- Razvi S, Shakoor A, Vanderpump M, Weaver JU, Pearce SH. The influence of age on the relationship between subclinical hypothyroidism and ischemic heart disease: a metaanalysis. J Clin Endocrinol Metab 2008; 93(8):2998–3007. doi:10.1210/jc.2008-0167
- Hamilton TE, Davis S, Onstad L, Kopecky KJ. Thyrotropin levels in a population with no clinical, autoantibody, or ultrasonographic evidence of thyroid disease: implications for the diagnosis of subclinical hypothyroidism. J Clin Endocrinol Metab 2008; 93(4):1224–1230. doi:10.1210/jc.2006-2300
- Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med 2000; 160(4):526–534. pmid:10695693
- Teng W, Shan Z, Teng X, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med 2006; 354(26):2783–2793. doi:10.1056/NEJMoa054022
- Negro R, Stagnaro-Green A. Diagnosis and management of subclinical hypothyroidism in pregnancy. BMJ 2014; 349:g4929. doi:10.1136/bmj.g4929
- Baumgartner C, Blum MR, Rodondi N. Subclinical hypothyroidism: summary of evidence in 2014. Swiss Med Wkly 2014; 144:w14058. doi:10.4414/smw.2014.14058
- Stedman TL. Stedman’s Medical Dictionary. 28th ed. Baltimore, MD: Lippincott Williams and Wilkins; 2006.
- Raza SA, Mahmood N. Subclinical hypothyroidism: controversies to consensus. Indian J Endocrinol Metab 2013; 17(suppl 3):S636–S642. doi:10.4103/2230-8210.123555
- Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab 2002; 87(7):3221–3226. doi:10.1210/jcem.87.7.8678
- Diez JJ, Iglesias P, Burman KD. Spontaneous normalization of thyrotropin concentrations in patients with subclinical hypothyroidism. J Clin Endocrinol Metab 2005; 90(7):4124–4127. doi:10.1210/jc.2005-0375
- Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol (Oxf) 1995; 43(1):55–68. pmid:7641412
- Li Y, Teng D, Shan Z, et al. Antithyroperoxidase and antithyroglobulin antibodies in a five-year follow-up survey of populations with different iodine intakes. J Clin Endocrinol Metab 2008; 93(5):1751–1757. doi:10.1210/jc.2007-2368
- Hennessey JV, Klein I, Woeber KA, Cobin R, Garber JR. Aggressive case finding: a clinical strategy for the documentation of thyroid dysfunction. Ann Intern Med 2015; 163(4):311–312. doi:10.7326/M15-0762
- Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: an evidence review for the US.Preventive Services Task Force. Ann Intern Med 2015; 162(1):35–45. doi:10.7326/M14-1456
- Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med 2000; 160(11):1573–1575. pmid:10847249
- Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 2012; 18(6):988–1028. doi:10.4158/EP12280.GL
- Jorde R, Waterloo K, Storhaug H, Nyrnes A, Sundsfjord J, Jenssen TG. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab 2006; 91(1):145–153. doi:10.1210/jc.2005-1775
- Joffe RT, Pearce EN, Hennessey JV, Ryan JJ, Stern RA. Subclinical hypothyroidism, mood, and cognition in older adults: a review. Int J Geriatr Psychiatry 2013; 28(2):111–118. doi:10.1002/gps.3796
- Cooper DS, Halpern R, Wood LC, Levin AA, Ridgway EC. L-thyroxine therapy in subclinical hypothyroidism. A double-blind, placebo-controlled trial. Ann Intern Med 1984; 101(1):18–24. pmid:6428290
- Nystrom E, Caidahl K, Fager G, Wikkelso C, Lundberg PA, Lindstedt G. A double-blind cross-over 12-month study of L-thyroxine treatment of women with ‘subclinical’ hypothyroidism. Clin Endocrinol (Oxf) 1988; 29(1):63–75. pmid:3073880
- Monzani F, Del Guerra P, Caraccio N, et al. Subclinical hypothyroidism: neurobehavioral features and beneficial effect of L-thyroxine treatment. Clin Investig 1993; 71(5):367–371. pmid:8508006
- Biondi B. Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab 2010; 95(8):3614–3617. doi:10.1210/jc.2010-1245
- Erdogan M, Canataroglu A, Ganidagli S, Kulaksizoglu M. Metabolic syndrome prevalence in subclinic and overt hypothyroid patients and the relation among metabolic syndrome parameters. J Endocrinol Invest 2011; 34(7):488–492. doi:10.3275/7202
- Javed Z, Sathyapalan T. Levothyroxine treatment of mild subclinical hypothyroidism: a review of potential risks and benefits. Ther Adv Endocrinol Metab 2016; 7(1):12–23. doi:10.1177/2042018815616543
- Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J 2013; 2(4):215–228. doi:10.1159/000356507
- Wang C. The relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res 2013; 2013:390534. doi:10.1155/2013/390534
- Razvi S, Weaver JU, Vanderpump MP, Pearce SH. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: reanalysis of the Whickham survey cohort. J Clin Endocrinol Metab 2010; 95(4):1734–1740. doi:10.1210/jc.2009-1749
- Bindels AJ, Westendorp RG, Frolich M, Seidell JC, Blokstra A, Smelt AH. The prevalence of subclinical hypothyroidism at different total plasma cholesterol levels in middle aged men and women: a need for case-finding? Clin Endocrinol (Oxf) 1999; 50(2):217–220. pmid:10396365
- Pearce EN. Hypothyroidism and dyslipidemia: modern concepts and approaches. Curr Cardiol Rep 2004; 6(6):451–456. pmid:15485607
- Pearce EN. Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab 2012; 97(2):326–333. doi:10.1210/jc.2011-2532
- Rizos CV, Elisaf MS, Liberopoulos EN. Effects of thyroid dysfunction on lipid profile. Open Cardiovasc Med J 2011; 5:76–84. doi:10.2174/1874192401105010076
- Peppa M, Betsi G, Dimitriadis G. Lipid abnormalities and cardiometabolic risk in patients with overt and subclinical thyroid disease. J Lipids 2011; 2011:575840. doi:10.1155/2011/575840
- Asvold BO, Vatten LJ, Nilsen TI, Bjoro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. the HUNT study. Eur J Endocrinol 2007;1 56(2):181–186. doi:10.1530/eje.1.02333
- Danese MD, Ladenson PW, Meinert CL, Powe NR. Clinical review 115: effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: a quantitative review of the literature. J Clin Endocrinol Metab 2000; 85(9):2993–3001. doi:10.1210/jcem.85.9.6841
- Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of L-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab 2007; 92(5):1715–1723. doi:10.1210/jc.2006-1869
- Abreu IM, Lau E, de Sousa Pinto B, Carvalho D. Subclinical hypothyroidism: to treat or not to treat, that is the question! A systematic review with meta-analysis on lipid profile. Endocr Connect 2017; 6(3):188–199. doi:10.1530/EC-17-0028
- Robison CD, Bair TL, Horne BD, et al. Hypothyroidism as a risk factor for statin intolerance. J Clin Lipidol 2014; 8(4):401–407. doi:10.1016/j.jacl.2014.05.005
- Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam study. Ann Intern Med 2000; 132(4):270–278. pmid:10681281
- Boekholdt SM, Titan SM, Wiersinga WM, et al. Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol (Oxf) 2010; 72(3):404–410. doi:10.1111/j.1365-2265.2009.03640.x
- Andersen MN, Olsen AM, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One 2015; 10(6):e0129793. doi:10.1371/journal.pone.0129793
- Biondi B. Cardiovascular effects of mild hypothyroidism. Thyroid 2007; 17(7):625–630. doi:10.1089/thy.2007.0158
- Brenta G, Mutti LA, Schnitman M, Fretes O, Perrone A, Matute ML. Assessment of left ventricular diastolic function by radionuclide ventriculography at rest and exercise in subclinical hypothyroidism, and its response to L-thyroxine therapy. Am J Cardiol 2003; 91(11):1327–1330. pmid:12767425
- Taddei S, Caraccio N, Virdis A, et al. Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: beneficial effect of levothyroxine therapy. J Clin Endocrinol Metab 2003; 88(8):3731–3737. doi:10.1210/jc.2003-030039
- Gao N, Zhang W, Zhang YZ, Yang Q, Chen SH. Carotid intima-media thickness in patients with subclinical hypothyroidism: a meta-analysis. Atherosclerosis 2013; 227(1):18–25. doi:10.1016/j.atherosclerosis.2012.10.070
- Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 2008; 29(1):76–131. doi:10.1210/er.2006-0043
- Chaker L, Baumgartner C, den Elzen WP, et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of stroke events and fatal stroke: an individual participant data analysis. J Clin Endocrinol Metab 2015; 100(6):2181–2191. doi:10.1210/jc.2015-1438
- Monzani F, Di Bello V, Caraccio N, et al. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. J Clin Endocrinol Metab 2001; 86(3):1110–1115. doi:10.1210/jcem.86.3.7291
- Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet 2001; 358(9285):861-865. doi:10.1016/S0140-6736(01)06067-6
- Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med 2012; 172(10):811–817. doi:10.1001/archinternmed.2012.1159
- Pasqualetti G, Tognini S, Polini A, Caraccio N, Monzani F. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab 2013; 98(6):2256–2266. doi:10.1210/jc.2012-3818
- Mooijaart SP, IEMO 80-plus Thyroid Trial Collaboration. Subclinical thyroid disorders. Lancet 2012; 380(9839):335. doi:10.1016/S0140-6736(12)61241-0
- Rodondi N, Bauer DC. Subclinical hypothyroidism and cardiovascular risk: how to end the controversy. J Clin Endocrinol Metab 2013; 98(6):2267–2269. doi:10.1210/jc.2013-1875
- Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med 2005; 165(21):2460–2466. doi:10.1001/archinte.165.21.2460
- Rodondi N, Bauer DC, Cappola AR, et al. Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure. The Cardiovascular Health study. J Am Coll Cardiol 2008; 52(14):1152–1159. doi:10.1016/j.jacc.2008.07.009
- Haggerty JJ Jr, Garbutt JC, Evans DL, et al. Subclinical hypothyroidism: a review of neuropsychiatric aspects. Int J Psychiatry Med 1990; 20(2):193–208. doi:10.2190/ADLY-1UU0-1A8L-HPXY
- Baldini IM, Vita A, Mauri MC, et al. Psychopathological and cognitive features in subclinical hypothyroidism. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21(6):925–935. pmid:9380789
- del Ser Quijano T, Delgado C, Martinez Espinosa S, Vazquez C. Cognitive deficiency in mild hypothyroidism. Neurologia 2000; 15(5):193–198. Spanish. pmid:10850118
- Correia N, Mullally S, Cooke G, et al. Evidence for a specific defect in hippocampal memory in overt and subclinical hypothyroidism. J Clin Endocrinol Metab 2009; 94(10):3789–3797. doi:10.1210/jc.2008-2702
- Aghili R, Khamseh ME, Malek M, et al. Changes of subtests of Wechsler memory scale and cognitive function in subjects with subclinical hypothyroidism following treatment with levothyroxine. Arch Med Sci 2012; 8(6):1096–1101. doi:10.5114/aoms.2012.32423
- Pasqualetti G, Pagano G, Rengo G, Ferrara N, Monzani F. Subclinical hypothyroidism and cognitive impairment: systematic review and meta-analysis. J Clin Endocrinol Metab 2015; 100(11):4240–4248. doi:10.1210/jc.2015-2046
- Christ-Crain M, Meier C, Huber PR, Staub J, Muller B. Effect of L-thyroxine replacement therapy on surrogate markers of skeletal and cardiac function in subclinical hypothyroidism. Endocrinologist 2004; 14(3):161–166. doi:10.1097/01.ten.0000127932.31710.4f
- Brennan MD, Powell C, Kaufman KR, Sun PC, Bahn RS, Nair KS. The impact of overt and subclinical hyperthyroidism on skeletal muscle. Thyroid 2006; 16(4):375–380. doi:10.1089/thy.2006.16.375
- Reuters VS, Teixeira Pde F, Vigario PS, et al. Functional capacity and muscular abnormalities in subclinical hypothyroidism. Am J Med Sci 2009; 338(4):259–263. doi:10.1097/MAJ.0b013e3181af7c7c
- Mainenti MR, Vigario PS, Teixeira PF, Maia MD, Oliveira FP, Vaisman M. Effect of levothyroxine replacement on exercise performance in subclinical hypothyroidism. J Endocrinol Invest 2009; 32(5):470–473. doi:10.3275/6106
- Lankhaar JA, de Vries WR, Jansen JA, Zelissen PM, Backx FJ. Impact of overt and subclinical hypothyroidism on exercise tolerance: a systematic review. Res Q Exerc Sport 2014; 85(3):365–389. doi:10.1080/02701367.2014.930405
- Lee JS, Buzkova P, Fink HA, et al. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch Intern Med 2010; 170(21):1876–1883. doi:10.1001/archinternmed.2010.424
- Svare A, Nilsen TI, Asvold BO, et al. Does thyroid function influence fracture risk? Prospective data from the HUNT2 study, Norway. Eur J Endocrinol 2013; 169(6):845–852. doi:10.1530/EJE-13-0546
- Di Mase R, Cerbone M, Improda N, et al. Bone health in children with long-term idiopathic subclinical hypothyroidism. Ital J Pediatr 2012; 38:56. doi:10.1186/1824-7288-38-56
- Boelaert K. The association between serum TSH concentration and thyroid cancer. Endocr Relat Cancer 2009; 16(4):1065–1072. doi:10.1677/ERC-09-0150
- Haymart MR, Glinberg SL, Liu J, Sippel RS, Jaume JC, Chen H. Higher serum TSH in thyroid cancer patients occurs independent of age and correlates with extrathyroidal extension. Clin Endocrinol (Oxf) 2009; 71(3):434–439. doi:10.1111/j.1365-2265.2008.03489.x
- Fiore E, Vitti P. Serum TSH and risk of papillary thyroid cancer in nodular thyroid disease. J Clin Endocrinol Metab 2012; 97(4):1134–1145. doi:10.1210/jc.2011-2735
- Fiore E, Rago T, Provenzale MA, et al. L-thyroxine-treated patients with nodular goiter have lower serum TSH and lower frequency of papillary thyroid cancer: results of a cross-sectional study on 27,914 patients. Endocr Relat Cancer 2010; 17(1):231–239. doi:10.1677/ERC-09-0251
- Hercbergs AH, Ashur-Fabian O, Garfield D. Thyroid hormones and cancer: clinical studies of hypothyroidism in oncology. Curr Opin Endocrinol Diabetes Obes 2010; 17(5):432–436. doi:10.1097/MED.0b013e32833d9710
- Thvilum M, Brandt F, Brix TH, Hegedus L. A review of the evidence for and against increased mortality in hypothyroidism. Nat Rev Endocrinol 2012; 8(7):417–424. doi:10.1038/nrendo.2012.29
- Stott DJ, Rodondi N, Kearney PM, et al; TRUST Study Group. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med 2017; 376(26):2534–2544. doi:10.1056/NEJMoa1603825
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril 2015; 104(3):545–753. doi:10.1016/j.fertnstert.2015.05.028
- Stagnaro-Green A, Abalovich M, Alexander E, et al; American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011; 21(10):1081–1125. doi:10.1089/thy.2011.0087
- Goldsmith RE, Sturgis SH, Lerman J, Stanbury JB. The menstrual pattern in thyroid disease. J Clin Endocrinol Metab. 1952; 12(7):846-855. doi:10.1210/jcem-12-7-846
- Plowden TC, Schisterman EF, Sjaarda LA, et al. Subclinical hypothyroidism and thyroid autoimmunity are not associated with fecundity, pregnancy loss, or live birth. J Clin Endocrinol Metab 2016; 101(6):2358–2365. doi:10.1210/jc.2016-1049
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017; 27(3):315–389. doi:10.1089/thy.2016.0457
- Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab 2006; 91(7):2587–2591. doi:10.1210/jc.2005-1603
- Panesar NS, Li CY, Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem 2001; 38(pt 4):329–332. doi:10.1258/0004563011900830
- Lepoutre T, Debieve F, Gruson D, Daumerie C. Reduction of miscarriages through universal screening and treatment of thyroid autoimmune diseases. Gynecol Obstet Invest 2012; 74(4):265–273. doi:10.1159/000343759
- De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97(8):2543–2565. doi:10.1210/jc.2011-2803
- Crawford NM, Steiner AZ. Thyroid autoimmunity and reproductive function. Semin Reprod Med 2016; 34(6):343–350. doi:10.1055/s-0036-1593485
- Maraka S, Ospina NM, O’Keeffe DT, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid 2016; 26(4):580–590. doi:10.1089/thy.2015.0418
- Wiles KS, Jarvis S, Nelson-Piercy C. Are we overtreating subclinical hypothyroidism in pregnancy? BMJ 2015; 351:h4726. doi:10.1136/bmj.h4726
- Tudela CM, Casey BM, McIntire DD, Cunningham FG. Relationship of subclinical thyroid disease to the incidence of gestational diabetes. Obstet Gynecol 2012; 119(5):983–988. doi:10.1097/AOG.0b013e318250aeeb
- Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European Thyroid Association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J 2014; 3(2):76–94. doi:10.1159/000362597
- Karakosta P, Alegakis D, Georgiou V, et al. Thyroid dysfunction and autoantibodies in early pregnancy are associated with increased risk of gestational diabetes and adverse birth outcomes. J Clin Endocrinol Metab 2012; 97(12):4464–4472. doi:10.1210/jc.2012-2540
- Toulis KA, Stagnaro-Green A, Negro R. Maternal subclinical hypothyroidsm and gestational diabetes mellitus: a meta-analysis. Endocr Pract 2014; 20(7):703–714. doi:10.4158/EP13440.RA
- van den Boogaard E, Vissenberg R, Land JA, et al. Significance of subclinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update 2011; 17(5):605–619. doi:10.1093/humupd/dmr024
- Wilson KL, Casey BM, McIntire DD, Halvorson LM, Cunningham FG. Subclinical thyroid disease and the incidence of hypertension in pregnancy. Obstet Gynecol 2012; 119(2 Pt 1):315–320. doi:10.1097/AOG.0b013e318240de6a
- Ashoor G, Maiz N, Rotas M, Jawdat F, Nicolaides KH. Maternal thyroid function at 11 to 13 weeks of gestation and subsequent fetal death. Thyroid 2010; 20(9):989–993. doi:10.1089/thy.2010.0058
- Casey BM, Dashe JS, Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol 2005; 105(2):239–245. doi:10.1097/01.AOG.0000152345.99421.22
- Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab 2010; 95(9):E44–E48. doi:10.1210/jc.2010-0340
- Su PY, Huang K, Hao JH, et al. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab 2011; 96(10):3234–3241. doi:10.1210/jc.2011-0274
- Allan WC, Haddow JE, Palomaki GE, et al. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen 2000; 7(3):127–130. doi:10.1136/jms.7.3.127
- Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol 2009; 160(6):985–991. doi:10.1530/EJE-08-0953
- Korevaar TI, Medici M, de Rijke YB, et al. Ethnic differences in maternal thyroid parameters during pregnancy: the generation R study. J Clin Endocrinol Metab 2013; 98(9):3678–3686. doi:10.1210/jc.2013-2005
- Cleary-Goldman J, Malone FD, Lambert-Messerlian G, et al. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol 2008; 112(1):85–92. doi:10.1097/AOG.0b013e3181788dd7
- Li Y, Shan Z, Teng W, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin Endocrinol (Oxf) 2010; 72(6):825–829. doi:10.1111/j.1365-2265.2009.03743.x
- Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 1999; 341(8):549–555. doi:10.1056/NEJM199908193410801
- Henrichs J, Bongers-Schokking JJ, Schenk JJ, et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab 2010; 95(9):4227–4234. doi:10.1210/jc.2010-0415
- Behrooz HG, Tohidi M, Mehrabi Y, Behrooz EG, Tehranidoost M, Azizi F. Subclinical hypothyroidism in pregnancy: intellectual development of offspring. Thyroid 2011; 21(10):1143–1147. doi:10.1089/thy.2011.0053
- Julvez J, Alvarez-Pedrerol M, Rebagliato M, et al. Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiology 2013; 24(1):150–157. doi:10.1097/EDE.0b013e318276ccd3
- Casey BM, Thom EA, Peaceman AM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med 2017; 376(9):815–825. doi:10.1056/NEJMoa1606205
- Burns RB, Bates CK, Hartzband P, Smetana GW. Should we treat for subclinical hypothyroidism?: Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med 2016; 164(11):764–770. doi:10.7326/M16-0857
- Kucukler FK, Akbaba G, Arduc A, Simsek Y, Guler S. Evaluation of the common mistakes made by patients in the use of levothyroxine. Eur J Intern Med 2014; 25(9):e107–e108. doi:10.1016/j.ejim.2014.09.002
- McMillan M, Rotenberg KS, Vora K, et al. Comorbidities, concomitant medications, and diet as factors affecting levothyroxine therapy: results of the CONTROL surveillance project. Drugs R D 2016; 16(1):53–68. doi:10.1007/s40268-015-0116-6
- Pollock MA, Sturrock A, Marshall K, et al. Thyroxine treatment in patients with symptoms of hypothyroidism but thyroid function tests within the reference range: Randomised double blind placebo controlled crossover trial. BMJ 2001; 323(7318):891–895. pmid:11668132
- Peeters RP. Subclinical hypothyroidism. N Engl J Med 2017; 376(26):2556–2565. doi:10.1056/NEJMcp1611144
- Cooper DS, Biondi B. Subclinical thyroid disease. Lancet 2012; 379(9821):1142–1154. doi:10.1016/S0140-6736(11)60276-6
- Fatourechi V. Subclinical hypothyroidism: an update for primary care physicians. Mayo Clin Proc 2009; 84(1):65–71. doi:10.4065/84.1.65
- Laurberg P, Andersen S, Carle A, Karmisholt J, Knudsen N, Pedersen IB. The TSH upper reference limit: where are we at? Nat Rev Endocrinol 2011; 7(4):232–239. doi:10.1038/nrendo.2011.13
- Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab 2005; 90(9):5483–5488. doi:10.1210/jc.2005-0455
- Spencer CA, Hollowell JG, Kazarosyan M, Braverman LE. National Health and Nutrition Examination Survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab 2007; 92(11):4236–4240. doi:10.1210/jc.2007-0287
- Fatourechi V, Klee GG, Grebe SK, et al. Effects of reducing the upper limit of normal TSH values. JAMA 2003; 290(24):3195–3196. doi:10.1001/jama.290.24.3195-a
- Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 2007; 92(12):4575–4582. doi:10.1210/jc.2007-1499
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002; 87(2):489–499. doi:10.1210/jcem.87.2.8182
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014; 24(12):1670–1751. doi:10.1089/thy.2014.0028
- Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc 2015; 63(8):1663–1673. doi:10.1111/jgs.13532
- Razvi S, Shakoor A, Vanderpump M, Weaver JU, Pearce SH. The influence of age on the relationship between subclinical hypothyroidism and ischemic heart disease: a metaanalysis. J Clin Endocrinol Metab 2008; 93(8):2998–3007. doi:10.1210/jc.2008-0167
- Hamilton TE, Davis S, Onstad L, Kopecky KJ. Thyrotropin levels in a population with no clinical, autoantibody, or ultrasonographic evidence of thyroid disease: implications for the diagnosis of subclinical hypothyroidism. J Clin Endocrinol Metab 2008; 93(4):1224–1230. doi:10.1210/jc.2006-2300
- Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med 2000; 160(4):526–534. pmid:10695693
- Teng W, Shan Z, Teng X, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med 2006; 354(26):2783–2793. doi:10.1056/NEJMoa054022
- Negro R, Stagnaro-Green A. Diagnosis and management of subclinical hypothyroidism in pregnancy. BMJ 2014; 349:g4929. doi:10.1136/bmj.g4929
- Baumgartner C, Blum MR, Rodondi N. Subclinical hypothyroidism: summary of evidence in 2014. Swiss Med Wkly 2014; 144:w14058. doi:10.4414/smw.2014.14058
- Stedman TL. Stedman’s Medical Dictionary. 28th ed. Baltimore, MD: Lippincott Williams and Wilkins; 2006.
- Raza SA, Mahmood N. Subclinical hypothyroidism: controversies to consensus. Indian J Endocrinol Metab 2013; 17(suppl 3):S636–S642. doi:10.4103/2230-8210.123555
- Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab 2002; 87(7):3221–3226. doi:10.1210/jcem.87.7.8678
- Diez JJ, Iglesias P, Burman KD. Spontaneous normalization of thyrotropin concentrations in patients with subclinical hypothyroidism. J Clin Endocrinol Metab 2005; 90(7):4124–4127. doi:10.1210/jc.2005-0375
- Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol (Oxf) 1995; 43(1):55–68. pmid:7641412
- Li Y, Teng D, Shan Z, et al. Antithyroperoxidase and antithyroglobulin antibodies in a five-year follow-up survey of populations with different iodine intakes. J Clin Endocrinol Metab 2008; 93(5):1751–1757. doi:10.1210/jc.2007-2368
- Hennessey JV, Klein I, Woeber KA, Cobin R, Garber JR. Aggressive case finding: a clinical strategy for the documentation of thyroid dysfunction. Ann Intern Med 2015; 163(4):311–312. doi:10.7326/M15-0762
- Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: an evidence review for the US.Preventive Services Task Force. Ann Intern Med 2015; 162(1):35–45. doi:10.7326/M14-1456
- Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med 2000; 160(11):1573–1575. pmid:10847249
- Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 2012; 18(6):988–1028. doi:10.4158/EP12280.GL
- Jorde R, Waterloo K, Storhaug H, Nyrnes A, Sundsfjord J, Jenssen TG. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab 2006; 91(1):145–153. doi:10.1210/jc.2005-1775
- Joffe RT, Pearce EN, Hennessey JV, Ryan JJ, Stern RA. Subclinical hypothyroidism, mood, and cognition in older adults: a review. Int J Geriatr Psychiatry 2013; 28(2):111–118. doi:10.1002/gps.3796
- Cooper DS, Halpern R, Wood LC, Levin AA, Ridgway EC. L-thyroxine therapy in subclinical hypothyroidism. A double-blind, placebo-controlled trial. Ann Intern Med 1984; 101(1):18–24. pmid:6428290
- Nystrom E, Caidahl K, Fager G, Wikkelso C, Lundberg PA, Lindstedt G. A double-blind cross-over 12-month study of L-thyroxine treatment of women with ‘subclinical’ hypothyroidism. Clin Endocrinol (Oxf) 1988; 29(1):63–75. pmid:3073880
- Monzani F, Del Guerra P, Caraccio N, et al. Subclinical hypothyroidism: neurobehavioral features and beneficial effect of L-thyroxine treatment. Clin Investig 1993; 71(5):367–371. pmid:8508006
- Biondi B. Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab 2010; 95(8):3614–3617. doi:10.1210/jc.2010-1245
- Erdogan M, Canataroglu A, Ganidagli S, Kulaksizoglu M. Metabolic syndrome prevalence in subclinic and overt hypothyroid patients and the relation among metabolic syndrome parameters. J Endocrinol Invest 2011; 34(7):488–492. doi:10.3275/7202
- Javed Z, Sathyapalan T. Levothyroxine treatment of mild subclinical hypothyroidism: a review of potential risks and benefits. Ther Adv Endocrinol Metab 2016; 7(1):12–23. doi:10.1177/2042018815616543
- Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J 2013; 2(4):215–228. doi:10.1159/000356507
- Wang C. The relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res 2013; 2013:390534. doi:10.1155/2013/390534
- Razvi S, Weaver JU, Vanderpump MP, Pearce SH. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: reanalysis of the Whickham survey cohort. J Clin Endocrinol Metab 2010; 95(4):1734–1740. doi:10.1210/jc.2009-1749
- Bindels AJ, Westendorp RG, Frolich M, Seidell JC, Blokstra A, Smelt AH. The prevalence of subclinical hypothyroidism at different total plasma cholesterol levels in middle aged men and women: a need for case-finding? Clin Endocrinol (Oxf) 1999; 50(2):217–220. pmid:10396365
- Pearce EN. Hypothyroidism and dyslipidemia: modern concepts and approaches. Curr Cardiol Rep 2004; 6(6):451–456. pmid:15485607
- Pearce EN. Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab 2012; 97(2):326–333. doi:10.1210/jc.2011-2532
- Rizos CV, Elisaf MS, Liberopoulos EN. Effects of thyroid dysfunction on lipid profile. Open Cardiovasc Med J 2011; 5:76–84. doi:10.2174/1874192401105010076
- Peppa M, Betsi G, Dimitriadis G. Lipid abnormalities and cardiometabolic risk in patients with overt and subclinical thyroid disease. J Lipids 2011; 2011:575840. doi:10.1155/2011/575840
- Asvold BO, Vatten LJ, Nilsen TI, Bjoro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. the HUNT study. Eur J Endocrinol 2007;1 56(2):181–186. doi:10.1530/eje.1.02333
- Danese MD, Ladenson PW, Meinert CL, Powe NR. Clinical review 115: effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: a quantitative review of the literature. J Clin Endocrinol Metab 2000; 85(9):2993–3001. doi:10.1210/jcem.85.9.6841
- Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of L-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab 2007; 92(5):1715–1723. doi:10.1210/jc.2006-1869
- Abreu IM, Lau E, de Sousa Pinto B, Carvalho D. Subclinical hypothyroidism: to treat or not to treat, that is the question! A systematic review with meta-analysis on lipid profile. Endocr Connect 2017; 6(3):188–199. doi:10.1530/EC-17-0028
- Robison CD, Bair TL, Horne BD, et al. Hypothyroidism as a risk factor for statin intolerance. J Clin Lipidol 2014; 8(4):401–407. doi:10.1016/j.jacl.2014.05.005
- Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam study. Ann Intern Med 2000; 132(4):270–278. pmid:10681281
- Boekholdt SM, Titan SM, Wiersinga WM, et al. Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol (Oxf) 2010; 72(3):404–410. doi:10.1111/j.1365-2265.2009.03640.x
- Andersen MN, Olsen AM, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One 2015; 10(6):e0129793. doi:10.1371/journal.pone.0129793
- Biondi B. Cardiovascular effects of mild hypothyroidism. Thyroid 2007; 17(7):625–630. doi:10.1089/thy.2007.0158
- Brenta G, Mutti LA, Schnitman M, Fretes O, Perrone A, Matute ML. Assessment of left ventricular diastolic function by radionuclide ventriculography at rest and exercise in subclinical hypothyroidism, and its response to L-thyroxine therapy. Am J Cardiol 2003; 91(11):1327–1330. pmid:12767425
- Taddei S, Caraccio N, Virdis A, et al. Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: beneficial effect of levothyroxine therapy. J Clin Endocrinol Metab 2003; 88(8):3731–3737. doi:10.1210/jc.2003-030039
- Gao N, Zhang W, Zhang YZ, Yang Q, Chen SH. Carotid intima-media thickness in patients with subclinical hypothyroidism: a meta-analysis. Atherosclerosis 2013; 227(1):18–25. doi:10.1016/j.atherosclerosis.2012.10.070
- Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 2008; 29(1):76–131. doi:10.1210/er.2006-0043
- Chaker L, Baumgartner C, den Elzen WP, et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of stroke events and fatal stroke: an individual participant data analysis. J Clin Endocrinol Metab 2015; 100(6):2181–2191. doi:10.1210/jc.2015-1438
- Monzani F, Di Bello V, Caraccio N, et al. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. J Clin Endocrinol Metab 2001; 86(3):1110–1115. doi:10.1210/jcem.86.3.7291
- Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet 2001; 358(9285):861-865. doi:10.1016/S0140-6736(01)06067-6
- Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med 2012; 172(10):811–817. doi:10.1001/archinternmed.2012.1159
- Pasqualetti G, Tognini S, Polini A, Caraccio N, Monzani F. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab 2013; 98(6):2256–2266. doi:10.1210/jc.2012-3818
- Mooijaart SP, IEMO 80-plus Thyroid Trial Collaboration. Subclinical thyroid disorders. Lancet 2012; 380(9839):335. doi:10.1016/S0140-6736(12)61241-0
- Rodondi N, Bauer DC. Subclinical hypothyroidism and cardiovascular risk: how to end the controversy. J Clin Endocrinol Metab 2013; 98(6):2267–2269. doi:10.1210/jc.2013-1875
- Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med 2005; 165(21):2460–2466. doi:10.1001/archinte.165.21.2460
- Rodondi N, Bauer DC, Cappola AR, et al. Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure. The Cardiovascular Health study. J Am Coll Cardiol 2008; 52(14):1152–1159. doi:10.1016/j.jacc.2008.07.009
- Haggerty JJ Jr, Garbutt JC, Evans DL, et al. Subclinical hypothyroidism: a review of neuropsychiatric aspects. Int J Psychiatry Med 1990; 20(2):193–208. doi:10.2190/ADLY-1UU0-1A8L-HPXY
- Baldini IM, Vita A, Mauri MC, et al. Psychopathological and cognitive features in subclinical hypothyroidism. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21(6):925–935. pmid:9380789
- del Ser Quijano T, Delgado C, Martinez Espinosa S, Vazquez C. Cognitive deficiency in mild hypothyroidism. Neurologia 2000; 15(5):193–198. Spanish. pmid:10850118
- Correia N, Mullally S, Cooke G, et al. Evidence for a specific defect in hippocampal memory in overt and subclinical hypothyroidism. J Clin Endocrinol Metab 2009; 94(10):3789–3797. doi:10.1210/jc.2008-2702
- Aghili R, Khamseh ME, Malek M, et al. Changes of subtests of Wechsler memory scale and cognitive function in subjects with subclinical hypothyroidism following treatment with levothyroxine. Arch Med Sci 2012; 8(6):1096–1101. doi:10.5114/aoms.2012.32423
- Pasqualetti G, Pagano G, Rengo G, Ferrara N, Monzani F. Subclinical hypothyroidism and cognitive impairment: systematic review and meta-analysis. J Clin Endocrinol Metab 2015; 100(11):4240–4248. doi:10.1210/jc.2015-2046
- Christ-Crain M, Meier C, Huber PR, Staub J, Muller B. Effect of L-thyroxine replacement therapy on surrogate markers of skeletal and cardiac function in subclinical hypothyroidism. Endocrinologist 2004; 14(3):161–166. doi:10.1097/01.ten.0000127932.31710.4f
- Brennan MD, Powell C, Kaufman KR, Sun PC, Bahn RS, Nair KS. The impact of overt and subclinical hyperthyroidism on skeletal muscle. Thyroid 2006; 16(4):375–380. doi:10.1089/thy.2006.16.375
- Reuters VS, Teixeira Pde F, Vigario PS, et al. Functional capacity and muscular abnormalities in subclinical hypothyroidism. Am J Med Sci 2009; 338(4):259–263. doi:10.1097/MAJ.0b013e3181af7c7c
- Mainenti MR, Vigario PS, Teixeira PF, Maia MD, Oliveira FP, Vaisman M. Effect of levothyroxine replacement on exercise performance in subclinical hypothyroidism. J Endocrinol Invest 2009; 32(5):470–473. doi:10.3275/6106
- Lankhaar JA, de Vries WR, Jansen JA, Zelissen PM, Backx FJ. Impact of overt and subclinical hypothyroidism on exercise tolerance: a systematic review. Res Q Exerc Sport 2014; 85(3):365–389. doi:10.1080/02701367.2014.930405
- Lee JS, Buzkova P, Fink HA, et al. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch Intern Med 2010; 170(21):1876–1883. doi:10.1001/archinternmed.2010.424
- Svare A, Nilsen TI, Asvold BO, et al. Does thyroid function influence fracture risk? Prospective data from the HUNT2 study, Norway. Eur J Endocrinol 2013; 169(6):845–852. doi:10.1530/EJE-13-0546
- Di Mase R, Cerbone M, Improda N, et al. Bone health in children with long-term idiopathic subclinical hypothyroidism. Ital J Pediatr 2012; 38:56. doi:10.1186/1824-7288-38-56
- Boelaert K. The association between serum TSH concentration and thyroid cancer. Endocr Relat Cancer 2009; 16(4):1065–1072. doi:10.1677/ERC-09-0150
- Haymart MR, Glinberg SL, Liu J, Sippel RS, Jaume JC, Chen H. Higher serum TSH in thyroid cancer patients occurs independent of age and correlates with extrathyroidal extension. Clin Endocrinol (Oxf) 2009; 71(3):434–439. doi:10.1111/j.1365-2265.2008.03489.x
- Fiore E, Vitti P. Serum TSH and risk of papillary thyroid cancer in nodular thyroid disease. J Clin Endocrinol Metab 2012; 97(4):1134–1145. doi:10.1210/jc.2011-2735
- Fiore E, Rago T, Provenzale MA, et al. L-thyroxine-treated patients with nodular goiter have lower serum TSH and lower frequency of papillary thyroid cancer: results of a cross-sectional study on 27,914 patients. Endocr Relat Cancer 2010; 17(1):231–239. doi:10.1677/ERC-09-0251
- Hercbergs AH, Ashur-Fabian O, Garfield D. Thyroid hormones and cancer: clinical studies of hypothyroidism in oncology. Curr Opin Endocrinol Diabetes Obes 2010; 17(5):432–436. doi:10.1097/MED.0b013e32833d9710
- Thvilum M, Brandt F, Brix TH, Hegedus L. A review of the evidence for and against increased mortality in hypothyroidism. Nat Rev Endocrinol 2012; 8(7):417–424. doi:10.1038/nrendo.2012.29
- Stott DJ, Rodondi N, Kearney PM, et al; TRUST Study Group. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med 2017; 376(26):2534–2544. doi:10.1056/NEJMoa1603825
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril 2015; 104(3):545–753. doi:10.1016/j.fertnstert.2015.05.028
- Stagnaro-Green A, Abalovich M, Alexander E, et al; American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011; 21(10):1081–1125. doi:10.1089/thy.2011.0087
- Goldsmith RE, Sturgis SH, Lerman J, Stanbury JB. The menstrual pattern in thyroid disease. J Clin Endocrinol Metab. 1952; 12(7):846-855. doi:10.1210/jcem-12-7-846
- Plowden TC, Schisterman EF, Sjaarda LA, et al. Subclinical hypothyroidism and thyroid autoimmunity are not associated with fecundity, pregnancy loss, or live birth. J Clin Endocrinol Metab 2016; 101(6):2358–2365. doi:10.1210/jc.2016-1049
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017; 27(3):315–389. doi:10.1089/thy.2016.0457
- Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab 2006; 91(7):2587–2591. doi:10.1210/jc.2005-1603
- Panesar NS, Li CY, Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem 2001; 38(pt 4):329–332. doi:10.1258/0004563011900830
- Lepoutre T, Debieve F, Gruson D, Daumerie C. Reduction of miscarriages through universal screening and treatment of thyroid autoimmune diseases. Gynecol Obstet Invest 2012; 74(4):265–273. doi:10.1159/000343759
- De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97(8):2543–2565. doi:10.1210/jc.2011-2803
- Crawford NM, Steiner AZ. Thyroid autoimmunity and reproductive function. Semin Reprod Med 2016; 34(6):343–350. doi:10.1055/s-0036-1593485
- Maraka S, Ospina NM, O’Keeffe DT, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid 2016; 26(4):580–590. doi:10.1089/thy.2015.0418
- Wiles KS, Jarvis S, Nelson-Piercy C. Are we overtreating subclinical hypothyroidism in pregnancy? BMJ 2015; 351:h4726. doi:10.1136/bmj.h4726
- Tudela CM, Casey BM, McIntire DD, Cunningham FG. Relationship of subclinical thyroid disease to the incidence of gestational diabetes. Obstet Gynecol 2012; 119(5):983–988. doi:10.1097/AOG.0b013e318250aeeb
- Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European Thyroid Association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J 2014; 3(2):76–94. doi:10.1159/000362597
- Karakosta P, Alegakis D, Georgiou V, et al. Thyroid dysfunction and autoantibodies in early pregnancy are associated with increased risk of gestational diabetes and adverse birth outcomes. J Clin Endocrinol Metab 2012; 97(12):4464–4472. doi:10.1210/jc.2012-2540
- Toulis KA, Stagnaro-Green A, Negro R. Maternal subclinical hypothyroidsm and gestational diabetes mellitus: a meta-analysis. Endocr Pract 2014; 20(7):703–714. doi:10.4158/EP13440.RA
- van den Boogaard E, Vissenberg R, Land JA, et al. Significance of subclinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update 2011; 17(5):605–619. doi:10.1093/humupd/dmr024
- Wilson KL, Casey BM, McIntire DD, Halvorson LM, Cunningham FG. Subclinical thyroid disease and the incidence of hypertension in pregnancy. Obstet Gynecol 2012; 119(2 Pt 1):315–320. doi:10.1097/AOG.0b013e318240de6a
- Ashoor G, Maiz N, Rotas M, Jawdat F, Nicolaides KH. Maternal thyroid function at 11 to 13 weeks of gestation and subsequent fetal death. Thyroid 2010; 20(9):989–993. doi:10.1089/thy.2010.0058
- Casey BM, Dashe JS, Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol 2005; 105(2):239–245. doi:10.1097/01.AOG.0000152345.99421.22
- Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab 2010; 95(9):E44–E48. doi:10.1210/jc.2010-0340
- Su PY, Huang K, Hao JH, et al. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab 2011; 96(10):3234–3241. doi:10.1210/jc.2011-0274
- Allan WC, Haddow JE, Palomaki GE, et al. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen 2000; 7(3):127–130. doi:10.1136/jms.7.3.127
- Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol 2009; 160(6):985–991. doi:10.1530/EJE-08-0953
- Korevaar TI, Medici M, de Rijke YB, et al. Ethnic differences in maternal thyroid parameters during pregnancy: the generation R study. J Clin Endocrinol Metab 2013; 98(9):3678–3686. doi:10.1210/jc.2013-2005
- Cleary-Goldman J, Malone FD, Lambert-Messerlian G, et al. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol 2008; 112(1):85–92. doi:10.1097/AOG.0b013e3181788dd7
- Li Y, Shan Z, Teng W, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin Endocrinol (Oxf) 2010; 72(6):825–829. doi:10.1111/j.1365-2265.2009.03743.x
- Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 1999; 341(8):549–555. doi:10.1056/NEJM199908193410801
- Henrichs J, Bongers-Schokking JJ, Schenk JJ, et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab 2010; 95(9):4227–4234. doi:10.1210/jc.2010-0415
- Behrooz HG, Tohidi M, Mehrabi Y, Behrooz EG, Tehranidoost M, Azizi F. Subclinical hypothyroidism in pregnancy: intellectual development of offspring. Thyroid 2011; 21(10):1143–1147. doi:10.1089/thy.2011.0053
- Julvez J, Alvarez-Pedrerol M, Rebagliato M, et al. Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiology 2013; 24(1):150–157. doi:10.1097/EDE.0b013e318276ccd3
- Casey BM, Thom EA, Peaceman AM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med 2017; 376(9):815–825. doi:10.1056/NEJMoa1606205
- Burns RB, Bates CK, Hartzband P, Smetana GW. Should we treat for subclinical hypothyroidism?: Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med 2016; 164(11):764–770. doi:10.7326/M16-0857
- Kucukler FK, Akbaba G, Arduc A, Simsek Y, Guler S. Evaluation of the common mistakes made by patients in the use of levothyroxine. Eur J Intern Med 2014; 25(9):e107–e108. doi:10.1016/j.ejim.2014.09.002
- McMillan M, Rotenberg KS, Vora K, et al. Comorbidities, concomitant medications, and diet as factors affecting levothyroxine therapy: results of the CONTROL surveillance project. Drugs R D 2016; 16(1):53–68. doi:10.1007/s40268-015-0116-6
- Pollock MA, Sturrock A, Marshall K, et al. Thyroxine treatment in patients with symptoms of hypothyroidism but thyroid function tests within the reference range: Randomised double blind placebo controlled crossover trial. BMJ 2001; 323(7318):891–895. pmid:11668132
- Peeters RP. Subclinical hypothyroidism. N Engl J Med 2017; 376(26):2556–2565. doi:10.1056/NEJMcp1611144
KEY POINTS
- From 4% to 20% of adults have subclinical hypothyroidism, with a higher prevalence in women, older people, and those with thyroid autoimmunity.
- Subclinical hypothyroidism can progress to overt hypothyroidism, especially if antithyroid antibodies are present, and has been associated with adverse metabolic, cardiovascular, reproductive, maternal-fetal, neuromuscular, and cognitive abnormalities and lower quality of life.
- Some studies have suggested that levothyroxine therapy is beneficial, but others have not, possibly owing to variability in study designs, sample sizes, and patient populations.
- Further trials are needed to clearly demonstrate the clinical impact of subclinical hypothyroidism and the effect of levothyroxine therapy.
There is more to the TSH than a number
At a quick read, the messages from these articles may seem contradictory. But the biology is more complex in the setting of endogenous production of T4 by the thyroid gland, which is regulated by TSH, which in turn is regulated in a feedback loop by the thyroid-produced T4. In the setting of a fixed replacement dose of exogenous levothyroxine, the provided hormone affects the pituitary production of TSH, which likely will have no significant subsequent effect on the T4 level. Thus, the feedback control loop is far simpler.
There has not been a definitive study demonstrating that thyroxine supplementation in patients with subclinical hypothyroidism results in a superior clinical outcome. There are hints that this may be the case, and Azim and Nasr cite some of these studies. Recognizing a few markedly different physiologic reasons why the TSH can be slightly elevated and the T4 normal helps explain the lack of uniform clinical success with supplementation therapy and provides rationales for some management strategies.
Any biological variability in the responsiveness of the thyroid gland to TSH may affect the relationship between the levels of TSH and thyroid gland-released T4. In theory, if the thyroid receptor has decreased affinity for TSH, a higher TSH concentration will be needed to get the thyroid gland to secrete the level of T4 that the pituitary sensing mechanism deems normal for that individual. If the receptor affinity was decreased due to a gene polymorphism, this relationship between TSH and T4 may be stable, and providing exogenous T4 will result in a lower, “normalized” TSH level but may disrupt the thyroid-pituitary crosstalk and may even produce clinical hyperthyroidism.
A similar scenario exists in the setting of early thyroid gland failure, such as in Hashimoto thyroiditis. But in the latter scenario, the TSH-to-T4 production relationship may be unstable over time, for as additional thyroid gland is destroyed, T4 production will continue to decrease, the TSH will increase, and the thyroid gland may ultimately fail and hypothyroidism will occur. Hence the recommendation that in the setting of subclinical hypothyroidism and antiperoxidase antibodies, T4 and TSH levels should be monitored regularly in order to detect early true thyroid gland failure when the T4 level can no longer be maintained despite the increased stimulation of the gland by the elevated TSH. Analogous to this may be subclinical hypothyroidism in the elderly, in whom thyroid gland failure may develop, despite an increased TSH, from senescence rather than autoimmunity. What I am suggesting is that the natural history of all patients with subclinical hypothyroidism is not alike, and it thus should not be surprising that there does not seem to be a one-size-fits-all approach to management.
Symptoms in patients with subclinical hypothyroidism have not uniformly improved with T4 treatment compared with placebo. Notably, most patients with subclinical hypothyroidism experience no symptoms. But consider the extremely common symptom of fatigue, which can be present for a myriad of defined and undefined reasons. This symptom may often lead physicians to check the TSH and, if that is even slightly elevated, to also check the T4. It may also lead some physicians to routinely check the T4. Subclinical hypothyroidism is also quite common; thus, by chance alone or because of the circadian timing of checking the TSH, a slightly elevated TSH and fatigue may coexist and yet be unrelated.
Additionally, a positive biochemical response to thyroxine supplementation, such as a lowering of cholesterol, does not prove that the patient was clinically hypothyroid prior to supplementation, any more than lowering a patient’s blood glucose with insulin proves that the patient was diabetic. The management of subclinical hypothyroidism should be nuanced and based on both clinical and laboratory parameters.
- Nasr C. Is a serum TSH measurement sufficient to monitor the treatment of primary hypothyroidism? Cleve Clin J Med 2016; 83(8):571–573. doi:10.3949/ccjm.83a.15165
- Mandell BF. Trust the thyroid thermostat. Cleve Clin J Med 2016; 83(8):552–553. doi:10.3949/ccjm.83b.08016
At a quick read, the messages from these articles may seem contradictory. But the biology is more complex in the setting of endogenous production of T4 by the thyroid gland, which is regulated by TSH, which in turn is regulated in a feedback loop by the thyroid-produced T4. In the setting of a fixed replacement dose of exogenous levothyroxine, the provided hormone affects the pituitary production of TSH, which likely will have no significant subsequent effect on the T4 level. Thus, the feedback control loop is far simpler.
There has not been a definitive study demonstrating that thyroxine supplementation in patients with subclinical hypothyroidism results in a superior clinical outcome. There are hints that this may be the case, and Azim and Nasr cite some of these studies. Recognizing a few markedly different physiologic reasons why the TSH can be slightly elevated and the T4 normal helps explain the lack of uniform clinical success with supplementation therapy and provides rationales for some management strategies.
Any biological variability in the responsiveness of the thyroid gland to TSH may affect the relationship between the levels of TSH and thyroid gland-released T4. In theory, if the thyroid receptor has decreased affinity for TSH, a higher TSH concentration will be needed to get the thyroid gland to secrete the level of T4 that the pituitary sensing mechanism deems normal for that individual. If the receptor affinity was decreased due to a gene polymorphism, this relationship between TSH and T4 may be stable, and providing exogenous T4 will result in a lower, “normalized” TSH level but may disrupt the thyroid-pituitary crosstalk and may even produce clinical hyperthyroidism.
A similar scenario exists in the setting of early thyroid gland failure, such as in Hashimoto thyroiditis. But in the latter scenario, the TSH-to-T4 production relationship may be unstable over time, for as additional thyroid gland is destroyed, T4 production will continue to decrease, the TSH will increase, and the thyroid gland may ultimately fail and hypothyroidism will occur. Hence the recommendation that in the setting of subclinical hypothyroidism and antiperoxidase antibodies, T4 and TSH levels should be monitored regularly in order to detect early true thyroid gland failure when the T4 level can no longer be maintained despite the increased stimulation of the gland by the elevated TSH. Analogous to this may be subclinical hypothyroidism in the elderly, in whom thyroid gland failure may develop, despite an increased TSH, from senescence rather than autoimmunity. What I am suggesting is that the natural history of all patients with subclinical hypothyroidism is not alike, and it thus should not be surprising that there does not seem to be a one-size-fits-all approach to management.
Symptoms in patients with subclinical hypothyroidism have not uniformly improved with T4 treatment compared with placebo. Notably, most patients with subclinical hypothyroidism experience no symptoms. But consider the extremely common symptom of fatigue, which can be present for a myriad of defined and undefined reasons. This symptom may often lead physicians to check the TSH and, if that is even slightly elevated, to also check the T4. It may also lead some physicians to routinely check the T4. Subclinical hypothyroidism is also quite common; thus, by chance alone or because of the circadian timing of checking the TSH, a slightly elevated TSH and fatigue may coexist and yet be unrelated.
Additionally, a positive biochemical response to thyroxine supplementation, such as a lowering of cholesterol, does not prove that the patient was clinically hypothyroid prior to supplementation, any more than lowering a patient’s blood glucose with insulin proves that the patient was diabetic. The management of subclinical hypothyroidism should be nuanced and based on both clinical and laboratory parameters.
At a quick read, the messages from these articles may seem contradictory. But the biology is more complex in the setting of endogenous production of T4 by the thyroid gland, which is regulated by TSH, which in turn is regulated in a feedback loop by the thyroid-produced T4. In the setting of a fixed replacement dose of exogenous levothyroxine, the provided hormone affects the pituitary production of TSH, which likely will have no significant subsequent effect on the T4 level. Thus, the feedback control loop is far simpler.
There has not been a definitive study demonstrating that thyroxine supplementation in patients with subclinical hypothyroidism results in a superior clinical outcome. There are hints that this may be the case, and Azim and Nasr cite some of these studies. Recognizing a few markedly different physiologic reasons why the TSH can be slightly elevated and the T4 normal helps explain the lack of uniform clinical success with supplementation therapy and provides rationales for some management strategies.
Any biological variability in the responsiveness of the thyroid gland to TSH may affect the relationship between the levels of TSH and thyroid gland-released T4. In theory, if the thyroid receptor has decreased affinity for TSH, a higher TSH concentration will be needed to get the thyroid gland to secrete the level of T4 that the pituitary sensing mechanism deems normal for that individual. If the receptor affinity was decreased due to a gene polymorphism, this relationship between TSH and T4 may be stable, and providing exogenous T4 will result in a lower, “normalized” TSH level but may disrupt the thyroid-pituitary crosstalk and may even produce clinical hyperthyroidism.
A similar scenario exists in the setting of early thyroid gland failure, such as in Hashimoto thyroiditis. But in the latter scenario, the TSH-to-T4 production relationship may be unstable over time, for as additional thyroid gland is destroyed, T4 production will continue to decrease, the TSH will increase, and the thyroid gland may ultimately fail and hypothyroidism will occur. Hence the recommendation that in the setting of subclinical hypothyroidism and antiperoxidase antibodies, T4 and TSH levels should be monitored regularly in order to detect early true thyroid gland failure when the T4 level can no longer be maintained despite the increased stimulation of the gland by the elevated TSH. Analogous to this may be subclinical hypothyroidism in the elderly, in whom thyroid gland failure may develop, despite an increased TSH, from senescence rather than autoimmunity. What I am suggesting is that the natural history of all patients with subclinical hypothyroidism is not alike, and it thus should not be surprising that there does not seem to be a one-size-fits-all approach to management.
Symptoms in patients with subclinical hypothyroidism have not uniformly improved with T4 treatment compared with placebo. Notably, most patients with subclinical hypothyroidism experience no symptoms. But consider the extremely common symptom of fatigue, which can be present for a myriad of defined and undefined reasons. This symptom may often lead physicians to check the TSH and, if that is even slightly elevated, to also check the T4. It may also lead some physicians to routinely check the T4. Subclinical hypothyroidism is also quite common; thus, by chance alone or because of the circadian timing of checking the TSH, a slightly elevated TSH and fatigue may coexist and yet be unrelated.
Additionally, a positive biochemical response to thyroxine supplementation, such as a lowering of cholesterol, does not prove that the patient was clinically hypothyroid prior to supplementation, any more than lowering a patient’s blood glucose with insulin proves that the patient was diabetic. The management of subclinical hypothyroidism should be nuanced and based on both clinical and laboratory parameters.
- Nasr C. Is a serum TSH measurement sufficient to monitor the treatment of primary hypothyroidism? Cleve Clin J Med 2016; 83(8):571–573. doi:10.3949/ccjm.83a.15165
- Mandell BF. Trust the thyroid thermostat. Cleve Clin J Med 2016; 83(8):552–553. doi:10.3949/ccjm.83b.08016
- Nasr C. Is a serum TSH measurement sufficient to monitor the treatment of primary hypothyroidism? Cleve Clin J Med 2016; 83(8):571–573. doi:10.3949/ccjm.83a.15165
- Mandell BF. Trust the thyroid thermostat. Cleve Clin J Med 2016; 83(8):552–553. doi:10.3949/ccjm.83b.08016
Aleukemic leukemia cutis
On examination, the numerous firm, indurated nodules ranged in size from 1 to 4 cm. There was no palpable lymphadenopathy.
Results of a peripheral blood cell count showed the following:
- Hemoglobin 12.5 g/dL (reference range 13.0–17.0)
- Platelet count 154 × 109/L (130–400)
- White blood cell count 5.0 × 109/L (4.0–11.0)
- Neutrophils 1.7 × 109/L (1.5–8.0)
- Lymphocytes 2.2 × 109/L (1.0–4.0)
- Monocytes 1.0 × 109/L (0.2–1.0)
- Eosinophils 0 (0–0.4)
- Basophils 0 (0–0.2)
- Blasts 0.
The findings were consistent with leukemic cells with monocytic differentiation. The infiltrate was unusual because leukemic infiltrates typically demonstrate a high nuclear-to-cytoplasmic ratio, but in this case the malignant cells had moderate amounts of cytoplasm due to the monocytic differentiation. Also, a grenz zone is more typically seen in B-cell lymphomas, and T cells more typically demonstrate epidermotropism.
Bone marrow aspiration was performed and revealed a hypercellular bone marrow with trilineage maturation with only 2% blasts. The fluorescence in situ hybridization testing for myelodysplastic syndrome and acute myeloid leukemia was normal. A diagnosis of aleukemic leukemia cutis was made.
After 2 months of chemotherapy with azacitidine, the nodules were less indurated. Treatment was briefly withdrawn due to the development of acute pneumonia, leading to a rapid progression of cutaneous involvement. Despite restarting chemotherapy, the patient died.
ALEUKEMIC LEUKEMIA CUTIS
The differential diagnosis of leukemia cutis is diverse and extensive. Patients often present with painless, firm, indurated nodules, papules, and plaques.1 The lesions can be small, involving a small amount of body surface area, but can also be very large and diffuse.
In our patient’s case, there were no new drugs or exposures to suggest a drug-related eruption, or pruritus or pain to suggest an inflammatory process. The rapid progression of the lesions suggested either an infectious or malignant process. The top 3 conditions in the differential diagnosis, based on his clinical presentation, were cutaneous T-cell lymphoma, cutaneous CD30+ anaplastic large-cell lymphoma, and a drug-induced cutaneous pseudolymphoma.
Skin biopsy is required to differentiate leukemia cutis from the other conditions. On skin biopsy study, leukemia cutis is characterized by infiltration of the skin by leukemic cells and is seen in 10% to 15% of patients with acute myeloid leukemia.2 In 5% of cases, leukemia cutis can present without bone marrow or peripheral signs of leukemia, hence the term aleukemic leukemia cutis.3 Cutaneous signs can occur before, after, or simultaneously with systemic leukemia.4
In the absence of systemic symptoms, the diagnosis is made when progressive cutaneous symptoms are present. The prognosis for aleukemic leukemia cutis is poor. Prompt diagnosis with skin biopsy is paramount to improve outcomes.
Acknowledgment: We would like to recognize Maanasa Devabhaktuni for her assistance in reporting this case.
- Yonal I, Hindilerden F, Coskun R, Dogan OI, Nalcaci M. Aleukemic leukemia cutis manifesting with disseminated nodular eruptions and a plaque preceding acute monocytic leukemia: a case report. Case Rep Oncol 2011; 4(3):547–554. doi:10.1159/000334745
- Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol 2008; 129(1):130–142. doi:10.1309/WYACYWF6NGM3WBRT
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci 2013; 28(4):614–619. doi:10.3346/jkms.2013.28.4.614
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis. Dermatol Online J 2015; 21(8)pii:13030/qt6m18g35f. pmid:26437164
On examination, the numerous firm, indurated nodules ranged in size from 1 to 4 cm. There was no palpable lymphadenopathy.
Results of a peripheral blood cell count showed the following:
- Hemoglobin 12.5 g/dL (reference range 13.0–17.0)
- Platelet count 154 × 109/L (130–400)
- White blood cell count 5.0 × 109/L (4.0–11.0)
- Neutrophils 1.7 × 109/L (1.5–8.0)
- Lymphocytes 2.2 × 109/L (1.0–4.0)
- Monocytes 1.0 × 109/L (0.2–1.0)
- Eosinophils 0 (0–0.4)
- Basophils 0 (0–0.2)
- Blasts 0.
The findings were consistent with leukemic cells with monocytic differentiation. The infiltrate was unusual because leukemic infiltrates typically demonstrate a high nuclear-to-cytoplasmic ratio, but in this case the malignant cells had moderate amounts of cytoplasm due to the monocytic differentiation. Also, a grenz zone is more typically seen in B-cell lymphomas, and T cells more typically demonstrate epidermotropism.
Bone marrow aspiration was performed and revealed a hypercellular bone marrow with trilineage maturation with only 2% blasts. The fluorescence in situ hybridization testing for myelodysplastic syndrome and acute myeloid leukemia was normal. A diagnosis of aleukemic leukemia cutis was made.
After 2 months of chemotherapy with azacitidine, the nodules were less indurated. Treatment was briefly withdrawn due to the development of acute pneumonia, leading to a rapid progression of cutaneous involvement. Despite restarting chemotherapy, the patient died.
ALEUKEMIC LEUKEMIA CUTIS
The differential diagnosis of leukemia cutis is diverse and extensive. Patients often present with painless, firm, indurated nodules, papules, and plaques.1 The lesions can be small, involving a small amount of body surface area, but can also be very large and diffuse.
In our patient’s case, there were no new drugs or exposures to suggest a drug-related eruption, or pruritus or pain to suggest an inflammatory process. The rapid progression of the lesions suggested either an infectious or malignant process. The top 3 conditions in the differential diagnosis, based on his clinical presentation, were cutaneous T-cell lymphoma, cutaneous CD30+ anaplastic large-cell lymphoma, and a drug-induced cutaneous pseudolymphoma.
Skin biopsy is required to differentiate leukemia cutis from the other conditions. On skin biopsy study, leukemia cutis is characterized by infiltration of the skin by leukemic cells and is seen in 10% to 15% of patients with acute myeloid leukemia.2 In 5% of cases, leukemia cutis can present without bone marrow or peripheral signs of leukemia, hence the term aleukemic leukemia cutis.3 Cutaneous signs can occur before, after, or simultaneously with systemic leukemia.4
In the absence of systemic symptoms, the diagnosis is made when progressive cutaneous symptoms are present. The prognosis for aleukemic leukemia cutis is poor. Prompt diagnosis with skin biopsy is paramount to improve outcomes.
Acknowledgment: We would like to recognize Maanasa Devabhaktuni for her assistance in reporting this case.
On examination, the numerous firm, indurated nodules ranged in size from 1 to 4 cm. There was no palpable lymphadenopathy.
Results of a peripheral blood cell count showed the following:
- Hemoglobin 12.5 g/dL (reference range 13.0–17.0)
- Platelet count 154 × 109/L (130–400)
- White blood cell count 5.0 × 109/L (4.0–11.0)
- Neutrophils 1.7 × 109/L (1.5–8.0)
- Lymphocytes 2.2 × 109/L (1.0–4.0)
- Monocytes 1.0 × 109/L (0.2–1.0)
- Eosinophils 0 (0–0.4)
- Basophils 0 (0–0.2)
- Blasts 0.
The findings were consistent with leukemic cells with monocytic differentiation. The infiltrate was unusual because leukemic infiltrates typically demonstrate a high nuclear-to-cytoplasmic ratio, but in this case the malignant cells had moderate amounts of cytoplasm due to the monocytic differentiation. Also, a grenz zone is more typically seen in B-cell lymphomas, and T cells more typically demonstrate epidermotropism.
Bone marrow aspiration was performed and revealed a hypercellular bone marrow with trilineage maturation with only 2% blasts. The fluorescence in situ hybridization testing for myelodysplastic syndrome and acute myeloid leukemia was normal. A diagnosis of aleukemic leukemia cutis was made.
After 2 months of chemotherapy with azacitidine, the nodules were less indurated. Treatment was briefly withdrawn due to the development of acute pneumonia, leading to a rapid progression of cutaneous involvement. Despite restarting chemotherapy, the patient died.
ALEUKEMIC LEUKEMIA CUTIS
The differential diagnosis of leukemia cutis is diverse and extensive. Patients often present with painless, firm, indurated nodules, papules, and plaques.1 The lesions can be small, involving a small amount of body surface area, but can also be very large and diffuse.
In our patient’s case, there were no new drugs or exposures to suggest a drug-related eruption, or pruritus or pain to suggest an inflammatory process. The rapid progression of the lesions suggested either an infectious or malignant process. The top 3 conditions in the differential diagnosis, based on his clinical presentation, were cutaneous T-cell lymphoma, cutaneous CD30+ anaplastic large-cell lymphoma, and a drug-induced cutaneous pseudolymphoma.
Skin biopsy is required to differentiate leukemia cutis from the other conditions. On skin biopsy study, leukemia cutis is characterized by infiltration of the skin by leukemic cells and is seen in 10% to 15% of patients with acute myeloid leukemia.2 In 5% of cases, leukemia cutis can present without bone marrow or peripheral signs of leukemia, hence the term aleukemic leukemia cutis.3 Cutaneous signs can occur before, after, or simultaneously with systemic leukemia.4
In the absence of systemic symptoms, the diagnosis is made when progressive cutaneous symptoms are present. The prognosis for aleukemic leukemia cutis is poor. Prompt diagnosis with skin biopsy is paramount to improve outcomes.
Acknowledgment: We would like to recognize Maanasa Devabhaktuni for her assistance in reporting this case.
- Yonal I, Hindilerden F, Coskun R, Dogan OI, Nalcaci M. Aleukemic leukemia cutis manifesting with disseminated nodular eruptions and a plaque preceding acute monocytic leukemia: a case report. Case Rep Oncol 2011; 4(3):547–554. doi:10.1159/000334745
- Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol 2008; 129(1):130–142. doi:10.1309/WYACYWF6NGM3WBRT
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci 2013; 28(4):614–619. doi:10.3346/jkms.2013.28.4.614
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis. Dermatol Online J 2015; 21(8)pii:13030/qt6m18g35f. pmid:26437164
- Yonal I, Hindilerden F, Coskun R, Dogan OI, Nalcaci M. Aleukemic leukemia cutis manifesting with disseminated nodular eruptions and a plaque preceding acute monocytic leukemia: a case report. Case Rep Oncol 2011; 4(3):547–554. doi:10.1159/000334745
- Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol 2008; 129(1):130–142. doi:10.1309/WYACYWF6NGM3WBRT
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci 2013; 28(4):614–619. doi:10.3346/jkms.2013.28.4.614
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis. Dermatol Online J 2015; 21(8)pii:13030/qt6m18g35f. pmid:26437164
Hip Fracture in Nursing Home Residents with Advanced Dementia: An Opportunity for Palliative Care
Study Overview
Objective. To compare clinical outcomes (mortality, pain, physical restraint use, pressure ulcer, antipsychotic drug use) in long-term care nursing home (NH) residents with advanced dementia and hip fracture who underwent surgical repair or nonsurgical management.
Design. A retrospective cohort study utilizing nationwide Medicare (Parts A, B, D and hospice) claims data linked with Centers for Medicare & Medicaid Services Minimum Data Set (MDS version 2.0) assessments.
Setting and participants. Long-stay NH residents older than 65 years with advanced dementia (defined as being assigned to Cognitive Performance Scale category 5 or 6 and a diagnosis of dementia or Alzheimer disease) and without a do not hospitalize (DNH) directive before hip fracture were identified by using MDS assessments completed from January 1, 2008 to December 31, 2013. Medicare (Part A – inpatient, or Part B – outpatient) claims data was then used to identify those residents who experienced a hip fracture within 2 years of the full MDS assessment using the International Classification of Diseases, Ninth Revision diagnostic codes. Procedure codes were used to determine whether a resident who experienced hip fracture underwent surgical repair.
Main outcome measures. The main outcome measure was all-cause mortality after hip fracture ascertained by the Medicare Enrollment File through 2013. The secondary outcome measures were documented pain, physical restraint use, pressure ulcers, antipsychotic drug use, and ambulatory status in NH residents who survived 6 months after hip fracture. These outcome measures were captured from the first MDS assessment completed between 120 and 240 days following the fracture or Medicare Part D claims. Documented pain was determined using a validated MDS 2.0 nursing assessment pain instrument within 7 days preceding MDS assessment. Physical restraint use was defined by the use of trunk, limb, or chair restraint within 7 days prior to MDS assessment. Pressure ulcer was defined as any stage 2 to 4 pressure ulcer. Antipsychotic drug use of any medication subclass was determined using Medicare Part D claims data and affirmative if drug was administered 180 days after hip fracture. Ambulatory status between 120 and 240 days following the fracture was determined in a subset of NH residents who were ambulatory before the hip fracture. The utilization of comfort-focused care after hip fracture was determined in NH residents who had a Medicare hospice claim or a new DNH directive in the 180 days after hip fracture.
The differences in survival among NH residents with advanced dementia and hip fracture were described by Kaplan-Meier curves. The association between surgical repair and survival was determined using multivariable Cox proportional hazards for all NH residents and stratified by pre-fracture ambulatory status. In those who survived 6 months after hip fracture, the associations between surgical repair and outcomes including documented pain, physical restraint use, pressure ulcers, antipsychotic drug use, and ambulatory status were determined using multivariable logistic regression models. Adjustment for differences in characteristics before hip fracture was performed using inverse probability of treatment weighting (IPTW) models.
Main results. 3083 long-stay NH residents with advanced dementia and hip fracture were included in the study. The cohort’s mean age was 84.2 ± 7.1 years, 79.2% were female (n = 2441), and 28.5% were ambulatory before hip fracture (n = 879). Of these NH residents, 84.8% (n = 2615) underwent surgical repair and 15.2% (n = 468) received nonsurgical management. At 6 months after hip fracture, mortality was 31.5% in the surgical group compared to 53.8% in the nonsurgical group. The greatest mortality difference between groups occurred in the first 30 days after hip fracture (11.5% in surgical group versus 30.6% in nonsurgical group). Surgical repair was associated with a decreased risk of death (Cox proportional hazard ratio) in the unadjusted (hazard ratio [HR], 0.55 [95% confidence interval {CI}, 0.49-0.61), multivariable adjusted (adjusted HR, 0.56 [95% CI, 0.49-0.63]), and IPTW (adjusted HR, 0.88 [95% CI, 0.79-0.98]) models. Similarly, surgically treated NH residents were less likely to die than those managed non-surgically when mortality was stratified by pre-fracture ambulatory status.
Among NH residents who survived 6 months after hip fracture, those who underwent surgical repair compared with those who received nonsurgical management had less documented pain (29.0% versus 30.9%), fewer pressure ulcers (11.2% versus 19.0%), greater physical restraint use (13.0% versus 11.1%), and greater antipsychotic drug use (29.5% versus 20.4%). In the adjusted IPTW models, surgical repair was associated with less pain (adjusted HR, 0.78 [95% CI, 0.61-0.99]) and fewer pressure ulcers (adjusted HR, 0.64 [95% CI, 0.47-0.86]).
Overall, 21.5% of NH residents utilized comfort-focused care within 6 months after hip fracture, with a mean time to utilization of hospice care of 56 ± 49 days. In those who were managed surgically, 19.3% utilized hospice care, as compared with 33.8% in those who did not receive surgical intervention. In NH residents who survived 6 months after hip fracture, only 1.1% in both groups acquired a DNH directive.
Conclusion. In older long-stay NH residents with advanced dementia and hip fracture, surgical repair was associated with lower all-cause mortality, less documented pain, and fewer pressure ulcers compared to nonsurgical management. However, adverse clinical outcomes such as pain, physical restraint use, pressure ulcers, and antipsychotic drug use were common regardless of treatment modality. The high incidence of these adverse outcomes and hazardous interventions, coupled with low utilization of comfort-focused care and DNH directive, highlight an opportunity to improve the quality of care in this vulnerable population.
Commentary
Hip fracture is very common in NH residents, with an overall incident rate of 2.3 per 100 person years and is associated with a high mortality rate of 36.2% by 6 months after fracture.1,2 Moreover, Neuman and colleagues have recently reported that among NH residents who have some degree of functional independence in locomotion prior to hip fracture, 54% either die or develop new total dependence in locomotion within 6 months of fracture and that severe cognitive impairment is a risk factor highly associated with these adverse outcomes.3 Despite this emerging knowledge, surgical repair of hip fracture remains the mainstay treatment in many NH residents in the hope of alleviating pain and improving mobility, and palliative care is considered only when patients are imminently dying or have deteriorated past the point of meaningful recovery. In cases of NH residents with advanced dementia whose life expectancy is limited and whose care goals may favor maintaining comfort, the health care proxies are frequently challenged with a difficult choice of either pursuing or foregoing surgical management—a complex medical decision to be made in the absence of sufficient evidence in this uniquely frail patient population.
The study reported by Berry and colleagues provides an important and timely investigation in examining associations of adverse clinical outcomes (mortality, pain, pressure ulcer) and hazardous interventions (physical restraint and antipsychotic drug use) in long-stay NH residents with advanced dementia and hip fracture who underwent surgical repair or nonsurgical management. The authors reported a 6-month mortality rate of 31.5% in NH residents who underwent surgical repair, an event rate similar to that reported by Neuman and colleagues. While surgical repair after hip fracture was associated with a decreased risk of death compared to nonsurgical management, high incidences of pain (29.0% to 30.9%) and pressure ulcers (11.2% to 19.0%), and frequent physical restraint use (11.1% to 13.0%) and antipsychotic drug use (20.4% to 29.5%) were noted in NH residents who survived 6 months after fracture regardless of treatment modality. These findings are consistent with the high rate of post-hip fracture functional disability previously reported by Neuman and colleagues, and highlight the trajectory of decline, frequent distressing symptoms, and prevalent use of pharmacologic and nonpharmacologic restraints in long-stay NH residents after hip fracture. Taken together, the low utilization of comfort-focused care (21.5%) and DNH directive (1.1%) in NH residents who survived 6 months suggest a missed opportunity to integrate palliative care in a patient population that stands to benefit from this intervention.
This study is the first to report the associations between hip fracture surgery and a reduction in adverse outcomes such as pain and pressure ulcer that commonly affect vulnerable NH residents with advanced dementia. This study was well designed and leveraged strengths of Medicare claims data linked with MDS assessments to capture outcome measures including pain, pressure ulcer, and restraint use that would otherwise be difficult to ascertain. However, as in all retrospective cohort design, there were limitations in this study. For instance, secondary outcomes were determined from a single time point (ie, first MDS assessment completed between 120 to 240 days following hip fracture) and thus data capture may be incomplete. Additionally, other conditions important to complex decision making in the care of frail older adults including postoperative complications (eg, delirium, infections, cardiac complications) and in-hospital mortality were not examined. Despite these limitations, this study has enhanced our understanding of the clinical course of long-term care NH residents with advanced dementia who endured hip fracture.
Applications for Clinical Practice
Patients’ goals of care should guide medical decision making in the management of hip fracture in NH residents with advanced dementia. The increased survival benefit of surgical repair of hip fracture in this patient population should be considered in the medical decision-making process if life-prolongation is preferred. However, palliative and hospice care need to be an important facet of discussion given the high rates of mortality, pain, pressure ulcer, and restraint use in this vulnerable subset of older adults.
—Fred Ko, MD, MS
1. Berry SD, Lee Y, Zullo AR, et al. Incidence of hip fracture in U.S. nursing homes. J Gerontol A Biol Sci Med Sci. 2016;71:1230-1234.
2. Neuman MD, Silber JH, Magaziner JS, et al. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern Med. 2014;174:1273-1280.
3. Berry SD, Rothbaum RR, Kiel DP, et al. Association of clinical outcomes with surgical repair of hip fractures vs nonsurgical management in nursing home residents with advanced dementia. JAMA Intern Med. 2018;178:774-780.
Study Overview
Objective. To compare clinical outcomes (mortality, pain, physical restraint use, pressure ulcer, antipsychotic drug use) in long-term care nursing home (NH) residents with advanced dementia and hip fracture who underwent surgical repair or nonsurgical management.
Design. A retrospective cohort study utilizing nationwide Medicare (Parts A, B, D and hospice) claims data linked with Centers for Medicare & Medicaid Services Minimum Data Set (MDS version 2.0) assessments.
Setting and participants. Long-stay NH residents older than 65 years with advanced dementia (defined as being assigned to Cognitive Performance Scale category 5 or 6 and a diagnosis of dementia or Alzheimer disease) and without a do not hospitalize (DNH) directive before hip fracture were identified by using MDS assessments completed from January 1, 2008 to December 31, 2013. Medicare (Part A – inpatient, or Part B – outpatient) claims data was then used to identify those residents who experienced a hip fracture within 2 years of the full MDS assessment using the International Classification of Diseases, Ninth Revision diagnostic codes. Procedure codes were used to determine whether a resident who experienced hip fracture underwent surgical repair.
Main outcome measures. The main outcome measure was all-cause mortality after hip fracture ascertained by the Medicare Enrollment File through 2013. The secondary outcome measures were documented pain, physical restraint use, pressure ulcers, antipsychotic drug use, and ambulatory status in NH residents who survived 6 months after hip fracture. These outcome measures were captured from the first MDS assessment completed between 120 and 240 days following the fracture or Medicare Part D claims. Documented pain was determined using a validated MDS 2.0 nursing assessment pain instrument within 7 days preceding MDS assessment. Physical restraint use was defined by the use of trunk, limb, or chair restraint within 7 days prior to MDS assessment. Pressure ulcer was defined as any stage 2 to 4 pressure ulcer. Antipsychotic drug use of any medication subclass was determined using Medicare Part D claims data and affirmative if drug was administered 180 days after hip fracture. Ambulatory status between 120 and 240 days following the fracture was determined in a subset of NH residents who were ambulatory before the hip fracture. The utilization of comfort-focused care after hip fracture was determined in NH residents who had a Medicare hospice claim or a new DNH directive in the 180 days after hip fracture.
The differences in survival among NH residents with advanced dementia and hip fracture were described by Kaplan-Meier curves. The association between surgical repair and survival was determined using multivariable Cox proportional hazards for all NH residents and stratified by pre-fracture ambulatory status. In those who survived 6 months after hip fracture, the associations between surgical repair and outcomes including documented pain, physical restraint use, pressure ulcers, antipsychotic drug use, and ambulatory status were determined using multivariable logistic regression models. Adjustment for differences in characteristics before hip fracture was performed using inverse probability of treatment weighting (IPTW) models.
Main results. 3083 long-stay NH residents with advanced dementia and hip fracture were included in the study. The cohort’s mean age was 84.2 ± 7.1 years, 79.2% were female (n = 2441), and 28.5% were ambulatory before hip fracture (n = 879). Of these NH residents, 84.8% (n = 2615) underwent surgical repair and 15.2% (n = 468) received nonsurgical management. At 6 months after hip fracture, mortality was 31.5% in the surgical group compared to 53.8% in the nonsurgical group. The greatest mortality difference between groups occurred in the first 30 days after hip fracture (11.5% in surgical group versus 30.6% in nonsurgical group). Surgical repair was associated with a decreased risk of death (Cox proportional hazard ratio) in the unadjusted (hazard ratio [HR], 0.55 [95% confidence interval {CI}, 0.49-0.61), multivariable adjusted (adjusted HR, 0.56 [95% CI, 0.49-0.63]), and IPTW (adjusted HR, 0.88 [95% CI, 0.79-0.98]) models. Similarly, surgically treated NH residents were less likely to die than those managed non-surgically when mortality was stratified by pre-fracture ambulatory status.
Among NH residents who survived 6 months after hip fracture, those who underwent surgical repair compared with those who received nonsurgical management had less documented pain (29.0% versus 30.9%), fewer pressure ulcers (11.2% versus 19.0%), greater physical restraint use (13.0% versus 11.1%), and greater antipsychotic drug use (29.5% versus 20.4%). In the adjusted IPTW models, surgical repair was associated with less pain (adjusted HR, 0.78 [95% CI, 0.61-0.99]) and fewer pressure ulcers (adjusted HR, 0.64 [95% CI, 0.47-0.86]).
Overall, 21.5% of NH residents utilized comfort-focused care within 6 months after hip fracture, with a mean time to utilization of hospice care of 56 ± 49 days. In those who were managed surgically, 19.3% utilized hospice care, as compared with 33.8% in those who did not receive surgical intervention. In NH residents who survived 6 months after hip fracture, only 1.1% in both groups acquired a DNH directive.
Conclusion. In older long-stay NH residents with advanced dementia and hip fracture, surgical repair was associated with lower all-cause mortality, less documented pain, and fewer pressure ulcers compared to nonsurgical management. However, adverse clinical outcomes such as pain, physical restraint use, pressure ulcers, and antipsychotic drug use were common regardless of treatment modality. The high incidence of these adverse outcomes and hazardous interventions, coupled with low utilization of comfort-focused care and DNH directive, highlight an opportunity to improve the quality of care in this vulnerable population.
Commentary
Hip fracture is very common in NH residents, with an overall incident rate of 2.3 per 100 person years and is associated with a high mortality rate of 36.2% by 6 months after fracture.1,2 Moreover, Neuman and colleagues have recently reported that among NH residents who have some degree of functional independence in locomotion prior to hip fracture, 54% either die or develop new total dependence in locomotion within 6 months of fracture and that severe cognitive impairment is a risk factor highly associated with these adverse outcomes.3 Despite this emerging knowledge, surgical repair of hip fracture remains the mainstay treatment in many NH residents in the hope of alleviating pain and improving mobility, and palliative care is considered only when patients are imminently dying or have deteriorated past the point of meaningful recovery. In cases of NH residents with advanced dementia whose life expectancy is limited and whose care goals may favor maintaining comfort, the health care proxies are frequently challenged with a difficult choice of either pursuing or foregoing surgical management—a complex medical decision to be made in the absence of sufficient evidence in this uniquely frail patient population.
The study reported by Berry and colleagues provides an important and timely investigation in examining associations of adverse clinical outcomes (mortality, pain, pressure ulcer) and hazardous interventions (physical restraint and antipsychotic drug use) in long-stay NH residents with advanced dementia and hip fracture who underwent surgical repair or nonsurgical management. The authors reported a 6-month mortality rate of 31.5% in NH residents who underwent surgical repair, an event rate similar to that reported by Neuman and colleagues. While surgical repair after hip fracture was associated with a decreased risk of death compared to nonsurgical management, high incidences of pain (29.0% to 30.9%) and pressure ulcers (11.2% to 19.0%), and frequent physical restraint use (11.1% to 13.0%) and antipsychotic drug use (20.4% to 29.5%) were noted in NH residents who survived 6 months after fracture regardless of treatment modality. These findings are consistent with the high rate of post-hip fracture functional disability previously reported by Neuman and colleagues, and highlight the trajectory of decline, frequent distressing symptoms, and prevalent use of pharmacologic and nonpharmacologic restraints in long-stay NH residents after hip fracture. Taken together, the low utilization of comfort-focused care (21.5%) and DNH directive (1.1%) in NH residents who survived 6 months suggest a missed opportunity to integrate palliative care in a patient population that stands to benefit from this intervention.
This study is the first to report the associations between hip fracture surgery and a reduction in adverse outcomes such as pain and pressure ulcer that commonly affect vulnerable NH residents with advanced dementia. This study was well designed and leveraged strengths of Medicare claims data linked with MDS assessments to capture outcome measures including pain, pressure ulcer, and restraint use that would otherwise be difficult to ascertain. However, as in all retrospective cohort design, there were limitations in this study. For instance, secondary outcomes were determined from a single time point (ie, first MDS assessment completed between 120 to 240 days following hip fracture) and thus data capture may be incomplete. Additionally, other conditions important to complex decision making in the care of frail older adults including postoperative complications (eg, delirium, infections, cardiac complications) and in-hospital mortality were not examined. Despite these limitations, this study has enhanced our understanding of the clinical course of long-term care NH residents with advanced dementia who endured hip fracture.
Applications for Clinical Practice
Patients’ goals of care should guide medical decision making in the management of hip fracture in NH residents with advanced dementia. The increased survival benefit of surgical repair of hip fracture in this patient population should be considered in the medical decision-making process if life-prolongation is preferred. However, palliative and hospice care need to be an important facet of discussion given the high rates of mortality, pain, pressure ulcer, and restraint use in this vulnerable subset of older adults.
—Fred Ko, MD, MS
Study Overview
Objective. To compare clinical outcomes (mortality, pain, physical restraint use, pressure ulcer, antipsychotic drug use) in long-term care nursing home (NH) residents with advanced dementia and hip fracture who underwent surgical repair or nonsurgical management.
Design. A retrospective cohort study utilizing nationwide Medicare (Parts A, B, D and hospice) claims data linked with Centers for Medicare & Medicaid Services Minimum Data Set (MDS version 2.0) assessments.
Setting and participants. Long-stay NH residents older than 65 years with advanced dementia (defined as being assigned to Cognitive Performance Scale category 5 or 6 and a diagnosis of dementia or Alzheimer disease) and without a do not hospitalize (DNH) directive before hip fracture were identified by using MDS assessments completed from January 1, 2008 to December 31, 2013. Medicare (Part A – inpatient, or Part B – outpatient) claims data was then used to identify those residents who experienced a hip fracture within 2 years of the full MDS assessment using the International Classification of Diseases, Ninth Revision diagnostic codes. Procedure codes were used to determine whether a resident who experienced hip fracture underwent surgical repair.
Main outcome measures. The main outcome measure was all-cause mortality after hip fracture ascertained by the Medicare Enrollment File through 2013. The secondary outcome measures were documented pain, physical restraint use, pressure ulcers, antipsychotic drug use, and ambulatory status in NH residents who survived 6 months after hip fracture. These outcome measures were captured from the first MDS assessment completed between 120 and 240 days following the fracture or Medicare Part D claims. Documented pain was determined using a validated MDS 2.0 nursing assessment pain instrument within 7 days preceding MDS assessment. Physical restraint use was defined by the use of trunk, limb, or chair restraint within 7 days prior to MDS assessment. Pressure ulcer was defined as any stage 2 to 4 pressure ulcer. Antipsychotic drug use of any medication subclass was determined using Medicare Part D claims data and affirmative if drug was administered 180 days after hip fracture. Ambulatory status between 120 and 240 days following the fracture was determined in a subset of NH residents who were ambulatory before the hip fracture. The utilization of comfort-focused care after hip fracture was determined in NH residents who had a Medicare hospice claim or a new DNH directive in the 180 days after hip fracture.
The differences in survival among NH residents with advanced dementia and hip fracture were described by Kaplan-Meier curves. The association between surgical repair and survival was determined using multivariable Cox proportional hazards for all NH residents and stratified by pre-fracture ambulatory status. In those who survived 6 months after hip fracture, the associations between surgical repair and outcomes including documented pain, physical restraint use, pressure ulcers, antipsychotic drug use, and ambulatory status were determined using multivariable logistic regression models. Adjustment for differences in characteristics before hip fracture was performed using inverse probability of treatment weighting (IPTW) models.
Main results. 3083 long-stay NH residents with advanced dementia and hip fracture were included in the study. The cohort’s mean age was 84.2 ± 7.1 years, 79.2% were female (n = 2441), and 28.5% were ambulatory before hip fracture (n = 879). Of these NH residents, 84.8% (n = 2615) underwent surgical repair and 15.2% (n = 468) received nonsurgical management. At 6 months after hip fracture, mortality was 31.5% in the surgical group compared to 53.8% in the nonsurgical group. The greatest mortality difference between groups occurred in the first 30 days after hip fracture (11.5% in surgical group versus 30.6% in nonsurgical group). Surgical repair was associated with a decreased risk of death (Cox proportional hazard ratio) in the unadjusted (hazard ratio [HR], 0.55 [95% confidence interval {CI}, 0.49-0.61), multivariable adjusted (adjusted HR, 0.56 [95% CI, 0.49-0.63]), and IPTW (adjusted HR, 0.88 [95% CI, 0.79-0.98]) models. Similarly, surgically treated NH residents were less likely to die than those managed non-surgically when mortality was stratified by pre-fracture ambulatory status.
Among NH residents who survived 6 months after hip fracture, those who underwent surgical repair compared with those who received nonsurgical management had less documented pain (29.0% versus 30.9%), fewer pressure ulcers (11.2% versus 19.0%), greater physical restraint use (13.0% versus 11.1%), and greater antipsychotic drug use (29.5% versus 20.4%). In the adjusted IPTW models, surgical repair was associated with less pain (adjusted HR, 0.78 [95% CI, 0.61-0.99]) and fewer pressure ulcers (adjusted HR, 0.64 [95% CI, 0.47-0.86]).
Overall, 21.5% of NH residents utilized comfort-focused care within 6 months after hip fracture, with a mean time to utilization of hospice care of 56 ± 49 days. In those who were managed surgically, 19.3% utilized hospice care, as compared with 33.8% in those who did not receive surgical intervention. In NH residents who survived 6 months after hip fracture, only 1.1% in both groups acquired a DNH directive.
Conclusion. In older long-stay NH residents with advanced dementia and hip fracture, surgical repair was associated with lower all-cause mortality, less documented pain, and fewer pressure ulcers compared to nonsurgical management. However, adverse clinical outcomes such as pain, physical restraint use, pressure ulcers, and antipsychotic drug use were common regardless of treatment modality. The high incidence of these adverse outcomes and hazardous interventions, coupled with low utilization of comfort-focused care and DNH directive, highlight an opportunity to improve the quality of care in this vulnerable population.
Commentary
Hip fracture is very common in NH residents, with an overall incident rate of 2.3 per 100 person years and is associated with a high mortality rate of 36.2% by 6 months after fracture.1,2 Moreover, Neuman and colleagues have recently reported that among NH residents who have some degree of functional independence in locomotion prior to hip fracture, 54% either die or develop new total dependence in locomotion within 6 months of fracture and that severe cognitive impairment is a risk factor highly associated with these adverse outcomes.3 Despite this emerging knowledge, surgical repair of hip fracture remains the mainstay treatment in many NH residents in the hope of alleviating pain and improving mobility, and palliative care is considered only when patients are imminently dying or have deteriorated past the point of meaningful recovery. In cases of NH residents with advanced dementia whose life expectancy is limited and whose care goals may favor maintaining comfort, the health care proxies are frequently challenged with a difficult choice of either pursuing or foregoing surgical management—a complex medical decision to be made in the absence of sufficient evidence in this uniquely frail patient population.
The study reported by Berry and colleagues provides an important and timely investigation in examining associations of adverse clinical outcomes (mortality, pain, pressure ulcer) and hazardous interventions (physical restraint and antipsychotic drug use) in long-stay NH residents with advanced dementia and hip fracture who underwent surgical repair or nonsurgical management. The authors reported a 6-month mortality rate of 31.5% in NH residents who underwent surgical repair, an event rate similar to that reported by Neuman and colleagues. While surgical repair after hip fracture was associated with a decreased risk of death compared to nonsurgical management, high incidences of pain (29.0% to 30.9%) and pressure ulcers (11.2% to 19.0%), and frequent physical restraint use (11.1% to 13.0%) and antipsychotic drug use (20.4% to 29.5%) were noted in NH residents who survived 6 months after fracture regardless of treatment modality. These findings are consistent with the high rate of post-hip fracture functional disability previously reported by Neuman and colleagues, and highlight the trajectory of decline, frequent distressing symptoms, and prevalent use of pharmacologic and nonpharmacologic restraints in long-stay NH residents after hip fracture. Taken together, the low utilization of comfort-focused care (21.5%) and DNH directive (1.1%) in NH residents who survived 6 months suggest a missed opportunity to integrate palliative care in a patient population that stands to benefit from this intervention.
This study is the first to report the associations between hip fracture surgery and a reduction in adverse outcomes such as pain and pressure ulcer that commonly affect vulnerable NH residents with advanced dementia. This study was well designed and leveraged strengths of Medicare claims data linked with MDS assessments to capture outcome measures including pain, pressure ulcer, and restraint use that would otherwise be difficult to ascertain. However, as in all retrospective cohort design, there were limitations in this study. For instance, secondary outcomes were determined from a single time point (ie, first MDS assessment completed between 120 to 240 days following hip fracture) and thus data capture may be incomplete. Additionally, other conditions important to complex decision making in the care of frail older adults including postoperative complications (eg, delirium, infections, cardiac complications) and in-hospital mortality were not examined. Despite these limitations, this study has enhanced our understanding of the clinical course of long-term care NH residents with advanced dementia who endured hip fracture.
Applications for Clinical Practice
Patients’ goals of care should guide medical decision making in the management of hip fracture in NH residents with advanced dementia. The increased survival benefit of surgical repair of hip fracture in this patient population should be considered in the medical decision-making process if life-prolongation is preferred. However, palliative and hospice care need to be an important facet of discussion given the high rates of mortality, pain, pressure ulcer, and restraint use in this vulnerable subset of older adults.
—Fred Ko, MD, MS
1. Berry SD, Lee Y, Zullo AR, et al. Incidence of hip fracture in U.S. nursing homes. J Gerontol A Biol Sci Med Sci. 2016;71:1230-1234.
2. Neuman MD, Silber JH, Magaziner JS, et al. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern Med. 2014;174:1273-1280.
3. Berry SD, Rothbaum RR, Kiel DP, et al. Association of clinical outcomes with surgical repair of hip fractures vs nonsurgical management in nursing home residents with advanced dementia. JAMA Intern Med. 2018;178:774-780.
1. Berry SD, Lee Y, Zullo AR, et al. Incidence of hip fracture in U.S. nursing homes. J Gerontol A Biol Sci Med Sci. 2016;71:1230-1234.
2. Neuman MD, Silber JH, Magaziner JS, et al. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern Med. 2014;174:1273-1280.
3. Berry SD, Rothbaum RR, Kiel DP, et al. Association of clinical outcomes with surgical repair of hip fractures vs nonsurgical management in nursing home residents with advanced dementia. JAMA Intern Med. 2018;178:774-780.
Phase 3 studies of antiamyloid Alzheimer’s drug crenezumab stopped
After a disappointing interim analysis, Roche and its collaborator AC Immune are halting two phase 3 trials of the antiamyloid antibody crenezumab.
CREAD 1 and CREAD 2 enrolled patients with prodromal-to-mild sporadic Alzheimer’s disease. The preplanned interim safety and efficacy analysis determined that neither study was likely to meet the primary endpoint of change from baseline on the Clinical Dementia Rating-sum of boxes score.
There were no unexpected safety signals associated with the drug, despite a quadrupling of the phase 3 dose from that used in phase 2. The company in its press release said that it will continue to conduct the Autosomal Dominant Alzheimer’s Disease (ADAD) trial as part of the Alzheimer’s Prevention Initiative (API). ADAD is a large South American trial of crenezumab in Colombian families with familial Alzheimer’s caused by mutations in the presenilin-1 gene (PSEN1).
Roche did not release any data but said the trial results will be discussed at an upcoming scientific meeting.
“While the results with crenezumab are disappointing, they meaningfully contribute to our understanding of Alzheimer’s disease,” Sandra Horning, MD, Roche’s chief medical officer and executive vice president for global development, said in an interview. “We gratefully acknowledge the participants in the CREAD trials and the efforts of everyone involved in this important program.”
The decision was not a surprise to researchers who have followed the antibody’s development. It advanced into phase 3 with lackluster phase 2 cognitive, imaging, and biomarker data. Its selection as the therapeutic agent for the ADAD trial was a key driver in its continued development, securing Roche $100 million in federal funds to help launch ADAD, the first-ever Alzheimer’s primary prevention study.
Despite its failure in sporadic Alzheimer’s, there is still some hope that crenezumab might benefit people with the PSEN1 mutation, said Richard Caselli, MD, professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
“The Colombian trial is aimed at dominantly-inherited AD due to a PSEN1 mutation, so it is different enough to imagine it still might make a difference in patients in whom amyloid metabolism is actually defective due to functionally altered amyloid precursor protein or gamma secretase,” he said in an interview. “Possibly some might argue that many of the patients in the crenezumab trial likely had additional pathologies so that even if the AD component responded, the overall clinical picture might not reflect it due to the other components. That would be interesting if proven and could even argue against equating young-onset with late-onset AD, at least for clinical purposes, as is currently envisioned.”
Michael Wolfe, PhD, had a different take on the matter.
“Although amyloid-beta [Abeta] production is not necessarily altered in sporadic AD, there is essentially the same pathology, presentation, and progression with familial and sporadic AD, suggesting a common molecular mechanism,” said Dr. Wolfe, who is the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, Lawrence. “It’s hard to say Abeta is the pathogenic species in familial but not sporadic AD.
“To me, the failures of the antiamyloid approaches are because the drugs are given too late, are targeting the wrong form of Abeta, or are targeting an enzyme [for example, beta secretase1] that has other important functions. Most likely it’s a combination of these reasons. One could argue that even if some form of Abeta is the pathogenic entity, it is not a practical target because intervention may need to be initiated many years before the onset of symptoms.”
Despite the long string of failed antiamyloid antibodies, it’s not yet time to give up on the approach, said James Kupiec, MD, chief medical officer at ProMIS Neurosciences of Toronto.
“I understand where the pessimism [around antiamyloid antibodies] is coming from, and I also understand the enthusiasm from these companies to pursue them,” said Dr. Kupiec, who formerly headed Pfizer’s neuroscience research unit. “Targeting plaque is clearly not going to do the job. But in my opinion, the deeper pathophysiologic questions have not been adequately addressed. I’m not willing to throw in the towel. The correct molecular species [of amyloid] has not been appropriately or adequately tested in studies with monoclonal antibodies.”
The antibodies that have been failing for 5 years now were designed in the early 2000s, Dr. Kupiec pointed out, when knowledge of the various amyloid species was still immature. Newer candidates can target specific conformations of the protein – monomers and oligomers – before they aggregate into insoluble sheets. “Solanezumab was the first of these, paving the way for this new generation of antibodies,” Dr. Kupiec said.
Because they target soluble Abeta, not amyloid plaques, these domain-specific antibodies are less likely to elicit ARIA (amyloid-related imaging abnormalities), the inflammatory reaction that’s been associated with plaque dissolution in other antibody trials. ARIA has been a dose-limiting step for antiamyloid antibodies – one that conformationally targeted antibodies could avoid, Dr. Kupiec said.
“There may be some limited success with the these, and there may be enough of a treatment effect to secure approval,” he said. “The question is: Can we generate a higher effect size with an antibody that is more selective to the toxic forms of Abeta?”
PMN310 is ProMIS’ attempt to thread this needle. In preclinical studies, the antibody did not bind to amyloid monomers, plaques, or vascular Abeta aggregates. The company expects to take this antibody into phase 1 trials later this year.
“If we have a molecule that doesn’t bind to monomers or to plaques, but only to the toxic oligomer, then that is an something well worth testing in the clinic,” he said.
Dr. Caselli and Dr. Wolfe have no financial disclosures.
On behalf of the millions of people living with Alzheimer’s disease and their families that we serve and represent, the Alzheimer’s Association is disappointed to learn that these trials have been stopped.
We learn something from every Alzheimer’s clinical trial. The Alzheimer’s Association looks forward to hearing details of these studies at an upcoming scientific meeting.
More important, we must redouble our efforts to better understand the causes of the disease, and to discover additional therapeutic targets. No stone can be left unturned in the pursuit of better treatments and effective preventions.
The Alzheimer’s Association is investing in research looking at a variety of novel targets for treatment and prevention, including brain inflammation, the life and death cycle of brain cells, how brain cells use different energy sources, and the impact of lifestyle.
• Lifestyle interventions include leading the U.S. POINTER Study.
• To further the study of blood pressure control on reducing risk of mild cognitive impairment and dementia, the Alzheimer’s Association recently announced seed funding of SPRINT MIND 2.0.
• Part The Cloud Translational Research program fills a gap in Alzheimer’s drug development by supporting more than 30 early phase clinical studies.
• The Association is also funding research into the causes of the disease.
The emotional and financial cost of Alzheimer’s is enormous. At the Alzheimer’s Association, we will not stop. We will not slow down in our fight against this terrible disease.
Maria Carrillo, PhD , is the Alzheimer’s Association’s chief science officer.
On behalf of the millions of people living with Alzheimer’s disease and their families that we serve and represent, the Alzheimer’s Association is disappointed to learn that these trials have been stopped.
We learn something from every Alzheimer’s clinical trial. The Alzheimer’s Association looks forward to hearing details of these studies at an upcoming scientific meeting.
More important, we must redouble our efforts to better understand the causes of the disease, and to discover additional therapeutic targets. No stone can be left unturned in the pursuit of better treatments and effective preventions.
The Alzheimer’s Association is investing in research looking at a variety of novel targets for treatment and prevention, including brain inflammation, the life and death cycle of brain cells, how brain cells use different energy sources, and the impact of lifestyle.
• Lifestyle interventions include leading the U.S. POINTER Study.
• To further the study of blood pressure control on reducing risk of mild cognitive impairment and dementia, the Alzheimer’s Association recently announced seed funding of SPRINT MIND 2.0.
• Part The Cloud Translational Research program fills a gap in Alzheimer’s drug development by supporting more than 30 early phase clinical studies.
• The Association is also funding research into the causes of the disease.
The emotional and financial cost of Alzheimer’s is enormous. At the Alzheimer’s Association, we will not stop. We will not slow down in our fight against this terrible disease.
Maria Carrillo, PhD , is the Alzheimer’s Association’s chief science officer.
On behalf of the millions of people living with Alzheimer’s disease and their families that we serve and represent, the Alzheimer’s Association is disappointed to learn that these trials have been stopped.
We learn something from every Alzheimer’s clinical trial. The Alzheimer’s Association looks forward to hearing details of these studies at an upcoming scientific meeting.
More important, we must redouble our efforts to better understand the causes of the disease, and to discover additional therapeutic targets. No stone can be left unturned in the pursuit of better treatments and effective preventions.
The Alzheimer’s Association is investing in research looking at a variety of novel targets for treatment and prevention, including brain inflammation, the life and death cycle of brain cells, how brain cells use different energy sources, and the impact of lifestyle.
• Lifestyle interventions include leading the U.S. POINTER Study.
• To further the study of blood pressure control on reducing risk of mild cognitive impairment and dementia, the Alzheimer’s Association recently announced seed funding of SPRINT MIND 2.0.
• Part The Cloud Translational Research program fills a gap in Alzheimer’s drug development by supporting more than 30 early phase clinical studies.
• The Association is also funding research into the causes of the disease.
The emotional and financial cost of Alzheimer’s is enormous. At the Alzheimer’s Association, we will not stop. We will not slow down in our fight against this terrible disease.
Maria Carrillo, PhD , is the Alzheimer’s Association’s chief science officer.
After a disappointing interim analysis, Roche and its collaborator AC Immune are halting two phase 3 trials of the antiamyloid antibody crenezumab.
CREAD 1 and CREAD 2 enrolled patients with prodromal-to-mild sporadic Alzheimer’s disease. The preplanned interim safety and efficacy analysis determined that neither study was likely to meet the primary endpoint of change from baseline on the Clinical Dementia Rating-sum of boxes score.
There were no unexpected safety signals associated with the drug, despite a quadrupling of the phase 3 dose from that used in phase 2. The company in its press release said that it will continue to conduct the Autosomal Dominant Alzheimer’s Disease (ADAD) trial as part of the Alzheimer’s Prevention Initiative (API). ADAD is a large South American trial of crenezumab in Colombian families with familial Alzheimer’s caused by mutations in the presenilin-1 gene (PSEN1).
Roche did not release any data but said the trial results will be discussed at an upcoming scientific meeting.
“While the results with crenezumab are disappointing, they meaningfully contribute to our understanding of Alzheimer’s disease,” Sandra Horning, MD, Roche’s chief medical officer and executive vice president for global development, said in an interview. “We gratefully acknowledge the participants in the CREAD trials and the efforts of everyone involved in this important program.”
The decision was not a surprise to researchers who have followed the antibody’s development. It advanced into phase 3 with lackluster phase 2 cognitive, imaging, and biomarker data. Its selection as the therapeutic agent for the ADAD trial was a key driver in its continued development, securing Roche $100 million in federal funds to help launch ADAD, the first-ever Alzheimer’s primary prevention study.
Despite its failure in sporadic Alzheimer’s, there is still some hope that crenezumab might benefit people with the PSEN1 mutation, said Richard Caselli, MD, professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
“The Colombian trial is aimed at dominantly-inherited AD due to a PSEN1 mutation, so it is different enough to imagine it still might make a difference in patients in whom amyloid metabolism is actually defective due to functionally altered amyloid precursor protein or gamma secretase,” he said in an interview. “Possibly some might argue that many of the patients in the crenezumab trial likely had additional pathologies so that even if the AD component responded, the overall clinical picture might not reflect it due to the other components. That would be interesting if proven and could even argue against equating young-onset with late-onset AD, at least for clinical purposes, as is currently envisioned.”
Michael Wolfe, PhD, had a different take on the matter.
“Although amyloid-beta [Abeta] production is not necessarily altered in sporadic AD, there is essentially the same pathology, presentation, and progression with familial and sporadic AD, suggesting a common molecular mechanism,” said Dr. Wolfe, who is the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, Lawrence. “It’s hard to say Abeta is the pathogenic species in familial but not sporadic AD.
“To me, the failures of the antiamyloid approaches are because the drugs are given too late, are targeting the wrong form of Abeta, or are targeting an enzyme [for example, beta secretase1] that has other important functions. Most likely it’s a combination of these reasons. One could argue that even if some form of Abeta is the pathogenic entity, it is not a practical target because intervention may need to be initiated many years before the onset of symptoms.”
Despite the long string of failed antiamyloid antibodies, it’s not yet time to give up on the approach, said James Kupiec, MD, chief medical officer at ProMIS Neurosciences of Toronto.
“I understand where the pessimism [around antiamyloid antibodies] is coming from, and I also understand the enthusiasm from these companies to pursue them,” said Dr. Kupiec, who formerly headed Pfizer’s neuroscience research unit. “Targeting plaque is clearly not going to do the job. But in my opinion, the deeper pathophysiologic questions have not been adequately addressed. I’m not willing to throw in the towel. The correct molecular species [of amyloid] has not been appropriately or adequately tested in studies with monoclonal antibodies.”
The antibodies that have been failing for 5 years now were designed in the early 2000s, Dr. Kupiec pointed out, when knowledge of the various amyloid species was still immature. Newer candidates can target specific conformations of the protein – monomers and oligomers – before they aggregate into insoluble sheets. “Solanezumab was the first of these, paving the way for this new generation of antibodies,” Dr. Kupiec said.
Because they target soluble Abeta, not amyloid plaques, these domain-specific antibodies are less likely to elicit ARIA (amyloid-related imaging abnormalities), the inflammatory reaction that’s been associated with plaque dissolution in other antibody trials. ARIA has been a dose-limiting step for antiamyloid antibodies – one that conformationally targeted antibodies could avoid, Dr. Kupiec said.
“There may be some limited success with the these, and there may be enough of a treatment effect to secure approval,” he said. “The question is: Can we generate a higher effect size with an antibody that is more selective to the toxic forms of Abeta?”
PMN310 is ProMIS’ attempt to thread this needle. In preclinical studies, the antibody did not bind to amyloid monomers, plaques, or vascular Abeta aggregates. The company expects to take this antibody into phase 1 trials later this year.
“If we have a molecule that doesn’t bind to monomers or to plaques, but only to the toxic oligomer, then that is an something well worth testing in the clinic,” he said.
Dr. Caselli and Dr. Wolfe have no financial disclosures.
After a disappointing interim analysis, Roche and its collaborator AC Immune are halting two phase 3 trials of the antiamyloid antibody crenezumab.
CREAD 1 and CREAD 2 enrolled patients with prodromal-to-mild sporadic Alzheimer’s disease. The preplanned interim safety and efficacy analysis determined that neither study was likely to meet the primary endpoint of change from baseline on the Clinical Dementia Rating-sum of boxes score.
There were no unexpected safety signals associated with the drug, despite a quadrupling of the phase 3 dose from that used in phase 2. The company in its press release said that it will continue to conduct the Autosomal Dominant Alzheimer’s Disease (ADAD) trial as part of the Alzheimer’s Prevention Initiative (API). ADAD is a large South American trial of crenezumab in Colombian families with familial Alzheimer’s caused by mutations in the presenilin-1 gene (PSEN1).
Roche did not release any data but said the trial results will be discussed at an upcoming scientific meeting.
“While the results with crenezumab are disappointing, they meaningfully contribute to our understanding of Alzheimer’s disease,” Sandra Horning, MD, Roche’s chief medical officer and executive vice president for global development, said in an interview. “We gratefully acknowledge the participants in the CREAD trials and the efforts of everyone involved in this important program.”
The decision was not a surprise to researchers who have followed the antibody’s development. It advanced into phase 3 with lackluster phase 2 cognitive, imaging, and biomarker data. Its selection as the therapeutic agent for the ADAD trial was a key driver in its continued development, securing Roche $100 million in federal funds to help launch ADAD, the first-ever Alzheimer’s primary prevention study.
Despite its failure in sporadic Alzheimer’s, there is still some hope that crenezumab might benefit people with the PSEN1 mutation, said Richard Caselli, MD, professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
“The Colombian trial is aimed at dominantly-inherited AD due to a PSEN1 mutation, so it is different enough to imagine it still might make a difference in patients in whom amyloid metabolism is actually defective due to functionally altered amyloid precursor protein or gamma secretase,” he said in an interview. “Possibly some might argue that many of the patients in the crenezumab trial likely had additional pathologies so that even if the AD component responded, the overall clinical picture might not reflect it due to the other components. That would be interesting if proven and could even argue against equating young-onset with late-onset AD, at least for clinical purposes, as is currently envisioned.”
Michael Wolfe, PhD, had a different take on the matter.
“Although amyloid-beta [Abeta] production is not necessarily altered in sporadic AD, there is essentially the same pathology, presentation, and progression with familial and sporadic AD, suggesting a common molecular mechanism,” said Dr. Wolfe, who is the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, Lawrence. “It’s hard to say Abeta is the pathogenic species in familial but not sporadic AD.
“To me, the failures of the antiamyloid approaches are because the drugs are given too late, are targeting the wrong form of Abeta, or are targeting an enzyme [for example, beta secretase1] that has other important functions. Most likely it’s a combination of these reasons. One could argue that even if some form of Abeta is the pathogenic entity, it is not a practical target because intervention may need to be initiated many years before the onset of symptoms.”
Despite the long string of failed antiamyloid antibodies, it’s not yet time to give up on the approach, said James Kupiec, MD, chief medical officer at ProMIS Neurosciences of Toronto.
“I understand where the pessimism [around antiamyloid antibodies] is coming from, and I also understand the enthusiasm from these companies to pursue them,” said Dr. Kupiec, who formerly headed Pfizer’s neuroscience research unit. “Targeting plaque is clearly not going to do the job. But in my opinion, the deeper pathophysiologic questions have not been adequately addressed. I’m not willing to throw in the towel. The correct molecular species [of amyloid] has not been appropriately or adequately tested in studies with monoclonal antibodies.”
The antibodies that have been failing for 5 years now were designed in the early 2000s, Dr. Kupiec pointed out, when knowledge of the various amyloid species was still immature. Newer candidates can target specific conformations of the protein – monomers and oligomers – before they aggregate into insoluble sheets. “Solanezumab was the first of these, paving the way for this new generation of antibodies,” Dr. Kupiec said.
Because they target soluble Abeta, not amyloid plaques, these domain-specific antibodies are less likely to elicit ARIA (amyloid-related imaging abnormalities), the inflammatory reaction that’s been associated with plaque dissolution in other antibody trials. ARIA has been a dose-limiting step for antiamyloid antibodies – one that conformationally targeted antibodies could avoid, Dr. Kupiec said.
“There may be some limited success with the these, and there may be enough of a treatment effect to secure approval,” he said. “The question is: Can we generate a higher effect size with an antibody that is more selective to the toxic forms of Abeta?”
PMN310 is ProMIS’ attempt to thread this needle. In preclinical studies, the antibody did not bind to amyloid monomers, plaques, or vascular Abeta aggregates. The company expects to take this antibody into phase 1 trials later this year.
“If we have a molecule that doesn’t bind to monomers or to plaques, but only to the toxic oligomer, then that is an something well worth testing in the clinic,” he said.
Dr. Caselli and Dr. Wolfe have no financial disclosures.
SPRINT MIND published: Extension trial to add 2 years’ follow-up
A new iteration of the SPRINT MIND hypertension trial will seek to prove conclusively the original study’s tantalizing suggestion: that intensive blood pressure control decreases the risk of developing mild cognitive impairment (MCI) and, eventually, dementia.
SPRINT MIND 2.0 will re-recruit SPRINT MIND subjects and enable another follow-up cognitive assessment and other clinical tests as they remain on their standard of care blood pressure regimen. It is largely funded by an $800,000 grant from the Alzheimer’s Association.
Initially released last July at the Alzheimer’s Association International Conference, the results of the SPRINT MIND have now appeared online in JAMA. Although it failed to meet its primary endpoint of reducing dementia incidence, the study did score on two secondary endpoints. Patients who reduced their systolic blood pressure to less than 120 mm Hg were 19% less likely to develop MCI and 17% less likely to be diagnosed with all-cause dementia than were those who achieved a hypertension target of less than 140 mm Hg.
The secondary results, and positive movement in the primary results, sparked excitement in the dementia research community last summer. They have suggested that the median 5-year follow-up just wasn’t long enough to show any significant effects on dementia, which can take years to fully manifest. Adding 2 more years with SPRINT MIND 2.0 should be long enough to discern those benefits, if indeed they exist.
“SPRINT MIND 2.0 and the work leading up to it offers genuine, concrete hope,” Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, said in a press statement. “MCI is a known risk factor for dementia, and everyone who experiences dementia passes through MCI. When you prevent new cases of MCI, you are preventing new cases of dementia. The Alzheimer’s Association finds these data to be compelling and is committed to getting clarity and certainty on the dementia outcome by following participants for a longer period of time.”
The study strengthens the new and energetic push to find ways to prevent dementia, which has proven itself intractable in every drug study to date.
“This study is in line with where the field of dementia research is going: preventing memory loss earlier,” said Laurie Ryan, PhD, chief of the dementias of aging branch in the National Institute on Aging. “Much like we have research-based interventions for heart health and cancer prevention, we hope to have guidance based on this and subsequent studies that will more definitively show how to slow or even stop dementia well before symptoms appear.”
NIA director Richard J. Hodes, MD, agreed.
“Dementia continues to be a large public health challenge, and based on the primary results of this study, we still have yet to find an intervention strategy proven to reduce the risk of dementia,” he said in a press statement. “Nevertheless, the secondary results showing that intensive lowering of blood pressure may reduce risk for MCI, a known risk factor for dementia, gives us additional avenues to explore on the path to prevention.”
SPRINT MIND was a substudy of the Systolic Blood Pressure Intervention Trial (SPRINT). It compared two strategies for managing hypertension in older adults. The intensive strategy had a target of less than 120 mm Hg, while standard care had a target of less than 140 mm Hg. SPRINT showed that more intensive blood pressure control produced a 25% reduction in the composite primary composite endpoint of cardiovascular events, stroke, and cardiovascular death. The intensive arm was so successful that SPRINT helped inform the 2017 high blood pressure clinical guidelines from the American Heart Association and American College of Cardiology.
The SPRINT MIND substudy, headed by Jeff D. Williamson, MD, of Wake Forest University, Winston-Salem, NC, asked whether intensive management had any effect on probable all-cause dementia or MCI, as well as imaging evidence of changes in white matter lesions and brain volume. It followed patients for up to 7 years and comprised 9,361 SPRINT subjects at least 50 years old (mean, 68 years) with at least one cardiovascular risk factor. Nearly a third (30%) were black, and 10% Hispanic. The primary outcome was incident probable dementia. Secondary outcomes were MCI and a composite of MCI and/or probable dementia. About a third had a SBP of 132 mm Hg or less, another third had a systolic pressure of 132-145 mm Hg, and the remainder had a systolic pressure greater than 145 mm Hg.
Physicians could use their choice of antihypertensive treatments. The study protocol encouraged, but did not mandate, thiazide-type diuretics as a first-line agent, followed by loop diuretics and beta-adrenergic blockers. Chlorthalidone was encouraged as the primary thiazide-type diuretic, and amlodipine as the preferred calcium-channel blocker.
The interventions did successfully control blood pressure, with a significant difference between the treatment groups. The mean SBP was 121.6 mm Hg in the intensive therapy group and 134.8 mm Hg in the standard group – a statistically significant difference of 13.3 mm Hg.
Dementia developed in 149 in the aggressive control group and 176 in the standard group – a nonsignificant difference of 17% (hazard ratio, 0.83). MCI developed in 287 in the intensive group and 353 in the standard treatment group. This amounted to a statistically significant 19% reduction. There was also a significant 15% reduction in the composite outcome of MCI or probable dementia in favor of intensive treatment.
As evidenced by the Alzheimer’s Association grant, dementia researchers chose to focus on SPRINT MIND’s positive secondary endpoints. At the AAIC meeting, Dr. Williamson even suggested that antihypertensive medications could be seen as disease-modifying agents for cognitive decline. Data support his claim: No dementia intervention yet tested has approached this level of success.
“I think we can say this is the first disease-modifying strategy to reduce the risk of MCI,” Dr. Williamson said during a press briefing. And although the primary endpoint – the 17% relative risk reduction for probable all-cause dementia – didn’t meet statistical significance, “It’s comforting to see that the benefit went in the same direction and was of the same magnitude..”
SOURCE: Williamson JD et al. JAMA 2019 Jan 28. doi:10.1001/jama.2018.21442.
SPRINT MIND offers hope that a very achievable blood pressure goal can dramatically alter the trajectory from mild cognitive impairment to dementia, Kristine Yaffe, MD, wrote in an accompanying editorial. But at this point, it’s impossible to make specific clinical recommendations.
Additionally it is not possible, right now, to know which hypertension treatment regimens were most effective in improved cognitive outcomes.
“Information necessary to compare the effects of classes of antihypertensive agents on cognitive outcomes is also not provided. SPRINT used a quasi-pragmatic approach with suggestions for treatment choice, but practitioners approached SBP control individually, and most participants were taking multiple drugs.”
Nevertheless, the positive secondary findings and the encouraging trajectory on dementia risk should fix blood pressure management squarely into a cornerstone of dementia prevention algorithms.
“The SPRINT MIND study may not be the final approach for prevention of AD or other cognitive impairment, but it represents a major leap forward in what has emerged as a marathon journey.”
Dr. Kristine Yaffe is professor of psychiatry, neurology and epidemiology and the Roy and Marie Scola Endowed Chair at the University of California, San Francisco.
SPRINT MIND offers hope that a very achievable blood pressure goal can dramatically alter the trajectory from mild cognitive impairment to dementia, Kristine Yaffe, MD, wrote in an accompanying editorial. But at this point, it’s impossible to make specific clinical recommendations.
Additionally it is not possible, right now, to know which hypertension treatment regimens were most effective in improved cognitive outcomes.
“Information necessary to compare the effects of classes of antihypertensive agents on cognitive outcomes is also not provided. SPRINT used a quasi-pragmatic approach with suggestions for treatment choice, but practitioners approached SBP control individually, and most participants were taking multiple drugs.”
Nevertheless, the positive secondary findings and the encouraging trajectory on dementia risk should fix blood pressure management squarely into a cornerstone of dementia prevention algorithms.
“The SPRINT MIND study may not be the final approach for prevention of AD or other cognitive impairment, but it represents a major leap forward in what has emerged as a marathon journey.”
Dr. Kristine Yaffe is professor of psychiatry, neurology and epidemiology and the Roy and Marie Scola Endowed Chair at the University of California, San Francisco.
SPRINT MIND offers hope that a very achievable blood pressure goal can dramatically alter the trajectory from mild cognitive impairment to dementia, Kristine Yaffe, MD, wrote in an accompanying editorial. But at this point, it’s impossible to make specific clinical recommendations.
Additionally it is not possible, right now, to know which hypertension treatment regimens were most effective in improved cognitive outcomes.
“Information necessary to compare the effects of classes of antihypertensive agents on cognitive outcomes is also not provided. SPRINT used a quasi-pragmatic approach with suggestions for treatment choice, but practitioners approached SBP control individually, and most participants were taking multiple drugs.”
Nevertheless, the positive secondary findings and the encouraging trajectory on dementia risk should fix blood pressure management squarely into a cornerstone of dementia prevention algorithms.
“The SPRINT MIND study may not be the final approach for prevention of AD or other cognitive impairment, but it represents a major leap forward in what has emerged as a marathon journey.”
Dr. Kristine Yaffe is professor of psychiatry, neurology and epidemiology and the Roy and Marie Scola Endowed Chair at the University of California, San Francisco.
A new iteration of the SPRINT MIND hypertension trial will seek to prove conclusively the original study’s tantalizing suggestion: that intensive blood pressure control decreases the risk of developing mild cognitive impairment (MCI) and, eventually, dementia.
SPRINT MIND 2.0 will re-recruit SPRINT MIND subjects and enable another follow-up cognitive assessment and other clinical tests as they remain on their standard of care blood pressure regimen. It is largely funded by an $800,000 grant from the Alzheimer’s Association.
Initially released last July at the Alzheimer’s Association International Conference, the results of the SPRINT MIND have now appeared online in JAMA. Although it failed to meet its primary endpoint of reducing dementia incidence, the study did score on two secondary endpoints. Patients who reduced their systolic blood pressure to less than 120 mm Hg were 19% less likely to develop MCI and 17% less likely to be diagnosed with all-cause dementia than were those who achieved a hypertension target of less than 140 mm Hg.
The secondary results, and positive movement in the primary results, sparked excitement in the dementia research community last summer. They have suggested that the median 5-year follow-up just wasn’t long enough to show any significant effects on dementia, which can take years to fully manifest. Adding 2 more years with SPRINT MIND 2.0 should be long enough to discern those benefits, if indeed they exist.
“SPRINT MIND 2.0 and the work leading up to it offers genuine, concrete hope,” Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, said in a press statement. “MCI is a known risk factor for dementia, and everyone who experiences dementia passes through MCI. When you prevent new cases of MCI, you are preventing new cases of dementia. The Alzheimer’s Association finds these data to be compelling and is committed to getting clarity and certainty on the dementia outcome by following participants for a longer period of time.”
The study strengthens the new and energetic push to find ways to prevent dementia, which has proven itself intractable in every drug study to date.
“This study is in line with where the field of dementia research is going: preventing memory loss earlier,” said Laurie Ryan, PhD, chief of the dementias of aging branch in the National Institute on Aging. “Much like we have research-based interventions for heart health and cancer prevention, we hope to have guidance based on this and subsequent studies that will more definitively show how to slow or even stop dementia well before symptoms appear.”
NIA director Richard J. Hodes, MD, agreed.
“Dementia continues to be a large public health challenge, and based on the primary results of this study, we still have yet to find an intervention strategy proven to reduce the risk of dementia,” he said in a press statement. “Nevertheless, the secondary results showing that intensive lowering of blood pressure may reduce risk for MCI, a known risk factor for dementia, gives us additional avenues to explore on the path to prevention.”
SPRINT MIND was a substudy of the Systolic Blood Pressure Intervention Trial (SPRINT). It compared two strategies for managing hypertension in older adults. The intensive strategy had a target of less than 120 mm Hg, while standard care had a target of less than 140 mm Hg. SPRINT showed that more intensive blood pressure control produced a 25% reduction in the composite primary composite endpoint of cardiovascular events, stroke, and cardiovascular death. The intensive arm was so successful that SPRINT helped inform the 2017 high blood pressure clinical guidelines from the American Heart Association and American College of Cardiology.
The SPRINT MIND substudy, headed by Jeff D. Williamson, MD, of Wake Forest University, Winston-Salem, NC, asked whether intensive management had any effect on probable all-cause dementia or MCI, as well as imaging evidence of changes in white matter lesions and brain volume. It followed patients for up to 7 years and comprised 9,361 SPRINT subjects at least 50 years old (mean, 68 years) with at least one cardiovascular risk factor. Nearly a third (30%) were black, and 10% Hispanic. The primary outcome was incident probable dementia. Secondary outcomes were MCI and a composite of MCI and/or probable dementia. About a third had a SBP of 132 mm Hg or less, another third had a systolic pressure of 132-145 mm Hg, and the remainder had a systolic pressure greater than 145 mm Hg.
Physicians could use their choice of antihypertensive treatments. The study protocol encouraged, but did not mandate, thiazide-type diuretics as a first-line agent, followed by loop diuretics and beta-adrenergic blockers. Chlorthalidone was encouraged as the primary thiazide-type diuretic, and amlodipine as the preferred calcium-channel blocker.
The interventions did successfully control blood pressure, with a significant difference between the treatment groups. The mean SBP was 121.6 mm Hg in the intensive therapy group and 134.8 mm Hg in the standard group – a statistically significant difference of 13.3 mm Hg.
Dementia developed in 149 in the aggressive control group and 176 in the standard group – a nonsignificant difference of 17% (hazard ratio, 0.83). MCI developed in 287 in the intensive group and 353 in the standard treatment group. This amounted to a statistically significant 19% reduction. There was also a significant 15% reduction in the composite outcome of MCI or probable dementia in favor of intensive treatment.
As evidenced by the Alzheimer’s Association grant, dementia researchers chose to focus on SPRINT MIND’s positive secondary endpoints. At the AAIC meeting, Dr. Williamson even suggested that antihypertensive medications could be seen as disease-modifying agents for cognitive decline. Data support his claim: No dementia intervention yet tested has approached this level of success.
“I think we can say this is the first disease-modifying strategy to reduce the risk of MCI,” Dr. Williamson said during a press briefing. And although the primary endpoint – the 17% relative risk reduction for probable all-cause dementia – didn’t meet statistical significance, “It’s comforting to see that the benefit went in the same direction and was of the same magnitude..”
SOURCE: Williamson JD et al. JAMA 2019 Jan 28. doi:10.1001/jama.2018.21442.
A new iteration of the SPRINT MIND hypertension trial will seek to prove conclusively the original study’s tantalizing suggestion: that intensive blood pressure control decreases the risk of developing mild cognitive impairment (MCI) and, eventually, dementia.
SPRINT MIND 2.0 will re-recruit SPRINT MIND subjects and enable another follow-up cognitive assessment and other clinical tests as they remain on their standard of care blood pressure regimen. It is largely funded by an $800,000 grant from the Alzheimer’s Association.
Initially released last July at the Alzheimer’s Association International Conference, the results of the SPRINT MIND have now appeared online in JAMA. Although it failed to meet its primary endpoint of reducing dementia incidence, the study did score on two secondary endpoints. Patients who reduced their systolic blood pressure to less than 120 mm Hg were 19% less likely to develop MCI and 17% less likely to be diagnosed with all-cause dementia than were those who achieved a hypertension target of less than 140 mm Hg.
The secondary results, and positive movement in the primary results, sparked excitement in the dementia research community last summer. They have suggested that the median 5-year follow-up just wasn’t long enough to show any significant effects on dementia, which can take years to fully manifest. Adding 2 more years with SPRINT MIND 2.0 should be long enough to discern those benefits, if indeed they exist.
“SPRINT MIND 2.0 and the work leading up to it offers genuine, concrete hope,” Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, said in a press statement. “MCI is a known risk factor for dementia, and everyone who experiences dementia passes through MCI. When you prevent new cases of MCI, you are preventing new cases of dementia. The Alzheimer’s Association finds these data to be compelling and is committed to getting clarity and certainty on the dementia outcome by following participants for a longer period of time.”
The study strengthens the new and energetic push to find ways to prevent dementia, which has proven itself intractable in every drug study to date.
“This study is in line with where the field of dementia research is going: preventing memory loss earlier,” said Laurie Ryan, PhD, chief of the dementias of aging branch in the National Institute on Aging. “Much like we have research-based interventions for heart health and cancer prevention, we hope to have guidance based on this and subsequent studies that will more definitively show how to slow or even stop dementia well before symptoms appear.”
NIA director Richard J. Hodes, MD, agreed.
“Dementia continues to be a large public health challenge, and based on the primary results of this study, we still have yet to find an intervention strategy proven to reduce the risk of dementia,” he said in a press statement. “Nevertheless, the secondary results showing that intensive lowering of blood pressure may reduce risk for MCI, a known risk factor for dementia, gives us additional avenues to explore on the path to prevention.”
SPRINT MIND was a substudy of the Systolic Blood Pressure Intervention Trial (SPRINT). It compared two strategies for managing hypertension in older adults. The intensive strategy had a target of less than 120 mm Hg, while standard care had a target of less than 140 mm Hg. SPRINT showed that more intensive blood pressure control produced a 25% reduction in the composite primary composite endpoint of cardiovascular events, stroke, and cardiovascular death. The intensive arm was so successful that SPRINT helped inform the 2017 high blood pressure clinical guidelines from the American Heart Association and American College of Cardiology.
The SPRINT MIND substudy, headed by Jeff D. Williamson, MD, of Wake Forest University, Winston-Salem, NC, asked whether intensive management had any effect on probable all-cause dementia or MCI, as well as imaging evidence of changes in white matter lesions and brain volume. It followed patients for up to 7 years and comprised 9,361 SPRINT subjects at least 50 years old (mean, 68 years) with at least one cardiovascular risk factor. Nearly a third (30%) were black, and 10% Hispanic. The primary outcome was incident probable dementia. Secondary outcomes were MCI and a composite of MCI and/or probable dementia. About a third had a SBP of 132 mm Hg or less, another third had a systolic pressure of 132-145 mm Hg, and the remainder had a systolic pressure greater than 145 mm Hg.
Physicians could use their choice of antihypertensive treatments. The study protocol encouraged, but did not mandate, thiazide-type diuretics as a first-line agent, followed by loop diuretics and beta-adrenergic blockers. Chlorthalidone was encouraged as the primary thiazide-type diuretic, and amlodipine as the preferred calcium-channel blocker.
The interventions did successfully control blood pressure, with a significant difference between the treatment groups. The mean SBP was 121.6 mm Hg in the intensive therapy group and 134.8 mm Hg in the standard group – a statistically significant difference of 13.3 mm Hg.
Dementia developed in 149 in the aggressive control group and 176 in the standard group – a nonsignificant difference of 17% (hazard ratio, 0.83). MCI developed in 287 in the intensive group and 353 in the standard treatment group. This amounted to a statistically significant 19% reduction. There was also a significant 15% reduction in the composite outcome of MCI or probable dementia in favor of intensive treatment.
As evidenced by the Alzheimer’s Association grant, dementia researchers chose to focus on SPRINT MIND’s positive secondary endpoints. At the AAIC meeting, Dr. Williamson even suggested that antihypertensive medications could be seen as disease-modifying agents for cognitive decline. Data support his claim: No dementia intervention yet tested has approached this level of success.
“I think we can say this is the first disease-modifying strategy to reduce the risk of MCI,” Dr. Williamson said during a press briefing. And although the primary endpoint – the 17% relative risk reduction for probable all-cause dementia – didn’t meet statistical significance, “It’s comforting to see that the benefit went in the same direction and was of the same magnitude..”
SOURCE: Williamson JD et al. JAMA 2019 Jan 28. doi:10.1001/jama.2018.21442.
FROM JAMA
Key clinical point: Keeping systolic blood pressure lower than 120 mm Hg did not significantly reduce the risk of all-cause dementia in patients with hypertension, but it did lower the risk of mild cognitive impairment and probable dementia.
Major finding: The intensively treated group had a nonsignificant 17% lower risk of dementia, and significant reductions in the risk of MCI (19%) and probable dementia (15%).
Study details: SPRINT MIND was a substudy of the SPRINT antihypertension trial.
Source: Williamson JD et al. JAMA 2019 Jan 28. doi:10.1001/jama.2018.21442.