User login
Quality of life with PAD follows function, not clinical markers
Focus on ability to perform functional tasks when designing interventions aimed at improving health-related quality of life for patients with symptomatic peripheral arterial disease (PAD), advise the authors of a study published in the Journal of Vascular Surgery.
Clinical markers of disease severity and comorbidities are often the primary targets of interventions in PAD patients, but health-related quality of life (HRQoL) based on their functional capabilities matters more to patients, according to Andrew W. Gardner, PhD, of Penn State University, Hershey, and his colleagues.
“Interventions designed to improve HRQoL should focus on improving the quality of executing functional tasks, such as walking more steadily without stumbling; completing ADLs [activities of daily living] that are not specific to walking, such as bathing and transferring; and improving patient-based ability to walk various distances and speeds and to climb stairs,” the researchers wrote.
They studied 216 PAD patients (mean age, 65 years) with ambulatory leg pain confirmed by treadmill exercise and ankle brachial index less than or equal to 0.90 at rest or less than or equal to 0.73 after exercise. Patient HRQoL was measured using the Medical Outcomes Study 36-Item Short Form Health Survey (SF-36). All patients performed a maximal treadmill test, a 6-minute walk test, and gait speed from a 4-meter walk test was measured. Their ambulatory activity was monitored for 7 days using a step monitor. In addition, patients self-assessed their ability to perform four lower-level ADLs, consisting of walking across a small room, bathing, transferring from a bed to a chair, and using the toilet. They also evaluated their ability to perform two higher-level ADLs consisting of walking up and down stairs to the second floor without help and walking a half-mile without help.
Approximately 10%-17% of the patients reported either having some difficulty with or being unable to perform basic ADLs, whereas the majority reported either having some difficulty with or being unable to perform higher-level ADLs consisting of walking up and down stairs (74%) and walking a half-mile without help (85%).
The primary novel finding, according to Dr. Gardner and his colleagues, was that patient-based measurements of physical function were the strongest predictors of both physical and mental subscales of HRQoL.
The significant predictors were Walking Impairment Questionnaire speed score (P less than .001), history of stumbling while walking (P less than .001), stair climbing score (P = .001), bathing (P = .001), 6-minute walking distance (P =.004), and daily walking cadence (P = .043). The significant predictors of the role limitations caused by emotional problems subscale of the SF-36 included a history of stumbling while walking (P less than .001), transferring from a bed to a chair (P less than .001), and the walking distance score (P = .022).
Noticeably, a history of stumbling while walking was considered particularly important to the patients. In contrast, objective measurements of physical function (6-minute walking distance and daily walking cadence) were predictive only of the physical function subscale. Comorbid conditions and objective measures of PAD severity, such as ankle brachial index, claudication onset time, and peak walking time, were not at all predictive of HRQoL, the researchers stated.
The authors reported that they had no conflicts of interest.
SOURCE: Gardner AW et al. J Vasc Surg. 2018;68:1126-34.
Focus on ability to perform functional tasks when designing interventions aimed at improving health-related quality of life for patients with symptomatic peripheral arterial disease (PAD), advise the authors of a study published in the Journal of Vascular Surgery.
Clinical markers of disease severity and comorbidities are often the primary targets of interventions in PAD patients, but health-related quality of life (HRQoL) based on their functional capabilities matters more to patients, according to Andrew W. Gardner, PhD, of Penn State University, Hershey, and his colleagues.
“Interventions designed to improve HRQoL should focus on improving the quality of executing functional tasks, such as walking more steadily without stumbling; completing ADLs [activities of daily living] that are not specific to walking, such as bathing and transferring; and improving patient-based ability to walk various distances and speeds and to climb stairs,” the researchers wrote.
They studied 216 PAD patients (mean age, 65 years) with ambulatory leg pain confirmed by treadmill exercise and ankle brachial index less than or equal to 0.90 at rest or less than or equal to 0.73 after exercise. Patient HRQoL was measured using the Medical Outcomes Study 36-Item Short Form Health Survey (SF-36). All patients performed a maximal treadmill test, a 6-minute walk test, and gait speed from a 4-meter walk test was measured. Their ambulatory activity was monitored for 7 days using a step monitor. In addition, patients self-assessed their ability to perform four lower-level ADLs, consisting of walking across a small room, bathing, transferring from a bed to a chair, and using the toilet. They also evaluated their ability to perform two higher-level ADLs consisting of walking up and down stairs to the second floor without help and walking a half-mile without help.
Approximately 10%-17% of the patients reported either having some difficulty with or being unable to perform basic ADLs, whereas the majority reported either having some difficulty with or being unable to perform higher-level ADLs consisting of walking up and down stairs (74%) and walking a half-mile without help (85%).
The primary novel finding, according to Dr. Gardner and his colleagues, was that patient-based measurements of physical function were the strongest predictors of both physical and mental subscales of HRQoL.
The significant predictors were Walking Impairment Questionnaire speed score (P less than .001), history of stumbling while walking (P less than .001), stair climbing score (P = .001), bathing (P = .001), 6-minute walking distance (P =.004), and daily walking cadence (P = .043). The significant predictors of the role limitations caused by emotional problems subscale of the SF-36 included a history of stumbling while walking (P less than .001), transferring from a bed to a chair (P less than .001), and the walking distance score (P = .022).
Noticeably, a history of stumbling while walking was considered particularly important to the patients. In contrast, objective measurements of physical function (6-minute walking distance and daily walking cadence) were predictive only of the physical function subscale. Comorbid conditions and objective measures of PAD severity, such as ankle brachial index, claudication onset time, and peak walking time, were not at all predictive of HRQoL, the researchers stated.
The authors reported that they had no conflicts of interest.
SOURCE: Gardner AW et al. J Vasc Surg. 2018;68:1126-34.
Focus on ability to perform functional tasks when designing interventions aimed at improving health-related quality of life for patients with symptomatic peripheral arterial disease (PAD), advise the authors of a study published in the Journal of Vascular Surgery.
Clinical markers of disease severity and comorbidities are often the primary targets of interventions in PAD patients, but health-related quality of life (HRQoL) based on their functional capabilities matters more to patients, according to Andrew W. Gardner, PhD, of Penn State University, Hershey, and his colleagues.
“Interventions designed to improve HRQoL should focus on improving the quality of executing functional tasks, such as walking more steadily without stumbling; completing ADLs [activities of daily living] that are not specific to walking, such as bathing and transferring; and improving patient-based ability to walk various distances and speeds and to climb stairs,” the researchers wrote.
They studied 216 PAD patients (mean age, 65 years) with ambulatory leg pain confirmed by treadmill exercise and ankle brachial index less than or equal to 0.90 at rest or less than or equal to 0.73 after exercise. Patient HRQoL was measured using the Medical Outcomes Study 36-Item Short Form Health Survey (SF-36). All patients performed a maximal treadmill test, a 6-minute walk test, and gait speed from a 4-meter walk test was measured. Their ambulatory activity was monitored for 7 days using a step monitor. In addition, patients self-assessed their ability to perform four lower-level ADLs, consisting of walking across a small room, bathing, transferring from a bed to a chair, and using the toilet. They also evaluated their ability to perform two higher-level ADLs consisting of walking up and down stairs to the second floor without help and walking a half-mile without help.
Approximately 10%-17% of the patients reported either having some difficulty with or being unable to perform basic ADLs, whereas the majority reported either having some difficulty with or being unable to perform higher-level ADLs consisting of walking up and down stairs (74%) and walking a half-mile without help (85%).
The primary novel finding, according to Dr. Gardner and his colleagues, was that patient-based measurements of physical function were the strongest predictors of both physical and mental subscales of HRQoL.
The significant predictors were Walking Impairment Questionnaire speed score (P less than .001), history of stumbling while walking (P less than .001), stair climbing score (P = .001), bathing (P = .001), 6-minute walking distance (P =.004), and daily walking cadence (P = .043). The significant predictors of the role limitations caused by emotional problems subscale of the SF-36 included a history of stumbling while walking (P less than .001), transferring from a bed to a chair (P less than .001), and the walking distance score (P = .022).
Noticeably, a history of stumbling while walking was considered particularly important to the patients. In contrast, objective measurements of physical function (6-minute walking distance and daily walking cadence) were predictive only of the physical function subscale. Comorbid conditions and objective measures of PAD severity, such as ankle brachial index, claudication onset time, and peak walking time, were not at all predictive of HRQoL, the researchers stated.
The authors reported that they had no conflicts of interest.
SOURCE: Gardner AW et al. J Vasc Surg. 2018;68:1126-34.
FROM THE JOURNAL OF VASCULAR SURGERY
Key clinical point: Patient assessment of functional status was the best predictor of health-related quality of life.
Major finding: Objective measures of peripheral arterial disease severity, such as ankle brachial index, claudication onset time, and peak walking time, were not predictive of health-related quality of life.
Study details: A clinical and survey study of 216 patients with peripheral arterial disease.
Disclosures: The authors reported that they had no financial conflicts of interest.
Source: Gardner AW et al. J Vasc Surg. 2018;68:1126-34.
Physical activity tied to lower depression risk among older adults
Meeting World Health Organization recommendations for levels of physical activity reduces the odds of prevalent depression by 40%, according to a study of more than 4,000 adults aged 50 years and older.
“To [our] knowledge, this is the first prospective cohort study to examine the protective effect of meeting [moderate to vigorous physical activity] guidelines, and different volumes of walking, on depression among a sample of adults,” Cillian P. McDowell, of the University of Limerick (Ireland), and his associates wrote in Experimental Gerontology.
The study drew on data from The Irish Longitudinal Study of Ageing and included 4,556 individuals, 56.7% of whom were female. The investigators created “dose categories” based on how much exercise participants performed each week. For moderate to vigorous physical activity, they assigned participants to low (0 to less than 600 metabolic equivalent [MET]–minutes per week), moderate (600 to less than 1,200 MET-min/week), and high (1,200 or more MET-min/week) categories. For walking, investigators divided participants among tertiles of minutes performed (0-110 min/week, 120-400 min/week, and 420 or more min/week). Symptoms of depression were assessed using the Center for Epidemiologic Studies Depression Scale, reported Mr. McDowell and his associates.
The odds of prevalent depression were 40% lower (odds ratio, 0.60; 95% confidence interval, 0.48-0.76) among participants who met the physical activity guidelines, 23% lower (OR, 0.77, 95% confidence interval, 0.49-1.21) among those who were in the moderate and high categories, and 43% lower (OR, 0.57; 95% CI, 0.45-0.73) among those who were in the moderate and high categories, Mr. McDowell and his associates wrote.
The study was not conducted to explore possible mechanisms underlying the ties between physical activity and depression. However, Mr. McDowell and his associates speculated that exercise training has both brain monoaminergic and neurotropic effects and might lower “inflammatory and oxidant markers. Further, physical activity may be associated with depression through psychological factors such as self-esteem.”
Mr. McDowell and his associates wrote. “Recent evidence has shown that people with [major depressive disorder] engage in higher levels of sedentary behavior, and that cross-sectionally sedentary behavior, is positively associated with depression,” they added. “Meeting WHO recommended [physical activity] levels could be recommended ... to prevent the onset of depression.”
The investigators pointed out that one of the major limitations of the study was that participants’ depression and activity were self-reported, which could predispose results to over- or underreporting. They also pointed out that a strength of the study was its large sample size.
Mr. McDowell and his associates reported no conflicts of interest. The sponsors of The Irish Longitudinal Study of Ageing played no role in this study’s design, methods, subject recruitment, data collection, analysis, or preparation.
SOURCE: McDowell CP et al. Exp Gerontol. 2018 Oct 2;112:68-75.
Meeting World Health Organization recommendations for levels of physical activity reduces the odds of prevalent depression by 40%, according to a study of more than 4,000 adults aged 50 years and older.
“To [our] knowledge, this is the first prospective cohort study to examine the protective effect of meeting [moderate to vigorous physical activity] guidelines, and different volumes of walking, on depression among a sample of adults,” Cillian P. McDowell, of the University of Limerick (Ireland), and his associates wrote in Experimental Gerontology.
The study drew on data from The Irish Longitudinal Study of Ageing and included 4,556 individuals, 56.7% of whom were female. The investigators created “dose categories” based on how much exercise participants performed each week. For moderate to vigorous physical activity, they assigned participants to low (0 to less than 600 metabolic equivalent [MET]–minutes per week), moderate (600 to less than 1,200 MET-min/week), and high (1,200 or more MET-min/week) categories. For walking, investigators divided participants among tertiles of minutes performed (0-110 min/week, 120-400 min/week, and 420 or more min/week). Symptoms of depression were assessed using the Center for Epidemiologic Studies Depression Scale, reported Mr. McDowell and his associates.
The odds of prevalent depression were 40% lower (odds ratio, 0.60; 95% confidence interval, 0.48-0.76) among participants who met the physical activity guidelines, 23% lower (OR, 0.77, 95% confidence interval, 0.49-1.21) among those who were in the moderate and high categories, and 43% lower (OR, 0.57; 95% CI, 0.45-0.73) among those who were in the moderate and high categories, Mr. McDowell and his associates wrote.
The study was not conducted to explore possible mechanisms underlying the ties between physical activity and depression. However, Mr. McDowell and his associates speculated that exercise training has both brain monoaminergic and neurotropic effects and might lower “inflammatory and oxidant markers. Further, physical activity may be associated with depression through psychological factors such as self-esteem.”
Mr. McDowell and his associates wrote. “Recent evidence has shown that people with [major depressive disorder] engage in higher levels of sedentary behavior, and that cross-sectionally sedentary behavior, is positively associated with depression,” they added. “Meeting WHO recommended [physical activity] levels could be recommended ... to prevent the onset of depression.”
The investigators pointed out that one of the major limitations of the study was that participants’ depression and activity were self-reported, which could predispose results to over- or underreporting. They also pointed out that a strength of the study was its large sample size.
Mr. McDowell and his associates reported no conflicts of interest. The sponsors of The Irish Longitudinal Study of Ageing played no role in this study’s design, methods, subject recruitment, data collection, analysis, or preparation.
SOURCE: McDowell CP et al. Exp Gerontol. 2018 Oct 2;112:68-75.
Meeting World Health Organization recommendations for levels of physical activity reduces the odds of prevalent depression by 40%, according to a study of more than 4,000 adults aged 50 years and older.
“To [our] knowledge, this is the first prospective cohort study to examine the protective effect of meeting [moderate to vigorous physical activity] guidelines, and different volumes of walking, on depression among a sample of adults,” Cillian P. McDowell, of the University of Limerick (Ireland), and his associates wrote in Experimental Gerontology.
The study drew on data from The Irish Longitudinal Study of Ageing and included 4,556 individuals, 56.7% of whom were female. The investigators created “dose categories” based on how much exercise participants performed each week. For moderate to vigorous physical activity, they assigned participants to low (0 to less than 600 metabolic equivalent [MET]–minutes per week), moderate (600 to less than 1,200 MET-min/week), and high (1,200 or more MET-min/week) categories. For walking, investigators divided participants among tertiles of minutes performed (0-110 min/week, 120-400 min/week, and 420 or more min/week). Symptoms of depression were assessed using the Center for Epidemiologic Studies Depression Scale, reported Mr. McDowell and his associates.
The odds of prevalent depression were 40% lower (odds ratio, 0.60; 95% confidence interval, 0.48-0.76) among participants who met the physical activity guidelines, 23% lower (OR, 0.77, 95% confidence interval, 0.49-1.21) among those who were in the moderate and high categories, and 43% lower (OR, 0.57; 95% CI, 0.45-0.73) among those who were in the moderate and high categories, Mr. McDowell and his associates wrote.
The study was not conducted to explore possible mechanisms underlying the ties between physical activity and depression. However, Mr. McDowell and his associates speculated that exercise training has both brain monoaminergic and neurotropic effects and might lower “inflammatory and oxidant markers. Further, physical activity may be associated with depression through psychological factors such as self-esteem.”
Mr. McDowell and his associates wrote. “Recent evidence has shown that people with [major depressive disorder] engage in higher levels of sedentary behavior, and that cross-sectionally sedentary behavior, is positively associated with depression,” they added. “Meeting WHO recommended [physical activity] levels could be recommended ... to prevent the onset of depression.”
The investigators pointed out that one of the major limitations of the study was that participants’ depression and activity were self-reported, which could predispose results to over- or underreporting. They also pointed out that a strength of the study was its large sample size.
Mr. McDowell and his associates reported no conflicts of interest. The sponsors of The Irish Longitudinal Study of Ageing played no role in this study’s design, methods, subject recruitment, data collection, analysis, or preparation.
SOURCE: McDowell CP et al. Exp Gerontol. 2018 Oct 2;112:68-75.
FROM EXPERIMENTAL GERONTOLOGY
Lay counseling effective for reducing late-life depression
Counseling delivered by trained lay community members can effectively treat depression and anxiety in older adults in low- and middle-income countries, a study shows.
“The [depression in later life] intervention, is to our knowledge, the first randomized clinical trial of indicated depression prevention in older adults living in a [low- and middle-income country] and as such addresses a previously unmet need in global health,” wrote Amit Dias, MD, and his colleagues. The findings show that the intervention could be a viable prevention option for older people living in those countries, which often lack the resources to provide prevention services for this population.
The study randomized 181 adults aged 60 years and older with subsyndromal depressive symptoms who attended rural and urban primary care clinics in Goa, India, to an intervention arm (n = 91) or to usual care (n = 90), reported Dr. Dias and his colleagues. The intervention arm was delivered by lay counselors (LCs) who were members of the local community, aged over 30 years, and graduates of any nonhealth-related field. The LCs, who received training, had weekly supervision and support from experts in the United States via Skype, reported Dr. Dias, of the department of preventive and social medicine at Goa Medical College in Bambolim, India, and his colleagues.
People in the intervention group also were given assistance with accessing medical and social programs. Six sessions lasting 30-40 minutes were delivered either in the patients’ homes or at a local center over a 6-10 week period.
Patients randomized to the control group received care as usual together with the same outcome assessments as the intervention group. Depressive episodes were measured using the Mini-International Neuropsychiatric Interview.
Results showed that 4.4% of participants in the intervention group had a major depressive episode, compared with 14.4% of those in the usual care group (number needed to treat, 9.95; 95% confidence interval, 5.12-182.43; P = 0.04), Dr. Dias and his colleagues wrote in JAMA Psychiatry. Kaplan-Meier estimates showed that 95.1% of patients in the intervention group were free of depression at 12 months, compared with 87.4% of those in the control arm.
The incidence of depression, as measured by General Health Questionnaire–12 scores, also was lower in the intervention group (12-month mean difference, –1.18; 95% CI, –2.03 to –0.31; P less than .001). The intervention also was associated with lower systolic blood pressure at 12 months (difference, –6.98; 95% CI, –11.96 to –2.01; group x time interaction, P less than 0.001) and a change in body mass index (difference, 0.23; 95% CI, –0.97 to 1.43; P = 0.04).
However, the intervention did not affect measures of functional status or cognition.
The researchers concluded that their findings extend earlier work (Lancet. 2010;376[9758]:2086-95)(Lancet. 2017:389[10065]:176-85), which also showed that LCs could effectively treat prevalent cases of depression and anxiety in primary care practice. “If the success of the [depression in later life] intervention in depression prevention can be replicated in other [low- and middle-income countries], then its utility and scalability would be further supported,” they concluded.
Dr. Dias and his colleagues cited several limitations. One is that people with mild cognitive impairment or dementia were excluded from the study.
The study was supported by grants from the U.S. National Institute of Mental Health. The authors reported no conflicts of interest.
SOURCE: Dias A et al. JAMA Psychiatry. 2018 Nov 7. doi: 10.1001/jamapsychaitry.2018.3048.
Depression occurring later in life is the most common mental health issue in the elderly and has been shown to have a negative impact on comorbidities and contribute to the risk for dementia and mortality. There is no doubt later-life depression poses a significant public health challenge. Low-income countries with limited resources can experience those challenges at a deeper level.
The current study contributes to the existing evidence, which shows that interventions carried out by nonhealth care professionals can be effective for addressing mental health conditions in low-resource settings. In addition, previous studies have shown that task sharing as a method is effective in tackling other health conditions such as HIV, hypertension, and tuberculosis in such settings.
However, it should be noted that, in the current study, the intervention was delivered by workers who received regular support. A logical next step, therefore, would be to examine the efficacy of interventions delivered by public health workers. Organizations that currently provide counseling services should be encouraged to adopt a structured approach demonstrated in the current study.
Jagadisha Thirthalli, MD, Palanimuthu T. Sivakumar, MD, and Bangalore N. Gangadhar, MD, are affiliated with the department of psychiatry at the National Institute of Mental Health and Neurosciences in Bengaluru, India. These comments are taken from an accompanying editorial (JAMA Psychiatry. 2018 Nov 7. doi: 10.1001/jamapsychiatry.2018.2898). No conflicts of interest were reported.
Depression occurring later in life is the most common mental health issue in the elderly and has been shown to have a negative impact on comorbidities and contribute to the risk for dementia and mortality. There is no doubt later-life depression poses a significant public health challenge. Low-income countries with limited resources can experience those challenges at a deeper level.
The current study contributes to the existing evidence, which shows that interventions carried out by nonhealth care professionals can be effective for addressing mental health conditions in low-resource settings. In addition, previous studies have shown that task sharing as a method is effective in tackling other health conditions such as HIV, hypertension, and tuberculosis in such settings.
However, it should be noted that, in the current study, the intervention was delivered by workers who received regular support. A logical next step, therefore, would be to examine the efficacy of interventions delivered by public health workers. Organizations that currently provide counseling services should be encouraged to adopt a structured approach demonstrated in the current study.
Jagadisha Thirthalli, MD, Palanimuthu T. Sivakumar, MD, and Bangalore N. Gangadhar, MD, are affiliated with the department of psychiatry at the National Institute of Mental Health and Neurosciences in Bengaluru, India. These comments are taken from an accompanying editorial (JAMA Psychiatry. 2018 Nov 7. doi: 10.1001/jamapsychiatry.2018.2898). No conflicts of interest were reported.
Depression occurring later in life is the most common mental health issue in the elderly and has been shown to have a negative impact on comorbidities and contribute to the risk for dementia and mortality. There is no doubt later-life depression poses a significant public health challenge. Low-income countries with limited resources can experience those challenges at a deeper level.
The current study contributes to the existing evidence, which shows that interventions carried out by nonhealth care professionals can be effective for addressing mental health conditions in low-resource settings. In addition, previous studies have shown that task sharing as a method is effective in tackling other health conditions such as HIV, hypertension, and tuberculosis in such settings.
However, it should be noted that, in the current study, the intervention was delivered by workers who received regular support. A logical next step, therefore, would be to examine the efficacy of interventions delivered by public health workers. Organizations that currently provide counseling services should be encouraged to adopt a structured approach demonstrated in the current study.
Jagadisha Thirthalli, MD, Palanimuthu T. Sivakumar, MD, and Bangalore N. Gangadhar, MD, are affiliated with the department of psychiatry at the National Institute of Mental Health and Neurosciences in Bengaluru, India. These comments are taken from an accompanying editorial (JAMA Psychiatry. 2018 Nov 7. doi: 10.1001/jamapsychiatry.2018.2898). No conflicts of interest were reported.
Counseling delivered by trained lay community members can effectively treat depression and anxiety in older adults in low- and middle-income countries, a study shows.
“The [depression in later life] intervention, is to our knowledge, the first randomized clinical trial of indicated depression prevention in older adults living in a [low- and middle-income country] and as such addresses a previously unmet need in global health,” wrote Amit Dias, MD, and his colleagues. The findings show that the intervention could be a viable prevention option for older people living in those countries, which often lack the resources to provide prevention services for this population.
The study randomized 181 adults aged 60 years and older with subsyndromal depressive symptoms who attended rural and urban primary care clinics in Goa, India, to an intervention arm (n = 91) or to usual care (n = 90), reported Dr. Dias and his colleagues. The intervention arm was delivered by lay counselors (LCs) who were members of the local community, aged over 30 years, and graduates of any nonhealth-related field. The LCs, who received training, had weekly supervision and support from experts in the United States via Skype, reported Dr. Dias, of the department of preventive and social medicine at Goa Medical College in Bambolim, India, and his colleagues.
People in the intervention group also were given assistance with accessing medical and social programs. Six sessions lasting 30-40 minutes were delivered either in the patients’ homes or at a local center over a 6-10 week period.
Patients randomized to the control group received care as usual together with the same outcome assessments as the intervention group. Depressive episodes were measured using the Mini-International Neuropsychiatric Interview.
Results showed that 4.4% of participants in the intervention group had a major depressive episode, compared with 14.4% of those in the usual care group (number needed to treat, 9.95; 95% confidence interval, 5.12-182.43; P = 0.04), Dr. Dias and his colleagues wrote in JAMA Psychiatry. Kaplan-Meier estimates showed that 95.1% of patients in the intervention group were free of depression at 12 months, compared with 87.4% of those in the control arm.
The incidence of depression, as measured by General Health Questionnaire–12 scores, also was lower in the intervention group (12-month mean difference, –1.18; 95% CI, –2.03 to –0.31; P less than .001). The intervention also was associated with lower systolic blood pressure at 12 months (difference, –6.98; 95% CI, –11.96 to –2.01; group x time interaction, P less than 0.001) and a change in body mass index (difference, 0.23; 95% CI, –0.97 to 1.43; P = 0.04).
However, the intervention did not affect measures of functional status or cognition.
The researchers concluded that their findings extend earlier work (Lancet. 2010;376[9758]:2086-95)(Lancet. 2017:389[10065]:176-85), which also showed that LCs could effectively treat prevalent cases of depression and anxiety in primary care practice. “If the success of the [depression in later life] intervention in depression prevention can be replicated in other [low- and middle-income countries], then its utility and scalability would be further supported,” they concluded.
Dr. Dias and his colleagues cited several limitations. One is that people with mild cognitive impairment or dementia were excluded from the study.
The study was supported by grants from the U.S. National Institute of Mental Health. The authors reported no conflicts of interest.
SOURCE: Dias A et al. JAMA Psychiatry. 2018 Nov 7. doi: 10.1001/jamapsychaitry.2018.3048.
Counseling delivered by trained lay community members can effectively treat depression and anxiety in older adults in low- and middle-income countries, a study shows.
“The [depression in later life] intervention, is to our knowledge, the first randomized clinical trial of indicated depression prevention in older adults living in a [low- and middle-income country] and as such addresses a previously unmet need in global health,” wrote Amit Dias, MD, and his colleagues. The findings show that the intervention could be a viable prevention option for older people living in those countries, which often lack the resources to provide prevention services for this population.
The study randomized 181 adults aged 60 years and older with subsyndromal depressive symptoms who attended rural and urban primary care clinics in Goa, India, to an intervention arm (n = 91) or to usual care (n = 90), reported Dr. Dias and his colleagues. The intervention arm was delivered by lay counselors (LCs) who were members of the local community, aged over 30 years, and graduates of any nonhealth-related field. The LCs, who received training, had weekly supervision and support from experts in the United States via Skype, reported Dr. Dias, of the department of preventive and social medicine at Goa Medical College in Bambolim, India, and his colleagues.
People in the intervention group also were given assistance with accessing medical and social programs. Six sessions lasting 30-40 minutes were delivered either in the patients’ homes or at a local center over a 6-10 week period.
Patients randomized to the control group received care as usual together with the same outcome assessments as the intervention group. Depressive episodes were measured using the Mini-International Neuropsychiatric Interview.
Results showed that 4.4% of participants in the intervention group had a major depressive episode, compared with 14.4% of those in the usual care group (number needed to treat, 9.95; 95% confidence interval, 5.12-182.43; P = 0.04), Dr. Dias and his colleagues wrote in JAMA Psychiatry. Kaplan-Meier estimates showed that 95.1% of patients in the intervention group were free of depression at 12 months, compared with 87.4% of those in the control arm.
The incidence of depression, as measured by General Health Questionnaire–12 scores, also was lower in the intervention group (12-month mean difference, –1.18; 95% CI, –2.03 to –0.31; P less than .001). The intervention also was associated with lower systolic blood pressure at 12 months (difference, –6.98; 95% CI, –11.96 to –2.01; group x time interaction, P less than 0.001) and a change in body mass index (difference, 0.23; 95% CI, –0.97 to 1.43; P = 0.04).
However, the intervention did not affect measures of functional status or cognition.
The researchers concluded that their findings extend earlier work (Lancet. 2010;376[9758]:2086-95)(Lancet. 2017:389[10065]:176-85), which also showed that LCs could effectively treat prevalent cases of depression and anxiety in primary care practice. “If the success of the [depression in later life] intervention in depression prevention can be replicated in other [low- and middle-income countries], then its utility and scalability would be further supported,” they concluded.
Dr. Dias and his colleagues cited several limitations. One is that people with mild cognitive impairment or dementia were excluded from the study.
The study was supported by grants from the U.S. National Institute of Mental Health. The authors reported no conflicts of interest.
SOURCE: Dias A et al. JAMA Psychiatry. 2018 Nov 7. doi: 10.1001/jamapsychaitry.2018.3048.
FROM JAMA PSYCHIATRY
Key clinical point: Lay counseling can be an effective intervention in reducing late-life depression in low- and middle-income countries.
Major finding: More than 4% of those in the intervention group had a major depressive episode, compared with 14.4% of those in the usual care group (number needed to treat, 9.95; 95% confidence interval, 5.12-182.43; P = 0.04).
Study details: Overall, 181 adults aged over 60 years with subsyndromal depressive symptoms who attended a rural and urban primary care clinics in Goa, India, who were randomized to an intervention arm (n = 91) or to usual care (n = 90).
Disclosures: The study was supported by grants from the U.S. National Institute of Mental Health. The authors reported no conflicts of interest.
Source: Dias A et al. JAMA Psychiatry. 2018 Nov 7. doi: 10.1001/jamapsychiatry.2018.3048.
Providing Rural Veterans With Access to Exercise Through Gerofit
Clinical video telehealth can be used to deliver functional circuit exercise training to older veterans in remote locations.
Exercise increases endurance, muscle strength, and functional performance with corresponding gains in mobility, survival, and quality of life.1 However, even with these benefits and improvements in clinical outcomes, only 15% of adults aged ≥ 65 years follow current guidelines for exercise.2 Despite their prior military training, the majority of veterans do not meet physical activity recommendations.3 Time, travel, and support are common barriers to exercise participation and adherence—barriers that are further amplified among older adults.
The Veterans Health Administration (VHA) is recognized as a world leader in telehealth service development. Currently, 677,000 veterans have received telehealth services, which represents 12% of the 5.6 million veterans under VHA care.4 Clinical video telehealth (CVT) is widely used within the VHA system to deliver health care that otherwise would not be available to veterans. Veterans who have difficulty traveling to the nearest US Department of Veteran Affairs (VA) medical center (VAMC) can access CVT programs at a participating VHA community-based outpatient clinic (CBOC). The VA has more than 45 CVT programs, including programs for mental health, weight management, cardiology, and dermatology. Outside the VA, cardiac exercise rehabilitation provided by CVT has been shown to be as effective as center-based programs in improving cardiovascular risk factors and functional capacity.5 A VHA exercise program that leveraged CVT resources and was dedicated to older adults with a wide range of comorbid conditions would have a high impact on the health and well-being of older veterans.
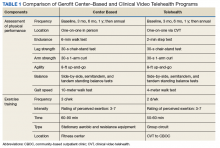
Gerofit is a VHA clinical demonstration program of supervised center-based exercise for veterans aged ≥ 65 years. Developed at the Durham VAMC Geriatric Research, Education, and Clinical Center (GRECC) in North Carolina, it has demonstrated improved clinical outcomes, including physical function, mobility, quality of life, and survival.6-10 The program offers veterans individualized exercise in a group setting that focuses on improving endurance, strength, and balance. The exercise prescription is based on the patient’s physical limitations as identified in a physical performance assessment.
With support from VHA Geriatric Extended Care (GEC) and the Office of Rural Health (ORH), Gerofit was implemented in 10 VAMCs across 8 VISNs. However, barriers such as travel time, distance, and transportation limit participation. Previously, we found that rural veterans lack access to exercise programs.11,12 Although some do aerobic exercise (AEX), most do not do resistance training (RT), though they are willing to learn. Access to Gerofit for rural veterans is expanding with recent support from the ORH Enterprise Wide Initiative. Rural program expansion includes several different Gerofit initiatives, many involving CBOCs.
The Salem VAMC Gerofit program sought to adapt the facility-based assessment and exercise procedures into a self-reliant CVT class for its CBOCs. This article describes the development of the Salem VAMC Gerofit CVT program, hereafter referred to as Tele-Gerofit.
Related: Expanding the Scope of Telemedicine in Gastroenterology
Program Design
Gerofit was established in 1986 at the Durham GRECC as an exercise and health promotion program for veterans aged ≥ 65 years.13 Its goal is to prevent or improve functional decline from physical inactivity and age-related conditions. Gerofit targets the geriatric patient population and thus extends beyond cardiac and pulmonary rehabilitation or weight loss programs. The primary exclusion criteria are based on safety issues in the context of a group exercise setting of older adults and include oxygen dependency, unstable cardiac disease, and moderate-to-severe cognitive impairment.
To participate in Gerofit, veterans must be able to perform activities of daily living and self-manage an exercise prescription developed by the exercise instructor based on physical performance testing. These physical performance tests include measures that are independent predictors of disability, loss of independent living, and death, as well as surrogate measures of exercise capacity (eg, strength, endurance, balance).14,15 A novel aspect of Gerofit is that the physical performance assessment is used not only to determine physical limitations, but also to individualize the exercise prescription based on the observed deficits in strength, endurance, or balance. These assessments are performed at initial enrollment; 3 months, 6 months, and 1 year later; and annually after that. Currently, the center-based Gerofit programs administer 5 items of the Senior Fitness Test: 6-minute walk, 10-meter walk (10-MWT), 30-second 1-arm curl, 30-second chair-stand test, and 8-foot up-and-go.15 The side-by-side, semitandem, and tandem standing balance tests from the short physical performance battery also are performed.16 In addition, participants complete a questionnaire that includes items from the physical functioning scale of the 12-Item Short Form Health Survey (SF-12).
After each assessment, the Gerofit exercise instructor reviews the results with the veteran and formulates an individualized exercise prescription along with goals for improvement. Veterans are encouraged to attend supervised center-based exercise sessions 3 times weekly. Classes are offered in a gym or fitness center at the VAMC or in leased space. Each patient uses a cue card that lists an exercise plan personalized for intensity and duration for aerobic exercise (AEX; eg, treadmill walking, stationary bicycling, arm ergometry), RT using dumbbells and weight equipment, and functional exercises for flexibility and balance. Some medical centers also offer yoga, tai chi, or dancing Gerofit classes.
For participants in the Durham Gerofit program, mortality decreased 25% over a decade (hazard ratio, 0.75; 95% CI, 0.61-0.91).9 A substudy that included the Psychological General Well-Being Index found that 81% of participants significantly increased their score after 1 year.7 Observed initial improvement in physical performance has been sustained over 5 years.10,17 One-year results from the recent Gerofit expansion to 6 other VAMCs showed clinically and statistically significantly improved physical performance from baseline to 3-, 6-, and 12-month follow-up.18
Adaptation of Gerofit to CVT Delivery
Initial work. The Greater Los Angeles VAMC Gerofit program conducted a pilot CVT exercise class of 6 veterans at the rural Bakersfield CBOC in California.19 Each week, an exercise instructor broadcast a 60-minute exercise class that included warm-up, RT with bands, progressive balance training, and flexibility. Trained student volunteers from California State University in Bakersfield kinesiology program were on site at the Bakersfield CBOC to perform the assessments and aid in exercises during the CVT sessions. Despite the lack of AEX per se, veterans showed significant improvement in endurance as measured by an increase in the number of steps completed in 2 minutes at the 3-month assessment (P = .049). Although exercises were not delivered in a circuit format, the improved endurance supported the potential for cardiovascular benefit from RT in older adults.
This pilot project also demonstrated that key components of the Gerofit program could be delivered safely by telehealth with onsite supervision. The Miami VA Healthcare System also offers CVT Gerofit exercise classes broadcast to the rural Florida CBOCs of Key Largo and Homestead.11 The exercise activities offered for the Miami CVT participants incorporate components of AEX (calisthenics) and RT (resistance bands). Veterans enjoyed the classes, and adherence was good. However, availability of staff and space are an ongoing challenge.
In Key Largo, 5 veterans participated before the CVT classes were placed on hold owing to the demands of other CVT programs and limited availability of the telehealth clinical technician (TCT). The Homestead CBOC continues to offer CVT Gerofit exercise classes and has 6 regular participants. Notably, the physical space at the Homestead CBOC is smaller than that at the Key Largo CBOC; the Homestead CBOC has adjusted by shifting to exercises performed while standing or sitting, ensuring participants’ safety and satisfaction.
The Baltimore, Maryland VAMC Gerofit program offers other innovative CVT exercise classes, including a tai chi class, and a class with exercise performed while sitting in a chair. Although the Baltimore VAMC CVT exercise classes do not have the scope of the center-based exercise prescriptions, they are unique in that they are broadcast not only to their affiliated CBOCs, but also other Gerofit programs in different VISNs.
Related: Telehealth for Rural Veterans With Neurologic Disorders
Salem VAMC Gerofit Program. The center-based Salem VAMC Gerofit program was established in July 2015. In fiscal year 2017, its dedicated exercise facility had more than 5,000 patient visits. Despite the program’s success, we prioritized establishing Tele-Gerofit because of the medical center’s rural location in southwest Virginia and the large number of veterans who receive care at CBOCs. Therefore, much as with the pilot CVT Gerofit classes in Los Angeles and Miami, the target setting was rural CBOCs. The goal for Salem VAMC Tele-Gerofit was to modify Gerofit delivery to the CVT format and a CBOC setting with minimal modification of the content and personnel requirements of both physical performance testing and exercise training procedures.
Adjustments for CBOC Setting. The enrollment process for Tele-Gerofit is the same as that for the center-based program. To start, a veteran’s primary care provider reviews the list of eligibility criteria and, if the veteran qualifies, places a consult. A Gerofit team member then contacts the veteran by phone to describe the program and schedule an assessment. At the baseline physical performance assessment, American College of Sports Medicine guidelines on exercise participation, health screening, and exercise intensity are used to evaluate veterans and rank them by their cardiovascular risk.20 All new program participants start with low-intensity exercise and gradually progress to recommended levels of exercise. Before starting an exercise class, participants are instructed on use of the 10-point rating of perceived exertion (RPE).
Each CBOC site is supplied with an RPE poster that is displayed for participants’ use. During a Tele-Gerofit class, the exercise instructor asks participants to periodically report their RPE. This class differs slightly from the center-based exercise sessions in which RPE is primarily assessed when a different exercise is introduced or the duration or intensity of an exercise is increased. The Gerofit instructor monitors exercise and treatment fidelity, but the onsite TCT observes for safety during class. The TCT also takes initial vital signs and sets up the room for the class. Emergency contacts and procedures are posted in each CBOC CVT room and are available to the center-based exercise instructor. Because the CBOCs are not inside medical facilities, some CBOC directors have asked that heart rate monitors be used as an extra safety precaution to ensure that high-risk participants do not exceed a heart rate limit that may be set by their cardiologists.
Modifications to Physical Performance Assessment. Physical performance testing had to be adapted to the small rooms available at the CBOCs. For measuring normal gait speed, the 10-MWT was replaced with the 4-meter walk test (4-MWT). The 4-MWT has excellent test–retest reliability with an intraclass correlation coefficient (ICC) of 0.93, but the discrepancy in gait speed between the 4-MWT and the 10-MWT is such that the tests cannot be used interchangeably.21 For measuring endurance, the 6-minute walk test was replaced with the 2-minute step test (2-MST). In older adults, the 2-MST has a moderate correlation with 6-minute walk distance (r = 0.36; P = .04) and high reliability (ICC, 0.90).15,22 The 30-second 1-arm curl, the 30-second chair-stand test, and the 8-foot up-and-go test are performed without modification and require only dumbbells, a chair without wheels, and a stopwatch.
The exercise instructor at the Salem VAMC conducts physical performance testing by 2-way videoconferencing with the veteran in a room at the CBOC. The TCT at the CBOC assists by measuring and demarcating 4 meters on the floor and a designated height on the wall for knee elevation for 4-MWT and 2-MST, respectively. The TCT remains in the room during the assessment visit. Except for taking vital signs before and after the physical performance assessment, the TCT does not participate in the testing. To date, more than 20 physical performance assessments have been conducted without difficulty at Salem-affiliated CBOCs. The primary challenge has been scheduling the room with CVT equipment (ie, camera and screen) for the 30-minute individual assessment session, which occurs on a rolling basis as individuals are enrolled and followed.
After the assessment is completed, the exercise instructor reviews the results with the participant and provides feedback on areas in need of improvement. However, these education sessions can be lengthy and are best supported by giving the patient a personalized handout.
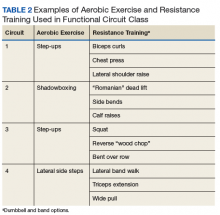
Functional Circuit Exercise. In Tele-Gerofit, exercise training is delivered by CVT broadcast from the Salem VAMC to veterans in a room (equipped with steps, dumbbells, chairs, and bands) at the CBOC. This type of exercise training, which uses only mobile equipment and plyometric (weight-bearing) exercises, is referred to as functional exercise. The AEX includes marching in place, moving on and off a raised step, and body-weight exercises, while RT uses dumbbells, resistance bands, and plyometric exercises (Table 2).
Progression of intensity is achieved by increasing the rate of stepping and the size of the steps (AEX) or the number of repetitions and the weight of the dumbbells or bands (RT). Each veteran exercises at an intensity level that is appropriate for his or her baseline limitations and medical conditions. The exercise instructor uses different forms of the same equipment (eg, heavier dumbbells, higher steps) to vary intensity among individuals while having them perform the same exercises as a group. The challenge is to adjust the pace of the AEX or the timing of the RT repetitions for individuals new to the class.
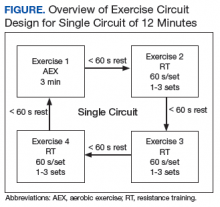
Delivery of exercise training in the form of circuits allows for a diverse exercise program in a setting with limited space. Circuit training is an exercise modality that consists of a series of different exercises, each usually completed in 30 to 60 seconds, with minimal rest between each type of exercise. Each Tele-Gerofit circuit has a mix of AEX and RT exercises performed for 3 minutes consecutively (Figure).
The design of the circuit training can be adjusted based on the number of individuals in the class. Larger classes can be split into 2 groups that alternate between exercise sets, while smaller classes have 1 group performing the same exercise set and then rotating to either the AEX or RT set. Total exercise time to complete the circuit depends on the number of different exercises, number of repetitions, and the rest between repetitions and the different exercises. In this way, total exercise time can be made shorter or longer depending on the veteran’s capacity.
Frequency. Tele-Gerofit exercise classes are currently offered twice weekly and last about 1 hour, which includes warm-up (8-10 minutes), functional circuit training (40 minutes), and cooldown/stretching (8-10 minutes). A challenge for the exercise instructor is the need to provide ongoing clear instructions both to the class and to individuals as needed. As the exercise prescription for each patient is based on physical performance testing, the exercise instructor for the training must be familiar with the test results. Derivation of the exercise prescription in Tele-Gerofit follows the same process as center-based Gerofit.
Each patient is given an exercise prescription written to address any impairments noted in the different domains of the physical performance assessment, scored using age and sex percentiles. For instance, individuals scoring poorly on lower body strength are given specific lower body strengthening exercises. Participants are given an exercise program that guides them toward achieving recommended physical activity guidelines using their RPE to modulate each exercise. Duration and intensity of each type of planned exercise are formally discussed after initial and follow-up assessments. In addition, exercise training is informally progressed throughout the program. For Tele-Gerofit, instructors must design each class with the group in mind while being prepared for modifications and specific changes for individuals.
Discussion
Tele-Gerofit adapts the well-established center-based Gerofit program to be executed without an exercise facility while maintaining the content of the evidence-based procedures. Physical performance testing and exercise training were modified, adding elements necessary for CVT assessments and classes to be broadcast from the Salem VAMC to its affiliated CBOCs. Tele-Gerofit exercises are performed in a circuit style that allows a veteran or small structured groups of veterans to move among exercises and requires less space than traditional group exercise does. Safety and monitoring concerns are addressed with a safety procedure that includes emergency plans for each site, prescreening of enrolled participants, and monitoring of exercise intensity in accordance with national guidelines.1 Similar to the center-based Gerofit program, the exercise prescription is tailored to each veteran’s physical limitations based on initial and ongoing assessment of physical performance. Tele-Gerofit physical performance testing fulfills the same need with only a few modifications using validated measures. Tele-Gerofit assessments are administered by CVT without the need for additional staff on site.
Adaptation of center-based Gerofit exercise classes to Tele-Gerofit is a major innovation. Use of a circuit exercise design was supported by findings in older adults that RT alone, when performed quickly with minimal rest between each set and exercise station, increases both aerobic capacity and strength.23,24 Older adult RT trials that compared circuit RT with traditional RT found that strength gains are comparable between circuit and traditional RT.24-26 Working with adults aged > 60 years, Takeshima and colleagues conducted a trial of circuit exercise with added callisthenic exercises performed in place between RT on exercise machines.27 This dual-modality (AEX+RT) circuit approach was well tolerated and effective, increasing aerobic capacity and strength. Unfortunately, the resistance exercise machines used in those circuit exercise studies and in the center-based Gerofit program are not an option for Tele-Gerofit.
The requirement for an exercise facility was removed by designing Tele-Gerofit exercise to include only functional exercises that rely on body weight or small mobile exercise equipment. Although popular among young adults, functional circuit exercise is understudied in older adults. Recently, a 12-week functional circuit exercise intervention in frail elderly adults demonstrated significant improvements in gait speed and the timed chair-stand test.28 A pilot observational study of Gerofit participants at the Canandaigua VAMC offered 27 veterans functional circuit exercise instead of their traditional exercise facility class and found larger increases in the timed chair-stand test and 6-minute walk distance compared with 11 Gerofit participants in the traditional program.29
This Tele-Gerofit exercise training combines functional and circuit exercise strategies into telehealth delivery. However, its effect on physical performance remains to be demonstrated. To address this question, we are conducting a single-arm pilot study of Tele-Gerofit with CVT broadcast to 3 Salem CBOC affiliates (Wytheville, Staunton, and Danville, Virginia). The goal is to determine the effect on physical performance and collect feasibility data, including attendance rate and patient satisfaction with the video broadcast. In addition, we are planning an effectiveness trial to compare the impact of functional circuit exercise delivered in person (center based, not CVT) with the parent Gerofit exercise program on direct measures of endurance and strength, in addition to physical performance.
Related: Setting and Method of Measurement Affect Blood Pressure Readings in Older Veterans
Implementation research is needed to determine how Tele-Gerofit can be disseminated to other VAMCs and community-based centers beyond CBOCs. Although the cost of the equipment used to implement Tele-Gerofit is minimal, the program requires dedicated and experienced exercise instructors, and the sharing of telehealth resources with other clinical programs. The authors expect that a diverse group of stakeholders is needed across service lines of primary care, geriatrics and extended care, physical medicine and rehabilitation, and telehealth. Of note, this multidisciplinary collaboration is a hallmark of the Gerofit program. The recent success of the implementation of center-based Gerofit in VAMCs across the US demonstrates the program’s flexibility and robust results.18
Plans also include refining strategies for physical performance testing and exercise monitoring. For instance, we would like to adapt telehealth technology for heart rate monitors that can be worn by high-risk veterans at the CBOC and viewed in real time by the exercise instructor.
Conclusion
Gerofit, which is designed to help older veterans maintain independent living and prevent disability, has been demonstrated to improve quality of life and survival. Our goal has been to adapt Gerofit to CVT and provide a supervised, individualized exercise program in a group setting—a program that can be widely disseminated. Salem VAMC Tele-Gerofit is an innovative and prescriptive program that delivers CVT functional circuit exercise training to remote locations without the need for stationary exercise equipment. This approach has the potential to become an effective and feasible exercise strategy for preventing and minimizing disability in the increasing population of older veterans. Work is needed to determine whether Tele-Gerofit provides a rapid translation of Gerofit to clinical practice and improved outcomes with substantial cost savings from reduced hospitalization and institutionalization.
Acknowledgments
Gerofit has been funded by the Veterans Health Affairs Office of Geriatrics and Extended Care Non-Institutional Long-Term Care Funding and Mentored Partnership Program, and the Veterans Health Affairs Office of Rural Health Rural Enterprise-Wide Initiative.
The authors thank Kim Birkett, MPH, for assistance in editing, references, and graphics and the staff at the Wytheville, Staunton, and Danville community-based outpatient clinics for their support.
1. American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510-1530.
2. Centers for Disease Control and Prevention. Adult participation in aerobic and muscle-strengthening physical activities—United States, 2011. MMWR Morb Mortal Wkly Rep. 2013;62(17):326-330.
3. Littman AJ, Forsberg CW, Koepsell TD. Physical activity in a national sample of veterans. Med Sci Sports Exerc. 2009;41(5):1006-1013.
4. US Department of Veterans Affairs, Office of Rural Health. Annual Report: Thrive 2015. https://www.ruralhealth.va.gov/docs/ORH_Annual_Report_2015_FINAL.pdf. Published 2015. Accessed July 16, 2018.
5. Rawstorn JC, Gant N, Direito A, Beckmann C, Maddison R. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart. 2016;102(15):1183-1192.
6. Morey MC. Celebrating 20 years of excellence in exercise for the older veteran. Fed Pract. 2007;24(10):49-65.
7. Cowper PA, Morey MC, Bearon LB, et al. The impact of supervised exercise on the psychological well-being and health status of older veterans. J Appl Gerontol. 1991;10(4):469-485.
8. Morey MC, Cowper PA, Feussner JR, et al. Evaluation of a supervised exercise program in a geriatric population. J Am Geriatr Soc. 1989;37(4):348-354.
9. Morey MC, Pieper CF, Crowley GM, Sullivan RJ, Puglisi CM. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50(12):1929-1933.
10. Morey MC, Pieper CF, Sullivan RJ Jr, Crowley GM, Cowper PA, Robbins MS. Five-year performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44(10):1226-1231.
11. Valencia WM, Botros D, Pendlebury D, et al. Proactive reach and telehealth monitoring (Gerofit) enhance resistance exercise at rural setting. Innovat Aging. 2017;1(suppl 1):225.12. Pendlebury D, Botros D VW. Proactive Reach: an innovative access approach to identify & deliver GEROFIT exercise telehealth counseling to rural veterans & enhance CBOC services. J Am Geriatr Soc. 2017(suppl 1):S208. Poster presented at: Annual Scientific Meeting of the American Geriatrics Society; May 18, 2017; San Antonio, TX.
13. Morey MC, Crowley GM, Robbins MS, Cowper PA, Sullivan RJ Jr. The Gerofit program: a VA innovation. South Med J. 1994;87(5):S83-S87.
14. Cooper R, Kuh D, Hardy R; Mortality Review Group; FALCon and HALCyon Study Teams. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467.
15. Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7(2):129-161.
16. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85-M94.
17. Morey MC, Cowper PA, Feussner JR, et al. Two-year trends in physical performance following supervised exercise among community-dwelling older veterans. J Am Geriatr Soc. 1991;39(10):986-992.
18. Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit program. J Am Geriatr Soc. 2018;66(5):1009-1016.
19. Blanchard E, Castle S, Ines E, et al. Delivering a clinical exercise program to rural veterans via video telehealth. Poster C167 presented at: Annual Scientific Meeting of the American Geriatrics Society; May 19-21, 2016; Long Beach, CA.
20. Riebe D, Ehrman JK, Liguori G, Magal M, eds; American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Philadelphia, PA: Wolters Kluwer Health; 2018.
21. Peters DM, Fritz SL, Krotish DE. Assessing the reliability and validity of a shorter walk test compared with the 10-meter walk test for measurements of gait speed in healthy, older adults. J Geriatr Phys Ther. 2013;36(1):24-30.
22. Pedrosa R, Holanda G. Correlation between the walk, 2-minute step and TUG tests among hypertensive older women. Rev Bras Fisioter. 2009;13(3):252-256.
23. Romero-Arenas S, Blazevich AJ, Martinez-Pascual M, et al. Effects of high-resistance circuit training in an elderly population. Exp Gerontol. 2013;48(3):334-340.
24. Brentano MA, Cadore EL, Da Silva EM, et al. Physiological adaptations to strength and circuit training in postmenopausal women with bone loss. J Strength Cond Res. 2008;22(6):1816-1825.
25. Romero-Arenas S, Martinez-Pascual M, Alcaraz PE. Impact of resistance circuit training on neuromuscular, cardiorespiratory and body composition adaptations in the elderly. Aging Dis. 2013;4(5):256-263.
26. Paoli A, Pacelli F, Bargossi AM, et al. Effects of three distinct protocols of fitness training on body composition, strength and blood lactate. J Sports Med Phys Fitness. 2010;50(1):43-51.
27. Takeshima N, Rogers ME, Islam MM, Yamauchi T, Watanabe E, Okada A. Effect of concurrent aerobic and resistance circuit exercise training on fitness in older adults. Eur J Appl Physiol. 2004;93(1-2):173-182.
28. Giné-Garriga M, Guerra M, Pagés E, Manini TM, Jiménez R, Unnithan VB. The effect of functional circuit training on physical frailty in frail older adults: a randomized controlled trial. J Aging Phys Act. 2010;18(4):401-424.
29. Biddle ED, Reynolds P, Kopp T, Cammarata H, Conway P. Implementation of functional training tools elicits improvements in aerobic fitness and lower body strength in older veterans. Poster C169 presented at: Annual Scientific Meeting of the American Geriatrics Society; May 19-21, 2016; Long Beach, CA.
Clinical video telehealth can be used to deliver functional circuit exercise training to older veterans in remote locations.
Clinical video telehealth can be used to deliver functional circuit exercise training to older veterans in remote locations.
Exercise increases endurance, muscle strength, and functional performance with corresponding gains in mobility, survival, and quality of life.1 However, even with these benefits and improvements in clinical outcomes, only 15% of adults aged ≥ 65 years follow current guidelines for exercise.2 Despite their prior military training, the majority of veterans do not meet physical activity recommendations.3 Time, travel, and support are common barriers to exercise participation and adherence—barriers that are further amplified among older adults.
The Veterans Health Administration (VHA) is recognized as a world leader in telehealth service development. Currently, 677,000 veterans have received telehealth services, which represents 12% of the 5.6 million veterans under VHA care.4 Clinical video telehealth (CVT) is widely used within the VHA system to deliver health care that otherwise would not be available to veterans. Veterans who have difficulty traveling to the nearest US Department of Veteran Affairs (VA) medical center (VAMC) can access CVT programs at a participating VHA community-based outpatient clinic (CBOC). The VA has more than 45 CVT programs, including programs for mental health, weight management, cardiology, and dermatology. Outside the VA, cardiac exercise rehabilitation provided by CVT has been shown to be as effective as center-based programs in improving cardiovascular risk factors and functional capacity.5 A VHA exercise program that leveraged CVT resources and was dedicated to older adults with a wide range of comorbid conditions would have a high impact on the health and well-being of older veterans.
Gerofit is a VHA clinical demonstration program of supervised center-based exercise for veterans aged ≥ 65 years. Developed at the Durham VAMC Geriatric Research, Education, and Clinical Center (GRECC) in North Carolina, it has demonstrated improved clinical outcomes, including physical function, mobility, quality of life, and survival.6-10 The program offers veterans individualized exercise in a group setting that focuses on improving endurance, strength, and balance. The exercise prescription is based on the patient’s physical limitations as identified in a physical performance assessment.
With support from VHA Geriatric Extended Care (GEC) and the Office of Rural Health (ORH), Gerofit was implemented in 10 VAMCs across 8 VISNs. However, barriers such as travel time, distance, and transportation limit participation. Previously, we found that rural veterans lack access to exercise programs.11,12 Although some do aerobic exercise (AEX), most do not do resistance training (RT), though they are willing to learn. Access to Gerofit for rural veterans is expanding with recent support from the ORH Enterprise Wide Initiative. Rural program expansion includes several different Gerofit initiatives, many involving CBOCs.
The Salem VAMC Gerofit program sought to adapt the facility-based assessment and exercise procedures into a self-reliant CVT class for its CBOCs. This article describes the development of the Salem VAMC Gerofit CVT program, hereafter referred to as Tele-Gerofit.
Related: Expanding the Scope of Telemedicine in Gastroenterology
Program Design
Gerofit was established in 1986 at the Durham GRECC as an exercise and health promotion program for veterans aged ≥ 65 years.13 Its goal is to prevent or improve functional decline from physical inactivity and age-related conditions. Gerofit targets the geriatric patient population and thus extends beyond cardiac and pulmonary rehabilitation or weight loss programs. The primary exclusion criteria are based on safety issues in the context of a group exercise setting of older adults and include oxygen dependency, unstable cardiac disease, and moderate-to-severe cognitive impairment.
To participate in Gerofit, veterans must be able to perform activities of daily living and self-manage an exercise prescription developed by the exercise instructor based on physical performance testing. These physical performance tests include measures that are independent predictors of disability, loss of independent living, and death, as well as surrogate measures of exercise capacity (eg, strength, endurance, balance).14,15 A novel aspect of Gerofit is that the physical performance assessment is used not only to determine physical limitations, but also to individualize the exercise prescription based on the observed deficits in strength, endurance, or balance. These assessments are performed at initial enrollment; 3 months, 6 months, and 1 year later; and annually after that. Currently, the center-based Gerofit programs administer 5 items of the Senior Fitness Test: 6-minute walk, 10-meter walk (10-MWT), 30-second 1-arm curl, 30-second chair-stand test, and 8-foot up-and-go.15 The side-by-side, semitandem, and tandem standing balance tests from the short physical performance battery also are performed.16 In addition, participants complete a questionnaire that includes items from the physical functioning scale of the 12-Item Short Form Health Survey (SF-12).
After each assessment, the Gerofit exercise instructor reviews the results with the veteran and formulates an individualized exercise prescription along with goals for improvement. Veterans are encouraged to attend supervised center-based exercise sessions 3 times weekly. Classes are offered in a gym or fitness center at the VAMC or in leased space. Each patient uses a cue card that lists an exercise plan personalized for intensity and duration for aerobic exercise (AEX; eg, treadmill walking, stationary bicycling, arm ergometry), RT using dumbbells and weight equipment, and functional exercises for flexibility and balance. Some medical centers also offer yoga, tai chi, or dancing Gerofit classes.
For participants in the Durham Gerofit program, mortality decreased 25% over a decade (hazard ratio, 0.75; 95% CI, 0.61-0.91).9 A substudy that included the Psychological General Well-Being Index found that 81% of participants significantly increased their score after 1 year.7 Observed initial improvement in physical performance has been sustained over 5 years.10,17 One-year results from the recent Gerofit expansion to 6 other VAMCs showed clinically and statistically significantly improved physical performance from baseline to 3-, 6-, and 12-month follow-up.18
Adaptation of Gerofit to CVT Delivery
Initial work. The Greater Los Angeles VAMC Gerofit program conducted a pilot CVT exercise class of 6 veterans at the rural Bakersfield CBOC in California.19 Each week, an exercise instructor broadcast a 60-minute exercise class that included warm-up, RT with bands, progressive balance training, and flexibility. Trained student volunteers from California State University in Bakersfield kinesiology program were on site at the Bakersfield CBOC to perform the assessments and aid in exercises during the CVT sessions. Despite the lack of AEX per se, veterans showed significant improvement in endurance as measured by an increase in the number of steps completed in 2 minutes at the 3-month assessment (P = .049). Although exercises were not delivered in a circuit format, the improved endurance supported the potential for cardiovascular benefit from RT in older adults.
This pilot project also demonstrated that key components of the Gerofit program could be delivered safely by telehealth with onsite supervision. The Miami VA Healthcare System also offers CVT Gerofit exercise classes broadcast to the rural Florida CBOCs of Key Largo and Homestead.11 The exercise activities offered for the Miami CVT participants incorporate components of AEX (calisthenics) and RT (resistance bands). Veterans enjoyed the classes, and adherence was good. However, availability of staff and space are an ongoing challenge.
In Key Largo, 5 veterans participated before the CVT classes were placed on hold owing to the demands of other CVT programs and limited availability of the telehealth clinical technician (TCT). The Homestead CBOC continues to offer CVT Gerofit exercise classes and has 6 regular participants. Notably, the physical space at the Homestead CBOC is smaller than that at the Key Largo CBOC; the Homestead CBOC has adjusted by shifting to exercises performed while standing or sitting, ensuring participants’ safety and satisfaction.
The Baltimore, Maryland VAMC Gerofit program offers other innovative CVT exercise classes, including a tai chi class, and a class with exercise performed while sitting in a chair. Although the Baltimore VAMC CVT exercise classes do not have the scope of the center-based exercise prescriptions, they are unique in that they are broadcast not only to their affiliated CBOCs, but also other Gerofit programs in different VISNs.
Related: Telehealth for Rural Veterans With Neurologic Disorders
Salem VAMC Gerofit Program. The center-based Salem VAMC Gerofit program was established in July 2015. In fiscal year 2017, its dedicated exercise facility had more than 5,000 patient visits. Despite the program’s success, we prioritized establishing Tele-Gerofit because of the medical center’s rural location in southwest Virginia and the large number of veterans who receive care at CBOCs. Therefore, much as with the pilot CVT Gerofit classes in Los Angeles and Miami, the target setting was rural CBOCs. The goal for Salem VAMC Tele-Gerofit was to modify Gerofit delivery to the CVT format and a CBOC setting with minimal modification of the content and personnel requirements of both physical performance testing and exercise training procedures.
Adjustments for CBOC Setting. The enrollment process for Tele-Gerofit is the same as that for the center-based program. To start, a veteran’s primary care provider reviews the list of eligibility criteria and, if the veteran qualifies, places a consult. A Gerofit team member then contacts the veteran by phone to describe the program and schedule an assessment. At the baseline physical performance assessment, American College of Sports Medicine guidelines on exercise participation, health screening, and exercise intensity are used to evaluate veterans and rank them by their cardiovascular risk.20 All new program participants start with low-intensity exercise and gradually progress to recommended levels of exercise. Before starting an exercise class, participants are instructed on use of the 10-point rating of perceived exertion (RPE).
Each CBOC site is supplied with an RPE poster that is displayed for participants’ use. During a Tele-Gerofit class, the exercise instructor asks participants to periodically report their RPE. This class differs slightly from the center-based exercise sessions in which RPE is primarily assessed when a different exercise is introduced or the duration or intensity of an exercise is increased. The Gerofit instructor monitors exercise and treatment fidelity, but the onsite TCT observes for safety during class. The TCT also takes initial vital signs and sets up the room for the class. Emergency contacts and procedures are posted in each CBOC CVT room and are available to the center-based exercise instructor. Because the CBOCs are not inside medical facilities, some CBOC directors have asked that heart rate monitors be used as an extra safety precaution to ensure that high-risk participants do not exceed a heart rate limit that may be set by their cardiologists.
Modifications to Physical Performance Assessment. Physical performance testing had to be adapted to the small rooms available at the CBOCs. For measuring normal gait speed, the 10-MWT was replaced with the 4-meter walk test (4-MWT). The 4-MWT has excellent test–retest reliability with an intraclass correlation coefficient (ICC) of 0.93, but the discrepancy in gait speed between the 4-MWT and the 10-MWT is such that the tests cannot be used interchangeably.21 For measuring endurance, the 6-minute walk test was replaced with the 2-minute step test (2-MST). In older adults, the 2-MST has a moderate correlation with 6-minute walk distance (r = 0.36; P = .04) and high reliability (ICC, 0.90).15,22 The 30-second 1-arm curl, the 30-second chair-stand test, and the 8-foot up-and-go test are performed without modification and require only dumbbells, a chair without wheels, and a stopwatch.
The exercise instructor at the Salem VAMC conducts physical performance testing by 2-way videoconferencing with the veteran in a room at the CBOC. The TCT at the CBOC assists by measuring and demarcating 4 meters on the floor and a designated height on the wall for knee elevation for 4-MWT and 2-MST, respectively. The TCT remains in the room during the assessment visit. Except for taking vital signs before and after the physical performance assessment, the TCT does not participate in the testing. To date, more than 20 physical performance assessments have been conducted without difficulty at Salem-affiliated CBOCs. The primary challenge has been scheduling the room with CVT equipment (ie, camera and screen) for the 30-minute individual assessment session, which occurs on a rolling basis as individuals are enrolled and followed.
After the assessment is completed, the exercise instructor reviews the results with the participant and provides feedback on areas in need of improvement. However, these education sessions can be lengthy and are best supported by giving the patient a personalized handout.
Functional Circuit Exercise. In Tele-Gerofit, exercise training is delivered by CVT broadcast from the Salem VAMC to veterans in a room (equipped with steps, dumbbells, chairs, and bands) at the CBOC. This type of exercise training, which uses only mobile equipment and plyometric (weight-bearing) exercises, is referred to as functional exercise. The AEX includes marching in place, moving on and off a raised step, and body-weight exercises, while RT uses dumbbells, resistance bands, and plyometric exercises (Table 2).
Progression of intensity is achieved by increasing the rate of stepping and the size of the steps (AEX) or the number of repetitions and the weight of the dumbbells or bands (RT). Each veteran exercises at an intensity level that is appropriate for his or her baseline limitations and medical conditions. The exercise instructor uses different forms of the same equipment (eg, heavier dumbbells, higher steps) to vary intensity among individuals while having them perform the same exercises as a group. The challenge is to adjust the pace of the AEX or the timing of the RT repetitions for individuals new to the class.
Delivery of exercise training in the form of circuits allows for a diverse exercise program in a setting with limited space. Circuit training is an exercise modality that consists of a series of different exercises, each usually completed in 30 to 60 seconds, with minimal rest between each type of exercise. Each Tele-Gerofit circuit has a mix of AEX and RT exercises performed for 3 minutes consecutively (Figure).
The design of the circuit training can be adjusted based on the number of individuals in the class. Larger classes can be split into 2 groups that alternate between exercise sets, while smaller classes have 1 group performing the same exercise set and then rotating to either the AEX or RT set. Total exercise time to complete the circuit depends on the number of different exercises, number of repetitions, and the rest between repetitions and the different exercises. In this way, total exercise time can be made shorter or longer depending on the veteran’s capacity.
Frequency. Tele-Gerofit exercise classes are currently offered twice weekly and last about 1 hour, which includes warm-up (8-10 minutes), functional circuit training (40 minutes), and cooldown/stretching (8-10 minutes). A challenge for the exercise instructor is the need to provide ongoing clear instructions both to the class and to individuals as needed. As the exercise prescription for each patient is based on physical performance testing, the exercise instructor for the training must be familiar with the test results. Derivation of the exercise prescription in Tele-Gerofit follows the same process as center-based Gerofit.
Each patient is given an exercise prescription written to address any impairments noted in the different domains of the physical performance assessment, scored using age and sex percentiles. For instance, individuals scoring poorly on lower body strength are given specific lower body strengthening exercises. Participants are given an exercise program that guides them toward achieving recommended physical activity guidelines using their RPE to modulate each exercise. Duration and intensity of each type of planned exercise are formally discussed after initial and follow-up assessments. In addition, exercise training is informally progressed throughout the program. For Tele-Gerofit, instructors must design each class with the group in mind while being prepared for modifications and specific changes for individuals.
Discussion
Tele-Gerofit adapts the well-established center-based Gerofit program to be executed without an exercise facility while maintaining the content of the evidence-based procedures. Physical performance testing and exercise training were modified, adding elements necessary for CVT assessments and classes to be broadcast from the Salem VAMC to its affiliated CBOCs. Tele-Gerofit exercises are performed in a circuit style that allows a veteran or small structured groups of veterans to move among exercises and requires less space than traditional group exercise does. Safety and monitoring concerns are addressed with a safety procedure that includes emergency plans for each site, prescreening of enrolled participants, and monitoring of exercise intensity in accordance with national guidelines.1 Similar to the center-based Gerofit program, the exercise prescription is tailored to each veteran’s physical limitations based on initial and ongoing assessment of physical performance. Tele-Gerofit physical performance testing fulfills the same need with only a few modifications using validated measures. Tele-Gerofit assessments are administered by CVT without the need for additional staff on site.
Adaptation of center-based Gerofit exercise classes to Tele-Gerofit is a major innovation. Use of a circuit exercise design was supported by findings in older adults that RT alone, when performed quickly with minimal rest between each set and exercise station, increases both aerobic capacity and strength.23,24 Older adult RT trials that compared circuit RT with traditional RT found that strength gains are comparable between circuit and traditional RT.24-26 Working with adults aged > 60 years, Takeshima and colleagues conducted a trial of circuit exercise with added callisthenic exercises performed in place between RT on exercise machines.27 This dual-modality (AEX+RT) circuit approach was well tolerated and effective, increasing aerobic capacity and strength. Unfortunately, the resistance exercise machines used in those circuit exercise studies and in the center-based Gerofit program are not an option for Tele-Gerofit.
The requirement for an exercise facility was removed by designing Tele-Gerofit exercise to include only functional exercises that rely on body weight or small mobile exercise equipment. Although popular among young adults, functional circuit exercise is understudied in older adults. Recently, a 12-week functional circuit exercise intervention in frail elderly adults demonstrated significant improvements in gait speed and the timed chair-stand test.28 A pilot observational study of Gerofit participants at the Canandaigua VAMC offered 27 veterans functional circuit exercise instead of their traditional exercise facility class and found larger increases in the timed chair-stand test and 6-minute walk distance compared with 11 Gerofit participants in the traditional program.29
This Tele-Gerofit exercise training combines functional and circuit exercise strategies into telehealth delivery. However, its effect on physical performance remains to be demonstrated. To address this question, we are conducting a single-arm pilot study of Tele-Gerofit with CVT broadcast to 3 Salem CBOC affiliates (Wytheville, Staunton, and Danville, Virginia). The goal is to determine the effect on physical performance and collect feasibility data, including attendance rate and patient satisfaction with the video broadcast. In addition, we are planning an effectiveness trial to compare the impact of functional circuit exercise delivered in person (center based, not CVT) with the parent Gerofit exercise program on direct measures of endurance and strength, in addition to physical performance.
Related: Setting and Method of Measurement Affect Blood Pressure Readings in Older Veterans
Implementation research is needed to determine how Tele-Gerofit can be disseminated to other VAMCs and community-based centers beyond CBOCs. Although the cost of the equipment used to implement Tele-Gerofit is minimal, the program requires dedicated and experienced exercise instructors, and the sharing of telehealth resources with other clinical programs. The authors expect that a diverse group of stakeholders is needed across service lines of primary care, geriatrics and extended care, physical medicine and rehabilitation, and telehealth. Of note, this multidisciplinary collaboration is a hallmark of the Gerofit program. The recent success of the implementation of center-based Gerofit in VAMCs across the US demonstrates the program’s flexibility and robust results.18
Plans also include refining strategies for physical performance testing and exercise monitoring. For instance, we would like to adapt telehealth technology for heart rate monitors that can be worn by high-risk veterans at the CBOC and viewed in real time by the exercise instructor.
Conclusion
Gerofit, which is designed to help older veterans maintain independent living and prevent disability, has been demonstrated to improve quality of life and survival. Our goal has been to adapt Gerofit to CVT and provide a supervised, individualized exercise program in a group setting—a program that can be widely disseminated. Salem VAMC Tele-Gerofit is an innovative and prescriptive program that delivers CVT functional circuit exercise training to remote locations without the need for stationary exercise equipment. This approach has the potential to become an effective and feasible exercise strategy for preventing and minimizing disability in the increasing population of older veterans. Work is needed to determine whether Tele-Gerofit provides a rapid translation of Gerofit to clinical practice and improved outcomes with substantial cost savings from reduced hospitalization and institutionalization.
Acknowledgments
Gerofit has been funded by the Veterans Health Affairs Office of Geriatrics and Extended Care Non-Institutional Long-Term Care Funding and Mentored Partnership Program, and the Veterans Health Affairs Office of Rural Health Rural Enterprise-Wide Initiative.
The authors thank Kim Birkett, MPH, for assistance in editing, references, and graphics and the staff at the Wytheville, Staunton, and Danville community-based outpatient clinics for their support.
Exercise increases endurance, muscle strength, and functional performance with corresponding gains in mobility, survival, and quality of life.1 However, even with these benefits and improvements in clinical outcomes, only 15% of adults aged ≥ 65 years follow current guidelines for exercise.2 Despite their prior military training, the majority of veterans do not meet physical activity recommendations.3 Time, travel, and support are common barriers to exercise participation and adherence—barriers that are further amplified among older adults.
The Veterans Health Administration (VHA) is recognized as a world leader in telehealth service development. Currently, 677,000 veterans have received telehealth services, which represents 12% of the 5.6 million veterans under VHA care.4 Clinical video telehealth (CVT) is widely used within the VHA system to deliver health care that otherwise would not be available to veterans. Veterans who have difficulty traveling to the nearest US Department of Veteran Affairs (VA) medical center (VAMC) can access CVT programs at a participating VHA community-based outpatient clinic (CBOC). The VA has more than 45 CVT programs, including programs for mental health, weight management, cardiology, and dermatology. Outside the VA, cardiac exercise rehabilitation provided by CVT has been shown to be as effective as center-based programs in improving cardiovascular risk factors and functional capacity.5 A VHA exercise program that leveraged CVT resources and was dedicated to older adults with a wide range of comorbid conditions would have a high impact on the health and well-being of older veterans.
Gerofit is a VHA clinical demonstration program of supervised center-based exercise for veterans aged ≥ 65 years. Developed at the Durham VAMC Geriatric Research, Education, and Clinical Center (GRECC) in North Carolina, it has demonstrated improved clinical outcomes, including physical function, mobility, quality of life, and survival.6-10 The program offers veterans individualized exercise in a group setting that focuses on improving endurance, strength, and balance. The exercise prescription is based on the patient’s physical limitations as identified in a physical performance assessment.
With support from VHA Geriatric Extended Care (GEC) and the Office of Rural Health (ORH), Gerofit was implemented in 10 VAMCs across 8 VISNs. However, barriers such as travel time, distance, and transportation limit participation. Previously, we found that rural veterans lack access to exercise programs.11,12 Although some do aerobic exercise (AEX), most do not do resistance training (RT), though they are willing to learn. Access to Gerofit for rural veterans is expanding with recent support from the ORH Enterprise Wide Initiative. Rural program expansion includes several different Gerofit initiatives, many involving CBOCs.
The Salem VAMC Gerofit program sought to adapt the facility-based assessment and exercise procedures into a self-reliant CVT class for its CBOCs. This article describes the development of the Salem VAMC Gerofit CVT program, hereafter referred to as Tele-Gerofit.
Related: Expanding the Scope of Telemedicine in Gastroenterology
Program Design
Gerofit was established in 1986 at the Durham GRECC as an exercise and health promotion program for veterans aged ≥ 65 years.13 Its goal is to prevent or improve functional decline from physical inactivity and age-related conditions. Gerofit targets the geriatric patient population and thus extends beyond cardiac and pulmonary rehabilitation or weight loss programs. The primary exclusion criteria are based on safety issues in the context of a group exercise setting of older adults and include oxygen dependency, unstable cardiac disease, and moderate-to-severe cognitive impairment.
To participate in Gerofit, veterans must be able to perform activities of daily living and self-manage an exercise prescription developed by the exercise instructor based on physical performance testing. These physical performance tests include measures that are independent predictors of disability, loss of independent living, and death, as well as surrogate measures of exercise capacity (eg, strength, endurance, balance).14,15 A novel aspect of Gerofit is that the physical performance assessment is used not only to determine physical limitations, but also to individualize the exercise prescription based on the observed deficits in strength, endurance, or balance. These assessments are performed at initial enrollment; 3 months, 6 months, and 1 year later; and annually after that. Currently, the center-based Gerofit programs administer 5 items of the Senior Fitness Test: 6-minute walk, 10-meter walk (10-MWT), 30-second 1-arm curl, 30-second chair-stand test, and 8-foot up-and-go.15 The side-by-side, semitandem, and tandem standing balance tests from the short physical performance battery also are performed.16 In addition, participants complete a questionnaire that includes items from the physical functioning scale of the 12-Item Short Form Health Survey (SF-12).
After each assessment, the Gerofit exercise instructor reviews the results with the veteran and formulates an individualized exercise prescription along with goals for improvement. Veterans are encouraged to attend supervised center-based exercise sessions 3 times weekly. Classes are offered in a gym or fitness center at the VAMC or in leased space. Each patient uses a cue card that lists an exercise plan personalized for intensity and duration for aerobic exercise (AEX; eg, treadmill walking, stationary bicycling, arm ergometry), RT using dumbbells and weight equipment, and functional exercises for flexibility and balance. Some medical centers also offer yoga, tai chi, or dancing Gerofit classes.
For participants in the Durham Gerofit program, mortality decreased 25% over a decade (hazard ratio, 0.75; 95% CI, 0.61-0.91).9 A substudy that included the Psychological General Well-Being Index found that 81% of participants significantly increased their score after 1 year.7 Observed initial improvement in physical performance has been sustained over 5 years.10,17 One-year results from the recent Gerofit expansion to 6 other VAMCs showed clinically and statistically significantly improved physical performance from baseline to 3-, 6-, and 12-month follow-up.18
Adaptation of Gerofit to CVT Delivery
Initial work. The Greater Los Angeles VAMC Gerofit program conducted a pilot CVT exercise class of 6 veterans at the rural Bakersfield CBOC in California.19 Each week, an exercise instructor broadcast a 60-minute exercise class that included warm-up, RT with bands, progressive balance training, and flexibility. Trained student volunteers from California State University in Bakersfield kinesiology program were on site at the Bakersfield CBOC to perform the assessments and aid in exercises during the CVT sessions. Despite the lack of AEX per se, veterans showed significant improvement in endurance as measured by an increase in the number of steps completed in 2 minutes at the 3-month assessment (P = .049). Although exercises were not delivered in a circuit format, the improved endurance supported the potential for cardiovascular benefit from RT in older adults.
This pilot project also demonstrated that key components of the Gerofit program could be delivered safely by telehealth with onsite supervision. The Miami VA Healthcare System also offers CVT Gerofit exercise classes broadcast to the rural Florida CBOCs of Key Largo and Homestead.11 The exercise activities offered for the Miami CVT participants incorporate components of AEX (calisthenics) and RT (resistance bands). Veterans enjoyed the classes, and adherence was good. However, availability of staff and space are an ongoing challenge.
In Key Largo, 5 veterans participated before the CVT classes were placed on hold owing to the demands of other CVT programs and limited availability of the telehealth clinical technician (TCT). The Homestead CBOC continues to offer CVT Gerofit exercise classes and has 6 regular participants. Notably, the physical space at the Homestead CBOC is smaller than that at the Key Largo CBOC; the Homestead CBOC has adjusted by shifting to exercises performed while standing or sitting, ensuring participants’ safety and satisfaction.
The Baltimore, Maryland VAMC Gerofit program offers other innovative CVT exercise classes, including a tai chi class, and a class with exercise performed while sitting in a chair. Although the Baltimore VAMC CVT exercise classes do not have the scope of the center-based exercise prescriptions, they are unique in that they are broadcast not only to their affiliated CBOCs, but also other Gerofit programs in different VISNs.
Related: Telehealth for Rural Veterans With Neurologic Disorders
Salem VAMC Gerofit Program. The center-based Salem VAMC Gerofit program was established in July 2015. In fiscal year 2017, its dedicated exercise facility had more than 5,000 patient visits. Despite the program’s success, we prioritized establishing Tele-Gerofit because of the medical center’s rural location in southwest Virginia and the large number of veterans who receive care at CBOCs. Therefore, much as with the pilot CVT Gerofit classes in Los Angeles and Miami, the target setting was rural CBOCs. The goal for Salem VAMC Tele-Gerofit was to modify Gerofit delivery to the CVT format and a CBOC setting with minimal modification of the content and personnel requirements of both physical performance testing and exercise training procedures.
Adjustments for CBOC Setting. The enrollment process for Tele-Gerofit is the same as that for the center-based program. To start, a veteran’s primary care provider reviews the list of eligibility criteria and, if the veteran qualifies, places a consult. A Gerofit team member then contacts the veteran by phone to describe the program and schedule an assessment. At the baseline physical performance assessment, American College of Sports Medicine guidelines on exercise participation, health screening, and exercise intensity are used to evaluate veterans and rank them by their cardiovascular risk.20 All new program participants start with low-intensity exercise and gradually progress to recommended levels of exercise. Before starting an exercise class, participants are instructed on use of the 10-point rating of perceived exertion (RPE).
Each CBOC site is supplied with an RPE poster that is displayed for participants’ use. During a Tele-Gerofit class, the exercise instructor asks participants to periodically report their RPE. This class differs slightly from the center-based exercise sessions in which RPE is primarily assessed when a different exercise is introduced or the duration or intensity of an exercise is increased. The Gerofit instructor monitors exercise and treatment fidelity, but the onsite TCT observes for safety during class. The TCT also takes initial vital signs and sets up the room for the class. Emergency contacts and procedures are posted in each CBOC CVT room and are available to the center-based exercise instructor. Because the CBOCs are not inside medical facilities, some CBOC directors have asked that heart rate monitors be used as an extra safety precaution to ensure that high-risk participants do not exceed a heart rate limit that may be set by their cardiologists.
Modifications to Physical Performance Assessment. Physical performance testing had to be adapted to the small rooms available at the CBOCs. For measuring normal gait speed, the 10-MWT was replaced with the 4-meter walk test (4-MWT). The 4-MWT has excellent test–retest reliability with an intraclass correlation coefficient (ICC) of 0.93, but the discrepancy in gait speed between the 4-MWT and the 10-MWT is such that the tests cannot be used interchangeably.21 For measuring endurance, the 6-minute walk test was replaced with the 2-minute step test (2-MST). In older adults, the 2-MST has a moderate correlation with 6-minute walk distance (r = 0.36; P = .04) and high reliability (ICC, 0.90).15,22 The 30-second 1-arm curl, the 30-second chair-stand test, and the 8-foot up-and-go test are performed without modification and require only dumbbells, a chair without wheels, and a stopwatch.
The exercise instructor at the Salem VAMC conducts physical performance testing by 2-way videoconferencing with the veteran in a room at the CBOC. The TCT at the CBOC assists by measuring and demarcating 4 meters on the floor and a designated height on the wall for knee elevation for 4-MWT and 2-MST, respectively. The TCT remains in the room during the assessment visit. Except for taking vital signs before and after the physical performance assessment, the TCT does not participate in the testing. To date, more than 20 physical performance assessments have been conducted without difficulty at Salem-affiliated CBOCs. The primary challenge has been scheduling the room with CVT equipment (ie, camera and screen) for the 30-minute individual assessment session, which occurs on a rolling basis as individuals are enrolled and followed.
After the assessment is completed, the exercise instructor reviews the results with the participant and provides feedback on areas in need of improvement. However, these education sessions can be lengthy and are best supported by giving the patient a personalized handout.
Functional Circuit Exercise. In Tele-Gerofit, exercise training is delivered by CVT broadcast from the Salem VAMC to veterans in a room (equipped with steps, dumbbells, chairs, and bands) at the CBOC. This type of exercise training, which uses only mobile equipment and plyometric (weight-bearing) exercises, is referred to as functional exercise. The AEX includes marching in place, moving on and off a raised step, and body-weight exercises, while RT uses dumbbells, resistance bands, and plyometric exercises (Table 2).
Progression of intensity is achieved by increasing the rate of stepping and the size of the steps (AEX) or the number of repetitions and the weight of the dumbbells or bands (RT). Each veteran exercises at an intensity level that is appropriate for his or her baseline limitations and medical conditions. The exercise instructor uses different forms of the same equipment (eg, heavier dumbbells, higher steps) to vary intensity among individuals while having them perform the same exercises as a group. The challenge is to adjust the pace of the AEX or the timing of the RT repetitions for individuals new to the class.
Delivery of exercise training in the form of circuits allows for a diverse exercise program in a setting with limited space. Circuit training is an exercise modality that consists of a series of different exercises, each usually completed in 30 to 60 seconds, with minimal rest between each type of exercise. Each Tele-Gerofit circuit has a mix of AEX and RT exercises performed for 3 minutes consecutively (Figure).
The design of the circuit training can be adjusted based on the number of individuals in the class. Larger classes can be split into 2 groups that alternate between exercise sets, while smaller classes have 1 group performing the same exercise set and then rotating to either the AEX or RT set. Total exercise time to complete the circuit depends on the number of different exercises, number of repetitions, and the rest between repetitions and the different exercises. In this way, total exercise time can be made shorter or longer depending on the veteran’s capacity.
Frequency. Tele-Gerofit exercise classes are currently offered twice weekly and last about 1 hour, which includes warm-up (8-10 minutes), functional circuit training (40 minutes), and cooldown/stretching (8-10 minutes). A challenge for the exercise instructor is the need to provide ongoing clear instructions both to the class and to individuals as needed. As the exercise prescription for each patient is based on physical performance testing, the exercise instructor for the training must be familiar with the test results. Derivation of the exercise prescription in Tele-Gerofit follows the same process as center-based Gerofit.
Each patient is given an exercise prescription written to address any impairments noted in the different domains of the physical performance assessment, scored using age and sex percentiles. For instance, individuals scoring poorly on lower body strength are given specific lower body strengthening exercises. Participants are given an exercise program that guides them toward achieving recommended physical activity guidelines using their RPE to modulate each exercise. Duration and intensity of each type of planned exercise are formally discussed after initial and follow-up assessments. In addition, exercise training is informally progressed throughout the program. For Tele-Gerofit, instructors must design each class with the group in mind while being prepared for modifications and specific changes for individuals.
Discussion
Tele-Gerofit adapts the well-established center-based Gerofit program to be executed without an exercise facility while maintaining the content of the evidence-based procedures. Physical performance testing and exercise training were modified, adding elements necessary for CVT assessments and classes to be broadcast from the Salem VAMC to its affiliated CBOCs. Tele-Gerofit exercises are performed in a circuit style that allows a veteran or small structured groups of veterans to move among exercises and requires less space than traditional group exercise does. Safety and monitoring concerns are addressed with a safety procedure that includes emergency plans for each site, prescreening of enrolled participants, and monitoring of exercise intensity in accordance with national guidelines.1 Similar to the center-based Gerofit program, the exercise prescription is tailored to each veteran’s physical limitations based on initial and ongoing assessment of physical performance. Tele-Gerofit physical performance testing fulfills the same need with only a few modifications using validated measures. Tele-Gerofit assessments are administered by CVT without the need for additional staff on site.
Adaptation of center-based Gerofit exercise classes to Tele-Gerofit is a major innovation. Use of a circuit exercise design was supported by findings in older adults that RT alone, when performed quickly with minimal rest between each set and exercise station, increases both aerobic capacity and strength.23,24 Older adult RT trials that compared circuit RT with traditional RT found that strength gains are comparable between circuit and traditional RT.24-26 Working with adults aged > 60 years, Takeshima and colleagues conducted a trial of circuit exercise with added callisthenic exercises performed in place between RT on exercise machines.27 This dual-modality (AEX+RT) circuit approach was well tolerated and effective, increasing aerobic capacity and strength. Unfortunately, the resistance exercise machines used in those circuit exercise studies and in the center-based Gerofit program are not an option for Tele-Gerofit.
The requirement for an exercise facility was removed by designing Tele-Gerofit exercise to include only functional exercises that rely on body weight or small mobile exercise equipment. Although popular among young adults, functional circuit exercise is understudied in older adults. Recently, a 12-week functional circuit exercise intervention in frail elderly adults demonstrated significant improvements in gait speed and the timed chair-stand test.28 A pilot observational study of Gerofit participants at the Canandaigua VAMC offered 27 veterans functional circuit exercise instead of their traditional exercise facility class and found larger increases in the timed chair-stand test and 6-minute walk distance compared with 11 Gerofit participants in the traditional program.29
This Tele-Gerofit exercise training combines functional and circuit exercise strategies into telehealth delivery. However, its effect on physical performance remains to be demonstrated. To address this question, we are conducting a single-arm pilot study of Tele-Gerofit with CVT broadcast to 3 Salem CBOC affiliates (Wytheville, Staunton, and Danville, Virginia). The goal is to determine the effect on physical performance and collect feasibility data, including attendance rate and patient satisfaction with the video broadcast. In addition, we are planning an effectiveness trial to compare the impact of functional circuit exercise delivered in person (center based, not CVT) with the parent Gerofit exercise program on direct measures of endurance and strength, in addition to physical performance.
Related: Setting and Method of Measurement Affect Blood Pressure Readings in Older Veterans
Implementation research is needed to determine how Tele-Gerofit can be disseminated to other VAMCs and community-based centers beyond CBOCs. Although the cost of the equipment used to implement Tele-Gerofit is minimal, the program requires dedicated and experienced exercise instructors, and the sharing of telehealth resources with other clinical programs. The authors expect that a diverse group of stakeholders is needed across service lines of primary care, geriatrics and extended care, physical medicine and rehabilitation, and telehealth. Of note, this multidisciplinary collaboration is a hallmark of the Gerofit program. The recent success of the implementation of center-based Gerofit in VAMCs across the US demonstrates the program’s flexibility and robust results.18
Plans also include refining strategies for physical performance testing and exercise monitoring. For instance, we would like to adapt telehealth technology for heart rate monitors that can be worn by high-risk veterans at the CBOC and viewed in real time by the exercise instructor.
Conclusion
Gerofit, which is designed to help older veterans maintain independent living and prevent disability, has been demonstrated to improve quality of life and survival. Our goal has been to adapt Gerofit to CVT and provide a supervised, individualized exercise program in a group setting—a program that can be widely disseminated. Salem VAMC Tele-Gerofit is an innovative and prescriptive program that delivers CVT functional circuit exercise training to remote locations without the need for stationary exercise equipment. This approach has the potential to become an effective and feasible exercise strategy for preventing and minimizing disability in the increasing population of older veterans. Work is needed to determine whether Tele-Gerofit provides a rapid translation of Gerofit to clinical practice and improved outcomes with substantial cost savings from reduced hospitalization and institutionalization.
Acknowledgments
Gerofit has been funded by the Veterans Health Affairs Office of Geriatrics and Extended Care Non-Institutional Long-Term Care Funding and Mentored Partnership Program, and the Veterans Health Affairs Office of Rural Health Rural Enterprise-Wide Initiative.
The authors thank Kim Birkett, MPH, for assistance in editing, references, and graphics and the staff at the Wytheville, Staunton, and Danville community-based outpatient clinics for their support.
1. American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510-1530.
2. Centers for Disease Control and Prevention. Adult participation in aerobic and muscle-strengthening physical activities—United States, 2011. MMWR Morb Mortal Wkly Rep. 2013;62(17):326-330.
3. Littman AJ, Forsberg CW, Koepsell TD. Physical activity in a national sample of veterans. Med Sci Sports Exerc. 2009;41(5):1006-1013.
4. US Department of Veterans Affairs, Office of Rural Health. Annual Report: Thrive 2015. https://www.ruralhealth.va.gov/docs/ORH_Annual_Report_2015_FINAL.pdf. Published 2015. Accessed July 16, 2018.
5. Rawstorn JC, Gant N, Direito A, Beckmann C, Maddison R. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart. 2016;102(15):1183-1192.
6. Morey MC. Celebrating 20 years of excellence in exercise for the older veteran. Fed Pract. 2007;24(10):49-65.
7. Cowper PA, Morey MC, Bearon LB, et al. The impact of supervised exercise on the psychological well-being and health status of older veterans. J Appl Gerontol. 1991;10(4):469-485.
8. Morey MC, Cowper PA, Feussner JR, et al. Evaluation of a supervised exercise program in a geriatric population. J Am Geriatr Soc. 1989;37(4):348-354.
9. Morey MC, Pieper CF, Crowley GM, Sullivan RJ, Puglisi CM. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50(12):1929-1933.
10. Morey MC, Pieper CF, Sullivan RJ Jr, Crowley GM, Cowper PA, Robbins MS. Five-year performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44(10):1226-1231.
11. Valencia WM, Botros D, Pendlebury D, et al. Proactive reach and telehealth monitoring (Gerofit) enhance resistance exercise at rural setting. Innovat Aging. 2017;1(suppl 1):225.12. Pendlebury D, Botros D VW. Proactive Reach: an innovative access approach to identify & deliver GEROFIT exercise telehealth counseling to rural veterans & enhance CBOC services. J Am Geriatr Soc. 2017(suppl 1):S208. Poster presented at: Annual Scientific Meeting of the American Geriatrics Society; May 18, 2017; San Antonio, TX.
13. Morey MC, Crowley GM, Robbins MS, Cowper PA, Sullivan RJ Jr. The Gerofit program: a VA innovation. South Med J. 1994;87(5):S83-S87.
14. Cooper R, Kuh D, Hardy R; Mortality Review Group; FALCon and HALCyon Study Teams. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467.
15. Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7(2):129-161.
16. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85-M94.
17. Morey MC, Cowper PA, Feussner JR, et al. Two-year trends in physical performance following supervised exercise among community-dwelling older veterans. J Am Geriatr Soc. 1991;39(10):986-992.
18. Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit program. J Am Geriatr Soc. 2018;66(5):1009-1016.
19. Blanchard E, Castle S, Ines E, et al. Delivering a clinical exercise program to rural veterans via video telehealth. Poster C167 presented at: Annual Scientific Meeting of the American Geriatrics Society; May 19-21, 2016; Long Beach, CA.
20. Riebe D, Ehrman JK, Liguori G, Magal M, eds; American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Philadelphia, PA: Wolters Kluwer Health; 2018.
21. Peters DM, Fritz SL, Krotish DE. Assessing the reliability and validity of a shorter walk test compared with the 10-meter walk test for measurements of gait speed in healthy, older adults. J Geriatr Phys Ther. 2013;36(1):24-30.
22. Pedrosa R, Holanda G. Correlation between the walk, 2-minute step and TUG tests among hypertensive older women. Rev Bras Fisioter. 2009;13(3):252-256.
23. Romero-Arenas S, Blazevich AJ, Martinez-Pascual M, et al. Effects of high-resistance circuit training in an elderly population. Exp Gerontol. 2013;48(3):334-340.
24. Brentano MA, Cadore EL, Da Silva EM, et al. Physiological adaptations to strength and circuit training in postmenopausal women with bone loss. J Strength Cond Res. 2008;22(6):1816-1825.
25. Romero-Arenas S, Martinez-Pascual M, Alcaraz PE. Impact of resistance circuit training on neuromuscular, cardiorespiratory and body composition adaptations in the elderly. Aging Dis. 2013;4(5):256-263.
26. Paoli A, Pacelli F, Bargossi AM, et al. Effects of three distinct protocols of fitness training on body composition, strength and blood lactate. J Sports Med Phys Fitness. 2010;50(1):43-51.
27. Takeshima N, Rogers ME, Islam MM, Yamauchi T, Watanabe E, Okada A. Effect of concurrent aerobic and resistance circuit exercise training on fitness in older adults. Eur J Appl Physiol. 2004;93(1-2):173-182.
28. Giné-Garriga M, Guerra M, Pagés E, Manini TM, Jiménez R, Unnithan VB. The effect of functional circuit training on physical frailty in frail older adults: a randomized controlled trial. J Aging Phys Act. 2010;18(4):401-424.
29. Biddle ED, Reynolds P, Kopp T, Cammarata H, Conway P. Implementation of functional training tools elicits improvements in aerobic fitness and lower body strength in older veterans. Poster C169 presented at: Annual Scientific Meeting of the American Geriatrics Society; May 19-21, 2016; Long Beach, CA.
1. American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510-1530.
2. Centers for Disease Control and Prevention. Adult participation in aerobic and muscle-strengthening physical activities—United States, 2011. MMWR Morb Mortal Wkly Rep. 2013;62(17):326-330.
3. Littman AJ, Forsberg CW, Koepsell TD. Physical activity in a national sample of veterans. Med Sci Sports Exerc. 2009;41(5):1006-1013.
4. US Department of Veterans Affairs, Office of Rural Health. Annual Report: Thrive 2015. https://www.ruralhealth.va.gov/docs/ORH_Annual_Report_2015_FINAL.pdf. Published 2015. Accessed July 16, 2018.
5. Rawstorn JC, Gant N, Direito A, Beckmann C, Maddison R. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart. 2016;102(15):1183-1192.
6. Morey MC. Celebrating 20 years of excellence in exercise for the older veteran. Fed Pract. 2007;24(10):49-65.
7. Cowper PA, Morey MC, Bearon LB, et al. The impact of supervised exercise on the psychological well-being and health status of older veterans. J Appl Gerontol. 1991;10(4):469-485.
8. Morey MC, Cowper PA, Feussner JR, et al. Evaluation of a supervised exercise program in a geriatric population. J Am Geriatr Soc. 1989;37(4):348-354.
9. Morey MC, Pieper CF, Crowley GM, Sullivan RJ, Puglisi CM. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50(12):1929-1933.
10. Morey MC, Pieper CF, Sullivan RJ Jr, Crowley GM, Cowper PA, Robbins MS. Five-year performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44(10):1226-1231.
11. Valencia WM, Botros D, Pendlebury D, et al. Proactive reach and telehealth monitoring (Gerofit) enhance resistance exercise at rural setting. Innovat Aging. 2017;1(suppl 1):225.12. Pendlebury D, Botros D VW. Proactive Reach: an innovative access approach to identify & deliver GEROFIT exercise telehealth counseling to rural veterans & enhance CBOC services. J Am Geriatr Soc. 2017(suppl 1):S208. Poster presented at: Annual Scientific Meeting of the American Geriatrics Society; May 18, 2017; San Antonio, TX.
13. Morey MC, Crowley GM, Robbins MS, Cowper PA, Sullivan RJ Jr. The Gerofit program: a VA innovation. South Med J. 1994;87(5):S83-S87.
14. Cooper R, Kuh D, Hardy R; Mortality Review Group; FALCon and HALCyon Study Teams. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467.
15. Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7(2):129-161.
16. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85-M94.
17. Morey MC, Cowper PA, Feussner JR, et al. Two-year trends in physical performance following supervised exercise among community-dwelling older veterans. J Am Geriatr Soc. 1991;39(10):986-992.
18. Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit program. J Am Geriatr Soc. 2018;66(5):1009-1016.
19. Blanchard E, Castle S, Ines E, et al. Delivering a clinical exercise program to rural veterans via video telehealth. Poster C167 presented at: Annual Scientific Meeting of the American Geriatrics Society; May 19-21, 2016; Long Beach, CA.
20. Riebe D, Ehrman JK, Liguori G, Magal M, eds; American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Philadelphia, PA: Wolters Kluwer Health; 2018.
21. Peters DM, Fritz SL, Krotish DE. Assessing the reliability and validity of a shorter walk test compared with the 10-meter walk test for measurements of gait speed in healthy, older adults. J Geriatr Phys Ther. 2013;36(1):24-30.
22. Pedrosa R, Holanda G. Correlation between the walk, 2-minute step and TUG tests among hypertensive older women. Rev Bras Fisioter. 2009;13(3):252-256.
23. Romero-Arenas S, Blazevich AJ, Martinez-Pascual M, et al. Effects of high-resistance circuit training in an elderly population. Exp Gerontol. 2013;48(3):334-340.
24. Brentano MA, Cadore EL, Da Silva EM, et al. Physiological adaptations to strength and circuit training in postmenopausal women with bone loss. J Strength Cond Res. 2008;22(6):1816-1825.
25. Romero-Arenas S, Martinez-Pascual M, Alcaraz PE. Impact of resistance circuit training on neuromuscular, cardiorespiratory and body composition adaptations in the elderly. Aging Dis. 2013;4(5):256-263.
26. Paoli A, Pacelli F, Bargossi AM, et al. Effects of three distinct protocols of fitness training on body composition, strength and blood lactate. J Sports Med Phys Fitness. 2010;50(1):43-51.
27. Takeshima N, Rogers ME, Islam MM, Yamauchi T, Watanabe E, Okada A. Effect of concurrent aerobic and resistance circuit exercise training on fitness in older adults. Eur J Appl Physiol. 2004;93(1-2):173-182.
28. Giné-Garriga M, Guerra M, Pagés E, Manini TM, Jiménez R, Unnithan VB. The effect of functional circuit training on physical frailty in frail older adults: a randomized controlled trial. J Aging Phys Act. 2010;18(4):401-424.
29. Biddle ED, Reynolds P, Kopp T, Cammarata H, Conway P. Implementation of functional training tools elicits improvements in aerobic fitness and lower body strength in older veterans. Poster C169 presented at: Annual Scientific Meeting of the American Geriatrics Society; May 19-21, 2016; Long Beach, CA.
Bisphosphonate-related atypical femoral fracture: Managing a rare but serious complication
Bisphosphonate therapy minimizes bone loss and reduces fracture risk by up to 50% in patients with osteoporosis,1 but it is also associated with increased risks of osteonecrosis of the jaw and atypical femoral fracture. Although atypical femoral fractures are rare, they can have a devastating effect. Patient concern about this complication has contributed to a decrease in bisphosphonate use by about half in the last decade or so,2,3 and we fear this could result in an increase in hip fracture rates.
In this article, we examine the evidence on bisphosphonate-associated atypical femoral fractures, including risks, pathogenesis, treatment, and prevention.
ATYPICAL FRACTURES INVOLVE THE FEMORAL SHAFT, NOT THE HEAD
An atypical femoral fracture is a transverse fracture of the femoral shaft (diaphysis), defined by both clinical criteria and radiographic appearance.
To be defined as atypical, a femoral fracture must meet 4 of the following 5 criteria4:
- Occurs with minimal or no trauma
- Has a predominantly transverse fracture line, originating at the lateral cortex and sometimes becoming oblique as it progresses medially across the femur
- Extends through both cortices and may be associated with a medial spike (complete fractures); or involves only the lateral cortex (incomplete fractures)
- Is noncomminuted or minimally comminuted
- Shows localized periosteal or endosteal thickening (termed “beaking” or “flaring”) of the lateral cortex at the fracture site.
Several minor features are also important but are not required, eg:
- Cortical thickening of the femoral shaft
- Unilateral or bilateral prodromal pain preceding the fracture
- Bilateral incomplete or complete femoral diaphysis fractures
- Delayed fracture healing.
Atypical femoral fracture can occur anywhere along the shaft, from just distal to the lesser trochanter to just proximal to the supracondylar flare. However, most occur in 2 areas, with 1 cluster centered at about 41 mm from the lesser trochanter (more common in relatively younger patients) and the other at 187 mm.5
ABSOLUTE RISK IS LOW BUT INCREASES WITH LONGER USE
Atypical femoral fractures are rare. Schilcher et al6 reviewed radiographs of 1,234 women who had a subtrochanteric or shaft fracture and found 59 (4.6%) of fractures were atypical. In a systematic review of 14 studies,7 the incidence ranged from 3.0 to 9.8 cases per 100,000 patient-years.
Furthermore, not all atypical femoral fractures are in bisphosphonate users: 7.4% were in nonusers in 1 series8 and 22% in another.9
Nevertheless, most studies show that bisphosphonate use increases the incidence of atypical femoral fracture, and the incidence increases with duration of use, especially after 3 years.7
An international task force of the American Society for Bone and Mineral Research listed the absolute risk as between 3.2 and 50 cases per 100,000 patient-years, with longer use (> 5 years) increasing the risk to about 100 per 100,000 patient-years.4 After stopping bisphosphonate therapy, the risk diminished by 70% per year.9
In another study, for 0.1 to 1.9 years of therapy, the age-adjusted atypical fracture rates were 1.78 per 100,000 per year (95% confidence interval [CI] 1.5–2.0), increasing to 113.1 per 100,000 per year (95% CI 69.3–156.8) with exposure from 8 to 9.9 years.10
A case-control study found that more than 5 years of bisphosphonate use increased the fracture risk by an odds ratio of 2.74 (95% CI 1.25–6.02).11
The incidence of typical femoral fracture was higher in those who adhered better to their oral bisphosphonate regimen in some studies,12 but the opposite was true in others.13
The benefits of bisphosphonate therapy in reducing fracture risk, however, outweigh the risk of atypical fracture.4
We do not know whether the rate of atypical femoral fracture is increasing. A review of Kaiser Permanente Northwest records found that the rates of atypical femoral shaft fracture had remained stable from 1996 to 2009. However, 61.9% of patients who met the strict radiographic criteria had taken oral bisphosphonates.14 These data suggest that bisphosphonate use has not increased the overall population-based risk for subtrochanteric and femoral shaft fractures, but that bisphosphonates and other risk factors may have increased the likelihood that such fractures will exhibit atypical radiographic features.
A population-based study in Denmark13 found that alendronate use longer than 10 years was associated with an adjusted 30% lower risk of hip fracture and no increase in the risk of subtrochanteric and femoral shaft fracture. In addition, the risk of subtrochanteric and femoral shaft fracture was lower with high adherence to alendronate treatment (based on medication possession ratio > 80%) compared with low adherence (ratio < 50%) (odds ratio 0.88, 95% CI 0.77–0.99). The risk was not increased in current vs past users.
The Danish study13 used the coding of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) to identify subtrochanteric and femoral shaft fractures without radiologic review for atypical radiographic features. The lack of specific ICD-10 coding for subtrochanteric and femoral shaft fractures with atypical radiographic features has limited our knowledge of their incidence.
Contralateral fracture in more than one-fourth of cases
After an atypical femoral fracture, patients have a significant risk of fracture on the contralateral side. In a case-control study, 28% of patients with atypical femoral fracture suffered a contralateral fracture, compared with 0.9% of patients presenting with a typical fracture pattern (odds ratio 42.6, 95% CI 12.8–142.4).15
Contralateral fracture occurs from 1 month to 4 years after the index atypical femoral fracture.16
There are reports of bisphosphonate-related low-impact fractures in other sites such as the tibia17 and forearm.18 However, they may be too rare to warrant screening.
Mortality rates
A Swedish database study found that patients with atypical femoral fractures, whether bisphosphonate users or nonusers, do not have higher mortality rates than patients with ordinary subtrochanteric or femoral shaft fractures.19 Furthermore, the mortality rates for those with atypical femoral fracture were similar to rates in the general population. In contrast, patients with an ordinary femoral fracture had a higher mortality risk than the general population.19
Other studies suggest that atypical femoral fracture may be associated with a less favorable prognosis in older patients,20 but this could be due to differences in demographics, treatment adherence, or postfracture care.21
In addition, functional outcomes as measured by independent mobility at discharge and at 3 months were comparable between patients with atypical fracture and those with typical fracture.22
IMAGING STUDIES
If a long-term bisphosphonate user presents with hip, thigh, or groin pain, imaging studies are recommended.
Plain radiography
Radiography is usually the first step and should include a frontal view of the pelvis (Figure 1) and 2 views of the full length of each femur. If radiography is not conclusive, bone scan or magnetic resonance imaging (MRI) should be considered.
A linear cortex transverse fracture pattern and focal lateral cortical thickening are the most sensitive and specific radiographic features.23,24 Because of the risk of fracture on the contralateral side, radiographic study of that side is recommended as well.
Computed tomography
Computed tomography (CT) is not sensitive for early stress fractures and, given the radiation burden, is not recommended in the workup of atypical fracture.
Bone scanning
Bone scanning using technetium 99m-labeled methylene diphosphonate with a gamma camera shows active bone turnover. Stress fractures and atypical femoral fractures are most easily identified in the third (delayed) phase of the bone scan. Although bone scanning is highly sensitive, the specificity is limited by lack of spatial resolution. Atypical femoral fracture appears as increased activity in the subtrochanteric region with a predilection for the lateral cortex.
Dual-energy x-ray absorptiometry
Conventional dual-energy x-ray absorptiometry (DXA) extends only to 1 to 2 cm below the lesser trochanter and can therefore miss atypical fractures, which usually occur farther down. The overall detection rate for DXA was 61% in a sample of 33 patients.25
Newer scanners can look at the entire femoral shaft.26 In addition, newer software can quantify focal thickening (beaking) of the lateral cortex and screen patients who have no symptoms. The results of serial measurements can be graphed so that the practitioner can view trends to help assess or rule out potential asymptomatic atypical femoral fracture.
A localized reaction (periosteal thickening of the lateral cortex or beaking) often precedes atypical femoral fracture. A 2017 study reported that patients with high localized reaction (mean height 3.3 mm) that was of the pointed type and was accompanied by prodromal pain had an increased risk of complete or incomplete atypical femoral fracture at that site.27 This finding is used by the newer DXA software. The predictive value of beaking on extended femoral DXA may be as high as 83%.26
Magnetic resonance imaging
The MRI characteristics of atypical femoral fracture are similar to those of other stress fractures except that there is a lateral-to-medial pattern rather than a medial pattern. The earliest findings include periosteal reaction about the lateral cortex with a normal marrow signal.
MRI may be of particular benefit in patients with known atypical femoral fracture to screen the contralateral leg. It should image the entire length of both femurs. Contrast enhancement is not needed.
Regardless of whether initial findings were discovered on conventional radiographs or DXA, MRI confirmation is needed. Radionuclide bone scanning is currently not recommended because it lacks specificity. Combination imaging is recommended, with either radiography plus MRI or DXA plus MRI.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of atypical femoral fracture includes stress fracture, pathologic fracture, hypophosphatasia, and osteogenesis imperfecta.28 Hypophosphatemic osteomalacia can cause Looser zones, which can be confused with atypical femoral fractures but usually occur on the medial side.4 Stress fracture of the femur can occur below the lesser trochanter but usually begins in the medial, not the lateral, cortex.
Pathologic fractures from underlying osseous lesions can mimic the cortical beaking of bisphosphonate-related fracture, but they usually show the associated underlying lucent lesion and poorly defined margins. A sinus tract along the region of a chronic osteomyelitis may also appear similar.
Hypophosphatasia is an inborn error of metabolism caused by a loss-of-function mutation in the gene encoding alkaline phosphatase, resulting in pyrophosphate accumulation and causing osteomalacia from impaired mineralization. This can result in femoral pseudofracture that is often bilateral and occurs in the subtrochanteric region.29
ADDITIONAL RISK FACTORS
Patients with atypical femoral fracture are generally a heterogeneous group, but there are risk factors to note other than bisphosphonate exposure.
Asian women had a risk 8 times higher than white women in 1 study.30
Bone geometry. Mahjoub et al8 reported that compared with controls, patients with atypical femoral fracture had greater offset of the femoral shaft from the center of rotation of the femoral head, a more acute angle between the femoral neck and shaft, and greater proximal cortical thickness.
Medications. In addition to bisphosphonates, other drugs associated with atypical femoral fracture include RANK-ligand inhibitors such as denosumab (another drug for osteoporosis),31 glucocorticoids,32,33 and proton pump inhibitors.32,33
Genetics. Three sisters with atypical femoral fracture were found to have 37 rare mutations in 34 genes, including one in the GGPS1 gene, which codes for geranylgeranyl pyrophosphate synthase—an enzyme that bisphosphonates inhibit.34
Medical conditions other than osteoporosis include collagen diseases, chronic pulmonary disease, asthma, rheumatoid arthritis, and diabetes.35
Clinical recommendations
Current recommendations are to reevaluate bisphosphonate use in patients with osteoporosis after 5 or more years of therapy.36
Given that patients with osteoporosis are at increased risk of typical fracture, those at higher risk should be considered for continued bisphosphonate therapy. Factors for high risk include the following:
- History of fracture on therapy
- Hip T score –2.5 or lower
- Older age (≥ 70)
- Other strong risk factors for fracture such as smoking, alcohol use, corticosteroid use, rheumatoid arthritis, and family history
- World Health Organization FRAX fracture risk score above the country-specific threshold.
Those at lower risk should be considered for a 2- to 3-year bisphosphonate holiday with periodic reevaluation of bone density and, possibly, bone markers.36
WHAT IS THE UNDERLYING PATHOPHYSIOLOGY?
The mechanism by which bisphosphonates increase the risk of atypical femoral fracture is not clear. These drugs work by suppressing bone turnover; however, in theory, prolonged use could suppress it too much and increase bone fragility.
One hypothesis is that bisphosphonates impair the toughening of cortical bone, an important barrier to clinical fracture. This is supported by a study that found bisphosphonate users with atypical femoral fracture had deficits in intrinsic and extrinsic bone toughness, perhaps due to treatment-related increases in matrix mineralization.37 Although this study and others showed an increase in matrix mineralization and reduced mineralization heterogeneity with bisphosphonate use,38,39 it is unclear whether such changes contributed to reduced toughness or to atypical femoral fracture.
Changes in the skeletal geometry of the lower limb such as femoral neck-shaft angle and femoral curvature alter the stresses and strains experienced by the femoral diaphysis with loading. Because the incidence of incomplete atypical femoral fracture is much greater than that of complete fracture, most incomplete atypical femoral fractures heal before the fracture progresses.
Ultimately, all fractures, including atypical femoral fractures, occur when mechanical stress and strain exceed bone strength.
Antiresorptive drugs such as bisphosphonates, estrogen, calcitonin, and RANK ligand inhibitors prevent hip fracture by increasing the strength of the proximal femur—perhaps at the expense of the strength (or toughness) of the subtrochanteric shaft. It is also possible that treatment-related increases in hip strength (and reduced hip fracture rates) promote or sustain the transfer of stress and strain to femoral regions that experience lesser or no increases in strength from treatment, which likely includes the shaft.40,41
CT studies in Japanese women with osteoporosis have shown that 2 years of zoledronate therapy had greater effects in the hip than in the femoral shaft, with significant increases in cortical thickness and volumetric bone mineral density at the femoral neck and intertrochanteric region compared with baseline.42 But zoledronate did not increase femoral shaft cortical thickness and caused only a minor increase in femoral shaft volumetric bone mineral density. Fracture patterns may have depended on damage and effects of bone turnover on mass and structure.
This hypothetical scenario portrays a possible “hip survival bias” mechanism for atypical femoral fracture, with the association with antiresorptive drugs arising from greater stress and strain in cortical regions where these fractures occur rather than from treatment-related reductions in cortical bone strength or toughness.
PRODROMAL PAIN IS COMMON
From 32% to 76% of patients who have incomplete or developing atypical femoral fracture present with a prodrome of groin or hip pain.4,43 Prodromal pain occurs any time from 2 weeks to several years before the fracture, presenting as pain in the anterior or lateral thigh or in the groin.
Prodromal pain in a patient on antiresorptive therapy should be a signal for the clinician to obtain a radiograph of the hip and to look for contralateral symptoms and fractures. The most common mechanism of injury appears to be a ground-level fall or even a nontraumatic activity such as walking or stepping off a curb.
MEDICAL MANAGEMENT
In bisphosphonate users with radiographic evidence of atypical femoral fracture, the bisphosphonate should be discontinued and the patient assessed for calcium and vitamin D deficiency, with supplements prescribed if needed.4
For patients with incomplete fracture and persistent pain after 3 months of medical management, prophylactic surgical nail fixation is recommended to prevent complete fracture.
Teriparatide, which has been associated with enhanced bone fracture healing, is a possible treatment to promote healing of atypical femoral fracture, either alone or as an adjunct to surgical fixation. A systematic review published in 2015 supported the use of teriparatide for enhancing fracture healing in atypical femoral fracture.44 In addition, a 10-patient series45 showed that incomplete fractures without radiolucent lines responded to teriparatide alone, whereas those with radiolucent lines needed intramedullary nailing.
These results suggest that teriparatide works best when the fracture site is stable, either inherently or with surgical fixation.
ORTHOPEDIC CARE
Orthopedic care for atypical femoral fracture differs depending on whether the patient experiences pain and whether the fracture is incomplete or complete. Figure 2 shows a treatment algorithm for atypical femoral fracture.
These are difficult fractures to manage, complicated by delayed healing in the elderly, complex displacement patterns, altered bone geometry, and risk of fracture in the opposite limb, all of which raise questions about recommending protected weight-bearing exercise.
Furthermore, atypical femoral fracture is often associated with increased anterolateral bowing of the femur, making it difficult to insert an intramedullary nail: the radius of curvature of the bone is shorter than that of a standard femoral nail. This mismatch can lead to intraoperative complications such as iatrogenic fracture during prophylactic nailing, malunion from excess straightening of the femur (which can itself lead to leg length discrepancy), and gapping of the fracture site, particularly on the medial side.
Intramedullary nailing for complete fracture
Intramedullary nailing is the first-line treatment for complete atypical femoral fracture, although the risk of delayed healing and revision surgery may be somewhat higher than with typical femoral fracture.46 Prophylactic intramedullary nailing should be considered for a patient with intractable pain.2
A radiograph of the opposite leg should be obtained routinely, looking for an asymptomatic fracture. Bisphosphonates should be discontinued and calcium and vitamin D continued. Teriparatide therapy can be considered as an alternative treatment.
Conservative management for incomplete fracture without pain
Incomplete atypical femoral fracture unaccompanied by pain can be followed conservatively.47 In addition to stopping antiresorptive therapy, patients need to avoid high-impact and repetitive-impact activities such as jumping or running. If pain occurs, patients should begin protected weight-bearing exercise.
Treatment is uncertain for incomplete fracture with pain
For patients with incomplete atypical femoral fracture and pain, treatment is controversial. Regimens that include 2 to 3 months of protected weight-bearing exercise, a full metabolic bone workup, calcium and vitamin D supplementation, and anabolic bone agents have produced some success. Some authors have reported poor results from conservative care, with few patients achieving pain relief or signs of complete healing.48,49 Additionally, if an incomplete fracture is found in the opposite femur, protected weight-bearing of both legs may not be possible.
Patients with incomplete fracture should be monitored regularly with radiography and physical examination. If there is progression of the fracture, escalation of pain, or failure to heal within 2 to 3 months, then surgical treatment is necessary.
Prophylactic placement of an intramedullary nail to prevent completion of the fracture and allow a return to full weight-bearing is generally advised.50 A long locking plate can be used if bone deformities make it difficult to place an intramedullary nail; however, nails are preferred because they allow formation of endochondral callus, which can be helpful in these difficult-to-heal fractures.
Results from retrospective reviews have shown that surgically treated patients with bisphosphonate-associated incomplete atypical femoral fracture were more likely than those treated nonsurgically to be pain-free (81% vs 64%) and have radiographic healing (100% vs 18% at final follow-up).46 Results have also been positive for those with complete atypical femoral fracture. At 6 months, 64% of surgically treated patients were pain-free and 98% were radiographically healed.51
The unusual geometry of the femur in patients with atypical femoral fracture and the presence of intramedullary cortical callus makes the placement of an intramedullary femoral rod more complex than in typical femoral fracture.8
Intramedullary nailing of atypical femoral fracture is a challenge for even the most experienced surgeon, and vigilance is imperative to avoid iatrogenic fracture and malunion.
MANY QUESTIONS REMAIN
We need more studies on the pathophysiology of bisphosphonate-associated atypical femoral fracture, the value of periodic screening with DXA, and which factors predict high risk (eg, Asian ethnicity, use of certain medications, femoral geometry). In addition, we need more data on the success of conservative management of incomplete fracture, including use of teriparatide.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996–2012: an ecological analysis. J Bone Miner Res 2015; 30(12):2179–2187. doi:10.1002/jbmr.2565
- Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in US patients between 2002 and 2011. J Bone Miner Res 2014; 29(9):1929–1937. doi:10.1002/jbmr.2202
- Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014; 29(1):1–23. doi:10.1002/jbmr.1998
- Koeppen VA, Schilcher J, Aspenberg P. Dichotomous location of 160 atypical femoral fractures. Acta Orthop 2013; 84(6):561–564. doi:10.3109/17453674.2013.866193
- Schilcher J, Koeppen V, Aspenberg P, Michäelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. Acta Orthop 2015; 86(1):100–107. doi:10.3109/17453674.2015.1004149
- Khow KS, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Epidemiology and postoperative outcomes of atypical femoral fractures in older adults: a systematic review. J Nutr Health Aging 2017; 21(1):83–91. doi:10.1007/s12603-015-0652-3
- Mahjoub Z, Jean S, Leclerc JT, et al. Incidence and characteristics of atypical femoral fractures: clinical and geometrical data. J Bone Miner Res 2016; 31(4):767–776. doi:10.1002/jbmr.2748
- Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011; 364(18):1728–1737. doi:10.1056/NEJMoa1010650
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res 2012; 27(12):2544–2550. doi:10.1002/jbmr.1719
- Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011; 305(8):783–789. doi:10.1001/jama.2011.190
- Wang Z, Ward MM, Chan L, Bhattacharyya T. Adherence to oral bisphosphonates and the risk of subtrochanteric and femoral shaft fractures among female Medicare beneficiaries. Osteoporos Int 2014; 25(8):2109–2116. doi:10.1007/s00198-014-2738-x
- Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. BMJ 2016; 353:i3365. doi:10.1136/bmj.i3365
- Feldstein AC, Black D, Perrin N, et al. Incidence and demography of femur fractures with and without atypical features. J Bone Miner Res 2012; 27(5):977–986. doi:10.1002/jbmr.1550
- Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med 2012; 172(12):930–936. doi:10.1001/archinternmed.2012.1796
- La Rocca Vieira R, Rosenberg ZS, Allison MB, Im SA, Babb J, Peck V. Frequency of incomplete atypical femoral fractures in asymptomatic patients on long term bisphosphonate therapy. AJR Am J Roentgenol 2012; 198(5):1144–1151. doi:10.2214/AJR.11.7442
- Bissonnette L, April PM, Dumais R, Boire G, Roux S. Atypical fracture of the tibial diaphysis associated with bisphosphonate therapy: a case report. Bone 2013; 56(2):406–409. doi:10.1016/j.bone.2013.07.012
- Moon J, Bither N, Lee T. Atypical forearm fractures associated with long-term use of bisphosphonate. Arch Orthop Trauma Surg 2013; 133(7):889–892. doi:10.1007/s00402-013-1760-3
- Kharazmi M, Hallberg P, Schilcher J, Aspenberg P, Michaëlsson K. Mortality after atypical femoral fractures: a cohort study. J Bone Miner Res 2016; 31(3):491–497. doi:10.1002/jbmr.2767
- Medin E, Goude F, Melberg HO, Tediosi F, Belicza E, Peltola M; EuroHOPE Study Group. European regional differences in all-cause mortality and length of stay for patients with hip fracture. Health Econ 2015; 24(suppl 2):53–64. doi:10.1002/hec.3278
- Abrahamsen B, Prieto-Alhambra D. Patients with atypical femur fractures have the same mortality as the background population-drug channeling bias, bisphosphonate effects and public health implications. J Bone Miner Res 2016; 31(3):488–490. doi:10.1002/jbmr.2801
- Khow KS, Paterson F, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Outcomes between older adults with atypical and typical femoral fractures are comparable. Injury 2017; 48(2):394–398. doi:10.1016/j.injury.2016.10.035
- Adams AL, Xue F, Chantra JQ, et al. Sensitivity and specificity of radiographic characteristics in atypical femoral fractures. Osteoporos Int 2017; 28(1):413–417. doi:10.1007/s00198-016-3809-y
- Rosenberg ZS, La Rocca Vieira R, Chan SS, et al. Bisphosphonate-related complete atypical subtrochanteric femoral fractures: diagnostic utility of radiography. AJR Am J Roentgenol 2011; 197(4):954–960. doi:10.2214/AJR.10.6262
- Kim S, Yang KH, Lim H, et al. Detection of prefracture hip lesions in atypical subtrochanteric fracture with dual-energy x-ray absorptiometry images. Radiology 2014; 270(2):487–495. doi:10.1148/radiol.13122691
- van de Laarschot DM, Smits AA, Buitendijk SK, Stegenga MT, Zillikens MC. Screening for atypical femur fractures using extended femur scans by DXA. J Bone Miner Res 2017; 32(8):1632–1639. doi:10.1002/jbmr.3164
- Sato H, Kondo N, Nakatsue T, et al. High and pointed type of femoral localized reaction frequently extends to complete an incomplete atypical femoral fracture in patients with autoimmune diseases on long-term glucocorticoids and bisphosphonates. Osteoporos Int 2017; 28(8):2367–2376. doi:10.1007/s00198-017-4038-8
- Giaconi JC, Watterson CT. Bisphosphonate-related atypical femur fractures and the radiographic features. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:107–124. doi:10.1007/978-3-319-23639-1
- Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 2009; 24(6):1132–1134. doi:10.1359/jbmr.081253
- Lo JC, Hui RL, Grimsrud CD, et al. The association of race/ethnicity and risk of atypical femoral fracture among older women receiving oral bisphosphonate therapy. Bone 2016; 85:142–147. doi:10.1016/j.bone.2016.01.002
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017; 5(7):513–523. doi:10.1016/S2213-8587(17)30138-9
- Koh JH, Myong JP, Yoo J, et al. Predisposing factors associated with atypical femur fracture among postmenopausal Korean women receiving bisphosphonate therapy: 8 years' experience in a single center. Osteoporos Int 2017; 28(11):3251–3259. doi:10.1007/s00198-017-4169-y
- Kim D, Sung YK, Cho SK, Han M, Kim YS. Factors associated with atypical femoral fracture. Rheumatol Int 2016; 36(1):65–71. doi:10.1007/s00296-015-3323-0
- Roca-Ayats N, Balcells S, Garcia-Giralt N, et al. GGPS1 mutation and atypical femoral fractures with bisphosphonates. N Engl J Med 2017; 376(18):1794–1795. doi:10.1056/NEJMc1612804
- Giusti A, Hamdy NA, Dekkers OM, Ramautar SR, Dijkstra S, Papapoulos SE. Atypical fractures and bisphosphonate therapy: a cohort study of patients with femoral fracture with radiographic adjudication of fracture site and features. Bone 2011; 48(5):966–971. doi:10.1016/j.bone.2010.12.033
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31(1):16–35. doi:10.1002/jbmr.2708
- Lloyd AA, Gludovatz B, Riedel C, et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc Natl Acad Sci USA 2017; 114(33):8722–8727. doi:10.1073/pnas.1704460114
- Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures; lessons from materials research. Bone 2013; 55(2):495–500. doi:10.1016/j.bone.2013.02.004
- Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone 2015; 71:58–62. doi:10.1016/j.bone.2014.10.010
- Sasaki S, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low-energy diaphyseal femoral fractures associated with bisphosphonate use and severe curved femur: a case series. J Bone Miner Metab 2012; 30(5):561–567. doi:10.1007/s00774-012-0358-0
- Pulkkinen P, Gluer C, Jamsa T. Investigation of differences between hip fracture types: a worthy strategy of improved risk assessment and fracture prevention. Bone 2011; 49(4):600–604. doi:10.1016/j.bone.2011.07.022
- Ito M, Sone T, Shiraki M, et al. The effect of once-yearly zoledronic acid on hip structural and biomechanical properties derived using computed tomography (CT) in Japanese women with osteoporosis. Bone 2018; 106:179–186. doi:10.1016/j.bone.2017.10.013
- Bogdan Y, Einhorn TA. Clinical presentation of atypical femur fractures. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:137–140. doi:10.1007/978-3-319-23639-1
- Im GI, Lee SH. Effect of teriparatide on healing of atypical femoral fractures: a systemic review. J Bone Metab 2015; 22(4):183–189. doi:10.11005/jbm.2015.22.4.183
- Saleh A, Hegde VV, Potty AG, Schneider R, Cornell CN, Lane JM. Management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. HSS J 2012; 8(2):103–110. doi:10.1007/s11420-012-9275-y
- Egol KA, Park JH, Prensky C, Rosenberg ZS, Peck V, Tejwani NC. Surgical treatment improves clinical and functional outcomes for patients who sustain incomplete bisphosphonate-related femur fractures. J Orthop Trauma 2013; 27(6):331–335. doi:10.1097/BOT.0b013e31827240ae
- Koh A, Guerado E, Giannoudis PV. Atypical femoral fractures related to bisphosphonate treatment: issues and controversies related to their surgical management. Bone Joint J 2017; 99-B(3):295–302. doi:10.1302/0301-620X.99B3.BJJ-2016-0276.R2
- Oh CW, Oh JK, Park KC, Kim JW, Yoon YC. Prophylactic nailing of incomplete atypical femoral fractures. ScientificWorldJournal 2013; 2013:450148. doi:10.1155/2013/450148
- Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin Orthop Relat Res 2010; 468(12):3393–3398. doi:10.1007/s11999-010-1583-2
- Tosounidis TH, Lampropoulou-Adamidou, Kanakaris NK. Intramedullary nailing of sequential bilateral atypical subtrochanteric fractures and the management of distal femoral intraoperative fracture. J Orthop Trauma 2015 Jun 11. Epub ahead of print. doi:10.1097/BOT.0000000000000370
- Egol KA, Park JH, Rosenberg ZS, Peck V, Tejwani NC. Healing delayed but generally reliable after bisphosphonate-associated complete femur fractures treated with IM nails. Clin Orthop Relat Res 2014; 472(9):2728–2734. doi:10.1007/s11999-013-2963-1
Bisphosphonate therapy minimizes bone loss and reduces fracture risk by up to 50% in patients with osteoporosis,1 but it is also associated with increased risks of osteonecrosis of the jaw and atypical femoral fracture. Although atypical femoral fractures are rare, they can have a devastating effect. Patient concern about this complication has contributed to a decrease in bisphosphonate use by about half in the last decade or so,2,3 and we fear this could result in an increase in hip fracture rates.
In this article, we examine the evidence on bisphosphonate-associated atypical femoral fractures, including risks, pathogenesis, treatment, and prevention.
ATYPICAL FRACTURES INVOLVE THE FEMORAL SHAFT, NOT THE HEAD
An atypical femoral fracture is a transverse fracture of the femoral shaft (diaphysis), defined by both clinical criteria and radiographic appearance.
To be defined as atypical, a femoral fracture must meet 4 of the following 5 criteria4:
- Occurs with minimal or no trauma
- Has a predominantly transverse fracture line, originating at the lateral cortex and sometimes becoming oblique as it progresses medially across the femur
- Extends through both cortices and may be associated with a medial spike (complete fractures); or involves only the lateral cortex (incomplete fractures)
- Is noncomminuted or minimally comminuted
- Shows localized periosteal or endosteal thickening (termed “beaking” or “flaring”) of the lateral cortex at the fracture site.
Several minor features are also important but are not required, eg:
- Cortical thickening of the femoral shaft
- Unilateral or bilateral prodromal pain preceding the fracture
- Bilateral incomplete or complete femoral diaphysis fractures
- Delayed fracture healing.
Atypical femoral fracture can occur anywhere along the shaft, from just distal to the lesser trochanter to just proximal to the supracondylar flare. However, most occur in 2 areas, with 1 cluster centered at about 41 mm from the lesser trochanter (more common in relatively younger patients) and the other at 187 mm.5
ABSOLUTE RISK IS LOW BUT INCREASES WITH LONGER USE
Atypical femoral fractures are rare. Schilcher et al6 reviewed radiographs of 1,234 women who had a subtrochanteric or shaft fracture and found 59 (4.6%) of fractures were atypical. In a systematic review of 14 studies,7 the incidence ranged from 3.0 to 9.8 cases per 100,000 patient-years.
Furthermore, not all atypical femoral fractures are in bisphosphonate users: 7.4% were in nonusers in 1 series8 and 22% in another.9
Nevertheless, most studies show that bisphosphonate use increases the incidence of atypical femoral fracture, and the incidence increases with duration of use, especially after 3 years.7
An international task force of the American Society for Bone and Mineral Research listed the absolute risk as between 3.2 and 50 cases per 100,000 patient-years, with longer use (> 5 years) increasing the risk to about 100 per 100,000 patient-years.4 After stopping bisphosphonate therapy, the risk diminished by 70% per year.9
In another study, for 0.1 to 1.9 years of therapy, the age-adjusted atypical fracture rates were 1.78 per 100,000 per year (95% confidence interval [CI] 1.5–2.0), increasing to 113.1 per 100,000 per year (95% CI 69.3–156.8) with exposure from 8 to 9.9 years.10
A case-control study found that more than 5 years of bisphosphonate use increased the fracture risk by an odds ratio of 2.74 (95% CI 1.25–6.02).11
The incidence of typical femoral fracture was higher in those who adhered better to their oral bisphosphonate regimen in some studies,12 but the opposite was true in others.13
The benefits of bisphosphonate therapy in reducing fracture risk, however, outweigh the risk of atypical fracture.4
We do not know whether the rate of atypical femoral fracture is increasing. A review of Kaiser Permanente Northwest records found that the rates of atypical femoral shaft fracture had remained stable from 1996 to 2009. However, 61.9% of patients who met the strict radiographic criteria had taken oral bisphosphonates.14 These data suggest that bisphosphonate use has not increased the overall population-based risk for subtrochanteric and femoral shaft fractures, but that bisphosphonates and other risk factors may have increased the likelihood that such fractures will exhibit atypical radiographic features.
A population-based study in Denmark13 found that alendronate use longer than 10 years was associated with an adjusted 30% lower risk of hip fracture and no increase in the risk of subtrochanteric and femoral shaft fracture. In addition, the risk of subtrochanteric and femoral shaft fracture was lower with high adherence to alendronate treatment (based on medication possession ratio > 80%) compared with low adherence (ratio < 50%) (odds ratio 0.88, 95% CI 0.77–0.99). The risk was not increased in current vs past users.
The Danish study13 used the coding of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) to identify subtrochanteric and femoral shaft fractures without radiologic review for atypical radiographic features. The lack of specific ICD-10 coding for subtrochanteric and femoral shaft fractures with atypical radiographic features has limited our knowledge of their incidence.
Contralateral fracture in more than one-fourth of cases
After an atypical femoral fracture, patients have a significant risk of fracture on the contralateral side. In a case-control study, 28% of patients with atypical femoral fracture suffered a contralateral fracture, compared with 0.9% of patients presenting with a typical fracture pattern (odds ratio 42.6, 95% CI 12.8–142.4).15
Contralateral fracture occurs from 1 month to 4 years after the index atypical femoral fracture.16
There are reports of bisphosphonate-related low-impact fractures in other sites such as the tibia17 and forearm.18 However, they may be too rare to warrant screening.
Mortality rates
A Swedish database study found that patients with atypical femoral fractures, whether bisphosphonate users or nonusers, do not have higher mortality rates than patients with ordinary subtrochanteric or femoral shaft fractures.19 Furthermore, the mortality rates for those with atypical femoral fracture were similar to rates in the general population. In contrast, patients with an ordinary femoral fracture had a higher mortality risk than the general population.19
Other studies suggest that atypical femoral fracture may be associated with a less favorable prognosis in older patients,20 but this could be due to differences in demographics, treatment adherence, or postfracture care.21
In addition, functional outcomes as measured by independent mobility at discharge and at 3 months were comparable between patients with atypical fracture and those with typical fracture.22
IMAGING STUDIES
If a long-term bisphosphonate user presents with hip, thigh, or groin pain, imaging studies are recommended.
Plain radiography
Radiography is usually the first step and should include a frontal view of the pelvis (Figure 1) and 2 views of the full length of each femur. If radiography is not conclusive, bone scan or magnetic resonance imaging (MRI) should be considered.
A linear cortex transverse fracture pattern and focal lateral cortical thickening are the most sensitive and specific radiographic features.23,24 Because of the risk of fracture on the contralateral side, radiographic study of that side is recommended as well.
Computed tomography
Computed tomography (CT) is not sensitive for early stress fractures and, given the radiation burden, is not recommended in the workup of atypical fracture.
Bone scanning
Bone scanning using technetium 99m-labeled methylene diphosphonate with a gamma camera shows active bone turnover. Stress fractures and atypical femoral fractures are most easily identified in the third (delayed) phase of the bone scan. Although bone scanning is highly sensitive, the specificity is limited by lack of spatial resolution. Atypical femoral fracture appears as increased activity in the subtrochanteric region with a predilection for the lateral cortex.
Dual-energy x-ray absorptiometry
Conventional dual-energy x-ray absorptiometry (DXA) extends only to 1 to 2 cm below the lesser trochanter and can therefore miss atypical fractures, which usually occur farther down. The overall detection rate for DXA was 61% in a sample of 33 patients.25
Newer scanners can look at the entire femoral shaft.26 In addition, newer software can quantify focal thickening (beaking) of the lateral cortex and screen patients who have no symptoms. The results of serial measurements can be graphed so that the practitioner can view trends to help assess or rule out potential asymptomatic atypical femoral fracture.
A localized reaction (periosteal thickening of the lateral cortex or beaking) often precedes atypical femoral fracture. A 2017 study reported that patients with high localized reaction (mean height 3.3 mm) that was of the pointed type and was accompanied by prodromal pain had an increased risk of complete or incomplete atypical femoral fracture at that site.27 This finding is used by the newer DXA software. The predictive value of beaking on extended femoral DXA may be as high as 83%.26
Magnetic resonance imaging
The MRI characteristics of atypical femoral fracture are similar to those of other stress fractures except that there is a lateral-to-medial pattern rather than a medial pattern. The earliest findings include periosteal reaction about the lateral cortex with a normal marrow signal.
MRI may be of particular benefit in patients with known atypical femoral fracture to screen the contralateral leg. It should image the entire length of both femurs. Contrast enhancement is not needed.
Regardless of whether initial findings were discovered on conventional radiographs or DXA, MRI confirmation is needed. Radionuclide bone scanning is currently not recommended because it lacks specificity. Combination imaging is recommended, with either radiography plus MRI or DXA plus MRI.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of atypical femoral fracture includes stress fracture, pathologic fracture, hypophosphatasia, and osteogenesis imperfecta.28 Hypophosphatemic osteomalacia can cause Looser zones, which can be confused with atypical femoral fractures but usually occur on the medial side.4 Stress fracture of the femur can occur below the lesser trochanter but usually begins in the medial, not the lateral, cortex.
Pathologic fractures from underlying osseous lesions can mimic the cortical beaking of bisphosphonate-related fracture, but they usually show the associated underlying lucent lesion and poorly defined margins. A sinus tract along the region of a chronic osteomyelitis may also appear similar.
Hypophosphatasia is an inborn error of metabolism caused by a loss-of-function mutation in the gene encoding alkaline phosphatase, resulting in pyrophosphate accumulation and causing osteomalacia from impaired mineralization. This can result in femoral pseudofracture that is often bilateral and occurs in the subtrochanteric region.29
ADDITIONAL RISK FACTORS
Patients with atypical femoral fracture are generally a heterogeneous group, but there are risk factors to note other than bisphosphonate exposure.
Asian women had a risk 8 times higher than white women in 1 study.30
Bone geometry. Mahjoub et al8 reported that compared with controls, patients with atypical femoral fracture had greater offset of the femoral shaft from the center of rotation of the femoral head, a more acute angle between the femoral neck and shaft, and greater proximal cortical thickness.
Medications. In addition to bisphosphonates, other drugs associated with atypical femoral fracture include RANK-ligand inhibitors such as denosumab (another drug for osteoporosis),31 glucocorticoids,32,33 and proton pump inhibitors.32,33
Genetics. Three sisters with atypical femoral fracture were found to have 37 rare mutations in 34 genes, including one in the GGPS1 gene, which codes for geranylgeranyl pyrophosphate synthase—an enzyme that bisphosphonates inhibit.34
Medical conditions other than osteoporosis include collagen diseases, chronic pulmonary disease, asthma, rheumatoid arthritis, and diabetes.35
Clinical recommendations
Current recommendations are to reevaluate bisphosphonate use in patients with osteoporosis after 5 or more years of therapy.36
Given that patients with osteoporosis are at increased risk of typical fracture, those at higher risk should be considered for continued bisphosphonate therapy. Factors for high risk include the following:
- History of fracture on therapy
- Hip T score –2.5 or lower
- Older age (≥ 70)
- Other strong risk factors for fracture such as smoking, alcohol use, corticosteroid use, rheumatoid arthritis, and family history
- World Health Organization FRAX fracture risk score above the country-specific threshold.
Those at lower risk should be considered for a 2- to 3-year bisphosphonate holiday with periodic reevaluation of bone density and, possibly, bone markers.36
WHAT IS THE UNDERLYING PATHOPHYSIOLOGY?
The mechanism by which bisphosphonates increase the risk of atypical femoral fracture is not clear. These drugs work by suppressing bone turnover; however, in theory, prolonged use could suppress it too much and increase bone fragility.
One hypothesis is that bisphosphonates impair the toughening of cortical bone, an important barrier to clinical fracture. This is supported by a study that found bisphosphonate users with atypical femoral fracture had deficits in intrinsic and extrinsic bone toughness, perhaps due to treatment-related increases in matrix mineralization.37 Although this study and others showed an increase in matrix mineralization and reduced mineralization heterogeneity with bisphosphonate use,38,39 it is unclear whether such changes contributed to reduced toughness or to atypical femoral fracture.
Changes in the skeletal geometry of the lower limb such as femoral neck-shaft angle and femoral curvature alter the stresses and strains experienced by the femoral diaphysis with loading. Because the incidence of incomplete atypical femoral fracture is much greater than that of complete fracture, most incomplete atypical femoral fractures heal before the fracture progresses.
Ultimately, all fractures, including atypical femoral fractures, occur when mechanical stress and strain exceed bone strength.
Antiresorptive drugs such as bisphosphonates, estrogen, calcitonin, and RANK ligand inhibitors prevent hip fracture by increasing the strength of the proximal femur—perhaps at the expense of the strength (or toughness) of the subtrochanteric shaft. It is also possible that treatment-related increases in hip strength (and reduced hip fracture rates) promote or sustain the transfer of stress and strain to femoral regions that experience lesser or no increases in strength from treatment, which likely includes the shaft.40,41
CT studies in Japanese women with osteoporosis have shown that 2 years of zoledronate therapy had greater effects in the hip than in the femoral shaft, with significant increases in cortical thickness and volumetric bone mineral density at the femoral neck and intertrochanteric region compared with baseline.42 But zoledronate did not increase femoral shaft cortical thickness and caused only a minor increase in femoral shaft volumetric bone mineral density. Fracture patterns may have depended on damage and effects of bone turnover on mass and structure.
This hypothetical scenario portrays a possible “hip survival bias” mechanism for atypical femoral fracture, with the association with antiresorptive drugs arising from greater stress and strain in cortical regions where these fractures occur rather than from treatment-related reductions in cortical bone strength or toughness.
PRODROMAL PAIN IS COMMON
From 32% to 76% of patients who have incomplete or developing atypical femoral fracture present with a prodrome of groin or hip pain.4,43 Prodromal pain occurs any time from 2 weeks to several years before the fracture, presenting as pain in the anterior or lateral thigh or in the groin.
Prodromal pain in a patient on antiresorptive therapy should be a signal for the clinician to obtain a radiograph of the hip and to look for contralateral symptoms and fractures. The most common mechanism of injury appears to be a ground-level fall or even a nontraumatic activity such as walking or stepping off a curb.
MEDICAL MANAGEMENT
In bisphosphonate users with radiographic evidence of atypical femoral fracture, the bisphosphonate should be discontinued and the patient assessed for calcium and vitamin D deficiency, with supplements prescribed if needed.4
For patients with incomplete fracture and persistent pain after 3 months of medical management, prophylactic surgical nail fixation is recommended to prevent complete fracture.
Teriparatide, which has been associated with enhanced bone fracture healing, is a possible treatment to promote healing of atypical femoral fracture, either alone or as an adjunct to surgical fixation. A systematic review published in 2015 supported the use of teriparatide for enhancing fracture healing in atypical femoral fracture.44 In addition, a 10-patient series45 showed that incomplete fractures without radiolucent lines responded to teriparatide alone, whereas those with radiolucent lines needed intramedullary nailing.
These results suggest that teriparatide works best when the fracture site is stable, either inherently or with surgical fixation.
ORTHOPEDIC CARE
Orthopedic care for atypical femoral fracture differs depending on whether the patient experiences pain and whether the fracture is incomplete or complete. Figure 2 shows a treatment algorithm for atypical femoral fracture.
These are difficult fractures to manage, complicated by delayed healing in the elderly, complex displacement patterns, altered bone geometry, and risk of fracture in the opposite limb, all of which raise questions about recommending protected weight-bearing exercise.
Furthermore, atypical femoral fracture is often associated with increased anterolateral bowing of the femur, making it difficult to insert an intramedullary nail: the radius of curvature of the bone is shorter than that of a standard femoral nail. This mismatch can lead to intraoperative complications such as iatrogenic fracture during prophylactic nailing, malunion from excess straightening of the femur (which can itself lead to leg length discrepancy), and gapping of the fracture site, particularly on the medial side.
Intramedullary nailing for complete fracture
Intramedullary nailing is the first-line treatment for complete atypical femoral fracture, although the risk of delayed healing and revision surgery may be somewhat higher than with typical femoral fracture.46 Prophylactic intramedullary nailing should be considered for a patient with intractable pain.2
A radiograph of the opposite leg should be obtained routinely, looking for an asymptomatic fracture. Bisphosphonates should be discontinued and calcium and vitamin D continued. Teriparatide therapy can be considered as an alternative treatment.
Conservative management for incomplete fracture without pain
Incomplete atypical femoral fracture unaccompanied by pain can be followed conservatively.47 In addition to stopping antiresorptive therapy, patients need to avoid high-impact and repetitive-impact activities such as jumping or running. If pain occurs, patients should begin protected weight-bearing exercise.
Treatment is uncertain for incomplete fracture with pain
For patients with incomplete atypical femoral fracture and pain, treatment is controversial. Regimens that include 2 to 3 months of protected weight-bearing exercise, a full metabolic bone workup, calcium and vitamin D supplementation, and anabolic bone agents have produced some success. Some authors have reported poor results from conservative care, with few patients achieving pain relief or signs of complete healing.48,49 Additionally, if an incomplete fracture is found in the opposite femur, protected weight-bearing of both legs may not be possible.
Patients with incomplete fracture should be monitored regularly with radiography and physical examination. If there is progression of the fracture, escalation of pain, or failure to heal within 2 to 3 months, then surgical treatment is necessary.
Prophylactic placement of an intramedullary nail to prevent completion of the fracture and allow a return to full weight-bearing is generally advised.50 A long locking plate can be used if bone deformities make it difficult to place an intramedullary nail; however, nails are preferred because they allow formation of endochondral callus, which can be helpful in these difficult-to-heal fractures.
Results from retrospective reviews have shown that surgically treated patients with bisphosphonate-associated incomplete atypical femoral fracture were more likely than those treated nonsurgically to be pain-free (81% vs 64%) and have radiographic healing (100% vs 18% at final follow-up).46 Results have also been positive for those with complete atypical femoral fracture. At 6 months, 64% of surgically treated patients were pain-free and 98% were radiographically healed.51
The unusual geometry of the femur in patients with atypical femoral fracture and the presence of intramedullary cortical callus makes the placement of an intramedullary femoral rod more complex than in typical femoral fracture.8
Intramedullary nailing of atypical femoral fracture is a challenge for even the most experienced surgeon, and vigilance is imperative to avoid iatrogenic fracture and malunion.
MANY QUESTIONS REMAIN
We need more studies on the pathophysiology of bisphosphonate-associated atypical femoral fracture, the value of periodic screening with DXA, and which factors predict high risk (eg, Asian ethnicity, use of certain medications, femoral geometry). In addition, we need more data on the success of conservative management of incomplete fracture, including use of teriparatide.
Bisphosphonate therapy minimizes bone loss and reduces fracture risk by up to 50% in patients with osteoporosis,1 but it is also associated with increased risks of osteonecrosis of the jaw and atypical femoral fracture. Although atypical femoral fractures are rare, they can have a devastating effect. Patient concern about this complication has contributed to a decrease in bisphosphonate use by about half in the last decade or so,2,3 and we fear this could result in an increase in hip fracture rates.
In this article, we examine the evidence on bisphosphonate-associated atypical femoral fractures, including risks, pathogenesis, treatment, and prevention.
ATYPICAL FRACTURES INVOLVE THE FEMORAL SHAFT, NOT THE HEAD
An atypical femoral fracture is a transverse fracture of the femoral shaft (diaphysis), defined by both clinical criteria and radiographic appearance.
To be defined as atypical, a femoral fracture must meet 4 of the following 5 criteria4:
- Occurs with minimal or no trauma
- Has a predominantly transverse fracture line, originating at the lateral cortex and sometimes becoming oblique as it progresses medially across the femur
- Extends through both cortices and may be associated with a medial spike (complete fractures); or involves only the lateral cortex (incomplete fractures)
- Is noncomminuted or minimally comminuted
- Shows localized periosteal or endosteal thickening (termed “beaking” or “flaring”) of the lateral cortex at the fracture site.
Several minor features are also important but are not required, eg:
- Cortical thickening of the femoral shaft
- Unilateral or bilateral prodromal pain preceding the fracture
- Bilateral incomplete or complete femoral diaphysis fractures
- Delayed fracture healing.
Atypical femoral fracture can occur anywhere along the shaft, from just distal to the lesser trochanter to just proximal to the supracondylar flare. However, most occur in 2 areas, with 1 cluster centered at about 41 mm from the lesser trochanter (more common in relatively younger patients) and the other at 187 mm.5
ABSOLUTE RISK IS LOW BUT INCREASES WITH LONGER USE
Atypical femoral fractures are rare. Schilcher et al6 reviewed radiographs of 1,234 women who had a subtrochanteric or shaft fracture and found 59 (4.6%) of fractures were atypical. In a systematic review of 14 studies,7 the incidence ranged from 3.0 to 9.8 cases per 100,000 patient-years.
Furthermore, not all atypical femoral fractures are in bisphosphonate users: 7.4% were in nonusers in 1 series8 and 22% in another.9
Nevertheless, most studies show that bisphosphonate use increases the incidence of atypical femoral fracture, and the incidence increases with duration of use, especially after 3 years.7
An international task force of the American Society for Bone and Mineral Research listed the absolute risk as between 3.2 and 50 cases per 100,000 patient-years, with longer use (> 5 years) increasing the risk to about 100 per 100,000 patient-years.4 After stopping bisphosphonate therapy, the risk diminished by 70% per year.9
In another study, for 0.1 to 1.9 years of therapy, the age-adjusted atypical fracture rates were 1.78 per 100,000 per year (95% confidence interval [CI] 1.5–2.0), increasing to 113.1 per 100,000 per year (95% CI 69.3–156.8) with exposure from 8 to 9.9 years.10
A case-control study found that more than 5 years of bisphosphonate use increased the fracture risk by an odds ratio of 2.74 (95% CI 1.25–6.02).11
The incidence of typical femoral fracture was higher in those who adhered better to their oral bisphosphonate regimen in some studies,12 but the opposite was true in others.13
The benefits of bisphosphonate therapy in reducing fracture risk, however, outweigh the risk of atypical fracture.4
We do not know whether the rate of atypical femoral fracture is increasing. A review of Kaiser Permanente Northwest records found that the rates of atypical femoral shaft fracture had remained stable from 1996 to 2009. However, 61.9% of patients who met the strict radiographic criteria had taken oral bisphosphonates.14 These data suggest that bisphosphonate use has not increased the overall population-based risk for subtrochanteric and femoral shaft fractures, but that bisphosphonates and other risk factors may have increased the likelihood that such fractures will exhibit atypical radiographic features.
A population-based study in Denmark13 found that alendronate use longer than 10 years was associated with an adjusted 30% lower risk of hip fracture and no increase in the risk of subtrochanteric and femoral shaft fracture. In addition, the risk of subtrochanteric and femoral shaft fracture was lower with high adherence to alendronate treatment (based on medication possession ratio > 80%) compared with low adherence (ratio < 50%) (odds ratio 0.88, 95% CI 0.77–0.99). The risk was not increased in current vs past users.
The Danish study13 used the coding of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) to identify subtrochanteric and femoral shaft fractures without radiologic review for atypical radiographic features. The lack of specific ICD-10 coding for subtrochanteric and femoral shaft fractures with atypical radiographic features has limited our knowledge of their incidence.
Contralateral fracture in more than one-fourth of cases
After an atypical femoral fracture, patients have a significant risk of fracture on the contralateral side. In a case-control study, 28% of patients with atypical femoral fracture suffered a contralateral fracture, compared with 0.9% of patients presenting with a typical fracture pattern (odds ratio 42.6, 95% CI 12.8–142.4).15
Contralateral fracture occurs from 1 month to 4 years after the index atypical femoral fracture.16
There are reports of bisphosphonate-related low-impact fractures in other sites such as the tibia17 and forearm.18 However, they may be too rare to warrant screening.
Mortality rates
A Swedish database study found that patients with atypical femoral fractures, whether bisphosphonate users or nonusers, do not have higher mortality rates than patients with ordinary subtrochanteric or femoral shaft fractures.19 Furthermore, the mortality rates for those with atypical femoral fracture were similar to rates in the general population. In contrast, patients with an ordinary femoral fracture had a higher mortality risk than the general population.19
Other studies suggest that atypical femoral fracture may be associated with a less favorable prognosis in older patients,20 but this could be due to differences in demographics, treatment adherence, or postfracture care.21
In addition, functional outcomes as measured by independent mobility at discharge and at 3 months were comparable between patients with atypical fracture and those with typical fracture.22
IMAGING STUDIES
If a long-term bisphosphonate user presents with hip, thigh, or groin pain, imaging studies are recommended.
Plain radiography
Radiography is usually the first step and should include a frontal view of the pelvis (Figure 1) and 2 views of the full length of each femur. If radiography is not conclusive, bone scan or magnetic resonance imaging (MRI) should be considered.
A linear cortex transverse fracture pattern and focal lateral cortical thickening are the most sensitive and specific radiographic features.23,24 Because of the risk of fracture on the contralateral side, radiographic study of that side is recommended as well.
Computed tomography
Computed tomography (CT) is not sensitive for early stress fractures and, given the radiation burden, is not recommended in the workup of atypical fracture.
Bone scanning
Bone scanning using technetium 99m-labeled methylene diphosphonate with a gamma camera shows active bone turnover. Stress fractures and atypical femoral fractures are most easily identified in the third (delayed) phase of the bone scan. Although bone scanning is highly sensitive, the specificity is limited by lack of spatial resolution. Atypical femoral fracture appears as increased activity in the subtrochanteric region with a predilection for the lateral cortex.
Dual-energy x-ray absorptiometry
Conventional dual-energy x-ray absorptiometry (DXA) extends only to 1 to 2 cm below the lesser trochanter and can therefore miss atypical fractures, which usually occur farther down. The overall detection rate for DXA was 61% in a sample of 33 patients.25
Newer scanners can look at the entire femoral shaft.26 In addition, newer software can quantify focal thickening (beaking) of the lateral cortex and screen patients who have no symptoms. The results of serial measurements can be graphed so that the practitioner can view trends to help assess or rule out potential asymptomatic atypical femoral fracture.
A localized reaction (periosteal thickening of the lateral cortex or beaking) often precedes atypical femoral fracture. A 2017 study reported that patients with high localized reaction (mean height 3.3 mm) that was of the pointed type and was accompanied by prodromal pain had an increased risk of complete or incomplete atypical femoral fracture at that site.27 This finding is used by the newer DXA software. The predictive value of beaking on extended femoral DXA may be as high as 83%.26
Magnetic resonance imaging
The MRI characteristics of atypical femoral fracture are similar to those of other stress fractures except that there is a lateral-to-medial pattern rather than a medial pattern. The earliest findings include periosteal reaction about the lateral cortex with a normal marrow signal.
MRI may be of particular benefit in patients with known atypical femoral fracture to screen the contralateral leg. It should image the entire length of both femurs. Contrast enhancement is not needed.
Regardless of whether initial findings were discovered on conventional radiographs or DXA, MRI confirmation is needed. Radionuclide bone scanning is currently not recommended because it lacks specificity. Combination imaging is recommended, with either radiography plus MRI or DXA plus MRI.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of atypical femoral fracture includes stress fracture, pathologic fracture, hypophosphatasia, and osteogenesis imperfecta.28 Hypophosphatemic osteomalacia can cause Looser zones, which can be confused with atypical femoral fractures but usually occur on the medial side.4 Stress fracture of the femur can occur below the lesser trochanter but usually begins in the medial, not the lateral, cortex.
Pathologic fractures from underlying osseous lesions can mimic the cortical beaking of bisphosphonate-related fracture, but they usually show the associated underlying lucent lesion and poorly defined margins. A sinus tract along the region of a chronic osteomyelitis may also appear similar.
Hypophosphatasia is an inborn error of metabolism caused by a loss-of-function mutation in the gene encoding alkaline phosphatase, resulting in pyrophosphate accumulation and causing osteomalacia from impaired mineralization. This can result in femoral pseudofracture that is often bilateral and occurs in the subtrochanteric region.29
ADDITIONAL RISK FACTORS
Patients with atypical femoral fracture are generally a heterogeneous group, but there are risk factors to note other than bisphosphonate exposure.
Asian women had a risk 8 times higher than white women in 1 study.30
Bone geometry. Mahjoub et al8 reported that compared with controls, patients with atypical femoral fracture had greater offset of the femoral shaft from the center of rotation of the femoral head, a more acute angle between the femoral neck and shaft, and greater proximal cortical thickness.
Medications. In addition to bisphosphonates, other drugs associated with atypical femoral fracture include RANK-ligand inhibitors such as denosumab (another drug for osteoporosis),31 glucocorticoids,32,33 and proton pump inhibitors.32,33
Genetics. Three sisters with atypical femoral fracture were found to have 37 rare mutations in 34 genes, including one in the GGPS1 gene, which codes for geranylgeranyl pyrophosphate synthase—an enzyme that bisphosphonates inhibit.34
Medical conditions other than osteoporosis include collagen diseases, chronic pulmonary disease, asthma, rheumatoid arthritis, and diabetes.35
Clinical recommendations
Current recommendations are to reevaluate bisphosphonate use in patients with osteoporosis after 5 or more years of therapy.36
Given that patients with osteoporosis are at increased risk of typical fracture, those at higher risk should be considered for continued bisphosphonate therapy. Factors for high risk include the following:
- History of fracture on therapy
- Hip T score –2.5 or lower
- Older age (≥ 70)
- Other strong risk factors for fracture such as smoking, alcohol use, corticosteroid use, rheumatoid arthritis, and family history
- World Health Organization FRAX fracture risk score above the country-specific threshold.
Those at lower risk should be considered for a 2- to 3-year bisphosphonate holiday with periodic reevaluation of bone density and, possibly, bone markers.36
WHAT IS THE UNDERLYING PATHOPHYSIOLOGY?
The mechanism by which bisphosphonates increase the risk of atypical femoral fracture is not clear. These drugs work by suppressing bone turnover; however, in theory, prolonged use could suppress it too much and increase bone fragility.
One hypothesis is that bisphosphonates impair the toughening of cortical bone, an important barrier to clinical fracture. This is supported by a study that found bisphosphonate users with atypical femoral fracture had deficits in intrinsic and extrinsic bone toughness, perhaps due to treatment-related increases in matrix mineralization.37 Although this study and others showed an increase in matrix mineralization and reduced mineralization heterogeneity with bisphosphonate use,38,39 it is unclear whether such changes contributed to reduced toughness or to atypical femoral fracture.
Changes in the skeletal geometry of the lower limb such as femoral neck-shaft angle and femoral curvature alter the stresses and strains experienced by the femoral diaphysis with loading. Because the incidence of incomplete atypical femoral fracture is much greater than that of complete fracture, most incomplete atypical femoral fractures heal before the fracture progresses.
Ultimately, all fractures, including atypical femoral fractures, occur when mechanical stress and strain exceed bone strength.
Antiresorptive drugs such as bisphosphonates, estrogen, calcitonin, and RANK ligand inhibitors prevent hip fracture by increasing the strength of the proximal femur—perhaps at the expense of the strength (or toughness) of the subtrochanteric shaft. It is also possible that treatment-related increases in hip strength (and reduced hip fracture rates) promote or sustain the transfer of stress and strain to femoral regions that experience lesser or no increases in strength from treatment, which likely includes the shaft.40,41
CT studies in Japanese women with osteoporosis have shown that 2 years of zoledronate therapy had greater effects in the hip than in the femoral shaft, with significant increases in cortical thickness and volumetric bone mineral density at the femoral neck and intertrochanteric region compared with baseline.42 But zoledronate did not increase femoral shaft cortical thickness and caused only a minor increase in femoral shaft volumetric bone mineral density. Fracture patterns may have depended on damage and effects of bone turnover on mass and structure.
This hypothetical scenario portrays a possible “hip survival bias” mechanism for atypical femoral fracture, with the association with antiresorptive drugs arising from greater stress and strain in cortical regions where these fractures occur rather than from treatment-related reductions in cortical bone strength or toughness.
PRODROMAL PAIN IS COMMON
From 32% to 76% of patients who have incomplete or developing atypical femoral fracture present with a prodrome of groin or hip pain.4,43 Prodromal pain occurs any time from 2 weeks to several years before the fracture, presenting as pain in the anterior or lateral thigh or in the groin.
Prodromal pain in a patient on antiresorptive therapy should be a signal for the clinician to obtain a radiograph of the hip and to look for contralateral symptoms and fractures. The most common mechanism of injury appears to be a ground-level fall or even a nontraumatic activity such as walking or stepping off a curb.
MEDICAL MANAGEMENT
In bisphosphonate users with radiographic evidence of atypical femoral fracture, the bisphosphonate should be discontinued and the patient assessed for calcium and vitamin D deficiency, with supplements prescribed if needed.4
For patients with incomplete fracture and persistent pain after 3 months of medical management, prophylactic surgical nail fixation is recommended to prevent complete fracture.
Teriparatide, which has been associated with enhanced bone fracture healing, is a possible treatment to promote healing of atypical femoral fracture, either alone or as an adjunct to surgical fixation. A systematic review published in 2015 supported the use of teriparatide for enhancing fracture healing in atypical femoral fracture.44 In addition, a 10-patient series45 showed that incomplete fractures without radiolucent lines responded to teriparatide alone, whereas those with radiolucent lines needed intramedullary nailing.
These results suggest that teriparatide works best when the fracture site is stable, either inherently or with surgical fixation.
ORTHOPEDIC CARE
Orthopedic care for atypical femoral fracture differs depending on whether the patient experiences pain and whether the fracture is incomplete or complete. Figure 2 shows a treatment algorithm for atypical femoral fracture.
These are difficult fractures to manage, complicated by delayed healing in the elderly, complex displacement patterns, altered bone geometry, and risk of fracture in the opposite limb, all of which raise questions about recommending protected weight-bearing exercise.
Furthermore, atypical femoral fracture is often associated with increased anterolateral bowing of the femur, making it difficult to insert an intramedullary nail: the radius of curvature of the bone is shorter than that of a standard femoral nail. This mismatch can lead to intraoperative complications such as iatrogenic fracture during prophylactic nailing, malunion from excess straightening of the femur (which can itself lead to leg length discrepancy), and gapping of the fracture site, particularly on the medial side.
Intramedullary nailing for complete fracture
Intramedullary nailing is the first-line treatment for complete atypical femoral fracture, although the risk of delayed healing and revision surgery may be somewhat higher than with typical femoral fracture.46 Prophylactic intramedullary nailing should be considered for a patient with intractable pain.2
A radiograph of the opposite leg should be obtained routinely, looking for an asymptomatic fracture. Bisphosphonates should be discontinued and calcium and vitamin D continued. Teriparatide therapy can be considered as an alternative treatment.
Conservative management for incomplete fracture without pain
Incomplete atypical femoral fracture unaccompanied by pain can be followed conservatively.47 In addition to stopping antiresorptive therapy, patients need to avoid high-impact and repetitive-impact activities such as jumping or running. If pain occurs, patients should begin protected weight-bearing exercise.
Treatment is uncertain for incomplete fracture with pain
For patients with incomplete atypical femoral fracture and pain, treatment is controversial. Regimens that include 2 to 3 months of protected weight-bearing exercise, a full metabolic bone workup, calcium and vitamin D supplementation, and anabolic bone agents have produced some success. Some authors have reported poor results from conservative care, with few patients achieving pain relief or signs of complete healing.48,49 Additionally, if an incomplete fracture is found in the opposite femur, protected weight-bearing of both legs may not be possible.
Patients with incomplete fracture should be monitored regularly with radiography and physical examination. If there is progression of the fracture, escalation of pain, or failure to heal within 2 to 3 months, then surgical treatment is necessary.
Prophylactic placement of an intramedullary nail to prevent completion of the fracture and allow a return to full weight-bearing is generally advised.50 A long locking plate can be used if bone deformities make it difficult to place an intramedullary nail; however, nails are preferred because they allow formation of endochondral callus, which can be helpful in these difficult-to-heal fractures.
Results from retrospective reviews have shown that surgically treated patients with bisphosphonate-associated incomplete atypical femoral fracture were more likely than those treated nonsurgically to be pain-free (81% vs 64%) and have radiographic healing (100% vs 18% at final follow-up).46 Results have also been positive for those with complete atypical femoral fracture. At 6 months, 64% of surgically treated patients were pain-free and 98% were radiographically healed.51
The unusual geometry of the femur in patients with atypical femoral fracture and the presence of intramedullary cortical callus makes the placement of an intramedullary femoral rod more complex than in typical femoral fracture.8
Intramedullary nailing of atypical femoral fracture is a challenge for even the most experienced surgeon, and vigilance is imperative to avoid iatrogenic fracture and malunion.
MANY QUESTIONS REMAIN
We need more studies on the pathophysiology of bisphosphonate-associated atypical femoral fracture, the value of periodic screening with DXA, and which factors predict high risk (eg, Asian ethnicity, use of certain medications, femoral geometry). In addition, we need more data on the success of conservative management of incomplete fracture, including use of teriparatide.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996–2012: an ecological analysis. J Bone Miner Res 2015; 30(12):2179–2187. doi:10.1002/jbmr.2565
- Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in US patients between 2002 and 2011. J Bone Miner Res 2014; 29(9):1929–1937. doi:10.1002/jbmr.2202
- Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014; 29(1):1–23. doi:10.1002/jbmr.1998
- Koeppen VA, Schilcher J, Aspenberg P. Dichotomous location of 160 atypical femoral fractures. Acta Orthop 2013; 84(6):561–564. doi:10.3109/17453674.2013.866193
- Schilcher J, Koeppen V, Aspenberg P, Michäelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. Acta Orthop 2015; 86(1):100–107. doi:10.3109/17453674.2015.1004149
- Khow KS, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Epidemiology and postoperative outcomes of atypical femoral fractures in older adults: a systematic review. J Nutr Health Aging 2017; 21(1):83–91. doi:10.1007/s12603-015-0652-3
- Mahjoub Z, Jean S, Leclerc JT, et al. Incidence and characteristics of atypical femoral fractures: clinical and geometrical data. J Bone Miner Res 2016; 31(4):767–776. doi:10.1002/jbmr.2748
- Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011; 364(18):1728–1737. doi:10.1056/NEJMoa1010650
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res 2012; 27(12):2544–2550. doi:10.1002/jbmr.1719
- Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011; 305(8):783–789. doi:10.1001/jama.2011.190
- Wang Z, Ward MM, Chan L, Bhattacharyya T. Adherence to oral bisphosphonates and the risk of subtrochanteric and femoral shaft fractures among female Medicare beneficiaries. Osteoporos Int 2014; 25(8):2109–2116. doi:10.1007/s00198-014-2738-x
- Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. BMJ 2016; 353:i3365. doi:10.1136/bmj.i3365
- Feldstein AC, Black D, Perrin N, et al. Incidence and demography of femur fractures with and without atypical features. J Bone Miner Res 2012; 27(5):977–986. doi:10.1002/jbmr.1550
- Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med 2012; 172(12):930–936. doi:10.1001/archinternmed.2012.1796
- La Rocca Vieira R, Rosenberg ZS, Allison MB, Im SA, Babb J, Peck V. Frequency of incomplete atypical femoral fractures in asymptomatic patients on long term bisphosphonate therapy. AJR Am J Roentgenol 2012; 198(5):1144–1151. doi:10.2214/AJR.11.7442
- Bissonnette L, April PM, Dumais R, Boire G, Roux S. Atypical fracture of the tibial diaphysis associated with bisphosphonate therapy: a case report. Bone 2013; 56(2):406–409. doi:10.1016/j.bone.2013.07.012
- Moon J, Bither N, Lee T. Atypical forearm fractures associated with long-term use of bisphosphonate. Arch Orthop Trauma Surg 2013; 133(7):889–892. doi:10.1007/s00402-013-1760-3
- Kharazmi M, Hallberg P, Schilcher J, Aspenberg P, Michaëlsson K. Mortality after atypical femoral fractures: a cohort study. J Bone Miner Res 2016; 31(3):491–497. doi:10.1002/jbmr.2767
- Medin E, Goude F, Melberg HO, Tediosi F, Belicza E, Peltola M; EuroHOPE Study Group. European regional differences in all-cause mortality and length of stay for patients with hip fracture. Health Econ 2015; 24(suppl 2):53–64. doi:10.1002/hec.3278
- Abrahamsen B, Prieto-Alhambra D. Patients with atypical femur fractures have the same mortality as the background population-drug channeling bias, bisphosphonate effects and public health implications. J Bone Miner Res 2016; 31(3):488–490. doi:10.1002/jbmr.2801
- Khow KS, Paterson F, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Outcomes between older adults with atypical and typical femoral fractures are comparable. Injury 2017; 48(2):394–398. doi:10.1016/j.injury.2016.10.035
- Adams AL, Xue F, Chantra JQ, et al. Sensitivity and specificity of radiographic characteristics in atypical femoral fractures. Osteoporos Int 2017; 28(1):413–417. doi:10.1007/s00198-016-3809-y
- Rosenberg ZS, La Rocca Vieira R, Chan SS, et al. Bisphosphonate-related complete atypical subtrochanteric femoral fractures: diagnostic utility of radiography. AJR Am J Roentgenol 2011; 197(4):954–960. doi:10.2214/AJR.10.6262
- Kim S, Yang KH, Lim H, et al. Detection of prefracture hip lesions in atypical subtrochanteric fracture with dual-energy x-ray absorptiometry images. Radiology 2014; 270(2):487–495. doi:10.1148/radiol.13122691
- van de Laarschot DM, Smits AA, Buitendijk SK, Stegenga MT, Zillikens MC. Screening for atypical femur fractures using extended femur scans by DXA. J Bone Miner Res 2017; 32(8):1632–1639. doi:10.1002/jbmr.3164
- Sato H, Kondo N, Nakatsue T, et al. High and pointed type of femoral localized reaction frequently extends to complete an incomplete atypical femoral fracture in patients with autoimmune diseases on long-term glucocorticoids and bisphosphonates. Osteoporos Int 2017; 28(8):2367–2376. doi:10.1007/s00198-017-4038-8
- Giaconi JC, Watterson CT. Bisphosphonate-related atypical femur fractures and the radiographic features. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:107–124. doi:10.1007/978-3-319-23639-1
- Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 2009; 24(6):1132–1134. doi:10.1359/jbmr.081253
- Lo JC, Hui RL, Grimsrud CD, et al. The association of race/ethnicity and risk of atypical femoral fracture among older women receiving oral bisphosphonate therapy. Bone 2016; 85:142–147. doi:10.1016/j.bone.2016.01.002
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017; 5(7):513–523. doi:10.1016/S2213-8587(17)30138-9
- Koh JH, Myong JP, Yoo J, et al. Predisposing factors associated with atypical femur fracture among postmenopausal Korean women receiving bisphosphonate therapy: 8 years' experience in a single center. Osteoporos Int 2017; 28(11):3251–3259. doi:10.1007/s00198-017-4169-y
- Kim D, Sung YK, Cho SK, Han M, Kim YS. Factors associated with atypical femoral fracture. Rheumatol Int 2016; 36(1):65–71. doi:10.1007/s00296-015-3323-0
- Roca-Ayats N, Balcells S, Garcia-Giralt N, et al. GGPS1 mutation and atypical femoral fractures with bisphosphonates. N Engl J Med 2017; 376(18):1794–1795. doi:10.1056/NEJMc1612804
- Giusti A, Hamdy NA, Dekkers OM, Ramautar SR, Dijkstra S, Papapoulos SE. Atypical fractures and bisphosphonate therapy: a cohort study of patients with femoral fracture with radiographic adjudication of fracture site and features. Bone 2011; 48(5):966–971. doi:10.1016/j.bone.2010.12.033
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31(1):16–35. doi:10.1002/jbmr.2708
- Lloyd AA, Gludovatz B, Riedel C, et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc Natl Acad Sci USA 2017; 114(33):8722–8727. doi:10.1073/pnas.1704460114
- Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures; lessons from materials research. Bone 2013; 55(2):495–500. doi:10.1016/j.bone.2013.02.004
- Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone 2015; 71:58–62. doi:10.1016/j.bone.2014.10.010
- Sasaki S, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low-energy diaphyseal femoral fractures associated with bisphosphonate use and severe curved femur: a case series. J Bone Miner Metab 2012; 30(5):561–567. doi:10.1007/s00774-012-0358-0
- Pulkkinen P, Gluer C, Jamsa T. Investigation of differences between hip fracture types: a worthy strategy of improved risk assessment and fracture prevention. Bone 2011; 49(4):600–604. doi:10.1016/j.bone.2011.07.022
- Ito M, Sone T, Shiraki M, et al. The effect of once-yearly zoledronic acid on hip structural and biomechanical properties derived using computed tomography (CT) in Japanese women with osteoporosis. Bone 2018; 106:179–186. doi:10.1016/j.bone.2017.10.013
- Bogdan Y, Einhorn TA. Clinical presentation of atypical femur fractures. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:137–140. doi:10.1007/978-3-319-23639-1
- Im GI, Lee SH. Effect of teriparatide on healing of atypical femoral fractures: a systemic review. J Bone Metab 2015; 22(4):183–189. doi:10.11005/jbm.2015.22.4.183
- Saleh A, Hegde VV, Potty AG, Schneider R, Cornell CN, Lane JM. Management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. HSS J 2012; 8(2):103–110. doi:10.1007/s11420-012-9275-y
- Egol KA, Park JH, Prensky C, Rosenberg ZS, Peck V, Tejwani NC. Surgical treatment improves clinical and functional outcomes for patients who sustain incomplete bisphosphonate-related femur fractures. J Orthop Trauma 2013; 27(6):331–335. doi:10.1097/BOT.0b013e31827240ae
- Koh A, Guerado E, Giannoudis PV. Atypical femoral fractures related to bisphosphonate treatment: issues and controversies related to their surgical management. Bone Joint J 2017; 99-B(3):295–302. doi:10.1302/0301-620X.99B3.BJJ-2016-0276.R2
- Oh CW, Oh JK, Park KC, Kim JW, Yoon YC. Prophylactic nailing of incomplete atypical femoral fractures. ScientificWorldJournal 2013; 2013:450148. doi:10.1155/2013/450148
- Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin Orthop Relat Res 2010; 468(12):3393–3398. doi:10.1007/s11999-010-1583-2
- Tosounidis TH, Lampropoulou-Adamidou, Kanakaris NK. Intramedullary nailing of sequential bilateral atypical subtrochanteric fractures and the management of distal femoral intraoperative fracture. J Orthop Trauma 2015 Jun 11. Epub ahead of print. doi:10.1097/BOT.0000000000000370
- Egol KA, Park JH, Rosenberg ZS, Peck V, Tejwani NC. Healing delayed but generally reliable after bisphosphonate-associated complete femur fractures treated with IM nails. Clin Orthop Relat Res 2014; 472(9):2728–2734. doi:10.1007/s11999-013-2963-1
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996–2012: an ecological analysis. J Bone Miner Res 2015; 30(12):2179–2187. doi:10.1002/jbmr.2565
- Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in US patients between 2002 and 2011. J Bone Miner Res 2014; 29(9):1929–1937. doi:10.1002/jbmr.2202
- Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014; 29(1):1–23. doi:10.1002/jbmr.1998
- Koeppen VA, Schilcher J, Aspenberg P. Dichotomous location of 160 atypical femoral fractures. Acta Orthop 2013; 84(6):561–564. doi:10.3109/17453674.2013.866193
- Schilcher J, Koeppen V, Aspenberg P, Michäelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. Acta Orthop 2015; 86(1):100–107. doi:10.3109/17453674.2015.1004149
- Khow KS, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Epidemiology and postoperative outcomes of atypical femoral fractures in older adults: a systematic review. J Nutr Health Aging 2017; 21(1):83–91. doi:10.1007/s12603-015-0652-3
- Mahjoub Z, Jean S, Leclerc JT, et al. Incidence and characteristics of atypical femoral fractures: clinical and geometrical data. J Bone Miner Res 2016; 31(4):767–776. doi:10.1002/jbmr.2748
- Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011; 364(18):1728–1737. doi:10.1056/NEJMoa1010650
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res 2012; 27(12):2544–2550. doi:10.1002/jbmr.1719
- Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011; 305(8):783–789. doi:10.1001/jama.2011.190
- Wang Z, Ward MM, Chan L, Bhattacharyya T. Adherence to oral bisphosphonates and the risk of subtrochanteric and femoral shaft fractures among female Medicare beneficiaries. Osteoporos Int 2014; 25(8):2109–2116. doi:10.1007/s00198-014-2738-x
- Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. BMJ 2016; 353:i3365. doi:10.1136/bmj.i3365
- Feldstein AC, Black D, Perrin N, et al. Incidence and demography of femur fractures with and without atypical features. J Bone Miner Res 2012; 27(5):977–986. doi:10.1002/jbmr.1550
- Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med 2012; 172(12):930–936. doi:10.1001/archinternmed.2012.1796
- La Rocca Vieira R, Rosenberg ZS, Allison MB, Im SA, Babb J, Peck V. Frequency of incomplete atypical femoral fractures in asymptomatic patients on long term bisphosphonate therapy. AJR Am J Roentgenol 2012; 198(5):1144–1151. doi:10.2214/AJR.11.7442
- Bissonnette L, April PM, Dumais R, Boire G, Roux S. Atypical fracture of the tibial diaphysis associated with bisphosphonate therapy: a case report. Bone 2013; 56(2):406–409. doi:10.1016/j.bone.2013.07.012
- Moon J, Bither N, Lee T. Atypical forearm fractures associated with long-term use of bisphosphonate. Arch Orthop Trauma Surg 2013; 133(7):889–892. doi:10.1007/s00402-013-1760-3
- Kharazmi M, Hallberg P, Schilcher J, Aspenberg P, Michaëlsson K. Mortality after atypical femoral fractures: a cohort study. J Bone Miner Res 2016; 31(3):491–497. doi:10.1002/jbmr.2767
- Medin E, Goude F, Melberg HO, Tediosi F, Belicza E, Peltola M; EuroHOPE Study Group. European regional differences in all-cause mortality and length of stay for patients with hip fracture. Health Econ 2015; 24(suppl 2):53–64. doi:10.1002/hec.3278
- Abrahamsen B, Prieto-Alhambra D. Patients with atypical femur fractures have the same mortality as the background population-drug channeling bias, bisphosphonate effects and public health implications. J Bone Miner Res 2016; 31(3):488–490. doi:10.1002/jbmr.2801
- Khow KS, Paterson F, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Outcomes between older adults with atypical and typical femoral fractures are comparable. Injury 2017; 48(2):394–398. doi:10.1016/j.injury.2016.10.035
- Adams AL, Xue F, Chantra JQ, et al. Sensitivity and specificity of radiographic characteristics in atypical femoral fractures. Osteoporos Int 2017; 28(1):413–417. doi:10.1007/s00198-016-3809-y
- Rosenberg ZS, La Rocca Vieira R, Chan SS, et al. Bisphosphonate-related complete atypical subtrochanteric femoral fractures: diagnostic utility of radiography. AJR Am J Roentgenol 2011; 197(4):954–960. doi:10.2214/AJR.10.6262
- Kim S, Yang KH, Lim H, et al. Detection of prefracture hip lesions in atypical subtrochanteric fracture with dual-energy x-ray absorptiometry images. Radiology 2014; 270(2):487–495. doi:10.1148/radiol.13122691
- van de Laarschot DM, Smits AA, Buitendijk SK, Stegenga MT, Zillikens MC. Screening for atypical femur fractures using extended femur scans by DXA. J Bone Miner Res 2017; 32(8):1632–1639. doi:10.1002/jbmr.3164
- Sato H, Kondo N, Nakatsue T, et al. High and pointed type of femoral localized reaction frequently extends to complete an incomplete atypical femoral fracture in patients with autoimmune diseases on long-term glucocorticoids and bisphosphonates. Osteoporos Int 2017; 28(8):2367–2376. doi:10.1007/s00198-017-4038-8
- Giaconi JC, Watterson CT. Bisphosphonate-related atypical femur fractures and the radiographic features. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:107–124. doi:10.1007/978-3-319-23639-1
- Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 2009; 24(6):1132–1134. doi:10.1359/jbmr.081253
- Lo JC, Hui RL, Grimsrud CD, et al. The association of race/ethnicity and risk of atypical femoral fracture among older women receiving oral bisphosphonate therapy. Bone 2016; 85:142–147. doi:10.1016/j.bone.2016.01.002
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017; 5(7):513–523. doi:10.1016/S2213-8587(17)30138-9
- Koh JH, Myong JP, Yoo J, et al. Predisposing factors associated with atypical femur fracture among postmenopausal Korean women receiving bisphosphonate therapy: 8 years' experience in a single center. Osteoporos Int 2017; 28(11):3251–3259. doi:10.1007/s00198-017-4169-y
- Kim D, Sung YK, Cho SK, Han M, Kim YS. Factors associated with atypical femoral fracture. Rheumatol Int 2016; 36(1):65–71. doi:10.1007/s00296-015-3323-0
- Roca-Ayats N, Balcells S, Garcia-Giralt N, et al. GGPS1 mutation and atypical femoral fractures with bisphosphonates. N Engl J Med 2017; 376(18):1794–1795. doi:10.1056/NEJMc1612804
- Giusti A, Hamdy NA, Dekkers OM, Ramautar SR, Dijkstra S, Papapoulos SE. Atypical fractures and bisphosphonate therapy: a cohort study of patients with femoral fracture with radiographic adjudication of fracture site and features. Bone 2011; 48(5):966–971. doi:10.1016/j.bone.2010.12.033
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31(1):16–35. doi:10.1002/jbmr.2708
- Lloyd AA, Gludovatz B, Riedel C, et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc Natl Acad Sci USA 2017; 114(33):8722–8727. doi:10.1073/pnas.1704460114
- Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures; lessons from materials research. Bone 2013; 55(2):495–500. doi:10.1016/j.bone.2013.02.004
- Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone 2015; 71:58–62. doi:10.1016/j.bone.2014.10.010
- Sasaki S, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low-energy diaphyseal femoral fractures associated with bisphosphonate use and severe curved femur: a case series. J Bone Miner Metab 2012; 30(5):561–567. doi:10.1007/s00774-012-0358-0
- Pulkkinen P, Gluer C, Jamsa T. Investigation of differences between hip fracture types: a worthy strategy of improved risk assessment and fracture prevention. Bone 2011; 49(4):600–604. doi:10.1016/j.bone.2011.07.022
- Ito M, Sone T, Shiraki M, et al. The effect of once-yearly zoledronic acid on hip structural and biomechanical properties derived using computed tomography (CT) in Japanese women with osteoporosis. Bone 2018; 106:179–186. doi:10.1016/j.bone.2017.10.013
- Bogdan Y, Einhorn TA. Clinical presentation of atypical femur fractures. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:137–140. doi:10.1007/978-3-319-23639-1
- Im GI, Lee SH. Effect of teriparatide on healing of atypical femoral fractures: a systemic review. J Bone Metab 2015; 22(4):183–189. doi:10.11005/jbm.2015.22.4.183
- Saleh A, Hegde VV, Potty AG, Schneider R, Cornell CN, Lane JM. Management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. HSS J 2012; 8(2):103–110. doi:10.1007/s11420-012-9275-y
- Egol KA, Park JH, Prensky C, Rosenberg ZS, Peck V, Tejwani NC. Surgical treatment improves clinical and functional outcomes for patients who sustain incomplete bisphosphonate-related femur fractures. J Orthop Trauma 2013; 27(6):331–335. doi:10.1097/BOT.0b013e31827240ae
- Koh A, Guerado E, Giannoudis PV. Atypical femoral fractures related to bisphosphonate treatment: issues and controversies related to their surgical management. Bone Joint J 2017; 99-B(3):295–302. doi:10.1302/0301-620X.99B3.BJJ-2016-0276.R2
- Oh CW, Oh JK, Park KC, Kim JW, Yoon YC. Prophylactic nailing of incomplete atypical femoral fractures. ScientificWorldJournal 2013; 2013:450148. doi:10.1155/2013/450148
- Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin Orthop Relat Res 2010; 468(12):3393–3398. doi:10.1007/s11999-010-1583-2
- Tosounidis TH, Lampropoulou-Adamidou, Kanakaris NK. Intramedullary nailing of sequential bilateral atypical subtrochanteric fractures and the management of distal femoral intraoperative fracture. J Orthop Trauma 2015 Jun 11. Epub ahead of print. doi:10.1097/BOT.0000000000000370
- Egol KA, Park JH, Rosenberg ZS, Peck V, Tejwani NC. Healing delayed but generally reliable after bisphosphonate-associated complete femur fractures treated with IM nails. Clin Orthop Relat Res 2014; 472(9):2728–2734. doi:10.1007/s11999-013-2963-1
KEY POINTS
- The benefits of bisphosphonate therapy in reducing fracture risk outweigh the risk of atypical fracture.
- Bisphosphonate use for longer than 5 years greatly increases the risk of atypical femoral fracture.
- Treatment of atypical femoral fracture varies depending on whether the patient has pain and whether the fracture is complete or incomplete.
Pulmonary infarction due to pulmonary embolism
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
A closer look at an ezetimibe discussion
Although I look forward to receiving JFP each month, I was initially disappointed in Dr. Jonathon M. Firnhaber’s article, “Newer cholesterol-lowering agents: What you must know” (J Fam Pract. 2018;67:339-341,344,345), because of what appeared to be a superficial discussion of the medication ezetimibe. The potential role of PCSK9 inhibitors in extremely high-risk individuals was well discussed, but my first read left me with the impression that ezetimibe should be used more widely.
It seemed that in the section for ezetimibe, the author was suggesting using it for primary prevention. The line, “Consider adding ezetimibe to maximally tolerated statin therapy for patients not meeting LDL-C goals with a statin alone” left me a bit confused, as the most widely used guideline (that by the American College of Cardiology/American Heart Association Task Force on Practice Guidelines) states that there is no goal low-density lipoprotein cholesterol (LDL-C) level for primary prevention in patients without known cardiovascular disease (CVD) because studies have not been done to support this concept.1
But upon rereading the article, I realized the statement was placed at the end of a section that discussed secondary prevention based on the IMPROVE-IT study.2 This trial included only patients with previous acute coronary syndrome, one of the populations at highest risk.
I write just to reinforce the importance of considering what evidence we have for primary prevention. Although there is a value to rechecking LDL-C levels to assess compliance, there really is no convincing evidence that we should treat to a goal LDL-C level in someone who does not already have CVD. So the addition of ezetimibe to a statin in these patients is not recommended. Thus, the often-quoted strategy: “Start them on the right statin, and don’t look back.”
Bill Crump, MD
Madisonville, Ky
1. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1-S45.
2. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
Continue to: Authors' response
Author’s response:
Thank you, Dr. Crump, for your feedback. I suspect that most clinicians would welcome more robust outcomes data on ezetimibe, but to date none have been published.
The IMPROVE-IT trial1 offers the best supportive evidence for the use of ezetimibe, but still finds only a 2% absolute risk reduction (ARR) in a composite endpoint (cardiovascular death, nonfatal myocardial infarction, unstable angina requiring rehospitalization, coronary revascularization ≥30 days after randomization, or nonfatal stroke), equating to a number needed to treat (NNT) of 50.
The largest meta-analysis of ezetimibe trials—published prior to IMPROVE-IT—combined 31,048 patients to find an ARR for myocardial infarction of 1.1% (NNT=91) and an ARR for stroke of 0.6% (NNT=167), with no difference in cardiovascular death.2
Because of its limited outcomes data, ezetimibe is best reserved for patients unable to tolerate statin therapy, for those in whom statin therapy is contraindicated, or for those not meeting LDL-C reduction goals with a statin alone. This position is also supported by the United Kingdom’s National Institute for Health and Care Excellence (NICE).3
Finally, you are correct that the 2013 American College of Cardiology/American Heart Association Guideline on the Assessment of Cardiovascular Risk does not advocate a number-driven LDL-C goal, but rather recommends a risk-based moderate (30%-50%) or high-intensity (>50%) LDL-C reduction goal.4
Jonathon Firnhaber, MD
Greenville, NC
1. Cannon C, Blazing M, Giugliano R, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
2. Savarese G, Ferrari G, Rosano G, et al. Safety and efficacy of ezetimibe: a meta-analysis. Int J Cardiol. 2015;201:247-252.
3. National Institute for Health and Care Excellence. Ezetimibe for treating primary heterozygous-familial and non-familial hypercholesterolaemia. Technology appraisal guidance [TA385]. February 24, 2016. www.nice.org.uk/guidance/ta385. Accessed September 12, 2018.
4. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935-2959.
Although I look forward to receiving JFP each month, I was initially disappointed in Dr. Jonathon M. Firnhaber’s article, “Newer cholesterol-lowering agents: What you must know” (J Fam Pract. 2018;67:339-341,344,345), because of what appeared to be a superficial discussion of the medication ezetimibe. The potential role of PCSK9 inhibitors in extremely high-risk individuals was well discussed, but my first read left me with the impression that ezetimibe should be used more widely.
It seemed that in the section for ezetimibe, the author was suggesting using it for primary prevention. The line, “Consider adding ezetimibe to maximally tolerated statin therapy for patients not meeting LDL-C goals with a statin alone” left me a bit confused, as the most widely used guideline (that by the American College of Cardiology/American Heart Association Task Force on Practice Guidelines) states that there is no goal low-density lipoprotein cholesterol (LDL-C) level for primary prevention in patients without known cardiovascular disease (CVD) because studies have not been done to support this concept.1
But upon rereading the article, I realized the statement was placed at the end of a section that discussed secondary prevention based on the IMPROVE-IT study.2 This trial included only patients with previous acute coronary syndrome, one of the populations at highest risk.
I write just to reinforce the importance of considering what evidence we have for primary prevention. Although there is a value to rechecking LDL-C levels to assess compliance, there really is no convincing evidence that we should treat to a goal LDL-C level in someone who does not already have CVD. So the addition of ezetimibe to a statin in these patients is not recommended. Thus, the often-quoted strategy: “Start them on the right statin, and don’t look back.”
Bill Crump, MD
Madisonville, Ky
1. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1-S45.
2. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
Continue to: Authors' response
Author’s response:
Thank you, Dr. Crump, for your feedback. I suspect that most clinicians would welcome more robust outcomes data on ezetimibe, but to date none have been published.
The IMPROVE-IT trial1 offers the best supportive evidence for the use of ezetimibe, but still finds only a 2% absolute risk reduction (ARR) in a composite endpoint (cardiovascular death, nonfatal myocardial infarction, unstable angina requiring rehospitalization, coronary revascularization ≥30 days after randomization, or nonfatal stroke), equating to a number needed to treat (NNT) of 50.
The largest meta-analysis of ezetimibe trials—published prior to IMPROVE-IT—combined 31,048 patients to find an ARR for myocardial infarction of 1.1% (NNT=91) and an ARR for stroke of 0.6% (NNT=167), with no difference in cardiovascular death.2
Because of its limited outcomes data, ezetimibe is best reserved for patients unable to tolerate statin therapy, for those in whom statin therapy is contraindicated, or for those not meeting LDL-C reduction goals with a statin alone. This position is also supported by the United Kingdom’s National Institute for Health and Care Excellence (NICE).3
Finally, you are correct that the 2013 American College of Cardiology/American Heart Association Guideline on the Assessment of Cardiovascular Risk does not advocate a number-driven LDL-C goal, but rather recommends a risk-based moderate (30%-50%) or high-intensity (>50%) LDL-C reduction goal.4
Jonathon Firnhaber, MD
Greenville, NC
1. Cannon C, Blazing M, Giugliano R, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
2. Savarese G, Ferrari G, Rosano G, et al. Safety and efficacy of ezetimibe: a meta-analysis. Int J Cardiol. 2015;201:247-252.
3. National Institute for Health and Care Excellence. Ezetimibe for treating primary heterozygous-familial and non-familial hypercholesterolaemia. Technology appraisal guidance [TA385]. February 24, 2016. www.nice.org.uk/guidance/ta385. Accessed September 12, 2018.
4. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935-2959.
Although I look forward to receiving JFP each month, I was initially disappointed in Dr. Jonathon M. Firnhaber’s article, “Newer cholesterol-lowering agents: What you must know” (J Fam Pract. 2018;67:339-341,344,345), because of what appeared to be a superficial discussion of the medication ezetimibe. The potential role of PCSK9 inhibitors in extremely high-risk individuals was well discussed, but my first read left me with the impression that ezetimibe should be used more widely.
It seemed that in the section for ezetimibe, the author was suggesting using it for primary prevention. The line, “Consider adding ezetimibe to maximally tolerated statin therapy for patients not meeting LDL-C goals with a statin alone” left me a bit confused, as the most widely used guideline (that by the American College of Cardiology/American Heart Association Task Force on Practice Guidelines) states that there is no goal low-density lipoprotein cholesterol (LDL-C) level for primary prevention in patients without known cardiovascular disease (CVD) because studies have not been done to support this concept.1
But upon rereading the article, I realized the statement was placed at the end of a section that discussed secondary prevention based on the IMPROVE-IT study.2 This trial included only patients with previous acute coronary syndrome, one of the populations at highest risk.
I write just to reinforce the importance of considering what evidence we have for primary prevention. Although there is a value to rechecking LDL-C levels to assess compliance, there really is no convincing evidence that we should treat to a goal LDL-C level in someone who does not already have CVD. So the addition of ezetimibe to a statin in these patients is not recommended. Thus, the often-quoted strategy: “Start them on the right statin, and don’t look back.”
Bill Crump, MD
Madisonville, Ky
1. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1-S45.
2. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
Continue to: Authors' response
Author’s response:
Thank you, Dr. Crump, for your feedback. I suspect that most clinicians would welcome more robust outcomes data on ezetimibe, but to date none have been published.
The IMPROVE-IT trial1 offers the best supportive evidence for the use of ezetimibe, but still finds only a 2% absolute risk reduction (ARR) in a composite endpoint (cardiovascular death, nonfatal myocardial infarction, unstable angina requiring rehospitalization, coronary revascularization ≥30 days after randomization, or nonfatal stroke), equating to a number needed to treat (NNT) of 50.
The largest meta-analysis of ezetimibe trials—published prior to IMPROVE-IT—combined 31,048 patients to find an ARR for myocardial infarction of 1.1% (NNT=91) and an ARR for stroke of 0.6% (NNT=167), with no difference in cardiovascular death.2
Because of its limited outcomes data, ezetimibe is best reserved for patients unable to tolerate statin therapy, for those in whom statin therapy is contraindicated, or for those not meeting LDL-C reduction goals with a statin alone. This position is also supported by the United Kingdom’s National Institute for Health and Care Excellence (NICE).3
Finally, you are correct that the 2013 American College of Cardiology/American Heart Association Guideline on the Assessment of Cardiovascular Risk does not advocate a number-driven LDL-C goal, but rather recommends a risk-based moderate (30%-50%) or high-intensity (>50%) LDL-C reduction goal.4
Jonathon Firnhaber, MD
Greenville, NC
1. Cannon C, Blazing M, Giugliano R, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
2. Savarese G, Ferrari G, Rosano G, et al. Safety and efficacy of ezetimibe: a meta-analysis. Int J Cardiol. 2015;201:247-252.
3. National Institute for Health and Care Excellence. Ezetimibe for treating primary heterozygous-familial and non-familial hypercholesterolaemia. Technology appraisal guidance [TA385]. February 24, 2016. www.nice.org.uk/guidance/ta385. Accessed September 12, 2018.
4. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935-2959.
Frailty tied to higher mortality after major vascular surgery
Frailty defined as functional dependence is a predictor of mortality risk in elderly patients having major vascular surgery, a meta-analysis of studies has found
“Functional dependency may be recommended for use in rapid screening for frailty in major vascular surgery because of the high quality of associated evidence. Additionally, information on central muscle mass also adds incremental predictive value to long-term survival of elderly patients after major vascular surgery,” the study investigaters stated. However, they pointed out that “other newly developed frailty tools require further validation in more studies” before they should be adopted.
The report, published in the European Journal of Vascular and Endovascular Surgery, evaluated the effect of frailty in major vascular surgery from a search of MEDLINE, Embase, Cochrane Database, and Scopus through May 2018. Data were extracted from the articles related to surgery for abdominal aortic aneurysms (AAA) and lower extremity artery disease (LEAD), and a modified Newcastle-Ottawa scale was used to assess the quality of the included studies, according to Jiarong Wang, MD, of the department of vascular surgery, Sichuan University, Sichuan Province, China, and colleagues. A total of 22 cohort studies and one randomized controlled trial was used in the final analysis. The reviewers expressed the impact of frailty on outcomes as odds ratios (OR) or hazard ratios (HR) using a random effects model.
The researchers found that frailty, in terms of functional dependence, was associated with a significantly increased 30-day mortality risk in patients with AAA without heterogeneity (OR 5.15) and also in LEAD patients (OR 3.29). Functionally dependent patients also had a significantly increased 30-day mortality risk, compared with independent patients (OR 4.49), and similar results were observed after stratifying those who underwent AAA repair (OR 5.14) or lower extremity revascularization (OR 4.18). Even for patients who underwent endovascular procedures rather than open surgery, functional dependency was also associated with a significantly increased 30-day mortality risk (OR 4.90). In addition, with regard to 30-day morbidity, frailty was associated with a significantly increased risk in both AAA (OR 2.79) and LEAD (OR 1.40) patients.
As far as long-term outcomes were concerned, frailty was associated with a significantly increased risk of long-term all-cause mortality in the overall studied population (HR 2.22), as well as in patients with AAA repair (HR 2.10) and LEAD revascularization (HR 2.46). Dr. Wang and colleagues found that central muscle mass was the only tool with moderate quality of evidence predicting long-term survival after major vascular surgery (HR .48), with other single-domain tools such as nutrition or cognition scoring being of low quality. The modified Frailty Index was the only multi-domain tool with moderate quality in predicting mortality for AAA, while others were scored as low or very low, the authors added.
“Future research is warranted to establish consensus on how to select the optimal frailty tool for certain clinical settings,” they concluded.
The authors reported that they had no conflicts of interest and no funding sources for the study.
SOURCE: Wang, J et al. Eur J Vasc Endovasc Surg. 2018;56:591-602.
Frailty defined as functional dependence is a predictor of mortality risk in elderly patients having major vascular surgery, a meta-analysis of studies has found
“Functional dependency may be recommended for use in rapid screening for frailty in major vascular surgery because of the high quality of associated evidence. Additionally, information on central muscle mass also adds incremental predictive value to long-term survival of elderly patients after major vascular surgery,” the study investigaters stated. However, they pointed out that “other newly developed frailty tools require further validation in more studies” before they should be adopted.
The report, published in the European Journal of Vascular and Endovascular Surgery, evaluated the effect of frailty in major vascular surgery from a search of MEDLINE, Embase, Cochrane Database, and Scopus through May 2018. Data were extracted from the articles related to surgery for abdominal aortic aneurysms (AAA) and lower extremity artery disease (LEAD), and a modified Newcastle-Ottawa scale was used to assess the quality of the included studies, according to Jiarong Wang, MD, of the department of vascular surgery, Sichuan University, Sichuan Province, China, and colleagues. A total of 22 cohort studies and one randomized controlled trial was used in the final analysis. The reviewers expressed the impact of frailty on outcomes as odds ratios (OR) or hazard ratios (HR) using a random effects model.
The researchers found that frailty, in terms of functional dependence, was associated with a significantly increased 30-day mortality risk in patients with AAA without heterogeneity (OR 5.15) and also in LEAD patients (OR 3.29). Functionally dependent patients also had a significantly increased 30-day mortality risk, compared with independent patients (OR 4.49), and similar results were observed after stratifying those who underwent AAA repair (OR 5.14) or lower extremity revascularization (OR 4.18). Even for patients who underwent endovascular procedures rather than open surgery, functional dependency was also associated with a significantly increased 30-day mortality risk (OR 4.90). In addition, with regard to 30-day morbidity, frailty was associated with a significantly increased risk in both AAA (OR 2.79) and LEAD (OR 1.40) patients.
As far as long-term outcomes were concerned, frailty was associated with a significantly increased risk of long-term all-cause mortality in the overall studied population (HR 2.22), as well as in patients with AAA repair (HR 2.10) and LEAD revascularization (HR 2.46). Dr. Wang and colleagues found that central muscle mass was the only tool with moderate quality of evidence predicting long-term survival after major vascular surgery (HR .48), with other single-domain tools such as nutrition or cognition scoring being of low quality. The modified Frailty Index was the only multi-domain tool with moderate quality in predicting mortality for AAA, while others were scored as low or very low, the authors added.
“Future research is warranted to establish consensus on how to select the optimal frailty tool for certain clinical settings,” they concluded.
The authors reported that they had no conflicts of interest and no funding sources for the study.
SOURCE: Wang, J et al. Eur J Vasc Endovasc Surg. 2018;56:591-602.
Frailty defined as functional dependence is a predictor of mortality risk in elderly patients having major vascular surgery, a meta-analysis of studies has found
“Functional dependency may be recommended for use in rapid screening for frailty in major vascular surgery because of the high quality of associated evidence. Additionally, information on central muscle mass also adds incremental predictive value to long-term survival of elderly patients after major vascular surgery,” the study investigaters stated. However, they pointed out that “other newly developed frailty tools require further validation in more studies” before they should be adopted.
The report, published in the European Journal of Vascular and Endovascular Surgery, evaluated the effect of frailty in major vascular surgery from a search of MEDLINE, Embase, Cochrane Database, and Scopus through May 2018. Data were extracted from the articles related to surgery for abdominal aortic aneurysms (AAA) and lower extremity artery disease (LEAD), and a modified Newcastle-Ottawa scale was used to assess the quality of the included studies, according to Jiarong Wang, MD, of the department of vascular surgery, Sichuan University, Sichuan Province, China, and colleagues. A total of 22 cohort studies and one randomized controlled trial was used in the final analysis. The reviewers expressed the impact of frailty on outcomes as odds ratios (OR) or hazard ratios (HR) using a random effects model.
The researchers found that frailty, in terms of functional dependence, was associated with a significantly increased 30-day mortality risk in patients with AAA without heterogeneity (OR 5.15) and also in LEAD patients (OR 3.29). Functionally dependent patients also had a significantly increased 30-day mortality risk, compared with independent patients (OR 4.49), and similar results were observed after stratifying those who underwent AAA repair (OR 5.14) or lower extremity revascularization (OR 4.18). Even for patients who underwent endovascular procedures rather than open surgery, functional dependency was also associated with a significantly increased 30-day mortality risk (OR 4.90). In addition, with regard to 30-day morbidity, frailty was associated with a significantly increased risk in both AAA (OR 2.79) and LEAD (OR 1.40) patients.
As far as long-term outcomes were concerned, frailty was associated with a significantly increased risk of long-term all-cause mortality in the overall studied population (HR 2.22), as well as in patients with AAA repair (HR 2.10) and LEAD revascularization (HR 2.46). Dr. Wang and colleagues found that central muscle mass was the only tool with moderate quality of evidence predicting long-term survival after major vascular surgery (HR .48), with other single-domain tools such as nutrition or cognition scoring being of low quality. The modified Frailty Index was the only multi-domain tool with moderate quality in predicting mortality for AAA, while others were scored as low or very low, the authors added.
“Future research is warranted to establish consensus on how to select the optimal frailty tool for certain clinical settings,” they concluded.
The authors reported that they had no conflicts of interest and no funding sources for the study.
SOURCE: Wang, J et al. Eur J Vasc Endovasc Surg. 2018;56:591-602.
FROM EUROPEAN JOURNAL OF VASCULAR AND ENDOVASCULAR SURGERY
Key clinical point: Frailty was associated with increased short- and long-term mortality in major vascular surgery.
Major finding: Frailty was associated with a fourfold increased risk of 30-day mortality and a doubled increased risk of long-term mortality after major vascular surgery.
Study details: A meta-analysis of 22 cohort studies and one randomized controlled trial.
Disclosures: The authors reported that they had no conflicts of interest and no funding sources for the study.
Source: Wang, J et al., 2018. Eur J Vasc Endovasc Surg. 56:591-602.
IDEAS study meets first aim of changing 3-month clinical management, health outcomes
BARCELONA – Amyloid PET brain imaging changed clinical management in 60% of patients with a diagnosis of mild cognitive impairment or dementia and confirmed a presumptive Alzheimer’s diagnosis in 95% of those with positive scans.
But the scans also benefited amyloid-negative patients, Gil Rabinovici, MD, said at the Clinical Trials on Alzheimer’s Disease conference. Before the test, 71% carried an Alzheimer’s disease (AD) diagnosis; after the test, just 10% did, opening the way for an accurate diagnosis and more effective treatment.
“These patients were saved from unnecessary treatment for Alzheimer’s,” said Dr. Rabinovici, the Edward Fein and Pearl Landrith Endowed Professor in Memory & Aging at the University of California, San Francisco. They received more suitable care plans because of the confirmation.
He presented final results of aim one of the IDEAS (Imaging Dementia–Evidence for Amyloid Scanning) study, which seeks to prove that amyloid imaging changes clinical management and improves health outcomes in Medicare beneficiaries who have been diagnosed with mild cognitive impairment (MCI) or dementia of uncertain cause. Its two aims are to show that amyloid PET imaging affects a patient’s care plan within 3 months of the scan and that this impacts major medical outcomes 12 months later. In diagnostically uncertain cases, investigators theorized, amyloid PET imaging would lead to significant changes in patient management, which would then translate into improved medical outcomes.
Ultimately, investigators hope the U.S.-wide, open-label study will prove the clinical value of amyloid PET scanning and convince the Centers for Medicare & Medicaid Services to make the test a fully covered service.
So far, IDEAS has accrued data on 11,409 patients and is quickly closing in on the 18,000-patient target. The patients reported on at CTAD were aged a mean of 75 years and were largely white; only 4% were black and 4% Hispanic. The mean Mini-Mental Scale Exam score was 26. AD was the leading suspect pathology in 73% of the 6,905 with MCI and in 83% of those with dementia of uncertain etiology. Overall, 44% were taking AD medications at baseline.
Scans were positive in 55% of those with MCI and in 70% of those with dementia. Overall, the scans changed clinical management in 61% (7,018), including 60% of those with MCI and 63% of those with dementia.
“We also asked physicians how much the scan results contributed to these changes, and 86.7% replied that they ‘contributed significantly,’ ” Dr. Rabinovici said.
Most changes involved adjustments to medication. AD drugs were started in 44% of MCI patients and in 45% of dementia patients, and non-AD drugs started in 22% and 25%, respectively. About a fifth of the patients received counseling in wake of the scan results.
Medication adjustments also varied by scan result. Among amyloid-positive MCI patients, AD drug use increased from 40% before imaging to 81% after; among amyloid-negative MCI patients, drug use decreased slightly from 27% to 24%. Among amyloid-positive dementia patients, AD drug use increased from 63% to 91%, and among amyloid-negative patients, it dropped from 50% to 44%. All these changes were statistically significant.
The primary diagnosis changed from AD to non-AD in 25%, and from non-AD to AD in 10%. Among amyloid-positive patients, the diagnosis prevalence jumped from 80.0% to 95.5%; among amyloid-negative patients, it dropped from 71% to just 10%.
“IDEAS now provides the strongest data we have supporting the beneficial impact of amyloid PET on patient management,” said Dr. Rabinovici. “Aim two, which is the 12-month health outcomes, we expect to be completed by the end of next year.”
The IDEAS team is also looking at a furthering the investigation with a study called, aptly, “NEW IDEAS.” That would reach out to recruit the minorities that were so underrepresented in the main study and include patients with early-onset MCI or dementia. Building up a library of DNA and blood plasma samples might also fit into the new project.
IDEAS is a funding collaboration of the CMS, the Alzheimer’s Association, Avid Radiopharmaceuticals/Eli Lilly, General Electric Healthcare, Piramal Imaging, and the American College of Radiology. Dr. Rabinovici had no financial disclosures.
BARCELONA – Amyloid PET brain imaging changed clinical management in 60% of patients with a diagnosis of mild cognitive impairment or dementia and confirmed a presumptive Alzheimer’s diagnosis in 95% of those with positive scans.
But the scans also benefited amyloid-negative patients, Gil Rabinovici, MD, said at the Clinical Trials on Alzheimer’s Disease conference. Before the test, 71% carried an Alzheimer’s disease (AD) diagnosis; after the test, just 10% did, opening the way for an accurate diagnosis and more effective treatment.
“These patients were saved from unnecessary treatment for Alzheimer’s,” said Dr. Rabinovici, the Edward Fein and Pearl Landrith Endowed Professor in Memory & Aging at the University of California, San Francisco. They received more suitable care plans because of the confirmation.
He presented final results of aim one of the IDEAS (Imaging Dementia–Evidence for Amyloid Scanning) study, which seeks to prove that amyloid imaging changes clinical management and improves health outcomes in Medicare beneficiaries who have been diagnosed with mild cognitive impairment (MCI) or dementia of uncertain cause. Its two aims are to show that amyloid PET imaging affects a patient’s care plan within 3 months of the scan and that this impacts major medical outcomes 12 months later. In diagnostically uncertain cases, investigators theorized, amyloid PET imaging would lead to significant changes in patient management, which would then translate into improved medical outcomes.
Ultimately, investigators hope the U.S.-wide, open-label study will prove the clinical value of amyloid PET scanning and convince the Centers for Medicare & Medicaid Services to make the test a fully covered service.
So far, IDEAS has accrued data on 11,409 patients and is quickly closing in on the 18,000-patient target. The patients reported on at CTAD were aged a mean of 75 years and were largely white; only 4% were black and 4% Hispanic. The mean Mini-Mental Scale Exam score was 26. AD was the leading suspect pathology in 73% of the 6,905 with MCI and in 83% of those with dementia of uncertain etiology. Overall, 44% were taking AD medications at baseline.
Scans were positive in 55% of those with MCI and in 70% of those with dementia. Overall, the scans changed clinical management in 61% (7,018), including 60% of those with MCI and 63% of those with dementia.
“We also asked physicians how much the scan results contributed to these changes, and 86.7% replied that they ‘contributed significantly,’ ” Dr. Rabinovici said.
Most changes involved adjustments to medication. AD drugs were started in 44% of MCI patients and in 45% of dementia patients, and non-AD drugs started in 22% and 25%, respectively. About a fifth of the patients received counseling in wake of the scan results.
Medication adjustments also varied by scan result. Among amyloid-positive MCI patients, AD drug use increased from 40% before imaging to 81% after; among amyloid-negative MCI patients, drug use decreased slightly from 27% to 24%. Among amyloid-positive dementia patients, AD drug use increased from 63% to 91%, and among amyloid-negative patients, it dropped from 50% to 44%. All these changes were statistically significant.
The primary diagnosis changed from AD to non-AD in 25%, and from non-AD to AD in 10%. Among amyloid-positive patients, the diagnosis prevalence jumped from 80.0% to 95.5%; among amyloid-negative patients, it dropped from 71% to just 10%.
“IDEAS now provides the strongest data we have supporting the beneficial impact of amyloid PET on patient management,” said Dr. Rabinovici. “Aim two, which is the 12-month health outcomes, we expect to be completed by the end of next year.”
The IDEAS team is also looking at a furthering the investigation with a study called, aptly, “NEW IDEAS.” That would reach out to recruit the minorities that were so underrepresented in the main study and include patients with early-onset MCI or dementia. Building up a library of DNA and blood plasma samples might also fit into the new project.
IDEAS is a funding collaboration of the CMS, the Alzheimer’s Association, Avid Radiopharmaceuticals/Eli Lilly, General Electric Healthcare, Piramal Imaging, and the American College of Radiology. Dr. Rabinovici had no financial disclosures.
BARCELONA – Amyloid PET brain imaging changed clinical management in 60% of patients with a diagnosis of mild cognitive impairment or dementia and confirmed a presumptive Alzheimer’s diagnosis in 95% of those with positive scans.
But the scans also benefited amyloid-negative patients, Gil Rabinovici, MD, said at the Clinical Trials on Alzheimer’s Disease conference. Before the test, 71% carried an Alzheimer’s disease (AD) diagnosis; after the test, just 10% did, opening the way for an accurate diagnosis and more effective treatment.
“These patients were saved from unnecessary treatment for Alzheimer’s,” said Dr. Rabinovici, the Edward Fein and Pearl Landrith Endowed Professor in Memory & Aging at the University of California, San Francisco. They received more suitable care plans because of the confirmation.
He presented final results of aim one of the IDEAS (Imaging Dementia–Evidence for Amyloid Scanning) study, which seeks to prove that amyloid imaging changes clinical management and improves health outcomes in Medicare beneficiaries who have been diagnosed with mild cognitive impairment (MCI) or dementia of uncertain cause. Its two aims are to show that amyloid PET imaging affects a patient’s care plan within 3 months of the scan and that this impacts major medical outcomes 12 months later. In diagnostically uncertain cases, investigators theorized, amyloid PET imaging would lead to significant changes in patient management, which would then translate into improved medical outcomes.
Ultimately, investigators hope the U.S.-wide, open-label study will prove the clinical value of amyloid PET scanning and convince the Centers for Medicare & Medicaid Services to make the test a fully covered service.
So far, IDEAS has accrued data on 11,409 patients and is quickly closing in on the 18,000-patient target. The patients reported on at CTAD were aged a mean of 75 years and were largely white; only 4% were black and 4% Hispanic. The mean Mini-Mental Scale Exam score was 26. AD was the leading suspect pathology in 73% of the 6,905 with MCI and in 83% of those with dementia of uncertain etiology. Overall, 44% were taking AD medications at baseline.
Scans were positive in 55% of those with MCI and in 70% of those with dementia. Overall, the scans changed clinical management in 61% (7,018), including 60% of those with MCI and 63% of those with dementia.
“We also asked physicians how much the scan results contributed to these changes, and 86.7% replied that they ‘contributed significantly,’ ” Dr. Rabinovici said.
Most changes involved adjustments to medication. AD drugs were started in 44% of MCI patients and in 45% of dementia patients, and non-AD drugs started in 22% and 25%, respectively. About a fifth of the patients received counseling in wake of the scan results.
Medication adjustments also varied by scan result. Among amyloid-positive MCI patients, AD drug use increased from 40% before imaging to 81% after; among amyloid-negative MCI patients, drug use decreased slightly from 27% to 24%. Among amyloid-positive dementia patients, AD drug use increased from 63% to 91%, and among amyloid-negative patients, it dropped from 50% to 44%. All these changes were statistically significant.
The primary diagnosis changed from AD to non-AD in 25%, and from non-AD to AD in 10%. Among amyloid-positive patients, the diagnosis prevalence jumped from 80.0% to 95.5%; among amyloid-negative patients, it dropped from 71% to just 10%.
“IDEAS now provides the strongest data we have supporting the beneficial impact of amyloid PET on patient management,” said Dr. Rabinovici. “Aim two, which is the 12-month health outcomes, we expect to be completed by the end of next year.”
The IDEAS team is also looking at a furthering the investigation with a study called, aptly, “NEW IDEAS.” That would reach out to recruit the minorities that were so underrepresented in the main study and include patients with early-onset MCI or dementia. Building up a library of DNA and blood plasma samples might also fit into the new project.
IDEAS is a funding collaboration of the CMS, the Alzheimer’s Association, Avid Radiopharmaceuticals/Eli Lilly, General Electric Healthcare, Piramal Imaging, and the American College of Radiology. Dr. Rabinovici had no financial disclosures.
REPORTING FROM CTAD
Key clinical point: Amyloid PET imaging can refine equivocal dementia diagnoses.
Major finding:
Study details: The IDEAS study has thus far accrued data on 11,409 subjects.
Disclosures: IDEAS is a funding collaboration of the Centers for Medicare & Medicaid Services, the Alzheimer’s Association, Avid Radiopharmaceuticals/Eli Lilly, General Electric Healthcare, Piramal Imaging, and the American College of Radiology. Dr. Rabinovici had no financial disclosures.
Stroke risk in elderly following AMI extends to 12 weeks
ATLANTA – Acute myocardial infarction is associated with a risk of stroke that extends beyond the 1-month time window currently considered the at-risk period, according to an analysis of Medicare data.
“The results of our study may allow clinicians to more accurately counsel patients regarding their stroke etiology and may allow refinement of stroke etiology classification systems and clinical trial selection criteria,” lead study author Alexander E. Merkler, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In an effort to better understand the duration of heightened stroke risk after acute myocardial infarction, Dr. Merkler, a neurologist at New York–based Weill Cornell Medicine, and his colleagues conducted a retrospective cohort study using inpatient and outpatient claims during 2008-2015 from a nationally representative 5% sample of Medicare beneficiaries who were at least 66 years old. They used previously validated ICD-9-CM diagnosis codes to ascertain the exposure variable of acute MI and the outcome of ischemic stroke but excluded strokes that occurred during an acute MI hospitalization.
Patients were censored at the time of ischemic stroke, death, end of Medicare coverage, or by Sept. 30, 2015. The researchers fit Cox regression models separately for the groups with and without acute MI to examine its association with ischemic stroke after adjusting for demographics, stroke risk factors, and Charlson comorbidities. Next, they used the corresponding survival probabilities to compute the hazard ratio (HR) in each 4-week interval after discharge, up to week 12. They also conducted a subgroup analysis to evaluate the duration of heightened ischemic stroke risk by MI type: ST-segment elevation MI (STEMI) versus non-STEMI (NSTEMI).
Dr. Merkler and his colleagues drew from data on 1.7 million eligible beneficiaries. Of these, 46,182 were hospitalized for acute MI and 80,466 for ischemic stroke. After they adjusted for demographics, stroke risk factors, and Charlson comorbidities, the researchers found that the risk of ischemic stroke was highest in the first 4 weeks after discharge from the MI hospitalization (HR, 2.7), yet remained elevated during weeks 5-8 (HR, 2.0) and weeks 9-12 (HR, 1.6). It was no longer significantly elevated afterward. The prolonged period of heightened ischemic stroke risk was evident in patients with both STEMI and NSTEMI.
“We were surprised by how long the risk of stroke lasts after MI,” Dr. Merkler said. He acknowledged certain limitations of the analysis, including the fact that patients were all over the age of 65 years. “In addition, we lack granular detail such as severity of MI [and] the extent of stroke work-up,” he said.
Dr. Merkler disclosed that he is supported by a grant from the National Institutes of Health and by the Leon Levy Foundation in Neuroscience. Most of his coauthors are also supported by NIH grants.
dbrunk@mdedge.com
Source: Ann Neurol. 2018;84[S22]:S146-7, Abstract M122.
ATLANTA – Acute myocardial infarction is associated with a risk of stroke that extends beyond the 1-month time window currently considered the at-risk period, according to an analysis of Medicare data.
“The results of our study may allow clinicians to more accurately counsel patients regarding their stroke etiology and may allow refinement of stroke etiology classification systems and clinical trial selection criteria,” lead study author Alexander E. Merkler, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In an effort to better understand the duration of heightened stroke risk after acute myocardial infarction, Dr. Merkler, a neurologist at New York–based Weill Cornell Medicine, and his colleagues conducted a retrospective cohort study using inpatient and outpatient claims during 2008-2015 from a nationally representative 5% sample of Medicare beneficiaries who were at least 66 years old. They used previously validated ICD-9-CM diagnosis codes to ascertain the exposure variable of acute MI and the outcome of ischemic stroke but excluded strokes that occurred during an acute MI hospitalization.
Patients were censored at the time of ischemic stroke, death, end of Medicare coverage, or by Sept. 30, 2015. The researchers fit Cox regression models separately for the groups with and without acute MI to examine its association with ischemic stroke after adjusting for demographics, stroke risk factors, and Charlson comorbidities. Next, they used the corresponding survival probabilities to compute the hazard ratio (HR) in each 4-week interval after discharge, up to week 12. They also conducted a subgroup analysis to evaluate the duration of heightened ischemic stroke risk by MI type: ST-segment elevation MI (STEMI) versus non-STEMI (NSTEMI).
Dr. Merkler and his colleagues drew from data on 1.7 million eligible beneficiaries. Of these, 46,182 were hospitalized for acute MI and 80,466 for ischemic stroke. After they adjusted for demographics, stroke risk factors, and Charlson comorbidities, the researchers found that the risk of ischemic stroke was highest in the first 4 weeks after discharge from the MI hospitalization (HR, 2.7), yet remained elevated during weeks 5-8 (HR, 2.0) and weeks 9-12 (HR, 1.6). It was no longer significantly elevated afterward. The prolonged period of heightened ischemic stroke risk was evident in patients with both STEMI and NSTEMI.
“We were surprised by how long the risk of stroke lasts after MI,” Dr. Merkler said. He acknowledged certain limitations of the analysis, including the fact that patients were all over the age of 65 years. “In addition, we lack granular detail such as severity of MI [and] the extent of stroke work-up,” he said.
Dr. Merkler disclosed that he is supported by a grant from the National Institutes of Health and by the Leon Levy Foundation in Neuroscience. Most of his coauthors are also supported by NIH grants.
dbrunk@mdedge.com
Source: Ann Neurol. 2018;84[S22]:S146-7, Abstract M122.
ATLANTA – Acute myocardial infarction is associated with a risk of stroke that extends beyond the 1-month time window currently considered the at-risk period, according to an analysis of Medicare data.
“The results of our study may allow clinicians to more accurately counsel patients regarding their stroke etiology and may allow refinement of stroke etiology classification systems and clinical trial selection criteria,” lead study author Alexander E. Merkler, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In an effort to better understand the duration of heightened stroke risk after acute myocardial infarction, Dr. Merkler, a neurologist at New York–based Weill Cornell Medicine, and his colleagues conducted a retrospective cohort study using inpatient and outpatient claims during 2008-2015 from a nationally representative 5% sample of Medicare beneficiaries who were at least 66 years old. They used previously validated ICD-9-CM diagnosis codes to ascertain the exposure variable of acute MI and the outcome of ischemic stroke but excluded strokes that occurred during an acute MI hospitalization.
Patients were censored at the time of ischemic stroke, death, end of Medicare coverage, or by Sept. 30, 2015. The researchers fit Cox regression models separately for the groups with and without acute MI to examine its association with ischemic stroke after adjusting for demographics, stroke risk factors, and Charlson comorbidities. Next, they used the corresponding survival probabilities to compute the hazard ratio (HR) in each 4-week interval after discharge, up to week 12. They also conducted a subgroup analysis to evaluate the duration of heightened ischemic stroke risk by MI type: ST-segment elevation MI (STEMI) versus non-STEMI (NSTEMI).
Dr. Merkler and his colleagues drew from data on 1.7 million eligible beneficiaries. Of these, 46,182 were hospitalized for acute MI and 80,466 for ischemic stroke. After they adjusted for demographics, stroke risk factors, and Charlson comorbidities, the researchers found that the risk of ischemic stroke was highest in the first 4 weeks after discharge from the MI hospitalization (HR, 2.7), yet remained elevated during weeks 5-8 (HR, 2.0) and weeks 9-12 (HR, 1.6). It was no longer significantly elevated afterward. The prolonged period of heightened ischemic stroke risk was evident in patients with both STEMI and NSTEMI.
“We were surprised by how long the risk of stroke lasts after MI,” Dr. Merkler said. He acknowledged certain limitations of the analysis, including the fact that patients were all over the age of 65 years. “In addition, we lack granular detail such as severity of MI [and] the extent of stroke work-up,” he said.
Dr. Merkler disclosed that he is supported by a grant from the National Institutes of Health and by the Leon Levy Foundation in Neuroscience. Most of his coauthors are also supported by NIH grants.
dbrunk@mdedge.com
Source: Ann Neurol. 2018;84[S22]:S146-7, Abstract M122.
AT ANA 2018
Key clinical point: .
Major finding: The risk of ischemic stroke was highest in the first 4 weeks after discharge from the MI hospitalization (HR, 2.7), yet remained elevated during weeks 5-8 (HR, 2.0) and weeks 9-12 (HR, 1.6).
Study details: An analysis of 46,182 Medicare beneficiaries who were hospitalized for acute MI and 80,466 who were hospitalized for ischemic stroke.
Disclosures: Dr. Merkler disclosed that he is supported by a grant from the National Institutes of Health and by the Leon Levy Foundation in Neuroscience. Most of his coauthors are also supported by NIH grants.
Source: Ann Neurol. 2018;84[S22]:S146-7, Abstract M122.