User login
Delirious after undergoing workup for stroke
CASE Altered mental status after stroke workup
Ms. L, age 91, is admitted to the hospital for a neurologic evaluation of a recent episode of left-sided weakness that occurred 1 week ago. This left-sided weakness resolved without intervention within 2 hours while at home. This presentation is typical of a transient ischemic attack (TIA). She has a history of hypertension, bradycardia, and pacemaker implantation. On initial evaluation, her memory is intact, and she is able to walk normally. Her score on the St. Louis University Mental Status (SLUMS) exam is 25, which suggests normal cognitive functioning for her academic background. A CT scan of the head reveals a subacute stroke of the right posterior limb of the internal capsule consistent with recent TIA.
Ms. L is admitted for a routine stroke workup and prepares to undergo a CT angiogram (CTA) with the use of the iodinated agent iopamidol (100 mL, 76%) to evaluate patency of cerebral vessels. Her baseline blood urea nitrogen (BUN) and creatinine levels are within normal limits.
A day after undergoing CTA, Ms. L starts mumbling to herself, has unpredictable mood outbursts, and is not oriented to time, place, or person.
[polldaddy:10199351]
The authors’ observations
Due to her acute altered mental status (AMS), Ms. L underwent an emergent CT scan of the head to rule out any acute intracranial hemorrhages or thromboembolic events. The results of this test were negative. Urinalysis, BUN, creatinine, basic chemistry, and complete blood count panels were unrevealing. On a repeat SLUMS exam, Ms. L scored 9, indicating cognitive impairment.
Ms. L also underwent a comprehensive metabolic profile, which excluded any electrolyte abnormalities, or any hepatic or renal causes of AMS. There was no sign of dehydration, acidosis, hypoglycemia, hypoxemia, hypotension, or bradycardia/tachycardia. A urinalysis, chest X-ray, complete blood count, and 2 blood cultures conducted 24 hours apart did not reveal any signs of infection. There were no recent changes in her medications and she was not taking any sleep medications or other psychiatric medications that might precipitate a withdrawal syndrome.
There have been multiple reports of contrast-induced nephropathy (CIN), which may be evidenced by high BUN-to-creatinine ratios and could cause AMS in geriatric patients. However, CIN was ruled out as a potential cause in our patient because her BUN-to-creatinine was unremarkable.
Continue to: Routine EEG was clinically...
Routine EEG was clinically inconclusive. Diffusion-weighted MRI may have been helpful to identify ischemic strokes that a CT scan of the head might miss,1 but we were unable to conduct this test because Ms. L had a pacemaker. Barber et al2 suggested that in the setting of acute stroke, the use of MRI may not have an added advantage over the CT scan of the head.
[polldaddy:10199352]
TREATMENT Rapid improvement with supportive therapy
Intravenous fluids are administered as supportive therapy to Ms. L for suspected contrast-induced encephalopathy (CIE). The next day, Ms. L experiences a notable improvement in cognition, beyond that attributed to IV hydration. By 3 days post-contrast injection, her SLUMS score increases to 15. By 72 hours after contrast administration, Ms. L’s cognition returns to baseline. She is monitored for 24 hours after returning to baseline cognitive functioning. After observing her to be in no physical or medical distress and at baseline functioning, she is discharged home under the care of her son with outpatient follow-up and rehab services.
The authors’ observations
For Ms. L, the differential diagnosis included post-ictal phenomenon, new-onset ischemic or hemorrhagic changes, hyperperfusion syndrome, and CIE.
Seizures were ruled out because EEG was inconclusive, and Ms. L did not have the clinical features one would expect in an ictal episode. Transient ischemic attack is, by definition, an ischemic event with clinical return to baseline within 24 hours. Although a CT scan of the head may not be the most sensitive way to detect early ischemic changes and small ischemic zones, the self-limiting course and complete resolution of Ms. L’s symptoms with return to baseline is indicative of a more benign pathology, such as CIE. New hemorrhagic conversions have a dramatic presentation on radiologic studies. Historically, CIE presentations on imaging have been closely associated with the hyperattentuation seen in subarachnoid hemorrhage (SAH). The absence of typical radiologic and clinical findings in our case ruled out SAH.
Continue to: Typical CT scan findings in CIE include...
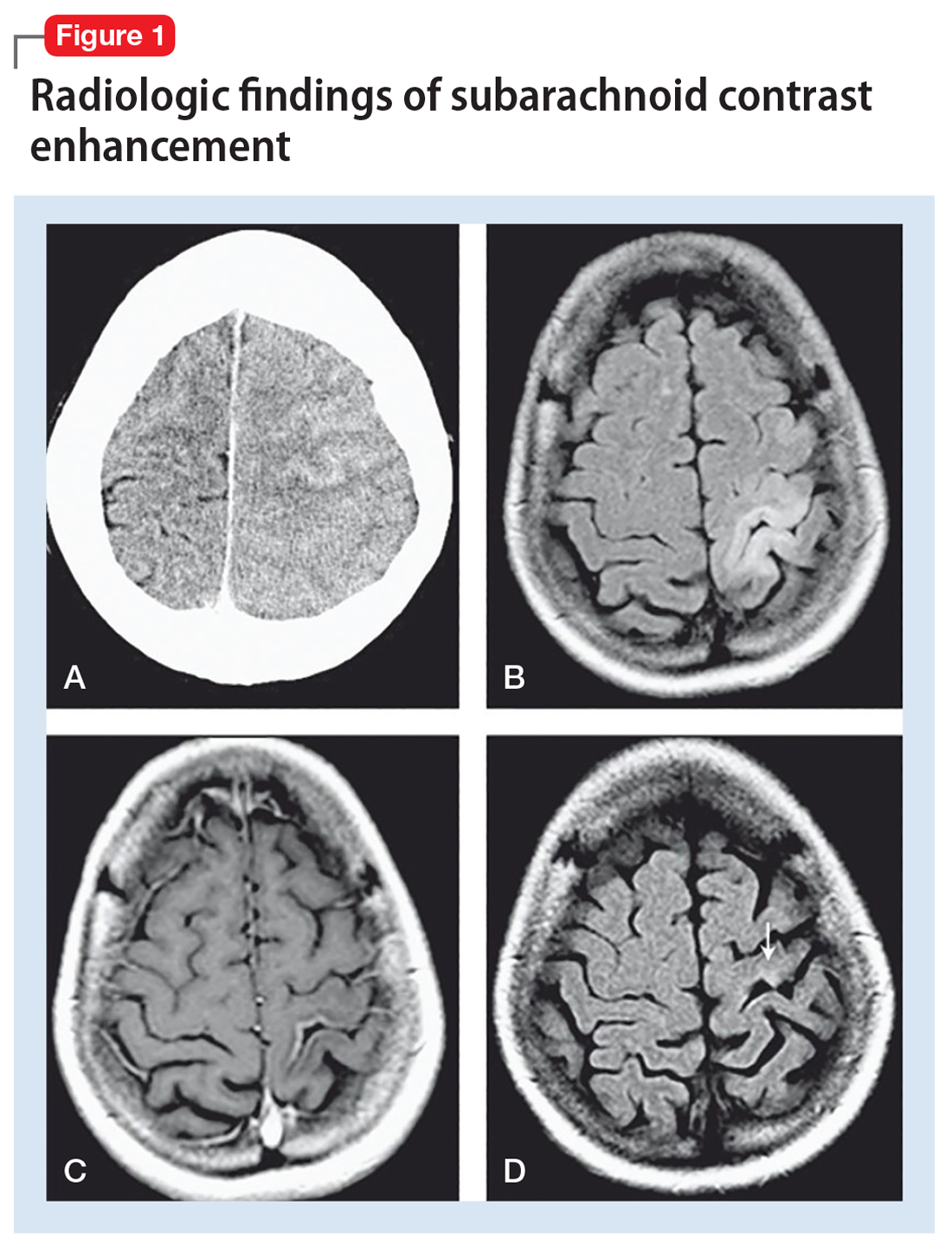
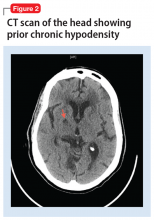
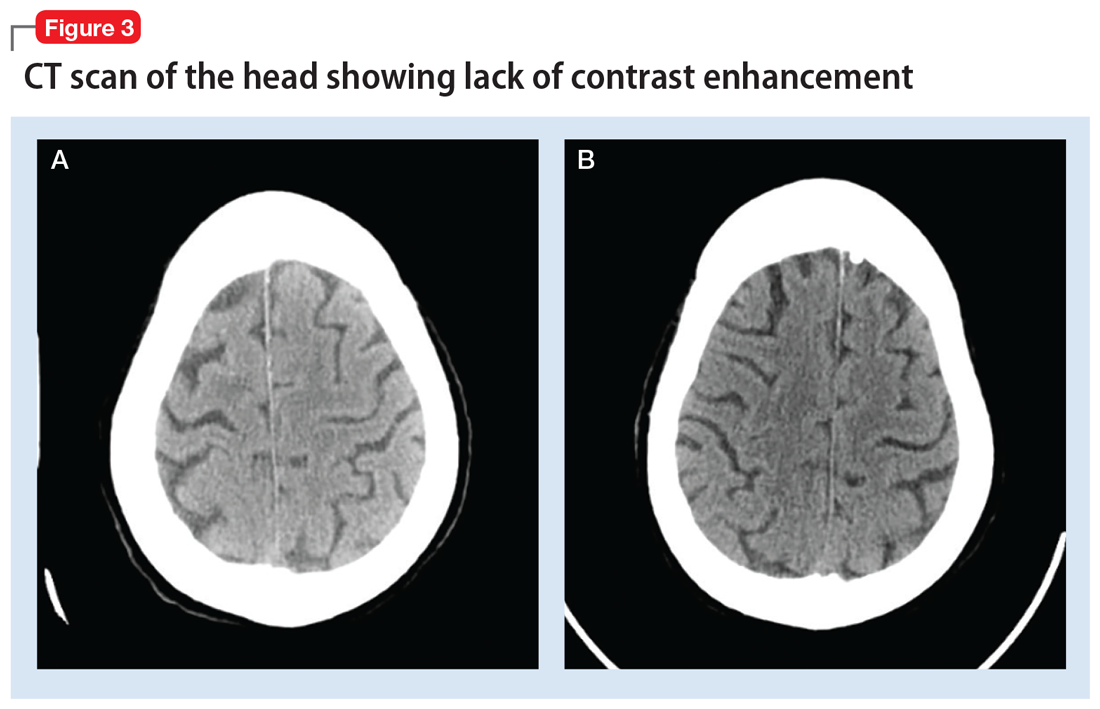
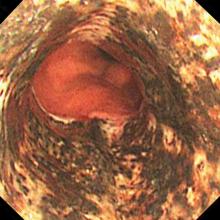
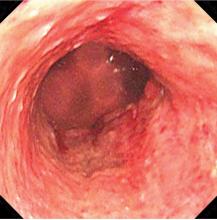
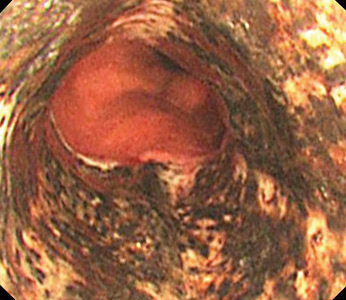
Typical CT scan findings in CIE include abnormal cortical contrast enhancement and edema, subarachnoid contrast enhancement, and striatal contrast enhancement (Figure 1, Figure 2, and Figure 3). Since the first clinical description, reports of 39 CT-/MRI-confirmed cases of CIE have been published in English language medical literature, with documented clinical follow-up3 and a median recovery time of 2.5 days. In a case report by Ito et al,4 there were no supportive radiographic findings. Ours is the second documented case that showed no radiologic signs of CIE. With a paucity of other etiologic evidence, negative lab tests for other causes of delirium, and the rapid resolution of Ms. L’s AMS after providing IV fluids as supportive treatment, a temporal correlation can be deduced, which implicates iodine-based contrast as the inciting factor.
Iodine-based contrast agents have been used since the 1920s. Today, >75 million procedures requiring iodine dyes are performed annually worldwide.5 This level of routine iodine contrast usage compels a mention of risk factors and complications from using such dyes. As a general rule, contrast agent reactions can be categorized as immediate (<1 day) or delayed (1 to 7 days after contrast administration). Immediate reactions are immunoglobulin E (IgE)-mediated anaphylactic reactions. Delayed reactions involve a T-cell mediated response that ranges from pruritus and urticaria (approximately 70%) to cardiac complications such as cardiovascular shock, arrhythmia, arrest, and Kounis syndrome. Other less prevalent complications include hypotension, bronchospasm, and CIN. Patients with the following factors may be at higher risk for contrast-induced reactions:
- asthma
- cardiac arrhythmias
- central myasthenia gravis
- >70 years of age
- pheochromocytoma
- sickle cell anemia
- hyperthyroidism
- dehydration
- hypotension.
Although some older literature reported correlations between seafood and shellfish allergies and iodine contrast reactions, more recent reports suggest there may not be a direct correlation, or any correlation at all.5,6
Iodinated CIE is a rare complication of contrast angiography. It was first reported in 1970 as transient cortical blindness after coronary angiography.7 Clinical manifestations include encephalopathy evidenced by AMS, affected orientation, and acute psychotic changes, including paranoia and hallucinations, seizures, cortical blindness, and focal neurologic deficits. Neuroimaging has been pivotal in confirming the diagnosis and in excluding thromboembolic and hemorrhagic complications of angiography.8
Encephalopathy has been documented after administration of
Continue to: Regardless of the mechanism...
Regardless of the mechanism, all the above-mentioned studies note a reversal of radiologic and neurologic findings without any deficits within 48 to 72 hours (median recovery time of 2.5 days).3 All reported cases of CIE, including ours, were found to be completely reversible without any neurologic or radiologic deficits after resolution (48 to 72 hours post-contrast administration).
Clinicians should have a high index of suspicion for CIE in patients with recent iodine-based contrast exposure. From a practical standpoint, such a mechanism could be easily missed because while use of a single-administration contrast agent may appear in procedure notes or medication administration records, it might not necessarily appear in documentation of currently administered medications. Also, such cases might not always present with unique radiologic findings, as illustrated by Ms. L’s case.
Bottom Line
Have a high index of suspicion for contrast-induced encephalopathy, especially in geriatric patients, even in the absence of radiologic findings. A full delirium/dementia workup is warranted to rule out other life-threatening causes of altered mental status. Timely recognition could enable implementation of medicationsparing approaches to the disorder, such as IV fluids and frequent reorientation.
Related Resources
- Donepudi B, Trottier S. A seizure and hemiplegia following contrast exposure: Understanding contrast-induced encephalopathy. Case Rep Med. 2018;2018:9278526. doi:10.1155/2018/9278526.
- Hamra M, Bakhit Y, Khan M, et al. Case report and literature review on contrast-induced encephalopathy. Future Cardiol. 2017;13(4):331-335.
Drug Brand Names
Iohexol • Omnipaque
Iopamidol • Isovue-370
Iopromide • Ultravist
Ioxilan • Oxilan
1. Moreau F, Asdaghi N, Modi J, et al. Magnetic resonance imaging versus computed tomography in transient ischemic attack and minor stroke: the more you see the more you know. Cerebrovasc Dis Extra. 2013;3(1):130-136.
2. Barber PA, Hill MD, Eliasziw M, et al. Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Neurosurg Psychiatry. 2005;76(11):1528-1533.
3. Leong S, Fanning NF. Persistent neurological deficit from iodinated contrast encephalopathy following intracranial aneurysm coiling: a case report and review of the literature. Interv Neuroradiol. 2012;18(1):33-41.
4. Ito N, Nishio R, Ozuki T, et al. A state of delirium (confusion) following cerebral angiography with ioxilan: a case report. Nihon Igaku Hoshasen Gakkai Zasshi. 2002; 62(7):370-371.
5. Bottinor W, Polkampally P, Jovin I. Adverse reactions to iodinated contrast media. Int J Angiol. 2013;22:149-154.
6. Cohan R. AHRQ Patient Safety Network Reaction to Dye. US Department of Health and Human Services Agency for Healthcare Research and Quality. https://psnet.ahrq.gov/webmm/case/75/reaction-to-dye. Published September 2004. Accessed March 5, 2017.
7. Fischer-Williams M, Gottschalk PG, Browell JN. Transient cortical blindness: an unusual complication of coronary angiography. Neurology. 1970;20(4):353-355.
8. Lantos G. Cortical blindness due to osmotic disruption of the blood-brain barrier by angiographic contrast material: CT and MRI studies. Neurology. 1989;39(4):567-571.
9. Kocabay G, Karabay CY. Iopromide-induced encephalopathy following coronary angioplasty. Perfusion. 2011;26:67-70.
10. Dangas G, Monsein LH, Laureno R, et al. Transient contrast encephalopathy after carotid artery stenting. Journal of Endovascular Therapy. 2001;8:111-113.
11. Sawaya RA, Hammoud R, Arnaout SJ, et al. Contrast induced encephalopathy following coronary angioplasty with iohexol. Southern Medical Journal. 2007;100(10):1054-1055.
CASE Altered mental status after stroke workup
Ms. L, age 91, is admitted to the hospital for a neurologic evaluation of a recent episode of left-sided weakness that occurred 1 week ago. This left-sided weakness resolved without intervention within 2 hours while at home. This presentation is typical of a transient ischemic attack (TIA). She has a history of hypertension, bradycardia, and pacemaker implantation. On initial evaluation, her memory is intact, and she is able to walk normally. Her score on the St. Louis University Mental Status (SLUMS) exam is 25, which suggests normal cognitive functioning for her academic background. A CT scan of the head reveals a subacute stroke of the right posterior limb of the internal capsule consistent with recent TIA.
Ms. L is admitted for a routine stroke workup and prepares to undergo a CT angiogram (CTA) with the use of the iodinated agent iopamidol (100 mL, 76%) to evaluate patency of cerebral vessels. Her baseline blood urea nitrogen (BUN) and creatinine levels are within normal limits.
A day after undergoing CTA, Ms. L starts mumbling to herself, has unpredictable mood outbursts, and is not oriented to time, place, or person.
[polldaddy:10199351]
The authors’ observations
Due to her acute altered mental status (AMS), Ms. L underwent an emergent CT scan of the head to rule out any acute intracranial hemorrhages or thromboembolic events. The results of this test were negative. Urinalysis, BUN, creatinine, basic chemistry, and complete blood count panels were unrevealing. On a repeat SLUMS exam, Ms. L scored 9, indicating cognitive impairment.
Ms. L also underwent a comprehensive metabolic profile, which excluded any electrolyte abnormalities, or any hepatic or renal causes of AMS. There was no sign of dehydration, acidosis, hypoglycemia, hypoxemia, hypotension, or bradycardia/tachycardia. A urinalysis, chest X-ray, complete blood count, and 2 blood cultures conducted 24 hours apart did not reveal any signs of infection. There were no recent changes in her medications and she was not taking any sleep medications or other psychiatric medications that might precipitate a withdrawal syndrome.
There have been multiple reports of contrast-induced nephropathy (CIN), which may be evidenced by high BUN-to-creatinine ratios and could cause AMS in geriatric patients. However, CIN was ruled out as a potential cause in our patient because her BUN-to-creatinine was unremarkable.
Continue to: Routine EEG was clinically...
Routine EEG was clinically inconclusive. Diffusion-weighted MRI may have been helpful to identify ischemic strokes that a CT scan of the head might miss,1 but we were unable to conduct this test because Ms. L had a pacemaker. Barber et al2 suggested that in the setting of acute stroke, the use of MRI may not have an added advantage over the CT scan of the head.
[polldaddy:10199352]
TREATMENT Rapid improvement with supportive therapy
Intravenous fluids are administered as supportive therapy to Ms. L for suspected contrast-induced encephalopathy (CIE). The next day, Ms. L experiences a notable improvement in cognition, beyond that attributed to IV hydration. By 3 days post-contrast injection, her SLUMS score increases to 15. By 72 hours after contrast administration, Ms. L’s cognition returns to baseline. She is monitored for 24 hours after returning to baseline cognitive functioning. After observing her to be in no physical or medical distress and at baseline functioning, she is discharged home under the care of her son with outpatient follow-up and rehab services.
The authors’ observations
For Ms. L, the differential diagnosis included post-ictal phenomenon, new-onset ischemic or hemorrhagic changes, hyperperfusion syndrome, and CIE.
Seizures were ruled out because EEG was inconclusive, and Ms. L did not have the clinical features one would expect in an ictal episode. Transient ischemic attack is, by definition, an ischemic event with clinical return to baseline within 24 hours. Although a CT scan of the head may not be the most sensitive way to detect early ischemic changes and small ischemic zones, the self-limiting course and complete resolution of Ms. L’s symptoms with return to baseline is indicative of a more benign pathology, such as CIE. New hemorrhagic conversions have a dramatic presentation on radiologic studies. Historically, CIE presentations on imaging have been closely associated with the hyperattentuation seen in subarachnoid hemorrhage (SAH). The absence of typical radiologic and clinical findings in our case ruled out SAH.
Continue to: Typical CT scan findings in CIE include...
Typical CT scan findings in CIE include abnormal cortical contrast enhancement and edema, subarachnoid contrast enhancement, and striatal contrast enhancement (Figure 1, Figure 2, and Figure 3). Since the first clinical description, reports of 39 CT-/MRI-confirmed cases of CIE have been published in English language medical literature, with documented clinical follow-up3 and a median recovery time of 2.5 days. In a case report by Ito et al,4 there were no supportive radiographic findings. Ours is the second documented case that showed no radiologic signs of CIE. With a paucity of other etiologic evidence, negative lab tests for other causes of delirium, and the rapid resolution of Ms. L’s AMS after providing IV fluids as supportive treatment, a temporal correlation can be deduced, which implicates iodine-based contrast as the inciting factor.
Iodine-based contrast agents have been used since the 1920s. Today, >75 million procedures requiring iodine dyes are performed annually worldwide.5 This level of routine iodine contrast usage compels a mention of risk factors and complications from using such dyes. As a general rule, contrast agent reactions can be categorized as immediate (<1 day) or delayed (1 to 7 days after contrast administration). Immediate reactions are immunoglobulin E (IgE)-mediated anaphylactic reactions. Delayed reactions involve a T-cell mediated response that ranges from pruritus and urticaria (approximately 70%) to cardiac complications such as cardiovascular shock, arrhythmia, arrest, and Kounis syndrome. Other less prevalent complications include hypotension, bronchospasm, and CIN. Patients with the following factors may be at higher risk for contrast-induced reactions:
- asthma
- cardiac arrhythmias
- central myasthenia gravis
- >70 years of age
- pheochromocytoma
- sickle cell anemia
- hyperthyroidism
- dehydration
- hypotension.
Although some older literature reported correlations between seafood and shellfish allergies and iodine contrast reactions, more recent reports suggest there may not be a direct correlation, or any correlation at all.5,6
Iodinated CIE is a rare complication of contrast angiography. It was first reported in 1970 as transient cortical blindness after coronary angiography.7 Clinical manifestations include encephalopathy evidenced by AMS, affected orientation, and acute psychotic changes, including paranoia and hallucinations, seizures, cortical blindness, and focal neurologic deficits. Neuroimaging has been pivotal in confirming the diagnosis and in excluding thromboembolic and hemorrhagic complications of angiography.8
Encephalopathy has been documented after administration of
Continue to: Regardless of the mechanism...
Regardless of the mechanism, all the above-mentioned studies note a reversal of radiologic and neurologic findings without any deficits within 48 to 72 hours (median recovery time of 2.5 days).3 All reported cases of CIE, including ours, were found to be completely reversible without any neurologic or radiologic deficits after resolution (48 to 72 hours post-contrast administration).
Clinicians should have a high index of suspicion for CIE in patients with recent iodine-based contrast exposure. From a practical standpoint, such a mechanism could be easily missed because while use of a single-administration contrast agent may appear in procedure notes or medication administration records, it might not necessarily appear in documentation of currently administered medications. Also, such cases might not always present with unique radiologic findings, as illustrated by Ms. L’s case.
Bottom Line
Have a high index of suspicion for contrast-induced encephalopathy, especially in geriatric patients, even in the absence of radiologic findings. A full delirium/dementia workup is warranted to rule out other life-threatening causes of altered mental status. Timely recognition could enable implementation of medicationsparing approaches to the disorder, such as IV fluids and frequent reorientation.
Related Resources
- Donepudi B, Trottier S. A seizure and hemiplegia following contrast exposure: Understanding contrast-induced encephalopathy. Case Rep Med. 2018;2018:9278526. doi:10.1155/2018/9278526.
- Hamra M, Bakhit Y, Khan M, et al. Case report and literature review on contrast-induced encephalopathy. Future Cardiol. 2017;13(4):331-335.
Drug Brand Names
Iohexol • Omnipaque
Iopamidol • Isovue-370
Iopromide • Ultravist
Ioxilan • Oxilan
CASE Altered mental status after stroke workup
Ms. L, age 91, is admitted to the hospital for a neurologic evaluation of a recent episode of left-sided weakness that occurred 1 week ago. This left-sided weakness resolved without intervention within 2 hours while at home. This presentation is typical of a transient ischemic attack (TIA). She has a history of hypertension, bradycardia, and pacemaker implantation. On initial evaluation, her memory is intact, and she is able to walk normally. Her score on the St. Louis University Mental Status (SLUMS) exam is 25, which suggests normal cognitive functioning for her academic background. A CT scan of the head reveals a subacute stroke of the right posterior limb of the internal capsule consistent with recent TIA.
Ms. L is admitted for a routine stroke workup and prepares to undergo a CT angiogram (CTA) with the use of the iodinated agent iopamidol (100 mL, 76%) to evaluate patency of cerebral vessels. Her baseline blood urea nitrogen (BUN) and creatinine levels are within normal limits.
A day after undergoing CTA, Ms. L starts mumbling to herself, has unpredictable mood outbursts, and is not oriented to time, place, or person.
[polldaddy:10199351]
The authors’ observations
Due to her acute altered mental status (AMS), Ms. L underwent an emergent CT scan of the head to rule out any acute intracranial hemorrhages or thromboembolic events. The results of this test were negative. Urinalysis, BUN, creatinine, basic chemistry, and complete blood count panels were unrevealing. On a repeat SLUMS exam, Ms. L scored 9, indicating cognitive impairment.
Ms. L also underwent a comprehensive metabolic profile, which excluded any electrolyte abnormalities, or any hepatic or renal causes of AMS. There was no sign of dehydration, acidosis, hypoglycemia, hypoxemia, hypotension, or bradycardia/tachycardia. A urinalysis, chest X-ray, complete blood count, and 2 blood cultures conducted 24 hours apart did not reveal any signs of infection. There were no recent changes in her medications and she was not taking any sleep medications or other psychiatric medications that might precipitate a withdrawal syndrome.
There have been multiple reports of contrast-induced nephropathy (CIN), which may be evidenced by high BUN-to-creatinine ratios and could cause AMS in geriatric patients. However, CIN was ruled out as a potential cause in our patient because her BUN-to-creatinine was unremarkable.
Continue to: Routine EEG was clinically...
Routine EEG was clinically inconclusive. Diffusion-weighted MRI may have been helpful to identify ischemic strokes that a CT scan of the head might miss,1 but we were unable to conduct this test because Ms. L had a pacemaker. Barber et al2 suggested that in the setting of acute stroke, the use of MRI may not have an added advantage over the CT scan of the head.
[polldaddy:10199352]
TREATMENT Rapid improvement with supportive therapy
Intravenous fluids are administered as supportive therapy to Ms. L for suspected contrast-induced encephalopathy (CIE). The next day, Ms. L experiences a notable improvement in cognition, beyond that attributed to IV hydration. By 3 days post-contrast injection, her SLUMS score increases to 15. By 72 hours after contrast administration, Ms. L’s cognition returns to baseline. She is monitored for 24 hours after returning to baseline cognitive functioning. After observing her to be in no physical or medical distress and at baseline functioning, she is discharged home under the care of her son with outpatient follow-up and rehab services.
The authors’ observations
For Ms. L, the differential diagnosis included post-ictal phenomenon, new-onset ischemic or hemorrhagic changes, hyperperfusion syndrome, and CIE.
Seizures were ruled out because EEG was inconclusive, and Ms. L did not have the clinical features one would expect in an ictal episode. Transient ischemic attack is, by definition, an ischemic event with clinical return to baseline within 24 hours. Although a CT scan of the head may not be the most sensitive way to detect early ischemic changes and small ischemic zones, the self-limiting course and complete resolution of Ms. L’s symptoms with return to baseline is indicative of a more benign pathology, such as CIE. New hemorrhagic conversions have a dramatic presentation on radiologic studies. Historically, CIE presentations on imaging have been closely associated with the hyperattentuation seen in subarachnoid hemorrhage (SAH). The absence of typical radiologic and clinical findings in our case ruled out SAH.
Continue to: Typical CT scan findings in CIE include...
Typical CT scan findings in CIE include abnormal cortical contrast enhancement and edema, subarachnoid contrast enhancement, and striatal contrast enhancement (Figure 1, Figure 2, and Figure 3). Since the first clinical description, reports of 39 CT-/MRI-confirmed cases of CIE have been published in English language medical literature, with documented clinical follow-up3 and a median recovery time of 2.5 days. In a case report by Ito et al,4 there were no supportive radiographic findings. Ours is the second documented case that showed no radiologic signs of CIE. With a paucity of other etiologic evidence, negative lab tests for other causes of delirium, and the rapid resolution of Ms. L’s AMS after providing IV fluids as supportive treatment, a temporal correlation can be deduced, which implicates iodine-based contrast as the inciting factor.
Iodine-based contrast agents have been used since the 1920s. Today, >75 million procedures requiring iodine dyes are performed annually worldwide.5 This level of routine iodine contrast usage compels a mention of risk factors and complications from using such dyes. As a general rule, contrast agent reactions can be categorized as immediate (<1 day) or delayed (1 to 7 days after contrast administration). Immediate reactions are immunoglobulin E (IgE)-mediated anaphylactic reactions. Delayed reactions involve a T-cell mediated response that ranges from pruritus and urticaria (approximately 70%) to cardiac complications such as cardiovascular shock, arrhythmia, arrest, and Kounis syndrome. Other less prevalent complications include hypotension, bronchospasm, and CIN. Patients with the following factors may be at higher risk for contrast-induced reactions:
- asthma
- cardiac arrhythmias
- central myasthenia gravis
- >70 years of age
- pheochromocytoma
- sickle cell anemia
- hyperthyroidism
- dehydration
- hypotension.
Although some older literature reported correlations between seafood and shellfish allergies and iodine contrast reactions, more recent reports suggest there may not be a direct correlation, or any correlation at all.5,6
Iodinated CIE is a rare complication of contrast angiography. It was first reported in 1970 as transient cortical blindness after coronary angiography.7 Clinical manifestations include encephalopathy evidenced by AMS, affected orientation, and acute psychotic changes, including paranoia and hallucinations, seizures, cortical blindness, and focal neurologic deficits. Neuroimaging has been pivotal in confirming the diagnosis and in excluding thromboembolic and hemorrhagic complications of angiography.8
Encephalopathy has been documented after administration of
Continue to: Regardless of the mechanism...
Regardless of the mechanism, all the above-mentioned studies note a reversal of radiologic and neurologic findings without any deficits within 48 to 72 hours (median recovery time of 2.5 days).3 All reported cases of CIE, including ours, were found to be completely reversible without any neurologic or radiologic deficits after resolution (48 to 72 hours post-contrast administration).
Clinicians should have a high index of suspicion for CIE in patients with recent iodine-based contrast exposure. From a practical standpoint, such a mechanism could be easily missed because while use of a single-administration contrast agent may appear in procedure notes or medication administration records, it might not necessarily appear in documentation of currently administered medications. Also, such cases might not always present with unique radiologic findings, as illustrated by Ms. L’s case.
Bottom Line
Have a high index of suspicion for contrast-induced encephalopathy, especially in geriatric patients, even in the absence of radiologic findings. A full delirium/dementia workup is warranted to rule out other life-threatening causes of altered mental status. Timely recognition could enable implementation of medicationsparing approaches to the disorder, such as IV fluids and frequent reorientation.
Related Resources
- Donepudi B, Trottier S. A seizure and hemiplegia following contrast exposure: Understanding contrast-induced encephalopathy. Case Rep Med. 2018;2018:9278526. doi:10.1155/2018/9278526.
- Hamra M, Bakhit Y, Khan M, et al. Case report and literature review on contrast-induced encephalopathy. Future Cardiol. 2017;13(4):331-335.
Drug Brand Names
Iohexol • Omnipaque
Iopamidol • Isovue-370
Iopromide • Ultravist
Ioxilan • Oxilan
1. Moreau F, Asdaghi N, Modi J, et al. Magnetic resonance imaging versus computed tomography in transient ischemic attack and minor stroke: the more you see the more you know. Cerebrovasc Dis Extra. 2013;3(1):130-136.
2. Barber PA, Hill MD, Eliasziw M, et al. Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Neurosurg Psychiatry. 2005;76(11):1528-1533.
3. Leong S, Fanning NF. Persistent neurological deficit from iodinated contrast encephalopathy following intracranial aneurysm coiling: a case report and review of the literature. Interv Neuroradiol. 2012;18(1):33-41.
4. Ito N, Nishio R, Ozuki T, et al. A state of delirium (confusion) following cerebral angiography with ioxilan: a case report. Nihon Igaku Hoshasen Gakkai Zasshi. 2002; 62(7):370-371.
5. Bottinor W, Polkampally P, Jovin I. Adverse reactions to iodinated contrast media. Int J Angiol. 2013;22:149-154.
6. Cohan R. AHRQ Patient Safety Network Reaction to Dye. US Department of Health and Human Services Agency for Healthcare Research and Quality. https://psnet.ahrq.gov/webmm/case/75/reaction-to-dye. Published September 2004. Accessed March 5, 2017.
7. Fischer-Williams M, Gottschalk PG, Browell JN. Transient cortical blindness: an unusual complication of coronary angiography. Neurology. 1970;20(4):353-355.
8. Lantos G. Cortical blindness due to osmotic disruption of the blood-brain barrier by angiographic contrast material: CT and MRI studies. Neurology. 1989;39(4):567-571.
9. Kocabay G, Karabay CY. Iopromide-induced encephalopathy following coronary angioplasty. Perfusion. 2011;26:67-70.
10. Dangas G, Monsein LH, Laureno R, et al. Transient contrast encephalopathy after carotid artery stenting. Journal of Endovascular Therapy. 2001;8:111-113.
11. Sawaya RA, Hammoud R, Arnaout SJ, et al. Contrast induced encephalopathy following coronary angioplasty with iohexol. Southern Medical Journal. 2007;100(10):1054-1055.
1. Moreau F, Asdaghi N, Modi J, et al. Magnetic resonance imaging versus computed tomography in transient ischemic attack and minor stroke: the more you see the more you know. Cerebrovasc Dis Extra. 2013;3(1):130-136.
2. Barber PA, Hill MD, Eliasziw M, et al. Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Neurosurg Psychiatry. 2005;76(11):1528-1533.
3. Leong S, Fanning NF. Persistent neurological deficit from iodinated contrast encephalopathy following intracranial aneurysm coiling: a case report and review of the literature. Interv Neuroradiol. 2012;18(1):33-41.
4. Ito N, Nishio R, Ozuki T, et al. A state of delirium (confusion) following cerebral angiography with ioxilan: a case report. Nihon Igaku Hoshasen Gakkai Zasshi. 2002; 62(7):370-371.
5. Bottinor W, Polkampally P, Jovin I. Adverse reactions to iodinated contrast media. Int J Angiol. 2013;22:149-154.
6. Cohan R. AHRQ Patient Safety Network Reaction to Dye. US Department of Health and Human Services Agency for Healthcare Research and Quality. https://psnet.ahrq.gov/webmm/case/75/reaction-to-dye. Published September 2004. Accessed March 5, 2017.
7. Fischer-Williams M, Gottschalk PG, Browell JN. Transient cortical blindness: an unusual complication of coronary angiography. Neurology. 1970;20(4):353-355.
8. Lantos G. Cortical blindness due to osmotic disruption of the blood-brain barrier by angiographic contrast material: CT and MRI studies. Neurology. 1989;39(4):567-571.
9. Kocabay G, Karabay CY. Iopromide-induced encephalopathy following coronary angioplasty. Perfusion. 2011;26:67-70.
10. Dangas G, Monsein LH, Laureno R, et al. Transient contrast encephalopathy after carotid artery stenting. Journal of Endovascular Therapy. 2001;8:111-113.
11. Sawaya RA, Hammoud R, Arnaout SJ, et al. Contrast induced encephalopathy following coronary angioplasty with iohexol. Southern Medical Journal. 2007;100(10):1054-1055.
Can lifestyle modifications delay or prevent Alzheimer’s disease?
Clinicians have devoted strenuous efforts to secondary prevention of Alzheimer’s disease (AD) by diagnosing and treating patients as early as possible. Unfortunately, there is no cure for AD, and the field has witnessed recurrent failures of several pharmacotherapy candidates with either symptomatic or disease-modifying properties.1 An estimated one-third of AD cases can be attributed to modifiable risk factors.2 Thus, implementing primary prevention measures by addressing modifiable risk factors thought to contribute to the disease, with the goal of reducing the risk of developing AD, or at least delaying its onset, is a crucial public health strategy.
Cardiovascular risk factors, such as hypertension, hyperlipidemia, diabetes, hyperhomocysteinemia, obesity, and smoking, have emerged as substantive risk factors for AD.3 Optimal management of these major risk factors, especially in mid-life, may be a preventive approach against AD. Although detailing the evidence on the impact of managing cardiovascular risk factors to delay or prevent AD is beyond the scope of this article, it is becoming clear that “what is good for the heart is good for the brain.”
Additional modifiable risk factors are related to lifestyle habits, such as physical exercise, mental and social activity, meditation/spiritual activity, and diet. This article reviews the importance of pursuing a healthy lifestyle in delaying AD, with the corresponding levels of evidence that support each specific lifestyle modification. The levels of evidence are defined in Table 1.4
Physical exercise
Twenty-one percent of AD cases in the United States are attributable to physical inactivity.5 In addition to its beneficial effect on metabolic syndrome, in animal and human research, regular exercise has been shown to have direct neuroprotective effects. High levels of physical activity increase hippocampal neurogenesis and neuroplasticity, increase vascular circulation in the brain regions implicated in AD, and modulate inflammatory mediators as well as brain growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1).6
The definition of regular physical exercise varies across the literature, but usually implies aerobic exercise—an ongoing activity sufficient to increase the heart rate and the need for oxygen, sustained for 20 to 30 minutes per session.7 Modalities include household activities and leisure-time activities. In a large prospective cohort study, Scarmeas et al8 categorized leisure-time activities into 3 types:
- light (walking, dancing, calisthenics, golfing, bowling, gardening, horseback riding)
- moderate (bicycling, swimming, hiking, playing tennis)
- vigorous (aerobic dancing, jogging, playing handball).
These types of physical exercise were weighed by the frequency of participation per week. Compared with being physically inactive, low levels of weekly physical activity (0.1 hours of vigorous, 0.8 hours of moderate, or 1.3 hours of light exercise) were associated with a 29% to 41% lower risk of developing AD, while higher weekly physical activity (1.3 hours of vigorous, 2.3 hours of moderate, or 3.8 hours of light exercise) were associated with a 37% to 50% lower risk (level III).8
In another 20-year cohort study, engaging in leisure-time physical activity at least twice a week in mid-life was significantly associated with a reduced risk of AD, after adjusting for age, sex, education, follow-up time, locomotor disorders, apolipoprotein E (ApoE) genotype, vascular disorders, smoking, and alcohol intake (level III).9 Moreover, a systematic review of 29 randomized controlled trials (RCTs) showed that aerobic exercise training, such as brisk walking, jogging, and biking, was associated with improvements in attention, processing speed, executive function, and memory among healthy older adults and those with mild cognitive impairment (MCI; level IA).10
Continue to: From a pathophysiological standpoint...
From a pathophysiological standpoint, higher levels of physical exercise in cognitively intact older adults have been associated with reduced brain amyloid beta deposits, especially in ApoE4 carriers.11 This inverse relationship also has been demonstrated in patients who are presymptomatic who carry 1 of the 3 known autosomal dominant mutations for the familial forms of AD.12
Overall, physicians should recommend that patients—especially those with cardiovascular risk factors that increase their risk for AD—exercise regularly by following the guidelines of the American Heart Association or the American College of Sports Medicine.13 These include muscle-strengthening activities (legs, hips, back, abdomen, shoulders, and arms) at least 2 days/week, in addition to either 30 minutes/day of moderate-intensity aerobic activity such as brisk walking, 5 days/week; or 25 minutes of vigorous aerobic activity such as jogging and running, 3 days/week14 (level IA evidence for overall improvement in cognitive function; level III evidence for AD delay/risk reduction). Neuromotor exercise, such as yoga and tai chi, and flexibility exercise such as muscle stretching, especially after a hot bath, 2 to 3 days/week are also recommended (level III).15
Mental activity
Nineteen percent of AD cases worldwide and 7% in the United States. can be attributed to low educational attainment, which is associated with low brain cognitive reserve.5 Cognitive resilience in later life may be enhanced by building brain reserves through intellectual stimulation, which affects neuronal branching and plasticity.16 Higher levels of complex mental activities measured across the lifespan, such as education, occupation, reading, and writing, are correlated with significantly less hippocampal volume shrinkage over time.17 Frequent participation in mentally stimulating activities—such as listening to the radio; reading newspapers, magazines, or books; playing games (cards, checkers, crosswords or other puzzles); and visiting museums—was associated with an up to 64% reduction in the odds of developing AD in a cohort of cognitively intact older adults followed for 4 years.18 The correlation between mental activity and AD was found to be independent of physical activity, social activity, or baseline cognitive function.19
In a large cohort of cognitively intact older adults (mean age 70), engaging in a mentally stimulating activity (craft activities, computer use, or going to the theater/movies) once to twice a week was significantly associated with a reduced incidence of amnestic MCI.20 Another prospective 21-year study demonstrated a significant reduction in AD risk in community-dwelling cognitively intact older adults (age 75 to 85) who participated in cognitively stimulating activities, such as reading books or newspapers, writing for pleasure, doing crossword puzzles, playing board games or cards, or playing musical instruments, several times/week.21
Growing scientific evidence also suggests that lifelong multilingualism can delay AD onset by 4 to 5 years.22 Multilingualism is associated with greater cognitive reserve, gray matter volume, functional connectivity and white matter density.23
Continue to: Physicians should encourage their patients...
Physicians should encourage their patients to engage in intellectually stimulating activities and creative leisure-time activities several times/week to enhance their cognitive reserves and delay AD onset (level III evidence with respect to AD risk reduction/delay).
Social activity
Social engagement may be an additional protective factor against AD. In a large 4-year prospective study, increased loneliness in cognitively intact older adults doubled the risk of AD.24 Data from the large French cohort PAQUID (Personnes Agées QUID) emphasized the importance of a patient’s social network as a protective factor against AD. In this cohort, the perception of reciprocity in relationships with others (the perception that a person had received more than he or she had given) was associated with a 53% reduction in AD risk (level III).25 In another longitudinal cohort study, social activity was found to decrease the incidence of subjective cognitive decline, which is a prodromal syndrome for MCI and AD (level III).26
A major confounder in studies assessing for social activity is the uncertainty if social withdrawal is a modifiable risk factor or an early manifestation of AD, since apathetic patients with AD tend to be socially withdrawn.27 Another limitation of measuring the impact of social activity relative to AD risk is the difficulty in isolating social activities from activities that have physical and mental activity components, such as leisure-time activities.28
Meditation/spiritual activity
Chronic psychological stress is believed to compromise limbic structures that regulate stress-related behaviors and the memory network, which might explain how being prone to psychological distress may be associated with MCI or AD.29 Cognitive stress may increase the oxidative stress and telomere shortening implicated in the neurodegenerative processes of AD.30 In one study, participants who were highly prone to psychological distress were found to be at 3 times increased risk for developing AD, after adjusting for depression symptoms and physical and mental activities (level III).31 By reducing chronic psychological stress, meditation techniques offer a promising preventive option against AD.
Mindfulness-based interventions (MBI) have gained increased attention in the past decade. They entail directing one’s attention towards the present moment, thereby decreasing ruminative thoughts and stress arousal.32 Recent RCTs have shown that MBI may promote brain health in older adults not only by improving psychological well-being but also by improving attentional control33 and functional connectivity in brain regions implicated in executive functioning,34 as well as by modulating inflammatory processes implicated in AD.35 Furthermore, an RCT of patients diagnosed with MCI found that compared with memory enhancement training, a weekly 60-minute yoga session improved memory and executive functioning.36
Continue to: Kirtan Kriya is a medication technique...
Kirtan Kriya is a meditation technique that is easy to learn and practice by older adults and can improve memory in patients at risk for developing AD.37 However, more rigorous RCTs conducted in larger samples of older adults are needed to better evaluate the effect of all meditation techniques for delaying or preventing AD (level IB with respect to improvement in cognitive functioning/level III for AD delay/risk reduction).38
Spiritual activities, such as going to places of worship or religious meditation, have been associated with a lower prevalence of AD. Attending religious services, gatherings, or retreats involves a social component because these activities often are practiced in groups. They also confer a method of dealing with psychological distress and depression. Additionally, frequent readings of religious texts represents a mentally stimulating activity that may also contribute to delaying/preventing AD (level III).39
Diet
In the past decade, a growing body of evidence has linked diet to cognition. Individuals with a higher intake of calories and fat are at higher risk for developing AD.40 The incidence of AD rose in Japan after the country transitioned to a more Westernized diet.41 A modern Western diet rich in saturated fatty acids and simple carbohydrates may negatively impact hippocampus-mediated functions such as memory and learning, and is associated with an increased risk of AD.42 In contrast with high-glycemic and fatty diets, a “healthy diet” is associated with a decrease in beta-amyloid burden, inflammation, and oxidative stress.43,44
Studies focusing on dietary patterns rather than a single nutrient for delaying or preventing AD have yielded more robust and consistent results.45 In a recent meta-analysis, adhering to a Mediterranean diet—which is rich in fruits and vegetables, whole grains, olive oil, and fish; moderate in some dairy products and wine; and low in red meat—was associated with a decreased risk of AD; this evidence was derived mostly from epidemiologic studies.46 Scarmeas et al8 found that high adherence to the Mediterranean diet was associated with 32% to 40% reduced risk of AD. Combining this diet with physical exercise was associated with an up to 67% reduced risk (level III). The Dietary Approaches to Stop Hypertension (DASH) diet, which is rich in total grains, fruits, vegetables, and dairy products, but low in sodium and sweets, correlated with neurocognitive improvement in patients with hypertension.47 Both the Mediterranean and DASH diets have been associated with better cognitive function48 and slower cognitive decline.49 Thus, an attempt to combine the neuroprotective components from both diets led to the creation of the MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) diet, which also has been associated with a lower incidence of AD.50
Besides specific diets, some food groups have also been found to promote brain health and may help delay or prevent AD. Berries have the highest amount of antioxidants of all fruit. Among vegetables, tomatoes and green leafy vegetables have the highest amount of nutrients for the brain. Nuts, such as walnuts, which are rich in omega-3 fatty acids, are also considered “power foods” for the brain; however, they should be consumed in moderation because they are also rich in fat. Monounsaturated fatty acids, which are found in olives and olive oil, are also beneficial for the brain. Among the 3 types of omega-3 fatty acids, the most important for cognition is docosahexaenoic acid (DHA) because it constitutes 40% of all fatty acids in the brain. Mainly found in oily fish, DHA has antioxidant and anti-inflammatory properties that may delay or prevent AD. Low levels of DHA have been found in patients with AD.51
Continue to: Curcumin, which is derived from...
Curcumin, which is derived from the curry spice turmeric, is a polyphenol with anti-inflammatory, antioxidant, and anti-amyloid properties that may have a promising role in preventing AD in cognitively intact individuals. Initial trials with curcumin have yielded mixed results on cognition, which was partly related to the low solubility and bioavailability of its formulation.52 However, a recent 18-month double-blind randomized placebo-controlled trial found positive effects on memory and attention, as well as reduction of amyloid plaques and tau tangles deposition in the brain, in non-demented older adults age 51 to 84 who took Theracumin, a highly absorptive oral form of curcumin dispersed with colloidal nanoparticles.53 A longer follow-up is required to determine if curcumin can delay or prevent AD.
Alcohol
The role of alcohol in AD prevention is controversial. Overall, data from prospective studies has shown that low to moderate alcohol consumption may be associated with a reduced risk of AD (level III).54 Alcohol drinking in mid-life showed a U-shaped relationship with cognitive impairment; both abstainers and heavy drinkers had an increased risk of cognitive decline compared with light to moderate drinkers (level III).55 Binge drinking significantly increased the odds of cognitive decline, even after controlling for total alcohol consumption per week.55
The definition of low-to-moderate drinking varies substantially among countries. In addition, the size and amount of alcohol contained in a standard drink may differ.56 According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA),57 moderate drinking is defined as up to 1 drink daily for women and 2 drinks daily for men. Binge drinking involves drinking >4 drinks for women and >5 drinks for men, in approximately 2 hours, at least monthly. In the United States, one standard drink contains 14 grams of pure alcohol, which is usually found in 12 ounces of regular beer, 5 ounces of wine, and 1.5 ounces of distilled spirits (vodka or whiskey).58
In a 5-year prospective Canadian study, having 1 drink weekly (especially wine) was associated with an up to 50% reduced risk of AD (level III).59 In the French cohort PAQUID, mild drinkers (<1 to 2 drinks/day) and moderate drinkers (3 to 4 drinks daily) had a reduced incidence of AD compared with non-drinkers. Wine was the most frequently consumed beverage in this study.60 Other studies have found cognitive benefits from mild to moderate drinking regardless of beverage type.54 However, a recent study that included a 30-year follow-up failed to find a significant protective effect of light drinking over abstinence in terms of hippocampal atrophy.61 Atrophy of the hippocampus was correlated with increasing alcohol amounts in a dose-dependent manner, starting at 7 to 14 drinks/week (level III).61
Research has shown that moderate and heavy alcohol use or misuse can directly induce microglial activation and inflammatory mediators’ release, which induce amyloid beta pathology and leads to brain atrophy.62 Hence, non-drinkers should not be advised to begin drinking, because of the lack of RCTs and the concern that beginning to drink may lead to heavy drinking. All drinkers should be advised to adhere to the NIAAA recommendations.13
Continue to: Coffee/tea
Coffee/tea
Although studies of caffeinated coffee have been heterogeneous and yielded mixed results (beneficial effect vs no effect on delaying cognitive decline), systematic reviews and meta-analyses of cross-sectional, case-control, and longitudinal cohort studies have found a general trend towards a favorable preventive role (level III).63-65 Caffeine exhibits its neuroprotective effect by increasing brain serotonin and acetylcholine, and by stabilizing blood-brain-barrier integrity.66 Moreover, in an animal study, mice given caffeine in their drinking water from young adulthood into older age had lower amyloid beta plasma levels compared with those given decaffeinated water.67 These findings suggest that in humans, 5 cups of regular caffeinated coffee daily, equivalent to 500 mg of caffeine,
An Italian study showed that older adults who don’t or rarely drink coffee (<1 cup daily) and those who recently increased their consumption pattern to >1 cup daily had a higher incidence of MCI than those who habitually consumed 1 to 2 cups daily.69 Therefore, it is not recommended to advise a change in coffee drinking pattern in old age. Older adults who are coffee drinkers should, however, be educated about the association between heavier caffeine intake and anxiety, insomnia, and cardiac arrhythmias.70
Despite its more modest caffeine levels, green tea is rich in polyphenols, which belong to the family of catechins and are characterized by antioxidant and anti-inflammatory properties.71 In a Japanese cohort, higher green tea consumption (up to 1 cup daily) was associated with a decreased incidence of MCI in older adults.72 More studies are needed to confirm its potential preventative role in AD.
Which lifestyle change is the most important?
Focusing on a single lifestyle change may be insufficient, especially because the bulk of evidence for individual interventions comes from population-based cohort studies (level III), rather than strong RCTs with a long follow-up. There is increasing evidence that combining multiple lifestyle modifications may yield better outcomes in maintaining or improving cognition.73
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a large, 2-year RCT that included community-dwelling older adults (age 60 to 77) with no diagnosis of major neurocognitive disorder, found that compared with regular health advice, multi-domain interventions reduced cognitive decline and improved overall cognition, executive functioning, and processing speed. The interventions evaluated in this study combined the following 4 modalities74:
- a healthy diet according to the Finnish nutrition recommendations (eating vegetables, fruits, and berries [minimum: 500 g/d], whole grain cereals [several times a day], and fish [2 to 3 times/week]; using low-salt products; consuming fat-free or low-fat milk products; and limiting red meat consumption to <500 g/week
- regular physical exercise tailored for improving muscle strength (1 to 3 times/week) coupled with aerobic exercise (2 to 5 times/week)
- cognitive training, including group sessions that have a social activity component and computer-based individual sessions 3 times/week that target episodic and working memory and executive functioning
- optimal management of cardiovascular risk factors.
Continue to: This multi-domain approach...
This multi-domain approach for lifestyle modification should be strongly recommended to cognitively intact older patients (level IB).
Modeled after the FINGER study, the Alzheimer’s Association U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER) is a 2-year, multicenter, controlled clinical trial aimed at testing the ability of a multidimensional lifestyle intervention to prevent AD in at-risk older adults (age 60 to 79, with established metabolic and cardiovascular risk factors). Interventions include a combination of physical exercise, nutritional counseling and management, cognitive and social stimulation, and improved management of cardiovascular risk factors. Recruitment for this large-scale trial was estimated to begin in January 2019 (NCT03688126).75
On a practical basis, Desai et al13 have proposed a checklist (Table 213) that physicians can use in their routine consultations to improve primary prevention of AD among their older patients.
Bottom Line
Advise patients that pursuing a healthy lifestyle is a key to delaying or preventing Alzheimer’s disease. This involves managing cardiovascular risk factors and a combination of staying physically, mentally, socially, and spiritually active, in addition to adhering to a healthy diet such as the Mediterranean diet.
Related Resources
- Anderson K, Grossberg GT. Brain games to slow cognitive decline in Alzheimer’s disease. J Am Med Dir Assoc. 2014;15(8):536-537.
- Small G, Vorgan G. The memory prescription: Dr. Garry Small’s 14-day plan to keep your brain and body young. New York, NY: Hyperion; 2004.
- Small G, Vorgan G. The Alzheimer’s prevention program; keep your brain healthy for the rest of your life. New York, NY: Workman Publishing Company, Inc.; 2012.
Drug Brand Name
Curcumin • Theracurmin
1. Mehta D, Jackson R, Paul G, et al. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin Investig Drugs. 2017;26(6):735-739.
2. Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794.
3. Meng XF, Yu JT, Wang HF, et al. Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42(4):1295-1310.
4. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
5. Barnes DE, Yaffe Y. The projected impact of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828.
6. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464-472.
7. Ahlskog JE, Geda YE, Graff-Radford NR, et al. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876-884.
8. Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer Disease. JAMA. 2009;302(6):627-637.
9. Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705-711.
10. Smith PJ et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239-252.
11. Brown BM, Peiffer JJ, Taddei K, et al. Physical activity and amyloid-beta plasma and brain levels: results from the Australian imaging, biomarkers and lifestyle study of ageing. Mol Psychiatry. 2013;18(8):875-881.
12. Brown BM, Sohrabi HR, Taddei K, et al. Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer’s disease. Alzheimers Dement. 2017;13(11):1197-1206.
13. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
14. Centers for Disease Control and Prevention. Physical activity: how much physical activity do older adults need?
15. Garber CE, Blissmer B, Deschenes MR, et al; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-1359.
16. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113);2673-2734.
17. Valenzuela MJ, Sachdev P, Wen W, et al. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3(7):e2598. doi.org/10.1371/journal.pone.0002598.
18. Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59(12):1910-1914.
19. Wilson RS, Scherr PA, Schneider JA, et al. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911-1920.
20. Krell-Roesch J, Vemuri P, Pink A, et al. Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the apoe ε4 genotype. JAMA Neurol. 2017;74(3):332-338.
21. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508-2516.
22. Klein RM, Christie J, Parkvall M. Does multilingualism affect the incidence of Alzheimer’s disease?: a worldwide analysis by country. SSM Popul Health. 2016;2:463-467.
23. Grundy JG, Anderson JAE, Bialystok E. Neural correlates of cognitive processing in monolinguals and bilinguals. Ann N Y Acad Sci. 2017;1396(1):183-201.
24. Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234-240.
25. Amieva H, Stoykova R, Matharan F, et al. What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosom Med. 2010;72(9):905-911.
26. Kuiper JS, Oude Voshaar RC, Zuidema SU, et al. The relationship between social functioning and subjective memory complaints in older persons: a population-based longitudinal cohort study. Int J Geriatr Psychiatry. 2017;32(10):1059-1071.
27. Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98-104.
28. Marioni RE, Proust-Lima C, Amieva H, et al. Social activity, cognitive decline and dementia risk: a 20-year prospective cohort study. BMC Public Health. 2015;15:1089.
29. Wilson RS, Schneider JA, Boyle PA, et al. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085-2092.
30. Cai Z, Yan LJ, Ratka A. Telomere shortening and Alzheimer’s disease. Neuromolecular Med. 2013;15(1):25-48.
31. Wilson RS, Arnold SE, Schneider JA, et al. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143-153.
32. Epel E, Daubenmier J, Moskowitz JT, et al. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34-53.
33. Malinowski P, Moore AW, Mead Br, et al. Mindful aging: the effects of regular brief mindfulness practice on electrophysiological markers of cognitive and affective processing in older adults. Mindfulness (N Y). 2017;8(1):78-94.
34. Taren AA, Gianaros PJ, Greco CM, et al. Mindfulness meditation training and executive control network resting state functional connectivity: a randomized controlled trial. Psychosom Med. 2017;79(6):674-683.
35. Fountain-Zaragoza S, Prakash RS. Mindfulness training for healthy aging: impact on attention, well-being, and inflammation. Front in Aging Neurosci. 2017;9:11.
36. Eyre HA, Siddarth P, Acevedo B, et al. A randomized controlled trial of Kundalini yoga in mild cognitive impairment. Int Psychogeriatr. 2017;29(4):557-567.
37. Khalsa DS. Stress, meditation, and Alzheimer’s disease prevention: where the evidence stands. J Alzheimers Dis. 2015;48(1):1-12.
38. Berk L, van Boxtel M, van Os J. Can mindfulness-based interventions influence cognitive functioning in older adults? A review and considerations for future research. Aging Ment Health. 2017;21(11):1113-1120.
39. Hosseini S, Chaurasia A, Oremus M. The effect of religion and spirituality on cognitive function: a systematic review. Gerontologist. 2017. doi: 10.1093/geront/gnx024.
40. Luchsinger JA, Tang MX, Shea S, et al. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59(8):1258-1263.
41. Grant WB. Trends in diet and Alzheimer’s disease during the nutrition transition in Japan and developing countries. J Alzheimers Dis. 2014;38(3):611-620.
42. Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59-68.
43. Hu N, Yu JT, Tan L, et al. Nutrition and the risk of Alzheimer’s disease. Biomed Res Int. 2013;2013:524820. doi: 10.1155/2013/524820.
44. Taylor MK, Sullivan DK, Swerdlow RH, et al. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106(6):1463-1470.
45. van de Rest O, Berendsen AM, Haveman-Nies A, et al. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154-168.
46. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. 2016;7(5):889-904.
47. Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331-1338.
48. Wengreen H, Munger RG, Cutler A, et al. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County study on memory, health and aging. Am J Clin Nutr. 2013;98(5):1263-1271.
49. Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410-1416.
50. Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007-1014.
51. Desai AK, Rush J, Naveen L, et al. Nutrition and nutritional supplements to promote brain health. In: Hartman-Stein PE, Rue AL, eds. Enhancing cognitive fitness in adults: a guide to the use and development of community-based programs. New York, NY: Springer; 2011:249-269.
52. Goozee KG, Shah TM, Sohrabi HR, et al. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr. 2016;115(3):449-465.
53. Small GW, Siddarth P, Li Z, et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatry. 2018;26(3):266-277.
54. Kim JW, Lee DY, Lee BC, et al. Alcohol and cognition in the elderly: a review. Psychiatry Investig. 2012;9(1):8-16.
55. Virtaa JJ, Järvenpää T, Heikkilä K, et al. Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis. 2010;22(3):939-948.
56. Kerr WC, Stockwell T. Understanding standard drinks and drinking guidelines. Drug and Alcohol Rev. 2012;31(2):200-205.
57. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Accessed December 9, 2017.
58. National Institute on Alcohol Abuse and Alcoholism. What is a standard drink? https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink. Accessed November 9, 2017.
59. Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol. 2002;156(5):445-453.
60. Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris). 1997;153(3):185-192.
61. Topiwala A, Allan CL, Valkanova V, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357.
62. Venkataraman A, Kalk N, Sewell G, et al. Alcohol and Alzheimer’s disease-does alcohol dependence contribute to beta-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol. 2017;52(2):151-158.
63. Ma QP, Huang C, Cui QY, et al. Meta-analysis of the association between tea intake and the risk of cognitive disorders. PLoS One. 2016;11(11):e0165861. doi: 10.1371/journal.pone.0165861.
64. Santos C, Costa J, Santos J, et al. Caffeine intake and dementia: systematic review and meta-analysis. J Alzheimers Dis. 2010;20(Suppl 1):S187-204.
65. Panza F, Solfrizzi V, Barulli MR, et al. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J Nutr Health Aging. 2015;19(3):313-328.
66. Wierzejska R. Can coffee consumption lower the risk of Alzheimer’s disease and Parkinson’s disease? A literature review. Arch Med Sci. 2017;13(3):507-514.
67. Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis. 2010;20 (Suppl 1):S117-S126.
68. Eskelinen MH, Ngandu T, Tuomilehto J, et al. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16(1):85-91.
69. Solfrizzi V, Panza F, Imbimbo BP, et al. Coffee consumption habits and the risk of mild cognitive impairment: the Italian longitudinal study on aging. J Alzheimers Dis. 2015;47(4):889-899.
70. Vittoria Mattioli. Beverages of daily life: impact of caffeine on atrial fibrillation. J Atr Fibrillation. 2014;7(2):1133.
71. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;5:13.
72. Noguchi-Shinohara M, Yuki S, Dohmoto C, et al. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS One. 2014;9(5):e96013. doi: 10.1371/journal.pone.0096013.
73. Schneider N, Yvon C. A review of multidomain interventions to support healthy cognitive ageing. J Nutr Health Aging. 2013;17(3):252-257.
74. Ngandu T, Lehitsalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
75. U.S. National Library of Medicing. ClinicalTrials.gov. U.S. study to protect brain health through lifestyle intervention to reduce risk (POINTER). https://clinicaltrials.gov/ct2/show/NCT03688126?term=pointer&cond=Alzheimer+Disease&rank=1. Published September 28, 2018. Accessed November 3, 2018.
Clinicians have devoted strenuous efforts to secondary prevention of Alzheimer’s disease (AD) by diagnosing and treating patients as early as possible. Unfortunately, there is no cure for AD, and the field has witnessed recurrent failures of several pharmacotherapy candidates with either symptomatic or disease-modifying properties.1 An estimated one-third of AD cases can be attributed to modifiable risk factors.2 Thus, implementing primary prevention measures by addressing modifiable risk factors thought to contribute to the disease, with the goal of reducing the risk of developing AD, or at least delaying its onset, is a crucial public health strategy.
Cardiovascular risk factors, such as hypertension, hyperlipidemia, diabetes, hyperhomocysteinemia, obesity, and smoking, have emerged as substantive risk factors for AD.3 Optimal management of these major risk factors, especially in mid-life, may be a preventive approach against AD. Although detailing the evidence on the impact of managing cardiovascular risk factors to delay or prevent AD is beyond the scope of this article, it is becoming clear that “what is good for the heart is good for the brain.”
Additional modifiable risk factors are related to lifestyle habits, such as physical exercise, mental and social activity, meditation/spiritual activity, and diet. This article reviews the importance of pursuing a healthy lifestyle in delaying AD, with the corresponding levels of evidence that support each specific lifestyle modification. The levels of evidence are defined in Table 1.4
Physical exercise
Twenty-one percent of AD cases in the United States are attributable to physical inactivity.5 In addition to its beneficial effect on metabolic syndrome, in animal and human research, regular exercise has been shown to have direct neuroprotective effects. High levels of physical activity increase hippocampal neurogenesis and neuroplasticity, increase vascular circulation in the brain regions implicated in AD, and modulate inflammatory mediators as well as brain growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1).6
The definition of regular physical exercise varies across the literature, but usually implies aerobic exercise—an ongoing activity sufficient to increase the heart rate and the need for oxygen, sustained for 20 to 30 minutes per session.7 Modalities include household activities and leisure-time activities. In a large prospective cohort study, Scarmeas et al8 categorized leisure-time activities into 3 types:
- light (walking, dancing, calisthenics, golfing, bowling, gardening, horseback riding)
- moderate (bicycling, swimming, hiking, playing tennis)
- vigorous (aerobic dancing, jogging, playing handball).
These types of physical exercise were weighed by the frequency of participation per week. Compared with being physically inactive, low levels of weekly physical activity (0.1 hours of vigorous, 0.8 hours of moderate, or 1.3 hours of light exercise) were associated with a 29% to 41% lower risk of developing AD, while higher weekly physical activity (1.3 hours of vigorous, 2.3 hours of moderate, or 3.8 hours of light exercise) were associated with a 37% to 50% lower risk (level III).8
In another 20-year cohort study, engaging in leisure-time physical activity at least twice a week in mid-life was significantly associated with a reduced risk of AD, after adjusting for age, sex, education, follow-up time, locomotor disorders, apolipoprotein E (ApoE) genotype, vascular disorders, smoking, and alcohol intake (level III).9 Moreover, a systematic review of 29 randomized controlled trials (RCTs) showed that aerobic exercise training, such as brisk walking, jogging, and biking, was associated with improvements in attention, processing speed, executive function, and memory among healthy older adults and those with mild cognitive impairment (MCI; level IA).10
Continue to: From a pathophysiological standpoint...
From a pathophysiological standpoint, higher levels of physical exercise in cognitively intact older adults have been associated with reduced brain amyloid beta deposits, especially in ApoE4 carriers.11 This inverse relationship also has been demonstrated in patients who are presymptomatic who carry 1 of the 3 known autosomal dominant mutations for the familial forms of AD.12
Overall, physicians should recommend that patients—especially those with cardiovascular risk factors that increase their risk for AD—exercise regularly by following the guidelines of the American Heart Association or the American College of Sports Medicine.13 These include muscle-strengthening activities (legs, hips, back, abdomen, shoulders, and arms) at least 2 days/week, in addition to either 30 minutes/day of moderate-intensity aerobic activity such as brisk walking, 5 days/week; or 25 minutes of vigorous aerobic activity such as jogging and running, 3 days/week14 (level IA evidence for overall improvement in cognitive function; level III evidence for AD delay/risk reduction). Neuromotor exercise, such as yoga and tai chi, and flexibility exercise such as muscle stretching, especially after a hot bath, 2 to 3 days/week are also recommended (level III).15
Mental activity
Nineteen percent of AD cases worldwide and 7% in the United States. can be attributed to low educational attainment, which is associated with low brain cognitive reserve.5 Cognitive resilience in later life may be enhanced by building brain reserves through intellectual stimulation, which affects neuronal branching and plasticity.16 Higher levels of complex mental activities measured across the lifespan, such as education, occupation, reading, and writing, are correlated with significantly less hippocampal volume shrinkage over time.17 Frequent participation in mentally stimulating activities—such as listening to the radio; reading newspapers, magazines, or books; playing games (cards, checkers, crosswords or other puzzles); and visiting museums—was associated with an up to 64% reduction in the odds of developing AD in a cohort of cognitively intact older adults followed for 4 years.18 The correlation between mental activity and AD was found to be independent of physical activity, social activity, or baseline cognitive function.19
In a large cohort of cognitively intact older adults (mean age 70), engaging in a mentally stimulating activity (craft activities, computer use, or going to the theater/movies) once to twice a week was significantly associated with a reduced incidence of amnestic MCI.20 Another prospective 21-year study demonstrated a significant reduction in AD risk in community-dwelling cognitively intact older adults (age 75 to 85) who participated in cognitively stimulating activities, such as reading books or newspapers, writing for pleasure, doing crossword puzzles, playing board games or cards, or playing musical instruments, several times/week.21
Growing scientific evidence also suggests that lifelong multilingualism can delay AD onset by 4 to 5 years.22 Multilingualism is associated with greater cognitive reserve, gray matter volume, functional connectivity and white matter density.23
Continue to: Physicians should encourage their patients...
Physicians should encourage their patients to engage in intellectually stimulating activities and creative leisure-time activities several times/week to enhance their cognitive reserves and delay AD onset (level III evidence with respect to AD risk reduction/delay).
Social activity
Social engagement may be an additional protective factor against AD. In a large 4-year prospective study, increased loneliness in cognitively intact older adults doubled the risk of AD.24 Data from the large French cohort PAQUID (Personnes Agées QUID) emphasized the importance of a patient’s social network as a protective factor against AD. In this cohort, the perception of reciprocity in relationships with others (the perception that a person had received more than he or she had given) was associated with a 53% reduction in AD risk (level III).25 In another longitudinal cohort study, social activity was found to decrease the incidence of subjective cognitive decline, which is a prodromal syndrome for MCI and AD (level III).26
A major confounder in studies assessing for social activity is the uncertainty if social withdrawal is a modifiable risk factor or an early manifestation of AD, since apathetic patients with AD tend to be socially withdrawn.27 Another limitation of measuring the impact of social activity relative to AD risk is the difficulty in isolating social activities from activities that have physical and mental activity components, such as leisure-time activities.28
Meditation/spiritual activity
Chronic psychological stress is believed to compromise limbic structures that regulate stress-related behaviors and the memory network, which might explain how being prone to psychological distress may be associated with MCI or AD.29 Cognitive stress may increase the oxidative stress and telomere shortening implicated in the neurodegenerative processes of AD.30 In one study, participants who were highly prone to psychological distress were found to be at 3 times increased risk for developing AD, after adjusting for depression symptoms and physical and mental activities (level III).31 By reducing chronic psychological stress, meditation techniques offer a promising preventive option against AD.
Mindfulness-based interventions (MBI) have gained increased attention in the past decade. They entail directing one’s attention towards the present moment, thereby decreasing ruminative thoughts and stress arousal.32 Recent RCTs have shown that MBI may promote brain health in older adults not only by improving psychological well-being but also by improving attentional control33 and functional connectivity in brain regions implicated in executive functioning,34 as well as by modulating inflammatory processes implicated in AD.35 Furthermore, an RCT of patients diagnosed with MCI found that compared with memory enhancement training, a weekly 60-minute yoga session improved memory and executive functioning.36
Continue to: Kirtan Kriya is a medication technique...
Kirtan Kriya is a meditation technique that is easy to learn and practice by older adults and can improve memory in patients at risk for developing AD.37 However, more rigorous RCTs conducted in larger samples of older adults are needed to better evaluate the effect of all meditation techniques for delaying or preventing AD (level IB with respect to improvement in cognitive functioning/level III for AD delay/risk reduction).38
Spiritual activities, such as going to places of worship or religious meditation, have been associated with a lower prevalence of AD. Attending religious services, gatherings, or retreats involves a social component because these activities often are practiced in groups. They also confer a method of dealing with psychological distress and depression. Additionally, frequent readings of religious texts represents a mentally stimulating activity that may also contribute to delaying/preventing AD (level III).39
Diet
In the past decade, a growing body of evidence has linked diet to cognition. Individuals with a higher intake of calories and fat are at higher risk for developing AD.40 The incidence of AD rose in Japan after the country transitioned to a more Westernized diet.41 A modern Western diet rich in saturated fatty acids and simple carbohydrates may negatively impact hippocampus-mediated functions such as memory and learning, and is associated with an increased risk of AD.42 In contrast with high-glycemic and fatty diets, a “healthy diet” is associated with a decrease in beta-amyloid burden, inflammation, and oxidative stress.43,44
Studies focusing on dietary patterns rather than a single nutrient for delaying or preventing AD have yielded more robust and consistent results.45 In a recent meta-analysis, adhering to a Mediterranean diet—which is rich in fruits and vegetables, whole grains, olive oil, and fish; moderate in some dairy products and wine; and low in red meat—was associated with a decreased risk of AD; this evidence was derived mostly from epidemiologic studies.46 Scarmeas et al8 found that high adherence to the Mediterranean diet was associated with 32% to 40% reduced risk of AD. Combining this diet with physical exercise was associated with an up to 67% reduced risk (level III). The Dietary Approaches to Stop Hypertension (DASH) diet, which is rich in total grains, fruits, vegetables, and dairy products, but low in sodium and sweets, correlated with neurocognitive improvement in patients with hypertension.47 Both the Mediterranean and DASH diets have been associated with better cognitive function48 and slower cognitive decline.49 Thus, an attempt to combine the neuroprotective components from both diets led to the creation of the MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) diet, which also has been associated with a lower incidence of AD.50
Besides specific diets, some food groups have also been found to promote brain health and may help delay or prevent AD. Berries have the highest amount of antioxidants of all fruit. Among vegetables, tomatoes and green leafy vegetables have the highest amount of nutrients for the brain. Nuts, such as walnuts, which are rich in omega-3 fatty acids, are also considered “power foods” for the brain; however, they should be consumed in moderation because they are also rich in fat. Monounsaturated fatty acids, which are found in olives and olive oil, are also beneficial for the brain. Among the 3 types of omega-3 fatty acids, the most important for cognition is docosahexaenoic acid (DHA) because it constitutes 40% of all fatty acids in the brain. Mainly found in oily fish, DHA has antioxidant and anti-inflammatory properties that may delay or prevent AD. Low levels of DHA have been found in patients with AD.51
Continue to: Curcumin, which is derived from...
Curcumin, which is derived from the curry spice turmeric, is a polyphenol with anti-inflammatory, antioxidant, and anti-amyloid properties that may have a promising role in preventing AD in cognitively intact individuals. Initial trials with curcumin have yielded mixed results on cognition, which was partly related to the low solubility and bioavailability of its formulation.52 However, a recent 18-month double-blind randomized placebo-controlled trial found positive effects on memory and attention, as well as reduction of amyloid plaques and tau tangles deposition in the brain, in non-demented older adults age 51 to 84 who took Theracumin, a highly absorptive oral form of curcumin dispersed with colloidal nanoparticles.53 A longer follow-up is required to determine if curcumin can delay or prevent AD.
Alcohol
The role of alcohol in AD prevention is controversial. Overall, data from prospective studies has shown that low to moderate alcohol consumption may be associated with a reduced risk of AD (level III).54 Alcohol drinking in mid-life showed a U-shaped relationship with cognitive impairment; both abstainers and heavy drinkers had an increased risk of cognitive decline compared with light to moderate drinkers (level III).55 Binge drinking significantly increased the odds of cognitive decline, even after controlling for total alcohol consumption per week.55
The definition of low-to-moderate drinking varies substantially among countries. In addition, the size and amount of alcohol contained in a standard drink may differ.56 According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA),57 moderate drinking is defined as up to 1 drink daily for women and 2 drinks daily for men. Binge drinking involves drinking >4 drinks for women and >5 drinks for men, in approximately 2 hours, at least monthly. In the United States, one standard drink contains 14 grams of pure alcohol, which is usually found in 12 ounces of regular beer, 5 ounces of wine, and 1.5 ounces of distilled spirits (vodka or whiskey).58
In a 5-year prospective Canadian study, having 1 drink weekly (especially wine) was associated with an up to 50% reduced risk of AD (level III).59 In the French cohort PAQUID, mild drinkers (<1 to 2 drinks/day) and moderate drinkers (3 to 4 drinks daily) had a reduced incidence of AD compared with non-drinkers. Wine was the most frequently consumed beverage in this study.60 Other studies have found cognitive benefits from mild to moderate drinking regardless of beverage type.54 However, a recent study that included a 30-year follow-up failed to find a significant protective effect of light drinking over abstinence in terms of hippocampal atrophy.61 Atrophy of the hippocampus was correlated with increasing alcohol amounts in a dose-dependent manner, starting at 7 to 14 drinks/week (level III).61
Research has shown that moderate and heavy alcohol use or misuse can directly induce microglial activation and inflammatory mediators’ release, which induce amyloid beta pathology and leads to brain atrophy.62 Hence, non-drinkers should not be advised to begin drinking, because of the lack of RCTs and the concern that beginning to drink may lead to heavy drinking. All drinkers should be advised to adhere to the NIAAA recommendations.13
Continue to: Coffee/tea
Coffee/tea
Although studies of caffeinated coffee have been heterogeneous and yielded mixed results (beneficial effect vs no effect on delaying cognitive decline), systematic reviews and meta-analyses of cross-sectional, case-control, and longitudinal cohort studies have found a general trend towards a favorable preventive role (level III).63-65 Caffeine exhibits its neuroprotective effect by increasing brain serotonin and acetylcholine, and by stabilizing blood-brain-barrier integrity.66 Moreover, in an animal study, mice given caffeine in their drinking water from young adulthood into older age had lower amyloid beta plasma levels compared with those given decaffeinated water.67 These findings suggest that in humans, 5 cups of regular caffeinated coffee daily, equivalent to 500 mg of caffeine,
An Italian study showed that older adults who don’t or rarely drink coffee (<1 cup daily) and those who recently increased their consumption pattern to >1 cup daily had a higher incidence of MCI than those who habitually consumed 1 to 2 cups daily.69 Therefore, it is not recommended to advise a change in coffee drinking pattern in old age. Older adults who are coffee drinkers should, however, be educated about the association between heavier caffeine intake and anxiety, insomnia, and cardiac arrhythmias.70
Despite its more modest caffeine levels, green tea is rich in polyphenols, which belong to the family of catechins and are characterized by antioxidant and anti-inflammatory properties.71 In a Japanese cohort, higher green tea consumption (up to 1 cup daily) was associated with a decreased incidence of MCI in older adults.72 More studies are needed to confirm its potential preventative role in AD.
Which lifestyle change is the most important?
Focusing on a single lifestyle change may be insufficient, especially because the bulk of evidence for individual interventions comes from population-based cohort studies (level III), rather than strong RCTs with a long follow-up. There is increasing evidence that combining multiple lifestyle modifications may yield better outcomes in maintaining or improving cognition.73
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a large, 2-year RCT that included community-dwelling older adults (age 60 to 77) with no diagnosis of major neurocognitive disorder, found that compared with regular health advice, multi-domain interventions reduced cognitive decline and improved overall cognition, executive functioning, and processing speed. The interventions evaluated in this study combined the following 4 modalities74:
- a healthy diet according to the Finnish nutrition recommendations (eating vegetables, fruits, and berries [minimum: 500 g/d], whole grain cereals [several times a day], and fish [2 to 3 times/week]; using low-salt products; consuming fat-free or low-fat milk products; and limiting red meat consumption to <500 g/week
- regular physical exercise tailored for improving muscle strength (1 to 3 times/week) coupled with aerobic exercise (2 to 5 times/week)
- cognitive training, including group sessions that have a social activity component and computer-based individual sessions 3 times/week that target episodic and working memory and executive functioning
- optimal management of cardiovascular risk factors.
Continue to: This multi-domain approach...
This multi-domain approach for lifestyle modification should be strongly recommended to cognitively intact older patients (level IB).
Modeled after the FINGER study, the Alzheimer’s Association U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER) is a 2-year, multicenter, controlled clinical trial aimed at testing the ability of a multidimensional lifestyle intervention to prevent AD in at-risk older adults (age 60 to 79, with established metabolic and cardiovascular risk factors). Interventions include a combination of physical exercise, nutritional counseling and management, cognitive and social stimulation, and improved management of cardiovascular risk factors. Recruitment for this large-scale trial was estimated to begin in January 2019 (NCT03688126).75
On a practical basis, Desai et al13 have proposed a checklist (Table 213) that physicians can use in their routine consultations to improve primary prevention of AD among their older patients.
Bottom Line
Advise patients that pursuing a healthy lifestyle is a key to delaying or preventing Alzheimer’s disease. This involves managing cardiovascular risk factors and a combination of staying physically, mentally, socially, and spiritually active, in addition to adhering to a healthy diet such as the Mediterranean diet.
Related Resources
- Anderson K, Grossberg GT. Brain games to slow cognitive decline in Alzheimer’s disease. J Am Med Dir Assoc. 2014;15(8):536-537.
- Small G, Vorgan G. The memory prescription: Dr. Garry Small’s 14-day plan to keep your brain and body young. New York, NY: Hyperion; 2004.
- Small G, Vorgan G. The Alzheimer’s prevention program; keep your brain healthy for the rest of your life. New York, NY: Workman Publishing Company, Inc.; 2012.
Drug Brand Name
Curcumin • Theracurmin
Clinicians have devoted strenuous efforts to secondary prevention of Alzheimer’s disease (AD) by diagnosing and treating patients as early as possible. Unfortunately, there is no cure for AD, and the field has witnessed recurrent failures of several pharmacotherapy candidates with either symptomatic or disease-modifying properties.1 An estimated one-third of AD cases can be attributed to modifiable risk factors.2 Thus, implementing primary prevention measures by addressing modifiable risk factors thought to contribute to the disease, with the goal of reducing the risk of developing AD, or at least delaying its onset, is a crucial public health strategy.
Cardiovascular risk factors, such as hypertension, hyperlipidemia, diabetes, hyperhomocysteinemia, obesity, and smoking, have emerged as substantive risk factors for AD.3 Optimal management of these major risk factors, especially in mid-life, may be a preventive approach against AD. Although detailing the evidence on the impact of managing cardiovascular risk factors to delay or prevent AD is beyond the scope of this article, it is becoming clear that “what is good for the heart is good for the brain.”
Additional modifiable risk factors are related to lifestyle habits, such as physical exercise, mental and social activity, meditation/spiritual activity, and diet. This article reviews the importance of pursuing a healthy lifestyle in delaying AD, with the corresponding levels of evidence that support each specific lifestyle modification. The levels of evidence are defined in Table 1.4
Physical exercise
Twenty-one percent of AD cases in the United States are attributable to physical inactivity.5 In addition to its beneficial effect on metabolic syndrome, in animal and human research, regular exercise has been shown to have direct neuroprotective effects. High levels of physical activity increase hippocampal neurogenesis and neuroplasticity, increase vascular circulation in the brain regions implicated in AD, and modulate inflammatory mediators as well as brain growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1).6
The definition of regular physical exercise varies across the literature, but usually implies aerobic exercise—an ongoing activity sufficient to increase the heart rate and the need for oxygen, sustained for 20 to 30 minutes per session.7 Modalities include household activities and leisure-time activities. In a large prospective cohort study, Scarmeas et al8 categorized leisure-time activities into 3 types:
- light (walking, dancing, calisthenics, golfing, bowling, gardening, horseback riding)
- moderate (bicycling, swimming, hiking, playing tennis)
- vigorous (aerobic dancing, jogging, playing handball).
These types of physical exercise were weighed by the frequency of participation per week. Compared with being physically inactive, low levels of weekly physical activity (0.1 hours of vigorous, 0.8 hours of moderate, or 1.3 hours of light exercise) were associated with a 29% to 41% lower risk of developing AD, while higher weekly physical activity (1.3 hours of vigorous, 2.3 hours of moderate, or 3.8 hours of light exercise) were associated with a 37% to 50% lower risk (level III).8
In another 20-year cohort study, engaging in leisure-time physical activity at least twice a week in mid-life was significantly associated with a reduced risk of AD, after adjusting for age, sex, education, follow-up time, locomotor disorders, apolipoprotein E (ApoE) genotype, vascular disorders, smoking, and alcohol intake (level III).9 Moreover, a systematic review of 29 randomized controlled trials (RCTs) showed that aerobic exercise training, such as brisk walking, jogging, and biking, was associated with improvements in attention, processing speed, executive function, and memory among healthy older adults and those with mild cognitive impairment (MCI; level IA).10
Continue to: From a pathophysiological standpoint...
From a pathophysiological standpoint, higher levels of physical exercise in cognitively intact older adults have been associated with reduced brain amyloid beta deposits, especially in ApoE4 carriers.11 This inverse relationship also has been demonstrated in patients who are presymptomatic who carry 1 of the 3 known autosomal dominant mutations for the familial forms of AD.12
Overall, physicians should recommend that patients—especially those with cardiovascular risk factors that increase their risk for AD—exercise regularly by following the guidelines of the American Heart Association or the American College of Sports Medicine.13 These include muscle-strengthening activities (legs, hips, back, abdomen, shoulders, and arms) at least 2 days/week, in addition to either 30 minutes/day of moderate-intensity aerobic activity such as brisk walking, 5 days/week; or 25 minutes of vigorous aerobic activity such as jogging and running, 3 days/week14 (level IA evidence for overall improvement in cognitive function; level III evidence for AD delay/risk reduction). Neuromotor exercise, such as yoga and tai chi, and flexibility exercise such as muscle stretching, especially after a hot bath, 2 to 3 days/week are also recommended (level III).15
Mental activity
Nineteen percent of AD cases worldwide and 7% in the United States. can be attributed to low educational attainment, which is associated with low brain cognitive reserve.5 Cognitive resilience in later life may be enhanced by building brain reserves through intellectual stimulation, which affects neuronal branching and plasticity.16 Higher levels of complex mental activities measured across the lifespan, such as education, occupation, reading, and writing, are correlated with significantly less hippocampal volume shrinkage over time.17 Frequent participation in mentally stimulating activities—such as listening to the radio; reading newspapers, magazines, or books; playing games (cards, checkers, crosswords or other puzzles); and visiting museums—was associated with an up to 64% reduction in the odds of developing AD in a cohort of cognitively intact older adults followed for 4 years.18 The correlation between mental activity and AD was found to be independent of physical activity, social activity, or baseline cognitive function.19
In a large cohort of cognitively intact older adults (mean age 70), engaging in a mentally stimulating activity (craft activities, computer use, or going to the theater/movies) once to twice a week was significantly associated with a reduced incidence of amnestic MCI.20 Another prospective 21-year study demonstrated a significant reduction in AD risk in community-dwelling cognitively intact older adults (age 75 to 85) who participated in cognitively stimulating activities, such as reading books or newspapers, writing for pleasure, doing crossword puzzles, playing board games or cards, or playing musical instruments, several times/week.21
Growing scientific evidence also suggests that lifelong multilingualism can delay AD onset by 4 to 5 years.22 Multilingualism is associated with greater cognitive reserve, gray matter volume, functional connectivity and white matter density.23
Continue to: Physicians should encourage their patients...
Physicians should encourage their patients to engage in intellectually stimulating activities and creative leisure-time activities several times/week to enhance their cognitive reserves and delay AD onset (level III evidence with respect to AD risk reduction/delay).
Social activity
Social engagement may be an additional protective factor against AD. In a large 4-year prospective study, increased loneliness in cognitively intact older adults doubled the risk of AD.24 Data from the large French cohort PAQUID (Personnes Agées QUID) emphasized the importance of a patient’s social network as a protective factor against AD. In this cohort, the perception of reciprocity in relationships with others (the perception that a person had received more than he or she had given) was associated with a 53% reduction in AD risk (level III).25 In another longitudinal cohort study, social activity was found to decrease the incidence of subjective cognitive decline, which is a prodromal syndrome for MCI and AD (level III).26
A major confounder in studies assessing for social activity is the uncertainty if social withdrawal is a modifiable risk factor or an early manifestation of AD, since apathetic patients with AD tend to be socially withdrawn.27 Another limitation of measuring the impact of social activity relative to AD risk is the difficulty in isolating social activities from activities that have physical and mental activity components, such as leisure-time activities.28
Meditation/spiritual activity
Chronic psychological stress is believed to compromise limbic structures that regulate stress-related behaviors and the memory network, which might explain how being prone to psychological distress may be associated with MCI or AD.29 Cognitive stress may increase the oxidative stress and telomere shortening implicated in the neurodegenerative processes of AD.30 In one study, participants who were highly prone to psychological distress were found to be at 3 times increased risk for developing AD, after adjusting for depression symptoms and physical and mental activities (level III).31 By reducing chronic psychological stress, meditation techniques offer a promising preventive option against AD.
Mindfulness-based interventions (MBI) have gained increased attention in the past decade. They entail directing one’s attention towards the present moment, thereby decreasing ruminative thoughts and stress arousal.32 Recent RCTs have shown that MBI may promote brain health in older adults not only by improving psychological well-being but also by improving attentional control33 and functional connectivity in brain regions implicated in executive functioning,34 as well as by modulating inflammatory processes implicated in AD.35 Furthermore, an RCT of patients diagnosed with MCI found that compared with memory enhancement training, a weekly 60-minute yoga session improved memory and executive functioning.36
Continue to: Kirtan Kriya is a medication technique...
Kirtan Kriya is a meditation technique that is easy to learn and practice by older adults and can improve memory in patients at risk for developing AD.37 However, more rigorous RCTs conducted in larger samples of older adults are needed to better evaluate the effect of all meditation techniques for delaying or preventing AD (level IB with respect to improvement in cognitive functioning/level III for AD delay/risk reduction).38
Spiritual activities, such as going to places of worship or religious meditation, have been associated with a lower prevalence of AD. Attending religious services, gatherings, or retreats involves a social component because these activities often are practiced in groups. They also confer a method of dealing with psychological distress and depression. Additionally, frequent readings of religious texts represents a mentally stimulating activity that may also contribute to delaying/preventing AD (level III).39
Diet
In the past decade, a growing body of evidence has linked diet to cognition. Individuals with a higher intake of calories and fat are at higher risk for developing AD.40 The incidence of AD rose in Japan after the country transitioned to a more Westernized diet.41 A modern Western diet rich in saturated fatty acids and simple carbohydrates may negatively impact hippocampus-mediated functions such as memory and learning, and is associated with an increased risk of AD.42 In contrast with high-glycemic and fatty diets, a “healthy diet” is associated with a decrease in beta-amyloid burden, inflammation, and oxidative stress.43,44
Studies focusing on dietary patterns rather than a single nutrient for delaying or preventing AD have yielded more robust and consistent results.45 In a recent meta-analysis, adhering to a Mediterranean diet—which is rich in fruits and vegetables, whole grains, olive oil, and fish; moderate in some dairy products and wine; and low in red meat—was associated with a decreased risk of AD; this evidence was derived mostly from epidemiologic studies.46 Scarmeas et al8 found that high adherence to the Mediterranean diet was associated with 32% to 40% reduced risk of AD. Combining this diet with physical exercise was associated with an up to 67% reduced risk (level III). The Dietary Approaches to Stop Hypertension (DASH) diet, which is rich in total grains, fruits, vegetables, and dairy products, but low in sodium and sweets, correlated with neurocognitive improvement in patients with hypertension.47 Both the Mediterranean and DASH diets have been associated with better cognitive function48 and slower cognitive decline.49 Thus, an attempt to combine the neuroprotective components from both diets led to the creation of the MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) diet, which also has been associated with a lower incidence of AD.50
Besides specific diets, some food groups have also been found to promote brain health and may help delay or prevent AD. Berries have the highest amount of antioxidants of all fruit. Among vegetables, tomatoes and green leafy vegetables have the highest amount of nutrients for the brain. Nuts, such as walnuts, which are rich in omega-3 fatty acids, are also considered “power foods” for the brain; however, they should be consumed in moderation because they are also rich in fat. Monounsaturated fatty acids, which are found in olives and olive oil, are also beneficial for the brain. Among the 3 types of omega-3 fatty acids, the most important for cognition is docosahexaenoic acid (DHA) because it constitutes 40% of all fatty acids in the brain. Mainly found in oily fish, DHA has antioxidant and anti-inflammatory properties that may delay or prevent AD. Low levels of DHA have been found in patients with AD.51
Continue to: Curcumin, which is derived from...
Curcumin, which is derived from the curry spice turmeric, is a polyphenol with anti-inflammatory, antioxidant, and anti-amyloid properties that may have a promising role in preventing AD in cognitively intact individuals. Initial trials with curcumin have yielded mixed results on cognition, which was partly related to the low solubility and bioavailability of its formulation.52 However, a recent 18-month double-blind randomized placebo-controlled trial found positive effects on memory and attention, as well as reduction of amyloid plaques and tau tangles deposition in the brain, in non-demented older adults age 51 to 84 who took Theracumin, a highly absorptive oral form of curcumin dispersed with colloidal nanoparticles.53 A longer follow-up is required to determine if curcumin can delay or prevent AD.
Alcohol
The role of alcohol in AD prevention is controversial. Overall, data from prospective studies has shown that low to moderate alcohol consumption may be associated with a reduced risk of AD (level III).54 Alcohol drinking in mid-life showed a U-shaped relationship with cognitive impairment; both abstainers and heavy drinkers had an increased risk of cognitive decline compared with light to moderate drinkers (level III).55 Binge drinking significantly increased the odds of cognitive decline, even after controlling for total alcohol consumption per week.55
The definition of low-to-moderate drinking varies substantially among countries. In addition, the size and amount of alcohol contained in a standard drink may differ.56 According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA),57 moderate drinking is defined as up to 1 drink daily for women and 2 drinks daily for men. Binge drinking involves drinking >4 drinks for women and >5 drinks for men, in approximately 2 hours, at least monthly. In the United States, one standard drink contains 14 grams of pure alcohol, which is usually found in 12 ounces of regular beer, 5 ounces of wine, and 1.5 ounces of distilled spirits (vodka or whiskey).58
In a 5-year prospective Canadian study, having 1 drink weekly (especially wine) was associated with an up to 50% reduced risk of AD (level III).59 In the French cohort PAQUID, mild drinkers (<1 to 2 drinks/day) and moderate drinkers (3 to 4 drinks daily) had a reduced incidence of AD compared with non-drinkers. Wine was the most frequently consumed beverage in this study.60 Other studies have found cognitive benefits from mild to moderate drinking regardless of beverage type.54 However, a recent study that included a 30-year follow-up failed to find a significant protective effect of light drinking over abstinence in terms of hippocampal atrophy.61 Atrophy of the hippocampus was correlated with increasing alcohol amounts in a dose-dependent manner, starting at 7 to 14 drinks/week (level III).61
Research has shown that moderate and heavy alcohol use or misuse can directly induce microglial activation and inflammatory mediators’ release, which induce amyloid beta pathology and leads to brain atrophy.62 Hence, non-drinkers should not be advised to begin drinking, because of the lack of RCTs and the concern that beginning to drink may lead to heavy drinking. All drinkers should be advised to adhere to the NIAAA recommendations.13
Continue to: Coffee/tea
Coffee/tea
Although studies of caffeinated coffee have been heterogeneous and yielded mixed results (beneficial effect vs no effect on delaying cognitive decline), systematic reviews and meta-analyses of cross-sectional, case-control, and longitudinal cohort studies have found a general trend towards a favorable preventive role (level III).63-65 Caffeine exhibits its neuroprotective effect by increasing brain serotonin and acetylcholine, and by stabilizing blood-brain-barrier integrity.66 Moreover, in an animal study, mice given caffeine in their drinking water from young adulthood into older age had lower amyloid beta plasma levels compared with those given decaffeinated water.67 These findings suggest that in humans, 5 cups of regular caffeinated coffee daily, equivalent to 500 mg of caffeine,
An Italian study showed that older adults who don’t or rarely drink coffee (<1 cup daily) and those who recently increased their consumption pattern to >1 cup daily had a higher incidence of MCI than those who habitually consumed 1 to 2 cups daily.69 Therefore, it is not recommended to advise a change in coffee drinking pattern in old age. Older adults who are coffee drinkers should, however, be educated about the association between heavier caffeine intake and anxiety, insomnia, and cardiac arrhythmias.70
Despite its more modest caffeine levels, green tea is rich in polyphenols, which belong to the family of catechins and are characterized by antioxidant and anti-inflammatory properties.71 In a Japanese cohort, higher green tea consumption (up to 1 cup daily) was associated with a decreased incidence of MCI in older adults.72 More studies are needed to confirm its potential preventative role in AD.
Which lifestyle change is the most important?
Focusing on a single lifestyle change may be insufficient, especially because the bulk of evidence for individual interventions comes from population-based cohort studies (level III), rather than strong RCTs with a long follow-up. There is increasing evidence that combining multiple lifestyle modifications may yield better outcomes in maintaining or improving cognition.73
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a large, 2-year RCT that included community-dwelling older adults (age 60 to 77) with no diagnosis of major neurocognitive disorder, found that compared with regular health advice, multi-domain interventions reduced cognitive decline and improved overall cognition, executive functioning, and processing speed. The interventions evaluated in this study combined the following 4 modalities74:
- a healthy diet according to the Finnish nutrition recommendations (eating vegetables, fruits, and berries [minimum: 500 g/d], whole grain cereals [several times a day], and fish [2 to 3 times/week]; using low-salt products; consuming fat-free or low-fat milk products; and limiting red meat consumption to <500 g/week
- regular physical exercise tailored for improving muscle strength (1 to 3 times/week) coupled with aerobic exercise (2 to 5 times/week)
- cognitive training, including group sessions that have a social activity component and computer-based individual sessions 3 times/week that target episodic and working memory and executive functioning
- optimal management of cardiovascular risk factors.
Continue to: This multi-domain approach...
This multi-domain approach for lifestyle modification should be strongly recommended to cognitively intact older patients (level IB).
Modeled after the FINGER study, the Alzheimer’s Association U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER) is a 2-year, multicenter, controlled clinical trial aimed at testing the ability of a multidimensional lifestyle intervention to prevent AD in at-risk older adults (age 60 to 79, with established metabolic and cardiovascular risk factors). Interventions include a combination of physical exercise, nutritional counseling and management, cognitive and social stimulation, and improved management of cardiovascular risk factors. Recruitment for this large-scale trial was estimated to begin in January 2019 (NCT03688126).75
On a practical basis, Desai et al13 have proposed a checklist (Table 213) that physicians can use in their routine consultations to improve primary prevention of AD among their older patients.
Bottom Line
Advise patients that pursuing a healthy lifestyle is a key to delaying or preventing Alzheimer’s disease. This involves managing cardiovascular risk factors and a combination of staying physically, mentally, socially, and spiritually active, in addition to adhering to a healthy diet such as the Mediterranean diet.
Related Resources
- Anderson K, Grossberg GT. Brain games to slow cognitive decline in Alzheimer’s disease. J Am Med Dir Assoc. 2014;15(8):536-537.
- Small G, Vorgan G. The memory prescription: Dr. Garry Small’s 14-day plan to keep your brain and body young. New York, NY: Hyperion; 2004.
- Small G, Vorgan G. The Alzheimer’s prevention program; keep your brain healthy for the rest of your life. New York, NY: Workman Publishing Company, Inc.; 2012.
Drug Brand Name
Curcumin • Theracurmin
1. Mehta D, Jackson R, Paul G, et al. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin Investig Drugs. 2017;26(6):735-739.
2. Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794.
3. Meng XF, Yu JT, Wang HF, et al. Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42(4):1295-1310.
4. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
5. Barnes DE, Yaffe Y. The projected impact of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828.
6. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464-472.
7. Ahlskog JE, Geda YE, Graff-Radford NR, et al. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876-884.
8. Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer Disease. JAMA. 2009;302(6):627-637.
9. Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705-711.
10. Smith PJ et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239-252.
11. Brown BM, Peiffer JJ, Taddei K, et al. Physical activity and amyloid-beta plasma and brain levels: results from the Australian imaging, biomarkers and lifestyle study of ageing. Mol Psychiatry. 2013;18(8):875-881.
12. Brown BM, Sohrabi HR, Taddei K, et al. Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer’s disease. Alzheimers Dement. 2017;13(11):1197-1206.
13. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
14. Centers for Disease Control and Prevention. Physical activity: how much physical activity do older adults need?
15. Garber CE, Blissmer B, Deschenes MR, et al; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-1359.
16. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113);2673-2734.
17. Valenzuela MJ, Sachdev P, Wen W, et al. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3(7):e2598. doi.org/10.1371/journal.pone.0002598.
18. Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59(12):1910-1914.
19. Wilson RS, Scherr PA, Schneider JA, et al. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911-1920.
20. Krell-Roesch J, Vemuri P, Pink A, et al. Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the apoe ε4 genotype. JAMA Neurol. 2017;74(3):332-338.
21. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508-2516.
22. Klein RM, Christie J, Parkvall M. Does multilingualism affect the incidence of Alzheimer’s disease?: a worldwide analysis by country. SSM Popul Health. 2016;2:463-467.
23. Grundy JG, Anderson JAE, Bialystok E. Neural correlates of cognitive processing in monolinguals and bilinguals. Ann N Y Acad Sci. 2017;1396(1):183-201.
24. Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234-240.
25. Amieva H, Stoykova R, Matharan F, et al. What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosom Med. 2010;72(9):905-911.
26. Kuiper JS, Oude Voshaar RC, Zuidema SU, et al. The relationship between social functioning and subjective memory complaints in older persons: a population-based longitudinal cohort study. Int J Geriatr Psychiatry. 2017;32(10):1059-1071.
27. Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98-104.
28. Marioni RE, Proust-Lima C, Amieva H, et al. Social activity, cognitive decline and dementia risk: a 20-year prospective cohort study. BMC Public Health. 2015;15:1089.
29. Wilson RS, Schneider JA, Boyle PA, et al. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085-2092.
30. Cai Z, Yan LJ, Ratka A. Telomere shortening and Alzheimer’s disease. Neuromolecular Med. 2013;15(1):25-48.
31. Wilson RS, Arnold SE, Schneider JA, et al. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143-153.
32. Epel E, Daubenmier J, Moskowitz JT, et al. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34-53.
33. Malinowski P, Moore AW, Mead Br, et al. Mindful aging: the effects of regular brief mindfulness practice on electrophysiological markers of cognitive and affective processing in older adults. Mindfulness (N Y). 2017;8(1):78-94.
34. Taren AA, Gianaros PJ, Greco CM, et al. Mindfulness meditation training and executive control network resting state functional connectivity: a randomized controlled trial. Psychosom Med. 2017;79(6):674-683.
35. Fountain-Zaragoza S, Prakash RS. Mindfulness training for healthy aging: impact on attention, well-being, and inflammation. Front in Aging Neurosci. 2017;9:11.
36. Eyre HA, Siddarth P, Acevedo B, et al. A randomized controlled trial of Kundalini yoga in mild cognitive impairment. Int Psychogeriatr. 2017;29(4):557-567.
37. Khalsa DS. Stress, meditation, and Alzheimer’s disease prevention: where the evidence stands. J Alzheimers Dis. 2015;48(1):1-12.
38. Berk L, van Boxtel M, van Os J. Can mindfulness-based interventions influence cognitive functioning in older adults? A review and considerations for future research. Aging Ment Health. 2017;21(11):1113-1120.
39. Hosseini S, Chaurasia A, Oremus M. The effect of religion and spirituality on cognitive function: a systematic review. Gerontologist. 2017. doi: 10.1093/geront/gnx024.
40. Luchsinger JA, Tang MX, Shea S, et al. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59(8):1258-1263.
41. Grant WB. Trends in diet and Alzheimer’s disease during the nutrition transition in Japan and developing countries. J Alzheimers Dis. 2014;38(3):611-620.
42. Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59-68.
43. Hu N, Yu JT, Tan L, et al. Nutrition and the risk of Alzheimer’s disease. Biomed Res Int. 2013;2013:524820. doi: 10.1155/2013/524820.
44. Taylor MK, Sullivan DK, Swerdlow RH, et al. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106(6):1463-1470.
45. van de Rest O, Berendsen AM, Haveman-Nies A, et al. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154-168.
46. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. 2016;7(5):889-904.
47. Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331-1338.
48. Wengreen H, Munger RG, Cutler A, et al. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County study on memory, health and aging. Am J Clin Nutr. 2013;98(5):1263-1271.
49. Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410-1416.
50. Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007-1014.
51. Desai AK, Rush J, Naveen L, et al. Nutrition and nutritional supplements to promote brain health. In: Hartman-Stein PE, Rue AL, eds. Enhancing cognitive fitness in adults: a guide to the use and development of community-based programs. New York, NY: Springer; 2011:249-269.
52. Goozee KG, Shah TM, Sohrabi HR, et al. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr. 2016;115(3):449-465.
53. Small GW, Siddarth P, Li Z, et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatry. 2018;26(3):266-277.
54. Kim JW, Lee DY, Lee BC, et al. Alcohol and cognition in the elderly: a review. Psychiatry Investig. 2012;9(1):8-16.
55. Virtaa JJ, Järvenpää T, Heikkilä K, et al. Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis. 2010;22(3):939-948.
56. Kerr WC, Stockwell T. Understanding standard drinks and drinking guidelines. Drug and Alcohol Rev. 2012;31(2):200-205.
57. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Accessed December 9, 2017.
58. National Institute on Alcohol Abuse and Alcoholism. What is a standard drink? https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink. Accessed November 9, 2017.
59. Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol. 2002;156(5):445-453.
60. Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris). 1997;153(3):185-192.
61. Topiwala A, Allan CL, Valkanova V, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357.
62. Venkataraman A, Kalk N, Sewell G, et al. Alcohol and Alzheimer’s disease-does alcohol dependence contribute to beta-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol. 2017;52(2):151-158.
63. Ma QP, Huang C, Cui QY, et al. Meta-analysis of the association between tea intake and the risk of cognitive disorders. PLoS One. 2016;11(11):e0165861. doi: 10.1371/journal.pone.0165861.
64. Santos C, Costa J, Santos J, et al. Caffeine intake and dementia: systematic review and meta-analysis. J Alzheimers Dis. 2010;20(Suppl 1):S187-204.
65. Panza F, Solfrizzi V, Barulli MR, et al. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J Nutr Health Aging. 2015;19(3):313-328.
66. Wierzejska R. Can coffee consumption lower the risk of Alzheimer’s disease and Parkinson’s disease? A literature review. Arch Med Sci. 2017;13(3):507-514.
67. Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis. 2010;20 (Suppl 1):S117-S126.
68. Eskelinen MH, Ngandu T, Tuomilehto J, et al. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16(1):85-91.
69. Solfrizzi V, Panza F, Imbimbo BP, et al. Coffee consumption habits and the risk of mild cognitive impairment: the Italian longitudinal study on aging. J Alzheimers Dis. 2015;47(4):889-899.
70. Vittoria Mattioli. Beverages of daily life: impact of caffeine on atrial fibrillation. J Atr Fibrillation. 2014;7(2):1133.
71. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;5:13.
72. Noguchi-Shinohara M, Yuki S, Dohmoto C, et al. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS One. 2014;9(5):e96013. doi: 10.1371/journal.pone.0096013.
73. Schneider N, Yvon C. A review of multidomain interventions to support healthy cognitive ageing. J Nutr Health Aging. 2013;17(3):252-257.
74. Ngandu T, Lehitsalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
75. U.S. National Library of Medicing. ClinicalTrials.gov. U.S. study to protect brain health through lifestyle intervention to reduce risk (POINTER). https://clinicaltrials.gov/ct2/show/NCT03688126?term=pointer&cond=Alzheimer+Disease&rank=1. Published September 28, 2018. Accessed November 3, 2018.
1. Mehta D, Jackson R, Paul G, et al. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin Investig Drugs. 2017;26(6):735-739.
2. Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794.
3. Meng XF, Yu JT, Wang HF, et al. Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42(4):1295-1310.
4. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
5. Barnes DE, Yaffe Y. The projected impact of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828.
6. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464-472.
7. Ahlskog JE, Geda YE, Graff-Radford NR, et al. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876-884.
8. Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer Disease. JAMA. 2009;302(6):627-637.
9. Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705-711.
10. Smith PJ et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239-252.
11. Brown BM, Peiffer JJ, Taddei K, et al. Physical activity and amyloid-beta plasma and brain levels: results from the Australian imaging, biomarkers and lifestyle study of ageing. Mol Psychiatry. 2013;18(8):875-881.
12. Brown BM, Sohrabi HR, Taddei K, et al. Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer’s disease. Alzheimers Dement. 2017;13(11):1197-1206.
13. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
14. Centers for Disease Control and Prevention. Physical activity: how much physical activity do older adults need?
15. Garber CE, Blissmer B, Deschenes MR, et al; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-1359.
16. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113);2673-2734.
17. Valenzuela MJ, Sachdev P, Wen W, et al. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3(7):e2598. doi.org/10.1371/journal.pone.0002598.
18. Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59(12):1910-1914.
19. Wilson RS, Scherr PA, Schneider JA, et al. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911-1920.
20. Krell-Roesch J, Vemuri P, Pink A, et al. Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the apoe ε4 genotype. JAMA Neurol. 2017;74(3):332-338.
21. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508-2516.
22. Klein RM, Christie J, Parkvall M. Does multilingualism affect the incidence of Alzheimer’s disease?: a worldwide analysis by country. SSM Popul Health. 2016;2:463-467.
23. Grundy JG, Anderson JAE, Bialystok E. Neural correlates of cognitive processing in monolinguals and bilinguals. Ann N Y Acad Sci. 2017;1396(1):183-201.
24. Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234-240.
25. Amieva H, Stoykova R, Matharan F, et al. What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosom Med. 2010;72(9):905-911.
26. Kuiper JS, Oude Voshaar RC, Zuidema SU, et al. The relationship between social functioning and subjective memory complaints in older persons: a population-based longitudinal cohort study. Int J Geriatr Psychiatry. 2017;32(10):1059-1071.
27. Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98-104.
28. Marioni RE, Proust-Lima C, Amieva H, et al. Social activity, cognitive decline and dementia risk: a 20-year prospective cohort study. BMC Public Health. 2015;15:1089.
29. Wilson RS, Schneider JA, Boyle PA, et al. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085-2092.
30. Cai Z, Yan LJ, Ratka A. Telomere shortening and Alzheimer’s disease. Neuromolecular Med. 2013;15(1):25-48.
31. Wilson RS, Arnold SE, Schneider JA, et al. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143-153.
32. Epel E, Daubenmier J, Moskowitz JT, et al. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34-53.
33. Malinowski P, Moore AW, Mead Br, et al. Mindful aging: the effects of regular brief mindfulness practice on electrophysiological markers of cognitive and affective processing in older adults. Mindfulness (N Y). 2017;8(1):78-94.
34. Taren AA, Gianaros PJ, Greco CM, et al. Mindfulness meditation training and executive control network resting state functional connectivity: a randomized controlled trial. Psychosom Med. 2017;79(6):674-683.
35. Fountain-Zaragoza S, Prakash RS. Mindfulness training for healthy aging: impact on attention, well-being, and inflammation. Front in Aging Neurosci. 2017;9:11.
36. Eyre HA, Siddarth P, Acevedo B, et al. A randomized controlled trial of Kundalini yoga in mild cognitive impairment. Int Psychogeriatr. 2017;29(4):557-567.
37. Khalsa DS. Stress, meditation, and Alzheimer’s disease prevention: where the evidence stands. J Alzheimers Dis. 2015;48(1):1-12.
38. Berk L, van Boxtel M, van Os J. Can mindfulness-based interventions influence cognitive functioning in older adults? A review and considerations for future research. Aging Ment Health. 2017;21(11):1113-1120.
39. Hosseini S, Chaurasia A, Oremus M. The effect of religion and spirituality on cognitive function: a systematic review. Gerontologist. 2017. doi: 10.1093/geront/gnx024.
40. Luchsinger JA, Tang MX, Shea S, et al. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59(8):1258-1263.
41. Grant WB. Trends in diet and Alzheimer’s disease during the nutrition transition in Japan and developing countries. J Alzheimers Dis. 2014;38(3):611-620.
42. Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59-68.
43. Hu N, Yu JT, Tan L, et al. Nutrition and the risk of Alzheimer’s disease. Biomed Res Int. 2013;2013:524820. doi: 10.1155/2013/524820.
44. Taylor MK, Sullivan DK, Swerdlow RH, et al. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106(6):1463-1470.
45. van de Rest O, Berendsen AM, Haveman-Nies A, et al. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154-168.
46. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. 2016;7(5):889-904.
47. Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331-1338.
48. Wengreen H, Munger RG, Cutler A, et al. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County study on memory, health and aging. Am J Clin Nutr. 2013;98(5):1263-1271.
49. Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410-1416.
50. Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007-1014.
51. Desai AK, Rush J, Naveen L, et al. Nutrition and nutritional supplements to promote brain health. In: Hartman-Stein PE, Rue AL, eds. Enhancing cognitive fitness in adults: a guide to the use and development of community-based programs. New York, NY: Springer; 2011:249-269.
52. Goozee KG, Shah TM, Sohrabi HR, et al. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr. 2016;115(3):449-465.
53. Small GW, Siddarth P, Li Z, et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatry. 2018;26(3):266-277.
54. Kim JW, Lee DY, Lee BC, et al. Alcohol and cognition in the elderly: a review. Psychiatry Investig. 2012;9(1):8-16.
55. Virtaa JJ, Järvenpää T, Heikkilä K, et al. Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis. 2010;22(3):939-948.
56. Kerr WC, Stockwell T. Understanding standard drinks and drinking guidelines. Drug and Alcohol Rev. 2012;31(2):200-205.
57. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Accessed December 9, 2017.
58. National Institute on Alcohol Abuse and Alcoholism. What is a standard drink? https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink. Accessed November 9, 2017.
59. Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol. 2002;156(5):445-453.
60. Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris). 1997;153(3):185-192.
61. Topiwala A, Allan CL, Valkanova V, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357.
62. Venkataraman A, Kalk N, Sewell G, et al. Alcohol and Alzheimer’s disease-does alcohol dependence contribute to beta-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol. 2017;52(2):151-158.
63. Ma QP, Huang C, Cui QY, et al. Meta-analysis of the association between tea intake and the risk of cognitive disorders. PLoS One. 2016;11(11):e0165861. doi: 10.1371/journal.pone.0165861.
64. Santos C, Costa J, Santos J, et al. Caffeine intake and dementia: systematic review and meta-analysis. J Alzheimers Dis. 2010;20(Suppl 1):S187-204.
65. Panza F, Solfrizzi V, Barulli MR, et al. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J Nutr Health Aging. 2015;19(3):313-328.
66. Wierzejska R. Can coffee consumption lower the risk of Alzheimer’s disease and Parkinson’s disease? A literature review. Arch Med Sci. 2017;13(3):507-514.
67. Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis. 2010;20 (Suppl 1):S117-S126.
68. Eskelinen MH, Ngandu T, Tuomilehto J, et al. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16(1):85-91.
69. Solfrizzi V, Panza F, Imbimbo BP, et al. Coffee consumption habits and the risk of mild cognitive impairment: the Italian longitudinal study on aging. J Alzheimers Dis. 2015;47(4):889-899.
70. Vittoria Mattioli. Beverages of daily life: impact of caffeine on atrial fibrillation. J Atr Fibrillation. 2014;7(2):1133.
71. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;5:13.
72. Noguchi-Shinohara M, Yuki S, Dohmoto C, et al. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS One. 2014;9(5):e96013. doi: 10.1371/journal.pone.0096013.
73. Schneider N, Yvon C. A review of multidomain interventions to support healthy cognitive ageing. J Nutr Health Aging. 2013;17(3):252-257.
74. Ngandu T, Lehitsalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
75. U.S. National Library of Medicing. ClinicalTrials.gov. U.S. study to protect brain health through lifestyle intervention to reduce risk (POINTER). https://clinicaltrials.gov/ct2/show/NCT03688126?term=pointer&cond=Alzheimer+Disease&rank=1. Published September 28, 2018. Accessed November 3, 2018.
ABIM sued over maintenance of certification
Also today, drug test results should not dictate treatment, duodenoscopes contain more bacteria than expected, and weight-loss medications may have a role following bariatric surgery.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, drug test results should not dictate treatment, duodenoscopes contain more bacteria than expected, and weight-loss medications may have a role following bariatric surgery.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, drug test results should not dictate treatment, duodenoscopes contain more bacteria than expected, and weight-loss medications may have a role following bariatric surgery.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
A Pharmacist-Led Transitional Care Program to Reduce Hospital Readmissions in Older Adults
Medication reconciliation and patient education during admission and after discharge helped older patients remain independent at home.
There will be 53 million older adults in the US by 2020.1 Increasing age often brings medical comorbidities and prescriptions for multiple medications. An increasing number of prescribed medications combined with age-related changes in the ability to metabolize drugs makes older adults highly vulnerable to adverse drug events (ADEs).2 In addition, older adults often have difficulty self-managing their medications and adhering to prescribed regimens.3 As a result, ADEs can lead to poor health outcomes, including hospitalizations, in older adults.
Medication errors and ADEs are particularly common during transitions from hospital to home and can lead to unnecessary readmissions,a major cause of wasteful health care spending in the US.4,5 More than $25 billion are estimated to be spent annually on hospital readmissions, with Medicare picking up the bill for $17 billion of the total.6,7 Researchers have found that the majority of ADEs following hospital discharge are either entirely preventable or at least ameliorable (ie, the negative impact or harm resulting from the ADE could have been reduced).8
To address these issues, we undertook a clinical demonstration project that implemented a new transitional care program to improve the quality of care for older veterans transitioning from the Audie L. Murphy Veterans Memorial Hospital of the South Texas Veterans Health Care System (STVHCS) in San Antonio to home. The Geriatrics Medication Education at Discharge project (GMED) falls under the auspices of the San Antonio Geriatrics Research Education and Clinical Center (GRECC). Clinical demonstration projects are mandated for US Department of Veterans Affairs (VA) GRECCs to create and promote innovative models of care for older veterans. Dissemination of successful clinical demonstration projects to other VA sites is strongly encouraged. The GMED program was modeled after the Boston GRECC Pharmacological Intervention in Late Life (PILL) program.9 The PILL program, which focuses on serving older veterans with cognitive impairment, demonstrated that a postdischarge pharmacist telephone visit for medication reconciliation leads to a reduction in readmission within 60 days of discharge.9 The goals of the GMED program were to reduce polypharmacy, inappropriate prescribing and 30-day readmissions.
Methods
The project was conducted when a full-time clinical pharmacy specialist (CPS) was available (May-September 2013 and April 2014-March 2015). This project was approved as nonresearch/quality improvement by the University of Texas Health Science Center Institutional Review Board, which serves the STVHCS. Consent was not required.
Eligibility
Patients were identified via a daily hospital database query of all adults aged ≥ 65 years admitted to the hospital through Inpatient Medicine, Neurology, or Cardiology services within the prior 24 hours. Patients meeting any of the following criteria based on review of the Computerized Patient Record System (CPRS) by the team geriatrician and CPS were considered eligible: (1) aged ≥ 70 years prescribed ≥ 12 outpatient medications; (2) aged ≥ 65 years with a medical history of dementia; (3) aged ≥ 65 years prescribed outpatient medications meeting Beers criteria10; (4) age ≥ 65 years with ≥ 2 hospital admissions (including the current, index admission) within the past calendar year; or (5) aged ≥ 65 years with ≥ 3 emergency department visits within the past calendar year. For the first polypharmacy criterion, patients aged ≥ 70 years were selected instead of aged ≥ 65 years so as not to exceed the capacity of 1 CPS. Twelve or more medications were used as a cutoff for polypharmacy based on prior quality improvement information gathered from our VA geriatrics clinic examining the average number of medications taken by older veterans in the outpatient setting.
Related: Reducing COPD Readmission Rates: Using a COPD Care Service During Care Transitions
Patients were excluded if they were expected to be discharged to any facility where the patient and/or the caregiver were not primarily responsible for medication administration after discharge. Patients who met eligibility criteria but were not seen by the transitional program pharmacist (due to staff capacity) were included in this analysis as a convenience comparison group of patients who received usual care. Patients were not randomized. All communication occurred in English, but this project did not exclude patients with limited English proficiency.
A program support assistant conducted the daily query of the hospital database. The pharmacist conducted the chart review to determine eligibility and delivered the intervention. Eligible patients were selected at random for the intervention with the intention of providing the intervention to as many veterans as possible.
The GMED Intervention
The GMED program included 2 phases, which were both conducted by a CPS with oversight from a senior CPS with geriatric pharmacology expertise and an internist/geriatrician.
The first phase of the transitional care program included an individual, face-to-face meeting between the CPS and the patient during the hospitalization. If a veteran was not present in the room at the time of an attempted visit, the pharmacist made 2 additional attempts (3 total) to include the patient in the transitional care program during the hospitalization.
The second component of the transitional care program included a telephone visit within 2 to 3 days of discharge, conducted by the same CPS who performed the face-to-face visit. The purpose of the telephone visit was to perform medication reconciliation, identify and rectify medication errors, provide further patient education, and assist in facilitating appropriate follow-up by the patient’s primary care provider (PCP), if required. At a minimum, veterans were asked a series of questions pertaining to their concerns about medication regimens, receipt of newly prescribed medications at discharge, additional education regarding medications after the CPS encounter during hospitalization, and whether the veteran required assistance with the medication regimen in the home setting. Follow-up questions were asked as needed to clarify and identify potential medication problems. All information from this telephone encounter was communicated to the PCP through CPRS documentation and by telephone as needed.
Related: Initiative to Minimize Pharmaceutical Risk in Older Veterans (IMPROVE) Polypharmacy Clinic
Data Collection
A standardized questionnaire was used prospectively for patients in the transitional care program group to assess patient education, primary residence, presence of a caregiver, fall history, medication adherence, and cognitive status (using Mini-Cog).13 Additional information (patient age, number of outpatient medications prior to and following the admission, presence of Beers criteria outpatient medications prior to and following the admission, new outpatient prescriptions, and changes to existing prescriptions as a result of the hospitalization) was gathered prospectively from patient interviews or from chart review.
For patients included in the comparison group, a retrospective administrative chart review was conducted to collect information such as age, sex, ethnic group, admission within 1 year prior to index admission, frailty, and Charlson Comorbidity Index (CCI) score, a method of categorizing comorbidities of patients based on the diagnosis codes found in administrative data.14 Each comorbidity category has an associated weight (from 1 to 6), based on the adjusted risk of mortality or resource use, and the sum of all the weights results in a single comorbidity score for a patient (0 indicates no comorbidities; higher scores predict greater risk of mortality or increased resource use).
We used the index developed from 17 disease categories. The range for CCI was 0 to 25. Frailty was defined as the presence of any of the following frailty-related diagnoses: anemia; fall, head injury, other injury; coagulopathy; electrolyte disturbance; or gait disorder. These diagnoses are either primary frailty characteristics within the frailty phenotype or have been shown in prior studies to be associated with the frailty phenotype.15-18 While more widely accepted frailty definitions exist,these other definitions require direct examination of the patient and could not be used in this project because we did not directly interact with the comparison group.16,19 The frailty definition used has been previously identified as a predictor of health care utilization and 30-day readmission in a veteran population.20 Whether or not the CPS detected a postdischarge medication error was recorded. All CPS recommendations were documented.
An index admission was defined as a hospital admission that occurred during the project period. Thirty-day readmission was defined as a hospital admission that occurred within 30 days of the discharge date of an index admission. Each index admission was considered individually for readmission (yes vs no) even if it occurred in the same patient over the project period. A 30-day readmission was not considered an index admission. An admission that occurred after a 30-day readmission was considered a subsequent index admission. Patients who died in the hospital were not included in this analysis, as they would not have participated in the entire intervention.
Statistical Analysis
We compared characteristics between patients who received GMED and patients who never received GMED (comparison group). Generalized estimating equations (GEE) were used to determine whether the rate of 30-day readmission (yes vs no) in the transitional care program group differed from that of the comparison group. In our GEE analysis, we assumed a binomial distribution and the logit link to model the log-odds of readmission as a linear function of transitional care program status (yes vs no) and other covariates, including age, frailty, hospital admission within 1 year prior to the index admission, and CCI score as covariates. Thirty-day readmission status associated with each index admission was coded as 1 for a readmission within 30 days of the discharge date of the index admission, or 0 for no readmission within 30 days.
Transitional care program status was determined whether or not the individual received the transitional care program for each index admission. This analysis allowed us to model repeated measures of index admissions as a function of the project period and whether the patient was seen by the GMED CPS during the index admission. The patient identifier was used as a cluster variable in the GEE analysis. Inverse propensity scores of receiving GMED at the index admission were adjusted as weights in the GEE analysis to minimize confounding and, hence, to strengthen the causal interpretation of the effect of the transitional care program. If there was ≥ 1 index admission, the GMED status (yes vs no) at the initial index admission was used as the dependent variable to calculate propensity scores. The propensity scores of transitional care program status were derived from the logistic regression analysis that modeled the log-odds of receiving the transitional care program at the index admission as a linear function of age, CCI, frailty, and prior hospitalization during the 1-year period prior to the index admission.
Related: Development and Implementation of a Geriatric Walking Clinic
Results
The GMED CPS saw 435 patients during the project period; 47 (10.8%) died prior to 30 days and were excluded, leaving 388 patients who received the transitional care program included in this evaluation.
Data from the CPS-patient interviews and chart reviews were available for 378 of the 388 patients (Table 2). Patients were primarily male, non-Hispanic white, with a high school education. More than half (65%) the patients were admitted for a new diagnosis or clinical condition.
The 30-day readmission rate was 15.6% for the transitional care program group and 21.9% for the comparison group. Three hundred seventy-one patients received the transitional care program only once, 16 patients received the transitional care program twice (ie, they had 2 index admissions during the study period and received the intervention both times), and 1 patient received the transitional care program 3 times.
In an unadjusted GEE model, the odds ratio (OR) for readmission in the transitional care program group was 0.74 (95% CI, 0.54-1.0, P = .06) compared with the usual care group (Table 3).
Thirty-five percent of patients had ≥ 1 CPS-recommended change in their treatment at the time of the inpatient admission (Table 4).
Discussion
We developed a transitional care program for hospitalized older veterans to improve the transition from hospital to home. After adjusting for clinical factors, GMED was associated with 26% lower odds of readmission within 30 days of discharge compared with that of the control group. The GMED CPS made changes to the medical regimen both during the inpatient admission as well as after discharge to correct medication errors and educate patients.
In addition, GMED led to a reduction in the number of prescribed medications, which impacts inappropriate polypharmacy—a significant problem in older adults, which contributes to ADEs.21 Our intervention was patient centered, as all decisions and education regarding medication management were tailored to each patient, taking into account medical and psychosocial factors.
Studies of similar programs have shown that a pharmacist-based program can improve outcomes in patients transitioning from hospital to home. A meta-analysis of 19 studies that evaluated the effectiveness of pharmacy-led medication reconciliation interventions at the time of a care transition showed that compared with usual care a pharmacist intervention led to reduced medication discrepancies.22 In this meta-analysis, medication discrepancies of higher clinical impact were more easily identified through pharmacy-led interventions than with usual care, suggesting improved safety. Although not all studies have shown a clear reduction in readmission rates or other health care utilization, the addition of clinical pharmacist services in the care of inpatients has generally resulted in improved care with no evidence of harm.23
Based on these findings and collaboration with another GRECC, we designed our program to focus on older adults with polypharmacy, cognitive impairment, high-risk medication usage, and/or a history of high health care use.9 Our findings add to the growing body of evidence that a CPS-led transitional care program results in reduced polypharmacy and reduced unnecessary hospital readmissions. Further, our findings have demonstrated the effectiveness of this type of program in a practical, clinical setting with veteran patients.
At the time of project inception, we believed that the majority of our interventions would occur postdischarge. We were somewhat surprised that a major component of GMED was suggested interventions by our pharmacist at the time of admission. We believe that because the CPS made suggestions during admission, we prevented postdischarge ADEs. A frequent intervention corrected the medication reconciliation on file at admission. This finding also was seen in another study by Gleason and colleagues, which examined medication errors at admission for 651 adult medicine inpatients.24 This study found that more than one-third of patients had medication reconciliation errors. Further, older age (≥ 65 years) was associated with increased odds of medication errors in this study.
Of note, a survey of hospital-based pharmacists indicated medication reconciliation is the most important role of the pharmacist in improving care transitions.25 The pharmacists stated that detection of errors at the time of admission is very important. The pharmacists further reported that additional education and counseling for patients with poor understanding of their medications was also important. Our findings support these findings and the use of a pharmacist as part of the medical team to improve medication reconciliation and education.
Limitations
A limitation of GMED is that we monitored only admissions to our hospital; therefore, we did not account for any hospitalizations that may have occurred outside the STVHCS. Another limitation is that this was not a randomized controlled trial, and we used a convenience sample of patients who met our criteria for eligibility but were not seen due to time constraints. This introduces potential bias such that patients admitted and discharged on nights or weekends when the CPS was not available were not included in the transitional care program group, and these patients may fundamentally differ from those admitted and discharged Monday through Friday.
However, Khanna and colleagues found that night or weekend admission was not associated with 30-day readmission or other worse outcomes (such as length of stay, 30-day emergency department visit, or intensive care unit transfer) in 857 general medicine admissions at a tertiary care hospital.26 Every effort was made to include as many eligible patients as possible in the transitional program group, and we were able to demonstrate that the patients in the 2 groups were similar. Frailty and prior hospital admission were more prevalent, although not significantly so, in the transitional program group, suggesting that any selection bias would have actually attenuated—not enhanced—the observed effect of the transitional program. Although the transitional program group patients were slightly younger by 0.3 years, they were similar in frailty status and CCI score.
Conclusion
The GMED program was associated with reduced 30-day hospital readmission, discontinuation of unnecessary medications, and corrected medication errors and discrepancies. We propose that a CPS-based transitional care program can improve the quality of care for older patients being discharged to home.
Acknowledgments
Supported by funding from the Veterans Health Administration T21 Non-Institutional Long-Term Care Initiative and VA Office of Rural Health and the San Antonio Geriatrics Research, Education, and Clinical Center. The sponsor did not have any role in the design, methods, data collection, or analysis, and preparation.
Author Contributions
R. Rottman-Sagebiel developed the transitional program concept and design and executed the program implementation, interpretation of data, and preparation of the manuscript. S. Pastewait, N. Cupples, A. Conde, M. Moris, and E. Gonzalez assisted with program design and implementation. S. Cope assisted with interpretation of data and preparation of the manuscript. H. Braden assisted with interpretation of data. D. MacCarthy assisted with data management and statistical analysis. C. Wang and S. Espinoza developed the program concept and design, performed statistical analysis and interpretation of data, and helped prepare the manuscript.
Advances in Geriatrics
Advances in Geriatrics features articles focused on quality improvement/quality assurance initiatives, pilot studies, best practices, research, patient education, and patient-centered care written by health care providers associated with Veteran Health Administration Geriatric Research Education and Clinical Centers. Interested authors can submit articles at editorialmanager.com/fedprac or send a brief 2 to 3 sentence abstract to fedprac@mdedge.com for feedback and publication recommendations.
1. Vincent GK, Velkoff VA. The Next Four Decades: The Older Population in the United States: 2010 to 2050. US Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2010.
2. Merle L, Laroche ML, Dantoine T, Charmes JP. Predicting and preventing adverse drug reactions in the very old. Drugs Aging. 2005;22(5):375-392.
3. Shi S, Mörike K, Klotz U. The clinical implications of ageing for rational drug therapy. Eur J Clin Pharmacol. 2008;64(2):183-199.
4. Coleman EA, Min Sj, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449-1465.
5. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-1516.
6. Price Waterhouse Coopers Health Research Institute. The Price of Excess: Identifying Waste in Healthcare Spending. Price Waterhouse Coopers Health Research Institute; 2008.
7. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
8. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167.
9. Paquin AM, Salow M, Rudolph JL. Pharmacist calls to older adults with cognitive difficulties after discharge in a Tertiary Veterans Administration Medical Center: a quality improvement program. J Am Geriatr Soc. 2015;63(3):571-577.
10. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
11. Greenwald JL, Halasyamani L, Greene J, et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477-485.
12. Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83.
13. Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini‐cog: a cognitive ‘vital signs’ measure for dementia screening in multi‐lingual elderly. Int J Geriatr Psychiatry. 2000;15(11):1021-1027.
14. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
15. Chaves PH, Semba RD, Leng SX, et al. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005;60(6):729-735.
16. Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M156.
17. Walston J, McBurnie MA, Newman A, et al; Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Int Med. 2002;162(20):2333-2341.
18. Stookey JD, Purser JL, Pieper CF, Cohen HJ. Plasma hypertonicity: another marker of frailty? J Am Geriatr Soc. 2004;52(8):1313-1320.
19. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722-727.
20. Pugh JA, Wang CP, Espinoza SE, et al. Influence of frailty‐related diagnoses, high‐risk prescribing in elderly adults, and primary care use on readmissions in fewer than 30 days for veterans aged 65 and older. J Am Geriatr Soc. 2014;62(2):291-298.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
22. Mekonnen AB, McLachlan AJ, Brien JA. Pharmacy‐led medication reconciliation programmes at hospital transitions: a systematic review and meta‐analysis. J Clin Pharm Ther. 2016;41(2):128-144.
23. Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Int Med. 2006;166(9):955-964.
24. Gleason KM, McDaniel MR, Feinglass J, et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441-447.
25. Haynes KT, Oberne A, Cawthon C, Kripalani S. Pharmacists’ recommendations to improve care transitions. Ann Pharmacother. 2012;46(9):1152-1159.
26. Khanna R, Wachsberg K, Marouni A, Feinglass J, Williams MV, Wayne DB. The association between night or weekend admission and hospitalization‐relevant patient outcomes. J Hosp Med. 2011;6(1):10-14.
Medication reconciliation and patient education during admission and after discharge helped older patients remain independent at home.
Medication reconciliation and patient education during admission and after discharge helped older patients remain independent at home.
There will be 53 million older adults in the US by 2020.1 Increasing age often brings medical comorbidities and prescriptions for multiple medications. An increasing number of prescribed medications combined with age-related changes in the ability to metabolize drugs makes older adults highly vulnerable to adverse drug events (ADEs).2 In addition, older adults often have difficulty self-managing their medications and adhering to prescribed regimens.3 As a result, ADEs can lead to poor health outcomes, including hospitalizations, in older adults.
Medication errors and ADEs are particularly common during transitions from hospital to home and can lead to unnecessary readmissions,a major cause of wasteful health care spending in the US.4,5 More than $25 billion are estimated to be spent annually on hospital readmissions, with Medicare picking up the bill for $17 billion of the total.6,7 Researchers have found that the majority of ADEs following hospital discharge are either entirely preventable or at least ameliorable (ie, the negative impact or harm resulting from the ADE could have been reduced).8
To address these issues, we undertook a clinical demonstration project that implemented a new transitional care program to improve the quality of care for older veterans transitioning from the Audie L. Murphy Veterans Memorial Hospital of the South Texas Veterans Health Care System (STVHCS) in San Antonio to home. The Geriatrics Medication Education at Discharge project (GMED) falls under the auspices of the San Antonio Geriatrics Research Education and Clinical Center (GRECC). Clinical demonstration projects are mandated for US Department of Veterans Affairs (VA) GRECCs to create and promote innovative models of care for older veterans. Dissemination of successful clinical demonstration projects to other VA sites is strongly encouraged. The GMED program was modeled after the Boston GRECC Pharmacological Intervention in Late Life (PILL) program.9 The PILL program, which focuses on serving older veterans with cognitive impairment, demonstrated that a postdischarge pharmacist telephone visit for medication reconciliation leads to a reduction in readmission within 60 days of discharge.9 The goals of the GMED program were to reduce polypharmacy, inappropriate prescribing and 30-day readmissions.
Methods
The project was conducted when a full-time clinical pharmacy specialist (CPS) was available (May-September 2013 and April 2014-March 2015). This project was approved as nonresearch/quality improvement by the University of Texas Health Science Center Institutional Review Board, which serves the STVHCS. Consent was not required.
Eligibility
Patients were identified via a daily hospital database query of all adults aged ≥ 65 years admitted to the hospital through Inpatient Medicine, Neurology, or Cardiology services within the prior 24 hours. Patients meeting any of the following criteria based on review of the Computerized Patient Record System (CPRS) by the team geriatrician and CPS were considered eligible: (1) aged ≥ 70 years prescribed ≥ 12 outpatient medications; (2) aged ≥ 65 years with a medical history of dementia; (3) aged ≥ 65 years prescribed outpatient medications meeting Beers criteria10; (4) age ≥ 65 years with ≥ 2 hospital admissions (including the current, index admission) within the past calendar year; or (5) aged ≥ 65 years with ≥ 3 emergency department visits within the past calendar year. For the first polypharmacy criterion, patients aged ≥ 70 years were selected instead of aged ≥ 65 years so as not to exceed the capacity of 1 CPS. Twelve or more medications were used as a cutoff for polypharmacy based on prior quality improvement information gathered from our VA geriatrics clinic examining the average number of medications taken by older veterans in the outpatient setting.
Related: Reducing COPD Readmission Rates: Using a COPD Care Service During Care Transitions
Patients were excluded if they were expected to be discharged to any facility where the patient and/or the caregiver were not primarily responsible for medication administration after discharge. Patients who met eligibility criteria but were not seen by the transitional program pharmacist (due to staff capacity) were included in this analysis as a convenience comparison group of patients who received usual care. Patients were not randomized. All communication occurred in English, but this project did not exclude patients with limited English proficiency.
A program support assistant conducted the daily query of the hospital database. The pharmacist conducted the chart review to determine eligibility and delivered the intervention. Eligible patients were selected at random for the intervention with the intention of providing the intervention to as many veterans as possible.
The GMED Intervention
The GMED program included 2 phases, which were both conducted by a CPS with oversight from a senior CPS with geriatric pharmacology expertise and an internist/geriatrician.
The first phase of the transitional care program included an individual, face-to-face meeting between the CPS and the patient during the hospitalization. If a veteran was not present in the room at the time of an attempted visit, the pharmacist made 2 additional attempts (3 total) to include the patient in the transitional care program during the hospitalization.
The second component of the transitional care program included a telephone visit within 2 to 3 days of discharge, conducted by the same CPS who performed the face-to-face visit. The purpose of the telephone visit was to perform medication reconciliation, identify and rectify medication errors, provide further patient education, and assist in facilitating appropriate follow-up by the patient’s primary care provider (PCP), if required. At a minimum, veterans were asked a series of questions pertaining to their concerns about medication regimens, receipt of newly prescribed medications at discharge, additional education regarding medications after the CPS encounter during hospitalization, and whether the veteran required assistance with the medication regimen in the home setting. Follow-up questions were asked as needed to clarify and identify potential medication problems. All information from this telephone encounter was communicated to the PCP through CPRS documentation and by telephone as needed.
Related: Initiative to Minimize Pharmaceutical Risk in Older Veterans (IMPROVE) Polypharmacy Clinic
Data Collection
A standardized questionnaire was used prospectively for patients in the transitional care program group to assess patient education, primary residence, presence of a caregiver, fall history, medication adherence, and cognitive status (using Mini-Cog).13 Additional information (patient age, number of outpatient medications prior to and following the admission, presence of Beers criteria outpatient medications prior to and following the admission, new outpatient prescriptions, and changes to existing prescriptions as a result of the hospitalization) was gathered prospectively from patient interviews or from chart review.
For patients included in the comparison group, a retrospective administrative chart review was conducted to collect information such as age, sex, ethnic group, admission within 1 year prior to index admission, frailty, and Charlson Comorbidity Index (CCI) score, a method of categorizing comorbidities of patients based on the diagnosis codes found in administrative data.14 Each comorbidity category has an associated weight (from 1 to 6), based on the adjusted risk of mortality or resource use, and the sum of all the weights results in a single comorbidity score for a patient (0 indicates no comorbidities; higher scores predict greater risk of mortality or increased resource use).
We used the index developed from 17 disease categories. The range for CCI was 0 to 25. Frailty was defined as the presence of any of the following frailty-related diagnoses: anemia; fall, head injury, other injury; coagulopathy; electrolyte disturbance; or gait disorder. These diagnoses are either primary frailty characteristics within the frailty phenotype or have been shown in prior studies to be associated with the frailty phenotype.15-18 While more widely accepted frailty definitions exist,these other definitions require direct examination of the patient and could not be used in this project because we did not directly interact with the comparison group.16,19 The frailty definition used has been previously identified as a predictor of health care utilization and 30-day readmission in a veteran population.20 Whether or not the CPS detected a postdischarge medication error was recorded. All CPS recommendations were documented.
An index admission was defined as a hospital admission that occurred during the project period. Thirty-day readmission was defined as a hospital admission that occurred within 30 days of the discharge date of an index admission. Each index admission was considered individually for readmission (yes vs no) even if it occurred in the same patient over the project period. A 30-day readmission was not considered an index admission. An admission that occurred after a 30-day readmission was considered a subsequent index admission. Patients who died in the hospital were not included in this analysis, as they would not have participated in the entire intervention.
Statistical Analysis
We compared characteristics between patients who received GMED and patients who never received GMED (comparison group). Generalized estimating equations (GEE) were used to determine whether the rate of 30-day readmission (yes vs no) in the transitional care program group differed from that of the comparison group. In our GEE analysis, we assumed a binomial distribution and the logit link to model the log-odds of readmission as a linear function of transitional care program status (yes vs no) and other covariates, including age, frailty, hospital admission within 1 year prior to the index admission, and CCI score as covariates. Thirty-day readmission status associated with each index admission was coded as 1 for a readmission within 30 days of the discharge date of the index admission, or 0 for no readmission within 30 days.
Transitional care program status was determined whether or not the individual received the transitional care program for each index admission. This analysis allowed us to model repeated measures of index admissions as a function of the project period and whether the patient was seen by the GMED CPS during the index admission. The patient identifier was used as a cluster variable in the GEE analysis. Inverse propensity scores of receiving GMED at the index admission were adjusted as weights in the GEE analysis to minimize confounding and, hence, to strengthen the causal interpretation of the effect of the transitional care program. If there was ≥ 1 index admission, the GMED status (yes vs no) at the initial index admission was used as the dependent variable to calculate propensity scores. The propensity scores of transitional care program status were derived from the logistic regression analysis that modeled the log-odds of receiving the transitional care program at the index admission as a linear function of age, CCI, frailty, and prior hospitalization during the 1-year period prior to the index admission.
Related: Development and Implementation of a Geriatric Walking Clinic
Results
The GMED CPS saw 435 patients during the project period; 47 (10.8%) died prior to 30 days and were excluded, leaving 388 patients who received the transitional care program included in this evaluation.
Data from the CPS-patient interviews and chart reviews were available for 378 of the 388 patients (Table 2). Patients were primarily male, non-Hispanic white, with a high school education. More than half (65%) the patients were admitted for a new diagnosis or clinical condition.
The 30-day readmission rate was 15.6% for the transitional care program group and 21.9% for the comparison group. Three hundred seventy-one patients received the transitional care program only once, 16 patients received the transitional care program twice (ie, they had 2 index admissions during the study period and received the intervention both times), and 1 patient received the transitional care program 3 times.
In an unadjusted GEE model, the odds ratio (OR) for readmission in the transitional care program group was 0.74 (95% CI, 0.54-1.0, P = .06) compared with the usual care group (Table 3).
Thirty-five percent of patients had ≥ 1 CPS-recommended change in their treatment at the time of the inpatient admission (Table 4).
Discussion
We developed a transitional care program for hospitalized older veterans to improve the transition from hospital to home. After adjusting for clinical factors, GMED was associated with 26% lower odds of readmission within 30 days of discharge compared with that of the control group. The GMED CPS made changes to the medical regimen both during the inpatient admission as well as after discharge to correct medication errors and educate patients.
In addition, GMED led to a reduction in the number of prescribed medications, which impacts inappropriate polypharmacy—a significant problem in older adults, which contributes to ADEs.21 Our intervention was patient centered, as all decisions and education regarding medication management were tailored to each patient, taking into account medical and psychosocial factors.
Studies of similar programs have shown that a pharmacist-based program can improve outcomes in patients transitioning from hospital to home. A meta-analysis of 19 studies that evaluated the effectiveness of pharmacy-led medication reconciliation interventions at the time of a care transition showed that compared with usual care a pharmacist intervention led to reduced medication discrepancies.22 In this meta-analysis, medication discrepancies of higher clinical impact were more easily identified through pharmacy-led interventions than with usual care, suggesting improved safety. Although not all studies have shown a clear reduction in readmission rates or other health care utilization, the addition of clinical pharmacist services in the care of inpatients has generally resulted in improved care with no evidence of harm.23
Based on these findings and collaboration with another GRECC, we designed our program to focus on older adults with polypharmacy, cognitive impairment, high-risk medication usage, and/or a history of high health care use.9 Our findings add to the growing body of evidence that a CPS-led transitional care program results in reduced polypharmacy and reduced unnecessary hospital readmissions. Further, our findings have demonstrated the effectiveness of this type of program in a practical, clinical setting with veteran patients.
At the time of project inception, we believed that the majority of our interventions would occur postdischarge. We were somewhat surprised that a major component of GMED was suggested interventions by our pharmacist at the time of admission. We believe that because the CPS made suggestions during admission, we prevented postdischarge ADEs. A frequent intervention corrected the medication reconciliation on file at admission. This finding also was seen in another study by Gleason and colleagues, which examined medication errors at admission for 651 adult medicine inpatients.24 This study found that more than one-third of patients had medication reconciliation errors. Further, older age (≥ 65 years) was associated with increased odds of medication errors in this study.
Of note, a survey of hospital-based pharmacists indicated medication reconciliation is the most important role of the pharmacist in improving care transitions.25 The pharmacists stated that detection of errors at the time of admission is very important. The pharmacists further reported that additional education and counseling for patients with poor understanding of their medications was also important. Our findings support these findings and the use of a pharmacist as part of the medical team to improve medication reconciliation and education.
Limitations
A limitation of GMED is that we monitored only admissions to our hospital; therefore, we did not account for any hospitalizations that may have occurred outside the STVHCS. Another limitation is that this was not a randomized controlled trial, and we used a convenience sample of patients who met our criteria for eligibility but were not seen due to time constraints. This introduces potential bias such that patients admitted and discharged on nights or weekends when the CPS was not available were not included in the transitional care program group, and these patients may fundamentally differ from those admitted and discharged Monday through Friday.
However, Khanna and colleagues found that night or weekend admission was not associated with 30-day readmission or other worse outcomes (such as length of stay, 30-day emergency department visit, or intensive care unit transfer) in 857 general medicine admissions at a tertiary care hospital.26 Every effort was made to include as many eligible patients as possible in the transitional program group, and we were able to demonstrate that the patients in the 2 groups were similar. Frailty and prior hospital admission were more prevalent, although not significantly so, in the transitional program group, suggesting that any selection bias would have actually attenuated—not enhanced—the observed effect of the transitional program. Although the transitional program group patients were slightly younger by 0.3 years, they were similar in frailty status and CCI score.
Conclusion
The GMED program was associated with reduced 30-day hospital readmission, discontinuation of unnecessary medications, and corrected medication errors and discrepancies. We propose that a CPS-based transitional care program can improve the quality of care for older patients being discharged to home.
Acknowledgments
Supported by funding from the Veterans Health Administration T21 Non-Institutional Long-Term Care Initiative and VA Office of Rural Health and the San Antonio Geriatrics Research, Education, and Clinical Center. The sponsor did not have any role in the design, methods, data collection, or analysis, and preparation.
Author Contributions
R. Rottman-Sagebiel developed the transitional program concept and design and executed the program implementation, interpretation of data, and preparation of the manuscript. S. Pastewait, N. Cupples, A. Conde, M. Moris, and E. Gonzalez assisted with program design and implementation. S. Cope assisted with interpretation of data and preparation of the manuscript. H. Braden assisted with interpretation of data. D. MacCarthy assisted with data management and statistical analysis. C. Wang and S. Espinoza developed the program concept and design, performed statistical analysis and interpretation of data, and helped prepare the manuscript.
Advances in Geriatrics
Advances in Geriatrics features articles focused on quality improvement/quality assurance initiatives, pilot studies, best practices, research, patient education, and patient-centered care written by health care providers associated with Veteran Health Administration Geriatric Research Education and Clinical Centers. Interested authors can submit articles at editorialmanager.com/fedprac or send a brief 2 to 3 sentence abstract to fedprac@mdedge.com for feedback and publication recommendations.
There will be 53 million older adults in the US by 2020.1 Increasing age often brings medical comorbidities and prescriptions for multiple medications. An increasing number of prescribed medications combined with age-related changes in the ability to metabolize drugs makes older adults highly vulnerable to adverse drug events (ADEs).2 In addition, older adults often have difficulty self-managing their medications and adhering to prescribed regimens.3 As a result, ADEs can lead to poor health outcomes, including hospitalizations, in older adults.
Medication errors and ADEs are particularly common during transitions from hospital to home and can lead to unnecessary readmissions,a major cause of wasteful health care spending in the US.4,5 More than $25 billion are estimated to be spent annually on hospital readmissions, with Medicare picking up the bill for $17 billion of the total.6,7 Researchers have found that the majority of ADEs following hospital discharge are either entirely preventable or at least ameliorable (ie, the negative impact or harm resulting from the ADE could have been reduced).8
To address these issues, we undertook a clinical demonstration project that implemented a new transitional care program to improve the quality of care for older veterans transitioning from the Audie L. Murphy Veterans Memorial Hospital of the South Texas Veterans Health Care System (STVHCS) in San Antonio to home. The Geriatrics Medication Education at Discharge project (GMED) falls under the auspices of the San Antonio Geriatrics Research Education and Clinical Center (GRECC). Clinical demonstration projects are mandated for US Department of Veterans Affairs (VA) GRECCs to create and promote innovative models of care for older veterans. Dissemination of successful clinical demonstration projects to other VA sites is strongly encouraged. The GMED program was modeled after the Boston GRECC Pharmacological Intervention in Late Life (PILL) program.9 The PILL program, which focuses on serving older veterans with cognitive impairment, demonstrated that a postdischarge pharmacist telephone visit for medication reconciliation leads to a reduction in readmission within 60 days of discharge.9 The goals of the GMED program were to reduce polypharmacy, inappropriate prescribing and 30-day readmissions.
Methods
The project was conducted when a full-time clinical pharmacy specialist (CPS) was available (May-September 2013 and April 2014-March 2015). This project was approved as nonresearch/quality improvement by the University of Texas Health Science Center Institutional Review Board, which serves the STVHCS. Consent was not required.
Eligibility
Patients were identified via a daily hospital database query of all adults aged ≥ 65 years admitted to the hospital through Inpatient Medicine, Neurology, or Cardiology services within the prior 24 hours. Patients meeting any of the following criteria based on review of the Computerized Patient Record System (CPRS) by the team geriatrician and CPS were considered eligible: (1) aged ≥ 70 years prescribed ≥ 12 outpatient medications; (2) aged ≥ 65 years with a medical history of dementia; (3) aged ≥ 65 years prescribed outpatient medications meeting Beers criteria10; (4) age ≥ 65 years with ≥ 2 hospital admissions (including the current, index admission) within the past calendar year; or (5) aged ≥ 65 years with ≥ 3 emergency department visits within the past calendar year. For the first polypharmacy criterion, patients aged ≥ 70 years were selected instead of aged ≥ 65 years so as not to exceed the capacity of 1 CPS. Twelve or more medications were used as a cutoff for polypharmacy based on prior quality improvement information gathered from our VA geriatrics clinic examining the average number of medications taken by older veterans in the outpatient setting.
Related: Reducing COPD Readmission Rates: Using a COPD Care Service During Care Transitions
Patients were excluded if they were expected to be discharged to any facility where the patient and/or the caregiver were not primarily responsible for medication administration after discharge. Patients who met eligibility criteria but were not seen by the transitional program pharmacist (due to staff capacity) were included in this analysis as a convenience comparison group of patients who received usual care. Patients were not randomized. All communication occurred in English, but this project did not exclude patients with limited English proficiency.
A program support assistant conducted the daily query of the hospital database. The pharmacist conducted the chart review to determine eligibility and delivered the intervention. Eligible patients were selected at random for the intervention with the intention of providing the intervention to as many veterans as possible.
The GMED Intervention
The GMED program included 2 phases, which were both conducted by a CPS with oversight from a senior CPS with geriatric pharmacology expertise and an internist/geriatrician.
The first phase of the transitional care program included an individual, face-to-face meeting between the CPS and the patient during the hospitalization. If a veteran was not present in the room at the time of an attempted visit, the pharmacist made 2 additional attempts (3 total) to include the patient in the transitional care program during the hospitalization.
The second component of the transitional care program included a telephone visit within 2 to 3 days of discharge, conducted by the same CPS who performed the face-to-face visit. The purpose of the telephone visit was to perform medication reconciliation, identify and rectify medication errors, provide further patient education, and assist in facilitating appropriate follow-up by the patient’s primary care provider (PCP), if required. At a minimum, veterans were asked a series of questions pertaining to their concerns about medication regimens, receipt of newly prescribed medications at discharge, additional education regarding medications after the CPS encounter during hospitalization, and whether the veteran required assistance with the medication regimen in the home setting. Follow-up questions were asked as needed to clarify and identify potential medication problems. All information from this telephone encounter was communicated to the PCP through CPRS documentation and by telephone as needed.
Related: Initiative to Minimize Pharmaceutical Risk in Older Veterans (IMPROVE) Polypharmacy Clinic
Data Collection
A standardized questionnaire was used prospectively for patients in the transitional care program group to assess patient education, primary residence, presence of a caregiver, fall history, medication adherence, and cognitive status (using Mini-Cog).13 Additional information (patient age, number of outpatient medications prior to and following the admission, presence of Beers criteria outpatient medications prior to and following the admission, new outpatient prescriptions, and changes to existing prescriptions as a result of the hospitalization) was gathered prospectively from patient interviews or from chart review.
For patients included in the comparison group, a retrospective administrative chart review was conducted to collect information such as age, sex, ethnic group, admission within 1 year prior to index admission, frailty, and Charlson Comorbidity Index (CCI) score, a method of categorizing comorbidities of patients based on the diagnosis codes found in administrative data.14 Each comorbidity category has an associated weight (from 1 to 6), based on the adjusted risk of mortality or resource use, and the sum of all the weights results in a single comorbidity score for a patient (0 indicates no comorbidities; higher scores predict greater risk of mortality or increased resource use).
We used the index developed from 17 disease categories. The range for CCI was 0 to 25. Frailty was defined as the presence of any of the following frailty-related diagnoses: anemia; fall, head injury, other injury; coagulopathy; electrolyte disturbance; or gait disorder. These diagnoses are either primary frailty characteristics within the frailty phenotype or have been shown in prior studies to be associated with the frailty phenotype.15-18 While more widely accepted frailty definitions exist,these other definitions require direct examination of the patient and could not be used in this project because we did not directly interact with the comparison group.16,19 The frailty definition used has been previously identified as a predictor of health care utilization and 30-day readmission in a veteran population.20 Whether or not the CPS detected a postdischarge medication error was recorded. All CPS recommendations were documented.
An index admission was defined as a hospital admission that occurred during the project period. Thirty-day readmission was defined as a hospital admission that occurred within 30 days of the discharge date of an index admission. Each index admission was considered individually for readmission (yes vs no) even if it occurred in the same patient over the project period. A 30-day readmission was not considered an index admission. An admission that occurred after a 30-day readmission was considered a subsequent index admission. Patients who died in the hospital were not included in this analysis, as they would not have participated in the entire intervention.
Statistical Analysis
We compared characteristics between patients who received GMED and patients who never received GMED (comparison group). Generalized estimating equations (GEE) were used to determine whether the rate of 30-day readmission (yes vs no) in the transitional care program group differed from that of the comparison group. In our GEE analysis, we assumed a binomial distribution and the logit link to model the log-odds of readmission as a linear function of transitional care program status (yes vs no) and other covariates, including age, frailty, hospital admission within 1 year prior to the index admission, and CCI score as covariates. Thirty-day readmission status associated with each index admission was coded as 1 for a readmission within 30 days of the discharge date of the index admission, or 0 for no readmission within 30 days.
Transitional care program status was determined whether or not the individual received the transitional care program for each index admission. This analysis allowed us to model repeated measures of index admissions as a function of the project period and whether the patient was seen by the GMED CPS during the index admission. The patient identifier was used as a cluster variable in the GEE analysis. Inverse propensity scores of receiving GMED at the index admission were adjusted as weights in the GEE analysis to minimize confounding and, hence, to strengthen the causal interpretation of the effect of the transitional care program. If there was ≥ 1 index admission, the GMED status (yes vs no) at the initial index admission was used as the dependent variable to calculate propensity scores. The propensity scores of transitional care program status were derived from the logistic regression analysis that modeled the log-odds of receiving the transitional care program at the index admission as a linear function of age, CCI, frailty, and prior hospitalization during the 1-year period prior to the index admission.
Related: Development and Implementation of a Geriatric Walking Clinic
Results
The GMED CPS saw 435 patients during the project period; 47 (10.8%) died prior to 30 days and were excluded, leaving 388 patients who received the transitional care program included in this evaluation.
Data from the CPS-patient interviews and chart reviews were available for 378 of the 388 patients (Table 2). Patients were primarily male, non-Hispanic white, with a high school education. More than half (65%) the patients were admitted for a new diagnosis or clinical condition.
The 30-day readmission rate was 15.6% for the transitional care program group and 21.9% for the comparison group. Three hundred seventy-one patients received the transitional care program only once, 16 patients received the transitional care program twice (ie, they had 2 index admissions during the study period and received the intervention both times), and 1 patient received the transitional care program 3 times.
In an unadjusted GEE model, the odds ratio (OR) for readmission in the transitional care program group was 0.74 (95% CI, 0.54-1.0, P = .06) compared with the usual care group (Table 3).
Thirty-five percent of patients had ≥ 1 CPS-recommended change in their treatment at the time of the inpatient admission (Table 4).
Discussion
We developed a transitional care program for hospitalized older veterans to improve the transition from hospital to home. After adjusting for clinical factors, GMED was associated with 26% lower odds of readmission within 30 days of discharge compared with that of the control group. The GMED CPS made changes to the medical regimen both during the inpatient admission as well as after discharge to correct medication errors and educate patients.
In addition, GMED led to a reduction in the number of prescribed medications, which impacts inappropriate polypharmacy—a significant problem in older adults, which contributes to ADEs.21 Our intervention was patient centered, as all decisions and education regarding medication management were tailored to each patient, taking into account medical and psychosocial factors.
Studies of similar programs have shown that a pharmacist-based program can improve outcomes in patients transitioning from hospital to home. A meta-analysis of 19 studies that evaluated the effectiveness of pharmacy-led medication reconciliation interventions at the time of a care transition showed that compared with usual care a pharmacist intervention led to reduced medication discrepancies.22 In this meta-analysis, medication discrepancies of higher clinical impact were more easily identified through pharmacy-led interventions than with usual care, suggesting improved safety. Although not all studies have shown a clear reduction in readmission rates or other health care utilization, the addition of clinical pharmacist services in the care of inpatients has generally resulted in improved care with no evidence of harm.23
Based on these findings and collaboration with another GRECC, we designed our program to focus on older adults with polypharmacy, cognitive impairment, high-risk medication usage, and/or a history of high health care use.9 Our findings add to the growing body of evidence that a CPS-led transitional care program results in reduced polypharmacy and reduced unnecessary hospital readmissions. Further, our findings have demonstrated the effectiveness of this type of program in a practical, clinical setting with veteran patients.
At the time of project inception, we believed that the majority of our interventions would occur postdischarge. We were somewhat surprised that a major component of GMED was suggested interventions by our pharmacist at the time of admission. We believe that because the CPS made suggestions during admission, we prevented postdischarge ADEs. A frequent intervention corrected the medication reconciliation on file at admission. This finding also was seen in another study by Gleason and colleagues, which examined medication errors at admission for 651 adult medicine inpatients.24 This study found that more than one-third of patients had medication reconciliation errors. Further, older age (≥ 65 years) was associated with increased odds of medication errors in this study.
Of note, a survey of hospital-based pharmacists indicated medication reconciliation is the most important role of the pharmacist in improving care transitions.25 The pharmacists stated that detection of errors at the time of admission is very important. The pharmacists further reported that additional education and counseling for patients with poor understanding of their medications was also important. Our findings support these findings and the use of a pharmacist as part of the medical team to improve medication reconciliation and education.
Limitations
A limitation of GMED is that we monitored only admissions to our hospital; therefore, we did not account for any hospitalizations that may have occurred outside the STVHCS. Another limitation is that this was not a randomized controlled trial, and we used a convenience sample of patients who met our criteria for eligibility but were not seen due to time constraints. This introduces potential bias such that patients admitted and discharged on nights or weekends when the CPS was not available were not included in the transitional care program group, and these patients may fundamentally differ from those admitted and discharged Monday through Friday.
However, Khanna and colleagues found that night or weekend admission was not associated with 30-day readmission or other worse outcomes (such as length of stay, 30-day emergency department visit, or intensive care unit transfer) in 857 general medicine admissions at a tertiary care hospital.26 Every effort was made to include as many eligible patients as possible in the transitional program group, and we were able to demonstrate that the patients in the 2 groups were similar. Frailty and prior hospital admission were more prevalent, although not significantly so, in the transitional program group, suggesting that any selection bias would have actually attenuated—not enhanced—the observed effect of the transitional program. Although the transitional program group patients were slightly younger by 0.3 years, they were similar in frailty status and CCI score.
Conclusion
The GMED program was associated with reduced 30-day hospital readmission, discontinuation of unnecessary medications, and corrected medication errors and discrepancies. We propose that a CPS-based transitional care program can improve the quality of care for older patients being discharged to home.
Acknowledgments
Supported by funding from the Veterans Health Administration T21 Non-Institutional Long-Term Care Initiative and VA Office of Rural Health and the San Antonio Geriatrics Research, Education, and Clinical Center. The sponsor did not have any role in the design, methods, data collection, or analysis, and preparation.
Author Contributions
R. Rottman-Sagebiel developed the transitional program concept and design and executed the program implementation, interpretation of data, and preparation of the manuscript. S. Pastewait, N. Cupples, A. Conde, M. Moris, and E. Gonzalez assisted with program design and implementation. S. Cope assisted with interpretation of data and preparation of the manuscript. H. Braden assisted with interpretation of data. D. MacCarthy assisted with data management and statistical analysis. C. Wang and S. Espinoza developed the program concept and design, performed statistical analysis and interpretation of data, and helped prepare the manuscript.
Advances in Geriatrics
Advances in Geriatrics features articles focused on quality improvement/quality assurance initiatives, pilot studies, best practices, research, patient education, and patient-centered care written by health care providers associated with Veteran Health Administration Geriatric Research Education and Clinical Centers. Interested authors can submit articles at editorialmanager.com/fedprac or send a brief 2 to 3 sentence abstract to fedprac@mdedge.com for feedback and publication recommendations.
1. Vincent GK, Velkoff VA. The Next Four Decades: The Older Population in the United States: 2010 to 2050. US Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2010.
2. Merle L, Laroche ML, Dantoine T, Charmes JP. Predicting and preventing adverse drug reactions in the very old. Drugs Aging. 2005;22(5):375-392.
3. Shi S, Mörike K, Klotz U. The clinical implications of ageing for rational drug therapy. Eur J Clin Pharmacol. 2008;64(2):183-199.
4. Coleman EA, Min Sj, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449-1465.
5. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-1516.
6. Price Waterhouse Coopers Health Research Institute. The Price of Excess: Identifying Waste in Healthcare Spending. Price Waterhouse Coopers Health Research Institute; 2008.
7. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
8. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167.
9. Paquin AM, Salow M, Rudolph JL. Pharmacist calls to older adults with cognitive difficulties after discharge in a Tertiary Veterans Administration Medical Center: a quality improvement program. J Am Geriatr Soc. 2015;63(3):571-577.
10. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
11. Greenwald JL, Halasyamani L, Greene J, et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477-485.
12. Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83.
13. Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini‐cog: a cognitive ‘vital signs’ measure for dementia screening in multi‐lingual elderly. Int J Geriatr Psychiatry. 2000;15(11):1021-1027.
14. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
15. Chaves PH, Semba RD, Leng SX, et al. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005;60(6):729-735.
16. Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M156.
17. Walston J, McBurnie MA, Newman A, et al; Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Int Med. 2002;162(20):2333-2341.
18. Stookey JD, Purser JL, Pieper CF, Cohen HJ. Plasma hypertonicity: another marker of frailty? J Am Geriatr Soc. 2004;52(8):1313-1320.
19. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722-727.
20. Pugh JA, Wang CP, Espinoza SE, et al. Influence of frailty‐related diagnoses, high‐risk prescribing in elderly adults, and primary care use on readmissions in fewer than 30 days for veterans aged 65 and older. J Am Geriatr Soc. 2014;62(2):291-298.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
22. Mekonnen AB, McLachlan AJ, Brien JA. Pharmacy‐led medication reconciliation programmes at hospital transitions: a systematic review and meta‐analysis. J Clin Pharm Ther. 2016;41(2):128-144.
23. Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Int Med. 2006;166(9):955-964.
24. Gleason KM, McDaniel MR, Feinglass J, et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441-447.
25. Haynes KT, Oberne A, Cawthon C, Kripalani S. Pharmacists’ recommendations to improve care transitions. Ann Pharmacother. 2012;46(9):1152-1159.
26. Khanna R, Wachsberg K, Marouni A, Feinglass J, Williams MV, Wayne DB. The association between night or weekend admission and hospitalization‐relevant patient outcomes. J Hosp Med. 2011;6(1):10-14.
1. Vincent GK, Velkoff VA. The Next Four Decades: The Older Population in the United States: 2010 to 2050. US Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2010.
2. Merle L, Laroche ML, Dantoine T, Charmes JP. Predicting and preventing adverse drug reactions in the very old. Drugs Aging. 2005;22(5):375-392.
3. Shi S, Mörike K, Klotz U. The clinical implications of ageing for rational drug therapy. Eur J Clin Pharmacol. 2008;64(2):183-199.
4. Coleman EA, Min Sj, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449-1465.
5. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-1516.
6. Price Waterhouse Coopers Health Research Institute. The Price of Excess: Identifying Waste in Healthcare Spending. Price Waterhouse Coopers Health Research Institute; 2008.
7. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
8. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167.
9. Paquin AM, Salow M, Rudolph JL. Pharmacist calls to older adults with cognitive difficulties after discharge in a Tertiary Veterans Administration Medical Center: a quality improvement program. J Am Geriatr Soc. 2015;63(3):571-577.
10. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
11. Greenwald JL, Halasyamani L, Greene J, et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477-485.
12. Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83.
13. Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini‐cog: a cognitive ‘vital signs’ measure for dementia screening in multi‐lingual elderly. Int J Geriatr Psychiatry. 2000;15(11):1021-1027.
14. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
15. Chaves PH, Semba RD, Leng SX, et al. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005;60(6):729-735.
16. Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M156.
17. Walston J, McBurnie MA, Newman A, et al; Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Int Med. 2002;162(20):2333-2341.
18. Stookey JD, Purser JL, Pieper CF, Cohen HJ. Plasma hypertonicity: another marker of frailty? J Am Geriatr Soc. 2004;52(8):1313-1320.
19. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722-727.
20. Pugh JA, Wang CP, Espinoza SE, et al. Influence of frailty‐related diagnoses, high‐risk prescribing in elderly adults, and primary care use on readmissions in fewer than 30 days for veterans aged 65 and older. J Am Geriatr Soc. 2014;62(2):291-298.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
22. Mekonnen AB, McLachlan AJ, Brien JA. Pharmacy‐led medication reconciliation programmes at hospital transitions: a systematic review and meta‐analysis. J Clin Pharm Ther. 2016;41(2):128-144.
23. Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Int Med. 2006;166(9):955-964.
24. Gleason KM, McDaniel MR, Feinglass J, et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441-447.
25. Haynes KT, Oberne A, Cawthon C, Kripalani S. Pharmacists’ recommendations to improve care transitions. Ann Pharmacother. 2012;46(9):1152-1159.
26. Khanna R, Wachsberg K, Marouni A, Feinglass J, Williams MV, Wayne DB. The association between night or weekend admission and hospitalization‐relevant patient outcomes. J Hosp Med. 2011;6(1):10-14.
Latest intranasal insulin results for Alzheimer’s muddied by malfunctioning inhaler
BARCELONA –
Instead of doing poorly, patients using the faulty device actually experienced better outcomes than did those who entered the study later and used a more reliable inhaler, Suzanne Craft, PhD, said at the Clinical Trials on Alzheimer’s Disease conference.
A secondary analysis of the ViaNase device subgroup “replicated findings in our original studies,” which used the same atomizer, said Dr. Craft, a professor of gerontology and geriatric medicine at Wake Forest University, Winston-Salem, N.C. “We remain optimistic, but clearly we are at the beginning of understanding optimal insulin doses and delivery techniques for this population.”
The 289-patient, placebo-controlled study was predicated by a prior successful study by Dr. Craft and her colleagues, published in 2012 in JAMA Neurology. That trial randomized 104 patients with amnestic mild cognitive impairment (MCI) or mild-moderate Alzheimer’s to placebo or intranasal insulin 20 or 40 IU. After 4 months, subjects in both insulin groups showed preserved cognition and functional abilities, as well as increased cerebral glucose metabolism.
The ViaNase device was manufactured by Kurve Technology. But the company redesigned it for the new trial, adding an electronic timing component, which Dr. Craft said, was supposed to increase ease of use.
“Unfortunately, there were frequent malfunctions of this mechanism for the first 49 patients – so much so that we had to discontinue using the device and switch to a newer one,” for the other 240 patients in the study. This intranasal drug-delivery system, called the Precisions Olfactory Delivery (POD) device, is made by Impel NeuroPharma. Dr. Craft’s trial is its first investigation in patients with Alzheimer’s disease.
The new study randomized 289 patients with MCI or mild Alzheimer’s to twice-daily sprays with a placebo device, or to intranasal insulin 40 IU for 12 months, followed by a 6-month, open-label period. The primary outcome was the Alzheimer’s Disease Assessment Scale-Cognition measure (ADAS-Cog 12). Secondary outcomes were the Clinical Dementia Rating Scale sum of boxes (CDR-sb) a memory composite measure, activities of daily living, cerebrospinal fluid biomarkers, and MRI of the hippocampus and entorhinal cortex.
Because of the device problems, Dr. Craft conducted separate analyses for the user groups. The primary was an intent-to-treat (ITT), mixed-model, repeat-measures analysis of the 240 using the POD device. The model controlled for age, sex, genetic risk status, and investigation site. An exploratory ITT analysis looked only at the ADAS-Cog 12 in the 49 who used the ViaNase device. Patients were a mean of 71 years old, with a mean Mini Mental State Exam score of 25. About 42% were positive for the high-risk apolipoprotein E epsilon-4 allele.
At 12 months, there was no between-group difference on the ADAS-Cog 12 measure; both groups increased by about 4 points, indicating worsening. Nor were there any changes in any of the Alzheimer’s-related biomarkers: amyloid-beta 40 and 42, total tau, or phosphorylated tau. There was a small but statistically significant difference in the sizes of the hippocampus and entorhinal cortex.
The ViaNase group fared somewhat better in the secondary analysis of the ADAS-Cog12. The measure increased by about 5 points in the placebo group, and about 2.5 points in the insulin group. The significant separation was evident at 3 months and continued to widen over the course of the study.
Compliance was very good in the larger group – around 85%. It was lower in the ViaNase group, probably because of the device’s unreliability. Retention was good in both groups. There were no significant differences in adverse events and no obvious safety issues.
The 6-month, open-label period will close out before the end of the year. In the meantime, Dr. Craft is conducting additional subgroup analyses on the 12-month data.
Dr. Craft has served as a consultant for GlaxoSmithKline and Accera.
SOURCE: Craft S et al. J Prev Alz Dis 2018;5(S1):S9, Abstract OC2.
BARCELONA –
Instead of doing poorly, patients using the faulty device actually experienced better outcomes than did those who entered the study later and used a more reliable inhaler, Suzanne Craft, PhD, said at the Clinical Trials on Alzheimer’s Disease conference.
A secondary analysis of the ViaNase device subgroup “replicated findings in our original studies,” which used the same atomizer, said Dr. Craft, a professor of gerontology and geriatric medicine at Wake Forest University, Winston-Salem, N.C. “We remain optimistic, but clearly we are at the beginning of understanding optimal insulin doses and delivery techniques for this population.”
The 289-patient, placebo-controlled study was predicated by a prior successful study by Dr. Craft and her colleagues, published in 2012 in JAMA Neurology. That trial randomized 104 patients with amnestic mild cognitive impairment (MCI) or mild-moderate Alzheimer’s to placebo or intranasal insulin 20 or 40 IU. After 4 months, subjects in both insulin groups showed preserved cognition and functional abilities, as well as increased cerebral glucose metabolism.
The ViaNase device was manufactured by Kurve Technology. But the company redesigned it for the new trial, adding an electronic timing component, which Dr. Craft said, was supposed to increase ease of use.
“Unfortunately, there were frequent malfunctions of this mechanism for the first 49 patients – so much so that we had to discontinue using the device and switch to a newer one,” for the other 240 patients in the study. This intranasal drug-delivery system, called the Precisions Olfactory Delivery (POD) device, is made by Impel NeuroPharma. Dr. Craft’s trial is its first investigation in patients with Alzheimer’s disease.
The new study randomized 289 patients with MCI or mild Alzheimer’s to twice-daily sprays with a placebo device, or to intranasal insulin 40 IU for 12 months, followed by a 6-month, open-label period. The primary outcome was the Alzheimer’s Disease Assessment Scale-Cognition measure (ADAS-Cog 12). Secondary outcomes were the Clinical Dementia Rating Scale sum of boxes (CDR-sb) a memory composite measure, activities of daily living, cerebrospinal fluid biomarkers, and MRI of the hippocampus and entorhinal cortex.
Because of the device problems, Dr. Craft conducted separate analyses for the user groups. The primary was an intent-to-treat (ITT), mixed-model, repeat-measures analysis of the 240 using the POD device. The model controlled for age, sex, genetic risk status, and investigation site. An exploratory ITT analysis looked only at the ADAS-Cog 12 in the 49 who used the ViaNase device. Patients were a mean of 71 years old, with a mean Mini Mental State Exam score of 25. About 42% were positive for the high-risk apolipoprotein E epsilon-4 allele.
At 12 months, there was no between-group difference on the ADAS-Cog 12 measure; both groups increased by about 4 points, indicating worsening. Nor were there any changes in any of the Alzheimer’s-related biomarkers: amyloid-beta 40 and 42, total tau, or phosphorylated tau. There was a small but statistically significant difference in the sizes of the hippocampus and entorhinal cortex.
The ViaNase group fared somewhat better in the secondary analysis of the ADAS-Cog12. The measure increased by about 5 points in the placebo group, and about 2.5 points in the insulin group. The significant separation was evident at 3 months and continued to widen over the course of the study.
Compliance was very good in the larger group – around 85%. It was lower in the ViaNase group, probably because of the device’s unreliability. Retention was good in both groups. There were no significant differences in adverse events and no obvious safety issues.
The 6-month, open-label period will close out before the end of the year. In the meantime, Dr. Craft is conducting additional subgroup analyses on the 12-month data.
Dr. Craft has served as a consultant for GlaxoSmithKline and Accera.
SOURCE: Craft S et al. J Prev Alz Dis 2018;5(S1):S9, Abstract OC2.
BARCELONA –
Instead of doing poorly, patients using the faulty device actually experienced better outcomes than did those who entered the study later and used a more reliable inhaler, Suzanne Craft, PhD, said at the Clinical Trials on Alzheimer’s Disease conference.
A secondary analysis of the ViaNase device subgroup “replicated findings in our original studies,” which used the same atomizer, said Dr. Craft, a professor of gerontology and geriatric medicine at Wake Forest University, Winston-Salem, N.C. “We remain optimistic, but clearly we are at the beginning of understanding optimal insulin doses and delivery techniques for this population.”
The 289-patient, placebo-controlled study was predicated by a prior successful study by Dr. Craft and her colleagues, published in 2012 in JAMA Neurology. That trial randomized 104 patients with amnestic mild cognitive impairment (MCI) or mild-moderate Alzheimer’s to placebo or intranasal insulin 20 or 40 IU. After 4 months, subjects in both insulin groups showed preserved cognition and functional abilities, as well as increased cerebral glucose metabolism.
The ViaNase device was manufactured by Kurve Technology. But the company redesigned it for the new trial, adding an electronic timing component, which Dr. Craft said, was supposed to increase ease of use.
“Unfortunately, there were frequent malfunctions of this mechanism for the first 49 patients – so much so that we had to discontinue using the device and switch to a newer one,” for the other 240 patients in the study. This intranasal drug-delivery system, called the Precisions Olfactory Delivery (POD) device, is made by Impel NeuroPharma. Dr. Craft’s trial is its first investigation in patients with Alzheimer’s disease.
The new study randomized 289 patients with MCI or mild Alzheimer’s to twice-daily sprays with a placebo device, or to intranasal insulin 40 IU for 12 months, followed by a 6-month, open-label period. The primary outcome was the Alzheimer’s Disease Assessment Scale-Cognition measure (ADAS-Cog 12). Secondary outcomes were the Clinical Dementia Rating Scale sum of boxes (CDR-sb) a memory composite measure, activities of daily living, cerebrospinal fluid biomarkers, and MRI of the hippocampus and entorhinal cortex.
Because of the device problems, Dr. Craft conducted separate analyses for the user groups. The primary was an intent-to-treat (ITT), mixed-model, repeat-measures analysis of the 240 using the POD device. The model controlled for age, sex, genetic risk status, and investigation site. An exploratory ITT analysis looked only at the ADAS-Cog 12 in the 49 who used the ViaNase device. Patients were a mean of 71 years old, with a mean Mini Mental State Exam score of 25. About 42% were positive for the high-risk apolipoprotein E epsilon-4 allele.
At 12 months, there was no between-group difference on the ADAS-Cog 12 measure; both groups increased by about 4 points, indicating worsening. Nor were there any changes in any of the Alzheimer’s-related biomarkers: amyloid-beta 40 and 42, total tau, or phosphorylated tau. There was a small but statistically significant difference in the sizes of the hippocampus and entorhinal cortex.
The ViaNase group fared somewhat better in the secondary analysis of the ADAS-Cog12. The measure increased by about 5 points in the placebo group, and about 2.5 points in the insulin group. The significant separation was evident at 3 months and continued to widen over the course of the study.
Compliance was very good in the larger group – around 85%. It was lower in the ViaNase group, probably because of the device’s unreliability. Retention was good in both groups. There were no significant differences in adverse events and no obvious safety issues.
The 6-month, open-label period will close out before the end of the year. In the meantime, Dr. Craft is conducting additional subgroup analyses on the 12-month data.
Dr. Craft has served as a consultant for GlaxoSmithKline and Accera.
SOURCE: Craft S et al. J Prev Alz Dis 2018;5(S1):S9, Abstract OC2.
REPORTING FROM CTAD
Geriatrics update 2018: Challenges in mental health, mobility, and postdischarge care
Unfortunately, recent research has not unveiled a breakthrough for preventing or treating cognitive impairment or Alzheimer disease. But several studies from the last 2 years are helping to drive the field of geriatrics forward, providing evidence of what does and does not help a variety of issues specific to the elderly.
Based on a search of the 2017 and 2018 literature, this article presents new evidence on preventing and treating cognitive impairment, managing dementia-associated behavioral disturbances and delirium, preventing falls, and improving inpatient mobility and posthospital care transitions.
COGNITIVE IMPAIRMENT, DEMENTIA: STILL NO SILVER BULLET
With the exception of oral anticoagulation treatment for atrial fibrillation, there is little evidence that pharmacologic or nonpharmacologic interventions slow the onset or progression of Alzheimer disease.
Nonpharmacologic interventions
Home occupational therapy. A 2-year home-based occupational therapy intervention1 showed no evidence of slowing functional decline in patients with Alzheimer disease. The randomized controlled trial involving 180 participants consisted of monthly sessions of an intensive, well-established collaborative-care management model that included fall prevention and other safety strategies, personalized training in activities of daily living, exercise, and education. Outcome measures for activities of daily living did not differ significantly between the treatment and control groups.1
Physical activity. Whether physical activity interventions slow cognitive decline and prevent dementia in cognitively intact adults was examined in a systematic review of 32 trials.2 Most of the trials followed patients for 6 months; a few stretched for 1 or 2 years.
Evidence was insufficient to prove cognitive benefit for short-term, single-component or multicomponent physical activity interventions. However, a multidomain physical activity intervention that also included dietary modifications and cognitive training did show a delay in cognitive decline, but only “low-strength” evidence.2
Nutritional supplements. The antioxidants vitamin E and selenium were studied for their possible cognitive benefit in the double-blind randomized Prevention of Alzheimer Disease by Vitamin E and Selenium trial3 in 3,786 asymptomatic men ages 60 and older. Neither supplement was found to prevent dementia over a 7-year follow-up period.
A review of 38 trials4 evaluated the effects on cognition of omega-3 fatty acids, soy, ginkgo biloba, B vitamins, vitamin D plus calcium, vitamin C, beta-carotene, and multi-ingredient supplements. It found insufficient evidence to recommend any over-the-counter supplement for cognitive protection in adults with normal cognition or mild cognitive impairment.
Pharmacologic treatments
Testosterone supplementation. The Testosterone Trials tested the effects of testosterone gel vs placebo for 1 year on 493 men over age 65 with low testosterone (< 275 ng/mL) and with subjective memory complaints and objective memory performance deficits. Treatment was not associated with improved memory or other cognitive functions compared with placebo.5
Antiamyloid drugs. A randomized, double-blind, placebo-controlled trial in nearly 2,000 patients evaluated verubecestat, an oral beta-site amyloid precursor protein-cleaving enzyme-1 inhibitor that reduces the amyloid-beta level in cerebrospinal fluid.6
Verubecestat did not reduce cognitive or functional decline in patients with mild-to-moderate Alzheimer disease, while adverse events including rashes, falls, injuries, sleep disturbances, suicidal ideation, weight loss, and hair color change were more common in the treatment groups. The trial was terminated early because of futility at 50 months.
And in a placebo-controlled trial of solanezumab, a monoclonal antibody directed against the amyloid beta peptide, no benefit was demonstrated at 80 weeks in more than 2,000 patients with Alzheimer disease.7
Multiple common agents. A well-conducted systematic review8 of 51 trials of at least a 6-month duration did not support the use of antihypertensive agents, diabetes medications, nonsteroidal anti-inflammatory drugs, aspirin, hormones, or lipid-lowering drugs for cognitive protection for people with normal cognition or mild cognitive impairment.
However, some studies found reassuring evidence that standard therapies for other conditions do not worsen cognitive decline and are protective for atrial fibrillation.8
Proton-pump inhibitors. Concern exists for a potential link between dementia risk and proton-pump inhibitors, which are widely used to treat acid-related gastrointestinal disorders.9
A prospective population-based cohort study10 of nearly 3,500 people ages 65 and older without baseline dementia screened participants for dementia every 2 years over a mean period of 7.5 years and provided further evaluation for those who screened positive. Use of proton-pump inhibitors was not found to be associated with dementia risk, even with high cumulative exposure.
Results from this study do not support avoiding proton-pump inhibitors out of concern for dementia risk, although long-term use is associated with other safety concerns.
Oral anticoagulation. The increased risk of dementia with atrial fibrillation is well documented.11
A retrospective study12 based on a Swedish health registry and using more than 444,000 patients covering more than 1.5 million years at risk found that oral anticoagulant treatment at baseline conferred a 29% lower risk of dementia in an intention-to-treat analysis and a 48% lower risk in on-treatment analysis compared with no oral anticoagulation therapy. No difference was found between new oral anticoagulants and warfarin.
Transcatheter aortic valve implantation is not associated with cognitive decline
For patients with severe aortic stenosis who are not surgical candidates, transcatheter aortic valve implantation is superior to standard medical therapy,13 but there are concerns of neurologic and cognitive changes after the procedure.14 A meta-analysis of 18 studies assessing cognitive performance in more than 1,000 patients (average age ≥ 80) after undergoing the procedure for severe aortic stenosis found no significant cognitive performance changes from baseline perioperatively or 3 or 6 months later.15
TREATING DEMENTIA-ASSOCIATED BEHAVIORAL DISTURBANCES
Behavioral and psychiatric symptoms often accompany dementia, but no drugs have yet been approved by the US Food and Drug Administration (FDA) to address them in this population. Nonpharmacologic interventions are recommended as first-line therapy.
Antipsychotics are not recommended
Antipsychotics are often prescribed,16 although they are associated with metabolic syndrome17 and increased risks of stroke and death.18 The FDA has issued black box warnings against using antipsychotics for behavioral management in patients with dementia. Further, the American Geriatrics Society and the American Psychiatric Association do not endorse using them as initial therapy for behavioral and psychological symptoms of dementia.16,19
The Centers for Medicare and Medicaid Services partnered with nursing homes to improve the quality of care for patients with dementia, with results measured as the rate of prescribing antipsychotic medications. Although the use of psychotropic medications declined after initiating the partnership, the use of mood stabilizers increased, possibly as a substitute for antipsychotics.20
Dextromethorphan-quinidine use is up, despite modest evidence of benefit
A consumer news report in 2017 stated that the use of dextromethorphan-quinidine in long-term care facilities increased by nearly 400% between 2012 and 2016.21
Evidence for its benefits comes from a 10-week, phase 2, randomized controlled trial conducted at 42 US study sites with 194 patients with probable Alzheimer disease. Compared with the placebo group, the active treatment group had mildly reduced agitation but an increased risk of falls, dizziness, and diarrhea. However, rates of adverse effects were low, and the authors concluded that treatment was generally well tolerated.22
Pimavanserin: No long-term benefit for psychosis
In a phase 2, randomized, double-blind, placebo-controlled trial in 181 patients with possible or probable Alzheimer disease and psychotic symptoms, pimavanserin was associated with improved symptoms as measured by the Neuropsychiatric Inventory–Nursing Home Version psychosis score at 6 weeks, but no difference was found compared with placebo at 12 weeks. The treatment group had more adverse events, including agitation, aggression, peripheral edema, anxiety, and symptoms of dementia, although the differences were not statistically significant.23
DELIRIUM: AVOID ANTIPSYCHOTICS
Delirium is common in hospitalized older adults, especially those who have baseline cognitive or functional impairment and are exposed to precipitating factors such as treatment with anticholinergic or narcotic medications, infection, surgery, or admission to an intensive care unit.24
Delirium at discharge predicts poor outcomes
In a prospective study of 152 hospitalized patients with delirium, those who either did not recover from delirium or had only partially recovered at discharge were more likely to visit the emergency department, be rehospitalized, or die during the subsequent 3 months than those who had fully recovered from delirium at discharge.25
Multicomponent, patient-centered approach can help
A randomized trial in 377 patients in Taiwan evaluated the use of a modified Hospital Elder Life Program, consisting of 3 protocols focused on orienting communication, oral and nutritional assistance, and early mobilization. Patients were at least 65 years old and undergoing elective abdominal surgery with expected length of hospital stay longer than 6 days. The program, administered daily during hospitalization, significantly lowered postoperative delirium by 56% and hospital stay by 2 days compared with usual care.26
Prophylactic haloperidol does not improve outcomes
In a multicenter randomized, double-blind, placebo-controlled trial, van den Boogaard et al studied prophylactic intravenous haloperidol in nearly 1,800 critically ill patients at high risk of delirium.27 Haloperidol did not improve survival at 28 days compared with placebo. For secondary outcomes, including delirium incidence, delirium-free and coma-free days, duration of mechanical ventilation, and hospital and intensive care department length of stay, treatment was not found to differ statistically from placebo.
Antipsychotics may worsen delirium
A double-blind, parallel-arm, dose-titrated randomized trial, conducted at 11 Australian hospices or hospitals with palliative care services, administered oral risperidone, haloperidol, or placebo to 247 patients with life-limiting illness and delirium. Both treatment groups had higher delirium symptom scores than the placebo group.28
In addition, a systematic review and meta-analysis of 19 studies found no benefit of antipsychotic medications for preventing or treating delirium in hospitalized adults.29
Antipsychotics are often continued indefinitely
A retrospective chart review at a US academic health system found30 that among 487 patients with a new antipsychotic medication prescribed during hospitalization, 147 (30.2%) were discharged on an antipsychotic. Of these, 121 (82.3%) had a diagnosis of delirium. Only 15 (12.4%) had discharge summaries that included instructions for discontinuing the drug.
Another US health system retrospectively reviewed antipsychotic use and found31 that out of 260 patients who were newly exposed to an antipsychotic drug during hospitalization, 146 (56.2%) were discharged on an antipsychotic drug, and 65% of these patients were still on the drug at the time of the next hospital admission.
EXERCISE, EXERCISE, EXERCISE
Exercise recommended, but not vitamin D, to prevent falls
In 2018, the US Preventive Services Task Force updated its recommendations for preventing falls in community-dwelling older adults.32 Based on the findings of several trials, the task force recommends exercise interventions for adults age 65 and older who are at increased risk for falls. Gait, balance, and functional training were studied in 17 trials, resistance training in 13, flexibility in 8, endurance training in 5, and tai chi in 3, with 5 studies including general physical activity. Exercise interventions most commonly took place for 3 sessions per week for 12 months (range 2–42 months).
The task force also recommends against vitamin D supplementation for fall prevention in community-dwelling adults age 65 or older who are not known to have osteoporosis or vitamin D deficiency.
Early mobilization helps inpatients
Hospitalized older adults usually spend most of their time in bed. Forty-five previously ambulatory patients (age ≥ 65 without dementia or delirium) in a Veterans Affairs hospital were monitored with wireless accelerometers and were found to spend, on average, 83% of the measured hospital stay in bed. Standing or walking time ranged from 0.2% to 21%, with a median of only 3% (43 minutes a day).33
Since falls with injury became a Centers for Medicare and Medicaid Services nonreimbursable hospital-acquired condition, tension has arisen between promoting mobility and preventing falls.34 Two studies evaluating the adoption of mobility-restricting approaches such as bed-alarms, “fall-alert” signs, supervision of patients in the bathroom, and ensuring patients’ walking aids are within reach, did not find a significant reduction in falls or fall-related injuries.35,36
A clinically significant loss of community mobility is common after hospitalization in older adults.37 Older adults who developed mobility impairment during hospitalization had a higher risk of death in a large, retrospective study.38 A large Canadian multisite intervention trial39 that promoted early mobilization in older patients who were admitted to general medical wards resulted in increased mobilization and significantly shorter hospital stays.
POSTHOSPITAL CARE NEEDS IMPROVEMENT
After hospitalization, older adults who have difficulty with activities of daily living or complex medical needs often require continued care.
About 20% of hospitalized Medicare beneficiaries in the United States are discharged to skilled nursing facilities.40 This is often a stressful transition, and most people have little guidance on selecting a facility and simply choose one based on its proximity to home.41
A program of frequent visits by hospital-employed physicians and advanced practice professionals at skilled nursing facilities resulted in a significantly lower 30-day readmission rate compared with nonparticipating skilled nursing facilities in the same geographic area.42
Home healthcare is recommended after hospital discharge at a rapidly increasing rate. Overall referral rates increased from 8.6% to 14.1% between 2001 and 2012, and from 14.3% to 24.0% for patients with heart failure.43 A qualitative study of home healthcare nurses found a need for improved care coordination between home healthcare agencies and discharging hospitals, including defining accountability for orders and enhancing communication.44
- Callahan CM, Boustani MA, Schmid AA, et al. Targeting functional decline in Alzheimer disease: a randomized trial. Ann Intern Med 2017; 166(3):164–171. doi:10.7326/M16-0830
- Brasure M, Desai P, Davila H, et al. Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):30–38. doi:10.7326/M17-1528
- Kryscio RJ, Abner EL, Caban-Holt A, et al. Association of antioxidant supplement use and dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol 2017; 74(5):567–573. doi:10.1001/jamaneurol.2016.5778
- Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):52–62. doi:10.7326/M17-1530
- Resnick SM, Matsumoto AM, Stephens-Shields AJ, et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA 2017; 317(7):717–727. doi:10.1001/jama.2016.21044
- Egan MF, Kost J, Tariot PN, et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med 2018; 378(18):1691–1703. doi:10.1056/NEJMoa1706441
- Honig LS, Vellas B, Woodward M, et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 2018; 378(4):321–330. doi:10.1056/NEJMoa1705971
- Fink HA, Jutkowitz E, McCarten JR, et al. Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):39–51. doi:10.7326/M17-1529
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73(4):410–416. doi:10.1001/jamaneurol.2015.4791
- Gray SL, Walker RL, Dublin S, et al. Proton pump inhibitor use and dementia risk: prospective population-based study. J Am Geriatr Soc 2018; 66(2):247–253. doi:10.1111/jgs.15073
- de Bruijn RF, Heeringa J, Wolters FJ, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol 2015; 72(11):1288–1294. doi:10.1001/jamaneurol.2015.2161
- Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2018; 39(6):453–460. doi:10.1093/eurheartj/ehx579
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363(17):1597–1607. doi:10.1056/NEJMoa1008232
- Haussig S, Mangner N, Dwyer MG, et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI randomized clinical trial. JAMA 2016; 316(6):592–601. doi:10.1001/jama.2016.10302
- Khan MM, Herrmann N, Gallagher D, et al. Cognitive outcomes after transcatheter aortic valve implantation: a metaanalysis. J Am Geriatr Soc 2018; 66(2):254–262. doi:10.1111/jgs.15123
- Choosing Wisely; ABIM Foundation. American Geriatrics Society: ten things physicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society. Accessed November 6, 2018.
- Lieberman JA 3rd. Metabolic changes associated with antipsychotic use. Prim Care Companion J Clin Psychiatry 2004; 6(suppl 2):8–13. pmid:16001095
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294(15):1934–1943. doi:10.1001/jama.294.15.1934
- Choosing Wisely; ABIM Foundation. American Psychiatric Association: five things physicians and patients should question. www.choosingwisely.org/societies/american-psychiatric-association. Accessed November 6, 2018.
- Maust DT, Kim HM, Chiang C, Kales HC. Association of the Centers for Medicare & Medicaid Services’ National Partnership to improve dementia care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med 2018; 178(5):640–647. doi:10.1001/jamainternmed.2018.0379
- CNN. The little red pill being pushed on the elderly. www.cnn.com/2017/10/12/health/nuedexta-nursing-homes-invs/index.html. Accessed November 6, 2018.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314(12):1242–1254. doi:10.1001/jama.2015.10214
- Ballard C, Banister C, Khan Z, et al; ADP Investigators. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol 2018; 17(3):213–222. doi:10.1016/S1474-4422(18)30039-5
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354(11):1157–1165. doi:10.1056/NEJMra052321
- Cole MG, McCusker J, Bailey R, et al. Partial and no recovery from delirium after hospital discharge predict increased adverse events. Age Ageing 2017; 46(1):90–95. doi:10.1093/ageing/afw153
- Chen CC, Li HC, Liang JT, et al. Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surg 2017; 152(9):827–834. doi:10.1001/jamasurg.2017.1083
- van den Boogaard M, Slooter AJC, Brüggemann RJM, et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA 2018; 319(7):680–690. doi:10.1001/jama.2018.0160
- Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med 2017; 177(1):34–42. doi:10.1001/jamainternmed.2016.7491
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 64(4):705–714. doi:10.1111/jgs.14076
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- Loh KP, Ramdass S, Garb JL, et al. Long-term outcomes of elders discharged on antipsychotics. J Hosp Med 2016; 11(8):550–555. doi:10.1002/jhm.2585
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation statement. JAMA 2018; 319(16):1696–1704. doi:10.1001/jama.2018.3097
- Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc 2009; 57(9):1660–1665. doi:10.1111/j.1532-5415.2009.02393.x
- Growdon ME, Shorr RI, Inouye SK. The tension between promoting mobility and preventing falls in the hospital. JAMA Intern Med 2017; 177(6):759–760. doi:10.1001/jamainternmed.2017.0840
- Barker AL, Morello RT, Wolfe R, et al. 6-PACK programme to decrease fall injuries in acute hospitals: cluster randomised controlled trial. BMJ 2016; 352:h6781. doi:10.1136/bmj.h6781
- Shorr RI, Chandler AM, Mion LC, et al. Effects of an intervention to increase bed alarm use to prevent falls in hospitalized patients: a cluster randomized trial. Ann Intern Med 2012; 157(10):692–699. doi:10.7326/0003-4819-157-10-201211200-00005
- Loyd C, Beasley TM, Miltner RS, Clark D, King B, Brown CJ. Trajectories of community mobility recovery after hospitalization in older adults. J Am Geriatr Soc 2018; 66(7):1399–1403. doi:10.1111/jgs.15397
- Valiani V, Chen Z, Lipori G, Pahor M, Sabbá C, Manini TM. Prognostic value of Braden Activity subscale for mobility status in hospitalized older adults. J Hosp Med 2017; 12(6):396–401. doi:10.12788/jhm.2748
- Liu B, Moore JE, Almaawiy U, et al; MOVE ON Collaboration. Outcomes of mobilisation of vulnerable elders in Ontario (MOVE ON): a multisite interrupted time series evaluation of an implementation intervention to increase patient mobilisation. Age Ageing 2018; 47(1):112–119. doi:10.1093/ageing/afx128
- Report to Congress: Medicare Payment Policy. Medicare Payment Advisory Commission 2016. www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed November 6, 2018.
- Gadbois EA, Tyler DA, Mor V. Selecting a skilled nursing facility for postacute care: individual and family perspectives. J Am Geriatr Soc 2017; 65(11):2459–2465. doi:10.1111/jgs.14988
- Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med 2017; 12(4):238–244. doi:10.12788/jhm.2710
- Jones CD, Ginde AA, Burke RE, Wald HL, Masoudi FA, Boxer RS. Increasing home healthcare referrals upon discharge from U.S. hospitals: 2001-2012. J Am Geriatr Soc 2015; 63(6):1265–1266. doi:10.1111/jgs.13467
- Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med 2017; 32(10):1114–1121. doi:10.1007/s11606-017-4104-0
Unfortunately, recent research has not unveiled a breakthrough for preventing or treating cognitive impairment or Alzheimer disease. But several studies from the last 2 years are helping to drive the field of geriatrics forward, providing evidence of what does and does not help a variety of issues specific to the elderly.
Based on a search of the 2017 and 2018 literature, this article presents new evidence on preventing and treating cognitive impairment, managing dementia-associated behavioral disturbances and delirium, preventing falls, and improving inpatient mobility and posthospital care transitions.
COGNITIVE IMPAIRMENT, DEMENTIA: STILL NO SILVER BULLET
With the exception of oral anticoagulation treatment for atrial fibrillation, there is little evidence that pharmacologic or nonpharmacologic interventions slow the onset or progression of Alzheimer disease.
Nonpharmacologic interventions
Home occupational therapy. A 2-year home-based occupational therapy intervention1 showed no evidence of slowing functional decline in patients with Alzheimer disease. The randomized controlled trial involving 180 participants consisted of monthly sessions of an intensive, well-established collaborative-care management model that included fall prevention and other safety strategies, personalized training in activities of daily living, exercise, and education. Outcome measures for activities of daily living did not differ significantly between the treatment and control groups.1
Physical activity. Whether physical activity interventions slow cognitive decline and prevent dementia in cognitively intact adults was examined in a systematic review of 32 trials.2 Most of the trials followed patients for 6 months; a few stretched for 1 or 2 years.
Evidence was insufficient to prove cognitive benefit for short-term, single-component or multicomponent physical activity interventions. However, a multidomain physical activity intervention that also included dietary modifications and cognitive training did show a delay in cognitive decline, but only “low-strength” evidence.2
Nutritional supplements. The antioxidants vitamin E and selenium were studied for their possible cognitive benefit in the double-blind randomized Prevention of Alzheimer Disease by Vitamin E and Selenium trial3 in 3,786 asymptomatic men ages 60 and older. Neither supplement was found to prevent dementia over a 7-year follow-up period.
A review of 38 trials4 evaluated the effects on cognition of omega-3 fatty acids, soy, ginkgo biloba, B vitamins, vitamin D plus calcium, vitamin C, beta-carotene, and multi-ingredient supplements. It found insufficient evidence to recommend any over-the-counter supplement for cognitive protection in adults with normal cognition or mild cognitive impairment.
Pharmacologic treatments
Testosterone supplementation. The Testosterone Trials tested the effects of testosterone gel vs placebo for 1 year on 493 men over age 65 with low testosterone (< 275 ng/mL) and with subjective memory complaints and objective memory performance deficits. Treatment was not associated with improved memory or other cognitive functions compared with placebo.5
Antiamyloid drugs. A randomized, double-blind, placebo-controlled trial in nearly 2,000 patients evaluated verubecestat, an oral beta-site amyloid precursor protein-cleaving enzyme-1 inhibitor that reduces the amyloid-beta level in cerebrospinal fluid.6
Verubecestat did not reduce cognitive or functional decline in patients with mild-to-moderate Alzheimer disease, while adverse events including rashes, falls, injuries, sleep disturbances, suicidal ideation, weight loss, and hair color change were more common in the treatment groups. The trial was terminated early because of futility at 50 months.
And in a placebo-controlled trial of solanezumab, a monoclonal antibody directed against the amyloid beta peptide, no benefit was demonstrated at 80 weeks in more than 2,000 patients with Alzheimer disease.7
Multiple common agents. A well-conducted systematic review8 of 51 trials of at least a 6-month duration did not support the use of antihypertensive agents, diabetes medications, nonsteroidal anti-inflammatory drugs, aspirin, hormones, or lipid-lowering drugs for cognitive protection for people with normal cognition or mild cognitive impairment.
However, some studies found reassuring evidence that standard therapies for other conditions do not worsen cognitive decline and are protective for atrial fibrillation.8
Proton-pump inhibitors. Concern exists for a potential link between dementia risk and proton-pump inhibitors, which are widely used to treat acid-related gastrointestinal disorders.9
A prospective population-based cohort study10 of nearly 3,500 people ages 65 and older without baseline dementia screened participants for dementia every 2 years over a mean period of 7.5 years and provided further evaluation for those who screened positive. Use of proton-pump inhibitors was not found to be associated with dementia risk, even with high cumulative exposure.
Results from this study do not support avoiding proton-pump inhibitors out of concern for dementia risk, although long-term use is associated with other safety concerns.
Oral anticoagulation. The increased risk of dementia with atrial fibrillation is well documented.11
A retrospective study12 based on a Swedish health registry and using more than 444,000 patients covering more than 1.5 million years at risk found that oral anticoagulant treatment at baseline conferred a 29% lower risk of dementia in an intention-to-treat analysis and a 48% lower risk in on-treatment analysis compared with no oral anticoagulation therapy. No difference was found between new oral anticoagulants and warfarin.
Transcatheter aortic valve implantation is not associated with cognitive decline
For patients with severe aortic stenosis who are not surgical candidates, transcatheter aortic valve implantation is superior to standard medical therapy,13 but there are concerns of neurologic and cognitive changes after the procedure.14 A meta-analysis of 18 studies assessing cognitive performance in more than 1,000 patients (average age ≥ 80) after undergoing the procedure for severe aortic stenosis found no significant cognitive performance changes from baseline perioperatively or 3 or 6 months later.15
TREATING DEMENTIA-ASSOCIATED BEHAVIORAL DISTURBANCES
Behavioral and psychiatric symptoms often accompany dementia, but no drugs have yet been approved by the US Food and Drug Administration (FDA) to address them in this population. Nonpharmacologic interventions are recommended as first-line therapy.
Antipsychotics are not recommended
Antipsychotics are often prescribed,16 although they are associated with metabolic syndrome17 and increased risks of stroke and death.18 The FDA has issued black box warnings against using antipsychotics for behavioral management in patients with dementia. Further, the American Geriatrics Society and the American Psychiatric Association do not endorse using them as initial therapy for behavioral and psychological symptoms of dementia.16,19
The Centers for Medicare and Medicaid Services partnered with nursing homes to improve the quality of care for patients with dementia, with results measured as the rate of prescribing antipsychotic medications. Although the use of psychotropic medications declined after initiating the partnership, the use of mood stabilizers increased, possibly as a substitute for antipsychotics.20
Dextromethorphan-quinidine use is up, despite modest evidence of benefit
A consumer news report in 2017 stated that the use of dextromethorphan-quinidine in long-term care facilities increased by nearly 400% between 2012 and 2016.21
Evidence for its benefits comes from a 10-week, phase 2, randomized controlled trial conducted at 42 US study sites with 194 patients with probable Alzheimer disease. Compared with the placebo group, the active treatment group had mildly reduced agitation but an increased risk of falls, dizziness, and diarrhea. However, rates of adverse effects were low, and the authors concluded that treatment was generally well tolerated.22
Pimavanserin: No long-term benefit for psychosis
In a phase 2, randomized, double-blind, placebo-controlled trial in 181 patients with possible or probable Alzheimer disease and psychotic symptoms, pimavanserin was associated with improved symptoms as measured by the Neuropsychiatric Inventory–Nursing Home Version psychosis score at 6 weeks, but no difference was found compared with placebo at 12 weeks. The treatment group had more adverse events, including agitation, aggression, peripheral edema, anxiety, and symptoms of dementia, although the differences were not statistically significant.23
DELIRIUM: AVOID ANTIPSYCHOTICS
Delirium is common in hospitalized older adults, especially those who have baseline cognitive or functional impairment and are exposed to precipitating factors such as treatment with anticholinergic or narcotic medications, infection, surgery, or admission to an intensive care unit.24
Delirium at discharge predicts poor outcomes
In a prospective study of 152 hospitalized patients with delirium, those who either did not recover from delirium or had only partially recovered at discharge were more likely to visit the emergency department, be rehospitalized, or die during the subsequent 3 months than those who had fully recovered from delirium at discharge.25
Multicomponent, patient-centered approach can help
A randomized trial in 377 patients in Taiwan evaluated the use of a modified Hospital Elder Life Program, consisting of 3 protocols focused on orienting communication, oral and nutritional assistance, and early mobilization. Patients were at least 65 years old and undergoing elective abdominal surgery with expected length of hospital stay longer than 6 days. The program, administered daily during hospitalization, significantly lowered postoperative delirium by 56% and hospital stay by 2 days compared with usual care.26
Prophylactic haloperidol does not improve outcomes
In a multicenter randomized, double-blind, placebo-controlled trial, van den Boogaard et al studied prophylactic intravenous haloperidol in nearly 1,800 critically ill patients at high risk of delirium.27 Haloperidol did not improve survival at 28 days compared with placebo. For secondary outcomes, including delirium incidence, delirium-free and coma-free days, duration of mechanical ventilation, and hospital and intensive care department length of stay, treatment was not found to differ statistically from placebo.
Antipsychotics may worsen delirium
A double-blind, parallel-arm, dose-titrated randomized trial, conducted at 11 Australian hospices or hospitals with palliative care services, administered oral risperidone, haloperidol, or placebo to 247 patients with life-limiting illness and delirium. Both treatment groups had higher delirium symptom scores than the placebo group.28
In addition, a systematic review and meta-analysis of 19 studies found no benefit of antipsychotic medications for preventing or treating delirium in hospitalized adults.29
Antipsychotics are often continued indefinitely
A retrospective chart review at a US academic health system found30 that among 487 patients with a new antipsychotic medication prescribed during hospitalization, 147 (30.2%) were discharged on an antipsychotic. Of these, 121 (82.3%) had a diagnosis of delirium. Only 15 (12.4%) had discharge summaries that included instructions for discontinuing the drug.
Another US health system retrospectively reviewed antipsychotic use and found31 that out of 260 patients who were newly exposed to an antipsychotic drug during hospitalization, 146 (56.2%) were discharged on an antipsychotic drug, and 65% of these patients were still on the drug at the time of the next hospital admission.
EXERCISE, EXERCISE, EXERCISE
Exercise recommended, but not vitamin D, to prevent falls
In 2018, the US Preventive Services Task Force updated its recommendations for preventing falls in community-dwelling older adults.32 Based on the findings of several trials, the task force recommends exercise interventions for adults age 65 and older who are at increased risk for falls. Gait, balance, and functional training were studied in 17 trials, resistance training in 13, flexibility in 8, endurance training in 5, and tai chi in 3, with 5 studies including general physical activity. Exercise interventions most commonly took place for 3 sessions per week for 12 months (range 2–42 months).
The task force also recommends against vitamin D supplementation for fall prevention in community-dwelling adults age 65 or older who are not known to have osteoporosis or vitamin D deficiency.
Early mobilization helps inpatients
Hospitalized older adults usually spend most of their time in bed. Forty-five previously ambulatory patients (age ≥ 65 without dementia or delirium) in a Veterans Affairs hospital were monitored with wireless accelerometers and were found to spend, on average, 83% of the measured hospital stay in bed. Standing or walking time ranged from 0.2% to 21%, with a median of only 3% (43 minutes a day).33
Since falls with injury became a Centers for Medicare and Medicaid Services nonreimbursable hospital-acquired condition, tension has arisen between promoting mobility and preventing falls.34 Two studies evaluating the adoption of mobility-restricting approaches such as bed-alarms, “fall-alert” signs, supervision of patients in the bathroom, and ensuring patients’ walking aids are within reach, did not find a significant reduction in falls or fall-related injuries.35,36
A clinically significant loss of community mobility is common after hospitalization in older adults.37 Older adults who developed mobility impairment during hospitalization had a higher risk of death in a large, retrospective study.38 A large Canadian multisite intervention trial39 that promoted early mobilization in older patients who were admitted to general medical wards resulted in increased mobilization and significantly shorter hospital stays.
POSTHOSPITAL CARE NEEDS IMPROVEMENT
After hospitalization, older adults who have difficulty with activities of daily living or complex medical needs often require continued care.
About 20% of hospitalized Medicare beneficiaries in the United States are discharged to skilled nursing facilities.40 This is often a stressful transition, and most people have little guidance on selecting a facility and simply choose one based on its proximity to home.41
A program of frequent visits by hospital-employed physicians and advanced practice professionals at skilled nursing facilities resulted in a significantly lower 30-day readmission rate compared with nonparticipating skilled nursing facilities in the same geographic area.42
Home healthcare is recommended after hospital discharge at a rapidly increasing rate. Overall referral rates increased from 8.6% to 14.1% between 2001 and 2012, and from 14.3% to 24.0% for patients with heart failure.43 A qualitative study of home healthcare nurses found a need for improved care coordination between home healthcare agencies and discharging hospitals, including defining accountability for orders and enhancing communication.44
Unfortunately, recent research has not unveiled a breakthrough for preventing or treating cognitive impairment or Alzheimer disease. But several studies from the last 2 years are helping to drive the field of geriatrics forward, providing evidence of what does and does not help a variety of issues specific to the elderly.
Based on a search of the 2017 and 2018 literature, this article presents new evidence on preventing and treating cognitive impairment, managing dementia-associated behavioral disturbances and delirium, preventing falls, and improving inpatient mobility and posthospital care transitions.
COGNITIVE IMPAIRMENT, DEMENTIA: STILL NO SILVER BULLET
With the exception of oral anticoagulation treatment for atrial fibrillation, there is little evidence that pharmacologic or nonpharmacologic interventions slow the onset or progression of Alzheimer disease.
Nonpharmacologic interventions
Home occupational therapy. A 2-year home-based occupational therapy intervention1 showed no evidence of slowing functional decline in patients with Alzheimer disease. The randomized controlled trial involving 180 participants consisted of monthly sessions of an intensive, well-established collaborative-care management model that included fall prevention and other safety strategies, personalized training in activities of daily living, exercise, and education. Outcome measures for activities of daily living did not differ significantly between the treatment and control groups.1
Physical activity. Whether physical activity interventions slow cognitive decline and prevent dementia in cognitively intact adults was examined in a systematic review of 32 trials.2 Most of the trials followed patients for 6 months; a few stretched for 1 or 2 years.
Evidence was insufficient to prove cognitive benefit for short-term, single-component or multicomponent physical activity interventions. However, a multidomain physical activity intervention that also included dietary modifications and cognitive training did show a delay in cognitive decline, but only “low-strength” evidence.2
Nutritional supplements. The antioxidants vitamin E and selenium were studied for their possible cognitive benefit in the double-blind randomized Prevention of Alzheimer Disease by Vitamin E and Selenium trial3 in 3,786 asymptomatic men ages 60 and older. Neither supplement was found to prevent dementia over a 7-year follow-up period.
A review of 38 trials4 evaluated the effects on cognition of omega-3 fatty acids, soy, ginkgo biloba, B vitamins, vitamin D plus calcium, vitamin C, beta-carotene, and multi-ingredient supplements. It found insufficient evidence to recommend any over-the-counter supplement for cognitive protection in adults with normal cognition or mild cognitive impairment.
Pharmacologic treatments
Testosterone supplementation. The Testosterone Trials tested the effects of testosterone gel vs placebo for 1 year on 493 men over age 65 with low testosterone (< 275 ng/mL) and with subjective memory complaints and objective memory performance deficits. Treatment was not associated with improved memory or other cognitive functions compared with placebo.5
Antiamyloid drugs. A randomized, double-blind, placebo-controlled trial in nearly 2,000 patients evaluated verubecestat, an oral beta-site amyloid precursor protein-cleaving enzyme-1 inhibitor that reduces the amyloid-beta level in cerebrospinal fluid.6
Verubecestat did not reduce cognitive or functional decline in patients with mild-to-moderate Alzheimer disease, while adverse events including rashes, falls, injuries, sleep disturbances, suicidal ideation, weight loss, and hair color change were more common in the treatment groups. The trial was terminated early because of futility at 50 months.
And in a placebo-controlled trial of solanezumab, a monoclonal antibody directed against the amyloid beta peptide, no benefit was demonstrated at 80 weeks in more than 2,000 patients with Alzheimer disease.7
Multiple common agents. A well-conducted systematic review8 of 51 trials of at least a 6-month duration did not support the use of antihypertensive agents, diabetes medications, nonsteroidal anti-inflammatory drugs, aspirin, hormones, or lipid-lowering drugs for cognitive protection for people with normal cognition or mild cognitive impairment.
However, some studies found reassuring evidence that standard therapies for other conditions do not worsen cognitive decline and are protective for atrial fibrillation.8
Proton-pump inhibitors. Concern exists for a potential link between dementia risk and proton-pump inhibitors, which are widely used to treat acid-related gastrointestinal disorders.9
A prospective population-based cohort study10 of nearly 3,500 people ages 65 and older without baseline dementia screened participants for dementia every 2 years over a mean period of 7.5 years and provided further evaluation for those who screened positive. Use of proton-pump inhibitors was not found to be associated with dementia risk, even with high cumulative exposure.
Results from this study do not support avoiding proton-pump inhibitors out of concern for dementia risk, although long-term use is associated with other safety concerns.
Oral anticoagulation. The increased risk of dementia with atrial fibrillation is well documented.11
A retrospective study12 based on a Swedish health registry and using more than 444,000 patients covering more than 1.5 million years at risk found that oral anticoagulant treatment at baseline conferred a 29% lower risk of dementia in an intention-to-treat analysis and a 48% lower risk in on-treatment analysis compared with no oral anticoagulation therapy. No difference was found between new oral anticoagulants and warfarin.
Transcatheter aortic valve implantation is not associated with cognitive decline
For patients with severe aortic stenosis who are not surgical candidates, transcatheter aortic valve implantation is superior to standard medical therapy,13 but there are concerns of neurologic and cognitive changes after the procedure.14 A meta-analysis of 18 studies assessing cognitive performance in more than 1,000 patients (average age ≥ 80) after undergoing the procedure for severe aortic stenosis found no significant cognitive performance changes from baseline perioperatively or 3 or 6 months later.15
TREATING DEMENTIA-ASSOCIATED BEHAVIORAL DISTURBANCES
Behavioral and psychiatric symptoms often accompany dementia, but no drugs have yet been approved by the US Food and Drug Administration (FDA) to address them in this population. Nonpharmacologic interventions are recommended as first-line therapy.
Antipsychotics are not recommended
Antipsychotics are often prescribed,16 although they are associated with metabolic syndrome17 and increased risks of stroke and death.18 The FDA has issued black box warnings against using antipsychotics for behavioral management in patients with dementia. Further, the American Geriatrics Society and the American Psychiatric Association do not endorse using them as initial therapy for behavioral and psychological symptoms of dementia.16,19
The Centers for Medicare and Medicaid Services partnered with nursing homes to improve the quality of care for patients with dementia, with results measured as the rate of prescribing antipsychotic medications. Although the use of psychotropic medications declined after initiating the partnership, the use of mood stabilizers increased, possibly as a substitute for antipsychotics.20
Dextromethorphan-quinidine use is up, despite modest evidence of benefit
A consumer news report in 2017 stated that the use of dextromethorphan-quinidine in long-term care facilities increased by nearly 400% between 2012 and 2016.21
Evidence for its benefits comes from a 10-week, phase 2, randomized controlled trial conducted at 42 US study sites with 194 patients with probable Alzheimer disease. Compared with the placebo group, the active treatment group had mildly reduced agitation but an increased risk of falls, dizziness, and diarrhea. However, rates of adverse effects were low, and the authors concluded that treatment was generally well tolerated.22
Pimavanserin: No long-term benefit for psychosis
In a phase 2, randomized, double-blind, placebo-controlled trial in 181 patients with possible or probable Alzheimer disease and psychotic symptoms, pimavanserin was associated with improved symptoms as measured by the Neuropsychiatric Inventory–Nursing Home Version psychosis score at 6 weeks, but no difference was found compared with placebo at 12 weeks. The treatment group had more adverse events, including agitation, aggression, peripheral edema, anxiety, and symptoms of dementia, although the differences were not statistically significant.23
DELIRIUM: AVOID ANTIPSYCHOTICS
Delirium is common in hospitalized older adults, especially those who have baseline cognitive or functional impairment and are exposed to precipitating factors such as treatment with anticholinergic or narcotic medications, infection, surgery, or admission to an intensive care unit.24
Delirium at discharge predicts poor outcomes
In a prospective study of 152 hospitalized patients with delirium, those who either did not recover from delirium or had only partially recovered at discharge were more likely to visit the emergency department, be rehospitalized, or die during the subsequent 3 months than those who had fully recovered from delirium at discharge.25
Multicomponent, patient-centered approach can help
A randomized trial in 377 patients in Taiwan evaluated the use of a modified Hospital Elder Life Program, consisting of 3 protocols focused on orienting communication, oral and nutritional assistance, and early mobilization. Patients were at least 65 years old and undergoing elective abdominal surgery with expected length of hospital stay longer than 6 days. The program, administered daily during hospitalization, significantly lowered postoperative delirium by 56% and hospital stay by 2 days compared with usual care.26
Prophylactic haloperidol does not improve outcomes
In a multicenter randomized, double-blind, placebo-controlled trial, van den Boogaard et al studied prophylactic intravenous haloperidol in nearly 1,800 critically ill patients at high risk of delirium.27 Haloperidol did not improve survival at 28 days compared with placebo. For secondary outcomes, including delirium incidence, delirium-free and coma-free days, duration of mechanical ventilation, and hospital and intensive care department length of stay, treatment was not found to differ statistically from placebo.
Antipsychotics may worsen delirium
A double-blind, parallel-arm, dose-titrated randomized trial, conducted at 11 Australian hospices or hospitals with palliative care services, administered oral risperidone, haloperidol, or placebo to 247 patients with life-limiting illness and delirium. Both treatment groups had higher delirium symptom scores than the placebo group.28
In addition, a systematic review and meta-analysis of 19 studies found no benefit of antipsychotic medications for preventing or treating delirium in hospitalized adults.29
Antipsychotics are often continued indefinitely
A retrospective chart review at a US academic health system found30 that among 487 patients with a new antipsychotic medication prescribed during hospitalization, 147 (30.2%) were discharged on an antipsychotic. Of these, 121 (82.3%) had a diagnosis of delirium. Only 15 (12.4%) had discharge summaries that included instructions for discontinuing the drug.
Another US health system retrospectively reviewed antipsychotic use and found31 that out of 260 patients who were newly exposed to an antipsychotic drug during hospitalization, 146 (56.2%) were discharged on an antipsychotic drug, and 65% of these patients were still on the drug at the time of the next hospital admission.
EXERCISE, EXERCISE, EXERCISE
Exercise recommended, but not vitamin D, to prevent falls
In 2018, the US Preventive Services Task Force updated its recommendations for preventing falls in community-dwelling older adults.32 Based on the findings of several trials, the task force recommends exercise interventions for adults age 65 and older who are at increased risk for falls. Gait, balance, and functional training were studied in 17 trials, resistance training in 13, flexibility in 8, endurance training in 5, and tai chi in 3, with 5 studies including general physical activity. Exercise interventions most commonly took place for 3 sessions per week for 12 months (range 2–42 months).
The task force also recommends against vitamin D supplementation for fall prevention in community-dwelling adults age 65 or older who are not known to have osteoporosis or vitamin D deficiency.
Early mobilization helps inpatients
Hospitalized older adults usually spend most of their time in bed. Forty-five previously ambulatory patients (age ≥ 65 without dementia or delirium) in a Veterans Affairs hospital were monitored with wireless accelerometers and were found to spend, on average, 83% of the measured hospital stay in bed. Standing or walking time ranged from 0.2% to 21%, with a median of only 3% (43 minutes a day).33
Since falls with injury became a Centers for Medicare and Medicaid Services nonreimbursable hospital-acquired condition, tension has arisen between promoting mobility and preventing falls.34 Two studies evaluating the adoption of mobility-restricting approaches such as bed-alarms, “fall-alert” signs, supervision of patients in the bathroom, and ensuring patients’ walking aids are within reach, did not find a significant reduction in falls or fall-related injuries.35,36
A clinically significant loss of community mobility is common after hospitalization in older adults.37 Older adults who developed mobility impairment during hospitalization had a higher risk of death in a large, retrospective study.38 A large Canadian multisite intervention trial39 that promoted early mobilization in older patients who were admitted to general medical wards resulted in increased mobilization and significantly shorter hospital stays.
POSTHOSPITAL CARE NEEDS IMPROVEMENT
After hospitalization, older adults who have difficulty with activities of daily living or complex medical needs often require continued care.
About 20% of hospitalized Medicare beneficiaries in the United States are discharged to skilled nursing facilities.40 This is often a stressful transition, and most people have little guidance on selecting a facility and simply choose one based on its proximity to home.41
A program of frequent visits by hospital-employed physicians and advanced practice professionals at skilled nursing facilities resulted in a significantly lower 30-day readmission rate compared with nonparticipating skilled nursing facilities in the same geographic area.42
Home healthcare is recommended after hospital discharge at a rapidly increasing rate. Overall referral rates increased from 8.6% to 14.1% between 2001 and 2012, and from 14.3% to 24.0% for patients with heart failure.43 A qualitative study of home healthcare nurses found a need for improved care coordination between home healthcare agencies and discharging hospitals, including defining accountability for orders and enhancing communication.44
- Callahan CM, Boustani MA, Schmid AA, et al. Targeting functional decline in Alzheimer disease: a randomized trial. Ann Intern Med 2017; 166(3):164–171. doi:10.7326/M16-0830
- Brasure M, Desai P, Davila H, et al. Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):30–38. doi:10.7326/M17-1528
- Kryscio RJ, Abner EL, Caban-Holt A, et al. Association of antioxidant supplement use and dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol 2017; 74(5):567–573. doi:10.1001/jamaneurol.2016.5778
- Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):52–62. doi:10.7326/M17-1530
- Resnick SM, Matsumoto AM, Stephens-Shields AJ, et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA 2017; 317(7):717–727. doi:10.1001/jama.2016.21044
- Egan MF, Kost J, Tariot PN, et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med 2018; 378(18):1691–1703. doi:10.1056/NEJMoa1706441
- Honig LS, Vellas B, Woodward M, et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 2018; 378(4):321–330. doi:10.1056/NEJMoa1705971
- Fink HA, Jutkowitz E, McCarten JR, et al. Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):39–51. doi:10.7326/M17-1529
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73(4):410–416. doi:10.1001/jamaneurol.2015.4791
- Gray SL, Walker RL, Dublin S, et al. Proton pump inhibitor use and dementia risk: prospective population-based study. J Am Geriatr Soc 2018; 66(2):247–253. doi:10.1111/jgs.15073
- de Bruijn RF, Heeringa J, Wolters FJ, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol 2015; 72(11):1288–1294. doi:10.1001/jamaneurol.2015.2161
- Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2018; 39(6):453–460. doi:10.1093/eurheartj/ehx579
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363(17):1597–1607. doi:10.1056/NEJMoa1008232
- Haussig S, Mangner N, Dwyer MG, et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI randomized clinical trial. JAMA 2016; 316(6):592–601. doi:10.1001/jama.2016.10302
- Khan MM, Herrmann N, Gallagher D, et al. Cognitive outcomes after transcatheter aortic valve implantation: a metaanalysis. J Am Geriatr Soc 2018; 66(2):254–262. doi:10.1111/jgs.15123
- Choosing Wisely; ABIM Foundation. American Geriatrics Society: ten things physicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society. Accessed November 6, 2018.
- Lieberman JA 3rd. Metabolic changes associated with antipsychotic use. Prim Care Companion J Clin Psychiatry 2004; 6(suppl 2):8–13. pmid:16001095
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294(15):1934–1943. doi:10.1001/jama.294.15.1934
- Choosing Wisely; ABIM Foundation. American Psychiatric Association: five things physicians and patients should question. www.choosingwisely.org/societies/american-psychiatric-association. Accessed November 6, 2018.
- Maust DT, Kim HM, Chiang C, Kales HC. Association of the Centers for Medicare & Medicaid Services’ National Partnership to improve dementia care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med 2018; 178(5):640–647. doi:10.1001/jamainternmed.2018.0379
- CNN. The little red pill being pushed on the elderly. www.cnn.com/2017/10/12/health/nuedexta-nursing-homes-invs/index.html. Accessed November 6, 2018.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314(12):1242–1254. doi:10.1001/jama.2015.10214
- Ballard C, Banister C, Khan Z, et al; ADP Investigators. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol 2018; 17(3):213–222. doi:10.1016/S1474-4422(18)30039-5
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354(11):1157–1165. doi:10.1056/NEJMra052321
- Cole MG, McCusker J, Bailey R, et al. Partial and no recovery from delirium after hospital discharge predict increased adverse events. Age Ageing 2017; 46(1):90–95. doi:10.1093/ageing/afw153
- Chen CC, Li HC, Liang JT, et al. Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surg 2017; 152(9):827–834. doi:10.1001/jamasurg.2017.1083
- van den Boogaard M, Slooter AJC, Brüggemann RJM, et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA 2018; 319(7):680–690. doi:10.1001/jama.2018.0160
- Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med 2017; 177(1):34–42. doi:10.1001/jamainternmed.2016.7491
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 64(4):705–714. doi:10.1111/jgs.14076
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- Loh KP, Ramdass S, Garb JL, et al. Long-term outcomes of elders discharged on antipsychotics. J Hosp Med 2016; 11(8):550–555. doi:10.1002/jhm.2585
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation statement. JAMA 2018; 319(16):1696–1704. doi:10.1001/jama.2018.3097
- Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc 2009; 57(9):1660–1665. doi:10.1111/j.1532-5415.2009.02393.x
- Growdon ME, Shorr RI, Inouye SK. The tension between promoting mobility and preventing falls in the hospital. JAMA Intern Med 2017; 177(6):759–760. doi:10.1001/jamainternmed.2017.0840
- Barker AL, Morello RT, Wolfe R, et al. 6-PACK programme to decrease fall injuries in acute hospitals: cluster randomised controlled trial. BMJ 2016; 352:h6781. doi:10.1136/bmj.h6781
- Shorr RI, Chandler AM, Mion LC, et al. Effects of an intervention to increase bed alarm use to prevent falls in hospitalized patients: a cluster randomized trial. Ann Intern Med 2012; 157(10):692–699. doi:10.7326/0003-4819-157-10-201211200-00005
- Loyd C, Beasley TM, Miltner RS, Clark D, King B, Brown CJ. Trajectories of community mobility recovery after hospitalization in older adults. J Am Geriatr Soc 2018; 66(7):1399–1403. doi:10.1111/jgs.15397
- Valiani V, Chen Z, Lipori G, Pahor M, Sabbá C, Manini TM. Prognostic value of Braden Activity subscale for mobility status in hospitalized older adults. J Hosp Med 2017; 12(6):396–401. doi:10.12788/jhm.2748
- Liu B, Moore JE, Almaawiy U, et al; MOVE ON Collaboration. Outcomes of mobilisation of vulnerable elders in Ontario (MOVE ON): a multisite interrupted time series evaluation of an implementation intervention to increase patient mobilisation. Age Ageing 2018; 47(1):112–119. doi:10.1093/ageing/afx128
- Report to Congress: Medicare Payment Policy. Medicare Payment Advisory Commission 2016. www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed November 6, 2018.
- Gadbois EA, Tyler DA, Mor V. Selecting a skilled nursing facility for postacute care: individual and family perspectives. J Am Geriatr Soc 2017; 65(11):2459–2465. doi:10.1111/jgs.14988
- Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med 2017; 12(4):238–244. doi:10.12788/jhm.2710
- Jones CD, Ginde AA, Burke RE, Wald HL, Masoudi FA, Boxer RS. Increasing home healthcare referrals upon discharge from U.S. hospitals: 2001-2012. J Am Geriatr Soc 2015; 63(6):1265–1266. doi:10.1111/jgs.13467
- Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med 2017; 32(10):1114–1121. doi:10.1007/s11606-017-4104-0
- Callahan CM, Boustani MA, Schmid AA, et al. Targeting functional decline in Alzheimer disease: a randomized trial. Ann Intern Med 2017; 166(3):164–171. doi:10.7326/M16-0830
- Brasure M, Desai P, Davila H, et al. Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):30–38. doi:10.7326/M17-1528
- Kryscio RJ, Abner EL, Caban-Holt A, et al. Association of antioxidant supplement use and dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol 2017; 74(5):567–573. doi:10.1001/jamaneurol.2016.5778
- Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):52–62. doi:10.7326/M17-1530
- Resnick SM, Matsumoto AM, Stephens-Shields AJ, et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA 2017; 317(7):717–727. doi:10.1001/jama.2016.21044
- Egan MF, Kost J, Tariot PN, et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med 2018; 378(18):1691–1703. doi:10.1056/NEJMoa1706441
- Honig LS, Vellas B, Woodward M, et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 2018; 378(4):321–330. doi:10.1056/NEJMoa1705971
- Fink HA, Jutkowitz E, McCarten JR, et al. Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):39–51. doi:10.7326/M17-1529
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73(4):410–416. doi:10.1001/jamaneurol.2015.4791
- Gray SL, Walker RL, Dublin S, et al. Proton pump inhibitor use and dementia risk: prospective population-based study. J Am Geriatr Soc 2018; 66(2):247–253. doi:10.1111/jgs.15073
- de Bruijn RF, Heeringa J, Wolters FJ, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol 2015; 72(11):1288–1294. doi:10.1001/jamaneurol.2015.2161
- Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2018; 39(6):453–460. doi:10.1093/eurheartj/ehx579
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363(17):1597–1607. doi:10.1056/NEJMoa1008232
- Haussig S, Mangner N, Dwyer MG, et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI randomized clinical trial. JAMA 2016; 316(6):592–601. doi:10.1001/jama.2016.10302
- Khan MM, Herrmann N, Gallagher D, et al. Cognitive outcomes after transcatheter aortic valve implantation: a metaanalysis. J Am Geriatr Soc 2018; 66(2):254–262. doi:10.1111/jgs.15123
- Choosing Wisely; ABIM Foundation. American Geriatrics Society: ten things physicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society. Accessed November 6, 2018.
- Lieberman JA 3rd. Metabolic changes associated with antipsychotic use. Prim Care Companion J Clin Psychiatry 2004; 6(suppl 2):8–13. pmid:16001095
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294(15):1934–1943. doi:10.1001/jama.294.15.1934
- Choosing Wisely; ABIM Foundation. American Psychiatric Association: five things physicians and patients should question. www.choosingwisely.org/societies/american-psychiatric-association. Accessed November 6, 2018.
- Maust DT, Kim HM, Chiang C, Kales HC. Association of the Centers for Medicare & Medicaid Services’ National Partnership to improve dementia care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med 2018; 178(5):640–647. doi:10.1001/jamainternmed.2018.0379
- CNN. The little red pill being pushed on the elderly. www.cnn.com/2017/10/12/health/nuedexta-nursing-homes-invs/index.html. Accessed November 6, 2018.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314(12):1242–1254. doi:10.1001/jama.2015.10214
- Ballard C, Banister C, Khan Z, et al; ADP Investigators. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol 2018; 17(3):213–222. doi:10.1016/S1474-4422(18)30039-5
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354(11):1157–1165. doi:10.1056/NEJMra052321
- Cole MG, McCusker J, Bailey R, et al. Partial and no recovery from delirium after hospital discharge predict increased adverse events. Age Ageing 2017; 46(1):90–95. doi:10.1093/ageing/afw153
- Chen CC, Li HC, Liang JT, et al. Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surg 2017; 152(9):827–834. doi:10.1001/jamasurg.2017.1083
- van den Boogaard M, Slooter AJC, Brüggemann RJM, et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA 2018; 319(7):680–690. doi:10.1001/jama.2018.0160
- Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med 2017; 177(1):34–42. doi:10.1001/jamainternmed.2016.7491
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 64(4):705–714. doi:10.1111/jgs.14076
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- Loh KP, Ramdass S, Garb JL, et al. Long-term outcomes of elders discharged on antipsychotics. J Hosp Med 2016; 11(8):550–555. doi:10.1002/jhm.2585
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation statement. JAMA 2018; 319(16):1696–1704. doi:10.1001/jama.2018.3097
- Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc 2009; 57(9):1660–1665. doi:10.1111/j.1532-5415.2009.02393.x
- Growdon ME, Shorr RI, Inouye SK. The tension between promoting mobility and preventing falls in the hospital. JAMA Intern Med 2017; 177(6):759–760. doi:10.1001/jamainternmed.2017.0840
- Barker AL, Morello RT, Wolfe R, et al. 6-PACK programme to decrease fall injuries in acute hospitals: cluster randomised controlled trial. BMJ 2016; 352:h6781. doi:10.1136/bmj.h6781
- Shorr RI, Chandler AM, Mion LC, et al. Effects of an intervention to increase bed alarm use to prevent falls in hospitalized patients: a cluster randomized trial. Ann Intern Med 2012; 157(10):692–699. doi:10.7326/0003-4819-157-10-201211200-00005
- Loyd C, Beasley TM, Miltner RS, Clark D, King B, Brown CJ. Trajectories of community mobility recovery after hospitalization in older adults. J Am Geriatr Soc 2018; 66(7):1399–1403. doi:10.1111/jgs.15397
- Valiani V, Chen Z, Lipori G, Pahor M, Sabbá C, Manini TM. Prognostic value of Braden Activity subscale for mobility status in hospitalized older adults. J Hosp Med 2017; 12(6):396–401. doi:10.12788/jhm.2748
- Liu B, Moore JE, Almaawiy U, et al; MOVE ON Collaboration. Outcomes of mobilisation of vulnerable elders in Ontario (MOVE ON): a multisite interrupted time series evaluation of an implementation intervention to increase patient mobilisation. Age Ageing 2018; 47(1):112–119. doi:10.1093/ageing/afx128
- Report to Congress: Medicare Payment Policy. Medicare Payment Advisory Commission 2016. www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed November 6, 2018.
- Gadbois EA, Tyler DA, Mor V. Selecting a skilled nursing facility for postacute care: individual and family perspectives. J Am Geriatr Soc 2017; 65(11):2459–2465. doi:10.1111/jgs.14988
- Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med 2017; 12(4):238–244. doi:10.12788/jhm.2710
- Jones CD, Ginde AA, Burke RE, Wald HL, Masoudi FA, Boxer RS. Increasing home healthcare referrals upon discharge from U.S. hospitals: 2001-2012. J Am Geriatr Soc 2015; 63(6):1265–1266. doi:10.1111/jgs.13467
- Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med 2017; 32(10):1114–1121. doi:10.1007/s11606-017-4104-0
KEY POINTS
- Oral anticoagulant treatment for atrial fibrillation helps preserve cognitive function.
- Antipsychotics are not recommended as initial therapy for dementia-associated behavioral disturbances or for hospitalization-induced delirium.
- A multicomponent inpatient program can help prevent postoperative delirium in hospitalized patients.
- The US Preventive Services Task Force recommends exercise to prevent falls.
- Early mobility should be encouraged for hospitalized patients.
- Better continuity of care between hospitals and skilled nursing facilities can reduce hospital readmission rates.
Acute necrotizing esophagitis
An 82-year-old man with poorly controlled diabetes mellitus presented to our emergency department with a 1-day history of confusion and coffee-ground emesis.
Biopsy study revealed necrosis of the esophageal mucosa. A diagnosis of acute necrotizing esophagitis was made.
ACUTE NECROTIZING ESOPHAGITIS
Acute necrotizing esophagitis is thought to arise from a combination of an ischemic insult to the esophagus, an impaired mucosal barrier system, and a backflow injury from chemical contents of gastric secretions.1 The tissue hypoperfusion derives from vasculopathy, hypotension, or malnutrition. It is associated with diabetes mellitus, diabetic ketoacidosis, lactic acidosis, alcohol abuse, cirrhosis, renal insufficiency, malignancy, antibiotic use, esophageal infections, and aortic dissection.
The esophagus has a diverse blood supply. The upper esophagus is supplied by the inferior thyroid arteries, the mid-esophagus by the bronchial, proper esophageal, and intercostal arteries, and the distal esophagus by the left gastric and left inferior phrenic arteries.1
KEY FEATURES AND DIAGNOSTIC CLUES
The necrotic changes are prominent in the distal esophagus, which is more susceptible to ischemia and mucosal injury. The characteristic endoscopic finding is a diffuse black esophagus with a sharp transition to normal mucosa at the gastroesophageal junction.
The differential diagnosis includes melanosis, pseudomelanosis, malignant melanoma, acanthosis nigricans, coal dust deposition, caustic ingestion, radiation esophagitis, and infectious esophagitis caused by cytomegalovirus, herpes simplex virus, Candida albicans, or Klebsiella pneumoniae.2–4
TREATMENT AND OUTCOME
Avoidance of oral intake and gastric acid suppression with intravenous proton pump inhibitors are recommended to prevent additional injury of the esophageal mucosa.
The condition generally resolves with restored blood flow and treatment of any coexisting illness. However, it may be complicated by perforation (6.8%), mediastinitis (5.7%), or subsequent development of esophageal stricture (10.2%).5 Patients with esophageal stricture require endoscopic dilation after mucosal recovery.
The overall risk of death in acute necrotizing esophagitis is high (31.8%) and most often due to the underlying disease, such as sepsis, malignancy, cardiogenic shock, or hypovolemic shock.5 The mortality rate directly attributed to complications of acute necrotizing esophagitis is much lower (5.7%).5
- Gurvits GE. Black esophagus: acute esophageal necrosis syndrome. World J Gastroenterol 2010; 16(26):3219–3225. pmid:20614476
- Khan HA. Coal dust deposition—rare cause of “black esophagus.” Am J Gastroenterol 1996; 91(10):2256. pmid:8855776
- Ertekin C, Alimoglu O, Akyildiz H, Guloglu R, Taviloglu K. The results of caustic ingestions. Hepatogastroenterology 2004; 51(59):1397–1400. pmid:15362762
- Kozlowski LM, Nigra TP. Esophageal acanthosis nigricans in association with adenocarcinoma from an unknown primary site. J Am Acad Dermatol 1992; 26(2 pt 2):348–351. pmid:1569256
- Gurvits GE, Shapsis A, Lau N, Gualtieri N, Robilotti JG. Acute esophageal necrosis: a rare syndrome. J Gastroenterol 2007; 42(1):29–38. doi:10.1007/s00535-006-1974-z
An 82-year-old man with poorly controlled diabetes mellitus presented to our emergency department with a 1-day history of confusion and coffee-ground emesis.
Biopsy study revealed necrosis of the esophageal mucosa. A diagnosis of acute necrotizing esophagitis was made.
ACUTE NECROTIZING ESOPHAGITIS
Acute necrotizing esophagitis is thought to arise from a combination of an ischemic insult to the esophagus, an impaired mucosal barrier system, and a backflow injury from chemical contents of gastric secretions.1 The tissue hypoperfusion derives from vasculopathy, hypotension, or malnutrition. It is associated with diabetes mellitus, diabetic ketoacidosis, lactic acidosis, alcohol abuse, cirrhosis, renal insufficiency, malignancy, antibiotic use, esophageal infections, and aortic dissection.
The esophagus has a diverse blood supply. The upper esophagus is supplied by the inferior thyroid arteries, the mid-esophagus by the bronchial, proper esophageal, and intercostal arteries, and the distal esophagus by the left gastric and left inferior phrenic arteries.1
KEY FEATURES AND DIAGNOSTIC CLUES
The necrotic changes are prominent in the distal esophagus, which is more susceptible to ischemia and mucosal injury. The characteristic endoscopic finding is a diffuse black esophagus with a sharp transition to normal mucosa at the gastroesophageal junction.
The differential diagnosis includes melanosis, pseudomelanosis, malignant melanoma, acanthosis nigricans, coal dust deposition, caustic ingestion, radiation esophagitis, and infectious esophagitis caused by cytomegalovirus, herpes simplex virus, Candida albicans, or Klebsiella pneumoniae.2–4
TREATMENT AND OUTCOME
Avoidance of oral intake and gastric acid suppression with intravenous proton pump inhibitors are recommended to prevent additional injury of the esophageal mucosa.
The condition generally resolves with restored blood flow and treatment of any coexisting illness. However, it may be complicated by perforation (6.8%), mediastinitis (5.7%), or subsequent development of esophageal stricture (10.2%).5 Patients with esophageal stricture require endoscopic dilation after mucosal recovery.
The overall risk of death in acute necrotizing esophagitis is high (31.8%) and most often due to the underlying disease, such as sepsis, malignancy, cardiogenic shock, or hypovolemic shock.5 The mortality rate directly attributed to complications of acute necrotizing esophagitis is much lower (5.7%).5
An 82-year-old man with poorly controlled diabetes mellitus presented to our emergency department with a 1-day history of confusion and coffee-ground emesis.
Biopsy study revealed necrosis of the esophageal mucosa. A diagnosis of acute necrotizing esophagitis was made.
ACUTE NECROTIZING ESOPHAGITIS
Acute necrotizing esophagitis is thought to arise from a combination of an ischemic insult to the esophagus, an impaired mucosal barrier system, and a backflow injury from chemical contents of gastric secretions.1 The tissue hypoperfusion derives from vasculopathy, hypotension, or malnutrition. It is associated with diabetes mellitus, diabetic ketoacidosis, lactic acidosis, alcohol abuse, cirrhosis, renal insufficiency, malignancy, antibiotic use, esophageal infections, and aortic dissection.
The esophagus has a diverse blood supply. The upper esophagus is supplied by the inferior thyroid arteries, the mid-esophagus by the bronchial, proper esophageal, and intercostal arteries, and the distal esophagus by the left gastric and left inferior phrenic arteries.1
KEY FEATURES AND DIAGNOSTIC CLUES
The necrotic changes are prominent in the distal esophagus, which is more susceptible to ischemia and mucosal injury. The characteristic endoscopic finding is a diffuse black esophagus with a sharp transition to normal mucosa at the gastroesophageal junction.
The differential diagnosis includes melanosis, pseudomelanosis, malignant melanoma, acanthosis nigricans, coal dust deposition, caustic ingestion, radiation esophagitis, and infectious esophagitis caused by cytomegalovirus, herpes simplex virus, Candida albicans, or Klebsiella pneumoniae.2–4
TREATMENT AND OUTCOME
Avoidance of oral intake and gastric acid suppression with intravenous proton pump inhibitors are recommended to prevent additional injury of the esophageal mucosa.
The condition generally resolves with restored blood flow and treatment of any coexisting illness. However, it may be complicated by perforation (6.8%), mediastinitis (5.7%), or subsequent development of esophageal stricture (10.2%).5 Patients with esophageal stricture require endoscopic dilation after mucosal recovery.
The overall risk of death in acute necrotizing esophagitis is high (31.8%) and most often due to the underlying disease, such as sepsis, malignancy, cardiogenic shock, or hypovolemic shock.5 The mortality rate directly attributed to complications of acute necrotizing esophagitis is much lower (5.7%).5
- Gurvits GE. Black esophagus: acute esophageal necrosis syndrome. World J Gastroenterol 2010; 16(26):3219–3225. pmid:20614476
- Khan HA. Coal dust deposition—rare cause of “black esophagus.” Am J Gastroenterol 1996; 91(10):2256. pmid:8855776
- Ertekin C, Alimoglu O, Akyildiz H, Guloglu R, Taviloglu K. The results of caustic ingestions. Hepatogastroenterology 2004; 51(59):1397–1400. pmid:15362762
- Kozlowski LM, Nigra TP. Esophageal acanthosis nigricans in association with adenocarcinoma from an unknown primary site. J Am Acad Dermatol 1992; 26(2 pt 2):348–351. pmid:1569256
- Gurvits GE, Shapsis A, Lau N, Gualtieri N, Robilotti JG. Acute esophageal necrosis: a rare syndrome. J Gastroenterol 2007; 42(1):29–38. doi:10.1007/s00535-006-1974-z
- Gurvits GE. Black esophagus: acute esophageal necrosis syndrome. World J Gastroenterol 2010; 16(26):3219–3225. pmid:20614476
- Khan HA. Coal dust deposition—rare cause of “black esophagus.” Am J Gastroenterol 1996; 91(10):2256. pmid:8855776
- Ertekin C, Alimoglu O, Akyildiz H, Guloglu R, Taviloglu K. The results of caustic ingestions. Hepatogastroenterology 2004; 51(59):1397–1400. pmid:15362762
- Kozlowski LM, Nigra TP. Esophageal acanthosis nigricans in association with adenocarcinoma from an unknown primary site. J Am Acad Dermatol 1992; 26(2 pt 2):348–351. pmid:1569256
- Gurvits GE, Shapsis A, Lau N, Gualtieri N, Robilotti JG. Acute esophageal necrosis: a rare syndrome. J Gastroenterol 2007; 42(1):29–38. doi:10.1007/s00535-006-1974-z
Prostate cancer screening
To the Editor: In their article on men’s health,1Chaitoff and colleagues present the scenario of a 60-year-old patient, with no other history given, whose recent screening prostate-specific antigen (PSA) level was 5.1 ng/mL, and who asks his doctor:
- Should I have agreed to the screening?
- How effective is the screening?
- What are the next steps?
These questions are consistent with the patient having read the latest US Preventive Services Task Force (USPSTF) report on PSA screening, which states: “Screening offers a small potential benefit of reducing the chance of death from prostate cancer in some men. However, many men will experience potential harms of screening, including false-positive results…”2
I would tell the patient that he can expect greater benefit from PSA screening than reported by the USPSTF simply by adhering to the screening protocol. Intention-to-treat analysis applied to the trial results diminished the apparent benefits of PSA screening by counting fatal prostate cancers experienced by nonadherent study participants as screening failures.3 In other words, screening works better in those who actually get screened!
The authors state1 that “in 2014, an estimated 172,258 men in the United States were diagnosed with prostate cancer, but only 28,343 men died of it.” Nevertheless, prostate cancer remains the second most common cause of cancer deaths in American men, after lung cancer.4 In addition to the reduction in prostate cancer-specific mortality with screening, patients should consider the reduction in morbidity from painful bone metastases and pathologic fractures, which are common in advanced prostate cancer.
A false-positive elevated PSA can be caused by reversible benign conditions, such as prostate infection or trauma, which can resolve over time, returning the PSA to its baseline level. Studies have demonstrated that simply repeating the PSA test a few weeks later will significantly reduce the number of false-positive PSA screening tests.5
Also, it is not optimal to screen for prostate cancer using a single PSA measurement. This patient’s PSA of 5.1 ng/mL cannot distinguish between chronic benign prostatic hyperplasia and a fast-growing but still curable malignancy. If the patient’s PSA had been tested annually and was known to be stable at its current level, a benign or indolent condition would be most likely, allowing for the possibility of continuing noninvasive observation. If his PSA was 1.1 ng/mL a year ago, and his PSA remains elevated when retested in a few weeks, the likelihood of malignancy would increase, increasing the yield of biopsy.
Lastly, consider false-negatives. A man with a PSA of 2.0 ng/mL would not have undergone biopsy in any of the trials, but if he had a history of several consecutive annual PSA levels less than 1.0 ng/mL, the doubling of his PSA during an interval less than or equal to 1 year could signal an early aggressive prostate cancer. Increases in PSA velocity can reveal the rapid proliferation of malignant prostate cells before the tumor is large enough to cross a static threshold PSA. We have zero data indicating how much benefit can be derived from the use of PSA velocity in this fashion. However, clinicians who carefully track serial PSA changes in each patient have anecdotes of success in early detection and cure of aggressive prostate cancers that would not have been detected by the trial protocols using fixed PSA thresholds. Until such trials are done, we can only tell patients that the ability to compute PSA velocity may be another source of benefit of annual screening of PSA.
- Chaitoff A, Killeen TC, Nielsen C. Men’s health 2018: BPH, prostate cancer, erectile dysfunction, supplements. Cleve Clin J Med 2018; 85(11):871–880. doi:10.3949/ccjm.85a.18011
- US Preventive Services Task Force. Prostate cancer: screening. May 2018. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening1?ds=1&s=PSA. Accessed November 6, 2018.
- Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res 2011; 2(3):109–112. doi:10.4103/2229-3485.83221
- Cancer.Net. Prostate cancer: statistics. www.cancer.net/cancer-types/prostate-cancer/statistics. Accessed November 6, 2018.
- Lavallée LT, Binette A, Witiuk K, et al. Reducing the harm of prostate cancer screening: repeated prostate-specific antigen testing. Mayo Clin Proc 2016; 91(1):17–22. doi:10.1016/j.mayocp.2015.07.030
To the Editor: In their article on men’s health,1Chaitoff and colleagues present the scenario of a 60-year-old patient, with no other history given, whose recent screening prostate-specific antigen (PSA) level was 5.1 ng/mL, and who asks his doctor:
- Should I have agreed to the screening?
- How effective is the screening?
- What are the next steps?
These questions are consistent with the patient having read the latest US Preventive Services Task Force (USPSTF) report on PSA screening, which states: “Screening offers a small potential benefit of reducing the chance of death from prostate cancer in some men. However, many men will experience potential harms of screening, including false-positive results…”2
I would tell the patient that he can expect greater benefit from PSA screening than reported by the USPSTF simply by adhering to the screening protocol. Intention-to-treat analysis applied to the trial results diminished the apparent benefits of PSA screening by counting fatal prostate cancers experienced by nonadherent study participants as screening failures.3 In other words, screening works better in those who actually get screened!
The authors state1 that “in 2014, an estimated 172,258 men in the United States were diagnosed with prostate cancer, but only 28,343 men died of it.” Nevertheless, prostate cancer remains the second most common cause of cancer deaths in American men, after lung cancer.4 In addition to the reduction in prostate cancer-specific mortality with screening, patients should consider the reduction in morbidity from painful bone metastases and pathologic fractures, which are common in advanced prostate cancer.
A false-positive elevated PSA can be caused by reversible benign conditions, such as prostate infection or trauma, which can resolve over time, returning the PSA to its baseline level. Studies have demonstrated that simply repeating the PSA test a few weeks later will significantly reduce the number of false-positive PSA screening tests.5
Also, it is not optimal to screen for prostate cancer using a single PSA measurement. This patient’s PSA of 5.1 ng/mL cannot distinguish between chronic benign prostatic hyperplasia and a fast-growing but still curable malignancy. If the patient’s PSA had been tested annually and was known to be stable at its current level, a benign or indolent condition would be most likely, allowing for the possibility of continuing noninvasive observation. If his PSA was 1.1 ng/mL a year ago, and his PSA remains elevated when retested in a few weeks, the likelihood of malignancy would increase, increasing the yield of biopsy.
Lastly, consider false-negatives. A man with a PSA of 2.0 ng/mL would not have undergone biopsy in any of the trials, but if he had a history of several consecutive annual PSA levels less than 1.0 ng/mL, the doubling of his PSA during an interval less than or equal to 1 year could signal an early aggressive prostate cancer. Increases in PSA velocity can reveal the rapid proliferation of malignant prostate cells before the tumor is large enough to cross a static threshold PSA. We have zero data indicating how much benefit can be derived from the use of PSA velocity in this fashion. However, clinicians who carefully track serial PSA changes in each patient have anecdotes of success in early detection and cure of aggressive prostate cancers that would not have been detected by the trial protocols using fixed PSA thresholds. Until such trials are done, we can only tell patients that the ability to compute PSA velocity may be another source of benefit of annual screening of PSA.
To the Editor: In their article on men’s health,1Chaitoff and colleagues present the scenario of a 60-year-old patient, with no other history given, whose recent screening prostate-specific antigen (PSA) level was 5.1 ng/mL, and who asks his doctor:
- Should I have agreed to the screening?
- How effective is the screening?
- What are the next steps?
These questions are consistent with the patient having read the latest US Preventive Services Task Force (USPSTF) report on PSA screening, which states: “Screening offers a small potential benefit of reducing the chance of death from prostate cancer in some men. However, many men will experience potential harms of screening, including false-positive results…”2
I would tell the patient that he can expect greater benefit from PSA screening than reported by the USPSTF simply by adhering to the screening protocol. Intention-to-treat analysis applied to the trial results diminished the apparent benefits of PSA screening by counting fatal prostate cancers experienced by nonadherent study participants as screening failures.3 In other words, screening works better in those who actually get screened!
The authors state1 that “in 2014, an estimated 172,258 men in the United States were diagnosed with prostate cancer, but only 28,343 men died of it.” Nevertheless, prostate cancer remains the second most common cause of cancer deaths in American men, after lung cancer.4 In addition to the reduction in prostate cancer-specific mortality with screening, patients should consider the reduction in morbidity from painful bone metastases and pathologic fractures, which are common in advanced prostate cancer.
A false-positive elevated PSA can be caused by reversible benign conditions, such as prostate infection or trauma, which can resolve over time, returning the PSA to its baseline level. Studies have demonstrated that simply repeating the PSA test a few weeks later will significantly reduce the number of false-positive PSA screening tests.5
Also, it is not optimal to screen for prostate cancer using a single PSA measurement. This patient’s PSA of 5.1 ng/mL cannot distinguish between chronic benign prostatic hyperplasia and a fast-growing but still curable malignancy. If the patient’s PSA had been tested annually and was known to be stable at its current level, a benign or indolent condition would be most likely, allowing for the possibility of continuing noninvasive observation. If his PSA was 1.1 ng/mL a year ago, and his PSA remains elevated when retested in a few weeks, the likelihood of malignancy would increase, increasing the yield of biopsy.
Lastly, consider false-negatives. A man with a PSA of 2.0 ng/mL would not have undergone biopsy in any of the trials, but if he had a history of several consecutive annual PSA levels less than 1.0 ng/mL, the doubling of his PSA during an interval less than or equal to 1 year could signal an early aggressive prostate cancer. Increases in PSA velocity can reveal the rapid proliferation of malignant prostate cells before the tumor is large enough to cross a static threshold PSA. We have zero data indicating how much benefit can be derived from the use of PSA velocity in this fashion. However, clinicians who carefully track serial PSA changes in each patient have anecdotes of success in early detection and cure of aggressive prostate cancers that would not have been detected by the trial protocols using fixed PSA thresholds. Until such trials are done, we can only tell patients that the ability to compute PSA velocity may be another source of benefit of annual screening of PSA.
- Chaitoff A, Killeen TC, Nielsen C. Men’s health 2018: BPH, prostate cancer, erectile dysfunction, supplements. Cleve Clin J Med 2018; 85(11):871–880. doi:10.3949/ccjm.85a.18011
- US Preventive Services Task Force. Prostate cancer: screening. May 2018. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening1?ds=1&s=PSA. Accessed November 6, 2018.
- Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res 2011; 2(3):109–112. doi:10.4103/2229-3485.83221
- Cancer.Net. Prostate cancer: statistics. www.cancer.net/cancer-types/prostate-cancer/statistics. Accessed November 6, 2018.
- Lavallée LT, Binette A, Witiuk K, et al. Reducing the harm of prostate cancer screening: repeated prostate-specific antigen testing. Mayo Clin Proc 2016; 91(1):17–22. doi:10.1016/j.mayocp.2015.07.030
- Chaitoff A, Killeen TC, Nielsen C. Men’s health 2018: BPH, prostate cancer, erectile dysfunction, supplements. Cleve Clin J Med 2018; 85(11):871–880. doi:10.3949/ccjm.85a.18011
- US Preventive Services Task Force. Prostate cancer: screening. May 2018. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening1?ds=1&s=PSA. Accessed November 6, 2018.
- Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res 2011; 2(3):109–112. doi:10.4103/2229-3485.83221
- Cancer.Net. Prostate cancer: statistics. www.cancer.net/cancer-types/prostate-cancer/statistics. Accessed November 6, 2018.
- Lavallée LT, Binette A, Witiuk K, et al. Reducing the harm of prostate cancer screening: repeated prostate-specific antigen testing. Mayo Clin Proc 2016; 91(1):17–22. doi:10.1016/j.mayocp.2015.07.030
Bundled Hospital-at-Home and Transitional Care Program Is Associated with Reduced Rate of Hospital Readmission
Study Overview
Objective. To examine the effect of a hospital-at-home (HaH) and transitional care program on clinical outcomes and patient experiences when compared with inpatient hospitalization.
Design. Cohort study with matched controls.
Setting and participants. The study was conducted in a single center and aimed to evaluate a HaH program bundled with a 30-day postacute period of home-based transitional care. The program is funded by the Center for Medicare and Medicaid Innovation of the Centers for Medicare and Medicaid Services (CMS) with the goal of establishing a new HaH program that provides acute hospital-level care in a patient’s home as a substitute for transitional inpatient care.
Patients were eligible for the program if they were aged 18 years or older, lived in Manhattan, New York, had fee-for-service Medicare or private insurer that had contracted for HaH services, and required inpatient hospital admission for eligible conditions. Eligible conditions included acute exacerbations of asthma or chronic obstructive pulmonary disease, congestive heart failure (CHF), urinary tract infections (UTI), community-acquired pneumonia (CAP), cellulitis of lower extremities, deep venous thrombosis, pulmonary embolism, hypertensive urgency, hyperglycemia, and dehydration; this list was later expanded to 19 conditions representing 65 diagnosis-related groups. Patients were excluded if they were clinically unstable, required cardiac monitoring or intensive care, or lived in an unsafe home environment. Patients were identified in the emergency department (ED) and approached for enrollment in the program. Patients who were eligible for admission but refused HaH admission, or those who were identified as eligible for admission but for whom HaH clinicians were not available were enrolled as control patients.
Intervention. The HaH intervention included physician or nurse practitioner visits at home to provide acute care services including physical examination, illness and vital signs monitoring, intravenous infusions, wound care, and education regarding the illness. Nurses visited patients once or more a day to provide most of the care, and a physician or nurse practitioner saw patients at least daily in person or via video call facilitated by the nurse. A social worker also visited each patient at least once. Medical equipment, phlebotomy, and home radiography were also provided at home as needed. Patients were discharged from acute care when their acute illness resolved; subsequently, nurses and social workers provided self-
Main outcome measures. Main study outcome measures include duration of the acute care period (length of stay [LOS]) and 30-day all-cause hospital readmissions or ED visits, transfer to a skilled nursing facility, and referral to a certified home health care agency. LOS was defined as being from the date the patient was listed for admission by an ED physician to the date that post-acute care was initiated (for HaH) or hospital discharge (for control patients). Other measures include patient’s rating of care measured using items in 6 of the 9 domains of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey that were most salient to care at home, including communication with nurses, communication with physicians, pain management, communication about medicines, discharge information, and overall hospital rating.
Main results. The HaH clinical team approached 460 patients and enrolled 295 to the program. A total of 212 patients who were admitted to the hospital were enrolled as control patients. HaH patients were older than control patients, with an average age of 76.9 years (SD, 16.6) and 71.5 years (SD 13.8), respectively, and more likely to have at least 1 functional limitation (71.5% vs. 55.5%). The most frequent admission diagnoses to HaH were UTIs, CAP, cellulitis, and CHF. HaH patients had a shorter hospitalization LOS (3.2 days) compared with the control group (5.5 days; 95% confidence interval [CI], –1.8 to –2.7 days). HaH patients were less likely to have 30-day all-cause hospital readmissions (8.6% vs. 15.6%; 95% CI, –12.9% to –1.1%) and 30-day ED revisits (5.8% vs. 11.7%) compared to controls. Analysis adjusted for age, sex, race, ethnicity, education, insurance type, physical function, general health, and admitting diagnosis found that HaH patients had lower odds of hospital readmission (odds ratio [OR], 0.43; 95% CI, 0.36-0.52) and lower odds of ED revisits (OR, 0.39; 95% CI, 0.31-0.49). HaH patients reported higher ratings for communication with nurses and physicians and communication about medicines when compared with controls; they were also more likely to report the highest rating for overall hospital care (68.8% vs. 45.3%). Scores for pain management were lower for HaH patients when compared with controls.
Conclusions. Patients receiving care through the HaH program were less likely to be readmitted at 30 days after hospital discharge, had lower hospital LOS and reported higher ratings of care when compared to patients receiving care in the hospital. The study demonstrated the potential benefits of the HaH model of care for adults who need inpatient hospitalization.
Commentary
This study adds to the literature on outcomes associated with HaH programs. The first study of the HaH model in the United States was published in 2005,1 and despite the early demonstration of its feasibility and outcomes in this and subsequent studies,2,3 HaH models have not been widely adopted, unlike in other countries with integrated health care systems.4 One of the primary reasons this model has not been adopted is the lack of a specific payment mechanism in Medicare fee for service for HaH. Implementation of the HaH program described in the current study was an effort funded by a CMS innovation award to test the effect of models of care with the potential of developing payment mechanisms that would support further dissemination of these models. The results from the current study were encouraging and have led to the Physician-Focused Payment Model Technical Advisory Committee’s unanimous recommendation to the U.S. Department of Health and Human Services for full implementation in 2017.
The current study does have certain limitations. It is not a randomized trial, and thus control group selection could be affected by selection bias. Also, the study was conducted in a single health system and thus may have limited generalizability. Nevertheless, this study was designed based on prior studies of HaH, including randomized and non-randomized studies, that have demonstrated benefits similar to the current study. The finding that HaH patients reported worse pain control than did patients hospitalized in the inpatient setting, where staff is available 24 hours a day, may suggest differences in care that is feasible at home versus in the inpatient setting. Finally, because it is a bundled program that includes both HaH and a post-discharge care transition program, it is unclear if the effects found in this evaluation can be attributed to specific components within the bundled program.
Applications for Clinical Practice
Patients, particularly older adults, may prefer to have hospital-level care delivered at home; clinicians may consider how HaH may allow patients to avoid potential hazards of hospitalization,5 such as inpatient falls, delirium, and other iatrogenic events. The HaH program is feasible and safe, and is associated with improved outcomes of care for patients.
—William W. Hung, MD, MPH
1. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
2. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital at home”. Med J Aust. 2012;197:512-519.
3. Mader SL, Medcraft MC, Joseph C, et al. Program at home: a Veteran Affairs healthcare program to deliver hospital care in the home. J Am Geriatr Soc. 2008;56: 2317-2322.
4. Montalto M. The 500-bed hospital that isn’t there: the Victorian Department of Health Review of the hospital in the home program. Med J Aust. 2010;193:598-601.
5. Creditor MC. Hazards of hospitalization. Ann Intern Med. 1993;118:219-223.
Study Overview
Objective. To examine the effect of a hospital-at-home (HaH) and transitional care program on clinical outcomes and patient experiences when compared with inpatient hospitalization.
Design. Cohort study with matched controls.
Setting and participants. The study was conducted in a single center and aimed to evaluate a HaH program bundled with a 30-day postacute period of home-based transitional care. The program is funded by the Center for Medicare and Medicaid Innovation of the Centers for Medicare and Medicaid Services (CMS) with the goal of establishing a new HaH program that provides acute hospital-level care in a patient’s home as a substitute for transitional inpatient care.
Patients were eligible for the program if they were aged 18 years or older, lived in Manhattan, New York, had fee-for-service Medicare or private insurer that had contracted for HaH services, and required inpatient hospital admission for eligible conditions. Eligible conditions included acute exacerbations of asthma or chronic obstructive pulmonary disease, congestive heart failure (CHF), urinary tract infections (UTI), community-acquired pneumonia (CAP), cellulitis of lower extremities, deep venous thrombosis, pulmonary embolism, hypertensive urgency, hyperglycemia, and dehydration; this list was later expanded to 19 conditions representing 65 diagnosis-related groups. Patients were excluded if they were clinically unstable, required cardiac monitoring or intensive care, or lived in an unsafe home environment. Patients were identified in the emergency department (ED) and approached for enrollment in the program. Patients who were eligible for admission but refused HaH admission, or those who were identified as eligible for admission but for whom HaH clinicians were not available were enrolled as control patients.
Intervention. The HaH intervention included physician or nurse practitioner visits at home to provide acute care services including physical examination, illness and vital signs monitoring, intravenous infusions, wound care, and education regarding the illness. Nurses visited patients once or more a day to provide most of the care, and a physician or nurse practitioner saw patients at least daily in person or via video call facilitated by the nurse. A social worker also visited each patient at least once. Medical equipment, phlebotomy, and home radiography were also provided at home as needed. Patients were discharged from acute care when their acute illness resolved; subsequently, nurses and social workers provided self-
Main outcome measures. Main study outcome measures include duration of the acute care period (length of stay [LOS]) and 30-day all-cause hospital readmissions or ED visits, transfer to a skilled nursing facility, and referral to a certified home health care agency. LOS was defined as being from the date the patient was listed for admission by an ED physician to the date that post-acute care was initiated (for HaH) or hospital discharge (for control patients). Other measures include patient’s rating of care measured using items in 6 of the 9 domains of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey that were most salient to care at home, including communication with nurses, communication with physicians, pain management, communication about medicines, discharge information, and overall hospital rating.
Main results. The HaH clinical team approached 460 patients and enrolled 295 to the program. A total of 212 patients who were admitted to the hospital were enrolled as control patients. HaH patients were older than control patients, with an average age of 76.9 years (SD, 16.6) and 71.5 years (SD 13.8), respectively, and more likely to have at least 1 functional limitation (71.5% vs. 55.5%). The most frequent admission diagnoses to HaH were UTIs, CAP, cellulitis, and CHF. HaH patients had a shorter hospitalization LOS (3.2 days) compared with the control group (5.5 days; 95% confidence interval [CI], –1.8 to –2.7 days). HaH patients were less likely to have 30-day all-cause hospital readmissions (8.6% vs. 15.6%; 95% CI, –12.9% to –1.1%) and 30-day ED revisits (5.8% vs. 11.7%) compared to controls. Analysis adjusted for age, sex, race, ethnicity, education, insurance type, physical function, general health, and admitting diagnosis found that HaH patients had lower odds of hospital readmission (odds ratio [OR], 0.43; 95% CI, 0.36-0.52) and lower odds of ED revisits (OR, 0.39; 95% CI, 0.31-0.49). HaH patients reported higher ratings for communication with nurses and physicians and communication about medicines when compared with controls; they were also more likely to report the highest rating for overall hospital care (68.8% vs. 45.3%). Scores for pain management were lower for HaH patients when compared with controls.
Conclusions. Patients receiving care through the HaH program were less likely to be readmitted at 30 days after hospital discharge, had lower hospital LOS and reported higher ratings of care when compared to patients receiving care in the hospital. The study demonstrated the potential benefits of the HaH model of care for adults who need inpatient hospitalization.
Commentary
This study adds to the literature on outcomes associated with HaH programs. The first study of the HaH model in the United States was published in 2005,1 and despite the early demonstration of its feasibility and outcomes in this and subsequent studies,2,3 HaH models have not been widely adopted, unlike in other countries with integrated health care systems.4 One of the primary reasons this model has not been adopted is the lack of a specific payment mechanism in Medicare fee for service for HaH. Implementation of the HaH program described in the current study was an effort funded by a CMS innovation award to test the effect of models of care with the potential of developing payment mechanisms that would support further dissemination of these models. The results from the current study were encouraging and have led to the Physician-Focused Payment Model Technical Advisory Committee’s unanimous recommendation to the U.S. Department of Health and Human Services for full implementation in 2017.
The current study does have certain limitations. It is not a randomized trial, and thus control group selection could be affected by selection bias. Also, the study was conducted in a single health system and thus may have limited generalizability. Nevertheless, this study was designed based on prior studies of HaH, including randomized and non-randomized studies, that have demonstrated benefits similar to the current study. The finding that HaH patients reported worse pain control than did patients hospitalized in the inpatient setting, where staff is available 24 hours a day, may suggest differences in care that is feasible at home versus in the inpatient setting. Finally, because it is a bundled program that includes both HaH and a post-discharge care transition program, it is unclear if the effects found in this evaluation can be attributed to specific components within the bundled program.
Applications for Clinical Practice
Patients, particularly older adults, may prefer to have hospital-level care delivered at home; clinicians may consider how HaH may allow patients to avoid potential hazards of hospitalization,5 such as inpatient falls, delirium, and other iatrogenic events. The HaH program is feasible and safe, and is associated with improved outcomes of care for patients.
—William W. Hung, MD, MPH
Study Overview
Objective. To examine the effect of a hospital-at-home (HaH) and transitional care program on clinical outcomes and patient experiences when compared with inpatient hospitalization.
Design. Cohort study with matched controls.
Setting and participants. The study was conducted in a single center and aimed to evaluate a HaH program bundled with a 30-day postacute period of home-based transitional care. The program is funded by the Center for Medicare and Medicaid Innovation of the Centers for Medicare and Medicaid Services (CMS) with the goal of establishing a new HaH program that provides acute hospital-level care in a patient’s home as a substitute for transitional inpatient care.
Patients were eligible for the program if they were aged 18 years or older, lived in Manhattan, New York, had fee-for-service Medicare or private insurer that had contracted for HaH services, and required inpatient hospital admission for eligible conditions. Eligible conditions included acute exacerbations of asthma or chronic obstructive pulmonary disease, congestive heart failure (CHF), urinary tract infections (UTI), community-acquired pneumonia (CAP), cellulitis of lower extremities, deep venous thrombosis, pulmonary embolism, hypertensive urgency, hyperglycemia, and dehydration; this list was later expanded to 19 conditions representing 65 diagnosis-related groups. Patients were excluded if they were clinically unstable, required cardiac monitoring or intensive care, or lived in an unsafe home environment. Patients were identified in the emergency department (ED) and approached for enrollment in the program. Patients who were eligible for admission but refused HaH admission, or those who were identified as eligible for admission but for whom HaH clinicians were not available were enrolled as control patients.
Intervention. The HaH intervention included physician or nurse practitioner visits at home to provide acute care services including physical examination, illness and vital signs monitoring, intravenous infusions, wound care, and education regarding the illness. Nurses visited patients once or more a day to provide most of the care, and a physician or nurse practitioner saw patients at least daily in person or via video call facilitated by the nurse. A social worker also visited each patient at least once. Medical equipment, phlebotomy, and home radiography were also provided at home as needed. Patients were discharged from acute care when their acute illness resolved; subsequently, nurses and social workers provided self-
Main outcome measures. Main study outcome measures include duration of the acute care period (length of stay [LOS]) and 30-day all-cause hospital readmissions or ED visits, transfer to a skilled nursing facility, and referral to a certified home health care agency. LOS was defined as being from the date the patient was listed for admission by an ED physician to the date that post-acute care was initiated (for HaH) or hospital discharge (for control patients). Other measures include patient’s rating of care measured using items in 6 of the 9 domains of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey that were most salient to care at home, including communication with nurses, communication with physicians, pain management, communication about medicines, discharge information, and overall hospital rating.
Main results. The HaH clinical team approached 460 patients and enrolled 295 to the program. A total of 212 patients who were admitted to the hospital were enrolled as control patients. HaH patients were older than control patients, with an average age of 76.9 years (SD, 16.6) and 71.5 years (SD 13.8), respectively, and more likely to have at least 1 functional limitation (71.5% vs. 55.5%). The most frequent admission diagnoses to HaH were UTIs, CAP, cellulitis, and CHF. HaH patients had a shorter hospitalization LOS (3.2 days) compared with the control group (5.5 days; 95% confidence interval [CI], –1.8 to –2.7 days). HaH patients were less likely to have 30-day all-cause hospital readmissions (8.6% vs. 15.6%; 95% CI, –12.9% to –1.1%) and 30-day ED revisits (5.8% vs. 11.7%) compared to controls. Analysis adjusted for age, sex, race, ethnicity, education, insurance type, physical function, general health, and admitting diagnosis found that HaH patients had lower odds of hospital readmission (odds ratio [OR], 0.43; 95% CI, 0.36-0.52) and lower odds of ED revisits (OR, 0.39; 95% CI, 0.31-0.49). HaH patients reported higher ratings for communication with nurses and physicians and communication about medicines when compared with controls; they were also more likely to report the highest rating for overall hospital care (68.8% vs. 45.3%). Scores for pain management were lower for HaH patients when compared with controls.
Conclusions. Patients receiving care through the HaH program were less likely to be readmitted at 30 days after hospital discharge, had lower hospital LOS and reported higher ratings of care when compared to patients receiving care in the hospital. The study demonstrated the potential benefits of the HaH model of care for adults who need inpatient hospitalization.
Commentary
This study adds to the literature on outcomes associated with HaH programs. The first study of the HaH model in the United States was published in 2005,1 and despite the early demonstration of its feasibility and outcomes in this and subsequent studies,2,3 HaH models have not been widely adopted, unlike in other countries with integrated health care systems.4 One of the primary reasons this model has not been adopted is the lack of a specific payment mechanism in Medicare fee for service for HaH. Implementation of the HaH program described in the current study was an effort funded by a CMS innovation award to test the effect of models of care with the potential of developing payment mechanisms that would support further dissemination of these models. The results from the current study were encouraging and have led to the Physician-Focused Payment Model Technical Advisory Committee’s unanimous recommendation to the U.S. Department of Health and Human Services for full implementation in 2017.
The current study does have certain limitations. It is not a randomized trial, and thus control group selection could be affected by selection bias. Also, the study was conducted in a single health system and thus may have limited generalizability. Nevertheless, this study was designed based on prior studies of HaH, including randomized and non-randomized studies, that have demonstrated benefits similar to the current study. The finding that HaH patients reported worse pain control than did patients hospitalized in the inpatient setting, where staff is available 24 hours a day, may suggest differences in care that is feasible at home versus in the inpatient setting. Finally, because it is a bundled program that includes both HaH and a post-discharge care transition program, it is unclear if the effects found in this evaluation can be attributed to specific components within the bundled program.
Applications for Clinical Practice
Patients, particularly older adults, may prefer to have hospital-level care delivered at home; clinicians may consider how HaH may allow patients to avoid potential hazards of hospitalization,5 such as inpatient falls, delirium, and other iatrogenic events. The HaH program is feasible and safe, and is associated with improved outcomes of care for patients.
—William W. Hung, MD, MPH
1. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
2. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital at home”. Med J Aust. 2012;197:512-519.
3. Mader SL, Medcraft MC, Joseph C, et al. Program at home: a Veteran Affairs healthcare program to deliver hospital care in the home. J Am Geriatr Soc. 2008;56: 2317-2322.
4. Montalto M. The 500-bed hospital that isn’t there: the Victorian Department of Health Review of the hospital in the home program. Med J Aust. 2010;193:598-601.
5. Creditor MC. Hazards of hospitalization. Ann Intern Med. 1993;118:219-223.
1. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
2. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital at home”. Med J Aust. 2012;197:512-519.
3. Mader SL, Medcraft MC, Joseph C, et al. Program at home: a Veteran Affairs healthcare program to deliver hospital care in the home. J Am Geriatr Soc. 2008;56: 2317-2322.
4. Montalto M. The 500-bed hospital that isn’t there: the Victorian Department of Health Review of the hospital in the home program. Med J Aust. 2010;193:598-601.
5. Creditor MC. Hazards of hospitalization. Ann Intern Med. 1993;118:219-223.
Three commonly used quick cognitive assessments often yield flawed results
a retrospective analysis has concluded.
The likelihood of a false-positive or false-negative result declined sharply when all three tests were given, however; only about 2% of patients were misclassified in all three, David Llewellyn, PhD, and his colleagues reported in Neurology: Clinical Practice.
The Mini Mental State Examination (MMSE), Memory Impairment Screen (MIS), and animal naming (AN) were susceptible to different measurement biases, wrote Dr. Llewellyn of the University of Exeter (U.K.).
Just one variable – an informant’s perception of the patient’s memory as unimpaired – consistently predicted inaccuracy in all three tests. Most of the patients in this category carried the diagnosis of cognitively impaired but not demented (CIND), a finding that has important clinical implications.
“These participants may be in the very early stages of conversion to dementia. ... Therefore, of those with low or borderline cognitive assessment results, reassessment to detect further decline may be appropriate.”
The study comprised 824 patients included in the Aging, Demographics and Memory Study, which is a subsample of the Health and Retirement Study. They completed the tests from 2001-2004, during which time they were a mean of 82 years old. A panel of experts adjudicated diagnoses, which they then parsed into all-cause dementia, CIND, or cognitively normal. The testing included a self and informant assessment of memory decline. The investigators also looked at 22 predictors of cognition, including patient characteristics, apolipoprotein E carriage (ApoE e4), and sociodemographic factors.
The prevalence of dementia was 35.3%; of the nondemented patients, 43% met the criteria for CIND. The team found that 35.7% of cases were misclassified by at least one test, 13.4% by two, and 1.7% by all three.
The MMSE was the least accurate, with a 21% misclassification rate, reflected in an 18.6% false-positive rate for those without dementia and a 2.4% rate of false-negative for those with dementia.
The MIS had a 16% misclassification rate, with a 9.5% rate of false-positive for those with no dementia and a 6.3% rate of false-negative for those without.
The AN had a 14% misclassification rate, with a 6.8% false-positive rate for those without dementia and a 7.7% false-negative rate for those with dementia.
For the MMSE, MIS, and AN, the number of participants with false-positives that met the criteria for CIND were 74.5%, 82.1%, and 82.1%, respectively.
In the final multivariate model, seven variables predicted misclassification, including black ethnicity for the MMSE; age, visual impairment, ApoeE4 noncarrier, and depression for the MIS; and no hyperlipidemia and normal informant memory assessment for the AN. Lower years of education and heart problems predicted misclassification on both the MMSE and AN.
An absence of informant-related poor memory predicted misclassification on all three tests.
“Failing to detect dementia can delay access to treatment and support, whereas false alarms lead to unnecessary investigations, causing pressure on health care systems,” Dr. Llewellyn said in a press statement. “Identifying people with dementia in a timely fashion is important, particularly as new methods of treatment come onstream. Our findings show that we desperately need more accurate and less biased ways of detecting dementia swiftly in clinic.”
The study was supported by the Halpin Trust, the Mary Kinross Charitable Trust, the Engineering and Physical Sciences Research Council, and the U.K. National Institute for Health Research. None of the authors reported any financial conflicts relevant to the work.
SOURCE: Llewellyn D et al. Neuro Clin Pract. 2019;1:1-9.
a retrospective analysis has concluded.
The likelihood of a false-positive or false-negative result declined sharply when all three tests were given, however; only about 2% of patients were misclassified in all three, David Llewellyn, PhD, and his colleagues reported in Neurology: Clinical Practice.
The Mini Mental State Examination (MMSE), Memory Impairment Screen (MIS), and animal naming (AN) were susceptible to different measurement biases, wrote Dr. Llewellyn of the University of Exeter (U.K.).
Just one variable – an informant’s perception of the patient’s memory as unimpaired – consistently predicted inaccuracy in all three tests. Most of the patients in this category carried the diagnosis of cognitively impaired but not demented (CIND), a finding that has important clinical implications.
“These participants may be in the very early stages of conversion to dementia. ... Therefore, of those with low or borderline cognitive assessment results, reassessment to detect further decline may be appropriate.”
The study comprised 824 patients included in the Aging, Demographics and Memory Study, which is a subsample of the Health and Retirement Study. They completed the tests from 2001-2004, during which time they were a mean of 82 years old. A panel of experts adjudicated diagnoses, which they then parsed into all-cause dementia, CIND, or cognitively normal. The testing included a self and informant assessment of memory decline. The investigators also looked at 22 predictors of cognition, including patient characteristics, apolipoprotein E carriage (ApoE e4), and sociodemographic factors.
The prevalence of dementia was 35.3%; of the nondemented patients, 43% met the criteria for CIND. The team found that 35.7% of cases were misclassified by at least one test, 13.4% by two, and 1.7% by all three.
The MMSE was the least accurate, with a 21% misclassification rate, reflected in an 18.6% false-positive rate for those without dementia and a 2.4% rate of false-negative for those with dementia.
The MIS had a 16% misclassification rate, with a 9.5% rate of false-positive for those with no dementia and a 6.3% rate of false-negative for those without.
The AN had a 14% misclassification rate, with a 6.8% false-positive rate for those without dementia and a 7.7% false-negative rate for those with dementia.
For the MMSE, MIS, and AN, the number of participants with false-positives that met the criteria for CIND were 74.5%, 82.1%, and 82.1%, respectively.
In the final multivariate model, seven variables predicted misclassification, including black ethnicity for the MMSE; age, visual impairment, ApoeE4 noncarrier, and depression for the MIS; and no hyperlipidemia and normal informant memory assessment for the AN. Lower years of education and heart problems predicted misclassification on both the MMSE and AN.
An absence of informant-related poor memory predicted misclassification on all three tests.
“Failing to detect dementia can delay access to treatment and support, whereas false alarms lead to unnecessary investigations, causing pressure on health care systems,” Dr. Llewellyn said in a press statement. “Identifying people with dementia in a timely fashion is important, particularly as new methods of treatment come onstream. Our findings show that we desperately need more accurate and less biased ways of detecting dementia swiftly in clinic.”
The study was supported by the Halpin Trust, the Mary Kinross Charitable Trust, the Engineering and Physical Sciences Research Council, and the U.K. National Institute for Health Research. None of the authors reported any financial conflicts relevant to the work.
SOURCE: Llewellyn D et al. Neuro Clin Pract. 2019;1:1-9.
a retrospective analysis has concluded.
The likelihood of a false-positive or false-negative result declined sharply when all three tests were given, however; only about 2% of patients were misclassified in all three, David Llewellyn, PhD, and his colleagues reported in Neurology: Clinical Practice.
The Mini Mental State Examination (MMSE), Memory Impairment Screen (MIS), and animal naming (AN) were susceptible to different measurement biases, wrote Dr. Llewellyn of the University of Exeter (U.K.).
Just one variable – an informant’s perception of the patient’s memory as unimpaired – consistently predicted inaccuracy in all three tests. Most of the patients in this category carried the diagnosis of cognitively impaired but not demented (CIND), a finding that has important clinical implications.
“These participants may be in the very early stages of conversion to dementia. ... Therefore, of those with low or borderline cognitive assessment results, reassessment to detect further decline may be appropriate.”
The study comprised 824 patients included in the Aging, Demographics and Memory Study, which is a subsample of the Health and Retirement Study. They completed the tests from 2001-2004, during which time they were a mean of 82 years old. A panel of experts adjudicated diagnoses, which they then parsed into all-cause dementia, CIND, or cognitively normal. The testing included a self and informant assessment of memory decline. The investigators also looked at 22 predictors of cognition, including patient characteristics, apolipoprotein E carriage (ApoE e4), and sociodemographic factors.
The prevalence of dementia was 35.3%; of the nondemented patients, 43% met the criteria for CIND. The team found that 35.7% of cases were misclassified by at least one test, 13.4% by two, and 1.7% by all three.
The MMSE was the least accurate, with a 21% misclassification rate, reflected in an 18.6% false-positive rate for those without dementia and a 2.4% rate of false-negative for those with dementia.
The MIS had a 16% misclassification rate, with a 9.5% rate of false-positive for those with no dementia and a 6.3% rate of false-negative for those without.
The AN had a 14% misclassification rate, with a 6.8% false-positive rate for those without dementia and a 7.7% false-negative rate for those with dementia.
For the MMSE, MIS, and AN, the number of participants with false-positives that met the criteria for CIND were 74.5%, 82.1%, and 82.1%, respectively.
In the final multivariate model, seven variables predicted misclassification, including black ethnicity for the MMSE; age, visual impairment, ApoeE4 noncarrier, and depression for the MIS; and no hyperlipidemia and normal informant memory assessment for the AN. Lower years of education and heart problems predicted misclassification on both the MMSE and AN.
An absence of informant-related poor memory predicted misclassification on all three tests.
“Failing to detect dementia can delay access to treatment and support, whereas false alarms lead to unnecessary investigations, causing pressure on health care systems,” Dr. Llewellyn said in a press statement. “Identifying people with dementia in a timely fashion is important, particularly as new methods of treatment come onstream. Our findings show that we desperately need more accurate and less biased ways of detecting dementia swiftly in clinic.”
The study was supported by the Halpin Trust, the Mary Kinross Charitable Trust, the Engineering and Physical Sciences Research Council, and the U.K. National Institute for Health Research. None of the authors reported any financial conflicts relevant to the work.
SOURCE: Llewellyn D et al. Neuro Clin Pract. 2019;1:1-9.
FROM NEUROLOGY: CLINICAL PRACTICE
Key clinical point: Used alone, the MMSE, Memory Impairment Screen, and animal naming tests may not correctly flag patients with memory problems.
Major finding: More than a third of patients received an inaccurate diagnosis from at least one of the tests.
Study details: The retrospective study comprised 824 patients.
Disclosures: The study was supported by the Halpin Trust, the Mary Kinross Charitable Trust, the Engineering and Physical Sciences Research Council, and the U.K. National Institute for Health Research. None of the authors reported any financial conflicts relevant to the work.Source: Llewellyn D et al. Neuro Clin Pract. 2019;9(1):1-9.